
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 167
ACETALDEHYDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Mrs. J. de Fouw, National Institute of Public
Health and Enviromental Protection, Bilthoven, Netherlands
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Acetaldehyde.
(Environmental health criteria ; 167)
1.Acetadehyde - adverse effects 2.Enviromental exposure I.Series
ISBN 92 4 157167 5 (NLM Classification: QU 99)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
Preamble
Introduction
1. SUMMARY
1.1 Identity, physical and chemical properties,
and analytical methods
1.2 Sources of human and environmental exposure
1.3 Environmental transport, distribution, and
transformation
1.4 Environmental levels and human exposure
1.5 Kinetics and metabolism
1.5.1 Absorption, distribution, and elimination
1.5.2 Metabolism
1.5.3 Reaction with other components
1.6 Effects on organisms in the environment
1.6.1 Aquatic organisms
1.6.2 Terrestrial organisms
1.7 Effects on experimental animals and in vitro test
systems
1.7.1 Single exposure
1.7.2 Short- and long-term exposures
1.7.3 Reproduction, embryotoxicity, and
teratogenicity
1.7.4 Mutagenicity and related end-points
1.7.5 Carcinogenicity
1.7.6 Special studies
1.8 Effects on humans
1.9 Evaluation of human health risks and effects on the
environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Anthropogenic sources
3.2.1 Production
3.2.1.1 Production levels and processes
3.2.1.2 Emissions
3.2.2 Uses
3.2.3 Waste disposal
3.2.4 Other sources
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.2 Abiotic degradation
4.3 Biodegradation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water
5.1.3 Soil
5.1.4 Food
5.1.5 Cigarette smoke
5.2 General population exposure
5.3 Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Distribution after inhalation
exposure
6.2.1.2 Distribution to the embryo and
fetus
6.2.1.3 Distribution to the brain
6.2.2 Human studies
6.3 Metabolism
6.3.1 Animal studies
6.3.1.1 Liver
6.3.1.2 Respiratory tract
6.3.1.3 Kidneys
6.3.1.4 Testes and ovaries
6.3.1.5 Embryonic tissue
6.3.1.6 Metabolism during pregnancy
6.3.2 Human studies
6.4 Elimination
6.5 Reaction with cellular macromolecules
6.5.1 Proteins
6.5.2 Nucleic acids
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.2 Terrestrial organisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 LD50 and LC50 values
8.2 Short-term exposure
8.2.1 Oral
8.2.2 Inhalation
8.2.3 Dermal
8.2.4 Parenteral
8.3 Skin and eye irritation; sensitization
8.4 Long-term exposure
8.4.1 Oral
8.4.2 Inhalation
8.5 Reproductive and developmental toxicity
8.6 Mutagenicity and related end-points
8.6.1 Bacteria
8.6.2 Non-mammalian eukaryotic systems
8.6.2.1 Gene mutation assays
8.6.2.2 Chromosome alterations
8.6.3 Cultured mammalian cells
8.6.3.1 Gene mutation assays
8.6.3.2 Chromosome alterations and sister
chromatid exchange
8.6.4 In vivo assays
8.6.4.1 Somatic cells
8.6.4.2 Germ cells
8.6.5 Other assays
8.6.5.1 DNA single-strand breaks
8.6.5.2 DNA cross-linking
8.6.6 Cell transformation
8.7 Carcinogenicity bioassays
8.7.1 Inhalation exposure
8.7.2 Co-carcinogenicity and promotion studies
8.8 Neurological effects
8.9 Immunological effects
8.9.1 Direct effects on immune cells
8.9.2 Generation of antibodies reacting with
acetaldehyde-modified proteins
8.9.3 Related immunological effects
8.10 Biochemical effects
9. EFFECTS ON HUMANS
9.1 General population exposure
9.2 Occupational exposure
9.2.1 General observations
9.2.2 Clinical studies
9.2.3 Epidemiological studies
9.3 Effects of endogenous acetaldehyde
9.3.1 Effects of ethanol possibly attributable to
acetaldehyde or acetaldehyde metabolism
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
10.1.2 Health effects
10.1.3 Approaches to risk assessment
10.2 Evaluation of effects on the environment
11. RECOMMENDATIONS FOR RESEARCH
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an everincreasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC,
JECFA, JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has
organized meetings of scientists to establish lists of priority
chemicals for subsequent evaluation. Such meetings have been held in:
Ispra, Italy, 1980; Oxford, United Kingdom, 1984; Berlin, Germany,
1987; and North Carolina, USA, 1995. The selection of chemicals has
been based on the following criteria: the existence of scientific
evidence that the substance presents a hazard to human health and/or
the environment; the possible use, persistence, accumulation or
degradation of the substance shows that there may be significant human
or environmental exposure; the size and nature of populations at risk
(both human and other species) and risks for environment;
international concern, i.e. the substance is of major interest to
several countries; adequate data on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
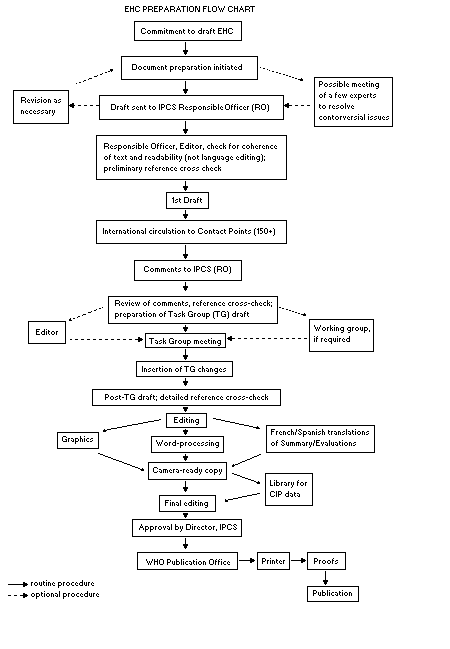
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart on page 13. A designated staff
member of IPCS, responsible for the scientific quality of the
document, serves as Responsible Officer (RO). The IPCS Editor is
responsible for layout and language. The first draft, prepared by
consultants or, more usually, staff from an IPCS Participating
Institution, is based initially on data provided from the
International Register of Potentially Toxic Chemicals, and reference
data bases such as Medline and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
Members
Mrs I. Arts, Department of Biological Toxicology, TNO Nutrition and
Food Research, Zeist, The Netherlands
Dr R.E. Barry, Faculty of Medicine, University of Bristol, Bristol
Royal Infirmary, Bristol, United Kingdom
Professor D. Beritic-Stahuljak, Andrija tampar School of Public
Health, Faculty of Medicine, University of Zagreb, Zagreb, Croatia
Dr Sai Mei Hou, Karolinska Institute, Huddinge, Sweden
Dr M.E. Meek, Environmental Health Directorate, Priority Substances
Section, Health & Welfare Canada, Tunney's Pasture, Ottawa, Canada
(Chairperson)
Professor M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
Professor G. Obe, University of Essen, Essen, Germany
Professor T.V.N. Persaud, Department of Anatomy, Faculty of
Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
Mr D. Renshaw, Department of Health, Elephant & Castle, London, United
Kingdom
Dr A. Smith, Health and Safety Executive, Toxicology Unit, Bootle,
Merseyside, United Kingdom (Co-rapporteur)
Professor A. Watanabe, Toyama Medical and Pharmaceutical University,
Faculty of Medicine, Toyama, Japan
Dr S. Worrall, Department of Biochemistry, University of Queensland,
Brisbane, Queensland, Australia
Representatives from other organizations
Dr V. Krutovskikh, Programme of Multistage Carcinogenesis,
International Agency for Research on Cancer, Lyon, France
Secretariat
Mrs J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
(Co-rapporteur)
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia (Responsible Officer and Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
A WHO Task Group on Environmental Health Criteria for
Acetaldehyde met in Geneva from 6 to 10 December 1993. Professor F.
Valic opened the meeting on behalf of the three cooperating
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to acetaldehyde.
The first draft of this monograph was prepared by Mrs J. de Fouw,
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands.
Professor F. Valic was responsible for the overall scientific
content of the monograph and for the organization of the meeting, and
Mrs M.O. Head of Oxford for the technical editing of the monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
INTRODUCTION
This monograph will deal mainly with the effects of direct
exposure to acetaldehyde. However, it should be borne in mind that
for most people exposure to acetaldehyde will occur through the
consumption of alcoholic beverages (IARC, 1988). These beverages
contain ethanol, which is metabolized to acetaldehyde by alcohol
dehydrogenase (ADH). ADH activity has been detected in nearly every
tissue including liver, kidney, muscle, intestine, ovary, and testes
(Buehler et al., 1983; Agarwal & Goedde, 1990).
However, data concerning metabolically formed acetaldehyde will
only be considered when no data are available on direct exposure.
The accurate determination of acetaldehyde in body fluid and
tissue samples is relatively difficult. Only the most recent
techniques take into account artifactual acetaldehyde formation in
biological samples, especially those containing ethanol (Eriksson &
Fukunaga 1993). As values for concentrations of acetaldehyde given in
older references may well have been overestimates, absolute values are
only given when necessary.
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Acetaldehyde is a colourless, volatile liquid with a pungent
suffocating odour. The reported odour threshold is 0.09 mg/m3.
Acetaldehyde is a highly flammable and reactive compound that is
miscible in water and most common solvents.
Analytical methods are available for the detection of
acetaldehyde in air (including breath) and water. The principal
method is based on the reaction of acetaldehyde with
2,4-dinitrophenylhydrazine and subsequent analysis of the hydrazone
derivatives by high pressure liquid chromatography or gas
chromatography.
1.2 Sources of human and environmental exposure
Acetaldehyde is a metabolic intermediate in humans and higher
plants and a product of alcohol fermentation. It has been identified
in food, beverages, and cigarette smoke. It is also present in
vehicle exhaust and in wastes from various industries. Degradation of
hydrocarbons, sewage, and solid biological wastes produces
acetaldehyde, as well as the open burning and incineration of gas,
fuel oil, and coal.
More than 80% of the acetaldehyde used commercially is produced
by the liquid-phase oxidation of ethylene with a catalytic solution of
palladium and copper chlorides. Production in Japan was 323 thousand
tonnes in 1981. In the USA, production was 281 thousand tonnes in 1982
while in Western Europe it was 706 thousand tonnes in 1983. Most
acetaldehyde produced commercially is used in the production of acetic
acid. It is also used in flavourings and foods.
The annual emission of acetaldehyde from all sources in the USA
is estimated to be 12.2 million kg.
1.3 Environmental transport, distribution, and transformation
Because of its high reactivity, intercompartmental transport of
acetaldehyde is expected to be limited. Some transfer of acetaldehyde
to air from water and soil is expected because of the high vapour
pressure and low sorption coefficient.
It is suggested that the photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Photolysis is
expected to contribute another substantial fraction to the removal
process. Both processes cause a reported daily loss of about 80% of
atmospheric acetaldehyde emissions. Reported half-lives of
acetaldehyde in water and air are 1.9 h and 10-60 h, respectively.
Acetaldehyde is readily biodegradable.
1.4 Environmental levels and human exposure
Levels of acetaldehyde in ambient air generally average
5 µg/m3. Concentrations in water are generally less than
0.1 µg/litre. Analysis of a wide range of foodstuffs in the
Netherlands showed that concentrations, generally less than 1 mg/kg,
occasionally ranged up to several 100 mg/kg, particularly in some
fruit juices and vinegar.
By far, the main source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure.
With respect to other media, the general population is exposed to
acetaldehyde principally from food and beverages, and, to a lesser
extent, from air. The contribution from drinking-water is negligible.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route of exposure is most likely inhalation with possible
dermal contact.
1.5 Kinetics and metabolism
1.5.1 Absorption, distribution, and elimination
Available studies on toxicity indicate that acetaldehyde is
absorbed through the lungs and gastrointestinal tract; however, no
adequate quantitative studies have been identified. Absorption
through the skin is probable.
Following inhalation by rats, acetaldehyde is distributed to the
blood, liver, kidney, spleen, heart, and other muscle tissues. Low
levels were detected in embryos after maternal intraperitoneal (ip)
injection of acetaldehyde (mouse) and following maternal exposure to
ethanol (mouse and rat). Potential production of acetaldehyde has
also been observed in rat fetuses and in the human placenta,
in vitro.
Distribution of acetaldehyde to brain interstitial fluid, but not
to brain cells, has been demonstrated following ip injection of
ethanol. A high affinity, low Km ALDHa may be important in
maintaining low levels of acetaldehyde in the brain during the
metabolism of ethanol.
Acetaldehyde is taken up by red blood cells and, following
ethanol consumption in humans and in baboons, in vivo,
intracellular levels can be 10 times higher than plasma levels.
Following oral administration, virtually no unchanged
acetaldehyde is excreted in the urine.
1.5.2 Metabolism
The major pathway for the metabolism of acetaldehyde is by
oxidation to acetate under the influence of NADb-dependent ALDH.
Acetate enters the citric acid cycle as acetyl-CoA. There are several
isoenzymes of ALDH with different kinetic and binding parameters that
influence acetaldehyde oxidation rates.
ALDH activity has been localized in the respiratory tract
epithelium (excluding olfactory epithelium) in rats, in the renal
cortex and tubules in the dog, rat, guinea-pig, and baboon, and, in
the testes in the mouse.
Acetaldehyde is metabolized by mouse and rat embryonic tissue
in vitro. Acetaldehyde crosses the rat placenta, in spite of
placental metabolism.
Though there is some metabolism of acetaldehyde in human renal
tubules, the liver is the most important metabolic site.
Several isoenzymic forms of ALDH have been identified in the
human liver and other tissues. There is polymorphism for the
mitochondrial ALDH. Subjects who are homozygous or heterozygous for a
point mutation in the mitochondrial ALDH corresponding gene have low
activity of this enzyme, metabolize acetaldehyde slowly, and are
intolerant of ethanol.
The metabolism of acetaldehyde can be inhibited by
crotonaldehyde, dimethylmaleate, phorone, disulfiram, and calcium
carbamide.
a ALDH = acetaldehyde dehydrogenase.
b NAD = nicotinamide adenine dinocleotide.
1.5.3 Reaction with other components
Acetaldehyde forms stable and unstable adducts with proteins.
This can impair protein function, as evidenced by inhibition of enzyme
activity, impaired histone-DNA binding, and inhibition of
polymerization of tubulin.
Unstable adducts of acetaldehyde of undetermined significance
occur in vitro with nucleic acids.
Acetaldehyde can react with various macromolecules in the body,
preferentially those containing lysine residues, which can lead to
marked alterations in the biological function of these molecules.
1.6 Effects on organisms in the environment
1.6.1 Aquatic organisms
LC50s in fish ranged from 35 (guppy) to 140 mg/litre (species
not specified). An EC5 of 82 mg/litre and an EC50 of 42 mg/litre
were reported for algae and Daphnia magna, respectively.
1.6.2 Terrestrial organisms
Acetaldehyde in air appears to be toxic for some microorganisms
at relatively low concentrations.
Aphids were killed when exposed to acetaldehyde at a
concentration of 0.36 µg/m3 for 3 or 4 h.
Median lethal values were 8.91 mg/litre per h and 7.69 mg/litre
per h for the slug species, Arion hortensis and Agriolimax
reticulatus, respectively.
Inhibition of seed germination in the onion, carrot, and tomato
by acetaldehyde (up to 1.52 mg/litre) was reversible, whereas
inhibition of Amaranthus palmeri, similarly exposed, was
irreversible. Acetaldehyde at 0.54 µg/m3 damaged lettuce.
1.7 Effects on experimental animals and in vitro test systems
1.7.1 Single exposure
LD50s in rats and mice and LC50s in rats and Syrian hamsters
showed that the acute toxicity of acetaldehyde is low. Acute dermal
studies were not identified.
1.7.2 Short- and long-term exposures
In repeated dose studies, by both the oral and inhalation routes,
toxic effects at relatively low concentrations were limited
principally to the sites of initial contact. In a 28-day study in
which acetaldehyde at 675 mg/kg body weight (no-observedeffect level
(NOEL): 125 mg/kg body weight) was administered in the drinking-water
to rats, effects were limited to slight focal hyperkeratosis of the
forestomach. Following administration of a single dose level (0.05%
in the drinking-water) for 6 months (estimated by the Task Group to be
approximately 40 mg/kg body weight) in a biochemical study,
acetaldehyde induced synthesis of rat liver collagen, an observation
that was supported by in vitro data.
Following inhalation, NOELs for respiratory effects were
275 mg/m3 in rats exposed for 4 weeks and 700 mg/m3 in hamsters
exposed for 13 weeks. At lowest-observed-effect levels, degenerative
changes were observed in the olfactory epithelium in rats
(437 mg/m3) and the trachea (2400 mg/m3) in hamsters.
Degenerative changes in the respiratory epithelium and larynx were
observed at higher concentrations. No repeated dose dermal studies
were identified.
1.7.3 Reproduction, embryotoxicity, and teratogenicity
In several studies, parenteral exposure of pregnant rats and mice
to acetaldehyde induced fetal malformations. In the majority of these
studies, maternal toxicity was not evaluated. No data on reproductive
toxicity were identified.
1.7.4 Mutagenicity and related end-points
Acetaldehyde is genotoxic in vitro, inducing gene mutations,
clastogenic effects, and sister-chromatid exchanges (SCEs) in
mammalian cells in the absence of exogenous metabolic activation.
However, negative results were reported in adequate tests on
Salmonella. Following intraperitoneal injection, acetaldehyde induced
SCEs in the bone marrow of Chinese hamsters and mice. However,
acetaldehyde administered intraperitoneally did not increase the
frequency of micronuclei in early mouse spermatids. There is indirect
evidence from in vitro and in vivo studies to suggest that
acetaldehyde can induce protein-DNA and DNA-DNA cross-links.
1.7.5 Carcinogenicity
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses). However, in hamsters,
increases in nasal and laryngeal carcinomas were non-significant. All
concentrations of acetaldehyde administered in the studies induced
chronic tissue damage in the respiratory tract.
1.7.6 Special studies
Adequate studies on the potential neuro- and immunotoxicity of
acetaldehyde were not identified.
1.8 Effects on humans
In limited studies on human volunteers, acetaldehyde was mildly
irritating to the eyes and upper respiratory tract following exposure
for very short periods to concentrations exceeding approximately 90
and 240 mg/m3, respectively. Cutaneous erythema was observed in
patch testing with acetaldehyde, in twelve subjects of "Oriental
ancestry".
One limited investigation in which the incidence of cancer was
examined in workers exposed to acetaldehyde and other compounds has
been reported.
On the basis of indirect evidence, acetaldehyde has been
implicated as the putatively toxic metabolite in the induction of
alcohol-associated liver damage, facial flushing, and developmental
effects.
1.9 Evaluation of human health risks and effects on the environment
The acute toxicity of acetaldehyde by the inhalation or oral
route in studies conducted on animals was low. According to studies
on humans and animals, acetaldehyde is mildly irritating to the eyes
and the upper respiratory tract. In limited studies on human
volunteers, acetaldehyde was mildly irritating to the eyes and upper
respiratory tract (section 1.8). Cutaneous erythema has also been
observed in the patch testing of humans. Although a possible mechanism
has been identified, available data are inadequate to assess the
potential of acetaldehyde to induce sensitization.
Available data on the effects of acetaldehyde following ingestion
are limited. Following oral administration of 675 mg/kg body weight
per day to rats, a borderline increase in hyper-keratosis of the
forestomach was observed (NOEL: 125 mg/kg body weight). In rats
exposed to a dose level of approximately 40 mg acetaldehyde/kg body
weight in the drinking-water for 6 months, there was an increase in
collagen synthesis in the liver, the significance of which is unclear.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In available
studies, the lowest concentration at which effects were observed was
437 mg/m3 following administration for 5 weeks. The NOELs
identified for respiratory effects were 275 mg/m3 in rats exposed
for 4 weeks and 700 mg/m3 in hamsters exposed for 13 weeks.
At concentrations that induced tissue damage in the respiratory
tract, increased incidences were observed of nasal adenocarcinomas and
squamous cell carcinomas in the rat and laryngeal and nasal carcinomas
in the hamster.
There is evidence to suggest that acetaldehyde causes genetic
damage to somatic cells in vivo.
Available data are inadequate for the assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in the general, or
occupationally exposed, populations.
On the basis of data on irritancy in humans, a tolerable
concentration of 2 mg/m3 has been derived. Since the mechanism of
induction of tumours by acetaldehyde has not been well studied, two
approaches were adopted for the provision of guidance with respect to
this end-point, i.e., the development of a tolerable concentration
based on division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, and, estimation of lifetime
cancer risk based on linear extrapolation. The tolerable
concentration is 0.3 mg/m3. The concentrations associated with a
10-5 excess lifetime risk are 11-65 µg/m3.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical formula: C2H4O
Chemical structure: CH3-CHO
Common name: acetaldehyde
Common synonyms: ethanal; acetic aldehyde; acetylaldehyde;
ethylaldehyde; diethylacetal;
1,1-diethyoxy ethane
CAS chemical name: acetaldehyde
CAS registry number: 75-07-0
RTECS registry number: AB 1925000
2.2 Physical and chemical properties
The most important physical and chemical properties of
acetaldehyde are given in Table 1.
Acetaldehyde is a volatile liquid with a pungent, suffocating
odour that is fruity in dilute concentrations. The odour threshold for
acetaldehyde is reported to be 0.09 mg/m3 (0.05 ppm). This was a
geometric average of all available literature data (Amoore & Hautala,
1983). In the case of carbon dioxide solutions in acetaldehyde, the
acetaldehyde odour is weakened by the carbon dioxide (Hagemeyer,
1978).
Acetaldehyde is a highly reactive compound that undergoes
numerous condensation, addition, and polymerization reactions. It
decomposes at temperatures above 400°C, forming principally methane
and carbon monoxide. Acetaldehyde is highly flammable when exposed to
heat or flame, and, in air, it can be explosive. Acetaldehyde can
react violently with acid anhydrides, alcohols, ketones, phenols,
NH3, HCN, H2S, P, halogens, isocyanates, strong alkalies, and
amines. It is miscible in all proportions with water and the most
common organic solvents. In aqueous solutions, acetaldehyde exists in
equilibrium with the hydrate, CH3 CH(OH)2. The enol form, vinyl
alcohol (CH2=CHOH) exists in equilibrium with acetaldehyde to the
extent of approximately one molecule per 30 000 (Hagemeyer, 1978).
Table 1. Physical and chemical properties of acetaldehydea
Colour colourless
Relative molecular mass 44.1
Boiling point at 101.3 kPa 20.2°C
Melting point -123.5°C
Octanol/water partition coefficient as 0.63
log Pow
Flash point, closed cup -38°C
Autoignition temperature 185-193°C
Explosion limits of mixtures with air 4.5-60.5 vol % acetaldehyde
Vapour pressure at -50°C 2.5 kPa
0°C 44.0 kPa
20.16°C 101.3 kPa
Specific gravity (20/4) 0.778
Relative vapour density 1.52
Refractive index 20/D 1.33113
Dissociation constant at 0°C, Ka 0.7 × 10-14
Solubility miscible in water and most
common solvents
a From: Hagemeyer (1978); IPCS/CEC (1990).
Commercial acetaldehyde should have the following typical
specifications: purity, 99% min; acidity (as acetic acid), 0.1% max,
and a specific gravity of 0.804-0.811 (0°/20°C) (US NRC, 1981).
2.3 Conversion factors
1 mg acetaldehyde/m3 air = 0.56 ppm at 25°C and 101.3 kPa
(760 mmHg).
1 ppm = 1.8 mg acetaldehyde/m3 air.
2.4 Analytical methods
Several analytical procedures used for the sampling and
determination of acetaldehyde in various media are summarized in
Table 2.
Table 2. Sampling, preparation, and determination of acetaldehydea
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in a midget HPLC with 18 µg/m3 20 litre designed for analysis of Lipari & Swarin
impinger containing 2,4-DNPH spectrophotometric automobile exhaust (1982)
in acetonitrile with detection
perchloric acid as catalyst
Air collection in a tube containing HPLC with 0.9 µg/m3 2 litre suitable for analysis of Jarke et al.
a thermal stable organic polymer spectrophotometric indoor and outdoor air (1981)
based on 2,6-diphenyl-p- detection
phenylene oxide
Air adsorption on a silica gel GC-FTD 0.09-0.45 50-100 suitable for analysis of Aoyama & Yashiro
treated with 2,4-DNPH µg/m3 litre smog and automobile (1983)
exhaust
Air collection in a 2,4-DNPH HPLC with < 18 µg/m3 < 20 litre suitable for long-term Tejada (1986)
coated Sep-PAK cartridge, spectrophotometric sampling at low µg/m3
acidified with HCl detection (ppb) levels in ambient
air, or, for short-term
sampling at low mg/m3
(ppm) levels in diluted
automotive exhaust
emissions
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in annular denuders HPLC with UV 0.36 µg/m3 100 litre suitable for analysis of Possanzini et
coated with 2,4-DNPH absorbance or outdoor and indoor al. (1987)
detection voltametric
pollution
Air collection and derivatization HPLC with UV 90 µg/m3 5 litre suitable for personal Binding et al.
on 2,4-DNPH coated detection monitoring of 5-min, (1986)
Chromosorb P short-term values as
well as for continuous
sampling over a whole
work shift
Air collection on DNPH-coated C18 HPLC with UV 12 ng per suitable for ambient Grosjean (1991)
cartridge detection cartridge monitoring
Air collection and derivatization HPLC with UV 32 mg/m3b 60 litre suitable for short-term US NIOSH (1987)
in midget bubblers containing detection exposure sampling;
Girard T solution interference with other
aldehydes and volatile
ketones should be
considered
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in a Chromosorb 104 GC-FID 0.1 µg/litre 1.5 litre suitable for monitoring Watanabe (1988)
tube installed in an automated of outdoor and indoor
sampler pollution
Air collection on a XAD-2 sorbent GC-FID 1.3 mg/m3c 3 litre suitable for short-term US NIOSH (1989)
coated with 2-(hydroxymethyl)- exposure sampling and for
piperidine analysis of field samples
Water derivatization in a two-phase HPLC with 21 µg -- designed for analysis of Facchini et
system by addition of 2,4-DNPH electrochemical fog and rain water al. (1986)
and isooctane detection
Water purging with nitrogen gas and sweeping by rapid 200 µg/ 5 ml suitable for analysis of Spingarn et
collection on a Tenax GC heating of trap litre aqueous solution and al. (1982)
sorbent and silica gel trap into GC-MS industrial effluent
Water derivatization with 2,4-DNPH HPLC; the reaction < 10 µg per 1 ml suitable for routinely Steinberg &
(in acetonitrile) mixture is analysed sample monitoring rain, fog, Kaplan (1984)
directly, without and mist samples
prior separation of
the DNPH-derivatives
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Water collection in a PTFE-cartridge HPLC with UV 0.3 µg/litre 500 ml designed for analysis of Takami et
packed with sulfonated cation detection water samples at the low al. (1985)
exchange resin charged with µg/litre levels
2,4-DNPH
Water collection of aqueous solution HS-GC-FID 25 µg/litre 10 ml designed for the Gramiccioni
in vials, no special treatments quantification of et al. (1986)
released from plastics acetaldehyde
into aqueous foods
Water collection on cyanogen bromide spectrophotometric 0.6 mg/ 30 µlitre immobilized aldehyde Almuaibed &
activated Sepharose 4B detection litre dehydrogenase makes the Townshend (1987)
containing aldehyde determination more
dehydrogenase; soluble aldehyde economic and simpler
dehydrogenase injected in the
sampler flow stream using a
double injection technique
Beverage collection of the 2,6- HPLC with 0.01 µg per 15 ml designed for analysis of Okamoto et
dimethylpyridine derivative spectrophotometric sample wine al. (1981)
on a 3-aminopropyl- detection
triethoxysiloxane or a
Nucleosil 5NH2 treated silica
gel with propionaldehyde as
internal standard
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Beverage steam distillation followed by HPLC with UV ± 5 µg/ 500 ml designed for analysis of Piendl et
liquid liquid extraction, detection litre beer al. (1981)
derivatization with p-nitrobenzyl-
oxyamine-hydrochloride with T-2
undecenal as internal standard
Beverage conversion of acetaldehyde HS-GC-FID ± 1 mg/ 5 ml designed as a rapid Jones et al.
acetals and bisulfite addition litre means by which the (1986)
products to free acetaldehyde by acetaldehyde production
a series of 1-min acid, base, and consumption
and iodine treatments followed pattern of different
by a 10-min equilibration period wines can be predicted
Breast collection of volatile compounds thermal -- 60 ml designed for Pellizari et
milk on a Tenax cartridge after desorption determination in breast al. (1982)
warming milk and purging with into GC-MS milk
helium
Blood precipitation of protein with GC headspace 4.4 µg per - designed for analysis of Eriksson et
perchloric acid analysis sample blood in order to reduce al. (1982)
artificial formation
of acetaldehyde
Blood derivatization with 2,4-DNPH HPLC with UV 4.4 ng per 2 ml designed for analysis of Pezzoli et
with butyraldehyde as internal detection sample blood al. (1984)
standard and perchloric acid
(for protein precipitation)
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Blood rapid separation plasma: HPLC with > 0.9 ng 0.5 ml designed for analysis of Di Padova et
plasma: deproteinization and spectrophotometric per sample plasma plasma and red blood al. (1986)
derivatization with 2,4-DNPH detection cells
haemolysate: deproteinization haemolysate:
and mixed with semicarbazide HS-GC-FID
hydrochloride
Blood separation of plasma and HPLC with 11 µg per 1 ml suitable for clinical Peterson &
haemolysate plasma: fluorescence sample plasma use Polizzi (1987)
1,3-cyclo-hexanedione and detection or RBC
isooctane haemolysate: haemolysate
1,3-cyclo-hexadione both in
presence of ammonium ion
Blood reaction with 1,3-cyclo-hexanone HPLC with 4.4 µg per 50-100 designed for microassays Ung-Chhun &
in the presence of ammonium ion fluorescence sample µlitre with negligible Collins (1987)
propionaldehyde used as internal detection interference
standard
Blood collection in an organic solution HPLC with > 0.13 µg 1 ml designed for analysis of Rideout et
of 2-diphenylacetyl- spectrofluometric per sample blood; with minor al. (1986)
1,3-indandione-1-hydrazone, and detection modifications also
forming fluorescent azine suitablefor analysis of
derivative-precipitation of beverages, breath, and
proteins tissue
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Blood reaction with methanolic solution HPLC; the 4.4 µg/litre 1 ml blood suitable for assessment Lynch et
and of 2,4-DNPH, with acetaldehyde adduct 1 g tissue of acetaldehyde levels in al. (1983)
tissue dinitrophenyl-[14C]-formaldehyde was identified by clinical and experimental
as internal standard co-chromatography studies of ethanol
with the authentic metabolism and alcoholic
derivative and by beverages
mass spectrometry
a 2,4-DNPH = 2,4-dinitrophenylhydrazine; HPLC = high pressure liquid chromatography; GC-FID = gas chromatography with flame ionization
detection; GC-FTD = gas chromatography with flame thermionic detection; GC-MS = gas chromatography with mass spectrometric detection;
HS-GC-FID = head space gas chromatography with flame ionization detection.
b minimum working range (estimated LOD: 1.6 mg/m3).
c minimum working range (estimated LOD: 0.67 mg/m3).
The most specific and sensitive analytical method, widely used to
date, is based on the reaction of acetaldehyde with
2,4-dinitro-phenylhydrazine (2,4-DNPH) and the subsequent analysis of
the hydrazone derivatives by high pressure liquid chromatography
(HPLC) or gas chromatography (GC). Methods mentioned by US NIOSH are
based on derivatization with Girard T solution followed by HPLC
analysis with UV detection (US NIOSH, 1987), or, on derivatization
with 2-(hydroxymethyl)piperidine followed by GC analysis with a flame
ionization detector (FID) (US NIOSH, 1989). In the method based on
Girard T derivation, other volatile aldehydes compete for the Girard T
reagent. Chromatographic conditions may be adjusted to resolve
acetaldehyde from other aldehydes.
Spingarn et al. (1982) determined volatile organic compounds in
aqueous solutions, including acetaldehyde, using a technique in which
the compounds were purged from the solution by bubbling with an inert
gas into a trap containing a Tenax sorbent and silica gel. The
analytes were separated by GC and detected with either specific
ionization detection or MS. An improvement in detection limits,
compared with those of the widely used spectrophotometric method of
analysing carbonyls in aqueous solution, was obtained by Facchini et
al. (1986) by means of an electrochemical detector.
In the determination of acetaldehyde in blood, two main
difficulties exist. The first is related to its disappearance from
blood prior to measurement and the second is related to the formation
of acetaldehyde in blood after collection. According to Pezzoli et
al. (1984), the addition of butyraldehyde to blood, as an internal
standard, immediately after withdrawal, obviates some of the
inconveniences in the determination of acetaldehyde in blood. The
addition of butyric acid makes it possible to obtain results both for
the interaction of the aldehyde group of the acetaldehyde with amino
groups, and for the formation and extraction of the derivative
compound. However, Di Padova et al. (1986) stated that the addition
of butyraldehyde was not specific for the determination of the
acetaldehyde but was related to the aldehyde group reactivity.
Therefore, they described an improved procedure for measuring
acetaldehyde in plasma, based on rapid separation, 2,4-DNPH
derivatization, and HPLC analysis, and a procedure for measuring
acetaldehyde in red blood cells, based on the use of a semicarbazide
solution and analysis by head space gas chromatography.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acetaldehyde is a metabolic intermediate in humans and higher
plants and it is a product of alcohol fermentation (IARC, 1985). It
has been identified as a volatile component of mature cotton leaves
and cotton blossoms (Berni & Stanley, 1982) and as a component in the
essential oil of alfalfa at a concentration of about 0.2% (Kami,
1983). It occurs in food, various fruits, and several spices (see
section 5.1.4) and in oak and tobacco leaves (Furia & Bellanca, 1975;
US NRC, 1985).
Acetaldehyde is formed in the atmosphere in a variety of ways.
It is generated by the oxidation of non-methane hydrocarbons both in
the background troposphere and in photochemical smog (Grosjean, 1982).
3.2 Anthropogenic sources
3.2.1 Production
3.2.1.1 Production levels and processes
Until 1968, most acetaldehyde produced in the USA was made by the
partial oxidation of ethanol over a silver catalyst; however,
currently less than 5% of US production is based on this process. The
liquid-phase oxidation of ethylene using a catalytic solution of
palladium and copper chlorides was first used commercially in the USA
in 1960 and more than 80% of the world production of acetaldehyde is
made by this process. The remainder is produced by the oxidation of
ethanol and the hydration of acetylene. Acetaldehyde is produced by a
limited number of companies over the world. The total production of
acetaldehyde in the USA in 1982 amounted to 281 thousand tonnes.
Total acetaldehyde production in Western Europe in 1982 was 706
thousand tonnes, and the production capacity was estimated to have
been nearly 1 million tonnes. In Japan, the estimated production in
1981 was 323 thousand tonnes (Hagemeyer, 1978; IARC, 1985).
3.2.1.2 Emissions
Eimutis et al. (1978) estimated that the annual atmospheric
emissions of acetaldehyde in the USA amounted to 12.2 thousand tonnes
(Table 3). Emissions of acetaldehyde in the Netherlands in the year
1980 were reported to be 584 tonnes (Guicherit & Schulting, 1985).
Table 3. Emission and sources of acetaldehyde in the USA
Source Emissions
(tonnes/year)
Residential external combustion of wood 5056.4
Coffee roasting 4411.4
Acetic acid manufacture 1460.9
Vinyl acetate manufacture from ethylene 1094.6
Ethanol manufacture 57.8
Acrylonitrile manufacture 51.6
Acetic acid manufacture from butane 20.8
Crotonaldehyde manufacture 4.5
Acetone and phenol manufacture from cumene 1.9
Acetaldehyde manufacture by hydration of ethylene 0.5
Polyvinyl chloride manufacture 0.2
Acetaldehyde manufacture by oxidation of ethanol 0.1
3.2.2 Uses
Acetaldehyde is an important intermediate in the production of
acetic acid, ethyl acetate, peracetic acid, pentaerythritol, chloral,
glyoxal, alkylamines, and pyridines (Hagemeyer, 1978). The use
pattern for the estimated 281 thousand tonnes of acetaldehyde produced
in the USA in 1982 was as follows: acetic acid 61%, pyridine and
pyrine bases 9%, peracetic acid 8%, pentaerythritol 7%, 1,3-butylene
glycol 2%, chloral 1%, and other applications (including use as a food
additive and exports) 12%. The use pattern for the estimated 706
thousand tonnes of acetaldehyde produced in Western Europe was as
follows: acetic acid 62%, ethyl acetate 19%, pentaerythritol 5%,
synthetic pyridines 3%, and all other uses 11% (IARC, 1985).
Acetaldehyde is used for the flavourings: berry, butter,
chocolate, apple, apricot, banana, grape, peach, black walnut, and
rum, and it is used in the following foods: beverages, ice cream and
ices, candy, baked goods, gelatin desserts, and chewing gum (Furia &
Bellanca, 1975; US NRC, 1981, 1985). Acetaldehyde is also used in
perfumes, aniline dyes, plastics, in the manufacture of synthetic
rubber, in the silvering of mirrors, in the hardening of gelatin
fibres, and in the laboratory (Verschueren, 1983).
3.2.3 Waste disposal
Degradation of hydrocarbons, sewage, and solid biological wastes
produces acetaldehyde. It has been detected in effluents from
sewage-treatment plants and chemical plants (US EPA, 1975; Shackelford
& Keith, 1976).
Acetaldehyde has been identified as a constituent in the wastes
from petroleum refining, coal processing, the oxidation of alcohols,
saturated hydrocarbons, or ethylene, and the hydration of acetylene
(IARC, 1985).
3.2.4 Other sources
Acetaldehyde is detected as a combustion product of plastics and
polycarbonate and polyurethane foams of western European origin
(Hagen, 1967; Boettner et al., 1973).
Acetaldehyde occurs in vehicle exhaust at levels of
1.4-8.8 mg/m3 in gasoline exhaust, about 5.8 mg/m3 in diesel
exhaust (Verschueren, 1983), and 51.6% acetaldehyde/ n-hexane GC peak
area ratio in exhaust gas oxygenates (Hugues & Hum, 1960). It also
occurs in the open burning and incineration of gas, fuel oil, and
coal, and evaporation products of perfumes (Verschueren, 1983).
Acetaldehyde has been identified in fresh tobacco leaves and in
tobacco smoke (concentrations ranging from 2.1 to 4.6 mg/litre smoke)
(Buyske et al., 1956; Osborne et al., 1956; Mold & McRae, 1957).
When Lipari et al. (1984) measured aldehyde emissions from
wood-burning fireplaces, they ranged from 0.08 to 0.20 g/kg of wood
burned, based on tests with cedar, jack pine, red oak, and green ash.
Acetaldehyde emissions from wood-burning furnaces and
stoves were also measured in a Swedish study (Rudling et al., 1981)
and in a Norwegian study (Ramdahl et al., 1982). In the Swedish
study, the emissions ranged from 1-72 mg/kg wood in prechamber ovens
to 9-710 mg/kg wood in fireplace stoves. In the Norwegian study, the
reported emissions from stoves were 14.4 mg/kg dry wood under normal
burning conditions and up to 992 mg/kg dry wood under low-efficiency
combustion.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Acetaldehyde can enter the atmosphere during production of the
compound itself, as a product of incomplete combustion, and also as a
by-product of fermentation (Grosjean, 1982).
Photochemical oxidation of acetaldehyde has been shown to be an
important process in the chemistry of photochemical smog (Bagnall &
Sidebottom, 1984; Leone & Seinfeld, 1984). Present theories ascribe
the importance of acetaldehyde to its being a precursor of
peroxyacetylnitrate (PAN) in polluted atmospheres (Kopczynski et al.,
1974; Grosjean et al., 1983; Bagnall & Sidebottom, 1984; Moortgat &
McQuigg, 1984). Acetaldehyde is likely to be a precursor of acetic
acid, which is a component of natural precipitation and contributes to
its acidity (Moortgat & McQuigg, 1984).
Intercompartmental transport of acetaldehyde is expected to be
limited, because of its high reactivity. However, because of the high
vapour pressure of acetaldehyde, some transfer to air from water and
soil can be expected.
The tendency of acetaldehyde to adsorb on soil particles can be
expressed in terms of Koc, the ratio of the amount of chemical
adsorbed per unit weight of organic carbon to the concentration of the
chemical in solution at equilibrium. On the basis of the available
empirical relationships derived for estimating Koc, a low soil
adsorption potential is expected (Lyman et al., 1982). Koch & Nagel
(1988) calculated a soil sorption coefficient of 0.90 for
acetaldehyde, and, therefore, acetaldehyde was classified as a
compound with a very low sorption tendency.
4.2 Abiotic degradation
It is suggested that photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Singh et al.
(1982) reported that photolysis and reaction with hydroxyl radicals
cause a daily loss rate of about 80% of atmospheric acetaldehyde
emissions. Grosjean et al. (1983) reported that the reaction with
hydroxyl radicals could remove 50-300 tonnes of carbonyls from the Los
Angeles air over a 12-h daytime period and, thus, is considered to be
a major removal process for all aldehydes. The absolute rate constant
for the reaction of the hydroxyl radical with acetaldehyde was
determined over a temperature range of 26-153°C by Atkinson & Pitts
(1978). At 26°C, they obtained a rate constant of (1.60 ± 0.16) ×
10-11 cm3 per molecule per second. This results in a half-life
for acetaldehyde of 10 h, using a 12-h daytime average hydroxyl
radical concentration of 2 × 10-15 mol/litre (Lyman et al., 1982).
Hustert & Parlar (1981) reported that 49.5% acetaldehyde was
photochemically degraded (reaction with hydroxyl radicals) after a 2-h
radiation (lambda > 230 nm) at 25°C, which, contrary to Atkinson &
Pitts (1978), shows a half-life of 2 h. Atkinson et al. (1984)
obtained a rate constant of (1.34 ± 0.28) × 10-15 for the gas-phase
reaction of nitrate radicals with acetaldehyde at 25°C. This results
in a half-life for acetaldehyde of 59.6 h using a 12-h nighttime
average nitrate radical concentration of 4.0 × 10-12 mol/litre
(Atkinson et al., 1987).
There is a considerable amount of evidence that acetaldehyde in
aqueous solution is in equilibrium with its hydrated form
CH3CH(OH)2. The degree of hydration decreases with increasing
temperature (e.g., at 0°C, the fraction of acetaldehyde hydrated is
0.73; at 25°C, it is 0.59) (Bell & Clunie, 1952).
Von Burg & Stout (1991) reported a half-life of 1.9 h for
acetaldehyde in river water; no other details were provided.
4.3 Biodegradation
Several studies have revealed significant degradation of
acetaldehyde by mixed cultures obtained from sludges and settled
sewage. Hatfield (1957) reported the ability of acclimatized sludge
to oxidize acetaldehyde (major portion of the biological and chemical
oxygen demand (BOD and COD) removed within a 4-h aeration period).
Ludzack & Ettinger (1960) determined the BOD for acetaldehyde in
activated sludge at 20°C and found that 93% of the acetaldehyde was
removed after an observation period of 1/3-5 days and an
acclimatization period of 30 days. Thom & Agg (1975) and Speece
(1983) also reported that acetaldehyde was easily biodegradable by
biological sewage treatment (additional information was not provided).
However, Gerhold & Malaney (1966) reported little degradation of
acetaldehyde by unacclimatized municipal sludge with a BOD of 27.6% as
a percentage of the theoretical oxygen demand in 24 h.
Acetaldehyde is also degraded by anaerobic biological treatment
with unacclimatized acetate-enriched cultures. A COD-removal of 97%
was obtained at the end of a 90-day acclimatization period in
completely mixed reactors with a 20-day hydraulic retention time, no
solids recycle, and a final daily feed concentration of
10 000 mg/litre (Chou & Speece, 1978).
Acetaldehyde is reported to be readily biodegradable using the
biodegradability MITI test, defined in OECD Guidelines for testing of
chemicals (OECD, 1992).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The concentrations of acetaldehyde in uncontaminated Arctic air
masses, determined over a 24-h period, ranged from not detected to
0.54 µg/m3 (Cavanagh et al., 1969).
In samples collected during April 1981, the levels of
acetaldehyde in the air in Pittsburg (PA) and Chicago (Il) were
0.36-4.68 µg/m3 and 1.62-6.12 µg/m3, respectively (Singh et al.,
1982). In samples collected at 7 other locations in the USA between
1975 and 1978, mean concentrations in ambient air were 5-124 µg/m3
(Brodzinsky & Singh, 1982).
Schulam et al.(1985) determined the levels of acetaldehyde in air
(June-August 1983) in the urban location of Schenectady (NY) and the
rural location of Whiteface Mountain (NY). Concentrations of
acetaldehyde were similar in the two locations (the levels of
acetaldehyde varied from 0.36 to 1.44 µg/m3, detection limit:
0.29 µg/m3).
The average ambient atmospheric level of acetaldehyde, measured
during the four seasons at Brookhaven National Laboratory (Upton, Long
Island, NY) from July 1982 to May 1983, was 5.2 µg/m3, with a mean
minimum concentration in winter of 1.8 µg/m3 and a mean maximum
value in summer of 15.1 µg/m3 (Tanner & Meng, 1984). Concentrations
of acetaldehyde in the air in Tulsa, OK (sampled in July 1978), Rio
Blanco County, CO (sampled in July 1978), and the Great Smoky
Mountains, TN (sampled in September 1978), ranged up to 14.9, 16.9,
and 23.9 µg/m3, respectively (Arntz & Meeks, 1981).
Mean concentrations of acetaldehyde in the air in Tokyo during
four seasons in 1985-86 ranged from 2.2 to 7.3 µg/m3 (Watanabe,
1987). Seasonal trends were not noted. Concentrations of
acetaldehyde in an environmental survey conducted by the Japan
Environment Agency in 1987 ranged from 0.9 to 22 µg/m3 (number of
sites sampled unspecified) (Japan Environment Agency, 1989).
The mean concentration of acetaldehyde was 2 µg/m3 at three
locations in the Netherlands, namely, the island of Terschelling (one
of the least polluted areas of the country), Delft (suburban), and
Vlaardingen (heavily industrialized area) (Guicherit & Schulting,
1985).
Grosjean (1991) reported levels of acetaldehyde in ambient air,
sampled every sixth day over a one-year period, at six locations in
Southern California between September 1988 and September 1989.
Concentrations ranged up to 23.3 µg/m3 (13 ppb) with average values
at the various locations ranging from 5.2 to 8.6 µg/m3
(2.9-4.8 ppb).
The mean concentration of acetaldehyde in fog samples taken in
November, 1985 in the Po Valley (Italy) was 21 µg/litre (Facchini et
al., 1986). At urban locations in California (Los Angeles) and Alaska
(Fairbanks), concentrations of acetaldehyde ranged from 0.007 to
0.13 µg/ml in ice fog (Alaska), 0 to 0.11 µg/ml in rain (CA), 0 to
0.59 µg/ml in cloud (CA), 0.10 to 0.11 µg/ml in mist (CA), and 0.006
to 0.17 µg/ml in fog (CA) (Grosjean & Wright, 1983).
5.1.2 Water
No quantitative data on concentrations of acetaldehyde in raw
water supplies were identified.
Acetaldehyde has been detected in drinking-water from
Philadelphia and Seattle at levels of up to 0.1 µg/litre (Keith et
al., 1976). No other information was provided.
5.1.3 Soil
Data on concentrations of acetaldehyde in soil were not
identified.
5.1.4 Food
Acetaldehyde has been detected in a wide range of foodstuffs (US
NRC, 1981, 1985; Horvath et al., 1983; Feron et al., 1991), though few
quantitative data are available. In a variety of foodstuffs analysed
in the Netherlands including fruits and juices, vegetables, milk
products, bread, eggs, fish, meat, and alcoholic beverages,
concentrations were generally less than 1 mg/kg, but occasionally
ranged up to several hundred mg/kg, particularly in some fruit juices
and alcoholic beverages; in vinegar, a maximum value of 1060 mg/kg was
reported (Maarse & Visscher, 1992). Acetaldehyde has been identified
in alcoholic beverages, such as beer and wine (Okamoto et al., 1981;
Piendl et al., 1981; Jones et al., 1986); levels in 18 English beers
ranged from 2.6 to 13.5 mg/litre (Delcour et al., 1982). Levels of
0.2 to 1.2 mg/litre were found in wine samples in Japan (Okamato et
al., 1981), while Margeri et al. (1984) reported levels of
acetaldehyde in wines ranging between about 30 and 80 µg/litre.
Acetaldehyde has been detected, but not quantified, in breast
milk in the USA (detection limit not reported) (Pellizari et al.,
1982).
5.1.5 Cigarette smoke
Acetaldehyde is present in tobacco leaves and in cigarette smoke
(Furia & Bellanca, 1975; US NRC, 1985). Hoffman et al. (1975)
detected acetaldehyde in the smoke of tobacco (980 µg per cigarette)
and marijuana (1200 µg/cigarette). The concentration in smoke from
several cigarettes ranged from 0.87 to 1.22 mg per cigarette or from
1.14 to 1.37 mg/cigarette, depending on the method of detection. The
concentration of acetaldehyde in three types of low-tar cigarettes
ranged from 0.09 to 0.27 mg/cigarette (Manning et al., 1983).
5.2 General population exposure
Acetaldehyde is a metabolic product of ethanol. On the basis of
the assumptions that a standard drink contains 10 g of ethanol and
that about 90% of imbibed alcohol is metabolized to acetaldehyde,
alcoholic beverages are generally by far the most significant source
of exposure to acetaldehyde for the general population.
On the basis of the content of acetaldehyde in cigarettes
reported in section 5.1.5, it is likely that cigarettes contribute
significantly to the total intake of acetaldehyde by smokers.
Assuming that smoke contains about 1 mg acetaldehyde per cigarette,
that 20 cigarettes are smoked per day, and a mean adult body weight of
64 kg (WHO, in press), intake from mainstream smoke would be about
300 µg/kg body weight per day.
On the basis of the average dietary intake of food groups in
different regions of the world (WHO, in press) and the contents of
acetaldehyde in foodstuffs and non-alcoholic beverages in the
Netherlands (Maarse & Visscher, 1992), food (particularly fruit
juices) may be one of the principal sources of exposure to
acetaldehyde in the general environment. More representative data on
mean concentrations in foodstuffs have not been identified, but, on
the basis of the ranges of concentrations determined in the Dutch
survey, intake in food is estimated to range from just less than 10 to
several hundred µg/kg body weight per day.
Data from recent studies in various locations in the world
indicate that mean concentrations of acetaldehyde in ambient air range
from 2 to 8.6 µg/m3 (Guicheret & Schulting, 1985; Watanabe, 1987;
Grosjean, 1991) (section 5.1.1). Data on concentrations of
acetaldehyde in indoor air were not identified. On the basis of a
daily inhalation volume for adults of 22 m3, a mean body weight for
males and females of 64 kg (WHO, in press), and the assumption that
mean concentrations are approximately 5 µg/m3, the mean intake of
acetaldehyde from ambient air for the general population is estimated
to be 1.7 µg/kg body weight per day.
Limited identified data on concentrations of acetaldehyde in
drinking-water indicate that they are generally less than 0.1 µg/litre
(Keith et al., 1976). Assuming a daily volume of ingestion for adults
of 1.4 litres and a mean body weight for males and females of 64 kg
(WHO, in press), and that levels are less than 0.1 µg/litre, the
estimated intake of acetaldehyde from drinking-water for the general
population would not exceed 0.002 µg/kg body weight per day.
5.3 Occupational exposure
Workers are exposed to acetaldehyde in the organic chemicals
industry and in the fabricated rubber, plastic, and fermentation
industries (US NIOSH, 1980, 1981). Concentrations of acetaldehyde
were below the detection limits (1-3.4 mg/m3) in five studies in
which the workroom air of plants, such as those in textile finishing,
propylene bottle production, and ureaformaldehyde foam-insulation
manufacturing, was monitored (Rosensteel & Tanaka, 1976; Ahrenholz &
Gorman, 1980; Herrick, 1980; Chrostek & Shoemaker, 1981; Chrostek,
1981). Bittersohl (1975) reported levels of acetaldehyde of
1-7 mg/m3 in the hydrogenation unit of a chemical factory after
equipment leakages.
Concentrations of acetaldehyde to which workers may be exposed
near aircraft with low-smoke combustor engines were found to range
from 139 to 394 µg/m3 (Miyamoto, 1986).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
No studies are available on animals or humans concerning the
absorption of acetaldehyde. However, the results of toxicity studies
indicate that absorption via the lungs and gastrointestinal tract does
occur. The physical and chemical properties of acetaldehyde indicate
that absorption via the skin is also possible.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Distribution after inhalation exposure
Distribution studies were conducted on overnight-starved, male
Sprague-Dawley rats exposed (whole-body) to unknown concentrations of
acetaldehyde vapour for 1 h. Acetaldehyde was recovered in total
blood, liver, kidneys, spleen, heart muscle, and skeletal muscle. No
other tissues were studied. The concentration of acetaldehyde in the
liver was relatively low (Hobara et al., 1985; Watanabe et al., 1986).
This can be attributed to rapid metabolism by hepatocytes.
6.2.1.2 Distribution to the embryo and fetus
No studies are available concerning routes of relevance to
humans.
Acetaldehyde was detected in the embryo up to 2 h after maternal
ip injection of 200 mg acetaldehyde/kg body weight in CD-1 mice on day
10 of gestation; acetaldehyde was measured within 5 min of injection.
Following maternal ip injection of 79 mg ethanol/kg body weight,
acetaldehyde was measured up to 12 h after injection; however, levels
were low and approached the limit of sensitivity (Blakley & Scott,
1984b).
Several other studies have demonstrated the presence of
acetaldehyde in the embryos of rats (Espinet & Argiles, 1984; Gordon
et al., 1985; Guerri & Sanchis, 1985; Clarke et al., 1986),
guinea-pigs, and ewes (Clarke, 1988) exposed to ethanol. Embryological
and cytogenic studies with ethanol and acetaldehyde in preimplantation
mouse embryos in vitro showed that acetaldehyde is three times more
toxic than ethanol. It has been suggested that the preimplantation
mouse embryo is able to convert ethanol to acetaldehyde, and that the
enzyme involved is alcohol dehydrogenase (ADH) (Lau et al., 1991)
6.2.1.3 Distribution to the brain
In the only study involving a route of relevance to humans,
following a single intragastric administration of 4500 mg ethanol/kg
body weight to male and female Wistar rats, acetaldehyde was detected
in the blood and in brain interstitial fluid collected from the
caudate nucleus and the thalamushypothalamus region. Following
administration of disulfiram (an inhibitor of the aldehyde
dehydrogenase (ALDH)-catalysed oxidation of acetaldehyde to acetate)
20 h prior to exposure to ethanol, there was a 6-fold increase in the
concentration of acetaldehyde in the blood and brain. Although
acetaldehyde was found in interstitial fluid, none was detected in
whole brain (Westcott et al., 1980).
In albino rats treated first with pyrazole, an inhibitor of ADH,
injected (ip) with a solution of acetaldehyde in saline (200 mg/kg
body weight per day) for 10 days, and then sacrificed 30 min after
receiving the last injection, acetaldehyde was detected in the brain,
liver, and blood (Prasanna & Ramakrishnan, 1984b, 1987).
A study by Pettersson & Kiessling (1977) indicated the importance
of ALDH activity, with a low Michaelis constant, in maintaining a low
level of brain acetaldehyde during ethanol metabolism. They detected
acetaldehyde and ethanol in the cerebrospinal fluid of rats after
intraperitoneal administration of ethanol alone or of ethanol followed
by acetaldehyde.
6.2.2 Human studies
The percentage of acetaldehyde retained by 8 volunteers inhaling
acetaldehyde vapour (100-800 mg/m3) from a recording respirometer
ranged from 45 to 70%, at different respiratory rates. Total
respiratory tract retention was the same whether the vapour was
inhaled through the nose or the mouth. A direct relationship was
found between the contact time and uptake, independent of rate. Thus,
the critical factor in determining acetaldehyde uptake is the duration
of the ventilatory cycle (Egle, 1970).
Baraona et al. (1987) used the blood of 5 healthy individuals, 6
alcoholic patients, and 2 baboons to show that, after alcohol
consumption, most of the blood acetaldehyde was found in the red blood
cells. In vivo, the acetaldehyde concentration in red cells was
about 10 times higher than that in the plasma. No significant
variations were seen between the 3 groups.
Studies using the perfused human placental cotyledon indicated
that the human placenta has the potential to produce acetaldehyde,
which can enter the fetal circulation. Furthermore, partial transfer
of acetaldehyde from maternal to fetal blood may occur (Karl et al.,
1988).
6.3 Metabolism
6.3.1 Animal studies
The main pathway for the metabolism of acetaldehyde is shown in
Fig. 1.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
Members
Mrs I. Arts, Department of Biological Toxicology, TNO Nutrition and
Food Research, Zeist, The Netherlands
Dr R.E. Barry, Faculty of Medicine, University of Bristol, Bristol
Royal Infirmary, Bristol, United Kingdom
Professor D. Beritic-Stahuljak, Andrija tampar School of Public
Health, Faculty of Medicine, University of Zagreb, Zagreb, Croatia
Dr Sai Mei Hou, Karolinska Institute, Huddinge, Sweden
Dr M.E. Meek, Environmental Health Directorate, Priority Substances
Section, Health & Welfare Canada, Tunney's Pasture, Ottawa, Canada
(Chairperson)
Professor M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
Professor G. Obe, University of Essen, Essen, Germany
Professor T.V.N. Persaud, Department of Anatomy, Faculty of
Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
Mr D. Renshaw, Department of Health, Elephant & Castle, London, United
Kingdom
Dr A. Smith, Health and Safety Executive, Toxicology Unit, Bootle,
Merseyside, United Kingdom (Co-rapporteur)
Professor A. Watanabe, Toyama Medical and Pharmaceutical University,
Faculty of Medicine, Toyama, Japan
Dr S. Worrall, Department of Biochemistry, University of Queensland,
Brisbane, Queensland, Australia
Representatives from other organizations
Dr V. Krutovskikh, Programme of Multistage Carcinogenesis,
International Agency for Research on Cancer, Lyon, France
Secretariat
Mrs J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
(Co-rapporteur)
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia (Responsible Officer and Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
A WHO Task Group on Environmental Health Criteria for
Acetaldehyde met in Geneva from 6 to 10 December 1993. Professor F.
Valic opened the meeting on behalf of the three cooperating
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to acetaldehyde.
The first draft of this monograph was prepared by Mrs J. de Fouw,
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands.
Professor F. Valic was responsible for the overall scientific
content of the monograph and for the organization of the meeting, and
Mrs M.O. Head of Oxford for the technical editing of the monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
INTRODUCTION
This monograph will deal mainly with the effects of direct
exposure to acetaldehyde. However, it should be borne in mind that
for most people exposure to acetaldehyde will occur through the
consumption of alcoholic beverages (IARC, 1988). These beverages
contain ethanol, which is metabolized to acetaldehyde by alcohol
dehydrogenase (ADH). ADH activity has been detected in nearly every
tissue including liver, kidney, muscle, intestine, ovary, and testes
(Buehler et al., 1983; Agarwal & Goedde, 1990).
However, data concerning metabolically formed acetaldehyde will
only be considered when no data are available on direct exposure.
The accurate determination of acetaldehyde in body fluid and
tissue samples is relatively difficult. Only the most recent
techniques take into account artifactual acetaldehyde formation in
biological samples, especially those containing ethanol (Eriksson &
Fukunaga 1993). As values for concentrations of acetaldehyde given in
older references may well have been overestimates, absolute values are
only given when necessary.
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Acetaldehyde is a colourless, volatile liquid with a pungent
suffocating odour. The reported odour threshold is 0.09 mg/m3.
Acetaldehyde is a highly flammable and reactive compound that is
miscible in water and most common solvents.
Analytical methods are available for the detection of
acetaldehyde in air (including breath) and water. The principal
method is based on the reaction of acetaldehyde with
2,4-dinitrophenylhydrazine and subsequent analysis of the hydrazone
derivatives by high pressure liquid chromatography or gas
chromatography.
1.2 Sources of human and environmental exposure
Acetaldehyde is a metabolic intermediate in humans and higher
plants and a product of alcohol fermentation. It has been identified
in food, beverages, and cigarette smoke. It is also present in
vehicle exhaust and in wastes from various industries. Degradation of
hydrocarbons, sewage, and solid biological wastes produces
acetaldehyde, as well as the open burning and incineration of gas,
fuel oil, and coal.
More than 80% of the acetaldehyde used commercially is produced
by the liquid-phase oxidation of ethylene with a catalytic solution of
palladium and copper chlorides. Production in Japan was 323 thousand
tonnes in 1981. In the USA, production was 281 thousand tonnes in 1982
while in Western Europe it was 706 thousand tonnes in 1983. Most
acetaldehyde produced commercially is used in the production of acetic
acid. It is also used in flavourings and foods.
The annual emission of acetaldehyde from all sources in the USA
is estimated to be 12.2 million kg.
1.3 Environmental transport, distribution, and transformation
Because of its high reactivity, intercompartmental transport of
acetaldehyde is expected to be limited. Some transfer of acetaldehyde
to air from water and soil is expected because of the high vapour
pressure and low sorption coefficient.
It is suggested that the photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Photolysis is
expected to contribute another substantial fraction to the removal
process. Both processes cause a reported daily loss of about 80% of
atmospheric acetaldehyde emissions. Reported half-lives of
acetaldehyde in water and air are 1.9 h and 10-60 h, respectively.
Acetaldehyde is readily biodegradable.
1.4 Environmental levels and human exposure
Levels of acetaldehyde in ambient air generally average
5 µg/m3. Concentrations in water are generally less than
0.1 µg/litre. Analysis of a wide range of foodstuffs in the
Netherlands showed that concentrations, generally less than 1 mg/kg,
occasionally ranged up to several 100 mg/kg, particularly in some
fruit juices and vinegar.
By far, the main source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure.
With respect to other media, the general population is exposed to
acetaldehyde principally from food and beverages, and, to a lesser
extent, from air. The contribution from drinking-water is negligible.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route of exposure is most likely inhalation with possible
dermal contact.
1.5 Kinetics and metabolism
1.5.1 Absorption, distribution, and elimination
Available studies on toxicity indicate that acetaldehyde is
absorbed through the lungs and gastrointestinal tract; however, no
adequate quantitative studies have been identified. Absorption
through the skin is probable.
Following inhalation by rats, acetaldehyde is distributed to the
blood, liver, kidney, spleen, heart, and other muscle tissues. Low
levels were detected in embryos after maternal intraperitoneal (ip)
injection of acetaldehyde (mouse) and following maternal exposure to
ethanol (mouse and rat). Potential production of acetaldehyde has
also been observed in rat fetuses and in the human placenta,
in vitro.
Distribution of acetaldehyde to brain interstitial fluid, but not
to brain cells, has been demonstrated following ip injection of
ethanol. A high affinity, low Km ALDHa may be important in
maintaining low levels of acetaldehyde in the brain during the
metabolism of ethanol.
Acetaldehyde is taken up by red blood cells and, following
ethanol consumption in humans and in baboons, in vivo,
intracellular levels can be 10 times higher than plasma levels.
Following oral administration, virtually no unchanged
acetaldehyde is excreted in the urine.
1.5.2 Metabolism
The major pathway for the metabolism of acetaldehyde is by
oxidation to acetate under the influence of NADb-dependent ALDH.
Acetate enters the citric acid cycle as acetyl-CoA. There are several
isoenzymes of ALDH with different kinetic and binding parameters that
influence acetaldehyde oxidation rates.
ALDH activity has been localized in the respiratory tract
epithelium (excluding olfactory epithelium) in rats, in the renal
cortex and tubules in the dog, rat, guinea-pig, and baboon, and, in
the testes in the mouse.
Acetaldehyde is metabolized by mouse and rat embryonic tissue
in vitro. Acetaldehyde crosses the rat placenta, in spite of
placental metabolism.
Though there is some metabolism of acetaldehyde in human renal
tubules, the liver is the most important metabolic site.
Several isoenzymic forms of ALDH have been identified in the
human liver and other tissues. There is polymorphism for the
mitochondrial ALDH. Subjects who are homozygous or heterozygous for a
point mutation in the mitochondrial ALDH corresponding gene have low
activity of this enzyme, metabolize acetaldehyde slowly, and are
intolerant of ethanol.
The metabolism of acetaldehyde can be inhibited by
crotonaldehyde, dimethylmaleate, phorone, disulfiram, and calcium
carbamide.
a ALDH = acetaldehyde dehydrogenase.
b NAD = nicotinamide adenine dinocleotide.
1.5.3 Reaction with other components
Acetaldehyde forms stable and unstable adducts with proteins.
This can impair protein function, as evidenced by inhibition of enzyme
activity, impaired histone-DNA binding, and inhibition of
polymerization of tubulin.
Unstable adducts of acetaldehyde of undetermined significance
occur in vitro with nucleic acids.
Acetaldehyde can react with various macromolecules in the body,
preferentially those containing lysine residues, which can lead to
marked alterations in the biological function of these molecules.
1.6 Effects on organisms in the environment
1.6.1 Aquatic organisms
LC50s in fish ranged from 35 (guppy) to 140 mg/litre (species
not specified). An EC5 of 82 mg/litre and an EC50 of 42 mg/litre
were reported for algae and Daphnia magna, respectively.
1.6.2 Terrestrial organisms
Acetaldehyde in air appears to be toxic for some microorganisms
at relatively low concentrations.
Aphids were killed when exposed to acetaldehyde at a
concentration of 0.36 µg/m3 for 3 or 4 h.
Median lethal values were 8.91 mg/litre per h and 7.69 mg/litre
per h for the slug species, Arion hortensis and Agriolimax
reticulatus, respectively.
Inhibition of seed germination in the onion, carrot, and tomato
by acetaldehyde (up to 1.52 mg/litre) was reversible, whereas
inhibition of Amaranthus palmeri, similarly exposed, was
irreversible. Acetaldehyde at 0.54 µg/m3 damaged lettuce.
1.7 Effects on experimental animals and in vitro test systems
1.7.1 Single exposure
LD50s in rats and mice and LC50s in rats and Syrian hamsters
showed that the acute toxicity of acetaldehyde is low. Acute dermal
studies were not identified.
1.7.2 Short- and long-term exposures
In repeated dose studies, by both the oral and inhalation routes,
toxic effects at relatively low concentrations were limited
principally to the sites of initial contact. In a 28-day study in
which acetaldehyde at 675 mg/kg body weight (no-observedeffect level
(NOEL): 125 mg/kg body weight) was administered in the drinking-water
to rats, effects were limited to slight focal hyperkeratosis of the
forestomach. Following administration of a single dose level (0.05%
in the drinking-water) for 6 months (estimated by the Task Group to be
approximately 40 mg/kg body weight) in a biochemical study,
acetaldehyde induced synthesis of rat liver collagen, an observation
that was supported by in vitro data.
Following inhalation, NOELs for respiratory effects were
275 mg/m3 in rats exposed for 4 weeks and 700 mg/m3 in hamsters
exposed for 13 weeks. At lowest-observed-effect levels, degenerative
changes were observed in the olfactory epithelium in rats
(437 mg/m3) and the trachea (2400 mg/m3) in hamsters.
Degenerative changes in the respiratory epithelium and larynx were
observed at higher concentrations. No repeated dose dermal studies
were identified.
1.7.3 Reproduction, embryotoxicity, and teratogenicity
In several studies, parenteral exposure of pregnant rats and mice
to acetaldehyde induced fetal malformations. In the majority of these
studies, maternal toxicity was not evaluated. No data on reproductive
toxicity were identified.
1.7.4 Mutagenicity and related end-points
Acetaldehyde is genotoxic in vitro, inducing gene mutations,
clastogenic effects, and sister-chromatid exchanges (SCEs) in
mammalian cells in the absence of exogenous metabolic activation.
However, negative results were reported in adequate tests on
Salmonella. Following intraperitoneal injection, acetaldehyde induced
SCEs in the bone marrow of Chinese hamsters and mice. However,
acetaldehyde administered intraperitoneally did not increase the
frequency of micronuclei in early mouse spermatids. There is indirect
evidence from in vitro and in vivo studies to suggest that
acetaldehyde can induce protein-DNA and DNA-DNA cross-links.
1.7.5 Carcinogenicity
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses). However, in hamsters,
increases in nasal and laryngeal carcinomas were non-significant. All
concentrations of acetaldehyde administered in the studies induced
chronic tissue damage in the respiratory tract.
1.7.6 Special studies
Adequate studies on the potential neuro- and immunotoxicity of
acetaldehyde were not identified.
1.8 Effects on humans
In limited studies on human volunteers, acetaldehyde was mildly
irritating to the eyes and upper respiratory tract following exposure
for very short periods to concentrations exceeding approximately 90
and 240 mg/m3, respectively. Cutaneous erythema was observed in
patch testing with acetaldehyde, in twelve subjects of "Oriental
ancestry".
One limited investigation in which the incidence of cancer was
examined in workers exposed to acetaldehyde and other compounds has
been reported.
On the basis of indirect evidence, acetaldehyde has been
implicated as the putatively toxic metabolite in the induction of
alcohol-associated liver damage, facial flushing, and developmental
effects.
1.9 Evaluation of human health risks and effects on the environment
The acute toxicity of acetaldehyde by the inhalation or oral
route in studies conducted on animals was low. According to studies
on humans and animals, acetaldehyde is mildly irritating to the eyes
and the upper respiratory tract. In limited studies on human
volunteers, acetaldehyde was mildly irritating to the eyes and upper
respiratory tract (section 1.8). Cutaneous erythema has also been
observed in the patch testing of humans. Although a possible mechanism
has been identified, available data are inadequate to assess the
potential of acetaldehyde to induce sensitization.
Available data on the effects of acetaldehyde following ingestion
are limited. Following oral administration of 675 mg/kg body weight
per day to rats, a borderline increase in hyper-keratosis of the
forestomach was observed (NOEL: 125 mg/kg body weight). In rats
exposed to a dose level of approximately 40 mg acetaldehyde/kg body
weight in the drinking-water for 6 months, there was an increase in
collagen synthesis in the liver, the significance of which is unclear.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In available
studies, the lowest concentration at which effects were observed was
437 mg/m3 following administration for 5 weeks. The NOELs
identified for respiratory effects were 275 mg/m3 in rats exposed
for 4 weeks and 700 mg/m3 in hamsters exposed for 13 weeks.
At concentrations that induced tissue damage in the respiratory
tract, increased incidences were observed of nasal adenocarcinomas and
squamous cell carcinomas in the rat and laryngeal and nasal carcinomas
in the hamster.
There is evidence to suggest that acetaldehyde causes genetic
damage to somatic cells in vivo.
Available data are inadequate for the assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in the general, or
occupationally exposed, populations.
On the basis of data on irritancy in humans, a tolerable
concentration of 2 mg/m3 has been derived. Since the mechanism of
induction of tumours by acetaldehyde has not been well studied, two
approaches were adopted for the provision of guidance with respect to
this end-point, i.e., the development of a tolerable concentration
based on division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, and, estimation of lifetime
cancer risk based on linear extrapolation. The tolerable
concentration is 0.3 mg/m3. The concentrations associated with a
10-5 excess lifetime risk are 11-65 µg/m3.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical formula: C2H4O
Chemical structure: CH3-CHO
Common name: acetaldehyde
Common synonyms: ethanal; acetic aldehyde; acetylaldehyde;
ethylaldehyde; diethylacetal;
1,1-diethyoxy ethane
CAS chemical name: acetaldehyde
CAS registry number: 75-07-0
RTECS registry number: AB 1925000
2.2 Physical and chemical properties
The most important physical and chemical properties of
acetaldehyde are given in Table 1.
Acetaldehyde is a volatile liquid with a pungent, suffocating
odour that is fruity in dilute concentrations. The odour threshold for
acetaldehyde is reported to be 0.09 mg/m3 (0.05 ppm). This was a
geometric average of all available literature data (Amoore & Hautala,
1983). In the case of carbon dioxide solutions in acetaldehyde, the
acetaldehyde odour is weakened by the carbon dioxide (Hagemeyer,
1978).
Acetaldehyde is a highly reactive compound that undergoes
numerous condensation, addition, and polymerization reactions. It
decomposes at temperatures above 400°C, forming principally methane
and carbon monoxide. Acetaldehyde is highly flammable when exposed to
heat or flame, and, in air, it can be explosive. Acetaldehyde can
react violently with acid anhydrides, alcohols, ketones, phenols,
NH3, HCN, H2S, P, halogens, isocyanates, strong alkalies, and
amines. It is miscible in all proportions with water and the most
common organic solvents. In aqueous solutions, acetaldehyde exists in
equilibrium with the hydrate, CH3 CH(OH)2. The enol form, vinyl
alcohol (CH2=CHOH) exists in equilibrium with acetaldehyde to the
extent of approximately one molecule per 30 000 (Hagemeyer, 1978).
Table 1. Physical and chemical properties of acetaldehydea
Colour colourless
Relative molecular mass 44.1
Boiling point at 101.3 kPa 20.2°C
Melting point -123.5°C
Octanol/water partition coefficient as 0.63
log Pow
Flash point, closed cup -38°C
Autoignition temperature 185-193°C
Explosion limits of mixtures with air 4.5-60.5 vol % acetaldehyde
Vapour pressure at -50°C 2.5 kPa
0°C 44.0 kPa
20.16°C 101.3 kPa
Specific gravity (20/4) 0.778
Relative vapour density 1.52
Refractive index 20/D 1.33113
Dissociation constant at 0°C, Ka 0.7 × 10-14
Solubility miscible in water and most
common solvents
a From: Hagemeyer (1978); IPCS/CEC (1990).
Commercial acetaldehyde should have the following typical
specifications: purity, 99% min; acidity (as acetic acid), 0.1% max,
and a specific gravity of 0.804-0.811 (0°/20°C) (US NRC, 1981).
2.3 Conversion factors
1 mg acetaldehyde/m3 air = 0.56 ppm at 25°C and 101.3 kPa
(760 mmHg).
1 ppm = 1.8 mg acetaldehyde/m3 air.
2.4 Analytical methods
Several analytical procedures used for the sampling and
determination of acetaldehyde in various media are summarized in
Table 2.
Table 2. Sampling, preparation, and determination of acetaldehydea
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in a midget HPLC with 18 µg/m3 20 litre designed for analysis of Lipari & Swarin
impinger containing 2,4-DNPH spectrophotometric automobile exhaust (1982)
in acetonitrile with detection
perchloric acid as catalyst
Air collection in a tube containing HPLC with 0.9 µg/m3 2 litre suitable for analysis of Jarke et al.
a thermal stable organic polymer spectrophotometric indoor and outdoor air (1981)
based on 2,6-diphenyl-p- detection
phenylene oxide
Air adsorption on a silica gel GC-FTD 0.09-0.45 50-100 suitable for analysis of Aoyama & Yashiro
treated with 2,4-DNPH µg/m3 litre smog and automobile (1983)
exhaust
Air collection in a 2,4-DNPH HPLC with < 18 µg/m3 < 20 litre suitable for long-term Tejada (1986)
coated Sep-PAK cartridge, spectrophotometric sampling at low µg/m3
acidified with HCl detection (ppb) levels in ambient
air, or, for short-term
sampling at low mg/m3
(ppm) levels in diluted
automotive exhaust
emissions
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in annular denuders HPLC with UV 0.36 µg/m3 100 litre suitable for analysis of Possanzini et
coated with 2,4-DNPH absorbance or outdoor and indoor al. (1987)
detection voltametric
pollution
Air collection and derivatization HPLC with UV 90 µg/m3 5 litre suitable for personal Binding et al.
on 2,4-DNPH coated detection monitoring of 5-min, (1986)
Chromosorb P short-term values as
well as for continuous
sampling over a whole
work shift
Air collection on DNPH-coated C18 HPLC with UV 12 ng per suitable for ambient Grosjean (1991)
cartridge detection cartridge monitoring
Air collection and derivatization HPLC with UV 32 mg/m3b 60 litre suitable for short-term US NIOSH (1987)
in midget bubblers containing detection exposure sampling;
Girard T solution interference with other
aldehydes and volatile
ketones should be
considered
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Air collection in a Chromosorb 104 GC-FID 0.1 µg/litre 1.5 litre suitable for monitoring Watanabe (1988)
tube installed in an automated of outdoor and indoor
sampler pollution
Air collection on a XAD-2 sorbent GC-FID 1.3 mg/m3c 3 litre suitable for short-term US NIOSH (1989)
coated with 2-(hydroxymethyl)- exposure sampling and for
piperidine analysis of field samples
Water derivatization in a two-phase HPLC with 21 µg -- designed for analysis of Facchini et
system by addition of 2,4-DNPH electrochemical fog and rain water al. (1986)
and isooctane detection
Water purging with nitrogen gas and sweeping by rapid 200 µg/ 5 ml suitable for analysis of Spingarn et
collection on a Tenax GC heating of trap litre aqueous solution and al. (1982)
sorbent and silica gel trap into GC-MS industrial effluent
Water derivatization with 2,4-DNPH HPLC; the reaction < 10 µg per 1 ml suitable for routinely Steinberg &
(in acetonitrile) mixture is analysed sample monitoring rain, fog, Kaplan (1984)
directly, without and mist samples
prior separation of
the DNPH-derivatives
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Water collection in a PTFE-cartridge HPLC with UV 0.3 µg/litre 500 ml designed for analysis of Takami et
packed with sulfonated cation detection water samples at the low al. (1985)
exchange resin charged with µg/litre levels
2,4-DNPH
Water collection of aqueous solution HS-GC-FID 25 µg/litre 10 ml designed for the Gramiccioni
in vials, no special treatments quantification of et al. (1986)
released from plastics acetaldehyde
into aqueous foods
Water collection on cyanogen bromide spectrophotometric 0.6 mg/ 30 µlitre immobilized aldehyde Almuaibed &
activated Sepharose 4B detection litre dehydrogenase makes the Townshend (1987)
containing aldehyde determination more
dehydrogenase; soluble aldehyde economic and simpler
dehydrogenase injected in the
sampler flow stream using a
double injection technique
Beverage collection of the 2,6- HPLC with 0.01 µg per 15 ml designed for analysis of Okamoto et
dimethylpyridine derivative spectrophotometric sample wine al. (1981)
on a 3-aminopropyl- detection
triethoxysiloxane or a
Nucleosil 5NH2 treated silica
gel with propionaldehyde as
internal standard
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Beverage steam distillation followed by HPLC with UV ± 5 µg/ 500 ml designed for analysis of Piendl et
liquid liquid extraction, detection litre beer al. (1981)
derivatization with p-nitrobenzyl-
oxyamine-hydrochloride with T-2
undecenal as internal standard
Beverage conversion of acetaldehyde HS-GC-FID ± 1 mg/ 5 ml designed as a rapid Jones et al.
acetals and bisulfite addition litre means by which the (1986)
products to free acetaldehyde by acetaldehyde production
a series of 1-min acid, base, and consumption
and iodine treatments followed pattern of different
by a 10-min equilibration period wines can be predicted
Breast collection of volatile compounds thermal -- 60 ml designed for Pellizari et
milk on a Tenax cartridge after desorption determination in breast al. (1982)
warming milk and purging with into GC-MS milk
helium
Blood precipitation of protein with GC headspace 4.4 µg per - designed for analysis of Eriksson et
perchloric acid analysis sample blood in order to reduce al. (1982)
artificial formation
of acetaldehyde
Blood derivatization with 2,4-DNPH HPLC with UV 4.4 ng per 2 ml designed for analysis of Pezzoli et
with butyraldehyde as internal detection sample blood al. (1984)
standard and perchloric acid
(for protein precipitation)
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Blood rapid separation plasma: HPLC with > 0.9 ng 0.5 ml designed for analysis of Di Padova et
plasma: deproteinization and spectrophotometric per sample plasma plasma and red blood al. (1986)
derivatization with 2,4-DNPH detection cells
haemolysate: deproteinization haemolysate:
and mixed with semicarbazide HS-GC-FID
hydrochloride
Blood separation of plasma and HPLC with 11 µg per 1 ml suitable for clinical Peterson &
haemolysate plasma: fluorescence sample plasma use Polizzi (1987)
1,3-cyclo-hexanedione and detection or RBC
isooctane haemolysate: haemolysate
1,3-cyclo-hexadione both in
presence of ammonium ion
Blood reaction with 1,3-cyclo-hexanone HPLC with 4.4 µg per 50-100 designed for microassays Ung-Chhun &
in the presence of ammonium ion fluorescence sample µlitre with negligible Collins (1987)
propionaldehyde used as internal detection interference
standard
Blood collection in an organic solution HPLC with > 0.13 µg 1 ml designed for analysis of Rideout et
of 2-diphenylacetyl- spectrofluometric per sample blood; with minor al. (1986)
1,3-indandione-1-hydrazone, and detection modifications also
forming fluorescent azine suitablefor analysis of
derivative-precipitation of beverages, breath, and
proteins tissue
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
Blood reaction with methanolic solution HPLC; the 4.4 µg/litre 1 ml blood suitable for assessment Lynch et
and of 2,4-DNPH, with acetaldehyde adduct 1 g tissue of acetaldehyde levels in al. (1983)
tissue dinitrophenyl-[14C]-formaldehyde was identified by clinical and experimental
as internal standard co-chromatography studies of ethanol
with the authentic metabolism and alcoholic
derivative and by beverages
mass spectrometry
a 2,4-DNPH = 2,4-dinitrophenylhydrazine; HPLC = high pressure liquid chromatography; GC-FID = gas chromatography with flame ionization
detection; GC-FTD = gas chromatography with flame thermionic detection; GC-MS = gas chromatography with mass spectrometric detection;
HS-GC-FID = head space gas chromatography with flame ionization detection.
b minimum working range (estimated LOD: 1.6 mg/m3).
c minimum working range (estimated LOD: 0.67 mg/m3).
The most specific and sensitive analytical method, widely used to
date, is based on the reaction of acetaldehyde with
2,4-dinitro-phenylhydrazine (2,4-DNPH) and the subsequent analysis of
the hydrazone derivatives by high pressure liquid chromatography
(HPLC) or gas chromatography (GC). Methods mentioned by US NIOSH are
based on derivatization with Girard T solution followed by HPLC
analysis with UV detection (US NIOSH, 1987), or, on derivatization
with 2-(hydroxymethyl)piperidine followed by GC analysis with a flame
ionization detector (FID) (US NIOSH, 1989). In the method based on
Girard T derivation, other volatile aldehydes compete for the Girard T
reagent. Chromatographic conditions may be adjusted to resolve
acetaldehyde from other aldehydes.
Spingarn et al. (1982) determined volatile organic compounds in
aqueous solutions, including acetaldehyde, using a technique in which
the compounds were purged from the solution by bubbling with an inert
gas into a trap containing a Tenax sorbent and silica gel. The
analytes were separated by GC and detected with either specific
ionization detection or MS. An improvement in detection limits,
compared with those of the widely used spectrophotometric method of
analysing carbonyls in aqueous solution, was obtained by Facchini et
al. (1986) by means of an electrochemical detector.
In the determination of acetaldehyde in blood, two main
difficulties exist. The first is related to its disappearance from
blood prior to measurement and the second is related to the formation
of acetaldehyde in blood after collection. According to Pezzoli et
al. (1984), the addition of butyraldehyde to blood, as an internal
standard, immediately after withdrawal, obviates some of the
inconveniences in the determination of acetaldehyde in blood. The
addition of butyric acid makes it possible to obtain results both for
the interaction of the aldehyde group of the acetaldehyde with amino
groups, and for the formation and extraction of the derivative
compound. However, Di Padova et al. (1986) stated that the addition
of butyraldehyde was not specific for the determination of the
acetaldehyde but was related to the aldehyde group reactivity.
Therefore, they described an improved procedure for measuring
acetaldehyde in plasma, based on rapid separation, 2,4-DNPH
derivatization, and HPLC analysis, and a procedure for measuring
acetaldehyde in red blood cells, based on the use of a semicarbazide
solution and analysis by head space gas chromatography.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Acetaldehyde is a metabolic intermediate in humans and higher
plants and it is a product of alcohol fermentation (IARC, 1985). It
has been identified as a volatile component of mature cotton leaves
and cotton blossoms (Berni & Stanley, 1982) and as a component in the
essential oil of alfalfa at a concentration of about 0.2% (Kami,
1983). It occurs in food, various fruits, and several spices (see
section 5.1.4) and in oak and tobacco leaves (Furia & Bellanca, 1975;
US NRC, 1985).
Acetaldehyde is formed in the atmosphere in a variety of ways.
It is generated by the oxidation of non-methane hydrocarbons both in
the background troposphere and in photochemical smog (Grosjean, 1982).
3.2 Anthropogenic sources
3.2.1 Production
3.2.1.1 Production levels and processes
Until 1968, most acetaldehyde produced in the USA was made by the
partial oxidation of ethanol over a silver catalyst; however,
currently less than 5% of US production is based on this process. The
liquid-phase oxidation of ethylene using a catalytic solution of
palladium and copper chlorides was first used commercially in the USA
in 1960 and more than 80% of the world production of acetaldehyde is
made by this process. The remainder is produced by the oxidation of
ethanol and the hydration of acetylene. Acetaldehyde is produced by a
limited number of companies over the world. The total production of
acetaldehyde in the USA in 1982 amounted to 281 thousand tonnes.
Total acetaldehyde production in Western Europe in 1982 was 706
thousand tonnes, and the production capacity was estimated to have
been nearly 1 million tonnes. In Japan, the estimated production in
1981 was 323 thousand tonnes (Hagemeyer, 1978; IARC, 1985).
3.2.1.2 Emissions
Eimutis et al. (1978) estimated that the annual atmospheric
emissions of acetaldehyde in the USA amounted to 12.2 thousand tonnes
(Table 3). Emissions of acetaldehyde in the Netherlands in the year
1980 were reported to be 584 tonnes (Guicherit & Schulting, 1985).
Table 3. Emission and sources of acetaldehyde in the USA
Source Emissions
(tonnes/year)
Residential external combustion of wood 5056.4
Coffee roasting 4411.4
Acetic acid manufacture 1460.9
Vinyl acetate manufacture from ethylene 1094.6
Ethanol manufacture 57.8
Acrylonitrile manufacture 51.6
Acetic acid manufacture from butane 20.8
Crotonaldehyde manufacture 4.5
Acetone and phenol manufacture from cumene 1.9
Acetaldehyde manufacture by hydration of ethylene 0.5
Polyvinyl chloride manufacture 0.2
Acetaldehyde manufacture by oxidation of ethanol 0.1
3.2.2 Uses
Acetaldehyde is an important intermediate in the production of
acetic acid, ethyl acetate, peracetic acid, pentaerythritol, chloral,
glyoxal, alkylamines, and pyridines (Hagemeyer, 1978). The use
pattern for the estimated 281 thousand tonnes of acetaldehyde produced
in the USA in 1982 was as follows: acetic acid 61%, pyridine and
pyrine bases 9%, peracetic acid 8%, pentaerythritol 7%, 1,3-butylene
glycol 2%, chloral 1%, and other applications (including use as a food
additive and exports) 12%. The use pattern for the estimated 706
thousand tonnes of acetaldehyde produced in Western Europe was as
follows: acetic acid 62%, ethyl acetate 19%, pentaerythritol 5%,
synthetic pyridines 3%, and all other uses 11% (IARC, 1985).
Acetaldehyde is used for the flavourings: berry, butter,
chocolate, apple, apricot, banana, grape, peach, black walnut, and
rum, and it is used in the following foods: beverages, ice cream and
ices, candy, baked goods, gelatin desserts, and chewing gum (Furia &
Bellanca, 1975; US NRC, 1981, 1985). Acetaldehyde is also used in
perfumes, aniline dyes, plastics, in the manufacture of synthetic
rubber, in the silvering of mirrors, in the hardening of gelatin
fibres, and in the laboratory (Verschueren, 1983).
3.2.3 Waste disposal
Degradation of hydrocarbons, sewage, and solid biological wastes
produces acetaldehyde. It has been detected in effluents from
sewage-treatment plants and chemical plants (US EPA, 1975; Shackelford
& Keith, 1976).
Acetaldehyde has been identified as a constituent in the wastes
from petroleum refining, coal processing, the oxidation of alcohols,
saturated hydrocarbons, or ethylene, and the hydration of acetylene
(IARC, 1985).
3.2.4 Other sources
Acetaldehyde is detected as a combustion product of plastics and
polycarbonate and polyurethane foams of western European origin
(Hagen, 1967; Boettner et al., 1973).
Acetaldehyde occurs in vehicle exhaust at levels of
1.4-8.8 mg/m3 in gasoline exhaust, about 5.8 mg/m3 in diesel
exhaust (Verschueren, 1983), and 51.6% acetaldehyde/ n-hexane GC peak
area ratio in exhaust gas oxygenates (Hugues & Hum, 1960). It also
occurs in the open burning and incineration of gas, fuel oil, and
coal, and evaporation products of perfumes (Verschueren, 1983).
Acetaldehyde has been identified in fresh tobacco leaves and in
tobacco smoke (concentrations ranging from 2.1 to 4.6 mg/litre smoke)
(Buyske et al., 1956; Osborne et al., 1956; Mold & McRae, 1957).
When Lipari et al. (1984) measured aldehyde emissions from
wood-burning fireplaces, they ranged from 0.08 to 0.20 g/kg of wood
burned, based on tests with cedar, jack pine, red oak, and green ash.
Acetaldehyde emissions from wood-burning furnaces and
stoves were also measured in a Swedish study (Rudling et al., 1981)
and in a Norwegian study (Ramdahl et al., 1982). In the Swedish
study, the emissions ranged from 1-72 mg/kg wood in prechamber ovens
to 9-710 mg/kg wood in fireplace stoves. In the Norwegian study, the
reported emissions from stoves were 14.4 mg/kg dry wood under normal
burning conditions and up to 992 mg/kg dry wood under low-efficiency
combustion.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Acetaldehyde can enter the atmosphere during production of the
compound itself, as a product of incomplete combustion, and also as a
by-product of fermentation (Grosjean, 1982).
Photochemical oxidation of acetaldehyde has been shown to be an
important process in the chemistry of photochemical smog (Bagnall &
Sidebottom, 1984; Leone & Seinfeld, 1984). Present theories ascribe
the importance of acetaldehyde to its being a precursor of
peroxyacetylnitrate (PAN) in polluted atmospheres (Kopczynski et al.,
1974; Grosjean et al., 1983; Bagnall & Sidebottom, 1984; Moortgat &
McQuigg, 1984). Acetaldehyde is likely to be a precursor of acetic
acid, which is a component of natural precipitation and contributes to
its acidity (Moortgat & McQuigg, 1984).
Intercompartmental transport of acetaldehyde is expected to be
limited, because of its high reactivity. However, because of the high
vapour pressure of acetaldehyde, some transfer to air from water and
soil can be expected.
The tendency of acetaldehyde to adsorb on soil particles can be
expressed in terms of Koc, the ratio of the amount of chemical
adsorbed per unit weight of organic carbon to the concentration of the
chemical in solution at equilibrium. On the basis of the available
empirical relationships derived for estimating Koc, a low soil
adsorption potential is expected (Lyman et al., 1982). Koch & Nagel
(1988) calculated a soil sorption coefficient of 0.90 for
acetaldehyde, and, therefore, acetaldehyde was classified as a
compound with a very low sorption tendency.
4.2 Abiotic degradation
It is suggested that photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Singh et al.
(1982) reported that photolysis and reaction with hydroxyl radicals
cause a daily loss rate of about 80% of atmospheric acetaldehyde
emissions. Grosjean et al. (1983) reported that the reaction with
hydroxyl radicals could remove 50-300 tonnes of carbonyls from the Los
Angeles air over a 12-h daytime period and, thus, is considered to be
a major removal process for all aldehydes. The absolute rate constant
for the reaction of the hydroxyl radical with acetaldehyde was
determined over a temperature range of 26-153°C by Atkinson & Pitts
(1978). At 26°C, they obtained a rate constant of (1.60 ± 0.16) ×
10-11 cm3 per molecule per second. This results in a half-life
for acetaldehyde of 10 h, using a 12-h daytime average hydroxyl
radical concentration of 2 × 10-15 mol/litre (Lyman et al., 1982).
Hustert & Parlar (1981) reported that 49.5% acetaldehyde was
photochemically degraded (reaction with hydroxyl radicals) after a 2-h
radiation (lambda > 230 nm) at 25°C, which, contrary to Atkinson &
Pitts (1978), shows a half-life of 2 h. Atkinson et al. (1984)
obtained a rate constant of (1.34 ± 0.28) × 10-15 for the gas-phase
reaction of nitrate radicals with acetaldehyde at 25°C. This results
in a half-life for acetaldehyde of 59.6 h using a 12-h nighttime
average nitrate radical concentration of 4.0 × 10-12 mol/litre
(Atkinson et al., 1987).
There is a considerable amount of evidence that acetaldehyde in
aqueous solution is in equilibrium with its hydrated form
CH3CH(OH)2. The degree of hydration decreases with increasing
temperature (e.g., at 0°C, the fraction of acetaldehyde hydrated is
0.73; at 25°C, it is 0.59) (Bell & Clunie, 1952).
Von Burg & Stout (1991) reported a half-life of 1.9 h for
acetaldehyde in river water; no other details were provided.
4.3 Biodegradation
Several studies have revealed significant degradation of
acetaldehyde by mixed cultures obtained from sludges and settled
sewage. Hatfield (1957) reported the ability of acclimatized sludge
to oxidize acetaldehyde (major portion of the biological and chemical
oxygen demand (BOD and COD) removed within a 4-h aeration period).
Ludzack & Ettinger (1960) determined the BOD for acetaldehyde in
activated sludge at 20°C and found that 93% of the acetaldehyde was
removed after an observation period of 1/3-5 days and an
acclimatization period of 30 days. Thom & Agg (1975) and Speece
(1983) also reported that acetaldehyde was easily biodegradable by
biological sewage treatment (additional information was not provided).
However, Gerhold & Malaney (1966) reported little degradation of
acetaldehyde by unacclimatized municipal sludge with a BOD of 27.6% as
a percentage of the theoretical oxygen demand in 24 h.
Acetaldehyde is also degraded by anaerobic biological treatment
with unacclimatized acetate-enriched cultures. A COD-removal of 97%
was obtained at the end of a 90-day acclimatization period in
completely mixed reactors with a 20-day hydraulic retention time, no
solids recycle, and a final daily feed concentration of
10 000 mg/litre (Chou & Speece, 1978).
Acetaldehyde is reported to be readily biodegradable using the
biodegradability MITI test, defined in OECD Guidelines for testing of
chemicals (OECD, 1992).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The concentrations of acetaldehyde in uncontaminated Arctic air
masses, determined over a 24-h period, ranged from not detected to
0.54 µg/m3 (Cavanagh et al., 1969).
In samples collected during April 1981, the levels of
acetaldehyde in the air in Pittsburg (PA) and Chicago (Il) were
0.36-4.68 µg/m3 and 1.62-6.12 µg/m3, respectively (Singh et al.,
1982). In samples collected at 7 other locations in the USA between
1975 and 1978, mean concentrations in ambient air were 5-124 µg/m3
(Brodzinsky & Singh, 1982).
Schulam et al.(1985) determined the levels of acetaldehyde in air
(June-August 1983) in the urban location of Schenectady (NY) and the
rural location of Whiteface Mountain (NY). Concentrations of
acetaldehyde were similar in the two locations (the levels of
acetaldehyde varied from 0.36 to 1.44 µg/m3, detection limit:
0.29 µg/m3).
The average ambient atmospheric level of acetaldehyde, measured
during the four seasons at Brookhaven National Laboratory (Upton, Long
Island, NY) from July 1982 to May 1983, was 5.2 µg/m3, with a mean
minimum concentration in winter of 1.8 µg/m3 and a mean maximum
value in summer of 15.1 µg/m3 (Tanner & Meng, 1984). Concentrations
of acetaldehyde in the air in Tulsa, OK (sampled in July 1978), Rio
Blanco County, CO (sampled in July 1978), and the Great Smoky
Mountains, TN (sampled in September 1978), ranged up to 14.9, 16.9,
and 23.9 µg/m3, respectively (Arntz & Meeks, 1981).
Mean concentrations of acetaldehyde in the air in Tokyo during
four seasons in 1985-86 ranged from 2.2 to 7.3 µg/m3 (Watanabe,
1987). Seasonal trends were not noted. Concentrations of
acetaldehyde in an environmental survey conducted by the Japan
Environment Agency in 1987 ranged from 0.9 to 22 µg/m3 (number of
sites sampled unspecified) (Japan Environment Agency, 1989).
The mean concentration of acetaldehyde was 2 µg/m3 at three
locations in the Netherlands, namely, the island of Terschelling (one
of the least polluted areas of the country), Delft (suburban), and
Vlaardingen (heavily industrialized area) (Guicherit & Schulting,
1985).
Grosjean (1991) reported levels of acetaldehyde in ambient air,
sampled every sixth day over a one-year period, at six locations in
Southern California between September 1988 and September 1989.
Concentrations ranged up to 23.3 µg/m3 (13 ppb) with average values
at the various locations ranging from 5.2 to 8.6 µg/m3
(2.9-4.8 ppb).
The mean concentration of acetaldehyde in fog samples taken in
November, 1985 in the Po Valley (Italy) was 21 µg/litre (Facchini et
al., 1986). At urban locations in California (Los Angeles) and Alaska
(Fairbanks), concentrations of acetaldehyde ranged from 0.007 to
0.13 µg/ml in ice fog (Alaska), 0 to 0.11 µg/ml in rain (CA), 0 to
0.59 µg/ml in cloud (CA), 0.10 to 0.11 µg/ml in mist (CA), and 0.006
to 0.17 µg/ml in fog (CA) (Grosjean & Wright, 1983).
5.1.2 Water
No quantitative data on concentrations of acetaldehyde in raw
water supplies were identified.
Acetaldehyde has been detected in drinking-water from
Philadelphia and Seattle at levels of up to 0.1 µg/litre (Keith et
al., 1976). No other information was provided.
5.1.3 Soil
Data on concentrations of acetaldehyde in soil were not
identified.
5.1.4 Food
Acetaldehyde has been detected in a wide range of foodstuffs (US
NRC, 1981, 1985; Horvath et al., 1983; Feron et al., 1991), though few
quantitative data are available. In a variety of foodstuffs analysed
in the Netherlands including fruits and juices, vegetables, milk
products, bread, eggs, fish, meat, and alcoholic beverages,
concentrations were generally less than 1 mg/kg, but occasionally
ranged up to several hundred mg/kg, particularly in some fruit juices
and alcoholic beverages; in vinegar, a maximum value of 1060 mg/kg was
reported (Maarse & Visscher, 1992). Acetaldehyde has been identified
in alcoholic beverages, such as beer and wine (Okamoto et al., 1981;
Piendl et al., 1981; Jones et al., 1986); levels in 18 English beers
ranged from 2.6 to 13.5 mg/litre (Delcour et al., 1982). Levels of
0.2 to 1.2 mg/litre were found in wine samples in Japan (Okamato et
al., 1981), while Margeri et al. (1984) reported levels of
acetaldehyde in wines ranging between about 30 and 80 µg/litre.
Acetaldehyde has been detected, but not quantified, in breast
milk in the USA (detection limit not reported) (Pellizari et al.,
1982).
5.1.5 Cigarette smoke
Acetaldehyde is present in tobacco leaves and in cigarette smoke
(Furia & Bellanca, 1975; US NRC, 1985). Hoffman et al. (1975)
detected acetaldehyde in the smoke of tobacco (980 µg per cigarette)
and marijuana (1200 µg/cigarette). The concentration in smoke from
several cigarettes ranged from 0.87 to 1.22 mg per cigarette or from
1.14 to 1.37 mg/cigarette, depending on the method of detection. The
concentration of acetaldehyde in three types of low-tar cigarettes
ranged from 0.09 to 0.27 mg/cigarette (Manning et al., 1983).
5.2 General population exposure
Acetaldehyde is a metabolic product of ethanol. On the basis of
the assumptions that a standard drink contains 10 g of ethanol and
that about 90% of imbibed alcohol is metabolized to acetaldehyde,
alcoholic beverages are generally by far the most significant source
of exposure to acetaldehyde for the general population.
On the basis of the content of acetaldehyde in cigarettes
reported in section 5.1.5, it is likely that cigarettes contribute
significantly to the total intake of acetaldehyde by smokers.
Assuming that smoke contains about 1 mg acetaldehyde per cigarette,
that 20 cigarettes are smoked per day, and a mean adult body weight of
64 kg (WHO, in press), intake from mainstream smoke would be about
300 µg/kg body weight per day.
On the basis of the average dietary intake of food groups in
different regions of the world (WHO, in press) and the contents of
acetaldehyde in foodstuffs and non-alcoholic beverages in the
Netherlands (Maarse & Visscher, 1992), food (particularly fruit
juices) may be one of the principal sources of exposure to
acetaldehyde in the general environment. More representative data on
mean concentrations in foodstuffs have not been identified, but, on
the basis of the ranges of concentrations determined in the Dutch
survey, intake in food is estimated to range from just less than 10 to
several hundred µg/kg body weight per day.
Data from recent studies in various locations in the world
indicate that mean concentrations of acetaldehyde in ambient air range
from 2 to 8.6 µg/m3 (Guicheret & Schulting, 1985; Watanabe, 1987;
Grosjean, 1991) (section 5.1.1). Data on concentrations of
acetaldehyde in indoor air were not identified. On the basis of a
daily inhalation volume for adults of 22 m3, a mean body weight for
males and females of 64 kg (WHO, in press), and the assumption that
mean concentrations are approximately 5 µg/m3, the mean intake of
acetaldehyde from ambient air for the general population is estimated
to be 1.7 µg/kg body weight per day.
Limited identified data on concentrations of acetaldehyde in
drinking-water indicate that they are generally less than 0.1 µg/litre
(Keith et al., 1976). Assuming a daily volume of ingestion for adults
of 1.4 litres and a mean body weight for males and females of 64 kg
(WHO, in press), and that levels are less than 0.1 µg/litre, the
estimated intake of acetaldehyde from drinking-water for the general
population would not exceed 0.002 µg/kg body weight per day.
5.3 Occupational exposure
Workers are exposed to acetaldehyde in the organic chemicals
industry and in the fabricated rubber, plastic, and fermentation
industries (US NIOSH, 1980, 1981). Concentrations of acetaldehyde
were below the detection limits (1-3.4 mg/m3) in five studies in
which the workroom air of plants, such as those in textile finishing,
propylene bottle production, and ureaformaldehyde foam-insulation
manufacturing, was monitored (Rosensteel & Tanaka, 1976; Ahrenholz &
Gorman, 1980; Herrick, 1980; Chrostek & Shoemaker, 1981; Chrostek,
1981). Bittersohl (1975) reported levels of acetaldehyde of
1-7 mg/m3 in the hydrogenation unit of a chemical factory after
equipment leakages.
Concentrations of acetaldehyde to which workers may be exposed
near aircraft with low-smoke combustor engines were found to range
from 139 to 394 µg/m3 (Miyamoto, 1986).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
No studies are available on animals or humans concerning the
absorption of acetaldehyde. However, the results of toxicity studies
indicate that absorption via the lungs and gastrointestinal tract does
occur. The physical and chemical properties of acetaldehyde indicate
that absorption via the skin is also possible.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Distribution after inhalation exposure
Distribution studies were conducted on overnight-starved, male
Sprague-Dawley rats exposed (whole-body) to unknown concentrations of
acetaldehyde vapour for 1 h. Acetaldehyde was recovered in total
blood, liver, kidneys, spleen, heart muscle, and skeletal muscle. No
other tissues were studied. The concentration of acetaldehyde in the
liver was relatively low (Hobara et al., 1985; Watanabe et al., 1986).
This can be attributed to rapid metabolism by hepatocytes.
6.2.1.2 Distribution to the embryo and fetus
No studies are available concerning routes of relevance to
humans.
Acetaldehyde was detected in the embryo up to 2 h after maternal
ip injection of 200 mg acetaldehyde/kg body weight in CD-1 mice on day
10 of gestation; acetaldehyde was measured within 5 min of injection.
Following maternal ip injection of 79 mg ethanol/kg body weight,
acetaldehyde was measured up to 12 h after injection; however, levels
were low and approached the limit of sensitivity (Blakley & Scott,
1984b).
Several other studies have demonstrated the presence of
acetaldehyde in the embryos of rats (Espinet & Argiles, 1984; Gordon
et al., 1985; Guerri & Sanchis, 1985; Clarke et al., 1986),
guinea-pigs, and ewes (Clarke, 1988) exposed to ethanol. Embryological
and cytogenic studies with ethanol and acetaldehyde in preimplantation
mouse embryos in vitro showed that acetaldehyde is three times more
toxic than ethanol. It has been suggested that the preimplantation
mouse embryo is able to convert ethanol to acetaldehyde, and that the
enzyme involved is alcohol dehydrogenase (ADH) (Lau et al., 1991)
6.2.1.3 Distribution to the brain
In the only study involving a route of relevance to humans,
following a single intragastric administration of 4500 mg ethanol/kg
body weight to male and female Wistar rats, acetaldehyde was detected
in the blood and in brain interstitial fluid collected from the
caudate nucleus and the thalamushypothalamus region. Following
administration of disulfiram (an inhibitor of the aldehyde
dehydrogenase (ALDH)-catalysed oxidation of acetaldehyde to acetate)
20 h prior to exposure to ethanol, there was a 6-fold increase in the
concentration of acetaldehyde in the blood and brain. Although
acetaldehyde was found in interstitial fluid, none was detected in
whole brain (Westcott et al., 1980).
In albino rats treated first with pyrazole, an inhibitor of ADH,
injected (ip) with a solution of acetaldehyde in saline (200 mg/kg
body weight per day) for 10 days, and then sacrificed 30 min after
receiving the last injection, acetaldehyde was detected in the brain,
liver, and blood (Prasanna & Ramakrishnan, 1984b, 1987).
A study by Pettersson & Kiessling (1977) indicated the importance
of ALDH activity, with a low Michaelis constant, in maintaining a low
level of brain acetaldehyde during ethanol metabolism. They detected
acetaldehyde and ethanol in the cerebrospinal fluid of rats after
intraperitoneal administration of ethanol alone or of ethanol followed
by acetaldehyde.
6.2.2 Human studies
The percentage of acetaldehyde retained by 8 volunteers inhaling
acetaldehyde vapour (100-800 mg/m3) from a recording respirometer
ranged from 45 to 70%, at different respiratory rates. Total
respiratory tract retention was the same whether the vapour was
inhaled through the nose or the mouth. A direct relationship was
found between the contact time and uptake, independent of rate. Thus,
the critical factor in determining acetaldehyde uptake is the duration
of the ventilatory cycle (Egle, 1970).
Baraona et al. (1987) used the blood of 5 healthy individuals, 6
alcoholic patients, and 2 baboons to show that, after alcohol
consumption, most of the blood acetaldehyde was found in the red blood
cells. In vivo, the acetaldehyde concentration in red cells was
about 10 times higher than that in the plasma. No significant
variations were seen between the 3 groups.
Studies using the perfused human placental cotyledon indicated
that the human placenta has the potential to produce acetaldehyde,
which can enter the fetal circulation. Furthermore, partial transfer
of acetaldehyde from maternal to fetal blood may occur (Karl et al.,
1988).
6.3 Metabolism
6.3.1 Animal studies
The main pathway for the metabolism of acetaldehyde is shown in
Fig. 1.
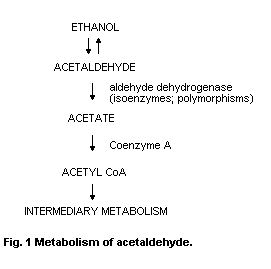 6.3.1.1 Liver
The main pathway for the metabolism of acetaldehyde is by rapid
oxidation to acetate, which enters the citric acid cycle in an
activated form as acetyl-CoA and is metabolized to CO2 and H2O.
Although catalase and other oxidases may contribute to metabolism
(Brien & Loomis, 1983), because of its high affinity, at least 90% of
acetaldehyde is oxidized by mitochondrial ALDH (Hellström-Lindahl &
Weiner, 1985) reducing NAD+ to NADH in the process. This step can
be blocked by disulfiram.
There are multiple molecular forms of ALDH with different kinetic
properties that influence the rate of removal of acetaldehyde
(Marjanen, 1973; Parilla et al., 1974; Teschke et al., 1977).
Acetaldehyde is a highly reactive molecule that can react with
many other large or small molecules by adduction, condensation, or
polymerization. These pathways may have little quantitative
significance in acetaldehyde metabolism, but the by-products may have
biological significance (Collins et al., 1979; Sorrell & Tuma, 1985).
Acetaldehyde is the primary metabolic product of ethanol
oxidation. Since ethanol is oxidized to acetaldehyde mole for mole,
and, since the exposure to exogenous acetaldehyde is small, endogenous
acetaldehyde resulting from the metabolism of ingested ethanol is
likely to be the most important source of exposure for most people.
Oxidation of ethanol to acetaldehyde occurs predominantly under the
influence of ADH, of which there are many isoenzymic forms. Like
ALDH, ADH is also NAD dependent. The inseparable metabolism of
ethanol and acetaldehyde results in the reduction of NAD+, thus,
affecting the redox state of the liver causing secondary metabolic
consequences.
6.3.1.2 Respiratory tract
ALDH localization in the respiratory tract of Fischer-344 rats
was studied by Bogdanffy et al. (1986). Histochemical studies
indicated activity principally in the nasal respiratory epithelium,
especially in the supranuclear cytoplasm of ciliated epithelial cells.
Activity was also high in the Clara cells of the lower bronchioles.
The tracheal epithelia possessed only low levels of ALDH. The
olfactory epithelium was almost devoid of ALDH activity.
Casanova-Schmitz et al. (1984) characterized at least 2
isoenzymes of ALDH in rat nasal mucosa homogenates.
6.3.1.3 Kidneys
In an in vitro study, Michoudet & Baverel (1987a,b) studied the
metabolism of acetaldehyde in isolated dog, rat, guinea-pig, and
baboon kidney-cortex tubules.
Acetaldehyde was found to be metabolized by the tubules at high
rates and in a dose-dependent manner in all four species. It was
noted that, at all acetaldehyde concentrations, most of the
acetaldehyde removed was recovered as acetate in dog, guinea-pig, and
baboon, but not in rat kidney tubules.
6.3.1.4 Testes and ovaries
There are no studies on the capacity of the testes or ovaries to
mediate the biotransformation of acetaldehyde. However, ALDH activity
has been identified in the testes of Swiss-Webster mice (Anderson et
al., 1985).
6.3.1.5 Embryonic tissue
In an in vitro study, the ability of CBA/beige mouse (10 days
old) and Wistar rat (12 days old) embryos to metabolize acetaldehyde
was reported by Priscott & Ford (1985).
6.3.1.6 Metabolism during pregnancy
After intravenous administration of acetaldehyde (10 mg/kg body
weight) blood acetaldehyde levels were higher in pregnant rats than in
virgin rats. Acetaldehyde at high concentrations was able to cross
the placental barrier very rapidly. At low maternal concentrations,
it was metabolized by aldehyde dehydrogenase activity in the placenta
and fetal liver, and acetaldehyde was not detected in fetal blood.
Above the acetaldehyde threshold, the metabolic capacity of the
feto-placental unit was surpassed and acetaldehyde was detected in
fetal blood (Zorzano & Herrera, 1989).
6.3.2 Human studies
No high quality studies of the in vivo metabolism of
acetaldehyde in humans have been identified. Accurate assays for
acetaldehyde in blood and tissues have only recently become available
(Harade et al., 1978a,b).
Human liver ALDH consists of at least 4 main isoenzymes, which
are also present in many other tissues (Koivula, 1975; Goedde et al.,
1979). Mitochondrial ALDH is inactive in at least 40% of the Oriental
population. The frequently observed intolerance to alcohol (the
"flushing" reaction) is linked to this deficiency, which is produced
by an inherited positive mutation in the corresponding gene (Yoshida
et al., 1984; Goedde & Agarwal, 1986, 1987; Hsu et al., 1988).
Subjects with phenotypic deficiency have always shown the presence of
at least one mutant gene (heterozygous or homozygous) (Crabb et al.,
1989; Goedde et al., 1989; Singh et al., 1989).
In vitro, acetaldehyde (0.04-0.88 g/litre) was metabolized at
high rates and in a dose-dependent manner in isolated human
kidney-cortex tubules (Michoudet & Baverel, 1987b).
6.4 Elimination
In dogs, urinary excretion of acetaldehyde was essentially
non-existent following administration of a single dose of 600 mg
acetaldehyde/kg body weight, via a stomach tube (Booze & Oehme, 1986).
6.5 Reaction with cellular macromolecules
6.5.1 Proteins
Acetaldehyde can react with nucleophilic groups, such as amino,
hydroxyl, and sulfydryl groups, through nucleophilic attack on the
carbonyl carbon atom of acetaldehyde to give both stable and unstable
adducts (Tuma et al., 1984). Several adduct structures, formed when
acetaldehyde reacts with proteins in vitro, have been identified,
but have not yet been described fully.
The best characterized nucleophiles able to form adducts with
acetaldehyde are amino groups, notably the alpha-amino terminus of
peptides and proteins and the epsilon-amino group on the side-chain of
lysine residues. These reactions are shown in Fig. 2.
The structure of 2-methylimidazolidin-4-one adducts has been
confirmed by proton NMR (Gidley et al., 1981) and 13C-NMR
spectroscopy (San George & Hoberman, 1986). N-ethylation lysine
residues have been demonstrated by Tuma et al. (1984).
In a series of studies on lysine-dependent enzymes, Mauch et al.
(1986, 1987) were able to demonstrate that incubation of purified
enzymes with acetaldehyde for 1 h at 37°C led to inhibition of their
catalytic activity. Lysine non-dependent enzymes were not affected by
this treatment. A similar study involving the incubation of rat liver
histone H1 with physiological concentrations of acetaldehyde showed
that spontaneously stable adducts were formed on lysine residues at
the carboxy terminus, a site crucial for its function as a eukaryotic
repressor (Niëmela et al., 1990). This acetaldehyde-modified histone
H1 showed impaired DNA binding activity.
Tuma et al. (1987) have characterized the interaction of
acetaldehyde with a "highly reactive" lysine residue in purified
alpha-tubulin, which is only available in the monomeric form. They
found that modification of this residue was the critical factor in the
inhibition of tubulin polymerization by acetaldehyde, and that
modification of 5% of these residues was enough to inhibit tubulin
polymerization completely in vitro. Crebelli et al. (1989) found
similar effects: 0.075% v/v (13.5 mmol/litre) acetaldehyde partially
inhibited the in vitro polymerization of cattle brain tubulin, and
0.15% (27 mmol/litre) caused complete inhibition.
Incubation of calf brain microtubular proteins also resulted in
decreased polymerization, in an analogous manner to tubulin (McKinnon
et al., 1987a,b). Thus, acetaldehyde modification can impair the
molecular function of macromolecules, which can lead to marked
alterations in biological function.
6.3.1.1 Liver
The main pathway for the metabolism of acetaldehyde is by rapid
oxidation to acetate, which enters the citric acid cycle in an
activated form as acetyl-CoA and is metabolized to CO2 and H2O.
Although catalase and other oxidases may contribute to metabolism
(Brien & Loomis, 1983), because of its high affinity, at least 90% of
acetaldehyde is oxidized by mitochondrial ALDH (Hellström-Lindahl &
Weiner, 1985) reducing NAD+ to NADH in the process. This step can
be blocked by disulfiram.
There are multiple molecular forms of ALDH with different kinetic
properties that influence the rate of removal of acetaldehyde
(Marjanen, 1973; Parilla et al., 1974; Teschke et al., 1977).
Acetaldehyde is a highly reactive molecule that can react with
many other large or small molecules by adduction, condensation, or
polymerization. These pathways may have little quantitative
significance in acetaldehyde metabolism, but the by-products may have
biological significance (Collins et al., 1979; Sorrell & Tuma, 1985).
Acetaldehyde is the primary metabolic product of ethanol
oxidation. Since ethanol is oxidized to acetaldehyde mole for mole,
and, since the exposure to exogenous acetaldehyde is small, endogenous
acetaldehyde resulting from the metabolism of ingested ethanol is
likely to be the most important source of exposure for most people.
Oxidation of ethanol to acetaldehyde occurs predominantly under the
influence of ADH, of which there are many isoenzymic forms. Like
ALDH, ADH is also NAD dependent. The inseparable metabolism of
ethanol and acetaldehyde results in the reduction of NAD+, thus,
affecting the redox state of the liver causing secondary metabolic
consequences.
6.3.1.2 Respiratory tract
ALDH localization in the respiratory tract of Fischer-344 rats
was studied by Bogdanffy et al. (1986). Histochemical studies
indicated activity principally in the nasal respiratory epithelium,
especially in the supranuclear cytoplasm of ciliated epithelial cells.
Activity was also high in the Clara cells of the lower bronchioles.
The tracheal epithelia possessed only low levels of ALDH. The
olfactory epithelium was almost devoid of ALDH activity.
Casanova-Schmitz et al. (1984) characterized at least 2
isoenzymes of ALDH in rat nasal mucosa homogenates.
6.3.1.3 Kidneys
In an in vitro study, Michoudet & Baverel (1987a,b) studied the
metabolism of acetaldehyde in isolated dog, rat, guinea-pig, and
baboon kidney-cortex tubules.
Acetaldehyde was found to be metabolized by the tubules at high
rates and in a dose-dependent manner in all four species. It was
noted that, at all acetaldehyde concentrations, most of the
acetaldehyde removed was recovered as acetate in dog, guinea-pig, and
baboon, but not in rat kidney tubules.
6.3.1.4 Testes and ovaries
There are no studies on the capacity of the testes or ovaries to
mediate the biotransformation of acetaldehyde. However, ALDH activity
has been identified in the testes of Swiss-Webster mice (Anderson et
al., 1985).
6.3.1.5 Embryonic tissue
In an in vitro study, the ability of CBA/beige mouse (10 days
old) and Wistar rat (12 days old) embryos to metabolize acetaldehyde
was reported by Priscott & Ford (1985).
6.3.1.6 Metabolism during pregnancy
After intravenous administration of acetaldehyde (10 mg/kg body
weight) blood acetaldehyde levels were higher in pregnant rats than in
virgin rats. Acetaldehyde at high concentrations was able to cross
the placental barrier very rapidly. At low maternal concentrations,
it was metabolized by aldehyde dehydrogenase activity in the placenta
and fetal liver, and acetaldehyde was not detected in fetal blood.
Above the acetaldehyde threshold, the metabolic capacity of the
feto-placental unit was surpassed and acetaldehyde was detected in
fetal blood (Zorzano & Herrera, 1989).
6.3.2 Human studies
No high quality studies of the in vivo metabolism of
acetaldehyde in humans have been identified. Accurate assays for
acetaldehyde in blood and tissues have only recently become available
(Harade et al., 1978a,b).
Human liver ALDH consists of at least 4 main isoenzymes, which
are also present in many other tissues (Koivula, 1975; Goedde et al.,
1979). Mitochondrial ALDH is inactive in at least 40% of the Oriental
population. The frequently observed intolerance to alcohol (the
"flushing" reaction) is linked to this deficiency, which is produced
by an inherited positive mutation in the corresponding gene (Yoshida
et al., 1984; Goedde & Agarwal, 1986, 1987; Hsu et al., 1988).
Subjects with phenotypic deficiency have always shown the presence of
at least one mutant gene (heterozygous or homozygous) (Crabb et al.,
1989; Goedde et al., 1989; Singh et al., 1989).
In vitro, acetaldehyde (0.04-0.88 g/litre) was metabolized at
high rates and in a dose-dependent manner in isolated human
kidney-cortex tubules (Michoudet & Baverel, 1987b).
6.4 Elimination
In dogs, urinary excretion of acetaldehyde was essentially
non-existent following administration of a single dose of 600 mg
acetaldehyde/kg body weight, via a stomach tube (Booze & Oehme, 1986).
6.5 Reaction with cellular macromolecules
6.5.1 Proteins
Acetaldehyde can react with nucleophilic groups, such as amino,
hydroxyl, and sulfydryl groups, through nucleophilic attack on the
carbonyl carbon atom of acetaldehyde to give both stable and unstable
adducts (Tuma et al., 1984). Several adduct structures, formed when
acetaldehyde reacts with proteins in vitro, have been identified,
but have not yet been described fully.
The best characterized nucleophiles able to form adducts with
acetaldehyde are amino groups, notably the alpha-amino terminus of
peptides and proteins and the epsilon-amino group on the side-chain of
lysine residues. These reactions are shown in Fig. 2.
The structure of 2-methylimidazolidin-4-one adducts has been
confirmed by proton NMR (Gidley et al., 1981) and 13C-NMR
spectroscopy (San George & Hoberman, 1986). N-ethylation lysine
residues have been demonstrated by Tuma et al. (1984).
In a series of studies on lysine-dependent enzymes, Mauch et al.
(1986, 1987) were able to demonstrate that incubation of purified
enzymes with acetaldehyde for 1 h at 37°C led to inhibition of their
catalytic activity. Lysine non-dependent enzymes were not affected by
this treatment. A similar study involving the incubation of rat liver
histone H1 with physiological concentrations of acetaldehyde showed
that spontaneously stable adducts were formed on lysine residues at
the carboxy terminus, a site crucial for its function as a eukaryotic
repressor (Niëmela et al., 1990). This acetaldehyde-modified histone
H1 showed impaired DNA binding activity.
Tuma et al. (1987) have characterized the interaction of
acetaldehyde with a "highly reactive" lysine residue in purified
alpha-tubulin, which is only available in the monomeric form. They
found that modification of this residue was the critical factor in the
inhibition of tubulin polymerization by acetaldehyde, and that
modification of 5% of these residues was enough to inhibit tubulin
polymerization completely in vitro. Crebelli et al. (1989) found
similar effects: 0.075% v/v (13.5 mmol/litre) acetaldehyde partially
inhibited the in vitro polymerization of cattle brain tubulin, and
0.15% (27 mmol/litre) caused complete inhibition.
Incubation of calf brain microtubular proteins also resulted in
decreased polymerization, in an analogous manner to tubulin (McKinnon
et al., 1987a,b). Thus, acetaldehyde modification can impair the
molecular function of macromolecules, which can lead to marked
alterations in biological function.
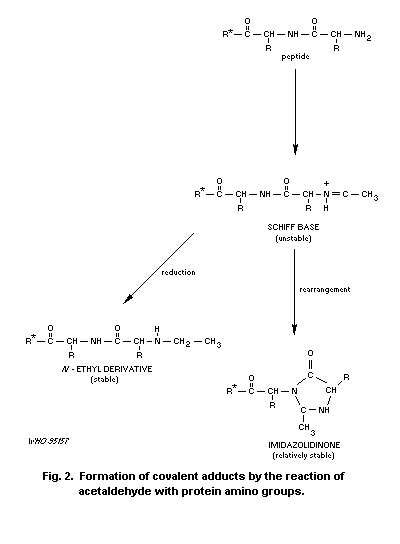 No data are available on the formation of acetaldehydemodified
proteins in animals or humans directly exposed to acetaldehyde.
However, some data are available on proteins modified by acetaldehyde
derived from ethanol metabolism. In these studies, proteins carrying
acetaldehyde adducts were shown to be present in the liver cytosol of
rats fed ethanol for periods of 3 weeks, 12 months, or 27 months
(Worrall et al., 1991a). Acetaldehyde-modified proteins have also been
detected in the plasma (Liu et al., 1990) and haemoglobin of
alcoholics (Niemela & Israel, 1992). Furthermore, a limited
immunohistochemical study has demonstrated the presence of
acetaldehyde-modified proteins in the livers of some alcoholics
(Niemela et al., 1991). These studies demonstrate that acetaldehyde
adducts can form in the body. The possible immunological consequences
of adduct formation will be discussed in section 8.9.
6.5.2 Nucleic acids
No data are available from in vivo studies on the generation of
DNA adducts.
Acetaldehyde reacts with nucleosides and deoxynucleosides at pH
6.5 and 37°C in vitro to form unstable adducts by binding to the
exocyclic amino groups of adenine, cytosine, and guanine (Hemminki &
Suni, 1984). Addition of a reducing agent (sodium borohydride) leads
to the formation of stable adducts, of which the main one was
identified as N2-ethylguanosine using mass spectrometry and NMR.
Similar data for the formation of unstable adducts formed by reacting
acetaldehyde with ribonucleosides and deoxyribonucleosides was
reported by Fraenkel-Conrat & Singer (1988). When ethanol was present
in the reaction mixture, a different type of adduct was formed, which
was identified by fast atom bombardment and proton NMR to be a mixed
acetal (-NH-CH(CH3)-OR). These adducts were found to have half-lives
varying from 2.5 to 24 h at pH 7.5 and 37°C, depending on the base
involved.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
An LC50 (semi-static study) of 35 mg/litre was found for
acetaldehyde in the guppy (Poecilia reticulata; 10 laboratory-reared
and acclimatized fish, 2-3 months old) (Deneer et al., 1988). Grahl
(1983) reported an LC50 (48-96 h) of 124 mg/litre for acetaldehyde
in fish (no additional information was provided). Juhnke & Luedemann
(1978) presented the results for fish obtained in the Golden Orfe
test, and found an LC50 of 140-124 mg/litre for acetaldehyde (no
additional information was provided). An LC50 (static conditions;
96-h) of 53 mg/litre was reported for the bluegill (Lepomis
macrochirus) by Von Burg & Stout (1991).
Acetaldehyde had a depressing effect on the aggressive behaviour
of the fish cichlid (Cichlasoma nigro fasciatum) at concentrations
that did not cause locomotor decrements in this species (Peeke &
Figler, 1981).
An EC5 (48-h; population growth) of 82 mg/litre and an EC50
(48-h; static conditions; immobilization) of 42 mg/litre were reported
for protozoa (Chilomonas paramecium) and the waterflea (Daphnia
magna), respectively (Von Burg & Stout, 1991).
7.2 Terrestrial organisms
Aharoni & Barkai-Golan (1973) studied the effects of acetaldehyde
vapours on the germination and colony-forming potential of two fungi
species, Alternaria tenuis and Stemphylium botryosum. The rate of
growth inhibition increased with both concentration and time of
exposure. The exposure of the spores was conducted at room
temperature. A. tenuis, the more sensitive species, was inactivated
by 0.54 µg acetaldehyde/m3 applied for 5 h, whereas 1.08 µg
acetaldehyde/m3 for 2 h, was needed to inactivate S. botryosum
spores.
Pittevils et al. (1979) reported the activity of acetaldehyde
against the fungi affecting stored apples and pears (Colletotrichum
gloeosporioides, Cryptosporiopsis malicorticis, Phlyctaena
vagabunda, Botrytis cinerea, and Alternaria tenuis). Acetaldehyde
was rapidly lethal at low concentrations: after a 24-h treatment
period, the lethal concentration of acetaldehyde ranged from
0.036 µg/m3 (A. tenuis) to 0.09 µg/m3 (C. gloeosporioides).
Acetaldehyde remained lethal for the five fungi, even when the
treatment lasted only 20 min (0.90 µg/m3 for P. vagabunda, C.
malicorticis, and A. tenuis, and 0.36 µg/m3 for C. gloeosporioides
and B. cinerea).
The fungi Botrytis cinerea, Penicillium expansum, Rhizopus
stolonifer, Monilinia fructicola, Erwinia carotovora, and
Pseudomonas fluorescens were killed, when exposed to
acetaldehyde vapours at concentrations ranging from 0.045 to
3.6 µg/m3, applied for 0.5 to 120 min at room temperature (Aharoni &
Stadelbacher, 1973).
Aharoni et al. (1979) studied acetaldehyde as a fumigant for
control of the green peach aphid (Myzus persicae) on head lettuce
(Lactuca sativa). When aphids were placed on the lettuce prior to
fumigation, 0.36 µg acetaldehyde/m3 and a 3-4 h exposure were
required for 100% mortality. A similar treatment (0.27-0.36 µg/m3
for 4 h) was found to cause 100% mortality of aphids on lettuce by
Stewart et al. (1980).
The fumigant effect of acetaldehyde was tested on the garden slug
(Arion hortensis; weight range, 0.2-0.5 g) and the grey field slug
(Agriolimax reticulatus; weight range, 0.3-0.6 g). It caused both
species to close the pulmonary aperture and to secrete excess
'irritation' mucus. Medial lethal values of 7.69 ± 0.21 mg/litre
per h for A. reticulatus and of 8.91 ± 0.81 mg/litre per h for
A. hortensis were found (Henderson, 1970).
The seed germination of the onion (Allium cepa L.), carrot
(Daucus carota L.), Palmer Amaranth (Amaranthus palmeri S Wats.),
and tomato (Lycopersicon esculentum Mill.) after exposure to
acetaldehyde (up to 1.52 mg/litre), was examined by Bradow & Connick
(1988). After a 3-day exposure, acetaldehyde inhibited the seed
germination of all four plants by more than 50%. Seeds inhibited by a
3-day exposure to acetaldehyde followed by a 4-day recovery period
germinated to the same extent as the controls after seven days, except
for the Palmer Amaranth, which remained inhibited.
Acetaldehyde at concentrations of 0.54-1.08 µg/m3 affected head
lettuce (Lactuca sativa), as evidenced by dark-green, water-soaked,
necrotic areas on the outer leaves of the lettuce. Concentrations of
up to 0.36 µg/m3 did not affect the lettuce (Aharoni et al., 1979;
Stewart et al., 1980).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 LD50 and LC50 values
Relevant data are summarized in Table 4.
Oral LD50s for acetaldehyde in rats and mice ranged from 660 to
1930 mg/kg body weight. LC50s (0.5-4 h) in rats and Syrian hamsters
ranged from 24 to 37 g/m3. It is, therefore, concluded that the
acute toxicity of acetaldehyde is low. LD50 values by the dermal
route were not available.
LD50s for intratracheal, subcutaneous, intraperitoneal, and
intravenous administration are also presented in Table 4.
Table 4. LD50/LC50 values for acetaldehyde
Species Route of LD50/LC50 Reference
administration
Rat oral 1930 mg/kg body weight Smyth et al. (1951)
Rat oral 660 mg/kg body weight Sprince et al. (1974)
Mouse oral 1230 mg/kg body weight US NRC (1977)
Dog oral > 600 mg/kg body weight Booze & Oehme (1986)
Rat inhalation 24 g/m3; 4 h Appelman et al. (1982)
Rat inhalation 37 g/m3; 0.5 h Skog (1950)
Syrian
hamster inhalation 31 g/m3; 4 h Kruysse (1970)
Syrian
hamster intratracheal 96.1 mg/kg body weight Feron & De Jong (1971)
Rat subcutaneous 640 mg/kg body weight Skog (1950)
Mouse subcutaneous 560 mg/kg body weight Skog (1950)
Mouse intraperitoneal 500 mg/kg body weight Truitt & Walsh (1971)
Mouse intravenous 165 mg/kg body weight O'Shea & Kaufman
(pregnant) (1979)
8.2 Short-term exposure
8.2.1 Oral
Oral administration in the drinking-water of 675 mg
acetaldehyde/kg body weight to Wistar rats, daily for 4 weeks,
resulted in slight to moderate focal hyperkeratosis of the forestomach
in 8/10 males and 8/10 females. No effects were observed at lower
dose levels of 25 and 125 mg/kg body weight. In the control group,
very slight focal hyperkeratosis of the forestomach was noted in 6/20
females and 3/20 males (1/20 slight). At the top dose (675 mg/kg),
relative kidney weights were slightly increased in males, urinary
production was decreased, and there were variations in serum
biochemistry, most of which were attributable to reduced water intake.
There were no effects in the liver. The no-observed-effect level
(NOEL) was 125 mg/kg, the lowest-observed-effect level (LOEL) was
675 mg/kg (Til et al., 1988).
8.2.2 Inhalation
Male Sprague-Dawley rats were continuously exposed to
acetaldehyde vapour for 22 days at levels gradually increasing from
750 mg/m3 for a few days up to 2500 mg/m3 for the last few days.
By gradually increasing the concentrations, mortality in the early
period following exposure to 2000-2500 mg/m3 was prevented,
presumably because of metabolic adaptation; sudden, high, blood
acetaldehyde levels inducing vagal reflex reactions may result in
respiratory inhibition, and, as a consequence, death (Lamboeuf et al.,
1987; Latge et al., 1987).
Groups of 10 male and 10 female Wistar rats were exposed to
acetaldehyde at 0, 720, 1800, 3950, or 9000 mg/m3 (0, 400, 1000,
2200, or 5000 ppm) for 6 h/day, 5 days/week, for 4 weeks. Mortality
was slightly increased at 3950 and 9000 mg/m3, whereas growth was
retarded at 1800 mg/m3 and above in males, and at 9000 mg/m3 in
females. At 9000 mg/m3, relative liver weight decreased, and
relative lung weight in males increased. No treatment-related
histopathological changes were observed in the liver. Degenerative
changes of the nose were observed after exposure to all concentrations
(720 mg/m3-9000 mg/m3), with hyperplasia and metaplasia occurring
at concentrations of 3950 mg/m3 or more. A NOEL was not identified
(LOEL: 720 mg/m3) (Appelman et al., 1982).
Groups of 10 male Wistar rats were exposed to acetaldehyde, for
6 h/day, 5 days/week, for 4 weeks, in three different patterns: (a)
as a continuous daily exposure of 6 h to 0, 270, or 900 mg/m3 (0,
150, or 500 ppm), (b) as two daily exposures of 3 h to similar
concentrations with an intervening 1.5-h period with no exposure, or
(c) as two daily 3-h periods of exposure to similar concentrations
with an intervening 1.5-h period with eight short (5-min) peaks of 6
times the basic concentration, resulting in time-weighted average
concentrations of 0, 255, or 1050 mg/m3, respectively. Though there
were no indications of toxicity following continuous or interrupted
exposures to 270 and 900 mg/m3 and intermittent high/low exposure to
255 mg/m3, intermittent high/low exposure to 1050 mg/m3 induced
growth retardation (Appelman et al., 1986).
At 900 mg/m3, the observed effects were very similar to the
ones reported earlier by Appelman et al. (1982) at 720 mg/m3.
Variation of the pattern of exposure, by including a 1.5-h break, or
by additionally including eight 5-min, 6-fold higher peak exposures,
did not alter the observed degenerative effects. No effects were
observed in Wistar rats exposed to a lower concentration, 5 days/week
for 4 weeks, either as a "continuous" (6 h/day) exposure of
270 mg/m3, or as a time-weighted average of 255 mg/m3 after the
described intermittent low-high exposure. The NOEL was 255 mg/m3,
6-h TWA (LOEL = 1050 mg/m3, 6-h time weighted average) (Appelman et
al., 1986).
In another study, groups of 12 male Wistar rats were exposed to 0
or 437 mg acetaldehyde/m3 (0 or 243 ppm), 8 h/day, 5 days per week,
for 5 weeks. Hyperplasia of the olfactory epithelium and nasal
inflammation were observed in exposed animals, and on the basis of
lung function tests, residual volume and functional residual capacity
were increased, indicating some (unspecified) damage of the distal
airways (Saldiva et al., 1985).
8.2.3 Dermal
No relevant studies were identified.
8.2.4 Parenteral
Effects in the liver have been reported in several studies, but
only at very high doses. Intraperitoneal injection of male albino
rats with 200 mg acetaldehyde/kg body weight, daily, for 10 days, with
additional pyrazole treatment to inhibit the conversion of
acetaldehyde to ethanol, caused fatty accumulation in the liver, as
indicated by accumulation of total lipids, triacyl glycerols, and
total cholesterol, increased glycogenolysis, and a shift in metabolism
from the citric acid cycle towards the pentose phosphate pathway in
the liver. Serum triacyl glycerol, total cholesterol, and free fatty
acid levels were also increased. Changes were similar in rats not
receiving pyrazole pretreatment (Prasanna & Ramakrishnan, 1984a,
1987). The same treatment altered thyroid function, as indicated by
lower serum T4 and decreased iodine uptake in male albino rats, though
these effects may have been secondary to the observed hepatic changes
(Prasanna et al., 1986) and histopathological changes of the pancreas,
with resulting changes in trypsinogen levels and amylase secretion and
activity in female Sprague-Dawley rats (Majumdar et al., 1986).
8.3 Skin and eye irritation; sensitization
No relevant data were identified.
8.4 Long-term exposure
8.4.1 Oral
In rats exposed to 0.05% acetaldehyde in drinking-water
(estimated by the Task Group to be approximately 40 mg/kg body weight)
for 6 months, there was an increase in collagen synthesis in the liver
(Bankowski et al., 1993). The toxicological significance of this
observation is not known; no other effects were examined.
8.4.2 Inhalation
Non-neoplastic effects observed in carcinogenicity studies are
discussed in section 8.7.1.
Groups of 20 Syrian hamsters were exposed to acetaldehyde vapour
at 0, 700, 2400, or 8200 mg/m3 (0, 390, 1340, or 4560 ppm) for
6 h/day, 5 days/week, for 13 weeks. Increased relative lung and heart
weights as well as growth retardation were reported after exposure to
8200 mg/m3, though there were no increases in mortality in any of
the exposed groups (Kruysse et al., 1975). At the highest
concentration, there were severe degenerative, hyperplastic, and
metaplastic changes in the epithelium as well as subepithelial glands
and turbinate bones. Rhinitis was observed, with abundant nasal
discharge and salivation. The epithelium of the larynx, trachea, and
lungs was damaged, with some focal hyperplasia and metaplasia,
accompanied by tracheitis and focal bronchopneumonia. Changes in the
tracheal epithelium were also observed at 2400 mg/m3. At
700 mg/m3, no significant effects were observed (NOEL: 700 mg/m3;
LOEL: 2400 mg/m3).
8.5 Reproductive and developmental toxicity
Studies on reproductive effects have not been identified. A
number of studies on developmental effects have been conducted,
primarily to investigate the role of acetaldehyde in ethanol-induced
teratogenicity. However, in all of these studies, acetaldehyde was
administered by injection rather than by the principal routes of
exposure in the occupational and general environments (i.e., ingestion
and inhalation). Results of identified studies in which acetaldehyde
was administered during gestation to rats and mice by intraperitoneal,
intravenous, or amniotic injection are presented in Table 5. Though
dose-related embryotoxic, fetotoxic, and teratogenic effects were
observed in most of these studies, particularly those on rats,
maternal toxicity was not adequately assessed or reported in any of
these investigations. Dose-related embryotoxic effects were also
observed in in vitro studies on rat embryos exposed to acetaldehyde
(Popov et al., 1981; Campbell & Fantel, 1983).
Effects on the placenta have been observed following
intraperitoneal injection of acetaldehyde into pregnant rats
(Sreenathan et al., 1984a). In an in vitro study on human placental
membrane vesicles, very high concentrations (approximately 100 times
higher than the highest reported levels in blood), inhibited L-alanine
transport (Asai et al., 1985).
8.6 Mutagenicity and related end-points
Relevant data are summarized in Table 6.
8.6.1 Bacteria
Acetaldehyde was not mutagenic when tested adequately in standard
preincubation assays with S. typhimurium.
Statistically significant mutagenic responses were induced in
E. coli WP2uvrA in preincubation assays without metabolic activation
at 37°C (Veghelyi et al., 1978) and 0°C (Igali & Gazso, 1980). In
contrast, in another study on the same strain, results were negative
(Hemminki et al., 1980).
8.6.2 Non-mammalian eukaryotic systems
8.6.2.1 Gene mutation assays
Acetaldehyde (0.1% or 1.0% for 2 h) induced mutations in
genes that affect the egg-laying system of Caenorhabditis elegans
(Greenwald & Horvitz, 1980).
Acetaldehyde induced sex-linked recessive lethal mutations in
Drosophila melanogaster (Woodruff et al., 1985).
8.6.2.2 Chromosome alterations
Acetaldehyde induced chromosome malsegregation and mitotic
cross-over (yA2 marker) in Aspergillus nidulans diploid strain P1
during early conidial germination (Crebelli et al., 1989).
There was a dose-related increase in chromosomal aberrations in
Vicia faba root tips exposed to acetaldehyde (Rieger & Michaelis,
1960). Acetaldehyde also induced chromosome aberrations, micronuclei,
and sister chromatid exchanges (SCEs) in the root meristem cells of
Allium cepa (Cortes et al., 1986).
Table 5. Developmental toxicity
Species Exposure Dose Effects Reference
Rat day 10, 11, or 12; 0, 50, 75, 100 mg/kg fetal resorption, malformations (oedema, microcephaly, Sreenathan
days 10-12 of body weight, ip micrognathia, micromelia, hydrocephaly, exencephaly), growth et al. (1982,
pregnancy; days retardation; reduced placental weight; 50 mg/kg body weight, 1984b)
8-15 (50 mg/kg days 8-15: delays in ossification; skeletal malformations,
body weight per such as wavy ribs; no discussion of maternal effects
day)
Rat days 8-15 of 50, 75, 100 or 150 increase in resorption, and fetal death (changes were dose Padmanabhan
pregnancy mg/kg body weight, dependent); malformations: oedema, microcephaly, micrognathia, et al. (1983)
ip micromelia, syndactyly, hydronephrosis and subcutaneous
haemorrhage in the face, fore- and hind paws; reduced mean
placental weight and umbilical cord length; increased amniotic
fluid weight
maternal toxicity: rel. reduced water, food consumption, rel.
reduced body weight gain, 50 mg and above
Rat day 13 of pregnancy 1% or 10% solution 10%: 100% mortality; 1%: 80% malformations versus 14% in Bariliak &
embryo amniotic injections controls Kozachuk
(1983)
Rat days 9-12 of pregancy 1% (100 mg/kg body reduction in head length; no effects on morphological Ali &
weight), ip scores or crown rump length Persaud (1988)
Table 5 (contd).
Species Exposure Dose Effects Reference
Rat days 8-13 of 10 or 50 mg/kg body reduced performance in surface righting, olfactory Schreiner
pregnancy weight, ip discrimination, and learning ability; lower startle amplitudes et al. (1987)
in an auditory startle habituation test; maternal toxicity not Abstract
addressed; age of testing of offspring not specified
Rat day 5 of gestation 0.03-0.04 µg/kg body retarded blastulation Checiu et
weight examination on al. (1984)
day 5 of gestation
Mouse day 7 or 8 of 0.32 g/kg, iv exencephaly, mandibular and maxillary hypoplasia; Webster et
pregnancy; day 9 polydactyly club foot; no discussion of maternal effects al. (1983)
or 10 of pregnancy
Mouse day 10 of pregnancy 1000 mg/kg, ip no increase in resorptions or malformed fetuses or decrease Blakley &
in fetal weight; no discussion of maternal effects Scott (1984a)
Mouse day 7, 8 or 9 of 31 or 62 mg/kg body dose-dependent embryolethality; non-closure of anterior or O'Shea &
pregnancy weight, iv; posterior neuropore; smaller embryos; no discussion of Kaufman
examination on day maternal effects (1979)
10 or 19 of gestation
Mouse day 9 of pregnancy 2, 4, 6% (8 ml/kg no intermediate cellular effeects Bannigan &
body weight), ip Burke (1982)
Mouse day 6, 7 or 8 of 62 mg/kg body weight, neural tube effects; embryonic mortality; no discussion O'Shea &
pregnancy; and days iv; examination on of maternal effects Kaufman
6-8, 7-8 or 7-9 of day 10 or 12 of (1981)
pregnancy gestation
Table 6. Tests for gene mutation, chromosomal damage, and sister chromatid exchanges induced by acetaldehyde
Test system Measured end-point Test conditions MA RES Reference
Prokaryotic systems
Escherichia coli WP2uvrA reverse mutations 40 µg/ml; preincubated in capped tubes - + Veghelyi et al. (1978)
Escherichia coli WP2uvrA reverse mutations 0.88-441 µg/ml; incubated in capped - - Hemminki et al. (1980)
tubes at 37°C
Escherichia coli Wp2uvrA reverse mutations 0.8 µg/ml; incubated in capped tubes - + Igali & Gazso (1980)
at 0°C
Salmonella typhimurium
TA102 reverse mutations 1014 µg/plate; liquid preincubation - - Marnett et al. (1985)
TA104 -
Salmonella typhimurium reverse mutations 33-10 000 µg/plate; liquid preincubation + - Mortelmans et al. (1986)
TA1535 - -
TA1537 + -
- -
TA97 + -
- -
TA98 + -
- -
TA100 + -
- -
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Salmonella typhimurium concentration not given; + - Sasaki & Endo (1978)
TA100 liquid preincubation
Non-mammalian eukaryotic systems
Allium cepa root chromosomal aberration 7.5-7500 µg/ml - + Cortés et al. (1986)
meristem cells and SCE +
Vicia faba root tips chromosomal aberration 220-2205 µg/ml for 24 h at 12°C - + Rieger & Michaelis (1960)
Caenorhabditis elegans gene mutation 794-7940 µg/ml for 2 h - + Greenwald & Horvitz
(1980)
Drosophila melanogaster sex-linked recessive 22 500 mg/litre in 10% ethanol by + Woodruff et al. (1985)
injection
Canton-S wild-type lethal mutations 25 000 mg/litre in 10% ethanol by -
feeding
Aspergilus nodulans chromosomal 198-2381 µg/ml + Crebelli et al. (1989)
Diploid P1 malsegregation
In vitro mammalian systems
Mouse lymphoma L5178Y forward mutation (tk 176-353 µg/ml for 4 h - + Wangenheim & Bolcsfoldi
cells locus) (1986, 1988)
Chinese hamster ovary chromosomal aberration 88-5000 µg/ml - + Au & Badr (1979)
cells
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Chinese hamster ovary SCE 1.3-13 µg/ml - + Brambilla et al. (1986)
cells
Chinese hamster ovary SCE 8-80 µg/ml for 1 h + + De Raat et al. (1983)
cells - +
Chinese hamster ovary SCE 2-12 µg/ml for 24 h - + Obe & Beek (1979)
cells
Chinese hamster ovary SCE 4-8 µg/ml for 8 days - + Obe & Ristow (1977)
cells
Chinese hamster ovary hyperploidy 0.35-1.05 mmol/litre - + Dulout & Furnus (1988)
cells hypoploidy +
Human lymphocytes forward mutations 26.5-106 µg/ml for 24 h - + He & Lambert (1990)
(hprt locus) 8.8-26.5 µg/ml for 48 h
Human lymphocytes chromosomal aberration 0.1-20 mmol/litre added + Obe et al. (1985)
every 12 h for 5 days
Human lymphocytes chromosomal aberration 8-15 µg/ml - + Obe et al. (1979)
(normal Franconis
anaemia)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 0.8 µg/ml 2x/day, 4 days - + Obe et al. (1978)
Human lymphocytes chromosomal aberration 4-48 µg/ml for 72 h - + Boehlke et al. (1983)
SCE +
Human (whole blood, chromosomal aberration 20-40 µg/ml + Badr & Hussain (1977)
alcoholics)
Human lymphocytes SCE 4.4-106 µg/ml for 1-70 h - + He & Lambert (1985)
Human lymphocytes SCE 4.4-13 µg/ml for 70 h - + Lambert & He (1988)
Human lymphocytes SCE 4.4-18 µg/ml for 72 h + Helander & Lindahl-
Kiessling (1991)
Human lymphocytes SCE 4.4-22 µg/ml for 48 h + Sipi et al. (1992)
Human lymphocytes SCE 4-8 µg/ml for 90 h - + Jansson (1982)
Human lymphocytes SCE 0.04-4.4 µg/ml for 55 h - + Knadle (1985)
Human lymphocytes SCE 5.5-88 µg/ml for 48 h - + Norppa et al. (1985)
Human lymphocytes SCE reaction mixture added to whole blood - + Obe et al. (1986)
samples in dialysis tube; + +
concentration not given
Human lymphocytes SCE 4-16 µg/ml for 24 h - + Ristow & Obe (1978)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 1.8-35 µg/ml for 72 h - + Véghelyi & Osztovics
(1978)
Human lymphocytes SCE 1.8-35 µg/ml for 72 h + Véghelyi et al. (1978)
Wistar rat (skin chromosomal aberration 4.4-44 µg/ml for 12, 24, or 48 h - + Bird et al. (1982)
fibroblast) (micronuclei)
Mouse embryo cell transformation 10-100 µg acetaldehyde/ml for 24 h - - Abernethy et al. (1982)
C3H/10T1/2 cells 10-100 µg/ml followed by 0.25 µg +
TPA/ml 2x/week
Rat (HRRT kidney cells) cell transformation acetaldehyde - - Eker & Sanner (1986)
acetaldehyde followed by TPA and PDD +
MA = Metabolic activation; RES = Result.
8.6.3 Cultured mammalian cells
8.6.3.1 Gene mutation assays
Acetaldehyde induced gene mutations at the hypoxanthineguanine
phosphoribosyl transferase (hprt) locus in human lymphocytes
in vitro; there was a statistically significant and dose-related
increase in the frequency of mutants (He & Lambert, 1990).
Acetaldehyde produced a significant dose-related increase in forward
mutations in a mouse lymphoma L5178Y thimidine kinase locus assay,
without exogenous metabolic activation (Wangenheim & Bolcsfoldi, 1986,
1988).
8.6.3.2 Chromosome alterations and sister chromatid exchange
Dose-dependent increased frequencies of chromosome aberrations
have been observed in human lymphocytes following incubation with
acetaldehyde (Badr & Hussain, 1977; Obe et al., 1978, 1979, 1985;
Veghelyi & Ostovics, 1978; Boehlke et al., 1983).
Acetaldehyde induced a dose-dependent increase in chromosomal
damage in Chinese hamster ovary cells, though it was reported that the
activity was reduced (not quantified) in the presence of liver
homogenate fraction (Au & Badr, 1979). Dose-related increases in
micronuclei and chromosome aberrations were observed in cultured rat
skin fibroblasts in the absence of metabolic activation (Bird et al.,
1982).
SCE was induced by acetaldehyde (1.3-106 µg/ml) in the absence of
exogenous metabolic activation in Chinese hamster ovary cells and
human lymphocyte cultures (Obe & Ristow, 1977; Ristow & Obe, 1978;
Veghelyi et al., 1978; Veghelyi & Osztovics, 1978; Obe & Beek, 1979;
Jansson, 1982; Boehlke et al., 1983; De Raat et al., 1983; He &
Lambert, 1985; Knadle, 1985; Norppa et al., 1985; Brambilla et al.,
1986; Obe et al., 1986; Lambert & He, 1988; Helander &
Lindahl-Kiessling, 1991; Sipi et al., 1992). The increase in SCEs in
Chinese hamster ovary cells was less pronounced after addition of an
exogenous metabolic activating system (De Raat et al., 1983).
Similarly, addition of NAD+ and ALDH to human lymphocyte cultures
exposed to acetaldehyde decreased SCE induction (Obe et al., 1986).
Addition of 1-amino-cyclopropanol, an inhibitor of ALDH, to human
lymphocytes enhanced SCE induction by acetaldehyde (Helander &
Lindahl-Kiessling, 1991). These observations suggest that ALDH may
reduce the genotoxic potential of acetaldehyde.
Acetaldehyde induced aneuploidy has been reported in Chinese
hamster ovary cells (Dulout & Furnus, 1988). However, mainly
hypoploid cells were increased and, therefore, no firm conclusion can
be drawn from these studies. Acetaldehyde induced SCE in
pre-implantation mouse embryos in vitro (Lau et al., 1991).
8.6.4 In vivo assays
Only limited data are available on the genotoxicity of
acetaldehyde in vivo.
8.6.4.1 Somatic cells
C57BL/6J mice (only 2 animals of unspecified sex at each dose
level) received daily intraperitoneal doses of 0, 6, or 12 mg
acetaldehyde/kg body weight per day, for 5 days, and blood samples
were collected on days 3-6. The frequencies of micronuclei in mature
peripheral erythrocytes on days 5 and 6 (combined) were significantly
increased at the low dose, but not at the high dose (Ma et al., 1985).
No information on the frequency in polychromatic erythrocytes was
provided, so the possibility that this result was due to increased
erythropoiesis instead of mutagenesis cannot be discounted. This
study was reported only in the form of an abstract.
Groups of 6-7 Chinese hamsters (male and female) received 0.01,
0.1, or 0.5 mg acetaldehyde/kg body weight in saline by single
intraperitoneal injection and were killed 24 h later. Doses of
0.6 mg/kg body weight were lethal in preliminary tests. The
frequencies of SCEs in bone-marrow metaphases were elevated at 0.5 mg
acetaldehyde/kg body weight, but not at the lower doses (Korte & Obe,
1981). These observations support earlier findings in a more limited
study at an embryotoxic dose in mice and indicate in vivo formation
of SCEs (Obe et al., 1979).
Female Wistar rats were injected with 0.02 ml of 1% acetaldehyde
intra-amniotically on day 13 of pregnancy. Embryonic cells were
obtained 24 h later for cytogenetic analysis. Exposed rat embryos had
a higher frequency of chromosomal aberrations (mostly chromatid gaps
and breaks) than controls (Barilyak & Kozachuk, 1983). There was no
increase in the frequency of aneuploid cells.
8.6.4.2 Germ cells
Hybrid male mice ((C57B1/6J × C3H/He)F1), 4/dose group, were
injected intraperitoneally with a single dose of 0, 125, 250, 375, or
500 mg acetaldehyde/kg body weight in saline. Thirteen days after
exposure, the frequency of micronuclei in early spermatids was not
increased, though there were adequate positive controls (Lahdetie,
1988).
8.6.5 Other assays
Relevant data are summarized in Table 7.
8.6.5.1 DNA single-strand breaks
No single-strand breaks were detected with the alkaline elution
assay in DNA from various sources, after exposure in vitro to
acetaldehyde (Sina et al., 1983; Marinari et al., 1984; Harris et al.,
1985; Lambert et al., 1985; Saladino et al., 1985).
8.6.5.2 DNA cross-linking
There is some indirect evidence suggesting DNA cross-linking by
acetaldehyde.
Ristow & Obe (1978) observed enhanced reannealing of heat
denatured, isolated, calf thymus DNA following incubation with
acetaldehyde. It was suggested that this may have been due to DNA-DNA
cross-linking, because thermally denatured DNA will not reanneal in
this assay, unless complimentary DNA strands are held together by
cross-linkings.
Altered alkaline elution patterns indicative of DNA-DNA
cross-links were observed with DNA isolated from cultured human
leukocytes and exposed initially to acetaldehyde and then to X-rays
(Lambert et al., 1985; Lambert & He, 1988). DNA-protein cross-linking
was not observed by Harris et al. (1985) or by Saladino et al. (1985)
after exposure of human bronchial epithelial cells in vitro, but it
was observed by Lam et al. (1986) after incubation of either calf
thymus nucleohistones or fresh homogenates of the nasal mucosa of rats
with acetaldehyde. The possibility that acetaldehyde induces
DNA-protein cross-links in male Fischer-344 rat nasal mucosa has been
indirectly studied by measuring the extractability of DNA from
insoluble proteins following in vitro and in vivo exposures.
Decreased extractability of DNA was observed in preparations from
tissue homogenates exposed to acetaldehyde at concentrations of 100
and 500 mmol per litre but not 10 mmol/litre. Similarly, decreased
extractability was observed in nasal mucosal preparations from rats
exposed for 6 h to 1000 or 3000 mg/litre (ppm) but not 100 or
300 mg/litre (ppm) (Lam et al., 1986).
Table 7. Other tests indicative of genetic damage induced by acetaldehyde
Test system Measured end-point Test conditions Results Reference
Calf thymus DNA DNA-DNA cross-links 44 100 µg/ml for 30 min + Ristow & Obe (1978)
Calf thymus nucleohistones DNA-protein cross-links 4410-44 100 µg/ml - Lam et al. (1986)
Chinese hamster ovary DNA single-strand breaks no details - Marinari et al. (1984)
K1-cells DNA-DNA cross-links +
Rat hepatocytes DNA single-strand breaks 1.3 µg/ml for 3 h - Sina et al. (1983)
Human bronchial epithelial DNA single-strand breaks unknown concentration; - Harris et al. (1985)
cells DNA-protein cross-links 6 h -
Human bronchial epithelial DNA single-strand breaks up to 44 µg/ml for 1 h - Saladino et al. (1985)
cells DNA-protein cross-links -
Human leukocytes DNA single-strand breaks 441-882 µg/ml for 4 h - Lambert et al. (1985)
DNA-DNA cross-links +
Human lymphocytes DNA-DNA cross-links 441 µg/ml + Lambert & He (1988)
8.6.6 Cell transformation
Acetaldehyde did not initiate cell transformation in cultured
C3H/10T1/2 cells in the presence of the tumour promotor
12- O-tetradecanoylphorbol-13-acetate (TPA) (Abernethy et al., 1982).
Acetaldehyde initiated cell transformation in a rat kidney cell
line (HRRT) after pretreatment with tumour promotors (Eker & Sanner,
1986).
8.7 Carcinogenicity bioassays
8.7.1 Inhalation exposure
Only one carcinogenicity study in which animals were exposed by
inhalation to acetaldehyde over a lifetime has been performed. In
other carcinogenicity bioassays, animals were exposed for shorter
periods.
Wistar rats (55/sex per dose) were exposed for life (6 h/day, 5
days/week, for 28 months) to acetaldehyde concentrations of 1350,
2700, or 1800-5400 mg/m3 (the last concentration was gradually
reduced from 5400 mg/m3 in week 20 to 1800 mg/m3 in week 52).
Satellite groups of 5-10 additional rats of each sex were killed at
13, 26, and 52 weeks. Growth retardation occurred throughout the
study at all dose levels. Mortality was greater than in controls in
all dose groups and all of the animals in the high-dose group had died
by week 102. At week 52, there were degenerative changes in the
olfactory nasal epithelium at all dose levels including slight to
severe hyperplasia and keratinized stratified metaplasia of the larynx
(high dose only) and degenerative changes of the upper respiratory
epithelium (including papillomatous hyperplasia at the top dose only).
In the trachea, there was focal flattening and irregular arrangement
of the epithelium in 3/10 top-dose males at 52 weeks. In satellite
groups of 30 rats per sex, for which there was a 26-week recovery
period after 52 weeks of exposure, there was evidence of partial
regeneration of the olfactory epithelium in the low- and mid-dose
groups; there was also progression from hyperplasia and metaplasia to
neoplasia in some animals. At 28 months, carcinomas of the nose
developed in all exposed groups (Table 8). Although tumour incidence
was dose-related, the latency period appeared to be independent of
concentration. First tumours in all groups appeared during the 12th
month of exposure. The incidence of tumours was not increased in the
lungs, larynx, and trachea.
In simultaneously exposed groups of 30 Wistar rats/sex per dose,
exposure was terminated after 52 weeks and the animals killed
following a recovery period of 26 weeks. After the recovery period,
both mortality and nasal tumour incidence were very similar to those
in the groups in which exposure had been continued for 26 more weeks
(Woutersen et al., 1984, 1986; Woutersen & Feron, 1987).
Groups of Syrian golden hamsters (36/sex per dose) were exposed
for 52 weeks (7 h/day, 5 days/week) to a concentration gradually
reduced from 4500 mg/m3 in week 9 to 2970 mg/m3 in week 52. At
week 52, six animals/sex per dose were killed; the remaining animals
(30/sex per dose) were killed after a recovery period of 29 weeks. At
the end of the recovery period, nasal carcinomas were observed in 1/26
males and 1/27 females versus 0/24 and 0/23 in controls, and the
incidence of laryngeal carcinomas was increased (5/23 in males, 3/20
in females, against 0/20 and 0/22 in controls). No tumours were
observed in bronchi or lungs (Feron et al., 1982).
Table 8. Incidence of nasal tumours in Wistar rats after 28 months
of exposurea,b
Types of tumour 0 1350 2700 2760c
mg/m3 mg/m3 mg/m3 mg/m3
Males
Squamous cell carcinoma 1/49 1/52 10/53* 16/49***
Adenocarcinoma 0/49 16/52*** 31/53*** 21/49***
Carcinoma in situ 0/49 0/52 0/53 1/49
Females
Squamous cell carcinoma 0/50 0/48 5/53 17/53***
Adenocarcinoma 0/50 6/48* 28/53*** 23/53***
Carcinoma in situ 0/50 0/48 3/53 5/53
a From: Woutersen et al. (1986).
b Total number of tumour-bearing animals not specified.
Significance: Fisher Exact Test *P < 0.05, **P < 0.01, ***P <
0.001.
c The highest concentration was gradually reduced from
5400 mg/m3 during the first 20 weeks to 1800 mg/m3 in week
52; the time-weighted average concentration for 28 months of
exposure was calculated by the Task Group to be 2760 mg/m3.
Groups of 35 male Syrian hamsters were exposed by inhalation to 0
or 2700 mg acetaldehyde/m3 (0 or 1500 ppm) for 7 h/day, 5 days/week
for 52 weeks. At 52 weeks, 5 animals were killed for pathological
examination. The remainder of the animals were maintained for a
further 26-week recovery period. Body weight gain was less in the
exposed animals and mortality was increased from week 52 onwards.
There was a slight, but significant, decrease in rbc, haemoglobin, and
haematocrit in exposed animals. Relative kidney weights were
increased and urinary levels of protein and GOT were elevated. There
were marked lesions in the nasal cavity of animals killed at 52 weeks:
flattened epithelial cells with bizarre nuclei, fewer subepithelial
glands, submucosal thickening, and keratinizing stratified squamous
metaplasia of olfactory and respiratory epithelium. There was also
slight focal hyperplasia and metaplasia of the epithelium of the
trachea. There was partial or complete recovery from these lesions in
the animals killed at the end of the recovery period. There were no
tumours of the respiratory tract in any of the animals exposed to
acetaldehyde (Feron, 1979).
Weekly intratracheal administration of 0.2 ml of 2 or 4%
acetaldehyde in saline during a period of 52 weeks, followed by a
recovery period of another 52 weeks, did not induce any tumours in the
respiratory tract of male and female Syrian golden hamsters (Feron,
1979).
8.7.2 Co-carcinogenicity and promotion studies
In a mid-term test (Ito Model), groups of 19-20 male F344 rats
received a single intraperitoneal injection of diethylnitrosamine and
then various concentrations of acetaldehyde (2.5% and 5%, associated
with a recorded daily intake of 1.66 and 2.75 mg/kg body weight) in
their drinking-water, from week 2 until termination in week 6. All
rats had a two-thirds partial hepatectomy in week 3, in order to
stimulate cell proliferation. At 6 weeks, there was a significant
decrease in liver and body weight in all exposed animals, but
acetaldehyde had no effect on the development of glutathione
S-transferase (placental type)-positive liver cell foci (Ikawa et
al., 1986).
There were laryngeal tumours in 7/31 male and 6/32 female Syrian
golden hamsters exposed to a mixture of isoprene (approximately
2.09 mg/m3 or 750 ppm), methyl chloride (approximately 1.94 mg/m3
or 950 ppm) methyl nitrite (approximately 0.49 mg/m3 or 195 ppm) and
acetaldehyde (approximately 2340 mg/m3 or 1300 ppm) for 6 h/day, 5
days/week, over 23 months. There were no tumours of the respiratory
tract in controls (Feron et al., 1985). The authors suggested that
the laryngeal effects observed in hamsters exposed to the vapour
mixture were most probably caused by acetaldehyde alone (presumably
based on comparison with results in studies on hamsters exposed to
acetaldehyde alone).
There was no evidence of co-carcinogenicity in Syrian golden
hamsters exposed either by simultaneous inhalation exposure to
acetaldehyde and intratracheal instillation of benzo(a)pyrene (Feron,
1979; Feron et al., 1982) or by intratracheal instillation of
diethylnitrosamine (Feron et al., 1982) for 52 weeks followed by
recovery periods of 26-28 weeks. Nor was there any evidence of the
co-carcinogenicity of acetaldehyde following simultaneous
intratracheal instillations of acetaldehyde and either benzo (a)pyrene
or diethylnitrosamine for 52 weeks, followed by a recovery period of
52 weeks (Feron et al., 1982).
8.8 Neurological effects
Secondary effects of acetaldehyde on the respiratory and
cardiovascular systems, attributed to its influence on the autonomic
nervous system, have been observed following acute exposure of
experimental animals to acetaldehyde, principally by infusion or
intravenous administration. In two identified studies in which
animals were exposed by a route more relevant to environmental or
occupational exposure (i.e., inhalation), a 50% decrease in
respiratory rate was observed in two strains of mice and in rats
during inhalation of 5000 mg/m3 for 10 min (Steinhagen & Barrow,
1984; Babiuk et al., 1985; Takahashi et al., 1986).
Neurological effects following exposure by routes most relevant
to environmental and occupational exposure (i.e., inhalation and
ingestion) were limited to biochemical changes in the brain in
short-term studies on rats and mice exposed to relatively high
concentrations of acetaldehyde (750-13 230 mg/m3). Reported effects
included changes in the phospholipid fractions and increases in the
concentrations of monoamines and Na+/K+ATPase (Ortiz et al., 1974;
Shiohara et al., 1985; Latge et al., 1987; Roumec et al., 1988).
Manifestations of neurotoxicity were not examined in any of these
studies.
In the only identified study in which histopathological effects
on the nervous system were reported, degeneration was observable using
both light and electron microscopy in the cerebral cortex of rats
receiving a single intraperitoneal injection of 5 mg acetaldehyde/kg
body weight (Phillips, 1987).
In a short-term study involving intravenous exposure (24-26 mg/kg
body weight per day, for 20 days), the level of salsolinol in the
brain of rats was increased (Myers et al., 1985). Biochemical effects
of acetaldehyde on the brain have also been observed in vitro in
cerebral cortical neurons and brain microsomal preparations (Lahti &
Majchrowicz, 1969; Cederbaum & Rubin, 1977; Kuriyama et al., 1987).
8.9 Immunological effects
8.9.1 Direct effects on immune cells
Only one study has been carried out on whole animals. In a study
on CD1 mice exposed to 324 mg/m3, 3 h/day for 5 days, the
bacteriocidal activity of alveolar macrophages was reduced by 15%.
However, acetaldehyde did not affect mortality following streptococcal
infection (Aranyi et al., 1986).
In in vitro studies, 0.01% acetaldehyde caused a significant
inhibition of human granulocyte chemotaxis (Schopf et al., 1985). At
0.03%, acetaldehyde inhibited the release of lysozyme from monocytes,
but granulocytes were unaffected. The generation of oxygen radicals
by zymosan particle-stimulated monocytes and granulocytes was
inhibited by acetaldehyde in a dose-dependent manner. In a similar
study in which peripheral mononuclear cells were incubated with
acetaldehyde, decreased lytic activity against K562 cells was observed
at concentrations higher than 12 µmol per litre (Fink & Dancygier,
1988). In another study, acetaldehyde decreased 3H thymidine
incorporation into phytohaemagglutinin or Concanavalin A - stimulated
human lymphocytes (Levallois et al., 1987).
8.9.2 Generation of antibodies reacting with acetaldehyde-modified
proteins
The modification of proteins has been described in section 6.5.1.
In several studies, these modified proteins induced an immune response
when injected into rabbits and mice (Israel et al., 1986; Worrall et
al., 1989). The reactivity of the resulting antibodies towards
proteins, modified by acetaldehyde in vitro, is independent of the
carrier protein. These studies demonstrate (a) that
acetaldehyde-modified proteins are immunogenic; and (b) that
antibodies raised against a single modified protein will cross-react
with any other protein modified in the same manner.
Passive immunization of rats with a monoclonal IgE
antiacetaldehyde adduct antibody, derived from mice immunized with
acetaldehyde-modified proteins, rendered these animals hypersensitive
to acetaldehyde-modified proteins (Israel et al., 1992).
No immunization studies analogous to those described above have
been carried out on humans. In several studies, the presence of
antibodies, reactive with proteins modified by acetaldehyde in vitro,
has been reported in the plasma of alcoholics (Niemela et al., 1987;
Hoerner et al., 1988; Worrall et al., 1990, 1991a,b). The results of
these studies suggest that acetaldehyde-modified proteins are
generated in humans.
8.9.3 Related immunological effects
In rat liver membrane vesicles, exposed to acetaldehyde in vitro,
superoxide anion production by neutrophils was significantly enhanced
(Williams & Barry, 1986). In a further study, human liver plasma
membranes, modified by acetaldehyde, activated complement protein C3
components (Barry & McGivan, 1985).
8.10 Biochemical effects
Biochemical effects have been observed in cultured hepatocytes,
liver homogenates and mitochondrial fractions, purified enzymes,
isolated hepatic lipid membranes and whole liver preparations,
including: metabolic effects on lipid peroxidation (Stege, 1982;
Mueller & Sies, 1987; Shaw & Jayatilleke, 1987), phospholipid
metabolism (Snyder, 1988), transaminase activity (Solomon, 1987;
Snyder, 1988), carbohydrate and lipid metabolism (Matsuzaki & Lieber,
1977; Cederbaum & Dicker, 1981), and mitochondrial respiration
(Cederbaum et al., 1974). Increased hepatic collagen synthesis has
also been observed (Savolainen et al., 1984; Brenner & Chojkier,
1987).
Metabolic effects have been observed in other in vitro systems
including: renal cortex tubules (Michoudet & Baverel, 1985: altered
pyruvate/lactate metabolism), kidney, and muscle microsomal
preparations (Cederbaum & Rubin, 1977: inhibition of pyruvate
dehydrogenase), perfused hearts and cardiac whole homogenates
(Schreiber et al., 1972, 1974: myocardial protein synthesis and
inhibition of cardiac microsomal protein synthesis; Rawat, 1979:
reduced protein synthesis; Segel, 1984: no effect on mitochondrial
respiration), myocardial cells (McCall & Ryan, 1987: no effect on
Na+/K+-ATPase), leukocytes (Green & Baron, 1986: inhibition of
Na+/K+-ATPase), erythrocytes (Helander & Tottmar, 1987: inhibition
of the disappearance rate of biogenic aldehydes; Ninfali et al., 1987:
induction of intracellular reduced state; Solomon, 1988: inhibition of
aldolase-mediated by acetaldehyde oxidation- and inhibition of the
aminotransferases-mediated by non-oxidative acetaldehyde
metabolization; Atukorala et al., 1988: inhibition of transketolase),
and leukocytes and platelets (Helander & Tottmar, 1987: inhibition of
the disappearance rate of biogenic aldehydes).
9. EFFECTS ON HUMANS
9.1 General population exposure
No specific studies were available.
9.2 Occupational exposure
9.2.1 General observations
Acute exposure to acetaldehyde vapours has resulted in irritation
of the eyes and mucous membranes, reddening of the skin, pulmonary
oedema, headache, and sore throat. Repeated exposure causes
dermatitis and conjunctivitis. Ingestion causes nausea, vomiting,
diarrhoea, narcosis, and respiratory failure. Liquid acetaldehyde was
reported to cause superficial injury of the cornea. These effects
were reported in reviews and not in documented studies (Grant, 1974;
Hagemeyer, 1978; Dreisbach, 1987). In addition, a statement was found
in a textbook stating that prolonged exposure to acetaldehyde caused a
decrease in red and white blood cells plus a sustained rise in blood
pressure; however, no further information was available (Hagemeyer,
1978).
9.2.2 Clinical studies
A group of 12 volunteers were exposed in a chamber to various
nominal concentrations of acetaldehyde vapour on 15 different
occasions. No atmospheric sampling was performed. At 90 mg/m3
(50 ppm), the majority of the group experienced some degree of eye
irritation. Several subjects were discomforted at a lower
concentration of 45 mg/m3 (25 ppm), but no details were provided
(Silverman et al., 1946).
A group of 14 "healthy male" volunteers, aged 18-45 years, were
exposed in a 100 m3 chamber to a measured concentration of
acetaldehyde vapour of 240 mg/m3 (134 ppm) for 30 min. This
concentration was said to be mildly irritating to the upper
respiratory tract. No other clinical signs were reported (Sim &
Pattle, 1957).
Twelve volunteers of Oriental ancestry were patch-tested with
acetaldehyde (75%), ethanol (75%), and ethyl acetate (25%). With
acetaldehyde treatment, cutaneous erythema was seen in all 12
subjects, whereas ethanol was positive in 5 subjects. No vascular
response was detected for the acetaldehyde metabolite, ethyl acetate
(Wilkin & Fortner, 1985).
In an additional study, increased heart rate, ventilation, and
calculated respiratory dead space, and a decrease in alveolar CO2
levels were reported, following intravenous infusion of acetaldehyde
(5% v/v) in young male volunteers (Asmussen et al., 1948).
9.2.3 Epidemiological studies
A very limited investigation was performed on an unspecified
number of people, selected from 150 workers who had been employed for
20 years or more in a chemical factory. Nine cases of cancer were
identified (all in male smokers) 5 of which were in the respiratory
tract, 2 in the oral cavity, 1 in the stomach, and 1 in the caecum.
The number of respiratory cancers was higher than expected compared
with the prevalence in the national population (not corrected for
smoking). In parts of the factory, there was exposure to a variety of
chemicals. In one area, 1-7 mg acetaldehyde/m3 had been measured,
together with 5-70 mg butyraldehyde/m3, 1-7 mg crotonaldehyde/m3,
2-6 mg n-butanol per m3, and about 15 mg ethylhexanol/m3. Other
areas of the factory were known to have higher concentrations of
vapours of aldehydes and aldol, which caused irritation of the eyes
and upper respiratory tract (Bittersohl, 1974, 1975).
9.3 Effects of endogenous acetaldehyde
9.3.1 Effects of ethanol possibly attributable to acetaldehyde or
acetaldehyde metabolism
There is no direct evidence to link acetaldehyde with liver
injury. However, data obtained from animal models and human subjects
suggest that acetaldehyde may play a role in liver damage, especially
that associated with ethanol (sections 3.1.1 and 8.10).
An increased sensitivity to ethanol with respect to facial
flushing was observed in certain human populations (especially of
Oriental origin), which was ascribed to genetic differences in
acetaldehyde elimination, due to aldehyde dehydrogenase polymorphism
(Goedde & Agarwal, 1986, 1987; Eriksson, 1987; see also section
6.2.2).
The fetal alcohol syndrome is a specific pattern of congenital
abnormalities found in children of mothers who drink heavily. The
abnormalities most typically associated with alcohol-induced
developmental effects can be grouped into four categories: central
nervous system dysfunction (mental retardation, microcephaly,
hyperactivity); growth deficiencies (reduced prenatal length and
especially weight, with no catch-up postnatal growth; reduced adipose
tissue, normal growth hormone, cortisol, and gonadotropins); a
characteristic cluster of facial abnormalities (short palpebral
fissures, short upturned nose, hypoplastic philtrum, thinned upper-lip
vermillion, flattened midface); and variable major and minor
malformations (such as, heart failure, anomalies of the genitalia,
joints, and palmar creases) (Clarren & Smith, 1978).
The mechanisms by which ethanol produces its developmental
effects are not completely understood, and no data are available with
respect to ethanol doses and/or blood levels needed for such effects.
It is generally assumed that acetaldehyde, as the primary metabolite,
may contribute to the developmental effects of ethanol. Several
animal studies have demonstrated the direct teratogenic effect of
acetaldehyde (Ali & Persaud, 1988; see also section 8.5); however, no
reports are available with respect to the direct teratogenicity of
acetaldehyde in humans.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
By far, the principal source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure for
smokers. With respect to other media, the general population is
exposed to acetaldehyde principally from food, and to a lesser extent
from air. Intake from drinking-water is negligible compared with that
from other media.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route is most likely inhalation and, possibly, dermal
contact.
10.1.2 Health effects
Acetaldehyde is a reactive molecule that adducts to
macromolecules and can also condense or polymerise with small
molecules. Although quantitatively unimportant in acetaldehyde
metabolism, these by-products may have important biological effects.
In studies conducted on animals, the acute toxicity of
acetaldehyde by the oral or inhalation routes was low. Oral LD50s
ranged from 660 to 1930 mg/kg body weight and LC50s (0.5-4 h) from
24 to 37 g/m3. RD50s in rats and mice are slightly greater than
5000 mg/m3.
Data on the irritant effects of acetaldehyde on the skin and eye
are restricted to those from limited studies on human volunteers. It
was mildly irritating to the eyes and upper respiratory tract
following acute exposure for very short periods, in limited studies on
human volunteers at concentrations exceeding approximately 90 and
240 mg/m3, respectively (Silverman et al., 1946; Sim & Pattle,
1957). Cutaneous erythema was observed in the patch testing of twelve
subjects of "Oriental ancestry" with acetaldehyde (Wilkin & Fortner,
1985). Although a possible mechanism has been identified, available
data are inadequate to assess the potential of acetaldehyde to induce
sensitization.
Available data relevant to the assessment of the potential
adverse effects in humans of exposure to acetaldehyde in the
occupational and general environments are limited to those on
irritation mentioned above. The remainder of this evaluation is,
therefore, based on studies on animals.
Effects of acetaldehyde following oral administration to animals
have been much less well studied than those following inhalation. In
one of the two available studies by the oral route, the
no-observed-effect level (NOEL) following four weeks of administration
of acetaldehyde to rats was 125 mg/kg body weight per day (Til et al.,
1988). At the next higher concentration (675 mg/kg body weight per
day), a borderline increase in hyperkeratosis of the forestomach was
observed. In rats exposed to 0.05% acetaldehyde (estimated by the
Task Group to be approximately 40 mg/kg body weight) in the
drinking-water for 6 months, there was an increase in collagen
synthesis in the liver (Bankowski et al., 1993). No other effects
were examined and the toxicological significance of this observation
is unknown.
Acetaldehyde has induced gene mutations, clastogenic effects, and
sister chromatid exchanges (SCE) in mammalian cells in vitro.
Though available data on genotoxicity in vivo are limited, increases
in SCE have been observed in hamsters and mice exposed
intraperitoneally to acetaldehyde (Obe et al., 1979; Korte & Obe,
1981). This suggests genetic damage to somatic cells in vivo.
Available data are inadequate to assess the potential of acetaldehyde
to induce genetic damage in mammalian germ cells in vivo.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In the
respiratory tract, degenerative changes of the olfactory epithelium in
rats and trachea in hamsters have been observed at the lowest
concentrations. Degenerative changes in the respiratory epithelium and
larynx have been observed at higher concentrations. In available
studies, the lowest concentration at which effects were observed
(degenerative changes in the olfactory epithelium of rats) was
437 mg/m3 following administration for 5 weeks (Saldiva et al.,
1985). The NOELs identified for respiratory effects were 275 mg/m3
in rats exposed for 4 weeks (Appelman et al., 1986) and 700 mg/m3 in
hamsters exposed for 13 weeks (Kruysse et al., 1975).
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses of 1350 mg/m3 and greater)
(Woutersen et al., 1986) and non significant increases in laryngeal
and nasal carcinomas in hamsters (Feron et al., 1982). All
concentrations of acetaldehyde administered in these studies induced
tissue damage in the respiratory tract. On the basis of limited
available data, no conclusions can be drawn concerning the potential
of acetaldehyde to promote tumours.
The distribution of nasal lesions induced in rats exposed to
acetaldehyde in inhalation studies correlates with regional
deficiencies in ALDH, observed in a different strain of rats,
prompting the authors to suggest that regional susceptibility to the
toxic effects may be due, at least in part, to a lack of ALDH in the
susceptible regions (Bogdanffy et al., 1986).
Available data are inadequate for assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in occupationally exposed or
general populations.
10.1.3 Approaches to risk assessment
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Though the
principal sources of exposure to acetaldehyde in the general
population are through the metabolism of alcohol, in cigarette smoke,
and food, air is believed to be the main route of exposure in the
occupational environment. In addition, available data are inadequate
to provide guidance concerning the potential risks associated with
oral exposure to acetaldehyde. On this basis, only air is addressed
here.
On the basis of data on irritancy in humans, a tolerable
concentration can be derived as follows:
45 mg/m3
Tolerable concentration = = 2 mg/m3 (2000 µg/m3)
20
where:
no effects were observed in a limited study on human volunteers at
45 mg/m3 (Silverman et al., 1946) and 20 is the uncertainty factor
(×10 for intraspecies variation and × 2 for the poor quality of the
data).
Data suggest that acetaldehyde causes genetic damage to
somatic cells in vivo. The irritancy of acetaldehyde may also play
an important role in the development of tumours in the nose and larynx
of rats and hamsters, respectively, exposed by inhalation, though all
concentrations of acetaldehyde administered in carcinogenesis
bioassays induced both irritancy and nasal tumours. Therefore, two
approaches were adopted for the provision of guidance with respect to
the potential carcinogenicity of acetaldehyde.
In the first, a tolerable concentration (TC) was derived on the
basis of division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, based on the principles
outlined in WHO (in press) and the assumption that there is a
threshold for acetaldehyde-induced cancer of the respiratory tract in
rodents exposed via inhalation. There is some support for this
approach on the basis of relevant data on the analogues of
acetaldehyde, i.e., formaldehyde and glutaraldehyde, which have
similar spectra of in vitro mutagenic effects, but are clearly not
mutagenic in vivo (IARC, 1985; WHO, 1989). Thus,
275 mg/m3
Tolerable concentration = = 0.3 mg/m3 (300 µg/m3)
1000
where:
275 mg/m3 was the NOEL for irritation in rats in a 4-week study
(Appelman et al., 1986) and 1000 is the uncertainty factor (×10 for
interspecies variation, ×10 for intraspecies variation and ×10 for a
less than long-term study and severity of effect, i.e.,
carcinogenicity associated with irritation).
Since the mechanism of induction of tumours by acetaldehyde has
not been well studied, lifetime cancer risk has also been estimated on
the basis of a default model (i.e., linearized multistage) (WHO, in
press). However, it is very likely that, since estimated risk is
based on tumour incidence at concentrations that induce irritancy in
the respiratory tract and no-observed-effect levels for irritancy are
well below these concentrations, the true cancer risk is most likely
much lower at concentrations generally present in the environment and
may, indeed, be zero.
Concentrations associated with a 10-5 excess lifetime risk (lower
95% confidence limits) for nasal tumours (adenocarcinomas, squamous
cell carcinomas, and carcinomas in situ) in male and female rats, in
the only carcinogenicity study in which animals were exposed via
inhalation to acetaldehyde over the lifetime (Woutersen et al., 1986),
calculated on the basis of the linearized multistage model (Global
82), are 11-65 µg/m3. The high-dose animal groups were excluded in
the derivation of these estimates because of early mortality. A body
surface area correction was not incorporated.
10.2 Evaluation of effects on the environment
Acetaldehyde enters the environment during industrial production,
as a product of incomplete combustion (e.g., in vehicle exhausts) and
as a product of alcohol fermentation. Once released,
intercompartmental transport of acetaldehyde is expected to be
limited, because of its reactivity. However, because of its high
vapour pressure and low tendency for sorption onto soil, it is most
likely to be present in air. Acetaldehyde is readily biodegradable;
approximate half-lives are 10-60 h and 1.9 h in air and water,
respectively.
No quantitative data on levels in ambient water were identified.
However, on the basis of concentrations measured in drinking-water,
levels in the aquatic environment are expected to be low (i.e., less
than 0.1 µg/litre). Concentrations in ambient air average about
5 µg/m3.
Acetaldehyde is slightly toxic for fish. The lowest reported
LC50 was 35 µg/litre (Poecilia reticulata). No long-term toxicity
tests have been performed. Acetaldehyde is toxic for micro-organisms
at relatively low concentrations. Seed germination of several plants
was inhibited by more than 50% after exposure to 1.52 mg/litre (3 h).
There were no effects on lettuce exposed to 0.36 µg/m3 acetaldehyde.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
11. RECOMMENDATIONS FOR RESEARCH
1. Additional studies on the fate in the environment and impact of
acetaldehyde on biota.
2. Additional information on exposure of workers in the occupational
environment and levels in the vicinity of industrial facilities.
3. Carcinogenesis bioassays by the inhalation route including doses
that do not induce cytotoxicity (e.g., 275 mg/m3).
4. Studies on the biological significance of byproducts of
acetaldehyde, such as acetaldehyde-modified proteins.
5. Studies on pathogenicity, reproductive and developmental
toxicity, and genotoxicity in vivo by relevant routes of
exposure.
6. Additional studies on toxicity following ingestion in
experimental animals.
7. Studies of irritation in workers exposed to acetaldehyde.
References
Abernethy DJ, Frazelle JH, & Boreiko CJ (1982) Effects of ethanol,
acetaldehyde and acetic acid in the C3H/10TI/2C18 cell transformation
system. Environ Mutagen, 4: 331.
Agarwal DP & Goedde HW (1990) Alcohol metabolism, alcohol intolerance,
and alcoholism. Berlin, Heidelberg, New York, Springer-Verlag, 184 pp.
Aharoni Y & Barkai-Golan R (1973) Sensitivity to acetaldehyde vapors
of Alterneria tenuis and Stemphylium botryosum. Phytopathol Z,
78: 57-61.
Aharoni Y & Stadelbacher GJ (1973) The toxicity of acetaldehyde vapors
to postharvest pathogens of fruits and vegetables. Phytopathology,
63: 544-545.
Aharoni Y, Stewart JK, Hartsell PL, & Young DK (1979) Acetaldehyde - a
potential fumigant for control of the Green peach aphid on harvested
head lettuce. J Econ Entomol, 72: 493-495.
Ahrenholz S & Gorman R (1980) Health and hazard evaluation
determination. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health (Report No. HE 80-2-727).
Ali F & Persaud TVN (1988) Mechanisms of fetal alcohol effects: role
of acetaldehyde. Exp Pathol, 33(1): 17-21.
Almuaibed AM & Townshend A (1987) Determination of acetaldehyde by
flow-injection analysis with soluble or immobilized aldehyde
dehydrogenase. Anal Chim Acta, 198: 37-44.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Anderson RA, Quigg JM, Oswald C, & Zaneveld LJD (1985) Demonstration
of a functional blood-testis barrier to acetaldehyde; evidence for
lack of acetaldehyde effect on ethanol-induced depression of
testosterone in vivo. Biochem Pharmacol, 34(5): 685-695.
Aoyama T & Yashiro T (1983) Analytical study of low-concentration
gases. Part 2: Collection of acetaldehyde at low concentrations in air
in a solid reaction tube and its analysis using a flame thermionic
detector. J Chromatogr, 265: 45-55.
Appelman LM, Woutersen RA, & Feron VJ (1982) Inhalation toxicity of
acetaldehyde in rats. I. Acute and subacute studies. Toxicology,
23: 293-307.
Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, & Notten WRF (1986)
Effect of variable versus fixed exposure levels on the toxicity of
acetaldehyde in rats. J Appl Toxicol, 6(5): 331-336.
Aranyi C, O'Shea WJ, Graham JA, & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6(4): 713-720.
Arntz RR & Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmos Environ, 15(9): 1643-1651.
Asai M, Narita O, & Kashiwamata S (1985) Effects of acetaldehyde
and/or ethanol on neutral amino acid transport systems in microvillous
brush border membrane vesicles prepared from human placenta.
Experientia (Basel), 41(12): 1566-1568.
Asmussen E, Hald J, & Larsen V (1948) The pharmacological action of
acetaldehyde on the human organism. Acta Pharmacol, 4: 311-320.
Atkinson R & Pitts JN (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-426
K. J Chem Phys, 68(8): 3581-3584.
Atkinson R, Plum CN, Carter WPL, Winer AM, & Pitts JN (1984) Rate
constants for the gas-phase reactions of nitrate radicals with a
series of organics in air at 298 +/- 1 K. J Phys Chem, 88: 1210-1215.
Atkinson R, Aschmann SM, & Goodman MA (1987) Kinetics of the gas-phase
reactions of NO radicals with a series of alkynes, haloalkenes, and
a,b-unsaturated aldehydes. Int J Chem Kinet, 19: 299-307.
Atukorala TMS, Duggleby RG, & Nixon PF (1988) Effects of acetaldehyde
upon catalysis by human erythrocyte transketolase. Biochem Pharmacol,
37(10): 2100-2101.
Au W & Badr FM (1979) Does ethanol induce chromosomal damage?
In Vitro, 15: 221.
Babiuk C, Steinhagen WH, & Barrow CS (1985) Sensory irritation
response to inhaled aldehydes after formaldehyde pretreatment. Toxicol
Appl Pharmacol, 79(1): 143-149.
Badr FM & Hussain F (1977) Action of ethanol and its metabolite
acetaldehyde in human lymphocytes. In vivo and in vitro study.
Genetics, 86: s2-s3.
Bagnall GN & Sidebottom HW (1984) Photooxidation of acetaldehyde. In:
Proceedings of the Third Symposium on Physical and Chemical Behaviour
of Atmospheric Pollutants, Varese, Italy. Luxembourg, Commission of
the European Communities, pp 188-193 (EUR 9436).
Bankowski E, Pawlicka E, & Sobolewski K (1993) Liver collagen of rats
submitted to chronic intoxication with acetaldehyde. Mol Cell Biochem,
121: 37-43.
Baraona E, Di Padova C, Tabasco J, & Lieber CS (1987) Red blood cells:
a new major modality for acetaldehyde transport from liver to other
tissues. Life Sci, 40: 253-258.
Barilyak IR & Kozachuk SY (1983) [Embryotoxic and mutagenic activity
of ethanol and acetaldehyde after intra-amniotic injection.] Citol
Genet, 17: 57-60 (in Russian).
Barry RE & McGivan JD (1985) Acetaldehyde alone may initiate
hepatocellular damage in acute alcoholic liver disease. Gut,
26(10): 1065-1069.
Bell RP & Clunie JC (1952) The hydration of acetaldehyde in aqueous
solution. Trans Faraday Soc, 48: 439-442.
Berni RJ & Stanley JB (1982) Volatiles from cotton plant parts. Text
Res J, 52: 539-541.
Binding N, Thiewens S, & Witting U (1986) [Specific determination of
formaldehyde and other aldehydes and ketones for stationary and
personal exposure monitoring.] Staub-Reinhalt Luft, 46(10): 444-446
(in German).
Bird RP, Draper HH, & Basrur PK (1982) Effect of malonaldehyde and
acetaldehyde on cultured mammalian cells. Production of micronuclei
and chromosomal aberrations. Mutat Res, 101: 237-246.
Bittersohl G (1974) [Epidemiologic investigations on cancer incidence
in workers contacted by acetaldol and other aliphatic aldehydes.] Arch
Geschwulstforsch, 43: 172-176 (in German).
Bittersohl G (1975) Epidemiological research on cancer risk by aldol
and aliphatic aldehydes. Environ Qual Saf, 4: 235-238.
Blakley PM & Scott WR Jr (1984a) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. I. Teratogenicity of
ethanol and acetaldehyde. Toxicol Appl Pharmacol, 72: 355-363.
Blakley PM & Scott WR Jr (1984b) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. II. Pharmacokinetics of
the placental transfer of ethanol and acetaldehyde. Toxicol Appl
Pharmacol, 72: 364-371.
Boehlke JU, Singh S, & Goedde HW (1983) Cytogenetic effects of
acetaldehyde in lymphocytes of Germans and Japanese: SCE, clastogenic
activity, and cell cycle delay. Hum Genet, 63: 285-289.
Boettner EA, Ball GL, & Weiss B (1973) Combustion products from the
incineration of plastics. Cincinnati, Ohio, US Environmental
Protection Agency, pp 1-5, 106-122 (EPA-670/2-73-049; PB-222.001).
Bogdanffy MS, Randall HW, & Morgan KT (1986) Histochemical
localization of aldehyde dehydrogenase in the respiratory tract of the
Fisher 344 rat. Toxicol Appl Pharmacol, 82: 560-567.
Booze TF & Oehme FW (1986) An investigation of metaldehyde and
acetaldehyde toxicities in dogs. Fundam Appl Toxicol, 6: 440-446.
Bradow JM & Connick WJ (1988) Seed-germination inhibition by volatile
alcohols and other compounds associated with Amaranthus palmeri
residues. J Chem Ecol, 14(7): 1633-1648.
Brambilla G, Sciaba L, Faggin P, Maura A, Marinari UM, Ferro M, &
Esterbauer H (1986) Cytotoxicity, DNA fragmentation and sister
chromatid exchange in Chinese hamster ovary cells exposed to the lipid
peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat
Res, 171: 169-176.
Brenner DA & Chojkier M (1987) Acetaldehyde increases collagen gene
transcription in cultured human fibroblasts. J Biol Chem,
262(36): 17690-17695.
Brien JF & Loomis CW (1983) Pharmacology of acetaldehyde. Can J
Physiol Pharmacol, 61: 1-22.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data. Final Report (Contract
No. 68-02-3542). Menlo Park, California, US Environmental Protection
Agency.
Buehler R, Pestalozzi D, Hess M, & Von Wartburg JP (1983)
Immunohistochemical localization of alcohol dehydrogenase in human
kidney, endocrine organs and brain. Pharmacol Biochem Behav,
18(Suppl 1): 55-59.
Buyske DA, Owen LH, Wilder P, & Hobbs ME (1956) Chromatography of the
2,4-dihydrophenyl-hydrazones of some aldehydes and ketones in tobacco
smoke. Ann Chem, 28: 910-913.
Campbell MA & Fantel AG (1983) Teratogenicity of acetaldehyde
in vitro: relevance to the fetal alcohol syndrome. Life Sci,
32: 2641-2647.
Casanova-Schmitz M, David RM, & Heck Hd'A (1984) Oxidation of
formaldehyde and acetaldehyde by NAD+-dependent dehydrogenases in rat
nasal mucosal homogenates. Biochem Pharmacol, 33(7): 1137-1142.
Cavanagh LA, Schadt CF, & Robinson E (1969) Atmospheric hydrocarbon
and carbon monoxide measurements at Point Barrow, Alaska. Environ Sci
Technol, 3(3): 251-257.
Cederbaum AI & Dicker E (1981) Effect of cyanamide on the metabolism
of ethanol and acetaldehyde and on gluconeogenesis by isolated rat
hepatocytes. Biochem Pharmacol, 30: 3079-3088.
Cederbaum AI & Rubin E (1977) Sensitivity to acetaldehyde of pyruvate
oxidation by mitochondria from liver, kidney, brain and muscle.
Biochem Pharmacol, 26: 1349-1353.
Cederbaum AI, Lieber CS, & Rubin E (1974) The effect of acetaldehyde
on mitochondrial function. Arch Biochem Biophys, 161: 26-39.
Checiu M, Sandor S, & Garban Z (1984) The effect of ethanol upon early
development in mice and rats. 6. In vivo effect of acetaldehyde upon
preimplantation stages in rats. Rev Roum Morphol Embryol Physiol
Morphol Embryol 30: 175-184.
Chou WL & Speece RE (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
Chrostek WJ (1981) Health hazard evaluation report No.
HETA-81-098-941. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Chrostek WJ & Schoemaker WE (1981) Health hazard evaluation report No.
HETA-81-298-944. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Clarke DW, Steenaart NAE, Slack CJ, & Brien JF (1986) Pharmacokinetics
of ethanol and its metabolite, acetaldehyde, and fetolethality in the
third-trimester pregnant guinea pig for oral administration of acute,
multiple-dose ethanol. Can J Physiol Pharmacol, 64: 1060-1067.
Clarke DW (1988) The disposition of ethanol and its proximate
metabolite, acetaldehyde, in the maternal-fetal unit of the nearterm
guinea pig and pregnant ewe. Diss Abstr Int, 48(10): 2932B-2933B.
Clarren SK & Smith DW (1978) The fetal alcohol syndrome. New Engl J
Med, 298: 1063-1067.
Collins MA, Nijm WP, Borge GF, Teas G, & Goldfarb C (1979)
Dopamine-related tetrahydroisoquinolines: significant urinary
excretion by alcoholics after alcohol consumption. Science,
206: 1184-1186.
Cortes F, Mateos S, & Escalza P (1986) Cytotoxic and genotoxic effects
of ethanol and acetaldehyde in root-meristem cells of Allium cepa.
Mutat Res, 171(2-3): 139-143.
Crabb DW, Edenberg HJ, Bosron WF, & Li TK (1989) Genotypes for
aldehyde dehydrogenase deficiency and alcohol sensitivity. J Clin
Invest, 83: 314-316.
Crebelli R, Conti G, Conti I, & Carere A (1989) A comparative study of
ethanol and acetaldehyde as inducers of chromosome malsegregation in
Aspergillus nidulans. Mutat Res, 215: 187-195.
Delcour JA, Caers JM, Dondeyne P, Delvaux F, & Robberechts E (1982) An
enzymic assay for the determination acetaldehyde in beers. J Inst
Brew, 88: 384-386.
Deneer JW, Seinen W, & Hermens JLM (1988) The acute toxicity of
aldehydes to the guppy. Aquat Toxicol, 12: 185-192.
De Raat WK, Davis PB, & Bakker GL (1983) Induction of sister-chromatid
exchanges by alcohol and alcoholic beverages after metabolic
activation by rat-liver homogenate. Mutat Res, 124: 85-90.
Di Padova C, Alderman J, & Lieber CS (1986) Improved methods for
measurement of acetaldehyde concentrations in plasma and red blood
cells. Alcohol Clin Exp Res, 10(1): 86-89.
Dreisbach RH (1987) Handbook of poisoning, 9th ed. Los Altos,
California, Lange, pp 185-188.
Dulout FN & Furnus CC (1988) Acetaldehyde-induced aneuploidy in
cultured Chinese hamster cells. Mutagenesis, 3(3): 207-211.
Egle JL (1970) Retention of inhaled acetaldehyde in man. J Pharmacol
Exp Ther, 174(1): 14-19.
Eimutis EC, Quill RP, & Rinaldi GM (1978) Source assessment
noncriteria pollutant emissions (1978 update). Research Triangle Park,
North Carolina, US Environmental Protection Agency, Industrial
Environmental Research Laboratory, pp 1-5 (PB-291 747).
Eker P & Sanner T (1986) Initiation of in vitro cell transformation
by formaldehyde and acetaldehyde as measured by attachment-independent
survival of cells in aggregates. Eur J Cancer Clin Oncol,
22(6): 671-676.
Eriksson CJP (1987) Genetic aspects of the relation between alcohol
metabolism and consumption in humans. Mutat Res, 186(3): 241-247.
Eriksson CJP & Fukunaga T (1993) Human blood acetaldehyde (update
1991). Alcohol Alcohol, 28(Suppl 2): 9-25.
Eriksson CJP, Mizoi Y, & Fukunaga T (1982) The determination of
acetaldehyde in human blood by the perchloric acid precipitation
method: The characterization and elimination of artefactual
acetaldehyde formation. Anal Biochem, 125: 259-263. Espinet C &
Argiles JM (1984) Ethanol and acetaldehyde concentrations in the rat
foeto-maternal system after an acute ethanol administration given to
the mother. Arch Int Physiol Biochim, 92: 339-344.
Facchini MC, Chiavari G, & Fuzzi S (1986) An improved HPCL method for
carbonyl compound speciation in the atmospheric liquid phase.
Chemosphere, 15(6): 667-674.
Feron VJ (1979) Effects of exposure to acetaldehyde in Syrian hamsters
simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Prog
Exp Tumor Res, 24: 162-176.
Feron VJ & De Jong D (1971) Acute intratracheal toxicity of
acetaldehyde in Syrian golden hamsters. Zeist, Central Institute for
Nutrition and Food Research, TNO (Report No. R 3600).
Feron VJ, Kruysse A, & Woutersen RA (1982) Respiratory tract tumors in
hamsters exposed to acetaldehyde vapour alone or simultaneously to
benzo[a]pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol,
18: 13-31.
Feron VJ, Kuper CF, Spit BJ, Renzel PGJ, & Woutersen RA (1985) Glass
fibers and vapor phase components of cigarette smoke as factors in
experimental respiratory tract carcinogenesis. In: Carcinogenesis. New
York, Raven Press, pp 93-118.
Feron VJ, Til HP, Vrijer F, Woutersen RA, Cassee FR, & van Bladeren PJ
(1991) Aldehydes: occurrence, carcinogenic potential, mechanism of
action and risk assessment. Mutat Res, 259: 363-385.
Fink R & Dancygier H (1988) Acetaldehyde inhibits the activity of
human natural killer cells. Gastroenterology, 94(5): A127.
Fraenkel-Conrat H & Singer B (1988) Nucleoside adducts are formed by
cooperative reaction of acetaldehyde and alcohols: possible mechanism
for the role of ethanol in carcinogenesis. Proc Natl Acad Sci (USA),
85: 3758-3761.
Furia TE & Bellanca N ed. (1975) Fenaroli's handbook of flavour
ingredients, 2nd ed. Cleveland, Ohio, CRC Press Inc., vol 2, p 3.
Gerhold RM & Malaney GW (1966) Structural determination in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38(4): 562-579.
Gidley MJ, Hall LD, Sanders JKM, & Summers MC (1981)
Acetaldehyde-enkephalins: structure proof and some conformational
deductions from one- and two-dimensional proton nuclear magnetic
resonance spectra. Biochemistry, 20: 3880-3883.
Goedde HW & Agarwal DP (1986) Aldehyde oxidation: ethnic variations in
metabolism and response. In: Kalow W, Goedde W, & Agarwal DP ed.
Ethnic differences in reactions to drugs and xenobiotics. New York,
Alan R. Liss Inc., pp 113-138.
Goedde HW & Agarwal DP (1987) Polymorphism of aldehyde dehydrogenase
and alcohol sensitivity. Enzyme, 37: 29-44.
Goedde HW, Agarwal DP, & Harada S (1979) Alcohol metabolizing enzymes:
studies of isozymes in human biopsies and cultured fibroblasts. Clin
Genet, 16: 29-33.
Goedde HW, Singh S, Agarwal DP, Frizte G, Stapel K, & Paik YK (1989)
Genotyping of mitochondrial aldehyde dehydrogenase in blood samples
using allele-specific oligonucleotides: comparison with phenotyping in
hair roots. Hum Genet, 81: 305-307.
Gordon BHJ, Baraona E, & Lieber CS (1985) Blood acetaldehyde response
to ethanol ingestion during the reproductive cycle of the female rat.
Alcohol, 2: 271-275.
Grahl K (1983) [Classification of substances in water on the basis of
their toxicity potential to aquatic organisms.] Acta Hydrochim
Hydrobiol, 11(1): 137-143 (in German).
Gramiccioni L, Milana MR, Di Marzio S, & Lorusso S (1986) GC
determination of traces of acetaldehyde in aqueous matrix: a rapid
headspace method. Chromatographia, 21(1): 9-11.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Thomas CT Publications, p 76.
Green RJ & Baron DN (1986) The acute in vitro effect of ethanol, its
metabolites and other toxic alcohols on ion flax in isolated human
leucocytes and erythrocytes. Biochem Pharmacol, 35(20): 3457-3464.
Greenwald IS & Horvitz HR (1980) Unc-93(e1500): a behavioral mutant of
Caenorhabditis elegans that defines a gene with a wild-type null
phenotype. Genetics, 96: 147-164.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D (1991) Ambient levels of formaldehyde, acetaldehyde and
fumaric acid in Southern California. Results of a one-year base-line
study. Environ Sci Technol, 25: 710-715.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos Environ, 17(10): 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Guerri C & Sanchis R (1985) Acetaldehyde and alcohol levels in
pregnant rats and their fetuses. Alcohol, 2: 267-270.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hagemeyer HJ (1978) Acetaldehyde. In: Grayson M ed. Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 1, pp 97-112.
Hagen E (1967) The composition of combustion gases of polyurethane
forms and polycarbonates. Plaste Kautsch, 6: 391-392.
Harade S, Agarwal DP, & Goedde HW (1978a) Human liver alcohol
dehydrogenase isoenzyme variations: improved separation methods using
prolonged high voltage starch gel electrophoresis and isoelectric
focusing. Hum Genet, 40: 215-220.
Harade S, Agarwal DP, & Goedde HW (1978b) Application of gel
isoelectric focusing and electrophoresis in the study of alcohol
dehydrogenase and acetaldehyde dehydrogenase isoenzymes of human
tissues and fibroblasts. In: Catsimoolas U ed. Electrophoresis 78.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 275-282.
Harris CC, Willey JC, Saladino AJ, & Grafstrom RC (1985) Effects of
tumor promotors, aldehydes, peroxides, and tobacco smoke condensate on
growth and differentiation of cultured normal and transformed human
bronchial cells. In: Mass MJ, Kaufman DG, Siegfried JM, Steele WE, &
Nesnow S, ed. Carcinogenesis, a comprehensive survey. Cancer of the
respiratory tract. Predisposing factors. New York, Raven Press, pp
159-171.
Hatfield R (1957) Biological oxidation of some organic compounds. Ind
Eng Chem, 49(2): 192-196.
He SM & Lambert B (1985) Induction and persistence of SCE-inducing
damage in human lymphocytes exposed to vinyl acetate and acetaldehyde
in vitro. Mutat Res, 158(3): 201-208.
He SM & Lambert B (1990) Acetaldehyde-induced mutation at the hprt
locus in human lymphocytes in vitro. Environ Mol Mutagen, 16: 57-63.
Helander A & Tottmar O (1987) Effects of ethanol, acetaldehyde and
disulfiram on the metabolism of biogenic aldehydes in isolated human
blood cells and platelets. Biochem Pharmacol, 36(22): 3981-3985.
Helander A & Lindahl-Kiessling K (1991) Increased frequency of
acetaldehyde-induced sister-chromatid exchanges in human lymphocytes
treated with an aldehyde dehydrogenase inhibitor. Mutat Res, 264:
103-107.
Hellström-Lindahl E & Weiner H (1985) Effects of disulfiram on the
oxidation of benzaldehyde and acetaldehyde in rats liver. Biochem
Pharmacol, 34(9): 1529-1535.
Hemminki K & Suni R (1984) Sites of reaction of glutaraldehyde and
acetaldehyde with nucleosides. Arch Toxicol, 55: 186-190.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rate
and mutagenicity of directly acting industrial and laboratory
chemicals. Arch Toxicol, 46: 277-285.
Henderson IF (1970) The fumigant effect of metaldehyde on slugs. Ann
Appl Biol, 65: 507-510.
Herrick RF (1980) Industrial hygiene survey report on urea
formaldehyde foam insulation manufacturing. Cincinnati, Ohio, US
National Institute of Occupational Safety and Health, pp 2-5, 9-12
(PB83-107797).
Hobara N, Watanabe A, Kobayashi M, Nakatsukasa H, Nagshima H, Fukuda
T, & Araki Y (1985) Tissue distribution of acetaldehyde inhalation and
intragastric ethanol administration. Bull Environ Contam Toxicol,
35: 393-396.
Hoerner M, Behrens UJ, Worner T, Blacksberg I, Braly LF, Schaffner F,
& Lieber CS (1988) The role of alcoholism and liver disease in the
appearance of serum antibodies against acetaldehyde adducts.
Hepatology, 8: 569-574.
Hoffman D, Brunnenman, KD, Gori GB, & Wynder EL (1975) On the
carcinogenicity of marijuana smoke. Recent Adv Phytochem, 9: 63-81.
Horvath RJ, Senter SD, & Dekazos ED (1983) GLC-MS analysis of volatile
constituents in rabbit eye blueberries. J Food Sci, 48: 278-279.
Hsu LC, Bendel RE, & Yoshida A (1978) Genomic structure of the human
mitochondrial aldehyde dehydrogenase gene. Genomics, 2(1): 57-65.
Hugues UJ & Hum RW (1960) A preliminary survey of hydrocarbon-derived
oxygenated material in automobile exhaust gases. J Air Pollut Control
Assoc, 10: 367-371.
Hustert K & Parlar H (1981) [A test for photochemical degradation of
environmental chemicals in the gas phase.] Chemosphere,
10(9): 1045-1050 (in German).
IARC (1985) Acetaldehyde. In: Allyl compounds, aldehydes, epoxides and
peroxides. Lyon, International Agency for Research on Cancer, pp
101-132 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 36).
IARC (1988) Alcohol drinking. Lyon, International Agency for Research
on Cancer, 416 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risks to Humans, Volume 44).
Igali S & Gazso L (1980) Mutagenic effects of alcohol and acetaldehyde
on Escherichia coli. Mutat Res, 74: 209-210.
Ikawa E, Tsuda H, Sakata T, Masui T, Sato K, & Ito N (1986)
Modification potentials of ethyl alcohol and acetaldehyde on
development of preneoplastic glutathione S-transferase
P-form-positive liver cell foci initiated by diethylnitrosamine in the
rat. Cancer Lett, 31(1): 53-60.
IPCS/CEC (1990) International Chemical Safety Card No. 0009:
Acetaldehyde. Luxembourg, Commission of the European Communities
(First series).
Israel Y, Hurwitz E, Niemela EO, & Arnon R (1986) Monoclonal and
polyclonal antibodies against acetaldehyde-containing epitopes in
acetaldehyde-protein adducts. Proc Natl Acad Sci (USA),
83(20): 7923-7927.
Israel Y, MacDonald A, Niemela O, Zemel D, Shami E, Zywulko M, Klajner
F, & Borgono C (1992) Hypersensitivity to acetaldehyde-protein
adducts. Mol Pharmacol, 42(4): 711-717.
Jansson T (1982) The frequency of sister chromatid exchanges in human
lymphocytes treated with ethanol and acetaldehyde. Hereditas,
97: 301-303.
Japan Environment Agency (1989) Report on environmental survey and
wildlife monitoring of chemicals. In: Chemicals in the environment.
Tokyo, Japan Environment Agency, Department of Environmental Health,
p 73.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Jones JS, Sadler GD, & Nelson PE (1986) Acetaldehyde and accelerated
storage of wine: a new rapid method for analysis. J Food Sci,
51(1): 229-230.
Juhnke I & Luedemann D (1978) [Results of investigation of acute
toxicity to fish of 200 chemical compounds by the "Golden Orfe" test.]
Z Wasser Abwasser Forschung, 11(5): 161-164 (in German).
Kami T (1983) Composition of the essential oil of alfalfa. J Agric
Food Chem, 31: 38-41.
Karl PI, Gordon BHJ, Lieber CS, & Fisher SE (1988) Acetaldehyde
production and transfer by perfused human placental cotyledon.
Science, 242: 273-275.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Identification and analysis of
organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Press,
pp 329-373.
Knadle S (1985) Synergistic interaction between hydroquinone and
acetaldehyde in the induction of sister chromatid exchange in human
lymphocytes in vitro. Cancer Res, 45(10): 4853-4857.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77: 269-276.
Koivula T (1975) Subcellular distribution and characterization of
human liver aldehyde dehydrogenase fractions. Life Sci, 16: 1563-1570.
Kopczynski SL, Altshuller AP, & Sutterfield FD (1974) Photochemical
reactivities of aldehyde-nitrogen oxide systems. Environ Sci Technol,
8(10): 909-918.
Korte A & Obe G (1981) Influence of chronic ethanol uptake and acute
acetaldehyde treatment on the chromosomes of bone-marrow cells and
peripheral lymphocytes of Chinese hamsters. Mutat Res, 88: 389-395.
Kruysse A (1970) Acute inhalation toxicity of acetaldehyde in
hamsters. Zeist, Central Institute for Nutrition and Food Research,
TNO (Report No. R 3270).
Kruysse A, Feron VJ, & Til HP (1975) Repeated exposure to acetaldehyde
vapor. Studies in Syrian golden hamsters. Arch Environ Health,
30: 449-452.
Kuriyama K, Ohkuma S, Tomono S, & Hirouchi M (1987) Effects of alcohol
and acetaldehyde on metabolism and function of neurotransmitter system
in cerebral cortical neurons in primary culture. Alcohol Alcohol,
22(Suppl 1): 685-689.
Lahdetie J (1988) Effects of vinyl acetate and acetaldehyde on sperm
morphology and meiotic micronuclei in mice. Mutat Res,
202(1): 171-178.
Lahti RA & Majchrowicz E (1969) Acetaldehyde - an inhibitor of the
enzymatic oxidation of 5-hydroxyindoleacetaldehyde. Biochem Pharmacol,
18: 535-538.
Lam CW, Casanova M, & Heck HD (1986) Decreased extractability of DNA
from proteins in the rat nasal mucosa after acetaldehyde exposure.
Fundam Appl Toxicol, 6(3): 541-550.
Lambert B, Chen Y, He SM, & Sten M (1985) DNA cross-links in human
leucocytes treated with vinyl acetate and acetaldehyde in vitro.
Mutat Res, 146(3): 301-303.
Lambert B & He SM (1988) DNA and chromosome damage induced by
acetaldehyde in human lymphocytes in vitro. Ann NY Acad Sci,
534: 369-376.
Lamboeuf Y, Latge C, Roumec C, & De Saint Blanquat G (1987) Ethanol
tolerance in the rat after inhalation of acetaldehyde for a period of
21 days. Alcohol Alcohol, 22(Suppl 1): 441-447.
Latge C, Lamboeuf Y, Roumec C, & De Saint Blanquat G (1987) Effect of
chronic acetaldehyde intoxication on ethanol tolerance and membrane
fatty acids. Drug Alcohol Depend, 20(1): 47-56.
Lau CF, Vogel R, Obe G, & Spielmann H (1991) Embryologic and
cytogenetic effects of ethanol on preimplantation mouse embryos
in vitro. Reprod Toxicol, 5: 405-410.
Leone JA & Seinfeld JH (1984) Analysis of the characteristics of
complex chemical reaction mechanisms: application to photochemical
smog chemistry. Environ Sci Technol, 18(4): 280-287.
Levallois C, Maini JC, & Balmes JL (1987) Sensitivity of human
lymphocytes to acetaldehyde: comparison between alcoholic and control
subjects. Drug Alcohol Depend, 20(2): 135-142.
Lipari F & Swarin SJ (1982) Determination of formaldehyde and other
aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. Chromatography, 247: 297-306.
Lipari F, Dasch JM, & Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environ Sci Technol, 18: 326-330.
Liu RC, Lumeng L, Shahidi S, Kelly T, & Pound DC (1990)
Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol
Clin Exp Res, 14: 438-443.
Ludzack JR & Ettinger MB (1960) Industrial wastes. Chemical structures
resistant to aerobic biochemical stabilization. J Water Pollut Control
Fed, 32(11): 1173-1200.
Lyman WJ, Rheel WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, McGraw-Hill Book Company.
Lynch C, Lim CK, Thomas M, & Peters TJ (1983) Assay of blood and
tissue aldehydes by HPLC analysis of their 2,4-dinitrophenylhydrazine
adducts. Clin Chim Acta, 130: 117-122.
Ma T, Miller TL, & Xu Z (1985) Mouse peripheral erythrocyte
micronucleus assay on the genotoxicity of acetaldehyde. Environ
Mutagen, 7(Suppl 3): 28.
Maarse H & Visscher CA (1992) Volatile compounds in food. Qualitative
and quantitative data. Supplement 3 and cumulative index. Zeist,
Central Institute for Nutrition and Food Research, TNO, Biotechnology
and Chemistry Institute, pp XXXIII-XLI.
McCall D & Ryan K (1987) The effect of ethanol and acetaldehyde on Na
pump function in cultured rat heart cells. J Mol Cell Cardiol,
19: 453-463.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987a)
Effects of acetaldehyde on polymerization of microtubule proteins.
Brain Res, 416: 90-99.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987b) The
reaction of acetaldehyde with brain microtubular proteins: formation
of stable adducts and inhibition of polymerization. Neurosci Lett,
79: 163-168.
Majumdar APN, Vesenka GD, Dubick MA, Yu GSM, DeMorrow JM, & Geokas MC
(1986) Morphological and biochemical changes of the pancreas in rats
treated with acetaldehyde. Am J Physiol 250(5): G598-G606.
Manning DL, Maskarinec MP, Jenkins RA, & Marshall AH (1983) High
performance liquid chromatographic determination of selected gas phase
carbonyls in tobacco smoke. J Assoc Off Anal Chem, 66(1): 8-12.
Margeri G, Versini G, Gianotti I, & Pellegrini R (1984) Acetaldehyde
in beverages. Riv Soc Ital Sci Aliment, 5: 401-406.
Marinari UM, Ferro M, Sciaba L, Finollo R, Bassi AM, & Brambilla G
(1984) DNA-damaging activity of biotic and xenobiotic aldehydes in
Chinese hamster ovary cells. Cell Biochem Funct, 2(4): 243-248.
Marjanen LA (1973) Comparison of aldehyde dehydrogenases from cytosol
and mitochondria of rat liver. Biochim Biophys Acta, 327: 238-246.
Marnett LJ, Hurd H, Hollstein M, Levin DE, Esterbauer H, & Ames BN
(1985) Natural occurring carbonyl compounds are mutagens in Salmonella
tester strain TA104. Mutat Res, 148: 25-34.
Matsuzaki S & Lieber CS (1977) Increased susceptibility of hepatic
mitochondria to the toxicity of acetaldehyde after chronic ethanol
consumption. Biochem Biophys Res Commun, 75(4): 1059-1065.
Mauch TJ, Donahue TM, Zetterman RK, Sorrell MF, & Tuma DJ (1986)
Covalent binding of acetaldehyde selectively inhibits the catalytic
activity of lysine-dependent enzymes. Hepatology, 6(2): 263-269.
Mauch TJ, Tuma DJ, & Sorrell MF (1987) The binding of acetaldehyde to
the active site of ribonuclease: alterations in catalytic activity and
effects of phosphate. Alcohol Alcohol, 22(2): 103-112.
Michoudet C & Baverel G (1985) Metabolism and metabolic effects of
acetaldehyde in dog, rat and guinea pig renal cortex. Dev Nephrol,
10: 143-149.
Michoudet C & Baverel G (1987a) Characteristics of acetaldehyde
metabolism in isolated dog, rat and guinea pig kidney tubules. Biochem
Pharmacol, 36(22): 3987-3991.
Michoudet C & Baverel G (1987b) Metabolism of acetaldehyde in human
and baboon renal cortex:ethanol synthesis by isolated baboon
kidney-cortex tubules. FEBS Lett, 216(1): 113-117.
Miyamoto Y (1986) Eye and respiratory irritants in jet engine exhaust.
Aviat Space Environ Med, 57: 1104-1108.
Mold JD & McRae TG (1957) The determination of some low molecular
weight aldehydes and ketones in cigarette smoke as the
2,4-dinitrophenylhydrazone. Tob Sci, 1: 40-46.
Moortgat GK & McQuigg RD (1984) A FTIR spectroscopic study of the
photooxidation of acetaldehyde in air. In: Proceedings of the Third
European Symposium on Physico-Chemical Behaviour of Atmospheric
Pollutants, Varese, Italy. Luxembourg, Commission of the European
Communities, pp 194-204 (EUR 9436).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing of
270 chemicals. Environ Mutagen, 8(Suppl 17): 1-119.
Mueller A & Sies H (1987) Alcohol, aldehydes and lipid peroxidation
current notions. Alcohol Alcohol, 22(Suppl 1): 67-74.
Myers WD, Ng KT, Singer G, Smythe GA, & Duncan MW (1985) Dopamine and
salsolinol levels in rat hypothalami and striatum after
schedule-induced self-injection (SISI) of ethanol and acetaldehyde.
Brain Res, 358(1-2): 122-128.
Niemela O & Israel Y (1992) Hemoglobin-acetaldehyde adducts in human
alcohol abusers. Lab Invest, 67(2): 246-252.
Niemela O, Klajner F, Orrego H, Vidins E, Blendis LM, & Israel Y
(1987) Antibodies against acetaldehyde-modified protein epitopes in
human alcoholics. Hepatology, 7: 1210-1214.
Niemela O, Mannermaa RM, & Oikarinen (1990) Impairment o histone H1
DNA binding by adduct formation with acetaldehyde. Life Sci,
47: 2241-2249.
Niemela O, Juvonen T, & Parkkila S (1991) Immunohistochemical
demonstration of acetaldehyde-modified epitopes in human liver after
alcohol consumption. J Clin Invest, 87: 1367-1374.
Ninfali P, Palma F, Piacentini MP, & Fornaini G (1987) Action of
acetaldehyde on glucose metabolism of newborn and adult erythrocytes.
Biol Neonate, 52(5):256-263.
Norppa H, Tursi F, Pfaeffli P, Maeki-Paakkanen J, & Jaerventaus H
(1985) Chromosome damage induced by vinyl acetate through in vitro
formation of acetaldehyde in human lymphocytes and Chinese hamster
ovary cells. Cancer Res, 45(10): 4816-4821.
Obe O & Beek B (1979) Mutagenic activity of aldehydes. Drug Alcohol
Depend, 4: 91-94.
Obe G & Ristow H (1977) Acetaldehyde, but not ethanol, induces sister
chromatid exchanges in Chinese hamster cells in vitro. Mutat Res,
56: 211-213.
Obe G, Ristow H, & Herha J (1978) Mutagenic activity of alcohol in
man. In: Mutations: their origin, nature and potential relevance to
genetic risk in man. Proceedings Yearly Conference 1977 of German
Research Society. Boppard, Harald Boldt, pp 151-161.
Obe G, Natarajan AT, Meyers M, & Den Hertog A (1979) Induction of
chromosomal aberrations in peripheral lymphocytes of human blood
in vitro, and of SCE's in bone-marrow cells of mice in vivo by
ethanol and its metabolite acetaldehyde. Mutat Res, 68: 291-294.
Obe G, Brodmann R, Fleischer R, Engeln H, Gobel D, & Herha J (1985)
[Mutagenic and carcinogenic activity of adduct products.] In: Keup W
ed. [Biology of addiction.] Berlin, Heidelberg, New York,
Springer-Verlag, pp 31-43 (in German).
Obe G, Jonas R, & Schmidt S (1986) Metabolism of ethanol in vitro
produces a compounds which induces sister-chromatid exchanges in human
peripheral lymphocytes in vitro: Acetaldehyde not ethanol is
mutagenic. Mutat Res, 174: 47-52.
OECD (1992) Guidelines for testing chemicals. Section 3. Degradation
and accumulation. 301 C. Paris, Organisation for Economic Cooperation
and Development, pp 1-21.
Okamoto M, Ohtsuka K, Imai J, & Yamada F (1981) High-performance
liquid chromatographic determination of acetaldehyde in wine as its
lutidine derivative. J Chromatogr, 219: 175-178.
Ortiz A, Griffth PJ, & Littleton JM (1974) A comparison of the effects
of chronic administration of ethanol and acetaldehyde to mice:
evidence for a role of acetaldehyde in ethanol dependence. J Pharm
Pharmacol, 26: 249-260.
Osborne JS, Adamek S, & Hobbs ME (1956) Some component of gas phase of
cigarette smoke. Ann Chem, 28: 211-215.
O'Shea KS & Kaufman MH (1979) The teratogenic effect of acetaldehyde:
implications for the study of the fetal alcohol syndrome. J Anat,
128: 65-76.
O'Shea KS & Kaufman MH (1981) Effect of acetaldehyde on the
neuroepithelium of early mouser embryos. J Anat, 132: 107-118.
Padmanabhan R, Sreenathan RN, & Singh S (1983) Studies on the lethal
and teratogenic effects of acetaldehyde in the rat. Congenit Anom,
23: 13-23.
Parrilla R, Ohkawa K, Lindros KO, Zimmernman UP, Kobayashi K, &
Williamson JR (1974) Functional compartmentation of acetaldehyde
oxidation in rat liver. J Biol Chem, 249: 4926-4933.
Peeke HV & Figler MH (1981) Modulation of aggressive behavior in fish
by alcohol and congeners. Pharmacol Biochem Behav, 14(Suppl 1): 79-84.
Pellizari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compound in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Peterson CM & Polizzi CM (1987) Improved method for acetaldehyde in
plasma and hemoglobin-associated acetaldehyde: results in teetotalers
and alcoholics reporting for treatment. Alcohol, 4: 477-480.
Pettersson H & Kiessling K (1977) Acetaldehyde occurrence in
cerebrospinal fluid during ethanol oxidation in rats and its
dependence on the blood level and on dietary factors. Biochem
Pharmacol, 26: 237-240.
Pezzoli C, Galli-Kienle M, Di Padova C, & Stramentinoli G (1984) HPLC
method for the evaluation of blood acetaldehyde without ethanol
interference. J Liq Chromatogr, 7(4): 765-788.
Phillips SC (1987) Can brain lesions occur in experimental animals by
administration of ethanol or acetaldehyde? Acta Med Scand,
71(Suppl 7): 67-72.
Piendl A, Westner H, & Geiger E (1981) [Detection and separation of
aldehydes in beer using high pressure liquid chromatography (HPLC).]
Braunwissenschaft, 34: 301-307 (in German).
Pittevils J, Vanparys L, & Soenen A (1979) Fungicide and fungistatic
action of fifteen different aromatic components on some important
storage diseases in vitro. Meded Fac Landbouwwet Rijksuniv Gent,
44(1): 445-457.
Popov VB, Vaisman BL, Puchkov VF, & Ignatyeva TV (1981) Toxic action
of ethanol and biotransformation products on postimplantation rat
embryos in culture. Bull Exp Biol Med, 92: 725-728.
Possanzini M, Ciccioli P, Di Paolo V, & Draisci R (1987) Determination
of low boiling aldehydes in air and exhaust gases by using annular
denuders combined with HPLC techniques. Chromatographia,
23(11): 829-834.
Prasanna CV & Ramakrishnan S (1984a) Effect of acetaldehyde on hepatic
glucose metabolism. Indian J Biochem Biophys, 21(1):62-64.
Prasanna CV & Ramakrishnan S (1984b) Effect of acetaldehyde on
carbohydrate metabolism in rat brain. Indian J Biochem Biophys,
21(2): 121-123.
Prasanna CV & Ramakrishnan S (1987) Effect of acetaldehyde on hepatic
and blood lipids. Indian J Med Res, 85: 206-211.
Prasanna CV, Namagiri T, & Ramakrishnan S (1986) Effect of
acetaldehyde on thyroid function. Indian J Exp Biol, 24(2): 77-78.
Priscott PK & Ford JR (1985) An in vitro model of acetaldehyde
metabolism by rodent conceptuses. in vitro Cell Dev Biol,
21(2): 88-92.
Ramdahl T, Alfheim I, Rustad S, & Olsen T (1982) Chemical and
biological characterization of emissions from small residential stoves
burning wood and charcoal. Chemosphere, 11(4): 601-611.
Rawat K (1979) Inhibition of cardiac protein synthesis by prolonged
ethanol administration. Res Commun Chem Pathol Pharmacol, 25: 89.
Rideout JM, Lim CK, & Peters TJ (1986) Assay of blood acetaldehyde by
HPLC with fluorescence detection of its 2-diphenylacetyl-1,3-
indandione-1-azine derivative. Clin Chim Acta, 161: 29-35.
Rieger R & Michaelis A (1960) [Chromosomal aberrations after action of
acetaldehyde upon the primary roots of Vicia faba.] Biol Zent.bl,
679: 1-5 (in German).
Ristow H & Obe G (1978) Acetaldehyde induces cross-links in DNA and
causes sister-chromatid exchanges in human cells. Mutat Res,
58: 115-119.
Rosensteel RE & Tanaka S (1976) Health hazard evaluation report
No. 75-89-344. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health.
Roumec C, Lamboeuf Y, & De Saint Blanquat G (1988) Sinaptosomal
phospholipids in rats chronically treated with acetaldehyde. Adv
Biosci, 71: 201-205.
Rudling L, Ahling B, & Loefroth G (1981) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in small furnaces and stoves. In: Proceedings of the International
Conference on Residential Solids Fuels, Environmental Impacts and
Solutions. Beaverton, Oregon, Oregon Graduate Center, pp 34-53.
Saladino AJ, Willey JC, Lechner JF, Grafstrom RC, Laveck M, & Harris
CC (1985) Effects of formaldehyde, acetaldehyde, benzoyl peroxide and
hydrogen peroxide on cultured normal human bronchial epithelial cells.
Cancer Res, 45(6): 2522-2526.
Saldiva PHN, Do Rio Caldeira MP, Massad CW, Calheiros DF, Cardoso LMN,
Bohm GM, & Saldiva CD (1985) Effects of formaldehyde and acetaldehyde
inhalation on rat pulmonary mechanics. J Appl Toxicol, 5(5): 288-292.
San George RC & Hoberman HD (1986) Reaction of acetaldehyde with
hemoglobin. J Biol Chem, 261: 6811-6821.
Sasaki Y & Endo R (1978) Mutagenicity of aldehydes in Salmonella.
Mutat Res, 54: 251-252.
Savolainen ER, Leo MA, Timpl R, & Lieber CS (1984) Acetaldehyde and
lactate stimulate collagen synthesis of cultured baboon liver
myofibroblasts. Gastroenterology, 87(4): 777-787.
Schopf RE, Tromperer M, Bork K, & Morsches B (1985) Effects of ethanol
and acetaldehyde on phagocytic functions. Arch Dermatol Res,
277(2): 131-137.
Schreiber SS, Briden K, Oratz M, & Rothschild MA (1972) Ethanol,
acetaldehyde, and myocardial protein synthesis. J Clin Invest,
51: 2820-2826.
Schreiber SS, Oratz M, Rothschild MA, Reff F, & Evans C (1974)
Alcoholic cardiomyopathy. II. The inhibition of cardiac microsomal
protein synthesis by acetaldehyde. J Mol Cell Cardiol, 6: 207-213.
Schreiner G, Ulbrich M, Senger-Weil M, & Bass R (1987) Effects of
prenatal ethanol or acetaldehyde exposure on postnatal development and
behavior in the rat. Teratology, 36(1): 31A.
Schulam P, Newbold R, & Hull LA (1985) Urban and rural ambient air
aldehyde levels in Schenectady, New York and on Whiteface Mountain,
New York. Atmos Environ, 19(4): 623-626.
Segel LD (1984) Mitochondrial respiration after cardiac perfusion with
ethanol or acetaldehyde. Alcohol Clin Exp Res, 8(6): 560-564.
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency, pp 7, 37, 46 (EPA-600/4/76/062).
Shaw S & Jayatilleke E (1987) Acetaldehyde-mediated hepatic lipid
peroxidation: role of superoxide and ferritin. Biochem Biophys Res
Commun, 143(3): 984-990.
Shiohara E, Sukada M, Chiba S, Yamazaki H, Nishiguchi K, Miyamoto R, &
Nakanishi S (1985) Effect of chronic administration of acetaldehyde by
inhalation on (NA+/K+)-activated adenosine triphosphatase activity of
rat brain membranes. Toxicology, 34(4): 277-284.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Sim VM & Pattle RE (1957) Effect of possible smoke irritation on human
subjects. J Am Med Assoc, 165: 1908-1913.
Sina JF, Bean CL, Dysart GR, Taylor VI, & Bradley MO (1983) Evaluation
of the alkaline elution/rat hepatocyte assay as a predictor of
carcinogenic/mutagenic potential. Mutat Res, 113: 357-391.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1982) Measurements of
hazardous organic chemicals in the ambient atmosphere. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Environmental Sciences Research Laboratory, pp 32-37, 70-79, 84-90
(SRI International Project 7774).
Singh S, Fritze G, Fang B, Harada S, Paik YK, Eckey R, Agarwal DP, &
Goedde HW (1989) Inheritance of mitochondrial aldehyde dehydrogenase:
genotyping in Chinese, Japanese, and South Korean families reveals
dominance of the mutant allele. Hum Genet, 83: 119-121.
Sipi P, Jarventaus H, & Norppa H (1992) Sister-chromatid exchanges
induced by vinyl esters and respective carboxylic acids in cultured
human lymphocytes. Mutat Res, 279: 75-82.
Skog E (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde, propionaldehyde
and butyraldehyde; as well as of acrolein and crotonaldehyde. Acta
Pharmacol, 6: 299-318.
Smyth HF, Carpenter CP, & Weils CS (1951) Range-finding toxicity data:
list IV. Am Med Assoc Arch Ind Health Occup Med, 4: 119.
Snyder JW (1988) The role of phospholipid metabolism in ethanol- and
acetaldehyde-initiated hepatocyte injury. Diss Abstr Int,
B48(10): 2937.
Solomon LR (1987) Studies on the mechanism of acetaldehyde-mediated
inhibition of rat liver transaminases. Clin Chim Acta,
168(2): 207-217.
Solomon LR (1988) Effects of acetaldehyde on human red cell
metabolism: evidence for the formation of enzyme inhibitors. Clin Chim
Acta, 175: 249-266.
Sorrell MF & Tuma DY (1985) Hypothesis: alcoholic liver injury and the
covalent binding of acetaldehyde. Alcohol Clin Exp Res, 9: 306-309.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17(9): 416A-427A.
Spingarn NE, Northington DJ, & Pressely T (1982) Analysis of volatile
hazardous substances by GS/MS. J Chromatogr Sci, 20: 287-288.
Sprince H, Parker CM, Smith GG, & Gonzales LJ (1974) Protection
against acetaldehyde toxicity in the rat by L-cysteine, thiamin and
L-2-methylthiazolidine-4-carboxylic acid. Agents Actions, 4: 125-130.
Sreenathan RN, Padmanabhan R, & Singh S (1982) Teratogenic effects of
acetaldehyde in the rat. Drug Alcohol Depend, 9: 339-350.
Sreenathan RN, Padmanabhan R, & Singh S (1984a) Structural changes of
placenta following maternal administration of acetaldehyde in rat. Z
Mikrosk-Anat Forsch (Leipzig), 98: 597-604.
Sreenathan RN, Singh S, & Padmanabhan R (1984b) Effect of acetaldehyde
on skeletogenesis in rats. Drug Alcohol Depend, 14(2): 165-174.
Stege TE (1982) Acetaldehyde-induced lipid peroxidation in isolated
hepatocytes. Chem Pathol Pharmacol, 36: 287-297.
Steinberg S & Kaplan IR (1984) The determination of low-molecular
weight aldehydes in rain, fog and mist by reserved-phase liquid
chromatography of the 2,4-dinitrophenyl-hydrazone derivatives. Int J
Environ Anal Chem, 18: 253-266.
Steinhagen WH & Barrow CS (1984) Sensory irritation structure-activity
study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol
Appl Pharmacol, 72(3): 495-503.
Stewart JK, Aharoni Y, Hastsell PL, & Young DK (1980) Symptoms of
acetaldehyde injury on head lettuce. HortScience, 15(2): 148-149.
Takahashi K, Tomaru A, Nishiyama N, & Horiguchi M (1986) Alcoholic
cardiomyopathy: direct and indirect effects of acetaldehyde.
Jpn Circ J, 50(6): 567.
Takami T, Kuwata K, Sugimae A, & Nakamoto M (1985) Trace determination
of aldehydes in water by high-performance liquid chromatography. Anal
Chem, 57: 243-245.
Tanner RL & Meng Z (1984) Seasonal variations in ambient atmospheric
levels of formaldehyde and acetaldehyde. Environ Sci Technol,
18: 723-726.
Tejada SB (1986) Evaluation of silica gel cartridges coated in situ
with acidified 2,4-dinitrophenylhydrazine for sampling aldehydes and
ketones in air. Int J Environ Anal Chem, 26: 167-185.
Teschke R, Hasumura Y, & Lieber CS (1977) Hepatic pathways of ethanol
and acetaldehyde metabolism and their role in the pathogenesis of
alcohol-induced liver injury. Nutr Metab, 21(Suppl 1): 144-147.
Thom NS & Agg AR (1975) The breakdown of synthetic organic compounds
in biological processes. Proc R Soc London, B189: 347-357.
Til HP, Woutersen RA, Feron VJ, & Clary JJ (1988) Evaluation of the
oral toxicity of acetaldehyde and formaldehyde in a 4-week
drinking-water study in rats. Fundam Chem Toxicol, 26(5): 447-452.
Truitt EB & Walsh MJ (1971) The role of acetaldehyde in the actions of
ethanol. In: Kissin B & Begleiter H ed. The biology of alcoholism.
Volume 1: Biochemistry. New York, London, Plenum Press, pp 161-195.
Tuma DJ, Donohue TM Jr, Medina VA, & Sorrell MF (1984) Enhancement of
acetaldehyde-protein adduct formation by L-ascorbate. Arch Biochem
Biophys, 234(2): 377-381.
Tuma DJ, Jennett RD, & Sorrell MF (1987) The interaction of
acetaldehyde with tubuline. Ann NY Acad Sci, 282: 227-286.
Ung-Chhun NS & Collins MA (1987) Estimation of blood acetaldehyde
during ethanol metabolism: a sensitive HPLC/fluorescence microassay
with negligible artifactual interference. Alcohol, 4: 473-476.
US EPA (1975) Identification of organic compounds in effluents from
industrial sources. Washington, DC, US Environmental Protection
Agency, pp C-2, 4-14, 4-15, 4-17 (EPA-560/3-75-002; PB-241 841).
US NIOSH (1980) Projected number of occupational exposures to chemical
and physical hazards. Cincinnati, Ohio, US National Institute for
Occupational Safety and Health.
US NIOSH (1981) National occupational hazards survey. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health, p 14.
US NIOSH (1987) Manual of analytical methods. Method 3507. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health.
US NIOSH (1989) Manual of analytical methods. Method 2538.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health.
US NRC (1977) Other organic constituents (Acetaldehyde). In: Drinking
water and health. Washington, DC, US National Research Council,
pp 680-687.
US NRC (1981) Food chemical codex, 3rd ed. Washington, DC, US National
Research Council, National Academy Press, pp 353-355, 583, 615.
US NRC (1985) Chemicals used on food processing. Washington, DC, US
National Research Council, National Academy of Sciences (Publication
No. 1274).
Veghelyi PV & Osztovics M (1978) The alcohol syndromes: the
intrarecombigenic effect of acetaldehyde. Experientia (Basel),
34: 195-196.
Veghelyi PV, Osztovics M, Kardos G, Leisztner L, Szaszovszky E, Igali
S, & Imrei J (1978) The fetal alcohol syndrome: symptoms and
pathogenesis. Acta Paediatr Acad Sci Hung, 19(3): 171-189.
Verschueren U (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 139-141.
Von Burg R & Stout T (1991) Toxicology update-acetaldehyde. J Appl
Toxicol, 11: 373-376.
Wangenheim J & Bocsfoldi G (1986) Mouse lymphoma tk-positive-negative
assay of 30 compounds. Environ Mutagen, 8(Suppl 6): 90.
Wangenheim J & Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3(3): 193-206.
Watanabe I (1987) [Distribution of lower oxygenated organic compounds
in urban air.] Preprint of 54th Annual Meeting of the Chemical Society
of Japan, vol 1, p 129 (in Japanese).
Watanabe I (1988) Sequential automated analysis system for lower
oxygenated organic compounds in ambient air. J Res Nat Bureau Stand,
93(3): 299-301.
Watanabe A, Hobara N, & Nagashima H (1986) Blood and liver
acetaldehyde concentration in rats following acetaldehyde inhalation
and intravenous and intragastric ethanol administration. Bull Environ
Contam Toxicol, 37: 513-516.
Webster WS, Walsh DA, McEwen SE, & Lipson AH (1983) Some teratogenic
properties of ethanol and acetaldehyde in C57BL/6J mice: implications
for the study of the fetal alcohol syndrome. Teratology, 27: 231-243.
Westcott JY, Weiner H, Schultz J, & Myers RD (1980) In vivo
acetaldehyde in the brain of the rat treated with ethanol. Biochem
Pharmacol, 29: 411-417.
WHO (1989) IPCS Environmental Health Criteria 89: Formaldehyde.
Geneva, World Health Organization, 219 pp.
WHO (in press) Environmental Health Criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Wilkin JK & Fortner G (1985) Cutaneous vascular sensitivity to lower
aliphatic alcohols and aldehydes in Orientals. Alcohol Clin Exp Res,
9(6): 522-525.
Williams AJ & Barry RE (1986) Superoxide anion production and
degranulation of rat neutrophils in response to acetaldehyde-altered
liver cell membranes. Clin Sci, 71(3): 313-318.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen,
7(5): 677-702.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1989) Antibodies
against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1990) Antibodies
against acetaldehyde-modified epitopes: presence in alcoholic,
nonalcoholic liver disease and control subjects. Alcohol Alcohol,
25(5): 509-517.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991a) Detection of
stable acetaldehyde-modified proteins in the livers of ethanol-fed
rats. Alcohol Alcohol, 26(4): 437-444.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991b) Antibodies of
S3 against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Woutersen RA & Feron VJ (1987) Inhalation toxicity of acetaldehyde in
rats. IV. Progression and regression of nasal lesions after
discontinuation. Toxicology, 47(3): 295-305.
Woutersen RA, Appelman LM, Feron VJ, & der Heijden CA (1984)
Inhalation toxicity of acetaldehyde in rats. II. Carcinogenicity
study: interim results after 15 months. Toxicology, 31: 123-133.
Woutersen RA, Appelman LM, Van Garderen-Hoetmer A, & Feron VJ (1986)
Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity
study. Toxicology, 41(2): 213-231.
Yoshida A, Huang IY, & Ikawa M (1984) Molecular abnormality of an
inactive aldehyde dehydrogenase variant commonly found in Orientals.
Proc Natl Acad Sci (USA), 81: 258-261.
Zorzano A & Herrera E (1989) Disposition of ethanol and acetaldehyde
in late pregnant rats and their fetuses. Paediatr Res, 25(1): 102-106.
Résumé
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
L'acétaldéhyde est un liquide incolore et volatil à l'odeur acre
et suffocante. D'après la littérature, son seuil olfactif est de
0,09 mg/m3. L'acétaldéhyde est un composé très inflammable et
réactif qui est miscible à l'eau et à la plupart des solvants
ordinaires.
Il existe un certain nombre de méthodes d'analyse pour la
recherche de l'acétaldéhyde dans l'air (y compris dans l'haleine) et
dans l'eau. La principale méthode repose sur la réaction de
l'acétaldéhyde avec la 2,4-dinitrophénylhydrazine, puis analyse de
l'hydrazone obtenue par chromatographie liquide à haute pression ou
chromatographie en phase gazeuse.
2. Sources d'exposition humaine et environnementale
L'acétaldéhyde est un intermédiaire métabolique chez l'homme et
les plantes supérieures; c'est également un produit de la fermentation
alcoolique. On a décelé sa présence dans certaines denrées
alimentaires et boissons ainsi que dans la fumée de cigarette. Il est
également présent dans les gaz d'échappement des véhicules à moteur
ainsi que dans divers déchets industriels. La décomposition des
hydrocarbures, des effluents d'égouts et des déchets biologiques
solides produit de l'acétaldéhyde, de même que la combustion à l'air
libre ou, selon le cas, l'incinération du gaz, du mazout et de la
houille.
Plus de 80% de l'acétaldéhyde utilisé à des fins commerciales est
produit par l'oxydation en phase liquide de l'éthylène en présence
d'une solution catalytique de chlorures de palladium et de cuivre. Au
Japon, la production était de 323.000 tonnes en 1981. Aux Etats-Unis
d'Amérique elle a été de 281.000 tonnes en 1982 et en Europe
occidentale de 706.000 tonnes en 1983. La majeure partie de
l'acétaldéhyde produit commercialement est utilisé pour la préparation
de l'acide acétique. On l'utilise également pour la préparation de
certains arômes et denrées alimentaires.
On estime qu'aux Etats-Unis d'Amérique, les émissions annuelles
d'acétaldéhyde de toutes origines atteignent 12,2 millions de kg.
3. Transport, distribution et transformation dans l'environnement
En raison de la forte réactivité de l'acétaldéhyde, le transport
intercompartimental de ce composé devrait être limité. On peut
s'attendre à un certain transfert d'acétaldéhyde de l'air vers l'eau
et le sol en raison de sa forte tension de vapeur et de son faible
coefficient de sorption.
On pense que l'élimination photo-induite de l'acétaldéhyde
atmosphérique passe essentiellement par la formation de radicaux. La
photolyse devrait jouer également un rôle important dans le processus
d'élimination. Environ 80% des émissions d'acétaldéhyde dans
l'atmosphère sont éliminés chaque jour par ces deux processus.
D'après la littérature, la demi-vie de l'acétaldéhyde dans l'eau et
l'air est respectivement égale à 1,9 heure et 10-60 heures.
L'acétaldéhyde est facilement biodégradable.
4. Concentrations dans l'environnement et exposition humaine
Les concentrations moyennes d'acétaldéhyde dans l'environnement
sont généralement égales à 5 µg/m3. Dans l'eau, elles sont
généralement inférieures à 0,1 µg/litre. L'analyse de denrées
alimentaires très diverses effectuées aux Pays-Bas a montré que leur
teneur en acétaldéhyde est généralement inférieure à 1 mg/kg, mais
peut atteindre occasionnellement jusqu'à plusieurs centaines de mg/kg,
en particulier dans certains jus de fruit et dans le vinaigre.
Pour la population générale, la principale source d'exposition à
l'acétaldéhyde est de loin le métabolisme de l'alcool. La fumée de
cigarette constitue également une importante source d'exposition. En
ce qui concerne les autres véhicules, la population générale est
exposée à l'acétaldéhyde essentiellement par l'intermédiaire de la
nourriture et des boissons et, dans une moindre mesure, de l'air.
L'eau de boisson est négligeable à cet égard.
Les données disponibles sont insuffisantes pour qu'on puisse
évaluer l'ampleur de l'exposition à l'acétaldéhyde sur les lieux de
travail. Les travailleurs peuvent y être exposés dans certaines
industries ainsi que lors de la fermentation de l'alcool; dans ces
conditions la principale voie d'exposition est très probablement
l'inhalation avec possibilité également de contacts cutanés.
5. Cinétique et métabolisme
5.1 Absorption, distribution et élimination
Les données toxicologiques dont on dispose indiquent que
l'acétaldéhyde est résorbé au niveau des poumons et des voies
digestives; toutefois on ne connaît pas d'études quantitatives
appropriées qui aient été consacrées à ce problème. Il est probable
qu'il y a également une résorption cutanée.
Après avoir fait inhaler de l'acétaldéhyde à des rats, on a
constaté qu'il se répartissait dans le sang, le foie, les reins, la
rate, le coeur et le tissu musculaire. De faibles quantités en ont
été décelées dans des embryons après injection intrapéritonéale à la
mère (souris) ainsi qu'après exposition de la mère à de l'éthanol
(souris et rat). Il pourrait également y avoir production
d'acétaldé-hyde in vitro chez les foetus de rat et dans le placenta
humain.
Après injection intrapéritonéale d'éthanol, on a pu mettre en
évidence la distribution de l'acétaldéhyde dans le liquide
interstitiel de l'encéphale, à l'exclusion des cellules cérébrales. Il
est possible qu'une acétaldéhyde-déshydrogénase de forte affinité et
de faible Km joue un rôle important dans le maintien de faibles
concentrations d'acétaldéhyde dans le cerveau au cours de la
métabolisation de l'éthanol.
On a constaté que chez l'homme et les babouins, l'acétaldéhyde
pouvait être fixé par les globules rouges in vivo, la concentration
intraglobulaire pouvant atteindre dix fois la concentration
plasmatique.
Après administration par voie orale, il n'y a pratiquement pas
d'acétaldéhyde qui soit excrété tel quel dans les urines.
5.2 Métabolisme
La principale voie métabolique de l'acétaldéhyde consiste dans
une oxydation en acétate sous l'action de l'ALDHa NADb-dépendante.
L'acétate entre dans le cycle de l'acide citrique sous forme
d'acétyl-CoA. Il existe plusieurs isoenzymes de l'ALDH présentant
divers paramètres cinétiques et paramètres de liaison qui influent sur
la vitesse d'oxydation de l'acétaldéhyde.
Chez le rat, on a pu localiser une activité ALDH dans
l'épithélium respiratoire (à l'exclusion de l'épithélium olfactif),
alors que cette localisation se situe au niveau du rein chez la souris
et au niveau du cortex et des tubules rénaux chez le chien, le rat, le
cobaye et le babouin.
Les tissus embryonnaires de souris et de rat métabolisent
l'acétaldéhyde in vitro. L'acétaldéhyde traverse le placenta du rat
malgré l'existence d'un métabolisme placentaire.
a acétaldéhyde-déshydrogénase
b nicotinamide-adénine-dinucléotide
Il y a un certaine métabolisation de l'acétaldéhyde au niveau des
tubules rénaux chez l'homme mais c'est le foie qui est le principal
site du métabolisme.
Chez l'homme, on a décelé la présence de plusieurs formes
isoenzymatiques d'ALDH, au niveau du foie et d'autres tissus. Il y a
également un polymorphisme de l'ALDH michocondrial. Les sujets qui
sont homozygotes ou hétérozygotes pour une mutation ponctuelle du gène
correspondant à l'ALDH mitrochondriale, présentent une faible activité
ALDH, métabolisent lentement l'acétaldéhyde et ne supportent pas
l'alcool éthylique.
Le crotonadéhyde, la maléate de diméthyle, la phorone, le
disulfirame et le carbamide calcique inhibent le métabolisme de
l'acétaldéhyde.
5.3 Réactions avec d'autres constituants
L'acétaldéhyde forme des adduits stables ou instables avec les
protéines. Ces adduits peuvent perturber la fonction de ces protéines
comme le montre d'ailleurs l'inhibition des enzymes, les difficultés
de liaison entre histones et ADN et l'inhibition de la polymérisation
de la tubuline.
Des adduits instables de l'acétaldéhyde dont on connaît mal
l'importance se forment in vitro avec les acides nucléiques.
L'acétaldéhyde peut réagir sur diverses macromolécules de
l'organisme, de préférence avec celles qui sont porteuses de résidus
de lysine; cette réaction peut conduire à des altérations importantes
de la fonction biologique de ces molécules.
6. Effets sur les êtres vivants dans leur milieu naturel
6.1 Organismes aquatiques
Chez les poissons la CL50 peut varier de 35 mg/litre (guppy) à
140 mg/litre (espèces non précisées). Une CE5 de 82 mg/litre et une
CE50 de 42 mg/litre ont été observées respectivement chez des algues
et chez Daphnia magna.
6.2 Organismes terrestres
La présence d'acétaldéhyde dans l'air se révèle toxique pour
certains microorganismes, à des concentrations relativement faibles.
Des aphidiens ont été tués par exposition de 3 à 4 heures à de
l'acétaldéhyde à une concentration de 0,36 µg/m3.
Chez deux espèces de limaces, Arion hortensis et Agriolimax
reticulatus, on a observé des concentrations létales médianes
respectivement égales à 8,91 mg/litre et par heure et 7,69 mg/litre et
par heure.
L'acétaldéhyde provoque une inhibition réversible de la
germination des oignons, des carottes et des tomates à des
concentrations allant jusqu'à 1,52 mg/litre; par contre cette
inhibition est irréversible pour Amaranthus palmeri, dans les mêmes
conditions d'exposition. A la concentration de 0.54 µg/m3,
l'acétaldéhyde a détérioré des laitues.
7. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Les valeurs de la DL50 pour le rat et la souris et de la CL50
pour le rat et le hamster doré montrent que la toxicité aiguë de
l'acétaldéhyde est faible. On n'a pas connaissance d'études relatives
à la toxicité cutanée aiguë de l'acétaldéhyde.
7.2 Exposition à court et à long terme
Lors d'études au cours desquelles des doses ont été administrées
à plusieurs reprises, tant par la voie orale que par la voie
respiratoire, les effets toxiques observés à des concentrations
relativement faibles se limitaient essentiellement aux points de
contact initiaux. Lors d'une étude de 28 jours au cours de laquelle
de l'acétaldéhyde a été administré à des rats, mélangé à leur eau de
boisson, à la dose de 675 mg/kg de poids corporel (dose sans effets
observables 125 mg/kg de poids corporel), on a constaté que les effets
se limitaient à une légère hyperkératose focale au niveau de la
portion cardiaque de l'estomac. Après administration d'acétaldéhyde à
concentration constante de 0,05% dans l'eau de boisson pendant une
durée de 6 mois (le Groupe de travail estime que cela correspond à peu
près à 40 mg/kg de poids corporel) au cours d'une étude biochimique,
on a constaté que l'acétaldéhyde provoquait la synthèse de collagène
au niveau du foie chez le rat, observation d'ailleurs corroborée par
les résultats obtenus in vitro.
Lors d'une étude d'inhalation de 4 semaines chez le rat et de 13
semaines chez le hamster, on a obtenu, pour la concentration sans
effets respiratoires observables, des valeurs respectivement égales à
275 mg/m3 et 700 mg/m3. Aux concentrations les plus faibles
produisant un effet, on a observé des altérations dégénératives au
niveau de l'épithélium olfactif chez le rat (437 mg/m3) et de la
trachée (2400 mg/m3) chez le hamster. Des altérations dégénératives
de l'épithélium respiratoire et du larynx ont été observées à des
concentrations plus élevées. On n'a pas connaissance d'études
relatives à l'administration répétée d'acétaldéhyde au niveau cutané.
7.3 Reproduction, embryotoxicité et tératogénicité
Plusieurs études montrent que des malformations foetales peuvent
survenir par suite de l'exposition parentérale de rattes et de souris
gravides à l'acétaldéhyde. Dans la plupart de ces études, on n'a pas
étudié la toxicité pour la mère. On n'a pas pu trouver de données
relatives à la toxicité de l'acétaldéhyde pour la fonction de
reproduction.
7.4 Mutagénicité et points d'aboutissement des effets correspondants
In vitro, l'acétaldéhyde est génotoxique et produit des
mutations géniques, des effets clastogènes ainsi que des échanges
entre chromatides soeurs dans des cultures de cellules mammaliennes en
l'absence d'activation métabolique exogène. Toutefois, des épreuves
effectuées dans les règles sur des salmonelles ont donné des résultats
négatifs. Après avoir injecté de l'acétaldéhyde par voie
intrapéritonéale à des hamsters chinois et à des souris, on a constaté
que ce composé produisait des échanges entre chromatides soeurs au
niveau de la moelle osseuse. Cependant, de l'acétaldéhyde administré
par la même voie n'a pas eu pour conséquence un accroissement de la
fréquence des micronoyaux dans les spermatides de souris récemment
formés. On peut déduire indirectement de certaines études in vitro
et in vivo que l'acétaldéhyde est susceptible de produire des
pontages protéines-ADN et ADN-ADN.
7.5 Cancérogénicité
Des études d'inhalation effectuées sur des rats et des hamsters
ont permis de constater un accroissement de l'incidence des tumeurs.
Chez le rat, il s'agissait d'une augmentation, liée à la dose, de la
fréquence des adénocarcinomes au niveau de la muqueuse nasale ainsi
que des carcinomes spino-cellulaires (accroissement significatif à
toutes les doses). Toutefois chez le hamster, l'accroissement constaté
de la fréquence des carcinomes du nez et du larynx n'était pas
significatif. A toutes les concentrations d'acétaldéhyde, on a
constaté l'apparition de lésions tissulaires chroniques au niveau des
voies respiratoires.
7.6 Etudes spéciales
On n'a pas connaissance d'études appropriées portant sur la
neuro- et l'immunotoxicité potentielle de l'acétaldéhyde.
8. Effets sur l'homme
Lors d'études limitées portant sur des volontaires humains, on a
constaté que l'acétaldéhyde avait un effet légèrement irritant sur les
yeux et les voies respiratoires supérieures, après exposition de très
brève durée à des concentrations respectivement supérieures à environ
90 et 240 mg/m3. Après l'application d'un timbre inbibé
d'acétaldéhyde, on a constaté la présence d'un érythème cutané chez 12
sujets "d'ascendance orientale".
On a signalé l'existence d'une enquête limitée au cours de
laquelle on a étudié l'incidence des cancers chez des travailleurs
exposés à de l'acétaldéhyde.
On possède des preuves indirectes que le métabolite toxique
supposé être à l'origine des lésions hépatiques imputables à l'alcool,
de bouffées de chaleur et d'effets sur la développement, est en fait
l'acétaldéhyde.
9. Evaluation des risques pour la santé humaine et des effets
sur l'environnement
Les études effectuées sur l'animal montrent que, par voie
respiratoire ou par voie orale, l'acétaldéhyde n'est que faiblement
toxique. D'après les études effectuées sur l'animal mais également
sur l'homme, on a constaté que ce composé était légèrement irritant
pour l'oeil et les voies respiratoires supérieures. Chez l'homme,
l'application d'un timbre cutané inbibé d'acétaldéhyde peut provoquer
l'apparition d'un érythème cutané. On pense avoir découvert le
mécanisme à l'origine de cet effet mais les données disponibles sont
encore insuffisantes pour qu'on puisse évaluer le pouvoir
sensibilisateur de l'acétaldéhyde.
On ne dispose que de données limitées sur les effets d'une
ingestion d'acétaldéhyde. Après administration d'acétaldéhyde par
voie orale à des rats à la dose quotidienne de 675 mg/kg de poids
corporel, on a constaté un accroissement limite de l'hyperkératose au
niveau de la portion cardiaque de l'estomac (dose sans effets nocifs
observables: 125 mg/kg de poids corporel). Après avoir fait boire à
des rats six mois durant de l'eau contenant environ 40 mg
d'acétaldéhyde/kg de poids corporel, on a constaté un accroissement de
la synthèse du collagène hépatique, effet dont la signification reste
obscure.
D'après des études effectuées sur des rats et des hamsters, ce
sont les voies respiratoires supérieures qui constituent l'organe
cible de l'acétaldéhyde lorsque ce composé est inhalé. D'après les
résultats obtenus, la concentration la plus faible à laquelle on a
commencé à observer des effets était de 437 mg/m3, après une période
d'administration de 5 semaines. Chez des rats exposés pendant 4
semaines, la dose sans effets respiratoires observables était de
275 mg/m3; elle était de 700 mg/m3 chez des hamsters exposés
pendant 13 semaines.
Aux concentrations qui produisaient des lésions tissulaires des
voies respiratoires, on a observé un accroissement de l'incidence des
adénocarcinomes du nez et des carcinomes spino-cellulaires chez le
rat; il y a également eu accroissement des carcinomes du larynx et du
nez chez le hamster.
On est fondé à penser qu' in vivo, l'acétaldéhyde provoque des
lésions génétiques dans les cellules somatiques.
les données disponibles sont insuffisantes pour qu'on puisse
apprécier les effets qu'une exposition à l'acétaldéhyde pourrait avoir
sur la reproduction, le développement ainsi que les fonctions
neurologiques et immunologiques de la population générale ou de la
population professionnellement exposées.
Pour ce qui est du pouvoir irritant de ce composé chez l'homme,
on estime que la concentration tolérable est de 2 mg/m3. Etant
donné que le mécanisme d'induction de tumeurs par l'acétaldéhyde n'a
pas été bien étudié, on a adopté deux approches concernant ce point
d'aboutissement de son action toxique, à savoir la fixation d'une
concentration tolérable qui serait obtenue en divisant la dose
irritante pour les voies respiratoires chez les rongeurs, par un
certain facteur d'incertitude et l'estimation d'un risque de cancer
sur toute la durée de l'existence, en procédant par extrapolation
linéaire. La concentration tolérable est de 0,3 mg/m3. La
concentration produisant un excès de risque de 10-5 sur toute la durée
de la vie se situe entre 11-65 µg/m3.
Les données limitées dont on dispose ne permettent pas de tirer
des conclusions définitives quant au risque que l'acétaldéhyde
pourrait représenter pour l'ensemble de la faune et de la flore.
Toutefois, si l'on considère la courte durée de vie de l'acétaldéhyde
dans l'air et dans l'eau et le fait qu'il est facilement
biodégradable, on est amené à penser que ce composé n'est guère
menaçant pour les organismes aquatiques ou terrestres, sauf peut-être
en cas de décharge ou de déversement de produits industriels.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El acetaldehído es un líquido incoloro, volátil y de olor acre y
sofocante. El umbral señalado para el olor es de 0,09 mg/m3. El
acetaldehído es un compuesto muy inflamable y reactivo, soluble en
agua y en la mayoría de los disolventes comunes.
Existen métodos de análisis para la detección del acetaldehído en
el aire (incluso en el aliento) y en el agua. El principal de ellos
se basa en la reacción del producto con 2,4-dinitrofenilhidracina y
ulterior análisis de los derivados de la hidrazona por cromatografía
de líquidos a alta presión o cromatografía de gases.
2. Fuentes de exposición humana y ambiental
El acetaldehído es un producto intermedio del metabolismo en los
hombres y en las plantas superiores, y también proviene de la
fermentación alcohólica. Ha sido identificado en los alimentos, las
bebidas y el humo de los cigarrillos. También se encuentra en los
gases de escape de los vehículos y en ciertos desechos industriales.
La degradación de los hidrocarburos, las aguas residuales y los
desechos biológicos sólidos produce acetaldehído, como también lo
hacen la combustión al aire libre y la incineración de gas, fuel oil y
carbón.
Más del 80% del acetaldehído usado comercialmente proviene de la
oxidación en fase líquida de etileno con una solución catalítica de
paladio y cloruros de cobre. En 1981, la producción japonesa fue de
323 000 toneladas. En los Estados Unidos de América fue de 281 000
toneladas en 1982 y en Europa occidental de 706 000 toneladas en 1983.
La mayoría del acetaldehído producido comercialmente se destina a la
fabricación de ácido acético. También se utiliza en sustancias
aromáticas y en alimentos.
En los Estados Unidos, la emisión anual de acetaldehído de todo
origen se calcula en 12,2 millones de kilogramos.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta reactividad, el transporte intercompartimental
de acetaldehído debe ser limitado. Es de suponer que hay cierta
transferencia de la sustancia al aire desde el agua y el suelo, como
consecuencia de la fuerte presión del vapor y el bajo coeficiente de
sorción.
Es posible que la eliminación fotoinducida del acetaldehído en la
atmósfera tenga lugar principalmente por formación de un radical. La
fotosíntesis puede también contribuir bastante al proceso de
eliminación. La pérdida resultante de acetaldehído proveniente de
emisiones en la atmósfera es de alrededor del 80%. La vida media de
la sustancia en el agua y en el aire es de 1,9 h y 10-60 h,
respectivamente.
El acetaldehído es muy biodegradable.
4. Niveles medioambientales y exposición humana
Los niveles de acetaldehído en el aire ambiente suelen ser por
término medio de 5 µg/m3. Las concentraciones en el agua no llegan
en general a 0,1 µg/litro. El análisis de diversos alimentos en los
Países Bajos demostró que las concentraciones, normalmente inferiores
a 1 mg/kg, a veces llegaban a 100 mg/kg, particularmente en algunos
zumos de frutas y en el vinagre.
La principal fuente, con mucho, de exposición al acetaldehído
para la mayoría de la población es el metabolismo del alcohol. El
humo del cigarrillo es también una fuente significativa. Aparte de
eso pueden mencionarse en segundo lugar los alimentos y bebidas y, en
menor grado, el aire. La contribución del agua de beber es
insignificante.
No existen datos suficientes para determinar la importancia de la
exposición al acetaldehído en el lugar de trabajo. El personal puede
estar expuesto en algunas industrias manufactureras y donde hay
fermentación con producción de alcohol, siendo la principal vía la
inhalación y posiblemente el contacto con la piel.
5. Cinética y metabolismo
5.1 Absorción, distribución y eliminación
Los estudios existentes sobre toxicidad indican que el
acetaldehído se absorbe por los pulmones y el conducto
gastrointestinal; sin embargo, no parecen existir análisis
cuantitativos adecuados. Es probable la absorción por la piel.
En las ratas, el acetaldehído inhalado se distribuye por la
sangre, pasando al hígado, el riñón, el bazo, el corazón y otros
tejidos musculares. Se han detectado bajos niveles en embriones de
ratón tras inyectar la sustancia a la madre por vía intraperitoneal o
tras exposición de ratas o ratones hembra a etanol. La producción
potencial de acetaldehído in vitro se ha observado en fetos de rata
y en la placenta humana.
Tras inyección intraperitoneal de etanol se ha demostrado la
distribución de acetaldehído en el líquido intersticial pero no en las
células del cerebro. Una alta afinidad y escaso Km ALDHa pueden ser
importantes para mantener bajos niveles de acetaldehído en el cerebro
durante el metabolismo del etanol.
El acetaldehído es absorbido por los eritrocitos y, tras consumo
de etanol, los niveles intracelulares en hombres y babuinos pueden ser
in vivo 10 veces más altos que los del plasma.
Después de la administración por vía oral, la excreción de
acetaldehído por la orina prácticamente no cambia.
5.2 Metabolismo
Un medio importante en el metabolismo del acetaldehído es la
oxidación para pasar a acetato bajo la influencia de una ALDH
dependiente de NADb. El acetato pasa al ciclo de ácido cítrico como
acetil-CoA. Hay varios isoenzimas de ALDH con distintos parámetros
cinéticos y de ligazón que influyen en la rapidez de la oxidación del
acetaldehído.
Se ha detectado actividad de la ALDH en el epitelio del tracto
respiratorio (excluido el de las vías olfativas) de ratas, en el
cortex renal y los túbulos del perro, la rata, el cobayo y el babuino,
y también en los testículos del ratón.
El acetaldehído es metabolizado in vitro por el tejido
embrionario del ratón y la rata. La sustancia atraviesa la placenta
del ratón, pese al metabolismo placentario.
Aunque hay algún metabolismo en los túbulos renales humanos, el
principal órgano metabolizador es el hígado.
En el hígado y otros tejidos humanos se han detectado varias
formas isoenzimáticas de ALDH. En las mitocondrias la ALDH presenta
polimorfismo. Los sujetos que son homozigóticos o heterozigóticos por
una mutación singular del gen correspondiente a la ALDH de las
mitocondrias presentan poca actividad de esta enzima, metabolizan el
acetaldehído lentamente y no toleran el etanol.
El metabolismo del acetaldehído puede ser inhibido por el
crotonaldehído, el dimetilmaleato, el forón, el disulfiram y la
carbamida de calcio.
5.3 Reacción con otros compuestos
El acetaldehído puede formar aductores estables e inestables con
las proteínas. Eso menoscaba la función proteínica, como lo demuestra
la inhibición de la actividad enzimática, la menor ligazón histona-ADN
y la polimerización inhibida de la tubulina.
Los aductores inestables, de importancia indeterminada, se
producen in vitro con ácidos nucleicos.
El acetaldehído puede reaccionar con diversas macromoléculas en
el organismo humano, de preferencia las que contienen residuos de
lisina, lo que puede conducir a notables alteraciones de la función
biológica de dichas moléculas.
6. Efectos sobre los organismos presentes en el medioambiente
6.1 Organismos acuáticos
La CL50 para los peces va de 35 (Lebistes reticulatus) a
140 mg/litro (especies no especificadas). Se ha señalado una CE5 de
82 mg/litro y una CE50 de 42 mg/litro para las algas y para Daphnia
magna, respectivamente.
6.2 Organismos terrestres
El acetaldehído atmosférico a concentraciones relativamente bajas
parece ser tóxico para algunos microorganismos.
Los afidios mueren cuando son expuestos a concentraciones de
0,36 µg/m3 durante tres o cuatro horas.
Para las especies de babosa Arion hortensis y Agriolimax
reticulatus se han registrado valores letales medios de
8,91 mg/litro y 7,69 mg/litro por hora, respectivamente.
La inhibición de la germinación para la semilla de cebolla,
zanahoria y tomate tratada con acetaldehído (hasta 1,52 mg/litro) es
reversible, mientras que la de Amaranthus palmeri expuesta del mismo
modo es irreversible. El acetaldehído a 0,54 µg/m3 es perjudicial
para la lechuga.
7. Efectos en animales experimentales y sistemas de prueba in vitro
7.1 Exposición única
El acetaldehído tiene una toxicidad aguda baja, con una DL50 en
ratas y ratones y una CL50 en ratas y hámsters sirios. No se han
encontrado estudios de toxicidad dérmica aguda.
7.2 Exposición a corto y a largo plazo
En estudios reiterados del efecto tóxico de dosis por vía oral y
por inhalación, los efectos tóxicos de concentraciones relativamente
bajas se limitaron más bien a los puntos de contacto inicial. En un
estudio de 28 días en el que se administró a ratas con el agua de
beber acetaldehído a razón de 675 mg/kg de peso corporal (nivel sin
efecto observado (NOEL) = 125 mg/kg de peso corporal) los efectos se
limitaron a una ligera hiperqueratosis focal en la parte anterior del
estómago. En un estudio bioquímico, tras la administración a ratas de
dosis singulares (0,05% en el agua de beber) durante seis meses
(equivalente según el Grupo Especial a alrededor de un 40 mg/kg de
peso corporal), el acetaldehído indujo la síntesis de colágeno
hepático, observación corroborada mediante pruebas in vitro.
El nivel sin efecto respiratorio observado (NOEL) fue de
275 mg/m3 para ratas expuestas a acetaldehído por inhalación durante
cuatro semanas y 700 mg/m3 en hámsters expuestos durante 13 semanas.
A los niveles más bajos de efecto observado se produjeron cambios
degenerativos del epitelio olfativo en ratas (437 mg/m3) y de la
tráquea (2400 mg/m3) en hámsters. A concentraciones superiores se
produjeron cambios degenerativos del epitelio respiratorio y de la
laringe. No se han identificado estudios dérmicos con dosis
repetidas.
7.3 Reproducción, embriotoxicidad y teratogenicidad
En varios estudios, la exposición parenteral de ratas y ratones
gestantes a acetaldehído produjo malformaciones fetales. En la
mayoría de esos estudios no se evaluó la toxicidad materna. Tampoco
se dispone de datos sobre la toxicidad reproductiva.
7.4 Mutagenicidad y otros efectos finales afines
El acetaldehído es genotóxico in vitro e induce mutación de los
genes, con efectos clastogénicos e intercambios de cromatidios
hermanos (SCE) en las células de mamíferos, en ausencia de activación
metabólica exógena. Sin embargo se han registrado resultados
negativos en pruebas adecuadas con Salmonella. Tras inyección
intraperitoneal, el acetaldehído indujo SCE en la médula ósea de
hámsters chinos y ratones pero, en cambio, no hizo aumentar la
frecuencia de los micronúcleos en las espermátides precoces de ratón.
Indirectamente, ciertos estudios in vitro e in vivo parecen
indicar que el acetaldehído puede inducir enlaces cruzados
proteína-ADN y ADN-ADN.
7.5 Carcinogenicidad
Se ha observado una mayor incidencia de tumores en ratas y
hámsters expuestos a acetaldehído por inhalación. En las ratas, los
adenocarcinomas nasales aumentaron según la dosis pero los carcinomas
de células escamosas fueron significativos independientemente de la
dosis. Por el contrario, en los hámsters, el aumento de los
carcinomas nasales y laríngeos fue insignificante. A todas las
concentraciones, el acetaldehído administrado en los estudios produjo
daños irreversibles del tejido respiratorio.
7.6 Estudios especiales
No se conocen estudios adecuados sobre la neurotoxicidad y la
inmunotoxicidad potencial del acetaldehído.
8. Efectos en el ser humano
En estudios limitados con voluntarios, el acetaldehído produjo
irritación moderada de los ojos y de las vías respiratorias superiores
tras exposición por muy breves periodos a concentraciones que excedían
algo de 90 y 240 mg/m3, respectivamente. Las pruebas de parche
efectuadas con acetaldehído produjeron eritema cutáneo en 12 sujetos
de «ascendencia oriental».
Se ha tenido conocimiento de una investigación limitada sobre la
incidencia del cáncer entre trabajadores expuestos a acetaldehído y
otras sustancias.
Según pruebas indirectas, el acetaldehído participa como
metabolito potencialmente tóxico en la inducción de las afecciones
hepáticas, la congestión facial y las alteraciones del desarrollo
asociadas con el alcohol.
9. Evaluación de los riesgos para la salud humana y los efectos en el
medio ambiente
Los estudios efectuados con animales indican que la toxicidad
aguda del acetaldehído por inhalación o por vía oral es baja. Según
las investigaciones con sujetos humanos y con animales, esa sustancia
es algo irritante para los ojos y las vías respiratorias superiores.
Esos mismos efectos se han apreciado en voluntarios a los que se
administró acetaldehído (sección 1.8). Se ha observado eritema
cutáneo consiguiente a las pruebas de parche en sujetos humanos.
Aunque se ha identificado un posible mecanismo, los datos de que se
dispone son insuficientes para calcular el potencial del acetaldehído
como inductor de sensibilización.
Existe poca información sobre los efectos del acetaldehído
ingerido. Tras administrar diariamente a ratas por vía oral 675 mg/kg
de peso corporal (NOEL = 125 mg/kg de peso corporal) se observó un
aumento liminar de la hiperqueratosis en la parte anterior del
estómago. En las ratas que ingirieron durante seis meses con el agua
de beber dosis aproximadas de 40 mg/kg de peso corporal hubo un
aumento de la síntesis de colágeno en el hígado, cuya significación
está por dilucidar.
Los estudios de inhalación con ratas y hámsters permiten afirmar
que el tejido sensible es el de las vías respiratorias superiores. La
concentración más baja a la que se observaron efectos fue 437 mg/m3
durante cinco semanas. El NOEL identificado del tracto respiratorio
fue 275 mg/m3 en ratas expuestas durante cuatro semanas y
700 mg/m3 en hámsters expuestos durante 13 semanas.
A las concentraciones que producen daños en los tejidos del
tracto respiratorio se observaron aumentos de la incidencia del
adenocarcinoma nasal y del carcinoma de células escamosas en la rata,
y de los carcinomas laríngeos y nasales en el hámster.
Hay indicios de que el acetaldehído produce in vivo
alteraciones genéticas en las células somáticas.
No se dispone de datos suficientes para determinar si el
acetaldehído tiene efectos en la reproducción y el desarrollo o en el
estado neurológico o inmunológico de la población general o de la que
está particularmente expuesta por razón de su trabajo.
A partir de datos sobre la acción irritante en sujetos humanos se
ha considerado que la concentración tolerable es de 2 mg/m3. El
mecanismo de inducción de tumores por el acetaldehído no está bien
estudiado. En consecuencia, se han adoptado dos criterios
orientativos a ese respecto; a saber, la determinación de una
concentración tolerable obtenida dividiendo un nivel de efecto
irritante en el tracto respiratorio de los roedores por un factor de
incertidumbre, y la estimación del riesgo de cáncer mediante
extrapolación linear. La concentración tolerable resulta ser de
0,3 mg/m3. Las concentraciones asociadas con un aumento del riesgo
permanente de 10-5 son de 11-65 µg/m3.
La escasez de datos impide llegar a conclusiones definitivas
sobre los riesgos potenciales del acetaldehído para la biota en el
medio ambiente. Sin embargo, basándose en la breve vida media del
acetaldehído en el aire y en el agua, asi como en el hecho de que es
fácilmente biodegradable, cabe afirmar que los efectos de esa
sustancia en los organismos terrestres y acuáticos son escasos,
excepto posiblemente con ocasión de descargas industriales o vertidos.
No data are available on the formation of acetaldehydemodified
proteins in animals or humans directly exposed to acetaldehyde.
However, some data are available on proteins modified by acetaldehyde
derived from ethanol metabolism. In these studies, proteins carrying
acetaldehyde adducts were shown to be present in the liver cytosol of
rats fed ethanol for periods of 3 weeks, 12 months, or 27 months
(Worrall et al., 1991a). Acetaldehyde-modified proteins have also been
detected in the plasma (Liu et al., 1990) and haemoglobin of
alcoholics (Niemela & Israel, 1992). Furthermore, a limited
immunohistochemical study has demonstrated the presence of
acetaldehyde-modified proteins in the livers of some alcoholics
(Niemela et al., 1991). These studies demonstrate that acetaldehyde
adducts can form in the body. The possible immunological consequences
of adduct formation will be discussed in section 8.9.
6.5.2 Nucleic acids
No data are available from in vivo studies on the generation of
DNA adducts.
Acetaldehyde reacts with nucleosides and deoxynucleosides at pH
6.5 and 37°C in vitro to form unstable adducts by binding to the
exocyclic amino groups of adenine, cytosine, and guanine (Hemminki &
Suni, 1984). Addition of a reducing agent (sodium borohydride) leads
to the formation of stable adducts, of which the main one was
identified as N2-ethylguanosine using mass spectrometry and NMR.
Similar data for the formation of unstable adducts formed by reacting
acetaldehyde with ribonucleosides and deoxyribonucleosides was
reported by Fraenkel-Conrat & Singer (1988). When ethanol was present
in the reaction mixture, a different type of adduct was formed, which
was identified by fast atom bombardment and proton NMR to be a mixed
acetal (-NH-CH(CH3)-OR). These adducts were found to have half-lives
varying from 2.5 to 24 h at pH 7.5 and 37°C, depending on the base
involved.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
An LC50 (semi-static study) of 35 mg/litre was found for
acetaldehyde in the guppy (Poecilia reticulata; 10 laboratory-reared
and acclimatized fish, 2-3 months old) (Deneer et al., 1988). Grahl
(1983) reported an LC50 (48-96 h) of 124 mg/litre for acetaldehyde
in fish (no additional information was provided). Juhnke & Luedemann
(1978) presented the results for fish obtained in the Golden Orfe
test, and found an LC50 of 140-124 mg/litre for acetaldehyde (no
additional information was provided). An LC50 (static conditions;
96-h) of 53 mg/litre was reported for the bluegill (Lepomis
macrochirus) by Von Burg & Stout (1991).
Acetaldehyde had a depressing effect on the aggressive behaviour
of the fish cichlid (Cichlasoma nigro fasciatum) at concentrations
that did not cause locomotor decrements in this species (Peeke &
Figler, 1981).
An EC5 (48-h; population growth) of 82 mg/litre and an EC50
(48-h; static conditions; immobilization) of 42 mg/litre were reported
for protozoa (Chilomonas paramecium) and the waterflea (Daphnia
magna), respectively (Von Burg & Stout, 1991).
7.2 Terrestrial organisms
Aharoni & Barkai-Golan (1973) studied the effects of acetaldehyde
vapours on the germination and colony-forming potential of two fungi
species, Alternaria tenuis and Stemphylium botryosum. The rate of
growth inhibition increased with both concentration and time of
exposure. The exposure of the spores was conducted at room
temperature. A. tenuis, the more sensitive species, was inactivated
by 0.54 µg acetaldehyde/m3 applied for 5 h, whereas 1.08 µg
acetaldehyde/m3 for 2 h, was needed to inactivate S. botryosum
spores.
Pittevils et al. (1979) reported the activity of acetaldehyde
against the fungi affecting stored apples and pears (Colletotrichum
gloeosporioides, Cryptosporiopsis malicorticis, Phlyctaena
vagabunda, Botrytis cinerea, and Alternaria tenuis). Acetaldehyde
was rapidly lethal at low concentrations: after a 24-h treatment
period, the lethal concentration of acetaldehyde ranged from
0.036 µg/m3 (A. tenuis) to 0.09 µg/m3 (C. gloeosporioides).
Acetaldehyde remained lethal for the five fungi, even when the
treatment lasted only 20 min (0.90 µg/m3 for P. vagabunda, C.
malicorticis, and A. tenuis, and 0.36 µg/m3 for C. gloeosporioides
and B. cinerea).
The fungi Botrytis cinerea, Penicillium expansum, Rhizopus
stolonifer, Monilinia fructicola, Erwinia carotovora, and
Pseudomonas fluorescens were killed, when exposed to
acetaldehyde vapours at concentrations ranging from 0.045 to
3.6 µg/m3, applied for 0.5 to 120 min at room temperature (Aharoni &
Stadelbacher, 1973).
Aharoni et al. (1979) studied acetaldehyde as a fumigant for
control of the green peach aphid (Myzus persicae) on head lettuce
(Lactuca sativa). When aphids were placed on the lettuce prior to
fumigation, 0.36 µg acetaldehyde/m3 and a 3-4 h exposure were
required for 100% mortality. A similar treatment (0.27-0.36 µg/m3
for 4 h) was found to cause 100% mortality of aphids on lettuce by
Stewart et al. (1980).
The fumigant effect of acetaldehyde was tested on the garden slug
(Arion hortensis; weight range, 0.2-0.5 g) and the grey field slug
(Agriolimax reticulatus; weight range, 0.3-0.6 g). It caused both
species to close the pulmonary aperture and to secrete excess
'irritation' mucus. Medial lethal values of 7.69 ± 0.21 mg/litre
per h for A. reticulatus and of 8.91 ± 0.81 mg/litre per h for
A. hortensis were found (Henderson, 1970).
The seed germination of the onion (Allium cepa L.), carrot
(Daucus carota L.), Palmer Amaranth (Amaranthus palmeri S Wats.),
and tomato (Lycopersicon esculentum Mill.) after exposure to
acetaldehyde (up to 1.52 mg/litre), was examined by Bradow & Connick
(1988). After a 3-day exposure, acetaldehyde inhibited the seed
germination of all four plants by more than 50%. Seeds inhibited by a
3-day exposure to acetaldehyde followed by a 4-day recovery period
germinated to the same extent as the controls after seven days, except
for the Palmer Amaranth, which remained inhibited.
Acetaldehyde at concentrations of 0.54-1.08 µg/m3 affected head
lettuce (Lactuca sativa), as evidenced by dark-green, water-soaked,
necrotic areas on the outer leaves of the lettuce. Concentrations of
up to 0.36 µg/m3 did not affect the lettuce (Aharoni et al., 1979;
Stewart et al., 1980).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 LD50 and LC50 values
Relevant data are summarized in Table 4.
Oral LD50s for acetaldehyde in rats and mice ranged from 660 to
1930 mg/kg body weight. LC50s (0.5-4 h) in rats and Syrian hamsters
ranged from 24 to 37 g/m3. It is, therefore, concluded that the
acute toxicity of acetaldehyde is low. LD50 values by the dermal
route were not available.
LD50s for intratracheal, subcutaneous, intraperitoneal, and
intravenous administration are also presented in Table 4.
Table 4. LD50/LC50 values for acetaldehyde
Species Route of LD50/LC50 Reference
administration
Rat oral 1930 mg/kg body weight Smyth et al. (1951)
Rat oral 660 mg/kg body weight Sprince et al. (1974)
Mouse oral 1230 mg/kg body weight US NRC (1977)
Dog oral > 600 mg/kg body weight Booze & Oehme (1986)
Rat inhalation 24 g/m3; 4 h Appelman et al. (1982)
Rat inhalation 37 g/m3; 0.5 h Skog (1950)
Syrian
hamster inhalation 31 g/m3; 4 h Kruysse (1970)
Syrian
hamster intratracheal 96.1 mg/kg body weight Feron & De Jong (1971)
Rat subcutaneous 640 mg/kg body weight Skog (1950)
Mouse subcutaneous 560 mg/kg body weight Skog (1950)
Mouse intraperitoneal 500 mg/kg body weight Truitt & Walsh (1971)
Mouse intravenous 165 mg/kg body weight O'Shea & Kaufman
(pregnant) (1979)
8.2 Short-term exposure
8.2.1 Oral
Oral administration in the drinking-water of 675 mg
acetaldehyde/kg body weight to Wistar rats, daily for 4 weeks,
resulted in slight to moderate focal hyperkeratosis of the forestomach
in 8/10 males and 8/10 females. No effects were observed at lower
dose levels of 25 and 125 mg/kg body weight. In the control group,
very slight focal hyperkeratosis of the forestomach was noted in 6/20
females and 3/20 males (1/20 slight). At the top dose (675 mg/kg),
relative kidney weights were slightly increased in males, urinary
production was decreased, and there were variations in serum
biochemistry, most of which were attributable to reduced water intake.
There were no effects in the liver. The no-observed-effect level
(NOEL) was 125 mg/kg, the lowest-observed-effect level (LOEL) was
675 mg/kg (Til et al., 1988).
8.2.2 Inhalation
Male Sprague-Dawley rats were continuously exposed to
acetaldehyde vapour for 22 days at levels gradually increasing from
750 mg/m3 for a few days up to 2500 mg/m3 for the last few days.
By gradually increasing the concentrations, mortality in the early
period following exposure to 2000-2500 mg/m3 was prevented,
presumably because of metabolic adaptation; sudden, high, blood
acetaldehyde levels inducing vagal reflex reactions may result in
respiratory inhibition, and, as a consequence, death (Lamboeuf et al.,
1987; Latge et al., 1987).
Groups of 10 male and 10 female Wistar rats were exposed to
acetaldehyde at 0, 720, 1800, 3950, or 9000 mg/m3 (0, 400, 1000,
2200, or 5000 ppm) for 6 h/day, 5 days/week, for 4 weeks. Mortality
was slightly increased at 3950 and 9000 mg/m3, whereas growth was
retarded at 1800 mg/m3 and above in males, and at 9000 mg/m3 in
females. At 9000 mg/m3, relative liver weight decreased, and
relative lung weight in males increased. No treatment-related
histopathological changes were observed in the liver. Degenerative
changes of the nose were observed after exposure to all concentrations
(720 mg/m3-9000 mg/m3), with hyperplasia and metaplasia occurring
at concentrations of 3950 mg/m3 or more. A NOEL was not identified
(LOEL: 720 mg/m3) (Appelman et al., 1982).
Groups of 10 male Wistar rats were exposed to acetaldehyde, for
6 h/day, 5 days/week, for 4 weeks, in three different patterns: (a)
as a continuous daily exposure of 6 h to 0, 270, or 900 mg/m3 (0,
150, or 500 ppm), (b) as two daily exposures of 3 h to similar
concentrations with an intervening 1.5-h period with no exposure, or
(c) as two daily 3-h periods of exposure to similar concentrations
with an intervening 1.5-h period with eight short (5-min) peaks of 6
times the basic concentration, resulting in time-weighted average
concentrations of 0, 255, or 1050 mg/m3, respectively. Though there
were no indications of toxicity following continuous or interrupted
exposures to 270 and 900 mg/m3 and intermittent high/low exposure to
255 mg/m3, intermittent high/low exposure to 1050 mg/m3 induced
growth retardation (Appelman et al., 1986).
At 900 mg/m3, the observed effects were very similar to the
ones reported earlier by Appelman et al. (1982) at 720 mg/m3.
Variation of the pattern of exposure, by including a 1.5-h break, or
by additionally including eight 5-min, 6-fold higher peak exposures,
did not alter the observed degenerative effects. No effects were
observed in Wistar rats exposed to a lower concentration, 5 days/week
for 4 weeks, either as a "continuous" (6 h/day) exposure of
270 mg/m3, or as a time-weighted average of 255 mg/m3 after the
described intermittent low-high exposure. The NOEL was 255 mg/m3,
6-h TWA (LOEL = 1050 mg/m3, 6-h time weighted average) (Appelman et
al., 1986).
In another study, groups of 12 male Wistar rats were exposed to 0
or 437 mg acetaldehyde/m3 (0 or 243 ppm), 8 h/day, 5 days per week,
for 5 weeks. Hyperplasia of the olfactory epithelium and nasal
inflammation were observed in exposed animals, and on the basis of
lung function tests, residual volume and functional residual capacity
were increased, indicating some (unspecified) damage of the distal
airways (Saldiva et al., 1985).
8.2.3 Dermal
No relevant studies were identified.
8.2.4 Parenteral
Effects in the liver have been reported in several studies, but
only at very high doses. Intraperitoneal injection of male albino
rats with 200 mg acetaldehyde/kg body weight, daily, for 10 days, with
additional pyrazole treatment to inhibit the conversion of
acetaldehyde to ethanol, caused fatty accumulation in the liver, as
indicated by accumulation of total lipids, triacyl glycerols, and
total cholesterol, increased glycogenolysis, and a shift in metabolism
from the citric acid cycle towards the pentose phosphate pathway in
the liver. Serum triacyl glycerol, total cholesterol, and free fatty
acid levels were also increased. Changes were similar in rats not
receiving pyrazole pretreatment (Prasanna & Ramakrishnan, 1984a,
1987). The same treatment altered thyroid function, as indicated by
lower serum T4 and decreased iodine uptake in male albino rats, though
these effects may have been secondary to the observed hepatic changes
(Prasanna et al., 1986) and histopathological changes of the pancreas,
with resulting changes in trypsinogen levels and amylase secretion and
activity in female Sprague-Dawley rats (Majumdar et al., 1986).
8.3 Skin and eye irritation; sensitization
No relevant data were identified.
8.4 Long-term exposure
8.4.1 Oral
In rats exposed to 0.05% acetaldehyde in drinking-water
(estimated by the Task Group to be approximately 40 mg/kg body weight)
for 6 months, there was an increase in collagen synthesis in the liver
(Bankowski et al., 1993). The toxicological significance of this
observation is not known; no other effects were examined.
8.4.2 Inhalation
Non-neoplastic effects observed in carcinogenicity studies are
discussed in section 8.7.1.
Groups of 20 Syrian hamsters were exposed to acetaldehyde vapour
at 0, 700, 2400, or 8200 mg/m3 (0, 390, 1340, or 4560 ppm) for
6 h/day, 5 days/week, for 13 weeks. Increased relative lung and heart
weights as well as growth retardation were reported after exposure to
8200 mg/m3, though there were no increases in mortality in any of
the exposed groups (Kruysse et al., 1975). At the highest
concentration, there were severe degenerative, hyperplastic, and
metaplastic changes in the epithelium as well as subepithelial glands
and turbinate bones. Rhinitis was observed, with abundant nasal
discharge and salivation. The epithelium of the larynx, trachea, and
lungs was damaged, with some focal hyperplasia and metaplasia,
accompanied by tracheitis and focal bronchopneumonia. Changes in the
tracheal epithelium were also observed at 2400 mg/m3. At
700 mg/m3, no significant effects were observed (NOEL: 700 mg/m3;
LOEL: 2400 mg/m3).
8.5 Reproductive and developmental toxicity
Studies on reproductive effects have not been identified. A
number of studies on developmental effects have been conducted,
primarily to investigate the role of acetaldehyde in ethanol-induced
teratogenicity. However, in all of these studies, acetaldehyde was
administered by injection rather than by the principal routes of
exposure in the occupational and general environments (i.e., ingestion
and inhalation). Results of identified studies in which acetaldehyde
was administered during gestation to rats and mice by intraperitoneal,
intravenous, or amniotic injection are presented in Table 5. Though
dose-related embryotoxic, fetotoxic, and teratogenic effects were
observed in most of these studies, particularly those on rats,
maternal toxicity was not adequately assessed or reported in any of
these investigations. Dose-related embryotoxic effects were also
observed in in vitro studies on rat embryos exposed to acetaldehyde
(Popov et al., 1981; Campbell & Fantel, 1983).
Effects on the placenta have been observed following
intraperitoneal injection of acetaldehyde into pregnant rats
(Sreenathan et al., 1984a). In an in vitro study on human placental
membrane vesicles, very high concentrations (approximately 100 times
higher than the highest reported levels in blood), inhibited L-alanine
transport (Asai et al., 1985).
8.6 Mutagenicity and related end-points
Relevant data are summarized in Table 6.
8.6.1 Bacteria
Acetaldehyde was not mutagenic when tested adequately in standard
preincubation assays with S. typhimurium.
Statistically significant mutagenic responses were induced in
E. coli WP2uvrA in preincubation assays without metabolic activation
at 37°C (Veghelyi et al., 1978) and 0°C (Igali & Gazso, 1980). In
contrast, in another study on the same strain, results were negative
(Hemminki et al., 1980).
8.6.2 Non-mammalian eukaryotic systems
8.6.2.1 Gene mutation assays
Acetaldehyde (0.1% or 1.0% for 2 h) induced mutations in
genes that affect the egg-laying system of Caenorhabditis elegans
(Greenwald & Horvitz, 1980).
Acetaldehyde induced sex-linked recessive lethal mutations in
Drosophila melanogaster (Woodruff et al., 1985).
8.6.2.2 Chromosome alterations
Acetaldehyde induced chromosome malsegregation and mitotic
cross-over (yA2 marker) in Aspergillus nidulans diploid strain P1
during early conidial germination (Crebelli et al., 1989).
There was a dose-related increase in chromosomal aberrations in
Vicia faba root tips exposed to acetaldehyde (Rieger & Michaelis,
1960). Acetaldehyde also induced chromosome aberrations, micronuclei,
and sister chromatid exchanges (SCEs) in the root meristem cells of
Allium cepa (Cortes et al., 1986).
Table 5. Developmental toxicity
Species Exposure Dose Effects Reference
Rat day 10, 11, or 12; 0, 50, 75, 100 mg/kg fetal resorption, malformations (oedema, microcephaly, Sreenathan
days 10-12 of body weight, ip micrognathia, micromelia, hydrocephaly, exencephaly), growth et al. (1982,
pregnancy; days retardation; reduced placental weight; 50 mg/kg body weight, 1984b)
8-15 (50 mg/kg days 8-15: delays in ossification; skeletal malformations,
body weight per such as wavy ribs; no discussion of maternal effects
day)
Rat days 8-15 of 50, 75, 100 or 150 increase in resorption, and fetal death (changes were dose Padmanabhan
pregnancy mg/kg body weight, dependent); malformations: oedema, microcephaly, micrognathia, et al. (1983)
ip micromelia, syndactyly, hydronephrosis and subcutaneous
haemorrhage in the face, fore- and hind paws; reduced mean
placental weight and umbilical cord length; increased amniotic
fluid weight
maternal toxicity: rel. reduced water, food consumption, rel.
reduced body weight gain, 50 mg and above
Rat day 13 of pregnancy 1% or 10% solution 10%: 100% mortality; 1%: 80% malformations versus 14% in Bariliak &
embryo amniotic injections controls Kozachuk
(1983)
Rat days 9-12 of pregancy 1% (100 mg/kg body reduction in head length; no effects on morphological Ali &
weight), ip scores or crown rump length Persaud (1988)
Table 5 (contd).
Species Exposure Dose Effects Reference
Rat days 8-13 of 10 or 50 mg/kg body reduced performance in surface righting, olfactory Schreiner
pregnancy weight, ip discrimination, and learning ability; lower startle amplitudes et al. (1987)
in an auditory startle habituation test; maternal toxicity not Abstract
addressed; age of testing of offspring not specified
Rat day 5 of gestation 0.03-0.04 µg/kg body retarded blastulation Checiu et
weight examination on al. (1984)
day 5 of gestation
Mouse day 7 or 8 of 0.32 g/kg, iv exencephaly, mandibular and maxillary hypoplasia; Webster et
pregnancy; day 9 polydactyly club foot; no discussion of maternal effects al. (1983)
or 10 of pregnancy
Mouse day 10 of pregnancy 1000 mg/kg, ip no increase in resorptions or malformed fetuses or decrease Blakley &
in fetal weight; no discussion of maternal effects Scott (1984a)
Mouse day 7, 8 or 9 of 31 or 62 mg/kg body dose-dependent embryolethality; non-closure of anterior or O'Shea &
pregnancy weight, iv; posterior neuropore; smaller embryos; no discussion of Kaufman
examination on day maternal effects (1979)
10 or 19 of gestation
Mouse day 9 of pregnancy 2, 4, 6% (8 ml/kg no intermediate cellular effeects Bannigan &
body weight), ip Burke (1982)
Mouse day 6, 7 or 8 of 62 mg/kg body weight, neural tube effects; embryonic mortality; no discussion O'Shea &
pregnancy; and days iv; examination on of maternal effects Kaufman
6-8, 7-8 or 7-9 of day 10 or 12 of (1981)
pregnancy gestation
Table 6. Tests for gene mutation, chromosomal damage, and sister chromatid exchanges induced by acetaldehyde
Test system Measured end-point Test conditions MA RES Reference
Prokaryotic systems
Escherichia coli WP2uvrA reverse mutations 40 µg/ml; preincubated in capped tubes - + Veghelyi et al. (1978)
Escherichia coli WP2uvrA reverse mutations 0.88-441 µg/ml; incubated in capped - - Hemminki et al. (1980)
tubes at 37°C
Escherichia coli Wp2uvrA reverse mutations 0.8 µg/ml; incubated in capped tubes - + Igali & Gazso (1980)
at 0°C
Salmonella typhimurium
TA102 reverse mutations 1014 µg/plate; liquid preincubation - - Marnett et al. (1985)
TA104 -
Salmonella typhimurium reverse mutations 33-10 000 µg/plate; liquid preincubation + - Mortelmans et al. (1986)
TA1535 - -
TA1537 + -
- -
TA97 + -
- -
TA98 + -
- -
TA100 + -
- -
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Salmonella typhimurium concentration not given; + - Sasaki & Endo (1978)
TA100 liquid preincubation
Non-mammalian eukaryotic systems
Allium cepa root chromosomal aberration 7.5-7500 µg/ml - + Cortés et al. (1986)
meristem cells and SCE +
Vicia faba root tips chromosomal aberration 220-2205 µg/ml for 24 h at 12°C - + Rieger & Michaelis (1960)
Caenorhabditis elegans gene mutation 794-7940 µg/ml for 2 h - + Greenwald & Horvitz
(1980)
Drosophila melanogaster sex-linked recessive 22 500 mg/litre in 10% ethanol by + Woodruff et al. (1985)
injection
Canton-S wild-type lethal mutations 25 000 mg/litre in 10% ethanol by -
feeding
Aspergilus nodulans chromosomal 198-2381 µg/ml + Crebelli et al. (1989)
Diploid P1 malsegregation
In vitro mammalian systems
Mouse lymphoma L5178Y forward mutation (tk 176-353 µg/ml for 4 h - + Wangenheim & Bolcsfoldi
cells locus) (1986, 1988)
Chinese hamster ovary chromosomal aberration 88-5000 µg/ml - + Au & Badr (1979)
cells
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Chinese hamster ovary SCE 1.3-13 µg/ml - + Brambilla et al. (1986)
cells
Chinese hamster ovary SCE 8-80 µg/ml for 1 h + + De Raat et al. (1983)
cells - +
Chinese hamster ovary SCE 2-12 µg/ml for 24 h - + Obe & Beek (1979)
cells
Chinese hamster ovary SCE 4-8 µg/ml for 8 days - + Obe & Ristow (1977)
cells
Chinese hamster ovary hyperploidy 0.35-1.05 mmol/litre - + Dulout & Furnus (1988)
cells hypoploidy +
Human lymphocytes forward mutations 26.5-106 µg/ml for 24 h - + He & Lambert (1990)
(hprt locus) 8.8-26.5 µg/ml for 48 h
Human lymphocytes chromosomal aberration 0.1-20 mmol/litre added + Obe et al. (1985)
every 12 h for 5 days
Human lymphocytes chromosomal aberration 8-15 µg/ml - + Obe et al. (1979)
(normal Franconis
anaemia)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 0.8 µg/ml 2x/day, 4 days - + Obe et al. (1978)
Human lymphocytes chromosomal aberration 4-48 µg/ml for 72 h - + Boehlke et al. (1983)
SCE +
Human (whole blood, chromosomal aberration 20-40 µg/ml + Badr & Hussain (1977)
alcoholics)
Human lymphocytes SCE 4.4-106 µg/ml for 1-70 h - + He & Lambert (1985)
Human lymphocytes SCE 4.4-13 µg/ml for 70 h - + Lambert & He (1988)
Human lymphocytes SCE 4.4-18 µg/ml for 72 h + Helander & Lindahl-
Kiessling (1991)
Human lymphocytes SCE 4.4-22 µg/ml for 48 h + Sipi et al. (1992)
Human lymphocytes SCE 4-8 µg/ml for 90 h - + Jansson (1982)
Human lymphocytes SCE 0.04-4.4 µg/ml for 55 h - + Knadle (1985)
Human lymphocytes SCE 5.5-88 µg/ml for 48 h - + Norppa et al. (1985)
Human lymphocytes SCE reaction mixture added to whole blood - + Obe et al. (1986)
samples in dialysis tube; + +
concentration not given
Human lymphocytes SCE 4-16 µg/ml for 24 h - + Ristow & Obe (1978)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 1.8-35 µg/ml for 72 h - + Véghelyi & Osztovics
(1978)
Human lymphocytes SCE 1.8-35 µg/ml for 72 h + Véghelyi et al. (1978)
Wistar rat (skin chromosomal aberration 4.4-44 µg/ml for 12, 24, or 48 h - + Bird et al. (1982)
fibroblast) (micronuclei)
Mouse embryo cell transformation 10-100 µg acetaldehyde/ml for 24 h - - Abernethy et al. (1982)
C3H/10T1/2 cells 10-100 µg/ml followed by 0.25 µg +
TPA/ml 2x/week
Rat (HRRT kidney cells) cell transformation acetaldehyde - - Eker & Sanner (1986)
acetaldehyde followed by TPA and PDD +
MA = Metabolic activation; RES = Result.
8.6.3 Cultured mammalian cells
8.6.3.1 Gene mutation assays
Acetaldehyde induced gene mutations at the hypoxanthineguanine
phosphoribosyl transferase (hprt) locus in human lymphocytes
in vitro; there was a statistically significant and dose-related
increase in the frequency of mutants (He & Lambert, 1990).
Acetaldehyde produced a significant dose-related increase in forward
mutations in a mouse lymphoma L5178Y thimidine kinase locus assay,
without exogenous metabolic activation (Wangenheim & Bolcsfoldi, 1986,
1988).
8.6.3.2 Chromosome alterations and sister chromatid exchange
Dose-dependent increased frequencies of chromosome aberrations
have been observed in human lymphocytes following incubation with
acetaldehyde (Badr & Hussain, 1977; Obe et al., 1978, 1979, 1985;
Veghelyi & Ostovics, 1978; Boehlke et al., 1983).
Acetaldehyde induced a dose-dependent increase in chromosomal
damage in Chinese hamster ovary cells, though it was reported that the
activity was reduced (not quantified) in the presence of liver
homogenate fraction (Au & Badr, 1979). Dose-related increases in
micronuclei and chromosome aberrations were observed in cultured rat
skin fibroblasts in the absence of metabolic activation (Bird et al.,
1982).
SCE was induced by acetaldehyde (1.3-106 µg/ml) in the absence of
exogenous metabolic activation in Chinese hamster ovary cells and
human lymphocyte cultures (Obe & Ristow, 1977; Ristow & Obe, 1978;
Veghelyi et al., 1978; Veghelyi & Osztovics, 1978; Obe & Beek, 1979;
Jansson, 1982; Boehlke et al., 1983; De Raat et al., 1983; He &
Lambert, 1985; Knadle, 1985; Norppa et al., 1985; Brambilla et al.,
1986; Obe et al., 1986; Lambert & He, 1988; Helander &
Lindahl-Kiessling, 1991; Sipi et al., 1992). The increase in SCEs in
Chinese hamster ovary cells was less pronounced after addition of an
exogenous metabolic activating system (De Raat et al., 1983).
Similarly, addition of NAD+ and ALDH to human lymphocyte cultures
exposed to acetaldehyde decreased SCE induction (Obe et al., 1986).
Addition of 1-amino-cyclopropanol, an inhibitor of ALDH, to human
lymphocytes enhanced SCE induction by acetaldehyde (Helander &
Lindahl-Kiessling, 1991). These observations suggest that ALDH may
reduce the genotoxic potential of acetaldehyde.
Acetaldehyde induced aneuploidy has been reported in Chinese
hamster ovary cells (Dulout & Furnus, 1988). However, mainly
hypoploid cells were increased and, therefore, no firm conclusion can
be drawn from these studies. Acetaldehyde induced SCE in
pre-implantation mouse embryos in vitro (Lau et al., 1991).
8.6.4 In vivo assays
Only limited data are available on the genotoxicity of
acetaldehyde in vivo.
8.6.4.1 Somatic cells
C57BL/6J mice (only 2 animals of unspecified sex at each dose
level) received daily intraperitoneal doses of 0, 6, or 12 mg
acetaldehyde/kg body weight per day, for 5 days, and blood samples
were collected on days 3-6. The frequencies of micronuclei in mature
peripheral erythrocytes on days 5 and 6 (combined) were significantly
increased at the low dose, but not at the high dose (Ma et al., 1985).
No information on the frequency in polychromatic erythrocytes was
provided, so the possibility that this result was due to increased
erythropoiesis instead of mutagenesis cannot be discounted. This
study was reported only in the form of an abstract.
Groups of 6-7 Chinese hamsters (male and female) received 0.01,
0.1, or 0.5 mg acetaldehyde/kg body weight in saline by single
intraperitoneal injection and were killed 24 h later. Doses of
0.6 mg/kg body weight were lethal in preliminary tests. The
frequencies of SCEs in bone-marrow metaphases were elevated at 0.5 mg
acetaldehyde/kg body weight, but not at the lower doses (Korte & Obe,
1981). These observations support earlier findings in a more limited
study at an embryotoxic dose in mice and indicate in vivo formation
of SCEs (Obe et al., 1979).
Female Wistar rats were injected with 0.02 ml of 1% acetaldehyde
intra-amniotically on day 13 of pregnancy. Embryonic cells were
obtained 24 h later for cytogenetic analysis. Exposed rat embryos had
a higher frequency of chromosomal aberrations (mostly chromatid gaps
and breaks) than controls (Barilyak & Kozachuk, 1983). There was no
increase in the frequency of aneuploid cells.
8.6.4.2 Germ cells
Hybrid male mice ((C57B1/6J × C3H/He)F1), 4/dose group, were
injected intraperitoneally with a single dose of 0, 125, 250, 375, or
500 mg acetaldehyde/kg body weight in saline. Thirteen days after
exposure, the frequency of micronuclei in early spermatids was not
increased, though there were adequate positive controls (Lahdetie,
1988).
8.6.5 Other assays
Relevant data are summarized in Table 7.
8.6.5.1 DNA single-strand breaks
No single-strand breaks were detected with the alkaline elution
assay in DNA from various sources, after exposure in vitro to
acetaldehyde (Sina et al., 1983; Marinari et al., 1984; Harris et al.,
1985; Lambert et al., 1985; Saladino et al., 1985).
8.6.5.2 DNA cross-linking
There is some indirect evidence suggesting DNA cross-linking by
acetaldehyde.
Ristow & Obe (1978) observed enhanced reannealing of heat
denatured, isolated, calf thymus DNA following incubation with
acetaldehyde. It was suggested that this may have been due to DNA-DNA
cross-linking, because thermally denatured DNA will not reanneal in
this assay, unless complimentary DNA strands are held together by
cross-linkings.
Altered alkaline elution patterns indicative of DNA-DNA
cross-links were observed with DNA isolated from cultured human
leukocytes and exposed initially to acetaldehyde and then to X-rays
(Lambert et al., 1985; Lambert & He, 1988). DNA-protein cross-linking
was not observed by Harris et al. (1985) or by Saladino et al. (1985)
after exposure of human bronchial epithelial cells in vitro, but it
was observed by Lam et al. (1986) after incubation of either calf
thymus nucleohistones or fresh homogenates of the nasal mucosa of rats
with acetaldehyde. The possibility that acetaldehyde induces
DNA-protein cross-links in male Fischer-344 rat nasal mucosa has been
indirectly studied by measuring the extractability of DNA from
insoluble proteins following in vitro and in vivo exposures.
Decreased extractability of DNA was observed in preparations from
tissue homogenates exposed to acetaldehyde at concentrations of 100
and 500 mmol per litre but not 10 mmol/litre. Similarly, decreased
extractability was observed in nasal mucosal preparations from rats
exposed for 6 h to 1000 or 3000 mg/litre (ppm) but not 100 or
300 mg/litre (ppm) (Lam et al., 1986).
Table 7. Other tests indicative of genetic damage induced by acetaldehyde
Test system Measured end-point Test conditions Results Reference
Calf thymus DNA DNA-DNA cross-links 44 100 µg/ml for 30 min + Ristow & Obe (1978)
Calf thymus nucleohistones DNA-protein cross-links 4410-44 100 µg/ml - Lam et al. (1986)
Chinese hamster ovary DNA single-strand breaks no details - Marinari et al. (1984)
K1-cells DNA-DNA cross-links +
Rat hepatocytes DNA single-strand breaks 1.3 µg/ml for 3 h - Sina et al. (1983)
Human bronchial epithelial DNA single-strand breaks unknown concentration; - Harris et al. (1985)
cells DNA-protein cross-links 6 h -
Human bronchial epithelial DNA single-strand breaks up to 44 µg/ml for 1 h - Saladino et al. (1985)
cells DNA-protein cross-links -
Human leukocytes DNA single-strand breaks 441-882 µg/ml for 4 h - Lambert et al. (1985)
DNA-DNA cross-links +
Human lymphocytes DNA-DNA cross-links 441 µg/ml + Lambert & He (1988)
8.6.6 Cell transformation
Acetaldehyde did not initiate cell transformation in cultured
C3H/10T1/2 cells in the presence of the tumour promotor
12- O-tetradecanoylphorbol-13-acetate (TPA) (Abernethy et al., 1982).
Acetaldehyde initiated cell transformation in a rat kidney cell
line (HRRT) after pretreatment with tumour promotors (Eker & Sanner,
1986).
8.7 Carcinogenicity bioassays
8.7.1 Inhalation exposure
Only one carcinogenicity study in which animals were exposed by
inhalation to acetaldehyde over a lifetime has been performed. In
other carcinogenicity bioassays, animals were exposed for shorter
periods.
Wistar rats (55/sex per dose) were exposed for life (6 h/day, 5
days/week, for 28 months) to acetaldehyde concentrations of 1350,
2700, or 1800-5400 mg/m3 (the last concentration was gradually
reduced from 5400 mg/m3 in week 20 to 1800 mg/m3 in week 52).
Satellite groups of 5-10 additional rats of each sex were killed at
13, 26, and 52 weeks. Growth retardation occurred throughout the
study at all dose levels. Mortality was greater than in controls in
all dose groups and all of the animals in the high-dose group had died
by week 102. At week 52, there were degenerative changes in the
olfactory nasal epithelium at all dose levels including slight to
severe hyperplasia and keratinized stratified metaplasia of the larynx
(high dose only) and degenerative changes of the upper respiratory
epithelium (including papillomatous hyperplasia at the top dose only).
In the trachea, there was focal flattening and irregular arrangement
of the epithelium in 3/10 top-dose males at 52 weeks. In satellite
groups of 30 rats per sex, for which there was a 26-week recovery
period after 52 weeks of exposure, there was evidence of partial
regeneration of the olfactory epithelium in the low- and mid-dose
groups; there was also progression from hyperplasia and metaplasia to
neoplasia in some animals. At 28 months, carcinomas of the nose
developed in all exposed groups (Table 8). Although tumour incidence
was dose-related, the latency period appeared to be independent of
concentration. First tumours in all groups appeared during the 12th
month of exposure. The incidence of tumours was not increased in the
lungs, larynx, and trachea.
In simultaneously exposed groups of 30 Wistar rats/sex per dose,
exposure was terminated after 52 weeks and the animals killed
following a recovery period of 26 weeks. After the recovery period,
both mortality and nasal tumour incidence were very similar to those
in the groups in which exposure had been continued for 26 more weeks
(Woutersen et al., 1984, 1986; Woutersen & Feron, 1987).
Groups of Syrian golden hamsters (36/sex per dose) were exposed
for 52 weeks (7 h/day, 5 days/week) to a concentration gradually
reduced from 4500 mg/m3 in week 9 to 2970 mg/m3 in week 52. At
week 52, six animals/sex per dose were killed; the remaining animals
(30/sex per dose) were killed after a recovery period of 29 weeks. At
the end of the recovery period, nasal carcinomas were observed in 1/26
males and 1/27 females versus 0/24 and 0/23 in controls, and the
incidence of laryngeal carcinomas was increased (5/23 in males, 3/20
in females, against 0/20 and 0/22 in controls). No tumours were
observed in bronchi or lungs (Feron et al., 1982).
Table 8. Incidence of nasal tumours in Wistar rats after 28 months
of exposurea,b
Types of tumour 0 1350 2700 2760c
mg/m3 mg/m3 mg/m3 mg/m3
Males
Squamous cell carcinoma 1/49 1/52 10/53* 16/49***
Adenocarcinoma 0/49 16/52*** 31/53*** 21/49***
Carcinoma in situ 0/49 0/52 0/53 1/49
Females
Squamous cell carcinoma 0/50 0/48 5/53 17/53***
Adenocarcinoma 0/50 6/48* 28/53*** 23/53***
Carcinoma in situ 0/50 0/48 3/53 5/53
a From: Woutersen et al. (1986).
b Total number of tumour-bearing animals not specified.
Significance: Fisher Exact Test *P < 0.05, **P < 0.01, ***P <
0.001.
c The highest concentration was gradually reduced from
5400 mg/m3 during the first 20 weeks to 1800 mg/m3 in week
52; the time-weighted average concentration for 28 months of
exposure was calculated by the Task Group to be 2760 mg/m3.
Groups of 35 male Syrian hamsters were exposed by inhalation to 0
or 2700 mg acetaldehyde/m3 (0 or 1500 ppm) for 7 h/day, 5 days/week
for 52 weeks. At 52 weeks, 5 animals were killed for pathological
examination. The remainder of the animals were maintained for a
further 26-week recovery period. Body weight gain was less in the
exposed animals and mortality was increased from week 52 onwards.
There was a slight, but significant, decrease in rbc, haemoglobin, and
haematocrit in exposed animals. Relative kidney weights were
increased and urinary levels of protein and GOT were elevated. There
were marked lesions in the nasal cavity of animals killed at 52 weeks:
flattened epithelial cells with bizarre nuclei, fewer subepithelial
glands, submucosal thickening, and keratinizing stratified squamous
metaplasia of olfactory and respiratory epithelium. There was also
slight focal hyperplasia and metaplasia of the epithelium of the
trachea. There was partial or complete recovery from these lesions in
the animals killed at the end of the recovery period. There were no
tumours of the respiratory tract in any of the animals exposed to
acetaldehyde (Feron, 1979).
Weekly intratracheal administration of 0.2 ml of 2 or 4%
acetaldehyde in saline during a period of 52 weeks, followed by a
recovery period of another 52 weeks, did not induce any tumours in the
respiratory tract of male and female Syrian golden hamsters (Feron,
1979).
8.7.2 Co-carcinogenicity and promotion studies
In a mid-term test (Ito Model), groups of 19-20 male F344 rats
received a single intraperitoneal injection of diethylnitrosamine and
then various concentrations of acetaldehyde (2.5% and 5%, associated
with a recorded daily intake of 1.66 and 2.75 mg/kg body weight) in
their drinking-water, from week 2 until termination in week 6. All
rats had a two-thirds partial hepatectomy in week 3, in order to
stimulate cell proliferation. At 6 weeks, there was a significant
decrease in liver and body weight in all exposed animals, but
acetaldehyde had no effect on the development of glutathione
S-transferase (placental type)-positive liver cell foci (Ikawa et
al., 1986).
There were laryngeal tumours in 7/31 male and 6/32 female Syrian
golden hamsters exposed to a mixture of isoprene (approximately
2.09 mg/m3 or 750 ppm), methyl chloride (approximately 1.94 mg/m3
or 950 ppm) methyl nitrite (approximately 0.49 mg/m3 or 195 ppm) and
acetaldehyde (approximately 2340 mg/m3 or 1300 ppm) for 6 h/day, 5
days/week, over 23 months. There were no tumours of the respiratory
tract in controls (Feron et al., 1985). The authors suggested that
the laryngeal effects observed in hamsters exposed to the vapour
mixture were most probably caused by acetaldehyde alone (presumably
based on comparison with results in studies on hamsters exposed to
acetaldehyde alone).
There was no evidence of co-carcinogenicity in Syrian golden
hamsters exposed either by simultaneous inhalation exposure to
acetaldehyde and intratracheal instillation of benzo(a)pyrene (Feron,
1979; Feron et al., 1982) or by intratracheal instillation of
diethylnitrosamine (Feron et al., 1982) for 52 weeks followed by
recovery periods of 26-28 weeks. Nor was there any evidence of the
co-carcinogenicity of acetaldehyde following simultaneous
intratracheal instillations of acetaldehyde and either benzo (a)pyrene
or diethylnitrosamine for 52 weeks, followed by a recovery period of
52 weeks (Feron et al., 1982).
8.8 Neurological effects
Secondary effects of acetaldehyde on the respiratory and
cardiovascular systems, attributed to its influence on the autonomic
nervous system, have been observed following acute exposure of
experimental animals to acetaldehyde, principally by infusion or
intravenous administration. In two identified studies in which
animals were exposed by a route more relevant to environmental or
occupational exposure (i.e., inhalation), a 50% decrease in
respiratory rate was observed in two strains of mice and in rats
during inhalation of 5000 mg/m3 for 10 min (Steinhagen & Barrow,
1984; Babiuk et al., 1985; Takahashi et al., 1986).
Neurological effects following exposure by routes most relevant
to environmental and occupational exposure (i.e., inhalation and
ingestion) were limited to biochemical changes in the brain in
short-term studies on rats and mice exposed to relatively high
concentrations of acetaldehyde (750-13 230 mg/m3). Reported effects
included changes in the phospholipid fractions and increases in the
concentrations of monoamines and Na+/K+ATPase (Ortiz et al., 1974;
Shiohara et al., 1985; Latge et al., 1987; Roumec et al., 1988).
Manifestations of neurotoxicity were not examined in any of these
studies.
In the only identified study in which histopathological effects
on the nervous system were reported, degeneration was observable using
both light and electron microscopy in the cerebral cortex of rats
receiving a single intraperitoneal injection of 5 mg acetaldehyde/kg
body weight (Phillips, 1987).
In a short-term study involving intravenous exposure (24-26 mg/kg
body weight per day, for 20 days), the level of salsolinol in the
brain of rats was increased (Myers et al., 1985). Biochemical effects
of acetaldehyde on the brain have also been observed in vitro in
cerebral cortical neurons and brain microsomal preparations (Lahti &
Majchrowicz, 1969; Cederbaum & Rubin, 1977; Kuriyama et al., 1987).
8.9 Immunological effects
8.9.1 Direct effects on immune cells
Only one study has been carried out on whole animals. In a study
on CD1 mice exposed to 324 mg/m3, 3 h/day for 5 days, the
bacteriocidal activity of alveolar macrophages was reduced by 15%.
However, acetaldehyde did not affect mortality following streptococcal
infection (Aranyi et al., 1986).
In in vitro studies, 0.01% acetaldehyde caused a significant
inhibition of human granulocyte chemotaxis (Schopf et al., 1985). At
0.03%, acetaldehyde inhibited the release of lysozyme from monocytes,
but granulocytes were unaffected. The generation of oxygen radicals
by zymosan particle-stimulated monocytes and granulocytes was
inhibited by acetaldehyde in a dose-dependent manner. In a similar
study in which peripheral mononuclear cells were incubated with
acetaldehyde, decreased lytic activity against K562 cells was observed
at concentrations higher than 12 µmol per litre (Fink & Dancygier,
1988). In another study, acetaldehyde decreased 3H thymidine
incorporation into phytohaemagglutinin or Concanavalin A - stimulated
human lymphocytes (Levallois et al., 1987).
8.9.2 Generation of antibodies reacting with acetaldehyde-modified
proteins
The modification of proteins has been described in section 6.5.1.
In several studies, these modified proteins induced an immune response
when injected into rabbits and mice (Israel et al., 1986; Worrall et
al., 1989). The reactivity of the resulting antibodies towards
proteins, modified by acetaldehyde in vitro, is independent of the
carrier protein. These studies demonstrate (a) that
acetaldehyde-modified proteins are immunogenic; and (b) that
antibodies raised against a single modified protein will cross-react
with any other protein modified in the same manner.
Passive immunization of rats with a monoclonal IgE
antiacetaldehyde adduct antibody, derived from mice immunized with
acetaldehyde-modified proteins, rendered these animals hypersensitive
to acetaldehyde-modified proteins (Israel et al., 1992).
No immunization studies analogous to those described above have
been carried out on humans. In several studies, the presence of
antibodies, reactive with proteins modified by acetaldehyde in vitro,
has been reported in the plasma of alcoholics (Niemela et al., 1987;
Hoerner et al., 1988; Worrall et al., 1990, 1991a,b). The results of
these studies suggest that acetaldehyde-modified proteins are
generated in humans.
8.9.3 Related immunological effects
In rat liver membrane vesicles, exposed to acetaldehyde in vitro,
superoxide anion production by neutrophils was significantly enhanced
(Williams & Barry, 1986). In a further study, human liver plasma
membranes, modified by acetaldehyde, activated complement protein C3
components (Barry & McGivan, 1985).
8.10 Biochemical effects
Biochemical effects have been observed in cultured hepatocytes,
liver homogenates and mitochondrial fractions, purified enzymes,
isolated hepatic lipid membranes and whole liver preparations,
including: metabolic effects on lipid peroxidation (Stege, 1982;
Mueller & Sies, 1987; Shaw & Jayatilleke, 1987), phospholipid
metabolism (Snyder, 1988), transaminase activity (Solomon, 1987;
Snyder, 1988), carbohydrate and lipid metabolism (Matsuzaki & Lieber,
1977; Cederbaum & Dicker, 1981), and mitochondrial respiration
(Cederbaum et al., 1974). Increased hepatic collagen synthesis has
also been observed (Savolainen et al., 1984; Brenner & Chojkier,
1987).
Metabolic effects have been observed in other in vitro systems
including: renal cortex tubules (Michoudet & Baverel, 1985: altered
pyruvate/lactate metabolism), kidney, and muscle microsomal
preparations (Cederbaum & Rubin, 1977: inhibition of pyruvate
dehydrogenase), perfused hearts and cardiac whole homogenates
(Schreiber et al., 1972, 1974: myocardial protein synthesis and
inhibition of cardiac microsomal protein synthesis; Rawat, 1979:
reduced protein synthesis; Segel, 1984: no effect on mitochondrial
respiration), myocardial cells (McCall & Ryan, 1987: no effect on
Na+/K+-ATPase), leukocytes (Green & Baron, 1986: inhibition of
Na+/K+-ATPase), erythrocytes (Helander & Tottmar, 1987: inhibition
of the disappearance rate of biogenic aldehydes; Ninfali et al., 1987:
induction of intracellular reduced state; Solomon, 1988: inhibition of
aldolase-mediated by acetaldehyde oxidation- and inhibition of the
aminotransferases-mediated by non-oxidative acetaldehyde
metabolization; Atukorala et al., 1988: inhibition of transketolase),
and leukocytes and platelets (Helander & Tottmar, 1987: inhibition of
the disappearance rate of biogenic aldehydes).
9. EFFECTS ON HUMANS
9.1 General population exposure
No specific studies were available.
9.2 Occupational exposure
9.2.1 General observations
Acute exposure to acetaldehyde vapours has resulted in irritation
of the eyes and mucous membranes, reddening of the skin, pulmonary
oedema, headache, and sore throat. Repeated exposure causes
dermatitis and conjunctivitis. Ingestion causes nausea, vomiting,
diarrhoea, narcosis, and respiratory failure. Liquid acetaldehyde was
reported to cause superficial injury of the cornea. These effects
were reported in reviews and not in documented studies (Grant, 1974;
Hagemeyer, 1978; Dreisbach, 1987). In addition, a statement was found
in a textbook stating that prolonged exposure to acetaldehyde caused a
decrease in red and white blood cells plus a sustained rise in blood
pressure; however, no further information was available (Hagemeyer,
1978).
9.2.2 Clinical studies
A group of 12 volunteers were exposed in a chamber to various
nominal concentrations of acetaldehyde vapour on 15 different
occasions. No atmospheric sampling was performed. At 90 mg/m3
(50 ppm), the majority of the group experienced some degree of eye
irritation. Several subjects were discomforted at a lower
concentration of 45 mg/m3 (25 ppm), but no details were provided
(Silverman et al., 1946).
A group of 14 "healthy male" volunteers, aged 18-45 years, were
exposed in a 100 m3 chamber to a measured concentration of
acetaldehyde vapour of 240 mg/m3 (134 ppm) for 30 min. This
concentration was said to be mildly irritating to the upper
respiratory tract. No other clinical signs were reported (Sim &
Pattle, 1957).
Twelve volunteers of Oriental ancestry were patch-tested with
acetaldehyde (75%), ethanol (75%), and ethyl acetate (25%). With
acetaldehyde treatment, cutaneous erythema was seen in all 12
subjects, whereas ethanol was positive in 5 subjects. No vascular
response was detected for the acetaldehyde metabolite, ethyl acetate
(Wilkin & Fortner, 1985).
In an additional study, increased heart rate, ventilation, and
calculated respiratory dead space, and a decrease in alveolar CO2
levels were reported, following intravenous infusion of acetaldehyde
(5% v/v) in young male volunteers (Asmussen et al., 1948).
9.2.3 Epidemiological studies
A very limited investigation was performed on an unspecified
number of people, selected from 150 workers who had been employed for
20 years or more in a chemical factory. Nine cases of cancer were
identified (all in male smokers) 5 of which were in the respiratory
tract, 2 in the oral cavity, 1 in the stomach, and 1 in the caecum.
The number of respiratory cancers was higher than expected compared
with the prevalence in the national population (not corrected for
smoking). In parts of the factory, there was exposure to a variety of
chemicals. In one area, 1-7 mg acetaldehyde/m3 had been measured,
together with 5-70 mg butyraldehyde/m3, 1-7 mg crotonaldehyde/m3,
2-6 mg n-butanol per m3, and about 15 mg ethylhexanol/m3. Other
areas of the factory were known to have higher concentrations of
vapours of aldehydes and aldol, which caused irritation of the eyes
and upper respiratory tract (Bittersohl, 1974, 1975).
9.3 Effects of endogenous acetaldehyde
9.3.1 Effects of ethanol possibly attributable to acetaldehyde or
acetaldehyde metabolism
There is no direct evidence to link acetaldehyde with liver
injury. However, data obtained from animal models and human subjects
suggest that acetaldehyde may play a role in liver damage, especially
that associated with ethanol (sections 3.1.1 and 8.10).
An increased sensitivity to ethanol with respect to facial
flushing was observed in certain human populations (especially of
Oriental origin), which was ascribed to genetic differences in
acetaldehyde elimination, due to aldehyde dehydrogenase polymorphism
(Goedde & Agarwal, 1986, 1987; Eriksson, 1987; see also section
6.2.2).
The fetal alcohol syndrome is a specific pattern of congenital
abnormalities found in children of mothers who drink heavily. The
abnormalities most typically associated with alcohol-induced
developmental effects can be grouped into four categories: central
nervous system dysfunction (mental retardation, microcephaly,
hyperactivity); growth deficiencies (reduced prenatal length and
especially weight, with no catch-up postnatal growth; reduced adipose
tissue, normal growth hormone, cortisol, and gonadotropins); a
characteristic cluster of facial abnormalities (short palpebral
fissures, short upturned nose, hypoplastic philtrum, thinned upper-lip
vermillion, flattened midface); and variable major and minor
malformations (such as, heart failure, anomalies of the genitalia,
joints, and palmar creases) (Clarren & Smith, 1978).
The mechanisms by which ethanol produces its developmental
effects are not completely understood, and no data are available with
respect to ethanol doses and/or blood levels needed for such effects.
It is generally assumed that acetaldehyde, as the primary metabolite,
may contribute to the developmental effects of ethanol. Several
animal studies have demonstrated the direct teratogenic effect of
acetaldehyde (Ali & Persaud, 1988; see also section 8.5); however, no
reports are available with respect to the direct teratogenicity of
acetaldehyde in humans.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
By far, the principal source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure for
smokers. With respect to other media, the general population is
exposed to acetaldehyde principally from food, and to a lesser extent
from air. Intake from drinking-water is negligible compared with that
from other media.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route is most likely inhalation and, possibly, dermal
contact.
10.1.2 Health effects
Acetaldehyde is a reactive molecule that adducts to
macromolecules and can also condense or polymerise with small
molecules. Although quantitatively unimportant in acetaldehyde
metabolism, these by-products may have important biological effects.
In studies conducted on animals, the acute toxicity of
acetaldehyde by the oral or inhalation routes was low. Oral LD50s
ranged from 660 to 1930 mg/kg body weight and LC50s (0.5-4 h) from
24 to 37 g/m3. RD50s in rats and mice are slightly greater than
5000 mg/m3.
Data on the irritant effects of acetaldehyde on the skin and eye
are restricted to those from limited studies on human volunteers. It
was mildly irritating to the eyes and upper respiratory tract
following acute exposure for very short periods, in limited studies on
human volunteers at concentrations exceeding approximately 90 and
240 mg/m3, respectively (Silverman et al., 1946; Sim & Pattle,
1957). Cutaneous erythema was observed in the patch testing of twelve
subjects of "Oriental ancestry" with acetaldehyde (Wilkin & Fortner,
1985). Although a possible mechanism has been identified, available
data are inadequate to assess the potential of acetaldehyde to induce
sensitization.
Available data relevant to the assessment of the potential
adverse effects in humans of exposure to acetaldehyde in the
occupational and general environments are limited to those on
irritation mentioned above. The remainder of this evaluation is,
therefore, based on studies on animals.
Effects of acetaldehyde following oral administration to animals
have been much less well studied than those following inhalation. In
one of the two available studies by the oral route, the
no-observed-effect level (NOEL) following four weeks of administration
of acetaldehyde to rats was 125 mg/kg body weight per day (Til et al.,
1988). At the next higher concentration (675 mg/kg body weight per
day), a borderline increase in hyperkeratosis of the forestomach was
observed. In rats exposed to 0.05% acetaldehyde (estimated by the
Task Group to be approximately 40 mg/kg body weight) in the
drinking-water for 6 months, there was an increase in collagen
synthesis in the liver (Bankowski et al., 1993). No other effects
were examined and the toxicological significance of this observation
is unknown.
Acetaldehyde has induced gene mutations, clastogenic effects, and
sister chromatid exchanges (SCE) in mammalian cells in vitro.
Though available data on genotoxicity in vivo are limited, increases
in SCE have been observed in hamsters and mice exposed
intraperitoneally to acetaldehyde (Obe et al., 1979; Korte & Obe,
1981). This suggests genetic damage to somatic cells in vivo.
Available data are inadequate to assess the potential of acetaldehyde
to induce genetic damage in mammalian germ cells in vivo.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In the
respiratory tract, degenerative changes of the olfactory epithelium in
rats and trachea in hamsters have been observed at the lowest
concentrations. Degenerative changes in the respiratory epithelium and
larynx have been observed at higher concentrations. In available
studies, the lowest concentration at which effects were observed
(degenerative changes in the olfactory epithelium of rats) was
437 mg/m3 following administration for 5 weeks (Saldiva et al.,
1985). The NOELs identified for respiratory effects were 275 mg/m3
in rats exposed for 4 weeks (Appelman et al., 1986) and 700 mg/m3 in
hamsters exposed for 13 weeks (Kruysse et al., 1975).
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses of 1350 mg/m3 and greater)
(Woutersen et al., 1986) and non significant increases in laryngeal
and nasal carcinomas in hamsters (Feron et al., 1982). All
concentrations of acetaldehyde administered in these studies induced
tissue damage in the respiratory tract. On the basis of limited
available data, no conclusions can be drawn concerning the potential
of acetaldehyde to promote tumours.
The distribution of nasal lesions induced in rats exposed to
acetaldehyde in inhalation studies correlates with regional
deficiencies in ALDH, observed in a different strain of rats,
prompting the authors to suggest that regional susceptibility to the
toxic effects may be due, at least in part, to a lack of ALDH in the
susceptible regions (Bogdanffy et al., 1986).
Available data are inadequate for assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in occupationally exposed or
general populations.
10.1.3 Approaches to risk assessment
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Though the
principal sources of exposure to acetaldehyde in the general
population are through the metabolism of alcohol, in cigarette smoke,
and food, air is believed to be the main route of exposure in the
occupational environment. In addition, available data are inadequate
to provide guidance concerning the potential risks associated with
oral exposure to acetaldehyde. On this basis, only air is addressed
here.
On the basis of data on irritancy in humans, a tolerable
concentration can be derived as follows:
45 mg/m3
Tolerable concentration = = 2 mg/m3 (2000 µg/m3)
20
where:
no effects were observed in a limited study on human volunteers at
45 mg/m3 (Silverman et al., 1946) and 20 is the uncertainty factor
(×10 for intraspecies variation and × 2 for the poor quality of the
data).
Data suggest that acetaldehyde causes genetic damage to
somatic cells in vivo. The irritancy of acetaldehyde may also play
an important role in the development of tumours in the nose and larynx
of rats and hamsters, respectively, exposed by inhalation, though all
concentrations of acetaldehyde administered in carcinogenesis
bioassays induced both irritancy and nasal tumours. Therefore, two
approaches were adopted for the provision of guidance with respect to
the potential carcinogenicity of acetaldehyde.
In the first, a tolerable concentration (TC) was derived on the
basis of division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, based on the principles
outlined in WHO (in press) and the assumption that there is a
threshold for acetaldehyde-induced cancer of the respiratory tract in
rodents exposed via inhalation. There is some support for this
approach on the basis of relevant data on the analogues of
acetaldehyde, i.e., formaldehyde and glutaraldehyde, which have
similar spectra of in vitro mutagenic effects, but are clearly not
mutagenic in vivo (IARC, 1985; WHO, 1989). Thus,
275 mg/m3
Tolerable concentration = = 0.3 mg/m3 (300 µg/m3)
1000
where:
275 mg/m3 was the NOEL for irritation in rats in a 4-week study
(Appelman et al., 1986) and 1000 is the uncertainty factor (×10 for
interspecies variation, ×10 for intraspecies variation and ×10 for a
less than long-term study and severity of effect, i.e.,
carcinogenicity associated with irritation).
Since the mechanism of induction of tumours by acetaldehyde has
not been well studied, lifetime cancer risk has also been estimated on
the basis of a default model (i.e., linearized multistage) (WHO, in
press). However, it is very likely that, since estimated risk is
based on tumour incidence at concentrations that induce irritancy in
the respiratory tract and no-observed-effect levels for irritancy are
well below these concentrations, the true cancer risk is most likely
much lower at concentrations generally present in the environment and
may, indeed, be zero.
Concentrations associated with a 10-5 excess lifetime risk (lower
95% confidence limits) for nasal tumours (adenocarcinomas, squamous
cell carcinomas, and carcinomas in situ) in male and female rats, in
the only carcinogenicity study in which animals were exposed via
inhalation to acetaldehyde over the lifetime (Woutersen et al., 1986),
calculated on the basis of the linearized multistage model (Global
82), are 11-65 µg/m3. The high-dose animal groups were excluded in
the derivation of these estimates because of early mortality. A body
surface area correction was not incorporated.
10.2 Evaluation of effects on the environment
Acetaldehyde enters the environment during industrial production,
as a product of incomplete combustion (e.g., in vehicle exhausts) and
as a product of alcohol fermentation. Once released,
intercompartmental transport of acetaldehyde is expected to be
limited, because of its reactivity. However, because of its high
vapour pressure and low tendency for sorption onto soil, it is most
likely to be present in air. Acetaldehyde is readily biodegradable;
approximate half-lives are 10-60 h and 1.9 h in air and water,
respectively.
No quantitative data on levels in ambient water were identified.
However, on the basis of concentrations measured in drinking-water,
levels in the aquatic environment are expected to be low (i.e., less
than 0.1 µg/litre). Concentrations in ambient air average about
5 µg/m3.
Acetaldehyde is slightly toxic for fish. The lowest reported
LC50 was 35 µg/litre (Poecilia reticulata). No long-term toxicity
tests have been performed. Acetaldehyde is toxic for micro-organisms
at relatively low concentrations. Seed germination of several plants
was inhibited by more than 50% after exposure to 1.52 mg/litre (3 h).
There were no effects on lettuce exposed to 0.36 µg/m3 acetaldehyde.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
11. RECOMMENDATIONS FOR RESEARCH
1. Additional studies on the fate in the environment and impact of
acetaldehyde on biota.
2. Additional information on exposure of workers in the occupational
environment and levels in the vicinity of industrial facilities.
3. Carcinogenesis bioassays by the inhalation route including doses
that do not induce cytotoxicity (e.g., 275 mg/m3).
4. Studies on the biological significance of byproducts of
acetaldehyde, such as acetaldehyde-modified proteins.
5. Studies on pathogenicity, reproductive and developmental
toxicity, and genotoxicity in vivo by relevant routes of
exposure.
6. Additional studies on toxicity following ingestion in
experimental animals.
7. Studies of irritation in workers exposed to acetaldehyde.
References
Abernethy DJ, Frazelle JH, & Boreiko CJ (1982) Effects of ethanol,
acetaldehyde and acetic acid in the C3H/10TI/2C18 cell transformation
system. Environ Mutagen, 4: 331.
Agarwal DP & Goedde HW (1990) Alcohol metabolism, alcohol intolerance,
and alcoholism. Berlin, Heidelberg, New York, Springer-Verlag, 184 pp.
Aharoni Y & Barkai-Golan R (1973) Sensitivity to acetaldehyde vapors
of Alterneria tenuis and Stemphylium botryosum. Phytopathol Z,
78: 57-61.
Aharoni Y & Stadelbacher GJ (1973) The toxicity of acetaldehyde vapors
to postharvest pathogens of fruits and vegetables. Phytopathology,
63: 544-545.
Aharoni Y, Stewart JK, Hartsell PL, & Young DK (1979) Acetaldehyde - a
potential fumigant for control of the Green peach aphid on harvested
head lettuce. J Econ Entomol, 72: 493-495.
Ahrenholz S & Gorman R (1980) Health and hazard evaluation
determination. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health (Report No. HE 80-2-727).
Ali F & Persaud TVN (1988) Mechanisms of fetal alcohol effects: role
of acetaldehyde. Exp Pathol, 33(1): 17-21.
Almuaibed AM & Townshend A (1987) Determination of acetaldehyde by
flow-injection analysis with soluble or immobilized aldehyde
dehydrogenase. Anal Chim Acta, 198: 37-44.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Anderson RA, Quigg JM, Oswald C, & Zaneveld LJD (1985) Demonstration
of a functional blood-testis barrier to acetaldehyde; evidence for
lack of acetaldehyde effect on ethanol-induced depression of
testosterone in vivo. Biochem Pharmacol, 34(5): 685-695.
Aoyama T & Yashiro T (1983) Analytical study of low-concentration
gases. Part 2: Collection of acetaldehyde at low concentrations in air
in a solid reaction tube and its analysis using a flame thermionic
detector. J Chromatogr, 265: 45-55.
Appelman LM, Woutersen RA, & Feron VJ (1982) Inhalation toxicity of
acetaldehyde in rats. I. Acute and subacute studies. Toxicology,
23: 293-307.
Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, & Notten WRF (1986)
Effect of variable versus fixed exposure levels on the toxicity of
acetaldehyde in rats. J Appl Toxicol, 6(5): 331-336.
Aranyi C, O'Shea WJ, Graham JA, & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6(4): 713-720.
Arntz RR & Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmos Environ, 15(9): 1643-1651.
Asai M, Narita O, & Kashiwamata S (1985) Effects of acetaldehyde
and/or ethanol on neutral amino acid transport systems in microvillous
brush border membrane vesicles prepared from human placenta.
Experientia (Basel), 41(12): 1566-1568.
Asmussen E, Hald J, & Larsen V (1948) The pharmacological action of
acetaldehyde on the human organism. Acta Pharmacol, 4: 311-320.
Atkinson R & Pitts JN (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-426
K. J Chem Phys, 68(8): 3581-3584.
Atkinson R, Plum CN, Carter WPL, Winer AM, & Pitts JN (1984) Rate
constants for the gas-phase reactions of nitrate radicals with a
series of organics in air at 298 +/- 1 K. J Phys Chem, 88: 1210-1215.
Atkinson R, Aschmann SM, & Goodman MA (1987) Kinetics of the gas-phase
reactions of NO radicals with a series of alkynes, haloalkenes, and
a,b-unsaturated aldehydes. Int J Chem Kinet, 19: 299-307.
Atukorala TMS, Duggleby RG, & Nixon PF (1988) Effects of acetaldehyde
upon catalysis by human erythrocyte transketolase. Biochem Pharmacol,
37(10): 2100-2101.
Au W & Badr FM (1979) Does ethanol induce chromosomal damage?
In Vitro, 15: 221.
Babiuk C, Steinhagen WH, & Barrow CS (1985) Sensory irritation
response to inhaled aldehydes after formaldehyde pretreatment. Toxicol
Appl Pharmacol, 79(1): 143-149.
Badr FM & Hussain F (1977) Action of ethanol and its metabolite
acetaldehyde in human lymphocytes. In vivo and in vitro study.
Genetics, 86: s2-s3.
Bagnall GN & Sidebottom HW (1984) Photooxidation of acetaldehyde. In:
Proceedings of the Third Symposium on Physical and Chemical Behaviour
of Atmospheric Pollutants, Varese, Italy. Luxembourg, Commission of
the European Communities, pp 188-193 (EUR 9436).
Bankowski E, Pawlicka E, & Sobolewski K (1993) Liver collagen of rats
submitted to chronic intoxication with acetaldehyde. Mol Cell Biochem,
121: 37-43.
Baraona E, Di Padova C, Tabasco J, & Lieber CS (1987) Red blood cells:
a new major modality for acetaldehyde transport from liver to other
tissues. Life Sci, 40: 253-258.
Barilyak IR & Kozachuk SY (1983) [Embryotoxic and mutagenic activity
of ethanol and acetaldehyde after intra-amniotic injection.] Citol
Genet, 17: 57-60 (in Russian).
Barry RE & McGivan JD (1985) Acetaldehyde alone may initiate
hepatocellular damage in acute alcoholic liver disease. Gut,
26(10): 1065-1069.
Bell RP & Clunie JC (1952) The hydration of acetaldehyde in aqueous
solution. Trans Faraday Soc, 48: 439-442.
Berni RJ & Stanley JB (1982) Volatiles from cotton plant parts. Text
Res J, 52: 539-541.
Binding N, Thiewens S, & Witting U (1986) [Specific determination of
formaldehyde and other aldehydes and ketones for stationary and
personal exposure monitoring.] Staub-Reinhalt Luft, 46(10): 444-446
(in German).
Bird RP, Draper HH, & Basrur PK (1982) Effect of malonaldehyde and
acetaldehyde on cultured mammalian cells. Production of micronuclei
and chromosomal aberrations. Mutat Res, 101: 237-246.
Bittersohl G (1974) [Epidemiologic investigations on cancer incidence
in workers contacted by acetaldol and other aliphatic aldehydes.] Arch
Geschwulstforsch, 43: 172-176 (in German).
Bittersohl G (1975) Epidemiological research on cancer risk by aldol
and aliphatic aldehydes. Environ Qual Saf, 4: 235-238.
Blakley PM & Scott WR Jr (1984a) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. I. Teratogenicity of
ethanol and acetaldehyde. Toxicol Appl Pharmacol, 72: 355-363.
Blakley PM & Scott WR Jr (1984b) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. II. Pharmacokinetics of
the placental transfer of ethanol and acetaldehyde. Toxicol Appl
Pharmacol, 72: 364-371.
Boehlke JU, Singh S, & Goedde HW (1983) Cytogenetic effects of
acetaldehyde in lymphocytes of Germans and Japanese: SCE, clastogenic
activity, and cell cycle delay. Hum Genet, 63: 285-289.
Boettner EA, Ball GL, & Weiss B (1973) Combustion products from the
incineration of plastics. Cincinnati, Ohio, US Environmental
Protection Agency, pp 1-5, 106-122 (EPA-670/2-73-049; PB-222.001).
Bogdanffy MS, Randall HW, & Morgan KT (1986) Histochemical
localization of aldehyde dehydrogenase in the respiratory tract of the
Fisher 344 rat. Toxicol Appl Pharmacol, 82: 560-567.
Booze TF & Oehme FW (1986) An investigation of metaldehyde and
acetaldehyde toxicities in dogs. Fundam Appl Toxicol, 6: 440-446.
Bradow JM & Connick WJ (1988) Seed-germination inhibition by volatile
alcohols and other compounds associated with Amaranthus palmeri
residues. J Chem Ecol, 14(7): 1633-1648.
Brambilla G, Sciaba L, Faggin P, Maura A, Marinari UM, Ferro M, &
Esterbauer H (1986) Cytotoxicity, DNA fragmentation and sister
chromatid exchange in Chinese hamster ovary cells exposed to the lipid
peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat
Res, 171: 169-176.
Brenner DA & Chojkier M (1987) Acetaldehyde increases collagen gene
transcription in cultured human fibroblasts. J Biol Chem,
262(36): 17690-17695.
Brien JF & Loomis CW (1983) Pharmacology of acetaldehyde. Can J
Physiol Pharmacol, 61: 1-22.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data. Final Report (Contract
No. 68-02-3542). Menlo Park, California, US Environmental Protection
Agency.
Buehler R, Pestalozzi D, Hess M, & Von Wartburg JP (1983)
Immunohistochemical localization of alcohol dehydrogenase in human
kidney, endocrine organs and brain. Pharmacol Biochem Behav,
18(Suppl 1): 55-59.
Buyske DA, Owen LH, Wilder P, & Hobbs ME (1956) Chromatography of the
2,4-dihydrophenyl-hydrazones of some aldehydes and ketones in tobacco
smoke. Ann Chem, 28: 910-913.
Campbell MA & Fantel AG (1983) Teratogenicity of acetaldehyde
in vitro: relevance to the fetal alcohol syndrome. Life Sci,
32: 2641-2647.
Casanova-Schmitz M, David RM, & Heck Hd'A (1984) Oxidation of
formaldehyde and acetaldehyde by NAD+-dependent dehydrogenases in rat
nasal mucosal homogenates. Biochem Pharmacol, 33(7): 1137-1142.
Cavanagh LA, Schadt CF, & Robinson E (1969) Atmospheric hydrocarbon
and carbon monoxide measurements at Point Barrow, Alaska. Environ Sci
Technol, 3(3): 251-257.
Cederbaum AI & Dicker E (1981) Effect of cyanamide on the metabolism
of ethanol and acetaldehyde and on gluconeogenesis by isolated rat
hepatocytes. Biochem Pharmacol, 30: 3079-3088.
Cederbaum AI & Rubin E (1977) Sensitivity to acetaldehyde of pyruvate
oxidation by mitochondria from liver, kidney, brain and muscle.
Biochem Pharmacol, 26: 1349-1353.
Cederbaum AI, Lieber CS, & Rubin E (1974) The effect of acetaldehyde
on mitochondrial function. Arch Biochem Biophys, 161: 26-39.
Checiu M, Sandor S, & Garban Z (1984) The effect of ethanol upon early
development in mice and rats. 6. In vivo effect of acetaldehyde upon
preimplantation stages in rats. Rev Roum Morphol Embryol Physiol
Morphol Embryol 30: 175-184.
Chou WL & Speece RE (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
Chrostek WJ (1981) Health hazard evaluation report No.
HETA-81-098-941. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Chrostek WJ & Schoemaker WE (1981) Health hazard evaluation report No.
HETA-81-298-944. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Clarke DW, Steenaart NAE, Slack CJ, & Brien JF (1986) Pharmacokinetics
of ethanol and its metabolite, acetaldehyde, and fetolethality in the
third-trimester pregnant guinea pig for oral administration of acute,
multiple-dose ethanol. Can J Physiol Pharmacol, 64: 1060-1067.
Clarke DW (1988) The disposition of ethanol and its proximate
metabolite, acetaldehyde, in the maternal-fetal unit of the nearterm
guinea pig and pregnant ewe. Diss Abstr Int, 48(10): 2932B-2933B.
Clarren SK & Smith DW (1978) The fetal alcohol syndrome. New Engl J
Med, 298: 1063-1067.
Collins MA, Nijm WP, Borge GF, Teas G, & Goldfarb C (1979)
Dopamine-related tetrahydroisoquinolines: significant urinary
excretion by alcoholics after alcohol consumption. Science,
206: 1184-1186.
Cortes F, Mateos S, & Escalza P (1986) Cytotoxic and genotoxic effects
of ethanol and acetaldehyde in root-meristem cells of Allium cepa.
Mutat Res, 171(2-3): 139-143.
Crabb DW, Edenberg HJ, Bosron WF, & Li TK (1989) Genotypes for
aldehyde dehydrogenase deficiency and alcohol sensitivity. J Clin
Invest, 83: 314-316.
Crebelli R, Conti G, Conti I, & Carere A (1989) A comparative study of
ethanol and acetaldehyde as inducers of chromosome malsegregation in
Aspergillus nidulans. Mutat Res, 215: 187-195.
Delcour JA, Caers JM, Dondeyne P, Delvaux F, & Robberechts E (1982) An
enzymic assay for the determination acetaldehyde in beers. J Inst
Brew, 88: 384-386.
Deneer JW, Seinen W, & Hermens JLM (1988) The acute toxicity of
aldehydes to the guppy. Aquat Toxicol, 12: 185-192.
De Raat WK, Davis PB, & Bakker GL (1983) Induction of sister-chromatid
exchanges by alcohol and alcoholic beverages after metabolic
activation by rat-liver homogenate. Mutat Res, 124: 85-90.
Di Padova C, Alderman J, & Lieber CS (1986) Improved methods for
measurement of acetaldehyde concentrations in plasma and red blood
cells. Alcohol Clin Exp Res, 10(1): 86-89.
Dreisbach RH (1987) Handbook of poisoning, 9th ed. Los Altos,
California, Lange, pp 185-188.
Dulout FN & Furnus CC (1988) Acetaldehyde-induced aneuploidy in
cultured Chinese hamster cells. Mutagenesis, 3(3): 207-211.
Egle JL (1970) Retention of inhaled acetaldehyde in man. J Pharmacol
Exp Ther, 174(1): 14-19.
Eimutis EC, Quill RP, & Rinaldi GM (1978) Source assessment
noncriteria pollutant emissions (1978 update). Research Triangle Park,
North Carolina, US Environmental Protection Agency, Industrial
Environmental Research Laboratory, pp 1-5 (PB-291 747).
Eker P & Sanner T (1986) Initiation of in vitro cell transformation
by formaldehyde and acetaldehyde as measured by attachment-independent
survival of cells in aggregates. Eur J Cancer Clin Oncol,
22(6): 671-676.
Eriksson CJP (1987) Genetic aspects of the relation between alcohol
metabolism and consumption in humans. Mutat Res, 186(3): 241-247.
Eriksson CJP & Fukunaga T (1993) Human blood acetaldehyde (update
1991). Alcohol Alcohol, 28(Suppl 2): 9-25.
Eriksson CJP, Mizoi Y, & Fukunaga T (1982) The determination of
acetaldehyde in human blood by the perchloric acid precipitation
method: The characterization and elimination of artefactual
acetaldehyde formation. Anal Biochem, 125: 259-263. Espinet C &
Argiles JM (1984) Ethanol and acetaldehyde concentrations in the rat
foeto-maternal system after an acute ethanol administration given to
the mother. Arch Int Physiol Biochim, 92: 339-344.
Facchini MC, Chiavari G, & Fuzzi S (1986) An improved HPCL method for
carbonyl compound speciation in the atmospheric liquid phase.
Chemosphere, 15(6): 667-674.
Feron VJ (1979) Effects of exposure to acetaldehyde in Syrian hamsters
simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Prog
Exp Tumor Res, 24: 162-176.
Feron VJ & De Jong D (1971) Acute intratracheal toxicity of
acetaldehyde in Syrian golden hamsters. Zeist, Central Institute for
Nutrition and Food Research, TNO (Report No. R 3600).
Feron VJ, Kruysse A, & Woutersen RA (1982) Respiratory tract tumors in
hamsters exposed to acetaldehyde vapour alone or simultaneously to
benzo[a]pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol,
18: 13-31.
Feron VJ, Kuper CF, Spit BJ, Renzel PGJ, & Woutersen RA (1985) Glass
fibers and vapor phase components of cigarette smoke as factors in
experimental respiratory tract carcinogenesis. In: Carcinogenesis. New
York, Raven Press, pp 93-118.
Feron VJ, Til HP, Vrijer F, Woutersen RA, Cassee FR, & van Bladeren PJ
(1991) Aldehydes: occurrence, carcinogenic potential, mechanism of
action and risk assessment. Mutat Res, 259: 363-385.
Fink R & Dancygier H (1988) Acetaldehyde inhibits the activity of
human natural killer cells. Gastroenterology, 94(5): A127.
Fraenkel-Conrat H & Singer B (1988) Nucleoside adducts are formed by
cooperative reaction of acetaldehyde and alcohols: possible mechanism
for the role of ethanol in carcinogenesis. Proc Natl Acad Sci (USA),
85: 3758-3761.
Furia TE & Bellanca N ed. (1975) Fenaroli's handbook of flavour
ingredients, 2nd ed. Cleveland, Ohio, CRC Press Inc., vol 2, p 3.
Gerhold RM & Malaney GW (1966) Structural determination in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38(4): 562-579.
Gidley MJ, Hall LD, Sanders JKM, & Summers MC (1981)
Acetaldehyde-enkephalins: structure proof and some conformational
deductions from one- and two-dimensional proton nuclear magnetic
resonance spectra. Biochemistry, 20: 3880-3883.
Goedde HW & Agarwal DP (1986) Aldehyde oxidation: ethnic variations in
metabolism and response. In: Kalow W, Goedde W, & Agarwal DP ed.
Ethnic differences in reactions to drugs and xenobiotics. New York,
Alan R. Liss Inc., pp 113-138.
Goedde HW & Agarwal DP (1987) Polymorphism of aldehyde dehydrogenase
and alcohol sensitivity. Enzyme, 37: 29-44.
Goedde HW, Agarwal DP, & Harada S (1979) Alcohol metabolizing enzymes:
studies of isozymes in human biopsies and cultured fibroblasts. Clin
Genet, 16: 29-33.
Goedde HW, Singh S, Agarwal DP, Frizte G, Stapel K, & Paik YK (1989)
Genotyping of mitochondrial aldehyde dehydrogenase in blood samples
using allele-specific oligonucleotides: comparison with phenotyping in
hair roots. Hum Genet, 81: 305-307.
Gordon BHJ, Baraona E, & Lieber CS (1985) Blood acetaldehyde response
to ethanol ingestion during the reproductive cycle of the female rat.
Alcohol, 2: 271-275.
Grahl K (1983) [Classification of substances in water on the basis of
their toxicity potential to aquatic organisms.] Acta Hydrochim
Hydrobiol, 11(1): 137-143 (in German).
Gramiccioni L, Milana MR, Di Marzio S, & Lorusso S (1986) GC
determination of traces of acetaldehyde in aqueous matrix: a rapid
headspace method. Chromatographia, 21(1): 9-11.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Thomas CT Publications, p 76.
Green RJ & Baron DN (1986) The acute in vitro effect of ethanol, its
metabolites and other toxic alcohols on ion flax in isolated human
leucocytes and erythrocytes. Biochem Pharmacol, 35(20): 3457-3464.
Greenwald IS & Horvitz HR (1980) Unc-93(e1500): a behavioral mutant of
Caenorhabditis elegans that defines a gene with a wild-type null
phenotype. Genetics, 96: 147-164.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D (1991) Ambient levels of formaldehyde, acetaldehyde and
fumaric acid in Southern California. Results of a one-year base-line
study. Environ Sci Technol, 25: 710-715.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos Environ, 17(10): 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Guerri C & Sanchis R (1985) Acetaldehyde and alcohol levels in
pregnant rats and their fetuses. Alcohol, 2: 267-270.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hagemeyer HJ (1978) Acetaldehyde. In: Grayson M ed. Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 1, pp 97-112.
Hagen E (1967) The composition of combustion gases of polyurethane
forms and polycarbonates. Plaste Kautsch, 6: 391-392.
Harade S, Agarwal DP, & Goedde HW (1978a) Human liver alcohol
dehydrogenase isoenzyme variations: improved separation methods using
prolonged high voltage starch gel electrophoresis and isoelectric
focusing. Hum Genet, 40: 215-220.
Harade S, Agarwal DP, & Goedde HW (1978b) Application of gel
isoelectric focusing and electrophoresis in the study of alcohol
dehydrogenase and acetaldehyde dehydrogenase isoenzymes of human
tissues and fibroblasts. In: Catsimoolas U ed. Electrophoresis 78.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 275-282.
Harris CC, Willey JC, Saladino AJ, & Grafstrom RC (1985) Effects of
tumor promotors, aldehydes, peroxides, and tobacco smoke condensate on
growth and differentiation of cultured normal and transformed human
bronchial cells. In: Mass MJ, Kaufman DG, Siegfried JM, Steele WE, &
Nesnow S, ed. Carcinogenesis, a comprehensive survey. Cancer of the
respiratory tract. Predisposing factors. New York, Raven Press, pp
159-171.
Hatfield R (1957) Biological oxidation of some organic compounds. Ind
Eng Chem, 49(2): 192-196.
He SM & Lambert B (1985) Induction and persistence of SCE-inducing
damage in human lymphocytes exposed to vinyl acetate and acetaldehyde
in vitro. Mutat Res, 158(3): 201-208.
He SM & Lambert B (1990) Acetaldehyde-induced mutation at the hprt
locus in human lymphocytes in vitro. Environ Mol Mutagen, 16: 57-63.
Helander A & Tottmar O (1987) Effects of ethanol, acetaldehyde and
disulfiram on the metabolism of biogenic aldehydes in isolated human
blood cells and platelets. Biochem Pharmacol, 36(22): 3981-3985.
Helander A & Lindahl-Kiessling K (1991) Increased frequency of
acetaldehyde-induced sister-chromatid exchanges in human lymphocytes
treated with an aldehyde dehydrogenase inhibitor. Mutat Res, 264:
103-107.
Hellström-Lindahl E & Weiner H (1985) Effects of disulfiram on the
oxidation of benzaldehyde and acetaldehyde in rats liver. Biochem
Pharmacol, 34(9): 1529-1535.
Hemminki K & Suni R (1984) Sites of reaction of glutaraldehyde and
acetaldehyde with nucleosides. Arch Toxicol, 55: 186-190.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rate
and mutagenicity of directly acting industrial and laboratory
chemicals. Arch Toxicol, 46: 277-285.
Henderson IF (1970) The fumigant effect of metaldehyde on slugs. Ann
Appl Biol, 65: 507-510.
Herrick RF (1980) Industrial hygiene survey report on urea
formaldehyde foam insulation manufacturing. Cincinnati, Ohio, US
National Institute of Occupational Safety and Health, pp 2-5, 9-12
(PB83-107797).
Hobara N, Watanabe A, Kobayashi M, Nakatsukasa H, Nagshima H, Fukuda
T, & Araki Y (1985) Tissue distribution of acetaldehyde inhalation and
intragastric ethanol administration. Bull Environ Contam Toxicol,
35: 393-396.
Hoerner M, Behrens UJ, Worner T, Blacksberg I, Braly LF, Schaffner F,
& Lieber CS (1988) The role of alcoholism and liver disease in the
appearance of serum antibodies against acetaldehyde adducts.
Hepatology, 8: 569-574.
Hoffman D, Brunnenman, KD, Gori GB, & Wynder EL (1975) On the
carcinogenicity of marijuana smoke. Recent Adv Phytochem, 9: 63-81.
Horvath RJ, Senter SD, & Dekazos ED (1983) GLC-MS analysis of volatile
constituents in rabbit eye blueberries. J Food Sci, 48: 278-279.
Hsu LC, Bendel RE, & Yoshida A (1978) Genomic structure of the human
mitochondrial aldehyde dehydrogenase gene. Genomics, 2(1): 57-65.
Hugues UJ & Hum RW (1960) A preliminary survey of hydrocarbon-derived
oxygenated material in automobile exhaust gases. J Air Pollut Control
Assoc, 10: 367-371.
Hustert K & Parlar H (1981) [A test for photochemical degradation of
environmental chemicals in the gas phase.] Chemosphere,
10(9): 1045-1050 (in German).
IARC (1985) Acetaldehyde. In: Allyl compounds, aldehydes, epoxides and
peroxides. Lyon, International Agency for Research on Cancer, pp
101-132 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 36).
IARC (1988) Alcohol drinking. Lyon, International Agency for Research
on Cancer, 416 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risks to Humans, Volume 44).
Igali S & Gazso L (1980) Mutagenic effects of alcohol and acetaldehyde
on Escherichia coli. Mutat Res, 74: 209-210.
Ikawa E, Tsuda H, Sakata T, Masui T, Sato K, & Ito N (1986)
Modification potentials of ethyl alcohol and acetaldehyde on
development of preneoplastic glutathione S-transferase
P-form-positive liver cell foci initiated by diethylnitrosamine in the
rat. Cancer Lett, 31(1): 53-60.
IPCS/CEC (1990) International Chemical Safety Card No. 0009:
Acetaldehyde. Luxembourg, Commission of the European Communities
(First series).
Israel Y, Hurwitz E, Niemela EO, & Arnon R (1986) Monoclonal and
polyclonal antibodies against acetaldehyde-containing epitopes in
acetaldehyde-protein adducts. Proc Natl Acad Sci (USA),
83(20): 7923-7927.
Israel Y, MacDonald A, Niemela O, Zemel D, Shami E, Zywulko M, Klajner
F, & Borgono C (1992) Hypersensitivity to acetaldehyde-protein
adducts. Mol Pharmacol, 42(4): 711-717.
Jansson T (1982) The frequency of sister chromatid exchanges in human
lymphocytes treated with ethanol and acetaldehyde. Hereditas,
97: 301-303.
Japan Environment Agency (1989) Report on environmental survey and
wildlife monitoring of chemicals. In: Chemicals in the environment.
Tokyo, Japan Environment Agency, Department of Environmental Health,
p 73.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Jones JS, Sadler GD, & Nelson PE (1986) Acetaldehyde and accelerated
storage of wine: a new rapid method for analysis. J Food Sci,
51(1): 229-230.
Juhnke I & Luedemann D (1978) [Results of investigation of acute
toxicity to fish of 200 chemical compounds by the "Golden Orfe" test.]
Z Wasser Abwasser Forschung, 11(5): 161-164 (in German).
Kami T (1983) Composition of the essential oil of alfalfa. J Agric
Food Chem, 31: 38-41.
Karl PI, Gordon BHJ, Lieber CS, & Fisher SE (1988) Acetaldehyde
production and transfer by perfused human placental cotyledon.
Science, 242: 273-275.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Identification and analysis of
organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Press,
pp 329-373.
Knadle S (1985) Synergistic interaction between hydroquinone and
acetaldehyde in the induction of sister chromatid exchange in human
lymphocytes in vitro. Cancer Res, 45(10): 4853-4857.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77: 269-276.
Koivula T (1975) Subcellular distribution and characterization of
human liver aldehyde dehydrogenase fractions. Life Sci, 16: 1563-1570.
Kopczynski SL, Altshuller AP, & Sutterfield FD (1974) Photochemical
reactivities of aldehyde-nitrogen oxide systems. Environ Sci Technol,
8(10): 909-918.
Korte A & Obe G (1981) Influence of chronic ethanol uptake and acute
acetaldehyde treatment on the chromosomes of bone-marrow cells and
peripheral lymphocytes of Chinese hamsters. Mutat Res, 88: 389-395.
Kruysse A (1970) Acute inhalation toxicity of acetaldehyde in
hamsters. Zeist, Central Institute for Nutrition and Food Research,
TNO (Report No. R 3270).
Kruysse A, Feron VJ, & Til HP (1975) Repeated exposure to acetaldehyde
vapor. Studies in Syrian golden hamsters. Arch Environ Health,
30: 449-452.
Kuriyama K, Ohkuma S, Tomono S, & Hirouchi M (1987) Effects of alcohol
and acetaldehyde on metabolism and function of neurotransmitter system
in cerebral cortical neurons in primary culture. Alcohol Alcohol,
22(Suppl 1): 685-689.
Lahdetie J (1988) Effects of vinyl acetate and acetaldehyde on sperm
morphology and meiotic micronuclei in mice. Mutat Res,
202(1): 171-178.
Lahti RA & Majchrowicz E (1969) Acetaldehyde - an inhibitor of the
enzymatic oxidation of 5-hydroxyindoleacetaldehyde. Biochem Pharmacol,
18: 535-538.
Lam CW, Casanova M, & Heck HD (1986) Decreased extractability of DNA
from proteins in the rat nasal mucosa after acetaldehyde exposure.
Fundam Appl Toxicol, 6(3): 541-550.
Lambert B, Chen Y, He SM, & Sten M (1985) DNA cross-links in human
leucocytes treated with vinyl acetate and acetaldehyde in vitro.
Mutat Res, 146(3): 301-303.
Lambert B & He SM (1988) DNA and chromosome damage induced by
acetaldehyde in human lymphocytes in vitro. Ann NY Acad Sci,
534: 369-376.
Lamboeuf Y, Latge C, Roumec C, & De Saint Blanquat G (1987) Ethanol
tolerance in the rat after inhalation of acetaldehyde for a period of
21 days. Alcohol Alcohol, 22(Suppl 1): 441-447.
Latge C, Lamboeuf Y, Roumec C, & De Saint Blanquat G (1987) Effect of
chronic acetaldehyde intoxication on ethanol tolerance and membrane
fatty acids. Drug Alcohol Depend, 20(1): 47-56.
Lau CF, Vogel R, Obe G, & Spielmann H (1991) Embryologic and
cytogenetic effects of ethanol on preimplantation mouse embryos
in vitro. Reprod Toxicol, 5: 405-410.
Leone JA & Seinfeld JH (1984) Analysis of the characteristics of
complex chemical reaction mechanisms: application to photochemical
smog chemistry. Environ Sci Technol, 18(4): 280-287.
Levallois C, Maini JC, & Balmes JL (1987) Sensitivity of human
lymphocytes to acetaldehyde: comparison between alcoholic and control
subjects. Drug Alcohol Depend, 20(2): 135-142.
Lipari F & Swarin SJ (1982) Determination of formaldehyde and other
aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. Chromatography, 247: 297-306.
Lipari F, Dasch JM, & Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environ Sci Technol, 18: 326-330.
Liu RC, Lumeng L, Shahidi S, Kelly T, & Pound DC (1990)
Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol
Clin Exp Res, 14: 438-443.
Ludzack JR & Ettinger MB (1960) Industrial wastes. Chemical structures
resistant to aerobic biochemical stabilization. J Water Pollut Control
Fed, 32(11): 1173-1200.
Lyman WJ, Rheel WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, McGraw-Hill Book Company.
Lynch C, Lim CK, Thomas M, & Peters TJ (1983) Assay of blood and
tissue aldehydes by HPLC analysis of their 2,4-dinitrophenylhydrazine
adducts. Clin Chim Acta, 130: 117-122.
Ma T, Miller TL, & Xu Z (1985) Mouse peripheral erythrocyte
micronucleus assay on the genotoxicity of acetaldehyde. Environ
Mutagen, 7(Suppl 3): 28.
Maarse H & Visscher CA (1992) Volatile compounds in food. Qualitative
and quantitative data. Supplement 3 and cumulative index. Zeist,
Central Institute for Nutrition and Food Research, TNO, Biotechnology
and Chemistry Institute, pp XXXIII-XLI.
McCall D & Ryan K (1987) The effect of ethanol and acetaldehyde on Na
pump function in cultured rat heart cells. J Mol Cell Cardiol,
19: 453-463.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987a)
Effects of acetaldehyde on polymerization of microtubule proteins.
Brain Res, 416: 90-99.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987b) The
reaction of acetaldehyde with brain microtubular proteins: formation
of stable adducts and inhibition of polymerization. Neurosci Lett,
79: 163-168.
Majumdar APN, Vesenka GD, Dubick MA, Yu GSM, DeMorrow JM, & Geokas MC
(1986) Morphological and biochemical changes of the pancreas in rats
treated with acetaldehyde. Am J Physiol 250(5): G598-G606.
Manning DL, Maskarinec MP, Jenkins RA, & Marshall AH (1983) High
performance liquid chromatographic determination of selected gas phase
carbonyls in tobacco smoke. J Assoc Off Anal Chem, 66(1): 8-12.
Margeri G, Versini G, Gianotti I, & Pellegrini R (1984) Acetaldehyde
in beverages. Riv Soc Ital Sci Aliment, 5: 401-406.
Marinari UM, Ferro M, Sciaba L, Finollo R, Bassi AM, & Brambilla G
(1984) DNA-damaging activity of biotic and xenobiotic aldehydes in
Chinese hamster ovary cells. Cell Biochem Funct, 2(4): 243-248.
Marjanen LA (1973) Comparison of aldehyde dehydrogenases from cytosol
and mitochondria of rat liver. Biochim Biophys Acta, 327: 238-246.
Marnett LJ, Hurd H, Hollstein M, Levin DE, Esterbauer H, & Ames BN
(1985) Natural occurring carbonyl compounds are mutagens in Salmonella
tester strain TA104. Mutat Res, 148: 25-34.
Matsuzaki S & Lieber CS (1977) Increased susceptibility of hepatic
mitochondria to the toxicity of acetaldehyde after chronic ethanol
consumption. Biochem Biophys Res Commun, 75(4): 1059-1065.
Mauch TJ, Donahue TM, Zetterman RK, Sorrell MF, & Tuma DJ (1986)
Covalent binding of acetaldehyde selectively inhibits the catalytic
activity of lysine-dependent enzymes. Hepatology, 6(2): 263-269.
Mauch TJ, Tuma DJ, & Sorrell MF (1987) The binding of acetaldehyde to
the active site of ribonuclease: alterations in catalytic activity and
effects of phosphate. Alcohol Alcohol, 22(2): 103-112.
Michoudet C & Baverel G (1985) Metabolism and metabolic effects of
acetaldehyde in dog, rat and guinea pig renal cortex. Dev Nephrol,
10: 143-149.
Michoudet C & Baverel G (1987a) Characteristics of acetaldehyde
metabolism in isolated dog, rat and guinea pig kidney tubules. Biochem
Pharmacol, 36(22): 3987-3991.
Michoudet C & Baverel G (1987b) Metabolism of acetaldehyde in human
and baboon renal cortex:ethanol synthesis by isolated baboon
kidney-cortex tubules. FEBS Lett, 216(1): 113-117.
Miyamoto Y (1986) Eye and respiratory irritants in jet engine exhaust.
Aviat Space Environ Med, 57: 1104-1108.
Mold JD & McRae TG (1957) The determination of some low molecular
weight aldehydes and ketones in cigarette smoke as the
2,4-dinitrophenylhydrazone. Tob Sci, 1: 40-46.
Moortgat GK & McQuigg RD (1984) A FTIR spectroscopic study of the
photooxidation of acetaldehyde in air. In: Proceedings of the Third
European Symposium on Physico-Chemical Behaviour of Atmospheric
Pollutants, Varese, Italy. Luxembourg, Commission of the European
Communities, pp 194-204 (EUR 9436).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing of
270 chemicals. Environ Mutagen, 8(Suppl 17): 1-119.
Mueller A & Sies H (1987) Alcohol, aldehydes and lipid peroxidation
current notions. Alcohol Alcohol, 22(Suppl 1): 67-74.
Myers WD, Ng KT, Singer G, Smythe GA, & Duncan MW (1985) Dopamine and
salsolinol levels in rat hypothalami and striatum after
schedule-induced self-injection (SISI) of ethanol and acetaldehyde.
Brain Res, 358(1-2): 122-128.
Niemela O & Israel Y (1992) Hemoglobin-acetaldehyde adducts in human
alcohol abusers. Lab Invest, 67(2): 246-252.
Niemela O, Klajner F, Orrego H, Vidins E, Blendis LM, & Israel Y
(1987) Antibodies against acetaldehyde-modified protein epitopes in
human alcoholics. Hepatology, 7: 1210-1214.
Niemela O, Mannermaa RM, & Oikarinen (1990) Impairment o histone H1
DNA binding by adduct formation with acetaldehyde. Life Sci,
47: 2241-2249.
Niemela O, Juvonen T, & Parkkila S (1991) Immunohistochemical
demonstration of acetaldehyde-modified epitopes in human liver after
alcohol consumption. J Clin Invest, 87: 1367-1374.
Ninfali P, Palma F, Piacentini MP, & Fornaini G (1987) Action of
acetaldehyde on glucose metabolism of newborn and adult erythrocytes.
Biol Neonate, 52(5):256-263.
Norppa H, Tursi F, Pfaeffli P, Maeki-Paakkanen J, & Jaerventaus H
(1985) Chromosome damage induced by vinyl acetate through in vitro
formation of acetaldehyde in human lymphocytes and Chinese hamster
ovary cells. Cancer Res, 45(10): 4816-4821.
Obe O & Beek B (1979) Mutagenic activity of aldehydes. Drug Alcohol
Depend, 4: 91-94.
Obe G & Ristow H (1977) Acetaldehyde, but not ethanol, induces sister
chromatid exchanges in Chinese hamster cells in vitro. Mutat Res,
56: 211-213.
Obe G, Ristow H, & Herha J (1978) Mutagenic activity of alcohol in
man. In: Mutations: their origin, nature and potential relevance to
genetic risk in man. Proceedings Yearly Conference 1977 of German
Research Society. Boppard, Harald Boldt, pp 151-161.
Obe G, Natarajan AT, Meyers M, & Den Hertog A (1979) Induction of
chromosomal aberrations in peripheral lymphocytes of human blood
in vitro, and of SCE's in bone-marrow cells of mice in vivo by
ethanol and its metabolite acetaldehyde. Mutat Res, 68: 291-294.
Obe G, Brodmann R, Fleischer R, Engeln H, Gobel D, & Herha J (1985)
[Mutagenic and carcinogenic activity of adduct products.] In: Keup W
ed. [Biology of addiction.] Berlin, Heidelberg, New York,
Springer-Verlag, pp 31-43 (in German).
Obe G, Jonas R, & Schmidt S (1986) Metabolism of ethanol in vitro
produces a compounds which induces sister-chromatid exchanges in human
peripheral lymphocytes in vitro: Acetaldehyde not ethanol is
mutagenic. Mutat Res, 174: 47-52.
OECD (1992) Guidelines for testing chemicals. Section 3. Degradation
and accumulation. 301 C. Paris, Organisation for Economic Cooperation
and Development, pp 1-21.
Okamoto M, Ohtsuka K, Imai J, & Yamada F (1981) High-performance
liquid chromatographic determination of acetaldehyde in wine as its
lutidine derivative. J Chromatogr, 219: 175-178.
Ortiz A, Griffth PJ, & Littleton JM (1974) A comparison of the effects
of chronic administration of ethanol and acetaldehyde to mice:
evidence for a role of acetaldehyde in ethanol dependence. J Pharm
Pharmacol, 26: 249-260.
Osborne JS, Adamek S, & Hobbs ME (1956) Some component of gas phase of
cigarette smoke. Ann Chem, 28: 211-215.
O'Shea KS & Kaufman MH (1979) The teratogenic effect of acetaldehyde:
implications for the study of the fetal alcohol syndrome. J Anat,
128: 65-76.
O'Shea KS & Kaufman MH (1981) Effect of acetaldehyde on the
neuroepithelium of early mouser embryos. J Anat, 132: 107-118.
Padmanabhan R, Sreenathan RN, & Singh S (1983) Studies on the lethal
and teratogenic effects of acetaldehyde in the rat. Congenit Anom,
23: 13-23.
Parrilla R, Ohkawa K, Lindros KO, Zimmernman UP, Kobayashi K, &
Williamson JR (1974) Functional compartmentation of acetaldehyde
oxidation in rat liver. J Biol Chem, 249: 4926-4933.
Peeke HV & Figler MH (1981) Modulation of aggressive behavior in fish
by alcohol and congeners. Pharmacol Biochem Behav, 14(Suppl 1): 79-84.
Pellizari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compound in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Peterson CM & Polizzi CM (1987) Improved method for acetaldehyde in
plasma and hemoglobin-associated acetaldehyde: results in teetotalers
and alcoholics reporting for treatment. Alcohol, 4: 477-480.
Pettersson H & Kiessling K (1977) Acetaldehyde occurrence in
cerebrospinal fluid during ethanol oxidation in rats and its
dependence on the blood level and on dietary factors. Biochem
Pharmacol, 26: 237-240.
Pezzoli C, Galli-Kienle M, Di Padova C, & Stramentinoli G (1984) HPLC
method for the evaluation of blood acetaldehyde without ethanol
interference. J Liq Chromatogr, 7(4): 765-788.
Phillips SC (1987) Can brain lesions occur in experimental animals by
administration of ethanol or acetaldehyde? Acta Med Scand,
71(Suppl 7): 67-72.
Piendl A, Westner H, & Geiger E (1981) [Detection and separation of
aldehydes in beer using high pressure liquid chromatography (HPLC).]
Braunwissenschaft, 34: 301-307 (in German).
Pittevils J, Vanparys L, & Soenen A (1979) Fungicide and fungistatic
action of fifteen different aromatic components on some important
storage diseases in vitro. Meded Fac Landbouwwet Rijksuniv Gent,
44(1): 445-457.
Popov VB, Vaisman BL, Puchkov VF, & Ignatyeva TV (1981) Toxic action
of ethanol and biotransformation products on postimplantation rat
embryos in culture. Bull Exp Biol Med, 92: 725-728.
Possanzini M, Ciccioli P, Di Paolo V, & Draisci R (1987) Determination
of low boiling aldehydes in air and exhaust gases by using annular
denuders combined with HPLC techniques. Chromatographia,
23(11): 829-834.
Prasanna CV & Ramakrishnan S (1984a) Effect of acetaldehyde on hepatic
glucose metabolism. Indian J Biochem Biophys, 21(1):62-64.
Prasanna CV & Ramakrishnan S (1984b) Effect of acetaldehyde on
carbohydrate metabolism in rat brain. Indian J Biochem Biophys,
21(2): 121-123.
Prasanna CV & Ramakrishnan S (1987) Effect of acetaldehyde on hepatic
and blood lipids. Indian J Med Res, 85: 206-211.
Prasanna CV, Namagiri T, & Ramakrishnan S (1986) Effect of
acetaldehyde on thyroid function. Indian J Exp Biol, 24(2): 77-78.
Priscott PK & Ford JR (1985) An in vitro model of acetaldehyde
metabolism by rodent conceptuses. in vitro Cell Dev Biol,
21(2): 88-92.
Ramdahl T, Alfheim I, Rustad S, & Olsen T (1982) Chemical and
biological characterization of emissions from small residential stoves
burning wood and charcoal. Chemosphere, 11(4): 601-611.
Rawat K (1979) Inhibition of cardiac protein synthesis by prolonged
ethanol administration. Res Commun Chem Pathol Pharmacol, 25: 89.
Rideout JM, Lim CK, & Peters TJ (1986) Assay of blood acetaldehyde by
HPLC with fluorescence detection of its 2-diphenylacetyl-1,3-
indandione-1-azine derivative. Clin Chim Acta, 161: 29-35.
Rieger R & Michaelis A (1960) [Chromosomal aberrations after action of
acetaldehyde upon the primary roots of Vicia faba.] Biol Zent.bl,
679: 1-5 (in German).
Ristow H & Obe G (1978) Acetaldehyde induces cross-links in DNA and
causes sister-chromatid exchanges in human cells. Mutat Res,
58: 115-119.
Rosensteel RE & Tanaka S (1976) Health hazard evaluation report
No. 75-89-344. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health.
Roumec C, Lamboeuf Y, & De Saint Blanquat G (1988) Sinaptosomal
phospholipids in rats chronically treated with acetaldehyde. Adv
Biosci, 71: 201-205.
Rudling L, Ahling B, & Loefroth G (1981) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in small furnaces and stoves. In: Proceedings of the International
Conference on Residential Solids Fuels, Environmental Impacts and
Solutions. Beaverton, Oregon, Oregon Graduate Center, pp 34-53.
Saladino AJ, Willey JC, Lechner JF, Grafstrom RC, Laveck M, & Harris
CC (1985) Effects of formaldehyde, acetaldehyde, benzoyl peroxide and
hydrogen peroxide on cultured normal human bronchial epithelial cells.
Cancer Res, 45(6): 2522-2526.
Saldiva PHN, Do Rio Caldeira MP, Massad CW, Calheiros DF, Cardoso LMN,
Bohm GM, & Saldiva CD (1985) Effects of formaldehyde and acetaldehyde
inhalation on rat pulmonary mechanics. J Appl Toxicol, 5(5): 288-292.
San George RC & Hoberman HD (1986) Reaction of acetaldehyde with
hemoglobin. J Biol Chem, 261: 6811-6821.
Sasaki Y & Endo R (1978) Mutagenicity of aldehydes in Salmonella.
Mutat Res, 54: 251-252.
Savolainen ER, Leo MA, Timpl R, & Lieber CS (1984) Acetaldehyde and
lactate stimulate collagen synthesis of cultured baboon liver
myofibroblasts. Gastroenterology, 87(4): 777-787.
Schopf RE, Tromperer M, Bork K, & Morsches B (1985) Effects of ethanol
and acetaldehyde on phagocytic functions. Arch Dermatol Res,
277(2): 131-137.
Schreiber SS, Briden K, Oratz M, & Rothschild MA (1972) Ethanol,
acetaldehyde, and myocardial protein synthesis. J Clin Invest,
51: 2820-2826.
Schreiber SS, Oratz M, Rothschild MA, Reff F, & Evans C (1974)
Alcoholic cardiomyopathy. II. The inhibition of cardiac microsomal
protein synthesis by acetaldehyde. J Mol Cell Cardiol, 6: 207-213.
Schreiner G, Ulbrich M, Senger-Weil M, & Bass R (1987) Effects of
prenatal ethanol or acetaldehyde exposure on postnatal development and
behavior in the rat. Teratology, 36(1): 31A.
Schulam P, Newbold R, & Hull LA (1985) Urban and rural ambient air
aldehyde levels in Schenectady, New York and on Whiteface Mountain,
New York. Atmos Environ, 19(4): 623-626.
Segel LD (1984) Mitochondrial respiration after cardiac perfusion with
ethanol or acetaldehyde. Alcohol Clin Exp Res, 8(6): 560-564.
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency, pp 7, 37, 46 (EPA-600/4/76/062).
Shaw S & Jayatilleke E (1987) Acetaldehyde-mediated hepatic lipid
peroxidation: role of superoxide and ferritin. Biochem Biophys Res
Commun, 143(3): 984-990.
Shiohara E, Sukada M, Chiba S, Yamazaki H, Nishiguchi K, Miyamoto R, &
Nakanishi S (1985) Effect of chronic administration of acetaldehyde by
inhalation on (NA+/K+)-activated adenosine triphosphatase activity of
rat brain membranes. Toxicology, 34(4): 277-284.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Sim VM & Pattle RE (1957) Effect of possible smoke irritation on human
subjects. J Am Med Assoc, 165: 1908-1913.
Sina JF, Bean CL, Dysart GR, Taylor VI, & Bradley MO (1983) Evaluation
of the alkaline elution/rat hepatocyte assay as a predictor of
carcinogenic/mutagenic potential. Mutat Res, 113: 357-391.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1982) Measurements of
hazardous organic chemicals in the ambient atmosphere. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Environmental Sciences Research Laboratory, pp 32-37, 70-79, 84-90
(SRI International Project 7774).
Singh S, Fritze G, Fang B, Harada S, Paik YK, Eckey R, Agarwal DP, &
Goedde HW (1989) Inheritance of mitochondrial aldehyde dehydrogenase:
genotyping in Chinese, Japanese, and South Korean families reveals
dominance of the mutant allele. Hum Genet, 83: 119-121.
Sipi P, Jarventaus H, & Norppa H (1992) Sister-chromatid exchanges
induced by vinyl esters and respective carboxylic acids in cultured
human lymphocytes. Mutat Res, 279: 75-82.
Skog E (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde, propionaldehyde
and butyraldehyde; as well as of acrolein and crotonaldehyde. Acta
Pharmacol, 6: 299-318.
Smyth HF, Carpenter CP, & Weils CS (1951) Range-finding toxicity data:
list IV. Am Med Assoc Arch Ind Health Occup Med, 4: 119.
Snyder JW (1988) The role of phospholipid metabolism in ethanol- and
acetaldehyde-initiated hepatocyte injury. Diss Abstr Int,
B48(10): 2937.
Solomon LR (1987) Studies on the mechanism of acetaldehyde-mediated
inhibition of rat liver transaminases. Clin Chim Acta,
168(2): 207-217.
Solomon LR (1988) Effects of acetaldehyde on human red cell
metabolism: evidence for the formation of enzyme inhibitors. Clin Chim
Acta, 175: 249-266.
Sorrell MF & Tuma DY (1985) Hypothesis: alcoholic liver injury and the
covalent binding of acetaldehyde. Alcohol Clin Exp Res, 9: 306-309.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17(9): 416A-427A.
Spingarn NE, Northington DJ, & Pressely T (1982) Analysis of volatile
hazardous substances by GS/MS. J Chromatogr Sci, 20: 287-288.
Sprince H, Parker CM, Smith GG, & Gonzales LJ (1974) Protection
against acetaldehyde toxicity in the rat by L-cysteine, thiamin and
L-2-methylthiazolidine-4-carboxylic acid. Agents Actions, 4: 125-130.
Sreenathan RN, Padmanabhan R, & Singh S (1982) Teratogenic effects of
acetaldehyde in the rat. Drug Alcohol Depend, 9: 339-350.
Sreenathan RN, Padmanabhan R, & Singh S (1984a) Structural changes of
placenta following maternal administration of acetaldehyde in rat. Z
Mikrosk-Anat Forsch (Leipzig), 98: 597-604.
Sreenathan RN, Singh S, & Padmanabhan R (1984b) Effect of acetaldehyde
on skeletogenesis in rats. Drug Alcohol Depend, 14(2): 165-174.
Stege TE (1982) Acetaldehyde-induced lipid peroxidation in isolated
hepatocytes. Chem Pathol Pharmacol, 36: 287-297.
Steinberg S & Kaplan IR (1984) The determination of low-molecular
weight aldehydes in rain, fog and mist by reserved-phase liquid
chromatography of the 2,4-dinitrophenyl-hydrazone derivatives. Int J
Environ Anal Chem, 18: 253-266.
Steinhagen WH & Barrow CS (1984) Sensory irritation structure-activity
study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol
Appl Pharmacol, 72(3): 495-503.
Stewart JK, Aharoni Y, Hastsell PL, & Young DK (1980) Symptoms of
acetaldehyde injury on head lettuce. HortScience, 15(2): 148-149.
Takahashi K, Tomaru A, Nishiyama N, & Horiguchi M (1986) Alcoholic
cardiomyopathy: direct and indirect effects of acetaldehyde.
Jpn Circ J, 50(6): 567.
Takami T, Kuwata K, Sugimae A, & Nakamoto M (1985) Trace determination
of aldehydes in water by high-performance liquid chromatography. Anal
Chem, 57: 243-245.
Tanner RL & Meng Z (1984) Seasonal variations in ambient atmospheric
levels of formaldehyde and acetaldehyde. Environ Sci Technol,
18: 723-726.
Tejada SB (1986) Evaluation of silica gel cartridges coated in situ
with acidified 2,4-dinitrophenylhydrazine for sampling aldehydes and
ketones in air. Int J Environ Anal Chem, 26: 167-185.
Teschke R, Hasumura Y, & Lieber CS (1977) Hepatic pathways of ethanol
and acetaldehyde metabolism and their role in the pathogenesis of
alcohol-induced liver injury. Nutr Metab, 21(Suppl 1): 144-147.
Thom NS & Agg AR (1975) The breakdown of synthetic organic compounds
in biological processes. Proc R Soc London, B189: 347-357.
Til HP, Woutersen RA, Feron VJ, & Clary JJ (1988) Evaluation of the
oral toxicity of acetaldehyde and formaldehyde in a 4-week
drinking-water study in rats. Fundam Chem Toxicol, 26(5): 447-452.
Truitt EB & Walsh MJ (1971) The role of acetaldehyde in the actions of
ethanol. In: Kissin B & Begleiter H ed. The biology of alcoholism.
Volume 1: Biochemistry. New York, London, Plenum Press, pp 161-195.
Tuma DJ, Donohue TM Jr, Medina VA, & Sorrell MF (1984) Enhancement of
acetaldehyde-protein adduct formation by L-ascorbate. Arch Biochem
Biophys, 234(2): 377-381.
Tuma DJ, Jennett RD, & Sorrell MF (1987) The interaction of
acetaldehyde with tubuline. Ann NY Acad Sci, 282: 227-286.
Ung-Chhun NS & Collins MA (1987) Estimation of blood acetaldehyde
during ethanol metabolism: a sensitive HPLC/fluorescence microassay
with negligible artifactual interference. Alcohol, 4: 473-476.
US EPA (1975) Identification of organic compounds in effluents from
industrial sources. Washington, DC, US Environmental Protection
Agency, pp C-2, 4-14, 4-15, 4-17 (EPA-560/3-75-002; PB-241 841).
US NIOSH (1980) Projected number of occupational exposures to chemical
and physical hazards. Cincinnati, Ohio, US National Institute for
Occupational Safety and Health.
US NIOSH (1981) National occupational hazards survey. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health, p 14.
US NIOSH (1987) Manual of analytical methods. Method 3507. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health.
US NIOSH (1989) Manual of analytical methods. Method 2538.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health.
US NRC (1977) Other organic constituents (Acetaldehyde). In: Drinking
water and health. Washington, DC, US National Research Council,
pp 680-687.
US NRC (1981) Food chemical codex, 3rd ed. Washington, DC, US National
Research Council, National Academy Press, pp 353-355, 583, 615.
US NRC (1985) Chemicals used on food processing. Washington, DC, US
National Research Council, National Academy of Sciences (Publication
No. 1274).
Veghelyi PV & Osztovics M (1978) The alcohol syndromes: the
intrarecombigenic effect of acetaldehyde. Experientia (Basel),
34: 195-196.
Veghelyi PV, Osztovics M, Kardos G, Leisztner L, Szaszovszky E, Igali
S, & Imrei J (1978) The fetal alcohol syndrome: symptoms and
pathogenesis. Acta Paediatr Acad Sci Hung, 19(3): 171-189.
Verschueren U (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 139-141.
Von Burg R & Stout T (1991) Toxicology update-acetaldehyde. J Appl
Toxicol, 11: 373-376.
Wangenheim J & Bocsfoldi G (1986) Mouse lymphoma tk-positive-negative
assay of 30 compounds. Environ Mutagen, 8(Suppl 6): 90.
Wangenheim J & Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3(3): 193-206.
Watanabe I (1987) [Distribution of lower oxygenated organic compounds
in urban air.] Preprint of 54th Annual Meeting of the Chemical Society
of Japan, vol 1, p 129 (in Japanese).
Watanabe I (1988) Sequential automated analysis system for lower
oxygenated organic compounds in ambient air. J Res Nat Bureau Stand,
93(3): 299-301.
Watanabe A, Hobara N, & Nagashima H (1986) Blood and liver
acetaldehyde concentration in rats following acetaldehyde inhalation
and intravenous and intragastric ethanol administration. Bull Environ
Contam Toxicol, 37: 513-516.
Webster WS, Walsh DA, McEwen SE, & Lipson AH (1983) Some teratogenic
properties of ethanol and acetaldehyde in C57BL/6J mice: implications
for the study of the fetal alcohol syndrome. Teratology, 27: 231-243.
Westcott JY, Weiner H, Schultz J, & Myers RD (1980) In vivo
acetaldehyde in the brain of the rat treated with ethanol. Biochem
Pharmacol, 29: 411-417.
WHO (1989) IPCS Environmental Health Criteria 89: Formaldehyde.
Geneva, World Health Organization, 219 pp.
WHO (in press) Environmental Health Criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Wilkin JK & Fortner G (1985) Cutaneous vascular sensitivity to lower
aliphatic alcohols and aldehydes in Orientals. Alcohol Clin Exp Res,
9(6): 522-525.
Williams AJ & Barry RE (1986) Superoxide anion production and
degranulation of rat neutrophils in response to acetaldehyde-altered
liver cell membranes. Clin Sci, 71(3): 313-318.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen,
7(5): 677-702.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1989) Antibodies
against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1990) Antibodies
against acetaldehyde-modified epitopes: presence in alcoholic,
nonalcoholic liver disease and control subjects. Alcohol Alcohol,
25(5): 509-517.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991a) Detection of
stable acetaldehyde-modified proteins in the livers of ethanol-fed
rats. Alcohol Alcohol, 26(4): 437-444.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991b) Antibodies of
S3 against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Woutersen RA & Feron VJ (1987) Inhalation toxicity of acetaldehyde in
rats. IV. Progression and regression of nasal lesions after
discontinuation. Toxicology, 47(3): 295-305.
Woutersen RA, Appelman LM, Feron VJ, & der Heijden CA (1984)
Inhalation toxicity of acetaldehyde in rats. II. Carcinogenicity
study: interim results after 15 months. Toxicology, 31: 123-133.
Woutersen RA, Appelman LM, Van Garderen-Hoetmer A, & Feron VJ (1986)
Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity
study. Toxicology, 41(2): 213-231.
Yoshida A, Huang IY, & Ikawa M (1984) Molecular abnormality of an
inactive aldehyde dehydrogenase variant commonly found in Orientals.
Proc Natl Acad Sci (USA), 81: 258-261.
Zorzano A & Herrera E (1989) Disposition of ethanol and acetaldehyde
in late pregnant rats and their fetuses. Paediatr Res, 25(1): 102-106.
Résumé
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
L'acétaldéhyde est un liquide incolore et volatil à l'odeur acre
et suffocante. D'après la littérature, son seuil olfactif est de
0,09 mg/m3. L'acétaldéhyde est un composé très inflammable et
réactif qui est miscible à l'eau et à la plupart des solvants
ordinaires.
Il existe un certain nombre de méthodes d'analyse pour la
recherche de l'acétaldéhyde dans l'air (y compris dans l'haleine) et
dans l'eau. La principale méthode repose sur la réaction de
l'acétaldéhyde avec la 2,4-dinitrophénylhydrazine, puis analyse de
l'hydrazone obtenue par chromatographie liquide à haute pression ou
chromatographie en phase gazeuse.
2. Sources d'exposition humaine et environnementale
L'acétaldéhyde est un intermédiaire métabolique chez l'homme et
les plantes supérieures; c'est également un produit de la fermentation
alcoolique. On a décelé sa présence dans certaines denrées
alimentaires et boissons ainsi que dans la fumée de cigarette. Il est
également présent dans les gaz d'échappement des véhicules à moteur
ainsi que dans divers déchets industriels. La décomposition des
hydrocarbures, des effluents d'égouts et des déchets biologiques
solides produit de l'acétaldéhyde, de même que la combustion à l'air
libre ou, selon le cas, l'incinération du gaz, du mazout et de la
houille.
Plus de 80% de l'acétaldéhyde utilisé à des fins commerciales est
produit par l'oxydation en phase liquide de l'éthylène en présence
d'une solution catalytique de chlorures de palladium et de cuivre. Au
Japon, la production était de 323.000 tonnes en 1981. Aux Etats-Unis
d'Amérique elle a été de 281.000 tonnes en 1982 et en Europe
occidentale de 706.000 tonnes en 1983. La majeure partie de
l'acétaldéhyde produit commercialement est utilisé pour la préparation
de l'acide acétique. On l'utilise également pour la préparation de
certains arômes et denrées alimentaires.
On estime qu'aux Etats-Unis d'Amérique, les émissions annuelles
d'acétaldéhyde de toutes origines atteignent 12,2 millions de kg.
3. Transport, distribution et transformation dans l'environnement
En raison de la forte réactivité de l'acétaldéhyde, le transport
intercompartimental de ce composé devrait être limité. On peut
s'attendre à un certain transfert d'acétaldéhyde de l'air vers l'eau
et le sol en raison de sa forte tension de vapeur et de son faible
coefficient de sorption.
On pense que l'élimination photo-induite de l'acétaldéhyde
atmosphérique passe essentiellement par la formation de radicaux. La
photolyse devrait jouer également un rôle important dans le processus
d'élimination. Environ 80% des émissions d'acétaldéhyde dans
l'atmosphère sont éliminés chaque jour par ces deux processus.
D'après la littérature, la demi-vie de l'acétaldéhyde dans l'eau et
l'air est respectivement égale à 1,9 heure et 10-60 heures.
L'acétaldéhyde est facilement biodégradable.
4. Concentrations dans l'environnement et exposition humaine
Les concentrations moyennes d'acétaldéhyde dans l'environnement
sont généralement égales à 5 µg/m3. Dans l'eau, elles sont
généralement inférieures à 0,1 µg/litre. L'analyse de denrées
alimentaires très diverses effectuées aux Pays-Bas a montré que leur
teneur en acétaldéhyde est généralement inférieure à 1 mg/kg, mais
peut atteindre occasionnellement jusqu'à plusieurs centaines de mg/kg,
en particulier dans certains jus de fruit et dans le vinaigre.
Pour la population générale, la principale source d'exposition à
l'acétaldéhyde est de loin le métabolisme de l'alcool. La fumée de
cigarette constitue également une importante source d'exposition. En
ce qui concerne les autres véhicules, la population générale est
exposée à l'acétaldéhyde essentiellement par l'intermédiaire de la
nourriture et des boissons et, dans une moindre mesure, de l'air.
L'eau de boisson est négligeable à cet égard.
Les données disponibles sont insuffisantes pour qu'on puisse
évaluer l'ampleur de l'exposition à l'acétaldéhyde sur les lieux de
travail. Les travailleurs peuvent y être exposés dans certaines
industries ainsi que lors de la fermentation de l'alcool; dans ces
conditions la principale voie d'exposition est très probablement
l'inhalation avec possibilité également de contacts cutanés.
5. Cinétique et métabolisme
5.1 Absorption, distribution et élimination
Les données toxicologiques dont on dispose indiquent que
l'acétaldéhyde est résorbé au niveau des poumons et des voies
digestives; toutefois on ne connaît pas d'études quantitatives
appropriées qui aient été consacrées à ce problème. Il est probable
qu'il y a également une résorption cutanée.
Après avoir fait inhaler de l'acétaldéhyde à des rats, on a
constaté qu'il se répartissait dans le sang, le foie, les reins, la
rate, le coeur et le tissu musculaire. De faibles quantités en ont
été décelées dans des embryons après injection intrapéritonéale à la
mère (souris) ainsi qu'après exposition de la mère à de l'éthanol
(souris et rat). Il pourrait également y avoir production
d'acétaldé-hyde in vitro chez les foetus de rat et dans le placenta
humain.
Après injection intrapéritonéale d'éthanol, on a pu mettre en
évidence la distribution de l'acétaldéhyde dans le liquide
interstitiel de l'encéphale, à l'exclusion des cellules cérébrales. Il
est possible qu'une acétaldéhyde-déshydrogénase de forte affinité et
de faible Km joue un rôle important dans le maintien de faibles
concentrations d'acétaldéhyde dans le cerveau au cours de la
métabolisation de l'éthanol.
On a constaté que chez l'homme et les babouins, l'acétaldéhyde
pouvait être fixé par les globules rouges in vivo, la concentration
intraglobulaire pouvant atteindre dix fois la concentration
plasmatique.
Après administration par voie orale, il n'y a pratiquement pas
d'acétaldéhyde qui soit excrété tel quel dans les urines.
5.2 Métabolisme
La principale voie métabolique de l'acétaldéhyde consiste dans
une oxydation en acétate sous l'action de l'ALDHa NADb-dépendante.
L'acétate entre dans le cycle de l'acide citrique sous forme
d'acétyl-CoA. Il existe plusieurs isoenzymes de l'ALDH présentant
divers paramètres cinétiques et paramètres de liaison qui influent sur
la vitesse d'oxydation de l'acétaldéhyde.
Chez le rat, on a pu localiser une activité ALDH dans
l'épithélium respiratoire (à l'exclusion de l'épithélium olfactif),
alors que cette localisation se situe au niveau du rein chez la souris
et au niveau du cortex et des tubules rénaux chez le chien, le rat, le
cobaye et le babouin.
Les tissus embryonnaires de souris et de rat métabolisent
l'acétaldéhyde in vitro. L'acétaldéhyde traverse le placenta du rat
malgré l'existence d'un métabolisme placentaire.
a acétaldéhyde-déshydrogénase
b nicotinamide-adénine-dinucléotide
Il y a un certaine métabolisation de l'acétaldéhyde au niveau des
tubules rénaux chez l'homme mais c'est le foie qui est le principal
site du métabolisme.
Chez l'homme, on a décelé la présence de plusieurs formes
isoenzymatiques d'ALDH, au niveau du foie et d'autres tissus. Il y a
également un polymorphisme de l'ALDH michocondrial. Les sujets qui
sont homozygotes ou hétérozygotes pour une mutation ponctuelle du gène
correspondant à l'ALDH mitrochondriale, présentent une faible activité
ALDH, métabolisent lentement l'acétaldéhyde et ne supportent pas
l'alcool éthylique.
Le crotonadéhyde, la maléate de diméthyle, la phorone, le
disulfirame et le carbamide calcique inhibent le métabolisme de
l'acétaldéhyde.
5.3 Réactions avec d'autres constituants
L'acétaldéhyde forme des adduits stables ou instables avec les
protéines. Ces adduits peuvent perturber la fonction de ces protéines
comme le montre d'ailleurs l'inhibition des enzymes, les difficultés
de liaison entre histones et ADN et l'inhibition de la polymérisation
de la tubuline.
Des adduits instables de l'acétaldéhyde dont on connaît mal
l'importance se forment in vitro avec les acides nucléiques.
L'acétaldéhyde peut réagir sur diverses macromolécules de
l'organisme, de préférence avec celles qui sont porteuses de résidus
de lysine; cette réaction peut conduire à des altérations importantes
de la fonction biologique de ces molécules.
6. Effets sur les êtres vivants dans leur milieu naturel
6.1 Organismes aquatiques
Chez les poissons la CL50 peut varier de 35 mg/litre (guppy) à
140 mg/litre (espèces non précisées). Une CE5 de 82 mg/litre et une
CE50 de 42 mg/litre ont été observées respectivement chez des algues
et chez Daphnia magna.
6.2 Organismes terrestres
La présence d'acétaldéhyde dans l'air se révèle toxique pour
certains microorganismes, à des concentrations relativement faibles.
Des aphidiens ont été tués par exposition de 3 à 4 heures à de
l'acétaldéhyde à une concentration de 0,36 µg/m3.
Chez deux espèces de limaces, Arion hortensis et Agriolimax
reticulatus, on a observé des concentrations létales médianes
respectivement égales à 8,91 mg/litre et par heure et 7,69 mg/litre et
par heure.
L'acétaldéhyde provoque une inhibition réversible de la
germination des oignons, des carottes et des tomates à des
concentrations allant jusqu'à 1,52 mg/litre; par contre cette
inhibition est irréversible pour Amaranthus palmeri, dans les mêmes
conditions d'exposition. A la concentration de 0.54 µg/m3,
l'acétaldéhyde a détérioré des laitues.
7. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Les valeurs de la DL50 pour le rat et la souris et de la CL50
pour le rat et le hamster doré montrent que la toxicité aiguë de
l'acétaldéhyde est faible. On n'a pas connaissance d'études relatives
à la toxicité cutanée aiguë de l'acétaldéhyde.
7.2 Exposition à court et à long terme
Lors d'études au cours desquelles des doses ont été administrées
à plusieurs reprises, tant par la voie orale que par la voie
respiratoire, les effets toxiques observés à des concentrations
relativement faibles se limitaient essentiellement aux points de
contact initiaux. Lors d'une étude de 28 jours au cours de laquelle
de l'acétaldéhyde a été administré à des rats, mélangé à leur eau de
boisson, à la dose de 675 mg/kg de poids corporel (dose sans effets
observables 125 mg/kg de poids corporel), on a constaté que les effets
se limitaient à une légère hyperkératose focale au niveau de la
portion cardiaque de l'estomac. Après administration d'acétaldéhyde à
concentration constante de 0,05% dans l'eau de boisson pendant une
durée de 6 mois (le Groupe de travail estime que cela correspond à peu
près à 40 mg/kg de poids corporel) au cours d'une étude biochimique,
on a constaté que l'acétaldéhyde provoquait la synthèse de collagène
au niveau du foie chez le rat, observation d'ailleurs corroborée par
les résultats obtenus in vitro.
Lors d'une étude d'inhalation de 4 semaines chez le rat et de 13
semaines chez le hamster, on a obtenu, pour la concentration sans
effets respiratoires observables, des valeurs respectivement égales à
275 mg/m3 et 700 mg/m3. Aux concentrations les plus faibles
produisant un effet, on a observé des altérations dégénératives au
niveau de l'épithélium olfactif chez le rat (437 mg/m3) et de la
trachée (2400 mg/m3) chez le hamster. Des altérations dégénératives
de l'épithélium respiratoire et du larynx ont été observées à des
concentrations plus élevées. On n'a pas connaissance d'études
relatives à l'administration répétée d'acétaldéhyde au niveau cutané.
7.3 Reproduction, embryotoxicité et tératogénicité
Plusieurs études montrent que des malformations foetales peuvent
survenir par suite de l'exposition parentérale de rattes et de souris
gravides à l'acétaldéhyde. Dans la plupart de ces études, on n'a pas
étudié la toxicité pour la mère. On n'a pas pu trouver de données
relatives à la toxicité de l'acétaldéhyde pour la fonction de
reproduction.
7.4 Mutagénicité et points d'aboutissement des effets correspondants
In vitro, l'acétaldéhyde est génotoxique et produit des
mutations géniques, des effets clastogènes ainsi que des échanges
entre chromatides soeurs dans des cultures de cellules mammaliennes en
l'absence d'activation métabolique exogène. Toutefois, des épreuves
effectuées dans les règles sur des salmonelles ont donné des résultats
négatifs. Après avoir injecté de l'acétaldéhyde par voie
intrapéritonéale à des hamsters chinois et à des souris, on a constaté
que ce composé produisait des échanges entre chromatides soeurs au
niveau de la moelle osseuse. Cependant, de l'acétaldéhyde administré
par la même voie n'a pas eu pour conséquence un accroissement de la
fréquence des micronoyaux dans les spermatides de souris récemment
formés. On peut déduire indirectement de certaines études in vitro
et in vivo que l'acétaldéhyde est susceptible de produire des
pontages protéines-ADN et ADN-ADN.
7.5 Cancérogénicité
Des études d'inhalation effectuées sur des rats et des hamsters
ont permis de constater un accroissement de l'incidence des tumeurs.
Chez le rat, il s'agissait d'une augmentation, liée à la dose, de la
fréquence des adénocarcinomes au niveau de la muqueuse nasale ainsi
que des carcinomes spino-cellulaires (accroissement significatif à
toutes les doses). Toutefois chez le hamster, l'accroissement constaté
de la fréquence des carcinomes du nez et du larynx n'était pas
significatif. A toutes les concentrations d'acétaldéhyde, on a
constaté l'apparition de lésions tissulaires chroniques au niveau des
voies respiratoires.
7.6 Etudes spéciales
On n'a pas connaissance d'études appropriées portant sur la
neuro- et l'immunotoxicité potentielle de l'acétaldéhyde.
8. Effets sur l'homme
Lors d'études limitées portant sur des volontaires humains, on a
constaté que l'acétaldéhyde avait un effet légèrement irritant sur les
yeux et les voies respiratoires supérieures, après exposition de très
brève durée à des concentrations respectivement supérieures à environ
90 et 240 mg/m3. Après l'application d'un timbre inbibé
d'acétaldéhyde, on a constaté la présence d'un érythème cutané chez 12
sujets "d'ascendance orientale".
On a signalé l'existence d'une enquête limitée au cours de
laquelle on a étudié l'incidence des cancers chez des travailleurs
exposés à de l'acétaldéhyde.
On possède des preuves indirectes que le métabolite toxique
supposé être à l'origine des lésions hépatiques imputables à l'alcool,
de bouffées de chaleur et d'effets sur la développement, est en fait
l'acétaldéhyde.
9. Evaluation des risques pour la santé humaine et des effets
sur l'environnement
Les études effectuées sur l'animal montrent que, par voie
respiratoire ou par voie orale, l'acétaldéhyde n'est que faiblement
toxique. D'après les études effectuées sur l'animal mais également
sur l'homme, on a constaté que ce composé était légèrement irritant
pour l'oeil et les voies respiratoires supérieures. Chez l'homme,
l'application d'un timbre cutané inbibé d'acétaldéhyde peut provoquer
l'apparition d'un érythème cutané. On pense avoir découvert le
mécanisme à l'origine de cet effet mais les données disponibles sont
encore insuffisantes pour qu'on puisse évaluer le pouvoir
sensibilisateur de l'acétaldéhyde.
On ne dispose que de données limitées sur les effets d'une
ingestion d'acétaldéhyde. Après administration d'acétaldéhyde par
voie orale à des rats à la dose quotidienne de 675 mg/kg de poids
corporel, on a constaté un accroissement limite de l'hyperkératose au
niveau de la portion cardiaque de l'estomac (dose sans effets nocifs
observables: 125 mg/kg de poids corporel). Après avoir fait boire à
des rats six mois durant de l'eau contenant environ 40 mg
d'acétaldéhyde/kg de poids corporel, on a constaté un accroissement de
la synthèse du collagène hépatique, effet dont la signification reste
obscure.
D'après des études effectuées sur des rats et des hamsters, ce
sont les voies respiratoires supérieures qui constituent l'organe
cible de l'acétaldéhyde lorsque ce composé est inhalé. D'après les
résultats obtenus, la concentration la plus faible à laquelle on a
commencé à observer des effets était de 437 mg/m3, après une période
d'administration de 5 semaines. Chez des rats exposés pendant 4
semaines, la dose sans effets respiratoires observables était de
275 mg/m3; elle était de 700 mg/m3 chez des hamsters exposés
pendant 13 semaines.
Aux concentrations qui produisaient des lésions tissulaires des
voies respiratoires, on a observé un accroissement de l'incidence des
adénocarcinomes du nez et des carcinomes spino-cellulaires chez le
rat; il y a également eu accroissement des carcinomes du larynx et du
nez chez le hamster.
On est fondé à penser qu' in vivo, l'acétaldéhyde provoque des
lésions génétiques dans les cellules somatiques.
les données disponibles sont insuffisantes pour qu'on puisse
apprécier les effets qu'une exposition à l'acétaldéhyde pourrait avoir
sur la reproduction, le développement ainsi que les fonctions
neurologiques et immunologiques de la population générale ou de la
population professionnellement exposées.
Pour ce qui est du pouvoir irritant de ce composé chez l'homme,
on estime que la concentration tolérable est de 2 mg/m3. Etant
donné que le mécanisme d'induction de tumeurs par l'acétaldéhyde n'a
pas été bien étudié, on a adopté deux approches concernant ce point
d'aboutissement de son action toxique, à savoir la fixation d'une
concentration tolérable qui serait obtenue en divisant la dose
irritante pour les voies respiratoires chez les rongeurs, par un
certain facteur d'incertitude et l'estimation d'un risque de cancer
sur toute la durée de l'existence, en procédant par extrapolation
linéaire. La concentration tolérable est de 0,3 mg/m3. La
concentration produisant un excès de risque de 10-5 sur toute la durée
de la vie se situe entre 11-65 µg/m3.
Les données limitées dont on dispose ne permettent pas de tirer
des conclusions définitives quant au risque que l'acétaldéhyde
pourrait représenter pour l'ensemble de la faune et de la flore.
Toutefois, si l'on considère la courte durée de vie de l'acétaldéhyde
dans l'air et dans l'eau et le fait qu'il est facilement
biodégradable, on est amené à penser que ce composé n'est guère
menaçant pour les organismes aquatiques ou terrestres, sauf peut-être
en cas de décharge ou de déversement de produits industriels.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El acetaldehído es un líquido incoloro, volátil y de olor acre y
sofocante. El umbral señalado para el olor es de 0,09 mg/m3. El
acetaldehído es un compuesto muy inflamable y reactivo, soluble en
agua y en la mayoría de los disolventes comunes.
Existen métodos de análisis para la detección del acetaldehído en
el aire (incluso en el aliento) y en el agua. El principal de ellos
se basa en la reacción del producto con 2,4-dinitrofenilhidracina y
ulterior análisis de los derivados de la hidrazona por cromatografía
de líquidos a alta presión o cromatografía de gases.
2. Fuentes de exposición humana y ambiental
El acetaldehído es un producto intermedio del metabolismo en los
hombres y en las plantas superiores, y también proviene de la
fermentación alcohólica. Ha sido identificado en los alimentos, las
bebidas y el humo de los cigarrillos. También se encuentra en los
gases de escape de los vehículos y en ciertos desechos industriales.
La degradación de los hidrocarburos, las aguas residuales y los
desechos biológicos sólidos produce acetaldehído, como también lo
hacen la combustión al aire libre y la incineración de gas, fuel oil y
carbón.
Más del 80% del acetaldehído usado comercialmente proviene de la
oxidación en fase líquida de etileno con una solución catalítica de
paladio y cloruros de cobre. En 1981, la producción japonesa fue de
323 000 toneladas. En los Estados Unidos de América fue de 281 000
toneladas en 1982 y en Europa occidental de 706 000 toneladas en 1983.
La mayoría del acetaldehído producido comercialmente se destina a la
fabricación de ácido acético. También se utiliza en sustancias
aromáticas y en alimentos.
En los Estados Unidos, la emisión anual de acetaldehído de todo
origen se calcula en 12,2 millones de kilogramos.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta reactividad, el transporte intercompartimental
de acetaldehído debe ser limitado. Es de suponer que hay cierta
transferencia de la sustancia al aire desde el agua y el suelo, como
consecuencia de la fuerte presión del vapor y el bajo coeficiente de
sorción.
Es posible que la eliminación fotoinducida del acetaldehído en la
atmósfera tenga lugar principalmente por formación de un radical. La
fotosíntesis puede también contribuir bastante al proceso de
eliminación. La pérdida resultante de acetaldehído proveniente de
emisiones en la atmósfera es de alrededor del 80%. La vida media de
la sustancia en el agua y en el aire es de 1,9 h y 10-60 h,
respectivamente.
El acetaldehído es muy biodegradable.
4. Niveles medioambientales y exposición humana
Los niveles de acetaldehído en el aire ambiente suelen ser por
término medio de 5 µg/m3. Las concentraciones en el agua no llegan
en general a 0,1 µg/litro. El análisis de diversos alimentos en los
Países Bajos demostró que las concentraciones, normalmente inferiores
a 1 mg/kg, a veces llegaban a 100 mg/kg, particularmente en algunos
zumos de frutas y en el vinagre.
La principal fuente, con mucho, de exposición al acetaldehído
para la mayoría de la población es el metabolismo del alcohol. El
humo del cigarrillo es también una fuente significativa. Aparte de
eso pueden mencionarse en segundo lugar los alimentos y bebidas y, en
menor grado, el aire. La contribución del agua de beber es
insignificante.
No existen datos suficientes para determinar la importancia de la
exposición al acetaldehído en el lugar de trabajo. El personal puede
estar expuesto en algunas industrias manufactureras y donde hay
fermentación con producción de alcohol, siendo la principal vía la
inhalación y posiblemente el contacto con la piel.
5. Cinética y metabolismo
5.1 Absorción, distribución y eliminación
Los estudios existentes sobre toxicidad indican que el
acetaldehído se absorbe por los pulmones y el conducto
gastrointestinal; sin embargo, no parecen existir análisis
cuantitativos adecuados. Es probable la absorción por la piel.
En las ratas, el acetaldehído inhalado se distribuye por la
sangre, pasando al hígado, el riñón, el bazo, el corazón y otros
tejidos musculares. Se han detectado bajos niveles en embriones de
ratón tras inyectar la sustancia a la madre por vía intraperitoneal o
tras exposición de ratas o ratones hembra a etanol. La producción
potencial de acetaldehído in vitro se ha observado en fetos de rata
y en la placenta humana.
Tras inyección intraperitoneal de etanol se ha demostrado la
distribución de acetaldehído en el líquido intersticial pero no en las
células del cerebro. Una alta afinidad y escaso Km ALDHa pueden ser
importantes para mantener bajos niveles de acetaldehído en el cerebro
durante el metabolismo del etanol.
El acetaldehído es absorbido por los eritrocitos y, tras consumo
de etanol, los niveles intracelulares en hombres y babuinos pueden ser
in vivo 10 veces más altos que los del plasma.
Después de la administración por vía oral, la excreción de
acetaldehído por la orina prácticamente no cambia.
5.2 Metabolismo
Un medio importante en el metabolismo del acetaldehído es la
oxidación para pasar a acetato bajo la influencia de una ALDH
dependiente de NADb. El acetato pasa al ciclo de ácido cítrico como
acetil-CoA. Hay varios isoenzimas de ALDH con distintos parámetros
cinéticos y de ligazón que influyen en la rapidez de la oxidación del
acetaldehído.
Se ha detectado actividad de la ALDH en el epitelio del tracto
respiratorio (excluido el de las vías olfativas) de ratas, en el
cortex renal y los túbulos del perro, la rata, el cobayo y el babuino,
y también en los testículos del ratón.
El acetaldehído es metabolizado in vitro por el tejido
embrionario del ratón y la rata. La sustancia atraviesa la placenta
del ratón, pese al metabolismo placentario.
Aunque hay algún metabolismo en los túbulos renales humanos, el
principal órgano metabolizador es el hígado.
En el hígado y otros tejidos humanos se han detectado varias
formas isoenzimáticas de ALDH. En las mitocondrias la ALDH presenta
polimorfismo. Los sujetos que son homozigóticos o heterozigóticos por
una mutación singular del gen correspondiente a la ALDH de las
mitocondrias presentan poca actividad de esta enzima, metabolizan el
acetaldehído lentamente y no toleran el etanol.
El metabolismo del acetaldehído puede ser inhibido por el
crotonaldehído, el dimetilmaleato, el forón, el disulfiram y la
carbamida de calcio.
5.3 Reacción con otros compuestos
El acetaldehído puede formar aductores estables e inestables con
las proteínas. Eso menoscaba la función proteínica, como lo demuestra
la inhibición de la actividad enzimática, la menor ligazón histona-ADN
y la polimerización inhibida de la tubulina.
Los aductores inestables, de importancia indeterminada, se
producen in vitro con ácidos nucleicos.
El acetaldehído puede reaccionar con diversas macromoléculas en
el organismo humano, de preferencia las que contienen residuos de
lisina, lo que puede conducir a notables alteraciones de la función
biológica de dichas moléculas.
6. Efectos sobre los organismos presentes en el medioambiente
6.1 Organismos acuáticos
La CL50 para los peces va de 35 (Lebistes reticulatus) a
140 mg/litro (especies no especificadas). Se ha señalado una CE5 de
82 mg/litro y una CE50 de 42 mg/litro para las algas y para Daphnia
magna, respectivamente.
6.2 Organismos terrestres
El acetaldehído atmosférico a concentraciones relativamente bajas
parece ser tóxico para algunos microorganismos.
Los afidios mueren cuando son expuestos a concentraciones de
0,36 µg/m3 durante tres o cuatro horas.
Para las especies de babosa Arion hortensis y Agriolimax
reticulatus se han registrado valores letales medios de
8,91 mg/litro y 7,69 mg/litro por hora, respectivamente.
La inhibición de la germinación para la semilla de cebolla,
zanahoria y tomate tratada con acetaldehído (hasta 1,52 mg/litro) es
reversible, mientras que la de Amaranthus palmeri expuesta del mismo
modo es irreversible. El acetaldehído a 0,54 µg/m3 es perjudicial
para la lechuga.
7. Efectos en animales experimentales y sistemas de prueba in vitro
7.1 Exposición única
El acetaldehído tiene una toxicidad aguda baja, con una DL50 en
ratas y ratones y una CL50 en ratas y hámsters sirios. No se han
encontrado estudios de toxicidad dérmica aguda.
7.2 Exposición a corto y a largo plazo
En estudios reiterados del efecto tóxico de dosis por vía oral y
por inhalación, los efectos tóxicos de concentraciones relativamente
bajas se limitaron más bien a los puntos de contacto inicial. En un
estudio de 28 días en el que se administró a ratas con el agua de
beber acetaldehído a razón de 675 mg/kg de peso corporal (nivel sin
efecto observado (NOEL) = 125 mg/kg de peso corporal) los efectos se
limitaron a una ligera hiperqueratosis focal en la parte anterior del
estómago. En un estudio bioquímico, tras la administración a ratas de
dosis singulares (0,05% en el agua de beber) durante seis meses
(equivalente según el Grupo Especial a alrededor de un 40 mg/kg de
peso corporal), el acetaldehído indujo la síntesis de colágeno
hepático, observación corroborada mediante pruebas in vitro.
El nivel sin efecto respiratorio observado (NOEL) fue de
275 mg/m3 para ratas expuestas a acetaldehído por inhalación durante
cuatro semanas y 700 mg/m3 en hámsters expuestos durante 13 semanas.
A los niveles más bajos de efecto observado se produjeron cambios
degenerativos del epitelio olfativo en ratas (437 mg/m3) y de la
tráquea (2400 mg/m3) en hámsters. A concentraciones superiores se
produjeron cambios degenerativos del epitelio respiratorio y de la
laringe. No se han identificado estudios dérmicos con dosis
repetidas.
7.3 Reproducción, embriotoxicidad y teratogenicidad
En varios estudios, la exposición parenteral de ratas y ratones
gestantes a acetaldehído produjo malformaciones fetales. En la
mayoría de esos estudios no se evaluó la toxicidad materna. Tampoco
se dispone de datos sobre la toxicidad reproductiva.
7.4 Mutagenicidad y otros efectos finales afines
El acetaldehído es genotóxico in vitro e induce mutación de los
genes, con efectos clastogénicos e intercambios de cromatidios
hermanos (SCE) en las células de mamíferos, en ausencia de activación
metabólica exógena. Sin embargo se han registrado resultados
negativos en pruebas adecuadas con Salmonella. Tras inyección
intraperitoneal, el acetaldehído indujo SCE en la médula ósea de
hámsters chinos y ratones pero, en cambio, no hizo aumentar la
frecuencia de los micronúcleos en las espermátides precoces de ratón.
Indirectamente, ciertos estudios in vitro e in vivo parecen
indicar que el acetaldehído puede inducir enlaces cruzados
proteína-ADN y ADN-ADN.
7.5 Carcinogenicidad
Se ha observado una mayor incidencia de tumores en ratas y
hámsters expuestos a acetaldehído por inhalación. En las ratas, los
adenocarcinomas nasales aumentaron según la dosis pero los carcinomas
de células escamosas fueron significativos independientemente de la
dosis. Por el contrario, en los hámsters, el aumento de los
carcinomas nasales y laríngeos fue insignificante. A todas las
concentraciones, el acetaldehído administrado en los estudios produjo
daños irreversibles del tejido respiratorio.
7.6 Estudios especiales
No se conocen estudios adecuados sobre la neurotoxicidad y la
inmunotoxicidad potencial del acetaldehído.
8. Efectos en el ser humano
En estudios limitados con voluntarios, el acetaldehído produjo
irritación moderada de los ojos y de las vías respiratorias superiores
tras exposición por muy breves periodos a concentraciones que excedían
algo de 90 y 240 mg/m3, respectivamente. Las pruebas de parche
efectuadas con acetaldehído produjeron eritema cutáneo en 12 sujetos
de «ascendencia oriental».
Se ha tenido conocimiento de una investigación limitada sobre la
incidencia del cáncer entre trabajadores expuestos a acetaldehído y
otras sustancias.
Según pruebas indirectas, el acetaldehído participa como
metabolito potencialmente tóxico en la inducción de las afecciones
hepáticas, la congestión facial y las alteraciones del desarrollo
asociadas con el alcohol.
9. Evaluación de los riesgos para la salud humana y los efectos en el
medio ambiente
Los estudios efectuados con animales indican que la toxicidad
aguda del acetaldehído por inhalación o por vía oral es baja. Según
las investigaciones con sujetos humanos y con animales, esa sustancia
es algo irritante para los ojos y las vías respiratorias superiores.
Esos mismos efectos se han apreciado en voluntarios a los que se
administró acetaldehído (sección 1.8). Se ha observado eritema
cutáneo consiguiente a las pruebas de parche en sujetos humanos.
Aunque se ha identificado un posible mecanismo, los datos de que se
dispone son insuficientes para calcular el potencial del acetaldehído
como inductor de sensibilización.
Existe poca información sobre los efectos del acetaldehído
ingerido. Tras administrar diariamente a ratas por vía oral 675 mg/kg
de peso corporal (NOEL = 125 mg/kg de peso corporal) se observó un
aumento liminar de la hiperqueratosis en la parte anterior del
estómago. En las ratas que ingirieron durante seis meses con el agua
de beber dosis aproximadas de 40 mg/kg de peso corporal hubo un
aumento de la síntesis de colágeno en el hígado, cuya significación
está por dilucidar.
Los estudios de inhalación con ratas y hámsters permiten afirmar
que el tejido sensible es el de las vías respiratorias superiores. La
concentración más baja a la que se observaron efectos fue 437 mg/m3
durante cinco semanas. El NOEL identificado del tracto respiratorio
fue 275 mg/m3 en ratas expuestas durante cuatro semanas y
700 mg/m3 en hámsters expuestos durante 13 semanas.
A las concentraciones que producen daños en los tejidos del
tracto respiratorio se observaron aumentos de la incidencia del
adenocarcinoma nasal y del carcinoma de células escamosas en la rata,
y de los carcinomas laríngeos y nasales en el hámster.
Hay indicios de que el acetaldehído produce in vivo
alteraciones genéticas en las células somáticas.
No se dispone de datos suficientes para determinar si el
acetaldehído tiene efectos en la reproducción y el desarrollo o en el
estado neurológico o inmunológico de la población general o de la que
está particularmente expuesta por razón de su trabajo.
A partir de datos sobre la acción irritante en sujetos humanos se
ha considerado que la concentración tolerable es de 2 mg/m3. El
mecanismo de inducción de tumores por el acetaldehído no está bien
estudiado. En consecuencia, se han adoptado dos criterios
orientativos a ese respecto; a saber, la determinación de una
concentración tolerable obtenida dividiendo un nivel de efecto
irritante en el tracto respiratorio de los roedores por un factor de
incertidumbre, y la estimación del riesgo de cáncer mediante
extrapolación linear. La concentración tolerable resulta ser de
0,3 mg/m3. Las concentraciones asociadas con un aumento del riesgo
permanente de 10-5 son de 11-65 µg/m3.
La escasez de datos impide llegar a conclusiones definitivas
sobre los riesgos potenciales del acetaldehído para la biota en el
medio ambiente. Sin embargo, basándose en la breve vida media del
acetaldehído en el aire y en el agua, asi como en el hecho de que es
fácilmente biodegradable, cabe afirmar que los efectos de esa
sustancia en los organismos terrestres y acuáticos son escasos,
excepto posiblemente con ocasión de descargas industriales o vertidos.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
Members
Mrs I. Arts, Department of Biological Toxicology, TNO Nutrition and
Food Research, Zeist, The Netherlands
Dr R.E. Barry, Faculty of Medicine, University of Bristol, Bristol
Royal Infirmary, Bristol, United Kingdom
Professor D. Beritic-Stahuljak, Andrija tampar School of Public
Health, Faculty of Medicine, University of Zagreb, Zagreb, Croatia
Dr Sai Mei Hou, Karolinska Institute, Huddinge, Sweden
Dr M.E. Meek, Environmental Health Directorate, Priority Substances
Section, Health & Welfare Canada, Tunney's Pasture, Ottawa, Canada
(Chairperson)
Professor M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
Professor G. Obe, University of Essen, Essen, Germany
Professor T.V.N. Persaud, Department of Anatomy, Faculty of
Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
Mr D. Renshaw, Department of Health, Elephant & Castle, London, United
Kingdom
Dr A. Smith, Health and Safety Executive, Toxicology Unit, Bootle,
Merseyside, United Kingdom (Co-rapporteur)
Professor A. Watanabe, Toyama Medical and Pharmaceutical University,
Faculty of Medicine, Toyama, Japan
Dr S. Worrall, Department of Biochemistry, University of Queensland,
Brisbane, Queensland, Australia
Representatives from other organizations
Dr V. Krutovskikh, Programme of Multistage Carcinogenesis,
International Agency for Research on Cancer, Lyon, France
Secretariat
Mrs J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
(Co-rapporteur)
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia (Responsible Officer and Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
A WHO Task Group on Environmental Health Criteria for
Acetaldehyde met in Geneva from 6 to 10 December 1993. Professor F.
Valic opened the meeting on behalf of the three cooperating
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to acetaldehyde.
The first draft of this monograph was prepared by Mrs J. de Fouw,
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands.
Professor F. Valic was responsible for the overall scientific
content of the monograph and for the organization of the meeting, and
Mrs M.O. Head of Oxford for the technical editing of the monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
INTRODUCTION
This monograph will deal mainly with the effects of direct
exposure to acetaldehyde. However, it should be borne in mind that
for most people exposure to acetaldehyde will occur through the
consumption of alcoholic beverages (IARC, 1988). These beverages
contain ethanol, which is metabolized to acetaldehyde by alcohol
dehydrogenase (ADH). ADH activity has been detected in nearly every
tissue including liver, kidney, muscle, intestine, ovary, and testes
(Buehler et al., 1983; Agarwal & Goedde, 1990).
However, data concerning metabolically formed acetaldehyde will
only be considered when no data are available on direct exposure.
The accurate determination of acetaldehyde in body fluid and
tissue samples is relatively difficult. Only the most recent
techniques take into account artifactual acetaldehyde formation in
biological samples, especially those containing ethanol (Eriksson &
Fukunaga 1993). As values for concentrations of acetaldehyde given in
older references may well have been overestimates, absolute values are
only given when necessary.
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Acetaldehyde is a colourless, volatile liquid with a pungent
suffocating odour. The reported odour threshold is 0.09 mg/m3.
Acetaldehyde is a highly flammable and reactive compound that is
miscible in water and most common solvents.
Analytical methods are available for the detection of
acetaldehyde in air (including breath) and water. The principal
method is based on the reaction of acetaldehyde with
2,4-dinitrophenylhydrazine and subsequent analysis of the hydrazone
derivatives by high pressure liquid chromatography or gas
chromatography.
1.2 Sources of human and environmental exposure
Acetaldehyde is a metabolic intermediate in humans and higher
plants and a product of alcohol fermentation. It has been identified
in food, beverages, and cigarette smoke. It is also present in
vehicle exhaust and in wastes from various industries. Degradation of
hydrocarbons, sewage, and solid biological wastes produces
acetaldehyde, as well as the open burning and incineration of gas,
fuel oil, and coal.
More than 80% of the acetaldehyde used commercially is produced
by the liquid-phase oxidation of ethylene with a catalytic solution of
palladium and copper chlorides. Production in Japan was 323 thousand
tonnes in 1981. In the USA, production was 281 thousand tonnes in 1982
while in Western Europe it was 706 thousand tonnes in 1983. Most
acetaldehyde produced commercially is used in the production of acetic
acid. It is also used in flavourings and foods.
The annual emission of acetaldehyde from all sources in the USA
is estimated to be 12.2 million kg.
1.3 Environmental transport, distribution, and transformation
Because of its high reactivity, intercompartmental transport of
acetaldehyde is expected to be limited. Some transfer of acetaldehyde
to air from water and soil is expected because of the high vapour
pressure and low sorption coefficient.
It is suggested that the photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Photolysis is
expected to contribute another substantial fraction to the removal
process. Both processes cause a reported daily loss of about 80% of
atmospheric acetaldehyde emissions. Reported half-lives of
acetaldehyde in water and air are 1.9 h and 10-60 h, respectively.
Acetaldehyde is readily biodegradable.
1.4 Environmental levels and human exposure
Levels of acetaldehyde in ambient air generally average
5 µg/m3. Concentrations in water are generally less than
0.1 µg/litre. Analysis of a wide range of foodstuffs in the
Netherlands showed that concentrations, generally less than 1 mg/kg,
occasionally ranged up to several 100 mg/kg, particularly in some
fruit juices and vinegar.
By far, the main source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure.
With respect to other media, the general population is exposed to
acetaldehyde principally from food and beverages, and, to a lesser
extent, from air. The contribution from drinking-water is negligible.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route of exposure is most likely inhalation with possible
dermal contact.
1.5 Kinetics and metabolism
1.5.1 Absorption, distribution, and elimination
Available studies on toxicity indicate that acetaldehyde is
absorbed through the lungs and gastrointestinal tract; however, no
adequate quantitative studies have been identified. Absorption
through the skin is probable.
Following inhalation by rats, acetaldehyde is distributed to the
blood, liver, kidney, spleen, heart, and other muscle tissues. Low
levels were detected in embryos after maternal intraperitoneal (ip)
injection of acetaldehyde (mouse) and following maternal exposure to
ethanol (mouse and rat). Potential production of acetaldehyde has
also been observed in rat fetuses and in the human placenta,
in vitro.
Distribution of acetaldehyde to brain interstitial fluid, but not
to brain cells, has been demonstrated following ip injection of
ethanol. A high affinity, low Km ALDHa may be important in
maintaining low levels of acetaldehyde in the brain during the
metabolism of ethanol.
Acetaldehyde is taken up by red blood cells and, following
ethanol consumption in humans and in baboons, in vivo,
intracellular levels can be 10 times higher than plasma levels.
Following oral administration, virtually no unchanged
acetaldehyde is excreted in the urine.
1.5.2 Metabolism
The major pathway for the metabolism of acetaldehyde is by
oxidation to acetate under the influence of NADb-dependent ALDH.
Acetate enters the citric acid cycle as acetyl-CoA. There are several
isoenzymes of ALDH with different kinetic and binding parameters that
influence acetaldehyde oxidation rates.
ALDH activity has been localized in the respiratory tract
epithelium (excluding olfactory epithelium) in rats, in the renal
cortex and tubules in the dog, rat, guinea-pig, and baboon, and, in
the testes in the mouse.
Acetaldehyde is metabolized by mouse and rat embryonic tissue
in vitro. Acetaldehyde crosses the rat placenta, in spite of
placental metabolism.
Though there is some metabolism of acetaldehyde in human renal
tubules, the liver is the most important metabolic site.
Several isoenzymic forms of ALDH have been identified in the
human liver and other tissues. There is polymorphism for the
mitochondrial ALDH. Subjects who are homozygous or heterozygous for a
point mutation in the mitochondrial ALDH corresponding gene have low
activity of this enzyme, metabolize acetaldehyde slowly, and are
intolerant of ethanol.
The metabolism of acetaldehyde can be inhibited by
crotonaldehyde, dimethylmaleate, phorone, disulfiram, and calcium
carbamide.
a ALDH = acetaldehyde dehydrogenase.
b NAD = nicotinamide adenine dinocleotide.
1.5.3 Reaction with other components
Acetaldehyde forms stable and unstable adducts with proteins.
This can impair protein function, as evidenced by inhibition of enzyme
activity, impaired histone-DNA binding, and inhibition of
polymerization of tubulin.
Unstable adducts of acetaldehyde of undetermined significance
occur in vitro with nucleic acids.
Acetaldehyde can react with various macromolecules in the body,
preferentially those containing lysine residues, which can lead to
marked alterations in the biological function of these molecules.
1.6 Effects on organisms in the environment
1.6.1 Aquatic organisms
LC50s in fish ranged from 35 (guppy) to 140 mg/litre (species
not specified). An EC5 of 82 mg/litre and an EC50 of 42 mg/litre
were reported for algae and Daphnia magna, respectively.
1.6.2 Terrestrial organisms
Acetaldehyde in air appears to be toxic for some microorganisms
at relatively low concentrations.
Aphids were killed when exposed to acetaldehyde at a
concentration of 0.36 µg/m3 for 3 or 4 h.
Median lethal values were 8.91 mg/litre per h and 7.69 mg/litre
per h for the slug species, Arion hortensis and Agriolimax
reticulatus, respectively.
Inhibition of seed germination in the onion, carrot, and tomato
by acetaldehyde (up to 1.52 mg/litre) was reversible, whereas
inhibition of Amaranthus palmeri, similarly exposed, was
irreversible. Acetaldehyde at 0.54 µg/m3 damaged lettuce.
1.7 Effects on experimental animals and in vitro test systems
1.7.1 Single exposure
LD50s in rats and mice and LC50s in rats and Syrian hamsters
showed that the acute toxicity of acetaldehyde is low. Acute dermal
studies were not identified.
1.7.2 Short- and long-term exposures
In repeated dose studies, by both the oral and inhalation routes,
toxic effects at relatively low concentrations were limited
principally to the sites of initial contact. In a 28-day study in
which acetaldehyde at 675 mg/kg body weight (no-observedeffect level
(NOEL): 125 mg/kg body weight) was administered in the drinking-water
to rats, effects were limited to slight focal hyperkeratosis of the
forestomach. Following administration of a single dose level (0.05%
in the drinking-water) for 6 months (estimated by the Task Group to be
approximately 40 mg/kg body weight) in a biochemical study,
acetaldehyde induced synthesis of rat liver collagen, an observation
that was supported by in vitro data.
Following inhalation, NOELs for respiratory effects were
275 mg/m3 in rats exposed for 4 weeks and 700 mg/m3 in hamsters
exposed for 13 weeks. At lowest-observed-effect levels, degenerative
changes were observed in the olfactory epithelium in rats
(437 mg/m3) and the trachea (2400 mg/m3) in hamsters.
Degenerative changes in the respiratory epithelium and larynx were
observed at higher concentrations. No repeated dose dermal studies
were identified.
1.7.3 Reproduction, embryotoxicity, and teratogenicity
In several studies, parenteral exposure of pregnant rats and mice
to acetaldehyde induced fetal malformations. In the majority of these
studies, maternal toxicity was not evaluated. No data on reproductive
toxicity were identified.
1.7.4 Mutagenicity and related end-points
Acetaldehyde is genotoxic in vitro, inducing gene mutations,
clastogenic effects, and sister-chromatid exchanges (SCEs) in
mammalian cells in the absence of exogenous metabolic activation.
However, negative results were reported in adequate tests on
Salmonella. Following intraperitoneal injection, acetaldehyde induced
SCEs in the bone marrow of Chinese hamsters and mice. However,
acetaldehyde administered intraperitoneally did not increase the
frequency of micronuclei in early mouse spermatids. There is indirect
evidence from in vitro and in vivo studies to suggest that
acetaldehyde can induce protein-DNA and DNA-DNA cross-links.
1.7.5 Carcinogenicity
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses). However, in hamsters,
increases in nasal and laryngeal carcinomas were non-significant. All
concentrations of acetaldehyde administered in the studies induced
chronic tissue damage in the respiratory tract.
1.7.6 Special studies
Adequate studies on the potential neuro- and immunotoxicity of
acetaldehyde were not identified.
1.8 Effects on humans
In limited studies on human volunteers, acetaldehyde was mildly
irritating to the eyes and upper respiratory tract following exposure
for very short periods to concentrations exceeding approximately 90
and 240 mg/m3, respectively. Cutaneous erythema was observed in
patch testing with acetaldehyde, in twelve subjects of "Oriental
ancestry".
One limited investigation in which the incidence of cancer was
examined in workers exposed to acetaldehyde and other compounds has
been reported.
On the basis of indirect evidence, acetaldehyde has been
implicated as the putatively toxic metabolite in the induction of
alcohol-associated liver damage, facial flushing, and developmental
effects.
1.9 Evaluation of human health risks and effects on the environment
The acute toxicity of acetaldehyde by the inhalation or oral
route in studies conducted on animals was low. According to studies
on humans and animals, acetaldehyde is mildly irritating to the eyes
and the upper respiratory tract. In limited studies on human
volunteers, acetaldehyde was mildly irritating to the eyes and upper
respiratory tract (section 1.8). Cutaneous erythema has also been
observed in the patch testing of humans. Although a possible mechanism
has been identified, available data are inadequate to assess the
potential of acetaldehyde to induce sensitization.
Available data on the effects of acetaldehyde following ingestion
are limited. Following oral administration of 675 mg/kg body weight
per day to rats, a borderline increase in hyper-keratosis of the
forestomach was observed (NOEL: 125 mg/kg body weight). In rats
exposed to a dose level of approximately 40 mg acetaldehyde/kg body
weight in the drinking-water for 6 months, there was an increase in
collagen synthesis in the liver, the significance of which is unclear.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In available
studies, the lowest concentration at which effects were observed was
437 mg/m3 following administration for 5 weeks. The NOELs
identified for respiratory effects were 275 mg/m3 in rats exposed
for 4 weeks and 700 mg/m3 in hamsters exposed for 13 weeks.
At concentrations that induced tissue damage in the respiratory
tract, increased incidences were observed of nasal adenocarcinomas and
squamous cell carcinomas in the rat and laryngeal and nasal carcinomas
in the hamster.
There is evidence to suggest that acetaldehyde causes genetic
damage to somatic cells in vivo.
Available data are inadequate for the assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in the general, or
occupationally exposed, populations.
On the basis of data on irritancy in humans, a tolerable
concentration of 2 mg/m3 has been derived. Since the mechanism of
induction of tumours by acetaldehyde has not been well studied, two
approaches were adopted for the provision of guidance with respect to
this end-point, i.e., the development of a tolerable concentration
based on division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, and, estimation of lifetime
cancer risk based on linear extrapolation. The tolerable
concentration is 0.3 mg/m3. The concentrations associated with a
10-5 excess lifetime risk are 11-65 µg/m3.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical formula: C2H4O
Chemical structure: CH3-CHO
Common name: acetaldehyde
Common synonyms: ethanal; acetic aldehyde; acetylaldehyde;
ethylaldehyde; diethylacetal;
1,1-diethyoxy ethane
CAS chemical name: acetaldehyde
CAS registry number:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
Members
Mrs I. Arts, Department of Biological Toxicology, TNO Nutrition and
Food Research, Zeist, The Netherlands
Dr R.E. Barry, Faculty of Medicine, University of Bristol, Bristol
Royal Infirmary, Bristol, United Kingdom
Professor D. Beritic-Stahuljak, Andrija tampar School of Public
Health, Faculty of Medicine, University of Zagreb, Zagreb, Croatia
Dr Sai Mei Hou, Karolinska Institute, Huddinge, Sweden
Dr M.E. Meek, Environmental Health Directorate, Priority Substances
Section, Health & Welfare Canada, Tunney's Pasture, Ottawa, Canada
(Chairperson)
Professor M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
Professor G. Obe, University of Essen, Essen, Germany
Professor T.V.N. Persaud, Department of Anatomy, Faculty of
Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
Mr D. Renshaw, Department of Health, Elephant & Castle, London, United
Kingdom
Dr A. Smith, Health and Safety Executive, Toxicology Unit, Bootle,
Merseyside, United Kingdom (Co-rapporteur)
Professor A. Watanabe, Toyama Medical and Pharmaceutical University,
Faculty of Medicine, Toyama, Japan
Dr S. Worrall, Department of Biochemistry, University of Queensland,
Brisbane, Queensland, Australia
Representatives from other organizations
Dr V. Krutovskikh, Programme of Multistage Carcinogenesis,
International Agency for Research on Cancer, Lyon, France
Secretariat
Mrs J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
(Co-rapporteur)
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia (Responsible Officer and Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR ACETALDEHYDE
A WHO Task Group on Environmental Health Criteria for
Acetaldehyde met in Geneva from 6 to 10 December 1993. Professor F.
Valic opened the meeting on behalf of the three cooperating
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to acetaldehyde.
The first draft of this monograph was prepared by Mrs J. de Fouw,
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands.
Professor F. Valic was responsible for the overall scientific
content of the monograph and for the organization of the meeting, and
Mrs M.O. Head of Oxford for the technical editing of the monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
INTRODUCTION
This monograph will deal mainly with the effects of direct
exposure to acetaldehyde. However, it should be borne in mind that
for most people exposure to acetaldehyde will occur through the
consumption of alcoholic beverages (IARC, 1988). These beverages
contain ethanol, which is metabolized to acetaldehyde by alcohol
dehydrogenase (ADH). ADH activity has been detected in nearly every
tissue including liver, kidney, muscle, intestine, ovary, and testes
(Buehler et al., 1983; Agarwal & Goedde, 1990).
However, data concerning metabolically formed acetaldehyde will
only be considered when no data are available on direct exposure.
The accurate determination of acetaldehyde in body fluid and
tissue samples is relatively difficult. Only the most recent
techniques take into account artifactual acetaldehyde formation in
biological samples, especially those containing ethanol (Eriksson &
Fukunaga 1993). As values for concentrations of acetaldehyde given in
older references may well have been overestimates, absolute values are
only given when necessary.
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Acetaldehyde is a colourless, volatile liquid with a pungent
suffocating odour. The reported odour threshold is 0.09 mg/m3.
Acetaldehyde is a highly flammable and reactive compound that is
miscible in water and most common solvents.
Analytical methods are available for the detection of
acetaldehyde in air (including breath) and water. The principal
method is based on the reaction of acetaldehyde with
2,4-dinitrophenylhydrazine and subsequent analysis of the hydrazone
derivatives by high pressure liquid chromatography or gas
chromatography.
1.2 Sources of human and environmental exposure
Acetaldehyde is a metabolic intermediate in humans and higher
plants and a product of alcohol fermentation. It has been identified
in food, beverages, and cigarette smoke. It is also present in
vehicle exhaust and in wastes from various industries. Degradation of
hydrocarbons, sewage, and solid biological wastes produces
acetaldehyde, as well as the open burning and incineration of gas,
fuel oil, and coal.
More than 80% of the acetaldehyde used commercially is produced
by the liquid-phase oxidation of ethylene with a catalytic solution of
palladium and copper chlorides. Production in Japan was 323 thousand
tonnes in 1981. In the USA, production was 281 thousand tonnes in 1982
while in Western Europe it was 706 thousand tonnes in 1983. Most
acetaldehyde produced commercially is used in the production of acetic
acid. It is also used in flavourings and foods.
The annual emission of acetaldehyde from all sources in the USA
is estimated to be 12.2 million kg.
1.3 Environmental transport, distribution, and transformation
Because of its high reactivity, intercompartmental transport of
acetaldehyde is expected to be limited. Some transfer of acetaldehyde
to air from water and soil is expected because of the high vapour
pressure and low sorption coefficient.
It is suggested that the photo-induced atmospheric removal of
acetaldehyde occurs predominantly via radical formation. Photolysis is
expected to contribute another substantial fraction to the removal
process. Both processes cause a reported daily loss of about 80% of
atmospheric acetaldehyde emissions. Reported half-lives of
acetaldehyde in water and air are 1.9 h and 10-60 h, respectively.
Acetaldehyde is readily biodegradable.
1.4 Environmental levels and human exposure
Levels of acetaldehyde in ambient air generally average
5 µg/m3. Concentrations in water are generally less than
0.1 µg/litre. Analysis of a wide range of foodstuffs in the
Netherlands showed that concentrations, generally less than 1 mg/kg,
occasionally ranged up to several 100 mg/kg, particularly in some
fruit juices and vinegar.
By far, the main source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure.
With respect to other media, the general population is exposed to
acetaldehyde principally from food and beverages, and, to a lesser
extent, from air. The contribution from drinking-water is negligible.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route of exposure is most likely inhalation with possible
dermal contact.
1.5 Kinetics and metabolism
1.5.1 Absorption, distribution, and elimination
Available studies on toxicity indicate that acetaldehyde is
absorbed through the lungs and gastrointestinal tract; however, no
adequate quantitative studies have been identified. Absorption
through the skin is probable.
Following inhalation by rats, acetaldehyde is distributed to the
blood, liver, kidney, spleen, heart, and other muscle tissues. Low
levels were detected in embryos after maternal intraperitoneal (ip)
injection of acetaldehyde (mouse) and following maternal exposure to
ethanol (mouse and rat). Potential production of acetaldehyde has
also been observed in rat fetuses and in the human placenta,
in vitro.
Distribution of acetaldehyde to brain interstitial fluid, but not
to brain cells, has been demonstrated following ip injection of
ethanol. A high affinity, low Km ALDHa may be important in
maintaining low levels of acetaldehyde in the brain during the
metabolism of ethanol.
Acetaldehyde is taken up by red blood cells and, following
ethanol consumption in humans and in baboons, in vivo,
intracellular levels can be 10 times higher than plasma levels.
Following oral administration, virtually no unchanged
acetaldehyde is excreted in the urine.
1.5.2 Metabolism
The major pathway for the metabolism of acetaldehyde is by
oxidation to acetate under the influence of NADb-dependent ALDH.
Acetate enters the citric acid cycle as acetyl-CoA. There are several
isoenzymes of ALDH with different kinetic and binding parameters that
influence acetaldehyde oxidation rates.
ALDH activity has been localized in the respiratory tract
epithelium (excluding olfactory epithelium) in rats, in the renal
cortex and tubules in the dog, rat, guinea-pig, and baboon, and, in
the testes in the mouse.
Acetaldehyde is metabolized by mouse and rat embryonic tissue
in vitro. Acetaldehyde crosses the rat placenta, in spite of
placental metabolism.
Though there is some metabolism of acetaldehyde in human renal
tubules, the liver is the most important metabolic site.
Several isoenzymic forms of ALDH have been identified in the
human liver and other tissues. There is polymorphism for the
mitochondrial ALDH. Subjects who are homozygous or heterozygous for a
point mutation in the mitochondrial ALDH corresponding gene have low
activity of this enzyme, metabolize acetaldehyde slowly, and are
intolerant of ethanol.
The metabolism of acetaldehyde can be inhibited by
crotonaldehyde, dimethylmaleate, phorone, disulfiram, and calcium
carbamide.
a ALDH = acetaldehyde dehydrogenase.
b NAD = nicotinamide adenine dinocleotide.
1.5.3 Reaction with other components
Acetaldehyde forms stable and unstable adducts with proteins.
This can impair protein function, as evidenced by inhibition of enzyme
activity, impaired histone-DNA binding, and inhibition of
polymerization of tubulin.
Unstable adducts of acetaldehyde of undetermined significance
occur in vitro with nucleic acids.
Acetaldehyde can react with various macromolecules in the body,
preferentially those containing lysine residues, which can lead to
marked alterations in the biological function of these molecules.
1.6 Effects on organisms in the environment
1.6.1 Aquatic organisms
LC50s in fish ranged from 35 (guppy) to 140 mg/litre (species
not specified). An EC5 of 82 mg/litre and an EC50 of 42 mg/litre
were reported for algae and Daphnia magna, respectively.
1.6.2 Terrestrial organisms
Acetaldehyde in air appears to be toxic for some microorganisms
at relatively low concentrations.
Aphids were killed when exposed to acetaldehyde at a
concentration of 0.36 µg/m3 for 3 or 4 h.
Median lethal values were 8.91 mg/litre per h and 7.69 mg/litre
per h for the slug species, Arion hortensis and Agriolimax
reticulatus, respectively.
Inhibition of seed germination in the onion, carrot, and tomato
by acetaldehyde (up to 1.52 mg/litre) was reversible, whereas
inhibition of Amaranthus palmeri, similarly exposed, was
irreversible. Acetaldehyde at 0.54 µg/m3 damaged lettuce.
1.7 Effects on experimental animals and in vitro test systems
1.7.1 Single exposure
LD50s in rats and mice and LC50s in rats and Syrian hamsters
showed that the acute toxicity of acetaldehyde is low. Acute dermal
studies were not identified.
1.7.2 Short- and long-term exposures
In repeated dose studies, by both the oral and inhalation routes,
toxic effects at relatively low concentrations were limited
principally to the sites of initial contact. In a 28-day study in
which acetaldehyde at 675 mg/kg body weight (no-observedeffect level
(NOEL): 125 mg/kg body weight) was administered in the drinking-water
to rats, effects were limited to slight focal hyperkeratosis of the
forestomach. Following administration of a single dose level (0.05%
in the drinking-water) for 6 months (estimated by the Task Group to be
approximately 40 mg/kg body weight) in a biochemical study,
acetaldehyde induced synthesis of rat liver collagen, an observation
that was supported by in vitro data.
Following inhalation, NOELs for respiratory effects were
275 mg/m3 in rats exposed for 4 weeks and 700 mg/m3 in hamsters
exposed for 13 weeks. At lowest-observed-effect levels, degenerative
changes were observed in the olfactory epithelium in rats
(437 mg/m3) and the trachea (2400 mg/m3) in hamsters.
Degenerative changes in the respiratory epithelium and larynx were
observed at higher concentrations. No repeated dose dermal studies
were identified.
1.7.3 Reproduction, embryotoxicity, and teratogenicity
In several studies, parenteral exposure of pregnant rats and mice
to acetaldehyde induced fetal malformations. In the majority of these
studies, maternal toxicity was not evaluated. No data on reproductive
toxicity were identified.
1.7.4 Mutagenicity and related end-points
Acetaldehyde is genotoxic in vitro, inducing gene mutations,
clastogenic effects, and sister-chromatid exchanges (SCEs) in
mammalian cells in the absence of exogenous metabolic activation.
However, negative results were reported in adequate tests on
Salmonella. Following intraperitoneal injection, acetaldehyde induced
SCEs in the bone marrow of Chinese hamsters and mice. However,
acetaldehyde administered intraperitoneally did not increase the
frequency of micronuclei in early mouse spermatids. There is indirect
evidence from in vitro and in vivo studies to suggest that
acetaldehyde can induce protein-DNA and DNA-DNA cross-links.
1.7.5 Carcinogenicity
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses). However, in hamsters,
increases in nasal and laryngeal carcinomas were non-significant. All
concentrations of acetaldehyde administered in the studies induced
chronic tissue damage in the respiratory tract.
1.7.6 Special studies
Adequate studies on the potential neuro- and immunotoxicity of
acetaldehyde were not identified.
1.8 Effects on humans
In limited studies on human volunteers, acetaldehyde was mildly
irritating to the eyes and upper respiratory tract following exposure
for very short periods to concentrations exceeding approximately 90
and 240 mg/m3, respectively. Cutaneous erythema was observed in
patch testing with acetaldehyde, in twelve subjects of "Oriental
ancestry".
One limited investigation in which the incidence of cancer was
examined in workers exposed to acetaldehyde and other compounds has
been reported.
On the basis of indirect evidence, acetaldehyde has been
implicated as the putatively toxic metabolite in the induction of
alcohol-associated liver damage, facial flushing, and developmental
effects.
1.9 Evaluation of human health risks and effects on the environment
The acute toxicity of acetaldehyde by the inhalation or oral
route in studies conducted on animals was low. According to studies
on humans and animals, acetaldehyde is mildly irritating to the eyes
and the upper respiratory tract. In limited studies on human
volunteers, acetaldehyde was mildly irritating to the eyes and upper
respiratory tract (section 1.8). Cutaneous erythema has also been
observed in the patch testing of humans. Although a possible mechanism
has been identified, available data are inadequate to assess the
potential of acetaldehyde to induce sensitization.
Available data on the effects of acetaldehyde following ingestion
are limited. Following oral administration of 675 mg/kg body weight
per day to rats, a borderline increase in hyper-keratosis of the
forestomach was observed (NOEL: 125 mg/kg body weight). In rats
exposed to a dose level of approximately 40 mg acetaldehyde/kg body
weight in the drinking-water for 6 months, there was an increase in
collagen synthesis in the liver, the significance of which is unclear.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In available
studies, the lowest concentration at which effects were observed was
437 mg/m3 following administration for 5 weeks. The NOELs
identified for respiratory effects were 275 mg/m3 in rats exposed
for 4 weeks and 700 mg/m3 in hamsters exposed for 13 weeks.
At concentrations that induced tissue damage in the respiratory
tract, increased incidences were observed of nasal adenocarcinomas and
squamous cell carcinomas in the rat and laryngeal and nasal carcinomas
in the hamster.
There is evidence to suggest that acetaldehyde causes genetic
damage to somatic cells in vivo.
Available data are inadequate for the assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in the general, or
occupationally exposed, populations.
On the basis of data on irritancy in humans, a tolerable
concentration of 2 mg/m3 has been derived. Since the mechanism of
induction of tumours by acetaldehyde has not been well studied, two
approaches were adopted for the provision of guidance with respect to
this end-point, i.e., the development of a tolerable concentration
based on division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, and, estimation of lifetime
cancer risk based on linear extrapolation. The tolerable
concentration is 0.3 mg/m3. The concentrations associated with a
10-5 excess lifetime risk are 11-65 µg/m3.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical formula: C2H4O
Chemical structure: CH3-CHO
Common name: acetaldehyde
Common synonyms: ethanal; acetic aldehyde; acetylaldehyde;
ethylaldehyde; diethylacetal;
1,1-diethyoxy ethane
CAS chemical name: acetaldehyde
CAS registry number:  6.3.1.1 Liver
The main pathway for the metabolism of acetaldehyde is by rapid
oxidation to acetate, which enters the citric acid cycle in an
activated form as acetyl-CoA and is metabolized to CO2 and H2O.
Although catalase and other oxidases may contribute to metabolism
(Brien & Loomis, 1983), because of its high affinity, at least 90% of
acetaldehyde is oxidized by mitochondrial ALDH (Hellström-Lindahl &
Weiner, 1985) reducing NAD+ to NADH in the process. This step can
be blocked by disulfiram.
There are multiple molecular forms of ALDH with different kinetic
properties that influence the rate of removal of acetaldehyde
(Marjanen, 1973; Parilla et al., 1974; Teschke et al., 1977).
Acetaldehyde is a highly reactive molecule that can react with
many other large or small molecules by adduction, condensation, or
polymerization. These pathways may have little quantitative
significance in acetaldehyde metabolism, but the by-products may have
biological significance (Collins et al., 1979; Sorrell & Tuma, 1985).
Acetaldehyde is the primary metabolic product of ethanol
oxidation. Since ethanol is oxidized to acetaldehyde mole for mole,
and, since the exposure to exogenous acetaldehyde is small, endogenous
acetaldehyde resulting from the metabolism of ingested ethanol is
likely to be the most important source of exposure for most people.
Oxidation of ethanol to acetaldehyde occurs predominantly under the
influence of ADH, of which there are many isoenzymic forms. Like
ALDH, ADH is also NAD dependent. The inseparable metabolism of
ethanol and acetaldehyde results in the reduction of NAD+, thus,
affecting the redox state of the liver causing secondary metabolic
consequences.
6.3.1.2 Respiratory tract
ALDH localization in the respiratory tract of Fischer-344 rats
was studied by Bogdanffy et al. (1986). Histochemical studies
indicated activity principally in the nasal respiratory epithelium,
especially in the supranuclear cytoplasm of ciliated epithelial cells.
Activity was also high in the Clara cells of the lower bronchioles.
The tracheal epithelia possessed only low levels of ALDH. The
olfactory epithelium was almost devoid of ALDH activity.
Casanova-Schmitz et al. (1984) characterized at least 2
isoenzymes of ALDH in rat nasal mucosa homogenates.
6.3.1.3 Kidneys
In an in vitro study, Michoudet & Baverel (1987a,b) studied the
metabolism of acetaldehyde in isolated dog, rat, guinea-pig, and
baboon kidney-cortex tubules.
Acetaldehyde was found to be metabolized by the tubules at high
rates and in a dose-dependent manner in all four species. It was
noted that, at all acetaldehyde concentrations, most of the
acetaldehyde removed was recovered as acetate in dog, guinea-pig, and
baboon, but not in rat kidney tubules.
6.3.1.4 Testes and ovaries
There are no studies on the capacity of the testes or ovaries to
mediate the biotransformation of acetaldehyde. However, ALDH activity
has been identified in the testes of Swiss-Webster mice (Anderson et
al., 1985).
6.3.1.5 Embryonic tissue
In an in vitro study, the ability of CBA/beige mouse (10 days
old) and Wistar rat (12 days old) embryos to metabolize acetaldehyde
was reported by Priscott & Ford (1985).
6.3.1.6 Metabolism during pregnancy
After intravenous administration of acetaldehyde (10 mg/kg body
weight) blood acetaldehyde levels were higher in pregnant rats than in
virgin rats. Acetaldehyde at high concentrations was able to cross
the placental barrier very rapidly. At low maternal concentrations,
it was metabolized by aldehyde dehydrogenase activity in the placenta
and fetal liver, and acetaldehyde was not detected in fetal blood.
Above the acetaldehyde threshold, the metabolic capacity of the
feto-placental unit was surpassed and acetaldehyde was detected in
fetal blood (Zorzano & Herrera, 1989).
6.3.2 Human studies
No high quality studies of the in vivo metabolism of
acetaldehyde in humans have been identified. Accurate assays for
acetaldehyde in blood and tissues have only recently become available
(Harade et al., 1978a,b).
Human liver ALDH consists of at least 4 main isoenzymes, which
are also present in many other tissues (Koivula, 1975; Goedde et al.,
1979). Mitochondrial ALDH is inactive in at least 40% of the Oriental
population. The frequently observed intolerance to alcohol (the
"flushing" reaction) is linked to this deficiency, which is produced
by an inherited positive mutation in the corresponding gene (Yoshida
et al., 1984; Goedde & Agarwal, 1986, 1987; Hsu et al., 1988).
Subjects with phenotypic deficiency have always shown the presence of
at least one mutant gene (heterozygous or homozygous) (Crabb et al.,
1989; Goedde et al., 1989; Singh et al., 1989).
In vitro, acetaldehyde (0.04-0.88 g/litre) was metabolized at
high rates and in a dose-dependent manner in isolated human
kidney-cortex tubules (Michoudet & Baverel, 1987b).
6.4 Elimination
In dogs, urinary excretion of acetaldehyde was essentially
non-existent following administration of a single dose of 600 mg
acetaldehyde/kg body weight, via a stomach tube (Booze & Oehme, 1986).
6.5 Reaction with cellular macromolecules
6.5.1 Proteins
Acetaldehyde can react with nucleophilic groups, such as amino,
hydroxyl, and sulfydryl groups, through nucleophilic attack on the
carbonyl carbon atom of acetaldehyde to give both stable and unstable
adducts (Tuma et al., 1984). Several adduct structures, formed when
acetaldehyde reacts with proteins in vitro, have been identified,
but have not yet been described fully.
The best characterized nucleophiles able to form adducts with
acetaldehyde are amino groups, notably the alpha-amino terminus of
peptides and proteins and the epsilon-amino group on the side-chain of
lysine residues. These reactions are shown in Fig. 2.
The structure of 2-methylimidazolidin-4-one adducts has been
confirmed by proton NMR (Gidley et al., 1981) and 13C-NMR
spectroscopy (San George & Hoberman, 1986). N-ethylation lysine
residues have been demonstrated by Tuma et al. (1984).
In a series of studies on lysine-dependent enzymes, Mauch et al.
(1986, 1987) were able to demonstrate that incubation of purified
enzymes with acetaldehyde for 1 h at 37°C led to inhibition of their
catalytic activity. Lysine non-dependent enzymes were not affected by
this treatment. A similar study involving the incubation of rat liver
histone H1 with physiological concentrations of acetaldehyde showed
that spontaneously stable adducts were formed on lysine residues at
the carboxy terminus, a site crucial for its function as a eukaryotic
repressor (Niëmela et al., 1990). This acetaldehyde-modified histone
H1 showed impaired DNA binding activity.
Tuma et al. (1987) have characterized the interaction of
acetaldehyde with a "highly reactive" lysine residue in purified
alpha-tubulin, which is only available in the monomeric form. They
found that modification of this residue was the critical factor in the
inhibition of tubulin polymerization by acetaldehyde, and that
modification of 5% of these residues was enough to inhibit tubulin
polymerization completely in vitro. Crebelli et al. (1989) found
similar effects: 0.075% v/v (13.5 mmol/litre) acetaldehyde partially
inhibited the in vitro polymerization of cattle brain tubulin, and
0.15% (27 mmol/litre) caused complete inhibition.
Incubation of calf brain microtubular proteins also resulted in
decreased polymerization, in an analogous manner to tubulin (McKinnon
et al., 1987a,b). Thus, acetaldehyde modification can impair the
molecular function of macromolecules, which can lead to marked
alterations in biological function.
6.3.1.1 Liver
The main pathway for the metabolism of acetaldehyde is by rapid
oxidation to acetate, which enters the citric acid cycle in an
activated form as acetyl-CoA and is metabolized to CO2 and H2O.
Although catalase and other oxidases may contribute to metabolism
(Brien & Loomis, 1983), because of its high affinity, at least 90% of
acetaldehyde is oxidized by mitochondrial ALDH (Hellström-Lindahl &
Weiner, 1985) reducing NAD+ to NADH in the process. This step can
be blocked by disulfiram.
There are multiple molecular forms of ALDH with different kinetic
properties that influence the rate of removal of acetaldehyde
(Marjanen, 1973; Parilla et al., 1974; Teschke et al., 1977).
Acetaldehyde is a highly reactive molecule that can react with
many other large or small molecules by adduction, condensation, or
polymerization. These pathways may have little quantitative
significance in acetaldehyde metabolism, but the by-products may have
biological significance (Collins et al., 1979; Sorrell & Tuma, 1985).
Acetaldehyde is the primary metabolic product of ethanol
oxidation. Since ethanol is oxidized to acetaldehyde mole for mole,
and, since the exposure to exogenous acetaldehyde is small, endogenous
acetaldehyde resulting from the metabolism of ingested ethanol is
likely to be the most important source of exposure for most people.
Oxidation of ethanol to acetaldehyde occurs predominantly under the
influence of ADH, of which there are many isoenzymic forms. Like
ALDH, ADH is also NAD dependent. The inseparable metabolism of
ethanol and acetaldehyde results in the reduction of NAD+, thus,
affecting the redox state of the liver causing secondary metabolic
consequences.
6.3.1.2 Respiratory tract
ALDH localization in the respiratory tract of Fischer-344 rats
was studied by Bogdanffy et al. (1986). Histochemical studies
indicated activity principally in the nasal respiratory epithelium,
especially in the supranuclear cytoplasm of ciliated epithelial cells.
Activity was also high in the Clara cells of the lower bronchioles.
The tracheal epithelia possessed only low levels of ALDH. The
olfactory epithelium was almost devoid of ALDH activity.
Casanova-Schmitz et al. (1984) characterized at least 2
isoenzymes of ALDH in rat nasal mucosa homogenates.
6.3.1.3 Kidneys
In an in vitro study, Michoudet & Baverel (1987a,b) studied the
metabolism of acetaldehyde in isolated dog, rat, guinea-pig, and
baboon kidney-cortex tubules.
Acetaldehyde was found to be metabolized by the tubules at high
rates and in a dose-dependent manner in all four species. It was
noted that, at all acetaldehyde concentrations, most of the
acetaldehyde removed was recovered as acetate in dog, guinea-pig, and
baboon, but not in rat kidney tubules.
6.3.1.4 Testes and ovaries
There are no studies on the capacity of the testes or ovaries to
mediate the biotransformation of acetaldehyde. However, ALDH activity
has been identified in the testes of Swiss-Webster mice (Anderson et
al., 1985).
6.3.1.5 Embryonic tissue
In an in vitro study, the ability of CBA/beige mouse (10 days
old) and Wistar rat (12 days old) embryos to metabolize acetaldehyde
was reported by Priscott & Ford (1985).
6.3.1.6 Metabolism during pregnancy
After intravenous administration of acetaldehyde (10 mg/kg body
weight) blood acetaldehyde levels were higher in pregnant rats than in
virgin rats. Acetaldehyde at high concentrations was able to cross
the placental barrier very rapidly. At low maternal concentrations,
it was metabolized by aldehyde dehydrogenase activity in the placenta
and fetal liver, and acetaldehyde was not detected in fetal blood.
Above the acetaldehyde threshold, the metabolic capacity of the
feto-placental unit was surpassed and acetaldehyde was detected in
fetal blood (Zorzano & Herrera, 1989).
6.3.2 Human studies
No high quality studies of the in vivo metabolism of
acetaldehyde in humans have been identified. Accurate assays for
acetaldehyde in blood and tissues have only recently become available
(Harade et al., 1978a,b).
Human liver ALDH consists of at least 4 main isoenzymes, which
are also present in many other tissues (Koivula, 1975; Goedde et al.,
1979). Mitochondrial ALDH is inactive in at least 40% of the Oriental
population. The frequently observed intolerance to alcohol (the
"flushing" reaction) is linked to this deficiency, which is produced
by an inherited positive mutation in the corresponding gene (Yoshida
et al., 1984; Goedde & Agarwal, 1986, 1987; Hsu et al., 1988).
Subjects with phenotypic deficiency have always shown the presence of
at least one mutant gene (heterozygous or homozygous) (Crabb et al.,
1989; Goedde et al., 1989; Singh et al., 1989).
In vitro, acetaldehyde (0.04-0.88 g/litre) was metabolized at
high rates and in a dose-dependent manner in isolated human
kidney-cortex tubules (Michoudet & Baverel, 1987b).
6.4 Elimination
In dogs, urinary excretion of acetaldehyde was essentially
non-existent following administration of a single dose of 600 mg
acetaldehyde/kg body weight, via a stomach tube (Booze & Oehme, 1986).
6.5 Reaction with cellular macromolecules
6.5.1 Proteins
Acetaldehyde can react with nucleophilic groups, such as amino,
hydroxyl, and sulfydryl groups, through nucleophilic attack on the
carbonyl carbon atom of acetaldehyde to give both stable and unstable
adducts (Tuma et al., 1984). Several adduct structures, formed when
acetaldehyde reacts with proteins in vitro, have been identified,
but have not yet been described fully.
The best characterized nucleophiles able to form adducts with
acetaldehyde are amino groups, notably the alpha-amino terminus of
peptides and proteins and the epsilon-amino group on the side-chain of
lysine residues. These reactions are shown in Fig. 2.
The structure of 2-methylimidazolidin-4-one adducts has been
confirmed by proton NMR (Gidley et al., 1981) and 13C-NMR
spectroscopy (San George & Hoberman, 1986). N-ethylation lysine
residues have been demonstrated by Tuma et al. (1984).
In a series of studies on lysine-dependent enzymes, Mauch et al.
(1986, 1987) were able to demonstrate that incubation of purified
enzymes with acetaldehyde for 1 h at 37°C led to inhibition of their
catalytic activity. Lysine non-dependent enzymes were not affected by
this treatment. A similar study involving the incubation of rat liver
histone H1 with physiological concentrations of acetaldehyde showed
that spontaneously stable adducts were formed on lysine residues at
the carboxy terminus, a site crucial for its function as a eukaryotic
repressor (Niëmela et al., 1990). This acetaldehyde-modified histone
H1 showed impaired DNA binding activity.
Tuma et al. (1987) have characterized the interaction of
acetaldehyde with a "highly reactive" lysine residue in purified
alpha-tubulin, which is only available in the monomeric form. They
found that modification of this residue was the critical factor in the
inhibition of tubulin polymerization by acetaldehyde, and that
modification of 5% of these residues was enough to inhibit tubulin
polymerization completely in vitro. Crebelli et al. (1989) found
similar effects: 0.075% v/v (13.5 mmol/litre) acetaldehyde partially
inhibited the in vitro polymerization of cattle brain tubulin, and
0.15% (27 mmol/litre) caused complete inhibition.
Incubation of calf brain microtubular proteins also resulted in
decreased polymerization, in an analogous manner to tubulin (McKinnon
et al., 1987a,b). Thus, acetaldehyde modification can impair the
molecular function of macromolecules, which can lead to marked
alterations in biological function.
 No data are available on the formation of acetaldehydemodified
proteins in animals or humans directly exposed to acetaldehyde.
However, some data are available on proteins modified by acetaldehyde
derived from ethanol metabolism. In these studies, proteins carrying
acetaldehyde adducts were shown to be present in the liver cytosol of
rats fed ethanol for periods of 3 weeks, 12 months, or 27 months
(Worrall et al., 1991a). Acetaldehyde-modified proteins have also been
detected in the plasma (Liu et al., 1990) and haemoglobin of
alcoholics (Niemela & Israel, 1992). Furthermore, a limited
immunohistochemical study has demonstrated the presence of
acetaldehyde-modified proteins in the livers of some alcoholics
(Niemela et al., 1991). These studies demonstrate that acetaldehyde
adducts can form in the body. The possible immunological consequences
of adduct formation will be discussed in section 8.9.
6.5.2 Nucleic acids
No data are available from in vivo studies on the generation of
DNA adducts.
Acetaldehyde reacts with nucleosides and deoxynucleosides at pH
6.5 and 37°C in vitro to form unstable adducts by binding to the
exocyclic amino groups of adenine, cytosine, and guanine (Hemminki &
Suni, 1984). Addition of a reducing agent (sodium borohydride) leads
to the formation of stable adducts, of which the main one was
identified as N2-ethylguanosine using mass spectrometry and NMR.
Similar data for the formation of unstable adducts formed by reacting
acetaldehyde with ribonucleosides and deoxyribonucleosides was
reported by Fraenkel-Conrat & Singer (1988). When ethanol was present
in the reaction mixture, a different type of adduct was formed, which
was identified by fast atom bombardment and proton NMR to be a mixed
acetal (-NH-CH(CH3)-OR). These adducts were found to have half-lives
varying from 2.5 to 24 h at pH 7.5 and 37°C, depending on the base
involved.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
An LC50 (semi-static study) of 35 mg/litre was found for
acetaldehyde in the guppy (Poecilia reticulata; 10 laboratory-reared
and acclimatized fish, 2-3 months old) (Deneer et al., 1988). Grahl
(1983) reported an LC50 (48-96 h) of 124 mg/litre for acetaldehyde
in fish (no additional information was provided). Juhnke & Luedemann
(1978) presented the results for fish obtained in the Golden Orfe
test, and found an LC50 of 140-124 mg/litre for acetaldehyde (no
additional information was provided). An LC50 (static conditions;
96-h) of 53 mg/litre was reported for the bluegill (Lepomis
macrochirus) by Von Burg & Stout (1991).
Acetaldehyde had a depressing effect on the aggressive behaviour
of the fish cichlid (Cichlasoma nigro fasciatum) at concentrations
that did not cause locomotor decrements in this species (Peeke &
Figler, 1981).
An EC5 (48-h; population growth) of 82 mg/litre and an EC50
(48-h; static conditions; immobilization) of 42 mg/litre were reported
for protozoa (Chilomonas paramecium) and the waterflea (Daphnia
magna), respectively (Von Burg & Stout, 1991).
7.2 Terrestrial organisms
Aharoni & Barkai-Golan (1973) studied the effects of acetaldehyde
vapours on the germination and colony-forming potential of two fungi
species, Alternaria tenuis and Stemphylium botryosum. The rate of
growth inhibition increased with both concentration and time of
exposure. The exposure of the spores was conducted at room
temperature. A. tenuis, the more sensitive species, was inactivated
by 0.54 µg acetaldehyde/m3 applied for 5 h, whereas 1.08 µg
acetaldehyde/m3 for 2 h, was needed to inactivate S. botryosum
spores.
Pittevils et al. (1979) reported the activity of acetaldehyde
against the fungi affecting stored apples and pears (Colletotrichum
gloeosporioides, Cryptosporiopsis malicorticis, Phlyctaena
vagabunda, Botrytis cinerea, and Alternaria tenuis). Acetaldehyde
was rapidly lethal at low concentrations: after a 24-h treatment
period, the lethal concentration of acetaldehyde ranged from
0.036 µg/m3 (A. tenuis) to 0.09 µg/m3 (C. gloeosporioides).
Acetaldehyde remained lethal for the five fungi, even when the
treatment lasted only 20 min (0.90 µg/m3 for P. vagabunda, C.
malicorticis, and A. tenuis, and 0.36 µg/m3 for C. gloeosporioides
and B. cinerea).
The fungi Botrytis cinerea, Penicillium expansum, Rhizopus
stolonifer, Monilinia fructicola, Erwinia carotovora, and
Pseudomonas fluorescens were killed, when exposed to
acetaldehyde vapours at concentrations ranging from 0.045 to
3.6 µg/m3, applied for 0.5 to 120 min at room temperature (Aharoni &
Stadelbacher, 1973).
Aharoni et al. (1979) studied acetaldehyde as a fumigant for
control of the green peach aphid (Myzus persicae) on head lettuce
(Lactuca sativa). When aphids were placed on the lettuce prior to
fumigation, 0.36 µg acetaldehyde/m3 and a 3-4 h exposure were
required for 100% mortality. A similar treatment (0.27-0.36 µg/m3
for 4 h) was found to cause 100% mortality of aphids on lettuce by
Stewart et al. (1980).
The fumigant effect of acetaldehyde was tested on the garden slug
(Arion hortensis; weight range, 0.2-0.5 g) and the grey field slug
(Agriolimax reticulatus; weight range, 0.3-0.6 g). It caused both
species to close the pulmonary aperture and to secrete excess
'irritation' mucus. Medial lethal values of 7.69 ± 0.21 mg/litre
per h for A. reticulatus and of 8.91 ± 0.81 mg/litre per h for
A. hortensis were found (Henderson, 1970).
The seed germination of the onion (Allium cepa L.), carrot
(Daucus carota L.), Palmer Amaranth (Amaranthus palmeri S Wats.),
and tomato (Lycopersicon esculentum Mill.) after exposure to
acetaldehyde (up to 1.52 mg/litre), was examined by Bradow & Connick
(1988). After a 3-day exposure, acetaldehyde inhibited the seed
germination of all four plants by more than 50%. Seeds inhibited by a
3-day exposure to acetaldehyde followed by a 4-day recovery period
germinated to the same extent as the controls after seven days, except
for the Palmer Amaranth, which remained inhibited.
Acetaldehyde at concentrations of 0.54-1.08 µg/m3 affected head
lettuce (Lactuca sativa), as evidenced by dark-green, water-soaked,
necrotic areas on the outer leaves of the lettuce. Concentrations of
up to 0.36 µg/m3 did not affect the lettuce (Aharoni et al., 1979;
Stewart et al., 1980).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 LD50 and LC50 values
Relevant data are summarized in Table 4.
Oral LD50s for acetaldehyde in rats and mice ranged from 660 to
1930 mg/kg body weight. LC50s (0.5-4 h) in rats and Syrian hamsters
ranged from 24 to 37 g/m3. It is, therefore, concluded that the
acute toxicity of acetaldehyde is low. LD50 values by the dermal
route were not available.
LD50s for intratracheal, subcutaneous, intraperitoneal, and
intravenous administration are also presented in Table 4.
Table 4. LD50/LC50 values for acetaldehyde
Species Route of LD50/LC50 Reference
administration
Rat oral 1930 mg/kg body weight Smyth et al. (1951)
Rat oral 660 mg/kg body weight Sprince et al. (1974)
Mouse oral 1230 mg/kg body weight US NRC (1977)
Dog oral > 600 mg/kg body weight Booze & Oehme (1986)
Rat inhalation 24 g/m3; 4 h Appelman et al. (1982)
Rat inhalation 37 g/m3; 0.5 h Skog (1950)
Syrian
hamster inhalation 31 g/m3; 4 h Kruysse (1970)
Syrian
hamster intratracheal 96.1 mg/kg body weight Feron & De Jong (1971)
Rat subcutaneous 640 mg/kg body weight Skog (1950)
Mouse subcutaneous 560 mg/kg body weight Skog (1950)
Mouse intraperitoneal 500 mg/kg body weight Truitt & Walsh (1971)
Mouse intravenous 165 mg/kg body weight O'Shea & Kaufman
(pregnant) (1979)
8.2 Short-term exposure
8.2.1 Oral
Oral administration in the drinking-water of 675 mg
acetaldehyde/kg body weight to Wistar rats, daily for 4 weeks,
resulted in slight to moderate focal hyperkeratosis of the forestomach
in 8/10 males and 8/10 females. No effects were observed at lower
dose levels of 25 and 125 mg/kg body weight. In the control group,
very slight focal hyperkeratosis of the forestomach was noted in 6/20
females and 3/20 males (1/20 slight). At the top dose (675 mg/kg),
relative kidney weights were slightly increased in males, urinary
production was decreased, and there were variations in serum
biochemistry, most of which were attributable to reduced water intake.
There were no effects in the liver. The no-observed-effect level
(NOEL) was 125 mg/kg, the lowest-observed-effect level (LOEL) was
675 mg/kg (Til et al., 1988).
8.2.2 Inhalation
Male Sprague-Dawley rats were continuously exposed to
acetaldehyde vapour for 22 days at levels gradually increasing from
750 mg/m3 for a few days up to 2500 mg/m3 for the last few days.
By gradually increasing the concentrations, mortality in the early
period following exposure to 2000-2500 mg/m3 was prevented,
presumably because of metabolic adaptation; sudden, high, blood
acetaldehyde levels inducing vagal reflex reactions may result in
respiratory inhibition, and, as a consequence, death (Lamboeuf et al.,
1987; Latge et al., 1987).
Groups of 10 male and 10 female Wistar rats were exposed to
acetaldehyde at 0, 720, 1800, 3950, or 9000 mg/m3 (0, 400, 1000,
2200, or 5000 ppm) for 6 h/day, 5 days/week, for 4 weeks. Mortality
was slightly increased at 3950 and 9000 mg/m3, whereas growth was
retarded at 1800 mg/m3 and above in males, and at 9000 mg/m3 in
females. At 9000 mg/m3, relative liver weight decreased, and
relative lung weight in males increased. No treatment-related
histopathological changes were observed in the liver. Degenerative
changes of the nose were observed after exposure to all concentrations
(720 mg/m3-9000 mg/m3), with hyperplasia and metaplasia occurring
at concentrations of 3950 mg/m3 or more. A NOEL was not identified
(LOEL: 720 mg/m3) (Appelman et al., 1982).
Groups of 10 male Wistar rats were exposed to acetaldehyde, for
6 h/day, 5 days/week, for 4 weeks, in three different patterns: (a)
as a continuous daily exposure of 6 h to 0, 270, or 900 mg/m3 (0,
150, or 500 ppm), (b) as two daily exposures of 3 h to similar
concentrations with an intervening 1.5-h period with no exposure, or
(c) as two daily 3-h periods of exposure to similar concentrations
with an intervening 1.5-h period with eight short (5-min) peaks of 6
times the basic concentration, resulting in time-weighted average
concentrations of 0, 255, or 1050 mg/m3, respectively. Though there
were no indications of toxicity following continuous or interrupted
exposures to 270 and 900 mg/m3 and intermittent high/low exposure to
255 mg/m3, intermittent high/low exposure to 1050 mg/m3 induced
growth retardation (Appelman et al., 1986).
At 900 mg/m3, the observed effects were very similar to the
ones reported earlier by Appelman et al. (1982) at 720 mg/m3.
Variation of the pattern of exposure, by including a 1.5-h break, or
by additionally including eight 5-min, 6-fold higher peak exposures,
did not alter the observed degenerative effects. No effects were
observed in Wistar rats exposed to a lower concentration, 5 days/week
for 4 weeks, either as a "continuous" (6 h/day) exposure of
270 mg/m3, or as a time-weighted average of 255 mg/m3 after the
described intermittent low-high exposure. The NOEL was 255 mg/m3,
6-h TWA (LOEL = 1050 mg/m3, 6-h time weighted average) (Appelman et
al., 1986).
In another study, groups of 12 male Wistar rats were exposed to 0
or 437 mg acetaldehyde/m3 (0 or 243 ppm), 8 h/day, 5 days per week,
for 5 weeks. Hyperplasia of the olfactory epithelium and nasal
inflammation were observed in exposed animals, and on the basis of
lung function tests, residual volume and functional residual capacity
were increased, indicating some (unspecified) damage of the distal
airways (Saldiva et al., 1985).
8.2.3 Dermal
No relevant studies were identified.
8.2.4 Parenteral
Effects in the liver have been reported in several studies, but
only at very high doses. Intraperitoneal injection of male albino
rats with 200 mg acetaldehyde/kg body weight, daily, for 10 days, with
additional pyrazole treatment to inhibit the conversion of
acetaldehyde to ethanol, caused fatty accumulation in the liver, as
indicated by accumulation of total lipids, triacyl glycerols, and
total cholesterol, increased glycogenolysis, and a shift in metabolism
from the citric acid cycle towards the pentose phosphate pathway in
the liver. Serum triacyl glycerol, total cholesterol, and free fatty
acid levels were also increased. Changes were similar in rats not
receiving pyrazole pretreatment (Prasanna & Ramakrishnan, 1984a,
1987). The same treatment altered thyroid function, as indicated by
lower serum T4 and decreased iodine uptake in male albino rats, though
these effects may have been secondary to the observed hepatic changes
(Prasanna et al., 1986) and histopathological changes of the pancreas,
with resulting changes in trypsinogen levels and amylase secretion and
activity in female Sprague-Dawley rats (Majumdar et al., 1986).
8.3 Skin and eye irritation; sensitization
No relevant data were identified.
8.4 Long-term exposure
8.4.1 Oral
In rats exposed to 0.05% acetaldehyde in drinking-water
(estimated by the Task Group to be approximately 40 mg/kg body weight)
for 6 months, there was an increase in collagen synthesis in the liver
(Bankowski et al., 1993). The toxicological significance of this
observation is not known; no other effects were examined.
8.4.2 Inhalation
Non-neoplastic effects observed in carcinogenicity studies are
discussed in section 8.7.1.
Groups of 20 Syrian hamsters were exposed to acetaldehyde vapour
at 0, 700, 2400, or 8200 mg/m3 (0, 390, 1340, or 4560 ppm) for
6 h/day, 5 days/week, for 13 weeks. Increased relative lung and heart
weights as well as growth retardation were reported after exposure to
8200 mg/m3, though there were no increases in mortality in any of
the exposed groups (Kruysse et al., 1975). At the highest
concentration, there were severe degenerative, hyperplastic, and
metaplastic changes in the epithelium as well as subepithelial glands
and turbinate bones. Rhinitis was observed, with abundant nasal
discharge and salivation. The epithelium of the larynx, trachea, and
lungs was damaged, with some focal hyperplasia and metaplasia,
accompanied by tracheitis and focal bronchopneumonia. Changes in the
tracheal epithelium were also observed at 2400 mg/m3. At
700 mg/m3, no significant effects were observed (NOEL: 700 mg/m3;
LOEL: 2400 mg/m3).
8.5 Reproductive and developmental toxicity
Studies on reproductive effects have not been identified. A
number of studies on developmental effects have been conducted,
primarily to investigate the role of acetaldehyde in ethanol-induced
teratogenicity. However, in all of these studies, acetaldehyde was
administered by injection rather than by the principal routes of
exposure in the occupational and general environments (i.e., ingestion
and inhalation). Results of identified studies in which acetaldehyde
was administered during gestation to rats and mice by intraperitoneal,
intravenous, or amniotic injection are presented in Table 5. Though
dose-related embryotoxic, fetotoxic, and teratogenic effects were
observed in most of these studies, particularly those on rats,
maternal toxicity was not adequately assessed or reported in any of
these investigations. Dose-related embryotoxic effects were also
observed in in vitro studies on rat embryos exposed to acetaldehyde
(Popov et al., 1981; Campbell & Fantel, 1983).
Effects on the placenta have been observed following
intraperitoneal injection of acetaldehyde into pregnant rats
(Sreenathan et al., 1984a). In an in vitro study on human placental
membrane vesicles, very high concentrations (approximately 100 times
higher than the highest reported levels in blood), inhibited L-alanine
transport (Asai et al., 1985).
8.6 Mutagenicity and related end-points
Relevant data are summarized in Table 6.
8.6.1 Bacteria
Acetaldehyde was not mutagenic when tested adequately in standard
preincubation assays with S. typhimurium.
Statistically significant mutagenic responses were induced in
E. coli WP2uvrA in preincubation assays without metabolic activation
at 37°C (Veghelyi et al., 1978) and 0°C (Igali & Gazso, 1980). In
contrast, in another study on the same strain, results were negative
(Hemminki et al., 1980).
8.6.2 Non-mammalian eukaryotic systems
8.6.2.1 Gene mutation assays
Acetaldehyde (0.1% or 1.0% for 2 h) induced mutations in
genes that affect the egg-laying system of Caenorhabditis elegans
(Greenwald & Horvitz, 1980).
Acetaldehyde induced sex-linked recessive lethal mutations in
Drosophila melanogaster (Woodruff et al., 1985).
8.6.2.2 Chromosome alterations
Acetaldehyde induced chromosome malsegregation and mitotic
cross-over (yA2 marker) in Aspergillus nidulans diploid strain P1
during early conidial germination (Crebelli et al., 1989).
There was a dose-related increase in chromosomal aberrations in
Vicia faba root tips exposed to acetaldehyde (Rieger & Michaelis,
1960). Acetaldehyde also induced chromosome aberrations, micronuclei,
and sister chromatid exchanges (SCEs) in the root meristem cells of
Allium cepa (Cortes et al., 1986).
Table 5. Developmental toxicity
Species Exposure Dose Effects Reference
Rat day 10, 11, or 12; 0, 50, 75, 100 mg/kg fetal resorption, malformations (oedema, microcephaly, Sreenathan
days 10-12 of body weight, ip micrognathia, micromelia, hydrocephaly, exencephaly), growth et al. (1982,
pregnancy; days retardation; reduced placental weight; 50 mg/kg body weight, 1984b)
8-15 (50 mg/kg days 8-15: delays in ossification; skeletal malformations,
body weight per such as wavy ribs; no discussion of maternal effects
day)
Rat days 8-15 of 50, 75, 100 or 150 increase in resorption, and fetal death (changes were dose Padmanabhan
pregnancy mg/kg body weight, dependent); malformations: oedema, microcephaly, micrognathia, et al. (1983)
ip micromelia, syndactyly, hydronephrosis and subcutaneous
haemorrhage in the face, fore- and hind paws; reduced mean
placental weight and umbilical cord length; increased amniotic
fluid weight
maternal toxicity: rel. reduced water, food consumption, rel.
reduced body weight gain, 50 mg and above
Rat day 13 of pregnancy 1% or 10% solution 10%: 100% mortality; 1%: 80% malformations versus 14% in Bariliak &
embryo amniotic injections controls Kozachuk
(1983)
Rat days 9-12 of pregancy 1% (100 mg/kg body reduction in head length; no effects on morphological Ali &
weight), ip scores or crown rump length Persaud (1988)
Table 5 (contd).
Species Exposure Dose Effects Reference
Rat days 8-13 of 10 or 50 mg/kg body reduced performance in surface righting, olfactory Schreiner
pregnancy weight, ip discrimination, and learning ability; lower startle amplitudes et al. (1987)
in an auditory startle habituation test; maternal toxicity not Abstract
addressed; age of testing of offspring not specified
Rat day 5 of gestation 0.03-0.04 µg/kg body retarded blastulation Checiu et
weight examination on al. (1984)
day 5 of gestation
Mouse day 7 or 8 of 0.32 g/kg, iv exencephaly, mandibular and maxillary hypoplasia; Webster et
pregnancy; day 9 polydactyly club foot; no discussion of maternal effects al. (1983)
or 10 of pregnancy
Mouse day 10 of pregnancy 1000 mg/kg, ip no increase in resorptions or malformed fetuses or decrease Blakley &
in fetal weight; no discussion of maternal effects Scott (1984a)
Mouse day 7, 8 or 9 of 31 or 62 mg/kg body dose-dependent embryolethality; non-closure of anterior or O'Shea &
pregnancy weight, iv; posterior neuropore; smaller embryos; no discussion of Kaufman
examination on day maternal effects (1979)
10 or 19 of gestation
Mouse day 9 of pregnancy 2, 4, 6% (8 ml/kg no intermediate cellular effeects Bannigan &
body weight), ip Burke (1982)
Mouse day 6, 7 or 8 of 62 mg/kg body weight, neural tube effects; embryonic mortality; no discussion O'Shea &
pregnancy; and days iv; examination on of maternal effects Kaufman
6-8, 7-8 or 7-9 of day 10 or 12 of (1981)
pregnancy gestation
Table 6. Tests for gene mutation, chromosomal damage, and sister chromatid exchanges induced by acetaldehyde
Test system Measured end-point Test conditions MA RES Reference
Prokaryotic systems
Escherichia coli WP2uvrA reverse mutations 40 µg/ml; preincubated in capped tubes - + Veghelyi et al. (1978)
Escherichia coli WP2uvrA reverse mutations 0.88-441 µg/ml; incubated in capped - - Hemminki et al. (1980)
tubes at 37°C
Escherichia coli Wp2uvrA reverse mutations 0.8 µg/ml; incubated in capped tubes - + Igali & Gazso (1980)
at 0°C
Salmonella typhimurium
TA102 reverse mutations 1014 µg/plate; liquid preincubation - - Marnett et al. (1985)
TA104 -
Salmonella typhimurium reverse mutations 33-10 000 µg/plate; liquid preincubation + - Mortelmans et al. (1986)
TA1535 - -
TA1537 + -
- -
TA97 + -
- -
TA98 + -
- -
TA100 + -
- -
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Salmonella typhimurium concentration not given; + - Sasaki & Endo (1978)
TA100 liquid preincubation
Non-mammalian eukaryotic systems
Allium cepa root chromosomal aberration 7.5-7500 µg/ml - + Cortés et al. (1986)
meristem cells and SCE +
Vicia faba root tips chromosomal aberration 220-2205 µg/ml for 24 h at 12°C - + Rieger & Michaelis (1960)
Caenorhabditis elegans gene mutation 794-7940 µg/ml for 2 h - + Greenwald & Horvitz
(1980)
Drosophila melanogaster sex-linked recessive 22 500 mg/litre in 10% ethanol by + Woodruff et al. (1985)
injection
Canton-S wild-type lethal mutations 25 000 mg/litre in 10% ethanol by -
feeding
Aspergilus nodulans chromosomal 198-2381 µg/ml + Crebelli et al. (1989)
Diploid P1 malsegregation
In vitro mammalian systems
Mouse lymphoma L5178Y forward mutation (tk 176-353 µg/ml for 4 h - + Wangenheim & Bolcsfoldi
cells locus) (1986, 1988)
Chinese hamster ovary chromosomal aberration 88-5000 µg/ml - + Au & Badr (1979)
cells
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Chinese hamster ovary SCE 1.3-13 µg/ml - + Brambilla et al. (1986)
cells
Chinese hamster ovary SCE 8-80 µg/ml for 1 h + + De Raat et al. (1983)
cells - +
Chinese hamster ovary SCE 2-12 µg/ml for 24 h - + Obe & Beek (1979)
cells
Chinese hamster ovary SCE 4-8 µg/ml for 8 days - + Obe & Ristow (1977)
cells
Chinese hamster ovary hyperploidy 0.35-1.05 mmol/litre - + Dulout & Furnus (1988)
cells hypoploidy +
Human lymphocytes forward mutations 26.5-106 µg/ml for 24 h - + He & Lambert (1990)
(hprt locus) 8.8-26.5 µg/ml for 48 h
Human lymphocytes chromosomal aberration 0.1-20 mmol/litre added + Obe et al. (1985)
every 12 h for 5 days
Human lymphocytes chromosomal aberration 8-15 µg/ml - + Obe et al. (1979)
(normal Franconis
anaemia)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 0.8 µg/ml 2x/day, 4 days - + Obe et al. (1978)
Human lymphocytes chromosomal aberration 4-48 µg/ml for 72 h - + Boehlke et al. (1983)
SCE +
Human (whole blood, chromosomal aberration 20-40 µg/ml + Badr & Hussain (1977)
alcoholics)
Human lymphocytes SCE 4.4-106 µg/ml for 1-70 h - + He & Lambert (1985)
Human lymphocytes SCE 4.4-13 µg/ml for 70 h - + Lambert & He (1988)
Human lymphocytes SCE 4.4-18 µg/ml for 72 h + Helander & Lindahl-
Kiessling (1991)
Human lymphocytes SCE 4.4-22 µg/ml for 48 h + Sipi et al. (1992)
Human lymphocytes SCE 4-8 µg/ml for 90 h - + Jansson (1982)
Human lymphocytes SCE 0.04-4.4 µg/ml for 55 h - + Knadle (1985)
Human lymphocytes SCE 5.5-88 µg/ml for 48 h - + Norppa et al. (1985)
Human lymphocytes SCE reaction mixture added to whole blood - + Obe et al. (1986)
samples in dialysis tube; + +
concentration not given
Human lymphocytes SCE 4-16 µg/ml for 24 h - + Ristow & Obe (1978)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 1.8-35 µg/ml for 72 h - + Véghelyi & Osztovics
(1978)
Human lymphocytes SCE 1.8-35 µg/ml for 72 h + Véghelyi et al. (1978)
Wistar rat (skin chromosomal aberration 4.4-44 µg/ml for 12, 24, or 48 h - + Bird et al. (1982)
fibroblast) (micronuclei)
Mouse embryo cell transformation 10-100 µg acetaldehyde/ml for 24 h - - Abernethy et al. (1982)
C3H/10T1/2 cells 10-100 µg/ml followed by 0.25 µg +
TPA/ml 2x/week
Rat (HRRT kidney cells) cell transformation acetaldehyde - - Eker & Sanner (1986)
acetaldehyde followed by TPA and PDD +
MA = Metabolic activation; RES = Result.
8.6.3 Cultured mammalian cells
8.6.3.1 Gene mutation assays
Acetaldehyde induced gene mutations at the hypoxanthineguanine
phosphoribosyl transferase (hprt) locus in human lymphocytes
in vitro; there was a statistically significant and dose-related
increase in the frequency of mutants (He & Lambert, 1990).
Acetaldehyde produced a significant dose-related increase in forward
mutations in a mouse lymphoma L5178Y thimidine kinase locus assay,
without exogenous metabolic activation (Wangenheim & Bolcsfoldi, 1986,
1988).
8.6.3.2 Chromosome alterations and sister chromatid exchange
Dose-dependent increased frequencies of chromosome aberrations
have been observed in human lymphocytes following incubation with
acetaldehyde (Badr & Hussain, 1977; Obe et al., 1978, 1979, 1985;
Veghelyi & Ostovics, 1978; Boehlke et al., 1983).
Acetaldehyde induced a dose-dependent increase in chromosomal
damage in Chinese hamster ovary cells, though it was reported that the
activity was reduced (not quantified) in the presence of liver
homogenate fraction (Au & Badr, 1979). Dose-related increases in
micronuclei and chromosome aberrations were observed in cultured rat
skin fibroblasts in the absence of metabolic activation (Bird et al.,
1982).
SCE was induced by acetaldehyde (1.3-106 µg/ml) in the absence of
exogenous metabolic activation in Chinese hamster ovary cells and
human lymphocyte cultures (Obe & Ristow, 1977; Ristow & Obe, 1978;
Veghelyi et al., 1978; Veghelyi & Osztovics, 1978; Obe & Beek, 1979;
Jansson, 1982; Boehlke et al., 1983; De Raat et al., 1983; He &
Lambert, 1985; Knadle, 1985; Norppa et al., 1985; Brambilla et al.,
1986; Obe et al., 1986; Lambert & He, 1988; Helander &
Lindahl-Kiessling, 1991; Sipi et al., 1992). The increase in SCEs in
Chinese hamster ovary cells was less pronounced after addition of an
exogenous metabolic activating system (De Raat et al., 1983).
Similarly, addition of NAD+ and ALDH to human lymphocyte cultures
exposed to acetaldehyde decreased SCE induction (Obe et al., 1986).
Addition of 1-amino-cyclopropanol, an inhibitor of ALDH, to human
lymphocytes enhanced SCE induction by acetaldehyde (Helander &
Lindahl-Kiessling, 1991). These observations suggest that ALDH may
reduce the genotoxic potential of acetaldehyde.
Acetaldehyde induced aneuploidy has been reported in Chinese
hamster ovary cells (Dulout & Furnus, 1988). However, mainly
hypoploid cells were increased and, therefore, no firm conclusion can
be drawn from these studies. Acetaldehyde induced SCE in
pre-implantation mouse embryos in vitro (Lau et al., 1991).
8.6.4 In vivo assays
Only limited data are available on the genotoxicity of
acetaldehyde in vivo.
8.6.4.1 Somatic cells
C57BL/6J mice (only 2 animals of unspecified sex at each dose
level) received daily intraperitoneal doses of 0, 6, or 12 mg
acetaldehyde/kg body weight per day, for 5 days, and blood samples
were collected on days 3-6. The frequencies of micronuclei in mature
peripheral erythrocytes on days 5 and 6 (combined) were significantly
increased at the low dose, but not at the high dose (Ma et al., 1985).
No information on the frequency in polychromatic erythrocytes was
provided, so the possibility that this result was due to increased
erythropoiesis instead of mutagenesis cannot be discounted. This
study was reported only in the form of an abstract.
Groups of 6-7 Chinese hamsters (male and female) received 0.01,
0.1, or 0.5 mg acetaldehyde/kg body weight in saline by single
intraperitoneal injection and were killed 24 h later. Doses of
0.6 mg/kg body weight were lethal in preliminary tests. The
frequencies of SCEs in bone-marrow metaphases were elevated at 0.5 mg
acetaldehyde/kg body weight, but not at the lower doses (Korte & Obe,
1981). These observations support earlier findings in a more limited
study at an embryotoxic dose in mice and indicate in vivo formation
of SCEs (Obe et al., 1979).
Female Wistar rats were injected with 0.02 ml of 1% acetaldehyde
intra-amniotically on day 13 of pregnancy. Embryonic cells were
obtained 24 h later for cytogenetic analysis. Exposed rat embryos had
a higher frequency of chromosomal aberrations (mostly chromatid gaps
and breaks) than controls (Barilyak & Kozachuk, 1983). There was no
increase in the frequency of aneuploid cells.
8.6.4.2 Germ cells
Hybrid male mice ((C57B1/6J × C3H/He)F1), 4/dose group, were
injected intraperitoneally with a single dose of 0, 125, 250, 375, or
500 mg acetaldehyde/kg body weight in saline. Thirteen days after
exposure, the frequency of micronuclei in early spermatids was not
increased, though there were adequate positive controls (Lahdetie,
1988).
8.6.5 Other assays
Relevant data are summarized in Table 7.
8.6.5.1 DNA single-strand breaks
No single-strand breaks were detected with the alkaline elution
assay in DNA from various sources, after exposure in vitro to
acetaldehyde (Sina et al., 1983; Marinari et al., 1984; Harris et al.,
1985; Lambert et al., 1985; Saladino et al., 1985).
8.6.5.2 DNA cross-linking
There is some indirect evidence suggesting DNA cross-linking by
acetaldehyde.
Ristow & Obe (1978) observed enhanced reannealing of heat
denatured, isolated, calf thymus DNA following incubation with
acetaldehyde. It was suggested that this may have been due to DNA-DNA
cross-linking, because thermally denatured DNA will not reanneal in
this assay, unless complimentary DNA strands are held together by
cross-linkings.
Altered alkaline elution patterns indicative of DNA-DNA
cross-links were observed with DNA isolated from cultured human
leukocytes and exposed initially to acetaldehyde and then to X-rays
(Lambert et al., 1985; Lambert & He, 1988). DNA-protein cross-linking
was not observed by Harris et al. (1985) or by Saladino et al. (1985)
after exposure of human bronchial epithelial cells in vitro, but it
was observed by Lam et al. (1986) after incubation of either calf
thymus nucleohistones or fresh homogenates of the nasal mucosa of rats
with acetaldehyde. The possibility that acetaldehyde induces
DNA-protein cross-links in male Fischer-344 rat nasal mucosa has been
indirectly studied by measuring the extractability of DNA from
insoluble proteins following in vitro and in vivo exposures.
Decreased extractability of DNA was observed in preparations from
tissue homogenates exposed to acetaldehyde at concentrations of 100
and 500 mmol per litre but not 10 mmol/litre. Similarly, decreased
extractability was observed in nasal mucosal preparations from rats
exposed for 6 h to 1000 or 3000 mg/litre (ppm) but not 100 or
300 mg/litre (ppm) (Lam et al., 1986).
Table 7. Other tests indicative of genetic damage induced by acetaldehyde
Test system Measured end-point Test conditions Results Reference
Calf thymus DNA DNA-DNA cross-links 44 100 µg/ml for 30 min + Ristow & Obe (1978)
Calf thymus nucleohistones DNA-protein cross-links 4410-44 100 µg/ml - Lam et al. (1986)
Chinese hamster ovary DNA single-strand breaks no details - Marinari et al. (1984)
K1-cells DNA-DNA cross-links +
Rat hepatocytes DNA single-strand breaks 1.3 µg/ml for 3 h - Sina et al. (1983)
Human bronchial epithelial DNA single-strand breaks unknown concentration; - Harris et al. (1985)
cells DNA-protein cross-links 6 h -
Human bronchial epithelial DNA single-strand breaks up to 44 µg/ml for 1 h - Saladino et al. (1985)
cells DNA-protein cross-links -
Human leukocytes DNA single-strand breaks 441-882 µg/ml for 4 h - Lambert et al. (1985)
DNA-DNA cross-links +
Human lymphocytes DNA-DNA cross-links 441 µg/ml + Lambert & He (1988)
8.6.6 Cell transformation
Acetaldehyde did not initiate cell transformation in cultured
C3H/10T1/2 cells in the presence of the tumour promotor
12- O-tetradecanoylphorbol-13-acetate (TPA) (Abernethy et al., 1982).
Acetaldehyde initiated cell transformation in a rat kidney cell
line (HRRT) after pretreatment with tumour promotors (Eker & Sanner,
1986).
8.7 Carcinogenicity bioassays
8.7.1 Inhalation exposure
Only one carcinogenicity study in which animals were exposed by
inhalation to acetaldehyde over a lifetime has been performed. In
other carcinogenicity bioassays, animals were exposed for shorter
periods.
Wistar rats (55/sex per dose) were exposed for life (6 h/day, 5
days/week, for 28 months) to acetaldehyde concentrations of 1350,
2700, or 1800-5400 mg/m3 (the last concentration was gradually
reduced from 5400 mg/m3 in week 20 to 1800 mg/m3 in week 52).
Satellite groups of 5-10 additional rats of each sex were killed at
13, 26, and 52 weeks. Growth retardation occurred throughout the
study at all dose levels. Mortality was greater than in controls in
all dose groups and all of the animals in the high-dose group had died
by week 102. At week 52, there were degenerative changes in the
olfactory nasal epithelium at all dose levels including slight to
severe hyperplasia and keratinized stratified metaplasia of the larynx
(high dose only) and degenerative changes of the upper respiratory
epithelium (including papillomatous hyperplasia at the top dose only).
In the trachea, there was focal flattening and irregular arrangement
of the epithelium in 3/10 top-dose males at 52 weeks. In satellite
groups of 30 rats per sex, for which there was a 26-week recovery
period after 52 weeks of exposure, there was evidence of partial
regeneration of the olfactory epithelium in the low- and mid-dose
groups; there was also progression from hyperplasia and metaplasia to
neoplasia in some animals. At 28 months, carcinomas of the nose
developed in all exposed groups (Table 8). Although tumour incidence
was dose-related, the latency period appeared to be independent of
concentration. First tumours in all groups appeared during the 12th
month of exposure. The incidence of tumours was not increased in the
lungs, larynx, and trachea.
In simultaneously exposed groups of 30 Wistar rats/sex per dose,
exposure was terminated after 52 weeks and the animals killed
following a recovery period of 26 weeks. After the recovery period,
both mortality and nasal tumour incidence were very similar to those
in the groups in which exposure had been continued for 26 more weeks
(Woutersen et al., 1984, 1986; Woutersen & Feron, 1987).
Groups of Syrian golden hamsters (36/sex per dose) were exposed
for 52 weeks (7 h/day, 5 days/week) to a concentration gradually
reduced from 4500 mg/m3 in week 9 to 2970 mg/m3 in week 52. At
week 52, six animals/sex per dose were killed; the remaining animals
(30/sex per dose) were killed after a recovery period of 29 weeks. At
the end of the recovery period, nasal carcinomas were observed in 1/26
males and 1/27 females versus 0/24 and 0/23 in controls, and the
incidence of laryngeal carcinomas was increased (5/23 in males, 3/20
in females, against 0/20 and 0/22 in controls). No tumours were
observed in bronchi or lungs (Feron et al., 1982).
Table 8. Incidence of nasal tumours in Wistar rats after 28 months
of exposurea,b
Types of tumour 0 1350 2700 2760c
mg/m3 mg/m3 mg/m3 mg/m3
Males
Squamous cell carcinoma 1/49 1/52 10/53* 16/49***
Adenocarcinoma 0/49 16/52*** 31/53*** 21/49***
Carcinoma in situ 0/49 0/52 0/53 1/49
Females
Squamous cell carcinoma 0/50 0/48 5/53 17/53***
Adenocarcinoma 0/50 6/48* 28/53*** 23/53***
Carcinoma in situ 0/50 0/48 3/53 5/53
a From: Woutersen et al. (1986).
b Total number of tumour-bearing animals not specified.
Significance: Fisher Exact Test *P < 0.05, **P < 0.01, ***P <
0.001.
c The highest concentration was gradually reduced from
5400 mg/m3 during the first 20 weeks to 1800 mg/m3 in week
52; the time-weighted average concentration for 28 months of
exposure was calculated by the Task Group to be 2760 mg/m3.
Groups of 35 male Syrian hamsters were exposed by inhalation to 0
or 2700 mg acetaldehyde/m3 (0 or 1500 ppm) for 7 h/day, 5 days/week
for 52 weeks. At 52 weeks, 5 animals were killed for pathological
examination. The remainder of the animals were maintained for a
further 26-week recovery period. Body weight gain was less in the
exposed animals and mortality was increased from week 52 onwards.
There was a slight, but significant, decrease in rbc, haemoglobin, and
haematocrit in exposed animals. Relative kidney weights were
increased and urinary levels of protein and GOT were elevated. There
were marked lesions in the nasal cavity of animals killed at 52 weeks:
flattened epithelial cells with bizarre nuclei, fewer subepithelial
glands, submucosal thickening, and keratinizing stratified squamous
metaplasia of olfactory and respiratory epithelium. There was also
slight focal hyperplasia and metaplasia of the epithelium of the
trachea. There was partial or complete recovery from these lesions in
the animals killed at the end of the recovery period. There were no
tumours of the respiratory tract in any of the animals exposed to
acetaldehyde (Feron, 1979).
Weekly intratracheal administration of 0.2 ml of 2 or 4%
acetaldehyde in saline during a period of 52 weeks, followed by a
recovery period of another 52 weeks, did not induce any tumours in the
respiratory tract of male and female Syrian golden hamsters (Feron,
1979).
8.7.2 Co-carcinogenicity and promotion studies
In a mid-term test (Ito Model), groups of 19-20 male F344 rats
received a single intraperitoneal injection of diethylnitrosamine and
then various concentrations of acetaldehyde (2.5% and 5%, associated
with a recorded daily intake of 1.66 and 2.75 mg/kg body weight) in
their drinking-water, from week 2 until termination in week 6. All
rats had a two-thirds partial hepatectomy in week 3, in order to
stimulate cell proliferation. At 6 weeks, there was a significant
decrease in liver and body weight in all exposed animals, but
acetaldehyde had no effect on the development of glutathione
S-transferase (placental type)-positive liver cell foci (Ikawa et
al., 1986).
There were laryngeal tumours in 7/31 male and 6/32 female Syrian
golden hamsters exposed to a mixture of isoprene (approximately
2.09 mg/m3 or 750 ppm), methyl chloride (approximately 1.94 mg/m3
or 950 ppm) methyl nitrite (approximately 0.49 mg/m3 or 195 ppm) and
acetaldehyde (approximately 2340 mg/m3 or 1300 ppm) for 6 h/day, 5
days/week, over 23 months. There were no tumours of the respiratory
tract in controls (Feron et al., 1985). The authors suggested that
the laryngeal effects observed in hamsters exposed to the vapour
mixture were most probably caused by acetaldehyde alone (presumably
based on comparison with results in studies on hamsters exposed to
acetaldehyde alone).
There was no evidence of co-carcinogenicity in Syrian golden
hamsters exposed either by simultaneous inhalation exposure to
acetaldehyde and intratracheal instillation of benzo(a)pyrene (Feron,
1979; Feron et al., 1982) or by intratracheal instillation of
diethylnitrosamine (Feron et al., 1982) for 52 weeks followed by
recovery periods of 26-28 weeks. Nor was there any evidence of the
co-carcinogenicity of acetaldehyde following simultaneous
intratracheal instillations of acetaldehyde and either benzo (a)pyrene
or diethylnitrosamine for 52 weeks, followed by a recovery period of
52 weeks (Feron et al., 1982).
8.8 Neurological effects
Secondary effects of acetaldehyde on the respiratory and
cardiovascular systems, attributed to its influence on the autonomic
nervous system, have been observed following acute exposure of
experimental animals to acetaldehyde, principally by infusion or
intravenous administration. In two identified studies in which
animals were exposed by a route more relevant to environmental or
occupational exposure (i.e., inhalation), a 50% decrease in
respiratory rate was observed in two strains of mice and in rats
during inhalation of 5000 mg/m3 for 10 min (Steinhagen & Barrow,
1984; Babiuk et al., 1985; Takahashi et al., 1986).
Neurological effects following exposure by routes most relevant
to environmental and occupational exposure (i.e., inhalation and
ingestion) were limited to biochemical changes in the brain in
short-term studies on rats and mice exposed to relatively high
concentrations of acetaldehyde (750-13 230 mg/m3). Reported effects
included changes in the phospholipid fractions and increases in the
concentrations of monoamines and Na+/K+ATPase (Ortiz et al., 1974;
Shiohara et al., 1985; Latge et al., 1987; Roumec et al., 1988).
Manifestations of neurotoxicity were not examined in any of these
studies.
In the only identified study in which histopathological effects
on the nervous system were reported, degeneration was observable using
both light and electron microscopy in the cerebral cortex of rats
receiving a single intraperitoneal injection of 5 mg acetaldehyde/kg
body weight (Phillips, 1987).
In a short-term study involving intravenous exposure (24-26 mg/kg
body weight per day, for 20 days), the level of salsolinol in the
brain of rats was increased (Myers et al., 1985). Biochemical effects
of acetaldehyde on the brain have also been observed in vitro in
cerebral cortical neurons and brain microsomal preparations (Lahti &
Majchrowicz, 1969; Cederbaum & Rubin, 1977; Kuriyama et al., 1987).
8.9 Immunological effects
8.9.1 Direct effects on immune cells
Only one study has been carried out on whole animals. In a study
on CD1 mice exposed to 324 mg/m3, 3 h/day for 5 days, the
bacteriocidal activity of alveolar macrophages was reduced by 15%.
However, acetaldehyde did not affect mortality following streptococcal
infection (Aranyi et al., 1986).
In in vitro studies, 0.01% acetaldehyde caused a significant
inhibition of human granulocyte chemotaxis (Schopf et al., 1985). At
0.03%, acetaldehyde inhibited the release of lysozyme from monocytes,
but granulocytes were unaffected. The generation of oxygen radicals
by zymosan particle-stimulated monocytes and granulocytes was
inhibited by acetaldehyde in a dose-dependent manner. In a similar
study in which peripheral mononuclear cells were incubated with
acetaldehyde, decreased lytic activity against K562 cells was observed
at concentrations higher than 12 µmol per litre (Fink & Dancygier,
1988). In another study, acetaldehyde decreased 3H thymidine
incorporation into phytohaemagglutinin or Concanavalin A - stimulated
human lymphocytes (Levallois et al., 1987).
8.9.2 Generation of antibodies reacting with acetaldehyde-modified
proteins
The modification of proteins has been described in section 6.5.1.
In several studies, these modified proteins induced an immune response
when injected into rabbits and mice (Israel et al., 1986; Worrall et
al., 1989). The reactivity of the resulting antibodies towards
proteins, modified by acetaldehyde in vitro, is independent of the
carrier protein. These studies demonstrate (a) that
acetaldehyde-modified proteins are immunogenic; and (b) that
antibodies raised against a single modified protein will cross-react
with any other protein modified in the same manner.
Passive immunization of rats with a monoclonal IgE
antiacetaldehyde adduct antibody, derived from mice immunized with
acetaldehyde-modified proteins, rendered these animals hypersensitive
to acetaldehyde-modified proteins (Israel et al., 1992).
No immunization studies analogous to those described above have
been carried out on humans. In several studies, the presence of
antibodies, reactive with proteins modified by acetaldehyde in vitro,
has been reported in the plasma of alcoholics (Niemela et al., 1987;
Hoerner et al., 1988; Worrall et al., 1990, 1991a,b). The results of
these studies suggest that acetaldehyde-modified proteins are
generated in humans.
8.9.3 Related immunological effects
In rat liver membrane vesicles, exposed to acetaldehyde in vitro,
superoxide anion production by neutrophils was significantly enhanced
(Williams & Barry, 1986). In a further study, human liver plasma
membranes, modified by acetaldehyde, activated complement protein C3
components (Barry & McGivan, 1985).
8.10 Biochemical effects
Biochemical effects have been observed in cultured hepatocytes,
liver homogenates and mitochondrial fractions, purified enzymes,
isolated hepatic lipid membranes and whole liver preparations,
including: metabolic effects on lipid peroxidation (Stege, 1982;
Mueller & Sies, 1987; Shaw & Jayatilleke, 1987), phospholipid
metabolism (Snyder, 1988), transaminase activity (Solomon, 1987;
Snyder, 1988), carbohydrate and lipid metabolism (Matsuzaki & Lieber,
1977; Cederbaum & Dicker, 1981), and mitochondrial respiration
(Cederbaum et al., 1974). Increased hepatic collagen synthesis has
also been observed (Savolainen et al., 1984; Brenner & Chojkier,
1987).
Metabolic effects have been observed in other in vitro systems
including: renal cortex tubules (Michoudet & Baverel, 1985: altered
pyruvate/lactate metabolism), kidney, and muscle microsomal
preparations (Cederbaum & Rubin, 1977: inhibition of pyruvate
dehydrogenase), perfused hearts and cardiac whole homogenates
(Schreiber et al., 1972, 1974: myocardial protein synthesis and
inhibition of cardiac microsomal protein synthesis; Rawat, 1979:
reduced protein synthesis; Segel, 1984: no effect on mitochondrial
respiration), myocardial cells (McCall & Ryan, 1987: no effect on
Na+/K+-ATPase), leukocytes (Green & Baron, 1986: inhibition of
Na+/K+-ATPase), erythrocytes (Helander & Tottmar, 1987: inhibition
of the disappearance rate of biogenic aldehydes; Ninfali et al., 1987:
induction of intracellular reduced state; Solomon, 1988: inhibition of
aldolase-mediated by acetaldehyde oxidation- and inhibition of the
aminotransferases-mediated by non-oxidative acetaldehyde
metabolization; Atukorala et al., 1988: inhibition of transketolase),
and leukocytes and platelets (Helander & Tottmar, 1987: inhibition of
the disappearance rate of biogenic aldehydes).
9. EFFECTS ON HUMANS
9.1 General population exposure
No specific studies were available.
9.2 Occupational exposure
9.2.1 General observations
Acute exposure to acetaldehyde vapours has resulted in irritation
of the eyes and mucous membranes, reddening of the skin, pulmonary
oedema, headache, and sore throat. Repeated exposure causes
dermatitis and conjunctivitis. Ingestion causes nausea, vomiting,
diarrhoea, narcosis, and respiratory failure. Liquid acetaldehyde was
reported to cause superficial injury of the cornea. These effects
were reported in reviews and not in documented studies (Grant, 1974;
Hagemeyer, 1978; Dreisbach, 1987). In addition, a statement was found
in a textbook stating that prolonged exposure to acetaldehyde caused a
decrease in red and white blood cells plus a sustained rise in blood
pressure; however, no further information was available (Hagemeyer,
1978).
9.2.2 Clinical studies
A group of 12 volunteers were exposed in a chamber to various
nominal concentrations of acetaldehyde vapour on 15 different
occasions. No atmospheric sampling was performed. At 90 mg/m3
(50 ppm), the majority of the group experienced some degree of eye
irritation. Several subjects were discomforted at a lower
concentration of 45 mg/m3 (25 ppm), but no details were provided
(Silverman et al., 1946).
A group of 14 "healthy male" volunteers, aged 18-45 years, were
exposed in a 100 m3 chamber to a measured concentration of
acetaldehyde vapour of 240 mg/m3 (134 ppm) for 30 min. This
concentration was said to be mildly irritating to the upper
respiratory tract. No other clinical signs were reported (Sim &
Pattle, 1957).
Twelve volunteers of Oriental ancestry were patch-tested with
acetaldehyde (75%), ethanol (75%), and ethyl acetate (25%). With
acetaldehyde treatment, cutaneous erythema was seen in all 12
subjects, whereas ethanol was positive in 5 subjects. No vascular
response was detected for the acetaldehyde metabolite, ethyl acetate
(Wilkin & Fortner, 1985).
In an additional study, increased heart rate, ventilation, and
calculated respiratory dead space, and a decrease in alveolar CO2
levels were reported, following intravenous infusion of acetaldehyde
(5% v/v) in young male volunteers (Asmussen et al., 1948).
9.2.3 Epidemiological studies
A very limited investigation was performed on an unspecified
number of people, selected from 150 workers who had been employed for
20 years or more in a chemical factory. Nine cases of cancer were
identified (all in male smokers) 5 of which were in the respiratory
tract, 2 in the oral cavity, 1 in the stomach, and 1 in the caecum.
The number of respiratory cancers was higher than expected compared
with the prevalence in the national population (not corrected for
smoking). In parts of the factory, there was exposure to a variety of
chemicals. In one area, 1-7 mg acetaldehyde/m3 had been measured,
together with 5-70 mg butyraldehyde/m3, 1-7 mg crotonaldehyde/m3,
2-6 mg n-butanol per m3, and about 15 mg ethylhexanol/m3. Other
areas of the factory were known to have higher concentrations of
vapours of aldehydes and aldol, which caused irritation of the eyes
and upper respiratory tract (Bittersohl, 1974, 1975).
9.3 Effects of endogenous acetaldehyde
9.3.1 Effects of ethanol possibly attributable to acetaldehyde or
acetaldehyde metabolism
There is no direct evidence to link acetaldehyde with liver
injury. However, data obtained from animal models and human subjects
suggest that acetaldehyde may play a role in liver damage, especially
that associated with ethanol (sections 3.1.1 and 8.10).
An increased sensitivity to ethanol with respect to facial
flushing was observed in certain human populations (especially of
Oriental origin), which was ascribed to genetic differences in
acetaldehyde elimination, due to aldehyde dehydrogenase polymorphism
(Goedde & Agarwal, 1986, 1987; Eriksson, 1987; see also section
6.2.2).
The fetal alcohol syndrome is a specific pattern of congenital
abnormalities found in children of mothers who drink heavily. The
abnormalities most typically associated with alcohol-induced
developmental effects can be grouped into four categories: central
nervous system dysfunction (mental retardation, microcephaly,
hyperactivity); growth deficiencies (reduced prenatal length and
especially weight, with no catch-up postnatal growth; reduced adipose
tissue, normal growth hormone, cortisol, and gonadotropins); a
characteristic cluster of facial abnormalities (short palpebral
fissures, short upturned nose, hypoplastic philtrum, thinned upper-lip
vermillion, flattened midface); and variable major and minor
malformations (such as, heart failure, anomalies of the genitalia,
joints, and palmar creases) (Clarren & Smith, 1978).
The mechanisms by which ethanol produces its developmental
effects are not completely understood, and no data are available with
respect to ethanol doses and/or blood levels needed for such effects.
It is generally assumed that acetaldehyde, as the primary metabolite,
may contribute to the developmental effects of ethanol. Several
animal studies have demonstrated the direct teratogenic effect of
acetaldehyde (Ali & Persaud, 1988; see also section 8.5); however, no
reports are available with respect to the direct teratogenicity of
acetaldehyde in humans.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
By far, the principal source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure for
smokers. With respect to other media, the general population is
exposed to acetaldehyde principally from food, and to a lesser extent
from air. Intake from drinking-water is negligible compared with that
from other media.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route is most likely inhalation and, possibly, dermal
contact.
10.1.2 Health effects
Acetaldehyde is a reactive molecule that adducts to
macromolecules and can also condense or polymerise with small
molecules. Although quantitatively unimportant in acetaldehyde
metabolism, these by-products may have important biological effects.
In studies conducted on animals, the acute toxicity of
acetaldehyde by the oral or inhalation routes was low. Oral LD50s
ranged from 660 to 1930 mg/kg body weight and LC50s (0.5-4 h) from
24 to 37 g/m3. RD50s in rats and mice are slightly greater than
5000 mg/m3.
Data on the irritant effects of acetaldehyde on the skin and eye
are restricted to those from limited studies on human volunteers. It
was mildly irritating to the eyes and upper respiratory tract
following acute exposure for very short periods, in limited studies on
human volunteers at concentrations exceeding approximately 90 and
240 mg/m3, respectively (Silverman et al., 1946; Sim & Pattle,
1957). Cutaneous erythema was observed in the patch testing of twelve
subjects of "Oriental ancestry" with acetaldehyde (Wilkin & Fortner,
1985). Although a possible mechanism has been identified, available
data are inadequate to assess the potential of acetaldehyde to induce
sensitization.
Available data relevant to the assessment of the potential
adverse effects in humans of exposure to acetaldehyde in the
occupational and general environments are limited to those on
irritation mentioned above. The remainder of this evaluation is,
therefore, based on studies on animals.
Effects of acetaldehyde following oral administration to animals
have been much less well studied than those following inhalation. In
one of the two available studies by the oral route, the
no-observed-effect level (NOEL) following four weeks of administration
of acetaldehyde to rats was 125 mg/kg body weight per day (Til et al.,
1988). At the next higher concentration (675 mg/kg body weight per
day), a borderline increase in hyperkeratosis of the forestomach was
observed. In rats exposed to 0.05% acetaldehyde (estimated by the
Task Group to be approximately 40 mg/kg body weight) in the
drinking-water for 6 months, there was an increase in collagen
synthesis in the liver (Bankowski et al., 1993). No other effects
were examined and the toxicological significance of this observation
is unknown.
Acetaldehyde has induced gene mutations, clastogenic effects, and
sister chromatid exchanges (SCE) in mammalian cells in vitro.
Though available data on genotoxicity in vivo are limited, increases
in SCE have been observed in hamsters and mice exposed
intraperitoneally to acetaldehyde (Obe et al., 1979; Korte & Obe,
1981). This suggests genetic damage to somatic cells in vivo.
Available data are inadequate to assess the potential of acetaldehyde
to induce genetic damage in mammalian germ cells in vivo.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In the
respiratory tract, degenerative changes of the olfactory epithelium in
rats and trachea in hamsters have been observed at the lowest
concentrations. Degenerative changes in the respiratory epithelium and
larynx have been observed at higher concentrations. In available
studies, the lowest concentration at which effects were observed
(degenerative changes in the olfactory epithelium of rats) was
437 mg/m3 following administration for 5 weeks (Saldiva et al.,
1985). The NOELs identified for respiratory effects were 275 mg/m3
in rats exposed for 4 weeks (Appelman et al., 1986) and 700 mg/m3 in
hamsters exposed for 13 weeks (Kruysse et al., 1975).
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses of 1350 mg/m3 and greater)
(Woutersen et al., 1986) and non significant increases in laryngeal
and nasal carcinomas in hamsters (Feron et al., 1982). All
concentrations of acetaldehyde administered in these studies induced
tissue damage in the respiratory tract. On the basis of limited
available data, no conclusions can be drawn concerning the potential
of acetaldehyde to promote tumours.
The distribution of nasal lesions induced in rats exposed to
acetaldehyde in inhalation studies correlates with regional
deficiencies in ALDH, observed in a different strain of rats,
prompting the authors to suggest that regional susceptibility to the
toxic effects may be due, at least in part, to a lack of ALDH in the
susceptible regions (Bogdanffy et al., 1986).
Available data are inadequate for assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in occupationally exposed or
general populations.
10.1.3 Approaches to risk assessment
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Though the
principal sources of exposure to acetaldehyde in the general
population are through the metabolism of alcohol, in cigarette smoke,
and food, air is believed to be the main route of exposure in the
occupational environment. In addition, available data are inadequate
to provide guidance concerning the potential risks associated with
oral exposure to acetaldehyde. On this basis, only air is addressed
here.
On the basis of data on irritancy in humans, a tolerable
concentration can be derived as follows:
45 mg/m3
Tolerable concentration = = 2 mg/m3 (2000 µg/m3)
20
where:
no effects were observed in a limited study on human volunteers at
45 mg/m3 (Silverman et al., 1946) and 20 is the uncertainty factor
(×10 for intraspecies variation and × 2 for the poor quality of the
data).
Data suggest that acetaldehyde causes genetic damage to
somatic cells in vivo. The irritancy of acetaldehyde may also play
an important role in the development of tumours in the nose and larynx
of rats and hamsters, respectively, exposed by inhalation, though all
concentrations of acetaldehyde administered in carcinogenesis
bioassays induced both irritancy and nasal tumours. Therefore, two
approaches were adopted for the provision of guidance with respect to
the potential carcinogenicity of acetaldehyde.
In the first, a tolerable concentration (TC) was derived on the
basis of division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, based on the principles
outlined in WHO (in press) and the assumption that there is a
threshold for acetaldehyde-induced cancer of the respiratory tract in
rodents exposed via inhalation. There is some support for this
approach on the basis of relevant data on the analogues of
acetaldehyde, i.e., formaldehyde and glutaraldehyde, which have
similar spectra of in vitro mutagenic effects, but are clearly not
mutagenic in vivo (IARC, 1985; WHO, 1989). Thus,
275 mg/m3
Tolerable concentration = = 0.3 mg/m3 (300 µg/m3)
1000
where:
275 mg/m3 was the NOEL for irritation in rats in a 4-week study
(Appelman et al., 1986) and 1000 is the uncertainty factor (×10 for
interspecies variation, ×10 for intraspecies variation and ×10 for a
less than long-term study and severity of effect, i.e.,
carcinogenicity associated with irritation).
Since the mechanism of induction of tumours by acetaldehyde has
not been well studied, lifetime cancer risk has also been estimated on
the basis of a default model (i.e., linearized multistage) (WHO, in
press). However, it is very likely that, since estimated risk is
based on tumour incidence at concentrations that induce irritancy in
the respiratory tract and no-observed-effect levels for irritancy are
well below these concentrations, the true cancer risk is most likely
much lower at concentrations generally present in the environment and
may, indeed, be zero.
Concentrations associated with a 10-5 excess lifetime risk (lower
95% confidence limits) for nasal tumours (adenocarcinomas, squamous
cell carcinomas, and carcinomas in situ) in male and female rats, in
the only carcinogenicity study in which animals were exposed via
inhalation to acetaldehyde over the lifetime (Woutersen et al., 1986),
calculated on the basis of the linearized multistage model (Global
82), are 11-65 µg/m3. The high-dose animal groups were excluded in
the derivation of these estimates because of early mortality. A body
surface area correction was not incorporated.
10.2 Evaluation of effects on the environment
Acetaldehyde enters the environment during industrial production,
as a product of incomplete combustion (e.g., in vehicle exhausts) and
as a product of alcohol fermentation. Once released,
intercompartmental transport of acetaldehyde is expected to be
limited, because of its reactivity. However, because of its high
vapour pressure and low tendency for sorption onto soil, it is most
likely to be present in air. Acetaldehyde is readily biodegradable;
approximate half-lives are 10-60 h and 1.9 h in air and water,
respectively.
No quantitative data on levels in ambient water were identified.
However, on the basis of concentrations measured in drinking-water,
levels in the aquatic environment are expected to be low (i.e., less
than 0.1 µg/litre). Concentrations in ambient air average about
5 µg/m3.
Acetaldehyde is slightly toxic for fish. The lowest reported
LC50 was 35 µg/litre (Poecilia reticulata). No long-term toxicity
tests have been performed. Acetaldehyde is toxic for micro-organisms
at relatively low concentrations. Seed germination of several plants
was inhibited by more than 50% after exposure to 1.52 mg/litre (3 h).
There were no effects on lettuce exposed to 0.36 µg/m3 acetaldehyde.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
11. RECOMMENDATIONS FOR RESEARCH
1. Additional studies on the fate in the environment and impact of
acetaldehyde on biota.
2. Additional information on exposure of workers in the occupational
environment and levels in the vicinity of industrial facilities.
3. Carcinogenesis bioassays by the inhalation route including doses
that do not induce cytotoxicity (e.g., 275 mg/m3).
4. Studies on the biological significance of byproducts of
acetaldehyde, such as acetaldehyde-modified proteins.
5. Studies on pathogenicity, reproductive and developmental
toxicity, and genotoxicity in vivo by relevant routes of
exposure.
6. Additional studies on toxicity following ingestion in
experimental animals.
7. Studies of irritation in workers exposed to acetaldehyde.
References
Abernethy DJ, Frazelle JH, & Boreiko CJ (1982) Effects of ethanol,
acetaldehyde and acetic acid in the C3H/10TI/2C18 cell transformation
system. Environ Mutagen, 4: 331.
Agarwal DP & Goedde HW (1990) Alcohol metabolism, alcohol intolerance,
and alcoholism. Berlin, Heidelberg, New York, Springer-Verlag, 184 pp.
Aharoni Y & Barkai-Golan R (1973) Sensitivity to acetaldehyde vapors
of Alterneria tenuis and Stemphylium botryosum. Phytopathol Z,
78: 57-61.
Aharoni Y & Stadelbacher GJ (1973) The toxicity of acetaldehyde vapors
to postharvest pathogens of fruits and vegetables. Phytopathology,
63: 544-545.
Aharoni Y, Stewart JK, Hartsell PL, & Young DK (1979) Acetaldehyde - a
potential fumigant for control of the Green peach aphid on harvested
head lettuce. J Econ Entomol, 72: 493-495.
Ahrenholz S & Gorman R (1980) Health and hazard evaluation
determination. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health (Report No. HE 80-2-727).
Ali F & Persaud TVN (1988) Mechanisms of fetal alcohol effects: role
of acetaldehyde. Exp Pathol, 33(1): 17-21.
Almuaibed AM & Townshend A (1987) Determination of acetaldehyde by
flow-injection analysis with soluble or immobilized aldehyde
dehydrogenase. Anal Chim Acta, 198: 37-44.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Anderson RA, Quigg JM, Oswald C, & Zaneveld LJD (1985) Demonstration
of a functional blood-testis barrier to acetaldehyde; evidence for
lack of acetaldehyde effect on ethanol-induced depression of
testosterone in vivo. Biochem Pharmacol, 34(5): 685-695.
Aoyama T & Yashiro T (1983) Analytical study of low-concentration
gases. Part 2: Collection of acetaldehyde at low concentrations in air
in a solid reaction tube and its analysis using a flame thermionic
detector. J Chromatogr, 265: 45-55.
Appelman LM, Woutersen RA, & Feron VJ (1982) Inhalation toxicity of
acetaldehyde in rats. I. Acute and subacute studies. Toxicology,
23: 293-307.
Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, & Notten WRF (1986)
Effect of variable versus fixed exposure levels on the toxicity of
acetaldehyde in rats. J Appl Toxicol, 6(5): 331-336.
Aranyi C, O'Shea WJ, Graham JA, & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6(4): 713-720.
Arntz RR & Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmos Environ, 15(9): 1643-1651.
Asai M, Narita O, & Kashiwamata S (1985) Effects of acetaldehyde
and/or ethanol on neutral amino acid transport systems in microvillous
brush border membrane vesicles prepared from human placenta.
Experientia (Basel), 41(12): 1566-1568.
Asmussen E, Hald J, & Larsen V (1948) The pharmacological action of
acetaldehyde on the human organism. Acta Pharmacol, 4: 311-320.
Atkinson R & Pitts JN (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-426
K. J Chem Phys, 68(8): 3581-3584.
Atkinson R, Plum CN, Carter WPL, Winer AM, & Pitts JN (1984) Rate
constants for the gas-phase reactions of nitrate radicals with a
series of organics in air at 298 +/- 1 K. J Phys Chem, 88: 1210-1215.
Atkinson R, Aschmann SM, & Goodman MA (1987) Kinetics of the gas-phase
reactions of NO radicals with a series of alkynes, haloalkenes, and
a,b-unsaturated aldehydes. Int J Chem Kinet, 19: 299-307.
Atukorala TMS, Duggleby RG, & Nixon PF (1988) Effects of acetaldehyde
upon catalysis by human erythrocyte transketolase. Biochem Pharmacol,
37(10): 2100-2101.
Au W & Badr FM (1979) Does ethanol induce chromosomal damage?
In Vitro, 15: 221.
Babiuk C, Steinhagen WH, & Barrow CS (1985) Sensory irritation
response to inhaled aldehydes after formaldehyde pretreatment. Toxicol
Appl Pharmacol, 79(1): 143-149.
Badr FM & Hussain F (1977) Action of ethanol and its metabolite
acetaldehyde in human lymphocytes. In vivo and in vitro study.
Genetics, 86: s2-s3.
Bagnall GN & Sidebottom HW (1984) Photooxidation of acetaldehyde. In:
Proceedings of the Third Symposium on Physical and Chemical Behaviour
of Atmospheric Pollutants, Varese, Italy. Luxembourg, Commission of
the European Communities, pp 188-193 (EUR 9436).
Bankowski E, Pawlicka E, & Sobolewski K (1993) Liver collagen of rats
submitted to chronic intoxication with acetaldehyde. Mol Cell Biochem,
121: 37-43.
Baraona E, Di Padova C, Tabasco J, & Lieber CS (1987) Red blood cells:
a new major modality for acetaldehyde transport from liver to other
tissues. Life Sci, 40: 253-258.
Barilyak IR & Kozachuk SY (1983) [Embryotoxic and mutagenic activity
of ethanol and acetaldehyde after intra-amniotic injection.] Citol
Genet, 17: 57-60 (in Russian).
Barry RE & McGivan JD (1985) Acetaldehyde alone may initiate
hepatocellular damage in acute alcoholic liver disease. Gut,
26(10): 1065-1069.
Bell RP & Clunie JC (1952) The hydration of acetaldehyde in aqueous
solution. Trans Faraday Soc, 48: 439-442.
Berni RJ & Stanley JB (1982) Volatiles from cotton plant parts. Text
Res J, 52: 539-541.
Binding N, Thiewens S, & Witting U (1986) [Specific determination of
formaldehyde and other aldehydes and ketones for stationary and
personal exposure monitoring.] Staub-Reinhalt Luft, 46(10): 444-446
(in German).
Bird RP, Draper HH, & Basrur PK (1982) Effect of malonaldehyde and
acetaldehyde on cultured mammalian cells. Production of micronuclei
and chromosomal aberrations. Mutat Res, 101: 237-246.
Bittersohl G (1974) [Epidemiologic investigations on cancer incidence
in workers contacted by acetaldol and other aliphatic aldehydes.] Arch
Geschwulstforsch, 43: 172-176 (in German).
Bittersohl G (1975) Epidemiological research on cancer risk by aldol
and aliphatic aldehydes. Environ Qual Saf, 4: 235-238.
Blakley PM & Scott WR Jr (1984a) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. I. Teratogenicity of
ethanol and acetaldehyde. Toxicol Appl Pharmacol, 72: 355-363.
Blakley PM & Scott WR Jr (1984b) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. II. Pharmacokinetics of
the placental transfer of ethanol and acetaldehyde. Toxicol Appl
Pharmacol, 72: 364-371.
Boehlke JU, Singh S, & Goedde HW (1983) Cytogenetic effects of
acetaldehyde in lymphocytes of Germans and Japanese: SCE, clastogenic
activity, and cell cycle delay. Hum Genet, 63: 285-289.
Boettner EA, Ball GL, & Weiss B (1973) Combustion products from the
incineration of plastics. Cincinnati, Ohio, US Environmental
Protection Agency, pp 1-5, 106-122 (EPA-670/2-73-049; PB-222.001).
Bogdanffy MS, Randall HW, & Morgan KT (1986) Histochemical
localization of aldehyde dehydrogenase in the respiratory tract of the
Fisher 344 rat. Toxicol Appl Pharmacol, 82: 560-567.
Booze TF & Oehme FW (1986) An investigation of metaldehyde and
acetaldehyde toxicities in dogs. Fundam Appl Toxicol, 6: 440-446.
Bradow JM & Connick WJ (1988) Seed-germination inhibition by volatile
alcohols and other compounds associated with Amaranthus palmeri
residues. J Chem Ecol, 14(7): 1633-1648.
Brambilla G, Sciaba L, Faggin P, Maura A, Marinari UM, Ferro M, &
Esterbauer H (1986) Cytotoxicity, DNA fragmentation and sister
chromatid exchange in Chinese hamster ovary cells exposed to the lipid
peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat
Res, 171: 169-176.
Brenner DA & Chojkier M (1987) Acetaldehyde increases collagen gene
transcription in cultured human fibroblasts. J Biol Chem,
262(36): 17690-17695.
Brien JF & Loomis CW (1983) Pharmacology of acetaldehyde. Can J
Physiol Pharmacol, 61: 1-22.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data. Final Report (Contract
No. 68-02-3542). Menlo Park, California, US Environmental Protection
Agency.
Buehler R, Pestalozzi D, Hess M, & Von Wartburg JP (1983)
Immunohistochemical localization of alcohol dehydrogenase in human
kidney, endocrine organs and brain. Pharmacol Biochem Behav,
18(Suppl 1): 55-59.
Buyske DA, Owen LH, Wilder P, & Hobbs ME (1956) Chromatography of the
2,4-dihydrophenyl-hydrazones of some aldehydes and ketones in tobacco
smoke. Ann Chem, 28: 910-913.
Campbell MA & Fantel AG (1983) Teratogenicity of acetaldehyde
in vitro: relevance to the fetal alcohol syndrome. Life Sci,
32: 2641-2647.
Casanova-Schmitz M, David RM, & Heck Hd'A (1984) Oxidation of
formaldehyde and acetaldehyde by NAD+-dependent dehydrogenases in rat
nasal mucosal homogenates. Biochem Pharmacol, 33(7): 1137-1142.
Cavanagh LA, Schadt CF, & Robinson E (1969) Atmospheric hydrocarbon
and carbon monoxide measurements at Point Barrow, Alaska. Environ Sci
Technol, 3(3): 251-257.
Cederbaum AI & Dicker E (1981) Effect of cyanamide on the metabolism
of ethanol and acetaldehyde and on gluconeogenesis by isolated rat
hepatocytes. Biochem Pharmacol, 30: 3079-3088.
Cederbaum AI & Rubin E (1977) Sensitivity to acetaldehyde of pyruvate
oxidation by mitochondria from liver, kidney, brain and muscle.
Biochem Pharmacol, 26: 1349-1353.
Cederbaum AI, Lieber CS, & Rubin E (1974) The effect of acetaldehyde
on mitochondrial function. Arch Biochem Biophys, 161: 26-39.
Checiu M, Sandor S, & Garban Z (1984) The effect of ethanol upon early
development in mice and rats. 6. In vivo effect of acetaldehyde upon
preimplantation stages in rats. Rev Roum Morphol Embryol Physiol
Morphol Embryol 30: 175-184.
Chou WL & Speece RE (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
Chrostek WJ (1981) Health hazard evaluation report No.
HETA-81-098-941. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Chrostek WJ & Schoemaker WE (1981) Health hazard evaluation report No.
HETA-81-298-944. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Clarke DW, Steenaart NAE, Slack CJ, & Brien JF (1986) Pharmacokinetics
of ethanol and its metabolite, acetaldehyde, and fetolethality in the
third-trimester pregnant guinea pig for oral administration of acute,
multiple-dose ethanol. Can J Physiol Pharmacol, 64: 1060-1067.
Clarke DW (1988) The disposition of ethanol and its proximate
metabolite, acetaldehyde, in the maternal-fetal unit of the nearterm
guinea pig and pregnant ewe. Diss Abstr Int, 48(10): 2932B-2933B.
Clarren SK & Smith DW (1978) The fetal alcohol syndrome. New Engl J
Med, 298: 1063-1067.
Collins MA, Nijm WP, Borge GF, Teas G, & Goldfarb C (1979)
Dopamine-related tetrahydroisoquinolines: significant urinary
excretion by alcoholics after alcohol consumption. Science,
206: 1184-1186.
Cortes F, Mateos S, & Escalza P (1986) Cytotoxic and genotoxic effects
of ethanol and acetaldehyde in root-meristem cells of Allium cepa.
Mutat Res, 171(2-3): 139-143.
Crabb DW, Edenberg HJ, Bosron WF, & Li TK (1989) Genotypes for
aldehyde dehydrogenase deficiency and alcohol sensitivity. J Clin
Invest, 83: 314-316.
Crebelli R, Conti G, Conti I, & Carere A (1989) A comparative study of
ethanol and acetaldehyde as inducers of chromosome malsegregation in
Aspergillus nidulans. Mutat Res, 215: 187-195.
Delcour JA, Caers JM, Dondeyne P, Delvaux F, & Robberechts E (1982) An
enzymic assay for the determination acetaldehyde in beers. J Inst
Brew, 88: 384-386.
Deneer JW, Seinen W, & Hermens JLM (1988) The acute toxicity of
aldehydes to the guppy. Aquat Toxicol, 12: 185-192.
De Raat WK, Davis PB, & Bakker GL (1983) Induction of sister-chromatid
exchanges by alcohol and alcoholic beverages after metabolic
activation by rat-liver homogenate. Mutat Res, 124: 85-90.
Di Padova C, Alderman J, & Lieber CS (1986) Improved methods for
measurement of acetaldehyde concentrations in plasma and red blood
cells. Alcohol Clin Exp Res, 10(1): 86-89.
Dreisbach RH (1987) Handbook of poisoning, 9th ed. Los Altos,
California, Lange, pp 185-188.
Dulout FN & Furnus CC (1988) Acetaldehyde-induced aneuploidy in
cultured Chinese hamster cells. Mutagenesis, 3(3): 207-211.
Egle JL (1970) Retention of inhaled acetaldehyde in man. J Pharmacol
Exp Ther, 174(1): 14-19.
Eimutis EC, Quill RP, & Rinaldi GM (1978) Source assessment
noncriteria pollutant emissions (1978 update). Research Triangle Park,
North Carolina, US Environmental Protection Agency, Industrial
Environmental Research Laboratory, pp 1-5 (PB-291 747).
Eker P & Sanner T (1986) Initiation of in vitro cell transformation
by formaldehyde and acetaldehyde as measured by attachment-independent
survival of cells in aggregates. Eur J Cancer Clin Oncol,
22(6): 671-676.
Eriksson CJP (1987) Genetic aspects of the relation between alcohol
metabolism and consumption in humans. Mutat Res, 186(3): 241-247.
Eriksson CJP & Fukunaga T (1993) Human blood acetaldehyde (update
1991). Alcohol Alcohol, 28(Suppl 2): 9-25.
Eriksson CJP, Mizoi Y, & Fukunaga T (1982) The determination of
acetaldehyde in human blood by the perchloric acid precipitation
method: The characterization and elimination of artefactual
acetaldehyde formation. Anal Biochem, 125: 259-263. Espinet C &
Argiles JM (1984) Ethanol and acetaldehyde concentrations in the rat
foeto-maternal system after an acute ethanol administration given to
the mother. Arch Int Physiol Biochim, 92: 339-344.
Facchini MC, Chiavari G, & Fuzzi S (1986) An improved HPCL method for
carbonyl compound speciation in the atmospheric liquid phase.
Chemosphere, 15(6): 667-674.
Feron VJ (1979) Effects of exposure to acetaldehyde in Syrian hamsters
simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Prog
Exp Tumor Res, 24: 162-176.
Feron VJ & De Jong D (1971) Acute intratracheal toxicity of
acetaldehyde in Syrian golden hamsters. Zeist, Central Institute for
Nutrition and Food Research, TNO (Report No. R 3600).
Feron VJ, Kruysse A, & Woutersen RA (1982) Respiratory tract tumors in
hamsters exposed to acetaldehyde vapour alone or simultaneously to
benzo[a]pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol,
18: 13-31.
Feron VJ, Kuper CF, Spit BJ, Renzel PGJ, & Woutersen RA (1985) Glass
fibers and vapor phase components of cigarette smoke as factors in
experimental respiratory tract carcinogenesis. In: Carcinogenesis. New
York, Raven Press, pp 93-118.
Feron VJ, Til HP, Vrijer F, Woutersen RA, Cassee FR, & van Bladeren PJ
(1991) Aldehydes: occurrence, carcinogenic potential, mechanism of
action and risk assessment. Mutat Res, 259: 363-385.
Fink R & Dancygier H (1988) Acetaldehyde inhibits the activity of
human natural killer cells. Gastroenterology, 94(5): A127.
Fraenkel-Conrat H & Singer B (1988) Nucleoside adducts are formed by
cooperative reaction of acetaldehyde and alcohols: possible mechanism
for the role of ethanol in carcinogenesis. Proc Natl Acad Sci (USA),
85: 3758-3761.
Furia TE & Bellanca N ed. (1975) Fenaroli's handbook of flavour
ingredients, 2nd ed. Cleveland, Ohio, CRC Press Inc., vol 2, p 3.
Gerhold RM & Malaney GW (1966) Structural determination in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38(4): 562-579.
Gidley MJ, Hall LD, Sanders JKM, & Summers MC (1981)
Acetaldehyde-enkephalins: structure proof and some conformational
deductions from one- and two-dimensional proton nuclear magnetic
resonance spectra. Biochemistry, 20: 3880-3883.
Goedde HW & Agarwal DP (1986) Aldehyde oxidation: ethnic variations in
metabolism and response. In: Kalow W, Goedde W, & Agarwal DP ed.
Ethnic differences in reactions to drugs and xenobiotics. New York,
Alan R. Liss Inc., pp 113-138.
Goedde HW & Agarwal DP (1987) Polymorphism of aldehyde dehydrogenase
and alcohol sensitivity. Enzyme, 37: 29-44.
Goedde HW, Agarwal DP, & Harada S (1979) Alcohol metabolizing enzymes:
studies of isozymes in human biopsies and cultured fibroblasts. Clin
Genet, 16: 29-33.
Goedde HW, Singh S, Agarwal DP, Frizte G, Stapel K, & Paik YK (1989)
Genotyping of mitochondrial aldehyde dehydrogenase in blood samples
using allele-specific oligonucleotides: comparison with phenotyping in
hair roots. Hum Genet, 81: 305-307.
Gordon BHJ, Baraona E, & Lieber CS (1985) Blood acetaldehyde response
to ethanol ingestion during the reproductive cycle of the female rat.
Alcohol, 2: 271-275.
Grahl K (1983) [Classification of substances in water on the basis of
their toxicity potential to aquatic organisms.] Acta Hydrochim
Hydrobiol, 11(1): 137-143 (in German).
Gramiccioni L, Milana MR, Di Marzio S, & Lorusso S (1986) GC
determination of traces of acetaldehyde in aqueous matrix: a rapid
headspace method. Chromatographia, 21(1): 9-11.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Thomas CT Publications, p 76.
Green RJ & Baron DN (1986) The acute in vitro effect of ethanol, its
metabolites and other toxic alcohols on ion flax in isolated human
leucocytes and erythrocytes. Biochem Pharmacol, 35(20): 3457-3464.
Greenwald IS & Horvitz HR (1980) Unc-93(e1500): a behavioral mutant of
Caenorhabditis elegans that defines a gene with a wild-type null
phenotype. Genetics, 96: 147-164.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D (1991) Ambient levels of formaldehyde, acetaldehyde and
fumaric acid in Southern California. Results of a one-year base-line
study. Environ Sci Technol, 25: 710-715.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos Environ, 17(10): 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Guerri C & Sanchis R (1985) Acetaldehyde and alcohol levels in
pregnant rats and their fetuses. Alcohol, 2: 267-270.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hagemeyer HJ (1978) Acetaldehyde. In: Grayson M ed. Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 1, pp 97-112.
Hagen E (1967) The composition of combustion gases of polyurethane
forms and polycarbonates. Plaste Kautsch, 6: 391-392.
Harade S, Agarwal DP, & Goedde HW (1978a) Human liver alcohol
dehydrogenase isoenzyme variations: improved separation methods using
prolonged high voltage starch gel electrophoresis and isoelectric
focusing. Hum Genet, 40: 215-220.
Harade S, Agarwal DP, & Goedde HW (1978b) Application of gel
isoelectric focusing and electrophoresis in the study of alcohol
dehydrogenase and acetaldehyde dehydrogenase isoenzymes of human
tissues and fibroblasts. In: Catsimoolas U ed. Electrophoresis 78.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 275-282.
Harris CC, Willey JC, Saladino AJ, & Grafstrom RC (1985) Effects of
tumor promotors, aldehydes, peroxides, and tobacco smoke condensate on
growth and differentiation of cultured normal and transformed human
bronchial cells. In: Mass MJ, Kaufman DG, Siegfried JM, Steele WE, &
Nesnow S, ed. Carcinogenesis, a comprehensive survey. Cancer of the
respiratory tract. Predisposing factors. New York, Raven Press, pp
159-171.
Hatfield R (1957) Biological oxidation of some organic compounds. Ind
Eng Chem, 49(2): 192-196.
He SM & Lambert B (1985) Induction and persistence of SCE-inducing
damage in human lymphocytes exposed to vinyl acetate and acetaldehyde
in vitro. Mutat Res, 158(3): 201-208.
He SM & Lambert B (1990) Acetaldehyde-induced mutation at the hprt
locus in human lymphocytes in vitro. Environ Mol Mutagen, 16: 57-63.
Helander A & Tottmar O (1987) Effects of ethanol, acetaldehyde and
disulfiram on the metabolism of biogenic aldehydes in isolated human
blood cells and platelets. Biochem Pharmacol, 36(22): 3981-3985.
Helander A & Lindahl-Kiessling K (1991) Increased frequency of
acetaldehyde-induced sister-chromatid exchanges in human lymphocytes
treated with an aldehyde dehydrogenase inhibitor. Mutat Res, 264:
103-107.
Hellström-Lindahl E & Weiner H (1985) Effects of disulfiram on the
oxidation of benzaldehyde and acetaldehyde in rats liver. Biochem
Pharmacol, 34(9): 1529-1535.
Hemminki K & Suni R (1984) Sites of reaction of glutaraldehyde and
acetaldehyde with nucleosides. Arch Toxicol, 55: 186-190.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rate
and mutagenicity of directly acting industrial and laboratory
chemicals. Arch Toxicol, 46: 277-285.
Henderson IF (1970) The fumigant effect of metaldehyde on slugs. Ann
Appl Biol, 65: 507-510.
Herrick RF (1980) Industrial hygiene survey report on urea
formaldehyde foam insulation manufacturing. Cincinnati, Ohio, US
National Institute of Occupational Safety and Health, pp 2-5, 9-12
(PB83-107797).
Hobara N, Watanabe A, Kobayashi M, Nakatsukasa H, Nagshima H, Fukuda
T, & Araki Y (1985) Tissue distribution of acetaldehyde inhalation and
intragastric ethanol administration. Bull Environ Contam Toxicol,
35: 393-396.
Hoerner M, Behrens UJ, Worner T, Blacksberg I, Braly LF, Schaffner F,
& Lieber CS (1988) The role of alcoholism and liver disease in the
appearance of serum antibodies against acetaldehyde adducts.
Hepatology, 8: 569-574.
Hoffman D, Brunnenman, KD, Gori GB, & Wynder EL (1975) On the
carcinogenicity of marijuana smoke. Recent Adv Phytochem, 9: 63-81.
Horvath RJ, Senter SD, & Dekazos ED (1983) GLC-MS analysis of volatile
constituents in rabbit eye blueberries. J Food Sci, 48: 278-279.
Hsu LC, Bendel RE, & Yoshida A (1978) Genomic structure of the human
mitochondrial aldehyde dehydrogenase gene. Genomics, 2(1): 57-65.
Hugues UJ & Hum RW (1960) A preliminary survey of hydrocarbon-derived
oxygenated material in automobile exhaust gases. J Air Pollut Control
Assoc, 10: 367-371.
Hustert K & Parlar H (1981) [A test for photochemical degradation of
environmental chemicals in the gas phase.] Chemosphere,
10(9): 1045-1050 (in German).
IARC (1985) Acetaldehyde. In: Allyl compounds, aldehydes, epoxides and
peroxides. Lyon, International Agency for Research on Cancer, pp
101-132 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 36).
IARC (1988) Alcohol drinking. Lyon, International Agency for Research
on Cancer, 416 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risks to Humans, Volume 44).
Igali S & Gazso L (1980) Mutagenic effects of alcohol and acetaldehyde
on Escherichia coli. Mutat Res, 74: 209-210.
Ikawa E, Tsuda H, Sakata T, Masui T, Sato K, & Ito N (1986)
Modification potentials of ethyl alcohol and acetaldehyde on
development of preneoplastic glutathione S-transferase
P-form-positive liver cell foci initiated by diethylnitrosamine in the
rat. Cancer Lett, 31(1): 53-60.
IPCS/CEC (1990) International Chemical Safety Card No. 0009:
Acetaldehyde. Luxembourg, Commission of the European Communities
(First series).
Israel Y, Hurwitz E, Niemela EO, & Arnon R (1986) Monoclonal and
polyclonal antibodies against acetaldehyde-containing epitopes in
acetaldehyde-protein adducts. Proc Natl Acad Sci (USA),
83(20): 7923-7927.
Israel Y, MacDonald A, Niemela O, Zemel D, Shami E, Zywulko M, Klajner
F, & Borgono C (1992) Hypersensitivity to acetaldehyde-protein
adducts. Mol Pharmacol, 42(4): 711-717.
Jansson T (1982) The frequency of sister chromatid exchanges in human
lymphocytes treated with ethanol and acetaldehyde. Hereditas,
97: 301-303.
Japan Environment Agency (1989) Report on environmental survey and
wildlife monitoring of chemicals. In: Chemicals in the environment.
Tokyo, Japan Environment Agency, Department of Environmental Health,
p 73.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Jones JS, Sadler GD, & Nelson PE (1986) Acetaldehyde and accelerated
storage of wine: a new rapid method for analysis. J Food Sci,
51(1): 229-230.
Juhnke I & Luedemann D (1978) [Results of investigation of acute
toxicity to fish of 200 chemical compounds by the "Golden Orfe" test.]
Z Wasser Abwasser Forschung, 11(5): 161-164 (in German).
Kami T (1983) Composition of the essential oil of alfalfa. J Agric
Food Chem, 31: 38-41.
Karl PI, Gordon BHJ, Lieber CS, & Fisher SE (1988) Acetaldehyde
production and transfer by perfused human placental cotyledon.
Science, 242: 273-275.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Identification and analysis of
organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Press,
pp 329-373.
Knadle S (1985) Synergistic interaction between hydroquinone and
acetaldehyde in the induction of sister chromatid exchange in human
lymphocytes in vitro. Cancer Res, 45(10): 4853-4857.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77: 269-276.
Koivula T (1975) Subcellular distribution and characterization of
human liver aldehyde dehydrogenase fractions. Life Sci, 16: 1563-1570.
Kopczynski SL, Altshuller AP, & Sutterfield FD (1974) Photochemical
reactivities of aldehyde-nitrogen oxide systems. Environ Sci Technol,
8(10): 909-918.
Korte A & Obe G (1981) Influence of chronic ethanol uptake and acute
acetaldehyde treatment on the chromosomes of bone-marrow cells and
peripheral lymphocytes of Chinese hamsters. Mutat Res, 88: 389-395.
Kruysse A (1970) Acute inhalation toxicity of acetaldehyde in
hamsters. Zeist, Central Institute for Nutrition and Food Research,
TNO (Report No. R 3270).
Kruysse A, Feron VJ, & Til HP (1975) Repeated exposure to acetaldehyde
vapor. Studies in Syrian golden hamsters. Arch Environ Health,
30: 449-452.
Kuriyama K, Ohkuma S, Tomono S, & Hirouchi M (1987) Effects of alcohol
and acetaldehyde on metabolism and function of neurotransmitter system
in cerebral cortical neurons in primary culture. Alcohol Alcohol,
22(Suppl 1): 685-689.
Lahdetie J (1988) Effects of vinyl acetate and acetaldehyde on sperm
morphology and meiotic micronuclei in mice. Mutat Res,
202(1): 171-178.
Lahti RA & Majchrowicz E (1969) Acetaldehyde - an inhibitor of the
enzymatic oxidation of 5-hydroxyindoleacetaldehyde. Biochem Pharmacol,
18: 535-538.
Lam CW, Casanova M, & Heck HD (1986) Decreased extractability of DNA
from proteins in the rat nasal mucosa after acetaldehyde exposure.
Fundam Appl Toxicol, 6(3): 541-550.
Lambert B, Chen Y, He SM, & Sten M (1985) DNA cross-links in human
leucocytes treated with vinyl acetate and acetaldehyde in vitro.
Mutat Res, 146(3): 301-303.
Lambert B & He SM (1988) DNA and chromosome damage induced by
acetaldehyde in human lymphocytes in vitro. Ann NY Acad Sci,
534: 369-376.
Lamboeuf Y, Latge C, Roumec C, & De Saint Blanquat G (1987) Ethanol
tolerance in the rat after inhalation of acetaldehyde for a period of
21 days. Alcohol Alcohol, 22(Suppl 1): 441-447.
Latge C, Lamboeuf Y, Roumec C, & De Saint Blanquat G (1987) Effect of
chronic acetaldehyde intoxication on ethanol tolerance and membrane
fatty acids. Drug Alcohol Depend, 20(1): 47-56.
Lau CF, Vogel R, Obe G, & Spielmann H (1991) Embryologic and
cytogenetic effects of ethanol on preimplantation mouse embryos
in vitro. Reprod Toxicol, 5: 405-410.
Leone JA & Seinfeld JH (1984) Analysis of the characteristics of
complex chemical reaction mechanisms: application to photochemical
smog chemistry. Environ Sci Technol, 18(4): 280-287.
Levallois C, Maini JC, & Balmes JL (1987) Sensitivity of human
lymphocytes to acetaldehyde: comparison between alcoholic and control
subjects. Drug Alcohol Depend, 20(2): 135-142.
Lipari F & Swarin SJ (1982) Determination of formaldehyde and other
aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. Chromatography, 247: 297-306.
Lipari F, Dasch JM, & Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environ Sci Technol, 18: 326-330.
Liu RC, Lumeng L, Shahidi S, Kelly T, & Pound DC (1990)
Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol
Clin Exp Res, 14: 438-443.
Ludzack JR & Ettinger MB (1960) Industrial wastes. Chemical structures
resistant to aerobic biochemical stabilization. J Water Pollut Control
Fed, 32(11): 1173-1200.
Lyman WJ, Rheel WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, McGraw-Hill Book Company.
Lynch C, Lim CK, Thomas M, & Peters TJ (1983) Assay of blood and
tissue aldehydes by HPLC analysis of their 2,4-dinitrophenylhydrazine
adducts. Clin Chim Acta, 130: 117-122.
Ma T, Miller TL, & Xu Z (1985) Mouse peripheral erythrocyte
micronucleus assay on the genotoxicity of acetaldehyde. Environ
Mutagen, 7(Suppl 3): 28.
Maarse H & Visscher CA (1992) Volatile compounds in food. Qualitative
and quantitative data. Supplement 3 and cumulative index. Zeist,
Central Institute for Nutrition and Food Research, TNO, Biotechnology
and Chemistry Institute, pp XXXIII-XLI.
McCall D & Ryan K (1987) The effect of ethanol and acetaldehyde on Na
pump function in cultured rat heart cells. J Mol Cell Cardiol,
19: 453-463.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987a)
Effects of acetaldehyde on polymerization of microtubule proteins.
Brain Res, 416: 90-99.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987b) The
reaction of acetaldehyde with brain microtubular proteins: formation
of stable adducts and inhibition of polymerization. Neurosci Lett,
79: 163-168.
Majumdar APN, Vesenka GD, Dubick MA, Yu GSM, DeMorrow JM, & Geokas MC
(1986) Morphological and biochemical changes of the pancreas in rats
treated with acetaldehyde. Am J Physiol 250(5): G598-G606.
Manning DL, Maskarinec MP, Jenkins RA, & Marshall AH (1983) High
performance liquid chromatographic determination of selected gas phase
carbonyls in tobacco smoke. J Assoc Off Anal Chem, 66(1): 8-12.
Margeri G, Versini G, Gianotti I, & Pellegrini R (1984) Acetaldehyde
in beverages. Riv Soc Ital Sci Aliment, 5: 401-406.
Marinari UM, Ferro M, Sciaba L, Finollo R, Bassi AM, & Brambilla G
(1984) DNA-damaging activity of biotic and xenobiotic aldehydes in
Chinese hamster ovary cells. Cell Biochem Funct, 2(4): 243-248.
Marjanen LA (1973) Comparison of aldehyde dehydrogenases from cytosol
and mitochondria of rat liver. Biochim Biophys Acta, 327: 238-246.
Marnett LJ, Hurd H, Hollstein M, Levin DE, Esterbauer H, & Ames BN
(1985) Natural occurring carbonyl compounds are mutagens in Salmonella
tester strain TA104. Mutat Res, 148: 25-34.
Matsuzaki S & Lieber CS (1977) Increased susceptibility of hepatic
mitochondria to the toxicity of acetaldehyde after chronic ethanol
consumption. Biochem Biophys Res Commun, 75(4): 1059-1065.
Mauch TJ, Donahue TM, Zetterman RK, Sorrell MF, & Tuma DJ (1986)
Covalent binding of acetaldehyde selectively inhibits the catalytic
activity of lysine-dependent enzymes. Hepatology, 6(2): 263-269.
Mauch TJ, Tuma DJ, & Sorrell MF (1987) The binding of acetaldehyde to
the active site of ribonuclease: alterations in catalytic activity and
effects of phosphate. Alcohol Alcohol, 22(2): 103-112.
Michoudet C & Baverel G (1985) Metabolism and metabolic effects of
acetaldehyde in dog, rat and guinea pig renal cortex. Dev Nephrol,
10: 143-149.
Michoudet C & Baverel G (1987a) Characteristics of acetaldehyde
metabolism in isolated dog, rat and guinea pig kidney tubules. Biochem
Pharmacol, 36(22): 3987-3991.
Michoudet C & Baverel G (1987b) Metabolism of acetaldehyde in human
and baboon renal cortex:ethanol synthesis by isolated baboon
kidney-cortex tubules. FEBS Lett, 216(1): 113-117.
Miyamoto Y (1986) Eye and respiratory irritants in jet engine exhaust.
Aviat Space Environ Med, 57: 1104-1108.
Mold JD & McRae TG (1957) The determination of some low molecular
weight aldehydes and ketones in cigarette smoke as the
2,4-dinitrophenylhydrazone. Tob Sci, 1: 40-46.
Moortgat GK & McQuigg RD (1984) A FTIR spectroscopic study of the
photooxidation of acetaldehyde in air. In: Proceedings of the Third
European Symposium on Physico-Chemical Behaviour of Atmospheric
Pollutants, Varese, Italy. Luxembourg, Commission of the European
Communities, pp 194-204 (EUR 9436).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing of
270 chemicals. Environ Mutagen, 8(Suppl 17): 1-119.
Mueller A & Sies H (1987) Alcohol, aldehydes and lipid peroxidation
current notions. Alcohol Alcohol, 22(Suppl 1): 67-74.
Myers WD, Ng KT, Singer G, Smythe GA, & Duncan MW (1985) Dopamine and
salsolinol levels in rat hypothalami and striatum after
schedule-induced self-injection (SISI) of ethanol and acetaldehyde.
Brain Res, 358(1-2): 122-128.
Niemela O & Israel Y (1992) Hemoglobin-acetaldehyde adducts in human
alcohol abusers. Lab Invest, 67(2): 246-252.
Niemela O, Klajner F, Orrego H, Vidins E, Blendis LM, & Israel Y
(1987) Antibodies against acetaldehyde-modified protein epitopes in
human alcoholics. Hepatology, 7: 1210-1214.
Niemela O, Mannermaa RM, & Oikarinen (1990) Impairment o histone H1
DNA binding by adduct formation with acetaldehyde. Life Sci,
47: 2241-2249.
Niemela O, Juvonen T, & Parkkila S (1991) Immunohistochemical
demonstration of acetaldehyde-modified epitopes in human liver after
alcohol consumption. J Clin Invest, 87: 1367-1374.
Ninfali P, Palma F, Piacentini MP, & Fornaini G (1987) Action of
acetaldehyde on glucose metabolism of newborn and adult erythrocytes.
Biol Neonate, 52(5):256-263.
Norppa H, Tursi F, Pfaeffli P, Maeki-Paakkanen J, & Jaerventaus H
(1985) Chromosome damage induced by vinyl acetate through in vitro
formation of acetaldehyde in human lymphocytes and Chinese hamster
ovary cells. Cancer Res, 45(10): 4816-4821.
Obe O & Beek B (1979) Mutagenic activity of aldehydes. Drug Alcohol
Depend, 4: 91-94.
Obe G & Ristow H (1977) Acetaldehyde, but not ethanol, induces sister
chromatid exchanges in Chinese hamster cells in vitro. Mutat Res,
56: 211-213.
Obe G, Ristow H, & Herha J (1978) Mutagenic activity of alcohol in
man. In: Mutations: their origin, nature and potential relevance to
genetic risk in man. Proceedings Yearly Conference 1977 of German
Research Society. Boppard, Harald Boldt, pp 151-161.
Obe G, Natarajan AT, Meyers M, & Den Hertog A (1979) Induction of
chromosomal aberrations in peripheral lymphocytes of human blood
in vitro, and of SCE's in bone-marrow cells of mice in vivo by
ethanol and its metabolite acetaldehyde. Mutat Res, 68: 291-294.
Obe G, Brodmann R, Fleischer R, Engeln H, Gobel D, & Herha J (1985)
[Mutagenic and carcinogenic activity of adduct products.] In: Keup W
ed. [Biology of addiction.] Berlin, Heidelberg, New York,
Springer-Verlag, pp 31-43 (in German).
Obe G, Jonas R, & Schmidt S (1986) Metabolism of ethanol in vitro
produces a compounds which induces sister-chromatid exchanges in human
peripheral lymphocytes in vitro: Acetaldehyde not ethanol is
mutagenic. Mutat Res, 174: 47-52.
OECD (1992) Guidelines for testing chemicals. Section 3. Degradation
and accumulation. 301 C. Paris, Organisation for Economic Cooperation
and Development, pp 1-21.
Okamoto M, Ohtsuka K, Imai J, & Yamada F (1981) High-performance
liquid chromatographic determination of acetaldehyde in wine as its
lutidine derivative. J Chromatogr, 219: 175-178.
Ortiz A, Griffth PJ, & Littleton JM (1974) A comparison of the effects
of chronic administration of ethanol and acetaldehyde to mice:
evidence for a role of acetaldehyde in ethanol dependence. J Pharm
Pharmacol, 26: 249-260.
Osborne JS, Adamek S, & Hobbs ME (1956) Some component of gas phase of
cigarette smoke. Ann Chem, 28: 211-215.
O'Shea KS & Kaufman MH (1979) The teratogenic effect of acetaldehyde:
implications for the study of the fetal alcohol syndrome. J Anat,
128: 65-76.
O'Shea KS & Kaufman MH (1981) Effect of acetaldehyde on the
neuroepithelium of early mouser embryos. J Anat, 132: 107-118.
Padmanabhan R, Sreenathan RN, & Singh S (1983) Studies on the lethal
and teratogenic effects of acetaldehyde in the rat. Congenit Anom,
23: 13-23.
Parrilla R, Ohkawa K, Lindros KO, Zimmernman UP, Kobayashi K, &
Williamson JR (1974) Functional compartmentation of acetaldehyde
oxidation in rat liver. J Biol Chem, 249: 4926-4933.
Peeke HV & Figler MH (1981) Modulation of aggressive behavior in fish
by alcohol and congeners. Pharmacol Biochem Behav, 14(Suppl 1): 79-84.
Pellizari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compound in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Peterson CM & Polizzi CM (1987) Improved method for acetaldehyde in
plasma and hemoglobin-associated acetaldehyde: results in teetotalers
and alcoholics reporting for treatment. Alcohol, 4: 477-480.
Pettersson H & Kiessling K (1977) Acetaldehyde occurrence in
cerebrospinal fluid during ethanol oxidation in rats and its
dependence on the blood level and on dietary factors. Biochem
Pharmacol, 26: 237-240.
Pezzoli C, Galli-Kienle M, Di Padova C, & Stramentinoli G (1984) HPLC
method for the evaluation of blood acetaldehyde without ethanol
interference. J Liq Chromatogr, 7(4): 765-788.
Phillips SC (1987) Can brain lesions occur in experimental animals by
administration of ethanol or acetaldehyde? Acta Med Scand,
71(Suppl 7): 67-72.
Piendl A, Westner H, & Geiger E (1981) [Detection and separation of
aldehydes in beer using high pressure liquid chromatography (HPLC).]
Braunwissenschaft, 34: 301-307 (in German).
Pittevils J, Vanparys L, & Soenen A (1979) Fungicide and fungistatic
action of fifteen different aromatic components on some important
storage diseases in vitro. Meded Fac Landbouwwet Rijksuniv Gent,
44(1): 445-457.
Popov VB, Vaisman BL, Puchkov VF, & Ignatyeva TV (1981) Toxic action
of ethanol and biotransformation products on postimplantation rat
embryos in culture. Bull Exp Biol Med, 92: 725-728.
Possanzini M, Ciccioli P, Di Paolo V, & Draisci R (1987) Determination
of low boiling aldehydes in air and exhaust gases by using annular
denuders combined with HPLC techniques. Chromatographia,
23(11): 829-834.
Prasanna CV & Ramakrishnan S (1984a) Effect of acetaldehyde on hepatic
glucose metabolism. Indian J Biochem Biophys, 21(1):62-64.
Prasanna CV & Ramakrishnan S (1984b) Effect of acetaldehyde on
carbohydrate metabolism in rat brain. Indian J Biochem Biophys,
21(2): 121-123.
Prasanna CV & Ramakrishnan S (1987) Effect of acetaldehyde on hepatic
and blood lipids. Indian J Med Res, 85: 206-211.
Prasanna CV, Namagiri T, & Ramakrishnan S (1986) Effect of
acetaldehyde on thyroid function. Indian J Exp Biol, 24(2): 77-78.
Priscott PK & Ford JR (1985) An in vitro model of acetaldehyde
metabolism by rodent conceptuses. in vitro Cell Dev Biol,
21(2): 88-92.
Ramdahl T, Alfheim I, Rustad S, & Olsen T (1982) Chemical and
biological characterization of emissions from small residential stoves
burning wood and charcoal. Chemosphere, 11(4): 601-611.
Rawat K (1979) Inhibition of cardiac protein synthesis by prolonged
ethanol administration. Res Commun Chem Pathol Pharmacol, 25: 89.
Rideout JM, Lim CK, & Peters TJ (1986) Assay of blood acetaldehyde by
HPLC with fluorescence detection of its 2-diphenylacetyl-1,3-
indandione-1-azine derivative. Clin Chim Acta, 161: 29-35.
Rieger R & Michaelis A (1960) [Chromosomal aberrations after action of
acetaldehyde upon the primary roots of Vicia faba.] Biol Zent.bl,
679: 1-5 (in German).
Ristow H & Obe G (1978) Acetaldehyde induces cross-links in DNA and
causes sister-chromatid exchanges in human cells. Mutat Res,
58: 115-119.
Rosensteel RE & Tanaka S (1976) Health hazard evaluation report
No. 75-89-344. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health.
Roumec C, Lamboeuf Y, & De Saint Blanquat G (1988) Sinaptosomal
phospholipids in rats chronically treated with acetaldehyde. Adv
Biosci, 71: 201-205.
Rudling L, Ahling B, & Loefroth G (1981) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in small furnaces and stoves. In: Proceedings of the International
Conference on Residential Solids Fuels, Environmental Impacts and
Solutions. Beaverton, Oregon, Oregon Graduate Center, pp 34-53.
Saladino AJ, Willey JC, Lechner JF, Grafstrom RC, Laveck M, & Harris
CC (1985) Effects of formaldehyde, acetaldehyde, benzoyl peroxide and
hydrogen peroxide on cultured normal human bronchial epithelial cells.
Cancer Res, 45(6): 2522-2526.
Saldiva PHN, Do Rio Caldeira MP, Massad CW, Calheiros DF, Cardoso LMN,
Bohm GM, & Saldiva CD (1985) Effects of formaldehyde and acetaldehyde
inhalation on rat pulmonary mechanics. J Appl Toxicol, 5(5): 288-292.
San George RC & Hoberman HD (1986) Reaction of acetaldehyde with
hemoglobin. J Biol Chem, 261: 6811-6821.
Sasaki Y & Endo R (1978) Mutagenicity of aldehydes in Salmonella.
Mutat Res, 54: 251-252.
Savolainen ER, Leo MA, Timpl R, & Lieber CS (1984) Acetaldehyde and
lactate stimulate collagen synthesis of cultured baboon liver
myofibroblasts. Gastroenterology, 87(4): 777-787.
Schopf RE, Tromperer M, Bork K, & Morsches B (1985) Effects of ethanol
and acetaldehyde on phagocytic functions. Arch Dermatol Res,
277(2): 131-137.
Schreiber SS, Briden K, Oratz M, & Rothschild MA (1972) Ethanol,
acetaldehyde, and myocardial protein synthesis. J Clin Invest,
51: 2820-2826.
Schreiber SS, Oratz M, Rothschild MA, Reff F, & Evans C (1974)
Alcoholic cardiomyopathy. II. The inhibition of cardiac microsomal
protein synthesis by acetaldehyde. J Mol Cell Cardiol, 6: 207-213.
Schreiner G, Ulbrich M, Senger-Weil M, & Bass R (1987) Effects of
prenatal ethanol or acetaldehyde exposure on postnatal development and
behavior in the rat. Teratology, 36(1): 31A.
Schulam P, Newbold R, & Hull LA (1985) Urban and rural ambient air
aldehyde levels in Schenectady, New York and on Whiteface Mountain,
New York. Atmos Environ, 19(4): 623-626.
Segel LD (1984) Mitochondrial respiration after cardiac perfusion with
ethanol or acetaldehyde. Alcohol Clin Exp Res, 8(6): 560-564.
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency, pp 7, 37, 46 (EPA-600/4/76/062).
Shaw S & Jayatilleke E (1987) Acetaldehyde-mediated hepatic lipid
peroxidation: role of superoxide and ferritin. Biochem Biophys Res
Commun, 143(3): 984-990.
Shiohara E, Sukada M, Chiba S, Yamazaki H, Nishiguchi K, Miyamoto R, &
Nakanishi S (1985) Effect of chronic administration of acetaldehyde by
inhalation on (NA+/K+)-activated adenosine triphosphatase activity of
rat brain membranes. Toxicology, 34(4): 277-284.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Sim VM & Pattle RE (1957) Effect of possible smoke irritation on human
subjects. J Am Med Assoc, 165: 1908-1913.
Sina JF, Bean CL, Dysart GR, Taylor VI, & Bradley MO (1983) Evaluation
of the alkaline elution/rat hepatocyte assay as a predictor of
carcinogenic/mutagenic potential. Mutat Res, 113: 357-391.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1982) Measurements of
hazardous organic chemicals in the ambient atmosphere. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Environmental Sciences Research Laboratory, pp 32-37, 70-79, 84-90
(SRI International Project 7774).
Singh S, Fritze G, Fang B, Harada S, Paik YK, Eckey R, Agarwal DP, &
Goedde HW (1989) Inheritance of mitochondrial aldehyde dehydrogenase:
genotyping in Chinese, Japanese, and South Korean families reveals
dominance of the mutant allele. Hum Genet, 83: 119-121.
Sipi P, Jarventaus H, & Norppa H (1992) Sister-chromatid exchanges
induced by vinyl esters and respective carboxylic acids in cultured
human lymphocytes. Mutat Res, 279: 75-82.
Skog E (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde, propionaldehyde
and butyraldehyde; as well as of acrolein and crotonaldehyde. Acta
Pharmacol, 6: 299-318.
Smyth HF, Carpenter CP, & Weils CS (1951) Range-finding toxicity data:
list IV. Am Med Assoc Arch Ind Health Occup Med, 4: 119.
Snyder JW (1988) The role of phospholipid metabolism in ethanol- and
acetaldehyde-initiated hepatocyte injury. Diss Abstr Int,
B48(10): 2937.
Solomon LR (1987) Studies on the mechanism of acetaldehyde-mediated
inhibition of rat liver transaminases. Clin Chim Acta,
168(2): 207-217.
Solomon LR (1988) Effects of acetaldehyde on human red cell
metabolism: evidence for the formation of enzyme inhibitors. Clin Chim
Acta, 175: 249-266.
Sorrell MF & Tuma DY (1985) Hypothesis: alcoholic liver injury and the
covalent binding of acetaldehyde. Alcohol Clin Exp Res, 9: 306-309.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17(9): 416A-427A.
Spingarn NE, Northington DJ, & Pressely T (1982) Analysis of volatile
hazardous substances by GS/MS. J Chromatogr Sci, 20: 287-288.
Sprince H, Parker CM, Smith GG, & Gonzales LJ (1974) Protection
against acetaldehyde toxicity in the rat by L-cysteine, thiamin and
L-2-methylthiazolidine-4-carboxylic acid. Agents Actions, 4: 125-130.
Sreenathan RN, Padmanabhan R, & Singh S (1982) Teratogenic effects of
acetaldehyde in the rat. Drug Alcohol Depend, 9: 339-350.
Sreenathan RN, Padmanabhan R, & Singh S (1984a) Structural changes of
placenta following maternal administration of acetaldehyde in rat. Z
Mikrosk-Anat Forsch (Leipzig), 98: 597-604.
Sreenathan RN, Singh S, & Padmanabhan R (1984b) Effect of acetaldehyde
on skeletogenesis in rats. Drug Alcohol Depend, 14(2): 165-174.
Stege TE (1982) Acetaldehyde-induced lipid peroxidation in isolated
hepatocytes. Chem Pathol Pharmacol, 36: 287-297.
Steinberg S & Kaplan IR (1984) The determination of low-molecular
weight aldehydes in rain, fog and mist by reserved-phase liquid
chromatography of the 2,4-dinitrophenyl-hydrazone derivatives. Int J
Environ Anal Chem, 18: 253-266.
Steinhagen WH & Barrow CS (1984) Sensory irritation structure-activity
study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol
Appl Pharmacol, 72(3): 495-503.
Stewart JK, Aharoni Y, Hastsell PL, & Young DK (1980) Symptoms of
acetaldehyde injury on head lettuce. HortScience, 15(2): 148-149.
Takahashi K, Tomaru A, Nishiyama N, & Horiguchi M (1986) Alcoholic
cardiomyopathy: direct and indirect effects of acetaldehyde.
Jpn Circ J, 50(6): 567.
Takami T, Kuwata K, Sugimae A, & Nakamoto M (1985) Trace determination
of aldehydes in water by high-performance liquid chromatography. Anal
Chem, 57: 243-245.
Tanner RL & Meng Z (1984) Seasonal variations in ambient atmospheric
levels of formaldehyde and acetaldehyde. Environ Sci Technol,
18: 723-726.
Tejada SB (1986) Evaluation of silica gel cartridges coated in situ
with acidified 2,4-dinitrophenylhydrazine for sampling aldehydes and
ketones in air. Int J Environ Anal Chem, 26: 167-185.
Teschke R, Hasumura Y, & Lieber CS (1977) Hepatic pathways of ethanol
and acetaldehyde metabolism and their role in the pathogenesis of
alcohol-induced liver injury. Nutr Metab, 21(Suppl 1): 144-147.
Thom NS & Agg AR (1975) The breakdown of synthetic organic compounds
in biological processes. Proc R Soc London, B189: 347-357.
Til HP, Woutersen RA, Feron VJ, & Clary JJ (1988) Evaluation of the
oral toxicity of acetaldehyde and formaldehyde in a 4-week
drinking-water study in rats. Fundam Chem Toxicol, 26(5): 447-452.
Truitt EB & Walsh MJ (1971) The role of acetaldehyde in the actions of
ethanol. In: Kissin B & Begleiter H ed. The biology of alcoholism.
Volume 1: Biochemistry. New York, London, Plenum Press, pp 161-195.
Tuma DJ, Donohue TM Jr, Medina VA, & Sorrell MF (1984) Enhancement of
acetaldehyde-protein adduct formation by L-ascorbate. Arch Biochem
Biophys, 234(2): 377-381.
Tuma DJ, Jennett RD, & Sorrell MF (1987) The interaction of
acetaldehyde with tubuline. Ann NY Acad Sci, 282: 227-286.
Ung-Chhun NS & Collins MA (1987) Estimation of blood acetaldehyde
during ethanol metabolism: a sensitive HPLC/fluorescence microassay
with negligible artifactual interference. Alcohol, 4: 473-476.
US EPA (1975) Identification of organic compounds in effluents from
industrial sources. Washington, DC, US Environmental Protection
Agency, pp C-2, 4-14, 4-15, 4-17 (EPA-560/3-75-002; PB-241 841).
US NIOSH (1980) Projected number of occupational exposures to chemical
and physical hazards. Cincinnati, Ohio, US National Institute for
Occupational Safety and Health.
US NIOSH (1981) National occupational hazards survey. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health, p 14.
US NIOSH (1987) Manual of analytical methods. Method 3507. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health.
US NIOSH (1989) Manual of analytical methods. Method 2538.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health.
US NRC (1977) Other organic constituents (Acetaldehyde). In: Drinking
water and health. Washington, DC, US National Research Council,
pp 680-687.
US NRC (1981) Food chemical codex, 3rd ed. Washington, DC, US National
Research Council, National Academy Press, pp 353-355, 583, 615.
US NRC (1985) Chemicals used on food processing. Washington, DC, US
National Research Council, National Academy of Sciences (Publication
No. 1274).
Veghelyi PV & Osztovics M (1978) The alcohol syndromes: the
intrarecombigenic effect of acetaldehyde. Experientia (Basel),
34: 195-196.
Veghelyi PV, Osztovics M, Kardos G, Leisztner L, Szaszovszky E, Igali
S, & Imrei J (1978) The fetal alcohol syndrome: symptoms and
pathogenesis. Acta Paediatr Acad Sci Hung, 19(3): 171-189.
Verschueren U (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 139-141.
Von Burg R & Stout T (1991) Toxicology update-acetaldehyde. J Appl
Toxicol, 11: 373-376.
Wangenheim J & Bocsfoldi G (1986) Mouse lymphoma tk-positive-negative
assay of 30 compounds. Environ Mutagen, 8(Suppl 6): 90.
Wangenheim J & Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3(3): 193-206.
Watanabe I (1987) [Distribution of lower oxygenated organic compounds
in urban air.] Preprint of 54th Annual Meeting of the Chemical Society
of Japan, vol 1, p 129 (in Japanese).
Watanabe I (1988) Sequential automated analysis system for lower
oxygenated organic compounds in ambient air. J Res Nat Bureau Stand,
93(3): 299-301.
Watanabe A, Hobara N, & Nagashima H (1986) Blood and liver
acetaldehyde concentration in rats following acetaldehyde inhalation
and intravenous and intragastric ethanol administration. Bull Environ
Contam Toxicol, 37: 513-516.
Webster WS, Walsh DA, McEwen SE, & Lipson AH (1983) Some teratogenic
properties of ethanol and acetaldehyde in C57BL/6J mice: implications
for the study of the fetal alcohol syndrome. Teratology, 27: 231-243.
Westcott JY, Weiner H, Schultz J, & Myers RD (1980) In vivo
acetaldehyde in the brain of the rat treated with ethanol. Biochem
Pharmacol, 29: 411-417.
WHO (1989) IPCS Environmental Health Criteria 89: Formaldehyde.
Geneva, World Health Organization, 219 pp.
WHO (in press) Environmental Health Criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Wilkin JK & Fortner G (1985) Cutaneous vascular sensitivity to lower
aliphatic alcohols and aldehydes in Orientals. Alcohol Clin Exp Res,
9(6): 522-525.
Williams AJ & Barry RE (1986) Superoxide anion production and
degranulation of rat neutrophils in response to acetaldehyde-altered
liver cell membranes. Clin Sci, 71(3): 313-318.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen,
7(5): 677-702.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1989) Antibodies
against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1990) Antibodies
against acetaldehyde-modified epitopes: presence in alcoholic,
nonalcoholic liver disease and control subjects. Alcohol Alcohol,
25(5): 509-517.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991a) Detection of
stable acetaldehyde-modified proteins in the livers of ethanol-fed
rats. Alcohol Alcohol, 26(4): 437-444.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991b) Antibodies of
S3 against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Woutersen RA & Feron VJ (1987) Inhalation toxicity of acetaldehyde in
rats. IV. Progression and regression of nasal lesions after
discontinuation. Toxicology, 47(3): 295-305.
Woutersen RA, Appelman LM, Feron VJ, & der Heijden CA (1984)
Inhalation toxicity of acetaldehyde in rats. II. Carcinogenicity
study: interim results after 15 months. Toxicology, 31: 123-133.
Woutersen RA, Appelman LM, Van Garderen-Hoetmer A, & Feron VJ (1986)
Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity
study. Toxicology, 41(2): 213-231.
Yoshida A, Huang IY, & Ikawa M (1984) Molecular abnormality of an
inactive aldehyde dehydrogenase variant commonly found in Orientals.
Proc Natl Acad Sci (USA), 81: 258-261.
Zorzano A & Herrera E (1989) Disposition of ethanol and acetaldehyde
in late pregnant rats and their fetuses. Paediatr Res, 25(1): 102-106.
Résumé
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
L'acétaldéhyde est un liquide incolore et volatil à l'odeur acre
et suffocante. D'après la littérature, son seuil olfactif est de
0,09 mg/m3. L'acétaldéhyde est un composé très inflammable et
réactif qui est miscible à l'eau et à la plupart des solvants
ordinaires.
Il existe un certain nombre de méthodes d'analyse pour la
recherche de l'acétaldéhyde dans l'air (y compris dans l'haleine) et
dans l'eau. La principale méthode repose sur la réaction de
l'acétaldéhyde avec la 2,4-dinitrophénylhydrazine, puis analyse de
l'hydrazone obtenue par chromatographie liquide à haute pression ou
chromatographie en phase gazeuse.
2. Sources d'exposition humaine et environnementale
L'acétaldéhyde est un intermédiaire métabolique chez l'homme et
les plantes supérieures; c'est également un produit de la fermentation
alcoolique. On a décelé sa présence dans certaines denrées
alimentaires et boissons ainsi que dans la fumée de cigarette. Il est
également présent dans les gaz d'échappement des véhicules à moteur
ainsi que dans divers déchets industriels. La décomposition des
hydrocarbures, des effluents d'égouts et des déchets biologiques
solides produit de l'acétaldéhyde, de même que la combustion à l'air
libre ou, selon le cas, l'incinération du gaz, du mazout et de la
houille.
Plus de 80% de l'acétaldéhyde utilisé à des fins commerciales est
produit par l'oxydation en phase liquide de l'éthylène en présence
d'une solution catalytique de chlorures de palladium et de cuivre. Au
Japon, la production était de 323.000 tonnes en 1981. Aux Etats-Unis
d'Amérique elle a été de 281.000 tonnes en 1982 et en Europe
occidentale de 706.000 tonnes en 1983. La majeure partie de
l'acétaldéhyde produit commercialement est utilisé pour la préparation
de l'acide acétique. On l'utilise également pour la préparation de
certains arômes et denrées alimentaires.
On estime qu'aux Etats-Unis d'Amérique, les émissions annuelles
d'acétaldéhyde de toutes origines atteignent 12,2 millions de kg.
3. Transport, distribution et transformation dans l'environnement
En raison de la forte réactivité de l'acétaldéhyde, le transport
intercompartimental de ce composé devrait être limité. On peut
s'attendre à un certain transfert d'acétaldéhyde de l'air vers l'eau
et le sol en raison de sa forte tension de vapeur et de son faible
coefficient de sorption.
On pense que l'élimination photo-induite de l'acétaldéhyde
atmosphérique passe essentiellement par la formation de radicaux. La
photolyse devrait jouer également un rôle important dans le processus
d'élimination. Environ 80% des émissions d'acétaldéhyde dans
l'atmosphère sont éliminés chaque jour par ces deux processus.
D'après la littérature, la demi-vie de l'acétaldéhyde dans l'eau et
l'air est respectivement égale à 1,9 heure et 10-60 heures.
L'acétaldéhyde est facilement biodégradable.
4. Concentrations dans l'environnement et exposition humaine
Les concentrations moyennes d'acétaldéhyde dans l'environnement
sont généralement égales à 5 µg/m3. Dans l'eau, elles sont
généralement inférieures à 0,1 µg/litre. L'analyse de denrées
alimentaires très diverses effectuées aux Pays-Bas a montré que leur
teneur en acétaldéhyde est généralement inférieure à 1 mg/kg, mais
peut atteindre occasionnellement jusqu'à plusieurs centaines de mg/kg,
en particulier dans certains jus de fruit et dans le vinaigre.
Pour la population générale, la principale source d'exposition à
l'acétaldéhyde est de loin le métabolisme de l'alcool. La fumée de
cigarette constitue également une importante source d'exposition. En
ce qui concerne les autres véhicules, la population générale est
exposée à l'acétaldéhyde essentiellement par l'intermédiaire de la
nourriture et des boissons et, dans une moindre mesure, de l'air.
L'eau de boisson est négligeable à cet égard.
Les données disponibles sont insuffisantes pour qu'on puisse
évaluer l'ampleur de l'exposition à l'acétaldéhyde sur les lieux de
travail. Les travailleurs peuvent y être exposés dans certaines
industries ainsi que lors de la fermentation de l'alcool; dans ces
conditions la principale voie d'exposition est très probablement
l'inhalation avec possibilité également de contacts cutanés.
5. Cinétique et métabolisme
5.1 Absorption, distribution et élimination
Les données toxicologiques dont on dispose indiquent que
l'acétaldéhyde est résorbé au niveau des poumons et des voies
digestives; toutefois on ne connaît pas d'études quantitatives
appropriées qui aient été consacrées à ce problème. Il est probable
qu'il y a également une résorption cutanée.
Après avoir fait inhaler de l'acétaldéhyde à des rats, on a
constaté qu'il se répartissait dans le sang, le foie, les reins, la
rate, le coeur et le tissu musculaire. De faibles quantités en ont
été décelées dans des embryons après injection intrapéritonéale à la
mère (souris) ainsi qu'après exposition de la mère à de l'éthanol
(souris et rat). Il pourrait également y avoir production
d'acétaldé-hyde in vitro chez les foetus de rat et dans le placenta
humain.
Après injection intrapéritonéale d'éthanol, on a pu mettre en
évidence la distribution de l'acétaldéhyde dans le liquide
interstitiel de l'encéphale, à l'exclusion des cellules cérébrales. Il
est possible qu'une acétaldéhyde-déshydrogénase de forte affinité et
de faible Km joue un rôle important dans le maintien de faibles
concentrations d'acétaldéhyde dans le cerveau au cours de la
métabolisation de l'éthanol.
On a constaté que chez l'homme et les babouins, l'acétaldéhyde
pouvait être fixé par les globules rouges in vivo, la concentration
intraglobulaire pouvant atteindre dix fois la concentration
plasmatique.
Après administration par voie orale, il n'y a pratiquement pas
d'acétaldéhyde qui soit excrété tel quel dans les urines.
5.2 Métabolisme
La principale voie métabolique de l'acétaldéhyde consiste dans
une oxydation en acétate sous l'action de l'ALDHa NADb-dépendante.
L'acétate entre dans le cycle de l'acide citrique sous forme
d'acétyl-CoA. Il existe plusieurs isoenzymes de l'ALDH présentant
divers paramètres cinétiques et paramètres de liaison qui influent sur
la vitesse d'oxydation de l'acétaldéhyde.
Chez le rat, on a pu localiser une activité ALDH dans
l'épithélium respiratoire (à l'exclusion de l'épithélium olfactif),
alors que cette localisation se situe au niveau du rein chez la souris
et au niveau du cortex et des tubules rénaux chez le chien, le rat, le
cobaye et le babouin.
Les tissus embryonnaires de souris et de rat métabolisent
l'acétaldéhyde in vitro. L'acétaldéhyde traverse le placenta du rat
malgré l'existence d'un métabolisme placentaire.
a acétaldéhyde-déshydrogénase
b nicotinamide-adénine-dinucléotide
Il y a un certaine métabolisation de l'acétaldéhyde au niveau des
tubules rénaux chez l'homme mais c'est le foie qui est le principal
site du métabolisme.
Chez l'homme, on a décelé la présence de plusieurs formes
isoenzymatiques d'ALDH, au niveau du foie et d'autres tissus. Il y a
également un polymorphisme de l'ALDH michocondrial. Les sujets qui
sont homozygotes ou hétérozygotes pour une mutation ponctuelle du gène
correspondant à l'ALDH mitrochondriale, présentent une faible activité
ALDH, métabolisent lentement l'acétaldéhyde et ne supportent pas
l'alcool éthylique.
Le crotonadéhyde, la maléate de diméthyle, la phorone, le
disulfirame et le carbamide calcique inhibent le métabolisme de
l'acétaldéhyde.
5.3 Réactions avec d'autres constituants
L'acétaldéhyde forme des adduits stables ou instables avec les
protéines. Ces adduits peuvent perturber la fonction de ces protéines
comme le montre d'ailleurs l'inhibition des enzymes, les difficultés
de liaison entre histones et ADN et l'inhibition de la polymérisation
de la tubuline.
Des adduits instables de l'acétaldéhyde dont on connaît mal
l'importance se forment in vitro avec les acides nucléiques.
L'acétaldéhyde peut réagir sur diverses macromolécules de
l'organisme, de préférence avec celles qui sont porteuses de résidus
de lysine; cette réaction peut conduire à des altérations importantes
de la fonction biologique de ces molécules.
6. Effets sur les êtres vivants dans leur milieu naturel
6.1 Organismes aquatiques
Chez les poissons la CL50 peut varier de 35 mg/litre (guppy) à
140 mg/litre (espèces non précisées). Une CE5 de 82 mg/litre et une
CE50 de 42 mg/litre ont été observées respectivement chez des algues
et chez Daphnia magna.
6.2 Organismes terrestres
La présence d'acétaldéhyde dans l'air se révèle toxique pour
certains microorganismes, à des concentrations relativement faibles.
Des aphidiens ont été tués par exposition de 3 à 4 heures à de
l'acétaldéhyde à une concentration de 0,36 µg/m3.
Chez deux espèces de limaces, Arion hortensis et Agriolimax
reticulatus, on a observé des concentrations létales médianes
respectivement égales à 8,91 mg/litre et par heure et 7,69 mg/litre et
par heure.
L'acétaldéhyde provoque une inhibition réversible de la
germination des oignons, des carottes et des tomates à des
concentrations allant jusqu'à 1,52 mg/litre; par contre cette
inhibition est irréversible pour Amaranthus palmeri, dans les mêmes
conditions d'exposition. A la concentration de 0.54 µg/m3,
l'acétaldéhyde a détérioré des laitues.
7. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Les valeurs de la DL50 pour le rat et la souris et de la CL50
pour le rat et le hamster doré montrent que la toxicité aiguë de
l'acétaldéhyde est faible. On n'a pas connaissance d'études relatives
à la toxicité cutanée aiguë de l'acétaldéhyde.
7.2 Exposition à court et à long terme
Lors d'études au cours desquelles des doses ont été administrées
à plusieurs reprises, tant par la voie orale que par la voie
respiratoire, les effets toxiques observés à des concentrations
relativement faibles se limitaient essentiellement aux points de
contact initiaux. Lors d'une étude de 28 jours au cours de laquelle
de l'acétaldéhyde a été administré à des rats, mélangé à leur eau de
boisson, à la dose de 675 mg/kg de poids corporel (dose sans effets
observables 125 mg/kg de poids corporel), on a constaté que les effets
se limitaient à une légère hyperkératose focale au niveau de la
portion cardiaque de l'estomac. Après administration d'acétaldéhyde à
concentration constante de 0,05% dans l'eau de boisson pendant une
durée de 6 mois (le Groupe de travail estime que cela correspond à peu
près à 40 mg/kg de poids corporel) au cours d'une étude biochimique,
on a constaté que l'acétaldéhyde provoquait la synthèse de collagène
au niveau du foie chez le rat, observation d'ailleurs corroborée par
les résultats obtenus in vitro.
Lors d'une étude d'inhalation de 4 semaines chez le rat et de 13
semaines chez le hamster, on a obtenu, pour la concentration sans
effets respiratoires observables, des valeurs respectivement égales à
275 mg/m3 et 700 mg/m3. Aux concentrations les plus faibles
produisant un effet, on a observé des altérations dégénératives au
niveau de l'épithélium olfactif chez le rat (437 mg/m3) et de la
trachée (2400 mg/m3) chez le hamster. Des altérations dégénératives
de l'épithélium respiratoire et du larynx ont été observées à des
concentrations plus élevées. On n'a pas connaissance d'études
relatives à l'administration répétée d'acétaldéhyde au niveau cutané.
7.3 Reproduction, embryotoxicité et tératogénicité
Plusieurs études montrent que des malformations foetales peuvent
survenir par suite de l'exposition parentérale de rattes et de souris
gravides à l'acétaldéhyde. Dans la plupart de ces études, on n'a pas
étudié la toxicité pour la mère. On n'a pas pu trouver de données
relatives à la toxicité de l'acétaldéhyde pour la fonction de
reproduction.
7.4 Mutagénicité et points d'aboutissement des effets correspondants
In vitro, l'acétaldéhyde est génotoxique et produit des
mutations géniques, des effets clastogènes ainsi que des échanges
entre chromatides soeurs dans des cultures de cellules mammaliennes en
l'absence d'activation métabolique exogène. Toutefois, des épreuves
effectuées dans les règles sur des salmonelles ont donné des résultats
négatifs. Après avoir injecté de l'acétaldéhyde par voie
intrapéritonéale à des hamsters chinois et à des souris, on a constaté
que ce composé produisait des échanges entre chromatides soeurs au
niveau de la moelle osseuse. Cependant, de l'acétaldéhyde administré
par la même voie n'a pas eu pour conséquence un accroissement de la
fréquence des micronoyaux dans les spermatides de souris récemment
formés. On peut déduire indirectement de certaines études in vitro
et in vivo que l'acétaldéhyde est susceptible de produire des
pontages protéines-ADN et ADN-ADN.
7.5 Cancérogénicité
Des études d'inhalation effectuées sur des rats et des hamsters
ont permis de constater un accroissement de l'incidence des tumeurs.
Chez le rat, il s'agissait d'une augmentation, liée à la dose, de la
fréquence des adénocarcinomes au niveau de la muqueuse nasale ainsi
que des carcinomes spino-cellulaires (accroissement significatif à
toutes les doses). Toutefois chez le hamster, l'accroissement constaté
de la fréquence des carcinomes du nez et du larynx n'était pas
significatif. A toutes les concentrations d'acétaldéhyde, on a
constaté l'apparition de lésions tissulaires chroniques au niveau des
voies respiratoires.
7.6 Etudes spéciales
On n'a pas connaissance d'études appropriées portant sur la
neuro- et l'immunotoxicité potentielle de l'acétaldéhyde.
8. Effets sur l'homme
Lors d'études limitées portant sur des volontaires humains, on a
constaté que l'acétaldéhyde avait un effet légèrement irritant sur les
yeux et les voies respiratoires supérieures, après exposition de très
brève durée à des concentrations respectivement supérieures à environ
90 et 240 mg/m3. Après l'application d'un timbre inbibé
d'acétaldéhyde, on a constaté la présence d'un érythème cutané chez 12
sujets "d'ascendance orientale".
On a signalé l'existence d'une enquête limitée au cours de
laquelle on a étudié l'incidence des cancers chez des travailleurs
exposés à de l'acétaldéhyde.
On possède des preuves indirectes que le métabolite toxique
supposé être à l'origine des lésions hépatiques imputables à l'alcool,
de bouffées de chaleur et d'effets sur la développement, est en fait
l'acétaldéhyde.
9. Evaluation des risques pour la santé humaine et des effets
sur l'environnement
Les études effectuées sur l'animal montrent que, par voie
respiratoire ou par voie orale, l'acétaldéhyde n'est que faiblement
toxique. D'après les études effectuées sur l'animal mais également
sur l'homme, on a constaté que ce composé était légèrement irritant
pour l'oeil et les voies respiratoires supérieures. Chez l'homme,
l'application d'un timbre cutané inbibé d'acétaldéhyde peut provoquer
l'apparition d'un érythème cutané. On pense avoir découvert le
mécanisme à l'origine de cet effet mais les données disponibles sont
encore insuffisantes pour qu'on puisse évaluer le pouvoir
sensibilisateur de l'acétaldéhyde.
On ne dispose que de données limitées sur les effets d'une
ingestion d'acétaldéhyde. Après administration d'acétaldéhyde par
voie orale à des rats à la dose quotidienne de 675 mg/kg de poids
corporel, on a constaté un accroissement limite de l'hyperkératose au
niveau de la portion cardiaque de l'estomac (dose sans effets nocifs
observables: 125 mg/kg de poids corporel). Après avoir fait boire à
des rats six mois durant de l'eau contenant environ 40 mg
d'acétaldéhyde/kg de poids corporel, on a constaté un accroissement de
la synthèse du collagène hépatique, effet dont la signification reste
obscure.
D'après des études effectuées sur des rats et des hamsters, ce
sont les voies respiratoires supérieures qui constituent l'organe
cible de l'acétaldéhyde lorsque ce composé est inhalé. D'après les
résultats obtenus, la concentration la plus faible à laquelle on a
commencé à observer des effets était de 437 mg/m3, après une période
d'administration de 5 semaines. Chez des rats exposés pendant 4
semaines, la dose sans effets respiratoires observables était de
275 mg/m3; elle était de 700 mg/m3 chez des hamsters exposés
pendant 13 semaines.
Aux concentrations qui produisaient des lésions tissulaires des
voies respiratoires, on a observé un accroissement de l'incidence des
adénocarcinomes du nez et des carcinomes spino-cellulaires chez le
rat; il y a également eu accroissement des carcinomes du larynx et du
nez chez le hamster.
On est fondé à penser qu' in vivo, l'acétaldéhyde provoque des
lésions génétiques dans les cellules somatiques.
les données disponibles sont insuffisantes pour qu'on puisse
apprécier les effets qu'une exposition à l'acétaldéhyde pourrait avoir
sur la reproduction, le développement ainsi que les fonctions
neurologiques et immunologiques de la population générale ou de la
population professionnellement exposées.
Pour ce qui est du pouvoir irritant de ce composé chez l'homme,
on estime que la concentration tolérable est de 2 mg/m3. Etant
donné que le mécanisme d'induction de tumeurs par l'acétaldéhyde n'a
pas été bien étudié, on a adopté deux approches concernant ce point
d'aboutissement de son action toxique, à savoir la fixation d'une
concentration tolérable qui serait obtenue en divisant la dose
irritante pour les voies respiratoires chez les rongeurs, par un
certain facteur d'incertitude et l'estimation d'un risque de cancer
sur toute la durée de l'existence, en procédant par extrapolation
linéaire. La concentration tolérable est de 0,3 mg/m3. La
concentration produisant un excès de risque de 10-5 sur toute la durée
de la vie se situe entre 11-65 µg/m3.
Les données limitées dont on dispose ne permettent pas de tirer
des conclusions définitives quant au risque que l'acétaldéhyde
pourrait représenter pour l'ensemble de la faune et de la flore.
Toutefois, si l'on considère la courte durée de vie de l'acétaldéhyde
dans l'air et dans l'eau et le fait qu'il est facilement
biodégradable, on est amené à penser que ce composé n'est guère
menaçant pour les organismes aquatiques ou terrestres, sauf peut-être
en cas de décharge ou de déversement de produits industriels.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El acetaldehído es un líquido incoloro, volátil y de olor acre y
sofocante. El umbral señalado para el olor es de 0,09 mg/m3. El
acetaldehído es un compuesto muy inflamable y reactivo, soluble en
agua y en la mayoría de los disolventes comunes.
Existen métodos de análisis para la detección del acetaldehído en
el aire (incluso en el aliento) y en el agua. El principal de ellos
se basa en la reacción del producto con 2,4-dinitrofenilhidracina y
ulterior análisis de los derivados de la hidrazona por cromatografía
de líquidos a alta presión o cromatografía de gases.
2. Fuentes de exposición humana y ambiental
El acetaldehído es un producto intermedio del metabolismo en los
hombres y en las plantas superiores, y también proviene de la
fermentación alcohólica. Ha sido identificado en los alimentos, las
bebidas y el humo de los cigarrillos. También se encuentra en los
gases de escape de los vehículos y en ciertos desechos industriales.
La degradación de los hidrocarburos, las aguas residuales y los
desechos biológicos sólidos produce acetaldehído, como también lo
hacen la combustión al aire libre y la incineración de gas, fuel oil y
carbón.
Más del 80% del acetaldehído usado comercialmente proviene de la
oxidación en fase líquida de etileno con una solución catalítica de
paladio y cloruros de cobre. En 1981, la producción japonesa fue de
323 000 toneladas. En los Estados Unidos de América fue de 281 000
toneladas en 1982 y en Europa occidental de 706 000 toneladas en 1983.
La mayoría del acetaldehído producido comercialmente se destina a la
fabricación de ácido acético. También se utiliza en sustancias
aromáticas y en alimentos.
En los Estados Unidos, la emisión anual de acetaldehído de todo
origen se calcula en 12,2 millones de kilogramos.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta reactividad, el transporte intercompartimental
de acetaldehído debe ser limitado. Es de suponer que hay cierta
transferencia de la sustancia al aire desde el agua y el suelo, como
consecuencia de la fuerte presión del vapor y el bajo coeficiente de
sorción.
Es posible que la eliminación fotoinducida del acetaldehído en la
atmósfera tenga lugar principalmente por formación de un radical. La
fotosíntesis puede también contribuir bastante al proceso de
eliminación. La pérdida resultante de acetaldehído proveniente de
emisiones en la atmósfera es de alrededor del 80%. La vida media de
la sustancia en el agua y en el aire es de 1,9 h y 10-60 h,
respectivamente.
El acetaldehído es muy biodegradable.
4. Niveles medioambientales y exposición humana
Los niveles de acetaldehído en el aire ambiente suelen ser por
término medio de 5 µg/m3. Las concentraciones en el agua no llegan
en general a 0,1 µg/litro. El análisis de diversos alimentos en los
Países Bajos demostró que las concentraciones, normalmente inferiores
a 1 mg/kg, a veces llegaban a 100 mg/kg, particularmente en algunos
zumos de frutas y en el vinagre.
La principal fuente, con mucho, de exposición al acetaldehído
para la mayoría de la población es el metabolismo del alcohol. El
humo del cigarrillo es también una fuente significativa. Aparte de
eso pueden mencionarse en segundo lugar los alimentos y bebidas y, en
menor grado, el aire. La contribución del agua de beber es
insignificante.
No existen datos suficientes para determinar la importancia de la
exposición al acetaldehído en el lugar de trabajo. El personal puede
estar expuesto en algunas industrias manufactureras y donde hay
fermentación con producción de alcohol, siendo la principal vía la
inhalación y posiblemente el contacto con la piel.
5. Cinética y metabolismo
5.1 Absorción, distribución y eliminación
Los estudios existentes sobre toxicidad indican que el
acetaldehído se absorbe por los pulmones y el conducto
gastrointestinal; sin embargo, no parecen existir análisis
cuantitativos adecuados. Es probable la absorción por la piel.
En las ratas, el acetaldehído inhalado se distribuye por la
sangre, pasando al hígado, el riñón, el bazo, el corazón y otros
tejidos musculares. Se han detectado bajos niveles en embriones de
ratón tras inyectar la sustancia a la madre por vía intraperitoneal o
tras exposición de ratas o ratones hembra a etanol. La producción
potencial de acetaldehído in vitro se ha observado en fetos de rata
y en la placenta humana.
Tras inyección intraperitoneal de etanol se ha demostrado la
distribución de acetaldehído en el líquido intersticial pero no en las
células del cerebro. Una alta afinidad y escaso Km ALDHa pueden ser
importantes para mantener bajos niveles de acetaldehído en el cerebro
durante el metabolismo del etanol.
El acetaldehído es absorbido por los eritrocitos y, tras consumo
de etanol, los niveles intracelulares en hombres y babuinos pueden ser
in vivo 10 veces más altos que los del plasma.
Después de la administración por vía oral, la excreción de
acetaldehído por la orina prácticamente no cambia.
5.2 Metabolismo
Un medio importante en el metabolismo del acetaldehído es la
oxidación para pasar a acetato bajo la influencia de una ALDH
dependiente de NADb. El acetato pasa al ciclo de ácido cítrico como
acetil-CoA. Hay varios isoenzimas de ALDH con distintos parámetros
cinéticos y de ligazón que influyen en la rapidez de la oxidación del
acetaldehído.
Se ha detectado actividad de la ALDH en el epitelio del tracto
respiratorio (excluido el de las vías olfativas) de ratas, en el
cortex renal y los túbulos del perro, la rata, el cobayo y el babuino,
y también en los testículos del ratón.
El acetaldehído es metabolizado in vitro por el tejido
embrionario del ratón y la rata. La sustancia atraviesa la placenta
del ratón, pese al metabolismo placentario.
Aunque hay algún metabolismo en los túbulos renales humanos, el
principal órgano metabolizador es el hígado.
En el hígado y otros tejidos humanos se han detectado varias
formas isoenzimáticas de ALDH. En las mitocondrias la ALDH presenta
polimorfismo. Los sujetos que son homozigóticos o heterozigóticos por
una mutación singular del gen correspondiente a la ALDH de las
mitocondrias presentan poca actividad de esta enzima, metabolizan el
acetaldehído lentamente y no toleran el etanol.
El metabolismo del acetaldehído puede ser inhibido por el
crotonaldehído, el dimetilmaleato, el forón, el disulfiram y la
carbamida de calcio.
5.3 Reacción con otros compuestos
El acetaldehído puede formar aductores estables e inestables con
las proteínas. Eso menoscaba la función proteínica, como lo demuestra
la inhibición de la actividad enzimática, la menor ligazón histona-ADN
y la polimerización inhibida de la tubulina.
Los aductores inestables, de importancia indeterminada, se
producen in vitro con ácidos nucleicos.
El acetaldehído puede reaccionar con diversas macromoléculas en
el organismo humano, de preferencia las que contienen residuos de
lisina, lo que puede conducir a notables alteraciones de la función
biológica de dichas moléculas.
6. Efectos sobre los organismos presentes en el medioambiente
6.1 Organismos acuáticos
La CL50 para los peces va de 35 (Lebistes reticulatus) a
140 mg/litro (especies no especificadas). Se ha señalado una CE5 de
82 mg/litro y una CE50 de 42 mg/litro para las algas y para Daphnia
magna, respectivamente.
6.2 Organismos terrestres
El acetaldehído atmosférico a concentraciones relativamente bajas
parece ser tóxico para algunos microorganismos.
Los afidios mueren cuando son expuestos a concentraciones de
0,36 µg/m3 durante tres o cuatro horas.
Para las especies de babosa Arion hortensis y Agriolimax
reticulatus se han registrado valores letales medios de
8,91 mg/litro y 7,69 mg/litro por hora, respectivamente.
La inhibición de la germinación para la semilla de cebolla,
zanahoria y tomate tratada con acetaldehído (hasta 1,52 mg/litro) es
reversible, mientras que la de Amaranthus palmeri expuesta del mismo
modo es irreversible. El acetaldehído a 0,54 µg/m3 es perjudicial
para la lechuga.
7. Efectos en animales experimentales y sistemas de prueba in vitro
7.1 Exposición única
El acetaldehído tiene una toxicidad aguda baja, con una DL50 en
ratas y ratones y una CL50 en ratas y hámsters sirios. No se han
encontrado estudios de toxicidad dérmica aguda.
7.2 Exposición a corto y a largo plazo
En estudios reiterados del efecto tóxico de dosis por vía oral y
por inhalación, los efectos tóxicos de concentraciones relativamente
bajas se limitaron más bien a los puntos de contacto inicial. En un
estudio de 28 días en el que se administró a ratas con el agua de
beber acetaldehído a razón de 675 mg/kg de peso corporal (nivel sin
efecto observado (NOEL) = 125 mg/kg de peso corporal) los efectos se
limitaron a una ligera hiperqueratosis focal en la parte anterior del
estómago. En un estudio bioquímico, tras la administración a ratas de
dosis singulares (0,05% en el agua de beber) durante seis meses
(equivalente según el Grupo Especial a alrededor de un 40 mg/kg de
peso corporal), el acetaldehído indujo la síntesis de colágeno
hepático, observación corroborada mediante pruebas in vitro.
El nivel sin efecto respiratorio observado (NOEL) fue de
275 mg/m3 para ratas expuestas a acetaldehído por inhalación durante
cuatro semanas y 700 mg/m3 en hámsters expuestos durante 13 semanas.
A los niveles más bajos de efecto observado se produjeron cambios
degenerativos del epitelio olfativo en ratas (437 mg/m3) y de la
tráquea (2400 mg/m3) en hámsters. A concentraciones superiores se
produjeron cambios degenerativos del epitelio respiratorio y de la
laringe. No se han identificado estudios dérmicos con dosis
repetidas.
7.3 Reproducción, embriotoxicidad y teratogenicidad
En varios estudios, la exposición parenteral de ratas y ratones
gestantes a acetaldehído produjo malformaciones fetales. En la
mayoría de esos estudios no se evaluó la toxicidad materna. Tampoco
se dispone de datos sobre la toxicidad reproductiva.
7.4 Mutagenicidad y otros efectos finales afines
El acetaldehído es genotóxico in vitro e induce mutación de los
genes, con efectos clastogénicos e intercambios de cromatidios
hermanos (SCE) en las células de mamíferos, en ausencia de activación
metabólica exógena. Sin embargo se han registrado resultados
negativos en pruebas adecuadas con Salmonella. Tras inyección
intraperitoneal, el acetaldehído indujo SCE en la médula ósea de
hámsters chinos y ratones pero, en cambio, no hizo aumentar la
frecuencia de los micronúcleos en las espermátides precoces de ratón.
Indirectamente, ciertos estudios in vitro e in vivo parecen
indicar que el acetaldehído puede inducir enlaces cruzados
proteína-ADN y ADN-ADN.
7.5 Carcinogenicidad
Se ha observado una mayor incidencia de tumores en ratas y
hámsters expuestos a acetaldehído por inhalación. En las ratas, los
adenocarcinomas nasales aumentaron según la dosis pero los carcinomas
de células escamosas fueron significativos independientemente de la
dosis. Por el contrario, en los hámsters, el aumento de los
carcinomas nasales y laríngeos fue insignificante. A todas las
concentraciones, el acetaldehído administrado en los estudios produjo
daños irreversibles del tejido respiratorio.
7.6 Estudios especiales
No se conocen estudios adecuados sobre la neurotoxicidad y la
inmunotoxicidad potencial del acetaldehído.
8. Efectos en el ser humano
En estudios limitados con voluntarios, el acetaldehído produjo
irritación moderada de los ojos y de las vías respiratorias superiores
tras exposición por muy breves periodos a concentraciones que excedían
algo de 90 y 240 mg/m3, respectivamente. Las pruebas de parche
efectuadas con acetaldehído produjeron eritema cutáneo en 12 sujetos
de «ascendencia oriental».
Se ha tenido conocimiento de una investigación limitada sobre la
incidencia del cáncer entre trabajadores expuestos a acetaldehído y
otras sustancias.
Según pruebas indirectas, el acetaldehído participa como
metabolito potencialmente tóxico en la inducción de las afecciones
hepáticas, la congestión facial y las alteraciones del desarrollo
asociadas con el alcohol.
9. Evaluación de los riesgos para la salud humana y los efectos en el
medio ambiente
Los estudios efectuados con animales indican que la toxicidad
aguda del acetaldehído por inhalación o por vía oral es baja. Según
las investigaciones con sujetos humanos y con animales, esa sustancia
es algo irritante para los ojos y las vías respiratorias superiores.
Esos mismos efectos se han apreciado en voluntarios a los que se
administró acetaldehído (sección 1.8). Se ha observado eritema
cutáneo consiguiente a las pruebas de parche en sujetos humanos.
Aunque se ha identificado un posible mecanismo, los datos de que se
dispone son insuficientes para calcular el potencial del acetaldehído
como inductor de sensibilización.
Existe poca información sobre los efectos del acetaldehído
ingerido. Tras administrar diariamente a ratas por vía oral 675 mg/kg
de peso corporal (NOEL = 125 mg/kg de peso corporal) se observó un
aumento liminar de la hiperqueratosis en la parte anterior del
estómago. En las ratas que ingirieron durante seis meses con el agua
de beber dosis aproximadas de 40 mg/kg de peso corporal hubo un
aumento de la síntesis de colágeno en el hígado, cuya significación
está por dilucidar.
Los estudios de inhalación con ratas y hámsters permiten afirmar
que el tejido sensible es el de las vías respiratorias superiores. La
concentración más baja a la que se observaron efectos fue 437 mg/m3
durante cinco semanas. El NOEL identificado del tracto respiratorio
fue 275 mg/m3 en ratas expuestas durante cuatro semanas y
700 mg/m3 en hámsters expuestos durante 13 semanas.
A las concentraciones que producen daños en los tejidos del
tracto respiratorio se observaron aumentos de la incidencia del
adenocarcinoma nasal y del carcinoma de células escamosas en la rata,
y de los carcinomas laríngeos y nasales en el hámster.
Hay indicios de que el acetaldehído produce in vivo
alteraciones genéticas en las células somáticas.
No se dispone de datos suficientes para determinar si el
acetaldehído tiene efectos en la reproducción y el desarrollo o en el
estado neurológico o inmunológico de la población general o de la que
está particularmente expuesta por razón de su trabajo.
A partir de datos sobre la acción irritante en sujetos humanos se
ha considerado que la concentración tolerable es de 2 mg/m3. El
mecanismo de inducción de tumores por el acetaldehído no está bien
estudiado. En consecuencia, se han adoptado dos criterios
orientativos a ese respecto; a saber, la determinación de una
concentración tolerable obtenida dividiendo un nivel de efecto
irritante en el tracto respiratorio de los roedores por un factor de
incertidumbre, y la estimación del riesgo de cáncer mediante
extrapolación linear. La concentración tolerable resulta ser de
0,3 mg/m3. Las concentraciones asociadas con un aumento del riesgo
permanente de 10-5 son de 11-65 µg/m3.
La escasez de datos impide llegar a conclusiones definitivas
sobre los riesgos potenciales del acetaldehído para la biota en el
medio ambiente. Sin embargo, basándose en la breve vida media del
acetaldehído en el aire y en el agua, asi como en el hecho de que es
fácilmente biodegradable, cabe afirmar que los efectos de esa
sustancia en los organismos terrestres y acuáticos son escasos,
excepto posiblemente con ocasión de descargas industriales o vertidos.
No data are available on the formation of acetaldehydemodified
proteins in animals or humans directly exposed to acetaldehyde.
However, some data are available on proteins modified by acetaldehyde
derived from ethanol metabolism. In these studies, proteins carrying
acetaldehyde adducts were shown to be present in the liver cytosol of
rats fed ethanol for periods of 3 weeks, 12 months, or 27 months
(Worrall et al., 1991a). Acetaldehyde-modified proteins have also been
detected in the plasma (Liu et al., 1990) and haemoglobin of
alcoholics (Niemela & Israel, 1992). Furthermore, a limited
immunohistochemical study has demonstrated the presence of
acetaldehyde-modified proteins in the livers of some alcoholics
(Niemela et al., 1991). These studies demonstrate that acetaldehyde
adducts can form in the body. The possible immunological consequences
of adduct formation will be discussed in section 8.9.
6.5.2 Nucleic acids
No data are available from in vivo studies on the generation of
DNA adducts.
Acetaldehyde reacts with nucleosides and deoxynucleosides at pH
6.5 and 37°C in vitro to form unstable adducts by binding to the
exocyclic amino groups of adenine, cytosine, and guanine (Hemminki &
Suni, 1984). Addition of a reducing agent (sodium borohydride) leads
to the formation of stable adducts, of which the main one was
identified as N2-ethylguanosine using mass spectrometry and NMR.
Similar data for the formation of unstable adducts formed by reacting
acetaldehyde with ribonucleosides and deoxyribonucleosides was
reported by Fraenkel-Conrat & Singer (1988). When ethanol was present
in the reaction mixture, a different type of adduct was formed, which
was identified by fast atom bombardment and proton NMR to be a mixed
acetal (-NH-CH(CH3)-OR). These adducts were found to have half-lives
varying from 2.5 to 24 h at pH 7.5 and 37°C, depending on the base
involved.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
An LC50 (semi-static study) of 35 mg/litre was found for
acetaldehyde in the guppy (Poecilia reticulata; 10 laboratory-reared
and acclimatized fish, 2-3 months old) (Deneer et al., 1988). Grahl
(1983) reported an LC50 (48-96 h) of 124 mg/litre for acetaldehyde
in fish (no additional information was provided). Juhnke & Luedemann
(1978) presented the results for fish obtained in the Golden Orfe
test, and found an LC50 of 140-124 mg/litre for acetaldehyde (no
additional information was provided). An LC50 (static conditions;
96-h) of 53 mg/litre was reported for the bluegill (Lepomis
macrochirus) by Von Burg & Stout (1991).
Acetaldehyde had a depressing effect on the aggressive behaviour
of the fish cichlid (Cichlasoma nigro fasciatum) at concentrations
that did not cause locomotor decrements in this species (Peeke &
Figler, 1981).
An EC5 (48-h; population growth) of 82 mg/litre and an EC50
(48-h; static conditions; immobilization) of 42 mg/litre were reported
for protozoa (Chilomonas paramecium) and the waterflea (Daphnia
magna), respectively (Von Burg & Stout, 1991).
7.2 Terrestrial organisms
Aharoni & Barkai-Golan (1973) studied the effects of acetaldehyde
vapours on the germination and colony-forming potential of two fungi
species, Alternaria tenuis and Stemphylium botryosum. The rate of
growth inhibition increased with both concentration and time of
exposure. The exposure of the spores was conducted at room
temperature. A. tenuis, the more sensitive species, was inactivated
by 0.54 µg acetaldehyde/m3 applied for 5 h, whereas 1.08 µg
acetaldehyde/m3 for 2 h, was needed to inactivate S. botryosum
spores.
Pittevils et al. (1979) reported the activity of acetaldehyde
against the fungi affecting stored apples and pears (Colletotrichum
gloeosporioides, Cryptosporiopsis malicorticis, Phlyctaena
vagabunda, Botrytis cinerea, and Alternaria tenuis). Acetaldehyde
was rapidly lethal at low concentrations: after a 24-h treatment
period, the lethal concentration of acetaldehyde ranged from
0.036 µg/m3 (A. tenuis) to 0.09 µg/m3 (C. gloeosporioides).
Acetaldehyde remained lethal for the five fungi, even when the
treatment lasted only 20 min (0.90 µg/m3 for P. vagabunda, C.
malicorticis, and A. tenuis, and 0.36 µg/m3 for C. gloeosporioides
and B. cinerea).
The fungi Botrytis cinerea, Penicillium expansum, Rhizopus
stolonifer, Monilinia fructicola, Erwinia carotovora, and
Pseudomonas fluorescens were killed, when exposed to
acetaldehyde vapours at concentrations ranging from 0.045 to
3.6 µg/m3, applied for 0.5 to 120 min at room temperature (Aharoni &
Stadelbacher, 1973).
Aharoni et al. (1979) studied acetaldehyde as a fumigant for
control of the green peach aphid (Myzus persicae) on head lettuce
(Lactuca sativa). When aphids were placed on the lettuce prior to
fumigation, 0.36 µg acetaldehyde/m3 and a 3-4 h exposure were
required for 100% mortality. A similar treatment (0.27-0.36 µg/m3
for 4 h) was found to cause 100% mortality of aphids on lettuce by
Stewart et al. (1980).
The fumigant effect of acetaldehyde was tested on the garden slug
(Arion hortensis; weight range, 0.2-0.5 g) and the grey field slug
(Agriolimax reticulatus; weight range, 0.3-0.6 g). It caused both
species to close the pulmonary aperture and to secrete excess
'irritation' mucus. Medial lethal values of 7.69 ± 0.21 mg/litre
per h for A. reticulatus and of 8.91 ± 0.81 mg/litre per h for
A. hortensis were found (Henderson, 1970).
The seed germination of the onion (Allium cepa L.), carrot
(Daucus carota L.), Palmer Amaranth (Amaranthus palmeri S Wats.),
and tomato (Lycopersicon esculentum Mill.) after exposure to
acetaldehyde (up to 1.52 mg/litre), was examined by Bradow & Connick
(1988). After a 3-day exposure, acetaldehyde inhibited the seed
germination of all four plants by more than 50%. Seeds inhibited by a
3-day exposure to acetaldehyde followed by a 4-day recovery period
germinated to the same extent as the controls after seven days, except
for the Palmer Amaranth, which remained inhibited.
Acetaldehyde at concentrations of 0.54-1.08 µg/m3 affected head
lettuce (Lactuca sativa), as evidenced by dark-green, water-soaked,
necrotic areas on the outer leaves of the lettuce. Concentrations of
up to 0.36 µg/m3 did not affect the lettuce (Aharoni et al., 1979;
Stewart et al., 1980).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 LD50 and LC50 values
Relevant data are summarized in Table 4.
Oral LD50s for acetaldehyde in rats and mice ranged from 660 to
1930 mg/kg body weight. LC50s (0.5-4 h) in rats and Syrian hamsters
ranged from 24 to 37 g/m3. It is, therefore, concluded that the
acute toxicity of acetaldehyde is low. LD50 values by the dermal
route were not available.
LD50s for intratracheal, subcutaneous, intraperitoneal, and
intravenous administration are also presented in Table 4.
Table 4. LD50/LC50 values for acetaldehyde
Species Route of LD50/LC50 Reference
administration
Rat oral 1930 mg/kg body weight Smyth et al. (1951)
Rat oral 660 mg/kg body weight Sprince et al. (1974)
Mouse oral 1230 mg/kg body weight US NRC (1977)
Dog oral > 600 mg/kg body weight Booze & Oehme (1986)
Rat inhalation 24 g/m3; 4 h Appelman et al. (1982)
Rat inhalation 37 g/m3; 0.5 h Skog (1950)
Syrian
hamster inhalation 31 g/m3; 4 h Kruysse (1970)
Syrian
hamster intratracheal 96.1 mg/kg body weight Feron & De Jong (1971)
Rat subcutaneous 640 mg/kg body weight Skog (1950)
Mouse subcutaneous 560 mg/kg body weight Skog (1950)
Mouse intraperitoneal 500 mg/kg body weight Truitt & Walsh (1971)
Mouse intravenous 165 mg/kg body weight O'Shea & Kaufman
(pregnant) (1979)
8.2 Short-term exposure
8.2.1 Oral
Oral administration in the drinking-water of 675 mg
acetaldehyde/kg body weight to Wistar rats, daily for 4 weeks,
resulted in slight to moderate focal hyperkeratosis of the forestomach
in 8/10 males and 8/10 females. No effects were observed at lower
dose levels of 25 and 125 mg/kg body weight. In the control group,
very slight focal hyperkeratosis of the forestomach was noted in 6/20
females and 3/20 males (1/20 slight). At the top dose (675 mg/kg),
relative kidney weights were slightly increased in males, urinary
production was decreased, and there were variations in serum
biochemistry, most of which were attributable to reduced water intake.
There were no effects in the liver. The no-observed-effect level
(NOEL) was 125 mg/kg, the lowest-observed-effect level (LOEL) was
675 mg/kg (Til et al., 1988).
8.2.2 Inhalation
Male Sprague-Dawley rats were continuously exposed to
acetaldehyde vapour for 22 days at levels gradually increasing from
750 mg/m3 for a few days up to 2500 mg/m3 for the last few days.
By gradually increasing the concentrations, mortality in the early
period following exposure to 2000-2500 mg/m3 was prevented,
presumably because of metabolic adaptation; sudden, high, blood
acetaldehyde levels inducing vagal reflex reactions may result in
respiratory inhibition, and, as a consequence, death (Lamboeuf et al.,
1987; Latge et al., 1987).
Groups of 10 male and 10 female Wistar rats were exposed to
acetaldehyde at 0, 720, 1800, 3950, or 9000 mg/m3 (0, 400, 1000,
2200, or 5000 ppm) for 6 h/day, 5 days/week, for 4 weeks. Mortality
was slightly increased at 3950 and 9000 mg/m3, whereas growth was
retarded at 1800 mg/m3 and above in males, and at 9000 mg/m3 in
females. At 9000 mg/m3, relative liver weight decreased, and
relative lung weight in males increased. No treatment-related
histopathological changes were observed in the liver. Degenerative
changes of the nose were observed after exposure to all concentrations
(720 mg/m3-9000 mg/m3), with hyperplasia and metaplasia occurring
at concentrations of 3950 mg/m3 or more. A NOEL was not identified
(LOEL: 720 mg/m3) (Appelman et al., 1982).
Groups of 10 male Wistar rats were exposed to acetaldehyde, for
6 h/day, 5 days/week, for 4 weeks, in three different patterns: (a)
as a continuous daily exposure of 6 h to 0, 270, or 900 mg/m3 (0,
150, or 500 ppm), (b) as two daily exposures of 3 h to similar
concentrations with an intervening 1.5-h period with no exposure, or
(c) as two daily 3-h periods of exposure to similar concentrations
with an intervening 1.5-h period with eight short (5-min) peaks of 6
times the basic concentration, resulting in time-weighted average
concentrations of 0, 255, or 1050 mg/m3, respectively. Though there
were no indications of toxicity following continuous or interrupted
exposures to 270 and 900 mg/m3 and intermittent high/low exposure to
255 mg/m3, intermittent high/low exposure to 1050 mg/m3 induced
growth retardation (Appelman et al., 1986).
At 900 mg/m3, the observed effects were very similar to the
ones reported earlier by Appelman et al. (1982) at 720 mg/m3.
Variation of the pattern of exposure, by including a 1.5-h break, or
by additionally including eight 5-min, 6-fold higher peak exposures,
did not alter the observed degenerative effects. No effects were
observed in Wistar rats exposed to a lower concentration, 5 days/week
for 4 weeks, either as a "continuous" (6 h/day) exposure of
270 mg/m3, or as a time-weighted average of 255 mg/m3 after the
described intermittent low-high exposure. The NOEL was 255 mg/m3,
6-h TWA (LOEL = 1050 mg/m3, 6-h time weighted average) (Appelman et
al., 1986).
In another study, groups of 12 male Wistar rats were exposed to 0
or 437 mg acetaldehyde/m3 (0 or 243 ppm), 8 h/day, 5 days per week,
for 5 weeks. Hyperplasia of the olfactory epithelium and nasal
inflammation were observed in exposed animals, and on the basis of
lung function tests, residual volume and functional residual capacity
were increased, indicating some (unspecified) damage of the distal
airways (Saldiva et al., 1985).
8.2.3 Dermal
No relevant studies were identified.
8.2.4 Parenteral
Effects in the liver have been reported in several studies, but
only at very high doses. Intraperitoneal injection of male albino
rats with 200 mg acetaldehyde/kg body weight, daily, for 10 days, with
additional pyrazole treatment to inhibit the conversion of
acetaldehyde to ethanol, caused fatty accumulation in the liver, as
indicated by accumulation of total lipids, triacyl glycerols, and
total cholesterol, increased glycogenolysis, and a shift in metabolism
from the citric acid cycle towards the pentose phosphate pathway in
the liver. Serum triacyl glycerol, total cholesterol, and free fatty
acid levels were also increased. Changes were similar in rats not
receiving pyrazole pretreatment (Prasanna & Ramakrishnan, 1984a,
1987). The same treatment altered thyroid function, as indicated by
lower serum T4 and decreased iodine uptake in male albino rats, though
these effects may have been secondary to the observed hepatic changes
(Prasanna et al., 1986) and histopathological changes of the pancreas,
with resulting changes in trypsinogen levels and amylase secretion and
activity in female Sprague-Dawley rats (Majumdar et al., 1986).
8.3 Skin and eye irritation; sensitization
No relevant data were identified.
8.4 Long-term exposure
8.4.1 Oral
In rats exposed to 0.05% acetaldehyde in drinking-water
(estimated by the Task Group to be approximately 40 mg/kg body weight)
for 6 months, there was an increase in collagen synthesis in the liver
(Bankowski et al., 1993). The toxicological significance of this
observation is not known; no other effects were examined.
8.4.2 Inhalation
Non-neoplastic effects observed in carcinogenicity studies are
discussed in section 8.7.1.
Groups of 20 Syrian hamsters were exposed to acetaldehyde vapour
at 0, 700, 2400, or 8200 mg/m3 (0, 390, 1340, or 4560 ppm) for
6 h/day, 5 days/week, for 13 weeks. Increased relative lung and heart
weights as well as growth retardation were reported after exposure to
8200 mg/m3, though there were no increases in mortality in any of
the exposed groups (Kruysse et al., 1975). At the highest
concentration, there were severe degenerative, hyperplastic, and
metaplastic changes in the epithelium as well as subepithelial glands
and turbinate bones. Rhinitis was observed, with abundant nasal
discharge and salivation. The epithelium of the larynx, trachea, and
lungs was damaged, with some focal hyperplasia and metaplasia,
accompanied by tracheitis and focal bronchopneumonia. Changes in the
tracheal epithelium were also observed at 2400 mg/m3. At
700 mg/m3, no significant effects were observed (NOEL: 700 mg/m3;
LOEL: 2400 mg/m3).
8.5 Reproductive and developmental toxicity
Studies on reproductive effects have not been identified. A
number of studies on developmental effects have been conducted,
primarily to investigate the role of acetaldehyde in ethanol-induced
teratogenicity. However, in all of these studies, acetaldehyde was
administered by injection rather than by the principal routes of
exposure in the occupational and general environments (i.e., ingestion
and inhalation). Results of identified studies in which acetaldehyde
was administered during gestation to rats and mice by intraperitoneal,
intravenous, or amniotic injection are presented in Table 5. Though
dose-related embryotoxic, fetotoxic, and teratogenic effects were
observed in most of these studies, particularly those on rats,
maternal toxicity was not adequately assessed or reported in any of
these investigations. Dose-related embryotoxic effects were also
observed in in vitro studies on rat embryos exposed to acetaldehyde
(Popov et al., 1981; Campbell & Fantel, 1983).
Effects on the placenta have been observed following
intraperitoneal injection of acetaldehyde into pregnant rats
(Sreenathan et al., 1984a). In an in vitro study on human placental
membrane vesicles, very high concentrations (approximately 100 times
higher than the highest reported levels in blood), inhibited L-alanine
transport (Asai et al., 1985).
8.6 Mutagenicity and related end-points
Relevant data are summarized in Table 6.
8.6.1 Bacteria
Acetaldehyde was not mutagenic when tested adequately in standard
preincubation assays with S. typhimurium.
Statistically significant mutagenic responses were induced in
E. coli WP2uvrA in preincubation assays without metabolic activation
at 37°C (Veghelyi et al., 1978) and 0°C (Igali & Gazso, 1980). In
contrast, in another study on the same strain, results were negative
(Hemminki et al., 1980).
8.6.2 Non-mammalian eukaryotic systems
8.6.2.1 Gene mutation assays
Acetaldehyde (0.1% or 1.0% for 2 h) induced mutations in
genes that affect the egg-laying system of Caenorhabditis elegans
(Greenwald & Horvitz, 1980).
Acetaldehyde induced sex-linked recessive lethal mutations in
Drosophila melanogaster (Woodruff et al., 1985).
8.6.2.2 Chromosome alterations
Acetaldehyde induced chromosome malsegregation and mitotic
cross-over (yA2 marker) in Aspergillus nidulans diploid strain P1
during early conidial germination (Crebelli et al., 1989).
There was a dose-related increase in chromosomal aberrations in
Vicia faba root tips exposed to acetaldehyde (Rieger & Michaelis,
1960). Acetaldehyde also induced chromosome aberrations, micronuclei,
and sister chromatid exchanges (SCEs) in the root meristem cells of
Allium cepa (Cortes et al., 1986).
Table 5. Developmental toxicity
Species Exposure Dose Effects Reference
Rat day 10, 11, or 12; 0, 50, 75, 100 mg/kg fetal resorption, malformations (oedema, microcephaly, Sreenathan
days 10-12 of body weight, ip micrognathia, micromelia, hydrocephaly, exencephaly), growth et al. (1982,
pregnancy; days retardation; reduced placental weight; 50 mg/kg body weight, 1984b)
8-15 (50 mg/kg days 8-15: delays in ossification; skeletal malformations,
body weight per such as wavy ribs; no discussion of maternal effects
day)
Rat days 8-15 of 50, 75, 100 or 150 increase in resorption, and fetal death (changes were dose Padmanabhan
pregnancy mg/kg body weight, dependent); malformations: oedema, microcephaly, micrognathia, et al. (1983)
ip micromelia, syndactyly, hydronephrosis and subcutaneous
haemorrhage in the face, fore- and hind paws; reduced mean
placental weight and umbilical cord length; increased amniotic
fluid weight
maternal toxicity: rel. reduced water, food consumption, rel.
reduced body weight gain, 50 mg and above
Rat day 13 of pregnancy 1% or 10% solution 10%: 100% mortality; 1%: 80% malformations versus 14% in Bariliak &
embryo amniotic injections controls Kozachuk
(1983)
Rat days 9-12 of pregancy 1% (100 mg/kg body reduction in head length; no effects on morphological Ali &
weight), ip scores or crown rump length Persaud (1988)
Table 5 (contd).
Species Exposure Dose Effects Reference
Rat days 8-13 of 10 or 50 mg/kg body reduced performance in surface righting, olfactory Schreiner
pregnancy weight, ip discrimination, and learning ability; lower startle amplitudes et al. (1987)
in an auditory startle habituation test; maternal toxicity not Abstract
addressed; age of testing of offspring not specified
Rat day 5 of gestation 0.03-0.04 µg/kg body retarded blastulation Checiu et
weight examination on al. (1984)
day 5 of gestation
Mouse day 7 or 8 of 0.32 g/kg, iv exencephaly, mandibular and maxillary hypoplasia; Webster et
pregnancy; day 9 polydactyly club foot; no discussion of maternal effects al. (1983)
or 10 of pregnancy
Mouse day 10 of pregnancy 1000 mg/kg, ip no increase in resorptions or malformed fetuses or decrease Blakley &
in fetal weight; no discussion of maternal effects Scott (1984a)
Mouse day 7, 8 or 9 of 31 or 62 mg/kg body dose-dependent embryolethality; non-closure of anterior or O'Shea &
pregnancy weight, iv; posterior neuropore; smaller embryos; no discussion of Kaufman
examination on day maternal effects (1979)
10 or 19 of gestation
Mouse day 9 of pregnancy 2, 4, 6% (8 ml/kg no intermediate cellular effeects Bannigan &
body weight), ip Burke (1982)
Mouse day 6, 7 or 8 of 62 mg/kg body weight, neural tube effects; embryonic mortality; no discussion O'Shea &
pregnancy; and days iv; examination on of maternal effects Kaufman
6-8, 7-8 or 7-9 of day 10 or 12 of (1981)
pregnancy gestation
Table 6. Tests for gene mutation, chromosomal damage, and sister chromatid exchanges induced by acetaldehyde
Test system Measured end-point Test conditions MA RES Reference
Prokaryotic systems
Escherichia coli WP2uvrA reverse mutations 40 µg/ml; preincubated in capped tubes - + Veghelyi et al. (1978)
Escherichia coli WP2uvrA reverse mutations 0.88-441 µg/ml; incubated in capped - - Hemminki et al. (1980)
tubes at 37°C
Escherichia coli Wp2uvrA reverse mutations 0.8 µg/ml; incubated in capped tubes - + Igali & Gazso (1980)
at 0°C
Salmonella typhimurium
TA102 reverse mutations 1014 µg/plate; liquid preincubation - - Marnett et al. (1985)
TA104 -
Salmonella typhimurium reverse mutations 33-10 000 µg/plate; liquid preincubation + - Mortelmans et al. (1986)
TA1535 - -
TA1537 + -
- -
TA97 + -
- -
TA98 + -
- -
TA100 + -
- -
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Salmonella typhimurium concentration not given; + - Sasaki & Endo (1978)
TA100 liquid preincubation
Non-mammalian eukaryotic systems
Allium cepa root chromosomal aberration 7.5-7500 µg/ml - + Cortés et al. (1986)
meristem cells and SCE +
Vicia faba root tips chromosomal aberration 220-2205 µg/ml for 24 h at 12°C - + Rieger & Michaelis (1960)
Caenorhabditis elegans gene mutation 794-7940 µg/ml for 2 h - + Greenwald & Horvitz
(1980)
Drosophila melanogaster sex-linked recessive 22 500 mg/litre in 10% ethanol by + Woodruff et al. (1985)
injection
Canton-S wild-type lethal mutations 25 000 mg/litre in 10% ethanol by -
feeding
Aspergilus nodulans chromosomal 198-2381 µg/ml + Crebelli et al. (1989)
Diploid P1 malsegregation
In vitro mammalian systems
Mouse lymphoma L5178Y forward mutation (tk 176-353 µg/ml for 4 h - + Wangenheim & Bolcsfoldi
cells locus) (1986, 1988)
Chinese hamster ovary chromosomal aberration 88-5000 µg/ml - + Au & Badr (1979)
cells
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Chinese hamster ovary SCE 1.3-13 µg/ml - + Brambilla et al. (1986)
cells
Chinese hamster ovary SCE 8-80 µg/ml for 1 h + + De Raat et al. (1983)
cells - +
Chinese hamster ovary SCE 2-12 µg/ml for 24 h - + Obe & Beek (1979)
cells
Chinese hamster ovary SCE 4-8 µg/ml for 8 days - + Obe & Ristow (1977)
cells
Chinese hamster ovary hyperploidy 0.35-1.05 mmol/litre - + Dulout & Furnus (1988)
cells hypoploidy +
Human lymphocytes forward mutations 26.5-106 µg/ml for 24 h - + He & Lambert (1990)
(hprt locus) 8.8-26.5 µg/ml for 48 h
Human lymphocytes chromosomal aberration 0.1-20 mmol/litre added + Obe et al. (1985)
every 12 h for 5 days
Human lymphocytes chromosomal aberration 8-15 µg/ml - + Obe et al. (1979)
(normal Franconis
anaemia)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 0.8 µg/ml 2x/day, 4 days - + Obe et al. (1978)
Human lymphocytes chromosomal aberration 4-48 µg/ml for 72 h - + Boehlke et al. (1983)
SCE +
Human (whole blood, chromosomal aberration 20-40 µg/ml + Badr & Hussain (1977)
alcoholics)
Human lymphocytes SCE 4.4-106 µg/ml for 1-70 h - + He & Lambert (1985)
Human lymphocytes SCE 4.4-13 µg/ml for 70 h - + Lambert & He (1988)
Human lymphocytes SCE 4.4-18 µg/ml for 72 h + Helander & Lindahl-
Kiessling (1991)
Human lymphocytes SCE 4.4-22 µg/ml for 48 h + Sipi et al. (1992)
Human lymphocytes SCE 4-8 µg/ml for 90 h - + Jansson (1982)
Human lymphocytes SCE 0.04-4.4 µg/ml for 55 h - + Knadle (1985)
Human lymphocytes SCE 5.5-88 µg/ml for 48 h - + Norppa et al. (1985)
Human lymphocytes SCE reaction mixture added to whole blood - + Obe et al. (1986)
samples in dialysis tube; + +
concentration not given
Human lymphocytes SCE 4-16 µg/ml for 24 h - + Ristow & Obe (1978)
Table 6 (contd).
Test system Measured end-point Test conditions MA RES Reference
Human lymphocytes chromosomal aberration 1.8-35 µg/ml for 72 h - + Véghelyi & Osztovics
(1978)
Human lymphocytes SCE 1.8-35 µg/ml for 72 h + Véghelyi et al. (1978)
Wistar rat (skin chromosomal aberration 4.4-44 µg/ml for 12, 24, or 48 h - + Bird et al. (1982)
fibroblast) (micronuclei)
Mouse embryo cell transformation 10-100 µg acetaldehyde/ml for 24 h - - Abernethy et al. (1982)
C3H/10T1/2 cells 10-100 µg/ml followed by 0.25 µg +
TPA/ml 2x/week
Rat (HRRT kidney cells) cell transformation acetaldehyde - - Eker & Sanner (1986)
acetaldehyde followed by TPA and PDD +
MA = Metabolic activation; RES = Result.
8.6.3 Cultured mammalian cells
8.6.3.1 Gene mutation assays
Acetaldehyde induced gene mutations at the hypoxanthineguanine
phosphoribosyl transferase (hprt) locus in human lymphocytes
in vitro; there was a statistically significant and dose-related
increase in the frequency of mutants (He & Lambert, 1990).
Acetaldehyde produced a significant dose-related increase in forward
mutations in a mouse lymphoma L5178Y thimidine kinase locus assay,
without exogenous metabolic activation (Wangenheim & Bolcsfoldi, 1986,
1988).
8.6.3.2 Chromosome alterations and sister chromatid exchange
Dose-dependent increased frequencies of chromosome aberrations
have been observed in human lymphocytes following incubation with
acetaldehyde (Badr & Hussain, 1977; Obe et al., 1978, 1979, 1985;
Veghelyi & Ostovics, 1978; Boehlke et al., 1983).
Acetaldehyde induced a dose-dependent increase in chromosomal
damage in Chinese hamster ovary cells, though it was reported that the
activity was reduced (not quantified) in the presence of liver
homogenate fraction (Au & Badr, 1979). Dose-related increases in
micronuclei and chromosome aberrations were observed in cultured rat
skin fibroblasts in the absence of metabolic activation (Bird et al.,
1982).
SCE was induced by acetaldehyde (1.3-106 µg/ml) in the absence of
exogenous metabolic activation in Chinese hamster ovary cells and
human lymphocyte cultures (Obe & Ristow, 1977; Ristow & Obe, 1978;
Veghelyi et al., 1978; Veghelyi & Osztovics, 1978; Obe & Beek, 1979;
Jansson, 1982; Boehlke et al., 1983; De Raat et al., 1983; He &
Lambert, 1985; Knadle, 1985; Norppa et al., 1985; Brambilla et al.,
1986; Obe et al., 1986; Lambert & He, 1988; Helander &
Lindahl-Kiessling, 1991; Sipi et al., 1992). The increase in SCEs in
Chinese hamster ovary cells was less pronounced after addition of an
exogenous metabolic activating system (De Raat et al., 1983).
Similarly, addition of NAD+ and ALDH to human lymphocyte cultures
exposed to acetaldehyde decreased SCE induction (Obe et al., 1986).
Addition of 1-amino-cyclopropanol, an inhibitor of ALDH, to human
lymphocytes enhanced SCE induction by acetaldehyde (Helander &
Lindahl-Kiessling, 1991). These observations suggest that ALDH may
reduce the genotoxic potential of acetaldehyde.
Acetaldehyde induced aneuploidy has been reported in Chinese
hamster ovary cells (Dulout & Furnus, 1988). However, mainly
hypoploid cells were increased and, therefore, no firm conclusion can
be drawn from these studies. Acetaldehyde induced SCE in
pre-implantation mouse embryos in vitro (Lau et al., 1991).
8.6.4 In vivo assays
Only limited data are available on the genotoxicity of
acetaldehyde in vivo.
8.6.4.1 Somatic cells
C57BL/6J mice (only 2 animals of unspecified sex at each dose
level) received daily intraperitoneal doses of 0, 6, or 12 mg
acetaldehyde/kg body weight per day, for 5 days, and blood samples
were collected on days 3-6. The frequencies of micronuclei in mature
peripheral erythrocytes on days 5 and 6 (combined) were significantly
increased at the low dose, but not at the high dose (Ma et al., 1985).
No information on the frequency in polychromatic erythrocytes was
provided, so the possibility that this result was due to increased
erythropoiesis instead of mutagenesis cannot be discounted. This
study was reported only in the form of an abstract.
Groups of 6-7 Chinese hamsters (male and female) received 0.01,
0.1, or 0.5 mg acetaldehyde/kg body weight in saline by single
intraperitoneal injection and were killed 24 h later. Doses of
0.6 mg/kg body weight were lethal in preliminary tests. The
frequencies of SCEs in bone-marrow metaphases were elevated at 0.5 mg
acetaldehyde/kg body weight, but not at the lower doses (Korte & Obe,
1981). These observations support earlier findings in a more limited
study at an embryotoxic dose in mice and indicate in vivo formation
of SCEs (Obe et al., 1979).
Female Wistar rats were injected with 0.02 ml of 1% acetaldehyde
intra-amniotically on day 13 of pregnancy. Embryonic cells were
obtained 24 h later for cytogenetic analysis. Exposed rat embryos had
a higher frequency of chromosomal aberrations (mostly chromatid gaps
and breaks) than controls (Barilyak & Kozachuk, 1983). There was no
increase in the frequency of aneuploid cells.
8.6.4.2 Germ cells
Hybrid male mice ((C57B1/6J × C3H/He)F1), 4/dose group, were
injected intraperitoneally with a single dose of 0, 125, 250, 375, or
500 mg acetaldehyde/kg body weight in saline. Thirteen days after
exposure, the frequency of micronuclei in early spermatids was not
increased, though there were adequate positive controls (Lahdetie,
1988).
8.6.5 Other assays
Relevant data are summarized in Table 7.
8.6.5.1 DNA single-strand breaks
No single-strand breaks were detected with the alkaline elution
assay in DNA from various sources, after exposure in vitro to
acetaldehyde (Sina et al., 1983; Marinari et al., 1984; Harris et al.,
1985; Lambert et al., 1985; Saladino et al., 1985).
8.6.5.2 DNA cross-linking
There is some indirect evidence suggesting DNA cross-linking by
acetaldehyde.
Ristow & Obe (1978) observed enhanced reannealing of heat
denatured, isolated, calf thymus DNA following incubation with
acetaldehyde. It was suggested that this may have been due to DNA-DNA
cross-linking, because thermally denatured DNA will not reanneal in
this assay, unless complimentary DNA strands are held together by
cross-linkings.
Altered alkaline elution patterns indicative of DNA-DNA
cross-links were observed with DNA isolated from cultured human
leukocytes and exposed initially to acetaldehyde and then to X-rays
(Lambert et al., 1985; Lambert & He, 1988). DNA-protein cross-linking
was not observed by Harris et al. (1985) or by Saladino et al. (1985)
after exposure of human bronchial epithelial cells in vitro, but it
was observed by Lam et al. (1986) after incubation of either calf
thymus nucleohistones or fresh homogenates of the nasal mucosa of rats
with acetaldehyde. The possibility that acetaldehyde induces
DNA-protein cross-links in male Fischer-344 rat nasal mucosa has been
indirectly studied by measuring the extractability of DNA from
insoluble proteins following in vitro and in vivo exposures.
Decreased extractability of DNA was observed in preparations from
tissue homogenates exposed to acetaldehyde at concentrations of 100
and 500 mmol per litre but not 10 mmol/litre. Similarly, decreased
extractability was observed in nasal mucosal preparations from rats
exposed for 6 h to 1000 or 3000 mg/litre (ppm) but not 100 or
300 mg/litre (ppm) (Lam et al., 1986).
Table 7. Other tests indicative of genetic damage induced by acetaldehyde
Test system Measured end-point Test conditions Results Reference
Calf thymus DNA DNA-DNA cross-links 44 100 µg/ml for 30 min + Ristow & Obe (1978)
Calf thymus nucleohistones DNA-protein cross-links 4410-44 100 µg/ml - Lam et al. (1986)
Chinese hamster ovary DNA single-strand breaks no details - Marinari et al. (1984)
K1-cells DNA-DNA cross-links +
Rat hepatocytes DNA single-strand breaks 1.3 µg/ml for 3 h - Sina et al. (1983)
Human bronchial epithelial DNA single-strand breaks unknown concentration; - Harris et al. (1985)
cells DNA-protein cross-links 6 h -
Human bronchial epithelial DNA single-strand breaks up to 44 µg/ml for 1 h - Saladino et al. (1985)
cells DNA-protein cross-links -
Human leukocytes DNA single-strand breaks 441-882 µg/ml for 4 h - Lambert et al. (1985)
DNA-DNA cross-links +
Human lymphocytes DNA-DNA cross-links 441 µg/ml + Lambert & He (1988)
8.6.6 Cell transformation
Acetaldehyde did not initiate cell transformation in cultured
C3H/10T1/2 cells in the presence of the tumour promotor
12- O-tetradecanoylphorbol-13-acetate (TPA) (Abernethy et al., 1982).
Acetaldehyde initiated cell transformation in a rat kidney cell
line (HRRT) after pretreatment with tumour promotors (Eker & Sanner,
1986).
8.7 Carcinogenicity bioassays
8.7.1 Inhalation exposure
Only one carcinogenicity study in which animals were exposed by
inhalation to acetaldehyde over a lifetime has been performed. In
other carcinogenicity bioassays, animals were exposed for shorter
periods.
Wistar rats (55/sex per dose) were exposed for life (6 h/day, 5
days/week, for 28 months) to acetaldehyde concentrations of 1350,
2700, or 1800-5400 mg/m3 (the last concentration was gradually
reduced from 5400 mg/m3 in week 20 to 1800 mg/m3 in week 52).
Satellite groups of 5-10 additional rats of each sex were killed at
13, 26, and 52 weeks. Growth retardation occurred throughout the
study at all dose levels. Mortality was greater than in controls in
all dose groups and all of the animals in the high-dose group had died
by week 102. At week 52, there were degenerative changes in the
olfactory nasal epithelium at all dose levels including slight to
severe hyperplasia and keratinized stratified metaplasia of the larynx
(high dose only) and degenerative changes of the upper respiratory
epithelium (including papillomatous hyperplasia at the top dose only).
In the trachea, there was focal flattening and irregular arrangement
of the epithelium in 3/10 top-dose males at 52 weeks. In satellite
groups of 30 rats per sex, for which there was a 26-week recovery
period after 52 weeks of exposure, there was evidence of partial
regeneration of the olfactory epithelium in the low- and mid-dose
groups; there was also progression from hyperplasia and metaplasia to
neoplasia in some animals. At 28 months, carcinomas of the nose
developed in all exposed groups (Table 8). Although tumour incidence
was dose-related, the latency period appeared to be independent of
concentration. First tumours in all groups appeared during the 12th
month of exposure. The incidence of tumours was not increased in the
lungs, larynx, and trachea.
In simultaneously exposed groups of 30 Wistar rats/sex per dose,
exposure was terminated after 52 weeks and the animals killed
following a recovery period of 26 weeks. After the recovery period,
both mortality and nasal tumour incidence were very similar to those
in the groups in which exposure had been continued for 26 more weeks
(Woutersen et al., 1984, 1986; Woutersen & Feron, 1987).
Groups of Syrian golden hamsters (36/sex per dose) were exposed
for 52 weeks (7 h/day, 5 days/week) to a concentration gradually
reduced from 4500 mg/m3 in week 9 to 2970 mg/m3 in week 52. At
week 52, six animals/sex per dose were killed; the remaining animals
(30/sex per dose) were killed after a recovery period of 29 weeks. At
the end of the recovery period, nasal carcinomas were observed in 1/26
males and 1/27 females versus 0/24 and 0/23 in controls, and the
incidence of laryngeal carcinomas was increased (5/23 in males, 3/20
in females, against 0/20 and 0/22 in controls). No tumours were
observed in bronchi or lungs (Feron et al., 1982).
Table 8. Incidence of nasal tumours in Wistar rats after 28 months
of exposurea,b
Types of tumour 0 1350 2700 2760c
mg/m3 mg/m3 mg/m3 mg/m3
Males
Squamous cell carcinoma 1/49 1/52 10/53* 16/49***
Adenocarcinoma 0/49 16/52*** 31/53*** 21/49***
Carcinoma in situ 0/49 0/52 0/53 1/49
Females
Squamous cell carcinoma 0/50 0/48 5/53 17/53***
Adenocarcinoma 0/50 6/48* 28/53*** 23/53***
Carcinoma in situ 0/50 0/48 3/53 5/53
a From: Woutersen et al. (1986).
b Total number of tumour-bearing animals not specified.
Significance: Fisher Exact Test *P < 0.05, **P < 0.01, ***P <
0.001.
c The highest concentration was gradually reduced from
5400 mg/m3 during the first 20 weeks to 1800 mg/m3 in week
52; the time-weighted average concentration for 28 months of
exposure was calculated by the Task Group to be 2760 mg/m3.
Groups of 35 male Syrian hamsters were exposed by inhalation to 0
or 2700 mg acetaldehyde/m3 (0 or 1500 ppm) for 7 h/day, 5 days/week
for 52 weeks. At 52 weeks, 5 animals were killed for pathological
examination. The remainder of the animals were maintained for a
further 26-week recovery period. Body weight gain was less in the
exposed animals and mortality was increased from week 52 onwards.
There was a slight, but significant, decrease in rbc, haemoglobin, and
haematocrit in exposed animals. Relative kidney weights were
increased and urinary levels of protein and GOT were elevated. There
were marked lesions in the nasal cavity of animals killed at 52 weeks:
flattened epithelial cells with bizarre nuclei, fewer subepithelial
glands, submucosal thickening, and keratinizing stratified squamous
metaplasia of olfactory and respiratory epithelium. There was also
slight focal hyperplasia and metaplasia of the epithelium of the
trachea. There was partial or complete recovery from these lesions in
the animals killed at the end of the recovery period. There were no
tumours of the respiratory tract in any of the animals exposed to
acetaldehyde (Feron, 1979).
Weekly intratracheal administration of 0.2 ml of 2 or 4%
acetaldehyde in saline during a period of 52 weeks, followed by a
recovery period of another 52 weeks, did not induce any tumours in the
respiratory tract of male and female Syrian golden hamsters (Feron,
1979).
8.7.2 Co-carcinogenicity and promotion studies
In a mid-term test (Ito Model), groups of 19-20 male F344 rats
received a single intraperitoneal injection of diethylnitrosamine and
then various concentrations of acetaldehyde (2.5% and 5%, associated
with a recorded daily intake of 1.66 and 2.75 mg/kg body weight) in
their drinking-water, from week 2 until termination in week 6. All
rats had a two-thirds partial hepatectomy in week 3, in order to
stimulate cell proliferation. At 6 weeks, there was a significant
decrease in liver and body weight in all exposed animals, but
acetaldehyde had no effect on the development of glutathione
S-transferase (placental type)-positive liver cell foci (Ikawa et
al., 1986).
There were laryngeal tumours in 7/31 male and 6/32 female Syrian
golden hamsters exposed to a mixture of isoprene (approximately
2.09 mg/m3 or 750 ppm), methyl chloride (approximately 1.94 mg/m3
or 950 ppm) methyl nitrite (approximately 0.49 mg/m3 or 195 ppm) and
acetaldehyde (approximately 2340 mg/m3 or 1300 ppm) for 6 h/day, 5
days/week, over 23 months. There were no tumours of the respiratory
tract in controls (Feron et al., 1985). The authors suggested that
the laryngeal effects observed in hamsters exposed to the vapour
mixture were most probably caused by acetaldehyde alone (presumably
based on comparison with results in studies on hamsters exposed to
acetaldehyde alone).
There was no evidence of co-carcinogenicity in Syrian golden
hamsters exposed either by simultaneous inhalation exposure to
acetaldehyde and intratracheal instillation of benzo(a)pyrene (Feron,
1979; Feron et al., 1982) or by intratracheal instillation of
diethylnitrosamine (Feron et al., 1982) for 52 weeks followed by
recovery periods of 26-28 weeks. Nor was there any evidence of the
co-carcinogenicity of acetaldehyde following simultaneous
intratracheal instillations of acetaldehyde and either benzo (a)pyrene
or diethylnitrosamine for 52 weeks, followed by a recovery period of
52 weeks (Feron et al., 1982).
8.8 Neurological effects
Secondary effects of acetaldehyde on the respiratory and
cardiovascular systems, attributed to its influence on the autonomic
nervous system, have been observed following acute exposure of
experimental animals to acetaldehyde, principally by infusion or
intravenous administration. In two identified studies in which
animals were exposed by a route more relevant to environmental or
occupational exposure (i.e., inhalation), a 50% decrease in
respiratory rate was observed in two strains of mice and in rats
during inhalation of 5000 mg/m3 for 10 min (Steinhagen & Barrow,
1984; Babiuk et al., 1985; Takahashi et al., 1986).
Neurological effects following exposure by routes most relevant
to environmental and occupational exposure (i.e., inhalation and
ingestion) were limited to biochemical changes in the brain in
short-term studies on rats and mice exposed to relatively high
concentrations of acetaldehyde (750-13 230 mg/m3). Reported effects
included changes in the phospholipid fractions and increases in the
concentrations of monoamines and Na+/K+ATPase (Ortiz et al., 1974;
Shiohara et al., 1985; Latge et al., 1987; Roumec et al., 1988).
Manifestations of neurotoxicity were not examined in any of these
studies.
In the only identified study in which histopathological effects
on the nervous system were reported, degeneration was observable using
both light and electron microscopy in the cerebral cortex of rats
receiving a single intraperitoneal injection of 5 mg acetaldehyde/kg
body weight (Phillips, 1987).
In a short-term study involving intravenous exposure (24-26 mg/kg
body weight per day, for 20 days), the level of salsolinol in the
brain of rats was increased (Myers et al., 1985). Biochemical effects
of acetaldehyde on the brain have also been observed in vitro in
cerebral cortical neurons and brain microsomal preparations (Lahti &
Majchrowicz, 1969; Cederbaum & Rubin, 1977; Kuriyama et al., 1987).
8.9 Immunological effects
8.9.1 Direct effects on immune cells
Only one study has been carried out on whole animals. In a study
on CD1 mice exposed to 324 mg/m3, 3 h/day for 5 days, the
bacteriocidal activity of alveolar macrophages was reduced by 15%.
However, acetaldehyde did not affect mortality following streptococcal
infection (Aranyi et al., 1986).
In in vitro studies, 0.01% acetaldehyde caused a significant
inhibition of human granulocyte chemotaxis (Schopf et al., 1985). At
0.03%, acetaldehyde inhibited the release of lysozyme from monocytes,
but granulocytes were unaffected. The generation of oxygen radicals
by zymosan particle-stimulated monocytes and granulocytes was
inhibited by acetaldehyde in a dose-dependent manner. In a similar
study in which peripheral mononuclear cells were incubated with
acetaldehyde, decreased lytic activity against K562 cells was observed
at concentrations higher than 12 µmol per litre (Fink & Dancygier,
1988). In another study, acetaldehyde decreased 3H thymidine
incorporation into phytohaemagglutinin or Concanavalin A - stimulated
human lymphocytes (Levallois et al., 1987).
8.9.2 Generation of antibodies reacting with acetaldehyde-modified
proteins
The modification of proteins has been described in section 6.5.1.
In several studies, these modified proteins induced an immune response
when injected into rabbits and mice (Israel et al., 1986; Worrall et
al., 1989). The reactivity of the resulting antibodies towards
proteins, modified by acetaldehyde in vitro, is independent of the
carrier protein. These studies demonstrate (a) that
acetaldehyde-modified proteins are immunogenic; and (b) that
antibodies raised against a single modified protein will cross-react
with any other protein modified in the same manner.
Passive immunization of rats with a monoclonal IgE
antiacetaldehyde adduct antibody, derived from mice immunized with
acetaldehyde-modified proteins, rendered these animals hypersensitive
to acetaldehyde-modified proteins (Israel et al., 1992).
No immunization studies analogous to those described above have
been carried out on humans. In several studies, the presence of
antibodies, reactive with proteins modified by acetaldehyde in vitro,
has been reported in the plasma of alcoholics (Niemela et al., 1987;
Hoerner et al., 1988; Worrall et al., 1990, 1991a,b). The results of
these studies suggest that acetaldehyde-modified proteins are
generated in humans.
8.9.3 Related immunological effects
In rat liver membrane vesicles, exposed to acetaldehyde in vitro,
superoxide anion production by neutrophils was significantly enhanced
(Williams & Barry, 1986). In a further study, human liver plasma
membranes, modified by acetaldehyde, activated complement protein C3
components (Barry & McGivan, 1985).
8.10 Biochemical effects
Biochemical effects have been observed in cultured hepatocytes,
liver homogenates and mitochondrial fractions, purified enzymes,
isolated hepatic lipid membranes and whole liver preparations,
including: metabolic effects on lipid peroxidation (Stege, 1982;
Mueller & Sies, 1987; Shaw & Jayatilleke, 1987), phospholipid
metabolism (Snyder, 1988), transaminase activity (Solomon, 1987;
Snyder, 1988), carbohydrate and lipid metabolism (Matsuzaki & Lieber,
1977; Cederbaum & Dicker, 1981), and mitochondrial respiration
(Cederbaum et al., 1974). Increased hepatic collagen synthesis has
also been observed (Savolainen et al., 1984; Brenner & Chojkier,
1987).
Metabolic effects have been observed in other in vitro systems
including: renal cortex tubules (Michoudet & Baverel, 1985: altered
pyruvate/lactate metabolism), kidney, and muscle microsomal
preparations (Cederbaum & Rubin, 1977: inhibition of pyruvate
dehydrogenase), perfused hearts and cardiac whole homogenates
(Schreiber et al., 1972, 1974: myocardial protein synthesis and
inhibition of cardiac microsomal protein synthesis; Rawat, 1979:
reduced protein synthesis; Segel, 1984: no effect on mitochondrial
respiration), myocardial cells (McCall & Ryan, 1987: no effect on
Na+/K+-ATPase), leukocytes (Green & Baron, 1986: inhibition of
Na+/K+-ATPase), erythrocytes (Helander & Tottmar, 1987: inhibition
of the disappearance rate of biogenic aldehydes; Ninfali et al., 1987:
induction of intracellular reduced state; Solomon, 1988: inhibition of
aldolase-mediated by acetaldehyde oxidation- and inhibition of the
aminotransferases-mediated by non-oxidative acetaldehyde
metabolization; Atukorala et al., 1988: inhibition of transketolase),
and leukocytes and platelets (Helander & Tottmar, 1987: inhibition of
the disappearance rate of biogenic aldehydes).
9. EFFECTS ON HUMANS
9.1 General population exposure
No specific studies were available.
9.2 Occupational exposure
9.2.1 General observations
Acute exposure to acetaldehyde vapours has resulted in irritation
of the eyes and mucous membranes, reddening of the skin, pulmonary
oedema, headache, and sore throat. Repeated exposure causes
dermatitis and conjunctivitis. Ingestion causes nausea, vomiting,
diarrhoea, narcosis, and respiratory failure. Liquid acetaldehyde was
reported to cause superficial injury of the cornea. These effects
were reported in reviews and not in documented studies (Grant, 1974;
Hagemeyer, 1978; Dreisbach, 1987). In addition, a statement was found
in a textbook stating that prolonged exposure to acetaldehyde caused a
decrease in red and white blood cells plus a sustained rise in blood
pressure; however, no further information was available (Hagemeyer,
1978).
9.2.2 Clinical studies
A group of 12 volunteers were exposed in a chamber to various
nominal concentrations of acetaldehyde vapour on 15 different
occasions. No atmospheric sampling was performed. At 90 mg/m3
(50 ppm), the majority of the group experienced some degree of eye
irritation. Several subjects were discomforted at a lower
concentration of 45 mg/m3 (25 ppm), but no details were provided
(Silverman et al., 1946).
A group of 14 "healthy male" volunteers, aged 18-45 years, were
exposed in a 100 m3 chamber to a measured concentration of
acetaldehyde vapour of 240 mg/m3 (134 ppm) for 30 min. This
concentration was said to be mildly irritating to the upper
respiratory tract. No other clinical signs were reported (Sim &
Pattle, 1957).
Twelve volunteers of Oriental ancestry were patch-tested with
acetaldehyde (75%), ethanol (75%), and ethyl acetate (25%). With
acetaldehyde treatment, cutaneous erythema was seen in all 12
subjects, whereas ethanol was positive in 5 subjects. No vascular
response was detected for the acetaldehyde metabolite, ethyl acetate
(Wilkin & Fortner, 1985).
In an additional study, increased heart rate, ventilation, and
calculated respiratory dead space, and a decrease in alveolar CO2
levels were reported, following intravenous infusion of acetaldehyde
(5% v/v) in young male volunteers (Asmussen et al., 1948).
9.2.3 Epidemiological studies
A very limited investigation was performed on an unspecified
number of people, selected from 150 workers who had been employed for
20 years or more in a chemical factory. Nine cases of cancer were
identified (all in male smokers) 5 of which were in the respiratory
tract, 2 in the oral cavity, 1 in the stomach, and 1 in the caecum.
The number of respiratory cancers was higher than expected compared
with the prevalence in the national population (not corrected for
smoking). In parts of the factory, there was exposure to a variety of
chemicals. In one area, 1-7 mg acetaldehyde/m3 had been measured,
together with 5-70 mg butyraldehyde/m3, 1-7 mg crotonaldehyde/m3,
2-6 mg n-butanol per m3, and about 15 mg ethylhexanol/m3. Other
areas of the factory were known to have higher concentrations of
vapours of aldehydes and aldol, which caused irritation of the eyes
and upper respiratory tract (Bittersohl, 1974, 1975).
9.3 Effects of endogenous acetaldehyde
9.3.1 Effects of ethanol possibly attributable to acetaldehyde or
acetaldehyde metabolism
There is no direct evidence to link acetaldehyde with liver
injury. However, data obtained from animal models and human subjects
suggest that acetaldehyde may play a role in liver damage, especially
that associated with ethanol (sections 3.1.1 and 8.10).
An increased sensitivity to ethanol with respect to facial
flushing was observed in certain human populations (especially of
Oriental origin), which was ascribed to genetic differences in
acetaldehyde elimination, due to aldehyde dehydrogenase polymorphism
(Goedde & Agarwal, 1986, 1987; Eriksson, 1987; see also section
6.2.2).
The fetal alcohol syndrome is a specific pattern of congenital
abnormalities found in children of mothers who drink heavily. The
abnormalities most typically associated with alcohol-induced
developmental effects can be grouped into four categories: central
nervous system dysfunction (mental retardation, microcephaly,
hyperactivity); growth deficiencies (reduced prenatal length and
especially weight, with no catch-up postnatal growth; reduced adipose
tissue, normal growth hormone, cortisol, and gonadotropins); a
characteristic cluster of facial abnormalities (short palpebral
fissures, short upturned nose, hypoplastic philtrum, thinned upper-lip
vermillion, flattened midface); and variable major and minor
malformations (such as, heart failure, anomalies of the genitalia,
joints, and palmar creases) (Clarren & Smith, 1978).
The mechanisms by which ethanol produces its developmental
effects are not completely understood, and no data are available with
respect to ethanol doses and/or blood levels needed for such effects.
It is generally assumed that acetaldehyde, as the primary metabolite,
may contribute to the developmental effects of ethanol. Several
animal studies have demonstrated the direct teratogenic effect of
acetaldehyde (Ali & Persaud, 1988; see also section 8.5); however, no
reports are available with respect to the direct teratogenicity of
acetaldehyde in humans.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
By far, the principal source of exposure to acetaldehyde for the
majority of the general population is through the metabolism of
alcohol. Cigarette smoke is also a significant source of exposure for
smokers. With respect to other media, the general population is
exposed to acetaldehyde principally from food, and to a lesser extent
from air. Intake from drinking-water is negligible compared with that
from other media.
Available data are inadequate to determine the extent of exposure
to acetaldehyde in the workplace. Workers may be exposed in some
manufacturing industries and during alcohol fermentation, where the
principal route is most likely inhalation and, possibly, dermal
contact.
10.1.2 Health effects
Acetaldehyde is a reactive molecule that adducts to
macromolecules and can also condense or polymerise with small
molecules. Although quantitatively unimportant in acetaldehyde
metabolism, these by-products may have important biological effects.
In studies conducted on animals, the acute toxicity of
acetaldehyde by the oral or inhalation routes was low. Oral LD50s
ranged from 660 to 1930 mg/kg body weight and LC50s (0.5-4 h) from
24 to 37 g/m3. RD50s in rats and mice are slightly greater than
5000 mg/m3.
Data on the irritant effects of acetaldehyde on the skin and eye
are restricted to those from limited studies on human volunteers. It
was mildly irritating to the eyes and upper respiratory tract
following acute exposure for very short periods, in limited studies on
human volunteers at concentrations exceeding approximately 90 and
240 mg/m3, respectively (Silverman et al., 1946; Sim & Pattle,
1957). Cutaneous erythema was observed in the patch testing of twelve
subjects of "Oriental ancestry" with acetaldehyde (Wilkin & Fortner,
1985). Although a possible mechanism has been identified, available
data are inadequate to assess the potential of acetaldehyde to induce
sensitization.
Available data relevant to the assessment of the potential
adverse effects in humans of exposure to acetaldehyde in the
occupational and general environments are limited to those on
irritation mentioned above. The remainder of this evaluation is,
therefore, based on studies on animals.
Effects of acetaldehyde following oral administration to animals
have been much less well studied than those following inhalation. In
one of the two available studies by the oral route, the
no-observed-effect level (NOEL) following four weeks of administration
of acetaldehyde to rats was 125 mg/kg body weight per day (Til et al.,
1988). At the next higher concentration (675 mg/kg body weight per
day), a borderline increase in hyperkeratosis of the forestomach was
observed. In rats exposed to 0.05% acetaldehyde (estimated by the
Task Group to be approximately 40 mg/kg body weight) in the
drinking-water for 6 months, there was an increase in collagen
synthesis in the liver (Bankowski et al., 1993). No other effects
were examined and the toxicological significance of this observation
is unknown.
Acetaldehyde has induced gene mutations, clastogenic effects, and
sister chromatid exchanges (SCE) in mammalian cells in vitro.
Though available data on genotoxicity in vivo are limited, increases
in SCE have been observed in hamsters and mice exposed
intraperitoneally to acetaldehyde (Obe et al., 1979; Korte & Obe,
1981). This suggests genetic damage to somatic cells in vivo.
Available data are inadequate to assess the potential of acetaldehyde
to induce genetic damage in mammalian germ cells in vivo.
On the basis of studies on rats and hamsters, the target tissue
in inhalation studies is the upper respiratory tract. In the
respiratory tract, degenerative changes of the olfactory epithelium in
rats and trachea in hamsters have been observed at the lowest
concentrations. Degenerative changes in the respiratory epithelium and
larynx have been observed at higher concentrations. In available
studies, the lowest concentration at which effects were observed
(degenerative changes in the olfactory epithelium of rats) was
437 mg/m3 following administration for 5 weeks (Saldiva et al.,
1985). The NOELs identified for respiratory effects were 275 mg/m3
in rats exposed for 4 weeks (Appelman et al., 1986) and 700 mg/m3 in
hamsters exposed for 13 weeks (Kruysse et al., 1975).
Increased incidences of tumours have been observed in inhalation
studies on rats and hamsters exposed to acetaldehyde. In rats, there
were dose-related increases in nasal adenocarcinomas and squamous cell
carcinomas (significant at all doses of 1350 mg/m3 and greater)
(Woutersen et al., 1986) and non significant increases in laryngeal
and nasal carcinomas in hamsters (Feron et al., 1982). All
concentrations of acetaldehyde administered in these studies induced
tissue damage in the respiratory tract. On the basis of limited
available data, no conclusions can be drawn concerning the potential
of acetaldehyde to promote tumours.
The distribution of nasal lesions induced in rats exposed to
acetaldehyde in inhalation studies correlates with regional
deficiencies in ALDH, observed in a different strain of rats,
prompting the authors to suggest that regional susceptibility to the
toxic effects may be due, at least in part, to a lack of ALDH in the
susceptible regions (Bogdanffy et al., 1986).
Available data are inadequate for assessment of the potential
reproductive, developmental, neurological, or immunological effects
associated with exposure to acetaldehyde in occupationally exposed or
general populations.
10.1.3 Approaches to risk assessment
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Though the
principal sources of exposure to acetaldehyde in the general
population are through the metabolism of alcohol, in cigarette smoke,
and food, air is believed to be the main route of exposure in the
occupational environment. In addition, available data are inadequate
to provide guidance concerning the potential risks associated with
oral exposure to acetaldehyde. On this basis, only air is addressed
here.
On the basis of data on irritancy in humans, a tolerable
concentration can be derived as follows:
45 mg/m3
Tolerable concentration = = 2 mg/m3 (2000 µg/m3)
20
where:
no effects were observed in a limited study on human volunteers at
45 mg/m3 (Silverman et al., 1946) and 20 is the uncertainty factor
(×10 for intraspecies variation and × 2 for the poor quality of the
data).
Data suggest that acetaldehyde causes genetic damage to
somatic cells in vivo. The irritancy of acetaldehyde may also play
an important role in the development of tumours in the nose and larynx
of rats and hamsters, respectively, exposed by inhalation, though all
concentrations of acetaldehyde administered in carcinogenesis
bioassays induced both irritancy and nasal tumours. Therefore, two
approaches were adopted for the provision of guidance with respect to
the potential carcinogenicity of acetaldehyde.
In the first, a tolerable concentration (TC) was derived on the
basis of division of an effect level for irritancy in the respiratory
tract of rodents by an uncertainty factor, based on the principles
outlined in WHO (in press) and the assumption that there is a
threshold for acetaldehyde-induced cancer of the respiratory tract in
rodents exposed via inhalation. There is some support for this
approach on the basis of relevant data on the analogues of
acetaldehyde, i.e., formaldehyde and glutaraldehyde, which have
similar spectra of in vitro mutagenic effects, but are clearly not
mutagenic in vivo (IARC, 1985; WHO, 1989). Thus,
275 mg/m3
Tolerable concentration = = 0.3 mg/m3 (300 µg/m3)
1000
where:
275 mg/m3 was the NOEL for irritation in rats in a 4-week study
(Appelman et al., 1986) and 1000 is the uncertainty factor (×10 for
interspecies variation, ×10 for intraspecies variation and ×10 for a
less than long-term study and severity of effect, i.e.,
carcinogenicity associated with irritation).
Since the mechanism of induction of tumours by acetaldehyde has
not been well studied, lifetime cancer risk has also been estimated on
the basis of a default model (i.e., linearized multistage) (WHO, in
press). However, it is very likely that, since estimated risk is
based on tumour incidence at concentrations that induce irritancy in
the respiratory tract and no-observed-effect levels for irritancy are
well below these concentrations, the true cancer risk is most likely
much lower at concentrations generally present in the environment and
may, indeed, be zero.
Concentrations associated with a 10-5 excess lifetime risk (lower
95% confidence limits) for nasal tumours (adenocarcinomas, squamous
cell carcinomas, and carcinomas in situ) in male and female rats, in
the only carcinogenicity study in which animals were exposed via
inhalation to acetaldehyde over the lifetime (Woutersen et al., 1986),
calculated on the basis of the linearized multistage model (Global
82), are 11-65 µg/m3. The high-dose animal groups were excluded in
the derivation of these estimates because of early mortality. A body
surface area correction was not incorporated.
10.2 Evaluation of effects on the environment
Acetaldehyde enters the environment during industrial production,
as a product of incomplete combustion (e.g., in vehicle exhausts) and
as a product of alcohol fermentation. Once released,
intercompartmental transport of acetaldehyde is expected to be
limited, because of its reactivity. However, because of its high
vapour pressure and low tendency for sorption onto soil, it is most
likely to be present in air. Acetaldehyde is readily biodegradable;
approximate half-lives are 10-60 h and 1.9 h in air and water,
respectively.
No quantitative data on levels in ambient water were identified.
However, on the basis of concentrations measured in drinking-water,
levels in the aquatic environment are expected to be low (i.e., less
than 0.1 µg/litre). Concentrations in ambient air average about
5 µg/m3.
Acetaldehyde is slightly toxic for fish. The lowest reported
LC50 was 35 µg/litre (Poecilia reticulata). No long-term toxicity
tests have been performed. Acetaldehyde is toxic for micro-organisms
at relatively low concentrations. Seed germination of several plants
was inhibited by more than 50% after exposure to 1.52 mg/litre (3 h).
There were no effects on lettuce exposed to 0.36 µg/m3 acetaldehyde.
The limited available data preclude definitive conclusions
concerning the potential risks of acetaldehyde for environmental
biota. However, on the basis of the short half-lives of acetaldehyde
in air and water and the fact that it is readily biodegradable, the
impact of acetaldehyde on organisms in the aquatic and terrestrial
environments is expected to be low, except, possibly, during
industrial discharges or spills.
11. RECOMMENDATIONS FOR RESEARCH
1. Additional studies on the fate in the environment and impact of
acetaldehyde on biota.
2. Additional information on exposure of workers in the occupational
environment and levels in the vicinity of industrial facilities.
3. Carcinogenesis bioassays by the inhalation route including doses
that do not induce cytotoxicity (e.g., 275 mg/m3).
4. Studies on the biological significance of byproducts of
acetaldehyde, such as acetaldehyde-modified proteins.
5. Studies on pathogenicity, reproductive and developmental
toxicity, and genotoxicity in vivo by relevant routes of
exposure.
6. Additional studies on toxicity following ingestion in
experimental animals.
7. Studies of irritation in workers exposed to acetaldehyde.
References
Abernethy DJ, Frazelle JH, & Boreiko CJ (1982) Effects of ethanol,
acetaldehyde and acetic acid in the C3H/10TI/2C18 cell transformation
system. Environ Mutagen, 4: 331.
Agarwal DP & Goedde HW (1990) Alcohol metabolism, alcohol intolerance,
and alcoholism. Berlin, Heidelberg, New York, Springer-Verlag, 184 pp.
Aharoni Y & Barkai-Golan R (1973) Sensitivity to acetaldehyde vapors
of Alterneria tenuis and Stemphylium botryosum. Phytopathol Z,
78: 57-61.
Aharoni Y & Stadelbacher GJ (1973) The toxicity of acetaldehyde vapors
to postharvest pathogens of fruits and vegetables. Phytopathology,
63: 544-545.
Aharoni Y, Stewart JK, Hartsell PL, & Young DK (1979) Acetaldehyde - a
potential fumigant for control of the Green peach aphid on harvested
head lettuce. J Econ Entomol, 72: 493-495.
Ahrenholz S & Gorman R (1980) Health and hazard evaluation
determination. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health (Report No. HE 80-2-727).
Ali F & Persaud TVN (1988) Mechanisms of fetal alcohol effects: role
of acetaldehyde. Exp Pathol, 33(1): 17-21.
Almuaibed AM & Townshend A (1987) Determination of acetaldehyde by
flow-injection analysis with soluble or immobilized aldehyde
dehydrogenase. Anal Chim Acta, 198: 37-44.
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3(6): 272-290.
Anderson RA, Quigg JM, Oswald C, & Zaneveld LJD (1985) Demonstration
of a functional blood-testis barrier to acetaldehyde; evidence for
lack of acetaldehyde effect on ethanol-induced depression of
testosterone in vivo. Biochem Pharmacol, 34(5): 685-695.
Aoyama T & Yashiro T (1983) Analytical study of low-concentration
gases. Part 2: Collection of acetaldehyde at low concentrations in air
in a solid reaction tube and its analysis using a flame thermionic
detector. J Chromatogr, 265: 45-55.
Appelman LM, Woutersen RA, & Feron VJ (1982) Inhalation toxicity of
acetaldehyde in rats. I. Acute and subacute studies. Toxicology,
23: 293-307.
Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, & Notten WRF (1986)
Effect of variable versus fixed exposure levels on the toxicity of
acetaldehyde in rats. J Appl Toxicol, 6(5): 331-336.
Aranyi C, O'Shea WJ, Graham JA, & Miller FJ (1986) The effects of
inhalation of organic chemical air contaminants on murine lung host
defenses. Fundam Appl Toxicol, 6(4): 713-720.
Arntz RR & Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmos Environ, 15(9): 1643-1651.
Asai M, Narita O, & Kashiwamata S (1985) Effects of acetaldehyde
and/or ethanol on neutral amino acid transport systems in microvillous
brush border membrane vesicles prepared from human placenta.
Experientia (Basel), 41(12): 1566-1568.
Asmussen E, Hald J, & Larsen V (1948) The pharmacological action of
acetaldehyde on the human organism. Acta Pharmacol, 4: 311-320.
Atkinson R & Pitts JN (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-426
K. J Chem Phys, 68(8): 3581-3584.
Atkinson R, Plum CN, Carter WPL, Winer AM, & Pitts JN (1984) Rate
constants for the gas-phase reactions of nitrate radicals with a
series of organics in air at 298 +/- 1 K. J Phys Chem, 88: 1210-1215.
Atkinson R, Aschmann SM, & Goodman MA (1987) Kinetics of the gas-phase
reactions of NO radicals with a series of alkynes, haloalkenes, and
a,b-unsaturated aldehydes. Int J Chem Kinet, 19: 299-307.
Atukorala TMS, Duggleby RG, & Nixon PF (1988) Effects of acetaldehyde
upon catalysis by human erythrocyte transketolase. Biochem Pharmacol,
37(10): 2100-2101.
Au W & Badr FM (1979) Does ethanol induce chromosomal damage?
In Vitro, 15: 221.
Babiuk C, Steinhagen WH, & Barrow CS (1985) Sensory irritation
response to inhaled aldehydes after formaldehyde pretreatment. Toxicol
Appl Pharmacol, 79(1): 143-149.
Badr FM & Hussain F (1977) Action of ethanol and its metabolite
acetaldehyde in human lymphocytes. In vivo and in vitro study.
Genetics, 86: s2-s3.
Bagnall GN & Sidebottom HW (1984) Photooxidation of acetaldehyde. In:
Proceedings of the Third Symposium on Physical and Chemical Behaviour
of Atmospheric Pollutants, Varese, Italy. Luxembourg, Commission of
the European Communities, pp 188-193 (EUR 9436).
Bankowski E, Pawlicka E, & Sobolewski K (1993) Liver collagen of rats
submitted to chronic intoxication with acetaldehyde. Mol Cell Biochem,
121: 37-43.
Baraona E, Di Padova C, Tabasco J, & Lieber CS (1987) Red blood cells:
a new major modality for acetaldehyde transport from liver to other
tissues. Life Sci, 40: 253-258.
Barilyak IR & Kozachuk SY (1983) [Embryotoxic and mutagenic activity
of ethanol and acetaldehyde after intra-amniotic injection.] Citol
Genet, 17: 57-60 (in Russian).
Barry RE & McGivan JD (1985) Acetaldehyde alone may initiate
hepatocellular damage in acute alcoholic liver disease. Gut,
26(10): 1065-1069.
Bell RP & Clunie JC (1952) The hydration of acetaldehyde in aqueous
solution. Trans Faraday Soc, 48: 439-442.
Berni RJ & Stanley JB (1982) Volatiles from cotton plant parts. Text
Res J, 52: 539-541.
Binding N, Thiewens S, & Witting U (1986) [Specific determination of
formaldehyde and other aldehydes and ketones for stationary and
personal exposure monitoring.] Staub-Reinhalt Luft, 46(10): 444-446
(in German).
Bird RP, Draper HH, & Basrur PK (1982) Effect of malonaldehyde and
acetaldehyde on cultured mammalian cells. Production of micronuclei
and chromosomal aberrations. Mutat Res, 101: 237-246.
Bittersohl G (1974) [Epidemiologic investigations on cancer incidence
in workers contacted by acetaldol and other aliphatic aldehydes.] Arch
Geschwulstforsch, 43: 172-176 (in German).
Bittersohl G (1975) Epidemiological research on cancer risk by aldol
and aliphatic aldehydes. Environ Qual Saf, 4: 235-238.
Blakley PM & Scott WR Jr (1984a) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. I. Teratogenicity of
ethanol and acetaldehyde. Toxicol Appl Pharmacol, 72: 355-363.
Blakley PM & Scott WR Jr (1984b) Determination of the proximate
teratogen of the mouse fetal alcohol syndrome. II. Pharmacokinetics of
the placental transfer of ethanol and acetaldehyde. Toxicol Appl
Pharmacol, 72: 364-371.
Boehlke JU, Singh S, & Goedde HW (1983) Cytogenetic effects of
acetaldehyde in lymphocytes of Germans and Japanese: SCE, clastogenic
activity, and cell cycle delay. Hum Genet, 63: 285-289.
Boettner EA, Ball GL, & Weiss B (1973) Combustion products from the
incineration of plastics. Cincinnati, Ohio, US Environmental
Protection Agency, pp 1-5, 106-122 (EPA-670/2-73-049; PB-222.001).
Bogdanffy MS, Randall HW, & Morgan KT (1986) Histochemical
localization of aldehyde dehydrogenase in the respiratory tract of the
Fisher 344 rat. Toxicol Appl Pharmacol, 82: 560-567.
Booze TF & Oehme FW (1986) An investigation of metaldehyde and
acetaldehyde toxicities in dogs. Fundam Appl Toxicol, 6: 440-446.
Bradow JM & Connick WJ (1988) Seed-germination inhibition by volatile
alcohols and other compounds associated with Amaranthus palmeri
residues. J Chem Ecol, 14(7): 1633-1648.
Brambilla G, Sciaba L, Faggin P, Maura A, Marinari UM, Ferro M, &
Esterbauer H (1986) Cytotoxicity, DNA fragmentation and sister
chromatid exchange in Chinese hamster ovary cells exposed to the lipid
peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat
Res, 171: 169-176.
Brenner DA & Chojkier M (1987) Acetaldehyde increases collagen gene
transcription in cultured human fibroblasts. J Biol Chem,
262(36): 17690-17695.
Brien JF & Loomis CW (1983) Pharmacology of acetaldehyde. Can J
Physiol Pharmacol, 61: 1-22.
Brodzinsky R & Singh HB (1982) Volatile organic chemicals in the
atmosphere: An assessment of available data. Final Report (Contract
No. 68-02-3542). Menlo Park, California, US Environmental Protection
Agency.
Buehler R, Pestalozzi D, Hess M, & Von Wartburg JP (1983)
Immunohistochemical localization of alcohol dehydrogenase in human
kidney, endocrine organs and brain. Pharmacol Biochem Behav,
18(Suppl 1): 55-59.
Buyske DA, Owen LH, Wilder P, & Hobbs ME (1956) Chromatography of the
2,4-dihydrophenyl-hydrazones of some aldehydes and ketones in tobacco
smoke. Ann Chem, 28: 910-913.
Campbell MA & Fantel AG (1983) Teratogenicity of acetaldehyde
in vitro: relevance to the fetal alcohol syndrome. Life Sci,
32: 2641-2647.
Casanova-Schmitz M, David RM, & Heck Hd'A (1984) Oxidation of
formaldehyde and acetaldehyde by NAD+-dependent dehydrogenases in rat
nasal mucosal homogenates. Biochem Pharmacol, 33(7): 1137-1142.
Cavanagh LA, Schadt CF, & Robinson E (1969) Atmospheric hydrocarbon
and carbon monoxide measurements at Point Barrow, Alaska. Environ Sci
Technol, 3(3): 251-257.
Cederbaum AI & Dicker E (1981) Effect of cyanamide on the metabolism
of ethanol and acetaldehyde and on gluconeogenesis by isolated rat
hepatocytes. Biochem Pharmacol, 30: 3079-3088.
Cederbaum AI & Rubin E (1977) Sensitivity to acetaldehyde of pyruvate
oxidation by mitochondria from liver, kidney, brain and muscle.
Biochem Pharmacol, 26: 1349-1353.
Cederbaum AI, Lieber CS, & Rubin E (1974) The effect of acetaldehyde
on mitochondrial function. Arch Biochem Biophys, 161: 26-39.
Checiu M, Sandor S, & Garban Z (1984) The effect of ethanol upon early
development in mice and rats. 6. In vivo effect of acetaldehyde upon
preimplantation stages in rats. Rev Roum Morphol Embryol Physiol
Morphol Embryol 30: 175-184.
Chou WL & Speece RE (1978) Acclimation and degradation of
petrochemical wastewater components by methane fermentation.
Biotechnol Bioeng Symp, 8: 391-414.
Chrostek WJ (1981) Health hazard evaluation report No.
HETA-81-098-941. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Chrostek WJ & Schoemaker WE (1981) Health hazard evaluation report No.
HETA-81-298-944. Cincinnati, Ohio, US National Institute of
Occupational Safety and Health.
Clarke DW, Steenaart NAE, Slack CJ, & Brien JF (1986) Pharmacokinetics
of ethanol and its metabolite, acetaldehyde, and fetolethality in the
third-trimester pregnant guinea pig for oral administration of acute,
multiple-dose ethanol. Can J Physiol Pharmacol, 64: 1060-1067.
Clarke DW (1988) The disposition of ethanol and its proximate
metabolite, acetaldehyde, in the maternal-fetal unit of the nearterm
guinea pig and pregnant ewe. Diss Abstr Int, 48(10): 2932B-2933B.
Clarren SK & Smith DW (1978) The fetal alcohol syndrome. New Engl J
Med, 298: 1063-1067.
Collins MA, Nijm WP, Borge GF, Teas G, & Goldfarb C (1979)
Dopamine-related tetrahydroisoquinolines: significant urinary
excretion by alcoholics after alcohol consumption. Science,
206: 1184-1186.
Cortes F, Mateos S, & Escalza P (1986) Cytotoxic and genotoxic effects
of ethanol and acetaldehyde in root-meristem cells of Allium cepa.
Mutat Res, 171(2-3): 139-143.
Crabb DW, Edenberg HJ, Bosron WF, & Li TK (1989) Genotypes for
aldehyde dehydrogenase deficiency and alcohol sensitivity. J Clin
Invest, 83: 314-316.
Crebelli R, Conti G, Conti I, & Carere A (1989) A comparative study of
ethanol and acetaldehyde as inducers of chromosome malsegregation in
Aspergillus nidulans. Mutat Res, 215: 187-195.
Delcour JA, Caers JM, Dondeyne P, Delvaux F, & Robberechts E (1982) An
enzymic assay for the determination acetaldehyde in beers. J Inst
Brew, 88: 384-386.
Deneer JW, Seinen W, & Hermens JLM (1988) The acute toxicity of
aldehydes to the guppy. Aquat Toxicol, 12: 185-192.
De Raat WK, Davis PB, & Bakker GL (1983) Induction of sister-chromatid
exchanges by alcohol and alcoholic beverages after metabolic
activation by rat-liver homogenate. Mutat Res, 124: 85-90.
Di Padova C, Alderman J, & Lieber CS (1986) Improved methods for
measurement of acetaldehyde concentrations in plasma and red blood
cells. Alcohol Clin Exp Res, 10(1): 86-89.
Dreisbach RH (1987) Handbook of poisoning, 9th ed. Los Altos,
California, Lange, pp 185-188.
Dulout FN & Furnus CC (1988) Acetaldehyde-induced aneuploidy in
cultured Chinese hamster cells. Mutagenesis, 3(3): 207-211.
Egle JL (1970) Retention of inhaled acetaldehyde in man. J Pharmacol
Exp Ther, 174(1): 14-19.
Eimutis EC, Quill RP, & Rinaldi GM (1978) Source assessment
noncriteria pollutant emissions (1978 update). Research Triangle Park,
North Carolina, US Environmental Protection Agency, Industrial
Environmental Research Laboratory, pp 1-5 (PB-291 747).
Eker P & Sanner T (1986) Initiation of in vitro cell transformation
by formaldehyde and acetaldehyde as measured by attachment-independent
survival of cells in aggregates. Eur J Cancer Clin Oncol,
22(6): 671-676.
Eriksson CJP (1987) Genetic aspects of the relation between alcohol
metabolism and consumption in humans. Mutat Res, 186(3): 241-247.
Eriksson CJP & Fukunaga T (1993) Human blood acetaldehyde (update
1991). Alcohol Alcohol, 28(Suppl 2): 9-25.
Eriksson CJP, Mizoi Y, & Fukunaga T (1982) The determination of
acetaldehyde in human blood by the perchloric acid precipitation
method: The characterization and elimination of artefactual
acetaldehyde formation. Anal Biochem, 125: 259-263. Espinet C &
Argiles JM (1984) Ethanol and acetaldehyde concentrations in the rat
foeto-maternal system after an acute ethanol administration given to
the mother. Arch Int Physiol Biochim, 92: 339-344.
Facchini MC, Chiavari G, & Fuzzi S (1986) An improved HPCL method for
carbonyl compound speciation in the atmospheric liquid phase.
Chemosphere, 15(6): 667-674.
Feron VJ (1979) Effects of exposure to acetaldehyde in Syrian hamsters
simultaneously treated with benzo[a]pyrene or diethylnitrosamine. Prog
Exp Tumor Res, 24: 162-176.
Feron VJ & De Jong D (1971) Acute intratracheal toxicity of
acetaldehyde in Syrian golden hamsters. Zeist, Central Institute for
Nutrition and Food Research, TNO (Report No. R 3600).
Feron VJ, Kruysse A, & Woutersen RA (1982) Respiratory tract tumors in
hamsters exposed to acetaldehyde vapour alone or simultaneously to
benzo[a]pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol,
18: 13-31.
Feron VJ, Kuper CF, Spit BJ, Renzel PGJ, & Woutersen RA (1985) Glass
fibers and vapor phase components of cigarette smoke as factors in
experimental respiratory tract carcinogenesis. In: Carcinogenesis. New
York, Raven Press, pp 93-118.
Feron VJ, Til HP, Vrijer F, Woutersen RA, Cassee FR, & van Bladeren PJ
(1991) Aldehydes: occurrence, carcinogenic potential, mechanism of
action and risk assessment. Mutat Res, 259: 363-385.
Fink R & Dancygier H (1988) Acetaldehyde inhibits the activity of
human natural killer cells. Gastroenterology, 94(5): A127.
Fraenkel-Conrat H & Singer B (1988) Nucleoside adducts are formed by
cooperative reaction of acetaldehyde and alcohols: possible mechanism
for the role of ethanol in carcinogenesis. Proc Natl Acad Sci (USA),
85: 3758-3761.
Furia TE & Bellanca N ed. (1975) Fenaroli's handbook of flavour
ingredients, 2nd ed. Cleveland, Ohio, CRC Press Inc., vol 2, p 3.
Gerhold RM & Malaney GW (1966) Structural determination in the
oxidation of aliphatic compounds by activated sludge. J Water Pollut
Control Fed, 38(4): 562-579.
Gidley MJ, Hall LD, Sanders JKM, & Summers MC (1981)
Acetaldehyde-enkephalins: structure proof and some conformational
deductions from one- and two-dimensional proton nuclear magnetic
resonance spectra. Biochemistry, 20: 3880-3883.
Goedde HW & Agarwal DP (1986) Aldehyde oxidation: ethnic variations in
metabolism and response. In: Kalow W, Goedde W, & Agarwal DP ed.
Ethnic differences in reactions to drugs and xenobiotics. New York,
Alan R. Liss Inc., pp 113-138.
Goedde HW & Agarwal DP (1987) Polymorphism of aldehyde dehydrogenase
and alcohol sensitivity. Enzyme, 37: 29-44.
Goedde HW, Agarwal DP, & Harada S (1979) Alcohol metabolizing enzymes:
studies of isozymes in human biopsies and cultured fibroblasts. Clin
Genet, 16: 29-33.
Goedde HW, Singh S, Agarwal DP, Frizte G, Stapel K, & Paik YK (1989)
Genotyping of mitochondrial aldehyde dehydrogenase in blood samples
using allele-specific oligonucleotides: comparison with phenotyping in
hair roots. Hum Genet, 81: 305-307.
Gordon BHJ, Baraona E, & Lieber CS (1985) Blood acetaldehyde response
to ethanol ingestion during the reproductive cycle of the female rat.
Alcohol, 2: 271-275.
Grahl K (1983) [Classification of substances in water on the basis of
their toxicity potential to aquatic organisms.] Acta Hydrochim
Hydrobiol, 11(1): 137-143 (in German).
Gramiccioni L, Milana MR, Di Marzio S, & Lorusso S (1986) GC
determination of traces of acetaldehyde in aqueous matrix: a rapid
headspace method. Chromatographia, 21(1): 9-11.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Thomas CT Publications, p 76.
Green RJ & Baron DN (1986) The acute in vitro effect of ethanol, its
metabolites and other toxic alcohols on ion flax in isolated human
leucocytes and erythrocytes. Biochem Pharmacol, 35(20): 3457-3464.
Greenwald IS & Horvitz HR (1980) Unc-93(e1500): a behavioral mutant of
Caenorhabditis elegans that defines a gene with a wild-type null
phenotype. Genetics, 96: 147-164.
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles
ambient air. Environ Sci Technol, 16: 254-262.
Grosjean D (1991) Ambient levels of formaldehyde, acetaldehyde and
fumaric acid in Southern California. Results of a one-year base-line
study. Environ Sci Technol, 25: 710-715.
Grosjean D & Wright B (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos Environ, 17(10): 2093-2096.
Grosjean D, Swanson RD, & Ellis C (1983) Carbonyls in Los Angeles air:
contribution of direct emissions and photochemistry. Sci Total
Environ, 29: 65-85.
Guerri C & Sanchis R (1985) Acetaldehyde and alcohol levels in
pregnant rats and their fetuses. Alcohol, 2: 267-270.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Hagemeyer HJ (1978) Acetaldehyde. In: Grayson M ed. Kirk-Othmer
encyclopedia of chemical technology, 3rd ed. New York, John Wiley and
Sons, vol 1, pp 97-112.
Hagen E (1967) The composition of combustion gases of polyurethane
forms and polycarbonates. Plaste Kautsch, 6: 391-392.
Harade S, Agarwal DP, & Goedde HW (1978a) Human liver alcohol
dehydrogenase isoenzyme variations: improved separation methods using
prolonged high voltage starch gel electrophoresis and isoelectric
focusing. Hum Genet, 40: 215-220.
Harade S, Agarwal DP, & Goedde HW (1978b) Application of gel
isoelectric focusing and electrophoresis in the study of alcohol
dehydrogenase and acetaldehyde dehydrogenase isoenzymes of human
tissues and fibroblasts. In: Catsimoolas U ed. Electrophoresis 78.
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp 275-282.
Harris CC, Willey JC, Saladino AJ, & Grafstrom RC (1985) Effects of
tumor promotors, aldehydes, peroxides, and tobacco smoke condensate on
growth and differentiation of cultured normal and transformed human
bronchial cells. In: Mass MJ, Kaufman DG, Siegfried JM, Steele WE, &
Nesnow S, ed. Carcinogenesis, a comprehensive survey. Cancer of the
respiratory tract. Predisposing factors. New York, Raven Press, pp
159-171.
Hatfield R (1957) Biological oxidation of some organic compounds. Ind
Eng Chem, 49(2): 192-196.
He SM & Lambert B (1985) Induction and persistence of SCE-inducing
damage in human lymphocytes exposed to vinyl acetate and acetaldehyde
in vitro. Mutat Res, 158(3): 201-208.
He SM & Lambert B (1990) Acetaldehyde-induced mutation at the hprt
locus in human lymphocytes in vitro. Environ Mol Mutagen, 16: 57-63.
Helander A & Tottmar O (1987) Effects of ethanol, acetaldehyde and
disulfiram on the metabolism of biogenic aldehydes in isolated human
blood cells and platelets. Biochem Pharmacol, 36(22): 3981-3985.
Helander A & Lindahl-Kiessling K (1991) Increased frequency of
acetaldehyde-induced sister-chromatid exchanges in human lymphocytes
treated with an aldehyde dehydrogenase inhibitor. Mutat Res, 264:
103-107.
Hellström-Lindahl E & Weiner H (1985) Effects of disulfiram on the
oxidation of benzaldehyde and acetaldehyde in rats liver. Biochem
Pharmacol, 34(9): 1529-1535.
Hemminki K & Suni R (1984) Sites of reaction of glutaraldehyde and
acetaldehyde with nucleosides. Arch Toxicol, 55: 186-190.
Hemminki K, Falck K, & Vainio H (1980) Comparison of alkylation rate
and mutagenicity of directly acting industrial and laboratory
chemicals. Arch Toxicol, 46: 277-285.
Henderson IF (1970) The fumigant effect of metaldehyde on slugs. Ann
Appl Biol, 65: 507-510.
Herrick RF (1980) Industrial hygiene survey report on urea
formaldehyde foam insulation manufacturing. Cincinnati, Ohio, US
National Institute of Occupational Safety and Health, pp 2-5, 9-12
(PB83-107797).
Hobara N, Watanabe A, Kobayashi M, Nakatsukasa H, Nagshima H, Fukuda
T, & Araki Y (1985) Tissue distribution of acetaldehyde inhalation and
intragastric ethanol administration. Bull Environ Contam Toxicol,
35: 393-396.
Hoerner M, Behrens UJ, Worner T, Blacksberg I, Braly LF, Schaffner F,
& Lieber CS (1988) The role of alcoholism and liver disease in the
appearance of serum antibodies against acetaldehyde adducts.
Hepatology, 8: 569-574.
Hoffman D, Brunnenman, KD, Gori GB, & Wynder EL (1975) On the
carcinogenicity of marijuana smoke. Recent Adv Phytochem, 9: 63-81.
Horvath RJ, Senter SD, & Dekazos ED (1983) GLC-MS analysis of volatile
constituents in rabbit eye blueberries. J Food Sci, 48: 278-279.
Hsu LC, Bendel RE, & Yoshida A (1978) Genomic structure of the human
mitochondrial aldehyde dehydrogenase gene. Genomics, 2(1): 57-65.
Hugues UJ & Hum RW (1960) A preliminary survey of hydrocarbon-derived
oxygenated material in automobile exhaust gases. J Air Pollut Control
Assoc, 10: 367-371.
Hustert K & Parlar H (1981) [A test for photochemical degradation of
environmental chemicals in the gas phase.] Chemosphere,
10(9): 1045-1050 (in German).
IARC (1985) Acetaldehyde. In: Allyl compounds, aldehydes, epoxides and
peroxides. Lyon, International Agency for Research on Cancer, pp
101-132 (IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, Volume 36).
IARC (1988) Alcohol drinking. Lyon, International Agency for Research
on Cancer, 416 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risks to Humans, Volume 44).
Igali S & Gazso L (1980) Mutagenic effects of alcohol and acetaldehyde
on Escherichia coli. Mutat Res, 74: 209-210.
Ikawa E, Tsuda H, Sakata T, Masui T, Sato K, & Ito N (1986)
Modification potentials of ethyl alcohol and acetaldehyde on
development of preneoplastic glutathione S-transferase
P-form-positive liver cell foci initiated by diethylnitrosamine in the
rat. Cancer Lett, 31(1): 53-60.
IPCS/CEC (1990) International Chemical Safety Card No. 0009:
Acetaldehyde. Luxembourg, Commission of the European Communities
(First series).
Israel Y, Hurwitz E, Niemela EO, & Arnon R (1986) Monoclonal and
polyclonal antibodies against acetaldehyde-containing epitopes in
acetaldehyde-protein adducts. Proc Natl Acad Sci (USA),
83(20): 7923-7927.
Israel Y, MacDonald A, Niemela O, Zemel D, Shami E, Zywulko M, Klajner
F, & Borgono C (1992) Hypersensitivity to acetaldehyde-protein
adducts. Mol Pharmacol, 42(4): 711-717.
Jansson T (1982) The frequency of sister chromatid exchanges in human
lymphocytes treated with ethanol and acetaldehyde. Hereditas,
97: 301-303.
Japan Environment Agency (1989) Report on environmental survey and
wildlife monitoring of chemicals. In: Chemicals in the environment.
Tokyo, Japan Environment Agency, Department of Environmental Health,
p 73.
Jarke FH, Dravnieks A, & Gordon SM (1981) Organic contaminants in
indoor air and their relation to outdoor contaminants. Am Soc Heat
Refrig Air-Cond Eng Trans, 87: 153-166.
Jones JS, Sadler GD, & Nelson PE (1986) Acetaldehyde and accelerated
storage of wine: a new rapid method for analysis. J Food Sci,
51(1): 229-230.
Juhnke I & Luedemann D (1978) [Results of investigation of acute
toxicity to fish of 200 chemical compounds by the "Golden Orfe" test.]
Z Wasser Abwasser Forschung, 11(5): 161-164 (in German).
Kami T (1983) Composition of the essential oil of alfalfa. J Agric
Food Chem, 31: 38-41.
Karl PI, Gordon BHJ, Lieber CS, & Fisher SE (1988) Acetaldehyde
production and transfer by perfused human placental cotyledon.
Science, 242: 273-275.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Identification and analysis of
organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Press,
pp 329-373.
Knadle S (1985) Synergistic interaction between hydroquinone and
acetaldehyde in the induction of sister chromatid exchange in human
lymphocytes in vitro. Cancer Res, 45(10): 4853-4857.
Koch R & Nagel M (1988) Quantitative structure activity relationships
in soil ecotoxicology. Sci Total Environ, 77: 269-276.
Koivula T (1975) Subcellular distribution and characterization of
human liver aldehyde dehydrogenase fractions. Life Sci, 16: 1563-1570.
Kopczynski SL, Altshuller AP, & Sutterfield FD (1974) Photochemical
reactivities of aldehyde-nitrogen oxide systems. Environ Sci Technol,
8(10): 909-918.
Korte A & Obe G (1981) Influence of chronic ethanol uptake and acute
acetaldehyde treatment on the chromosomes of bone-marrow cells and
peripheral lymphocytes of Chinese hamsters. Mutat Res, 88: 389-395.
Kruysse A (1970) Acute inhalation toxicity of acetaldehyde in
hamsters. Zeist, Central Institute for Nutrition and Food Research,
TNO (Report No. R 3270).
Kruysse A, Feron VJ, & Til HP (1975) Repeated exposure to acetaldehyde
vapor. Studies in Syrian golden hamsters. Arch Environ Health,
30: 449-452.
Kuriyama K, Ohkuma S, Tomono S, & Hirouchi M (1987) Effects of alcohol
and acetaldehyde on metabolism and function of neurotransmitter system
in cerebral cortical neurons in primary culture. Alcohol Alcohol,
22(Suppl 1): 685-689.
Lahdetie J (1988) Effects of vinyl acetate and acetaldehyde on sperm
morphology and meiotic micronuclei in mice. Mutat Res,
202(1): 171-178.
Lahti RA & Majchrowicz E (1969) Acetaldehyde - an inhibitor of the
enzymatic oxidation of 5-hydroxyindoleacetaldehyde. Biochem Pharmacol,
18: 535-538.
Lam CW, Casanova M, & Heck HD (1986) Decreased extractability of DNA
from proteins in the rat nasal mucosa after acetaldehyde exposure.
Fundam Appl Toxicol, 6(3): 541-550.
Lambert B, Chen Y, He SM, & Sten M (1985) DNA cross-links in human
leucocytes treated with vinyl acetate and acetaldehyde in vitro.
Mutat Res, 146(3): 301-303.
Lambert B & He SM (1988) DNA and chromosome damage induced by
acetaldehyde in human lymphocytes in vitro. Ann NY Acad Sci,
534: 369-376.
Lamboeuf Y, Latge C, Roumec C, & De Saint Blanquat G (1987) Ethanol
tolerance in the rat after inhalation of acetaldehyde for a period of
21 days. Alcohol Alcohol, 22(Suppl 1): 441-447.
Latge C, Lamboeuf Y, Roumec C, & De Saint Blanquat G (1987) Effect of
chronic acetaldehyde intoxication on ethanol tolerance and membrane
fatty acids. Drug Alcohol Depend, 20(1): 47-56.
Lau CF, Vogel R, Obe G, & Spielmann H (1991) Embryologic and
cytogenetic effects of ethanol on preimplantation mouse embryos
in vitro. Reprod Toxicol, 5: 405-410.
Leone JA & Seinfeld JH (1984) Analysis of the characteristics of
complex chemical reaction mechanisms: application to photochemical
smog chemistry. Environ Sci Technol, 18(4): 280-287.
Levallois C, Maini JC, & Balmes JL (1987) Sensitivity of human
lymphocytes to acetaldehyde: comparison between alcoholic and control
subjects. Drug Alcohol Depend, 20(2): 135-142.
Lipari F & Swarin SJ (1982) Determination of formaldehyde and other
aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. Chromatography, 247: 297-306.
Lipari F, Dasch JM, & Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environ Sci Technol, 18: 326-330.
Liu RC, Lumeng L, Shahidi S, Kelly T, & Pound DC (1990)
Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol
Clin Exp Res, 14: 438-443.
Ludzack JR & Ettinger MB (1960) Industrial wastes. Chemical structures
resistant to aerobic biochemical stabilization. J Water Pollut Control
Fed, 32(11): 1173-1200.
Lyman WJ, Rheel WF, & Rosenblatt DH (1982) Handbook of chemical
property estimation methods. New York, McGraw-Hill Book Company.
Lynch C, Lim CK, Thomas M, & Peters TJ (1983) Assay of blood and
tissue aldehydes by HPLC analysis of their 2,4-dinitrophenylhydrazine
adducts. Clin Chim Acta, 130: 117-122.
Ma T, Miller TL, & Xu Z (1985) Mouse peripheral erythrocyte
micronucleus assay on the genotoxicity of acetaldehyde. Environ
Mutagen, 7(Suppl 3): 28.
Maarse H & Visscher CA (1992) Volatile compounds in food. Qualitative
and quantitative data. Supplement 3 and cumulative index. Zeist,
Central Institute for Nutrition and Food Research, TNO, Biotechnology
and Chemistry Institute, pp XXXIII-XLI.
McCall D & Ryan K (1987) The effect of ethanol and acetaldehyde on Na
pump function in cultured rat heart cells. J Mol Cell Cardiol,
19: 453-463.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987a)
Effects of acetaldehyde on polymerization of microtubule proteins.
Brain Res, 416: 90-99.
McKinnon G, Davidson M, De Jersey J, Shanley B, & Ward L (1987b) The
reaction of acetaldehyde with brain microtubular proteins: formation
of stable adducts and inhibition of polymerization. Neurosci Lett,
79: 163-168.
Majumdar APN, Vesenka GD, Dubick MA, Yu GSM, DeMorrow JM, & Geokas MC
(1986) Morphological and biochemical changes of the pancreas in rats
treated with acetaldehyde. Am J Physiol 250(5): G598-G606.
Manning DL, Maskarinec MP, Jenkins RA, & Marshall AH (1983) High
performance liquid chromatographic determination of selected gas phase
carbonyls in tobacco smoke. J Assoc Off Anal Chem, 66(1): 8-12.
Margeri G, Versini G, Gianotti I, & Pellegrini R (1984) Acetaldehyde
in beverages. Riv Soc Ital Sci Aliment, 5: 401-406.
Marinari UM, Ferro M, Sciaba L, Finollo R, Bassi AM, & Brambilla G
(1984) DNA-damaging activity of biotic and xenobiotic aldehydes in
Chinese hamster ovary cells. Cell Biochem Funct, 2(4): 243-248.
Marjanen LA (1973) Comparison of aldehyde dehydrogenases from cytosol
and mitochondria of rat liver. Biochim Biophys Acta, 327: 238-246.
Marnett LJ, Hurd H, Hollstein M, Levin DE, Esterbauer H, & Ames BN
(1985) Natural occurring carbonyl compounds are mutagens in Salmonella
tester strain TA104. Mutat Res, 148: 25-34.
Matsuzaki S & Lieber CS (1977) Increased susceptibility of hepatic
mitochondria to the toxicity of acetaldehyde after chronic ethanol
consumption. Biochem Biophys Res Commun, 75(4): 1059-1065.
Mauch TJ, Donahue TM, Zetterman RK, Sorrell MF, & Tuma DJ (1986)
Covalent binding of acetaldehyde selectively inhibits the catalytic
activity of lysine-dependent enzymes. Hepatology, 6(2): 263-269.
Mauch TJ, Tuma DJ, & Sorrell MF (1987) The binding of acetaldehyde to
the active site of ribonuclease: alterations in catalytic activity and
effects of phosphate. Alcohol Alcohol, 22(2): 103-112.
Michoudet C & Baverel G (1985) Metabolism and metabolic effects of
acetaldehyde in dog, rat and guinea pig renal cortex. Dev Nephrol,
10: 143-149.
Michoudet C & Baverel G (1987a) Characteristics of acetaldehyde
metabolism in isolated dog, rat and guinea pig kidney tubules. Biochem
Pharmacol, 36(22): 3987-3991.
Michoudet C & Baverel G (1987b) Metabolism of acetaldehyde in human
and baboon renal cortex:ethanol synthesis by isolated baboon
kidney-cortex tubules. FEBS Lett, 216(1): 113-117.
Miyamoto Y (1986) Eye and respiratory irritants in jet engine exhaust.
Aviat Space Environ Med, 57: 1104-1108.
Mold JD & McRae TG (1957) The determination of some low molecular
weight aldehydes and ketones in cigarette smoke as the
2,4-dinitrophenylhydrazone. Tob Sci, 1: 40-46.
Moortgat GK & McQuigg RD (1984) A FTIR spectroscopic study of the
photooxidation of acetaldehyde in air. In: Proceedings of the Third
European Symposium on Physico-Chemical Behaviour of Atmospheric
Pollutants, Varese, Italy. Luxembourg, Commission of the European
Communities, pp 194-204 (EUR 9436).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing of
270 chemicals. Environ Mutagen, 8(Suppl 17): 1-119.
Mueller A & Sies H (1987) Alcohol, aldehydes and lipid peroxidation
current notions. Alcohol Alcohol, 22(Suppl 1): 67-74.
Myers WD, Ng KT, Singer G, Smythe GA, & Duncan MW (1985) Dopamine and
salsolinol levels in rat hypothalami and striatum after
schedule-induced self-injection (SISI) of ethanol and acetaldehyde.
Brain Res, 358(1-2): 122-128.
Niemela O & Israel Y (1992) Hemoglobin-acetaldehyde adducts in human
alcohol abusers. Lab Invest, 67(2): 246-252.
Niemela O, Klajner F, Orrego H, Vidins E, Blendis LM, & Israel Y
(1987) Antibodies against acetaldehyde-modified protein epitopes in
human alcoholics. Hepatology, 7: 1210-1214.
Niemela O, Mannermaa RM, & Oikarinen (1990) Impairment o histone H1
DNA binding by adduct formation with acetaldehyde. Life Sci,
47: 2241-2249.
Niemela O, Juvonen T, & Parkkila S (1991) Immunohistochemical
demonstration of acetaldehyde-modified epitopes in human liver after
alcohol consumption. J Clin Invest, 87: 1367-1374.
Ninfali P, Palma F, Piacentini MP, & Fornaini G (1987) Action of
acetaldehyde on glucose metabolism of newborn and adult erythrocytes.
Biol Neonate, 52(5):256-263.
Norppa H, Tursi F, Pfaeffli P, Maeki-Paakkanen J, & Jaerventaus H
(1985) Chromosome damage induced by vinyl acetate through in vitro
formation of acetaldehyde in human lymphocytes and Chinese hamster
ovary cells. Cancer Res, 45(10): 4816-4821.
Obe O & Beek B (1979) Mutagenic activity of aldehydes. Drug Alcohol
Depend, 4: 91-94.
Obe G & Ristow H (1977) Acetaldehyde, but not ethanol, induces sister
chromatid exchanges in Chinese hamster cells in vitro. Mutat Res,
56: 211-213.
Obe G, Ristow H, & Herha J (1978) Mutagenic activity of alcohol in
man. In: Mutations: their origin, nature and potential relevance to
genetic risk in man. Proceedings Yearly Conference 1977 of German
Research Society. Boppard, Harald Boldt, pp 151-161.
Obe G, Natarajan AT, Meyers M, & Den Hertog A (1979) Induction of
chromosomal aberrations in peripheral lymphocytes of human blood
in vitro, and of SCE's in bone-marrow cells of mice in vivo by
ethanol and its metabolite acetaldehyde. Mutat Res, 68: 291-294.
Obe G, Brodmann R, Fleischer R, Engeln H, Gobel D, & Herha J (1985)
[Mutagenic and carcinogenic activity of adduct products.] In: Keup W
ed. [Biology of addiction.] Berlin, Heidelberg, New York,
Springer-Verlag, pp 31-43 (in German).
Obe G, Jonas R, & Schmidt S (1986) Metabolism of ethanol in vitro
produces a compounds which induces sister-chromatid exchanges in human
peripheral lymphocytes in vitro: Acetaldehyde not ethanol is
mutagenic. Mutat Res, 174: 47-52.
OECD (1992) Guidelines for testing chemicals. Section 3. Degradation
and accumulation. 301 C. Paris, Organisation for Economic Cooperation
and Development, pp 1-21.
Okamoto M, Ohtsuka K, Imai J, & Yamada F (1981) High-performance
liquid chromatographic determination of acetaldehyde in wine as its
lutidine derivative. J Chromatogr, 219: 175-178.
Ortiz A, Griffth PJ, & Littleton JM (1974) A comparison of the effects
of chronic administration of ethanol and acetaldehyde to mice:
evidence for a role of acetaldehyde in ethanol dependence. J Pharm
Pharmacol, 26: 249-260.
Osborne JS, Adamek S, & Hobbs ME (1956) Some component of gas phase of
cigarette smoke. Ann Chem, 28: 211-215.
O'Shea KS & Kaufman MH (1979) The teratogenic effect of acetaldehyde:
implications for the study of the fetal alcohol syndrome. J Anat,
128: 65-76.
O'Shea KS & Kaufman MH (1981) Effect of acetaldehyde on the
neuroepithelium of early mouser embryos. J Anat, 132: 107-118.
Padmanabhan R, Sreenathan RN, & Singh S (1983) Studies on the lethal
and teratogenic effects of acetaldehyde in the rat. Congenit Anom,
23: 13-23.
Parrilla R, Ohkawa K, Lindros KO, Zimmernman UP, Kobayashi K, &
Williamson JR (1974) Functional compartmentation of acetaldehyde
oxidation in rat liver. J Biol Chem, 249: 4926-4933.
Peeke HV & Figler MH (1981) Modulation of aggressive behavior in fish
by alcohol and congeners. Pharmacol Biochem Behav, 14(Suppl 1): 79-84.
Pellizari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compound in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Peterson CM & Polizzi CM (1987) Improved method for acetaldehyde in
plasma and hemoglobin-associated acetaldehyde: results in teetotalers
and alcoholics reporting for treatment. Alcohol, 4: 477-480.
Pettersson H & Kiessling K (1977) Acetaldehyde occurrence in
cerebrospinal fluid during ethanol oxidation in rats and its
dependence on the blood level and on dietary factors. Biochem
Pharmacol, 26: 237-240.
Pezzoli C, Galli-Kienle M, Di Padova C, & Stramentinoli G (1984) HPLC
method for the evaluation of blood acetaldehyde without ethanol
interference. J Liq Chromatogr, 7(4): 765-788.
Phillips SC (1987) Can brain lesions occur in experimental animals by
administration of ethanol or acetaldehyde? Acta Med Scand,
71(Suppl 7): 67-72.
Piendl A, Westner H, & Geiger E (1981) [Detection and separation of
aldehydes in beer using high pressure liquid chromatography (HPLC).]
Braunwissenschaft, 34: 301-307 (in German).
Pittevils J, Vanparys L, & Soenen A (1979) Fungicide and fungistatic
action of fifteen different aromatic components on some important
storage diseases in vitro. Meded Fac Landbouwwet Rijksuniv Gent,
44(1): 445-457.
Popov VB, Vaisman BL, Puchkov VF, & Ignatyeva TV (1981) Toxic action
of ethanol and biotransformation products on postimplantation rat
embryos in culture. Bull Exp Biol Med, 92: 725-728.
Possanzini M, Ciccioli P, Di Paolo V, & Draisci R (1987) Determination
of low boiling aldehydes in air and exhaust gases by using annular
denuders combined with HPLC techniques. Chromatographia,
23(11): 829-834.
Prasanna CV & Ramakrishnan S (1984a) Effect of acetaldehyde on hepatic
glucose metabolism. Indian J Biochem Biophys, 21(1):62-64.
Prasanna CV & Ramakrishnan S (1984b) Effect of acetaldehyde on
carbohydrate metabolism in rat brain. Indian J Biochem Biophys,
21(2): 121-123.
Prasanna CV & Ramakrishnan S (1987) Effect of acetaldehyde on hepatic
and blood lipids. Indian J Med Res, 85: 206-211.
Prasanna CV, Namagiri T, & Ramakrishnan S (1986) Effect of
acetaldehyde on thyroid function. Indian J Exp Biol, 24(2): 77-78.
Priscott PK & Ford JR (1985) An in vitro model of acetaldehyde
metabolism by rodent conceptuses. in vitro Cell Dev Biol,
21(2): 88-92.
Ramdahl T, Alfheim I, Rustad S, & Olsen T (1982) Chemical and
biological characterization of emissions from small residential stoves
burning wood and charcoal. Chemosphere, 11(4): 601-611.
Rawat K (1979) Inhibition of cardiac protein synthesis by prolonged
ethanol administration. Res Commun Chem Pathol Pharmacol, 25: 89.
Rideout JM, Lim CK, & Peters TJ (1986) Assay of blood acetaldehyde by
HPLC with fluorescence detection of its 2-diphenylacetyl-1,3-
indandione-1-azine derivative. Clin Chim Acta, 161: 29-35.
Rieger R & Michaelis A (1960) [Chromosomal aberrations after action of
acetaldehyde upon the primary roots of Vicia faba.] Biol Zent.bl,
679: 1-5 (in German).
Ristow H & Obe G (1978) Acetaldehyde induces cross-links in DNA and
causes sister-chromatid exchanges in human cells. Mutat Res,
58: 115-119.
Rosensteel RE & Tanaka S (1976) Health hazard evaluation report
No. 75-89-344. Cincinnati, Ohio, US National Institute of Occupational
Safety and Health.
Roumec C, Lamboeuf Y, & De Saint Blanquat G (1988) Sinaptosomal
phospholipids in rats chronically treated with acetaldehyde. Adv
Biosci, 71: 201-205.
Rudling L, Ahling B, & Loefroth G (1981) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in small furnaces and stoves. In: Proceedings of the International
Conference on Residential Solids Fuels, Environmental Impacts and
Solutions. Beaverton, Oregon, Oregon Graduate Center, pp 34-53.
Saladino AJ, Willey JC, Lechner JF, Grafstrom RC, Laveck M, & Harris
CC (1985) Effects of formaldehyde, acetaldehyde, benzoyl peroxide and
hydrogen peroxide on cultured normal human bronchial epithelial cells.
Cancer Res, 45(6): 2522-2526.
Saldiva PHN, Do Rio Caldeira MP, Massad CW, Calheiros DF, Cardoso LMN,
Bohm GM, & Saldiva CD (1985) Effects of formaldehyde and acetaldehyde
inhalation on rat pulmonary mechanics. J Appl Toxicol, 5(5): 288-292.
San George RC & Hoberman HD (1986) Reaction of acetaldehyde with
hemoglobin. J Biol Chem, 261: 6811-6821.
Sasaki Y & Endo R (1978) Mutagenicity of aldehydes in Salmonella.
Mutat Res, 54: 251-252.
Savolainen ER, Leo MA, Timpl R, & Lieber CS (1984) Acetaldehyde and
lactate stimulate collagen synthesis of cultured baboon liver
myofibroblasts. Gastroenterology, 87(4): 777-787.
Schopf RE, Tromperer M, Bork K, & Morsches B (1985) Effects of ethanol
and acetaldehyde on phagocytic functions. Arch Dermatol Res,
277(2): 131-137.
Schreiber SS, Briden K, Oratz M, & Rothschild MA (1972) Ethanol,
acetaldehyde, and myocardial protein synthesis. J Clin Invest,
51: 2820-2826.
Schreiber SS, Oratz M, Rothschild MA, Reff F, & Evans C (1974)
Alcoholic cardiomyopathy. II. The inhibition of cardiac microsomal
protein synthesis by acetaldehyde. J Mol Cell Cardiol, 6: 207-213.
Schreiner G, Ulbrich M, Senger-Weil M, & Bass R (1987) Effects of
prenatal ethanol or acetaldehyde exposure on postnatal development and
behavior in the rat. Teratology, 36(1): 31A.
Schulam P, Newbold R, & Hull LA (1985) Urban and rural ambient air
aldehyde levels in Schenectady, New York and on Whiteface Mountain,
New York. Atmos Environ, 19(4): 623-626.
Segel LD (1984) Mitochondrial respiration after cardiac perfusion with
ethanol or acetaldehyde. Alcohol Clin Exp Res, 8(6): 560-564.
Shackelford WM & Keith LH (1976) Frequency of organic compounds
identified in water. Washington, DC, US Environmental Protection
Agency, pp 7, 37, 46 (EPA-600/4/76/062).
Shaw S & Jayatilleke E (1987) Acetaldehyde-mediated hepatic lipid
peroxidation: role of superoxide and ferritin. Biochem Biophys Res
Commun, 143(3): 984-990.
Shiohara E, Sukada M, Chiba S, Yamazaki H, Nishiguchi K, Miyamoto R, &
Nakanishi S (1985) Effect of chronic administration of acetaldehyde by
inhalation on (NA+/K+)-activated adenosine triphosphatase activity of
rat brain membranes. Toxicology, 34(4): 277-284.
Silverman L, Schulte HF, & First MW (1946) Further studies on sensory
response to certain industrial solvent vapors. J Ind Hyg Toxicol,
28: 262-266.
Sim VM & Pattle RE (1957) Effect of possible smoke irritation on human
subjects. J Am Med Assoc, 165: 1908-1913.
Sina JF, Bean CL, Dysart GR, Taylor VI, & Bradley MO (1983) Evaluation
of the alkaline elution/rat hepatocyte assay as a predictor of
carcinogenic/mutagenic potential. Mutat Res, 113: 357-391.
Singh HB, Salas LJ, Stiles R, & Shigeishi H (1982) Measurements of
hazardous organic chemicals in the ambient atmosphere. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Environmental Sciences Research Laboratory, pp 32-37, 70-79, 84-90
(SRI International Project 7774).
Singh S, Fritze G, Fang B, Harada S, Paik YK, Eckey R, Agarwal DP, &
Goedde HW (1989) Inheritance of mitochondrial aldehyde dehydrogenase:
genotyping in Chinese, Japanese, and South Korean families reveals
dominance of the mutant allele. Hum Genet, 83: 119-121.
Sipi P, Jarventaus H, & Norppa H (1992) Sister-chromatid exchanges
induced by vinyl esters and respective carboxylic acids in cultured
human lymphocytes. Mutat Res, 279: 75-82.
Skog E (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde, propionaldehyde
and butyraldehyde; as well as of acrolein and crotonaldehyde. Acta
Pharmacol, 6: 299-318.
Smyth HF, Carpenter CP, & Weils CS (1951) Range-finding toxicity data:
list IV. Am Med Assoc Arch Ind Health Occup Med, 4: 119.
Snyder JW (1988) The role of phospholipid metabolism in ethanol- and
acetaldehyde-initiated hepatocyte injury. Diss Abstr Int,
B48(10): 2937.
Solomon LR (1987) Studies on the mechanism of acetaldehyde-mediated
inhibition of rat liver transaminases. Clin Chim Acta,
168(2): 207-217.
Solomon LR (1988) Effects of acetaldehyde on human red cell
metabolism: evidence for the formation of enzyme inhibitors. Clin Chim
Acta, 175: 249-266.
Sorrell MF & Tuma DY (1985) Hypothesis: alcoholic liver injury and the
covalent binding of acetaldehyde. Alcohol Clin Exp Res, 9: 306-309.
Speece RE (1983) Anaerobic biotechnology for industrial wastewater
treatment. Environ Sci Technol, 17(9): 416A-427A.
Spingarn NE, Northington DJ, & Pressely T (1982) Analysis of volatile
hazardous substances by GS/MS. J Chromatogr Sci, 20: 287-288.
Sprince H, Parker CM, Smith GG, & Gonzales LJ (1974) Protection
against acetaldehyde toxicity in the rat by L-cysteine, thiamin and
L-2-methylthiazolidine-4-carboxylic acid. Agents Actions, 4: 125-130.
Sreenathan RN, Padmanabhan R, & Singh S (1982) Teratogenic effects of
acetaldehyde in the rat. Drug Alcohol Depend, 9: 339-350.
Sreenathan RN, Padmanabhan R, & Singh S (1984a) Structural changes of
placenta following maternal administration of acetaldehyde in rat. Z
Mikrosk-Anat Forsch (Leipzig), 98: 597-604.
Sreenathan RN, Singh S, & Padmanabhan R (1984b) Effect of acetaldehyde
on skeletogenesis in rats. Drug Alcohol Depend, 14(2): 165-174.
Stege TE (1982) Acetaldehyde-induced lipid peroxidation in isolated
hepatocytes. Chem Pathol Pharmacol, 36: 287-297.
Steinberg S & Kaplan IR (1984) The determination of low-molecular
weight aldehydes in rain, fog and mist by reserved-phase liquid
chromatography of the 2,4-dinitrophenyl-hydrazone derivatives. Int J
Environ Anal Chem, 18: 253-266.
Steinhagen WH & Barrow CS (1984) Sensory irritation structure-activity
study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol
Appl Pharmacol, 72(3): 495-503.
Stewart JK, Aharoni Y, Hastsell PL, & Young DK (1980) Symptoms of
acetaldehyde injury on head lettuce. HortScience, 15(2): 148-149.
Takahashi K, Tomaru A, Nishiyama N, & Horiguchi M (1986) Alcoholic
cardiomyopathy: direct and indirect effects of acetaldehyde.
Jpn Circ J, 50(6): 567.
Takami T, Kuwata K, Sugimae A, & Nakamoto M (1985) Trace determination
of aldehydes in water by high-performance liquid chromatography. Anal
Chem, 57: 243-245.
Tanner RL & Meng Z (1984) Seasonal variations in ambient atmospheric
levels of formaldehyde and acetaldehyde. Environ Sci Technol,
18: 723-726.
Tejada SB (1986) Evaluation of silica gel cartridges coated in situ
with acidified 2,4-dinitrophenylhydrazine for sampling aldehydes and
ketones in air. Int J Environ Anal Chem, 26: 167-185.
Teschke R, Hasumura Y, & Lieber CS (1977) Hepatic pathways of ethanol
and acetaldehyde metabolism and their role in the pathogenesis of
alcohol-induced liver injury. Nutr Metab, 21(Suppl 1): 144-147.
Thom NS & Agg AR (1975) The breakdown of synthetic organic compounds
in biological processes. Proc R Soc London, B189: 347-357.
Til HP, Woutersen RA, Feron VJ, & Clary JJ (1988) Evaluation of the
oral toxicity of acetaldehyde and formaldehyde in a 4-week
drinking-water study in rats. Fundam Chem Toxicol, 26(5): 447-452.
Truitt EB & Walsh MJ (1971) The role of acetaldehyde in the actions of
ethanol. In: Kissin B & Begleiter H ed. The biology of alcoholism.
Volume 1: Biochemistry. New York, London, Plenum Press, pp 161-195.
Tuma DJ, Donohue TM Jr, Medina VA, & Sorrell MF (1984) Enhancement of
acetaldehyde-protein adduct formation by L-ascorbate. Arch Biochem
Biophys, 234(2): 377-381.
Tuma DJ, Jennett RD, & Sorrell MF (1987) The interaction of
acetaldehyde with tubuline. Ann NY Acad Sci, 282: 227-286.
Ung-Chhun NS & Collins MA (1987) Estimation of blood acetaldehyde
during ethanol metabolism: a sensitive HPLC/fluorescence microassay
with negligible artifactual interference. Alcohol, 4: 473-476.
US EPA (1975) Identification of organic compounds in effluents from
industrial sources. Washington, DC, US Environmental Protection
Agency, pp C-2, 4-14, 4-15, 4-17 (EPA-560/3-75-002; PB-241 841).
US NIOSH (1980) Projected number of occupational exposures to chemical
and physical hazards. Cincinnati, Ohio, US National Institute for
Occupational Safety and Health.
US NIOSH (1981) National occupational hazards survey. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health, p 14.
US NIOSH (1987) Manual of analytical methods. Method 3507. Cincinnati,
Ohio, US National Institute for Occupational Safety and Health.
US NIOSH (1989) Manual of analytical methods. Method 2538.
Cincinnati, Ohio, US National Institute for Occupational Safety and
Health.
US NRC (1977) Other organic constituents (Acetaldehyde). In: Drinking
water and health. Washington, DC, US National Research Council,
pp 680-687.
US NRC (1981) Food chemical codex, 3rd ed. Washington, DC, US National
Research Council, National Academy Press, pp 353-355, 583, 615.
US NRC (1985) Chemicals used on food processing. Washington, DC, US
National Research Council, National Academy of Sciences (Publication
No. 1274).
Veghelyi PV & Osztovics M (1978) The alcohol syndromes: the
intrarecombigenic effect of acetaldehyde. Experientia (Basel),
34: 195-196.
Veghelyi PV, Osztovics M, Kardos G, Leisztner L, Szaszovszky E, Igali
S, & Imrei J (1978) The fetal alcohol syndrome: symptoms and
pathogenesis. Acta Paediatr Acad Sci Hung, 19(3): 171-189.
Verschueren U (1983) Handbook of environmental data on organic
chemicals, 2nd ed. New York, Van Nostrand Reinhold Company,
pp 139-141.
Von Burg R & Stout T (1991) Toxicology update-acetaldehyde. J Appl
Toxicol, 11: 373-376.
Wangenheim J & Bocsfoldi G (1986) Mouse lymphoma tk-positive-negative
assay of 30 compounds. Environ Mutagen, 8(Suppl 6): 90.
Wangenheim J & Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine
kinase locus assay of 50 compounds. Mutagenesis, 3(3): 193-206.
Watanabe I (1987) [Distribution of lower oxygenated organic compounds
in urban air.] Preprint of 54th Annual Meeting of the Chemical Society
of Japan, vol 1, p 129 (in Japanese).
Watanabe I (1988) Sequential automated analysis system for lower
oxygenated organic compounds in ambient air. J Res Nat Bureau Stand,
93(3): 299-301.
Watanabe A, Hobara N, & Nagashima H (1986) Blood and liver
acetaldehyde concentration in rats following acetaldehyde inhalation
and intravenous and intragastric ethanol administration. Bull Environ
Contam Toxicol, 37: 513-516.
Webster WS, Walsh DA, McEwen SE, & Lipson AH (1983) Some teratogenic
properties of ethanol and acetaldehyde in C57BL/6J mice: implications
for the study of the fetal alcohol syndrome. Teratology, 27: 231-243.
Westcott JY, Weiner H, Schultz J, & Myers RD (1980) In vivo
acetaldehyde in the brain of the rat treated with ethanol. Biochem
Pharmacol, 29: 411-417.
WHO (1989) IPCS Environmental Health Criteria 89: Formaldehyde.
Geneva, World Health Organization, 219 pp.
WHO (in press) Environmental Health Criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization.
Wilkin JK & Fortner G (1985) Cutaneous vascular sensitivity to lower
aliphatic alcohols and aldehydes in Orientals. Alcohol Clin Exp Res,
9(6): 522-525.
Williams AJ & Barry RE (1986) Superoxide anion production and
degranulation of rat neutrophils in response to acetaldehyde-altered
liver cell membranes. Clin Sci, 71(3): 313-318.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen,
7(5): 677-702.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1989) Antibodies
against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1990) Antibodies
against acetaldehyde-modified epitopes: presence in alcoholic,
nonalcoholic liver disease and control subjects. Alcohol Alcohol,
25(5): 509-517.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991a) Detection of
stable acetaldehyde-modified proteins in the livers of ethanol-fed
rats. Alcohol Alcohol, 26(4): 437-444.
Worrall S, De Jersey J, Shanley BC, & Wilce PA (1991b) Antibodies of
S3 against acetaldehyde-modified epitopes: an elevated IgA response in
alcoholics. Eur J Clin Invest, 21: 90-95.
Woutersen RA & Feron VJ (1987) Inhalation toxicity of acetaldehyde in
rats. IV. Progression and regression of nasal lesions after
discontinuation. Toxicology, 47(3): 295-305.
Woutersen RA, Appelman LM, Feron VJ, & der Heijden CA (1984)
Inhalation toxicity of acetaldehyde in rats. II. Carcinogenicity
study: interim results after 15 months. Toxicology, 31: 123-133.
Woutersen RA, Appelman LM, Van Garderen-Hoetmer A, & Feron VJ (1986)
Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity
study. Toxicology, 41(2): 213-231.
Yoshida A, Huang IY, & Ikawa M (1984) Molecular abnormality of an
inactive aldehyde dehydrogenase variant commonly found in Orientals.
Proc Natl Acad Sci (USA), 81: 258-261.
Zorzano A & Herrera E (1989) Disposition of ethanol and acetaldehyde
in late pregnant rats and their fetuses. Paediatr Res, 25(1): 102-106.
Résumé
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
L'acétaldéhyde est un liquide incolore et volatil à l'odeur acre
et suffocante. D'après la littérature, son seuil olfactif est de
0,09 mg/m3. L'acétaldéhyde est un composé très inflammable et
réactif qui est miscible à l'eau et à la plupart des solvants
ordinaires.
Il existe un certain nombre de méthodes d'analyse pour la
recherche de l'acétaldéhyde dans l'air (y compris dans l'haleine) et
dans l'eau. La principale méthode repose sur la réaction de
l'acétaldéhyde avec la 2,4-dinitrophénylhydrazine, puis analyse de
l'hydrazone obtenue par chromatographie liquide à haute pression ou
chromatographie en phase gazeuse.
2. Sources d'exposition humaine et environnementale
L'acétaldéhyde est un intermédiaire métabolique chez l'homme et
les plantes supérieures; c'est également un produit de la fermentation
alcoolique. On a décelé sa présence dans certaines denrées
alimentaires et boissons ainsi que dans la fumée de cigarette. Il est
également présent dans les gaz d'échappement des véhicules à moteur
ainsi que dans divers déchets industriels. La décomposition des
hydrocarbures, des effluents d'égouts et des déchets biologiques
solides produit de l'acétaldéhyde, de même que la combustion à l'air
libre ou, selon le cas, l'incinération du gaz, du mazout et de la
houille.
Plus de 80% de l'acétaldéhyde utilisé à des fins commerciales est
produit par l'oxydation en phase liquide de l'éthylène en présence
d'une solution catalytique de chlorures de palladium et de cuivre. Au
Japon, la production était de 323.000 tonnes en 1981. Aux Etats-Unis
d'Amérique elle a été de 281.000 tonnes en 1982 et en Europe
occidentale de 706.000 tonnes en 1983. La majeure partie de
l'acétaldéhyde produit commercialement est utilisé pour la préparation
de l'acide acétique. On l'utilise également pour la préparation de
certains arômes et denrées alimentaires.
On estime qu'aux Etats-Unis d'Amérique, les émissions annuelles
d'acétaldéhyde de toutes origines atteignent 12,2 millions de kg.
3. Transport, distribution et transformation dans l'environnement
En raison de la forte réactivité de l'acétaldéhyde, le transport
intercompartimental de ce composé devrait être limité. On peut
s'attendre à un certain transfert d'acétaldéhyde de l'air vers l'eau
et le sol en raison de sa forte tension de vapeur et de son faible
coefficient de sorption.
On pense que l'élimination photo-induite de l'acétaldéhyde
atmosphérique passe essentiellement par la formation de radicaux. La
photolyse devrait jouer également un rôle important dans le processus
d'élimination. Environ 80% des émissions d'acétaldéhyde dans
l'atmosphère sont éliminés chaque jour par ces deux processus.
D'après la littérature, la demi-vie de l'acétaldéhyde dans l'eau et
l'air est respectivement égale à 1,9 heure et 10-60 heures.
L'acétaldéhyde est facilement biodégradable.
4. Concentrations dans l'environnement et exposition humaine
Les concentrations moyennes d'acétaldéhyde dans l'environnement
sont généralement égales à 5 µg/m3. Dans l'eau, elles sont
généralement inférieures à 0,1 µg/litre. L'analyse de denrées
alimentaires très diverses effectuées aux Pays-Bas a montré que leur
teneur en acétaldéhyde est généralement inférieure à 1 mg/kg, mais
peut atteindre occasionnellement jusqu'à plusieurs centaines de mg/kg,
en particulier dans certains jus de fruit et dans le vinaigre.
Pour la population générale, la principale source d'exposition à
l'acétaldéhyde est de loin le métabolisme de l'alcool. La fumée de
cigarette constitue également une importante source d'exposition. En
ce qui concerne les autres véhicules, la population générale est
exposée à l'acétaldéhyde essentiellement par l'intermédiaire de la
nourriture et des boissons et, dans une moindre mesure, de l'air.
L'eau de boisson est négligeable à cet égard.
Les données disponibles sont insuffisantes pour qu'on puisse
évaluer l'ampleur de l'exposition à l'acétaldéhyde sur les lieux de
travail. Les travailleurs peuvent y être exposés dans certaines
industries ainsi que lors de la fermentation de l'alcool; dans ces
conditions la principale voie d'exposition est très probablement
l'inhalation avec possibilité également de contacts cutanés.
5. Cinétique et métabolisme
5.1 Absorption, distribution et élimination
Les données toxicologiques dont on dispose indiquent que
l'acétaldéhyde est résorbé au niveau des poumons et des voies
digestives; toutefois on ne connaît pas d'études quantitatives
appropriées qui aient été consacrées à ce problème. Il est probable
qu'il y a également une résorption cutanée.
Après avoir fait inhaler de l'acétaldéhyde à des rats, on a
constaté qu'il se répartissait dans le sang, le foie, les reins, la
rate, le coeur et le tissu musculaire. De faibles quantités en ont
été décelées dans des embryons après injection intrapéritonéale à la
mère (souris) ainsi qu'après exposition de la mère à de l'éthanol
(souris et rat). Il pourrait également y avoir production
d'acétaldé-hyde in vitro chez les foetus de rat et dans le placenta
humain.
Après injection intrapéritonéale d'éthanol, on a pu mettre en
évidence la distribution de l'acétaldéhyde dans le liquide
interstitiel de l'encéphale, à l'exclusion des cellules cérébrales. Il
est possible qu'une acétaldéhyde-déshydrogénase de forte affinité et
de faible Km joue un rôle important dans le maintien de faibles
concentrations d'acétaldéhyde dans le cerveau au cours de la
métabolisation de l'éthanol.
On a constaté que chez l'homme et les babouins, l'acétaldéhyde
pouvait être fixé par les globules rouges in vivo, la concentration
intraglobulaire pouvant atteindre dix fois la concentration
plasmatique.
Après administration par voie orale, il n'y a pratiquement pas
d'acétaldéhyde qui soit excrété tel quel dans les urines.
5.2 Métabolisme
La principale voie métabolique de l'acétaldéhyde consiste dans
une oxydation en acétate sous l'action de l'ALDHa NADb-dépendante.
L'acétate entre dans le cycle de l'acide citrique sous forme
d'acétyl-CoA. Il existe plusieurs isoenzymes de l'ALDH présentant
divers paramètres cinétiques et paramètres de liaison qui influent sur
la vitesse d'oxydation de l'acétaldéhyde.
Chez le rat, on a pu localiser une activité ALDH dans
l'épithélium respiratoire (à l'exclusion de l'épithélium olfactif),
alors que cette localisation se situe au niveau du rein chez la souris
et au niveau du cortex et des tubules rénaux chez le chien, le rat, le
cobaye et le babouin.
Les tissus embryonnaires de souris et de rat métabolisent
l'acétaldéhyde in vitro. L'acétaldéhyde traverse le placenta du rat
malgré l'existence d'un métabolisme placentaire.
a acétaldéhyde-déshydrogénase
b nicotinamide-adénine-dinucléotide
Il y a un certaine métabolisation de l'acétaldéhyde au niveau des
tubules rénaux chez l'homme mais c'est le foie qui est le principal
site du métabolisme.
Chez l'homme, on a décelé la présence de plusieurs formes
isoenzymatiques d'ALDH, au niveau du foie et d'autres tissus. Il y a
également un polymorphisme de l'ALDH michocondrial. Les sujets qui
sont homozygotes ou hétérozygotes pour une mutation ponctuelle du gène
correspondant à l'ALDH mitrochondriale, présentent une faible activité
ALDH, métabolisent lentement l'acétaldéhyde et ne supportent pas
l'alcool éthylique.
Le crotonadéhyde, la maléate de diméthyle, la phorone, le
disulfirame et le carbamide calcique inhibent le métabolisme de
l'acétaldéhyde.
5.3 Réactions avec d'autres constituants
L'acétaldéhyde forme des adduits stables ou instables avec les
protéines. Ces adduits peuvent perturber la fonction de ces protéines
comme le montre d'ailleurs l'inhibition des enzymes, les difficultés
de liaison entre histones et ADN et l'inhibition de la polymérisation
de la tubuline.
Des adduits instables de l'acétaldéhyde dont on connaît mal
l'importance se forment in vitro avec les acides nucléiques.
L'acétaldéhyde peut réagir sur diverses macromolécules de
l'organisme, de préférence avec celles qui sont porteuses de résidus
de lysine; cette réaction peut conduire à des altérations importantes
de la fonction biologique de ces molécules.
6. Effets sur les êtres vivants dans leur milieu naturel
6.1 Organismes aquatiques
Chez les poissons la CL50 peut varier de 35 mg/litre (guppy) à
140 mg/litre (espèces non précisées). Une CE5 de 82 mg/litre et une
CE50 de 42 mg/litre ont été observées respectivement chez des algues
et chez Daphnia magna.
6.2 Organismes terrestres
La présence d'acétaldéhyde dans l'air se révèle toxique pour
certains microorganismes, à des concentrations relativement faibles.
Des aphidiens ont été tués par exposition de 3 à 4 heures à de
l'acétaldéhyde à une concentration de 0,36 µg/m3.
Chez deux espèces de limaces, Arion hortensis et Agriolimax
reticulatus, on a observé des concentrations létales médianes
respectivement égales à 8,91 mg/litre et par heure et 7,69 mg/litre et
par heure.
L'acétaldéhyde provoque une inhibition réversible de la
germination des oignons, des carottes et des tomates à des
concentrations allant jusqu'à 1,52 mg/litre; par contre cette
inhibition est irréversible pour Amaranthus palmeri, dans les mêmes
conditions d'exposition. A la concentration de 0.54 µg/m3,
l'acétaldéhyde a détérioré des laitues.
7. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Les valeurs de la DL50 pour le rat et la souris et de la CL50
pour le rat et le hamster doré montrent que la toxicité aiguë de
l'acétaldéhyde est faible. On n'a pas connaissance d'études relatives
à la toxicité cutanée aiguë de l'acétaldéhyde.
7.2 Exposition à court et à long terme
Lors d'études au cours desquelles des doses ont été administrées
à plusieurs reprises, tant par la voie orale que par la voie
respiratoire, les effets toxiques observés à des concentrations
relativement faibles se limitaient essentiellement aux points de
contact initiaux. Lors d'une étude de 28 jours au cours de laquelle
de l'acétaldéhyde a été administré à des rats, mélangé à leur eau de
boisson, à la dose de 675 mg/kg de poids corporel (dose sans effets
observables 125 mg/kg de poids corporel), on a constaté que les effets
se limitaient à une légère hyperkératose focale au niveau de la
portion cardiaque de l'estomac. Après administration d'acétaldéhyde à
concentration constante de 0,05% dans l'eau de boisson pendant une
durée de 6 mois (le Groupe de travail estime que cela correspond à peu
près à 40 mg/kg de poids corporel) au cours d'une étude biochimique,
on a constaté que l'acétaldéhyde provoquait la synthèse de collagène
au niveau du foie chez le rat, observation d'ailleurs corroborée par
les résultats obtenus in vitro.
Lors d'une étude d'inhalation de 4 semaines chez le rat et de 13
semaines chez le hamster, on a obtenu, pour la concentration sans
effets respiratoires observables, des valeurs respectivement égales à
275 mg/m3 et 700 mg/m3. Aux concentrations les plus faibles
produisant un effet, on a observé des altérations dégénératives au
niveau de l'épithélium olfactif chez le rat (437 mg/m3) et de la
trachée (2400 mg/m3) chez le hamster. Des altérations dégénératives
de l'épithélium respiratoire et du larynx ont été observées à des
concentrations plus élevées. On n'a pas connaissance d'études
relatives à l'administration répétée d'acétaldéhyde au niveau cutané.
7.3 Reproduction, embryotoxicité et tératogénicité
Plusieurs études montrent que des malformations foetales peuvent
survenir par suite de l'exposition parentérale de rattes et de souris
gravides à l'acétaldéhyde. Dans la plupart de ces études, on n'a pas
étudié la toxicité pour la mère. On n'a pas pu trouver de données
relatives à la toxicité de l'acétaldéhyde pour la fonction de
reproduction.
7.4 Mutagénicité et points d'aboutissement des effets correspondants
In vitro, l'acétaldéhyde est génotoxique et produit des
mutations géniques, des effets clastogènes ainsi que des échanges
entre chromatides soeurs dans des cultures de cellules mammaliennes en
l'absence d'activation métabolique exogène. Toutefois, des épreuves
effectuées dans les règles sur des salmonelles ont donné des résultats
négatifs. Après avoir injecté de l'acétaldéhyde par voie
intrapéritonéale à des hamsters chinois et à des souris, on a constaté
que ce composé produisait des échanges entre chromatides soeurs au
niveau de la moelle osseuse. Cependant, de l'acétaldéhyde administré
par la même voie n'a pas eu pour conséquence un accroissement de la
fréquence des micronoyaux dans les spermatides de souris récemment
formés. On peut déduire indirectement de certaines études in vitro
et in vivo que l'acétaldéhyde est susceptible de produire des
pontages protéines-ADN et ADN-ADN.
7.5 Cancérogénicité
Des études d'inhalation effectuées sur des rats et des hamsters
ont permis de constater un accroissement de l'incidence des tumeurs.
Chez le rat, il s'agissait d'une augmentation, liée à la dose, de la
fréquence des adénocarcinomes au niveau de la muqueuse nasale ainsi
que des carcinomes spino-cellulaires (accroissement significatif à
toutes les doses). Toutefois chez le hamster, l'accroissement constaté
de la fréquence des carcinomes du nez et du larynx n'était pas
significatif. A toutes les concentrations d'acétaldéhyde, on a
constaté l'apparition de lésions tissulaires chroniques au niveau des
voies respiratoires.
7.6 Etudes spéciales
On n'a pas connaissance d'études appropriées portant sur la
neuro- et l'immunotoxicité potentielle de l'acétaldéhyde.
8. Effets sur l'homme
Lors d'études limitées portant sur des volontaires humains, on a
constaté que l'acétaldéhyde avait un effet légèrement irritant sur les
yeux et les voies respiratoires supérieures, après exposition de très
brève durée à des concentrations respectivement supérieures à environ
90 et 240 mg/m3. Après l'application d'un timbre inbibé
d'acétaldéhyde, on a constaté la présence d'un érythème cutané chez 12
sujets "d'ascendance orientale".
On a signalé l'existence d'une enquête limitée au cours de
laquelle on a étudié l'incidence des cancers chez des travailleurs
exposés à de l'acétaldéhyde.
On possède des preuves indirectes que le métabolite toxique
supposé être à l'origine des lésions hépatiques imputables à l'alcool,
de bouffées de chaleur et d'effets sur la développement, est en fait
l'acétaldéhyde.
9. Evaluation des risques pour la santé humaine et des effets
sur l'environnement
Les études effectuées sur l'animal montrent que, par voie
respiratoire ou par voie orale, l'acétaldéhyde n'est que faiblement
toxique. D'après les études effectuées sur l'animal mais également
sur l'homme, on a constaté que ce composé était légèrement irritant
pour l'oeil et les voies respiratoires supérieures. Chez l'homme,
l'application d'un timbre cutané inbibé d'acétaldéhyde peut provoquer
l'apparition d'un érythème cutané. On pense avoir découvert le
mécanisme à l'origine de cet effet mais les données disponibles sont
encore insuffisantes pour qu'on puisse évaluer le pouvoir
sensibilisateur de l'acétaldéhyde.
On ne dispose que de données limitées sur les effets d'une
ingestion d'acétaldéhyde. Après administration d'acétaldéhyde par
voie orale à des rats à la dose quotidienne de 675 mg/kg de poids
corporel, on a constaté un accroissement limite de l'hyperkératose au
niveau de la portion cardiaque de l'estomac (dose sans effets nocifs
observables: 125 mg/kg de poids corporel). Après avoir fait boire à
des rats six mois durant de l'eau contenant environ 40 mg
d'acétaldéhyde/kg de poids corporel, on a constaté un accroissement de
la synthèse du collagène hépatique, effet dont la signification reste
obscure.
D'après des études effectuées sur des rats et des hamsters, ce
sont les voies respiratoires supérieures qui constituent l'organe
cible de l'acétaldéhyde lorsque ce composé est inhalé. D'après les
résultats obtenus, la concentration la plus faible à laquelle on a
commencé à observer des effets était de 437 mg/m3, après une période
d'administration de 5 semaines. Chez des rats exposés pendant 4
semaines, la dose sans effets respiratoires observables était de
275 mg/m3; elle était de 700 mg/m3 chez des hamsters exposés
pendant 13 semaines.
Aux concentrations qui produisaient des lésions tissulaires des
voies respiratoires, on a observé un accroissement de l'incidence des
adénocarcinomes du nez et des carcinomes spino-cellulaires chez le
rat; il y a également eu accroissement des carcinomes du larynx et du
nez chez le hamster.
On est fondé à penser qu' in vivo, l'acétaldéhyde provoque des
lésions génétiques dans les cellules somatiques.
les données disponibles sont insuffisantes pour qu'on puisse
apprécier les effets qu'une exposition à l'acétaldéhyde pourrait avoir
sur la reproduction, le développement ainsi que les fonctions
neurologiques et immunologiques de la population générale ou de la
population professionnellement exposées.
Pour ce qui est du pouvoir irritant de ce composé chez l'homme,
on estime que la concentration tolérable est de 2 mg/m3. Etant
donné que le mécanisme d'induction de tumeurs par l'acétaldéhyde n'a
pas été bien étudié, on a adopté deux approches concernant ce point
d'aboutissement de son action toxique, à savoir la fixation d'une
concentration tolérable qui serait obtenue en divisant la dose
irritante pour les voies respiratoires chez les rongeurs, par un
certain facteur d'incertitude et l'estimation d'un risque de cancer
sur toute la durée de l'existence, en procédant par extrapolation
linéaire. La concentration tolérable est de 0,3 mg/m3. La
concentration produisant un excès de risque de 10-5 sur toute la durée
de la vie se situe entre 11-65 µg/m3.
Les données limitées dont on dispose ne permettent pas de tirer
des conclusions définitives quant au risque que l'acétaldéhyde
pourrait représenter pour l'ensemble de la faune et de la flore.
Toutefois, si l'on considère la courte durée de vie de l'acétaldéhyde
dans l'air et dans l'eau et le fait qu'il est facilement
biodégradable, on est amené à penser que ce composé n'est guère
menaçant pour les organismes aquatiques ou terrestres, sauf peut-être
en cas de décharge ou de déversement de produits industriels.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El acetaldehído es un líquido incoloro, volátil y de olor acre y
sofocante. El umbral señalado para el olor es de 0,09 mg/m3. El
acetaldehído es un compuesto muy inflamable y reactivo, soluble en
agua y en la mayoría de los disolventes comunes.
Existen métodos de análisis para la detección del acetaldehído en
el aire (incluso en el aliento) y en el agua. El principal de ellos
se basa en la reacción del producto con 2,4-dinitrofenilhidracina y
ulterior análisis de los derivados de la hidrazona por cromatografía
de líquidos a alta presión o cromatografía de gases.
2. Fuentes de exposición humana y ambiental
El acetaldehído es un producto intermedio del metabolismo en los
hombres y en las plantas superiores, y también proviene de la
fermentación alcohólica. Ha sido identificado en los alimentos, las
bebidas y el humo de los cigarrillos. También se encuentra en los
gases de escape de los vehículos y en ciertos desechos industriales.
La degradación de los hidrocarburos, las aguas residuales y los
desechos biológicos sólidos produce acetaldehído, como también lo
hacen la combustión al aire libre y la incineración de gas, fuel oil y
carbón.
Más del 80% del acetaldehído usado comercialmente proviene de la
oxidación en fase líquida de etileno con una solución catalítica de
paladio y cloruros de cobre. En 1981, la producción japonesa fue de
323 000 toneladas. En los Estados Unidos de América fue de 281 000
toneladas en 1982 y en Europa occidental de 706 000 toneladas en 1983.
La mayoría del acetaldehído producido comercialmente se destina a la
fabricación de ácido acético. También se utiliza en sustancias
aromáticas y en alimentos.
En los Estados Unidos, la emisión anual de acetaldehído de todo
origen se calcula en 12,2 millones de kilogramos.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta reactividad, el transporte intercompartimental
de acetaldehído debe ser limitado. Es de suponer que hay cierta
transferencia de la sustancia al aire desde el agua y el suelo, como
consecuencia de la fuerte presión del vapor y el bajo coeficiente de
sorción.
Es posible que la eliminación fotoinducida del acetaldehído en la
atmósfera tenga lugar principalmente por formación de un radical. La
fotosíntesis puede también contribuir bastante al proceso de
eliminación. La pérdida resultante de acetaldehído proveniente de
emisiones en la atmósfera es de alrededor del 80%. La vida media de
la sustancia en el agua y en el aire es de 1,9 h y 10-60 h,
respectivamente.
El acetaldehído es muy biodegradable.
4. Niveles medioambientales y exposición humana
Los niveles de acetaldehído en el aire ambiente suelen ser por
término medio de 5 µg/m3. Las concentraciones en el agua no llegan
en general a 0,1 µg/litro. El análisis de diversos alimentos en los
Países Bajos demostró que las concentraciones, normalmente inferiores
a 1 mg/kg, a veces llegaban a 100 mg/kg, particularmente en algunos
zumos de frutas y en el vinagre.
La principal fuente, con mucho, de exposición al acetaldehído
para la mayoría de la población es el metabolismo del alcohol. El
humo del cigarrillo es también una fuente significativa. Aparte de
eso pueden mencionarse en segundo lugar los alimentos y bebidas y, en
menor grado, el aire. La contribución del agua de beber es
insignificante.
No existen datos suficientes para determinar la importancia de la
exposición al acetaldehído en el lugar de trabajo. El personal puede
estar expuesto en algunas industrias manufactureras y donde hay
fermentación con producción de alcohol, siendo la principal vía la
inhalación y posiblemente el contacto con la piel.
5. Cinética y metabolismo
5.1 Absorción, distribución y eliminación
Los estudios existentes sobre toxicidad indican que el
acetaldehído se absorbe por los pulmones y el conducto
gastrointestinal; sin embargo, no parecen existir análisis
cuantitativos adecuados. Es probable la absorción por la piel.
En las ratas, el acetaldehído inhalado se distribuye por la
sangre, pasando al hígado, el riñón, el bazo, el corazón y otros
tejidos musculares. Se han detectado bajos niveles en embriones de
ratón tras inyectar la sustancia a la madre por vía intraperitoneal o
tras exposición de ratas o ratones hembra a etanol. La producción
potencial de acetaldehído in vitro se ha observado en fetos de rata
y en la placenta humana.
Tras inyección intraperitoneal de etanol se ha demostrado la
distribución de acetaldehído en el líquido intersticial pero no en las
células del cerebro. Una alta afinidad y escaso Km ALDHa pueden ser
importantes para mantener bajos niveles de acetaldehído en el cerebro
durante el metabolismo del etanol.
El acetaldehído es absorbido por los eritrocitos y, tras consumo
de etanol, los niveles intracelulares en hombres y babuinos pueden ser
in vivo 10 veces más altos que los del plasma.
Después de la administración por vía oral, la excreción de
acetaldehído por la orina prácticamente no cambia.
5.2 Metabolismo
Un medio importante en el metabolismo del acetaldehído es la
oxidación para pasar a acetato bajo la influencia de una ALDH
dependiente de NADb. El acetato pasa al ciclo de ácido cítrico como
acetil-CoA. Hay varios isoenzimas de ALDH con distintos parámetros
cinéticos y de ligazón que influyen en la rapidez de la oxidación del
acetaldehído.
Se ha detectado actividad de la ALDH en el epitelio del tracto
respiratorio (excluido el de las vías olfativas) de ratas, en el
cortex renal y los túbulos del perro, la rata, el cobayo y el babuino,
y también en los testículos del ratón.
El acetaldehído es metabolizado in vitro por el tejido
embrionario del ratón y la rata. La sustancia atraviesa la placenta
del ratón, pese al metabolismo placentario.
Aunque hay algún metabolismo en los túbulos renales humanos, el
principal órgano metabolizador es el hígado.
En el hígado y otros tejidos humanos se han detectado varias
formas isoenzimáticas de ALDH. En las mitocondrias la ALDH presenta
polimorfismo. Los sujetos que son homozigóticos o heterozigóticos por
una mutación singular del gen correspondiente a la ALDH de las
mitocondrias presentan poca actividad de esta enzima, metabolizan el
acetaldehído lentamente y no toleran el etanol.
El metabolismo del acetaldehído puede ser inhibido por el
crotonaldehído, el dimetilmaleato, el forón, el disulfiram y la
carbamida de calcio.
5.3 Reacción con otros compuestos
El acetaldehído puede formar aductores estables e inestables con
las proteínas. Eso menoscaba la función proteínica, como lo demuestra
la inhibición de la actividad enzimática, la menor ligazón histona-ADN
y la polimerización inhibida de la tubulina.
Los aductores inestables, de importancia indeterminada, se
producen in vitro con ácidos nucleicos.
El acetaldehído puede reaccionar con diversas macromoléculas en
el organismo humano, de preferencia las que contienen residuos de
lisina, lo que puede conducir a notables alteraciones de la función
biológica de dichas moléculas.
6. Efectos sobre los organismos presentes en el medioambiente
6.1 Organismos acuáticos
La CL50 para los peces va de 35 (Lebistes reticulatus) a
140 mg/litro (especies no especificadas). Se ha señalado una CE5 de
82 mg/litro y una CE50 de 42 mg/litro para las algas y para Daphnia
magna, respectivamente.
6.2 Organismos terrestres
El acetaldehído atmosférico a concentraciones relativamente bajas
parece ser tóxico para algunos microorganismos.
Los afidios mueren cuando son expuestos a concentraciones de
0,36 µg/m3 durante tres o cuatro horas.
Para las especies de babosa Arion hortensis y Agriolimax
reticulatus se han registrado valores letales medios de
8,91 mg/litro y 7,69 mg/litro por hora, respectivamente.
La inhibición de la germinación para la semilla de cebolla,
zanahoria y tomate tratada con acetaldehído (hasta 1,52 mg/litro) es
reversible, mientras que la de Amaranthus palmeri expuesta del mismo
modo es irreversible. El acetaldehído a 0,54 µg/m3 es perjudicial
para la lechuga.
7. Efectos en animales experimentales y sistemas de prueba in vitro
7.1 Exposición única
El acetaldehído tiene una toxicidad aguda baja, con una DL50 en
ratas y ratones y una CL50 en ratas y hámsters sirios. No se han
encontrado estudios de toxicidad dérmica aguda.
7.2 Exposición a corto y a largo plazo
En estudios reiterados del efecto tóxico de dosis por vía oral y
por inhalación, los efectos tóxicos de concentraciones relativamente
bajas se limitaron más bien a los puntos de contacto inicial. En un
estudio de 28 días en el que se administró a ratas con el agua de
beber acetaldehído a razón de 675 mg/kg de peso corporal (nivel sin
efecto observado (NOEL) = 125 mg/kg de peso corporal) los efectos se
limitaron a una ligera hiperqueratosis focal en la parte anterior del
estómago. En un estudio bioquímico, tras la administración a ratas de
dosis singulares (0,05% en el agua de beber) durante seis meses
(equivalente según el Grupo Especial a alrededor de un 40 mg/kg de
peso corporal), el acetaldehído indujo la síntesis de colágeno
hepático, observación corroborada mediante pruebas in vitro.
El nivel sin efecto respiratorio observado (NOEL) fue de
275 mg/m3 para ratas expuestas a acetaldehído por inhalación durante
cuatro semanas y 700 mg/m3 en hámsters expuestos durante 13 semanas.
A los niveles más bajos de efecto observado se produjeron cambios
degenerativos del epitelio olfativo en ratas (437 mg/m3) y de la
tráquea (2400 mg/m3) en hámsters. A concentraciones superiores se
produjeron cambios degenerativos del epitelio respiratorio y de la
laringe. No se han identificado estudios dérmicos con dosis
repetidas.
7.3 Reproducción, embriotoxicidad y teratogenicidad
En varios estudios, la exposición parenteral de ratas y ratones
gestantes a acetaldehído produjo malformaciones fetales. En la
mayoría de esos estudios no se evaluó la toxicidad materna. Tampoco
se dispone de datos sobre la toxicidad reproductiva.
7.4 Mutagenicidad y otros efectos finales afines
El acetaldehído es genotóxico in vitro e induce mutación de los
genes, con efectos clastogénicos e intercambios de cromatidios
hermanos (SCE) en las células de mamíferos, en ausencia de activación
metabólica exógena. Sin embargo se han registrado resultados
negativos en pruebas adecuadas con Salmonella. Tras inyección
intraperitoneal, el acetaldehído indujo SCE en la médula ósea de
hámsters chinos y ratones pero, en cambio, no hizo aumentar la
frecuencia de los micronúcleos en las espermátides precoces de ratón.
Indirectamente, ciertos estudios in vitro e in vivo parecen
indicar que el acetaldehído puede inducir enlaces cruzados
proteína-ADN y ADN-ADN.
7.5 Carcinogenicidad
Se ha observado una mayor incidencia de tumores en ratas y
hámsters expuestos a acetaldehído por inhalación. En las ratas, los
adenocarcinomas nasales aumentaron según la dosis pero los carcinomas
de células escamosas fueron significativos independientemente de la
dosis. Por el contrario, en los hámsters, el aumento de los
carcinomas nasales y laríngeos fue insignificante. A todas las
concentraciones, el acetaldehído administrado en los estudios produjo
daños irreversibles del tejido respiratorio.
7.6 Estudios especiales
No se conocen estudios adecuados sobre la neurotoxicidad y la
inmunotoxicidad potencial del acetaldehído.
8. Efectos en el ser humano
En estudios limitados con voluntarios, el acetaldehído produjo
irritación moderada de los ojos y de las vías respiratorias superiores
tras exposición por muy breves periodos a concentraciones que excedían
algo de 90 y 240 mg/m3, respectivamente. Las pruebas de parche
efectuadas con acetaldehído produjeron eritema cutáneo en 12 sujetos
de «ascendencia oriental».
Se ha tenido conocimiento de una investigación limitada sobre la
incidencia del cáncer entre trabajadores expuestos a acetaldehído y
otras sustancias.
Según pruebas indirectas, el acetaldehído participa como
metabolito potencialmente tóxico en la inducción de las afecciones
hepáticas, la congestión facial y las alteraciones del desarrollo
asociadas con el alcohol.
9. Evaluación de los riesgos para la salud humana y los efectos en el
medio ambiente
Los estudios efectuados con animales indican que la toxicidad
aguda del acetaldehído por inhalación o por vía oral es baja. Según
las investigaciones con sujetos humanos y con animales, esa sustancia
es algo irritante para los ojos y las vías respiratorias superiores.
Esos mismos efectos se han apreciado en voluntarios a los que se
administró acetaldehído (sección 1.8). Se ha observado eritema
cutáneo consiguiente a las pruebas de parche en sujetos humanos.
Aunque se ha identificado un posible mecanismo, los datos de que se
dispone son insuficientes para calcular el potencial del acetaldehído
como inductor de sensibilización.
Existe poca información sobre los efectos del acetaldehído
ingerido. Tras administrar diariamente a ratas por vía oral 675 mg/kg
de peso corporal (NOEL = 125 mg/kg de peso corporal) se observó un
aumento liminar de la hiperqueratosis en la parte anterior del
estómago. En las ratas que ingirieron durante seis meses con el agua
de beber dosis aproximadas de 40 mg/kg de peso corporal hubo un
aumento de la síntesis de colágeno en el hígado, cuya significación
está por dilucidar.
Los estudios de inhalación con ratas y hámsters permiten afirmar
que el tejido sensible es el de las vías respiratorias superiores. La
concentración más baja a la que se observaron efectos fue 437 mg/m3
durante cinco semanas. El NOEL identificado del tracto respiratorio
fue 275 mg/m3 en ratas expuestas durante cuatro semanas y
700 mg/m3 en hámsters expuestos durante 13 semanas.
A las concentraciones que producen daños en los tejidos del
tracto respiratorio se observaron aumentos de la incidencia del
adenocarcinoma nasal y del carcinoma de células escamosas en la rata,
y de los carcinomas laríngeos y nasales en el hámster.
Hay indicios de que el acetaldehído produce in vivo
alteraciones genéticas en las células somáticas.
No se dispone de datos suficientes para determinar si el
acetaldehído tiene efectos en la reproducción y el desarrollo o en el
estado neurológico o inmunológico de la población general o de la que
está particularmente expuesta por razón de su trabajo.
A partir de datos sobre la acción irritante en sujetos humanos se
ha considerado que la concentración tolerable es de 2 mg/m3. El
mecanismo de inducción de tumores por el acetaldehído no está bien
estudiado. En consecuencia, se han adoptado dos criterios
orientativos a ese respecto; a saber, la determinación de una
concentración tolerable obtenida dividiendo un nivel de efecto
irritante en el tracto respiratorio de los roedores por un factor de
incertidumbre, y la estimación del riesgo de cáncer mediante
extrapolación linear. La concentración tolerable resulta ser de
0,3 mg/m3. Las concentraciones asociadas con un aumento del riesgo
permanente de 10-5 son de 11-65 µg/m3.
La escasez de datos impide llegar a conclusiones definitivas
sobre los riesgos potenciales del acetaldehído para la biota en el
medio ambiente. Sin embargo, basándose en la breve vida media del
acetaldehído en el aire y en el agua, asi como en el hecho de que es
fácilmente biodegradable, cabe afirmar que los efectos de esa
sustancia en los organismos terrestres y acuáticos son escasos,
excepto posiblemente con ocasión de descargas industriales o vertidos.
