
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 156
HEXACHLOROBUTADIENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. T. Vermeire,
National Institute of Public Health and
Environmental Protection, Bilthoven,
The Netherlands
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Hexachlorobutadiene.
(Environmental health criteria: 156)
1. Butadienes - toxicity 2. Environmental exposure
I.Series
ISBN 92 4 157126 X (NLM Classification QV 305.H7)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland,
Which will be glad to provide the latest information on any changes
made to the text, plans for new editions, and reprints and
translations already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the impression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of every country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBUTADIENE
1. SUMMARY
1.1. Identity, physical and chemical properties,
analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and
transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on organisms in the environment
1.7. Effects on experimental animals and
in vitro test systems
1.7.1. General toxicity
1.7.2. Reproduction, embryotoxicity and
teratogenicity
1.7.3. Genotoxicity and carcinogenicity
1.7.4. Mechanisms of toxicity
1.8. Effects on humans
1.9. Evaluation of human health risks and
effects on the environment
1.9.1. Evaluation of human health risks
1.9.2. Evaluation of effects on the
environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Waste disposal
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.2.1. Photolysis
4.2.2. Photooxidation
4.2.3. Hydrolysis
4.3. Biodegradation
4.4. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil and sediment
5.1.4. Biota
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption and distribution
6.2. Metabolism
6.2.1. In vitro studies
6.2.2. In vivo studies
6.3. Reaction with body components
6.4. Excretion
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic organisms
7.1.1. Short-term toxicity
7.1.2. Long-term toxicity
7.2. Terrestrial organisms
7.2.1. Short-term toxicity
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.1.1. Inhalation exposure
8.1.1.1 Mortality
8.1.1.2 Systemic effects
8.1.2. Oral exposure
8.1.2.1 Mortality
8.1.2.2 Systemic effects
8.1.3. Dermal exposure
8.1.3.1 Mortality
8.1.3.2 Systemic effects
8.1.4. Other routes of exposure
8.2. Short-term exposure
8.2.1. Inhalation exposure
8.2.2. Oral exposure
8.2.2.1 Rats
8.2.2.2 Mice
8.3. Long-term exposure
8.4. Skin and eye irritation; sensitization
8.4.1. Irritation
8.4.2. Sensitization
8.5. Reproduction, embryotoxicity and
teratogenicity
8.5.1. Reproduction
8.5.2. Embryotoxicity and teratogenicity
8.6. Mutagenicity and related end-points
8.6.1. In vitro effects
8.6.2. In vivo effects
8.7. Carcinogenicity/long-term toxicity
8.7.1. Inhalation exposure
8.7.2. Oral exposure
8.7.3. Dermal exposure
8.7.4. Exposure by other routes
8.8. Other special studies
8.8.1. Effects on the nervous system
8.8.2. Effects on the liver
8.8.2.1 Acute effects
8.8.2.2 Short-term effects
8.8.3. Effects on the kidneys
8.8.3.1 Acute effects
8.8.3.2 Short- and long-term effects
8.9. Factors modifying toxicity; toxicity of
metabolites
8.9.1. Factors modifying toxicity
8.9.1.1 Surgery
8.9.1.2 Inhibitors and inducers of
mixed-function oxidases (MFO)
8.9.1.3 Inhibitors of gamma-glutamyltrans-
peptidase (EC 2.3.2.2)
8.9.1.4 Inhibitors of cysteine conjugate
ß-lyase
8.9.1.5 Inhibitors of organic anion
transport
8.9.1.6 Non-protein sylfhydryl scavengers
8.9.2. Toxicity of metabolites
8.9.2.1 In vitro studies
8.9.2.2 In vivo studies
8.10. Mechanisms of toxicity - mode of action
8.10.1. Mechanisms of toxicity
8.10.2. Mode of action
9. EFFECTS ON HUMANS
9.1. General population exposure
9.2. Occupational exposure
9.3. In vitro metabolism studies
9.4. Extrapolation of NOAEL from animals to
humans
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Hazard identification
10.1.2. Exposure
10.1.3. Hazard evaluation
10.2. Evaluation of effects on the environment
10.2.1. Hazard identification
10.2.2. Exposure
10.2.3. Hazard evaluation
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
HEXACHLOROBUTADIENE
Members
Dr T.M. Crisp, Reproductive and Development Toxicology Branch, Human
Health Assessment Group, Office of Health and Environmental
Assessment, Environmental Protection Agency, Washington, DC, USA
(Joint Rapporteur)
Professor W. Dekant, Toxicology Institute, Würzburg University,
Würzburg, Germany
Dr I.V. German, Ukrainian Institute for Ecohygiene and Toxicology of
Chemicals, Kiev, Ukraine
Dr B. Gilbert, Fundaçao Oswaldo Cruz, Ministry of Health,
Manguinhos, Rio de Janeiro, Brazil (Joint Rapporteur)
Ms E. Kuempel, Document Development Branch, National Institute for
Occupational Safety and Health, Robert A. Taft Laboratories,
Cincinnati, Ohio, USA
Dr E.A. Lock, Biochemical Toxicology Section, Imperial Chemical
Industries, Central Toxicological Laboratory, Alderly Park,
Macclesfield, Cheshire, United Kingdom
Professor M.H. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
(Chairman)
Dr A. Smith, Toxicology Unit, Health and Safety Executive, Bootle,
Merseyside, United Kingdom
Secretariat
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia (Responsible Officer and Secretary)
Dr T. Vermeire, National Institute of Public Health and
Environmental Protection, Toxicology Advisory Centre, Bilthoven,
The Netherlands
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROBUTADIENE
A Task Group on Environmental Health Criteria for
Hexachlorobutadiene met at the Institute of Hygiene and
Epidemiology, Brussels, Belgium, from 10 to 15 December 1992. Dr C.
Vleminckx welcomed the participants on behalf of the host
institution and Professor F. Valic opened the meeting on behalf of
the three cooperating organizations of the IPCS (UNEP/ILO/WHO). The
Task Group reviewed and revised the draft monograph and made an
evaluation of the risks for human health and the environment from
exposure to hexachlorobutadiene.
The first draft of this monograph was prepared by Dr T.
Vermeire, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands.
Professor F. Valic was responsible for the overall scientific
content of the monograph and for the organization of the meeting,
and Dr P.G. Jenkins, IPCS, for the technical editing of the
monograph.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
ABBREVIATIONS
ACPB 1-( N-acetylcystein- S-yl)-1,2,3,4,4-pentachloro-1,3-
butadiene
BCTB 1,4-(bis-cystein- S-yl)-1,2,3,4-tetrachloro-1,3-
butadiene BGTB 1,4-(bis-glutathion- S-yl)-1,2,3,4-
tetrachloro-1,3-butadiene
CMTPB 1-carboxymethylthio-1,2,3,4,4-pentachloro-1,3-
butadiene
CPB 1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene
GPB 1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-1,3-
butadiene
GSH reduced glutathione
HCBD hexachlorobutadiene
ip intraperitoneal
iv intravenous
MTPB 1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene
NIOSH National Institute of Occupational Safety and Health
NOAEL no-observed-adverse-effect level
OECD Organisation for Economic Co-operation and Development
PATPB 1-(pyruvic acid thiol)-1,2,3,4,4-pentachloro-1,3-
butadiene
PBSA 1,2,3,4,4-pentachloro-1,3-butadienyl sulfenic acid
TBA 2,3,4,4-tetrachloro-1,3-butenoic acid
TPA 12- o-tetradecanoylphorbol-13-acetate
TPB 1-thiol-1,2,3,4,4-pentachloro-1,3-butadiene
UDS unscheduled DNA synthesis
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
Hexachlorobutadiene is a non-flammable, incombustible, clear,
oily and colourless liquid at ordinary temperature and pressure. It
is poorly soluble in water but miscible with ether and ethanol.
The substance can be detected and determined quantitatively by
gas chromatographic methods. The detection limits are 0.03 µg/m3
in air, 0.001 µg/litre in water, 0.7 µg/kg wet weight in soil or
sediment, and 0.02 µg/litre in blood. A level of 0.47 µg/kg wet
weight has been determined in tissue.
1.2 Sources of human and environmental exposure
Hexachlorobutadiene is not reported to occur as a natural
product. It is chiefly produced as a by-product of the manufacture
of chlorinated hydrocarbons where it occurs in the heavy fractions
(Hex-waste). The world annual production of the compound in heavy
fractions was estimated in 1982 to be 10 000 tonnes.
Hexachlorobutadiene can be used for recovery of
chlorine-containing gas in chlorine plants and as a wash liquor for
removing certain volatile organic compounds from gas streams. It has
further been used as a fluid in gyroscopes, as heat transfer,
transformer, insulating and hydraulic fluids, as a solvent for
elastomers, and as an intermediate and fumigant.
1.3 Environmental transport, distribution and transformation
The main pathways of entry into the environment are emissions
from waste and dispersive use. Intercompartmental transport will
chiefly occur by volatilization, adsorption to particulate matter,
and subsequent deposition or sedimentation. Hexachlorobutadiene does
not migrate rapidly in soil and accumulates in sediment. In water,
it is considered persistent unless there is high turbulence.
Hydrolysis does not occur. The substance seems to be readily
biodegradable aerobically, though biodegradability has not been
investigated thoroughly. Hexachlorobutadiene photolyses on surfaces.
In addition to deposition, reaction with hydroxyl radicals is
assumed to be an important sink of hexachlorobutadiene in the
troposphere, and the estimated atmospheric half-life is up to 2.3
years. The substance has a high bioaccumulating potential as has
been confirmed by both laboratory and field observations. Average
steady-state bioconcentration factors of 5800 and 17 000, based on
wet weight, have been determined experimentally in rainbow trout.
Biomagnification has not been observed either in the laboratory or
in the field.
1.4 Environmental levels and human exposure
Hexachlorobutadiene has been measured in urban air: in all
cases levels were below 0.5 µg/m3. Concentrations in remote areas
are less than 1 pg/m3. In lake and river water in Europe
concentrations of up to 2 µg/litre have been recorded, but mean
levels are usually below 100 ng/litre. In the Great Lakes area of
Canada, much lower levels (around 1 ng/litre) were measured. Bottom
sediment levels here can be as high as 120 µg/kg dry weight. Older
sediment layers from around 1960 contained higher concen-trations
(up to 550 µg/kg wet weight). The sediment concentration was
demonstrated to increase with particle size in the sediment.
Concentrations of hexachlorobutadiene in aquatic organisms,
birds and mammals indicate bioaccumulation but not biomagnification.
In polluted waters, levels of over 1000 µg/kg wet weight have been
measured in several species and 120 mg/kg (lipid base) in one
species. Present levels generally remain below 100 µg/kg wet weight
away from industrial outflows.
The compound has been detected in human urine, blood and
tissues. Certain food items containing a high lipid fraction have
been found to contain up to about 40 µg/kg and, in one case, over
1000 µg/kg.
One study reported occupational exposures of 1.6-12.2 mg/m3
and urine levels of up to 20 mg/litre.
1.5 Kinetics and metabolism
Hexachlorobutadiene is rapidly absorbed following oral
administration to experimental animals, but the rate of absorption
following inhalation or dermal exposure has not been investigated.
In rats and mice, the compound distributes mainly to the liver,
kidneys and adipose tissue. It is rapidly excreted. Binding to liver
and kidney protein and nucleic acids has been demonstrated.
The biotransformation of the compound in experimental animals
appears to be a saturable process. This process proceeds mainly
through a glutathione-mediated pathway in which hexachlorobutadiene
is initially converted to S-glutathione conjugates. These
conjugates can be metabolized further, especially in the
brush-border membrane of renal tubular cells, to a reactive sulfur
metabolite, which probably accounts for the observed nephrotoxicity,
genotoxicity and carcinogenicity.
1.6 Effects on organisms in the environment
Hexachlorobutadiene is moderately to very toxic to aquatic
organisms. Fish species and crustaceans were found to be the most
sensitive, 96-h LC50 values ranging from 0.032 to 1.2 and 0.09 to
approximately 1.7 mg/litre for crustaceans and fish, respectively.
The kidney was demonstrated to be an important target organ in fish.
Based on several long-term tests with algae and fish species, a
no-observed-effect level (NOEL) of 0.003 mg/litre was established;
this classifies the compound as very toxic to aquatic species.
End-points investigated include general toxicity, neurotoxicity,
biochemistry, haematology, pathology, and reproductive parameters.
In one 28-day early-lifestage test with fathead minnows,
reproduction was unaffected at concentrations of up to
0.017 mg/litre, whereas increased mortality and a decreased body
weight were observed at 0.013 and 0.017 mg/litre. The NOEL was
0.0065 mg/litre.
Only one reliable test with terrestrial organisms has been
described. In a 90-day test with Japanese quail, receiving a diet
containing the compound at concentrations from 0.3 to 30 mg/kg diet,
the survival of chicks was decreased at 10 mg/kg diet only.
1.7 Effects on experimental animals and in vitro test systems
1.7.1 General toxicity
Hexachlorobutadiene is slightly to moderately toxic to adult
rats, moderately toxic to male weanling rats, and highly toxic to
female weanling rats following a single oral dose. The major target
organs are the kidney and, to a much lesser extent, the liver.
Based on animal data, the vapour of hexachlorobutadiene is
irritating to mucous membranes and the liquid is corrosive. The
substance should be regarded as a sensitizing agent.
In the kidneys of rats, mice and rabbits, hexachlorobutadiene
causes a dose-dependent necrosis of the renal proximal tubules.
Adult male rats are less sensitive to renal toxicity than adult
females or young males. Young mice are more susceptible than adults,
no sex difference being apparent. In adult female rats the lowest
single intraperitoneal dose at which renal necrosis was observed was
25 mg/kg body weight, and in adult male and female mice it was
6.3 mg/kg body weight. Biochemical changes and distinct functional
alterations in the kidneys occurred at doses similar to or higher
than those at which necrosis occurred.
In six short-term oral tests, two reproductive studies and one
long-term diet study with rats, the kidney was also the major target
organ. Dose-related effects included a decreased relative kidney
weight and tubular epithelial degeneration. The no-observed-
adverse-effect level (NOAEL) for renal toxicity in rats in a 2-year
study was 0.2 mg/kg body weight per day. In mice the NOAEL in a
13-week study was 0.2 mg/kg body weight per day. In both species,
adult females were more susceptible than adult males.
In one short-term inhalation test (6 h/day for 12 days),
similar effects on the kidneys were observed with a nominal vapour
concentration of 267 mg/m3, at which concentration respiratory
difficulties and cortical degeneration in the adrenal glands were
also observed.
1.7.2 Reproduction, embryotoxicity and teratogenicity
Two reproduction diet studies in rats at doses up to 20 and
75 mg/kg body weight per day, respectively, revealed reduced birth
weight and neonatal weight gain at maternally toxic doses of 20 and
7.5 mg/kg body weight, respectively. The highly toxic dose of
75 mg/kg body weight per day was sufficient to prevent conception
and uterine implantation. Skeletal abnormalities were not observed.
In two teratogenicity tests, where rats were exposed either to
hexachlorobutadiene vapour at concentrations between 21 and
160 mg/m3 for 6 h/day (from days 6 to 20 of pregnancy) or
intraperitoneally to 10 mg/kg body weight per day (from days 1 to 15
of pregnancy), fetuses demonstrated developmental toxicity,
including reduced birth weight, delay in heart development and
dilated ureters, but no gross malformations. The retarded
development was observed at levels which were also toxic to the
dams.
1.7.3 Genotoxicity and carcinogenicity
Hexachlorobutadiene induces gene mutations in the Ames
Salmonella test under special conditions favouring the formation of
glutathione conjugation products. It induced chromosomal aberrations
in one in vivo study but not in two in vitro studies. In one in
vitro test the frequency of sister chromatid exchanges was
increased in Chinese hamster ovary cells. High mutagenic potency by
sulfur metabolites of hexachlorobutadiene was reported. In in vitro
studies, the compound induced unscheduled DNA synthesis in Syrian
hamster embryo fibroblast cultures but not in rat hepatocyte
cultures. It induced unscheduled DNA synthesis in rats in vivo,
but did not induce sex-linked recessive lethal mutations in
Drosophila melanogaster.
In the only long-term (2 years) study, in which rats received a
diet containing hexachlorobutadiene at doses of 0.2, 2 or 20 mg/kg
body weight per day, an increased incidence of renal tubular
neoplasms was observed only at the highest dose level.
1.7.4 Mechanisms of toxicity
The nephrotoxicity, mutagenicity and carcinogenicity of
hexachlorobutadiene is dependent on the biosynthesis of the toxic
sulfur conjugate 1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-
1,3-butadiene (GPB). This conjugate is mainly synthesised in the
liver and is further metabolized in the bile, gut and kidneys to
1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (CPB). The
activation of CPB, dependent on cysteine conjugate ß-lyase, to a
reactive thioketene in the proximal tubular cells finally results in
covalent binding to cellular macromolecules.
1.8 Effects on humans
No pathogenic effects in the general population have been
described.
There have been two reports of disorders among agricultural
workers using hexachlorobutadiene as a fumigant, but they were also
exposed to other substances. An increased frequency of chromosomal
aberrations was found in the lymphocytes of peripheral blood of
workers engaged in the production of hexachlorobutadiene and
reported to be exposed to concentrations of 1.6-12.2 mg/m3.
1.9 Evaluation of human health risks and effects on the environment
1.9.1 Evaluation of human health risks
As there have been very few human studies, the evaluation is
mainly based on studies in experimental animals. However, limited
human in vitro data suggest that the metabolism of
hexachlorobutadiene in humans is similar to that observed in
animals.
Hexachlorobutadiene vapour is considered to be irritating to
the mucous membranes of humans, and the liquid is corrosive. The
compound should also be regarded a sensitizing agent.
The main target organs for toxicity are the kidney and, to a
much lesser extent, the liver. On the basis of short- and long-term
oral studies in rats and mice, the NOAEL is 0.2 mg/kg body weight
per day. In one short-term inhalation study in rats (12 days,
6 h/day), the NOAEL was 53 mg/m3.
Reduced birth weight and neonatal weight gain was observed only
at maternally toxic doses, as was developmental toxicity.
Hexachlorobutadiene has been found to induce gene mutations,
chromosomal aberrations, increased sister chromatid exchanges and
unscheduled DNA synthesis, although some studies have reported
negative results. There is limited evidence for the genotoxicity of
hexachlorobutadiene in animals, and insufficient evidence in humans.
Long-term oral administration of hexachlorobutadiene to rats
was found to induce an increased frequency of renal tubular
neoplasms, but only at a high dose level causing marked
nephrotoxicity. There is limited evidence for carcinogenicity in
animals and insufficient evidence in humans.
On the basis of the NOAEL for mice or rats of 0.2 mg/kg body
weight per day, a NOAEL of 0.03-0.05 mg/kg body weight per day has
been estimated for humans. There is a margin of safety of 150
between the estimated NOAEL and the estimated maximum total daily
intake assuming absorption of the compound via contaminated
drinking-water and food of high lipid content.
1.9.2 Evaluation of effects on the environment
Hexachlorobutadiene is moderately to highly toxic to aquatic
organisms; crustaceans and fish are the most sensitive species. An
environmental concern level of 0.1 µg/litre has been established. It
is estimated that the maximum predicted environmental concentration
away from point sources is twice the extrapolated environmental
concern level and, consequently, aquatic organisms may be at risk in
polluted surface waters. Adverse effects on benthic organisms cannot
be excluded.
Considering the toxicity of hexachlorobutadiene to mammals,
consumption of benthic or aquatic organisms by other species may
cause concern.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL
METHODS
2.1 Identity
Chemical formula: C4Cl6
Chemical structure:
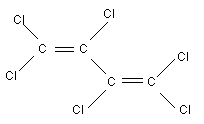 Common name: hexachlorobutadiene
Common synonyms: 1,3-hexachlorobutadiene, 1,1,2,3,4,4-
hexachloro-1,3-butadiene, perchloro-
butadiene
Common trade names: C-46, Dolen-pur, GP40-66: 120, UN2279
Common abbreviation: HCBD
CAS registry number: 87-68-3
RTECS registry number: EJ 0700000
Relative molecular mass: 260.8
2.2 Physical and chemical properties
Hexachlorobutadiene is a non-flammable, incombustible, clear,
colourless and oily liquid at ordinary temperature and pressure. Its
odour is described as turpentine-like. The odour threshold for the
compound in air is reported to be 12 mg/m3 (Ruth, 1986). In water
an odour threshold of 0.006 mg/litre has been reported (US EPA,
1980). The compound is poorly soluble in water but is miscible with
ether and ethanol.
Hexachlorobutadiene is very stable to acid and alkali in the
absence of an appropriate solvent and has no tendency to polymerize
even under high pressure. It reacts with chlorine under severe
reaction conditions, often with cleavage of the carbon skeleton
(Ullmann, 1986).
Some physical and chemical data on hexachlorobutadiene are
presented in Table 1.
Table 1. Some physical and chemical properties of
hexachlorobutadienea
Physical state liquid
Colour clear, colourless
Melting point -18 °C
Boiling point 212 °C at 101.3 kPa
Water solubility 3.2 mg/litre at 25 °Cb
Log n-octanol-water partition
coefficient (Kow) 4.78b, 4.90c
Density 1.68 g/cm3 at 20 °C
Relative vapour density 9.0
Vapour pressure 20 Pa (0.15 mmHg) at 20 °Cd
Autoignition temperature 610 °C
a Unless otherwise stated, the data are selected from secondary
sources.
b Experimentally derived by Banerjee et al. (1980)
c Experimentally derived by Chiou (1985)
d McConnell et al. (1975)
2.3 Conversion factors
1 ppm = 10.67 mg/m3 air at 25 °C and 101.3 kPa (760 mmHg)
1 mg/m3 air = 0.094 ppm.
2.4 Analytical methods
A summary of relevant methods of sampling and gas
chromatographic analysis is presented in Table 2.
The analytical method for air, reported by Dillon (1979) and
Boyd et al. (1981) has been approved by NIOSH and was published in
the NIOSH Manual of Analytical Methods (NIOSH, 1979, 1990).
Table 2. Sampling, preparation and analysis of hexachlorobutadiene
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Air adsorption on Chromosorb gas chromatography 360 litre developed for personal Mann et al.
101; extraction by hexane with electron capture sampling in industry (1974)
detection
Air adsorption on Amberlite gas chromatography 10 µg/m3 3 litre suitable for personal Boyd et al.
XAD-2; extraction by with electron capture and area monitoring; (1981); Dillon
hexane detection validation range (1979)
10-2000 µg/m3
Air adsorption on Tenax-GC; gas chromatography 11 µg/m3 2 litre suitable for Melcher &
purging of water vapour, with flame ionization continuous area Caldecourt
oxygen, etc., by nitrogen; detection monitoring (1980)
desorption by heating
Air adsorption on Tenax-GC; gas chromatography 0.03 µg/m3 a developed for the Krost et al.
desorption by heating (capillary column) analysis of ambient (1982); Pellizari
under a helium flow; with mass air (1982); Barkley
cryofocussing spectro-metric et al. (1980)
detection
Water extraction by hexane; gas chromatography 0.05 µg/litre 16 litre developed for the Oliver & Nicol
concentration; drying with (capillary column) analysis of surface (1982)
Na2SO4; clean-up by silica with electron water
gel chromatography capture detection
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Water extraction by gas chromatography 0.0014 µg/litre 0.8-1 litre US EPA Method Lopez-Avila
dichloro-methane-acetone; with electron capture 8120 et al. (1989)
drying; concentration detection
by N2 stream
Water extraction by gas chromatography 0.001 µg/litre 12 litre developed for Zogorski (1984)
dichloro-methane; with electron capture monitoring of
drying; concentration detection domestic and process
waters
Water extraction by gas chromatography 0.34 µg/litre 1 litre US EPA Method 612; US EPA (1984a)
dichloro-methane; with electron capture developed for the
drying; concentration detection analysis of municipal
and exchange to and industrial
hexane; clean-up by discharges
fluorisil chromatography
Water extraction by gas chromatography 0.9 µg/litre 1 litre US EPA Method 625; US EPA (1984b)
dichloro-methane at pH developed for the
>11, then at pH <2; analysis of municipal
drying; concentration and industrial
discharges
Water purging by helium; gas chromatography 0.4 µg/litre 0.1 litre developed for the Otson & Chan
trapping; desorption by (capillary column) analysis of volatile (1987);
heating with mass organics in waters Eichelberger
spectro-metric et al. (1990)
detection
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Soil, extraction by gas chromatography 0.7 µg/kg Laseter et
sediment acetone-benzene with electron capture wet weight al. (1976)
detection
Soil add water; adjust to pH gas chromatography developed for Kiang & Grob
>12; extraction by (capillary column) screening of soil (1986)
dichloromethane; with flame ionization for priority
centrifugation; drying; and mass pollutants
concentration spectro-metric
detection
Sediment add water; adjust to pH gas chromatography developed for Lopez-Avila et
> 11; extraction by (capillary column) screening of al. (1983)
dichloromethane; with flame/electron sediment for
centrifugation; drying; capture/mass priority pollutants
concentration; spectro-metric
clean-up by silica detection
gel chromatography
Sediment extraction by gas chromatography 13 µg/kga 10-15 g dry Oliver & Nicol
hexane-acetone; removal (capillary column) weight (1982)
of acetone by with electron capture
water extraction; drying; detection
concentration; clean-up
by silica gel
chromatography and
agitation with mercury
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Biota homogenization; filtration; gas chromatography 0.7 µg/kg method applied to Laseter et
separation; extraction by with electron capture analysis of fish al. (1976)
hexane; clean-up by detection
fluorisil chromatography
Biota grind and mix edible gas chromatography 0.005 mg/kg 25 g (eggs) wet weight Yurawecz et
tissue; extraction; with electron capture wet weight 50 g (fish) wet weight al. (1976)
clean-up by fluorisil detection or 0.04 3 g (milk fat)
chromatography mg/kg fat 100 g (vegetables)
wet weight
Biota grinding with Na2SO4; gas chomatography 0.47 µg/kga 15 g method applied to Oliver &
Nicol
extraction by (capillary column) analysis of fish (1982)
hexane-acetone; with electron capture
back-extraction of acetone detection
by water; concentration;
clean-up by silica
gel chromatography
Biota extraction by gas chromatography 1 µg/kg 2 g method applied to Mes et al.
benzene-acetone; with electron capture wet weighta analysis of (1982; 1985;
filtration; concentration; detection chlorinated 1986)
redissolution in hexane; hydrocarbon residues
clean-up including in human adipose
fluorisil-silicic tissue and human milk
acid chromatography
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Biota extraction by hexane gas chromatography 0.0182 µg/litre 100 mg method applied to Kastl & Hermann
containing an internal with electron capture whole (rat) blood (1983)
standard; centrifugation; detection analysis
direct injection
a lowest reported level measured
The method was validated for the concentration range of
10-2000µg/m3 in 3 litre air samples. The lowest detectable
quantity for this method was reported to be 20 ng, the desorption
efficiency 98%, and the relative standard deviation 9%. Melcher &
Caldecourt (1980) described a gas chromatographic method for the
direct determination of organic compounds in air using a collection
precolumn from which the compounds are directly injected into the
analytical column by rapid heating of the precolumn. The method was
reported to be suitable for the analysis of aqueous samples by
purging the precolumn following injection of the sample
(0.01-0.2 cm3). The analytical method developed for volatile
halogenated compounds by Krost et al. (1982) was applied by
Pellizari (1982) and Barkley et al. (1980). Barkley et al.
(1980) also described the analysis of volatile halogenated compounds
in water, blood and urine using a modification of this method: the
substances are recovered from water by heating and from biological
matrices by heating and purging and are subsequently trapped on a
Tenax column.
A spectrophotometric method for the determination of
hexachlorobutadiene in blood and urine has been reported. The method
involves extraction by heptane and determination by either UV
spectroscopy or colorimetry after derivatization with pyridine.
Reported detection limits were 0.05 mg/litre for the UV method and
5 mg/litre for the colorimetric method (Gauntley et al., 1975).
Interference by other chlorinated hydrocarbons can be expected.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Hexachlorobutadiene has not been reported to occur as a natural
product.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
The available data are in general of poor quality and not
up-to-date. Commercial production of hexachlorobutadiene was
reported to occur in Germany and Austria (SRI, 1984). In the USA,
commercial production was apparently terminated around 1970 (Mumma &
Lawless, 1975). The compound was and is chiefly produced as
by-product of the manufacture of chlorinated hydrocarbons, often in
association with hexachlorobenzene. In the USA, the manufacture of
tetrachloroethene, trichloroethene and carbon tetrachloride
accounted in 1972 for over 99% of this production of
hexachlorobutadiene in heavy fractions, the so-called Hex-waste, and
amounted to 3310-6580 tonnes (Brown et al., 1975; Mumma & Lawless,
1975; Yurawecz et al., 1976; see also section 3.2.3). It was also
reported to be a by-product of the manufacture of vinyl chloride,
allyl chloride and epichlorohydrin by chlorinolysis processes (Kusz
et al., 1984). Hexachlorobutadiene has been identified in the
effluents of sewage treatment plants (section 5.2) and as a
by-product of the pyrolysis of trichloro-ethene (Yasuhara & Morita,
1990) and plastics (Singh et al., 1982). The annual world
production of hexachlorobutadiene in heavy fractions was estimated
in 1982 to be 10 000 tonnes (Hutzinger, 1982). No data have been
found regarding the amount of hexachlorobutadiene, if any, which is
now recovered from this waste.
Apart from the possible commercial production of
hexachloro-butadiene by recovery from Hex-waste, three pathways for
chemical synthesis are known: the chlorination and
dehydro-chlorination of hexachlorobutene; the chlorination of
polychlorobutanes; and the catalytic chlorination of butadiene
(Mumma & Lawless, 1975; CESARS, 1981). There is no evidence,
however, that the latter reactions have ever been used commercially.
The fraction of hexachlorobutadiene released to the environment
during its industrial life cycle (not defined) has been estimated to
be between 1 and 3% (SRI, 1984). The fraction of hexachlorobutadiene
lost to the environment during its production at a tetrachloroethene
manufacturing plant in the USA was estimated to be 1.5% (Brown
et al., 1975). Using a simple model describing the troposphere,
the global annual emission rate was calculated to be 3000 tonnes of
hexachlorobutadiene based on air sampling data of 1985 (Class &
Ballschmiter, 1987; see also section 4.2.2).
3.2.2 Uses
Hexachlorobutadiene can be used for the recovery of "snift",
which is chlorine-containing gas in chlorine plants, and as a wash
liquor for removing volatile organic compounds from gas streams. It
can be used as a fluid in gyroscopes, as heat transfer, transformer,
insulating and hydraulic fluids, and as solvent for elastomers. It
can be an intermediate in the manufacture of lubricants and rubber
compounds. In the ex-USSR, the substance was reported to find
widespread application as a fumigant for treating Phylloxera on
grapes, and 600-800 tonnes was used for this purpose in 1975 (Brown
et al., 1975; Mumma & Lawless, 1975).
3.2.3 Waste disposal
Hex-waste containing hexachlorobutadiene may be destroyed by
incineration, placed in landfill, or simply stored. Another
procedure involves recycling the compound by catalytic chlorination
and subsequent high temperature chlorinolysis to carbon
tetrachloride and tetrachloroethene (Markovec & Magee, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The main pathways for entry of hexachlorobutadiene into the
environment are its emission via industrial waste (section 3.2.3)
and following dispersive use (section 3.2.2). The compound may enter
surface and ground water, soil and air. In view of its physical
properties, intercompartmental transport of hexachloro-butadiene is
expected to occur by volatilization and adsorption to suspended
particulate matter.
Considering the vapour pressure of the compound, i.e. 20 Pa at
20 °C (McConnell et al., 1975), transfer across soil-air
boundaries may be significant. Depending on the soil type,
adsorption will hinder this transport (see below). In a field study
in the ex-USSR, concentrations of hexachlorobutadiene in air above a
vineyard were found to be 0.08 and 0.003 mg/m3 at 1 day and 3
months, respectively, following a spring application of 250 kg/ha.
The method of analysis was not reported. Volatilization of the
compound from light soils was more rapid than from heavy soils
(Litvinov & Gorenshtein, 1982).
The Henry coefficient of hexachlorobutadiene is 0.43 (1040
Pa.m3.mol-1) at 25 °C (Shen, 1982) and 0.3 at 22 °C Hellmann,
1987a). These values are comparable to those of other chlorinated
aliphatic alkenes. They indicate possible transfer of the compound
across water-air boundaries leading to a wide distribution, with
aerial transport playing a major role (McConnell et al., 1975). In
a model experiment, hexachlorobutadiene was allowed to evaporate
from a 20-mg/litre aqueous-methanolic solution, containing 10%
methanol, in a porcelain basin with slow magnetic stirring at 22 °C.
UV spectrophotometry recorded a 25% loss within 28 min. It was shown
that methanol decreased the disappearance time. For the transfer of
this and other model results to flowing waters, a reduction factor
of 30 was proposed for the rate of evaporation on the basis of
limited data for two compounds (Hellmann, 1987a).
In a model experiment, UV spectrophotometric analysis of
solutions of hexachlorobutadiene in deionized water to which
1 g/litre of clay mineral (Fuller's earth) was added revealed a
clay-water partition coefficient of 500 litre/kg, showing limited
adsorption to pure clay minerals comparable to that of other
chlorinated alkenes (Hellmann, 1987b). Based on the log
octanol-water partition coefficient (log Kow) of 4.78-4.90
(Table 1), hexa-chlorobutadiene is expected to adsorb strongly to
organic matter. The organic carbon-water partition coefficient
(Koc) can be estimated to be 25 120 litre/kg on the basis of a log
Kow of 4.8 using the semi-empirical equation of Karickhoff (1981).
Oliver & Charlton (1984) determined a Koc value of 158 500
litre/kg on the basis of sediment and water concentrations in the
Niagara River, USA. Partition coefficients of approximately
200-260 litre/kg were found for two unspecified types of soil in
model experiments employing gas chromatographic analysis of
solutions of hexa-chlorobutadiene in water (Leeuwangh et al.,
1975; Laseter et al., 1976). In field experiments conducted along
the Mississippi river in the USA in 1974-1975, some water samples
were found to contain 1.0-1.5 µg/litre, whereas levee soil samples
at the same sites contained 62-1001 µg/kg dry weight. At a more
polluted site near a Hex-waste landfill, water samples contained
0.04-4.6 µg/litre and mud samples 270-2370 µg/kg dry weight. These
studies show that soil-water partition coefficients can range over 2
to 4 orders of magnitude assuming equilibrium (Laseter et al.,
1976). It can be concluded that the compound does not migrate
rapidly in soils and will accumulate in sediment. It should be noted
that the micro-particles onto which hexachlorobutadiene is absorbed
may themselves migrate in the sub-surface resulting in facilitated
transport. The degree of adsorption to soil is highly dependent on
the content of organic matter and is less pronounced in sandy soils.
On the basis of data for Dutch surface waters, the half-lives
of hexachlorobutadiene were estimated to be 3-30 days in rivers and
30-300 days in lakes and ground water. This suggests that
turbulence, and therefore increased aerobic biodegradation,
volatilization and adsorption, account for the shorter half-lives in
river water, that the compound is difficult to degrade both
biologically and chemically (see below), and that, overall, the
compound is persistent in water (Zoeteman et al., 1980).
4.2 Abiotic degradation
4.2.1 Photolysis
Hexachlorobutadiene absorbs light within the solar spectrum.
Irradiation of a solution of hexachlorobutadiene in benzene at
254 nm for 15 min resulted in the formation of numerous products
having a relative molecular mass greater than that of
hexachloro-butadiene itself (Laseter et al., 1976). The extent of
mineralization of the compound adsorbed to silica gel and exposed to
oxygen was examined following irradiation with ultraviolet light
filtered by quartz (wavelength < 290 nm) or by pyrex (simulating
tropospheric UV with a wavelength > 290 nm). After 6 days, 50-90%
mineralization to hydrogen chloride and/or chlorine, and carbon
dioxide was observed (Gb et al., 1977). These experiments indicate
that hexachlorobutadiene present as a virtual monolayer on silica
gel undergoes quite rapid photolysis.
4.2.2 Photooxidation
Using a steady-state mathematical model for the troposphere
(describing it as 2 boxes one north one south of the equator) and on
the basis of gas chromatographic analysis of air samples from sites
far away from anthropogenic sources, the tropospheric lifetime of
hexachlorobutadiene was estimated to be 2.3 years for the northern
hemisphere and 0.8 years for the southern hemisphere. It was assumed
that the reaction with hydroxyl radicals in the troposphere is the
main sink for hexachloro-butadiene, by analogy with other
halocarbons. The calculated lifetimes at -8 °C correspond to a
pseudo-first order rate constant of (2 ± 1) x 10-14
cm3.molecules-1.sec-1 at estimated hydroxyl radical
concentrations of 7 x 105 molecules.cm-3 for the northern
hemisphere and 17 x 105 for the southern hemisphere (Class &
Ballschmiter, 1987). Experimentally, a half-life of 1 week was
determined when hexachlorobutadiene was exposed to air in flasks
outdoors. This relatively short disappearance time was possibly due
to heterogeneous reactions on the vessel walls, as suggested by the
authors of the report. Hydrogen chloride was found to be the main
degradation product after exposure of samples to xenon arc
radiations (wavelength > 290 nm) (Pearson & McConnell, 1975).
4.2.3 Hydrolysis
Hexachlorobutadiene is highly resistant to chemical degradation
by strong acids and alkalis in the absence of appropriate solvents,
although it is readily degraded by ethanolic alkali (Roedig &
Bernemann, 1956). Based on the measured hydrolysis rate of the
compound in a 1:1 acetone-water mixture, a half-life of over 1800 h
was calculated (Hermens et al., 1985).
4.3 Biodegradation
Hexachlorobutadiene, at concentrations of 5 or 10 mg/litre, was
completely degraded by adapted aerobic microorganisms within 7 days
in a static-culture flask screening procedure at 25 °C, as shown by
gas chromatography and by determination of total and dissolved
organic carbon. The inoculum was taken from settled domestic waste
water (Tabak et al., 1981). Approximately 70% adsorption to sludge
and 10% degradation was found to occur within 8 days in a pilot
low-loaded biological sewage treatment plant (Schröder, 1987).
Anaerobic degradation of hexachlorobutadiene at 100 mg/litre
was not observed in 48-h batch assays at 37 °C using an inoculum
from a laboratory digester (Johnson & Young, 1983).
4.4 Bioaccumulation
Considering the low water solubility of 3.2 mg/litre and the
high log Kow of 4.78-4.90 (Table 1), a strong bioaccumulating
potential would be expected. Both laboratory and field data support
this prediction. In flow-through laboratory tests with algae,
crustaceans, molluscs and fish in fresh or marine waters,
bioconcentration factors (on a wet weight basis) were between 71 and
17 000. The results appear to be highly dependent on the exposure
period and there is great variability between organisms (Leeuwangh
et al., 1975; Pearson & McConnell, 1975; Laseter et al., 1976;
Oliver & Niimi, 1983). Steady state was clearly demonstrated to be
reached in only one of these tests. Oliver & Niimi (1983) exposed
rainbow trout (Salmo gairdnerii) to aqueous solutions of
hexachlorobutadiene at 0.10 and 3.4 ng/litre and found average
bioconcentration factors of 5800 and 17 000, steady states having
been reached after 69 and 7 days, respectively. When Oligochaete
worms were exposed via spiked Lake Ontario sediments to a pore water
concentration of 32 ng/litre in a flow-through system, steady state
was reached within 4 to 11 days and the average bioconcentration
factor was 29 000, based on dry weight of which about 8% is lipid
(Oliver, 1987). Biomagnifi-cation, the concentrating of a substance
through a food chain, was not observed for hexachlorobutadiene in
two limited laboratory experiments with fish fed contaminated food
(Pearson & McConnell, 1975; Laseter et al., 1976).
The bioaccumulation factors found in plankton, crustaceans,
molluscs, insects and fish in surface waters are comparable to those
observed in the laboratory: available bioaccumulation factors based
on wet weight range between 33 and 11 700 (Goldbach et al., 1976;
Laseter et al., 1976). No biomagnification was observed when
levels in fish were compared with those of detritus and several
invertebrates (Goldbach et al., 1976). The latter was confirmed by
a trophodynamic analysis in the Lake Ontario ecosystem (Oliver &
Niimi, 1988).
Limited bioaccumulation of hexachlorobutadiene was observed in
the fat of rats following exposure for 4 to 12 weeks to a mixture of
this compound and 1,2,3,4-tetrachlorobenzene, hexachloroben-zene,
1,3,5-trichlorobenzene, o-dichlorobenzene and
gamma-hexa-chlorocyclohexane in food (each compound at 2 or 4 mg/kg
body weight per day). Fat concentrations of up to 8 mg/kg were
observed at the higher dose rates (Jacobs et al., 1974).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Concentrations of hexachlorobutadiene measured in air at
different locations are summarized in Table 3.
5.1.2 Water
Concentrations of hexachlorobutadiene measured in water at
different locations are summarized in Table 4.
5.1.3 Soil and sediment
Concentrations of hexachlorobutadiene measured in soil and
sediment at different locations are summarized in Table 5.
5.1.4 Biota
Concentrations of hexachlorobutadiene measured in aquatic
organisms, birds and mammals are summarized in Table 6.
5.2 General population exposure
Levels of hexachlorobutadiene encountered in the food and
drinking-water of the general population are summarized in Table 7.
Hexachlorobutadiene was not detected in the urine or blood of
nine individuals living near Old Love Canal, USA, whereas trace
levels were found in the breath of one of them (Barkley et al.,
1980). In another investigation the compound could not be detected
in the blood of 36 Love Canal area residents (Bristol et al.,
1982). Hexachlorobutadiene was found at levels of 0.8-4 µg/kg wet
weight (fat) and 1.2-13.7 µg/kg wet weight (liver) in postmortem
tissues from 6 out of 8 United Kingdom residents in 1970 (McConnell
et al., 1975). In the adipose tissue of accident victims in Canada
(1976), levels of 1 to 8 µg/kg wet weight were measured in 128 out
of 135 samples (Mes et al., 1982, 1985). In Canada (1982),
hexachlorobutadiene could not be detected in any of 210 samples of
breast milk (Mes et al., 1986).
When 15 samples of hazardous waste from incineration facilities
in the USA were analysed, 4 sites were found to contain
hexa-chlorobutadiene, but the levels were reported to be below
10 mg/kg (Demarini et al., 1987). In sewage sludge, Alberti &
Ploger (1986) measured levels of below 1 µg/kg dry weight (3 samples
of municipal or municipal/industrial sludge), up to 0.6 µg/kg dry
weight (1 sample of municipal/industrial sludge), and 15 µg/kg dry
weight (1 sample of industrial sludge).
5.3 Occupational exposure
Hexachlorobutadiene levels of 1.6-12.2 mg/m3 air have been
measured in the workplace, resulting in reported urine levels of up
to 20 mg/litre in workers at the end of the day (German & Viter,
1985).
Table 3. Levels of hexachlorobutadiene in environmental air
Type of Year Location Detection Levels determineda Reference
air limit (ng/m3) (ng/m3)
Ambient 1985 Atlantic Ocean, lower 0.0001-0.0004 (r) Class & Ballschmiter
troposphere in a south-north 0.0003 (m, north) (1987)
cross section, 8 sites 0.0001 (m, south)
Urban 1978 USA, Niagara Falls, inside nd (n=9) Barkley et al. (1980)
homes near dump site
USA, Niagara Falls, outside nd (n=6)
homes near dump site trace (n=3)
USA, Niagara Falls area nd (n=3)
trace (n=1)
50-390 (r, n=2)
Urban 1980- USA, 7 cities nd-117 (r,m) Singh et al. (1982)
1981 nd-251 (r)
Table 3 (contd).
Type of Year Location Detection Levels determineda Reference
air limit (ng/m3) (ng/m3)
Polluted 1975 USA, 9 sites with chemical nd-460 000 (r) Li et al. (1976)b
industries, on plant property
USA, 9 sites with chemical nd-22 000 (r)
industries, off plant property
Polluted 1978 USA, Niagara Falls, household < 45 (n=1) Barkley et al.
basement near dump site (1980)
Polluted 1978 idem 30-410 (r, n=4) Pellizari (1982)
Polluted 1982 USA, liquid waste lagoon 2 nd (n=2) Guzewich et al.
3-160 (n=4) (1983)
a nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; n = number of samples
b The highest levels were associated with the production of tetrachloroethene and trichloroethene. At other plants,
levels of hexachlorobutadiene remained below 3 ng/m3. Waste holding areas (especially when involving open storage)
were often the most significant sources of hexachlorobutadiene, contaminated soil being a secondary source. The
total number of samples examined was 405.
Table 4. Levels of hexachlorobutadiene in environmental water
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface Canada, Niagara River 50 1.5 Oliver & Nicol (1982)
Surface 1982 Canada, Niagara River 0.82 (m, n=5) Oliver & Charlton (1984)
Surface 1981-1983 Canada, Niagara River 0.01 0.78 (m, n=104) Oliver & Nicol (1984)
0.67 (median)
0.27-3.2 (r)
Surface 1981 Canada, Niagara River nd-0.6 (n=1) Fox et al. (1983)
Surface 1972-1973 Netherlands, River IJssel, 50-130 (r, n=5) Goldbach et al. (1976)
Ketelmeer, IJsselmeer
Surface 1976-1978 Netherlands, River Rhine 1000-2000 Zoeteman et al. (1980)
Surface 1975 USA, 9 sites with chemical nd-240 000 (r) Li et al. (1976)
industries, on plant property
idem, off plant property nd-23 000 (r)
Table 4 (contd).
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface 1976 Germany, River Rhine, 865 km 10 10 (50-percentile) Alberti (1983)
180 (90-percentile)
1978 Germany, idem 10 20 (50-percentile)
60 (90-percentile)
1981 Germany, idem 10 < 10 (50-percentile)
40 (90-percentile)
1980-1981 Germany, 4 River Rhine 10 nd
tributaries
Germany, River Lippe 10 40-200
Surface 1979-1981 Germany, River Rhine, < 50 Haberer et al. (1988)
1979-1981 Germany, River Main < 1000
Surface 1983 Netherlands, River Rhine, River Lek < 100 (m, n=52) Meijers (1988)
idem, before dune infiltration 70 (m, n=13)
Table 4 (contd).
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface 1984-1985 Germany, River Rhine 10-20 Petersen (1986)
Germany, River Elbe 10-150
Estuarine USA, Calcasieu River estuary, 1298 Pereira et al. (1988)
vicinity of industrial outfall
Sea 1972-1973 United Kingdom, Liverpool Bay 1 4 (m, n=150) Pearson & McConnell
nd-30 (r) (1975)
Sea 1977 USA, Gulf of Mexico, Sauer (1981)
open ocean 1 nd (n=4)
coast 1 nd-15 (n=4)
Ground Switzerland, aquifer contaminated 200-300 (r) Giger & Schaffner (1981)
water by leachate from a chemical waste
disposal site
a nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; n = number of samples;
x percentile = x percent of samples with values up to that given
Table 5. Levels of hexachlorobutadiene in soil and sediment
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Soil, vineyards infected with Phylloxera < 7300 (8 mo) Vorobyeva (1980)
agricultural and treated at 250 kg/ha < 2990 (32 mo)
Soil 1975 USA, 9 sites with chemical nd-980 000 (r)b Li et al. (1976)
industries, on plant property
idem, off plant property nd-110 (r)b
Sediment 1975 idem, on plant property nd-33 000 (r)b Li et al. (1976)
idem, off plant property nd-40 (r)b
Sediment, United Kingdom, Liverpool Bay < 1 (n=110) Pearson & McConnell
marine > 1 (n=30) (1975)
Sediment, Canada, Niagara Falls 18 Oliver & Nicol (1982)
river/lake
Sediment, 1980 Canada, Lake Ontario nd (n=9) Kaminsky et al. (1983)
lake trace (n=3)
8.7 (n=1)
Table 5 (contd).
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Sediment, 1981 Canada, Niagara River, downstream 9.6-37 (n=5, dwt)c Fox et al. (1983)
river idem, upstream nd (n=1, dwt)
Sediment, 1982 Germany, River Rhine, 707 km 0.002 (dwt) Alberti (1983)
river idem, 815 km 0.005 (dwt)
Sediment, 1981 Canada, Lake Ontario 12-120 (n=5, dwt)
lake
Sediment, 1968-1978 Canada, Niagara Falls sediment nd Durham & Oliver (1983)
lake 1959-1962 core near Niagara River 550
1980-1981 18
1868-1981 nd-550
Sediment, 1980-1982 Canada, lakes 0.04-9.3 (r, n=57) Oliver & Bourbonniere
lake 1980 Canada, Lake Huron 0.08 (m, n=9, dwt) (1985)
1982 Canada, Lake St. Clair 7.3 (m, n=2, dwt)
1982 Canada, Lake Erie 0.2-1.6 (r,m, n=46, dwt)
Sediment, 1982 Canada, Niagara Falls, settling nd (n=1) Oliver & Charlton (1984)
lake particulates at 20 m depth 2.9-11 (r, n=5), 5.9 (m)
idem, settling particulates at
68 m depth 7.4 (m)
bottom sediment 32 (m, n=12)
Table 5 (contd).
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Sediment, lake Canada, Lake Ontario 0.1-75 (r, n=3) Oliver (1984)
Sediment, USA, Eagle Harbour, creosote < 0.79 (m, n=15, dwt) Malins et al. (1985)
sea harbour contaminated sediment, 3 sites
Sediment, USA, President Point, 1 < 2.0 (n=1, dwt)
sea harbour reference site
Sediment USA, Calcasieu River estuary, 85 (bottom) Pereira et al. (1988)
estuarine vicinity of industrial outfall 1.7 (suspended)
a dwt = dry weight; nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; mo = months after treatment;
n = number of samples
b The highest levels were associated with the production of tetrachloroethene and trichloroethene. Waste holding areas (especially when
involving open storage) were often the most significant sources of hexachlorobutadiene, contaminated soil being a secondary source.
c surficial sediment; the sediment concentration increased with fraction size
d surficial sediment
Table 6. Concentrations of hexachlorobutadiene in aquatic organisms, birds and mammals
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Detritus (bottom) 1972-1976 Netherlands, surface water 200 Goldbach et al. (1976)
Detritus (floating) 220
Invertebrates
Plankton 1972-1973 United Kingdom, sea water nd-2.0 Pearson & McConnell (1975)
Ragworm,
Nereis diversicolor 0.06
Mussel,
Mytilus edulis nd-3.8
Crab,
Cancer pagarus nd-1.1
Others nd
Cerastoderma edule
Ostrea edulis
Buccinum undatum
Crepidula fornicata
Carcinus maenus
Eupagurus bernhardus
Crangon crangon
Asterias rubens
Solaster sp.
Echinus esculentus
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Snail 1972-1976 Netherlands, surface water Goldbach et al. (1976)
Lymnaea peregra 30, 1670
Clam,
Sphaerium sp. 2410
Oligochaetes 0.3 (m, n=3)
Oligochaetes 1981 Canada, Lake Ontario nd-37 (dwt) Fox et al. (1983)
Amphipods 7.5-62 (dwt)
Mysids 6 (dwt)
Benthic organisms in 1983-1984 USA, sea water < 5 (dwt) Malins et al. (1985)
stomachs of fish
Clam, 1982-1983 Canada, Great Lakes area Kauss & Hamdy (1985)
E. complanatus nd-83 (r, n=34)
Marine algae 1972-1973 United Kingdom, sea water Pearson & McConnell (1975)
Enteromorpha compressa nd
Ulva lactuca nd
Fucus vesiculosis 8.9
Fucus serratus 0.6
Fucus spiralis 0.6
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Fish
Ray, 1972-1973 United Kingdom, sea water Pearson & McConnell (1975)
Raja clavata (flesh) 0.1-0.4
Raja clavata (liver) 0.2-1.5
Plaice,
Pleuronectes platessa (flesh) nd-0.4
Pleuronectes platessa (liver) 0.2-1.2
Dab,
Limanda limanda (flesh) < 0.1
Limanda limanda (liver) nd
Mackerel,
Scomber scombrus (flesh) nd-2.6
Cod,
Gadus morrhua (flesh) < 0.1
Gadus morrhua (air bladder) 0.35
Others (liver and/or flesh), nd
Platycthus flesus
Solea solea
Aspitrigla cuculus
Trachurus trachurus
Trisopterus luscus
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Trout, USA, Niagara River, Lake 0.47 Oliver & Nicol (1982)
Salmo gairdneri Ontario
Trout 1981 Canada, Lake Ontario 1.3 (dwt) Fox et al. (1983)
Catfish (flesh) 1973 USA, surface water near trace-4600 Yurawecz et al. (1976)
Gaspergoo (flesh) chemical plants manufacturing 200
Buffalo fish (flesh) tetrachloroethene 100
Mullet (flesh) trace
Sea trout (flesh) trace
Sheepshead minnow (flesh) trace
Catfish 1973 USA, < 40 km from tetrachloro- 10-1200 Yip (1976)
Carp ethene or trichloroethene 62
Gaspergoo manufacturing plants 12-30
Buffalo fish 120
Whiting 20
Drum 10
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Pike perch, Goldbach et al. (1976)
Stizostedion lucioperca 1972-1976 Netherlands, Ketelmeer (lake) 440 (m, n=8)
Netherlands, IJsselmeer (lake) 23 (m, n=4)
Perch,
Perca fluviatilis Netherlands, Ketelmeer 130, 400 (n=2)
Pike,
Esox lucius Netherlands, Ketelmeer 260
Tench,
Tinca tinca Netherlands, Ketelmeer 950
Common bream,
Abramis brama Netherlands, Ketelmeer 1520 (m, n=5)
Netherlands, IJsselmeer 33 (m, n=5)
White bream
Blicca bjoerkna Netherlands, Ketelmeer 360 (m, n=3)
Roach,
Rutilis rutilis Netherlands, Ketelmeer 910 (m, n=10)
Netherlands, IJsselmeer 61 (m, n=4)
Eel,
Anguilla anguilla Netherlands, IJsselmeer 33 (m, n=4)
Smelt
Osmerus eperlanus Netherlands, IJsselmeer 43 (m, n=3)
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
English sole (liver) 1983-1984 USA, sea water < 9 (dwt) Malins et al. (1985)
English sole (muscle) < 0.2 (dwt)
Catfish USA, vicinity of industrial 46 000-120 000 Pereira et al. (1988)
outfall in Calcasieu River (lipid base)
estuary
Atlantic croaker idem, in Calcasieu River 41 000 (lipid base)
Blue crab 12 000 (lipid base)
Spotted sea trout 15 000 (lipid base)
Blue catfish 46 000 (lipid base)
Coho salmon 1980 USA, Great Lakes nd (n=31) Clark et al. (1984)
trace-10 (r, n=5)
Several species 1983 USA, 14 Lake Michigan nd Camanzo et al. (1987)
tributaries and embayments
Birds
Guillemot, 1972-1973 United Kingdom Pearson & McConnell (1975)
Uria aalge (eggs) 1.6-9.9
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Swan, Pearson & McConnell (1975)
Cygnus olor (liver) 5.2
Cygnus olor (kidney) nd
Moorhen, 1972-1973 United Kingdom Pearson & McConnell (1975)
Gallinula chloropus (liver) 0.8
Gallinula chloropus (muscle) 2.6
Gallinula chloropus (eggs) nd
Others nd
Sula bassana (liver, eggs)
Phalacrocerax aristotelis (eggs)
Alca torda (eggs)
Rissa tridactyla (eggs)
Anas platyrhyncos (eggs)
Mammals 1972-1973 United Kingdom Pearson & McConnell (1975)
Grey seal,
Halichoerus grypus (blubber) 0.4-3.6
Halichoerus grypus (liver) nd-0.8
Common shrew,
Sorex araneus nd
a dwt = dry weight; r = range of individual values; m = mean of individueal values; n = number of samples; nd = not detectable; wwt = wet
weight
Table 7. Levels of hexachlorobutadiene in food and drinking-water
Type of food Year Location Levels determineda Reference
or drinking-water (µg/kg wwt or µg/litre)
Tap water 1978 USA, houses bordering Old nd-trace (r, n=3) Barkley et al. (1980)
Love Canal, Niagara Falls 0.06-0.17 (r, n=6)
Well water 1978 USA, Tennessee, contaminated nd-2.53 (r, n=28) Clark et al. (1982)
by leachate from waste dump 0.15 (m, n=22)
Fresh milk United Kingdom 0.08 McConnell et al. (1975)
Butter 2
Cheese, eggs nd
Meat (3 types) nd
Oils/fats (4 out of 5 types) nd
Vegetable cooking oil 0.2
Beverages (5 out of 6 types) nd
Light ale 0.2
Fruits/vegetables (5 out of 7 types) nd
Tomatoes United Kingdom, reclaimed lagoon 0.8
Black grapes United Kingdom, import 3.7
Table 7 (contd).
Type of food Year Location Levels determineda Reference
or drinking-water (µg/kg wwt or µg/litre)
Fresh bread United Kingdom nd
Eggs 1973 USA, < 40 km from tetrachloro- nd (n=15) Yip (1976)
Milk ethylene or trichloroethylene nd (n=19)
manufacturing plants, 6-7 sites 1320 (n=1, fat basis)
Vegetables (7 types) nd (n=20)
Condensed milk 1975 Germany, Bonn 4 Kotzias et al. (1975)
Milk (products) (2 types) nd
Eggs (white) nd
Eggs (yolk) 42
Meats (4 types) nd
Tinned fish (2 types) nd
Onion bread nd
Chicken feed 39
Chicken meal 2
a nd = not detected; m = mean of individual values; n = number of samples; r = range; wwt = wet weight
6. KINETICS AND METABOLISM
6.1 Absorption and distribution
Whole body autoradiography of longitudinal sagittal sections of
male rats after administration of a single oral dose of 200 mg
uniformly labelled hexachlorobutadiene/kg body weight in corn oil
demonstrated that intestinal absorption of the parent compound was
virtually complete by 16 h. The radioactivity in the
gastrointestinal tract at this point in time was mainly due to
water-soluble metabolites, whereas 85% of the radioactivity in the
small intestine was still present as unchanged hexachlorobutadiene
4 h after the administration. At all points in time radioactivity
levels in the stomach were low compared to those in the intestines.
The autoradiogram showed a specific distribution of radioactivity,
especially in the outer medulla of the kidney (Nash et al., 1984).
Reichert et al. (1985) orally administered 1 or 50 mg of
labelled hexachlorobutadiene/kg body weight in tricaprylin to female
rats and recovered, at 72 h, approximately 7% of the label in
carcass and tissues, mainly liver, brain and kidneys. Most of the
label was excreted via urine or faeces within this time period
(section 6.4). In mice given 30 mg of labelled hexachlorobutadiene
per kg body weight in corn oil, over 85% of the label was excreted
within 72 h (section 6.4); 6.7-13.6% was found in the carcass,
especially in adipose tissue (Dekant et al., 1988a). This report
on mice supports the study by Reichert et al. (1985) on rats with
respect to the amount of labelled hexachlorobutadiene absorbed.
6.2 Metabolism
The extent of metabolic transformation and the identity of
excretion products found in studies with rodents are summarized in
Table 8. The available evidence suggests that hexachloro-butadiene
is metabolized in a glutathione-dependent reaction to toxic sulfur
metabolites. The glutathione- S-conjugate 1-(glutathion-S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (GPB) is formed in the liver and
excreted with bile. GPB is reabsorbed from the gut both intact and
after degradation to 1-(cystein- S-yl)-1,2,3,4,4-pentachloro-
1,3-butadiene (CPB). Finally, these sulfur conjugates and the
corresponding mercapturic acid 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB) are delivered to the
kidney. In the kidney, high concentrations of CPB are present due to
renal accumulation, enzymes with acylase activity and
gamma-glutamyltranspeptidase. CPB is finally cleaved by renal
cysteine conjugate ß-lyase to the electrophile
trichlorovinyl-chlorothioketene. The renal accumulation of sulfur
conjugates and the location of ß-lyase along the nephron (MacFarlane
et al., 1989) explain the organ- and site-specific toxicity of
hexachlorobutadiene (Lock, 1987a,b; Anders et al., 1987; Dekant
et al., 1990a,b; Koob & Dekant, 1991).
Table 8. Tracer studies with [14C] hexachlorobutadiene
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
Rat ip 0.1 urine total 29 48 Davis et al. (1980)
water-soluble 25 48
faeces total 40 48
300.1 urine total 7 48
water-soluble 6 48
faeces total 7 48
Rat oral 200 urine total 11 120 Nash et al. (1984)
PBSA 1 120
non-ether soluble 7 120
faeces total 39 120
Rat oral 1 expired air total 8.9 72 Reichert et al. (1985)
HCBD 5.3 72
CO2 3.6 72
urine total 30.6 72
faeces total 42.1 72
50 expired air total 6.6 72
HCBD 5.4 72
Table 8 (contd).
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
CO2 1.2 72
urine total 11.0 72
faeces total 69 72
Rat oral 100 urine total 5.4 24 Reichert et al. (1985);
S-containing ca 4.3 24 Reichert & Schutz (1986)
ACPB }
MTPB } 0.5 24
CMTPB}
faeces total 60 72
oral 1 expired air total 7.45 72
C2 2.2
urine total 17.5
faeces & gitb total 61.8
carcass total 10.5
100 expired air total 7.57 72
CO2 0.7
urine total 9.0
faeces & gitb total 72.1
carcass total 5.8
Table 8 (contd).
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
Rat iv 1 expired air total 8.54 72 Payan et al. (1991)
CO2 2.6
urine total 21.1
faeces & gitb total 59.3
carcass total 12.9
100 expired air total 8.11 72
CO2 0.9
urine total 9.2
faeces & gitb total 71.5
carcass total 11.1
Mouse oral 30 expired air total = HCBD 4.5 72 Dekant et al. (1988a)
urine total 7.2 72
faeces total 72.0 72
HCBD > 57 72
GPB 7.2 72
a For abbreviations see Fig. 1; "total" indicates that no individual chemicals were specified
b git = gastrointestinal tract
6.2.1 In vitro studies
Incubation of hexachlorobutadiene with rat or mouse liver or
kidney subcellular fractions caused a depletion of non-protein
sulfhydryl groups, which was not due to oxidation (Kluwe et al.,
1981).
The formation of GPB and of 1,4-(bis-glutathion- S-yl)-
1,2,3,4-tetrachloro-1,3-butadiene (BGTB) is catalysed by
glutathione- S-transferase in rat and mouse liver microsomes and
cytosol (Wolf et al., 1984; Wallin et al., 1988; Dekant et al.,
1988a,b). GPB formation has also been observed in human liver
microsomes and those from several other species (Oesch & Wolf, 1989;
McLellen et al., 1989). Conjugation in mouse liver microsomes, but
not in those from rat liver, is significantly faster in females than
in males (Wolf et al., 1984; Dekant et al., 1988a).
GPB formation has also been demonstrated in the isolated
perfused rat liver; in this system, GPB formed in the liver was
almost exclusively excreted with bile by a carrier-mediated active
transport mechanism; only after infusing very high concentrations of
hexachlorobutadiene was sinusoidal excretion of GPB into the
perfusate observed (Gietl & Anders, 1991).
A large number of studies have used GPB, CPB and ACPB to
further delineate the fate of hexachlorobutadiene in the organism.
These studies have demonstrated that CPB is the penultimate
intermediate in hexachlorobutadiene metabolism. CPB is a substrate
for renal cysteine conjugate ß-lyase and is metabolized by this
enzyme to 2,3,4,4-tetrachlorobutenoic acid and
2,3,4,4-tetrachlorothionobutenoic acid (Dekant et al., 1988a).
Trichloro-vinyl-chlorothioketene has been identified as the ultimate
reactive intermediate in hexachlorobutadiene metabolism catalysed by
ß-lyase (Dekant et al., 1991). ACPB accumulated by the renal
organic anion transporter is cleaved to CPB by renal acylases
(Vamvakas et al., 1987; Pratt & Lock, 1988).
6.2.2 In vivo studies
In in vivo studies, hexachlorobutadiene caused a marked,
dose-related depletion of renal nonprotein sulfhydryl (NP-SH) in
mice at single intraperitoneal doses of 33-50 mg/kg body weight but
little or no decrease in hepatic NP-SH (Kluwe et al., 1981; Lock
et al., 1984). This pattern was also observed in female rats at
single intraperitoneal doses from 300 mg/kg body weight (Hook
et al., 1983). Conversely, the compound caused a marked,
dose-related depletion of hepatic NP-SH in male rats from 300 mg/kg
body weight intraperitoneally, but no decrease (or even an increase)
in renal NP-SH (Kluwe et al., 1981, 1982; Lock & Ishmael, 1981;
Baggett & Berndt, 1984).
When cannulated male rats were given intravenously either a
tracer dose of 0.071 mg radiolabelled hexachlorobutadiene/kg body
weight or the same dose at 24 h after an intraperitoneal nephrotoxic
dose of 300 mg/kg body weight in corn oil, 13 and 10% of the label
was recovered in the bile, respectively, within the 3 h following
the tracer dose. The labelled material was completely water soluble
(Davis et al., 1980).
In a study by Payan et al. (1991), rats with cannulated bile
ducts received once, either orally or intravenously, 1 or 100 mg of
radiolabelled hexachlorobutadiene/kg body weight. At 72 h after
exposure, fractional urinary excretion (7.5% of the dose) was
independent of the dose and route of administration, in contrast to
the situation in intact rats (see section 6.4). Fractional biliary
excretion decreased with increasing dose following oral
administration (66.8% versus 58%) and intravenous injection (88.7%
versus 72%). Fractional faecal excretion was minimal following
intravenous injection (3.1% following the low oral dose and 16.2%
following the high oral dose). In a group of bile duct-duodenum
cannula-linked rats given one dose of 100 mg/kg body weight, all
tissue concentrations (kidney, liver, plasma, carcass) and the
urinary excretions at 30 h after dosing were higher in bile donor
rats than in recipient rats. The biliary contribution to both
urinary and tissue concentrations was calculated to be 40%. Of the
biliary metabolites entering the recipients, 80% was found to be
reabsorbed.
Nash et al. (1984) administered 200 mg labelled
hexachloro-butadiene in corn oil/kg body weight to male rats with
exteriorized bile flow. They recovered 35% of the label in the bile
during the 48 h following treatment, 40% of which was identified as
GPB (Fig. 1) and 12% as CPB. In another investigation into the
identity of biliary excretion products, male rats were given
intravenously an aqueous suspension of 0.026 mg of labelled
hexachlorobutadiene. During the next two hours over 30% of the label
was recovered in bile; 35% of this radioactivity was identified as
GPB and 6% as BGTB (Fig. 1), but the remaining labelled material was
not identified. Since some of the unidentified peaks disappeared
after treatment of bile with inhibitors of
gamma-glutamyltranspeptidase, they probably represent degradation
products of GPB and BGTP (Jones et al., 1985).
Common name: hexachlorobutadiene
Common synonyms: 1,3-hexachlorobutadiene, 1,1,2,3,4,4-
hexachloro-1,3-butadiene, perchloro-
butadiene
Common trade names: C-46, Dolen-pur, GP40-66: 120, UN2279
Common abbreviation: HCBD
CAS registry number: 87-68-3
RTECS registry number: EJ 0700000
Relative molecular mass: 260.8
2.2 Physical and chemical properties
Hexachlorobutadiene is a non-flammable, incombustible, clear,
colourless and oily liquid at ordinary temperature and pressure. Its
odour is described as turpentine-like. The odour threshold for the
compound in air is reported to be 12 mg/m3 (Ruth, 1986). In water
an odour threshold of 0.006 mg/litre has been reported (US EPA,
1980). The compound is poorly soluble in water but is miscible with
ether and ethanol.
Hexachlorobutadiene is very stable to acid and alkali in the
absence of an appropriate solvent and has no tendency to polymerize
even under high pressure. It reacts with chlorine under severe
reaction conditions, often with cleavage of the carbon skeleton
(Ullmann, 1986).
Some physical and chemical data on hexachlorobutadiene are
presented in Table 1.
Table 1. Some physical and chemical properties of
hexachlorobutadienea
Physical state liquid
Colour clear, colourless
Melting point -18 °C
Boiling point 212 °C at 101.3 kPa
Water solubility 3.2 mg/litre at 25 °Cb
Log n-octanol-water partition
coefficient (Kow) 4.78b, 4.90c
Density 1.68 g/cm3 at 20 °C
Relative vapour density 9.0
Vapour pressure 20 Pa (0.15 mmHg) at 20 °Cd
Autoignition temperature 610 °C
a Unless otherwise stated, the data are selected from secondary
sources.
b Experimentally derived by Banerjee et al. (1980)
c Experimentally derived by Chiou (1985)
d McConnell et al. (1975)
2.3 Conversion factors
1 ppm = 10.67 mg/m3 air at 25 °C and 101.3 kPa (760 mmHg)
1 mg/m3 air = 0.094 ppm.
2.4 Analytical methods
A summary of relevant methods of sampling and gas
chromatographic analysis is presented in Table 2.
The analytical method for air, reported by Dillon (1979) and
Boyd et al. (1981) has been approved by NIOSH and was published in
the NIOSH Manual of Analytical Methods (NIOSH, 1979, 1990).
Table 2. Sampling, preparation and analysis of hexachlorobutadiene
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Air adsorption on Chromosorb gas chromatography 360 litre developed for personal Mann et al.
101; extraction by hexane with electron capture sampling in industry (1974)
detection
Air adsorption on Amberlite gas chromatography 10 µg/m3 3 litre suitable for personal Boyd et al.
XAD-2; extraction by with electron capture and area monitoring; (1981); Dillon
hexane detection validation range (1979)
10-2000 µg/m3
Air adsorption on Tenax-GC; gas chromatography 11 µg/m3 2 litre suitable for Melcher &
purging of water vapour, with flame ionization continuous area Caldecourt
oxygen, etc., by nitrogen; detection monitoring (1980)
desorption by heating
Air adsorption on Tenax-GC; gas chromatography 0.03 µg/m3 a developed for the Krost et al.
desorption by heating (capillary column) analysis of ambient (1982); Pellizari
under a helium flow; with mass air (1982); Barkley
cryofocussing spectro-metric et al. (1980)
detection
Water extraction by hexane; gas chromatography 0.05 µg/litre 16 litre developed for the Oliver & Nicol
concentration; drying with (capillary column) analysis of surface (1982)
Na2SO4; clean-up by silica with electron water
gel chromatography capture detection
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Water extraction by gas chromatography 0.0014 µg/litre 0.8-1 litre US EPA Method Lopez-Avila
dichloro-methane-acetone; with electron capture 8120 et al. (1989)
drying; concentration detection
by N2 stream
Water extraction by gas chromatography 0.001 µg/litre 12 litre developed for Zogorski (1984)
dichloro-methane; with electron capture monitoring of
drying; concentration detection domestic and process
waters
Water extraction by gas chromatography 0.34 µg/litre 1 litre US EPA Method 612; US EPA (1984a)
dichloro-methane; with electron capture developed for the
drying; concentration detection analysis of municipal
and exchange to and industrial
hexane; clean-up by discharges
fluorisil chromatography
Water extraction by gas chromatography 0.9 µg/litre 1 litre US EPA Method 625; US EPA (1984b)
dichloro-methane at pH developed for the
>11, then at pH <2; analysis of municipal
drying; concentration and industrial
discharges
Water purging by helium; gas chromatography 0.4 µg/litre 0.1 litre developed for the Otson & Chan
trapping; desorption by (capillary column) analysis of volatile (1987);
heating with mass organics in waters Eichelberger
spectro-metric et al. (1990)
detection
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Soil, extraction by gas chromatography 0.7 µg/kg Laseter et
sediment acetone-benzene with electron capture wet weight al. (1976)
detection
Soil add water; adjust to pH gas chromatography developed for Kiang & Grob
>12; extraction by (capillary column) screening of soil (1986)
dichloromethane; with flame ionization for priority
centrifugation; drying; and mass pollutants
concentration spectro-metric
detection
Sediment add water; adjust to pH gas chromatography developed for Lopez-Avila et
> 11; extraction by (capillary column) screening of al. (1983)
dichloromethane; with flame/electron sediment for
centrifugation; drying; capture/mass priority pollutants
concentration; spectro-metric
clean-up by silica detection
gel chromatography
Sediment extraction by gas chromatography 13 µg/kga 10-15 g dry Oliver & Nicol
hexane-acetone; removal (capillary column) weight (1982)
of acetone by with electron capture
water extraction; drying; detection
concentration; clean-up
by silica gel
chromatography and
agitation with mercury
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Biota homogenization; filtration; gas chromatography 0.7 µg/kg method applied to Laseter et
separation; extraction by with electron capture analysis of fish al. (1976)
hexane; clean-up by detection
fluorisil chromatography
Biota grind and mix edible gas chromatography 0.005 mg/kg 25 g (eggs) wet weight Yurawecz et
tissue; extraction; with electron capture wet weight 50 g (fish) wet weight al. (1976)
clean-up by fluorisil detection or 0.04 3 g (milk fat)
chromatography mg/kg fat 100 g (vegetables)
wet weight
Biota grinding with Na2SO4; gas chomatography 0.47 µg/kga 15 g method applied to Oliver &
Nicol
extraction by (capillary column) analysis of fish (1982)
hexane-acetone; with electron capture
back-extraction of acetone detection
by water; concentration;
clean-up by silica
gel chromatography
Biota extraction by gas chromatography 1 µg/kg 2 g method applied to Mes et al.
benzene-acetone; with electron capture wet weighta analysis of (1982; 1985;
filtration; concentration; detection chlorinated 1986)
redissolution in hexane; hydrocarbon residues
clean-up including in human adipose
fluorisil-silicic tissue and human milk
acid chromatography
Table 2 (contd).
Medium Sampling method Analytical method Detection limit Sample size Comments Reference
Biota extraction by hexane gas chromatography 0.0182 µg/litre 100 mg method applied to Kastl & Hermann
containing an internal with electron capture whole (rat) blood (1983)
standard; centrifugation; detection analysis
direct injection
a lowest reported level measured
The method was validated for the concentration range of
10-2000µg/m3 in 3 litre air samples. The lowest detectable
quantity for this method was reported to be 20 ng, the desorption
efficiency 98%, and the relative standard deviation 9%. Melcher &
Caldecourt (1980) described a gas chromatographic method for the
direct determination of organic compounds in air using a collection
precolumn from which the compounds are directly injected into the
analytical column by rapid heating of the precolumn. The method was
reported to be suitable for the analysis of aqueous samples by
purging the precolumn following injection of the sample
(0.01-0.2 cm3). The analytical method developed for volatile
halogenated compounds by Krost et al. (1982) was applied by
Pellizari (1982) and Barkley et al. (1980). Barkley et al.
(1980) also described the analysis of volatile halogenated compounds
in water, blood and urine using a modification of this method: the
substances are recovered from water by heating and from biological
matrices by heating and purging and are subsequently trapped on a
Tenax column.
A spectrophotometric method for the determination of
hexachlorobutadiene in blood and urine has been reported. The method
involves extraction by heptane and determination by either UV
spectroscopy or colorimetry after derivatization with pyridine.
Reported detection limits were 0.05 mg/litre for the UV method and
5 mg/litre for the colorimetric method (Gauntley et al., 1975).
Interference by other chlorinated hydrocarbons can be expected.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Hexachlorobutadiene has not been reported to occur as a natural
product.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
The available data are in general of poor quality and not
up-to-date. Commercial production of hexachlorobutadiene was
reported to occur in Germany and Austria (SRI, 1984). In the USA,
commercial production was apparently terminated around 1970 (Mumma &
Lawless, 1975). The compound was and is chiefly produced as
by-product of the manufacture of chlorinated hydrocarbons, often in
association with hexachlorobenzene. In the USA, the manufacture of
tetrachloroethene, trichloroethene and carbon tetrachloride
accounted in 1972 for over 99% of this production of
hexachlorobutadiene in heavy fractions, the so-called Hex-waste, and
amounted to 3310-6580 tonnes (Brown et al., 1975; Mumma & Lawless,
1975; Yurawecz et al., 1976; see also section 3.2.3). It was also
reported to be a by-product of the manufacture of vinyl chloride,
allyl chloride and epichlorohydrin by chlorinolysis processes (Kusz
et al., 1984). Hexachlorobutadiene has been identified in the
effluents of sewage treatment plants (section 5.2) and as a
by-product of the pyrolysis of trichloro-ethene (Yasuhara & Morita,
1990) and plastics (Singh et al., 1982). The annual world
production of hexachlorobutadiene in heavy fractions was estimated
in 1982 to be 10 000 tonnes (Hutzinger, 1982). No data have been
found regarding the amount of hexachlorobutadiene, if any, which is
now recovered from this waste.
Apart from the possible commercial production of
hexachloro-butadiene by recovery from Hex-waste, three pathways for
chemical synthesis are known: the chlorination and
dehydro-chlorination of hexachlorobutene; the chlorination of
polychlorobutanes; and the catalytic chlorination of butadiene
(Mumma & Lawless, 1975; CESARS, 1981). There is no evidence,
however, that the latter reactions have ever been used commercially.
The fraction of hexachlorobutadiene released to the environment
during its industrial life cycle (not defined) has been estimated to
be between 1 and 3% (SRI, 1984). The fraction of hexachlorobutadiene
lost to the environment during its production at a tetrachloroethene
manufacturing plant in the USA was estimated to be 1.5% (Brown
et al., 1975). Using a simple model describing the troposphere,
the global annual emission rate was calculated to be 3000 tonnes of
hexachlorobutadiene based on air sampling data of 1985 (Class &
Ballschmiter, 1987; see also section 4.2.2).
3.2.2 Uses
Hexachlorobutadiene can be used for the recovery of "snift",
which is chlorine-containing gas in chlorine plants, and as a wash
liquor for removing volatile organic compounds from gas streams. It
can be used as a fluid in gyroscopes, as heat transfer, transformer,
insulating and hydraulic fluids, and as solvent for elastomers. It
can be an intermediate in the manufacture of lubricants and rubber
compounds. In the ex-USSR, the substance was reported to find
widespread application as a fumigant for treating Phylloxera on
grapes, and 600-800 tonnes was used for this purpose in 1975 (Brown
et al., 1975; Mumma & Lawless, 1975).
3.2.3 Waste disposal
Hex-waste containing hexachlorobutadiene may be destroyed by
incineration, placed in landfill, or simply stored. Another
procedure involves recycling the compound by catalytic chlorination
and subsequent high temperature chlorinolysis to carbon
tetrachloride and tetrachloroethene (Markovec & Magee, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The main pathways for entry of hexachlorobutadiene into the
environment are its emission via industrial waste (section 3.2.3)
and following dispersive use (section 3.2.2). The compound may enter
surface and ground water, soil and air. In view of its physical
properties, intercompartmental transport of hexachloro-butadiene is
expected to occur by volatilization and adsorption to suspended
particulate matter.
Considering the vapour pressure of the compound, i.e. 20 Pa at
20 °C (McConnell et al., 1975), transfer across soil-air
boundaries may be significant. Depending on the soil type,
adsorption will hinder this transport (see below). In a field study
in the ex-USSR, concentrations of hexachlorobutadiene in air above a
vineyard were found to be 0.08 and 0.003 mg/m3 at 1 day and 3
months, respectively, following a spring application of 250 kg/ha.
The method of analysis was not reported. Volatilization of the
compound from light soils was more rapid than from heavy soils
(Litvinov & Gorenshtein, 1982).
The Henry coefficient of hexachlorobutadiene is 0.43 (1040
Pa.m3.mol-1) at 25 °C (Shen, 1982) and 0.3 at 22 °C Hellmann,
1987a). These values are comparable to those of other chlorinated
aliphatic alkenes. They indicate possible transfer of the compound
across water-air boundaries leading to a wide distribution, with
aerial transport playing a major role (McConnell et al., 1975). In
a model experiment, hexachlorobutadiene was allowed to evaporate
from a 20-mg/litre aqueous-methanolic solution, containing 10%
methanol, in a porcelain basin with slow magnetic stirring at 22 °C.
UV spectrophotometry recorded a 25% loss within 28 min. It was shown
that methanol decreased the disappearance time. For the transfer of
this and other model results to flowing waters, a reduction factor
of 30 was proposed for the rate of evaporation on the basis of
limited data for two compounds (Hellmann, 1987a).
In a model experiment, UV spectrophotometric analysis of
solutions of hexachlorobutadiene in deionized water to which
1 g/litre of clay mineral (Fuller's earth) was added revealed a
clay-water partition coefficient of 500 litre/kg, showing limited
adsorption to pure clay minerals comparable to that of other
chlorinated alkenes (Hellmann, 1987b). Based on the log
octanol-water partition coefficient (log Kow) of 4.78-4.90
(Table 1), hexa-chlorobutadiene is expected to adsorb strongly to
organic matter. The organic carbon-water partition coefficient
(Koc) can be estimated to be 25 120 litre/kg on the basis of a log
Kow of 4.8 using the semi-empirical equation of Karickhoff (1981).
Oliver & Charlton (1984) determined a Koc value of 158 500
litre/kg on the basis of sediment and water concentrations in the
Niagara River, USA. Partition coefficients of approximately
200-260 litre/kg were found for two unspecified types of soil in
model experiments employing gas chromatographic analysis of
solutions of hexa-chlorobutadiene in water (Leeuwangh et al.,
1975; Laseter et al., 1976). In field experiments conducted along
the Mississippi river in the USA in 1974-1975, some water samples
were found to contain 1.0-1.5 µg/litre, whereas levee soil samples
at the same sites contained 62-1001 µg/kg dry weight. At a more
polluted site near a Hex-waste landfill, water samples contained
0.04-4.6 µg/litre and mud samples 270-2370 µg/kg dry weight. These
studies show that soil-water partition coefficients can range over 2
to 4 orders of magnitude assuming equilibrium (Laseter et al.,
1976). It can be concluded that the compound does not migrate
rapidly in soils and will accumulate in sediment. It should be noted
that the micro-particles onto which hexachlorobutadiene is absorbed
may themselves migrate in the sub-surface resulting in facilitated
transport. The degree of adsorption to soil is highly dependent on
the content of organic matter and is less pronounced in sandy soils.
On the basis of data for Dutch surface waters, the half-lives
of hexachlorobutadiene were estimated to be 3-30 days in rivers and
30-300 days in lakes and ground water. This suggests that
turbulence, and therefore increased aerobic biodegradation,
volatilization and adsorption, account for the shorter half-lives in
river water, that the compound is difficult to degrade both
biologically and chemically (see below), and that, overall, the
compound is persistent in water (Zoeteman et al., 1980).
4.2 Abiotic degradation
4.2.1 Photolysis
Hexachlorobutadiene absorbs light within the solar spectrum.
Irradiation of a solution of hexachlorobutadiene in benzene at
254 nm for 15 min resulted in the formation of numerous products
having a relative molecular mass greater than that of
hexachloro-butadiene itself (Laseter et al., 1976). The extent of
mineralization of the compound adsorbed to silica gel and exposed to
oxygen was examined following irradiation with ultraviolet light
filtered by quartz (wavelength < 290 nm) or by pyrex (simulating
tropospheric UV with a wavelength > 290 nm). After 6 days, 50-90%
mineralization to hydrogen chloride and/or chlorine, and carbon
dioxide was observed (Gb et al., 1977). These experiments indicate
that hexachlorobutadiene present as a virtual monolayer on silica
gel undergoes quite rapid photolysis.
4.2.2 Photooxidation
Using a steady-state mathematical model for the troposphere
(describing it as 2 boxes one north one south of the equator) and on
the basis of gas chromatographic analysis of air samples from sites
far away from anthropogenic sources, the tropospheric lifetime of
hexachlorobutadiene was estimated to be 2.3 years for the northern
hemisphere and 0.8 years for the southern hemisphere. It was assumed
that the reaction with hydroxyl radicals in the troposphere is the
main sink for hexachloro-butadiene, by analogy with other
halocarbons. The calculated lifetimes at -8 °C correspond to a
pseudo-first order rate constant of (2 ± 1) x 10-14
cm3.molecules-1.sec-1 at estimated hydroxyl radical
concentrations of 7 x 105 molecules.cm-3 for the northern
hemisphere and 17 x 105 for the southern hemisphere (Class &
Ballschmiter, 1987). Experimentally, a half-life of 1 week was
determined when hexachlorobutadiene was exposed to air in flasks
outdoors. This relatively short disappearance time was possibly due
to heterogeneous reactions on the vessel walls, as suggested by the
authors of the report. Hydrogen chloride was found to be the main
degradation product after exposure of samples to xenon arc
radiations (wavelength > 290 nm) (Pearson & McConnell, 1975).
4.2.3 Hydrolysis
Hexachlorobutadiene is highly resistant to chemical degradation
by strong acids and alkalis in the absence of appropriate solvents,
although it is readily degraded by ethanolic alkali (Roedig &
Bernemann, 1956). Based on the measured hydrolysis rate of the
compound in a 1:1 acetone-water mixture, a half-life of over 1800 h
was calculated (Hermens et al., 1985).
4.3 Biodegradation
Hexachlorobutadiene, at concentrations of 5 or 10 mg/litre, was
completely degraded by adapted aerobic microorganisms within 7 days
in a static-culture flask screening procedure at 25 °C, as shown by
gas chromatography and by determination of total and dissolved
organic carbon. The inoculum was taken from settled domestic waste
water (Tabak et al., 1981). Approximately 70% adsorption to sludge
and 10% degradation was found to occur within 8 days in a pilot
low-loaded biological sewage treatment plant (Schröder, 1987).
Anaerobic degradation of hexachlorobutadiene at 100 mg/litre
was not observed in 48-h batch assays at 37 °C using an inoculum
from a laboratory digester (Johnson & Young, 1983).
4.4 Bioaccumulation
Considering the low water solubility of 3.2 mg/litre and the
high log Kow of 4.78-4.90 (Table 1), a strong bioaccumulating
potential would be expected. Both laboratory and field data support
this prediction. In flow-through laboratory tests with algae,
crustaceans, molluscs and fish in fresh or marine waters,
bioconcentration factors (on a wet weight basis) were between 71 and
17 000. The results appear to be highly dependent on the exposure
period and there is great variability between organisms (Leeuwangh
et al., 1975; Pearson & McConnell, 1975; Laseter et al., 1976;
Oliver & Niimi, 1983). Steady state was clearly demonstrated to be
reached in only one of these tests. Oliver & Niimi (1983) exposed
rainbow trout (Salmo gairdnerii) to aqueous solutions of
hexachlorobutadiene at 0.10 and 3.4 ng/litre and found average
bioconcentration factors of 5800 and 17 000, steady states having
been reached after 69 and 7 days, respectively. When Oligochaete
worms were exposed via spiked Lake Ontario sediments to a pore water
concentration of 32 ng/litre in a flow-through system, steady state
was reached within 4 to 11 days and the average bioconcentration
factor was 29 000, based on dry weight of which about 8% is lipid
(Oliver, 1987). Biomagnifi-cation, the concentrating of a substance
through a food chain, was not observed for hexachlorobutadiene in
two limited laboratory experiments with fish fed contaminated food
(Pearson & McConnell, 1975; Laseter et al., 1976).
The bioaccumulation factors found in plankton, crustaceans,
molluscs, insects and fish in surface waters are comparable to those
observed in the laboratory: available bioaccumulation factors based
on wet weight range between 33 and 11 700 (Goldbach et al., 1976;
Laseter et al., 1976). No biomagnification was observed when
levels in fish were compared with those of detritus and several
invertebrates (Goldbach et al., 1976). The latter was confirmed by
a trophodynamic analysis in the Lake Ontario ecosystem (Oliver &
Niimi, 1988).
Limited bioaccumulation of hexachlorobutadiene was observed in
the fat of rats following exposure for 4 to 12 weeks to a mixture of
this compound and 1,2,3,4-tetrachlorobenzene, hexachloroben-zene,
1,3,5-trichlorobenzene, o-dichlorobenzene and
gamma-hexa-chlorocyclohexane in food (each compound at 2 or 4 mg/kg
body weight per day). Fat concentrations of up to 8 mg/kg were
observed at the higher dose rates (Jacobs et al., 1974).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Concentrations of hexachlorobutadiene measured in air at
different locations are summarized in Table 3.
5.1.2 Water
Concentrations of hexachlorobutadiene measured in water at
different locations are summarized in Table 4.
5.1.3 Soil and sediment
Concentrations of hexachlorobutadiene measured in soil and
sediment at different locations are summarized in Table 5.
5.1.4 Biota
Concentrations of hexachlorobutadiene measured in aquatic
organisms, birds and mammals are summarized in Table 6.
5.2 General population exposure
Levels of hexachlorobutadiene encountered in the food and
drinking-water of the general population are summarized in Table 7.
Hexachlorobutadiene was not detected in the urine or blood of
nine individuals living near Old Love Canal, USA, whereas trace
levels were found in the breath of one of them (Barkley et al.,
1980). In another investigation the compound could not be detected
in the blood of 36 Love Canal area residents (Bristol et al.,
1982). Hexachlorobutadiene was found at levels of 0.8-4 µg/kg wet
weight (fat) and 1.2-13.7 µg/kg wet weight (liver) in postmortem
tissues from 6 out of 8 United Kingdom residents in 1970 (McConnell
et al., 1975). In the adipose tissue of accident victims in Canada
(1976), levels of 1 to 8 µg/kg wet weight were measured in 128 out
of 135 samples (Mes et al., 1982, 1985). In Canada (1982),
hexachlorobutadiene could not be detected in any of 210 samples of
breast milk (Mes et al., 1986).
When 15 samples of hazardous waste from incineration facilities
in the USA were analysed, 4 sites were found to contain
hexa-chlorobutadiene, but the levels were reported to be below
10 mg/kg (Demarini et al., 1987). In sewage sludge, Alberti &
Ploger (1986) measured levels of below 1 µg/kg dry weight (3 samples
of municipal or municipal/industrial sludge), up to 0.6 µg/kg dry
weight (1 sample of municipal/industrial sludge), and 15 µg/kg dry
weight (1 sample of industrial sludge).
5.3 Occupational exposure
Hexachlorobutadiene levels of 1.6-12.2 mg/m3 air have been
measured in the workplace, resulting in reported urine levels of up
to 20 mg/litre in workers at the end of the day (German & Viter,
1985).
Table 3. Levels of hexachlorobutadiene in environmental air
Type of Year Location Detection Levels determineda Reference
air limit (ng/m3) (ng/m3)
Ambient 1985 Atlantic Ocean, lower 0.0001-0.0004 (r) Class & Ballschmiter
troposphere in a south-north 0.0003 (m, north) (1987)
cross section, 8 sites 0.0001 (m, south)
Urban 1978 USA, Niagara Falls, inside nd (n=9) Barkley et al. (1980)
homes near dump site
USA, Niagara Falls, outside nd (n=6)
homes near dump site trace (n=3)
USA, Niagara Falls area nd (n=3)
trace (n=1)
50-390 (r, n=2)
Urban 1980- USA, 7 cities nd-117 (r,m) Singh et al. (1982)
1981 nd-251 (r)
Table 3 (contd).
Type of Year Location Detection Levels determineda Reference
air limit (ng/m3) (ng/m3)
Polluted 1975 USA, 9 sites with chemical nd-460 000 (r) Li et al. (1976)b
industries, on plant property
USA, 9 sites with chemical nd-22 000 (r)
industries, off plant property
Polluted 1978 USA, Niagara Falls, household < 45 (n=1) Barkley et al.
basement near dump site (1980)
Polluted 1978 idem 30-410 (r, n=4) Pellizari (1982)
Polluted 1982 USA, liquid waste lagoon 2 nd (n=2) Guzewich et al.
3-160 (n=4) (1983)
a nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; n = number of samples
b The highest levels were associated with the production of tetrachloroethene and trichloroethene. At other plants,
levels of hexachlorobutadiene remained below 3 ng/m3. Waste holding areas (especially when involving open storage)
were often the most significant sources of hexachlorobutadiene, contaminated soil being a secondary source. The
total number of samples examined was 405.
Table 4. Levels of hexachlorobutadiene in environmental water
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface Canada, Niagara River 50 1.5 Oliver & Nicol (1982)
Surface 1982 Canada, Niagara River 0.82 (m, n=5) Oliver & Charlton (1984)
Surface 1981-1983 Canada, Niagara River 0.01 0.78 (m, n=104) Oliver & Nicol (1984)
0.67 (median)
0.27-3.2 (r)
Surface 1981 Canada, Niagara River nd-0.6 (n=1) Fox et al. (1983)
Surface 1972-1973 Netherlands, River IJssel, 50-130 (r, n=5) Goldbach et al. (1976)
Ketelmeer, IJsselmeer
Surface 1976-1978 Netherlands, River Rhine 1000-2000 Zoeteman et al. (1980)
Surface 1975 USA, 9 sites with chemical nd-240 000 (r) Li et al. (1976)
industries, on plant property
idem, off plant property nd-23 000 (r)
Table 4 (contd).
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface 1976 Germany, River Rhine, 865 km 10 10 (50-percentile) Alberti (1983)
180 (90-percentile)
1978 Germany, idem 10 20 (50-percentile)
60 (90-percentile)
1981 Germany, idem 10 < 10 (50-percentile)
40 (90-percentile)
1980-1981 Germany, 4 River Rhine 10 nd
tributaries
Germany, River Lippe 10 40-200
Surface 1979-1981 Germany, River Rhine, < 50 Haberer et al. (1988)
1979-1981 Germany, River Main < 1000
Surface 1983 Netherlands, River Rhine, River Lek < 100 (m, n=52) Meijers (1988)
idem, before dune infiltration 70 (m, n=13)
Table 4 (contd).
Type of Year Location Detection limit Levels determineda Reference
water (ng/litre) (ng/litre)
Surface 1984-1985 Germany, River Rhine 10-20 Petersen (1986)
Germany, River Elbe 10-150
Estuarine USA, Calcasieu River estuary, 1298 Pereira et al. (1988)
vicinity of industrial outfall
Sea 1972-1973 United Kingdom, Liverpool Bay 1 4 (m, n=150) Pearson & McConnell
nd-30 (r) (1975)
Sea 1977 USA, Gulf of Mexico, Sauer (1981)
open ocean 1 nd (n=4)
coast 1 nd-15 (n=4)
Ground Switzerland, aquifer contaminated 200-300 (r) Giger & Schaffner (1981)
water by leachate from a chemical waste
disposal site
a nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; n = number of samples;
x percentile = x percent of samples with values up to that given
Table 5. Levels of hexachlorobutadiene in soil and sediment
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Soil, vineyards infected with Phylloxera < 7300 (8 mo) Vorobyeva (1980)
agricultural and treated at 250 kg/ha < 2990 (32 mo)
Soil 1975 USA, 9 sites with chemical nd-980 000 (r)b Li et al. (1976)
industries, on plant property
idem, off plant property nd-110 (r)b
Sediment 1975 idem, on plant property nd-33 000 (r)b Li et al. (1976)
idem, off plant property nd-40 (r)b
Sediment, United Kingdom, Liverpool Bay < 1 (n=110) Pearson & McConnell
marine > 1 (n=30) (1975)
Sediment, Canada, Niagara Falls 18 Oliver & Nicol (1982)
river/lake
Sediment, 1980 Canada, Lake Ontario nd (n=9) Kaminsky et al. (1983)
lake trace (n=3)
8.7 (n=1)
Table 5 (contd).
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Sediment, 1981 Canada, Niagara River, downstream 9.6-37 (n=5, dwt)c Fox et al. (1983)
river idem, upstream nd (n=1, dwt)
Sediment, 1982 Germany, River Rhine, 707 km 0.002 (dwt) Alberti (1983)
river idem, 815 km 0.005 (dwt)
Sediment, 1981 Canada, Lake Ontario 12-120 (n=5, dwt)
lake
Sediment, 1968-1978 Canada, Niagara Falls sediment nd Durham & Oliver (1983)
lake 1959-1962 core near Niagara River 550
1980-1981 18
1868-1981 nd-550
Sediment, 1980-1982 Canada, lakes 0.04-9.3 (r, n=57) Oliver & Bourbonniere
lake 1980 Canada, Lake Huron 0.08 (m, n=9, dwt) (1985)
1982 Canada, Lake St. Clair 7.3 (m, n=2, dwt)
1982 Canada, Lake Erie 0.2-1.6 (r,m, n=46, dwt)
Sediment, 1982 Canada, Niagara Falls, settling nd (n=1) Oliver & Charlton (1984)
lake particulates at 20 m depth 2.9-11 (r, n=5), 5.9 (m)
idem, settling particulates at
68 m depth 7.4 (m)
bottom sediment 32 (m, n=12)
Table 5 (contd).
Type of soil Year Location Levels determineda Reference
or sediment (µg/kg)
Sediment, lake Canada, Lake Ontario 0.1-75 (r, n=3) Oliver (1984)
Sediment, USA, Eagle Harbour, creosote < 0.79 (m, n=15, dwt) Malins et al. (1985)
sea harbour contaminated sediment, 3 sites
Sediment, USA, President Point, 1 < 2.0 (n=1, dwt)
sea harbour reference site
Sediment USA, Calcasieu River estuary, 85 (bottom) Pereira et al. (1988)
estuarine vicinity of industrial outfall 1.7 (suspended)
a dwt = dry weight; nd = not detectable; r = range of individual values; r,m = range of mean values; m = mean; mo = months after treatment;
n = number of samples
b The highest levels were associated with the production of tetrachloroethene and trichloroethene. Waste holding areas (especially when
involving open storage) were often the most significant sources of hexachlorobutadiene, contaminated soil being a secondary source.
c surficial sediment; the sediment concentration increased with fraction size
d surficial sediment
Table 6. Concentrations of hexachlorobutadiene in aquatic organisms, birds and mammals
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Detritus (bottom) 1972-1976 Netherlands, surface water 200 Goldbach et al. (1976)
Detritus (floating) 220
Invertebrates
Plankton 1972-1973 United Kingdom, sea water nd-2.0 Pearson & McConnell (1975)
Ragworm,
Nereis diversicolor 0.06
Mussel,
Mytilus edulis nd-3.8
Crab,
Cancer pagarus nd-1.1
Others nd
Cerastoderma edule
Ostrea edulis
Buccinum undatum
Crepidula fornicata
Carcinus maenus
Eupagurus bernhardus
Crangon crangon
Asterias rubens
Solaster sp.
Echinus esculentus
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Snail 1972-1976 Netherlands, surface water Goldbach et al. (1976)
Lymnaea peregra 30, 1670
Clam,
Sphaerium sp. 2410
Oligochaetes 0.3 (m, n=3)
Oligochaetes 1981 Canada, Lake Ontario nd-37 (dwt) Fox et al. (1983)
Amphipods 7.5-62 (dwt)
Mysids 6 (dwt)
Benthic organisms in 1983-1984 USA, sea water < 5 (dwt) Malins et al. (1985)
stomachs of fish
Clam, 1982-1983 Canada, Great Lakes area Kauss & Hamdy (1985)
E. complanatus nd-83 (r, n=34)
Marine algae 1972-1973 United Kingdom, sea water Pearson & McConnell (1975)
Enteromorpha compressa nd
Ulva lactuca nd
Fucus vesiculosis 8.9
Fucus serratus 0.6
Fucus spiralis 0.6
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Fish
Ray, 1972-1973 United Kingdom, sea water Pearson & McConnell (1975)
Raja clavata (flesh) 0.1-0.4
Raja clavata (liver) 0.2-1.5
Plaice,
Pleuronectes platessa (flesh) nd-0.4
Pleuronectes platessa (liver) 0.2-1.2
Dab,
Limanda limanda (flesh) < 0.1
Limanda limanda (liver) nd
Mackerel,
Scomber scombrus (flesh) nd-2.6
Cod,
Gadus morrhua (flesh) < 0.1
Gadus morrhua (air bladder) 0.35
Others (liver and/or flesh), nd
Platycthus flesus
Solea solea
Aspitrigla cuculus
Trachurus trachurus
Trisopterus luscus
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Trout, USA, Niagara River, Lake 0.47 Oliver & Nicol (1982)
Salmo gairdneri Ontario
Trout 1981 Canada, Lake Ontario 1.3 (dwt) Fox et al. (1983)
Catfish (flesh) 1973 USA, surface water near trace-4600 Yurawecz et al. (1976)
Gaspergoo (flesh) chemical plants manufacturing 200
Buffalo fish (flesh) tetrachloroethene 100
Mullet (flesh) trace
Sea trout (flesh) trace
Sheepshead minnow (flesh) trace
Catfish 1973 USA, < 40 km from tetrachloro- 10-1200 Yip (1976)
Carp ethene or trichloroethene 62
Gaspergoo manufacturing plants 12-30
Buffalo fish 120
Whiting 20
Drum 10
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Pike perch, Goldbach et al. (1976)
Stizostedion lucioperca 1972-1976 Netherlands, Ketelmeer (lake) 440 (m, n=8)
Netherlands, IJsselmeer (lake) 23 (m, n=4)
Perch,
Perca fluviatilis Netherlands, Ketelmeer 130, 400 (n=2)
Pike,
Esox lucius Netherlands, Ketelmeer 260
Tench,
Tinca tinca Netherlands, Ketelmeer 950
Common bream,
Abramis brama Netherlands, Ketelmeer 1520 (m, n=5)
Netherlands, IJsselmeer 33 (m, n=5)
White bream
Blicca bjoerkna Netherlands, Ketelmeer 360 (m, n=3)
Roach,
Rutilis rutilis Netherlands, Ketelmeer 910 (m, n=10)
Netherlands, IJsselmeer 61 (m, n=4)
Eel,
Anguilla anguilla Netherlands, IJsselmeer 33 (m, n=4)
Smelt
Osmerus eperlanus Netherlands, IJsselmeer 43 (m, n=3)
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
English sole (liver) 1983-1984 USA, sea water < 9 (dwt) Malins et al. (1985)
English sole (muscle) < 0.2 (dwt)
Catfish USA, vicinity of industrial 46 000-120 000 Pereira et al. (1988)
outfall in Calcasieu River (lipid base)
estuary
Atlantic croaker idem, in Calcasieu River 41 000 (lipid base)
Blue crab 12 000 (lipid base)
Spotted sea trout 15 000 (lipid base)
Blue catfish 46 000 (lipid base)
Coho salmon 1980 USA, Great Lakes nd (n=31) Clark et al. (1984)
trace-10 (r, n=5)
Several species 1983 USA, 14 Lake Michigan nd Camanzo et al. (1987)
tributaries and embayments
Birds
Guillemot, 1972-1973 United Kingdom Pearson & McConnell (1975)
Uria aalge (eggs) 1.6-9.9
Table 6 (contd).
Type of biota Year Location Levels determineda Reference
(µg/kg wwt)
Swan, Pearson & McConnell (1975)
Cygnus olor (liver) 5.2
Cygnus olor (kidney) nd
Moorhen, 1972-1973 United Kingdom Pearson & McConnell (1975)
Gallinula chloropus (liver) 0.8
Gallinula chloropus (muscle) 2.6
Gallinula chloropus (eggs) nd
Others nd
Sula bassana (liver, eggs)
Phalacrocerax aristotelis (eggs)
Alca torda (eggs)
Rissa tridactyla (eggs)
Anas platyrhyncos (eggs)
Mammals 1972-1973 United Kingdom Pearson & McConnell (1975)
Grey seal,
Halichoerus grypus (blubber) 0.4-3.6
Halichoerus grypus (liver) nd-0.8
Common shrew,
Sorex araneus nd
a dwt = dry weight; r = range of individual values; m = mean of individueal values; n = number of samples; nd = not detectable; wwt = wet
weight
Table 7. Levels of hexachlorobutadiene in food and drinking-water
Type of food Year Location Levels determineda Reference
or drinking-water (µg/kg wwt or µg/litre)
Tap water 1978 USA, houses bordering Old nd-trace (r, n=3) Barkley et al. (1980)
Love Canal, Niagara Falls 0.06-0.17 (r, n=6)
Well water 1978 USA, Tennessee, contaminated nd-2.53 (r, n=28) Clark et al. (1982)
by leachate from waste dump 0.15 (m, n=22)
Fresh milk United Kingdom 0.08 McConnell et al. (1975)
Butter 2
Cheese, eggs nd
Meat (3 types) nd
Oils/fats (4 out of 5 types) nd
Vegetable cooking oil 0.2
Beverages (5 out of 6 types) nd
Light ale 0.2
Fruits/vegetables (5 out of 7 types) nd
Tomatoes United Kingdom, reclaimed lagoon 0.8
Black grapes United Kingdom, import 3.7
Table 7 (contd).
Type of food Year Location Levels determineda Reference
or drinking-water (µg/kg wwt or µg/litre)
Fresh bread United Kingdom nd
Eggs 1973 USA, < 40 km from tetrachloro- nd (n=15) Yip (1976)
Milk ethylene or trichloroethylene nd (n=19)
manufacturing plants, 6-7 sites 1320 (n=1, fat basis)
Vegetables (7 types) nd (n=20)
Condensed milk 1975 Germany, Bonn 4 Kotzias et al. (1975)
Milk (products) (2 types) nd
Eggs (white) nd
Eggs (yolk) 42
Meats (4 types) nd
Tinned fish (2 types) nd
Onion bread nd
Chicken feed 39
Chicken meal 2
a nd = not detected; m = mean of individual values; n = number of samples; r = range; wwt = wet weight
6. KINETICS AND METABOLISM
6.1 Absorption and distribution
Whole body autoradiography of longitudinal sagittal sections of
male rats after administration of a single oral dose of 200 mg
uniformly labelled hexachlorobutadiene/kg body weight in corn oil
demonstrated that intestinal absorption of the parent compound was
virtually complete by 16 h. The radioactivity in the
gastrointestinal tract at this point in time was mainly due to
water-soluble metabolites, whereas 85% of the radioactivity in the
small intestine was still present as unchanged hexachlorobutadiene
4 h after the administration. At all points in time radioactivity
levels in the stomach were low compared to those in the intestines.
The autoradiogram showed a specific distribution of radioactivity,
especially in the outer medulla of the kidney (Nash et al., 1984).
Reichert et al. (1985) orally administered 1 or 50 mg of
labelled hexachlorobutadiene/kg body weight in tricaprylin to female
rats and recovered, at 72 h, approximately 7% of the label in
carcass and tissues, mainly liver, brain and kidneys. Most of the
label was excreted via urine or faeces within this time period
(section 6.4). In mice given 30 mg of labelled hexachlorobutadiene
per kg body weight in corn oil, over 85% of the label was excreted
within 72 h (section 6.4); 6.7-13.6% was found in the carcass,
especially in adipose tissue (Dekant et al., 1988a). This report
on mice supports the study by Reichert et al. (1985) on rats with
respect to the amount of labelled hexachlorobutadiene absorbed.
6.2 Metabolism
The extent of metabolic transformation and the identity of
excretion products found in studies with rodents are summarized in
Table 8. The available evidence suggests that hexachloro-butadiene
is metabolized in a glutathione-dependent reaction to toxic sulfur
metabolites. The glutathione- S-conjugate 1-(glutathion-S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (GPB) is formed in the liver and
excreted with bile. GPB is reabsorbed from the gut both intact and
after degradation to 1-(cystein- S-yl)-1,2,3,4,4-pentachloro-
1,3-butadiene (CPB). Finally, these sulfur conjugates and the
corresponding mercapturic acid 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB) are delivered to the
kidney. In the kidney, high concentrations of CPB are present due to
renal accumulation, enzymes with acylase activity and
gamma-glutamyltranspeptidase. CPB is finally cleaved by renal
cysteine conjugate ß-lyase to the electrophile
trichlorovinyl-chlorothioketene. The renal accumulation of sulfur
conjugates and the location of ß-lyase along the nephron (MacFarlane
et al., 1989) explain the organ- and site-specific toxicity of
hexachlorobutadiene (Lock, 1987a,b; Anders et al., 1987; Dekant
et al., 1990a,b; Koob & Dekant, 1991).
Table 8. Tracer studies with [14C] hexachlorobutadiene
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
Rat ip 0.1 urine total 29 48 Davis et al. (1980)
water-soluble 25 48
faeces total 40 48
300.1 urine total 7 48
water-soluble 6 48
faeces total 7 48
Rat oral 200 urine total 11 120 Nash et al. (1984)
PBSA 1 120
non-ether soluble 7 120
faeces total 39 120
Rat oral 1 expired air total 8.9 72 Reichert et al. (1985)
HCBD 5.3 72
CO2 3.6 72
urine total 30.6 72
faeces total 42.1 72
50 expired air total 6.6 72
HCBD 5.4 72
Table 8 (contd).
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
CO2 1.2 72
urine total 11.0 72
faeces total 69 72
Rat oral 100 urine total 5.4 24 Reichert et al. (1985);
S-containing ca 4.3 24 Reichert & Schutz (1986)
ACPB }
MTPB } 0.5 24
CMTPB}
faeces total 60 72
oral 1 expired air total 7.45 72
C2 2.2
urine total 17.5
faeces & gitb total 61.8
carcass total 10.5
100 expired air total 7.57 72
CO2 0.7
urine total 9.0
faeces & gitb total 72.1
carcass total 5.8
Table 8 (contd).
Species Route Dose (mg/kg Medium Metabolitea Fraction of Time after Reference
body weight) dose (%) dosing (h)
Rat iv 1 expired air total 8.54 72 Payan et al. (1991)
CO2 2.6
urine total 21.1
faeces & gitb total 59.3
carcass total 12.9
100 expired air total 8.11 72
CO2 0.9
urine total 9.2
faeces & gitb total 71.5
carcass total 11.1
Mouse oral 30 expired air total = HCBD 4.5 72 Dekant et al. (1988a)
urine total 7.2 72
faeces total 72.0 72
HCBD > 57 72
GPB 7.2 72
a For abbreviations see Fig. 1; "total" indicates that no individual chemicals were specified
b git = gastrointestinal tract
6.2.1 In vitro studies
Incubation of hexachlorobutadiene with rat or mouse liver or
kidney subcellular fractions caused a depletion of non-protein
sulfhydryl groups, which was not due to oxidation (Kluwe et al.,
1981).
The formation of GPB and of 1,4-(bis-glutathion- S-yl)-
1,2,3,4-tetrachloro-1,3-butadiene (BGTB) is catalysed by
glutathione- S-transferase in rat and mouse liver microsomes and
cytosol (Wolf et al., 1984; Wallin et al., 1988; Dekant et al.,
1988a,b). GPB formation has also been observed in human liver
microsomes and those from several other species (Oesch & Wolf, 1989;
McLellen et al., 1989). Conjugation in mouse liver microsomes, but
not in those from rat liver, is significantly faster in females than
in males (Wolf et al., 1984; Dekant et al., 1988a).
GPB formation has also been demonstrated in the isolated
perfused rat liver; in this system, GPB formed in the liver was
almost exclusively excreted with bile by a carrier-mediated active
transport mechanism; only after infusing very high concentrations of
hexachlorobutadiene was sinusoidal excretion of GPB into the
perfusate observed (Gietl & Anders, 1991).
A large number of studies have used GPB, CPB and ACPB to
further delineate the fate of hexachlorobutadiene in the organism.
These studies have demonstrated that CPB is the penultimate
intermediate in hexachlorobutadiene metabolism. CPB is a substrate
for renal cysteine conjugate ß-lyase and is metabolized by this
enzyme to 2,3,4,4-tetrachlorobutenoic acid and
2,3,4,4-tetrachlorothionobutenoic acid (Dekant et al., 1988a).
Trichloro-vinyl-chlorothioketene has been identified as the ultimate
reactive intermediate in hexachlorobutadiene metabolism catalysed by
ß-lyase (Dekant et al., 1991). ACPB accumulated by the renal
organic anion transporter is cleaved to CPB by renal acylases
(Vamvakas et al., 1987; Pratt & Lock, 1988).
6.2.2 In vivo studies
In in vivo studies, hexachlorobutadiene caused a marked,
dose-related depletion of renal nonprotein sulfhydryl (NP-SH) in
mice at single intraperitoneal doses of 33-50 mg/kg body weight but
little or no decrease in hepatic NP-SH (Kluwe et al., 1981; Lock
et al., 1984). This pattern was also observed in female rats at
single intraperitoneal doses from 300 mg/kg body weight (Hook
et al., 1983). Conversely, the compound caused a marked,
dose-related depletion of hepatic NP-SH in male rats from 300 mg/kg
body weight intraperitoneally, but no decrease (or even an increase)
in renal NP-SH (Kluwe et al., 1981, 1982; Lock & Ishmael, 1981;
Baggett & Berndt, 1984).
When cannulated male rats were given intravenously either a
tracer dose of 0.071 mg radiolabelled hexachlorobutadiene/kg body
weight or the same dose at 24 h after an intraperitoneal nephrotoxic
dose of 300 mg/kg body weight in corn oil, 13 and 10% of the label
was recovered in the bile, respectively, within the 3 h following
the tracer dose. The labelled material was completely water soluble
(Davis et al., 1980).
In a study by Payan et al. (1991), rats with cannulated bile
ducts received once, either orally or intravenously, 1 or 100 mg of
radiolabelled hexachlorobutadiene/kg body weight. At 72 h after
exposure, fractional urinary excretion (7.5% of the dose) was
independent of the dose and route of administration, in contrast to
the situation in intact rats (see section 6.4). Fractional biliary
excretion decreased with increasing dose following oral
administration (66.8% versus 58%) and intravenous injection (88.7%
versus 72%). Fractional faecal excretion was minimal following
intravenous injection (3.1% following the low oral dose and 16.2%
following the high oral dose). In a group of bile duct-duodenum
cannula-linked rats given one dose of 100 mg/kg body weight, all
tissue concentrations (kidney, liver, plasma, carcass) and the
urinary excretions at 30 h after dosing were higher in bile donor
rats than in recipient rats. The biliary contribution to both
urinary and tissue concentrations was calculated to be 40%. Of the
biliary metabolites entering the recipients, 80% was found to be
reabsorbed.
Nash et al. (1984) administered 200 mg labelled
hexachloro-butadiene in corn oil/kg body weight to male rats with
exteriorized bile flow. They recovered 35% of the label in the bile
during the 48 h following treatment, 40% of which was identified as
GPB (Fig. 1) and 12% as CPB. In another investigation into the
identity of biliary excretion products, male rats were given
intravenously an aqueous suspension of 0.026 mg of labelled
hexachlorobutadiene. During the next two hours over 30% of the label
was recovered in bile; 35% of this radioactivity was identified as
GPB and 6% as BGTB (Fig. 1), but the remaining labelled material was
not identified. Since some of the unidentified peaks disappeared
after treatment of bile with inhibitors of
gamma-glutamyltranspeptidase, they probably represent degradation
products of GPB and BGTP (Jones et al., 1985).
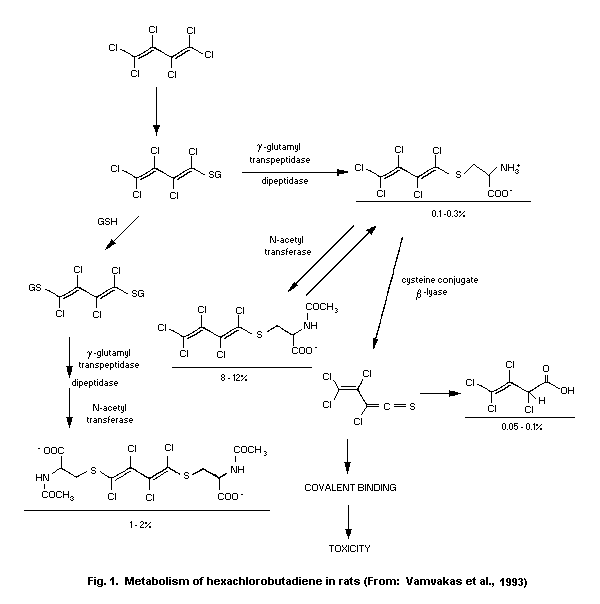 The intestinal absorption of GPB and CPB was studied in rats by
infusing the compounds into the intestine via a biliary cannula.
When GPB was infused, both GPB and CPB were found in the blood in
approximately equal concentrations. Higher blood CPB concentrations
were found after CPB infusion than after GPB infusion (Gietl
et al., 1991).
In studies with radiolabelled hexachlorobutadiene, several
urinary metabolites were identified. The structure of these
metabolites further supported the hypothesis that
hexachloro-butadiene is bioactivated by glutathione conjugation.
ACPB was found to be the main metabolite (representing
approximately 80% of the radioactivity present in urine) excreted
after the administration of [14C] hexachlorobutadiene (200 mg/kg)
in female rats (Reichert & Schütz, 1986). The same authors also
identified 1-carboxymethylthion-1,2,3,4,4-pentachlorobuta-1,3-diene
and 1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) as
urinary metabolites (Reichert et al., 1985). It is the opinion of
the Task Group that the identification of MTPB by diazomethane
treatment of the urinary extract is questionable.
In male rats, 1,2,3,4,4-pentachloro-1,3-butadienyl sulfenic
acid (PBSA) is the only metabolite excreted in urine that has so far
been identified. The data presented suggest that ACPB is not a major
urinary metabolite of hexachlorobutadiene in male rats (Nash
et al., 1984). In urine of mice exposed to radiolabelled
hexachlorobutadiene (30 mg/kg), CPB, ACPB and
2,3,4,4-tetra-chlorobutenoic acid were identified as urinary
metabolites (Dekant et al., 1988a).
It is probable that 2,3,4,4-tetrachlorobutenoic acid is formed
by reaction of the intermediate thioketene with water and further
hydrolysis of the thionol acid thus formed (Dekant et al., 1988a).
The weight of evidence suggests that oxidative reactions
involving cytochrome P-450 have little role in the metabolism of
hexachlorobutadiene (Wolf et al., 1984; Dekant et al., 1988a).
6.3 Reaction with body components
The covalent binding of [14C]-hexachlorobutadiene-related
radioactivity to tissue proteins has been shown to be time
dependent, with the highest level occurring during the first 6 h
after treatment. The half-life of hexachlorobutadiene binding was
22 h in both liver and kidney (Reichert, 1983; Reichert et al.,
1985).
In a DNA binding study, rats received a single oral dose of
20 mg [14C]-hexachlorobutadiene, and DNA was isolated from the
kidneys of these rats at 6, 18.5 and 30 h after dose administration.
Although the results have been reported only in summary form,
various levels of radioactivity were recovered with the DNA, but
there was a marked variation in the level of radioactivity between
samples. Furthermore complete analysis of the DNA was not performed
and protein may have been associated with the DNA (Stott et al.,
1981).
Covalent binding to mouse liver and kidney DNA was demonstrated
after the oral administration of radiolabelled hexachlorobutadiene
(30 mg/kg body weight) in corn oil (Schrenk & Dekant, 1989). In the
liver and kidneys, the binding capacity of mitochondrial DNA was
significantly higher than that of nuclear DNA. The level of binding
to nuclear DNA in the liver was indistinguishable from that of
controls. HPLC separation of the hydrolysed DNA indicated the
presence of three distinct peaks of radioactivity.
6.4 Excretion
Following oral administration in rats and mice of single doses
of hexachlorobutadiene up to 100 mg/kg body weight, the total
excretion within 72 h was at least 65% of the dose. In mice, less
than half of a dose of 30 mg/kg body weight was metabolized (Dekant
et al., 1988a). In rats, assuming that the faeces mostly contain
unchanged compound and no non-resorbed conjugates, 44% of an orally
administered low dose of hexachlorobutadiene (1 mg/kg body weight)
was metabolized (Reichert et al., 1985). At higher doses the
percentage of hexachlorobutadiene metabolized decreased
dramatically. The biotransformation of hexachloro-butadiene in rats
appears to be a saturable process considering the reduced excretion
of carbon dioxide and renal metabolites at increasing doses (Davis
et al., 1980; Reichert et al., 1985; Reichert & Scuhtz, 1986;
Payan et al., 1991). This could be explained by saturation of the
gastrointestinal absorption, which was observed by Reichert et al.
(1985). It should be noted, however, that the observed increase in
the amount of unchanged hexachloro-butadiene in faeces with
increasing dose applies only up to 100 mg/kg body weight. At higher
dose levels, the amount of unchanged hexachlorobutadiene in faeces
decreases, probably due to a decrease in faecal output (Davis
et al., 1980; Nash et al., 1984).
The results of studies of Payan et al. (1991) on bile-duct
cannulated (see section 6.2.2) and intact (see Table 8) rats show
that saturation of gastrointestinal absorption indeed occurs
following oral administration.
Pharmacokinetic data concerning the fate of
hexachloro-butadiene in organisms were not available to the Task
Group.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Short-term toxicity
A summary of short-term aquatic toxicity data is presented in
Table 9. In most of these studies the concentration of
hexachloro-butadiene was not reported. Therefore, the actual effect
concentrations may be lower than the nominal ones. In several cases
these nominal values far exceed the solubility limits. Based on
these data the substance is moderately to highly toxic to aquatic
organisms (Canton et al., 1990).
Adverse effects reported in some of these acute tests included
loss of equilibrium, erratic swimming (Leeuwangh et al., 1975;
Laseter et al., 1976), decreased activity, increased rate of
opercular movement, jumping (Leeuwangh et al., 1975), inverted
positions, fin fibrillation and muscle tetany (Laseter et al.,
1976). In a special investigation into kidney pathology, groups of
five goldfish (Carassius auratus) received one intraperitoneal
injection of hexachlorobutadiene at a dose level of 500 mg/kg body
weight in corn oil. They were subsequently fasted for up to 6 days
and sacrificed at different points in time up to day 7. Controls
received corn oil only. The temperature was kept between 18 and
21 °C. By 24 h the fish showed decreased activity, swam with dorsal
fins down, and had difficulty in following food. By day 4
exophthalmos, distended abdomen and ascites were observed. These
signs of toxicity were all reversible. Relative kidney weights were
elevated on day 4 only. From 12 h after exposure, P2 and P3
renal epithelial cells exhibited marked vacuolation and necrosis,
which persisted up to day 7. Increased gamma-glutamyl-transferase
(EC 2.3.2.2) staining was seen in P2 and P3 segments
(Reimschüssel et al., 1989).
In a report of this experiment, the fish were sacrificed at
different points in time up to day 70 after exposure. In one of the
two experiments the fish also received 5-bromo-2'-deoxyuridine 4 h
prior to sacrifice. Morphometric analysis of developing nephrons
showed an increase in the percentage of volume occupied by
basophilia clusters and developing nephrons from day 14 onwards. The
apparent number of basophilic clusters and developing nephrons per
unit surface area was also increased from day 14 (Reimschüssel
et al., 1990).
Table 9. Short-term aquatic toxicity of hexachlorobutadiene
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fresh water
Algae Haematococcus 20 stat, closed 24 EC10b > 2 Knie et al.
pluvialis (1983)
Bacteria Pseudomonas putida 25 7.0 stat, closed 16 TTc > 25 Bringmann &
Kuhn (1977)
Bacteria Pseudomonas putida 20 7.2 stat, open 0.5 EC10b > 0.9 Knie et al.
(1983)
Protozoans Chilomonas 20 6.9 stat, closed 48 TTc > 10 Bringmann et al.
paramecium (1980)
Molluscs great pond snail, 19 stat,d closed 24 LC50 0.21 Leeuwangh et
Lymnaea stagnalis 96 LC50 0.21 al. (1975)e
Crustaceans water flea, 20 7 250 stat, open 24 EC50 0.5 Knie et al.
Daphnia magna (1983)
aquatic sowbug, 19 stat,d closed 96 LC50 0.13 Leeuwangh et
Asellus aquaticus al. (1975)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fish goldfish, 17.5 stat,d open 96 LC50 0.09 Leeuwangh et
Carassius auratus al. (1975)e
Fish zebrafish, 20 8.0 9.0 180 flow, closed 48 LC50 1 Slooff (1979)
Brachydanio rerio
Fish rainbow trout, LC50 0.320 US EPA (1980)
Salmo gairdnerii
Fish bluegill sunfish, LC50 0.326 US EPA (1980)
Lepomis macrochirus
Fish sheepshead minnow, LC50 0.557 US EPA (1980)
Cyprinodon variegatus
Fish golden orfe, 20 8 270 open 48 LC50 3 Knie et al.
Leuciscus idus (1983)e
Fish fathead minnow, 25 6.7-7.6 8.0 45 flow, open 96 LC50 0.10 Walbridge et
Pimephales promelas al. (1983)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Marine
Crustaceans harpacticoid 21 7.9 > 5 stat, open 96 LC50 1.2 Bengtsson &
copepod Tarkpea (1983)
grass shrimp, LC50 0.032 US EPA (1980)
Palaemonetes pugio
Mysid shrimp, LC50 0.059 US EPA (1980)
Mysidopsis bahia
Fish sailfin molly, 22-24 6.6-7.9 8-9 flow, open 26 LC50 4.2 Laseter et
Poecilia latipinna 30 LC50 4.5 al. (1976)e,f
77 LC50 1.4-1.9
115 LC50 1.7
138 LC50 1.2
pinfish, Lagodon LC50 0.399 US EPA (1980)
rhomboides
a static or flow-through test, open or closed system d semi-static (daily renewal) test
b effect is 10% reduction in oxygen consumption e analysis for hexachlorobutadiene was reported
c TT = toxic threshold for inhibition of cell multiplication f salinity was 0.25%, 96-h LC50 was calculated to be 1.6 mg/litre
7.1.2 Long-term toxicity
The cell multiplication of green algae (Scenedesmus
quadricauda) was not inhibited after 8 days of static exposure to
a nominal concentration of 25 mg/litre (well above pure water
solubility) in a closed system at 27 °C and a pH of 7 (Bringmann &
Kühn, 1977). A 14-day LC50 of 0.4 mg/litre was determined for
2- to 3-month old guppies (Poecilia reticulata) in a semi-static
test using an open system at 22 °C, a water hardness of 25 mg
CaCO3/litre, and a dissolved oxygen concentration of >
5 mg/litre. No analysis for hexachlorobutadiene was reported
(Könemann, 1981). In the same test under the same conditions, but
with analysis for the compound, the 14-day LC50 was 0.16 mg/litre
(Hermens et al., 1985).
In a study by Leeuwangh et al. (1975), groups of six goldfish
(Carassius auratus) were each exposed to hexachlorobutadiene in
tap water at measured concentrations of 0, 0.0003, 0.003, 0.0096 or
0.03 mg/litre for 49 and 67 days. The static test in an open system
was conducted at 19 °C, a pH of 7.6, and a dissolved oxygen
concentration between 3.2 and 6.3 mg/litre. Body weights were
decreased after 49 days at 0.03 mg/litre, and body weight gain was
still reduced at 67 days. Abnormal behaviour, jumping,
incoordination, increased opercular movement and overall immobility
were noted at 0.0096 mg/litre. After 49 days at 0.0096 mg/litre (no
data at 0.03 mg/litre), relative liver weights were increased, and
the activity of liver glucose-6-phosphatase (EC 3.1.3.9) was
decreased, whereas the activity of liver glucose-6-phosphate
dehydrogenase (EC 1.1.1.49) was increased. After 67 days the
activity of liver phenylalanine hydroxylase (EC 1.14.16.1) showed a
concentration-related increase. No effects were found on haemoglobin
concentration and haematocrit after 49 days or on the activity of
serum alanine aminotransferase (EC 2.6.2.1) and serum alkaline
phosphatase (EC 3.1.3.1) after 67 days.
Groups of 12 largemouth bass (Micropterus salmoides) were
each exposed to hexachlorobutadiene for 10 days at measured
concentrations of 0.00343 and 0.03195 mg/litre in filtered tap water
with a salinity of 0.08-0.1%, at 22-24 °C, a pH of 6.6-7.9 and a
dissolved oxygen concentration of 7.6-8.5 mg/litre. A control group
comprised 12 water and 12 vehicle (acetone) controls. Plasma
cortisol levels were increased at both concentrations, but
haematocrit values were not affected. At the higher concentration
there was leukocytic infiltration in the kidneys of one of the fish
and paleness and accentuated lobulation of parenchyma in the livers
(Laseter et al., 1976).
In an early lifestage test, four replicate groups, each of 30
fathead minnow (Pimephales promelas) eggs, 2-4 h after spawning,
were exposed to measured hexachlorobutadiene concentrations in
sand-filtered and sterilized lake water of 0.0017, 0.0032, 0.0065,
0.013 and 0.017 mg/litre in an open system. Following hatching
(4-5 days after spawning), four replicate groups of 15 larvae
continued to be exposed for 28 days. Control groups of equal size
were exposed to slightly contaminated water containing
0.00008 mg/litre. The temperature was 25 °C, pH was 7.4, dissolved
oxygen concentration 7 mg/litre, and water hardness 45 mg
CaCO3/litre. The hatchability of embryos and the percentage of
normal larvae at hatch were not affected. An increased fish
mortality and a concentration-related decrease of body weight were
observed at the two highest concentrations at the end of the
exposure period (Benoit et al., 1982).
7.2 Terrestrial organisms
7.2.1 Short-term toxicity
Except for one test with birds, reliable tests with terrestrial
organisms have not been reported.
Groups of 12 female and four male Japanese quails (Coturnix
coturnix japonica) were exposed to a diet containing
hexachloro-butadiene at levels of 0, 0.3, 3, 10 or 30 mg/kg diet for
90 days. Each cage contained three females and one male of the same
dose group. Feed analysis indicated levels close to the nominal
values. Adults were all histopathologically examined. Eggs were
collected on days 37-46, 64-73, and 81-90. Six adults died during
the study: 4 at 0.3 mg/kg, 1 at 10 mg/kg, and 1 at 30 mg/kg, but
this was not considered to be related to treatment. The survival of
chicks from eggs collected on days 81-90 was decreased at 10 mg/kg
only. Egg production, the percentage of fertile eggs, the percentage
of hatchable eggs, and eggshell thickness were unaffected compared
to controls (Schwetz et al., 1974).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The available mortality data are summarized in Table 10.
8.1.1 Inhalation exposure
8.1.1.1 Mortality
In the only reported study of the mortality of
hexachloro-butadiene following inhalation, groups of 20 SPF mice of
the OF1 strain were exposed for 6 h (Gage, 1970). The vapour
concentrations were measured by gas chromatography, and the
concentrations were within 10% of the nominal value. Results are
shown in Table 10.
8.1.1.2 Systemic effects
The decrease in respiratory rate (reflex bradypnoea) in groups
of six male Swiss OF1 mice was measured following exposure for
15 min to hexachlorobutadiene vapour at concentrations between 886
and 2625 mg/m3. The vapour concentrations were checked by gas
chromatography. The mice were restrained in a body plethysmograph,
while the heads were extended into an inhalation chamber. The 50%
effect level, calculated from the concentration-effect curve, was
2250 mg/m3 (De Ceaurriz et al., 1988).
8.1.2 Oral exposure
8.1.2.1 Mortality
Based on acute mortality data, hexachlorobutadiene is slightly
to moderately toxic to adult rats, moderately toxic to male weanling
rats, and highly toxic to female weanling rats following ingestion.
Young rats are far more sensitive than adult rats (Kociba et al.,
1977a). Gradiski et al. (1975) observed delayed mortality (after
24 h) in oral LD50 studies on rats and mice.
8.1.2.2 Systemic effects
Hexachlorobutadiene mainly affects the kidneys and, to a lesser
extent, the liver. The effects on these organs and the related
biochemical findings are discussed extensively in sections 8.8.2
(liver) and 8.8.3 (kidney). In oral LD50 studies on rats and mice,
Gradiski et al. (1975) observed hyper-reactivity just after
exposure, followed by decreased activity and staggering.
Table 10. Mortality of hexachlorobutadiene from single exposure
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Mouse oral 87 (78.1-95.9) Murzakayev (1963)b
OF1 adult female inhalation (6 h) 14 107 (102-113) Gage (1970)
OF1 adult female oral 14 65 (60-70) Gradiski et al. (1975)c
OF1 adult male oral 14 80 (75-85) Gradiski et al. (1975)c
Alderley Park adult male intraperitoneal 14 67 (53-85) Lock et al. (1984)e
Alderley Park adult female intraperitoneal 14 85 (65-111)
C57BL/10J adult male intraperitoneal 14 57 (41-81)
C3H adult male intraperitoneal 14 25-75
BALB/c adult male intraperitoneal 14 32-40
DBA/2J adult male intraperitoneal 14 53 (36-76)
Rat oral 350 (323-377) Murzakayev (1963)b
OF2 rat adult female oral 14 270 (250-290) Gradiski et al. (1975)c
OF2 rat adult male oral 14 250 (220-280) Gradiski et al. (1975)c
Sprague-Dawley adult female oral 200-400 Kociba et al. (1977a)d
Sprague-Dawley adult male oral 580 (504-667) Kociba et al. (1977a)d
Sprague-Dawley weanling female oral 46 (26-81) Kociba et al. (1977a)d
Sprague-Dawley weanling male oral 65 (46-91) Kociba et al. (1977a)d
Table 10 (contd).
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Alderley Park weanling male intraperitoneal 7 57 (38-87) Hook et al. (1983)e
Alderley Park 29 days male intraperitoneal 7 96 (72-128)
Alderley Park adult male intraperitoneal 7 360 (325-396)
Guinea-pig oral 90 (81.5-98.5) Murzakayev (1963)b
Rabbit
New Zealand adult female dermal (8 h) 14 1120 (890-1400) Duprat & Gradiski (1978)f
a When available, 95% confidence limits are reported between brackets.
b Observation period, strain, sex, age (or body weight) and vehicle were not reported.
c Vehicle was olive oil.
d Confidence limits not calculable; observation until no toxicity was observed any longer; vehicle was corn oil.
e Vehicle was corn oil.
f Application undiluted using glass vials (3.6 cm2).
8.1.3 Dermal exposure
8.1.3.1 Mortality
Hexachlorobutadiene was harmful to rabbits following acute
dermal exposure (LD50 = 1120 mg/kg body weight; (range,
890-1400 mg/kg; Table 10). After a dose of 780 mg/kg body weight,
death occurred within 24 h from respiratory and cardiac failure
(Duprat & Gradiski, 1978).
8.1.3.2 Systemic effects
New Zealand rabbits dermally exposed to undiluted
hexa-chlorobutadiene at doses of 1170 and 1550 mg/kg body weight
exhibited stupor, dyspnoea and cyanosis (Duprat & Gradiski, 1978).
8.1.4 Other routes of exposure
Hexachlorobutadiene has been shown to be toxic to various
strains of mice after intraperitoneal injection (Lock et al.,
1984) and harmful to adult rats (Hook et al., 1983). The compound
was considerably more toxic to young and weanling rats (Table 10).
Rats intraperitoneally exposed to single lethal doses of
hexachlorobutadiene from 500 to 1000 mg/kg in corn oil exhibited
piloerection, sedation, hunching, incoordination, loss of muscle
tone and hypothermia (Lock & Ishmael, 1979). A macroscopic and
haematological investigation of rats intraperitoneally exposed to
doses between 121 and 336 mg/kg body weight in olive oil did not
reveal any damage to the gastrointestinal tract, spleen, heart or
gonads. In the lungs, congestion, haemorrhage, and oedema were
observed, but these were attributed by the authors to ether
anaesthesia. At dose levels of 213 mg/kg body weight or more,
lymphopenia and related neutrophilia were induced (Gradiski et al.,
1975).
8.2 Short-term exposure
8.2.1 Inhalation exposure
In the only inhalation study published, groups of four adult
Alderley Park SPF rats of each sex were dynamically exposed to
nominal concentrations of 53, 107 or 267 mg/m3 6 h/day for 15
days, 1067 mg/m3 6 h/day for 12 days, or 2668 mg/m3 4 h/day for
2 days. Petroleum ether was used as a solvent for concentrations
below 1067 mg/m3. Many other chemicals were tested similarly, and
batches of control rats of unknown size were maintained at intervals
of 2 months during the whole experimental period. No analysis for
hexachlorobutadiene was carried out. Two of the four female rats
exposed to 1067 mg/m3 died, and autopsy revealed pale, enlarged
kidneys, adrenal regeneration and renal cortical tubular
degeneration with epithelial regeneration. Rats of both sexes lost
weight at 1067 mg/m3 and the weight gain of females was reduced at
107 and 267 mg/m3. Irritation of eyes and nose was observed at the
two highest levels. At 267 and 1067 mg/m3 rats were in a poor
condition, females being more affected than males. Respiratory
difficulties were seen at and above 267 mg/m3. Haematological
examination at the end of the exposure period showed slight anaemia
in females at 1067 mg/m3. Urinalysis did not reveal abnormalities
at any of the exposure levels. Macroscopically enlarged, pale
kidneys were found at 267 and 1067 mg/m3 and enlarged adrenals at
1067 mg/m3. Histopatho-logical investigations revealed proximal
tubular degeneration in the kidneys and cortical degeneration in
adrenals at concentrations of 267 mg/m3 or more. No toxic signs
were observed at the lowest exposure level and autopsy revealed no
gross abnormalities (Gage, 1970).
8.2.2 Oral exposure
8.2.2.1 Rats
Groups of five adult male Sprague-Dawley rats were exposed to
daily oral doses of hexachlorobutadiene (0, 0.2 or 20 mg/kg body
weight) in corn oil for 3 weeks. Only the kidneys were examined
histologically. At the higher dose level, body weight gain was
decreased and relative kidney weight increased. Histopathological
examination of the kidneys revealed damage in the middle and inner
cortical region, including loss of cytoplasm, nuclear pyknosis,
increased basophilia and mitotic activity, and increased cellular
debris. No toxic signs were observed at a dosage of 0.2 mg/kg per day
(Stott et al., 1981).
In a study by Kociba et al. (1971), groups of four female
Sprague-Dawley rats consumed for 30 days a diet containing
hexachlorobutadiene, which resulted in ingested nominal daily doses
of 0, 1, 3, 10, 30, 65 and 100 mg/kg body weight. Analysis of the
compound in the feed was not reported. Body weights were decreased
at the two highest dose levels. At 10 mg/kg or more, a dose-related
decrease in body weight gain was observed along with a decrease in
food consumption. There was also an increase in haemoglobin
concentrations, which, although significant, was not clearly dose
related. There was no effect on serum alanine aminotransferase
activity (EC 2.6.1.2). A dose-related increase in relative kidney
weight was observed at dose levels of 3 mg/kg or more.
Histopathological examination, which was restricted to liver and
kidneys, showed proximal tubular degeneration, individual cell
necrosis, and regenerative changes in the kidneys at doses of
10 mg/kg or more. Hepatocellular swelling was seen at 100 mg/kg. The
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight per
day.
In a 2-week experiment, groups of six weanling Wistar-derived
rats of each sex were exposed to measured dietary
hexachlorobutadiene levels of 0, 73, 182 or 447 mg/kg (the Task
Group considered this equivalent to doses of 0, 7.3, 18.2 and
44.7 mg/kg body weight per day, respectively). At all dose levels,
body weight and food conversion efficiency (g of weight gain/g of
food) were decreased in a dose-related manner. Food consumption per
g of body weight was decreased at 44.7 mg/kg body weight. Relative
kidney weights were increased at the two highest dose levels. At all
dose levels a dose-related degeneration of kidney tubular cells was
observed, especially in the straight limbs of the proximal tubules
located in the outer medulla. No toxic signs were observed in the
liver. A NOAEL was not found (Harleman & Seinen, 1979).
In a follow-up to the dietary study, groups of 10 weanling
Wistar-derived rats of each sex received daily doses by gavage of 0,
0.4, 1, 2.5, 6.3 or 15.6 mg/kg body weight in peanut oil for 13
weeks (Harleman & Seinen, 1979). Body weight gain, food consumption
and food utilization efficiency were decreased at 6.3 and
15.6 mg/kg. Polyuria was observed in females at these dose levels
after week 10, while a dose-related decrease in urine osmolarity
occurred at dose levels of 2.5 mg/kg or more. In males, the latter
effect was observed at 15.6 mg/kg. No other changes were observed in
urinalysis (after week 10) and haematological investigations (after
week 8). A dose-related increase in relative kidney weight was
measured in males of all treatment groups but only at 6.3 mg/kg or
more in females. Dose-related increases in the relative weight of
liver and spleen were measured at 6.3 mg/kg or more.
Histopathological examinations revealed changes in liver and
kidneys. In the livers of males dosed with 6.3 mg/kg or more, an
increased basophilic, flocky granulation was observed. At dose
levels of 6.3 mg/kg or more in males and 2.5 mg/kg or more in
females, there was a dose-related degeneration of the renal proximal
tubules, as shown by hyperchromatic nuclei, hypercellularity,
vacuolation and focal necrosis of epithelial cells and a diminished
brush border. No adverse effects were observed at daily doses of
1 mg/kg in females or 2.5 mg/kg in males (Harleman & Seinen, 1979).
8.2.2.2 Mice
In a two-week study, groups of five B6C3F1 mice of each sex
were fed a diet containing hexachlorobutadiene at nominal doses of
0, 30, 100, 300, 1000 or 3000 mg/kg feed for 15 days (calculated by
the Task Group to be equivalent to 0, 4.3, 14.3, 43, 143 and
430 mg/kg body weight per day, respectively, using standard values
for average body weight and food consumption in mice). Analysis of
the feed was carried out by gas chromatography, and no more than 9%
loss of the chemical was observed in one day (feed was replaced
every 2 days). All mice given 143 or 430 mg/kg body weight died or
were sacrificed in a moribund condition within 7 days. A
dose-related growth retardation was observed. At the two highest
doses, the observed toxic effects included renal tubular necrosis,
hepatic cytoplasmic vacuolization, and testicular degeneration
characterized by the presence of syncytial giant cell formation of
spermatocytes. At dose levels of 43 mg/kg body weight or more,
minimal to mild depletion of bone marrow (characterized by a
decrease in the haematopoietic cells) was seen in two out of five
mice of both sexes per dose group. At dose levels of 4.3, 14.3 and
43 mg/kg body weight, at which all animals survived to the end of
the study, renal tubular cell regeneration was observed (Yang
et al., 1989; Yang, 1991).
In a 13-week study, groups of 10 B6C3F1 mice of each sex were
fed a diet containing hexachlorobutadiene at concentrations of 0, 1,
3, 10, 30 or 100 mg/kg feed. Using measurements of food consumption
and body weight, the authors determined doses of 0, 0.1, 0.4, 1.5,
4.9 or 16.8 mg/kg body weight per day for males, and 0, 0.2, 0.5,
1.8, 4.5 or 19.2 mg/kg body weight per day for females. Analysis of
the compound was carried out as in the two-week study. No
treatment-related clinical signs or deaths were observed. The
motility of sperm from treated mice was significantly lower than in
controls, although this effect was not dose related (Yang et al.,
1989; Yang, 1991). Body weight gain was reduced at dose levels of
4.5 and 19.2 mg/kg body weight in females. Reductions in kidney
weight occurred at dose levels of 1.5 mg/kg or more in males and at
19.2 mg/kg body weight in females, and reductions in heart weight
occurred at 19.2 mg/kg body weight in males. Necropsy revealed a
treatment-related increase in renal tubular regeneration (prominent
in the outer stripe of the medulla) at dose levels of 0.2 mg/kg body
weight or more in females (Table 11). Although the author concluded
that a NOAEL was not observed for females, the Task Group noted that
the occurrence of renal tubular regeneration in one out of ten
female mice in the 0.2-mg/kg body weight group is insufficient
evidence of an adverse effect at this dose level in females.
Table 11. Incidences of renal tubular regeneration in 13-week
feed studies on B6C3F1 micea
Concentration Dose (mg/kg body Number of mice with
(mg/kg feed) weight per day) lesions/number examined
Male Female Male Female
0 0 0 0/10 0/10
1 0.1 0.2 0/10 1/10
3 0.4 0.5 0/10 9/10
10 1.5 1.8 0/9 10/10
30 4.9 4.5 10/10 10/10
100 16.8 19.2 10/10 10/10
a Modified from: Yang (1991)
8.3 Long-term exposure
No long-term inhalation studies have been reported. A study
describing long-term exposure of mice by the dermal route is
presented in section 8.7.3.
An oral toxicity/carcinogenicity test in rats has been reported
(see section 8.7.2).
8.4 Skin and eye irritation; sensitization
8.4.1 Irritation
The vapour of hexachlorobutadiene has been found to be
irritating to the eyes and nose of rats (Gage, 1970; see section
8.2.1).
Groups of six New Zealand rabbits received either 0.78 g
(0.5 ml) of undiluted hexachlorobutadiene on the intact or abraded
skin for 24 h, or 0.15 g (0.1 ml) in the conjuctival sac of the left
eye. Assessment of the degree of irritation was conducted according
to Draize et al. (1944) and by calculating the primary irritation
index. Hexachlorobutadiene was moderately irritating for the skin
(primary irritation index 4) but not irritating for the eyes
(primary irritating index 1.5). Moderate conjunctivitis, epithelial
abrasion and, at day 7, epithelial keratitis were observed in the
eyes (Duprat et al., 1976).
Duprat & Gradiski (1978) applied undiluted hexachloro-butadiene
to New Zealand rabbits at doses of 0.39, 0.78, 1.17 and 1.55 mg/kg
body weight (0.25, 0.50, 0.75 and 1.00 ml, respectively) under
occluded conditions, using glass vials, for 8 h. The observation
period was 14 days. The skin was histopathologically examined in all
dead animals, in half the survivors at day 15, and in the remaining
survivors at day 36. After 12 h of exposure to the two highest
doses, epidermis and subcutaneous tissue revealed oedema and
polymorphonuclear leukocyte infiltration. In the epidermal cells,
degeneration with pyknosis of nuclei and perinuclear oedema, and
focal separation from the corium with vesicle formation were seen.
After 3 to 5 days of exposure to the three highest doses, dermal
necrosis was observed, leading to eschar formation and partial
destruction of hair follicles. The effects increased with time, not
with dose. Two to five weeks after application, repair was apparent
at all dose levels, with scarring and upper dermis fibrosis, and
epidermal acanthosis with focal dyskeratosis. Diffuse mononuclear
infiltrate was seen in the dermis.
8.4.2 Sensitization
A group of 20 Hartley guinea-pigs were treated according to the
Magnusson-Kligman protocol by intradermal injections of 5%
hexachlorobutadiene in peanut oil and, after one week and subsequent
treatment by sodium lauryl sulfate, by a 48-h dermal application of
a 25% suspension of the chemical in vaseline. The challenge was
performed by dermal application of a 20% suspension in vaseline. A
group of five controls was induced similarly and challenged by
vaseline only. All exposed animals, but none of the controls, showed
a positive reaction. The test was repeated in the same fashion
without adjuvant in five guinea-pigs: all animals showed positive
reactions (Gradiski et al., 1975).
8.5 Reproduction, embryotoxicity and teratogenicity
8.5.1 Reproduction
A group of female albino rats was exposed to one dose of
hexachlorobutadiene (20 mg/kg body weight) administered
subcutaneously before mating. Within 90 days after exposure, all 86
newborn rats had died, compared with 13 of the 61 controls. The
offspring from exposed dams were reported to show excitation,
disturbances of motor coordination, a decrease in appetite and a
loss of weight, lymphocytosis, neutropenia, myelocytes, Jolly's and
Cabeau's bodies, pneumonia, bronchitis, granular dystrophy of renal
cells, glomerulonephritis, inflammatory destructive lesions of the
gastrointestinal tract and vascular hyperaemia (Poteryayeva, 1966).
The Task Group noted major deficiencies and incomplete reporting of
the experiment, the unusual route of administration, and the high
percentage of mortality in control rats.
Groups of 10-12 male and 20-24 female Sprague-Dawley rats
received a diet containing hexachlorobutadiene at dose levels of
0.2, 2.0 or 20 mg/kg body weight per day for 90 days prior to
mating, 15 days during mating, and subsequently throughout gestation
and lactation. In the mating period, two females were placed with
one male of the same dose group. The control group consisted of
17 males and 34 females. The diets were reportedly analysed for the
test compound. No mortality was observed. At 20 mg/kg, adults showed
decreased food consumption and body weight gain. Blood urea
nitrogen, serum alanine aminotransferase (EC 2.6.1.2) and serum
creatinine were unchanged compared to controls. The dams had an
increased relative brain weight and the male rats had an increased
relative liver weight at 20 mg/kg. The relative kidney weights were
increased in both sexes at 20 mg/kg. At 2 and 20 mg/kg the kidneys
of adult rats revealed dose-related tubular dilatation and
hypertrophy with foci of epithelial degeneration and regeneration;
however, there was no effect at 0.2 mg/kg. The only adverse
reproductive effect in neonates was a decreased weanling weight at
20 mg/kg. There was no detectable effect on the percentage
pregnancy, the period from first cohabitation to delivery, survival
indices, sex ratio, histopathology of weanlings, and the incidence
of skeletal alterations and abnormalities in neonates (Schwetz
et al., 1977).
In a third reproductive study, groups of six female SPF
Wistar-derived rats, 10 weeks of age, received a diet containing
hexa-chlorobutadiene at levels of 0, 150 and 1500 mg/kg diet
(estimated by the Task Group to be equivalent to 0, 7.5 and 75 mg/kg
body weight per day) for 3 weeks prior to mating, 3 weeks during
mating, and subsequently throughout gestation and lactation. In the
mating period, two untreated males were placed with the females,
after which the females were housed individually. The 75-mg/kg
female adults were killed in week 10, while those given 0 or
7.5 mg/kg were killed in week 18. Food analysis at the low dose
level revealed hexachlorobutadiene levels within 96% of nominal
values after 1 week and within 81% of nominal values after 2 weeks.
Diets were prepared weekly. There was a reduced body weight gain by
female rats in the two groups receiving hexachlorobutadiene.
Weakness of hind legs, unsteady gait, incoordination and ataxia were
seen at 75 mg/kg. The relative kidney weight was increased at both
dose levels. Histopathological investigations revealed
hypercellularity of epithelial cells, hydropic degeneration, and
necrosis of proximal tubules in the kidneys at 7.5 mg/kg. At
75 mg/kg, slight proliferation of bile duct epithelial cells,
fragmentation and demyelination of single fibres of the femoral
nerve, and extensive renal degeneration were observed. Again at
75 mg/kg, no conceptions occurred, the ovaries showing little
follicular activity, and there was no uterine implantation sites. At
7.5 mg/kg fertility and litter size were reduced, but not
significantly. In both the control and 7.5-mg/kg groups, the
resorption quotient was low. Compared to controls, pup weights were
reduced significantly on days 0, 10 and 20 in the 7.5-mg/kg group.
No gross abnormalities were observed (Harleman & Seinen, 1979).
8.5.2 Embryotoxicity and teratogenicity
In a teratology study, groups of 24-25 female rats were exposed
to hexachlorobutadiene vapour at measured concentrations of 0, 21,
53, 107 or 160 mg/m3 for 6 h per day from days 6 to 20 of
pregnancy. The breathing zone atmosphere was analysed by gas
chromatography. Maternal weight gain decreased at 53 and
160 mg/m3. At the other two exposure levels, the slight decrease
in maternal weight was not significant. The mean number of
implantation sites, total fetal losses, resorptions, live fetuses,
incidences of pregnancy, and sex ratio were not affected by exposure
to hexachlorobutadiene, compared to controls. Fetal body weight was
reduced in both sexes at 160 mg/m3. The incidences of external,
visceral, and skeletal alterations were not significantly increased
(Saillenfait et al., 1989).
In a study by Hardin et al. (1981), groups of 10-15 mated
Sprague-Dawley rats received hexachlorobutadiene in corn oil by
intraperitoneal injection at a dose level of 10 mg/kg body weight
per day from days 1 to 15 of gestation. It was reported (without
further details) that at least two maternal organ weights were
changed and that pre- or postimplantation survival was reduced.
Maternal tissues did not reveal histopathological effects. Fetuses
had a reduced weight or length, a 1-2 day delay in heart
development, and dilated ureters. No grossly visible external or
internal malformations were observed (Hardin et al., 1981).
It was reported briefly by Badaeva et al. (1985) that daily
oral administration of hexachlorobutadiene to pregnant rats at a
dose level of 8.1 mg/kg body weight per day resulted in
histopathologi-cal changes of nerve cells and myelin fibres of the
spinal cord in the dams and their offspring.
8.6 Mutagenicity and related end-points
8.6.1 In vitro effects
Purified hexachlorobutadiene induces gene mutations in the Ames
Salmonella test when specific incubation conditions are employed.
In preincubation assays adapted to include rat liver microsomes
and additional reduced glutathione, hexachlorobutadiene induced
point mutations in Salmonella typhimurium TA100 (Vamvakas et al.,
1988a). Assays lacking specialized metabolic activation conditions
have generally yielded negative results (Table 12).
Data from bacterial mutagenicity assays are consistent with the
proposed scheme for the biotransformation of hexachlorobutadiene in
animals (section 6.3; Fig. 1). Activity in S. typhimurium TA100,
mediated by subcellular fractions of rat kidney, was inhibited by
the addition of the ß-lyase inhibitor, AOAA (Vamvakas et al.,
1988a, 1989a) and the gamma-glutamyltranspeptidase inhibitor,
acivicin (Vamvakas et al., 1989a).
Several of the proposed metabolites of hexachlorobutadiene have
been assayed for mutagenic activity in S. typhimurium TA100
(Table 13). The mono-glutathione (GPB) and mono-cysteine (CPB)
conjugates were mutagenic in the presence or absence of rat kidney
S9. Rat liver microsomes and mitochondria that exhibit high
gamma-glutamyltranspeptidase activities strongly enhanced the
mutagenic potency of GPB in the presence of additional glutathione,
in contrast to liver microsomes that exhibit lower
gamma-glutamyltranspeptidase activity. Furthermore, AOAA and
acivicin both inhibit the activation of GPB mediated by kidney
fractions. The di-glutathione (BGTB) and di-cysteine (BCTB)
conjugates of hexachlorobutadiene were not mutagenic either in the
presence or absence of rat kidney S9 (Green & Odum, 1985; Dekant
et al., 1986; Vamvakas et al., 1988a, 1989a).
The mercapturic acid, ACPB, was mutagenic both in the presence
and absence of rat liver S9. It has been suggested that the
metabolism of ACPB in animals is catalysed by an N-deacetylase and
by ß-lyase (section 6.3) (Reichert et al., 1984). The Task Group
considered that S. typhimurium possesses both of these enzymes
activities. Both MTPB and CMTPB gave negative results in tests with
S. typhimurium TA100 and are considered to be detoxified
metabolites of hexachlorobutadiene (Wild et al., 1986).
Table 12. Studies on mutagenicity of hexachlorobutadiene
Test description Species/strain/cell type Conditionsa Resultc Reference
Reverse mutations Salmonella typhimurium TA98, +/- rat liver S9, purity 98%, - De Meester et al.
TA100, TA1530, TA1535, TA1538 plate incorporation (1981)
S. typhimurium TA100 +/- rat liver S9, purity > 99%, - Stott et al. (1981)
plate incorporation
S. typhimurium TA98, TA100 +/- rat liver S9, purity not - Reichert et al. (1983)
reported, suspension testb
S. typhimurium TA98, TA100, +/- rat liver S9, purity not - Haworth et al. (1983)
TA1535, TA1537 reported, preincubation test
+ rat liver S9*, purity > 99.5%, +
preincubation test
S. typhimurium TA100, TA1535 +/- rat liver S9, purity not - Chudin et al. (1985)
TA1538 reported, plate incorporation
S. typhimurium TA100 +/- rat liver S9, - Reichert et al. (1984)
- rat liver S9* +
S. typhimurium TA100 + rat liver S9*, purity >99.5%, +d Wild et al. (1986)
preincubation test
S. typhimurium TA100 no activation, purity 98% + Vamvakas et al. (1988a)
no activation, purity > 99.5%, -
preincubation test
Table 12 (contd).
Test description Species/strain/cell type Conditionsa Resultc Reference
S. typhimurium TA100 + rat liver microsomes - Vamvakas et al. (1988a)
without additional GSH
+ rat liver microsomes and +e
additional GSH, purity > 99.5%,
plate incorporation
Sex-linked lethals Drosophila melanogaster feeding or injection - Woodruff et al. (1985)
Chromosome aberrations CHO cells +/- rat liver S9 - Galloway et al. (1987)
Chromosome aberrations human lymphocytes - rat liver S9 - German (1988)
Sister chromatid exchanges CHO cells +/- rat liver S9 + Galloway et al. (1987)
Chromosome aberrations mouse bone marrow cells inhalation, 4 h + German (1988)
Chromosome aberrations mouse bone marrow cells oral gavage + German (1988)
a S9* = a fortified S9 mix containing 3 times the normal protein concentration; GSH = reduced glutathione
b The extreme toxicity of the compound without S9 was supposed to exclude testing in this system
c + = > twice the background rate or, in the case of bacterial studies, a reproducible dose-related increase in the number of
revertants per plate; - = negative
d 0.23 revertants per nmol
e Addition of rat kidney microsomes further increased the number of revertants; positive results were inhibited by the ß-lyase inhibitor
aminooxyacetic acid
Chinese hamster ovary (CHO) cells were exposed to between 5 and
24 mg hexachlorobutadiene/litre for 2 h in the presence of rat liver
S9 and throughout the incubation period (8-26 h, depending on cell
cycle delay) in the absence of rat liver S9. In comparison with
concurrent controls, no significant increase in chromosome
aberration frequency was observed (Galloway et al., 1987). In a
further study, in which human lymphocyte cultures were exposed to
between 0.01 and 0.001 mg hexachlorobutadiene per litre in the
absence of S9 for 27 h, there was also no clastogenic effect. At the
highest dose level there was a reduction of approximately 60% in the
mitotic index of human lymphocyte cultures (German 1988). However,
hexachlorobutadiene at a dose level of at least 4 mg/litre did cause
a significant increase in the frequency of sister chromatid exchange
in CHO cells in both the presence and absence of rat liver S9
(Galloway et al., 1987).
Hexachlorobutadiene was found to induce unscheduled DNA
synthesis (UDS) in Syrian hamster embryo fibroblast cultures.
Moreover, the magnitude of the response was increased when a
preincubation period with rat liver S15 was employed (Schiffman et
al., 1984). However, there was no induction of UDS in a study
using rat hepatocyte cultures (Stott et al., 1981).
In summary, the Task Group concluded that hexachloro-butadiene
was genotoxic in vitro and that the negative results reported in
some studies may have resulted from the use of inappropriate
conditions for metabolic activation.
8.6.2 In vivo effects
Hexachlorobutadiene induced a significant increase in the
frequency of chromosomal aberrations in mouse bone marrow cells
following the administration of acute oral doses of 2 or 10 mg/kg
body weight or acute inhalation exposure to 10 mg/m3 for 4 h. Both
experiments used six mice per dose group, and the animals were
sacrificed after 24 h (German, 1988).
Six hours after the administration of a single oral dose of
20 mg hexachlorobutadiene/kg body weight to two groups of five male
Sprague-Dawley rats, there were statistically significant increases
in kidney UDS of 27% and 54% above concurrent control levels.
Administration of a positive control substance,
dimethyl-nitrosamine, resulted in an increase of 187% over controls
(Stott et al., 1981).
As described in section 6.3, radiolabelled nucleotides were
recovered from the kidneys of rats and mice administered
14C-labelled hexachlorobutadiene by gavage (Stott et al., 1981;
Schrenk & Dekant, 1989). The Task Group concluded that these studies
indicated covalent binding of hexachlorobutadiene or its metabolites
Table 13. Tests for reverse mutations in Salmonella typhimurium TA100 by proposed metabolites of hexachlorobutadiene
Metabolite and abbreviationa Conditionsb Resultc Reference
1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (GPB) no activation - Green & Odum (1985)
+ rat kidney S9 +
+/- rat kidney fractions +d Vamvakas et al. (1988a)
1,4-(bis-glutathion- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BGTB)
1,4-(bis-cystein- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BCTB)
1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (CPB) +/- rat kidney S9 +e Green & Odum (1985)
no activation +f Dekant et al. (1986)
1-( N-acetylcystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene - rat liver S9 - Wild et al. (1986)
(ACPB) + rat liver S9 +g
1-carboxymethylthio-1,2,3,4,4-pentachloro-1,3-butadiene + rat liver S9* - Wild et al. (1986)
(CMTPB)
Table 13 (contd).
Metabolite and abbreviationa Conditionsb Resultc Reference
1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) + rat liver S9 - Wild et al. (1986)
2,2,3,4,4-pentachloro-3-butenoic acid (PBA) +/- rat liver S9 + Reichert et al. (1984)
2,2,3,4,4-pentachloro-3-butenoic acid chloride (PBAC) +/- rat liver S9 + Reichert et al. (1984)
a See also Figure 1
b Plate incorporation assays with, except in the case of the tests by Green & Odum, preincubation; S9* = a fortified S9 mix containing
3 times the normal protein concentration
c + = > twice background rate; - = negative
d Mutagenic potency enhanced by rat kidney microsomes or mitochondria and less so by cytosol; positive results were inhibited by the
ß-lyase inhibitor aminooxyacetic acid
e Mutagenic potency enhanced by rat kidney S9
f Positive results were inhibited by the ß-lyase inhibitor aminooxyacetic acid
g 18.7 revertants per nmol; mutagenic potency decreased by addition of pyridoxal phospate; activation by cytosol, with and without cofactors,
had the same results as S9 mix; microsome mix was inactive
to kidney DNA in vivo. The study with mice showed that the level
of binding to mitochondrial DNA was greater than that to nuclear
DNA. In addition, radioactivity was recovered in mitochondrial DNA,
but not nuclear DNA, from mouse liver (Schrenk & Dekant, 1989).
Hexachlorobutadiene did not induce sex-linked recessive lethal
mutations in Drosophila melanogaster following treatment of adults
either via the diet or by injection (Woodruff et al., 1985).
8.7 Carcinogenicity/long-term toxicity
8.7.1 Inhalation exposure
No long-term carcinogenicity studies, where inhalation was the
route of exposure, have been reported.
8.7.2 Oral exposure
In a study by Kociba et al. (1977a,b), groups of 39-40
adult Sprague-Dawley rats of each sex received a diet containing
hexachlorobutadiene at 0.2, 2 or 20 mg/kg body weight per day for 22
(males) or 24 (females) months. Control groups comprised 90 rats of
each sex. Analysis for the compound was not reported. An increased
mortality was observed in males at 20 mg/kg. Hexachlorobutadiene
caused a depression of the body weight gain in both sexes at the
highest dose level without any effect on food consumption.
Haematological investigations performed at 12-14 and 22-24 months,
revealed a slight, but statistically significant, depression in the
red blood cell count of males at 20 mg/kg (22 months). Urinalysis at
12-14 months and 22-24 months did not reveal effects except for a
small increase in coproporphyrin excretion. The analysis of the
clinical chemistry parameters of blood urea nitrogen, serum alanine
aminotransferase (EC 2.6.1.2) and serum alkaline phosphatase
(EC 3.1.3.1) at 12 months revealed no treatment-related effects,
except for statistically significant decreases in serum alanine
aminotransferase in males of the 20-mg/kg dose group and females of
the 0.2- or 20-mg/kg dose groups. These changes were considered by
the authors to be of questionable toxicological significance. The
relative kidney weights were elevated at 20 mg/kg for both sexes, as
were the relative weights of the brain in females and of the testes
in males. In both sexes, an extensive histopathological examination
revealed tubular epithelial hyperplasia at 2 and 20 mg/kg, but not
at 0.2 mg/kg, and an increased incidence of renal tubular neoplasms
at 20 mg/kg (see Table 14) (Kociba et al., 1977a,b).
8.7.3 Dermal exposure
In a study by Van Duuren et al. (1979), groups of 30 female
Ha:ICR Swiss mice received 6.0 mg hexachlorobutadiene in acetone
applied 3 times per week to the shaven dorsal skin for between 144
and 594 days. A group of 100 untreated females were included in the
study, together with 30 controls which received acetone only. The
study duration was described as being between 440 and 594 days.
Sections of skin, liver, stomach and kidney were sampled at autopsy,
but no increase in the number of distant tumours was observed.
In a two-stage initiation-promotion experiment, each of
20 female Swiss mice received one application of 15.0 mg
hexa-chlorobutadiene in acetone to the dorsal skin. After 14 days,
the mice similarly received 5 µg of the tumour promoter
12- o-tetra-decanoylphorbol-13-acetate (TPA) three times weekly for
between 428 and 576 days. Hexachlorobutadiene administration did not
induce a significant increase in the fraction of mice developing
skin papillomas in this study (Van Duuren et al., 1979).
Table 14. Renal tubular neoplasms in rats after long-term
exposure to hexachlorobutadienea
Dose (mg/kg Sex Incidence of renal tubular neoplasms
body weight
per day) adenoma adenocarcinoma total
0 males 1/90 0/90 1/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 2/39 7/39 9/39 (P < 0.05)
0 females 0/90 0/90 0/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 3/40 3/40b 6/40 (P < 0.05)
a From: Kociba et al. (1977a)
b One of these was an undifferentiated carcinoma
8.7.4 Exposure by other routes
In a study of repeated exposure to hexachlorobutadiene by ip
injection, groups of 20 A/St strain male mice (from 6 to 8 weeks of
age) received 12 or 13 ip injections of hexachlorobutadiene (4 or
8 mg/kg body weight) in tricaprylin, respectively. The purity of the
hexachlorobutadiene was stated to exceed 99.9%. Urethane was used as
a positive control for carcinogenesis, and a negative control group
of 50 mice receiving tricaprylin only. Survival was 95% for mice
receiving 4 mg/kg and 70% for mice receiving 8 mg/kg, compared to
92% for controls. Mice were sacrificed at 24 weeks after the first
injection, and the number of surface adenomas in the lungs was
counted. No significant increase in adenomas, compared to the
vehicle-treated control, was observed (Theiss et al., 1977). The
Task Group noted major deficiencies of this study; including the
choice of a sensitive strain of mice, the short duration of both the
exposure period (4 weeks) and the follow-up period (24 weeks), the
small group sizes of the experimental animals, the unusual route of
administration, and the limited histopathology. The strain A mice
used in this study are highly predisposed to spontaneous lung
cancer, which is likely to have further compromised the value of the
study.
8.8 Other special studies
8.8.1 Effects on the nervous system
Acute high exposure to hexachlorobutadiene has a depressant
effect on the central nervous system (see sections 8.1.1.2 and
8.1.2).
Subchronic exposure of rats at high dose levels (1500 mg/kg
diet for 13 weeks) also produced some signs of neurotoxicity, which
was associated with demyelinization and fragmentation of femoral
nerve fibres (Harleman & Seinén, 1979; see also Badaeva et al.,
1985, section 8.5.2).
8.8.2 Effects on the liver
8.8.2.1 Acute effects
Hexachlorobutadiene causes hydropic changes in the liver of
rats (Gradiski et al., 1975; Lock & Ishmael, 1981; Lock et al.,
1982), mice (Lock et al., 1985), and rabbits (Duprat & Gradiski,
1978), sometimes accompanied by fat accumulation (Duprat & Gradiski,
1978; Lock & Ishmael, 1981; Lock et al., 1982).
Male rats, exposed to a single intraperitoneal dose of
hexachlorobutadiene (200 or 300 mg/kg body weight) in corn oil
showed increased relative liver weights, mitochondrial swelling in
liver and bile duct, proliferation of smooth endoplasmic reticulum,
lipid accumulation, and increased water content in the liver.
Biochemical changes in the liver were a decrease, followed after 1
day by an increase, in non-protein sulfhydryl (NP-SH) concentration,
and an increase in potassium content. All effects were reversible
within 10 days. Increases in plasma urea and alkaline phosphatase
(EC 3.1.3.1.) were also reported. In a separate experiment, the
highest dose administered by ip injection which did not cause an
increased water content in the liver was 25 mg/kg body weight (Lock
et al., 1982).
Male rats exposed to single intraperitoneal doses up to
100 mg/kg body weight showed an increase in serum bile acids and
bilirubin (Bai et al., 1992).
Male mice, exposed to single intraperitoneal doses of 50, 100
and 200 mg/kg body weight in corn oil, showed a dose-related
increase in relative liver weight at 100 and 200 mg/kg, and, at all
dose levels, dose-related, reversible changes in the liver
(mitochondrial swelling, proliferation of smooth endoplasmic
reticulum, and an increased water content). Reversible biochemical
changes included increases in sodium and potassium content, NP-SH
concentration in the liver, and serum alanine aminotransferase
activity (EC 2.6.1.2) at 50 mg/kg (Lock et al., 1985).
8.8.2.2 Short-term effects
As discussed in section 8.2.2.1, slight hepatotoxic effects
have been observed following oral exposure of rats (Kociba
et al., 1971; Harleman & Seinen, 1979).
8.8.3 Effects on the kidneys
This section will describe the main features of the renal
toxicity induced by hexachlorobutadiene. For more detail the reader
is referred to the reviews of Rush et al. (1984), Lock (1988),
Yang (1988) and Dekant et al. (1990a).
8.8.3.1 Acute effects
Inhalation exposure of rats produces renal tubular necrosis
(Gage, 1970; see section 8.2.1). Enzyme histochemical
investi-gations were performed on groups of 10 male Swiss OF1 mice
24 h after whole-body inhalation exposure for 4 h to
hexachloro-butadiene at measured concentrations of 29.3, 53.4, 106.7
or 266.8 mg/m3. A concentration-related increase in the percentage
of damaged kidney tubules, which had been stained for alkaline
phosphatase (EC 3.1.3.1), was observed at all exposure levels. The
EC50 was calculated to be 76.8 mg/m3 (De Ceaurriz et al.,
1988).
A single oral dose of hexachlorobutadiene (200 mg/kg body
weight) in polyethylene glycol caused an increase in plasma urea
concentration, a decrease in plasma alanine aminotransferase
activity, and, in urine, increases in the levels of glucose,
protein, alanine aminotransferase, N-acetyl-ß-D-glucosaminidase,
gamma-glutamyltranspeptidase (EC 2.3.2.2) and alanine aminopeptidase
(EC 3.4.11.12) (Nash et al., 1984).
Following in vivo administration, hexachlorobutadiene caused
dose-dependent necrosis of the renal proximal tubules in rats
(Gradiski et al., 1975; Lock & Ishmael, 1979, 1981; Kluwe et al.,
1982; Hook et al., 1982, 1983; Ishmael et al., 1982; Ishmael &
Lock, 1986), mice (Ishmael et al., 1984) and rabbits (Duprat &
Gradiski, 1978). In rats, the lesions were restricted to the pars
recta (S3-segment) and were macroscopically observed as a distinct
band of damage in the outer stripe of the medulla (Lock & Ishmael,
1979; Ishmael et al., 1982). In mice and rabbits both the pars
recta and the pars convoluta of the proximal convoluted tubules were
damaged (Duprat & Gradiski, 1978; Ishmael et al., 1984). The
lesion is characterized microscopically by necrotic epithelial
cells, most of which are devoid of nuclei. The few remaining nuclei
show karyorrhexis, and the cytoplasm is strongly eosinophilic. Many
renal tubules contained cellular debris (Duprat & Gradiski, 1978;
Lock & Ishmael, 1979; Lock et al., 1984; Ishmael et al., 1984).
Vacuolation of the pars convoluta was observed (Duprat & Gradiski,
1978; Ishmael et al., 1982, 1984). Mitochondrial swelling and loss
of brush-borders were prominent ultrastructural findings (Ishmael
et al., 1982, 1984).
Adult male rats have been found to be less sensitive to the
renal toxicity induced by hexachlorobutadiene than adult females and
young males (Hook et al., 1983; Kuo & Hook, 1983). When male rats
were dosed intraperitoneally with a single dose of 300 mg/kg in corn
oil, the earliest pathological change was mitochondrial swelling in
proximal tubular cells observed after 1-2 h. Extensive necrosis was
evident between days 1 and 4, and active regeneration by day 5
(Ishmael et al., 1982). Similar renal toxicity was seen at a dose
level of 25 or 50 mg/kg body weight in female rats and young males,
respectively. A similar pattern of pathological changes with
comparable intensity was observed in mice at an intraperitoneal dose
of 50 mg/kg body weight (Ishmael et al., 1984). In a study by Lock
et al. (1984), young mice were found to be more susceptible than
adults, but no sex difference was apparent. In both rats and mice,
differences in strain susceptibility were observed (Hook et al.,
1983; Lock et al., 1984). The lowest intraperitoneal dose at which
renal necrosis was observed in adult female rats was 25 mg/kg body
weight (Lock & Ishmael, 1985) and in adult male and female mice was
6.3 mg/kg body weight (Lock et al., 1984).
Biochemical changes found following intraperitoneal exposure in
both rats and mice were increases in renal water content (Kluwe et
al., 1982; Ishmael et al., 1982, 1984; Gartland et al., 1989),
plasma urea (Lock & Ishmael, 1979, 1981; Ishmael et al., 1982,
1984; Hook et al., 1983; Lock et al., 1984; Ishmael & Lock,
1986; Stonard et al., 1987; Gartland et al., 1989), plasma
alkaline phosphatase (EC 3.1.3.1.) (Lock & Ishmael, 1981), serum
alanine aminotransferase (EC 2.6.1.2) (Gradiski et al., 1975; Kuo
& Hook, 1983) and serum aspartate aminotransferase (Gradiski et
al., 1975; Davis et al., 1980). In the urine of rats, increases
in urinary protein, glucose and ketones have been measured (Lock &
Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al., 1980;
Stonard et al., 1987), as well as increases in the activities of
alkaline phosphatase and N-acetyl-ß-D-glucosaminidase (EC
3.2.1.50) (Lock & Ishmael, 1979; Stonard et al., 1987) and in
lactic acid level (Gartland et al., 1989). In the kidneys of rats,
increased sodium concentrations were accompanied by equally
decreased potassium concentrations (Davis et al., 1980). All these
changes occurred at similar or higher intraperitoneal doses than
those at which renal necrosis was observed.
Distinct renal functional changes in adult rats have been
observed at intraperitoneal doses of 100-400 mg/kg body weight.
These include a decrease in urine-concentrating ability (polyuria)
(Lock & Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al.,
1980; Stonard et al., 1987), a reduced glomerular filtration rate
(Davis et al., 1980) and a reduction of in vivo renal clearance
of inulin, urea, p-aminohippuric acid (PAH), tetraethyl-ammonium
bromide (TEA) (Lock & Ishmael, 1979) and imipramine (Davis et al.,
1980). When organic ion transport was assessed in vitro in renal
cortical slices of rats and mice that had been exposed to an
intraperitoneal dose of 100 mg/kg, 200 mg/kg body weight or more,
the transport of anions (PAH) was found to be reduced, but the
transport of the cation (TEA), aminoisobutyrate was not (or was only
slightly reduced) in rats (Lock & Ishmael, 1979; Berndt & Mehendale,
1979; Kluwe et al., 1982; Hook et al., 1982, 1983). In male
adult mice, transport of PAH and TEA was reduced from
intraperitoneal doses of 12.5 and 25.0 mg/kg body weight,
respectively. The anion transport was reduced in adult females (Lock
et al., 1984).
8.8.3.2 Short- and long-term effects
The short- and long-term effects of hexachlorobutadiene on the
kidneys of experimental animals have already been discussed in
sections 8.2 , 8.5 and 8.7. Based on the studies of Kociba et al.
(1971, 1977a,b), Schwetz et al. (1977), Harleman & Seinen (1979),
Stott et al. (1981) and Yang et al. (1989), the oral NOAEL for
renal toxicity is 0.2 mg/kg body weight per day. Results of the
studies are summarized in Table 15. Female rats and mice were found
to be distinctly more susceptible than males upon oral exposure for
13 weeks (Yang et al., 1989; Yang, 1991); this was also observed
in the single-exposure mortality studies (section 8.1).
8.9 Factors modifying toxicity; toxicity of metabolites
8.9.1 Factors modifying toxicity
8.9.1.1 Surgery
Complete protection from the nephrotoxic effects of
hexachlorobutadiene was observed in rats that had been fitted with a
biliary cannula before being given a single oral dose of 200 mg/kg
body weight. Administration of bile, collected from rats dosed
orally with the compound, to naive rats produced marked renal
toxicity but no liver toxicity (Nash et al., 1984).
8.9.1.2 Inhibitors and inducers of mixed-function oxidases (MFO)
In the majority of studies, the effects of MFO inhibitors
(piperonyl butoxide, SKF 525A) and MFO inducers (Aroclor 1254,
isosafrole, ß-naphthoflavone, phenobarbitone) on the nephro-toxicity
induced by hexachlorobutadiene in rats and mice were absent or
negligible (Lock & Ishmael, 1981; Hook et al., 1982; Lock et al.,
1984; Davis, 1984). Furthermore, phenobarbitone pretreatment for 7
days at 0.05% in drinking-water enhanced the renal toxicity induced
by intraperitoneal doses of hexachloro-butadiene in weanling rats
(Hook et al., 1983).
8.9.1.3 Inhibitors of gamma-glutamyltranspeptidase (EC 2.3.2.2)
Male rats pretreated with Acivicin (L-(alphaS, 5S)-
alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid), an
inhibitor of gamma-glutamyltranspeptidase (down to 3% of control
activity in this study), and subsequently exposed intraperitoneally
to hexachlorobutadiene, did not show a decrease in nephrotoxicity
compared to rats treated with hexachlorobutadiene alone. It was
concluded that gamma-glutamyltranspeptidase inhibition did not limit
the formation of nephrotoxic metabolites (Davis, 1988).
Male Swiss OF1 mice, pretreated with Acivicin and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group noted that only one marker for nephrotoxicity was employed in
this study.
Table 15. No-observed-adverse-effect level (NOAEL) calculated from short-term and long-term
studies of exposure to hexachlorobutadiene by oral administration
Species (Strain) Age Sex Number of Duration of Dose (mg/kg NOAEL (mg/kg References
animals study body weight body weight
per group per day) per day)
Rat (Wistar-derived) weanling male 6 2 weeks 7.3, 18.2, 44.7 < 7.3 Harleman & Seinen
female 6 (1979)
Rat (Sprague-Dawley) adult male 5 3 weeks 0.2, 20 0.2 Stott et al. (1981)
Rat (Sprague-Dawley) adult female 4 30 days 1, 3, 10, 30, 65, 100 3 Kociba et al. (1971)
Rat (Sprague-Dawley) adult male 10-12 3 months 0.2, 2.0, 20 0.2 Schwetz et al.
female 20-24 (1977)
Rat (Sprague-Dawley) adult male 39-40 24 months 0.2, 2.0, 20 0.2 Kociba et al.
female 39-40 (1977a,b)
Mouse (B6C3F1) adult male 5 2 weeks 4.3, 14.3, 43, 143, 430 < 4.3 Yang et al.
female 5 (1989); Yang (1991)
Mouse (B6C3F1) adult male 10 13 weeks 0.1, 0.4, 1.5, 4.9, 18.8 1.5 Yang et al.
female 10 0.2, 0.5, 1.8, 4.5, 19.2 < 0.2 (1989); Yang (1991)
8.9.1.4 Inhibitors of cysteine conjugate ß-lyase
Male Swiss OF1 mice, pretreated with the two ß-lyase inhibitors
amino-oxyacetic acid (AOAA) and DL-propargylglycine (PPG) and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group again noted that only one marker for nephrotoxicity was
employed in this study.
8.9.1.5 Inhibitors of organic anion transport
Pre-treatment of male rats with probenecid
[(4-(dipropyl-amino)sulfonyl)] benzoic acid (105 µmol/kg body
weight), an inhibitor of organic anion transport, did not alter the
increase in plasma urea or decrease in renal clearance of
p-aminohippuric acid induced by hexachlorobutadiene (Hook et al.,
1982). However, in female rats, a higher dose (500 µmol/kg body
weight) of probenecid totally protected against the renal toxicity,
both functional and morphological, produced by ACPB (Lock & Ishmael,
1985). In addition this dose of probenecid protected female rats
against the toxic effects produced by CPB and GPBN as well as the
parent chemical (Lock & Ishmael, 1985). Male mice pre-treated with
probenecid were also protected against the nephrotoxicity produced
by hexachlorobutadiene (Ban & De Ceaurriz, 1988). The Task Group
noted that this latter study used only one marker for
nephrotoxicity.
8.9.1.6 Non-protein sulfhydryl scavengers
Depletion of hepatic and renal non-protein sulfhydryl content
(glutathione) by diethylmaleate in rats appears to potentiate the
nephrotoxicity of hexachlorobutadiene as measured by a number of
functional markers such as plasma urea (Hook et al., 1982; Baggett
& Berndt, 1984, Davis et al., 1986). However, the Task Group noted
that no information was available on the metabolism of
hexachlorobutadiene to help interpret these studies.
8.9.2 Toxicity of metabolites
This section discusses the renal toxicity of some metabolites
of hexachlorobutadiene, the formation of which was discussed in
section 6.2. These metabolites are 1-(glutathion- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (GPB), 1-(cystein- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (CPB), and 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB). Their mutagenic activity
was described along with that of hexachlorobutadiene in section 8.6.
8.9.2.1 In vitro studies
GPB decreased the viability of isolated renal epithelial cells
of male rats, as measured by leakage of lactate dehydrogenase (EC
1.1.1.27), with a very steep dose-response curve and a lag period of
30 min. No cytotoxicity was observed when the GPB metabolism was
blocked by anthglutin, an inhibitor of gamma-glutamyl-transpeptidase
(EC 2.3.2.2) or amino-oxyacetic acid (AOAA), an inhibitor of renal
cysteine conjugate ß-lyase (EC 4.4.1.13). The cytotoxicity of GPB
was related to an impairment of mitochondrial function, as shown by
loss of mitochondrial Ca2+ and ATP and inhibition of respiration
and thiol depletion (Jones et al., 1986b). Likewise, GPB produced
a concentration-dependent nephro-toxicity in the isolated perfused
rat kidney, as indicated by the appearance in the urine of alkaline
phosphatase, gamma-glutamyl-transpeptidase and glucose (Jones et
al., 1986a). These changes were prevented by Acivicin and by AOAA
(Schrenk et al., 1988a).
In the study of Schrenk et al. (1988a), CPB also caused a
marked nephrotoxicity in the isolated perfused kidney, which could
be prevented by AOAA. In isolated rabbit renal tubules, CPB was
observed to decrease the accumulation of p-amino-hippuric acid and
tetraethylammonium (Jaffe et al., 1983), to affect mitochondrial
function as shown by effects on cell respiration, and to decrease
the glutathione content and, after a lag period of 60 min, cell
viability (Schnellmann et al., 1987). The effects on respiration
resulted initially from the uncoupling of oxidative phosphorylation,
followed later by inhibition of state 3 respiration (Schnellmann et
al., 1987). Impaired mitochondrial function was observed in
CPB-exposed isolated rat renal cortical mitochondria as an inability
to retain Ca2+, collapse of the membrane potential, impaired state
3 respiration with succinate as substrate, and nonenzymatic
depletion of thiol content. The latter effect was blocked by AOAA.
From these results it was concluded that the reactive intermediate
formed from CPB interacts with the inner mitochondrial membrane
(Wallin et al., 1987). CPB also inhibited rat kidney mitochondrial
DNA, RNA and protein synthesis, and AOAA blocked this effect.
Moreover, CPB converted supercoiled DNA to relaxed circular DNA and
shorter linear fragments (Banki & Anders, 1989). Chen et al.
(1990) observed a decreased viability of isolated human renal
proximal tubular cells upon exposure to CPB, which was again blocked
by AOAA. Using radiolabelled ACPB and rat renal cortical slices, it
was established that ACPB is transported by the same renal
mechanisms involved in the movement of many organic anions into
tubular fluid. This carrier-mediated transport is reduced by
specific inhibitors like probenecid and sulfinpyrazone, a
competitive and metabolic inhibitor like 2,4-dinitrophenol, and the
transport substrate p-aminohippuric acid (Lock et al., 1986).
This was confirmed by recent studies on the mechanism of uptake of
GPB and CPB in the isolated perfused rat kidney (Schrenk et al.,
1988b). Probenecid has also been reported to protect renal proximal
tubular cells against ACPB-induced cytotoxicity, as determined by
monitoring proline incorporation into renal proteins (Bach et al.,
1986).
8.9.2.2 In vivo studies
A single oral dose of 138 mg/kg body weight (0.27 mmol) of GPB
or a single equimolar oral dose of 100 mg ACPB/kg body weight in
polyethylene glycol to male rats caused marked nephrotoxicity
similar in both biochemical and histopathological aspects to that
observed with an oral dose of 200 mg/kg body weight (0.97 mmol) of
hexachlorobutadiene (Nash et al., 1984). When rats received
intraperitoneally GPB, CPB or ACPB in polyethylene glycol at single
doses between 6.25 and 100 mg/kg body weight, increases in plasma
urea level and renal proximal tubular necrosis were observed at dose
levels of > 6.25 mg/kg body weight in females and 10 or 12.5 mg/kg
body weight in males. The conjugates exhibited a similar pattern of
nephrotoxicity at equimolar doses and were more nephrotoxic than the
parent compound (Lock & Ishmael, 1985; Ishmael & Lock, 1986). All
compounds tested were more toxic to female rats than males (Ishmael
& Lock, 1986). Probenecid pretreatment protected the rats against
the nephrotoxicity of these metabolites. Probenecid was shown to
block the active tubular secretion of ACPB and to reduce the extent
of covalent binding to renal protein (Lock & Ishmael, 1985). In
mice, GPB and ACPB were also shown to be more toxic than the parent
compound: renal necrosis was found following single intraperitoneal
doses of 5.0 mg hexachlorobutadiene/kg body weight, 3.1 mg GPB/kg
body weight and 3.0 mg ACPB/kg body weight in corn oil, which were
the lowest doses tested (Lock et al., 1984). A single
intraperitoneal dose of 10 mg CPB/kg body weight in DMSO and water
caused dose-related damage in the pars recta of renal proximal
tubules in male mice (Jaffe et al., 1983).
The nephrotoxicity of some structural analogues of the
above-mentioned conjugates, e.g. S-(1,2-dichlorovinyl)-L-cysteine,
has been investigated extensively and has revealed a remarkable
similarity (Anders et al., 1987; Lock, 1988).
8.10 Mechanisms of toxicity - mode of action
8.10.1 Mechanisms of toxicity
The following evidence supports the hypothesis that the
nephrotoxicity, mutagenicity and carcinogenicity of
hexachloro-butadiene is dependent on the biosynthesis of the toxic
sulfur conjugate GPB. This conjugate is mainly synthetized in the
liver and further metabolized in the bile, gut, and kidneys to the
CPB. Cysteine conjugate ß-lyase-dependent activation of CPB to a
reactive thioketene in the proximal tubular cells finally results in
covalent binding to cellular macromolecules.
1. The nephrotoxicity of hexachlorobutadiene in rats was prevented
by the implantation of a biliary cannula; administration of
bile from hexachlorobutadiene-treated rats to naive rats
resulted in nephrotoxicity identical to the nephrotoxicity
caused by hexachlorobutadiene (see section 8.9.1.1).
2. Inhibitors of renal organic anion transport protected rats
against the nephrotoxicity of hexachlorobutadiene and its
sulfur conjugates. Inhibition of the organic anion transport
also protected isolated kidney cells against the nephrotoxicity
of hexachlorobutadiene-derived sulfur-conjugates (see sections
8.9.1.3 and 8.9.2.1).
3. Anthglutin, Acivicin and aminooxyacetic acid, specific
inhibitors of gamma-glutamyltranspeptidase and cysteine
conjugate ß-lyase protected against the cytotoxicity of
hexachloro-butadiene-derived sulfur-conjugates in freshly
isolated rat renal proximal tubular cells (see section
8.9.2.1).
4. Synthetic sulfur-conjugates of hexachlorobutadiene show a
higher nephrotoxicity than the parent compounds in rats and
mice and produce renal damage identical to the renal damage
induced by hexachlorobutadiene, based on clinical chemistry and
histopathological examination (see section 8.9.2.2).
5. Hexachlorobutadiene and its sulfur-conjugates are genotoxic in
bacteria; bioactivation by glutathione conjugation is required
for hexachlorobutadiene genotoxicity. The ultimate mutagen is
formed by cysteine conjugate ß-lyase-dependent cleavage of CPB
(see section 8.6).
6. Hexachlorobutadiene induces renal tumours in rats only at doses
that produce marked nephrotoxicity (see sections 8.7 and
8.8.2.2).
8.10.2 Mode of action
The in vitro studies of Jones et al. (1986b), Wallin et
al. (1987) and Schnellmann et al. (1987) on the cytotoxicity of
sulfur-conjugates to renal tubular cells (section 8.9.1.2) point to
renal cortical mitochondria as the major target for
sulfur-conjugates of hexachlorobutadiene, analogous to that
established for close structural analogues (Dekant et al., 1990b).
The hypothesis proposes an interaction of the reactive metabolite
with the inner mitochondrial membrane, which ultimately causes
respiratory insufficiency.
9. EFFECTS ON HUMANS
9.1 General population exposure
Hexachlorobutadiene has been found in postmortem examinations,
but not in living persons. No pathogenic effects have been recorded
(see section 5.2).
9.2 Occupational exposure
Two reports on certain disorders among agricultural workers in
vineyards where hexachlorobutadiene has been used as a fumigant
(Krasniuk et al., 1969; Burkatskaya et al., 1982) cannot be
evaluated, since such workers are known to be occupationally exposed
to additional substances.
In two cytogenetic studies of occupationally exposed workers
from the same plant engaged in the production of
hexachloro-butadiene, an increase in the frequency of chromosomal
aberrations in peripheral blood lymphocytes was observed (German,
1986). The workers were exposed to hexachloro-butadiene
concentrations that ranged from 1.6 to 16.9 mg/m3. The Task Group
noted that exposure concentrations were determined by the factory
and that the frequency of chromosome aberrations was not associated
with the period of employment.
9.3 In vitro metabolism studies
The following studies have been reported:
a) Purified human liver microsomal glutathione- S-transferase
and human liver cytosol metabolize hexachlorobutadiene to form
GPB (McLellan et al., 1989; Oesch & Wolf, 1989).
b) The enzyme cysteine conjugate ß-lyase has been isolated and
purified from human kidney cytosol (Lash et al., 1990). The
activity of the human cytosolic enzymes with a structurally
related compound (1,2,2-trichlorovinyl-L-cysteine) is about
10-fold lower than that of rat renal cytosol (Green et al.,
1990).
c) Studies in isolated human proximal tubular cells have shown
that CPB causes a ß-lyase-dependant cytotoxicity (Chen et al.,
1990).
These limited studies suggest that humans have the ability to
metabolize hexachlorobutadiene to toxic metabolites.
9.4 Extrapolation of NOAEL from animals to humans
Conversion of equivalent doses across species can utilize
allometric relationships that relate physiological and anatomical
variables across species. Physiological and metabolic rates have
been shown to relate closely to body weight to the power 0.75
(Boxenbaum, 1982). The equivalent NOAEL in humans (mg/kg body weight
per day) can be determined from the following equation:
Wa
dh = da (--)0.25
Wh
where
d = dose rate (mg/kg body weight per day) in humans (dh) or
animals (da)
wa = weight (kg) of animals (mice 30 g; rats 400 g)
wh = weight of humans (70 kg)
The NOAEL for humans, based on the NOAEL in mice, is:
dh= (0.2 mg/kg body weight per day) (0.03)0.25
70
= 0.03 mg/kg body weight per day
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Hazard identification
The following evaluation is based on toxicity studies in
experimental animals; however, there are some human in vitro data
which indicate that hexachlorobutadiene metabolism can occur by a
similar route to that shown in experimental animals.
Hexachlorobutadiene is slightly to moderately toxic based on
acute oral experiments with adult rats, and moderately to highly
toxic based on acute oral experiments with weanling rats (based on
the WHO pesticide toxicity classification). The specific toxicity of
the compound to the kidneys is higher for females than males.
Following acute dermal exposure of rabbits, the compound was found
to be weakly toxic.
Regardless of species studied and the route of exposure (ip,
oral, inhalation, dermal) in both short- and long-term studies, the
target organ for toxicity is the kidney. Bioactivation to produce a
reactive sulfur metabolite occurs following conjugation with
glutathione. The monoglutathione conjugate of hexachloro-butadiene
is processed to the cysteine- S-conjugate, which is then a
substrate for renal cysteine conjugate ß-lyase. Hexachloro-butadiene
produces a dose-dependent necrosis of the renal proximal tubules,
which is followed by regenerative and/or proliferative changes. On
the basis of both short- and long-term studies in rats and mice
orally exposed to hexachlorobutadiene, the no-observed-adverse-
effect level (NOAEL) is 0.2 mg/kg body weight per day. In one
short-term inhalation study (12 days, 6 h/day), the NOAEL was
53 mg/m3.
The vapour of hexachlorobutadiene was found to be irritating to
the eyes and nose of rats in one short-term inhalation study. The
undiluted compound appeared corrosive in an experiment with rabbits.
Based on these limited data, the vapour should be regarded as
irritating to human mucous membranes and the liquid should be
regarded as corrosive.
In a well-conducted Magnusson-Kligman test hexachloro-butadiene
was a sensitizing agent both with and without adjuvant. Therefore,
the compound should be regarded as a sensitizing agent for humans.
In reproductive studies, reduced birth weight and neonatal
weight gain in rats were observed, but these effects may be
attributed to maternal toxicity. Developmental toxicity to rat
fetuses was observed in two teratogenicity tests, but again only at
levels that were also toxic to the dams. This developmental toxicity
included reduced birth weight, a 1- to 2-day delay in heart
development, and dilated ureters, but no gross abnormalities were
observed.
In vitro studies have shown that hexachlorobutadiene and, to
a much greater extent, its sulfur metabolites induce mutations in
Salmonella typhimurium. In one study of exposure to
hexachloro-butadiene by inhalation or oral administration, an
increased frequency of chromosomal aberrations was observed in mouse
bone marrow cells. There is limited evidence for the genotoxicity of
hexachlorobutadiene in animals, and insufficient evidence in humans.
The long-term oral administration of hexachlorobutadiene to
rats induced an increased frequency of renal tubular neoplasms, but
only at doses that caused marked nephrotoxicity; at the lowest dose,
no adverse effects were observed.
The Task Group concluded that there is limited evidence for the
carcinogenicity of hexachlorobutadiene in animals (one study in one
rodent strain) and insufficient evidence in humans.
10.1.2 Exposure
Hexachlorobutadiene is mainly a waste product. As such, it can
be encountered in different environmental compartments, but
predominantly in sediment and biota (see also 10.2.1). Exposure of
the general public therefore mainly occurs indirectly via
drinking-water and food of high lipid content. Assuming a maximum
concentration of 2.5 µg/litre in contaminated drinking-water and
10 µg/kg wet weight in contaminated fatty food items (meat, fish,
milk) and daily intakes of 2 litres drinking-water, 0.3 kg meat,
0.2 kg fish and 0.5 kg milk, a maximum total daily intake of
0.2 µg/kg body weight can be calculated for a 70-kg person.
10.1.3 Hazard evaluation
The NOAEL for mice or rats exposed to hexachlorobutadiene is
0.2 mg/kg body weight per day (see Table 15), from which a NOAEL of
0.03-0.05 mg/kg body weight day has been derived for humans (see
section 9.4).
The Task Group considered the margin of safety of 150 between
the estimated NOAEL in humans and the maximum total daily intake
(see section 10.1.2) to be sufficient to protect the general
population against the adverse effects of hexachlorobutadiene.
10.2 Evaluation of effects on the environment
10.2.1 Hazard identification
Hexachlorobutadiene is a chemically stable compound. Complete
aerobic biodegradation has been observed following adaptation of the
inoculum. Partial biodegradation was found to occur in a pilot
sewage treatment plant. Based on these observations and the chemical
structure, it can be concluded that hexachlorobutadiene is not
readily biodegradable, but can be considered to be inherently
biodegradable. Experimental photolysis of hexachlorobutadiene in the
presence of a surface was rapid, but in the absence of a surface the
compound is believed to be persistent. Degradation in the atmosphere
is assumed to occur by a rather slow reaction with hydroxyl
radicals. A half-life of up to 2.3 years has been calculated.
Once hexachlorobutadiene is released into the environment,
intercompartmental transport will occur chiefly by volatilization
from water and soil, adsorption to particulate matter in water and
air, and subsequent sedimentation or deposition. In view of a strong
adsorption potential to organic matter, the compound accumulates in
sediment and will not migrate rapidly in soils. Both field and
laboratory exposure data support these conclusions.
Field and laboratory data also support the high
bioaccumu-lation potential in aquatic and benthic organisms which
can be expected on the basis of the lipophilic nature of the
compound. However, no evidence has been obtained for
biomagnification.
Hexachlorobutadiene is moderately to highly toxic to aquatic
organisms; crustaceans and fish are the most sensitive species. The
lowest E(L)C50 for freshwater organisms is 0.09 mg/litre
(goldfish). The lowest chronic NOEC is 3 µg/litre (goldfish).
Applying the preliminary effect assessment extrapolation procedure,
as adopted in the OECD Workshop on Aquatic Effect Assessment (OECD,
1990), an Environmental Concern Level of 0.1 µg/litre can be
established.
The toxicity data on terrestrial organisms are insufficient to
establish any toxicity threshold.
10.2.2 Exposure
Current environmental levels in surface waters are generally
below 0.2 µg/litre, rising to 1.3 µg/litre in highly polluted
rivers. Levels in the upper sediment can be as high as 120 µg/kg in
heavily polluted rivers or estuaries. In older sediment layers much
higher concentrations can be measured. The concentrations in
freshwater biota measured since 1980 generally do not exceed
100 µg/kg fresh weight, but in a polluted area can reach 120 mg/kg
in the lipid of fish.
10.2.3 Hazard evaluation
It can be concluded that away from point sources the maximum
predicted environmental concentration (PEC) is twice the
extrapolated Environmental Concern Level of 0.1 µg/litre. Aquatic
organisms therefore may be at risk in polluted surface waters.
In view of the rather high concentrations of the compound
measured in some sediments, adverse effects on benthic organisms
cannot be excluded.
Considering the toxicity of the substance to mammals (the NOAEL
for rats or mice is 0.2 mg/kg body weight per day) and its high
bioaccumulating potential, the consumption of benthic or aquatic
organisms in polluted surface water by other species may give cause
for concern. For example, an otter weighing 10 kg and consuming 1 kg
fish per day in waters containing 0.2 µg hexachlorobutadiene/litre
could ingest 1200 µg/day (assuming a bioconcentration factor for
fish of 6000, leading to a concentration of 1200 µg/kg wet weight)
or 120 µg/kg body weight per day, which is above the calculated
NOAEL value for the otter (calculated as in section 9.4).
11. FURTHER RESEARCH
Hexachlorobutadiene is primarily a waste product and hence an
environmental contaminant having only limited use as a fumigant in
some parts of the world. The Task Group identified the following
areas for which additional information is needed:
a) the degradation of hexachlorobutadiene in the environment
focusing on photodegradation and biodegradation;
b) the terrestrial toxicity of hexachlorobutadiene including tests
on benthic organisms;
c) the genotoxic activity of hexachlorobutadiene in vivo. A
further test for micronucleus or chromosome aberration
induction in mouse bone marrow cells would strengthen the
available data;
d) the metabolism of hexachlorobutadiene and its glutathione-
derived conjugates by human liver and renal enzymes and
inter-individual variability.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risk of hexachlorobutadiene was evaluated by
the International Agency for Research on Cancer in 1979 (IARC,
1979).
The summary of data reported and the evaluation of the IARC
monograph on hexachlorobutadiene is reproduced here.
Experimental data
Hexachlorobutadiene was tested in one experiment in rats by
oral administration: it produced benign and malignant tumours in the
kidneys of animals of both sexes. It was tested inadequately in one
experiment in mice by intraperitoneal injection.
Human data
No case reports of epidemiological studies were available to
the Working Group.
The occurrence of hexachlorobutadiene as a by-product in the
production of various chlorinated hydrocarbons for over 50 years and
its use in some areas as a pesticide indicate that widespread human
exposure in both the occupational and general environment occurs.
This is confirmed by reports of its occurrence in the environment.
Evaluation
There is limited evidence that hexachlorobutadiene is
carcinogenic in rats.
REFERENCES
Alberti J (1983) [Organic contaminants in river sediment.] Vom
Wasser, 61: 149-154 (in German).
Alberti J & Plöger E (1986) [Hazardous organic chemicals in sewage
sludge.] Gewasserschutz Wasser Abwasser, 85: 483-498 (in German).
Anders MW, Elfaeea AA, & Lash LH (1987) Cellular effects of reactive
intermediates: nephrotoxicity of S-conjugates of amino acids. Arch
Toxicol, 60: 103-108.
Bach PH, Ketley CP, Ahmed I, & Dixit M (1986) The mechanisms of
target cell injury by nephrotoxins. Food Chem Toxicol, 24: 775-779.
Badaeva LN, Ovsyannikova LM, & Kiseleva NI (1985) [Manifestation of
neurotoxic effect of chloroorganic pesticide hexachlorobutadiene
during postnatal period of ontogenesis in rats.] Arch Anat Gistol
Embriol, 89: 44-49 (in Russian).
Baggett JM & Berndt WO (1984) Renal and hepatic glutathione
concentrations in rats after treatment with hexachloro-1-3-butadiene
and citrinin. Arch Toxicol, 56: 46-49.
Bai CL, Canfield PJ, & Stacey NH (1992) Effects of
hexachloro-1,3-butadiene and 1,1,2,2-tetrachloroethylene on
individual serum bile acids. Toxicol Ind Health, 8: 191-203.
Ban M & De Ceaurriz J (1988) Probenecid-induced protection against
acute hexachloro-1,2-butadiene and methyl mercury toxicity to the
mouse kidney. Toxicol Lett, 40: 71-76.
Banerjee S, Yalkowsky SH, & Valvani SC (1980) Water solubility and
octanol/water partition coefficients of organics. Limitations of the
solubility-partition coefficient correlation. Environ Sci Technol,
14: 1227-1229.
Banki K & Anders MW (1989) Inhibition of rat kidney mitochondrial
DNA, RNA and protein synthesis by halogenated cysteine S-conjugates.
Carcinogenesis, 10: 767-772.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - a
multimedia environmental study. Biomed Mass Spectrom, 7: 139-146.
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow
Pinephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berndt WO & Mehendale HM (1979) Effects of hexachlorobutadiene
(HCBD) on renal function and renal organic ion transport in the rat.
Toxicology, 14: 55-65.
Boxenbaum H (1982) Inter-species scaling, allometry, physiologic
time, and the ground plan of pharmacokinetics. J Pharmacokinet
Biopharm, 10: 201-227.
Bristol DW, Crist HL, Lewis RG, MacLeod KE, & Sovocool GW (1982)
Chemical analysis of human blood for assessment of environmental
exposure to semivolatile organochlorine chemical contaminants. J
Anal Toxicol, 6: 269-275.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds. ACS
Symp Ser, 149: 49-64.
Bringmann G & Kühn R (1977) [Limiting values of the harmful action
of water-endangering substances on bacteria (Pseudomonas putida)
and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z Wasser-Abwasser Forsch, 10: 87-98
(in German).
Brown SL, Chan FY, Jones JL, Liu DH, McCaleb KE, Mill T, Sapios KN,
& Schendel DE (1975) Research program on hazard priority ranking of
manufactured chemicals (chemicals 1-20). Menlo Park, California,
Stanford Research Institute (NSF/RA/E-75/190A; PB 263161).
Burkatskaya EN, Viter VF, Ivanova ZV, Kaskevitch LM, Gorskaya NZ,
Kolpakov IE, & Deineka KA (1982) [Clinico-hygienic data on working
conditions during use of hexachlorobutadiene in vineyards.] Vrach
Delo, 11: 99-102 (in Russian).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13: 296-309.
Canton JH, Linders JBHJ, Luttik R, Mensink BJWG, Panman E, Van de
Plassche EJ, Sparenburg PM, & Tuinstra J (1990) Catching-up
manoeuvre old pesticides: an integration. Bilthoven, The
Netherlands, National Institute of Health and Environmental
Protection (Report No. 678801001).
CESARS (198l) Hexachlorobutadiene, latest update June 1981.
Baltimore, Maryland, Chemical Evaluation Search and Retrieval
System, Inc.
Chen JC, Stevens JL, Trifillis AL, & Jones TW (1990) Renal cysteine
conjugate betalyase-mediated toxicity studied with primary cultures
of human proximal tubular cells. Toxicol Appl Pharmacol, 103:
463-473.
Chiou CT (1985) Partition coefficients of organic compounds in
lipid-water systems and correlations with fish bioconcentration
factors. Environ Sci Technol, 19: 57-62.
Chudin VA, Gafieva ZA, Koshurnikova NA, Nifatov AP, & Revina VS
(1985) [Evaluating the mutagenicity and carcinogenicity of
hexachlorobutadiene.] Gig i Sanit, 5: 79-80 (in Russian).
Clark CS, Meyer CR, Gartside PS, Majeti VA, Specker B, Balistreri
WF, & Elia VJ (1982) An environmental health survey of drinking
water contamination by leachate from a pesticide waste dump in
Hardeman County, Tennessee. Arch Environ Health, 37: 9-18.
Clark JR, Devault D, Bowden RJ, & Weishaar JA (1984) Contaminant
analysis of fillets from Great Lakes coho salmon. J Great Lakes Res,
10: 38-47.
Class T & Ballschmiter K (1987) Global baseline pollution studies.
X. Atmospheric halocarbons: global budget estimations for
tetrachloroethene, 1,2-dichloroethane, 1,1,1,2-tetrachloroethane,
hexachloroethane and hexachlorobutadiene. Estimation of the hydroxyl
radical concentrations in the troposphere of the northern and
southern hemisphere. Fresenius Z Anal Chem, 327: 198-225.
Davis ME (1984) Changes of hexachlorobutadiene nephrotoxicity after
piperonyl butoxide treatment. Toxicology, 30: 217-225.
Davis ME (1988) Effects of AT-125 on the nephrotoxicity of
hexachloro-1,2-butadiene in rats. Toxicol Appl Pharmacol, 95: 44-52.
Davis ME, Berndt WO, & Mehendale HM (1980) Disposition
nephrotoxicity of hexachloro-1,3-butadiene. Toxicology, 16: 179-191.
Davis ME, Berndt WO, & Mehendale HM (1986) Effects of cysteine and
diethylmalate pretreatments on renal function and response to a
nephrotoxicant. Arch Toxicol, 59: 7-11.
De Ceaurriz J & Ban M (1990) Role of gamma-glutamyltranspeptidase
and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and
methyl mercury in mice. Toxicol Lett, 50: 249-256.
De Ceaurriz J, Gagnaire F, Ban M, & Bonnet P (1988) Assessment of
the relative hazard involved with airborne irritants with additional
hepatotoxic or nephrotoxic properties in mice. J Appl Toxicol, 8:
417-422.
Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, & Henschler D
(1986) Bacterial beta-lyase mediated cleavage and mutagenicity of
cysteine conjugates derived from the nephrocarcinogenic alkenes
trichloroethylene, tetrachloroethylene and hexachloro-butadiene.
Chem-Biol Interact, 60: 31-45.
Dekant W, Schrenk D, Vamvakas S, & Henschler D (1988a) Metabolism of
hexachloro-1,3-butadiene in mice: in vivo and in vitro evidence
for activation by glutathione conjugation. Xenobiotica, 18: 803-816.
Dekant W, Vamvakas S, Henschler D, & Anders MW (1988b) Enzymatic
conjugation of hexachloro-1,3-butadiene with glutathione. Formation
of 1-(glutathion- S-yl)-1,2,3,4,4-pentachlorobuta-1,3-diene and
1,4-bis(glutathion-S-yl)-1,2,3,4-tetrachloro-1,3-butadiene. Drug
Metab Dispos, 16: 701-706.
Dekant W, Berthold K, Vamvakas S, & Henschler D (1988c)
Thioacylating agents as ultimate intermediates in the beta-lyase
catalysed metabolism of S-(pentachloro-butadienyl)-L-cysteine.
Chem-Biol Interact, 67: 139-148.
Dekant W, Vamvakas S, & Anders MW (1990a) Bioactivation of
hexachlorobutadiene by glutathione conjugation. Food Chem Toxicol,
28: 285-293.
Dekant W, Vamvakas S, Koob M, Kochling A, Kanhai W, Muller D, &
Henschler D (1990b) A mechanism of haloalkene-induced renal
carcinogenesis. Environ Health Perspect, 88: 107-110.
Dekant W, Urban G, Gorsman C, & Anders MW (1991) Thioketene
formation from alpha-haloalkenyl 2-nitrophenyl disulfides: models
for biological reactive intermediates of cytotoxic S-conjugates. J
Am Chem Soc, 113: 5120-5122.
Demarini DM, Inmon JP, Simmons JE, Berman E, Pasley TC, Warren SH, &
Williams RW (1987) Mutagenicity in Salmonella of hazardous wastes
and urine from rats fed these wastes. Mutat Res, 189: 205-216.
De Meester C, Mercier M, & Poncelet F (1981) Mutagenic activity of
butadiene, hexachlorobutadiene, and isoprene. In: Gut I, Cifert M, &
Plaa GL ed. Industrial environmental xenobiotics. Proceedings of an
International Conference, 1980. Berlin, Heidelberg, New York,
Springer Verlag, pp 196-203.
Dillon HK (1979) Development of air sampling and analytical methods
for toxic chlorinated organic compounds: Research report for
hexachlorobutadiene. Birmingham, Alabama, Southern Research
Institute (SORI-EAS-79-658; PB 87-126934/AS).
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Duprat P & Gradiski D (1978) Percutaneous toxicity of
hexachlorobutadiene. Acta Pharmacol Toxicol, 43: 346-353.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur J Toxicol, 9: 171-177.
Durham RW & Oliver BG (1983) History of Lake Ontario contamination
from the Niagara river by sediment radiodating and chlorinated
hydrocarbon analysis. J Great Lakes Res, 9: 160-168.
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with US EPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 29:
460.467.
Fox ME, Carey JH, & Oliver BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the western
basin of Lake Ontario. J Great Lakes Res, 9: 287-294.
Gäb S, Schmitzer J, Thamm HW, Parlar H, & Korte F (1977)
Photomineralization rate of organic compounds adsorbed on
particulate matter. Nature (Lond), 270: 331-333.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gartland KPR, Bonner FW, & Nicholson JK (1989) Investigations into
the biochemical effects of region-specific nephrotoxins. Mol
Pharmacol, 35: 242-250.
Gauntley P, Magadur JL, Morel G, Chaumont P, & Canel F (1975) Dosage
de l'hexachlorobutadiène dans les milieux biologiques. Eur J
Toxicol, 8: 152-158.
German IV (1986) [Level of chromosome aberrations in worker coming
in contact with hexachlorobutadiene during production.] Gig Tr Prof
Zabol, 5: 57-59 (in Russian).
German IV (1988) [On mutagenic activity of the pesticide
hexachlorobutadiene.] Citol Gen, 22(4): 40-42 (in Russian).
German IV & Viter VF (1985) Evaluation of worker's health in Dactal
and hexachlorobutadiene production processes. Hyg Employ Toxicol
Pestic Polym, 15: 32-34.
Gietl YS & Anders MW (1991) Biosynthesis and biliary excretion of
S-conjugates of hexachlorobuta-1,3-diene in the perfused rat liver.
Drug Metab Dispos, 19: 274-277. Gietl YS, Vamvakas S, & Anders MW
(1991) Intestinal absorption of S-(pentachloro-butadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine,
S-conjugates of hexachlorobuta-1,3-diene. Drug Metab Dispos, 19:
703-707.
Giger W & Schaffner C (1981) Groundwater pollution by volatile
organic chemicals. In: Van Duijvenbooden W, Glasbergen P, & Van
Lelyveld H ed. Quality of groundwater. Proceedings of an
International Symposium, Noordwijkerhout, The Netherlands, 23-27
March 1981. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 517-522 (Studies in Environmental Sciences, Volume
17).
Goldbach RW, Van Genderen H, & Leeuwangh P (1976)
Hexachlorobutadiene residues in aquatic fauna from surface water fed
by the river Rhine. Sci Total Environ, 6: 31-40.
Gradiski D, Duprat P, Magadur JL, & Fayein E (1975) Etude
toxicologique expérimentale de l'hexachlorobutadiène. Eur J Toxicol,
8: 180-187.
Green T & Odum J (1985) Structure/activity studies of the
nephrotoxic and mutagenic action of cysteine conjugates of chloro-
and fluoroalkenes. Chem-Biol Interact, 54: 15-31.
Green T, Odum J, Nash JA, & Foster JR (1990)
Perchloroethylene-induced rat kidney tumors: an investigation of the
mechanisms involved and their relevance to humans. Toxicol Appl
Pharmacol, 103: 80-89.
Guzewich DC, Curtis AB, Valis R, Vindpal JH, & Deeter DP (1983) Air
sampling around a hazardous liquid surface impoundment. 76th Annual
Meeting of the Air Pollution Control Association, Atlanta, USA.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Haberer K, Normann S, & Drews M (1988) [Tests on certain trace
substances from the Rhine and Main with a view to obtaining
drinking-water.] Wasser Abwasser, 129: 45-49 (in German).
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harleman JH & Seinen W (1979) Short-term toxicity and reproduction
studies in rats with hexachloro-(1,3)-butadiene. Toxicol Appl
Pharmacol, 47: 1-14.
Haworth S, Lawloer T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hellmann H (1987a) [Model tests on volatilization of organic trace
substances in surface waters.] Fresenius Z Anal Chem, 328: 475-479
(in German).
Hellmann H (1987b) [Investigation of the degree of concentration of
organic trace substances on clay minerals.] Fresenius Z Anal Chem,
327: 524-529 (in German).
Hermens J, Busser F, Leeuwang P, & Musch A (1985) Quantitative
correlation studies between the acute lethal toxicity of 15 organic
halides to the guppy (Poecilia reticulata) and chemical reactivity
towards 4-nitrobenzylpyridine. Toxicol Environ Chem, 9: 219-236.
Hook JB, Rose MS, & Lock EA (1982) The nephrotoxicity of
hexachloro-1,3-butadiene in the rat: studies of organic anion and
cation transport in renal slices and the effect of monoxygenase
inducers. Toxicol Appl Pharmacol, 65: 373-382.
Hook JB, Ishmael J, & Lock EA (1983) Nephrotoxicity of
hexachloro-1,3-butadiene in the rat: the effect of age, sex, and
strain. Toxicol Appl Pharmacol, 67: 122-131.
Hutzinger O (1982) Handbook of environmental chemistry: 3B.
Anthropogenic sources. Berlin, Heidelberg, New York, Springer
Verlag, pp 69-87.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer, pp 179-189 (IARC Monograph on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
20).
Ishmael J & Lock EA (1986) Nephrotoxicity of hexachlorobutadiene and
its glutathione-derived conjugates. Toxicol Pathol, 14: 258-262.
Ishmael J, Pratt I, & Lock EA (1982) Necrosis of the pars recta (S3
segment) of the rat kidney produced by hexachloro-1,3-butadiene. J
Pathol, 138: 99-113.
Ishmael J, Pratt I, & Lock EA (1984) Hexachloro-1,3-butadiene-
induced renal tubular necrosis in the mouse. J Pathol, 142: 195-203.
Jaffe DR, Hassall CD, Brendel K, & Gandolfi AJ (1983) in vivo and
in vitro nephrotoxicity of the cysteine conjugate of
hexachlorobutadiene. J Toxicol Environ Health, 11: 857-867.
Jacobs A, Blangetti M, & Hellmund E (1974) [Accumulation of
chlorinated contaminants of the Rhine in fat tissue of rats.] Vom
Wasser, 43: 259-274 (in German).
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed, 55:
1441-1449.
Jones TW, Gerdes RG, Ormstad K, & Orrenius S (1985) The formation of
both a mono-and a bis-substituted glutathione conjugate of
hexachlorobutadiene by isolated hepatocytes and following in vivo
administration to the rat. Chem-Biol Interact, 56: 251-267.
Jones TW, Wallin A, Thor H, Gerdes RG, Ormstad K, & Orrenius S
(1986a) The mechanism of pentachlorobutadienyl-glutathione
nephrotoxicity studies with isolated rat renal epithelial cells.
Arch Biochem Biophys, 251: 504-513.
Jones TW, Thor H, & Orrenius S (1986b) In vitro studies of
mechanisms of cytotoxicity. Food Chem Toxicol, 24: 769-773.
Jones TW, Quin C, Schaeffer VH, & Stevens JL (1988)
Immunohistochemical localization of glutamine transaminase K, a rat
kidney cysteine conjugate beta-lyase, and the relationship to the
segment specificity of cysteine conjugate nephrotoxicity. Mol
Pharmacol, 34(5): 621-627.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites n Lake Ontario. J Great Lakes Res, 9:
183-189.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soil. Chemosphere,
10: 833-846.
Kastl PE & Hermann BA (1983) Quantitative gas chromatographic
determination of hexachloro-1,3-butadiene in whole rat blood at part
per trillion levels. J Chromatogr, 280(2): 390-393.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St Clair and Detroit rivers using introduced
clams Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci
Health, A21: 15-53.
Kluwe WM, McNish R, Smithson K, & Hook JB (198l) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane,
tris(2,3-dibromopropyl)phosphate, and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and non-target
organs. Biochem Pharmacol, 30: 2265-2271.
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and
in vitro. J Pharmacol Exp Ther, 230: 597-603.
Kociba RJ, Gehring PJ, Humiston CG, & Sparschu GL (1971) Toxicologic
study of female rats administered hexachlorobutadiene or
hexachlorobenzene for thirty days. Midland, Michigan, Dow Chemical
Company, Chemical Biology Research (Unpublished report).
Kociba RJ, Keyes DG, Jersey GC, Ballard JJ, Dittenber DA, Quast JF,
Wade CE, Humiston CG, & Schwetz BA (1977a) Results of a two year
chronic toxicity study with hexachlorobutadiene in rats. Am Ind Hyg
Assoc J, 38: 589-602.
Kociba RJ, Schwetz BA, Keyes DG, Jersey GC, Ballard JJ, Dittenber
DA, Quast JF, Wade CE, & Humiston CG (1977b) Chronic toxicity and
reproduction studies of hexachloro-butadiene in rats. Environ Health
Perspect, 21: 49-53.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part I: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Koob M & Dekant W (1991) Bioactivation of xenobiotics by formation
of toxic glutathione conjugates. Chem-Biol Interact, 77: 107-136.
Kotzias D, Lay JP, Klein W, & Korte F (1975) [Analysis of residues
of hexachloro-butadiene in foodstuffs and poultry.] Chemosphere, 4:
247-250 (in German).
Krasniuk EP, Ziritskaya LA, Bioko VG, Voitenko GA, & Matokhniuk LA
(1969) [Health condition of vine-growers contacting with fumigants
hexachlorobutadiene and polychlorbutan-80.] Vrach Delo, 7: 111-115
(in Russian).
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuo CH, & Hook JB (1983) Effects of age and sex on
hexachloro-1,3-butadiene toxicity in the Fischer 344 rat. Life Sci,
33: 517-523.
Kusz P, Andriysiak A, & Pokorska Z (1984) Gas chromatographic
monitoring of the chlorolysis processes of some byproducts from
vinyl chloride, allyl chloride and epichlorohydrin production. J
Chromatogr, 286: 287-291.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW,
& Evans RL (1976) An ecological study of hexachlorobutadiene (HCBD).
New Orleans, Louisiana, University of New Orleans, Department of
Biological Sciences (Prepared for the US Environmental Protection
Agency, Office of Toxic Substances, Washington (EPA 560/6-76-010; PB
252671).
Lash LH, Nelson RM, Dyke RA, & Anders MW (1990) Purification and
characterization of human kidney cytosolic cysteine conjugate
beta-lyase activity. Drug Metab Dispos, 18: 50-54.
Leeuwangh P, Bult H, & Schneiders L (1975) Toxicity of
hexachlorobutadiene in aquatic organisms. In: Koeman JH & Strik
JJTWA ed. Sublethal effects of toxic chemicals on aquatic animals.
Proceedings of the Swedish-Netherlands Symposium, Waningen, The
Netherlands, 2-5 September 1975. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 167-176.
Li RT, Going JE, & Spigarelli JL (1976) Sampling and analysis of
selected toxic substances: Task I B. Hexachlorobutadiene. Kansas
City, Missouri, Midwest Research Institute (EPA Contract No.
68-01-2646).
Litvinov PI & Gorenshtein RS (1982) [Migration of
hexachlorobutadiene.] Zashch Rast (Moscow), 8: 33-34 (in Russian).
Lock EA (1987a) Metabolic activation of halogenated chemicals and
its relevance to nephrotoxicity. In: Bach PH & Lock EA ed.
Nephrotoxicity in the experimental and clinical situation.
Dordrecht, Martinus Nijhoff Publishers, pp 429-461.
Lock EA (1987b) The nephrotoxicity of haloalkane and haloalkene
glutathione conjugates. In: DeMatteis F & Lock EA ed. Selectivity
and molecular mechanisms of toxicity. New York, McMillan Publishing
Company, pp 59-83.
Lock EA (1988) Studies on the mechanism of nephrotoxicity and
nephrocarcinogenicity of halogenated alkenes. CRC Crit Rev Toxicol,
19: 23-42.
Lock EA & Ishmael J (1979) The acute toxic effects of
hexachloro-l,3-butadiene on the rat kidney. Arch Toxicol, 43: 47-57.
Lock EA & Ishmael J (1981) Hepatic and renal nonprotein sulfhydryl
concentration following toxic doses of hexachloro-1,3-butadiene in
the rat: the effect of Aroclor 1254, phenobarbitone, or SKF 525A
treatment. Toxicol Appl Pharmacol, 57: 79-87.
Lock EA & Ishmael J (1985) Effect of the organic acid transport
inhibitor probenecid on renal cortical uptake and proximal tubular
toxicity of hexachloro-1,3-butadiene and its conjugates. Toxicol
Appl Pharmacol, 81: 32-42.
Lock EA, Ishmael J, & Pratt I (1982) Hydropic change in rat liver
induced by hexachloro-1,3-butadiene. J Appl Toxicol, 2: 315-320.
Lock EA, Ishmael J, & Hook JB (1984) Nephrotoxicity of
hexachloro-1,3-butadiene in the mouse: the effect of age, sex,
strain, monooxygenase modifiers, and the role of glutathione.
Toxicol Appl Pharmacol, 72: 484-494.
Lock EA, Pratt I, & Ishmael J (1985)
Hexachloro-1,3-butadiene-induced hydropic change in mouse liver. J
Appl Toxicol, 5: 74-79.
Lock EA, Odum J, & Ormond P (1986) Transport of
N-acetyl-S-pentachloro-1,3-butadi-ethylcysteine by renal cortex.
Arch Toxicol, 59: 12-15.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55: 881-889.
Lopez-Avila V, Dodhiwala NS, Milanes J, & Beckert W (1989)
Evaluation of EPA method 8120 for determination of chlorinated
hydrocarbons in environmental samples. J Assoc Off Anal Chem, 72:
593-602.
McConnell G, Ferguson DM, & Pearson CR (1975) Chlorinated
hydrocarbon and the environment. Endeavour, 34: 13-18.
McFarlane M, Foster JR, Gibson GG, King LJ, & Lock EA (1989)
Cysteine conjugate beta-lyase of rat kidney cytosol:
characterization, immunocytochemical localization, and correlation
with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol, 98:
185-197.
McLellan LI, Wolf CR, & Hayes JD (1989) Human microsomal glutathione
S-transferase. Its involvement in the conjugation of
hexachlorobuta-1,3-diene with glutathione. Biochem J, 258: 87-93.
Malins DC, Krahn MM, Myers MS, Rhoses LD, Brown DW, Krone CA, McCain
BB, & Chan SI (1985) Toxic chemicals in sediments and biota from a
creasote-polluted harbor: relationships with hepatic neoplasms and
other hepatic lesions in English sole (Parophrys vetulus).
Carcinogenesis, 6: 1463-1469.
Mann JB, Enos HF, Gonzales J, & Thompson JF (1974) Development of
sampling and analytical procedure for determining hexachlorobenzene
and hexachloro-1,3-butadiene in air. Environ Sci Technol, 8:
584-585.
Markovec LM & Magee RJ (1984) Identification of major
perchloroaromatic compounds in waste products from the production of
carbon tetrachloride and tetrachloroethylene. Analyst, 109: 497-501.
Meijers AP (1988) [Organic trace substances in Rhine water and in
filtrate from the banks of the Rhine.] Wasser Abwasser, 129:
208-211.
Melcher RG & Caldecourt VJ (1980) Delayed injection-preconcentration
gas chromatographic technique for parts-per-billion determination of
organic compounds in air and water. Anal Chem, 52: 875-881.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and
other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, & Turton D (1985) Environmental contaminants in
human fat: comparison between accidental and nonaccidental causes of
death. Ecotoxicol Environ Saf, 10: 70-74.
Mes J, Davies DJ, Turton D, & Sun WF (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mumma CE & Lawless EW (1975) Survey of industrial processing data:
Task I. Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processing. Kansas City, Missouri, Midwest Research
Institute (Prepared for the US Environmental Protection Agency,
Office of Toxic Substances, Washington) (EPA 560/3-75-003; PB
243641).
Murzakayev FG (1963) [Data for substantiating maximal permissible
concentrations of hexachlorobutadiene and polychlorobutane in water
basins.] Gig i Sanit, 28: 9-14 (in Russian).
Nash JA, King LJ, Lock EA, & Green T (1984) The metabolism and
disposition of hexachloro-1,3-butadiene in the rat and its relevance
for nephrotoxicity. Toxicol Appl Pharmacol, 73: 124-137.
NIOSH (1979) Manual of analytical methods: Method 308. Cincinnati,
Ohio, National Institute of Occupational Safety and Health, vol 5.
NIOSH (1990) Manual of analytical methods: Method No. P & CAM 307.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
NPHEP (National Institute of Public Health and Environmental
Protection, The Netherlands) (1990) A proposal for guideline
concerning the estimation of environmental concern levels for the
modified EPA method. In: Report of the OECD Workshop on
Ecotoxicological Effects Assessment, Arlington, USA, December 1990.
Paris, Organisation for Economic Co-operation and Development.
Oesch F & Wolf CR (1989) Properties of the microsomal and cytosolic
glutathione transferases involved in hexachloro-1,3-butadiene
conjugation. Biochem Pharmacol, 38: 353-359.
OECD (1990) Report of the OECD Workshop of Ecotoxicological Effect
Assessment, Arlington, USA, December 1990. Paris, Organisation for
Economic co-operation and Development.
Oliver BG (1984) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Biouptake of chlorinated hydrocarbons from
laboratory-spiked and field sediments by oligochaete worms. Environ
Sci Technol, 21: 785-790.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St Clair, and Erie: implications
regarding sources along the St Clair and Detroit Rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Nicol KD (1982) Gas chromatographic determination of
chlorobenzenes and other chlorinated hydrocarbons in environmental
samples using fused silica capillary columns. Chromatographia, 16:
336-340.
Oliver BG & Nicol KD (1984) Chlorinated contaminants in the Niagara
River (198l-1983). Sci Total Environ, 34: 57-70.
Oliver BG & Niimi AJ (1983) Bioconcentration of chlorobenzenes from
water by Rainbow trout: correlations with partition coefficients and
environmental residues. Environ Sci Technol, 17: 287-291.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of
polychlorinated biphenyl congeners and other chlorinated
hydrocarbons in the Lake Ontario ecosystems. Environ Sci Technol,
22: 388-397.
Otson R & Chan C (1987) Sample handling and analysis for 51 volatile
organics by an adapted urge and trap GC-MS technique. Int J Environ
Anal Chem, 30: 275-287.
Payan JP, Fabry JP, Beydon D, & De Ceaurriz J (1991) Biliary
excretion of hexachloro-1,3-butadiene and its relevance to tissue
uptake and renal excretion in male rats. J Appl Toxicol, 11:
437-442.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16: 781-785.
Pereira WE, Rostad CE, Chiou CT, Brinton TI, Barber LB, Demcheck DK,
& Demas CR (1988) Contamination of estuarine water, biota, and
sediment by halogenated organic compounds: a field study. Environ
Sci Technol, 22: 772-778.
Petersen VP (1986) [Analysis of moderately volatile organochlorine
compounds and their concentration distribution in different streams
of the Federal Republic of Germany.] Dtsch Gewässerkd Mitt, 30:
10-15 (in German).
Poteryayeva GE (1966) [The effect of hexachlorobutadiene on
offspring of albino rats.] Gig i Sanit, 31: 33-36 (in Russian).
Pratt IS & Lock EA (1988) Deacylation and further metabolism of the
mercapturic acid of hexachloro-1,3-butadiene by rat kidney cytosol
in vitro. Arch Toxicol, 62: 341-345.
Rapson W, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24: 590-596.
Reichert D (1983) Metabolism and disposition of
hexachloro(1,3)butadiene in rats. Dev Toxicol Environ Sci, 5:
411-414.
Reichert D & Schütz S (1986) Mercapturic acid formation is an
activation and intermediary step in metabolism of
hexachlorobutadiene. Biochem Pharmacol, 35: 1271-1275.
Reichert D, Neudecker T, Spengler U, & Henschler D (1983)
Mutagenicity of dichloroacetylene, trichloroacryloyl chloride and
hexachlorobutadiene. Mutat Res, 117: 21-29.
Reichert D, Neudecker T, & Schütz S (1984) Mutagenicity of
hexachlorobutadiene, perchlorobutenoic acid and perchlorobutenoic
acid chloride. Mutat Res, 137: 89-93.
Reichert D, Schütz S, & Metzler M (1985) Excretion pattern and
metabolism of hexachlorobutadiene in rats. Evidence for metabolic
activation by conjugation reactions. Biochem Pharmacol, 34: 499-505.
Reimschüssel R, Bennet RO, May EB, & Lipsky MM (1989) Renal
histopathological changes in the goldfish (Carassius auratus)
after sublethal exposure to hexachlorobutadiene. Aquat Toxicol, 15:
169-180.
Reimschüssel R, Bennett RO, May EB, & Lipsky MM (1990) Development
of newly formed nephrons in the goldfish kidney following
hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol, 18:
32-38.
Roedig A & Bernemann P (1956) [Data on the highly chlorinated
vinylether 1-ethoxy-pentachloro-(1,3)-butadiene from
perchlorobutadiene.] Justus Liebigs Ann Chem, 600: 1-11 (in German).
Rush GF, Smith JH, Newton JF, & Hook JB (1984) Chemically induced
nephrotoxicity: role of metabolic activation. CRC Crit Rev Toxicol,
13: 99-156.
Ruth JH (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A142-A151.
Saillenfait AM, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Inhalation teratology study on hexachloro-1,3-butadiene in rats.
Toxicol Lett, 47: 235-240.
Sauer T Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Schiffmann D, Reichert D, & Henschler D (1984) Induction of
morphological transformation and unscheduled DNA synthesis in Syrian
hamster embryo fibroblasts by hexachlorobutadiene and its putative
metabolite pentachlorobutenoic acid. Cancer Lett, 23: 297-305.
Schnellmann RG, Lock EA, & Mandel LJ (1987) A mechanism of
S-(1,2,3,4,4-pentachloro-1,3-butadienyl)L-cysteine toxicity to
rabbit renal proximal tubules. Toxicol Appl Pharmacol, 90: 513-521.
Schrenk D & Dekant W (1989) Covalent binding of hexachlorobutadiene
metabolites to renal and hepatic mitochondrial DNA. Carcinogenesis,
10: 1139-1141.
Schrenk D, Dekant W, Wunsch PH, & Henschler D (1988a) Role of
metabolic activation in the toxicity of S-(pentachlorobutadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine in the
isolated perfused rat kidney. Toxicol in Vitro, 2: 283-290.
Schrenk D, Dekant W, & Henschler D (1988b) Metabolism and excretion
of S-conjugates derived from hexachlorobutadiene in the isolated
perfused rat kidney. Mol Pharmacol, 34: 407-412.
Schröder HF (1987) Chlorinated hydrocarbons in biological sewage
purification - Fate and difficulties in balancing. Water Sci
Technol, 19: 429-438.
Schwetz BA, Norris JM, Kociba RJ, Keeler PA, Cornier RF, & Gehring
PJ (1974) Reproduction study in Japanese quail fed
hexachlorobutadiene for 90 days. Toxicol Appl Pharmacol, 30:
255-265.
Schwetz BA, Smith FA, Humiston CG, Quast JF, & Kociba RJ (1977)
Results of a reproduction study in rats fed diets containing
hexachlorobutadiene. Toxicol Appl Pharmacol, 42: 387-398.
Shen TT (1982) Air quality assessment for land disposal of
industrial wastes. Environ Manage, 6: 297-305.
Singh HB, Salas LJ, & Stiles R (1982) Distribution of selected
gaseous organic mutagens and suspected carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
SRI (1984) Directory of chemical producers, Western Europe. Menlo
Park, California, Stanford Research Institute.
Stonard MD, Gore CW, Oliver GJA, & Smith IK (1987) Urinary enzymes
and protein pattern as indicators of injury to different regions of
the kidney. Fundam Appl Toxicol, 9: 339-351.
Stott WT, Quast JF, & Watanabe PG (1981) Differentiation of the
mechanisms of oncogenicity of 1,4-dioxane and
1,3-hexachlorobutadiene in the rat. Toxicol Appl Pharmacol, 60:
287-300.
Tabak HH, Quave SA, Mashni CE, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1517.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res, 37:
2717-2720.
Ullmann F (1986) Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH-Verlagsgesellschaft, vol A6, pp 322-323, 375, 391,
398.
US EPA (1980) Ambient water quality criteria for
hexachlorobutadiene. Washington, DC, US Environmental Protection
Agency, Criteria and Standards Division (EPA-440/5-80-053;
PB-81-117640).
UP EPA (1984a) A method for determination of certain chlorinated
hydrocarbons. EPA method No. 612. Fed Reg, 491(209): 29-210.
US EPA (1984b) A gas chromatographic method for determination of
certain chlorinated hydrocarbons. EPA method No. 612. Fed Reg,
491(209): 29-210.
Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, & Henschler D
(1987) Enzymatic transformation of mercapturic acids derived from
halogenated alkenes to reactive and mutagenic intermediates. Biochem
Pharmacol, 36: 2741-2748.
Vamvakas S, Kordowich FJ, Dekant W, Neudecker T, & Henschler D
(1988a) Mutagenicity of hexachloro-1,3-butadiene and its
S-conjugates in the Ames test - role of activation by the
mercapturic acid pathway in its nephrocarcinogenicity.
Carcinogenesis, 9: 907-910.
Vamvakas S, Muller DA, Dekant W, & Henschler D (1988b) DNA binding
of sulfur containing metabolites from 35S-(pentachlorobutadienyl)-
L-cysteine in bacteria and isolated renal tubular cells. Drug Metab
Drug Interact, 6: 349-358.
Vamvakas S, Dekant W, & Henschler D (1989a) Genotoxicity of
haloalkene and haloalkane glutathione S-conjugates in porcine kidney
cells. Toxicol in Vitro, 3: 151-156.
Vamvakas S, Kochling A, Berthold K, & Dekant W (1989b)
Cytotoxicity of cysteine S-conjugates: structure-activity
relationships. Chem-Biol Interact, 71: 79-90.
Vamvakas S, Dekant W, & Henschler D (1993) Nephrocarcinogenicity of
haloalkenes and alkynes. In: Renal disposition and nephrotoxicity of
xenobiotics. New York, Academic Press, pp 323-342.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchlonne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63:
1433-1439.
Vorobyeva TN (1980) [Enpared residues in grapes.] Khim Sel'sk Khoz,
18(10): 41-42 (in Russian).
Wallin A, Jones TW, Vercesi AE, Cotgreave I, Ormstad K, & Orrenius S
(1987) Toxicity of S-pentachlorobutadienyl-L-cysteine studied with
isolated rat renal cortical mitochondria. Arch Biochem Biophys, 258:
365-372.
Wallin A, Gerdes RG, Morgenstern R, Jones TW, & Ormstad K (1988)
Features of microsomal and cytosolic glutathione conjugation of
hexachlorobutadiene in rat liver. Chem-Biol Interact, 68: 1-11.
Wild D, Schütz S, & Reichert D (1986) Mutagenicity of the
mercapturic acid and other S-containing derivatives of
hexachloro-1,3-butadiene. Carcinogenesis, 7: 431-434.
Wolf CR, Berry PN, Nash JA, Green T, & Lock EA (1984) Role of
microsomal and cytosolic glutathione S-transferases in the
conjugation of hexachloro-1,3-butadiene and its possible relevance
to toxicity. J Pharmacol Exp Toxicol, 228: 202-208.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen, 7:
677-702.
Yang RSH (1988) Hexachloro-1,3-butadiene: toxicology, metabolism,
and mechanisms of toxicity. Rev Environ Contam Toxicol, 101:
121-137.
Yang RSH (1991) Toxicity studies of hexachloro-1,3-butadiene in
B6C3F1 mice (feed studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP TOX 1) (NIH Publication
No. 91-3120).
Yang RSH, Abdo KM, Elwell MR, Levy AC, & Brennecke LH (1989)
Subchronic toxicology studies of hexachloro-1,3-butadiene (HCBD) in
B6C3F1 mice by dietary incorporation. J Environ Pathol Toxicol
Oncol, 9: 323-332.
Yasuhara A & Morita M (1990) Formation of chlorinated compounds in
pyrolysis of trichloroethylene. Chemosphere, 21: 479-486.
Yip G (1976) Survey for hexachloro-1,3-butadiene in fish, eggs,
milk, and vegetables. J Assoc Anal Chem, 59: 559-561.
Yurawecz MP, Dreifuss PA, & Kamps LR (1976) Determination of
hexachloro-1,3-butadiene in spinach, eggs, fish, and milk by
electron capture gas-liquid chromatography. J Assoc Anal Chem, 59:
552-558.
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of The
Netherlands. Chemosphere, 9: 231-249.
Zogorski JS (1984) Experience in monitoring domestic water sources
and process waters for trace organics. J Environ Sci Health, A19:
233-249.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
L'hexachlorobutadiène est un liquide ininflammable,
incombustible, limpide et huileux à la température et la pression
ordinaires. Il est peu soluble dans l'eau mais miscible à l'éther et
à l'éthanol.
On peut le mettre en évidence et le doser par chromatographie
en phase gazeuse. Les limites de détection sont de 0,03 µg/m3 dans
l'air, 0,001 µg/litre dans l'eau, de 0,7 µg/kg de matière humide
dans le sol ou les sédiments et de 0,02 µg/litre dans le sang. Dans
les tissus, cette limite est de 0,47 µg/kg de tissus frais.
2. Sources d'exposition humaine et environnementale
L'hexachlorobutadiène n'existe pas à l'état naturel. C'est
essentiellement un sous-produit de la fabrication des hydro-carbures
chlorés que l'on retrouve dans les fractions lourdes. La production
annuelle mondiale (dans les fractions lourdes) a été estimée à
10 000 tonnes en 1982. L'hexachlorobutadiène peut être utilisé pour
la récupération des gaz contenant du chlore dans les ateliers de
fabrication du chlore et comme liquide de lavage pour éliminer du
courant gazeux certains composés organiques volatils. On l'utilise
également dans les gyroscopes, comme fluide calo-porteur, dans les
transformateurs, comme liquide isolant ou liquide hydraulique ainsi
que comme solvant des élastomères, comme intermédiaire et comme
fumigant.
3. Transport, distribution et transformation dans l'environnement
Les principales voies de pénétration dans l'environnement sont
les émissions résultant des déchets et les utilisations qui
entraînent la dispersion du produit. Le transport
inter-compartimental s'effectue principalement par volatilisation,
adsorption sur les matières particulaires puis dépôt ou
sédimentation. L'hexachloro-butadiène ne migre pas facilement dans
le sol et s'accumule dans les sédiments. Dans l'eau, on le considère
comme persistant, sauf turbulences importantes. Il n'y a pas
d'hydrolyse. Le produit semble être facilement biodégradable par
voie aérobie, encore que le phénomène n'ait pas été étudié à fond.
L'hexachlorobutadiène présent sur les surfaces subit une photolyse.
Outre le dépôt, on estime que la réaction de l'hexachlorobutadiène
avec les radicaux hydroxyles constitue un mode de piégeage important
de ce composé dans la troposphère, la demi-vie atmosphérique
estimative de l'hexachlorobutadiène pouvant aller jusqu'à 2,3 ans.
Le produit a un potentiel élevé de bioaccumulation, comme l'ont
confirmé les observations en laboratoire et sur le terrain. Ainsi,
on a trouvé des facteurs de bioconcentration à l'état stationnaire
(obtenus expérimentalement par rapport au poids de tissus frais)
respectivement égaux en moyenne à 5800 et 17 000 chez la truite
arc-en-ciel. On n'a pas observé d'amplification biologique au
laboratoire ou sur le terrain.
4. Niveaux dans l'environnement et exposition humaine
Le dosage de l'hexachlorobutadiène dans l'air des villes a
donné dans tous les cas des valeurs inférieures à 0,5 µg/m3. Dans
les régions écartées, les concentrations sont inférieures à
1 pg/m3. Dans les lacs et les cours d'eau d'Europe, on a
enregistré des concentrations pouvant aller jusqu'à 2 µg/litre mais
les valeurs moyennes sont généralement inférieures à 100 ng/litre.
Dans la région des grands lacs au Canada, on a obtenu des valeurs
beaucoup plus faibles (autour de 1 ng/litre). En revanche la teneur
des sédiments du fond peut, dans cette zone, atteindre 120 µg/kg de
poids sec. Les couches sédimentaires plus anciennes, remontant aux
environs de 1960, présentaient des teneurs plus élevées (jusqu'à
550 µg/kg de matière humide). On a montré que la concentration dans
les sédiments augmentait avec la granulométrie des particules.
A en juger par la concentration de l'hexachlorobutadiène dans
les organismes aquatiques, les oiseaux et les mammifères, le composé
s'accumule mais ne subit pas d'amplification biologique. Dans les
eaux polluées, on a relevé des concentrations dépassant 1000 µg/kg
de tissus frais chez plusieurs espèces et même 120 mg/kg (par
rapport aux lipides) chez une espèce. Les concentrations actuelles
restent généralement inférieures à 1000 µg/kg de poids frais à
distance des points de décharge industrielle.
On a décelé la présence du composé dans l'urine, le sang et les
tissus humains. Dans certaines denrées alimentaires ayant une
fraction lipidique importante, on en a relevé jusqu'à 40 µg/kg et
dans un cas, plus de 1000 µg/kg.
D'après une étude, le niveau d'exposition pourrait atteindre
1,6 à 12,2 mg/m3 et les concentrations urinaires, 20 mg/litre.
5. Cinétique et métabolisme
Après administration par voie orale, l'hexachlorobutadiène est
rapidement absorbé chez l'animal de laboratoire mais le taux de
résorption après inhalation ou exposition par voie cutanée n'a pas
été étudié. Chez le rat et la souris, le composé se répartit
principalement dans le foie, les reins et les tissus adipeux. Il est
rapidement excrété. On a mis en évidence une fixation aux protéines
et aux acides nucléiques dans le foie et les reins.
La biotransformation du composé chez l'animal de laboratoire se
révèle être un processus saturable. Elle s'effectue principalement
par l'intermédiaire du glutathion, l'hexachlorobutadiène étant
d'abord transformé en conjugué du S-glutathion. La métabolisation
de ce conjugué se poursuit ensuite, en particulier au niveau de la
membrane constituant la bordure en brosse des cellules des tubules
rénaux, pour donner un métabolite sulfuré réactif qui est
probablement responsable des effets néphrotoxiques, génotoxiques et
cancérogènes observés.
6. Effets sur les êtres vivants dans leur milieu naturel
L'hexachlorobutadiène est modérément à très toxique pour les
organismes aquatiques. Certaines espèces de poissons et de crustacés
se sont révélées être les plus sensibles, les valeurs de la CL50 à
96 h. allant de 0,032 à 1,2 et de 0,09 à environ 1,7 mg/litre,
respectivement pour les crustacés et les poissons. Chez les
poissons, le rein est organe-cible important.
On a établi la valeur de la dose sans effets observables à
0,003 mg/litre, à partir des résultats d'un certain nombre
d'épreuves à long terme sur certaines espèces d'algues et de
poissons; cela permet de considérer ce composé comme très toxique
pour les organismes aquatiques. Parmi les points d'aboutissement
biologiques étudiés figuraient la toxicité générale, la
neurotoxicité, la biochimie, l'hématologie, l'anatomopathologie et
la reproduction. Lors d'une étude de 28 jours portant sur les
premiers stades de la vie de Pimephales promelas, une espèce de
vairon, on a observé que la reproduction n'était pas affectée à des
concentrations allant jusqu'à 0,017 mg/litre, alors qu'à 0,013 et
0,017 mg/litre il y avait accroissement de la mortalité et réduction
du poids du corps. La dose sans effets observables était de
0,0065 mg/litre.
On n'a décrit qu'une seule épreuve fiable portant sur des
organismes terrestres. Lors d'une épreuve de 90 jours sur des
cailles japonaises qui recevaient une alimentation contenant ce
composé à des concentrations allant de 0,3 à 30 mg/kg de nourriture,
on a constaté que la survie des oisillons n'était réduite qu'à
partir de 10 mg/kg de nourriture.
7. Effets sur les animaux de laboratoire et les systèmes
d'épreuves in vitro
7.1 Toxicité générale
L'hexachlorobutadiène est légèrement à modérément toxique pour
le rat adulte, modérément toxique pour le raton juste sevré et
extrêmement toxique pour les rattes juste sevrées après
administration d'une seule dose par voie buccale. Les principaux
organes-cibles sont le rein et dans une bien moindre mesure, le
foie.
D'après les données obtenues sur l'animal d'expérience, les
vapeurs d'hexachlorobutadiène sont irritantes pour les muqueuses et
le liquide est corrosif. On peut considérer ce composé comme un
agent sensibilisateur.
Chez le rat, la souris et le lapin, l'hexachlorobutadiène
provoque une nécrose, liée à la dose, des tubules proximaux du rein.
Les rats mâles adultes sont moins sensibles à la néphrotoxicité que
les femelles ou les jeunes mâles. Les souriceaux sont plus sensibles
que les souris adultes sans qu'on puisse observer de différences
entre les deux sexes. Chez la ratte adulte, la dose intrapéritonéale
unique la plus faible à laquelle on ait observé une nécrose rénale
était de 25 mg/kg de poids corporel; elle était de 6,3 mg/kg de
poids corporel chez les souris adultes mâles et femelles. A des
doses égales ou supérieures à celles qui entraînaient une nécrose,
on a observé des modifications biochimiques et une nette
amélioration de la fonction rénale.
Lors de six épreuves à court terme où le composé a été
administré par la voie orale, deux études de reproduction et une
étude d'alimentation à long terme portant sur des rats, c'est
également le rein qui s'est révélé être l'organe-cible. Parmi les
effets liés à la dose, on notait une diminution du poids relatif des
reins et une dégénérescence de l'épithélium des tubules. La dose
sans effets nocifs observables au niveau des reins, tirée d'une
étude de deux ans sur le rat, était de 0,2 mg/kg de poids corporel
et par jour. Une étude de 13 semaines sur des souris a montré que
cette dose était de 0,2 mg/kg de poids corporel et par jour pour cet
animal. Chez les deux espèces, les femelles adultes étaient plus
sensibles que les mâles adultes.
Lors d'une étude d'inhalation à court terme (six heures par
jour pendant 12 jours) on a observé des effets analogues au niveau
des reins avec une concentration nominale de vapeur
d'hexa-chlorobutadiène égale à 267 mg/m3; cette concentration a
également entraîné des difficultés respiratoires, ainsi qu'une
dégénérescence des corticosurrénales.
7.2 Reproduction, embryotoxicité et teratogétogénicité
Deux études d'alimentation portant sur la reproduction ont été
effectuée sur des rats à des doses quotidiennes allant jusqu'à 20 et
75 mg/kg de poids corporel respectivement; elles ont fait ressortir
une réduction du poids de naissance et du gain de poids néonatal aux
doses toxiques pour la mère. La dose quotidienne de 75 mg/kg de
poids corporel, qui était hautement toxique, s'est révélée
suffisante pour empêcher la conception et la nidation intra-utérine.
On n'a pas observé d'anomalies du squelette.
Lors de deux études de tératogénicité, des rats ont été exposés
soit à des vapeurs d'hexachlorobutadiène à des concentrations allant
de 21 à 160 mg/m3, six heures par jour du sixième au vingtième
jour de la gestation, soit par voie intrapéritonéale à une dose
quotidienne de 10 mg/kg de poids corporel (du premier au quinzième
jour de la gestation). Des effets nocifs ont été notés sur le
développement des foetus, qui consistaient en une réduction du poids
de naissance, un retard dans le développement cardiaque, une
dilatation des uretères, mais pas de malformations macroscopiques.
Le retard de développement a été observé à des doses qui étaient
également toxiques pour les mères.
7.3 Génotoxicité et cancérogénicité
L'hexachlorobutadiène provoque des mutations géniques dans
l'épreuve d'Ames sur salmonelle dans des conditions particulières
qui favorisent la formation de produits de conjugaison avec le
glutathion. Lors d'une étude in vivo on a observé des aberrations
chromosomiques qui n'ont en revanche pas été constatées lors de deux
autres études in vitro. Une étude in vitro portant sur des
cellules ovariennes de hamster chinois a révélé une augmentation de
la fréquence des échanges entre chromatides soeurs. On a fait état
de la très forte mutagénicité des métabolites sulfurés de
l'hexachlorobutadiène. D'autres études in vitro ont montré que ce
composé provoquait une synthèse non programmée de l'ADN dans des
cultures de fibroblastes embryonnaires de hamsters de Syrie, effets
qui n'étaient pas observés dans des cultures d'hépatocytes de rats.
Le composé provoque également une synthèse non programmée de l'ADN
in vivo mais n'induit pas de mutations létales récessives liées au
sexe chez Drosophila melanogaster.
Lors de la seule étude à long terme (deux ans) qui ait été
effectuée, des rats ont reçu une alimentation contenant de
l'hexachlorobutadiène à des doses quotidiennes respectives de 0,2, 2
ou 20 mg/kg de poids corporel et seule la dose la plus élevée a
provoqué un accroissement de l'incidence des tumeurs malignes au
niveau des tubules rénaux.
7.4 Mécanismes de la toxicité
La néphrotoxicité, la mutagénicité et la cancérogénicité de
l'hexachlorobutadiène sont liées à la biosynthèse d'un conjugué
sulfuré toxique, le 1-glutathion- S-yl-1,2,3,4,4-pentachloro-
butadiène. Ce conjugué est principalement synthétisé dans le foie et
métabolisé ensuite dans la bile, l'intestin et les reins en
1-cystéine- S-yl-1,2,3,4,4-pentachlorobutadiène (CPB). L'activation
du CPB en thiocétène réactif (qui dépend de la cystéine-conjuguée-
béta lyase) au niveau des cellules des tubules proximaux, aboutit en
définitive à la formation de liaisons covalentes avec les
macromolécules cellulaires.
8. Effets sur l'homme
On n'a pas décrit d'effets pathogènes sur la population dans
son ensemble.
On possède deux rapports faisant état de troubles chez des
ouvriers agricoles qui utilisaient de l'hexachlorobutadiène comme
fumigant mais il est vrai qu'ils avaient également été exposés à
d'autres substances. On a également observé un accroissement de la
fréquence des aberrations chromosomiques dans les lymphocytes du
sang périphériques de travailleurs employés à la production
d'hexachlorobutadiène et qui avaient été exposés à des
concentrations de 1,6 à 12,2 mg/m3.
9. Evaluation des risques pour la santé humaine et des
effets sur l'environnement
9.1 Evaluation des risques pour la santé humaine
Comme très peu d'études ont été effectuées sur l'homme,
l'évaluation repose essentiellement sur les animaux de laboratoire.
Toutefois les données in vitro limitées dont on dispose au sujet
de l'homme incitent à penser que le métabolisme de
l'hexachloro-butadiène est analogue chez l'homme et l'animal.
On estime que les vapeurs d'hexachlorobutadiène sont irritantes
pour les muqueuses et que le liquide est corrosif. Ce composé doit
également être considéré comme un agent sensibilisateur.
Les principaux organes-cibles de son action toxique sont les
reins et dans une mesure bien moindre, le foie. Sur la base des
études à court et à long terme effectuées sur des rats et des
souris, la dose quotidienne sans effets nocifs observables est
évaluée à 0,2 mg/kg de poids corporel. On l'a estimée à 53 mg/m3
lors d'une étude d'inhalation à court terme chez le rat (12 jours,
six heures par jour).
L'action toxique sur le développement, de même que la réduction
du poids de naissance et du gain de poids néonatal n'ont été
observés qu'à des doses toxiques pour la mère.
On a observé que l'hexachlorobutadiène produisait des mutations
géniques, des aberrations chromosomiques, un accroissement des
échanges entre chromatides soeurs et une synthèse non programmée de
l'ADN, encore que certaines études aient donné des résultats
négatifs. On ne possède que des indices limités en faveur d'une
génotoxicité de l'hexachlorobutadiène chez l'animal, indices qui
sont insuffisants en ce qui concerne l'homme.
On a constaté que l'administration d'hexachlorobutadiène par
voie orale pendant une longue période à des rats accroissait la
fréquence des tumeurs malignes au niveau des tubules rénaux, mais il
s'agissait uniquement de doses élevées fortement néphrotoxiques. En
ce qui concerne la cancérogénicité de cette substance, les indices
sont limités chez l'animal et insuffisants chez l'homme.
En se basant sur la dose quotidienne sans effets nocifs
observables estimée à 0,2 mg/kg de poids corporel chez la souris ou
le rat, on a fixé à 0,03-0,05 mg/kg de poids corporel la dose
quotidienne sans effets nocifs observables chez l'homme. La marge de
sécurité entre la dose estimative sans effets nocifs observables et
la dose journalière maximale totale ingérée estimée en se basant sur
une absorption du composé par l'intermédiaire d'une eau de boisson
et de produits alimentaires contaminés à forte teneur en lipides,
est égale à 150.
9.2 Evaluation des effets sur l'environnement
L'hexachlorobutadiène est modérément à fortement toxique pour
les organismes aquatiques: les crustacés et les poissons sont les
espèces les plus sensibles. On a fixé à 0,1 g/litre la
concentration écologiquement préoccupante. On estime que la
concentration maximale prévisible dans l'environnement à distance
des sources ponctuelles de pollution est égale à deux fois la dose
écologique-ment préoccupante extrapolée et, par voie de conséquence,
que les organismes aquatiques peuvent être menacés dans les eaux de
surface polluées. On ne peut exclure des effets nocifs sur le
benthos.
Compte tenu de la toxicité de l'hexachlorobutadiène pour les
mammifères, la consommation de benthos ou d'organismes aquatiques
par d'autres espèces pourrait être préoccupante.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos de análisis
El hexaclorobutadieno es un líquido no inflamable,
incombus-tible, claro, oleoso e incoloro a temperatura y presión
ordinarias. Es poco soluble en el agua, pero miscible con éter y
etanol.
La sustancia puede detectarse y determinarse cuantitativamente
por métodos de cromatografía de gases. Los límites de detección son
de 0,03 µg/m3 de aire, 0,001 µg/litro de agua, 0,7 µg/kg de peso
húmedo en el suelo o en sedimentos y de 0,02 µg/litro de sangre. Se
ha determinado un nivel de 0,47 µg/kg de peso húmedo de tejido.
2. Fuentes de exposición humana y ambiental
No hay indicaciones de que el hexaclorobutadieno exista como
producto natural. Es principalmente un subproducto de la fabricación
de hidrocarburos clorados y se presenta en las fracciones pesadas
(como residuo). La producción anual mundial del compuesto en las
fracciones pesadas en 1982 se estimó en 10 000 toneladas.
El hexaclorobutadieno puede utilizarse para recuperar gas que
contiene cloro en plantas productoras de cloro y como líquido de
lavado para eliminar ciertos compuestos orgánicos volátiles de las
corrientes de gases. También se ha utilizado como fluido en
giróscopos, como transmisor de calor, transformador, fluido aislante
y fluido hidráulico, disolvente para elastómeros y como
intermediario y sustancia para fumigar.
3. Transporte, distribución y transformación en el medio ambiente
Las principales vías de ingreso en el medio ambiente son las
emisiones de residuos y el uso dispersivo. El paso de un entorno a
otro ocurre principalmente por volatilización, adsorción a
corpúsculos de materia y subsiguiente deposición o sedimentación. El
hexaclorabutadieno no migra rápidamente en el suelo y se acumula en
el sedimento. Se considera persistente en el agua a menos que haya
mucha turbulencia. No produce hidrólisis. La sustancia parece ser
fácilmente biodegradable aeróbicamente, aunque su biodegradabilidad
no se ha investigado a fondo. El hexaclorobutadieno se fotoliza en
las superficies. Se supone que, además de la deposición, la reacción
con radicales hidroxilo es un importante sumidero de
hexaclorobutadieno en la troposfera y su semivida atmosférica
estimada es de hasta 2,3 años. La sustancia tiene un elevado
potencial de bioacumulación, que se ha comprobado mediante
observaciones en laboratorio y sobre el terreno. En la trucha arco
iris se han determinado experimentalmente factores de
bioconcentración en estado estacionario de 5800 y 17 000 como
promedio, sobre la base del peso húmedo. No se ha observado
biomagnificación en laboratorio ni sobre el terreno.
4. Niveles ambientales y exposición humana
Se ha determinado la presencia de hexaclorubutadieno en el aire
urbano; en todos los casos, los niveles eran inferiores a
0,5 µg/m3. Las concentraciones en lugares aislados son inferiores
a 7 pg/m3. En las aguas de lagos y ríos de Europa se han
registrado concentraciones de hasta 2 µg/litro, pero los niveles
medios son generalmente inferiores a 100 ng/litro. En la región de
los Grandes Lagos del Canadá se han detectado niveles muy inferiores
(de aproximadamente 1 ng/litro). Allí los niveles en el sedimento
del fondo pueden ser de 120 µg/kg de peso en seco. En capas más
antiguas de sedimento, de 1960 aproximadamente, se encontraron
concentraciones más elevadas (de hasta 550 µg/kg de peso húmedo). Se
ha demostrado que la concentración en el sedimento aumenta con el
tamaño de la partícula de sedimento.
Las concentraciones de hexaclorobutadieno en organismos
acuáticos, aves y mamíferos indican bioacumulación pero no
biomagnificación. En las aguas contaminadas se han detectado niveles
de más de 1000 µg/kg de peso húmedo en varias especies y de
120 mg/kg (base grasa) en una especie. Lejos de los efluentes
industriales, los niveles actuales se mantienen en general por
debajo de 100 µg/kg de peso húmedo.
Se ha detectado la presencia del compuesto en la orina, en la
sangre y en tejidos humanos. En ciertos alimentos que contienen una
elevada fracción lipídica se han encontrado hasta unos 40 µg/kg y,
en un caso, más de 1000 µg/kg.
Un estudio señala exposiciones ocupacionales de
1,6-12,2 mg/m3 y en la orina niveles de hasta 20 mg/litro.
5. Cinética y metabolismo
Se ha observado que los animales de laboratorio absorben
rápidamente el hexaclorobutadieno después de la administración oral,
pero no se ha investigado la velocidad de absorción después de la
inhalación o de la exposición dérmica. En ratas y ratones, el
compuesto se distribuye principalmente al hígado, a los riñones y al
tejido adiposo. Se excreta rápidamente. Se ha demostrado que se fija
a las proteínas y ácidos nucleicos del hígado y de los riñones.
La biotransformación del compuesto en animales de
experimentación parece ser un proceso saturable. Se produce
principalmente a través de una vía mediada por el glutatión, en la
cual el hexaclorobutadieno se convierte inicialmente en conjugados
de S-glutatión. Estos conjugados pueden seguir metabolizándose,
especialmente en el ribete en cepillo de las membranas de las
células de los tubos renales, produciendo un metabolito sulfuroso
reactivo que probablemente explique la nefrotoxicidad, genotoxicidad
y carcinogenicidad observadas.
6. Efectos en organismos presentes en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos. Los más sensibles que se hayan observado han
sido especies de peces y crustáceos; los valores de la CL50 en
96 horas oscilan entre 0,032 y 1,2 mg/litro en crustáceos y entre
0,09 y 1,7 mg/litro en peces. Se ha demostrado que el riñón es un
órgano muy afectado en los peces.
Sobre la base de varias pruebas a largo plazo con especies de
algas y de peces, se ha establecido un nivel sin efectos observados
de 0,003 mg/litro; así pues, el compuesto se clasifica como muy
tóxico para las especies acuáticas. Los valores extremos
investigados comprenden parámetros de toxicidad general,
neurotoxicidad, bioquímicos, hematológicos, patológicos y
relacionados con la reproducción. En una prueba de 28 días de
duración en la que se examinaron las primeras fases de la vida de
carpas se observó que la reproducción no se veía afectada con
concentraciones de hasta 0,017 mg/litro, mientras que con
concentraciones de 0,013 y 0,017 mg/litro se observaron un aumento
de la mortalidad y una disminución del peso corporal. El nivel sin
efectos observados era de 0,0065 mg por litro.
Se ha descrito una sola prueba fiable con organismos
terrestres. En una prueba de 90 días con codornices japonesas
alimentadas con una dieta que contenía el compuesto en
concentraciones de 0,3 a 30 mg/kg de dieta se observó que la
supervivencia de los polluelos disminuía a partir de 10 mg/kg de
dieta.
7. Efectos en animales de experimentación y en sistemas de
prueba in vitro
7.1 Toxicidad general
Después de la ingestión de una dosis oral única, el
hexaclorobutadieno es de levemente a moderadamente tóxico para las
ratas adultas, moderadamente tóxico para las ratas macho destetadas
y muy tóxico para las ratas hembras destetadas. Los principales
órganos afectados son el riñón y, en grado mucho menor, el hígado.
Los datos obtenidos con animales indican que el vapor de
hexaclorobutadieno es irritante para las membranas mucosas y el
líquido es corrosivo. La sustancia debe considerarse como un agente
sensibilizador.
En los riñones de ratas, ratones y conejos, el
hexacloro-butadieno causa en los tubos proximales del riñón una
necrosis que depende de la dosis. Las ratas macho adultas son menos
vulnerables a la toxicidad renal que las hembras adultas y que los
machos jóvenes. Los ratones jóvenes son más vulnerables que los
adultos y no se observaron diferencias entre un sexo y otro. En las
ratas hembra adultas la dosis intraperitoneal única más baja con la
cual se observó necrosis renal fue de 25 mg/kg de peso corporal y en
ratones adultos, machos y hembras, fue de 6,3 mg/kg de peso
corporal. Se observaron cambios bioquímicos y alteraciones
funcionales marcados en los riñones con dosis iguales o mayores que
las asociadas con necrosis.
Asimismo, en seis pruebas orales de corto plazo, dos estudios
sobre reproducción y un estudio de largo plazo sobre la dieta
realizados con ratas, el riñón fue el principal órgano afectado. Los
efectos relacionados con la dosis comprenden una reducción del peso
relativo del riñón y una degeneración del epitelio de los tubos. El
nivel sin efectos nocivos observados de toxicidad renal en ratas en
un estudio de dos años fue de 0,2 mg/kg de peso corporal por día. En
un estudio de 13 semanas efectuado en ratones se obtuvo un nivel sin
efectos nocivos observados de 0,2 mg/kg de peso corporal por día.
Las hembras adultas de ambas especies eran más vulnerables que los
machos adultos.
En una prueba de inhalación de corto plazo (6 horas por día
durante 12 días) se observaron efectos semejantes en los riñones con
una concentración de vapor nominal de 267 mg/m3, con la cual
también se observaron trastornos respiratorios y degeneración de la
corteza suprarrenal.
7.2 Reproducción, embriotoxicidad y teratogenicidad
Dos estudios sobre dieta y reproducción en ratas con dosis de
hasta 20 y 75 mg/kg de peso corporal por día, respectivamente,
mostraron una reducción del peso al nacer y un aumento del peso
neonatal cuando se administraban a la madre dosis tóxicas de 20 y
7,5 mg/kg de peso corporal, respectivamente. La dosis altamente
tóxica de 75 mg/kg de peso corporal por día fue suficiente para
impedir la concepción y la implantación uterina. No se observaron
anormalidades del esqueleto.
En dos pruebas de teratogenicidad en las que se expuso a las
ratas o bien a vapor de hexaclorobutadieno en concentraciones que
oscilaban entre 21 y 160 mg/m3 durante 6 horas diarias (desde el
6° hasta el 20° día del embarazo) o bien a la administración
intraperitoneal de 10 mg/kg de peso corporal por día (desde el 1° al
15° día de embarazo) se observaron en el desarrollo del feto efectos
tóxicos tales como una reducción del peso al nacer, un retraso del
desarrollo del corazón y uréteres dilatados pero sin grandes
malformaciones. El retraso del desarrollo se observó en niveles que
también eran tóxicos para las madres.
7.3 Genotoxicidad y carcinogenicidad
En la prueba de Ames Salmonella se ha observado que el
hexaclorobutadieno induce mutaciones genéticas en condiciones
especiales que favorecen la formación de productos de conjugación
con el glutatión. En un estudio in vivo se observó que había
inducido aberraciones cromosómicas, pero no se observaron tales
aberraciones en dos estudios in vitro. En una prueba in vitro se
observó que la frecuencia de los intercambios entre cromátidas
hermanas había aumentado en las células ováricas de hámsters de
China. Se ha señalado el gran potencial mutagénico de los
metabolitos sulfurosos del hexaclorobutadieno. En estudios in
vitro, el compuesto indujo síntesis imprevistas de ADN en cultivos
de fibroblastos de embriones de hámsters de Siria, pero no en
cultivos de hepatocitos. Indujo síntesis imprevistas de ADN en ratas
in vivo, pero no indujo mutaciones letales recesivas ligadas al
sexo en Drosophila melanogaster.
En el único estudio de largo plazo (dos años), en el cual las
ratas recibieron una dieta que contenía hexaclorobutadieno en dosis
de 0,2, 2 ó 20 mg/kg de peso corporal por día, se observó una mayor
incidencia de neoplasias de los tubos renales únicamente con la
dosis más elevada.
7.4 Mecanismos de toxicidad
La nefrotoxicidad, mutagenicidad y carcinogenicidad del
hexaclorobutadieno depende de la biosíntesis del conjugado sulfuroso
tóxico 1-glutatión- S-yl-1,2,3,4,4-pentaclorobutadieno. Este
conjugado se sintetiza principalmente en el hígado y se metaboliza
luego en la bilis, el intestino y los riñones convirtiéndose en
1-cisteína- S-yl-1,2,3,4,4-pentaclorobutadieno (CPB). La activación
de CPB, que depende del conjugado de cisteína beta-lyasa, en una
tiocetena reactiva en las células de los tubos proximales finalmente
da lugar a un enlace covalente con macromoléculas celulares.
8. Efectos en el ser humano
No se han descrito efectos patogénicos en la población en
general.
Se conocen dos casos de trastornos padecidos por trabajadores
agrícolas que utilizaban el hexaclorobutadieno como fumigante, pero
esas personas también habían estado expuestas a otras sustancias. En
los linfocitos de la sangre periférica de operarios que trabajaban
en la producción de hexaclorobutadieno y estaban expuestos, según se
informa, a concentraciones de 1,6 a 12,2 mg/m3 se observó una
frecuencia mayor de aberraciones cromosómicas.
9. Evaluación de los riesgos para la salud humana y de los
efectos en el medio ambiente
9.1 Evaluación de los riesgos para la salud humana
Como se han hecho muy pocos estudios en el ser humano, la
evaluación se basa principalmente en estudios efectuados en animales
de laboratorio. Sin embargo, los limitados datos existentes sobre el
ser humano, obtenidos in vitro, sugieren que el metabolismo del
hexaclorobutadieno en el ser humano es semejante al observado en
animales.
Se considera que el vapor de hexaclorobutadieno irrita las
membranas mucosas del ser humano y que en estado líquido es
corrosivo. El compuesto también debe considerarse como un agente
sensibilizador.
Los principales órganos afectados por la toxicidad son los
riñones y, en mucho menor grado, el hígado. En base a estudios de
corto y largo plazo de ingestión por vía oral por ratas y ratones,
se determinó un nivel sin efectos adversos observados de 0,2 mg/kg
de peso corporal por día. En un estudio de inhalación en el corto
plazo en ratas (12 días, a razón de 6 horas por día), el nivel sin
efectos adversos observados fue de 53 mg/m3.
Se observaron una reducción del peso al nacer y un aumento de
peso neonatal únicamente con dosis tóxicas para la madre; lo mismo
puede decirse de los efectos tóxicos para el desarrollo.
Se ha observado que el hexaclorobutadieno induce mutaciones
genéticas, aberraciones cromosómicas, aumento de los intercambios
entre cromátidas hermanas y síntesis imprevistas de ADN, aunque
algunos estudios han dado resultados negativos. Con respecto a la
genotoxicidad del hexaclorubutadieno, las observaciones realizadas
en animales son limitadas y las efectuadas en el ser humano
insuficientes.
Tras la administración oral a largo plazo del
hexacloro-butadieno a ratas, se ha observado una mayor frecuencia de
neoplasias de los tubos renales, pero solamente con dosis elevadas
causantes de nefrotoxicidad notable. Hay indicios limitados de
carcinogenicidad en animales e indicios insuficientes en el ser
humano.
En base al nivel sin efectos adversos observados en ratones y
ratas, que es de 0,2 mg/kg de peso corporal por día, se ha estimado
un nivel sin efectos adversos observados en el ser humano, que es de
0,03 a 0,05 mg/kg de peso corporal por día. Hay un margen de
seguridad de 150 entre el nivel sin efectos adversos observados
estimado y la ingesta diaria total máxima estimada, suponiendo que
el compuesto se absorba a través del agua de bebida contaminada y de
alimentos con elevado contenido de lípidos.
9.2 Evaluación de los efectos en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos; los crustáceos y peces son los más
vulnerables. Se ha establecido un nivel de riesgo para el medio
ambiente de 0,1 µg/litro. Se estima que la concentración ambiental
prevista máxima lejos de las fuentes equivale al nivel de riesgo
ambiental extrapolado multiplicado por dos y, por consiguiente, los
organismos acuáticos tal vez estén en peligro en las aguas de
superficie contaminadas. No pueden excluirse efectos adversos en
organismos bentónicos.
En vista de la toxicidad del hexaclorobutadieno para los
mamíferos, el consumo de organismos bentónicos o acuáticos por otras
especies tal vez sea motivo de inquietud.
The intestinal absorption of GPB and CPB was studied in rats by
infusing the compounds into the intestine via a biliary cannula.
When GPB was infused, both GPB and CPB were found in the blood in
approximately equal concentrations. Higher blood CPB concentrations
were found after CPB infusion than after GPB infusion (Gietl
et al., 1991).
In studies with radiolabelled hexachlorobutadiene, several
urinary metabolites were identified. The structure of these
metabolites further supported the hypothesis that
hexachloro-butadiene is bioactivated by glutathione conjugation.
ACPB was found to be the main metabolite (representing
approximately 80% of the radioactivity present in urine) excreted
after the administration of [14C] hexachlorobutadiene (200 mg/kg)
in female rats (Reichert & Schütz, 1986). The same authors also
identified 1-carboxymethylthion-1,2,3,4,4-pentachlorobuta-1,3-diene
and 1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) as
urinary metabolites (Reichert et al., 1985). It is the opinion of
the Task Group that the identification of MTPB by diazomethane
treatment of the urinary extract is questionable.
In male rats, 1,2,3,4,4-pentachloro-1,3-butadienyl sulfenic
acid (PBSA) is the only metabolite excreted in urine that has so far
been identified. The data presented suggest that ACPB is not a major
urinary metabolite of hexachlorobutadiene in male rats (Nash
et al., 1984). In urine of mice exposed to radiolabelled
hexachlorobutadiene (30 mg/kg), CPB, ACPB and
2,3,4,4-tetra-chlorobutenoic acid were identified as urinary
metabolites (Dekant et al., 1988a).
It is probable that 2,3,4,4-tetrachlorobutenoic acid is formed
by reaction of the intermediate thioketene with water and further
hydrolysis of the thionol acid thus formed (Dekant et al., 1988a).
The weight of evidence suggests that oxidative reactions
involving cytochrome P-450 have little role in the metabolism of
hexachlorobutadiene (Wolf et al., 1984; Dekant et al., 1988a).
6.3 Reaction with body components
The covalent binding of [14C]-hexachlorobutadiene-related
radioactivity to tissue proteins has been shown to be time
dependent, with the highest level occurring during the first 6 h
after treatment. The half-life of hexachlorobutadiene binding was
22 h in both liver and kidney (Reichert, 1983; Reichert et al.,
1985).
In a DNA binding study, rats received a single oral dose of
20 mg [14C]-hexachlorobutadiene, and DNA was isolated from the
kidneys of these rats at 6, 18.5 and 30 h after dose administration.
Although the results have been reported only in summary form,
various levels of radioactivity were recovered with the DNA, but
there was a marked variation in the level of radioactivity between
samples. Furthermore complete analysis of the DNA was not performed
and protein may have been associated with the DNA (Stott et al.,
1981).
Covalent binding to mouse liver and kidney DNA was demonstrated
after the oral administration of radiolabelled hexachlorobutadiene
(30 mg/kg body weight) in corn oil (Schrenk & Dekant, 1989). In the
liver and kidneys, the binding capacity of mitochondrial DNA was
significantly higher than that of nuclear DNA. The level of binding
to nuclear DNA in the liver was indistinguishable from that of
controls. HPLC separation of the hydrolysed DNA indicated the
presence of three distinct peaks of radioactivity.
6.4 Excretion
Following oral administration in rats and mice of single doses
of hexachlorobutadiene up to 100 mg/kg body weight, the total
excretion within 72 h was at least 65% of the dose. In mice, less
than half of a dose of 30 mg/kg body weight was metabolized (Dekant
et al., 1988a). In rats, assuming that the faeces mostly contain
unchanged compound and no non-resorbed conjugates, 44% of an orally
administered low dose of hexachlorobutadiene (1 mg/kg body weight)
was metabolized (Reichert et al., 1985). At higher doses the
percentage of hexachlorobutadiene metabolized decreased
dramatically. The biotransformation of hexachloro-butadiene in rats
appears to be a saturable process considering the reduced excretion
of carbon dioxide and renal metabolites at increasing doses (Davis
et al., 1980; Reichert et al., 1985; Reichert & Scuhtz, 1986;
Payan et al., 1991). This could be explained by saturation of the
gastrointestinal absorption, which was observed by Reichert et al.
(1985). It should be noted, however, that the observed increase in
the amount of unchanged hexachloro-butadiene in faeces with
increasing dose applies only up to 100 mg/kg body weight. At higher
dose levels, the amount of unchanged hexachlorobutadiene in faeces
decreases, probably due to a decrease in faecal output (Davis
et al., 1980; Nash et al., 1984).
The results of studies of Payan et al. (1991) on bile-duct
cannulated (see section 6.2.2) and intact (see Table 8) rats show
that saturation of gastrointestinal absorption indeed occurs
following oral administration.
Pharmacokinetic data concerning the fate of
hexachloro-butadiene in organisms were not available to the Task
Group.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Short-term toxicity
A summary of short-term aquatic toxicity data is presented in
Table 9. In most of these studies the concentration of
hexachloro-butadiene was not reported. Therefore, the actual effect
concentrations may be lower than the nominal ones. In several cases
these nominal values far exceed the solubility limits. Based on
these data the substance is moderately to highly toxic to aquatic
organisms (Canton et al., 1990).
Adverse effects reported in some of these acute tests included
loss of equilibrium, erratic swimming (Leeuwangh et al., 1975;
Laseter et al., 1976), decreased activity, increased rate of
opercular movement, jumping (Leeuwangh et al., 1975), inverted
positions, fin fibrillation and muscle tetany (Laseter et al.,
1976). In a special investigation into kidney pathology, groups of
five goldfish (Carassius auratus) received one intraperitoneal
injection of hexachlorobutadiene at a dose level of 500 mg/kg body
weight in corn oil. They were subsequently fasted for up to 6 days
and sacrificed at different points in time up to day 7. Controls
received corn oil only. The temperature was kept between 18 and
21 °C. By 24 h the fish showed decreased activity, swam with dorsal
fins down, and had difficulty in following food. By day 4
exophthalmos, distended abdomen and ascites were observed. These
signs of toxicity were all reversible. Relative kidney weights were
elevated on day 4 only. From 12 h after exposure, P2 and P3
renal epithelial cells exhibited marked vacuolation and necrosis,
which persisted up to day 7. Increased gamma-glutamyl-transferase
(EC 2.3.2.2) staining was seen in P2 and P3 segments
(Reimschüssel et al., 1989).
In a report of this experiment, the fish were sacrificed at
different points in time up to day 70 after exposure. In one of the
two experiments the fish also received 5-bromo-2'-deoxyuridine 4 h
prior to sacrifice. Morphometric analysis of developing nephrons
showed an increase in the percentage of volume occupied by
basophilia clusters and developing nephrons from day 14 onwards. The
apparent number of basophilic clusters and developing nephrons per
unit surface area was also increased from day 14 (Reimschüssel
et al., 1990).
Table 9. Short-term aquatic toxicity of hexachlorobutadiene
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fresh water
Algae Haematococcus 20 stat, closed 24 EC10b > 2 Knie et al.
pluvialis (1983)
Bacteria Pseudomonas putida 25 7.0 stat, closed 16 TTc > 25 Bringmann &
Kuhn (1977)
Bacteria Pseudomonas putida 20 7.2 stat, open 0.5 EC10b > 0.9 Knie et al.
(1983)
Protozoans Chilomonas 20 6.9 stat, closed 48 TTc > 10 Bringmann et al.
paramecium (1980)
Molluscs great pond snail, 19 stat,d closed 24 LC50 0.21 Leeuwangh et
Lymnaea stagnalis 96 LC50 0.21 al. (1975)e
Crustaceans water flea, 20 7 250 stat, open 24 EC50 0.5 Knie et al.
Daphnia magna (1983)
aquatic sowbug, 19 stat,d closed 96 LC50 0.13 Leeuwangh et
Asellus aquaticus al. (1975)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fish goldfish, 17.5 stat,d open 96 LC50 0.09 Leeuwangh et
Carassius auratus al. (1975)e
Fish zebrafish, 20 8.0 9.0 180 flow, closed 48 LC50 1 Slooff (1979)
Brachydanio rerio
Fish rainbow trout, LC50 0.320 US EPA (1980)
Salmo gairdnerii
Fish bluegill sunfish, LC50 0.326 US EPA (1980)
Lepomis macrochirus
Fish sheepshead minnow, LC50 0.557 US EPA (1980)
Cyprinodon variegatus
Fish golden orfe, 20 8 270 open 48 LC50 3 Knie et al.
Leuciscus idus (1983)e
Fish fathead minnow, 25 6.7-7.6 8.0 45 flow, open 96 LC50 0.10 Walbridge et
Pimephales promelas al. (1983)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Marine
Crustaceans harpacticoid 21 7.9 > 5 stat, open 96 LC50 1.2 Bengtsson &
copepod Tarkpea (1983)
grass shrimp, LC50 0.032 US EPA (1980)
Palaemonetes pugio
Mysid shrimp, LC50 0.059 US EPA (1980)
Mysidopsis bahia
Fish sailfin molly, 22-24 6.6-7.9 8-9 flow, open 26 LC50 4.2 Laseter et
Poecilia latipinna 30 LC50 4.5 al. (1976)e,f
77 LC50 1.4-1.9
115 LC50 1.7
138 LC50 1.2
pinfish, Lagodon LC50 0.399 US EPA (1980)
rhomboides
a static or flow-through test, open or closed system d semi-static (daily renewal) test
b effect is 10% reduction in oxygen consumption e analysis for hexachlorobutadiene was reported
c TT = toxic threshold for inhibition of cell multiplication f salinity was 0.25%, 96-h LC50 was calculated to be 1.6 mg/litre
7.1.2 Long-term toxicity
The cell multiplication of green algae (Scenedesmus
quadricauda) was not inhibited after 8 days of static exposure to
a nominal concentration of 25 mg/litre (well above pure water
solubility) in a closed system at 27 °C and a pH of 7 (Bringmann &
Kühn, 1977). A 14-day LC50 of 0.4 mg/litre was determined for
2- to 3-month old guppies (Poecilia reticulata) in a semi-static
test using an open system at 22 °C, a water hardness of 25 mg
CaCO3/litre, and a dissolved oxygen concentration of >
5 mg/litre. No analysis for hexachlorobutadiene was reported
(Könemann, 1981). In the same test under the same conditions, but
with analysis for the compound, the 14-day LC50 was 0.16 mg/litre
(Hermens et al., 1985).
In a study by Leeuwangh et al. (1975), groups of six goldfish
(Carassius auratus) were each exposed to hexachlorobutadiene in
tap water at measured concentrations of 0, 0.0003, 0.003, 0.0096 or
0.03 mg/litre for 49 and 67 days. The static test in an open system
was conducted at 19 °C, a pH of 7.6, and a dissolved oxygen
concentration between 3.2 and 6.3 mg/litre. Body weights were
decreased after 49 days at 0.03 mg/litre, and body weight gain was
still reduced at 67 days. Abnormal behaviour, jumping,
incoordination, increased opercular movement and overall immobility
were noted at 0.0096 mg/litre. After 49 days at 0.0096 mg/litre (no
data at 0.03 mg/litre), relative liver weights were increased, and
the activity of liver glucose-6-phosphatase (EC 3.1.3.9) was
decreased, whereas the activity of liver glucose-6-phosphate
dehydrogenase (EC 1.1.1.49) was increased. After 67 days the
activity of liver phenylalanine hydroxylase (EC 1.14.16.1) showed a
concentration-related increase. No effects were found on haemoglobin
concentration and haematocrit after 49 days or on the activity of
serum alanine aminotransferase (EC 2.6.2.1) and serum alkaline
phosphatase (EC 3.1.3.1) after 67 days.
Groups of 12 largemouth bass (Micropterus salmoides) were
each exposed to hexachlorobutadiene for 10 days at measured
concentrations of 0.00343 and 0.03195 mg/litre in filtered tap water
with a salinity of 0.08-0.1%, at 22-24 °C, a pH of 6.6-7.9 and a
dissolved oxygen concentration of 7.6-8.5 mg/litre. A control group
comprised 12 water and 12 vehicle (acetone) controls. Plasma
cortisol levels were increased at both concentrations, but
haematocrit values were not affected. At the higher concentration
there was leukocytic infiltration in the kidneys of one of the fish
and paleness and accentuated lobulation of parenchyma in the livers
(Laseter et al., 1976).
In an early lifestage test, four replicate groups, each of 30
fathead minnow (Pimephales promelas) eggs, 2-4 h after spawning,
were exposed to measured hexachlorobutadiene concentrations in
sand-filtered and sterilized lake water of 0.0017, 0.0032, 0.0065,
0.013 and 0.017 mg/litre in an open system. Following hatching
(4-5 days after spawning), four replicate groups of 15 larvae
continued to be exposed for 28 days. Control groups of equal size
were exposed to slightly contaminated water containing
0.00008 mg/litre. The temperature was 25 °C, pH was 7.4, dissolved
oxygen concentration 7 mg/litre, and water hardness 45 mg
CaCO3/litre. The hatchability of embryos and the percentage of
normal larvae at hatch were not affected. An increased fish
mortality and a concentration-related decrease of body weight were
observed at the two highest concentrations at the end of the
exposure period (Benoit et al., 1982).
7.2 Terrestrial organisms
7.2.1 Short-term toxicity
Except for one test with birds, reliable tests with terrestrial
organisms have not been reported.
Groups of 12 female and four male Japanese quails (Coturnix
coturnix japonica) were exposed to a diet containing
hexachloro-butadiene at levels of 0, 0.3, 3, 10 or 30 mg/kg diet for
90 days. Each cage contained three females and one male of the same
dose group. Feed analysis indicated levels close to the nominal
values. Adults were all histopathologically examined. Eggs were
collected on days 37-46, 64-73, and 81-90. Six adults died during
the study: 4 at 0.3 mg/kg, 1 at 10 mg/kg, and 1 at 30 mg/kg, but
this was not considered to be related to treatment. The survival of
chicks from eggs collected on days 81-90 was decreased at 10 mg/kg
only. Egg production, the percentage of fertile eggs, the percentage
of hatchable eggs, and eggshell thickness were unaffected compared
to controls (Schwetz et al., 1974).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The available mortality data are summarized in Table 10.
8.1.1 Inhalation exposure
8.1.1.1 Mortality
In the only reported study of the mortality of
hexachloro-butadiene following inhalation, groups of 20 SPF mice of
the OF1 strain were exposed for 6 h (Gage, 1970). The vapour
concentrations were measured by gas chromatography, and the
concentrations were within 10% of the nominal value. Results are
shown in Table 10.
8.1.1.2 Systemic effects
The decrease in respiratory rate (reflex bradypnoea) in groups
of six male Swiss OF1 mice was measured following exposure for
15 min to hexachlorobutadiene vapour at concentrations between 886
and 2625 mg/m3. The vapour concentrations were checked by gas
chromatography. The mice were restrained in a body plethysmograph,
while the heads were extended into an inhalation chamber. The 50%
effect level, calculated from the concentration-effect curve, was
2250 mg/m3 (De Ceaurriz et al., 1988).
8.1.2 Oral exposure
8.1.2.1 Mortality
Based on acute mortality data, hexachlorobutadiene is slightly
to moderately toxic to adult rats, moderately toxic to male weanling
rats, and highly toxic to female weanling rats following ingestion.
Young rats are far more sensitive than adult rats (Kociba et al.,
1977a). Gradiski et al. (1975) observed delayed mortality (after
24 h) in oral LD50 studies on rats and mice.
8.1.2.2 Systemic effects
Hexachlorobutadiene mainly affects the kidneys and, to a lesser
extent, the liver. The effects on these organs and the related
biochemical findings are discussed extensively in sections 8.8.2
(liver) and 8.8.3 (kidney). In oral LD50 studies on rats and mice,
Gradiski et al. (1975) observed hyper-reactivity just after
exposure, followed by decreased activity and staggering.
Table 10. Mortality of hexachlorobutadiene from single exposure
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Mouse oral 87 (78.1-95.9) Murzakayev (1963)b
OF1 adult female inhalation (6 h) 14 107 (102-113) Gage (1970)
OF1 adult female oral 14 65 (60-70) Gradiski et al. (1975)c
OF1 adult male oral 14 80 (75-85) Gradiski et al. (1975)c
Alderley Park adult male intraperitoneal 14 67 (53-85) Lock et al. (1984)e
Alderley Park adult female intraperitoneal 14 85 (65-111)
C57BL/10J adult male intraperitoneal 14 57 (41-81)
C3H adult male intraperitoneal 14 25-75
BALB/c adult male intraperitoneal 14 32-40
DBA/2J adult male intraperitoneal 14 53 (36-76)
Rat oral 350 (323-377) Murzakayev (1963)b
OF2 rat adult female oral 14 270 (250-290) Gradiski et al. (1975)c
OF2 rat adult male oral 14 250 (220-280) Gradiski et al. (1975)c
Sprague-Dawley adult female oral 200-400 Kociba et al. (1977a)d
Sprague-Dawley adult male oral 580 (504-667) Kociba et al. (1977a)d
Sprague-Dawley weanling female oral 46 (26-81) Kociba et al. (1977a)d
Sprague-Dawley weanling male oral 65 (46-91) Kociba et al. (1977a)d
Table 10 (contd).
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Alderley Park weanling male intraperitoneal 7 57 (38-87) Hook et al. (1983)e
Alderley Park 29 days male intraperitoneal 7 96 (72-128)
Alderley Park adult male intraperitoneal 7 360 (325-396)
Guinea-pig oral 90 (81.5-98.5) Murzakayev (1963)b
Rabbit
New Zealand adult female dermal (8 h) 14 1120 (890-1400) Duprat & Gradiski (1978)f
a When available, 95% confidence limits are reported between brackets.
b Observation period, strain, sex, age (or body weight) and vehicle were not reported.
c Vehicle was olive oil.
d Confidence limits not calculable; observation until no toxicity was observed any longer; vehicle was corn oil.
e Vehicle was corn oil.
f Application undiluted using glass vials (3.6 cm2).
8.1.3 Dermal exposure
8.1.3.1 Mortality
Hexachlorobutadiene was harmful to rabbits following acute
dermal exposure (LD50 = 1120 mg/kg body weight; (range,
890-1400 mg/kg; Table 10). After a dose of 780 mg/kg body weight,
death occurred within 24 h from respiratory and cardiac failure
(Duprat & Gradiski, 1978).
8.1.3.2 Systemic effects
New Zealand rabbits dermally exposed to undiluted
hexa-chlorobutadiene at doses of 1170 and 1550 mg/kg body weight
exhibited stupor, dyspnoea and cyanosis (Duprat & Gradiski, 1978).
8.1.4 Other routes of exposure
Hexachlorobutadiene has been shown to be toxic to various
strains of mice after intraperitoneal injection (Lock et al.,
1984) and harmful to adult rats (Hook et al., 1983). The compound
was considerably more toxic to young and weanling rats (Table 10).
Rats intraperitoneally exposed to single lethal doses of
hexachlorobutadiene from 500 to 1000 mg/kg in corn oil exhibited
piloerection, sedation, hunching, incoordination, loss of muscle
tone and hypothermia (Lock & Ishmael, 1979). A macroscopic and
haematological investigation of rats intraperitoneally exposed to
doses between 121 and 336 mg/kg body weight in olive oil did not
reveal any damage to the gastrointestinal tract, spleen, heart or
gonads. In the lungs, congestion, haemorrhage, and oedema were
observed, but these were attributed by the authors to ether
anaesthesia. At dose levels of 213 mg/kg body weight or more,
lymphopenia and related neutrophilia were induced (Gradiski et al.,
1975).
8.2 Short-term exposure
8.2.1 Inhalation exposure
In the only inhalation study published, groups of four adult
Alderley Park SPF rats of each sex were dynamically exposed to
nominal concentrations of 53, 107 or 267 mg/m3 6 h/day for 15
days, 1067 mg/m3 6 h/day for 12 days, or 2668 mg/m3 4 h/day for
2 days. Petroleum ether was used as a solvent for concentrations
below 1067 mg/m3. Many other chemicals were tested similarly, and
batches of control rats of unknown size were maintained at intervals
of 2 months during the whole experimental period. No analysis for
hexachlorobutadiene was carried out. Two of the four female rats
exposed to 1067 mg/m3 died, and autopsy revealed pale, enlarged
kidneys, adrenal regeneration and renal cortical tubular
degeneration with epithelial regeneration. Rats of both sexes lost
weight at 1067 mg/m3 and the weight gain of females was reduced at
107 and 267 mg/m3. Irritation of eyes and nose was observed at the
two highest levels. At 267 and 1067 mg/m3 rats were in a poor
condition, females being more affected than males. Respiratory
difficulties were seen at and above 267 mg/m3. Haematological
examination at the end of the exposure period showed slight anaemia
in females at 1067 mg/m3. Urinalysis did not reveal abnormalities
at any of the exposure levels. Macroscopically enlarged, pale
kidneys were found at 267 and 1067 mg/m3 and enlarged adrenals at
1067 mg/m3. Histopatho-logical investigations revealed proximal
tubular degeneration in the kidneys and cortical degeneration in
adrenals at concentrations of 267 mg/m3 or more. No toxic signs
were observed at the lowest exposure level and autopsy revealed no
gross abnormalities (Gage, 1970).
8.2.2 Oral exposure
8.2.2.1 Rats
Groups of five adult male Sprague-Dawley rats were exposed to
daily oral doses of hexachlorobutadiene (0, 0.2 or 20 mg/kg body
weight) in corn oil for 3 weeks. Only the kidneys were examined
histologically. At the higher dose level, body weight gain was
decreased and relative kidney weight increased. Histopathological
examination of the kidneys revealed damage in the middle and inner
cortical region, including loss of cytoplasm, nuclear pyknosis,
increased basophilia and mitotic activity, and increased cellular
debris. No toxic signs were observed at a dosage of 0.2 mg/kg per day
(Stott et al., 1981).
In a study by Kociba et al. (1971), groups of four female
Sprague-Dawley rats consumed for 30 days a diet containing
hexachlorobutadiene, which resulted in ingested nominal daily doses
of 0, 1, 3, 10, 30, 65 and 100 mg/kg body weight. Analysis of the
compound in the feed was not reported. Body weights were decreased
at the two highest dose levels. At 10 mg/kg or more, a dose-related
decrease in body weight gain was observed along with a decrease in
food consumption. There was also an increase in haemoglobin
concentrations, which, although significant, was not clearly dose
related. There was no effect on serum alanine aminotransferase
activity (EC 2.6.1.2). A dose-related increase in relative kidney
weight was observed at dose levels of 3 mg/kg or more.
Histopathological examination, which was restricted to liver and
kidneys, showed proximal tubular degeneration, individual cell
necrosis, and regenerative changes in the kidneys at doses of
10 mg/kg or more. Hepatocellular swelling was seen at 100 mg/kg. The
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight per
day.
In a 2-week experiment, groups of six weanling Wistar-derived
rats of each sex were exposed to measured dietary
hexachlorobutadiene levels of 0, 73, 182 or 447 mg/kg (the Task
Group considered this equivalent to doses of 0, 7.3, 18.2 and
44.7 mg/kg body weight per day, respectively). At all dose levels,
body weight and food conversion efficiency (g of weight gain/g of
food) were decreased in a dose-related manner. Food consumption per
g of body weight was decreased at 44.7 mg/kg body weight. Relative
kidney weights were increased at the two highest dose levels. At all
dose levels a dose-related degeneration of kidney tubular cells was
observed, especially in the straight limbs of the proximal tubules
located in the outer medulla. No toxic signs were observed in the
liver. A NOAEL was not found (Harleman & Seinen, 1979).
In a follow-up to the dietary study, groups of 10 weanling
Wistar-derived rats of each sex received daily doses by gavage of 0,
0.4, 1, 2.5, 6.3 or 15.6 mg/kg body weight in peanut oil for 13
weeks (Harleman & Seinen, 1979). Body weight gain, food consumption
and food utilization efficiency were decreased at 6.3 and
15.6 mg/kg. Polyuria was observed in females at these dose levels
after week 10, while a dose-related decrease in urine osmolarity
occurred at dose levels of 2.5 mg/kg or more. In males, the latter
effect was observed at 15.6 mg/kg. No other changes were observed in
urinalysis (after week 10) and haematological investigations (after
week 8). A dose-related increase in relative kidney weight was
measured in males of all treatment groups but only at 6.3 mg/kg or
more in females. Dose-related increases in the relative weight of
liver and spleen were measured at 6.3 mg/kg or more.
Histopathological examinations revealed changes in liver and
kidneys. In the livers of males dosed with 6.3 mg/kg or more, an
increased basophilic, flocky granulation was observed. At dose
levels of 6.3 mg/kg or more in males and 2.5 mg/kg or more in
females, there was a dose-related degeneration of the renal proximal
tubules, as shown by hyperchromatic nuclei, hypercellularity,
vacuolation and focal necrosis of epithelial cells and a diminished
brush border. No adverse effects were observed at daily doses of
1 mg/kg in females or 2.5 mg/kg in males (Harleman & Seinen, 1979).
8.2.2.2 Mice
In a two-week study, groups of five B6C3F1 mice of each sex
were fed a diet containing hexachlorobutadiene at nominal doses of
0, 30, 100, 300, 1000 or 3000 mg/kg feed for 15 days (calculated by
the Task Group to be equivalent to 0, 4.3, 14.3, 43, 143 and
430 mg/kg body weight per day, respectively, using standard values
for average body weight and food consumption in mice). Analysis of
the feed was carried out by gas chromatography, and no more than 9%
loss of the chemical was observed in one day (feed was replaced
every 2 days). All mice given 143 or 430 mg/kg body weight died or
were sacrificed in a moribund condition within 7 days. A
dose-related growth retardation was observed. At the two highest
doses, the observed toxic effects included renal tubular necrosis,
hepatic cytoplasmic vacuolization, and testicular degeneration
characterized by the presence of syncytial giant cell formation of
spermatocytes. At dose levels of 43 mg/kg body weight or more,
minimal to mild depletion of bone marrow (characterized by a
decrease in the haematopoietic cells) was seen in two out of five
mice of both sexes per dose group. At dose levels of 4.3, 14.3 and
43 mg/kg body weight, at which all animals survived to the end of
the study, renal tubular cell regeneration was observed (Yang
et al., 1989; Yang, 1991).
In a 13-week study, groups of 10 B6C3F1 mice of each sex were
fed a diet containing hexachlorobutadiene at concentrations of 0, 1,
3, 10, 30 or 100 mg/kg feed. Using measurements of food consumption
and body weight, the authors determined doses of 0, 0.1, 0.4, 1.5,
4.9 or 16.8 mg/kg body weight per day for males, and 0, 0.2, 0.5,
1.8, 4.5 or 19.2 mg/kg body weight per day for females. Analysis of
the compound was carried out as in the two-week study. No
treatment-related clinical signs or deaths were observed. The
motility of sperm from treated mice was significantly lower than in
controls, although this effect was not dose related (Yang et al.,
1989; Yang, 1991). Body weight gain was reduced at dose levels of
4.5 and 19.2 mg/kg body weight in females. Reductions in kidney
weight occurred at dose levels of 1.5 mg/kg or more in males and at
19.2 mg/kg body weight in females, and reductions in heart weight
occurred at 19.2 mg/kg body weight in males. Necropsy revealed a
treatment-related increase in renal tubular regeneration (prominent
in the outer stripe of the medulla) at dose levels of 0.2 mg/kg body
weight or more in females (Table 11). Although the author concluded
that a NOAEL was not observed for females, the Task Group noted that
the occurrence of renal tubular regeneration in one out of ten
female mice in the 0.2-mg/kg body weight group is insufficient
evidence of an adverse effect at this dose level in females.
Table 11. Incidences of renal tubular regeneration in 13-week
feed studies on B6C3F1 micea
Concentration Dose (mg/kg body Number of mice with
(mg/kg feed) weight per day) lesions/number examined
Male Female Male Female
0 0 0 0/10 0/10
1 0.1 0.2 0/10 1/10
3 0.4 0.5 0/10 9/10
10 1.5 1.8 0/9 10/10
30 4.9 4.5 10/10 10/10
100 16.8 19.2 10/10 10/10
a Modified from: Yang (1991)
8.3 Long-term exposure
No long-term inhalation studies have been reported. A study
describing long-term exposure of mice by the dermal route is
presented in section 8.7.3.
An oral toxicity/carcinogenicity test in rats has been reported
(see section 8.7.2).
8.4 Skin and eye irritation; sensitization
8.4.1 Irritation
The vapour of hexachlorobutadiene has been found to be
irritating to the eyes and nose of rats (Gage, 1970; see section
8.2.1).
Groups of six New Zealand rabbits received either 0.78 g
(0.5 ml) of undiluted hexachlorobutadiene on the intact or abraded
skin for 24 h, or 0.15 g (0.1 ml) in the conjuctival sac of the left
eye. Assessment of the degree of irritation was conducted according
to Draize et al. (1944) and by calculating the primary irritation
index. Hexachlorobutadiene was moderately irritating for the skin
(primary irritation index 4) but not irritating for the eyes
(primary irritating index 1.5). Moderate conjunctivitis, epithelial
abrasion and, at day 7, epithelial keratitis were observed in the
eyes (Duprat et al., 1976).
Duprat & Gradiski (1978) applied undiluted hexachloro-butadiene
to New Zealand rabbits at doses of 0.39, 0.78, 1.17 and 1.55 mg/kg
body weight (0.25, 0.50, 0.75 and 1.00 ml, respectively) under
occluded conditions, using glass vials, for 8 h. The observation
period was 14 days. The skin was histopathologically examined in all
dead animals, in half the survivors at day 15, and in the remaining
survivors at day 36. After 12 h of exposure to the two highest
doses, epidermis and subcutaneous tissue revealed oedema and
polymorphonuclear leukocyte infiltration. In the epidermal cells,
degeneration with pyknosis of nuclei and perinuclear oedema, and
focal separation from the corium with vesicle formation were seen.
After 3 to 5 days of exposure to the three highest doses, dermal
necrosis was observed, leading to eschar formation and partial
destruction of hair follicles. The effects increased with time, not
with dose. Two to five weeks after application, repair was apparent
at all dose levels, with scarring and upper dermis fibrosis, and
epidermal acanthosis with focal dyskeratosis. Diffuse mononuclear
infiltrate was seen in the dermis.
8.4.2 Sensitization
A group of 20 Hartley guinea-pigs were treated according to the
Magnusson-Kligman protocol by intradermal injections of 5%
hexachlorobutadiene in peanut oil and, after one week and subsequent
treatment by sodium lauryl sulfate, by a 48-h dermal application of
a 25% suspension of the chemical in vaseline. The challenge was
performed by dermal application of a 20% suspension in vaseline. A
group of five controls was induced similarly and challenged by
vaseline only. All exposed animals, but none of the controls, showed
a positive reaction. The test was repeated in the same fashion
without adjuvant in five guinea-pigs: all animals showed positive
reactions (Gradiski et al., 1975).
8.5 Reproduction, embryotoxicity and teratogenicity
8.5.1 Reproduction
A group of female albino rats was exposed to one dose of
hexachlorobutadiene (20 mg/kg body weight) administered
subcutaneously before mating. Within 90 days after exposure, all 86
newborn rats had died, compared with 13 of the 61 controls. The
offspring from exposed dams were reported to show excitation,
disturbances of motor coordination, a decrease in appetite and a
loss of weight, lymphocytosis, neutropenia, myelocytes, Jolly's and
Cabeau's bodies, pneumonia, bronchitis, granular dystrophy of renal
cells, glomerulonephritis, inflammatory destructive lesions of the
gastrointestinal tract and vascular hyperaemia (Poteryayeva, 1966).
The Task Group noted major deficiencies and incomplete reporting of
the experiment, the unusual route of administration, and the high
percentage of mortality in control rats.
Groups of 10-12 male and 20-24 female Sprague-Dawley rats
received a diet containing hexachlorobutadiene at dose levels of
0.2, 2.0 or 20 mg/kg body weight per day for 90 days prior to
mating, 15 days during mating, and subsequently throughout gestation
and lactation. In the mating period, two females were placed with
one male of the same dose group. The control group consisted of
17 males and 34 females. The diets were reportedly analysed for the
test compound. No mortality was observed. At 20 mg/kg, adults showed
decreased food consumption and body weight gain. Blood urea
nitrogen, serum alanine aminotransferase (EC 2.6.1.2) and serum
creatinine were unchanged compared to controls. The dams had an
increased relative brain weight and the male rats had an increased
relative liver weight at 20 mg/kg. The relative kidney weights were
increased in both sexes at 20 mg/kg. At 2 and 20 mg/kg the kidneys
of adult rats revealed dose-related tubular dilatation and
hypertrophy with foci of epithelial degeneration and regeneration;
however, there was no effect at 0.2 mg/kg. The only adverse
reproductive effect in neonates was a decreased weanling weight at
20 mg/kg. There was no detectable effect on the percentage
pregnancy, the period from first cohabitation to delivery, survival
indices, sex ratio, histopathology of weanlings, and the incidence
of skeletal alterations and abnormalities in neonates (Schwetz
et al., 1977).
In a third reproductive study, groups of six female SPF
Wistar-derived rats, 10 weeks of age, received a diet containing
hexa-chlorobutadiene at levels of 0, 150 and 1500 mg/kg diet
(estimated by the Task Group to be equivalent to 0, 7.5 and 75 mg/kg
body weight per day) for 3 weeks prior to mating, 3 weeks during
mating, and subsequently throughout gestation and lactation. In the
mating period, two untreated males were placed with the females,
after which the females were housed individually. The 75-mg/kg
female adults were killed in week 10, while those given 0 or
7.5 mg/kg were killed in week 18. Food analysis at the low dose
level revealed hexachlorobutadiene levels within 96% of nominal
values after 1 week and within 81% of nominal values after 2 weeks.
Diets were prepared weekly. There was a reduced body weight gain by
female rats in the two groups receiving hexachlorobutadiene.
Weakness of hind legs, unsteady gait, incoordination and ataxia were
seen at 75 mg/kg. The relative kidney weight was increased at both
dose levels. Histopathological investigations revealed
hypercellularity of epithelial cells, hydropic degeneration, and
necrosis of proximal tubules in the kidneys at 7.5 mg/kg. At
75 mg/kg, slight proliferation of bile duct epithelial cells,
fragmentation and demyelination of single fibres of the femoral
nerve, and extensive renal degeneration were observed. Again at
75 mg/kg, no conceptions occurred, the ovaries showing little
follicular activity, and there was no uterine implantation sites. At
7.5 mg/kg fertility and litter size were reduced, but not
significantly. In both the control and 7.5-mg/kg groups, the
resorption quotient was low. Compared to controls, pup weights were
reduced significantly on days 0, 10 and 20 in the 7.5-mg/kg group.
No gross abnormalities were observed (Harleman & Seinen, 1979).
8.5.2 Embryotoxicity and teratogenicity
In a teratology study, groups of 24-25 female rats were exposed
to hexachlorobutadiene vapour at measured concentrations of 0, 21,
53, 107 or 160 mg/m3 for 6 h per day from days 6 to 20 of
pregnancy. The breathing zone atmosphere was analysed by gas
chromatography. Maternal weight gain decreased at 53 and
160 mg/m3. At the other two exposure levels, the slight decrease
in maternal weight was not significant. The mean number of
implantation sites, total fetal losses, resorptions, live fetuses,
incidences of pregnancy, and sex ratio were not affected by exposure
to hexachlorobutadiene, compared to controls. Fetal body weight was
reduced in both sexes at 160 mg/m3. The incidences of external,
visceral, and skeletal alterations were not significantly increased
(Saillenfait et al., 1989).
In a study by Hardin et al. (1981), groups of 10-15 mated
Sprague-Dawley rats received hexachlorobutadiene in corn oil by
intraperitoneal injection at a dose level of 10 mg/kg body weight
per day from days 1 to 15 of gestation. It was reported (without
further details) that at least two maternal organ weights were
changed and that pre- or postimplantation survival was reduced.
Maternal tissues did not reveal histopathological effects. Fetuses
had a reduced weight or length, a 1-2 day delay in heart
development, and dilated ureters. No grossly visible external or
internal malformations were observed (Hardin et al., 1981).
It was reported briefly by Badaeva et al. (1985) that daily
oral administration of hexachlorobutadiene to pregnant rats at a
dose level of 8.1 mg/kg body weight per day resulted in
histopathologi-cal changes of nerve cells and myelin fibres of the
spinal cord in the dams and their offspring.
8.6 Mutagenicity and related end-points
8.6.1 In vitro effects
Purified hexachlorobutadiene induces gene mutations in the Ames
Salmonella test when specific incubation conditions are employed.
In preincubation assays adapted to include rat liver microsomes
and additional reduced glutathione, hexachlorobutadiene induced
point mutations in Salmonella typhimurium TA100 (Vamvakas et al.,
1988a). Assays lacking specialized metabolic activation conditions
have generally yielded negative results (Table 12).
Data from bacterial mutagenicity assays are consistent with the
proposed scheme for the biotransformation of hexachlorobutadiene in
animals (section 6.3; Fig. 1). Activity in S. typhimurium TA100,
mediated by subcellular fractions of rat kidney, was inhibited by
the addition of the ß-lyase inhibitor, AOAA (Vamvakas et al.,
1988a, 1989a) and the gamma-glutamyltranspeptidase inhibitor,
acivicin (Vamvakas et al., 1989a).
Several of the proposed metabolites of hexachlorobutadiene have
been assayed for mutagenic activity in S. typhimurium TA100
(Table 13). The mono-glutathione (GPB) and mono-cysteine (CPB)
conjugates were mutagenic in the presence or absence of rat kidney
S9. Rat liver microsomes and mitochondria that exhibit high
gamma-glutamyltranspeptidase activities strongly enhanced the
mutagenic potency of GPB in the presence of additional glutathione,
in contrast to liver microsomes that exhibit lower
gamma-glutamyltranspeptidase activity. Furthermore, AOAA and
acivicin both inhibit the activation of GPB mediated by kidney
fractions. The di-glutathione (BGTB) and di-cysteine (BCTB)
conjugates of hexachlorobutadiene were not mutagenic either in the
presence or absence of rat kidney S9 (Green & Odum, 1985; Dekant
et al., 1986; Vamvakas et al., 1988a, 1989a).
The mercapturic acid, ACPB, was mutagenic both in the presence
and absence of rat liver S9. It has been suggested that the
metabolism of ACPB in animals is catalysed by an N-deacetylase and
by ß-lyase (section 6.3) (Reichert et al., 1984). The Task Group
considered that S. typhimurium possesses both of these enzymes
activities. Both MTPB and CMTPB gave negative results in tests with
S. typhimurium TA100 and are considered to be detoxified
metabolites of hexachlorobutadiene (Wild et al., 1986).
Table 12. Studies on mutagenicity of hexachlorobutadiene
Test description Species/strain/cell type Conditionsa Resultc Reference
Reverse mutations Salmonella typhimurium TA98, +/- rat liver S9, purity 98%, - De Meester et al.
TA100, TA1530, TA1535, TA1538 plate incorporation (1981)
S. typhimurium TA100 +/- rat liver S9, purity > 99%, - Stott et al. (1981)
plate incorporation
S. typhimurium TA98, TA100 +/- rat liver S9, purity not - Reichert et al. (1983)
reported, suspension testb
S. typhimurium TA98, TA100, +/- rat liver S9, purity not - Haworth et al. (1983)
TA1535, TA1537 reported, preincubation test
+ rat liver S9*, purity > 99.5%, +
preincubation test
S. typhimurium TA100, TA1535 +/- rat liver S9, purity not - Chudin et al. (1985)
TA1538 reported, plate incorporation
S. typhimurium TA100 +/- rat liver S9, - Reichert et al. (1984)
- rat liver S9* +
S. typhimurium TA100 + rat liver S9*, purity >99.5%, +d Wild et al. (1986)
preincubation test
S. typhimurium TA100 no activation, purity 98% + Vamvakas et al. (1988a)
no activation, purity > 99.5%, -
preincubation test
Table 12 (contd).
Test description Species/strain/cell type Conditionsa Resultc Reference
S. typhimurium TA100 + rat liver microsomes - Vamvakas et al. (1988a)
without additional GSH
+ rat liver microsomes and +e
additional GSH, purity > 99.5%,
plate incorporation
Sex-linked lethals Drosophila melanogaster feeding or injection - Woodruff et al. (1985)
Chromosome aberrations CHO cells +/- rat liver S9 - Galloway et al. (1987)
Chromosome aberrations human lymphocytes - rat liver S9 - German (1988)
Sister chromatid exchanges CHO cells +/- rat liver S9 + Galloway et al. (1987)
Chromosome aberrations mouse bone marrow cells inhalation, 4 h + German (1988)
Chromosome aberrations mouse bone marrow cells oral gavage + German (1988)
a S9* = a fortified S9 mix containing 3 times the normal protein concentration; GSH = reduced glutathione
b The extreme toxicity of the compound without S9 was supposed to exclude testing in this system
c + = > twice the background rate or, in the case of bacterial studies, a reproducible dose-related increase in the number of
revertants per plate; - = negative
d 0.23 revertants per nmol
e Addition of rat kidney microsomes further increased the number of revertants; positive results were inhibited by the ß-lyase inhibitor
aminooxyacetic acid
Chinese hamster ovary (CHO) cells were exposed to between 5 and
24 mg hexachlorobutadiene/litre for 2 h in the presence of rat liver
S9 and throughout the incubation period (8-26 h, depending on cell
cycle delay) in the absence of rat liver S9. In comparison with
concurrent controls, no significant increase in chromosome
aberration frequency was observed (Galloway et al., 1987). In a
further study, in which human lymphocyte cultures were exposed to
between 0.01 and 0.001 mg hexachlorobutadiene per litre in the
absence of S9 for 27 h, there was also no clastogenic effect. At the
highest dose level there was a reduction of approximately 60% in the
mitotic index of human lymphocyte cultures (German 1988). However,
hexachlorobutadiene at a dose level of at least 4 mg/litre did cause
a significant increase in the frequency of sister chromatid exchange
in CHO cells in both the presence and absence of rat liver S9
(Galloway et al., 1987).
Hexachlorobutadiene was found to induce unscheduled DNA
synthesis (UDS) in Syrian hamster embryo fibroblast cultures.
Moreover, the magnitude of the response was increased when a
preincubation period with rat liver S15 was employed (Schiffman et
al., 1984). However, there was no induction of UDS in a study
using rat hepatocyte cultures (Stott et al., 1981).
In summary, the Task Group concluded that hexachloro-butadiene
was genotoxic in vitro and that the negative results reported in
some studies may have resulted from the use of inappropriate
conditions for metabolic activation.
8.6.2 In vivo effects
Hexachlorobutadiene induced a significant increase in the
frequency of chromosomal aberrations in mouse bone marrow cells
following the administration of acute oral doses of 2 or 10 mg/kg
body weight or acute inhalation exposure to 10 mg/m3 for 4 h. Both
experiments used six mice per dose group, and the animals were
sacrificed after 24 h (German, 1988).
Six hours after the administration of a single oral dose of
20 mg hexachlorobutadiene/kg body weight to two groups of five male
Sprague-Dawley rats, there were statistically significant increases
in kidney UDS of 27% and 54% above concurrent control levels.
Administration of a positive control substance,
dimethyl-nitrosamine, resulted in an increase of 187% over controls
(Stott et al., 1981).
As described in section 6.3, radiolabelled nucleotides were
recovered from the kidneys of rats and mice administered
14C-labelled hexachlorobutadiene by gavage (Stott et al., 1981;
Schrenk & Dekant, 1989). The Task Group concluded that these studies
indicated covalent binding of hexachlorobutadiene or its metabolites
Table 13. Tests for reverse mutations in Salmonella typhimurium TA100 by proposed metabolites of hexachlorobutadiene
Metabolite and abbreviationa Conditionsb Resultc Reference
1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (GPB) no activation - Green & Odum (1985)
+ rat kidney S9 +
+/- rat kidney fractions +d Vamvakas et al. (1988a)
1,4-(bis-glutathion- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BGTB)
1,4-(bis-cystein- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BCTB)
1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (CPB) +/- rat kidney S9 +e Green & Odum (1985)
no activation +f Dekant et al. (1986)
1-( N-acetylcystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene - rat liver S9 - Wild et al. (1986)
(ACPB) + rat liver S9 +g
1-carboxymethylthio-1,2,3,4,4-pentachloro-1,3-butadiene + rat liver S9* - Wild et al. (1986)
(CMTPB)
Table 13 (contd).
Metabolite and abbreviationa Conditionsb Resultc Reference
1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) + rat liver S9 - Wild et al. (1986)
2,2,3,4,4-pentachloro-3-butenoic acid (PBA) +/- rat liver S9 + Reichert et al. (1984)
2,2,3,4,4-pentachloro-3-butenoic acid chloride (PBAC) +/- rat liver S9 + Reichert et al. (1984)
a See also Figure 1
b Plate incorporation assays with, except in the case of the tests by Green & Odum, preincubation; S9* = a fortified S9 mix containing
3 times the normal protein concentration
c + = > twice background rate; - = negative
d Mutagenic potency enhanced by rat kidney microsomes or mitochondria and less so by cytosol; positive results were inhibited by the
ß-lyase inhibitor aminooxyacetic acid
e Mutagenic potency enhanced by rat kidney S9
f Positive results were inhibited by the ß-lyase inhibitor aminooxyacetic acid
g 18.7 revertants per nmol; mutagenic potency decreased by addition of pyridoxal phospate; activation by cytosol, with and without cofactors,
had the same results as S9 mix; microsome mix was inactive
to kidney DNA in vivo. The study with mice showed that the level
of binding to mitochondrial DNA was greater than that to nuclear
DNA. In addition, radioactivity was recovered in mitochondrial DNA,
but not nuclear DNA, from mouse liver (Schrenk & Dekant, 1989).
Hexachlorobutadiene did not induce sex-linked recessive lethal
mutations in Drosophila melanogaster following treatment of adults
either via the diet or by injection (Woodruff et al., 1985).
8.7 Carcinogenicity/long-term toxicity
8.7.1 Inhalation exposure
No long-term carcinogenicity studies, where inhalation was the
route of exposure, have been reported.
8.7.2 Oral exposure
In a study by Kociba et al. (1977a,b), groups of 39-40
adult Sprague-Dawley rats of each sex received a diet containing
hexachlorobutadiene at 0.2, 2 or 20 mg/kg body weight per day for 22
(males) or 24 (females) months. Control groups comprised 90 rats of
each sex. Analysis for the compound was not reported. An increased
mortality was observed in males at 20 mg/kg. Hexachlorobutadiene
caused a depression of the body weight gain in both sexes at the
highest dose level without any effect on food consumption.
Haematological investigations performed at 12-14 and 22-24 months,
revealed a slight, but statistically significant, depression in the
red blood cell count of males at 20 mg/kg (22 months). Urinalysis at
12-14 months and 22-24 months did not reveal effects except for a
small increase in coproporphyrin excretion. The analysis of the
clinical chemistry parameters of blood urea nitrogen, serum alanine
aminotransferase (EC 2.6.1.2) and serum alkaline phosphatase
(EC 3.1.3.1) at 12 months revealed no treatment-related effects,
except for statistically significant decreases in serum alanine
aminotransferase in males of the 20-mg/kg dose group and females of
the 0.2- or 20-mg/kg dose groups. These changes were considered by
the authors to be of questionable toxicological significance. The
relative kidney weights were elevated at 20 mg/kg for both sexes, as
were the relative weights of the brain in females and of the testes
in males. In both sexes, an extensive histopathological examination
revealed tubular epithelial hyperplasia at 2 and 20 mg/kg, but not
at 0.2 mg/kg, and an increased incidence of renal tubular neoplasms
at 20 mg/kg (see Table 14) (Kociba et al., 1977a,b).
8.7.3 Dermal exposure
In a study by Van Duuren et al. (1979), groups of 30 female
Ha:ICR Swiss mice received 6.0 mg hexachlorobutadiene in acetone
applied 3 times per week to the shaven dorsal skin for between 144
and 594 days. A group of 100 untreated females were included in the
study, together with 30 controls which received acetone only. The
study duration was described as being between 440 and 594 days.
Sections of skin, liver, stomach and kidney were sampled at autopsy,
but no increase in the number of distant tumours was observed.
In a two-stage initiation-promotion experiment, each of
20 female Swiss mice received one application of 15.0 mg
hexa-chlorobutadiene in acetone to the dorsal skin. After 14 days,
the mice similarly received 5 µg of the tumour promoter
12- o-tetra-decanoylphorbol-13-acetate (TPA) three times weekly for
between 428 and 576 days. Hexachlorobutadiene administration did not
induce a significant increase in the fraction of mice developing
skin papillomas in this study (Van Duuren et al., 1979).
Table 14. Renal tubular neoplasms in rats after long-term
exposure to hexachlorobutadienea
Dose (mg/kg Sex Incidence of renal tubular neoplasms
body weight
per day) adenoma adenocarcinoma total
0 males 1/90 0/90 1/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 2/39 7/39 9/39 (P < 0.05)
0 females 0/90 0/90 0/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 3/40 3/40b 6/40 (P < 0.05)
a From: Kociba et al. (1977a)
b One of these was an undifferentiated carcinoma
8.7.4 Exposure by other routes
In a study of repeated exposure to hexachlorobutadiene by ip
injection, groups of 20 A/St strain male mice (from 6 to 8 weeks of
age) received 12 or 13 ip injections of hexachlorobutadiene (4 or
8 mg/kg body weight) in tricaprylin, respectively. The purity of the
hexachlorobutadiene was stated to exceed 99.9%. Urethane was used as
a positive control for carcinogenesis, and a negative control group
of 50 mice receiving tricaprylin only. Survival was 95% for mice
receiving 4 mg/kg and 70% for mice receiving 8 mg/kg, compared to
92% for controls. Mice were sacrificed at 24 weeks after the first
injection, and the number of surface adenomas in the lungs was
counted. No significant increase in adenomas, compared to the
vehicle-treated control, was observed (Theiss et al., 1977). The
Task Group noted major deficiencies of this study; including the
choice of a sensitive strain of mice, the short duration of both the
exposure period (4 weeks) and the follow-up period (24 weeks), the
small group sizes of the experimental animals, the unusual route of
administration, and the limited histopathology. The strain A mice
used in this study are highly predisposed to spontaneous lung
cancer, which is likely to have further compromised the value of the
study.
8.8 Other special studies
8.8.1 Effects on the nervous system
Acute high exposure to hexachlorobutadiene has a depressant
effect on the central nervous system (see sections 8.1.1.2 and
8.1.2).
Subchronic exposure of rats at high dose levels (1500 mg/kg
diet for 13 weeks) also produced some signs of neurotoxicity, which
was associated with demyelinization and fragmentation of femoral
nerve fibres (Harleman & Seinén, 1979; see also Badaeva et al.,
1985, section 8.5.2).
8.8.2 Effects on the liver
8.8.2.1 Acute effects
Hexachlorobutadiene causes hydropic changes in the liver of
rats (Gradiski et al., 1975; Lock & Ishmael, 1981; Lock et al.,
1982), mice (Lock et al., 1985), and rabbits (Duprat & Gradiski,
1978), sometimes accompanied by fat accumulation (Duprat & Gradiski,
1978; Lock & Ishmael, 1981; Lock et al., 1982).
Male rats, exposed to a single intraperitoneal dose of
hexachlorobutadiene (200 or 300 mg/kg body weight) in corn oil
showed increased relative liver weights, mitochondrial swelling in
liver and bile duct, proliferation of smooth endoplasmic reticulum,
lipid accumulation, and increased water content in the liver.
Biochemical changes in the liver were a decrease, followed after 1
day by an increase, in non-protein sulfhydryl (NP-SH) concentration,
and an increase in potassium content. All effects were reversible
within 10 days. Increases in plasma urea and alkaline phosphatase
(EC 3.1.3.1.) were also reported. In a separate experiment, the
highest dose administered by ip injection which did not cause an
increased water content in the liver was 25 mg/kg body weight (Lock
et al., 1982).
Male rats exposed to single intraperitoneal doses up to
100 mg/kg body weight showed an increase in serum bile acids and
bilirubin (Bai et al., 1992).
Male mice, exposed to single intraperitoneal doses of 50, 100
and 200 mg/kg body weight in corn oil, showed a dose-related
increase in relative liver weight at 100 and 200 mg/kg, and, at all
dose levels, dose-related, reversible changes in the liver
(mitochondrial swelling, proliferation of smooth endoplasmic
reticulum, and an increased water content). Reversible biochemical
changes included increases in sodium and potassium content, NP-SH
concentration in the liver, and serum alanine aminotransferase
activity (EC 2.6.1.2) at 50 mg/kg (Lock et al., 1985).
8.8.2.2 Short-term effects
As discussed in section 8.2.2.1, slight hepatotoxic effects
have been observed following oral exposure of rats (Kociba
et al., 1971; Harleman & Seinen, 1979).
8.8.3 Effects on the kidneys
This section will describe the main features of the renal
toxicity induced by hexachlorobutadiene. For more detail the reader
is referred to the reviews of Rush et al. (1984), Lock (1988),
Yang (1988) and Dekant et al. (1990a).
8.8.3.1 Acute effects
Inhalation exposure of rats produces renal tubular necrosis
(Gage, 1970; see section 8.2.1). Enzyme histochemical
investi-gations were performed on groups of 10 male Swiss OF1 mice
24 h after whole-body inhalation exposure for 4 h to
hexachloro-butadiene at measured concentrations of 29.3, 53.4, 106.7
or 266.8 mg/m3. A concentration-related increase in the percentage
of damaged kidney tubules, which had been stained for alkaline
phosphatase (EC 3.1.3.1), was observed at all exposure levels. The
EC50 was calculated to be 76.8 mg/m3 (De Ceaurriz et al.,
1988).
A single oral dose of hexachlorobutadiene (200 mg/kg body
weight) in polyethylene glycol caused an increase in plasma urea
concentration, a decrease in plasma alanine aminotransferase
activity, and, in urine, increases in the levels of glucose,
protein, alanine aminotransferase, N-acetyl-ß-D-glucosaminidase,
gamma-glutamyltranspeptidase (EC 2.3.2.2) and alanine aminopeptidase
(EC 3.4.11.12) (Nash et al., 1984).
Following in vivo administration, hexachlorobutadiene caused
dose-dependent necrosis of the renal proximal tubules in rats
(Gradiski et al., 1975; Lock & Ishmael, 1979, 1981; Kluwe et al.,
1982; Hook et al., 1982, 1983; Ishmael et al., 1982; Ishmael &
Lock, 1986), mice (Ishmael et al., 1984) and rabbits (Duprat &
Gradiski, 1978). In rats, the lesions were restricted to the pars
recta (S3-segment) and were macroscopically observed as a distinct
band of damage in the outer stripe of the medulla (Lock & Ishmael,
1979; Ishmael et al., 1982). In mice and rabbits both the pars
recta and the pars convoluta of the proximal convoluted tubules were
damaged (Duprat & Gradiski, 1978; Ishmael et al., 1984). The
lesion is characterized microscopically by necrotic epithelial
cells, most of which are devoid of nuclei. The few remaining nuclei
show karyorrhexis, and the cytoplasm is strongly eosinophilic. Many
renal tubules contained cellular debris (Duprat & Gradiski, 1978;
Lock & Ishmael, 1979; Lock et al., 1984; Ishmael et al., 1984).
Vacuolation of the pars convoluta was observed (Duprat & Gradiski,
1978; Ishmael et al., 1982, 1984). Mitochondrial swelling and loss
of brush-borders were prominent ultrastructural findings (Ishmael
et al., 1982, 1984).
Adult male rats have been found to be less sensitive to the
renal toxicity induced by hexachlorobutadiene than adult females and
young males (Hook et al., 1983; Kuo & Hook, 1983). When male rats
were dosed intraperitoneally with a single dose of 300 mg/kg in corn
oil, the earliest pathological change was mitochondrial swelling in
proximal tubular cells observed after 1-2 h. Extensive necrosis was
evident between days 1 and 4, and active regeneration by day 5
(Ishmael et al., 1982). Similar renal toxicity was seen at a dose
level of 25 or 50 mg/kg body weight in female rats and young males,
respectively. A similar pattern of pathological changes with
comparable intensity was observed in mice at an intraperitoneal dose
of 50 mg/kg body weight (Ishmael et al., 1984). In a study by Lock
et al. (1984), young mice were found to be more susceptible than
adults, but no sex difference was apparent. In both rats and mice,
differences in strain susceptibility were observed (Hook et al.,
1983; Lock et al., 1984). The lowest intraperitoneal dose at which
renal necrosis was observed in adult female rats was 25 mg/kg body
weight (Lock & Ishmael, 1985) and in adult male and female mice was
6.3 mg/kg body weight (Lock et al., 1984).
Biochemical changes found following intraperitoneal exposure in
both rats and mice were increases in renal water content (Kluwe et
al., 1982; Ishmael et al., 1982, 1984; Gartland et al., 1989),
plasma urea (Lock & Ishmael, 1979, 1981; Ishmael et al., 1982,
1984; Hook et al., 1983; Lock et al., 1984; Ishmael & Lock,
1986; Stonard et al., 1987; Gartland et al., 1989), plasma
alkaline phosphatase (EC 3.1.3.1.) (Lock & Ishmael, 1981), serum
alanine aminotransferase (EC 2.6.1.2) (Gradiski et al., 1975; Kuo
& Hook, 1983) and serum aspartate aminotransferase (Gradiski et
al., 1975; Davis et al., 1980). In the urine of rats, increases
in urinary protein, glucose and ketones have been measured (Lock &
Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al., 1980;
Stonard et al., 1987), as well as increases in the activities of
alkaline phosphatase and N-acetyl-ß-D-glucosaminidase (EC
3.2.1.50) (Lock & Ishmael, 1979; Stonard et al., 1987) and in
lactic acid level (Gartland et al., 1989). In the kidneys of rats,
increased sodium concentrations were accompanied by equally
decreased potassium concentrations (Davis et al., 1980). All these
changes occurred at similar or higher intraperitoneal doses than
those at which renal necrosis was observed.
Distinct renal functional changes in adult rats have been
observed at intraperitoneal doses of 100-400 mg/kg body weight.
These include a decrease in urine-concentrating ability (polyuria)
(Lock & Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al.,
1980; Stonard et al., 1987), a reduced glomerular filtration rate
(Davis et al., 1980) and a reduction of in vivo renal clearance
of inulin, urea, p-aminohippuric acid (PAH), tetraethyl-ammonium
bromide (TEA) (Lock & Ishmael, 1979) and imipramine (Davis et al.,
1980). When organic ion transport was assessed in vitro in renal
cortical slices of rats and mice that had been exposed to an
intraperitoneal dose of 100 mg/kg, 200 mg/kg body weight or more,
the transport of anions (PAH) was found to be reduced, but the
transport of the cation (TEA), aminoisobutyrate was not (or was only
slightly reduced) in rats (Lock & Ishmael, 1979; Berndt & Mehendale,
1979; Kluwe et al., 1982; Hook et al., 1982, 1983). In male
adult mice, transport of PAH and TEA was reduced from
intraperitoneal doses of 12.5 and 25.0 mg/kg body weight,
respectively. The anion transport was reduced in adult females (Lock
et al., 1984).
8.8.3.2 Short- and long-term effects
The short- and long-term effects of hexachlorobutadiene on the
kidneys of experimental animals have already been discussed in
sections 8.2 , 8.5 and 8.7. Based on the studies of Kociba et al.
(1971, 1977a,b), Schwetz et al. (1977), Harleman & Seinen (1979),
Stott et al. (1981) and Yang et al. (1989), the oral NOAEL for
renal toxicity is 0.2 mg/kg body weight per day. Results of the
studies are summarized in Table 15. Female rats and mice were found
to be distinctly more susceptible than males upon oral exposure for
13 weeks (Yang et al., 1989; Yang, 1991); this was also observed
in the single-exposure mortality studies (section 8.1).
8.9 Factors modifying toxicity; toxicity of metabolites
8.9.1 Factors modifying toxicity
8.9.1.1 Surgery
Complete protection from the nephrotoxic effects of
hexachlorobutadiene was observed in rats that had been fitted with a
biliary cannula before being given a single oral dose of 200 mg/kg
body weight. Administration of bile, collected from rats dosed
orally with the compound, to naive rats produced marked renal
toxicity but no liver toxicity (Nash et al., 1984).
8.9.1.2 Inhibitors and inducers of mixed-function oxidases (MFO)
In the majority of studies, the effects of MFO inhibitors
(piperonyl butoxide, SKF 525A) and MFO inducers (Aroclor 1254,
isosafrole, ß-naphthoflavone, phenobarbitone) on the nephro-toxicity
induced by hexachlorobutadiene in rats and mice were absent or
negligible (Lock & Ishmael, 1981; Hook et al., 1982; Lock et al.,
1984; Davis, 1984). Furthermore, phenobarbitone pretreatment for 7
days at 0.05% in drinking-water enhanced the renal toxicity induced
by intraperitoneal doses of hexachloro-butadiene in weanling rats
(Hook et al., 1983).
8.9.1.3 Inhibitors of gamma-glutamyltranspeptidase (EC 2.3.2.2)
Male rats pretreated with Acivicin (L-(alphaS, 5S)-
alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid), an
inhibitor of gamma-glutamyltranspeptidase (down to 3% of control
activity in this study), and subsequently exposed intraperitoneally
to hexachlorobutadiene, did not show a decrease in nephrotoxicity
compared to rats treated with hexachlorobutadiene alone. It was
concluded that gamma-glutamyltranspeptidase inhibition did not limit
the formation of nephrotoxic metabolites (Davis, 1988).
Male Swiss OF1 mice, pretreated with Acivicin and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group noted that only one marker for nephrotoxicity was employed in
this study.
Table 15. No-observed-adverse-effect level (NOAEL) calculated from short-term and long-term
studies of exposure to hexachlorobutadiene by oral administration
Species (Strain) Age Sex Number of Duration of Dose (mg/kg NOAEL (mg/kg References
animals study body weight body weight
per group per day) per day)
Rat (Wistar-derived) weanling male 6 2 weeks 7.3, 18.2, 44.7 < 7.3 Harleman & Seinen
female 6 (1979)
Rat (Sprague-Dawley) adult male 5 3 weeks 0.2, 20 0.2 Stott et al. (1981)
Rat (Sprague-Dawley) adult female 4 30 days 1, 3, 10, 30, 65, 100 3 Kociba et al. (1971)
Rat (Sprague-Dawley) adult male 10-12 3 months 0.2, 2.0, 20 0.2 Schwetz et al.
female 20-24 (1977)
Rat (Sprague-Dawley) adult male 39-40 24 months 0.2, 2.0, 20 0.2 Kociba et al.
female 39-40 (1977a,b)
Mouse (B6C3F1) adult male 5 2 weeks 4.3, 14.3, 43, 143, 430 < 4.3 Yang et al.
female 5 (1989); Yang (1991)
Mouse (B6C3F1) adult male 10 13 weeks 0.1, 0.4, 1.5, 4.9, 18.8 1.5 Yang et al.
female 10 0.2, 0.5, 1.8, 4.5, 19.2 < 0.2 (1989); Yang (1991)
8.9.1.4 Inhibitors of cysteine conjugate ß-lyase
Male Swiss OF1 mice, pretreated with the two ß-lyase inhibitors
amino-oxyacetic acid (AOAA) and DL-propargylglycine (PPG) and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group again noted that only one marker for nephrotoxicity was
employed in this study.
8.9.1.5 Inhibitors of organic anion transport
Pre-treatment of male rats with probenecid
[(4-(dipropyl-amino)sulfonyl)] benzoic acid (105 µmol/kg body
weight), an inhibitor of organic anion transport, did not alter the
increase in plasma urea or decrease in renal clearance of
p-aminohippuric acid induced by hexachlorobutadiene (Hook et al.,
1982). However, in female rats, a higher dose (500 µmol/kg body
weight) of probenecid totally protected against the renal toxicity,
both functional and morphological, produced by ACPB (Lock & Ishmael,
1985). In addition this dose of probenecid protected female rats
against the toxic effects produced by CPB and GPBN as well as the
parent chemical (Lock & Ishmael, 1985). Male mice pre-treated with
probenecid were also protected against the nephrotoxicity produced
by hexachlorobutadiene (Ban & De Ceaurriz, 1988). The Task Group
noted that this latter study used only one marker for
nephrotoxicity.
8.9.1.6 Non-protein sulfhydryl scavengers
Depletion of hepatic and renal non-protein sulfhydryl content
(glutathione) by diethylmaleate in rats appears to potentiate the
nephrotoxicity of hexachlorobutadiene as measured by a number of
functional markers such as plasma urea (Hook et al., 1982; Baggett
& Berndt, 1984, Davis et al., 1986). However, the Task Group noted
that no information was available on the metabolism of
hexachlorobutadiene to help interpret these studies.
8.9.2 Toxicity of metabolites
This section discusses the renal toxicity of some metabolites
of hexachlorobutadiene, the formation of which was discussed in
section 6.2. These metabolites are 1-(glutathion- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (GPB), 1-(cystein- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (CPB), and 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB). Their mutagenic activity
was described along with that of hexachlorobutadiene in section 8.6.
8.9.2.1 In vitro studies
GPB decreased the viability of isolated renal epithelial cells
of male rats, as measured by leakage of lactate dehydrogenase (EC
1.1.1.27), with a very steep dose-response curve and a lag period of
30 min. No cytotoxicity was observed when the GPB metabolism was
blocked by anthglutin, an inhibitor of gamma-glutamyl-transpeptidase
(EC 2.3.2.2) or amino-oxyacetic acid (AOAA), an inhibitor of renal
cysteine conjugate ß-lyase (EC 4.4.1.13). The cytotoxicity of GPB
was related to an impairment of mitochondrial function, as shown by
loss of mitochondrial Ca2+ and ATP and inhibition of respiration
and thiol depletion (Jones et al., 1986b). Likewise, GPB produced
a concentration-dependent nephro-toxicity in the isolated perfused
rat kidney, as indicated by the appearance in the urine of alkaline
phosphatase, gamma-glutamyl-transpeptidase and glucose (Jones et
al., 1986a). These changes were prevented by Acivicin and by AOAA
(Schrenk et al., 1988a).
In the study of Schrenk et al. (1988a), CPB also caused a
marked nephrotoxicity in the isolated perfused kidney, which could
be prevented by AOAA. In isolated rabbit renal tubules, CPB was
observed to decrease the accumulation of p-amino-hippuric acid and
tetraethylammonium (Jaffe et al., 1983), to affect mitochondrial
function as shown by effects on cell respiration, and to decrease
the glutathione content and, after a lag period of 60 min, cell
viability (Schnellmann et al., 1987). The effects on respiration
resulted initially from the uncoupling of oxidative phosphorylation,
followed later by inhibition of state 3 respiration (Schnellmann et
al., 1987). Impaired mitochondrial function was observed in
CPB-exposed isolated rat renal cortical mitochondria as an inability
to retain Ca2+, collapse of the membrane potential, impaired state
3 respiration with succinate as substrate, and nonenzymatic
depletion of thiol content. The latter effect was blocked by AOAA.
From these results it was concluded that the reactive intermediate
formed from CPB interacts with the inner mitochondrial membrane
(Wallin et al., 1987). CPB also inhibited rat kidney mitochondrial
DNA, RNA and protein synthesis, and AOAA blocked this effect.
Moreover, CPB converted supercoiled DNA to relaxed circular DNA and
shorter linear fragments (Banki & Anders, 1989). Chen et al.
(1990) observed a decreased viability of isolated human renal
proximal tubular cells upon exposure to CPB, which was again blocked
by AOAA. Using radiolabelled ACPB and rat renal cortical slices, it
was established that ACPB is transported by the same renal
mechanisms involved in the movement of many organic anions into
tubular fluid. This carrier-mediated transport is reduced by
specific inhibitors like probenecid and sulfinpyrazone, a
competitive and metabolic inhibitor like 2,4-dinitrophenol, and the
transport substrate p-aminohippuric acid (Lock et al., 1986).
This was confirmed by recent studies on the mechanism of uptake of
GPB and CPB in the isolated perfused rat kidney (Schrenk et al.,
1988b). Probenecid has also been reported to protect renal proximal
tubular cells against ACPB-induced cytotoxicity, as determined by
monitoring proline incorporation into renal proteins (Bach et al.,
1986).
8.9.2.2 In vivo studies
A single oral dose of 138 mg/kg body weight (0.27 mmol) of GPB
or a single equimolar oral dose of 100 mg ACPB/kg body weight in
polyethylene glycol to male rats caused marked nephrotoxicity
similar in both biochemical and histopathological aspects to that
observed with an oral dose of 200 mg/kg body weight (0.97 mmol) of
hexachlorobutadiene (Nash et al., 1984). When rats received
intraperitoneally GPB, CPB or ACPB in polyethylene glycol at single
doses between 6.25 and 100 mg/kg body weight, increases in plasma
urea level and renal proximal tubular necrosis were observed at dose
levels of > 6.25 mg/kg body weight in females and 10 or 12.5 mg/kg
body weight in males. The conjugates exhibited a similar pattern of
nephrotoxicity at equimolar doses and were more nephrotoxic than the
parent compound (Lock & Ishmael, 1985; Ishmael & Lock, 1986). All
compounds tested were more toxic to female rats than males (Ishmael
& Lock, 1986). Probenecid pretreatment protected the rats against
the nephrotoxicity of these metabolites. Probenecid was shown to
block the active tubular secretion of ACPB and to reduce the extent
of covalent binding to renal protein (Lock & Ishmael, 1985). In
mice, GPB and ACPB were also shown to be more toxic than the parent
compound: renal necrosis was found following single intraperitoneal
doses of 5.0 mg hexachlorobutadiene/kg body weight, 3.1 mg GPB/kg
body weight and 3.0 mg ACPB/kg body weight in corn oil, which were
the lowest doses tested (Lock et al., 1984). A single
intraperitoneal dose of 10 mg CPB/kg body weight in DMSO and water
caused dose-related damage in the pars recta of renal proximal
tubules in male mice (Jaffe et al., 1983).
The nephrotoxicity of some structural analogues of the
above-mentioned conjugates, e.g. S-(1,2-dichlorovinyl)-L-cysteine,
has been investigated extensively and has revealed a remarkable
similarity (Anders et al., 1987; Lock, 1988).
8.10 Mechanisms of toxicity - mode of action
8.10.1 Mechanisms of toxicity
The following evidence supports the hypothesis that the
nephrotoxicity, mutagenicity and carcinogenicity of
hexachloro-butadiene is dependent on the biosynthesis of the toxic
sulfur conjugate GPB. This conjugate is mainly synthetized in the
liver and further metabolized in the bile, gut, and kidneys to the
CPB. Cysteine conjugate ß-lyase-dependent activation of CPB to a
reactive thioketene in the proximal tubular cells finally results in
covalent binding to cellular macromolecules.
1. The nephrotoxicity of hexachlorobutadiene in rats was prevented
by the implantation of a biliary cannula; administration of
bile from hexachlorobutadiene-treated rats to naive rats
resulted in nephrotoxicity identical to the nephrotoxicity
caused by hexachlorobutadiene (see section 8.9.1.1).
2. Inhibitors of renal organic anion transport protected rats
against the nephrotoxicity of hexachlorobutadiene and its
sulfur conjugates. Inhibition of the organic anion transport
also protected isolated kidney cells against the nephrotoxicity
of hexachlorobutadiene-derived sulfur-conjugates (see sections
8.9.1.3 and 8.9.2.1).
3. Anthglutin, Acivicin and aminooxyacetic acid, specific
inhibitors of gamma-glutamyltranspeptidase and cysteine
conjugate ß-lyase protected against the cytotoxicity of
hexachloro-butadiene-derived sulfur-conjugates in freshly
isolated rat renal proximal tubular cells (see section
8.9.2.1).
4. Synthetic sulfur-conjugates of hexachlorobutadiene show a
higher nephrotoxicity than the parent compounds in rats and
mice and produce renal damage identical to the renal damage
induced by hexachlorobutadiene, based on clinical chemistry and
histopathological examination (see section 8.9.2.2).
5. Hexachlorobutadiene and its sulfur-conjugates are genotoxic in
bacteria; bioactivation by glutathione conjugation is required
for hexachlorobutadiene genotoxicity. The ultimate mutagen is
formed by cysteine conjugate ß-lyase-dependent cleavage of CPB
(see section 8.6).
6. Hexachlorobutadiene induces renal tumours in rats only at doses
that produce marked nephrotoxicity (see sections 8.7 and
8.8.2.2).
8.10.2 Mode of action
The in vitro studies of Jones et al. (1986b), Wallin et
al. (1987) and Schnellmann et al. (1987) on the cytotoxicity of
sulfur-conjugates to renal tubular cells (section 8.9.1.2) point to
renal cortical mitochondria as the major target for
sulfur-conjugates of hexachlorobutadiene, analogous to that
established for close structural analogues (Dekant et al., 1990b).
The hypothesis proposes an interaction of the reactive metabolite
with the inner mitochondrial membrane, which ultimately causes
respiratory insufficiency.
9. EFFECTS ON HUMANS
9.1 General population exposure
Hexachlorobutadiene has been found in postmortem examinations,
but not in living persons. No pathogenic effects have been recorded
(see section 5.2).
9.2 Occupational exposure
Two reports on certain disorders among agricultural workers in
vineyards where hexachlorobutadiene has been used as a fumigant
(Krasniuk et al., 1969; Burkatskaya et al., 1982) cannot be
evaluated, since such workers are known to be occupationally exposed
to additional substances.
In two cytogenetic studies of occupationally exposed workers
from the same plant engaged in the production of
hexachloro-butadiene, an increase in the frequency of chromosomal
aberrations in peripheral blood lymphocytes was observed (German,
1986). The workers were exposed to hexachloro-butadiene
concentrations that ranged from 1.6 to 16.9 mg/m3. The Task Group
noted that exposure concentrations were determined by the factory
and that the frequency of chromosome aberrations was not associated
with the period of employment.
9.3 In vitro metabolism studies
The following studies have been reported:
a) Purified human liver microsomal glutathione- S-transferase
and human liver cytosol metabolize hexachlorobutadiene to form
GPB (McLellan et al., 1989; Oesch & Wolf, 1989).
b) The enzyme cysteine conjugate ß-lyase has been isolated and
purified from human kidney cytosol (Lash et al., 1990). The
activity of the human cytosolic enzymes with a structurally
related compound (1,2,2-trichlorovinyl-L-cysteine) is about
10-fold lower than that of rat renal cytosol (Green et al.,
1990).
c) Studies in isolated human proximal tubular cells have shown
that CPB causes a ß-lyase-dependant cytotoxicity (Chen et al.,
1990).
These limited studies suggest that humans have the ability to
metabolize hexachlorobutadiene to toxic metabolites.
9.4 Extrapolation of NOAEL from animals to humans
Conversion of equivalent doses across species can utilize
allometric relationships that relate physiological and anatomical
variables across species. Physiological and metabolic rates have
been shown to relate closely to body weight to the power 0.75
(Boxenbaum, 1982). The equivalent NOAEL in humans (mg/kg body weight
per day) can be determined from the following equation:
Wa
dh = da (--)0.25
Wh
where
d = dose rate (mg/kg body weight per day) in humans (dh) or
animals (da)
wa = weight (kg) of animals (mice 30 g; rats 400 g)
wh = weight of humans (70 kg)
The NOAEL for humans, based on the NOAEL in mice, is:
dh= (0.2 mg/kg body weight per day) (0.03)0.25
70
= 0.03 mg/kg body weight per day
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Hazard identification
The following evaluation is based on toxicity studies in
experimental animals; however, there are some human in vitro data
which indicate that hexachlorobutadiene metabolism can occur by a
similar route to that shown in experimental animals.
Hexachlorobutadiene is slightly to moderately toxic based on
acute oral experiments with adult rats, and moderately to highly
toxic based on acute oral experiments with weanling rats (based on
the WHO pesticide toxicity classification). The specific toxicity of
the compound to the kidneys is higher for females than males.
Following acute dermal exposure of rabbits, the compound was found
to be weakly toxic.
Regardless of species studied and the route of exposure (ip,
oral, inhalation, dermal) in both short- and long-term studies, the
target organ for toxicity is the kidney. Bioactivation to produce a
reactive sulfur metabolite occurs following conjugation with
glutathione. The monoglutathione conjugate of hexachloro-butadiene
is processed to the cysteine- S-conjugate, which is then a
substrate for renal cysteine conjugate ß-lyase. Hexachloro-butadiene
produces a dose-dependent necrosis of the renal proximal tubules,
which is followed by regenerative and/or proliferative changes. On
the basis of both short- and long-term studies in rats and mice
orally exposed to hexachlorobutadiene, the no-observed-adverse-
effect level (NOAEL) is 0.2 mg/kg body weight per day. In one
short-term inhalation study (12 days, 6 h/day), the NOAEL was
53 mg/m3.
The vapour of hexachlorobutadiene was found to be irritating to
the eyes and nose of rats in one short-term inhalation study. The
undiluted compound appeared corrosive in an experiment with rabbits.
Based on these limited data, the vapour should be regarded as
irritating to human mucous membranes and the liquid should be
regarded as corrosive.
In a well-conducted Magnusson-Kligman test hexachloro-butadiene
was a sensitizing agent both with and without adjuvant. Therefore,
the compound should be regarded as a sensitizing agent for humans.
In reproductive studies, reduced birth weight and neonatal
weight gain in rats were observed, but these effects may be
attributed to maternal toxicity. Developmental toxicity to rat
fetuses was observed in two teratogenicity tests, but again only at
levels that were also toxic to the dams. This developmental toxicity
included reduced birth weight, a 1- to 2-day delay in heart
development, and dilated ureters, but no gross abnormalities were
observed.
In vitro studies have shown that hexachlorobutadiene and, to
a much greater extent, its sulfur metabolites induce mutations in
Salmonella typhimurium. In one study of exposure to
hexachloro-butadiene by inhalation or oral administration, an
increased frequency of chromosomal aberrations was observed in mouse
bone marrow cells. There is limited evidence for the genotoxicity of
hexachlorobutadiene in animals, and insufficient evidence in humans.
The long-term oral administration of hexachlorobutadiene to
rats induced an increased frequency of renal tubular neoplasms, but
only at doses that caused marked nephrotoxicity; at the lowest dose,
no adverse effects were observed.
The Task Group concluded that there is limited evidence for the
carcinogenicity of hexachlorobutadiene in animals (one study in one
rodent strain) and insufficient evidence in humans.
10.1.2 Exposure
Hexachlorobutadiene is mainly a waste product. As such, it can
be encountered in different environmental compartments, but
predominantly in sediment and biota (see also 10.2.1). Exposure of
the general public therefore mainly occurs indirectly via
drinking-water and food of high lipid content. Assuming a maximum
concentration of 2.5 µg/litre in contaminated drinking-water and
10 µg/kg wet weight in contaminated fatty food items (meat, fish,
milk) and daily intakes of 2 litres drinking-water, 0.3 kg meat,
0.2 kg fish and 0.5 kg milk, a maximum total daily intake of
0.2 µg/kg body weight can be calculated for a 70-kg person.
10.1.3 Hazard evaluation
The NOAEL for mice or rats exposed to hexachlorobutadiene is
0.2 mg/kg body weight per day (see Table 15), from which a NOAEL of
0.03-0.05 mg/kg body weight day has been derived for humans (see
section 9.4).
The Task Group considered the margin of safety of 150 between
the estimated NOAEL in humans and the maximum total daily intake
(see section 10.1.2) to be sufficient to protect the general
population against the adverse effects of hexachlorobutadiene.
10.2 Evaluation of effects on the environment
10.2.1 Hazard identification
Hexachlorobutadiene is a chemically stable compound. Complete
aerobic biodegradation has been observed following adaptation of the
inoculum. Partial biodegradation was found to occur in a pilot
sewage treatment plant. Based on these observations and the chemical
structure, it can be concluded that hexachlorobutadiene is not
readily biodegradable, but can be considered to be inherently
biodegradable. Experimental photolysis of hexachlorobutadiene in the
presence of a surface was rapid, but in the absence of a surface the
compound is believed to be persistent. Degradation in the atmosphere
is assumed to occur by a rather slow reaction with hydroxyl
radicals. A half-life of up to 2.3 years has been calculated.
Once hexachlorobutadiene is released into the environment,
intercompartmental transport will occur chiefly by volatilization
from water and soil, adsorption to particulate matter in water and
air, and subsequent sedimentation or deposition. In view of a strong
adsorption potential to organic matter, the compound accumulates in
sediment and will not migrate rapidly in soils. Both field and
laboratory exposure data support these conclusions.
Field and laboratory data also support the high
bioaccumu-lation potential in aquatic and benthic organisms which
can be expected on the basis of the lipophilic nature of the
compound. However, no evidence has been obtained for
biomagnification.
Hexachlorobutadiene is moderately to highly toxic to aquatic
organisms; crustaceans and fish are the most sensitive species. The
lowest E(L)C50 for freshwater organisms is 0.09 mg/litre
(goldfish). The lowest chronic NOEC is 3 µg/litre (goldfish).
Applying the preliminary effect assessment extrapolation procedure,
as adopted in the OECD Workshop on Aquatic Effect Assessment (OECD,
1990), an Environmental Concern Level of 0.1 µg/litre can be
established.
The toxicity data on terrestrial organisms are insufficient to
establish any toxicity threshold.
10.2.2 Exposure
Current environmental levels in surface waters are generally
below 0.2 µg/litre, rising to 1.3 µg/litre in highly polluted
rivers. Levels in the upper sediment can be as high as 120 µg/kg in
heavily polluted rivers or estuaries. In older sediment layers much
higher concentrations can be measured. The concentrations in
freshwater biota measured since 1980 generally do not exceed
100 µg/kg fresh weight, but in a polluted area can reach 120 mg/kg
in the lipid of fish.
10.2.3 Hazard evaluation
It can be concluded that away from point sources the maximum
predicted environmental concentration (PEC) is twice the
extrapolated Environmental Concern Level of 0.1 µg/litre. Aquatic
organisms therefore may be at risk in polluted surface waters.
In view of the rather high concentrations of the compound
measured in some sediments, adverse effects on benthic organisms
cannot be excluded.
Considering the toxicity of the substance to mammals (the NOAEL
for rats or mice is 0.2 mg/kg body weight per day) and its high
bioaccumulating potential, the consumption of benthic or aquatic
organisms in polluted surface water by other species may give cause
for concern. For example, an otter weighing 10 kg and consuming 1 kg
fish per day in waters containing 0.2 µg hexachlorobutadiene/litre
could ingest 1200 µg/day (assuming a bioconcentration factor for
fish of 6000, leading to a concentration of 1200 µg/kg wet weight)
or 120 µg/kg body weight per day, which is above the calculated
NOAEL value for the otter (calculated as in section 9.4).
11. FURTHER RESEARCH
Hexachlorobutadiene is primarily a waste product and hence an
environmental contaminant having only limited use as a fumigant in
some parts of the world. The Task Group identified the following
areas for which additional information is needed:
a) the degradation of hexachlorobutadiene in the environment
focusing on photodegradation and biodegradation;
b) the terrestrial toxicity of hexachlorobutadiene including tests
on benthic organisms;
c) the genotoxic activity of hexachlorobutadiene in vivo. A
further test for micronucleus or chromosome aberration
induction in mouse bone marrow cells would strengthen the
available data;
d) the metabolism of hexachlorobutadiene and its glutathione-
derived conjugates by human liver and renal enzymes and
inter-individual variability.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risk of hexachlorobutadiene was evaluated by
the International Agency for Research on Cancer in 1979 (IARC,
1979).
The summary of data reported and the evaluation of the IARC
monograph on hexachlorobutadiene is reproduced here.
Experimental data
Hexachlorobutadiene was tested in one experiment in rats by
oral administration: it produced benign and malignant tumours in the
kidneys of animals of both sexes. It was tested inadequately in one
experiment in mice by intraperitoneal injection.
Human data
No case reports of epidemiological studies were available to
the Working Group.
The occurrence of hexachlorobutadiene as a by-product in the
production of various chlorinated hydrocarbons for over 50 years and
its use in some areas as a pesticide indicate that widespread human
exposure in both the occupational and general environment occurs.
This is confirmed by reports of its occurrence in the environment.
Evaluation
There is limited evidence that hexachlorobutadiene is
carcinogenic in rats.
REFERENCES
Alberti J (1983) [Organic contaminants in river sediment.] Vom
Wasser, 61: 149-154 (in German).
Alberti J & Plöger E (1986) [Hazardous organic chemicals in sewage
sludge.] Gewasserschutz Wasser Abwasser, 85: 483-498 (in German).
Anders MW, Elfaeea AA, & Lash LH (1987) Cellular effects of reactive
intermediates: nephrotoxicity of S-conjugates of amino acids. Arch
Toxicol, 60: 103-108.
Bach PH, Ketley CP, Ahmed I, & Dixit M (1986) The mechanisms of
target cell injury by nephrotoxins. Food Chem Toxicol, 24: 775-779.
Badaeva LN, Ovsyannikova LM, & Kiseleva NI (1985) [Manifestation of
neurotoxic effect of chloroorganic pesticide hexachlorobutadiene
during postnatal period of ontogenesis in rats.] Arch Anat Gistol
Embriol, 89: 44-49 (in Russian).
Baggett JM & Berndt WO (1984) Renal and hepatic glutathione
concentrations in rats after treatment with hexachloro-1-3-butadiene
and citrinin. Arch Toxicol, 56: 46-49.
Bai CL, Canfield PJ, & Stacey NH (1992) Effects of
hexachloro-1,3-butadiene and 1,1,2,2-tetrachloroethylene on
individual serum bile acids. Toxicol Ind Health, 8: 191-203.
Ban M & De Ceaurriz J (1988) Probenecid-induced protection against
acute hexachloro-1,2-butadiene and methyl mercury toxicity to the
mouse kidney. Toxicol Lett, 40: 71-76.
Banerjee S, Yalkowsky SH, & Valvani SC (1980) Water solubility and
octanol/water partition coefficients of organics. Limitations of the
solubility-partition coefficient correlation. Environ Sci Technol,
14: 1227-1229.
Banki K & Anders MW (1989) Inhibition of rat kidney mitochondrial
DNA, RNA and protein synthesis by halogenated cysteine S-conjugates.
Carcinogenesis, 10: 767-772.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - a
multimedia environmental study. Biomed Mass Spectrom, 7: 139-146.
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow
Pinephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berndt WO & Mehendale HM (1979) Effects of hexachlorobutadiene
(HCBD) on renal function and renal organic ion transport in the rat.
Toxicology, 14: 55-65.
Boxenbaum H (1982) Inter-species scaling, allometry, physiologic
time, and the ground plan of pharmacokinetics. J Pharmacokinet
Biopharm, 10: 201-227.
Bristol DW, Crist HL, Lewis RG, MacLeod KE, & Sovocool GW (1982)
Chemical analysis of human blood for assessment of environmental
exposure to semivolatile organochlorine chemical contaminants. J
Anal Toxicol, 6: 269-275.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds. ACS
Symp Ser, 149: 49-64.
Bringmann G & Kühn R (1977) [Limiting values of the harmful action
of water-endangering substances on bacteria (Pseudomonas putida)
and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z Wasser-Abwasser Forsch, 10: 87-98
(in German).
Brown SL, Chan FY, Jones JL, Liu DH, McCaleb KE, Mill T, Sapios KN,
& Schendel DE (1975) Research program on hazard priority ranking of
manufactured chemicals (chemicals 1-20). Menlo Park, California,
Stanford Research Institute (NSF/RA/E-75/190A; PB 263161).
Burkatskaya EN, Viter VF, Ivanova ZV, Kaskevitch LM, Gorskaya NZ,
Kolpakov IE, & Deineka KA (1982) [Clinico-hygienic data on working
conditions during use of hexachlorobutadiene in vineyards.] Vrach
Delo, 11: 99-102 (in Russian).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13: 296-309.
Canton JH, Linders JBHJ, Luttik R, Mensink BJWG, Panman E, Van de
Plassche EJ, Sparenburg PM, & Tuinstra J (1990) Catching-up
manoeuvre old pesticides: an integration. Bilthoven, The
Netherlands, National Institute of Health and Environmental
Protection (Report No. 678801001).
CESARS (198l) Hexachlorobutadiene, latest update June 1981.
Baltimore, Maryland, Chemical Evaluation Search and Retrieval
System, Inc.
Chen JC, Stevens JL, Trifillis AL, & Jones TW (1990) Renal cysteine
conjugate betalyase-mediated toxicity studied with primary cultures
of human proximal tubular cells. Toxicol Appl Pharmacol, 103:
463-473.
Chiou CT (1985) Partition coefficients of organic compounds in
lipid-water systems and correlations with fish bioconcentration
factors. Environ Sci Technol, 19: 57-62.
Chudin VA, Gafieva ZA, Koshurnikova NA, Nifatov AP, & Revina VS
(1985) [Evaluating the mutagenicity and carcinogenicity of
hexachlorobutadiene.] Gig i Sanit, 5: 79-80 (in Russian).
Clark CS, Meyer CR, Gartside PS, Majeti VA, Specker B, Balistreri
WF, & Elia VJ (1982) An environmental health survey of drinking
water contamination by leachate from a pesticide waste dump in
Hardeman County, Tennessee. Arch Environ Health, 37: 9-18.
Clark JR, Devault D, Bowden RJ, & Weishaar JA (1984) Contaminant
analysis of fillets from Great Lakes coho salmon. J Great Lakes Res,
10: 38-47.
Class T & Ballschmiter K (1987) Global baseline pollution studies.
X. Atmospheric halocarbons: global budget estimations for
tetrachloroethene, 1,2-dichloroethane, 1,1,1,2-tetrachloroethane,
hexachloroethane and hexachlorobutadiene. Estimation of the hydroxyl
radical concentrations in the troposphere of the northern and
southern hemisphere. Fresenius Z Anal Chem, 327: 198-225.
Davis ME (1984) Changes of hexachlorobutadiene nephrotoxicity after
piperonyl butoxide treatment. Toxicology, 30: 217-225.
Davis ME (1988) Effects of AT-125 on the nephrotoxicity of
hexachloro-1,2-butadiene in rats. Toxicol Appl Pharmacol, 95: 44-52.
Davis ME, Berndt WO, & Mehendale HM (1980) Disposition
nephrotoxicity of hexachloro-1,3-butadiene. Toxicology, 16: 179-191.
Davis ME, Berndt WO, & Mehendale HM (1986) Effects of cysteine and
diethylmalate pretreatments on renal function and response to a
nephrotoxicant. Arch Toxicol, 59: 7-11.
De Ceaurriz J & Ban M (1990) Role of gamma-glutamyltranspeptidase
and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and
methyl mercury in mice. Toxicol Lett, 50: 249-256.
De Ceaurriz J, Gagnaire F, Ban M, & Bonnet P (1988) Assessment of
the relative hazard involved with airborne irritants with additional
hepatotoxic or nephrotoxic properties in mice. J Appl Toxicol, 8:
417-422.
Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, & Henschler D
(1986) Bacterial beta-lyase mediated cleavage and mutagenicity of
cysteine conjugates derived from the nephrocarcinogenic alkenes
trichloroethylene, tetrachloroethylene and hexachloro-butadiene.
Chem-Biol Interact, 60: 31-45.
Dekant W, Schrenk D, Vamvakas S, & Henschler D (1988a) Metabolism of
hexachloro-1,3-butadiene in mice: in vivo and in vitro evidence
for activation by glutathione conjugation. Xenobiotica, 18: 803-816.
Dekant W, Vamvakas S, Henschler D, & Anders MW (1988b) Enzymatic
conjugation of hexachloro-1,3-butadiene with glutathione. Formation
of 1-(glutathion- S-yl)-1,2,3,4,4-pentachlorobuta-1,3-diene and
1,4-bis(glutathion-S-yl)-1,2,3,4-tetrachloro-1,3-butadiene. Drug
Metab Dispos, 16: 701-706.
Dekant W, Berthold K, Vamvakas S, & Henschler D (1988c)
Thioacylating agents as ultimate intermediates in the beta-lyase
catalysed metabolism of S-(pentachloro-butadienyl)-L-cysteine.
Chem-Biol Interact, 67: 139-148.
Dekant W, Vamvakas S, & Anders MW (1990a) Bioactivation of
hexachlorobutadiene by glutathione conjugation. Food Chem Toxicol,
28: 285-293.
Dekant W, Vamvakas S, Koob M, Kochling A, Kanhai W, Muller D, &
Henschler D (1990b) A mechanism of haloalkene-induced renal
carcinogenesis. Environ Health Perspect, 88: 107-110.
Dekant W, Urban G, Gorsman C, & Anders MW (1991) Thioketene
formation from alpha-haloalkenyl 2-nitrophenyl disulfides: models
for biological reactive intermediates of cytotoxic S-conjugates. J
Am Chem Soc, 113: 5120-5122.
Demarini DM, Inmon JP, Simmons JE, Berman E, Pasley TC, Warren SH, &
Williams RW (1987) Mutagenicity in Salmonella of hazardous wastes
and urine from rats fed these wastes. Mutat Res, 189: 205-216.
De Meester C, Mercier M, & Poncelet F (1981) Mutagenic activity of
butadiene, hexachlorobutadiene, and isoprene. In: Gut I, Cifert M, &
Plaa GL ed. Industrial environmental xenobiotics. Proceedings of an
International Conference, 1980. Berlin, Heidelberg, New York,
Springer Verlag, pp 196-203.
Dillon HK (1979) Development of air sampling and analytical methods
for toxic chlorinated organic compounds: Research report for
hexachlorobutadiene. Birmingham, Alabama, Southern Research
Institute (SORI-EAS-79-658; PB 87-126934/AS).
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Duprat P & Gradiski D (1978) Percutaneous toxicity of
hexachlorobutadiene. Acta Pharmacol Toxicol, 43: 346-353.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur J Toxicol, 9: 171-177.
Durham RW & Oliver BG (1983) History of Lake Ontario contamination
from the Niagara river by sediment radiodating and chlorinated
hydrocarbon analysis. J Great Lakes Res, 9: 160-168.
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with US EPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 29:
460.467.
Fox ME, Carey JH, & Oliver BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the western
basin of Lake Ontario. J Great Lakes Res, 9: 287-294.
Gäb S, Schmitzer J, Thamm HW, Parlar H, & Korte F (1977)
Photomineralization rate of organic compounds adsorbed on
particulate matter. Nature (Lond), 270: 331-333.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gartland KPR, Bonner FW, & Nicholson JK (1989) Investigations into
the biochemical effects of region-specific nephrotoxins. Mol
Pharmacol, 35: 242-250.
Gauntley P, Magadur JL, Morel G, Chaumont P, & Canel F (1975) Dosage
de l'hexachlorobutadiène dans les milieux biologiques. Eur J
Toxicol, 8: 152-158.
German IV (1986) [Level of chromosome aberrations in worker coming
in contact with hexachlorobutadiene during production.] Gig Tr Prof
Zabol, 5: 57-59 (in Russian).
German IV (1988) [On mutagenic activity of the pesticide
hexachlorobutadiene.] Citol Gen, 22(4): 40-42 (in Russian).
German IV & Viter VF (1985) Evaluation of worker's health in Dactal
and hexachlorobutadiene production processes. Hyg Employ Toxicol
Pestic Polym, 15: 32-34.
Gietl YS & Anders MW (1991) Biosynthesis and biliary excretion of
S-conjugates of hexachlorobuta-1,3-diene in the perfused rat liver.
Drug Metab Dispos, 19: 274-277. Gietl YS, Vamvakas S, & Anders MW
(1991) Intestinal absorption of S-(pentachloro-butadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine,
S-conjugates of hexachlorobuta-1,3-diene. Drug Metab Dispos, 19:
703-707.
Giger W & Schaffner C (1981) Groundwater pollution by volatile
organic chemicals. In: Van Duijvenbooden W, Glasbergen P, & Van
Lelyveld H ed. Quality of groundwater. Proceedings of an
International Symposium, Noordwijkerhout, The Netherlands, 23-27
March 1981. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 517-522 (Studies in Environmental Sciences, Volume
17).
Goldbach RW, Van Genderen H, & Leeuwangh P (1976)
Hexachlorobutadiene residues in aquatic fauna from surface water fed
by the river Rhine. Sci Total Environ, 6: 31-40.
Gradiski D, Duprat P, Magadur JL, & Fayein E (1975) Etude
toxicologique expérimentale de l'hexachlorobutadiène. Eur J Toxicol,
8: 180-187.
Green T & Odum J (1985) Structure/activity studies of the
nephrotoxic and mutagenic action of cysteine conjugates of chloro-
and fluoroalkenes. Chem-Biol Interact, 54: 15-31.
Green T, Odum J, Nash JA, & Foster JR (1990)
Perchloroethylene-induced rat kidney tumors: an investigation of the
mechanisms involved and their relevance to humans. Toxicol Appl
Pharmacol, 103: 80-89.
Guzewich DC, Curtis AB, Valis R, Vindpal JH, & Deeter DP (1983) Air
sampling around a hazardous liquid surface impoundment. 76th Annual
Meeting of the Air Pollution Control Association, Atlanta, USA.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Haberer K, Normann S, & Drews M (1988) [Tests on certain trace
substances from the Rhine and Main with a view to obtaining
drinking-water.] Wasser Abwasser, 129: 45-49 (in German).
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harleman JH & Seinen W (1979) Short-term toxicity and reproduction
studies in rats with hexachloro-(1,3)-butadiene. Toxicol Appl
Pharmacol, 47: 1-14.
Haworth S, Lawloer T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hellmann H (1987a) [Model tests on volatilization of organic trace
substances in surface waters.] Fresenius Z Anal Chem, 328: 475-479
(in German).
Hellmann H (1987b) [Investigation of the degree of concentration of
organic trace substances on clay minerals.] Fresenius Z Anal Chem,
327: 524-529 (in German).
Hermens J, Busser F, Leeuwang P, & Musch A (1985) Quantitative
correlation studies between the acute lethal toxicity of 15 organic
halides to the guppy (Poecilia reticulata) and chemical reactivity
towards 4-nitrobenzylpyridine. Toxicol Environ Chem, 9: 219-236.
Hook JB, Rose MS, & Lock EA (1982) The nephrotoxicity of
hexachloro-1,3-butadiene in the rat: studies of organic anion and
cation transport in renal slices and the effect of monoxygenase
inducers. Toxicol Appl Pharmacol, 65: 373-382.
Hook JB, Ishmael J, & Lock EA (1983) Nephrotoxicity of
hexachloro-1,3-butadiene in the rat: the effect of age, sex, and
strain. Toxicol Appl Pharmacol, 67: 122-131.
Hutzinger O (1982) Handbook of environmental chemistry: 3B.
Anthropogenic sources. Berlin, Heidelberg, New York, Springer
Verlag, pp 69-87.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer, pp 179-189 (IARC Monograph on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
20).
Ishmael J & Lock EA (1986) Nephrotoxicity of hexachlorobutadiene and
its glutathione-derived conjugates. Toxicol Pathol, 14: 258-262.
Ishmael J, Pratt I, & Lock EA (1982) Necrosis of the pars recta (S3
segment) of the rat kidney produced by hexachloro-1,3-butadiene. J
Pathol, 138: 99-113.
Ishmael J, Pratt I, & Lock EA (1984) Hexachloro-1,3-butadiene-
induced renal tubular necrosis in the mouse. J Pathol, 142: 195-203.
Jaffe DR, Hassall CD, Brendel K, & Gandolfi AJ (1983) in vivo and
in vitro nephrotoxicity of the cysteine conjugate of
hexachlorobutadiene. J Toxicol Environ Health, 11: 857-867.
Jacobs A, Blangetti M, & Hellmund E (1974) [Accumulation of
chlorinated contaminants of the Rhine in fat tissue of rats.] Vom
Wasser, 43: 259-274 (in German).
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed, 55:
1441-1449.
Jones TW, Gerdes RG, Ormstad K, & Orrenius S (1985) The formation of
both a mono-and a bis-substituted glutathione conjugate of
hexachlorobutadiene by isolated hepatocytes and following in vivo
administration to the rat. Chem-Biol Interact, 56: 251-267.
Jones TW, Wallin A, Thor H, Gerdes RG, Ormstad K, & Orrenius S
(1986a) The mechanism of pentachlorobutadienyl-glutathione
nephrotoxicity studies with isolated rat renal epithelial cells.
Arch Biochem Biophys, 251: 504-513.
Jones TW, Thor H, & Orrenius S (1986b) In vitro studies of
mechanisms of cytotoxicity. Food Chem Toxicol, 24: 769-773.
Jones TW, Quin C, Schaeffer VH, & Stevens JL (1988)
Immunohistochemical localization of glutamine transaminase K, a rat
kidney cysteine conjugate beta-lyase, and the relationship to the
segment specificity of cysteine conjugate nephrotoxicity. Mol
Pharmacol, 34(5): 621-627.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites n Lake Ontario. J Great Lakes Res, 9:
183-189.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soil. Chemosphere,
10: 833-846.
Kastl PE & Hermann BA (1983) Quantitative gas chromatographic
determination of hexachloro-1,3-butadiene in whole rat blood at part
per trillion levels. J Chromatogr, 280(2): 390-393.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St Clair and Detroit rivers using introduced
clams Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci
Health, A21: 15-53.
Kluwe WM, McNish R, Smithson K, & Hook JB (198l) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane,
tris(2,3-dibromopropyl)phosphate, and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and non-target
organs. Biochem Pharmacol, 30: 2265-2271.
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and
in vitro. J Pharmacol Exp Ther, 230: 597-603.
Kociba RJ, Gehring PJ, Humiston CG, & Sparschu GL (1971) Toxicologic
study of female rats administered hexachlorobutadiene or
hexachlorobenzene for thirty days. Midland, Michigan, Dow Chemical
Company, Chemical Biology Research (Unpublished report).
Kociba RJ, Keyes DG, Jersey GC, Ballard JJ, Dittenber DA, Quast JF,
Wade CE, Humiston CG, & Schwetz BA (1977a) Results of a two year
chronic toxicity study with hexachlorobutadiene in rats. Am Ind Hyg
Assoc J, 38: 589-602.
Kociba RJ, Schwetz BA, Keyes DG, Jersey GC, Ballard JJ, Dittenber
DA, Quast JF, Wade CE, & Humiston CG (1977b) Chronic toxicity and
reproduction studies of hexachloro-butadiene in rats. Environ Health
Perspect, 21: 49-53.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part I: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Koob M & Dekant W (1991) Bioactivation of xenobiotics by formation
of toxic glutathione conjugates. Chem-Biol Interact, 77: 107-136.
Kotzias D, Lay JP, Klein W, & Korte F (1975) [Analysis of residues
of hexachloro-butadiene in foodstuffs and poultry.] Chemosphere, 4:
247-250 (in German).
Krasniuk EP, Ziritskaya LA, Bioko VG, Voitenko GA, & Matokhniuk LA
(1969) [Health condition of vine-growers contacting with fumigants
hexachlorobutadiene and polychlorbutan-80.] Vrach Delo, 7: 111-115
(in Russian).
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuo CH, & Hook JB (1983) Effects of age and sex on
hexachloro-1,3-butadiene toxicity in the Fischer 344 rat. Life Sci,
33: 517-523.
Kusz P, Andriysiak A, & Pokorska Z (1984) Gas chromatographic
monitoring of the chlorolysis processes of some byproducts from
vinyl chloride, allyl chloride and epichlorohydrin production. J
Chromatogr, 286: 287-291.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW,
& Evans RL (1976) An ecological study of hexachlorobutadiene (HCBD).
New Orleans, Louisiana, University of New Orleans, Department of
Biological Sciences (Prepared for the US Environmental Protection
Agency, Office of Toxic Substances, Washington (EPA 560/6-76-010; PB
252671).
Lash LH, Nelson RM, Dyke RA, & Anders MW (1990) Purification and
characterization of human kidney cytosolic cysteine conjugate
beta-lyase activity. Drug Metab Dispos, 18: 50-54.
Leeuwangh P, Bult H, & Schneiders L (1975) Toxicity of
hexachlorobutadiene in aquatic organisms. In: Koeman JH & Strik
JJTWA ed. Sublethal effects of toxic chemicals on aquatic animals.
Proceedings of the Swedish-Netherlands Symposium, Waningen, The
Netherlands, 2-5 September 1975. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 167-176.
Li RT, Going JE, & Spigarelli JL (1976) Sampling and analysis of
selected toxic substances: Task I B. Hexachlorobutadiene. Kansas
City, Missouri, Midwest Research Institute (EPA Contract No.
68-01-2646).
Litvinov PI & Gorenshtein RS (1982) [Migration of
hexachlorobutadiene.] Zashch Rast (Moscow), 8: 33-34 (in Russian).
Lock EA (1987a) Metabolic activation of halogenated chemicals and
its relevance to nephrotoxicity. In: Bach PH & Lock EA ed.
Nephrotoxicity in the experimental and clinical situation.
Dordrecht, Martinus Nijhoff Publishers, pp 429-461.
Lock EA (1987b) The nephrotoxicity of haloalkane and haloalkene
glutathione conjugates. In: DeMatteis F & Lock EA ed. Selectivity
and molecular mechanisms of toxicity. New York, McMillan Publishing
Company, pp 59-83.
Lock EA (1988) Studies on the mechanism of nephrotoxicity and
nephrocarcinogenicity of halogenated alkenes. CRC Crit Rev Toxicol,
19: 23-42.
Lock EA & Ishmael J (1979) The acute toxic effects of
hexachloro-l,3-butadiene on the rat kidney. Arch Toxicol, 43: 47-57.
Lock EA & Ishmael J (1981) Hepatic and renal nonprotein sulfhydryl
concentration following toxic doses of hexachloro-1,3-butadiene in
the rat: the effect of Aroclor 1254, phenobarbitone, or SKF 525A
treatment. Toxicol Appl Pharmacol, 57: 79-87.
Lock EA & Ishmael J (1985) Effect of the organic acid transport
inhibitor probenecid on renal cortical uptake and proximal tubular
toxicity of hexachloro-1,3-butadiene and its conjugates. Toxicol
Appl Pharmacol, 81: 32-42.
Lock EA, Ishmael J, & Pratt I (1982) Hydropic change in rat liver
induced by hexachloro-1,3-butadiene. J Appl Toxicol, 2: 315-320.
Lock EA, Ishmael J, & Hook JB (1984) Nephrotoxicity of
hexachloro-1,3-butadiene in the mouse: the effect of age, sex,
strain, monooxygenase modifiers, and the role of glutathione.
Toxicol Appl Pharmacol, 72: 484-494.
Lock EA, Pratt I, & Ishmael J (1985)
Hexachloro-1,3-butadiene-induced hydropic change in mouse liver. J
Appl Toxicol, 5: 74-79.
Lock EA, Odum J, & Ormond P (1986) Transport of
N-acetyl-S-pentachloro-1,3-butadi-ethylcysteine by renal cortex.
Arch Toxicol, 59: 12-15.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55: 881-889.
Lopez-Avila V, Dodhiwala NS, Milanes J, & Beckert W (1989)
Evaluation of EPA method 8120 for determination of chlorinated
hydrocarbons in environmental samples. J Assoc Off Anal Chem, 72:
593-602.
McConnell G, Ferguson DM, & Pearson CR (1975) Chlorinated
hydrocarbon and the environment. Endeavour, 34: 13-18.
McFarlane M, Foster JR, Gibson GG, King LJ, & Lock EA (1989)
Cysteine conjugate beta-lyase of rat kidney cytosol:
characterization, immunocytochemical localization, and correlation
with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol, 98:
185-197.
McLellan LI, Wolf CR, & Hayes JD (1989) Human microsomal glutathione
S-transferase. Its involvement in the conjugation of
hexachlorobuta-1,3-diene with glutathione. Biochem J, 258: 87-93.
Malins DC, Krahn MM, Myers MS, Rhoses LD, Brown DW, Krone CA, McCain
BB, & Chan SI (1985) Toxic chemicals in sediments and biota from a
creasote-polluted harbor: relationships with hepatic neoplasms and
other hepatic lesions in English sole (Parophrys vetulus).
Carcinogenesis, 6: 1463-1469.
Mann JB, Enos HF, Gonzales J, & Thompson JF (1974) Development of
sampling and analytical procedure for determining hexachlorobenzene
and hexachloro-1,3-butadiene in air. Environ Sci Technol, 8:
584-585.
Markovec LM & Magee RJ (1984) Identification of major
perchloroaromatic compounds in waste products from the production of
carbon tetrachloride and tetrachloroethylene. Analyst, 109: 497-501.
Meijers AP (1988) [Organic trace substances in Rhine water and in
filtrate from the banks of the Rhine.] Wasser Abwasser, 129:
208-211.
Melcher RG & Caldecourt VJ (1980) Delayed injection-preconcentration
gas chromatographic technique for parts-per-billion determination of
organic compounds in air and water. Anal Chem, 52: 875-881.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and
other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, & Turton D (1985) Environmental contaminants in
human fat: comparison between accidental and nonaccidental causes of
death. Ecotoxicol Environ Saf, 10: 70-74.
Mes J, Davies DJ, Turton D, & Sun WF (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mumma CE & Lawless EW (1975) Survey of industrial processing data:
Task I. Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processing. Kansas City, Missouri, Midwest Research
Institute (Prepared for the US Environmental Protection Agency,
Office of Toxic Substances, Washington) (EPA 560/3-75-003; PB
243641).
Murzakayev FG (1963) [Data for substantiating maximal permissible
concentrations of hexachlorobutadiene and polychlorobutane in water
basins.] Gig i Sanit, 28: 9-14 (in Russian).
Nash JA, King LJ, Lock EA, & Green T (1984) The metabolism and
disposition of hexachloro-1,3-butadiene in the rat and its relevance
for nephrotoxicity. Toxicol Appl Pharmacol, 73: 124-137.
NIOSH (1979) Manual of analytical methods: Method 308. Cincinnati,
Ohio, National Institute of Occupational Safety and Health, vol 5.
NIOSH (1990) Manual of analytical methods: Method No. P & CAM 307.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
NPHEP (National Institute of Public Health and Environmental
Protection, The Netherlands) (1990) A proposal for guideline
concerning the estimation of environmental concern levels for the
modified EPA method. In: Report of the OECD Workshop on
Ecotoxicological Effects Assessment, Arlington, USA, December 1990.
Paris, Organisation for Economic Co-operation and Development.
Oesch F & Wolf CR (1989) Properties of the microsomal and cytosolic
glutathione transferases involved in hexachloro-1,3-butadiene
conjugation. Biochem Pharmacol, 38: 353-359.
OECD (1990) Report of the OECD Workshop of Ecotoxicological Effect
Assessment, Arlington, USA, December 1990. Paris, Organisation for
Economic co-operation and Development.
Oliver BG (1984) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Biouptake of chlorinated hydrocarbons from
laboratory-spiked and field sediments by oligochaete worms. Environ
Sci Technol, 21: 785-790.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St Clair, and Erie: implications
regarding sources along the St Clair and Detroit Rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Nicol KD (1982) Gas chromatographic determination of
chlorobenzenes and other chlorinated hydrocarbons in environmental
samples using fused silica capillary columns. Chromatographia, 16:
336-340.
Oliver BG & Nicol KD (1984) Chlorinated contaminants in the Niagara
River (198l-1983). Sci Total Environ, 34: 57-70.
Oliver BG & Niimi AJ (1983) Bioconcentration of chlorobenzenes from
water by Rainbow trout: correlations with partition coefficients and
environmental residues. Environ Sci Technol, 17: 287-291.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of
polychlorinated biphenyl congeners and other chlorinated
hydrocarbons in the Lake Ontario ecosystems. Environ Sci Technol,
22: 388-397.
Otson R & Chan C (1987) Sample handling and analysis for 51 volatile
organics by an adapted urge and trap GC-MS technique. Int J Environ
Anal Chem, 30: 275-287.
Payan JP, Fabry JP, Beydon D, & De Ceaurriz J (1991) Biliary
excretion of hexachloro-1,3-butadiene and its relevance to tissue
uptake and renal excretion in male rats. J Appl Toxicol, 11:
437-442.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16: 781-785.
Pereira WE, Rostad CE, Chiou CT, Brinton TI, Barber LB, Demcheck DK,
& Demas CR (1988) Contamination of estuarine water, biota, and
sediment by halogenated organic compounds: a field study. Environ
Sci Technol, 22: 772-778.
Petersen VP (1986) [Analysis of moderately volatile organochlorine
compounds and their concentration distribution in different streams
of the Federal Republic of Germany.] Dtsch Gewässerkd Mitt, 30:
10-15 (in German).
Poteryayeva GE (1966) [The effect of hexachlorobutadiene on
offspring of albino rats.] Gig i Sanit, 31: 33-36 (in Russian).
Pratt IS & Lock EA (1988) Deacylation and further metabolism of the
mercapturic acid of hexachloro-1,3-butadiene by rat kidney cytosol
in vitro. Arch Toxicol, 62: 341-345.
Rapson W, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24: 590-596.
Reichert D (1983) Metabolism and disposition of
hexachloro(1,3)butadiene in rats. Dev Toxicol Environ Sci, 5:
411-414.
Reichert D & Schütz S (1986) Mercapturic acid formation is an
activation and intermediary step in metabolism of
hexachlorobutadiene. Biochem Pharmacol, 35: 1271-1275.
Reichert D, Neudecker T, Spengler U, & Henschler D (1983)
Mutagenicity of dichloroacetylene, trichloroacryloyl chloride and
hexachlorobutadiene. Mutat Res, 117: 21-29.
Reichert D, Neudecker T, & Schütz S (1984) Mutagenicity of
hexachlorobutadiene, perchlorobutenoic acid and perchlorobutenoic
acid chloride. Mutat Res, 137: 89-93.
Reichert D, Schütz S, & Metzler M (1985) Excretion pattern and
metabolism of hexachlorobutadiene in rats. Evidence for metabolic
activation by conjugation reactions. Biochem Pharmacol, 34: 499-505.
Reimschüssel R, Bennet RO, May EB, & Lipsky MM (1989) Renal
histopathological changes in the goldfish (Carassius auratus)
after sublethal exposure to hexachlorobutadiene. Aquat Toxicol, 15:
169-180.
Reimschüssel R, Bennett RO, May EB, & Lipsky MM (1990) Development
of newly formed nephrons in the goldfish kidney following
hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol, 18:
32-38.
Roedig A & Bernemann P (1956) [Data on the highly chlorinated
vinylether 1-ethoxy-pentachloro-(1,3)-butadiene from
perchlorobutadiene.] Justus Liebigs Ann Chem, 600: 1-11 (in German).
Rush GF, Smith JH, Newton JF, & Hook JB (1984) Chemically induced
nephrotoxicity: role of metabolic activation. CRC Crit Rev Toxicol,
13: 99-156.
Ruth JH (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A142-A151.
Saillenfait AM, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Inhalation teratology study on hexachloro-1,3-butadiene in rats.
Toxicol Lett, 47: 235-240.
Sauer T Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Schiffmann D, Reichert D, & Henschler D (1984) Induction of
morphological transformation and unscheduled DNA synthesis in Syrian
hamster embryo fibroblasts by hexachlorobutadiene and its putative
metabolite pentachlorobutenoic acid. Cancer Lett, 23: 297-305.
Schnellmann RG, Lock EA, & Mandel LJ (1987) A mechanism of
S-(1,2,3,4,4-pentachloro-1,3-butadienyl)L-cysteine toxicity to
rabbit renal proximal tubules. Toxicol Appl Pharmacol, 90: 513-521.
Schrenk D & Dekant W (1989) Covalent binding of hexachlorobutadiene
metabolites to renal and hepatic mitochondrial DNA. Carcinogenesis,
10: 1139-1141.
Schrenk D, Dekant W, Wunsch PH, & Henschler D (1988a) Role of
metabolic activation in the toxicity of S-(pentachlorobutadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine in the
isolated perfused rat kidney. Toxicol in Vitro, 2: 283-290.
Schrenk D, Dekant W, & Henschler D (1988b) Metabolism and excretion
of S-conjugates derived from hexachlorobutadiene in the isolated
perfused rat kidney. Mol Pharmacol, 34: 407-412.
Schröder HF (1987) Chlorinated hydrocarbons in biological sewage
purification - Fate and difficulties in balancing. Water Sci
Technol, 19: 429-438.
Schwetz BA, Norris JM, Kociba RJ, Keeler PA, Cornier RF, & Gehring
PJ (1974) Reproduction study in Japanese quail fed
hexachlorobutadiene for 90 days. Toxicol Appl Pharmacol, 30:
255-265.
Schwetz BA, Smith FA, Humiston CG, Quast JF, & Kociba RJ (1977)
Results of a reproduction study in rats fed diets containing
hexachlorobutadiene. Toxicol Appl Pharmacol, 42: 387-398.
Shen TT (1982) Air quality assessment for land disposal of
industrial wastes. Environ Manage, 6: 297-305.
Singh HB, Salas LJ, & Stiles R (1982) Distribution of selected
gaseous organic mutagens and suspected carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
SRI (1984) Directory of chemical producers, Western Europe. Menlo
Park, California, Stanford Research Institute.
Stonard MD, Gore CW, Oliver GJA, & Smith IK (1987) Urinary enzymes
and protein pattern as indicators of injury to different regions of
the kidney. Fundam Appl Toxicol, 9: 339-351.
Stott WT, Quast JF, & Watanabe PG (1981) Differentiation of the
mechanisms of oncogenicity of 1,4-dioxane and
1,3-hexachlorobutadiene in the rat. Toxicol Appl Pharmacol, 60:
287-300.
Tabak HH, Quave SA, Mashni CE, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1517.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res, 37:
2717-2720.
Ullmann F (1986) Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH-Verlagsgesellschaft, vol A6, pp 322-323, 375, 391,
398.
US EPA (1980) Ambient water quality criteria for
hexachlorobutadiene. Washington, DC, US Environmental Protection
Agency, Criteria and Standards Division (EPA-440/5-80-053;
PB-81-117640).
UP EPA (1984a) A method for determination of certain chlorinated
hydrocarbons. EPA method No. 612. Fed Reg, 491(209): 29-210.
US EPA (1984b) A gas chromatographic method for determination of
certain chlorinated hydrocarbons. EPA method No. 612. Fed Reg,
491(209): 29-210.
Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, & Henschler D
(1987) Enzymatic transformation of mercapturic acids derived from
halogenated alkenes to reactive and mutagenic intermediates. Biochem
Pharmacol, 36: 2741-2748.
Vamvakas S, Kordowich FJ, Dekant W, Neudecker T, & Henschler D
(1988a) Mutagenicity of hexachloro-1,3-butadiene and its
S-conjugates in the Ames test - role of activation by the
mercapturic acid pathway in its nephrocarcinogenicity.
Carcinogenesis, 9: 907-910.
Vamvakas S, Muller DA, Dekant W, & Henschler D (1988b) DNA binding
of sulfur containing metabolites from 35S-(pentachlorobutadienyl)-
L-cysteine in bacteria and isolated renal tubular cells. Drug Metab
Drug Interact, 6: 349-358.
Vamvakas S, Dekant W, & Henschler D (1989a) Genotoxicity of
haloalkene and haloalkane glutathione S-conjugates in porcine kidney
cells. Toxicol in Vitro, 3: 151-156.
Vamvakas S, Kochling A, Berthold K, & Dekant W (1989b)
Cytotoxicity of cysteine S-conjugates: structure-activity
relationships. Chem-Biol Interact, 71: 79-90.
Vamvakas S, Dekant W, & Henschler D (1993) Nephrocarcinogenicity of
haloalkenes and alkynes. In: Renal disposition and nephrotoxicity of
xenobiotics. New York, Academic Press, pp 323-342.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchlonne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63:
1433-1439.
Vorobyeva TN (1980) [Enpared residues in grapes.] Khim Sel'sk Khoz,
18(10): 41-42 (in Russian).
Wallin A, Jones TW, Vercesi AE, Cotgreave I, Ormstad K, & Orrenius S
(1987) Toxicity of S-pentachlorobutadienyl-L-cysteine studied with
isolated rat renal cortical mitochondria. Arch Biochem Biophys, 258:
365-372.
Wallin A, Gerdes RG, Morgenstern R, Jones TW, & Ormstad K (1988)
Features of microsomal and cytosolic glutathione conjugation of
hexachlorobutadiene in rat liver. Chem-Biol Interact, 68: 1-11.
Wild D, Schütz S, & Reichert D (1986) Mutagenicity of the
mercapturic acid and other S-containing derivatives of
hexachloro-1,3-butadiene. Carcinogenesis, 7: 431-434.
Wolf CR, Berry PN, Nash JA, Green T, & Lock EA (1984) Role of
microsomal and cytosolic glutathione S-transferases in the
conjugation of hexachloro-1,3-butadiene and its possible relevance
to toxicity. J Pharmacol Exp Toxicol, 228: 202-208.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen, 7:
677-702.
Yang RSH (1988) Hexachloro-1,3-butadiene: toxicology, metabolism,
and mechanisms of toxicity. Rev Environ Contam Toxicol, 101:
121-137.
Yang RSH (1991) Toxicity studies of hexachloro-1,3-butadiene in
B6C3F1 mice (feed studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP TOX 1) (NIH Publication
No. 91-3120).
Yang RSH, Abdo KM, Elwell MR, Levy AC, & Brennecke LH (1989)
Subchronic toxicology studies of hexachloro-1,3-butadiene (HCBD) in
B6C3F1 mice by dietary incorporation. J Environ Pathol Toxicol
Oncol, 9: 323-332.
Yasuhara A & Morita M (1990) Formation of chlorinated compounds in
pyrolysis of trichloroethylene. Chemosphere, 21: 479-486.
Yip G (1976) Survey for hexachloro-1,3-butadiene in fish, eggs,
milk, and vegetables. J Assoc Anal Chem, 59: 559-561.
Yurawecz MP, Dreifuss PA, & Kamps LR (1976) Determination of
hexachloro-1,3-butadiene in spinach, eggs, fish, and milk by
electron capture gas-liquid chromatography. J Assoc Anal Chem, 59:
552-558.
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of The
Netherlands. Chemosphere, 9: 231-249.
Zogorski JS (1984) Experience in monitoring domestic water sources
and process waters for trace organics. J Environ Sci Health, A19:
233-249.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
L'hexachlorobutadiène est un liquide ininflammable,
incombustible, limpide et huileux à la température et la pression
ordinaires. Il est peu soluble dans l'eau mais miscible à l'éther et
à l'éthanol.
On peut le mettre en évidence et le doser par chromatographie
en phase gazeuse. Les limites de détection sont de 0,03 µg/m3 dans
l'air, 0,001 µg/litre dans l'eau, de 0,7 µg/kg de matière humide
dans le sol ou les sédiments et de 0,02 µg/litre dans le sang. Dans
les tissus, cette limite est de 0,47 µg/kg de tissus frais.
2. Sources d'exposition humaine et environnementale
L'hexachlorobutadiène n'existe pas à l'état naturel. C'est
essentiellement un sous-produit de la fabrication des hydro-carbures
chlorés que l'on retrouve dans les fractions lourdes. La production
annuelle mondiale (dans les fractions lourdes) a été estimée à
10 000 tonnes en 1982. L'hexachlorobutadiène peut être utilisé pour
la récupération des gaz contenant du chlore dans les ateliers de
fabrication du chlore et comme liquide de lavage pour éliminer du
courant gazeux certains composés organiques volatils. On l'utilise
également dans les gyroscopes, comme fluide calo-porteur, dans les
transformateurs, comme liquide isolant ou liquide hydraulique ainsi
que comme solvant des élastomères, comme intermédiaire et comme
fumigant.
3. Transport, distribution et transformation dans l'environnement
Les principales voies de pénétration dans l'environnement sont
les émissions résultant des déchets et les utilisations qui
entraînent la dispersion du produit. Le transport
inter-compartimental s'effectue principalement par volatilisation,
adsorption sur les matières particulaires puis dépôt ou
sédimentation. L'hexachloro-butadiène ne migre pas facilement dans
le sol et s'accumule dans les sédiments. Dans l'eau, on le considère
comme persistant, sauf turbulences importantes. Il n'y a pas
d'hydrolyse. Le produit semble être facilement biodégradable par
voie aérobie, encore que le phénomène n'ait pas été étudié à fond.
L'hexachlorobutadiène présent sur les surfaces subit une photolyse.
Outre le dépôt, on estime que la réaction de l'hexachlorobutadiène
avec les radicaux hydroxyles constitue un mode de piégeage important
de ce composé dans la troposphère, la demi-vie atmosphérique
estimative de l'hexachlorobutadiène pouvant aller jusqu'à 2,3 ans.
Le produit a un potentiel élevé de bioaccumulation, comme l'ont
confirmé les observations en laboratoire et sur le terrain. Ainsi,
on a trouvé des facteurs de bioconcentration à l'état stationnaire
(obtenus expérimentalement par rapport au poids de tissus frais)
respectivement égaux en moyenne à 5800 et 17 000 chez la truite
arc-en-ciel. On n'a pas observé d'amplification biologique au
laboratoire ou sur le terrain.
4. Niveaux dans l'environnement et exposition humaine
Le dosage de l'hexachlorobutadiène dans l'air des villes a
donné dans tous les cas des valeurs inférieures à 0,5 µg/m3. Dans
les régions écartées, les concentrations sont inférieures à
1 pg/m3. Dans les lacs et les cours d'eau d'Europe, on a
enregistré des concentrations pouvant aller jusqu'à 2 µg/litre mais
les valeurs moyennes sont généralement inférieures à 100 ng/litre.
Dans la région des grands lacs au Canada, on a obtenu des valeurs
beaucoup plus faibles (autour de 1 ng/litre). En revanche la teneur
des sédiments du fond peut, dans cette zone, atteindre 120 µg/kg de
poids sec. Les couches sédimentaires plus anciennes, remontant aux
environs de 1960, présentaient des teneurs plus élevées (jusqu'à
550 µg/kg de matière humide). On a montré que la concentration dans
les sédiments augmentait avec la granulométrie des particules.
A en juger par la concentration de l'hexachlorobutadiène dans
les organismes aquatiques, les oiseaux et les mammifères, le composé
s'accumule mais ne subit pas d'amplification biologique. Dans les
eaux polluées, on a relevé des concentrations dépassant 1000 µg/kg
de tissus frais chez plusieurs espèces et même 120 mg/kg (par
rapport aux lipides) chez une espèce. Les concentrations actuelles
restent généralement inférieures à 1000 µg/kg de poids frais à
distance des points de décharge industrielle.
On a décelé la présence du composé dans l'urine, le sang et les
tissus humains. Dans certaines denrées alimentaires ayant une
fraction lipidique importante, on en a relevé jusqu'à 40 µg/kg et
dans un cas, plus de 1000 µg/kg.
D'après une étude, le niveau d'exposition pourrait atteindre
1,6 à 12,2 mg/m3 et les concentrations urinaires, 20 mg/litre.
5. Cinétique et métabolisme
Après administration par voie orale, l'hexachlorobutadiène est
rapidement absorbé chez l'animal de laboratoire mais le taux de
résorption après inhalation ou exposition par voie cutanée n'a pas
été étudié. Chez le rat et la souris, le composé se répartit
principalement dans le foie, les reins et les tissus adipeux. Il est
rapidement excrété. On a mis en évidence une fixation aux protéines
et aux acides nucléiques dans le foie et les reins.
La biotransformation du composé chez l'animal de laboratoire se
révèle être un processus saturable. Elle s'effectue principalement
par l'intermédiaire du glutathion, l'hexachlorobutadiène étant
d'abord transformé en conjugué du S-glutathion. La métabolisation
de ce conjugué se poursuit ensuite, en particulier au niveau de la
membrane constituant la bordure en brosse des cellules des tubules
rénaux, pour donner un métabolite sulfuré réactif qui est
probablement responsable des effets néphrotoxiques, génotoxiques et
cancérogènes observés.
6. Effets sur les êtres vivants dans leur milieu naturel
L'hexachlorobutadiène est modérément à très toxique pour les
organismes aquatiques. Certaines espèces de poissons et de crustacés
se sont révélées être les plus sensibles, les valeurs de la CL50 à
96 h. allant de 0,032 à 1,2 et de 0,09 à environ 1,7 mg/litre,
respectivement pour les crustacés et les poissons. Chez les
poissons, le rein est organe-cible important.
On a établi la valeur de la dose sans effets observables à
0,003 mg/litre, à partir des résultats d'un certain nombre
d'épreuves à long terme sur certaines espèces d'algues et de
poissons; cela permet de considérer ce composé comme très toxique
pour les organismes aquatiques. Parmi les points d'aboutissement
biologiques étudiés figuraient la toxicité générale, la
neurotoxicité, la biochimie, l'hématologie, l'anatomopathologie et
la reproduction. Lors d'une étude de 28 jours portant sur les
premiers stades de la vie de Pimephales promelas, une espèce de
vairon, on a observé que la reproduction n'était pas affectée à des
concentrations allant jusqu'à 0,017 mg/litre, alors qu'à 0,013 et
0,017 mg/litre il y avait accroissement de la mortalité et réduction
du poids du corps. La dose sans effets observables était de
0,0065 mg/litre.
On n'a décrit qu'une seule épreuve fiable portant sur des
organismes terrestres. Lors d'une épreuve de 90 jours sur des
cailles japonaises qui recevaient une alimentation contenant ce
composé à des concentrations allant de 0,3 à 30 mg/kg de nourriture,
on a constaté que la survie des oisillons n'était réduite qu'à
partir de 10 mg/kg de nourriture.
7. Effets sur les animaux de laboratoire et les systèmes
d'épreuves in vitro
7.1 Toxicité générale
L'hexachlorobutadiène est légèrement à modérément toxique pour
le rat adulte, modérément toxique pour le raton juste sevré et
extrêmement toxique pour les rattes juste sevrées après
administration d'une seule dose par voie buccale. Les principaux
organes-cibles sont le rein et dans une bien moindre mesure, le
foie.
D'après les données obtenues sur l'animal d'expérience, les
vapeurs d'hexachlorobutadiène sont irritantes pour les muqueuses et
le liquide est corrosif. On peut considérer ce composé comme un
agent sensibilisateur.
Chez le rat, la souris et le lapin, l'hexachlorobutadiène
provoque une nécrose, liée à la dose, des tubules proximaux du rein.
Les rats mâles adultes sont moins sensibles à la néphrotoxicité que
les femelles ou les jeunes mâles. Les souriceaux sont plus sensibles
que les souris adultes sans qu'on puisse observer de différences
entre les deux sexes. Chez la ratte adulte, la dose intrapéritonéale
unique la plus faible à laquelle on ait observé une nécrose rénale
était de 25 mg/kg de poids corporel; elle était de 6,3 mg/kg de
poids corporel chez les souris adultes mâles et femelles. A des
doses égales ou supérieures à celles qui entraînaient une nécrose,
on a observé des modifications biochimiques et une nette
amélioration de la fonction rénale.
Lors de six épreuves à court terme où le composé a été
administré par la voie orale, deux études de reproduction et une
étude d'alimentation à long terme portant sur des rats, c'est
également le rein qui s'est révélé être l'organe-cible. Parmi les
effets liés à la dose, on notait une diminution du poids relatif des
reins et une dégénérescence de l'épithélium des tubules. La dose
sans effets nocifs observables au niveau des reins, tirée d'une
étude de deux ans sur le rat, était de 0,2 mg/kg de poids corporel
et par jour. Une étude de 13 semaines sur des souris a montré que
cette dose était de 0,2 mg/kg de poids corporel et par jour pour cet
animal. Chez les deux espèces, les femelles adultes étaient plus
sensibles que les mâles adultes.
Lors d'une étude d'inhalation à court terme (six heures par
jour pendant 12 jours) on a observé des effets analogues au niveau
des reins avec une concentration nominale de vapeur
d'hexa-chlorobutadiène égale à 267 mg/m3; cette concentration a
également entraîné des difficultés respiratoires, ainsi qu'une
dégénérescence des corticosurrénales.
7.2 Reproduction, embryotoxicité et teratogétogénicité
Deux études d'alimentation portant sur la reproduction ont été
effectuée sur des rats à des doses quotidiennes allant jusqu'à 20 et
75 mg/kg de poids corporel respectivement; elles ont fait ressortir
une réduction du poids de naissance et du gain de poids néonatal aux
doses toxiques pour la mère. La dose quotidienne de 75 mg/kg de
poids corporel, qui était hautement toxique, s'est révélée
suffisante pour empêcher la conception et la nidation intra-utérine.
On n'a pas observé d'anomalies du squelette.
Lors de deux études de tératogénicité, des rats ont été exposés
soit à des vapeurs d'hexachlorobutadiène à des concentrations allant
de 21 à 160 mg/m3, six heures par jour du sixième au vingtième
jour de la gestation, soit par voie intrapéritonéale à une dose
quotidienne de 10 mg/kg de poids corporel (du premier au quinzième
jour de la gestation). Des effets nocifs ont été notés sur le
développement des foetus, qui consistaient en une réduction du poids
de naissance, un retard dans le développement cardiaque, une
dilatation des uretères, mais pas de malformations macroscopiques.
Le retard de développement a été observé à des doses qui étaient
également toxiques pour les mères.
7.3 Génotoxicité et cancérogénicité
L'hexachlorobutadiène provoque des mutations géniques dans
l'épreuve d'Ames sur salmonelle dans des conditions particulières
qui favorisent la formation de produits de conjugaison avec le
glutathion. Lors d'une étude in vivo on a observé des aberrations
chromosomiques qui n'ont en revanche pas été constatées lors de deux
autres études in vitro. Une étude in vitro portant sur des
cellules ovariennes de hamster chinois a révélé une augmentation de
la fréquence des échanges entre chromatides soeurs. On a fait état
de la très forte mutagénicité des métabolites sulfurés de
l'hexachlorobutadiène. D'autres études in vitro ont montré que ce
composé provoquait une synthèse non programmée de l'ADN dans des
cultures de fibroblastes embryonnaires de hamsters de Syrie, effets
qui n'étaient pas observés dans des cultures d'hépatocytes de rats.
Le composé provoque également une synthèse non programmée de l'ADN
in vivo mais n'induit pas de mutations létales récessives liées au
sexe chez Drosophila melanogaster.
Lors de la seule étude à long terme (deux ans) qui ait été
effectuée, des rats ont reçu une alimentation contenant de
l'hexachlorobutadiène à des doses quotidiennes respectives de 0,2, 2
ou 20 mg/kg de poids corporel et seule la dose la plus élevée a
provoqué un accroissement de l'incidence des tumeurs malignes au
niveau des tubules rénaux.
7.4 Mécanismes de la toxicité
La néphrotoxicité, la mutagénicité et la cancérogénicité de
l'hexachlorobutadiène sont liées à la biosynthèse d'un conjugué
sulfuré toxique, le 1-glutathion- S-yl-1,2,3,4,4-pentachloro-
butadiène. Ce conjugué est principalement synthétisé dans le foie et
métabolisé ensuite dans la bile, l'intestin et les reins en
1-cystéine- S-yl-1,2,3,4,4-pentachlorobutadiène (CPB). L'activation
du CPB en thiocétène réactif (qui dépend de la cystéine-conjuguée-
béta lyase) au niveau des cellules des tubules proximaux, aboutit en
définitive à la formation de liaisons covalentes avec les
macromolécules cellulaires.
8. Effets sur l'homme
On n'a pas décrit d'effets pathogènes sur la population dans
son ensemble.
On possède deux rapports faisant état de troubles chez des
ouvriers agricoles qui utilisaient de l'hexachlorobutadiène comme
fumigant mais il est vrai qu'ils avaient également été exposés à
d'autres substances. On a également observé un accroissement de la
fréquence des aberrations chromosomiques dans les lymphocytes du
sang périphériques de travailleurs employés à la production
d'hexachlorobutadiène et qui avaient été exposés à des
concentrations de 1,6 à 12,2 mg/m3.
9. Evaluation des risques pour la santé humaine et des
effets sur l'environnement
9.1 Evaluation des risques pour la santé humaine
Comme très peu d'études ont été effectuées sur l'homme,
l'évaluation repose essentiellement sur les animaux de laboratoire.
Toutefois les données in vitro limitées dont on dispose au sujet
de l'homme incitent à penser que le métabolisme de
l'hexachloro-butadiène est analogue chez l'homme et l'animal.
On estime que les vapeurs d'hexachlorobutadiène sont irritantes
pour les muqueuses et que le liquide est corrosif. Ce composé doit
également être considéré comme un agent sensibilisateur.
Les principaux organes-cibles de son action toxique sont les
reins et dans une mesure bien moindre, le foie. Sur la base des
études à court et à long terme effectuées sur des rats et des
souris, la dose quotidienne sans effets nocifs observables est
évaluée à 0,2 mg/kg de poids corporel. On l'a estimée à 53 mg/m3
lors d'une étude d'inhalation à court terme chez le rat (12 jours,
six heures par jour).
L'action toxique sur le développement, de même que la réduction
du poids de naissance et du gain de poids néonatal n'ont été
observés qu'à des doses toxiques pour la mère.
On a observé que l'hexachlorobutadiène produisait des mutations
géniques, des aberrations chromosomiques, un accroissement des
échanges entre chromatides soeurs et une synthèse non programmée de
l'ADN, encore que certaines études aient donné des résultats
négatifs. On ne possède que des indices limités en faveur d'une
génotoxicité de l'hexachlorobutadiène chez l'animal, indices qui
sont insuffisants en ce qui concerne l'homme.
On a constaté que l'administration d'hexachlorobutadiène par
voie orale pendant une longue période à des rats accroissait la
fréquence des tumeurs malignes au niveau des tubules rénaux, mais il
s'agissait uniquement de doses élevées fortement néphrotoxiques. En
ce qui concerne la cancérogénicité de cette substance, les indices
sont limités chez l'animal et insuffisants chez l'homme.
En se basant sur la dose quotidienne sans effets nocifs
observables estimée à 0,2 mg/kg de poids corporel chez la souris ou
le rat, on a fixé à 0,03-0,05 mg/kg de poids corporel la dose
quotidienne sans effets nocifs observables chez l'homme. La marge de
sécurité entre la dose estimative sans effets nocifs observables et
la dose journalière maximale totale ingérée estimée en se basant sur
une absorption du composé par l'intermédiaire d'une eau de boisson
et de produits alimentaires contaminés à forte teneur en lipides,
est égale à 150.
9.2 Evaluation des effets sur l'environnement
L'hexachlorobutadiène est modérément à fortement toxique pour
les organismes aquatiques: les crustacés et les poissons sont les
espèces les plus sensibles. On a fixé à 0,1 g/litre la
concentration écologiquement préoccupante. On estime que la
concentration maximale prévisible dans l'environnement à distance
des sources ponctuelles de pollution est égale à deux fois la dose
écologique-ment préoccupante extrapolée et, par voie de conséquence,
que les organismes aquatiques peuvent être menacés dans les eaux de
surface polluées. On ne peut exclure des effets nocifs sur le
benthos.
Compte tenu de la toxicité de l'hexachlorobutadiène pour les
mammifères, la consommation de benthos ou d'organismes aquatiques
par d'autres espèces pourrait être préoccupante.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos de análisis
El hexaclorobutadieno es un líquido no inflamable,
incombus-tible, claro, oleoso e incoloro a temperatura y presión
ordinarias. Es poco soluble en el agua, pero miscible con éter y
etanol.
La sustancia puede detectarse y determinarse cuantitativamente
por métodos de cromatografía de gases. Los límites de detección son
de 0,03 µg/m3 de aire, 0,001 µg/litro de agua, 0,7 µg/kg de peso
húmedo en el suelo o en sedimentos y de 0,02 µg/litro de sangre. Se
ha determinado un nivel de 0,47 µg/kg de peso húmedo de tejido.
2. Fuentes de exposición humana y ambiental
No hay indicaciones de que el hexaclorobutadieno exista como
producto natural. Es principalmente un subproducto de la fabricación
de hidrocarburos clorados y se presenta en las fracciones pesadas
(como residuo). La producción anual mundial del compuesto en las
fracciones pesadas en 1982 se estimó en 10 000 toneladas.
El hexaclorobutadieno puede utilizarse para recuperar gas que
contiene cloro en plantas productoras de cloro y como líquido de
lavado para eliminar ciertos compuestos orgánicos volátiles de las
corrientes de gases. También se ha utilizado como fluido en
giróscopos, como transmisor de calor, transformador, fluido aislante
y fluido hidráulico, disolvente para elastómeros y como
intermediario y sustancia para fumigar.
3. Transporte, distribución y transformación en el medio ambiente
Las principales vías de ingreso en el medio ambiente son las
emisiones de residuos y el uso dispersivo. El paso de un entorno a
otro ocurre principalmente por volatilización, adsorción a
corpúsculos de materia y subsiguiente deposición o sedimentación. El
hexaclorabutadieno no migra rápidamente en el suelo y se acumula en
el sedimento. Se considera persistente en el agua a menos que haya
mucha turbulencia. No produce hidrólisis. La sustancia parece ser
fácilmente biodegradable aeróbicamente, aunque su biodegradabilidad
no se ha investigado a fondo. El hexaclorobutadieno se fotoliza en
las superficies. Se supone que, además de la deposición, la reacción
con radicales hidroxilo es un importante sumidero de
hexaclorobutadieno en la troposfera y su semivida atmosférica
estimada es de hasta 2,3 años. La sustancia tiene un elevado
potencial de bioacumulación, que se ha comprobado mediante
observaciones en laboratorio y sobre el terreno. En la trucha arco
iris se han determinado experimentalmente factores de
bioconcentración en estado estacionario de 5800 y 17 000 como
promedio, sobre la base del peso húmedo. No se ha observado
biomagnificación en laboratorio ni sobre el terreno.
4. Niveles ambientales y exposición humana
Se ha determinado la presencia de hexaclorubutadieno en el aire
urbano; en todos los casos, los niveles eran inferiores a
0,5 µg/m3. Las concentraciones en lugares aislados son inferiores
a 7 pg/m3. En las aguas de lagos y ríos de Europa se han
registrado concentraciones de hasta 2 µg/litro, pero los niveles
medios son generalmente inferiores a 100 ng/litro. En la región de
los Grandes Lagos del Canadá se han detectado niveles muy inferiores
(de aproximadamente 1 ng/litro). Allí los niveles en el sedimento
del fondo pueden ser de 120 µg/kg de peso en seco. En capas más
antiguas de sedimento, de 1960 aproximadamente, se encontraron
concentraciones más elevadas (de hasta 550 µg/kg de peso húmedo). Se
ha demostrado que la concentración en el sedimento aumenta con el
tamaño de la partícula de sedimento.
Las concentraciones de hexaclorobutadieno en organismos
acuáticos, aves y mamíferos indican bioacumulación pero no
biomagnificación. En las aguas contaminadas se han detectado niveles
de más de 1000 µg/kg de peso húmedo en varias especies y de
120 mg/kg (base grasa) en una especie. Lejos de los efluentes
industriales, los niveles actuales se mantienen en general por
debajo de 100 µg/kg de peso húmedo.
Se ha detectado la presencia del compuesto en la orina, en la
sangre y en tejidos humanos. En ciertos alimentos que contienen una
elevada fracción lipídica se han encontrado hasta unos 40 µg/kg y,
en un caso, más de 1000 µg/kg.
Un estudio señala exposiciones ocupacionales de
1,6-12,2 mg/m3 y en la orina niveles de hasta 20 mg/litro.
5. Cinética y metabolismo
Se ha observado que los animales de laboratorio absorben
rápidamente el hexaclorobutadieno después de la administración oral,
pero no se ha investigado la velocidad de absorción después de la
inhalación o de la exposición dérmica. En ratas y ratones, el
compuesto se distribuye principalmente al hígado, a los riñones y al
tejido adiposo. Se excreta rápidamente. Se ha demostrado que se fija
a las proteínas y ácidos nucleicos del hígado y de los riñones.
La biotransformación del compuesto en animales de
experimentación parece ser un proceso saturable. Se produce
principalmente a través de una vía mediada por el glutatión, en la
cual el hexaclorobutadieno se convierte inicialmente en conjugados
de S-glutatión. Estos conjugados pueden seguir metabolizándose,
especialmente en el ribete en cepillo de las membranas de las
células de los tubos renales, produciendo un metabolito sulfuroso
reactivo que probablemente explique la nefrotoxicidad, genotoxicidad
y carcinogenicidad observadas.
6. Efectos en organismos presentes en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos. Los más sensibles que se hayan observado han
sido especies de peces y crustáceos; los valores de la CL50 en
96 horas oscilan entre 0,032 y 1,2 mg/litro en crustáceos y entre
0,09 y 1,7 mg/litro en peces. Se ha demostrado que el riñón es un
órgano muy afectado en los peces.
Sobre la base de varias pruebas a largo plazo con especies de
algas y de peces, se ha establecido un nivel sin efectos observados
de 0,003 mg/litro; así pues, el compuesto se clasifica como muy
tóxico para las especies acuáticas. Los valores extremos
investigados comprenden parámetros de toxicidad general,
neurotoxicidad, bioquímicos, hematológicos, patológicos y
relacionados con la reproducción. En una prueba de 28 días de
duración en la que se examinaron las primeras fases de la vida de
carpas se observó que la reproducción no se veía afectada con
concentraciones de hasta 0,017 mg/litro, mientras que con
concentraciones de 0,013 y 0,017 mg/litro se observaron un aumento
de la mortalidad y una disminución del peso corporal. El nivel sin
efectos observados era de 0,0065 mg por litro.
Se ha descrito una sola prueba fiable con organismos
terrestres. En una prueba de 90 días con codornices japonesas
alimentadas con una dieta que contenía el compuesto en
concentraciones de 0,3 a 30 mg/kg de dieta se observó que la
supervivencia de los polluelos disminuía a partir de 10 mg/kg de
dieta.
7. Efectos en animales de experimentación y en sistemas de
prueba in vitro
7.1 Toxicidad general
Después de la ingestión de una dosis oral única, el
hexaclorobutadieno es de levemente a moderadamente tóxico para las
ratas adultas, moderadamente tóxico para las ratas macho destetadas
y muy tóxico para las ratas hembras destetadas. Los principales
órganos afectados son el riñón y, en grado mucho menor, el hígado.
Los datos obtenidos con animales indican que el vapor de
hexaclorobutadieno es irritante para las membranas mucosas y el
líquido es corrosivo. La sustancia debe considerarse como un agente
sensibilizador.
En los riñones de ratas, ratones y conejos, el
hexacloro-butadieno causa en los tubos proximales del riñón una
necrosis que depende de la dosis. Las ratas macho adultas son menos
vulnerables a la toxicidad renal que las hembras adultas y que los
machos jóvenes. Los ratones jóvenes son más vulnerables que los
adultos y no se observaron diferencias entre un sexo y otro. En las
ratas hembra adultas la dosis intraperitoneal única más baja con la
cual se observó necrosis renal fue de 25 mg/kg de peso corporal y en
ratones adultos, machos y hembras, fue de 6,3 mg/kg de peso
corporal. Se observaron cambios bioquímicos y alteraciones
funcionales marcados en los riñones con dosis iguales o mayores que
las asociadas con necrosis.
Asimismo, en seis pruebas orales de corto plazo, dos estudios
sobre reproducción y un estudio de largo plazo sobre la dieta
realizados con ratas, el riñón fue el principal órgano afectado. Los
efectos relacionados con la dosis comprenden una reducción del peso
relativo del riñón y una degeneración del epitelio de los tubos. El
nivel sin efectos nocivos observados de toxicidad renal en ratas en
un estudio de dos años fue de 0,2 mg/kg de peso corporal por día. En
un estudio de 13 semanas efectuado en ratones se obtuvo un nivel sin
efectos nocivos observados de 0,2 mg/kg de peso corporal por día.
Las hembras adultas de ambas especies eran más vulnerables que los
machos adultos.
En una prueba de inhalación de corto plazo (6 horas por día
durante 12 días) se observaron efectos semejantes en los riñones con
una concentración de vapor nominal de 267 mg/m3, con la cual
también se observaron trastornos respiratorios y degeneración de la
corteza suprarrenal.
7.2 Reproducción, embriotoxicidad y teratogenicidad
Dos estudios sobre dieta y reproducción en ratas con dosis de
hasta 20 y 75 mg/kg de peso corporal por día, respectivamente,
mostraron una reducción del peso al nacer y un aumento del peso
neonatal cuando se administraban a la madre dosis tóxicas de 20 y
7,5 mg/kg de peso corporal, respectivamente. La dosis altamente
tóxica de 75 mg/kg de peso corporal por día fue suficiente para
impedir la concepción y la implantación uterina. No se observaron
anormalidades del esqueleto.
En dos pruebas de teratogenicidad en las que se expuso a las
ratas o bien a vapor de hexaclorobutadieno en concentraciones que
oscilaban entre 21 y 160 mg/m3 durante 6 horas diarias (desde el
6° hasta el 20° día del embarazo) o bien a la administración
intraperitoneal de 10 mg/kg de peso corporal por día (desde el 1° al
15° día de embarazo) se observaron en el desarrollo del feto efectos
tóxicos tales como una reducción del peso al nacer, un retraso del
desarrollo del corazón y uréteres dilatados pero sin grandes
malformaciones. El retraso del desarrollo se observó en niveles que
también eran tóxicos para las madres.
7.3 Genotoxicidad y carcinogenicidad
En la prueba de Ames Salmonella se ha observado que el
hexaclorobutadieno induce mutaciones genéticas en condiciones
especiales que favorecen la formación de productos de conjugación
con el glutatión. En un estudio in vivo se observó que había
inducido aberraciones cromosómicas, pero no se observaron tales
aberraciones en dos estudios in vitro. En una prueba in vitro se
observó que la frecuencia de los intercambios entre cromátidas
hermanas había aumentado en las células ováricas de hámsters de
China. Se ha señalado el gran potencial mutagénico de los
metabolitos sulfurosos del hexaclorobutadieno. En estudios in
vitro, el compuesto indujo síntesis imprevistas de ADN en cultivos
de fibroblastos de embriones de hámsters de Siria, pero no en
cultivos de hepatocitos. Indujo síntesis imprevistas de ADN en ratas
in vivo, pero no indujo mutaciones letales recesivas ligadas al
sexo en Drosophila melanogaster.
En el único estudio de largo plazo (dos años), en el cual las
ratas recibieron una dieta que contenía hexaclorobutadieno en dosis
de 0,2, 2 ó 20 mg/kg de peso corporal por día, se observó una mayor
incidencia de neoplasias de los tubos renales únicamente con la
dosis más elevada.
7.4 Mecanismos de toxicidad
La nefrotoxicidad, mutagenicidad y carcinogenicidad del
hexaclorobutadieno depende de la biosíntesis del conjugado sulfuroso
tóxico 1-glutatión- S-yl-1,2,3,4,4-pentaclorobutadieno. Este
conjugado se sintetiza principalmente en el hígado y se metaboliza
luego en la bilis, el intestino y los riñones convirtiéndose en
1-cisteína- S-yl-1,2,3,4,4-pentaclorobutadieno (CPB). La activación
de CPB, que depende del conjugado de cisteína beta-lyasa, en una
tiocetena reactiva en las células de los tubos proximales finalmente
da lugar a un enlace covalente con macromoléculas celulares.
8. Efectos en el ser humano
No se han descrito efectos patogénicos en la población en
general.
Se conocen dos casos de trastornos padecidos por trabajadores
agrícolas que utilizaban el hexaclorobutadieno como fumigante, pero
esas personas también habían estado expuestas a otras sustancias. En
los linfocitos de la sangre periférica de operarios que trabajaban
en la producción de hexaclorobutadieno y estaban expuestos, según se
informa, a concentraciones de 1,6 a 12,2 mg/m3 se observó una
frecuencia mayor de aberraciones cromosómicas.
9. Evaluación de los riesgos para la salud humana y de los
efectos en el medio ambiente
9.1 Evaluación de los riesgos para la salud humana
Como se han hecho muy pocos estudios en el ser humano, la
evaluación se basa principalmente en estudios efectuados en animales
de laboratorio. Sin embargo, los limitados datos existentes sobre el
ser humano, obtenidos in vitro, sugieren que el metabolismo del
hexaclorobutadieno en el ser humano es semejante al observado en
animales.
Se considera que el vapor de hexaclorobutadieno irrita las
membranas mucosas del ser humano y que en estado líquido es
corrosivo. El compuesto también debe considerarse como un agente
sensibilizador.
Los principales órganos afectados por la toxicidad son los
riñones y, en mucho menor grado, el hígado. En base a estudios de
corto y largo plazo de ingestión por vía oral por ratas y ratones,
se determinó un nivel sin efectos adversos observados de 0,2 mg/kg
de peso corporal por día. En un estudio de inhalación en el corto
plazo en ratas (12 días, a razón de 6 horas por día), el nivel sin
efectos adversos observados fue de 53 mg/m3.
Se observaron una reducción del peso al nacer y un aumento de
peso neonatal únicamente con dosis tóxicas para la madre; lo mismo
puede decirse de los efectos tóxicos para el desarrollo.
Se ha observado que el hexaclorobutadieno induce mutaciones
genéticas, aberraciones cromosómicas, aumento de los intercambios
entre cromátidas hermanas y síntesis imprevistas de ADN, aunque
algunos estudios han dado resultados negativos. Con respecto a la
genotoxicidad del hexaclorubutadieno, las observaciones realizadas
en animales son limitadas y las efectuadas en el ser humano
insuficientes.
Tras la administración oral a largo plazo del
hexacloro-butadieno a ratas, se ha observado una mayor frecuencia de
neoplasias de los tubos renales, pero solamente con dosis elevadas
causantes de nefrotoxicidad notable. Hay indicios limitados de
carcinogenicidad en animales e indicios insuficientes en el ser
humano.
En base al nivel sin efectos adversos observados en ratones y
ratas, que es de 0,2 mg/kg de peso corporal por día, se ha estimado
un nivel sin efectos adversos observados en el ser humano, que es de
0,03 a 0,05 mg/kg de peso corporal por día. Hay un margen de
seguridad de 150 entre el nivel sin efectos adversos observados
estimado y la ingesta diaria total máxima estimada, suponiendo que
el compuesto se absorba a través del agua de bebida contaminada y de
alimentos con elevado contenido de lípidos.
9.2 Evaluación de los efectos en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos; los crustáceos y peces son los más
vulnerables. Se ha establecido un nivel de riesgo para el medio
ambiente de 0,1 µg/litro. Se estima que la concentración ambiental
prevista máxima lejos de las fuentes equivale al nivel de riesgo
ambiental extrapolado multiplicado por dos y, por consiguiente, los
organismos acuáticos tal vez estén en peligro en las aguas de
superficie contaminadas. No pueden excluirse efectos adversos en
organismos bentónicos.
En vista de la toxicidad del hexaclorobutadieno para los
mamíferos, el consumo de organismos bentónicos o acuáticos por otras
especies tal vez sea motivo de inquietud.
 Common name: hexachlorobutadiene
Common synonyms: 1,3-hexachlorobutadiene, 1,1,2,3,4,4-
hexachloro-1,3-butadiene, perchloro-
butadiene
Common trade names: C-46, Dolen-pur, GP40-66: 120, UN2279
Common abbreviation: HCBD
CAS registry number:
Common name: hexachlorobutadiene
Common synonyms: 1,3-hexachlorobutadiene, 1,1,2,3,4,4-
hexachloro-1,3-butadiene, perchloro-
butadiene
Common trade names: C-46, Dolen-pur, GP40-66: 120, UN2279
Common abbreviation: HCBD
CAS registry number:  The intestinal absorption of GPB and CPB was studied in rats by
infusing the compounds into the intestine via a biliary cannula.
When GPB was infused, both GPB and CPB were found in the blood in
approximately equal concentrations. Higher blood CPB concentrations
were found after CPB infusion than after GPB infusion (Gietl
et al., 1991).
In studies with radiolabelled hexachlorobutadiene, several
urinary metabolites were identified. The structure of these
metabolites further supported the hypothesis that
hexachloro-butadiene is bioactivated by glutathione conjugation.
ACPB was found to be the main metabolite (representing
approximately 80% of the radioactivity present in urine) excreted
after the administration of [14C] hexachlorobutadiene (200 mg/kg)
in female rats (Reichert & Schütz, 1986). The same authors also
identified 1-carboxymethylthion-1,2,3,4,4-pentachlorobuta-1,3-diene
and 1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) as
urinary metabolites (Reichert et al., 1985). It is the opinion of
the Task Group that the identification of MTPB by diazomethane
treatment of the urinary extract is questionable.
In male rats, 1,2,3,4,4-pentachloro-1,3-butadienyl sulfenic
acid (PBSA) is the only metabolite excreted in urine that has so far
been identified. The data presented suggest that ACPB is not a major
urinary metabolite of hexachlorobutadiene in male rats (Nash
et al., 1984). In urine of mice exposed to radiolabelled
hexachlorobutadiene (30 mg/kg), CPB, ACPB and
2,3,4,4-tetra-chlorobutenoic acid were identified as urinary
metabolites (Dekant et al., 1988a).
It is probable that 2,3,4,4-tetrachlorobutenoic acid is formed
by reaction of the intermediate thioketene with water and further
hydrolysis of the thionol acid thus formed (Dekant et al., 1988a).
The weight of evidence suggests that oxidative reactions
involving cytochrome P-450 have little role in the metabolism of
hexachlorobutadiene (Wolf et al., 1984; Dekant et al., 1988a).
6.3 Reaction with body components
The covalent binding of [14C]-hexachlorobutadiene-related
radioactivity to tissue proteins has been shown to be time
dependent, with the highest level occurring during the first 6 h
after treatment. The half-life of hexachlorobutadiene binding was
22 h in both liver and kidney (Reichert, 1983; Reichert et al.,
1985).
In a DNA binding study, rats received a single oral dose of
20 mg [14C]-hexachlorobutadiene, and DNA was isolated from the
kidneys of these rats at 6, 18.5 and 30 h after dose administration.
Although the results have been reported only in summary form,
various levels of radioactivity were recovered with the DNA, but
there was a marked variation in the level of radioactivity between
samples. Furthermore complete analysis of the DNA was not performed
and protein may have been associated with the DNA (Stott et al.,
1981).
Covalent binding to mouse liver and kidney DNA was demonstrated
after the oral administration of radiolabelled hexachlorobutadiene
(30 mg/kg body weight) in corn oil (Schrenk & Dekant, 1989). In the
liver and kidneys, the binding capacity of mitochondrial DNA was
significantly higher than that of nuclear DNA. The level of binding
to nuclear DNA in the liver was indistinguishable from that of
controls. HPLC separation of the hydrolysed DNA indicated the
presence of three distinct peaks of radioactivity.
6.4 Excretion
Following oral administration in rats and mice of single doses
of hexachlorobutadiene up to 100 mg/kg body weight, the total
excretion within 72 h was at least 65% of the dose. In mice, less
than half of a dose of 30 mg/kg body weight was metabolized (Dekant
et al., 1988a). In rats, assuming that the faeces mostly contain
unchanged compound and no non-resorbed conjugates, 44% of an orally
administered low dose of hexachlorobutadiene (1 mg/kg body weight)
was metabolized (Reichert et al., 1985). At higher doses the
percentage of hexachlorobutadiene metabolized decreased
dramatically. The biotransformation of hexachloro-butadiene in rats
appears to be a saturable process considering the reduced excretion
of carbon dioxide and renal metabolites at increasing doses (Davis
et al., 1980; Reichert et al., 1985; Reichert & Scuhtz, 1986;
Payan et al., 1991). This could be explained by saturation of the
gastrointestinal absorption, which was observed by Reichert et al.
(1985). It should be noted, however, that the observed increase in
the amount of unchanged hexachloro-butadiene in faeces with
increasing dose applies only up to 100 mg/kg body weight. At higher
dose levels, the amount of unchanged hexachlorobutadiene in faeces
decreases, probably due to a decrease in faecal output (Davis
et al., 1980; Nash et al., 1984).
The results of studies of Payan et al. (1991) on bile-duct
cannulated (see section 6.2.2) and intact (see Table 8) rats show
that saturation of gastrointestinal absorption indeed occurs
following oral administration.
Pharmacokinetic data concerning the fate of
hexachloro-butadiene in organisms were not available to the Task
Group.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Short-term toxicity
A summary of short-term aquatic toxicity data is presented in
Table 9. In most of these studies the concentration of
hexachloro-butadiene was not reported. Therefore, the actual effect
concentrations may be lower than the nominal ones. In several cases
these nominal values far exceed the solubility limits. Based on
these data the substance is moderately to highly toxic to aquatic
organisms (Canton et al., 1990).
Adverse effects reported in some of these acute tests included
loss of equilibrium, erratic swimming (Leeuwangh et al., 1975;
Laseter et al., 1976), decreased activity, increased rate of
opercular movement, jumping (Leeuwangh et al., 1975), inverted
positions, fin fibrillation and muscle tetany (Laseter et al.,
1976). In a special investigation into kidney pathology, groups of
five goldfish (Carassius auratus) received one intraperitoneal
injection of hexachlorobutadiene at a dose level of 500 mg/kg body
weight in corn oil. They were subsequently fasted for up to 6 days
and sacrificed at different points in time up to day 7. Controls
received corn oil only. The temperature was kept between 18 and
21 °C. By 24 h the fish showed decreased activity, swam with dorsal
fins down, and had difficulty in following food. By day 4
exophthalmos, distended abdomen and ascites were observed. These
signs of toxicity were all reversible. Relative kidney weights were
elevated on day 4 only. From 12 h after exposure, P2 and P3
renal epithelial cells exhibited marked vacuolation and necrosis,
which persisted up to day 7. Increased gamma-glutamyl-transferase
(EC 2.3.2.2) staining was seen in P2 and P3 segments
(Reimschüssel et al., 1989).
In a report of this experiment, the fish were sacrificed at
different points in time up to day 70 after exposure. In one of the
two experiments the fish also received 5-bromo-2'-deoxyuridine 4 h
prior to sacrifice. Morphometric analysis of developing nephrons
showed an increase in the percentage of volume occupied by
basophilia clusters and developing nephrons from day 14 onwards. The
apparent number of basophilic clusters and developing nephrons per
unit surface area was also increased from day 14 (Reimschüssel
et al., 1990).
Table 9. Short-term aquatic toxicity of hexachlorobutadiene
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fresh water
Algae Haematococcus 20 stat, closed 24 EC10b > 2 Knie et al.
pluvialis (1983)
Bacteria Pseudomonas putida 25 7.0 stat, closed 16 TTc > 25 Bringmann &
Kuhn (1977)
Bacteria Pseudomonas putida 20 7.2 stat, open 0.5 EC10b > 0.9 Knie et al.
(1983)
Protozoans Chilomonas 20 6.9 stat, closed 48 TTc > 10 Bringmann et al.
paramecium (1980)
Molluscs great pond snail, 19 stat,d closed 24 LC50 0.21 Leeuwangh et
Lymnaea stagnalis 96 LC50 0.21 al. (1975)e
Crustaceans water flea, 20 7 250 stat, open 24 EC50 0.5 Knie et al.
Daphnia magna (1983)
aquatic sowbug, 19 stat,d closed 96 LC50 0.13 Leeuwangh et
Asellus aquaticus al. (1975)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fish goldfish, 17.5 stat,d open 96 LC50 0.09 Leeuwangh et
Carassius auratus al. (1975)e
Fish zebrafish, 20 8.0 9.0 180 flow, closed 48 LC50 1 Slooff (1979)
Brachydanio rerio
Fish rainbow trout, LC50 0.320 US EPA (1980)
Salmo gairdnerii
Fish bluegill sunfish, LC50 0.326 US EPA (1980)
Lepomis macrochirus
Fish sheepshead minnow, LC50 0.557 US EPA (1980)
Cyprinodon variegatus
Fish golden orfe, 20 8 270 open 48 LC50 3 Knie et al.
Leuciscus idus (1983)e
Fish fathead minnow, 25 6.7-7.6 8.0 45 flow, open 96 LC50 0.10 Walbridge et
Pimephales promelas al. (1983)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Marine
Crustaceans harpacticoid 21 7.9 > 5 stat, open 96 LC50 1.2 Bengtsson &
copepod Tarkpea (1983)
grass shrimp, LC50 0.032 US EPA (1980)
Palaemonetes pugio
Mysid shrimp, LC50 0.059 US EPA (1980)
Mysidopsis bahia
Fish sailfin molly, 22-24 6.6-7.9 8-9 flow, open 26 LC50 4.2 Laseter et
Poecilia latipinna 30 LC50 4.5 al. (1976)e,f
77 LC50 1.4-1.9
115 LC50 1.7
138 LC50 1.2
pinfish, Lagodon LC50 0.399 US EPA (1980)
rhomboides
a static or flow-through test, open or closed system d semi-static (daily renewal) test
b effect is 10% reduction in oxygen consumption e analysis for hexachlorobutadiene was reported
c TT = toxic threshold for inhibition of cell multiplication f salinity was 0.25%, 96-h LC50 was calculated to be 1.6 mg/litre
7.1.2 Long-term toxicity
The cell multiplication of green algae (Scenedesmus
quadricauda) was not inhibited after 8 days of static exposure to
a nominal concentration of 25 mg/litre (well above pure water
solubility) in a closed system at 27 °C and a pH of 7 (Bringmann &
Kühn, 1977). A 14-day LC50 of 0.4 mg/litre was determined for
2- to 3-month old guppies (Poecilia reticulata) in a semi-static
test using an open system at 22 °C, a water hardness of 25 mg
CaCO3/litre, and a dissolved oxygen concentration of >
5 mg/litre. No analysis for hexachlorobutadiene was reported
(Könemann, 1981). In the same test under the same conditions, but
with analysis for the compound, the 14-day LC50 was 0.16 mg/litre
(Hermens et al., 1985).
In a study by Leeuwangh et al. (1975), groups of six goldfish
(Carassius auratus) were each exposed to hexachlorobutadiene in
tap water at measured concentrations of 0, 0.0003, 0.003, 0.0096 or
0.03 mg/litre for 49 and 67 days. The static test in an open system
was conducted at 19 °C, a pH of 7.6, and a dissolved oxygen
concentration between 3.2 and 6.3 mg/litre. Body weights were
decreased after 49 days at 0.03 mg/litre, and body weight gain was
still reduced at 67 days. Abnormal behaviour, jumping,
incoordination, increased opercular movement and overall immobility
were noted at 0.0096 mg/litre. After 49 days at 0.0096 mg/litre (no
data at 0.03 mg/litre), relative liver weights were increased, and
the activity of liver glucose-6-phosphatase (EC 3.1.3.9) was
decreased, whereas the activity of liver glucose-6-phosphate
dehydrogenase (EC 1.1.1.49) was increased. After 67 days the
activity of liver phenylalanine hydroxylase (EC 1.14.16.1) showed a
concentration-related increase. No effects were found on haemoglobin
concentration and haematocrit after 49 days or on the activity of
serum alanine aminotransferase (EC 2.6.2.1) and serum alkaline
phosphatase (EC 3.1.3.1) after 67 days.
Groups of 12 largemouth bass (Micropterus salmoides) were
each exposed to hexachlorobutadiene for 10 days at measured
concentrations of 0.00343 and 0.03195 mg/litre in filtered tap water
with a salinity of 0.08-0.1%, at 22-24 °C, a pH of 6.6-7.9 and a
dissolved oxygen concentration of 7.6-8.5 mg/litre. A control group
comprised 12 water and 12 vehicle (acetone) controls. Plasma
cortisol levels were increased at both concentrations, but
haematocrit values were not affected. At the higher concentration
there was leukocytic infiltration in the kidneys of one of the fish
and paleness and accentuated lobulation of parenchyma in the livers
(Laseter et al., 1976).
In an early lifestage test, four replicate groups, each of 30
fathead minnow (Pimephales promelas) eggs, 2-4 h after spawning,
were exposed to measured hexachlorobutadiene concentrations in
sand-filtered and sterilized lake water of 0.0017, 0.0032, 0.0065,
0.013 and 0.017 mg/litre in an open system. Following hatching
(4-5 days after spawning), four replicate groups of 15 larvae
continued to be exposed for 28 days. Control groups of equal size
were exposed to slightly contaminated water containing
0.00008 mg/litre. The temperature was 25 °C, pH was 7.4, dissolved
oxygen concentration 7 mg/litre, and water hardness 45 mg
CaCO3/litre. The hatchability of embryos and the percentage of
normal larvae at hatch were not affected. An increased fish
mortality and a concentration-related decrease of body weight were
observed at the two highest concentrations at the end of the
exposure period (Benoit et al., 1982).
7.2 Terrestrial organisms
7.2.1 Short-term toxicity
Except for one test with birds, reliable tests with terrestrial
organisms have not been reported.
Groups of 12 female and four male Japanese quails (Coturnix
coturnix japonica) were exposed to a diet containing
hexachloro-butadiene at levels of 0, 0.3, 3, 10 or 30 mg/kg diet for
90 days. Each cage contained three females and one male of the same
dose group. Feed analysis indicated levels close to the nominal
values. Adults were all histopathologically examined. Eggs were
collected on days 37-46, 64-73, and 81-90. Six adults died during
the study: 4 at 0.3 mg/kg, 1 at 10 mg/kg, and 1 at 30 mg/kg, but
this was not considered to be related to treatment. The survival of
chicks from eggs collected on days 81-90 was decreased at 10 mg/kg
only. Egg production, the percentage of fertile eggs, the percentage
of hatchable eggs, and eggshell thickness were unaffected compared
to controls (Schwetz et al., 1974).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The available mortality data are summarized in Table 10.
8.1.1 Inhalation exposure
8.1.1.1 Mortality
In the only reported study of the mortality of
hexachloro-butadiene following inhalation, groups of 20 SPF mice of
the OF1 strain were exposed for 6 h (Gage, 1970). The vapour
concentrations were measured by gas chromatography, and the
concentrations were within 10% of the nominal value. Results are
shown in Table 10.
8.1.1.2 Systemic effects
The decrease in respiratory rate (reflex bradypnoea) in groups
of six male Swiss OF1 mice was measured following exposure for
15 min to hexachlorobutadiene vapour at concentrations between 886
and 2625 mg/m3. The vapour concentrations were checked by gas
chromatography. The mice were restrained in a body plethysmograph,
while the heads were extended into an inhalation chamber. The 50%
effect level, calculated from the concentration-effect curve, was
2250 mg/m3 (De Ceaurriz et al., 1988).
8.1.2 Oral exposure
8.1.2.1 Mortality
Based on acute mortality data, hexachlorobutadiene is slightly
to moderately toxic to adult rats, moderately toxic to male weanling
rats, and highly toxic to female weanling rats following ingestion.
Young rats are far more sensitive than adult rats (Kociba et al.,
1977a). Gradiski et al. (1975) observed delayed mortality (after
24 h) in oral LD50 studies on rats and mice.
8.1.2.2 Systemic effects
Hexachlorobutadiene mainly affects the kidneys and, to a lesser
extent, the liver. The effects on these organs and the related
biochemical findings are discussed extensively in sections 8.8.2
(liver) and 8.8.3 (kidney). In oral LD50 studies on rats and mice,
Gradiski et al. (1975) observed hyper-reactivity just after
exposure, followed by decreased activity and staggering.
Table 10. Mortality of hexachlorobutadiene from single exposure
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Mouse oral 87 (78.1-95.9) Murzakayev (1963)b
OF1 adult female inhalation (6 h) 14 107 (102-113) Gage (1970)
OF1 adult female oral 14 65 (60-70) Gradiski et al. (1975)c
OF1 adult male oral 14 80 (75-85) Gradiski et al. (1975)c
Alderley Park adult male intraperitoneal 14 67 (53-85) Lock et al. (1984)e
Alderley Park adult female intraperitoneal 14 85 (65-111)
C57BL/10J adult male intraperitoneal 14 57 (41-81)
C3H adult male intraperitoneal 14 25-75
BALB/c adult male intraperitoneal 14 32-40
DBA/2J adult male intraperitoneal 14 53 (36-76)
Rat oral 350 (323-377) Murzakayev (1963)b
OF2 rat adult female oral 14 270 (250-290) Gradiski et al. (1975)c
OF2 rat adult male oral 14 250 (220-280) Gradiski et al. (1975)c
Sprague-Dawley adult female oral 200-400 Kociba et al. (1977a)d
Sprague-Dawley adult male oral 580 (504-667) Kociba et al. (1977a)d
Sprague-Dawley weanling female oral 46 (26-81) Kociba et al. (1977a)d
Sprague-Dawley weanling male oral 65 (46-91) Kociba et al. (1977a)d
Table 10 (contd).
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Alderley Park weanling male intraperitoneal 7 57 (38-87) Hook et al. (1983)e
Alderley Park 29 days male intraperitoneal 7 96 (72-128)
Alderley Park adult male intraperitoneal 7 360 (325-396)
Guinea-pig oral 90 (81.5-98.5) Murzakayev (1963)b
Rabbit
New Zealand adult female dermal (8 h) 14 1120 (890-1400) Duprat & Gradiski (1978)f
a When available, 95% confidence limits are reported between brackets.
b Observation period, strain, sex, age (or body weight) and vehicle were not reported.
c Vehicle was olive oil.
d Confidence limits not calculable; observation until no toxicity was observed any longer; vehicle was corn oil.
e Vehicle was corn oil.
f Application undiluted using glass vials (3.6 cm2).
8.1.3 Dermal exposure
8.1.3.1 Mortality
Hexachlorobutadiene was harmful to rabbits following acute
dermal exposure (LD50 = 1120 mg/kg body weight; (range,
890-1400 mg/kg; Table 10). After a dose of 780 mg/kg body weight,
death occurred within 24 h from respiratory and cardiac failure
(Duprat & Gradiski, 1978).
8.1.3.2 Systemic effects
New Zealand rabbits dermally exposed to undiluted
hexa-chlorobutadiene at doses of 1170 and 1550 mg/kg body weight
exhibited stupor, dyspnoea and cyanosis (Duprat & Gradiski, 1978).
8.1.4 Other routes of exposure
Hexachlorobutadiene has been shown to be toxic to various
strains of mice after intraperitoneal injection (Lock et al.,
1984) and harmful to adult rats (Hook et al., 1983). The compound
was considerably more toxic to young and weanling rats (Table 10).
Rats intraperitoneally exposed to single lethal doses of
hexachlorobutadiene from 500 to 1000 mg/kg in corn oil exhibited
piloerection, sedation, hunching, incoordination, loss of muscle
tone and hypothermia (Lock & Ishmael, 1979). A macroscopic and
haematological investigation of rats intraperitoneally exposed to
doses between 121 and 336 mg/kg body weight in olive oil did not
reveal any damage to the gastrointestinal tract, spleen, heart or
gonads. In the lungs, congestion, haemorrhage, and oedema were
observed, but these were attributed by the authors to ether
anaesthesia. At dose levels of 213 mg/kg body weight or more,
lymphopenia and related neutrophilia were induced (Gradiski et al.,
1975).
8.2 Short-term exposure
8.2.1 Inhalation exposure
In the only inhalation study published, groups of four adult
Alderley Park SPF rats of each sex were dynamically exposed to
nominal concentrations of 53, 107 or 267 mg/m3 6 h/day for 15
days, 1067 mg/m3 6 h/day for 12 days, or 2668 mg/m3 4 h/day for
2 days. Petroleum ether was used as a solvent for concentrations
below 1067 mg/m3. Many other chemicals were tested similarly, and
batches of control rats of unknown size were maintained at intervals
of 2 months during the whole experimental period. No analysis for
hexachlorobutadiene was carried out. Two of the four female rats
exposed to 1067 mg/m3 died, and autopsy revealed pale, enlarged
kidneys, adrenal regeneration and renal cortical tubular
degeneration with epithelial regeneration. Rats of both sexes lost
weight at 1067 mg/m3 and the weight gain of females was reduced at
107 and 267 mg/m3. Irritation of eyes and nose was observed at the
two highest levels. At 267 and 1067 mg/m3 rats were in a poor
condition, females being more affected than males. Respiratory
difficulties were seen at and above 267 mg/m3. Haematological
examination at the end of the exposure period showed slight anaemia
in females at 1067 mg/m3. Urinalysis did not reveal abnormalities
at any of the exposure levels. Macroscopically enlarged, pale
kidneys were found at 267 and 1067 mg/m3 and enlarged adrenals at
1067 mg/m3. Histopatho-logical investigations revealed proximal
tubular degeneration in the kidneys and cortical degeneration in
adrenals at concentrations of 267 mg/m3 or more. No toxic signs
were observed at the lowest exposure level and autopsy revealed no
gross abnormalities (Gage, 1970).
8.2.2 Oral exposure
8.2.2.1 Rats
Groups of five adult male Sprague-Dawley rats were exposed to
daily oral doses of hexachlorobutadiene (0, 0.2 or 20 mg/kg body
weight) in corn oil for 3 weeks. Only the kidneys were examined
histologically. At the higher dose level, body weight gain was
decreased and relative kidney weight increased. Histopathological
examination of the kidneys revealed damage in the middle and inner
cortical region, including loss of cytoplasm, nuclear pyknosis,
increased basophilia and mitotic activity, and increased cellular
debris. No toxic signs were observed at a dosage of 0.2 mg/kg per day
(Stott et al., 1981).
In a study by Kociba et al. (1971), groups of four female
Sprague-Dawley rats consumed for 30 days a diet containing
hexachlorobutadiene, which resulted in ingested nominal daily doses
of 0, 1, 3, 10, 30, 65 and 100 mg/kg body weight. Analysis of the
compound in the feed was not reported. Body weights were decreased
at the two highest dose levels. At 10 mg/kg or more, a dose-related
decrease in body weight gain was observed along with a decrease in
food consumption. There was also an increase in haemoglobin
concentrations, which, although significant, was not clearly dose
related. There was no effect on serum alanine aminotransferase
activity (EC 2.6.1.2). A dose-related increase in relative kidney
weight was observed at dose levels of 3 mg/kg or more.
Histopathological examination, which was restricted to liver and
kidneys, showed proximal tubular degeneration, individual cell
necrosis, and regenerative changes in the kidneys at doses of
10 mg/kg or more. Hepatocellular swelling was seen at 100 mg/kg. The
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight per
day.
In a 2-week experiment, groups of six weanling Wistar-derived
rats of each sex were exposed to measured dietary
hexachlorobutadiene levels of 0, 73, 182 or 447 mg/kg (the Task
Group considered this equivalent to doses of 0, 7.3, 18.2 and
44.7 mg/kg body weight per day, respectively). At all dose levels,
body weight and food conversion efficiency (g of weight gain/g of
food) were decreased in a dose-related manner. Food consumption per
g of body weight was decreased at 44.7 mg/kg body weight. Relative
kidney weights were increased at the two highest dose levels. At all
dose levels a dose-related degeneration of kidney tubular cells was
observed, especially in the straight limbs of the proximal tubules
located in the outer medulla. No toxic signs were observed in the
liver. A NOAEL was not found (Harleman & Seinen, 1979).
In a follow-up to the dietary study, groups of 10 weanling
Wistar-derived rats of each sex received daily doses by gavage of 0,
0.4, 1, 2.5, 6.3 or 15.6 mg/kg body weight in peanut oil for 13
weeks (Harleman & Seinen, 1979). Body weight gain, food consumption
and food utilization efficiency were decreased at 6.3 and
15.6 mg/kg. Polyuria was observed in females at these dose levels
after week 10, while a dose-related decrease in urine osmolarity
occurred at dose levels of 2.5 mg/kg or more. In males, the latter
effect was observed at 15.6 mg/kg. No other changes were observed in
urinalysis (after week 10) and haematological investigations (after
week 8). A dose-related increase in relative kidney weight was
measured in males of all treatment groups but only at 6.3 mg/kg or
more in females. Dose-related increases in the relative weight of
liver and spleen were measured at 6.3 mg/kg or more.
Histopathological examinations revealed changes in liver and
kidneys. In the livers of males dosed with 6.3 mg/kg or more, an
increased basophilic, flocky granulation was observed. At dose
levels of 6.3 mg/kg or more in males and 2.5 mg/kg or more in
females, there was a dose-related degeneration of the renal proximal
tubules, as shown by hyperchromatic nuclei, hypercellularity,
vacuolation and focal necrosis of epithelial cells and a diminished
brush border. No adverse effects were observed at daily doses of
1 mg/kg in females or 2.5 mg/kg in males (Harleman & Seinen, 1979).
8.2.2.2 Mice
In a two-week study, groups of five B6C3F1 mice of each sex
were fed a diet containing hexachlorobutadiene at nominal doses of
0, 30, 100, 300, 1000 or 3000 mg/kg feed for 15 days (calculated by
the Task Group to be equivalent to 0, 4.3, 14.3, 43, 143 and
430 mg/kg body weight per day, respectively, using standard values
for average body weight and food consumption in mice). Analysis of
the feed was carried out by gas chromatography, and no more than 9%
loss of the chemical was observed in one day (feed was replaced
every 2 days). All mice given 143 or 430 mg/kg body weight died or
were sacrificed in a moribund condition within 7 days. A
dose-related growth retardation was observed. At the two highest
doses, the observed toxic effects included renal tubular necrosis,
hepatic cytoplasmic vacuolization, and testicular degeneration
characterized by the presence of syncytial giant cell formation of
spermatocytes. At dose levels of 43 mg/kg body weight or more,
minimal to mild depletion of bone marrow (characterized by a
decrease in the haematopoietic cells) was seen in two out of five
mice of both sexes per dose group. At dose levels of 4.3, 14.3 and
43 mg/kg body weight, at which all animals survived to the end of
the study, renal tubular cell regeneration was observed (Yang
et al., 1989; Yang, 1991).
In a 13-week study, groups of 10 B6C3F1 mice of each sex were
fed a diet containing hexachlorobutadiene at concentrations of 0, 1,
3, 10, 30 or 100 mg/kg feed. Using measurements of food consumption
and body weight, the authors determined doses of 0, 0.1, 0.4, 1.5,
4.9 or 16.8 mg/kg body weight per day for males, and 0, 0.2, 0.5,
1.8, 4.5 or 19.2 mg/kg body weight per day for females. Analysis of
the compound was carried out as in the two-week study. No
treatment-related clinical signs or deaths were observed. The
motility of sperm from treated mice was significantly lower than in
controls, although this effect was not dose related (Yang et al.,
1989; Yang, 1991). Body weight gain was reduced at dose levels of
4.5 and 19.2 mg/kg body weight in females. Reductions in kidney
weight occurred at dose levels of 1.5 mg/kg or more in males and at
19.2 mg/kg body weight in females, and reductions in heart weight
occurred at 19.2 mg/kg body weight in males. Necropsy revealed a
treatment-related increase in renal tubular regeneration (prominent
in the outer stripe of the medulla) at dose levels of 0.2 mg/kg body
weight or more in females (Table 11). Although the author concluded
that a NOAEL was not observed for females, the Task Group noted that
the occurrence of renal tubular regeneration in one out of ten
female mice in the 0.2-mg/kg body weight group is insufficient
evidence of an adverse effect at this dose level in females.
Table 11. Incidences of renal tubular regeneration in 13-week
feed studies on B6C3F1 micea
Concentration Dose (mg/kg body Number of mice with
(mg/kg feed) weight per day) lesions/number examined
Male Female Male Female
0 0 0 0/10 0/10
1 0.1 0.2 0/10 1/10
3 0.4 0.5 0/10 9/10
10 1.5 1.8 0/9 10/10
30 4.9 4.5 10/10 10/10
100 16.8 19.2 10/10 10/10
a Modified from: Yang (1991)
8.3 Long-term exposure
No long-term inhalation studies have been reported. A study
describing long-term exposure of mice by the dermal route is
presented in section 8.7.3.
An oral toxicity/carcinogenicity test in rats has been reported
(see section 8.7.2).
8.4 Skin and eye irritation; sensitization
8.4.1 Irritation
The vapour of hexachlorobutadiene has been found to be
irritating to the eyes and nose of rats (Gage, 1970; see section
8.2.1).
Groups of six New Zealand rabbits received either 0.78 g
(0.5 ml) of undiluted hexachlorobutadiene on the intact or abraded
skin for 24 h, or 0.15 g (0.1 ml) in the conjuctival sac of the left
eye. Assessment of the degree of irritation was conducted according
to Draize et al. (1944) and by calculating the primary irritation
index. Hexachlorobutadiene was moderately irritating for the skin
(primary irritation index 4) but not irritating for the eyes
(primary irritating index 1.5). Moderate conjunctivitis, epithelial
abrasion and, at day 7, epithelial keratitis were observed in the
eyes (Duprat et al., 1976).
Duprat & Gradiski (1978) applied undiluted hexachloro-butadiene
to New Zealand rabbits at doses of 0.39, 0.78, 1.17 and 1.55 mg/kg
body weight (0.25, 0.50, 0.75 and 1.00 ml, respectively) under
occluded conditions, using glass vials, for 8 h. The observation
period was 14 days. The skin was histopathologically examined in all
dead animals, in half the survivors at day 15, and in the remaining
survivors at day 36. After 12 h of exposure to the two highest
doses, epidermis and subcutaneous tissue revealed oedema and
polymorphonuclear leukocyte infiltration. In the epidermal cells,
degeneration with pyknosis of nuclei and perinuclear oedema, and
focal separation from the corium with vesicle formation were seen.
After 3 to 5 days of exposure to the three highest doses, dermal
necrosis was observed, leading to eschar formation and partial
destruction of hair follicles. The effects increased with time, not
with dose. Two to five weeks after application, repair was apparent
at all dose levels, with scarring and upper dermis fibrosis, and
epidermal acanthosis with focal dyskeratosis. Diffuse mononuclear
infiltrate was seen in the dermis.
8.4.2 Sensitization
A group of 20 Hartley guinea-pigs were treated according to the
Magnusson-Kligman protocol by intradermal injections of 5%
hexachlorobutadiene in peanut oil and, after one week and subsequent
treatment by sodium lauryl sulfate, by a 48-h dermal application of
a 25% suspension of the chemical in vaseline. The challenge was
performed by dermal application of a 20% suspension in vaseline. A
group of five controls was induced similarly and challenged by
vaseline only. All exposed animals, but none of the controls, showed
a positive reaction. The test was repeated in the same fashion
without adjuvant in five guinea-pigs: all animals showed positive
reactions (Gradiski et al., 1975).
8.5 Reproduction, embryotoxicity and teratogenicity
8.5.1 Reproduction
A group of female albino rats was exposed to one dose of
hexachlorobutadiene (20 mg/kg body weight) administered
subcutaneously before mating. Within 90 days after exposure, all 86
newborn rats had died, compared with 13 of the 61 controls. The
offspring from exposed dams were reported to show excitation,
disturbances of motor coordination, a decrease in appetite and a
loss of weight, lymphocytosis, neutropenia, myelocytes, Jolly's and
Cabeau's bodies, pneumonia, bronchitis, granular dystrophy of renal
cells, glomerulonephritis, inflammatory destructive lesions of the
gastrointestinal tract and vascular hyperaemia (Poteryayeva, 1966).
The Task Group noted major deficiencies and incomplete reporting of
the experiment, the unusual route of administration, and the high
percentage of mortality in control rats.
Groups of 10-12 male and 20-24 female Sprague-Dawley rats
received a diet containing hexachlorobutadiene at dose levels of
0.2, 2.0 or 20 mg/kg body weight per day for 90 days prior to
mating, 15 days during mating, and subsequently throughout gestation
and lactation. In the mating period, two females were placed with
one male of the same dose group. The control group consisted of
17 males and 34 females. The diets were reportedly analysed for the
test compound. No mortality was observed. At 20 mg/kg, adults showed
decreased food consumption and body weight gain. Blood urea
nitrogen, serum alanine aminotransferase (EC 2.6.1.2) and serum
creatinine were unchanged compared to controls. The dams had an
increased relative brain weight and the male rats had an increased
relative liver weight at 20 mg/kg. The relative kidney weights were
increased in both sexes at 20 mg/kg. At 2 and 20 mg/kg the kidneys
of adult rats revealed dose-related tubular dilatation and
hypertrophy with foci of epithelial degeneration and regeneration;
however, there was no effect at 0.2 mg/kg. The only adverse
reproductive effect in neonates was a decreased weanling weight at
20 mg/kg. There was no detectable effect on the percentage
pregnancy, the period from first cohabitation to delivery, survival
indices, sex ratio, histopathology of weanlings, and the incidence
of skeletal alterations and abnormalities in neonates (Schwetz
et al., 1977).
In a third reproductive study, groups of six female SPF
Wistar-derived rats, 10 weeks of age, received a diet containing
hexa-chlorobutadiene at levels of 0, 150 and 1500 mg/kg diet
(estimated by the Task Group to be equivalent to 0, 7.5 and 75 mg/kg
body weight per day) for 3 weeks prior to mating, 3 weeks during
mating, and subsequently throughout gestation and lactation. In the
mating period, two untreated males were placed with the females,
after which the females were housed individually. The 75-mg/kg
female adults were killed in week 10, while those given 0 or
7.5 mg/kg were killed in week 18. Food analysis at the low dose
level revealed hexachlorobutadiene levels within 96% of nominal
values after 1 week and within 81% of nominal values after 2 weeks.
Diets were prepared weekly. There was a reduced body weight gain by
female rats in the two groups receiving hexachlorobutadiene.
Weakness of hind legs, unsteady gait, incoordination and ataxia were
seen at 75 mg/kg. The relative kidney weight was increased at both
dose levels. Histopathological investigations revealed
hypercellularity of epithelial cells, hydropic degeneration, and
necrosis of proximal tubules in the kidneys at 7.5 mg/kg. At
75 mg/kg, slight proliferation of bile duct epithelial cells,
fragmentation and demyelination of single fibres of the femoral
nerve, and extensive renal degeneration were observed. Again at
75 mg/kg, no conceptions occurred, the ovaries showing little
follicular activity, and there was no uterine implantation sites. At
7.5 mg/kg fertility and litter size were reduced, but not
significantly. In both the control and 7.5-mg/kg groups, the
resorption quotient was low. Compared to controls, pup weights were
reduced significantly on days 0, 10 and 20 in the 7.5-mg/kg group.
No gross abnormalities were observed (Harleman & Seinen, 1979).
8.5.2 Embryotoxicity and teratogenicity
In a teratology study, groups of 24-25 female rats were exposed
to hexachlorobutadiene vapour at measured concentrations of 0, 21,
53, 107 or 160 mg/m3 for 6 h per day from days 6 to 20 of
pregnancy. The breathing zone atmosphere was analysed by gas
chromatography. Maternal weight gain decreased at 53 and
160 mg/m3. At the other two exposure levels, the slight decrease
in maternal weight was not significant. The mean number of
implantation sites, total fetal losses, resorptions, live fetuses,
incidences of pregnancy, and sex ratio were not affected by exposure
to hexachlorobutadiene, compared to controls. Fetal body weight was
reduced in both sexes at 160 mg/m3. The incidences of external,
visceral, and skeletal alterations were not significantly increased
(Saillenfait et al., 1989).
In a study by Hardin et al. (1981), groups of 10-15 mated
Sprague-Dawley rats received hexachlorobutadiene in corn oil by
intraperitoneal injection at a dose level of 10 mg/kg body weight
per day from days 1 to 15 of gestation. It was reported (without
further details) that at least two maternal organ weights were
changed and that pre- or postimplantation survival was reduced.
Maternal tissues did not reveal histopathological effects. Fetuses
had a reduced weight or length, a 1-2 day delay in heart
development, and dilated ureters. No grossly visible external or
internal malformations were observed (Hardin et al., 1981).
It was reported briefly by Badaeva et al. (1985) that daily
oral administration of hexachlorobutadiene to pregnant rats at a
dose level of 8.1 mg/kg body weight per day resulted in
histopathologi-cal changes of nerve cells and myelin fibres of the
spinal cord in the dams and their offspring.
8.6 Mutagenicity and related end-points
8.6.1 In vitro effects
Purified hexachlorobutadiene induces gene mutations in the Ames
Salmonella test when specific incubation conditions are employed.
In preincubation assays adapted to include rat liver microsomes
and additional reduced glutathione, hexachlorobutadiene induced
point mutations in Salmonella typhimurium TA100 (Vamvakas et al.,
1988a). Assays lacking specialized metabolic activation conditions
have generally yielded negative results (Table 12).
Data from bacterial mutagenicity assays are consistent with the
proposed scheme for the biotransformation of hexachlorobutadiene in
animals (section 6.3; Fig. 1). Activity in S. typhimurium TA100,
mediated by subcellular fractions of rat kidney, was inhibited by
the addition of the ß-lyase inhibitor, AOAA (Vamvakas et al.,
1988a, 1989a) and the gamma-glutamyltranspeptidase inhibitor,
acivicin (Vamvakas et al., 1989a).
Several of the proposed metabolites of hexachlorobutadiene have
been assayed for mutagenic activity in S. typhimurium TA100
(Table 13). The mono-glutathione (GPB) and mono-cysteine (CPB)
conjugates were mutagenic in the presence or absence of rat kidney
S9. Rat liver microsomes and mitochondria that exhibit high
gamma-glutamyltranspeptidase activities strongly enhanced the
mutagenic potency of GPB in the presence of additional glutathione,
in contrast to liver microsomes that exhibit lower
gamma-glutamyltranspeptidase activity. Furthermore, AOAA and
acivicin both inhibit the activation of GPB mediated by kidney
fractions. The di-glutathione (BGTB) and di-cysteine (BCTB)
conjugates of hexachlorobutadiene were not mutagenic either in the
presence or absence of rat kidney S9 (Green & Odum, 1985; Dekant
et al., 1986; Vamvakas et al., 1988a, 1989a).
The mercapturic acid, ACPB, was mutagenic both in the presence
and absence of rat liver S9. It has been suggested that the
metabolism of ACPB in animals is catalysed by an N-deacetylase and
by ß-lyase (section 6.3) (Reichert et al., 1984). The Task Group
considered that S. typhimurium possesses both of these enzymes
activities. Both MTPB and CMTPB gave negative results in tests with
S. typhimurium TA100 and are considered to be detoxified
metabolites of hexachlorobutadiene (Wild et al., 1986).
Table 12. Studies on mutagenicity of hexachlorobutadiene
Test description Species/strain/cell type Conditionsa Resultc Reference
Reverse mutations Salmonella typhimurium TA98, +/- rat liver S9, purity 98%, - De Meester et al.
TA100, TA1530, TA1535, TA1538 plate incorporation (1981)
S. typhimurium TA100 +/- rat liver S9, purity > 99%, - Stott et al. (1981)
plate incorporation
S. typhimurium TA98, TA100 +/- rat liver S9, purity not - Reichert et al. (1983)
reported, suspension testb
S. typhimurium TA98, TA100, +/- rat liver S9, purity not - Haworth et al. (1983)
TA1535, TA1537 reported, preincubation test
+ rat liver S9*, purity > 99.5%, +
preincubation test
S. typhimurium TA100, TA1535 +/- rat liver S9, purity not - Chudin et al. (1985)
TA1538 reported, plate incorporation
S. typhimurium TA100 +/- rat liver S9, - Reichert et al. (1984)
- rat liver S9* +
S. typhimurium TA100 + rat liver S9*, purity >99.5%, +d Wild et al. (1986)
preincubation test
S. typhimurium TA100 no activation, purity 98% + Vamvakas et al. (1988a)
no activation, purity > 99.5%, -
preincubation test
Table 12 (contd).
Test description Species/strain/cell type Conditionsa Resultc Reference
S. typhimurium TA100 + rat liver microsomes - Vamvakas et al. (1988a)
without additional GSH
+ rat liver microsomes and +e
additional GSH, purity > 99.5%,
plate incorporation
Sex-linked lethals Drosophila melanogaster feeding or injection - Woodruff et al. (1985)
Chromosome aberrations CHO cells +/- rat liver S9 - Galloway et al. (1987)
Chromosome aberrations human lymphocytes - rat liver S9 - German (1988)
Sister chromatid exchanges CHO cells +/- rat liver S9 + Galloway et al. (1987)
Chromosome aberrations mouse bone marrow cells inhalation, 4 h + German (1988)
Chromosome aberrations mouse bone marrow cells oral gavage + German (1988)
a S9* = a fortified S9 mix containing 3 times the normal protein concentration; GSH = reduced glutathione
b The extreme toxicity of the compound without S9 was supposed to exclude testing in this system
c + = > twice the background rate or, in the case of bacterial studies, a reproducible dose-related increase in the number of
revertants per plate; - = negative
d 0.23 revertants per nmol
e Addition of rat kidney microsomes further increased the number of revertants; positive results were inhibited by the ß-lyase inhibitor
aminooxyacetic acid
Chinese hamster ovary (CHO) cells were exposed to between 5 and
24 mg hexachlorobutadiene/litre for 2 h in the presence of rat liver
S9 and throughout the incubation period (8-26 h, depending on cell
cycle delay) in the absence of rat liver S9. In comparison with
concurrent controls, no significant increase in chromosome
aberration frequency was observed (Galloway et al., 1987). In a
further study, in which human lymphocyte cultures were exposed to
between 0.01 and 0.001 mg hexachlorobutadiene per litre in the
absence of S9 for 27 h, there was also no clastogenic effect. At the
highest dose level there was a reduction of approximately 60% in the
mitotic index of human lymphocyte cultures (German 1988). However,
hexachlorobutadiene at a dose level of at least 4 mg/litre did cause
a significant increase in the frequency of sister chromatid exchange
in CHO cells in both the presence and absence of rat liver S9
(Galloway et al., 1987).
Hexachlorobutadiene was found to induce unscheduled DNA
synthesis (UDS) in Syrian hamster embryo fibroblast cultures.
Moreover, the magnitude of the response was increased when a
preincubation period with rat liver S15 was employed (Schiffman et
al., 1984). However, there was no induction of UDS in a study
using rat hepatocyte cultures (Stott et al., 1981).
In summary, the Task Group concluded that hexachloro-butadiene
was genotoxic in vitro and that the negative results reported in
some studies may have resulted from the use of inappropriate
conditions for metabolic activation.
8.6.2 In vivo effects
Hexachlorobutadiene induced a significant increase in the
frequency of chromosomal aberrations in mouse bone marrow cells
following the administration of acute oral doses of 2 or 10 mg/kg
body weight or acute inhalation exposure to 10 mg/m3 for 4 h. Both
experiments used six mice per dose group, and the animals were
sacrificed after 24 h (German, 1988).
Six hours after the administration of a single oral dose of
20 mg hexachlorobutadiene/kg body weight to two groups of five male
Sprague-Dawley rats, there were statistically significant increases
in kidney UDS of 27% and 54% above concurrent control levels.
Administration of a positive control substance,
dimethyl-nitrosamine, resulted in an increase of 187% over controls
(Stott et al., 1981).
As described in section 6.3, radiolabelled nucleotides were
recovered from the kidneys of rats and mice administered
14C-labelled hexachlorobutadiene by gavage (Stott et al., 1981;
Schrenk & Dekant, 1989). The Task Group concluded that these studies
indicated covalent binding of hexachlorobutadiene or its metabolites
Table 13. Tests for reverse mutations in Salmonella typhimurium TA100 by proposed metabolites of hexachlorobutadiene
Metabolite and abbreviationa Conditionsb Resultc Reference
1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (GPB) no activation - Green & Odum (1985)
+ rat kidney S9 +
+/- rat kidney fractions +d Vamvakas et al. (1988a)
1,4-(bis-glutathion- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BGTB)
1,4-(bis-cystein- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BCTB)
1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (CPB) +/- rat kidney S9 +e Green & Odum (1985)
no activation +f Dekant et al. (1986)
1-( N-acetylcystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene - rat liver S9 - Wild et al. (1986)
(ACPB) + rat liver S9 +g
1-carboxymethylthio-1,2,3,4,4-pentachloro-1,3-butadiene + rat liver S9* - Wild et al. (1986)
(CMTPB)
Table 13 (contd).
Metabolite and abbreviationa Conditionsb Resultc Reference
1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) + rat liver S9 - Wild et al. (1986)
2,2,3,4,4-pentachloro-3-butenoic acid (PBA) +/- rat liver S9 + Reichert et al. (1984)
2,2,3,4,4-pentachloro-3-butenoic acid chloride (PBAC) +/- rat liver S9 + Reichert et al. (1984)
a See also Figure 1
b Plate incorporation assays with, except in the case of the tests by Green & Odum, preincubation; S9* = a fortified S9 mix containing
3 times the normal protein concentration
c + = > twice background rate; - = negative
d Mutagenic potency enhanced by rat kidney microsomes or mitochondria and less so by cytosol; positive results were inhibited by the
ß-lyase inhibitor aminooxyacetic acid
e Mutagenic potency enhanced by rat kidney S9
f Positive results were inhibited by the ß-lyase inhibitor aminooxyacetic acid
g 18.7 revertants per nmol; mutagenic potency decreased by addition of pyridoxal phospate; activation by cytosol, with and without cofactors,
had the same results as S9 mix; microsome mix was inactive
to kidney DNA in vivo. The study with mice showed that the level
of binding to mitochondrial DNA was greater than that to nuclear
DNA. In addition, radioactivity was recovered in mitochondrial DNA,
but not nuclear DNA, from mouse liver (Schrenk & Dekant, 1989).
Hexachlorobutadiene did not induce sex-linked recessive lethal
mutations in Drosophila melanogaster following treatment of adults
either via the diet or by injection (Woodruff et al., 1985).
8.7 Carcinogenicity/long-term toxicity
8.7.1 Inhalation exposure
No long-term carcinogenicity studies, where inhalation was the
route of exposure, have been reported.
8.7.2 Oral exposure
In a study by Kociba et al. (1977a,b), groups of 39-40
adult Sprague-Dawley rats of each sex received a diet containing
hexachlorobutadiene at 0.2, 2 or 20 mg/kg body weight per day for 22
(males) or 24 (females) months. Control groups comprised 90 rats of
each sex. Analysis for the compound was not reported. An increased
mortality was observed in males at 20 mg/kg. Hexachlorobutadiene
caused a depression of the body weight gain in both sexes at the
highest dose level without any effect on food consumption.
Haematological investigations performed at 12-14 and 22-24 months,
revealed a slight, but statistically significant, depression in the
red blood cell count of males at 20 mg/kg (22 months). Urinalysis at
12-14 months and 22-24 months did not reveal effects except for a
small increase in coproporphyrin excretion. The analysis of the
clinical chemistry parameters of blood urea nitrogen, serum alanine
aminotransferase (EC 2.6.1.2) and serum alkaline phosphatase
(EC 3.1.3.1) at 12 months revealed no treatment-related effects,
except for statistically significant decreases in serum alanine
aminotransferase in males of the 20-mg/kg dose group and females of
the 0.2- or 20-mg/kg dose groups. These changes were considered by
the authors to be of questionable toxicological significance. The
relative kidney weights were elevated at 20 mg/kg for both sexes, as
were the relative weights of the brain in females and of the testes
in males. In both sexes, an extensive histopathological examination
revealed tubular epithelial hyperplasia at 2 and 20 mg/kg, but not
at 0.2 mg/kg, and an increased incidence of renal tubular neoplasms
at 20 mg/kg (see Table 14) (Kociba et al., 1977a,b).
8.7.3 Dermal exposure
In a study by Van Duuren et al. (1979), groups of 30 female
Ha:ICR Swiss mice received 6.0 mg hexachlorobutadiene in acetone
applied 3 times per week to the shaven dorsal skin for between 144
and 594 days. A group of 100 untreated females were included in the
study, together with 30 controls which received acetone only. The
study duration was described as being between 440 and 594 days.
Sections of skin, liver, stomach and kidney were sampled at autopsy,
but no increase in the number of distant tumours was observed.
In a two-stage initiation-promotion experiment, each of
20 female Swiss mice received one application of 15.0 mg
hexa-chlorobutadiene in acetone to the dorsal skin. After 14 days,
the mice similarly received 5 µg of the tumour promoter
12- o-tetra-decanoylphorbol-13-acetate (TPA) three times weekly for
between 428 and 576 days. Hexachlorobutadiene administration did not
induce a significant increase in the fraction of mice developing
skin papillomas in this study (Van Duuren et al., 1979).
Table 14. Renal tubular neoplasms in rats after long-term
exposure to hexachlorobutadienea
Dose (mg/kg Sex Incidence of renal tubular neoplasms
body weight
per day) adenoma adenocarcinoma total
0 males 1/90 0/90 1/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 2/39 7/39 9/39 (P < 0.05)
0 females 0/90 0/90 0/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 3/40 3/40b 6/40 (P < 0.05)
a From: Kociba et al. (1977a)
b One of these was an undifferentiated carcinoma
8.7.4 Exposure by other routes
In a study of repeated exposure to hexachlorobutadiene by ip
injection, groups of 20 A/St strain male mice (from 6 to 8 weeks of
age) received 12 or 13 ip injections of hexachlorobutadiene (4 or
8 mg/kg body weight) in tricaprylin, respectively. The purity of the
hexachlorobutadiene was stated to exceed 99.9%. Urethane was used as
a positive control for carcinogenesis, and a negative control group
of 50 mice receiving tricaprylin only. Survival was 95% for mice
receiving 4 mg/kg and 70% for mice receiving 8 mg/kg, compared to
92% for controls. Mice were sacrificed at 24 weeks after the first
injection, and the number of surface adenomas in the lungs was
counted. No significant increase in adenomas, compared to the
vehicle-treated control, was observed (Theiss et al., 1977). The
Task Group noted major deficiencies of this study; including the
choice of a sensitive strain of mice, the short duration of both the
exposure period (4 weeks) and the follow-up period (24 weeks), the
small group sizes of the experimental animals, the unusual route of
administration, and the limited histopathology. The strain A mice
used in this study are highly predisposed to spontaneous lung
cancer, which is likely to have further compromised the value of the
study.
8.8 Other special studies
8.8.1 Effects on the nervous system
Acute high exposure to hexachlorobutadiene has a depressant
effect on the central nervous system (see sections 8.1.1.2 and
8.1.2).
Subchronic exposure of rats at high dose levels (1500 mg/kg
diet for 13 weeks) also produced some signs of neurotoxicity, which
was associated with demyelinization and fragmentation of femoral
nerve fibres (Harleman & Seinén, 1979; see also Badaeva et al.,
1985, section 8.5.2).
8.8.2 Effects on the liver
8.8.2.1 Acute effects
Hexachlorobutadiene causes hydropic changes in the liver of
rats (Gradiski et al., 1975; Lock & Ishmael, 1981; Lock et al.,
1982), mice (Lock et al., 1985), and rabbits (Duprat & Gradiski,
1978), sometimes accompanied by fat accumulation (Duprat & Gradiski,
1978; Lock & Ishmael, 1981; Lock et al., 1982).
Male rats, exposed to a single intraperitoneal dose of
hexachlorobutadiene (200 or 300 mg/kg body weight) in corn oil
showed increased relative liver weights, mitochondrial swelling in
liver and bile duct, proliferation of smooth endoplasmic reticulum,
lipid accumulation, and increased water content in the liver.
Biochemical changes in the liver were a decrease, followed after 1
day by an increase, in non-protein sulfhydryl (NP-SH) concentration,
and an increase in potassium content. All effects were reversible
within 10 days. Increases in plasma urea and alkaline phosphatase
(EC 3.1.3.1.) were also reported. In a separate experiment, the
highest dose administered by ip injection which did not cause an
increased water content in the liver was 25 mg/kg body weight (Lock
et al., 1982).
Male rats exposed to single intraperitoneal doses up to
100 mg/kg body weight showed an increase in serum bile acids and
bilirubin (Bai et al., 1992).
Male mice, exposed to single intraperitoneal doses of 50, 100
and 200 mg/kg body weight in corn oil, showed a dose-related
increase in relative liver weight at 100 and 200 mg/kg, and, at all
dose levels, dose-related, reversible changes in the liver
(mitochondrial swelling, proliferation of smooth endoplasmic
reticulum, and an increased water content). Reversible biochemical
changes included increases in sodium and potassium content, NP-SH
concentration in the liver, and serum alanine aminotransferase
activity (EC 2.6.1.2) at 50 mg/kg (Lock et al., 1985).
8.8.2.2 Short-term effects
As discussed in section 8.2.2.1, slight hepatotoxic effects
have been observed following oral exposure of rats (Kociba
et al., 1971; Harleman & Seinen, 1979).
8.8.3 Effects on the kidneys
This section will describe the main features of the renal
toxicity induced by hexachlorobutadiene. For more detail the reader
is referred to the reviews of Rush et al. (1984), Lock (1988),
Yang (1988) and Dekant et al. (1990a).
8.8.3.1 Acute effects
Inhalation exposure of rats produces renal tubular necrosis
(Gage, 1970; see section 8.2.1). Enzyme histochemical
investi-gations were performed on groups of 10 male Swiss OF1 mice
24 h after whole-body inhalation exposure for 4 h to
hexachloro-butadiene at measured concentrations of 29.3, 53.4, 106.7
or 266.8 mg/m3. A concentration-related increase in the percentage
of damaged kidney tubules, which had been stained for alkaline
phosphatase (EC 3.1.3.1), was observed at all exposure levels. The
EC50 was calculated to be 76.8 mg/m3 (De Ceaurriz et al.,
1988).
A single oral dose of hexachlorobutadiene (200 mg/kg body
weight) in polyethylene glycol caused an increase in plasma urea
concentration, a decrease in plasma alanine aminotransferase
activity, and, in urine, increases in the levels of glucose,
protein, alanine aminotransferase, N-acetyl-ß-D-glucosaminidase,
gamma-glutamyltranspeptidase (EC 2.3.2.2) and alanine aminopeptidase
(EC 3.4.11.12) (Nash et al., 1984).
Following in vivo administration, hexachlorobutadiene caused
dose-dependent necrosis of the renal proximal tubules in rats
(Gradiski et al., 1975; Lock & Ishmael, 1979, 1981; Kluwe et al.,
1982; Hook et al., 1982, 1983; Ishmael et al., 1982; Ishmael &
Lock, 1986), mice (Ishmael et al., 1984) and rabbits (Duprat &
Gradiski, 1978). In rats, the lesions were restricted to the pars
recta (S3-segment) and were macroscopically observed as a distinct
band of damage in the outer stripe of the medulla (Lock & Ishmael,
1979; Ishmael et al., 1982). In mice and rabbits both the pars
recta and the pars convoluta of the proximal convoluted tubules were
damaged (Duprat & Gradiski, 1978; Ishmael et al., 1984). The
lesion is characterized microscopically by necrotic epithelial
cells, most of which are devoid of nuclei. The few remaining nuclei
show karyorrhexis, and the cytoplasm is strongly eosinophilic. Many
renal tubules contained cellular debris (Duprat & Gradiski, 1978;
Lock & Ishmael, 1979; Lock et al., 1984; Ishmael et al., 1984).
Vacuolation of the pars convoluta was observed (Duprat & Gradiski,
1978; Ishmael et al., 1982, 1984). Mitochondrial swelling and loss
of brush-borders were prominent ultrastructural findings (Ishmael
et al., 1982, 1984).
Adult male rats have been found to be less sensitive to the
renal toxicity induced by hexachlorobutadiene than adult females and
young males (Hook et al., 1983; Kuo & Hook, 1983). When male rats
were dosed intraperitoneally with a single dose of 300 mg/kg in corn
oil, the earliest pathological change was mitochondrial swelling in
proximal tubular cells observed after 1-2 h. Extensive necrosis was
evident between days 1 and 4, and active regeneration by day 5
(Ishmael et al., 1982). Similar renal toxicity was seen at a dose
level of 25 or 50 mg/kg body weight in female rats and young males,
respectively. A similar pattern of pathological changes with
comparable intensity was observed in mice at an intraperitoneal dose
of 50 mg/kg body weight (Ishmael et al., 1984). In a study by Lock
et al. (1984), young mice were found to be more susceptible than
adults, but no sex difference was apparent. In both rats and mice,
differences in strain susceptibility were observed (Hook et al.,
1983; Lock et al., 1984). The lowest intraperitoneal dose at which
renal necrosis was observed in adult female rats was 25 mg/kg body
weight (Lock & Ishmael, 1985) and in adult male and female mice was
6.3 mg/kg body weight (Lock et al., 1984).
Biochemical changes found following intraperitoneal exposure in
both rats and mice were increases in renal water content (Kluwe et
al., 1982; Ishmael et al., 1982, 1984; Gartland et al., 1989),
plasma urea (Lock & Ishmael, 1979, 1981; Ishmael et al., 1982,
1984; Hook et al., 1983; Lock et al., 1984; Ishmael & Lock,
1986; Stonard et al., 1987; Gartland et al., 1989), plasma
alkaline phosphatase (EC 3.1.3.1.) (Lock & Ishmael, 1981), serum
alanine aminotransferase (EC 2.6.1.2) (Gradiski et al., 1975; Kuo
& Hook, 1983) and serum aspartate aminotransferase (Gradiski et
al., 1975; Davis et al., 1980). In the urine of rats, increases
in urinary protein, glucose and ketones have been measured (Lock &
Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al., 1980;
Stonard et al., 1987), as well as increases in the activities of
alkaline phosphatase and N-acetyl-ß-D-glucosaminidase (EC
3.2.1.50) (Lock & Ishmael, 1979; Stonard et al., 1987) and in
lactic acid level (Gartland et al., 1989). In the kidneys of rats,
increased sodium concentrations were accompanied by equally
decreased potassium concentrations (Davis et al., 1980). All these
changes occurred at similar or higher intraperitoneal doses than
those at which renal necrosis was observed.
Distinct renal functional changes in adult rats have been
observed at intraperitoneal doses of 100-400 mg/kg body weight.
These include a decrease in urine-concentrating ability (polyuria)
(Lock & Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al.,
1980; Stonard et al., 1987), a reduced glomerular filtration rate
(Davis et al., 1980) and a reduction of in vivo renal clearance
of inulin, urea, p-aminohippuric acid (PAH), tetraethyl-ammonium
bromide (TEA) (Lock & Ishmael, 1979) and imipramine (Davis et al.,
1980). When organic ion transport was assessed in vitro in renal
cortical slices of rats and mice that had been exposed to an
intraperitoneal dose of 100 mg/kg, 200 mg/kg body weight or more,
the transport of anions (PAH) was found to be reduced, but the
transport of the cation (TEA), aminoisobutyrate was not (or was only
slightly reduced) in rats (Lock & Ishmael, 1979; Berndt & Mehendale,
1979; Kluwe et al., 1982; Hook et al., 1982, 1983). In male
adult mice, transport of PAH and TEA was reduced from
intraperitoneal doses of 12.5 and 25.0 mg/kg body weight,
respectively. The anion transport was reduced in adult females (Lock
et al., 1984).
8.8.3.2 Short- and long-term effects
The short- and long-term effects of hexachlorobutadiene on the
kidneys of experimental animals have already been discussed in
sections 8.2 , 8.5 and 8.7. Based on the studies of Kociba et al.
(1971, 1977a,b), Schwetz et al. (1977), Harleman & Seinen (1979),
Stott et al. (1981) and Yang et al. (1989), the oral NOAEL for
renal toxicity is 0.2 mg/kg body weight per day. Results of the
studies are summarized in Table 15. Female rats and mice were found
to be distinctly more susceptible than males upon oral exposure for
13 weeks (Yang et al., 1989; Yang, 1991); this was also observed
in the single-exposure mortality studies (section 8.1).
8.9 Factors modifying toxicity; toxicity of metabolites
8.9.1 Factors modifying toxicity
8.9.1.1 Surgery
Complete protection from the nephrotoxic effects of
hexachlorobutadiene was observed in rats that had been fitted with a
biliary cannula before being given a single oral dose of 200 mg/kg
body weight. Administration of bile, collected from rats dosed
orally with the compound, to naive rats produced marked renal
toxicity but no liver toxicity (Nash et al., 1984).
8.9.1.2 Inhibitors and inducers of mixed-function oxidases (MFO)
In the majority of studies, the effects of MFO inhibitors
(piperonyl butoxide, SKF 525A) and MFO inducers (Aroclor 1254,
isosafrole, ß-naphthoflavone, phenobarbitone) on the nephro-toxicity
induced by hexachlorobutadiene in rats and mice were absent or
negligible (Lock & Ishmael, 1981; Hook et al., 1982; Lock et al.,
1984; Davis, 1984). Furthermore, phenobarbitone pretreatment for 7
days at 0.05% in drinking-water enhanced the renal toxicity induced
by intraperitoneal doses of hexachloro-butadiene in weanling rats
(Hook et al., 1983).
8.9.1.3 Inhibitors of gamma-glutamyltranspeptidase (EC 2.3.2.2)
Male rats pretreated with Acivicin (L-(alphaS, 5S)-
alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid), an
inhibitor of gamma-glutamyltranspeptidase (down to 3% of control
activity in this study), and subsequently exposed intraperitoneally
to hexachlorobutadiene, did not show a decrease in nephrotoxicity
compared to rats treated with hexachlorobutadiene alone. It was
concluded that gamma-glutamyltranspeptidase inhibition did not limit
the formation of nephrotoxic metabolites (Davis, 1988).
Male Swiss OF1 mice, pretreated with Acivicin and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group noted that only one marker for nephrotoxicity was employed in
this study.
Table 15. No-observed-adverse-effect level (NOAEL) calculated from short-term and long-term
studies of exposure to hexachlorobutadiene by oral administration
Species (Strain) Age Sex Number of Duration of Dose (mg/kg NOAEL (mg/kg References
animals study body weight body weight
per group per day) per day)
Rat (Wistar-derived) weanling male 6 2 weeks 7.3, 18.2, 44.7 < 7.3 Harleman & Seinen
female 6 (1979)
Rat (Sprague-Dawley) adult male 5 3 weeks 0.2, 20 0.2 Stott et al. (1981)
Rat (Sprague-Dawley) adult female 4 30 days 1, 3, 10, 30, 65, 100 3 Kociba et al. (1971)
Rat (Sprague-Dawley) adult male 10-12 3 months 0.2, 2.0, 20 0.2 Schwetz et al.
female 20-24 (1977)
Rat (Sprague-Dawley) adult male 39-40 24 months 0.2, 2.0, 20 0.2 Kociba et al.
female 39-40 (1977a,b)
Mouse (B6C3F1) adult male 5 2 weeks 4.3, 14.3, 43, 143, 430 < 4.3 Yang et al.
female 5 (1989); Yang (1991)
Mouse (B6C3F1) adult male 10 13 weeks 0.1, 0.4, 1.5, 4.9, 18.8 1.5 Yang et al.
female 10 0.2, 0.5, 1.8, 4.5, 19.2 < 0.2 (1989); Yang (1991)
8.9.1.4 Inhibitors of cysteine conjugate ß-lyase
Male Swiss OF1 mice, pretreated with the two ß-lyase inhibitors
amino-oxyacetic acid (AOAA) and DL-propargylglycine (PPG) and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group again noted that only one marker for nephrotoxicity was
employed in this study.
8.9.1.5 Inhibitors of organic anion transport
Pre-treatment of male rats with probenecid
[(4-(dipropyl-amino)sulfonyl)] benzoic acid (105 µmol/kg body
weight), an inhibitor of organic anion transport, did not alter the
increase in plasma urea or decrease in renal clearance of
p-aminohippuric acid induced by hexachlorobutadiene (Hook et al.,
1982). However, in female rats, a higher dose (500 µmol/kg body
weight) of probenecid totally protected against the renal toxicity,
both functional and morphological, produced by ACPB (Lock & Ishmael,
1985). In addition this dose of probenecid protected female rats
against the toxic effects produced by CPB and GPBN as well as the
parent chemical (Lock & Ishmael, 1985). Male mice pre-treated with
probenecid were also protected against the nephrotoxicity produced
by hexachlorobutadiene (Ban & De Ceaurriz, 1988). The Task Group
noted that this latter study used only one marker for
nephrotoxicity.
8.9.1.6 Non-protein sulfhydryl scavengers
Depletion of hepatic and renal non-protein sulfhydryl content
(glutathione) by diethylmaleate in rats appears to potentiate the
nephrotoxicity of hexachlorobutadiene as measured by a number of
functional markers such as plasma urea (Hook et al., 1982; Baggett
& Berndt, 1984, Davis et al., 1986). However, the Task Group noted
that no information was available on the metabolism of
hexachlorobutadiene to help interpret these studies.
8.9.2 Toxicity of metabolites
This section discusses the renal toxicity of some metabolites
of hexachlorobutadiene, the formation of which was discussed in
section 6.2. These metabolites are 1-(glutathion- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (GPB), 1-(cystein- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (CPB), and 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB). Their mutagenic activity
was described along with that of hexachlorobutadiene in section 8.6.
8.9.2.1 In vitro studies
GPB decreased the viability of isolated renal epithelial cells
of male rats, as measured by leakage of lactate dehydrogenase (EC
1.1.1.27), with a very steep dose-response curve and a lag period of
30 min. No cytotoxicity was observed when the GPB metabolism was
blocked by anthglutin, an inhibitor of gamma-glutamyl-transpeptidase
(EC 2.3.2.2) or amino-oxyacetic acid (AOAA), an inhibitor of renal
cysteine conjugate ß-lyase (EC 4.4.1.13). The cytotoxicity of GPB
was related to an impairment of mitochondrial function, as shown by
loss of mitochondrial Ca2+ and ATP and inhibition of respiration
and thiol depletion (Jones et al., 1986b). Likewise, GPB produced
a concentration-dependent nephro-toxicity in the isolated perfused
rat kidney, as indicated by the appearance in the urine of alkaline
phosphatase, gamma-glutamyl-transpeptidase and glucose (Jones et
al., 1986a). These changes were prevented by Acivicin and by AOAA
(Schrenk et al., 1988a).
In the study of Schrenk et al. (1988a), CPB also caused a
marked nephrotoxicity in the isolated perfused kidney, which could
be prevented by AOAA. In isolated rabbit renal tubules, CPB was
observed to decrease the accumulation of p-amino-hippuric acid and
tetraethylammonium (Jaffe et al., 1983), to affect mitochondrial
function as shown by effects on cell respiration, and to decrease
the glutathione content and, after a lag period of 60 min, cell
viability (Schnellmann et al., 1987). The effects on respiration
resulted initially from the uncoupling of oxidative phosphorylation,
followed later by inhibition of state 3 respiration (Schnellmann et
al., 1987). Impaired mitochondrial function was observed in
CPB-exposed isolated rat renal cortical mitochondria as an inability
to retain Ca2+, collapse of the membrane potential, impaired state
3 respiration with succinate as substrate, and nonenzymatic
depletion of thiol content. The latter effect was blocked by AOAA.
From these results it was concluded that the reactive intermediate
formed from CPB interacts with the inner mitochondrial membrane
(Wallin et al., 1987). CPB also inhibited rat kidney mitochondrial
DNA, RNA and protein synthesis, and AOAA blocked this effect.
Moreover, CPB converted supercoiled DNA to relaxed circular DNA and
shorter linear fragments (Banki & Anders, 1989). Chen et al.
(1990) observed a decreased viability of isolated human renal
proximal tubular cells upon exposure to CPB, which was again blocked
by AOAA. Using radiolabelled ACPB and rat renal cortical slices, it
was established that ACPB is transported by the same renal
mechanisms involved in the movement of many organic anions into
tubular fluid. This carrier-mediated transport is reduced by
specific inhibitors like probenecid and sulfinpyrazone, a
competitive and metabolic inhibitor like 2,4-dinitrophenol, and the
transport substrate p-aminohippuric acid (Lock et al., 1986).
This was confirmed by recent studies on the mechanism of uptake of
GPB and CPB in the isolated perfused rat kidney (Schrenk et al.,
1988b). Probenecid has also been reported to protect renal proximal
tubular cells against ACPB-induced cytotoxicity, as determined by
monitoring proline incorporation into renal proteins (Bach et al.,
1986).
8.9.2.2 In vivo studies
A single oral dose of 138 mg/kg body weight (0.27 mmol) of GPB
or a single equimolar oral dose of 100 mg ACPB/kg body weight in
polyethylene glycol to male rats caused marked nephrotoxicity
similar in both biochemical and histopathological aspects to that
observed with an oral dose of 200 mg/kg body weight (0.97 mmol) of
hexachlorobutadiene (Nash et al., 1984). When rats received
intraperitoneally GPB, CPB or ACPB in polyethylene glycol at single
doses between 6.25 and 100 mg/kg body weight, increases in plasma
urea level and renal proximal tubular necrosis were observed at dose
levels of > 6.25 mg/kg body weight in females and 10 or 12.5 mg/kg
body weight in males. The conjugates exhibited a similar pattern of
nephrotoxicity at equimolar doses and were more nephrotoxic than the
parent compound (Lock & Ishmael, 1985; Ishmael & Lock, 1986). All
compounds tested were more toxic to female rats than males (Ishmael
& Lock, 1986). Probenecid pretreatment protected the rats against
the nephrotoxicity of these metabolites. Probenecid was shown to
block the active tubular secretion of ACPB and to reduce the extent
of covalent binding to renal protein (Lock & Ishmael, 1985). In
mice, GPB and ACPB were also shown to be more toxic than the parent
compound: renal necrosis was found following single intraperitoneal
doses of 5.0 mg hexachlorobutadiene/kg body weight, 3.1 mg GPB/kg
body weight and 3.0 mg ACPB/kg body weight in corn oil, which were
the lowest doses tested (Lock et al., 1984). A single
intraperitoneal dose of 10 mg CPB/kg body weight in DMSO and water
caused dose-related damage in the pars recta of renal proximal
tubules in male mice (Jaffe et al., 1983).
The nephrotoxicity of some structural analogues of the
above-mentioned conjugates, e.g. S-(1,2-dichlorovinyl)-L-cysteine,
has been investigated extensively and has revealed a remarkable
similarity (Anders et al., 1987; Lock, 1988).
8.10 Mechanisms of toxicity - mode of action
8.10.1 Mechanisms of toxicity
The following evidence supports the hypothesis that the
nephrotoxicity, mutagenicity and carcinogenicity of
hexachloro-butadiene is dependent on the biosynthesis of the toxic
sulfur conjugate GPB. This conjugate is mainly synthetized in the
liver and further metabolized in the bile, gut, and kidneys to the
CPB. Cysteine conjugate ß-lyase-dependent activation of CPB to a
reactive thioketene in the proximal tubular cells finally results in
covalent binding to cellular macromolecules.
1. The nephrotoxicity of hexachlorobutadiene in rats was prevented
by the implantation of a biliary cannula; administration of
bile from hexachlorobutadiene-treated rats to naive rats
resulted in nephrotoxicity identical to the nephrotoxicity
caused by hexachlorobutadiene (see section 8.9.1.1).
2. Inhibitors of renal organic anion transport protected rats
against the nephrotoxicity of hexachlorobutadiene and its
sulfur conjugates. Inhibition of the organic anion transport
also protected isolated kidney cells against the nephrotoxicity
of hexachlorobutadiene-derived sulfur-conjugates (see sections
8.9.1.3 and 8.9.2.1).
3. Anthglutin, Acivicin and aminooxyacetic acid, specific
inhibitors of gamma-glutamyltranspeptidase and cysteine
conjugate ß-lyase protected against the cytotoxicity of
hexachloro-butadiene-derived sulfur-conjugates in freshly
isolated rat renal proximal tubular cells (see section
8.9.2.1).
4. Synthetic sulfur-conjugates of hexachlorobutadiene show a
higher nephrotoxicity than the parent compounds in rats and
mice and produce renal damage identical to the renal damage
induced by hexachlorobutadiene, based on clinical chemistry and
histopathological examination (see section 8.9.2.2).
5. Hexachlorobutadiene and its sulfur-conjugates are genotoxic in
bacteria; bioactivation by glutathione conjugation is required
for hexachlorobutadiene genotoxicity. The ultimate mutagen is
formed by cysteine conjugate ß-lyase-dependent cleavage of CPB
(see section 8.6).
6. Hexachlorobutadiene induces renal tumours in rats only at doses
that produce marked nephrotoxicity (see sections 8.7 and
8.8.2.2).
8.10.2 Mode of action
The in vitro studies of Jones et al. (1986b), Wallin et
al. (1987) and Schnellmann et al. (1987) on the cytotoxicity of
sulfur-conjugates to renal tubular cells (section 8.9.1.2) point to
renal cortical mitochondria as the major target for
sulfur-conjugates of hexachlorobutadiene, analogous to that
established for close structural analogues (Dekant et al., 1990b).
The hypothesis proposes an interaction of the reactive metabolite
with the inner mitochondrial membrane, which ultimately causes
respiratory insufficiency.
9. EFFECTS ON HUMANS
9.1 General population exposure
Hexachlorobutadiene has been found in postmortem examinations,
but not in living persons. No pathogenic effects have been recorded
(see section 5.2).
9.2 Occupational exposure
Two reports on certain disorders among agricultural workers in
vineyards where hexachlorobutadiene has been used as a fumigant
(Krasniuk et al., 1969; Burkatskaya et al., 1982) cannot be
evaluated, since such workers are known to be occupationally exposed
to additional substances.
In two cytogenetic studies of occupationally exposed workers
from the same plant engaged in the production of
hexachloro-butadiene, an increase in the frequency of chromosomal
aberrations in peripheral blood lymphocytes was observed (German,
1986). The workers were exposed to hexachloro-butadiene
concentrations that ranged from 1.6 to 16.9 mg/m3. The Task Group
noted that exposure concentrations were determined by the factory
and that the frequency of chromosome aberrations was not associated
with the period of employment.
9.3 In vitro metabolism studies
The following studies have been reported:
a) Purified human liver microsomal glutathione- S-transferase
and human liver cytosol metabolize hexachlorobutadiene to form
GPB (McLellan et al., 1989; Oesch & Wolf, 1989).
b) The enzyme cysteine conjugate ß-lyase has been isolated and
purified from human kidney cytosol (Lash et al., 1990). The
activity of the human cytosolic enzymes with a structurally
related compound (1,2,2-trichlorovinyl-L-cysteine) is about
10-fold lower than that of rat renal cytosol (Green et al.,
1990).
c) Studies in isolated human proximal tubular cells have shown
that CPB causes a ß-lyase-dependant cytotoxicity (Chen et al.,
1990).
These limited studies suggest that humans have the ability to
metabolize hexachlorobutadiene to toxic metabolites.
9.4 Extrapolation of NOAEL from animals to humans
Conversion of equivalent doses across species can utilize
allometric relationships that relate physiological and anatomical
variables across species. Physiological and metabolic rates have
been shown to relate closely to body weight to the power 0.75
(Boxenbaum, 1982). The equivalent NOAEL in humans (mg/kg body weight
per day) can be determined from the following equation:
Wa
dh = da (--)0.25
Wh
where
d = dose rate (mg/kg body weight per day) in humans (dh) or
animals (da)
wa = weight (kg) of animals (mice 30 g; rats 400 g)
wh = weight of humans (70 kg)
The NOAEL for humans, based on the NOAEL in mice, is:
dh= (0.2 mg/kg body weight per day) (0.03)0.25
70
= 0.03 mg/kg body weight per day
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Hazard identification
The following evaluation is based on toxicity studies in
experimental animals; however, there are some human in vitro data
which indicate that hexachlorobutadiene metabolism can occur by a
similar route to that shown in experimental animals.
Hexachlorobutadiene is slightly to moderately toxic based on
acute oral experiments with adult rats, and moderately to highly
toxic based on acute oral experiments with weanling rats (based on
the WHO pesticide toxicity classification). The specific toxicity of
the compound to the kidneys is higher for females than males.
Following acute dermal exposure of rabbits, the compound was found
to be weakly toxic.
Regardless of species studied and the route of exposure (ip,
oral, inhalation, dermal) in both short- and long-term studies, the
target organ for toxicity is the kidney. Bioactivation to produce a
reactive sulfur metabolite occurs following conjugation with
glutathione. The monoglutathione conjugate of hexachloro-butadiene
is processed to the cysteine- S-conjugate, which is then a
substrate for renal cysteine conjugate ß-lyase. Hexachloro-butadiene
produces a dose-dependent necrosis of the renal proximal tubules,
which is followed by regenerative and/or proliferative changes. On
the basis of both short- and long-term studies in rats and mice
orally exposed to hexachlorobutadiene, the no-observed-adverse-
effect level (NOAEL) is 0.2 mg/kg body weight per day. In one
short-term inhalation study (12 days, 6 h/day), the NOAEL was
53 mg/m3.
The vapour of hexachlorobutadiene was found to be irritating to
the eyes and nose of rats in one short-term inhalation study. The
undiluted compound appeared corrosive in an experiment with rabbits.
Based on these limited data, the vapour should be regarded as
irritating to human mucous membranes and the liquid should be
regarded as corrosive.
In a well-conducted Magnusson-Kligman test hexachloro-butadiene
was a sensitizing agent both with and without adjuvant. Therefore,
the compound should be regarded as a sensitizing agent for humans.
In reproductive studies, reduced birth weight and neonatal
weight gain in rats were observed, but these effects may be
attributed to maternal toxicity. Developmental toxicity to rat
fetuses was observed in two teratogenicity tests, but again only at
levels that were also toxic to the dams. This developmental toxicity
included reduced birth weight, a 1- to 2-day delay in heart
development, and dilated ureters, but no gross abnormalities were
observed.
In vitro studies have shown that hexachlorobutadiene and, to
a much greater extent, its sulfur metabolites induce mutations in
Salmonella typhimurium. In one study of exposure to
hexachloro-butadiene by inhalation or oral administration, an
increased frequency of chromosomal aberrations was observed in mouse
bone marrow cells. There is limited evidence for the genotoxicity of
hexachlorobutadiene in animals, and insufficient evidence in humans.
The long-term oral administration of hexachlorobutadiene to
rats induced an increased frequency of renal tubular neoplasms, but
only at doses that caused marked nephrotoxicity; at the lowest dose,
no adverse effects were observed.
The Task Group concluded that there is limited evidence for the
carcinogenicity of hexachlorobutadiene in animals (one study in one
rodent strain) and insufficient evidence in humans.
10.1.2 Exposure
Hexachlorobutadiene is mainly a waste product. As such, it can
be encountered in different environmental compartments, but
predominantly in sediment and biota (see also 10.2.1). Exposure of
the general public therefore mainly occurs indirectly via
drinking-water and food of high lipid content. Assuming a maximum
concentration of 2.5 µg/litre in contaminated drinking-water and
10 µg/kg wet weight in contaminated fatty food items (meat, fish,
milk) and daily intakes of 2 litres drinking-water, 0.3 kg meat,
0.2 kg fish and 0.5 kg milk, a maximum total daily intake of
0.2 µg/kg body weight can be calculated for a 70-kg person.
10.1.3 Hazard evaluation
The NOAEL for mice or rats exposed to hexachlorobutadiene is
0.2 mg/kg body weight per day (see Table 15), from which a NOAEL of
0.03-0.05 mg/kg body weight day has been derived for humans (see
section 9.4).
The Task Group considered the margin of safety of 150 between
the estimated NOAEL in humans and the maximum total daily intake
(see section 10.1.2) to be sufficient to protect the general
population against the adverse effects of hexachlorobutadiene.
10.2 Evaluation of effects on the environment
10.2.1 Hazard identification
Hexachlorobutadiene is a chemically stable compound. Complete
aerobic biodegradation has been observed following adaptation of the
inoculum. Partial biodegradation was found to occur in a pilot
sewage treatment plant. Based on these observations and the chemical
structure, it can be concluded that hexachlorobutadiene is not
readily biodegradable, but can be considered to be inherently
biodegradable. Experimental photolysis of hexachlorobutadiene in the
presence of a surface was rapid, but in the absence of a surface the
compound is believed to be persistent. Degradation in the atmosphere
is assumed to occur by a rather slow reaction with hydroxyl
radicals. A half-life of up to 2.3 years has been calculated.
Once hexachlorobutadiene is released into the environment,
intercompartmental transport will occur chiefly by volatilization
from water and soil, adsorption to particulate matter in water and
air, and subsequent sedimentation or deposition. In view of a strong
adsorption potential to organic matter, the compound accumulates in
sediment and will not migrate rapidly in soils. Both field and
laboratory exposure data support these conclusions.
Field and laboratory data also support the high
bioaccumu-lation potential in aquatic and benthic organisms which
can be expected on the basis of the lipophilic nature of the
compound. However, no evidence has been obtained for
biomagnification.
Hexachlorobutadiene is moderately to highly toxic to aquatic
organisms; crustaceans and fish are the most sensitive species. The
lowest E(L)C50 for freshwater organisms is 0.09 mg/litre
(goldfish). The lowest chronic NOEC is 3 µg/litre (goldfish).
Applying the preliminary effect assessment extrapolation procedure,
as adopted in the OECD Workshop on Aquatic Effect Assessment (OECD,
1990), an Environmental Concern Level of 0.1 µg/litre can be
established.
The toxicity data on terrestrial organisms are insufficient to
establish any toxicity threshold.
10.2.2 Exposure
Current environmental levels in surface waters are generally
below 0.2 µg/litre, rising to 1.3 µg/litre in highly polluted
rivers. Levels in the upper sediment can be as high as 120 µg/kg in
heavily polluted rivers or estuaries. In older sediment layers much
higher concentrations can be measured. The concentrations in
freshwater biota measured since 1980 generally do not exceed
100 µg/kg fresh weight, but in a polluted area can reach 120 mg/kg
in the lipid of fish.
10.2.3 Hazard evaluation
It can be concluded that away from point sources the maximum
predicted environmental concentration (PEC) is twice the
extrapolated Environmental Concern Level of 0.1 µg/litre. Aquatic
organisms therefore may be at risk in polluted surface waters.
In view of the rather high concentrations of the compound
measured in some sediments, adverse effects on benthic organisms
cannot be excluded.
Considering the toxicity of the substance to mammals (the NOAEL
for rats or mice is 0.2 mg/kg body weight per day) and its high
bioaccumulating potential, the consumption of benthic or aquatic
organisms in polluted surface water by other species may give cause
for concern. For example, an otter weighing 10 kg and consuming 1 kg
fish per day in waters containing 0.2 µg hexachlorobutadiene/litre
could ingest 1200 µg/day (assuming a bioconcentration factor for
fish of 6000, leading to a concentration of 1200 µg/kg wet weight)
or 120 µg/kg body weight per day, which is above the calculated
NOAEL value for the otter (calculated as in section 9.4).
11. FURTHER RESEARCH
Hexachlorobutadiene is primarily a waste product and hence an
environmental contaminant having only limited use as a fumigant in
some parts of the world. The Task Group identified the following
areas for which additional information is needed:
a) the degradation of hexachlorobutadiene in the environment
focusing on photodegradation and biodegradation;
b) the terrestrial toxicity of hexachlorobutadiene including tests
on benthic organisms;
c) the genotoxic activity of hexachlorobutadiene in vivo. A
further test for micronucleus or chromosome aberration
induction in mouse bone marrow cells would strengthen the
available data;
d) the metabolism of hexachlorobutadiene and its glutathione-
derived conjugates by human liver and renal enzymes and
inter-individual variability.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risk of hexachlorobutadiene was evaluated by
the International Agency for Research on Cancer in 1979 (IARC,
1979).
The summary of data reported and the evaluation of the IARC
monograph on hexachlorobutadiene is reproduced here.
Experimental data
Hexachlorobutadiene was tested in one experiment in rats by
oral administration: it produced benign and malignant tumours in the
kidneys of animals of both sexes. It was tested inadequately in one
experiment in mice by intraperitoneal injection.
Human data
No case reports of epidemiological studies were available to
the Working Group.
The occurrence of hexachlorobutadiene as a by-product in the
production of various chlorinated hydrocarbons for over 50 years and
its use in some areas as a pesticide indicate that widespread human
exposure in both the occupational and general environment occurs.
This is confirmed by reports of its occurrence in the environment.
Evaluation
There is limited evidence that hexachlorobutadiene is
carcinogenic in rats.
REFERENCES
Alberti J (1983) [Organic contaminants in river sediment.] Vom
Wasser, 61: 149-154 (in German).
Alberti J & Plöger E (1986) [Hazardous organic chemicals in sewage
sludge.] Gewasserschutz Wasser Abwasser, 85: 483-498 (in German).
Anders MW, Elfaeea AA, & Lash LH (1987) Cellular effects of reactive
intermediates: nephrotoxicity of S-conjugates of amino acids. Arch
Toxicol, 60: 103-108.
Bach PH, Ketley CP, Ahmed I, & Dixit M (1986) The mechanisms of
target cell injury by nephrotoxins. Food Chem Toxicol, 24: 775-779.
Badaeva LN, Ovsyannikova LM, & Kiseleva NI (1985) [Manifestation of
neurotoxic effect of chloroorganic pesticide hexachlorobutadiene
during postnatal period of ontogenesis in rats.] Arch Anat Gistol
Embriol, 89: 44-49 (in Russian).
Baggett JM & Berndt WO (1984) Renal and hepatic glutathione
concentrations in rats after treatment with hexachloro-1-3-butadiene
and citrinin. Arch Toxicol, 56: 46-49.
Bai CL, Canfield PJ, & Stacey NH (1992) Effects of
hexachloro-1,3-butadiene and 1,1,2,2-tetrachloroethylene on
individual serum bile acids. Toxicol Ind Health, 8: 191-203.
Ban M & De Ceaurriz J (1988) Probenecid-induced protection against
acute hexachloro-1,2-butadiene and methyl mercury toxicity to the
mouse kidney. Toxicol Lett, 40: 71-76.
Banerjee S, Yalkowsky SH, & Valvani SC (1980) Water solubility and
octanol/water partition coefficients of organics. Limitations of the
solubility-partition coefficient correlation. Environ Sci Technol,
14: 1227-1229.
Banki K & Anders MW (1989) Inhibition of rat kidney mitochondrial
DNA, RNA and protein synthesis by halogenated cysteine S-conjugates.
Carcinogenesis, 10: 767-772.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - a
multimedia environmental study. Biomed Mass Spectrom, 7: 139-146.
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow
Pinephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berndt WO & Mehendale HM (1979) Effects of hexachlorobutadiene
(HCBD) on renal function and renal organic ion transport in the rat.
Toxicology, 14: 55-65.
Boxenbaum H (1982) Inter-species scaling, allometry, physiologic
time, and the ground plan of pharmacokinetics. J Pharmacokinet
Biopharm, 10: 201-227.
Bristol DW, Crist HL, Lewis RG, MacLeod KE, & Sovocool GW (1982)
Chemical analysis of human blood for assessment of environmental
exposure to semivolatile organochlorine chemical contaminants. J
Anal Toxicol, 6: 269-275.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds. ACS
Symp Ser, 149: 49-64.
Bringmann G & Kühn R (1977) [Limiting values of the harmful action
of water-endangering substances on bacteria (Pseudomonas putida)
and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z Wasser-Abwasser Forsch, 10: 87-98
(in German).
Brown SL, Chan FY, Jones JL, Liu DH, McCaleb KE, Mill T, Sapios KN,
& Schendel DE (1975) Research program on hazard priority ranking of
manufactured chemicals (chemicals 1-20). Menlo Park, California,
Stanford Research Institute (NSF/RA/E-75/190A; PB 263161).
Burkatskaya EN, Viter VF, Ivanova ZV, Kaskevitch LM, Gorskaya NZ,
Kolpakov IE, & Deineka KA (1982) [Clinico-hygienic data on working
conditions during use of hexachlorobutadiene in vineyards.] Vrach
Delo, 11: 99-102 (in Russian).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13: 296-309.
Canton JH, Linders JBHJ, Luttik R, Mensink BJWG, Panman E, Van de
Plassche EJ, Sparenburg PM, & Tuinstra J (1990) Catching-up
manoeuvre old pesticides: an integration. Bilthoven, The
Netherlands, National Institute of Health and Environmental
Protection (Report No. 678801001).
CESARS (198l) Hexachlorobutadiene, latest update June 1981.
Baltimore, Maryland, Chemical Evaluation Search and Retrieval
System, Inc.
Chen JC, Stevens JL, Trifillis AL, & Jones TW (1990) Renal cysteine
conjugate betalyase-mediated toxicity studied with primary cultures
of human proximal tubular cells. Toxicol Appl Pharmacol, 103:
463-473.
Chiou CT (1985) Partition coefficients of organic compounds in
lipid-water systems and correlations with fish bioconcentration
factors. Environ Sci Technol, 19: 57-62.
Chudin VA, Gafieva ZA, Koshurnikova NA, Nifatov AP, & Revina VS
(1985) [Evaluating the mutagenicity and carcinogenicity of
hexachlorobutadiene.] Gig i Sanit, 5: 79-80 (in Russian).
Clark CS, Meyer CR, Gartside PS, Majeti VA, Specker B, Balistreri
WF, & Elia VJ (1982) An environmental health survey of drinking
water contamination by leachate from a pesticide waste dump in
Hardeman County, Tennessee. Arch Environ Health, 37: 9-18.
Clark JR, Devault D, Bowden RJ, & Weishaar JA (1984) Contaminant
analysis of fillets from Great Lakes coho salmon. J Great Lakes Res,
10: 38-47.
Class T & Ballschmiter K (1987) Global baseline pollution studies.
X. Atmospheric halocarbons: global budget estimations for
tetrachloroethene, 1,2-dichloroethane, 1,1,1,2-tetrachloroethane,
hexachloroethane and hexachlorobutadiene. Estimation of the hydroxyl
radical concentrations in the troposphere of the northern and
southern hemisphere. Fresenius Z Anal Chem, 327: 198-225.
Davis ME (1984) Changes of hexachlorobutadiene nephrotoxicity after
piperonyl butoxide treatment. Toxicology, 30: 217-225.
Davis ME (1988) Effects of AT-125 on the nephrotoxicity of
hexachloro-1,2-butadiene in rats. Toxicol Appl Pharmacol, 95: 44-52.
Davis ME, Berndt WO, & Mehendale HM (1980) Disposition
nephrotoxicity of hexachloro-1,3-butadiene. Toxicology, 16: 179-191.
Davis ME, Berndt WO, & Mehendale HM (1986) Effects of cysteine and
diethylmalate pretreatments on renal function and response to a
nephrotoxicant. Arch Toxicol, 59: 7-11.
De Ceaurriz J & Ban M (1990) Role of gamma-glutamyltranspeptidase
and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and
methyl mercury in mice. Toxicol Lett, 50: 249-256.
De Ceaurriz J, Gagnaire F, Ban M, & Bonnet P (1988) Assessment of
the relative hazard involved with airborne irritants with additional
hepatotoxic or nephrotoxic properties in mice. J Appl Toxicol, 8:
417-422.
Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, & Henschler D
(1986) Bacterial beta-lyase mediated cleavage and mutagenicity of
cysteine conjugates derived from the nephrocarcinogenic alkenes
trichloroethylene, tetrachloroethylene and hexachloro-butadiene.
Chem-Biol Interact, 60: 31-45.
Dekant W, Schrenk D, Vamvakas S, & Henschler D (1988a) Metabolism of
hexachloro-1,3-butadiene in mice: in vivo and in vitro evidence
for activation by glutathione conjugation. Xenobiotica, 18: 803-816.
Dekant W, Vamvakas S, Henschler D, & Anders MW (1988b) Enzymatic
conjugation of hexachloro-1,3-butadiene with glutathione. Formation
of 1-(glutathion- S-yl)-1,2,3,4,4-pentachlorobuta-1,3-diene and
1,4-bis(glutathion-S-yl)-1,2,3,4-tetrachloro-1,3-butadiene. Drug
Metab Dispos, 16: 701-706.
Dekant W, Berthold K, Vamvakas S, & Henschler D (1988c)
Thioacylating agents as ultimate intermediates in the beta-lyase
catalysed metabolism of S-(pentachloro-butadienyl)-L-cysteine.
Chem-Biol Interact, 67: 139-148.
Dekant W, Vamvakas S, & Anders MW (1990a) Bioactivation of
hexachlorobutadiene by glutathione conjugation. Food Chem Toxicol,
28: 285-293.
Dekant W, Vamvakas S, Koob M, Kochling A, Kanhai W, Muller D, &
Henschler D (1990b) A mechanism of haloalkene-induced renal
carcinogenesis. Environ Health Perspect, 88: 107-110.
Dekant W, Urban G, Gorsman C, & Anders MW (1991) Thioketene
formation from alpha-haloalkenyl 2-nitrophenyl disulfides: models
for biological reactive intermediates of cytotoxic S-conjugates. J
Am Chem Soc, 113: 5120-5122.
Demarini DM, Inmon JP, Simmons JE, Berman E, Pasley TC, Warren SH, &
Williams RW (1987) Mutagenicity in Salmonella of hazardous wastes
and urine from rats fed these wastes. Mutat Res, 189: 205-216.
De Meester C, Mercier M, & Poncelet F (1981) Mutagenic activity of
butadiene, hexachlorobutadiene, and isoprene. In: Gut I, Cifert M, &
Plaa GL ed. Industrial environmental xenobiotics. Proceedings of an
International Conference, 1980. Berlin, Heidelberg, New York,
Springer Verlag, pp 196-203.
Dillon HK (1979) Development of air sampling and analytical methods
for toxic chlorinated organic compounds: Research report for
hexachlorobutadiene. Birmingham, Alabama, Southern Research
Institute (SORI-EAS-79-658; PB 87-126934/AS).
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Duprat P & Gradiski D (1978) Percutaneous toxicity of
hexachlorobutadiene. Acta Pharmacol Toxicol, 43: 346-353.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur J Toxicol, 9: 171-177.
Durham RW & Oliver BG (1983) History of Lake Ontario contamination
from the Niagara river by sediment radiodating and chlorinated
hydrocarbon analysis. J Great Lakes Res, 9: 160-168.
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with US EPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 29:
460.467.
Fox ME, Carey JH, & Oliver BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the western
basin of Lake Ontario. J Great Lakes Res, 9: 287-294.
Gäb S, Schmitzer J, Thamm HW, Parlar H, & Korte F (1977)
Photomineralization rate of organic compounds adsorbed on
particulate matter. Nature (Lond), 270: 331-333.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gartland KPR, Bonner FW, & Nicholson JK (1989) Investigations into
the biochemical effects of region-specific nephrotoxins. Mol
Pharmacol, 35: 242-250.
Gauntley P, Magadur JL, Morel G, Chaumont P, & Canel F (1975) Dosage
de l'hexachlorobutadiène dans les milieux biologiques. Eur J
Toxicol, 8: 152-158.
German IV (1986) [Level of chromosome aberrations in worker coming
in contact with hexachlorobutadiene during production.] Gig Tr Prof
Zabol, 5: 57-59 (in Russian).
German IV (1988) [On mutagenic activity of the pesticide
hexachlorobutadiene.] Citol Gen, 22(4): 40-42 (in Russian).
German IV & Viter VF (1985) Evaluation of worker's health in Dactal
and hexachlorobutadiene production processes. Hyg Employ Toxicol
Pestic Polym, 15: 32-34.
Gietl YS & Anders MW (1991) Biosynthesis and biliary excretion of
S-conjugates of hexachlorobuta-1,3-diene in the perfused rat liver.
Drug Metab Dispos, 19: 274-277. Gietl YS, Vamvakas S, & Anders MW
(1991) Intestinal absorption of S-(pentachloro-butadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine,
S-conjugates of hexachlorobuta-1,3-diene. Drug Metab Dispos, 19:
703-707.
Giger W & Schaffner C (1981) Groundwater pollution by volatile
organic chemicals. In: Van Duijvenbooden W, Glasbergen P, & Van
Lelyveld H ed. Quality of groundwater. Proceedings of an
International Symposium, Noordwijkerhout, The Netherlands, 23-27
March 1981. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 517-522 (Studies in Environmental Sciences, Volume
17).
Goldbach RW, Van Genderen H, & Leeuwangh P (1976)
Hexachlorobutadiene residues in aquatic fauna from surface water fed
by the river Rhine. Sci Total Environ, 6: 31-40.
Gradiski D, Duprat P, Magadur JL, & Fayein E (1975) Etude
toxicologique expérimentale de l'hexachlorobutadiène. Eur J Toxicol,
8: 180-187.
Green T & Odum J (1985) Structure/activity studies of the
nephrotoxic and mutagenic action of cysteine conjugates of chloro-
and fluoroalkenes. Chem-Biol Interact, 54: 15-31.
Green T, Odum J, Nash JA, & Foster JR (1990)
Perchloroethylene-induced rat kidney tumors: an investigation of the
mechanisms involved and their relevance to humans. Toxicol Appl
Pharmacol, 103: 80-89.
Guzewich DC, Curtis AB, Valis R, Vindpal JH, & Deeter DP (1983) Air
sampling around a hazardous liquid surface impoundment. 76th Annual
Meeting of the Air Pollution Control Association, Atlanta, USA.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Haberer K, Normann S, & Drews M (1988) [Tests on certain trace
substances from the Rhine and Main with a view to obtaining
drinking-water.] Wasser Abwasser, 129: 45-49 (in German).
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harleman JH & Seinen W (1979) Short-term toxicity and reproduction
studies in rats with hexachloro-(1,3)-butadiene. Toxicol Appl
Pharmacol, 47: 1-14.
Haworth S, Lawloer T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hellmann H (1987a) [Model tests on volatilization of organic trace
substances in surface waters.] Fresenius Z Anal Chem, 328: 475-479
(in German).
Hellmann H (1987b) [Investigation of the degree of concentration of
organic trace substances on clay minerals.] Fresenius Z Anal Chem,
327: 524-529 (in German).
Hermens J, Busser F, Leeuwang P, & Musch A (1985) Quantitative
correlation studies between the acute lethal toxicity of 15 organic
halides to the guppy (Poecilia reticulata) and chemical reactivity
towards 4-nitrobenzylpyridine. Toxicol Environ Chem, 9: 219-236.
Hook JB, Rose MS, & Lock EA (1982) The nephrotoxicity of
hexachloro-1,3-butadiene in the rat: studies of organic anion and
cation transport in renal slices and the effect of monoxygenase
inducers. Toxicol Appl Pharmacol, 65: 373-382.
Hook JB, Ishmael J, & Lock EA (1983) Nephrotoxicity of
hexachloro-1,3-butadiene in the rat: the effect of age, sex, and
strain. Toxicol Appl Pharmacol, 67: 122-131.
Hutzinger O (1982) Handbook of environmental chemistry: 3B.
Anthropogenic sources. Berlin, Heidelberg, New York, Springer
Verlag, pp 69-87.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer, pp 179-189 (IARC Monograph on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
20).
Ishmael J & Lock EA (1986) Nephrotoxicity of hexachlorobutadiene and
its glutathione-derived conjugates. Toxicol Pathol, 14: 258-262.
Ishmael J, Pratt I, & Lock EA (1982) Necrosis of the pars recta (S3
segment) of the rat kidney produced by hexachloro-1,3-butadiene. J
Pathol, 138: 99-113.
Ishmael J, Pratt I, & Lock EA (1984) Hexachloro-1,3-butadiene-
induced renal tubular necrosis in the mouse. J Pathol, 142: 195-203.
Jaffe DR, Hassall CD, Brendel K, & Gandolfi AJ (1983) in vivo and
in vitro nephrotoxicity of the cysteine conjugate of
hexachlorobutadiene. J Toxicol Environ Health, 11: 857-867.
Jacobs A, Blangetti M, & Hellmund E (1974) [Accumulation of
chlorinated contaminants of the Rhine in fat tissue of rats.] Vom
Wasser, 43: 259-274 (in German).
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed, 55:
1441-1449.
Jones TW, Gerdes RG, Ormstad K, & Orrenius S (1985) The formation of
both a mono-and a bis-substituted glutathione conjugate of
hexachlorobutadiene by isolated hepatocytes and following in vivo
administration to the rat. Chem-Biol Interact, 56: 251-267.
Jones TW, Wallin A, Thor H, Gerdes RG, Ormstad K, & Orrenius S
(1986a) The mechanism of pentachlorobutadienyl-glutathione
nephrotoxicity studies with isolated rat renal epithelial cells.
Arch Biochem Biophys, 251: 504-513.
Jones TW, Thor H, & Orrenius S (1986b) In vitro studies of
mechanisms of cytotoxicity. Food Chem Toxicol, 24: 769-773.
Jones TW, Quin C, Schaeffer VH, & Stevens JL (1988)
Immunohistochemical localization of glutamine transaminase K, a rat
kidney cysteine conjugate beta-lyase, and the relationship to the
segment specificity of cysteine conjugate nephrotoxicity. Mol
Pharmacol, 34(5): 621-627.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites n Lake Ontario. J Great Lakes Res, 9:
183-189.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soil. Chemosphere,
10: 833-846.
Kastl PE & Hermann BA (1983) Quantitative gas chromatographic
determination of hexachloro-1,3-butadiene in whole rat blood at part
per trillion levels. J Chromatogr, 280(2): 390-393.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St Clair and Detroit rivers using introduced
clams Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci
Health, A21: 15-53.
Kluwe WM, McNish R, Smithson K, & Hook JB (198l) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane,
tris(2,3-dibromopropyl)phosphate, and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and non-target
organs. Biochem Pharmacol, 30: 2265-2271.
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and
in vitro. J Pharmacol Exp Ther, 230: 597-603.
Kociba RJ, Gehring PJ, Humiston CG, & Sparschu GL (1971) Toxicologic
study of female rats administered hexachlorobutadiene or
hexachlorobenzene for thirty days. Midland, Michigan, Dow Chemical
Company, Chemical Biology Research (Unpublished report).
Kociba RJ, Keyes DG, Jersey GC, Ballard JJ, Dittenber DA, Quast JF,
Wade CE, Humiston CG, & Schwetz BA (1977a) Results of a two year
chronic toxicity study with hexachlorobutadiene in rats. Am Ind Hyg
Assoc J, 38: 589-602.
Kociba RJ, Schwetz BA, Keyes DG, Jersey GC, Ballard JJ, Dittenber
DA, Quast JF, Wade CE, & Humiston CG (1977b) Chronic toxicity and
reproduction studies of hexachloro-butadiene in rats. Environ Health
Perspect, 21: 49-53.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part I: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Koob M & Dekant W (1991) Bioactivation of xenobiotics by formation
of toxic glutathione conjugates. Chem-Biol Interact, 77: 107-136.
Kotzias D, Lay JP, Klein W, & Korte F (1975) [Analysis of residues
of hexachloro-butadiene in foodstuffs and poultry.] Chemosphere, 4:
247-250 (in German).
Krasniuk EP, Ziritskaya LA, Bioko VG, Voitenko GA, & Matokhniuk LA
(1969) [Health condition of vine-growers contacting with fumigants
hexachlorobutadiene and polychlorbutan-80.] Vrach Delo, 7: 111-115
(in Russian).
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuo CH, & Hook JB (1983) Effects of age and sex on
hexachloro-1,3-butadiene toxicity in the Fischer 344 rat. Life Sci,
33: 517-523.
Kusz P, Andriysiak A, & Pokorska Z (1984) Gas chromatographic
monitoring of the chlorolysis processes of some byproducts from
vinyl chloride, allyl chloride and epichlorohydrin production. J
Chromatogr, 286: 287-291.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW,
& Evans RL (1976) An ecological study of hexachlorobutadiene (HCBD).
New Orleans, Louisiana, University of New Orleans, Department of
Biological Sciences (Prepared for the US Environmental Protection
Agency, Office of Toxic Substances, Washington (EPA 560/6-76-010; PB
252671).
Lash LH, Nelson RM, Dyke RA, & Anders MW (1990) Purification and
characterization of human kidney cytosolic cysteine conjugate
beta-lyase activity. Drug Metab Dispos, 18: 50-54.
Leeuwangh P, Bult H, & Schneiders L (1975) Toxicity of
hexachlorobutadiene in aquatic organisms. In: Koeman JH & Strik
JJTWA ed. Sublethal effects of toxic chemicals on aquatic animals.
Proceedings of the Swedish-Netherlands Symposium, Waningen, The
Netherlands, 2-5 September 1975. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 167-176.
Li RT, Going JE, & Spigarelli JL (1976) Sampling and analysis of
selected toxic substances: Task I B. Hexachlorobutadiene. Kansas
City, Missouri, Midwest Research Institute (EPA Contract No.
68-01-2646).
Litvinov PI & Gorenshtein RS (1982) [Migration of
hexachlorobutadiene.] Zashch Rast (Moscow), 8: 33-34 (in Russian).
Lock EA (1987a) Metabolic activation of halogenated chemicals and
its relevance to nephrotoxicity. In: Bach PH & Lock EA ed.
Nephrotoxicity in the experimental and clinical situation.
Dordrecht, Martinus Nijhoff Publishers, pp 429-461.
Lock EA (1987b) The nephrotoxicity of haloalkane and haloalkene
glutathione conjugates. In: DeMatteis F & Lock EA ed. Selectivity
and molecular mechanisms of toxicity. New York, McMillan Publishing
Company, pp 59-83.
Lock EA (1988) Studies on the mechanism of nephrotoxicity and
nephrocarcinogenicity of halogenated alkenes. CRC Crit Rev Toxicol,
19: 23-42.
Lock EA & Ishmael J (1979) The acute toxic effects of
hexachloro-l,3-butadiene on the rat kidney. Arch Toxicol, 43: 47-57.
Lock EA & Ishmael J (1981) Hepatic and renal nonprotein sulfhydryl
concentration following toxic doses of hexachloro-1,3-butadiene in
the rat: the effect of Aroclor 1254, phenobarbitone, or SKF 525A
treatment. Toxicol Appl Pharmacol, 57: 79-87.
Lock EA & Ishmael J (1985) Effect of the organic acid transport
inhibitor probenecid on renal cortical uptake and proximal tubular
toxicity of hexachloro-1,3-butadiene and its conjugates. Toxicol
Appl Pharmacol, 81: 32-42.
Lock EA, Ishmael J, & Pratt I (1982) Hydropic change in rat liver
induced by hexachloro-1,3-butadiene. J Appl Toxicol, 2: 315-320.
Lock EA, Ishmael J, & Hook JB (1984) Nephrotoxicity of
hexachloro-1,3-butadiene in the mouse: the effect of age, sex,
strain, monooxygenase modifiers, and the role of glutathione.
Toxicol Appl Pharmacol, 72: 484-494.
Lock EA, Pratt I, & Ishmael J (1985)
Hexachloro-1,3-butadiene-induced hydropic change in mouse liver. J
Appl Toxicol, 5: 74-79.
Lock EA, Odum J, & Ormond P (1986) Transport of
N-acetyl-S-pentachloro-1,3-butadi-ethylcysteine by renal cortex.
Arch Toxicol, 59: 12-15.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55: 881-889.
Lopez-Avila V, Dodhiwala NS, Milanes J, & Beckert W (1989)
Evaluation of EPA method 8120 for determination of chlorinated
hydrocarbons in environmental samples. J Assoc Off Anal Chem, 72:
593-602.
McConnell G, Ferguson DM, & Pearson CR (1975) Chlorinated
hydrocarbon and the environment. Endeavour, 34: 13-18.
McFarlane M, Foster JR, Gibson GG, King LJ, & Lock EA (1989)
Cysteine conjugate beta-lyase of rat kidney cytosol:
characterization, immunocytochemical localization, and correlation
with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol, 98:
185-197.
McLellan LI, Wolf CR, & Hayes JD (1989) Human microsomal glutathione
S-transferase. Its involvement in the conjugation of
hexachlorobuta-1,3-diene with glutathione. Biochem J, 258: 87-93.
Malins DC, Krahn MM, Myers MS, Rhoses LD, Brown DW, Krone CA, McCain
BB, & Chan SI (1985) Toxic chemicals in sediments and biota from a
creasote-polluted harbor: relationships with hepatic neoplasms and
other hepatic lesions in English sole (Parophrys vetulus).
Carcinogenesis, 6: 1463-1469.
Mann JB, Enos HF, Gonzales J, & Thompson JF (1974) Development of
sampling and analytical procedure for determining hexachlorobenzene
and hexachloro-1,3-butadiene in air. Environ Sci Technol, 8:
584-585.
Markovec LM & Magee RJ (1984) Identification of major
perchloroaromatic compounds in waste products from the production of
carbon tetrachloride and tetrachloroethylene. Analyst, 109: 497-501.
Meijers AP (1988) [Organic trace substances in Rhine water and in
filtrate from the banks of the Rhine.] Wasser Abwasser, 129:
208-211.
Melcher RG & Caldecourt VJ (1980) Delayed injection-preconcentration
gas chromatographic technique for parts-per-billion determination of
organic compounds in air and water. Anal Chem, 52: 875-881.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and
other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, & Turton D (1985) Environmental contaminants in
human fat: comparison between accidental and nonaccidental causes of
death. Ecotoxicol Environ Saf, 10: 70-74.
Mes J, Davies DJ, Turton D, & Sun WF (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mumma CE & Lawless EW (1975) Survey of industrial processing data:
Task I. Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processing. Kansas City, Missouri, Midwest Research
Institute (Prepared for the US Environmental Protection Agency,
Office of Toxic Substances, Washington) (EPA 560/3-75-003; PB
243641).
Murzakayev FG (1963) [Data for substantiating maximal permissible
concentrations of hexachlorobutadiene and polychlorobutane in water
basins.] Gig i Sanit, 28: 9-14 (in Russian).
Nash JA, King LJ, Lock EA, & Green T (1984) The metabolism and
disposition of hexachloro-1,3-butadiene in the rat and its relevance
for nephrotoxicity. Toxicol Appl Pharmacol, 73: 124-137.
NIOSH (1979) Manual of analytical methods: Method 308. Cincinnati,
Ohio, National Institute of Occupational Safety and Health, vol 5.
NIOSH (1990) Manual of analytical methods: Method No. P & CAM 307.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
NPHEP (National Institute of Public Health and Environmental
Protection, The Netherlands) (1990) A proposal for guideline
concerning the estimation of environmental concern levels for the
modified EPA method. In: Report of the OECD Workshop on
Ecotoxicological Effects Assessment, Arlington, USA, December 1990.
Paris, Organisation for Economic Co-operation and Development.
Oesch F & Wolf CR (1989) Properties of the microsomal and cytosolic
glutathione transferases involved in hexachloro-1,3-butadiene
conjugation. Biochem Pharmacol, 38: 353-359.
OECD (1990) Report of the OECD Workshop of Ecotoxicological Effect
Assessment, Arlington, USA, December 1990. Paris, Organisation for
Economic co-operation and Development.
Oliver BG (1984) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Biouptake of chlorinated hydrocarbons from
laboratory-spiked and field sediments by oligochaete worms. Environ
Sci Technol, 21: 785-790.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St Clair, and Erie: implications
regarding sources along the St Clair and Detroit Rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Nicol KD (1982) Gas chromatographic determination of
chlorobenzenes and other chlorinated hydrocarbons in environmental
samples using fused silica capillary columns. Chromatographia, 16:
336-340.
Oliver BG & Nicol KD (1984) Chlorinated contaminants in the Niagara
River (198l-1983). Sci Total Environ, 34: 57-70.
Oliver BG & Niimi AJ (1983) Bioconcentration of chlorobenzenes from
water by Rainbow trout: correlations with partition coefficients and
environmental residues. Environ Sci Technol, 17: 287-291.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of
polychlorinated biphenyl congeners and other chlorinated
hydrocarbons in the Lake Ontario ecosystems. Environ Sci Technol,
22: 388-397.
Otson R & Chan C (1987) Sample handling and analysis for 51 volatile
organics by an adapted urge and trap GC-MS technique. Int J Environ
Anal Chem, 30: 275-287.
Payan JP, Fabry JP, Beydon D, & De Ceaurriz J (1991) Biliary
excretion of hexachloro-1,3-butadiene and its relevance to tissue
uptake and renal excretion in male rats. J Appl Toxicol, 11:
437-442.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16: 781-785.
Pereira WE, Rostad CE, Chiou CT, Brinton TI, Barber LB, Demcheck DK,
& Demas CR (1988) Contamination of estuarine water, biota, and
sediment by halogenated organic compounds: a field study. Environ
Sci Technol, 22: 772-778.
Petersen VP (1986) [Analysis of moderately volatile organochlorine
compounds and their concentration distribution in different streams
of the Federal Republic of Germany.] Dtsch Gewässerkd Mitt, 30:
10-15 (in German).
Poteryayeva GE (1966) [The effect of hexachlorobutadiene on
offspring of albino rats.] Gig i Sanit, 31: 33-36 (in Russian).
Pratt IS & Lock EA (1988) Deacylation and further metabolism of the
mercapturic acid of hexachloro-1,3-butadiene by rat kidney cytosol
in vitro. Arch Toxicol, 62: 341-345.
Rapson W, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24: 590-596.
Reichert D (1983) Metabolism and disposition of
hexachloro(1,3)butadiene in rats. Dev Toxicol Environ Sci, 5:
411-414.
Reichert D & Schütz S (1986) Mercapturic acid formation is an
activation and intermediary step in metabolism of
hexachlorobutadiene. Biochem Pharmacol, 35: 1271-1275.
Reichert D, Neudecker T, Spengler U, & Henschler D (1983)
Mutagenicity of dichloroacetylene, trichloroacryloyl chloride and
hexachlorobutadiene. Mutat Res, 117: 21-29.
Reichert D, Neudecker T, & Schütz S (1984) Mutagenicity of
hexachlorobutadiene, perchlorobutenoic acid and perchlorobutenoic
acid chloride. Mutat Res, 137: 89-93.
Reichert D, Schütz S, & Metzler M (1985) Excretion pattern and
metabolism of hexachlorobutadiene in rats. Evidence for metabolic
activation by conjugation reactions. Biochem Pharmacol, 34: 499-505.
Reimschüssel R, Bennet RO, May EB, & Lipsky MM (1989) Renal
histopathological changes in the goldfish (Carassius auratus)
after sublethal exposure to hexachlorobutadiene. Aquat Toxicol, 15:
169-180.
Reimschüssel R, Bennett RO, May EB, & Lipsky MM (1990) Development
of newly formed nephrons in the goldfish kidney following
hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol, 18:
32-38.
Roedig A & Bernemann P (1956) [Data on the highly chlorinated
vinylether 1-ethoxy-pentachloro-(1,3)-butadiene from
perchlorobutadiene.] Justus Liebigs Ann Chem, 600: 1-11 (in German).
Rush GF, Smith JH, Newton JF, & Hook JB (1984) Chemically induced
nephrotoxicity: role of metabolic activation. CRC Crit Rev Toxicol,
13: 99-156.
Ruth JH (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A142-A151.
Saillenfait AM, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Inhalation teratology study on hexachloro-1,3-butadiene in rats.
Toxicol Lett, 47: 235-240.
Sauer T Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Schiffmann D, Reichert D, & Henschler D (1984) Induction of
morphological transformation and unscheduled DNA synthesis in Syrian
hamster embryo fibroblasts by hexachlorobutadiene and its putative
metabolite pentachlorobutenoic acid. Cancer Lett, 23: 297-305.
Schnellmann RG, Lock EA, & Mandel LJ (1987) A mechanism of
S-(1,2,3,4,4-pentachloro-1,3-butadienyl)L-cysteine toxicity to
rabbit renal proximal tubules. Toxicol Appl Pharmacol, 90: 513-521.
Schrenk D & Dekant W (1989) Covalent binding of hexachlorobutadiene
metabolites to renal and hepatic mitochondrial DNA. Carcinogenesis,
10: 1139-1141.
Schrenk D, Dekant W, Wunsch PH, & Henschler D (1988a) Role of
metabolic activation in the toxicity of S-(pentachlorobutadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine in the
isolated perfused rat kidney. Toxicol in Vitro, 2: 283-290.
Schrenk D, Dekant W, & Henschler D (1988b) Metabolism and excretion
of S-conjugates derived from hexachlorobutadiene in the isolated
perfused rat kidney. Mol Pharmacol, 34: 407-412.
Schröder HF (1987) Chlorinated hydrocarbons in biological sewage
purification - Fate and difficulties in balancing. Water Sci
Technol, 19: 429-438.
Schwetz BA, Norris JM, Kociba RJ, Keeler PA, Cornier RF, & Gehring
PJ (1974) Reproduction study in Japanese quail fed
hexachlorobutadiene for 90 days. Toxicol Appl Pharmacol, 30:
255-265.
Schwetz BA, Smith FA, Humiston CG, Quast JF, & Kociba RJ (1977)
Results of a reproduction study in rats fed diets containing
hexachlorobutadiene. Toxicol Appl Pharmacol, 42: 387-398.
Shen TT (1982) Air quality assessment for land disposal of
industrial wastes. Environ Manage, 6: 297-305.
Singh HB, Salas LJ, & Stiles R (1982) Distribution of selected
gaseous organic mutagens and suspected carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
SRI (1984) Directory of chemical producers, Western Europe. Menlo
Park, California, Stanford Research Institute.
Stonard MD, Gore CW, Oliver GJA, & Smith IK (1987) Urinary enzymes
and protein pattern as indicators of injury to different regions of
the kidney. Fundam Appl Toxicol, 9: 339-351.
Stott WT, Quast JF, & Watanabe PG (1981) Differentiation of the
mechanisms of oncogenicity of 1,4-dioxane and
1,3-hexachlorobutadiene in the rat. Toxicol Appl Pharmacol, 60:
287-300.
Tabak HH, Quave SA, Mashni CE, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1517.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res, 37:
2717-2720.
Ullmann F (1986) Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH-Verlagsgesellschaft, vol A6, pp 322-323, 375, 391,
398.
US EPA (1980) Ambient water quality criteria for
hexachlorobutadiene. Washington, DC, US Environmental Protection
Agency, Criteria and Standards Division (EPA-440/5-80-053;
PB-81-117640).
UP EPA (1984a) A method for determination of certain chlorinated
hydrocarbons. EPA method No. 612. Fed Reg, 491(209): 29-210.
US EPA (1984b) A gas chromatographic method for determination of
certain chlorinated hydrocarbons. EPA method No. 612. Fed Reg,
491(209): 29-210.
Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, & Henschler D
(1987) Enzymatic transformation of mercapturic acids derived from
halogenated alkenes to reactive and mutagenic intermediates. Biochem
Pharmacol, 36: 2741-2748.
Vamvakas S, Kordowich FJ, Dekant W, Neudecker T, & Henschler D
(1988a) Mutagenicity of hexachloro-1,3-butadiene and its
S-conjugates in the Ames test - role of activation by the
mercapturic acid pathway in its nephrocarcinogenicity.
Carcinogenesis, 9: 907-910.
Vamvakas S, Muller DA, Dekant W, & Henschler D (1988b) DNA binding
of sulfur containing metabolites from 35S-(pentachlorobutadienyl)-
L-cysteine in bacteria and isolated renal tubular cells. Drug Metab
Drug Interact, 6: 349-358.
Vamvakas S, Dekant W, & Henschler D (1989a) Genotoxicity of
haloalkene and haloalkane glutathione S-conjugates in porcine kidney
cells. Toxicol in Vitro, 3: 151-156.
Vamvakas S, Kochling A, Berthold K, & Dekant W (1989b)
Cytotoxicity of cysteine S-conjugates: structure-activity
relationships. Chem-Biol Interact, 71: 79-90.
Vamvakas S, Dekant W, & Henschler D (1993) Nephrocarcinogenicity of
haloalkenes and alkynes. In: Renal disposition and nephrotoxicity of
xenobiotics. New York, Academic Press, pp 323-342.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchlonne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63:
1433-1439.
Vorobyeva TN (1980) [Enpared residues in grapes.] Khim Sel'sk Khoz,
18(10): 41-42 (in Russian).
Wallin A, Jones TW, Vercesi AE, Cotgreave I, Ormstad K, & Orrenius S
(1987) Toxicity of S-pentachlorobutadienyl-L-cysteine studied with
isolated rat renal cortical mitochondria. Arch Biochem Biophys, 258:
365-372.
Wallin A, Gerdes RG, Morgenstern R, Jones TW, & Ormstad K (1988)
Features of microsomal and cytosolic glutathione conjugation of
hexachlorobutadiene in rat liver. Chem-Biol Interact, 68: 1-11.
Wild D, Schütz S, & Reichert D (1986) Mutagenicity of the
mercapturic acid and other S-containing derivatives of
hexachloro-1,3-butadiene. Carcinogenesis, 7: 431-434.
Wolf CR, Berry PN, Nash JA, Green T, & Lock EA (1984) Role of
microsomal and cytosolic glutathione S-transferases in the
conjugation of hexachloro-1,3-butadiene and its possible relevance
to toxicity. J Pharmacol Exp Toxicol, 228: 202-208.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen, 7:
677-702.
Yang RSH (1988) Hexachloro-1,3-butadiene: toxicology, metabolism,
and mechanisms of toxicity. Rev Environ Contam Toxicol, 101:
121-137.
Yang RSH (1991) Toxicity studies of hexachloro-1,3-butadiene in
B6C3F1 mice (feed studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP TOX 1) (NIH Publication
No. 91-3120).
Yang RSH, Abdo KM, Elwell MR, Levy AC, & Brennecke LH (1989)
Subchronic toxicology studies of hexachloro-1,3-butadiene (HCBD) in
B6C3F1 mice by dietary incorporation. J Environ Pathol Toxicol
Oncol, 9: 323-332.
Yasuhara A & Morita M (1990) Formation of chlorinated compounds in
pyrolysis of trichloroethylene. Chemosphere, 21: 479-486.
Yip G (1976) Survey for hexachloro-1,3-butadiene in fish, eggs,
milk, and vegetables. J Assoc Anal Chem, 59: 559-561.
Yurawecz MP, Dreifuss PA, & Kamps LR (1976) Determination of
hexachloro-1,3-butadiene in spinach, eggs, fish, and milk by
electron capture gas-liquid chromatography. J Assoc Anal Chem, 59:
552-558.
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of The
Netherlands. Chemosphere, 9: 231-249.
Zogorski JS (1984) Experience in monitoring domestic water sources
and process waters for trace organics. J Environ Sci Health, A19:
233-249.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
L'hexachlorobutadiène est un liquide ininflammable,
incombustible, limpide et huileux à la température et la pression
ordinaires. Il est peu soluble dans l'eau mais miscible à l'éther et
à l'éthanol.
On peut le mettre en évidence et le doser par chromatographie
en phase gazeuse. Les limites de détection sont de 0,03 µg/m3 dans
l'air, 0,001 µg/litre dans l'eau, de 0,7 µg/kg de matière humide
dans le sol ou les sédiments et de 0,02 µg/litre dans le sang. Dans
les tissus, cette limite est de 0,47 µg/kg de tissus frais.
2. Sources d'exposition humaine et environnementale
L'hexachlorobutadiène n'existe pas à l'état naturel. C'est
essentiellement un sous-produit de la fabrication des hydro-carbures
chlorés que l'on retrouve dans les fractions lourdes. La production
annuelle mondiale (dans les fractions lourdes) a été estimée à
10 000 tonnes en 1982. L'hexachlorobutadiène peut être utilisé pour
la récupération des gaz contenant du chlore dans les ateliers de
fabrication du chlore et comme liquide de lavage pour éliminer du
courant gazeux certains composés organiques volatils. On l'utilise
également dans les gyroscopes, comme fluide calo-porteur, dans les
transformateurs, comme liquide isolant ou liquide hydraulique ainsi
que comme solvant des élastomères, comme intermédiaire et comme
fumigant.
3. Transport, distribution et transformation dans l'environnement
Les principales voies de pénétration dans l'environnement sont
les émissions résultant des déchets et les utilisations qui
entraînent la dispersion du produit. Le transport
inter-compartimental s'effectue principalement par volatilisation,
adsorption sur les matières particulaires puis dépôt ou
sédimentation. L'hexachloro-butadiène ne migre pas facilement dans
le sol et s'accumule dans les sédiments. Dans l'eau, on le considère
comme persistant, sauf turbulences importantes. Il n'y a pas
d'hydrolyse. Le produit semble être facilement biodégradable par
voie aérobie, encore que le phénomène n'ait pas été étudié à fond.
L'hexachlorobutadiène présent sur les surfaces subit une photolyse.
Outre le dépôt, on estime que la réaction de l'hexachlorobutadiène
avec les radicaux hydroxyles constitue un mode de piégeage important
de ce composé dans la troposphère, la demi-vie atmosphérique
estimative de l'hexachlorobutadiène pouvant aller jusqu'à 2,3 ans.
Le produit a un potentiel élevé de bioaccumulation, comme l'ont
confirmé les observations en laboratoire et sur le terrain. Ainsi,
on a trouvé des facteurs de bioconcentration à l'état stationnaire
(obtenus expérimentalement par rapport au poids de tissus frais)
respectivement égaux en moyenne à 5800 et 17 000 chez la truite
arc-en-ciel. On n'a pas observé d'amplification biologique au
laboratoire ou sur le terrain.
4. Niveaux dans l'environnement et exposition humaine
Le dosage de l'hexachlorobutadiène dans l'air des villes a
donné dans tous les cas des valeurs inférieures à 0,5 µg/m3. Dans
les régions écartées, les concentrations sont inférieures à
1 pg/m3. Dans les lacs et les cours d'eau d'Europe, on a
enregistré des concentrations pouvant aller jusqu'à 2 µg/litre mais
les valeurs moyennes sont généralement inférieures à 100 ng/litre.
Dans la région des grands lacs au Canada, on a obtenu des valeurs
beaucoup plus faibles (autour de 1 ng/litre). En revanche la teneur
des sédiments du fond peut, dans cette zone, atteindre 120 µg/kg de
poids sec. Les couches sédimentaires plus anciennes, remontant aux
environs de 1960, présentaient des teneurs plus élevées (jusqu'à
550 µg/kg de matière humide). On a montré que la concentration dans
les sédiments augmentait avec la granulométrie des particules.
A en juger par la concentration de l'hexachlorobutadiène dans
les organismes aquatiques, les oiseaux et les mammifères, le composé
s'accumule mais ne subit pas d'amplification biologique. Dans les
eaux polluées, on a relevé des concentrations dépassant 1000 µg/kg
de tissus frais chez plusieurs espèces et même 120 mg/kg (par
rapport aux lipides) chez une espèce. Les concentrations actuelles
restent généralement inférieures à 1000 µg/kg de poids frais à
distance des points de décharge industrielle.
On a décelé la présence du composé dans l'urine, le sang et les
tissus humains. Dans certaines denrées alimentaires ayant une
fraction lipidique importante, on en a relevé jusqu'à 40 µg/kg et
dans un cas, plus de 1000 µg/kg.
D'après une étude, le niveau d'exposition pourrait atteindre
1,6 à 12,2 mg/m3 et les concentrations urinaires, 20 mg/litre.
5. Cinétique et métabolisme
Après administration par voie orale, l'hexachlorobutadiène est
rapidement absorbé chez l'animal de laboratoire mais le taux de
résorption après inhalation ou exposition par voie cutanée n'a pas
été étudié. Chez le rat et la souris, le composé se répartit
principalement dans le foie, les reins et les tissus adipeux. Il est
rapidement excrété. On a mis en évidence une fixation aux protéines
et aux acides nucléiques dans le foie et les reins.
La biotransformation du composé chez l'animal de laboratoire se
révèle être un processus saturable. Elle s'effectue principalement
par l'intermédiaire du glutathion, l'hexachlorobutadiène étant
d'abord transformé en conjugué du S-glutathion. La métabolisation
de ce conjugué se poursuit ensuite, en particulier au niveau de la
membrane constituant la bordure en brosse des cellules des tubules
rénaux, pour donner un métabolite sulfuré réactif qui est
probablement responsable des effets néphrotoxiques, génotoxiques et
cancérogènes observés.
6. Effets sur les êtres vivants dans leur milieu naturel
L'hexachlorobutadiène est modérément à très toxique pour les
organismes aquatiques. Certaines espèces de poissons et de crustacés
se sont révélées être les plus sensibles, les valeurs de la CL50 à
96 h. allant de 0,032 à 1,2 et de 0,09 à environ 1,7 mg/litre,
respectivement pour les crustacés et les poissons. Chez les
poissons, le rein est organe-cible important.
On a établi la valeur de la dose sans effets observables à
0,003 mg/litre, à partir des résultats d'un certain nombre
d'épreuves à long terme sur certaines espèces d'algues et de
poissons; cela permet de considérer ce composé comme très toxique
pour les organismes aquatiques. Parmi les points d'aboutissement
biologiques étudiés figuraient la toxicité générale, la
neurotoxicité, la biochimie, l'hématologie, l'anatomopathologie et
la reproduction. Lors d'une étude de 28 jours portant sur les
premiers stades de la vie de Pimephales promelas, une espèce de
vairon, on a observé que la reproduction n'était pas affectée à des
concentrations allant jusqu'à 0,017 mg/litre, alors qu'à 0,013 et
0,017 mg/litre il y avait accroissement de la mortalité et réduction
du poids du corps. La dose sans effets observables était de
0,0065 mg/litre.
On n'a décrit qu'une seule épreuve fiable portant sur des
organismes terrestres. Lors d'une épreuve de 90 jours sur des
cailles japonaises qui recevaient une alimentation contenant ce
composé à des concentrations allant de 0,3 à 30 mg/kg de nourriture,
on a constaté que la survie des oisillons n'était réduite qu'à
partir de 10 mg/kg de nourriture.
7. Effets sur les animaux de laboratoire et les systèmes
d'épreuves in vitro
7.1 Toxicité générale
L'hexachlorobutadiène est légèrement à modérément toxique pour
le rat adulte, modérément toxique pour le raton juste sevré et
extrêmement toxique pour les rattes juste sevrées après
administration d'une seule dose par voie buccale. Les principaux
organes-cibles sont le rein et dans une bien moindre mesure, le
foie.
D'après les données obtenues sur l'animal d'expérience, les
vapeurs d'hexachlorobutadiène sont irritantes pour les muqueuses et
le liquide est corrosif. On peut considérer ce composé comme un
agent sensibilisateur.
Chez le rat, la souris et le lapin, l'hexachlorobutadiène
provoque une nécrose, liée à la dose, des tubules proximaux du rein.
Les rats mâles adultes sont moins sensibles à la néphrotoxicité que
les femelles ou les jeunes mâles. Les souriceaux sont plus sensibles
que les souris adultes sans qu'on puisse observer de différences
entre les deux sexes. Chez la ratte adulte, la dose intrapéritonéale
unique la plus faible à laquelle on ait observé une nécrose rénale
était de 25 mg/kg de poids corporel; elle était de 6,3 mg/kg de
poids corporel chez les souris adultes mâles et femelles. A des
doses égales ou supérieures à celles qui entraînaient une nécrose,
on a observé des modifications biochimiques et une nette
amélioration de la fonction rénale.
Lors de six épreuves à court terme où le composé a été
administré par la voie orale, deux études de reproduction et une
étude d'alimentation à long terme portant sur des rats, c'est
également le rein qui s'est révélé être l'organe-cible. Parmi les
effets liés à la dose, on notait une diminution du poids relatif des
reins et une dégénérescence de l'épithélium des tubules. La dose
sans effets nocifs observables au niveau des reins, tirée d'une
étude de deux ans sur le rat, était de 0,2 mg/kg de poids corporel
et par jour. Une étude de 13 semaines sur des souris a montré que
cette dose était de 0,2 mg/kg de poids corporel et par jour pour cet
animal. Chez les deux espèces, les femelles adultes étaient plus
sensibles que les mâles adultes.
Lors d'une étude d'inhalation à court terme (six heures par
jour pendant 12 jours) on a observé des effets analogues au niveau
des reins avec une concentration nominale de vapeur
d'hexa-chlorobutadiène égale à 267 mg/m3; cette concentration a
également entraîné des difficultés respiratoires, ainsi qu'une
dégénérescence des corticosurrénales.
7.2 Reproduction, embryotoxicité et teratogétogénicité
Deux études d'alimentation portant sur la reproduction ont été
effectuée sur des rats à des doses quotidiennes allant jusqu'à 20 et
75 mg/kg de poids corporel respectivement; elles ont fait ressortir
une réduction du poids de naissance et du gain de poids néonatal aux
doses toxiques pour la mère. La dose quotidienne de 75 mg/kg de
poids corporel, qui était hautement toxique, s'est révélée
suffisante pour empêcher la conception et la nidation intra-utérine.
On n'a pas observé d'anomalies du squelette.
Lors de deux études de tératogénicité, des rats ont été exposés
soit à des vapeurs d'hexachlorobutadiène à des concentrations allant
de 21 à 160 mg/m3, six heures par jour du sixième au vingtième
jour de la gestation, soit par voie intrapéritonéale à une dose
quotidienne de 10 mg/kg de poids corporel (du premier au quinzième
jour de la gestation). Des effets nocifs ont été notés sur le
développement des foetus, qui consistaient en une réduction du poids
de naissance, un retard dans le développement cardiaque, une
dilatation des uretères, mais pas de malformations macroscopiques.
Le retard de développement a été observé à des doses qui étaient
également toxiques pour les mères.
7.3 Génotoxicité et cancérogénicité
L'hexachlorobutadiène provoque des mutations géniques dans
l'épreuve d'Ames sur salmonelle dans des conditions particulières
qui favorisent la formation de produits de conjugaison avec le
glutathion. Lors d'une étude in vivo on a observé des aberrations
chromosomiques qui n'ont en revanche pas été constatées lors de deux
autres études in vitro. Une étude in vitro portant sur des
cellules ovariennes de hamster chinois a révélé une augmentation de
la fréquence des échanges entre chromatides soeurs. On a fait état
de la très forte mutagénicité des métabolites sulfurés de
l'hexachlorobutadiène. D'autres études in vitro ont montré que ce
composé provoquait une synthèse non programmée de l'ADN dans des
cultures de fibroblastes embryonnaires de hamsters de Syrie, effets
qui n'étaient pas observés dans des cultures d'hépatocytes de rats.
Le composé provoque également une synthèse non programmée de l'ADN
in vivo mais n'induit pas de mutations létales récessives liées au
sexe chez Drosophila melanogaster.
Lors de la seule étude à long terme (deux ans) qui ait été
effectuée, des rats ont reçu une alimentation contenant de
l'hexachlorobutadiène à des doses quotidiennes respectives de 0,2, 2
ou 20 mg/kg de poids corporel et seule la dose la plus élevée a
provoqué un accroissement de l'incidence des tumeurs malignes au
niveau des tubules rénaux.
7.4 Mécanismes de la toxicité
La néphrotoxicité, la mutagénicité et la cancérogénicité de
l'hexachlorobutadiène sont liées à la biosynthèse d'un conjugué
sulfuré toxique, le 1-glutathion- S-yl-1,2,3,4,4-pentachloro-
butadiène. Ce conjugué est principalement synthétisé dans le foie et
métabolisé ensuite dans la bile, l'intestin et les reins en
1-cystéine- S-yl-1,2,3,4,4-pentachlorobutadiène (CPB). L'activation
du CPB en thiocétène réactif (qui dépend de la cystéine-conjuguée-
béta lyase) au niveau des cellules des tubules proximaux, aboutit en
définitive à la formation de liaisons covalentes avec les
macromolécules cellulaires.
8. Effets sur l'homme
On n'a pas décrit d'effets pathogènes sur la population dans
son ensemble.
On possède deux rapports faisant état de troubles chez des
ouvriers agricoles qui utilisaient de l'hexachlorobutadiène comme
fumigant mais il est vrai qu'ils avaient également été exposés à
d'autres substances. On a également observé un accroissement de la
fréquence des aberrations chromosomiques dans les lymphocytes du
sang périphériques de travailleurs employés à la production
d'hexachlorobutadiène et qui avaient été exposés à des
concentrations de 1,6 à 12,2 mg/m3.
9. Evaluation des risques pour la santé humaine et des
effets sur l'environnement
9.1 Evaluation des risques pour la santé humaine
Comme très peu d'études ont été effectuées sur l'homme,
l'évaluation repose essentiellement sur les animaux de laboratoire.
Toutefois les données in vitro limitées dont on dispose au sujet
de l'homme incitent à penser que le métabolisme de
l'hexachloro-butadiène est analogue chez l'homme et l'animal.
On estime que les vapeurs d'hexachlorobutadiène sont irritantes
pour les muqueuses et que le liquide est corrosif. Ce composé doit
également être considéré comme un agent sensibilisateur.
Les principaux organes-cibles de son action toxique sont les
reins et dans une mesure bien moindre, le foie. Sur la base des
études à court et à long terme effectuées sur des rats et des
souris, la dose quotidienne sans effets nocifs observables est
évaluée à 0,2 mg/kg de poids corporel. On l'a estimée à 53 mg/m3
lors d'une étude d'inhalation à court terme chez le rat (12 jours,
six heures par jour).
L'action toxique sur le développement, de même que la réduction
du poids de naissance et du gain de poids néonatal n'ont été
observés qu'à des doses toxiques pour la mère.
On a observé que l'hexachlorobutadiène produisait des mutations
géniques, des aberrations chromosomiques, un accroissement des
échanges entre chromatides soeurs et une synthèse non programmée de
l'ADN, encore que certaines études aient donné des résultats
négatifs. On ne possède que des indices limités en faveur d'une
génotoxicité de l'hexachlorobutadiène chez l'animal, indices qui
sont insuffisants en ce qui concerne l'homme.
On a constaté que l'administration d'hexachlorobutadiène par
voie orale pendant une longue période à des rats accroissait la
fréquence des tumeurs malignes au niveau des tubules rénaux, mais il
s'agissait uniquement de doses élevées fortement néphrotoxiques. En
ce qui concerne la cancérogénicité de cette substance, les indices
sont limités chez l'animal et insuffisants chez l'homme.
En se basant sur la dose quotidienne sans effets nocifs
observables estimée à 0,2 mg/kg de poids corporel chez la souris ou
le rat, on a fixé à 0,03-0,05 mg/kg de poids corporel la dose
quotidienne sans effets nocifs observables chez l'homme. La marge de
sécurité entre la dose estimative sans effets nocifs observables et
la dose journalière maximale totale ingérée estimée en se basant sur
une absorption du composé par l'intermédiaire d'une eau de boisson
et de produits alimentaires contaminés à forte teneur en lipides,
est égale à 150.
9.2 Evaluation des effets sur l'environnement
L'hexachlorobutadiène est modérément à fortement toxique pour
les organismes aquatiques: les crustacés et les poissons sont les
espèces les plus sensibles. On a fixé à 0,1 g/litre la
concentration écologiquement préoccupante. On estime que la
concentration maximale prévisible dans l'environnement à distance
des sources ponctuelles de pollution est égale à deux fois la dose
écologique-ment préoccupante extrapolée et, par voie de conséquence,
que les organismes aquatiques peuvent être menacés dans les eaux de
surface polluées. On ne peut exclure des effets nocifs sur le
benthos.
Compte tenu de la toxicité de l'hexachlorobutadiène pour les
mammifères, la consommation de benthos ou d'organismes aquatiques
par d'autres espèces pourrait être préoccupante.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos de análisis
El hexaclorobutadieno es un líquido no inflamable,
incombus-tible, claro, oleoso e incoloro a temperatura y presión
ordinarias. Es poco soluble en el agua, pero miscible con éter y
etanol.
La sustancia puede detectarse y determinarse cuantitativamente
por métodos de cromatografía de gases. Los límites de detección son
de 0,03 µg/m3 de aire, 0,001 µg/litro de agua, 0,7 µg/kg de peso
húmedo en el suelo o en sedimentos y de 0,02 µg/litro de sangre. Se
ha determinado un nivel de 0,47 µg/kg de peso húmedo de tejido.
2. Fuentes de exposición humana y ambiental
No hay indicaciones de que el hexaclorobutadieno exista como
producto natural. Es principalmente un subproducto de la fabricación
de hidrocarburos clorados y se presenta en las fracciones pesadas
(como residuo). La producción anual mundial del compuesto en las
fracciones pesadas en 1982 se estimó en 10 000 toneladas.
El hexaclorobutadieno puede utilizarse para recuperar gas que
contiene cloro en plantas productoras de cloro y como líquido de
lavado para eliminar ciertos compuestos orgánicos volátiles de las
corrientes de gases. También se ha utilizado como fluido en
giróscopos, como transmisor de calor, transformador, fluido aislante
y fluido hidráulico, disolvente para elastómeros y como
intermediario y sustancia para fumigar.
3. Transporte, distribución y transformación en el medio ambiente
Las principales vías de ingreso en el medio ambiente son las
emisiones de residuos y el uso dispersivo. El paso de un entorno a
otro ocurre principalmente por volatilización, adsorción a
corpúsculos de materia y subsiguiente deposición o sedimentación. El
hexaclorabutadieno no migra rápidamente en el suelo y se acumula en
el sedimento. Se considera persistente en el agua a menos que haya
mucha turbulencia. No produce hidrólisis. La sustancia parece ser
fácilmente biodegradable aeróbicamente, aunque su biodegradabilidad
no se ha investigado a fondo. El hexaclorobutadieno se fotoliza en
las superficies. Se supone que, además de la deposición, la reacción
con radicales hidroxilo es un importante sumidero de
hexaclorobutadieno en la troposfera y su semivida atmosférica
estimada es de hasta 2,3 años. La sustancia tiene un elevado
potencial de bioacumulación, que se ha comprobado mediante
observaciones en laboratorio y sobre el terreno. En la trucha arco
iris se han determinado experimentalmente factores de
bioconcentración en estado estacionario de 5800 y 17 000 como
promedio, sobre la base del peso húmedo. No se ha observado
biomagnificación en laboratorio ni sobre el terreno.
4. Niveles ambientales y exposición humana
Se ha determinado la presencia de hexaclorubutadieno en el aire
urbano; en todos los casos, los niveles eran inferiores a
0,5 µg/m3. Las concentraciones en lugares aislados son inferiores
a 7 pg/m3. En las aguas de lagos y ríos de Europa se han
registrado concentraciones de hasta 2 µg/litro, pero los niveles
medios son generalmente inferiores a 100 ng/litro. En la región de
los Grandes Lagos del Canadá se han detectado niveles muy inferiores
(de aproximadamente 1 ng/litro). Allí los niveles en el sedimento
del fondo pueden ser de 120 µg/kg de peso en seco. En capas más
antiguas de sedimento, de 1960 aproximadamente, se encontraron
concentraciones más elevadas (de hasta 550 µg/kg de peso húmedo). Se
ha demostrado que la concentración en el sedimento aumenta con el
tamaño de la partícula de sedimento.
Las concentraciones de hexaclorobutadieno en organismos
acuáticos, aves y mamíferos indican bioacumulación pero no
biomagnificación. En las aguas contaminadas se han detectado niveles
de más de 1000 µg/kg de peso húmedo en varias especies y de
120 mg/kg (base grasa) en una especie. Lejos de los efluentes
industriales, los niveles actuales se mantienen en general por
debajo de 100 µg/kg de peso húmedo.
Se ha detectado la presencia del compuesto en la orina, en la
sangre y en tejidos humanos. En ciertos alimentos que contienen una
elevada fracción lipídica se han encontrado hasta unos 40 µg/kg y,
en un caso, más de 1000 µg/kg.
Un estudio señala exposiciones ocupacionales de
1,6-12,2 mg/m3 y en la orina niveles de hasta 20 mg/litro.
5. Cinética y metabolismo
Se ha observado que los animales de laboratorio absorben
rápidamente el hexaclorobutadieno después de la administración oral,
pero no se ha investigado la velocidad de absorción después de la
inhalación o de la exposición dérmica. En ratas y ratones, el
compuesto se distribuye principalmente al hígado, a los riñones y al
tejido adiposo. Se excreta rápidamente. Se ha demostrado que se fija
a las proteínas y ácidos nucleicos del hígado y de los riñones.
La biotransformación del compuesto en animales de
experimentación parece ser un proceso saturable. Se produce
principalmente a través de una vía mediada por el glutatión, en la
cual el hexaclorobutadieno se convierte inicialmente en conjugados
de S-glutatión. Estos conjugados pueden seguir metabolizándose,
especialmente en el ribete en cepillo de las membranas de las
células de los tubos renales, produciendo un metabolito sulfuroso
reactivo que probablemente explique la nefrotoxicidad, genotoxicidad
y carcinogenicidad observadas.
6. Efectos en organismos presentes en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos. Los más sensibles que se hayan observado han
sido especies de peces y crustáceos; los valores de la CL50 en
96 horas oscilan entre 0,032 y 1,2 mg/litro en crustáceos y entre
0,09 y 1,7 mg/litro en peces. Se ha demostrado que el riñón es un
órgano muy afectado en los peces.
Sobre la base de varias pruebas a largo plazo con especies de
algas y de peces, se ha establecido un nivel sin efectos observados
de 0,003 mg/litro; así pues, el compuesto se clasifica como muy
tóxico para las especies acuáticas. Los valores extremos
investigados comprenden parámetros de toxicidad general,
neurotoxicidad, bioquímicos, hematológicos, patológicos y
relacionados con la reproducción. En una prueba de 28 días de
duración en la que se examinaron las primeras fases de la vida de
carpas se observó que la reproducción no se veía afectada con
concentraciones de hasta 0,017 mg/litro, mientras que con
concentraciones de 0,013 y 0,017 mg/litro se observaron un aumento
de la mortalidad y una disminución del peso corporal. El nivel sin
efectos observados era de 0,0065 mg por litro.
Se ha descrito una sola prueba fiable con organismos
terrestres. En una prueba de 90 días con codornices japonesas
alimentadas con una dieta que contenía el compuesto en
concentraciones de 0,3 a 30 mg/kg de dieta se observó que la
supervivencia de los polluelos disminuía a partir de 10 mg/kg de
dieta.
7. Efectos en animales de experimentación y en sistemas de
prueba in vitro
7.1 Toxicidad general
Después de la ingestión de una dosis oral única, el
hexaclorobutadieno es de levemente a moderadamente tóxico para las
ratas adultas, moderadamente tóxico para las ratas macho destetadas
y muy tóxico para las ratas hembras destetadas. Los principales
órganos afectados son el riñón y, en grado mucho menor, el hígado.
Los datos obtenidos con animales indican que el vapor de
hexaclorobutadieno es irritante para las membranas mucosas y el
líquido es corrosivo. La sustancia debe considerarse como un agente
sensibilizador.
En los riñones de ratas, ratones y conejos, el
hexacloro-butadieno causa en los tubos proximales del riñón una
necrosis que depende de la dosis. Las ratas macho adultas son menos
vulnerables a la toxicidad renal que las hembras adultas y que los
machos jóvenes. Los ratones jóvenes son más vulnerables que los
adultos y no se observaron diferencias entre un sexo y otro. En las
ratas hembra adultas la dosis intraperitoneal única más baja con la
cual se observó necrosis renal fue de 25 mg/kg de peso corporal y en
ratones adultos, machos y hembras, fue de 6,3 mg/kg de peso
corporal. Se observaron cambios bioquímicos y alteraciones
funcionales marcados en los riñones con dosis iguales o mayores que
las asociadas con necrosis.
Asimismo, en seis pruebas orales de corto plazo, dos estudios
sobre reproducción y un estudio de largo plazo sobre la dieta
realizados con ratas, el riñón fue el principal órgano afectado. Los
efectos relacionados con la dosis comprenden una reducción del peso
relativo del riñón y una degeneración del epitelio de los tubos. El
nivel sin efectos nocivos observados de toxicidad renal en ratas en
un estudio de dos años fue de 0,2 mg/kg de peso corporal por día. En
un estudio de 13 semanas efectuado en ratones se obtuvo un nivel sin
efectos nocivos observados de 0,2 mg/kg de peso corporal por día.
Las hembras adultas de ambas especies eran más vulnerables que los
machos adultos.
En una prueba de inhalación de corto plazo (6 horas por día
durante 12 días) se observaron efectos semejantes en los riñones con
una concentración de vapor nominal de 267 mg/m3, con la cual
también se observaron trastornos respiratorios y degeneración de la
corteza suprarrenal.
7.2 Reproducción, embriotoxicidad y teratogenicidad
Dos estudios sobre dieta y reproducción en ratas con dosis de
hasta 20 y 75 mg/kg de peso corporal por día, respectivamente,
mostraron una reducción del peso al nacer y un aumento del peso
neonatal cuando se administraban a la madre dosis tóxicas de 20 y
7,5 mg/kg de peso corporal, respectivamente. La dosis altamente
tóxica de 75 mg/kg de peso corporal por día fue suficiente para
impedir la concepción y la implantación uterina. No se observaron
anormalidades del esqueleto.
En dos pruebas de teratogenicidad en las que se expuso a las
ratas o bien a vapor de hexaclorobutadieno en concentraciones que
oscilaban entre 21 y 160 mg/m3 durante 6 horas diarias (desde el
6° hasta el 20° día del embarazo) o bien a la administración
intraperitoneal de 10 mg/kg de peso corporal por día (desde el 1° al
15° día de embarazo) se observaron en el desarrollo del feto efectos
tóxicos tales como una reducción del peso al nacer, un retraso del
desarrollo del corazón y uréteres dilatados pero sin grandes
malformaciones. El retraso del desarrollo se observó en niveles que
también eran tóxicos para las madres.
7.3 Genotoxicidad y carcinogenicidad
En la prueba de Ames Salmonella se ha observado que el
hexaclorobutadieno induce mutaciones genéticas en condiciones
especiales que favorecen la formación de productos de conjugación
con el glutatión. En un estudio in vivo se observó que había
inducido aberraciones cromosómicas, pero no se observaron tales
aberraciones en dos estudios in vitro. En una prueba in vitro se
observó que la frecuencia de los intercambios entre cromátidas
hermanas había aumentado en las células ováricas de hámsters de
China. Se ha señalado el gran potencial mutagénico de los
metabolitos sulfurosos del hexaclorobutadieno. En estudios in
vitro, el compuesto indujo síntesis imprevistas de ADN en cultivos
de fibroblastos de embriones de hámsters de Siria, pero no en
cultivos de hepatocitos. Indujo síntesis imprevistas de ADN en ratas
in vivo, pero no indujo mutaciones letales recesivas ligadas al
sexo en Drosophila melanogaster.
En el único estudio de largo plazo (dos años), en el cual las
ratas recibieron una dieta que contenía hexaclorobutadieno en dosis
de 0,2, 2 ó 20 mg/kg de peso corporal por día, se observó una mayor
incidencia de neoplasias de los tubos renales únicamente con la
dosis más elevada.
7.4 Mecanismos de toxicidad
La nefrotoxicidad, mutagenicidad y carcinogenicidad del
hexaclorobutadieno depende de la biosíntesis del conjugado sulfuroso
tóxico 1-glutatión- S-yl-1,2,3,4,4-pentaclorobutadieno. Este
conjugado se sintetiza principalmente en el hígado y se metaboliza
luego en la bilis, el intestino y los riñones convirtiéndose en
1-cisteína- S-yl-1,2,3,4,4-pentaclorobutadieno (CPB). La activación
de CPB, que depende del conjugado de cisteína beta-lyasa, en una
tiocetena reactiva en las células de los tubos proximales finalmente
da lugar a un enlace covalente con macromoléculas celulares.
8. Efectos en el ser humano
No se han descrito efectos patogénicos en la población en
general.
Se conocen dos casos de trastornos padecidos por trabajadores
agrícolas que utilizaban el hexaclorobutadieno como fumigante, pero
esas personas también habían estado expuestas a otras sustancias. En
los linfocitos de la sangre periférica de operarios que trabajaban
en la producción de hexaclorobutadieno y estaban expuestos, según se
informa, a concentraciones de 1,6 a 12,2 mg/m3 se observó una
frecuencia mayor de aberraciones cromosómicas.
9. Evaluación de los riesgos para la salud humana y de los
efectos en el medio ambiente
9.1 Evaluación de los riesgos para la salud humana
Como se han hecho muy pocos estudios en el ser humano, la
evaluación se basa principalmente en estudios efectuados en animales
de laboratorio. Sin embargo, los limitados datos existentes sobre el
ser humano, obtenidos in vitro, sugieren que el metabolismo del
hexaclorobutadieno en el ser humano es semejante al observado en
animales.
Se considera que el vapor de hexaclorobutadieno irrita las
membranas mucosas del ser humano y que en estado líquido es
corrosivo. El compuesto también debe considerarse como un agente
sensibilizador.
Los principales órganos afectados por la toxicidad son los
riñones y, en mucho menor grado, el hígado. En base a estudios de
corto y largo plazo de ingestión por vía oral por ratas y ratones,
se determinó un nivel sin efectos adversos observados de 0,2 mg/kg
de peso corporal por día. En un estudio de inhalación en el corto
plazo en ratas (12 días, a razón de 6 horas por día), el nivel sin
efectos adversos observados fue de 53 mg/m3.
Se observaron una reducción del peso al nacer y un aumento de
peso neonatal únicamente con dosis tóxicas para la madre; lo mismo
puede decirse de los efectos tóxicos para el desarrollo.
Se ha observado que el hexaclorobutadieno induce mutaciones
genéticas, aberraciones cromosómicas, aumento de los intercambios
entre cromátidas hermanas y síntesis imprevistas de ADN, aunque
algunos estudios han dado resultados negativos. Con respecto a la
genotoxicidad del hexaclorubutadieno, las observaciones realizadas
en animales son limitadas y las efectuadas en el ser humano
insuficientes.
Tras la administración oral a largo plazo del
hexacloro-butadieno a ratas, se ha observado una mayor frecuencia de
neoplasias de los tubos renales, pero solamente con dosis elevadas
causantes de nefrotoxicidad notable. Hay indicios limitados de
carcinogenicidad en animales e indicios insuficientes en el ser
humano.
En base al nivel sin efectos adversos observados en ratones y
ratas, que es de 0,2 mg/kg de peso corporal por día, se ha estimado
un nivel sin efectos adversos observados en el ser humano, que es de
0,03 a 0,05 mg/kg de peso corporal por día. Hay un margen de
seguridad de 150 entre el nivel sin efectos adversos observados
estimado y la ingesta diaria total máxima estimada, suponiendo que
el compuesto se absorba a través del agua de bebida contaminada y de
alimentos con elevado contenido de lípidos.
9.2 Evaluación de los efectos en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos; los crustáceos y peces son los más
vulnerables. Se ha establecido un nivel de riesgo para el medio
ambiente de 0,1 µg/litro. Se estima que la concentración ambiental
prevista máxima lejos de las fuentes equivale al nivel de riesgo
ambiental extrapolado multiplicado por dos y, por consiguiente, los
organismos acuáticos tal vez estén en peligro en las aguas de
superficie contaminadas. No pueden excluirse efectos adversos en
organismos bentónicos.
En vista de la toxicidad del hexaclorobutadieno para los
mamíferos, el consumo de organismos bentónicos o acuáticos por otras
especies tal vez sea motivo de inquietud.
The intestinal absorption of GPB and CPB was studied in rats by
infusing the compounds into the intestine via a biliary cannula.
When GPB was infused, both GPB and CPB were found in the blood in
approximately equal concentrations. Higher blood CPB concentrations
were found after CPB infusion than after GPB infusion (Gietl
et al., 1991).
In studies with radiolabelled hexachlorobutadiene, several
urinary metabolites were identified. The structure of these
metabolites further supported the hypothesis that
hexachloro-butadiene is bioactivated by glutathione conjugation.
ACPB was found to be the main metabolite (representing
approximately 80% of the radioactivity present in urine) excreted
after the administration of [14C] hexachlorobutadiene (200 mg/kg)
in female rats (Reichert & Schütz, 1986). The same authors also
identified 1-carboxymethylthion-1,2,3,4,4-pentachlorobuta-1,3-diene
and 1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) as
urinary metabolites (Reichert et al., 1985). It is the opinion of
the Task Group that the identification of MTPB by diazomethane
treatment of the urinary extract is questionable.
In male rats, 1,2,3,4,4-pentachloro-1,3-butadienyl sulfenic
acid (PBSA) is the only metabolite excreted in urine that has so far
been identified. The data presented suggest that ACPB is not a major
urinary metabolite of hexachlorobutadiene in male rats (Nash
et al., 1984). In urine of mice exposed to radiolabelled
hexachlorobutadiene (30 mg/kg), CPB, ACPB and
2,3,4,4-tetra-chlorobutenoic acid were identified as urinary
metabolites (Dekant et al., 1988a).
It is probable that 2,3,4,4-tetrachlorobutenoic acid is formed
by reaction of the intermediate thioketene with water and further
hydrolysis of the thionol acid thus formed (Dekant et al., 1988a).
The weight of evidence suggests that oxidative reactions
involving cytochrome P-450 have little role in the metabolism of
hexachlorobutadiene (Wolf et al., 1984; Dekant et al., 1988a).
6.3 Reaction with body components
The covalent binding of [14C]-hexachlorobutadiene-related
radioactivity to tissue proteins has been shown to be time
dependent, with the highest level occurring during the first 6 h
after treatment. The half-life of hexachlorobutadiene binding was
22 h in both liver and kidney (Reichert, 1983; Reichert et al.,
1985).
In a DNA binding study, rats received a single oral dose of
20 mg [14C]-hexachlorobutadiene, and DNA was isolated from the
kidneys of these rats at 6, 18.5 and 30 h after dose administration.
Although the results have been reported only in summary form,
various levels of radioactivity were recovered with the DNA, but
there was a marked variation in the level of radioactivity between
samples. Furthermore complete analysis of the DNA was not performed
and protein may have been associated with the DNA (Stott et al.,
1981).
Covalent binding to mouse liver and kidney DNA was demonstrated
after the oral administration of radiolabelled hexachlorobutadiene
(30 mg/kg body weight) in corn oil (Schrenk & Dekant, 1989). In the
liver and kidneys, the binding capacity of mitochondrial DNA was
significantly higher than that of nuclear DNA. The level of binding
to nuclear DNA in the liver was indistinguishable from that of
controls. HPLC separation of the hydrolysed DNA indicated the
presence of three distinct peaks of radioactivity.
6.4 Excretion
Following oral administration in rats and mice of single doses
of hexachlorobutadiene up to 100 mg/kg body weight, the total
excretion within 72 h was at least 65% of the dose. In mice, less
than half of a dose of 30 mg/kg body weight was metabolized (Dekant
et al., 1988a). In rats, assuming that the faeces mostly contain
unchanged compound and no non-resorbed conjugates, 44% of an orally
administered low dose of hexachlorobutadiene (1 mg/kg body weight)
was metabolized (Reichert et al., 1985). At higher doses the
percentage of hexachlorobutadiene metabolized decreased
dramatically. The biotransformation of hexachloro-butadiene in rats
appears to be a saturable process considering the reduced excretion
of carbon dioxide and renal metabolites at increasing doses (Davis
et al., 1980; Reichert et al., 1985; Reichert & Scuhtz, 1986;
Payan et al., 1991). This could be explained by saturation of the
gastrointestinal absorption, which was observed by Reichert et al.
(1985). It should be noted, however, that the observed increase in
the amount of unchanged hexachloro-butadiene in faeces with
increasing dose applies only up to 100 mg/kg body weight. At higher
dose levels, the amount of unchanged hexachlorobutadiene in faeces
decreases, probably due to a decrease in faecal output (Davis
et al., 1980; Nash et al., 1984).
The results of studies of Payan et al. (1991) on bile-duct
cannulated (see section 6.2.2) and intact (see Table 8) rats show
that saturation of gastrointestinal absorption indeed occurs
following oral administration.
Pharmacokinetic data concerning the fate of
hexachloro-butadiene in organisms were not available to the Task
Group.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic organisms
7.1.1 Short-term toxicity
A summary of short-term aquatic toxicity data is presented in
Table 9. In most of these studies the concentration of
hexachloro-butadiene was not reported. Therefore, the actual effect
concentrations may be lower than the nominal ones. In several cases
these nominal values far exceed the solubility limits. Based on
these data the substance is moderately to highly toxic to aquatic
organisms (Canton et al., 1990).
Adverse effects reported in some of these acute tests included
loss of equilibrium, erratic swimming (Leeuwangh et al., 1975;
Laseter et al., 1976), decreased activity, increased rate of
opercular movement, jumping (Leeuwangh et al., 1975), inverted
positions, fin fibrillation and muscle tetany (Laseter et al.,
1976). In a special investigation into kidney pathology, groups of
five goldfish (Carassius auratus) received one intraperitoneal
injection of hexachlorobutadiene at a dose level of 500 mg/kg body
weight in corn oil. They were subsequently fasted for up to 6 days
and sacrificed at different points in time up to day 7. Controls
received corn oil only. The temperature was kept between 18 and
21 °C. By 24 h the fish showed decreased activity, swam with dorsal
fins down, and had difficulty in following food. By day 4
exophthalmos, distended abdomen and ascites were observed. These
signs of toxicity were all reversible. Relative kidney weights were
elevated on day 4 only. From 12 h after exposure, P2 and P3
renal epithelial cells exhibited marked vacuolation and necrosis,
which persisted up to day 7. Increased gamma-glutamyl-transferase
(EC 2.3.2.2) staining was seen in P2 and P3 segments
(Reimschüssel et al., 1989).
In a report of this experiment, the fish were sacrificed at
different points in time up to day 70 after exposure. In one of the
two experiments the fish also received 5-bromo-2'-deoxyuridine 4 h
prior to sacrifice. Morphometric analysis of developing nephrons
showed an increase in the percentage of volume occupied by
basophilia clusters and developing nephrons from day 14 onwards. The
apparent number of basophilic clusters and developing nephrons per
unit surface area was also increased from day 14 (Reimschüssel
et al., 1990).
Table 9. Short-term aquatic toxicity of hexachlorobutadiene
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fresh water
Algae Haematococcus 20 stat, closed 24 EC10b > 2 Knie et al.
pluvialis (1983)
Bacteria Pseudomonas putida 25 7.0 stat, closed 16 TTc > 25 Bringmann &
Kuhn (1977)
Bacteria Pseudomonas putida 20 7.2 stat, open 0.5 EC10b > 0.9 Knie et al.
(1983)
Protozoans Chilomonas 20 6.9 stat, closed 48 TTc > 10 Bringmann et al.
paramecium (1980)
Molluscs great pond snail, 19 stat,d closed 24 LC50 0.21 Leeuwangh et
Lymnaea stagnalis 96 LC50 0.21 al. (1975)e
Crustaceans water flea, 20 7 250 stat, open 24 EC50 0.5 Knie et al.
Daphnia magna (1983)
aquatic sowbug, 19 stat,d closed 96 LC50 0.13 Leeuwangh et
Asellus aquaticus al. (1975)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Fish goldfish, 17.5 stat,d open 96 LC50 0.09 Leeuwangh et
Carassius auratus al. (1975)e
Fish zebrafish, 20 8.0 9.0 180 flow, closed 48 LC50 1 Slooff (1979)
Brachydanio rerio
Fish rainbow trout, LC50 0.320 US EPA (1980)
Salmo gairdnerii
Fish bluegill sunfish, LC50 0.326 US EPA (1980)
Lepomis macrochirus
Fish sheepshead minnow, LC50 0.557 US EPA (1980)
Cyprinodon variegatus
Fish golden orfe, 20 8 270 open 48 LC50 3 Knie et al.
Leuciscus idus (1983)e
Fish fathead minnow, 25 6.7-7.6 8.0 45 flow, open 96 LC50 0.10 Walbridge et
Pimephales promelas al. (1983)e
Table 9 (contd).
Organisms Species Temperature pH Dissolved Hardness Stat/flowa Exposure Parameter Concentration Reference
(°C) oxygen (mg CaCO3 open/closed period (mg/litre)
(mg/litre) per litre) (h)
Marine
Crustaceans harpacticoid 21 7.9 > 5 stat, open 96 LC50 1.2 Bengtsson &
copepod Tarkpea (1983)
grass shrimp, LC50 0.032 US EPA (1980)
Palaemonetes pugio
Mysid shrimp, LC50 0.059 US EPA (1980)
Mysidopsis bahia
Fish sailfin molly, 22-24 6.6-7.9 8-9 flow, open 26 LC50 4.2 Laseter et
Poecilia latipinna 30 LC50 4.5 al. (1976)e,f
77 LC50 1.4-1.9
115 LC50 1.7
138 LC50 1.2
pinfish, Lagodon LC50 0.399 US EPA (1980)
rhomboides
a static or flow-through test, open or closed system d semi-static (daily renewal) test
b effect is 10% reduction in oxygen consumption e analysis for hexachlorobutadiene was reported
c TT = toxic threshold for inhibition of cell multiplication f salinity was 0.25%, 96-h LC50 was calculated to be 1.6 mg/litre
7.1.2 Long-term toxicity
The cell multiplication of green algae (Scenedesmus
quadricauda) was not inhibited after 8 days of static exposure to
a nominal concentration of 25 mg/litre (well above pure water
solubility) in a closed system at 27 °C and a pH of 7 (Bringmann &
Kühn, 1977). A 14-day LC50 of 0.4 mg/litre was determined for
2- to 3-month old guppies (Poecilia reticulata) in a semi-static
test using an open system at 22 °C, a water hardness of 25 mg
CaCO3/litre, and a dissolved oxygen concentration of >
5 mg/litre. No analysis for hexachlorobutadiene was reported
(Könemann, 1981). In the same test under the same conditions, but
with analysis for the compound, the 14-day LC50 was 0.16 mg/litre
(Hermens et al., 1985).
In a study by Leeuwangh et al. (1975), groups of six goldfish
(Carassius auratus) were each exposed to hexachlorobutadiene in
tap water at measured concentrations of 0, 0.0003, 0.003, 0.0096 or
0.03 mg/litre for 49 and 67 days. The static test in an open system
was conducted at 19 °C, a pH of 7.6, and a dissolved oxygen
concentration between 3.2 and 6.3 mg/litre. Body weights were
decreased after 49 days at 0.03 mg/litre, and body weight gain was
still reduced at 67 days. Abnormal behaviour, jumping,
incoordination, increased opercular movement and overall immobility
were noted at 0.0096 mg/litre. After 49 days at 0.0096 mg/litre (no
data at 0.03 mg/litre), relative liver weights were increased, and
the activity of liver glucose-6-phosphatase (EC 3.1.3.9) was
decreased, whereas the activity of liver glucose-6-phosphate
dehydrogenase (EC 1.1.1.49) was increased. After 67 days the
activity of liver phenylalanine hydroxylase (EC 1.14.16.1) showed a
concentration-related increase. No effects were found on haemoglobin
concentration and haematocrit after 49 days or on the activity of
serum alanine aminotransferase (EC 2.6.2.1) and serum alkaline
phosphatase (EC 3.1.3.1) after 67 days.
Groups of 12 largemouth bass (Micropterus salmoides) were
each exposed to hexachlorobutadiene for 10 days at measured
concentrations of 0.00343 and 0.03195 mg/litre in filtered tap water
with a salinity of 0.08-0.1%, at 22-24 °C, a pH of 6.6-7.9 and a
dissolved oxygen concentration of 7.6-8.5 mg/litre. A control group
comprised 12 water and 12 vehicle (acetone) controls. Plasma
cortisol levels were increased at both concentrations, but
haematocrit values were not affected. At the higher concentration
there was leukocytic infiltration in the kidneys of one of the fish
and paleness and accentuated lobulation of parenchyma in the livers
(Laseter et al., 1976).
In an early lifestage test, four replicate groups, each of 30
fathead minnow (Pimephales promelas) eggs, 2-4 h after spawning,
were exposed to measured hexachlorobutadiene concentrations in
sand-filtered and sterilized lake water of 0.0017, 0.0032, 0.0065,
0.013 and 0.017 mg/litre in an open system. Following hatching
(4-5 days after spawning), four replicate groups of 15 larvae
continued to be exposed for 28 days. Control groups of equal size
were exposed to slightly contaminated water containing
0.00008 mg/litre. The temperature was 25 °C, pH was 7.4, dissolved
oxygen concentration 7 mg/litre, and water hardness 45 mg
CaCO3/litre. The hatchability of embryos and the percentage of
normal larvae at hatch were not affected. An increased fish
mortality and a concentration-related decrease of body weight were
observed at the two highest concentrations at the end of the
exposure period (Benoit et al., 1982).
7.2 Terrestrial organisms
7.2.1 Short-term toxicity
Except for one test with birds, reliable tests with terrestrial
organisms have not been reported.
Groups of 12 female and four male Japanese quails (Coturnix
coturnix japonica) were exposed to a diet containing
hexachloro-butadiene at levels of 0, 0.3, 3, 10 or 30 mg/kg diet for
90 days. Each cage contained three females and one male of the same
dose group. Feed analysis indicated levels close to the nominal
values. Adults were all histopathologically examined. Eggs were
collected on days 37-46, 64-73, and 81-90. Six adults died during
the study: 4 at 0.3 mg/kg, 1 at 10 mg/kg, and 1 at 30 mg/kg, but
this was not considered to be related to treatment. The survival of
chicks from eggs collected on days 81-90 was decreased at 10 mg/kg
only. Egg production, the percentage of fertile eggs, the percentage
of hatchable eggs, and eggshell thickness were unaffected compared
to controls (Schwetz et al., 1974).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The available mortality data are summarized in Table 10.
8.1.1 Inhalation exposure
8.1.1.1 Mortality
In the only reported study of the mortality of
hexachloro-butadiene following inhalation, groups of 20 SPF mice of
the OF1 strain were exposed for 6 h (Gage, 1970). The vapour
concentrations were measured by gas chromatography, and the
concentrations were within 10% of the nominal value. Results are
shown in Table 10.
8.1.1.2 Systemic effects
The decrease in respiratory rate (reflex bradypnoea) in groups
of six male Swiss OF1 mice was measured following exposure for
15 min to hexachlorobutadiene vapour at concentrations between 886
and 2625 mg/m3. The vapour concentrations were checked by gas
chromatography. The mice were restrained in a body plethysmograph,
while the heads were extended into an inhalation chamber. The 50%
effect level, calculated from the concentration-effect curve, was
2250 mg/m3 (De Ceaurriz et al., 1988).
8.1.2 Oral exposure
8.1.2.1 Mortality
Based on acute mortality data, hexachlorobutadiene is slightly
to moderately toxic to adult rats, moderately toxic to male weanling
rats, and highly toxic to female weanling rats following ingestion.
Young rats are far more sensitive than adult rats (Kociba et al.,
1977a). Gradiski et al. (1975) observed delayed mortality (after
24 h) in oral LD50 studies on rats and mice.
8.1.2.2 Systemic effects
Hexachlorobutadiene mainly affects the kidneys and, to a lesser
extent, the liver. The effects on these organs and the related
biochemical findings are discussed extensively in sections 8.8.2
(liver) and 8.8.3 (kidney). In oral LD50 studies on rats and mice,
Gradiski et al. (1975) observed hyper-reactivity just after
exposure, followed by decreased activity and staggering.
Table 10. Mortality of hexachlorobutadiene from single exposure
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Mouse oral 87 (78.1-95.9) Murzakayev (1963)b
OF1 adult female inhalation (6 h) 14 107 (102-113) Gage (1970)
OF1 adult female oral 14 65 (60-70) Gradiski et al. (1975)c
OF1 adult male oral 14 80 (75-85) Gradiski et al. (1975)c
Alderley Park adult male intraperitoneal 14 67 (53-85) Lock et al. (1984)e
Alderley Park adult female intraperitoneal 14 85 (65-111)
C57BL/10J adult male intraperitoneal 14 57 (41-81)
C3H adult male intraperitoneal 14 25-75
BALB/c adult male intraperitoneal 14 32-40
DBA/2J adult male intraperitoneal 14 53 (36-76)
Rat oral 350 (323-377) Murzakayev (1963)b
OF2 rat adult female oral 14 270 (250-290) Gradiski et al. (1975)c
OF2 rat adult male oral 14 250 (220-280) Gradiski et al. (1975)c
Sprague-Dawley adult female oral 200-400 Kociba et al. (1977a)d
Sprague-Dawley adult male oral 580 (504-667) Kociba et al. (1977a)d
Sprague-Dawley weanling female oral 46 (26-81) Kociba et al. (1977a)d
Sprague-Dawley weanling male oral 65 (46-91) Kociba et al. (1977a)d
Table 10 (contd).
Species/strain Age Sex Route of exposure Observation LD50 (mg/kg Reference
period (days) body weight)
or LC50 (mg/m3)a
Alderley Park weanling male intraperitoneal 7 57 (38-87) Hook et al. (1983)e
Alderley Park 29 days male intraperitoneal 7 96 (72-128)
Alderley Park adult male intraperitoneal 7 360 (325-396)
Guinea-pig oral 90 (81.5-98.5) Murzakayev (1963)b
Rabbit
New Zealand adult female dermal (8 h) 14 1120 (890-1400) Duprat & Gradiski (1978)f
a When available, 95% confidence limits are reported between brackets.
b Observation period, strain, sex, age (or body weight) and vehicle were not reported.
c Vehicle was olive oil.
d Confidence limits not calculable; observation until no toxicity was observed any longer; vehicle was corn oil.
e Vehicle was corn oil.
f Application undiluted using glass vials (3.6 cm2).
8.1.3 Dermal exposure
8.1.3.1 Mortality
Hexachlorobutadiene was harmful to rabbits following acute
dermal exposure (LD50 = 1120 mg/kg body weight; (range,
890-1400 mg/kg; Table 10). After a dose of 780 mg/kg body weight,
death occurred within 24 h from respiratory and cardiac failure
(Duprat & Gradiski, 1978).
8.1.3.2 Systemic effects
New Zealand rabbits dermally exposed to undiluted
hexa-chlorobutadiene at doses of 1170 and 1550 mg/kg body weight
exhibited stupor, dyspnoea and cyanosis (Duprat & Gradiski, 1978).
8.1.4 Other routes of exposure
Hexachlorobutadiene has been shown to be toxic to various
strains of mice after intraperitoneal injection (Lock et al.,
1984) and harmful to adult rats (Hook et al., 1983). The compound
was considerably more toxic to young and weanling rats (Table 10).
Rats intraperitoneally exposed to single lethal doses of
hexachlorobutadiene from 500 to 1000 mg/kg in corn oil exhibited
piloerection, sedation, hunching, incoordination, loss of muscle
tone and hypothermia (Lock & Ishmael, 1979). A macroscopic and
haematological investigation of rats intraperitoneally exposed to
doses between 121 and 336 mg/kg body weight in olive oil did not
reveal any damage to the gastrointestinal tract, spleen, heart or
gonads. In the lungs, congestion, haemorrhage, and oedema were
observed, but these were attributed by the authors to ether
anaesthesia. At dose levels of 213 mg/kg body weight or more,
lymphopenia and related neutrophilia were induced (Gradiski et al.,
1975).
8.2 Short-term exposure
8.2.1 Inhalation exposure
In the only inhalation study published, groups of four adult
Alderley Park SPF rats of each sex were dynamically exposed to
nominal concentrations of 53, 107 or 267 mg/m3 6 h/day for 15
days, 1067 mg/m3 6 h/day for 12 days, or 2668 mg/m3 4 h/day for
2 days. Petroleum ether was used as a solvent for concentrations
below 1067 mg/m3. Many other chemicals were tested similarly, and
batches of control rats of unknown size were maintained at intervals
of 2 months during the whole experimental period. No analysis for
hexachlorobutadiene was carried out. Two of the four female rats
exposed to 1067 mg/m3 died, and autopsy revealed pale, enlarged
kidneys, adrenal regeneration and renal cortical tubular
degeneration with epithelial regeneration. Rats of both sexes lost
weight at 1067 mg/m3 and the weight gain of females was reduced at
107 and 267 mg/m3. Irritation of eyes and nose was observed at the
two highest levels. At 267 and 1067 mg/m3 rats were in a poor
condition, females being more affected than males. Respiratory
difficulties were seen at and above 267 mg/m3. Haematological
examination at the end of the exposure period showed slight anaemia
in females at 1067 mg/m3. Urinalysis did not reveal abnormalities
at any of the exposure levels. Macroscopically enlarged, pale
kidneys were found at 267 and 1067 mg/m3 and enlarged adrenals at
1067 mg/m3. Histopatho-logical investigations revealed proximal
tubular degeneration in the kidneys and cortical degeneration in
adrenals at concentrations of 267 mg/m3 or more. No toxic signs
were observed at the lowest exposure level and autopsy revealed no
gross abnormalities (Gage, 1970).
8.2.2 Oral exposure
8.2.2.1 Rats
Groups of five adult male Sprague-Dawley rats were exposed to
daily oral doses of hexachlorobutadiene (0, 0.2 or 20 mg/kg body
weight) in corn oil for 3 weeks. Only the kidneys were examined
histologically. At the higher dose level, body weight gain was
decreased and relative kidney weight increased. Histopathological
examination of the kidneys revealed damage in the middle and inner
cortical region, including loss of cytoplasm, nuclear pyknosis,
increased basophilia and mitotic activity, and increased cellular
debris. No toxic signs were observed at a dosage of 0.2 mg/kg per day
(Stott et al., 1981).
In a study by Kociba et al. (1971), groups of four female
Sprague-Dawley rats consumed for 30 days a diet containing
hexachlorobutadiene, which resulted in ingested nominal daily doses
of 0, 1, 3, 10, 30, 65 and 100 mg/kg body weight. Analysis of the
compound in the feed was not reported. Body weights were decreased
at the two highest dose levels. At 10 mg/kg or more, a dose-related
decrease in body weight gain was observed along with a decrease in
food consumption. There was also an increase in haemoglobin
concentrations, which, although significant, was not clearly dose
related. There was no effect on serum alanine aminotransferase
activity (EC 2.6.1.2). A dose-related increase in relative kidney
weight was observed at dose levels of 3 mg/kg or more.
Histopathological examination, which was restricted to liver and
kidneys, showed proximal tubular degeneration, individual cell
necrosis, and regenerative changes in the kidneys at doses of
10 mg/kg or more. Hepatocellular swelling was seen at 100 mg/kg. The
no-observed-adverse-effect level (NOAEL) was 1 mg/kg body weight per
day.
In a 2-week experiment, groups of six weanling Wistar-derived
rats of each sex were exposed to measured dietary
hexachlorobutadiene levels of 0, 73, 182 or 447 mg/kg (the Task
Group considered this equivalent to doses of 0, 7.3, 18.2 and
44.7 mg/kg body weight per day, respectively). At all dose levels,
body weight and food conversion efficiency (g of weight gain/g of
food) were decreased in a dose-related manner. Food consumption per
g of body weight was decreased at 44.7 mg/kg body weight. Relative
kidney weights were increased at the two highest dose levels. At all
dose levels a dose-related degeneration of kidney tubular cells was
observed, especially in the straight limbs of the proximal tubules
located in the outer medulla. No toxic signs were observed in the
liver. A NOAEL was not found (Harleman & Seinen, 1979).
In a follow-up to the dietary study, groups of 10 weanling
Wistar-derived rats of each sex received daily doses by gavage of 0,
0.4, 1, 2.5, 6.3 or 15.6 mg/kg body weight in peanut oil for 13
weeks (Harleman & Seinen, 1979). Body weight gain, food consumption
and food utilization efficiency were decreased at 6.3 and
15.6 mg/kg. Polyuria was observed in females at these dose levels
after week 10, while a dose-related decrease in urine osmolarity
occurred at dose levels of 2.5 mg/kg or more. In males, the latter
effect was observed at 15.6 mg/kg. No other changes were observed in
urinalysis (after week 10) and haematological investigations (after
week 8). A dose-related increase in relative kidney weight was
measured in males of all treatment groups but only at 6.3 mg/kg or
more in females. Dose-related increases in the relative weight of
liver and spleen were measured at 6.3 mg/kg or more.
Histopathological examinations revealed changes in liver and
kidneys. In the livers of males dosed with 6.3 mg/kg or more, an
increased basophilic, flocky granulation was observed. At dose
levels of 6.3 mg/kg or more in males and 2.5 mg/kg or more in
females, there was a dose-related degeneration of the renal proximal
tubules, as shown by hyperchromatic nuclei, hypercellularity,
vacuolation and focal necrosis of epithelial cells and a diminished
brush border. No adverse effects were observed at daily doses of
1 mg/kg in females or 2.5 mg/kg in males (Harleman & Seinen, 1979).
8.2.2.2 Mice
In a two-week study, groups of five B6C3F1 mice of each sex
were fed a diet containing hexachlorobutadiene at nominal doses of
0, 30, 100, 300, 1000 or 3000 mg/kg feed for 15 days (calculated by
the Task Group to be equivalent to 0, 4.3, 14.3, 43, 143 and
430 mg/kg body weight per day, respectively, using standard values
for average body weight and food consumption in mice). Analysis of
the feed was carried out by gas chromatography, and no more than 9%
loss of the chemical was observed in one day (feed was replaced
every 2 days). All mice given 143 or 430 mg/kg body weight died or
were sacrificed in a moribund condition within 7 days. A
dose-related growth retardation was observed. At the two highest
doses, the observed toxic effects included renal tubular necrosis,
hepatic cytoplasmic vacuolization, and testicular degeneration
characterized by the presence of syncytial giant cell formation of
spermatocytes. At dose levels of 43 mg/kg body weight or more,
minimal to mild depletion of bone marrow (characterized by a
decrease in the haematopoietic cells) was seen in two out of five
mice of both sexes per dose group. At dose levels of 4.3, 14.3 and
43 mg/kg body weight, at which all animals survived to the end of
the study, renal tubular cell regeneration was observed (Yang
et al., 1989; Yang, 1991).
In a 13-week study, groups of 10 B6C3F1 mice of each sex were
fed a diet containing hexachlorobutadiene at concentrations of 0, 1,
3, 10, 30 or 100 mg/kg feed. Using measurements of food consumption
and body weight, the authors determined doses of 0, 0.1, 0.4, 1.5,
4.9 or 16.8 mg/kg body weight per day for males, and 0, 0.2, 0.5,
1.8, 4.5 or 19.2 mg/kg body weight per day for females. Analysis of
the compound was carried out as in the two-week study. No
treatment-related clinical signs or deaths were observed. The
motility of sperm from treated mice was significantly lower than in
controls, although this effect was not dose related (Yang et al.,
1989; Yang, 1991). Body weight gain was reduced at dose levels of
4.5 and 19.2 mg/kg body weight in females. Reductions in kidney
weight occurred at dose levels of 1.5 mg/kg or more in males and at
19.2 mg/kg body weight in females, and reductions in heart weight
occurred at 19.2 mg/kg body weight in males. Necropsy revealed a
treatment-related increase in renal tubular regeneration (prominent
in the outer stripe of the medulla) at dose levels of 0.2 mg/kg body
weight or more in females (Table 11). Although the author concluded
that a NOAEL was not observed for females, the Task Group noted that
the occurrence of renal tubular regeneration in one out of ten
female mice in the 0.2-mg/kg body weight group is insufficient
evidence of an adverse effect at this dose level in females.
Table 11. Incidences of renal tubular regeneration in 13-week
feed studies on B6C3F1 micea
Concentration Dose (mg/kg body Number of mice with
(mg/kg feed) weight per day) lesions/number examined
Male Female Male Female
0 0 0 0/10 0/10
1 0.1 0.2 0/10 1/10
3 0.4 0.5 0/10 9/10
10 1.5 1.8 0/9 10/10
30 4.9 4.5 10/10 10/10
100 16.8 19.2 10/10 10/10
a Modified from: Yang (1991)
8.3 Long-term exposure
No long-term inhalation studies have been reported. A study
describing long-term exposure of mice by the dermal route is
presented in section 8.7.3.
An oral toxicity/carcinogenicity test in rats has been reported
(see section 8.7.2).
8.4 Skin and eye irritation; sensitization
8.4.1 Irritation
The vapour of hexachlorobutadiene has been found to be
irritating to the eyes and nose of rats (Gage, 1970; see section
8.2.1).
Groups of six New Zealand rabbits received either 0.78 g
(0.5 ml) of undiluted hexachlorobutadiene on the intact or abraded
skin for 24 h, or 0.15 g (0.1 ml) in the conjuctival sac of the left
eye. Assessment of the degree of irritation was conducted according
to Draize et al. (1944) and by calculating the primary irritation
index. Hexachlorobutadiene was moderately irritating for the skin
(primary irritation index 4) but not irritating for the eyes
(primary irritating index 1.5). Moderate conjunctivitis, epithelial
abrasion and, at day 7, epithelial keratitis were observed in the
eyes (Duprat et al., 1976).
Duprat & Gradiski (1978) applied undiluted hexachloro-butadiene
to New Zealand rabbits at doses of 0.39, 0.78, 1.17 and 1.55 mg/kg
body weight (0.25, 0.50, 0.75 and 1.00 ml, respectively) under
occluded conditions, using glass vials, for 8 h. The observation
period was 14 days. The skin was histopathologically examined in all
dead animals, in half the survivors at day 15, and in the remaining
survivors at day 36. After 12 h of exposure to the two highest
doses, epidermis and subcutaneous tissue revealed oedema and
polymorphonuclear leukocyte infiltration. In the epidermal cells,
degeneration with pyknosis of nuclei and perinuclear oedema, and
focal separation from the corium with vesicle formation were seen.
After 3 to 5 days of exposure to the three highest doses, dermal
necrosis was observed, leading to eschar formation and partial
destruction of hair follicles. The effects increased with time, not
with dose. Two to five weeks after application, repair was apparent
at all dose levels, with scarring and upper dermis fibrosis, and
epidermal acanthosis with focal dyskeratosis. Diffuse mononuclear
infiltrate was seen in the dermis.
8.4.2 Sensitization
A group of 20 Hartley guinea-pigs were treated according to the
Magnusson-Kligman protocol by intradermal injections of 5%
hexachlorobutadiene in peanut oil and, after one week and subsequent
treatment by sodium lauryl sulfate, by a 48-h dermal application of
a 25% suspension of the chemical in vaseline. The challenge was
performed by dermal application of a 20% suspension in vaseline. A
group of five controls was induced similarly and challenged by
vaseline only. All exposed animals, but none of the controls, showed
a positive reaction. The test was repeated in the same fashion
without adjuvant in five guinea-pigs: all animals showed positive
reactions (Gradiski et al., 1975).
8.5 Reproduction, embryotoxicity and teratogenicity
8.5.1 Reproduction
A group of female albino rats was exposed to one dose of
hexachlorobutadiene (20 mg/kg body weight) administered
subcutaneously before mating. Within 90 days after exposure, all 86
newborn rats had died, compared with 13 of the 61 controls. The
offspring from exposed dams were reported to show excitation,
disturbances of motor coordination, a decrease in appetite and a
loss of weight, lymphocytosis, neutropenia, myelocytes, Jolly's and
Cabeau's bodies, pneumonia, bronchitis, granular dystrophy of renal
cells, glomerulonephritis, inflammatory destructive lesions of the
gastrointestinal tract and vascular hyperaemia (Poteryayeva, 1966).
The Task Group noted major deficiencies and incomplete reporting of
the experiment, the unusual route of administration, and the high
percentage of mortality in control rats.
Groups of 10-12 male and 20-24 female Sprague-Dawley rats
received a diet containing hexachlorobutadiene at dose levels of
0.2, 2.0 or 20 mg/kg body weight per day for 90 days prior to
mating, 15 days during mating, and subsequently throughout gestation
and lactation. In the mating period, two females were placed with
one male of the same dose group. The control group consisted of
17 males and 34 females. The diets were reportedly analysed for the
test compound. No mortality was observed. At 20 mg/kg, adults showed
decreased food consumption and body weight gain. Blood urea
nitrogen, serum alanine aminotransferase (EC 2.6.1.2) and serum
creatinine were unchanged compared to controls. The dams had an
increased relative brain weight and the male rats had an increased
relative liver weight at 20 mg/kg. The relative kidney weights were
increased in both sexes at 20 mg/kg. At 2 and 20 mg/kg the kidneys
of adult rats revealed dose-related tubular dilatation and
hypertrophy with foci of epithelial degeneration and regeneration;
however, there was no effect at 0.2 mg/kg. The only adverse
reproductive effect in neonates was a decreased weanling weight at
20 mg/kg. There was no detectable effect on the percentage
pregnancy, the period from first cohabitation to delivery, survival
indices, sex ratio, histopathology of weanlings, and the incidence
of skeletal alterations and abnormalities in neonates (Schwetz
et al., 1977).
In a third reproductive study, groups of six female SPF
Wistar-derived rats, 10 weeks of age, received a diet containing
hexa-chlorobutadiene at levels of 0, 150 and 1500 mg/kg diet
(estimated by the Task Group to be equivalent to 0, 7.5 and 75 mg/kg
body weight per day) for 3 weeks prior to mating, 3 weeks during
mating, and subsequently throughout gestation and lactation. In the
mating period, two untreated males were placed with the females,
after which the females were housed individually. The 75-mg/kg
female adults were killed in week 10, while those given 0 or
7.5 mg/kg were killed in week 18. Food analysis at the low dose
level revealed hexachlorobutadiene levels within 96% of nominal
values after 1 week and within 81% of nominal values after 2 weeks.
Diets were prepared weekly. There was a reduced body weight gain by
female rats in the two groups receiving hexachlorobutadiene.
Weakness of hind legs, unsteady gait, incoordination and ataxia were
seen at 75 mg/kg. The relative kidney weight was increased at both
dose levels. Histopathological investigations revealed
hypercellularity of epithelial cells, hydropic degeneration, and
necrosis of proximal tubules in the kidneys at 7.5 mg/kg. At
75 mg/kg, slight proliferation of bile duct epithelial cells,
fragmentation and demyelination of single fibres of the femoral
nerve, and extensive renal degeneration were observed. Again at
75 mg/kg, no conceptions occurred, the ovaries showing little
follicular activity, and there was no uterine implantation sites. At
7.5 mg/kg fertility and litter size were reduced, but not
significantly. In both the control and 7.5-mg/kg groups, the
resorption quotient was low. Compared to controls, pup weights were
reduced significantly on days 0, 10 and 20 in the 7.5-mg/kg group.
No gross abnormalities were observed (Harleman & Seinen, 1979).
8.5.2 Embryotoxicity and teratogenicity
In a teratology study, groups of 24-25 female rats were exposed
to hexachlorobutadiene vapour at measured concentrations of 0, 21,
53, 107 or 160 mg/m3 for 6 h per day from days 6 to 20 of
pregnancy. The breathing zone atmosphere was analysed by gas
chromatography. Maternal weight gain decreased at 53 and
160 mg/m3. At the other two exposure levels, the slight decrease
in maternal weight was not significant. The mean number of
implantation sites, total fetal losses, resorptions, live fetuses,
incidences of pregnancy, and sex ratio were not affected by exposure
to hexachlorobutadiene, compared to controls. Fetal body weight was
reduced in both sexes at 160 mg/m3. The incidences of external,
visceral, and skeletal alterations were not significantly increased
(Saillenfait et al., 1989).
In a study by Hardin et al. (1981), groups of 10-15 mated
Sprague-Dawley rats received hexachlorobutadiene in corn oil by
intraperitoneal injection at a dose level of 10 mg/kg body weight
per day from days 1 to 15 of gestation. It was reported (without
further details) that at least two maternal organ weights were
changed and that pre- or postimplantation survival was reduced.
Maternal tissues did not reveal histopathological effects. Fetuses
had a reduced weight or length, a 1-2 day delay in heart
development, and dilated ureters. No grossly visible external or
internal malformations were observed (Hardin et al., 1981).
It was reported briefly by Badaeva et al. (1985) that daily
oral administration of hexachlorobutadiene to pregnant rats at a
dose level of 8.1 mg/kg body weight per day resulted in
histopathologi-cal changes of nerve cells and myelin fibres of the
spinal cord in the dams and their offspring.
8.6 Mutagenicity and related end-points
8.6.1 In vitro effects
Purified hexachlorobutadiene induces gene mutations in the Ames
Salmonella test when specific incubation conditions are employed.
In preincubation assays adapted to include rat liver microsomes
and additional reduced glutathione, hexachlorobutadiene induced
point mutations in Salmonella typhimurium TA100 (Vamvakas et al.,
1988a). Assays lacking specialized metabolic activation conditions
have generally yielded negative results (Table 12).
Data from bacterial mutagenicity assays are consistent with the
proposed scheme for the biotransformation of hexachlorobutadiene in
animals (section 6.3; Fig. 1). Activity in S. typhimurium TA100,
mediated by subcellular fractions of rat kidney, was inhibited by
the addition of the ß-lyase inhibitor, AOAA (Vamvakas et al.,
1988a, 1989a) and the gamma-glutamyltranspeptidase inhibitor,
acivicin (Vamvakas et al., 1989a).
Several of the proposed metabolites of hexachlorobutadiene have
been assayed for mutagenic activity in S. typhimurium TA100
(Table 13). The mono-glutathione (GPB) and mono-cysteine (CPB)
conjugates were mutagenic in the presence or absence of rat kidney
S9. Rat liver microsomes and mitochondria that exhibit high
gamma-glutamyltranspeptidase activities strongly enhanced the
mutagenic potency of GPB in the presence of additional glutathione,
in contrast to liver microsomes that exhibit lower
gamma-glutamyltranspeptidase activity. Furthermore, AOAA and
acivicin both inhibit the activation of GPB mediated by kidney
fractions. The di-glutathione (BGTB) and di-cysteine (BCTB)
conjugates of hexachlorobutadiene were not mutagenic either in the
presence or absence of rat kidney S9 (Green & Odum, 1985; Dekant
et al., 1986; Vamvakas et al., 1988a, 1989a).
The mercapturic acid, ACPB, was mutagenic both in the presence
and absence of rat liver S9. It has been suggested that the
metabolism of ACPB in animals is catalysed by an N-deacetylase and
by ß-lyase (section 6.3) (Reichert et al., 1984). The Task Group
considered that S. typhimurium possesses both of these enzymes
activities. Both MTPB and CMTPB gave negative results in tests with
S. typhimurium TA100 and are considered to be detoxified
metabolites of hexachlorobutadiene (Wild et al., 1986).
Table 12. Studies on mutagenicity of hexachlorobutadiene
Test description Species/strain/cell type Conditionsa Resultc Reference
Reverse mutations Salmonella typhimurium TA98, +/- rat liver S9, purity 98%, - De Meester et al.
TA100, TA1530, TA1535, TA1538 plate incorporation (1981)
S. typhimurium TA100 +/- rat liver S9, purity > 99%, - Stott et al. (1981)
plate incorporation
S. typhimurium TA98, TA100 +/- rat liver S9, purity not - Reichert et al. (1983)
reported, suspension testb
S. typhimurium TA98, TA100, +/- rat liver S9, purity not - Haworth et al. (1983)
TA1535, TA1537 reported, preincubation test
+ rat liver S9*, purity > 99.5%, +
preincubation test
S. typhimurium TA100, TA1535 +/- rat liver S9, purity not - Chudin et al. (1985)
TA1538 reported, plate incorporation
S. typhimurium TA100 +/- rat liver S9, - Reichert et al. (1984)
- rat liver S9* +
S. typhimurium TA100 + rat liver S9*, purity >99.5%, +d Wild et al. (1986)
preincubation test
S. typhimurium TA100 no activation, purity 98% + Vamvakas et al. (1988a)
no activation, purity > 99.5%, -
preincubation test
Table 12 (contd).
Test description Species/strain/cell type Conditionsa Resultc Reference
S. typhimurium TA100 + rat liver microsomes - Vamvakas et al. (1988a)
without additional GSH
+ rat liver microsomes and +e
additional GSH, purity > 99.5%,
plate incorporation
Sex-linked lethals Drosophila melanogaster feeding or injection - Woodruff et al. (1985)
Chromosome aberrations CHO cells +/- rat liver S9 - Galloway et al. (1987)
Chromosome aberrations human lymphocytes - rat liver S9 - German (1988)
Sister chromatid exchanges CHO cells +/- rat liver S9 + Galloway et al. (1987)
Chromosome aberrations mouse bone marrow cells inhalation, 4 h + German (1988)
Chromosome aberrations mouse bone marrow cells oral gavage + German (1988)
a S9* = a fortified S9 mix containing 3 times the normal protein concentration; GSH = reduced glutathione
b The extreme toxicity of the compound without S9 was supposed to exclude testing in this system
c + = > twice the background rate or, in the case of bacterial studies, a reproducible dose-related increase in the number of
revertants per plate; - = negative
d 0.23 revertants per nmol
e Addition of rat kidney microsomes further increased the number of revertants; positive results were inhibited by the ß-lyase inhibitor
aminooxyacetic acid
Chinese hamster ovary (CHO) cells were exposed to between 5 and
24 mg hexachlorobutadiene/litre for 2 h in the presence of rat liver
S9 and throughout the incubation period (8-26 h, depending on cell
cycle delay) in the absence of rat liver S9. In comparison with
concurrent controls, no significant increase in chromosome
aberration frequency was observed (Galloway et al., 1987). In a
further study, in which human lymphocyte cultures were exposed to
between 0.01 and 0.001 mg hexachlorobutadiene per litre in the
absence of S9 for 27 h, there was also no clastogenic effect. At the
highest dose level there was a reduction of approximately 60% in the
mitotic index of human lymphocyte cultures (German 1988). However,
hexachlorobutadiene at a dose level of at least 4 mg/litre did cause
a significant increase in the frequency of sister chromatid exchange
in CHO cells in both the presence and absence of rat liver S9
(Galloway et al., 1987).
Hexachlorobutadiene was found to induce unscheduled DNA
synthesis (UDS) in Syrian hamster embryo fibroblast cultures.
Moreover, the magnitude of the response was increased when a
preincubation period with rat liver S15 was employed (Schiffman et
al., 1984). However, there was no induction of UDS in a study
using rat hepatocyte cultures (Stott et al., 1981).
In summary, the Task Group concluded that hexachloro-butadiene
was genotoxic in vitro and that the negative results reported in
some studies may have resulted from the use of inappropriate
conditions for metabolic activation.
8.6.2 In vivo effects
Hexachlorobutadiene induced a significant increase in the
frequency of chromosomal aberrations in mouse bone marrow cells
following the administration of acute oral doses of 2 or 10 mg/kg
body weight or acute inhalation exposure to 10 mg/m3 for 4 h. Both
experiments used six mice per dose group, and the animals were
sacrificed after 24 h (German, 1988).
Six hours after the administration of a single oral dose of
20 mg hexachlorobutadiene/kg body weight to two groups of five male
Sprague-Dawley rats, there were statistically significant increases
in kidney UDS of 27% and 54% above concurrent control levels.
Administration of a positive control substance,
dimethyl-nitrosamine, resulted in an increase of 187% over controls
(Stott et al., 1981).
As described in section 6.3, radiolabelled nucleotides were
recovered from the kidneys of rats and mice administered
14C-labelled hexachlorobutadiene by gavage (Stott et al., 1981;
Schrenk & Dekant, 1989). The Task Group concluded that these studies
indicated covalent binding of hexachlorobutadiene or its metabolites
Table 13. Tests for reverse mutations in Salmonella typhimurium TA100 by proposed metabolites of hexachlorobutadiene
Metabolite and abbreviationa Conditionsb Resultc Reference
1-(glutathion- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (GPB) no activation - Green & Odum (1985)
+ rat kidney S9 +
+/- rat kidney fractions +d Vamvakas et al. (1988a)
1,4-(bis-glutathion- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BGTB)
1,4-(bis-cystein- S-yl)-1,2,3,4-tetrachloro-1,3-butadiene +/- rat kidney fractions - Vamvakas et al. (1988a)
(BCTB)
1-(cystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene (CPB) +/- rat kidney S9 +e Green & Odum (1985)
no activation +f Dekant et al. (1986)
1-( N-acetylcystein- S-yl)-1,2,3,4,4-pentachloro-1,3-butadiene - rat liver S9 - Wild et al. (1986)
(ACPB) + rat liver S9 +g
1-carboxymethylthio-1,2,3,4,4-pentachloro-1,3-butadiene + rat liver S9* - Wild et al. (1986)
(CMTPB)
Table 13 (contd).
Metabolite and abbreviationa Conditionsb Resultc Reference
1-methylthio-1,2,3,4,4-pentachloro-1,3-butadiene (MTPB) + rat liver S9 - Wild et al. (1986)
2,2,3,4,4-pentachloro-3-butenoic acid (PBA) +/- rat liver S9 + Reichert et al. (1984)
2,2,3,4,4-pentachloro-3-butenoic acid chloride (PBAC) +/- rat liver S9 + Reichert et al. (1984)
a See also Figure 1
b Plate incorporation assays with, except in the case of the tests by Green & Odum, preincubation; S9* = a fortified S9 mix containing
3 times the normal protein concentration
c + = > twice background rate; - = negative
d Mutagenic potency enhanced by rat kidney microsomes or mitochondria and less so by cytosol; positive results were inhibited by the
ß-lyase inhibitor aminooxyacetic acid
e Mutagenic potency enhanced by rat kidney S9
f Positive results were inhibited by the ß-lyase inhibitor aminooxyacetic acid
g 18.7 revertants per nmol; mutagenic potency decreased by addition of pyridoxal phospate; activation by cytosol, with and without cofactors,
had the same results as S9 mix; microsome mix was inactive
to kidney DNA in vivo. The study with mice showed that the level
of binding to mitochondrial DNA was greater than that to nuclear
DNA. In addition, radioactivity was recovered in mitochondrial DNA,
but not nuclear DNA, from mouse liver (Schrenk & Dekant, 1989).
Hexachlorobutadiene did not induce sex-linked recessive lethal
mutations in Drosophila melanogaster following treatment of adults
either via the diet or by injection (Woodruff et al., 1985).
8.7 Carcinogenicity/long-term toxicity
8.7.1 Inhalation exposure
No long-term carcinogenicity studies, where inhalation was the
route of exposure, have been reported.
8.7.2 Oral exposure
In a study by Kociba et al. (1977a,b), groups of 39-40
adult Sprague-Dawley rats of each sex received a diet containing
hexachlorobutadiene at 0.2, 2 or 20 mg/kg body weight per day for 22
(males) or 24 (females) months. Control groups comprised 90 rats of
each sex. Analysis for the compound was not reported. An increased
mortality was observed in males at 20 mg/kg. Hexachlorobutadiene
caused a depression of the body weight gain in both sexes at the
highest dose level without any effect on food consumption.
Haematological investigations performed at 12-14 and 22-24 months,
revealed a slight, but statistically significant, depression in the
red blood cell count of males at 20 mg/kg (22 months). Urinalysis at
12-14 months and 22-24 months did not reveal effects except for a
small increase in coproporphyrin excretion. The analysis of the
clinical chemistry parameters of blood urea nitrogen, serum alanine
aminotransferase (EC 2.6.1.2) and serum alkaline phosphatase
(EC 3.1.3.1) at 12 months revealed no treatment-related effects,
except for statistically significant decreases in serum alanine
aminotransferase in males of the 20-mg/kg dose group and females of
the 0.2- or 20-mg/kg dose groups. These changes were considered by
the authors to be of questionable toxicological significance. The
relative kidney weights were elevated at 20 mg/kg for both sexes, as
were the relative weights of the brain in females and of the testes
in males. In both sexes, an extensive histopathological examination
revealed tubular epithelial hyperplasia at 2 and 20 mg/kg, but not
at 0.2 mg/kg, and an increased incidence of renal tubular neoplasms
at 20 mg/kg (see Table 14) (Kociba et al., 1977a,b).
8.7.3 Dermal exposure
In a study by Van Duuren et al. (1979), groups of 30 female
Ha:ICR Swiss mice received 6.0 mg hexachlorobutadiene in acetone
applied 3 times per week to the shaven dorsal skin for between 144
and 594 days. A group of 100 untreated females were included in the
study, together with 30 controls which received acetone only. The
study duration was described as being between 440 and 594 days.
Sections of skin, liver, stomach and kidney were sampled at autopsy,
but no increase in the number of distant tumours was observed.
In a two-stage initiation-promotion experiment, each of
20 female Swiss mice received one application of 15.0 mg
hexa-chlorobutadiene in acetone to the dorsal skin. After 14 days,
the mice similarly received 5 µg of the tumour promoter
12- o-tetra-decanoylphorbol-13-acetate (TPA) three times weekly for
between 428 and 576 days. Hexachlorobutadiene administration did not
induce a significant increase in the fraction of mice developing
skin papillomas in this study (Van Duuren et al., 1979).
Table 14. Renal tubular neoplasms in rats after long-term
exposure to hexachlorobutadienea
Dose (mg/kg Sex Incidence of renal tubular neoplasms
body weight
per day) adenoma adenocarcinoma total
0 males 1/90 0/90 1/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 2/39 7/39 9/39 (P < 0.05)
0 females 0/90 0/90 0/90
0.2 0/40 0/40 0/40
2.0 0/40 0/40 0/40
20 3/40 3/40b 6/40 (P < 0.05)
a From: Kociba et al. (1977a)
b One of these was an undifferentiated carcinoma
8.7.4 Exposure by other routes
In a study of repeated exposure to hexachlorobutadiene by ip
injection, groups of 20 A/St strain male mice (from 6 to 8 weeks of
age) received 12 or 13 ip injections of hexachlorobutadiene (4 or
8 mg/kg body weight) in tricaprylin, respectively. The purity of the
hexachlorobutadiene was stated to exceed 99.9%. Urethane was used as
a positive control for carcinogenesis, and a negative control group
of 50 mice receiving tricaprylin only. Survival was 95% for mice
receiving 4 mg/kg and 70% for mice receiving 8 mg/kg, compared to
92% for controls. Mice were sacrificed at 24 weeks after the first
injection, and the number of surface adenomas in the lungs was
counted. No significant increase in adenomas, compared to the
vehicle-treated control, was observed (Theiss et al., 1977). The
Task Group noted major deficiencies of this study; including the
choice of a sensitive strain of mice, the short duration of both the
exposure period (4 weeks) and the follow-up period (24 weeks), the
small group sizes of the experimental animals, the unusual route of
administration, and the limited histopathology. The strain A mice
used in this study are highly predisposed to spontaneous lung
cancer, which is likely to have further compromised the value of the
study.
8.8 Other special studies
8.8.1 Effects on the nervous system
Acute high exposure to hexachlorobutadiene has a depressant
effect on the central nervous system (see sections 8.1.1.2 and
8.1.2).
Subchronic exposure of rats at high dose levels (1500 mg/kg
diet for 13 weeks) also produced some signs of neurotoxicity, which
was associated with demyelinization and fragmentation of femoral
nerve fibres (Harleman & Seinén, 1979; see also Badaeva et al.,
1985, section 8.5.2).
8.8.2 Effects on the liver
8.8.2.1 Acute effects
Hexachlorobutadiene causes hydropic changes in the liver of
rats (Gradiski et al., 1975; Lock & Ishmael, 1981; Lock et al.,
1982), mice (Lock et al., 1985), and rabbits (Duprat & Gradiski,
1978), sometimes accompanied by fat accumulation (Duprat & Gradiski,
1978; Lock & Ishmael, 1981; Lock et al., 1982).
Male rats, exposed to a single intraperitoneal dose of
hexachlorobutadiene (200 or 300 mg/kg body weight) in corn oil
showed increased relative liver weights, mitochondrial swelling in
liver and bile duct, proliferation of smooth endoplasmic reticulum,
lipid accumulation, and increased water content in the liver.
Biochemical changes in the liver were a decrease, followed after 1
day by an increase, in non-protein sulfhydryl (NP-SH) concentration,
and an increase in potassium content. All effects were reversible
within 10 days. Increases in plasma urea and alkaline phosphatase
(EC 3.1.3.1.) were also reported. In a separate experiment, the
highest dose administered by ip injection which did not cause an
increased water content in the liver was 25 mg/kg body weight (Lock
et al., 1982).
Male rats exposed to single intraperitoneal doses up to
100 mg/kg body weight showed an increase in serum bile acids and
bilirubin (Bai et al., 1992).
Male mice, exposed to single intraperitoneal doses of 50, 100
and 200 mg/kg body weight in corn oil, showed a dose-related
increase in relative liver weight at 100 and 200 mg/kg, and, at all
dose levels, dose-related, reversible changes in the liver
(mitochondrial swelling, proliferation of smooth endoplasmic
reticulum, and an increased water content). Reversible biochemical
changes included increases in sodium and potassium content, NP-SH
concentration in the liver, and serum alanine aminotransferase
activity (EC 2.6.1.2) at 50 mg/kg (Lock et al., 1985).
8.8.2.2 Short-term effects
As discussed in section 8.2.2.1, slight hepatotoxic effects
have been observed following oral exposure of rats (Kociba
et al., 1971; Harleman & Seinen, 1979).
8.8.3 Effects on the kidneys
This section will describe the main features of the renal
toxicity induced by hexachlorobutadiene. For more detail the reader
is referred to the reviews of Rush et al. (1984), Lock (1988),
Yang (1988) and Dekant et al. (1990a).
8.8.3.1 Acute effects
Inhalation exposure of rats produces renal tubular necrosis
(Gage, 1970; see section 8.2.1). Enzyme histochemical
investi-gations were performed on groups of 10 male Swiss OF1 mice
24 h after whole-body inhalation exposure for 4 h to
hexachloro-butadiene at measured concentrations of 29.3, 53.4, 106.7
or 266.8 mg/m3. A concentration-related increase in the percentage
of damaged kidney tubules, which had been stained for alkaline
phosphatase (EC 3.1.3.1), was observed at all exposure levels. The
EC50 was calculated to be 76.8 mg/m3 (De Ceaurriz et al.,
1988).
A single oral dose of hexachlorobutadiene (200 mg/kg body
weight) in polyethylene glycol caused an increase in plasma urea
concentration, a decrease in plasma alanine aminotransferase
activity, and, in urine, increases in the levels of glucose,
protein, alanine aminotransferase, N-acetyl-ß-D-glucosaminidase,
gamma-glutamyltranspeptidase (EC 2.3.2.2) and alanine aminopeptidase
(EC 3.4.11.12) (Nash et al., 1984).
Following in vivo administration, hexachlorobutadiene caused
dose-dependent necrosis of the renal proximal tubules in rats
(Gradiski et al., 1975; Lock & Ishmael, 1979, 1981; Kluwe et al.,
1982; Hook et al., 1982, 1983; Ishmael et al., 1982; Ishmael &
Lock, 1986), mice (Ishmael et al., 1984) and rabbits (Duprat &
Gradiski, 1978). In rats, the lesions were restricted to the pars
recta (S3-segment) and were macroscopically observed as a distinct
band of damage in the outer stripe of the medulla (Lock & Ishmael,
1979; Ishmael et al., 1982). In mice and rabbits both the pars
recta and the pars convoluta of the proximal convoluted tubules were
damaged (Duprat & Gradiski, 1978; Ishmael et al., 1984). The
lesion is characterized microscopically by necrotic epithelial
cells, most of which are devoid of nuclei. The few remaining nuclei
show karyorrhexis, and the cytoplasm is strongly eosinophilic. Many
renal tubules contained cellular debris (Duprat & Gradiski, 1978;
Lock & Ishmael, 1979; Lock et al., 1984; Ishmael et al., 1984).
Vacuolation of the pars convoluta was observed (Duprat & Gradiski,
1978; Ishmael et al., 1982, 1984). Mitochondrial swelling and loss
of brush-borders were prominent ultrastructural findings (Ishmael
et al., 1982, 1984).
Adult male rats have been found to be less sensitive to the
renal toxicity induced by hexachlorobutadiene than adult females and
young males (Hook et al., 1983; Kuo & Hook, 1983). When male rats
were dosed intraperitoneally with a single dose of 300 mg/kg in corn
oil, the earliest pathological change was mitochondrial swelling in
proximal tubular cells observed after 1-2 h. Extensive necrosis was
evident between days 1 and 4, and active regeneration by day 5
(Ishmael et al., 1982). Similar renal toxicity was seen at a dose
level of 25 or 50 mg/kg body weight in female rats and young males,
respectively. A similar pattern of pathological changes with
comparable intensity was observed in mice at an intraperitoneal dose
of 50 mg/kg body weight (Ishmael et al., 1984). In a study by Lock
et al. (1984), young mice were found to be more susceptible than
adults, but no sex difference was apparent. In both rats and mice,
differences in strain susceptibility were observed (Hook et al.,
1983; Lock et al., 1984). The lowest intraperitoneal dose at which
renal necrosis was observed in adult female rats was 25 mg/kg body
weight (Lock & Ishmael, 1985) and in adult male and female mice was
6.3 mg/kg body weight (Lock et al., 1984).
Biochemical changes found following intraperitoneal exposure in
both rats and mice were increases in renal water content (Kluwe et
al., 1982; Ishmael et al., 1982, 1984; Gartland et al., 1989),
plasma urea (Lock & Ishmael, 1979, 1981; Ishmael et al., 1982,
1984; Hook et al., 1983; Lock et al., 1984; Ishmael & Lock,
1986; Stonard et al., 1987; Gartland et al., 1989), plasma
alkaline phosphatase (EC 3.1.3.1.) (Lock & Ishmael, 1981), serum
alanine aminotransferase (EC 2.6.1.2) (Gradiski et al., 1975; Kuo
& Hook, 1983) and serum aspartate aminotransferase (Gradiski et
al., 1975; Davis et al., 1980). In the urine of rats, increases
in urinary protein, glucose and ketones have been measured (Lock &
Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al., 1980;
Stonard et al., 1987), as well as increases in the activities of
alkaline phosphatase and N-acetyl-ß-D-glucosaminidase (EC
3.2.1.50) (Lock & Ishmael, 1979; Stonard et al., 1987) and in
lactic acid level (Gartland et al., 1989). In the kidneys of rats,
increased sodium concentrations were accompanied by equally
decreased potassium concentrations (Davis et al., 1980). All these
changes occurred at similar or higher intraperitoneal doses than
those at which renal necrosis was observed.
Distinct renal functional changes in adult rats have been
observed at intraperitoneal doses of 100-400 mg/kg body weight.
These include a decrease in urine-concentrating ability (polyuria)
(Lock & Ishmael, 1979; Berndt & Mehendale, 1979; Davis et al.,
1980; Stonard et al., 1987), a reduced glomerular filtration rate
(Davis et al., 1980) and a reduction of in vivo renal clearance
of inulin, urea, p-aminohippuric acid (PAH), tetraethyl-ammonium
bromide (TEA) (Lock & Ishmael, 1979) and imipramine (Davis et al.,
1980). When organic ion transport was assessed in vitro in renal
cortical slices of rats and mice that had been exposed to an
intraperitoneal dose of 100 mg/kg, 200 mg/kg body weight or more,
the transport of anions (PAH) was found to be reduced, but the
transport of the cation (TEA), aminoisobutyrate was not (or was only
slightly reduced) in rats (Lock & Ishmael, 1979; Berndt & Mehendale,
1979; Kluwe et al., 1982; Hook et al., 1982, 1983). In male
adult mice, transport of PAH and TEA was reduced from
intraperitoneal doses of 12.5 and 25.0 mg/kg body weight,
respectively. The anion transport was reduced in adult females (Lock
et al., 1984).
8.8.3.2 Short- and long-term effects
The short- and long-term effects of hexachlorobutadiene on the
kidneys of experimental animals have already been discussed in
sections 8.2 , 8.5 and 8.7. Based on the studies of Kociba et al.
(1971, 1977a,b), Schwetz et al. (1977), Harleman & Seinen (1979),
Stott et al. (1981) and Yang et al. (1989), the oral NOAEL for
renal toxicity is 0.2 mg/kg body weight per day. Results of the
studies are summarized in Table 15. Female rats and mice were found
to be distinctly more susceptible than males upon oral exposure for
13 weeks (Yang et al., 1989; Yang, 1991); this was also observed
in the single-exposure mortality studies (section 8.1).
8.9 Factors modifying toxicity; toxicity of metabolites
8.9.1 Factors modifying toxicity
8.9.1.1 Surgery
Complete protection from the nephrotoxic effects of
hexachlorobutadiene was observed in rats that had been fitted with a
biliary cannula before being given a single oral dose of 200 mg/kg
body weight. Administration of bile, collected from rats dosed
orally with the compound, to naive rats produced marked renal
toxicity but no liver toxicity (Nash et al., 1984).
8.9.1.2 Inhibitors and inducers of mixed-function oxidases (MFO)
In the majority of studies, the effects of MFO inhibitors
(piperonyl butoxide, SKF 525A) and MFO inducers (Aroclor 1254,
isosafrole, ß-naphthoflavone, phenobarbitone) on the nephro-toxicity
induced by hexachlorobutadiene in rats and mice were absent or
negligible (Lock & Ishmael, 1981; Hook et al., 1982; Lock et al.,
1984; Davis, 1984). Furthermore, phenobarbitone pretreatment for 7
days at 0.05% in drinking-water enhanced the renal toxicity induced
by intraperitoneal doses of hexachloro-butadiene in weanling rats
(Hook et al., 1983).
8.9.1.3 Inhibitors of gamma-glutamyltranspeptidase (EC 2.3.2.2)
Male rats pretreated with Acivicin (L-(alphaS, 5S)-
alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid), an
inhibitor of gamma-glutamyltranspeptidase (down to 3% of control
activity in this study), and subsequently exposed intraperitoneally
to hexachlorobutadiene, did not show a decrease in nephrotoxicity
compared to rats treated with hexachlorobutadiene alone. It was
concluded that gamma-glutamyltranspeptidase inhibition did not limit
the formation of nephrotoxic metabolites (Davis, 1988).
Male Swiss OF1 mice, pretreated with Acivicin and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group noted that only one marker for nephrotoxicity was employed in
this study.
Table 15. No-observed-adverse-effect level (NOAEL) calculated from short-term and long-term
studies of exposure to hexachlorobutadiene by oral administration
Species (Strain) Age Sex Number of Duration of Dose (mg/kg NOAEL (mg/kg References
animals study body weight body weight
per group per day) per day)
Rat (Wistar-derived) weanling male 6 2 weeks 7.3, 18.2, 44.7 < 7.3 Harleman & Seinen
female 6 (1979)
Rat (Sprague-Dawley) adult male 5 3 weeks 0.2, 20 0.2 Stott et al. (1981)
Rat (Sprague-Dawley) adult female 4 30 days 1, 3, 10, 30, 65, 100 3 Kociba et al. (1971)
Rat (Sprague-Dawley) adult male 10-12 3 months 0.2, 2.0, 20 0.2 Schwetz et al.
female 20-24 (1977)
Rat (Sprague-Dawley) adult male 39-40 24 months 0.2, 2.0, 20 0.2 Kociba et al.
female 39-40 (1977a,b)
Mouse (B6C3F1) adult male 5 2 weeks 4.3, 14.3, 43, 143, 430 < 4.3 Yang et al.
female 5 (1989); Yang (1991)
Mouse (B6C3F1) adult male 10 13 weeks 0.1, 0.4, 1.5, 4.9, 18.8 1.5 Yang et al.
female 10 0.2, 0.5, 1.8, 4.5, 19.2 < 0.2 (1989); Yang (1991)
8.9.1.4 Inhibitors of cysteine conjugate ß-lyase
Male Swiss OF1 mice, pretreated with the two ß-lyase inhibitors
amino-oxyacetic acid (AOAA) and DL-propargylglycine (PPG) and
subsequently exposed to a single oral dose of hexachlorobutadiene
(80 mg/kg body weight), showed a decrease in nephrotoxicity compared
to mice treated with hexachlorobutadiene alone, as measured by
alkaline phosphatase staining (De Ceaurriz & Ban, 1990). The Task
Group again noted that only one marker for nephrotoxicity was
employed in this study.
8.9.1.5 Inhibitors of organic anion transport
Pre-treatment of male rats with probenecid
[(4-(dipropyl-amino)sulfonyl)] benzoic acid (105 µmol/kg body
weight), an inhibitor of organic anion transport, did not alter the
increase in plasma urea or decrease in renal clearance of
p-aminohippuric acid induced by hexachlorobutadiene (Hook et al.,
1982). However, in female rats, a higher dose (500 µmol/kg body
weight) of probenecid totally protected against the renal toxicity,
both functional and morphological, produced by ACPB (Lock & Ishmael,
1985). In addition this dose of probenecid protected female rats
against the toxic effects produced by CPB and GPBN as well as the
parent chemical (Lock & Ishmael, 1985). Male mice pre-treated with
probenecid were also protected against the nephrotoxicity produced
by hexachlorobutadiene (Ban & De Ceaurriz, 1988). The Task Group
noted that this latter study used only one marker for
nephrotoxicity.
8.9.1.6 Non-protein sulfhydryl scavengers
Depletion of hepatic and renal non-protein sulfhydryl content
(glutathione) by diethylmaleate in rats appears to potentiate the
nephrotoxicity of hexachlorobutadiene as measured by a number of
functional markers such as plasma urea (Hook et al., 1982; Baggett
& Berndt, 1984, Davis et al., 1986). However, the Task Group noted
that no information was available on the metabolism of
hexachlorobutadiene to help interpret these studies.
8.9.2 Toxicity of metabolites
This section discusses the renal toxicity of some metabolites
of hexachlorobutadiene, the formation of which was discussed in
section 6.2. These metabolites are 1-(glutathion- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (GPB), 1-(cystein- S-yl)-1,2,3,4,4-
pentachloro-1,3-butadiene (CPB), and 1-( N-acetylcystein- S-yl)-
1,2,3,4,4-pentachloro-1,3-butadiene (ACPB). Their mutagenic activity
was described along with that of hexachlorobutadiene in section 8.6.
8.9.2.1 In vitro studies
GPB decreased the viability of isolated renal epithelial cells
of male rats, as measured by leakage of lactate dehydrogenase (EC
1.1.1.27), with a very steep dose-response curve and a lag period of
30 min. No cytotoxicity was observed when the GPB metabolism was
blocked by anthglutin, an inhibitor of gamma-glutamyl-transpeptidase
(EC 2.3.2.2) or amino-oxyacetic acid (AOAA), an inhibitor of renal
cysteine conjugate ß-lyase (EC 4.4.1.13). The cytotoxicity of GPB
was related to an impairment of mitochondrial function, as shown by
loss of mitochondrial Ca2+ and ATP and inhibition of respiration
and thiol depletion (Jones et al., 1986b). Likewise, GPB produced
a concentration-dependent nephro-toxicity in the isolated perfused
rat kidney, as indicated by the appearance in the urine of alkaline
phosphatase, gamma-glutamyl-transpeptidase and glucose (Jones et
al., 1986a). These changes were prevented by Acivicin and by AOAA
(Schrenk et al., 1988a).
In the study of Schrenk et al. (1988a), CPB also caused a
marked nephrotoxicity in the isolated perfused kidney, which could
be prevented by AOAA. In isolated rabbit renal tubules, CPB was
observed to decrease the accumulation of p-amino-hippuric acid and
tetraethylammonium (Jaffe et al., 1983), to affect mitochondrial
function as shown by effects on cell respiration, and to decrease
the glutathione content and, after a lag period of 60 min, cell
viability (Schnellmann et al., 1987). The effects on respiration
resulted initially from the uncoupling of oxidative phosphorylation,
followed later by inhibition of state 3 respiration (Schnellmann et
al., 1987). Impaired mitochondrial function was observed in
CPB-exposed isolated rat renal cortical mitochondria as an inability
to retain Ca2+, collapse of the membrane potential, impaired state
3 respiration with succinate as substrate, and nonenzymatic
depletion of thiol content. The latter effect was blocked by AOAA.
From these results it was concluded that the reactive intermediate
formed from CPB interacts with the inner mitochondrial membrane
(Wallin et al., 1987). CPB also inhibited rat kidney mitochondrial
DNA, RNA and protein synthesis, and AOAA blocked this effect.
Moreover, CPB converted supercoiled DNA to relaxed circular DNA and
shorter linear fragments (Banki & Anders, 1989). Chen et al.
(1990) observed a decreased viability of isolated human renal
proximal tubular cells upon exposure to CPB, which was again blocked
by AOAA. Using radiolabelled ACPB and rat renal cortical slices, it
was established that ACPB is transported by the same renal
mechanisms involved in the movement of many organic anions into
tubular fluid. This carrier-mediated transport is reduced by
specific inhibitors like probenecid and sulfinpyrazone, a
competitive and metabolic inhibitor like 2,4-dinitrophenol, and the
transport substrate p-aminohippuric acid (Lock et al., 1986).
This was confirmed by recent studies on the mechanism of uptake of
GPB and CPB in the isolated perfused rat kidney (Schrenk et al.,
1988b). Probenecid has also been reported to protect renal proximal
tubular cells against ACPB-induced cytotoxicity, as determined by
monitoring proline incorporation into renal proteins (Bach et al.,
1986).
8.9.2.2 In vivo studies
A single oral dose of 138 mg/kg body weight (0.27 mmol) of GPB
or a single equimolar oral dose of 100 mg ACPB/kg body weight in
polyethylene glycol to male rats caused marked nephrotoxicity
similar in both biochemical and histopathological aspects to that
observed with an oral dose of 200 mg/kg body weight (0.97 mmol) of
hexachlorobutadiene (Nash et al., 1984). When rats received
intraperitoneally GPB, CPB or ACPB in polyethylene glycol at single
doses between 6.25 and 100 mg/kg body weight, increases in plasma
urea level and renal proximal tubular necrosis were observed at dose
levels of > 6.25 mg/kg body weight in females and 10 or 12.5 mg/kg
body weight in males. The conjugates exhibited a similar pattern of
nephrotoxicity at equimolar doses and were more nephrotoxic than the
parent compound (Lock & Ishmael, 1985; Ishmael & Lock, 1986). All
compounds tested were more toxic to female rats than males (Ishmael
& Lock, 1986). Probenecid pretreatment protected the rats against
the nephrotoxicity of these metabolites. Probenecid was shown to
block the active tubular secretion of ACPB and to reduce the extent
of covalent binding to renal protein (Lock & Ishmael, 1985). In
mice, GPB and ACPB were also shown to be more toxic than the parent
compound: renal necrosis was found following single intraperitoneal
doses of 5.0 mg hexachlorobutadiene/kg body weight, 3.1 mg GPB/kg
body weight and 3.0 mg ACPB/kg body weight in corn oil, which were
the lowest doses tested (Lock et al., 1984). A single
intraperitoneal dose of 10 mg CPB/kg body weight in DMSO and water
caused dose-related damage in the pars recta of renal proximal
tubules in male mice (Jaffe et al., 1983).
The nephrotoxicity of some structural analogues of the
above-mentioned conjugates, e.g. S-(1,2-dichlorovinyl)-L-cysteine,
has been investigated extensively and has revealed a remarkable
similarity (Anders et al., 1987; Lock, 1988).
8.10 Mechanisms of toxicity - mode of action
8.10.1 Mechanisms of toxicity
The following evidence supports the hypothesis that the
nephrotoxicity, mutagenicity and carcinogenicity of
hexachloro-butadiene is dependent on the biosynthesis of the toxic
sulfur conjugate GPB. This conjugate is mainly synthetized in the
liver and further metabolized in the bile, gut, and kidneys to the
CPB. Cysteine conjugate ß-lyase-dependent activation of CPB to a
reactive thioketene in the proximal tubular cells finally results in
covalent binding to cellular macromolecules.
1. The nephrotoxicity of hexachlorobutadiene in rats was prevented
by the implantation of a biliary cannula; administration of
bile from hexachlorobutadiene-treated rats to naive rats
resulted in nephrotoxicity identical to the nephrotoxicity
caused by hexachlorobutadiene (see section 8.9.1.1).
2. Inhibitors of renal organic anion transport protected rats
against the nephrotoxicity of hexachlorobutadiene and its
sulfur conjugates. Inhibition of the organic anion transport
also protected isolated kidney cells against the nephrotoxicity
of hexachlorobutadiene-derived sulfur-conjugates (see sections
8.9.1.3 and 8.9.2.1).
3. Anthglutin, Acivicin and aminooxyacetic acid, specific
inhibitors of gamma-glutamyltranspeptidase and cysteine
conjugate ß-lyase protected against the cytotoxicity of
hexachloro-butadiene-derived sulfur-conjugates in freshly
isolated rat renal proximal tubular cells (see section
8.9.2.1).
4. Synthetic sulfur-conjugates of hexachlorobutadiene show a
higher nephrotoxicity than the parent compounds in rats and
mice and produce renal damage identical to the renal damage
induced by hexachlorobutadiene, based on clinical chemistry and
histopathological examination (see section 8.9.2.2).
5. Hexachlorobutadiene and its sulfur-conjugates are genotoxic in
bacteria; bioactivation by glutathione conjugation is required
for hexachlorobutadiene genotoxicity. The ultimate mutagen is
formed by cysteine conjugate ß-lyase-dependent cleavage of CPB
(see section 8.6).
6. Hexachlorobutadiene induces renal tumours in rats only at doses
that produce marked nephrotoxicity (see sections 8.7 and
8.8.2.2).
8.10.2 Mode of action
The in vitro studies of Jones et al. (1986b), Wallin et
al. (1987) and Schnellmann et al. (1987) on the cytotoxicity of
sulfur-conjugates to renal tubular cells (section 8.9.1.2) point to
renal cortical mitochondria as the major target for
sulfur-conjugates of hexachlorobutadiene, analogous to that
established for close structural analogues (Dekant et al., 1990b).
The hypothesis proposes an interaction of the reactive metabolite
with the inner mitochondrial membrane, which ultimately causes
respiratory insufficiency.
9. EFFECTS ON HUMANS
9.1 General population exposure
Hexachlorobutadiene has been found in postmortem examinations,
but not in living persons. No pathogenic effects have been recorded
(see section 5.2).
9.2 Occupational exposure
Two reports on certain disorders among agricultural workers in
vineyards where hexachlorobutadiene has been used as a fumigant
(Krasniuk et al., 1969; Burkatskaya et al., 1982) cannot be
evaluated, since such workers are known to be occupationally exposed
to additional substances.
In two cytogenetic studies of occupationally exposed workers
from the same plant engaged in the production of
hexachloro-butadiene, an increase in the frequency of chromosomal
aberrations in peripheral blood lymphocytes was observed (German,
1986). The workers were exposed to hexachloro-butadiene
concentrations that ranged from 1.6 to 16.9 mg/m3. The Task Group
noted that exposure concentrations were determined by the factory
and that the frequency of chromosome aberrations was not associated
with the period of employment.
9.3 In vitro metabolism studies
The following studies have been reported:
a) Purified human liver microsomal glutathione- S-transferase
and human liver cytosol metabolize hexachlorobutadiene to form
GPB (McLellan et al., 1989; Oesch & Wolf, 1989).
b) The enzyme cysteine conjugate ß-lyase has been isolated and
purified from human kidney cytosol (Lash et al., 1990). The
activity of the human cytosolic enzymes with a structurally
related compound (1,2,2-trichlorovinyl-L-cysteine) is about
10-fold lower than that of rat renal cytosol (Green et al.,
1990).
c) Studies in isolated human proximal tubular cells have shown
that CPB causes a ß-lyase-dependant cytotoxicity (Chen et al.,
1990).
These limited studies suggest that humans have the ability to
metabolize hexachlorobutadiene to toxic metabolites.
9.4 Extrapolation of NOAEL from animals to humans
Conversion of equivalent doses across species can utilize
allometric relationships that relate physiological and anatomical
variables across species. Physiological and metabolic rates have
been shown to relate closely to body weight to the power 0.75
(Boxenbaum, 1982). The equivalent NOAEL in humans (mg/kg body weight
per day) can be determined from the following equation:
Wa
dh = da (--)0.25
Wh
where
d = dose rate (mg/kg body weight per day) in humans (dh) or
animals (da)
wa = weight (kg) of animals (mice 30 g; rats 400 g)
wh = weight of humans (70 kg)
The NOAEL for humans, based on the NOAEL in mice, is:
dh= (0.2 mg/kg body weight per day) (0.03)0.25
70
= 0.03 mg/kg body weight per day
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Hazard identification
The following evaluation is based on toxicity studies in
experimental animals; however, there are some human in vitro data
which indicate that hexachlorobutadiene metabolism can occur by a
similar route to that shown in experimental animals.
Hexachlorobutadiene is slightly to moderately toxic based on
acute oral experiments with adult rats, and moderately to highly
toxic based on acute oral experiments with weanling rats (based on
the WHO pesticide toxicity classification). The specific toxicity of
the compound to the kidneys is higher for females than males.
Following acute dermal exposure of rabbits, the compound was found
to be weakly toxic.
Regardless of species studied and the route of exposure (ip,
oral, inhalation, dermal) in both short- and long-term studies, the
target organ for toxicity is the kidney. Bioactivation to produce a
reactive sulfur metabolite occurs following conjugation with
glutathione. The monoglutathione conjugate of hexachloro-butadiene
is processed to the cysteine- S-conjugate, which is then a
substrate for renal cysteine conjugate ß-lyase. Hexachloro-butadiene
produces a dose-dependent necrosis of the renal proximal tubules,
which is followed by regenerative and/or proliferative changes. On
the basis of both short- and long-term studies in rats and mice
orally exposed to hexachlorobutadiene, the no-observed-adverse-
effect level (NOAEL) is 0.2 mg/kg body weight per day. In one
short-term inhalation study (12 days, 6 h/day), the NOAEL was
53 mg/m3.
The vapour of hexachlorobutadiene was found to be irritating to
the eyes and nose of rats in one short-term inhalation study. The
undiluted compound appeared corrosive in an experiment with rabbits.
Based on these limited data, the vapour should be regarded as
irritating to human mucous membranes and the liquid should be
regarded as corrosive.
In a well-conducted Magnusson-Kligman test hexachloro-butadiene
was a sensitizing agent both with and without adjuvant. Therefore,
the compound should be regarded as a sensitizing agent for humans.
In reproductive studies, reduced birth weight and neonatal
weight gain in rats were observed, but these effects may be
attributed to maternal toxicity. Developmental toxicity to rat
fetuses was observed in two teratogenicity tests, but again only at
levels that were also toxic to the dams. This developmental toxicity
included reduced birth weight, a 1- to 2-day delay in heart
development, and dilated ureters, but no gross abnormalities were
observed.
In vitro studies have shown that hexachlorobutadiene and, to
a much greater extent, its sulfur metabolites induce mutations in
Salmonella typhimurium. In one study of exposure to
hexachloro-butadiene by inhalation or oral administration, an
increased frequency of chromosomal aberrations was observed in mouse
bone marrow cells. There is limited evidence for the genotoxicity of
hexachlorobutadiene in animals, and insufficient evidence in humans.
The long-term oral administration of hexachlorobutadiene to
rats induced an increased frequency of renal tubular neoplasms, but
only at doses that caused marked nephrotoxicity; at the lowest dose,
no adverse effects were observed.
The Task Group concluded that there is limited evidence for the
carcinogenicity of hexachlorobutadiene in animals (one study in one
rodent strain) and insufficient evidence in humans.
10.1.2 Exposure
Hexachlorobutadiene is mainly a waste product. As such, it can
be encountered in different environmental compartments, but
predominantly in sediment and biota (see also 10.2.1). Exposure of
the general public therefore mainly occurs indirectly via
drinking-water and food of high lipid content. Assuming a maximum
concentration of 2.5 µg/litre in contaminated drinking-water and
10 µg/kg wet weight in contaminated fatty food items (meat, fish,
milk) and daily intakes of 2 litres drinking-water, 0.3 kg meat,
0.2 kg fish and 0.5 kg milk, a maximum total daily intake of
0.2 µg/kg body weight can be calculated for a 70-kg person.
10.1.3 Hazard evaluation
The NOAEL for mice or rats exposed to hexachlorobutadiene is
0.2 mg/kg body weight per day (see Table 15), from which a NOAEL of
0.03-0.05 mg/kg body weight day has been derived for humans (see
section 9.4).
The Task Group considered the margin of safety of 150 between
the estimated NOAEL in humans and the maximum total daily intake
(see section 10.1.2) to be sufficient to protect the general
population against the adverse effects of hexachlorobutadiene.
10.2 Evaluation of effects on the environment
10.2.1 Hazard identification
Hexachlorobutadiene is a chemically stable compound. Complete
aerobic biodegradation has been observed following adaptation of the
inoculum. Partial biodegradation was found to occur in a pilot
sewage treatment plant. Based on these observations and the chemical
structure, it can be concluded that hexachlorobutadiene is not
readily biodegradable, but can be considered to be inherently
biodegradable. Experimental photolysis of hexachlorobutadiene in the
presence of a surface was rapid, but in the absence of a surface the
compound is believed to be persistent. Degradation in the atmosphere
is assumed to occur by a rather slow reaction with hydroxyl
radicals. A half-life of up to 2.3 years has been calculated.
Once hexachlorobutadiene is released into the environment,
intercompartmental transport will occur chiefly by volatilization
from water and soil, adsorption to particulate matter in water and
air, and subsequent sedimentation or deposition. In view of a strong
adsorption potential to organic matter, the compound accumulates in
sediment and will not migrate rapidly in soils. Both field and
laboratory exposure data support these conclusions.
Field and laboratory data also support the high
bioaccumu-lation potential in aquatic and benthic organisms which
can be expected on the basis of the lipophilic nature of the
compound. However, no evidence has been obtained for
biomagnification.
Hexachlorobutadiene is moderately to highly toxic to aquatic
organisms; crustaceans and fish are the most sensitive species. The
lowest E(L)C50 for freshwater organisms is 0.09 mg/litre
(goldfish). The lowest chronic NOEC is 3 µg/litre (goldfish).
Applying the preliminary effect assessment extrapolation procedure,
as adopted in the OECD Workshop on Aquatic Effect Assessment (OECD,
1990), an Environmental Concern Level of 0.1 µg/litre can be
established.
The toxicity data on terrestrial organisms are insufficient to
establish any toxicity threshold.
10.2.2 Exposure
Current environmental levels in surface waters are generally
below 0.2 µg/litre, rising to 1.3 µg/litre in highly polluted
rivers. Levels in the upper sediment can be as high as 120 µg/kg in
heavily polluted rivers or estuaries. In older sediment layers much
higher concentrations can be measured. The concentrations in
freshwater biota measured since 1980 generally do not exceed
100 µg/kg fresh weight, but in a polluted area can reach 120 mg/kg
in the lipid of fish.
10.2.3 Hazard evaluation
It can be concluded that away from point sources the maximum
predicted environmental concentration (PEC) is twice the
extrapolated Environmental Concern Level of 0.1 µg/litre. Aquatic
organisms therefore may be at risk in polluted surface waters.
In view of the rather high concentrations of the compound
measured in some sediments, adverse effects on benthic organisms
cannot be excluded.
Considering the toxicity of the substance to mammals (the NOAEL
for rats or mice is 0.2 mg/kg body weight per day) and its high
bioaccumulating potential, the consumption of benthic or aquatic
organisms in polluted surface water by other species may give cause
for concern. For example, an otter weighing 10 kg and consuming 1 kg
fish per day in waters containing 0.2 µg hexachlorobutadiene/litre
could ingest 1200 µg/day (assuming a bioconcentration factor for
fish of 6000, leading to a concentration of 1200 µg/kg wet weight)
or 120 µg/kg body weight per day, which is above the calculated
NOAEL value for the otter (calculated as in section 9.4).
11. FURTHER RESEARCH
Hexachlorobutadiene is primarily a waste product and hence an
environmental contaminant having only limited use as a fumigant in
some parts of the world. The Task Group identified the following
areas for which additional information is needed:
a) the degradation of hexachlorobutadiene in the environment
focusing on photodegradation and biodegradation;
b) the terrestrial toxicity of hexachlorobutadiene including tests
on benthic organisms;
c) the genotoxic activity of hexachlorobutadiene in vivo. A
further test for micronucleus or chromosome aberration
induction in mouse bone marrow cells would strengthen the
available data;
d) the metabolism of hexachlorobutadiene and its glutathione-
derived conjugates by human liver and renal enzymes and
inter-individual variability.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenic risk of hexachlorobutadiene was evaluated by
the International Agency for Research on Cancer in 1979 (IARC,
1979).
The summary of data reported and the evaluation of the IARC
monograph on hexachlorobutadiene is reproduced here.
Experimental data
Hexachlorobutadiene was tested in one experiment in rats by
oral administration: it produced benign and malignant tumours in the
kidneys of animals of both sexes. It was tested inadequately in one
experiment in mice by intraperitoneal injection.
Human data
No case reports of epidemiological studies were available to
the Working Group.
The occurrence of hexachlorobutadiene as a by-product in the
production of various chlorinated hydrocarbons for over 50 years and
its use in some areas as a pesticide indicate that widespread human
exposure in both the occupational and general environment occurs.
This is confirmed by reports of its occurrence in the environment.
Evaluation
There is limited evidence that hexachlorobutadiene is
carcinogenic in rats.
REFERENCES
Alberti J (1983) [Organic contaminants in river sediment.] Vom
Wasser, 61: 149-154 (in German).
Alberti J & Plöger E (1986) [Hazardous organic chemicals in sewage
sludge.] Gewasserschutz Wasser Abwasser, 85: 483-498 (in German).
Anders MW, Elfaeea AA, & Lash LH (1987) Cellular effects of reactive
intermediates: nephrotoxicity of S-conjugates of amino acids. Arch
Toxicol, 60: 103-108.
Bach PH, Ketley CP, Ahmed I, & Dixit M (1986) The mechanisms of
target cell injury by nephrotoxins. Food Chem Toxicol, 24: 775-779.
Badaeva LN, Ovsyannikova LM, & Kiseleva NI (1985) [Manifestation of
neurotoxic effect of chloroorganic pesticide hexachlorobutadiene
during postnatal period of ontogenesis in rats.] Arch Anat Gistol
Embriol, 89: 44-49 (in Russian).
Baggett JM & Berndt WO (1984) Renal and hepatic glutathione
concentrations in rats after treatment with hexachloro-1-3-butadiene
and citrinin. Arch Toxicol, 56: 46-49.
Bai CL, Canfield PJ, & Stacey NH (1992) Effects of
hexachloro-1,3-butadiene and 1,1,2,2-tetrachloroethylene on
individual serum bile acids. Toxicol Ind Health, 8: 191-203.
Ban M & De Ceaurriz J (1988) Probenecid-induced protection against
acute hexachloro-1,2-butadiene and methyl mercury toxicity to the
mouse kidney. Toxicol Lett, 40: 71-76.
Banerjee S, Yalkowsky SH, & Valvani SC (1980) Water solubility and
octanol/water partition coefficients of organics. Limitations of the
solubility-partition coefficient correlation. Environ Sci Technol,
14: 1227-1229.
Banki K & Anders MW (1989) Inhibition of rat kidney mitochondrial
DNA, RNA and protein synthesis by halogenated cysteine S-conjugates.
Carcinogenesis, 10: 767-772.
Barkley J, Bunch J, Bursey JT, Castillo N, Cooper SD, Davis JM,
Erickson MD, Harris BSH III, Kirkpatrick M, Michael LC, Parks SP,
Pellizari ED, Ray M, Smith D, Tomer KB, Wagner R, & Zweidinger RA
(1980) Gas chromatography mass spectrometry computer analysis of
volatile halogenated hydrocarbons in man and his environment - a
multimedia environmental study. Biomed Mass Spectrom, 7: 139-146.
Benoit DA, Puglisi FA, & Olson DL (1982) A fathead minnow
Pinephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ Pollut,
A28: 189-197.
Berndt WO & Mehendale HM (1979) Effects of hexachlorobutadiene
(HCBD) on renal function and renal organic ion transport in the rat.
Toxicology, 14: 55-65.
Boxenbaum H (1982) Inter-species scaling, allometry, physiologic
time, and the ground plan of pharmacokinetics. J Pharmacokinet
Biopharm, 10: 201-227.
Bristol DW, Crist HL, Lewis RG, MacLeod KE, & Sovocool GW (1982)
Chemical analysis of human blood for assessment of environmental
exposure to semivolatile organochlorine chemical contaminants. J
Anal Toxicol, 6: 269-275.
Boyd KW, Emory MB, & Dillon HK (1981) Development of personal
sampling and analytical methods for organochlorine compounds. ACS
Symp Ser, 149: 49-64.
Bringmann G & Kühn R (1977) [Limiting values of the harmful action
of water-endangering substances on bacteria (Pseudomonas putida)
and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z Wasser-Abwasser Forsch, 10: 87-98
(in German).
Brown SL, Chan FY, Jones JL, Liu DH, McCaleb KE, Mill T, Sapios KN,
& Schendel DE (1975) Research program on hazard priority ranking of
manufactured chemicals (chemicals 1-20). Menlo Park, California,
Stanford Research Institute (NSF/RA/E-75/190A; PB 263161).
Burkatskaya EN, Viter VF, Ivanova ZV, Kaskevitch LM, Gorskaya NZ,
Kolpakov IE, & Deineka KA (1982) [Clinico-hygienic data on working
conditions during use of hexachlorobutadiene in vineyards.] Vrach
Delo, 11: 99-102 (in Russian).
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in nearshore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13: 296-309.
Canton JH, Linders JBHJ, Luttik R, Mensink BJWG, Panman E, Van de
Plassche EJ, Sparenburg PM, & Tuinstra J (1990) Catching-up
manoeuvre old pesticides: an integration. Bilthoven, The
Netherlands, National Institute of Health and Environmental
Protection (Report No. 678801001).
CESARS (198l) Hexachlorobutadiene, latest update June 1981.
Baltimore, Maryland, Chemical Evaluation Search and Retrieval
System, Inc.
Chen JC, Stevens JL, Trifillis AL, & Jones TW (1990) Renal cysteine
conjugate betalyase-mediated toxicity studied with primary cultures
of human proximal tubular cells. Toxicol Appl Pharmacol, 103:
463-473.
Chiou CT (1985) Partition coefficients of organic compounds in
lipid-water systems and correlations with fish bioconcentration
factors. Environ Sci Technol, 19: 57-62.
Chudin VA, Gafieva ZA, Koshurnikova NA, Nifatov AP, & Revina VS
(1985) [Evaluating the mutagenicity and carcinogenicity of
hexachlorobutadiene.] Gig i Sanit, 5: 79-80 (in Russian).
Clark CS, Meyer CR, Gartside PS, Majeti VA, Specker B, Balistreri
WF, & Elia VJ (1982) An environmental health survey of drinking
water contamination by leachate from a pesticide waste dump in
Hardeman County, Tennessee. Arch Environ Health, 37: 9-18.
Clark JR, Devault D, Bowden RJ, & Weishaar JA (1984) Contaminant
analysis of fillets from Great Lakes coho salmon. J Great Lakes Res,
10: 38-47.
Class T & Ballschmiter K (1987) Global baseline pollution studies.
X. Atmospheric halocarbons: global budget estimations for
tetrachloroethene, 1,2-dichloroethane, 1,1,1,2-tetrachloroethane,
hexachloroethane and hexachlorobutadiene. Estimation of the hydroxyl
radical concentrations in the troposphere of the northern and
southern hemisphere. Fresenius Z Anal Chem, 327: 198-225.
Davis ME (1984) Changes of hexachlorobutadiene nephrotoxicity after
piperonyl butoxide treatment. Toxicology, 30: 217-225.
Davis ME (1988) Effects of AT-125 on the nephrotoxicity of
hexachloro-1,2-butadiene in rats. Toxicol Appl Pharmacol, 95: 44-52.
Davis ME, Berndt WO, & Mehendale HM (1980) Disposition
nephrotoxicity of hexachloro-1,3-butadiene. Toxicology, 16: 179-191.
Davis ME, Berndt WO, & Mehendale HM (1986) Effects of cysteine and
diethylmalate pretreatments on renal function and response to a
nephrotoxicant. Arch Toxicol, 59: 7-11.
De Ceaurriz J & Ban M (1990) Role of gamma-glutamyltranspeptidase
and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and
methyl mercury in mice. Toxicol Lett, 50: 249-256.
De Ceaurriz J, Gagnaire F, Ban M, & Bonnet P (1988) Assessment of
the relative hazard involved with airborne irritants with additional
hepatotoxic or nephrotoxic properties in mice. J Appl Toxicol, 8:
417-422.
Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, & Henschler D
(1986) Bacterial beta-lyase mediated cleavage and mutagenicity of
cysteine conjugates derived from the nephrocarcinogenic alkenes
trichloroethylene, tetrachloroethylene and hexachloro-butadiene.
Chem-Biol Interact, 60: 31-45.
Dekant W, Schrenk D, Vamvakas S, & Henschler D (1988a) Metabolism of
hexachloro-1,3-butadiene in mice: in vivo and in vitro evidence
for activation by glutathione conjugation. Xenobiotica, 18: 803-816.
Dekant W, Vamvakas S, Henschler D, & Anders MW (1988b) Enzymatic
conjugation of hexachloro-1,3-butadiene with glutathione. Formation
of 1-(glutathion- S-yl)-1,2,3,4,4-pentachlorobuta-1,3-diene and
1,4-bis(glutathion-S-yl)-1,2,3,4-tetrachloro-1,3-butadiene. Drug
Metab Dispos, 16: 701-706.
Dekant W, Berthold K, Vamvakas S, & Henschler D (1988c)
Thioacylating agents as ultimate intermediates in the beta-lyase
catalysed metabolism of S-(pentachloro-butadienyl)-L-cysteine.
Chem-Biol Interact, 67: 139-148.
Dekant W, Vamvakas S, & Anders MW (1990a) Bioactivation of
hexachlorobutadiene by glutathione conjugation. Food Chem Toxicol,
28: 285-293.
Dekant W, Vamvakas S, Koob M, Kochling A, Kanhai W, Muller D, &
Henschler D (1990b) A mechanism of haloalkene-induced renal
carcinogenesis. Environ Health Perspect, 88: 107-110.
Dekant W, Urban G, Gorsman C, & Anders MW (1991) Thioketene
formation from alpha-haloalkenyl 2-nitrophenyl disulfides: models
for biological reactive intermediates of cytotoxic S-conjugates. J
Am Chem Soc, 113: 5120-5122.
Demarini DM, Inmon JP, Simmons JE, Berman E, Pasley TC, Warren SH, &
Williams RW (1987) Mutagenicity in Salmonella of hazardous wastes
and urine from rats fed these wastes. Mutat Res, 189: 205-216.
De Meester C, Mercier M, & Poncelet F (1981) Mutagenic activity of
butadiene, hexachlorobutadiene, and isoprene. In: Gut I, Cifert M, &
Plaa GL ed. Industrial environmental xenobiotics. Proceedings of an
International Conference, 1980. Berlin, Heidelberg, New York,
Springer Verlag, pp 196-203.
Dillon HK (1979) Development of air sampling and analytical methods
for toxic chlorinated organic compounds: Research report for
hexachlorobutadiene. Birmingham, Alabama, Southern Research
Institute (SORI-EAS-79-658; PB 87-126934/AS).
Draize JH, Woodard G, & Calvery HO (1944) Methods for the study of
irritation and toxicity of substances applied topically to the skin
and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Duprat P & Gradiski D (1978) Percutaneous toxicity of
hexachlorobutadiene. Acta Pharmacol Toxicol, 43: 346-353.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvants chlorés aliphatiques sur la peau et les
muqueuses oculaires du lapin. Eur J Toxicol, 9: 171-177.
Durham RW & Oliver BG (1983) History of Lake Ontario contamination
from the Niagara river by sediment radiodating and chlorinated
hydrocarbon analysis. J Great Lakes Res, 9: 160-168.
Eichelberger JW, Bellar TA, Donnelly JP, & Budde WL (1990)
Determination of volatile organics in drinking water with US EPA
method 524.2 and the ion trap detector. J Chromatogr Sci, 29:
460.467.
Fox ME, Carey JH, & Oliver BG (1983) Compartmental distribution of
organochlorine contaminants in the Niagara River and the western
basin of Lake Ontario. J Great Lakes Res, 9: 287-294.
Gäb S, Schmitzer J, Thamm HW, Parlar H, & Korte F (1977)
Photomineralization rate of organic compounds adsorbed on
particulate matter. Nature (Lond), 270: 331-333.
Gage JC (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br J Ind Med, 27: 1-18.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10(Suppl): 1-175.
Gartland KPR, Bonner FW, & Nicholson JK (1989) Investigations into
the biochemical effects of region-specific nephrotoxins. Mol
Pharmacol, 35: 242-250.
Gauntley P, Magadur JL, Morel G, Chaumont P, & Canel F (1975) Dosage
de l'hexachlorobutadiène dans les milieux biologiques. Eur J
Toxicol, 8: 152-158.
German IV (1986) [Level of chromosome aberrations in worker coming
in contact with hexachlorobutadiene during production.] Gig Tr Prof
Zabol, 5: 57-59 (in Russian).
German IV (1988) [On mutagenic activity of the pesticide
hexachlorobutadiene.] Citol Gen, 22(4): 40-42 (in Russian).
German IV & Viter VF (1985) Evaluation of worker's health in Dactal
and hexachlorobutadiene production processes. Hyg Employ Toxicol
Pestic Polym, 15: 32-34.
Gietl YS & Anders MW (1991) Biosynthesis and biliary excretion of
S-conjugates of hexachlorobuta-1,3-diene in the perfused rat liver.
Drug Metab Dispos, 19: 274-277. Gietl YS, Vamvakas S, & Anders MW
(1991) Intestinal absorption of S-(pentachloro-butadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine,
S-conjugates of hexachlorobuta-1,3-diene. Drug Metab Dispos, 19:
703-707.
Giger W & Schaffner C (1981) Groundwater pollution by volatile
organic chemicals. In: Van Duijvenbooden W, Glasbergen P, & Van
Lelyveld H ed. Quality of groundwater. Proceedings of an
International Symposium, Noordwijkerhout, The Netherlands, 23-27
March 1981. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 517-522 (Studies in Environmental Sciences, Volume
17).
Goldbach RW, Van Genderen H, & Leeuwangh P (1976)
Hexachlorobutadiene residues in aquatic fauna from surface water fed
by the river Rhine. Sci Total Environ, 6: 31-40.
Gradiski D, Duprat P, Magadur JL, & Fayein E (1975) Etude
toxicologique expérimentale de l'hexachlorobutadiène. Eur J Toxicol,
8: 180-187.
Green T & Odum J (1985) Structure/activity studies of the
nephrotoxic and mutagenic action of cysteine conjugates of chloro-
and fluoroalkenes. Chem-Biol Interact, 54: 15-31.
Green T, Odum J, Nash JA, & Foster JR (1990)
Perchloroethylene-induced rat kidney tumors: an investigation of the
mechanisms involved and their relevance to humans. Toxicol Appl
Pharmacol, 103: 80-89.
Guzewich DC, Curtis AB, Valis R, Vindpal JH, & Deeter DP (1983) Air
sampling around a hazardous liquid surface impoundment. 76th Annual
Meeting of the Air Pollution Control Association, Atlanta, USA.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Haberer K, Normann S, & Drews M (1988) [Tests on certain trace
substances from the Rhine and Main with a view to obtaining
drinking-water.] Wasser Abwasser, 129: 45-49 (in German).
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7(Suppl 4): 66-75.
Harleman JH & Seinen W (1979) Short-term toxicity and reproduction
studies in rats with hexachloro-(1,3)-butadiene. Toxicol Appl
Pharmacol, 47: 1-14.
Haworth S, Lawloer T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, Suppl 1: 3-142.
Hellmann H (1987a) [Model tests on volatilization of organic trace
substances in surface waters.] Fresenius Z Anal Chem, 328: 475-479
(in German).
Hellmann H (1987b) [Investigation of the degree of concentration of
organic trace substances on clay minerals.] Fresenius Z Anal Chem,
327: 524-529 (in German).
Hermens J, Busser F, Leeuwang P, & Musch A (1985) Quantitative
correlation studies between the acute lethal toxicity of 15 organic
halides to the guppy (Poecilia reticulata) and chemical reactivity
towards 4-nitrobenzylpyridine. Toxicol Environ Chem, 9: 219-236.
Hook JB, Rose MS, & Lock EA (1982) The nephrotoxicity of
hexachloro-1,3-butadiene in the rat: studies of organic anion and
cation transport in renal slices and the effect of monoxygenase
inducers. Toxicol Appl Pharmacol, 65: 373-382.
Hook JB, Ishmael J, & Lock EA (1983) Nephrotoxicity of
hexachloro-1,3-butadiene in the rat: the effect of age, sex, and
strain. Toxicol Appl Pharmacol, 67: 122-131.
Hutzinger O (1982) Handbook of environmental chemistry: 3B.
Anthropogenic sources. Berlin, Heidelberg, New York, Springer
Verlag, pp 69-87.
IARC (1979) Some halogenated hydrocarbons. Lyon, International
Agency for Research on Cancer, pp 179-189 (IARC Monograph on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
20).
Ishmael J & Lock EA (1986) Nephrotoxicity of hexachlorobutadiene and
its glutathione-derived conjugates. Toxicol Pathol, 14: 258-262.
Ishmael J, Pratt I, & Lock EA (1982) Necrosis of the pars recta (S3
segment) of the rat kidney produced by hexachloro-1,3-butadiene. J
Pathol, 138: 99-113.
Ishmael J, Pratt I, & Lock EA (1984) Hexachloro-1,3-butadiene-
induced renal tubular necrosis in the mouse. J Pathol, 142: 195-203.
Jaffe DR, Hassall CD, Brendel K, & Gandolfi AJ (1983) in vivo and
in vitro nephrotoxicity of the cysteine conjugate of
hexachlorobutadiene. J Toxicol Environ Health, 11: 857-867.
Jacobs A, Blangetti M, & Hellmund E (1974) [Accumulation of
chlorinated contaminants of the Rhine in fat tissue of rats.] Vom
Wasser, 43: 259-274 (in German).
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed, 55:
1441-1449.
Jones TW, Gerdes RG, Ormstad K, & Orrenius S (1985) The formation of
both a mono-and a bis-substituted glutathione conjugate of
hexachlorobutadiene by isolated hepatocytes and following in vivo
administration to the rat. Chem-Biol Interact, 56: 251-267.
Jones TW, Wallin A, Thor H, Gerdes RG, Ormstad K, & Orrenius S
(1986a) The mechanism of pentachlorobutadienyl-glutathione
nephrotoxicity studies with isolated rat renal epithelial cells.
Arch Biochem Biophys, 251: 504-513.
Jones TW, Thor H, & Orrenius S (1986b) In vitro studies of
mechanisms of cytotoxicity. Food Chem Toxicol, 24: 769-773.
Jones TW, Quin C, Schaeffer VH, & Stevens JL (1988)
Immunohistochemical localization of glutamine transaminase K, a rat
kidney cysteine conjugate beta-lyase, and the relationship to the
segment specificity of cysteine conjugate nephrotoxicity. Mol
Pharmacol, 34(5): 621-627.
Kaminsky R, Kaiser KLE, & Hites RA (1983) Fates of organic compounds
from Niagara Falls dumpsites n Lake Ontario. J Great Lakes Res, 9:
183-189.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soil. Chemosphere,
10: 833-846.
Kastl PE & Hermann BA (1983) Quantitative gas chromatographic
determination of hexachloro-1,3-butadiene in whole rat blood at part
per trillion levels. J Chromatogr, 280(2): 390-393.
Kauss PB & Hamdy YS (1985) Biological monitoring of organochlorine
contaminants in the St Clair and Detroit rivers using introduced
clams Elliptio complanatus. J Great Lakes Res, 11: 247-263.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci
Health, A21: 15-53.
Kluwe WM, McNish R, Smithson K, & Hook JB (198l) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane,
tris(2,3-dibromopropyl)phosphate, and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and non-target
organs. Biochem Pharmacol, 30: 2265-2271.
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and
in vitro. J Pharmacol Exp Ther, 230: 597-603.
Kociba RJ, Gehring PJ, Humiston CG, & Sparschu GL (1971) Toxicologic
study of female rats administered hexachlorobutadiene or
hexachlorobenzene for thirty days. Midland, Michigan, Dow Chemical
Company, Chemical Biology Research (Unpublished report).
Kociba RJ, Keyes DG, Jersey GC, Ballard JJ, Dittenber DA, Quast JF,
Wade CE, Humiston CG, & Schwetz BA (1977a) Results of a two year
chronic toxicity study with hexachlorobutadiene in rats. Am Ind Hyg
Assoc J, 38: 589-602.
Kociba RJ, Schwetz BA, Keyes DG, Jersey GC, Ballard JJ, Dittenber
DA, Quast JF, Wade CE, & Humiston CG (1977b) Chronic toxicity and
reproduction studies of hexachloro-butadiene in rats. Environ Health
Perspect, 21: 49-53.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies. Part I: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Koob M & Dekant W (1991) Bioactivation of xenobiotics by formation
of toxic glutathione conjugates. Chem-Biol Interact, 77: 107-136.
Kotzias D, Lay JP, Klein W, & Korte F (1975) [Analysis of residues
of hexachloro-butadiene in foodstuffs and poultry.] Chemosphere, 4:
247-250 (in German).
Krasniuk EP, Ziritskaya LA, Bioko VG, Voitenko GA, & Matokhniuk LA
(1969) [Health condition of vine-growers contacting with fumigants
hexachlorobutadiene and polychlorbutan-80.] Vrach Delo, 7: 111-115
(in Russian).
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Kuo CH, & Hook JB (1983) Effects of age and sex on
hexachloro-1,3-butadiene toxicity in the Fischer 344 rat. Life Sci,
33: 517-523.
Kusz P, Andriysiak A, & Pokorska Z (1984) Gas chromatographic
monitoring of the chlorolysis processes of some byproducts from
vinyl chloride, allyl chloride and epichlorohydrin production. J
Chromatogr, 286: 287-291.
Laseter JL, Bartell CK, Laska AL, Holmquist DG, Condie DB, Brown JW,
& Evans RL (1976) An ecological study of hexachlorobutadiene (HCBD).
New Orleans, Louisiana, University of New Orleans, Department of
Biological Sciences (Prepared for the US Environmental Protection
Agency, Office of Toxic Substances, Washington (EPA 560/6-76-010; PB
252671).
Lash LH, Nelson RM, Dyke RA, & Anders MW (1990) Purification and
characterization of human kidney cytosolic cysteine conjugate
beta-lyase activity. Drug Metab Dispos, 18: 50-54.
Leeuwangh P, Bult H, & Schneiders L (1975) Toxicity of
hexachlorobutadiene in aquatic organisms. In: Koeman JH & Strik
JJTWA ed. Sublethal effects of toxic chemicals on aquatic animals.
Proceedings of the Swedish-Netherlands Symposium, Waningen, The
Netherlands, 2-5 September 1975. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 167-176.
Li RT, Going JE, & Spigarelli JL (1976) Sampling and analysis of
selected toxic substances: Task I B. Hexachlorobutadiene. Kansas
City, Missouri, Midwest Research Institute (EPA Contract No.
68-01-2646).
Litvinov PI & Gorenshtein RS (1982) [Migration of
hexachlorobutadiene.] Zashch Rast (Moscow), 8: 33-34 (in Russian).
Lock EA (1987a) Metabolic activation of halogenated chemicals and
its relevance to nephrotoxicity. In: Bach PH & Lock EA ed.
Nephrotoxicity in the experimental and clinical situation.
Dordrecht, Martinus Nijhoff Publishers, pp 429-461.
Lock EA (1987b) The nephrotoxicity of haloalkane and haloalkene
glutathione conjugates. In: DeMatteis F & Lock EA ed. Selectivity
and molecular mechanisms of toxicity. New York, McMillan Publishing
Company, pp 59-83.
Lock EA (1988) Studies on the mechanism of nephrotoxicity and
nephrocarcinogenicity of halogenated alkenes. CRC Crit Rev Toxicol,
19: 23-42.
Lock EA & Ishmael J (1979) The acute toxic effects of
hexachloro-l,3-butadiene on the rat kidney. Arch Toxicol, 43: 47-57.
Lock EA & Ishmael J (1981) Hepatic and renal nonprotein sulfhydryl
concentration following toxic doses of hexachloro-1,3-butadiene in
the rat: the effect of Aroclor 1254, phenobarbitone, or SKF 525A
treatment. Toxicol Appl Pharmacol, 57: 79-87.
Lock EA & Ishmael J (1985) Effect of the organic acid transport
inhibitor probenecid on renal cortical uptake and proximal tubular
toxicity of hexachloro-1,3-butadiene and its conjugates. Toxicol
Appl Pharmacol, 81: 32-42.
Lock EA, Ishmael J, & Pratt I (1982) Hydropic change in rat liver
induced by hexachloro-1,3-butadiene. J Appl Toxicol, 2: 315-320.
Lock EA, Ishmael J, & Hook JB (1984) Nephrotoxicity of
hexachloro-1,3-butadiene in the mouse: the effect of age, sex,
strain, monooxygenase modifiers, and the role of glutathione.
Toxicol Appl Pharmacol, 72: 484-494.
Lock EA, Pratt I, & Ishmael J (1985)
Hexachloro-1,3-butadiene-induced hydropic change in mouse liver. J
Appl Toxicol, 5: 74-79.
Lock EA, Odum J, & Ormond P (1986) Transport of
N-acetyl-S-pentachloro-1,3-butadi-ethylcysteine by renal cortex.
Arch Toxicol, 59: 12-15.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, & Billets S (1983)
Determination of 51 priority organic compounds after extraction from
standard reference materials. Anal Chem, 55: 881-889.
Lopez-Avila V, Dodhiwala NS, Milanes J, & Beckert W (1989)
Evaluation of EPA method 8120 for determination of chlorinated
hydrocarbons in environmental samples. J Assoc Off Anal Chem, 72:
593-602.
McConnell G, Ferguson DM, & Pearson CR (1975) Chlorinated
hydrocarbon and the environment. Endeavour, 34: 13-18.
McFarlane M, Foster JR, Gibson GG, King LJ, & Lock EA (1989)
Cysteine conjugate beta-lyase of rat kidney cytosol:
characterization, immunocytochemical localization, and correlation
with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol, 98:
185-197.
McLellan LI, Wolf CR, & Hayes JD (1989) Human microsomal glutathione
S-transferase. Its involvement in the conjugation of
hexachlorobuta-1,3-diene with glutathione. Biochem J, 258: 87-93.
Malins DC, Krahn MM, Myers MS, Rhoses LD, Brown DW, Krone CA, McCain
BB, & Chan SI (1985) Toxic chemicals in sediments and biota from a
creasote-polluted harbor: relationships with hepatic neoplasms and
other hepatic lesions in English sole (Parophrys vetulus).
Carcinogenesis, 6: 1463-1469.
Mann JB, Enos HF, Gonzales J, & Thompson JF (1974) Development of
sampling and analytical procedure for determining hexachlorobenzene
and hexachloro-1,3-butadiene in air. Environ Sci Technol, 8:
584-585.
Markovec LM & Magee RJ (1984) Identification of major
perchloroaromatic compounds in waste products from the production of
carbon tetrachloride and tetrachloroethylene. Analyst, 109: 497-501.
Meijers AP (1988) [Organic trace substances in Rhine water and in
filtrate from the banks of the Rhine.] Wasser Abwasser, 129:
208-211.
Melcher RG & Caldecourt VJ (1980) Delayed injection-preconcentration
gas chromatographic technique for parts-per-billion determination of
organic compounds in air and water. Anal Chem, 52: 875-881.
Mes J, Davies DJ, & Turton D (1982) Polychlorinated biphenyl and
other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull Environ Contam Toxicol, 28: 97-104.
Mes J, Davies DJ, & Turton D (1985) Environmental contaminants in
human fat: comparison between accidental and nonaccidental causes of
death. Ecotoxicol Environ Saf, 10: 70-74.
Mes J, Davies DJ, Turton D, & Sun WF (1986) Levels and trends of
chlorinated hydrocarbon contaminants in the breast milk of Canadian
women. Food Addit Contam, 3: 313-322.
Mumma CE & Lawless EW (1975) Survey of industrial processing data:
Task I. Hexachlorobenzene and hexachlorobutadiene pollution from
chlorocarbon processing. Kansas City, Missouri, Midwest Research
Institute (Prepared for the US Environmental Protection Agency,
Office of Toxic Substances, Washington) (EPA 560/3-75-003; PB
243641).
Murzakayev FG (1963) [Data for substantiating maximal permissible
concentrations of hexachlorobutadiene and polychlorobutane in water
basins.] Gig i Sanit, 28: 9-14 (in Russian).
Nash JA, King LJ, Lock EA, & Green T (1984) The metabolism and
disposition of hexachloro-1,3-butadiene in the rat and its relevance
for nephrotoxicity. Toxicol Appl Pharmacol, 73: 124-137.
NIOSH (1979) Manual of analytical methods: Method 308. Cincinnati,
Ohio, National Institute of Occupational Safety and Health, vol 5.
NIOSH (1990) Manual of analytical methods: Method No. P & CAM 307.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
NPHEP (National Institute of Public Health and Environmental
Protection, The Netherlands) (1990) A proposal for guideline
concerning the estimation of environmental concern levels for the
modified EPA method. In: Report of the OECD Workshop on
Ecotoxicological Effects Assessment, Arlington, USA, December 1990.
Paris, Organisation for Economic Co-operation and Development.
Oesch F & Wolf CR (1989) Properties of the microsomal and cytosolic
glutathione transferases involved in hexachloro-1,3-butadiene
conjugation. Biochem Pharmacol, 38: 353-359.
OECD (1990) Report of the OECD Workshop of Ecotoxicological Effect
Assessment, Arlington, USA, December 1990. Paris, Organisation for
Economic co-operation and Development.
Oliver BG (1984) Uptake of chlorinated organics from
anthropogenically contaminated sediments by oligochaete worms. Can J
Fish Aquat Sci, 41: 878-883.
Oliver BG (1987) Biouptake of chlorinated hydrocarbons from
laboratory-spiked and field sediments by oligochaete worms. Environ
Sci Technol, 21: 785-790.
Oliver BG & Bourbonniere RA (1985) Chlorinated contaminants in
surficial sediments of Lakes Huron, St Clair, and Erie: implications
regarding sources along the St Clair and Detroit Rivers. J Great
Lakes Res, 11: 366-372.
Oliver BG & Charlton MN (1984) Chlorinated organic contaminants on
settling particulates in the Niagara River vicinity of Lake Ontario.
Environ Sci Technol, 18: 903-908.
Oliver BG & Nicol KD (1982) Gas chromatographic determination of
chlorobenzenes and other chlorinated hydrocarbons in environmental
samples using fused silica capillary columns. Chromatographia, 16:
336-340.
Oliver BG & Nicol KD (1984) Chlorinated contaminants in the Niagara
River (198l-1983). Sci Total Environ, 34: 57-70.
Oliver BG & Niimi AJ (1983) Bioconcentration of chlorobenzenes from
water by Rainbow trout: correlations with partition coefficients and
environmental residues. Environ Sci Technol, 17: 287-291.
Oliver BG & Niimi AJ (1988) Trophodynamic analysis of
polychlorinated biphenyl congeners and other chlorinated
hydrocarbons in the Lake Ontario ecosystems. Environ Sci Technol,
22: 388-397.
Otson R & Chan C (1987) Sample handling and analysis for 51 volatile
organics by an adapted urge and trap GC-MS technique. Int J Environ
Anal Chem, 30: 275-287.
Payan JP, Fabry JP, Beydon D, & De Ceaurriz J (1991) Biliary
excretion of hexachloro-1,3-butadiene and its relevance to tissue
uptake and renal excretion in male rats. J Appl Toxicol, 11:
437-442.
Pearson CR & McConnell G (1975) Chlorinated C1 and C2 hydrocarbons
in the marine environment. Proc R Soc (Lond), B189: 305-332.
Pellizari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16: 781-785.
Pereira WE, Rostad CE, Chiou CT, Brinton TI, Barber LB, Demcheck DK,
& Demas CR (1988) Contamination of estuarine water, biota, and
sediment by halogenated organic compounds: a field study. Environ
Sci Technol, 22: 772-778.
Petersen VP (1986) [Analysis of moderately volatile organochlorine
compounds and their concentration distribution in different streams
of the Federal Republic of Germany.] Dtsch Gewässerkd Mitt, 30:
10-15 (in German).
Poteryayeva GE (1966) [The effect of hexachlorobutadiene on
offspring of albino rats.] Gig i Sanit, 31: 33-36 (in Russian).
Pratt IS & Lock EA (1988) Deacylation and further metabolism of the
mercapturic acid of hexachloro-1,3-butadiene by rat kidney cytosol
in vitro. Arch Toxicol, 62: 341-345.
Rapson W, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24: 590-596.
Reichert D (1983) Metabolism and disposition of
hexachloro(1,3)butadiene in rats. Dev Toxicol Environ Sci, 5:
411-414.
Reichert D & Schütz S (1986) Mercapturic acid formation is an
activation and intermediary step in metabolism of
hexachlorobutadiene. Biochem Pharmacol, 35: 1271-1275.
Reichert D, Neudecker T, Spengler U, & Henschler D (1983)
Mutagenicity of dichloroacetylene, trichloroacryloyl chloride and
hexachlorobutadiene. Mutat Res, 117: 21-29.
Reichert D, Neudecker T, & Schütz S (1984) Mutagenicity of
hexachlorobutadiene, perchlorobutenoic acid and perchlorobutenoic
acid chloride. Mutat Res, 137: 89-93.
Reichert D, Schütz S, & Metzler M (1985) Excretion pattern and
metabolism of hexachlorobutadiene in rats. Evidence for metabolic
activation by conjugation reactions. Biochem Pharmacol, 34: 499-505.
Reimschüssel R, Bennet RO, May EB, & Lipsky MM (1989) Renal
histopathological changes in the goldfish (Carassius auratus)
after sublethal exposure to hexachlorobutadiene. Aquat Toxicol, 15:
169-180.
Reimschüssel R, Bennett RO, May EB, & Lipsky MM (1990) Development
of newly formed nephrons in the goldfish kidney following
hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol, 18:
32-38.
Roedig A & Bernemann P (1956) [Data on the highly chlorinated
vinylether 1-ethoxy-pentachloro-(1,3)-butadiene from
perchlorobutadiene.] Justus Liebigs Ann Chem, 600: 1-11 (in German).
Rush GF, Smith JH, Newton JF, & Hook JB (1984) Chemically induced
nephrotoxicity: role of metabolic activation. CRC Crit Rev Toxicol,
13: 99-156.
Ruth JH (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A142-A151.
Saillenfait AM, Bonnet P, Guenier JP, & De Ceaurriz J (1989)
Inhalation teratology study on hexachloro-1,3-butadiene in rats.
Toxicol Lett, 47: 235-240.
Sauer T Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Schiffmann D, Reichert D, & Henschler D (1984) Induction of
morphological transformation and unscheduled DNA synthesis in Syrian
hamster embryo fibroblasts by hexachlorobutadiene and its putative
metabolite pentachlorobutenoic acid. Cancer Lett, 23: 297-305.
Schnellmann RG, Lock EA, & Mandel LJ (1987) A mechanism of
S-(1,2,3,4,4-pentachloro-1,3-butadienyl)L-cysteine toxicity to
rabbit renal proximal tubules. Toxicol Appl Pharmacol, 90: 513-521.
Schrenk D & Dekant W (1989) Covalent binding of hexachlorobutadiene
metabolites to renal and hepatic mitochondrial DNA. Carcinogenesis,
10: 1139-1141.
Schrenk D, Dekant W, Wunsch PH, & Henschler D (1988a) Role of
metabolic activation in the toxicity of S-(pentachlorobutadienyl)
glutathione and S-(pentachlorobutadienyl)-L-cysteine in the
isolated perfused rat kidney. Toxicol in Vitro, 2: 283-290.
Schrenk D, Dekant W, & Henschler D (1988b) Metabolism and excretion
of S-conjugates derived from hexachlorobutadiene in the isolated
perfused rat kidney. Mol Pharmacol, 34: 407-412.
Schröder HF (1987) Chlorinated hydrocarbons in biological sewage
purification - Fate and difficulties in balancing. Water Sci
Technol, 19: 429-438.
Schwetz BA, Norris JM, Kociba RJ, Keeler PA, Cornier RF, & Gehring
PJ (1974) Reproduction study in Japanese quail fed
hexachlorobutadiene for 90 days. Toxicol Appl Pharmacol, 30:
255-265.
Schwetz BA, Smith FA, Humiston CG, Quast JF, & Kociba RJ (1977)
Results of a reproduction study in rats fed diets containing
hexachlorobutadiene. Toxicol Appl Pharmacol, 42: 387-398.
Shen TT (1982) Air quality assessment for land disposal of
industrial wastes. Environ Manage, 6: 297-305.
Singh HB, Salas LJ, & Stiles R (1982) Distribution of selected
gaseous organic mutagens and suspected carcinogens in ambient air.
Environ Sci Technol, 16: 872-880.
SRI (1984) Directory of chemical producers, Western Europe. Menlo
Park, California, Stanford Research Institute.
Stonard MD, Gore CW, Oliver GJA, & Smith IK (1987) Urinary enzymes
and protein pattern as indicators of injury to different regions of
the kidney. Fundam Appl Toxicol, 9: 339-351.
Stott WT, Quast JF, & Watanabe PG (1981) Differentiation of the
mechanisms of oncogenicity of 1,4-dioxane and
1,3-hexachlorobutadiene in the rat. Toxicol Appl Pharmacol, 60:
287-300.
Tabak HH, Quave SA, Mashni CE, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1517.
Theiss JC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer Res, 37:
2717-2720.
Ullmann F (1986) Ullmann's encyclopedia of industrial chemistry.
Weinheim, VCH-Verlagsgesellschaft, vol A6, pp 322-323, 375, 391,
398.
US EPA (1980) Ambient water quality criteria for
hexachlorobutadiene. Washington, DC, US Environmental Protection
Agency, Criteria and Standards Division (EPA-440/5-80-053;
PB-81-117640).
UP EPA (1984a) A method for determination of certain chlorinated
hydrocarbons. EPA method No. 612. Fed Reg, 491(209): 29-210.
US EPA (1984b) A gas chromatographic method for determination of
certain chlorinated hydrocarbons. EPA method No. 612. Fed Reg,
491(209): 29-210.
Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, & Henschler D
(1987) Enzymatic transformation of mercapturic acids derived from
halogenated alkenes to reactive and mutagenic intermediates. Biochem
Pharmacol, 36: 2741-2748.
Vamvakas S, Kordowich FJ, Dekant W, Neudecker T, & Henschler D
(1988a) Mutagenicity of hexachloro-1,3-butadiene and its
S-conjugates in the Ames test - role of activation by the
mercapturic acid pathway in its nephrocarcinogenicity.
Carcinogenesis, 9: 907-910.
Vamvakas S, Muller DA, Dekant W, & Henschler D (1988b) DNA binding
of sulfur containing metabolites from 35S-(pentachlorobutadienyl)-
L-cysteine in bacteria and isolated renal tubular cells. Drug Metab
Drug Interact, 6: 349-358.
Vamvakas S, Dekant W, & Henschler D (1989a) Genotoxicity of
haloalkene and haloalkane glutathione S-conjugates in porcine kidney
cells. Toxicol in Vitro, 3: 151-156.
Vamvakas S, Kochling A, Berthold K, & Dekant W (1989b)
Cytotoxicity of cysteine S-conjugates: structure-activity
relationships. Chem-Biol Interact, 71: 79-90.
Vamvakas S, Dekant W, & Henschler D (1993) Nephrocarcinogenicity of
haloalkenes and alkynes. In: Renal disposition and nephrotoxicity of
xenobiotics. New York, Academic Press, pp 323-342.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchlonne S,
Seldman I, & Roth D (1979) Carcinogenicity of halogenated olefinic
and aliphatic hydrocarbons in mice. J Natl Cancer Inst, 63:
1433-1439.
Vorobyeva TN (1980) [Enpared residues in grapes.] Khim Sel'sk Khoz,
18(10): 41-42 (in Russian).
Wallin A, Jones TW, Vercesi AE, Cotgreave I, Ormstad K, & Orrenius S
(1987) Toxicity of S-pentachlorobutadienyl-L-cysteine studied with
isolated rat renal cortical mitochondria. Arch Biochem Biophys, 258:
365-372.
Wallin A, Gerdes RG, Morgenstern R, Jones TW, & Ormstad K (1988)
Features of microsomal and cytosolic glutathione conjugation of
hexachlorobutadiene in rat liver. Chem-Biol Interact, 68: 1-11.
Wild D, Schütz S, & Reichert D (1986) Mutagenicity of the
mercapturic acid and other S-containing derivatives of
hexachloro-1,3-butadiene. Carcinogenesis, 7: 431-434.
Wolf CR, Berry PN, Nash JA, Green T, & Lock EA (1984) Role of
microsomal and cytosolic glutathione S-transferases in the
conjugation of hexachloro-1,3-butadiene and its possible relevance
to toxicity. J Pharmacol Exp Toxicol, 228: 202-208.
Woodruff RC, Mason JM, Valencia R, & Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environ Mutagen, 7:
677-702.
Yang RSH (1988) Hexachloro-1,3-butadiene: toxicology, metabolism,
and mechanisms of toxicity. Rev Environ Contam Toxicol, 101:
121-137.
Yang RSH (1991) Toxicity studies of hexachloro-1,3-butadiene in
B6C3F1 mice (feed studies). Research Triangle Park, North
Carolina, National Toxicology Program (NTP TOX 1) (NIH Publication
No. 91-3120).
Yang RSH, Abdo KM, Elwell MR, Levy AC, & Brennecke LH (1989)
Subchronic toxicology studies of hexachloro-1,3-butadiene (HCBD) in
B6C3F1 mice by dietary incorporation. J Environ Pathol Toxicol
Oncol, 9: 323-332.
Yasuhara A & Morita M (1990) Formation of chlorinated compounds in
pyrolysis of trichloroethylene. Chemosphere, 21: 479-486.
Yip G (1976) Survey for hexachloro-1,3-butadiene in fish, eggs,
milk, and vegetables. J Assoc Anal Chem, 59: 559-561.
Yurawecz MP, Dreifuss PA, & Kamps LR (1976) Determination of
hexachloro-1,3-butadiene in spinach, eggs, fish, and milk by
electron capture gas-liquid chromatography. J Assoc Anal Chem, 59:
552-558.
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of The
Netherlands. Chemosphere, 9: 231-249.
Zogorski JS (1984) Experience in monitoring domestic water sources
and process waters for trace organics. J Environ Sci Health, A19:
233-249.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
L'hexachlorobutadiène est un liquide ininflammable,
incombustible, limpide et huileux à la température et la pression
ordinaires. Il est peu soluble dans l'eau mais miscible à l'éther et
à l'éthanol.
On peut le mettre en évidence et le doser par chromatographie
en phase gazeuse. Les limites de détection sont de 0,03 µg/m3 dans
l'air, 0,001 µg/litre dans l'eau, de 0,7 µg/kg de matière humide
dans le sol ou les sédiments et de 0,02 µg/litre dans le sang. Dans
les tissus, cette limite est de 0,47 µg/kg de tissus frais.
2. Sources d'exposition humaine et environnementale
L'hexachlorobutadiène n'existe pas à l'état naturel. C'est
essentiellement un sous-produit de la fabrication des hydro-carbures
chlorés que l'on retrouve dans les fractions lourdes. La production
annuelle mondiale (dans les fractions lourdes) a été estimée à
10 000 tonnes en 1982. L'hexachlorobutadiène peut être utilisé pour
la récupération des gaz contenant du chlore dans les ateliers de
fabrication du chlore et comme liquide de lavage pour éliminer du
courant gazeux certains composés organiques volatils. On l'utilise
également dans les gyroscopes, comme fluide calo-porteur, dans les
transformateurs, comme liquide isolant ou liquide hydraulique ainsi
que comme solvant des élastomères, comme intermédiaire et comme
fumigant.
3. Transport, distribution et transformation dans l'environnement
Les principales voies de pénétration dans l'environnement sont
les émissions résultant des déchets et les utilisations qui
entraînent la dispersion du produit. Le transport
inter-compartimental s'effectue principalement par volatilisation,
adsorption sur les matières particulaires puis dépôt ou
sédimentation. L'hexachloro-butadiène ne migre pas facilement dans
le sol et s'accumule dans les sédiments. Dans l'eau, on le considère
comme persistant, sauf turbulences importantes. Il n'y a pas
d'hydrolyse. Le produit semble être facilement biodégradable par
voie aérobie, encore que le phénomène n'ait pas été étudié à fond.
L'hexachlorobutadiène présent sur les surfaces subit une photolyse.
Outre le dépôt, on estime que la réaction de l'hexachlorobutadiène
avec les radicaux hydroxyles constitue un mode de piégeage important
de ce composé dans la troposphère, la demi-vie atmosphérique
estimative de l'hexachlorobutadiène pouvant aller jusqu'à 2,3 ans.
Le produit a un potentiel élevé de bioaccumulation, comme l'ont
confirmé les observations en laboratoire et sur le terrain. Ainsi,
on a trouvé des facteurs de bioconcentration à l'état stationnaire
(obtenus expérimentalement par rapport au poids de tissus frais)
respectivement égaux en moyenne à 5800 et 17 000 chez la truite
arc-en-ciel. On n'a pas observé d'amplification biologique au
laboratoire ou sur le terrain.
4. Niveaux dans l'environnement et exposition humaine
Le dosage de l'hexachlorobutadiène dans l'air des villes a
donné dans tous les cas des valeurs inférieures à 0,5 µg/m3. Dans
les régions écartées, les concentrations sont inférieures à
1 pg/m3. Dans les lacs et les cours d'eau d'Europe, on a
enregistré des concentrations pouvant aller jusqu'à 2 µg/litre mais
les valeurs moyennes sont généralement inférieures à 100 ng/litre.
Dans la région des grands lacs au Canada, on a obtenu des valeurs
beaucoup plus faibles (autour de 1 ng/litre). En revanche la teneur
des sédiments du fond peut, dans cette zone, atteindre 120 µg/kg de
poids sec. Les couches sédimentaires plus anciennes, remontant aux
environs de 1960, présentaient des teneurs plus élevées (jusqu'à
550 µg/kg de matière humide). On a montré que la concentration dans
les sédiments augmentait avec la granulométrie des particules.
A en juger par la concentration de l'hexachlorobutadiène dans
les organismes aquatiques, les oiseaux et les mammifères, le composé
s'accumule mais ne subit pas d'amplification biologique. Dans les
eaux polluées, on a relevé des concentrations dépassant 1000 µg/kg
de tissus frais chez plusieurs espèces et même 120 mg/kg (par
rapport aux lipides) chez une espèce. Les concentrations actuelles
restent généralement inférieures à 1000 µg/kg de poids frais à
distance des points de décharge industrielle.
On a décelé la présence du composé dans l'urine, le sang et les
tissus humains. Dans certaines denrées alimentaires ayant une
fraction lipidique importante, on en a relevé jusqu'à 40 µg/kg et
dans un cas, plus de 1000 µg/kg.
D'après une étude, le niveau d'exposition pourrait atteindre
1,6 à 12,2 mg/m3 et les concentrations urinaires, 20 mg/litre.
5. Cinétique et métabolisme
Après administration par voie orale, l'hexachlorobutadiène est
rapidement absorbé chez l'animal de laboratoire mais le taux de
résorption après inhalation ou exposition par voie cutanée n'a pas
été étudié. Chez le rat et la souris, le composé se répartit
principalement dans le foie, les reins et les tissus adipeux. Il est
rapidement excrété. On a mis en évidence une fixation aux protéines
et aux acides nucléiques dans le foie et les reins.
La biotransformation du composé chez l'animal de laboratoire se
révèle être un processus saturable. Elle s'effectue principalement
par l'intermédiaire du glutathion, l'hexachlorobutadiène étant
d'abord transformé en conjugué du S-glutathion. La métabolisation
de ce conjugué se poursuit ensuite, en particulier au niveau de la
membrane constituant la bordure en brosse des cellules des tubules
rénaux, pour donner un métabolite sulfuré réactif qui est
probablement responsable des effets néphrotoxiques, génotoxiques et
cancérogènes observés.
6. Effets sur les êtres vivants dans leur milieu naturel
L'hexachlorobutadiène est modérément à très toxique pour les
organismes aquatiques. Certaines espèces de poissons et de crustacés
se sont révélées être les plus sensibles, les valeurs de la CL50 à
96 h. allant de 0,032 à 1,2 et de 0,09 à environ 1,7 mg/litre,
respectivement pour les crustacés et les poissons. Chez les
poissons, le rein est organe-cible important.
On a établi la valeur de la dose sans effets observables à
0,003 mg/litre, à partir des résultats d'un certain nombre
d'épreuves à long terme sur certaines espèces d'algues et de
poissons; cela permet de considérer ce composé comme très toxique
pour les organismes aquatiques. Parmi les points d'aboutissement
biologiques étudiés figuraient la toxicité générale, la
neurotoxicité, la biochimie, l'hématologie, l'anatomopathologie et
la reproduction. Lors d'une étude de 28 jours portant sur les
premiers stades de la vie de Pimephales promelas, une espèce de
vairon, on a observé que la reproduction n'était pas affectée à des
concentrations allant jusqu'à 0,017 mg/litre, alors qu'à 0,013 et
0,017 mg/litre il y avait accroissement de la mortalité et réduction
du poids du corps. La dose sans effets observables était de
0,0065 mg/litre.
On n'a décrit qu'une seule épreuve fiable portant sur des
organismes terrestres. Lors d'une épreuve de 90 jours sur des
cailles japonaises qui recevaient une alimentation contenant ce
composé à des concentrations allant de 0,3 à 30 mg/kg de nourriture,
on a constaté que la survie des oisillons n'était réduite qu'à
partir de 10 mg/kg de nourriture.
7. Effets sur les animaux de laboratoire et les systèmes
d'épreuves in vitro
7.1 Toxicité générale
L'hexachlorobutadiène est légèrement à modérément toxique pour
le rat adulte, modérément toxique pour le raton juste sevré et
extrêmement toxique pour les rattes juste sevrées après
administration d'une seule dose par voie buccale. Les principaux
organes-cibles sont le rein et dans une bien moindre mesure, le
foie.
D'après les données obtenues sur l'animal d'expérience, les
vapeurs d'hexachlorobutadiène sont irritantes pour les muqueuses et
le liquide est corrosif. On peut considérer ce composé comme un
agent sensibilisateur.
Chez le rat, la souris et le lapin, l'hexachlorobutadiène
provoque une nécrose, liée à la dose, des tubules proximaux du rein.
Les rats mâles adultes sont moins sensibles à la néphrotoxicité que
les femelles ou les jeunes mâles. Les souriceaux sont plus sensibles
que les souris adultes sans qu'on puisse observer de différences
entre les deux sexes. Chez la ratte adulte, la dose intrapéritonéale
unique la plus faible à laquelle on ait observé une nécrose rénale
était de 25 mg/kg de poids corporel; elle était de 6,3 mg/kg de
poids corporel chez les souris adultes mâles et femelles. A des
doses égales ou supérieures à celles qui entraînaient une nécrose,
on a observé des modifications biochimiques et une nette
amélioration de la fonction rénale.
Lors de six épreuves à court terme où le composé a été
administré par la voie orale, deux études de reproduction et une
étude d'alimentation à long terme portant sur des rats, c'est
également le rein qui s'est révélé être l'organe-cible. Parmi les
effets liés à la dose, on notait une diminution du poids relatif des
reins et une dégénérescence de l'épithélium des tubules. La dose
sans effets nocifs observables au niveau des reins, tirée d'une
étude de deux ans sur le rat, était de 0,2 mg/kg de poids corporel
et par jour. Une étude de 13 semaines sur des souris a montré que
cette dose était de 0,2 mg/kg de poids corporel et par jour pour cet
animal. Chez les deux espèces, les femelles adultes étaient plus
sensibles que les mâles adultes.
Lors d'une étude d'inhalation à court terme (six heures par
jour pendant 12 jours) on a observé des effets analogues au niveau
des reins avec une concentration nominale de vapeur
d'hexa-chlorobutadiène égale à 267 mg/m3; cette concentration a
également entraîné des difficultés respiratoires, ainsi qu'une
dégénérescence des corticosurrénales.
7.2 Reproduction, embryotoxicité et teratogétogénicité
Deux études d'alimentation portant sur la reproduction ont été
effectuée sur des rats à des doses quotidiennes allant jusqu'à 20 et
75 mg/kg de poids corporel respectivement; elles ont fait ressortir
une réduction du poids de naissance et du gain de poids néonatal aux
doses toxiques pour la mère. La dose quotidienne de 75 mg/kg de
poids corporel, qui était hautement toxique, s'est révélée
suffisante pour empêcher la conception et la nidation intra-utérine.
On n'a pas observé d'anomalies du squelette.
Lors de deux études de tératogénicité, des rats ont été exposés
soit à des vapeurs d'hexachlorobutadiène à des concentrations allant
de 21 à 160 mg/m3, six heures par jour du sixième au vingtième
jour de la gestation, soit par voie intrapéritonéale à une dose
quotidienne de 10 mg/kg de poids corporel (du premier au quinzième
jour de la gestation). Des effets nocifs ont été notés sur le
développement des foetus, qui consistaient en une réduction du poids
de naissance, un retard dans le développement cardiaque, une
dilatation des uretères, mais pas de malformations macroscopiques.
Le retard de développement a été observé à des doses qui étaient
également toxiques pour les mères.
7.3 Génotoxicité et cancérogénicité
L'hexachlorobutadiène provoque des mutations géniques dans
l'épreuve d'Ames sur salmonelle dans des conditions particulières
qui favorisent la formation de produits de conjugaison avec le
glutathion. Lors d'une étude in vivo on a observé des aberrations
chromosomiques qui n'ont en revanche pas été constatées lors de deux
autres études in vitro. Une étude in vitro portant sur des
cellules ovariennes de hamster chinois a révélé une augmentation de
la fréquence des échanges entre chromatides soeurs. On a fait état
de la très forte mutagénicité des métabolites sulfurés de
l'hexachlorobutadiène. D'autres études in vitro ont montré que ce
composé provoquait une synthèse non programmée de l'ADN dans des
cultures de fibroblastes embryonnaires de hamsters de Syrie, effets
qui n'étaient pas observés dans des cultures d'hépatocytes de rats.
Le composé provoque également une synthèse non programmée de l'ADN
in vivo mais n'induit pas de mutations létales récessives liées au
sexe chez Drosophila melanogaster.
Lors de la seule étude à long terme (deux ans) qui ait été
effectuée, des rats ont reçu une alimentation contenant de
l'hexachlorobutadiène à des doses quotidiennes respectives de 0,2, 2
ou 20 mg/kg de poids corporel et seule la dose la plus élevée a
provoqué un accroissement de l'incidence des tumeurs malignes au
niveau des tubules rénaux.
7.4 Mécanismes de la toxicité
La néphrotoxicité, la mutagénicité et la cancérogénicité de
l'hexachlorobutadiène sont liées à la biosynthèse d'un conjugué
sulfuré toxique, le 1-glutathion- S-yl-1,2,3,4,4-pentachloro-
butadiène. Ce conjugué est principalement synthétisé dans le foie et
métabolisé ensuite dans la bile, l'intestin et les reins en
1-cystéine- S-yl-1,2,3,4,4-pentachlorobutadiène (CPB). L'activation
du CPB en thiocétène réactif (qui dépend de la cystéine-conjuguée-
béta lyase) au niveau des cellules des tubules proximaux, aboutit en
définitive à la formation de liaisons covalentes avec les
macromolécules cellulaires.
8. Effets sur l'homme
On n'a pas décrit d'effets pathogènes sur la population dans
son ensemble.
On possède deux rapports faisant état de troubles chez des
ouvriers agricoles qui utilisaient de l'hexachlorobutadiène comme
fumigant mais il est vrai qu'ils avaient également été exposés à
d'autres substances. On a également observé un accroissement de la
fréquence des aberrations chromosomiques dans les lymphocytes du
sang périphériques de travailleurs employés à la production
d'hexachlorobutadiène et qui avaient été exposés à des
concentrations de 1,6 à 12,2 mg/m3.
9. Evaluation des risques pour la santé humaine et des
effets sur l'environnement
9.1 Evaluation des risques pour la santé humaine
Comme très peu d'études ont été effectuées sur l'homme,
l'évaluation repose essentiellement sur les animaux de laboratoire.
Toutefois les données in vitro limitées dont on dispose au sujet
de l'homme incitent à penser que le métabolisme de
l'hexachloro-butadiène est analogue chez l'homme et l'animal.
On estime que les vapeurs d'hexachlorobutadiène sont irritantes
pour les muqueuses et que le liquide est corrosif. Ce composé doit
également être considéré comme un agent sensibilisateur.
Les principaux organes-cibles de son action toxique sont les
reins et dans une mesure bien moindre, le foie. Sur la base des
études à court et à long terme effectuées sur des rats et des
souris, la dose quotidienne sans effets nocifs observables est
évaluée à 0,2 mg/kg de poids corporel. On l'a estimée à 53 mg/m3
lors d'une étude d'inhalation à court terme chez le rat (12 jours,
six heures par jour).
L'action toxique sur le développement, de même que la réduction
du poids de naissance et du gain de poids néonatal n'ont été
observés qu'à des doses toxiques pour la mère.
On a observé que l'hexachlorobutadiène produisait des mutations
géniques, des aberrations chromosomiques, un accroissement des
échanges entre chromatides soeurs et une synthèse non programmée de
l'ADN, encore que certaines études aient donné des résultats
négatifs. On ne possède que des indices limités en faveur d'une
génotoxicité de l'hexachlorobutadiène chez l'animal, indices qui
sont insuffisants en ce qui concerne l'homme.
On a constaté que l'administration d'hexachlorobutadiène par
voie orale pendant une longue période à des rats accroissait la
fréquence des tumeurs malignes au niveau des tubules rénaux, mais il
s'agissait uniquement de doses élevées fortement néphrotoxiques. En
ce qui concerne la cancérogénicité de cette substance, les indices
sont limités chez l'animal et insuffisants chez l'homme.
En se basant sur la dose quotidienne sans effets nocifs
observables estimée à 0,2 mg/kg de poids corporel chez la souris ou
le rat, on a fixé à 0,03-0,05 mg/kg de poids corporel la dose
quotidienne sans effets nocifs observables chez l'homme. La marge de
sécurité entre la dose estimative sans effets nocifs observables et
la dose journalière maximale totale ingérée estimée en se basant sur
une absorption du composé par l'intermédiaire d'une eau de boisson
et de produits alimentaires contaminés à forte teneur en lipides,
est égale à 150.
9.2 Evaluation des effets sur l'environnement
L'hexachlorobutadiène est modérément à fortement toxique pour
les organismes aquatiques: les crustacés et les poissons sont les
espèces les plus sensibles. On a fixé à 0,1 g/litre la
concentration écologiquement préoccupante. On estime que la
concentration maximale prévisible dans l'environnement à distance
des sources ponctuelles de pollution est égale à deux fois la dose
écologique-ment préoccupante extrapolée et, par voie de conséquence,
que les organismes aquatiques peuvent être menacés dans les eaux de
surface polluées. On ne peut exclure des effets nocifs sur le
benthos.
Compte tenu de la toxicité de l'hexachlorobutadiène pour les
mammifères, la consommation de benthos ou d'organismes aquatiques
par d'autres espèces pourrait être préoccupante.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos de análisis
El hexaclorobutadieno es un líquido no inflamable,
incombus-tible, claro, oleoso e incoloro a temperatura y presión
ordinarias. Es poco soluble en el agua, pero miscible con éter y
etanol.
La sustancia puede detectarse y determinarse cuantitativamente
por métodos de cromatografía de gases. Los límites de detección son
de 0,03 µg/m3 de aire, 0,001 µg/litro de agua, 0,7 µg/kg de peso
húmedo en el suelo o en sedimentos y de 0,02 µg/litro de sangre. Se
ha determinado un nivel de 0,47 µg/kg de peso húmedo de tejido.
2. Fuentes de exposición humana y ambiental
No hay indicaciones de que el hexaclorobutadieno exista como
producto natural. Es principalmente un subproducto de la fabricación
de hidrocarburos clorados y se presenta en las fracciones pesadas
(como residuo). La producción anual mundial del compuesto en las
fracciones pesadas en 1982 se estimó en 10 000 toneladas.
El hexaclorobutadieno puede utilizarse para recuperar gas que
contiene cloro en plantas productoras de cloro y como líquido de
lavado para eliminar ciertos compuestos orgánicos volátiles de las
corrientes de gases. También se ha utilizado como fluido en
giróscopos, como transmisor de calor, transformador, fluido aislante
y fluido hidráulico, disolvente para elastómeros y como
intermediario y sustancia para fumigar.
3. Transporte, distribución y transformación en el medio ambiente
Las principales vías de ingreso en el medio ambiente son las
emisiones de residuos y el uso dispersivo. El paso de un entorno a
otro ocurre principalmente por volatilización, adsorción a
corpúsculos de materia y subsiguiente deposición o sedimentación. El
hexaclorabutadieno no migra rápidamente en el suelo y se acumula en
el sedimento. Se considera persistente en el agua a menos que haya
mucha turbulencia. No produce hidrólisis. La sustancia parece ser
fácilmente biodegradable aeróbicamente, aunque su biodegradabilidad
no se ha investigado a fondo. El hexaclorobutadieno se fotoliza en
las superficies. Se supone que, además de la deposición, la reacción
con radicales hidroxilo es un importante sumidero de
hexaclorobutadieno en la troposfera y su semivida atmosférica
estimada es de hasta 2,3 años. La sustancia tiene un elevado
potencial de bioacumulación, que se ha comprobado mediante
observaciones en laboratorio y sobre el terreno. En la trucha arco
iris se han determinado experimentalmente factores de
bioconcentración en estado estacionario de 5800 y 17 000 como
promedio, sobre la base del peso húmedo. No se ha observado
biomagnificación en laboratorio ni sobre el terreno.
4. Niveles ambientales y exposición humana
Se ha determinado la presencia de hexaclorubutadieno en el aire
urbano; en todos los casos, los niveles eran inferiores a
0,5 µg/m3. Las concentraciones en lugares aislados son inferiores
a 7 pg/m3. En las aguas de lagos y ríos de Europa se han
registrado concentraciones de hasta 2 µg/litro, pero los niveles
medios son generalmente inferiores a 100 ng/litro. En la región de
los Grandes Lagos del Canadá se han detectado niveles muy inferiores
(de aproximadamente 1 ng/litro). Allí los niveles en el sedimento
del fondo pueden ser de 120 µg/kg de peso en seco. En capas más
antiguas de sedimento, de 1960 aproximadamente, se encontraron
concentraciones más elevadas (de hasta 550 µg/kg de peso húmedo). Se
ha demostrado que la concentración en el sedimento aumenta con el
tamaño de la partícula de sedimento.
Las concentraciones de hexaclorobutadieno en organismos
acuáticos, aves y mamíferos indican bioacumulación pero no
biomagnificación. En las aguas contaminadas se han detectado niveles
de más de 1000 µg/kg de peso húmedo en varias especies y de
120 mg/kg (base grasa) en una especie. Lejos de los efluentes
industriales, los niveles actuales se mantienen en general por
debajo de 100 µg/kg de peso húmedo.
Se ha detectado la presencia del compuesto en la orina, en la
sangre y en tejidos humanos. En ciertos alimentos que contienen una
elevada fracción lipídica se han encontrado hasta unos 40 µg/kg y,
en un caso, más de 1000 µg/kg.
Un estudio señala exposiciones ocupacionales de
1,6-12,2 mg/m3 y en la orina niveles de hasta 20 mg/litro.
5. Cinética y metabolismo
Se ha observado que los animales de laboratorio absorben
rápidamente el hexaclorobutadieno después de la administración oral,
pero no se ha investigado la velocidad de absorción después de la
inhalación o de la exposición dérmica. En ratas y ratones, el
compuesto se distribuye principalmente al hígado, a los riñones y al
tejido adiposo. Se excreta rápidamente. Se ha demostrado que se fija
a las proteínas y ácidos nucleicos del hígado y de los riñones.
La biotransformación del compuesto en animales de
experimentación parece ser un proceso saturable. Se produce
principalmente a través de una vía mediada por el glutatión, en la
cual el hexaclorobutadieno se convierte inicialmente en conjugados
de S-glutatión. Estos conjugados pueden seguir metabolizándose,
especialmente en el ribete en cepillo de las membranas de las
células de los tubos renales, produciendo un metabolito sulfuroso
reactivo que probablemente explique la nefrotoxicidad, genotoxicidad
y carcinogenicidad observadas.
6. Efectos en organismos presentes en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos. Los más sensibles que se hayan observado han
sido especies de peces y crustáceos; los valores de la CL50 en
96 horas oscilan entre 0,032 y 1,2 mg/litro en crustáceos y entre
0,09 y 1,7 mg/litro en peces. Se ha demostrado que el riñón es un
órgano muy afectado en los peces.
Sobre la base de varias pruebas a largo plazo con especies de
algas y de peces, se ha establecido un nivel sin efectos observados
de 0,003 mg/litro; así pues, el compuesto se clasifica como muy
tóxico para las especies acuáticas. Los valores extremos
investigados comprenden parámetros de toxicidad general,
neurotoxicidad, bioquímicos, hematológicos, patológicos y
relacionados con la reproducción. En una prueba de 28 días de
duración en la que se examinaron las primeras fases de la vida de
carpas se observó que la reproducción no se veía afectada con
concentraciones de hasta 0,017 mg/litro, mientras que con
concentraciones de 0,013 y 0,017 mg/litro se observaron un aumento
de la mortalidad y una disminución del peso corporal. El nivel sin
efectos observados era de 0,0065 mg por litro.
Se ha descrito una sola prueba fiable con organismos
terrestres. En una prueba de 90 días con codornices japonesas
alimentadas con una dieta que contenía el compuesto en
concentraciones de 0,3 a 30 mg/kg de dieta se observó que la
supervivencia de los polluelos disminuía a partir de 10 mg/kg de
dieta.
7. Efectos en animales de experimentación y en sistemas de
prueba in vitro
7.1 Toxicidad general
Después de la ingestión de una dosis oral única, el
hexaclorobutadieno es de levemente a moderadamente tóxico para las
ratas adultas, moderadamente tóxico para las ratas macho destetadas
y muy tóxico para las ratas hembras destetadas. Los principales
órganos afectados son el riñón y, en grado mucho menor, el hígado.
Los datos obtenidos con animales indican que el vapor de
hexaclorobutadieno es irritante para las membranas mucosas y el
líquido es corrosivo. La sustancia debe considerarse como un agente
sensibilizador.
En los riñones de ratas, ratones y conejos, el
hexacloro-butadieno causa en los tubos proximales del riñón una
necrosis que depende de la dosis. Las ratas macho adultas son menos
vulnerables a la toxicidad renal que las hembras adultas y que los
machos jóvenes. Los ratones jóvenes son más vulnerables que los
adultos y no se observaron diferencias entre un sexo y otro. En las
ratas hembra adultas la dosis intraperitoneal única más baja con la
cual se observó necrosis renal fue de 25 mg/kg de peso corporal y en
ratones adultos, machos y hembras, fue de 6,3 mg/kg de peso
corporal. Se observaron cambios bioquímicos y alteraciones
funcionales marcados en los riñones con dosis iguales o mayores que
las asociadas con necrosis.
Asimismo, en seis pruebas orales de corto plazo, dos estudios
sobre reproducción y un estudio de largo plazo sobre la dieta
realizados con ratas, el riñón fue el principal órgano afectado. Los
efectos relacionados con la dosis comprenden una reducción del peso
relativo del riñón y una degeneración del epitelio de los tubos. El
nivel sin efectos nocivos observados de toxicidad renal en ratas en
un estudio de dos años fue de 0,2 mg/kg de peso corporal por día. En
un estudio de 13 semanas efectuado en ratones se obtuvo un nivel sin
efectos nocivos observados de 0,2 mg/kg de peso corporal por día.
Las hembras adultas de ambas especies eran más vulnerables que los
machos adultos.
En una prueba de inhalación de corto plazo (6 horas por día
durante 12 días) se observaron efectos semejantes en los riñones con
una concentración de vapor nominal de 267 mg/m3, con la cual
también se observaron trastornos respiratorios y degeneración de la
corteza suprarrenal.
7.2 Reproducción, embriotoxicidad y teratogenicidad
Dos estudios sobre dieta y reproducción en ratas con dosis de
hasta 20 y 75 mg/kg de peso corporal por día, respectivamente,
mostraron una reducción del peso al nacer y un aumento del peso
neonatal cuando se administraban a la madre dosis tóxicas de 20 y
7,5 mg/kg de peso corporal, respectivamente. La dosis altamente
tóxica de 75 mg/kg de peso corporal por día fue suficiente para
impedir la concepción y la implantación uterina. No se observaron
anormalidades del esqueleto.
En dos pruebas de teratogenicidad en las que se expuso a las
ratas o bien a vapor de hexaclorobutadieno en concentraciones que
oscilaban entre 21 y 160 mg/m3 durante 6 horas diarias (desde el
6° hasta el 20° día del embarazo) o bien a la administración
intraperitoneal de 10 mg/kg de peso corporal por día (desde el 1° al
15° día de embarazo) se observaron en el desarrollo del feto efectos
tóxicos tales como una reducción del peso al nacer, un retraso del
desarrollo del corazón y uréteres dilatados pero sin grandes
malformaciones. El retraso del desarrollo se observó en niveles que
también eran tóxicos para las madres.
7.3 Genotoxicidad y carcinogenicidad
En la prueba de Ames Salmonella se ha observado que el
hexaclorobutadieno induce mutaciones genéticas en condiciones
especiales que favorecen la formación de productos de conjugación
con el glutatión. En un estudio in vivo se observó que había
inducido aberraciones cromosómicas, pero no se observaron tales
aberraciones en dos estudios in vitro. En una prueba in vitro se
observó que la frecuencia de los intercambios entre cromátidas
hermanas había aumentado en las células ováricas de hámsters de
China. Se ha señalado el gran potencial mutagénico de los
metabolitos sulfurosos del hexaclorobutadieno. En estudios in
vitro, el compuesto indujo síntesis imprevistas de ADN en cultivos
de fibroblastos de embriones de hámsters de Siria, pero no en
cultivos de hepatocitos. Indujo síntesis imprevistas de ADN en ratas
in vivo, pero no indujo mutaciones letales recesivas ligadas al
sexo en Drosophila melanogaster.
En el único estudio de largo plazo (dos años), en el cual las
ratas recibieron una dieta que contenía hexaclorobutadieno en dosis
de 0,2, 2 ó 20 mg/kg de peso corporal por día, se observó una mayor
incidencia de neoplasias de los tubos renales únicamente con la
dosis más elevada.
7.4 Mecanismos de toxicidad
La nefrotoxicidad, mutagenicidad y carcinogenicidad del
hexaclorobutadieno depende de la biosíntesis del conjugado sulfuroso
tóxico 1-glutatión- S-yl-1,2,3,4,4-pentaclorobutadieno. Este
conjugado se sintetiza principalmente en el hígado y se metaboliza
luego en la bilis, el intestino y los riñones convirtiéndose en
1-cisteína- S-yl-1,2,3,4,4-pentaclorobutadieno (CPB). La activación
de CPB, que depende del conjugado de cisteína beta-lyasa, en una
tiocetena reactiva en las células de los tubos proximales finalmente
da lugar a un enlace covalente con macromoléculas celulares.
8. Efectos en el ser humano
No se han descrito efectos patogénicos en la población en
general.
Se conocen dos casos de trastornos padecidos por trabajadores
agrícolas que utilizaban el hexaclorobutadieno como fumigante, pero
esas personas también habían estado expuestas a otras sustancias. En
los linfocitos de la sangre periférica de operarios que trabajaban
en la producción de hexaclorobutadieno y estaban expuestos, según se
informa, a concentraciones de 1,6 a 12,2 mg/m3 se observó una
frecuencia mayor de aberraciones cromosómicas.
9. Evaluación de los riesgos para la salud humana y de los
efectos en el medio ambiente
9.1 Evaluación de los riesgos para la salud humana
Como se han hecho muy pocos estudios en el ser humano, la
evaluación se basa principalmente en estudios efectuados en animales
de laboratorio. Sin embargo, los limitados datos existentes sobre el
ser humano, obtenidos in vitro, sugieren que el metabolismo del
hexaclorobutadieno en el ser humano es semejante al observado en
animales.
Se considera que el vapor de hexaclorobutadieno irrita las
membranas mucosas del ser humano y que en estado líquido es
corrosivo. El compuesto también debe considerarse como un agente
sensibilizador.
Los principales órganos afectados por la toxicidad son los
riñones y, en mucho menor grado, el hígado. En base a estudios de
corto y largo plazo de ingestión por vía oral por ratas y ratones,
se determinó un nivel sin efectos adversos observados de 0,2 mg/kg
de peso corporal por día. En un estudio de inhalación en el corto
plazo en ratas (12 días, a razón de 6 horas por día), el nivel sin
efectos adversos observados fue de 53 mg/m3.
Se observaron una reducción del peso al nacer y un aumento de
peso neonatal únicamente con dosis tóxicas para la madre; lo mismo
puede decirse de los efectos tóxicos para el desarrollo.
Se ha observado que el hexaclorobutadieno induce mutaciones
genéticas, aberraciones cromosómicas, aumento de los intercambios
entre cromátidas hermanas y síntesis imprevistas de ADN, aunque
algunos estudios han dado resultados negativos. Con respecto a la
genotoxicidad del hexaclorubutadieno, las observaciones realizadas
en animales son limitadas y las efectuadas en el ser humano
insuficientes.
Tras la administración oral a largo plazo del
hexacloro-butadieno a ratas, se ha observado una mayor frecuencia de
neoplasias de los tubos renales, pero solamente con dosis elevadas
causantes de nefrotoxicidad notable. Hay indicios limitados de
carcinogenicidad en animales e indicios insuficientes en el ser
humano.
En base al nivel sin efectos adversos observados en ratones y
ratas, que es de 0,2 mg/kg de peso corporal por día, se ha estimado
un nivel sin efectos adversos observados en el ser humano, que es de
0,03 a 0,05 mg/kg de peso corporal por día. Hay un margen de
seguridad de 150 entre el nivel sin efectos adversos observados
estimado y la ingesta diaria total máxima estimada, suponiendo que
el compuesto se absorba a través del agua de bebida contaminada y de
alimentos con elevado contenido de lípidos.
9.2 Evaluación de los efectos en el medio ambiente
El hexaclorobutadieno es de moderadamente a muy tóxico para los
organismos acuáticos; los crustáceos y peces son los más
vulnerables. Se ha establecido un nivel de riesgo para el medio
ambiente de 0,1 µg/litro. Se estima que la concentración ambiental
prevista máxima lejos de las fuentes equivale al nivel de riesgo
ambiental extrapolado multiplicado por dos y, por consiguiente, los
organismos acuáticos tal vez estén en peligro en las aguas de
superficie contaminadas. No pueden excluirse efectos adversos en
organismos bentónicos.
En vista de la toxicidad del hexaclorobutadieno para los
mamíferos, el consumo de organismos bentónicos o acuáticos por otras
especies tal vez sea motivo de inquietud.
