
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 162
BROMINATED DIPHENYL ETHERS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr G.J. van Esch, Bilthoven, Netherlands
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Brominated diphenylethers.
(Environmental health criteria; 162)
1.Phenyl ethers -- adverse effects 2.Environmental exposure
3.Occupational exposure I.Series
ISBN 92 4 157162 4 (NLM Classification: QD 341.E7)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
GLOSSARY
BROMINATED DIPHENYL ETHERS -- GENERAL INTRODUCTION
1. GENERAL REMARKS
2. GENERAL INFORMATION ON BROMINATED DIPHENYL ETHERS
2.1. Analytical methods
2.2. Production levels and processes
2.3. Resins, polymers and substrates in which PBDE are used
3. FORMATION OF BROMINATED DIBENZOFURANS DIPHENYL ETHERS
3.1. General
3.2. Additional data on pyrolysis of non-specified PBDE and/or
polymers containing non-specified PBDE
4. WORKPLACE EXPOSURE STUDIES
4.1. Exposure to PBDE
4.2. Exposure to PBDF/PBDD
5. EXPOSURE OF THE GENERAL POPULATION
5.1. General population
5.2. Possible exposure to PBDE and PBDF/PBDD
5.2.1. Television sets
5.2.2. Fire tests and fire accidents
6. ENVIRONMENTAL POLLUTION BY PBDE
6.1. Ultimate fate following use
6.2. Air
6.3. Soil
6.4. Water
6.5. Sediments and sewage sludge
6.6. Aquatic vertebrates
6.7. Aquatic mammals
6.8. Terrestrial vertebrates
6.8.1. Birds
6.8.2. Humans
DECABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties
1.1.2. Production and uses
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in labortory animals and
humans
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions
1.2.1. DeBDE
1.2.2. Breakdown products
1.3. Recommendations
1.3.1. General
1.3.2. Further studies
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Pure substance
2.1.2. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Extraction from polymers
4.2. Biotransformation
4.3. Abiotic degradation
4.3.1. Photodegradation
4.3.2. Pyrolysis
4.3.3. Combustion of DeBDE and polymers containing DeBDE
4.3.3.1 Pyrolysis studies
4.3.3.2 Workplace exposure studies
4.4. Ultimate fate following use
4.4.1. General
4.4.2. Exposure of the general population
4.5. Fire accident
4.6. Simulated fire conditions
4.7. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Aquatic sediments
5.1.4. Aquatic and terrestrial organisms
5.2. Exposure of humans
5.2.1. Occurrence of DeBDE in human tissues
5.2.2. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption and elimination
6.2. Distribution
6.3. Retention and turnover
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral: Rat
7.1.2. Dermal: Rabbit
7.1.3. Inhalation: Rat
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Mouse
7.2.1.2 Rat
7.2.2. Inhalation
7.2.2.1 Rat
7.3. Long-term exposure
7.3.1. Oral
7.3.1.1 Mouse
7.3.1.2 Rat
7.4. Skin and eye irritation; sensitization
7.4.1. Skin irritation
7.4.2. Eye irritation
7.4.3. Sensitization
7.4.4. Chloracnegenic activity
7.5. Reproductive toxicity, embryotoxicity, and teratogenicity
7.5.1. Reproductive toxicity
7.5.2. Teratogenicity
7.6. Mutagenicity and related end-points
7.6.1. Mutation
7.6.2. Chromosomal effects
7.7. Carcinogenicity
7.7.1. Oral
7.7.1.1 Mouse
7.7.1.2 Rat
7.8. Other special studies
7.8.1. Liver
7.8.2. Miscellaneous
7.8.3. Toxicity of soot, char, and other waste products
from combustion of DeBDE-containing polymers
7.8.3.1 Acute oral toxicity
7.8.3.2 Skin irritation and comedogenicity
7.8.3.3 Eye irritation
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Skin sensitization
8.2.2. Neurotoxicity
8.2.3. Epidemiological studies
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
NONABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
OCTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties
1.1.2. Production and uses
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals and
humans
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions
1.2.1. OBDE
1.2.2. Breakdown products
1.3. Recommendations
1.3.1. General
1.3.2. Further studies
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Technical product
2.3. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTALEXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Biotransformation
4.2. Abiotic degradation
4.2.1. Pyrolysis of octabromodiphenyl ether
4.2.2. Pyrolysis studies with polymers containing
octabromodiphenyl ether
4.2.3. Behaviour of octabromodiphenyl ether during
processing
4.3. Bioaccumulation
4.4. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Aquatic sediments
5.1.3. Aquatic and terrestrial organisms
5.2. Exposure of the general population
5.3. Occupational exposure during manufacture, formulation or
use
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1. Single exposure
6.1.1. Oral: Rat
6.1.2. Dermal: Rabbit
6.1.3. Inhalation: Rat
6.2. Short-term exposure
6.2.1. Oral: Rat
6.2.2. Inhalation: Rat
6.3. Long-term exposure
6.4. Skin and eye irritation; sensitization
6.4.1. Skin irritation
6.4.2. Eye irritation
6.5. Teratogenicity, reproductive toxicity,
and embryotoxicity
6.5.1. Teratogenicity
6.5.1.1 Oral: Rat
6.5.1.2 Oral: Rabbit
6.6. Mutagenicity and related end-points
6.6.1. DNA damage
6.6.2. Mutation
6.6.3. Chromosomal effects
6.7. Carcinogenicity
6.8. Other special studies
6.8.1. Liver
6.9. Appraisal
HEPTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1. Single exposure
6.2. Skin and eye irritation; sensitization
HEXABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Levels in the environment
5.1.1. Water
5.1.2. Aquatic sediments
5.1.3. Aquatic and terrestrial organisms
5.2. General population exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
PENTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties
1.1.2. Production and uses
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals and
humans
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions
1.2.1. PeBDE
1.2.2. Breakdown products
1.3. Recommendations
1.3.1. General
1.3.2. Further studies
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Pyrolysis
4.2. Workplace exposure studies
4.3. Bioaccumulation
4.4. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Levels in the environment
5.1.1. Sediment and sewage sludge
5.1.2. Fish and shellfish
5.1.3. Aquatic mammals
5.1.4. Terrestrial mammals
5.1.5. Birds
5.2. General population
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposures
7.1.1. Oral
7.1.2. Dermal
7.1.3. Inhalation
7.2. Short-term exposure
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.4.1. Skin irritation
7.4.2. Eye irritation
7.5. Reproductive toxicity, embryotoxicity and teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Other special studies
TETRABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties
1.1.2. Production and uses
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Effects on laboratory mammals and in vitro test
systems
1.1.6. Kinetics and metabolism in laboratory animals and
humans
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions
1.2.1. TeBDE
1.2.2. Breakdown products
1.3. Recommendations
1.3.1. General
1.3.2. Further studies
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Pyrolysis
4.2. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Soil and sediment
5.1.2. Fish and shellfish
5.1.3. Birds
5.1.4. Aquatic mammals
5.1.5. Terrestrial mammals
5.2. General population exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
TRIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental levels
4.1.1. Birds
DIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Soil/sediment
5.1.3. Birds
5.2. General population exposure
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1. Single exposure
6.2. Other special studies
6.2.1. Liver
MONOBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Physical and chemical properties
1.1.2. Production and uses
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals and
humans
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
4.2.1. Biodegradation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Soil/Sediment
5.1.3. Aquatic organisms
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1. Reproductive toxicity, embryotoxicity,
teratogenicity
6.2. Carcinogenicity
7. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN, EVALUACION, CONCLUSIONES Y RECOMENDACIONES
GLOSSARY
PBDE polybrominated diphenyl ethers
MBDE monobromodiphenyl ethers
DiBDE dibromodiphenyl ethers
TrBDE tribromodiphenyl ethers
TeBDE tetrabromodiphenyl ethers
PeBDE pentabromodiphenyl ethers
HxBDE hexabromodiphenyl ethers
HpBDE heptabromodiphenyl ethers
OBDE octabromodiphenyl ethers
NBDE nonabromodiphenyl ethers
DeBDE decabromodiphenyl ethers
PBDF polybrominated dibenzofurans
TeBDF tetrabromodibenzofurans
PeBDF pentabromodibenzofurans
HxBDF hexabromodibenzofurans
HpBDF heptabromodibenzofurans
PBDD polybrominated dibenzodioxins
TeBDD tetrabromodibenzodioxins
PeBDD pentabromodibenzodioxins
HxBDD hexabromodibenzodioxins
HpBDD heptabromodibenzodioxins
PBBz polybrominated benzenes
PBP polybrominated phenols
PBN polybrominated naphthalenes
PBB polybrominated biphenyls
PCB polychlorinated biphenyls
THP Tetrakis(hydroxymethyl)phosphonium salts
ABS acrylonitrile-butadiene-styrene
BASF Badische Anilin und Soda Fabrik
BFRIP Brominated Flame Retardant Industry Panel
BOD biochemical oxygen demand
CEFIC Conseil Européen de l'Industrie Chimique (European
Chemical Industry Council)
DTA differential thermal analysis
EBFRIP European Brominated Flame Retardant Industry Panel
EEC European Economic Community
ER epoxy resin
FY Fiscal Year
GC/ECD gas chromatography/electron capture detector
GC/MS gas chromatography/mass spectrometry
HIPS high impact polystyrene
HPLC high pressure liquid chromatography
HRGC/MS high resolution gas chromatography/mass spectrometry
IG ignition loss
NCI negative chemical ionization
NHATS National Human Adipose Tissue Survey
NIOSH National Institute of Occupational Safety and Health
PA polyamide
PAN polyacrylonitrile
PBT polybutylene terephthalate
PE polyethylene
PET polyethylene terephthalate
PP polypropylene
PR phenolic resin
PS polystyrene
PUR polyurethane
PVC polyvinylchloride
SIM selective ion monitoring
TGA thermal gravimetric analysis
UPE unsaturated (Thermoset) polyesters
US EPA United States Environmental Protection Agency
US NTP United States National Toxicology Program
XPE cross-linked polyethylene
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BROMINATED
DIPHENYL ETHERS
Members
Dr L.A. Albert, Consultores Ambientales Asociados, S.C., Xalapa,
Veracruz, Mexico (Vice-Chairman)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Cambridgeshire, United Kingdom
Professor B. Jansson, Institute of Applied Environmental Research,
Stockholm University, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany
Dr M. Luotamo, Finnish Institute of Occupational Health, Helsinki,
Finland
Professor Wai-On Phoon, Worksafe Australia, and University of Sydney,
Sydney, Australia (Chairman)
Mr J. Rea, Department of Environment, London, United Kingdom
Dr S. Sleight, Department of Pathology, Michigan State University,
East Lansing, USA
Observers
Dr M.L. Hardy, Health and Environment, Ethyl Corporation, Baton Rouge,
USA
Dr D.L. McAllister, Quality Assurance and Research Services,
Great Lakes Chemical Corporation, West Lafayette, Indiana, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr G.J. van Esch, Bilthoven, The Netherlands (Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case Postale
356, 1219 Chatelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
NOTE: The proprietary information contained in this document cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route, and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR BROMINATED DIPHENYL ETHERS
A WHO Task Group on Environmental Health Criteria for Brominated
Diphenyl Ethers met at the World Health Organization, Geneva, from
28 June to 2 July 1993. Dr K.W. Jager, of the IPCS, welcomed the
participants on behalf of Dr M. Mercier, Director IPCS, and the three
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to brominated
diphenyl ethers.
The first draft of the monograph was prepared by Dr G.J. van Esch
of the Netherlands, who also prepared the second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph, and Mrs M.O. Head of Oxford,
England, for the editing.
The fact that industry made proprietary toxicological information
available to the IPCS and the Task Group on the products under
discussion is gratefully acknowledged. This allowed the Task Group to
make its evaluation on a more complete data base.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
BROMINATED DIPHENYL ETHERS GENERAL INTRODUCTION
1. GENERAL REMARKS
This Environmental Health Criteria monograph on brominated
diphenyl ethers has been prepared as part of an overview on the impact
of a number of flame retardants on human health and the environment.
The group of polybrominated diphenyl ethers (PBDE) has been selected
as a priority because of the recent interest in these substances. Only
products based on penta-, octa-, and decabromodiphenyl ethers are of
commercial interest.
The general chemical formula of brominated diphenyl ethers is:
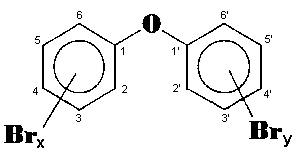 Polybrominated diphenyl ethers (PBDE) have a large number of
congeners, depending on the number and position of the bromine atoms
on the two phenyl rings. The total number of possible congeners is
209, and the numbers of isomers for mono-, di-, tri- up to
decabromodiphenyl ethers are: 3, 12, 24, 42, 46, 42, 24, 12, 3, and 1,
respectively.
The commercial PBDE are produced by the bromination of diphenyl
oxide under certain conditions, which result in products containing
mixtures of brominated diphenyl ethers (see the individual PBDE). The
compositions of commercial DeBDE, OBDE, and PeBDE are given in
Table 1.
No, or virtually no, data are available on dibromo-, tribromo-,
hexabromo-, heptabromo-, and nonabromodiphenyl ether (DiBDE, TrBDE,
HxBDE, HpBDE, and NBDE, respectively). Flame retardants containing
predominantly penta-, octa- and decabromodiphenyl ethers are
commercially produced (with tetrabromodiphenyl ether as a major
component of "pentabromo-diphenyl ether", which is a mixture).
The commercial PBDE are rather stable compounds with boiling
points ranging between 310 and 425 °C and with low vapour pressures,
e.g., 3.85 up to 13.3 Pa at 20-25 °C; they are lipophilic substances.
Their solubility in water is very poor, especially that of the higher
brominated diphenyl ethers, and the n-octanol/water partition
coefficients (log Pow) range between 4.28 and 9.9.
Polybrominated diphenyl ethers have not been reported to occur
naturally in the environment, but other types of brominated diphenyl
ethers have been found in marine organisms (Carte & Faulkner, 1981;
Faulkner, 1990).
The presence in the environment of some of the brominated diphenyl
ethers has been documented, the highest concentration being 1 g/kg
sediment in streams or ponds in the vicinity of a manufacturing
facility.
Data on environmental fate, although limited to MBDE, DiBDE, and
DeBDE, suggest that biodegradation is not an important degradation
pathway for the PBDE, but that photodegradation may play a significant
role.
Table 1. Composition of commercial brominated diphenyl ethers
Product Composition
PBDEa TrBDE TeBDE PeBDE HxBDE HpBDE OBDE NBDE DeBDE
DeBDE 0.3-3% 97-98%
OBDE 10-12% 43-44% 31-35% 9-11% 0-1%
PeBDE 0-1% 24-38% 50-62% 4-8%
TeBDEb 7.6% -- 41-41.7% 44.4-45% 6-7%
a Unknown structure.
b No longer commercially produced. Analysis of one single sample.
Many reports have appeared in the literature describing the
behaviour of brominated flame retardants under pyrolytic conditions.
In general, these reports have indicated that maximum concentrations
of PBDF and/or PBDD were observed at temperatures of 400-800 °C and
that the 2,3,7,8-substituted compounds were seen only in very low
concentrations.
Processing of the polymers under abusive or extreme conditions
produced higher levels of PBDF, but the concentrations were
significantly lower than the values previously reported from
laboratory pyrolysis studies. 2,3,7,8-Brominated isomers were only
found at low levels in a sample abusively processed. The
2,3,7,8-brominated isomers, which are of concern for toxicological and
regulatory reasons, were not detected under normal processing
conditions. The results of the laboratory pyrolysis experiments with
PBDE, showed that PBDF and/or PBDD were formed in various
concentrations, depending on the type of PBDE, the polymer matrix, the
specific processing conditions (temperature, presence of oxygen, etc.)
and equipment used, and the presence of Sb2O3. Behaviour of PBDE is
strongly dependent upon the polymer matrix and upon the specific
processing conditions mentioned above, thus laboratory pyrolysis
experiments can hardly be used as reliable models to predict behaviour
in commercial moulding operations.
2. GENERAL INFORMATION ON BROMONATED DIPHENYL ETHERS
2.1 Analytical methods
Several methods to determine residues of PBDE in various media
(air, sewage sludge, sediment, human adipose tissue, marine organisms,
fish, and feed) as well as in commercial products have been reported.
For details, see Table 2.
In general, sample extraction and clean-up techniques for the
analysis of PBDE residues in biological samples are similar to those
developed for PBB (see EHC 152: Polybrominated biphenyls), though
the chromatographic conditions have to be modified in view of the long
retention times of the highly brominated PBDE. Temperature programming
and the use of capillary columns have been found to be very useful for
the separation of the different congeners of PBDE. Recovery for the
different PBDE is generally higher than 80%. Most methods are based on
extraction with organic solvents, such as hexane/acetone,
hexane/diphenyl ether, acetone, etc, purification of the extracts by
gel permeation or adsorption chromatography, and determination mainly
by gas chromatography, either with electron capture detection (ECD),
or, coupled with mass spectrometry (MS). A multi-residue method has
also been developed that includes a multi-step separation enabling the
determination of several polychlorinated and polybrominated pollutants
in biological samples (Jansson et al., 1991).
Table 2. Analytical methods for PBDE
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Sewage extract with chloroform; evaporate and dissolve GC/MS 0.06 mg/kg Kaart & Kokk
residue in ethanol (1987)
Sediment extract with acetone; clean-up on Florisil NAA; < 5 µg/kg Watanabe et al.
GC/EC < 5 µg/kg (1987b)
Fish extract with acetone-hexane + hexane-ethyl ether; GC/EC; limit of Andersson &
treatment with sulfuric acid or clean-up on alumina; GC/MS detection Blomkvist
chromatography on silica gel 0.1 mg/kg fat (1981)
Animal tissues homogenize; extract with n-hexane-acetone; GC/MS (NCl) 10 ng/kg Jansson et al.
(Multi-residue treatment with sulfuric acid; gel permeation (1991)
method) chromatography; chromatography or silica gel;
chromatography or activated charcoal
Rat liver extract with tetrahydrofuran HPLC Rogers & Hill
(1980)
Table 2 (continued)
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Fish extract freeze-dried powdered sample with pet. ether; GC/MS < 5 µg/kg fat Kruger (1988)
gel permeation chromatography; clean-up on Florisil; (NCl/SIM)
elute with hexane
Cow's milk centrifuge; gel permeation chromatography; clean-up GC/MS < 2.5 µg/kg fat Kruger (1988)
on Florisil; elute with hexane (NCl/SIM)
Human milk extract with potassium oxalate/ethanol/diethyl GC/MS < 0.6 µg/kg fat Kruger (1988)
ether/pentane; gel permeation chromatography; (NCl/SIM)
clean-up on Florisil; elute with hexane
Human adipose extract with methylene chloride; evaporate; clean-up HRGC/HRMSa limit of Cramer et al.
tissue on silica gel followed by clean-up on alumina and on detection (1990a,b)
a carbon/silica gel column 0.73-120 ng/kg
(different
congeners)
Commercial PBDE homogenize and dissolve in tetrachloromethane for HPLC; GC/MS; -- deKok et al.
HPLC and GC/MS or n-hexane for TLC/UV TLC/UV (1979)
a High resolution gas chromatography/high resolution mass spectrometry.
2.2 Production levels and processes
According to the information given by the European Brominated
Flame Retardant Industry Panel (EBFRIP), eight manufacturers are
currently producing polybrominated diphenyl ethers. They are: Dead Sea
Bromines/Eurobrome (The Netherlands); Atochem (France); Ethyl
Corporation (USA); Great Lakes Chemical Corporation (USA); Tosoh
(Japan); Matsunaga (Japan); Nippo (Japan); Great Lakes Chemical Ltd
(United Kingdom).
The annual global consumption of PBDE is 40 000 tonnes (30 000
tonnes of DeBDE; 6000 tonnes OBDE and 4000 tonnes PeBDE) (Arias,
1992).
It has been reported that the use of brominated flame retardants
in Japan increased from 2500 tonnes in 1975 to 22 100 tonnes in 1987
(Watanabe & Tatsukawa, 1990).
The production and import figures for the European Economic
Community (EEC) are given in Table 3.
Table 3. Production and import quantities of PBDE in metric
tonnes in the EECa
1986 1987 1988 1989
Production 4276 3624 4066 3843
Import 4310 3492 4955 7103
Total 8586 7116 9021 10 946
aFrom: EBFRIP (1990).
Data on the usage of PBDE are available for some individual
European countries. Germany uses 3000-5000 tonnes/year, Sweden
1400-2000 tonnes/year, and The Netherlands 3300-3700 tonnes/year
(OECD, 1991; van Zorge, 1992), but Pijnenburg & Everts (1991) and
Pijnenburg et al. (1992) reported a level of 2500 tonnes PBDE for the
last country. In the United Kingdom, up to 2000 tonnes per year are
used (UK DOE, 1993).
Because of the significant reduction in the fire hazard for the
public achieved by the use of PBDE in a wide range of applications,
particularly in the furniture industry, and electrical/computer
components and housing, the consumption of PBDE has significantly
increased over the last years (EBFRIP, 1990).
2.3 Resins, polymers, and substrates in which PBDE are used
The major uses of the polybrominated diphenyl ethers in descending
order of importance are: high-impact polystyrene, ABS, flexible
polyurethane foam, textile coatings (not clothing), wire and cable
insulation, electrical/electronic connectors and other interior parts.
These applications account for at least 80-90% of the consumption of
brominated diphenyl ethers in the USA.
Brominated diphenyl ethers are used as additive flame retardants.
Additive flame retardants are incorporated into the plastic matrix
like other additives, such as plasticizers. The ideal additive is
inexpensive, colourless, easily blended, compatible, heat and light
stable, efficient, permanent, and has no deleterious effect on the
properties of the base polymer. The most important limitations are
incompatibilities that affect the physical properties of the polymers
and the tendency for additives to be fugitive. These additive flame
retardants are much more prone to leaching or escape from the finished
polymer product than the reactive flame retardants (Hutzinger et al.,
1976; Hutzinger & Thoma, 1987; Larsen, 1980).
The uses of penta-, octa-, and decabromodiphenyl ethers in the
different resins, polymers, and substrates are shown in Table 4. The
principal applications of these PBDE-containing substances are shown
in Table 5.
PBDE are used in the different resins, polymers, and substrates at
levels ranging from 5 up to 30%. The quantities used for each
application are not publicly available. In consumer products, resins
containing PBDE are typically used in interior parts, minimizing the
potential for exposure of the public. The incorporation of the PBDE
into the polymer matrix further reduces the possibilities of exposure
(EBFRIP, 1990).
Table 4. Use of penta- (PeBDE), octa- (OBDE), and decabromodiphenyl ethers
(DeBDE) in resins, polymers, and substratesa
Resins/polymers/substrates DeBDE OBDE PeBDE
ABS X
Epoxy-resins X
Phenolic resins X X
PAN X
PA X X
PBT X X
PE/XPE X
PET X
PP X
PS, HIPS X X
PVC X X
PUR X
UPE X X
Rubber X X
Paints/lacquers X X
Textiles X X
aFrom: EBFRIP (1990); UK Department of Environment (1992).
Table 5. The various applications of resins in which PBDE are used are listed belowa
Polymer Principal applications Examples of final products
ABS Moulded parts TV-sets/business machines,
computer housings, household
appliances (hairdryer, curler),
automotive parts, electronics,
telecommunications
EPOXY Circuit boards, Computers, ship interiors,
protective coatings electronic parts
PAINTS/ Coatings Marine and industry lacquers
LACQUERS for protection of containers
PHENOLICS Printed circuit boards Paper laminates/glass prepregs
for printed circuit boards
PAN Panels, electrical Lighting panels for elevators
components and rooms, housing of electrical
appliances
PA Electrical connectors, Computers, connectors,
automative interior housing in electrical industry,
parts board, electrical connectors,
automotive industry,
transportation
PBT Electrical connectors Switches, fuse, switch box,
and components computer housings, switchboard
electrical connectors,
stereos, business machines,
military electronics
PE/XPE Cross-linked wire and Major application: power cable
cable, foam tubing, with cross-linked low density
weather protection PE; also used for conduit for
and moisture barriers building with high density PE;
Final uses: portable apparatus
building control, instrument,
shipboard, automotive, marine
appliances, insulation of heating
tubes
PET Electrical Boxes, relays, coils, bobbins
components
Table 5. (cont'd).
Polymer Principal applications Examples of final products
PP Conduits, electronic TV and electronic devices, such
devices as yoke, housings, circuit board
hangers, conduits; Final uses:
electro-mechanical parts TV,
hot waste water pipes,
underground junction boxes
PS, HIPS TV cabinets and back TV back panels, computer
covers, electrical covers and housings of
appliance housings electrical appliances, office
machines, smoke detectors
PVC Cable sheets Wire end cables, floor mats,
industrial sheets
PUR Cushioning materials, Furniture, sound insulation
packaging, padding panels, wood imitations,
transportation
RUBBER Transportation Conveyor belts, foamed pipes
for insulation
TEXTILES Coatings Back coatings, impregnation:
carpets, automotive seating,
furniture in homes and official
buildings, aircraft, undergrounds,
tents, trains, and military
safety clothing
UPE Circuit boards, Electrical equipment, coatings
coatings for chemical processing plants
mouldings, military and marine
applications: construction
panels
aFrom: EBFRIP (1990).
3. FORMATION OF BROMINATED DIBENZOFURANS AND DIBENZODIOXINS FROM
POLYBROMINATED DIPHENYL ETHERS
3.1 General
Polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) can be formed from polybrominated diphenyl
ethers, polybrominated phenols, and polybrominated biphenyls under
different conditions, including heating (combustion). Laboratory
experiments have also demonstrated the formation of PBDF and PBDD
during the pyrolysis of certain other brominated flame retardants (see
the EHC on Brominated flame retardants, in preparation). As
discussed in EHC 88: Polychlorinated dibenzo-para-dioxins and
dibenzofurans, there are hundreds of possible congeners of
halogenated dibenzofurans and dibenzo-dioxins. However, only congeners
with substituents in the 2,3,7,8-positions are of toxicological
significance. In many reports, only the total levels of PBDF and PBDD
are given, without regard to substitution pattern; such totals are of
limited value in the estimation of possible risk.
Hutzinger & co-workers investigated the pyrolysis of brominated
flame retardants and flame retardant polymer systems and several
publications have appeared. In general, the results reported showed
that brominated dibenzofurans were observed at 700-800 °C and that the
2,3,7,8-substituted compounds were seen in only low concentrations, if
at all (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987; Dumler et al.,
1989).
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF, and Hoechst) and American
industries independently reported the results of combustion and
pyrolysis experiments with flame retarded polymers (BFRIP, 1990).
Recently, brominated aromatic compounds have also attracted
attention, since reports have appeared about emissions of PBDD and
PBDF and other brominated and mixed halogenated aromatic compounds in
accidental fires and from the combustion of waste (see section 4 of
both DeBDE and OBDE).
For more information on these pyrolysis experiments, see the
different sections relating to the individual brominated diphenyl
ethers, e.g., PeBDE, OBDE, and DeBDE.
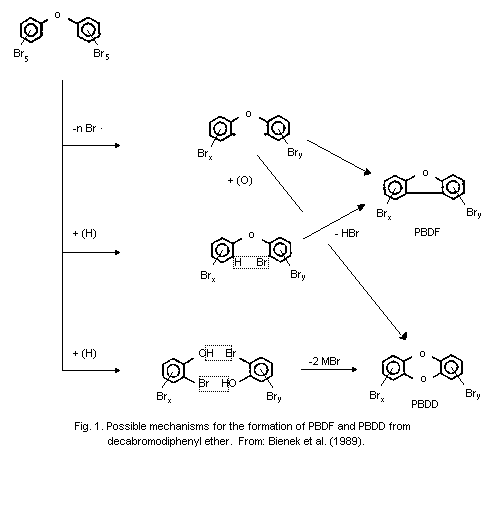
As an example, the formation of PBDF and PBDD from
decabromodiphenyl ether is illustrated in Fig. 1.
Polybrominated diphenyl ethers (PBDE) have a large number of
congeners, depending on the number and position of the bromine atoms
on the two phenyl rings. The total number of possible congeners is
209, and the numbers of isomers for mono-, di-, tri- up to
decabromodiphenyl ethers are: 3, 12, 24, 42, 46, 42, 24, 12, 3, and 1,
respectively.
The commercial PBDE are produced by the bromination of diphenyl
oxide under certain conditions, which result in products containing
mixtures of brominated diphenyl ethers (see the individual PBDE). The
compositions of commercial DeBDE, OBDE, and PeBDE are given in
Table 1.
No, or virtually no, data are available on dibromo-, tribromo-,
hexabromo-, heptabromo-, and nonabromodiphenyl ether (DiBDE, TrBDE,
HxBDE, HpBDE, and NBDE, respectively). Flame retardants containing
predominantly penta-, octa- and decabromodiphenyl ethers are
commercially produced (with tetrabromodiphenyl ether as a major
component of "pentabromo-diphenyl ether", which is a mixture).
The commercial PBDE are rather stable compounds with boiling
points ranging between 310 and 425 °C and with low vapour pressures,
e.g., 3.85 up to 13.3 Pa at 20-25 °C; they are lipophilic substances.
Their solubility in water is very poor, especially that of the higher
brominated diphenyl ethers, and the n-octanol/water partition
coefficients (log Pow) range between 4.28 and 9.9.
Polybrominated diphenyl ethers have not been reported to occur
naturally in the environment, but other types of brominated diphenyl
ethers have been found in marine organisms (Carte & Faulkner, 1981;
Faulkner, 1990).
The presence in the environment of some of the brominated diphenyl
ethers has been documented, the highest concentration being 1 g/kg
sediment in streams or ponds in the vicinity of a manufacturing
facility.
Data on environmental fate, although limited to MBDE, DiBDE, and
DeBDE, suggest that biodegradation is not an important degradation
pathway for the PBDE, but that photodegradation may play a significant
role.
Table 1. Composition of commercial brominated diphenyl ethers
Product Composition
PBDEa TrBDE TeBDE PeBDE HxBDE HpBDE OBDE NBDE DeBDE
DeBDE 0.3-3% 97-98%
OBDE 10-12% 43-44% 31-35% 9-11% 0-1%
PeBDE 0-1% 24-38% 50-62% 4-8%
TeBDEb 7.6% -- 41-41.7% 44.4-45% 6-7%
a Unknown structure.
b No longer commercially produced. Analysis of one single sample.
Many reports have appeared in the literature describing the
behaviour of brominated flame retardants under pyrolytic conditions.
In general, these reports have indicated that maximum concentrations
of PBDF and/or PBDD were observed at temperatures of 400-800 °C and
that the 2,3,7,8-substituted compounds were seen only in very low
concentrations.
Processing of the polymers under abusive or extreme conditions
produced higher levels of PBDF, but the concentrations were
significantly lower than the values previously reported from
laboratory pyrolysis studies. 2,3,7,8-Brominated isomers were only
found at low levels in a sample abusively processed. The
2,3,7,8-brominated isomers, which are of concern for toxicological and
regulatory reasons, were not detected under normal processing
conditions. The results of the laboratory pyrolysis experiments with
PBDE, showed that PBDF and/or PBDD were formed in various
concentrations, depending on the type of PBDE, the polymer matrix, the
specific processing conditions (temperature, presence of oxygen, etc.)
and equipment used, and the presence of Sb2O3. Behaviour of PBDE is
strongly dependent upon the polymer matrix and upon the specific
processing conditions mentioned above, thus laboratory pyrolysis
experiments can hardly be used as reliable models to predict behaviour
in commercial moulding operations.
2. GENERAL INFORMATION ON BROMONATED DIPHENYL ETHERS
2.1 Analytical methods
Several methods to determine residues of PBDE in various media
(air, sewage sludge, sediment, human adipose tissue, marine organisms,
fish, and feed) as well as in commercial products have been reported.
For details, see Table 2.
In general, sample extraction and clean-up techniques for the
analysis of PBDE residues in biological samples are similar to those
developed for PBB (see EHC 152: Polybrominated biphenyls), though
the chromatographic conditions have to be modified in view of the long
retention times of the highly brominated PBDE. Temperature programming
and the use of capillary columns have been found to be very useful for
the separation of the different congeners of PBDE. Recovery for the
different PBDE is generally higher than 80%. Most methods are based on
extraction with organic solvents, such as hexane/acetone,
hexane/diphenyl ether, acetone, etc, purification of the extracts by
gel permeation or adsorption chromatography, and determination mainly
by gas chromatography, either with electron capture detection (ECD),
or, coupled with mass spectrometry (MS). A multi-residue method has
also been developed that includes a multi-step separation enabling the
determination of several polychlorinated and polybrominated pollutants
in biological samples (Jansson et al., 1991).
Table 2. Analytical methods for PBDE
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Sewage extract with chloroform; evaporate and dissolve GC/MS 0.06 mg/kg Kaart & Kokk
residue in ethanol (1987)
Sediment extract with acetone; clean-up on Florisil NAA; < 5 µg/kg Watanabe et al.
GC/EC < 5 µg/kg (1987b)
Fish extract with acetone-hexane + hexane-ethyl ether; GC/EC; limit of Andersson &
treatment with sulfuric acid or clean-up on alumina; GC/MS detection Blomkvist
chromatography on silica gel 0.1 mg/kg fat (1981)
Animal tissues homogenize; extract with n-hexane-acetone; GC/MS (NCl) 10 ng/kg Jansson et al.
(Multi-residue treatment with sulfuric acid; gel permeation (1991)
method) chromatography; chromatography or silica gel;
chromatography or activated charcoal
Rat liver extract with tetrahydrofuran HPLC Rogers & Hill
(1980)
Table 2 (continued)
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Fish extract freeze-dried powdered sample with pet. ether; GC/MS < 5 µg/kg fat Kruger (1988)
gel permeation chromatography; clean-up on Florisil; (NCl/SIM)
elute with hexane
Cow's milk centrifuge; gel permeation chromatography; clean-up GC/MS < 2.5 µg/kg fat Kruger (1988)
on Florisil; elute with hexane (NCl/SIM)
Human milk extract with potassium oxalate/ethanol/diethyl GC/MS < 0.6 µg/kg fat Kruger (1988)
ether/pentane; gel permeation chromatography; (NCl/SIM)
clean-up on Florisil; elute with hexane
Human adipose extract with methylene chloride; evaporate; clean-up HRGC/HRMSa limit of Cramer et al.
tissue on silica gel followed by clean-up on alumina and on detection (1990a,b)
a carbon/silica gel column 0.73-120 ng/kg
(different
congeners)
Commercial PBDE homogenize and dissolve in tetrachloromethane for HPLC; GC/MS; -- deKok et al.
HPLC and GC/MS or n-hexane for TLC/UV TLC/UV (1979)
a High resolution gas chromatography/high resolution mass spectrometry.
2.2 Production levels and processes
According to the information given by the European Brominated
Flame Retardant Industry Panel (EBFRIP), eight manufacturers are
currently producing polybrominated diphenyl ethers. They are: Dead Sea
Bromines/Eurobrome (The Netherlands); Atochem (France); Ethyl
Corporation (USA); Great Lakes Chemical Corporation (USA); Tosoh
(Japan); Matsunaga (Japan); Nippo (Japan); Great Lakes Chemical Ltd
(United Kingdom).
The annual global consumption of PBDE is 40 000 tonnes (30 000
tonnes of DeBDE; 6000 tonnes OBDE and 4000 tonnes PeBDE) (Arias,
1992).
It has been reported that the use of brominated flame retardants
in Japan increased from 2500 tonnes in 1975 to 22 100 tonnes in 1987
(Watanabe & Tatsukawa, 1990).
The production and import figures for the European Economic
Community (EEC) are given in Table 3.
Table 3. Production and import quantities of PBDE in metric
tonnes in the EECa
1986 1987 1988 1989
Production 4276 3624 4066 3843
Import 4310 3492 4955 7103
Total 8586 7116 9021 10 946
aFrom: EBFRIP (1990).
Data on the usage of PBDE are available for some individual
European countries. Germany uses 3000-5000 tonnes/year, Sweden
1400-2000 tonnes/year, and The Netherlands 3300-3700 tonnes/year
(OECD, 1991; van Zorge, 1992), but Pijnenburg & Everts (1991) and
Pijnenburg et al. (1992) reported a level of 2500 tonnes PBDE for the
last country. In the United Kingdom, up to 2000 tonnes per year are
used (UK DOE, 1993).
Because of the significant reduction in the fire hazard for the
public achieved by the use of PBDE in a wide range of applications,
particularly in the furniture industry, and electrical/computer
components and housing, the consumption of PBDE has significantly
increased over the last years (EBFRIP, 1990).
2.3 Resins, polymers, and substrates in which PBDE are used
The major uses of the polybrominated diphenyl ethers in descending
order of importance are: high-impact polystyrene, ABS, flexible
polyurethane foam, textile coatings (not clothing), wire and cable
insulation, electrical/electronic connectors and other interior parts.
These applications account for at least 80-90% of the consumption of
brominated diphenyl ethers in the USA.
Brominated diphenyl ethers are used as additive flame retardants.
Additive flame retardants are incorporated into the plastic matrix
like other additives, such as plasticizers. The ideal additive is
inexpensive, colourless, easily blended, compatible, heat and light
stable, efficient, permanent, and has no deleterious effect on the
properties of the base polymer. The most important limitations are
incompatibilities that affect the physical properties of the polymers
and the tendency for additives to be fugitive. These additive flame
retardants are much more prone to leaching or escape from the finished
polymer product than the reactive flame retardants (Hutzinger et al.,
1976; Hutzinger & Thoma, 1987; Larsen, 1980).
The uses of penta-, octa-, and decabromodiphenyl ethers in the
different resins, polymers, and substrates are shown in Table 4. The
principal applications of these PBDE-containing substances are shown
in Table 5.
PBDE are used in the different resins, polymers, and substrates at
levels ranging from 5 up to 30%. The quantities used for each
application are not publicly available. In consumer products, resins
containing PBDE are typically used in interior parts, minimizing the
potential for exposure of the public. The incorporation of the PBDE
into the polymer matrix further reduces the possibilities of exposure
(EBFRIP, 1990).
Table 4. Use of penta- (PeBDE), octa- (OBDE), and decabromodiphenyl ethers
(DeBDE) in resins, polymers, and substratesa
Resins/polymers/substrates DeBDE OBDE PeBDE
ABS X
Epoxy-resins X
Phenolic resins X X
PAN X
PA X X
PBT X X
PE/XPE X
PET X
PP X
PS, HIPS X X
PVC X X
PUR X
UPE X X
Rubber X X
Paints/lacquers X X
Textiles X X
aFrom: EBFRIP (1990); UK Department of Environment (1992).
Table 5. The various applications of resins in which PBDE are used are listed belowa
Polymer Principal applications Examples of final products
ABS Moulded parts TV-sets/business machines,
computer housings, household
appliances (hairdryer, curler),
automotive parts, electronics,
telecommunications
EPOXY Circuit boards, Computers, ship interiors,
protective coatings electronic parts
PAINTS/ Coatings Marine and industry lacquers
LACQUERS for protection of containers
PHENOLICS Printed circuit boards Paper laminates/glass prepregs
for printed circuit boards
PAN Panels, electrical Lighting panels for elevators
components and rooms, housing of electrical
appliances
PA Electrical connectors, Computers, connectors,
automative interior housing in electrical industry,
parts board, electrical connectors,
automotive industry,
transportation
PBT Electrical connectors Switches, fuse, switch box,
and components computer housings, switchboard
electrical connectors,
stereos, business machines,
military electronics
PE/XPE Cross-linked wire and Major application: power cable
cable, foam tubing, with cross-linked low density
weather protection PE; also used for conduit for
and moisture barriers building with high density PE;
Final uses: portable apparatus
building control, instrument,
shipboard, automotive, marine
appliances, insulation of heating
tubes
PET Electrical Boxes, relays, coils, bobbins
components
Table 5. (cont'd).
Polymer Principal applications Examples of final products
PP Conduits, electronic TV and electronic devices, such
devices as yoke, housings, circuit board
hangers, conduits; Final uses:
electro-mechanical parts TV,
hot waste water pipes,
underground junction boxes
PS, HIPS TV cabinets and back TV back panels, computer
covers, electrical covers and housings of
appliance housings electrical appliances, office
machines, smoke detectors
PVC Cable sheets Wire end cables, floor mats,
industrial sheets
PUR Cushioning materials, Furniture, sound insulation
packaging, padding panels, wood imitations,
transportation
RUBBER Transportation Conveyor belts, foamed pipes
for insulation
TEXTILES Coatings Back coatings, impregnation:
carpets, automotive seating,
furniture in homes and official
buildings, aircraft, undergrounds,
tents, trains, and military
safety clothing
UPE Circuit boards, Electrical equipment, coatings
coatings for chemical processing plants
mouldings, military and marine
applications: construction
panels
aFrom: EBFRIP (1990).
3. FORMATION OF BROMINATED DIBENZOFURANS AND DIBENZODIOXINS FROM
POLYBROMINATED DIPHENYL ETHERS
3.1 General
Polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) can be formed from polybrominated diphenyl
ethers, polybrominated phenols, and polybrominated biphenyls under
different conditions, including heating (combustion). Laboratory
experiments have also demonstrated the formation of PBDF and PBDD
during the pyrolysis of certain other brominated flame retardants (see
the EHC on Brominated flame retardants, in preparation). As
discussed in EHC 88: Polychlorinated dibenzo-para-dioxins and
dibenzofurans, there are hundreds of possible congeners of
halogenated dibenzofurans and dibenzo-dioxins. However, only congeners
with substituents in the 2,3,7,8-positions are of toxicological
significance. In many reports, only the total levels of PBDF and PBDD
are given, without regard to substitution pattern; such totals are of
limited value in the estimation of possible risk.
Hutzinger & co-workers investigated the pyrolysis of brominated
flame retardants and flame retardant polymer systems and several
publications have appeared. In general, the results reported showed
that brominated dibenzofurans were observed at 700-800 °C and that the
2,3,7,8-substituted compounds were seen in only low concentrations, if
at all (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987; Dumler et al.,
1989).
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF, and Hoechst) and American
industries independently reported the results of combustion and
pyrolysis experiments with flame retarded polymers (BFRIP, 1990).
Recently, brominated aromatic compounds have also attracted
attention, since reports have appeared about emissions of PBDD and
PBDF and other brominated and mixed halogenated aromatic compounds in
accidental fires and from the combustion of waste (see section 4 of
both DeBDE and OBDE).
For more information on these pyrolysis experiments, see the
different sections relating to the individual brominated diphenyl
ethers, e.g., PeBDE, OBDE, and DeBDE.
As an example, the formation of PBDF and PBDD from
decabromodiphenyl ether is illustrated in Fig. 1.
 3.2 Additional data on the pyrolysis of non-specified PBDE and/or
polymers containing non-specified PBDE
The earliest published work on the pyrolysis of brominated flame
retardants was that of Buser, whose first paper appeared in 1986
(Buser, 1986). Buser pyrolysed three technical PBDE mixtures with
different degrees of bromination from commercial sources (pentabromo-,
71% bromine; octabromo-, 79% bromine (predominantly hexa- to
nonabrominated PBDE) and decabromo-, 83% bromine, 97% DeBDE) at
510-630 °C in small quartz vials. The vials were placed in a heated
oven for about one minute, and the contents analysed.
A range of PBDD and PBDF was found with a total yield of up to 10%.
HRGG/MS analysis revealed the formation of reasonably simple mixtures
of reaction products with often one or two main PBDF- and
PBDD-isomers. Debromination reactions lead to lower brominated PBDF
and PBDD congeners. In general, the higher brominated PBDE lead to
higher brominated PBDF and PBDD. Most likely, the PBDF and PBDD are
formed in intramolecular cyclization reactions involving the attack by
oxygen on the diphenyl ether system (Fig. 1) (Buser, 1986; Bieniek et
al., 1989).
The next report to appear in the literature was also in 1986 from
the laboratory of Hutzinger. Hutzinger's group pyrolysed penta- and
decabromodiphenyl ethers at 700, 800, and 900 °C, in a quartz tube
oven, for about 10 min. They did not provide any isomer-specific
results, but they reported the formation of PBDF and PBDD. Hutzinger
continued to investigate the pyrolysis of brominated flame retardants
and brominated flame retardant polymer systems, and several
publications appeared (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987;
Dumler et al., 1989a). In general, the results reported in these
publications were consistent with those of earlier work in that
maximum concentrations of PBDF were observed at 700-800 °C and
2,3,7,8-substituted compounds were seen only in very low
concentrations, if at all.
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF and Hoechst) independently
reported the results of combustion and pyrolysis experiments with
flame retarded polymers.
In the German work, pyrolysis studies were conducted with
high-impact polystyrene/DeBDE, polypropylene/DeBDE, and ABS/OBDE
(Neupert et al., 1989) (see also individual flame retardants). In all
of these studies, the pyrolysis residues were analysed for the
presence of PBDD and PBDF. While brominated PBDF were identified, only
very small quantities of 2,3,7,8-TeBDF were observed (see Table 6)
(BFRIP, 1990).
Table 6. Analytical results from the pyrolysis products of ABS/OBDE
(Bayer)a
Compound Concentration
Test 1 (ppm) Test 2 (ppm)
Brominated NDb NDb
dibenzodioxins
Brominated
dibenzofurans:
MBDF 115 60
DiBDF 10 000 7500
TrBDF 8000 2500
TeBDF 2000 2500
2,3,7,8-TeBDF < 0.1 < 8
PeBDF 1700 2000
HxBDF 530 470
HpBDF < 1.4 32
OBDF < 3 < 2.5
aFrom: BFRIP(1990).
bND = Not detectable.
Other brominated pyrolysis products of PBDE may be formed by
cleavage and oxidation, including PBBz, phenol, and some naphthalenes.
PBDF, however, may also be formed from small reactive species
generated during PBDE cleavage (Umweltbundesamt, 1989; Buser, 1986)
(see also the individual brominated diphenyl ethers).
Striebich et al. (1991) examined gas phase oxidative and pyrolysis
thermal decomposition of a 1:1 percentage weight mixture of two
commercial polybrominated diphenyl ether products (tri- through
deca-bromination). The gas phase material was quantitatively
transported to a quartz thermal reactor and subjected to a series of
controlled time/temperature exposures (300-800 °C for 2.0 seconds) in
either air or a nitrogen atmosphere. Thermal decomposition products
were identified. Isomers with higher levels of bromination were
generally more stable than lower brominated diphenyl ethers, under
both oxidative and pyrolytic conditions. Table 7 shows the approximate
yields of products from brominated diphenyl ethers. At 800 °C, all
products were decomposed to HBr or non-detectable products, in both
air and nitrogen. Neither PBDF/PBDD nor any parent material could be
detected at this temperature.
Table 7. Thermal decomposition products from a mixture of two
commercial PBDE (1:1 w/w)a
Product Maximum yield (%)
Nitrogen (650 °C) Air (625 °C)
Dibromobenzenes 0.35 NDc
Tribromobenzenes 0.64 0.92
Tetrabromobenzenes 0.43 0.95
Unknown (pentabromobenzenes?) 0.04 0.24
Brominated alkanes, alkenes, 1.40 0.77
and other PICsb
DiBDF 0.03 NDc
TrDBF 0.03 0.03
TeBDF 0.03 0.03
DiBDD NDc 0.04
TrBDD NDc 0.04
TeBDD NDc 0.01
aFrom: Striebich et al. (1991).
bPICs = Products of incomplete combustion.
cND = Not detectable.
4. WORKPLACE EXPOSURE STUDIES
4.1 Exposure to PBDE
Inhalation exposure to brominated diphenyl ethers is expected to
be low, since the vapour pressure of these chemicals is in the range
of 10-7 mmHg. Particulates in the respirable range are expected to be
formed during the grinding of solids. As inhalation of dust is
possible, the use of dust respirators and gloves/goggles is
recommended in areas of potential exposure.
Dermal exposure may occur during filtration, drying,
drumming/bagging, size reduction, and maintenance (US EPA, 1986).
Exposure to these compounds can also take place during processing
(incorporation into various polymers) and the use of the polymer blend
to fabricate the final articles. After processing, the resin is
generally in the form of pellets rather than powder. Exposure is
expected to be low at fabrication sites because of the low vapour
pressure and of ventilation controls (US EPA, 1986).
4.2 Exposure to PBDF/PBDD
Workers may be exposed to PBDF/PBDD during the production and
processing of plastics containing PBDE as flame retardants and of
products made from them. In addition, workers and the general
population may be exposed to PBDF/PBDD when products, particularly
from the electrical, electronic, and computer industries, emit
PBDF/PBDD during normal operations (see section 4 of both DeBDE and
OBDE).
The PBDF/PBDD contents of component parts taken from 6 electrical
appliances (including printers, TV sets, and computer terminals) as
well as two casings were determined. PBDF/PBDD were detected in 16 of
the materials; mainly higher brominated PBDF at concentrations of
between 0.007 and 4.2 mg/kg (sum of MBDF to HxBDF/MBDD to HxBDD) were
found (Hamm & Theisen. 1992).
Determination of PBDE and PBDF concentrations in air and dust
samples were made in offices having a large number of TV or computer
monitors in operation: the police traffic control office in Hamburg
(47 monitors; room 100 m2, 6 m high, 23 °C) and three rooms of a
television company with monitors (20 °C).
In the police traffic control office, air samples were taken for 3
days at a level of 1.5 m above the floor, a total of 84 m3 air being
drawn, and analysed. Dust samples were taken from the monitors from a
total surface of 3 × 10 m (39 g).
In the first room of a television company (50 m2), where 58
monitors were in use, a total of 129 m3 air was taken over 5 days. In
the second room (40 m2),where 38 monitors were in use, 126 m3 air
was taken, while in the third room (30 m2), where 42 monitors were in
use, 145 m3 air was taken. Dust samples were also collected once a
day from all rooms using a vacuum cleaner.
The concentrations in air of the police station and the television
company ranged between 0.29 and 1.27 pg PBDF/m3 and 97 pg PBDE/m3.
Indoor dust contained PBDF at low ppb and PBDE at high ppb levels.
2,3,7,8-substituted PBDF were not detected (limit of determination
between 0.3 and 0.1 µg/m3) (Ball et al., 1992).
5. EXPOSURE OF THE GENERAL POPULATION
5.1 General population
Limited information is available on the exposure of the general
population to brominated diphenyl ethers. Uptake of TeBDE and PeBDE
may occur in humans via the foodchain, e.g., by consuming fish. In
Germany, PBDE has been detected in human and cow's milk at levels of
2.6 and 3 µg/kg fat, respectively (Kruger, 1988).
Remmers et al. (1990) found evidence of the occurrence of
polychlorinated diphenyl ethers (PCDE) and PBDE in human adipose
tissue specimens from the USA, during the analysis of these tissues
for dioxins and furans. The results showed the presence of HxBDE/HxCDE
through to DeBDE/DeCDE in the tissues analysed.
The presence of brominated diphenyl ethers was indicated in all of
the 47 samples analysed (Cramer et al., 1990a,b). The human samples
were composites derived from all parts of the USA and covering ages
ranging from birth to > 45 years. Additional work is needed to
confirm the presence of these compounds, which have been found
provisionally in the following frequencies and concentrations; HxBDE
(72%; nd-1000 ng/kg); HpBDE (100%; 1-2000 ng/kg) and OBDE (60%;
nd-8000 ng/kg). DeBDE was found in only a few samples at
concentrations of 0.4-0.7 ng/kg.
Exposure may also mainly occur through skin contact (flame retardants
in polymers used in textiles), but also via inhalation (release of
flame retardants from the polymer matrix) (US EPA, 1986).
5.2 Possible exposure to PBDE and PBDF/PBDD
5.2.1 Television sets
Studies were carried out to determine whether PBDF escape from TV
sets. Four air samples (2 parallel to each other) were taken over 3
days in a closed room (volume 26.8 m3), where a new TV set was
operating for 17 h/day. The surface temperature of the TV set (back
panel) was 38-40 °C. One sampling was performed above the TV set,
while the others were carried out in the centre of the room (2.2 m
from the TV set). The levels in the centre of the room of tri-,
tetra-, penta-, and hexabromo-dibenzofurans were 25, 2.7, 0.5, and
0.1 µg/m3, respectively (levels in outdoor air ranged from < 0.05 to
0.16 µg/m3). Above the TV set, the concentrations of the 4 PBDF were
143, 11, 0.5, and < 0.1 µg/m3. Hepta- and octabromodibenzofuran, and
poly-brominated dibenzodioxins were not found (limits of determination
0.1 and 0.2 µg/m3, respectively) (Bruckmann et al., 1990).
An investigation was conducted to determine the emissions of PBDE
and PBDF from plastics in two TV sets, one colour and one monochrome,
two computer monitors, and three printers, under conditions of use.
Analytical methods were refined to obtain a reliable determination of
PBDF. Each appliance was placed, under conditions of use, in a test
chamber. The volume of the steel chamber was 1.17 m3 (1.5 × 1.07 ×
0.82m). For three days, pure air was continuously drawn through the
chambers at a rate of 1.5 m3/h; the emitted compounds were absorbed
on a sampler for the subsequent extraction and determination of PBDE
and PBDF with 4 or more bromines. PBDF concentrations were found to
vary between not detected (limit of detection 3-10 pg) and 1799 pg per
appliance tested and PBDE concentrations, between 0.4 and
889 ng/appliance; 2,3,7,8 isomers (1070 µg/appliance) were detected
only from the colour TV set (Ball et al., 1991).
Three new television sets were placed in a 1.81 m3 test chamber.
Two of the cabinets were made from polystyrene, which was flame
retarded with 11.5% DeBDE. The third television set was made of
high-impact polystyrene, treated with DeBDE/Sb2O3 as a flame
retardant. PBDF and PBDD concentrations were determined in air
collected over 3 days while the two television sets were operating and
during one day when the third TV set was operating. The concentrations
of TeBDD, PeBDD, TeBDF, and PeBDF ranged between 0.09 and 1.52 µg/m3
(Ranken et al., 1990).
5.2.2 Fire tests and fire accidents
Six appliances and 2 casings were burned in a fire test room
(floor area 21 m2, volume 48 m3), which was kept closed during the
fire tests and slowly ventilated after extinguishing the fires (worst
case conditions). After the fire test, samples of combustion residues
and smoke condensate were taken. Smoke was collected in 5 tests. The
combustion residues showed the presence of PBDF and PBDD in
concentrations ranging between 1 and 1930 mg/kg and from the casing
components almost 1%. Smoke condensate from contaminated surfaces
contained levels of between 6 and 1610 µg monobromo- up to
hexabromodibenzofuran/dibenzodioxin per m2. Smoke contained
11-1700 µg monobromo- up to hexabromo- dibenzofuran/dibenzodioxin per
m3 (see Table 8) (Hamm & Theisen, 1992).
Residues and smoke condensates resulting from actual fire
accidents with 9 TV sets were examined. PBDF/PBDD concentrations in
the residues were mainly in the µg/kg range, one value being
107 mg/kg. Close to the fire site, the PBDF/PBDD area contamination
concentrations were between 0.1 and 13.1 µg/m3 (see Table 9) (Hamm &
Theisen, 1992).
It was concluded that the levels of PBDF/PBDD produced in real
fires are much lower than those produced under fire-test conditions.
Table 8. PBDF/D concentrations in original components of electrical appliances and in samples from fire tests with these
appliances or with their casingsa
Object of investigation Original components Fire test samples
Casings Printed circuit Combustion Smoke Smoke
boards residues condensate
Total mono- to Total mono- to Total mono- to Total mono- to Total mono-to
hexaBDF/D µg/g hexaBDF/D µg/g hexaBDF/D hexaBDF/D hexaBDF/D
(ppm) (ppm) µg/g (ppm) µg/m2 µg/m3
Casing of electrical appliance 1 0.63 -b 8700 177 -b
Casing of electrical appliance 2 0.64 -b 7750 1610 -b
Electrical appliance 3 -c 1.77 468 106 456
Electrical appliance 4 0.06 3.44 43 260 355
Electrical appliance 5 0.81 1.98 18 396 1700
Electrical appliance 6 4.20 0.35 1930 234 1350
Etectrlcal appliance 7 -c 0.13 1 6 11
Electrical appliance 8 1.26 0.007 24 323 -b
aFrom: Hamm & Thiesen (1992).
b = Not determined.
c = Not detectable.
Table 9. PBDF/D-concentrations in residues and smoke condensates from
real fire accidents with television setsa
Fire accident Combustion Smoke condensates
(Case number) residues
Close to fire site At some
distance from
fire site
Total mono- to Total mono- to Total mono- to
hexaBDF/D hexaBDF/D hexaBDF/D
µg/g (ppm) µg/m2 µg/m2
I 0.235 10.7 0.665
II 0.004 0.134 0.102
III 0.209 13.1 0.382
IV 0.009 NDb NDb
V 0.001 4.82 1.39
VI 0.017 0.759 0.425
VII 0.001 0.021 0.008
VIII 0.001 10.5 5.37
IX 107 7.47 0.847
aFrom: Hamm & Thiesen (1992).
bND = Not detectable.
6. ENVIRONMENTAL POLLUTION BY PBDE
6.1 Ultimate fate following use
Products containing PBDE are disposed of in the normal domestic
waste stream (landfill and incineration).
No studies are available on the fate of PBDE-containing products
in landfills, but there is concern that the PBDE may eventually leach
out. Bearing in mind that PBDE, at least the congeners with more than
3 bromine atoms, are persistent in the environment, the introduction
of such chemicals into widespread products may be a considerable
long-term diffuse source of emissions of these compounds to the
environment. This type of source is difficult to control and the
unnecessary use of persistent organic compounds should be avoided.
Formation of PBDF and/or PBDD as a result of landfill fires is
also a possibility, though no data are available on the scale of this
source. The results of pyrolysis experiments showed that PBDE can form
PBDF and PBDD (in much smaller quantities) under a wide range of
heating conditions (see General Introduction sections 3.1 and 3.2). If
chlorine is present, mixed halogenated furans/dioxins can also be
generated (Oberg et al., 1987; Zier et al., 1991). Unless sufficiently
high temperatures and long residence times are maintained, PBDF/PBDD
can be generated during the incineration of products containing PBDE.
They can also result from poorly-controlled combustion gas cooling.
Modern, properly operated municipal waste incineration (MWI) should
not emit significant quantities of PBDF/PBDD, regardless of the
composition of the municipal waste.
Lahl et al. (1991) reported increases in dibenzofuran and
dibenzodioxin levels in filter dust, when products containing PBDE
were added to the feed-stock. Riggs et al. (1990) reported PBDF
generation when a flame retarded resin was burnt under simulated MWI
conditions. However, Oberg et al. (1987) reported no increased
emissions of dibenzodioxins when the bromine content of an
incineration feed-stock was increased. Monobromodichloro-dibenzofuran
levels were slightly increased. Oberg & Bergström (1990) conducted
further experiments with a hazardous waste incinerator, to study the
relationship between bromine levels in municipal waste and incinerator
dibenzodioxin and dibenzofuran emissions. They concluded that no
unacceptable environmental risks were associated with the incineration
of brominated compounds in plants with good combustion conditions
equipped with efficient flue gas cleaning. They further noted that
only 0.0125% of the feed to Swedish MWIs was brominated waste.
6.2 Air
Watanabe et al. (1992) reported on the presence of PBDE in the air
in Taiwan and Japan. The concentrations in the air samples collected
in Taiwan from a recycling plant in January 1991 were, in general,
higher than those in Japan; 3 samples were analysed in Taiwan, and 5
in Japan. Tribromo-, tetrabromo-, pentabromo-, and hexabromodiphenyl
ethers were present in the following mean concentrations: Taiwan, 32,
52, 23, and 31 µg/m3, and, Japan, 7.1, 21, 8.9, and 21 µg/m3,
respectively.
6.3 Soil
Two ash and two soil samples were collected in Taiwan from a
recycling plant in January 1991 and analysed for the presence of PBDE.
Tri-, tetra-, penta-, hexa-, and decabromodiphenyl ethers were present
in ash in the following concentrations 20-20, 130, 78-110, 47-54, and
510-2500 µg/kg, respectively; the concentrations in soil were 38-40,
75-104, 41-84, 20-23 and 260-330 µg/kg, respectively. Hepta- and
octabromodiphenyl ether were not found (Watanabe et al., undated).
6.4 Water
Marine, estuarine, and river water samples were analysed for the
presence of the different PBDE. Except for monobromo-diphenyl ether,
levels of all the higher brominated PBDE were below the detection
limit. MBDE was mainly found in the surroundings of manufacturing
plants in the USA (US EPA, 1986).
6.5 Sediments and sewage sludge
In Japan, Spain, Sweden, and the USA, studies were carried out to
determine the presence of the different PBDE in marine, estuarine, or
river sediment. PBDE were mainly found in river sediment. In general,
the levels were below 100 µg/kg dry weight, except in rivers in the
vicinity of manufacturing plants. In these cases, the concentrations
were much higher. In a river in Sweden, concentrations of 11.5 mg
DeBDE, 0.8 mg TeBDE, and 2.8 mg PeBDE/kg dry weight were found. In the
USA, at a manufacturing plant, as much as 1 g DeBDE/kg was found
(Zweidinger et al., 1978; DeCarlo, 1979; Environment Agency Japan,
1983, 1989, 1991; Watanabe et al., 1986, 1987b; Fernandez et al.,
1992).
The upper layers in a laminated sediment core from the Baltic Sea
(Bornholm Deep) contained higher levels of TeBDE and PeBDE than the
deeper layers, indicating an increasing burden of these compounds
(Nylund et al., 1992).
A series of samples of sewage sludges from municipal waste water
treatment plants in Germany were analysed for poly-halogenated
compounds, such as halogenated diphenyl ethers. Tribromo- to
heptabromodiphenyl ethers were found at relatively high concentrations
(Hagenmaier et al., 1991). Sewage sludge was analysed in Sweden for
the presence of TeBDE and PeBDE. Concentrations of 15 and 19 µg/kg,
respectively, were found (Sellström et al., 1990a,b).
6.6 Aquatic vertebrates
The presence of PBDE depends mainly on the degree of bromination.
DeBDE, OBDE, and HxBDE were not found in mussel and fish samples
collected in Japan. No data are available for HpBDE. However, PeBDE
was found in mussel and fish species in concentrations of < 3 µg/kg
wet weight in Japan. Concentrations of 22 µg 2,2',4,4',5-PeBDE/kg wet
weight were found in cod liver collected in the North Sea, and
concentrations of up to 64 µg/kg on a fat basis were found in fish
collected in Sweden. The concentrations were much higher in fish
collected in the vicinity of industrial areas, e.g., up to 9.4 mg/kg
on a fat basis (Jansson et al., in press). Levels for TeBDE, mainly
2,2',4,4'-TeBDE, were comparable but generally higher. Mussels and
fish in Japan contained up to 14.6 µg/kg wet weight, cod liver
collected in the North Sea, 360 µg/kg, and eel from the Netherlands,
up to 1700 µg/kg fat. Different species of fish collected in Sweden
contained up to 88 mg/kg fat (Andersson & Blomkvist, 1981; Watanabe,
1987; Watanabe et al., 1987b; De Boer, 1989, 1990). An increasing
trend was observed in PeBDE and TeBDE levels in freshwater fish in
Sweden. Only limited data are available concerning lower brominated
PBDE (Jansson et al., in press).
Thirty-five samples of 18 freshwater fish collected in German
rivers, and 17 samples collected from the Baltic Sea and the North Sea
contained 18.2-983.6 and 0.6-119.9 µg PBDE/kg fat (determined as
Bromkal 70-SDE), respectively (Kruger, 1988).
6.7 Aquatic mammals
Three bottle-nose dolphins (Tursiops truncatus), collected
during the 1987/88 mass mortality event along the central and south
Atlantic coast of the USA, were analysed for brominated diphenyl
ethers. The concentrations of PBDE were 200, 220, and 180 µg/kg lipid
(Kuehl et al., 1991).
Limited data are available on the presence of PBDE in aquatic
mammals. 2,2'4,4'5-PeBDE was found in ringed and grey seals, collected
in Sweden, in concentrations of 1.7 and 40 µg/kg fat, respectively.
TeBDE, mainly 2,2',4,4'-TeBDE, was also found in the blubber of these
2 species in concentrations of 47 and 650 µg/kg fat, respectively
(Jansson et al., in press). Seals collected at Spitzbergen contained
approximately 10 µg PBDE/kg fat, determined as Bromkal 75DE (Kruger,
1988).
6.8 Terrestrial vertebrates
Pooled samples of rabbits, moose, and reindeer, collected in
Sweden, contained PeBDE and TeBDE in concentrations of <0.3, 0.64,
and 0.26 µg 2,2',4,4',5-PeBDE/kg and <2, 0.82, and 0.18 µg
2,2',4,4'-TeBDE/kg lipid, respectively (Jansson et al., in press).
Four samples of cow's milk were analysed in Germany for the
presence of PBDE. The average concentration was 3.572 µg/kg fat (range
2.536-4.539 µg/kg) determined as Bromkal 70-5DE. The main component
was HxBDE (Kruger, 1988).
6.8.1 Birds
Limited data are available on the presence of PBDE in birds. In
Sweden, 2,2',4,4',5-PeBDE was found in the muscle tissue of osprey, in
newborn starlings, and in guillemot eggs in concentrations of 140,
2.3-4.2, and 24-260 µg/kg lipid, respectively. A trend towards
increasing concentrations of PeBDE and TeBDE in guillemot eggs from
the Baltic Sea was observed. 2,2',4,4'-TeBDE was found in the muscle
tissue of osprey in concentrations of up to 1800 µg/kg. Guillemots
collected from the Baltic Sea, the North Sea, and Spitzbergen
contained 370, 80, and 130 µg/kg on a fat basis, respectively (Jansson
et al., 1987, 1993).
In the USA, indications were found that dibromodiphenyl ether was
present in the eggs of fish-eating birds, but it was not quantified
(Stafford, 1983).
6.8.2 Humans
In Germany, 25 samples of breast milk were analysed for the
presence of PBDE. The ages of the women ranged between 24 and 36 years
and most of them were breast-feeding their first or second child. The
samples contained 0.6-11.1 µg PBDE/kg fat, determined as Bromkal
70-5DE. The main component was HxBDE. One sample from a Chinese woman
showed 7.7 µg PBDE/kg fat; a sample from another woman, exposed
occupationally to hydraulic fluids and transformer oils, contained
50 µg PBDE/kg fat. This last value was excluded from the given range
and average. (Kruger, 1988).
DECABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Typically, commercial DeBDE has a purity of 97-98%, with 0.3-3.0% of
nona- and/or octabrominated diphenyl ethers. Nonabromodiphenyl ether
(NBDE) is the major impurity. In contrast to the other polybrominated
diphenyl ethers there is only one isomer of DeBDE.
The melting point of DeBDE is approximately 300 °C and decomposition
occurs above 400 °C. Solubility in water is 20-30 µg/litre and the log
of the n-octanol/water partition coefficient is greater than 5.
Vapour pressure is < 10-6 mmHg at 20 °C.
1.1.2 Production and uses
Among the brominated diphenyl ethers (mono- to deca-),
deca-bromodiphenyl ether is the most important commercial product with
regard to production and use.
Commercial DeBDE has been produced in increasing degrees of purity
since the late 1970s. The global production of DeBDE is approximately
30 000 tonnes/year. It is used as an additive flame retardant in many
plastics, especially high-impact polystyrene, and in the treatment of
textiles used in soft furnishing, automobile fabrics, and tents.
1.1.3 Environmental transport, distribution, and transformation
Photodegradation of DeBDE occurs in organic solvents under
ultraviolet radiation (UVR) or sunlight; lower brominated diphenyl
ethers and brominated dibenzofurans are formed. Photodegradation also
occurs, to a lesser extent, in water with sunlight; however, lower
brominated diphenyl ethers and brominated dibenzofurans have not been
found.
Levels of DeBDE extracted from polymers are close to, or below,
the limit of detection, depending on the polymer type and extraction
solvent.
Because of its extremely low water solubility and vapour pressure,
DeBDE is likely to be transported primarily by adsorption to
particulate matter. It is persistent and likely to accumulate in
sediment and soil.
No data are available on its bioavailability from sediment and
soil. A study on rainbow trout did not show any bioaccumulation in
flesh, skin, or viscera, over 48 h. DeBDE is unlikely to bioaccumulate
because of its high relative molecular mass.
Products containing commercial DeBDE will eventually be disposed
of by landfill or incineration. DeBDE may eventually leach from
landfills. Polybrominated dibenzofurans (PBDF) and mixed
halogen-dibenzofurans and -dibenzodioxins may result from landfill
fires and inefficient incineration. Products containing commercial
DeBDE may contribute to these emissions.
Pyrolysis of both commercial DeBDE itself and polymers (HIPS, PBT,
industrial polypropylene) containing DeBDE, in the presence of oxygen,
produced PBDF, polybrominated dibenzodioxins (PBDD) being found to a
lesser extent. The maximum formation' of PBDF occurs at 400-500 °C,
but it can occur at temperatures up to 800 °C, and Sb2O3 plays a
catalytic role in the formation of PBDF and PBDD.
The formation, and amounts found, of PBDF and PBDD depend on
temperature, oxygen content, and length of pyrolysis. In the absence
of oxygen, mainly polybromobenzenes and polybromonaphthalenes are
formed.
1.1.4 Environmental levels and human exposure
DeBDE has been identified in air in the vicinity of manufacturing
plants at concentrations of up to 25 µg/m3. DeBDE was not detected in
water samples collected in Japan in the period 1977-91. However, it
was detected in river and estuarine sediment, collected in Japan in
the same period, at concentrations of up to approximately 12 mg/kg dry
weight. DeBDE (up to 1 g/kg) was also found in the USA in river
sediment close to one manufacturing plant. DeBDE was not detected in
fish samples collected in Japan, but, in one mussel sample, a level
just above the level of detection was found. DeBDE was not detected in
human adipose tissue samples collected in Japan, but, in the USA,
DeBDE was found in 3 out of 5 samples of human adipose tissue.
Human exposure to DeBDE can occur in the course of manufacture and
formulation into polymers. Exposure of the general population to DeBDE
is insignificant.
Determination of occupational exposure to the breakdown products
of DeBDE during manufacture, formulation, or use, showed that air
samples close to the extruder head contained high concentrations of
PBDF. Lower levels were found in the air of the workroom. PBDF was
also found in wipe samples. The application of good engineering
techniques has been shown to reduce occupational exposure to PBDF.
Exposure of, the general population to PBDF impurities in flame
retarded polymers is unlikely to be of significance.
1.1.5 Kinetics and metabolism in laboratory animals and humans
DeBDE is poorly absorbed from the gastrointestinal tract and is
rapidly excreted following injection.
The results of metabolic studies on the rat, using 14C labelled
DeBDE, indicated a half-life for the disappearance from the body of
less than 24 h and that the principal route of elimination following
oral ingestion was via the faeces. No appreciable 14C activity (less
than 1%) was found in either urine or expired air.
Rats fed 0.1 mg/kg body weight per day, for up to two years,
showed no accumulation of DeBDE in serum, kidneys, muscle, or testes,
as estimated from total bromine determination. Bromine accumulation in
the liver plateaued at 30 days and was cleared within 10 days
following treatment. After 180 days of treatment, the bromine level in
the liver of treated rats was no greater than that in control rats.
Adipose tissue accumulated low levels of total bromine, which remained
after 90 days of clean diet; the nature of the retained "bromine" is
not known. Since DeBDE accounted for only 77% of the commercial
mixture used, "bromine" could have been derived from NBDE or OBDE.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of DeBDE for laboratory animals is low. The
substance is not an irritant to the skin or eyes of rabbits. It is not
chloracnegenic on the skin of rabbits and is not a human skin
sensitizer.
The combustion products of flame retarded polystyrene containing
DeBDE and Sb203 were tested for acute toxicity and comedogenicity.
The rat oral LD50 of the soot and char was >2000 mg/kg body weight.
In short-term toxicity studies on rats and mice, DeBDE (purity
>97%) at dietary levels of 100 g/kg (4 weeks) or 50 g/kg (13 weeks;
equivalent to 2500 mg/kg body weight for the rat) did not induce
adverse effects. A one-generation reproduction study on rats showed no
adverse effects with dose levels of 100 mg/kg body weight. DeBDE did
not cause any teratogenic effects in the fetuses of rats administered
a dose level of 100 mg/kg body weight. With 1000 mg/kg body weight,
malformations, such as delayed ossification, were seen. DeBDE was not
shown to be mutagenic in a number of tests.
In a carcinogenicity study on rats and mice, DeBDE (purity 94-99%)
was administered at dietary levels of up to 50 g/kg. An increase in
the incidence of adenomas (but not carcinomas) was found in the livers
of male rats receiving 25 g/kg and female rats receiving 50 g
DeBDE/kg. In male mice, increased incidences of hepatocellular
adenomas and/or carcinomas (combined) were found at 25 g/kg and an
increase in thyroid follicular cell adenomas/ carcinomas (combined) at
both dose levels. Female mice did not show any increase in tumour
incidence. There was equivocal evidence for carcinogenicity in male
and female rats and male mice only at dose levels of 25-50 g DeBDE/kg
diet. As the results of all mutagenicity tests have been negative, it
can be concluded that DeBDE is not a genotoxic carcinogen. IARC (1990)
concluded that there was limited evidence for the carcinogenicity of
DeBDE in experimental animals. The very high dose levels, lack of
genotoxicity, and minimal evidence for carcinogenicity indicate that
DeBDE, at the present exposure levels, does not present a carcinogenic
risk for humans.
1.1.7 Effects on humans
No evidence for skin sensitization was found in 200 human subjects
exposed to DeBDE in a sensitization test.
A morbidity study of extruder personnel blending
polybutyl-eneterephthalate containing DeBDE, with consequently
potential exposure to PBDD and PBDF for 13 years, did not reveal any
deleterious effects, even though 2,3,7,8-TeBDF and -TeBDD were
detected in the blood. Results of immunological studies showed that
the immune system of the exposed persons was not adversely affected in
13 years.
1.1.8 Effects on other organisms in the laboratory and field
The EC50s for the growth of 3 marine unicellular algae were
greater than 1 mg DeBDE/litre. No further information is available on
the effects of DeBDE on other organisms in the laboratory and field.
1.2 Conclusions
1.2.1 DeBDE
DeBDE is widely used incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure is very low since the DeBDE is not
readily extracted from polymers. The acute toxicity of DeBDE is very
low and there is minimal absorption from the gastrointestinal tract.
Thus, risk to the general population from DeBDE is considered to be
insignificant.
Occupational exposure is to DeBDE in particulate form. The control
of dust during manufacture and use will adequately reduce the risk for
workers.
DeBDE is persistent and binds to particulate matter in the
environment; it is likely to accumulate in sediment. It is unlikely to
bioaccumulate. Current evidence suggests that environmental
photodegradation in water does not lead to the formation of lower
brominated diphenylethers or brominated dibenzofurans, but little is
known about degradation in other media.
There is minimal information on the toxicity of DeBDE for
organisms in the environment.
1.2.2 Breakdown products
Formation of PBDF and, to some extent, PBDD may occur when DeBDE,
or products containing it, are heated to 300-800 °C. The possible
hazards associated with this have to be addressed.
Properly controlled incineration does not lead to the emission of
significant quantities of brominated dioxins and -furans. Any
uncontrolled combustion of products containing DeBDE can lead to an
unquantified generation of PBDF/PBDD. The significance of this for
both humans and the environment will be addressed in a future
Environmental Health Criteria on PBDF/PBDD.
PBDF have been found in the blood of workers involved in the
production of plastics containing DeBDE. No adverse health effects
have been associated with this exposure. Good engineering controls can
prevent worker exposure to PBDF.
1.3 Recommendations
1.3.1 General
* Workers involved in the manufacture of DeBDE and products
containing the compound should be protected from exposure through
the application of appropriate industrial hygiene measures, the
monitoring of occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled, to minimize environmental
contamination with this persistent material and its breakdown
products.
* Manufacturers should minimize levels of impurities in commercial
DeBDE products, using the best available techniques. A purity of
97% or higher is recommended.
* Incineration should only be carried out in properly constituted
incinerators, running at consistently optimal conditions. Burning
by any other means may lead to the production of PBDF and/or PBDD.
1.3.2 Further studies
* Further studies on the bioavailability and toxicity of
sediment-bound DeBDE should be performed on relevant organisms.
* Continued monitoring of environmental levels is required.
* The generation of PBDF under real fire conditions should be
further investigated.
* Environmental biodegradation, and photodegradation in compartments
other than water, should be further studied.
* Investigation into possible methods and consequences of recycling
of DeBDE-containing polymers should be made.
* Analytical methods for DeBDE in various matrices should be
validated.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Pure substance
Chemical structure:
3.2 Additional data on the pyrolysis of non-specified PBDE and/or
polymers containing non-specified PBDE
The earliest published work on the pyrolysis of brominated flame
retardants was that of Buser, whose first paper appeared in 1986
(Buser, 1986). Buser pyrolysed three technical PBDE mixtures with
different degrees of bromination from commercial sources (pentabromo-,
71% bromine; octabromo-, 79% bromine (predominantly hexa- to
nonabrominated PBDE) and decabromo-, 83% bromine, 97% DeBDE) at
510-630 °C in small quartz vials. The vials were placed in a heated
oven for about one minute, and the contents analysed.
A range of PBDD and PBDF was found with a total yield of up to 10%.
HRGG/MS analysis revealed the formation of reasonably simple mixtures
of reaction products with often one or two main PBDF- and
PBDD-isomers. Debromination reactions lead to lower brominated PBDF
and PBDD congeners. In general, the higher brominated PBDE lead to
higher brominated PBDF and PBDD. Most likely, the PBDF and PBDD are
formed in intramolecular cyclization reactions involving the attack by
oxygen on the diphenyl ether system (Fig. 1) (Buser, 1986; Bieniek et
al., 1989).
The next report to appear in the literature was also in 1986 from
the laboratory of Hutzinger. Hutzinger's group pyrolysed penta- and
decabromodiphenyl ethers at 700, 800, and 900 °C, in a quartz tube
oven, for about 10 min. They did not provide any isomer-specific
results, but they reported the formation of PBDF and PBDD. Hutzinger
continued to investigate the pyrolysis of brominated flame retardants
and brominated flame retardant polymer systems, and several
publications appeared (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987;
Dumler et al., 1989a). In general, the results reported in these
publications were consistent with those of earlier work in that
maximum concentrations of PBDF were observed at 700-800 °C and
2,3,7,8-substituted compounds were seen only in very low
concentrations, if at all.
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF and Hoechst) independently
reported the results of combustion and pyrolysis experiments with
flame retarded polymers.
In the German work, pyrolysis studies were conducted with
high-impact polystyrene/DeBDE, polypropylene/DeBDE, and ABS/OBDE
(Neupert et al., 1989) (see also individual flame retardants). In all
of these studies, the pyrolysis residues were analysed for the
presence of PBDD and PBDF. While brominated PBDF were identified, only
very small quantities of 2,3,7,8-TeBDF were observed (see Table 6)
(BFRIP, 1990).
Table 6. Analytical results from the pyrolysis products of ABS/OBDE
(Bayer)a
Compound Concentration
Test 1 (ppm) Test 2 (ppm)
Brominated NDb NDb
dibenzodioxins
Brominated
dibenzofurans:
MBDF 115 60
DiBDF 10 000 7500
TrBDF 8000 2500
TeBDF 2000 2500
2,3,7,8-TeBDF < 0.1 < 8
PeBDF 1700 2000
HxBDF 530 470
HpBDF < 1.4 32
OBDF < 3 < 2.5
aFrom: BFRIP(1990).
bND = Not detectable.
Other brominated pyrolysis products of PBDE may be formed by
cleavage and oxidation, including PBBz, phenol, and some naphthalenes.
PBDF, however, may also be formed from small reactive species
generated during PBDE cleavage (Umweltbundesamt, 1989; Buser, 1986)
(see also the individual brominated diphenyl ethers).
Striebich et al. (1991) examined gas phase oxidative and pyrolysis
thermal decomposition of a 1:1 percentage weight mixture of two
commercial polybrominated diphenyl ether products (tri- through
deca-bromination). The gas phase material was quantitatively
transported to a quartz thermal reactor and subjected to a series of
controlled time/temperature exposures (300-800 °C for 2.0 seconds) in
either air or a nitrogen atmosphere. Thermal decomposition products
were identified. Isomers with higher levels of bromination were
generally more stable than lower brominated diphenyl ethers, under
both oxidative and pyrolytic conditions. Table 7 shows the approximate
yields of products from brominated diphenyl ethers. At 800 °C, all
products were decomposed to HBr or non-detectable products, in both
air and nitrogen. Neither PBDF/PBDD nor any parent material could be
detected at this temperature.
Table 7. Thermal decomposition products from a mixture of two
commercial PBDE (1:1 w/w)a
Product Maximum yield (%)
Nitrogen (650 °C) Air (625 °C)
Dibromobenzenes 0.35 NDc
Tribromobenzenes 0.64 0.92
Tetrabromobenzenes 0.43 0.95
Unknown (pentabromobenzenes?) 0.04 0.24
Brominated alkanes, alkenes, 1.40 0.77
and other PICsb
DiBDF 0.03 NDc
TrDBF 0.03 0.03
TeBDF 0.03 0.03
DiBDD NDc 0.04
TrBDD NDc 0.04
TeBDD NDc 0.01
aFrom: Striebich et al. (1991).
bPICs = Products of incomplete combustion.
cND = Not detectable.
4. WORKPLACE EXPOSURE STUDIES
4.1 Exposure to PBDE
Inhalation exposure to brominated diphenyl ethers is expected to
be low, since the vapour pressure of these chemicals is in the range
of 10-7 mmHg. Particulates in the respirable range are expected to be
formed during the grinding of solids. As inhalation of dust is
possible, the use of dust respirators and gloves/goggles is
recommended in areas of potential exposure.
Dermal exposure may occur during filtration, drying,
drumming/bagging, size reduction, and maintenance (US EPA, 1986).
Exposure to these compounds can also take place during processing
(incorporation into various polymers) and the use of the polymer blend
to fabricate the final articles. After processing, the resin is
generally in the form of pellets rather than powder. Exposure is
expected to be low at fabrication sites because of the low vapour
pressure and of ventilation controls (US EPA, 1986).
4.2 Exposure to PBDF/PBDD
Workers may be exposed to PBDF/PBDD during the production and
processing of plastics containing PBDE as flame retardants and of
products made from them. In addition, workers and the general
population may be exposed to PBDF/PBDD when products, particularly
from the electrical, electronic, and computer industries, emit
PBDF/PBDD during normal operations (see section 4 of both DeBDE and
OBDE).
The PBDF/PBDD contents of component parts taken from 6 electrical
appliances (including printers, TV sets, and computer terminals) as
well as two casings were determined. PBDF/PBDD were detected in 16 of
the materials; mainly higher brominated PBDF at concentrations of
between 0.007 and 4.2 mg/kg (sum of MBDF to HxBDF/MBDD to HxBDD) were
found (Hamm & Theisen. 1992).
Determination of PBDE and PBDF concentrations in air and dust
samples were made in offices having a large number of TV or computer
monitors in operation: the police traffic control office in Hamburg
(47 monitors; room 100 m2, 6 m high, 23 °C) and three rooms of a
television company with monitors (20 °C).
In the police traffic control office, air samples were taken for 3
days at a level of 1.5 m above the floor, a total of 84 m3 air being
drawn, and analysed. Dust samples were taken from the monitors from a
total surface of 3 × 10 m (39 g).
In the first room of a television company (50 m2), where 58
monitors were in use, a total of 129 m3 air was taken over 5 days. In
the second room (40 m2),where 38 monitors were in use, 126 m3 air
was taken, while in the third room (30 m2), where 42 monitors were in
use, 145 m3 air was taken. Dust samples were also collected once a
day from all rooms using a vacuum cleaner.
The concentrations in air of the police station and the television
company ranged between 0.29 and 1.27 pg PBDF/m3 and 97 pg PBDE/m3.
Indoor dust contained PBDF at low ppb and PBDE at high ppb levels.
2,3,7,8-substituted PBDF were not detected (limit of determination
between 0.3 and 0.1 µg/m3) (Ball et al., 1992).
5. EXPOSURE OF THE GENERAL POPULATION
5.1 General population
Limited information is available on the exposure of the general
population to brominated diphenyl ethers. Uptake of TeBDE and PeBDE
may occur in humans via the foodchain, e.g., by consuming fish. In
Germany, PBDE has been detected in human and cow's milk at levels of
2.6 and 3 µg/kg fat, respectively (Kruger, 1988).
Remmers et al. (1990) found evidence of the occurrence of
polychlorinated diphenyl ethers (PCDE) and PBDE in human adipose
tissue specimens from the USA, during the analysis of these tissues
for dioxins and furans. The results showed the presence of HxBDE/HxCDE
through to DeBDE/DeCDE in the tissues analysed.
The presence of brominated diphenyl ethers was indicated in all of
the 47 samples analysed (Cramer et al., 1990a,b). The human samples
were composites derived from all parts of the USA and covering ages
ranging from birth to > 45 years. Additional work is needed to
confirm the presence of these compounds, which have been found
provisionally in the following frequencies and concentrations; HxBDE
(72%; nd-1000 ng/kg); HpBDE (100%; 1-2000 ng/kg) and OBDE (60%;
nd-8000 ng/kg). DeBDE was found in only a few samples at
concentrations of 0.4-0.7 ng/kg.
Exposure may also mainly occur through skin contact (flame retardants
in polymers used in textiles), but also via inhalation (release of
flame retardants from the polymer matrix) (US EPA, 1986).
5.2 Possible exposure to PBDE and PBDF/PBDD
5.2.1 Television sets
Studies were carried out to determine whether PBDF escape from TV
sets. Four air samples (2 parallel to each other) were taken over 3
days in a closed room (volume 26.8 m3), where a new TV set was
operating for 17 h/day. The surface temperature of the TV set (back
panel) was 38-40 °C. One sampling was performed above the TV set,
while the others were carried out in the centre of the room (2.2 m
from the TV set). The levels in the centre of the room of tri-,
tetra-, penta-, and hexabromo-dibenzofurans were 25, 2.7, 0.5, and
0.1 µg/m3, respectively (levels in outdoor air ranged from < 0.05 to
0.16 µg/m3). Above the TV set, the concentrations of the 4 PBDF were
143, 11, 0.5, and < 0.1 µg/m3. Hepta- and octabromodibenzofuran, and
poly-brominated dibenzodioxins were not found (limits of determination
0.1 and 0.2 µg/m3, respectively) (Bruckmann et al., 1990).
An investigation was conducted to determine the emissions of PBDE
and PBDF from plastics in two TV sets, one colour and one monochrome,
two computer monitors, and three printers, under conditions of use.
Analytical methods were refined to obtain a reliable determination of
PBDF. Each appliance was placed, under conditions of use, in a test
chamber. The volume of the steel chamber was 1.17 m3 (1.5 × 1.07 ×
0.82m). For three days, pure air was continuously drawn through the
chambers at a rate of 1.5 m3/h; the emitted compounds were absorbed
on a sampler for the subsequent extraction and determination of PBDE
and PBDF with 4 or more bromines. PBDF concentrations were found to
vary between not detected (limit of detection 3-10 pg) and 1799 pg per
appliance tested and PBDE concentrations, between 0.4 and
889 ng/appliance; 2,3,7,8 isomers (1070 µg/appliance) were detected
only from the colour TV set (Ball et al., 1991).
Three new television sets were placed in a 1.81 m3 test chamber.
Two of the cabinets were made from polystyrene, which was flame
retarded with 11.5% DeBDE. The third television set was made of
high-impact polystyrene, treated with DeBDE/Sb2O3 as a flame
retardant. PBDF and PBDD concentrations were determined in air
collected over 3 days while the two television sets were operating and
during one day when the third TV set was operating. The concentrations
of TeBDD, PeBDD, TeBDF, and PeBDF ranged between 0.09 and 1.52 µg/m3
(Ranken et al., 1990).
5.2.2 Fire tests and fire accidents
Six appliances and 2 casings were burned in a fire test room
(floor area 21 m2, volume 48 m3), which was kept closed during the
fire tests and slowly ventilated after extinguishing the fires (worst
case conditions). After the fire test, samples of combustion residues
and smoke condensate were taken. Smoke was collected in 5 tests. The
combustion residues showed the presence of PBDF and PBDD in
concentrations ranging between 1 and 1930 mg/kg and from the casing
components almost 1%. Smoke condensate from contaminated surfaces
contained levels of between 6 and 1610 µg monobromo- up to
hexabromodibenzofuran/dibenzodioxin per m2. Smoke contained
11-1700 µg monobromo- up to hexabromo- dibenzofuran/dibenzodioxin per
m3 (see Table 8) (Hamm & Theisen, 1992).
Residues and smoke condensates resulting from actual fire
accidents with 9 TV sets were examined. PBDF/PBDD concentrations in
the residues were mainly in the µg/kg range, one value being
107 mg/kg. Close to the fire site, the PBDF/PBDD area contamination
concentrations were between 0.1 and 13.1 µg/m3 (see Table 9) (Hamm &
Theisen, 1992).
It was concluded that the levels of PBDF/PBDD produced in real
fires are much lower than those produced under fire-test conditions.
Table 8. PBDF/D concentrations in original components of electrical appliances and in samples from fire tests with these
appliances or with their casingsa
Object of investigation Original components Fire test samples
Casings Printed circuit Combustion Smoke Smoke
boards residues condensate
Total mono- to Total mono- to Total mono- to Total mono- to Total mono-to
hexaBDF/D µg/g hexaBDF/D µg/g hexaBDF/D hexaBDF/D hexaBDF/D
(ppm) (ppm) µg/g (ppm) µg/m2 µg/m3
Casing of electrical appliance 1 0.63 -b 8700 177 -b
Casing of electrical appliance 2 0.64 -b 7750 1610 -b
Electrical appliance 3 -c 1.77 468 106 456
Electrical appliance 4 0.06 3.44 43 260 355
Electrical appliance 5 0.81 1.98 18 396 1700
Electrical appliance 6 4.20 0.35 1930 234 1350
Etectrlcal appliance 7 -c 0.13 1 6 11
Electrical appliance 8 1.26 0.007 24 323 -b
aFrom: Hamm & Thiesen (1992).
b = Not determined.
c = Not detectable.
Table 9. PBDF/D-concentrations in residues and smoke condensates from
real fire accidents with television setsa
Fire accident Combustion Smoke condensates
(Case number) residues
Close to fire site At some
distance from
fire site
Total mono- to Total mono- to Total mono- to
hexaBDF/D hexaBDF/D hexaBDF/D
µg/g (ppm) µg/m2 µg/m2
I 0.235 10.7 0.665
II 0.004 0.134 0.102
III 0.209 13.1 0.382
IV 0.009 NDb NDb
V 0.001 4.82 1.39
VI 0.017 0.759 0.425
VII 0.001 0.021 0.008
VIII 0.001 10.5 5.37
IX 107 7.47 0.847
aFrom: Hamm & Thiesen (1992).
bND = Not detectable.
6. ENVIRONMENTAL POLLUTION BY PBDE
6.1 Ultimate fate following use
Products containing PBDE are disposed of in the normal domestic
waste stream (landfill and incineration).
No studies are available on the fate of PBDE-containing products
in landfills, but there is concern that the PBDE may eventually leach
out. Bearing in mind that PBDE, at least the congeners with more than
3 bromine atoms, are persistent in the environment, the introduction
of such chemicals into widespread products may be a considerable
long-term diffuse source of emissions of these compounds to the
environment. This type of source is difficult to control and the
unnecessary use of persistent organic compounds should be avoided.
Formation of PBDF and/or PBDD as a result of landfill fires is
also a possibility, though no data are available on the scale of this
source. The results of pyrolysis experiments showed that PBDE can form
PBDF and PBDD (in much smaller quantities) under a wide range of
heating conditions (see General Introduction sections 3.1 and 3.2). If
chlorine is present, mixed halogenated furans/dioxins can also be
generated (Oberg et al., 1987; Zier et al., 1991). Unless sufficiently
high temperatures and long residence times are maintained, PBDF/PBDD
can be generated during the incineration of products containing PBDE.
They can also result from poorly-controlled combustion gas cooling.
Modern, properly operated municipal waste incineration (MWI) should
not emit significant quantities of PBDF/PBDD, regardless of the
composition of the municipal waste.
Lahl et al. (1991) reported increases in dibenzofuran and
dibenzodioxin levels in filter dust, when products containing PBDE
were added to the feed-stock. Riggs et al. (1990) reported PBDF
generation when a flame retarded resin was burnt under simulated MWI
conditions. However, Oberg et al. (1987) reported no increased
emissions of dibenzodioxins when the bromine content of an
incineration feed-stock was increased. Monobromodichloro-dibenzofuran
levels were slightly increased. Oberg & Bergström (1990) conducted
further experiments with a hazardous waste incinerator, to study the
relationship between bromine levels in municipal waste and incinerator
dibenzodioxin and dibenzofuran emissions. They concluded that no
unacceptable environmental risks were associated with the incineration
of brominated compounds in plants with good combustion conditions
equipped with efficient flue gas cleaning. They further noted that
only 0.0125% of the feed to Swedish MWIs was brominated waste.
6.2 Air
Watanabe et al. (1992) reported on the presence of PBDE in the air
in Taiwan and Japan. The concentrations in the air samples collected
in Taiwan from a recycling plant in January 1991 were, in general,
higher than those in Japan; 3 samples were analysed in Taiwan, and 5
in Japan. Tribromo-, tetrabromo-, pentabromo-, and hexabromodiphenyl
ethers were present in the following mean concentrations: Taiwan, 32,
52, 23, and 31 µg/m3, and, Japan, 7.1, 21, 8.9, and 21 µg/m3,
respectively.
6.3 Soil
Two ash and two soil samples were collected in Taiwan from a
recycling plant in January 1991 and analysed for the presence of PBDE.
Tri-, tetra-, penta-, hexa-, and decabromodiphenyl ethers were present
in ash in the following concentrations 20-20, 130, 78-110, 47-54, and
510-2500 µg/kg, respectively; the concentrations in soil were 38-40,
75-104, 41-84, 20-23 and 260-330 µg/kg, respectively. Hepta- and
octabromodiphenyl ether were not found (Watanabe et al., undated).
6.4 Water
Marine, estuarine, and river water samples were analysed for the
presence of the different PBDE. Except for monobromo-diphenyl ether,
levels of all the higher brominated PBDE were below the detection
limit. MBDE was mainly found in the surroundings of manufacturing
plants in the USA (US EPA, 1986).
6.5 Sediments and sewage sludge
In Japan, Spain, Sweden, and the USA, studies were carried out to
determine the presence of the different PBDE in marine, estuarine, or
river sediment. PBDE were mainly found in river sediment. In general,
the levels were below 100 µg/kg dry weight, except in rivers in the
vicinity of manufacturing plants. In these cases, the concentrations
were much higher. In a river in Sweden, concentrations of 11.5 mg
DeBDE, 0.8 mg TeBDE, and 2.8 mg PeBDE/kg dry weight were found. In the
USA, at a manufacturing plant, as much as 1 g DeBDE/kg was found
(Zweidinger et al., 1978; DeCarlo, 1979; Environment Agency Japan,
1983, 1989, 1991; Watanabe et al., 1986, 1987b; Fernandez et al.,
1992).
The upper layers in a laminated sediment core from the Baltic Sea
(Bornholm Deep) contained higher levels of TeBDE and PeBDE than the
deeper layers, indicating an increasing burden of these compounds
(Nylund et al., 1992).
A series of samples of sewage sludges from municipal waste water
treatment plants in Germany were analysed for poly-halogenated
compounds, such as halogenated diphenyl ethers. Tribromo- to
heptabromodiphenyl ethers were found at relatively high concentrations
(Hagenmaier et al., 1991). Sewage sludge was analysed in Sweden for
the presence of TeBDE and PeBDE. Concentrations of 15 and 19 µg/kg,
respectively, were found (Sellström et al., 1990a,b).
6.6 Aquatic vertebrates
The presence of PBDE depends mainly on the degree of bromination.
DeBDE, OBDE, and HxBDE were not found in mussel and fish samples
collected in Japan. No data are available for HpBDE. However, PeBDE
was found in mussel and fish species in concentrations of < 3 µg/kg
wet weight in Japan. Concentrations of 22 µg 2,2',4,4',5-PeBDE/kg wet
weight were found in cod liver collected in the North Sea, and
concentrations of up to 64 µg/kg on a fat basis were found in fish
collected in Sweden. The concentrations were much higher in fish
collected in the vicinity of industrial areas, e.g., up to 9.4 mg/kg
on a fat basis (Jansson et al., in press). Levels for TeBDE, mainly
2,2',4,4'-TeBDE, were comparable but generally higher. Mussels and
fish in Japan contained up to 14.6 µg/kg wet weight, cod liver
collected in the North Sea, 360 µg/kg, and eel from the Netherlands,
up to 1700 µg/kg fat. Different species of fish collected in Sweden
contained up to 88 mg/kg fat (Andersson & Blomkvist, 1981; Watanabe,
1987; Watanabe et al., 1987b; De Boer, 1989, 1990). An increasing
trend was observed in PeBDE and TeBDE levels in freshwater fish in
Sweden. Only limited data are available concerning lower brominated
PBDE (Jansson et al., in press).
Thirty-five samples of 18 freshwater fish collected in German
rivers, and 17 samples collected from the Baltic Sea and the North Sea
contained 18.2-983.6 and 0.6-119.9 µg PBDE/kg fat (determined as
Bromkal 70-SDE), respectively (Kruger, 1988).
6.7 Aquatic mammals
Three bottle-nose dolphins (Tursiops truncatus), collected
during the 1987/88 mass mortality event along the central and south
Atlantic coast of the USA, were analysed for brominated diphenyl
ethers. The concentrations of PBDE were 200, 220, and 180 µg/kg lipid
(Kuehl et al., 1991).
Limited data are available on the presence of PBDE in aquatic
mammals. 2,2'4,4'5-PeBDE was found in ringed and grey seals, collected
in Sweden, in concentrations of 1.7 and 40 µg/kg fat, respectively.
TeBDE, mainly 2,2',4,4'-TeBDE, was also found in the blubber of these
2 species in concentrations of 47 and 650 µg/kg fat, respectively
(Jansson et al., in press). Seals collected at Spitzbergen contained
approximately 10 µg PBDE/kg fat, determined as Bromkal 75DE (Kruger,
1988).
6.8 Terrestrial vertebrates
Pooled samples of rabbits, moose, and reindeer, collected in
Sweden, contained PeBDE and TeBDE in concentrations of <0.3, 0.64,
and 0.26 µg 2,2',4,4',5-PeBDE/kg and <2, 0.82, and 0.18 µg
2,2',4,4'-TeBDE/kg lipid, respectively (Jansson et al., in press).
Four samples of cow's milk were analysed in Germany for the
presence of PBDE. The average concentration was 3.572 µg/kg fat (range
2.536-4.539 µg/kg) determined as Bromkal 70-5DE. The main component
was HxBDE (Kruger, 1988).
6.8.1 Birds
Limited data are available on the presence of PBDE in birds. In
Sweden, 2,2',4,4',5-PeBDE was found in the muscle tissue of osprey, in
newborn starlings, and in guillemot eggs in concentrations of 140,
2.3-4.2, and 24-260 µg/kg lipid, respectively. A trend towards
increasing concentrations of PeBDE and TeBDE in guillemot eggs from
the Baltic Sea was observed. 2,2',4,4'-TeBDE was found in the muscle
tissue of osprey in concentrations of up to 1800 µg/kg. Guillemots
collected from the Baltic Sea, the North Sea, and Spitzbergen
contained 370, 80, and 130 µg/kg on a fat basis, respectively (Jansson
et al., 1987, 1993).
In the USA, indications were found that dibromodiphenyl ether was
present in the eggs of fish-eating birds, but it was not quantified
(Stafford, 1983).
6.8.2 Humans
In Germany, 25 samples of breast milk were analysed for the
presence of PBDE. The ages of the women ranged between 24 and 36 years
and most of them were breast-feeding their first or second child. The
samples contained 0.6-11.1 µg PBDE/kg fat, determined as Bromkal
70-5DE. The main component was HxBDE. One sample from a Chinese woman
showed 7.7 µg PBDE/kg fat; a sample from another woman, exposed
occupationally to hydraulic fluids and transformer oils, contained
50 µg PBDE/kg fat. This last value was excluded from the given range
and average. (Kruger, 1988).
DECABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Typically, commercial DeBDE has a purity of 97-98%, with 0.3-3.0% of
nona- and/or octabrominated diphenyl ethers. Nonabromodiphenyl ether
(NBDE) is the major impurity. In contrast to the other polybrominated
diphenyl ethers there is only one isomer of DeBDE.
The melting point of DeBDE is approximately 300 °C and decomposition
occurs above 400 °C. Solubility in water is 20-30 µg/litre and the log
of the n-octanol/water partition coefficient is greater than 5.
Vapour pressure is < 10-6 mmHg at 20 °C.
1.1.2 Production and uses
Among the brominated diphenyl ethers (mono- to deca-),
deca-bromodiphenyl ether is the most important commercial product with
regard to production and use.
Commercial DeBDE has been produced in increasing degrees of purity
since the late 1970s. The global production of DeBDE is approximately
30 000 tonnes/year. It is used as an additive flame retardant in many
plastics, especially high-impact polystyrene, and in the treatment of
textiles used in soft furnishing, automobile fabrics, and tents.
1.1.3 Environmental transport, distribution, and transformation
Photodegradation of DeBDE occurs in organic solvents under
ultraviolet radiation (UVR) or sunlight; lower brominated diphenyl
ethers and brominated dibenzofurans are formed. Photodegradation also
occurs, to a lesser extent, in water with sunlight; however, lower
brominated diphenyl ethers and brominated dibenzofurans have not been
found.
Levels of DeBDE extracted from polymers are close to, or below,
the limit of detection, depending on the polymer type and extraction
solvent.
Because of its extremely low water solubility and vapour pressure,
DeBDE is likely to be transported primarily by adsorption to
particulate matter. It is persistent and likely to accumulate in
sediment and soil.
No data are available on its bioavailability from sediment and
soil. A study on rainbow trout did not show any bioaccumulation in
flesh, skin, or viscera, over 48 h. DeBDE is unlikely to bioaccumulate
because of its high relative molecular mass.
Products containing commercial DeBDE will eventually be disposed
of by landfill or incineration. DeBDE may eventually leach from
landfills. Polybrominated dibenzofurans (PBDF) and mixed
halogen-dibenzofurans and -dibenzodioxins may result from landfill
fires and inefficient incineration. Products containing commercial
DeBDE may contribute to these emissions.
Pyrolysis of both commercial DeBDE itself and polymers (HIPS, PBT,
industrial polypropylene) containing DeBDE, in the presence of oxygen,
produced PBDF, polybrominated dibenzodioxins (PBDD) being found to a
lesser extent. The maximum formation' of PBDF occurs at 400-500 °C,
but it can occur at temperatures up to 800 °C, and Sb2O3 plays a
catalytic role in the formation of PBDF and PBDD.
The formation, and amounts found, of PBDF and PBDD depend on
temperature, oxygen content, and length of pyrolysis. In the absence
of oxygen, mainly polybromobenzenes and polybromonaphthalenes are
formed.
1.1.4 Environmental levels and human exposure
DeBDE has been identified in air in the vicinity of manufacturing
plants at concentrations of up to 25 µg/m3. DeBDE was not detected in
water samples collected in Japan in the period 1977-91. However, it
was detected in river and estuarine sediment, collected in Japan in
the same period, at concentrations of up to approximately 12 mg/kg dry
weight. DeBDE (up to 1 g/kg) was also found in the USA in river
sediment close to one manufacturing plant. DeBDE was not detected in
fish samples collected in Japan, but, in one mussel sample, a level
just above the level of detection was found. DeBDE was not detected in
human adipose tissue samples collected in Japan, but, in the USA,
DeBDE was found in 3 out of 5 samples of human adipose tissue.
Human exposure to DeBDE can occur in the course of manufacture and
formulation into polymers. Exposure of the general population to DeBDE
is insignificant.
Determination of occupational exposure to the breakdown products
of DeBDE during manufacture, formulation, or use, showed that air
samples close to the extruder head contained high concentrations of
PBDF. Lower levels were found in the air of the workroom. PBDF was
also found in wipe samples. The application of good engineering
techniques has been shown to reduce occupational exposure to PBDF.
Exposure of, the general population to PBDF impurities in flame
retarded polymers is unlikely to be of significance.
1.1.5 Kinetics and metabolism in laboratory animals and humans
DeBDE is poorly absorbed from the gastrointestinal tract and is
rapidly excreted following injection.
The results of metabolic studies on the rat, using 14C labelled
DeBDE, indicated a half-life for the disappearance from the body of
less than 24 h and that the principal route of elimination following
oral ingestion was via the faeces. No appreciable 14C activity (less
than 1%) was found in either urine or expired air.
Rats fed 0.1 mg/kg body weight per day, for up to two years,
showed no accumulation of DeBDE in serum, kidneys, muscle, or testes,
as estimated from total bromine determination. Bromine accumulation in
the liver plateaued at 30 days and was cleared within 10 days
following treatment. After 180 days of treatment, the bromine level in
the liver of treated rats was no greater than that in control rats.
Adipose tissue accumulated low levels of total bromine, which remained
after 90 days of clean diet; the nature of the retained "bromine" is
not known. Since DeBDE accounted for only 77% of the commercial
mixture used, "bromine" could have been derived from NBDE or OBDE.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of DeBDE for laboratory animals is low. The
substance is not an irritant to the skin or eyes of rabbits. It is not
chloracnegenic on the skin of rabbits and is not a human skin
sensitizer.
The combustion products of flame retarded polystyrene containing
DeBDE and Sb203 were tested for acute toxicity and comedogenicity.
The rat oral LD50 of the soot and char was >2000 mg/kg body weight.
In short-term toxicity studies on rats and mice, DeBDE (purity
>97%) at dietary levels of 100 g/kg (4 weeks) or 50 g/kg (13 weeks;
equivalent to 2500 mg/kg body weight for the rat) did not induce
adverse effects. A one-generation reproduction study on rats showed no
adverse effects with dose levels of 100 mg/kg body weight. DeBDE did
not cause any teratogenic effects in the fetuses of rats administered
a dose level of 100 mg/kg body weight. With 1000 mg/kg body weight,
malformations, such as delayed ossification, were seen. DeBDE was not
shown to be mutagenic in a number of tests.
In a carcinogenicity study on rats and mice, DeBDE (purity 94-99%)
was administered at dietary levels of up to 50 g/kg. An increase in
the incidence of adenomas (but not carcinomas) was found in the livers
of male rats receiving 25 g/kg and female rats receiving 50 g
DeBDE/kg. In male mice, increased incidences of hepatocellular
adenomas and/or carcinomas (combined) were found at 25 g/kg and an
increase in thyroid follicular cell adenomas/ carcinomas (combined) at
both dose levels. Female mice did not show any increase in tumour
incidence. There was equivocal evidence for carcinogenicity in male
and female rats and male mice only at dose levels of 25-50 g DeBDE/kg
diet. As the results of all mutagenicity tests have been negative, it
can be concluded that DeBDE is not a genotoxic carcinogen. IARC (1990)
concluded that there was limited evidence for the carcinogenicity of
DeBDE in experimental animals. The very high dose levels, lack of
genotoxicity, and minimal evidence for carcinogenicity indicate that
DeBDE, at the present exposure levels, does not present a carcinogenic
risk for humans.
1.1.7 Effects on humans
No evidence for skin sensitization was found in 200 human subjects
exposed to DeBDE in a sensitization test.
A morbidity study of extruder personnel blending
polybutyl-eneterephthalate containing DeBDE, with consequently
potential exposure to PBDD and PBDF for 13 years, did not reveal any
deleterious effects, even though 2,3,7,8-TeBDF and -TeBDD were
detected in the blood. Results of immunological studies showed that
the immune system of the exposed persons was not adversely affected in
13 years.
1.1.8 Effects on other organisms in the laboratory and field
The EC50s for the growth of 3 marine unicellular algae were
greater than 1 mg DeBDE/litre. No further information is available on
the effects of DeBDE on other organisms in the laboratory and field.
1.2 Conclusions
1.2.1 DeBDE
DeBDE is widely used incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure is very low since the DeBDE is not
readily extracted from polymers. The acute toxicity of DeBDE is very
low and there is minimal absorption from the gastrointestinal tract.
Thus, risk to the general population from DeBDE is considered to be
insignificant.
Occupational exposure is to DeBDE in particulate form. The control
of dust during manufacture and use will adequately reduce the risk for
workers.
DeBDE is persistent and binds to particulate matter in the
environment; it is likely to accumulate in sediment. It is unlikely to
bioaccumulate. Current evidence suggests that environmental
photodegradation in water does not lead to the formation of lower
brominated diphenylethers or brominated dibenzofurans, but little is
known about degradation in other media.
There is minimal information on the toxicity of DeBDE for
organisms in the environment.
1.2.2 Breakdown products
Formation of PBDF and, to some extent, PBDD may occur when DeBDE,
or products containing it, are heated to 300-800 °C. The possible
hazards associated with this have to be addressed.
Properly controlled incineration does not lead to the emission of
significant quantities of brominated dioxins and -furans. Any
uncontrolled combustion of products containing DeBDE can lead to an
unquantified generation of PBDF/PBDD. The significance of this for
both humans and the environment will be addressed in a future
Environmental Health Criteria on PBDF/PBDD.
PBDF have been found in the blood of workers involved in the
production of plastics containing DeBDE. No adverse health effects
have been associated with this exposure. Good engineering controls can
prevent worker exposure to PBDF.
1.3 Recommendations
1.3.1 General
* Workers involved in the manufacture of DeBDE and products
containing the compound should be protected from exposure through
the application of appropriate industrial hygiene measures, the
monitoring of occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled, to minimize environmental
contamination with this persistent material and its breakdown
products.
* Manufacturers should minimize levels of impurities in commercial
DeBDE products, using the best available techniques. A purity of
97% or higher is recommended.
* Incineration should only be carried out in properly constituted
incinerators, running at consistently optimal conditions. Burning
by any other means may lead to the production of PBDF and/or PBDD.
1.3.2 Further studies
* Further studies on the bioavailability and toxicity of
sediment-bound DeBDE should be performed on relevant organisms.
* Continued monitoring of environmental levels is required.
* The generation of PBDF under real fire conditions should be
further investigated.
* Environmental biodegradation, and photodegradation in compartments
other than water, should be further studied.
* Investigation into possible methods and consequences of recycling
of DeBDE-containing polymers should be made.
* Analytical methods for DeBDE in various matrices should be
validated.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Pure substance
Chemical structure:
 Chemical formula: C12Br10O
Relative molecular mass: 959.22
Chemical name: decabromodiphenyl ether
(DeBDE); decabromodiphenyl
oxide
CAS registry number: 1163-19-5
(61345-53-7, mixture of
decabromodiphenyl oxide and
Sb2O3)
CAS name: 1,1'-oxybis[2,3,4,5,6-pentabromo]-
benzene
IUPAC name: bis(pentabromophenyl) ether
EINECS registry number: 214604
MITI number: 3-2846
Synonyms: decabromobiphenyl ether;
decabromobiphenyl oxide;
Decabrom; ether,
bis(pentabromophenyl);
ether, decabromodiphenyl
From: US EPA (1984); Ethyl Corp. (1992a).
On the basis of the chemical structure, decabromodiphenyl ether is
fully brominated and there is only one congener.
2.1.2 Technical product
Trade names: FR-300 BA; DE-83-RTM; Saytex
102; Saytex 102E; FR-1210;
Adine 505; AFR 1021;
Berkflam B10E; BR55N;
Bromkal 81; Bromkal 82-ODE;
Bromkal 83-10 DE; Caliban
F/R-P 39P; Caliban F/R-P 44;
Chemflam 011; DE 83; DP 10F;
EB 10FP; EBR 700; Flame Cut
BR 100; FR 300BA; FR P-39;
FRP 53; FR-PE; FR-PE(H);
Planelon DB 100; Tardex 100;
Trade names (contd) NC-1085; HFO-102; Hexcel PF1;
Phoscon Br-250; NCI-C55287
Caliban-F/RP-44 is a DeBDE
mixture with antimony oxide,
and, F/RP-53 contains 60% DeBDE,
which is used in conjunction with
THP-salts finishes and an acrylic
binder.
Commercial DeBDE is typically composed of 97-98% decabromodiphenyl
ether with 0.3-3.0% other brominated diphenyl ethers (BFRIP, 1990)
(see Table 1). Nonabromodiphenyl ether isomers are the major
impurities. The commercial product typically contains a minimum of
81-83% bromine (IARC, 1990) (83% theoretical; Ethyl Corp. 1992a).
Differences in manufacturing processes affect the nature and
amounts of impurities in the product (Larsen, 1980). Today's
commercial product is considerably purer than that manufactured in the
past. Isomers of nonabromodiphenyl ether and octabromodiphenyl ether
have been reported as impurities in DeBDE (Timmons & Brown, 1988).
FR-300-BA, produced in the early 1970s (no longer a commercial
product), was composed of 77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE
(Norris et al., 1975c). Later production of DeBDE, by the same
manufacturer, ranged in composition from 94 to 99% DeBDE with 0.3-4.5%
impurities (NBDE isomers were identified as the major impurities)
(NTP, 1986). Other DeBDE products, e.g., DE-83, Saytex 102E, and
Bromkal 82-ODE have a purity of approximately 93 to 98.5% with
different quantities of impurities (Dow Chem. Comp., 1978; De Kok et
al., 1979; Davidson & Ariano, 1986).
The availability of a technical product (possibly FR-1208) of
88.1% purity containing 11% nona-, and 0.5% octabromodiphenyl ether,
and 0.1% hexabromobenzene has been reported (Klusmeier et al., 1988).
In Japan, a DeBDE is produced containing about 3% of
nonabromodiphenyl ether as an impurity (Watanabe & Tatsukawa, 1987).
2.2 Physical and chemical properties
Commercial DeBDE is a free-flowing, odourless, off-white powder,
with a bromine content of 81-83% and a high melting point.
Melting point: 290-306 °C
Decomposition point, >320, >400, and 425 °C
DTA (different products)
Volatility: 1% 319 °C
TGA (% weight loss) 5% 353 °C
10% 370 °C
50% 414 °C
90% 436 °C
Specific gravity: 3.0, 3.25 at 20 °C
Decabromodiphenyl ether
Vapour pressure: <10-6 20 °C
(mmHg) <1 250 °C
2.03 278 °C
5.03 306 °C
Solubility: water 20-30 µg/litre
(at 25 °C) cottonseed oil 600 mg/litre
saturated copra oil 920 mg/litre
acetone 0.5, 1.0 g/litre
benzene 1.0, 4.8 g/litre
chlorobenzene 6.0 g/litre
methylene bromide 4.2 g/litre
methylene chloride 1.0, 4.9 g/litre
o-xylene 8.7 g/litre
methanol 1 g/litre
toluene 2 g/litre
methyl ethyl ketone 1 g/litre
pentane <1 g/litre
styrene <1 g/litre
Stability: stable under normal temperatures and
pressures
Flash-point: none
Flammability: non-flammable
Autoignition point: not applicable
n-Octanol/water
partition
coefficient
(log Pow): 5.24; 9.97*
From: Norris et al. (1973, 1974, 1975a,c); Tabor & Bergman (1975);
US EPA (1986); Chemag. (1988); Great Lakes Chemical Corporation
(1990b); IARC (1990); Kopp (1990); Watanabe & Tatsukawa (1990)*;
Bromine Compounds Ltd. (1992); Ethyl Corp. (1992a).
2.3 Analytical methods
The detection and quantification of DeBDE have been investigated
by several authors. The methods are based on gas-liquid
chromatographic separation using different detection methods, such as
electron capture detection and mass spectrometry (see General
Introduction, section 2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
DeBDE has not been reported to occur naturally (see General
Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
DeBDE is produced by the bromination of diphenyl oxide in the
presence of a Friedel-Crafts catalyst (Larsen, 1978). It is
manufactured in a batch process in enclosed vessels during both the
reaction and the drying cycle (US EPA, 1988; IARC, 1990).
Commercial production of DeBDE in the USA began in 1976. Among
brominated flame retardants, the quantities produced rank second only
to the quantities of tetrabromobisphenol A. There are 2 manufacturers
in the USA (BFRIP, 1992). IARC (1990) reported 2 manufacturers in
Belgium, 1 each in Switzerland, the United Kingdom, and Israel, and 5
in Japan (US EPA, 1988).
About 30 000 tonnes of DeBDE are used annually throughout the
world. About 40% of this total is used (in combination with antimony
trioxide) in high-impact polystyrene applications, such as television
and radio cabinets. Textile applications, such as a polyester fibre
additives and coatings for automobile fabric, tarpaulins, and tents
account for about 900 tonnes (IARC, 1990; OECD, 1991; Arias, 1992).
The annual consumption of DeBDE in Japan was 1000 tonnes in 1976,
2900 tonnes in 1984, 4000 tonnes in 1987, and 9800 tonnes in 1991,
mainly used for polystyrene, polyester, and polypropylene (Watanabe,
1987; Watanabe et al., 1987b; Watanabe et al., undated). Recently
published figures from a Japanese study showed that the consumption of
PBDE (mainly DeBDE) in Japan was about 20-30% of the total consumption
of brominated flame retardants (OECD, 1991).
In the Federal Republic of Germany, 1800-2000 tonnes were used in
plastics in 1988. DeBDE mixed with antimony trioxide and DeBDE used in
conjunction with tetrakis (hydroxymethyl) phosphonium (THP) salt
finishes and an acrylic binder are used to blend 50/50 and 65/35 with
polyester/cotton (Ulsamer et al., 1980).
An estimation of the use of decabromodiphenyl ether in the
Netherlands in 1988 was 1100-1300 tonnes (Anon, 1989).
3.2.2 Uses
DeBDE is a non-reactive, additive flame retardant widely used for
its high bromine content, thermal stability, and cost effectiveness.
It is used in thermoplastic resins, thermoset resins, textiles,
adhesives, and coatings. The major applications are for high-impact
polystyrene, cross-linked polyethylene polybutyl-eneterephthalate,
glass-reinforced thermoset and thermoplastic polyester moulding
resins, low density polyethylene extrusion coatings, non-drip
polypropylene (homo and copolymers), acrylo-nitrile-butadiene-styrene
rubber (ABS), nylon, adhesives, epoxy resins, polyvinylchloride, and
elastomers. The concentrations of DeBDE in the polymers range from 6
to 22% (Tabor & Bergman, 1975; Flick, 1986; NTP, 1986; Kaart & Kokk,
1987; IARC 1990).
A mixture of DeBDE and antimony trioxide has been used to treat
nylon and polyester/cotton fabrics for industrial safety apparel and
tents (LeBlanc, 1979). DeBDE is also used in the insulating materials
for wire and electrical cable (IARC, 1990).
In the United Kingdom, approximately 1000-1200 tonnes DeBDE is
used per year in the textile industry (back coatings on synthetic
fibres) (United Kingdom Department of Environment, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Extraction from polymers
Pellets of ABS (acrylonitrile-butadiene-styrene) terpolymer and
polystyrene containing 10% DeBDE, were placed in 2 litres of water and
shaken mechanically. Total bromine in water was estimated after 3 h
and up to 187 h. Extraction of bromine took place from the ABS, during
the first 43 h, in concentrations ranging from <1.0 to 3.7 mg/litre.
No bromine was extracted from polystyrene (limit of determination
<0.5 mg/litre). Because there was no increase in bromine
concentration with time, it was suggested by the authors that the
levels found were due to erosion and not to extraction. Extraction
studies were also carried out under static conditions with pellets of
ABS containing 4.25% DeBDE. Water, acetic acid, and cottonseed oil
were used as extraction solvents at temperatures of approximately 50
or 60 °C, during 1 or 7 days. Extraction occurred only with cottonseed
oil (7 days, 60 °C) at 1 mg DeBDE/litre (limit of determination
0.075 mg/litre) (Norris et al., 1973, 1974, 1975a).
4.2 Biotransformation
No data are available.
4.3 Abiotic degradation
4.3.1 Photodegradation
Studies have been performed on the photodegradation of DeBDE in
organic solvents and water. Organic solvents were used in the initial
photodegradation studies because of the extremely low water solubility
of DeBDE. In xylene, DeBDE was photodegraded by reductive
debromination with a half-life of 15 h (Norris et al., 1973, 1975a).
A commercial mixture of DeBDE containing traces of a
nonabromodiphenyl ether, was irradiated in hexane solution with UVR
and sunlight. A complicated mixture of tri- to octabromo-diphenyl
ether congeners was detected. Furthermore, a large number of PBDF
containing 1-6 bromo atoms was formed. The yield of the PBDF under the
experimental conditions was approximately 20% of the total amount of
DeBDE, after 16 h of irradiation by UVR and approximately 10% by
sunlight. The formation and distribution of photoproducts by sunlight
were similar to those of UVR, though a few differences could be
recognized in both, i.e., the decomposition rate of DeBDE and the
total amount of, and kinds of, PBDF formed. Polybromobenzenes were
also found in minor quantities in this experiment. The formation of
PBDF appeared to occur secondarily from debrominated diphenyl ethers
as photoproducts of DeBDE, but not directly from DeBDE (Watanabe &
Tatsukawa, 1987).
The xylene studies showed that DeBDE photodegraded readily, but
did not provide any indication of the nature of the stability of the
decomposition products of DeBDE in an aqueous environment. The
proposed stepwise photoreduction of DeBDE in xylene could lead to the
formation of lower brominated diphenyl ethers, which might be more
stable to UVR. However, in water, photohydroxylation would be the
favoured route and the hydroxyl substituted degradation products would
decompose rapidly via increased UV absorption. This was demonstrated
when DeBDE in water was exposed to actual sunlight over a 3-month
period (10 g DeBDE in 8 litres of water). This study was conducted to
determine whether stable lower brominated diphenyl ethers were formed
that would show increased persistence over DeBDE. Analysis (by GC/MS)
of the exposed water solution after 31, 66, and 98 days showed a
significant increase in bromine concentration relative to the
unexposed 98-day sample. After 98 days, the bromine concentration
corresponded to the breakdown of about 300 times the initial amount of
DeBDE soluble in water. Xylene extracts of the water phase (if both
98-day samples were analysed using electron capture gas
chromatography. Levels of 4-mono-bromodiphenyl ether and
4,4'-dibromodiphenyl ether were quantified and found to be <5 and
<2 ppb, respectively. In no case did the exposed sample show an
increase in any peak, with retention times similar to those of the
lower brominated diphenyl ethers.
The products of the photodegradation of DeBDE in water are not
lower brominated diphenyl ethers (Norris et al., 1973, 1975a).
4.3.2 Pyrolysis
A commercial DeBDE, FR 300 BA (77% DeBDE), was heated in a quartz
tube at 700, 800, and 900 °C and the concentrations of PBDD and PBDF
determined. The residues contained tetra- to octabromodibenzofurans
together with hepta- and octabromodioxins; the optimal formation
temperatures were 800 and 900 °C (Thoma et al., 1987a; Zacharewski et
al., 1988).
FR 300 BA (77%) was pyrolysed at 600, 700, 800, and 900 °C in the
absence of oxygen in a SGE pyrojector and the residues were analysed
using GC/MS in an on-line operation. Polybromobenzenes (PBB),
polybromonaphthalenes (PBN), and polybrominated dibenzofurans were
detected in the pyrolysate. Sixty percent of the starting products
were decomposed at 600 °C, hexabromobenzene being formed as the main
component together with traces of pentabromobenzenes and
nonabromodiphenyl ether. At a higher temperature (700 °C), the
pentabromobenzene concentration increased and tetrabromobenzenes were
also formed. Maximum amounts of hepta- and octabromodibenzofurans and
hexabromonaphthalenes were produced at this temperature. At 800 °C and
higher, an increase in levels of tetra- and pentabromobenzenes was
observed. During these reactions, the C-O bond is cleaved followed by
the attachment of a bromine atom. C-C ring closure leading to
dibenzofuran formation is very strongly suppressed by DeBDE (Thoma &
Hutzinger, 1987, 1989).
In the case of decabromodiphenyl ether (DeBDE), a total amount of
1-2% PBDF and PBDD was formed on thermolysis of DeBDE. At a
temperature of 630 °C, 90% of DeBDE was decomposed. The major products
were PBBz (tri- to hexabrominated). A heptabromodibenzofuran was a
main product, though tetra- to heptabromodibenzofurans and tetra- to
octabromodibenzodioxins were also found. In these experiments, PBDF
and PBDD were formed, which contained at least one or two,
respectively, bromine substituents less than the PBDE in the technical
product; often the same major isomers were present (Buser, 1986).
4.3.3 Combustion of polymers containing DeBDE
4.3.3.1 Pyrolysis studies
Clausen et al. (1987), Bieniek et al. (1989), and Lahaniatis (1989)
studied the influence of the combustion temperature on the formation
of PBDD and PBDF from plastics containing DeBDE. The results of the
experiments with PBT with 10% DeBDE/6% Sb2O3 at temperatures of 400,
500, 600, 700, and 800 °C in the presence of air, showed the formation
of PBDFs at mg/kg (ppm) concentrations. 2,3,7,8-TeBDD was not found
with a limit of determination of 10 mg/kg. Maximum formation of TeBDF
(4000 mg/kg) occurred at 400 °C, and, with increasing temperature,
concentrations decreased. No PBDF were formed in the thermolysis of
PBT with DeBDE but without Sb2O3. The results are summarized in
Table 10.
Comparable experiments were reported by Lahaniatis et al. (1991).
The formation of 2,3,7,8-tetrabromodibenzodioxin and -furan from
various polymers with DeBDE or PBDE were studied in thermolysis
experiments at 400, 600, and 800 °C. The polymers studied were PBT
with 10% DeBDE with, or without, 6% Sb2O3, epoxide resin (ER) with
3-6% DeBDE, and phenol resin (PR) with 3-6% of PBDE and copper. The
concentrations of the TeBDD and TeBDF at the 3 temperatures are given
in Table 11. The 2,3,7,8-TeBDD concentrations ranged from 0.01 to
7 mg/kg and the 2,3,7,8-TeBDF concentrations from 0.01 to 52 mg/kg.
The concentrations decreased with increasing temperature, and were low
at 800 °C.
Pure DeBDE and commercial polybutyleneterephthalate polymer
samples containing DeBDE and antimony trioxide have been pyrolysed at
temperatures ranging from 300 to 800 °C. The experiments were carried
out in a vertical combustion apparatus. The polymer/DeBDE samples
were: polybutylene-terephthalate (PBT) with 11% DeBDE and 5.5%
Sb2O3, PBT with 9% DeBDE and 7% Sb2O3, and PBT with 11% DeBDE and
2.7% Sb2O3 (Dumler et al., 1989a,b). The results of the pyrolysis
studies are given in Table 12. TeBDF were formed, with yields of up to
16%, from the 3 polymer samples. PBDF were mainly formed during
pyrolysis in the presence of DeBDE and Sb2O3. At 300 °C, the
conversion of DeBDE to PBDF was low, while highly brominated compounds
were formed. By increasing the temperature, the yield of
polybrominated compounds increased and more lower brominated PBDF were
formed. The maximum formation of PBDF occurred between 400 and 500 °C.
With increasing temperature, not only ring-closure reactions but also
debromination reactions with the predominant formation of tri- and
tetra- brominated PBDF may occur, The same reactions probably occur at
600 °C. At these elevated temperatures, PBDD were also detected to a
minor extent. The polymers with the lowest amount of Sb2O3 gave the
lowest PBDF concentrations. With increasing amounts of Sb2O3, the
concentration of PBDF increased, which suggests that antimony trioxide
plays a catalytic role. Pure DeBDE, pyrolysed under the same
conditions, produced significantly lower PBDF concentrations than
polymers with the flame retardants. In the case of pure DeBDE, the
temperature for the maximum formation of PBDF shifted to 700 °C.
Table 10. Thermolysis products of PBT with 10% decabromodiphenyl ether and 6% Sb2O3a
400°C 500°C 600°C 700°C 800°C
Bromodiphenyl ether
MBDE 10 50 -- -- --
DiBDE 30 100 -- -- --
TrBDE 100 200 50 -- --
TeBDE 500 300 -- -- --
PeBDE 800 400 -- -- --
HeBDE 1000 400 -- -- --
HpBDE 500 400 -- -- --
OBDE (500) -- -- -- --
Bromodibenzofuran
MBDF 100 300 100 50 --
DiBDF 500 400 200 10 --
TrBDF 3000 2000 400 -- --
TeBDF 4000 3000 600 -- --
PeBDF 4000 1000 200 -- --
HxBDF 1000 200 -- -- --
HpBDF (500) -- -- -- --
a From: Clausen et al. (1987); Bieniek at al. (1989).
Semiquantitative values in mg/kg based on the sample -- not confirmed.
2,3,7,8-Tetrabromodibenzofuran was not found (limit of determination 20 mg/kg).
2,3,7,8-Tetrabromodibenzodioxin was not found (limit of determination 10 mg/kg).
Table 11. Generation of 2,3,7,8-TeBDD/TeBDF by thermolysis of plastics with flame
retardants as additives in mg/kga,b
Compound 2,3,7,8-TeBDD 2,3,7,8-TeBDF
Sample 400 °C 600 °C 800 °C 400 °C 600 °C 800 °C
PBT/DeBDE/Sb2O3 0.02 0.01 --c 52 5.7 --
PBT/DeBDE --c --c --c 2.5 4.2 0.08
Five samples of
ER/DeBDE
minimum 0.05 0.3 0.01 0.4 0.6 0.01
maximum 0.3 0.8 0.03 1.0 2.5 0.04
PR/DeBDE/Cu --d 7 --d --d 5.7 --d
a From: Lahaniatis et al. (1991).
b The mg/kg values are based on the material used.
c Non-detected, detection limit 0.01 mg/kg.
d = Not determined.
Table 12. Results of pyrolysis studies
A. Polymer Sample A: Polybutyleneterephthalate with 11% decabromodiphenyl ether and 5.5%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)a
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- 754 3012 5551 3513 3076
DiBDF 9 2357 10 219 15 343 8445 1547
TrBDF 9 10 747 37 911 32 751 28 592 1274
TaBDF 9 14 979 52 634 37 437 35 963 1511
PeBDF 55 2293 18 391 20 666 13 504 555
HxBDF 703 127 3713 10 438 2639 109
HpBDF 1320 -- 246 946 491 --
OBDF -- -- -- -- -- --
B. Polymer Sample B: Polybutyleneterephthalate with 9% decabromodiphenyl ether and 7%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- -- 13 088 7633 8510 1144
DiBDF -- -- 15 754 10 643 9721 244
TrBDF 47 456 34 408 24 842 19 276 44
TeBDF 1472 4544 48 762 35 230 23 353 367
PeBDF 5560 18 132 24 753 16 154 6988 156
HxBDF 2886 24446 18 587 8832 1633 22
HpBDF 420 6910 2877 922 100 --
OBDF -- -- -- -- -- --
a From: Dumler et al. (1989b).
Table 12. (cont'd).
C. Polymer Sample C: Polybutyleneterephthalate with 11% decabromodiphenyl ether and 2.7%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- 2202 3413 2129 610 18
DiBDF -- 5187 6124 1674 82 --
TrBDF -- 15 033 15 952 1738 36 15
TeBDF -- 17 836 17 463 901 9 12
PeBDF -- 9127 3349 39 -- --
HxBDF 464 2457 901 82 -- --
HpBDF 2375 1329 246 36 -- --
OBDF traces traces -- -- -- --
D. Decabromodiphenyl ether (yields in mg/kg)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- -- -- -- -- 2
DiBDF 4 8 -- 3 2 1
TrBDF 4 13 -- 4 25 3
TeBDF -- 15 11 -- 100 102
PeBDF -- 16 218 380 591 218
HxBDF -- 42 109 61 1965 988
HpBDF -- -- 1081 1734 4539 418
OBDF -- -- -- -- -- --
A sample (1 g) of industrial polypropylene containing 125 g
DeBDE/kg and 75 g Sb2O3/kg, and a pure DeBDE sample, without
additives, were pyrolysed in a DIN-oven or in a sealed quartz ampoule
at temperatures of 400, 600, or 800 °C. Combustion products obtained
with the pyrolysis of pure DeBDE (50 mg) in the DIN-oven were mainly
hexabromobenzenes and the yields at 400, 600, and 800 °C, were 123,
568, and 6 g/kg, respectively. PBDF/PBDD were found in concentrations
of 1, 3, and 2 g/kg at 400, 600, and 800 °C, respectively. At 400 and
600 °C, HxBDF up to OBDF were found in concentrations of between 96
and 1449 mg/kg. At 800 °C, lower PBDF, e.g., TrBDF, TeBDF, and PeBDF,
were also present in concentrations ranging between 11 and 35 mg/kg.
The concentrations of HxBDF and HpBDF were 81 mg and 959 mg/kg,
respectively, but no OBDF was found. The concentrations of PBDD at
400-600 °C for HxBDD up to OBDD were between 6 and 329 mg/kg; at
800 °C, lower brominated compounds, e.g., TrBDD, TeBDD, and PeBDD were
also found at concentrations of between 8 and 35 mg/kg. HxBDD up to
OBDD were present in concentrations of between 74 and 452 mg/kg. The
combustion products of the samples of polypropylene containing
DeBDE/Sb2O3 were mainly PBDF and PBDD and the concentrations were
much higher than from the combustion of the pure DeBDE. MBDF up to
HpBDF were present at concentrations of between 4762 and
107 517 mg/kg, 1033 and 49 677 mg/kg, and 353 and 29 147 mg/kg at 400,
600, and 800 °C, respectively. At all 3 temperatures, TeBDF were found
in the highest concentrations. The results with polypropylene
containing DeBDE/Sb2O3, pyrolysed in a sealed quartz ampoule at
600 °C, showed only MBDF up to PeBDF at concentrations ranging between
8860 and 142 940 mg/kg. The highest concentrations were found for
TrBDF and TeBDF. PBDD were not found. From this study, it is clear
that pure DeBDE gives much lower PBDF values than polypropylene
containing DeBDE/Sb2O3 (Dumler et al., 1990).
The formation of PBDD and PBDF during the pyrolysis of PBT
containing DeBDE was studied at different temperatures and carrier gas
compositions. PBDF were formed at ppm levels. Even when oxygen was
available in the carrier gas, PBDD were formed at a much lower level
than PBDF. In the presence of 10% oxygen, the maximum yield of tetra-
to octabromodibenzofurans was 70 ppm at 600°C. The yields of
2,3,7,8-TeBDF and 1,2,3,7,8-PeBDF were less than 4.5 ppm. The thermal
degradation processes of the polymer were investigated using
thermogravimetric analysis. The flame retardant did not exert any
influence on the elementary chemical degradation processes. The flame
retardant activity of DeBDE consists of the emission of brominated
species in the gas phase, which scavenge the propagation radicals and
reduce flammability. The mechanisms of formation of PBDD and PBDF from
DeBDE in PBT consist of a combination of a condensed phase and a gas
phase mechanism (Luijk & Govers, 1992).
Macro-pyrolysis experiments were performed in a quartz tube
reactor. The polymer sample, PBT, was inserted in the pre-heated tube
and exposed at 400-700 °C for 20 min. The carrier gas was nitrogen, or
nitrogen combined with 5% or 10% oxygen. The results are given in
Tables 13 and 14. PBDF were formed in all tests with the different
carrier gases, but the yield of PBDD was at least two orders of
magnitude lower than that of the PBDF. In a nitrogen atmosphere, no
PBDD were detected (Luijk & Govers, 1992).
Using GC/MS, Sovocool et al. (1990) analysed pyrolysates of PBT
resins (flame retarded with DeBDE) which had been exposed to
temperatures of 400 and 600 °C in the presence of oxygen. They found
brominated dioxins, brominated dibenzofurans. brominated naphthalenes,
brominated benzenes and brominated toluenes, brominated biphenyls, and
brominated methyldibenzofurans (Me-PBDF). Besides the
methyldibenzofurans, other (C2 and C3) alkyl-PBDF were found in the
pyrolysates. Numerous congeners of ethyl- (and/or dimethyl)-PBDF and a
few propyl (and/or trimethyl or ethylmethyl)-PBDF were detected.
The C2- and C3-alkyl-PBDF found in the pyrolysates exhibited
relatively intense (M-CH3)+ fragments, supporting the argument for
ethyl-and propyl or ethyl-methyl substitutions rather than dimethyl
and trimethyl substitution patterns.
Early in 1987, the General Electric Company (GE) began to
investigate the pyrolysis of polybutyleneterephthalate (PBT) flame
retardant systems. The initial work was carried out at temperatures of
600-900 °C, but subsequent efforts were at processing temperatures in
the range of 200-500 °C. The results showed little or no formation of
PBDD at temperatures of 250-400 °C; concentrations of PBDF in the
pyrolysed products were as high as about 6000 mg/kg. The
concentrations of 2,3,7,8-substituted dibenzofurans were much lower,
not exceeding a maximum of 150 mg/kg. The results for PBDD and PBDF
were much higher than would be expected, based on the previous work by
Buser and Hutzinger. The results were also inconsistent with what was
observed in combustion experiments. The explanation was that the
exposure times and conditions used did not represent a good model of
what would actually occur in moulding equipment (BFRIP, 1990).
In the GE experiments at the Fresenius Institute, an apparatus was
used in which a small sample of the polymer in the form of a finely
ground powder was exposed to the pyrolysis temperature in a flowing
air stream. The pyrolysis time for these samples varied from 10 min at
the high temperatures to as much as 1.5 h at the low temperatures.
This is in contrast to extrusion and moulding operations in which the
polymer is in the form of a molten mass, air is largely excluded, and
exposure lasts less than 1 min.
Table 13. The yield of PBDF during the pyrolysis of PBT/DeBDE in ppm relative to blend, under different carrier gas conditionsa
Temperature TrBDF TeBDF 2,3,7,8- PeBDF 1,2,3,7,8- HxBDF HpBDF NBDF
[°C] TeBDF PeBDF
Nitrogen
400 6.7 (0.8) 3.9 (2.4) 0.15 (0.09) 8.7 (0.5) 0.2 (NS)b 10.6 (1.9) 3.7 (0.4) 0.05 (0.00)
500 15.9 (2.8) 14.5 (0.8) 0.6 (0.1) 13.3 (2.5) 0.1 (NS)b 9.8 (1.1) 4.8 (0.9) 0.1 (0.1)
600 6.8 (0.4) 4.9 (0.2) 0.2 (0.0) 4.5 (1.8) 0.1 (NS)b 2.9 (0.5) 0.7 (0.2) NDc
700 4.2 (0.8) 1.2 (0.1) 0.1 (NS) 0.5 (0.0) (NS)b 0.2 (0.0) 0.06 (0.02) NDc
Nitrogen + 5% oxygen
400 4.7 (1.4) 3.6 (1.1) 0.2 (0.1) 3.6 (1.1) 0.1 (NS)b 2.9 (1.2) 0.7 (0.3) NDc
500 7.7 (0.7) 6.4 (0.4) 0.4 (0.1) 5.3 (0.1) 0.1 (0.0) 5.3 (0.1) 1.1 (0.0) NDc
600 22.1 (2.7) 17.2 (1.8) 2.4 (0.3) 17.7 (3.2) 0.8 (0.2) 16.8 (1.3) 4.8 (0.3) 0.3 (0.0)
700 15.7 (0.3) 7.6 (0.1) 1.2 (0.0) 6.6 (1.2) 0.4 (0.1) 5.7 (0.2) 1.3 (0.3) 0.08 (0.03)
Nitrogen + 10% oxygen
400 9.6 (0.7) 7.5 (1.0) 0.3 (0.1) 7.7 (1.5) (NS)b 5.9 (3.0) 1.8 (1.0) NDc
500 11.0 (0.3) 8.7 (0.5) 0.6 (0.1) 8.8 (0.4) 0.2 (0.0) 8.7 (0.8) 3.7 (0.3) NDc
600 28.8 (3.2) 20.8 (1.8) 3.4 (0.5) 21.6 (3.4) 1.2 (0.2) 17.8 (1.3) 7.6 (1.1) 0.8 (0.1)
700 17.5 (0.7) 10.3 (1.4) 2.0 (0.2) 8.9 (0.3) 0.6 (0.0) 5.3 (0.3) 2.1 (0.3) 0.02 (0.02)
a From: Luijk & Govers (1992).
b NS = not separable, due to interferences.
c ND = not detected (below detection limit).
Table 14. The yield of PBDD during the pyrolysis of PBT/DeBDE in ppb relative to blend, under different carrier gas conditionsa
Temperature TrBDD TeBDD 2,3,7,8 PeBDD 1,2,3,7,8- HxBDD HpBDD NBDD
[°C] TeBDD PeBDD
Nitrogen + 5% oxygen
400 NSb NSb NSb NSb NSb NDc NDc NDc
500 NSb NSb NSb NSb NSb NSb 7 (2) 4 (4)
600 NSb 8 (NSb) NSb 44 (6) NSb 110 (10) 43 (0) 13 (1)
700 NSb 5 (1) NSb 19 (6) NSb 40 (5) 8 (2) NDc
Nitrogen + 10% oxygen
400 NSb NSb NSb NSb NSb NDc NDc NDc
500 6 (NSb) NSb NSb 3 (NSb) NSb 48 (NSb) 16 (NSb) NDc
600 44 (NSb) 29 (7) NSb 75 (NSb) NSb 106 (11) 46 (4) 16 (0)
700 32 (18) 24 (NSb) NSb 21 (NSb) NSb 19 (3) 4 (4) NDc
a From: Luijk & Govers (1992).
b NS = Not separable, due to interferences.
c ND = Not detected (below detection limit).
Dumler et al. (1989b) have recently published data from studies on
the pyrolysis of PBT/DeBDE resins. This work was conducted in
apparatus identical to that used by GE, thus, the results can be
compared. In the report by Hutzinger and coworkers, concentrations of
PBDF were calculated relative to the flame retardant only.
Recalculation of the results by BFRIP (1990), on the basis of the
total polymer, showed general agreement with the GE results
(Table 15).
Hutzinger's recent work was summarized in a report by Pohle of the
Umwelbundesamt (Umweltbundesamt, 1989). In this work, the tube furnace
(DIN Apparatus) and the vertical quartz oven (VCI Apparatus; as used
by the Fresenius Institute) were used to pyrolyse polymers containing
PBDE at temperatures ranging from 600 to 750 °C. The results showed
that PBDF were formed under these conditions, but that 2,3,7,8-TeBDF
was only a small fraction of the total. In general, the results were
similar to those reported earlier by Hutzinger and GE.
Because of the lack of good data collected under actual operating
conditions, BFRIP met with 6 major polymer producers in 1988, to
design a series of experiments to investigate the behaviour of DeBDE
and OBDE in HIPS, ABS, and PBT under controlled moulding or extrusion
conditions. The experiments are outlined in Tables 16 and 25 (in OBDE
section 4.2.3). For the three polymer systems, HIPS/DeBDE, ABS/OBDE,
and PBT/ DeBDE, samples were moulded in the application laboratories
of polymer producers. For the moulding operations, the severity of
operations ranged from normal to extreme. Normal conditions were those
in the middle range recommended by the resin manufacturers for
processing. The abusive conditions were at, or slightly above, the
maximum recommended exposure time and temperature. The extreme
conditions were well above those recommended by the resin manufacturer
and resulted in samples with severely degraded physical properties
and/or colour.
After moulding, all the samples were analysed by Triangle
Laboratories, Research Triangle Park, NC (TLI). Samples were also sent
to the US EPA Laboratory in Las Vegas for confirming analyses. The
results of these analyses are summarized in Tables 17 and 26 (in OBDE
section 4.2.3) and are discussed more fully in the report of this
study made at Dioxin'90 (McAllister et al., 1990; BFRIP, 1990). With
the exception of a single sample, PBDD were not identified in any of
the samples and 2,3,7,8-TeBDF were seen only in this one sample at
very low concentrations. In general, the Triangle Park and US EPA
results agreed fairly well for the TBDF and TBDD.
Table 15. Comparison of GE and Hutzinger results on pyrolysis of PBT/DeBDE Resin (PBT/11% DeBDE/2.7% antimony trioxide)
All concentrations in mg/kg relative to total polymera
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
Compound General Dumler General Dumler General Dumler General Dumler General Dumler General Dumler
Electric et al. Electric et al. Electric et al. Electric et al. Electric et al Electric et al.
USA (1989) USA (1989) USA (1989) USA (1989) USA (1989) USA (1989)
MBDF NAb - 240 370 230 70 NDc
DiBDF - - 570 673 180 9 -
TrBDF - - 1650 1750 190 36 NDc
TeBDF 5200 - 2000 1960 1200 1920 490 100 24 4 0.03 NAb
2,3,7,8- 150 - 64 - 120 - 19 - 0.4 - 0.005 -
TeBDF
PeBDF 5900 - 1200 1003 1300 370 350 40 8 - 0.006
1,2,3,7,8- NDc - NDc - NDc - NDc - NDc - NDc -
PeBDF
HpBDF NDc 260 NDc 150 50 40 3 4 0.1 - NDc -
OBDF - Tr - Tr - NDc - NDc - - - -
Total 13 450 310 4100 3383 3220 2430 943 234 35 4 0.04 NAb
TeBDF-
HpBDF
a From: BFRIP (1990).
b NA = not analysed.
c ND = not detected.
Table 16. Moulding studies with two polymer/FR systems at
different processing temperaturesa
Polymer/FRb Severity Conditions
HIPS/DeBDE None Base resin, not moulded
Normal 215-220 °C, 30- second cycle
Abusive 235-245 °C, 5-min cycle
Extreme 265-270 °C, 7-min cycle
PBT/DeBDE Normal 255 °C, 23-second cycle
Abusive 255 °C, 5-min cycle
Extreme 255 °C, 10-min cycle
a From: McAllister et al. (1990).
b HIPS/DeBDE High impact polystyrene/ 84.3%
DeBDE + 12.0%
antimony trioxide 3.7%
PBT/DeBDE Polybutyleneterepthalate/ 88.0%
DeBDE + 6.5%
antimony trioxide 5.5%
Different interpretations of the analytical results of the 2
laboratories resulted in larger differences in values for hexa-through
octa-brominated furans. These data showed that the processing of the
resin systems under normal conditions did not change the composition
compared with that of the base resin formulation. Even under abusive
conditions, there was little generation of PBDF or PBDD. In fact, PBDD
were observed in only 2 of the samples under abusive or extreme
exposures. 2,3,7,8-Substituted dibenzofurans were not observed, except
in one sample in which very low ppb levels were seen (Tables 17 and 26
in OBDE section 4.2.3).
4.3.3.2 Workplace exposure studies
The occurrence of PBDF and PBDD during the thermal processing of
glass-filled PBT resin containing DeBDE at 70 g/kg and Sb2O3 at
62 g/kg or no flame retardant was studied. No PBDD (limit of
determination 2 ng) were found. PBDF were present and isomers
containing bromine at the 2,3,7,8-position represented less than 10%
of the total PBDF. Fumes evolved during thermal processing of the
resins were collected at the die-head during extrusion.
Table 17. Comparison of Triangle Laboratories Inc. (TLI) (Research Triangle Park) and US EPA Laboratory (Las Vegas) results
on moulded polymer samples containing various flame retardants. (All concentrations are in mg/kg)a
High impact polystyrene with decabromodiphenyl ether
Base resin 1222-16-0 Normal 1222-16-1 Abusive 1222-16-2 Extreme 1222-16-3
Compound TLI US EPA TLI US EPAc TLI US EPAd TLI EPA
Total TBDF 0.01 ND 0.01 0.01 0.002 0.02 ND
PeBDF 0.04 0.004 0.05 0.06 0.02 0.2 0.009
HxBDF < 5.3 0.95 < 14.3 < 5.5 0.11 < 34.1 0.2
HpBDFb < 0.6 0.72 < 3.5 < 2.1 0.08 < 10.6 2.1
PBDFb < 4.1 0.15 < 9.3 < 7.0 ND < 35.7 3.2
Table 17. (cont'd).
PBT with decabromodiphenyl ether
Base resin Normal 1222-16-6 Abusive 1222-16-7 Extreme 1222-16-8
Compound TLI US EPA TLI US EPAd TLI US EPA TLI US EPAe
Total TeBDD < 0.001 < 0.0002 < 0.002 0.001
Total PeBDD < 0.001 < 0.0002 < 0.013 0.006
Total TeBDF 0.003 0.003 0.007 0.03 0.2 1.0
Total PeBDF 0.02 0.002 0.09 > 7.8 > 25 > 54
Total HxBDF 0.11 0.013 1.1 > 16.1 > 120 > 7.0
Total HpBDFb 0.31 < 4.3 3.0 < 4.6 58.5 < 12.1
Total OBDFb 0.95 < 16.9 3.2 < 9.9 7.5 < 68.8
aFrom: BFRIP (1990). Values preceeded by < are maximum possible. Analytical signals met identification criteria, but
may include contribution from interferences. US EPA and TLI used different identification criteria, resulting in
differences in reported values.
b Concentrations of HpBDF and OBDF from TLI are not validated because of lack of standards.
Concentrations are reported for comparison only.
c Sample 1222-16-1 was spilled during workup.
d Average of 2 analyses of same sample.
e US EPA results not validated.
The temperature in the 4 temperature zones was between 216 and
250 °C. It was found that thermal processing of resin with
DeBDE/Sb2O3 yielded higher concentrations of PBDF in the fumes than
that of resin without the flame retardants. The concentrations found
in two samples of resin with flame retardant and one sample without
flame retardant are given in Table 18.
In addition to these pyrolysis and moulding experiments, studies
have been performed to determine the possibility of the workplace
exposure to PBDF and PBDD of workers in extrusion or moulding
facilities. The first of these studies was a simulation sponsored by
General Electric (GE) at Battelle Laboratories in Columbus, OH (BFRIP,
1990). For this work, an experimental extrusion apparatus was used in
which the total volatiles from the extruder could be collected and
analysed. A typical commercial PBT/DeBDE resin was extruded, the
volatiles were collected and analysed at the Fresenius Institute. The
results of the analysis were used to estimate a probable workplace
concentration of 0.76 ng/m3 as TCDD equivalents.
The following experiment was carried out to determine the risks to
employees working in the actual production of PBT resin, flame
retarded with DeBDE, at Hoechst Celanese facility at Bishop, Texas
(Hoechst Celanese Corp., 1988). High-volume samples were taken above
the extruder die-head, the extruder vent port, the fibreglass addition
port, and the raw material feeder, because these were the areas where
off-gassing was most likely to occur and where employees could be
exposed to the off-gases from extrusion. Source samples can be
considered as worst-case situations, as samplers were placed directly
in the off-gas streams. A fifth sample was taken in an unused guard
house, 90 m from the extruder building, as a field control. Full-shift
personal samples were taken on all operators and helpers on each shift
to determine the actual employee exposure. Each sampler was worn by
employees for the entire 24-h period. The operating temperature was
240-260 °C. Personal samples are regarded as more representative than
the area/source samples, since they were worn by employees during the
production run.
Table 18. Concentrations of PBDF in resins in ng per sample componenta
Substance Two samples with flame retardant One sample without
flame retardant
TeBDF 201 1310 76
2,3,7,8-TeBDF 15 114 6
PeBDF 131 820 43
1,2,3,7,8- ND (< 2) ND (< 5) ND (< 2)
PeDBF
HxBDF 80 425 23
1,2,3,4,7,8-
HxBDF < 6 49 2
HpBDF < 11 51 2
Total PBDF 433 2606 144
aFrom: (Vinci & Craig, 1988).
The values are the total quantity of PBDF (in ng) found in the 2 sample
components, while the values of the specific isomers are given as values
present in the total PBDF. For example: 15 ng of 2,3,7,8-TeBDF present in
201 ng total PBDF.
No PBDD was detected in any of these samples (limit of determination, 2 ng).
bND = not detected.
The concentrations (expressed as TCDD equivalents) in ng/m3 in
the different air samples were:
Fibreglass port 0.027
Extruder die-head 3.589
Extruder vent port 0.191
Raw material feeder 0.015
Guard house 0.0004
Personal (three) samples 0.019, 0.011,
0.122.
BFRIP sponsored a study at a commercial PBT extrusion facility in
1988. This study was designed and conducted with input from the
National Institute of Occupational Safety and Health (NIOSH) and the
US EPA. In this study, both area samples and personnel monitors were
used to collect information on potential exposures. Area samples were
collected from locations expected to show maximum levels of volatile
components. Personnel samples were collected with pumps worn by
workers, and represented the average levels to which workers were
exposed. In all cases, exposures were calculated in terms of TeCDD
equivalents, using the concept of "Toxicity Equivalent Factors" (TEF)
(the concentration of a particular chlorinated (brominated)
dibenzodioxin or dibenzofuran is converted to an equivalent
concentration of 2,3,7,8-TeCDD by multiplying by the appropriate
factor). It should be noted that this conversion of exposure values
for PBDF to TeCDD toxicity equivalents is only an estimate and is by
no means generally accepted. The results from the area samples are
summarized in Table 19 and from personnel samples in Table 20. The
maximum worker exposure for PBDF was 0.1 ng/m3, expressed as TeCDD
toxicity equivalents (BFR/CEM Working Group, 1989; BFRIP, 1990;
EBFRIP, 1990).
Determination of the occupational exposure during extrusion of PBT
containing DeBDE at 90 g/kg and antimony trioxide at 80 mg/kg, was
carried out by BASF, in Germany. In air, the total PBDF concentration
close to the extruder head was 72.9 µg/m3 and that in the rooms
ranged from 0.169-0.989 µg/m3. Concentrations of hexabromo- and
heptabromodibenzofurans were the highest, followed by penta- and
octabromodibenzofuran. The total PBDD concentration in the room was
28.12 ng/m3, being mainly octa- and hexabromodibenzodioxins. The
tetrabromodibenzodioxin concentration was 2.04 ng/m3 (Kraus, 1990).
Umweltbundesamt (1989) reported the results from the workplace
study conducted by BASF in a PBT extrusion facility. The results from
the BASF study are summarized in Table 21. The higher level observed
in the vicinity of the extruder head was removed from the workplace by
local ventilation. According to the BASF report, workers spent most of
their time in the "Production room" or at the "Bagging station". Only
if there were problems with the equipment did they work near the
extruder. The actual worker exposure would be approximately the levels
observed in the production room or at the bagging station.
The results of the BASF and BFRIP studies are summarized in
Table 22. With the exception of the BASF sample collected at the
extruder head, the results are generally consistent between the two
studies. The higher value for this sample, compared with that from the
extruder die-head from the BFRIP, could be a result of a difference in
the placement of the sampler (BFRIP, 1990).
Table 19. Results from BFRIP air sampling testsa
Compound Toxicity Area Samples
equivalent Fibreglass port Extruder die head Extruder vent port Feed hopper Guard gate
factor
CONCb TCDD CONCb TCDD CONCb TCDD CONCb TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc equiv.c
2,3,7,8-TeBDD 1.00000 0.0001 0.0001 0.0033 0.0033 0.0016 0.0016 0.0000 0.0000 0.0001 0.0001
1,2,3,7,8-PeBDD 0.50000 0.0001 0.0000 0.0059 0.0030 0.003 0.0016 0.0006 0.0003 0.0001 0.0001
1,2,3,4,7,8-HxBDD 0.10000 0.0002 0.0000 0.6220 0.0622 0.202 0.0202 0.0002 0.0000 0.0006 0.0001
1,2,3,6,7,8-HxBDD 0.10000 0.0002 0.0000 0.6221 0.0622 0.202 0.0202 0.0002 0.0000 0.0006 0.0001
OBDD 0.00100 0.0006 0.0000 0.0382 0.0000 0.020 0.0000 0.0009 0.0000 0.0023 0.0000
2,3,7,8-TeBDF 0.10000 0.0118 0.0012 0.0017 0.0002 0.104 0.0104 0.0055 0.0005 0.0001 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0154 0.0008 0.0072 0.0004 0.005 0.0005 0.0092 0.0005 0.0002 0.0000
1,2,3,4,7,8-HxBDF 0.10000 1.0770 0.1077 126.8512 12.6851 1.962 0.1962 0.6036 0.0604 0.0006 0.0001
Total TeBDD 0.00000 0.0356 0.0000 1.9360 0.0000 2.199 0.0000 0.0412 0.0000 0.0001 0.0000
Total PeBDD 0.00000 0.0067 0.0000 0.3079 0.0000 0.003 0.0000 0.0048 0.0000 0.0001 0.0000
Total HxBDD 0.00000 0.0073 0.0000 36.9172 0.0000 0.448 0.0000 0.0084 0.0000 0.0006 0.0000
Total TeBDF 0.00000 3.4764 0.0000 394.2480 0.0000 13.735 0.0000 1.4432 0.0000 0.0620 0.0000
Total PeDBF 0.00000 7.4785 0.0000 1412.8761 0.0000 55.384 0.0000 3.5971 0.0000 0.0000 0.0000
Total HxBDF 0.00000 2.6888 0.0000 4544.6098 0.0000 146.321 0.0000 0.5275 0.0000 0.0000 0.0000
Air concentration 0.1098 12.8163 0.2503 0.0618 0.0004
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
Table 20. Results from BFRIP air sampling testsa
Compound Toxicity Personnel monitors
equivalent
6559 12524 49834
factor
CONCc TCDD CONCb TCDD CONCb TCDD
equivalentc equivalentc equivalentc
2,3,7,8-TeBDD 1.00000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
1,2,3,7,8-PeBDD 0.50000 0.0000 0.0000 0.0005 0.0002 0.0000 0.0000
1,2,3,4,7,8-HxBDD 0.10000 0.0000 0.0000 0.0025 0.0002 0.0000 0.0000
1,2,3,6,7,8-HxBDD 0.10000 0.0000 0.0000 0.0025 0.0002 0.0000 0.0000
OBDD 0.00100 0.0000 0.0000 0.0093 0.0000 0.0000 0.0000
2,3,7,8-TeBDF 0.10000 0.0132 0.0013 0.0000 0.0000 0.0000 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0365 0.0018 0.0012 0.0001 0.1855 0.0093
1,2,3,4,7,8-HxBDF 0.10000 0.3543 0.0354 0.1405 0.0140 5.4102 0.5410
Total TeBDD 0.00000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
Total PeBDD 0.00000 0.0000 0.0000 0.0005 0.0000 0.0000 0.0000
Total HxBDD 0.00000 0.0000 0.0000 0.0025 0.0000 0,0000 0.0000
Total TeBDF 0.00000 1.4065 0.0000 1.1929 0.0000 7.6914 0.0000
Total PeDBF 0.00000 5.0801 0.0000 5.9643 0.0000 38.8162 0.0000
Total HxBDF 0.00000 6.5995 0.0000 61.9528 0.0000 70.1965 0.0000
Air concentration 0.0386 0.0149 0.5503
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
Table 21. Results from BASF air sampling tests
Compound Toxicity Extruder
equivalent
factor Production room Work station Bagging station Extruder head
CONCb TCDD CONCb TCDD CONCc TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc
2,3,7,B-TeBDD 1.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,7,8-PeBDD 0.50000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,4,7,8-HxBDD 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,6,7,8-HxBDD 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
OBDD 0.00100 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
2,3,7,8 TeBDF 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0 0.0000 1.3 0.0650 0.0 0.0000 87.0 4.3500
1,2,3,4,7,8-HxBDF 0.10000 0.0 0.0000 2.6 0.2600 0.3 0.0300 1186.0 118.6000
Total TeBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Total PeBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Table 21. (cont'd).
Compound Toxicity Extruder
equivalent
factor Production room Work station Bagging station Extruder head
CONCb TCDD CONCb TCDD CONCc TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc
Total HxBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Total DiBDF 0.00000 1.1 0.0000 1.3 0.0000 0.2 0.0000 322.0 0.0000
Total TriBDF 0.00000 13.0 0.0000 7.4 0.0000 1.1 0.0000 705.0 0.0000
Total TeBDF 0.00000 20.0 0.0000 33.7 0.0000 5.1 0.0000 980.0 0.0000
Total PeBDF 0.00000 98.0 0.0000 7143.0 0.0000 8.6 0.0000 3910.0 0.0000
Total HxBDF 0.00000 594.0 0.0000 554.0 0.0000 13.0 0.0000 22 162.0 0.0000
Total HpBDF 0.00000 260.0 0.0000 200.0 0.0000 88.0 0.0000 39 550.0 0.0000
Total OBDF 0.00100 0.0 0.0000 0.0 0.0000 7.0 0.0070 5275.0 5.2750
Air concentration 0.0000 0.3250 0.0370 128.2250
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
An occupational exposure limit has not been established for any
PBDD or PBDF, but a recent paper by Leung et al. (1988) provides
guidance. Applying a 100-fold safety factor to the no-observed-effect
level in animal studies, an occupational exposure limit of 0.2 ng/m3
is obtained. For other compounds that behave similarly to TeCDD in
animals tests, safety factors from 2 to 10 have been used to establish
the occupational exposure limit. Using a safety factor of 10, the
occupational exposure limit for TeCDD becomes 2 ng/m3. With the
exception of the two samples from the extruder die-heads, all of the
values from the BASF and BFRIP studies were below 2 ng/m3. The
studies also indicate that workers are unlikely to be routinely
exposed to levels higher than 0.2 ng/m3.
BASF has submitted to the US EPA a report of the biological
monitoring of 5 workers, who were employed in the extrusion of PBT
containing DeBDE as a flame retardant (BFRIP, 1990). The results
indicated blood concentrations of 2,3,7,8-TeBDF and 2,3,7,8-TeBDD of
100-500 ng/litre. All workers appeared in good health. The finding of
2,3,7,8-TeBDD in the workers was particularly surprising, since air
samples had not revealed the presence of brominated dioxins. These
findings have not yet been confirmed, but a follow-up study is in
progress.
Table 22. Summary of BASF and BFRIP air sampling testsa
Location Study Concentration (ng/m3
air) TCDD equivalent
Personnel-1 BFRIP 0.039
Personnel-2 BFRIP 0.015
Personnel-3 BFRIP 0.550
Room air BASF 0.000
Work station BASF 0.325
Bagging station BASF 0.037
Extruder head BASF 128.225
Fibre glass addition port BFRIP 0.110
Extruder die head BFRIP 12.816
Extruder vent port BFRIP 0.250
Feed hopper BFRIP 0.062
Guard gate BFRIP 0.000
aFrom: EBFRIP (1990).
Investigations by plastic producers have shown that PBDF and PBDD
can be formed, not only on combustion of plastics containing PBDE, but
also during the blending of the flame retardant into a polymer. This
has been substantiated by BASF, who measured the workplace atmosphere
around extruders.
4.4 Ultimate fate following use
4.4.1 General
For the ultimate fate of DeBDE following use, and its effects on
the environment, see section 6.1. of the General Introduction.
4.4.2 Exposure of the general population
No data are available concerning the direct exposure of the
general population to DeBDE.
In a study by Ranken et al. (1990) to investigate the possibility
of exposure to PBDD/PBDF from TV sets, three new TV sets were placed
in an 8.81 m3 test chamber. Sets A and B were purchased locally,
while set C was provided by the manufacturer. The rear portion of each
television set cabinet was constructed from polystyrene. The purchased
televisions were flame retarded with 11.5% DeBDE. Set C was made of
high-impact polystyrene, flame retarded with DeBDE/Sb203. In order
to determine background levels of PBDD/PBDF, air was pulled through
the empty chamber and through a sampler for 8 h/day, for 3 days. Two
purchased sets were placed in the chamber. Air was pulled through the
chamber for 8 h/day, for 3 days. The experiment was repeated for
another 3 days. The television set (C) was placed in the room for 3
days, but the television was not in operation. In all the described
experiments, no PBDD/PBDF were found. Finally, the air was monitored
for 24 h while the television set C was operating. No PBDD/PBDF were
found. On the basis of the limits of determination, PBDD/PBDF
emissions from the operating television sets can be calculated to be
less than 0.17-1.52 pg TeBDD/m3, 0.35-0.39 pg PeBDD/m3, 0.09-0.33 pg
TeBDF/m3, and 0.14-0.19 pg PeBDF/m3 of air. The authors concluded
that, in actual practice, these values would be lower by a factor of
10-100 because of the dilution effect of a normal room size and the
expected air turnover.
4.5 Fire accident
Bruckmann et al. (1989) reported about an accidental fire in a
stock-house in which, according to an inventory file, 2.5 tonnes of
flame retardants (a mixture of DeBDE and antimony trioxide (Traflam
PO 80)) was stored. Four wipe samples and 6 solid samples of partly
burnt material were taken several hours after the fire. At the time of
sampling, it was discovered that only a minor quantity of the bags
with the flame retardant had been in contact with the fire. The 4 wipe
samples contained tetra-, penta-, hexa-, hepta-, and
octabromodibenzofurans in the following concentrations; 4.0-123,
0.82-77.6, 4.5-51.7, 0.25-12.8, and 0.53-8.5 ng/m2. PBDD were not
found (limit of determination 1 ng/m2 per isomer). The PBDF contents
of the solid samples were low with the highest concentration at
1 µg/kg.
4.6 Simulated fire conditions
In the experiments conducted by the BFRIP, samples of high-impact
polystyrene (HIPS) and HIPS/DeBDE/Sb203 were burned in a Mass Burning
Rate Apparatus with 21% oxygen to simulate a real fire scenario. The
temperatures ranged from 500 to 800 °C. Soot and char were collected
and analysed for PBDD and PBDF (Table 23).
Table 23. Analytical results from combustion products of
HIPS/DeBDEa
Compound Concentration
Char Soot
(mg/kg) (mg/kg)
Brominated dibenzodioxins NDb NDb
Brominated dibenzofurans:
MBDF 0.64 556
DiBDF 0.54 641
TrBDF 0.23 352
TeBDF < 0.1 73
2,3,7,8-TeBDF < 0.1 1.8
PeBDF < 0.1 3.5
HxBDF-OBDF < 0.1 < 0.1
aFrom: McAllister et al. (1990); Pinkerton et al. (1989).
bND = Not detected.
A single study on a mixed range of PBDE, between HxBDE and
DeBDE, indicated little bioconcentration in carp with a
bioconcentration factor of < 4 after 8 weeks of exposure (CBC, 1982).
No PBDD or PBDF were found in the HIPS without flame retardant.
Low levels of bromodibenzofurans were found in the char of the
flame-retarded HIPS, however, the levels of brominated furans ranged
from 3.5 mg penta- to 641 mg dibromodibenzofurans/kg. No PBDD were
found in char and soot. A maximum of 1.8 mg/kg of 2,3,7,8-TeBDF was
present in soot (Pinkerton et al., 1989; McAllister et al., 1990).
4.7 Bioaccumulation
A bioconcentration study was carried out with rainbow trout under
static conditions. The concentration in the water was 20 µg
14C-DeBDE/litre. Fish were exposed for 0, 1/2, 1, 2, 4, 6, 12, 24, or
48 h. There was no measurable accumulation of DeBDE in flesh, skin, or
viscera (Brosier et al., 1972; Norris et al., 1973, 1974, 1975a).
A single study on mixed PBDE between HxBDE and DeBDE indicated
little bioconcentration in carp with a bioconcentration factor of <4
after 8 weeks of exposure (CBC, 1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Decabromodiphenyl ether was identified in 10 samples of air
collected in the vicinity of two manufacturing facilities in
concentrations ranging from 0.016 to 25 µg/m3. The compound was
present in air in the form of particulates (Zweidinger et al., 1977).
5.1.2 Water
DeBDE was determined in 15 water samples collected in Japan in
1977. DeBDE was not present in any of the samples (limit of
determination 0.2-2.5 µg/litre) (Environment Agency Japan, 1983).
DeBDE was not detected in 75 water samples (limit of determination
0.1 µg/litre) in 1987 and was not detected in 141 samples collected at
47 locations (limit of determination 0.06 µg/litre) in 1988-89 in
Japan (Environment Agency Japan, 1989, 1991).
Yamamoto et al. (1991) analysed water from the Kino River basin
for the presence of DeBDE. In 12 samples of river water, the
concentrations of DeBDE were all below 0.1 µg/litre water.
5.1.3 Aquatic sediments
DeBDE was determined in 15 sediment samples collected in Japan in
1977. DeBDE was not found in any of the samples (limit of
determination 25-870 µg/kg dry weight) (Environment Agency Japan,
1983).
Marine, estuarine, and river sediment samples were collected at
different places in Japan in the years 1981-83 and analysed for DeBDE.
The DeBDE occurred in 6 river and 1 estuarine sediment sample (7 out
of 15 samples) in the range of 20-375 µg/kg, during this period
(Watanabe et al., 1987b).
DeBDE was identified at 20 µg/kg (dry weight) in one sample of 3
estuarine sediment samples from Osaka but was not detected in samples
from Tokyo, Matsuyama, or Hiroshima (Watanabe et al., 1987b).
In 1987, an environmental survey was conducted concerning DeBDE in
sediment in Japan. DeBDE was detected in 16 out of 60 samples at
concentrations ranging from 10 to 1370 µg/kg (limit of determination
7 µg/kg dry weight) in 1987 and was detected in 39 out of 129 samples
collected at 43 locations at concentrations ranging from 4 to
6000 µg/kg (limit of determination 4 µg/kg dry weight) in 1988-89
(Environment Agency Japan, 1989, 1991).
A sample was collected using a dredger, from the upper sediment
layer of the Second Neya River in Osaka, Japan, in 1983. The sample
contained approximately 0.2 mg DeBDE/kg dry weight (Watanabe et al.
(1986).
Yamamoto et al. (1991) analysed 20 sediment samples collected from
the Kino River in Japan and found that DeBDE was present in all the
samples. The concentrations ranged from 0.003 to 11.6 mg/kg dry
weight.
Zweidinger et al. (1978) found DeBDE at levels ranging from not
detectable to 1 g/kg in sediment samples in the vicinity of a flame
retardant manufacturing facility in the USA.
DeBDE (limit of determination < 10 µg/kg) was found in sediment
in the vicinity of bromine facilities in El Dorado and Magnolia,
Arkansas (DeCarlo, 1979) and also in sludge samples from a
discharge-treatment zone of a DeBDE facility in Bayonne, New Jersey
(US EPA, 1988; IARC, 1990).
5.1.4 Aquatic and terrestrial organisms
One of 3 mussel samples, collected in 1981-85, from Osaka Bay,
Japan, contained 1.4 µg DeBDE/kg (wet weight basis) (Watanabe, 1987;
Watanabe et al., 1987a,b). DeBDE was not found in mullet, goby, sea
bass, horse mackerel, sardine, mackerel, or hairtail from this area or
in mussel, mullet, goby, sardine, mackerel, or hairtail from other
locations (limit of determination < 0.5 µg/kg). A total of 17 samples
were analysed.
In 1987, an environmental survey was conducted concerning DeBDE in
fish. It was not detected in 75 fish samples in 1987 and was not
detected in 138 fish samples collected at 46 locations in 1988-89
(limit of determination 5 µg/kg wet weight) (Environment Agency Japan,
1989, 1991).
5.2 Exposure of humans
5.2.1 Occurrence of DeBDE in human tissues
DeBDE was not found in 5 human adipose tissue samples obtained
from a hospital in Osaka in 1985-86. The limit of determination was
< 50 µg/kg fat (Watanabe, 1987; Watanabe et al., 1987a).
DeBDE was detected at concentrations of up to 5 µg/kg in 2 out of
40 samples of human hair obtained from barber shops in El Dorado and
Magnolia, Arkansas (where the chemical is manufactured) in 1978
(DeCarlo, 1979).
In the USA, Cramer et al. (1990a,b) studied PBDD/PBDF levels in
human fat tissue samples during 1987 (National Human Adipose Tissue
Survey). The 865 specimens were combined to form 48 composites based
on 9 census divisions and their age groups. The PBDD/PBDF analysis was
carried out using HRGC/HRMS. No PBDD/PBDF were found (detection limit
of 1-40 ng/kg, depending on congener). Identification of PBDE was
based on comparison of full scan mass spectra of the samples with
available standards, application of SIM techniques to compare
theoretical ion ratios with observed ion ratios, and measurement of
fragment losses from molecular ion clusters. Five samples were also
monitored for DeBDE. No DeBDE was detected in 2 out of 5 samples; a
weak DeBDE response was found in 1 out of 5 samples, and, in 2 out of
5 samples, DeBDE was estimated at 400 and 700 ng/kg, respectively.
5.2.2 Occupational exposure
Wipe samples collected during an industrial hygiene survey in a
DeBDE manufacturing plant in the USA, indicated that workers in the
reactor area were exposed to 3.6 mg DeBDE/cm2. Personal samples
collected from workers in the mill area indicated that the airborne
levels of DeBDE ranged from 0.08 to 0.21 mg/m3, as a 8-h
time-weighted average. Following a spill in the mill area, personal
airborne levels ranged from 1.3 to 1.9 mg/m3 (Bialik, 1982).
Human exposure to DeBDE occurs in the course of manufacture and
use. Surveys have determined employee time-weighted average exposures
of 1-4 mg/m3 in air with excursions up to 42 mg/m3 during short
tasks. More than 90% of the particles in air were smaller than 10 µm
in diameter. On the basis of its classification as a nuisance material
by OSHA, the recommended workplace environmental exposure level in air
is 5 mg/m3 (8-h time-weighted average for a 40-h week) (NTP, 1986)
(see also section 4.2).
Studies on worker exposure to PBDF/PBDD during the production of
polymers containing PBDE are given in section 4.3.3.2.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption and elimination
Three male and 3 female Sprague-Dawley rats were administered
orally 14C-labelled DeBDE suspended in corn oil. The elimination in
urine and expired air was measured at 24-h intervals over a 16-day
period. Less than 1% was found in urine and expired air. The principal
route of elimination was via the faeces. Within 24 h, 90.6% of the
14C activity was excreted and all the 14C was accounted for by day 2
of the study. The data indicate that there is minimal absorption of
DeBDE by the gastrointestinal tract (Norris et al., 1973, 1974,
1975a,c; Dieter, 1979).
As a supplement to the carcinogenicity studies (sections 7.3 and
7.7), additional experiments were conducted to quantify DeBDE
absorption from the gastrointestinal tract of male rats and to
determine the effect of dose on absorption. Radiolabelled 14C-DeBDE
(97.9-99.2%) was diluted with unlabelled DeBDE to yield the desired
concentrations. DeBDE was mixed in the diet at approximate
concentrations of 250, 500, 5000, 25 000, or 50 000 mg/kg, or was
administered by intravenous injection. Animals were preconditioned by
being fed diets containing the respective dose of unlabelled DeBDE for
7 days before being fed diets containing 14C-DeBDE for 1 day, then
being returned to diets containing unlabelled material for the
remainder of the holding period. Results indicated that, after
exposure to all doses in the diet, more than 99% of the radioactivity
recovered was eliminated in the faeces within 72 h. Excretion in the
urine accounted for approximately 0.01% or less of the dose. The high
14C-DeBDE content of the gastrointestinal tissues was attributed to
intimate contact with the diet. Concentrations of DeBDE in other
tissues were near the limits of determination.
After an intravenous dose of 1 mg/kg, 61% of the recovered
radioactivity was eliminated in the faeces in 72 h and approximately
0.1% in the urine.
Estimates indicate that 0.33% ± 0.19% of the 50 000 mg/kg dose was
absorbed. Data for the 25 000 mg/kg diet showed that the percentage of
the dose absorbed was not significantly different from that of the
highest dose. Radioactivity present in the lives following exposure in
the diet was confirmed as DeBDE. However, the minimal absorption of
DeBDE from the gastrointestinal tract, and, presumably, from other
potential routes of exposure may explain the low toxicity of DeBDE
(NTP, 1986; El Dareer et al., 1987).
6.2 Distribution
The distribution of radioactivity was measured in various tissues
by Norris et al. (1973, 1975a,c). On day 16, no 14C-activity was
found in the tissues, with the exception of 0.01% of the administered
dose/g in the adrenal glands and 0.06% in the spleen.
In studies in which concentrations of 14C-labelled DeBDE of
250-50 000 mg/kg diet were fed to male Fischer 344 rats, more than 99%
of the radioactivity was recovered in the faeces and intestinal
contents (see section 6.1). The liver contained approximately 0.5% of
the consumed dose, 24 h after feeding, and this declined to 0.016%
after 72 h. Labelled material was extracted from the liver and found
to be mainly unchanged DeBDE. Trace amounts of label were found in the
kidneys, spleen, lung, brain, muscle, fat, and skin. Seventy-two hours
following an intravenous dose of "C-labelled DeBDE, the faeces and gut
contents contained 74% of the dose, suggesting significant binary
excretion. Of the extracted faecal label, 63% were metabolites of
DeBDE and 37% unchanged DeBDE. Labelled materials were found in the
liver, kidneys, and lungs and in lower concentrations in muscle, skin,
and fat (NTP, 1986; El Dareer et al., 1987).
Rats were administered DeBDE in the diet at 0, 25, or 50 g/kg to
determine the concentrations of DeBDE in the liver. The mean
concentrations in the livers were 0.21 ± 0.43 (9), 2.09 ± 0.89 (8),
and 8.6 ± 2.83 mg/kg liver (20), respectively. The number of livers
studied is given in brackets (Rogers & Hill, 1980) (no details
available).
6.3 Retention and turnover
A 2-year tissue accumulation study on the rat was carried out.
Groups of 3 male and 3 female Sprague-Dawley rats were maintained on
diets providing 0, 0.01, 0.1, or 1.0 mg DeBDE (FR-300 BA)/kg body
weight per day for designated periods of time. The tissues/organs
analysed for total bromine content were serum, adipose tissue, liver,
kidneys, skeletal muscle, and testes (see section 7.3.1.2). Interim
results after 10, 30, 90, 180 days, and 12 and 18 months of exposure
showed that the total bromine values in serum, liver, kidneys,
skeletal muscle, and testes were comparable with the control values.
The mean total bromine content of adipose tissue showed a time- and
dose-related (significant) increase, especially in the groups
receiving 0.1 and 1.0 mg DeBDE/kg per day. Although there was a
continuing increase in total bromine content, the concentrations in
adipose tissue of rats ingesting 0.01, and 0.1 mg DeBDE/kg per day for
23-24 months were 2.8 ± 0.9, and 7.5 ± 3.0 mg/kg, respectively,
compared with 2.0 ± 0.2 mg/kg for the control rats. In the liver, the
concentrations for the controls and the rats treated with 1.0 mg/kg
were 2.9 ± 0.2 and 5.9 ± 1.1, respectively. In serum, after 23-24
months, the concentrations for the 4 groups were 8.0 ± 1.2 (control),
9.1 ± 1.1 (0.01 mg/kg), 7.9 ± 0.6 (0.1 mg/kg) and 8.1 ± 1.0
(1.0 mg/kg) (Norris et al., 1974, 1975a; Kociba et al., 1975, 1975a).
The elimination of bromine from tissues was studied in male
Sprague-Dawley rats maintained for 90 days on a diet providing a dose
of 1.0 mg DeBDE/kg per day, and then on a control diet. Four rats out
of each group were sacrificed on the last day of exposure or after a
recovery period of 0, 10, 30, 60, or 90 days. Serum, kidneys, adipose
tissue, and liver were analysed. The low level of total bromine
accumulated in adipose tissue remained unaffected during 90 days of
recovery. Bromine was cleared from the liver within 10 days of
recovery (Norris et al., 1974, 1975a,c).
The half-life for the disappearance of 14C-activity from the body
of DeBDE-treated rats was less than 24 h. The principal route of
elimination was the faeces. No sex-related differences were found.
14C-activity was rapidly cleared in DeBDE-treated rats.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
The toxicological information on DeBDE has been summarized in
several publications including: Norris et al. (1973, 1974, 1975a,b,c),
AIHA (1981), and NTP (1986).
7.1 Single exposure
7.1.1 Oral: Rat
Intragastric intubation of single doses of 126-2000 mg commercial
DeBDE/kg body weight, as a 10% corn oil suspension, to female
Sprague-Dawley rats (Spartan strain), did not produce any indications
of toxicity or gross pathological changes during a 14-day period
(Norris et al., 1973, 1974, 1975a,c).
Groups of 5 male Spartan rats received single oral doses of up to
5 g/kg body weight, by gavage, as suspensions in corn oil. No deaths
occurred and the animals showed normal weight gain during a 14-day
observation time. The acute oral LD50 in rats was >5 g/kg body
weight (Great Lakes Chemical Corporation, undated b).
7.1.2 Dermal Rabbit
Commercial DeBDE was applied and to the occluded, clipped, intact
skin of 2 male and 2 female New Zealand white rabbits, each at a
dosage of 200 or 2000 mg/kg body weight, for 24 h. Observation time
was 14 days. No mortality occurred. The acute dermal LD50 in rabbits
was >2 g/kg body weight (Great Lakes Chem. Corp., undated b).
7.1.3 Inhalation: Rat
Groups of 10 male and 10 female Spartan rats were exposed for 1 h
to concentrations of commercial DeBDE of 2 or 48.2 mg/litre air and
subsequently observed for 14 days. All rats survived. At 2 mg/litre,
salivation was noted in 2 rats on the first day, but, from there on,
all the rats in this group appeared normal, except one with
respiratory difficulties and another with ocular discharge during the
observation period. At 48.1 mg/litre, eye squint and increased motor
activity were noted in the animals up to day 4. Respiratory
difficulties were noted in 2 rats on days 3-6 and in one rat on day 8
and one on day 7. A few rats showed eye squint and ocular discharge on
days 7-12. All rats were normal on day 14. The acute inhalation LD50
in rats was >48.2 mg/litre (Great Lakes Chemical Corporation,
undated b).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Mouse
In a 14-day study, groups of 5 B6C3F1 mice, of each sex, were
exposed to DeBDE (99%) in the diet at concentrations of 0, 20, 50, or
100 g/kg. No effects on health, survival, or body weights were
observed and no compound-related clinical signs or gross pathological
effects were reported (NTP, 1986).
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were administered DeBDE (two lots of DeBDE were used; one of 99% and
the other > 97%) in the diet at 0, 31, 62, 12.5, 25, or 50 g/kg, for
13 weeks. No evidence was found for compound-related effects on
physical appearance, behaviour, food consumption, body weight gain,
survival, and gross and microscopic pathology. This study was used to
establish the dose levels for the long-term study (Rutter & Machotka,
1979; NTP, 1986).
7.2.1.2 Rat
In 3 separate, 28-day feeding studies, each with groups of 10 male
and 10 female Charles River CD rats, commercial DeBDE was administered
at 0, 100, or 1000 mg/kg diet. The rats were observed for appearance,
mortality, food consumption, body weight gain, and organ weights.
After sacrifice, gross pathological and microscopic examinations of
the liver, kidneys, and thyroid were carried out. Adverse effects or
lesions associated with DeBDE administration were not found in these 3
rat studies. The total bromine contents of the liver, and adipose
tissue were determined and a slight increase in bromine concentration
was found at the highest dose level (Great Lakes Chemical Corporation,
undated b) (no details available).
Groups of 5 male Sprague-Dawley rats were administered a diet
containing 0, 0.01, 0.1, or 1.0% [equivalent to 0, 8, 80, or 800 mg
DeBDE (77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE)/kg body weight per
day], for 30 days. No influence on food intake or body weight gain was
found. No haematological changes were observed in the 8 and 80 mg/kg
dose groups, but, at 800 mg/kg, there were decreases in packed cell
volume and red blood cell count. Urinalysis parameters were not
affected at any dose level. No differences in heart, testes, brain,
and kidney weights were observed. Enlarged livers were found in rats
exposed to 80 and 800 mg DeBDE/kg. The liver lesions consisted of
centrolobular cytoplasmic vacuolization. Furthermore, in the rats
receiving 80 and 800 mg/kg, hyaline degenerative cytoplasmic changes
in the kidneys and thyroid hyperplasia were found. At a dietary level
of 0.1 g/kg (equivalent to 8 mg/kg body weight), no treatment-related
changes in the liver or thyroid, or other changes were found. (Remark:
limited histopathology was carried out) (Sparschu et al., 1971; Norris
et al., 1973, 1974, 1975a; Kociba et al., 1975a).
In a 14-day study with DeBDE (99%) administered in the diet to
Fischer 344/N rats at concentrations of 0, 5, 20, 50, or 100 g/kg, no
effects were seen on health, survival, and body weight, and no
compound-related clinical signs or gross pathological effects were
observed. Similarly, no toxic effects were seen in a 90-day study in
which doses of DeBDE (>97%) of 0, 3.1, 6.2, 12.5, 25, or 50 g/kg were
administered in the diet. No effects on survival, body weight, or feed
consumption were observed. No gross or microscopic effects were
reported. Liver weights were not recorded in these studies, but, in
subsequent studies, liver weights were significantly increased in
Fischer 344/N rats at dose levels of 25 and 50g DeBDE (92%)/kg diet,
for 14 days (NTP, 1986).
7.2.2 Inhalation
7.2.2.1 Rat
Pulmonary tissue response and pulmonary clearance of commercial
DeBDE were evaluated following intratracheal administration of the
dust to rats. The study was conducted to evaluate the possible health
hazards for man from inhalation of the dust generated during the
production and handling of DeBDE. Fifty male, Sprague-Dawley rats were
given an intratracheal injection of 20 mg DeBDE (77.4%) dust (length
mean diameter 2.65 µm, surface mean diameter 2.91 µm, and volume mean
diameter 3.17 µm) suspended in 1 ml of rat serum. A control group of
35 rats received only the serum vehicle. The rats were observed for
appearance, demeanour, and body weights. Groups of 5 treated and 2
control rats were killed on days 3, 10, 30, 91, and 365 for
determination of total bromine content in the lungs. The calculated
DeBDE equivalent values were used to determine the half-life of DeBDE
in lungs, which was determined to be 150 days. To assess the
respiratory tissue response, gross and histopathological examinations
were conducted on rats killed on days 10, 30, 416, and 556 and on rats
that died. No untoward effects were observed, except on days 10 and
556 (but not on days 30 and 416): the lungs of treated rats contained
scattered focal aggregates of alveolar macrophages showing clear,
angulated, cytoplasmic vacuoles or spaces, which probably represented
the location of the dust particles. A very slight focal thickening of
the interalveolar septae was noted in 2 out of 5 rats. Particles were
not present in the regional lymph nodes. No evidence of fibrosis or
other proliferative response was detected in the lungs or regional
lymph nodes (Jersey et al., 1976).
7.3 Long-term exposure
7.3.1 Oral
7.3.1.1 Mouse
A two-year study was carried out on B6C3F1 mice. The animals were
administered DeBDE in the diet at concentrations of 0, 25, or 50 g/kg,
for 113 weeks (NTP, 1986) (for details of the study see section
7.7.1.1).
7.3.1.2 Rat
A long-term/carcinogenicity study was carried out on
Sprague-Dawley rats administered daily doses of 0, 0.01, 0.1, or
1.0 mg DeBDE/kg body weight in the diet for 2 years (Kociba et al.,
1975, 1975a; Norris et al., 1975a,b). In another 2-year study, Fischer
344 rats were administered DeBDE at 0, 25, or 50 g/kg diet (NTP, 1986)
(for details of these 2 studies see section 7.7.1.2).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
Skin irritation studies conducted with commercial DeBDE, applied
as a dry solid (500 mg) on the shaved skin (under occlusion) of 2
groups of 3 New Zealand white rabbits caused no irritation on intact
skin and no, or only slight, erythematous and oedematous responses on
abraded skin, after a single exposure for 24 h and an observation
period of 72 h. A comparable study on 3 male and 3 female New Zealand
white rabbits in which 500 mg DeBDE was applied on intact or abraded
skin showed no skin irritation. Repeating the exposures of intact skin
for 5 days/week for 2 weeks or to abraded skin for 3 days did not
alter the response (Norris et al., 1973, 1974, 1975a,c; Great Lakes
Chemical Corporation, undated c).
7.4.2 Eye irritation
Eye irritation studies on 3 male and 3 female New Zealand White
rabbits showed that 100 mg Saytex 102/eye, applied as a dry solid,
caused only transient irritation (redness and chemosis) of the
conjunctival membranes. The cornea, iris, and lens were unaffected
(sodium fluorescein and UVR were used). After 24 h, the eyes had
recovered. The observations were made at 1, 24, 48, 72 h, and 7 days
after treatment (Norris et al., 1973, 1974, 1975a,c; Ethyl Corp.,
1986; Mallory et al., 1986; Great Lakes Chemical Corporation,
undated c).
7.4.3 Sensitization
Repeated application of a suspension of 5% DeBDE (77.4% DeBDE,
21.8% NBDE, 0.8% OBDE) in petrolatum, 3 times a week for 3 weeks, to
the skin of 50 human volunteers did not result in skin sensitization
reactions during the sensitizing period or, on challenge 2 weeks
following the last application. Skin irritation was observed in 14 out
of the total 450 applications (11 of the reactions were classified as
very slight and 3 as mild erythema). These reactions were seen in 9
out of the 50 persons (Norris et al., 1974, 1975a,c; Dow Chemical
Comp. USA, 1978).
7.4.4 Chloracnegenic activity
Chloracnegenic activity was studied on the ear of each of 4 New
Zealand White male and female rabbits. The test material (Saytex 102)
was administered once daily at 0.1 ml/day, 5 times per week, for 4
weeks, at concentrations of 1, 10, 100, or 1000 g/kg, in chloroform.
Observations were recorded prior to the initial dose and at 7, 14, 21,
and 28 days of dosing. Saytex 102 as a 10% chloroform solution caused
a slight erythematous response and slight exfoliation, but no
chloracne was observed during the study (Naismith & Matthews 1981).
In the period 1971-74, approximately 40 samples of DeBDE (pilot
plant samples), mother liquor, mother liquor still pot residue, and
still bottom samples were studied for their chloracnegenic activity.
In these studies, the samples (0.1 ml) were applied as such, or as a 5
or 10% solution in chloroform, on the rabbit ear, 5 days per week for
4 weeks. The samples of DeBDE did not induce any responses, but
responses to the mother liquor and still bottom samples were positive,
except in a few cases where the result was equivocal (Rampy, 1971-74).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
7.5.1 Reproductive toxicity
A one-generation reproduction study was performed in which 10
male, and 20 female, Sprague-Dawley rats at the two lower dose levels
(3 and 30 mg/kg body weight) and 15 male and 30 female rats at the
higher dose level (100 mg/kg body weight) were given commercial DeBDE
with the diet (weekly adjustment), for 60 days prior to mating, 15
days during mating, and subsequently throughout gestation and
lactation. Twenty male and 40 female rats served as controls. No signs
of toxicity were observed in the adult rats or the neonates during the
study or at necropsy. Unaffected parameters included body weight gain
and food consumption by adults, reproductive parameters (the
percentage pregnant and neonatal growth, survival, and development),
preterminal urinalysis and clinical chemistry in adult rats, gross
examination of all adult and weanling rats, and microscopic
examination of tissues from both age groups. No cytogenetic changes
were observed in the bone-marrow of parents and weanling rats. Thus,
no toxicological manifestations were associated with the ingestion of
100 mg DeBDE/kg in this reproduction study (Norris et al., 1975c;
Schwetz et al., 1975).
7.5.2 Teratogenicity
Pregnant female rats were given 0, 10, 100, or 1000 mg commercial
DeBDE/kg body weight, suspended in corn oil, by intragastric gavage,
on days 6-15 of gestation.
Maternal food consumption and body weight did not differ from
those of the controls. Liver weights of the mothers were comparable
with those of the control animals. The position and number of fetuses
in utero, the number of corpora lutea, individual pup weight,
crown-rump length, and sex ratio, were similar to those of the
controls.. A significant increase in resorptions was found at low dose
levels, but not at the high dose level. No gross external
abnormalities were seen in the fetuses of dams treated at any dose
level. Soft tissue and skeletal examinations revealed an increased
number of litters with subcutaneous oedema and delayed ossification of
bones of the skull of fetuses of dams treated with 1000 mg/kg, but not
with 100 mg/kg body weight. Analysis of maternal and fetal livers for
total bromine revealed a significantly increased concentration in
maternal livers of rats treated with 1000 mg/kg. Treatment with
100 mg/kg or less did not produce any increase in total bromine
contents. No increase in total bromine contents was observed in the
livers of fetuses from dams receiving any of the dose levels of DeBDE
(Norris et al., 1973, 1974, 1975a; Hanley, 1985; US EPA, 1989).
7.6 Mutagenicity and related end-points
7.6.1 Mutation
A technical product, HFO 102, was tested in a Salmonella
typhimurium assay with the strains TA 98, TA 100, TA 1535, and TA
1537 with, or without, metabolic activation. The HFO 102 was not
completely soluble in DMSO at a concentration of 10 mg/litre. However,
the suspension was used for the test and for preparation of the
solutions. The dose levels that were tested were 0.4, 4.0, 40, and
1000 µg/plate. No evidence for mutagenic activity was found (Shoichet
& Ehrlich, 1977).
Results of studies with Saccharomyces cerevisiae with, or
without, liver microsomal enzyme preparations, were also negative (no
details) (Great Lakes Chemical Corporation, undated b).
Commercial DeBDE in concentrations of 100-10 000 µg/plate was not
mutagenic in Salmonella typhimurium, TA 100, TA 1535, TA 1537, and
TA 98 strains, in the presence, or absence, of an exogenous metabolic
system from Aroclor 1254-induced male rat liver S9 and male Syrian
hamster liver S9 (NTP, 1986).
7.6.2 Chromosomal effects
Cytogenetic examination of bone marrow cells, taken at necropsy
from the femur of the parent animals from a reproduction study (see
section 7.5.1) as well as from the neonates at weaning, showed no
increase in cytogenetic aberrations compared with the controls (Norris
et al., 1975c) (no details).
Commercial DeBDE was not mutagenic in the mouse lymphoma
L5178Y/TK+/- assay in the presence, or absence, of Aroclor
1254-induced male F344 rat liver S9. Tests for cytogenic effects in
Chinese hamster ovary cells indicated that commercial DeBDE does not
cause chromosomal aberrations or sister-chromatid exchanges in either
the presence or absence of S9, prepared from livers of Aroclor
1254-induced male Sprague-Dawley rats (NTP, 1986).
7.7 Carcinogenicity
7.7.1 Oral
7.7.1.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (nine weeks old) were
fed 0, 25, or 50 g DeBDE (purity 94-99%; no brominated dioxins or
furans were found)/kg diet for 103 weeks; all survivors were killed in
weeks 112-113. The average daily consumption of DeBDE was estimated to
be 3200 mg/kg for low-dose and 6650 mg/kg for high-dose male mice and
3760 mg/kg for low-dose and 7780 mg/kg for high-dose female mice. Body
weights and survival of treated animals were comparable with those of
the controls. Non-neoplastic lesions observed in treated mice were
granulomas in the liver of low-dose males and hypertrophy in the liver
of low- and high-dose males. The combined incidence of hepatocellular
adenomas and carcinomas was significantly increased in males: control
8/50, low-dose 22/50, and high-dose 18/50 (but not increased in
comparison with historical control groups); the combined incidence of
thyroid gland follicular-cell adenomas and carcinomas was increased,
but not significantly, in treated males: control 0/50, low-dose 4/50,
high-dose 3/50; females: control 1/50, low-dose 3/50, high-dose 3/50.
Follicular-cell hyperplasia of the thyroid gland was increased in both
groups of treated male and female mice (NTP, 1986; Huff et al., 1989).
7.7.1.2 Rat
Groups of 25 male, and 25 female, Sprague-Dawley rats, 6-7 weeks
of age, were fed 0, 0.01, 0.1, or 1.0 mg DeBDE/kg body weight (purity
DeBDE 77.4%, nonabromodiphenyl ether 21.8%, octabromodiphenyl ether
0.8%) in the diet for 100-105 weeks. Additional groups of 10-34
rats/sex per dose level were killed at various times during the study
to investigate the accumulation of total bromine in the tissues.
Ingestion of up to 1.0 mg DeBDE/kg did not influence survival rates;
appearance, mean body weights, feed consumption, haematology,
urinalysis, clinical chemistry, and organ weights of treated groups
were similar to those of controls. No discernible toxicological
effects were produced by DeBDE and no significant differences in the
number of rats developing tumours, the total number of tumours, or the
specific type of tumours were observed between treated and control
groups, when evaluated by the Fisher's exact probability test. Adrenal
phaeochromocytomas were the most frequent tumours in the male rats in
all groups. During the study, there was a slight build up of the total
bromine content in the tissues of rats ingesting the 2 highest dose
levels, as measured by neutron activation analysis. However, the
source of bromine may, or may not, have been DeBDE, because NBDE and
OBDE were also present in the product. Serum, muscle, and kidneys did
not show any increase in total bromine content. In the liver,
low-level, steady-state conditions were attained by 12 months. Adipose
tissue showed a time- and dose-related increase in total bromine
content, subsequent to ingestion of 0.1 or 1.0 mg/kg. The total
bromine content of adipose tissue of rats ingesting 0.01 mg/kg for 2
years was 2.8 ± 0.9 mg/kg compared with a control value of 2.0 ±
0.2 mg/kg (section 6.3) (Kociba et al., 1975, 1975a; Norris et al.,
1975a,b). [The IARC Working Group (1990) noted the very low dose
levels used].
Groups of 50 male, and 50 female, Fischer 344/N rats, 7-8 weeks of
age, were fed DeBDE (purity 94-99%; no brominated dioxins or furans
were found) at 0, 25 or 50 g/kg diet, for 103 weeks; all survivors
were killed in weeks 111-112. The average daily consumption of DeBDE
was estimated to be 1120 and 2240 mg/kg for low-dose and high-dose
male rats, respectively, and 1200 and 2550 mg/kg for low-dose and
high-dose female rats, respectively. Body weights of treated rats were
not significantly different from those of the controls. Thrombosis and
degeneration of the liver, fibrosis of the spleen, and lymphoid
hyperplasia were observed in high-dose male rats. Significant
increases were observed in the incidence of neoplastic nodules of the
liver (adenomas) in treated males: control 1/50; low-dose 7/50;
high-dose 15/49 and females: control 1/50; low-dose 3/49; high-dose
9/50. No differences in the incidence of hepatocellular carcinomas
were seen among the groups. The incidence of acinar-cell adenomas of
the pancreas in males was: control 0/49; low-dose 0/50; high-dose
4/49. The difference between the controls and the high-dose group was
not significant. A high incidence of mononuclear-cell leukaemia was
observed in treated and control rats of both sexes (NTP, 1986; Huff et
al., 1989).
7.8 Other special studies
7.8.1 liver
Carlson (1980a) administered commercial DeBDE (in corn oil), by
gavage, at a dose of 0.1 mmol/kg body weight to male Sprague-Dawley
rats (200-250 g) for 14 days. Twenty-four hours after the last
(seventh) dose, the liver/body weight ratio increased. Koster et al.
(1980) found no evidence of a porphyrinogenic action after exposure of
cultures of chick embryo liver cells to a concentration of 5 µg DeBDE
(in DMSO)/ml medium with, or without, pretreatment with
naphthoflavone, an inducer of P450, P448, and of delta-levulinic acid
synthethase.
A sample of DeBDE, obtained from the NTP repository, was
administered by gavage (vehicle not clear) at 1500 mg/kg body weight
to 9, three-month-old, female Sprague-Dawley (CD) rats, 21 h and 4 h
before they were killed. No changes were observed in hepatic
cytochrome P450 content, hepatic DNA damage, as determined by alkaline
elution, hepatic ornithine decarboxylase activity, or serum alanine
aminotransferase activity (Kitchin et al., 1992).
7.8.2 Miscellaneous
Hefner (1973) studied, in an in vitro model, the rupture of
erythrocyte membranes (haemolysis) induced by dust particles. The test
approximates the in vivo rupture of the secondary lysosomal
membranes. Fibrogenic dusts are capable of rupturing the erythrocyte
membrane, while non-fibrogenic dusts are not. On the basis of the
in vitro model, DeBDE of the particle size studied (shown by optical
microscopy to have a 2.65 µm number length mean diameter with 94.5% of
the particles being 3.77 µm and below) appears to be non-fibrogenic
(only 0.1% haemolysis) at 115 mg dust/ml suspension and time from 15
to 120 min.
7.8.3 Toxicity of soot, char, and other waste products from
combustion of DeBDE-containing polymers
7.8.3.1 Acute oral toxicity
Groups of 5 male, and 5 female, Sprague-Dawley rats were
administered a mixture of soot and char (61/39%), generated from the
combustion of high-impact polystyrene, as a single dose, by garage at
0(vehicle), 0.5, 5.0, 50, 500, or 2000 mg/kg body weight. These
amounts were given in 10 ml 1% methyl cellulose/kg body weight.
Observation time was 28 days. Body weight gain, mortality, and weights
of 10 organs were studied, and these organs in the control and 2000 mg
groups were also examined microscopically. No overt signs of toxicity
were observed. The acute oral LD50 was >2 g/kg body weight (Fulfs &
Dahlgren, 1987a).
The acute toxicity of the combustion products of a matrix composed
of high-impact polystyrene, DeBDE, and antimony trioxide was
investigated. Six dose groups of 5 male and 5 female rats were
treated, by gavage, with 0, 0.5, 5, 50, 500, or 2000 mg/kg of combined
soot and char, generated from the combustion of the flame retarded
high-impact polystyrene (HIPS) suspended in 1% methyl cellulose and
observed for 28 days. No animals died during the course of the study
and no clinical signs of toxicity were observed. No histological
lesions were detected in the following organs examined: thyroid,
parathyroid, and adrenal glands, spleen, gonads, heart, liver, lung,
brain, kidneys, and thymus. The LD50 was > 2000 mg/kg body weight
(Fulfs & Dahlgren, 1987b; Pinkerton et al., 1989).
Undiluted solids from mother liquor (waste tars) were tested for
acute oral toxicity in rats. No acute oral lethality was seen with
0.126 g/kg body weight in corn oil, but 100% mortality was found, with
0.5 g in water. The animals displayed tremors (Norris, 1971).
7.8.3.2 Skin irritation and comedogenicity
Soot and char generated from the combustion of high-impact
polystyrene retarded with, or without, DeBDE and Sb2O3 were tested
for their comedogenicity using a New Zealand albino rabbit ear
bioassay (Draize, 1979). The soot and char samples were mixed in
different ratios. The dose levels were; 0.001, 0.003, 0.005, 0.008,
0.01, 0.03, 0.05, 0.08, and 0.1 g. The highest dose level (0.1 g) of
the mixture was actually in excess of the amount that remained on the
ear. The materials were applied in 0.1 ml of water. Five male rabbits,
with 2 healthy ears each, were randomly selected for these studies and
the original design required 10 exposure levels, 1 per ear. Dosing was
continued for 5 consecutive days. Ears were checked to grade the
erythema on day -1 and day 0, and then daily, and the final grading
was done on the day following the last dosing. The results showed
that, without flame retardants, very slight to well defined erythema
occurred with doses of 0.005 g and higher. In cases where the polymer
was flame retarded, the effect was stronger, very slight to
moderate/severe erythema occurred with doses of 0.001-0.003 g or more
(see Table 24) (Fulfs 1987a,b).
Table 24. Skin irritation by soot or char generated from the combination of
HIPS with, or without, DeBDE
Soot/char Mixture Dose Erythema score
soot/char
High impact 61/39% 0.005 g and score 1-2 (increasing
polystyrene higher with dose level from
day 2 onwards)
High impact 47/63% 0.003 g and score 1-3 (increasing
polystyrene with higher with close level, from
DeBDE and antimony day 1)
trioxide
0.001 g and same result, increasing
higher from day 3
Two groups of four (mainly 2 male and 2 female) New Zealand white
rabbits were used to test a mixture of soot and char (61/39%),
generated from the combustion of high-impact polystyrene treated
without flame retardants, in a rabbit ear comedogenicity bioassay.
Daily dose levels of 2, 5, 8, 20, or 50 mg of the mixture were
administered. Each daily dose was rubbed with 0.1 ml of water on the
inner surface of the pinna of one ear of the respective rabbit. The
animals were dosed 5 days per week for a total of 4 weeks. The total
cumulative dose levels were 40, 100, 160, 400, and 1000 mg. The ears
were graded for irritation (Draize test) and for hyperkeratosis (Adams
test). During the study, dermal irritation was found in all treated
groups with a score of 1-2. No comedogenic (score 0) responses were
observed on any of the ears. A slight increase in hyperkeratosis of
the sebaceous follicles was seen during histopathological examination
of the skin in the animals treated with the 2 highest dose levels. No
evidence of overt toxicity was seen among any of the animals tested
(Fulfs & Dahlgren, 1987c; Pinkerton et al., 1989).
A similar study was carried out with a combined soot and char
(47/53%) mixture, generated from the combustion of HIPS flame retarded
with DeBDE and antimony trioxide. The results were comparable with
those of the combustion products of HIPS without flame retardants.
There was no microscopic evidence of any significant hyperkeratotic
response (Fulfs & Dahlgren, 1987d).
Undiluted solids from mother liquor (waste tars) were tested for
skin irritation in rabbits. Slight skin irritation was observed at a
dose of 1.1 g/day for 3 days (Norris, 1971).
7.8.3.3 Eye irritation
Solids from mother liquor (waste tars) were tested for eye
irritation. The material is a mixture of brominated DeBDE (Br = 7-10).
A dose of 0.1 g of the mixture caused severe eye irritation and slight
corneal injury (Norris, 1971).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available.
8.2 Occupational exposure
8.2.1 Skin sensitization
Human volunteers (80 males and 120 females) were treated with 9
induction patches of 2 batches of DeBDE, identified as DBDO-1 and XD
8186.02. The first sample was evaluated as such, and the second as a
2% (w/v) aqueous solution. The upper arm was used in the males and the
upper back in the females. The patches were applied once every 2 days
and the substance left in contact with the skin for 24 h, after which
it was removed. After the application of 9 patches, there followed a
non-patching period of 12 days, after which the challenge patch was
applied to detect sensitization. A new skin site was used for this
24-h patch. After removal of the patch, reactions were observed after
24 and 48 h. This study did not reveal any evidence of skin
sensitization with either test material in any of the subjects
(Industrial Bio-test Laboratories, 1975).
8.2.2 Neurotoxicity
A health assessment of workers exposed to polybrominated biphenyls
and polybromodiphenyl ethers, e.g., DeBDE, during manufacture revealed
a higher than normal prevalence of primary hypothyroidism and a
significant reduction in sensory and fibula motor velocities; no other
dermatological or neurological effects were seen. The authors could
not conclude whether these effects were caused by DeBDE or by PBB,
which was also produced (earlier) in the plant. DeBDE was not detected
in the serum of the workers (Bahn et al., 1980).
8.2.3 Epidemiological studies
The health of workers in facilities where flame retarded polymers
were extruded has been evaluated in 3 studies, 2 at Celanese and 1 at
General Electric (BFRIP, 1990).
A study was conducted by Celanese Corporation in the late 1970s at
their Bishop, Texas, facility. The primary purpose of this study,
conducted by Tabershaw Associates, was to investigate the possible
effects of employee exposure to formaldehyde. However, brominated
flame retardants have been used at the facility since 1970, and,
therefore, significant effects resulting from the use of these
materials might also have been revealed by the study. No effects that
might be attributable to the use of the brominated flame retardants
were observed (EBFRIP, 1990).
A more complete study was conducted by General Electric Plastics
at their Mt. Vernon, Indiana, facility early in 1988. In the
conclusions of the study, it is stated that "there appears to be no
evidence that the workers in the study zone display any symptoms
associated with exposure to dioxins or related substances. More
critically, as a statistical group, the general medical examinations
for these workers compare very favorably with the examination for the
workers in the control zone or with published values for the general
population". It is finally concluded that "the study gives reasons to
continue to be observant but no reason to be alarmed" (EBFRIP, 1990).
A comprehensive medical evaluation of workers involved in the
processing of polymers containing brominated flame retardants was
undertaken at the BASF facility in Ludwigshafen, Germany. In this
study, some 40 potentially exposed workers and an equivalent number of
workers from a control group were subjected to complete medical and
psychological examination. The health of the workers compared
favourably with workers in the control zones. None of the workers
displayed symptoms associated with exposure to dioxin or related
substances, therefore, it was concluded that the workers did not show
any symptoms that could be associated with PBDD or PBDF emitted during
the polymer processing. It should be kept in mind that there might
also be exposure during the formulation of PBDE- containing polymers
and from products made of these polymers. Furthermore, exposure to
dusts may also occur (EBFRIP, 1990).
Zober et al. (1992) carried out a morbidity study of extruder
personnel with potential exposure to brominated dioxins and brominated
furans. The presence of PBDF/PBDD in air was established through
air-monitoring during the extrusion blending of
polybutyleneterephthalate with DeBDE. Biomonitoring results of 42
workers (exposed during the period 1975-88) and immunological tests
for exposed and 42 control employees were presented. Among potentially
exposed men, 2,3,7,8-TeBDF/TeBDD concentrations in blood lipid ranged
from nd to 112 and from nd to 478 ng/kg, respectively. The control
workers had concentrations of 7 and 7-48 ng/kg respectively. Results
of the immunological studies showed that the immune system of exposed
workers was not adversely affected at these burdens of TeBDF/TeBDD for
up to 13 years.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Marine unicellular algae, Skeletonema costatum, Thalassiosira
pseudonana, and Chlorella sp. were exposed to commercial DeBDE in 6
algal growth media. The duration of the exposure was 72 h for
S. costatum and T. pseudonana and 96 h for Chlorella sp. The
population density was estimated by cell counts using a
haemocytometer. Inhibition by 1 mg DeBDE/litre acetone was less than
50% in all species (Walsh et al., 1987).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation by IARC (1990) concluded that there is limited
evidence for the carcinogenicity of DeBDE in experimental animals. No
data were available from studies on the carcinogenicity of DeBDE in
humans. Overall evaluation: DeBDE is not classifiable as to its
carcinogenicity for humans (Group 3).
NONABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Nonabromodiphenyl ether is not manufactured or used. No data are
available on the following topics:
* Environmental transport, distribution, and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Levels of contamination of commercial brominated flame retardants
with nonabromodiphenyl ether should be minimized to avoid
contamination of the environment and exposure of humans.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12Br10O
Relative molecular mass: 959.22
Chemical name: decabromodiphenyl ether
(DeBDE); decabromodiphenyl
oxide
CAS registry number: 1163-19-5
(61345-53-7, mixture of
decabromodiphenyl oxide and
Sb2O3)
CAS name: 1,1'-oxybis[2,3,4,5,6-pentabromo]-
benzene
IUPAC name: bis(pentabromophenyl) ether
EINECS registry number: 214604
MITI number: 3-2846
Synonyms: decabromobiphenyl ether;
decabromobiphenyl oxide;
Decabrom; ether,
bis(pentabromophenyl);
ether, decabromodiphenyl
From: US EPA (1984); Ethyl Corp. (1992a).
On the basis of the chemical structure, decabromodiphenyl ether is
fully brominated and there is only one congener.
2.1.2 Technical product
Trade names: FR-300 BA; DE-83-RTM; Saytex
102; Saytex 102E; FR-1210;
Adine 505; AFR 1021;
Berkflam B10E; BR55N;
Bromkal 81; Bromkal 82-ODE;
Bromkal 83-10 DE; Caliban
F/R-P 39P; Caliban F/R-P 44;
Chemflam 011; DE 83; DP 10F;
EB 10FP; EBR 700; Flame Cut
BR 100; FR 300BA; FR P-39;
FRP 53; FR-PE; FR-PE(H);
Planelon DB 100; Tardex 100;
Trade names (contd) NC-1085; HFO-102; Hexcel PF1;
Phoscon Br-250; NCI-C55287
Caliban-F/RP-44 is a DeBDE
mixture with antimony oxide,
and, F/RP-53 contains 60% DeBDE,
which is used in conjunction with
THP-salts finishes and an acrylic
binder.
Commercial DeBDE is typically composed of 97-98% decabromodiphenyl
ether with 0.3-3.0% other brominated diphenyl ethers (BFRIP, 1990)
(see Table 1). Nonabromodiphenyl ether isomers are the major
impurities. The commercial product typically contains a minimum of
81-83% bromine (IARC, 1990) (83% theoretical; Ethyl Corp. 1992a).
Differences in manufacturing processes affect the nature and
amounts of impurities in the product (Larsen, 1980). Today's
commercial product is considerably purer than that manufactured in the
past. Isomers of nonabromodiphenyl ether and octabromodiphenyl ether
have been reported as impurities in DeBDE (Timmons & Brown, 1988).
FR-300-BA, produced in the early 1970s (no longer a commercial
product), was composed of 77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE
(Norris et al., 1975c). Later production of DeBDE, by the same
manufacturer, ranged in composition from 94 to 99% DeBDE with 0.3-4.5%
impurities (NBDE isomers were identified as the major impurities)
(NTP, 1986). Other DeBDE products, e.g., DE-83, Saytex 102E, and
Bromkal 82-ODE have a purity of approximately 93 to 98.5% with
different quantities of impurities (Dow Chem. Comp., 1978; De Kok et
al., 1979; Davidson & Ariano, 1986).
The availability of a technical product (possibly FR-1208) of
88.1% purity containing 11% nona-, and 0.5% octabromodiphenyl ether,
and 0.1% hexabromobenzene has been reported (Klusmeier et al., 1988).
In Japan, a DeBDE is produced containing about 3% of
nonabromodiphenyl ether as an impurity (Watanabe & Tatsukawa, 1987).
2.2 Physical and chemical properties
Commercial DeBDE is a free-flowing, odourless, off-white powder,
with a bromine content of 81-83% and a high melting point.
Melting point: 290-306 °C
Decomposition point, >320, >400, and 425 °C
DTA (different products)
Volatility: 1% 319 °C
TGA (% weight loss) 5% 353 °C
10% 370 °C
50% 414 °C
90% 436 °C
Specific gravity: 3.0, 3.25 at 20 °C
Decabromodiphenyl ether
Vapour pressure: <10-6 20 °C
(mmHg) <1 250 °C
2.03 278 °C
5.03 306 °C
Solubility: water 20-30 µg/litre
(at 25 °C) cottonseed oil 600 mg/litre
saturated copra oil 920 mg/litre
acetone 0.5, 1.0 g/litre
benzene 1.0, 4.8 g/litre
chlorobenzene 6.0 g/litre
methylene bromide 4.2 g/litre
methylene chloride 1.0, 4.9 g/litre
o-xylene 8.7 g/litre
methanol 1 g/litre
toluene 2 g/litre
methyl ethyl ketone 1 g/litre
pentane <1 g/litre
styrene <1 g/litre
Stability: stable under normal temperatures and
pressures
Flash-point: none
Flammability: non-flammable
Autoignition point: not applicable
n-Octanol/water
partition
coefficient
(log Pow): 5.24; 9.97*
From: Norris et al. (1973, 1974, 1975a,c); Tabor & Bergman (1975);
US EPA (1986); Chemag. (1988); Great Lakes Chemical Corporation
(1990b); IARC (1990); Kopp (1990); Watanabe & Tatsukawa (1990)*;
Bromine Compounds Ltd. (1992); Ethyl Corp. (1992a).
2.3 Analytical methods
The detection and quantification of DeBDE have been investigated
by several authors. The methods are based on gas-liquid
chromatographic separation using different detection methods, such as
electron capture detection and mass spectrometry (see General
Introduction, section 2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
DeBDE has not been reported to occur naturally (see General
Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
DeBDE is produced by the bromination of diphenyl oxide in the
presence of a Friedel-Crafts catalyst (Larsen, 1978). It is
manufactured in a batch process in enclosed vessels during both the
reaction and the drying cycle (US EPA, 1988; IARC, 1990).
Commercial production of DeBDE in the USA began in 1976. Among
brominated flame retardants, the quantities produced rank second only
to the quantities of tetrabromobisphenol A. There are 2 manufacturers
in the USA (BFRIP, 1992). IARC (1990) reported 2 manufacturers in
Belgium, 1 each in Switzerland, the United Kingdom, and Israel, and 5
in Japan (US EPA, 1988).
About 30 000 tonnes of DeBDE are used annually throughout the
world. About 40% of this total is used (in combination with antimony
trioxide) in high-impact polystyrene applications, such as television
and radio cabinets. Textile applications, such as a polyester fibre
additives and coatings for automobile fabric, tarpaulins, and tents
account for about 900 tonnes (IARC, 1990; OECD, 1991; Arias, 1992).
The annual consumption of DeBDE in Japan was 1000 tonnes in 1976,
2900 tonnes in 1984, 4000 tonnes in 1987, and 9800 tonnes in 1991,
mainly used for polystyrene, polyester, and polypropylene (Watanabe,
1987; Watanabe et al., 1987b; Watanabe et al., undated). Recently
published figures from a Japanese study showed that the consumption of
PBDE (mainly DeBDE) in Japan was about 20-30% of the total consumption
of brominated flame retardants (OECD, 1991).
In the Federal Republic of Germany, 1800-2000 tonnes were used in
plastics in 1988. DeBDE mixed with antimony trioxide and DeBDE used in
conjunction with tetrakis (hydroxymethyl) phosphonium (THP) salt
finishes and an acrylic binder are used to blend 50/50 and 65/35 with
polyester/cotton (Ulsamer et al., 1980).
An estimation of the use of decabromodiphenyl ether in the
Netherlands in 1988 was 1100-1300 tonnes (Anon, 1989).
3.2.2 Uses
DeBDE is a non-reactive, additive flame retardant widely used for
its high bromine content, thermal stability, and cost effectiveness.
It is used in thermoplastic resins, thermoset resins, textiles,
adhesives, and coatings. The major applications are for high-impact
polystyrene, cross-linked polyethylene polybutyl-eneterephthalate,
glass-reinforced thermoset and thermoplastic polyester moulding
resins, low density polyethylene extrusion coatings, non-drip
polypropylene (homo and copolymers), acrylo-nitrile-butadiene-styrene
rubber (ABS), nylon, adhesives, epoxy resins, polyvinylchloride, and
elastomers. The concentrations of DeBDE in the polymers range from 6
to 22% (Tabor & Bergman, 1975; Flick, 1986; NTP, 1986; Kaart & Kokk,
1987; IARC 1990).
A mixture of DeBDE and antimony trioxide has been used to treat
nylon and polyester/cotton fabrics for industrial safety apparel and
tents (LeBlanc, 1979). DeBDE is also used in the insulating materials
for wire and electrical cable (IARC, 1990).
In the United Kingdom, approximately 1000-1200 tonnes DeBDE is
used per year in the textile industry (back coatings on synthetic
fibres) (United Kingdom Department of Environment, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Extraction from polymers
Pellets of ABS (acrylonitrile-butadiene-styrene) terpolymer and
polystyrene containing 10% DeBDE, were placed in 2 litres of water and
shaken mechanically. Total bromine in water was estimated after 3 h
and up to 187 h. Extraction of bromine took place from the ABS, during
the first 43 h, in concentrations ranging from <1.0 to 3.7 mg/litre.
No bromine was extracted from polystyrene (limit of determination
<0.5 mg/litre). Because there was no increase in bromine
concentration with time, it was suggested by the authors that the
levels found were due to erosion and not to extraction. Extraction
studies were also carried out under static conditions with pellets of
ABS containing 4.25% DeBDE. Water, acetic acid, and cottonseed oil
were used as extraction solvents at temperatures of approximately 50
or 60 °C, during 1 or 7 days. Extraction occurred only with cottonseed
oil (7 days, 60 °C) at 1 mg DeBDE/litre (limit of determination
0.075 mg/litre) (Norris et al., 1973, 1974, 1975a).
4.2 Biotransformation
No data are available.
4.3 Abiotic degradation
4.3.1 Photodegradation
Studies have been performed on the photodegradation of DeBDE in
organic solvents and water. Organic solvents were used in the initial
photodegradation studies because of the extremely low water solubility
of DeBDE. In xylene, DeBDE was photodegraded by reductive
debromination with a half-life of 15 h (Norris et al., 1973, 1975a).
A commercial mixture of DeBDE containing traces of a
nonabromodiphenyl ether, was irradiated in hexane solution with UVR
and sunlight. A complicated mixture of tri- to octabromo-diphenyl
ether congeners was detected. Furthermore, a large number of PBDF
containing 1-6 bromo atoms was formed. The yield of the PBDF under the
experimental conditions was approximately 20% of the total amount of
DeBDE, after 16 h of irradiation by UVR and approximately 10% by
sunlight. The formation and distribution of photoproducts by sunlight
were similar to those of UVR, though a few differences could be
recognized in both, i.e., the decomposition rate of DeBDE and the
total amount of, and kinds of, PBDF formed. Polybromobenzenes were
also found in minor quantities in this experiment. The formation of
PBDF appeared to occur secondarily from debrominated diphenyl ethers
as photoproducts of DeBDE, but not directly from DeBDE (Watanabe &
Tatsukawa, 1987).
The xylene studies showed that DeBDE photodegraded readily, but
did not provide any indication of the nature of the stability of the
decomposition products of DeBDE in an aqueous environment. The
proposed stepwise photoreduction of DeBDE in xylene could lead to the
formation of lower brominated diphenyl ethers, which might be more
stable to UVR. However, in water, photohydroxylation would be the
favoured route and the hydroxyl substituted degradation products would
decompose rapidly via increased UV absorption. This was demonstrated
when DeBDE in water was exposed to actual sunlight over a 3-month
period (10 g DeBDE in 8 litres of water). This study was conducted to
determine whether stable lower brominated diphenyl ethers were formed
that would show increased persistence over DeBDE. Analysis (by GC/MS)
of the exposed water solution after 31, 66, and 98 days showed a
significant increase in bromine concentration relative to the
unexposed 98-day sample. After 98 days, the bromine concentration
corresponded to the breakdown of about 300 times the initial amount of
DeBDE soluble in water. Xylene extracts of the water phase (if both
98-day samples were analysed using electron capture gas
chromatography. Levels of 4-mono-bromodiphenyl ether and
4,4'-dibromodiphenyl ether were quantified and found to be <5 and
<2 ppb, respectively. In no case did the exposed sample show an
increase in any peak, with retention times similar to those of the
lower brominated diphenyl ethers.
The products of the photodegradation of DeBDE in water are not
lower brominated diphenyl ethers (Norris et al., 1973, 1975a).
4.3.2 Pyrolysis
A commercial DeBDE, FR 300 BA (77% DeBDE), was heated in a quartz
tube at 700, 800, and 900 °C and the concentrations of PBDD and PBDF
determined. The residues contained tetra- to octabromodibenzofurans
together with hepta- and octabromodioxins; the optimal formation
temperatures were 800 and 900 °C (Thoma et al., 1987a; Zacharewski et
al., 1988).
FR 300 BA (77%) was pyrolysed at 600, 700, 800, and 900 °C in the
absence of oxygen in a SGE pyrojector and the residues were analysed
using GC/MS in an on-line operation. Polybromobenzenes (PBB),
polybromonaphthalenes (PBN), and polybrominated dibenzofurans were
detected in the pyrolysate. Sixty percent of the starting products
were decomposed at 600 °C, hexabromobenzene being formed as the main
component together with traces of pentabromobenzenes and
nonabromodiphenyl ether. At a higher temperature (700 °C), the
pentabromobenzene concentration increased and tetrabromobenzenes were
also formed. Maximum amounts of hepta- and octabromodibenzofurans and
hexabromonaphthalenes were produced at this temperature. At 800 °C and
higher, an increase in levels of tetra- and pentabromobenzenes was
observed. During these reactions, the C-O bond is cleaved followed by
the attachment of a bromine atom. C-C ring closure leading to
dibenzofuran formation is very strongly suppressed by DeBDE (Thoma &
Hutzinger, 1987, 1989).
In the case of decabromodiphenyl ether (DeBDE), a total amount of
1-2% PBDF and PBDD was formed on thermolysis of DeBDE. At a
temperature of 630 °C, 90% of DeBDE was decomposed. The major products
were PBBz (tri- to hexabrominated). A heptabromodibenzofuran was a
main product, though tetra- to heptabromodibenzofurans and tetra- to
octabromodibenzodioxins were also found. In these experiments, PBDF
and PBDD were formed, which contained at least one or two,
respectively, bromine substituents less than the PBDE in the technical
product; often the same major isomers were present (Buser, 1986).
4.3.3 Combustion of polymers containing DeBDE
4.3.3.1 Pyrolysis studies
Clausen et al. (1987), Bieniek et al. (1989), and Lahaniatis (1989)
studied the influence of the combustion temperature on the formation
of PBDD and PBDF from plastics containing DeBDE. The results of the
experiments with PBT with 10% DeBDE/6% Sb2O3 at temperatures of 400,
500, 600, 700, and 800 °C in the presence of air, showed the formation
of PBDFs at mg/kg (ppm) concentrations. 2,3,7,8-TeBDD was not found
with a limit of determination of 10 mg/kg. Maximum formation of TeBDF
(4000 mg/kg) occurred at 400 °C, and, with increasing temperature,
concentrations decreased. No PBDF were formed in the thermolysis of
PBT with DeBDE but without Sb2O3. The results are summarized in
Table 10.
Comparable experiments were reported by Lahaniatis et al. (1991).
The formation of 2,3,7,8-tetrabromodibenzodioxin and -furan from
various polymers with DeBDE or PBDE were studied in thermolysis
experiments at 400, 600, and 800 °C. The polymers studied were PBT
with 10% DeBDE with, or without, 6% Sb2O3, epoxide resin (ER) with
3-6% DeBDE, and phenol resin (PR) with 3-6% of PBDE and copper. The
concentrations of the TeBDD and TeBDF at the 3 temperatures are given
in Table 11. The 2,3,7,8-TeBDD concentrations ranged from 0.01 to
7 mg/kg and the 2,3,7,8-TeBDF concentrations from 0.01 to 52 mg/kg.
The concentrations decreased with increasing temperature, and were low
at 800 °C.
Pure DeBDE and commercial polybutyleneterephthalate polymer
samples containing DeBDE and antimony trioxide have been pyrolysed at
temperatures ranging from 300 to 800 °C. The experiments were carried
out in a vertical combustion apparatus. The polymer/DeBDE samples
were: polybutylene-terephthalate (PBT) with 11% DeBDE and 5.5%
Sb2O3, PBT with 9% DeBDE and 7% Sb2O3, and PBT with 11% DeBDE and
2.7% Sb2O3 (Dumler et al., 1989a,b). The results of the pyrolysis
studies are given in Table 12. TeBDF were formed, with yields of up to
16%, from the 3 polymer samples. PBDF were mainly formed during
pyrolysis in the presence of DeBDE and Sb2O3. At 300 °C, the
conversion of DeBDE to PBDF was low, while highly brominated compounds
were formed. By increasing the temperature, the yield of
polybrominated compounds increased and more lower brominated PBDF were
formed. The maximum formation of PBDF occurred between 400 and 500 °C.
With increasing temperature, not only ring-closure reactions but also
debromination reactions with the predominant formation of tri- and
tetra- brominated PBDF may occur, The same reactions probably occur at
600 °C. At these elevated temperatures, PBDD were also detected to a
minor extent. The polymers with the lowest amount of Sb2O3 gave the
lowest PBDF concentrations. With increasing amounts of Sb2O3, the
concentration of PBDF increased, which suggests that antimony trioxide
plays a catalytic role. Pure DeBDE, pyrolysed under the same
conditions, produced significantly lower PBDF concentrations than
polymers with the flame retardants. In the case of pure DeBDE, the
temperature for the maximum formation of PBDF shifted to 700 °C.
Table 10. Thermolysis products of PBT with 10% decabromodiphenyl ether and 6% Sb2O3a
400°C 500°C 600°C 700°C 800°C
Bromodiphenyl ether
MBDE 10 50 -- -- --
DiBDE 30 100 -- -- --
TrBDE 100 200 50 -- --
TeBDE 500 300 -- -- --
PeBDE 800 400 -- -- --
HeBDE 1000 400 -- -- --
HpBDE 500 400 -- -- --
OBDE (500) -- -- -- --
Bromodibenzofuran
MBDF 100 300 100 50 --
DiBDF 500 400 200 10 --
TrBDF 3000 2000 400 -- --
TeBDF 4000 3000 600 -- --
PeBDF 4000 1000 200 -- --
HxBDF 1000 200 -- -- --
HpBDF (500) -- -- -- --
a From: Clausen et al. (1987); Bieniek at al. (1989).
Semiquantitative values in mg/kg based on the sample -- not confirmed.
2,3,7,8-Tetrabromodibenzofuran was not found (limit of determination 20 mg/kg).
2,3,7,8-Tetrabromodibenzodioxin was not found (limit of determination 10 mg/kg).
Table 11. Generation of 2,3,7,8-TeBDD/TeBDF by thermolysis of plastics with flame
retardants as additives in mg/kga,b
Compound 2,3,7,8-TeBDD 2,3,7,8-TeBDF
Sample 400 °C 600 °C 800 °C 400 °C 600 °C 800 °C
PBT/DeBDE/Sb2O3 0.02 0.01 --c 52 5.7 --
PBT/DeBDE --c --c --c 2.5 4.2 0.08
Five samples of
ER/DeBDE
minimum 0.05 0.3 0.01 0.4 0.6 0.01
maximum 0.3 0.8 0.03 1.0 2.5 0.04
PR/DeBDE/Cu --d 7 --d --d 5.7 --d
a From: Lahaniatis et al. (1991).
b The mg/kg values are based on the material used.
c Non-detected, detection limit 0.01 mg/kg.
d = Not determined.
Table 12. Results of pyrolysis studies
A. Polymer Sample A: Polybutyleneterephthalate with 11% decabromodiphenyl ether and 5.5%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)a
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- 754 3012 5551 3513 3076
DiBDF 9 2357 10 219 15 343 8445 1547
TrBDF 9 10 747 37 911 32 751 28 592 1274
TaBDF 9 14 979 52 634 37 437 35 963 1511
PeBDF 55 2293 18 391 20 666 13 504 555
HxBDF 703 127 3713 10 438 2639 109
HpBDF 1320 -- 246 946 491 --
OBDF -- -- -- -- -- --
B. Polymer Sample B: Polybutyleneterephthalate with 9% decabromodiphenyl ether and 7%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- -- 13 088 7633 8510 1144
DiBDF -- -- 15 754 10 643 9721 244
TrBDF 47 456 34 408 24 842 19 276 44
TeBDF 1472 4544 48 762 35 230 23 353 367
PeBDF 5560 18 132 24 753 16 154 6988 156
HxBDF 2886 24446 18 587 8832 1633 22
HpBDF 420 6910 2877 922 100 --
OBDF -- -- -- -- -- --
a From: Dumler et al. (1989b).
Table 12. (cont'd).
C. Polymer Sample C: Polybutyleneterephthalate with 11% decabromodiphenyl ether and 2.7%
antimony (III) oxide (yields in mg/kg, relative to the flame retardant)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- 2202 3413 2129 610 18
DiBDF -- 5187 6124 1674 82 --
TrBDF -- 15 033 15 952 1738 36 15
TeBDF -- 17 836 17 463 901 9 12
PeBDF -- 9127 3349 39 -- --
HxBDF 464 2457 901 82 -- --
HpBDF 2375 1329 246 36 -- --
OBDF traces traces -- -- -- --
D. Decabromodiphenyl ether (yields in mg/kg)
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
MBDF -- -- -- -- -- 2
DiBDF 4 8 -- 3 2 1
TrBDF 4 13 -- 4 25 3
TeBDF -- 15 11 -- 100 102
PeBDF -- 16 218 380 591 218
HxBDF -- 42 109 61 1965 988
HpBDF -- -- 1081 1734 4539 418
OBDF -- -- -- -- -- --
A sample (1 g) of industrial polypropylene containing 125 g
DeBDE/kg and 75 g Sb2O3/kg, and a pure DeBDE sample, without
additives, were pyrolysed in a DIN-oven or in a sealed quartz ampoule
at temperatures of 400, 600, or 800 °C. Combustion products obtained
with the pyrolysis of pure DeBDE (50 mg) in the DIN-oven were mainly
hexabromobenzenes and the yields at 400, 600, and 800 °C, were 123,
568, and 6 g/kg, respectively. PBDF/PBDD were found in concentrations
of 1, 3, and 2 g/kg at 400, 600, and 800 °C, respectively. At 400 and
600 °C, HxBDF up to OBDF were found in concentrations of between 96
and 1449 mg/kg. At 800 °C, lower PBDF, e.g., TrBDF, TeBDF, and PeBDF,
were also present in concentrations ranging between 11 and 35 mg/kg.
The concentrations of HxBDF and HpBDF were 81 mg and 959 mg/kg,
respectively, but no OBDF was found. The concentrations of PBDD at
400-600 °C for HxBDD up to OBDD were between 6 and 329 mg/kg; at
800 °C, lower brominated compounds, e.g., TrBDD, TeBDD, and PeBDD were
also found at concentrations of between 8 and 35 mg/kg. HxBDD up to
OBDD were present in concentrations of between 74 and 452 mg/kg. The
combustion products of the samples of polypropylene containing
DeBDE/Sb2O3 were mainly PBDF and PBDD and the concentrations were
much higher than from the combustion of the pure DeBDE. MBDF up to
HpBDF were present at concentrations of between 4762 and
107 517 mg/kg, 1033 and 49 677 mg/kg, and 353 and 29 147 mg/kg at 400,
600, and 800 °C, respectively. At all 3 temperatures, TeBDF were found
in the highest concentrations. The results with polypropylene
containing DeBDE/Sb2O3, pyrolysed in a sealed quartz ampoule at
600 °C, showed only MBDF up to PeBDF at concentrations ranging between
8860 and 142 940 mg/kg. The highest concentrations were found for
TrBDF and TeBDF. PBDD were not found. From this study, it is clear
that pure DeBDE gives much lower PBDF values than polypropylene
containing DeBDE/Sb2O3 (Dumler et al., 1990).
The formation of PBDD and PBDF during the pyrolysis of PBT
containing DeBDE was studied at different temperatures and carrier gas
compositions. PBDF were formed at ppm levels. Even when oxygen was
available in the carrier gas, PBDD were formed at a much lower level
than PBDF. In the presence of 10% oxygen, the maximum yield of tetra-
to octabromodibenzofurans was 70 ppm at 600°C. The yields of
2,3,7,8-TeBDF and 1,2,3,7,8-PeBDF were less than 4.5 ppm. The thermal
degradation processes of the polymer were investigated using
thermogravimetric analysis. The flame retardant did not exert any
influence on the elementary chemical degradation processes. The flame
retardant activity of DeBDE consists of the emission of brominated
species in the gas phase, which scavenge the propagation radicals and
reduce flammability. The mechanisms of formation of PBDD and PBDF from
DeBDE in PBT consist of a combination of a condensed phase and a gas
phase mechanism (Luijk & Govers, 1992).
Macro-pyrolysis experiments were performed in a quartz tube
reactor. The polymer sample, PBT, was inserted in the pre-heated tube
and exposed at 400-700 °C for 20 min. The carrier gas was nitrogen, or
nitrogen combined with 5% or 10% oxygen. The results are given in
Tables 13 and 14. PBDF were formed in all tests with the different
carrier gases, but the yield of PBDD was at least two orders of
magnitude lower than that of the PBDF. In a nitrogen atmosphere, no
PBDD were detected (Luijk & Govers, 1992).
Using GC/MS, Sovocool et al. (1990) analysed pyrolysates of PBT
resins (flame retarded with DeBDE) which had been exposed to
temperatures of 400 and 600 °C in the presence of oxygen. They found
brominated dioxins, brominated dibenzofurans. brominated naphthalenes,
brominated benzenes and brominated toluenes, brominated biphenyls, and
brominated methyldibenzofurans (Me-PBDF). Besides the
methyldibenzofurans, other (C2 and C3) alkyl-PBDF were found in the
pyrolysates. Numerous congeners of ethyl- (and/or dimethyl)-PBDF and a
few propyl (and/or trimethyl or ethylmethyl)-PBDF were detected.
The C2- and C3-alkyl-PBDF found in the pyrolysates exhibited
relatively intense (M-CH3)+ fragments, supporting the argument for
ethyl-and propyl or ethyl-methyl substitutions rather than dimethyl
and trimethyl substitution patterns.
Early in 1987, the General Electric Company (GE) began to
investigate the pyrolysis of polybutyleneterephthalate (PBT) flame
retardant systems. The initial work was carried out at temperatures of
600-900 °C, but subsequent efforts were at processing temperatures in
the range of 200-500 °C. The results showed little or no formation of
PBDD at temperatures of 250-400 °C; concentrations of PBDF in the
pyrolysed products were as high as about 6000 mg/kg. The
concentrations of 2,3,7,8-substituted dibenzofurans were much lower,
not exceeding a maximum of 150 mg/kg. The results for PBDD and PBDF
were much higher than would be expected, based on the previous work by
Buser and Hutzinger. The results were also inconsistent with what was
observed in combustion experiments. The explanation was that the
exposure times and conditions used did not represent a good model of
what would actually occur in moulding equipment (BFRIP, 1990).
In the GE experiments at the Fresenius Institute, an apparatus was
used in which a small sample of the polymer in the form of a finely
ground powder was exposed to the pyrolysis temperature in a flowing
air stream. The pyrolysis time for these samples varied from 10 min at
the high temperatures to as much as 1.5 h at the low temperatures.
This is in contrast to extrusion and moulding operations in which the
polymer is in the form of a molten mass, air is largely excluded, and
exposure lasts less than 1 min.
Table 13. The yield of PBDF during the pyrolysis of PBT/DeBDE in ppm relative to blend, under different carrier gas conditionsa
Temperature TrBDF TeBDF 2,3,7,8- PeBDF 1,2,3,7,8- HxBDF HpBDF NBDF
[°C] TeBDF PeBDF
Nitrogen
400 6.7 (0.8) 3.9 (2.4) 0.15 (0.09) 8.7 (0.5) 0.2 (NS)b 10.6 (1.9) 3.7 (0.4) 0.05 (0.00)
500 15.9 (2.8) 14.5 (0.8) 0.6 (0.1) 13.3 (2.5) 0.1 (NS)b 9.8 (1.1) 4.8 (0.9) 0.1 (0.1)
600 6.8 (0.4) 4.9 (0.2) 0.2 (0.0) 4.5 (1.8) 0.1 (NS)b 2.9 (0.5) 0.7 (0.2) NDc
700 4.2 (0.8) 1.2 (0.1) 0.1 (NS) 0.5 (0.0) (NS)b 0.2 (0.0) 0.06 (0.02) NDc
Nitrogen + 5% oxygen
400 4.7 (1.4) 3.6 (1.1) 0.2 (0.1) 3.6 (1.1) 0.1 (NS)b 2.9 (1.2) 0.7 (0.3) NDc
500 7.7 (0.7) 6.4 (0.4) 0.4 (0.1) 5.3 (0.1) 0.1 (0.0) 5.3 (0.1) 1.1 (0.0) NDc
600 22.1 (2.7) 17.2 (1.8) 2.4 (0.3) 17.7 (3.2) 0.8 (0.2) 16.8 (1.3) 4.8 (0.3) 0.3 (0.0)
700 15.7 (0.3) 7.6 (0.1) 1.2 (0.0) 6.6 (1.2) 0.4 (0.1) 5.7 (0.2) 1.3 (0.3) 0.08 (0.03)
Nitrogen + 10% oxygen
400 9.6 (0.7) 7.5 (1.0) 0.3 (0.1) 7.7 (1.5) (NS)b 5.9 (3.0) 1.8 (1.0) NDc
500 11.0 (0.3) 8.7 (0.5) 0.6 (0.1) 8.8 (0.4) 0.2 (0.0) 8.7 (0.8) 3.7 (0.3) NDc
600 28.8 (3.2) 20.8 (1.8) 3.4 (0.5) 21.6 (3.4) 1.2 (0.2) 17.8 (1.3) 7.6 (1.1) 0.8 (0.1)
700 17.5 (0.7) 10.3 (1.4) 2.0 (0.2) 8.9 (0.3) 0.6 (0.0) 5.3 (0.3) 2.1 (0.3) 0.02 (0.02)
a From: Luijk & Govers (1992).
b NS = not separable, due to interferences.
c ND = not detected (below detection limit).
Table 14. The yield of PBDD during the pyrolysis of PBT/DeBDE in ppb relative to blend, under different carrier gas conditionsa
Temperature TrBDD TeBDD 2,3,7,8 PeBDD 1,2,3,7,8- HxBDD HpBDD NBDD
[°C] TeBDD PeBDD
Nitrogen + 5% oxygen
400 NSb NSb NSb NSb NSb NDc NDc NDc
500 NSb NSb NSb NSb NSb NSb 7 (2) 4 (4)
600 NSb 8 (NSb) NSb 44 (6) NSb 110 (10) 43 (0) 13 (1)
700 NSb 5 (1) NSb 19 (6) NSb 40 (5) 8 (2) NDc
Nitrogen + 10% oxygen
400 NSb NSb NSb NSb NSb NDc NDc NDc
500 6 (NSb) NSb NSb 3 (NSb) NSb 48 (NSb) 16 (NSb) NDc
600 44 (NSb) 29 (7) NSb 75 (NSb) NSb 106 (11) 46 (4) 16 (0)
700 32 (18) 24 (NSb) NSb 21 (NSb) NSb 19 (3) 4 (4) NDc
a From: Luijk & Govers (1992).
b NS = Not separable, due to interferences.
c ND = Not detected (below detection limit).
Dumler et al. (1989b) have recently published data from studies on
the pyrolysis of PBT/DeBDE resins. This work was conducted in
apparatus identical to that used by GE, thus, the results can be
compared. In the report by Hutzinger and coworkers, concentrations of
PBDF were calculated relative to the flame retardant only.
Recalculation of the results by BFRIP (1990), on the basis of the
total polymer, showed general agreement with the GE results
(Table 15).
Hutzinger's recent work was summarized in a report by Pohle of the
Umwelbundesamt (Umweltbundesamt, 1989). In this work, the tube furnace
(DIN Apparatus) and the vertical quartz oven (VCI Apparatus; as used
by the Fresenius Institute) were used to pyrolyse polymers containing
PBDE at temperatures ranging from 600 to 750 °C. The results showed
that PBDF were formed under these conditions, but that 2,3,7,8-TeBDF
was only a small fraction of the total. In general, the results were
similar to those reported earlier by Hutzinger and GE.
Because of the lack of good data collected under actual operating
conditions, BFRIP met with 6 major polymer producers in 1988, to
design a series of experiments to investigate the behaviour of DeBDE
and OBDE in HIPS, ABS, and PBT under controlled moulding or extrusion
conditions. The experiments are outlined in Tables 16 and 25 (in OBDE
section 4.2.3). For the three polymer systems, HIPS/DeBDE, ABS/OBDE,
and PBT/ DeBDE, samples were moulded in the application laboratories
of polymer producers. For the moulding operations, the severity of
operations ranged from normal to extreme. Normal conditions were those
in the middle range recommended by the resin manufacturers for
processing. The abusive conditions were at, or slightly above, the
maximum recommended exposure time and temperature. The extreme
conditions were well above those recommended by the resin manufacturer
and resulted in samples with severely degraded physical properties
and/or colour.
After moulding, all the samples were analysed by Triangle
Laboratories, Research Triangle Park, NC (TLI). Samples were also sent
to the US EPA Laboratory in Las Vegas for confirming analyses. The
results of these analyses are summarized in Tables 17 and 26 (in OBDE
section 4.2.3) and are discussed more fully in the report of this
study made at Dioxin'90 (McAllister et al., 1990; BFRIP, 1990). With
the exception of a single sample, PBDD were not identified in any of
the samples and 2,3,7,8-TeBDF were seen only in this one sample at
very low concentrations. In general, the Triangle Park and US EPA
results agreed fairly well for the TBDF and TBDD.
Table 15. Comparison of GE and Hutzinger results on pyrolysis of PBT/DeBDE Resin (PBT/11% DeBDE/2.7% antimony trioxide)
All concentrations in mg/kg relative to total polymera
300 °C 400 °C 500 °C 600 °C 700 °C 800 °C
Compound General Dumler General Dumler General Dumler General Dumler General Dumler General Dumler
Electric et al. Electric et al. Electric et al. Electric et al. Electric et al Electric et al.
USA (1989) USA (1989) USA (1989) USA (1989) USA (1989) USA (1989)
MBDF NAb - 240 370 230 70 NDc
DiBDF - - 570 673 180 9 -
TrBDF - - 1650 1750 190 36 NDc
TeBDF 5200 - 2000 1960 1200 1920 490 100 24 4 0.03 NAb
2,3,7,8- 150 - 64 - 120 - 19 - 0.4 - 0.005 -
TeBDF
PeBDF 5900 - 1200 1003 1300 370 350 40 8 - 0.006
1,2,3,7,8- NDc - NDc - NDc - NDc - NDc - NDc -
PeBDF
HpBDF NDc 260 NDc 150 50 40 3 4 0.1 - NDc -
OBDF - Tr - Tr - NDc - NDc - - - -
Total 13 450 310 4100 3383 3220 2430 943 234 35 4 0.04 NAb
TeBDF-
HpBDF
a From: BFRIP (1990).
b NA = not analysed.
c ND = not detected.
Table 16. Moulding studies with two polymer/FR systems at
different processing temperaturesa
Polymer/FRb Severity Conditions
HIPS/DeBDE None Base resin, not moulded
Normal 215-220 °C, 30- second cycle
Abusive 235-245 °C, 5-min cycle
Extreme 265-270 °C, 7-min cycle
PBT/DeBDE Normal 255 °C, 23-second cycle
Abusive 255 °C, 5-min cycle
Extreme 255 °C, 10-min cycle
a From: McAllister et al. (1990).
b HIPS/DeBDE High impact polystyrene/ 84.3%
DeBDE + 12.0%
antimony trioxide 3.7%
PBT/DeBDE Polybutyleneterepthalate/ 88.0%
DeBDE + 6.5%
antimony trioxide 5.5%
Different interpretations of the analytical results of the 2
laboratories resulted in larger differences in values for hexa-through
octa-brominated furans. These data showed that the processing of the
resin systems under normal conditions did not change the composition
compared with that of the base resin formulation. Even under abusive
conditions, there was little generation of PBDF or PBDD. In fact, PBDD
were observed in only 2 of the samples under abusive or extreme
exposures. 2,3,7,8-Substituted dibenzofurans were not observed, except
in one sample in which very low ppb levels were seen (Tables 17 and 26
in OBDE section 4.2.3).
4.3.3.2 Workplace exposure studies
The occurrence of PBDF and PBDD during the thermal processing of
glass-filled PBT resin containing DeBDE at 70 g/kg and Sb2O3 at
62 g/kg or no flame retardant was studied. No PBDD (limit of
determination 2 ng) were found. PBDF were present and isomers
containing bromine at the 2,3,7,8-position represented less than 10%
of the total PBDF. Fumes evolved during thermal processing of the
resins were collected at the die-head during extrusion.
Table 17. Comparison of Triangle Laboratories Inc. (TLI) (Research Triangle Park) and US EPA Laboratory (Las Vegas) results
on moulded polymer samples containing various flame retardants. (All concentrations are in mg/kg)a
High impact polystyrene with decabromodiphenyl ether
Base resin 1222-16-0 Normal 1222-16-1 Abusive 1222-16-2 Extreme 1222-16-3
Compound TLI US EPA TLI US EPAc TLI US EPAd TLI EPA
Total TBDF 0.01 ND 0.01 0.01 0.002 0.02 ND
PeBDF 0.04 0.004 0.05 0.06 0.02 0.2 0.009
HxBDF < 5.3 0.95 < 14.3 < 5.5 0.11 < 34.1 0.2
HpBDFb < 0.6 0.72 < 3.5 < 2.1 0.08 < 10.6 2.1
PBDFb < 4.1 0.15 < 9.3 < 7.0 ND < 35.7 3.2
Table 17. (cont'd).
PBT with decabromodiphenyl ether
Base resin Normal 1222-16-6 Abusive 1222-16-7 Extreme 1222-16-8
Compound TLI US EPA TLI US EPAd TLI US EPA TLI US EPAe
Total TeBDD < 0.001 < 0.0002 < 0.002 0.001
Total PeBDD < 0.001 < 0.0002 < 0.013 0.006
Total TeBDF 0.003 0.003 0.007 0.03 0.2 1.0
Total PeBDF 0.02 0.002 0.09 > 7.8 > 25 > 54
Total HxBDF 0.11 0.013 1.1 > 16.1 > 120 > 7.0
Total HpBDFb 0.31 < 4.3 3.0 < 4.6 58.5 < 12.1
Total OBDFb 0.95 < 16.9 3.2 < 9.9 7.5 < 68.8
aFrom: BFRIP (1990). Values preceeded by < are maximum possible. Analytical signals met identification criteria, but
may include contribution from interferences. US EPA and TLI used different identification criteria, resulting in
differences in reported values.
b Concentrations of HpBDF and OBDF from TLI are not validated because of lack of standards.
Concentrations are reported for comparison only.
c Sample 1222-16-1 was spilled during workup.
d Average of 2 analyses of same sample.
e US EPA results not validated.
The temperature in the 4 temperature zones was between 216 and
250 °C. It was found that thermal processing of resin with
DeBDE/Sb2O3 yielded higher concentrations of PBDF in the fumes than
that of resin without the flame retardants. The concentrations found
in two samples of resin with flame retardant and one sample without
flame retardant are given in Table 18.
In addition to these pyrolysis and moulding experiments, studies
have been performed to determine the possibility of the workplace
exposure to PBDF and PBDD of workers in extrusion or moulding
facilities. The first of these studies was a simulation sponsored by
General Electric (GE) at Battelle Laboratories in Columbus, OH (BFRIP,
1990). For this work, an experimental extrusion apparatus was used in
which the total volatiles from the extruder could be collected and
analysed. A typical commercial PBT/DeBDE resin was extruded, the
volatiles were collected and analysed at the Fresenius Institute. The
results of the analysis were used to estimate a probable workplace
concentration of 0.76 ng/m3 as TCDD equivalents.
The following experiment was carried out to determine the risks to
employees working in the actual production of PBT resin, flame
retarded with DeBDE, at Hoechst Celanese facility at Bishop, Texas
(Hoechst Celanese Corp., 1988). High-volume samples were taken above
the extruder die-head, the extruder vent port, the fibreglass addition
port, and the raw material feeder, because these were the areas where
off-gassing was most likely to occur and where employees could be
exposed to the off-gases from extrusion. Source samples can be
considered as worst-case situations, as samplers were placed directly
in the off-gas streams. A fifth sample was taken in an unused guard
house, 90 m from the extruder building, as a field control. Full-shift
personal samples were taken on all operators and helpers on each shift
to determine the actual employee exposure. Each sampler was worn by
employees for the entire 24-h period. The operating temperature was
240-260 °C. Personal samples are regarded as more representative than
the area/source samples, since they were worn by employees during the
production run.
Table 18. Concentrations of PBDF in resins in ng per sample componenta
Substance Two samples with flame retardant One sample without
flame retardant
TeBDF 201 1310 76
2,3,7,8-TeBDF 15 114 6
PeBDF 131 820 43
1,2,3,7,8- ND (< 2) ND (< 5) ND (< 2)
PeDBF
HxBDF 80 425 23
1,2,3,4,7,8-
HxBDF < 6 49 2
HpBDF < 11 51 2
Total PBDF 433 2606 144
aFrom: (Vinci & Craig, 1988).
The values are the total quantity of PBDF (in ng) found in the 2 sample
components, while the values of the specific isomers are given as values
present in the total PBDF. For example: 15 ng of 2,3,7,8-TeBDF present in
201 ng total PBDF.
No PBDD was detected in any of these samples (limit of determination, 2 ng).
bND = not detected.
The concentrations (expressed as TCDD equivalents) in ng/m3 in
the different air samples were:
Fibreglass port 0.027
Extruder die-head 3.589
Extruder vent port 0.191
Raw material feeder 0.015
Guard house 0.0004
Personal (three) samples 0.019, 0.011,
0.122.
BFRIP sponsored a study at a commercial PBT extrusion facility in
1988. This study was designed and conducted with input from the
National Institute of Occupational Safety and Health (NIOSH) and the
US EPA. In this study, both area samples and personnel monitors were
used to collect information on potential exposures. Area samples were
collected from locations expected to show maximum levels of volatile
components. Personnel samples were collected with pumps worn by
workers, and represented the average levels to which workers were
exposed. In all cases, exposures were calculated in terms of TeCDD
equivalents, using the concept of "Toxicity Equivalent Factors" (TEF)
(the concentration of a particular chlorinated (brominated)
dibenzodioxin or dibenzofuran is converted to an equivalent
concentration of 2,3,7,8-TeCDD by multiplying by the appropriate
factor). It should be noted that this conversion of exposure values
for PBDF to TeCDD toxicity equivalents is only an estimate and is by
no means generally accepted. The results from the area samples are
summarized in Table 19 and from personnel samples in Table 20. The
maximum worker exposure for PBDF was 0.1 ng/m3, expressed as TeCDD
toxicity equivalents (BFR/CEM Working Group, 1989; BFRIP, 1990;
EBFRIP, 1990).
Determination of the occupational exposure during extrusion of PBT
containing DeBDE at 90 g/kg and antimony trioxide at 80 mg/kg, was
carried out by BASF, in Germany. In air, the total PBDF concentration
close to the extruder head was 72.9 µg/m3 and that in the rooms
ranged from 0.169-0.989 µg/m3. Concentrations of hexabromo- and
heptabromodibenzofurans were the highest, followed by penta- and
octabromodibenzofuran. The total PBDD concentration in the room was
28.12 ng/m3, being mainly octa- and hexabromodibenzodioxins. The
tetrabromodibenzodioxin concentration was 2.04 ng/m3 (Kraus, 1990).
Umweltbundesamt (1989) reported the results from the workplace
study conducted by BASF in a PBT extrusion facility. The results from
the BASF study are summarized in Table 21. The higher level observed
in the vicinity of the extruder head was removed from the workplace by
local ventilation. According to the BASF report, workers spent most of
their time in the "Production room" or at the "Bagging station". Only
if there were problems with the equipment did they work near the
extruder. The actual worker exposure would be approximately the levels
observed in the production room or at the bagging station.
The results of the BASF and BFRIP studies are summarized in
Table 22. With the exception of the BASF sample collected at the
extruder head, the results are generally consistent between the two
studies. The higher value for this sample, compared with that from the
extruder die-head from the BFRIP, could be a result of a difference in
the placement of the sampler (BFRIP, 1990).
Table 19. Results from BFRIP air sampling testsa
Compound Toxicity Area Samples
equivalent Fibreglass port Extruder die head Extruder vent port Feed hopper Guard gate
factor
CONCb TCDD CONCb TCDD CONCb TCDD CONCb TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc equiv.c
2,3,7,8-TeBDD 1.00000 0.0001 0.0001 0.0033 0.0033 0.0016 0.0016 0.0000 0.0000 0.0001 0.0001
1,2,3,7,8-PeBDD 0.50000 0.0001 0.0000 0.0059 0.0030 0.003 0.0016 0.0006 0.0003 0.0001 0.0001
1,2,3,4,7,8-HxBDD 0.10000 0.0002 0.0000 0.6220 0.0622 0.202 0.0202 0.0002 0.0000 0.0006 0.0001
1,2,3,6,7,8-HxBDD 0.10000 0.0002 0.0000 0.6221 0.0622 0.202 0.0202 0.0002 0.0000 0.0006 0.0001
OBDD 0.00100 0.0006 0.0000 0.0382 0.0000 0.020 0.0000 0.0009 0.0000 0.0023 0.0000
2,3,7,8-TeBDF 0.10000 0.0118 0.0012 0.0017 0.0002 0.104 0.0104 0.0055 0.0005 0.0001 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0154 0.0008 0.0072 0.0004 0.005 0.0005 0.0092 0.0005 0.0002 0.0000
1,2,3,4,7,8-HxBDF 0.10000 1.0770 0.1077 126.8512 12.6851 1.962 0.1962 0.6036 0.0604 0.0006 0.0001
Total TeBDD 0.00000 0.0356 0.0000 1.9360 0.0000 2.199 0.0000 0.0412 0.0000 0.0001 0.0000
Total PeBDD 0.00000 0.0067 0.0000 0.3079 0.0000 0.003 0.0000 0.0048 0.0000 0.0001 0.0000
Total HxBDD 0.00000 0.0073 0.0000 36.9172 0.0000 0.448 0.0000 0.0084 0.0000 0.0006 0.0000
Total TeBDF 0.00000 3.4764 0.0000 394.2480 0.0000 13.735 0.0000 1.4432 0.0000 0.0620 0.0000
Total PeDBF 0.00000 7.4785 0.0000 1412.8761 0.0000 55.384 0.0000 3.5971 0.0000 0.0000 0.0000
Total HxBDF 0.00000 2.6888 0.0000 4544.6098 0.0000 146.321 0.0000 0.5275 0.0000 0.0000 0.0000
Air concentration 0.1098 12.8163 0.2503 0.0618 0.0004
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
Table 20. Results from BFRIP air sampling testsa
Compound Toxicity Personnel monitors
equivalent
6559 12524 49834
factor
CONCc TCDD CONCb TCDD CONCb TCDD
equivalentc equivalentc equivalentc
2,3,7,8-TeBDD 1.00000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
1,2,3,7,8-PeBDD 0.50000 0.0000 0.0000 0.0005 0.0002 0.0000 0.0000
1,2,3,4,7,8-HxBDD 0.10000 0.0000 0.0000 0.0025 0.0002 0.0000 0.0000
1,2,3,6,7,8-HxBDD 0.10000 0.0000 0.0000 0.0025 0.0002 0.0000 0.0000
OBDD 0.00100 0.0000 0.0000 0.0093 0.0000 0.0000 0.0000
2,3,7,8-TeBDF 0.10000 0.0132 0.0013 0.0000 0.0000 0.0000 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0365 0.0018 0.0012 0.0001 0.1855 0.0093
1,2,3,4,7,8-HxBDF 0.10000 0.3543 0.0354 0.1405 0.0140 5.4102 0.5410
Total TeBDD 0.00000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
Total PeBDD 0.00000 0.0000 0.0000 0.0005 0.0000 0.0000 0.0000
Total HxBDD 0.00000 0.0000 0.0000 0.0025 0.0000 0,0000 0.0000
Total TeBDF 0.00000 1.4065 0.0000 1.1929 0.0000 7.6914 0.0000
Total PeDBF 0.00000 5.0801 0.0000 5.9643 0.0000 38.8162 0.0000
Total HxBDF 0.00000 6.5995 0.0000 61.9528 0.0000 70.1965 0.0000
Air concentration 0.0386 0.0149 0.5503
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
Table 21. Results from BASF air sampling tests
Compound Toxicity Extruder
equivalent
factor Production room Work station Bagging station Extruder head
CONCb TCDD CONCb TCDD CONCc TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc
2,3,7,B-TeBDD 1.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,7,8-PeBDD 0.50000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,4,7,8-HxBDD 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
1,2,3,6,7,8-HxBDD 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
OBDD 0.00100 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
2,3,7,8 TeBDF 0.10000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0000
1,2,3,7,8-PeBDF 0.05000 0.0 0.0000 1.3 0.0650 0.0 0.0000 87.0 4.3500
1,2,3,4,7,8-HxBDF 0.10000 0.0 0.0000 2.6 0.2600 0.3 0.0300 1186.0 118.6000
Total TeBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Total PeBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Table 21. (cont'd).
Compound Toxicity Extruder
equivalent
factor Production room Work station Bagging station Extruder head
CONCb TCDD CONCb TCDD CONCc TCDD CONCb TCDD
equivalentc equivalentc equivalentc equivalentc
Total HxBDD 0.00000 0.0 0.0000 0.0 0.0000 0.0 0.0000 0.0 0.0000
Total DiBDF 0.00000 1.1 0.0000 1.3 0.0000 0.2 0.0000 322.0 0.0000
Total TriBDF 0.00000 13.0 0.0000 7.4 0.0000 1.1 0.0000 705.0 0.0000
Total TeBDF 0.00000 20.0 0.0000 33.7 0.0000 5.1 0.0000 980.0 0.0000
Total PeBDF 0.00000 98.0 0.0000 7143.0 0.0000 8.6 0.0000 3910.0 0.0000
Total HxBDF 0.00000 594.0 0.0000 554.0 0.0000 13.0 0.0000 22 162.0 0.0000
Total HpBDF 0.00000 260.0 0.0000 200.0 0.0000 88.0 0.0000 39 550.0 0.0000
Total OBDF 0.00100 0.0 0.0000 0.0 0.0000 7.0 0.0070 5275.0 5.2750
Air concentration 0.0000 0.3250 0.0370 128.2250
ng/m3
a From: EBFRIP (1990).
b All concentrations are expressed as ng/m3.
c Toxicity equivalent factors are the international values adopted by the US EPA in 1989.
An occupational exposure limit has not been established for any
PBDD or PBDF, but a recent paper by Leung et al. (1988) provides
guidance. Applying a 100-fold safety factor to the no-observed-effect
level in animal studies, an occupational exposure limit of 0.2 ng/m3
is obtained. For other compounds that behave similarly to TeCDD in
animals tests, safety factors from 2 to 10 have been used to establish
the occupational exposure limit. Using a safety factor of 10, the
occupational exposure limit for TeCDD becomes 2 ng/m3. With the
exception of the two samples from the extruder die-heads, all of the
values from the BASF and BFRIP studies were below 2 ng/m3. The
studies also indicate that workers are unlikely to be routinely
exposed to levels higher than 0.2 ng/m3.
BASF has submitted to the US EPA a report of the biological
monitoring of 5 workers, who were employed in the extrusion of PBT
containing DeBDE as a flame retardant (BFRIP, 1990). The results
indicated blood concentrations of 2,3,7,8-TeBDF and 2,3,7,8-TeBDD of
100-500 ng/litre. All workers appeared in good health. The finding of
2,3,7,8-TeBDD in the workers was particularly surprising, since air
samples had not revealed the presence of brominated dioxins. These
findings have not yet been confirmed, but a follow-up study is in
progress.
Table 22. Summary of BASF and BFRIP air sampling testsa
Location Study Concentration (ng/m3
air) TCDD equivalent
Personnel-1 BFRIP 0.039
Personnel-2 BFRIP 0.015
Personnel-3 BFRIP 0.550
Room air BASF 0.000
Work station BASF 0.325
Bagging station BASF 0.037
Extruder head BASF 128.225
Fibre glass addition port BFRIP 0.110
Extruder die head BFRIP 12.816
Extruder vent port BFRIP 0.250
Feed hopper BFRIP 0.062
Guard gate BFRIP 0.000
aFrom: EBFRIP (1990).
Investigations by plastic producers have shown that PBDF and PBDD
can be formed, not only on combustion of plastics containing PBDE, but
also during the blending of the flame retardant into a polymer. This
has been substantiated by BASF, who measured the workplace atmosphere
around extruders.
4.4 Ultimate fate following use
4.4.1 General
For the ultimate fate of DeBDE following use, and its effects on
the environment, see section 6.1. of the General Introduction.
4.4.2 Exposure of the general population
No data are available concerning the direct exposure of the
general population to DeBDE.
In a study by Ranken et al. (1990) to investigate the possibility
of exposure to PBDD/PBDF from TV sets, three new TV sets were placed
in an 8.81 m3 test chamber. Sets A and B were purchased locally,
while set C was provided by the manufacturer. The rear portion of each
television set cabinet was constructed from polystyrene. The purchased
televisions were flame retarded with 11.5% DeBDE. Set C was made of
high-impact polystyrene, flame retarded with DeBDE/Sb203. In order
to determine background levels of PBDD/PBDF, air was pulled through
the empty chamber and through a sampler for 8 h/day, for 3 days. Two
purchased sets were placed in the chamber. Air was pulled through the
chamber for 8 h/day, for 3 days. The experiment was repeated for
another 3 days. The television set (C) was placed in the room for 3
days, but the television was not in operation. In all the described
experiments, no PBDD/PBDF were found. Finally, the air was monitored
for 24 h while the television set C was operating. No PBDD/PBDF were
found. On the basis of the limits of determination, PBDD/PBDF
emissions from the operating television sets can be calculated to be
less than 0.17-1.52 pg TeBDD/m3, 0.35-0.39 pg PeBDD/m3, 0.09-0.33 pg
TeBDF/m3, and 0.14-0.19 pg PeBDF/m3 of air. The authors concluded
that, in actual practice, these values would be lower by a factor of
10-100 because of the dilution effect of a normal room size and the
expected air turnover.
4.5 Fire accident
Bruckmann et al. (1989) reported about an accidental fire in a
stock-house in which, according to an inventory file, 2.5 tonnes of
flame retardants (a mixture of DeBDE and antimony trioxide (Traflam
PO 80)) was stored. Four wipe samples and 6 solid samples of partly
burnt material were taken several hours after the fire. At the time of
sampling, it was discovered that only a minor quantity of the bags
with the flame retardant had been in contact with the fire. The 4 wipe
samples contained tetra-, penta-, hexa-, hepta-, and
octabromodibenzofurans in the following concentrations; 4.0-123,
0.82-77.6, 4.5-51.7, 0.25-12.8, and 0.53-8.5 ng/m2. PBDD were not
found (limit of determination 1 ng/m2 per isomer). The PBDF contents
of the solid samples were low with the highest concentration at
1 µg/kg.
4.6 Simulated fire conditions
In the experiments conducted by the BFRIP, samples of high-impact
polystyrene (HIPS) and HIPS/DeBDE/Sb203 were burned in a Mass Burning
Rate Apparatus with 21% oxygen to simulate a real fire scenario. The
temperatures ranged from 500 to 800 °C. Soot and char were collected
and analysed for PBDD and PBDF (Table 23).
Table 23. Analytical results from combustion products of
HIPS/DeBDEa
Compound Concentration
Char Soot
(mg/kg) (mg/kg)
Brominated dibenzodioxins NDb NDb
Brominated dibenzofurans:
MBDF 0.64 556
DiBDF 0.54 641
TrBDF 0.23 352
TeBDF < 0.1 73
2,3,7,8-TeBDF < 0.1 1.8
PeBDF < 0.1 3.5
HxBDF-OBDF < 0.1 < 0.1
aFrom: McAllister et al. (1990); Pinkerton et al. (1989).
bND = Not detected.
A single study on a mixed range of PBDE, between HxBDE and
DeBDE, indicated little bioconcentration in carp with a
bioconcentration factor of < 4 after 8 weeks of exposure (CBC, 1982).
No PBDD or PBDF were found in the HIPS without flame retardant.
Low levels of bromodibenzofurans were found in the char of the
flame-retarded HIPS, however, the levels of brominated furans ranged
from 3.5 mg penta- to 641 mg dibromodibenzofurans/kg. No PBDD were
found in char and soot. A maximum of 1.8 mg/kg of 2,3,7,8-TeBDF was
present in soot (Pinkerton et al., 1989; McAllister et al., 1990).
4.7 Bioaccumulation
A bioconcentration study was carried out with rainbow trout under
static conditions. The concentration in the water was 20 µg
14C-DeBDE/litre. Fish were exposed for 0, 1/2, 1, 2, 4, 6, 12, 24, or
48 h. There was no measurable accumulation of DeBDE in flesh, skin, or
viscera (Brosier et al., 1972; Norris et al., 1973, 1974, 1975a).
A single study on mixed PBDE between HxBDE and DeBDE indicated
little bioconcentration in carp with a bioconcentration factor of <4
after 8 weeks of exposure (CBC, 1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Decabromodiphenyl ether was identified in 10 samples of air
collected in the vicinity of two manufacturing facilities in
concentrations ranging from 0.016 to 25 µg/m3. The compound was
present in air in the form of particulates (Zweidinger et al., 1977).
5.1.2 Water
DeBDE was determined in 15 water samples collected in Japan in
1977. DeBDE was not present in any of the samples (limit of
determination 0.2-2.5 µg/litre) (Environment Agency Japan, 1983).
DeBDE was not detected in 75 water samples (limit of determination
0.1 µg/litre) in 1987 and was not detected in 141 samples collected at
47 locations (limit of determination 0.06 µg/litre) in 1988-89 in
Japan (Environment Agency Japan, 1989, 1991).
Yamamoto et al. (1991) analysed water from the Kino River basin
for the presence of DeBDE. In 12 samples of river water, the
concentrations of DeBDE were all below 0.1 µg/litre water.
5.1.3 Aquatic sediments
DeBDE was determined in 15 sediment samples collected in Japan in
1977. DeBDE was not found in any of the samples (limit of
determination 25-870 µg/kg dry weight) (Environment Agency Japan,
1983).
Marine, estuarine, and river sediment samples were collected at
different places in Japan in the years 1981-83 and analysed for DeBDE.
The DeBDE occurred in 6 river and 1 estuarine sediment sample (7 out
of 15 samples) in the range of 20-375 µg/kg, during this period
(Watanabe et al., 1987b).
DeBDE was identified at 20 µg/kg (dry weight) in one sample of 3
estuarine sediment samples from Osaka but was not detected in samples
from Tokyo, Matsuyama, or Hiroshima (Watanabe et al., 1987b).
In 1987, an environmental survey was conducted concerning DeBDE in
sediment in Japan. DeBDE was detected in 16 out of 60 samples at
concentrations ranging from 10 to 1370 µg/kg (limit of determination
7 µg/kg dry weight) in 1987 and was detected in 39 out of 129 samples
collected at 43 locations at concentrations ranging from 4 to
6000 µg/kg (limit of determination 4 µg/kg dry weight) in 1988-89
(Environment Agency Japan, 1989, 1991).
A sample was collected using a dredger, from the upper sediment
layer of the Second Neya River in Osaka, Japan, in 1983. The sample
contained approximately 0.2 mg DeBDE/kg dry weight (Watanabe et al.
(1986).
Yamamoto et al. (1991) analysed 20 sediment samples collected from
the Kino River in Japan and found that DeBDE was present in all the
samples. The concentrations ranged from 0.003 to 11.6 mg/kg dry
weight.
Zweidinger et al. (1978) found DeBDE at levels ranging from not
detectable to 1 g/kg in sediment samples in the vicinity of a flame
retardant manufacturing facility in the USA.
DeBDE (limit of determination < 10 µg/kg) was found in sediment
in the vicinity of bromine facilities in El Dorado and Magnolia,
Arkansas (DeCarlo, 1979) and also in sludge samples from a
discharge-treatment zone of a DeBDE facility in Bayonne, New Jersey
(US EPA, 1988; IARC, 1990).
5.1.4 Aquatic and terrestrial organisms
One of 3 mussel samples, collected in 1981-85, from Osaka Bay,
Japan, contained 1.4 µg DeBDE/kg (wet weight basis) (Watanabe, 1987;
Watanabe et al., 1987a,b). DeBDE was not found in mullet, goby, sea
bass, horse mackerel, sardine, mackerel, or hairtail from this area or
in mussel, mullet, goby, sardine, mackerel, or hairtail from other
locations (limit of determination < 0.5 µg/kg). A total of 17 samples
were analysed.
In 1987, an environmental survey was conducted concerning DeBDE in
fish. It was not detected in 75 fish samples in 1987 and was not
detected in 138 fish samples collected at 46 locations in 1988-89
(limit of determination 5 µg/kg wet weight) (Environment Agency Japan,
1989, 1991).
5.2 Exposure of humans
5.2.1 Occurrence of DeBDE in human tissues
DeBDE was not found in 5 human adipose tissue samples obtained
from a hospital in Osaka in 1985-86. The limit of determination was
< 50 µg/kg fat (Watanabe, 1987; Watanabe et al., 1987a).
DeBDE was detected at concentrations of up to 5 µg/kg in 2 out of
40 samples of human hair obtained from barber shops in El Dorado and
Magnolia, Arkansas (where the chemical is manufactured) in 1978
(DeCarlo, 1979).
In the USA, Cramer et al. (1990a,b) studied PBDD/PBDF levels in
human fat tissue samples during 1987 (National Human Adipose Tissue
Survey). The 865 specimens were combined to form 48 composites based
on 9 census divisions and their age groups. The PBDD/PBDF analysis was
carried out using HRGC/HRMS. No PBDD/PBDF were found (detection limit
of 1-40 ng/kg, depending on congener). Identification of PBDE was
based on comparison of full scan mass spectra of the samples with
available standards, application of SIM techniques to compare
theoretical ion ratios with observed ion ratios, and measurement of
fragment losses from molecular ion clusters. Five samples were also
monitored for DeBDE. No DeBDE was detected in 2 out of 5 samples; a
weak DeBDE response was found in 1 out of 5 samples, and, in 2 out of
5 samples, DeBDE was estimated at 400 and 700 ng/kg, respectively.
5.2.2 Occupational exposure
Wipe samples collected during an industrial hygiene survey in a
DeBDE manufacturing plant in the USA, indicated that workers in the
reactor area were exposed to 3.6 mg DeBDE/cm2. Personal samples
collected from workers in the mill area indicated that the airborne
levels of DeBDE ranged from 0.08 to 0.21 mg/m3, as a 8-h
time-weighted average. Following a spill in the mill area, personal
airborne levels ranged from 1.3 to 1.9 mg/m3 (Bialik, 1982).
Human exposure to DeBDE occurs in the course of manufacture and
use. Surveys have determined employee time-weighted average exposures
of 1-4 mg/m3 in air with excursions up to 42 mg/m3 during short
tasks. More than 90% of the particles in air were smaller than 10 µm
in diameter. On the basis of its classification as a nuisance material
by OSHA, the recommended workplace environmental exposure level in air
is 5 mg/m3 (8-h time-weighted average for a 40-h week) (NTP, 1986)
(see also section 4.2).
Studies on worker exposure to PBDF/PBDD during the production of
polymers containing PBDE are given in section 4.3.3.2.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption and elimination
Three male and 3 female Sprague-Dawley rats were administered
orally 14C-labelled DeBDE suspended in corn oil. The elimination in
urine and expired air was measured at 24-h intervals over a 16-day
period. Less than 1% was found in urine and expired air. The principal
route of elimination was via the faeces. Within 24 h, 90.6% of the
14C activity was excreted and all the 14C was accounted for by day 2
of the study. The data indicate that there is minimal absorption of
DeBDE by the gastrointestinal tract (Norris et al., 1973, 1974,
1975a,c; Dieter, 1979).
As a supplement to the carcinogenicity studies (sections 7.3 and
7.7), additional experiments were conducted to quantify DeBDE
absorption from the gastrointestinal tract of male rats and to
determine the effect of dose on absorption. Radiolabelled 14C-DeBDE
(97.9-99.2%) was diluted with unlabelled DeBDE to yield the desired
concentrations. DeBDE was mixed in the diet at approximate
concentrations of 250, 500, 5000, 25 000, or 50 000 mg/kg, or was
administered by intravenous injection. Animals were preconditioned by
being fed diets containing the respective dose of unlabelled DeBDE for
7 days before being fed diets containing 14C-DeBDE for 1 day, then
being returned to diets containing unlabelled material for the
remainder of the holding period. Results indicated that, after
exposure to all doses in the diet, more than 99% of the radioactivity
recovered was eliminated in the faeces within 72 h. Excretion in the
urine accounted for approximately 0.01% or less of the dose. The high
14C-DeBDE content of the gastrointestinal tissues was attributed to
intimate contact with the diet. Concentrations of DeBDE in other
tissues were near the limits of determination.
After an intravenous dose of 1 mg/kg, 61% of the recovered
radioactivity was eliminated in the faeces in 72 h and approximately
0.1% in the urine.
Estimates indicate that 0.33% ± 0.19% of the 50 000 mg/kg dose was
absorbed. Data for the 25 000 mg/kg diet showed that the percentage of
the dose absorbed was not significantly different from that of the
highest dose. Radioactivity present in the lives following exposure in
the diet was confirmed as DeBDE. However, the minimal absorption of
DeBDE from the gastrointestinal tract, and, presumably, from other
potential routes of exposure may explain the low toxicity of DeBDE
(NTP, 1986; El Dareer et al., 1987).
6.2 Distribution
The distribution of radioactivity was measured in various tissues
by Norris et al. (1973, 1975a,c). On day 16, no 14C-activity was
found in the tissues, with the exception of 0.01% of the administered
dose/g in the adrenal glands and 0.06% in the spleen.
In studies in which concentrations of 14C-labelled DeBDE of
250-50 000 mg/kg diet were fed to male Fischer 344 rats, more than 99%
of the radioactivity was recovered in the faeces and intestinal
contents (see section 6.1). The liver contained approximately 0.5% of
the consumed dose, 24 h after feeding, and this declined to 0.016%
after 72 h. Labelled material was extracted from the liver and found
to be mainly unchanged DeBDE. Trace amounts of label were found in the
kidneys, spleen, lung, brain, muscle, fat, and skin. Seventy-two hours
following an intravenous dose of "C-labelled DeBDE, the faeces and gut
contents contained 74% of the dose, suggesting significant binary
excretion. Of the extracted faecal label, 63% were metabolites of
DeBDE and 37% unchanged DeBDE. Labelled materials were found in the
liver, kidneys, and lungs and in lower concentrations in muscle, skin,
and fat (NTP, 1986; El Dareer et al., 1987).
Rats were administered DeBDE in the diet at 0, 25, or 50 g/kg to
determine the concentrations of DeBDE in the liver. The mean
concentrations in the livers were 0.21 ± 0.43 (9), 2.09 ± 0.89 (8),
and 8.6 ± 2.83 mg/kg liver (20), respectively. The number of livers
studied is given in brackets (Rogers & Hill, 1980) (no details
available).
6.3 Retention and turnover
A 2-year tissue accumulation study on the rat was carried out.
Groups of 3 male and 3 female Sprague-Dawley rats were maintained on
diets providing 0, 0.01, 0.1, or 1.0 mg DeBDE (FR-300 BA)/kg body
weight per day for designated periods of time. The tissues/organs
analysed for total bromine content were serum, adipose tissue, liver,
kidneys, skeletal muscle, and testes (see section 7.3.1.2). Interim
results after 10, 30, 90, 180 days, and 12 and 18 months of exposure
showed that the total bromine values in serum, liver, kidneys,
skeletal muscle, and testes were comparable with the control values.
The mean total bromine content of adipose tissue showed a time- and
dose-related (significant) increase, especially in the groups
receiving 0.1 and 1.0 mg DeBDE/kg per day. Although there was a
continuing increase in total bromine content, the concentrations in
adipose tissue of rats ingesting 0.01, and 0.1 mg DeBDE/kg per day for
23-24 months were 2.8 ± 0.9, and 7.5 ± 3.0 mg/kg, respectively,
compared with 2.0 ± 0.2 mg/kg for the control rats. In the liver, the
concentrations for the controls and the rats treated with 1.0 mg/kg
were 2.9 ± 0.2 and 5.9 ± 1.1, respectively. In serum, after 23-24
months, the concentrations for the 4 groups were 8.0 ± 1.2 (control),
9.1 ± 1.1 (0.01 mg/kg), 7.9 ± 0.6 (0.1 mg/kg) and 8.1 ± 1.0
(1.0 mg/kg) (Norris et al., 1974, 1975a; Kociba et al., 1975, 1975a).
The elimination of bromine from tissues was studied in male
Sprague-Dawley rats maintained for 90 days on a diet providing a dose
of 1.0 mg DeBDE/kg per day, and then on a control diet. Four rats out
of each group were sacrificed on the last day of exposure or after a
recovery period of 0, 10, 30, 60, or 90 days. Serum, kidneys, adipose
tissue, and liver were analysed. The low level of total bromine
accumulated in adipose tissue remained unaffected during 90 days of
recovery. Bromine was cleared from the liver within 10 days of
recovery (Norris et al., 1974, 1975a,c).
The half-life for the disappearance of 14C-activity from the body
of DeBDE-treated rats was less than 24 h. The principal route of
elimination was the faeces. No sex-related differences were found.
14C-activity was rapidly cleared in DeBDE-treated rats.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
The toxicological information on DeBDE has been summarized in
several publications including: Norris et al. (1973, 1974, 1975a,b,c),
AIHA (1981), and NTP (1986).
7.1 Single exposure
7.1.1 Oral: Rat
Intragastric intubation of single doses of 126-2000 mg commercial
DeBDE/kg body weight, as a 10% corn oil suspension, to female
Sprague-Dawley rats (Spartan strain), did not produce any indications
of toxicity or gross pathological changes during a 14-day period
(Norris et al., 1973, 1974, 1975a,c).
Groups of 5 male Spartan rats received single oral doses of up to
5 g/kg body weight, by gavage, as suspensions in corn oil. No deaths
occurred and the animals showed normal weight gain during a 14-day
observation time. The acute oral LD50 in rats was >5 g/kg body
weight (Great Lakes Chemical Corporation, undated b).
7.1.2 Dermal Rabbit
Commercial DeBDE was applied and to the occluded, clipped, intact
skin of 2 male and 2 female New Zealand white rabbits, each at a
dosage of 200 or 2000 mg/kg body weight, for 24 h. Observation time
was 14 days. No mortality occurred. The acute dermal LD50 in rabbits
was >2 g/kg body weight (Great Lakes Chem. Corp., undated b).
7.1.3 Inhalation: Rat
Groups of 10 male and 10 female Spartan rats were exposed for 1 h
to concentrations of commercial DeBDE of 2 or 48.2 mg/litre air and
subsequently observed for 14 days. All rats survived. At 2 mg/litre,
salivation was noted in 2 rats on the first day, but, from there on,
all the rats in this group appeared normal, except one with
respiratory difficulties and another with ocular discharge during the
observation period. At 48.1 mg/litre, eye squint and increased motor
activity were noted in the animals up to day 4. Respiratory
difficulties were noted in 2 rats on days 3-6 and in one rat on day 8
and one on day 7. A few rats showed eye squint and ocular discharge on
days 7-12. All rats were normal on day 14. The acute inhalation LD50
in rats was >48.2 mg/litre (Great Lakes Chemical Corporation,
undated b).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Mouse
In a 14-day study, groups of 5 B6C3F1 mice, of each sex, were
exposed to DeBDE (99%) in the diet at concentrations of 0, 20, 50, or
100 g/kg. No effects on health, survival, or body weights were
observed and no compound-related clinical signs or gross pathological
effects were reported (NTP, 1986).
In a 13-week study, groups of 10 male and 10 female B6C3F1 mice
were administered DeBDE (two lots of DeBDE were used; one of 99% and
the other > 97%) in the diet at 0, 31, 62, 12.5, 25, or 50 g/kg, for
13 weeks. No evidence was found for compound-related effects on
physical appearance, behaviour, food consumption, body weight gain,
survival, and gross and microscopic pathology. This study was used to
establish the dose levels for the long-term study (Rutter & Machotka,
1979; NTP, 1986).
7.2.1.2 Rat
In 3 separate, 28-day feeding studies, each with groups of 10 male
and 10 female Charles River CD rats, commercial DeBDE was administered
at 0, 100, or 1000 mg/kg diet. The rats were observed for appearance,
mortality, food consumption, body weight gain, and organ weights.
After sacrifice, gross pathological and microscopic examinations of
the liver, kidneys, and thyroid were carried out. Adverse effects or
lesions associated with DeBDE administration were not found in these 3
rat studies. The total bromine contents of the liver, and adipose
tissue were determined and a slight increase in bromine concentration
was found at the highest dose level (Great Lakes Chemical Corporation,
undated b) (no details available).
Groups of 5 male Sprague-Dawley rats were administered a diet
containing 0, 0.01, 0.1, or 1.0% [equivalent to 0, 8, 80, or 800 mg
DeBDE (77.4% DeBDE, 21.8% NBDE, and 0.8% OBDE)/kg body weight per
day], for 30 days. No influence on food intake or body weight gain was
found. No haematological changes were observed in the 8 and 80 mg/kg
dose groups, but, at 800 mg/kg, there were decreases in packed cell
volume and red blood cell count. Urinalysis parameters were not
affected at any dose level. No differences in heart, testes, brain,
and kidney weights were observed. Enlarged livers were found in rats
exposed to 80 and 800 mg DeBDE/kg. The liver lesions consisted of
centrolobular cytoplasmic vacuolization. Furthermore, in the rats
receiving 80 and 800 mg/kg, hyaline degenerative cytoplasmic changes
in the kidneys and thyroid hyperplasia were found. At a dietary level
of 0.1 g/kg (equivalent to 8 mg/kg body weight), no treatment-related
changes in the liver or thyroid, or other changes were found. (Remark:
limited histopathology was carried out) (Sparschu et al., 1971; Norris
et al., 1973, 1974, 1975a; Kociba et al., 1975a).
In a 14-day study with DeBDE (99%) administered in the diet to
Fischer 344/N rats at concentrations of 0, 5, 20, 50, or 100 g/kg, no
effects were seen on health, survival, and body weight, and no
compound-related clinical signs or gross pathological effects were
observed. Similarly, no toxic effects were seen in a 90-day study in
which doses of DeBDE (>97%) of 0, 3.1, 6.2, 12.5, 25, or 50 g/kg were
administered in the diet. No effects on survival, body weight, or feed
consumption were observed. No gross or microscopic effects were
reported. Liver weights were not recorded in these studies, but, in
subsequent studies, liver weights were significantly increased in
Fischer 344/N rats at dose levels of 25 and 50g DeBDE (92%)/kg diet,
for 14 days (NTP, 1986).
7.2.2 Inhalation
7.2.2.1 Rat
Pulmonary tissue response and pulmonary clearance of commercial
DeBDE were evaluated following intratracheal administration of the
dust to rats. The study was conducted to evaluate the possible health
hazards for man from inhalation of the dust generated during the
production and handling of DeBDE. Fifty male, Sprague-Dawley rats were
given an intratracheal injection of 20 mg DeBDE (77.4%) dust (length
mean diameter 2.65 µm, surface mean diameter 2.91 µm, and volume mean
diameter 3.17 µm) suspended in 1 ml of rat serum. A control group of
35 rats received only the serum vehicle. The rats were observed for
appearance, demeanour, and body weights. Groups of 5 treated and 2
control rats were killed on days 3, 10, 30, 91, and 365 for
determination of total bromine content in the lungs. The calculated
DeBDE equivalent values were used to determine the half-life of DeBDE
in lungs, which was determined to be 150 days. To assess the
respiratory tissue response, gross and histopathological examinations
were conducted on rats killed on days 10, 30, 416, and 556 and on rats
that died. No untoward effects were observed, except on days 10 and
556 (but not on days 30 and 416): the lungs of treated rats contained
scattered focal aggregates of alveolar macrophages showing clear,
angulated, cytoplasmic vacuoles or spaces, which probably represented
the location of the dust particles. A very slight focal thickening of
the interalveolar septae was noted in 2 out of 5 rats. Particles were
not present in the regional lymph nodes. No evidence of fibrosis or
other proliferative response was detected in the lungs or regional
lymph nodes (Jersey et al., 1976).
7.3 Long-term exposure
7.3.1 Oral
7.3.1.1 Mouse
A two-year study was carried out on B6C3F1 mice. The animals were
administered DeBDE in the diet at concentrations of 0, 25, or 50 g/kg,
for 113 weeks (NTP, 1986) (for details of the study see section
7.7.1.1).
7.3.1.2 Rat
A long-term/carcinogenicity study was carried out on
Sprague-Dawley rats administered daily doses of 0, 0.01, 0.1, or
1.0 mg DeBDE/kg body weight in the diet for 2 years (Kociba et al.,
1975, 1975a; Norris et al., 1975a,b). In another 2-year study, Fischer
344 rats were administered DeBDE at 0, 25, or 50 g/kg diet (NTP, 1986)
(for details of these 2 studies see section 7.7.1.2).
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
Skin irritation studies conducted with commercial DeBDE, applied
as a dry solid (500 mg) on the shaved skin (under occlusion) of 2
groups of 3 New Zealand white rabbits caused no irritation on intact
skin and no, or only slight, erythematous and oedematous responses on
abraded skin, after a single exposure for 24 h and an observation
period of 72 h. A comparable study on 3 male and 3 female New Zealand
white rabbits in which 500 mg DeBDE was applied on intact or abraded
skin showed no skin irritation. Repeating the exposures of intact skin
for 5 days/week for 2 weeks or to abraded skin for 3 days did not
alter the response (Norris et al., 1973, 1974, 1975a,c; Great Lakes
Chemical Corporation, undated c).
7.4.2 Eye irritation
Eye irritation studies on 3 male and 3 female New Zealand White
rabbits showed that 100 mg Saytex 102/eye, applied as a dry solid,
caused only transient irritation (redness and chemosis) of the
conjunctival membranes. The cornea, iris, and lens were unaffected
(sodium fluorescein and UVR were used). After 24 h, the eyes had
recovered. The observations were made at 1, 24, 48, 72 h, and 7 days
after treatment (Norris et al., 1973, 1974, 1975a,c; Ethyl Corp.,
1986; Mallory et al., 1986; Great Lakes Chemical Corporation,
undated c).
7.4.3 Sensitization
Repeated application of a suspension of 5% DeBDE (77.4% DeBDE,
21.8% NBDE, 0.8% OBDE) in petrolatum, 3 times a week for 3 weeks, to
the skin of 50 human volunteers did not result in skin sensitization
reactions during the sensitizing period or, on challenge 2 weeks
following the last application. Skin irritation was observed in 14 out
of the total 450 applications (11 of the reactions were classified as
very slight and 3 as mild erythema). These reactions were seen in 9
out of the 50 persons (Norris et al., 1974, 1975a,c; Dow Chemical
Comp. USA, 1978).
7.4.4 Chloracnegenic activity
Chloracnegenic activity was studied on the ear of each of 4 New
Zealand White male and female rabbits. The test material (Saytex 102)
was administered once daily at 0.1 ml/day, 5 times per week, for 4
weeks, at concentrations of 1, 10, 100, or 1000 g/kg, in chloroform.
Observations were recorded prior to the initial dose and at 7, 14, 21,
and 28 days of dosing. Saytex 102 as a 10% chloroform solution caused
a slight erythematous response and slight exfoliation, but no
chloracne was observed during the study (Naismith & Matthews 1981).
In the period 1971-74, approximately 40 samples of DeBDE (pilot
plant samples), mother liquor, mother liquor still pot residue, and
still bottom samples were studied for their chloracnegenic activity.
In these studies, the samples (0.1 ml) were applied as such, or as a 5
or 10% solution in chloroform, on the rabbit ear, 5 days per week for
4 weeks. The samples of DeBDE did not induce any responses, but
responses to the mother liquor and still bottom samples were positive,
except in a few cases where the result was equivocal (Rampy, 1971-74).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
7.5.1 Reproductive toxicity
A one-generation reproduction study was performed in which 10
male, and 20 female, Sprague-Dawley rats at the two lower dose levels
(3 and 30 mg/kg body weight) and 15 male and 30 female rats at the
higher dose level (100 mg/kg body weight) were given commercial DeBDE
with the diet (weekly adjustment), for 60 days prior to mating, 15
days during mating, and subsequently throughout gestation and
lactation. Twenty male and 40 female rats served as controls. No signs
of toxicity were observed in the adult rats or the neonates during the
study or at necropsy. Unaffected parameters included body weight gain
and food consumption by adults, reproductive parameters (the
percentage pregnant and neonatal growth, survival, and development),
preterminal urinalysis and clinical chemistry in adult rats, gross
examination of all adult and weanling rats, and microscopic
examination of tissues from both age groups. No cytogenetic changes
were observed in the bone-marrow of parents and weanling rats. Thus,
no toxicological manifestations were associated with the ingestion of
100 mg DeBDE/kg in this reproduction study (Norris et al., 1975c;
Schwetz et al., 1975).
7.5.2 Teratogenicity
Pregnant female rats were given 0, 10, 100, or 1000 mg commercial
DeBDE/kg body weight, suspended in corn oil, by intragastric gavage,
on days 6-15 of gestation.
Maternal food consumption and body weight did not differ from
those of the controls. Liver weights of the mothers were comparable
with those of the control animals. The position and number of fetuses
in utero, the number of corpora lutea, individual pup weight,
crown-rump length, and sex ratio, were similar to those of the
controls.. A significant increase in resorptions was found at low dose
levels, but not at the high dose level. No gross external
abnormalities were seen in the fetuses of dams treated at any dose
level. Soft tissue and skeletal examinations revealed an increased
number of litters with subcutaneous oedema and delayed ossification of
bones of the skull of fetuses of dams treated with 1000 mg/kg, but not
with 100 mg/kg body weight. Analysis of maternal and fetal livers for
total bromine revealed a significantly increased concentration in
maternal livers of rats treated with 1000 mg/kg. Treatment with
100 mg/kg or less did not produce any increase in total bromine
contents. No increase in total bromine contents was observed in the
livers of fetuses from dams receiving any of the dose levels of DeBDE
(Norris et al., 1973, 1974, 1975a; Hanley, 1985; US EPA, 1989).
7.6 Mutagenicity and related end-points
7.6.1 Mutation
A technical product, HFO 102, was tested in a Salmonella
typhimurium assay with the strains TA 98, TA 100, TA 1535, and TA
1537 with, or without, metabolic activation. The HFO 102 was not
completely soluble in DMSO at a concentration of 10 mg/litre. However,
the suspension was used for the test and for preparation of the
solutions. The dose levels that were tested were 0.4, 4.0, 40, and
1000 µg/plate. No evidence for mutagenic activity was found (Shoichet
& Ehrlich, 1977).
Results of studies with Saccharomyces cerevisiae with, or
without, liver microsomal enzyme preparations, were also negative (no
details) (Great Lakes Chemical Corporation, undated b).
Commercial DeBDE in concentrations of 100-10 000 µg/plate was not
mutagenic in Salmonella typhimurium, TA 100, TA 1535, TA 1537, and
TA 98 strains, in the presence, or absence, of an exogenous metabolic
system from Aroclor 1254-induced male rat liver S9 and male Syrian
hamster liver S9 (NTP, 1986).
7.6.2 Chromosomal effects
Cytogenetic examination of bone marrow cells, taken at necropsy
from the femur of the parent animals from a reproduction study (see
section 7.5.1) as well as from the neonates at weaning, showed no
increase in cytogenetic aberrations compared with the controls (Norris
et al., 1975c) (no details).
Commercial DeBDE was not mutagenic in the mouse lymphoma
L5178Y/TK+/- assay in the presence, or absence, of Aroclor
1254-induced male F344 rat liver S9. Tests for cytogenic effects in
Chinese hamster ovary cells indicated that commercial DeBDE does not
cause chromosomal aberrations or sister-chromatid exchanges in either
the presence or absence of S9, prepared from livers of Aroclor
1254-induced male Sprague-Dawley rats (NTP, 1986).
7.7 Carcinogenicity
7.7.1 Oral
7.7.1.1 Mouse
Groups of 50 male and 50 female B6C3F1 mice (nine weeks old) were
fed 0, 25, or 50 g DeBDE (purity 94-99%; no brominated dioxins or
furans were found)/kg diet for 103 weeks; all survivors were killed in
weeks 112-113. The average daily consumption of DeBDE was estimated to
be 3200 mg/kg for low-dose and 6650 mg/kg for high-dose male mice and
3760 mg/kg for low-dose and 7780 mg/kg for high-dose female mice. Body
weights and survival of treated animals were comparable with those of
the controls. Non-neoplastic lesions observed in treated mice were
granulomas in the liver of low-dose males and hypertrophy in the liver
of low- and high-dose males. The combined incidence of hepatocellular
adenomas and carcinomas was significantly increased in males: control
8/50, low-dose 22/50, and high-dose 18/50 (but not increased in
comparison with historical control groups); the combined incidence of
thyroid gland follicular-cell adenomas and carcinomas was increased,
but not significantly, in treated males: control 0/50, low-dose 4/50,
high-dose 3/50; females: control 1/50, low-dose 3/50, high-dose 3/50.
Follicular-cell hyperplasia of the thyroid gland was increased in both
groups of treated male and female mice (NTP, 1986; Huff et al., 1989).
7.7.1.2 Rat
Groups of 25 male, and 25 female, Sprague-Dawley rats, 6-7 weeks
of age, were fed 0, 0.01, 0.1, or 1.0 mg DeBDE/kg body weight (purity
DeBDE 77.4%, nonabromodiphenyl ether 21.8%, octabromodiphenyl ether
0.8%) in the diet for 100-105 weeks. Additional groups of 10-34
rats/sex per dose level were killed at various times during the study
to investigate the accumulation of total bromine in the tissues.
Ingestion of up to 1.0 mg DeBDE/kg did not influence survival rates;
appearance, mean body weights, feed consumption, haematology,
urinalysis, clinical chemistry, and organ weights of treated groups
were similar to those of controls. No discernible toxicological
effects were produced by DeBDE and no significant differences in the
number of rats developing tumours, the total number of tumours, or the
specific type of tumours were observed between treated and control
groups, when evaluated by the Fisher's exact probability test. Adrenal
phaeochromocytomas were the most frequent tumours in the male rats in
all groups. During the study, there was a slight build up of the total
bromine content in the tissues of rats ingesting the 2 highest dose
levels, as measured by neutron activation analysis. However, the
source of bromine may, or may not, have been DeBDE, because NBDE and
OBDE were also present in the product. Serum, muscle, and kidneys did
not show any increase in total bromine content. In the liver,
low-level, steady-state conditions were attained by 12 months. Adipose
tissue showed a time- and dose-related increase in total bromine
content, subsequent to ingestion of 0.1 or 1.0 mg/kg. The total
bromine content of adipose tissue of rats ingesting 0.01 mg/kg for 2
years was 2.8 ± 0.9 mg/kg compared with a control value of 2.0 ±
0.2 mg/kg (section 6.3) (Kociba et al., 1975, 1975a; Norris et al.,
1975a,b). [The IARC Working Group (1990) noted the very low dose
levels used].
Groups of 50 male, and 50 female, Fischer 344/N rats, 7-8 weeks of
age, were fed DeBDE (purity 94-99%; no brominated dioxins or furans
were found) at 0, 25 or 50 g/kg diet, for 103 weeks; all survivors
were killed in weeks 111-112. The average daily consumption of DeBDE
was estimated to be 1120 and 2240 mg/kg for low-dose and high-dose
male rats, respectively, and 1200 and 2550 mg/kg for low-dose and
high-dose female rats, respectively. Body weights of treated rats were
not significantly different from those of the controls. Thrombosis and
degeneration of the liver, fibrosis of the spleen, and lymphoid
hyperplasia were observed in high-dose male rats. Significant
increases were observed in the incidence of neoplastic nodules of the
liver (adenomas) in treated males: control 1/50; low-dose 7/50;
high-dose 15/49 and females: control 1/50; low-dose 3/49; high-dose
9/50. No differences in the incidence of hepatocellular carcinomas
were seen among the groups. The incidence of acinar-cell adenomas of
the pancreas in males was: control 0/49; low-dose 0/50; high-dose
4/49. The difference between the controls and the high-dose group was
not significant. A high incidence of mononuclear-cell leukaemia was
observed in treated and control rats of both sexes (NTP, 1986; Huff et
al., 1989).
7.8 Other special studies
7.8.1 liver
Carlson (1980a) administered commercial DeBDE (in corn oil), by
gavage, at a dose of 0.1 mmol/kg body weight to male Sprague-Dawley
rats (200-250 g) for 14 days. Twenty-four hours after the last
(seventh) dose, the liver/body weight ratio increased. Koster et al.
(1980) found no evidence of a porphyrinogenic action after exposure of
cultures of chick embryo liver cells to a concentration of 5 µg DeBDE
(in DMSO)/ml medium with, or without, pretreatment with
naphthoflavone, an inducer of P450, P448, and of delta-levulinic acid
synthethase.
A sample of DeBDE, obtained from the NTP repository, was
administered by gavage (vehicle not clear) at 1500 mg/kg body weight
to 9, three-month-old, female Sprague-Dawley (CD) rats, 21 h and 4 h
before they were killed. No changes were observed in hepatic
cytochrome P450 content, hepatic DNA damage, as determined by alkaline
elution, hepatic ornithine decarboxylase activity, or serum alanine
aminotransferase activity (Kitchin et al., 1992).
7.8.2 Miscellaneous
Hefner (1973) studied, in an in vitro model, the rupture of
erythrocyte membranes (haemolysis) induced by dust particles. The test
approximates the in vivo rupture of the secondary lysosomal
membranes. Fibrogenic dusts are capable of rupturing the erythrocyte
membrane, while non-fibrogenic dusts are not. On the basis of the
in vitro model, DeBDE of the particle size studied (shown by optical
microscopy to have a 2.65 µm number length mean diameter with 94.5% of
the particles being 3.77 µm and below) appears to be non-fibrogenic
(only 0.1% haemolysis) at 115 mg dust/ml suspension and time from 15
to 120 min.
7.8.3 Toxicity of soot, char, and other waste products from
combustion of DeBDE-containing polymers
7.8.3.1 Acute oral toxicity
Groups of 5 male, and 5 female, Sprague-Dawley rats were
administered a mixture of soot and char (61/39%), generated from the
combustion of high-impact polystyrene, as a single dose, by garage at
0(vehicle), 0.5, 5.0, 50, 500, or 2000 mg/kg body weight. These
amounts were given in 10 ml 1% methyl cellulose/kg body weight.
Observation time was 28 days. Body weight gain, mortality, and weights
of 10 organs were studied, and these organs in the control and 2000 mg
groups were also examined microscopically. No overt signs of toxicity
were observed. The acute oral LD50 was >2 g/kg body weight (Fulfs &
Dahlgren, 1987a).
The acute toxicity of the combustion products of a matrix composed
of high-impact polystyrene, DeBDE, and antimony trioxide was
investigated. Six dose groups of 5 male and 5 female rats were
treated, by gavage, with 0, 0.5, 5, 50, 500, or 2000 mg/kg of combined
soot and char, generated from the combustion of the flame retarded
high-impact polystyrene (HIPS) suspended in 1% methyl cellulose and
observed for 28 days. No animals died during the course of the study
and no clinical signs of toxicity were observed. No histological
lesions were detected in the following organs examined: thyroid,
parathyroid, and adrenal glands, spleen, gonads, heart, liver, lung,
brain, kidneys, and thymus. The LD50 was > 2000 mg/kg body weight
(Fulfs & Dahlgren, 1987b; Pinkerton et al., 1989).
Undiluted solids from mother liquor (waste tars) were tested for
acute oral toxicity in rats. No acute oral lethality was seen with
0.126 g/kg body weight in corn oil, but 100% mortality was found, with
0.5 g in water. The animals displayed tremors (Norris, 1971).
7.8.3.2 Skin irritation and comedogenicity
Soot and char generated from the combustion of high-impact
polystyrene retarded with, or without, DeBDE and Sb2O3 were tested
for their comedogenicity using a New Zealand albino rabbit ear
bioassay (Draize, 1979). The soot and char samples were mixed in
different ratios. The dose levels were; 0.001, 0.003, 0.005, 0.008,
0.01, 0.03, 0.05, 0.08, and 0.1 g. The highest dose level (0.1 g) of
the mixture was actually in excess of the amount that remained on the
ear. The materials were applied in 0.1 ml of water. Five male rabbits,
with 2 healthy ears each, were randomly selected for these studies and
the original design required 10 exposure levels, 1 per ear. Dosing was
continued for 5 consecutive days. Ears were checked to grade the
erythema on day -1 and day 0, and then daily, and the final grading
was done on the day following the last dosing. The results showed
that, without flame retardants, very slight to well defined erythema
occurred with doses of 0.005 g and higher. In cases where the polymer
was flame retarded, the effect was stronger, very slight to
moderate/severe erythema occurred with doses of 0.001-0.003 g or more
(see Table 24) (Fulfs 1987a,b).
Table 24. Skin irritation by soot or char generated from the combination of
HIPS with, or without, DeBDE
Soot/char Mixture Dose Erythema score
soot/char
High impact 61/39% 0.005 g and score 1-2 (increasing
polystyrene higher with dose level from
day 2 onwards)
High impact 47/63% 0.003 g and score 1-3 (increasing
polystyrene with higher with close level, from
DeBDE and antimony day 1)
trioxide
0.001 g and same result, increasing
higher from day 3
Two groups of four (mainly 2 male and 2 female) New Zealand white
rabbits were used to test a mixture of soot and char (61/39%),
generated from the combustion of high-impact polystyrene treated
without flame retardants, in a rabbit ear comedogenicity bioassay.
Daily dose levels of 2, 5, 8, 20, or 50 mg of the mixture were
administered. Each daily dose was rubbed with 0.1 ml of water on the
inner surface of the pinna of one ear of the respective rabbit. The
animals were dosed 5 days per week for a total of 4 weeks. The total
cumulative dose levels were 40, 100, 160, 400, and 1000 mg. The ears
were graded for irritation (Draize test) and for hyperkeratosis (Adams
test). During the study, dermal irritation was found in all treated
groups with a score of 1-2. No comedogenic (score 0) responses were
observed on any of the ears. A slight increase in hyperkeratosis of
the sebaceous follicles was seen during histopathological examination
of the skin in the animals treated with the 2 highest dose levels. No
evidence of overt toxicity was seen among any of the animals tested
(Fulfs & Dahlgren, 1987c; Pinkerton et al., 1989).
A similar study was carried out with a combined soot and char
(47/53%) mixture, generated from the combustion of HIPS flame retarded
with DeBDE and antimony trioxide. The results were comparable with
those of the combustion products of HIPS without flame retardants.
There was no microscopic evidence of any significant hyperkeratotic
response (Fulfs & Dahlgren, 1987d).
Undiluted solids from mother liquor (waste tars) were tested for
skin irritation in rabbits. Slight skin irritation was observed at a
dose of 1.1 g/day for 3 days (Norris, 1971).
7.8.3.3 Eye irritation
Solids from mother liquor (waste tars) were tested for eye
irritation. The material is a mixture of brominated DeBDE (Br = 7-10).
A dose of 0.1 g of the mixture caused severe eye irritation and slight
corneal injury (Norris, 1971).
8. EFFECTS ON HUMANS
8.1 General population exposure
No data are available.
8.2 Occupational exposure
8.2.1 Skin sensitization
Human volunteers (80 males and 120 females) were treated with 9
induction patches of 2 batches of DeBDE, identified as DBDO-1 and XD
8186.02. The first sample was evaluated as such, and the second as a
2% (w/v) aqueous solution. The upper arm was used in the males and the
upper back in the females. The patches were applied once every 2 days
and the substance left in contact with the skin for 24 h, after which
it was removed. After the application of 9 patches, there followed a
non-patching period of 12 days, after which the challenge patch was
applied to detect sensitization. A new skin site was used for this
24-h patch. After removal of the patch, reactions were observed after
24 and 48 h. This study did not reveal any evidence of skin
sensitization with either test material in any of the subjects
(Industrial Bio-test Laboratories, 1975).
8.2.2 Neurotoxicity
A health assessment of workers exposed to polybrominated biphenyls
and polybromodiphenyl ethers, e.g., DeBDE, during manufacture revealed
a higher than normal prevalence of primary hypothyroidism and a
significant reduction in sensory and fibula motor velocities; no other
dermatological or neurological effects were seen. The authors could
not conclude whether these effects were caused by DeBDE or by PBB,
which was also produced (earlier) in the plant. DeBDE was not detected
in the serum of the workers (Bahn et al., 1980).
8.2.3 Epidemiological studies
The health of workers in facilities where flame retarded polymers
were extruded has been evaluated in 3 studies, 2 at Celanese and 1 at
General Electric (BFRIP, 1990).
A study was conducted by Celanese Corporation in the late 1970s at
their Bishop, Texas, facility. The primary purpose of this study,
conducted by Tabershaw Associates, was to investigate the possible
effects of employee exposure to formaldehyde. However, brominated
flame retardants have been used at the facility since 1970, and,
therefore, significant effects resulting from the use of these
materials might also have been revealed by the study. No effects that
might be attributable to the use of the brominated flame retardants
were observed (EBFRIP, 1990).
A more complete study was conducted by General Electric Plastics
at their Mt. Vernon, Indiana, facility early in 1988. In the
conclusions of the study, it is stated that "there appears to be no
evidence that the workers in the study zone display any symptoms
associated with exposure to dioxins or related substances. More
critically, as a statistical group, the general medical examinations
for these workers compare very favorably with the examination for the
workers in the control zone or with published values for the general
population". It is finally concluded that "the study gives reasons to
continue to be observant but no reason to be alarmed" (EBFRIP, 1990).
A comprehensive medical evaluation of workers involved in the
processing of polymers containing brominated flame retardants was
undertaken at the BASF facility in Ludwigshafen, Germany. In this
study, some 40 potentially exposed workers and an equivalent number of
workers from a control group were subjected to complete medical and
psychological examination. The health of the workers compared
favourably with workers in the control zones. None of the workers
displayed symptoms associated with exposure to dioxin or related
substances, therefore, it was concluded that the workers did not show
any symptoms that could be associated with PBDD or PBDF emitted during
the polymer processing. It should be kept in mind that there might
also be exposure during the formulation of PBDE- containing polymers
and from products made of these polymers. Furthermore, exposure to
dusts may also occur (EBFRIP, 1990).
Zober et al. (1992) carried out a morbidity study of extruder
personnel with potential exposure to brominated dioxins and brominated
furans. The presence of PBDF/PBDD in air was established through
air-monitoring during the extrusion blending of
polybutyleneterephthalate with DeBDE. Biomonitoring results of 42
workers (exposed during the period 1975-88) and immunological tests
for exposed and 42 control employees were presented. Among potentially
exposed men, 2,3,7,8-TeBDF/TeBDD concentrations in blood lipid ranged
from nd to 112 and from nd to 478 ng/kg, respectively. The control
workers had concentrations of 7 and 7-48 ng/kg respectively. Results
of the immunological studies showed that the immune system of exposed
workers was not adversely affected at these burdens of TeBDF/TeBDD for
up to 13 years.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Marine unicellular algae, Skeletonema costatum, Thalassiosira
pseudonana, and Chlorella sp. were exposed to commercial DeBDE in 6
algal growth media. The duration of the exposure was 72 h for
S. costatum and T. pseudonana and 96 h for Chlorella sp. The
population density was estimated by cell counts using a
haemocytometer. Inhibition by 1 mg DeBDE/litre acetone was less than
50% in all species (Walsh et al., 1987).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An evaluation by IARC (1990) concluded that there is limited
evidence for the carcinogenicity of DeBDE in experimental animals. No
data were available from studies on the carcinogenicity of DeBDE in
humans. Overall evaluation: DeBDE is not classifiable as to its
carcinogenicity for humans (Group 3).
NONABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Nonabromodiphenyl ether is not manufactured or used. No data are
available on the following topics:
* Environmental transport, distribution, and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Levels of contamination of commercial brominated flame retardants
with nonabromodiphenyl ether should be minimized to avoid
contamination of the environment and exposure of humans.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
 Chemical formula: C12 H Br9 O
CAS registry number: 63936-56-1
Chemical name: pentabromo(2,3,4,5-tetrabromophenoxy)-
benzene
Common name: nonabromodiphenyl ether (NBDE);
nonabromodiphenyloxide
Relative molecular mass: 880.37
Based on the chemical structure, there are three possible isomers
of nonabromodiphenyl ether.
From: US EPA (1984, 1986).
2.2 Physical and chemical properties
No data are available.
2.3 Analytical methods
No specific data are available (see General Introduction section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
NBDE has not been reported to occur naturally (see General
Introduction section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Nonabromodiphenyl ether has been prepared from decabromodiphenyl
ether by heating with NaSH in xylene at 130 °C for 2 h. The removal of
the reactive Br in the ortho-position of decabromodiphenyl ether led
to the photostability of the product (Noguchi et al., 1977).
3.2.2 Uses
Nonabromodiphenyl ether is not used commercially as a flame
retardant. It is present as an impurity in OBDE at a concentration of
10%.
OCTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Pure octabromodiphenyl ether is not manufactured or used. No data
are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial OBDE is a mixture of approximately 11% PeBDE/HxBDE, 44%
HpBDE, 31-35% OBDE, 10% NBDE, and 0.5% DeBDE. On the basis of the
chemical structure, there are 12 possible isomers of OBDE and 24
possible isomers of HpBDE.
The melting point varies from about 80 °C to >200 °C. The vapour
pressure is < 10-7 mmHg. The solubility in water is low and the
n-octanol/water partition coefficient (log Pow) >5.5. The above
variations in physical data may be explained by the differences in
composition of the mixtures tested.
1.1.2 Production and uses
The worldwide consumption of commercial OBDE per year is 6000
tonnes, 70% of which is used as a flame retardant in ABS for the
production of computers and business cabinets. OBDE is the second most
widely used PBDE flame retardant.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial OBDE have been found in aquatic sediment
and human fat. Some lower brominated components (HxBDE and PeBDE) of
commercial OBDE have been found in biota. OBDE has not been detected,
but HpBDE and NBDE have generally not been looked for. Commercial OBDE
components are likely to be persistent but, as bromination levels rise
beyond HxBDE, they are increasingly unlikely to bioaccumulate. A
bioaccumulation factor of less than 2 has been found in carp for a
commercial OBDE product.
Pyrolysis of commercial OBDE, as such, or of polymers with OBDE as
a flame retardant (with, or without, Sb2O3) at 600 °C has been shown
to produce PBDF and, in much lower concentrations, PBDD. Processing of
ABS with OBDE/Sb2O3 under different conditions, showed that under
normal processing conditions, only low levels of PBDF were formed.
Under abusive conditions, the concentrations were much higher. PBDD
concentrations were low in both cases.
1.1.4 Environmental levels and human exposure
OBDE and the lower brominated components of commercial OBDE were
not detected in water samples collected in Japan in 1987 and 1988.
Sediment samples were also analysed and, in approximately 2-6% of the
samples, OBDE was detected in concentrations ranging from 8 to
22 µg/kg dry weight. Lower brominated components were also found in
the sediment.
OBDE was not detected in fish samples collected in Japan in 1987
and 1988.
In the USA, samples of human fat were investigated for the
presence of PBDF and PBDD in 1987. The samples were derived from 865
specimens combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. In these
samples, PBDE were also identified and preliminary evidence showed the
presence of OBDE at a frequency of 60% and an estimated concentration
of up to 8000 ng/kg.
1.1.5 Kinetics and metabolism in laboratory animals and humans
No data are available.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of commercial OBDE for laboratory mammals is
low. The substance is not irritant to the skin and gives only slight
eye irritation in rabbits. In short-term toxicity studies (4-week and
13-week), rats administered dietary levels of 100 mg/kg had increased
liver weights and microscopic changes characterized by enlarged
centrolobular and midzonal liver parenchymal cells containing granular
structures. These liver changes were more severe at higher dose
levels, i.e., 1000 and 10 000 mg/kg diet. In addition, hyperplasia of
the thyroid was seen. Total bromine content in the tissues increased
during the study and decreased slowly during a recovery period. The
liver changes were reversible. In an inhalation study with micronized
dust of OBDE (8 h/day for 14 consecutive days), no effects were
observed with exposure to 1.2 mg/m3, but a level of 12 mg/m3 caused
the liver changes described in the oral studies.
In rats, commercial OBDE at relatively low doses increased
cytochrome P450 and induced hepatic microsomal enzymes, such as
UDP-glucuronyl transferase and benz[a]pyrene hydroxylase. Commercial
OBDE induced a porphyrinogenic effect in cultures of chick embryo
liver cells.
OBDE was tested for teratogenic potential in rats; at high dose
levels (25.0 and 50.0 mg/kg body weight), resorptions, or delayed
ossification of different bones and fetal malformations were observed.
The malformations observed with doses of 25 mg/kg body weight and
higher were most likely associated with maternal toxicity. These
changes were not seen at dose levels of 15.0 mg/kg body weight or
less. In rabbits, there was no evidence for teratogenic activity, but
fetotoxicity was seen at a maternally toxic dose level of 15 mg/kg
body weight. Teratogenicity studies showed a no-effect level of
2.5 mg/kg body weight.
In 28-day and 90-day rat studies, 100 mg OBDE per kg diet
(equivalent to 5 mg/kg body weight) induced minimal effects in the
liver. No no-effect level was established.
Results of the mutagenicity tests including an unscheduled DNA
assay, in vitro microbial assays, and an assay for sister chromatid
exchange with Chinese hamster ovary cells were all negative.
No long-term/carcinogenicity test results are available.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
Minimal data are available.
1.2 Conclusions
1.2.1 OBDE
Commercial OBDE is a mixture of hexa-, bepta-, octa-, and
nonabromodiphenyl ether, all of which persist in the environment,
largely bound to sediment.
OBDE is widely incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure by extraction from polymers is unlikely.
The acute toxicity of OBDE is low. There is no information on
uptake and loss in mammals. OBDE is not teratogenic or mutagenic.
Long-term toxicity and carcinogenicity studies are not available.
Several components of commercial OBDE have been identified in
human adipose tissue. The acute risk for the general population is
likely to be low. Risk assessment of long-term exposure is not
possible, because of the lack of relevant toxicity studies.
No information is available to draw conclusions on occupational
exposure to, or effects of, OBDE.
Limited information is available on the toxicity of OBDE for
organisms in the environment. Components of the commercial OBDE
mixture with lower levels of bromination may bioaccumulate in
organisms.
1.2.2 Breakdown products
Formation of PBDF, and to some extent PBDD, may occur when OBDE,
or products containing it, are heated to 400-800 °C. The possible
hazards associated with this have to be addressed.
Exposure of the general population to PBDF impurities in
flame-retarded polymers is unlikely to be significant. Properly
controlled incineration should not lead to the emission of significant
quantities of brominated dioxins and -furans. Any uncontrolled
combustion of products containing commercial OBDE can lead to the
generation of unquantified amounts of PBDF/PBDD. The significance of
this for both humans and the environment will be addressed in a future
EHC on PBDF/PBDD.
1.3 Recommendations
1.3.1 General
* Best available techniques should be used in the manufacture of
commercial OBDE, to minimize levels of hexa- and lower brominated
congeners, because of their bioaccumulation potential in the
environment.
* Workers involved in the manufacture of OBDE, and products
containing the compound, should be protected from exposure using
appropriate industrial hygiene measures, monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent material and its breakdown
products.
* Incineration of materials, flame retarded with OBDE, should only
be in properly constituted incinerators running under consistently
optimal conditions. Burning by any other means may lead to
production of PBDF and/or PBDD.
1.3.2 Further studies
Because the present toxicological database is inadequate to
evaluate the hazards of commercial OBDE for humans and the
environment, and, to support its use, the following studies should be
carried out:
* Investigations on the bioavailability and ecotoxicity of
sediment-bound components of commercial OBDE using the relevant
organisms
* Extended monitoring of environmental levels of components of
commercial OBDE
* Long-term toxicity and carcinogenicity studies of commercial OBDE
* Monitoring of occupational exposure to commercial OBDE
* Further investigations on the generation of PBDF under real fire
conditions
* Further studies on environmental biodegradation and
photodegradation in compartments other than water
* Investigation of possible methods and consequences of recycling of
OBDE-containing polymers
* Validation of analytical methods for OBDE in various matrices
* Investigations on the possibility of migration from different
types of polymers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12 H Br9 O
CAS registry number: 63936-56-1
Chemical name: pentabromo(2,3,4,5-tetrabromophenoxy)-
benzene
Common name: nonabromodiphenyl ether (NBDE);
nonabromodiphenyloxide
Relative molecular mass: 880.37
Based on the chemical structure, there are three possible isomers
of nonabromodiphenyl ether.
From: US EPA (1984, 1986).
2.2 Physical and chemical properties
No data are available.
2.3 Analytical methods
No specific data are available (see General Introduction section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
NBDE has not been reported to occur naturally (see General
Introduction section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Nonabromodiphenyl ether has been prepared from decabromodiphenyl
ether by heating with NaSH in xylene at 130 °C for 2 h. The removal of
the reactive Br in the ortho-position of decabromodiphenyl ether led
to the photostability of the product (Noguchi et al., 1977).
3.2.2 Uses
Nonabromodiphenyl ether is not used commercially as a flame
retardant. It is present as an impurity in OBDE at a concentration of
10%.
OCTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Pure octabromodiphenyl ether is not manufactured or used. No data
are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial OBDE is a mixture of approximately 11% PeBDE/HxBDE, 44%
HpBDE, 31-35% OBDE, 10% NBDE, and 0.5% DeBDE. On the basis of the
chemical structure, there are 12 possible isomers of OBDE and 24
possible isomers of HpBDE.
The melting point varies from about 80 °C to >200 °C. The vapour
pressure is < 10-7 mmHg. The solubility in water is low and the
n-octanol/water partition coefficient (log Pow) >5.5. The above
variations in physical data may be explained by the differences in
composition of the mixtures tested.
1.1.2 Production and uses
The worldwide consumption of commercial OBDE per year is 6000
tonnes, 70% of which is used as a flame retardant in ABS for the
production of computers and business cabinets. OBDE is the second most
widely used PBDE flame retardant.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial OBDE have been found in aquatic sediment
and human fat. Some lower brominated components (HxBDE and PeBDE) of
commercial OBDE have been found in biota. OBDE has not been detected,
but HpBDE and NBDE have generally not been looked for. Commercial OBDE
components are likely to be persistent but, as bromination levels rise
beyond HxBDE, they are increasingly unlikely to bioaccumulate. A
bioaccumulation factor of less than 2 has been found in carp for a
commercial OBDE product.
Pyrolysis of commercial OBDE, as such, or of polymers with OBDE as
a flame retardant (with, or without, Sb2O3) at 600 °C has been shown
to produce PBDF and, in much lower concentrations, PBDD. Processing of
ABS with OBDE/Sb2O3 under different conditions, showed that under
normal processing conditions, only low levels of PBDF were formed.
Under abusive conditions, the concentrations were much higher. PBDD
concentrations were low in both cases.
1.1.4 Environmental levels and human exposure
OBDE and the lower brominated components of commercial OBDE were
not detected in water samples collected in Japan in 1987 and 1988.
Sediment samples were also analysed and, in approximately 2-6% of the
samples, OBDE was detected in concentrations ranging from 8 to
22 µg/kg dry weight. Lower brominated components were also found in
the sediment.
OBDE was not detected in fish samples collected in Japan in 1987
and 1988.
In the USA, samples of human fat were investigated for the
presence of PBDF and PBDD in 1987. The samples were derived from 865
specimens combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. In these
samples, PBDE were also identified and preliminary evidence showed the
presence of OBDE at a frequency of 60% and an estimated concentration
of up to 8000 ng/kg.
1.1.5 Kinetics and metabolism in laboratory animals and humans
No data are available.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of commercial OBDE for laboratory mammals is
low. The substance is not irritant to the skin and gives only slight
eye irritation in rabbits. In short-term toxicity studies (4-week and
13-week), rats administered dietary levels of 100 mg/kg had increased
liver weights and microscopic changes characterized by enlarged
centrolobular and midzonal liver parenchymal cells containing granular
structures. These liver changes were more severe at higher dose
levels, i.e., 1000 and 10 000 mg/kg diet. In addition, hyperplasia of
the thyroid was seen. Total bromine content in the tissues increased
during the study and decreased slowly during a recovery period. The
liver changes were reversible. In an inhalation study with micronized
dust of OBDE (8 h/day for 14 consecutive days), no effects were
observed with exposure to 1.2 mg/m3, but a level of 12 mg/m3 caused
the liver changes described in the oral studies.
In rats, commercial OBDE at relatively low doses increased
cytochrome P450 and induced hepatic microsomal enzymes, such as
UDP-glucuronyl transferase and benz[a]pyrene hydroxylase. Commercial
OBDE induced a porphyrinogenic effect in cultures of chick embryo
liver cells.
OBDE was tested for teratogenic potential in rats; at high dose
levels (25.0 and 50.0 mg/kg body weight), resorptions, or delayed
ossification of different bones and fetal malformations were observed.
The malformations observed with doses of 25 mg/kg body weight and
higher were most likely associated with maternal toxicity. These
changes were not seen at dose levels of 15.0 mg/kg body weight or
less. In rabbits, there was no evidence for teratogenic activity, but
fetotoxicity was seen at a maternally toxic dose level of 15 mg/kg
body weight. Teratogenicity studies showed a no-effect level of
2.5 mg/kg body weight.
In 28-day and 90-day rat studies, 100 mg OBDE per kg diet
(equivalent to 5 mg/kg body weight) induced minimal effects in the
liver. No no-effect level was established.
Results of the mutagenicity tests including an unscheduled DNA
assay, in vitro microbial assays, and an assay for sister chromatid
exchange with Chinese hamster ovary cells were all negative.
No long-term/carcinogenicity test results are available.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
Minimal data are available.
1.2 Conclusions
1.2.1 OBDE
Commercial OBDE is a mixture of hexa-, bepta-, octa-, and
nonabromodiphenyl ether, all of which persist in the environment,
largely bound to sediment.
OBDE is widely incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure by extraction from polymers is unlikely.
The acute toxicity of OBDE is low. There is no information on
uptake and loss in mammals. OBDE is not teratogenic or mutagenic.
Long-term toxicity and carcinogenicity studies are not available.
Several components of commercial OBDE have been identified in
human adipose tissue. The acute risk for the general population is
likely to be low. Risk assessment of long-term exposure is not
possible, because of the lack of relevant toxicity studies.
No information is available to draw conclusions on occupational
exposure to, or effects of, OBDE.
Limited information is available on the toxicity of OBDE for
organisms in the environment. Components of the commercial OBDE
mixture with lower levels of bromination may bioaccumulate in
organisms.
1.2.2 Breakdown products
Formation of PBDF, and to some extent PBDD, may occur when OBDE,
or products containing it, are heated to 400-800 °C. The possible
hazards associated with this have to be addressed.
Exposure of the general population to PBDF impurities in
flame-retarded polymers is unlikely to be significant. Properly
controlled incineration should not lead to the emission of significant
quantities of brominated dioxins and -furans. Any uncontrolled
combustion of products containing commercial OBDE can lead to the
generation of unquantified amounts of PBDF/PBDD. The significance of
this for both humans and the environment will be addressed in a future
EHC on PBDF/PBDD.
1.3 Recommendations
1.3.1 General
* Best available techniques should be used in the manufacture of
commercial OBDE, to minimize levels of hexa- and lower brominated
congeners, because of their bioaccumulation potential in the
environment.
* Workers involved in the manufacture of OBDE, and products
containing the compound, should be protected from exposure using
appropriate industrial hygiene measures, monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent material and its breakdown
products.
* Incineration of materials, flame retarded with OBDE, should only
be in properly constituted incinerators running under consistently
optimal conditions. Burning by any other means may lead to
production of PBDF and/or PBDD.
1.3.2 Further studies
Because the present toxicological database is inadequate to
evaluate the hazards of commercial OBDE for humans and the
environment, and, to support its use, the following studies should be
carried out:
* Investigations on the bioavailability and ecotoxicity of
sediment-bound components of commercial OBDE using the relevant
organisms
* Extended monitoring of environmental levels of components of
commercial OBDE
* Long-term toxicity and carcinogenicity studies of commercial OBDE
* Monitoring of occupational exposure to commercial OBDE
* Further investigations on the generation of PBDF under real fire
conditions
* Further studies on environmental biodegradation and
photodegradation in compartments other than water
* Investigation of possible methods and consequences of recycling of
OBDE-containing polymers
* Validation of analytical methods for OBDE in various matrices
* Investigations on the possibility of migration from different
types of polymers.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
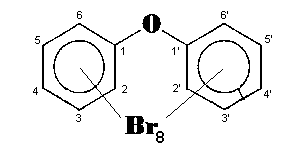 Chemical formula: C12H2Br8O
Relative molecular mass: 801.47
Common names: octabromodiphenyl ether (OBDE)
octabromodiphenyl oxide
CAS registry number: 32536-52-0
EINECS number: 2510879
MITI number: 3-3716
CAS name: 1, 1'-oxy(bis)-octabromo-benzene
Synonyms: octabromodiphenyl ether; benzene,
1,1'-oxy-bis-,octabromo-phenyl
ether
Based on the chemical structure, there are 12 possible isomers of
octabromodiphenyl ether.
From: IRPTC (1988); US EPA (1984); Ethyl Corp. (1992b).
2.1.1 Technical product
Trade names: Bromkal 79-8 DE; DE-79TM; FR 143;
Tardex 80; FR 1208; Adine 404;
Saytex 111
The commercial product is a mixture of polybrominated diphenyl
ethers with the following typical composition (see Table 1):
0-0.7% decabromodiphenyl ether
9.5-11.3% nonabromodiphenyl ether
31.3-35.3% octabromodiphenyl ether
43.7-44.5% heptabromodiphenyl ether
10.5-12.0% hexabromo- and pentabromodiphenyl ether.
Commercial OBDE has also been reported to be a mixture of 4%
hexabromo-, 62% hepta-, and 34% octabromodiphenyl ether (De Kok et
al., 1979).
2.2 Physical and chemical properties
Commercial products based on OBDE are off-white powders with a
faint odour, and a bromine content of 79-81%. In case of fire, and, in
the presence of fuels, hydrogen bromide and/or bromine may be
liberated (Kopp, 1990).
Melting point: 200 (167-257) °C, Ethyl Corp. (1992b);
79-87 °C; 75-125 °C, Kopp (1990); and
170-220 °C, De Kok et al. (1979)
Vapour pressure: < 10-7 mmHg at 25 °C
Solubility at 25 °C in g/litre:
water <1
methanol 2(7)
methylene chloride 110
toluene 190 (353)
benzene 200
styrene 250
methyl ethyl ketone 40
acetone 20 (122)
(From: Great Lakes Chemical Corporation, 1987, 1990a)
Specific gravity: 2.76 (2.63)
(From: US EPA, 1989, 1986)
n-Octanol/water partition
coefficient (log Pow): 5.5; 8.35-8.90
(From: Watanabe & Tatsukawa, 1990; Ethyl Corporation, 1992b).
2.3 Analytical methods
No specific data are available (see General Introduction section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Octabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Octabromodiphenyl ether is synthesized by treating diphenyl ether
with 8 equivalents of Br2 in the presence of Al2Cl6/A12Br6, first
at 35 °C and then at 120 °C (US EPA, 1986). No data are available on
production levels.
The annual consumption of OBDE in Japan was 150 tonnes in 1983,
and 1000 tonnes in 1987, mainly used in the preparation of ABS
(Watanabe, 1987; Watanabe et al., 1987b). Consumption in Germany was
600-800 tonnes/year in plastics in 1988 (CEM, 1989). Estimation of
total use of octabromodiphenyl ether in the Netherlands in 1988 was
600-800 tonnes.
The actual worldwide consumption of OBDE per year is 6000 tonnes
(Arias, 1992).
3.2.2 Uses
The high bromine content and its broad melting range make OBDE the
material of choice for a large variety of thermoplastics. Its use is
recommended for injection mouldings, especially when high surface
quality is desirable. Applications: ABS, nylon, high impact
polystyrene, low density polyethylene, polypropylene random copolymer
(Flick, 1986). It is also used in adhesives and coatings.
The major use of OBDE is in computer and business equipment
cabinets (Personal communication, McAllister).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Biotransformation
No data are available.
4.2 Abiotic degradation
4.2.1 Pyrolysis of octabromodiphenyl ether
Pyrolysis of octabromodiphenyl ether (OBDE) at 630 °C, in air,
resulted in approximately 96% decomposition leading to the formation
of polybrominated benzenes (PBBz) and about 5% of PBDF and PBDD,
mainly two pentabromodibenzodioxins and a hexabromodibenzofuran. Small
amounts of tri- to heptabromo-dibenzodioxins and tetra- to
heptabromodibenzofurans were present (Buser, 1986).
4.2.2 Pyrolysis studies with polymers containing octabromodiphenyl
ether
Pyrolysis studies were conducted on ABS containing OBDE (15%) and
antimony trioxide (6%) at 600 °C (Neupert et al., 1989). Brominated
dibenzofurans and brominated dibenzodioxins were identified in the
pyrolysis residues, however, the brominated dibenzodioxins and
2,3,7,8-TeBDF were present in very low quantities.
The pyrolysis of Cycolac, flame retarded with OBDE in combination
with antimony trioxide, gave high levels of brominated dibenzodioxins
and dibenzofurans and significant levels (9.3 mg/kg) of
2,3,7,8-isomers at 600 °C. Pyrolysis temperatures were 200, 250, and
600 °C during 30 min. The total PBDF were 110.8, 666, and 1631 mg/kg,
respectively. The values for PBDD were 1.75, 0.75, and 31.5 mg/kg,
respectively (Fresenius Institute, 1990).
4.2.3 Behaviour of octabromodiphenyl ether during processing
BFRIP designed an experiment to investigate the behaviour of OBDE
in ABS under controlled moulding or extrusion conditions. The polymer
system ABS/OBDE was operated under different conditions (Table 25).
After moulding, all the samples were analysed for brominated
dibenzodioxins and dibenzofurans. With the exception of a single
sample, brominated dibenzodioxins were not identified in any of the
samples. In this sample, the 2,3,7,8-brominated dibenzofurans were
seen only at very low concentrations (Table 26). The data show that
processing of the resin systems under normal conditions (225 °C; 1-min
cycle) resulted in no change in composition from that of the base
resin formulation (BFRIP, 1990; McAllister et al., 1990).
Table 25. Moulding study with ABS/OBDE system at different processing temperaturesa
Polymer/FR Severity Conditions
ABS/OBDE normal 225 °C, 1 min cycle
abusive 245 °C, 10 min cycle
ABS/OBDE acrylonitrile-butadiene- 79.8%
styrene/OBDE + 16.0%
antimony trioxide 4.2%
aFrom: McAllister et al. (1990).
Craig et al. (1989) measured the PBDF and PBDD contents of pre-
and post-extruded Cycolac resin treated with OBDE and antimony
trioxide. Two samples were tested at extrusion temperatures around
220 °C. PBDF levels varied from one another by a factor of 4 in the
pre-extrusion samples and by a factor of 2 in the post-extrusion
samples. The higher levels of total PBDF were 38 300 µg/kg in the
pre-extruded and 84 500 µg/kg in the post-extruded resins. PBDD was
not detected in the pre-extruded resin, but, in one post-extruded
sample, it was found at a level of 112 µg/kg. The fumes emitted during
extrusion were also analysed and a level of 1850 µg/kg was found. PBDD
was found in a concentration of 0.54 µg/kg. (Fume data expressed as
µg/kg of extruded resin).
4.3 Bioaccumulation
A single study on a mixture of PBDE including HxBDE to DeBDE
indicated little bioaccumulation in carp with a bio-concentration
factor of < 4 after 8 weeks of exposure (CBC, 1982).
4.4 Ultimate fate following use
For the ultimate fate of OBDE in the environment see General
Introduction, section 6.1.
Table 26. Comparison of Triangle Laboratories Inc. (TLI) (Research Triangle
Park) and US EPA Laboratory (Las Vegas) results on moulded polymer
samples containing various flame retardants. (All concentrations
are in mg/kg)a
ABS with octabromodiphenyl ether
Normal 1222-16-4 Abusive 1222-16-5
Compound TLI US TLI US
EPAc EPA
1,2,3,7,8-PeBDD < 0.002 0.02
2,3,7,8-TeBDF < 0.002 0.004
Total TeBDD < 0.001 0.01
Total PeBDD 0.03 < 0.13
Total TeBDF 0.003 0.003 0.17 0.16
Total PeBDF 1.1 1.3 < 14.0 31.5
Total HxBDF < 135.0 2.2 < 118.0 9.1
Total HpBDFb < 6.6 0.6 < 2.9 0.7
Total OBDFb < 34.5 0.04 < 13.9 0.02
a From: BFRIP (1990). Values preceded by < are maximum possible. Analytical signals
met identification criteria, but may include contribution from interferences.
US EPA and TLI used different identification criteria resulting in differences
in reported values.
b Concentration of HpBDF and OBDF from TLI are not validated because of lack of
standards. Concentrations are reported for comparison only.
c Average of two analysis of same sample.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In Japan, OBDE was not detected in 75 water samples (limit of
determination 0.1 µg/litre) in 1987 and was not detected in 147 water
samples collected at 49 areas (limit of determination, 0.07 µg/litre)
in 1988-89 (Environment Agency Japan, 1989, 1991).
5.1.2 Aquatic sediments
In 1987, environmental surveys were conducted concerning OBDE in
bottom sediment. OBDE was detected in 3 out of 51 samples at
concentrations ranging from 8 to 21 µg/kg in 1987 (limit of
determination 7 µg/kg dry weight) and was detected in 3 out of 135
samples, collected at 45 areas, at concentrations ranging from 15 to
22 µg/kg dry weight (limit of determination 5 µg/kg) in 1988-89
(Environment Agency Japan, 1989, 1991).
5.1.3 Aquatic and terrestrial organisms
In Japan, OBDE was not detected in 75 fish samples in 1987 and was
not found in 144 fish samples collected in 48 areas in 1988-89 (limit
of determination 5 µg/kg wet weight (Environment Agency Japan, 1989,
1991).
5.2 Exposure of the general population
In the USA, Cramer et al. (1990a,b) studied the presence of
PBDD/PBDF in human adipose tissue samples of the fiscal year 1987
(National Human Adipose Tissue Survey). The samples were derived from
865 specimen combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. The analysis
was carried out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD
was found (limits of determination, 10-40 ng/kg, depending on
congeners). Identification of the PBDE was based on comparison of full
scan mass spectra of the samples with the available standards,
application of SIM techniques to compare theoretical ion ratios with
observed ion ratios for characteristic ions, and measurement of
fragment losses from the molecular ion clusters. Preliminary evidence
for the presence of OBDE was found (frequency, 60%) with an estimated
concentration range of ND-8000 ng OBDE/kg (Cramer et al., 1990a,b;
Stanley et al., 1991).
5.3 Occupational exposure during manufacture, formulation, or use
No data are available.
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1 Single exposure
6.1.1 Oral: Rat
The acute oral LD50 in the rat is >28 g/kg body weight (Kalk,
1982); >5 g/kg body weight (Kopp, 1990; Great Lakes Chemical
Corporation, 1990a).
Male Charles River CD rats were administered commercial OBDE,
suspended in corn oil, by intubation, at doses of 50, 500, or
5000 mg/kg body weight. The observation period was 14 days. The rats
showed normal weight gain and no animals died (Great Lakes Chem Corp.,
1987; 1990a).
6.1.2 Dermal: Rabbit
Commercial OBDE was applied to the clipped, abraded, or intact
skin of male and female albino rabbits at concentrations of 200 or
2000 mg/kg body weight for 24 h. During the observation period of 14
days, none of the rabbits died and all had normal weight gain. The
dermal LD50 for rabbits is >2 g/kg body weight (Great Lakes Chemical
Corporation, 1987, 1990a).
6.1.3 Inhalation: Rat
The inhalation LC50 of Saytex 111 in rats was > 50 mg/litre (US
EPA, 1986).
Male and female Charles River CD rats were exposed for 1 h to
concentrations of commercial OBDE in air of 2 or 60 mg/litre. The
physical nature of the substance precluded administration at higher
levels than 60 mg/litre. Rats exposed to 2 and 60 mg/litre had
decreased motor activity, erythema, and eye squint, during exposure.
The animals exposed to 60 mg/litre also had tachypnea. During the
14-day observation period, the animals appeared normal, and exhibited
normal body weight gain, except for one rat at the highest dose level,
which showed salivation on days 5 and 7 of the observation period
(Great Lakes Chemical Corporation, 1987) (no further details).
6.2 Short-term exposure
6.2.1 Oral: Rat
Charles River CD rats were fed commercial OBDE at daily, dietary
dose levels of 0, 100, or 1000 mg/kg for 28 days. There were 10 male
and 10 female rats in each dose group. No changes in appearance,
behaviour, mortality, feed consumption, or body weight gain were
observed. Results of haematology, blood chemistry, and urinalysis were
similar to those in the controls. Absolute and relative liver weights
were significantly increased in female rats given 100 mg/kg and in
rats of both sexes given 1000 mg/kg diet. Other increases in organ
weights were not considered compound-related. No gross pathological
lesions were noted in rats in any of the groups. Compound-related
histopathological liver lesions were observed in both treated groups
of rats. They consisted of enlarged centrolobular and midzonal liver
parenchymal cells in which the cytoplasm had large areas of finely
granular structures containing eosinophilic "round bodies". These
changes occurred more frequently and with greater severity in the male
animals. Their incidence and severity were dose-related. Rats given
1000 mg/kg had a slight to moderate hyperplasia of the thyroid, but it
was not clear whether this was compound-related. Dose-related
increases in total bromine levels of the liver were noted in both
males and females and ranged from about 6 to 137 times the levels
found in controls (Great Lakes Chemical Corporation, 1987).
Another 28-day feeding study was carried out on 10 male and 10
female Charles River CD rats per group, which were administered a diet
containing 100, 1000, or 10000 mg commercial OBDE/kg. The control
group consisted of 35 male and 35 female rats from the 90-day feeding
study, described below. At the end of the study, 5 rats of each
sex/group were sacrificed and the remaining 5 animals/sex per group
were maintained on normal diets for 4 weeks (recovery period). No
changes in appearance, behaviour, or mortality were observed. Food
consumption and body weight gain were slightly higher in the control
group. Haematology values were normal, but serum urea nitrogen levels
were slightly elevated in some of the rats given 10 000 mg/kg diet. An
increase in absolute and relative liver weights was observed in some
of the rats given 1000 and 10 000 mg/kg. At necropsy, the livers of
some animals in the 10 000 mg/kg group showed accentuated lobulization
and discoloration. The livers of rats from all 3 dose levels showed
enlargement of the centrolobular and midzonal hepatocytes, the
cytoplasm of which had large areas of finely granular appearance and
frequently contained eosinophilic "round bodies". In the 10 000 mg/kg
group, vacuolization of hepatocytes and necrosis of scattered
individual hepatocytes were seen. In all 3 treated groups, the liver
lesions were less severe after the 4-week recovery period than during
the administration of the compound. A dose-related increase in liver
total bromine content was seen in rats in all treated groups after 4
weeks of treatment, but these total bromine levels decreased rapidly
in the recovery period. Only in the rats receiving the lowest dose
level did the liver total bromine concentration approach control
levels after a 4-week withdrawal period (Great Lakes Chemical
Corporation, 1987).
Charles River CD rats were fed commercial OBDE at dietary levels
of 0, 100, 1000, or 10 000 mg/kg for up to 13 weeks. There were 35
male and 35 female rats in each dose group. Behaviour, appearance,
body weight, food consumption, haematology, blood chemistry, and
urinalysis were studied after 1 and 2 months, and, at the end of the
study, in groups of 5 rats/sex per group. The remaining 20 rats per
group were used to study the recovery and 5 rats/sex per group were
sacrificed after 13 and 21 weeks and 6 months after withdrawal. During
the study, a few rats out of each group died, but without any
dose-relationship, the majority of these deaths occurring after the
collection of blood.
In the 100 mg/kg diet group, the only effect that was seen was an
increase in absolute and relative liver weights. Microscopic changes
were seen in 4 out of 10 rats, and were typified by granular
cytoplasmic changes, Liver total bromine contents increased during the
13-week treatment, but decreased during the recovery period.
In the 1000 mg/kg group, a decrease in body weight gain was
observed, but haematology, blood chemistry, and urinalysis results
were comparable with the controls. Increases in absolute and relative
liver and thyroid weights were observed, but these effects were not
seen in the animals sacrificed during the recovery period. Microscopic
lesions were seen in the cytoplasm of the centrolobular and midzonal
hepatocytes and included vacuolization, and hyaline intracytoplasmic
inclusions. A hyperplastic nodule was found, after 6 months
withdrawal, in one rat each from the 1000 and 10 000 mg/kg groups.
In the 10 000 mg/kg group, a decrease in body weight gain was
observed, even during the withdrawal period. Decreases in haemoglobin,
haematocrit, and erythrocyte counts were also observed. One female
animal had hypochromia, polychromia, and anisocytosis of the
erythrocytes. Glucose levels in the blood were slightly lower than in
the controls. Orange colouration of the urine was observed during
weeks 13-39. A significant increase in absolute and relative liver,
kidney, and thyroid weights was observed. At autopsy, there was
accentuated lobulation and yellowish mottling of the livers and
brownish discoloration of the liver and kidneys. After one year of
recovery, no such gross changes were observed. Histopathologically,
the liver changes consisted of granular cytoplasmic changes,
cytoplasmic vacuolization (possibly representing fatty degeneration),
necrosis of scattered parenchymal cells or of centrolobular cells,
centrolobular fibrosis, and pigmented Kupfer cells. In the kidneys,
the changes were characterized by the occurrence of small to moderate
numbers of cortical regenerative tubules. One rat had a severe tubular
nephrosis. Cellular changes in the thyroid were probably
compound-related. During the recovery period, the histological changes
decreased in severity and frequency.
The total bromine content of the liver increased during the 13
weeks of treatment and decreased during the recovery period, but
remained higher (not significantly) than the control values after one
year (Great Lakes Chemical Corporation, 1987).
6.2.2 Inhalation: Rat
Charles River CD rats were exposed (whole-body) to a micronized
dust of commercial OBDE, introduced into inhalation chambers at
nominal concentrations of 1.2, 12, 120, and 1200 mg/m3 for 8 h/day on
14 consecutive days. Actual airborne dust concentrations were 15-45%
of the nominal values. It was not possible to measure the extent of
oral intake of the substance. There were 5 male and 5 female rats in
each dose group and in the controls. No animals died during the test
period nor were there any changes in appearance or general behaviour
in the 1.2 and 12 mg/m3 groups. By the end of the 8-h exposure
period, all animals in the 1200 mg/m3 group and part of the
120 mg/m3 group exhibited a fast breathing pattern, which had
disappeared by the morning following exposure. Food consumption, body
weight gain, haematology, blood chemistry, and urinalysis in all of
the dose groups were normal. The total bromine concentrations in the
lung, liver, and fat were significantly higher than in the controls.
At autopsy, the average total bromine concentrations in the lung and
fat were about 1.5-12.5 times higher than in the liver. The relative
liver weights of the animals in the 12, 120, and 1200 mg/m3 dose
groups were significantly increased in a dose-related manner. These
changes were accompanied by histopathological lesions consisting of
focal to multifocal cytoplasmic enlargement of the hepatocytes, and
focal acidophilic degeneration of individual, and small groups of,
liver cells. At the 2 highest dose levels, the enlargement of the
hepatocytes was multifocal to diffuse in distribution and small to
large areas had necrosis in the centrolobular regions of the affected
liver lobules, especially in the 1200 mg/m3 group. No other
compound-related effects were observed (Great Lakes Chemical
Corporation, 1987).
6.3 Long-term exposure
No data are available.
6.4 Skin and eye irritation; sensitization
6.4.1 Skin irritation
Commercial OBDE was applied to the clipped and occluded intact, or
abraded, skin of male and female albino rabbits at a dose of 500 mg.
After 24 h, the skin was washed and examined for irritation. The
examination was repeated after 72 h. One rabbit had slight erythema at
72 h; the remaining rabbits did not have skin changes. It was
concluded that OBDE is not a primary skin irritant (Great Lakes
Chemical Corporation, 1987).
6.4.2 Eye irritation
Single applications of 100 mg commercial OBDE were made into the
conjunctival sac of the eye of 3 male and 3 female New Zealand white
rabbits. Examinations were made at 24, 48, and 72 h, and 7 days. A
slight discharge was noted from the eyes of 2 rabbits at 24 h and a
slight redness was noted in the eye of one rabbit at 48 h. No ocular
irritation or corneal damage (sodium fluorescein and UVR were used)
was observed (Great Lakes Chemical Corporation, 1987).
6.5 Teratogenicity, reproductive toxicity, and embryotoxicity
6.5.1 Teratogenicity
6.5.1.1 Oral: Rat
In a range-finding study, female rats (number not specified) were
dosed daily, by gavage, from days 6 to 15 of gestation with 2.5, 10.0,
15.0, 25.0, or 50.0 mg commercial OBDE (DE-79)/kg body weight. All
animals survived until gestation day 20, when they were sacrificed. At
25.0 mg/kg, increased serum bromine levels were observed. Mean
maternal body weight gain was reduced in the 50.0 mg/kg group during
gestation. Increased numbers of late resorptions and significantly
reduced mean fetal weights were observed at the highest dose level.
The cholesterol level was slightly increased in the dams given
50.0 mg/kg. No compound-related microscopic findings were observed in
the liver and kidneys of the mothers. No compound-related effects were
observed at 15.0 mg/kg or lower. The malformations and developmental
variations observed in the 50.0 mg/kg group were associated with
maternal toxicity. Fetal anasarca and bent limb bones were observed at
this dose level. Mean fetal body weight and increased post
implantation loss due to !ate resorptions was also observed at this
level, but the losses were not statistically significant, compared
with the controls. Reduced ossification of the skull, various
unossified bones, and two instances of bent ribs were noted at this
dose level, and were probably secondary to maternal toxicity (Great
Lakes Chemical Corporation, 1987) (abstract).
Four groups of 25 pregnant Charles-River Crb:COBS CD (SD) BR rats
were administered corn-oil suspensions of Saytex 111, by gavage, at
doses of 0, 2.5, 10.0, or 25 mg/kg body weight per day on days 6-15 of
gestation. The dams were sacrificed on day 20 of gestation and the
fetuses were examined for gross visceral and skeletal abnormalities.
The substance was more toxic to the conceptus than to the dam. At
25.0 mg/kg, dose-dependant effects on the conceptus were observed.
These included reduced average fetal body weight, increased
embryo/fetal deaths (resorptions), fetal malformations, such as
enlarged heart, rear limb malformation, and delayed skeletal
ossification. At 10 mg/kg, the only observed effect was a
statistically insignificant reduction in average fetal body weight (US
EPA, 1986).
6.5.1.2 Oral: Rabbit
Groups of 26 inseminated adult New Zealand White rabbits (weight
3.5-4.5 kg) were treated with 0 (corn oil), 2.0, 5.0, or 15 mg Saytex
111/kg body weight per day, by gavage, on days 7-19 of gestation.
Saytex 111 was a mixture containing; 0.2% PeBDE, 8.6% HxBDE, 45.0%
HpBDE, 33.5% OBDE, 11.2% NBDE, and 1.4% DeBDE. Body weight gain was
recorded on gestation days 0, 7, 10, 13, 16, 20, and 28. In addition,
maternal liver, kidneys, and gravid uterine weights were measured at
the time of hysterectomy. The offspring were examined on day 28 of
gestation. A statistically significant increase in liver weight and a
decrease in body weight gain were observed in the 15 mg/kg group.
There was no statistically significant deviation in maternal
mortality, number of pregnancies, number of litters with viable pups,
corpora lutea/dam, implantations/dam, live fetuses/litter, percentage
of resorptions, and fetal body weight. Slight fetal toxicity was
observed in the 15 mg/kg group, as evidenced by a significant increase
in delayed ossification of the sternebrae. There was an increase in
the incidence of retrocaval ureter in the 5 and 15 mg/kg group and
fused sternebrae in the 5 mg/kg group. These increases were not dose
related. It was concluded by the authors that there was no evidence
for teratogenic activity but that there was slight letotoxicity at
maternally toxic dose levels, e.g., 15 mg/kg body weight (Breslin et
al., 1989).
6.6 Mutagenicity and related end-points
6.6.1 DNA damage
An unscheduled DNA synthesis (UDS) assay, a test to induce DNA
damage followed by repair in mammalian cells, was carried out with
monolayers of WI-38 human fibroblast cells, which were exposed to
commercial OBDE in the presence of radiolabelled thymidine. OBDE was
tested at 5 concentrations ranging from 60 to 300 µg/ml. UDS was not
induced by OBDE either in the presence or in the absence of a
metabolic activation system (Great Lakes Chemical Corporation, 1987).
6.6.2 Mutation
Commercial OBDE was examined for mutagenic activity at a number of
concentrations in in vitro microbial assays using Salmonella
typhimurium and Saccharomyces cerevisiae with, and without, liver
microsomal enzyme preparations from Aroclor-induced rats. The results
of these tests were all negative (Great Lakes Chemical Corporation,
1987) (no details).
6.6.3 Chromosomal effects
In an assay for sister chromatid exchanges, Chinese hamster ovary
cells were exposed to concentrations of commercial OBDE of 7.5, 25,
75, 250, or 750 µg/ml (in DMSO), for 2 h, in the presence, or absence,
of a metabolic activation system. The exposure period was followed by
a 24-h expression period. No statistically significant increases in
the number of exchanges per chromosome or the number of exchanges per
cell were seen at any of the levels tested (Great Lakes Chemical
Corporation, 1987).
6.7 Carcinogenicity
No data are available.
6.8 Other special studies
6.8.1 Liver
Commercial OBDE in corn oil was administered by gavage to six male
Sprague-Dawley rats (200-250 g) for 90 days. When extensive induction
was revealed at all the original doses of 6.25, 12.5, and 25 µmol/kg
per day, the study was repeated at doses of 0.78, 1.56, and
3.13 µmol/kg per day. OBDE did not cause induction of NADPH cytochrome
c reductase and cytochrome P450 at the lower doses. However, a dose of
0.78/µmol/kg per day caused increases in both O-ethyl-
O-p-nitrophenyl phenyl-phosphonothioate (EPN) detoxification and
p-nitroanisole demethylation; larger increases were seen with
increasing dose levels. After a 30-day recovery period, evidence of
the maintenance of an induced state was seen only in animals receiving
3.13 µmol/kg per day. These elevations were still observable 60 days
after the last dose. No histological liver abnormalities were observed
in rats treated with 3.13 µmol/kg body weight or less (Carlson,
1980b).
In another study on male rats (200-250g) administered commercial
OBDE, by gavage, at 0.1 mmol/kg per day in corn oil for 14 days, the
above increase in enzyme activity was accompanied by an increase in
activity of UDP-glucuronyl transferase and benzo[a]pyrene hydroxylase
24 h after the seventh dose (Carlson, 1980a). Measurements made on
days 30 and 60 of recovery after 90 days exposure showed that the
indicators of induced xenobiotic metabolism returned slowly to control
values.
Koster et al. (1980) found a strong porphyrinogenic effect in
cultures of chick embryo liver cells at concentrations of 10 µg
commercial OBDE (in DMSO)/ml medium, with, and without, pretreatment
of ß-napthoflavone, an inducer of P450, P448, and delta-aminolevulinic
acid synthethase. The effect was determined semiquantitatively with
fluorescence microscopy, 24 h after addition of the flame retardant.
6.9 Appraisal
In 28-day and 90-day rat studies, 100 mg OBDE per kg diet
(equivalent to 5 mg/kg body weight) induced minimal effects in the
liver. No no-effect level was established. In a 14-day inhalation
study with micronized dust of OBDE, no effects were found with
exposure to 12 mg/m3. Teratogenicity studies showed a no-effect level
of 2.5 mg/kg body weight.
At the highest reported value of OBDE of 8000 ng/kg, from the
Human Adipose Tissue Sample Study, assuming 15% adipose tissue in the
adult male, a "stored" dose of 1.2 µg/kg can be calculated. Assuming
1% of the administered dose was absorbed, this would extrapolate to a
total dose of 120 µg/kg. With the further assumption that the total
exposure occurred over one year, a daily dose of 0.38 µg/kg per day
can be calculated. This dose is approximately 4 orders of magnitude
below the most sensitive endpoint in mammalian toxicity testing on
OBDE (NOEL = 2.5 mg/kg body weight in a teratology study).
HEPTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Heptabromodiphenyl ether is not manufactured or used.
There is no database on pure HpBDE on which to make an evaluation.
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
Because HpBDE is the major component of commercial
octabromodiphenyl ether, the summary, evaluation, conclusions, and
recommendations on commercial OBDE, are relevant to "commercial"
HpBDE.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12H2Br8O
Relative molecular mass: 801.47
Common names: octabromodiphenyl ether (OBDE)
octabromodiphenyl oxide
CAS registry number: 32536-52-0
EINECS number: 2510879
MITI number: 3-3716
CAS name: 1, 1'-oxy(bis)-octabromo-benzene
Synonyms: octabromodiphenyl ether; benzene,
1,1'-oxy-bis-,octabromo-phenyl
ether
Based on the chemical structure, there are 12 possible isomers of
octabromodiphenyl ether.
From: IRPTC (1988); US EPA (1984); Ethyl Corp. (1992b).
2.1.1 Technical product
Trade names: Bromkal 79-8 DE; DE-79TM; FR 143;
Tardex 80; FR 1208; Adine 404;
Saytex 111
The commercial product is a mixture of polybrominated diphenyl
ethers with the following typical composition (see Table 1):
0-0.7% decabromodiphenyl ether
9.5-11.3% nonabromodiphenyl ether
31.3-35.3% octabromodiphenyl ether
43.7-44.5% heptabromodiphenyl ether
10.5-12.0% hexabromo- and pentabromodiphenyl ether.
Commercial OBDE has also been reported to be a mixture of 4%
hexabromo-, 62% hepta-, and 34% octabromodiphenyl ether (De Kok et
al., 1979).
2.2 Physical and chemical properties
Commercial products based on OBDE are off-white powders with a
faint odour, and a bromine content of 79-81%. In case of fire, and, in
the presence of fuels, hydrogen bromide and/or bromine may be
liberated (Kopp, 1990).
Melting point: 200 (167-257) °C, Ethyl Corp. (1992b);
79-87 °C; 75-125 °C, Kopp (1990); and
170-220 °C, De Kok et al. (1979)
Vapour pressure: < 10-7 mmHg at 25 °C
Solubility at 25 °C in g/litre:
water <1
methanol 2(7)
methylene chloride 110
toluene 190 (353)
benzene 200
styrene 250
methyl ethyl ketone 40
acetone 20 (122)
(From: Great Lakes Chemical Corporation, 1987, 1990a)
Specific gravity: 2.76 (2.63)
(From: US EPA, 1989, 1986)
n-Octanol/water partition
coefficient (log Pow): 5.5; 8.35-8.90
(From: Watanabe & Tatsukawa, 1990; Ethyl Corporation, 1992b).
2.3 Analytical methods
No specific data are available (see General Introduction section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Octabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Octabromodiphenyl ether is synthesized by treating diphenyl ether
with 8 equivalents of Br2 in the presence of Al2Cl6/A12Br6, first
at 35 °C and then at 120 °C (US EPA, 1986). No data are available on
production levels.
The annual consumption of OBDE in Japan was 150 tonnes in 1983,
and 1000 tonnes in 1987, mainly used in the preparation of ABS
(Watanabe, 1987; Watanabe et al., 1987b). Consumption in Germany was
600-800 tonnes/year in plastics in 1988 (CEM, 1989). Estimation of
total use of octabromodiphenyl ether in the Netherlands in 1988 was
600-800 tonnes.
The actual worldwide consumption of OBDE per year is 6000 tonnes
(Arias, 1992).
3.2.2 Uses
The high bromine content and its broad melting range make OBDE the
material of choice for a large variety of thermoplastics. Its use is
recommended for injection mouldings, especially when high surface
quality is desirable. Applications: ABS, nylon, high impact
polystyrene, low density polyethylene, polypropylene random copolymer
(Flick, 1986). It is also used in adhesives and coatings.
The major use of OBDE is in computer and business equipment
cabinets (Personal communication, McAllister).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Biotransformation
No data are available.
4.2 Abiotic degradation
4.2.1 Pyrolysis of octabromodiphenyl ether
Pyrolysis of octabromodiphenyl ether (OBDE) at 630 °C, in air,
resulted in approximately 96% decomposition leading to the formation
of polybrominated benzenes (PBBz) and about 5% of PBDF and PBDD,
mainly two pentabromodibenzodioxins and a hexabromodibenzofuran. Small
amounts of tri- to heptabromo-dibenzodioxins and tetra- to
heptabromodibenzofurans were present (Buser, 1986).
4.2.2 Pyrolysis studies with polymers containing octabromodiphenyl
ether
Pyrolysis studies were conducted on ABS containing OBDE (15%) and
antimony trioxide (6%) at 600 °C (Neupert et al., 1989). Brominated
dibenzofurans and brominated dibenzodioxins were identified in the
pyrolysis residues, however, the brominated dibenzodioxins and
2,3,7,8-TeBDF were present in very low quantities.
The pyrolysis of Cycolac, flame retarded with OBDE in combination
with antimony trioxide, gave high levels of brominated dibenzodioxins
and dibenzofurans and significant levels (9.3 mg/kg) of
2,3,7,8-isomers at 600 °C. Pyrolysis temperatures were 200, 250, and
600 °C during 30 min. The total PBDF were 110.8, 666, and 1631 mg/kg,
respectively. The values for PBDD were 1.75, 0.75, and 31.5 mg/kg,
respectively (Fresenius Institute, 1990).
4.2.3 Behaviour of octabromodiphenyl ether during processing
BFRIP designed an experiment to investigate the behaviour of OBDE
in ABS under controlled moulding or extrusion conditions. The polymer
system ABS/OBDE was operated under different conditions (Table 25).
After moulding, all the samples were analysed for brominated
dibenzodioxins and dibenzofurans. With the exception of a single
sample, brominated dibenzodioxins were not identified in any of the
samples. In this sample, the 2,3,7,8-brominated dibenzofurans were
seen only at very low concentrations (Table 26). The data show that
processing of the resin systems under normal conditions (225 °C; 1-min
cycle) resulted in no change in composition from that of the base
resin formulation (BFRIP, 1990; McAllister et al., 1990).
Table 25. Moulding study with ABS/OBDE system at different processing temperaturesa
Polymer/FR Severity Conditions
ABS/OBDE normal 225 °C, 1 min cycle
abusive 245 °C, 10 min cycle
ABS/OBDE acrylonitrile-butadiene- 79.8%
styrene/OBDE + 16.0%
antimony trioxide 4.2%
aFrom: McAllister et al. (1990).
Craig et al. (1989) measured the PBDF and PBDD contents of pre-
and post-extruded Cycolac resin treated with OBDE and antimony
trioxide. Two samples were tested at extrusion temperatures around
220 °C. PBDF levels varied from one another by a factor of 4 in the
pre-extrusion samples and by a factor of 2 in the post-extrusion
samples. The higher levels of total PBDF were 38 300 µg/kg in the
pre-extruded and 84 500 µg/kg in the post-extruded resins. PBDD was
not detected in the pre-extruded resin, but, in one post-extruded
sample, it was found at a level of 112 µg/kg. The fumes emitted during
extrusion were also analysed and a level of 1850 µg/kg was found. PBDD
was found in a concentration of 0.54 µg/kg. (Fume data expressed as
µg/kg of extruded resin).
4.3 Bioaccumulation
A single study on a mixture of PBDE including HxBDE to DeBDE
indicated little bioaccumulation in carp with a bio-concentration
factor of < 4 after 8 weeks of exposure (CBC, 1982).
4.4 Ultimate fate following use
For the ultimate fate of OBDE in the environment see General
Introduction, section 6.1.
Table 26. Comparison of Triangle Laboratories Inc. (TLI) (Research Triangle
Park) and US EPA Laboratory (Las Vegas) results on moulded polymer
samples containing various flame retardants. (All concentrations
are in mg/kg)a
ABS with octabromodiphenyl ether
Normal 1222-16-4 Abusive 1222-16-5
Compound TLI US TLI US
EPAc EPA
1,2,3,7,8-PeBDD < 0.002 0.02
2,3,7,8-TeBDF < 0.002 0.004
Total TeBDD < 0.001 0.01
Total PeBDD 0.03 < 0.13
Total TeBDF 0.003 0.003 0.17 0.16
Total PeBDF 1.1 1.3 < 14.0 31.5
Total HxBDF < 135.0 2.2 < 118.0 9.1
Total HpBDFb < 6.6 0.6 < 2.9 0.7
Total OBDFb < 34.5 0.04 < 13.9 0.02
a From: BFRIP (1990). Values preceded by < are maximum possible. Analytical signals
met identification criteria, but may include contribution from interferences.
US EPA and TLI used different identification criteria resulting in differences
in reported values.
b Concentration of HpBDF and OBDF from TLI are not validated because of lack of
standards. Concentrations are reported for comparison only.
c Average of two analysis of same sample.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In Japan, OBDE was not detected in 75 water samples (limit of
determination 0.1 µg/litre) in 1987 and was not detected in 147 water
samples collected at 49 areas (limit of determination, 0.07 µg/litre)
in 1988-89 (Environment Agency Japan, 1989, 1991).
5.1.2 Aquatic sediments
In 1987, environmental surveys were conducted concerning OBDE in
bottom sediment. OBDE was detected in 3 out of 51 samples at
concentrations ranging from 8 to 21 µg/kg in 1987 (limit of
determination 7 µg/kg dry weight) and was detected in 3 out of 135
samples, collected at 45 areas, at concentrations ranging from 15 to
22 µg/kg dry weight (limit of determination 5 µg/kg) in 1988-89
(Environment Agency Japan, 1989, 1991).
5.1.3 Aquatic and terrestrial organisms
In Japan, OBDE was not detected in 75 fish samples in 1987 and was
not found in 144 fish samples collected in 48 areas in 1988-89 (limit
of determination 5 µg/kg wet weight (Environment Agency Japan, 1989,
1991).
5.2 Exposure of the general population
In the USA, Cramer et al. (1990a,b) studied the presence of
PBDD/PBDF in human adipose tissue samples of the fiscal year 1987
(National Human Adipose Tissue Survey). The samples were derived from
865 specimen combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. The analysis
was carried out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD
was found (limits of determination, 10-40 ng/kg, depending on
congeners). Identification of the PBDE was based on comparison of full
scan mass spectra of the samples with the available standards,
application of SIM techniques to compare theoretical ion ratios with
observed ion ratios for characteristic ions, and measurement of
fragment losses from the molecular ion clusters. Preliminary evidence
for the presence of OBDE was found (frequency, 60%) with an estimated
concentration range of ND-8000 ng OBDE/kg (Cramer et al., 1990a,b;
Stanley et al., 1991).
5.3 Occupational exposure during manufacture, formulation, or use
No data are available.
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
6.1 Single exposure
6.1.1 Oral: Rat
The acute oral LD50 in the rat is >28 g/kg body weight (Kalk,
1982); >5 g/kg body weight (Kopp, 1990; Great Lakes Chemical
Corporation, 1990a).
Male Charles River CD rats were administered commercial OBDE,
suspended in corn oil, by intubation, at doses of 50, 500, or
5000 mg/kg body weight. The observation period was 14 days. The rats
showed normal weight gain and no animals died (Great Lakes Chem Corp.,
1987; 1990a).
6.1.2 Dermal: Rabbit
Commercial OBDE was applied to the clipped, abraded, or intact
skin of male and female albino rabbits at concentrations of 200 or
2000 mg/kg body weight for 24 h. During the observation period of 14
days, none of the rabbits died and all had normal weight gain. The
dermal LD50 for rabbits is >2 g/kg body weight (Great Lakes Chemical
Corporation, 1987, 1990a).
6.1.3 Inhalation: Rat
The inhalation LC50 of Saytex 111 in rats was > 50 mg/litre (US
EPA, 1986).
Male and female Charles River CD rats were exposed for 1 h to
concentrations of commercial OBDE in air of 2 or 60 mg/litre. The
physical nature of the substance precluded administration at higher
levels than 60 mg/litre. Rats exposed to 2 and 60 mg/litre had
decreased motor activity, erythema, and eye squint, during exposure.
The animals exposed to 60 mg/litre also had tachypnea. During the
14-day observation period, the animals appeared normal, and exhibited
normal body weight gain, except for one rat at the highest dose level,
which showed salivation on days 5 and 7 of the observation period
(Great Lakes Chemical Corporation, 1987) (no further details).
6.2 Short-term exposure
6.2.1 Oral: Rat
Charles River CD rats were fed commercial OBDE at daily, dietary
dose levels of 0, 100, or 1000 mg/kg for 28 days. There were 10 male
and 10 female rats in each dose group. No changes in appearance,
behaviour, mortality, feed consumption, or body weight gain were
observed. Results of haematology, blood chemistry, and urinalysis were
similar to those in the controls. Absolute and relative liver weights
were significantly increased in female rats given 100 mg/kg and in
rats of both sexes given 1000 mg/kg diet. Other increases in organ
weights were not considered compound-related. No gross pathological
lesions were noted in rats in any of the groups. Compound-related
histopathological liver lesions were observed in both treated groups
of rats. They consisted of enlarged centrolobular and midzonal liver
parenchymal cells in which the cytoplasm had large areas of finely
granular structures containing eosinophilic "round bodies". These
changes occurred more frequently and with greater severity in the male
animals. Their incidence and severity were dose-related. Rats given
1000 mg/kg had a slight to moderate hyperplasia of the thyroid, but it
was not clear whether this was compound-related. Dose-related
increases in total bromine levels of the liver were noted in both
males and females and ranged from about 6 to 137 times the levels
found in controls (Great Lakes Chemical Corporation, 1987).
Another 28-day feeding study was carried out on 10 male and 10
female Charles River CD rats per group, which were administered a diet
containing 100, 1000, or 10000 mg commercial OBDE/kg. The control
group consisted of 35 male and 35 female rats from the 90-day feeding
study, described below. At the end of the study, 5 rats of each
sex/group were sacrificed and the remaining 5 animals/sex per group
were maintained on normal diets for 4 weeks (recovery period). No
changes in appearance, behaviour, or mortality were observed. Food
consumption and body weight gain were slightly higher in the control
group. Haematology values were normal, but serum urea nitrogen levels
were slightly elevated in some of the rats given 10 000 mg/kg diet. An
increase in absolute and relative liver weights was observed in some
of the rats given 1000 and 10 000 mg/kg. At necropsy, the livers of
some animals in the 10 000 mg/kg group showed accentuated lobulization
and discoloration. The livers of rats from all 3 dose levels showed
enlargement of the centrolobular and midzonal hepatocytes, the
cytoplasm of which had large areas of finely granular appearance and
frequently contained eosinophilic "round bodies". In the 10 000 mg/kg
group, vacuolization of hepatocytes and necrosis of scattered
individual hepatocytes were seen. In all 3 treated groups, the liver
lesions were less severe after the 4-week recovery period than during
the administration of the compound. A dose-related increase in liver
total bromine content was seen in rats in all treated groups after 4
weeks of treatment, but these total bromine levels decreased rapidly
in the recovery period. Only in the rats receiving the lowest dose
level did the liver total bromine concentration approach control
levels after a 4-week withdrawal period (Great Lakes Chemical
Corporation, 1987).
Charles River CD rats were fed commercial OBDE at dietary levels
of 0, 100, 1000, or 10 000 mg/kg for up to 13 weeks. There were 35
male and 35 female rats in each dose group. Behaviour, appearance,
body weight, food consumption, haematology, blood chemistry, and
urinalysis were studied after 1 and 2 months, and, at the end of the
study, in groups of 5 rats/sex per group. The remaining 20 rats per
group were used to study the recovery and 5 rats/sex per group were
sacrificed after 13 and 21 weeks and 6 months after withdrawal. During
the study, a few rats out of each group died, but without any
dose-relationship, the majority of these deaths occurring after the
collection of blood.
In the 100 mg/kg diet group, the only effect that was seen was an
increase in absolute and relative liver weights. Microscopic changes
were seen in 4 out of 10 rats, and were typified by granular
cytoplasmic changes, Liver total bromine contents increased during the
13-week treatment, but decreased during the recovery period.
In the 1000 mg/kg group, a decrease in body weight gain was
observed, but haematology, blood chemistry, and urinalysis results
were comparable with the controls. Increases in absolute and relative
liver and thyroid weights were observed, but these effects were not
seen in the animals sacrificed during the recovery period. Microscopic
lesions were seen in the cytoplasm of the centrolobular and midzonal
hepatocytes and included vacuolization, and hyaline intracytoplasmic
inclusions. A hyperplastic nodule was found, after 6 months
withdrawal, in one rat each from the 1000 and 10 000 mg/kg groups.
In the 10 000 mg/kg group, a decrease in body weight gain was
observed, even during the withdrawal period. Decreases in haemoglobin,
haematocrit, and erythrocyte counts were also observed. One female
animal had hypochromia, polychromia, and anisocytosis of the
erythrocytes. Glucose levels in the blood were slightly lower than in
the controls. Orange colouration of the urine was observed during
weeks 13-39. A significant increase in absolute and relative liver,
kidney, and thyroid weights was observed. At autopsy, there was
accentuated lobulation and yellowish mottling of the livers and
brownish discoloration of the liver and kidneys. After one year of
recovery, no such gross changes were observed. Histopathologically,
the liver changes consisted of granular cytoplasmic changes,
cytoplasmic vacuolization (possibly representing fatty degeneration),
necrosis of scattered parenchymal cells or of centrolobular cells,
centrolobular fibrosis, and pigmented Kupfer cells. In the kidneys,
the changes were characterized by the occurrence of small to moderate
numbers of cortical regenerative tubules. One rat had a severe tubular
nephrosis. Cellular changes in the thyroid were probably
compound-related. During the recovery period, the histological changes
decreased in severity and frequency.
The total bromine content of the liver increased during the 13
weeks of treatment and decreased during the recovery period, but
remained higher (not significantly) than the control values after one
year (Great Lakes Chemical Corporation, 1987).
6.2.2 Inhalation: Rat
Charles River CD rats were exposed (whole-body) to a micronized
dust of commercial OBDE, introduced into inhalation chambers at
nominal concentrations of 1.2, 12, 120, and 1200 mg/m3 for 8 h/day on
14 consecutive days. Actual airborne dust concentrations were 15-45%
of the nominal values. It was not possible to measure the extent of
oral intake of the substance. There were 5 male and 5 female rats in
each dose group and in the controls. No animals died during the test
period nor were there any changes in appearance or general behaviour
in the 1.2 and 12 mg/m3 groups. By the end of the 8-h exposure
period, all animals in the 1200 mg/m3 group and part of the
120 mg/m3 group exhibited a fast breathing pattern, which had
disappeared by the morning following exposure. Food consumption, body
weight gain, haematology, blood chemistry, and urinalysis in all of
the dose groups were normal. The total bromine concentrations in the
lung, liver, and fat were significantly higher than in the controls.
At autopsy, the average total bromine concentrations in the lung and
fat were about 1.5-12.5 times higher than in the liver. The relative
liver weights of the animals in the 12, 120, and 1200 mg/m3 dose
groups were significantly increased in a dose-related manner. These
changes were accompanied by histopathological lesions consisting of
focal to multifocal cytoplasmic enlargement of the hepatocytes, and
focal acidophilic degeneration of individual, and small groups of,
liver cells. At the 2 highest dose levels, the enlargement of the
hepatocytes was multifocal to diffuse in distribution and small to
large areas had necrosis in the centrolobular regions of the affected
liver lobules, especially in the 1200 mg/m3 group. No other
compound-related effects were observed (Great Lakes Chemical
Corporation, 1987).
6.3 Long-term exposure
No data are available.
6.4 Skin and eye irritation; sensitization
6.4.1 Skin irritation
Commercial OBDE was applied to the clipped and occluded intact, or
abraded, skin of male and female albino rabbits at a dose of 500 mg.
After 24 h, the skin was washed and examined for irritation. The
examination was repeated after 72 h. One rabbit had slight erythema at
72 h; the remaining rabbits did not have skin changes. It was
concluded that OBDE is not a primary skin irritant (Great Lakes
Chemical Corporation, 1987).
6.4.2 Eye irritation
Single applications of 100 mg commercial OBDE were made into the
conjunctival sac of the eye of 3 male and 3 female New Zealand white
rabbits. Examinations were made at 24, 48, and 72 h, and 7 days. A
slight discharge was noted from the eyes of 2 rabbits at 24 h and a
slight redness was noted in the eye of one rabbit at 48 h. No ocular
irritation or corneal damage (sodium fluorescein and UVR were used)
was observed (Great Lakes Chemical Corporation, 1987).
6.5 Teratogenicity, reproductive toxicity, and embryotoxicity
6.5.1 Teratogenicity
6.5.1.1 Oral: Rat
In a range-finding study, female rats (number not specified) were
dosed daily, by gavage, from days 6 to 15 of gestation with 2.5, 10.0,
15.0, 25.0, or 50.0 mg commercial OBDE (DE-79)/kg body weight. All
animals survived until gestation day 20, when they were sacrificed. At
25.0 mg/kg, increased serum bromine levels were observed. Mean
maternal body weight gain was reduced in the 50.0 mg/kg group during
gestation. Increased numbers of late resorptions and significantly
reduced mean fetal weights were observed at the highest dose level.
The cholesterol level was slightly increased in the dams given
50.0 mg/kg. No compound-related microscopic findings were observed in
the liver and kidneys of the mothers. No compound-related effects were
observed at 15.0 mg/kg or lower. The malformations and developmental
variations observed in the 50.0 mg/kg group were associated with
maternal toxicity. Fetal anasarca and bent limb bones were observed at
this dose level. Mean fetal body weight and increased post
implantation loss due to !ate resorptions was also observed at this
level, but the losses were not statistically significant, compared
with the controls. Reduced ossification of the skull, various
unossified bones, and two instances of bent ribs were noted at this
dose level, and were probably secondary to maternal toxicity (Great
Lakes Chemical Corporation, 1987) (abstract).
Four groups of 25 pregnant Charles-River Crb:COBS CD (SD) BR rats
were administered corn-oil suspensions of Saytex 111, by gavage, at
doses of 0, 2.5, 10.0, or 25 mg/kg body weight per day on days 6-15 of
gestation. The dams were sacrificed on day 20 of gestation and the
fetuses were examined for gross visceral and skeletal abnormalities.
The substance was more toxic to the conceptus than to the dam. At
25.0 mg/kg, dose-dependant effects on the conceptus were observed.
These included reduced average fetal body weight, increased
embryo/fetal deaths (resorptions), fetal malformations, such as
enlarged heart, rear limb malformation, and delayed skeletal
ossification. At 10 mg/kg, the only observed effect was a
statistically insignificant reduction in average fetal body weight (US
EPA, 1986).
6.5.1.2 Oral: Rabbit
Groups of 26 inseminated adult New Zealand White rabbits (weight
3.5-4.5 kg) were treated with 0 (corn oil), 2.0, 5.0, or 15 mg Saytex
111/kg body weight per day, by gavage, on days 7-19 of gestation.
Saytex 111 was a mixture containing; 0.2% PeBDE, 8.6% HxBDE, 45.0%
HpBDE, 33.5% OBDE, 11.2% NBDE, and 1.4% DeBDE. Body weight gain was
recorded on gestation days 0, 7, 10, 13, 16, 20, and 28. In addition,
maternal liver, kidneys, and gravid uterine weights were measured at
the time of hysterectomy. The offspring were examined on day 28 of
gestation. A statistically significant increase in liver weight and a
decrease in body weight gain were observed in the 15 mg/kg group.
There was no statistically significant deviation in maternal
mortality, number of pregnancies, number of litters with viable pups,
corpora lutea/dam, implantations/dam, live fetuses/litter, percentage
of resorptions, and fetal body weight. Slight fetal toxicity was
observed in the 15 mg/kg group, as evidenced by a significant increase
in delayed ossification of the sternebrae. There was an increase in
the incidence of retrocaval ureter in the 5 and 15 mg/kg group and
fused sternebrae in the 5 mg/kg group. These increases were not dose
related. It was concluded by the authors that there was no evidence
for teratogenic activity but that there was slight letotoxicity at
maternally toxic dose levels, e.g., 15 mg/kg body weight (Breslin et
al., 1989).
6.6 Mutagenicity and related end-points
6.6.1 DNA damage
An unscheduled DNA synthesis (UDS) assay, a test to induce DNA
damage followed by repair in mammalian cells, was carried out with
monolayers of WI-38 human fibroblast cells, which were exposed to
commercial OBDE in the presence of radiolabelled thymidine. OBDE was
tested at 5 concentrations ranging from 60 to 300 µg/ml. UDS was not
induced by OBDE either in the presence or in the absence of a
metabolic activation system (Great Lakes Chemical Corporation, 1987).
6.6.2 Mutation
Commercial OBDE was examined for mutagenic activity at a number of
concentrations in in vitro microbial assays using Salmonella
typhimurium and Saccharomyces cerevisiae with, and without, liver
microsomal enzyme preparations from Aroclor-induced rats. The results
of these tests were all negative (Great Lakes Chemical Corporation,
1987) (no details).
6.6.3 Chromosomal effects
In an assay for sister chromatid exchanges, Chinese hamster ovary
cells were exposed to concentrations of commercial OBDE of 7.5, 25,
75, 250, or 750 µg/ml (in DMSO), for 2 h, in the presence, or absence,
of a metabolic activation system. The exposure period was followed by
a 24-h expression period. No statistically significant increases in
the number of exchanges per chromosome or the number of exchanges per
cell were seen at any of the levels tested (Great Lakes Chemical
Corporation, 1987).
6.7 Carcinogenicity
No data are available.
6.8 Other special studies
6.8.1 Liver
Commercial OBDE in corn oil was administered by gavage to six male
Sprague-Dawley rats (200-250 g) for 90 days. When extensive induction
was revealed at all the original doses of 6.25, 12.5, and 25 µmol/kg
per day, the study was repeated at doses of 0.78, 1.56, and
3.13 µmol/kg per day. OBDE did not cause induction of NADPH cytochrome
c reductase and cytochrome P450 at the lower doses. However, a dose of
0.78/µmol/kg per day caused increases in both O-ethyl-
O-p-nitrophenyl phenyl-phosphonothioate (EPN) detoxification and
p-nitroanisole demethylation; larger increases were seen with
increasing dose levels. After a 30-day recovery period, evidence of
the maintenance of an induced state was seen only in animals receiving
3.13 µmol/kg per day. These elevations were still observable 60 days
after the last dose. No histological liver abnormalities were observed
in rats treated with 3.13 µmol/kg body weight or less (Carlson,
1980b).
In another study on male rats (200-250g) administered commercial
OBDE, by gavage, at 0.1 mmol/kg per day in corn oil for 14 days, the
above increase in enzyme activity was accompanied by an increase in
activity of UDP-glucuronyl transferase and benzo[a]pyrene hydroxylase
24 h after the seventh dose (Carlson, 1980a). Measurements made on
days 30 and 60 of recovery after 90 days exposure showed that the
indicators of induced xenobiotic metabolism returned slowly to control
values.
Koster et al. (1980) found a strong porphyrinogenic effect in
cultures of chick embryo liver cells at concentrations of 10 µg
commercial OBDE (in DMSO)/ml medium, with, and without, pretreatment
of ß-napthoflavone, an inducer of P450, P448, and delta-aminolevulinic
acid synthethase. The effect was determined semiquantitatively with
fluorescence microscopy, 24 h after addition of the flame retardant.
6.9 Appraisal
In 28-day and 90-day rat studies, 100 mg OBDE per kg diet
(equivalent to 5 mg/kg body weight) induced minimal effects in the
liver. No no-effect level was established. In a 14-day inhalation
study with micronized dust of OBDE, no effects were found with
exposure to 12 mg/m3. Teratogenicity studies showed a no-effect level
of 2.5 mg/kg body weight.
At the highest reported value of OBDE of 8000 ng/kg, from the
Human Adipose Tissue Sample Study, assuming 15% adipose tissue in the
adult male, a "stored" dose of 1.2 µg/kg can be calculated. Assuming
1% of the administered dose was absorbed, this would extrapolate to a
total dose of 120 µg/kg. With the further assumption that the total
exposure occurred over one year, a daily dose of 0.38 µg/kg per day
can be calculated. This dose is approximately 4 orders of magnitude
below the most sensitive endpoint in mammalian toxicity testing on
OBDE (NOEL = 2.5 mg/kg body weight in a teratology study).
HEPTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Heptabromodiphenyl ether is not manufactured or used.
There is no database on pure HpBDE on which to make an evaluation.
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
Because HpBDE is the major component of commercial
octabromodiphenyl ether, the summary, evaluation, conclusions, and
recommendations on commercial OBDE, are relevant to "commercial"
HpBDE.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Chemical structure:
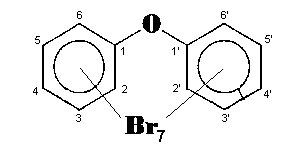 Chemical formula: C12 H3 Br7 O
Relative molecular mass: 722.3
CAS registry number: 68928-80-3
CAS name: 1,1'-oxy-bis(heptabromobenzene)
Common name: heptabromodiphenyl
ether (HpBDE)
From: US EPA (1986).
On the basis of the chemical structure, there are 24 possible
isomers of heptabromodiphenyl ether.
2.2 Physical and chemical properties
Melting point: 70-150 °C
(decomposition > 232 °C)
Density: 2.6 at 20 °C
Vapour pressure: <13.3 Pa at 20 °C
n-Octanol/water partition
coefficient (log Pow): not available
From: US EPA (1986).
2.3 Analytical methods
No specific data are available (See General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Heptabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Heptabromodiphenyl ether is not produced commercially or used, but
octabromodiphenyl ether contains approximately 44% HpBDE (see General
Introduction, Table 1).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A single study on mixed PBDE ranging from HxBDE and DeBDE
indicated lime bioaccumulation in carp with a bioconcentration factor
of <4, after 8 weeks of exposure (CBC, 1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Cramer et al. (1990a,b) studied the presence of PBDD/PBDF in human
adipose tissue samples in the USA in the fiscal year 1987 (National
Human Adipose Tissue Survey). The samples were derived from 865
specimens combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. The analysis
was carried out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD
were found, the limit of determination ranging from 10 to 40 ng/kg,
depending on the congeners. Identification of the PBDE was based on
comparison of full scan mass spectra of the samples with the available
standards, the application of SIM techniques to compare theoretical
ion ratios with observed ion ratios for characteristic ions, and the
measurement of fragment losses from the molecular ion clusters.
Preliminary evidence for the presence of heptabromodiphenyl ether was
found at a frequency of 100%, with an estimated concentration range of
1-2000 ng HpBDE/kg (Cramer et al., 1990a,b; Stanley et al., 1991).
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Short-term exposure
* Long-term exposure
* Reproductive toxicity, embryotoxicity, and teratogenicity
* Mutagenicity
* Carcinogenicity.
6.1 Single exposure
The acute oral LD50 for the rat is > 5 g/kg and the dermal LD50
for the rabbit, >2 g/kg body weight (Kopp, 1990).
6.2 Skin and eye irritation; sensitization
The substance is not irritant for either the skin or eyes (Kopp,
1990).
HEXABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Hexabromodiphenyl ether is not manufactured or used, but occurs as
a contaminant of commercial brominated diphenyl ethers. Such levels of
hexabromodiphenyl ether should be minimized to avoid contamination of
the environment and exposure of humans.
There is no database on which to make an evaluation.
No data are available on the following topics:
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12 H3 Br7 O
Relative molecular mass: 722.3
CAS registry number: 68928-80-3
CAS name: 1,1'-oxy-bis(heptabromobenzene)
Common name: heptabromodiphenyl
ether (HpBDE)
From: US EPA (1986).
On the basis of the chemical structure, there are 24 possible
isomers of heptabromodiphenyl ether.
2.2 Physical and chemical properties
Melting point: 70-150 °C
(decomposition > 232 °C)
Density: 2.6 at 20 °C
Vapour pressure: <13.3 Pa at 20 °C
n-Octanol/water partition
coefficient (log Pow): not available
From: US EPA (1986).
2.3 Analytical methods
No specific data are available (See General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Heptabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Heptabromodiphenyl ether is not produced commercially or used, but
octabromodiphenyl ether contains approximately 44% HpBDE (see General
Introduction, Table 1).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A single study on mixed PBDE ranging from HxBDE and DeBDE
indicated lime bioaccumulation in carp with a bioconcentration factor
of <4, after 8 weeks of exposure (CBC, 1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Cramer et al. (1990a,b) studied the presence of PBDD/PBDF in human
adipose tissue samples in the USA in the fiscal year 1987 (National
Human Adipose Tissue Survey). The samples were derived from 865
specimens combined to form 48 composite analogues. The composite
design was based on 9 census divisions and 3 age groups. The analysis
was carried out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD
were found, the limit of determination ranging from 10 to 40 ng/kg,
depending on the congeners. Identification of the PBDE was based on
comparison of full scan mass spectra of the samples with the available
standards, the application of SIM techniques to compare theoretical
ion ratios with observed ion ratios for characteristic ions, and the
measurement of fragment losses from the molecular ion clusters.
Preliminary evidence for the presence of heptabromodiphenyl ether was
found at a frequency of 100%, with an estimated concentration range of
1-2000 ng HpBDE/kg (Cramer et al., 1990a,b; Stanley et al., 1991).
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Short-term exposure
* Long-term exposure
* Reproductive toxicity, embryotoxicity, and teratogenicity
* Mutagenicity
* Carcinogenicity.
6.1 Single exposure
The acute oral LD50 for the rat is > 5 g/kg and the dermal LD50
for the rabbit, >2 g/kg body weight (Kopp, 1990).
6.2 Skin and eye irritation; sensitization
The substance is not irritant for either the skin or eyes (Kopp,
1990).
HEXABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Hexabromodiphenyl ether is not manufactured or used, but occurs as
a contaminant of commercial brominated diphenyl ethers. Such levels of
hexabromodiphenyl ether should be minimized to avoid contamination of
the environment and exposure of humans.
There is no database on which to make an evaluation.
No data are available on the following topics:
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
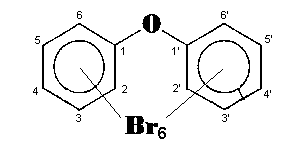 Chemical formula: C12 H4 Br6O
Relative molecular mass: 643.62
CAS registry number: 36483-60-0
CAS name: 1.1'-oxy-bis-hexabromobenzene
Common names: hexabromodiphenyl ether (HxBDE)
hexabromodiphenyl oxide
From: (US EPA, 1984, 1986).
On the basis of the chemical structure, there are of 42 possible
isomers of hexabromodiphenyl ether.
2.1.1 Technical product
Trade names: BR 33N
2.2 Physical and chemical properties
Vapour pressure: 0.95-0.99 kPa at 25 °C
n-Octanol/water partition
coefficient (log Pow): 6.86-7.92
From: Pijnenburg & Everts (1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Hexabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
As far as is known, hexabromodiphenyl ether has not been produced
commercially or used, but it is a component of TeBDE, PeBDE, and OBDE
at concentrations ranging from 4 to 12% (see General Introduction,
Table 1).
3.3 Uses
Total use of penta- and hexabromodiphenyl ethers in the Netherlands in
1988 was estimated to be 350 tonnes (Anon, 1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A single study on a mixture of PBDE, ranging between HxBDE and
DeBDE, indicated little bioaccumulation in carp with a
bioconcentration factor of < 4, after 8 weeks of exposure (CBC,
1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
5.1.1 Water
In Japan, HxBDE was not detected in 75 samples of water in 1987 or
in 150 samples collected in 50 areas in 1988-89 (in both cases the
limit of determination was 0.04 µg/litre) (Environment Agency Japan,
1989, 1991).
5.1.2 Aquatic sediments
Marine, estuarine, and river sediment samples were collected at
different places in Japan in 1981-83 and analysed for HxBDE. Five out
of 15 samples contained 9-26 µg/kg (Watanabe, 1987; Watanabe et al.,
1987b).
In 1987, environmental surveys were conducted on HxBDE levels in
sediment in Japan. HxBDE was detected in 4 out of 69 samples at
concentrations ranging from 7 to 77 µg/kg dry weight (limit of
determination 5.1 µg/kg dry weight) in 1987, and, in 4 out of 141
samples collected in 47 areas in 1988-89, at concentrations ranging
from 4.5 to 18 µg/kg dry weight (limit of determination 3.5 µg/kg dry
weight) (Environment Agency Japan, 1989, 1991).
5.1.3 Aquatic and terrestrial organisms
Mussel and a few species of fish (mullet, goby, and Japanese sea
bass) were collected from different seashores in Japan from 1981 to
1985. Other fish species were purchased at a wholesale commercial
source in Osaka Prefecture in 1981. HxBDE could not be detected
(<0.2 µg/kg wet weight) in any of the 5 mussel samples or in the 12
fish samples (Watanabe, 1987; Watanabe et al., 1987b).
In 1987, environmental surveys were conducted on HxBDE levels in
fish in Japan. HxBDE was detected in 5 out of 75 fish samples at
concentrations ranging from 3.8 to 14 µg/kg wet weight in 1987 and was
detected in 5 out of 144 samples collected at 48 areas at
concentrations ranging from 2 to 6 µg/kg wet weight in 1988-89 (limit
of determination 2 µg/kg wet weight) (Environ. Agency Japan, 1989,
1991).
5.2 General population exposure
In the USA, Cramer et al. (1990a,b) studied the levels of
PBDD/PBDF in human adipose tissue samples in 1987 (National Human
Adipose Tissue Survey). The samples were derived from 865 specimens
combined to form 48 composite analogues. The composite design was
based on 9 census divisions and 3 age groups. The analysis was carried
out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD were found,
the limit of determination ranging from 10 to 40 ng/kg, depending on
the congeners. Identification of the PBDE was based on comparison of
full scan mass spectra of the samples with the available standards,
application of SIM techniques to compare theoretical ion ratios with
observed ion ratios, for characteristic ions, and measurement of
fragment losses from the molecular ion clusters. Preliminary evidence
for the presence of HxBDE was found at a frequency of 72%, in an
estimated concentration range of ND to 1000 ng HxBDE/kg (Cramer et
al., 1990a,b; Stanley et al., 1991).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) was
investigated in the perirenal fat of groups of 3 male and 3 female
Wistar rats (weight 160-180 g), following a single oral dose of
300 mg/kg body weight in peanut oil. The groups were killed on days 1,
2, 3, 4, and 7 and then weekly for 10 weeks. Perirenal fat was
collected and analysed. Half-lives in male and female rats of 2
hexabromodiphenyl ethers (HxBDE(1) and HxBDE(2)) were: for female
rats, 44.6 (37.4-51.9) days and 90.0 (78.7-103.6) days, and, for male
rats, 55.1 (48.4-61.7) and 119.1 (102.8-136.1) days, respectively (Von
Meyerinck et al., 1990).
PENTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Pentabromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by other international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial pentabromodiphenyl ether (PeBDE) is a mixture of
tetra-, penta-, and hexabromodiphenyl ethers. It contains
approximately 50-60% PeBDE and 24-38% TeBDE. On the basis of the
chemical structure, there are 46 possible isomers of PeBDE and 42
possible isomers of TeBDE. The commercial products seem to contain 3
main components, i.e., 2,2',4,4',5-PeBDE, 2,2',4,4'-TeBDE, and an
unidentified congener containing 5 bromines.
The melting point is -7 to -3 °C and the boiling point, above
200 °C. The vapour pressure is low: <10-7 mmHg and the solubility
in water is negligible. The n-octanol/water partition coefficient
(log Pow) >6.
1.1.2 Production and uses
PeBDE is used as an additive in epoxy resins, phenol resins,
polyesters and polyurethane, and textiles. Worldwide consumption is
approximately 4000 tonnes per year. It is one of the major commercial
brominated diphenyl ether flame retardants.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial PeBDE have been found in biota, sediment,
and sewage sludge samples. Commercial PeBDE EHC 162: Brominated
diphenyl ethers components are likely to be persistent and
bioaccumulate. A bioconcentration factor of over 10 000 has been found
in carp for PeBDE.
Pyrolysis studies with commercial PeBDE showed that PBDF and PBDD
are formed. The optimal temperature for the formation of the PBDF and
PBDD was between 700-800 °C. When PeBDE was pyrolysed in the absence
of oxygen, polybromobenzenes, polybromophenols, and PBDF were formed.
1.1.4 Environmental levels and human exposure
Sediment samples taken from rivers and estuaries in Japan showed
levels ranging from no PeBDE (<2 µg/kg) up to 28 µg/kg dry weight. In
Sweden, the concentrations in sediment samples of certain rivers were
up to 1200 µg 2,2',4,4,'5-PeBDE/kg. Sewage sludge, analysed in Sweden
also contained this PeBDE.
In mussel and fish collected from different seashores in Japan in
the period 1981-85, concentrations of 0.4 and 2.8 µg PeBDE/kg wet
weight were found in 2 out of 5 mussel samples. No PeBDE was detected
in fish (limit of determination < 0.2 µg/kg). Concentrations of
1.9-22 µg/kg, on a fresh weight basis, were reported in liver samples
from cod from the North Sea. In Sweden, concentrations of between 7.2
and 64 µg 2,2',4,4',5-PeBDE/kg fat were found in freshwater whitefish
and herring collected at different places.
Pooled blubber of ringed seal and of grey seal collected in Sweden
in 1979-85 contained average concentrations of 1.7 µg and 40 µg
2,2',4'4',5-PeBDE/kg fat, respectively.
Pooled samples of muscle of rabbits, moose, and suet samples of
reindeer collected in 1985-86 in Sweden contained < 0.3 µg, 0.64 µg,
and 0.26 µg 2,2',4,4',5-PeBDE/kg fat, respectively.
Muscle samples of osprey, collected in Sweden in 1982-86,
contained an average concentration of 140 µg 2,2',4,4',5-PeBDE/kg fat.
The levels of 2 PeBDE isomers in guillemot eggs from the Baltic
have increased by one order of magnitude during the last decades. The
levels of these isomers in pike from a lake in Southern Sweden also
showed an increase (by a factor of about 4).
Baltic sediments representing different sampling years also
indicate a considerable increase during the last decade.
There is minimal information on human exposure, but a rough
estimate of exposure of the Swedish population through fish
consumption would suggest an intake of 0.1 µg PeBDE/person per day.
1.1.5 Kinetics and metabolism in laboratory animals and humans
The half-life of PeBDE has only been investigated in the perirenal
fat in rats. The average half-life was between 25 and 47 days,
depending on the sex of the animal and the type of isomer determined.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of commercial PeBDE is low in rats; the
dermal toxicity in rabbits is also low. Short-term inhalation exposure
in rats and the application of PeBDE to the conjunctival sac in
rabbits caused only mild, transient effects.
In short-term toxicity studies on rats (4-week and 13-week),
dietary concentrations of 100 mg/kg increased liver weights and caused
slight histological alterations. Changes consisted of the enlargement
of hepatic parenchymal cells, which had a granular appearance and
contained eosinophilic "round bodies". Dose-related increases in total
bromine content in the liver occurred and levels remained elevated for
as long as 24 weeks. A mild degree of thyroid hyperplasia, which was
reversible, was observed.
Hepatic enzyme induction and increases in cytochrome P450 c
occurred after oral administration of daily doses of PeBDE as low as
0.78 µmol/kg body weight. The results of tests for teratogenicity and
mutagenicity were negative.
No long-term/carcinogenicity studies have been reported.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
Minimal data are available.
1.2 Conclusions
1.2.1 PeBDE
Commercial PeBDE (a mixture of 24-38% tetra-, 50-60% penta-, and
4-8% hexabromodiphenyl ether) is persistent and accumulates in
organisms in the environment.
Commercial PeBDE is widely used, incorporated in polymers as an
additive flame retardant. Contact of the general population is with
products made from these polymers. Exposure by extraction from
polymers is unlikely. Human exposure to PeBDE via the food chain may
occur, since the substance has been detected in organisms in the
environment that are human food items, such as fish, shellfish, etc.
In fish and birds from Sweden, increasing levels have been measured
over the last 2 decades.
The acute toxicity of commercial PeBDE is low. There is no
information on uptake and loss in mammals. Reproduction, long-term
toxicity, and carcinogenicity studies are not available.
The risk to the general population cannot be determined from the
available data.
No information is available to draw conclusions on occupational
exposure levels or the effects of commercial PeBDE.
Limited information is available on the toxicity of commercial
PeBDE for organisms in the environment.
1.2.2 Breakdown products
PBDF and, to some extent, PBDD are formed when PeBDE (or products
containing it) are heated to 400-800 °C. The possible hazards
associated with this have to be addressed.
Exposure of the general population to PBDF in polymers flame
retarded with PeBDE is unlikely to be of significance. Properly
controlled incineration does not lead to the emission of significant
quantities of brominated dioxins and -furans. Any uncontrolled
combustion of products containing PeBDE can lead to the generation of
unquantified amounts of PBDF/PBDD. The significance of this for both
humans and the environment will be addressed in a future EHC on
PBDF/PBDD.
1.3 Recommendations
1.3.1 General
Persistence in the environment and accumulation in organisms
suggest that commercial PeBDE should not be used. However, if use
continues, the following points should be taken into account:
* Workers involved in the manufacture of PeBDE and products
containing the compound should be protected from exposure using
appropriate industrial hygiene measures, the monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent and accumulating material and
its breakdown products.
* Incineration of materials flame retarded with PeBDE should only be
carried out in properly constituted incinerators running
consistently under optimal conditions. Burning by any other means
will lead to production of toxic breakdown products.
1.3.2 Further studies
* Continued monitoring of environmental levels is required.
* Methods for the determination of PeBDE in various matrices should
be validated.
* Because the present toxicological database is inadequate to
evaluate the hazards of commercial PeBDE for humans and the
environment, and to support its use, the following studies should
be done:
- additional toxicological, carcinogenicity, and
ecotoxicological studies;
- further investigations on the generation of PBDF under real
fire conditions;
- investigations on possible methods and consequences of
recycling of PeBDE-containing polymers;
- studies on the possibilities of migration from flame-retarded
products.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12 H4 Br6O
Relative molecular mass: 643.62
CAS registry number: 36483-60-0
CAS name: 1.1'-oxy-bis-hexabromobenzene
Common names: hexabromodiphenyl ether (HxBDE)
hexabromodiphenyl oxide
From: (US EPA, 1984, 1986).
On the basis of the chemical structure, there are of 42 possible
isomers of hexabromodiphenyl ether.
2.1.1 Technical product
Trade names: BR 33N
2.2 Physical and chemical properties
Vapour pressure: 0.95-0.99 kPa at 25 °C
n-Octanol/water partition
coefficient (log Pow): 6.86-7.92
From: Pijnenburg & Everts (1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Hexabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
As far as is known, hexabromodiphenyl ether has not been produced
commercially or used, but it is a component of TeBDE, PeBDE, and OBDE
at concentrations ranging from 4 to 12% (see General Introduction,
Table 1).
3.3 Uses
Total use of penta- and hexabromodiphenyl ethers in the Netherlands in
1988 was estimated to be 350 tonnes (Anon, 1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A single study on a mixture of PBDE, ranging between HxBDE and
DeBDE, indicated little bioaccumulation in carp with a
bioconcentration factor of < 4, after 8 weeks of exposure (CBC,
1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
5.1.1 Water
In Japan, HxBDE was not detected in 75 samples of water in 1987 or
in 150 samples collected in 50 areas in 1988-89 (in both cases the
limit of determination was 0.04 µg/litre) (Environment Agency Japan,
1989, 1991).
5.1.2 Aquatic sediments
Marine, estuarine, and river sediment samples were collected at
different places in Japan in 1981-83 and analysed for HxBDE. Five out
of 15 samples contained 9-26 µg/kg (Watanabe, 1987; Watanabe et al.,
1987b).
In 1987, environmental surveys were conducted on HxBDE levels in
sediment in Japan. HxBDE was detected in 4 out of 69 samples at
concentrations ranging from 7 to 77 µg/kg dry weight (limit of
determination 5.1 µg/kg dry weight) in 1987, and, in 4 out of 141
samples collected in 47 areas in 1988-89, at concentrations ranging
from 4.5 to 18 µg/kg dry weight (limit of determination 3.5 µg/kg dry
weight) (Environment Agency Japan, 1989, 1991).
5.1.3 Aquatic and terrestrial organisms
Mussel and a few species of fish (mullet, goby, and Japanese sea
bass) were collected from different seashores in Japan from 1981 to
1985. Other fish species were purchased at a wholesale commercial
source in Osaka Prefecture in 1981. HxBDE could not be detected
(<0.2 µg/kg wet weight) in any of the 5 mussel samples or in the 12
fish samples (Watanabe, 1987; Watanabe et al., 1987b).
In 1987, environmental surveys were conducted on HxBDE levels in
fish in Japan. HxBDE was detected in 5 out of 75 fish samples at
concentrations ranging from 3.8 to 14 µg/kg wet weight in 1987 and was
detected in 5 out of 144 samples collected at 48 areas at
concentrations ranging from 2 to 6 µg/kg wet weight in 1988-89 (limit
of determination 2 µg/kg wet weight) (Environ. Agency Japan, 1989,
1991).
5.2 General population exposure
In the USA, Cramer et al. (1990a,b) studied the levels of
PBDD/PBDF in human adipose tissue samples in 1987 (National Human
Adipose Tissue Survey). The samples were derived from 865 specimens
combined to form 48 composite analogues. The composite design was
based on 9 census divisions and 3 age groups. The analysis was carried
out using HRGC/HRMS to determine PBDD/PBDF. No PBDF/PBDD were found,
the limit of determination ranging from 10 to 40 ng/kg, depending on
the congeners. Identification of the PBDE was based on comparison of
full scan mass spectra of the samples with the available standards,
application of SIM techniques to compare theoretical ion ratios with
observed ion ratios, for characteristic ions, and measurement of
fragment losses from the molecular ion clusters. Preliminary evidence
for the presence of HxBDE was found at a frequency of 72%, in an
estimated concentration range of ND to 1000 ng HxBDE/kg (Cramer et
al., 1990a,b; Stanley et al., 1991).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) was
investigated in the perirenal fat of groups of 3 male and 3 female
Wistar rats (weight 160-180 g), following a single oral dose of
300 mg/kg body weight in peanut oil. The groups were killed on days 1,
2, 3, 4, and 7 and then weekly for 10 weeks. Perirenal fat was
collected and analysed. Half-lives in male and female rats of 2
hexabromodiphenyl ethers (HxBDE(1) and HxBDE(2)) were: for female
rats, 44.6 (37.4-51.9) days and 90.0 (78.7-103.6) days, and, for male
rats, 55.1 (48.4-61.7) and 119.1 (102.8-136.1) days, respectively (Von
Meyerinck et al., 1990).
PENTABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Pentabromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by other international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial pentabromodiphenyl ether (PeBDE) is a mixture of
tetra-, penta-, and hexabromodiphenyl ethers. It contains
approximately 50-60% PeBDE and 24-38% TeBDE. On the basis of the
chemical structure, there are 46 possible isomers of PeBDE and 42
possible isomers of TeBDE. The commercial products seem to contain 3
main components, i.e., 2,2',4,4',5-PeBDE, 2,2',4,4'-TeBDE, and an
unidentified congener containing 5 bromines.
The melting point is -7 to -3 °C and the boiling point, above
200 °C. The vapour pressure is low: <10-7 mmHg and the solubility
in water is negligible. The n-octanol/water partition coefficient
(log Pow) >6.
1.1.2 Production and uses
PeBDE is used as an additive in epoxy resins, phenol resins,
polyesters and polyurethane, and textiles. Worldwide consumption is
approximately 4000 tonnes per year. It is one of the major commercial
brominated diphenyl ether flame retardants.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial PeBDE have been found in biota, sediment,
and sewage sludge samples. Commercial PeBDE EHC 162: Brominated
diphenyl ethers components are likely to be persistent and
bioaccumulate. A bioconcentration factor of over 10 000 has been found
in carp for PeBDE.
Pyrolysis studies with commercial PeBDE showed that PBDF and PBDD
are formed. The optimal temperature for the formation of the PBDF and
PBDD was between 700-800 °C. When PeBDE was pyrolysed in the absence
of oxygen, polybromobenzenes, polybromophenols, and PBDF were formed.
1.1.4 Environmental levels and human exposure
Sediment samples taken from rivers and estuaries in Japan showed
levels ranging from no PeBDE (<2 µg/kg) up to 28 µg/kg dry weight. In
Sweden, the concentrations in sediment samples of certain rivers were
up to 1200 µg 2,2',4,4,'5-PeBDE/kg. Sewage sludge, analysed in Sweden
also contained this PeBDE.
In mussel and fish collected from different seashores in Japan in
the period 1981-85, concentrations of 0.4 and 2.8 µg PeBDE/kg wet
weight were found in 2 out of 5 mussel samples. No PeBDE was detected
in fish (limit of determination < 0.2 µg/kg). Concentrations of
1.9-22 µg/kg, on a fresh weight basis, were reported in liver samples
from cod from the North Sea. In Sweden, concentrations of between 7.2
and 64 µg 2,2',4,4',5-PeBDE/kg fat were found in freshwater whitefish
and herring collected at different places.
Pooled blubber of ringed seal and of grey seal collected in Sweden
in 1979-85 contained average concentrations of 1.7 µg and 40 µg
2,2',4'4',5-PeBDE/kg fat, respectively.
Pooled samples of muscle of rabbits, moose, and suet samples of
reindeer collected in 1985-86 in Sweden contained < 0.3 µg, 0.64 µg,
and 0.26 µg 2,2',4,4',5-PeBDE/kg fat, respectively.
Muscle samples of osprey, collected in Sweden in 1982-86,
contained an average concentration of 140 µg 2,2',4,4',5-PeBDE/kg fat.
The levels of 2 PeBDE isomers in guillemot eggs from the Baltic
have increased by one order of magnitude during the last decades. The
levels of these isomers in pike from a lake in Southern Sweden also
showed an increase (by a factor of about 4).
Baltic sediments representing different sampling years also
indicate a considerable increase during the last decade.
There is minimal information on human exposure, but a rough
estimate of exposure of the Swedish population through fish
consumption would suggest an intake of 0.1 µg PeBDE/person per day.
1.1.5 Kinetics and metabolism in laboratory animals and humans
The half-life of PeBDE has only been investigated in the perirenal
fat in rats. The average half-life was between 25 and 47 days,
depending on the sex of the animal and the type of isomer determined.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute oral toxicity of commercial PeBDE is low in rats; the
dermal toxicity in rabbits is also low. Short-term inhalation exposure
in rats and the application of PeBDE to the conjunctival sac in
rabbits caused only mild, transient effects.
In short-term toxicity studies on rats (4-week and 13-week),
dietary concentrations of 100 mg/kg increased liver weights and caused
slight histological alterations. Changes consisted of the enlargement
of hepatic parenchymal cells, which had a granular appearance and
contained eosinophilic "round bodies". Dose-related increases in total
bromine content in the liver occurred and levels remained elevated for
as long as 24 weeks. A mild degree of thyroid hyperplasia, which was
reversible, was observed.
Hepatic enzyme induction and increases in cytochrome P450 c
occurred after oral administration of daily doses of PeBDE as low as
0.78 µmol/kg body weight. The results of tests for teratogenicity and
mutagenicity were negative.
No long-term/carcinogenicity studies have been reported.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
Minimal data are available.
1.2 Conclusions
1.2.1 PeBDE
Commercial PeBDE (a mixture of 24-38% tetra-, 50-60% penta-, and
4-8% hexabromodiphenyl ether) is persistent and accumulates in
organisms in the environment.
Commercial PeBDE is widely used, incorporated in polymers as an
additive flame retardant. Contact of the general population is with
products made from these polymers. Exposure by extraction from
polymers is unlikely. Human exposure to PeBDE via the food chain may
occur, since the substance has been detected in organisms in the
environment that are human food items, such as fish, shellfish, etc.
In fish and birds from Sweden, increasing levels have been measured
over the last 2 decades.
The acute toxicity of commercial PeBDE is low. There is no
information on uptake and loss in mammals. Reproduction, long-term
toxicity, and carcinogenicity studies are not available.
The risk to the general population cannot be determined from the
available data.
No information is available to draw conclusions on occupational
exposure levels or the effects of commercial PeBDE.
Limited information is available on the toxicity of commercial
PeBDE for organisms in the environment.
1.2.2 Breakdown products
PBDF and, to some extent, PBDD are formed when PeBDE (or products
containing it) are heated to 400-800 °C. The possible hazards
associated with this have to be addressed.
Exposure of the general population to PBDF in polymers flame
retarded with PeBDE is unlikely to be of significance. Properly
controlled incineration does not lead to the emission of significant
quantities of brominated dioxins and -furans. Any uncontrolled
combustion of products containing PeBDE can lead to the generation of
unquantified amounts of PBDF/PBDD. The significance of this for both
humans and the environment will be addressed in a future EHC on
PBDF/PBDD.
1.3 Recommendations
1.3.1 General
Persistence in the environment and accumulation in organisms
suggest that commercial PeBDE should not be used. However, if use
continues, the following points should be taken into account:
* Workers involved in the manufacture of PeBDE and products
containing the compound should be protected from exposure using
appropriate industrial hygiene measures, the monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent and accumulating material and
its breakdown products.
* Incineration of materials flame retarded with PeBDE should only be
carried out in properly constituted incinerators running
consistently under optimal conditions. Burning by any other means
will lead to production of toxic breakdown products.
1.3.2 Further studies
* Continued monitoring of environmental levels is required.
* Methods for the determination of PeBDE in various matrices should
be validated.
* Because the present toxicological database is inadequate to
evaluate the hazards of commercial PeBDE for humans and the
environment, and to support its use, the following studies should
be done:
- additional toxicological, carcinogenicity, and
ecotoxicological studies;
- further investigations on the generation of PBDF under real
fire conditions;
- investigations on possible methods and consequences of
recycling of PeBDE-containing polymers;
- studies on the possibilities of migration from flame-retarded
products.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
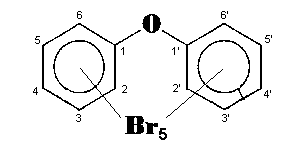 Chemical formula: C12H5Br5O
Relative molecular mass: 564.75
Common name: pentabromodiphenyl ether (PeBDE);
pentabromodiphenyl oxide
CAS registry number: 32534-81-9
CAS name: 1,1'-oxybis-pentabromo-benzene,
benzene, 1,1'-oxybis-, pentabromo
On the basis of the chemical structure, there are 46 possible
isomers of pentabromodiphenyl ether.
From: IRPTC (1988).
2.1.1 Technical product
Trade name DE 71; Bromkal 70-5 DE;
FR 1205/1215;
Bromkal 70; Bromkal G1;
Pentabromprop;
DE-60 F is a mixture of 85%
PeBDE and 15% of an aromatic phosphate
Commercial pentabromodiphenyl ether is a mixture of polybrominated
diphenyl ethers with the following typical composition (see Table 1):
0-1% tribromodiphenyl ether, 24-38% tetrabromodiphenyl ether, 50-62%
pentabromodiphenyl ether, 4-8% hexabromodiphenyl ether (Arias, 1992).
DE-71 is primarily a mixture of tetra-, penta-, and
hexabromodiphenyl ethers containing low levels of tribromodiphenyl
ether (< 1%) and heptabromodiphenyl ether (< 2%) (McAllister &
Ariano, 1982). Pentabromprop is a mixture of 39% tetra-, 61% penta-,
and 9% hexabromodiphenyl ethers. Bromkal 70-5 DE is a mixture of 34.2%
tetra-, 59.8% penta-, 5.8% hexa-, and 0.2% heptabromodiphenyl ethers,
but 41.7% tetra- and 45% pentabromodiphenyl ethers, and 7% of a second
pentabromodiphenyl ether have also been reported (Nylund et al.,
1992). Another manufacturer produces a mixture of 35% tetra-, 58%
penta-, and 4% higher brominated diphenyl ethers under the name of
Pentabromodiphenyl ether. Bromkal 70 is a mixture containing 36%
tetrabromo- and 74% pentabromodiphenyl ether. The bromine contents of
the mixtures range from 67 to 71.8% (De Kok et al., 1979). Bromkal
70-5, a mixture of brominated diphenyl ethers containing 67-71%
bromine, and an average of about 5 bromines per molecule, also
contained various isomers of tri-, tetra-, penta-, and
hexabromodiphenyl ethers. However, it is no longer produced
commercially (McAllister, 1991).
Sundström & Hutzinger (1976) identified 2,2',4,4'-tetra- and
2,2',4,4',5,-PeBDE as the major components of Bromkal 70-5 DE.
2.2 Physical and chemical properties
Pentabromodiphenyl ether (PeBDE) is a clear, amber to pale yellow,
highly viscous liquid, with an organic smell. Under conditions of
fire, hydrogen bromide and/or bromine occur.
Melting point -7 to -3 °Ca
Boiling point > 300 °C (decomposition starts
above 200 °C)
Specific gravity 2.28 at 25 °C; 1.78 at 40 °C
Vapour pressure 9.3 mmHg at 22 °Cb
(6.26-6.66 Torr at 25 °C)
Solubility Insoluble in water (9 × 10-7 mg/litre
at 20 °C), methanol 10 g/litre at 25 °C,
soluble in other organic solvents
such as chloroform, benzene, toluene,
acetone, carbon-tetrachloride, and
methylene chloride
Viscosity at 50 °C 1-6 Pa (150 000 cp at 25 °C;
1500 cp at 60 °C)
n-Octanol/water partition
coefficient (log Pow) 6.64-6.97
From: Great Lakes Chemical Corporation (undated a); Kalk (1982);
Kopp (1990); US EPA (1989); Hallenbeck (1993); US Testing Comp.
(1985).
2.3 Analytical methods
McAllister & Ariano (1982) developed a method for the
determination of the arbitrarily defined "bromination level" of PeBDE
(DE-71), both as the neat material and as a formulation in DE-60 F,
and for the quantitative determination of aromatic phosphate ester in
DE-60 F. The sample is dissolved in dibromomethane containing an
internal standard and the components separated by gas chromatography.
A multiresidue method has been developed for the determination of
PBDE residues in environmental samples (see General Introduction,
section 2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Pentabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Pentabromodiphenyl ether is synthesized by treating diphenyl ether
with 5 equivalents of Br2 at 30-65 °C in the presence of powdered
iron (US EPA, 1986).
The actual worldwide consumption of PeBDE per year is 4000 tonnes.
In the Federal Republic of Germany, in 1988, the approximate level of
use in plastics was 200-400 tonnes/year. The total use of penta- and
hexabromodiphenyl ethers in the Netherlands in 1988 was estimated to
be 350 tonnes (Anon., 1989b).
Production levels are not available.
3.2.2 Uses
PeBDE is used as an additive in epoxy resins, phenol resins,
unsaturated polyesters, polyurethane flexible, and textiles.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Pyrolysis
PeBDE (Bromkal 70, 70-5 DE, G1) were heated in a quartz tube at
700, 800, or 900 °C and the concentrations of PBDD and PBDF
determined. Monobromo- to pentabromodibenzofurans, as well as
monobromo- to tetrabromodioxins, were found in yields of up to 90% in
all 3 Bromkal samples. The optimal temperatures of formation were
between 700 and 800 °C (Thoma et al., 1987a).
Bromkal 70-5-DE was pyrolysed at 600, 700, 800, and 900 °C, in the
absence of oxygen, in a SGE pyrojector and the residues analysed by
GC/MS in an on-line operation. The pyrolysis of Bromkal 70-5-DE
yielded polybromobenzenes (PBBz), polybromophenois (PBP), and
brominated dibenzofurans. Because this pyrolysis is performed in a
pyrojector with a helium current, incorporation of oxygen is
impossible, consequently dioxins do not form (Thoma & Hutzinger, 1987,
1989).
4.2 Workplace exposure studies
In the processing of PBT, finished with pentabromodiphenyl ether
at 300 °C, PBDF were found in both the air at the workplace and the
machine extractor. PBDF were also found in air samples taken from a
vessel in which the granulate was stored. Since the bulk of the PBDF
formed during processing remain in the product, it can be assumed that
parts made from the polymers, such as computer casings, television
sets, etc., are contaminated with PBDF (CEM, 1989).
4.3 Bioaccumulation
Carp exposed for 8 weeks to commercial pentabromodiphenyl ether at
10 or 100 µg/litre showed bioconcentration factors of more than 10 000
(CBC, 1982).
4.4 Ultimate fate following use
For the ultimate fate of PeBDE following use see section 6.1 of
the General Introduction.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
5.1.1 Sediment and sewage sludge
Sediment samples were taken from the rivers near the centre of
Osaka City and marine sediments, from different estuaries in Japan and
from Osaka Bay, during the period 1981-83. No PeBDE was found
(<2 µg/kg dry weight in 9 estuarine or marine sediment samples. PeBDE
was present in 5 out of 6 river sediment samples at concentrations of
9-28 µg/kg on a dry weight basis (Watanabe, 1987; Watanabe et al.,
1987b).
Organic extracts, made from coastal sediments, collected offshore
from Barcelona, were analysed for the presence of organohalogenated
chemicals. A diversity of organohalogenated compounds were found,
among them PeBDE (Fernandez et al., 1992).
Sellström et al. (1990a,b) analysed sediment samples, taken
upstream and downstream from a factory, for the presence of
tetrabromobisphenol-A and derivatives. Both tetrabromobisphenol A and
2,2',4,4',5-pentabromodiphenyl ether were found. The levels found in
upstream and downstream sediments were 8.2 and 1200 µg/kg (ign loss),
respectively.
A laminated sediment core collected in the southern part of the
Baltic Proper (Bornholm Deep) was analysed for 2,2',4,4',5-PeBDE. The
core was cut into 5 mm slices down to 50 mm depth and in 10 mm slices
from 50 to 90 mm depth. The concentration of PeBDE at a depth of 5 mm
was 0.98 µg/kg IG and decreased more or less gradually in the deeper
layers to approximately 0.05 µg/kg at a depth of 40 mm. Below this
depth, levels were mostly not detectable (Nylund et al., 1992).
Sellström et al. (1990a,b) analysed sewage sludge and found 19 µg
2,2',4,4',5-pentabromodiphenyl ether/kg ign loss.
Two sewage samples were collected from a Swedish treatment plant
(Gothenburg) in September 1988 anti analysed for PBDE. One homogenate
was composed of samples (40 g/day) taken over a 32-day period with
little rain. Another homogenate was composed of samples (100 g/day)
taken during a rainy period of 7 days. Both TeBDE and PeBDE were
found. The concentration of 2,2',4,4',5-PeBDE was 19 µg/kg;
concentrations of PeBDE of unknown structures were 3.4 and 3.7 µg/kg
and total PeBDE, 37-38 µg/kg IG (Nylund et al., 1992).
5.1.2 Fish and shellfish
Mussels and some species of fish, e.g., mullet, goby, and Japanese
sea bass, were collected from different seashores in Japan from 1981
to 1985. Other fish species were purchased from a commercial wholesale
source in Osaka Prefecture in 1981. In 2 out of 5 mussel samples,
levels of 0.4 and 2.8 µg PeBDE/kg wet weight were found. In 12 fish
samples, no PeBDE (< 0.2 µg/kg wet weight) was found (Watanabe, 1987;
Watanabe et al., 1987b).
2,2',4,4',5'-Pentabromodiphenyl ether (PeBDE) was found in cod
liver (2 samples from each region) from the southern, central, and
northern regions of the North Sea, in 1982-87. Levels were 3.6 and
22.0 µg/kg; 4.9 and 7.2 µg/kg; and 1.9 and 6.5 µg/kg, respectively, on
a product basis (De Boer, 1989).
A multiresidue analytical method was applied to pooled muscle
samples of 35 freshwater whitefish (Coregonus sp.), 15 samples of
arctic char (Salvelinus alpinus), and a total of 260 samples of
herring (Clupea harengus), collected at different places in Sweden
during the period 1986-87. The average concentrations of
2,2',4,4',5-pentabromodiphenyl ether were 7.2, 64, and 9.8-46 µg/kg
lipid, respectively (Jansson et al., 1993).
Samples of bream, pike, perch, and trout from Sweden were shown to
contain 2,2',4,4',5-PeBDE in concentrations of 2.3-2.4 µg/kg,
60-1100 µg/kg, 380-9400 µg/kg, and 130-590 µg/kg lipid, respectively.
Another PeBDE isomer of unknown structure was also found in all fish
samples at concentrations of 11-37 µg/kg, 25-640 µg/kg,
230-3500 µg/kg, and 33-150 µg/kg lipid, respectively. The samples
represent both background and industrialized areas (Sellström et al.,
1993a).
A study of TeBDE and PeBDE in banked samples of pike from a lake
in Southern Sweden revealed concentrations increasing from 40 µg/kg
lipid in 1974 to 180/µg/kg lipid in 1991 (Sellström et al., 1993b).
Kruger (1988) sampled 40 freshwater fish of various species from
the waters of North-Rhine Westfalia in Germany and found PBDE in
concentrations ranging from 18 to 983 µg/kg fat, measured as Bromkal
70-5DE. Concentrations in 6 sea fish from the Baltic Sea ranged from
12 to 57 µg PBDE/kg fat and those in 11 fish from the North Sea, from
1 to 120 µg/kg fat, measured as Bromkal 70-5DE.
5.1.3 Aquatic mammals
Pooled samples of blubber of 7 ringed seals (Pusa hispida),
collected in 1981, and of 8 grey seals (Halichoerus grypus),
collected in 1979-85, in Sweden were analysed using a multiresidue
analytical method. The average concentrations of 2,2',4,4',5-
pentabromodiphenyl ether were 1.7 and 40 µg/kg lipid, respectively
(Jansson et al., 1993).
5.1.4 Terrestrial mammals
Pooled samples of muscle of 15 rabbits (Orytolagus cuniculus),
13 samples of moose (Alces alces), and 31 suet samples of reindeer,
collected in the period 1985-86, in Sweden, were analysed using a
multiresidue method. The average concentrations for rabbits, moose,
and reindeer, were < 0.3, 0.64, and 0.26 µg 2,2',4,4',5-penta-
bromodiphenyl ether/kg lipid, respectively (Jansson et al., 1993).
Concentrations of 10 µg PBDE/kg fat, measured as Bromkal 70-DE,
were detected in samples from 8 seals from Spitzbergen (Kruger, 1988).
PBDE, measured as Bromkal 70-DE, was detected in 4 samples of
cow's milk at concentrations ranging from 2.5 to 4.5 µg/kg fat
(Kruger, 1988).
5.1.5 Birds
Pooled samples of muscle of 35 osprey (Pandion haliaetus),
collected in Sweden in the period 1982-86, were analysed using a
multiresidue method, for 2,2',4,4',5-pentabromodiphenyl ether. The
average concentration was 140 µg/kg lipid (Jansson et al., 1993).
Newborn starlings from different places in Sweden have been shown
to contain 2.34.2 µg 2,2',4,4',5-PeBDE/kg lipid and an unidentified
PeBDE at 0.62-1.1 µg/kg lipid (Sellström et al., 1993a). The same
authors reported concentrations of the same two PeBDE isomers in
guillemot eggs from the Baltic Sea, caught during the period 1970-89
(see Table 27).
Table 27. Average PeBDE concentrations µg/kg lipid) in guillemot
eggs from the Baltic Seaa
Sampling year 2,2',4,4',5-PeBDE PeBDE
(unknown structure)
1970 24 4.2
1974 48 8.5
1975 33 4.6
1976 130 32
1979 130 37
1982 200 44
1983 210 49
1986 260 48
1987 160 40
1989 240 61
a From: Sellstrom et al.(1993a),
5.2 General population
Samples of human milk from 26 women in North Rhine Westfalia,
Germany, were analysed for PBDE, measured as Bromkal 70-DE.
Concentrations in the milk from 25 of the women ranged from 0.62 to
11.1 µg/kg fat. In one Chinese woman, a level of 50 µg/kg fat was
found (Kruger, 1988).
In Sweden, the major human exposure is via fish-related food and
the levels of PeBDE in fish may be used to estimate this exposure
route. Normal fish intake in Sweden is about 30 g/day, and, if herring
is used as a model fish, this will give an estimate of intake of about
0.1 µg PeBDE/person per day (Personal Communication, Jansson).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) in
perirenal fat was investigated in groups of 3 male and 3 female Wistar
rats (weight 160-180 g) following a single oral dose of 300 mg/kg body
weight in peanut oil. Animals from the groups were killed on days 1,
2, 3, 4, and 7 and then at weekly intervals for 10 weeks. The
perirenal fat was collected and analysed. The half-lives of 2 PeBDE
isomers, following extraction and separation with HPLC (containing
also tetrabromo- and hexabromodiphenyl ethers), are summarized in
Table 28 (Von Meyerinck et al., 1990).
Table 28. Half-lives of PeBDE in male and female ratsa
PBDE Half-lives in female rats Half-lives in male rats
in days in days
PeBDE(1)b 47.4 (42.5-52.4) 36.8 (33.7-40.0)
PeBDE(2)b 25.4 (22.6-28.4) 24.9 (22.6-27.1)
a From: von Meyerinck et al. (1990).
b (1) and (2) means two different isomars. Confidence interval,
P=0.05.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposures
7.1.1 Oral
Groups of 5 male, albino Charles River CD rats were administered
(by gavage) 50, 500, or 5000 mg commercial PeBDE (in corn oil)/kg body
weight and observed for 14 days. The rats receiving 50 and 500 mg/kg
survived and exhibited normal body weight gain. Four out of 5 rats
dosed with 5000 mg/kg died within 5 days. The remaining rats survived
and showed normal growth (Great Lakes Chemical Corporation,
undated a).
Groups of 5 male and 5 female Wistar rats were administered 2400,
4800, 6048, 7621, or 9600 mg commercial PeBDE/kg body weight. The
substance was given by gavage as an 80% w/v suspension in maize oil
after which the rats were observed for 44 days. The acute oral LD50
was 7400 mg/kg for males and 5800 mg/kg body weight for females. The
symptoms seen were decrease in growth, diarrhoea, piloerection,
reduced activity, clonic persistent tremors of the fore limbs, and red
staining around eyes and nose. A continual chewing movement of the
jaws was also seen. Post-mortem examination revealed pale (mottled),
enlarged, necrotic livers and multiple small ulcerations of the
gastric mucosa (Great Lakes Chemical Corporation undated a).
7.1.2 Dermal
Commercial PeBDE was applied to the clipped intact or abraded skin
of groups of 2 male and 2 female New Zealand white rabbits at doses of
200 or 2000 mg/kg body weight, for 24 h, under an occlusive dressing.
The rabbits were observed for 14 days. No animals died during the
observation period.
At 200 and 2000 mg/kg, normal body weight gain or only a slight
decrease in growth was seen (Great Lakes Chemical Corporation,
undated a).
7.1.3 Inhalation
The inhalation LC50 for the rat is >200 mg/litre (Kopp, 1990).
Groups of 10 male and 10 female Charles River CD rats were exposed for
1 h to an aerosol mist of commercial PeBDE mixed with corn oil at
concentrations of 2 or 200 mg/litre of air and subsequently observed
for 14 days. No rats died during the study. The rats exposed to
2 mg/litre exhibited increased and then decreased motor activity,
erythema, and eye squint during the exposure and for the following
24 h. After 24 h, and up to the end of the study, the animals appeared
normal. During exposure to 200 mg/litre, the same signs were observed
but also lacrimation, salivation, and tachypnoea. At 24 h and 48 h, 2
rats exhibited nasal congestion and one rat showed respiratory
congestion after 72 h. From day 4 to day 14, the animals appeared
normal and showed normal body weight gain (Great Lakes Chemical
Corporation, undated a).
7.2 Short-term exposure
Charles River CD rats were fed dietary levels of 0, 100, or
1000 mg commercial PeBDE (dissolved in corn oil)/kg daily for 28 days.
There were 10 male and 10 female animals in each group. No changes
were noted in behaviour, appearance, food consumption, or body weight
gain. Absolute and relative liver weights were significantly increased
in female rats fed with 100 mg/kg and in male and female rats fed with
1000 mg/kg. The liver lesions were more prevalent in male rats and
increased with dose. A significant decrease in the relative weights of
the pituitary and adrenal glands was found at the highest dose level.
No compound-related, gross pathological lesions were noted.
Microscopically, enlargement of the centrolobular and midzonal liver
parenchyma cells was seen, and the cytoplasm included areas with a
finely granular appearance; eosinophilic "round bodies" in enlarged
hepatocytes were seen in the animals at both dose levels. Several rats
from both the 100 mg and the 1000 mg/kg groups had slight to moderate
hyperplasia of the thyroid, but control animals also had thyroid
glands that could be considered hyperplastic. In thyroid glands
designated as hyperplastic, most follicles were very small, devoid of
colloid, and lined by basophilic columnar follicular epithelium.
Whether these thyroid changes were compound-related is not clear.
Dose-related increases in total bromide levels in liver tissues from
the (pooled) treated rats were 6-12 times higher than those in the
controls (Great Lakes Chemical Corporation, undated a).
Commercial PeBDE (DE-71) was given in the diet to 3 groups of 30
male and 30 female CD Sprague-Dawley rats. Dosage levels of 0, 2, 10,
or 100 mg/kg per day were administered for 90 days. Ten animals per
sex were sacrificed after 4 weeks and after 90 days, 5 animals were
sacrificed after a 6-week recovery period, and 5 animals, after a
24-week recovery period. No increased mortality or clinical effects
were observed. Decrease in food consumption was seen in high-dose
females and a decrease in body weight was observed in high-dose males
and females. Haematology parameters and liver function were normal,
but increased cholesterol values were observed for high-dose animals.
Tri-iodothyronine (T3) levels were normal, but tetraiodothyronine (T4)
levels were decreased (not dose-related) in the 10 mg and 100 mg/kg
group; an increase in serum total bromide levels was observed in these
2 groups after 4 and 13 weeks. Compound-related increases in liver and
urine porphyrins were observed in the high-dose animals after 13
weeks. Urine porphyrin levels were 13 times higher in females and up
to 8 times higher in males and liver porphyrin levels were almost 400
times higher than those of the controls. Compound-related increases in
tissue total bromine levels were noted in all tissues for males and
females at both the low- and high-dose levels (mid-dose levels not
determined). During the recovery period, a slow decrease in the total
bromine levels was noted, but, even after 24 weeks, the levels did not
reach the control values, especially in the highest dose group.
Relative liver weights in the 10 and 100 mg/kg groups were increased,
but, during the recovery period, liver weights that were still higher
after 6 weeks were normal after 24 weeks. Microscopic examination
revealed hepatocytomegaly and thyroid hyperplasia. The thyroid
hyperplasia was reversible in 24 weeks recovery period, but the liver
still showed slight hepatocytomegaly in the 10 and 100 mg/kg group. At
the lowest dose level (2 mg/kg), the only effect observed after 24
weeks' recovery, i.e., liver cell degeneration and necrosis, was seen
in females, but not in males (Great Lakes Chemical Corporation,
undated a).
7.3 Long-term exposure
No data are available.
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
Commercial PeBDE was applied, under occlusion, to the clipped
intact or abraded skin of groups of 3 male and 3 female New Zealand
white rabbits at a dose of 0.5 ml (approximately 1135 mg). After 24 h,
the wrappings were removed and the backs of the rabbits washed and
examined for signs of irritation. The examinations were repeated at
72 h. At 24 and 72 h, no, or only very slight, erythema was noted. No
oedema was seen (Great Lakes Chemical Corporation, undated a).
7.4.2 Eye irritation
A single application of 0.1 ml commercial PeBDE was instilled into
the conjunctival sac of the eyes of 3 male, and 3 female, New Zealand
white rabbits. Examinations were carried out at 24, 48, and 72 h, and
at 7 days. At 24 h, all rabbits showed slight redness, slight
chemosis, and slight discharge of the conjunctivae. These symptoms
subsided during 7 days. At 7 days, slight alopecia around the eyelid
was seen in 2 of the 6 animals. No irritation of the iris was
observed. Examination at 72 h revealed slight evidence of corneal
damage in one of the 6 animals (Great Lakes Chemical Corporation,
undated a).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Pregnant female rats were given corn-oil suspensions of commercial
PeBDE, by gavage, at dosages of 0, 10, 100, or 200 mg/kg body weight
per day, on days 6-15 of gestation. The material was not teratogenic.
The maternal no-effect level was 10 mg/kg and the embryo/fetal
no-effect level was 100 mg/kg body weight. Inhibition of maternal body
weight gain occurred at doses of 100 or 200 mg/kg. A slight,
nonstatistically significant, reduction in average fetal body weight
per litter was found at the highest dose level (BFRIP, 1990) (no
further details).
7.6 Mutagenicity and related endpoints
Commercial PeBDE was examined for mutagenic activity at a number
of concentrations in a series of in vitro microbial assays using
Salmonella typhimurium TA98, TA100, TA1535, and TA1537 and
Saccharomyces cerevisae in the presence, and absence, of liver
microsomal enzyme preparations from Aroclor-induced rats. A negative
result was obtained with and without microsomal activation (Great
Lakes Chemical Corporation, undated a).
7.7 Carcinogenicity
No data are available.
7.8 Other special studies
Commercial PeBDE in corn oil was administered, by gavage, to 6
male Sprague-Dawley rats (200-250 g) for 90 days. When the original
doses of 6.25, 12.5, and 25 µmol/kg per day revealed extensive
induction, the study was repeated at doses of 0.78, 1.56, and
3.13 µmol/kg per day. When the lower dose levels were tested, even the
lowest dose of PeBDE (0.78 µmol/kg) caused increases in
O-ethyl- O-p-nitrophenyl phenylphosphonothiate (EPN)
detoxiFication, p-nitroanisole demethylation, and NADPH cytochrome c
reductase and cytochrome P450 activity. A clear dose-response
relationship was found for EPN detoxification and p-nitroanisole
demethylation. When the animals were allowed a 30-day recovery period
following the last (90th) dose, elevations in these two measurements
were still observable at all doses, though increased NADPH cytochrome
c reductase activity was found only at the highest dose. Within this
period, the cytochrome P450 content had returned to normal. Even after
60 days recovery, elevations were noted in EPN detoxification and
p-nitroanisole demethylation at the 1.56 and 3.13 µmol/kg levels. No
histological liver abnormalities were observed in rats treated with
doses of 3.13 µmol/kg or less (Carlson, 1980b).
In another study, rats (200-250g) were administered commercial
PeBDE at 0.1 mmol/kg per day, in corn oil, by gavage, for 14 days. In
addition to the above described effects on the enzymes, increases in
the activities of UDP-glucuronyl-transferase and benzo[ a]pyrene-
hydroxylase were found 24 h after the seventh dose (Carlson, 1980a).
Von Meyerinck et al. (1990) studied the hepatic microsomal enzyme
inducing potential of Bromkal 70 in Wistar rats. A series of dosing
regimes was used, which varied from single oral doses of up to
300 mg/kg body weight to 28 daily oral doses of 50 mg/kg body weight.
In all cases, the vehicle was peanut oil (1 ml/kg), which was also
administered to the vehicle control group. There were 3 male and 3
female rats per group, except in the 28-day study in which 4 male and
4 female rats were used. The treatments resulted in 31-53% increases
in liver weight, 2.3-3.9-fold increases in cytochrome P450 c levels,
up to 2-fold increases in benzphetamine N-demethylation activity, and
2.2-5.3-fold increases in benzo( a)pyrene oxidation. Increases in
ethoxyresorufin O-deethylation activity ranged from not detectable
to 0.35 nmol/min mg microsomal protein in control groups to between
4.1 and 16.6 nmol/min mg microsomal protein in treatment groups. These
activities were sex and dose dependent. The most sensitive parameter
was ethoxyresorufin O-deethylase induction, which showed no
significant response in rats dosed once with 3 mg PeBDE/kg and killed
3 days later.
Commercial PeBDE (Bromkal 70-5 DE) showed the induction of
ethoxyresorufin O-deethylase activity in H-4-II E cells (Hanberg et
al., 1991).
TETRABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Tetrabromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial tetrabromodiphenyl ether, consisted of 41% tetra-, 45%
penta-, and 7% hexabromodiphenyl ethers and about 7% PBDE of unknown
structure. On the basis of the chemical structure, there are 42
possible isomers of tetrabromodiphenyl ether. Virtually no data are
available on physical and chemical properties, except that the
n-octanol/water partition coefficient (log Pow) is 5.87-6.16.
1.1.2 Production and uses
There is a report of the production (use) of about 1000 tonnes of
TeBDE in Japan in 1987. There is no known current production under the
name of tetrabromodiphenyl ether, but TeBDE is present in quantities
of from 24 to 38% in commercial pentabromodiphenyl ether.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial TeBDE have been found in biota, sediment,
and sewage sludge samples. Commercial TeBDE components (containing
approximately equal quantities of PeBDE) are likely to be persistent
and to bioaccumulate.
Pyrolysis studies with commercial TeBDE showed that PBDF and PBDD
are formed at 800 °C. Higher PBDF and PBDD were not found.
1.1.4 Environmental levels and human exposure
TeBDE was found, in Japan, in river sediment at concentrations of
12-31 µg/kg dry weight and, in Sweden, at concentrations of up to
840 µg/kg ign. loss, respectively. TeBDE was also found in sewage
sludge in Sweden, at a concentration of 15 µg/kg.
Mussels and fish, collected at different places in Japan,
contained TeBDE in concentrations ranging from < 0.1 to 14.6 µg
2,2',4,4'-TeBDE/kg wet weight. In Sweden, different types of fish were
collected from rivers and analysed for 2,2',4,4'-TeBDE. The mean
concentrations ranged from ND (< 0.1 mg/kg) to 110 mg/kg fat. The
analysis indicated that there was at least one local source of
pollution in a certain river. Whitefish, arctic char, and herring,
collected at different places in Sweden in 1986-87, contained
concentrations of 15, 400, and 59-450 µg 2,2',4,4'-TeBDE/kg fat,
respectively. Fish collected from rivers in Germany contained up to
1 mg TeBDE/kg fat.
In herring and in the liver of cod, collected in the southern,
central, and northern North Sea, in the period 1983-89, a decreasing
trend in the concentrations of TeBDE was found from the southern
region to the northern region. In the herring, concentrations of
8.4-100 µg 2,2',4,4'-TeBDE/kg, on a fat basis, were found.
The muscle tissue of birds nesting and wintering in the Baltic
Sea, the North Sea, and Spitzbergen, contained from 80 to 370 µg
2,2',4,4'-TeBDE/kg, on a fat basis. Osprey collected in Sweden in the
period 1982-86, contained average concentrations of 1800 µg/kg fat.
Increasing trends in the concentrations of 2,2',4,4'-TeBDE have
been indicated for Baltic sediments, freshwater fish, and sea bird
eggs from Sweden.
The blubber of seals collected in the Baltic Sea and Spitzbergen
showed concentrations of 10-730 µg 2,2',4,4'-TeBDE/kg, on a fat basis.
The chromatographic pattern of the PBDE was similar to that of Bromkal
70-5. Pooled samples of the blubber of ringed seals and grey seals,
collected in Sweden in 1979-85 showed concentrations of 47 µg and
650 µg 2,2',4,4'-TeBDE/kg fat, respectively.
Pooled muscle samples of terrestrial mammals, e.g., rabbits, moose
and reindeer, collected in 1985-86 in Sweden, showed average
concentrations of <2, 0.82, and 0.18 µg 2,2',4,4'-TeBDE/kg fat,
respectively.
Levels of 2.5-4.5 µg PBDE/kg fat, measured as Bromkal 70DE, were
found in 4 samples of cow's milk in Germany. PBDE, as Bromkal 70DE,
was found in the milk of 25 women in Germany at concentrations ranging
from 0.62 to 11.1 µg/kg fat.
A rough estimate of exposure via fish consumption among the
Swedish population would suggest an intake of 0.3 µg TeBDE/ person per
day.
1.1.5 Effects on laboratory mammals and in vitro test systems
There are no data on TeBDE itself, but acute and short-term data
are available for commercial PeBDE containing 41% TeBDE.
1.1.6 Kinetics and metabolism in laboratory animals and humans
Minimal data are available.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
No data are available.
1.2 Conclusions
1.2.1 TeBDE
Components of commercial TeBDE (a mixture of 41% 2,2',4,4'-tetra-;
45% 2,2',4,4',5'-penta-; 7% hexa-, and 7-8% polybrominated diphenyl
ethers with an unknown structure) are persistent and accumulate in
organisms in the environment.
TeBDE as a component of pentabromodiphenyl ether is widely
incorporated in polymers as an additive flame retardant. Contact of
the general population is with products made from these polymers.
Exposure by extraction from polymers is unlikely. Human exposure to
TeBDE, via the food chain, may occur, because the substance has been
detected in organisms in the environment that are human food items,
such as fish, shellfish, etc. In fish and birds from Sweden,
increasing levels have been measured over the last two decades.
There is a lack of information concerning short-, long-term
toxicity/carcinogenicity, and reproduction studies. Furthermore,
information on kinetics and metabolism in laboratory animals and
humans is not available.
The risk for the general population cannot be determined on the
basis of available data.
No information is available to draw conclusions on occupational
exposure levels or the effects of TeBDE.
No data are available on the toxicity of commercial TeBDE for
organisms in the environment.
1.2.2 Breakdown products
PBDF and PBDD are formed when TeBDE are heated to 800 °C. The
possible hazards associated with this have to be addressed.
Exposure of the general population to PBDF in polymers, flame
retarded with TeBDE, is unlikely to be of significance. Properly
controlled incineration does not lead to the emission of significant
quantities of brominated dioxins and furans. Any uncontrolled
combustion of products containing TeBDE can lead to the generation of
unquantified amounts of PBDF/PBDD. The significance of this for both
humans and the environment will be addressed in a future EHC on
PBDF/PBDD.
1.3 Recommendations
1.3.1 General
Because of their persistence in the environment and accumulation in
organisms, it is recommended that TeBDE should not be used. However,
should use continue, the following points must be taken into account:
* Workers involved in the manufacture of TeBDE and products
containing the compound should be protected from exposure using
appropriate industrial hygiene measures, the monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent and accumulating compound and
its breakdown products.
* Incineration of materials, flame retarded with TeBDE, should only
be carried out in properly constituted incinerators running under
optimal conditions. Burning by any other means will lead to the
production of furan breakdown products.
1.3.2 Further studies
* Continued monitoring of environmental levels is required.
* Analytical methods for TeBDE in various matrices should be
validated.
* Because the present toxicological data base is inadequate to
evaluate the hazards of commercial TeBDE for humans and the
environment, if use is continued, the following studies should be
done:
- additional toxicological, carcinogenicity, and
ecotoxicological studies;
- further investigations on the generation of PBDF under real
fire conditions;
- investigation into possible methods and consequences of
recycling of TeBDE-containing polymers;
- investigations of the possibility of migration from
flame-retarded products.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12H5Br5O
Relative molecular mass: 564.75
Common name: pentabromodiphenyl ether (PeBDE);
pentabromodiphenyl oxide
CAS registry number: 32534-81-9
CAS name: 1,1'-oxybis-pentabromo-benzene,
benzene, 1,1'-oxybis-, pentabromo
On the basis of the chemical structure, there are 46 possible
isomers of pentabromodiphenyl ether.
From: IRPTC (1988).
2.1.1 Technical product
Trade name DE 71; Bromkal 70-5 DE;
FR 1205/1215;
Bromkal 70; Bromkal G1;
Pentabromprop;
DE-60 F is a mixture of 85%
PeBDE and 15% of an aromatic phosphate
Commercial pentabromodiphenyl ether is a mixture of polybrominated
diphenyl ethers with the following typical composition (see Table 1):
0-1% tribromodiphenyl ether, 24-38% tetrabromodiphenyl ether, 50-62%
pentabromodiphenyl ether, 4-8% hexabromodiphenyl ether (Arias, 1992).
DE-71 is primarily a mixture of tetra-, penta-, and
hexabromodiphenyl ethers containing low levels of tribromodiphenyl
ether (< 1%) and heptabromodiphenyl ether (< 2%) (McAllister &
Ariano, 1982). Pentabromprop is a mixture of 39% tetra-, 61% penta-,
and 9% hexabromodiphenyl ethers. Bromkal 70-5 DE is a mixture of 34.2%
tetra-, 59.8% penta-, 5.8% hexa-, and 0.2% heptabromodiphenyl ethers,
but 41.7% tetra- and 45% pentabromodiphenyl ethers, and 7% of a second
pentabromodiphenyl ether have also been reported (Nylund et al.,
1992). Another manufacturer produces a mixture of 35% tetra-, 58%
penta-, and 4% higher brominated diphenyl ethers under the name of
Pentabromodiphenyl ether. Bromkal 70 is a mixture containing 36%
tetrabromo- and 74% pentabromodiphenyl ether. The bromine contents of
the mixtures range from 67 to 71.8% (De Kok et al., 1979). Bromkal
70-5, a mixture of brominated diphenyl ethers containing 67-71%
bromine, and an average of about 5 bromines per molecule, also
contained various isomers of tri-, tetra-, penta-, and
hexabromodiphenyl ethers. However, it is no longer produced
commercially (McAllister, 1991).
Sundström & Hutzinger (1976) identified 2,2',4,4'-tetra- and
2,2',4,4',5,-PeBDE as the major components of Bromkal 70-5 DE.
2.2 Physical and chemical properties
Pentabromodiphenyl ether (PeBDE) is a clear, amber to pale yellow,
highly viscous liquid, with an organic smell. Under conditions of
fire, hydrogen bromide and/or bromine occur.
Melting point -7 to -3 °Ca
Boiling point > 300 °C (decomposition starts
above 200 °C)
Specific gravity 2.28 at 25 °C; 1.78 at 40 °C
Vapour pressure 9.3 mmHg at 22 °Cb
(6.26-6.66 Torr at 25 °C)
Solubility Insoluble in water (9 × 10-7 mg/litre
at 20 °C), methanol 10 g/litre at 25 °C,
soluble in other organic solvents
such as chloroform, benzene, toluene,
acetone, carbon-tetrachloride, and
methylene chloride
Viscosity at 50 °C 1-6 Pa (150 000 cp at 25 °C;
1500 cp at 60 °C)
n-Octanol/water partition
coefficient (log Pow) 6.64-6.97
From: Great Lakes Chemical Corporation (undated a); Kalk (1982);
Kopp (1990); US EPA (1989); Hallenbeck (1993); US Testing Comp.
(1985).
2.3 Analytical methods
McAllister & Ariano (1982) developed a method for the
determination of the arbitrarily defined "bromination level" of PeBDE
(DE-71), both as the neat material and as a formulation in DE-60 F,
and for the quantitative determination of aromatic phosphate ester in
DE-60 F. The sample is dissolved in dibromomethane containing an
internal standard and the components separated by gas chromatography.
A multiresidue method has been developed for the determination of
PBDE residues in environmental samples (see General Introduction,
section 2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Pentabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Pentabromodiphenyl ether is synthesized by treating diphenyl ether
with 5 equivalents of Br2 at 30-65 °C in the presence of powdered
iron (US EPA, 1986).
The actual worldwide consumption of PeBDE per year is 4000 tonnes.
In the Federal Republic of Germany, in 1988, the approximate level of
use in plastics was 200-400 tonnes/year. The total use of penta- and
hexabromodiphenyl ethers in the Netherlands in 1988 was estimated to
be 350 tonnes (Anon., 1989b).
Production levels are not available.
3.2.2 Uses
PeBDE is used as an additive in epoxy resins, phenol resins,
unsaturated polyesters, polyurethane flexible, and textiles.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Pyrolysis
PeBDE (Bromkal 70, 70-5 DE, G1) were heated in a quartz tube at
700, 800, or 900 °C and the concentrations of PBDD and PBDF
determined. Monobromo- to pentabromodibenzofurans, as well as
monobromo- to tetrabromodioxins, were found in yields of up to 90% in
all 3 Bromkal samples. The optimal temperatures of formation were
between 700 and 800 °C (Thoma et al., 1987a).
Bromkal 70-5-DE was pyrolysed at 600, 700, 800, and 900 °C, in the
absence of oxygen, in a SGE pyrojector and the residues analysed by
GC/MS in an on-line operation. The pyrolysis of Bromkal 70-5-DE
yielded polybromobenzenes (PBBz), polybromophenois (PBP), and
brominated dibenzofurans. Because this pyrolysis is performed in a
pyrojector with a helium current, incorporation of oxygen is
impossible, consequently dioxins do not form (Thoma & Hutzinger, 1987,
1989).
4.2 Workplace exposure studies
In the processing of PBT, finished with pentabromodiphenyl ether
at 300 °C, PBDF were found in both the air at the workplace and the
machine extractor. PBDF were also found in air samples taken from a
vessel in which the granulate was stored. Since the bulk of the PBDF
formed during processing remain in the product, it can be assumed that
parts made from the polymers, such as computer casings, television
sets, etc., are contaminated with PBDF (CEM, 1989).
4.3 Bioaccumulation
Carp exposed for 8 weeks to commercial pentabromodiphenyl ether at
10 or 100 µg/litre showed bioconcentration factors of more than 10 000
(CBC, 1982).
4.4 Ultimate fate following use
For the ultimate fate of PeBDE following use see section 6.1 of
the General Introduction.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Levels in the environment
5.1.1 Sediment and sewage sludge
Sediment samples were taken from the rivers near the centre of
Osaka City and marine sediments, from different estuaries in Japan and
from Osaka Bay, during the period 1981-83. No PeBDE was found
(<2 µg/kg dry weight in 9 estuarine or marine sediment samples. PeBDE
was present in 5 out of 6 river sediment samples at concentrations of
9-28 µg/kg on a dry weight basis (Watanabe, 1987; Watanabe et al.,
1987b).
Organic extracts, made from coastal sediments, collected offshore
from Barcelona, were analysed for the presence of organohalogenated
chemicals. A diversity of organohalogenated compounds were found,
among them PeBDE (Fernandez et al., 1992).
Sellström et al. (1990a,b) analysed sediment samples, taken
upstream and downstream from a factory, for the presence of
tetrabromobisphenol-A and derivatives. Both tetrabromobisphenol A and
2,2',4,4',5-pentabromodiphenyl ether were found. The levels found in
upstream and downstream sediments were 8.2 and 1200 µg/kg (ign loss),
respectively.
A laminated sediment core collected in the southern part of the
Baltic Proper (Bornholm Deep) was analysed for 2,2',4,4',5-PeBDE. The
core was cut into 5 mm slices down to 50 mm depth and in 10 mm slices
from 50 to 90 mm depth. The concentration of PeBDE at a depth of 5 mm
was 0.98 µg/kg IG and decreased more or less gradually in the deeper
layers to approximately 0.05 µg/kg at a depth of 40 mm. Below this
depth, levels were mostly not detectable (Nylund et al., 1992).
Sellström et al. (1990a,b) analysed sewage sludge and found 19 µg
2,2',4,4',5-pentabromodiphenyl ether/kg ign loss.
Two sewage samples were collected from a Swedish treatment plant
(Gothenburg) in September 1988 anti analysed for PBDE. One homogenate
was composed of samples (40 g/day) taken over a 32-day period with
little rain. Another homogenate was composed of samples (100 g/day)
taken during a rainy period of 7 days. Both TeBDE and PeBDE were
found. The concentration of 2,2',4,4',5-PeBDE was 19 µg/kg;
concentrations of PeBDE of unknown structures were 3.4 and 3.7 µg/kg
and total PeBDE, 37-38 µg/kg IG (Nylund et al., 1992).
5.1.2 Fish and shellfish
Mussels and some species of fish, e.g., mullet, goby, and Japanese
sea bass, were collected from different seashores in Japan from 1981
to 1985. Other fish species were purchased from a commercial wholesale
source in Osaka Prefecture in 1981. In 2 out of 5 mussel samples,
levels of 0.4 and 2.8 µg PeBDE/kg wet weight were found. In 12 fish
samples, no PeBDE (< 0.2 µg/kg wet weight) was found (Watanabe, 1987;
Watanabe et al., 1987b).
2,2',4,4',5'-Pentabromodiphenyl ether (PeBDE) was found in cod
liver (2 samples from each region) from the southern, central, and
northern regions of the North Sea, in 1982-87. Levels were 3.6 and
22.0 µg/kg; 4.9 and 7.2 µg/kg; and 1.9 and 6.5 µg/kg, respectively, on
a product basis (De Boer, 1989).
A multiresidue analytical method was applied to pooled muscle
samples of 35 freshwater whitefish (Coregonus sp.), 15 samples of
arctic char (Salvelinus alpinus), and a total of 260 samples of
herring (Clupea harengus), collected at different places in Sweden
during the period 1986-87. The average concentrations of
2,2',4,4',5-pentabromodiphenyl ether were 7.2, 64, and 9.8-46 µg/kg
lipid, respectively (Jansson et al., 1993).
Samples of bream, pike, perch, and trout from Sweden were shown to
contain 2,2',4,4',5-PeBDE in concentrations of 2.3-2.4 µg/kg,
60-1100 µg/kg, 380-9400 µg/kg, and 130-590 µg/kg lipid, respectively.
Another PeBDE isomer of unknown structure was also found in all fish
samples at concentrations of 11-37 µg/kg, 25-640 µg/kg,
230-3500 µg/kg, and 33-150 µg/kg lipid, respectively. The samples
represent both background and industrialized areas (Sellström et al.,
1993a).
A study of TeBDE and PeBDE in banked samples of pike from a lake
in Southern Sweden revealed concentrations increasing from 40 µg/kg
lipid in 1974 to 180/µg/kg lipid in 1991 (Sellström et al., 1993b).
Kruger (1988) sampled 40 freshwater fish of various species from
the waters of North-Rhine Westfalia in Germany and found PBDE in
concentrations ranging from 18 to 983 µg/kg fat, measured as Bromkal
70-5DE. Concentrations in 6 sea fish from the Baltic Sea ranged from
12 to 57 µg PBDE/kg fat and those in 11 fish from the North Sea, from
1 to 120 µg/kg fat, measured as Bromkal 70-5DE.
5.1.3 Aquatic mammals
Pooled samples of blubber of 7 ringed seals (Pusa hispida),
collected in 1981, and of 8 grey seals (Halichoerus grypus),
collected in 1979-85, in Sweden were analysed using a multiresidue
analytical method. The average concentrations of 2,2',4,4',5-
pentabromodiphenyl ether were 1.7 and 40 µg/kg lipid, respectively
(Jansson et al., 1993).
5.1.4 Terrestrial mammals
Pooled samples of muscle of 15 rabbits (Orytolagus cuniculus),
13 samples of moose (Alces alces), and 31 suet samples of reindeer,
collected in the period 1985-86, in Sweden, were analysed using a
multiresidue method. The average concentrations for rabbits, moose,
and reindeer, were < 0.3, 0.64, and 0.26 µg 2,2',4,4',5-penta-
bromodiphenyl ether/kg lipid, respectively (Jansson et al., 1993).
Concentrations of 10 µg PBDE/kg fat, measured as Bromkal 70-DE,
were detected in samples from 8 seals from Spitzbergen (Kruger, 1988).
PBDE, measured as Bromkal 70-DE, was detected in 4 samples of
cow's milk at concentrations ranging from 2.5 to 4.5 µg/kg fat
(Kruger, 1988).
5.1.5 Birds
Pooled samples of muscle of 35 osprey (Pandion haliaetus),
collected in Sweden in the period 1982-86, were analysed using a
multiresidue method, for 2,2',4,4',5-pentabromodiphenyl ether. The
average concentration was 140 µg/kg lipid (Jansson et al., 1993).
Newborn starlings from different places in Sweden have been shown
to contain 2.34.2 µg 2,2',4,4',5-PeBDE/kg lipid and an unidentified
PeBDE at 0.62-1.1 µg/kg lipid (Sellström et al., 1993a). The same
authors reported concentrations of the same two PeBDE isomers in
guillemot eggs from the Baltic Sea, caught during the period 1970-89
(see Table 27).
Table 27. Average PeBDE concentrations µg/kg lipid) in guillemot
eggs from the Baltic Seaa
Sampling year 2,2',4,4',5-PeBDE PeBDE
(unknown structure)
1970 24 4.2
1974 48 8.5
1975 33 4.6
1976 130 32
1979 130 37
1982 200 44
1983 210 49
1986 260 48
1987 160 40
1989 240 61
a From: Sellstrom et al.(1993a),
5.2 General population
Samples of human milk from 26 women in North Rhine Westfalia,
Germany, were analysed for PBDE, measured as Bromkal 70-DE.
Concentrations in the milk from 25 of the women ranged from 0.62 to
11.1 µg/kg fat. In one Chinese woman, a level of 50 µg/kg fat was
found (Kruger, 1988).
In Sweden, the major human exposure is via fish-related food and
the levels of PeBDE in fish may be used to estimate this exposure
route. Normal fish intake in Sweden is about 30 g/day, and, if herring
is used as a model fish, this will give an estimate of intake of about
0.1 µg PeBDE/person per day (Personal Communication, Jansson).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) in
perirenal fat was investigated in groups of 3 male and 3 female Wistar
rats (weight 160-180 g) following a single oral dose of 300 mg/kg body
weight in peanut oil. Animals from the groups were killed on days 1,
2, 3, 4, and 7 and then at weekly intervals for 10 weeks. The
perirenal fat was collected and analysed. The half-lives of 2 PeBDE
isomers, following extraction and separation with HPLC (containing
also tetrabromo- and hexabromodiphenyl ethers), are summarized in
Table 28 (Von Meyerinck et al., 1990).
Table 28. Half-lives of PeBDE in male and female ratsa
PBDE Half-lives in female rats Half-lives in male rats
in days in days
PeBDE(1)b 47.4 (42.5-52.4) 36.8 (33.7-40.0)
PeBDE(2)b 25.4 (22.6-28.4) 24.9 (22.6-27.1)
a From: von Meyerinck et al. (1990).
b (1) and (2) means two different isomars. Confidence interval,
P=0.05.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposures
7.1.1 Oral
Groups of 5 male, albino Charles River CD rats were administered
(by gavage) 50, 500, or 5000 mg commercial PeBDE (in corn oil)/kg body
weight and observed for 14 days. The rats receiving 50 and 500 mg/kg
survived and exhibited normal body weight gain. Four out of 5 rats
dosed with 5000 mg/kg died within 5 days. The remaining rats survived
and showed normal growth (Great Lakes Chemical Corporation,
undated a).
Groups of 5 male and 5 female Wistar rats were administered 2400,
4800, 6048, 7621, or 9600 mg commercial PeBDE/kg body weight. The
substance was given by gavage as an 80% w/v suspension in maize oil
after which the rats were observed for 44 days. The acute oral LD50
was 7400 mg/kg for males and 5800 mg/kg body weight for females. The
symptoms seen were decrease in growth, diarrhoea, piloerection,
reduced activity, clonic persistent tremors of the fore limbs, and red
staining around eyes and nose. A continual chewing movement of the
jaws was also seen. Post-mortem examination revealed pale (mottled),
enlarged, necrotic livers and multiple small ulcerations of the
gastric mucosa (Great Lakes Chemical Corporation undated a).
7.1.2 Dermal
Commercial PeBDE was applied to the clipped intact or abraded skin
of groups of 2 male and 2 female New Zealand white rabbits at doses of
200 or 2000 mg/kg body weight, for 24 h, under an occlusive dressing.
The rabbits were observed for 14 days. No animals died during the
observation period.
At 200 and 2000 mg/kg, normal body weight gain or only a slight
decrease in growth was seen (Great Lakes Chemical Corporation,
undated a).
7.1.3 Inhalation
The inhalation LC50 for the rat is >200 mg/litre (Kopp, 1990).
Groups of 10 male and 10 female Charles River CD rats were exposed for
1 h to an aerosol mist of commercial PeBDE mixed with corn oil at
concentrations of 2 or 200 mg/litre of air and subsequently observed
for 14 days. No rats died during the study. The rats exposed to
2 mg/litre exhibited increased and then decreased motor activity,
erythema, and eye squint during the exposure and for the following
24 h. After 24 h, and up to the end of the study, the animals appeared
normal. During exposure to 200 mg/litre, the same signs were observed
but also lacrimation, salivation, and tachypnoea. At 24 h and 48 h, 2
rats exhibited nasal congestion and one rat showed respiratory
congestion after 72 h. From day 4 to day 14, the animals appeared
normal and showed normal body weight gain (Great Lakes Chemical
Corporation, undated a).
7.2 Short-term exposure
Charles River CD rats were fed dietary levels of 0, 100, or
1000 mg commercial PeBDE (dissolved in corn oil)/kg daily for 28 days.
There were 10 male and 10 female animals in each group. No changes
were noted in behaviour, appearance, food consumption, or body weight
gain. Absolute and relative liver weights were significantly increased
in female rats fed with 100 mg/kg and in male and female rats fed with
1000 mg/kg. The liver lesions were more prevalent in male rats and
increased with dose. A significant decrease in the relative weights of
the pituitary and adrenal glands was found at the highest dose level.
No compound-related, gross pathological lesions were noted.
Microscopically, enlargement of the centrolobular and midzonal liver
parenchyma cells was seen, and the cytoplasm included areas with a
finely granular appearance; eosinophilic "round bodies" in enlarged
hepatocytes were seen in the animals at both dose levels. Several rats
from both the 100 mg and the 1000 mg/kg groups had slight to moderate
hyperplasia of the thyroid, but control animals also had thyroid
glands that could be considered hyperplastic. In thyroid glands
designated as hyperplastic, most follicles were very small, devoid of
colloid, and lined by basophilic columnar follicular epithelium.
Whether these thyroid changes were compound-related is not clear.
Dose-related increases in total bromide levels in liver tissues from
the (pooled) treated rats were 6-12 times higher than those in the
controls (Great Lakes Chemical Corporation, undated a).
Commercial PeBDE (DE-71) was given in the diet to 3 groups of 30
male and 30 female CD Sprague-Dawley rats. Dosage levels of 0, 2, 10,
or 100 mg/kg per day were administered for 90 days. Ten animals per
sex were sacrificed after 4 weeks and after 90 days, 5 animals were
sacrificed after a 6-week recovery period, and 5 animals, after a
24-week recovery period. No increased mortality or clinical effects
were observed. Decrease in food consumption was seen in high-dose
females and a decrease in body weight was observed in high-dose males
and females. Haematology parameters and liver function were normal,
but increased cholesterol values were observed for high-dose animals.
Tri-iodothyronine (T3) levels were normal, but tetraiodothyronine (T4)
levels were decreased (not dose-related) in the 10 mg and 100 mg/kg
group; an increase in serum total bromide levels was observed in these
2 groups after 4 and 13 weeks. Compound-related increases in liver and
urine porphyrins were observed in the high-dose animals after 13
weeks. Urine porphyrin levels were 13 times higher in females and up
to 8 times higher in males and liver porphyrin levels were almost 400
times higher than those of the controls. Compound-related increases in
tissue total bromine levels were noted in all tissues for males and
females at both the low- and high-dose levels (mid-dose levels not
determined). During the recovery period, a slow decrease in the total
bromine levels was noted, but, even after 24 weeks, the levels did not
reach the control values, especially in the highest dose group.
Relative liver weights in the 10 and 100 mg/kg groups were increased,
but, during the recovery period, liver weights that were still higher
after 6 weeks were normal after 24 weeks. Microscopic examination
revealed hepatocytomegaly and thyroid hyperplasia. The thyroid
hyperplasia was reversible in 24 weeks recovery period, but the liver
still showed slight hepatocytomegaly in the 10 and 100 mg/kg group. At
the lowest dose level (2 mg/kg), the only effect observed after 24
weeks' recovery, i.e., liver cell degeneration and necrosis, was seen
in females, but not in males (Great Lakes Chemical Corporation,
undated a).
7.3 Long-term exposure
No data are available.
7.4 Skin and eye irritation; sensitization
7.4.1 Skin irritation
Commercial PeBDE was applied, under occlusion, to the clipped
intact or abraded skin of groups of 3 male and 3 female New Zealand
white rabbits at a dose of 0.5 ml (approximately 1135 mg). After 24 h,
the wrappings were removed and the backs of the rabbits washed and
examined for signs of irritation. The examinations were repeated at
72 h. At 24 and 72 h, no, or only very slight, erythema was noted. No
oedema was seen (Great Lakes Chemical Corporation, undated a).
7.4.2 Eye irritation
A single application of 0.1 ml commercial PeBDE was instilled into
the conjunctival sac of the eyes of 3 male, and 3 female, New Zealand
white rabbits. Examinations were carried out at 24, 48, and 72 h, and
at 7 days. At 24 h, all rabbits showed slight redness, slight
chemosis, and slight discharge of the conjunctivae. These symptoms
subsided during 7 days. At 7 days, slight alopecia around the eyelid
was seen in 2 of the 6 animals. No irritation of the iris was
observed. Examination at 72 h revealed slight evidence of corneal
damage in one of the 6 animals (Great Lakes Chemical Corporation,
undated a).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Pregnant female rats were given corn-oil suspensions of commercial
PeBDE, by gavage, at dosages of 0, 10, 100, or 200 mg/kg body weight
per day, on days 6-15 of gestation. The material was not teratogenic.
The maternal no-effect level was 10 mg/kg and the embryo/fetal
no-effect level was 100 mg/kg body weight. Inhibition of maternal body
weight gain occurred at doses of 100 or 200 mg/kg. A slight,
nonstatistically significant, reduction in average fetal body weight
per litter was found at the highest dose level (BFRIP, 1990) (no
further details).
7.6 Mutagenicity and related endpoints
Commercial PeBDE was examined for mutagenic activity at a number
of concentrations in a series of in vitro microbial assays using
Salmonella typhimurium TA98, TA100, TA1535, and TA1537 and
Saccharomyces cerevisae in the presence, and absence, of liver
microsomal enzyme preparations from Aroclor-induced rats. A negative
result was obtained with and without microsomal activation (Great
Lakes Chemical Corporation, undated a).
7.7 Carcinogenicity
No data are available.
7.8 Other special studies
Commercial PeBDE in corn oil was administered, by gavage, to 6
male Sprague-Dawley rats (200-250 g) for 90 days. When the original
doses of 6.25, 12.5, and 25 µmol/kg per day revealed extensive
induction, the study was repeated at doses of 0.78, 1.56, and
3.13 µmol/kg per day. When the lower dose levels were tested, even the
lowest dose of PeBDE (0.78 µmol/kg) caused increases in
O-ethyl- O-p-nitrophenyl phenylphosphonothiate (EPN)
detoxiFication, p-nitroanisole demethylation, and NADPH cytochrome c
reductase and cytochrome P450 activity. A clear dose-response
relationship was found for EPN detoxification and p-nitroanisole
demethylation. When the animals were allowed a 30-day recovery period
following the last (90th) dose, elevations in these two measurements
were still observable at all doses, though increased NADPH cytochrome
c reductase activity was found only at the highest dose. Within this
period, the cytochrome P450 content had returned to normal. Even after
60 days recovery, elevations were noted in EPN detoxification and
p-nitroanisole demethylation at the 1.56 and 3.13 µmol/kg levels. No
histological liver abnormalities were observed in rats treated with
doses of 3.13 µmol/kg or less (Carlson, 1980b).
In another study, rats (200-250g) were administered commercial
PeBDE at 0.1 mmol/kg per day, in corn oil, by gavage, for 14 days. In
addition to the above described effects on the enzymes, increases in
the activities of UDP-glucuronyl-transferase and benzo[ a]pyrene-
hydroxylase were found 24 h after the seventh dose (Carlson, 1980a).
Von Meyerinck et al. (1990) studied the hepatic microsomal enzyme
inducing potential of Bromkal 70 in Wistar rats. A series of dosing
regimes was used, which varied from single oral doses of up to
300 mg/kg body weight to 28 daily oral doses of 50 mg/kg body weight.
In all cases, the vehicle was peanut oil (1 ml/kg), which was also
administered to the vehicle control group. There were 3 male and 3
female rats per group, except in the 28-day study in which 4 male and
4 female rats were used. The treatments resulted in 31-53% increases
in liver weight, 2.3-3.9-fold increases in cytochrome P450 c levels,
up to 2-fold increases in benzphetamine N-demethylation activity, and
2.2-5.3-fold increases in benzo( a)pyrene oxidation. Increases in
ethoxyresorufin O-deethylation activity ranged from not detectable
to 0.35 nmol/min mg microsomal protein in control groups to between
4.1 and 16.6 nmol/min mg microsomal protein in treatment groups. These
activities were sex and dose dependent. The most sensitive parameter
was ethoxyresorufin O-deethylase induction, which showed no
significant response in rats dosed once with 3 mg PeBDE/kg and killed
3 days later.
Commercial PeBDE (Bromkal 70-5 DE) showed the induction of
ethoxyresorufin O-deethylase activity in H-4-II E cells (Hanberg et
al., 1991).
TETRABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Tetrabromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Commercial tetrabromodiphenyl ether, consisted of 41% tetra-, 45%
penta-, and 7% hexabromodiphenyl ethers and about 7% PBDE of unknown
structure. On the basis of the chemical structure, there are 42
possible isomers of tetrabromodiphenyl ether. Virtually no data are
available on physical and chemical properties, except that the
n-octanol/water partition coefficient (log Pow) is 5.87-6.16.
1.1.2 Production and uses
There is a report of the production (use) of about 1000 tonnes of
TeBDE in Japan in 1987. There is no known current production under the
name of tetrabromodiphenyl ether, but TeBDE is present in quantities
of from 24 to 38% in commercial pentabromodiphenyl ether.
1.1.3 Environmental transport, distribution, and transformation
Components of commercial TeBDE have been found in biota, sediment,
and sewage sludge samples. Commercial TeBDE components (containing
approximately equal quantities of PeBDE) are likely to be persistent
and to bioaccumulate.
Pyrolysis studies with commercial TeBDE showed that PBDF and PBDD
are formed at 800 °C. Higher PBDF and PBDD were not found.
1.1.4 Environmental levels and human exposure
TeBDE was found, in Japan, in river sediment at concentrations of
12-31 µg/kg dry weight and, in Sweden, at concentrations of up to
840 µg/kg ign. loss, respectively. TeBDE was also found in sewage
sludge in Sweden, at a concentration of 15 µg/kg.
Mussels and fish, collected at different places in Japan,
contained TeBDE in concentrations ranging from < 0.1 to 14.6 µg
2,2',4,4'-TeBDE/kg wet weight. In Sweden, different types of fish were
collected from rivers and analysed for 2,2',4,4'-TeBDE. The mean
concentrations ranged from ND (< 0.1 mg/kg) to 110 mg/kg fat. The
analysis indicated that there was at least one local source of
pollution in a certain river. Whitefish, arctic char, and herring,
collected at different places in Sweden in 1986-87, contained
concentrations of 15, 400, and 59-450 µg 2,2',4,4'-TeBDE/kg fat,
respectively. Fish collected from rivers in Germany contained up to
1 mg TeBDE/kg fat.
In herring and in the liver of cod, collected in the southern,
central, and northern North Sea, in the period 1983-89, a decreasing
trend in the concentrations of TeBDE was found from the southern
region to the northern region. In the herring, concentrations of
8.4-100 µg 2,2',4,4'-TeBDE/kg, on a fat basis, were found.
The muscle tissue of birds nesting and wintering in the Baltic
Sea, the North Sea, and Spitzbergen, contained from 80 to 370 µg
2,2',4,4'-TeBDE/kg, on a fat basis. Osprey collected in Sweden in the
period 1982-86, contained average concentrations of 1800 µg/kg fat.
Increasing trends in the concentrations of 2,2',4,4'-TeBDE have
been indicated for Baltic sediments, freshwater fish, and sea bird
eggs from Sweden.
The blubber of seals collected in the Baltic Sea and Spitzbergen
showed concentrations of 10-730 µg 2,2',4,4'-TeBDE/kg, on a fat basis.
The chromatographic pattern of the PBDE was similar to that of Bromkal
70-5. Pooled samples of the blubber of ringed seals and grey seals,
collected in Sweden in 1979-85 showed concentrations of 47 µg and
650 µg 2,2',4,4'-TeBDE/kg fat, respectively.
Pooled muscle samples of terrestrial mammals, e.g., rabbits, moose
and reindeer, collected in 1985-86 in Sweden, showed average
concentrations of <2, 0.82, and 0.18 µg 2,2',4,4'-TeBDE/kg fat,
respectively.
Levels of 2.5-4.5 µg PBDE/kg fat, measured as Bromkal 70DE, were
found in 4 samples of cow's milk in Germany. PBDE, as Bromkal 70DE,
was found in the milk of 25 women in Germany at concentrations ranging
from 0.62 to 11.1 µg/kg fat.
A rough estimate of exposure via fish consumption among the
Swedish population would suggest an intake of 0.3 µg TeBDE/ person per
day.
1.1.5 Effects on laboratory mammals and in vitro test systems
There are no data on TeBDE itself, but acute and short-term data
are available for commercial PeBDE containing 41% TeBDE.
1.1.6 Kinetics and metabolism in laboratory animals and humans
Minimal data are available.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
No data are available.
1.2 Conclusions
1.2.1 TeBDE
Components of commercial TeBDE (a mixture of 41% 2,2',4,4'-tetra-;
45% 2,2',4,4',5'-penta-; 7% hexa-, and 7-8% polybrominated diphenyl
ethers with an unknown structure) are persistent and accumulate in
organisms in the environment.
TeBDE as a component of pentabromodiphenyl ether is widely
incorporated in polymers as an additive flame retardant. Contact of
the general population is with products made from these polymers.
Exposure by extraction from polymers is unlikely. Human exposure to
TeBDE, via the food chain, may occur, because the substance has been
detected in organisms in the environment that are human food items,
such as fish, shellfish, etc. In fish and birds from Sweden,
increasing levels have been measured over the last two decades.
There is a lack of information concerning short-, long-term
toxicity/carcinogenicity, and reproduction studies. Furthermore,
information on kinetics and metabolism in laboratory animals and
humans is not available.
The risk for the general population cannot be determined on the
basis of available data.
No information is available to draw conclusions on occupational
exposure levels or the effects of TeBDE.
No data are available on the toxicity of commercial TeBDE for
organisms in the environment.
1.2.2 Breakdown products
PBDF and PBDD are formed when TeBDE are heated to 800 °C. The
possible hazards associated with this have to be addressed.
Exposure of the general population to PBDF in polymers, flame
retarded with TeBDE, is unlikely to be of significance. Properly
controlled incineration does not lead to the emission of significant
quantities of brominated dioxins and furans. Any uncontrolled
combustion of products containing TeBDE can lead to the generation of
unquantified amounts of PBDF/PBDD. The significance of this for both
humans and the environment will be addressed in a future EHC on
PBDF/PBDD.
1.3 Recommendations
1.3.1 General
Because of their persistence in the environment and accumulation in
organisms, it is recommended that TeBDE should not be used. However,
should use continue, the following points must be taken into account:
* Workers involved in the manufacture of TeBDE and products
containing the compound should be protected from exposure using
appropriate industrial hygiene measures, the monitoring of
occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled to minimize environmental
contamination with this persistent and accumulating compound and
its breakdown products.
* Incineration of materials, flame retarded with TeBDE, should only
be carried out in properly constituted incinerators running under
optimal conditions. Burning by any other means will lead to the
production of furan breakdown products.
1.3.2 Further studies
* Continued monitoring of environmental levels is required.
* Analytical methods for TeBDE in various matrices should be
validated.
* Because the present toxicological data base is inadequate to
evaluate the hazards of commercial TeBDE for humans and the
environment, if use is continued, the following studies should be
done:
- additional toxicological, carcinogenicity, and
ecotoxicological studies;
- further investigations on the generation of PBDF under real
fire conditions;
- investigation into possible methods and consequences of
recycling of TeBDE-containing polymers;
- investigations of the possibility of migration from
flame-retarded products.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
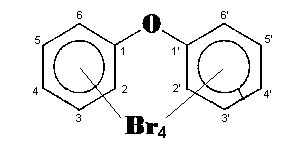 Chemical formula: C12H6Br4O
Relative molecular mass: 485.82
Common names: tetrabromodiphenyl ether (TeBDE)
tetrabromodiphenyl oxide
CAS registry number: 40088-47-9
CAS name: 1.1'-oxybis-tetrabromo-benzene
On the basis of the chemical structure, there are 42 possible
isomers of tetrabromodiphenyl ether.
2.2 Physical and chemical properties
The technical product consist of 41.7% (41%) of
2,2',4,4'-tetrabromodiphenyl ether, 44.4% (45%) 2,4,5,2',4'-
pentabromo-diphenyl ether, 6% (7%) hexabromodiphenyl ether and
7.6% PBDE of an unknown structure (see also pentabromodiphenyl ether
and Table 1) (Sundström & Hutzinger, 1976; De Kok et al., 1979;
US EPA, 1984; De Boer, 1989).
n-Octanol/water partition
coefficient (log Pow): 5.87-6.16
From: Pijnenburg & Everts (1991).
2.3 Analytical methods
A multiresidue method has been developed for the analysis of PBDE
residues in environmental samples (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Tetrabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
See sections 2 and 3 of Pentabromodiphenyl ether.
3.2.2 Uses
A thousand tonnes of tetrabromodiphenyl ether (TeBDE) were used in
Japan in 1987 (Watanabe, 1987c).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Pyrolysis
The pyrolysis of Bromkal 70-DE (a mixture of tetrabromodiphenyl
ether and pentabromodiphenyl ether) resulted in the formation of high
levels of PBDF and PBDD: approximately 60%. The substance was
pyrolysed at 800 °C in a quartz tube for 10 min (Thoma et al., 1986).
Bromkal 70-DE produced complex mixtures of mono- to
pentabromodibenzofurans and of mono- to tetrabromodibenzodioxins.
Higher PBDF and PBDD were not found (Zacharewski et al., 1988).
4.2 Ultimate fate following use
For the ultimate fate of TeBDE following use in the environment
see section 6.1. of the General Introduction.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Soil and sediment
Watanabe et al. (1987b) determined the residues of TeBDE in 9
marine and/or estuarine sediment samples and 6 fresh water river
sediment samples collected at different sites in Osaka during 1981-83.
The nine marine and/or estuarine samples did not contain TeBDE (limit
of determination < 2 µg/kg). Five out of 6 river sediment samples
contained 12-31 µg TeBDE/kg.
Sediment samples taken upstream and downstream from a factory were
analysed for the presence of tetrabromobisphenol A (TBBP-A) and its
dimethylated derivative. Besides TBBP-A, 2,2',4,4'-tetrabromodiphenyl
ether was also found. The levels found upstream and downstream were
3.5 and 840 µg/kg (IG), respectively (Sellström et al., 1990a,b).
A laminated sediment core collected in the southern part of the
Baltic Proper (Bornholm deep) was analysed for 2,2',4,4'-TeBDE. The
core was cut into 5 mm slices down to 50 mm depth and in 10 mm slices
from 50 to 90 mm depth. The concentration of TeBDE at 5 mm depth was
1.6 µg/kg IG and decreased gradually to 0.13 µg/kg at 40 mm depth. At
a depth of 90 mm, the concentration was 0.06 µg/kg (Nylund et al.,
1992).
Sellström et al. (1990b) analysed sewage sludge and found 15 µg
2,2',4,4'-tetrabromodiphenyl ether/kg IG.
Two sewage samples were collected from a Swedish treatment plant
(Gothenburg) in September 1988 and analysed for PBDE. One homogenate
was composed of samples (40 g/day) taken during a 32-day period with
little rain. Another homogenate was composed of samples (100 g/day)
taken during a rainy period of 7 days. TeBDE and PeBDE were found. The
concentration of 2,2',4,4'-TeBDE was 15 µg/kg IG (Nylund et al.,
1992).
5.1.2 Fish and shellfish
Watanabe et al. (1987b) determined the levels of TeBDE in fish and
mussels collected from different places in Japan in 1981-85. In total,
42 samples of mussel, mullet, goby, sardine, Japanese sea bass, horse
mackerel, and hairtail, were analysed. Seven out of 17 fish and
shellfish samples collected in the Osaka area contained 0.1-14.6 µg
TeBDE/kg and 3 samples of mussels from Osaka bay contained
1.6-14.6 µg/kg (wet weight). Single samples of sardine, Japanese sea
bass, mackerel and hairtail contained levels ranging from 0.1 to
0.8 µg TeBDE/kg. The other species did not contain TeBDE (limit of
determination <0.1 µg/kg).
De Boer (1989, 1990) measured the concentration of 2,2',4,4'-
tetrabromodiphenyl ether in the liver of cod (Gadus morhua),
collected in the southern, central, and northern North Sea, in the
period 1983-89. Each sample consisted of 25 fishes. The highest levels
(up to 360 µg/kg) were found in the liver of cod from the southern
part and the lowest levels (up to 68 µg/kg on a fat basis) in the
northern part of the North Sea. The concentrations showed a decreasing
trend from the southern region to the northern region. Herring
(Clupea harengus) collected in the 3 areas and the Straits of Dover
in 1985, contained average concentrations of 8.4-100 µg
2,2',4,4'-TeBDE/kg on a fat basis. Eels (Anguilla anguilla)
collected in Dutch freshwater (rivers and lakes) at 10 places were
analysed for the presence of 2,2',4,4'-TeBDE, during the period
1983-89. The concentrations ranged from <20 to 1700 µg/kg, on a fat
basis. In these marine and freshwater fish, 2,2',4,4'-TeBDE was always
found as the main component, i.e., 70% of the total brominated
diphenyl ethers.
Fish were collected from 5 different localities along the Viskan
and Haggan river system and the Klosterfjorden bay in Sweden. Muscle
and, in some cases, also the liver of bream (Abramis brama), eel
(Anguilla anguilla), pike (Esox lucius), sea trout (Salmo ocla),
and tench (Tinca tinca) were analysed. Expressed on a fat weight
basis, the highest level found was 110 mg PBDE/kg in the liver of a
pike. This pike specimen contained 27 mg PBDE/kg fat in the muscle
tissue. In addition to the 3 main components (1 tetrabromo- and 2
pentabromo-isomers), another tetrabromo- as well as 1 tribromo- and 2
hexabromo-isomers were identified by GC/MS. The maximum PBDE level
obtained in the muscle of eel was 17 mg/kg fat. The analysis indicated
that there was at least one local source of pollution along the River
Haggan. 2,2',4,4'-TeBDE was the most abundant PBDE-component. In most
samples, this compound accounted for 70-80% of total PBDE. The mean
concentration of PBDE ranged from ND (0.1 mg/kg) to 88 mg/kg fat. Eel
caught upstream, midstream, and in the Klosterfjorden bay contained
mean concentrations of ND (0.1 mg/kg), 4.3-16, and 0.9-1.4 mg PBDE/kg
fat, respectively (Andersson & Blomkvist, 1981).
Muscle tissue from bream, pike, and perch from the Viskan and
Haggan rivers in Sweden contained levels of 2,2',4,4'-TeBDE of between
1 and 23 mg/kg (on a fat basis). Perch from the Viskan river had the
highest levels (23 mg/kg) (Sellström et al., 1990b).
A method for the multiresidue analysis of organic pollutants was
applied to pooled muscle samples of 35 fresh water whitefish
(Coregonus sp.), 15 samples of arctic char (Salvelinus alpinus),
and a total of 260 samples of herring (Clupea harengus), collected
at different places in Sweden during the period 1986-87. The average
concentrations of 2,2',4,4'-tetrabromodiphenyl ether for whitefish,
arctic char, and herring were 15, 400, and 59-450 µg/kg lipid,
respectively (Jansson et al., 1993).
Bream, pike, perch, and trout from Swedish waters contained
250-750, 2000-6500, 2200-24 000, and 120-460 µg 2,2',4,4'-TeBDE/kg
lipid, respectively. These samples were from background and
industrialized areas (Sellström et al., 1993a). Pike samples from a
Swedish lake indicated a 4-fold increase in the same isomer from 1974
to 1991 (Sellström et al., 1993b).
5.1.3 Birds
Jansson et al. (1987) analysed pectoral muscle tissue of adult
guillemot (Uria aalge) nestling and wintering in the Baltic Sea,
adult birds of the same species nestling and wintering in the North
Sea, and adult guillemot collected at Spitzbergen. The pectoral muscle
of a single, adult, white tailed sea eagle (Haliaetus albicilla)
from the Baltic Sea was also analysed. The concentrations of
polybrominated diphenyl ethers (PBDE), calculated as Bromkal 70-5D, in
the muscle of the guillemots of the Baltic, North Sea, and Spitzbergen
were 370, 80 and 130 µg/kg, respectively, and that for the Baltic
eagle, 350 µg/kg on a fat basis. The chromatographic pattern of the
PBDE was similar to that of the Bromkal 70-5 product used as a
reference substance.
A method for multiresidue analysis was applied to pooled muscle
samples of 35 osprey (Pandion haliaetus), collected in Sweden in the
period 1982-86. The average concentration of 2,2'4,4'-tetra-
bromodiphenyl ether in osprey was 1800 µg/kg lipid (Jansson et al.,
1993).
A 10-fold increase in 2,2',4,4'-TeBDE has been indicated in
guillemot eggs from the Baltic Sea (Sellström et al., 1993a) (Table
29).
5.1.4 Aquatic mammals
Blubber samples of harbour seals (Phoca vitulina), collected in
the southern Baltic Sea and the Kattegat, and a ringed seal (Pusa
hispida), collected at Spitzbergen, were analysed by Jansson et al.
(1987). The concentrations of PBDE were 90, 10, and 40 µg/kg, on a fat
basis, respectively. The chromatographic pattern of the PBDE was
similar to that of the Bromkal 70-5 product used as a reference
substance. Sellström et al. (1990b) reported a concentration of 730 µg
PBDE/kg, on a fat basis, in a grey seal from the Baltic Sea.
Seven pooled samples of blubber of ringed seal (Pusa hispida),
collected in 1981, and 8 pooled samples of blubber of grey seals
(Halichoerus grypus), collected in Sweden in 1979-85, were analysed
using a multiresidue analytical method. The average concentrations of
2,2'4,4'-tetrabromodiphenyl ether were 47 and 650 µg/kg lipid,
respectively (Jansson et al., 1993).
Table 29. Average concentrations of 2,2',4,4'-TeBDE in guillemot eggs
from the Baltic Sea (in µg/kg lipid)a
Sampling year 2,2',4,4'-TeBDE
1970 130
1974 170
1975 130
1976 600
1979 640
1982 820
1983 880
1986 1200
1987 650
1989 1500
aFrom: Sellstrom et el. (1993a).
5.1.5 Terrestrial mammals
Multiresidue analysis of organic pollutants was applied to pooled
muscle samples of 15 rabbits (Oryctolagus cuniculus), 13 samples of
moose (Alces alces), and 31 samples of suet of reindeer (Rangifer
tarandus). The samples were collected in Sweden in the period
1985-86. The average concentrations of 2,2',4,4'-tetrabromodiphenyl
ether were <2, 0.82, 0.17 µg/kg lipid, for rabbit, moose, and
reindeer, respectively Jansson et al., 1993).
5.2 General population exposure
No measurements are available of human exposure to TeBDE. In
Sweden, the major human exposure is via fish-related food, and the
levels of TeBDE in fish may be used to estimate this route of
exposure. Normal fish intake in Sweden is about 30 g/day, and, if
herring is used as a model fish, this will give an estimated intake of
approximately 0.3 µg TeBDE/person per day (Personal Communication,
Jansson).
No data are available on occupational exposure during manufacture,
formulation, or use.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) was
investigated in the perirenal fat of groups of 3 male and 3 female
Wistar rats (weight 160-180g) following a single dose of 300 mg/kg
body weight in peanut oil. Animals from the groups were killed on days
1, 2, 3, 4, and 7, and then once a week for 10 weeks. Perirenal fat
was collected and analysed. The half-life of TeBDE was 19.1 days for
male rats and 29.9 days for female rats (Meyerinck et al., 1990).
TRIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Tribromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Environmental transport, distribution, and transformation
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Levels of contamination of commercial products with
tribromodiphenyl ether should be minimized to avoid contamination of
the environment and the exposure of humans.
Use of such commercial products leading to environmental
contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12H6Br4O
Relative molecular mass: 485.82
Common names: tetrabromodiphenyl ether (TeBDE)
tetrabromodiphenyl oxide
CAS registry number: 40088-47-9
CAS name: 1.1'-oxybis-tetrabromo-benzene
On the basis of the chemical structure, there are 42 possible
isomers of tetrabromodiphenyl ether.
2.2 Physical and chemical properties
The technical product consist of 41.7% (41%) of
2,2',4,4'-tetrabromodiphenyl ether, 44.4% (45%) 2,4,5,2',4'-
pentabromo-diphenyl ether, 6% (7%) hexabromodiphenyl ether and
7.6% PBDE of an unknown structure (see also pentabromodiphenyl ether
and Table 1) (Sundström & Hutzinger, 1976; De Kok et al., 1979;
US EPA, 1984; De Boer, 1989).
n-Octanol/water partition
coefficient (log Pow): 5.87-6.16
From: Pijnenburg & Everts (1991).
2.3 Analytical methods
A multiresidue method has been developed for the analysis of PBDE
residues in environmental samples (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Tetrabromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
See sections 2 and 3 of Pentabromodiphenyl ether.
3.2.2 Uses
A thousand tonnes of tetrabromodiphenyl ether (TeBDE) were used in
Japan in 1987 (Watanabe, 1987c).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Pyrolysis
The pyrolysis of Bromkal 70-DE (a mixture of tetrabromodiphenyl
ether and pentabromodiphenyl ether) resulted in the formation of high
levels of PBDF and PBDD: approximately 60%. The substance was
pyrolysed at 800 °C in a quartz tube for 10 min (Thoma et al., 1986).
Bromkal 70-DE produced complex mixtures of mono- to
pentabromodibenzofurans and of mono- to tetrabromodibenzodioxins.
Higher PBDF and PBDD were not found (Zacharewski et al., 1988).
4.2 Ultimate fate following use
For the ultimate fate of TeBDE following use in the environment
see section 6.1. of the General Introduction.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Soil and sediment
Watanabe et al. (1987b) determined the residues of TeBDE in 9
marine and/or estuarine sediment samples and 6 fresh water river
sediment samples collected at different sites in Osaka during 1981-83.
The nine marine and/or estuarine samples did not contain TeBDE (limit
of determination < 2 µg/kg). Five out of 6 river sediment samples
contained 12-31 µg TeBDE/kg.
Sediment samples taken upstream and downstream from a factory were
analysed for the presence of tetrabromobisphenol A (TBBP-A) and its
dimethylated derivative. Besides TBBP-A, 2,2',4,4'-tetrabromodiphenyl
ether was also found. The levels found upstream and downstream were
3.5 and 840 µg/kg (IG), respectively (Sellström et al., 1990a,b).
A laminated sediment core collected in the southern part of the
Baltic Proper (Bornholm deep) was analysed for 2,2',4,4'-TeBDE. The
core was cut into 5 mm slices down to 50 mm depth and in 10 mm slices
from 50 to 90 mm depth. The concentration of TeBDE at 5 mm depth was
1.6 µg/kg IG and decreased gradually to 0.13 µg/kg at 40 mm depth. At
a depth of 90 mm, the concentration was 0.06 µg/kg (Nylund et al.,
1992).
Sellström et al. (1990b) analysed sewage sludge and found 15 µg
2,2',4,4'-tetrabromodiphenyl ether/kg IG.
Two sewage samples were collected from a Swedish treatment plant
(Gothenburg) in September 1988 and analysed for PBDE. One homogenate
was composed of samples (40 g/day) taken during a 32-day period with
little rain. Another homogenate was composed of samples (100 g/day)
taken during a rainy period of 7 days. TeBDE and PeBDE were found. The
concentration of 2,2',4,4'-TeBDE was 15 µg/kg IG (Nylund et al.,
1992).
5.1.2 Fish and shellfish
Watanabe et al. (1987b) determined the levels of TeBDE in fish and
mussels collected from different places in Japan in 1981-85. In total,
42 samples of mussel, mullet, goby, sardine, Japanese sea bass, horse
mackerel, and hairtail, were analysed. Seven out of 17 fish and
shellfish samples collected in the Osaka area contained 0.1-14.6 µg
TeBDE/kg and 3 samples of mussels from Osaka bay contained
1.6-14.6 µg/kg (wet weight). Single samples of sardine, Japanese sea
bass, mackerel and hairtail contained levels ranging from 0.1 to
0.8 µg TeBDE/kg. The other species did not contain TeBDE (limit of
determination <0.1 µg/kg).
De Boer (1989, 1990) measured the concentration of 2,2',4,4'-
tetrabromodiphenyl ether in the liver of cod (Gadus morhua),
collected in the southern, central, and northern North Sea, in the
period 1983-89. Each sample consisted of 25 fishes. The highest levels
(up to 360 µg/kg) were found in the liver of cod from the southern
part and the lowest levels (up to 68 µg/kg on a fat basis) in the
northern part of the North Sea. The concentrations showed a decreasing
trend from the southern region to the northern region. Herring
(Clupea harengus) collected in the 3 areas and the Straits of Dover
in 1985, contained average concentrations of 8.4-100 µg
2,2',4,4'-TeBDE/kg on a fat basis. Eels (Anguilla anguilla)
collected in Dutch freshwater (rivers and lakes) at 10 places were
analysed for the presence of 2,2',4,4'-TeBDE, during the period
1983-89. The concentrations ranged from <20 to 1700 µg/kg, on a fat
basis. In these marine and freshwater fish, 2,2',4,4'-TeBDE was always
found as the main component, i.e., 70% of the total brominated
diphenyl ethers.
Fish were collected from 5 different localities along the Viskan
and Haggan river system and the Klosterfjorden bay in Sweden. Muscle
and, in some cases, also the liver of bream (Abramis brama), eel
(Anguilla anguilla), pike (Esox lucius), sea trout (Salmo ocla),
and tench (Tinca tinca) were analysed. Expressed on a fat weight
basis, the highest level found was 110 mg PBDE/kg in the liver of a
pike. This pike specimen contained 27 mg PBDE/kg fat in the muscle
tissue. In addition to the 3 main components (1 tetrabromo- and 2
pentabromo-isomers), another tetrabromo- as well as 1 tribromo- and 2
hexabromo-isomers were identified by GC/MS. The maximum PBDE level
obtained in the muscle of eel was 17 mg/kg fat. The analysis indicated
that there was at least one local source of pollution along the River
Haggan. 2,2',4,4'-TeBDE was the most abundant PBDE-component. In most
samples, this compound accounted for 70-80% of total PBDE. The mean
concentration of PBDE ranged from ND (0.1 mg/kg) to 88 mg/kg fat. Eel
caught upstream, midstream, and in the Klosterfjorden bay contained
mean concentrations of ND (0.1 mg/kg), 4.3-16, and 0.9-1.4 mg PBDE/kg
fat, respectively (Andersson & Blomkvist, 1981).
Muscle tissue from bream, pike, and perch from the Viskan and
Haggan rivers in Sweden contained levels of 2,2',4,4'-TeBDE of between
1 and 23 mg/kg (on a fat basis). Perch from the Viskan river had the
highest levels (23 mg/kg) (Sellström et al., 1990b).
A method for the multiresidue analysis of organic pollutants was
applied to pooled muscle samples of 35 fresh water whitefish
(Coregonus sp.), 15 samples of arctic char (Salvelinus alpinus),
and a total of 260 samples of herring (Clupea harengus), collected
at different places in Sweden during the period 1986-87. The average
concentrations of 2,2',4,4'-tetrabromodiphenyl ether for whitefish,
arctic char, and herring were 15, 400, and 59-450 µg/kg lipid,
respectively (Jansson et al., 1993).
Bream, pike, perch, and trout from Swedish waters contained
250-750, 2000-6500, 2200-24 000, and 120-460 µg 2,2',4,4'-TeBDE/kg
lipid, respectively. These samples were from background and
industrialized areas (Sellström et al., 1993a). Pike samples from a
Swedish lake indicated a 4-fold increase in the same isomer from 1974
to 1991 (Sellström et al., 1993b).
5.1.3 Birds
Jansson et al. (1987) analysed pectoral muscle tissue of adult
guillemot (Uria aalge) nestling and wintering in the Baltic Sea,
adult birds of the same species nestling and wintering in the North
Sea, and adult guillemot collected at Spitzbergen. The pectoral muscle
of a single, adult, white tailed sea eagle (Haliaetus albicilla)
from the Baltic Sea was also analysed. The concentrations of
polybrominated diphenyl ethers (PBDE), calculated as Bromkal 70-5D, in
the muscle of the guillemots of the Baltic, North Sea, and Spitzbergen
were 370, 80 and 130 µg/kg, respectively, and that for the Baltic
eagle, 350 µg/kg on a fat basis. The chromatographic pattern of the
PBDE was similar to that of the Bromkal 70-5 product used as a
reference substance.
A method for multiresidue analysis was applied to pooled muscle
samples of 35 osprey (Pandion haliaetus), collected in Sweden in the
period 1982-86. The average concentration of 2,2'4,4'-tetra-
bromodiphenyl ether in osprey was 1800 µg/kg lipid (Jansson et al.,
1993).
A 10-fold increase in 2,2',4,4'-TeBDE has been indicated in
guillemot eggs from the Baltic Sea (Sellström et al., 1993a) (Table
29).
5.1.4 Aquatic mammals
Blubber samples of harbour seals (Phoca vitulina), collected in
the southern Baltic Sea and the Kattegat, and a ringed seal (Pusa
hispida), collected at Spitzbergen, were analysed by Jansson et al.
(1987). The concentrations of PBDE were 90, 10, and 40 µg/kg, on a fat
basis, respectively. The chromatographic pattern of the PBDE was
similar to that of the Bromkal 70-5 product used as a reference
substance. Sellström et al. (1990b) reported a concentration of 730 µg
PBDE/kg, on a fat basis, in a grey seal from the Baltic Sea.
Seven pooled samples of blubber of ringed seal (Pusa hispida),
collected in 1981, and 8 pooled samples of blubber of grey seals
(Halichoerus grypus), collected in Sweden in 1979-85, were analysed
using a multiresidue analytical method. The average concentrations of
2,2'4,4'-tetrabromodiphenyl ether were 47 and 650 µg/kg lipid,
respectively (Jansson et al., 1993).
Table 29. Average concentrations of 2,2',4,4'-TeBDE in guillemot eggs
from the Baltic Sea (in µg/kg lipid)a
Sampling year 2,2',4,4'-TeBDE
1970 130
1974 170
1975 130
1976 600
1979 640
1982 820
1983 880
1986 1200
1987 650
1989 1500
aFrom: Sellstrom et el. (1993a).
5.1.5 Terrestrial mammals
Multiresidue analysis of organic pollutants was applied to pooled
muscle samples of 15 rabbits (Oryctolagus cuniculus), 13 samples of
moose (Alces alces), and 31 samples of suet of reindeer (Rangifer
tarandus). The samples were collected in Sweden in the period
1985-86. The average concentrations of 2,2',4,4'-tetrabromodiphenyl
ether were <2, 0.82, 0.17 µg/kg lipid, for rabbit, moose, and
reindeer, respectively Jansson et al., 1993).
5.2 General population exposure
No measurements are available of human exposure to TeBDE. In
Sweden, the major human exposure is via fish-related food, and the
levels of TeBDE in fish may be used to estimate this route of
exposure. Normal fish intake in Sweden is about 30 g/day, and, if
herring is used as a model fish, this will give an estimated intake of
approximately 0.3 µg TeBDE/person per day (Personal Communication,
Jansson).
No data are available on occupational exposure during manufacture,
formulation, or use.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The half-life of pentabromodiphenyl ether (Bromkal 70) was
investigated in the perirenal fat of groups of 3 male and 3 female
Wistar rats (weight 160-180g) following a single dose of 300 mg/kg
body weight in peanut oil. Animals from the groups were killed on days
1, 2, 3, 4, and 7, and then once a week for 10 weeks. Perirenal fat
was collected and analysed. The half-life of TeBDE was 19.1 days for
male rats and 29.9 days for female rats (Meyerinck et al., 1990).
TRIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
Tribromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Environmental transport, distribution, and transformation
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Levels of contamination of commercial products with
tribromodiphenyl ether should be minimized to avoid contamination of
the environment and the exposure of humans.
Use of such commercial products leading to environmental
contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
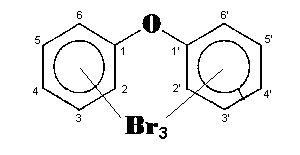 Chemical formula: C12 H7 Br3 O
Relative molecular mass 407.1
Common names tribromodiphenyl ether (TrBDE);
tribromodiphenyl oxide
CAS registry number 49690-94-0
CAS name 1,1'-oxybis-tribromo-benzene
On the basis of the chemical structure, there are 24 possible
isomers of tribromodiphenyl ether.
2.2 Physical and chemical properties
Vapour pressure 4.70-4.95 Pa at 25 °C
n-Octanol/water partition
coefficient (log Pow) 5.47-5.58
From: US EPA (1984, 1986); Watanabe (1987c); Pijnenburg & Everts
(1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Tribromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Environmental levels
4.1.1 Birds
Stafford (1983) found tribromodiphenyl ether (TrBDE) in the eggs
of the fish-eating bird, the black skimmer (Rynchos niger),
collected from various nesting sites in Texas and Louisiana in the
period 1980-81. The residues were not quantified.
DIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
Dibromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Contamination of commercial products with dibromodiphenyl ether
should be minimized to avoid contamination of the environment and
exposure of humans.
Use of such commercial products leading to environmental
contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12 H7 Br3 O
Relative molecular mass 407.1
Common names tribromodiphenyl ether (TrBDE);
tribromodiphenyl oxide
CAS registry number 49690-94-0
CAS name 1,1'-oxybis-tribromo-benzene
On the basis of the chemical structure, there are 24 possible
isomers of tribromodiphenyl ether.
2.2 Physical and chemical properties
Vapour pressure 4.70-4.95 Pa at 25 °C
n-Octanol/water partition
coefficient (log Pow) 5.47-5.58
From: US EPA (1984, 1986); Watanabe (1987c); Pijnenburg & Everts
(1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Tribromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Environmental levels
4.1.1 Birds
Stafford (1983) found tribromodiphenyl ether (TrBDE) in the eggs
of the fish-eating bird, the black skimmer (Rynchos niger),
collected from various nesting sites in Texas and Louisiana in the
period 1980-81. The residues were not quantified.
DIBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
Dibromodiphenyl ether is not manufactured or used.
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Effects on other organisms in the laboratory and field
* Previous evaluations by international bodies.
1.1 Summary and evaluation
There is no database on which to make an evaluation.
1.2 Recommendations
Contamination of commercial products with dibromodiphenyl ether
should be minimized to avoid contamination of the environment and
exposure of humans.
Use of such commercial products leading to environmental
contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
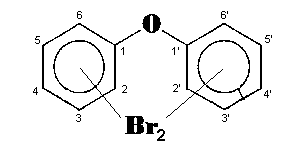 Chemical formula: C12 H8 Br2 O
Relative molecular mass: 328.02
Common name: dibromodiphenyl ether (DiBDE)
dibromodiphenyl oxide
CAS registry number: 2050-47-7
Synonyms: bis(bromophenyl) ether,
1.1'-oxybis (bromo), benzene
On the basis of the chemical structure, there are 12 possible
isomers of dibromodiphenyl ether, and p,p'-dibromodiphenyl ether is
one of them.
2.2 Physical and chemical properties
p,p'-Dibromodiphenyl ether (DiBDE) is a crystalline compound.
Melting point: 60.5 °C (58-60 °C)
Boiling point: 338-340 °C
Vapour pressure: 3.85-4.02 Pa at 25 °C
Specific gravity: 1.8 (solution)
Solubility: very soluble in benzene, soluble in
alcohol, and ethyl ether
n-Octanol/water partition
coefficient (log Pow): 5.03
From: US EPA (1984, 1986); Environment Agency Japan (1987); Pijnenburg
& Everts (1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1, and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Dibromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels end processes
p,p'-Dibromodiphenyl ether is prepared from p-phenoxyaniline
by sequential treatment with HBr + NaNO2 and Br2 + HBr followed by
warming in acetic acid (US EPA, 1986).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The in vitro microbial degradation of dibromodiphenyl ether was
studied with a soil isolate, strain S93B1, identified as Pseudomonas
cruciviae. After enrichment and isolation, the bacteria was
cultivated in an agar-slant at 30 °C for 12-17 days and growth was
determined. Strain S93B1 did not grow on DiBDE as the sole source of
carbon (Takase et al., 1986).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In Japan, p,p'-dibromodiphenyl ether was not detected in 27
water samples (limit of determination 0.01-0.03 µg/litre) in an
environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.2 Soil/sediment
In Japan, p,p'-dibromodiphenyl ether was not detected in 27
sediment samples (limit of determination 0.05-13 µg/kg dry weight) in
an environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.3 Birds
Stafford (1983) found p,p'-dibromodiphenyl ether in eggs of the
fish-eating bird, the black-skimmer (Rynchops niger), collected from
various nesting sites in Texas and Louisiana in the period 1980-81.
The residues were not quantified.
5.2 General population exposure
No data are available.
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Short-and long-term toxicity
* Skin and eye irritation, sensitization
* Reproductive toxicity, embryotoxicity, teratogenicity,
mutagenicity, and carcinogenicity.
6.1 Single exposure
The acute intraperitoneal LD50 in mice is 125 mg/kg body weight
(US EPA, 1984).
6.2 Other special studies
6.2.1 Liver
Carlson (1980a) and Kociba (undated) tested p,p'-dibromo-
diphenyl ether in a dose of 0.1 mmol/kg body weight per day, by
gavage, in corn oil, in male Sprague Dawley rats (200-250 g)
for 14 days. Twenty-four hours after the last (seventh) dose,
increases in liver/body weight ratio, NADPH cytochrome c-reductase,
and cytochrome P-450 were found.
MONOBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Physical and chemical properties
There are 3 possible isomers of monobromodiphenyl ether.
p-Bromodiphenyl ether is a liquid at ambient temperature with a
boiling point of 305-310 °C. Its solubility in water is calculated to
be 48 mg/litre. The log n-octanol water partition coefficient is
between 4 and 5. Vapour pressure at 20 °C is 0.0015 mmHg.
1.1.2 Production and uses
MBDE is not used as a flame retardant. A report of production
appeared in 1977, but the use is unknown.
1.1.3 Environmental transport, distribution, and transformation
The half-life of volatilization from water is in the range of
hundreds of days.
MBDE did not significantly biodegrade in a 7-day culture with
microorganisms from domestic waste water, but it has been reported to
degrade by 95% in activated sewage sludge. A single study showed a
strain of soil bacteria incapable of degrading MBDE as a sole carbon
source.
1.1.4 Environmental levels and human exposure
MBDE has been detected in surface water samples taken near
industrial sites in the USA but was not found in a similar survey in
Japan. It was also detected in soil water close to an industrial plant
in the USA. MBDE has been detected in aquatic sediment and aquatic
biota in the USA.
1.1.5 Kinetics and metabolism in laboratory animals and humans
No data are available.
1.1.6 Effects on laboratory mammals and in vitro test systems
MBDE is not teratogenic, but there are no data on the acute,
short-term, or long-term toxicity of MBDE and therefore no evaluation
can be made.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
A 96-h LC50 for bluegill sunfish has been reported at
4.9 mg/litre with a no-observed-effect concentration at less than
2.8 mg/litre. The 48-h LC50 for waterflea was 0.36 mg/litre with a
NOEC at less than 0.046 mg/litre.
1.2 Conclusions and recommendations
Monobromodiphenyl ether does not have any flame retardant
properties. It can accumulate in organisms in the environment and has
been detected in different environmental compartments. There is some
evidence that it can be biodegraded.
The limited information means that conclusions concerning exposure
levels and effects on the general population and organisms in the
environment cannot be reached.
There is no toxicological data base to support its use.
Uses leading to environmental contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
Chemical formula: C12 H8 Br2 O
Relative molecular mass: 328.02
Common name: dibromodiphenyl ether (DiBDE)
dibromodiphenyl oxide
CAS registry number: 2050-47-7
Synonyms: bis(bromophenyl) ether,
1.1'-oxybis (bromo), benzene
On the basis of the chemical structure, there are 12 possible
isomers of dibromodiphenyl ether, and p,p'-dibromodiphenyl ether is
one of them.
2.2 Physical and chemical properties
p,p'-Dibromodiphenyl ether (DiBDE) is a crystalline compound.
Melting point: 60.5 °C (58-60 °C)
Boiling point: 338-340 °C
Vapour pressure: 3.85-4.02 Pa at 25 °C
Specific gravity: 1.8 (solution)
Solubility: very soluble in benzene, soluble in
alcohol, and ethyl ether
n-Octanol/water partition
coefficient (log Pow): 5.03
From: US EPA (1984, 1986); Environment Agency Japan (1987); Pijnenburg
& Everts (1991).
2.3 Analytical methods
No specific data are available (see General Introduction, section
2.1, and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Dibromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels end processes
p,p'-Dibromodiphenyl ether is prepared from p-phenoxyaniline
by sequential treatment with HBr + NaNO2 and Br2 + HBr followed by
warming in acetic acid (US EPA, 1986).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The in vitro microbial degradation of dibromodiphenyl ether was
studied with a soil isolate, strain S93B1, identified as Pseudomonas
cruciviae. After enrichment and isolation, the bacteria was
cultivated in an agar-slant at 30 °C for 12-17 days and growth was
determined. Strain S93B1 did not grow on DiBDE as the sole source of
carbon (Takase et al., 1986).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
In Japan, p,p'-dibromodiphenyl ether was not detected in 27
water samples (limit of determination 0.01-0.03 µg/litre) in an
environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.2 Soil/sediment
In Japan, p,p'-dibromodiphenyl ether was not detected in 27
sediment samples (limit of determination 0.05-13 µg/kg dry weight) in
an environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.3 Birds
Stafford (1983) found p,p'-dibromodiphenyl ether in eggs of the
fish-eating bird, the black-skimmer (Rynchops niger), collected from
various nesting sites in Texas and Louisiana in the period 1980-81.
The residues were not quantified.
5.2 General population exposure
No data are available.
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Short-and long-term toxicity
* Skin and eye irritation, sensitization
* Reproductive toxicity, embryotoxicity, teratogenicity,
mutagenicity, and carcinogenicity.
6.1 Single exposure
The acute intraperitoneal LD50 in mice is 125 mg/kg body weight
(US EPA, 1984).
6.2 Other special studies
6.2.1 Liver
Carlson (1980a) and Kociba (undated) tested p,p'-dibromo-
diphenyl ether in a dose of 0.1 mmol/kg body weight per day, by
gavage, in corn oil, in male Sprague Dawley rats (200-250 g)
for 14 days. Twenty-four hours after the last (seventh) dose,
increases in liver/body weight ratio, NADPH cytochrome c-reductase,
and cytochrome P-450 were found.
MONOBROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
No data are available on the following topics:
* Kinetics and metabolism in laboratory animals and humans
* Effects on humans
* Previous evaluations by international bodies.
1.1 Summary and evaluation
1.1.1 Physical and chemical properties
There are 3 possible isomers of monobromodiphenyl ether.
p-Bromodiphenyl ether is a liquid at ambient temperature with a
boiling point of 305-310 °C. Its solubility in water is calculated to
be 48 mg/litre. The log n-octanol water partition coefficient is
between 4 and 5. Vapour pressure at 20 °C is 0.0015 mmHg.
1.1.2 Production and uses
MBDE is not used as a flame retardant. A report of production
appeared in 1977, but the use is unknown.
1.1.3 Environmental transport, distribution, and transformation
The half-life of volatilization from water is in the range of
hundreds of days.
MBDE did not significantly biodegrade in a 7-day culture with
microorganisms from domestic waste water, but it has been reported to
degrade by 95% in activated sewage sludge. A single study showed a
strain of soil bacteria incapable of degrading MBDE as a sole carbon
source.
1.1.4 Environmental levels and human exposure
MBDE has been detected in surface water samples taken near
industrial sites in the USA but was not found in a similar survey in
Japan. It was also detected in soil water close to an industrial plant
in the USA. MBDE has been detected in aquatic sediment and aquatic
biota in the USA.
1.1.5 Kinetics and metabolism in laboratory animals and humans
No data are available.
1.1.6 Effects on laboratory mammals and in vitro test systems
MBDE is not teratogenic, but there are no data on the acute,
short-term, or long-term toxicity of MBDE and therefore no evaluation
can be made.
1.1.7 Effects on humans
No data are available.
1.1.8 Effects on other organisms in the laboratory and field
A 96-h LC50 for bluegill sunfish has been reported at
4.9 mg/litre with a no-observed-effect concentration at less than
2.8 mg/litre. The 48-h LC50 for waterflea was 0.36 mg/litre with a
NOEC at less than 0.046 mg/litre.
1.2 Conclusions and recommendations
Monobromodiphenyl ether does not have any flame retardant
properties. It can accumulate in organisms in the environment and has
been detected in different environmental compartments. There is some
evidence that it can be biodegraded.
The limited information means that conclusions concerning exposure
levels and effects on the general population and organisms in the
environment cannot be reached.
There is no toxicological data base to support its use.
Uses leading to environmental contamination should be avoided.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
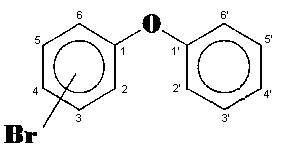 Chemical formula: C12H9BrO
Relative molecular mass: 249.11
Common names: monobromodiphenyl ether (MBDE)
mono bromodiphenyl oxide
CAS registry number: 101-55-3
CAS name: bromo-4-phenoxybenzene
Synonyms: bromophenylphenyl ether, bromodiphenyl
ether, bromophenyl ether,
bromophenoxybenzene
Trade names: HSDB 2747; NSC
On the basis of the chemical structure, there are 3 possible isomers
of monobromodiphenyl ether. p-Monobromodiphenyl ether is one of
them.
2.2 Physical and chemical properties
p-Bromodiphenyl ether is a liquid at common ambient
temperatures.
Melting point: 18.72 °C
Boiling point: 310.14 °C (305 °C
Vapour pressure at 20 °C: 0.0015 mmHg
Specific gravity: 1.449 (1.4208 at 20 °C)
Refractive index, n 20/D: 1.607
Flash point: >230°F
Solubility at 25 °C: 4.8 mg/litre water (calculated),
soluble in ethyl ether
n-Octanol/water partition
coefficient (log Pow): 4.28 (4.08-4.94)
From: US EPA (1984, 1986).
2.3 Analytical methods
p-Monobromodiphenyl ether (MBDE) can be determined by the
standard US EPA method 611-Haloethers. Chromatographic conditions are
described in US EPA (1986). The limit of determination in municipal
and industrial waste waters is 2.3 µg/litre. Zogorski (1984) reported
a limit of determination of 0.01 µg/litre using a gas chromatograph
equipped with an electron capture detector. McMahon (1983) applied
GC-MS (US EPA method 625) using a halide specific detector. Gurka et
al. (1982) used GC/Fourier transform infrared spectroscopy to detect
p-monobromodiphenyl ether (see also General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Monobromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
p-Monobromodiphenyl ether is prepared by brominating diphenyl
ether with Br2 at 95-100 °C in carbon tetrachloride (US EPA, 1986).
The production range (includes importation volumes) statistics
from the 1977 TSCA Inventory were up to 450 kg of p-mono-
bromodiphenyl ether (US EPA, 1986).
3.2.2 Uses
No data are available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
The half-life of p-monobromodiphenyl ether with respect to
volatilization has been estimated to be within the range of hundreds
of days (MacKay & Leinonen, 1975).
In contrast, by analogy to p-monochlorodiphenyl, Callahan et al.
(1979) estimated the half-life of p-monobromodiphenyl ether to be
10 h. However, sorption of the agent by organic material in water will
prolong its evaporative half-life in natural bodies of water. This
value should be considered a minimum.
4.2 Biotransformation
4.2.1 Biodegradation
p-Monobromodiphenyl ether was not significantly biodegradable in
a 7-day static-culture flask-screening procedure of Bunch and Chambers
utilizing biochemical oxygen demand (BOD) dilution water containing
5 mg of yeast extract/litre, as the synthetic medium; 5 and
10 mg/litre concentrations of the test compound, a 7-day static
incubation of 25 °C in the dark, followed by three weekly subcultures
(totalling 28 days of incubation), and incorporating settled domestic
waste water as microbial inoculum. At a test compound concentration of
5 mg/litre, only 2 out of 4 cultures showed any biodegradation in 7
days (19 and 36%), and, at a concentration of 10 mg/litre, only one
out of 4 showed biodegradation (19%) (Tabak et al., 1981).
p-Monobromodiphenyl ether was not able to support the growth of
Alcaligenes BM2, a strain of soil bacteria capable of using PCB
(dichloro-) mixtures as the sole source of carbon (Yagi & Sudo, 1980).
However, p-monobromodiphenyl ether was reported to be 95%
biodegradable when introduced as a pollutant (360 µg/litre) in a
full-scale activated sludge treatment system (US EPA, 1986).
The in vitro microbial degradation of p-monobromodiphenyl
ether was studied with a soil isolate, strain S93B1, identified as
Pseudomonas crucivae. After enrichment and isolation, the bacteria
were cultivated in an agar-slant at 30 °C for 12-17 days and growth
was determined. Strain S93B1 did not grow on the biphenyl ether as the
sole source of carbon (Takase et al., 1986).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data are available on general population exposure or on
occupational exposure during manufacture, formulation, or use.
5.1 Environmental levels
5.1.1 Water
According to US EPA (1986), there are monitoring data for
p-monobromodiphenyl ether in water in the USA. The mean
concentration was 0.2 mg/litre (range 0-202.7 mg/litre, 2193 listings)
in water. Most of these listings concerned river water samples taken
near industrial sites. Only a few groundwater and community
drinking-water samples were included (no further details were
available). Plumb (1991) reported the presence of p-monobromo-
diphenyl ether in groundwater from only 1 out of 479 disposal sites
that were analysed in the USA.
In Japan, p-monobromodiphenyl ether was not detected in 27 water
samples (limit of determination 0.15-0.5 µg/litre) in an environmental
survey in 1984 (Environment Agency Japan, 1987).
5.1.2 Soil/sediment
According to US EPA (1986), there are numerous US monitoring data
for p-monobromodiphenyl ether in sediments. The mean concentration
was 3.5 mg/kg (range 0-380 mg/kg, 585 listings) (no further details
are available).
In Japan, p-monobromodiphenyl ether was not detected in 27
sediment samples (limit of determination 2.5-120 µg/kg dry weight) in
an environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.3 Aquatic organisms
According to US EPA (1986) there are numerous US monitoring data
for p-monobromodiphenyl ether in tissue from aquatic organisms. The
concentrations were 2.0 mg/kg (range 070.0 mg/kg, 346 listings) (no
further details are available).
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Single exposure
* Short-term exposure
* Long-term exposure
* Skin and eye irritation; sensitization
* Mutagenicity and related endpoints.
6.1 Reproductive toxicity, embryotoxicity, and teratogenicity
Outbred Swiss (CD-1) mice (age 60 days) were treated with
p-monobromodiphenyl ether (95%). There were 20 and 21 mice
respectively in these treatment groups and 51 and 55 mice in untreated
and vehicle control (1 ml corn oil/kg) groups, respectively. Two
females per group were mated per male, until a copulation plug was
found, or for maximum 5 days. MBDE in corn oil was administered by
gavage in dose levels of 0, 100 or 1.000 mg/kg body weight/day, from
day 5 up to day 14 of gestation. Pups were counted and weighed (by
litter) on postnatal days 1, 3, 5, 10 and 15; they were sexed and
weaned on day 21 and autopsied on days 25 and 30 to determine gross
abnormalities and the weights of Harderian glands, liver, and kidneys.
No adverse effects on any of these parameters were observed (Francis,
1989).
6.2 Carcinogenicity
Theiss et al. (1977) tested p-monobromodiphenyl ether (MBDE) in
a short-term screening assay: the strain A mouse pulmonary tumour
assay. Four groups of 20 A/St male mice (6-8 weeks old) were given 24
intraperitoneal injections of tricaprylin (solvent-control), 23 i.p.
injections of 40 mg (low-dose), 17 injections of 100 mg (mid-dose) and
18 injections of 200 mg MBDE/kg body weight (high-dose), three times
per week. Total doses administered in the four groups were 0, 920,
1700, and 3600 mg/kg body weight, respectively. Twenty-four weeks
after the first injection, the mice were sacrificed and the lungs
examined. The number of lung tumours/mouse and survival rates of the
treated animals were not significantly different from the control
animals. The number of lung tumours/mouse were; control 0.39 ± 0.06;
low-dose 0.18 ± 0.10, mid-dose 0.15 ± 0.10 and high-dose 0.31 ± 0.15.
MBDE was negative in this pulmonary assay.
7. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The effects of acute exposure to p-monobromodiphenyl ether have
been studied in several species of aquatic invertebrates and fish. The
results are summarized in Table 30. The water flea (Daphnia magna),
is more sensitive than fish to p-monobromodiphenyl ether (US EPA,
1984).
In an early-life-stage (embryo-larval) test on fathead minnow,
conducted with p-monobromodiphenyl ether, adverse effects on
survival and growth were produced. The geometric mean of the highest
no-effect level was 0.122 mg/litre (US EPA, 1984).
Table 30. Acute toxic effects of p-monobromodiphenyl ether on aquatic organismsa
Species Exposure Mean Effect Reference
duration Method concentration
(h) (mg/litre)
Rainbow trout 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Oncorhynchus mykiss)b
Bluegill sunfish 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Lepomis macrochirus)
Bluegill sunfish 24 static 50.9c LC50 US EPA (1978);
(Lepomis macrochirus)
Buccafusco et al. (1981)
48 static 9.62 LC50 US EPA (1978)
72 static 4.94 LC50 US EPA (1978)
96 static 4.94 LC50 US EPA (1978)
96 static < 2.80 no effect US EPA (1978)
See lamprey 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Petromyzon marinus)
Water flea 24 static 0.46 LC50 US EPA (1978); LeBlanc (1980)
(Daphnia magna) 48 static 0.36 LC50 US EPA (1978); LeBlanc (1980)
48 static < 0.046 no effect US EPA (1978)
a From: US EPA (1984).
b Old name = Salmo Gairdneri.
c This dose exceeds the solubility of the test compound in water at 25 °C (4.6 mg/litre).
REFERENCES
AIHA (1981) Decabromo-diphenyloxide. Workplace environmental exposure
level guide. Am Ind Hyg Assoc J, 42: A76-A77.
Andersson O & Blomkvist G (1981) Polybrominated aromatic pollutants
found in fish in Sweden. Chemosphere, 10(9): 1051-1060.
Anon (1989) Draft evaluation report on flame retardants; PBB's and
PBBO's (in Dutch).
Applegate VC, Howell JH, Hall AE, & Smith MA (1957) Toxicity of 4,346
chemicals to larval lampreys and fish. Washington, DC, US Department
of the Interior, Fish and Wildlife Service (Special Scientific Report,
Fish No. 207).
Arias (1992) Brominated diphenyloxides as flame retardants; Bromine
based chemicals. Consultant report to the OECD, Paris.
Bahn A, Bialik O, Oler J, Houten L, & Landau E (1980) Health
assessment of occupational exposure to polybrominated biphenyl (PBB)
and polybrominated biphenyloxide (PBBO). Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 72 pp (ISS EPA 560/6-80-001; NTIS No. PB81-159675).
Ball M, Päpke O, & Lis A (1991) [Further investigation on the
formation of polybrominated dioxins and furans during thermal stress
of flameproof plastics and textiles. Sub-project 1.] Berlin, Federal
Office for the Environment (Research report No. 10403364/01; UBA-FB
91-082) (in German).
Ball M, Päpke O, & Lis A (1992) [Further investigation on the
formation of polybrominated dioxins and furans during thermal stress
of flameproof plastics and textiles. Sub-projects 1 and 2.] Berlin,
Federal Office for the Environment (Research report Nos. 10403364/01 &
/02; UBA-FB 91-082 & 92-097) (in German).
BFRIP (1990) Brominated flame retardants. A review of recent research
(Compiled by The Brominated Flame Retardant Industry Panel and The
European Brominated Flame Retardant Industry Panel). West Lafayette,
Indiana, USA, BFRIP (Unpublished report No. III/4143/90, submitted to
WHO by BFRIP).
Bialik O (1982) Endocrine function of workers exposed to PBB and PBBO:
Terminal progress report. Cincinnati, Ohio, National Institute for
Occupational Safety and Health.
Bieniek D, Bahadir M, & Korte F (1989) Formation of heterocyclic
hazardous compounds by thermal degradation of organic compounds.
Heterocycles, 28:719-722.
Breslin WJ, Kirk HD, & Zimmer MA (1989) Teratogenic evaluation of a
polybromodiphenyl oxide mixture in New Zealand White rabbits following
oral exposure. Fundam Appl Toxicol, 12: 151-157.
Bromine Compounds Ltd (1992) Material safety data sheet FR-1210. Beer
Sheva, Israel, Bromine Compounds Ltd, pp 1-5.
Brosier JS, Blanchard FA, & Takahashi IT (1972) Concentrations of
octabromodiphenyl and decabromodiphenyloxide in aquaria water and
trout flesh. Midland, Michigan, Dow Chemical Company (Unpublished
report No. AL 37448, submitted to WHO by BFRIP).
Bruckmann P, Hackhe K, Ball M, Lis A, & Papke O (1990) Degassing of
PBDD/PBDFs from a television set - PBDD/PBDF levels after a fire in a
stock house two case studies. In: Freij L ed. Proceedings of the
Workshop on Brominated Aromatic Flame Retardants, Skokloster, Sweden,
24-26 October 1989. Solna, National Chemicals Inspectorate (KEMI),
pp 99-104.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26(4): 446-452.
Buser HR (1986) Polybrominated dibenzofurans and dibenzo- p-dioxins:
Thermal reaction products of polybrominated diphenylether flame
retardants. Environ Sci Technol, 20(4): 404-408.
Carlson GP (1980a) Induction of xenobiotic metabolism in rats by
short-term administration of brominated diphenylethers. Toxicol Lett,
5: 19-25.
Carlson GP (1980b) Induction of xenobiotic metabolism in rats by
brominated diphenylethers administered for 90 days. Toxicol Lett,
6: 207-212.
Carte B & Faulkner DJ (1981) Polybrominated diphenyl ethers from
Dysidea herbacea. Dysidea chlorea and Phyllospongia foliascens.
Tetrahedron, 37: 2335-2339.
CBC (1982) The bioaccumulation of compound S-511 by carp. Tokyo,
Chemicals Inspection and Testing Institute, Chemical Biotesting Center
(Unpublished report).
CEM (1989) Polybrominated dibenzodioxins and dibenzofurans
(PBDDs/PBDFs) from flame retardants containing bromine. Assessment of
risk and proposed measures. Report by the Brominated flame retardants
CEM Working Group to the Conference of Environment Ministers, Bonn,
September 1989. (Report III/4299/89. Appendix).
Chemag (1988) Information sheet on Chemflam 011. Frankfurt am Main,
Chemag Aktiengesellschaft, p 1.
Clausen E, Lahaniatis ES, Bahadir M, & Bieniek D (1987) [Determination
of brominated dibenzofurans formed during the thermolysis of polymers
with decabromodiphenylether as flame-proofing agent.] Fresenius Z Anal
Chem, 327: 297300 (in German).
Craig DK, Mitchum RK, Bauer MR, Yancey MF, Peters AC, & Ioiner RL
(1989) Determination of polybrominated dibenzo- p-dioxins and
polybrominated dibenzofurans in CYCOLAC plastic resins and the fumes
evolved during normal thermal processing. Final report. Columbus.
Ohio, Battelle (Report to General Electric Company, Mt. Vernon,
Indiana, submitted to WHO by BFRIP).
Cramer PH, Stanley JS, & Thornburg KR (1990a) Mass spectral
confirmation of chlorinated and brominated diphenyl ether in human
adipose tissues. Kansas City, Missouri, Midwest Research Institute
(Prepared for the US Environmental Protection Agency, Washington) (US
NTIS report PB91-159699).
Cramer PH, Ayling RE, Thornburg KR, Stanley JS, Remmers JC, Breen JJ,
& Schwemberger J (1990b) Evaluation of an analytical method for the
determination of polybrominated dibenzo- p-dioxins/dibenzofurans
(PBDD/PBDF) in human adipose. Chemosphere, 20(7-9): 821-827.
Davidson JS & Ariano JM (1986) Decabromodiphenylether (DE-83):
Determination of area percent assay. Analytical method No. QC-86-02.
West Lafayette, Indiana, Great Lakes Chemical Corporation (Report
submitted to WHO by BFRIP).
De Boer J (1989) Organochlorine compounds and bromediphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere, 18(11/12): 2131-2140.
De Boer J (1990) Brominated diphenylethers in Dutch freshwater and
marine fish. In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar.
Tenth International Symposium on Chlorinated Dioxins and Related
Compounds. Volume 2: Organohalogenated compounds (Short papers).
Bayreuth, Germany, Ecoinforma Press, pp 315-318.
DeCarlo VJ (1979) Studies on brominated chemicals in the environment.
Ann NY Acad Sci, 320: 678-681.
Dieter MP (1979) Special studies protocol. Washington, Memorandum of
the Department of Health, Education and Welfare. Public Health
Service, National Institute of Health, National Cancer Institute.
Dow Chemical Company (1978) Compound: Decabromodiphenyl oxide
(Analytical method). Midland, Michigan, Dow Chemical Company,
pp 609-620 (Report prepared by Tracor Jitco, Inc., Rockville,
Maryland) (Report No. 3250, submitted to WHO by BFRIP).
Dumler R, Lenoir D, & Hutzinger O (1990) Formation of brominated
dibenzofurans and -dioxins from the combustion of the flame retardant
decabromodiphenyl ether under different conditions. In: Hutzinger O &
Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth International Symposium
on Chlorinated Dioxins and Related Compounds. Volume 2:
Organohalogenated compounds (short papers). Bayreuth, Germany,
Ecoinforma Press, pp 325-328.
Dumler R, Thoma H, Lenoir D, & Hutzinger O (1989a) Thermal formation
of polybrominated dibenzodioxins (PBDD) and dibenzofurans (PBDF) from
bromine containing flame retardants. Chemosphere, 19(1-6): 305-308.
Dumler R, Lenoir D, Thoma H, & Hutzinger O (1989b) Thermal formation
of polybrominated dibenzofurans from decabromodiphenyl ether in a
polybutylene-terephthalate polymer matrix. J Anal Appl Pyrolysis,
16: 153-158.
EBFRIP (1990) Information on polybrominated diphenylethers (PBDE's).
Economic, technical and regulatory assessment of the European
Brominated Flame Retardant Industry Panel - Responses to questions
submitted by EEC, Brussels. Rijswijk (NL), The European Brominated
Flame Retardant Industry Panel.
El Dareer SM, Kalin JR, Tillcry KF, & Hill DL (1987) Disposition of
decabromodiphenyl ether in rats dosed intravenously or by feeding.
J Toxicol Environ Health, 22: 405-415.
Environment Agency Japan (1983) Environmental monitoring of chemicals.
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Environment
Agency Japan, Department of Environmental Health, Office of Health
Studies.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Environment Agency Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Environment Agency Japan (1991) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1988 and 1989. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Ethyl Corporation (1986) Laboratory report: Primary eye irritation.
Saytex 102. Baton Rouge, Louisiana, Ethyl Corporation (Report
No. PH 421-ET-010-86) (Report submitted to WHO by BFRIP).
Ethyl Corporation (1992a) Information sheet from Ethyl Corporation on
Saytex 102E. Baton Rouge, Louisiana, Ethyl Corporation (Report
submitted to WHO by BFRIP).
Ethyl Corporation (1992b) Information sheet from Ethyl Corporation on
Saytex 111. Baton Rouge, Louisiana, Ethyl Corporation (Report
submitted to WHO by BFRIP).
Faulkner DJ (1988) Brominated marine natural products. In: Price D,
Iddon B, & Wakefield BJ ed. Bromine Compound: Chem. Appl.,
(Proceedings, International Conference on Chemical Applications of
Bromine and its Compounds.) Amsterdam, Oxford, New York, Elsevier
Science Publishers, Chapter 2, pp 121-144.
Faulkner DJ (1990) Naturally-occurring brominated compounds. In: Freij
L ed. Proceedings of the Workshop on Brominated Aromatic Flame
Retardants, Skoldoster, Sweden, 24-26 October 1989. Solna, National
Chemicals Inspectorate, pp 121-144.
Fernandez P, Grifoll M, Solahas AM, Bayona JM, & Albaiges J (1992)
Bioasssay-directed chemical analysis of genotoxic components in
coastal sediments. Environ Sci Technol, 26: 817-829.
Flick EW (1986) Plastics additives: An industrial guide. Section IX -
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Francis BM (1989) Relative developmental toxicities of nine diphenyl
ethers related to nitrofen. Environ Toxicol Chem, 8: 681-688.
Fresenius Institute (1990) Summary results on pyrolysis of different
types of ABS. Battelle report on contents and vapour-emission of PBDD
resp. PBDF's. Memorandum from Leeuwenburgh W.F., General Electric
Plastics ABS, BV. Amsterdam. 12 March 1990 (Report submitted to WHO by
BFRIP).
Fulfs JC (1987a) Rabbit ear bioassay for comedogenicity, dose-range
finding study for soot and char generated from the combustion of high
impact polystyrene. Fort Collins, CO, USA, Inhausen Research
Institute, Inc. (Report to Ethyl Corporation, Baton Rouge, Florida,
submitted to WHO by BFRIP).
Fulfs JC (1987b) Rabbit ear bioassay for comedogenicity, dose-range
finding study for soot and char generated from the combustion of high
impact polystyrene flame retarded with decabromodiphenyloxide and
antimony oxide. Fort Collins, CO, USA, Inhausen Research Institute,
Inc. (Report to Ethyl Corporation, Baton Rouge, Florida, submitted to
WHO by BFRIP).
Fulfs JC & Dahlgren RR (1987a) Acute oral toxicity in the rat of soot
and char generated from the combustion of high impact polystyrene
(HIPS). Fort Collins, CO, USA, Inhausen Research Institute, Inc.
(Report to Ethyl Corporation, Baton Rouge, Florida, submitted to WHO
by BFRIP).
Fulfs JC & Dahlgren RR (1987b) Acute oral toxicity in the rat of soot
and char generated from the combustion of high impact polystyrene
flame retarded with decabromodiphenyloxide and antimony trioxide (HIPS
FR). Fort Collins, CO, USA, Inhausen Research Institute, Inc. (Report
to Ethyl Corporation, Baton Rouge, Florida, submitted to WHO by
BFRIP).
Fulfs JC & Dahlgren RR (1987c) Rabbit ear biGassay for comedogemcity:
Multiple-dose-level definitive study for soot and char generated from
the combustion of high impact polystyrene. Fort Collins, CO, USA,
Inhausen Research Institute, Inc. (Report to Ethyl Corporation, Baton
Rouge, Florida, submitted to WHO by BFRIP).
Fulfs JC & Dahlgren RR (1987d) Rabbit ear bioassay for comedogenicity,
multiple-dose-level definitive study for soot and char generated from
the combustion of high impact polystyrene flame retarded with
decabromo-diphenyloxide and antimony trioxide. Fort Collins, CO, USA,
Inhausen Research Institute, Inc. (Report to Ethyl Corporation, Baton
Rouge, Florida, submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1987) Toxicity data of
octabromo-diphenyloxide (DE-79). West Lafayette, Indiana, Great Lakes
Chemical Corporation (Unpublished data submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1990a) Great Lakes DE-79tm: Product
information. West Lafayette, Indiana, Great Lakes Chemical Corporation
(Report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1990b) Great Lakes DE-83Rtm: Product
information. West Lafayette, Indiana, Great Lakes Chemical Corporation
(Report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (Undated a) Toxicity data of
pentabromo-diphenyloxide. West Lafayette, Indiana, Great Lakes
Chemical Corporation (Unpublished report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (Undated b) Toxicity data of
decabromo-diphenyloxide. West Lafayette, Indiana, Great Lakes Chemical
Corporation (Unpublished report submitted to WHO by BFRIP).
Gurka DF, Laska PR, & Titus R (1982) The capability of GC/FT-IR to
identify toxic substances in environmental sample extracts.
J Chromatogr Sci, 20: 145-154.
Hagenmaier H, She J, Dawidowsky N, Thomas B, & Dusterholt L (1991)
Analysis of sewage sludges for polyhalogenated dibenzo- p-dioxins,
dibenzofurans and diphenylethers. In: Dioxin'91. Abstract from the
11th International Symposium on Chlorinated Dioxins and Related
Compounds, Research Triangle Park, North Carolina, 23-27 September
1991, p 112.
Hallenbeck D (1993) Freezing point of DE-71. Inter-Office memorandum,
Great Lakes Chemical Corporation. June 29, 1993 (Report submitted to
WHO by BFRIP).
Hamm S & Theisen J (1992) Formation of polybrominated dibenzofurans
and polybrominated dibenzo(p)dioxins at fires of electrical
appliances. Poster presented at Dioxin'92. 12th International
Symposium on Dioxins and Related Compounds, Tampere, Finland, 24-28
August 1992. Munster, Society for Workplace and Environmental Analysis
GmbH.
Hanberg A, Stahlberg M, Georgellis A, De Wit C, & Ahlborg UG (1991)
Swedish dioxin survey: Evaluation of the H-4-II E bioassy for
screening environmental samples for dioxin:like enzyme induction.
Pharmacol Toxicol, 69: 442-449.
Hanley TR Jr (1985) Decabromodiphenyloxide: A summary of an oral
teratology study in Sprague-Dawley rats. Midland, Michigan, Dow
Chemical Company (Unpublished report submitted to WHO by BFRIP).
Hefner RE Jr (1973) A study of the rate of hemolysis induced by
decabromo-diphenoxide (DBDPO) particles with washed rat erythrocytes
in vitro. Midland, Michigan, Dow Chemical Company (Unpublished
report submitted to WHO by BFRIP).
Hoechst Celanese Corporation (1988) Report of air sampling for the
presence of polybrominated dibenzodioxins and dibenzofurans during the
production of polybutylene terephthalate resin with decabromodiphenyl
oxide (Unpublished report submitted to WHO by BFRIP).
Huff JE, Eustis SL, & Haseman JK (1989) Occurrence and relevance of
chemically induced benign neoplasms in long-term carcinogenicity
studies. Cancer Metastasis, 8: 121.
Hutzinger O & Thoma H (1987) Polybrominated dibenzo- p-dioxins and
dibenzofurans: The flame retardant issue. Chemosphere,
16(8/9): 1877-1880.
Hutzinger O, Sundstrom G, & Safe S (1976) Environmental chemistry of
flame retardants. Part. I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer, 345 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 48).
Industrial Bio-Test Laboratories (1975) Human repeated insult patch
test with DBDO-1 and XD 8186.02. Northbrook, Illinois, USA. (Report to
Dow Chemical Company, Midland, Michigan) (Report No. IBT 636-0654,
submitted to WHO by BFRIP).
IRPTC (1988) Data profiles on flame retardants; Deca-, octa- and
pentabromodiphenyloxide. Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme.
Jansson B, Asplund L, & olsson M (1987) Brominated flame retardants --
Ubiquitous environmental pollutants? Chemosphere,
16(10-12): 2343-2349.
Jansson B, Andersson R, Asplund L, Bergman A, Litzen K, Nylund K,
Reutergardh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjo T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Accepted for publication in Environ.
Toxicol. Chem., 12(7): 1163-1174.
Jersey G-C, Frauson LE, & Schuetz DJ (1976) Pulmonary clearance and
tissue response following a single intratracheal injection of
decabromo-diphenyloxide (DBDPO) dust in male rats. Midland, Michigan,
Dow Chemical Company (Unpublished report No. HET K-47298-(21),
submitted to WHO by BFRIP).
Kaart KS & Kokk KY (1987) Spectrometric determination of
decabromodiphenyl oxide in industrial sewage. Ind Lab, 53: 289-290.
Kalk (1982) [CFK Bromkal(R) - fire protection equipment]. Cologne,
Kalk Chemical Factory (Information sheet 3000-7/82) (in German).
Kitchin KT, Brown JL, & Kulkarni P (1992) Predictive assay for rodent
carcinogenicity using in vivo biochemical parameters: operational
characteristics and complementarity. Mutat Res, 266: 253-272.
Klusmeier W, Voegler P, Ohrbach KH, Weber H, & Kettrup A (1988)
Thermal decomposition of decabromodiphenyl ether. J Anal Appl
Pyrolysis, 13: 277-285.
Kociba RJ (undated) Comparative biologic activity and toxicity of
brominated derivatives of diphenylether (oxide). (Unpublished report
submitted to WHO by BFRIP).
Kociba RJ, Frauson LO, Humiston CG, Norris JM, Wade CE, Lisowe RW,
Quast IF, Jersey GC, & Jewett GL (1975a) Results of a two-year dietary
feeding study with decabromodiphenyl oxide (DBDPO) in rats. Midland,
Michigan, Dow Chemical Company (Unpublished report submitted to WHO by
BFRIP).
Kociba RJ, Frauson LO, Humiston CG, Norris JM, Wade CE, Lisowe RW,
Quast JF, Jersey GC, & Jewett GL (1975b) Results of a two-year dietary
feeding study with decabromodiphenyl oxide (DBDPO) in rats. Combust
Toxicol, 2: 267-285.
De Kok JJ, De Kok A, Brinkman UATh, & Kok RM (1977) Analysis of
polybrominated biphenyls. J Chromatogr, 142: 367-383.
De Kok JJ, De Kok A, & Brinkman UATh (1979) Analysis of polybrominated
aromatic ethers. J Chromatogr, 171: 269-278.
Kopp A (1990) [Documentation on fire-proofing agents containing
bromine.] Bonn, Ministry of Environment, Nature Conservation and
Nuclear Safety (Report to the European Economic Community, Brussels)
(in German).
Koster P, Debets FMH, & Strik JJTWA (1980) Porphyrinogenic action of
fire retardants. Bull Environ Contain Toxicol, 25:313-315.
Kraus HW (1990) Polybrominated dibenzodioxines and dibenzofurans
(PBDDs/PBDFs) from flame retardants containing bromine. Bonn, Ministry
for Environment, Nature Conservation and Nuclear Safety (Report to the
Organisation for Economic Co-operation and Development, Paris).
Kruger C (1988) [Polybrominated biphenyls and polybrominated diphenyl
ethers detection and quantitation in selected foods.] Munster,
University of Munster (Thesis) (in German).
Kuehl DW, Haebler R, & Potter C (1991) Chemical residues in dolphins
from the U.S. Atlantic coast including Atlantic bottlenose obtained
during the 1987/88 mass mortality. Chemosphere, 22(11): 1071-1084.
Lahaniatis ES, Bergheim W, & Rainer C (1989) Hazardous halogenated
substances formed during combustion processes. Toxicol Environ Chem,
20-21: 501-506.
Lahaniatis ES, Bergheim W, & Bieniek D (1991) Formation of
2,3,7,8-tetrabromodibenzodioxin and -furan by thermolysis of polymers
containing brominated flame retardants. Toxicol Environ Chem,
31/32: 521-526.
Lahl U, Wilken M, & Wiebe A (1991) [Polybrominated diphenyl ethers in
waste incineration.] Mull Abfall, 23:83-87 (in German).
Larsen ER (1978) Flame retardants. Halogenated fire retardants. In:
Mark HF, Othmer DF, Overberger CG, Seaborg GT, & Grayson M ed.
Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, vol 10,
pp 373-395.
Leblanc RB (1979) What's available for FR textiles. Text Ind,
143: 78-83.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24(5): 684-691.
Leung HW, Murray FJ, & Paustenbach DJ (1988) A proposed occupational
exposure limit for 2,3,7,8-tetrachlorodibenzo- p-dioxin. Am Ind Hyg
Assoc J, 49(9): 466-474.
Luijk R & Govers HAJ (1992) The formation of polybrominated
dibenzo- p-dioxins (PBDDs) and dibenzofurans (PBDFs) during pyrolysis
of polymer blends containing brominated flame retardants. Chemosphere,
25(3): 361-374.
McAllister DL & Ariano JIM (1982) DE-71/DE-60F. Determination of
bromination level and aromatic phosphate ester concentration.
Analytical method No. QCS-82-25. West Lafayette, Indiana, Great Lakes
Chemical Corporation (Report submitted to WHO by BFRIP).
McAllister DL, Mazac CJ, Gorsich R, Freiberg M, & Tondeur Y (1990)
Analysis of polymers containing brominated diphenyl ethers as flame
retardants after molding under various conditions. Chemospbere,
20(10-12): 1537-1541.
McAllister DL (1991) Letter of Great Lakes Chemical Corporation, West
Lafayette to the IPCS/WHO, dated November 25, 1991.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9(13): 1178-1180.
McMahon LW (1983) Organic priority pollutants in wastewater. In:
Proceedings of the 1982 UCC-ND/GATT Environmental Protection Seminar,
April, 5-7, 1982. Oak Ridge, TN. Oak Ridge National Laboratory/Union
Carbide Corp. US (DOE Contract No. W-7405-eng-26) (US EPA, 1986).
Mallory VT, Naismith RW, & Matthews RJ (1986) Primary eye irritation
(PH 421-ET-010-86) Saytex 102. Baton Rouge, Louisiana, Ethyl
Corporation (Unpublished report submitted to WHO by BFRIP).
Naismith RW & Matthews RJ (1981) Assay of comedogenicity in the rabbit
ear (PH 425-ET-001-81) Saytex 102. Baton Rouge, Louisiana, Ethyl
Corporation (Unpublished report submitted to WHO by BFRIP).
Neupert M, Weis H, Thies J, & Stock B (1989) Analytical procedures in
connection with acute animal toxicity studies. II. Pyrolysis products
obtained from an ABS copolymer containing octabromodiphenylether as a
flame retardant. Chemosphere, 19(16): 219-224.
Noguchi Y, Noda E, Toyoshima K, & Mitsui Toatsu Chemicals Inc. (1977)
Photostabilized polybromobiphenyl ethers. Japan Kokai 77 07,932,
January 21, 1977. (as reported in Chem. Abstr. 87: 134511x) (US EPA,
1986).
Norris JM (1971) Decabromodiphenyloxide solids from mother liquor
(waste tars). Midland, Michigan, Dow Chemical Company (Unpublished
report TOX-No. T2.TX-1767-1, submitted to WHO by BFRIP).
Norris JM, Ehrmantraut JW, Gibbons CL, Kociba RJ, Schwetz BA, Rose JQ,
Humiston CG, Jewett GL, Crummett WB, Gehring PJ, Tirsell JB, & Brosier
JS (1973) Toxicological and environmental factors involved in the
selection of decabromodiphenyl oxide as a fire retardant chemical.
Appl Polymer Symp, 22: 195-219.
Norris JM, Ehrmantraut JW, Gibbons CL, Kociba RJ, Schwetz BA, Rose JQ,
Humiston CO, Jewett GL, Crummett WB, Gehring Pl, Tirsell JB, & Brosier
JS (1974) Toxicological and environmental factors involved in the
selection of decabromodiphenyloxide as a fire retardant chemical.
J Fire Flamm Combust Toxicol, 1, 52-77.
Norris JM, Ehrmantraut JW, Kociba RJ, Schwetz BA, Rose JQ, Humiston
CO, Jewett OL, Crummet WB, Gehring PJ, Tirsell JB, & Brosier JS
(1975a) Evaluation of deca-bromodiphenyloxide as a flame-retardant
chemical. Chem Hum Health Environ, 1: 100-116.
Norris JM, Kociba RJ, Humiston CG, & Gehring PJ (1975b) The toxicity
of deca-bromodiphenyloxide and octabromodiphenyl as determined by
subacute and chronic dietary feeding studies in rats. Toxicol Appl
Pharmacol, 33(1): 170 (abstract).
Norris JM, Kociba RJ, Schwetz BA, Rose JQ, Humiston CG, Jewett GL,
Gehring PJ, & Mailhes JB (1975c) Toxicology of octabromodiphenyl and
decabromodiphenyloxide. Environ Health Perspect, 11: 153-161.
NTP (1986) Toxicology and carcinogenesis studies of decabromodiphenyl
oxide (CAS No. 1163-19-5) in F344/N rats and B6C3F1 mice (feed
studies). Research Triangle Park, North Carolina, US Department of
Health and Human Services, National Toxicology Program (NTP Technical
Report Series No. 309).
Nylund K, Asplurid L, Jansson B, Jonsson P, Litzen K, & Sellström U
(1992) Analysis of some polyhalogenated organic pollutants in sediment
and sewage sludge. Chemosphere, 24(12): 1721-1730.
Oberg T & Bergstrom J (1990) Bromine and waste incineration - An
environmental risk? In: Hutzinger O & Fiedler H ed. Dioxin'90.
EPRI-Seminar. Tenth International Symposium on Chlorinated Dioxins and
Related Compounds. Volume 2: Organo-halogenated compounds (short
papers). Bayreuth, Germany, Ecoinforma Press, pp 339-342.
Oberg T, Warman K, & Bergström J (1987) Brominated aromatics from
combustion. Chemosphere, 16(10-12): 2451-2465.
OECD (1991) Co-operation on existing chemicals: Risk reductions. Lead
country report on brominated flame retardants. Sixteenth Joint Meeting
of the Chemicals Group and Management Committee, 28-30 May 1991.
Paris, Organisation for Economic Cooperation and Development (Report
ENV/MC/CHEM (91)7).
Pijnenburg AMCM & Everts JW (1991) [Flame retardams: Occurrence and
toxicity.] The Hague, Ministry of Transport and Public Works (Note
No. GWAO-91.001) (in Dutch).
Pijnenburg AMCM, Everts JW, De Boer J, & Boon JP (1992) Polyhrominated
biphenyl (PBB) and diphenylether (PBDE) flame retardants: Analysis,
toxicity and occurrence in aquatic environments. Manuscript for
discussion in 1992 Annual Meeting of the ICES Marine Chemistry Working
Group (MCWG) and the Working Group on Biological Effects of
Contaminants (WGBEC). The Hague, Ministry of Transport and Public
Works (Unpublished report).
Pinkerton MN, Kociba RJ, Petrella RV, McAllister DL, Willis ML, Fulfs
JC, Thoma H, & Hutzinger O (1989) A preliminary report on the
investigation of the comparative toxicity of combustion products of
high impact polystyrene with and without decabromodiphenyloxide/
antimony trioxide as a flame retardant using 2,3,7,8-tetrabromo-
dibenzo- p-dioxin and 2,3,7,8-tetrabromodibenzofuran as positive
controls. Chemosphere, 18(1-6): 1243-1249.
Plumb RH Jr (1991) The occurrence of Appendix IX organic constituents
in disposal site ground water. Groundw Monit Rev, 11(2): 157-164.
Rampy LW (1971-1974) Chloracne studies on DBDPO and DBDPO mother
liquor. A series of working protocols with results from the Chemical
Biology Research Department of Dow Chemical USA, Midland, Michigan
(The work was carried out mainly by Rampy alone or with co-workers in
the period 1971-1974) (Report submitted to WHO by BFRIP).
Ranken PF, Ricks GM, Lynam DR, & Ariano JM (1990) Is watching
television toxic? BFRIP-sponsored study on the emission of PBDDs/PBDFs
from operating television sets. In: Hutzinger O & Fiedler H ed.
Dioxin'90. EPRI-Seminar. Tenth International Symposium on Chlorinated
Dioxins and Related Compounds. Volume 2: Organohalogenated compounds
(short papers). Bayreuth, Germany, Ecoinforma Press, pp 343-346.
Remmers JC, Breen JJ, Schwemberger J, Stanley JS, Cramer PH, &
Thornburg KR (1990) Mass spectral confirmat!on of chlorinated and
brominated diphenyl ethers in human adipose tissues. In: Hutzinger O &
Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth International Symposium
on Chlorinated Dioxins and Related Compounds. Volume 2:
Organohalogenated compounds (short papers). Bayreuth, Germany,
Ecoinforma Press, pp 347-350.
Riggs KB, Pitts GE, White JS, Mitchum RK, Reuther JJ, & Glatz JA
(1990) Polybrominated dibenzo- p-dioxin and polybrominated
dibenzofuran emissions from incineration of flame-retarded resins in a
simulated municipal waste incinerator. In: Hutzinger O & Fiedler H ed.
Dioxin'90. EPRI-Seminar. Tenth International Symposium on Chlorinated
Dioxins and Related Compounds. Volume 2: Organohalogenated compounds
(short papers). Bayreuth, Germany, Ecoinforma Press, pp 351-356.
Rogers J & Hill JT (1980) Final report. Determination of
decabromodiphenyl oxide (DBDPO) in rat livers. Vienna, Virginia,
Hazleton Laboratories America, Inc. (Unpublished report to Tracor
Jitco, Rockville, Maryland, submitted to WHO by BFRIP).
Rutter HA & Machotka S (1979) Final Report. Decabromodiphenyloxide:
13-week sub-chronic feeding study -- mice. Vienna, Virginia, Hazleton
Laboratories America, Inc. (Unpublished report to Tracor Jitco,
Rockville, Maryland, submitted to WHO by BFRIP).
Schwetz BA, Smith FA, Nitschke KD, Humiston CG, Jersey GC, & Kociba RJ
(1975) Results of a reproduction study in rats maintained on diets
containing decabromodiphenyloxide. Midland, Michigan, Dow Chemical
Company (Unpublished report No. HET K47298-(14), submitted to WHO by
BFRIP).
Sellström U, Andersson R, Asplund L, Jansson B, Jonsson P, Litzen K,
Nylund K, Uvemo U-B, & Wideqvist U (1990b) Anthropogenic brominated
aromatics in the Swedish environment. In: Freij L ed. Proceedings of
the Workshop on Brominated Aromatic Flame Retardants, Skokloster,
Sweden, 24-26 October 1989. Solna, National Chemicals Inspectorate
CKEMI), pp 73-78.
Sellström U, Jansson B, Ionsson P, Nylund K, Odsjö T, & Olsson M
(1990a) Anthropogenic brominated aromatics in the Swedish environment.
In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth
International Symposium on Chlorinated Dioxins and Related Compounds.
Volume 2: Organohalogenated compounds (short papers). Bayreuth,
Germany, Ecoinforma Press, pp 357-360.
Sellström U, Jansson B, Kierkegaard A, & De Wit C (1993a)
Polybrominated diphenyl ethers (PBDE) in biological samples from the
Swedish environment. Chemosphere, 26(9): 1703-1718.
Sellström U, Kierkegaard A, De Wit C, Jansson B, & Olsson M (1993b)
Time trend studies of polybrominated diphenyl ethers (PBDE) in
biological material from the Swedish environment. Paper presented at
Dioxin'93, Vienna, 1 September 1993.
Shoichet A & Ehrlich K (1977) Mutagenicity testing of HFO 102.
(Unpublished proprietary report from Gulf South Research Institute,
New Orleans, Louisiana to Hexel Fine Organics (Report submitted to WHO
by BFRIP).
Sovocool GW, Donnelly JR, Grange All, Simmons RD, & Munslow WD (1990)
Brominated dioxins and other bromoaromatics in plastic pyrolysates.
In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth
International Symposium on Chlorinated Dioxins and Related Compounds.
Volume 2: Organohalogenated compounds (short papers). Bayreuth,
Germany, Ecoinforma Press, pp 365-368.
Sparschu GL, Kociba RJ, & Clashman A (1971) Results of 30 day rat
dietary feeding studies on octabromobiphenyl SA-1902 and
decabromodiphenyl oxide SA-1892.1. Midland, Michigan, Dow Chemical
Company (Unpublished report submitted to WHO by BFRIP).
Stafford CJ (1983) Halogenated diphenylethers identified in avian
tissues and eggs by GC/MS. Chemosphere, 12(11/12): 1487-1495.
Stanley JS, Cramer PH, Thornburg KR, Remmers JC, Breen JJ, &
Schwemberger J (1991) Mass spectral confirmation of chlorinated and
brominated diphenylethers in human adipose tissues. Chemosphere,
23(8-10): 1185-1195.
Striebich RC, Rubey WA, Tirey DA, & Dellinger B (1991) High
temperature degradation of polybrominated flame retardant materials.
Chemosphere, 23(8-10): 1197-1204.
Sundström G & Hutzinger O (1976) Environmental chemistry of flame
retardants. V. The composition of Bromkal (R) 70-5 DE -- A
pentabromodiphenyl ether preparation. Chemosphere, 3: 187-190.
Tabak HH, Quave SA, Mashni Cl, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabor TE & Bergman S (1975) Decabromodiphenyl oxide. A new fire
retardant additive for plastics. In: Fire retardants. Proceedings of
1974 International Symposium on Flammability and Fire Retardants,
Cornwall, Ontario, 1-2 May 1974. Westport, Connecticut, Technomic
Publishing, pp 162-179.
Takase I, Omori T, & Midona Y (1986) Microbial degradation products
from biphenyl-related compounds. Agric Biol Chem, 50(3): 681-686.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Thoma H & Hutzinger O (1987) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemopshere,
16(6): 1353-1360.
Thoma H & Hutzinger O (1989) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemosphere,
18(5): 1047-1050.
Thoma H, Hauschulz G, Knorr E, & Hutzinger O (1987a) Polybrominated
dibenzofurans (PBDF) and dibenzodioxins (PBDD) from the pyrolysis of
neat brominated diphenylethers, biphenyls and plastic mixtures of
these compounds. Chemosphere, 16(1): 277-285.
Thoma H, Hauschulz G, & Hutzinger O (1987b) PVC-induced
chlorine-bromine exchange in the pyrolysis of polybrominated diphenyl
ethers, -biphenyls, dibenzodioxins and dibenzofurans. Chemosphere,
16(1): 297-307.
Timmons LL & Brown RD (1988) Analysis of the brominated fire retardant
decabromdiphenyloxide for low and trace levels impurities.
Chemosphere, 17: 217-234.
UK DOE (1992) Risk benefit analysis of hazardous chemicals. London, UK
Department of Environment.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
UMWELTBUNDESAMT (1989) Status report: Polybrominated dibenzodioxins
and polybrominated dibenzofurans. Berlin.
US EPA (1978) Chemical screening. Initial evaluations of substantial
risk notices: section 8(e), January 1, 1977 to June 39, 1979.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances, Office of Testing and Evaluation,
vol I, pp 39-40 (EPA 560/11-80-008).
US EPA (1984) Health and environmental effects profile for brominated
diphenyl ethers. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office CEPA/600/X-84/133).
US EPA (1986) Brominated diphenylethers. Chemical hazard information
profile. Washington, DC, US Environmental Protection Agency.
US EPA (1988) Information review: Brominated diphenyl ethers.
Washington, DC, US Environmental Protection Agency, TSCA, Interagency
Testing Committee (Report No. IR-516).
US EPA (1989) Letter from Director Office of Toxic Substances,
Washington, to Great Lakes Chemical Corporation, dated July 27, 1989.
US Testing Company (1985) Determination of vapour pressure and melting
point. Chemical Services Division. Hoboken, New Jersey. Letter to
Great Lakes Chemical Corporation. 7 October 1985 (Report submitted to
WHO by BFRIP).
Vinci TM & Craig DK (1988) Characterization of fume components
generated during the extrusion of flame-retarded polyester resins.
Columbus, Ohio, Battelle (Unpublished report to GE Plastics, Mt.
Vernon, Indiana, submitted to WHO by BFRIP).
Von Meyerinck L, Hufnagel B, Schmoldt A, & Benthe HF (1990) Induction
of rat liver microsomal cytochrome P-450 by the pentabromodiphenyl
ether Bromkal 70 and half lives of its components in the adipose
tissue. Toxicology, 61: 259-274.
Walsh GE, Yoder MI, McClaughlin LL, & Lores EM (1987) Responses of
marine unicellular algae to brominated organic compounds in six growth
media. Ecotoxicol Environ Saf, 14: 215-222.
Watanabe I (1987) Text from undated slide presentation.
Watanabe I & Tatsukawa R (1987) Formation of brominated dibenzofurans
from the photolysis of flame retardant decabromodiphenyl ether in
hexane solution by UV and sunlight. Bull Environ Contain Toxicol,
39: 953-959.
Watanabe I & Tatsukawa R (1990) Anthropogenic brominated aromatics in
the Japanese environment. In: Freij L ed. Proceedings of the Workshop
on Brominated Aromatic Flame Retardants, Skoldoster, Sweden, 24-26
October 1989. Solna, National Chemicals Inspectorate (KEMI), pp 63-71.
Watanabe I, Kashimoto T, & Tatsukawa R (1986) Confirmation of the
presence of the flame retardant decabromodiphenyl ether in river
sediment from Osaka, Japan. Bull Environ Contam Toxicol, 36: 839-842.
Watanabe I, Kashimoto T, Kawano M, & Tatsukawa R (1987a) A study of
organic bound halogens in human adipose tissue, marine organisms and
sediment by neutro-activation and gas chromatographic analysis.
Chemosphere, 16(4): 849-857.
Watanabe I, Kashimoto T, & Tatsukawa R (1987b) Polybrominated
biphenylethers in marine fish, shellfish and river and marine
sediments in Japan. Chemopshere, 16(10/12): 2389-2396.
Watanabe I, Kawano M, Wang Y, Chen Y, & Tatsukawa R (1992)
Polybrominated dibenzo- p-dioxins (PBDDs) and -dibenzofurans (PBDFs)
in atmospheric air in Taiwan and Japan. In: Dioxin'92. Twelfth
International Symposium on Dioxins and Related Compounds, Tampere,
Finland, 24-28 August 1992. Organohalogen Compounds Volume 9: Sources
of exposure.
Watanabe I, Kawano M, & Tatsukawa R (undated) Consumption trend and
environmental research on brominated flame retardants in Japan and the
formation of polyhalogenated dibenzofurans at the metal reclamation
factory. Document distributed at the OECD Workshop on the Risk
Reduction of Brominated Flame Retardants, Neuchatel, 1993.
WHO (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo- p-dioxins and bibenzofurans. Geneva, World Health
Organization.
WHO (1994) Environmental Health Criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization.
Yagi O & Sudo R (1980) Degradation of polychlorinated biphenyls by
microorganisms. JWPCF 52(5), 1035-1043 and US EPA, 1986.
Yamamoto K, Mori Y, Uehira S, Tanaka T, Koyama T, Katuyama K,
Taniguchi Y, & Sakamoto T (1991) Content of presence of the flame
retardant decabromodiphenyl ether in Kino river Basin. Wakayama-ken
Eisei Kogai Kenkyu Senta Nenpo, 37:116-122.
Zacharewski T, Harris M, Safe S, Thoma H, & Hutzinger O (1988)
Applications of the in vitro aryl hydrocarbon hydroxylase induction
assay for determining "2,3,7,8-tetrachlorodibenzo- p-dioxin
equivalents": pyrolyzed brominated flame retardants. Toxicology,
51: 177-189.
Zier B, Lenoir D, Lahaniatis ES, & Kettrup A (1991) Surface catalyzed
halogenation-dehalogenation reactions of aromatic bromine compounds
adsorbed on fly ash. Chemosphere, 22(12): 1121-1129.
Zober MA, Ott MG, Pfipke O, Senft K, & Germann C (1992) Morbidity
study of extruder personnel with potential exposure to brominated
dioxins and furans. I. Results of blood monitoring and immunological
tests. Br J Ind Med, 49: 532-544.
Zogorski JS (1984) Experience in monitoring domestic water sources and
process waters for trace organics. J Environ Sci Health,
A19(2): 233-249.
Van Zorge JA, Bruijnes C, Van den Berg EV, & Sprong W (1992)
Brominated flame retardants: Polybrominated biphenyls (PBBs) and
polybrominated diphenyloxides (PBDOs). OECD Cooperative risk reduction
activities for certain dangerous chemicals, Paris. Fifth draft, May
26th, 1992.
Zweidinger RA, Cooper SD, Erickson MD, Michael LC, & Pellizzari ED
(1977) Sampling and analysis for semivolatile brominated organics in
ambient air. In: Schuetzle D ed. Monitoring toxic substances.
Washington, DC, American Chemical Society, pp 217-231 (ACS Symposium
Series No. 94).
Zweidinger RA, Cooper SD, & Pellizzari ED (1978) Identification and
quantitation of brominated fire retardants. In: Measurement of organic
pollutants in water and waste water. Symposium of the American Society
for Testing and Materials, Denver, Colorado, 19-20 June 1978.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 234-250 (ASTM Technical Publication No. 686).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
DECABROMODIPHENYLETHER
1 Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le DeBDE du commerce se caractérise par une pureté de 97 à 98%, et
une teneur en nona- et/ou octabromodiphényléthers de 0,3 à 3,0%. Le
nonabromodiphényléther (NBDE) en est l'impureté principale.
Contrairement aux autres polybromo-diphényléthers, le DeBDE n'a qu'un
isomère.
Le point de fusion du DeBDE est d'environ 300°C et il se décompose
au dessus de 400°C. Sa solubilité dans l'eau est de 20-30/µg/litre et
le logarithme de son coefficient de partage n-octanol/eau est
supérieur à 5. Sa tension de vapeur est <10-6 mmHg à 20°C.
1.2 Production et usages
De tous les bromodiphényléthers (mono- à déca-) le
décabromodiphényléther est, de par sa production et son usage, le plus
important du point de vue commercial.
On le produit depuis la fin des années 70 à un degré de plus en
plus élevé de pureté. La production mondiale de DeBDE est d'environ
30 000 tonnes par an. On l'utilise comme retardateur de flamme dans de
nombreuses matières plastiques, notamment dans le polystyrène choc,
ainsi que pour le traitement des tissus d'ameublement, ou encore des
textiles utilisés en sellerie automobile ou pour la confection de
tentes.
1.3 Transport, distribution et transformation dans l'environnement
En solution dans des solvants organiques, le DeBDE subit une
photodécomposition sous l'influence du rayonnement ultra-violet ou de
la lumière solaire; cette réaction conduit à la formation de
bromodiphényléthers moins substitués et de bromodibenzofuranes. Cette
photodécomposition se produit également, quoique dans une moindre
mesure, en solution aqueuse, sous l'action du rayonnement solaire; on
ne retrouve plus alors d'homologues inférieurs du DeBDE ni de
bromodibenzofuranes.
Les quantités de DeBDE que l'on peut extraire des polymères sont
inférieures à la limite de détection ou très proches de cette limite,
selon le type de polymère et le solvant d'extraction.
Du fait que sa solubilité dans l'eau et sa tension de vapeur sont
très faibles, le DeBDE est vraisemblablement transporté
essentiellement par adsorption sur des particules. Il est persistant
et s'accumule probablement dans les sédiments et le sol.
On en dispose d'aucune donnée sur sa biodisponibilité à partir des
sédiments et du sol. Une étude sur des truites arc-en-ciel n'a pas
révélé de bioaccumulation dans la chair, la peau ou les viscères de
ces poissons, sur une période de 48 heures. Il est improbable que le
DeBDE subisse une bioaccumulation en raison de sa masse moléculaire
relative élevée.
Les rebuts qui contiennent du DeBDE commercial finissent dans les
décharges contrôlées ou les incinérateurs. Il se peut que du DeBDE
s'échappe de ces décharges par lessivage. Le brûlage sur les décharges
ou une incinération inefficace peuvent conduire à la formation de
polybromodibenzofuranes (PBDF) et d'un mélange d'halogénodi-
benzofuranes et d'halogénodibenzodioxines. Les produits qui
contiennent du DeBDE commercial peuvent contribuer à ces émissions.
La pyrolyse, en présence d'oxygène, de DeBDE commercial et de
polymères contenant de ce produit (polystyrène choc, PBT,
polypropylène industriel) produit des polybromodibenzofuranes et en
moindre quantités, des polybromodibenzodioxines. C'est aux
températures de 400 à 500°C que les PBDF sont produits en quantités
maximales mais ils peuvent également se former à des températures
allant jusqu'à 800°C et Sb2O3 en catalytise la formation.
La formation des PBDF et des PBDD ainsi que leurs proportions
respectives, dépendent de la température, de la teneur en oxygène et
de la durée de la pyrolyse. En l'absence d'oxygène, il se forme
principalement des polybromobenzènes et des polybromonaphtalènes.
1.4 Concentrations dans l'environnement et exposition humaine
On a trouvé du DeBDE dans l'air à proximité d'unités de
production, à des concentrations allant jusqu'à 25 µg/m3. En
revanche, on n'a pas décelé de DeBDE dans des échantillons d'eau
prélevés au Japon entre 1977 et 1991. Cependant, on en a trouvé dans
des sédiments de rivières, également prélevés au Japon au cours de la
même période, à des déconcentrations allant jusqu'à environ 12 mg/kg
de poids sec. On a également décelé la présence (jusqu'à 1 g/kg) de
DeBDE dans des sédiments de cours d'eau aux Etats-Unis, à proximité
d'une unité de production. On n'a pas décelé de DeBDE dans des
échantillons de poissons recueillis au Japon, mais, dans un
échantillon de moules on en a trouvé à des teneurs juste supérieures
au seuil de détection. Le produit n'a pas été décelé dans des
échantillons de tissus adipeux humains prélevés au Japon; en revanche,
aux Etats-Unis, on en a trouvé dans trois échantillons sur cinq du
même type de tissus.
Au cours de la production de DeBDE et de son adjonction aux
polymères, il peut y avoir exposition humaine. En revanche, cette
exposition est négligeable pour la population générale.
En cherchant à déterminer l'exposition professionnelle aux
produits de décomposition du DeBDE lors de sa fabrication et de son
incorporation par extrusion à des polymères, on a constaté la présence
de fortes concentrations de PBDF dans des échantillons d'air prélevés
au niveau de la filière de l'extrudeuse. Les concentrations étaient
moindres dans l'air de l'atelier. Des PBDF ont été également retrouvés
dans des échantillons obtenus lors de l'essuyage. Moyennant une bonne
technique de travail, on peut réduire l'exposition professionnelle aux
PBDF.
L'exposition de la population générale aux PBDF présents comme
impuretés dans des polymères contenant des retardateurs de flamme, est
vraisemblablement négligeable.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Le DeBDE est faiblement résorbé dans les voies digestives et après
injection, il est rapidement excrété.
D'après les résultats d'études de métabolisme chez le rat, au
moyen de DeBDE marqué au 14C, la demi-vie d'élimination de ce composé
serait inférieure à 24 heures et la principale voie d'élimination
après ingestion, serait la voie fécale. On n'a pas constaté la
présence d'une activité appréciable de 14C (moins de 1%) dans l'urine
ou l'air expiré.
Des rats à qui l'on avait administré pendant des périodes allant
jusqu'à deux ans, une dose quotidienne de 0,1 mg/kg de poids corporel
de ce composé, n'ont présenté aucun accumulation de DeBDE dans leur
sérum, leurs reins, leurs muscles ou leurs testicules, d'après le
dosage du brome total. L'accumulation du brome dans le foie a atteint
un palier au bout de 30 jours et l'élimination s'est effectuée dans
les 10 jours suivants le traitement. Après 180 jours, la concentration
de brome dans le foie n'était pas plus élevée chez les rats traités
que chez les rats témoins. Dans les tissus adipeux, on a noté une
faible accumulation de brome total, qui a subsisté après 90 jours de
nourriture sans DeBDE; on ignore quelle est la nature du "brome"
retenu. Etant donné que le DeBDE ne représentait que 77% du mélange
commercial utilisé, ce "brome" pourrait provenir du NBDE ou de l'OBDE.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Pour les animaux de laboratoire, la toxicité aiguë du DeBDE est
faible. Le produit n'est pas irritant pour la peau ou les yeux des
lapins. Il ne provoque pas de chloracné sur la peau des lapins et n'a
pas d'effet sensibilisateur sur la peau humaine.
Les produits de combustion du polystyrène contenant un retardateur
de flamme à base de DeBDE et de Sb2O3 ont été étudiés afin d'en
déterminer la toxicité aiguë et la comédogénicité. Chez le rat, la
DL50 par voie orale de la suie et du résidu de carbonisation était
>2000 mg/kg de poids corporel.
Lors d'études de toxicité à long terme effectuées sur des rats et
des souris, du DeBDe (pureté >97%) a été administré aux animaux mêlé
à leur nourriture aux doses de 100 g/kg de nourriture (4 semaines) ou
50g/kg (13 semaines, soit l'équivalent de 2500 mg/kg de poids corporel
en ce qui concerne le rat) sans produire d'effets nocifs. Une étude de
reproduction portant sur une génération de rats n'a pas révélé
d'effets nocifs aux doses de 100 mg/kg de poids corporel. Le DeBDE n'a
pas provoqué d'effets tératogènes chez des foetus de rats ayant reçu
une dose de 100 mg/kg de poids corporel. A la dose de 1000 mg/kg de
poids corporel, on a observé des malformations, par exemple un retard
d'ossification. Soumis à un certain nombre d'épreuves, le DeBDE ne
s'est pas révélé mutagène.
Lors d'une étude de cancérogénicité sur des rats et des souris, du
DeBDE (pureté 94-99%) a été administré, mêlé à leur nourriture, à des
doses allant jusqu'à 50 g/kg. On a observé une augmentation dans
l'incidence des adénomes (mais pas des carcinomes) au niveau du foie
chez les rats mâles qui en avaient reçu 25 g/kg et chez les femelles
qui en avaient reçu 50 g/kg. Chez les souris mâles, on a observé une
augmentation de l'incidence des adénomes et/ou des carcinomes
hépatocellulaires (les deux ensemble) à la dose de 25 g/kg et une
augmentation des adénomes et des cancers (les deux ensemble)
médullaires de la thyroïde à ces deux doses. Chez les souris femelles,
on n'a pas relevé d'augmentation dans l'incidence des tumeurs. C'est
uniquement aux doses de 25 à 50 g de DeBDE/kg de nourriture que chez
les rats mâles et femelles d'une part, et chez les souris mâles
d'autre part, on a constaté l'existence de signes équivoques de
cancérogénicité. Etant donné que les résultats de toutes les épreuves
de mutagénicité sont restés négatifs, on peut en conclure que le DeBDE
n'est pas un cancérogène génotoxique. Le CIRC (1990) est parvenu à la
conclusion que les preuves d'une cancérogénicité du DeBDE chez les
animaux de laboratoire sont limitées. Du fait des très fortes doses
utilisées dans ces études, de l'absence de génotoxicité et des
éléments de preuve très minimes en faveur d'une cancérogénicité de ce
composé, on peut conclure que le DeBDE, aux taux d'exposition actuels,
ne présente pas de risque cancérogène pour l'homme.
1.7 Effets sur l'homme
Chez des sujets humains exposés à du DeBDE lors d'une épreuve de
sensibilisation, on n'a noté aucun signe de sensibilisation cutanée.
Une étude de morbidité sur des ouvriers qui procédaient à
l'extrusion de polybutylène-téréphtalate contenant du DeBDE (avec par
conséquent un risque potentiel d'exposition aux PBDD et aux PBDF
pendant 13 ans) n'a pas révélé le moindre effet délétère, malgré la
présence dans leur sang de 2,3,7,8-TeBDF et de 2,3,7,8-TeBDD. Les
études immunologiques effectuées ont montré que le système immunitaire
des personnes exposées n'avait pas souffert au cours de ces 13 années.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
La CE50 relative à la croissance de trois algues marines
unicellulaires s'est révélée supérieure à 1 mg de DeBDE par litre. On
ne dispose d'aucune autre information concernant les effets du DeBDE
sur les autres êtres vivants au laboratoire ou dans leur milieu
naturel.
2 Conclusions
2.1 DeBDE
Le DeBDE est largement utilisé comme retardateur de flamme dans
les polymères. S'il entre en contact avec la population générale,
c'est par l'intermédiaire des produits fabriqués à partir de ces
polymères. L'exposition est très faible étant donné que le DeBDE n'est
pas facile à extraire de ces polymères. La toxicité aiguë du DeBDE est
très faible et son absorption au niveau des voies digestives est
minime. On peut donc considérer comme insignifiant le risque que le
DeBDE représente pour la population générale.
C'est sous forme particulaire que le DeBDE peut donner lieu à une
exposition professionnelle. En se prémunissant contre les poussières
au cours de la fabrication et de l'utilisation de ce produit, on peut
efficacement en réduire les risques pour les travailleurs.
Le DeBDE est persistant et il se fixe sur les particules de
matière présentes dans l'environnement; il est vraisemblable qu'il
s'accumule dans les sédiments. En revanche, sa bioaccumulation est
improbable. D'après les données disponibles, la photodécomposition du
DeBDE en milieu aqueux dans l'environnement ne conduit pas à la
formation de diphényléthers bromés inférieurs ni de
bromodibenzofuranes, mais on est mal renseigné sur la décomposition de
ce composé dans d'autres milieux.
On est également très peu renseigné sur la toxicité du DeBDE pour
les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Il peut se former des PBDF et, dans une certaine mesure, des PBDD
lorsque du DeBDE ou des produits qui en contiennent sont chauffés à
300-800°C. Il faut se préoccuper des dangers que ces produits
pourraient représenter,.
Lorsqu'elle est correctement conduite, l'incinération n'entraîne
pas l'émission de quantités importantes de bromodioxines ou de
bromodibenzofuranes. Si la combustion s'effectue dans des conditions
non contrôlées, des PBDF et des PBDD peuvent prendre naissance en
quantités indéterminées. Les risques qui pourraient en résulter pour
l'homme et l'environnement seront étudiés dans un prochain Critère
d'hygiène de l'environnement sur les PBDF et les PBDD.
On a observé la présence de PBDF dans le sang de travailleurs
employés à la production de matières plastiques contenant du DeBDE.
Toutefois aucun effet délétère sur leur santé n'a pu être attribué à
ce type d'exposition. Moyennant des techniques appropriées, il est
possible d'éviter l'exposition des travailleurs aux PBDF.
3 Recommandations
3.1 Recommandations générales
* Il convient que les travailleurs qui sont employés à la
fabrication de DeBDE et de produits qui en contiennent soient
protégés contre toute exposition par l'application de mesures
appropriées d'hygiène industrielle, la surveillance de
l'exposition professionnelle et des moyens techniques appropriés.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions produits par
les industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé persistant et ses
produits de décomposition.
* Les fabricants doivent faire en sorte que le DeBDE commercial
contienne le minimum d'impuretés, en utilisant pour cela les
meilleures techniques existantes. Une pureté d'au moins 97% est
recommandée.
* On ne doit procéder à l'incinération qu'au moyen d'incinérateurs
convenables, qui fonctionnent toujours dans les conditions
optimales. Le brûlage des déchets par tout autre moyen risque
d'entraîner la formation de PBDF et/ou de PBDD.
3.2 Etudes à effectuer
* Il conviendrait d'effectuer, sur les organismes appropriés, des
études sur la biodisponibilité et la toxicité du DeBDE lié aux
sédiments.
* Il importe d'assurer une surveillance permanente des
concentrations dans l'environnement.
* La production de PBDF en situation réelle d'incendie doit faire
l'objet d'études plus approfondies.
* Il importe d'étudier plus à fond la biodécomposition dans
l'environnement ainsi que la photodécomposition de ce composé dans
des milieux autres que l'eau.
* Il conviendrait d'étudier des méthodes pour le recyclage des
polymères contenant du DeBDE et leurs conséquences.
* Il faudrait valider les méthodes d'analyse du DeBDE dans diverses
matrices.
NONABROMODIPHENYLETHER
Le nonabromodiphényléther n'est ni produit ni utilisé. On ne
dispose d'aucune donnée sur les points suivants:
* Transport, distribution et transformation dans l 'environnement
* Concentrations dans l'environnement et exposition humaine
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe aucune base de données sur laquelle se fonder pour une
évaluation.
2 Recommandations
Il importe de réduire au minimum le taux de contamination des
retardateurs de flamme bromés du commerce par le
nonabromodiphényléther afin d'éviter une pollution de l'environnement
et l'exposition humaine.
OCTABROMODIPHENYLETHER
L'octabromodiphényléther n'est ni fabriqué ni utilisé à l'état
pur. On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1. Résumé et évaluation
1.1 Identité et propriétés physiques et chimiques
L'OBDE du commerce se présente sous la forme d'un mélange
d'environ 11% de PeBDE/HxBDE, 44% de HpBDE, 31-35% d'OBDE, 10% de NBDE
et 0,5% de DeBDE. D'après la structure chimique, il y a thé-oriquement
12 isomères de l'OBDE et 24 isomères de l'HpBDE.
Le point de fusion varie d'environ 80°C à >200°C. La tension de
vapeur est <10-7 mmHg, la solubilité dans l'eau est faible et le
logarithme du coefficient de partage n-octanol/eau est >5,5. Ces
variations dans les propriétés physiques s'expliquent par des
différences de composition des mélanges étudiés.
1.2 Production et usages
La consommation mondiale annuelle d'OBDE commercial est de 6000
tonnes, dont 70% sont utilisés comme retardateurs de flamme dans les
résines ABS qui servent à la fabrication d'ordinateurs et d'armoires
de bureau. L'OBDE vient au second rang des retardateurs de flamme dans
l'ordre d'utilisation.
1.3 Transport, distribution et transformation dans l'environnement
On a trouvé des constituants de l'OBDE du commerce dans des
sédiments aquatiques et des tissus adipeux humains. Certains de ces
constituants à plus faible teneur en brome (HxBDE et PeBDE) ont été
observés dans des biotes. On n'a pas décelé d'OBDE, mais on n'a
cherché généralement ni le HpBDE ni le NBDE. Les constituants de
l'OBDE commercial sont vraisemblablement persistants mais, lorsque le
degré de substitution par le brome dépasse 6, la bioaccumulation
devient de plus en plus improbable. On a trouvé chez la carpe un
facteur de bioaccumulation de moins de 2 pour un OBDE commercial.
La pyrolyse de l'OBDE commercial, soit tel quel, soit ajouté à des
polymères comme retardateur de flamme (avec ou sans Sb2O3) à 600°C,
produit des PBDF et, à beaucoup plus faibles concentrations, des PBDD.
Le traitement des résines ABS avec de l'OBDE et du Sb2O3 dans
différentes conditions révèle, que dans les conditions normales, il ne
se forme que de faibles concentrations de PBDF. Lorsque les conditions
sont mauvaises, les concentrations sont beaucoup plus élevées. En
revanche, les concentrations de PBDD sont faibles dans les deux cas.
1.4 Concentrations dans l'environnement et exposition humaine
Dans des échantillons d'eau recueillis au Japon en 1987 et 1988,
on n'a pas pu déceler d'OBDE ni de constituants de l'OBDE du commerce
à plus faible teneur en brome. On a également analysé des échantillons
de sédiments et dans environ 2-6% d'entre eux, on a observé la
présence d'OBDE à des concentrations de 8-22 µg/kg de poids sec. Dans
le sédiment, on a également trouvé des constituants moins substitués
en brome.
Chez des poissons capturés au Japon en 1987 et en 1988, on n'a pas
décelé d'OBDE.
Aux Etats-Unis d'Amérique, on a analysé en 1987 des échantillons
de tissus adipeux humains à la recherche de PBDF et de PBDD. Les
échantillons provenaient de 865 prélèvements associés de manière à
former 48 homologues composites. Ce schéma d'échantillonnage composite
reposait sur 9 divisions censitaires et 3 groupes d'âge. Dans ces
échantillons, on a également constaté la présence de PBDE et les
premiers résultats ont montré que de l'OBDE était également présent
dans 60% des cas avec une concentration estimative allant jusqu'à
8000 ng/kg.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Aucune donnée disponible.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
L'OBDE du commerce présente une faible toxicité aiguë pour les
mammifères de laboratoire. Il n'est pas irritant pour la peau et ne
provoque qu'une légère irritation oculaire chez le lapin. Lors
d'études toxicologiques à court terme (respectivement 4 et 13
semaines), on a observé, chez des rats à qui l'on en avait administré
dans leur nourriture à raison de 100 mg/kg, une augmentation du poids
du foie et des altérations microscopiques caractérisées par la
présence, dans la région centro- et médio-lobulaire, de cellules
parenchymateuses hypertrophiées contenant des structures granulaires.
La gravité de ces lésions hépatiques était plus marquée aux doses
élevées, c'est-à-dire 1000 et 10 000 mg/kg de nourriture. En outre, on
a observé une hyperplasie de la thyroïde. La teneur en brome total de
ces tissus a augmenté au cours de l'étude pour diminuer ensuite
lentement au cours de la période de récupération. Les anomalies
hépatiques étaient réversibles. Lors d'une étude toxicologique par
inhalation au cours de laquelle on a fait respirer de la poussière
micronisée d'OBDE aux animaux (8 h/jour, 14 jours de suite) on n'a pas
observé d'effets à la concentration de 1,2 mg/m3 mais à celle de
12 mg/m3, on a observé des lésions hépatiques analogues à celles qui
avaient été décelées après ingestion du produit.
Chez le rat, des doses relativement faibles d'OBDE du commerce
accroissent d'activité du cytochrome P-450 et induisent les enzymes
des microsomes hépatiques, comme l'UDP-glucuronyl-transférase et la
benz[a]pyrène-hydroxylase. On a également constaté que ce produit
avait un effet porphyrinogène sur les cultures de cellules hépatiques
d'embryon de poulet.
L'étude du pouvoir tératogène de l'OBDE chez le rat a montré qu'à
fortes doses (25,0 et 50,0 mg/kg de poids corporel), ce produit
provoquait des résorptions ou un retard d'ossification en différents
points du squelette ainsi que des malformations foetales. Les
malformations observées aux doses de 25 mg/kg de poids corporel et
au-delà étaient très vraisemblablement liées à la toxicité du produit
pour la mère. Ces malformations n'ont pas été observées aux doses
inférieures ou égaies à 15,0 mg/kg de poids corporel. Chez le lapin,
aucun signe d'activité tératogène n'a été observé, en revanche on a
constaté une certaine foetotoxicité à la dose de 15 mg/kg de poids
corporel qui était également toxique pour la mère. D'après les études
de tératogénicité, la dose sans effets nocifs observables est de
2,5 mg/kg de poids corporel.
Lors de deux études, l'une de 28 jours et l'autre de 90 jours, on
a constaté qu'une dose de 100 mg d'OBDE/kg de nourriture (soit
l'équivalent de 5 mg/kg de poids corporel) ne produisait que des
effets minimes sur le foie. On n'a pas cherché à établir la dose sans
effets observables.
Toutes les épreuves de mutagénicité: synthèse non programmée de
l'ADN, tests sur microorganismes in vitro et recherche d'échanges
entre chromatides-soeurs sur cellules ovariennes de hamster chinois se
sont révélées négatives.
On ne possède aucune donnée résultant d'études de cancéro-génicité
ou d'études à long terme.
1.7 Effets sur l'homme
Aucune donnée disponible.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Données minimales.
2 Conclusions
2.1 OBDE
L'OBDE du commerce est un mélange d'hexa-, d'hepta-, d'octa- et de
nonabromodiphényléthers, qui sont tous des composés rémanents, en
grande partie fixée aux sédiments.
L'OBDE est très largement utilisé comme retardateur de flamme dans
les polymères. Il peut y avoir contact avec la population générale par
l'intermédiaire de produits fabriqués à partir de ces polymères. Il
est peu probable qu'on puisse être exposé à de l'OBDE qui aurait été
extrait de ces polymères.
L'OBDE présente une faible toxicité aiguë. On ne dispose d'aucune
donnée sur sa fixation ou son élimination par l'organisme des
mammifères. Il n'est ni tératogène ni mutagène. On ne dispose d'aucune
donnée sur sa toxicité à long terme ni sur sa cancéro-génicité.
On a relevé la présence, dans des tissus adipeux humains, de
plusieurs constituants de l'OBDE commercial. Pour la population dans
son ensemble, le risque d'intoxication aiguë est vraisemblablement
faible. Il n'est pas possible d'évaluer les risques d'une exposition à
long terme en raison de l'absence d'études toxicologiques pertinentes.
On ne dispose non plus d'aucune information qui permette de tirer
des conclusions quant à l'exposition professionnelle à l'OBDE et à ses
effets.
Les données relatives à la toxicité de l'OBDE pour les êtres
vivants dans leur milieu naturel demeurent limitées. Il est possible
que les constituants peu substitués en brome du mélange commercial
subissent une bioaccumulation.
2.2 Produits de décomposition
Lors du chauffage de l'OBDE ou de produits qui en contiennent à
des températures de 400-800°C, il peut se former des PBDF et, dans une
certaine mesure, des PBDD. Il importe de se préoccuper des dangers qui
pourraient en résulter.
Il est improbable que la population générale soit exposée de façon
notable aux impuretés de type PBDF, présentes dans les polymères
contenant des retardateurs de flamme. Moyennant une incinération dans
les règles, il ne devrait pas y avoir d'émission importante de
bromodioxines ni de bromofuranes. Le brûlage sans précautions de
produits qui contiennent de l'OBDE du commerce, peut donner naissance
à des PBDF et des PBDD en quantités indéterminées. Ce problème et ses
conséquences pour la santé humaine et l'environnement, seront abordés
dans un prochain Critère d'hygiène de l'environnement consacré aux
PBDF et aux PBDD.
3 Recommandations
3.1 Recommandations générales
* Il convient d'utiliser les meilleurs techniques possibles pour la
fabrication de l'OBDE du commerce, afin de réduire au minimum la
teneur en homologues hexabromés ou moins substitués, du fait de
leur possibilité de bioaccumulation dans l'environnement.
* Les travailleurs qui sont employés à la fabrication de l'OBDE ou
de produits qui en contiennent, doivent être protégés contre
l'exposition à ces dérivés par des mesures d'hygiène industrielle,
une surveillance de l'exposition professionnelle et des moyens
techniques.
* Il convient de limiter au maximum la pollution de l'environnement
grâce à un traitement approprié des effluents et des émissions
provenant d'industries qui utilisent le composé ou des produits
qui en contiennent. Le rejet de produits industriels et de
produits de consommation devra être réglementé de façon à réduire
au minimum la pollution de l'environnement par ces composés
rémanents et leurs produits de décomposition.
* L'incinération de produits qui contiennent des retardateurs de
flamme à base d'OBDE, ne doit s'effectuer que dans des
incinérateurs appropriés fonctionnant toujours dans des conditions
optimales. Le brûlage par tout autre moyen risque de donner
naissance à des PBDF ou des PBDD.
3.2 Etudes à effectuer
Comme la base de données toxicologiques actuelle est insuffisante
pour permettre d'évaluer les dangers que représente l'OBDE du commerce
pour l'homme et l'environnement, et pour en faciliter l'utilisation,
il est recommandé d'entreprendre les études suivantes:
* Etudes, sur des organismes appropriés, relatives à la
biodisponibilité et à l'écotoxicité des constituants de l'OBDE du
commerce liés aux sédiments
* Surveillance généralisée de la concentration des constituants de
l'OBDE du commerce dans l'environnement
* Etudes toxicologiques à long terme et études de cancéro-génicité
portant sur l'OBDE du commerce
* Surveillance de l'exposition professionnelle à l'OBDE du commerce
* Poursuite des études sur la formation de PBDF dans des conditions
réelles d'incendie
* Poursuite des études sur la biodécomposition et la
photodécomposition environnementales dans des milieux non aqueux
* Etudes de méthodes de recyclage des polymères contenant de l'OBDE
et de ses conséquences
* Validation des méthodes d'analyse de l'OBDE dans diverses matrices
* Etudes sur la possibilité de migration à partir de divers types de
polymères.
HEPTABROMODIPHENYLETHER
L'heptabromodiphényléther n'est ni produit ni utilisé.
Il n'existe pas de base de données relatives à l'HpBDE pur sur
laquelle fonder une évaluation.
On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
Du fait que l'HpBDE est le principal constituant de
l'octabromodiphényléther du commerce, le résumé, l'évaluation, les
conclusions et les recommandations relatives à l'OBDE du commerce,
valent pour l'HpBDE "du commerce".
HEXABROMODIPHENYLETHER
L'hexabromodiphényléther n'est ni produit ni utilisé mais il
constitue une impureté des bromodiphényléthers du commerce. Il
convient d'en réduire la concentration au minimum afin d'éviter la
contamination de l'environnement et l'exposition de l'homme.
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
On ne dispose d'aucune donnée sur les points suivants:
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
PENTABROMODIPHENYLETHER
Le pentabromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques
Le pentabromodiphényléther du commerce (PeBDE) est un mélange de
tétra-, penta- et hexabromodiphényléther. Il contient
approximativement 50-60% de PeBDE et 24-38% de TeBDE. D'après la
structure chimique, il existe théoriquement 46 isomères du PeBDE et 42
isomères du TeBDE. Les principaux constituants des produits du
commerce semblent être au nombre de 3, à savoir le 2,2',4,4',5'-PeBDE,
le 2,2',4,4'-TeBDE et un analogue non identifié à 5 atomes de brome.
Le point de fusion se situe entre -7 et -3°C et le point
d'ébullition au-delà de 200°C. La tension de vapeur est faible:
<10-7 mmHg et la solubilité dans l'eau, négligeable. Le logarithme
du coefficient de partage n-octanol/eau est >6.
1.2 Production et usage
Le PeBDE est utilisé comme additif dans les résines époxy, les
résines phénoliques, les polyesters et le polyuréthanne ainsi que les
textiles. La consommation mondiale est d'environ 4000 tonnes par an.
C'est l'un des principaux bromodiphényléthers du commerce utilisés
comme retardateurs de flamme.
1.3 Transport, distribution et transformation dans l'environnement
On trouve des constituants du PeBDE du commerce dans les biotes,
les sédiments et les boues d'égouts. Il est vraisemblable que ces
constituants soient rémanents et qu'ils aient tendance à la
bioaccumulation. C'est ainsi que l'on a trouvé chez la carpe un
facteur de bioconcentration de plus de 10 000.
L'étude de la pyrolyse du PeBDE du commerce a montré qu'il se
forme au cours de ce processus des PBDF et des PBDD. La température
optimale de formation de ces composés se situe entre 700 et 800°C.
Lorsque la pyrolyse du PeBDE s'effectue en l'absence d'oxygène, il y a
formation de polybromobenzènes, de polybromophénols et de PBDF.
1.4 Concentrations dans l'environnement et exposition humaine
Dans des échantillons de sédiments prélevés au Japon dans des
cours d'eau et des estuaires, on a relevé des concentrations allant de
0 (<2 µg/kg)jusqu'à 28 µg/kg de poids sec de PeBDE. En Suède, les
sédiments de certains cours d'eau accusaient des concentrations allant
jusqu'à 1200 µg de 2,2',4,4',5'-PeBDE/kg. Des boues d'égouts,
analysées en Suède, contenaient également de ce PeBDE.
Des moules et poissons ont été prélevés aux fins d'analyse dans
diverses zones littorales du Japon entre 1981 et 1985; dans deux
échantillons de moules sur cinq, on a trouvé des concentrations de
PeBDE qui étaient respectivement égaies à 0,4 et 2,8 µg/kg de poids
humide. En revanche, dans le poisson, on n'a pas décelé de PeBDE
(limite de dosage <0,2 µg/kg). On a fait état de concentrations de
1,9-22 µg/kg de poids frais dans des échantillons de foie de morue
provenant de la mer du Nord. En Suède, on a trouvé des concentrations
qui se situaient entre 7,2 et 64 µg de 2,2',4,4',5'-PeBDE/kg de
graisse dans des poissons d'eau douce du genre corégone et dans des
harengs de différentes provenances.
Du gras de phoque ( Phoca hispida et phoque gris) prélevé en
Suède en 1979-85, s'est révélé contenir des concentrations moyennes de
2,2',4,4',5'-PeBDE respectivement égaies à 1,7 µg et 40 µg/kg de poids
corporel.
En Suède, dans un mélange d'échantillons de muscles de lapins et
d'élans ainsi que dans de la graisse de rognon de renne, on a
constaté, en 1985-86, la présence de concentrations respectivement
égaies à <0,3 µg, 0,64 µg et 0,26 µg de 2,2',4,4',5'-PeBDE/kg de
graisse.
Dans des échantillons de muscles de balbuzard, obtenus en Suède en
1982-86, on a noté une concentration moyenne de 140 µg de
2,2,',4,4',5'-PeBDE/kg de graisse.
On a constaté qu'au cours des dernières décennies, la
concentration de deux isomères du PeBDE avait augmenté d'un facteur 10
dans les oeufs de guillemots de la Baltique. On a également constaté
que la concentration de ces isomères avait augmenté d'un facteur 4
dans des brochets provenant d'un lac du sud de la Suède. L'analyse de
sédiments de la Baltique représentatifs de plusieurs années
d'échantillonnage indique également qu'il y a eu une augmentation
considérable de la pollution au cours de la dernière décennie.
On est très peu renseigné sur l'exposition humaine mais on peut
estimer en gros, d'après la consommation de poisson des suédois, que
la population de ce pays absorbe individuellement environ 0, 1 µg de
PeBDE par jour.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
La demi-vie du PeBDE n'a été étudiée qu'au niveau de la graisse
périrénale chez le rat. On a ainsi obtenu une valeur comprise entre 25
et 47 jours, en fonction du sexe de l'animal et du type d'isomère en
cause.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le PeBDE du commerce présente une faible toxicité aiguë par voie
orale chez le rat; la toxicité par voie percutanée est également
faible chez le lapin. En exposant pendant une brève durée des rats par
la voie respiratoire et en instillant du PeBDE à des lapins dans le
sac conjonctival, on n'a obtenu que des effets modérés et passagers.
Des études toxicologiques à court terme sur des rats (4 semaines
et 13 semaines respectivement) au cours desquelles du PeBDE a été
administré aux animaux à des concentrations de 100 mg/kg de
nourriture, ont révélé une augmentation du poids du foie et donné lieu
à de légères anomalies histologiques. Ces anomalies consistaient en
une hypertrophie des cellules du parenchyme hépatique, qui avaient un
aspect granulaire et contenaient des "inclusions rondes" éosinophiles.
On a constaté que la teneur en brome total du foie augmentait en
fonction de la dose administrée et restait élevée pendant des périodes
pouvant aller jusqu'à 24 semaines. On a également observé une légère
hyperplasie thyroïdienne réversible.
Après administration par voie orale de doses quotidiennes de PeBDE
ne dépassant pas 0,78 µmol/kg de poids corporel, on a observé
l'induction des enzymes hépatiques et un accroissement de l'activité
du cytochrome P-450 c. Les résultats des épreuves de tératogénicité et
de mutagénicité se sont révélés négatifs.
Aucune étude toxicologique à long terme ou étude de
cancérogénicité n'a été publiée.
1.7 Effets sur l'homme
Pas de données disponibles.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On ne dispose que d'un minimum de données.
2 Conclusions
2.1 PeBDE
Le PeBDE du commerce (mélange de 24-38% de tétra-, 50-60% de
penta- et 4-8% d'hexabromodiphényléther) est rémanent et il s'accumule
chez les êtres vivants dans leur milieu naturel.
Le PeBDE du commerce est largement utilisé, incorporé à des
polymères, comme retardateur de flamme. Lorsqu'il entre en contact
avec la population, c'est par l'intermédiaire de produits fabriqués à
partir de ces polymères. Il est improbable qu'il puisse y avoir
exposition après extraction du produit de ces polymères. Il peut y
avoir exposition de l'homme au PeBDE par l'intermédiaire de la chaîne
alimentaire, étant donné que la substance a été décelée chez divers
animaux vivant dans leur milieu naturel, animaux qui servent de
nourriture à l'homme, comme les poissons, les fruits de mer, etc. On
constate depuis au moins deux décennies que les concentrations
augmentent chez les poissons et les oiseaux de Suède.
Le PeBDE du commerce présente une faible toxicité aiguë. On ne
possède aucune donnée sur la fixation et l'élimination de ce produit
chez les mammifères. On ne dispose pas non plus d'études sur la
reproduction d'animaux exposés à cette substance, ni sur sa toxicité à
long terme ou sa cancérogénicité.
Il n'est pas possible, à partir des données disponibles, d'évaluer
le risque qu'il représente pour la population générale.
On ne possède pas non plus de données qui permettent d'établir le
niveau d'exposition professionnelle ou de se prononcer sur les effets
du PeBDE ?? du commerce.
On ne dispose que de données limitées sur la toxicité du PeBDE du
commerce pour les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Lorsqu'on chauffe du PeBDE ou des produits qui en contiennent à
une température de 400-800°C, il se forme des PBDF et, dans une
certaine mesure, des PBDD. Il faudra examiner les risques qui
pourraient en découler.
Il est peu probable que la population générale soit exposée de
façon notable aux PBDF produits par des polymères contenant du PeBDE
comme retardateur de flamme. Si elle est convenablement effectuée,
l'incinération n'entraîne pas l'émission de quantités importantes de
bromodioxines ou de bromofuranes. En revanche, le brûlage inconsidéré
de produits qui en contiennent peut donner naissance à des quantités
indéterminées de PBDF ou de PBDD. Dans un futur Critère d'hygiène de
l'environnement consacré à ces deux substances, l'importance de ce
problème pour l'homme et l'environnement sera examinée.
3 Recommandations
3.1 Recommandations générales
La rémanence du PeBDE du commerce dans l'environnement et son
accumulation dans les êtres vivants militent contre l'utilisation de
ce produit. Toutefois, s'il l'on continue à l'utiliser, il faudra
prendre les précautions suivantes:
* Les travailleurs qui sont employés à la production de PeBDE ou de
polymères qui en contiennent, doivent être protégés contre toute
exposition, par des mesures appropriées d'hygiène industrielle, la
surveillance de l'exposition professionnelle et des moyens
techniques convenables.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions produits par
les industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé qui persiste et
s'accumule et par ses produits de décomposition.
* On ne doit procéder à l'incinération qu'au moyen d'incinérateurs
convenables fonctionnant toujours dans des conditions optimales.
Le brûlage des déchets par tout autre moyen risque d'entraîner la
formation de produits de décomposition toxiques.
3.2 Etudes à effectuer
* Il faut poursuivre la surveillance des concentrations dans
l'environnement.
* Il conviendrait de valider les méthodes de dosage du PeBDE dans
diverses matrices.
* Du fait que la base de données toxicologiques actuelle est
insuffisante pour permettre d'évaluer des risques pour l'homme et
l'environnement du PeBDE du commerce et en justifier
l'utilisation, il conviendrait d'entreprendre les éludes
suivantes:
- études complémentaires de cancérogénicité et études
toxicologiques et écotoxicologiques:
- poursuite des travaux sur la formation de PBDF dans les
conditions réelles d'incendie;
- étude de méthodes pour le recyclage des polymères contenant du
PeBDE et de leurs conséquences;
- études sur les possibilités de migration à partir de produits
contenant des retardateurs de flamme.
TETRABROMODIPHENYLETHER
Le tétrabromodiphényléther n'est ni produit ni utilisé.
Il n'existe aucune donnée sur les points suivants:
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le tétrabromodiphényléther du commerce est constitué d'un mélange
de 41% de tétra-, 45% de penta-, et 7% d'hexabromo-diphényléthers plus
environ 7% d'un PBDE de structure inconnue. D'après la structure
chimique, le tétrabromodiphényléther a théoriquement 42 isomères. On
ne dispose pratiquement d'aucune donnée sur les propriétés physiques
et chimiques de ce composé, à part le logarithme du coefficient de
partage n-octanol/eau, qui est 5,87-6,16.
1.2 Production et usages
D'après un rapport, la production (et l'usage) du TeBDE aurait été
d'environ 1000 tonnes en 1987 au Japon. Autant qu'on sache, il
n'existe actuellement aucune production sous le nom de
tétra-bromodiphényléther, mais le composé est présent dans la
proportion de 24 à 38% dans le pentabromodiphényléther du commerce.
1.3 Transport, distribution et transformation dans l'environnement
On a trouvé des constituants du TeBDE du commerce dans des biotes,
des sédiments et des boues d'égouts. Il est probable que les
constituants du TeBDE du commerce (qui contiennent des quantités à peu
près équivalentes de PeBDE) aient un caractère rémanent et subissent
une bioaccumulation.
L'étude de la pyrolyse du TeBDE du commerce montre qu'à 800°C, il
se forme des PBDF et des PBDD, mais uniquement les homologues
inférieurs.
1.4 Concentrations dans l'environnement et exposition humaine
Au Japon, on a trouvé du TeBDE dans les sédiments de cours d'eau à
des concentrations de 12-31 µg/kg de poids sec et en Suède, à des
concentrations allant jusqu'à 840 µg/kg (perte au feu). La présence de
TeBDE a été également constatée en Suède dans des boues d'égouts à la
concentration de 15 µg/kg.
Au Japon, on a constaté, dans des moules et des poissons de
différentes provenances, la présence de TeBDE à des concentrations
allant de <0, 1 à 14,6 µg de 2,2',4,4'-TeBDE/kg de poids humide. En
Suède, on a capturé dans des cours d'eau diverses espèces de poissons
que l'on a analysés à la recherche de 2,2',4,4'-TeBDE. Les
concentrations moyennes allaient de 0 (<0,1 mg/kg) à 100 mg/kg de
graisse. L'analyse a montré qu'il existait au moins une source de
pollution locale dans un certain cours d'eau. Des corégones, des
ombles arctiques et des harengs pêchés en différents lieux de Suède
entre 1986 et 1987 se sont révélés contenir des concentrations de
TeBDE respectivement égaies à 15, 400 et 59-450 µg de
2,2',4,4'-TeBDE/kg de graisse. En Allemagne, des poissons provenant de
différents cours d'eau en contenaient jusqu'à 1 mg/kg de graisse.
Dans les harengs et les foies de morues pêchés dans la partie
méridionale, centrale et septentrionale de la mer du Nord, au cours de
la période 1983-89, on a noté une tendance à la diminution des
concentrations de TeBDE de la région méridionale à la région
septentrionale. Dans les harengs, la concentration allait de 8,4 à
100 µg de 2,2',4,4'-TeBDE/kg de graisse.
Dans le tissu musculaire d'oiseaux nichant et hivernant en mer
Baltique, en mer du Nord et au Spitzberg, on a relevé des
concentrations de 2,2',4,4'-TeBDE de 80-370 µg/kg de graisse. Chez des
balbuzards capturés en Suède au cours de la période 1982-86, on a
mesuré des concentrations moyennes de 1800 µg/kg de graisse.
En Suède, on constate une tendance à l'augmentation des
concentrations de 2,2',4,4'-TeBDE dans les sédiments de la Baltique,
les poissons d'eau douce et les oeufs d'oiseaux de mer.
Dans la graisse de phoques de la mer Baltique et du Spitzberg on a
observé des concentrations de 10-730 µg/kg de graisse.
Chromatographiquement, le PBDE est analogue au Bromkal 70-5. Sur des
échantillons groupés de graisse de phoques de l'espèce Phoca hispida
et de phoques gris, prélevés en Suède au cours de la période 1979-85,
on a mesuré des concentrations respectivement égaies à 47 µg et 650 µg
de 2,2',4,4'-TeBDE/kg.
Dans des échantillons groupés de muscles de mammifères terrestres,
par exemple des lapins, des élans et des rennes, obtenus en 1985-86 en
Suède, on a observé des concentrations moyennes respectivement égaies
à <2, 0,82, et 0,18 µg de 2,2',4,4'-TeBDE/kg de graisse.
En Allemagne, on a trouvé dans quatre échantillons de lait de
vache, des concentrations de 2,5-4,5 µg de PBDE/kg de matière grasse,
sous la forme de Bromkal 70DE. Dans le même pays et sous cette même
forme, on a retrouvé du PBDE dans le lait de 25 femmes à des
concentrations de 6,2-11,1 µg/kg de matière grasse.
Une estimation approximative de l'exposition de la population
suédoise, calculée en fonction de la consommation de poisson en Suède,
donne une absorption individuelle de 0,3/µg de TeBDE/jour.
1.5 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Il n'existe pas de données sur le TeBDE lui-même, cependant on
dispose de données de toxicité aiguë et de toxicité à court terme pour
le PeBDE du commerce qui contient 41% de TeBDE.
1.6 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
On ne possède qu'un minimum de données.
1.7 Effets sur l'homme
Il n'existe aucune donnée.
1.8 Effets sur les autres vivants au laboratoire et dans leur milieu
naturel
Il n'existe aucune donnée.
2 Conclusions
2.1 TeBDE
Les constituants du TeBDE du commerce (mélange à 41% de
2,2',4,4'-tétra-; 45% de 2,2',4,4',5'-penta; 7% d'hexa-, et 7-8% de
polybromodiphényléther de structure inconnue) persistent dans
l'environnement et s'accumulent chez les êtres vivants dans leur
milieu naturel.
Constituant du pentabromodiphényléther, le TeBDE est très souvent
incorporé à des polymères comme retardateur de flamme. La population
générale peut entrer en contact avec cette substance par
l'intermédiaire de produits fabriqués à partir de ces polymères. Il
est improbable qu'il puisse y avoir exposition par suite de
l'extraction du composé de ces polymères. Il peut y avoir exposition
humaine a TeBDE par l'intermédiaire de la chaîne alimentaire car on a
décelé la présence de ce composé chez des animaux dans leur milieu
naturel, animaux qui servent de nourriture à l'homme comme les
poissons, les fruits de mer, etc. En Suède, on observe depuis les deux
dernières décennies une augmentation des concentrations de ce composé
chez les poissons et les oiseaux.
On manque d'information au sujet des études toxicologiques à court
et à long terme, des éludes de cancérogénicité ou sur la reproduction
qui auraient pu être faites. En outre, on ne dispose pas de données
sur la cinétique et le métabolisme du produit chez les animaux de
laboratoire et l'homme.
Il n'est pas possible d'évaluer le risque pour la population
générale sur la base des données disponibles.
On ne dispose pas non plus de données suffisantes pour déterminer
les niveaux d'exposition professionnelle ni pour se prononcer sur les
effets du TeBDE.
Il n'existe aucune donnée sur la toxicité de TeBDE du commerce
pour les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Lorsqu'on chauffe du TeBDE à 800°C, il se forme des PBDF et des
PBDD. Il faudra étudier les dangers que cela peut comporter.
Il n'est guère probable que la population générale soit exposée de
façon importante aux PBDF qui se forment lorsque l'on chauffe des
polymères contenant du TeBDE comme retardateur de flamme. Si
l'incinération est effectuée correctement, elle n'entraîne pas
l'émission de bromodioxines ni de bromofuranes en quantités
importantes. Le brûlage inconsidéré de produits contenant du TeBDE
peut conduire à la formation de PBDF ou de PBDD en quantités
indéterminées. Dans un futur Critère d'hygiène de l'environnement
consacré aux PBDF et aux PBDD, l'importance de ce problème pour
l'homme et l'environnement sera examinée.
3 Recommandations
3.1 Recommandations générales
Du fait de sa rémanence dans l'environnement et de son
accumulation chez les êtres vivants, il est recommandé de ne pas
utiliser le TeBDE. Cependant si son usage se poursuit, il faudra
prendre les précautions suivantes:
* Les travailleurs qui sont employés à la production de TeBDE et de
produits qui en contiennent, doivent être protégés contre toute
exposition grâce à des mesures appropriées d'hygiène industrielle,
par la surveillance de l'exposition professionnelle et par des
mesures techniques adéquates.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions provenant des
industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé persistant et
cumulatif et ses produits de décomposition.
* On ne doit procéder à l'incinération de produits contenant un
retardateur de flamme à base TeBDE qu'au moyen d'incinérateurs
convenables fonctionnant toujours dans des conditions optimales.
Le brûlage des déchets par tout autre moyen risque d'entraîner la
formation de produits de décomposition principalement formés de
furanes.
3.2 Etudes à effectuer
* Il importe d'assurer une surveillance permanente des
concentrations dans l'environnement.
* Il faudrait valider les méthodes d'analyse du TeBDE dans diverses
matrices.
* Comme la base de données toxicologiques actuelle est insuffisante
pour permettre d'évaluer le danger que le TeBDE représente pour
l'homme et l'environnement, il faudra, si on continue à utiliser
ce produit, effectuer les travaux suivants:
- études toxicologiques et écotoxicologiques supplémentaires et
études de cancérogénicité;
- poursuite des études sur la formation de PBDF dans les
conditions réelles d'incendie;
- étude des méthodes de recyclage des polymères contenant du
TeBDE et de ses conséquences;
- recherches sur la possibilité de migration à partir de
produits contenant des retardateurs de flamme.
TRIBROMODIPHENYLETHER
Le tribromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Transport, distribution et transformation dans l'environnement
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
2 Recommandations
Il convient de réduire au minimum la contamination des produits du
commerce par du tribromodiphényléther afin d'éviter la pollution de
l'environnement et l'exposition humaine.
Il faut éviter d'utiliser des produits commerciaux susceptibles
d'entraîner la pollution du milieu.
DIBROMODIPHENYLETHER
Le dibromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
2 Recommandations
Il convient de réduire au minimum la contamination des produits du
commerce par du dibromodiphényléther afin d'éviter la pollution de
l'environnement et l'exposition humaine.
Il faut éviter d'utiliser des produits commerciaux susceptibles
d'entraîner la pollution du milieu.
MONOBROMODIPHENYLETHER
Il n'existe aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Evaluations antérieures par des organismes internationaux.
1. Résumé et évaluation
1.1 Propriétés physiques et chimiques
Il y a trois isomères possible du monobromodiphényléther.
Le p-bromodiphényléther se présente à la température ambiante
sous la forme d'un liquide dont le point d'ébullition est de
305-310°C. Le calcul montre que sa solubilité dans l'eau est de
48 mg/litre. Le logarithme de son coefficient de partage
n-octanol/eau se situe entre 4 et. 5. Sa tension de vapeur est de
0,0015 mmHg à 20°C.
1.2 Production et usages
Le MBDE n'est pas utilisé comme retardateur de flamme. Un rapport
sur sa production est paru en 1977, mais on ne lui connaît pas
d'usage.
1.3 Transport, distribution et transformation dans l'environnement
Sa demi-vie d'évaporation à partir de l'eau est de l'ordre de
plusieurs centaines de jours.
Placé dans une culture de 7jours ensemencé avec des micro-
organismes présents dans des eaux usées domestiques, il ne subit
pas de biodécomposition importante, mais il est dégradé à 90% dans des
boues d'égouts activées. D'après la seule étude consacrée à sa
dégradation par les bactéries terricoles, il semblerait que celles-ci,
tout au moins une souche particulière, soient incapables d'utiliser le
MBDE comme seule source de carbone.
1.4 Concentrations dans r environnement et exposition humaine
On a décelé la présence de MBDE dans des échantillons d'eau
superficielle prélevés à proximité de sites industriels aux Etats-Unis
d'Amérique; toutefois, une enquête analogue menée au Japon n'a pas
donné de résultats. On en a également décelé aux Etats-Unis d'Amérique
dans des eaux souterraines à proximité d'une usine. Aux Etats-Unis
d'Amérique, on a décelé la présence de MBDE dans des sédiments et des
biotes aquatiques.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Il n'existe pas de donnée.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le MBDE n'est pas tératogène, mais on ne possède aucune donnée sur
la toxicité aiguë ni la toxicité à court et à long terme de ce
composé; aussi est-il impossible de procéder à une évaluation.
1.7 Effets sur l'homme
Il n'existe aucune donnée.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Chez un poisson, Lepomis macrochirus, la CL50 à 96 heures
serait de 4,9 mg/litre, avec une concentration sans effets observables
de moins de 2,8 mg/litre. La CL50 à 48 heures pour la puce d'eau est
de 0,36 mg/litre avec une concentration sans effets observables de
moins de 0,046 mg/litre.
2 Conclusions et recommandations
Le monobromodiphényléther n'agit pas comme retardateur de flamme.
Il peut s'accumuler chez les êtres vivants dans leur milieu naturel et
on en a décelé la présence dans divers secteurs de l'environnement. Il
semblerait qu'il puisse subir une biodécomposition.
Ces données sont trop limitées pour qu'on puisse parvenir à des
conclusions quant aux niveaux d'exposition et aux effets que ce
composé peut avoir sur la population générale et les êtres vivants
dans leur milieu naturel.
Il n'existe aucune base de données toxicologiques qui plaide en
faveur de l'utilisation de ce produit.
Il faut éviter toute utilisation susceptible de conduire à une
pollution de l'environnement.
RESUMEN, EVALUACIN, CONCLUSIONES Y RECOMENDACIONES
ÉTER DE DECABROMODIFENILO
1. Resumen y evaluación
1.1 Identidad y propiedades fisicas y quimicas
El éter de decabromodifenilo (DeBDE) comercial suele tener una
pureza del 97-98%, con un 0,3-3,0% de éteres de difenilo nona- y
octabromados. La impureza mas importante es el éter de
nonabromodifenilo (NBDE). A diferencia de los otros éteres de difenilo
polibromados, el DeBDE tiene un solo isomero.
El punto de fusion es de unos 300°C y la descomposicion se produce
por encima de los 400°C. La solubilidad en agua es de 20-30 µg/litro y
el logaritmo del coeficiente de reparto n-octanol/agua es superior a
5. La presion de vapor es <10-6 mm de Hg a 20°C.
1.2 Produccion y aplicaciones
Entre los éteres de difenilo bromados (del mono- al deca-), el
éter de decabromodifenilo es el mas importante de fabricacion
comercial en cuanto a produccion y aplicaciones.
A partir del decenio de 1970 se ha elaborado DeBDE comercial cada
vez mas puro. Su produccion mundial es de unas 300 000 toneladas al
ano. Se utiliza como aditivo pirorretardante en numerosos plasticos,
sobre todo en el poliestireno de gran resistencia al impacto, en el
tratamiento de tejidos utilizados en pasamaneria, telas de automoviles
y tiendas de campana.
1.3 Transporte, distribucion y transformacion en el medio ambiente
La fotodegradacion del DeBDE tiene lugar en disolventes organicos
con radiacion ultravioleta (RUV) o la luz del sol y produce éteres de
difenilo con un numero menor de atomos de bromo y dibenzofuranos
bromados. También se produce fotodegradacion, en menor grado, en el
agua bajo la accion de la luz del sol; sin embargo, no se ha detectado
la presencia de éteres de difenilo menos bromados ni tampoco de
dibenzofuranos bromados.
En funcion del tipo de polimero y del disolvente de extraccion se
obtiene una concentracion de DeBDE que esta casi en el limite de
deteccion o es inferior a él.
Debido a que la solubilidad en agua y la presion de vapor son
extremadamente bajas, es probable que el transporte del DeBDE se
produzca sobre todo mediante adsorcion sobre particulas. Es
persistente y se acumula facilmente en el sedimento y en el suelo.
No se dispone de datos acerca de su biodisponibilidad a partir del
sedimento y del suelo. En un estudio realizado en la trucha irisada no
se detecto bioacumulacion en la carne, la piel o las visceras durante
mas de 48 horas. No es probable la bioacumulacion del DeBDE, debido a
su peso molecular relativamente alto.
A la larga, los productos que contienen DeBDE comercial se
eliminan en vertederos o mediante incineracion. El DeBDE, en ultimo
término, se puede lixiviar de los vertederos. Se pueden producir
dibenzofuranos polibromados (PBDF) y mezclas de dibenzofuranos y
dibenzodioxinas halogenados en incendios de los vertederos o por una
incineracion incompleta. Los productos que contienen DeBDE comercial
pueden contribuir a estas emisiones.
La pirolisis del propio DeBDE comercial y de los polimeros (HIPS,
PBT, polipropileno industrial) con DeBDE en presencia de oxigeno
produjo PBDF, detectandose en menor grado dibenzodioxinas polibromadas
(PBDD). La formacion maxima de PBDF tiene lugar a 400-500°C, pero se
puede producir a temperaturas de hasta 800°C; el Sb2O3 ejerce una
funcion catalitica en la formacion de PBDF y PBDD.
La formacion de PBDF y PBDD y las cantidades encontradas dependen
de la temperatura, el contenido de oxigeno y la duracion de la
pirolisis. En ausencia de oxigeno, se forman sobre todo
polibromobencenos y polibromonaftalenos.
1.4 Niveles medioambientales y exposicion humana
En la vecindad de las instalaciones de fabricacion se han
identificado concentraciones de DeBDE de hasta 25 µg/m3. No se
detecto en las muestras de agua recogidas en el Japon durante el
periodo de 1977-91. Sin embargo, se observo en el sedimento de rios
recogido en el Japon durante el mismo periodo a concentraciones de
hasta unos 12 mg/kg de peso seco. En los Estados Unidos se encontro
DeBDE (hasta 1 g/kg) en el sedimento de los rios cercanos a una
fabrica. No se detecto su presencia en las muestras de peces recogidas
en el Japon, aunque en una muestra de mejillones se observo una
concentracion ligeramente superior al nivel de deteccion. Aunque no se
encontro en las muestras de tejido adiposo humano recogidas en el
Japon, en los Estados Unidos se observo en 3 de 5 muestras de este
tipo de tejido.
La exposicion humana al DeBDE se puede producir en el curso de la
fabricacion y la formulacion en polimeros. La exposicion de la
poblacion general al DeBDE es insignificante.
En la determinacion de la exposicion en el trabajo a los productos
de degradacion del DeBDE durante la fabricacion, formulacion o
utilizacion se puso de manifiesto que las muestras de aire proximas al
cabezal del extrusor contenian concentraciones elevadas de PBDF. En el
aire de la sala de trabajo se encontraron niveles mas bajos. Se
detecto asimismo en muestras del material de limpieza. Se ha
demostrado que la aplicacion de buenas técnicas industriales reduce la
exposicion ocupacional al PBDF.
No es probable una exposicion significativa de la poblacion
general a las impurezas de PBDF de los polimeros con caracteristicas
pirorretardantes.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
El DeBDE tiene una escasa absorcion desde el tracto
gastrointestinal y se excreta con rapidez tras su inyeccion.
Los resultados de los estudios metabolicos en ratas utilizando
DeBDF marcado con 14C indicaron una semivida para su desaparicion del
organismo de menos de 24 horas, siendo la ruta principal de
eliminacion tras la ingestion oral la via fecal. En la orina o el aire
expirado no se encontro una actividad apreciable de 14C (inferior al
1%).
Las ratas alimentadas con 0,1 mg/kg de peso corporal al dia,
durante un periodo de hasta dos anos, no mostraron acumulacion de
DeBDF en el suero, los rinones, los musculos o los testiculos, como se
comprobo mediante una determinacion del bromo total. La acumulacion de
bromo en el higado alcanzo un nivel estacionarlo a los 30 dias y
desaparecio en un periodo de 10 dias después del tratamiento. Tras
180 dias de tratamiento, el nivel de bromo en el higado de las ratas
tratadas no era superior al de las testigo. En el tejido adiposo se
acumulo una concentracion baja de bromo total, que se mantenia tras
90 dias con una alimentacion sin DeBDE; se desconoce la naturaleza del
bromo acumulado. Habida cuenta de que el DeBDE representaba solo el
77% de la mezcla comercial utilizada, el "bromo" podria proceder del
NBDE o el OBDE.
1.6 Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
La toxicidad aguda del DeBDE en los animales de laboratorio es
baja. La sustancia no es irritante para la piel o los ojos de los
conejos. No es cloracnegénico en la piel de los conejos, ni tampoco
sensibilizador cutaneo humano.
Se realizaron pruebas de toxicidad aguda y de comedogenicidad de
los productos de la combustion del poliestireno pirorretardante con
DeBDE y Sb2O3. La DL50 por via oral del hollin y la carbonilla fue
> 2000 mg/kg de peso corporal.
No se observo induccion de efectos adversos en los estudios de
toxicidad a cono plazo realizados en ratas y ratones con niveles de
DeBDE (pureza > 97%) de 100 g/kg (4 semanas) o 50 g/kg de alimentos
(13 semanas, equivalente a 2500 mg/kg de peso corporal de la rata). En
un estudio de reproduccion de una sola generacion en ratas no se
observaron efectos adversos con niveles de dosis de 100 mg/kg de peso
corporal. El DeBDE no produjo efectos teratogénicos en los fetos de
las ratas que recibieron dosis de 100 mg/kg de peso corporal. Con
1000 mg/kg de peso corporal se detectaron malformaciones como el
retraso de la osificacion. En varias pruebas realizadas no se ha
observado que tenga efectos mutagénicos.
En un estudio de carcinogenicidad en ratas y ratones, se
administraron concentraciones de DeBDE (pureza 94-99%) de hasta
50 g/kg de alimentos. Se observo un aumento en el numero de adenomas
hepaticos (pero no de carcinomas) de las ratas macho que recibieron
25 g/kg y en las ratas hembra con 50 g/kg. En ratones macho tratados
con 25 g/kg se detecto una frecuencia mayor de adenomas y carcinomas
hepatocelulares (combinados) y con ambos niveles de dosis un aumento
de adenomas/carcinomas (combinados) de las células foliculares del
tiroides. En los ratones hembra no se aprecio ningun incremento de la
frecuencia de tumores. Se obtuvo una prueba equivoca de
carcinogenicidad en ratas macho y hembra y en ratones macho solo con
dosis de 25-50 g de DeBDE/kg de alimentos. Dado que los resultados de
todas las pruebas de mutagenicidad han sido negativos, se puede
concluir que el DeBDE carece de carcinogenicidad genotoxica. IARC
(1990) concluyo que las pruebas de carcinogenicidad del DeBDE en los
animales de experimentacion eran limitadas. Los niveles de
dosificacion muy elevados, la falta de genotoxicidad y las pruebas
minimas de carcinogenicidad ponen de manifiesto que el DeBDE, a las
concentraciones de exposicion actuales, no representa un riesgo de
carcinogénesis para el ser humano.
1.7 Efectos en el ser humano
No se detectaron signos de sensibilizacion cutanea en una prueba
realizada con 200 personas expuestas al DeBDE.
En un estudio de morbilidad realizado con el personal del extrusor
que mezcla polibutilenterftalato que contiene DeBDE, con la
consiguiente exposicion potencial al PBDD y al PBDF durante 13 anos,
no se observaron efectos nocivos, aun cuando se detectaron en sangre
2,3,7,8-TeBDF y -TeBDD. Los resultados de los estudios inmunologicos
pusieron de manifiesto que el sistema inmunitario de las personas
expuestas no se habia visto negativamente afectado después de 13 anos.
1.8 Efectos en otros organismos en d laboratorio y en el medio
ambiente
Las CE50s para el crecimiento de tres algas unicelulares marinas
fueron superiores a 1 mg de DeBDE/litro. No se dispone de mas
informacion sobre los efectos del DeBDE en otros organismos en el
laboratorio y en el medio ambiente.
2 Conclusiones
2.1 DeBDE
El DeBDE se utiliza ampliamente en polimeros como aditivo
pirorretardante. El contacto de la poblacion general se produce a
través de productos fabricados con dichos polimeros. La exposicion es
muy escasa, puesto que no es facil extraer el DeBDE de los polimeros.
La toxicidad aguda es muy baja y la absorcion del tracto
gastrointestinal minima. Asi pues, el riesgo para la poblacion general
se puede considerar insignificante.
La exposicion en el trabajo se da con el DeBDE en forma de
particulas. El control del polvo. durante la fabricacion y el empleo
reducira el riesgo para los trabajadores de manera suficiente.
El DeBDE es persistente y se une a particulas del medio ambiente;
es facil que se acumule en el sedimento, pero no es probable su
bioacumulacion. Aunque las pruebas disponibles indican que la
fotodegradacion ambiental en agua no producen éteres de difenilo o
dibenzofuranos con menor numero de atomos de bromo, apenas se conoce
nada sobre la degradacion en otros medios.
La informacion sobre la toxicidad del DeBDE para los organismos
del medio ambiente es minima.
2.2 Productos de degradacion
Se puede producir PBDF y, en cierta medida, PBDD cuando se somete
el DeBDE, o los productos que lo contienen, a temperaturas de
300-800°C. Hay que estudiar los posibles peligros asociados a estos
productos.
La incineracion controlada de manera adecuada no produce una
emision de cantidades significativas de dioxinas o furanos bromados.
Cualquier combustion no controlada de productos con DeBDE puede dar
lugar a una formacion no cuantificada de PBDF/PBDD. De su importancia
para el ser humano y el medio ambiente se ocupara un futuro numero de
Criterios de Salud Ambiental.
Se ha detectado PBDR en la sangre de las personas que trabajan en
la produccion de plasticos con DeBDE. No se han observado efectos
adversos relacionados con esta exposicion. Con un buen control técnico
se puede evitar la exposicion de los trabajadores al PBDF.
3 Recomendaciones
3.1 Generales
* Las personas que trabajan en la fabricacion de DeBDE y productos
que lo contienen deben estar protegidas de la exposicion mediante
la aplicacion de medidas adecuadas de higiene industrial, la
vigilancia de la exposicion en el trabajo y controles técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones industriales que
contienen el compuesto o sus productos. Se debe controlar la
eliminacion de los residuos industriales y los productos de
consumo para que sea minima la contaminacion del medio ambiente
con esta sustancia persistente y sus productos de degradacion.
* Los fabricantes deben reducir al minimo los niveles de impurezas
en los productos comerciales de DeBDE, utilizando las mejores
técnicas disponibles. Se recomienda una pureza del 97% o superior.
* La incineracion solo se debe realizar en incineradores adecuados
que funcionen siempre en condiciones optimas. La quema por otros
medios puede dar lugar a la formacion de PBDF y PBDD.
3.2 Otros estudios
* Se deberan realizar en los organismos pertinentes nuevos estudios
sobre la biodisponibilidad y la toxicidad del DeBDE unido al
sedimento.
* Hay que mantener una vigilancia constante de los niveles en el
medio ambiente.
* Se debe investigar con mas detalle la formacion de PBDF en
incendios reales.
* Hay que estudiar mas a fondo la biodegradacion en el medio
ambiente y la fotodegradacion en compartimentos distintos del
agua.
* Se debe investigar los posibles métodos y las consecuencias del
reciclaje de polimeros que contienen DeBDE.
* Hay que validar métodos analiticos para el DeBDE en diversos
aglomerantes.
ÉTER DE NONABROMODIFENILO
El éter de nonabromodifenilo no se fabrica ni se utiliza. No se
dispone de datos sobre los aspectos siguientes:
* Transporte, distribucion y transformacion en el medio ambiente
* Niveles medioambientales y exposicion humana
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay ninguna base de datos sobre la cual realizar una
evaluacion.
2 Recomendaciones
Los niveles de contaminacion de los pirorretardantes bromados
comerciales que contienen éter de nonabromodifenilo deberian ser
minimos a fin de evitar la contaminacion del medio ambiente y la
exposicion humana.
ÉTER DE OCTABROMODIFENILO
El éter de octabromodifenilo puro no se fabrica ni se utiliza. No
se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de octabromodifenilo (OBDE) comercial es una mezcla
formada por alrededor del 11% de PeBDE/HxBDE, 44% de HpBDE, 31-35% de
OBDE, 10% de NBDE y 0,5% de DeBDE. En funcion de su estructura
quimica, existen 12 posibles isomeros del OBDE y 24 del HpBDE.
El punto de fusion oscila entre unos 80°C y >200°C. La presion de
vapor es <10-7 mm de Hg. La solubilidad en agua es baja y el
logaritmo del coeficiente de reparto n-octanol/agua es >5,5. Las
variaciones anteriores obtenidas en los datos fisicos pueden deberse a
diferencias de composicion en las mezclas probadas.
1.2 Produccion y aplicaciones
El consumo anual de OBDE comercial en todo el mundo es de 6000
toneladas, el 70% del cual se utiliza como pirorretardante en ABS para
la produccion de computadores y cabinas comerciales. El OBDE es el
segundo mas utilizado de los pirorretardantes de PBDE.
1.3 Transporte, distribucion y transformacion en el medio ambiente
Se han detectado componentes de OBDE comercial en el sedimento
acuatico y en la grasa humana. En la biota se han encontrado algunos
componentes de menor numero de atomos de bromo (HxBDE y PeBDE) del
producto comercial. No se ha observado OBDE, pero normalmente no se ha
investigado la presencia de HpBDE y NBDE. Es facil que persistan los
componentes comerciales de OBDE, pero a medida que el numero de atomos
de bromo aumenta por encima del HxBDE disminuye la probabilidad de
bioacumulacion. En la carpa se ha encontrado un factor de
bioacumulacion inferior a 2 para un producto de OBDE comercial.
En la pirolisis a 600°C del producto comercial como tal o de
polimeros que lo contienen como aditivo pirorretardante (con Sb2O3 o
sin él) se ha puesto de manifiesto la produccion de PBDF y, en grado
mucho menor, PBDD. El tratamiento de ABS con OBDE/Sb2O3 en
diferentes condiciones permitio demostrar que, en el caso de
tratamiento normal, solo se formaban pequenas concentraciones de PBDF.
En condiciones de uso excesivo, las concentraciones fueron mucho mas
elevadas. Las concentraciones de PBDD fueron bajas en ambos casos.
1.4 Niveles medioambientales y exposicion humana
En una serie de muestras recogidas en el Japon durante 1987 y 1988
no se detecto OBDE ni los componentes de menor numero de atomos de
bromo del producto comercial. También se analizaron muestras del
sedimento y se encontro OBDE en alrededor del 2-6% de las muestras a
concentraciones que oscilaban entre 8 y 22 µg/kg de peso seco. En el
sedimento se observo asimismo la presencia de componentes de menor
numero de atomos de bromo
No se detecto OBDE en las muestras de peces recogidas en el Japon
durante 1987 y 1988.
En los Estados Unidos se investigo la presencia de PBDF Y PBDD en
muestras de grasa humana recogidas en 1987. Las muestras procedian de
865 personas combinadas para formar 48 grupos compuestos analogos. La
composicion se baso en 9 divisiones de censo y 3 grupos de edad. En
estas muestras también se identifico PBDE y los datos preliminares
indicaron la presencia de OBDE con una frecuencia del 60% y una
concentracion estimada de hasta 8000 ng/kg.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
No se dispone de datos.
1.6 Efectos en los animales de laboratorio y en los
sistemas de prueba in vitro
La toxicidad aguda del producto comercial en los animales de
laboratorio es baja. No es irritante cutaneo y solo produce una ligera
irritacion ocular en los conejos. En los estudios de toxicidad a corto
plazo (4 semanas y 13 semanas) se observo que en las ratas que
recibieron dosis de 100 mg/kg de alimentos se produjo un mayor peso
del higado y cambios microscopicos caracterizados por un aumento de
tamano de las células parenquimaticas hepaticas de la zona
centrolobular y media que contienen estructuras granulares. Estos
cambios hepaticos fueron mas graves con dosis mas elevadas, es decir,
1000 y 10 000 mg/kg de alimentos. A esto hay que anadir que se observo
hiperplasia en el tiroides. El contenido total de bromo en los tejidos
aumento durante el estudio y disminuyo lentamente durante un periodo
de recuperacion. Los cambios hepaticos fueron reversibles. En un
estudio de inhalacion con polvo de OBDE micronizado (8 horas al dia
durante 14 dias consecutivos) no se observaron efectos con
exposiciones a 1,2 mg/m3, pero a 12 mg/m3 se produjeron en el higado
los cambios descritos en los estudios de administracion oral.
El OBDE comercial a dosis relativamente bajas produjo en ratas un
aumento del citocromo P450 y la induccion de enzimas microsomicas
hepaticas, como la UDP-glucuronil transferasa y la benzo[alpha]pireno
hidroxilasa. El OBDE comercial indujo efectos porfirinogénicos en
cultivos de células hepaticas de embrion de pollo.
Su potencial teratogénico se probo en ratas; con dosis elevadas
(25,0 y 50,0 mg/kg de peso corporal) se detectaron procesos de
resorcion y osificacion retardada de diferentes huesos, asi como
malformaciones fetales. Las malformaciones observadas tras la
administracion de dosis de 25 mg/kg de peso corporal y superiores
probablemente se debieron en buena parte a la toxicidad materna. Estos
cambios no se advirtieron a dosis de 15,0 mg/kg de peso corporal o
inferiores. No se obtuvieron pruebas de actividad teratogénica en los
conejos, pero se observo fetotoxicidad a una dosis toxica para las
madres de 15 mg/kg de peso corporal. En los estudios de
teratogenicidad se puso de manifiesto la ausencia de efectos a una
dosis de 2,5 mg/kg de peso corporal.
En estudios de 28 dias y 90 dias realizados en ratas se observo
que 100 mg de OBDE por kg de alimentos (equivalentes a 5 mg/kg de peso
corporal) producian efectos minimos en el higado. No se establecio un
nivel sin efectos. Los resultados de las pruebas de mutagenicidad,
incluso con un ensayo de ADN fuera de programa, los ensayos
microbiologicos in vitro y un ensayo de intercambio de cromatidas
hermanas con células de ovario de hamster chino fueron negativos.
No se dispone de resultados de pruebas de carcinogenicidad a largo
plazo.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Los datos disponibles son minimos.
2 Conclusiones
2.1 OBDE
El OBDE comercial es una mezcla de éteres de hexa-,hepta-, octa-,
y nonabromodifenilo, todos ellos persistentes en el medio ambiente,
fundamentalmente unidos al sedimento.
El OBDE se anade con mucha frecuencia a los polimeros como aditivo
pirorretardante. El contacto de la poblacion general tiene lugar
mediante los productos fabricados con estos polimeros. No es probable
la exposicion por extraccion de los polimeros.
La toxicidad aguda del OBDE es baja. No se dispone de informacion
sobre su absorcion y eliminacion en los mamiferos. No es teratogénico
ni mutagénico. No se dispone de estudios de toxicidad y
carcinogenicidad a largo plazo.
En el tejido adiposo humano se han identificado varios componentes
del OBDE comercial. El riesgo grave para la poblacion general
probablemente es bajo. No es posible evaluar el riesgo de exposicion
prolongada, debido a la falta de los estudios de toxicidad
correspondientes.
La falta de informacion no permite sacar conclusiones sobre la
exposicion en el trabajo al OBDE o sus efectos.
La informacion sobre la toxicidad del OBDE para los organismos en
el medio ambiente es limitada. Los componentes de la mezcla de OBDE
comercial con menor numero de atomos de bromo podrian dar lugar a una
bioacumulacion en los organismos.
2.2 Productos de degradacion
Puede formarse PBDF, y en cierto grado PBDD, cuando el OBDE o los
productos que lo contienen se calientan a 400-800°C. Hay que
investigar los posibles peligros relacionados con esta transformacion.
No es probable que la exposicion de la poblacion general a las
impurezas de PBDF en los polimeros pirorretardantes sea significativa.
La incineracion adecuadamente controlada no deberia producir la
emision de cantidades importantes de dioxinas y furanos bromados.
Cualquier combustion no controlada de productos que contienen OBDE
comercial puede dar lugar a la produccion de cantidades no
cuantificadas de PBDF/PBDD. Su importancia para el ser humano y el
medio ambiente sera objeto de estudio en un futuro EHC sobre el
PBDF/PBDD.
3 Recomendaciones
3.1 Generales
* En la fabricacion de OBDE comercial se deben utilizar las mejores
técnicas disponibles, a fin de reducir al minimo los niveles de
hexabromodifenilo y los compuestos analogos de menor numero de
atomos de bromo, debido a su posible bioacumulacion en el medio
ambiente.
* Los trabajadores que intervienen en la fabricacion de OBDE y de
los productos que contienen el compuesto deben estar protegidos de
la exposicion mediante la aplicacion de medidas de higiene
industrial adecuadas, la vigilancia de la exposicion en el trabajo
y controles técnicos.
* Se debe reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de los efluentes y las emisiones en las
industrias que utilizan el compuesto o sus productos. Hay que
controlar la eliminacion de desechos industriales y de los
productos de consumo, a fin de reducir al minimo la contaminacion
del medio ambiente con este material persistente y los productos
de su degradacion.
* La incineracion de materiales con aditivos pirorretardantes a base
de OBDE se debe realizar solo en incineradores adecuados que
funcionen siempre en condiciones optimas. La combustion por
cualquier otro medio puede dar lugar a la produccion de PBDF y
PBDD.
3.2 Otros estudios
Habida cuenta de que la base de datos toxicologica actual no es
adecuada para evaluar los peligros del OBDE comercial para el ser
humano y el medio ambiente y a fin de mejorar su uso se deberian
realizar los siguientes estudios:
* Investigaciones sobre la biodisponibilidad y la ecotoxicidad de
los componentes del OBDE comercial unidos al sedimento utilizando
los organismos pertinentes.
* Mayor vigilancia de los niveles en el medio ambiente de los
componentes de OBDE comercial.
* Estudios de toxicidad y carcinogenicidad a largo plazo del OBDE
comercial.
* Vigilancia de la exposicion en el trabajo al OBDE comercial.
* Otras investigaciones sobre la formacion de PBDF en incendios
reales.
* Nuevos estudios sobre biodegradacion y fotodegradacion en el medio
ambiente en compartimentos diferentes del acuatico.
* Investigacion de posibles métodos y consecuencias del reciclaje de
polimeros que contienen OBDE.
* Validacion de métodos analiticos para el OBDE en varios
aglomerantes.
* Investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE HEPTABROMODIFENILO
El éter de heptabromodifenilo no se fabrica ni se utiliza.
No existe una base de datos sobre la que hacer una evaluacion del
HpBDE.
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
Dado que el HpBDE es el principal componente del éter de
octabromodifenilo comercial, el resumen, evaluacion, conclusiones y
recomendaciones del OBDE comercial son aplicables al HpBDE
"comercial".
ÉTER DE HEXABROMODIFENILO
Aunque el éter de hexabromodifenilo no se fabrica ni se utiliza,
es posible encontrarlo como contaminante de los éteres bromados del
difenilo comerciales. Se debe reducir al minimo tales niveles del éter
de hexabromodifenilo para evitar la contaminacion del medio ambiente y
la exposicion humana.
No existe una base de datos sobre la que hacer una evaluacion.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
ÉTER DE PENTABROMODIFENILO
El éter de pentabromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de pentabromodifenilo comercial (PeBDE) es una mezcla de
éteres de tetra-, penta-, y hexabromodifenilo. Contiene
aproximadamente un 50-60% de PeBDE y un 24-38% de TeBDE. Habida cuenta
de su estructura quimica, hay seis posibles isomeros del PeBDE y 42
del TeBDE. Los productos comerciales parecen estar formados por tres
componentes principales, a saber, 2,2',4,4',5'-PeBDE, 2,2',4,4'-TeBDE
y un compuesto analogo no identificado con cinco atomos de bromo.
El punto de fusion es de -7 a -3°C y el de ebullicion superior a
200°C. La presion de vapor es baja: <10-7 mm de Hg; la solubilidad
en agua es insignificante. El log del coeficiente de reparto
n-octanol/agua es >6.
1.2 Produccion y aplicaciones
El PeBDE se utiliza como aditivo de resinas epoxi, resinas
fenolicas, poliésteres y poliuretano, asi como de materiales textiles.
El consumo mundial es de unas 4000 toneladas anuales. Es uno de los
principales éteres bromados del difenilo pirorretardantes comerciales.
1.3 Transporte, distribucion y transformacion en el medio ambiente
Se han detectado componentes del PeBDE comercial en muestras de
biota, sedimento y fangos cloacales. Es facil que sean persistentes y
tengan capacidad de bioacumulacion. En las carpas se ha encontrado un
factor de bioacumulacion para el PeBDE superior a 10 000.
En los estudios de pirolisis con PeBDE comercial se observo la
formacion de PBDF y PBDD. La temperatura optima de formacion oscilo
entre 700-800°C. Cuando la pirolisis de PeBDE tenia lugar en ausencia
de oxigeno se formaban polibromobencenos, polibromofenoles y PBDF.
1.4 Niveles medioambientales y exposicion humana
En muestras obtenidas de rios y estuarios del Japon se detectaron
niveles que variaban entre la ausencia de PeBDE (<2 µg/kg) y 28 µg/kg
de peso seco. En Suecia, la concentracion en muestras de sedimento de
algunos rios alcanzo un valor de hasta 1200 µg de
2,2',4,4',5'-PeBDE/kg. También en el analisis de fangos cloacales de
Suecia se puso de manifiesto la presencia de PeBDE.
En los mejillones y peces recogidos en diferentes costas del Japon
durante el periodo de 1981-85 se encontraron concentraciones de 0,4 y
2,8 µg de PeBDE/kg de peso humedo de dos de cinco muestras de
mejillones. No se detecto PeBDE en los peces (limite de determinacion
<0,2 µg/kg). Se informo de concentraciones de 1,922 µg/kg de peso
fresco en muestras de higado de bacalao procedente del mar del Norte.
En Suecia se encontraron concentraciones de 7,2 a 64 µg de
2,2',4,4',5'-PeBDE/kg de grasa en corégonos de agua dulce y en
arenques recogidos en diversos lugares.
En una mezcla de grasa de focas oceladas y locas grises
recolectada en Suecia entre 1979 y 1985 se encontro una concentracion
media de 1,7 µg y 40 µg de 2,2',4,4',5'-PeBDE/kg de grasa,
respectivamente.
En mezclas de muestras de musculo de conejo y raton y en muestras
de sebo de reno recogidas en Suecia de 1985 a 1986 se determino una
concentracion <0,3 µg, 0,64 µg y 0,26 µg de 2,2',4,4',5'-PeBDE/kg de
grasa, respectivamente.
Las muestras de musculo de quebrantahuesos recogidas en Suecia en
1982-86 contenian una concentracion media de 140 µg de
2,2',4,4',5'-PeBDE/kg de grasa.
En los ultimos decenios se han multiplicado por diez los niveles
de dos isomeros del PeBDE en los huevos de arao del Baltico. La
concentracion de dichos isomeros en los lucios de un lago de la region
meridional de Suecia también ha experimentado un aumento (del
cuadruple). Se ha observado asimismo un aumento considerable en los
muestreos realizados en el sedimento del Baltico en distintos anos
durante el ultimo decenio.
Aunque se dispone de una informacion muy escasa sobre la
exposicion humana, una estimacion aproximada de la exposicion de la
poblacion sueca a través del consumo de pescado parece indicar una
absorcion diaria de 0,1 µg de PeBDE/persona.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
La semivida del PeBDE solo se ha investigado en la grasa
perirrenal de ratas. La semivida media oscilo entre 25 y 47 dias, en
funcion del sexo del animal y del tipo de isomero.
1.6 Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda por via oral del PeBDE comercial en ratas es
baja; la toxicidad dérmica en conejos es también escasa. La exposicion
de ratas a inhalacion durante periodos breves y la aplicacion de PeBDE
al saco conjuntival de conejos produjo solo efectos transitorios
leves.
En los estudios de toxicidad a corto plazo realizados en ratas
(4 y 13 semanas), las concentraciones de 100 mg/kg de alimentos
produjeron un aumento del peso del higado y ligeras alteraciones
histologicas. Los cambios consistieron en el agrandamiento de las
células parenquimaticas hepaticas, que tenian un aspecto granular y
contenian "cuerpos redondos" eosinofilos. Se produjo en el higado un
aumento de la cantidad total de bromo dependiente de la dosis y los
niveles se mantuvieron durante un periodo largo, de hasta 24 semanas.
Se observo una hiperplasia tiroidea ligera de caracter reversible.
Tras la administracion oral de PeBDE en dosis diarias bajas, del
orden de 0,78 µmoles/kg de peso corporal, se detecto la induccion de
enzimas hepaticas y un aumento de concentracion del citocromo P450.
Los resultados de la pruebas de teratogenicidad y mutagenicidad fueron
negativos.
No se ha informado de estudios de carcinogenicidad a largo plazo.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Se dispone de muy pocos datos.
2 Conclusiones
2.1 PeBDE
El PeBDE comercial (mezcla de varios éteres: 24-38% de tetra-,
50-60% de penta- y 4-8% de hexabromodifenilo) es persistente y se
acumula en los organismos y en el medio ambiente.
El PeBDE comercial de utiliza ampliamente incorporado a polimeros
como aditivo pirorretardante. El contacto de la poblacion general
tiene lugar a través de los productos fabricados con estos polimeros.
No es probable la exposicion debida a su extraccion de ellos. Se puede
producir exposicion humana al PeBDE mediante la cadena alimentaria,
puesto que se ha detectado su presencia en organismos del medio
ambiente que son articulos alimenticios humanos, como peces,
crustaceos, etc. En los dos ultimos decenios se han determinado
cantidades crecientes en los peces y las aves de Suecia.
La toxicidad aguda del PeBDE comercial es baja. No se dispone de
informacion acerca de su absorcion y eliminacion en los mamiferos.
Tampoco se han realizado estudios sobre la reproduccion, la toxicidad
a largo plazo y la carcinogenicidad.
De los datos disponibles no se puede determinar el riesgo de la
poblacion general.
Se carece de la informacion necesaria para sacar conclusiones
sobre los niveles de exposicion en el trabajo o los efectos del PeBDE
comercial.
Se dispone de una informacion limitada sobre la toxicidad del
PeBDE comercial para los organismos en el medio ambiente.
2.2 Productos de degradacion
Al calentar el PeBDE (o los productos que lo contienen) a
400-800°C se producen PBDF, y en cierta medida el PBDD. Habria que
ocuparse de los posibles peligros derivados de su formacion.
La exposicion de la poblacion general al PBDF de los polimeros
pirorretardantes con PeBDE probablemente carece de importancia. La
incineracion controlada de forma adecuada no produce una emision de
cantidades significativas de dioxinas y furanos bromados. La
combustion no controlada de productos con PeBDE puede dar lugar a la
formacion de cantidades no cuantificadas de PBDF/PBDD. En un futuro
EHC sobre PBDF y PBDD se prestara atencion a su importancia para el
ser humano y el medio ambiente.
3 Recomendaciones
3.1 Generales
Dada su persistencia en el medio ambiente y la acumulacion en
organismos no se deberia utilizar PeBDE comercial. Sin embargo, si se
va a seguir usando hay que tener en cuenta los puntos siguientes:
* Se debe proteger de la exposicion a los trabajadores que
intervienen en la fabricacion de PeBDE y de los productos que
contienen el compuesto mediante medidas adecuadas de higiene
industrial, vigilancia de la exposicion en el trabajo y controles
técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones de las industrias
que utilizan el compuesto o sus productos. Se debe controlar la
eliminacion de desechos industriales y de productos de consumo
para evitar en lo posible la contaminacion del medio ambiente con
este producto persistente y acumulable, asi como con sus productos
de degradacion.
* La incineracion de materiales pirorretardantes con PeBDE solo se
debe realizar en incineradores adecuados que funcionen siempre en
condiciones optimas. La quema por otros medios dara lugar a la
formacion de productos de descomposicion toxicos.
3.2 Otros estudios
* Se requiere una vigilancia permanente de sus niveles en el medio
ambiente.
* Se deben validar métodos de determinacion del PeBDE en diversos
aglomerantes.
* Habida cuenta de que la base de datos toxicologicos actual no es
adecuada para evaluar los peligros del PeBDE comercial para el ser
humano y el medio ambiente y, a fin de mejorar su uso, se deberian
realizar los siguientes estudios:
- estudios adicionales toxicologicos, carcinogénicos y
ecotoxicologicos;
- nuevas investigaciones sobre la formacion de PBDF en
condiciones de incendios reales;
- investigacion de posibles métodos de reciclaje de polimeros
que contienen PeBDE y sus consecuencias;
- investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE TETRABROMODIFENILO
El éter de tetrabromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de tetrabromodifenilo comercial esta formado por una
mezcla de éteres: 41% de tetra-, 45% de penta- y 7% de
hexabromodifenilo y alrededor del 7% de PBDE de estructura
desconocida. En funcion de su estructura quimica, hay 42 posibles
isomeros del éter de tetrabromodifenilo. Practicamente se carece de
datos sobre sus propiedades fisicas y quimicas, a excepcion del
logaritmo del coeficiente de reparto n-octanol/agua, que es
5,87-6,16.
1.2 Produccion y aplicaciones
Hay un informe de la produccion (utilizacion) de unas 1000
toneladas de TeBDE en el Japon en 1987. No se tiene conocimiento de
produccion alguna en la actualidad como éter de tetrabromodifenilo,
pero esta presente en el éter de pentabromodifenilo comercial en
cantidades que oscilan entre el 24 y el 38%.
1.3 Transporte, distribucion y transformacion en el medio ambiente
En muestras de biota, sedimento y fangos cloacales se han
encontrado componentes del TeBDE comercial. Es probable que éstos (con
cantidades aproximadamente iguales de PeBDE) sean persistentes y
bioacumulables.
En estudios de pirolisis con TeBDE comercial se puso de manifiesto
que a 800°C se formaban PBDF y PBDD. No se encontraron estos
compuestos con mas atomos de bromo.
1.4 Niveles medioambientales y exposicion humana
Se detecto TeBDE en el sedimento de rios del Japon en
concentraciones de 12-31 µg/kg de peso seco y en Suecia a niveles de
hasta 840 µg/kg de pérdida por incineracion. También se encontro TeBDE
en fangos cloacales de Suecia a concentraciones de 15 µg/kg.
Los mejillones y peces recogidos en diferentes lugares del Japon
contenian TeBDE en concentraciones que oscilaban entre <0,1 y 14,6 µg
de 2,2',4,4'-TeBDE/kg de peso seco. En Suecia se recogieron diferentes
tipos de peces en diversos rios para analizar la concentracion de
2,2',4,4'-TeBDE. Las concentraciones medias variaron entre la no
detectable (<0,1 mg/kg) y 110 mg/kg de grasa. En los analisis se puso
de manifiesto la existencia de por lo menos una fuente local de
contaminacion en un rio determinado. Los corégonos, las truchas
alpinas y los arenques recogidos en distintos lugares de Suecia en
1986-87 contenian concentraciones de 15, 400 y 59-450 µg de
2,2',4,4'-TeBDE/kg de grasa respectivamente. Los peces recogidos en
rios de Alemania contenian hasta 1 mg de TeBDE/kg de grasa.
En el arenque y en el higado de bacalao procedentes de las
regiones meridional, central y septentrional del mar del Norte durante
el periodo 1983-89, se encontro una tendencia decreciente en la
concentracion del TeBDE en direccion sur-norte. En el arenque se
detectaron concentraciones de 8,4-100 µg de 2,2',4,4'-TeBDE/kg de
grasa.
En el tejido muscular de aves que se reproducen e invernan en el
mar Baltico, el mar del Norte y Spitzbergen se determinaron
concentraciones de 80 a 370 µg de 2,2',4,4'-TeBDE/kg de grasa. Los
quebrantahuesos recogidos en Suecia entre 1982 y 1986 contenian
concentraciones medias de 1800 µg/kg de grasa.
Se ha indicado que hay una tendencia creciente de las
concentraciones de 2,2',4,4'-TeBDE en los sedimentos del mar Baltico,
los peces de agua dulce y los huevos de aves marinas de Suecia.
En la grasa de las focas recogidas en el mar Baltico y en
Spitzbergen se determinaron concentraciones de 10-73 µg de
2,2',4,4'-TeBDE/kg de grasa. El perfil cromatografico del PBDE fue
semejante al del Bromkal 70-5. En mezclas de muestras de grasa de
focas oceladas y focas grises recogidas en Suecia en 1979-85 se
encontraron concentraciones de 47 µg y 650 µg de 2,2',4,4'-TeBDE/kg de
grasa, respectivamente.
Las mezclas de muestras de musculo de mamiferos terrestres, por
ejemplo conejos, ratones y renos, recogidas en Suecia en 1985 y 1986
pusieron de manifiesto concentraciones medias de <2, 0,82 y 0,18 µg
de 2,2',4,4'-TeBDE/kg de grasa, respectivamente.
En cuatro muestras de leche de vaca recogidas en Alemania se
encontraron niveles de 2,5-4,5 µg de PBDE/kg de grasa, medida como
Bromkal 70DE. En la leche de 25 mujeres de Alemania se encontro PBDE,
como Bromkal 70DE, en concentraciones que oscilaron entre
6,2 y 11,1 µg/kg de grasa.
Una estimacion aproximada de la exposicion a través del consumo de
pescado entre la poblacion sueca parece indicar una absorcion diaria
de 0,3 µg de TeBDE/persona.
1.5 Efectos en los mamiferos de laboratorio y en los sistemas de
ensayo in vitro
No hay datos sobre el propio TeBDE, pero se dispone de datos sobre
toxicidad aguda y a corto plazo del PeBDE comercial con un contenido
de TeBDE del 41%.
1.6 Cinética y metabolismo en animales de laboratorio y en el ser
humano
Se dispone de muy pocos datos.
1.7 Efectos en el ser humano
Se carece de datos.
1.8 Efectos en otros organismos de laboratorio y del medio ambiente
Se carece de datos.
2 Conclusiones
2.1 TeBDE
Los componentes del TeBDE comercial (una mezcla de un 41% de
2,2',4,4'-tetra-; 45% de 2,2',4,4',5'-penta-; 7% de hexa-; y 7-8% de
éteres de difenilo polibromados de estructura desconocida) son
persistentes y se acumulan en organismos del medio ambiente.
El TeBDE, como componente del éter de pentabromodifenilo, se
incorpora con profusion a polimeros como aditivo pirorretardante. El
contacto de la poblacion general se produce a través de productos
fabricados con estos polimeros. No es probable la exposicion por
extraccion a partir de los mismos. Es posible la exposicion humana al
TeBDE, mediante la cadena alimentaria, porque se ha detectado su
presencia en organismos del medio ambiente que forman parte de la
alimentacion humana, como por ejemplo peces, crustaceos, etc. Durante
los dos ultimos decenios se han determinado concentraciones crecientes
en los peces y las aves de Suecia.
Apenas se dispone de informacion acerca de la
toxicidad/carcinogenicidad a corto y largo plazo y de estudios de
reproduccion.
No se puede determinar el riesgo para la poblacion general en
funcion de los datos disponibles.
No se dispone de la informacion necesaria para poder sacar
conclusiones sobre los niveles de exposicion en el trabajo o los
efectos del TeBDE.
Tampoco hay datos acerca de la toxicidad del TeBDE comercial para
los organismos del medio ambiente.
2.2 Productos de degradacion
Cuando el TeBDE se calienta a 800°C se forman PBDF y PBDD. Hay que
prestar atencion a los posibles peligros relacionados con éstos.
La exposicion de la poblacion general al PBDF de los polimeros
pirorretardantes con TeBDE probablemente carece de importancia. La
incineracion con los controles adecuados no produce una emision de
cantidades importantes de dioxinas y furanos bromados. Cualquier
combustion sin control de productos que contienen TeBDE puede dar
lugar a la formacion de cantidades no cuantificadas de PBDF/PBDD. Un
futuro numero de EHC se ocupara de su importancia para el ser humano y
el medio ambiente.
3 Recomendaciones
3.1 Generales
Dada su persistencia en el medio ambiente y la acumulacion en
organismos, se recomienda que no se utilice TeBDE. Sin embargo, si se
va a seguir usando hay que tener en cuenta los puntos siguientes:
* Se debe proteger de la exposicion a los trabajadores que
intervienen en la fabricacion de TeBDE y de los productos que
contienen el compuesto, mediante medidas adecuadas de higiene
industrial, vigilancia de la exposicion en el trabajo y controles
técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones de las industrias
que utilizan el compuesto o sus productos. Se debe controlar la
eliminacion de desechos industriales y de productos de consumo
para evitar en lo posible la contaminacion del medio ambiente con
este producto persistente y acumulable, asi como con sus productos
de degradacion.
* La incineracion de materiales pirorretardantes con TeBDE solo se
debe realizar en incineradores adecuados que funcionen siempre en
condiciones optimas. La quema por otros medios dara lugar a la
formacion de productos de degradacion del furano toxicos.
3.2 Otros estudios
* Se requiere una vigilancia permanente de sus niveles en el medio
ambiente.
* Se deben validar métodos de determinacion del TeBDE en diversos
aglomerantes.
* Habida cuenta de que la base de datos toxicologicos actual no es
adecuada para evaluar los peligros del TeBDE comercial para el ser
humano y el medio ambiente, si se va a continuar con su uso se
deberian realizar los siguientes estudios:
- estudios adicionales toxicologicos, carcinogénicos y
ecotoxicologicos;
- nuevas investigaciones sobre la formacion de PBDF en
condiciones de incendios reales;
- investigacion de posibles métodos de reciclaje de polimeros
que contienen TeBDE y sus consecuencias;
- investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE TRIBROMODIFENILO
El éter de tribromodifenilo no se fabrica ni se utiliza. No se
dispone de datos sobre los aspectos siguientes:
* Transporte, distribucion y transformacion en el medio ambiente
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay una base de datos sobre la cual realizar una evaluacion.
2 Recomendaciones
Se debe reducir al minimo la contaminacion de los productos
comerciales con éter de tribromodifenilo, a fin de evitar la del medio
ambiente y la exposicion humana.
Hay que evitar el uso de los productos comerciales capaces de
contaminar el medio ambiente.
ÉTER DE DIBROMODIFENILO
El éter de dibromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay una base de datos sobre la cual realizar una evaluacion.
2 Recomendaciones
Se debe reducir al minimo la contaminacion de los productos
comerciales con éter de dibromodifenilo, a fin de evitar la del medio
ambiente y la exposicion humana.
Hay que evitar el uso de los productos comerciales capaces de
contaminar el medio ambiente.
ÉTER DE MONOBROMODIFENILO
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano.
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Propiedades fisicas y quimicas
El éter de monobromodifenilo tiene tres posibles isomeros.
El éter de p-bromodifenilo a temperatura ambiente es un liquido
con un punto de ebullicion de 305-310°C. Se estima que su solubilidad
en agua es de 48 mg/litro. El log del coeficiente de reparto
n-octanol/agua es de 4 a 5. La presion de vapor a 20°C es de
0,0015 mm de Hg.
1.2 Produccion y aplicaciones
El MBDE no se utiliza como pirorretardante. En 1977 aparecio un
informe sobre su produccion, pero se desconoce su empleo.
1.3 Transporte, distribucion y transformacion en el medio ambiente
La semivida de volatizacion a partir del agua es del orden de
centenares de dias.
Aunque el MBDE no se degrado de manera significativa en un cultivo
de siete dias con microorganismos de las aguas residuales domésticas,
se ha informado que en los fangos cloacales activados lo hace en un
95%. En un estudio aislado se puso de manifiesto que una cepa de
bacterias del suelo no era capaz de degradar el MBDE como unica fuente
de carbono.
1.4 Niveles medioambientales y exposicion humana
Se ha detectado MBDE en muestras de agua superficial recogidas en
las cercanias de zonas industriales de los Estados Unidos, pero no se
obtuvieron estos resultados en un estudio semejante realizado en el
Japon. Se encontro asimismo en agua del suelo de un lugar proximo a
una instalacion industrial de los Estados Unidos. También se ha
detectado en el sedimento acuatico y en la biota acuatica de los
Estados Unidos.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
No se dispone de datos.
1.6 Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
El MBDE no es teratogénico, pero se carece de datos sobre su
toxicidad aguda, a corto plazo y a largo plazo, y por consiguiente no
se puede realizar su evaluacion.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Se ha informado que la CL50 en 96 h para Lepomis macrochirus es
de 4,9 mg/litro, con una concentracion sin efectos observados de menos
de 2,8 mg/litro. La CL50 en 48 horas para la pulga de agua fue de
0,36 mg/litro, con un NOEC de menos de 0,046 mg/litro.
2 Conclusiones y recomendaciones
El éter de monobromodifenilo no tiene propiedades
pirorretardantes. Puede acumularse en los organismos del medio
ambiente y se ha detectado en diferentes compartimentos ambientales.
Algunas pruebas indican que es biodegradable.
La reducida informacion de que se dispone impide sacar
conclusiones sobre los niveles de exposicion y los efectos sobre la
poblacion general y los organismos.
No existe una base de datos toxicologicos que apoye su uso.
Se debe evitar todo empleo que provoque la contaminacion del medio
ambiente.
Chemical formula: C12H9BrO
Relative molecular mass: 249.11
Common names: monobromodiphenyl ether (MBDE)
mono bromodiphenyl oxide
CAS registry number: 101-55-3
CAS name: bromo-4-phenoxybenzene
Synonyms: bromophenylphenyl ether, bromodiphenyl
ether, bromophenyl ether,
bromophenoxybenzene
Trade names: HSDB 2747; NSC
On the basis of the chemical structure, there are 3 possible isomers
of monobromodiphenyl ether. p-Monobromodiphenyl ether is one of
them.
2.2 Physical and chemical properties
p-Bromodiphenyl ether is a liquid at common ambient
temperatures.
Melting point: 18.72 °C
Boiling point: 310.14 °C (305 °C
Vapour pressure at 20 °C: 0.0015 mmHg
Specific gravity: 1.449 (1.4208 at 20 °C)
Refractive index, n 20/D: 1.607
Flash point: >230°F
Solubility at 25 °C: 4.8 mg/litre water (calculated),
soluble in ethyl ether
n-Octanol/water partition
coefficient (log Pow): 4.28 (4.08-4.94)
From: US EPA (1984, 1986).
2.3 Analytical methods
p-Monobromodiphenyl ether (MBDE) can be determined by the
standard US EPA method 611-Haloethers. Chromatographic conditions are
described in US EPA (1986). The limit of determination in municipal
and industrial waste waters is 2.3 µg/litre. Zogorski (1984) reported
a limit of determination of 0.01 µg/litre using a gas chromatograph
equipped with an electron capture detector. McMahon (1983) applied
GC-MS (US EPA method 625) using a halide specific detector. Gurka et
al. (1982) used GC/Fourier transform infrared spectroscopy to detect
p-monobromodiphenyl ether (see also General Introduction, section
2.1 and Table 2).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Monobromodiphenyl ether has not been reported to occur naturally
(see General Introduction, section 1.1).
3.2 Anthropogenic sources
3.2.1 Production levels and processes
p-Monobromodiphenyl ether is prepared by brominating diphenyl
ether with Br2 at 95-100 °C in carbon tetrachloride (US EPA, 1986).
The production range (includes importation volumes) statistics
from the 1977 TSCA Inventory were up to 450 kg of p-mono-
bromodiphenyl ether (US EPA, 1986).
3.2.2 Uses
No data are available.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
The half-life of p-monobromodiphenyl ether with respect to
volatilization has been estimated to be within the range of hundreds
of days (MacKay & Leinonen, 1975).
In contrast, by analogy to p-monochlorodiphenyl, Callahan et al.
(1979) estimated the half-life of p-monobromodiphenyl ether to be
10 h. However, sorption of the agent by organic material in water will
prolong its evaporative half-life in natural bodies of water. This
value should be considered a minimum.
4.2 Biotransformation
4.2.1 Biodegradation
p-Monobromodiphenyl ether was not significantly biodegradable in
a 7-day static-culture flask-screening procedure of Bunch and Chambers
utilizing biochemical oxygen demand (BOD) dilution water containing
5 mg of yeast extract/litre, as the synthetic medium; 5 and
10 mg/litre concentrations of the test compound, a 7-day static
incubation of 25 °C in the dark, followed by three weekly subcultures
(totalling 28 days of incubation), and incorporating settled domestic
waste water as microbial inoculum. At a test compound concentration of
5 mg/litre, only 2 out of 4 cultures showed any biodegradation in 7
days (19 and 36%), and, at a concentration of 10 mg/litre, only one
out of 4 showed biodegradation (19%) (Tabak et al., 1981).
p-Monobromodiphenyl ether was not able to support the growth of
Alcaligenes BM2, a strain of soil bacteria capable of using PCB
(dichloro-) mixtures as the sole source of carbon (Yagi & Sudo, 1980).
However, p-monobromodiphenyl ether was reported to be 95%
biodegradable when introduced as a pollutant (360 µg/litre) in a
full-scale activated sludge treatment system (US EPA, 1986).
The in vitro microbial degradation of p-monobromodiphenyl
ether was studied with a soil isolate, strain S93B1, identified as
Pseudomonas crucivae. After enrichment and isolation, the bacteria
were cultivated in an agar-slant at 30 °C for 12-17 days and growth
was determined. Strain S93B1 did not grow on the biphenyl ether as the
sole source of carbon (Takase et al., 1986).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data are available on general population exposure or on
occupational exposure during manufacture, formulation, or use.
5.1 Environmental levels
5.1.1 Water
According to US EPA (1986), there are monitoring data for
p-monobromodiphenyl ether in water in the USA. The mean
concentration was 0.2 mg/litre (range 0-202.7 mg/litre, 2193 listings)
in water. Most of these listings concerned river water samples taken
near industrial sites. Only a few groundwater and community
drinking-water samples were included (no further details were
available). Plumb (1991) reported the presence of p-monobromo-
diphenyl ether in groundwater from only 1 out of 479 disposal sites
that were analysed in the USA.
In Japan, p-monobromodiphenyl ether was not detected in 27 water
samples (limit of determination 0.15-0.5 µg/litre) in an environmental
survey in 1984 (Environment Agency Japan, 1987).
5.1.2 Soil/sediment
According to US EPA (1986), there are numerous US monitoring data
for p-monobromodiphenyl ether in sediments. The mean concentration
was 3.5 mg/kg (range 0-380 mg/kg, 585 listings) (no further details
are available).
In Japan, p-monobromodiphenyl ether was not detected in 27
sediment samples (limit of determination 2.5-120 µg/kg dry weight) in
an environmental survey in 1984 (Environment Agency Japan, 1987).
5.1.3 Aquatic organisms
According to US EPA (1986) there are numerous US monitoring data
for p-monobromodiphenyl ether in tissue from aquatic organisms. The
concentrations were 2.0 mg/kg (range 070.0 mg/kg, 346 listings) (no
further details are available).
6. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
No data are available on the following topics:
* Single exposure
* Short-term exposure
* Long-term exposure
* Skin and eye irritation; sensitization
* Mutagenicity and related endpoints.
6.1 Reproductive toxicity, embryotoxicity, and teratogenicity
Outbred Swiss (CD-1) mice (age 60 days) were treated with
p-monobromodiphenyl ether (95%). There were 20 and 21 mice
respectively in these treatment groups and 51 and 55 mice in untreated
and vehicle control (1 ml corn oil/kg) groups, respectively. Two
females per group were mated per male, until a copulation plug was
found, or for maximum 5 days. MBDE in corn oil was administered by
gavage in dose levels of 0, 100 or 1.000 mg/kg body weight/day, from
day 5 up to day 14 of gestation. Pups were counted and weighed (by
litter) on postnatal days 1, 3, 5, 10 and 15; they were sexed and
weaned on day 21 and autopsied on days 25 and 30 to determine gross
abnormalities and the weights of Harderian glands, liver, and kidneys.
No adverse effects on any of these parameters were observed (Francis,
1989).
6.2 Carcinogenicity
Theiss et al. (1977) tested p-monobromodiphenyl ether (MBDE) in
a short-term screening assay: the strain A mouse pulmonary tumour
assay. Four groups of 20 A/St male mice (6-8 weeks old) were given 24
intraperitoneal injections of tricaprylin (solvent-control), 23 i.p.
injections of 40 mg (low-dose), 17 injections of 100 mg (mid-dose) and
18 injections of 200 mg MBDE/kg body weight (high-dose), three times
per week. Total doses administered in the four groups were 0, 920,
1700, and 3600 mg/kg body weight, respectively. Twenty-four weeks
after the first injection, the mice were sacrificed and the lungs
examined. The number of lung tumours/mouse and survival rates of the
treated animals were not significantly different from the control
animals. The number of lung tumours/mouse were; control 0.39 ± 0.06;
low-dose 0.18 ± 0.10, mid-dose 0.15 ± 0.10 and high-dose 0.31 ± 0.15.
MBDE was negative in this pulmonary assay.
7. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The effects of acute exposure to p-monobromodiphenyl ether have
been studied in several species of aquatic invertebrates and fish. The
results are summarized in Table 30. The water flea (Daphnia magna),
is more sensitive than fish to p-monobromodiphenyl ether (US EPA,
1984).
In an early-life-stage (embryo-larval) test on fathead minnow,
conducted with p-monobromodiphenyl ether, adverse effects on
survival and growth were produced. The geometric mean of the highest
no-effect level was 0.122 mg/litre (US EPA, 1984).
Table 30. Acute toxic effects of p-monobromodiphenyl ether on aquatic organismsa
Species Exposure Mean Effect Reference
duration Method concentration
(h) (mg/litre)
Rainbow trout 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Oncorhynchus mykiss)b
Bluegill sunfish 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Lepomis macrochirus)
Bluegill sunfish 24 static 50.9c LC50 US EPA (1978);
(Lepomis macrochirus)
Buccafusco et al. (1981)
48 static 9.62 LC50 US EPA (1978)
72 static 4.94 LC50 US EPA (1978)
96 static 4.94 LC50 US EPA (1978)
96 static < 2.80 no effect US EPA (1978)
See lamprey 24 static-aerated 5.0 stress observed Applegate et al. (1957)
(Petromyzon marinus)
Water flea 24 static 0.46 LC50 US EPA (1978); LeBlanc (1980)
(Daphnia magna) 48 static 0.36 LC50 US EPA (1978); LeBlanc (1980)
48 static < 0.046 no effect US EPA (1978)
a From: US EPA (1984).
b Old name = Salmo Gairdneri.
c This dose exceeds the solubility of the test compound in water at 25 °C (4.6 mg/litre).
REFERENCES
AIHA (1981) Decabromo-diphenyloxide. Workplace environmental exposure
level guide. Am Ind Hyg Assoc J, 42: A76-A77.
Andersson O & Blomkvist G (1981) Polybrominated aromatic pollutants
found in fish in Sweden. Chemosphere, 10(9): 1051-1060.
Anon (1989) Draft evaluation report on flame retardants; PBB's and
PBBO's (in Dutch).
Applegate VC, Howell JH, Hall AE, & Smith MA (1957) Toxicity of 4,346
chemicals to larval lampreys and fish. Washington, DC, US Department
of the Interior, Fish and Wildlife Service (Special Scientific Report,
Fish No. 207).
Arias (1992) Brominated diphenyloxides as flame retardants; Bromine
based chemicals. Consultant report to the OECD, Paris.
Bahn A, Bialik O, Oler J, Houten L, & Landau E (1980) Health
assessment of occupational exposure to polybrominated biphenyl (PBB)
and polybrominated biphenyloxide (PBBO). Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 72 pp (ISS EPA 560/6-80-001; NTIS No. PB81-159675).
Ball M, Päpke O, & Lis A (1991) [Further investigation on the
formation of polybrominated dioxins and furans during thermal stress
of flameproof plastics and textiles. Sub-project 1.] Berlin, Federal
Office for the Environment (Research report No. 10403364/01; UBA-FB
91-082) (in German).
Ball M, Päpke O, & Lis A (1992) [Further investigation on the
formation of polybrominated dioxins and furans during thermal stress
of flameproof plastics and textiles. Sub-projects 1 and 2.] Berlin,
Federal Office for the Environment (Research report Nos. 10403364/01 &
/02; UBA-FB 91-082 & 92-097) (in German).
BFRIP (1990) Brominated flame retardants. A review of recent research
(Compiled by The Brominated Flame Retardant Industry Panel and The
European Brominated Flame Retardant Industry Panel). West Lafayette,
Indiana, USA, BFRIP (Unpublished report No. III/4143/90, submitted to
WHO by BFRIP).
Bialik O (1982) Endocrine function of workers exposed to PBB and PBBO:
Terminal progress report. Cincinnati, Ohio, National Institute for
Occupational Safety and Health.
Bieniek D, Bahadir M, & Korte F (1989) Formation of heterocyclic
hazardous compounds by thermal degradation of organic compounds.
Heterocycles, 28:719-722.
Breslin WJ, Kirk HD, & Zimmer MA (1989) Teratogenic evaluation of a
polybromodiphenyl oxide mixture in New Zealand White rabbits following
oral exposure. Fundam Appl Toxicol, 12: 151-157.
Bromine Compounds Ltd (1992) Material safety data sheet FR-1210. Beer
Sheva, Israel, Bromine Compounds Ltd, pp 1-5.
Brosier JS, Blanchard FA, & Takahashi IT (1972) Concentrations of
octabromodiphenyl and decabromodiphenyloxide in aquaria water and
trout flesh. Midland, Michigan, Dow Chemical Company (Unpublished
report No. AL 37448, submitted to WHO by BFRIP).
Bruckmann P, Hackhe K, Ball M, Lis A, & Papke O (1990) Degassing of
PBDD/PBDFs from a television set - PBDD/PBDF levels after a fire in a
stock house two case studies. In: Freij L ed. Proceedings of the
Workshop on Brominated Aromatic Flame Retardants, Skokloster, Sweden,
24-26 October 1989. Solna, National Chemicals Inspectorate (KEMI),
pp 99-104.
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26(4): 446-452.
Buser HR (1986) Polybrominated dibenzofurans and dibenzo- p-dioxins:
Thermal reaction products of polybrominated diphenylether flame
retardants. Environ Sci Technol, 20(4): 404-408.
Carlson GP (1980a) Induction of xenobiotic metabolism in rats by
short-term administration of brominated diphenylethers. Toxicol Lett,
5: 19-25.
Carlson GP (1980b) Induction of xenobiotic metabolism in rats by
brominated diphenylethers administered for 90 days. Toxicol Lett,
6: 207-212.
Carte B & Faulkner DJ (1981) Polybrominated diphenyl ethers from
Dysidea herbacea. Dysidea chlorea and Phyllospongia foliascens.
Tetrahedron, 37: 2335-2339.
CBC (1982) The bioaccumulation of compound S-511 by carp. Tokyo,
Chemicals Inspection and Testing Institute, Chemical Biotesting Center
(Unpublished report).
CEM (1989) Polybrominated dibenzodioxins and dibenzofurans
(PBDDs/PBDFs) from flame retardants containing bromine. Assessment of
risk and proposed measures. Report by the Brominated flame retardants
CEM Working Group to the Conference of Environment Ministers, Bonn,
September 1989. (Report III/4299/89. Appendix).
Chemag (1988) Information sheet on Chemflam 011. Frankfurt am Main,
Chemag Aktiengesellschaft, p 1.
Clausen E, Lahaniatis ES, Bahadir M, & Bieniek D (1987) [Determination
of brominated dibenzofurans formed during the thermolysis of polymers
with decabromodiphenylether as flame-proofing agent.] Fresenius Z Anal
Chem, 327: 297300 (in German).
Craig DK, Mitchum RK, Bauer MR, Yancey MF, Peters AC, & Ioiner RL
(1989) Determination of polybrominated dibenzo- p-dioxins and
polybrominated dibenzofurans in CYCOLAC plastic resins and the fumes
evolved during normal thermal processing. Final report. Columbus.
Ohio, Battelle (Report to General Electric Company, Mt. Vernon,
Indiana, submitted to WHO by BFRIP).
Cramer PH, Stanley JS, & Thornburg KR (1990a) Mass spectral
confirmation of chlorinated and brominated diphenyl ether in human
adipose tissues. Kansas City, Missouri, Midwest Research Institute
(Prepared for the US Environmental Protection Agency, Washington) (US
NTIS report PB91-159699).
Cramer PH, Ayling RE, Thornburg KR, Stanley JS, Remmers JC, Breen JJ,
& Schwemberger J (1990b) Evaluation of an analytical method for the
determination of polybrominated dibenzo- p-dioxins/dibenzofurans
(PBDD/PBDF) in human adipose. Chemosphere, 20(7-9): 821-827.
Davidson JS & Ariano JM (1986) Decabromodiphenylether (DE-83):
Determination of area percent assay. Analytical method No. QC-86-02.
West Lafayette, Indiana, Great Lakes Chemical Corporation (Report
submitted to WHO by BFRIP).
De Boer J (1989) Organochlorine compounds and bromediphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere, 18(11/12): 2131-2140.
De Boer J (1990) Brominated diphenylethers in Dutch freshwater and
marine fish. In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar.
Tenth International Symposium on Chlorinated Dioxins and Related
Compounds. Volume 2: Organohalogenated compounds (Short papers).
Bayreuth, Germany, Ecoinforma Press, pp 315-318.
DeCarlo VJ (1979) Studies on brominated chemicals in the environment.
Ann NY Acad Sci, 320: 678-681.
Dieter MP (1979) Special studies protocol. Washington, Memorandum of
the Department of Health, Education and Welfare. Public Health
Service, National Institute of Health, National Cancer Institute.
Dow Chemical Company (1978) Compound: Decabromodiphenyl oxide
(Analytical method). Midland, Michigan, Dow Chemical Company,
pp 609-620 (Report prepared by Tracor Jitco, Inc., Rockville,
Maryland) (Report No. 3250, submitted to WHO by BFRIP).
Dumler R, Lenoir D, & Hutzinger O (1990) Formation of brominated
dibenzofurans and -dioxins from the combustion of the flame retardant
decabromodiphenyl ether under different conditions. In: Hutzinger O &
Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth International Symposium
on Chlorinated Dioxins and Related Compounds. Volume 2:
Organohalogenated compounds (short papers). Bayreuth, Germany,
Ecoinforma Press, pp 325-328.
Dumler R, Thoma H, Lenoir D, & Hutzinger O (1989a) Thermal formation
of polybrominated dibenzodioxins (PBDD) and dibenzofurans (PBDF) from
bromine containing flame retardants. Chemosphere, 19(1-6): 305-308.
Dumler R, Lenoir D, Thoma H, & Hutzinger O (1989b) Thermal formation
of polybrominated dibenzofurans from decabromodiphenyl ether in a
polybutylene-terephthalate polymer matrix. J Anal Appl Pyrolysis,
16: 153-158.
EBFRIP (1990) Information on polybrominated diphenylethers (PBDE's).
Economic, technical and regulatory assessment of the European
Brominated Flame Retardant Industry Panel - Responses to questions
submitted by EEC, Brussels. Rijswijk (NL), The European Brominated
Flame Retardant Industry Panel.
El Dareer SM, Kalin JR, Tillcry KF, & Hill DL (1987) Disposition of
decabromodiphenyl ether in rats dosed intravenously or by feeding.
J Toxicol Environ Health, 22: 405-415.
Environment Agency Japan (1983) Environmental monitoring of chemicals.
Environmental survey report of F.Y. 1980 and 1981. Tokyo, Environment
Agency Japan, Department of Environmental Health, Office of Health
Studies.
Environment Agency Japan (1987) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1984 and 1985. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Environment Agency Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Environment Agency Japan (1991) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1988 and 1989. Tokyo, Environment Agency Japan, Department of
Environmental Health, Office of Health Studies.
Ethyl Corporation (1986) Laboratory report: Primary eye irritation.
Saytex 102. Baton Rouge, Louisiana, Ethyl Corporation (Report
No. PH 421-ET-010-86) (Report submitted to WHO by BFRIP).
Ethyl Corporation (1992a) Information sheet from Ethyl Corporation on
Saytex 102E. Baton Rouge, Louisiana, Ethyl Corporation (Report
submitted to WHO by BFRIP).
Ethyl Corporation (1992b) Information sheet from Ethyl Corporation on
Saytex 111. Baton Rouge, Louisiana, Ethyl Corporation (Report
submitted to WHO by BFRIP).
Faulkner DJ (1988) Brominated marine natural products. In: Price D,
Iddon B, & Wakefield BJ ed. Bromine Compound: Chem. Appl.,
(Proceedings, International Conference on Chemical Applications of
Bromine and its Compounds.) Amsterdam, Oxford, New York, Elsevier
Science Publishers, Chapter 2, pp 121-144.
Faulkner DJ (1990) Naturally-occurring brominated compounds. In: Freij
L ed. Proceedings of the Workshop on Brominated Aromatic Flame
Retardants, Skoldoster, Sweden, 24-26 October 1989. Solna, National
Chemicals Inspectorate, pp 121-144.
Fernandez P, Grifoll M, Solahas AM, Bayona JM, & Albaiges J (1992)
Bioasssay-directed chemical analysis of genotoxic components in
coastal sediments. Environ Sci Technol, 26: 817-829.
Flick EW (1986) Plastics additives: An industrial guide. Section IX -
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Francis BM (1989) Relative developmental toxicities of nine diphenyl
ethers related to nitrofen. Environ Toxicol Chem, 8: 681-688.
Fresenius Institute (1990) Summary results on pyrolysis of different
types of ABS. Battelle report on contents and vapour-emission of PBDD
resp. PBDF's. Memorandum from Leeuwenburgh W.F., General Electric
Plastics ABS, BV. Amsterdam. 12 March 1990 (Report submitted to WHO by
BFRIP).
Fulfs JC (1987a) Rabbit ear bioassay for comedogenicity, dose-range
finding study for soot and char generated from the combustion of high
impact polystyrene. Fort Collins, CO, USA, Inhausen Research
Institute, Inc. (Report to Ethyl Corporation, Baton Rouge, Florida,
submitted to WHO by BFRIP).
Fulfs JC (1987b) Rabbit ear bioassay for comedogenicity, dose-range
finding study for soot and char generated from the combustion of high
impact polystyrene flame retarded with decabromodiphenyloxide and
antimony oxide. Fort Collins, CO, USA, Inhausen Research Institute,
Inc. (Report to Ethyl Corporation, Baton Rouge, Florida, submitted to
WHO by BFRIP).
Fulfs JC & Dahlgren RR (1987a) Acute oral toxicity in the rat of soot
and char generated from the combustion of high impact polystyrene
(HIPS). Fort Collins, CO, USA, Inhausen Research Institute, Inc.
(Report to Ethyl Corporation, Baton Rouge, Florida, submitted to WHO
by BFRIP).
Fulfs JC & Dahlgren RR (1987b) Acute oral toxicity in the rat of soot
and char generated from the combustion of high impact polystyrene
flame retarded with decabromodiphenyloxide and antimony trioxide (HIPS
FR). Fort Collins, CO, USA, Inhausen Research Institute, Inc. (Report
to Ethyl Corporation, Baton Rouge, Florida, submitted to WHO by
BFRIP).
Fulfs JC & Dahlgren RR (1987c) Rabbit ear biGassay for comedogemcity:
Multiple-dose-level definitive study for soot and char generated from
the combustion of high impact polystyrene. Fort Collins, CO, USA,
Inhausen Research Institute, Inc. (Report to Ethyl Corporation, Baton
Rouge, Florida, submitted to WHO by BFRIP).
Fulfs JC & Dahlgren RR (1987d) Rabbit ear bioassay for comedogenicity,
multiple-dose-level definitive study for soot and char generated from
the combustion of high impact polystyrene flame retarded with
decabromo-diphenyloxide and antimony trioxide. Fort Collins, CO, USA,
Inhausen Research Institute, Inc. (Report to Ethyl Corporation, Baton
Rouge, Florida, submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1987) Toxicity data of
octabromo-diphenyloxide (DE-79). West Lafayette, Indiana, Great Lakes
Chemical Corporation (Unpublished data submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1990a) Great Lakes DE-79tm: Product
information. West Lafayette, Indiana, Great Lakes Chemical Corporation
(Report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (1990b) Great Lakes DE-83Rtm: Product
information. West Lafayette, Indiana, Great Lakes Chemical Corporation
(Report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (Undated a) Toxicity data of
pentabromo-diphenyloxide. West Lafayette, Indiana, Great Lakes
Chemical Corporation (Unpublished report submitted to WHO by BFRIP).
Great Lakes Chemical Corporation (Undated b) Toxicity data of
decabromo-diphenyloxide. West Lafayette, Indiana, Great Lakes Chemical
Corporation (Unpublished report submitted to WHO by BFRIP).
Gurka DF, Laska PR, & Titus R (1982) The capability of GC/FT-IR to
identify toxic substances in environmental sample extracts.
J Chromatogr Sci, 20: 145-154.
Hagenmaier H, She J, Dawidowsky N, Thomas B, & Dusterholt L (1991)
Analysis of sewage sludges for polyhalogenated dibenzo- p-dioxins,
dibenzofurans and diphenylethers. In: Dioxin'91. Abstract from the
11th International Symposium on Chlorinated Dioxins and Related
Compounds, Research Triangle Park, North Carolina, 23-27 September
1991, p 112.
Hallenbeck D (1993) Freezing point of DE-71. Inter-Office memorandum,
Great Lakes Chemical Corporation. June 29, 1993 (Report submitted to
WHO by BFRIP).
Hamm S & Theisen J (1992) Formation of polybrominated dibenzofurans
and polybrominated dibenzo(p)dioxins at fires of electrical
appliances. Poster presented at Dioxin'92. 12th International
Symposium on Dioxins and Related Compounds, Tampere, Finland, 24-28
August 1992. Munster, Society for Workplace and Environmental Analysis
GmbH.
Hanberg A, Stahlberg M, Georgellis A, De Wit C, & Ahlborg UG (1991)
Swedish dioxin survey: Evaluation of the H-4-II E bioassy for
screening environmental samples for dioxin:like enzyme induction.
Pharmacol Toxicol, 69: 442-449.
Hanley TR Jr (1985) Decabromodiphenyloxide: A summary of an oral
teratology study in Sprague-Dawley rats. Midland, Michigan, Dow
Chemical Company (Unpublished report submitted to WHO by BFRIP).
Hefner RE Jr (1973) A study of the rate of hemolysis induced by
decabromo-diphenoxide (DBDPO) particles with washed rat erythrocytes
in vitro. Midland, Michigan, Dow Chemical Company (Unpublished
report submitted to WHO by BFRIP).
Hoechst Celanese Corporation (1988) Report of air sampling for the
presence of polybrominated dibenzodioxins and dibenzofurans during the
production of polybutylene terephthalate resin with decabromodiphenyl
oxide (Unpublished report submitted to WHO by BFRIP).
Huff JE, Eustis SL, & Haseman JK (1989) Occurrence and relevance of
chemically induced benign neoplasms in long-term carcinogenicity
studies. Cancer Metastasis, 8: 121.
Hutzinger O & Thoma H (1987) Polybrominated dibenzo- p-dioxins and
dibenzofurans: The flame retardant issue. Chemosphere,
16(8/9): 1877-1880.
Hutzinger O, Sundstrom G, & Safe S (1976) Environmental chemistry of
flame retardants. Part. I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer, 345 pp (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 48).
Industrial Bio-Test Laboratories (1975) Human repeated insult patch
test with DBDO-1 and XD 8186.02. Northbrook, Illinois, USA. (Report to
Dow Chemical Company, Midland, Michigan) (Report No. IBT 636-0654,
submitted to WHO by BFRIP).
IRPTC (1988) Data profiles on flame retardants; Deca-, octa- and
pentabromodiphenyloxide. Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme.
Jansson B, Asplund L, & olsson M (1987) Brominated flame retardants --
Ubiquitous environmental pollutants? Chemosphere,
16(10-12): 2343-2349.
Jansson B, Andersson R, Asplund L, Bergman A, Litzen K, Nylund K,
Reutergardh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjo T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in biological
samples from the environment. Accepted for publication in Environ.
Toxicol. Chem., 12(7): 1163-1174.
Jersey G-C, Frauson LE, & Schuetz DJ (1976) Pulmonary clearance and
tissue response following a single intratracheal injection of
decabromo-diphenyloxide (DBDPO) dust in male rats. Midland, Michigan,
Dow Chemical Company (Unpublished report No. HET K-47298-(21),
submitted to WHO by BFRIP).
Kaart KS & Kokk KY (1987) Spectrometric determination of
decabromodiphenyl oxide in industrial sewage. Ind Lab, 53: 289-290.
Kalk (1982) [CFK Bromkal(R) - fire protection equipment]. Cologne,
Kalk Chemical Factory (Information sheet 3000-7/82) (in German).
Kitchin KT, Brown JL, & Kulkarni P (1992) Predictive assay for rodent
carcinogenicity using in vivo biochemical parameters: operational
characteristics and complementarity. Mutat Res, 266: 253-272.
Klusmeier W, Voegler P, Ohrbach KH, Weber H, & Kettrup A (1988)
Thermal decomposition of decabromodiphenyl ether. J Anal Appl
Pyrolysis, 13: 277-285.
Kociba RJ (undated) Comparative biologic activity and toxicity of
brominated derivatives of diphenylether (oxide). (Unpublished report
submitted to WHO by BFRIP).
Kociba RJ, Frauson LO, Humiston CG, Norris JM, Wade CE, Lisowe RW,
Quast IF, Jersey GC, & Jewett GL (1975a) Results of a two-year dietary
feeding study with decabromodiphenyl oxide (DBDPO) in rats. Midland,
Michigan, Dow Chemical Company (Unpublished report submitted to WHO by
BFRIP).
Kociba RJ, Frauson LO, Humiston CG, Norris JM, Wade CE, Lisowe RW,
Quast JF, Jersey GC, & Jewett GL (1975b) Results of a two-year dietary
feeding study with decabromodiphenyl oxide (DBDPO) in rats. Combust
Toxicol, 2: 267-285.
De Kok JJ, De Kok A, Brinkman UATh, & Kok RM (1977) Analysis of
polybrominated biphenyls. J Chromatogr, 142: 367-383.
De Kok JJ, De Kok A, & Brinkman UATh (1979) Analysis of polybrominated
aromatic ethers. J Chromatogr, 171: 269-278.
Kopp A (1990) [Documentation on fire-proofing agents containing
bromine.] Bonn, Ministry of Environment, Nature Conservation and
Nuclear Safety (Report to the European Economic Community, Brussels)
(in German).
Koster P, Debets FMH, & Strik JJTWA (1980) Porphyrinogenic action of
fire retardants. Bull Environ Contain Toxicol, 25:313-315.
Kraus HW (1990) Polybrominated dibenzodioxines and dibenzofurans
(PBDDs/PBDFs) from flame retardants containing bromine. Bonn, Ministry
for Environment, Nature Conservation and Nuclear Safety (Report to the
Organisation for Economic Co-operation and Development, Paris).
Kruger C (1988) [Polybrominated biphenyls and polybrominated diphenyl
ethers detection and quantitation in selected foods.] Munster,
University of Munster (Thesis) (in German).
Kuehl DW, Haebler R, & Potter C (1991) Chemical residues in dolphins
from the U.S. Atlantic coast including Atlantic bottlenose obtained
during the 1987/88 mass mortality. Chemosphere, 22(11): 1071-1084.
Lahaniatis ES, Bergheim W, & Rainer C (1989) Hazardous halogenated
substances formed during combustion processes. Toxicol Environ Chem,
20-21: 501-506.
Lahaniatis ES, Bergheim W, & Bieniek D (1991) Formation of
2,3,7,8-tetrabromodibenzodioxin and -furan by thermolysis of polymers
containing brominated flame retardants. Toxicol Environ Chem,
31/32: 521-526.
Lahl U, Wilken M, & Wiebe A (1991) [Polybrominated diphenyl ethers in
waste incineration.] Mull Abfall, 23:83-87 (in German).
Larsen ER (1978) Flame retardants. Halogenated fire retardants. In:
Mark HF, Othmer DF, Overberger CG, Seaborg GT, & Grayson M ed.
Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
Chichester, Brisbane, Toronto, John Wiley and Sons, vol 10,
pp 373-395.
Leblanc RB (1979) What's available for FR textiles. Text Ind,
143: 78-83.
Leblanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24(5): 684-691.
Leung HW, Murray FJ, & Paustenbach DJ (1988) A proposed occupational
exposure limit for 2,3,7,8-tetrachlorodibenzo- p-dioxin. Am Ind Hyg
Assoc J, 49(9): 466-474.
Luijk R & Govers HAJ (1992) The formation of polybrominated
dibenzo- p-dioxins (PBDDs) and dibenzofurans (PBDFs) during pyrolysis
of polymer blends containing brominated flame retardants. Chemosphere,
25(3): 361-374.
McAllister DL & Ariano JIM (1982) DE-71/DE-60F. Determination of
bromination level and aromatic phosphate ester concentration.
Analytical method No. QCS-82-25. West Lafayette, Indiana, Great Lakes
Chemical Corporation (Report submitted to WHO by BFRIP).
McAllister DL, Mazac CJ, Gorsich R, Freiberg M, & Tondeur Y (1990)
Analysis of polymers containing brominated diphenyl ethers as flame
retardants after molding under various conditions. Chemospbere,
20(10-12): 1537-1541.
McAllister DL (1991) Letter of Great Lakes Chemical Corporation, West
Lafayette to the IPCS/WHO, dated November 25, 1991.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
9(13): 1178-1180.
McMahon LW (1983) Organic priority pollutants in wastewater. In:
Proceedings of the 1982 UCC-ND/GATT Environmental Protection Seminar,
April, 5-7, 1982. Oak Ridge, TN. Oak Ridge National Laboratory/Union
Carbide Corp. US (DOE Contract No. W-7405-eng-26) (US EPA, 1986).
Mallory VT, Naismith RW, & Matthews RJ (1986) Primary eye irritation
(PH 421-ET-010-86) Saytex 102. Baton Rouge, Louisiana, Ethyl
Corporation (Unpublished report submitted to WHO by BFRIP).
Naismith RW & Matthews RJ (1981) Assay of comedogenicity in the rabbit
ear (PH 425-ET-001-81) Saytex 102. Baton Rouge, Louisiana, Ethyl
Corporation (Unpublished report submitted to WHO by BFRIP).
Neupert M, Weis H, Thies J, & Stock B (1989) Analytical procedures in
connection with acute animal toxicity studies. II. Pyrolysis products
obtained from an ABS copolymer containing octabromodiphenylether as a
flame retardant. Chemosphere, 19(16): 219-224.
Noguchi Y, Noda E, Toyoshima K, & Mitsui Toatsu Chemicals Inc. (1977)
Photostabilized polybromobiphenyl ethers. Japan Kokai 77 07,932,
January 21, 1977. (as reported in Chem. Abstr. 87: 134511x) (US EPA,
1986).
Norris JM (1971) Decabromodiphenyloxide solids from mother liquor
(waste tars). Midland, Michigan, Dow Chemical Company (Unpublished
report TOX-No. T2.TX-1767-1, submitted to WHO by BFRIP).
Norris JM, Ehrmantraut JW, Gibbons CL, Kociba RJ, Schwetz BA, Rose JQ,
Humiston CG, Jewett GL, Crummett WB, Gehring PJ, Tirsell JB, & Brosier
JS (1973) Toxicological and environmental factors involved in the
selection of decabromodiphenyl oxide as a fire retardant chemical.
Appl Polymer Symp, 22: 195-219.
Norris JM, Ehrmantraut JW, Gibbons CL, Kociba RJ, Schwetz BA, Rose JQ,
Humiston CO, Jewett GL, Crummett WB, Gehring Pl, Tirsell JB, & Brosier
JS (1974) Toxicological and environmental factors involved in the
selection of decabromodiphenyloxide as a fire retardant chemical.
J Fire Flamm Combust Toxicol, 1, 52-77.
Norris JM, Ehrmantraut JW, Kociba RJ, Schwetz BA, Rose JQ, Humiston
CO, Jewett OL, Crummet WB, Gehring PJ, Tirsell JB, & Brosier JS
(1975a) Evaluation of deca-bromodiphenyloxide as a flame-retardant
chemical. Chem Hum Health Environ, 1: 100-116.
Norris JM, Kociba RJ, Humiston CG, & Gehring PJ (1975b) The toxicity
of deca-bromodiphenyloxide and octabromodiphenyl as determined by
subacute and chronic dietary feeding studies in rats. Toxicol Appl
Pharmacol, 33(1): 170 (abstract).
Norris JM, Kociba RJ, Schwetz BA, Rose JQ, Humiston CG, Jewett GL,
Gehring PJ, & Mailhes JB (1975c) Toxicology of octabromodiphenyl and
decabromodiphenyloxide. Environ Health Perspect, 11: 153-161.
NTP (1986) Toxicology and carcinogenesis studies of decabromodiphenyl
oxide (CAS No. 1163-19-5) in F344/N rats and B6C3F1 mice (feed
studies). Research Triangle Park, North Carolina, US Department of
Health and Human Services, National Toxicology Program (NTP Technical
Report Series No. 309).
Nylund K, Asplurid L, Jansson B, Jonsson P, Litzen K, & Sellström U
(1992) Analysis of some polyhalogenated organic pollutants in sediment
and sewage sludge. Chemosphere, 24(12): 1721-1730.
Oberg T & Bergstrom J (1990) Bromine and waste incineration - An
environmental risk? In: Hutzinger O & Fiedler H ed. Dioxin'90.
EPRI-Seminar. Tenth International Symposium on Chlorinated Dioxins and
Related Compounds. Volume 2: Organo-halogenated compounds (short
papers). Bayreuth, Germany, Ecoinforma Press, pp 339-342.
Oberg T, Warman K, & Bergström J (1987) Brominated aromatics from
combustion. Chemosphere, 16(10-12): 2451-2465.
OECD (1991) Co-operation on existing chemicals: Risk reductions. Lead
country report on brominated flame retardants. Sixteenth Joint Meeting
of the Chemicals Group and Management Committee, 28-30 May 1991.
Paris, Organisation for Economic Cooperation and Development (Report
ENV/MC/CHEM (91)7).
Pijnenburg AMCM & Everts JW (1991) [Flame retardams: Occurrence and
toxicity.] The Hague, Ministry of Transport and Public Works (Note
No. GWAO-91.001) (in Dutch).
Pijnenburg AMCM, Everts JW, De Boer J, & Boon JP (1992) Polyhrominated
biphenyl (PBB) and diphenylether (PBDE) flame retardants: Analysis,
toxicity and occurrence in aquatic environments. Manuscript for
discussion in 1992 Annual Meeting of the ICES Marine Chemistry Working
Group (MCWG) and the Working Group on Biological Effects of
Contaminants (WGBEC). The Hague, Ministry of Transport and Public
Works (Unpublished report).
Pinkerton MN, Kociba RJ, Petrella RV, McAllister DL, Willis ML, Fulfs
JC, Thoma H, & Hutzinger O (1989) A preliminary report on the
investigation of the comparative toxicity of combustion products of
high impact polystyrene with and without decabromodiphenyloxide/
antimony trioxide as a flame retardant using 2,3,7,8-tetrabromo-
dibenzo- p-dioxin and 2,3,7,8-tetrabromodibenzofuran as positive
controls. Chemosphere, 18(1-6): 1243-1249.
Plumb RH Jr (1991) The occurrence of Appendix IX organic constituents
in disposal site ground water. Groundw Monit Rev, 11(2): 157-164.
Rampy LW (1971-1974) Chloracne studies on DBDPO and DBDPO mother
liquor. A series of working protocols with results from the Chemical
Biology Research Department of Dow Chemical USA, Midland, Michigan
(The work was carried out mainly by Rampy alone or with co-workers in
the period 1971-1974) (Report submitted to WHO by BFRIP).
Ranken PF, Ricks GM, Lynam DR, & Ariano JM (1990) Is watching
television toxic? BFRIP-sponsored study on the emission of PBDDs/PBDFs
from operating television sets. In: Hutzinger O & Fiedler H ed.
Dioxin'90. EPRI-Seminar. Tenth International Symposium on Chlorinated
Dioxins and Related Compounds. Volume 2: Organohalogenated compounds
(short papers). Bayreuth, Germany, Ecoinforma Press, pp 343-346.
Remmers JC, Breen JJ, Schwemberger J, Stanley JS, Cramer PH, &
Thornburg KR (1990) Mass spectral confirmat!on of chlorinated and
brominated diphenyl ethers in human adipose tissues. In: Hutzinger O &
Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth International Symposium
on Chlorinated Dioxins and Related Compounds. Volume 2:
Organohalogenated compounds (short papers). Bayreuth, Germany,
Ecoinforma Press, pp 347-350.
Riggs KB, Pitts GE, White JS, Mitchum RK, Reuther JJ, & Glatz JA
(1990) Polybrominated dibenzo- p-dioxin and polybrominated
dibenzofuran emissions from incineration of flame-retarded resins in a
simulated municipal waste incinerator. In: Hutzinger O & Fiedler H ed.
Dioxin'90. EPRI-Seminar. Tenth International Symposium on Chlorinated
Dioxins and Related Compounds. Volume 2: Organohalogenated compounds
(short papers). Bayreuth, Germany, Ecoinforma Press, pp 351-356.
Rogers J & Hill JT (1980) Final report. Determination of
decabromodiphenyl oxide (DBDPO) in rat livers. Vienna, Virginia,
Hazleton Laboratories America, Inc. (Unpublished report to Tracor
Jitco, Rockville, Maryland, submitted to WHO by BFRIP).
Rutter HA & Machotka S (1979) Final Report. Decabromodiphenyloxide:
13-week sub-chronic feeding study -- mice. Vienna, Virginia, Hazleton
Laboratories America, Inc. (Unpublished report to Tracor Jitco,
Rockville, Maryland, submitted to WHO by BFRIP).
Schwetz BA, Smith FA, Nitschke KD, Humiston CG, Jersey GC, & Kociba RJ
(1975) Results of a reproduction study in rats maintained on diets
containing decabromodiphenyloxide. Midland, Michigan, Dow Chemical
Company (Unpublished report No. HET K47298-(14), submitted to WHO by
BFRIP).
Sellström U, Andersson R, Asplund L, Jansson B, Jonsson P, Litzen K,
Nylund K, Uvemo U-B, & Wideqvist U (1990b) Anthropogenic brominated
aromatics in the Swedish environment. In: Freij L ed. Proceedings of
the Workshop on Brominated Aromatic Flame Retardants, Skokloster,
Sweden, 24-26 October 1989. Solna, National Chemicals Inspectorate
CKEMI), pp 73-78.
Sellström U, Jansson B, Ionsson P, Nylund K, Odsjö T, & Olsson M
(1990a) Anthropogenic brominated aromatics in the Swedish environment.
In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth
International Symposium on Chlorinated Dioxins and Related Compounds.
Volume 2: Organohalogenated compounds (short papers). Bayreuth,
Germany, Ecoinforma Press, pp 357-360.
Sellström U, Jansson B, Kierkegaard A, & De Wit C (1993a)
Polybrominated diphenyl ethers (PBDE) in biological samples from the
Swedish environment. Chemosphere, 26(9): 1703-1718.
Sellström U, Kierkegaard A, De Wit C, Jansson B, & Olsson M (1993b)
Time trend studies of polybrominated diphenyl ethers (PBDE) in
biological material from the Swedish environment. Paper presented at
Dioxin'93, Vienna, 1 September 1993.
Shoichet A & Ehrlich K (1977) Mutagenicity testing of HFO 102.
(Unpublished proprietary report from Gulf South Research Institute,
New Orleans, Louisiana to Hexel Fine Organics (Report submitted to WHO
by BFRIP).
Sovocool GW, Donnelly JR, Grange All, Simmons RD, & Munslow WD (1990)
Brominated dioxins and other bromoaromatics in plastic pyrolysates.
In: Hutzinger O & Fiedler H ed. Dioxin'90. EPRI-Seminar. Tenth
International Symposium on Chlorinated Dioxins and Related Compounds.
Volume 2: Organohalogenated compounds (short papers). Bayreuth,
Germany, Ecoinforma Press, pp 365-368.
Sparschu GL, Kociba RJ, & Clashman A (1971) Results of 30 day rat
dietary feeding studies on octabromobiphenyl SA-1902 and
decabromodiphenyl oxide SA-1892.1. Midland, Michigan, Dow Chemical
Company (Unpublished report submitted to WHO by BFRIP).
Stafford CJ (1983) Halogenated diphenylethers identified in avian
tissues and eggs by GC/MS. Chemosphere, 12(11/12): 1487-1495.
Stanley JS, Cramer PH, Thornburg KR, Remmers JC, Breen JJ, &
Schwemberger J (1991) Mass spectral confirmation of chlorinated and
brominated diphenylethers in human adipose tissues. Chemosphere,
23(8-10): 1185-1195.
Striebich RC, Rubey WA, Tirey DA, & Dellinger B (1991) High
temperature degradation of polybrominated flame retardant materials.
Chemosphere, 23(8-10): 1197-1204.
Sundström G & Hutzinger O (1976) Environmental chemistry of flame
retardants. V. The composition of Bromkal (R) 70-5 DE -- A
pentabromodiphenyl ether preparation. Chemosphere, 3: 187-190.
Tabak HH, Quave SA, Mashni Cl, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Tabor TE & Bergman S (1975) Decabromodiphenyl oxide. A new fire
retardant additive for plastics. In: Fire retardants. Proceedings of
1974 International Symposium on Flammability and Fire Retardants,
Cornwall, Ontario, 1-2 May 1974. Westport, Connecticut, Technomic
Publishing, pp 162-179.
Takase I, Omori T, & Midona Y (1986) Microbial degradation products
from biphenyl-related compounds. Agric Biol Chem, 50(3): 681-686.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res,
37: 2717-2720.
Thoma H & Hutzinger O (1987) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemopshere,
16(6): 1353-1360.
Thoma H & Hutzinger O (1989) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemosphere,
18(5): 1047-1050.
Thoma H, Hauschulz G, Knorr E, & Hutzinger O (1987a) Polybrominated
dibenzofurans (PBDF) and dibenzodioxins (PBDD) from the pyrolysis of
neat brominated diphenylethers, biphenyls and plastic mixtures of
these compounds. Chemosphere, 16(1): 277-285.
Thoma H, Hauschulz G, & Hutzinger O (1987b) PVC-induced
chlorine-bromine exchange in the pyrolysis of polybrominated diphenyl
ethers, -biphenyls, dibenzodioxins and dibenzofurans. Chemosphere,
16(1): 297-307.
Timmons LL & Brown RD (1988) Analysis of the brominated fire retardant
decabromdiphenyloxide for low and trace levels impurities.
Chemosphere, 17: 217-234.
UK DOE (1992) Risk benefit analysis of hazardous chemicals. London, UK
Department of Environment.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
UMWELTBUNDESAMT (1989) Status report: Polybrominated dibenzodioxins
and polybrominated dibenzofurans. Berlin.
US EPA (1978) Chemical screening. Initial evaluations of substantial
risk notices: section 8(e), January 1, 1977 to June 39, 1979.
Washington, DC, US Environmental Protection Agency, Office of
Pesticides and Toxic Substances, Office of Testing and Evaluation,
vol I, pp 39-40 (EPA 560/11-80-008).
US EPA (1984) Health and environmental effects profile for brominated
diphenyl ethers. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office CEPA/600/X-84/133).
US EPA (1986) Brominated diphenylethers. Chemical hazard information
profile. Washington, DC, US Environmental Protection Agency.
US EPA (1988) Information review: Brominated diphenyl ethers.
Washington, DC, US Environmental Protection Agency, TSCA, Interagency
Testing Committee (Report No. IR-516).
US EPA (1989) Letter from Director Office of Toxic Substances,
Washington, to Great Lakes Chemical Corporation, dated July 27, 1989.
US Testing Company (1985) Determination of vapour pressure and melting
point. Chemical Services Division. Hoboken, New Jersey. Letter to
Great Lakes Chemical Corporation. 7 October 1985 (Report submitted to
WHO by BFRIP).
Vinci TM & Craig DK (1988) Characterization of fume components
generated during the extrusion of flame-retarded polyester resins.
Columbus, Ohio, Battelle (Unpublished report to GE Plastics, Mt.
Vernon, Indiana, submitted to WHO by BFRIP).
Von Meyerinck L, Hufnagel B, Schmoldt A, & Benthe HF (1990) Induction
of rat liver microsomal cytochrome P-450 by the pentabromodiphenyl
ether Bromkal 70 and half lives of its components in the adipose
tissue. Toxicology, 61: 259-274.
Walsh GE, Yoder MI, McClaughlin LL, & Lores EM (1987) Responses of
marine unicellular algae to brominated organic compounds in six growth
media. Ecotoxicol Environ Saf, 14: 215-222.
Watanabe I (1987) Text from undated slide presentation.
Watanabe I & Tatsukawa R (1987) Formation of brominated dibenzofurans
from the photolysis of flame retardant decabromodiphenyl ether in
hexane solution by UV and sunlight. Bull Environ Contain Toxicol,
39: 953-959.
Watanabe I & Tatsukawa R (1990) Anthropogenic brominated aromatics in
the Japanese environment. In: Freij L ed. Proceedings of the Workshop
on Brominated Aromatic Flame Retardants, Skoldoster, Sweden, 24-26
October 1989. Solna, National Chemicals Inspectorate (KEMI), pp 63-71.
Watanabe I, Kashimoto T, & Tatsukawa R (1986) Confirmation of the
presence of the flame retardant decabromodiphenyl ether in river
sediment from Osaka, Japan. Bull Environ Contam Toxicol, 36: 839-842.
Watanabe I, Kashimoto T, Kawano M, & Tatsukawa R (1987a) A study of
organic bound halogens in human adipose tissue, marine organisms and
sediment by neutro-activation and gas chromatographic analysis.
Chemosphere, 16(4): 849-857.
Watanabe I, Kashimoto T, & Tatsukawa R (1987b) Polybrominated
biphenylethers in marine fish, shellfish and river and marine
sediments in Japan. Chemopshere, 16(10/12): 2389-2396.
Watanabe I, Kawano M, Wang Y, Chen Y, & Tatsukawa R (1992)
Polybrominated dibenzo- p-dioxins (PBDDs) and -dibenzofurans (PBDFs)
in atmospheric air in Taiwan and Japan. In: Dioxin'92. Twelfth
International Symposium on Dioxins and Related Compounds, Tampere,
Finland, 24-28 August 1992. Organohalogen Compounds Volume 9: Sources
of exposure.
Watanabe I, Kawano M, & Tatsukawa R (undated) Consumption trend and
environmental research on brominated flame retardants in Japan and the
formation of polyhalogenated dibenzofurans at the metal reclamation
factory. Document distributed at the OECD Workshop on the Risk
Reduction of Brominated Flame Retardants, Neuchatel, 1993.
WHO (1989) Environmental Health Criteria 88: Polychlorinated
dibenzo- p-dioxins and bibenzofurans. Geneva, World Health
Organization.
WHO (1994) Environmental Health Criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization.
Yagi O & Sudo R (1980) Degradation of polychlorinated biphenyls by
microorganisms. JWPCF 52(5), 1035-1043 and US EPA, 1986.
Yamamoto K, Mori Y, Uehira S, Tanaka T, Koyama T, Katuyama K,
Taniguchi Y, & Sakamoto T (1991) Content of presence of the flame
retardant decabromodiphenyl ether in Kino river Basin. Wakayama-ken
Eisei Kogai Kenkyu Senta Nenpo, 37:116-122.
Zacharewski T, Harris M, Safe S, Thoma H, & Hutzinger O (1988)
Applications of the in vitro aryl hydrocarbon hydroxylase induction
assay for determining "2,3,7,8-tetrachlorodibenzo- p-dioxin
equivalents": pyrolyzed brominated flame retardants. Toxicology,
51: 177-189.
Zier B, Lenoir D, Lahaniatis ES, & Kettrup A (1991) Surface catalyzed
halogenation-dehalogenation reactions of aromatic bromine compounds
adsorbed on fly ash. Chemosphere, 22(12): 1121-1129.
Zober MA, Ott MG, Pfipke O, Senft K, & Germann C (1992) Morbidity
study of extruder personnel with potential exposure to brominated
dioxins and furans. I. Results of blood monitoring and immunological
tests. Br J Ind Med, 49: 532-544.
Zogorski JS (1984) Experience in monitoring domestic water sources and
process waters for trace organics. J Environ Sci Health,
A19(2): 233-249.
Van Zorge JA, Bruijnes C, Van den Berg EV, & Sprong W (1992)
Brominated flame retardants: Polybrominated biphenyls (PBBs) and
polybrominated diphenyloxides (PBDOs). OECD Cooperative risk reduction
activities for certain dangerous chemicals, Paris. Fifth draft, May
26th, 1992.
Zweidinger RA, Cooper SD, Erickson MD, Michael LC, & Pellizzari ED
(1977) Sampling and analysis for semivolatile brominated organics in
ambient air. In: Schuetzle D ed. Monitoring toxic substances.
Washington, DC, American Chemical Society, pp 217-231 (ACS Symposium
Series No. 94).
Zweidinger RA, Cooper SD, & Pellizzari ED (1978) Identification and
quantitation of brominated fire retardants. In: Measurement of organic
pollutants in water and waste water. Symposium of the American Society
for Testing and Materials, Denver, Colorado, 19-20 June 1978.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 234-250 (ASTM Technical Publication No. 686).
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
DECABROMODIPHENYLETHER
1 Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le DeBDE du commerce se caractérise par une pureté de 97 à 98%, et
une teneur en nona- et/ou octabromodiphényléthers de 0,3 à 3,0%. Le
nonabromodiphényléther (NBDE) en est l'impureté principale.
Contrairement aux autres polybromo-diphényléthers, le DeBDE n'a qu'un
isomère.
Le point de fusion du DeBDE est d'environ 300°C et il se décompose
au dessus de 400°C. Sa solubilité dans l'eau est de 20-30/µg/litre et
le logarithme de son coefficient de partage n-octanol/eau est
supérieur à 5. Sa tension de vapeur est <10-6 mmHg à 20°C.
1.2 Production et usages
De tous les bromodiphényléthers (mono- à déca-) le
décabromodiphényléther est, de par sa production et son usage, le plus
important du point de vue commercial.
On le produit depuis la fin des années 70 à un degré de plus en
plus élevé de pureté. La production mondiale de DeBDE est d'environ
30 000 tonnes par an. On l'utilise comme retardateur de flamme dans de
nombreuses matières plastiques, notamment dans le polystyrène choc,
ainsi que pour le traitement des tissus d'ameublement, ou encore des
textiles utilisés en sellerie automobile ou pour la confection de
tentes.
1.3 Transport, distribution et transformation dans l'environnement
En solution dans des solvants organiques, le DeBDE subit une
photodécomposition sous l'influence du rayonnement ultra-violet ou de
la lumière solaire; cette réaction conduit à la formation de
bromodiphényléthers moins substitués et de bromodibenzofuranes. Cette
photodécomposition se produit également, quoique dans une moindre
mesure, en solution aqueuse, sous l'action du rayonnement solaire; on
ne retrouve plus alors d'homologues inférieurs du DeBDE ni de
bromodibenzofuranes.
Les quantités de DeBDE que l'on peut extraire des polymères sont
inférieures à la limite de détection ou très proches de cette limite,
selon le type de polymère et le solvant d'extraction.
Du fait que sa solubilité dans l'eau et sa tension de vapeur sont
très faibles, le DeBDE est vraisemblablement transporté
essentiellement par adsorption sur des particules. Il est persistant
et s'accumule probablement dans les sédiments et le sol.
On en dispose d'aucune donnée sur sa biodisponibilité à partir des
sédiments et du sol. Une étude sur des truites arc-en-ciel n'a pas
révélé de bioaccumulation dans la chair, la peau ou les viscères de
ces poissons, sur une période de 48 heures. Il est improbable que le
DeBDE subisse une bioaccumulation en raison de sa masse moléculaire
relative élevée.
Les rebuts qui contiennent du DeBDE commercial finissent dans les
décharges contrôlées ou les incinérateurs. Il se peut que du DeBDE
s'échappe de ces décharges par lessivage. Le brûlage sur les décharges
ou une incinération inefficace peuvent conduire à la formation de
polybromodibenzofuranes (PBDF) et d'un mélange d'halogénodi-
benzofuranes et d'halogénodibenzodioxines. Les produits qui
contiennent du DeBDE commercial peuvent contribuer à ces émissions.
La pyrolyse, en présence d'oxygène, de DeBDE commercial et de
polymères contenant de ce produit (polystyrène choc, PBT,
polypropylène industriel) produit des polybromodibenzofuranes et en
moindre quantités, des polybromodibenzodioxines. C'est aux
températures de 400 à 500°C que les PBDF sont produits en quantités
maximales mais ils peuvent également se former à des températures
allant jusqu'à 800°C et Sb2O3 en catalytise la formation.
La formation des PBDF et des PBDD ainsi que leurs proportions
respectives, dépendent de la température, de la teneur en oxygène et
de la durée de la pyrolyse. En l'absence d'oxygène, il se forme
principalement des polybromobenzènes et des polybromonaphtalènes.
1.4 Concentrations dans l'environnement et exposition humaine
On a trouvé du DeBDE dans l'air à proximité d'unités de
production, à des concentrations allant jusqu'à 25 µg/m3. En
revanche, on n'a pas décelé de DeBDE dans des échantillons d'eau
prélevés au Japon entre 1977 et 1991. Cependant, on en a trouvé dans
des sédiments de rivières, également prélevés au Japon au cours de la
même période, à des déconcentrations allant jusqu'à environ 12 mg/kg
de poids sec. On a également décelé la présence (jusqu'à 1 g/kg) de
DeBDE dans des sédiments de cours d'eau aux Etats-Unis, à proximité
d'une unité de production. On n'a pas décelé de DeBDE dans des
échantillons de poissons recueillis au Japon, mais, dans un
échantillon de moules on en a trouvé à des teneurs juste supérieures
au seuil de détection. Le produit n'a pas été décelé dans des
échantillons de tissus adipeux humains prélevés au Japon; en revanche,
aux Etats-Unis, on en a trouvé dans trois échantillons sur cinq du
même type de tissus.
Au cours de la production de DeBDE et de son adjonction aux
polymères, il peut y avoir exposition humaine. En revanche, cette
exposition est négligeable pour la population générale.
En cherchant à déterminer l'exposition professionnelle aux
produits de décomposition du DeBDE lors de sa fabrication et de son
incorporation par extrusion à des polymères, on a constaté la présence
de fortes concentrations de PBDF dans des échantillons d'air prélevés
au niveau de la filière de l'extrudeuse. Les concentrations étaient
moindres dans l'air de l'atelier. Des PBDF ont été également retrouvés
dans des échantillons obtenus lors de l'essuyage. Moyennant une bonne
technique de travail, on peut réduire l'exposition professionnelle aux
PBDF.
L'exposition de la population générale aux PBDF présents comme
impuretés dans des polymères contenant des retardateurs de flamme, est
vraisemblablement négligeable.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Le DeBDE est faiblement résorbé dans les voies digestives et après
injection, il est rapidement excrété.
D'après les résultats d'études de métabolisme chez le rat, au
moyen de DeBDE marqué au 14C, la demi-vie d'élimination de ce composé
serait inférieure à 24 heures et la principale voie d'élimination
après ingestion, serait la voie fécale. On n'a pas constaté la
présence d'une activité appréciable de 14C (moins de 1%) dans l'urine
ou l'air expiré.
Des rats à qui l'on avait administré pendant des périodes allant
jusqu'à deux ans, une dose quotidienne de 0,1 mg/kg de poids corporel
de ce composé, n'ont présenté aucun accumulation de DeBDE dans leur
sérum, leurs reins, leurs muscles ou leurs testicules, d'après le
dosage du brome total. L'accumulation du brome dans le foie a atteint
un palier au bout de 30 jours et l'élimination s'est effectuée dans
les 10 jours suivants le traitement. Après 180 jours, la concentration
de brome dans le foie n'était pas plus élevée chez les rats traités
que chez les rats témoins. Dans les tissus adipeux, on a noté une
faible accumulation de brome total, qui a subsisté après 90 jours de
nourriture sans DeBDE; on ignore quelle est la nature du "brome"
retenu. Etant donné que le DeBDE ne représentait que 77% du mélange
commercial utilisé, ce "brome" pourrait provenir du NBDE ou de l'OBDE.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Pour les animaux de laboratoire, la toxicité aiguë du DeBDE est
faible. Le produit n'est pas irritant pour la peau ou les yeux des
lapins. Il ne provoque pas de chloracné sur la peau des lapins et n'a
pas d'effet sensibilisateur sur la peau humaine.
Les produits de combustion du polystyrène contenant un retardateur
de flamme à base de DeBDE et de Sb2O3 ont été étudiés afin d'en
déterminer la toxicité aiguë et la comédogénicité. Chez le rat, la
DL50 par voie orale de la suie et du résidu de carbonisation était
>2000 mg/kg de poids corporel.
Lors d'études de toxicité à long terme effectuées sur des rats et
des souris, du DeBDe (pureté >97%) a été administré aux animaux mêlé
à leur nourriture aux doses de 100 g/kg de nourriture (4 semaines) ou
50g/kg (13 semaines, soit l'équivalent de 2500 mg/kg de poids corporel
en ce qui concerne le rat) sans produire d'effets nocifs. Une étude de
reproduction portant sur une génération de rats n'a pas révélé
d'effets nocifs aux doses de 100 mg/kg de poids corporel. Le DeBDE n'a
pas provoqué d'effets tératogènes chez des foetus de rats ayant reçu
une dose de 100 mg/kg de poids corporel. A la dose de 1000 mg/kg de
poids corporel, on a observé des malformations, par exemple un retard
d'ossification. Soumis à un certain nombre d'épreuves, le DeBDE ne
s'est pas révélé mutagène.
Lors d'une étude de cancérogénicité sur des rats et des souris, du
DeBDE (pureté 94-99%) a été administré, mêlé à leur nourriture, à des
doses allant jusqu'à 50 g/kg. On a observé une augmentation dans
l'incidence des adénomes (mais pas des carcinomes) au niveau du foie
chez les rats mâles qui en avaient reçu 25 g/kg et chez les femelles
qui en avaient reçu 50 g/kg. Chez les souris mâles, on a observé une
augmentation de l'incidence des adénomes et/ou des carcinomes
hépatocellulaires (les deux ensemble) à la dose de 25 g/kg et une
augmentation des adénomes et des cancers (les deux ensemble)
médullaires de la thyroïde à ces deux doses. Chez les souris femelles,
on n'a pas relevé d'augmentation dans l'incidence des tumeurs. C'est
uniquement aux doses de 25 à 50 g de DeBDE/kg de nourriture que chez
les rats mâles et femelles d'une part, et chez les souris mâles
d'autre part, on a constaté l'existence de signes équivoques de
cancérogénicité. Etant donné que les résultats de toutes les épreuves
de mutagénicité sont restés négatifs, on peut en conclure que le DeBDE
n'est pas un cancérogène génotoxique. Le CIRC (1990) est parvenu à la
conclusion que les preuves d'une cancérogénicité du DeBDE chez les
animaux de laboratoire sont limitées. Du fait des très fortes doses
utilisées dans ces études, de l'absence de génotoxicité et des
éléments de preuve très minimes en faveur d'une cancérogénicité de ce
composé, on peut conclure que le DeBDE, aux taux d'exposition actuels,
ne présente pas de risque cancérogène pour l'homme.
1.7 Effets sur l'homme
Chez des sujets humains exposés à du DeBDE lors d'une épreuve de
sensibilisation, on n'a noté aucun signe de sensibilisation cutanée.
Une étude de morbidité sur des ouvriers qui procédaient à
l'extrusion de polybutylène-téréphtalate contenant du DeBDE (avec par
conséquent un risque potentiel d'exposition aux PBDD et aux PBDF
pendant 13 ans) n'a pas révélé le moindre effet délétère, malgré la
présence dans leur sang de 2,3,7,8-TeBDF et de 2,3,7,8-TeBDD. Les
études immunologiques effectuées ont montré que le système immunitaire
des personnes exposées n'avait pas souffert au cours de ces 13 années.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
La CE50 relative à la croissance de trois algues marines
unicellulaires s'est révélée supérieure à 1 mg de DeBDE par litre. On
ne dispose d'aucune autre information concernant les effets du DeBDE
sur les autres êtres vivants au laboratoire ou dans leur milieu
naturel.
2 Conclusions
2.1 DeBDE
Le DeBDE est largement utilisé comme retardateur de flamme dans
les polymères. S'il entre en contact avec la population générale,
c'est par l'intermédiaire des produits fabriqués à partir de ces
polymères. L'exposition est très faible étant donné que le DeBDE n'est
pas facile à extraire de ces polymères. La toxicité aiguë du DeBDE est
très faible et son absorption au niveau des voies digestives est
minime. On peut donc considérer comme insignifiant le risque que le
DeBDE représente pour la population générale.
C'est sous forme particulaire que le DeBDE peut donner lieu à une
exposition professionnelle. En se prémunissant contre les poussières
au cours de la fabrication et de l'utilisation de ce produit, on peut
efficacement en réduire les risques pour les travailleurs.
Le DeBDE est persistant et il se fixe sur les particules de
matière présentes dans l'environnement; il est vraisemblable qu'il
s'accumule dans les sédiments. En revanche, sa bioaccumulation est
improbable. D'après les données disponibles, la photodécomposition du
DeBDE en milieu aqueux dans l'environnement ne conduit pas à la
formation de diphényléthers bromés inférieurs ni de
bromodibenzofuranes, mais on est mal renseigné sur la décomposition de
ce composé dans d'autres milieux.
On est également très peu renseigné sur la toxicité du DeBDE pour
les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Il peut se former des PBDF et, dans une certaine mesure, des PBDD
lorsque du DeBDE ou des produits qui en contiennent sont chauffés à
300-800°C. Il faut se préoccuper des dangers que ces produits
pourraient représenter,.
Lorsqu'elle est correctement conduite, l'incinération n'entraîne
pas l'émission de quantités importantes de bromodioxines ou de
bromodibenzofuranes. Si la combustion s'effectue dans des conditions
non contrôlées, des PBDF et des PBDD peuvent prendre naissance en
quantités indéterminées. Les risques qui pourraient en résulter pour
l'homme et l'environnement seront étudiés dans un prochain Critère
d'hygiène de l'environnement sur les PBDF et les PBDD.
On a observé la présence de PBDF dans le sang de travailleurs
employés à la production de matières plastiques contenant du DeBDE.
Toutefois aucun effet délétère sur leur santé n'a pu être attribué à
ce type d'exposition. Moyennant des techniques appropriées, il est
possible d'éviter l'exposition des travailleurs aux PBDF.
3 Recommandations
3.1 Recommandations générales
* Il convient que les travailleurs qui sont employés à la
fabrication de DeBDE et de produits qui en contiennent soient
protégés contre toute exposition par l'application de mesures
appropriées d'hygiène industrielle, la surveillance de
l'exposition professionnelle et des moyens techniques appropriés.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions produits par
les industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé persistant et ses
produits de décomposition.
* Les fabricants doivent faire en sorte que le DeBDE commercial
contienne le minimum d'impuretés, en utilisant pour cela les
meilleures techniques existantes. Une pureté d'au moins 97% est
recommandée.
* On ne doit procéder à l'incinération qu'au moyen d'incinérateurs
convenables, qui fonctionnent toujours dans les conditions
optimales. Le brûlage des déchets par tout autre moyen risque
d'entraîner la formation de PBDF et/ou de PBDD.
3.2 Etudes à effectuer
* Il conviendrait d'effectuer, sur les organismes appropriés, des
études sur la biodisponibilité et la toxicité du DeBDE lié aux
sédiments.
* Il importe d'assurer une surveillance permanente des
concentrations dans l'environnement.
* La production de PBDF en situation réelle d'incendie doit faire
l'objet d'études plus approfondies.
* Il importe d'étudier plus à fond la biodécomposition dans
l'environnement ainsi que la photodécomposition de ce composé dans
des milieux autres que l'eau.
* Il conviendrait d'étudier des méthodes pour le recyclage des
polymères contenant du DeBDE et leurs conséquences.
* Il faudrait valider les méthodes d'analyse du DeBDE dans diverses
matrices.
NONABROMODIPHENYLETHER
Le nonabromodiphényléther n'est ni produit ni utilisé. On ne
dispose d'aucune donnée sur les points suivants:
* Transport, distribution et transformation dans l 'environnement
* Concentrations dans l'environnement et exposition humaine
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe aucune base de données sur laquelle se fonder pour une
évaluation.
2 Recommandations
Il importe de réduire au minimum le taux de contamination des
retardateurs de flamme bromés du commerce par le
nonabromodiphényléther afin d'éviter une pollution de l'environnement
et l'exposition humaine.
OCTABROMODIPHENYLETHER
L'octabromodiphényléther n'est ni fabriqué ni utilisé à l'état
pur. On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1. Résumé et évaluation
1.1 Identité et propriétés physiques et chimiques
L'OBDE du commerce se présente sous la forme d'un mélange
d'environ 11% de PeBDE/HxBDE, 44% de HpBDE, 31-35% d'OBDE, 10% de NBDE
et 0,5% de DeBDE. D'après la structure chimique, il y a thé-oriquement
12 isomères de l'OBDE et 24 isomères de l'HpBDE.
Le point de fusion varie d'environ 80°C à >200°C. La tension de
vapeur est <10-7 mmHg, la solubilité dans l'eau est faible et le
logarithme du coefficient de partage n-octanol/eau est >5,5. Ces
variations dans les propriétés physiques s'expliquent par des
différences de composition des mélanges étudiés.
1.2 Production et usages
La consommation mondiale annuelle d'OBDE commercial est de 6000
tonnes, dont 70% sont utilisés comme retardateurs de flamme dans les
résines ABS qui servent à la fabrication d'ordinateurs et d'armoires
de bureau. L'OBDE vient au second rang des retardateurs de flamme dans
l'ordre d'utilisation.
1.3 Transport, distribution et transformation dans l'environnement
On a trouvé des constituants de l'OBDE du commerce dans des
sédiments aquatiques et des tissus adipeux humains. Certains de ces
constituants à plus faible teneur en brome (HxBDE et PeBDE) ont été
observés dans des biotes. On n'a pas décelé d'OBDE, mais on n'a
cherché généralement ni le HpBDE ni le NBDE. Les constituants de
l'OBDE commercial sont vraisemblablement persistants mais, lorsque le
degré de substitution par le brome dépasse 6, la bioaccumulation
devient de plus en plus improbable. On a trouvé chez la carpe un
facteur de bioaccumulation de moins de 2 pour un OBDE commercial.
La pyrolyse de l'OBDE commercial, soit tel quel, soit ajouté à des
polymères comme retardateur de flamme (avec ou sans Sb2O3) à 600°C,
produit des PBDF et, à beaucoup plus faibles concentrations, des PBDD.
Le traitement des résines ABS avec de l'OBDE et du Sb2O3 dans
différentes conditions révèle, que dans les conditions normales, il ne
se forme que de faibles concentrations de PBDF. Lorsque les conditions
sont mauvaises, les concentrations sont beaucoup plus élevées. En
revanche, les concentrations de PBDD sont faibles dans les deux cas.
1.4 Concentrations dans l'environnement et exposition humaine
Dans des échantillons d'eau recueillis au Japon en 1987 et 1988,
on n'a pas pu déceler d'OBDE ni de constituants de l'OBDE du commerce
à plus faible teneur en brome. On a également analysé des échantillons
de sédiments et dans environ 2-6% d'entre eux, on a observé la
présence d'OBDE à des concentrations de 8-22 µg/kg de poids sec. Dans
le sédiment, on a également trouvé des constituants moins substitués
en brome.
Chez des poissons capturés au Japon en 1987 et en 1988, on n'a pas
décelé d'OBDE.
Aux Etats-Unis d'Amérique, on a analysé en 1987 des échantillons
de tissus adipeux humains à la recherche de PBDF et de PBDD. Les
échantillons provenaient de 865 prélèvements associés de manière à
former 48 homologues composites. Ce schéma d'échantillonnage composite
reposait sur 9 divisions censitaires et 3 groupes d'âge. Dans ces
échantillons, on a également constaté la présence de PBDE et les
premiers résultats ont montré que de l'OBDE était également présent
dans 60% des cas avec une concentration estimative allant jusqu'à
8000 ng/kg.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Aucune donnée disponible.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
L'OBDE du commerce présente une faible toxicité aiguë pour les
mammifères de laboratoire. Il n'est pas irritant pour la peau et ne
provoque qu'une légère irritation oculaire chez le lapin. Lors
d'études toxicologiques à court terme (respectivement 4 et 13
semaines), on a observé, chez des rats à qui l'on en avait administré
dans leur nourriture à raison de 100 mg/kg, une augmentation du poids
du foie et des altérations microscopiques caractérisées par la
présence, dans la région centro- et médio-lobulaire, de cellules
parenchymateuses hypertrophiées contenant des structures granulaires.
La gravité de ces lésions hépatiques était plus marquée aux doses
élevées, c'est-à-dire 1000 et 10 000 mg/kg de nourriture. En outre, on
a observé une hyperplasie de la thyroïde. La teneur en brome total de
ces tissus a augmenté au cours de l'étude pour diminuer ensuite
lentement au cours de la période de récupération. Les anomalies
hépatiques étaient réversibles. Lors d'une étude toxicologique par
inhalation au cours de laquelle on a fait respirer de la poussière
micronisée d'OBDE aux animaux (8 h/jour, 14 jours de suite) on n'a pas
observé d'effets à la concentration de 1,2 mg/m3 mais à celle de
12 mg/m3, on a observé des lésions hépatiques analogues à celles qui
avaient été décelées après ingestion du produit.
Chez le rat, des doses relativement faibles d'OBDE du commerce
accroissent d'activité du cytochrome P-450 et induisent les enzymes
des microsomes hépatiques, comme l'UDP-glucuronyl-transférase et la
benz[a]pyrène-hydroxylase. On a également constaté que ce produit
avait un effet porphyrinogène sur les cultures de cellules hépatiques
d'embryon de poulet.
L'étude du pouvoir tératogène de l'OBDE chez le rat a montré qu'à
fortes doses (25,0 et 50,0 mg/kg de poids corporel), ce produit
provoquait des résorptions ou un retard d'ossification en différents
points du squelette ainsi que des malformations foetales. Les
malformations observées aux doses de 25 mg/kg de poids corporel et
au-delà étaient très vraisemblablement liées à la toxicité du produit
pour la mère. Ces malformations n'ont pas été observées aux doses
inférieures ou égaies à 15,0 mg/kg de poids corporel. Chez le lapin,
aucun signe d'activité tératogène n'a été observé, en revanche on a
constaté une certaine foetotoxicité à la dose de 15 mg/kg de poids
corporel qui était également toxique pour la mère. D'après les études
de tératogénicité, la dose sans effets nocifs observables est de
2,5 mg/kg de poids corporel.
Lors de deux études, l'une de 28 jours et l'autre de 90 jours, on
a constaté qu'une dose de 100 mg d'OBDE/kg de nourriture (soit
l'équivalent de 5 mg/kg de poids corporel) ne produisait que des
effets minimes sur le foie. On n'a pas cherché à établir la dose sans
effets observables.
Toutes les épreuves de mutagénicité: synthèse non programmée de
l'ADN, tests sur microorganismes in vitro et recherche d'échanges
entre chromatides-soeurs sur cellules ovariennes de hamster chinois se
sont révélées négatives.
On ne possède aucune donnée résultant d'études de cancéro-génicité
ou d'études à long terme.
1.7 Effets sur l'homme
Aucune donnée disponible.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Données minimales.
2 Conclusions
2.1 OBDE
L'OBDE du commerce est un mélange d'hexa-, d'hepta-, d'octa- et de
nonabromodiphényléthers, qui sont tous des composés rémanents, en
grande partie fixée aux sédiments.
L'OBDE est très largement utilisé comme retardateur de flamme dans
les polymères. Il peut y avoir contact avec la population générale par
l'intermédiaire de produits fabriqués à partir de ces polymères. Il
est peu probable qu'on puisse être exposé à de l'OBDE qui aurait été
extrait de ces polymères.
L'OBDE présente une faible toxicité aiguë. On ne dispose d'aucune
donnée sur sa fixation ou son élimination par l'organisme des
mammifères. Il n'est ni tératogène ni mutagène. On ne dispose d'aucune
donnée sur sa toxicité à long terme ni sur sa cancéro-génicité.
On a relevé la présence, dans des tissus adipeux humains, de
plusieurs constituants de l'OBDE commercial. Pour la population dans
son ensemble, le risque d'intoxication aiguë est vraisemblablement
faible. Il n'est pas possible d'évaluer les risques d'une exposition à
long terme en raison de l'absence d'études toxicologiques pertinentes.
On ne dispose non plus d'aucune information qui permette de tirer
des conclusions quant à l'exposition professionnelle à l'OBDE et à ses
effets.
Les données relatives à la toxicité de l'OBDE pour les êtres
vivants dans leur milieu naturel demeurent limitées. Il est possible
que les constituants peu substitués en brome du mélange commercial
subissent une bioaccumulation.
2.2 Produits de décomposition
Lors du chauffage de l'OBDE ou de produits qui en contiennent à
des températures de 400-800°C, il peut se former des PBDF et, dans une
certaine mesure, des PBDD. Il importe de se préoccuper des dangers qui
pourraient en résulter.
Il est improbable que la population générale soit exposée de façon
notable aux impuretés de type PBDF, présentes dans les polymères
contenant des retardateurs de flamme. Moyennant une incinération dans
les règles, il ne devrait pas y avoir d'émission importante de
bromodioxines ni de bromofuranes. Le brûlage sans précautions de
produits qui contiennent de l'OBDE du commerce, peut donner naissance
à des PBDF et des PBDD en quantités indéterminées. Ce problème et ses
conséquences pour la santé humaine et l'environnement, seront abordés
dans un prochain Critère d'hygiène de l'environnement consacré aux
PBDF et aux PBDD.
3 Recommandations
3.1 Recommandations générales
* Il convient d'utiliser les meilleurs techniques possibles pour la
fabrication de l'OBDE du commerce, afin de réduire au minimum la
teneur en homologues hexabromés ou moins substitués, du fait de
leur possibilité de bioaccumulation dans l'environnement.
* Les travailleurs qui sont employés à la fabrication de l'OBDE ou
de produits qui en contiennent, doivent être protégés contre
l'exposition à ces dérivés par des mesures d'hygiène industrielle,
une surveillance de l'exposition professionnelle et des moyens
techniques.
* Il convient de limiter au maximum la pollution de l'environnement
grâce à un traitement approprié des effluents et des émissions
provenant d'industries qui utilisent le composé ou des produits
qui en contiennent. Le rejet de produits industriels et de
produits de consommation devra être réglementé de façon à réduire
au minimum la pollution de l'environnement par ces composés
rémanents et leurs produits de décomposition.
* L'incinération de produits qui contiennent des retardateurs de
flamme à base d'OBDE, ne doit s'effectuer que dans des
incinérateurs appropriés fonctionnant toujours dans des conditions
optimales. Le brûlage par tout autre moyen risque de donner
naissance à des PBDF ou des PBDD.
3.2 Etudes à effectuer
Comme la base de données toxicologiques actuelle est insuffisante
pour permettre d'évaluer les dangers que représente l'OBDE du commerce
pour l'homme et l'environnement, et pour en faciliter l'utilisation,
il est recommandé d'entreprendre les études suivantes:
* Etudes, sur des organismes appropriés, relatives à la
biodisponibilité et à l'écotoxicité des constituants de l'OBDE du
commerce liés aux sédiments
* Surveillance généralisée de la concentration des constituants de
l'OBDE du commerce dans l'environnement
* Etudes toxicologiques à long terme et études de cancéro-génicité
portant sur l'OBDE du commerce
* Surveillance de l'exposition professionnelle à l'OBDE du commerce
* Poursuite des études sur la formation de PBDF dans des conditions
réelles d'incendie
* Poursuite des études sur la biodécomposition et la
photodécomposition environnementales dans des milieux non aqueux
* Etudes de méthodes de recyclage des polymères contenant de l'OBDE
et de ses conséquences
* Validation des méthodes d'analyse de l'OBDE dans diverses matrices
* Etudes sur la possibilité de migration à partir de divers types de
polymères.
HEPTABROMODIPHENYLETHER
L'heptabromodiphényléther n'est ni produit ni utilisé.
Il n'existe pas de base de données relatives à l'HpBDE pur sur
laquelle fonder une évaluation.
On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
Du fait que l'HpBDE est le principal constituant de
l'octabromodiphényléther du commerce, le résumé, l'évaluation, les
conclusions et les recommandations relatives à l'OBDE du commerce,
valent pour l'HpBDE "du commerce".
HEXABROMODIPHENYLETHER
L'hexabromodiphényléther n'est ni produit ni utilisé mais il
constitue une impureté des bromodiphényléthers du commerce. Il
convient d'en réduire la concentration au minimum afin d'éviter la
contamination de l'environnement et l'exposition de l'homme.
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
On ne dispose d'aucune donnée sur les points suivants:
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
PENTABROMODIPHENYLETHER
Le pentabromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
1.1.1 Identité, propriétés physiques et chimiques
Le pentabromodiphényléther du commerce (PeBDE) est un mélange de
tétra-, penta- et hexabromodiphényléther. Il contient
approximativement 50-60% de PeBDE et 24-38% de TeBDE. D'après la
structure chimique, il existe théoriquement 46 isomères du PeBDE et 42
isomères du TeBDE. Les principaux constituants des produits du
commerce semblent être au nombre de 3, à savoir le 2,2',4,4',5'-PeBDE,
le 2,2',4,4'-TeBDE et un analogue non identifié à 5 atomes de brome.
Le point de fusion se situe entre -7 et -3°C et le point
d'ébullition au-delà de 200°C. La tension de vapeur est faible:
<10-7 mmHg et la solubilité dans l'eau, négligeable. Le logarithme
du coefficient de partage n-octanol/eau est >6.
1.2 Production et usage
Le PeBDE est utilisé comme additif dans les résines époxy, les
résines phénoliques, les polyesters et le polyuréthanne ainsi que les
textiles. La consommation mondiale est d'environ 4000 tonnes par an.
C'est l'un des principaux bromodiphényléthers du commerce utilisés
comme retardateurs de flamme.
1.3 Transport, distribution et transformation dans l'environnement
On trouve des constituants du PeBDE du commerce dans les biotes,
les sédiments et les boues d'égouts. Il est vraisemblable que ces
constituants soient rémanents et qu'ils aient tendance à la
bioaccumulation. C'est ainsi que l'on a trouvé chez la carpe un
facteur de bioconcentration de plus de 10 000.
L'étude de la pyrolyse du PeBDE du commerce a montré qu'il se
forme au cours de ce processus des PBDF et des PBDD. La température
optimale de formation de ces composés se situe entre 700 et 800°C.
Lorsque la pyrolyse du PeBDE s'effectue en l'absence d'oxygène, il y a
formation de polybromobenzènes, de polybromophénols et de PBDF.
1.4 Concentrations dans l'environnement et exposition humaine
Dans des échantillons de sédiments prélevés au Japon dans des
cours d'eau et des estuaires, on a relevé des concentrations allant de
0 (<2 µg/kg)jusqu'à 28 µg/kg de poids sec de PeBDE. En Suède, les
sédiments de certains cours d'eau accusaient des concentrations allant
jusqu'à 1200 µg de 2,2',4,4',5'-PeBDE/kg. Des boues d'égouts,
analysées en Suède, contenaient également de ce PeBDE.
Des moules et poissons ont été prélevés aux fins d'analyse dans
diverses zones littorales du Japon entre 1981 et 1985; dans deux
échantillons de moules sur cinq, on a trouvé des concentrations de
PeBDE qui étaient respectivement égaies à 0,4 et 2,8 µg/kg de poids
humide. En revanche, dans le poisson, on n'a pas décelé de PeBDE
(limite de dosage <0,2 µg/kg). On a fait état de concentrations de
1,9-22 µg/kg de poids frais dans des échantillons de foie de morue
provenant de la mer du Nord. En Suède, on a trouvé des concentrations
qui se situaient entre 7,2 et 64 µg de 2,2',4,4',5'-PeBDE/kg de
graisse dans des poissons d'eau douce du genre corégone et dans des
harengs de différentes provenances.
Du gras de phoque ( Phoca hispida et phoque gris) prélevé en
Suède en 1979-85, s'est révélé contenir des concentrations moyennes de
2,2',4,4',5'-PeBDE respectivement égaies à 1,7 µg et 40 µg/kg de poids
corporel.
En Suède, dans un mélange d'échantillons de muscles de lapins et
d'élans ainsi que dans de la graisse de rognon de renne, on a
constaté, en 1985-86, la présence de concentrations respectivement
égaies à <0,3 µg, 0,64 µg et 0,26 µg de 2,2',4,4',5'-PeBDE/kg de
graisse.
Dans des échantillons de muscles de balbuzard, obtenus en Suède en
1982-86, on a noté une concentration moyenne de 140 µg de
2,2,',4,4',5'-PeBDE/kg de graisse.
On a constaté qu'au cours des dernières décennies, la
concentration de deux isomères du PeBDE avait augmenté d'un facteur 10
dans les oeufs de guillemots de la Baltique. On a également constaté
que la concentration de ces isomères avait augmenté d'un facteur 4
dans des brochets provenant d'un lac du sud de la Suède. L'analyse de
sédiments de la Baltique représentatifs de plusieurs années
d'échantillonnage indique également qu'il y a eu une augmentation
considérable de la pollution au cours de la dernière décennie.
On est très peu renseigné sur l'exposition humaine mais on peut
estimer en gros, d'après la consommation de poisson des suédois, que
la population de ce pays absorbe individuellement environ 0, 1 µg de
PeBDE par jour.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
La demi-vie du PeBDE n'a été étudiée qu'au niveau de la graisse
périrénale chez le rat. On a ainsi obtenu une valeur comprise entre 25
et 47 jours, en fonction du sexe de l'animal et du type d'isomère en
cause.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le PeBDE du commerce présente une faible toxicité aiguë par voie
orale chez le rat; la toxicité par voie percutanée est également
faible chez le lapin. En exposant pendant une brève durée des rats par
la voie respiratoire et en instillant du PeBDE à des lapins dans le
sac conjonctival, on n'a obtenu que des effets modérés et passagers.
Des études toxicologiques à court terme sur des rats (4 semaines
et 13 semaines respectivement) au cours desquelles du PeBDE a été
administré aux animaux à des concentrations de 100 mg/kg de
nourriture, ont révélé une augmentation du poids du foie et donné lieu
à de légères anomalies histologiques. Ces anomalies consistaient en
une hypertrophie des cellules du parenchyme hépatique, qui avaient un
aspect granulaire et contenaient des "inclusions rondes" éosinophiles.
On a constaté que la teneur en brome total du foie augmentait en
fonction de la dose administrée et restait élevée pendant des périodes
pouvant aller jusqu'à 24 semaines. On a également observé une légère
hyperplasie thyroïdienne réversible.
Après administration par voie orale de doses quotidiennes de PeBDE
ne dépassant pas 0,78 µmol/kg de poids corporel, on a observé
l'induction des enzymes hépatiques et un accroissement de l'activité
du cytochrome P-450 c. Les résultats des épreuves de tératogénicité et
de mutagénicité se sont révélés négatifs.
Aucune étude toxicologique à long terme ou étude de
cancérogénicité n'a été publiée.
1.7 Effets sur l'homme
Pas de données disponibles.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
On ne dispose que d'un minimum de données.
2 Conclusions
2.1 PeBDE
Le PeBDE du commerce (mélange de 24-38% de tétra-, 50-60% de
penta- et 4-8% d'hexabromodiphényléther) est rémanent et il s'accumule
chez les êtres vivants dans leur milieu naturel.
Le PeBDE du commerce est largement utilisé, incorporé à des
polymères, comme retardateur de flamme. Lorsqu'il entre en contact
avec la population, c'est par l'intermédiaire de produits fabriqués à
partir de ces polymères. Il est improbable qu'il puisse y avoir
exposition après extraction du produit de ces polymères. Il peut y
avoir exposition de l'homme au PeBDE par l'intermédiaire de la chaîne
alimentaire, étant donné que la substance a été décelée chez divers
animaux vivant dans leur milieu naturel, animaux qui servent de
nourriture à l'homme, comme les poissons, les fruits de mer, etc. On
constate depuis au moins deux décennies que les concentrations
augmentent chez les poissons et les oiseaux de Suède.
Le PeBDE du commerce présente une faible toxicité aiguë. On ne
possède aucune donnée sur la fixation et l'élimination de ce produit
chez les mammifères. On ne dispose pas non plus d'études sur la
reproduction d'animaux exposés à cette substance, ni sur sa toxicité à
long terme ou sa cancérogénicité.
Il n'est pas possible, à partir des données disponibles, d'évaluer
le risque qu'il représente pour la population générale.
On ne possède pas non plus de données qui permettent d'établir le
niveau d'exposition professionnelle ou de se prononcer sur les effets
du PeBDE ?? du commerce.
On ne dispose que de données limitées sur la toxicité du PeBDE du
commerce pour les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Lorsqu'on chauffe du PeBDE ou des produits qui en contiennent à
une température de 400-800°C, il se forme des PBDF et, dans une
certaine mesure, des PBDD. Il faudra examiner les risques qui
pourraient en découler.
Il est peu probable que la population générale soit exposée de
façon notable aux PBDF produits par des polymères contenant du PeBDE
comme retardateur de flamme. Si elle est convenablement effectuée,
l'incinération n'entraîne pas l'émission de quantités importantes de
bromodioxines ou de bromofuranes. En revanche, le brûlage inconsidéré
de produits qui en contiennent peut donner naissance à des quantités
indéterminées de PBDF ou de PBDD. Dans un futur Critère d'hygiène de
l'environnement consacré à ces deux substances, l'importance de ce
problème pour l'homme et l'environnement sera examinée.
3 Recommandations
3.1 Recommandations générales
La rémanence du PeBDE du commerce dans l'environnement et son
accumulation dans les êtres vivants militent contre l'utilisation de
ce produit. Toutefois, s'il l'on continue à l'utiliser, il faudra
prendre les précautions suivantes:
* Les travailleurs qui sont employés à la production de PeBDE ou de
polymères qui en contiennent, doivent être protégés contre toute
exposition, par des mesures appropriées d'hygiène industrielle, la
surveillance de l'exposition professionnelle et des moyens
techniques convenables.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions produits par
les industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé qui persiste et
s'accumule et par ses produits de décomposition.
* On ne doit procéder à l'incinération qu'au moyen d'incinérateurs
convenables fonctionnant toujours dans des conditions optimales.
Le brûlage des déchets par tout autre moyen risque d'entraîner la
formation de produits de décomposition toxiques.
3.2 Etudes à effectuer
* Il faut poursuivre la surveillance des concentrations dans
l'environnement.
* Il conviendrait de valider les méthodes de dosage du PeBDE dans
diverses matrices.
* Du fait que la base de données toxicologiques actuelle est
insuffisante pour permettre d'évaluer des risques pour l'homme et
l'environnement du PeBDE du commerce et en justifier
l'utilisation, il conviendrait d'entreprendre les éludes
suivantes:
- études complémentaires de cancérogénicité et études
toxicologiques et écotoxicologiques:
- poursuite des travaux sur la formation de PBDF dans les
conditions réelles d'incendie;
- étude de méthodes pour le recyclage des polymères contenant du
PeBDE et de leurs conséquences;
- études sur les possibilités de migration à partir de produits
contenant des retardateurs de flamme.
TETRABROMODIPHENYLETHER
Le tétrabromodiphényléther n'est ni produit ni utilisé.
Il n'existe aucune donnée sur les points suivants:
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques
Le tétrabromodiphényléther du commerce est constitué d'un mélange
de 41% de tétra-, 45% de penta-, et 7% d'hexabromo-diphényléthers plus
environ 7% d'un PBDE de structure inconnue. D'après la structure
chimique, le tétrabromodiphényléther a théoriquement 42 isomères. On
ne dispose pratiquement d'aucune donnée sur les propriétés physiques
et chimiques de ce composé, à part le logarithme du coefficient de
partage n-octanol/eau, qui est 5,87-6,16.
1.2 Production et usages
D'après un rapport, la production (et l'usage) du TeBDE aurait été
d'environ 1000 tonnes en 1987 au Japon. Autant qu'on sache, il
n'existe actuellement aucune production sous le nom de
tétra-bromodiphényléther, mais le composé est présent dans la
proportion de 24 à 38% dans le pentabromodiphényléther du commerce.
1.3 Transport, distribution et transformation dans l'environnement
On a trouvé des constituants du TeBDE du commerce dans des biotes,
des sédiments et des boues d'égouts. Il est probable que les
constituants du TeBDE du commerce (qui contiennent des quantités à peu
près équivalentes de PeBDE) aient un caractère rémanent et subissent
une bioaccumulation.
L'étude de la pyrolyse du TeBDE du commerce montre qu'à 800°C, il
se forme des PBDF et des PBDD, mais uniquement les homologues
inférieurs.
1.4 Concentrations dans l'environnement et exposition humaine
Au Japon, on a trouvé du TeBDE dans les sédiments de cours d'eau à
des concentrations de 12-31 µg/kg de poids sec et en Suède, à des
concentrations allant jusqu'à 840 µg/kg (perte au feu). La présence de
TeBDE a été également constatée en Suède dans des boues d'égouts à la
concentration de 15 µg/kg.
Au Japon, on a constaté, dans des moules et des poissons de
différentes provenances, la présence de TeBDE à des concentrations
allant de <0, 1 à 14,6 µg de 2,2',4,4'-TeBDE/kg de poids humide. En
Suède, on a capturé dans des cours d'eau diverses espèces de poissons
que l'on a analysés à la recherche de 2,2',4,4'-TeBDE. Les
concentrations moyennes allaient de 0 (<0,1 mg/kg) à 100 mg/kg de
graisse. L'analyse a montré qu'il existait au moins une source de
pollution locale dans un certain cours d'eau. Des corégones, des
ombles arctiques et des harengs pêchés en différents lieux de Suède
entre 1986 et 1987 se sont révélés contenir des concentrations de
TeBDE respectivement égaies à 15, 400 et 59-450 µg de
2,2',4,4'-TeBDE/kg de graisse. En Allemagne, des poissons provenant de
différents cours d'eau en contenaient jusqu'à 1 mg/kg de graisse.
Dans les harengs et les foies de morues pêchés dans la partie
méridionale, centrale et septentrionale de la mer du Nord, au cours de
la période 1983-89, on a noté une tendance à la diminution des
concentrations de TeBDE de la région méridionale à la région
septentrionale. Dans les harengs, la concentration allait de 8,4 à
100 µg de 2,2',4,4'-TeBDE/kg de graisse.
Dans le tissu musculaire d'oiseaux nichant et hivernant en mer
Baltique, en mer du Nord et au Spitzberg, on a relevé des
concentrations de 2,2',4,4'-TeBDE de 80-370 µg/kg de graisse. Chez des
balbuzards capturés en Suède au cours de la période 1982-86, on a
mesuré des concentrations moyennes de 1800 µg/kg de graisse.
En Suède, on constate une tendance à l'augmentation des
concentrations de 2,2',4,4'-TeBDE dans les sédiments de la Baltique,
les poissons d'eau douce et les oeufs d'oiseaux de mer.
Dans la graisse de phoques de la mer Baltique et du Spitzberg on a
observé des concentrations de 10-730 µg/kg de graisse.
Chromatographiquement, le PBDE est analogue au Bromkal 70-5. Sur des
échantillons groupés de graisse de phoques de l'espèce Phoca hispida
et de phoques gris, prélevés en Suède au cours de la période 1979-85,
on a mesuré des concentrations respectivement égaies à 47 µg et 650 µg
de 2,2',4,4'-TeBDE/kg.
Dans des échantillons groupés de muscles de mammifères terrestres,
par exemple des lapins, des élans et des rennes, obtenus en 1985-86 en
Suède, on a observé des concentrations moyennes respectivement égaies
à <2, 0,82, et 0,18 µg de 2,2',4,4'-TeBDE/kg de graisse.
En Allemagne, on a trouvé dans quatre échantillons de lait de
vache, des concentrations de 2,5-4,5 µg de PBDE/kg de matière grasse,
sous la forme de Bromkal 70DE. Dans le même pays et sous cette même
forme, on a retrouvé du PBDE dans le lait de 25 femmes à des
concentrations de 6,2-11,1 µg/kg de matière grasse.
Une estimation approximative de l'exposition de la population
suédoise, calculée en fonction de la consommation de poisson en Suède,
donne une absorption individuelle de 0,3/µg de TeBDE/jour.
1.5 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Il n'existe pas de données sur le TeBDE lui-même, cependant on
dispose de données de toxicité aiguë et de toxicité à court terme pour
le PeBDE du commerce qui contient 41% de TeBDE.
1.6 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
On ne possède qu'un minimum de données.
1.7 Effets sur l'homme
Il n'existe aucune donnée.
1.8 Effets sur les autres vivants au laboratoire et dans leur milieu
naturel
Il n'existe aucune donnée.
2 Conclusions
2.1 TeBDE
Les constituants du TeBDE du commerce (mélange à 41% de
2,2',4,4'-tétra-; 45% de 2,2',4,4',5'-penta; 7% d'hexa-, et 7-8% de
polybromodiphényléther de structure inconnue) persistent dans
l'environnement et s'accumulent chez les êtres vivants dans leur
milieu naturel.
Constituant du pentabromodiphényléther, le TeBDE est très souvent
incorporé à des polymères comme retardateur de flamme. La population
générale peut entrer en contact avec cette substance par
l'intermédiaire de produits fabriqués à partir de ces polymères. Il
est improbable qu'il puisse y avoir exposition par suite de
l'extraction du composé de ces polymères. Il peut y avoir exposition
humaine a TeBDE par l'intermédiaire de la chaîne alimentaire car on a
décelé la présence de ce composé chez des animaux dans leur milieu
naturel, animaux qui servent de nourriture à l'homme comme les
poissons, les fruits de mer, etc. En Suède, on observe depuis les deux
dernières décennies une augmentation des concentrations de ce composé
chez les poissons et les oiseaux.
On manque d'information au sujet des études toxicologiques à court
et à long terme, des éludes de cancérogénicité ou sur la reproduction
qui auraient pu être faites. En outre, on ne dispose pas de données
sur la cinétique et le métabolisme du produit chez les animaux de
laboratoire et l'homme.
Il n'est pas possible d'évaluer le risque pour la population
générale sur la base des données disponibles.
On ne dispose pas non plus de données suffisantes pour déterminer
les niveaux d'exposition professionnelle ni pour se prononcer sur les
effets du TeBDE.
Il n'existe aucune donnée sur la toxicité de TeBDE du commerce
pour les êtres vivants dans leur milieu naturel.
2.2 Produits de décomposition
Lorsqu'on chauffe du TeBDE à 800°C, il se forme des PBDF et des
PBDD. Il faudra étudier les dangers que cela peut comporter.
Il n'est guère probable que la population générale soit exposée de
façon importante aux PBDF qui se forment lorsque l'on chauffe des
polymères contenant du TeBDE comme retardateur de flamme. Si
l'incinération est effectuée correctement, elle n'entraîne pas
l'émission de bromodioxines ni de bromofuranes en quantités
importantes. Le brûlage inconsidéré de produits contenant du TeBDE
peut conduire à la formation de PBDF ou de PBDD en quantités
indéterminées. Dans un futur Critère d'hygiène de l'environnement
consacré aux PBDF et aux PBDD, l'importance de ce problème pour
l'homme et l'environnement sera examinée.
3 Recommandations
3.1 Recommandations générales
Du fait de sa rémanence dans l'environnement et de son
accumulation chez les êtres vivants, il est recommandé de ne pas
utiliser le TeBDE. Cependant si son usage se poursuit, il faudra
prendre les précautions suivantes:
* Les travailleurs qui sont employés à la production de TeBDE et de
produits qui en contiennent, doivent être protégés contre toute
exposition grâce à des mesures appropriées d'hygiène industrielle,
par la surveillance de l'exposition professionnelle et par des
mesures techniques adéquates.
* Il convient de minimiser l'exposition environnementale par un
traitement approprié des effluents et des émissions provenant des
industries qui utilisent ce composé ou des produits qui en
contiennent. Le rejet des déchets industriels et des produits de
consommation doit être réglementé afin de réduire au minimum la
contamination de l'environnement par ce composé persistant et
cumulatif et ses produits de décomposition.
* On ne doit procéder à l'incinération de produits contenant un
retardateur de flamme à base TeBDE qu'au moyen d'incinérateurs
convenables fonctionnant toujours dans des conditions optimales.
Le brûlage des déchets par tout autre moyen risque d'entraîner la
formation de produits de décomposition principalement formés de
furanes.
3.2 Etudes à effectuer
* Il importe d'assurer une surveillance permanente des
concentrations dans l'environnement.
* Il faudrait valider les méthodes d'analyse du TeBDE dans diverses
matrices.
* Comme la base de données toxicologiques actuelle est insuffisante
pour permettre d'évaluer le danger que le TeBDE représente pour
l'homme et l'environnement, il faudra, si on continue à utiliser
ce produit, effectuer les travaux suivants:
- études toxicologiques et écotoxicologiques supplémentaires et
études de cancérogénicité;
- poursuite des études sur la formation de PBDF dans les
conditions réelles d'incendie;
- étude des méthodes de recyclage des polymères contenant du
TeBDE et de ses conséquences;
- recherches sur la possibilité de migration à partir de
produits contenant des retardateurs de flamme.
TRIBROMODIPHENYLETHER
Le tribromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Transport, distribution et transformation dans l'environnement
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
2 Recommandations
Il convient de réduire au minimum la contamination des produits du
commerce par du tribromodiphényléther afin d'éviter la pollution de
l'environnement et l'exposition humaine.
Il faut éviter d'utiliser des produits commerciaux susceptibles
d'entraîner la pollution du milieu.
DIBROMODIPHENYLETHER
Le dibromodiphényléther n'est ni produit ni utilisé.
On ne dispose d'aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
* Evaluations antérieures par des organismes internationaux.
1 Résumé et évaluation
Il n'existe pas de base de données sur laquelle fonder une
évaluation.
2 Recommandations
Il convient de réduire au minimum la contamination des produits du
commerce par du dibromodiphényléther afin d'éviter la pollution de
l'environnement et l'exposition humaine.
Il faut éviter d'utiliser des produits commerciaux susceptibles
d'entraîner la pollution du milieu.
MONOBROMODIPHENYLETHER
Il n'existe aucune donnée sur les points suivants:
* Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
* Effets sur l'homme
* Evaluations antérieures par des organismes internationaux.
1. Résumé et évaluation
1.1 Propriétés physiques et chimiques
Il y a trois isomères possible du monobromodiphényléther.
Le p-bromodiphényléther se présente à la température ambiante
sous la forme d'un liquide dont le point d'ébullition est de
305-310°C. Le calcul montre que sa solubilité dans l'eau est de
48 mg/litre. Le logarithme de son coefficient de partage
n-octanol/eau se situe entre 4 et. 5. Sa tension de vapeur est de
0,0015 mmHg à 20°C.
1.2 Production et usages
Le MBDE n'est pas utilisé comme retardateur de flamme. Un rapport
sur sa production est paru en 1977, mais on ne lui connaît pas
d'usage.
1.3 Transport, distribution et transformation dans l'environnement
Sa demi-vie d'évaporation à partir de l'eau est de l'ordre de
plusieurs centaines de jours.
Placé dans une culture de 7jours ensemencé avec des micro-
organismes présents dans des eaux usées domestiques, il ne subit
pas de biodécomposition importante, mais il est dégradé à 90% dans des
boues d'égouts activées. D'après la seule étude consacrée à sa
dégradation par les bactéries terricoles, il semblerait que celles-ci,
tout au moins une souche particulière, soient incapables d'utiliser le
MBDE comme seule source de carbone.
1.4 Concentrations dans r environnement et exposition humaine
On a décelé la présence de MBDE dans des échantillons d'eau
superficielle prélevés à proximité de sites industriels aux Etats-Unis
d'Amérique; toutefois, une enquête analogue menée au Japon n'a pas
donné de résultats. On en a également décelé aux Etats-Unis d'Amérique
dans des eaux souterraines à proximité d'une usine. Aux Etats-Unis
d'Amérique, on a décelé la présence de MBDE dans des sédiments et des
biotes aquatiques.
1.5 Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Il n'existe pas de donnée.
1.6 Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Le MBDE n'est pas tératogène, mais on ne possède aucune donnée sur
la toxicité aiguë ni la toxicité à court et à long terme de ce
composé; aussi est-il impossible de procéder à une évaluation.
1.7 Effets sur l'homme
Il n'existe aucune donnée.
1.8 Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Chez un poisson, Lepomis macrochirus, la CL50 à 96 heures
serait de 4,9 mg/litre, avec une concentration sans effets observables
de moins de 2,8 mg/litre. La CL50 à 48 heures pour la puce d'eau est
de 0,36 mg/litre avec une concentration sans effets observables de
moins de 0,046 mg/litre.
2 Conclusions et recommandations
Le monobromodiphényléther n'agit pas comme retardateur de flamme.
Il peut s'accumuler chez les êtres vivants dans leur milieu naturel et
on en a décelé la présence dans divers secteurs de l'environnement. Il
semblerait qu'il puisse subir une biodécomposition.
Ces données sont trop limitées pour qu'on puisse parvenir à des
conclusions quant aux niveaux d'exposition et aux effets que ce
composé peut avoir sur la population générale et les êtres vivants
dans leur milieu naturel.
Il n'existe aucune base de données toxicologiques qui plaide en
faveur de l'utilisation de ce produit.
Il faut éviter toute utilisation susceptible de conduire à une
pollution de l'environnement.
RESUMEN, EVALUACIN, CONCLUSIONES Y RECOMENDACIONES
ÉTER DE DECABROMODIFENILO
1. Resumen y evaluación
1.1 Identidad y propiedades fisicas y quimicas
El éter de decabromodifenilo (DeBDE) comercial suele tener una
pureza del 97-98%, con un 0,3-3,0% de éteres de difenilo nona- y
octabromados. La impureza mas importante es el éter de
nonabromodifenilo (NBDE). A diferencia de los otros éteres de difenilo
polibromados, el DeBDE tiene un solo isomero.
El punto de fusion es de unos 300°C y la descomposicion se produce
por encima de los 400°C. La solubilidad en agua es de 20-30 µg/litro y
el logaritmo del coeficiente de reparto n-octanol/agua es superior a
5. La presion de vapor es <10-6 mm de Hg a 20°C.
1.2 Produccion y aplicaciones
Entre los éteres de difenilo bromados (del mono- al deca-), el
éter de decabromodifenilo es el mas importante de fabricacion
comercial en cuanto a produccion y aplicaciones.
A partir del decenio de 1970 se ha elaborado DeBDE comercial cada
vez mas puro. Su produccion mundial es de unas 300 000 toneladas al
ano. Se utiliza como aditivo pirorretardante en numerosos plasticos,
sobre todo en el poliestireno de gran resistencia al impacto, en el
tratamiento de tejidos utilizados en pasamaneria, telas de automoviles
y tiendas de campana.
1.3 Transporte, distribucion y transformacion en el medio ambiente
La fotodegradacion del DeBDE tiene lugar en disolventes organicos
con radiacion ultravioleta (RUV) o la luz del sol y produce éteres de
difenilo con un numero menor de atomos de bromo y dibenzofuranos
bromados. También se produce fotodegradacion, en menor grado, en el
agua bajo la accion de la luz del sol; sin embargo, no se ha detectado
la presencia de éteres de difenilo menos bromados ni tampoco de
dibenzofuranos bromados.
En funcion del tipo de polimero y del disolvente de extraccion se
obtiene una concentracion de DeBDE que esta casi en el limite de
deteccion o es inferior a él.
Debido a que la solubilidad en agua y la presion de vapor son
extremadamente bajas, es probable que el transporte del DeBDE se
produzca sobre todo mediante adsorcion sobre particulas. Es
persistente y se acumula facilmente en el sedimento y en el suelo.
No se dispone de datos acerca de su biodisponibilidad a partir del
sedimento y del suelo. En un estudio realizado en la trucha irisada no
se detecto bioacumulacion en la carne, la piel o las visceras durante
mas de 48 horas. No es probable la bioacumulacion del DeBDE, debido a
su peso molecular relativamente alto.
A la larga, los productos que contienen DeBDE comercial se
eliminan en vertederos o mediante incineracion. El DeBDE, en ultimo
término, se puede lixiviar de los vertederos. Se pueden producir
dibenzofuranos polibromados (PBDF) y mezclas de dibenzofuranos y
dibenzodioxinas halogenados en incendios de los vertederos o por una
incineracion incompleta. Los productos que contienen DeBDE comercial
pueden contribuir a estas emisiones.
La pirolisis del propio DeBDE comercial y de los polimeros (HIPS,
PBT, polipropileno industrial) con DeBDE en presencia de oxigeno
produjo PBDF, detectandose en menor grado dibenzodioxinas polibromadas
(PBDD). La formacion maxima de PBDF tiene lugar a 400-500°C, pero se
puede producir a temperaturas de hasta 800°C; el Sb2O3 ejerce una
funcion catalitica en la formacion de PBDF y PBDD.
La formacion de PBDF y PBDD y las cantidades encontradas dependen
de la temperatura, el contenido de oxigeno y la duracion de la
pirolisis. En ausencia de oxigeno, se forman sobre todo
polibromobencenos y polibromonaftalenos.
1.4 Niveles medioambientales y exposicion humana
En la vecindad de las instalaciones de fabricacion se han
identificado concentraciones de DeBDE de hasta 25 µg/m3. No se
detecto en las muestras de agua recogidas en el Japon durante el
periodo de 1977-91. Sin embargo, se observo en el sedimento de rios
recogido en el Japon durante el mismo periodo a concentraciones de
hasta unos 12 mg/kg de peso seco. En los Estados Unidos se encontro
DeBDE (hasta 1 g/kg) en el sedimento de los rios cercanos a una
fabrica. No se detecto su presencia en las muestras de peces recogidas
en el Japon, aunque en una muestra de mejillones se observo una
concentracion ligeramente superior al nivel de deteccion. Aunque no se
encontro en las muestras de tejido adiposo humano recogidas en el
Japon, en los Estados Unidos se observo en 3 de 5 muestras de este
tipo de tejido.
La exposicion humana al DeBDE se puede producir en el curso de la
fabricacion y la formulacion en polimeros. La exposicion de la
poblacion general al DeBDE es insignificante.
En la determinacion de la exposicion en el trabajo a los productos
de degradacion del DeBDE durante la fabricacion, formulacion o
utilizacion se puso de manifiesto que las muestras de aire proximas al
cabezal del extrusor contenian concentraciones elevadas de PBDF. En el
aire de la sala de trabajo se encontraron niveles mas bajos. Se
detecto asimismo en muestras del material de limpieza. Se ha
demostrado que la aplicacion de buenas técnicas industriales reduce la
exposicion ocupacional al PBDF.
No es probable una exposicion significativa de la poblacion
general a las impurezas de PBDF de los polimeros con caracteristicas
pirorretardantes.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
El DeBDE tiene una escasa absorcion desde el tracto
gastrointestinal y se excreta con rapidez tras su inyeccion.
Los resultados de los estudios metabolicos en ratas utilizando
DeBDF marcado con 14C indicaron una semivida para su desaparicion del
organismo de menos de 24 horas, siendo la ruta principal de
eliminacion tras la ingestion oral la via fecal. En la orina o el aire
expirado no se encontro una actividad apreciable de 14C (inferior al
1%).
Las ratas alimentadas con 0,1 mg/kg de peso corporal al dia,
durante un periodo de hasta dos anos, no mostraron acumulacion de
DeBDF en el suero, los rinones, los musculos o los testiculos, como se
comprobo mediante una determinacion del bromo total. La acumulacion de
bromo en el higado alcanzo un nivel estacionarlo a los 30 dias y
desaparecio en un periodo de 10 dias después del tratamiento. Tras
180 dias de tratamiento, el nivel de bromo en el higado de las ratas
tratadas no era superior al de las testigo. En el tejido adiposo se
acumulo una concentracion baja de bromo total, que se mantenia tras
90 dias con una alimentacion sin DeBDE; se desconoce la naturaleza del
bromo acumulado. Habida cuenta de que el DeBDE representaba solo el
77% de la mezcla comercial utilizada, el "bromo" podria proceder del
NBDE o el OBDE.
1.6 Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
La toxicidad aguda del DeBDE en los animales de laboratorio es
baja. La sustancia no es irritante para la piel o los ojos de los
conejos. No es cloracnegénico en la piel de los conejos, ni tampoco
sensibilizador cutaneo humano.
Se realizaron pruebas de toxicidad aguda y de comedogenicidad de
los productos de la combustion del poliestireno pirorretardante con
DeBDE y Sb2O3. La DL50 por via oral del hollin y la carbonilla fue
> 2000 mg/kg de peso corporal.
No se observo induccion de efectos adversos en los estudios de
toxicidad a cono plazo realizados en ratas y ratones con niveles de
DeBDE (pureza > 97%) de 100 g/kg (4 semanas) o 50 g/kg de alimentos
(13 semanas, equivalente a 2500 mg/kg de peso corporal de la rata). En
un estudio de reproduccion de una sola generacion en ratas no se
observaron efectos adversos con niveles de dosis de 100 mg/kg de peso
corporal. El DeBDE no produjo efectos teratogénicos en los fetos de
las ratas que recibieron dosis de 100 mg/kg de peso corporal. Con
1000 mg/kg de peso corporal se detectaron malformaciones como el
retraso de la osificacion. En varias pruebas realizadas no se ha
observado que tenga efectos mutagénicos.
En un estudio de carcinogenicidad en ratas y ratones, se
administraron concentraciones de DeBDE (pureza 94-99%) de hasta
50 g/kg de alimentos. Se observo un aumento en el numero de adenomas
hepaticos (pero no de carcinomas) de las ratas macho que recibieron
25 g/kg y en las ratas hembra con 50 g/kg. En ratones macho tratados
con 25 g/kg se detecto una frecuencia mayor de adenomas y carcinomas
hepatocelulares (combinados) y con ambos niveles de dosis un aumento
de adenomas/carcinomas (combinados) de las células foliculares del
tiroides. En los ratones hembra no se aprecio ningun incremento de la
frecuencia de tumores. Se obtuvo una prueba equivoca de
carcinogenicidad en ratas macho y hembra y en ratones macho solo con
dosis de 25-50 g de DeBDE/kg de alimentos. Dado que los resultados de
todas las pruebas de mutagenicidad han sido negativos, se puede
concluir que el DeBDE carece de carcinogenicidad genotoxica. IARC
(1990) concluyo que las pruebas de carcinogenicidad del DeBDE en los
animales de experimentacion eran limitadas. Los niveles de
dosificacion muy elevados, la falta de genotoxicidad y las pruebas
minimas de carcinogenicidad ponen de manifiesto que el DeBDE, a las
concentraciones de exposicion actuales, no representa un riesgo de
carcinogénesis para el ser humano.
1.7 Efectos en el ser humano
No se detectaron signos de sensibilizacion cutanea en una prueba
realizada con 200 personas expuestas al DeBDE.
En un estudio de morbilidad realizado con el personal del extrusor
que mezcla polibutilenterftalato que contiene DeBDE, con la
consiguiente exposicion potencial al PBDD y al PBDF durante 13 anos,
no se observaron efectos nocivos, aun cuando se detectaron en sangre
2,3,7,8-TeBDF y -TeBDD. Los resultados de los estudios inmunologicos
pusieron de manifiesto que el sistema inmunitario de las personas
expuestas no se habia visto negativamente afectado después de 13 anos.
1.8 Efectos en otros organismos en d laboratorio y en el medio
ambiente
Las CE50s para el crecimiento de tres algas unicelulares marinas
fueron superiores a 1 mg de DeBDE/litro. No se dispone de mas
informacion sobre los efectos del DeBDE en otros organismos en el
laboratorio y en el medio ambiente.
2 Conclusiones
2.1 DeBDE
El DeBDE se utiliza ampliamente en polimeros como aditivo
pirorretardante. El contacto de la poblacion general se produce a
través de productos fabricados con dichos polimeros. La exposicion es
muy escasa, puesto que no es facil extraer el DeBDE de los polimeros.
La toxicidad aguda es muy baja y la absorcion del tracto
gastrointestinal minima. Asi pues, el riesgo para la poblacion general
se puede considerar insignificante.
La exposicion en el trabajo se da con el DeBDE en forma de
particulas. El control del polvo. durante la fabricacion y el empleo
reducira el riesgo para los trabajadores de manera suficiente.
El DeBDE es persistente y se une a particulas del medio ambiente;
es facil que se acumule en el sedimento, pero no es probable su
bioacumulacion. Aunque las pruebas disponibles indican que la
fotodegradacion ambiental en agua no producen éteres de difenilo o
dibenzofuranos con menor numero de atomos de bromo, apenas se conoce
nada sobre la degradacion en otros medios.
La informacion sobre la toxicidad del DeBDE para los organismos
del medio ambiente es minima.
2.2 Productos de degradacion
Se puede producir PBDF y, en cierta medida, PBDD cuando se somete
el DeBDE, o los productos que lo contienen, a temperaturas de
300-800°C. Hay que estudiar los posibles peligros asociados a estos
productos.
La incineracion controlada de manera adecuada no produce una
emision de cantidades significativas de dioxinas o furanos bromados.
Cualquier combustion no controlada de productos con DeBDE puede dar
lugar a una formacion no cuantificada de PBDF/PBDD. De su importancia
para el ser humano y el medio ambiente se ocupara un futuro numero de
Criterios de Salud Ambiental.
Se ha detectado PBDR en la sangre de las personas que trabajan en
la produccion de plasticos con DeBDE. No se han observado efectos
adversos relacionados con esta exposicion. Con un buen control técnico
se puede evitar la exposicion de los trabajadores al PBDF.
3 Recomendaciones
3.1 Generales
* Las personas que trabajan en la fabricacion de DeBDE y productos
que lo contienen deben estar protegidas de la exposicion mediante
la aplicacion de medidas adecuadas de higiene industrial, la
vigilancia de la exposicion en el trabajo y controles técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones industriales que
contienen el compuesto o sus productos. Se debe controlar la
eliminacion de los residuos industriales y los productos de
consumo para que sea minima la contaminacion del medio ambiente
con esta sustancia persistente y sus productos de degradacion.
* Los fabricantes deben reducir al minimo los niveles de impurezas
en los productos comerciales de DeBDE, utilizando las mejores
técnicas disponibles. Se recomienda una pureza del 97% o superior.
* La incineracion solo se debe realizar en incineradores adecuados
que funcionen siempre en condiciones optimas. La quema por otros
medios puede dar lugar a la formacion de PBDF y PBDD.
3.2 Otros estudios
* Se deberan realizar en los organismos pertinentes nuevos estudios
sobre la biodisponibilidad y la toxicidad del DeBDE unido al
sedimento.
* Hay que mantener una vigilancia constante de los niveles en el
medio ambiente.
* Se debe investigar con mas detalle la formacion de PBDF en
incendios reales.
* Hay que estudiar mas a fondo la biodegradacion en el medio
ambiente y la fotodegradacion en compartimentos distintos del
agua.
* Se debe investigar los posibles métodos y las consecuencias del
reciclaje de polimeros que contienen DeBDE.
* Hay que validar métodos analiticos para el DeBDE en diversos
aglomerantes.
ÉTER DE NONABROMODIFENILO
El éter de nonabromodifenilo no se fabrica ni se utiliza. No se
dispone de datos sobre los aspectos siguientes:
* Transporte, distribucion y transformacion en el medio ambiente
* Niveles medioambientales y exposicion humana
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay ninguna base de datos sobre la cual realizar una
evaluacion.
2 Recomendaciones
Los niveles de contaminacion de los pirorretardantes bromados
comerciales que contienen éter de nonabromodifenilo deberian ser
minimos a fin de evitar la contaminacion del medio ambiente y la
exposicion humana.
ÉTER DE OCTABROMODIFENILO
El éter de octabromodifenilo puro no se fabrica ni se utiliza. No
se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de octabromodifenilo (OBDE) comercial es una mezcla
formada por alrededor del 11% de PeBDE/HxBDE, 44% de HpBDE, 31-35% de
OBDE, 10% de NBDE y 0,5% de DeBDE. En funcion de su estructura
quimica, existen 12 posibles isomeros del OBDE y 24 del HpBDE.
El punto de fusion oscila entre unos 80°C y >200°C. La presion de
vapor es <10-7 mm de Hg. La solubilidad en agua es baja y el
logaritmo del coeficiente de reparto n-octanol/agua es >5,5. Las
variaciones anteriores obtenidas en los datos fisicos pueden deberse a
diferencias de composicion en las mezclas probadas.
1.2 Produccion y aplicaciones
El consumo anual de OBDE comercial en todo el mundo es de 6000
toneladas, el 70% del cual se utiliza como pirorretardante en ABS para
la produccion de computadores y cabinas comerciales. El OBDE es el
segundo mas utilizado de los pirorretardantes de PBDE.
1.3 Transporte, distribucion y transformacion en el medio ambiente
Se han detectado componentes de OBDE comercial en el sedimento
acuatico y en la grasa humana. En la biota se han encontrado algunos
componentes de menor numero de atomos de bromo (HxBDE y PeBDE) del
producto comercial. No se ha observado OBDE, pero normalmente no se ha
investigado la presencia de HpBDE y NBDE. Es facil que persistan los
componentes comerciales de OBDE, pero a medida que el numero de atomos
de bromo aumenta por encima del HxBDE disminuye la probabilidad de
bioacumulacion. En la carpa se ha encontrado un factor de
bioacumulacion inferior a 2 para un producto de OBDE comercial.
En la pirolisis a 600°C del producto comercial como tal o de
polimeros que lo contienen como aditivo pirorretardante (con Sb2O3 o
sin él) se ha puesto de manifiesto la produccion de PBDF y, en grado
mucho menor, PBDD. El tratamiento de ABS con OBDE/Sb2O3 en
diferentes condiciones permitio demostrar que, en el caso de
tratamiento normal, solo se formaban pequenas concentraciones de PBDF.
En condiciones de uso excesivo, las concentraciones fueron mucho mas
elevadas. Las concentraciones de PBDD fueron bajas en ambos casos.
1.4 Niveles medioambientales y exposicion humana
En una serie de muestras recogidas en el Japon durante 1987 y 1988
no se detecto OBDE ni los componentes de menor numero de atomos de
bromo del producto comercial. También se analizaron muestras del
sedimento y se encontro OBDE en alrededor del 2-6% de las muestras a
concentraciones que oscilaban entre 8 y 22 µg/kg de peso seco. En el
sedimento se observo asimismo la presencia de componentes de menor
numero de atomos de bromo
No se detecto OBDE en las muestras de peces recogidas en el Japon
durante 1987 y 1988.
En los Estados Unidos se investigo la presencia de PBDF Y PBDD en
muestras de grasa humana recogidas en 1987. Las muestras procedian de
865 personas combinadas para formar 48 grupos compuestos analogos. La
composicion se baso en 9 divisiones de censo y 3 grupos de edad. En
estas muestras también se identifico PBDE y los datos preliminares
indicaron la presencia de OBDE con una frecuencia del 60% y una
concentracion estimada de hasta 8000 ng/kg.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
No se dispone de datos.
1.6 Efectos en los animales de laboratorio y en los
sistemas de prueba in vitro
La toxicidad aguda del producto comercial en los animales de
laboratorio es baja. No es irritante cutaneo y solo produce una ligera
irritacion ocular en los conejos. En los estudios de toxicidad a corto
plazo (4 semanas y 13 semanas) se observo que en las ratas que
recibieron dosis de 100 mg/kg de alimentos se produjo un mayor peso
del higado y cambios microscopicos caracterizados por un aumento de
tamano de las células parenquimaticas hepaticas de la zona
centrolobular y media que contienen estructuras granulares. Estos
cambios hepaticos fueron mas graves con dosis mas elevadas, es decir,
1000 y 10 000 mg/kg de alimentos. A esto hay que anadir que se observo
hiperplasia en el tiroides. El contenido total de bromo en los tejidos
aumento durante el estudio y disminuyo lentamente durante un periodo
de recuperacion. Los cambios hepaticos fueron reversibles. En un
estudio de inhalacion con polvo de OBDE micronizado (8 horas al dia
durante 14 dias consecutivos) no se observaron efectos con
exposiciones a 1,2 mg/m3, pero a 12 mg/m3 se produjeron en el higado
los cambios descritos en los estudios de administracion oral.
El OBDE comercial a dosis relativamente bajas produjo en ratas un
aumento del citocromo P450 y la induccion de enzimas microsomicas
hepaticas, como la UDP-glucuronil transferasa y la benzo[alpha]pireno
hidroxilasa. El OBDE comercial indujo efectos porfirinogénicos en
cultivos de células hepaticas de embrion de pollo.
Su potencial teratogénico se probo en ratas; con dosis elevadas
(25,0 y 50,0 mg/kg de peso corporal) se detectaron procesos de
resorcion y osificacion retardada de diferentes huesos, asi como
malformaciones fetales. Las malformaciones observadas tras la
administracion de dosis de 25 mg/kg de peso corporal y superiores
probablemente se debieron en buena parte a la toxicidad materna. Estos
cambios no se advirtieron a dosis de 15,0 mg/kg de peso corporal o
inferiores. No se obtuvieron pruebas de actividad teratogénica en los
conejos, pero se observo fetotoxicidad a una dosis toxica para las
madres de 15 mg/kg de peso corporal. En los estudios de
teratogenicidad se puso de manifiesto la ausencia de efectos a una
dosis de 2,5 mg/kg de peso corporal.
En estudios de 28 dias y 90 dias realizados en ratas se observo
que 100 mg de OBDE por kg de alimentos (equivalentes a 5 mg/kg de peso
corporal) producian efectos minimos en el higado. No se establecio un
nivel sin efectos. Los resultados de las pruebas de mutagenicidad,
incluso con un ensayo de ADN fuera de programa, los ensayos
microbiologicos in vitro y un ensayo de intercambio de cromatidas
hermanas con células de ovario de hamster chino fueron negativos.
No se dispone de resultados de pruebas de carcinogenicidad a largo
plazo.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Los datos disponibles son minimos.
2 Conclusiones
2.1 OBDE
El OBDE comercial es una mezcla de éteres de hexa-,hepta-, octa-,
y nonabromodifenilo, todos ellos persistentes en el medio ambiente,
fundamentalmente unidos al sedimento.
El OBDE se anade con mucha frecuencia a los polimeros como aditivo
pirorretardante. El contacto de la poblacion general tiene lugar
mediante los productos fabricados con estos polimeros. No es probable
la exposicion por extraccion de los polimeros.
La toxicidad aguda del OBDE es baja. No se dispone de informacion
sobre su absorcion y eliminacion en los mamiferos. No es teratogénico
ni mutagénico. No se dispone de estudios de toxicidad y
carcinogenicidad a largo plazo.
En el tejido adiposo humano se han identificado varios componentes
del OBDE comercial. El riesgo grave para la poblacion general
probablemente es bajo. No es posible evaluar el riesgo de exposicion
prolongada, debido a la falta de los estudios de toxicidad
correspondientes.
La falta de informacion no permite sacar conclusiones sobre la
exposicion en el trabajo al OBDE o sus efectos.
La informacion sobre la toxicidad del OBDE para los organismos en
el medio ambiente es limitada. Los componentes de la mezcla de OBDE
comercial con menor numero de atomos de bromo podrian dar lugar a una
bioacumulacion en los organismos.
2.2 Productos de degradacion
Puede formarse PBDF, y en cierto grado PBDD, cuando el OBDE o los
productos que lo contienen se calientan a 400-800°C. Hay que
investigar los posibles peligros relacionados con esta transformacion.
No es probable que la exposicion de la poblacion general a las
impurezas de PBDF en los polimeros pirorretardantes sea significativa.
La incineracion adecuadamente controlada no deberia producir la
emision de cantidades importantes de dioxinas y furanos bromados.
Cualquier combustion no controlada de productos que contienen OBDE
comercial puede dar lugar a la produccion de cantidades no
cuantificadas de PBDF/PBDD. Su importancia para el ser humano y el
medio ambiente sera objeto de estudio en un futuro EHC sobre el
PBDF/PBDD.
3 Recomendaciones
3.1 Generales
* En la fabricacion de OBDE comercial se deben utilizar las mejores
técnicas disponibles, a fin de reducir al minimo los niveles de
hexabromodifenilo y los compuestos analogos de menor numero de
atomos de bromo, debido a su posible bioacumulacion en el medio
ambiente.
* Los trabajadores que intervienen en la fabricacion de OBDE y de
los productos que contienen el compuesto deben estar protegidos de
la exposicion mediante la aplicacion de medidas de higiene
industrial adecuadas, la vigilancia de la exposicion en el trabajo
y controles técnicos.
* Se debe reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de los efluentes y las emisiones en las
industrias que utilizan el compuesto o sus productos. Hay que
controlar la eliminacion de desechos industriales y de los
productos de consumo, a fin de reducir al minimo la contaminacion
del medio ambiente con este material persistente y los productos
de su degradacion.
* La incineracion de materiales con aditivos pirorretardantes a base
de OBDE se debe realizar solo en incineradores adecuados que
funcionen siempre en condiciones optimas. La combustion por
cualquier otro medio puede dar lugar a la produccion de PBDF y
PBDD.
3.2 Otros estudios
Habida cuenta de que la base de datos toxicologica actual no es
adecuada para evaluar los peligros del OBDE comercial para el ser
humano y el medio ambiente y a fin de mejorar su uso se deberian
realizar los siguientes estudios:
* Investigaciones sobre la biodisponibilidad y la ecotoxicidad de
los componentes del OBDE comercial unidos al sedimento utilizando
los organismos pertinentes.
* Mayor vigilancia de los niveles en el medio ambiente de los
componentes de OBDE comercial.
* Estudios de toxicidad y carcinogenicidad a largo plazo del OBDE
comercial.
* Vigilancia de la exposicion en el trabajo al OBDE comercial.
* Otras investigaciones sobre la formacion de PBDF en incendios
reales.
* Nuevos estudios sobre biodegradacion y fotodegradacion en el medio
ambiente en compartimentos diferentes del acuatico.
* Investigacion de posibles métodos y consecuencias del reciclaje de
polimeros que contienen OBDE.
* Validacion de métodos analiticos para el OBDE en varios
aglomerantes.
* Investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE HEPTABROMODIFENILO
El éter de heptabromodifenilo no se fabrica ni se utiliza.
No existe una base de datos sobre la que hacer una evaluacion del
HpBDE.
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
Dado que el HpBDE es el principal componente del éter de
octabromodifenilo comercial, el resumen, evaluacion, conclusiones y
recomendaciones del OBDE comercial son aplicables al HpBDE
"comercial".
ÉTER DE HEXABROMODIFENILO
Aunque el éter de hexabromodifenilo no se fabrica ni se utiliza,
es posible encontrarlo como contaminante de los éteres bromados del
difenilo comerciales. Se debe reducir al minimo tales niveles del éter
de hexabromodifenilo para evitar la contaminacion del medio ambiente y
la exposicion humana.
No existe una base de datos sobre la que hacer una evaluacion.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
ÉTER DE PENTABROMODIFENILO
El éter de pentabromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de pentabromodifenilo comercial (PeBDE) es una mezcla de
éteres de tetra-, penta-, y hexabromodifenilo. Contiene
aproximadamente un 50-60% de PeBDE y un 24-38% de TeBDE. Habida cuenta
de su estructura quimica, hay seis posibles isomeros del PeBDE y 42
del TeBDE. Los productos comerciales parecen estar formados por tres
componentes principales, a saber, 2,2',4,4',5'-PeBDE, 2,2',4,4'-TeBDE
y un compuesto analogo no identificado con cinco atomos de bromo.
El punto de fusion es de -7 a -3°C y el de ebullicion superior a
200°C. La presion de vapor es baja: <10-7 mm de Hg; la solubilidad
en agua es insignificante. El log del coeficiente de reparto
n-octanol/agua es >6.
1.2 Produccion y aplicaciones
El PeBDE se utiliza como aditivo de resinas epoxi, resinas
fenolicas, poliésteres y poliuretano, asi como de materiales textiles.
El consumo mundial es de unas 4000 toneladas anuales. Es uno de los
principales éteres bromados del difenilo pirorretardantes comerciales.
1.3 Transporte, distribucion y transformacion en el medio ambiente
Se han detectado componentes del PeBDE comercial en muestras de
biota, sedimento y fangos cloacales. Es facil que sean persistentes y
tengan capacidad de bioacumulacion. En las carpas se ha encontrado un
factor de bioacumulacion para el PeBDE superior a 10 000.
En los estudios de pirolisis con PeBDE comercial se observo la
formacion de PBDF y PBDD. La temperatura optima de formacion oscilo
entre 700-800°C. Cuando la pirolisis de PeBDE tenia lugar en ausencia
de oxigeno se formaban polibromobencenos, polibromofenoles y PBDF.
1.4 Niveles medioambientales y exposicion humana
En muestras obtenidas de rios y estuarios del Japon se detectaron
niveles que variaban entre la ausencia de PeBDE (<2 µg/kg) y 28 µg/kg
de peso seco. En Suecia, la concentracion en muestras de sedimento de
algunos rios alcanzo un valor de hasta 1200 µg de
2,2',4,4',5'-PeBDE/kg. También en el analisis de fangos cloacales de
Suecia se puso de manifiesto la presencia de PeBDE.
En los mejillones y peces recogidos en diferentes costas del Japon
durante el periodo de 1981-85 se encontraron concentraciones de 0,4 y
2,8 µg de PeBDE/kg de peso humedo de dos de cinco muestras de
mejillones. No se detecto PeBDE en los peces (limite de determinacion
<0,2 µg/kg). Se informo de concentraciones de 1,922 µg/kg de peso
fresco en muestras de higado de bacalao procedente del mar del Norte.
En Suecia se encontraron concentraciones de 7,2 a 64 µg de
2,2',4,4',5'-PeBDE/kg de grasa en corégonos de agua dulce y en
arenques recogidos en diversos lugares.
En una mezcla de grasa de focas oceladas y locas grises
recolectada en Suecia entre 1979 y 1985 se encontro una concentracion
media de 1,7 µg y 40 µg de 2,2',4,4',5'-PeBDE/kg de grasa,
respectivamente.
En mezclas de muestras de musculo de conejo y raton y en muestras
de sebo de reno recogidas en Suecia de 1985 a 1986 se determino una
concentracion <0,3 µg, 0,64 µg y 0,26 µg de 2,2',4,4',5'-PeBDE/kg de
grasa, respectivamente.
Las muestras de musculo de quebrantahuesos recogidas en Suecia en
1982-86 contenian una concentracion media de 140 µg de
2,2',4,4',5'-PeBDE/kg de grasa.
En los ultimos decenios se han multiplicado por diez los niveles
de dos isomeros del PeBDE en los huevos de arao del Baltico. La
concentracion de dichos isomeros en los lucios de un lago de la region
meridional de Suecia también ha experimentado un aumento (del
cuadruple). Se ha observado asimismo un aumento considerable en los
muestreos realizados en el sedimento del Baltico en distintos anos
durante el ultimo decenio.
Aunque se dispone de una informacion muy escasa sobre la
exposicion humana, una estimacion aproximada de la exposicion de la
poblacion sueca a través del consumo de pescado parece indicar una
absorcion diaria de 0,1 µg de PeBDE/persona.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
La semivida del PeBDE solo se ha investigado en la grasa
perirrenal de ratas. La semivida media oscilo entre 25 y 47 dias, en
funcion del sexo del animal y del tipo de isomero.
1.6 Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
La toxicidad aguda por via oral del PeBDE comercial en ratas es
baja; la toxicidad dérmica en conejos es también escasa. La exposicion
de ratas a inhalacion durante periodos breves y la aplicacion de PeBDE
al saco conjuntival de conejos produjo solo efectos transitorios
leves.
En los estudios de toxicidad a corto plazo realizados en ratas
(4 y 13 semanas), las concentraciones de 100 mg/kg de alimentos
produjeron un aumento del peso del higado y ligeras alteraciones
histologicas. Los cambios consistieron en el agrandamiento de las
células parenquimaticas hepaticas, que tenian un aspecto granular y
contenian "cuerpos redondos" eosinofilos. Se produjo en el higado un
aumento de la cantidad total de bromo dependiente de la dosis y los
niveles se mantuvieron durante un periodo largo, de hasta 24 semanas.
Se observo una hiperplasia tiroidea ligera de caracter reversible.
Tras la administracion oral de PeBDE en dosis diarias bajas, del
orden de 0,78 µmoles/kg de peso corporal, se detecto la induccion de
enzimas hepaticas y un aumento de concentracion del citocromo P450.
Los resultados de la pruebas de teratogenicidad y mutagenicidad fueron
negativos.
No se ha informado de estudios de carcinogenicidad a largo plazo.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Se dispone de muy pocos datos.
2 Conclusiones
2.1 PeBDE
El PeBDE comercial (mezcla de varios éteres: 24-38% de tetra-,
50-60% de penta- y 4-8% de hexabromodifenilo) es persistente y se
acumula en los organismos y en el medio ambiente.
El PeBDE comercial de utiliza ampliamente incorporado a polimeros
como aditivo pirorretardante. El contacto de la poblacion general
tiene lugar a través de los productos fabricados con estos polimeros.
No es probable la exposicion debida a su extraccion de ellos. Se puede
producir exposicion humana al PeBDE mediante la cadena alimentaria,
puesto que se ha detectado su presencia en organismos del medio
ambiente que son articulos alimenticios humanos, como peces,
crustaceos, etc. En los dos ultimos decenios se han determinado
cantidades crecientes en los peces y las aves de Suecia.
La toxicidad aguda del PeBDE comercial es baja. No se dispone de
informacion acerca de su absorcion y eliminacion en los mamiferos.
Tampoco se han realizado estudios sobre la reproduccion, la toxicidad
a largo plazo y la carcinogenicidad.
De los datos disponibles no se puede determinar el riesgo de la
poblacion general.
Se carece de la informacion necesaria para sacar conclusiones
sobre los niveles de exposicion en el trabajo o los efectos del PeBDE
comercial.
Se dispone de una informacion limitada sobre la toxicidad del
PeBDE comercial para los organismos en el medio ambiente.
2.2 Productos de degradacion
Al calentar el PeBDE (o los productos que lo contienen) a
400-800°C se producen PBDF, y en cierta medida el PBDD. Habria que
ocuparse de los posibles peligros derivados de su formacion.
La exposicion de la poblacion general al PBDF de los polimeros
pirorretardantes con PeBDE probablemente carece de importancia. La
incineracion controlada de forma adecuada no produce una emision de
cantidades significativas de dioxinas y furanos bromados. La
combustion no controlada de productos con PeBDE puede dar lugar a la
formacion de cantidades no cuantificadas de PBDF/PBDD. En un futuro
EHC sobre PBDF y PBDD se prestara atencion a su importancia para el
ser humano y el medio ambiente.
3 Recomendaciones
3.1 Generales
Dada su persistencia en el medio ambiente y la acumulacion en
organismos no se deberia utilizar PeBDE comercial. Sin embargo, si se
va a seguir usando hay que tener en cuenta los puntos siguientes:
* Se debe proteger de la exposicion a los trabajadores que
intervienen en la fabricacion de PeBDE y de los productos que
contienen el compuesto mediante medidas adecuadas de higiene
industrial, vigilancia de la exposicion en el trabajo y controles
técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones de las industrias
que utilizan el compuesto o sus productos. Se debe controlar la
eliminacion de desechos industriales y de productos de consumo
para evitar en lo posible la contaminacion del medio ambiente con
este producto persistente y acumulable, asi como con sus productos
de degradacion.
* La incineracion de materiales pirorretardantes con PeBDE solo se
debe realizar en incineradores adecuados que funcionen siempre en
condiciones optimas. La quema por otros medios dara lugar a la
formacion de productos de descomposicion toxicos.
3.2 Otros estudios
* Se requiere una vigilancia permanente de sus niveles en el medio
ambiente.
* Se deben validar métodos de determinacion del PeBDE en diversos
aglomerantes.
* Habida cuenta de que la base de datos toxicologicos actual no es
adecuada para evaluar los peligros del PeBDE comercial para el ser
humano y el medio ambiente y, a fin de mejorar su uso, se deberian
realizar los siguientes estudios:
- estudios adicionales toxicologicos, carcinogénicos y
ecotoxicologicos;
- nuevas investigaciones sobre la formacion de PBDF en
condiciones de incendios reales;
- investigacion de posibles métodos de reciclaje de polimeros
que contienen PeBDE y sus consecuencias;
- investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE TETRABROMODIFENILO
El éter de tetrabromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones previas por parte de organos internacionales
1 Resumen y evaluacion
1.1 Identificacion y propiedades fisicas y quimicas
El éter de tetrabromodifenilo comercial esta formado por una
mezcla de éteres: 41% de tetra-, 45% de penta- y 7% de
hexabromodifenilo y alrededor del 7% de PBDE de estructura
desconocida. En funcion de su estructura quimica, hay 42 posibles
isomeros del éter de tetrabromodifenilo. Practicamente se carece de
datos sobre sus propiedades fisicas y quimicas, a excepcion del
logaritmo del coeficiente de reparto n-octanol/agua, que es
5,87-6,16.
1.2 Produccion y aplicaciones
Hay un informe de la produccion (utilizacion) de unas 1000
toneladas de TeBDE en el Japon en 1987. No se tiene conocimiento de
produccion alguna en la actualidad como éter de tetrabromodifenilo,
pero esta presente en el éter de pentabromodifenilo comercial en
cantidades que oscilan entre el 24 y el 38%.
1.3 Transporte, distribucion y transformacion en el medio ambiente
En muestras de biota, sedimento y fangos cloacales se han
encontrado componentes del TeBDE comercial. Es probable que éstos (con
cantidades aproximadamente iguales de PeBDE) sean persistentes y
bioacumulables.
En estudios de pirolisis con TeBDE comercial se puso de manifiesto
que a 800°C se formaban PBDF y PBDD. No se encontraron estos
compuestos con mas atomos de bromo.
1.4 Niveles medioambientales y exposicion humana
Se detecto TeBDE en el sedimento de rios del Japon en
concentraciones de 12-31 µg/kg de peso seco y en Suecia a niveles de
hasta 840 µg/kg de pérdida por incineracion. También se encontro TeBDE
en fangos cloacales de Suecia a concentraciones de 15 µg/kg.
Los mejillones y peces recogidos en diferentes lugares del Japon
contenian TeBDE en concentraciones que oscilaban entre <0,1 y 14,6 µg
de 2,2',4,4'-TeBDE/kg de peso seco. En Suecia se recogieron diferentes
tipos de peces en diversos rios para analizar la concentracion de
2,2',4,4'-TeBDE. Las concentraciones medias variaron entre la no
detectable (<0,1 mg/kg) y 110 mg/kg de grasa. En los analisis se puso
de manifiesto la existencia de por lo menos una fuente local de
contaminacion en un rio determinado. Los corégonos, las truchas
alpinas y los arenques recogidos en distintos lugares de Suecia en
1986-87 contenian concentraciones de 15, 400 y 59-450 µg de
2,2',4,4'-TeBDE/kg de grasa respectivamente. Los peces recogidos en
rios de Alemania contenian hasta 1 mg de TeBDE/kg de grasa.
En el arenque y en el higado de bacalao procedentes de las
regiones meridional, central y septentrional del mar del Norte durante
el periodo 1983-89, se encontro una tendencia decreciente en la
concentracion del TeBDE en direccion sur-norte. En el arenque se
detectaron concentraciones de 8,4-100 µg de 2,2',4,4'-TeBDE/kg de
grasa.
En el tejido muscular de aves que se reproducen e invernan en el
mar Baltico, el mar del Norte y Spitzbergen se determinaron
concentraciones de 80 a 370 µg de 2,2',4,4'-TeBDE/kg de grasa. Los
quebrantahuesos recogidos en Suecia entre 1982 y 1986 contenian
concentraciones medias de 1800 µg/kg de grasa.
Se ha indicado que hay una tendencia creciente de las
concentraciones de 2,2',4,4'-TeBDE en los sedimentos del mar Baltico,
los peces de agua dulce y los huevos de aves marinas de Suecia.
En la grasa de las focas recogidas en el mar Baltico y en
Spitzbergen se determinaron concentraciones de 10-73 µg de
2,2',4,4'-TeBDE/kg de grasa. El perfil cromatografico del PBDE fue
semejante al del Bromkal 70-5. En mezclas de muestras de grasa de
focas oceladas y focas grises recogidas en Suecia en 1979-85 se
encontraron concentraciones de 47 µg y 650 µg de 2,2',4,4'-TeBDE/kg de
grasa, respectivamente.
Las mezclas de muestras de musculo de mamiferos terrestres, por
ejemplo conejos, ratones y renos, recogidas en Suecia en 1985 y 1986
pusieron de manifiesto concentraciones medias de <2, 0,82 y 0,18 µg
de 2,2',4,4'-TeBDE/kg de grasa, respectivamente.
En cuatro muestras de leche de vaca recogidas en Alemania se
encontraron niveles de 2,5-4,5 µg de PBDE/kg de grasa, medida como
Bromkal 70DE. En la leche de 25 mujeres de Alemania se encontro PBDE,
como Bromkal 70DE, en concentraciones que oscilaron entre
6,2 y 11,1 µg/kg de grasa.
Una estimacion aproximada de la exposicion a través del consumo de
pescado entre la poblacion sueca parece indicar una absorcion diaria
de 0,3 µg de TeBDE/persona.
1.5 Efectos en los mamiferos de laboratorio y en los sistemas de
ensayo in vitro
No hay datos sobre el propio TeBDE, pero se dispone de datos sobre
toxicidad aguda y a corto plazo del PeBDE comercial con un contenido
de TeBDE del 41%.
1.6 Cinética y metabolismo en animales de laboratorio y en el ser
humano
Se dispone de muy pocos datos.
1.7 Efectos en el ser humano
Se carece de datos.
1.8 Efectos en otros organismos de laboratorio y del medio ambiente
Se carece de datos.
2 Conclusiones
2.1 TeBDE
Los componentes del TeBDE comercial (una mezcla de un 41% de
2,2',4,4'-tetra-; 45% de 2,2',4,4',5'-penta-; 7% de hexa-; y 7-8% de
éteres de difenilo polibromados de estructura desconocida) son
persistentes y se acumulan en organismos del medio ambiente.
El TeBDE, como componente del éter de pentabromodifenilo, se
incorpora con profusion a polimeros como aditivo pirorretardante. El
contacto de la poblacion general se produce a través de productos
fabricados con estos polimeros. No es probable la exposicion por
extraccion a partir de los mismos. Es posible la exposicion humana al
TeBDE, mediante la cadena alimentaria, porque se ha detectado su
presencia en organismos del medio ambiente que forman parte de la
alimentacion humana, como por ejemplo peces, crustaceos, etc. Durante
los dos ultimos decenios se han determinado concentraciones crecientes
en los peces y las aves de Suecia.
Apenas se dispone de informacion acerca de la
toxicidad/carcinogenicidad a corto y largo plazo y de estudios de
reproduccion.
No se puede determinar el riesgo para la poblacion general en
funcion de los datos disponibles.
No se dispone de la informacion necesaria para poder sacar
conclusiones sobre los niveles de exposicion en el trabajo o los
efectos del TeBDE.
Tampoco hay datos acerca de la toxicidad del TeBDE comercial para
los organismos del medio ambiente.
2.2 Productos de degradacion
Cuando el TeBDE se calienta a 800°C se forman PBDF y PBDD. Hay que
prestar atencion a los posibles peligros relacionados con éstos.
La exposicion de la poblacion general al PBDF de los polimeros
pirorretardantes con TeBDE probablemente carece de importancia. La
incineracion con los controles adecuados no produce una emision de
cantidades importantes de dioxinas y furanos bromados. Cualquier
combustion sin control de productos que contienen TeBDE puede dar
lugar a la formacion de cantidades no cuantificadas de PBDF/PBDD. Un
futuro numero de EHC se ocupara de su importancia para el ser humano y
el medio ambiente.
3 Recomendaciones
3.1 Generales
Dada su persistencia en el medio ambiente y la acumulacion en
organismos, se recomienda que no se utilice TeBDE. Sin embargo, si se
va a seguir usando hay que tener en cuenta los puntos siguientes:
* Se debe proteger de la exposicion a los trabajadores que
intervienen en la fabricacion de TeBDE y de los productos que
contienen el compuesto, mediante medidas adecuadas de higiene
industrial, vigilancia de la exposicion en el trabajo y controles
técnicos.
* Hay que reducir al minimo la exposicion ambiental mediante el
tratamiento adecuado de efluentes y emisiones de las industrias
que utilizan el compuesto o sus productos. Se debe controlar la
eliminacion de desechos industriales y de productos de consumo
para evitar en lo posible la contaminacion del medio ambiente con
este producto persistente y acumulable, asi como con sus productos
de degradacion.
* La incineracion de materiales pirorretardantes con TeBDE solo se
debe realizar en incineradores adecuados que funcionen siempre en
condiciones optimas. La quema por otros medios dara lugar a la
formacion de productos de degradacion del furano toxicos.
3.2 Otros estudios
* Se requiere una vigilancia permanente de sus niveles en el medio
ambiente.
* Se deben validar métodos de determinacion del TeBDE en diversos
aglomerantes.
* Habida cuenta de que la base de datos toxicologicos actual no es
adecuada para evaluar los peligros del TeBDE comercial para el ser
humano y el medio ambiente, si se va a continuar con su uso se
deberian realizar los siguientes estudios:
- estudios adicionales toxicologicos, carcinogénicos y
ecotoxicologicos;
- nuevas investigaciones sobre la formacion de PBDF en
condiciones de incendios reales;
- investigacion de posibles métodos de reciclaje de polimeros
que contienen TeBDE y sus consecuencias;
- investigaciones sobre la posibilidad de migracion a partir de
diferentes tipos de polimeros.
ÉTER DE TRIBROMODIFENILO
El éter de tribromodifenilo no se fabrica ni se utiliza. No se
dispone de datos sobre los aspectos siguientes:
* Transporte, distribucion y transformacion en el medio ambiente
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay una base de datos sobre la cual realizar una evaluacion.
2 Recomendaciones
Se debe reducir al minimo la contaminacion de los productos
comerciales con éter de tribromodifenilo, a fin de evitar la del medio
ambiente y la exposicion humana.
Hay que evitar el uso de los productos comerciales capaces de
contaminar el medio ambiente.
ÉTER DE DIBROMODIFENILO
El éter de dibromodifenilo no se fabrica ni se utiliza.
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano
* Efectos en otros organismos en el laboratorio y en el medio
ambiente
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
No hay una base de datos sobre la cual realizar una evaluacion.
2 Recomendaciones
Se debe reducir al minimo la contaminacion de los productos
comerciales con éter de dibromodifenilo, a fin de evitar la del medio
ambiente y la exposicion humana.
Hay que evitar el uso de los productos comerciales capaces de
contaminar el medio ambiente.
ÉTER DE MONOBROMODIFENILO
No se dispone de datos sobre los aspectos siguientes:
* Cinética y metabolismo en animales de laboratorio y en el ser
humano
* Efectos en el ser humano.
* Evaluaciones anteriores por parte de organos internacionales.
1 Resumen y evaluacion
1.1 Propiedades fisicas y quimicas
El éter de monobromodifenilo tiene tres posibles isomeros.
El éter de p-bromodifenilo a temperatura ambiente es un liquido
con un punto de ebullicion de 305-310°C. Se estima que su solubilidad
en agua es de 48 mg/litro. El log del coeficiente de reparto
n-octanol/agua es de 4 a 5. La presion de vapor a 20°C es de
0,0015 mm de Hg.
1.2 Produccion y aplicaciones
El MBDE no se utiliza como pirorretardante. En 1977 aparecio un
informe sobre su produccion, pero se desconoce su empleo.
1.3 Transporte, distribucion y transformacion en el medio ambiente
La semivida de volatizacion a partir del agua es del orden de
centenares de dias.
Aunque el MBDE no se degrado de manera significativa en un cultivo
de siete dias con microorganismos de las aguas residuales domésticas,
se ha informado que en los fangos cloacales activados lo hace en un
95%. En un estudio aislado se puso de manifiesto que una cepa de
bacterias del suelo no era capaz de degradar el MBDE como unica fuente
de carbono.
1.4 Niveles medioambientales y exposicion humana
Se ha detectado MBDE en muestras de agua superficial recogidas en
las cercanias de zonas industriales de los Estados Unidos, pero no se
obtuvieron estos resultados en un estudio semejante realizado en el
Japon. Se encontro asimismo en agua del suelo de un lugar proximo a
una instalacion industrial de los Estados Unidos. También se ha
detectado en el sedimento acuatico y en la biota acuatica de los
Estados Unidos.
1.5 Cinética y metabolismo en animales de laboratorio y en el ser
humano
No se dispone de datos.
1.6 Efectos en mamiferos de laboratorio y en sistemas de ensayo
in vitro
El MBDE no es teratogénico, pero se carece de datos sobre su
toxicidad aguda, a corto plazo y a largo plazo, y por consiguiente no
se puede realizar su evaluacion.
1.7 Efectos en el ser humano
No se dispone de datos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Se ha informado que la CL50 en 96 h para Lepomis macrochirus es
de 4,9 mg/litro, con una concentracion sin efectos observados de menos
de 2,8 mg/litro. La CL50 en 48 horas para la pulga de agua fue de
0,36 mg/litro, con un NOEC de menos de 0,046 mg/litro.
2 Conclusiones y recomendaciones
El éter de monobromodifenilo no tiene propiedades
pirorretardantes. Puede acumularse en los organismos del medio
ambiente y se ha detectado en diferentes compartimentos ambientales.
Algunas pruebas indican que es biodegradable.
La reducida informacion de que se dispone impide sacar
conclusiones sobre los niveles de exposicion y los efectos sobre la
poblacion general y los organismos.
No existe una base de datos toxicologicos que apoye su uso.
Se debe evitar todo empleo que provoque la contaminacion del medio
ambiente.
 Polybrominated diphenyl ethers (PBDE) have a large number of
congeners, depending on the number and position of the bromine atoms
on the two phenyl rings. The total number of possible congeners is
209, and the numbers of isomers for mono-, di-, tri- up to
decabromodiphenyl ethers are: 3, 12, 24, 42, 46, 42, 24, 12, 3, and 1,
respectively.
The commercial PBDE are produced by the bromination of diphenyl
oxide under certain conditions, which result in products containing
mixtures of brominated diphenyl ethers (see the individual PBDE). The
compositions of commercial DeBDE, OBDE, and PeBDE are given in
Table 1.
No, or virtually no, data are available on dibromo-, tribromo-,
hexabromo-, heptabromo-, and nonabromodiphenyl ether (DiBDE, TrBDE,
HxBDE, HpBDE, and NBDE, respectively). Flame retardants containing
predominantly penta-, octa- and decabromodiphenyl ethers are
commercially produced (with tetrabromodiphenyl ether as a major
component of "pentabromo-diphenyl ether", which is a mixture).
The commercial PBDE are rather stable compounds with boiling
points ranging between 310 and 425 °C and with low vapour pressures,
e.g., 3.85 up to 13.3 Pa at 20-25 °C; they are lipophilic substances.
Their solubility in water is very poor, especially that of the higher
brominated diphenyl ethers, and the n-octanol/water partition
coefficients (log Pow) range between 4.28 and 9.9.
Polybrominated diphenyl ethers have not been reported to occur
naturally in the environment, but other types of brominated diphenyl
ethers have been found in marine organisms (Carte & Faulkner, 1981;
Faulkner, 1990).
The presence in the environment of some of the brominated diphenyl
ethers has been documented, the highest concentration being 1 g/kg
sediment in streams or ponds in the vicinity of a manufacturing
facility.
Data on environmental fate, although limited to MBDE, DiBDE, and
DeBDE, suggest that biodegradation is not an important degradation
pathway for the PBDE, but that photodegradation may play a significant
role.
Table 1. Composition of commercial brominated diphenyl ethers
Product Composition
PBDEa TrBDE TeBDE PeBDE HxBDE HpBDE OBDE NBDE DeBDE
DeBDE 0.3-3% 97-98%
OBDE 10-12% 43-44% 31-35% 9-11% 0-1%
PeBDE 0-1% 24-38% 50-62% 4-8%
TeBDEb 7.6% -- 41-41.7% 44.4-45% 6-7%
a Unknown structure.
b No longer commercially produced. Analysis of one single sample.
Many reports have appeared in the literature describing the
behaviour of brominated flame retardants under pyrolytic conditions.
In general, these reports have indicated that maximum concentrations
of PBDF and/or PBDD were observed at temperatures of 400-800 °C and
that the 2,3,7,8-substituted compounds were seen only in very low
concentrations.
Processing of the polymers under abusive or extreme conditions
produced higher levels of PBDF, but the concentrations were
significantly lower than the values previously reported from
laboratory pyrolysis studies. 2,3,7,8-Brominated isomers were only
found at low levels in a sample abusively processed. The
2,3,7,8-brominated isomers, which are of concern for toxicological and
regulatory reasons, were not detected under normal processing
conditions. The results of the laboratory pyrolysis experiments with
PBDE, showed that PBDF and/or PBDD were formed in various
concentrations, depending on the type of PBDE, the polymer matrix, the
specific processing conditions (temperature, presence of oxygen, etc.)
and equipment used, and the presence of Sb2O3. Behaviour of PBDE is
strongly dependent upon the polymer matrix and upon the specific
processing conditions mentioned above, thus laboratory pyrolysis
experiments can hardly be used as reliable models to predict behaviour
in commercial moulding operations.
2. GENERAL INFORMATION ON BROMONATED DIPHENYL ETHERS
2.1 Analytical methods
Several methods to determine residues of PBDE in various media
(air, sewage sludge, sediment, human adipose tissue, marine organisms,
fish, and feed) as well as in commercial products have been reported.
For details, see Table 2.
In general, sample extraction and clean-up techniques for the
analysis of PBDE residues in biological samples are similar to those
developed for PBB (see EHC 152: Polybrominated biphenyls), though
the chromatographic conditions have to be modified in view of the long
retention times of the highly brominated PBDE. Temperature programming
and the use of capillary columns have been found to be very useful for
the separation of the different congeners of PBDE. Recovery for the
different PBDE is generally higher than 80%. Most methods are based on
extraction with organic solvents, such as hexane/acetone,
hexane/diphenyl ether, acetone, etc, purification of the extracts by
gel permeation or adsorption chromatography, and determination mainly
by gas chromatography, either with electron capture detection (ECD),
or, coupled with mass spectrometry (MS). A multi-residue method has
also been developed that includes a multi-step separation enabling the
determination of several polychlorinated and polybrominated pollutants
in biological samples (Jansson et al., 1991).
Table 2. Analytical methods for PBDE
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Sewage extract with chloroform; evaporate and dissolve GC/MS 0.06 mg/kg Kaart & Kokk
residue in ethanol (1987)
Sediment extract with acetone; clean-up on Florisil NAA; < 5 µg/kg Watanabe et al.
GC/EC < 5 µg/kg (1987b)
Fish extract with acetone-hexane + hexane-ethyl ether; GC/EC; limit of Andersson &
treatment with sulfuric acid or clean-up on alumina; GC/MS detection Blomkvist
chromatography on silica gel 0.1 mg/kg fat (1981)
Animal tissues homogenize; extract with n-hexane-acetone; GC/MS (NCl) 10 ng/kg Jansson et al.
(Multi-residue treatment with sulfuric acid; gel permeation (1991)
method) chromatography; chromatography or silica gel;
chromatography or activated charcoal
Rat liver extract with tetrahydrofuran HPLC Rogers & Hill
(1980)
Table 2 (continued)
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Fish extract freeze-dried powdered sample with pet. ether; GC/MS < 5 µg/kg fat Kruger (1988)
gel permeation chromatography; clean-up on Florisil; (NCl/SIM)
elute with hexane
Cow's milk centrifuge; gel permeation chromatography; clean-up GC/MS < 2.5 µg/kg fat Kruger (1988)
on Florisil; elute with hexane (NCl/SIM)
Human milk extract with potassium oxalate/ethanol/diethyl GC/MS < 0.6 µg/kg fat Kruger (1988)
ether/pentane; gel permeation chromatography; (NCl/SIM)
clean-up on Florisil; elute with hexane
Human adipose extract with methylene chloride; evaporate; clean-up HRGC/HRMSa limit of Cramer et al.
tissue on silica gel followed by clean-up on alumina and on detection (1990a,b)
a carbon/silica gel column 0.73-120 ng/kg
(different
congeners)
Commercial PBDE homogenize and dissolve in tetrachloromethane for HPLC; GC/MS; -- deKok et al.
HPLC and GC/MS or n-hexane for TLC/UV TLC/UV (1979)
a High resolution gas chromatography/high resolution mass spectrometry.
2.2 Production levels and processes
According to the information given by the European Brominated
Flame Retardant Industry Panel (EBFRIP), eight manufacturers are
currently producing polybrominated diphenyl ethers. They are: Dead Sea
Bromines/Eurobrome (The Netherlands); Atochem (France); Ethyl
Corporation (USA); Great Lakes Chemical Corporation (USA); Tosoh
(Japan); Matsunaga (Japan); Nippo (Japan); Great Lakes Chemical Ltd
(United Kingdom).
The annual global consumption of PBDE is 40 000 tonnes (30 000
tonnes of DeBDE; 6000 tonnes OBDE and 4000 tonnes PeBDE) (Arias,
1992).
It has been reported that the use of brominated flame retardants
in Japan increased from 2500 tonnes in 1975 to 22 100 tonnes in 1987
(Watanabe & Tatsukawa, 1990).
The production and import figures for the European Economic
Community (EEC) are given in Table 3.
Table 3. Production and import quantities of PBDE in metric
tonnes in the EECa
1986 1987 1988 1989
Production 4276 3624 4066 3843
Import 4310 3492 4955 7103
Total 8586 7116 9021 10 946
aFrom: EBFRIP (1990).
Data on the usage of PBDE are available for some individual
European countries. Germany uses 3000-5000 tonnes/year, Sweden
1400-2000 tonnes/year, and The Netherlands 3300-3700 tonnes/year
(OECD, 1991; van Zorge, 1992), but Pijnenburg & Everts (1991) and
Pijnenburg et al. (1992) reported a level of 2500 tonnes PBDE for the
last country. In the United Kingdom, up to 2000 tonnes per year are
used (UK DOE, 1993).
Because of the significant reduction in the fire hazard for the
public achieved by the use of PBDE in a wide range of applications,
particularly in the furniture industry, and electrical/computer
components and housing, the consumption of PBDE has significantly
increased over the last years (EBFRIP, 1990).
2.3 Resins, polymers, and substrates in which PBDE are used
The major uses of the polybrominated diphenyl ethers in descending
order of importance are: high-impact polystyrene, ABS, flexible
polyurethane foam, textile coatings (not clothing), wire and cable
insulation, electrical/electronic connectors and other interior parts.
These applications account for at least 80-90% of the consumption of
brominated diphenyl ethers in the USA.
Brominated diphenyl ethers are used as additive flame retardants.
Additive flame retardants are incorporated into the plastic matrix
like other additives, such as plasticizers. The ideal additive is
inexpensive, colourless, easily blended, compatible, heat and light
stable, efficient, permanent, and has no deleterious effect on the
properties of the base polymer. The most important limitations are
incompatibilities that affect the physical properties of the polymers
and the tendency for additives to be fugitive. These additive flame
retardants are much more prone to leaching or escape from the finished
polymer product than the reactive flame retardants (Hutzinger et al.,
1976; Hutzinger & Thoma, 1987; Larsen, 1980).
The uses of penta-, octa-, and decabromodiphenyl ethers in the
different resins, polymers, and substrates are shown in Table 4. The
principal applications of these PBDE-containing substances are shown
in Table 5.
PBDE are used in the different resins, polymers, and substrates at
levels ranging from 5 up to 30%. The quantities used for each
application are not publicly available. In consumer products, resins
containing PBDE are typically used in interior parts, minimizing the
potential for exposure of the public. The incorporation of the PBDE
into the polymer matrix further reduces the possibilities of exposure
(EBFRIP, 1990).
Table 4. Use of penta- (PeBDE), octa- (OBDE), and decabromodiphenyl ethers
(DeBDE) in resins, polymers, and substratesa
Resins/polymers/substrates DeBDE OBDE PeBDE
ABS X
Epoxy-resins X
Phenolic resins X X
PAN X
PA X X
PBT X X
PE/XPE X
PET X
PP X
PS, HIPS X X
PVC X X
PUR X
UPE X X
Rubber X X
Paints/lacquers X X
Textiles X X
aFrom: EBFRIP (1990); UK Department of Environment (1992).
Table 5. The various applications of resins in which PBDE are used are listed belowa
Polymer Principal applications Examples of final products
ABS Moulded parts TV-sets/business machines,
computer housings, household
appliances (hairdryer, curler),
automotive parts, electronics,
telecommunications
EPOXY Circuit boards, Computers, ship interiors,
protective coatings electronic parts
PAINTS/ Coatings Marine and industry lacquers
LACQUERS for protection of containers
PHENOLICS Printed circuit boards Paper laminates/glass prepregs
for printed circuit boards
PAN Panels, electrical Lighting panels for elevators
components and rooms, housing of electrical
appliances
PA Electrical connectors, Computers, connectors,
automative interior housing in electrical industry,
parts board, electrical connectors,
automotive industry,
transportation
PBT Electrical connectors Switches, fuse, switch box,
and components computer housings, switchboard
electrical connectors,
stereos, business machines,
military electronics
PE/XPE Cross-linked wire and Major application: power cable
cable, foam tubing, with cross-linked low density
weather protection PE; also used for conduit for
and moisture barriers building with high density PE;
Final uses: portable apparatus
building control, instrument,
shipboard, automotive, marine
appliances, insulation of heating
tubes
PET Electrical Boxes, relays, coils, bobbins
components
Table 5. (cont'd).
Polymer Principal applications Examples of final products
PP Conduits, electronic TV and electronic devices, such
devices as yoke, housings, circuit board
hangers, conduits; Final uses:
electro-mechanical parts TV,
hot waste water pipes,
underground junction boxes
PS, HIPS TV cabinets and back TV back panels, computer
covers, electrical covers and housings of
appliance housings electrical appliances, office
machines, smoke detectors
PVC Cable sheets Wire end cables, floor mats,
industrial sheets
PUR Cushioning materials, Furniture, sound insulation
packaging, padding panels, wood imitations,
transportation
RUBBER Transportation Conveyor belts, foamed pipes
for insulation
TEXTILES Coatings Back coatings, impregnation:
carpets, automotive seating,
furniture in homes and official
buildings, aircraft, undergrounds,
tents, trains, and military
safety clothing
UPE Circuit boards, Electrical equipment, coatings
coatings for chemical processing plants
mouldings, military and marine
applications: construction
panels
aFrom: EBFRIP (1990).
3. FORMATION OF BROMINATED DIBENZOFURANS AND DIBENZODIOXINS FROM
POLYBROMINATED DIPHENYL ETHERS
3.1 General
Polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) can be formed from polybrominated diphenyl
ethers, polybrominated phenols, and polybrominated biphenyls under
different conditions, including heating (combustion). Laboratory
experiments have also demonstrated the formation of PBDF and PBDD
during the pyrolysis of certain other brominated flame retardants (see
the EHC on Brominated flame retardants, in preparation). As
discussed in EHC 88: Polychlorinated dibenzo-para-dioxins and
dibenzofurans, there are hundreds of possible congeners of
halogenated dibenzofurans and dibenzo-dioxins. However, only congeners
with substituents in the 2,3,7,8-positions are of toxicological
significance. In many reports, only the total levels of PBDF and PBDD
are given, without regard to substitution pattern; such totals are of
limited value in the estimation of possible risk.
Hutzinger & co-workers investigated the pyrolysis of brominated
flame retardants and flame retardant polymer systems and several
publications have appeared. In general, the results reported showed
that brominated dibenzofurans were observed at 700-800 °C and that the
2,3,7,8-substituted compounds were seen in only low concentrations, if
at all (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987; Dumler et al.,
1989).
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF, and Hoechst) and American
industries independently reported the results of combustion and
pyrolysis experiments with flame retarded polymers (BFRIP, 1990).
Recently, brominated aromatic compounds have also attracted
attention, since reports have appeared about emissions of PBDD and
PBDF and other brominated and mixed halogenated aromatic compounds in
accidental fires and from the combustion of waste (see section 4 of
both DeBDE and OBDE).
For more information on these pyrolysis experiments, see the
different sections relating to the individual brominated diphenyl
ethers, e.g., PeBDE, OBDE, and DeBDE.
As an example, the formation of PBDF and PBDD from
decabromodiphenyl ether is illustrated in Fig. 1.
Polybrominated diphenyl ethers (PBDE) have a large number of
congeners, depending on the number and position of the bromine atoms
on the two phenyl rings. The total number of possible congeners is
209, and the numbers of isomers for mono-, di-, tri- up to
decabromodiphenyl ethers are: 3, 12, 24, 42, 46, 42, 24, 12, 3, and 1,
respectively.
The commercial PBDE are produced by the bromination of diphenyl
oxide under certain conditions, which result in products containing
mixtures of brominated diphenyl ethers (see the individual PBDE). The
compositions of commercial DeBDE, OBDE, and PeBDE are given in
Table 1.
No, or virtually no, data are available on dibromo-, tribromo-,
hexabromo-, heptabromo-, and nonabromodiphenyl ether (DiBDE, TrBDE,
HxBDE, HpBDE, and NBDE, respectively). Flame retardants containing
predominantly penta-, octa- and decabromodiphenyl ethers are
commercially produced (with tetrabromodiphenyl ether as a major
component of "pentabromo-diphenyl ether", which is a mixture).
The commercial PBDE are rather stable compounds with boiling
points ranging between 310 and 425 °C and with low vapour pressures,
e.g., 3.85 up to 13.3 Pa at 20-25 °C; they are lipophilic substances.
Their solubility in water is very poor, especially that of the higher
brominated diphenyl ethers, and the n-octanol/water partition
coefficients (log Pow) range between 4.28 and 9.9.
Polybrominated diphenyl ethers have not been reported to occur
naturally in the environment, but other types of brominated diphenyl
ethers have been found in marine organisms (Carte & Faulkner, 1981;
Faulkner, 1990).
The presence in the environment of some of the brominated diphenyl
ethers has been documented, the highest concentration being 1 g/kg
sediment in streams or ponds in the vicinity of a manufacturing
facility.
Data on environmental fate, although limited to MBDE, DiBDE, and
DeBDE, suggest that biodegradation is not an important degradation
pathway for the PBDE, but that photodegradation may play a significant
role.
Table 1. Composition of commercial brominated diphenyl ethers
Product Composition
PBDEa TrBDE TeBDE PeBDE HxBDE HpBDE OBDE NBDE DeBDE
DeBDE 0.3-3% 97-98%
OBDE 10-12% 43-44% 31-35% 9-11% 0-1%
PeBDE 0-1% 24-38% 50-62% 4-8%
TeBDEb 7.6% -- 41-41.7% 44.4-45% 6-7%
a Unknown structure.
b No longer commercially produced. Analysis of one single sample.
Many reports have appeared in the literature describing the
behaviour of brominated flame retardants under pyrolytic conditions.
In general, these reports have indicated that maximum concentrations
of PBDF and/or PBDD were observed at temperatures of 400-800 °C and
that the 2,3,7,8-substituted compounds were seen only in very low
concentrations.
Processing of the polymers under abusive or extreme conditions
produced higher levels of PBDF, but the concentrations were
significantly lower than the values previously reported from
laboratory pyrolysis studies. 2,3,7,8-Brominated isomers were only
found at low levels in a sample abusively processed. The
2,3,7,8-brominated isomers, which are of concern for toxicological and
regulatory reasons, were not detected under normal processing
conditions. The results of the laboratory pyrolysis experiments with
PBDE, showed that PBDF and/or PBDD were formed in various
concentrations, depending on the type of PBDE, the polymer matrix, the
specific processing conditions (temperature, presence of oxygen, etc.)
and equipment used, and the presence of Sb2O3. Behaviour of PBDE is
strongly dependent upon the polymer matrix and upon the specific
processing conditions mentioned above, thus laboratory pyrolysis
experiments can hardly be used as reliable models to predict behaviour
in commercial moulding operations.
2. GENERAL INFORMATION ON BROMONATED DIPHENYL ETHERS
2.1 Analytical methods
Several methods to determine residues of PBDE in various media
(air, sewage sludge, sediment, human adipose tissue, marine organisms,
fish, and feed) as well as in commercial products have been reported.
For details, see Table 2.
In general, sample extraction and clean-up techniques for the
analysis of PBDE residues in biological samples are similar to those
developed for PBB (see EHC 152: Polybrominated biphenyls), though
the chromatographic conditions have to be modified in view of the long
retention times of the highly brominated PBDE. Temperature programming
and the use of capillary columns have been found to be very useful for
the separation of the different congeners of PBDE. Recovery for the
different PBDE is generally higher than 80%. Most methods are based on
extraction with organic solvents, such as hexane/acetone,
hexane/diphenyl ether, acetone, etc, purification of the extracts by
gel permeation or adsorption chromatography, and determination mainly
by gas chromatography, either with electron capture detection (ECD),
or, coupled with mass spectrometry (MS). A multi-residue method has
also been developed that includes a multi-step separation enabling the
determination of several polychlorinated and polybrominated pollutants
in biological samples (Jansson et al., 1991).
Table 2. Analytical methods for PBDE
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Sewage extract with chloroform; evaporate and dissolve GC/MS 0.06 mg/kg Kaart & Kokk
residue in ethanol (1987)
Sediment extract with acetone; clean-up on Florisil NAA; < 5 µg/kg Watanabe et al.
GC/EC < 5 µg/kg (1987b)
Fish extract with acetone-hexane + hexane-ethyl ether; GC/EC; limit of Andersson &
treatment with sulfuric acid or clean-up on alumina; GC/MS detection Blomkvist
chromatography on silica gel 0.1 mg/kg fat (1981)
Animal tissues homogenize; extract with n-hexane-acetone; GC/MS (NCl) 10 ng/kg Jansson et al.
(Multi-residue treatment with sulfuric acid; gel permeation (1991)
method) chromatography; chromatography or silica gel;
chromatography or activated charcoal
Rat liver extract with tetrahydrofuran HPLC Rogers & Hill
(1980)
Table 2 (continued)
Sample Extraction and clean-up Separation and Limit of Reference
detection determination
Fish extract freeze-dried powdered sample with pet. ether; GC/MS < 5 µg/kg fat Kruger (1988)
gel permeation chromatography; clean-up on Florisil; (NCl/SIM)
elute with hexane
Cow's milk centrifuge; gel permeation chromatography; clean-up GC/MS < 2.5 µg/kg fat Kruger (1988)
on Florisil; elute with hexane (NCl/SIM)
Human milk extract with potassium oxalate/ethanol/diethyl GC/MS < 0.6 µg/kg fat Kruger (1988)
ether/pentane; gel permeation chromatography; (NCl/SIM)
clean-up on Florisil; elute with hexane
Human adipose extract with methylene chloride; evaporate; clean-up HRGC/HRMSa limit of Cramer et al.
tissue on silica gel followed by clean-up on alumina and on detection (1990a,b)
a carbon/silica gel column 0.73-120 ng/kg
(different
congeners)
Commercial PBDE homogenize and dissolve in tetrachloromethane for HPLC; GC/MS; -- deKok et al.
HPLC and GC/MS or n-hexane for TLC/UV TLC/UV (1979)
a High resolution gas chromatography/high resolution mass spectrometry.
2.2 Production levels and processes
According to the information given by the European Brominated
Flame Retardant Industry Panel (EBFRIP), eight manufacturers are
currently producing polybrominated diphenyl ethers. They are: Dead Sea
Bromines/Eurobrome (The Netherlands); Atochem (France); Ethyl
Corporation (USA); Great Lakes Chemical Corporation (USA); Tosoh
(Japan); Matsunaga (Japan); Nippo (Japan); Great Lakes Chemical Ltd
(United Kingdom).
The annual global consumption of PBDE is 40 000 tonnes (30 000
tonnes of DeBDE; 6000 tonnes OBDE and 4000 tonnes PeBDE) (Arias,
1992).
It has been reported that the use of brominated flame retardants
in Japan increased from 2500 tonnes in 1975 to 22 100 tonnes in 1987
(Watanabe & Tatsukawa, 1990).
The production and import figures for the European Economic
Community (EEC) are given in Table 3.
Table 3. Production and import quantities of PBDE in metric
tonnes in the EECa
1986 1987 1988 1989
Production 4276 3624 4066 3843
Import 4310 3492 4955 7103
Total 8586 7116 9021 10 946
aFrom: EBFRIP (1990).
Data on the usage of PBDE are available for some individual
European countries. Germany uses 3000-5000 tonnes/year, Sweden
1400-2000 tonnes/year, and The Netherlands 3300-3700 tonnes/year
(OECD, 1991; van Zorge, 1992), but Pijnenburg & Everts (1991) and
Pijnenburg et al. (1992) reported a level of 2500 tonnes PBDE for the
last country. In the United Kingdom, up to 2000 tonnes per year are
used (UK DOE, 1993).
Because of the significant reduction in the fire hazard for the
public achieved by the use of PBDE in a wide range of applications,
particularly in the furniture industry, and electrical/computer
components and housing, the consumption of PBDE has significantly
increased over the last years (EBFRIP, 1990).
2.3 Resins, polymers, and substrates in which PBDE are used
The major uses of the polybrominated diphenyl ethers in descending
order of importance are: high-impact polystyrene, ABS, flexible
polyurethane foam, textile coatings (not clothing), wire and cable
insulation, electrical/electronic connectors and other interior parts.
These applications account for at least 80-90% of the consumption of
brominated diphenyl ethers in the USA.
Brominated diphenyl ethers are used as additive flame retardants.
Additive flame retardants are incorporated into the plastic matrix
like other additives, such as plasticizers. The ideal additive is
inexpensive, colourless, easily blended, compatible, heat and light
stable, efficient, permanent, and has no deleterious effect on the
properties of the base polymer. The most important limitations are
incompatibilities that affect the physical properties of the polymers
and the tendency for additives to be fugitive. These additive flame
retardants are much more prone to leaching or escape from the finished
polymer product than the reactive flame retardants (Hutzinger et al.,
1976; Hutzinger & Thoma, 1987; Larsen, 1980).
The uses of penta-, octa-, and decabromodiphenyl ethers in the
different resins, polymers, and substrates are shown in Table 4. The
principal applications of these PBDE-containing substances are shown
in Table 5.
PBDE are used in the different resins, polymers, and substrates at
levels ranging from 5 up to 30%. The quantities used for each
application are not publicly available. In consumer products, resins
containing PBDE are typically used in interior parts, minimizing the
potential for exposure of the public. The incorporation of the PBDE
into the polymer matrix further reduces the possibilities of exposure
(EBFRIP, 1990).
Table 4. Use of penta- (PeBDE), octa- (OBDE), and decabromodiphenyl ethers
(DeBDE) in resins, polymers, and substratesa
Resins/polymers/substrates DeBDE OBDE PeBDE
ABS X
Epoxy-resins X
Phenolic resins X X
PAN X
PA X X
PBT X X
PE/XPE X
PET X
PP X
PS, HIPS X X
PVC X X
PUR X
UPE X X
Rubber X X
Paints/lacquers X X
Textiles X X
aFrom: EBFRIP (1990); UK Department of Environment (1992).
Table 5. The various applications of resins in which PBDE are used are listed belowa
Polymer Principal applications Examples of final products
ABS Moulded parts TV-sets/business machines,
computer housings, household
appliances (hairdryer, curler),
automotive parts, electronics,
telecommunications
EPOXY Circuit boards, Computers, ship interiors,
protective coatings electronic parts
PAINTS/ Coatings Marine and industry lacquers
LACQUERS for protection of containers
PHENOLICS Printed circuit boards Paper laminates/glass prepregs
for printed circuit boards
PAN Panels, electrical Lighting panels for elevators
components and rooms, housing of electrical
appliances
PA Electrical connectors, Computers, connectors,
automative interior housing in electrical industry,
parts board, electrical connectors,
automotive industry,
transportation
PBT Electrical connectors Switches, fuse, switch box,
and components computer housings, switchboard
electrical connectors,
stereos, business machines,
military electronics
PE/XPE Cross-linked wire and Major application: power cable
cable, foam tubing, with cross-linked low density
weather protection PE; also used for conduit for
and moisture barriers building with high density PE;
Final uses: portable apparatus
building control, instrument,
shipboard, automotive, marine
appliances, insulation of heating
tubes
PET Electrical Boxes, relays, coils, bobbins
components
Table 5. (cont'd).
Polymer Principal applications Examples of final products
PP Conduits, electronic TV and electronic devices, such
devices as yoke, housings, circuit board
hangers, conduits; Final uses:
electro-mechanical parts TV,
hot waste water pipes,
underground junction boxes
PS, HIPS TV cabinets and back TV back panels, computer
covers, electrical covers and housings of
appliance housings electrical appliances, office
machines, smoke detectors
PVC Cable sheets Wire end cables, floor mats,
industrial sheets
PUR Cushioning materials, Furniture, sound insulation
packaging, padding panels, wood imitations,
transportation
RUBBER Transportation Conveyor belts, foamed pipes
for insulation
TEXTILES Coatings Back coatings, impregnation:
carpets, automotive seating,
furniture in homes and official
buildings, aircraft, undergrounds,
tents, trains, and military
safety clothing
UPE Circuit boards, Electrical equipment, coatings
coatings for chemical processing plants
mouldings, military and marine
applications: construction
panels
aFrom: EBFRIP (1990).
3. FORMATION OF BROMINATED DIBENZOFURANS AND DIBENZODIOXINS FROM
POLYBROMINATED DIPHENYL ETHERS
3.1 General
Polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) can be formed from polybrominated diphenyl
ethers, polybrominated phenols, and polybrominated biphenyls under
different conditions, including heating (combustion). Laboratory
experiments have also demonstrated the formation of PBDF and PBDD
during the pyrolysis of certain other brominated flame retardants (see
the EHC on Brominated flame retardants, in preparation). As
discussed in EHC 88: Polychlorinated dibenzo-para-dioxins and
dibenzofurans, there are hundreds of possible congeners of
halogenated dibenzofurans and dibenzo-dioxins. However, only congeners
with substituents in the 2,3,7,8-positions are of toxicological
significance. In many reports, only the total levels of PBDF and PBDD
are given, without regard to substitution pattern; such totals are of
limited value in the estimation of possible risk.
Hutzinger & co-workers investigated the pyrolysis of brominated
flame retardants and flame retardant polymer systems and several
publications have appeared. In general, the results reported showed
that brominated dibenzofurans were observed at 700-800 °C and that the
2,3,7,8-substituted compounds were seen in only low concentrations, if
at all (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987; Dumler et al.,
1989).
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF, and Hoechst) and American
industries independently reported the results of combustion and
pyrolysis experiments with flame retarded polymers (BFRIP, 1990).
Recently, brominated aromatic compounds have also attracted
attention, since reports have appeared about emissions of PBDD and
PBDF and other brominated and mixed halogenated aromatic compounds in
accidental fires and from the combustion of waste (see section 4 of
both DeBDE and OBDE).
For more information on these pyrolysis experiments, see the
different sections relating to the individual brominated diphenyl
ethers, e.g., PeBDE, OBDE, and DeBDE.
As an example, the formation of PBDF and PBDD from
decabromodiphenyl ether is illustrated in Fig. 1.
 3.2 Additional data on the pyrolysis of non-specified PBDE and/or
polymers containing non-specified PBDE
The earliest published work on the pyrolysis of brominated flame
retardants was that of Buser, whose first paper appeared in 1986
(Buser, 1986). Buser pyrolysed three technical PBDE mixtures with
different degrees of bromination from commercial sources (pentabromo-,
71% bromine; octabromo-, 79% bromine (predominantly hexa- to
nonabrominated PBDE) and decabromo-, 83% bromine, 97% DeBDE) at
510-630 °C in small quartz vials. The vials were placed in a heated
oven for about one minute, and the contents analysed.
A range of PBDD and PBDF was found with a total yield of up to 10%.
HRGG/MS analysis revealed the formation of reasonably simple mixtures
of reaction products with often one or two main PBDF- and
PBDD-isomers. Debromination reactions lead to lower brominated PBDF
and PBDD congeners. In general, the higher brominated PBDE lead to
higher brominated PBDF and PBDD. Most likely, the PBDF and PBDD are
formed in intramolecular cyclization reactions involving the attack by
oxygen on the diphenyl ether system (Fig. 1) (Buser, 1986; Bieniek et
al., 1989).
The next report to appear in the literature was also in 1986 from
the laboratory of Hutzinger. Hutzinger's group pyrolysed penta- and
decabromodiphenyl ethers at 700, 800, and 900 °C, in a quartz tube
oven, for about 10 min. They did not provide any isomer-specific
results, but they reported the formation of PBDF and PBDD. Hutzinger
continued to investigate the pyrolysis of brominated flame retardants
and brominated flame retardant polymer systems, and several
publications appeared (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987;
Dumler et al., 1989a). In general, the results reported in these
publications were consistent with those of earlier work in that
maximum concentrations of PBDF were observed at 700-800 °C and
2,3,7,8-substituted compounds were seen only in very low
concentrations, if at all.
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF and Hoechst) independently
reported the results of combustion and pyrolysis experiments with
flame retarded polymers.
In the German work, pyrolysis studies were conducted with
high-impact polystyrene/DeBDE, polypropylene/DeBDE, and ABS/OBDE
(Neupert et al., 1989) (see also individual flame retardants). In all
of these studies, the pyrolysis residues were analysed for the
presence of PBDD and PBDF. While brominated PBDF were identified, only
very small quantities of 2,3,7,8-TeBDF were observed (see Table 6)
(BFRIP, 1990).
Table 6. Analytical results from the pyrolysis products of ABS/OBDE
(Bayer)a
Compound Concentration
Test 1 (ppm) Test 2 (ppm)
Brominated NDb NDb
dibenzodioxins
Brominated
dibenzofurans:
MBDF 115 60
DiBDF 10 000 7500
TrBDF 8000 2500
TeBDF 2000 2500
2,3,7,8-TeBDF < 0.1 < 8
PeBDF 1700 2000
HxBDF 530 470
HpBDF < 1.4 32
OBDF < 3 < 2.5
aFrom: BFRIP(1990).
bND = Not detectable.
Other brominated pyrolysis products of PBDE may be formed by
cleavage and oxidation, including PBBz, phenol, and some naphthalenes.
PBDF, however, may also be formed from small reactive species
generated during PBDE cleavage (Umweltbundesamt, 1989; Buser, 1986)
(see also the individual brominated diphenyl ethers).
Striebich et al. (1991) examined gas phase oxidative and pyrolysis
thermal decomposition of a 1:1 percentage weight mixture of two
commercial polybrominated diphenyl ether products (tri- through
deca-bromination). The gas phase material was quantitatively
transported to a quartz thermal reactor and subjected to a series of
controlled time/temperature exposures (300-800 °C for 2.0 seconds) in
either air or a nitrogen atmosphere. Thermal decomposition products
were identified. Isomers with higher levels of bromination were
generally more stable than lower brominated diphenyl ethers, under
both oxidative and pyrolytic conditions. Table 7 shows the approximate
yields of products from brominated diphenyl ethers. At 800 °C, all
products were decomposed to HBr or non-detectable products, in both
air and nitrogen. Neither PBDF/PBDD nor any parent material could be
detected at this temperature.
Table 7. Thermal decomposition products from a mixture of two
commercial PBDE (1:1 w/w)a
Product Maximum yield (%)
Nitrogen (650 °C) Air (625 °C)
Dibromobenzenes 0.35 NDc
Tribromobenzenes 0.64 0.92
Tetrabromobenzenes 0.43 0.95
Unknown (pentabromobenzenes?) 0.04 0.24
Brominated alkanes, alkenes, 1.40 0.77
and other PICsb
DiBDF 0.03 NDc
TrDBF 0.03 0.03
TeBDF 0.03 0.03
DiBDD NDc 0.04
TrBDD NDc 0.04
TeBDD NDc 0.01
aFrom: Striebich et al. (1991).
bPICs = Products of incomplete combustion.
cND = Not detectable.
4. WORKPLACE EXPOSURE STUDIES
4.1 Exposure to PBDE
Inhalation exposure to brominated diphenyl ethers is expected to
be low, since the vapour pressure of these chemicals is in the range
of 10-7 mmHg. Particulates in the respirable range are expected to be
formed during the grinding of solids. As inhalation of dust is
possible, the use of dust respirators and gloves/goggles is
recommended in areas of potential exposure.
Dermal exposure may occur during filtration, drying,
drumming/bagging, size reduction, and maintenance (US EPA, 1986).
Exposure to these compounds can also take place during processing
(incorporation into various polymers) and the use of the polymer blend
to fabricate the final articles. After processing, the resin is
generally in the form of pellets rather than powder. Exposure is
expected to be low at fabrication sites because of the low vapour
pressure and of ventilation controls (US EPA, 1986).
4.2 Exposure to PBDF/PBDD
Workers may be exposed to PBDF/PBDD during the production and
processing of plastics containing PBDE as flame retardants and of
products made from them. In addition, workers and the general
population may be exposed to PBDF/PBDD when products, particularly
from the electrical, electronic, and computer industries, emit
PBDF/PBDD during normal operations (see section 4 of both DeBDE and
OBDE).
The PBDF/PBDD contents of component parts taken from 6 electrical
appliances (including printers, TV sets, and computer terminals) as
well as two casings were determined. PBDF/PBDD were detected in 16 of
the materials; mainly higher brominated PBDF at concentrations of
between 0.007 and 4.2 mg/kg (sum of MBDF to HxBDF/MBDD to HxBDD) were
found (Hamm & Theisen. 1992).
Determination of PBDE and PBDF concentrations in air and dust
samples were made in offices having a large number of TV or computer
monitors in operation: the police traffic control office in Hamburg
(47 monitors; room 100 m2, 6 m high, 23 °C) and three rooms of a
television company with monitors (20 °C).
In the police traffic control office, air samples were taken for 3
days at a level of 1.5 m above the floor, a total of 84 m3 air being
drawn, and analysed. Dust samples were taken from the monitors from a
total surface of 3 × 10 m (39 g).
In the first room of a television company (50 m2), where 58
monitors were in use, a total of 129 m3 air was taken over 5 days. In
the second room (40 m2),where 38 monitors were in use, 126 m3 air
was taken, while in the third room (30 m2), where 42 monitors were in
use, 145 m3 air was taken. Dust samples were also collected once a
day from all rooms using a vacuum cleaner.
The concentrations in air of the police station and the television
company ranged between 0.29 and 1.27 pg PBDF/m3 and 97 pg PBDE/m3.
Indoor dust contained PBDF at low ppb and PBDE at high ppb levels.
2,3,7,8-substituted PBDF were not detected (limit of determination
between 0.3 and 0.1 µg/m3) (Ball et al., 1992).
5. EXPOSURE OF THE GENERAL POPULATION
5.1 General population
Limited information is available on the exposure of the general
population to brominated diphenyl ethers. Uptake of TeBDE and PeBDE
may occur in humans via the foodchain, e.g., by consuming fish. In
Germany, PBDE has been detected in human and cow's milk at levels of
2.6 and 3 µg/kg fat, respectively (Kruger, 1988).
Remmers et al. (1990) found evidence of the occurrence of
polychlorinated diphenyl ethers (PCDE) and PBDE in human adipose
tissue specimens from the USA, during the analysis of these tissues
for dioxins and furans. The results showed the presence of HxBDE/HxCDE
through to DeBDE/DeCDE in the tissues analysed.
The presence of brominated diphenyl ethers was indicated in all of
the 47 samples analysed (Cramer et al., 1990a,b). The human samples
were composites derived from all parts of the USA and covering ages
ranging from birth to > 45 years. Additional work is needed to
confirm the presence of these compounds, which have been found
provisionally in the following frequencies and concentrations; HxBDE
(72%; nd-1000 ng/kg); HpBDE (100%; 1-2000 ng/kg) and OBDE (60%;
nd-8000 ng/kg). DeBDE was found in only a few samples at
concentrations of 0.4-0.7 ng/kg.
Exposure may also mainly occur through skin contact (flame retardants
in polymers used in textiles), but also via inhalation (release of
flame retardants from the polymer matrix) (US EPA, 1986).
5.2 Possible exposure to PBDE and PBDF/PBDD
5.2.1 Television sets
Studies were carried out to determine whether PBDF escape from TV
sets. Four air samples (2 parallel to each other) were taken over 3
days in a closed room (volume 26.8 m3), where a new TV set was
operating for 17 h/day. The surface temperature of the TV set (back
panel) was 38-40 °C. One sampling was performed above the TV set,
while the others were carried out in the centre of the room (2.2 m
from the TV set). The levels in the centre of the room of tri-,
tetra-, penta-, and hexabromo-dibenzofurans were 25, 2.7, 0.5, and
0.1 µg/m3, respectively (levels in outdoor air ranged from < 0.05 to
0.16 µg/m3). Above the TV set, the concentrations of the 4 PBDF were
143, 11, 0.5, and < 0.1 µg/m3. Hepta- and octabromodibenzofuran, and
poly-brominated dibenzodioxins were not found (limits of determination
0.1 and 0.2 µg/m3, respectively) (Bruckmann et al., 1990).
An investigation was conducted to determine the emissions of PBDE
and PBDF from plastics in two TV sets, one colour and one monochrome,
two computer monitors, and three printers, under conditions of use.
Analytical methods were refined to obtain a reliable determination of
PBDF. Each appliance was placed, under conditions of use, in a test
chamber. The volume of the steel chamber was 1.17 m3 (1.5 × 1.07 ×
0.82m). For three days, pure air was continuously drawn through the
chambers at a rate of 1.5 m3/h; the emitted compounds were absorbed
on a sampler for the subsequent extraction and determination of PBDE
and PBDF with 4 or more bromines. PBDF concentrations were found to
vary between not detected (limit of detection 3-10 pg) and 1799 pg per
appliance tested and PBDE concentrations, between 0.4 and
889 ng/appliance; 2,3,7,8 isomers (1070 µg/appliance) were detected
only from the colour TV set (Ball et al., 1991).
Three new television sets were placed in a 1.81 m3 test chamber.
Two of the cabinets were made from polystyrene, which was flame
retarded with 11.5% DeBDE. The third television set was made of
high-impact polystyrene, treated with DeBDE/Sb2O3 as a flame
retardant. PBDF and PBDD concentrations were determined in air
collected over 3 days while the two television sets were operating and
during one day when the third TV set was operating. The concentrations
of TeBDD, PeBDD, TeBDF, and PeBDF ranged between 0.09 and 1.52 µg/m3
(Ranken et al., 1990).
5.2.2 Fire tests and fire accidents
Six appliances and 2 casings were burned in a fire test room
(floor area 21 m2, volume 48 m3), which was kept closed during the
fire tests and slowly ventilated after extinguishing the fires (worst
case conditions). After the fire test, samples of combustion residues
and smoke condensate were taken. Smoke was collected in 5 tests. The
combustion residues showed the presence of PBDF and PBDD in
concentrations ranging between 1 and 1930 mg/kg and from the casing
components almost 1%. Smoke condensate from contaminated surfaces
contained levels of between 6 and 1610 µg monobromo- up to
hexabromodibenzofuran/dibenzodioxin per m2. Smoke contained
11-1700 µg monobromo- up to hexabromo- dibenzofuran/dibenzodioxin per
m3 (see Table 8) (Hamm & Theisen, 1992).
Residues and smoke condensates resulting from actual fire
accidents with 9 TV sets were examined. PBDF/PBDD concentrations in
the residues were mainly in the µg/kg range, one value being
107 mg/kg. Close to the fire site, the PBDF/PBDD area contamination
concentrations were between 0.1 and 13.1 µg/m3 (see Table 9) (Hamm &
Theisen, 1992).
It was concluded that the levels of PBDF/PBDD produced in real
fires are much lower than those produced under fire-test conditions.
Table 8. PBDF/D concentrations in original components of electrical appliances and in samples from fire tests with these
appliances or with their casingsa
Object of investigation Original components Fire test samples
Casings Printed circuit Combustion Smoke Smoke
boards residues condensate
Total mono- to Total mono- to Total mono- to Total mono- to Total mono-to
hexaBDF/D µg/g hexaBDF/D µg/g hexaBDF/D hexaBDF/D hexaBDF/D
(ppm) (ppm) µg/g (ppm) µg/m2 µg/m3
Casing of electrical appliance 1 0.63 -b 8700 177 -b
Casing of electrical appliance 2 0.64 -b 7750 1610 -b
Electrical appliance 3 -c 1.77 468 106 456
Electrical appliance 4 0.06 3.44 43 260 355
Electrical appliance 5 0.81 1.98 18 396 1700
Electrical appliance 6 4.20 0.35 1930 234 1350
Etectrlcal appliance 7 -c 0.13 1 6 11
Electrical appliance 8 1.26 0.007 24 323 -b
aFrom: Hamm & Thiesen (1992).
b = Not determined.
c = Not detectable.
Table 9. PBDF/D-concentrations in residues and smoke condensates from
real fire accidents with television setsa
Fire accident Combustion Smoke condensates
(Case number) residues
Close to fire site At some
distance from
fire site
Total mono- to Total mono- to Total mono- to
hexaBDF/D hexaBDF/D hexaBDF/D
µg/g (ppm) µg/m2 µg/m2
I 0.235 10.7 0.665
II 0.004 0.134 0.102
III 0.209 13.1 0.382
IV 0.009 NDb NDb
V 0.001 4.82 1.39
VI 0.017 0.759 0.425
VII 0.001 0.021 0.008
VIII 0.001 10.5 5.37
IX 107 7.47 0.847
aFrom: Hamm & Thiesen (1992).
bND = Not detectable.
6. ENVIRONMENTAL POLLUTION BY PBDE
6.1 Ultimate fate following use
Products containing PBDE are disposed of in the normal domestic
waste stream (landfill and incineration).
No studies are available on the fate of PBDE-containing products
in landfills, but there is concern that the PBDE may eventually leach
out. Bearing in mind that PBDE, at least the congeners with more than
3 bromine atoms, are persistent in the environment, the introduction
of such chemicals into widespread products may be a considerable
long-term diffuse source of emissions of these compounds to the
environment. This type of source is difficult to control and the
unnecessary use of persistent organic compounds should be avoided.
Formation of PBDF and/or PBDD as a result of landfill fires is
also a possibility, though no data are available on the scale of this
source. The results of pyrolysis experiments showed that PBDE can form
PBDF and PBDD (in much smaller quantities) under a wide range of
heating conditions (see General Introduction sections 3.1 and 3.2). If
chlorine is present, mixed halogenated furans/dioxins can also be
generated (Oberg et al., 1987; Zier et al., 1991). Unless sufficiently
high temperatures and long residence times are maintained, PBDF/PBDD
can be generated during the incineration of products containing PBDE.
They can also result from poorly-controlled combustion gas cooling.
Modern, properly operated municipal waste incineration (MWI) should
not emit significant quantities of PBDF/PBDD, regardless of the
composition of the municipal waste.
Lahl et al. (1991) reported increases in dibenzofuran and
dibenzodioxin levels in filter dust, when products containing PBDE
were added to the feed-stock. Riggs et al. (1990) reported PBDF
generation when a flame retarded resin was burnt under simulated MWI
conditions. However, Oberg et al. (1987) reported no increased
emissions of dibenzodioxins when the bromine content of an
incineration feed-stock was increased. Monobromodichloro-dibenzofuran
levels were slightly increased. Oberg & Bergström (1990) conducted
further experiments with a hazardous waste incinerator, to study the
relationship between bromine levels in municipal waste and incinerator
dibenzodioxin and dibenzofuran emissions. They concluded that no
unacceptable environmental risks were associated with the incineration
of brominated compounds in plants with good combustion conditions
equipped with efficient flue gas cleaning. They further noted that
only 0.0125% of the feed to Swedish MWIs was brominated waste.
6.2 Air
Watanabe et al. (1992) reported on the presence of PBDE in the air
in Taiwan and Japan. The concentrations in the air samples collected
in Taiwan from a recycling plant in January 1991 were, in general,
higher than those in Japan; 3 samples were analysed in Taiwan, and 5
in Japan. Tribromo-, tetrabromo-, pentabromo-, and hexabromodiphenyl
ethers were present in the following mean concentrations: Taiwan, 32,
52, 23, and 31 µg/m3, and, Japan, 7.1, 21, 8.9, and 21 µg/m3,
respectively.
6.3 Soil
Two ash and two soil samples were collected in Taiwan from a
recycling plant in January 1991 and analysed for the presence of PBDE.
Tri-, tetra-, penta-, hexa-, and decabromodiphenyl ethers were present
in ash in the following concentrations 20-20, 130, 78-110, 47-54, and
510-2500 µg/kg, respectively; the concentrations in soil were 38-40,
75-104, 41-84, 20-23 and 260-330 µg/kg, respectively. Hepta- and
octabromodiphenyl ether were not found (Watanabe et al., undated).
6.4 Water
Marine, estuarine, and river water samples were analysed for the
presence of the different PBDE. Except for monobromo-diphenyl ether,
levels of all the higher brominated PBDE were below the detection
limit. MBDE was mainly found in the surroundings of manufacturing
plants in the USA (US EPA, 1986).
6.5 Sediments and sewage sludge
In Japan, Spain, Sweden, and the USA, studies were carried out to
determine the presence of the different PBDE in marine, estuarine, or
river sediment. PBDE were mainly found in river sediment. In general,
the levels were below 100 µg/kg dry weight, except in rivers in the
vicinity of manufacturing plants. In these cases, the concentrations
were much higher. In a river in Sweden, concentrations of 11.5 mg
DeBDE, 0.8 mg TeBDE, and 2.8 mg PeBDE/kg dry weight were found. In the
USA, at a manufacturing plant, as much as 1 g DeBDE/kg was found
(Zweidinger et al., 1978; DeCarlo, 1979; Environment Agency Japan,
1983, 1989, 1991; Watanabe et al., 1986, 1987b; Fernandez et al.,
1992).
The upper layers in a laminated sediment core from the Baltic Sea
(Bornholm Deep) contained higher levels of TeBDE and PeBDE than the
deeper layers, indicating an increasing burden of these compounds
(Nylund et al., 1992).
A series of samples of sewage sludges from municipal waste water
treatment plants in Germany were analysed for poly-halogenated
compounds, such as halogenated diphenyl ethers. Tribromo- to
heptabromodiphenyl ethers were found at relatively high concentrations
(Hagenmaier et al., 1991). Sewage sludge was analysed in Sweden for
the presence of TeBDE and PeBDE. Concentrations of 15 and 19 µg/kg,
respectively, were found (Sellström et al., 1990a,b).
6.6 Aquatic vertebrates
The presence of PBDE depends mainly on the degree of bromination.
DeBDE, OBDE, and HxBDE were not found in mussel and fish samples
collected in Japan. No data are available for HpBDE. However, PeBDE
was found in mussel and fish species in concentrations of < 3 µg/kg
wet weight in Japan. Concentrations of 22 µg 2,2',4,4',5-PeBDE/kg wet
weight were found in cod liver collected in the North Sea, and
concentrations of up to 64 µg/kg on a fat basis were found in fish
collected in Sweden. The concentrations were much higher in fish
collected in the vicinity of industrial areas, e.g., up to 9.4 mg/kg
on a fat basis (Jansson et al., in press). Levels for TeBDE, mainly
2,2',4,4'-TeBDE, were comparable but generally higher. Mussels and
fish in Japan contained up to 14.6 µg/kg wet weight, cod liver
collected in the North Sea, 360 µg/kg, and eel from the Netherlands,
up to 1700 µg/kg fat. Different species of fish collected in Sweden
contained up to 88 mg/kg fat (Andersson & Blomkvist, 1981; Watanabe,
1987; Watanabe et al., 1987b; De Boer, 1989, 1990). An increasing
trend was observed in PeBDE and TeBDE levels in freshwater fish in
Sweden. Only limited data are available concerning lower brominated
PBDE (Jansson et al., in press).
Thirty-five samples of 18 freshwater fish collected in German
rivers, and 17 samples collected from the Baltic Sea and the North Sea
contained 18.2-983.6 and 0.6-119.9 µg PBDE/kg fat (determined as
Bromkal 70-SDE), respectively (Kruger, 1988).
6.7 Aquatic mammals
Three bottle-nose dolphins (Tursiops truncatus), collected
during the 1987/88 mass mortality event along the central and south
Atlantic coast of the USA, were analysed for brominated diphenyl
ethers. The concentrations of PBDE were 200, 220, and 180 µg/kg lipid
(Kuehl et al., 1991).
Limited data are available on the presence of PBDE in aquatic
mammals. 2,2'4,4'5-PeBDE was found in ringed and grey seals, collected
in Sweden, in concentrations of 1.7 and 40 µg/kg fat, respectively.
TeBDE, mainly 2,2',4,4'-TeBDE, was also found in the blubber of these
2 species in concentrations of 47 and 650 µg/kg fat, respectively
(Jansson et al., in press). Seals collected at Spitzbergen contained
approximately 10 µg PBDE/kg fat, determined as Bromkal 75DE (Kruger,
1988).
6.8 Terrestrial vertebrates
Pooled samples of rabbits, moose, and reindeer, collected in
Sweden, contained PeBDE and TeBDE in concentrations of <0.3, 0.64,
and 0.26 µg 2,2',4,4',5-PeBDE/kg and <2, 0.82, and 0.18 µg
2,2',4,4'-TeBDE/kg lipid, respectively (Jansson et al., in press).
Four samples of cow's milk were analysed in Germany for the
presence of PBDE. The average concentration was 3.572 µg/kg fat (range
2.536-4.539 µg/kg) determined as Bromkal 70-5DE. The main component
was HxBDE (Kruger, 1988).
6.8.1 Birds
Limited data are available on the presence of PBDE in birds. In
Sweden, 2,2',4,4',5-PeBDE was found in the muscle tissue of osprey, in
newborn starlings, and in guillemot eggs in concentrations of 140,
2.3-4.2, and 24-260 µg/kg lipid, respectively. A trend towards
increasing concentrations of PeBDE and TeBDE in guillemot eggs from
the Baltic Sea was observed. 2,2',4,4'-TeBDE was found in the muscle
tissue of osprey in concentrations of up to 1800 µg/kg. Guillemots
collected from the Baltic Sea, the North Sea, and Spitzbergen
contained 370, 80, and 130 µg/kg on a fat basis, respectively (Jansson
et al., 1987, 1993).
In the USA, indications were found that dibromodiphenyl ether was
present in the eggs of fish-eating birds, but it was not quantified
(Stafford, 1983).
6.8.2 Humans
In Germany, 25 samples of breast milk were analysed for the
presence of PBDE. The ages of the women ranged between 24 and 36 years
and most of them were breast-feeding their first or second child. The
samples contained 0.6-11.1 µg PBDE/kg fat, determined as Bromkal
70-5DE. The main component was HxBDE. One sample from a Chinese woman
showed 7.7 µg PBDE/kg fat; a sample from another woman, exposed
occupationally to hydraulic fluids and transformer oils, contained
50 µg PBDE/kg fat. This last value was excluded from the given range
and average. (Kruger, 1988).
DECABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Typically, commercial DeBDE has a purity of 97-98%, with 0.3-3.0% of
nona- and/or octabrominated diphenyl ethers. Nonabromodiphenyl ether
(NBDE) is the major impurity. In contrast to the other polybrominated
diphenyl ethers there is only one isomer of DeBDE.
The melting point of DeBDE is approximately 300 °C and decomposition
occurs above 400 °C. Solubility in water is 20-30 µg/litre and the log
of the n-octanol/water partition coefficient is greater than 5.
Vapour pressure is < 10-6 mmHg at 20 °C.
1.1.2 Production and uses
Among the brominated diphenyl ethers (mono- to deca-),
deca-bromodiphenyl ether is the most important commercial product with
regard to production and use.
Commercial DeBDE has been produced in increasing degrees of purity
since the late 1970s. The global production of DeBDE is approximately
30 000 tonnes/year. It is used as an additive flame retardant in many
plastics, especially high-impact polystyrene, and in the treatment of
textiles used in soft furnishing, automobile fabrics, and tents.
1.1.3 Environmental transport, distribution, and transformation
Photodegradation of DeBDE occurs in organic solvents under
ultraviolet radiation (UVR) or sunlight; lower brominated diphenyl
ethers and brominated dibenzofurans are formed. Photodegradation also
occurs, to a lesser extent, in water with sunlight; however, lower
brominated diphenyl ethers and brominated dibenzofurans have not been
found.
Levels of DeBDE extracted from polymers are close to, or below,
the limit of detection, depending on the polymer type and extraction
solvent.
Because of its extremely low water solubility and vapour pressure,
DeBDE is likely to be transported primarily by adsorption to
particulate matter. It is persistent and likely to accumulate in
sediment and soil.
No data are available on its bioavailability from sediment and
soil. A study on rainbow trout did not show any bioaccumulation in
flesh, skin, or viscera, over 48 h. DeBDE is unlikely to bioaccumulate
because of its high relative molecular mass.
Products containing commercial DeBDE will eventually be disposed
of by landfill or incineration. DeBDE may eventually leach from
landfills. Polybrominated dibenzofurans (PBDF) and mixed
halogen-dibenzofurans and -dibenzodioxins may result from landfill
fires and inefficient incineration. Products containing commercial
DeBDE may contribute to these emissions.
Pyrolysis of both commercial DeBDE itself and polymers (HIPS, PBT,
industrial polypropylene) containing DeBDE, in the presence of oxygen,
produced PBDF, polybrominated dibenzodioxins (PBDD) being found to a
lesser extent. The maximum formation' of PBDF occurs at 400-500 °C,
but it can occur at temperatures up to 800 °C, and Sb2O3 plays a
catalytic role in the formation of PBDF and PBDD.
The formation, and amounts found, of PBDF and PBDD depend on
temperature, oxygen content, and length of pyrolysis. In the absence
of oxygen, mainly polybromobenzenes and polybromonaphthalenes are
formed.
1.1.4 Environmental levels and human exposure
DeBDE has been identified in air in the vicinity of manufacturing
plants at concentrations of up to 25 µg/m3. DeBDE was not detected in
water samples collected in Japan in the period 1977-91. However, it
was detected in river and estuarine sediment, collected in Japan in
the same period, at concentrations of up to approximately 12 mg/kg dry
weight. DeBDE (up to 1 g/kg) was also found in the USA in river
sediment close to one manufacturing plant. DeBDE was not detected in
fish samples collected in Japan, but, in one mussel sample, a level
just above the level of detection was found. DeBDE was not detected in
human adipose tissue samples collected in Japan, but, in the USA,
DeBDE was found in 3 out of 5 samples of human adipose tissue.
Human exposure to DeBDE can occur in the course of manufacture and
formulation into polymers. Exposure of the general population to DeBDE
is insignificant.
Determination of occupational exposure to the breakdown products
of DeBDE during manufacture, formulation, or use, showed that air
samples close to the extruder head contained high concentrations of
PBDF. Lower levels were found in the air of the workroom. PBDF was
also found in wipe samples. The application of good engineering
techniques has been shown to reduce occupational exposure to PBDF.
Exposure of, the general population to PBDF impurities in flame
retarded polymers is unlikely to be of significance.
1.1.5 Kinetics and metabolism in laboratory animals and humans
DeBDE is poorly absorbed from the gastrointestinal tract and is
rapidly excreted following injection.
The results of metabolic studies on the rat, using 14C labelled
DeBDE, indicated a half-life for the disappearance from the body of
less than 24 h and that the principal route of elimination following
oral ingestion was via the faeces. No appreciable 14C activity (less
than 1%) was found in either urine or expired air.
Rats fed 0.1 mg/kg body weight per day, for up to two years,
showed no accumulation of DeBDE in serum, kidneys, muscle, or testes,
as estimated from total bromine determination. Bromine accumulation in
the liver plateaued at 30 days and was cleared within 10 days
following treatment. After 180 days of treatment, the bromine level in
the liver of treated rats was no greater than that in control rats.
Adipose tissue accumulated low levels of total bromine, which remained
after 90 days of clean diet; the nature of the retained "bromine" is
not known. Since DeBDE accounted for only 77% of the commercial
mixture used, "bromine" could have been derived from NBDE or OBDE.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of DeBDE for laboratory animals is low. The
substance is not an irritant to the skin or eyes of rabbits. It is not
chloracnegenic on the skin of rabbits and is not a human skin
sensitizer.
The combustion products of flame retarded polystyrene containing
DeBDE and Sb203 were tested for acute toxicity and comedogenicity.
The rat oral LD50 of the soot and char was >2000 mg/kg body weight.
In short-term toxicity studies on rats and mice, DeBDE (purity
>97%) at dietary levels of 100 g/kg (4 weeks) or 50 g/kg (13 weeks;
equivalent to 2500 mg/kg body weight for the rat) did not induce
adverse effects. A one-generation reproduction study on rats showed no
adverse effects with dose levels of 100 mg/kg body weight. DeBDE did
not cause any teratogenic effects in the fetuses of rats administered
a dose level of 100 mg/kg body weight. With 1000 mg/kg body weight,
malformations, such as delayed ossification, were seen. DeBDE was not
shown to be mutagenic in a number of tests.
In a carcinogenicity study on rats and mice, DeBDE (purity 94-99%)
was administered at dietary levels of up to 50 g/kg. An increase in
the incidence of adenomas (but not carcinomas) was found in the livers
of male rats receiving 25 g/kg and female rats receiving 50 g
DeBDE/kg. In male mice, increased incidences of hepatocellular
adenomas and/or carcinomas (combined) were found at 25 g/kg and an
increase in thyroid follicular cell adenomas/ carcinomas (combined) at
both dose levels. Female mice did not show any increase in tumour
incidence. There was equivocal evidence for carcinogenicity in male
and female rats and male mice only at dose levels of 25-50 g DeBDE/kg
diet. As the results of all mutagenicity tests have been negative, it
can be concluded that DeBDE is not a genotoxic carcinogen. IARC (1990)
concluded that there was limited evidence for the carcinogenicity of
DeBDE in experimental animals. The very high dose levels, lack of
genotoxicity, and minimal evidence for carcinogenicity indicate that
DeBDE, at the present exposure levels, does not present a carcinogenic
risk for humans.
1.1.7 Effects on humans
No evidence for skin sensitization was found in 200 human subjects
exposed to DeBDE in a sensitization test.
A morbidity study of extruder personnel blending
polybutyl-eneterephthalate containing DeBDE, with consequently
potential exposure to PBDD and PBDF for 13 years, did not reveal any
deleterious effects, even though 2,3,7,8-TeBDF and -TeBDD were
detected in the blood. Results of immunological studies showed that
the immune system of the exposed persons was not adversely affected in
13 years.
1.1.8 Effects on other organisms in the laboratory and field
The EC50s for the growth of 3 marine unicellular algae were
greater than 1 mg DeBDE/litre. No further information is available on
the effects of DeBDE on other organisms in the laboratory and field.
1.2 Conclusions
1.2.1 DeBDE
DeBDE is widely used incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure is very low since the DeBDE is not
readily extracted from polymers. The acute toxicity of DeBDE is very
low and there is minimal absorption from the gastrointestinal tract.
Thus, risk to the general population from DeBDE is considered to be
insignificant.
Occupational exposure is to DeBDE in particulate form. The control
of dust during manufacture and use will adequately reduce the risk for
workers.
DeBDE is persistent and binds to particulate matter in the
environment; it is likely to accumulate in sediment. It is unlikely to
bioaccumulate. Current evidence suggests that environmental
photodegradation in water does not lead to the formation of lower
brominated diphenylethers or brominated dibenzofurans, but little is
known about degradation in other media.
There is minimal information on the toxicity of DeBDE for
organisms in the environment.
1.2.2 Breakdown products
Formation of PBDF and, to some extent, PBDD may occur when DeBDE,
or products containing it, are heated to 300-800 °C. The possible
hazards associated with this have to be addressed.
Properly controlled incineration does not lead to the emission of
significant quantities of brominated dioxins and -furans. Any
uncontrolled combustion of products containing DeBDE can lead to an
unquantified generation of PBDF/PBDD. The significance of this for
both humans and the environment will be addressed in a future
Environmental Health Criteria on PBDF/PBDD.
PBDF have been found in the blood of workers involved in the
production of plastics containing DeBDE. No adverse health effects
have been associated with this exposure. Good engineering controls can
prevent worker exposure to PBDF.
1.3 Recommendations
1.3.1 General
* Workers involved in the manufacture of DeBDE and products
containing the compound should be protected from exposure through
the application of appropriate industrial hygiene measures, the
monitoring of occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled, to minimize environmental
contamination with this persistent material and its breakdown
products.
* Manufacturers should minimize levels of impurities in commercial
DeBDE products, using the best available techniques. A purity of
97% or higher is recommended.
* Incineration should only be carried out in properly constituted
incinerators, running at consistently optimal conditions. Burning
by any other means may lead to the production of PBDF and/or PBDD.
1.3.2 Further studies
* Further studies on the bioavailability and toxicity of
sediment-bound DeBDE should be performed on relevant organisms.
* Continued monitoring of environmental levels is required.
* The generation of PBDF under real fire conditions should be
further investigated.
* Environmental biodegradation, and photodegradation in compartments
other than water, should be further studied.
* Investigation into possible methods and consequences of recycling
of DeBDE-containing polymers should be made.
* Analytical methods for DeBDE in various matrices should be
validated.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Pure substance
Chemical structure:
3.2 Additional data on the pyrolysis of non-specified PBDE and/or
polymers containing non-specified PBDE
The earliest published work on the pyrolysis of brominated flame
retardants was that of Buser, whose first paper appeared in 1986
(Buser, 1986). Buser pyrolysed three technical PBDE mixtures with
different degrees of bromination from commercial sources (pentabromo-,
71% bromine; octabromo-, 79% bromine (predominantly hexa- to
nonabrominated PBDE) and decabromo-, 83% bromine, 97% DeBDE) at
510-630 °C in small quartz vials. The vials were placed in a heated
oven for about one minute, and the contents analysed.
A range of PBDD and PBDF was found with a total yield of up to 10%.
HRGG/MS analysis revealed the formation of reasonably simple mixtures
of reaction products with often one or two main PBDF- and
PBDD-isomers. Debromination reactions lead to lower brominated PBDF
and PBDD congeners. In general, the higher brominated PBDE lead to
higher brominated PBDF and PBDD. Most likely, the PBDF and PBDD are
formed in intramolecular cyclization reactions involving the attack by
oxygen on the diphenyl ether system (Fig. 1) (Buser, 1986; Bieniek et
al., 1989).
The next report to appear in the literature was also in 1986 from
the laboratory of Hutzinger. Hutzinger's group pyrolysed penta- and
decabromodiphenyl ethers at 700, 800, and 900 °C, in a quartz tube
oven, for about 10 min. They did not provide any isomer-specific
results, but they reported the formation of PBDF and PBDD. Hutzinger
continued to investigate the pyrolysis of brominated flame retardants
and brominated flame retardant polymer systems, and several
publications appeared (Thoma et al., 1987a,b; Thoma & Hutzinger, 1987;
Dumler et al., 1989a). In general, the results reported in these
publications were consistent with those of earlier work in that
maximum concentrations of PBDF were observed at 700-800 °C and
2,3,7,8-substituted compounds were seen only in very low
concentrations, if at all.
Shortly after the initial reports of Buser and Hutzinger, BFRIP
and German chemical companies (Bayer, BASF and Hoechst) independently
reported the results of combustion and pyrolysis experiments with
flame retarded polymers.
In the German work, pyrolysis studies were conducted with
high-impact polystyrene/DeBDE, polypropylene/DeBDE, and ABS/OBDE
(Neupert et al., 1989) (see also individual flame retardants). In all
of these studies, the pyrolysis residues were analysed for the
presence of PBDD and PBDF. While brominated PBDF were identified, only
very small quantities of 2,3,7,8-TeBDF were observed (see Table 6)
(BFRIP, 1990).
Table 6. Analytical results from the pyrolysis products of ABS/OBDE
(Bayer)a
Compound Concentration
Test 1 (ppm) Test 2 (ppm)
Brominated NDb NDb
dibenzodioxins
Brominated
dibenzofurans:
MBDF 115 60
DiBDF 10 000 7500
TrBDF 8000 2500
TeBDF 2000 2500
2,3,7,8-TeBDF < 0.1 < 8
PeBDF 1700 2000
HxBDF 530 470
HpBDF < 1.4 32
OBDF < 3 < 2.5
aFrom: BFRIP(1990).
bND = Not detectable.
Other brominated pyrolysis products of PBDE may be formed by
cleavage and oxidation, including PBBz, phenol, and some naphthalenes.
PBDF, however, may also be formed from small reactive species
generated during PBDE cleavage (Umweltbundesamt, 1989; Buser, 1986)
(see also the individual brominated diphenyl ethers).
Striebich et al. (1991) examined gas phase oxidative and pyrolysis
thermal decomposition of a 1:1 percentage weight mixture of two
commercial polybrominated diphenyl ether products (tri- through
deca-bromination). The gas phase material was quantitatively
transported to a quartz thermal reactor and subjected to a series of
controlled time/temperature exposures (300-800 °C for 2.0 seconds) in
either air or a nitrogen atmosphere. Thermal decomposition products
were identified. Isomers with higher levels of bromination were
generally more stable than lower brominated diphenyl ethers, under
both oxidative and pyrolytic conditions. Table 7 shows the approximate
yields of products from brominated diphenyl ethers. At 800 °C, all
products were decomposed to HBr or non-detectable products, in both
air and nitrogen. Neither PBDF/PBDD nor any parent material could be
detected at this temperature.
Table 7. Thermal decomposition products from a mixture of two
commercial PBDE (1:1 w/w)a
Product Maximum yield (%)
Nitrogen (650 °C) Air (625 °C)
Dibromobenzenes 0.35 NDc
Tribromobenzenes 0.64 0.92
Tetrabromobenzenes 0.43 0.95
Unknown (pentabromobenzenes?) 0.04 0.24
Brominated alkanes, alkenes, 1.40 0.77
and other PICsb
DiBDF 0.03 NDc
TrDBF 0.03 0.03
TeBDF 0.03 0.03
DiBDD NDc 0.04
TrBDD NDc 0.04
TeBDD NDc 0.01
aFrom: Striebich et al. (1991).
bPICs = Products of incomplete combustion.
cND = Not detectable.
4. WORKPLACE EXPOSURE STUDIES
4.1 Exposure to PBDE
Inhalation exposure to brominated diphenyl ethers is expected to
be low, since the vapour pressure of these chemicals is in the range
of 10-7 mmHg. Particulates in the respirable range are expected to be
formed during the grinding of solids. As inhalation of dust is
possible, the use of dust respirators and gloves/goggles is
recommended in areas of potential exposure.
Dermal exposure may occur during filtration, drying,
drumming/bagging, size reduction, and maintenance (US EPA, 1986).
Exposure to these compounds can also take place during processing
(incorporation into various polymers) and the use of the polymer blend
to fabricate the final articles. After processing, the resin is
generally in the form of pellets rather than powder. Exposure is
expected to be low at fabrication sites because of the low vapour
pressure and of ventilation controls (US EPA, 1986).
4.2 Exposure to PBDF/PBDD
Workers may be exposed to PBDF/PBDD during the production and
processing of plastics containing PBDE as flame retardants and of
products made from them. In addition, workers and the general
population may be exposed to PBDF/PBDD when products, particularly
from the electrical, electronic, and computer industries, emit
PBDF/PBDD during normal operations (see section 4 of both DeBDE and
OBDE).
The PBDF/PBDD contents of component parts taken from 6 electrical
appliances (including printers, TV sets, and computer terminals) as
well as two casings were determined. PBDF/PBDD were detected in 16 of
the materials; mainly higher brominated PBDF at concentrations of
between 0.007 and 4.2 mg/kg (sum of MBDF to HxBDF/MBDD to HxBDD) were
found (Hamm & Theisen. 1992).
Determination of PBDE and PBDF concentrations in air and dust
samples were made in offices having a large number of TV or computer
monitors in operation: the police traffic control office in Hamburg
(47 monitors; room 100 m2, 6 m high, 23 °C) and three rooms of a
television company with monitors (20 °C).
In the police traffic control office, air samples were taken for 3
days at a level of 1.5 m above the floor, a total of 84 m3 air being
drawn, and analysed. Dust samples were taken from the monitors from a
total surface of 3 × 10 m (39 g).
In the first room of a television company (50 m2), where 58
monitors were in use, a total of 129 m3 air was taken over 5 days. In
the second room (40 m2),where 38 monitors were in use, 126 m3 air
was taken, while in the third room (30 m2), where 42 monitors were in
use, 145 m3 air was taken. Dust samples were also collected once a
day from all rooms using a vacuum cleaner.
The concentrations in air of the police station and the television
company ranged between 0.29 and 1.27 pg PBDF/m3 and 97 pg PBDE/m3.
Indoor dust contained PBDF at low ppb and PBDE at high ppb levels.
2,3,7,8-substituted PBDF were not detected (limit of determination
between 0.3 and 0.1 µg/m3) (Ball et al., 1992).
5. EXPOSURE OF THE GENERAL POPULATION
5.1 General population
Limited information is available on the exposure of the general
population to brominated diphenyl ethers. Uptake of TeBDE and PeBDE
may occur in humans via the foodchain, e.g., by consuming fish. In
Germany, PBDE has been detected in human and cow's milk at levels of
2.6 and 3 µg/kg fat, respectively (Kruger, 1988).
Remmers et al. (1990) found evidence of the occurrence of
polychlorinated diphenyl ethers (PCDE) and PBDE in human adipose
tissue specimens from the USA, during the analysis of these tissues
for dioxins and furans. The results showed the presence of HxBDE/HxCDE
through to DeBDE/DeCDE in the tissues analysed.
The presence of brominated diphenyl ethers was indicated in all of
the 47 samples analysed (Cramer et al., 1990a,b). The human samples
were composites derived from all parts of the USA and covering ages
ranging from birth to > 45 years. Additional work is needed to
confirm the presence of these compounds, which have been found
provisionally in the following frequencies and concentrations; HxBDE
(72%; nd-1000 ng/kg); HpBDE (100%; 1-2000 ng/kg) and OBDE (60%;
nd-8000 ng/kg). DeBDE was found in only a few samples at
concentrations of 0.4-0.7 ng/kg.
Exposure may also mainly occur through skin contact (flame retardants
in polymers used in textiles), but also via inhalation (release of
flame retardants from the polymer matrix) (US EPA, 1986).
5.2 Possible exposure to PBDE and PBDF/PBDD
5.2.1 Television sets
Studies were carried out to determine whether PBDF escape from TV
sets. Four air samples (2 parallel to each other) were taken over 3
days in a closed room (volume 26.8 m3), where a new TV set was
operating for 17 h/day. The surface temperature of the TV set (back
panel) was 38-40 °C. One sampling was performed above the TV set,
while the others were carried out in the centre of the room (2.2 m
from the TV set). The levels in the centre of the room of tri-,
tetra-, penta-, and hexabromo-dibenzofurans were 25, 2.7, 0.5, and
0.1 µg/m3, respectively (levels in outdoor air ranged from < 0.05 to
0.16 µg/m3). Above the TV set, the concentrations of the 4 PBDF were
143, 11, 0.5, and < 0.1 µg/m3. Hepta- and octabromodibenzofuran, and
poly-brominated dibenzodioxins were not found (limits of determination
0.1 and 0.2 µg/m3, respectively) (Bruckmann et al., 1990).
An investigation was conducted to determine the emissions of PBDE
and PBDF from plastics in two TV sets, one colour and one monochrome,
two computer monitors, and three printers, under conditions of use.
Analytical methods were refined to obtain a reliable determination of
PBDF. Each appliance was placed, under conditions of use, in a test
chamber. The volume of the steel chamber was 1.17 m3 (1.5 × 1.07 ×
0.82m). For three days, pure air was continuously drawn through the
chambers at a rate of 1.5 m3/h; the emitted compounds were absorbed
on a sampler for the subsequent extraction and determination of PBDE
and PBDF with 4 or more bromines. PBDF concentrations were found to
vary between not detected (limit of detection 3-10 pg) and 1799 pg per
appliance tested and PBDE concentrations, between 0.4 and
889 ng/appliance; 2,3,7,8 isomers (1070 µg/appliance) were detected
only from the colour TV set (Ball et al., 1991).
Three new television sets were placed in a 1.81 m3 test chamber.
Two of the cabinets were made from polystyrene, which was flame
retarded with 11.5% DeBDE. The third television set was made of
high-impact polystyrene, treated with DeBDE/Sb2O3 as a flame
retardant. PBDF and PBDD concentrations were determined in air
collected over 3 days while the two television sets were operating and
during one day when the third TV set was operating. The concentrations
of TeBDD, PeBDD, TeBDF, and PeBDF ranged between 0.09 and 1.52 µg/m3
(Ranken et al., 1990).
5.2.2 Fire tests and fire accidents
Six appliances and 2 casings were burned in a fire test room
(floor area 21 m2, volume 48 m3), which was kept closed during the
fire tests and slowly ventilated after extinguishing the fires (worst
case conditions). After the fire test, samples of combustion residues
and smoke condensate were taken. Smoke was collected in 5 tests. The
combustion residues showed the presence of PBDF and PBDD in
concentrations ranging between 1 and 1930 mg/kg and from the casing
components almost 1%. Smoke condensate from contaminated surfaces
contained levels of between 6 and 1610 µg monobromo- up to
hexabromodibenzofuran/dibenzodioxin per m2. Smoke contained
11-1700 µg monobromo- up to hexabromo- dibenzofuran/dibenzodioxin per
m3 (see Table 8) (Hamm & Theisen, 1992).
Residues and smoke condensates resulting from actual fire
accidents with 9 TV sets were examined. PBDF/PBDD concentrations in
the residues were mainly in the µg/kg range, one value being
107 mg/kg. Close to the fire site, the PBDF/PBDD area contamination
concentrations were between 0.1 and 13.1 µg/m3 (see Table 9) (Hamm &
Theisen, 1992).
It was concluded that the levels of PBDF/PBDD produced in real
fires are much lower than those produced under fire-test conditions.
Table 8. PBDF/D concentrations in original components of electrical appliances and in samples from fire tests with these
appliances or with their casingsa
Object of investigation Original components Fire test samples
Casings Printed circuit Combustion Smoke Smoke
boards residues condensate
Total mono- to Total mono- to Total mono- to Total mono- to Total mono-to
hexaBDF/D µg/g hexaBDF/D µg/g hexaBDF/D hexaBDF/D hexaBDF/D
(ppm) (ppm) µg/g (ppm) µg/m2 µg/m3
Casing of electrical appliance 1 0.63 -b 8700 177 -b
Casing of electrical appliance 2 0.64 -b 7750 1610 -b
Electrical appliance 3 -c 1.77 468 106 456
Electrical appliance 4 0.06 3.44 43 260 355
Electrical appliance 5 0.81 1.98 18 396 1700
Electrical appliance 6 4.20 0.35 1930 234 1350
Etectrlcal appliance 7 -c 0.13 1 6 11
Electrical appliance 8 1.26 0.007 24 323 -b
aFrom: Hamm & Thiesen (1992).
b = Not determined.
c = Not detectable.
Table 9. PBDF/D-concentrations in residues and smoke condensates from
real fire accidents with television setsa
Fire accident Combustion Smoke condensates
(Case number) residues
Close to fire site At some
distance from
fire site
Total mono- to Total mono- to Total mono- to
hexaBDF/D hexaBDF/D hexaBDF/D
µg/g (ppm) µg/m2 µg/m2
I 0.235 10.7 0.665
II 0.004 0.134 0.102
III 0.209 13.1 0.382
IV 0.009 NDb NDb
V 0.001 4.82 1.39
VI 0.017 0.759 0.425
VII 0.001 0.021 0.008
VIII 0.001 10.5 5.37
IX 107 7.47 0.847
aFrom: Hamm & Thiesen (1992).
bND = Not detectable.
6. ENVIRONMENTAL POLLUTION BY PBDE
6.1 Ultimate fate following use
Products containing PBDE are disposed of in the normal domestic
waste stream (landfill and incineration).
No studies are available on the fate of PBDE-containing products
in landfills, but there is concern that the PBDE may eventually leach
out. Bearing in mind that PBDE, at least the congeners with more than
3 bromine atoms, are persistent in the environment, the introduction
of such chemicals into widespread products may be a considerable
long-term diffuse source of emissions of these compounds to the
environment. This type of source is difficult to control and the
unnecessary use of persistent organic compounds should be avoided.
Formation of PBDF and/or PBDD as a result of landfill fires is
also a possibility, though no data are available on the scale of this
source. The results of pyrolysis experiments showed that PBDE can form
PBDF and PBDD (in much smaller quantities) under a wide range of
heating conditions (see General Introduction sections 3.1 and 3.2). If
chlorine is present, mixed halogenated furans/dioxins can also be
generated (Oberg et al., 1987; Zier et al., 1991). Unless sufficiently
high temperatures and long residence times are maintained, PBDF/PBDD
can be generated during the incineration of products containing PBDE.
They can also result from poorly-controlled combustion gas cooling.
Modern, properly operated municipal waste incineration (MWI) should
not emit significant quantities of PBDF/PBDD, regardless of the
composition of the municipal waste.
Lahl et al. (1991) reported increases in dibenzofuran and
dibenzodioxin levels in filter dust, when products containing PBDE
were added to the feed-stock. Riggs et al. (1990) reported PBDF
generation when a flame retarded resin was burnt under simulated MWI
conditions. However, Oberg et al. (1987) reported no increased
emissions of dibenzodioxins when the bromine content of an
incineration feed-stock was increased. Monobromodichloro-dibenzofuran
levels were slightly increased. Oberg & Bergström (1990) conducted
further experiments with a hazardous waste incinerator, to study the
relationship between bromine levels in municipal waste and incinerator
dibenzodioxin and dibenzofuran emissions. They concluded that no
unacceptable environmental risks were associated with the incineration
of brominated compounds in plants with good combustion conditions
equipped with efficient flue gas cleaning. They further noted that
only 0.0125% of the feed to Swedish MWIs was brominated waste.
6.2 Air
Watanabe et al. (1992) reported on the presence of PBDE in the air
in Taiwan and Japan. The concentrations in the air samples collected
in Taiwan from a recycling plant in January 1991 were, in general,
higher than those in Japan; 3 samples were analysed in Taiwan, and 5
in Japan. Tribromo-, tetrabromo-, pentabromo-, and hexabromodiphenyl
ethers were present in the following mean concentrations: Taiwan, 32,
52, 23, and 31 µg/m3, and, Japan, 7.1, 21, 8.9, and 21 µg/m3,
respectively.
6.3 Soil
Two ash and two soil samples were collected in Taiwan from a
recycling plant in January 1991 and analysed for the presence of PBDE.
Tri-, tetra-, penta-, hexa-, and decabromodiphenyl ethers were present
in ash in the following concentrations 20-20, 130, 78-110, 47-54, and
510-2500 µg/kg, respectively; the concentrations in soil were 38-40,
75-104, 41-84, 20-23 and 260-330 µg/kg, respectively. Hepta- and
octabromodiphenyl ether were not found (Watanabe et al., undated).
6.4 Water
Marine, estuarine, and river water samples were analysed for the
presence of the different PBDE. Except for monobromo-diphenyl ether,
levels of all the higher brominated PBDE were below the detection
limit. MBDE was mainly found in the surroundings of manufacturing
plants in the USA (US EPA, 1986).
6.5 Sediments and sewage sludge
In Japan, Spain, Sweden, and the USA, studies were carried out to
determine the presence of the different PBDE in marine, estuarine, or
river sediment. PBDE were mainly found in river sediment. In general,
the levels were below 100 µg/kg dry weight, except in rivers in the
vicinity of manufacturing plants. In these cases, the concentrations
were much higher. In a river in Sweden, concentrations of 11.5 mg
DeBDE, 0.8 mg TeBDE, and 2.8 mg PeBDE/kg dry weight were found. In the
USA, at a manufacturing plant, as much as 1 g DeBDE/kg was found
(Zweidinger et al., 1978; DeCarlo, 1979; Environment Agency Japan,
1983, 1989, 1991; Watanabe et al., 1986, 1987b; Fernandez et al.,
1992).
The upper layers in a laminated sediment core from the Baltic Sea
(Bornholm Deep) contained higher levels of TeBDE and PeBDE than the
deeper layers, indicating an increasing burden of these compounds
(Nylund et al., 1992).
A series of samples of sewage sludges from municipal waste water
treatment plants in Germany were analysed for poly-halogenated
compounds, such as halogenated diphenyl ethers. Tribromo- to
heptabromodiphenyl ethers were found at relatively high concentrations
(Hagenmaier et al., 1991). Sewage sludge was analysed in Sweden for
the presence of TeBDE and PeBDE. Concentrations of 15 and 19 µg/kg,
respectively, were found (Sellström et al., 1990a,b).
6.6 Aquatic vertebrates
The presence of PBDE depends mainly on the degree of bromination.
DeBDE, OBDE, and HxBDE were not found in mussel and fish samples
collected in Japan. No data are available for HpBDE. However, PeBDE
was found in mussel and fish species in concentrations of < 3 µg/kg
wet weight in Japan. Concentrations of 22 µg 2,2',4,4',5-PeBDE/kg wet
weight were found in cod liver collected in the North Sea, and
concentrations of up to 64 µg/kg on a fat basis were found in fish
collected in Sweden. The concentrations were much higher in fish
collected in the vicinity of industrial areas, e.g., up to 9.4 mg/kg
on a fat basis (Jansson et al., in press). Levels for TeBDE, mainly
2,2',4,4'-TeBDE, were comparable but generally higher. Mussels and
fish in Japan contained up to 14.6 µg/kg wet weight, cod liver
collected in the North Sea, 360 µg/kg, and eel from the Netherlands,
up to 1700 µg/kg fat. Different species of fish collected in Sweden
contained up to 88 mg/kg fat (Andersson & Blomkvist, 1981; Watanabe,
1987; Watanabe et al., 1987b; De Boer, 1989, 1990). An increasing
trend was observed in PeBDE and TeBDE levels in freshwater fish in
Sweden. Only limited data are available concerning lower brominated
PBDE (Jansson et al., in press).
Thirty-five samples of 18 freshwater fish collected in German
rivers, and 17 samples collected from the Baltic Sea and the North Sea
contained 18.2-983.6 and 0.6-119.9 µg PBDE/kg fat (determined as
Bromkal 70-SDE), respectively (Kruger, 1988).
6.7 Aquatic mammals
Three bottle-nose dolphins (Tursiops truncatus), collected
during the 1987/88 mass mortality event along the central and south
Atlantic coast of the USA, were analysed for brominated diphenyl
ethers. The concentrations of PBDE were 200, 220, and 180 µg/kg lipid
(Kuehl et al., 1991).
Limited data are available on the presence of PBDE in aquatic
mammals. 2,2'4,4'5-PeBDE was found in ringed and grey seals, collected
in Sweden, in concentrations of 1.7 and 40 µg/kg fat, respectively.
TeBDE, mainly 2,2',4,4'-TeBDE, was also found in the blubber of these
2 species in concentrations of 47 and 650 µg/kg fat, respectively
(Jansson et al., in press). Seals collected at Spitzbergen contained
approximately 10 µg PBDE/kg fat, determined as Bromkal 75DE (Kruger,
1988).
6.8 Terrestrial vertebrates
Pooled samples of rabbits, moose, and reindeer, collected in
Sweden, contained PeBDE and TeBDE in concentrations of <0.3, 0.64,
and 0.26 µg 2,2',4,4',5-PeBDE/kg and <2, 0.82, and 0.18 µg
2,2',4,4'-TeBDE/kg lipid, respectively (Jansson et al., in press).
Four samples of cow's milk were analysed in Germany for the
presence of PBDE. The average concentration was 3.572 µg/kg fat (range
2.536-4.539 µg/kg) determined as Bromkal 70-5DE. The main component
was HxBDE (Kruger, 1988).
6.8.1 Birds
Limited data are available on the presence of PBDE in birds. In
Sweden, 2,2',4,4',5-PeBDE was found in the muscle tissue of osprey, in
newborn starlings, and in guillemot eggs in concentrations of 140,
2.3-4.2, and 24-260 µg/kg lipid, respectively. A trend towards
increasing concentrations of PeBDE and TeBDE in guillemot eggs from
the Baltic Sea was observed. 2,2',4,4'-TeBDE was found in the muscle
tissue of osprey in concentrations of up to 1800 µg/kg. Guillemots
collected from the Baltic Sea, the North Sea, and Spitzbergen
contained 370, 80, and 130 µg/kg on a fat basis, respectively (Jansson
et al., 1987, 1993).
In the USA, indications were found that dibromodiphenyl ether was
present in the eggs of fish-eating birds, but it was not quantified
(Stafford, 1983).
6.8.2 Humans
In Germany, 25 samples of breast milk were analysed for the
presence of PBDE. The ages of the women ranged between 24 and 36 years
and most of them were breast-feeding their first or second child. The
samples contained 0.6-11.1 µg PBDE/kg fat, determined as Bromkal
70-5DE. The main component was HxBDE. One sample from a Chinese woman
showed 7.7 µg PBDE/kg fat; a sample from another woman, exposed
occupationally to hydraulic fluids and transformer oils, contained
50 µg PBDE/kg fat. This last value was excluded from the given range
and average. (Kruger, 1988).
DECABROMODIPHENYL ETHER
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties
Typically, commercial DeBDE has a purity of 97-98%, with 0.3-3.0% of
nona- and/or octabrominated diphenyl ethers. Nonabromodiphenyl ether
(NBDE) is the major impurity. In contrast to the other polybrominated
diphenyl ethers there is only one isomer of DeBDE.
The melting point of DeBDE is approximately 300 °C and decomposition
occurs above 400 °C. Solubility in water is 20-30 µg/litre and the log
of the n-octanol/water partition coefficient is greater than 5.
Vapour pressure is < 10-6 mmHg at 20 °C.
1.1.2 Production and uses
Among the brominated diphenyl ethers (mono- to deca-),
deca-bromodiphenyl ether is the most important commercial product with
regard to production and use.
Commercial DeBDE has been produced in increasing degrees of purity
since the late 1970s. The global production of DeBDE is approximately
30 000 tonnes/year. It is used as an additive flame retardant in many
plastics, especially high-impact polystyrene, and in the treatment of
textiles used in soft furnishing, automobile fabrics, and tents.
1.1.3 Environmental transport, distribution, and transformation
Photodegradation of DeBDE occurs in organic solvents under
ultraviolet radiation (UVR) or sunlight; lower brominated diphenyl
ethers and brominated dibenzofurans are formed. Photodegradation also
occurs, to a lesser extent, in water with sunlight; however, lower
brominated diphenyl ethers and brominated dibenzofurans have not been
found.
Levels of DeBDE extracted from polymers are close to, or below,
the limit of detection, depending on the polymer type and extraction
solvent.
Because of its extremely low water solubility and vapour pressure,
DeBDE is likely to be transported primarily by adsorption to
particulate matter. It is persistent and likely to accumulate in
sediment and soil.
No data are available on its bioavailability from sediment and
soil. A study on rainbow trout did not show any bioaccumulation in
flesh, skin, or viscera, over 48 h. DeBDE is unlikely to bioaccumulate
because of its high relative molecular mass.
Products containing commercial DeBDE will eventually be disposed
of by landfill or incineration. DeBDE may eventually leach from
landfills. Polybrominated dibenzofurans (PBDF) and mixed
halogen-dibenzofurans and -dibenzodioxins may result from landfill
fires and inefficient incineration. Products containing commercial
DeBDE may contribute to these emissions.
Pyrolysis of both commercial DeBDE itself and polymers (HIPS, PBT,
industrial polypropylene) containing DeBDE, in the presence of oxygen,
produced PBDF, polybrominated dibenzodioxins (PBDD) being found to a
lesser extent. The maximum formation' of PBDF occurs at 400-500 °C,
but it can occur at temperatures up to 800 °C, and Sb2O3 plays a
catalytic role in the formation of PBDF and PBDD.
The formation, and amounts found, of PBDF and PBDD depend on
temperature, oxygen content, and length of pyrolysis. In the absence
of oxygen, mainly polybromobenzenes and polybromonaphthalenes are
formed.
1.1.4 Environmental levels and human exposure
DeBDE has been identified in air in the vicinity of manufacturing
plants at concentrations of up to 25 µg/m3. DeBDE was not detected in
water samples collected in Japan in the period 1977-91. However, it
was detected in river and estuarine sediment, collected in Japan in
the same period, at concentrations of up to approximately 12 mg/kg dry
weight. DeBDE (up to 1 g/kg) was also found in the USA in river
sediment close to one manufacturing plant. DeBDE was not detected in
fish samples collected in Japan, but, in one mussel sample, a level
just above the level of detection was found. DeBDE was not detected in
human adipose tissue samples collected in Japan, but, in the USA,
DeBDE was found in 3 out of 5 samples of human adipose tissue.
Human exposure to DeBDE can occur in the course of manufacture and
formulation into polymers. Exposure of the general population to DeBDE
is insignificant.
Determination of occupational exposure to the breakdown products
of DeBDE during manufacture, formulation, or use, showed that air
samples close to the extruder head contained high concentrations of
PBDF. Lower levels were found in the air of the workroom. PBDF was
also found in wipe samples. The application of good engineering
techniques has been shown to reduce occupational exposure to PBDF.
Exposure of, the general population to PBDF impurities in flame
retarded polymers is unlikely to be of significance.
1.1.5 Kinetics and metabolism in laboratory animals and humans
DeBDE is poorly absorbed from the gastrointestinal tract and is
rapidly excreted following injection.
The results of metabolic studies on the rat, using 14C labelled
DeBDE, indicated a half-life for the disappearance from the body of
less than 24 h and that the principal route of elimination following
oral ingestion was via the faeces. No appreciable 14C activity (less
than 1%) was found in either urine or expired air.
Rats fed 0.1 mg/kg body weight per day, for up to two years,
showed no accumulation of DeBDE in serum, kidneys, muscle, or testes,
as estimated from total bromine determination. Bromine accumulation in
the liver plateaued at 30 days and was cleared within 10 days
following treatment. After 180 days of treatment, the bromine level in
the liver of treated rats was no greater than that in control rats.
Adipose tissue accumulated low levels of total bromine, which remained
after 90 days of clean diet; the nature of the retained "bromine" is
not known. Since DeBDE accounted for only 77% of the commercial
mixture used, "bromine" could have been derived from NBDE or OBDE.
1.1.6 Effects on laboratory mammals and in vitro test systems
The acute toxicity of DeBDE for laboratory animals is low. The
substance is not an irritant to the skin or eyes of rabbits. It is not
chloracnegenic on the skin of rabbits and is not a human skin
sensitizer.
The combustion products of flame retarded polystyrene containing
DeBDE and Sb203 were tested for acute toxicity and comedogenicity.
The rat oral LD50 of the soot and char was >2000 mg/kg body weight.
In short-term toxicity studies on rats and mice, DeBDE (purity
>97%) at dietary levels of 100 g/kg (4 weeks) or 50 g/kg (13 weeks;
equivalent to 2500 mg/kg body weight for the rat) did not induce
adverse effects. A one-generation reproduction study on rats showed no
adverse effects with dose levels of 100 mg/kg body weight. DeBDE did
not cause any teratogenic effects in the fetuses of rats administered
a dose level of 100 mg/kg body weight. With 1000 mg/kg body weight,
malformations, such as delayed ossification, were seen. DeBDE was not
shown to be mutagenic in a number of tests.
In a carcinogenicity study on rats and mice, DeBDE (purity 94-99%)
was administered at dietary levels of up to 50 g/kg. An increase in
the incidence of adenomas (but not carcinomas) was found in the livers
of male rats receiving 25 g/kg and female rats receiving 50 g
DeBDE/kg. In male mice, increased incidences of hepatocellular
adenomas and/or carcinomas (combined) were found at 25 g/kg and an
increase in thyroid follicular cell adenomas/ carcinomas (combined) at
both dose levels. Female mice did not show any increase in tumour
incidence. There was equivocal evidence for carcinogenicity in male
and female rats and male mice only at dose levels of 25-50 g DeBDE/kg
diet. As the results of all mutagenicity tests have been negative, it
can be concluded that DeBDE is not a genotoxic carcinogen. IARC (1990)
concluded that there was limited evidence for the carcinogenicity of
DeBDE in experimental animals. The very high dose levels, lack of
genotoxicity, and minimal evidence for carcinogenicity indicate that
DeBDE, at the present exposure levels, does not present a carcinogenic
risk for humans.
1.1.7 Effects on humans
No evidence for skin sensitization was found in 200 human subjects
exposed to DeBDE in a sensitization test.
A morbidity study of extruder personnel blending
polybutyl-eneterephthalate containing DeBDE, with consequently
potential exposure to PBDD and PBDF for 13 years, did not reveal any
deleterious effects, even though 2,3,7,8-TeBDF and -TeBDD were
detected in the blood. Results of immunological studies showed that
the immune system of the exposed persons was not adversely affected in
13 years.
1.1.8 Effects on other organisms in the laboratory and field
The EC50s for the growth of 3 marine unicellular algae were
greater than 1 mg DeBDE/litre. No further information is available on
the effects of DeBDE on other organisms in the laboratory and field.
1.2 Conclusions
1.2.1 DeBDE
DeBDE is widely used incorporated in polymers as an additive flame
retardant. Contact of the general population is with products made
from these polymers. Exposure is very low since the DeBDE is not
readily extracted from polymers. The acute toxicity of DeBDE is very
low and there is minimal absorption from the gastrointestinal tract.
Thus, risk to the general population from DeBDE is considered to be
insignificant.
Occupational exposure is to DeBDE in particulate form. The control
of dust during manufacture and use will adequately reduce the risk for
workers.
DeBDE is persistent and binds to particulate matter in the
environment; it is likely to accumulate in sediment. It is unlikely to
bioaccumulate. Current evidence suggests that environmental
photodegradation in water does not lead to the formation of lower
brominated diphenylethers or brominated dibenzofurans, but little is
known about degradation in other media.
There is minimal information on the toxicity of DeBDE for
organisms in the environment.
1.2.2 Breakdown products
Formation of PBDF and, to some extent, PBDD may occur when DeBDE,
or products containing it, are heated to 300-800 °C. The possible
hazards associated with this have to be addressed.
Properly controlled incineration does not lead to the emission of
significant quantities of brominated dioxins and -furans. Any
uncontrolled combustion of products containing DeBDE can lead to an
unquantified generation of PBDF/PBDD. The significance of this for
both humans and the environment will be addressed in a future
Environmental Health Criteria on PBDF/PBDD.
PBDF have been found in the blood of workers involved in the
production of plastics containing DeBDE. No adverse health effects
have been associated with this exposure. Good engineering controls can
prevent worker exposure to PBDF.
1.3 Recommendations
1.3.1 General
* Workers involved in the manufacture of DeBDE and products
containing the compound should be protected from exposure through
the application of appropriate industrial hygiene measures, the
monitoring of occupational exposure, and engineering controls.
* Environmental exposure should be minimized through the appropriate
treatment of effluents and emissions in industries using the
compound or products. Disposal of industrial wastes and consumer
products should be controlled, to minimize environmental
contamination with this persistent material and its breakdown
products.
* Manufacturers should minimize levels of impurities in commercial
DeBDE products, using the best available techniques. A purity of
97% or higher is recommended.
* Incineration should only be carried out in properly constituted
incinerators, running at consistently optimal conditions. Burning
by any other means may lead to the production of PBDF and/or PBDD.
1.3.2 Further studies
* Further studies on the bioavailability and toxicity of
sediment-bound DeBDE should be performed on relevant organisms.
* Continued monitoring of environmental levels is required.
* The generation of PBDF under real fire conditions should be
further investigated.
* Environmental biodegradation, and photodegradation in compartments
other than water, should be further studied.
* Investigation into possible methods and consequences of recycling
of DeBDE-containing polymers should be made.
* Analytical methods for DeBDE in various matrices should be
validated.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Pure substance
Chemical structure:
 Chemical formula: C12Br10O
Relative molecular mass: 959.22
Chemical name: decabromodiphenyl ether
(DeBDE); decabromodiphenyl
oxide
CAS registry number:
Chemical formula: C12Br10O
Relative molecular mass: 959.22
Chemical name: decabromodiphenyl ether
(DeBDE); decabromodiphenyl
oxide
CAS registry number:  Chemical formula: C12 H Br9 O
CAS registry number:
Chemical formula: C12 H Br9 O
CAS registry number:  Chemical formula: C12H2Br8O
Relative molecular mass: 801.47
Common names: octabromodiphenyl ether (OBDE)
octabromodiphenyl oxide
CAS registry number:
Chemical formula: C12H2Br8O
Relative molecular mass: 801.47
Common names: octabromodiphenyl ether (OBDE)
octabromodiphenyl oxide
CAS registry number:  Chemical formula: C12 H3 Br7 O
Relative molecular mass: 722.3
CAS registry number:
Chemical formula: C12 H3 Br7 O
Relative molecular mass: 722.3
CAS registry number:  Chemical formula: C12 H4 Br6O
Relative molecular mass: 643.62
CAS registry number:
Chemical formula: C12 H4 Br6O
Relative molecular mass: 643.62
CAS registry number:  Chemical formula: C12H5Br5O
Relative molecular mass: 564.75
Common name: pentabromodiphenyl ether (PeBDE);
pentabromodiphenyl oxide
CAS registry number:
Chemical formula: C12H5Br5O
Relative molecular mass: 564.75
Common name: pentabromodiphenyl ether (PeBDE);
pentabromodiphenyl oxide
CAS registry number:  Chemical formula: C12H6Br4O
Relative molecular mass: 485.82
Common names: tetrabromodiphenyl ether (TeBDE)
tetrabromodiphenyl oxide
CAS registry number:
Chemical formula: C12H6Br4O
Relative molecular mass: 485.82
Common names: tetrabromodiphenyl ether (TeBDE)
tetrabromodiphenyl oxide
CAS registry number:  Chemical formula: C12 H7 Br3 O
Relative molecular mass 407.1
Common names tribromodiphenyl ether (TrBDE);
tribromodiphenyl oxide
CAS registry number
Chemical formula: C12 H7 Br3 O
Relative molecular mass 407.1
Common names tribromodiphenyl ether (TrBDE);
tribromodiphenyl oxide
CAS registry number  Chemical formula: C12 H8 Br2 O
Relative molecular mass: 328.02
Common name: dibromodiphenyl ether (DiBDE)
dibromodiphenyl oxide
CAS registry number:
Chemical formula: C12 H8 Br2 O
Relative molecular mass: 328.02
Common name: dibromodiphenyl ether (DiBDE)
dibromodiphenyl oxide
CAS registry number:  Chemical formula: C12H9BrO
Relative molecular mass: 249.11
Common names: monobromodiphenyl ether (MBDE)
mono bromodiphenyl oxide
CAS registry number:
Chemical formula: C12H9BrO
Relative molecular mass: 249.11
Common names: monobromodiphenyl ether (MBDE)
mono bromodiphenyl oxide
CAS registry number: 