
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 152
POLYBROMINATED BIPHENYLS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. W. Gross, Dr. J. Kielhorn
and Dr. C. Melber, Fraunhofer Institute for
Toxicology and Aerosol Research, Hanover, Germany
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Hexachlorobutadiene.
(Environmental health criteria: 152)
1. Polybromobiphenyl compounds - adverse effects
2. Polybromobiphenyl compounds - toxicity
3. Environmental exposure
4. Environmental pollutants I.Series
ISBN 92 4 157152 7 (NLM Classification QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland,
Which will be glad to provide the latest information on any changes
made to the text, plans for new editions, and reprints and
translations already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the impression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of every country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR POLYBROMINATED BIPHENYLS (PBBs)
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties,
analytical methods
1.1.2. Sources of human and environmental exposure
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism
1.1.6. Effects on organisms in the environment
1.1.7. Effects on experimental animals and
in vitro test systems
1.1.8. Effects on humans
1.1.9. Overall evaluation of toxicity and
carcinogenicity
1.2. Conclusions
1.3. Recommendations
1.3.1. General
1.3.2. Future research
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituents
2.1.2. Technical products
2.1.2.1 Major trade names
2.1.2.2 Composition of the technical products
2.2. Physical and chemical properties
2.2.1. Physical and chemical properties of individual
congeners
2.3. Conversion factors for PBB in air
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes
3.2.1.3 Loss into the environment during
normal production
3.2.1.4 Methods of transport, accidental
release, and disposal of production
wastes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Biota
4.1.4.1 Terrestrial ecosystems
4.1.4.2 Aquatic ecosystems
4.1.4.3 Accidental contamination of the
food chain
4.2. Degradation
4.2.1. Photolytic degradation
4.2.2. Microbial degradation
4.2.3. Degradation by plants and animals
4.2.4. Bioaccumulation
4.2.4.1 Aquatic organisms
4.2.4.2 Terrestrial organisms
4.3. Ultimate fate following use
4.3.1. Disposal of PBB-contaminated animals
and wastes from the Michigan disaster
4.3.2. Thermal decomposition of PBBs
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water and sediments
5.1.2.1 Surface waters
5.1.2.2 Sediments
5.1.2.3 Groundwater
5.1.3. Soil
5.1.4. Feed and food
5.1.4.1 Feed
5.1.4.2 Food
5.1.5. Other products
5.1.6. Terrestrial and aquatic organisms
5.1.6.1 Aquatic and terrestrial plants
5.1.6.2 Animals
5.2. General population exposure
5.2.1. Quantified data on human exposure
5.2.1.1 Worldwide
5.2.1.2 The Michigan accident
5.2.2. Human monitoring methods for PBBs
5.2.3. Human monitoring data
5.2.4. Subpopulations at special risk
5.3. Occupational exposure during manufacture, formulation, or
use
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Animal studies
6.1.1.1 Gastrointestinal absorption
6.1.1.2 Dermal and inhalation absorption
6.1.2. Human studies
6.2. Distribution
6.2.1. Animal studies
6.2.1.1 Levels in organs and blood
6.2.1.2 Transfer to offspring
6.2.2. Human studies
6.3. Metabolic transformation
6.3.1. In vitro studies
6.3.2. In vivo studies
6.3.3. Metabolic pathway
6.4. Elimination and excretion in expired air, faeces,
urine
6.4.1. Animal studies
6.4.2. Human studies
6.5. Retention and turnover
6.5.1. Animal studies
6.5.1.1 Time trends, retention:
2,2',4,4',5,5'-hexabromobiphenyl
(BB 153)
6.5.1.2 Biological half-lives
6.5.1.3 Differences between individual
congeners
6.5.1.4 Octabromobiphenyl
6.5.2. Human studies
6.6. Reaction with body components
6.6.1. Animal studies
6.6.2. Human studies
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.3. Terrestrial organisms
7.3.1. Wildlife
7.3.2. Farm animals
7.3.2.1 Cattle
7.3.2.2 Other farm animals
7.4. Population and ecosystem effects
7.5. Effects on the abiotic environment
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Lethality
8.2. Single and short-term exposures: general signs of
toxicity
8.2.1. PBB mixtures
8.2.1.1 Overt clinical signs, food intake,
and body weight changes
8.2.1.2 Haematology and clinical chemistry
8.2.1.3 Morphological and histopathological
changes
8.2.2. Individual PBB congeners and comparative
studies
8.2.2.1 Food intake, overt clinical signs,
body weight changes
8.2.2.2 Haematology and clinical chemistry
8.2.2.3 Morphological and histopathological
changes
8.3. Skin and eye irritation, sensitization, dermal
lesions, and acne
8.4. Long-term toxicity
8.4.1. Rat
8.4.1.1 Overt clinical signs, body weight
changes, food intake
8.4.1.2 Haematology and clinical chemistry
8.4.1.3 Morphological changes
8.4.1.4 Histopathological changes
8.4.2. Mouse
8.4.3. Cattle
8.4.4. Mink
8.4.5. Rhesus monkey
8.4.6. Pre- and perinatal exposure
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. PBB mixtures
8.5.1.1 Mammals
8.5.1.2 Avian species
8.5.2. Individual PBB congeners
8.6. Mutagenicity and related end-points
8.7. Carcinogenicity
8.7.1. Carcinogenicity in long-term toxicity studies
8.7.2. Mechanisms of carcinogenicity
8.7.2.1 Tumour initiation
8.7.2.2 Tumour promotion
8.7.2.3 PBBs acting as complete carcinogens
8.8. Biochemical toxicity
8.8.1. Induction of microsomal enzymes
8.8.1.1 Commercial PBB mixtures
8.8.1.2 Individual PBB congeners
8.8.2. Endocrine interactions
8.8.2.1 Thyroid hormones
8.8.2.2 Sex hormones
8.8.2.3 Prostaglandins
8.8.3. Interaction with drugs and toxicants
8.8.4. Effect on vitamin A storage
8.8.5. Porphyria
8.8.6. Miscellaneous effects
8.9. Effects on intercellular communication
8.10. Immunotoxicity
8.11. Neurotoxicity
8.11.1. Exposure of adult animals
8.11.2. Perinatal exposure
8.12. Factors modifying toxicity, toxicity of metabolites
8.12.1. Contaminants affecting toxicity
8.12.1.1 Polybrominated naphthalenes (PBNs)
8.12.1.2 Mixed polybromo-chlorobiphenyls
8.12.2. Toxicity of metabolites
8.12.3. Toxicity of photolysis and pyrolysis products
8.12.3.1 Photolysis products
8.12.3.2 Pyrolysis products
8.13. Mechanism of toxicity including carcinogenicity
9. EFFECTS ON HUMANS
9.1. General population exposure
9.1.1. Acute toxicity-poisoning incidents
9.1.2. Epidemiological studies
9.1.2.1 Studies conducted by the Michigan
Department of Public Health
(MDPH studies)
9.1.2.2 Studies conducted by the
Environmental Science Laboratory,
Mount Sinai School of Medicine,
New York (ESL studies)
9.1.3. Special studies
9.1.3.1 Examination of subjects with
complaints
9.1.3.2 Cutaneous effects
9.1.3.3 Effects on liver function
9.1.3.4 Porphyria
9.1.3.5 Effects on spermatogenesis
9.1.3.6 Paediatric aspects
9.1.3.7 Neurological and neuropsychiatric
aspects
9.1.3.8 Lymphocyte and immune function
9.1.3.9 Carcinogenic embryonic antigen
plasma levels
9.1.3.10 Biochemical effects
9.2. Occupational exposure
9.2.1. Epidemiological studies
9.2.2. Clinical studies
9.2.3. Special studies
9.2.3.1 Cutaneous effects
9.2.3.2 Memory performance
9.2.3.3 Thyroid effects
9.2.3.4 Reproductive effects
9.2.3.5 Lymphocyte function
9.2.3.6 Mortality
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX 1
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH
CRITERIA FOR POLYBROMINATED BIPHENYLS
Members
Dr L. Albert, Consultores Ambientales Asociados, S.C., Xalapa,
Veracruz, Mexico
Dr J. Alexander, Department of Toxicology, National Institute of
Public Health, Oslo, Norway
Dr W. Gross, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany
Dr R.F. Hertel, Federal Health Department CV 2.1, Berlin,
Germany (Co-Rapporteur)
Dr B. Jansson, Swedish Environmental Protection Agency,
Environmental Impact Assessment Department, Solna, Sweden
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany
Dr R.D. Kimbrough, Institute for Evaluating Health Risks (IEHR),
Washington, DC, USA (Chairman)
Dr C. Melber, Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany (Co-Rapporteur)
Dr K. Mitsumori, Division of Pathology, Biological Safety
Research Center, National Institute of Hygienic Sciences,
Tokyo, Japan
Dr S. Sleight, Department of Pathology, Michigan State
University, East Lansing, Michigan, USA
Professor P. Yao, Institute of Occupational Medicine, Chinese
Academy of Preventive Medicine, Beijing, People's Republic of
China (Vice-Chairman)
Observers
Dr B. Savanne, ELF ATOCHEM, Paris La Défense, France
Mr S. Tsuda, Environmental Health and Safety Division,
Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Secretariat
Dr H. Galal-Gorchev, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
(Secretary)
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone
No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR POLYBROMINATED BIPHENYLS
A WHO Task Group on Environmental Health Criteria for
Polybrominated biphenyls (PBBs) met at the Fraunhofer Institute for
Toxicology and Aerosol Research, Hanover, Germany, from 22 to 26
June 1992. Dr H. Galal-Gorchev, IPCS, welcomed the participants on
behalf of Dr M. Mercier, Director of the IPCS, and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft and made an evaluation of the risks for human
health and the environment from exposure to PBBs.
The first draft was prepared by Dr W. Gross, Dr J. Kielhorn
and Dr C. Melber of the Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Germany, who also prepared the second
draft, incorporating comments received following circulation of the
first drafts to the IPCS Contact Points for Environmental Health
Criteria monographs.
Dr H. Galal-Gorchev and Dr K.W. Jager of the IPCS Central Unit
were responsible for the scientific content of the monograph, and
Mrs M.O. Head of Oxford for the technical editing.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, physical and chemical properties, analytical
methods
The term polybrominated biphenyls or polybromobiphenyls (PBBs)
refers to a group of halogenated hydrocarbons, formed by
substituting hydrogen by bromine in biphenyl. PBBs are not known to
occur as natural products. They have a molecular formula of C12
H(10-x-y)Br(x+y) where both x and y = 1 to 5. Theoretically 209
congeners are possible. Only a few have been synthesized
individually and characterized. PBBs, manufactured for commercial
use, consist mainly of hexa-, octa-, nona-, and decabromobiphenyls,
but also contain other homologues. They are additive type flame
retardants, and when blended with the dry solid or liquid polymeric
material, provide filter-type, flame retardant action with the
chemical release of hydrogen bromide if ignited.
PBBs are manufactured using a Friedel-Crafts type reaction in
which biphenyl is reacted with bromine with, or without, an
organic solvent, using, e.g., aluminium chloride, aluminium
bromide, or iron as catalyst.
Most research has been carried out on FireMaster BP-6 and
FF-1, which were involved in the Michigan disaster when this
compound was inadvertently added to animal feed instead of
magnesium oxide. The ensuing contamination of farm animals resulted
in the destruction of thousands of cattle, pigs, and sheep, and
millions of chickens.
The composition of FireMaster(R) changes from batch to
batch, but its main constituents are
2,2',4,4',5,5'-hexabromobiphenyl (60-80%), and
2,2',3,4,4',5,5'-heptabromobiphenyl (12-25%) together with lower
brominated compounds because of incomplete bromination reaction.
Mixed bromochlorobiphenyls and polybrominated naphthalenes have
also been observed as minor components of FireMaster(R).
FireMaster FF-1 (white powder) is FireMaster BP-6 (brown flakes) to
which 2% calcium silicate has been added as an anti-caking agent.
PBBs are solids with a low volatility that decreases with
increasing bromine number. PBBs are virtually insoluble in water,
soluble in fat, and slightly to highly soluble in various organic
solvents; solubility also decreases with increasing bromine number.
These compounds are relatively stable and chemically unreactive,
though highly brominated PBB mixtures are photodegraded with
reductive debromination upon exposure to ultraviolet radiation
(UVR).
The products of the experimental thermal decomposition of PBBs
depend on the temperature, the amount of oxygen present, and a
number of other factors. Investigations into the pyrolysis of
FireMaster BP-6 in the absence of oxygen (600-900 °C) have shown
that bromobenzenes and lower brominated biphenyls are formed, but
no polybrominated furans. In contrast, pyrolysis in the presence of
oxygen (700-900 °C) yielded some di- to heptabromodibenzofurans. In
the presence of polystyrene and polyethylene, higher levels were
found. Pyrolysis of FireMaster BP-6 with PVC at 800 °C yielded
mixed bromochlorobiphenyls. There is no information on the nature
of the products of incineration of PBB-containing material. Little
is known about the toxicities of brominated and
brominated/chlorinated dioxins and furans, but they are estimated
to be of about the same order as those of chlorinated dioxins and
furans.
The primary analytical technique used for the biological
monitoring of PBBs in environmental samples and biological tissues
and fluids, after the Michigan disaster, was gas chromatography
with electron capture detection. Individual congeners can be
determined by capillary gas chromatography and more specific
detection can be obtained with selected ion monitoring mass
spectrometry. Because of the large numbers of congeners possible,
investigations are hampered by lack of suitable synthetic
standards. Methods for extracting PBBs from biological samples have
been based on those for pesticides. PBBs are extracted with the
fat, and then purified.
The recent finding of PBB congeners in background biological
samples does not necessarily mean that concentrations are
increasing in the environment. The development of more sensitive
analytical techniques, such as negative ion chemical ionization
mass spectrometry, may be the explanation. Thus, the need for
retrospective studies is urgent. With improved clean-up methods, it
is possible to carry out specific analyses of the toxic co-planar
PBB congeners and such data are also needed.
1.1.2 Sources of human and environmental exposure
The commercial production of FireMaster(R) was started in
the USA in 1970. After the Michigan disaster, production was
discontinued (November 1974). The estimated production of PBBs in
the USA between 1970 and 1976 was 6000 tonnes (commercial
quantities). Octabromobiphenyl and decabromobiphenyl were produced
in the USA until 1979. A mixture of highly brominated PBBs called
Bromkal 80-9 D was produced in Germany until mid- 1985. Technical
grade decabromobiphenyl (Adine 0102) is currently produced in
France. As far as is known, this is the only current production of
PBBs.
PBBs were introduced as flame retardants in the early 1970s.
Prior to November 1974, hexabromobiphenyl was the most commercially
significant PBB in the USA and was incorporated into
acrylonitrile-butadiene-styrene (ABS) plastics (PBB content 10%),
used mainly in small appliance and automotive applications,
coatings, lacquers, and polyurethane foam. The other PBB flame
retardants have similar uses.
Losses of PBBs into the environment during normal production
can occur through emission into the air, waste waters, losses into
the soil, and to landfills, and have been found to be generally
low.
These chemicals can also enter the environment during
shipping and handling, and accidentally, as occurred in Michigan.
There is also the possibility of their entrance into the
environment as a result of the incineration of materials containing
PBBs as well as during accidental fires with the formation of other
toxic chemicals, such as polybromodibenzofurans or mixed
bromochloro derivatives.
The major part of the total volume of these compounds produced
will ultimately enter into the environment, as such, or as
breakdown products.
1.1.3 Environmental transport, distribution, and transformation
Long-range transport of PBBs in the atmosphere has not been
proven, but the presence of these compounds in Arctic seal samples
indicates a wide geographical distribution.
The principal known routes of PBBs into the aquatic
environment are from industrial waste discharge and leachates from
industrial dumping sites into receiving waters and from erosion of
polluted soils. PBBs are almost insoluble in water and are
primarily found in sediments of polluted lakes and rivers.
Pollution of soils can originate from point sources, such as
PBB plant areas and waste dumps. Once introduced into the soil,
PBBs do not appear to be translocated readily. PBBs have been found
to be 200 times more soluble in a landfill leachate than in
distilled water; this may result in a wider distribution in the
environment. The hydrophobic properties of PBBs make them easily
adsorbed from aqueous solutions onto soils. Preferential adsorption
of PBB congeners was noted, depending on the characteristics of the
soil (e.g., organic content) and the degree and position of bromine
substitution.
PBBs are stable and persistent, lipophilic, and only slightly
soluble in water; some of the congeners are poorly metabolized and
therefore accumulate in lipid compartments of biota. Once they have
been released into the environment, they can reach the food chain,
where they are concentrated.
PBBs have been detected in fish from several regions.
Ingestion of fish is a source of PBB transfer to mammals and birds.
Degradation of PBBs by purely abiotic chemical reactions
(excluding photochemical reactions) is considered unlikely. The
persistence of PBBs under field conditions has been reported. Soil
samples from a former PBB manufacturing site, analysed several
years after the Michigan incident, still contained PBBs though the
PBB congener composition was different, indicating a partial
degradation of the PBB residues in the soil sample.
Under laboratory conditions, PBBs are easily degraded by UVR.
Photodegradation of the commercial FireMaster(R) mixture led to
diminished concentrations of the more highly substituted PBB
congeners. The rates and extent of photolytic reactions of PBBs in
the environment have not been determined in detail, though field
observations indicate a high persistence of the original PBBs, or
a partial degradation to the less brominated congeners.
In laboratory investigations, mixtures of PBBs appear to be
fairly resistant to microbial degradation.
Neither uptake nor degradation of PBBs by plants has been
recorded. In contrast, PBBs are easily absorbed by animals and
though they have been found to be very persistent in animals, small
amounts of PBB metabolites have been detected. The main metabolic
products were hydroxy-derivatives, and, in some cases, there was
evidence of partially debrominated PBBs. No investigation of
sulfur-containing metabolites analogous to those of PCBs have been
reported.
The bioaccumulation of PBBs in fish has been investigated.
Bioaccumulation of PBBs in terrestrial animals has been
investigated in avian and mammalian species. Data were obtained
through field observations, evaluation of the Michigan disaster and
through controlled feeding studies. Generally, the accumulation of
PBBs in body fat depended on the dosage and duration of exposure.
Bioaccumulation of individual PBB congeners has been found to
increase with degree of bromination up to at least tetrabromo
biphenyls. Higher brominated congeners can be expected to
accumulate to an even greater extent. However, no information is
available for decabromobiphenyl; it is possible that it is poorly
absorbed.
Brominated dibenzofurans or partially debrominated PBBs have
been reported as products of the thermal decomposition of PBBs.
Their formation depends on several variables (e.g., temperature,
oxygen).
1.1.4 Environmental levels and human exposure
Only one report is available on PBB levels in air. In this
study, concentrations in the vicinity of three PBB-manufacturing or
PBB-processing plants in the USA were measured.
Levels in surface waters in the same vicinity and in the
Gratiot County landfill (Michigan, USA), which received over a
hundred thousand kg of waste containing 60-70% PBBs between 1971
and 1973, were monitored.
Groundwater monitoring data from the Gratiot County landfill
showed trace levels of PBBs even outside the landfill area,
however, PBBs were not detected in drinking-water wells in the
area.
Data on soil pollution by PBBs are available for areas of
manufacture, use, or disposal of PBBs, and for soils from fields of
the PBB-contaminated Michigan farms.
In the Michigan disaster, FireMaster(R) was inadvertently
added to animal feed. It was almost a year later that the mixing
error was discovered and the analyses indicated that PBBs were
responsible. During this time (summer 1973 - May 1974),
contaminated animals and their produce entered the human food
supply and the environment of the state of Michigan. Hundreds of
farms were affected, thousands of animals had to be slaughtered and
buried, as well as thousands of tons of farm produce.
Most data available on the PBB-contamination of wildlife refer
to fish and birds in the USA and Europe, primarily waterfowl, in
the vicinity of industrial sites, and marine mammals.
Recent reports on the PBB-contamination of fish, terrestrial
and marine mammals, and birds in the USA and Europe indicate a wide
distribution of these compounds. The congener pattern found in fish
samples is quite different from that found in commercial products.
Many of the major peaks could well be the result of the
photochemical debromination of decabromobiphenyl (BB 209), but this
has not been confirmed.
Occupational exposure was found in employees in chemical
plants in the USA, and in farm workers, as a result of the Michigan
PBB incident. Median serum and adipose tissue PBB levels were
higher among chemical workers. Information from other
countries/companies on occupational exposure associated with
manufacturing, formulation, and commercial uses is not available.
For most human populations, direct data on exposure to PBBs
from various sources have not been documented. Widespread human
exposure resulting from direct contact with contaminated feed and,
primarily, from the consumption of PBBs in meat, eggs, and dairy
products has been reported from Michigan, USA. At least 2000
families (primarily farmers and their neighbours) received heavy
exposure. Recently, PBBs have been detected in cows' milk and human
milk in Germany.
The congener patterns in these samples are different from that
in fish. The relative concentration of BB 153 is higher in human
milk than in fish.
The routes of exposure of the general population to PBBs are
not well known. Present knowledge indicates that ambient air and
water do not contain high levels. Lipid-rich food, especially from
contaminated waters, is probably of great importance. There is no
information on levels of exposure in indoor air and dermal exposure
levels from materials containing PBB flame retardants.
The PBB congener pattern found in human milk, collected in
Germany, resembled that found in cows' milk from the same region,
but levels in the human samples were substantially higher.
An estimate of the daily intake of PBB via food by the general
population has to be based on very few data. If it is assumed that
fish contains 20 µg PBB/kg lipid and 5% lipid and that a 60-kg
person eats 100 g fish/day, the intake will be 0.002 µg/kg body
weight per day. A PBB concentration of 0.05 µg/kg lipid in milk
(4% lipid) and a milk consumption of 500 ml/day will give the same
person a PBB intake of about 0.00002 µg/kg body weight per day.
An infant of 6 kg body weight consuming 800 ml human milk
(3.5% lipid) per day will have an intake of 0.01 µg PBB/kg body
weight per day, if the milk contains 2 µg PBB/kg lipid.
1.1.5 Kinetics and metabolism
Gastrointestinal absorption of PBBs varies according to the
degree of bromination, the lower brominated compounds being more
easily absorbed.
There is inadequate information on the absorption of DeBB and
OcBB/NoBB.
PBBs are distributed throughout the animal species and human
beings, the highest equilibrium concentrations being in adipose
tissues. Relatively high levels have also been found in the liver,
particularly of the more toxic congeners, which appear to be
concentrated in the liver. The partitioning ratios of the various
PBB congeners appear to differ between several tissues. Generally,
there is a marked tendency for bioaccumulation. In mammals,
transfer of PBBs to offspring occurs through transplacental and
milk routes. Human milk was found to contain levels of
2,2',4,4',5,5'-hexabromobiphenyl that were more than 100 times the
maternal serum levels. During a multigeneration study on rats,
administration of PBBs to a single generation resulted in
detectable residues in more than two subsequent generations. Eggs
of avian species were also affected by maternal PBB body burden.
Many PBB congeners are persistent in biological systems. There
was no evidence for significant metabolism or excretion of the more
abundant components of the FireMaster(R) mixture or for octa- and
decabromobiphenyl. In vitro-metabolism studies showed that
structure-activity relationships exist for the metabolism of PBBs.
PBBs could be metabolized by PB (phenobarbital)-induced microsomes
only if they possessed adjacent non-brominated carbons, meta and
para to the biphenyl bridge on at least one ring. Metabolism by
MC (3-methylcholanthrene)-induced microsomes required adjacent
non-brominated ortho and meta positions on at least one ring of
lower substituted congeners and higher bromination appeared to
prevent metabolism. Hydroxylated derivatives as major in vitro-
and in vivo-metabolism products of lower brominated biphenyls
have been identified in vertebrates. The metabolic yield was
relatively low. The hydroxylation reaction probably proceeds via
both arene oxide intermediates and by direct hydroxylation.
Humans, rats, rhesus monkeys, pigs, cows, and chickens
eliminate PBBs mainly in the faeces. In most cases, excretion rates
seem to be slow. Concentrations of 2,2',4,4',5,5'-hexabromobiphenyl
observed in the bile and faeces of humans were about 1/2 to 7/10 of
the serum levels and approximately 0.5% of the adipose levels.
Treatment to enhance elimination of PBBs in animals or humans had
no, or little, success. Another pathway of elimination is excretion
through milk.
Complex and varied relationships were found in PBB tissue
concentrations with time after PBB administration to rats and other
animals. They are described by several compartmental models. A
half-life of approximately 69 weeks was calculated for the
elimination of 2,2',4,4',5,5'-hexabromobiphenyl from the body fat
of rats. A half-life of more than 4 years was found in rhesus
monkeys. Average half-lives in humans have been estimated to be
between 8 and 12 years for 2,2',4,4',5,5'-hexabromobiphenyl. Ranges
of 5-95 years have been suggested in the literature. There are some
differences in retention and turnover between individual PBB
congeners. Results of analyses of serum from farmers and chemical
workers for 2,3',4,4',5-pentabromobiphenyl were inconsistent. This
inconsistency was probably because of the different sources of
exposure. The workers were exposed to all compounds of
FireMaster(R), while the Michigan population was exposed to
contaminated meat and milk containing a different PBB mixture as a
result of metabolic processes in farm animals. Bromine levels did
not decrease in the adipose tissue of rats, when technical
octabromobiphenyl was given. No information is available on the
retention of decabromobiphenyl.
Humans may have a greater tendency to retain certain PBB
congeners than experimental animals. This factor should be taken
into consideration in evaluating the human health hazards from
these chemicals.
In conclusion, all available data indicate that PBBs have a
marked tendency to bioaccumulate and persist. Metabolism is poor
and half-lives in humans are of the order of 8-12 years or longer.
1.1.6 Effects on organisms in the environment
Only few data are available on the effects of PBBs on
organisms in the environment. They refer to microorganisms, water
fleas, waterbirds, and farm animals.
Waterbirds nesting on islands in northwestern Lake Michigan
were studied to see if environmental contaminants were producing
effects on reproduction. Seventeen contaminants, including PBBs,
were measured, but none seemed to have a pronounced effect on
reproduction.
Farm animals that ingested feed inadvertently containing
Firemaster(R) FF-1 instead of magnesium oxide became sick. The
estimated average exposure of cows on the first identified highly
contaminated farm was 250 mg/kg body weight. The clinical signs of
toxicity were a 50% reduction in feed consumption (anorexia) and a
40% decrease in milk production, a few weeks after ingestion of the
contaminated feed. Although the supplemented feed was discontinued
within 16 days, milk production was not restored. Some cows showed
an increased frequency of urination, and lacrimation, and developed
haematomas, abscesses, abnormal hoof growth, lameness, alopecia,
hyperkeratosis, and cachexia; several died within 6 months of
exposure. Altogether, the death rate on this farm was 24/400. The
death rate of 6- to 18-month- old calves was much higher. About 50%
died within 6 weeks, only 2 out of 12 surviving after 5 months.
They developed hyper keratosis over their entire bodies. There were
also a variety of reproductive problems.
Necropsy findings have been reported for some of the mature
cows that died in the 6 months following exposure.
Histopathological studies revealed variable liver and kidney
changes.
Several clinical signs and pathological changes noted above
were later confirmed in controlled feeding studies (anorexia,
dehydration, excessive lacrimation, emaciation, hyperkeratosis,
reproductive difficulties, some clinical chemistry changes, and
renal damage).
A drop in production and sterility were reported in herds with
low-level contamination. This contrasts with results of controlled
studies, which did not show any significant differences between
herds with low-level contamination and control herds.
Although it was cattle feed that was originally involved in
the accidental substitution, other animal feeds became involved by
cross contamination, e.g., in the mixing machinery of feed
companies. It is likely that the exposure was not as high as that
of cattle. Although other animals (poultry, swine, horses, rabbits,
goats, and sheep) were reported as being contaminated and were
killed, details of ill effects were not recorded.
No information is available on the effects of PBBs on the
ecosystem.
1.1.7 Effects on experimental animals and in vitro test systems
The LD50 values of commercial mixtures show a relatively low
order of acute toxicity (LD50 > 1 g/kg body weight) in rats,
rabbits, and quails, following oral or dermal administration.
Deaths and acute manifestations of toxicity were delayed after
administration of PBB. The total dose administered determined the
extent of toxicity, whether given as a single dose or as repeated
doses over short periods (up to 50 days). The toxicity of PBBs was
higher with multiple-dose rather than single-dose administration.
Deaths after exposure to PBBs are delayed.
The few studies performed with commercial octa- and deca
bromobiphenyl mixtures did not result in mortality in rats and
fish. Of the individual PBB congeners, only three hexa isomers have
been tested, 3,3',4,4',5,5'-HxBB; and 2,3',4,4',5,5'-HxBB being
more toxic for rats than 2,2',4,4',5,5'-HxBB. On the basis of
limited, available data, OcBB and DeBB appear to be less toxic than
the PBB mixtures and less well absorbed.
In many acute and short-term studies, signs of PBB (mostly
FireMaster) toxicity have included reductions in feed consump tion.
At lethal doses, the cause of death cannot be ascribed to pathology
in a particular organ but rather to a "wasting syndrome" that the
animals develop as a first indication of toxicity. At death, the
loss in body weight can be as great as 30-40%. The few studies with
technical OcBB and DeBB did not show any such effects.
Morphological and histopathological changes, caused by PBB
exposure, are most prominent in the liver. Enlargement of the liver
frequently occurred at doses lower than those required to produce
body weight changes. The principal histopathological alterations in
rodent species may consist of extensive swelling and vacuolation of
hepatocytes, proliferation of smooth endoplasmatic reticulum, and
single-cell necrosis. The severity of the lesions depends on the
dose and the composition of the PBB material given.
Decreases in thymus weights were observed in rats, mice, and
cattle after doses of FireMaster(R), but not OcBB or DeBB.
There are some reports of increase in thyroid weight and
histological changes in the thyroid of rats, which have been
observed at low concentrations.
It is evident that individual PBB congeners differ in their
pattern of toxicity. The more toxic isomers and congeners cause a
decrease in thymus and/or body weight and produce pronounced
histological changes in the liver and thymus. Categorization of
halogenated biphenyls has been made on a structural basis.
Category 1 comprises isomers and congeners lacking ortho-
substituents (coplanar PBBs). Mono-ortho-substituted derivatives
constitute the second category. Other PBBs (mainly those with two
or more ortho-bromines) have been organized into the third
category. Congeners of Category 1 tend to elicit the most severe
effects, while the congeners of the second and third categories
show decreasing toxicological changes. Within the category, the
degree of bromination may also influence toxicity.
In all combinations tested, 3,3',4,4',5,5'-HxBB was found to
be the most toxic PBB. This congener is present in low
concentrations as a constituent of FireMaster(R). Of the major
FireMaster(R) constituents, 2,3,3',4,4',5-HxBB appeared to be the
most toxic one followed by 2,3',4,4',5,5'-HxBB and
2,3',4,4',5-PeBB. The main component of the FireMaster(R)
mixture, 2,2',4,4',5,5'-HxBB was relatively non-toxic as was
2,2',3,4,4',5,5'-HpBB, the second most abundant constituent.
The toxicity of technical OcBB and DeBB mixtures in relation
to their contents of various PBB congeners (and other possible
contaminants) is not so well elucidated.
Common skin and eye irritation tests and sensitization tests
resulted in no, or only mild, reactions to the technical PBB
mixtures tested (OcBB and DeBB). However, hyperkeratosis and hair
loss were seen in cattle, and lesions resembling chloracne were
seen in Rhesus monkeys, following the ingestion of a
FireMaster(R) mixture. Hyperkeratosis of the inner surface of the
rabbit ear was produced by FireMaster, but not by its main
components (2,2',4,4',5,5'-HxBB and 2,2',3,4,4'5,5'-HpBB).
Fractionation of FireMaster(R) revealed that most activity was
associated with the more polar fractions containing minor
components. Treatment with sunlight-irradiated HxBB caused severe
hyperkeratosis in rabbit ears.
Low dose, long-term feeding of technical OcBB to rats did not
affect food consumption and body weight, but an increase in the
relative liver weights of exposed rats was found at 2.5 mg/kg body
weight for 7 months. Long-term feeding of FireMaster(R) to rats
at doses of 10 mg/kg body weight for 6 months did not affect food
consumption. Doses of 1 mg/kg body weight over a 6-month period
affected liver weight. The thymus weight was decreased in female
rats administered 0.3 mg/kg body weight. Histopathological changes
were also noted. Controlled, long-term feeding studies on cattle
exposed to low doses of FireMaster(R) did not reveal any adverse
effects as indicated by food intake, clinical signs,
clinicopathological changes, or performance. Minks, guinea-pigs,
and monkeys appeared to be more susceptible to PBB toxicity.
Long-term effects related to the retention of administered
PBBs following pre- or perinatal exposure to high doses of
FireMaster(R) have been recorded in rats.
The most common adverse effects on reproduction were fetal
wastage and decrease in viability of offspring. Some effects were
still noted in mink at concentrations of 1 mg/kg diet. Decreases in
the viability of the offspring were observed in Rhesus monkeys
following a 12.5 month exposure to FireMaster(R) (0.3 mg/diet).
The monkeys received a daily dose of 0.01 mg/kg body weight and a
total dose of 3.8 mg/kg body weight. Reproduction and
neurobehavioural studies on monkeys and rats with low-level
exposure could not be evaluated since insufficient information was
given in the published papers on the experimental design of the
studies. A weak teratogenic potential was seen in rodents at high
doses that may have caused some maternal toxicity.
PBBs interact with the endocrine system. Rats and pigs showed
dose-related decreases in serum thyroxine and triiodo-thyronine.
PBBs have also been reported to affect the levels of steroid
hormones in most cases. The extent depends on the species as well
as the dose and time administered.
PBBs produced porphyria in rats and male mice at doses as low
as 0.3 mg/kg body weight per day. The no-effect level was 0.1 mg/kg
body weight per day. There was a pronounced influence of PBBs on
vitamin A storage as well as effects on the intermediary
metabolism.
Atrophy of the thymus was a frequent observation following PBB
exposure, and other lymphoid tissues have been shown to be
affected. Further indicators of a suppressed immune function have
also been demonstrated for FireMaster(R). Data on OcBB, NoBB,
DeBB, or individual PBB congeners are lacking.
One of the most intensively studied effects of PBBs is their
induction of mixed function oxidase (MFO) enzymes. Consistently,
FireMaster(R) was found to be a mixed-type inducer of hepatic
microsomal enzymes in rats and all other animal species tested.
Induction was also found to a lesser extent in other tissues. The
ability to induce hepatic microsomal enzymes differed for
individual PBB congeners. Correlations between structure and
microsomal enzyme inducing activity have been demonstrated.
Several studies have revealed that PBBs are able to alter the
biological activity of a variety of drugs and toxic substances.
This may partly be because of the ability of PBBs to induce
microsomal enzymes involved in the activation or deactivation of
xenobiotics.
The FireMaster(R) mixture, and some of its major components,
were found to be capable of inhibiting intercellular communication
in vitro. This inhibition occurs at non-cytotoxic concentrations.
Both the cytotoxicity and metabolic cooperation-inhibiting
properties of PBB congeners seem to be related to their structure,
i.e., presence or lack of ortho-substitution.
In vitro and in vivo assays (microbial and mammalian cell
mutagenesis, mammalian cell chromosomal damage, mammalian cell
transformation, and DNA damage and repair) have failed to indicate
any mutagenicity or genotoxicity of individual PBB congeners or
commercial mixtures.
Long-term toxicity studies have shown the liver to be the
principal site of the carcinogenic effects of PBB. The incidences
of hepatocellular carcinoma were significantly increased in both
male and female mice and rats receiving oral doses of the
FireMaster(R) mixture. Carcinogenic effects in the liver have
been reported in mice receiving diets containing Bromkal 80-9D
(technical nonabromobiphenyl) at 100 mg/kg (5 mg/kg body weight per
day) or more for 18 months. The lowest dose of PBB that produced
tumours (mostly adenomas) in rodents was 0.5 mg/kg body weight per
day for 2 years. The rats receiving 0.15 mg/kg body weight per day
in addition to pre- and perinatal exposure did not suffer any
adverse effects. The carcinogenicity of technical octabromobiphenyl
and decabromobiphenyl has not been studied.
Neither Firemaster BP-6 nor 2,2',4,4',5,5'-hexabromobiphenyl
showed tumour-initiating (using TPA as promotor) or
tumour-promoting (using DMBA as initiator) activity in a mouse skin
bioassay. However, in other mouse skin models (using DMBA or MNNG
as initiators), FM FF-1, 3,3',4,4',5,5'-hexabromobiphenyl, but not
2,2',4,4',5,5'-hexabromobiphenyl, showed tumour promoting activity.
In a two-stage rat liver bioassay using phenobarbital as promotor,
3,3',4,4'-tetrabromobiphenyl showed a weak initiating activity. In
the two-stage rat liver model using diethylnitrosamine and partial
hepatectomy, FM, 3,3',4,4'-tetra bromobiphenyl, and
2,2',4,4',5,5'-hexabromobiphenyl, but not
3,3,',4,4',5,5'-hexabromobiphenyl, showed tumour promoting
activity.
The results of the studies on cell communication, the negative
results of studies on genotoxicity and mutagenicity, and the
results of tumour promotion assays indicate that the mixtures and
congeners studied cause cancer by epigenetic mechanisms. No
information is available on technical octa-, nona-, or decabromo
biphenyl.
The mechanisms of action underlying the many manifestations of
the toxicity of PBBs and related compounds are not known. However,
some of the effects, such as the wasting syndrome, thymus atrophy,
hepatotoxicity, skin disorders, and reproductive toxicity may be
related to interaction with the so-called Ah- or TCDD-receptor
causing alteration in the expression of a number of genes.
Different PBB congeners vary in their interaction with the
receptor, the coplanar congeners being more active.
Many of the effects of PBB are seen after long-term exposure.
The reason for this may be the pronounced accumulation of some PBB
congeners and the poor ability of the body to metabolize and
eliminate them. This results in a build-up of the chemical in the
body overcoming compensatory mechanisms leading to adverse effects.
Some polybrominated naphthalenes (PBNs), known contaminants of
the FireMaster(R) mixture, are potent toxic substances and
teratogens. Although PBNs are only present at low levels in the
FireMaster(R) mixture, it is possible that they may contribute to
its toxicity.
Studies on the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-HxBB showed that the photolysis products were more
toxic than the original PBB. The pyrolysis products of FM caused
MFO enzyme induction, body weight loss, and thymic atrophy. Liver
enlargement was observed with pyrolysis products of technical OcBB.
1.1.8 Effects on humans
There was no example of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures following
the poisoning incident in Michigan, USA, 1973. The main
epidemiological studies were conducted by the Michigan Department
of Public Health (MDPH) and the Environmental Science Laboratory,
Mount Sinai School of Medicine, New York (ESL).
It was estimated that the most highly exposed people consumed
5-15 g PBB over a 230-day period through milk. Some additional
exposure may have occurred through meat. The exposure levels among
some of the farmers and most of the general population in Michigan
were much lower, i.e., the total exposure was 9-10 mg. However,
some people in this group may have received a total exposure of
about 800-900 mg. (A total dose of 9 mg corresponds to 0.15 mg/kg
body weight, and 900 mg-15 mg/kg body weight for a 60-kg average
adult; the dose/kg body weight would be higher for children).
In 1974, the first MDPH study compared the health status of
people on quarantined farms with people on non-quarantined farms in
the same area. Although a variety of symptoms were reported by both
groups, there was no pattern of differences between the groups. No
unusual abnormalities of the heart, liver, spleen, nervous system,
urinanalysis, blood counts, or any other medical conditions
examined could be found. In a later comprehensive MDPH study
including groups with different levels of exposure, there was no
positive association between serum concentrations of PBB and
reported symptom or disease frequencies. The ESL studies involved
about 990 farm residents, 55 chemical workers, and a group of
Wisconsin dairy farmers who were used as a control. The incidence
of symptoms in Michigan farmers was greater than the incidence in
Wisconsin farmers. The greatest differences were in the broad
classification of neurologi cal and musculoskeletal symptoms.
Elevated serum concentrations of some liver enzymes and
carcinoembryonic antigen were more prevalent in Michigan farmers
than in Wisconsin farmers. Chemical workers had a higher prevalence
of chest and skin symptoms and a lower prevalence of
musculoskeletal symptoms than farmers.
Although results of ESL studies were at times interpreted
differently from results of comparable studies, there was one area
of consistent agreement. Neither sets of studies demonstrated a
positive dose-response relationship between PBB levels in serum or
adipose tissue and the prevalence of symptoms or abnormal clinical
measurements. Several clinical areas were investigated using more
intensive special studies. Examination of neurological aspects by
means of objective performance tests revealed in one study a
negative correlation of serum PBB levels with performance test
scores, particularly in males in older age groups. The other
studies showed no association between serum or fat concentrations
of PBBs and performance in a battery of tests measuring memory,
motor strength, coordination, cortical-sensory perception,
personality, higher cognitive functioning, and other functions.
Paediatric aspects of PBB exposure were examined in families
of the ESL studies. Although many symptoms were reported, physical
examination failed to reveal any objective alteration that could be
attributed to PBB. There were different views about the more subtle
neuropsychological effects in the offspring and the results of
investigations of developmental abilities remain controversial,
too. The same is true for the investigation of lymphocyte and
immune function. One set of authors found no differences in
lymphocyte count or functions between groups with high and low
serum PBB levels, the other found a significant decrease in T- and
B-lymphocyte subpopulations in about 40% of an exposed Michigan
group, compared with unexposed groups, and impaired lymphocyte
function, i.e., decreased response to mitogens.
In the epidemiological studies reviewed, efforts have been
made to evaluate the relationship between PBB exposure and a large
number of adverse effects including behavioural effects and
subjective complaints. However, most studies suffer from major
failures in design introducing confounders that make it difficult,
or impossible, to draw conclusions about the relationship between
PBB exposure and possible health effects. The follow-up time has
not been long enough to evaluate possible carcinogenic effects.
Two small groups of workers with occupational exposure to a
mixture of PBBs or to DeBB and DBBO were identified. Lesions
resembling chloracne were found in 13% of the workers exposed to
the PBB mixture, such lesions were not seen in the DeBB- exposed
workers. However, a significantly higher prevalence of
hypothyroidism was seen in the latter group.
1.1.9 Overall evaluation of toxicity and carcinogenicity
The only lifetime study with a PBB mixture was conducted on
rats and mice in a recent NTP bioassay. The lowest dose tested that
still produced carcinogenic effects was 0.5 mg/kg body weight per
day (liver tumours in rodents). In other carcinogenicity studies,
3 mg/kg body weight per day given for 6 months resulted in a
carcinogenic response. The 6-month study demonstrates that less
than lifetime exposure at similar doses will also result in similar
adverse effects. Effects on reproduction in subhuman primates and
mink may occur at lower doses.
In addition, in the 2-year NTP rat study, a daily dose of
0.15 mg/kg body weight per day and prenatal and perinatal exposure
of the dam to 0.05 mg/kg body weight per day did not result in any
adverse effects. Thus, the total daily intake from food, water,
air, and soil should be less than 0.15 µg/kg body weight per day,
extrapolating from a NOAEL (no-observed- adverse-effect level) of
a positive carcinogenicity study, using an uncertainty (safety)
factor of 1000, since these compounds probably produce cancer by an
epigenetic mechanism.
The total dose received by the subpopulation in Michigan was
estimated to have ranged from 0.15 to 15 mg/kg body weight over a
230-day period. For this population, dividing the doses over a
lifetime for the average human being would be equivalent to a daily
dose ranging from 6 ng to 0.6 µg/kg body weight per day.
A total intake of 2 ng PBB/kg body weight per day, from known
sources, has been estimated for adults in the general population
and 10 ng/kg body weight per day for infants receiving human milk.
It should be kept in mind that these estimates are based on a very
limited and regional data base.
These calculations assume that a steady state for PBBs would
not be reached over a lifetime and that short-term higher exposure
can be substituted for long-term lower exposures, since these
compounds are extremely poorly metabolized and excreted.
Insufficient information is available for OcBB, NoBB, and DeBB
to calculate a total daily intake that would not result in adverse
effects.
1.2 Conclusions
Most of the PBB congeners found in commercial flame retardants
are lipophilic, persistent, and bioaccumulating. These compounds
are biomagnified in environmental food webs and pose a threat,
especially to organisms in the higher levels of these webs.
Furthermore, some PBB products are precursors to toxic
polybrominated dibenzofurans in combustion processes.
In addition to emissions during manufacture and use, PBB will
enter the environment from the widespread use of flame retardant
products. A considerable part of the PBB produced will ultimately
reach the environment because of the high stability of these
compounds.
PBBs are also found in environmental and human samples from
places far from known point sources. The congener pattern in the
environmental samples does not match those found in the technical
products, which indicates an environmental alteration, possibly a
photochemical debromination.
Very little information is available at present on the extent
of the exposure of the general population to PBBs. However, in the
few instances where measurements were made, trace amounts of PBBs
were identified. At present, this exposure does not give rise to
concern, but further build-up should be avoided. Human data from
the Michigan episode suggest that exposures in Michigan were
several order of magnitude higher than the exposure of the general
population. No definitive health effects that could be correlated
with PBB exposure in the Michigan population have been identified,
though the follow-up period has not been long enough for the
development of cancer. Since PBB levels in adipose tissue and serum
remain high in the Michigan population, their internal exposure
continues. In contrast, toxicity was observed in cattle in
Michigan. This discrepancy is explained by differences in the
extent of the exposure of the cattle.
Occupational exposure has only been examined in two plants in
the USA. It appears that chloracne-like lesions may develop in
workers producing PBB, and hypothyroidism in workers exposed to
DeBB. No studies have been conducted on workers incorporating deca-
or octa-/nona-bromobiphenyl into commercial products.
PBBs are extremely persistent in living organisms and have
been shown to produce chronic toxicity and cancer in animals.
Although the acute toxicity was low, cancer was induced at a dose
of 0.5 mg/kg body weight per day and the no-observed-effect level
was 0.15 mg/kg body weight per day. A number of chronic toxic
effects have been observed in experimental animals at doses of
around 1 mg/kg body weight per day following long-term exposure.
1.3 Recommendations
1.3.1 General
The Task Group is of the opinion that human beings and the
environment should not be exposed to PBBs in view of their high
persistence and bioaccumulation and potential adverse effects at
very low levels after long-term exposure. Therefore, PBBs should no
longer be used commercially.
Because of the limited toxicity data on DeBB and OcBB, their
extreme persistence and their potential break-down in the
environment, and the more toxic persistent compounds formed through
combustion, they should not be used commercially, unless their
safety has been demonstrated.
It is known that observations on the Michigan cohort are still
continuing. Publication of these data is required.
1.3.2 Future research
Future human and environmental PBB monitoring, including
workplace monitoring in the manufacture and user industries, should
be expanded, should be congener specific, and should include
OcBB/NoBB and DeBB. These compounds should be included in
monitoring programmes in progress for other halogenated compounds.
The time trends and geographical distribution of PBB levels in the
environment should continue to be monitored. Release of PBBs into
the environment from waste disposal sites should be surveyed.
Thermolysis experiments simulating conditions of accidental
fires and municipal incineration should be conducted. Additional
research should be continued on the mechanisms of toxicity and
carcinogenicity of PBBs and related compounds. PBBs may serve as
model compounds for such mechanistic research. Purified congeners
should be used in these studies.
The effects of PBBs on reproduction are not well elucidated.
Therefore, well-designed, long-term, reproductive studies at low
doses, using a sensitive species, should be performed.
There is also a need for more information on the
bioavailability and toxicokinetics of OcBB/NoBB, DeBB, and selected
congeners.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituents
The term "polybrominated biphenyls" or "polybromobiphe nyls"
(PBBs) refers to a group of halogenated hydrocarbons, formed by
substituting hydrogen by bromine in biphenyl (Fig. 1).
Molecular formula C12H(10-x-y)Brx+y
(x and y = 1 to 5)
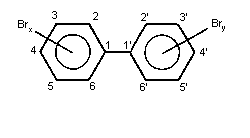 Molecular (empirical) formulae for PBB components of different
degrees of substitution and their relative molecular masses are
given in Table 1.
Theoretically, there can be 209 different forms (congeners) of
a brominated biphenyl, depending on the number and position of the
bromine (see Table 2).
At present, 101 individual PBB congeners are listed in the
Chemical Abstracts Service (CAS) registry. Because bromobiphe nyls
are produced commercially by the bromination of biphenyl, the
existence of any of the 209 congeners is possible in any commercial
mixture (Aust et al., 1983). Some PBBs exist primarily as
metabolites or accumulation or degradation products of the original
mixture. With increasing advance in analysis techniques, the number
of actually identified PBB compounds is growing.
Table 1. PBBs: molecular formula and relative molecular mass
PBB Formula Relative
molecular mass
Monobromobiphenyl C12H9Br 232.9
Dibromobiphenyl C12H8Br2 311.8
Tribromobiphenyl C12H7Br3 390.7
Tetrabromobiphenyl C12H6Br4 469.6
Pentabromobiphenyl C12H5Br5 548.5
Hexabromobiphenyl C12H4Br6 627.4
Heptabromobiphenyl C12H3Br7 706.3
Octabromobiphenyl C12H2Br8 785.2
Nonabromobiphenyl C12HBr9 864.1
Decabromobiphenyl C12Br10 943.0
Table 2. Multiplicity of PBB isomers and congenersa
Number of
Br Substituent 1 2 3 4 5 6 7 8 9 10
Number of
Isomers 3 12 24 42 46 42 24 12 3 1
a Modified from: Safe (1984).
The synthesis of pure congeners for use as standards is a
prerequisite for advances in chemical analysis, as well as research
into the toxicological and biological effects of PBBs. Some routes
for the synthesis of PBB congeners have been described by Sundström
et al. (1976b), Robertson et al. (1980, 1982a, 1984a), Höfler et al.
(1988), and Kubiczak et al. (1989).
Table 3 gives a list of all 209 possible congeners and their
CAS numbers, if already designated. The CAS names are designated as
follows:
1,1'-Biphenyl, .......... bromo-
e.g., 1,1'-Biphenyl, 2,2',4,4',5,5'-hexabromo- or
2,2',4,4',5,5'-hexabromo-1,1'-biphenyl (BB-153).
2.1.2 Technical products
2.1.2.1 Major trade names
The PBBs produced for commercial use include mixtures mainly
containing hexa-, octa-/nona-, and decabromobiphenyls. Data on past
and present trade names and manufacturers are summarized in Table 4
(for further details see section 3.2.1).
2.1.2.2 Composition of the technical products
Commercial PBB products are mixtures of various brominated
biphenyls. Several structural isomers of each of these brominated
compounds are possible and may be present in the product. All
mixtures are relatively highly brominated, with bromine contents
ranging from about 76% for hexabromobiphenyls to 81-85% for octa- to
decabromobiphenyl mixtures (Brinkman & de Kok, 1980).
Data on the composition of PBB mixtures are given in Table 5.
As shown in Table 5, the analytical results concerning the various
products are rather divergent. It indicates that the exact
composition of the mixtures varies between batches, and also within
each batch according to the sampling and analytical method. It can
be seen that samples of "octabromobiphenyl" often contained a larger
proportion of nona- than of octa-substituted PBBs. In this
monograph, these compounds are also referred to as "octa/nona"
bromobiphenyls.
Information on the isomeric composition of the octa- to deca-
mixtures is scarce. In an analysis of Bromkal 80, three isomers of
octabromobiphenyl were found to be present at 14, 16, and 42%
(Norström et al., 1976). A comparison of the isomeric composition of
an "octabromobiphenyl"-mixture with the FireMaster(R)- mixture has
been given by Moore & Aust (1978). De Kok et al. (1977) analysed
various "octabromobiphenyl"-mixtures and Bromkal 80-9D and discussed
the structures of isomers. Furthermore, two isomeric octa- and
three hexa-bromobiphenyls of a commercial decabromobiphenyl mixture
(RFR) have been reported (de Kok et al., 1977).
Table 3. Systematic numbering of PBB compounds and their CAS numbers
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
Monobromobiphenyls (26264-10-8) 17 2,2',4
18 2,2',5 59080-34-1
1 2 2052-07-7 19 2,2',6
2 3 2113-57-7 20 2,3,3'
3 4 92-66-0 21 2,3,4
22 2,3,4'
Dibromobiphenyls (27479-65-8) 23 2,3,5
24 2,3,6
4 2,2' 13029-09-9 25 2,3',4
5 2,3 115245-06-2 26 2,3',5 59080-35-2
6 2,3' 49602-90-6 27 2,3',6
7 2,4 53592-10-2 28 2,4,4' 6430-90-6
8 2,4 49602-91-7 29 2,4,5 115245-07-3
9 2,5 57422-77-2 30 2,4,6 59080-33-0
10 2,6 59080-32-9 31 2,4',5 59080-36-3
11 3,3' 16400-51-4 32 2,4',6 64258-03-3
12 3,4 60108-72-7 33 2',3,4
13 3,4' 57186-90-0 34 2',3,5
14 3,5 16372-96-6 35 3,3',4
15 4,4' 92-86-4 36 3,3',5
37 3,4,4' 6683-35-8
Tribromobiphenyls (51202-79-0) 38 3,4,5 115245-08-4
39 3,4',5 72416-87-6
16 2,2',3
Tetrabromobiphenyls 40088-45-7 65 2,3,5,6
66 2,3',4,4' 84303-45-7
40 2,2',3,3' 67 2,3',4,5
41 2,2',3,4 68 2,3',4,5'
43 2,2',3,5 69 2,3',4,6
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
44 2,2',3,5' 70 2,3',4',5 59080-38-5
45 2,2',3,6 71 2,3',4',6
46 2,2',3,6' 72 2,3',5,5'
47 2,2',4,4' 66115-57-9 73 2,3',5',6
48 2,2',4,5 74 2,4,4',5
49 2,2',4,5' 60044-24-8 75 2,4,4',6 64258-02-2
50 2,2',4,6 76 2',3,4,5
51 2,2',4,6' 97038-95-4 77 3,3',4,4' 77102-82-0
52 2,2',5,5' 59080-37-4 78 3,3',4,5
53 2,2',5,6' 60044-25-9 79 3,3',4,5' 97038-98-7
54 2,2',6,6' 97038-96-5 80 3,3',5,5' 16400-50-3
55 2,3,3',4 97038-99-8 81 3,4,4',5 59589-92-3
56 2,3,3',4'
57 2,3,3',5 Pentabromobiphenyls (56307-79-0)
58 2,3,3',5'
59 2,3,3',6 82 2,2',3,3',4
60 2,3,4,4' 83 2,2',3,3',5
61 2,3,4,5 115245-09-5 84 2,2',3,3',6
62 2,3,4,6 115245-10-8 85 2,2',3,4,4'
63 2,3,4',5 86 2,2',3,4,5
64 2,3,4',6 87 2,2',3,4,5'
88 2,2',3,4,6 77910-04-4 111 2,3,3',5,5'
89 2,2',3,4,6' 112 2,3,3',5,6
90 2,2',3,4',5 113 2,3,3',5',6
91 2,2',3,4',6 114 2,3,4,4',5 96551-70-1
92 2,2',3,5,5' 115 2,3,4,4',6
93 2,2',3,5,6 116 2,3,4,5,6 38421-62-4
94 2,2',3,5,6' 117 2,3,4',5,6
95 2,2',3,5',6 88700-05-4 118 2,3',4,4',5 67888-97-5
96 2,2',3,6,6' 119 2,3',4,4',6 86029-64-3
97 2,2',3',4,5 120 2,3',4,5,5' 80407-70-1
98 2,2',3',4,6 121 2,3',4,5',6
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
99 2,2',4,4',5 81397-99-1 122 2',3,3',4,5
100 2,2',4,4',6 97038-97-6 123 2',3,4,4',5 74114-77-5
101 2,2',4,5,5' 67888-96-4 124 2',3,4,5,5'
102 2,2',4,5,6' 80274-92-6 125 2',3,4,5,6'
103 2,2',4,5',6 59080-39-6 126 3,3',4,4',5 84303-46-8
104 2,2',4,6,6' 97063-75-7 127 3,3',4,5,5' 81902-33-2
105 2,3,3',4,4'
106 2,3,3',4,5 Hexabromobiphenyls (36355-01-8)
107 2,3,3',4',5
108 2,3,3',4,5' 128 2,2',3,3',4,4' 82865-89-2
109 2,3,3',4,6 129 2,2',3,3',4,5
110 2,3,3',4',6 130 2,2',3,3',4,5' 82865-90-5
131 2,2',3,3',4,6 155 2,2',4,4',6,6' 59261-08-4
132 2,2',3,3',4,6' 119264-50-5 156 2,3,3',4,4',5 77607-09-1
133 2,2',3,3',5,5' 55066-76-7 157 2,3,3',4,4',5' 84303-47-9
134 2,2',3,3',5,6 158 2,3,3',4,4',6
135 2,2',3,3',5,6' 119264-51-6 159 2,3,3',4,5,5' 120991-48-2
136 2,2',3,3',6,6' 160 2,3,3',4,5,6
137 2,2',3,4,4',5 81381-52-4 161 2,3,3',4,5',6
138 2,2',3,4,4',5' 67888-98-6 162 2,3,3',4',5,5'
139 2,2'3,4,4',6 163 2,3,3',4',5,6
140 2,2',3,4,4',6 164 2,3,3',4',5',6 82865-91-6
141 2,2',3,4,5,5' 120991-47-1 165 2,3,3',5,5',6
142 2,2',3,4,5,6 166 2,3,4,4',5,6
143 2,2',3,4,5,6' 167 2,3',4,4',5,5' 67888-99-7
144 2,2',3,4,5',6 119264-52-7 168 2,3',4,4',5',6 84303-48-0
145 2,2',3,4,6,6' 169 3,3',4,4',5,5' 60044-26-0
146 2,2',3,4',5,5'
147 2,2',3,4',5,6 Heptabromobiphenyl (35194-78-6)
148 2,2',3,4',5,6'
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
149 2,2',3,4',5',6 69278-59-7 170 2,2',3,3',4,4',5 69278-60-0
150 2,2',3,4',6,6' 93261-83-7 171 2,2',3,3',4,4',6
151 2,2',3,5,5',6 119264-53-8 172 2,2',3,3',4,5,5' 82865-92-7
152 2,2',3,5,6,6' 173 2,2',3,3',4,5,6
153 2,2',4,4',5,5' 59080-40-9 174 2,2',3,3',4,5,6' 88700-04-3
154 2,2',4,4',5,6' 36402-15-0 175 2,2',3,3',4,5',6
176 2,2',3,3',4,6,6' 195 2,2',3,3',4,4',5,6
177 2,2',3,3',4,5,6' 196 2,2',3,3',4,4',5',6
178 2,2',3,3',5,5',6 119264-54-9 197 2,2',3,3',4,4',6,6' 119264-59-4
179 2,2',3,3',5,6,6' 198 2,2',3,3',4,5,5',6
180 2,2',3,4,4',5,5' 67733-52-2 199 2,2',3,3',4,5,6,6'
181 2,2',3,4,4',5,6 200 2,2',3,3'4,5',6,6' 119264-60-7
182 2,2',3,4,4',5,6' 119264-55-0 201 2,2',3,3',4',5,5',6 69887-11-2
183 2,2',3,4,4',5',6 202 2,2',3,3',5,5',6,6' 59080-41-0
184 2,2',3,4,4',6,6' 119264-56-1 203 2,2',3,4,4',5,5',6
185 2,2',3,4,5,5',6 204 2,2',3,4,4',5,6,6' 119264-61-8
186 2,2',3,4,5,6,6' 119264-57-2 205 2,3,3',4,4',5,5',6
187 2,2',3,4',5,5',6 84303-49-1
188 2,3',3,4',5,6,6' 119264-58-3 Nonabromobiphenyls (27753-52-2)
189 2,3,3',4,4',5,5' 88700-06-5
190 2,3,3',4,4',5,6 79682-25-0 206 2,2',3,3',4,4',5,5',6 69278-62-2
191 2,3,3',4,4',5',6 207 2,2',3,3',4,4',5,6,6' 119264-62-9
192 2,3,3',4,5,5',6 208 2,2',3,3',4,5,5',6,6' 119264-63-0
193 2,3,3',4',5,5',6
Decabromobiphenyl
Octabromobiphenyls (27858-07-7)
209 2,2',3,3',4,4',5,5',6,6' 13654-09-6
194 2,2',3,3',4,4',5,5' 67889-00-3
a The Nos 1-209 correspond to those used by Ballschmiter & Zell (1980) for PCBs (January 1990).
Table 4. Major trade names and manufacturers of technical-grade PBBs and
commercial PBB mixturesa
PBB mixture Manufacturer CAS No.
Hexa-PBBs
FireMaster(R) BP-6 Michigan Chemical Corp. (St. Louis, Mich.) 59536-65-1
FireMaster(R) FF-1b Michigan Chemical Corp. (St. Louis, Mich.) 67774-32-7
Octa/nona-PBBs
Bromkal 80-9D Chemische Fabrik Kalk (Cologne, Germany) 61288-13-9
Technical
octabromobiphenyl White Chemical Corp. (Bayonne, New Jersey)
Octabromobiphenyl
FR 250 13A Dow Chemical Co. (Midland, Mich.)
Deca-PBB
Adine 0102 Ugine Kuhlmann now Atochem (Paris, France) 13654-09-6
Berkflam B 10 Berk (London, United Kingdom)
Flammex B-10 Berk (London, United Kingdom)
Technical
decabromobiphenyl White Chemical Corp. (Bayonne, New Jersey)
HFO 101 Hexcel (Basildon, United Kingdom)
a Adapted from: Brinkman & de Kok (1980).
b A pulverized form of FireMaster BP-6 containing 2% calcium polysilicate
to prevent caking. It was produced in limited quantities as a
development-product in 1971 and 1972.
Most research has been conducted with the hexabromobiphenyl
mixture FireMaster(R), which accounts for most of the manu
factured products and most of the environmental contamination
(Di Carlo et al., 1978). The main constituent of FireMaster(R) is
2,2',4,4',5,5'-hexabromobiphenyl. Its identification was reported by
Andersson et al. (1975), Jacobs et al. (1976), and Sundström et al.
(1976a). The second major component is heptabromobiphenyl containing
bromine at positions 2,2',3,4,4',5,5' (Hass et al., 1978; Moore
et al., 1978c). Accordingly, these two congeners account for about
75% of the mixture (e.g., Dannan et al., 1982d). Data on the
isomeric composition of FireMaster(R) found in the literature are
given in Table 6. The ranges of relative abundances of some
FireMaster(R) constituents are compiled in Table 7. Altogether at
least sixty compounds have been detected in FireMaster(R) (Orti
et al., 1983). About twelve of them are major PBB-components (Aust
et al., 1981), the others belong to the minor components (< 1%).
Table 5. Survey of literature on the composition of PBB mixturesa
PBB mixture (manufacturer) Weight of Weight of different homologus groups Reference
bromine (%)
Br10 Br9 Br8 Br7 Br6 Br5 Br4
"Hexabromobiphenyl"
FM BP-6 (Michigan Chemical) 75 13.8 62.8 10.6 2 de Kok et al.
(1977)c
" [Lot RP-158 (1971)] 12.5 72.5 9 4 Willett & Irving
(1976)
" [Lot 6244A (1974)] 13 77.5 5 4.5 Willett & Irving
(1976)
" 90 10 Norström et al.
(1976)
" 1 18 73 8 de Kok et al.
(1977)
" 33 63 4 Hass et al.
(1978)
" 7.7 74.5 5.6 Robertson et al.
(1984b)
" 24.5 79 6 Krüger (1988)
2,2',4,4',6,6' (RFR) 12 84 1 de Kok et al.
(1977)
2,2',4,4',6,6' (Aldrich) 2 24 70 4 de Kok et al.
(1977)
"Hexabromobiphenyl" (RFR) 25 67 4
(12-25) (60-80) (1-11) (2-5)b de Kok et al.
(1977)
Table 5 (contd).
PBB mixture (manufacturer) Weight of Weight of different homologus groups Reference
bromine (%)
Br10 Br9 Br8 Br7 Br6 Br5 Br4
Octanonabromobiphenyl
Bromkal 80-9D (Kalk) 81-82.5 9 65 1 de Kok et al.
(1977)
Bromkal 80 72 27 1 Norström et al.
(1976)
XN-1902 (Dow Chemical)c 82 6 47 45 2 Norris et al. (1973)
XN-1902 (Dow Chemical)c 2 34 57 7 de Kok et al. (1977)
Lot 102-7-72 (Dow Chemical)c 6 60 33 1 Waritz et al. (1977)
"Octabromobiphenyl" (RFR) 4 54 38 2 de Kok et al. (1977)
2,2',3,3',5,5',6,6' (RFR) 1 28 46 23 2 de Kok et al. (1977)
FR 250 13A (Dow Chemical) 8 49 31 1 Krüger (1988)
Decabromobiphenyl
HFO 101 (Hexcel) 84 96 2 de Kok et al. (1977)
Adine 0102 (Ugine Kuhlmann) 83-85 96 4 de Kok et al. (1977)
Adine 0102 (Ugine Kuhlmann) 96.8 2.9 0.3 Millischer et al.
(1979)
"Decabromobiphenyl" (RFR) 71 11 7 4 4 de Kok et al. (1977)
"DBB": Flammex B 10 (Berk)c 96.8 2.9 0.3 Di Carlo et al
(1978)
a Adapted from: Brinkman & de Kok (1980).
b Range of above readings with the exception of that of Norström et al. (1976), which differs greatly from the others.
c According to de Kok et al. (1977), these have never been marked.
Table 6. Identified PBB congeners in FireMaster(R)
BB No.a Structure % Composition of References
FM BP-6 FF-1
Dibromobiphenyls
4 2,2'- 0.02 Moore et al. (1979a)
Tribromobiphenyls
18 2,2'5- 0.050 Robertson et al. (1984b)
26 2,2',5- 0.024
31 2,4',5- 0.015
37 3,4,4'- 0.021
Tetrabromobiphenyls
49 2,2',4,5'- 0.025
52 2,2',5,5'- 0.052
66 2,3',4,4'- 0.028
70 2,3',4',5- 0.017
77b 3,3',4,4'- < 0.08 Orti et al. (1983)
0.159 Robertson et al. (1984b)
Pentabromobiphenyls
95 2,2',3,5',6- 0.02 Orti et al. (1983)
99 2,2'4,4',5- < 0.08
101 2,2',4,5,5'- 2.69 Robertson et al. (1984b)
4.5 3.7 Aust et al. (1981)
1.54 Orti et al. (1983)
2.6 Krüger (1988)
118 2,3',4,4',5- 2.94 Robertson et al. (1984b)
0.7 Aust et al. (1981)
3.2 Krüger (1988)
0.8 Orti et al. (1983)
126b 3,3',4,4',5- < 0.01
0.079 Robertson et al. (1984b)
Hexabromobiphenyls
132 2,2'.3.3',4,6'- 1 Krüger (1988)
138 2,2',3,4,4',5'- 12.3 Robertson et al. (1984b)
Table 6. cont'd
BB No.a Structure % Composition of References
FM BP-6 FF-1
12 8.6 Aust et al. (1981)
5.23 Orti et al. (1983)
10.6 Krüger (1988)
149 2,2',3,4',5',6- 2.24 Robertson et al. (1984b)
1.4 1.3 Aust et al. (1981)
0.78 Orti et al. (1983)
153 2,2'4,4',5,5'- 53.9 Robertson et al. (1984b)
47.8 47.1 Aust et al. (1981)
55.2 Orti et al. (1983)
58.5 Krüger (1988)
155 2,2',4,4',6,6'- 0.5
156 2,3,3',4,4',5- 0.980 Robertson et al. (1984b)
5.0 Aust et al. (1981)
0.37 Orti et al. (1983)
1.0 Krüger (1988)
157 2,3,3',4,4',5'- 0.05 Orti et al. (1983)
0.526 Robertson et al. (1984b)
0.5 Krüger (1988)
167 2,3',4,4',5,5'- 5.5 3.3 Aust et al. (1981)
3.37 Orti et al. (1983)
< 0.3
7.95 Robertson et al. (1984b)
5.5 Krüger (1988)
169b 3,3',4,4',5,5'- 0.294 Robertson et al. (1984b)
Heptabromobiphenyls
170 2,2',3,3',4,4',5- 0.256
1.1 1.5 Aust et al. (1981)
1.66 Orti et al. (1983)
2.4 Krüger (1988)
180 2,2',3,4,4',5,5'- 6.97 Robertson et al. (1984b)
24.7 Aust et al. (1981)
23.5 Orti et al. (1983) 20.8 Krüger (1988)
172 2,2',3,3',4,5,5'- < 0.30 Orti et al. (1983)
174 2,2',3,3',4,5,6'- 0.24
178 2,2',3,3',5,5',6- 0.3 Krüger (1988)
187 2,2',3,4',5,5',6- 0.392 Robertson et al. (1984b)
1.0 Krüger (1988)
189 2,3,3',4,4',5,5'- 0.51 Orti et al. (1983)
Table 6. cont'd
BB No.a Structure % Composition of References
FM BP-6 FF-1
Octabromobiphenyls
194 2,2',3,3',4,4', 0.9 2.4 Aust et al. (1981)
5,5'-
1.65 Orti et al. (1983)
possible structures for two
minor Br8 peaks:
196 2,2',3,3',4,4', Moore et al. (1980);
5,6'-
201 2,2',3,3',4,5, Orti et al. (1983)
5',6'-
203 2,2',3,4,4',5,
5'6-
a From: Ballschmiter & Zell (1980).
b These coplanar congeners are the most toxic congeners identified in
FireMaster BP-6 (Robertson et al., 1984b).
Table 7. Range of relative abundance of some PBB constituents
of Firemaster(R) FF-1 and BP-6a
Structure No.b BB No.c Abundance (%)
2,2',4',5,5'- 1 101 1.5-4.5
2,3',4,4',5,- 2 118 0.7-4.2
2,2',3,4',5',6- 3 149 0.8-2.2
2,2',4,4',5,5'- 4 153 47.1-59
2,2',3,4,4',5'- 5 138 5.2-12.3
2,3',4,4',5,5'- 6 167 3.3-8.0
2,3,3',4,4',5- 7 168 0.4-5.0
2,2',3,4,4',5,5'- 8 180 7.0-24.7
2,2',3,3',4,4',5- 9 170 0.3-2.4
2,2',3,3',4,4',5,5'- 12 194 0.9-2.4
a For references, see Table 6.
b Congener designation made on the basis of the gas chromatographic
elution sequence of the FireMaster(R) mixture.
c Congener designation according to Ballschmiter & Zall (1980).
Variations are due to differences in batches and analytical
techniques. In many cases, the differing electron capture responses
of the various congeners within the mixture were not taken into
account. Thus, values in Table 7 only give an approximate range of
composition and it is not possible to provide a precise composition
for the material that was introduced into the Michigan environment
(Fries, 1985b).
Both formulations of FireMaster(R) mixture, BP-6 and FF-1
have a similar isomeric composition. However, FireMaster BP-6
contains roughly 10% more of the relatively minor congeners (Dannan
et al., 1982b).
As can be concluded from the composition of the commercial
mixtures (Table 5), the major source of impurity that occurs in PBBs
results from the spread in the degree of bromination. For example,
FireMaster(R) BP-6 has been marketed as a hexabromin ated
biphenyl, but more than one quarter of the product consists of lower
brominated biphenyls because of incomplete bromination reaction
(Neufeld et al., 1977).
However, a producer of decabromobiphenyl has reported that
their material has a degree of purity of more than 98%, the
remaining 2% being nonabromobiphenyl. It is manufactured by a
special proprietary process rendering no brominated by-products
(Neufeld et al., 1977).
It is noteworthy that mixed polybromochlorobiphenyls (PCBs)
have been observed as minor contaminants in FireMaster(R). For
example, monochloropentabromobiphenyl (CAS No. 88703-30-4) was added
to the list of detected impurities (Domino & Domino, 1980; Tondeur
et al., 1984). Such compounds probably result from contamination of
commercial bromine by chlorine (Domino & Domino, 1980).
Polybrominated naphthalenes (PBNs) (Fig. 2) have been
identified as minor components in commercial PBB mixtures (see
Table 8). The isomeric composition of PBNs in FireMaster(R) is
unknown, but studies on this subject have been started (Robertson
et al., 1984a). It is assumed that naphthalene, present as an
impurity in industrial-grade biphenyl, is brominated during the
production of FireMaster(R), and that the presence of numerous
isomers and congeners of PBNs in FireMaster(R) is possible
(Robertson et al., 1984b).
Table 8. Occurrence of polybrominated naphthalenes (PBNs) in FireMaster(R)-mixtures
PBN CAS-Registry FireMaster(R) Concentration Reference
Number mixture
Tetrabromonaphthalene 88703-31-5 BP-6 or FF-1 no information Tondeur et al.
available (1984)
Pentabromonaphthalene 56448-55-6 BP-6 or FF-1 no information Tondeur et al.
available (1984)
FF-1 1 mg/kg O'Keefe (1979)
BP-6 150 mg/kg Hass et al.
(1978)
Hexabromonaphthalene 56480-06-9 BP-6 or FF-1 no information Tondeur et al.
available (1984)
FF-1 25 mg/kg O'Keefe (1979)
BP-6 70 mg/kg Hass et al.
(1978)
It has been shown that synthesis of hexa-bromonaphthalenes by
direct bromination results in a mixture of two isomers (Birnbaum
et al., 1983; Birnbaum & McKinney, 1985). The major isomer,
1,2,3,4,6,7-HBN, can be metabolized and excreted, while the minor
isomer, 2,3,4,5,6,7-HBN, is extremely persistent (Birnbaum &
McKinney, 1985).
Molecular (empirical) formulae for PBB components of different
degrees of substitution and their relative molecular masses are
given in Table 1.
Theoretically, there can be 209 different forms (congeners) of
a brominated biphenyl, depending on the number and position of the
bromine (see Table 2).
At present, 101 individual PBB congeners are listed in the
Chemical Abstracts Service (CAS) registry. Because bromobiphe nyls
are produced commercially by the bromination of biphenyl, the
existence of any of the 209 congeners is possible in any commercial
mixture (Aust et al., 1983). Some PBBs exist primarily as
metabolites or accumulation or degradation products of the original
mixture. With increasing advance in analysis techniques, the number
of actually identified PBB compounds is growing.
Table 1. PBBs: molecular formula and relative molecular mass
PBB Formula Relative
molecular mass
Monobromobiphenyl C12H9Br 232.9
Dibromobiphenyl C12H8Br2 311.8
Tribromobiphenyl C12H7Br3 390.7
Tetrabromobiphenyl C12H6Br4 469.6
Pentabromobiphenyl C12H5Br5 548.5
Hexabromobiphenyl C12H4Br6 627.4
Heptabromobiphenyl C12H3Br7 706.3
Octabromobiphenyl C12H2Br8 785.2
Nonabromobiphenyl C12HBr9 864.1
Decabromobiphenyl C12Br10 943.0
Table 2. Multiplicity of PBB isomers and congenersa
Number of
Br Substituent 1 2 3 4 5 6 7 8 9 10
Number of
Isomers 3 12 24 42 46 42 24 12 3 1
a Modified from: Safe (1984).
The synthesis of pure congeners for use as standards is a
prerequisite for advances in chemical analysis, as well as research
into the toxicological and biological effects of PBBs. Some routes
for the synthesis of PBB congeners have been described by Sundström
et al. (1976b), Robertson et al. (1980, 1982a, 1984a), Höfler et al.
(1988), and Kubiczak et al. (1989).
Table 3 gives a list of all 209 possible congeners and their
CAS numbers, if already designated. The CAS names are designated as
follows:
1,1'-Biphenyl, .......... bromo-
e.g., 1,1'-Biphenyl, 2,2',4,4',5,5'-hexabromo- or
2,2',4,4',5,5'-hexabromo-1,1'-biphenyl (BB-153).
2.1.2 Technical products
2.1.2.1 Major trade names
The PBBs produced for commercial use include mixtures mainly
containing hexa-, octa-/nona-, and decabromobiphenyls. Data on past
and present trade names and manufacturers are summarized in Table 4
(for further details see section 3.2.1).
2.1.2.2 Composition of the technical products
Commercial PBB products are mixtures of various brominated
biphenyls. Several structural isomers of each of these brominated
compounds are possible and may be present in the product. All
mixtures are relatively highly brominated, with bromine contents
ranging from about 76% for hexabromobiphenyls to 81-85% for octa- to
decabromobiphenyl mixtures (Brinkman & de Kok, 1980).
Data on the composition of PBB mixtures are given in Table 5.
As shown in Table 5, the analytical results concerning the various
products are rather divergent. It indicates that the exact
composition of the mixtures varies between batches, and also within
each batch according to the sampling and analytical method. It can
be seen that samples of "octabromobiphenyl" often contained a larger
proportion of nona- than of octa-substituted PBBs. In this
monograph, these compounds are also referred to as "octa/nona"
bromobiphenyls.
Information on the isomeric composition of the octa- to deca-
mixtures is scarce. In an analysis of Bromkal 80, three isomers of
octabromobiphenyl were found to be present at 14, 16, and 42%
(Norström et al., 1976). A comparison of the isomeric composition of
an "octabromobiphenyl"-mixture with the FireMaster(R)- mixture has
been given by Moore & Aust (1978). De Kok et al. (1977) analysed
various "octabromobiphenyl"-mixtures and Bromkal 80-9D and discussed
the structures of isomers. Furthermore, two isomeric octa- and
three hexa-bromobiphenyls of a commercial decabromobiphenyl mixture
(RFR) have been reported (de Kok et al., 1977).
Table 3. Systematic numbering of PBB compounds and their CAS numbers
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
Monobromobiphenyls (26264-10-8) 17 2,2',4
18 2,2',5 59080-34-1
1 2 2052-07-7 19 2,2',6
2 3 2113-57-7 20 2,3,3'
3 4 92-66-0 21 2,3,4
22 2,3,4'
Dibromobiphenyls (27479-65-8) 23 2,3,5
24 2,3,6
4 2,2' 13029-09-9 25 2,3',4
5 2,3 115245-06-2 26 2,3',5 59080-35-2
6 2,3' 49602-90-6 27 2,3',6
7 2,4 53592-10-2 28 2,4,4' 6430-90-6
8 2,4 49602-91-7 29 2,4,5 115245-07-3
9 2,5 57422-77-2 30 2,4,6 59080-33-0
10 2,6 59080-32-9 31 2,4',5 59080-36-3
11 3,3' 16400-51-4 32 2,4',6 64258-03-3
12 3,4 60108-72-7 33 2',3,4
13 3,4' 57186-90-0 34 2',3,5
14 3,5 16372-96-6 35 3,3',4
15 4,4' 92-86-4 36 3,3',5
37 3,4,4' 6683-35-8
Tribromobiphenyls (51202-79-0) 38 3,4,5 115245-08-4
39 3,4',5 72416-87-6
16 2,2',3
Tetrabromobiphenyls 40088-45-7 65 2,3,5,6
66 2,3',4,4' 84303-45-7
40 2,2',3,3' 67 2,3',4,5
41 2,2',3,4 68 2,3',4,5'
43 2,2',3,5 69 2,3',4,6
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
44 2,2',3,5' 70 2,3',4',5 59080-38-5
45 2,2',3,6 71 2,3',4',6
46 2,2',3,6' 72 2,3',5,5'
47 2,2',4,4' 66115-57-9 73 2,3',5',6
48 2,2',4,5 74 2,4,4',5
49 2,2',4,5' 60044-24-8 75 2,4,4',6 64258-02-2
50 2,2',4,6 76 2',3,4,5
51 2,2',4,6' 97038-95-4 77 3,3',4,4' 77102-82-0
52 2,2',5,5' 59080-37-4 78 3,3',4,5
53 2,2',5,6' 60044-25-9 79 3,3',4,5' 97038-98-7
54 2,2',6,6' 97038-96-5 80 3,3',5,5' 16400-50-3
55 2,3,3',4 97038-99-8 81 3,4,4',5 59589-92-3
56 2,3,3',4'
57 2,3,3',5 Pentabromobiphenyls (56307-79-0)
58 2,3,3',5'
59 2,3,3',6 82 2,2',3,3',4
60 2,3,4,4' 83 2,2',3,3',5
61 2,3,4,5 115245-09-5 84 2,2',3,3',6
62 2,3,4,6 115245-10-8 85 2,2',3,4,4'
63 2,3,4',5 86 2,2',3,4,5
64 2,3,4',6 87 2,2',3,4,5'
88 2,2',3,4,6 77910-04-4 111 2,3,3',5,5'
89 2,2',3,4,6' 112 2,3,3',5,6
90 2,2',3,4',5 113 2,3,3',5',6
91 2,2',3,4',6 114 2,3,4,4',5 96551-70-1
92 2,2',3,5,5' 115 2,3,4,4',6
93 2,2',3,5,6 116 2,3,4,5,6 38421-62-4
94 2,2',3,5,6' 117 2,3,4',5,6
95 2,2',3,5',6 88700-05-4 118 2,3',4,4',5 67888-97-5
96 2,2',3,6,6' 119 2,3',4,4',6 86029-64-3
97 2,2',3',4,5 120 2,3',4,5,5' 80407-70-1
98 2,2',3',4,6 121 2,3',4,5',6
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
99 2,2',4,4',5 81397-99-1 122 2',3,3',4,5
100 2,2',4,4',6 97038-97-6 123 2',3,4,4',5 74114-77-5
101 2,2',4,5,5' 67888-96-4 124 2',3,4,5,5'
102 2,2',4,5,6' 80274-92-6 125 2',3,4,5,6'
103 2,2',4,5',6 59080-39-6 126 3,3',4,4',5 84303-46-8
104 2,2',4,6,6' 97063-75-7 127 3,3',4,5,5' 81902-33-2
105 2,3,3',4,4'
106 2,3,3',4,5 Hexabromobiphenyls (36355-01-8)
107 2,3,3',4',5
108 2,3,3',4,5' 128 2,2',3,3',4,4' 82865-89-2
109 2,3,3',4,6 129 2,2',3,3',4,5
110 2,3,3',4',6 130 2,2',3,3',4,5' 82865-90-5
131 2,2',3,3',4,6 155 2,2',4,4',6,6' 59261-08-4
132 2,2',3,3',4,6' 119264-50-5 156 2,3,3',4,4',5 77607-09-1
133 2,2',3,3',5,5' 55066-76-7 157 2,3,3',4,4',5' 84303-47-9
134 2,2',3,3',5,6 158 2,3,3',4,4',6
135 2,2',3,3',5,6' 119264-51-6 159 2,3,3',4,5,5' 120991-48-2
136 2,2',3,3',6,6' 160 2,3,3',4,5,6
137 2,2',3,4,4',5 81381-52-4 161 2,3,3',4,5',6
138 2,2',3,4,4',5' 67888-98-6 162 2,3,3',4',5,5'
139 2,2'3,4,4',6 163 2,3,3',4',5,6
140 2,2',3,4,4',6 164 2,3,3',4',5',6 82865-91-6
141 2,2',3,4,5,5' 120991-47-1 165 2,3,3',5,5',6
142 2,2',3,4,5,6 166 2,3,4,4',5,6
143 2,2',3,4,5,6' 167 2,3',4,4',5,5' 67888-99-7
144 2,2',3,4,5',6 119264-52-7 168 2,3',4,4',5',6 84303-48-0
145 2,2',3,4,6,6' 169 3,3',4,4',5,5' 60044-26-0
146 2,2',3,4',5,5'
147 2,2',3,4',5,6 Heptabromobiphenyl (35194-78-6)
148 2,2',3,4',5,6'
Table 3. cont'd
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
149 2,2',3,4',5',6 69278-59-7 170 2,2',3,3',4,4',5 69278-60-0
150 2,2',3,4',6,6' 93261-83-7 171 2,2',3,3',4,4',6
151 2,2',3,5,5',6 119264-53-8 172 2,2',3,3',4,5,5' 82865-92-7
152 2,2',3,5,6,6' 173 2,2',3,3',4,5,6
153 2,2',4,4',5,5' 59080-40-9 174 2,2',3,3',4,5,6' 88700-04-3
154 2,2',4,4',5,6' 36402-15-0 175 2,2',3,3',4,5',6
176 2,2',3,3',4,6,6' 195 2,2',3,3',4,4',5,6
177 2,2',3,3',4,5,6' 196 2,2',3,3',4,4',5',6
178 2,2',3,3',5,5',6 119264-54-9 197 2,2',3,3',4,4',6,6' 119264-59-4
179 2,2',3,3',5,6,6' 198 2,2',3,3',4,5,5',6
180 2,2',3,4,4',5,5' 67733-52-2 199 2,2',3,3',4,5,6,6'
181 2,2',3,4,4',5,6 200 2,2',3,3'4,5',6,6' 119264-60-7
182 2,2',3,4,4',5,6' 119264-55-0 201 2,2',3,3',4',5,5',6 69887-11-2
183 2,2',3,4,4',5',6 202 2,2',3,3',5,5',6,6' 59080-41-0
184 2,2',3,4,4',6,6' 119264-56-1 203 2,2',3,4,4',5,5',6
185 2,2',3,4,5,5',6 204 2,2',3,4,4',5,6,6' 119264-61-8
186 2,2',3,4,5,6,6' 119264-57-2 205 2,3,3',4,4',5,5',6
187 2,2',3,4',5,5',6 84303-49-1
188 2,3',3,4',5,6,6' 119264-58-3 Nonabromobiphenyls (27753-52-2)
189 2,3,3',4,4',5,5' 88700-06-5
190 2,3,3',4,4',5,6 79682-25-0 206 2,2',3,3',4,4',5,5',6 69278-62-2
191 2,3,3',4,4',5',6 207 2,2',3,3',4,4',5,6,6' 119264-62-9
192 2,3,3',4,5,5',6 208 2,2',3,3',4,5,5',6,6' 119264-63-0
193 2,3,3',4',5,5',6
Decabromobiphenyl
Octabromobiphenyls (27858-07-7)
209 2,2',3,3',4,4',5,5',6,6' 13654-09-6
194 2,2',3,3',4,4',5,5' 67889-00-3
a The Nos 1-209 correspond to those used by Ballschmiter & Zell (1980) for PCBs (January 1990).
Table 4. Major trade names and manufacturers of technical-grade PBBs and
commercial PBB mixturesa
PBB mixture Manufacturer CAS No.
Hexa-PBBs
FireMaster(R) BP-6 Michigan Chemical Corp. (St. Louis, Mich.) 59536-65-1
FireMaster(R) FF-1b Michigan Chemical Corp. (St. Louis, Mich.) 67774-32-7
Octa/nona-PBBs
Bromkal 80-9D Chemische Fabrik Kalk (Cologne, Germany) 61288-13-9
Technical
octabromobiphenyl White Chemical Corp. (Bayonne, New Jersey)
Octabromobiphenyl
FR 250 13A Dow Chemical Co. (Midland, Mich.)
Deca-PBB
Adine 0102 Ugine Kuhlmann now Atochem (Paris, France) 13654-09-6
Berkflam B 10 Berk (London, United Kingdom)
Flammex B-10 Berk (London, United Kingdom)
Technical
decabromobiphenyl White Chemical Corp. (Bayonne, New Jersey)
HFO 101 Hexcel (Basildon, United Kingdom)
a Adapted from: Brinkman & de Kok (1980).
b A pulverized form of FireMaster BP-6 containing 2% calcium polysilicate
to prevent caking. It was produced in limited quantities as a
development-product in 1971 and 1972.
Most research has been conducted with the hexabromobiphenyl
mixture FireMaster(R), which accounts for most of the manu
factured products and most of the environmental contamination
(Di Carlo et al., 1978). The main constituent of FireMaster(R) is
2,2',4,4',5,5'-hexabromobiphenyl. Its identification was reported by
Andersson et al. (1975), Jacobs et al. (1976), and Sundström et al.
(1976a). The second major component is heptabromobiphenyl containing
bromine at positions 2,2',3,4,4',5,5' (Hass et al., 1978; Moore
et al., 1978c). Accordingly, these two congeners account for about
75% of the mixture (e.g., Dannan et al., 1982d). Data on the
isomeric composition of FireMaster(R) found in the literature are
given in Table 6. The ranges of relative abundances of some
FireMaster(R) constituents are compiled in Table 7. Altogether at
least sixty compounds have been detected in FireMaster(R) (Orti
et al., 1983). About twelve of them are major PBB-components (Aust
et al., 1981), the others belong to the minor components (< 1%).
Table 5. Survey of literature on the composition of PBB mixturesa
PBB mixture (manufacturer) Weight of Weight of different homologus groups Reference
bromine (%)
Br10 Br9 Br8 Br7 Br6 Br5 Br4
"Hexabromobiphenyl"
FM BP-6 (Michigan Chemical) 75 13.8 62.8 10.6 2 de Kok et al.
(1977)c
" [Lot RP-158 (1971)] 12.5 72.5 9 4 Willett & Irving
(1976)
" [Lot 6244A (1974)] 13 77.5 5 4.5 Willett & Irving
(1976)
" 90 10 Norström et al.
(1976)
" 1 18 73 8 de Kok et al.
(1977)
" 33 63 4 Hass et al.
(1978)
" 7.7 74.5 5.6 Robertson et al.
(1984b)
" 24.5 79 6 Krüger (1988)
2,2',4,4',6,6' (RFR) 12 84 1 de Kok et al.
(1977)
2,2',4,4',6,6' (Aldrich) 2 24 70 4 de Kok et al.
(1977)
"Hexabromobiphenyl" (RFR) 25 67 4
(12-25) (60-80) (1-11) (2-5)b de Kok et al.
(1977)
Table 5 (contd).
PBB mixture (manufacturer) Weight of Weight of different homologus groups Reference
bromine (%)
Br10 Br9 Br8 Br7 Br6 Br5 Br4
Octanonabromobiphenyl
Bromkal 80-9D (Kalk) 81-82.5 9 65 1 de Kok et al.
(1977)
Bromkal 80 72 27 1 Norström et al.
(1976)
XN-1902 (Dow Chemical)c 82 6 47 45 2 Norris et al. (1973)
XN-1902 (Dow Chemical)c 2 34 57 7 de Kok et al. (1977)
Lot 102-7-72 (Dow Chemical)c 6 60 33 1 Waritz et al. (1977)
"Octabromobiphenyl" (RFR) 4 54 38 2 de Kok et al. (1977)
2,2',3,3',5,5',6,6' (RFR) 1 28 46 23 2 de Kok et al. (1977)
FR 250 13A (Dow Chemical) 8 49 31 1 Krüger (1988)
Decabromobiphenyl
HFO 101 (Hexcel) 84 96 2 de Kok et al. (1977)
Adine 0102 (Ugine Kuhlmann) 83-85 96 4 de Kok et al. (1977)
Adine 0102 (Ugine Kuhlmann) 96.8 2.9 0.3 Millischer et al.
(1979)
"Decabromobiphenyl" (RFR) 71 11 7 4 4 de Kok et al. (1977)
"DBB": Flammex B 10 (Berk)c 96.8 2.9 0.3 Di Carlo et al
(1978)
a Adapted from: Brinkman & de Kok (1980).
b Range of above readings with the exception of that of Norström et al. (1976), which differs greatly from the others.
c According to de Kok et al. (1977), these have never been marked.
Table 6. Identified PBB congeners in FireMaster(R)
BB No.a Structure % Composition of References
FM BP-6 FF-1
Dibromobiphenyls
4 2,2'- 0.02 Moore et al. (1979a)
Tribromobiphenyls
18 2,2'5- 0.050 Robertson et al. (1984b)
26 2,2',5- 0.024
31 2,4',5- 0.015
37 3,4,4'- 0.021
Tetrabromobiphenyls
49 2,2',4,5'- 0.025
52 2,2',5,5'- 0.052
66 2,3',4,4'- 0.028
70 2,3',4',5- 0.017
77b 3,3',4,4'- < 0.08 Orti et al. (1983)
0.159 Robertson et al. (1984b)
Pentabromobiphenyls
95 2,2',3,5',6- 0.02 Orti et al. (1983)
99 2,2'4,4',5- < 0.08
101 2,2',4,5,5'- 2.69 Robertson et al. (1984b)
4.5 3.7 Aust et al. (1981)
1.54 Orti et al. (1983)
2.6 Krüger (1988)
118 2,3',4,4',5- 2.94 Robertson et al. (1984b)
0.7 Aust et al. (1981)
3.2 Krüger (1988)
0.8 Orti et al. (1983)
126b 3,3',4,4',5- < 0.01
0.079 Robertson et al. (1984b)
Hexabromobiphenyls
132 2,2'.3.3',4,6'- 1 Krüger (1988)
138 2,2',3,4,4',5'- 12.3 Robertson et al. (1984b)
Table 6. cont'd
BB No.a Structure % Composition of References
FM BP-6 FF-1
12 8.6 Aust et al. (1981)
5.23 Orti et al. (1983)
10.6 Krüger (1988)
149 2,2',3,4',5',6- 2.24 Robertson et al. (1984b)
1.4 1.3 Aust et al. (1981)
0.78 Orti et al. (1983)
153 2,2'4,4',5,5'- 53.9 Robertson et al. (1984b)
47.8 47.1 Aust et al. (1981)
55.2 Orti et al. (1983)
58.5 Krüger (1988)
155 2,2',4,4',6,6'- 0.5
156 2,3,3',4,4',5- 0.980 Robertson et al. (1984b)
5.0 Aust et al. (1981)
0.37 Orti et al. (1983)
1.0 Krüger (1988)
157 2,3,3',4,4',5'- 0.05 Orti et al. (1983)
0.526 Robertson et al. (1984b)
0.5 Krüger (1988)
167 2,3',4,4',5,5'- 5.5 3.3 Aust et al. (1981)
3.37 Orti et al. (1983)
< 0.3
7.95 Robertson et al. (1984b)
5.5 Krüger (1988)
169b 3,3',4,4',5,5'- 0.294 Robertson et al. (1984b)
Heptabromobiphenyls
170 2,2',3,3',4,4',5- 0.256
1.1 1.5 Aust et al. (1981)
1.66 Orti et al. (1983)
2.4 Krüger (1988)
180 2,2',3,4,4',5,5'- 6.97 Robertson et al. (1984b)
24.7 Aust et al. (1981)
23.5 Orti et al. (1983) 20.8 Krüger (1988)
172 2,2',3,3',4,5,5'- < 0.30 Orti et al. (1983)
174 2,2',3,3',4,5,6'- 0.24
178 2,2',3,3',5,5',6- 0.3 Krüger (1988)
187 2,2',3,4',5,5',6- 0.392 Robertson et al. (1984b)
1.0 Krüger (1988)
189 2,3,3',4,4',5,5'- 0.51 Orti et al. (1983)
Table 6. cont'd
BB No.a Structure % Composition of References
FM BP-6 FF-1
Octabromobiphenyls
194 2,2',3,3',4,4', 0.9 2.4 Aust et al. (1981)
5,5'-
1.65 Orti et al. (1983)
possible structures for two
minor Br8 peaks:
196 2,2',3,3',4,4', Moore et al. (1980);
5,6'-
201 2,2',3,3',4,5, Orti et al. (1983)
5',6'-
203 2,2',3,4,4',5,
5'6-
a From: Ballschmiter & Zell (1980).
b These coplanar congeners are the most toxic congeners identified in
FireMaster BP-6 (Robertson et al., 1984b).
Table 7. Range of relative abundance of some PBB constituents
of Firemaster(R) FF-1 and BP-6a
Structure No.b BB No.c Abundance (%)
2,2',4',5,5'- 1 101 1.5-4.5
2,3',4,4',5,- 2 118 0.7-4.2
2,2',3,4',5',6- 3 149 0.8-2.2
2,2',4,4',5,5'- 4 153 47.1-59
2,2',3,4,4',5'- 5 138 5.2-12.3
2,3',4,4',5,5'- 6 167 3.3-8.0
2,3,3',4,4',5- 7 168 0.4-5.0
2,2',3,4,4',5,5'- 8 180 7.0-24.7
2,2',3,3',4,4',5- 9 170 0.3-2.4
2,2',3,3',4,4',5,5'- 12 194 0.9-2.4
a For references, see Table 6.
b Congener designation made on the basis of the gas chromatographic
elution sequence of the FireMaster(R) mixture.
c Congener designation according to Ballschmiter & Zall (1980).
Variations are due to differences in batches and analytical
techniques. In many cases, the differing electron capture responses
of the various congeners within the mixture were not taken into
account. Thus, values in Table 7 only give an approximate range of
composition and it is not possible to provide a precise composition
for the material that was introduced into the Michigan environment
(Fries, 1985b).
Both formulations of FireMaster(R) mixture, BP-6 and FF-1
have a similar isomeric composition. However, FireMaster BP-6
contains roughly 10% more of the relatively minor congeners (Dannan
et al., 1982b).
As can be concluded from the composition of the commercial
mixtures (Table 5), the major source of impurity that occurs in PBBs
results from the spread in the degree of bromination. For example,
FireMaster(R) BP-6 has been marketed as a hexabromin ated
biphenyl, but more than one quarter of the product consists of lower
brominated biphenyls because of incomplete bromination reaction
(Neufeld et al., 1977).
However, a producer of decabromobiphenyl has reported that
their material has a degree of purity of more than 98%, the
remaining 2% being nonabromobiphenyl. It is manufactured by a
special proprietary process rendering no brominated by-products
(Neufeld et al., 1977).
It is noteworthy that mixed polybromochlorobiphenyls (PCBs)
have been observed as minor contaminants in FireMaster(R). For
example, monochloropentabromobiphenyl (CAS No. 88703-30-4) was added
to the list of detected impurities (Domino & Domino, 1980; Tondeur
et al., 1984). Such compounds probably result from contamination of
commercial bromine by chlorine (Domino & Domino, 1980).
Polybrominated naphthalenes (PBNs) (Fig. 2) have been
identified as minor components in commercial PBB mixtures (see
Table 8). The isomeric composition of PBNs in FireMaster(R) is
unknown, but studies on this subject have been started (Robertson
et al., 1984a). It is assumed that naphthalene, present as an
impurity in industrial-grade biphenyl, is brominated during the
production of FireMaster(R), and that the presence of numerous
isomers and congeners of PBNs in FireMaster(R) is possible
(Robertson et al., 1984b).
Table 8. Occurrence of polybrominated naphthalenes (PBNs) in FireMaster(R)-mixtures
PBN CAS-Registry FireMaster(R) Concentration Reference
Number mixture
Tetrabromonaphthalene 88703-31-5 BP-6 or FF-1 no information Tondeur et al.
available (1984)
Pentabromonaphthalene 56448-55-6 BP-6 or FF-1 no information Tondeur et al.
available (1984)
FF-1 1 mg/kg O'Keefe (1979)
BP-6 150 mg/kg Hass et al.
(1978)
Hexabromonaphthalene 56480-06-9 BP-6 or FF-1 no information Tondeur et al.
available (1984)
FF-1 25 mg/kg O'Keefe (1979)
BP-6 70 mg/kg Hass et al.
(1978)
It has been shown that synthesis of hexa-bromonaphthalenes by
direct bromination results in a mixture of two isomers (Birnbaum
et al., 1983; Birnbaum & McKinney, 1985). The major isomer,
1,2,3,4,6,7-HBN, can be metabolized and excreted, while the minor
isomer, 2,3,4,5,6,7-HBN, is extremely persistent (Birnbaum &
McKinney, 1985).
 Polybrominated benzenes and a possible methylbrominated furan
have also been reported to occur in FireMaster(R) (Brinkman & de
Kok, 1980).
Approximately 20 compounds, other than PBBs, were either
tentatively identified in FireMaster(R) or partially characterized
by Hass et al. (1978).
Polybromodibenzo- p-dioxins and polybromodibenzofurans were
searched for, because of their extreme toxicity and because
chlorinated dibenzofurans had been detected in commercial PCBs
(Nagayama et al., 1976). If present, their concentrations did not
exceed 0.5 mg/kg (Hass et al., 1978, O'Keefe, 1979). Polybromo
dibenzodioxins and polybromodibenzofurans were determined in a
sample of Adine 0102 (decabromobiphenyl). Monobromobenzo difurans
were present at a level of 1 mg/kg (1 ppm), otherwise all other
polybromodibenzodioxins and polybromodibenzofurans were present only
at less than 0.01 mg/kg (Atochem, 1990).
So far, phenoxyphenols and hydroxybiphenyls, which might be
intermediates in the formation of brominated dibenzo- p-dioxins and
brominated dibenzofurans, respectively, have not been identified
(O'Keefe, 1979).
Some impurities in PBBs result from impurities in the original
biphenyl material. According to two major manufacturers, their
biphenyl grade used for bromination contained less than 5 mg/kg and
5000 mg/kg, respectively, of impurities, e.g., toluene, naphthalene,
methylene biphenyl (fluorene), and various methyl biphenyls (Neufeld
et al., 1977).
2.2 Physical and chemical properties
In general, PBBs show an unusual chemical stability and
resistance to breakdown by acids, bases, heat, and reducing and
oxidizing agents (Safe, 1984).
PBBs can be compared chemically to the PCBs. Bromine, however,
is a better leaving group in chemical reactions than chlorine.
Unlike PCBs, the reactivity of PBBs has not been well studied and
documented in the literature (Pomerantz et al., 1978). Like PCBs
their chemical stability is dependent, in part, on the degree of
bromination and the specific substitution patterns (Safe, 1984). All
highly brominated PBB-mixtures are known to degrade rather rapidly
with UV irradiation (Brinkman & de Kok, 1980).
The technical mixtures typically are white, off-white, or beige
powdered solids. Some physical data on commercial PBB mixtures are
given in Table 9. It can be seen that there are discrepancies in the
values for the solubility of commercial PBBs in water (given in
Table 9) as well as those calculated for various PBB congeners
(Table 10). The source and quality of the water is important.
Determinations of water solubility of these very hydrophobic
compounds are also difficult to perform. Adsorption effects on
particles and glass surfaces may influence the results. PBBs were
found to be 200 times more soluble in landfill leachate than in
distilled water (Griffin & Chou, 1981a). In general, it can be said
that PBBs are only slightly soluble in water and that the solubility
decreases with increasing bromination.
For details of thermal decomposition, see section 4.3.2.
2.2.1 Physical and chemical properties of individual congeners
PBBs show a wide range of volatility (Farrell, 1980). Partition
coefficients between water/ n-hexane and water/1-octanol, as well
as aqueous solubilities for some individual PBB congeners are given
in Table 10. Correlations for predicting aqueous solubility and
partition coefficients for PBBs based on molecular structure have
been proposed (Patil, 1991). The solubility of PBBs in n-hexane
decreases rapidly with increasing bromine content (de Kok et al.,
1977).
Data on the melting points and UV absorption of individual PBB
congeners are summarized in Table 11. The main band in these spectra
is caused by pi -> pi* electron transitions, while the k band is
generally attributed to the conjugated biphenyl system with the
contribution of both biphenyl rings. With the k band, the
introduction of bromine atoms in positions meta or para to the
phenyl-phenyl bond induces a shift in kmax towards the visible
region, as is illustrated by 3,3',5,5'-tetra- and 3,3',4,4',5,5'-
hexabromobiphenyl. On the other hand, ortho substitution, which
causes a considerable hindrance for free rotation of the rings and,
thus, a loss in coplanarity, effects a sharp decrease in the
extinction coefficient of the k band (de Kok et al., 1977).
Data on NMR spectra are given by Orti et al. (1983), Robertson
et al. (1984b), and Kubiczak et al. (1989), and on mass spectrometry
(MS) by Erickson et al. (1980), Roboz et al. (1980), Buser (1986),
and Sovocool et al. (1987a,b). The "ortho" effect, observed for PBBs
and PCBs having 2,2'-; 2,2',6- or 2,2',6,6'- halogens can be
combined with GC retention index for isomer specific identifications
by gas chromatography and mass spectrometry (GC/MS) (Sovocool
et al., 1987a).
Table 9. Some physical data on commercial PBB mixturesa
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Melting point (°C) 72 200-250 220-290 380-386b
360-380
385
Lambda max (nm) 219e 225e 224e 227e
Density (g/cm3) at 2.6 - 3.2 3.2
room temperature
Solubility in water 11f 20-30 < 30
(µg/litre) at 25 °C 30f (pure 2,2', 4,4', 5,5'-)
610f
0.06g (deionized)
0.32g (distilled) insoluble
Solubility in organic
solvents (g/kg
solvent) at 28 °C
petroleum ether 20 18 insoluble in common
acetone 60 organic solvents
carbon tetrachloride 300 10e
chloroform 400
benzene 750 81
toluene 970
dioxane 1150
copra oil (37 °C) 0.8
Table 9 (contd).
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Vapour pressure (Pa)
25 °C 0.000007h < 0.000006
90 °C 0.01 (temperature not given)
140 °C 1
220 °C 100
Volatility (% weight loss) < 1% at 250 °C 1-2% at 300 °C < 5% at 341 °C
< 10% at 330 °C < 10% at 363 °C
< 50% at 350 °C < 25% at 388 °C
log Pow < 7 (calculated)d 8.6 (calculated)
Decomposition temperature 300-400 °C 435 °C 435 °C 395 °C
> 400° C
a Mumma & Wallace 1975).
b Norris et al. (1973).
c Kerscher (1979); CFK (1982).
d Atochem (1990).
e Brinkman & de Kok (1980).
f Filonow et al. (1976).
g Griffin & Chou (1981a,b).
h Jacobs et al. (1976).
Table 10. Partition coefficients between water and n-hexane (KHW) and 1-octanol
(Kow) and aqueous solubilities (Sw) for some individual PBB congeners (all
aqueous solubility measurements were carried out by the generator method)
Log KHW Log KOW Sw mol per Sw µg/litred
litre x 10-9 (25 °C)
(25 °C)
2-bromobiphenyl 4.59a
3-bromobiphenyl 4.85a
4-bromobiphenyl 4.96a 2800 650
3,5-dibromobiphenyl 5.78c
4,4'-dibromobiphneyl 5.61 5.72 18.4 5.7
2,4,6-tribromobiphenyl 6.21 6.03 41.1 16
3,4',5-tribromobiphenyl 6.42c
2,2',5,5',tetrabromobiphenyl 6.72 6.50 8.6 4
3,3',5,5'-tetrabromobiphenyl 7.42c
2,2',4,5,5'-pentabromobiphenyl 7.10a 0.8b 0.4
2,2',4,4',6,6'-hexabromobiphenyl 7.52 7.20 0.9 0.56
decabromobiphenyl 8.58a
Values from Gobas et al. (1988) with the exception of:
a From: Doucette & Andren (1987).
b From: Doucette & Andren (1988).
c From: Sugiura et al. (1978).
d calculated.
Table 11. Melting points and UV spectral data for some PBB congenersa
UV conditions: solutions in n-hexane; Beckman Acta CIII spectrometer
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
Biphenyl 71 201 4.66 246 4.26
1 2- e 201 4.51 240 3.90
2 3- e 205 4.60 248 4.21
3 4- e 200 4.67 254 4.38
4 2,2'- 81 198 4.64 220-230 e
9 2,5- e 203 4.49 226 4.38
15 4,4'- 164 201 4.64 261 4.43
20 2,4,6- 65-66 213 4.70 220-230 e
21 2,2',5- 78 200 4.66 235-245 e
26 2,3',5- e 213 4.57 e e
31 2,4',5- 78 205 4.60 245-255 e
49 2,2',4,5'- 84 207 4.66 235-245 e
52 2,2',5,5'- 143 204 4.67 235-240 e
80 3,3',5,5'- 188 220 4.76 255 4.18
114b 2,3,4,4',5- 128 222.6 (54.8) 258 e
137b 2,2',3,4,4',5- 124 223.1 (35.4) e e
141b 2,2',3,4,5,5'- 127 223.4 (191) e e
153 2,2',4,4',5,5'- (159-160)f 216 4.66 e e
156b 2,3,3',4,4',5- 178 224.9 (229) 259 e
159b 2,3,3',4,5,5'- 195 226.1 (61.4) 258 e
167 2,3',4,4',5,5'- (165-166)f e e e e
Table 11 (contd).
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
169 3,3',4,4',5,5'- 248 227 4.76 272 4.34
180b 2,2',3,4,4',5,5'- 166(165-166)f 224.1 (62.4) e e
189b 2,3,3',4,4',5,5'- 219 230.7 (102) 265 e
194b 2,2',3,3',4,4',5,5'- 235(232-233)f 223.7 (51.6) e e
202 2,2',3,3',5,5',6,6'- e 224 4.85 e e
206b 2,2',3,3',4,4',5,5',6- 262(263-264)f,g 225.2 (131) e e
Nona-(unidentified) e 225 5.18 e e
Deca- 378 227 5.11 e e
a Adapted from: de Kok et al. (1977), with the exception of the congeners marked with b.
b Congener data, including melting points are taken from Kubiczak et al. (1989). UV measurements: in n-heptane.
c No. according to Ballschmiter & Zell (1980).
d Melting points from Sundström et al. (1976b) but confirmed by de Kok et al. (1977), unless otherwise stated.
e No data available.
f From: Moore & Aust (1978).
2.3 Conversion factors for PBB in air
1 ppm = 26.1 mg/m3 for hexabromobiphenyl at 20 °C and
101.3 kPa.
1 mg/m3 = 0.038 ppm.
2.4 Analytical methods
Analytical methods for the determination of PBBs, which have
been reviewed by de Kok et al. (1977), Pomerantz et al. (1978), and
Fries (1985b), were adapted from established methods for chlorinated
hydrocarbon insecticides and PCBs (AOAC, 1975). The chronological
development of analytical methods for the detection and
quantification of PBB mixtures and congeners is summarized in
Table 12. In the wake of the Michigan disaster, methods were
described for the analysis of: contaminated feed, milk, and milk
products (Fehringer, 1975a,b); animal blood plasma, faeces, milk,
and bile (Willett et al., 1978) and liver and fat (Fawkes et al.,
1982). The methods were developed using tissue from animals fed with
PBBs of known composition. Needham and coworkers developed a method
to determine PBBs in human blood serum (Burse et al., 1980; Needham
et al., 1981) which was thoroughly tested in several laboratories,
but, even here, only the main components of FireMaster(R) were
determined. Similarly, the investigation by Eyster et al. (1983)
into the levels of PBBs in fat, serum, faeces, milk, and placenta
were not isomer specific. Thus, reported values may not reflect the
hazard of the residue because, for example, some congeners are more
toxic than the prominent 2,2',4,4',5,5'-HBB. Most samples of
biological origin have congener distributions that differ from those
of the original material (Fries, 1985b).
Concentrations of PBBs as low as 10 µg/kg in fatty foods
(Fehringer, 1975a), 3 µg/kg in dry feeds (Fehringer, 1975b) and
1 µg/litre in blood serum (Needham et al., 1981) can be detected and
quantified using routine methods. Coefficients of variation become
large as concentrations approach the limits of sensitivity of the
method; thus, values near the limit must be treated with caution
(Fries, 1985b). PBBs adsorb to glass more tenaciously than other
halogenated hydrocarbons, and are not easy to remove by the usual
cleaning methods (Willett et al., 1978). This can lead to erroneous
values, particularly when concentrations in samples are low and
there is a carry over from samples of high concentration. This
problem can be solved by using disposable glassware (Willett et al.,
1978).
Table 12. Analysis of commercial mixtures and individual PBB congeners: A chronological surveya
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) recrystallization GC FID no data identification of Sundström
BP-6 from ethanol/ given BB 153 and a HpBB as et al.
isopropanol major components (1976a)
PBB congeners no data given GC ECD no data routes of synthesis, Sundström
given melting points, relative et al.
retention times, electron (1976b)
capture responses for
some PBB congeners
Commercial - solubility of HPLC, TLC, MS no data survey of analysis for de Kok
mixtures FR 250 13A PBBs in n-hexane UV, GC given PBBs et al.
(octabromobipheyl) decreases rapidly with 1H- & 13C-NMR (1977)
FireMaster(R) BP-6 increasing bromine
and PBB congeners content; PBBs dissolved
in warm CCl4
Commercial sample hexane GC, 13C-NMR, ECD no data identification of Moore
of 1H-NMR, IR given BB 180 et al.
octabromobiphenyl heptabromobiphenyl (1978)
FireMaster(R) methylene chloride; GC, NMR, MS 0.5 mg/kg contains at least 13 Hass
BP-6 hexane HPLC SIM different PBBs and et al.
bromonaphthalene (no (1978)
bromodibenzofurans or
bromodibenzo-p-dioxins
found)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC, NMR MS no data purification and Moore &
FF-1 or BP-6 and given structural characterization Aust
octabromobiphenyl of 6 further PBB congeners (1978)
FireMaster(R) hexane GC ECD 0.03 ng absolute and relative Domino
FF-1 retention times of the 8 et al.
major constituents using (1980a)
tetrabromobiphenyl
as an internal standard
FireMaster(R) hexane GC MS no data mass spectra of major Domino &
FF-1 given PBBs in FireMaster; mixed Domino
poly-bromo and (1980)
chlorobiphenyls detected
FireMaster(R) GC ECD no data comparison of packed and Farrel
BP-6 given capillary columns; solves (1980)
some problems with lower
brominated biphenyls, but
has no great advantages
over packed columns for
more highly substituted
biphenyls
FireMaster(R) no data given GC PED 2.8 mg comparison with ECD; not Mulligan
BP-6 (cf 1.5 ng quite so sensitive, but et al.
ECD) is selective (1980)
Individual PBB toluene GC MS, SIM < 1 ng mono-deca PBB congeners Erickson
congeners et al.
(1980)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
22 individual hexane GC ECD, micro- 40 pg retention times given Sweetman &
PBBs FireMaster(R) (preceeded coulometric for 23 congeners response Boettner
FF-1 by HPLC) GC-detector increases with degree of (1982)
MS bromination, increased
detection temperature gives
improved sensitivity
FireMaster(R) carbon preparative FID, MS polar and unpolar hexane Needham
FF-1 tetrachloride; HPLC and fractions were also et al.
hexane GC 1H-NMR tested for hyperkeratotic (1982)
activity
FireMaster(R) hexane GC NCI, SIM 0.6 ng evaluation of halogen anion Greaves
BP-6 formation by polybrominated et al.
compounds in NCI-MS; SIM of (1982)
bromine anions has greater
specificity than ECD
FireMaster(R) fractionation by GC ECD, MS no data seven congeners were Dannan
BP-6 preferential given purified et al.
acetone (1982d)
solubilization,
repeated
crystallization,
alumina
adsorption
column
chromatography,
reversed phase
Lipidex-500
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) see Needham et al. preparative FID, MS at least 60 components Orti
FF-1 (1982) HPLC, GC, observed; isolated/ et al.
lot FH 7042 GC 1H-NMR determined structure of (1983)
10 minor components of
FireMaster (most are very
polar, later eluting
fractions)
PBB hexane GC helium 230 pg simultaneous monitoring Eckhoff
(unspecified) plasma of 4 atomic emission et al.
atomic wave-lengths; PBB mentioned (1983)
emission
spectrometric
detection
FireMaster(R) no data given GC MS, ECD identity of over 91% of Robertson
BP-6 1H-NMR PBB components in et al.
FireMaster using 22 (1984b)
individual PBB congeners
as standards; identification
of 7 additional PBBs
including 3 very toxic
coplanar PBBs
FireMaster(R) hexane GC PED, rapid multi-element quantification Zerezghi
BP-6 scanning et al.
plasma (1984)
emission
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC SIM, MS determination of suspected Tondeur
FF-1 toxic impurities et al.
(1984)
PBB photolysis hexane GC FID, ECD, purification of PBB congener Barnhart
mixture MS 2 using charcoal pretreatment et al.
and RPLC (1984)
Benzenes, hexane GC NCI-MS 0.1 pg especially valuable for Buser
biphenyls measuring trace levels in (1986)
dibenzodioxins, biological and environmental
dibenzofurans, samples; must be two Br;
diphenylethers, structural information is
benzofurans, partly lost
phenols
PBB congeners no data given GC MS no data use of 'ortho' effect for Sovocool
given PBB and isomer & Wilson
identification; accurate (1982);
structure assignments Sovocool
without use of multiple GC (1987a)
determinations
Various PBB hexane GC, HPLC FID no data relationship between Höfler
congeners given recorded retention data et al.
from HPLC and GC and (1988)
molecular surface area
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
Nine synthetic products purified GC MS 1 ng synthesis of 2,3,4,5- Kubiczak
PBBs; by alumina/Florisil; substituted PBBs and et al.
FireMaster(R) recrystallization characterization (1989)
FF-1 and BP-6 from methanol or
methylene chloride
Mono- and no data given 1H-NMR, no data no data Anklam
poly-brominated 13C-NMR given given (1989)
biphenyls
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NMR = Nuclear magnetic resonance.
FID = Flame ionization detector. PED = Microwave-induced plasma emission detector.
GC = Gas chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
GPC = Gel permeation chromatography. RPLC = Reverse-phase liquid chromatography.
HPLC = High pressure liquid chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatograpy.
MS = Mass spectrometry. Unitrex = Universal Trace Residue Extractor.
NAA = Neutron activation analysis. UV = Ultraviolet.
NCI = Negative ion chemical ionization mass
spectrometry.
Recovery of PBBs using established methods is in the range of
80-90% (Fries, 1985b). The solvent system that is used for sample
extraction can affect recovery. Poor recoveries were often found
with hexane but the optimal solvent conditions depend on the source
of the medium sample.
For extraction conditions see Table 13 (environmental samples),
Table 14 (food/feed), Table 15 (biological tissues and fluids (a)
serum/blood (b) adipose and other tissues).
In soil, Griffin & Chou (1981a) found that a polar organic
solvent was important and obtained the best results with
hexane/acetone (9:1).
For serum and blood, the standard extraction method given by
Burse et al. (1980) has been used by most workers.
Extraction of PBBs from adipose and other tissues presents
greater problems. PBBs are readily soluble in fat. They can
therefore be extracted with the fat out of the tissue/sample but,
afterwards, an intensive clean-up procedure for PBBs is necessary.
Various methods, such as adsorption chromatography with Florisil,
gel permeation chromatography, Florisil cartridges (Chiang et al.,
1987), and Unitrex (Head & Burse, 1987) have been proposed.
The sample extraction and clean-up techniques for the
determination of PBBs are similar to those used for PCBs (Krüger
et al., 1988; Jansson et al., 1991). The lipids can be removed from
the extract by gel permeation (Krüger, 1988) or by hydrolysis
(Jansson et al., 1991). Usually PBBs and PCBs are separated from
more polar compounds by adsorption chromatography on silica gel or
Florisil. If the coplanar compounds are to be determined, they have
to be isolated from the major compounds in the extract. This can be
done using activated charcoal, which adsorbs the planar molecules
more strongly than the non-planar. Brominated naphthalenes, dioxins,
and furans will also be separated from the major PBB components in
this step. HPLC methods are now being adopted for these separations
and both charcoal and modified silica gel columns are available for
HPLC separations of coplanar compounds.
Table 13. Determination of PBBs in environmental samplesa
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
Soil, grass, benzene/ Florisil GC ECD, FID 0.1 µg/kg BB 153, two PeBB Jacobs
carrots 2-propanol MS dry weight (soil) isomers, three et al.
13C-NMR 10 µg/kg additional HxBB (1976)
wet weight (plant) isomers, two HpBB
isomers detected
Soil leachate benzene/ GC ECD 0.1 µg/kg laboratory experiments Filonow
2-propanol dry weight et al.
(1976)
Soil, plant hexane/ Florisil GC ECD 0.1 µg/kg field and laboratory Jacobs
samples acetone dry weight (soil) experiments; no et al.
TLC ECD 0.3 µg/kg significant (1978)
wet weight (plant) degradation of PBBs
after 1 year
Effluent river hexane/ no data GC ECD 0.1 µg/litre environmental Hesse (1975)
water diethyl given (later 0.01 µg/ samples Hesse &
ether litre) Powers
(1978)
Sediment hexane/ no data GC ECD 100 µg/kg environmental Hesse &
acetone given samples Powers
(1978)
Soil hexane/ no data GC ECD, FID, separation of 30 PBB Stratton &
acetone 9:1 given MS congeners tested Whitlock
optimum conditions (1979)
Table 13 (contd).
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
for extraction of
PBBs from soil;
polar organic solvent
important
98 environmental hexane, Florisil GC MS 0.2 µg/kg analysed for hexa-, Stratton
samples Soxhlet hepta-, octa-, nona-, et al.
(fish, sediment, decabromobiphenyls; (1979)
soils, HxBB in 84% of samples
vegetation)
Soil, sediment, hexane Florisil GC MS (SIM) 0.2 µg/kg congeners detected Griffin &
sludge, Chou (1981a)
vegetation
Soil hexane/ Florisil GC FID, ECD degradation of PBBs Hill et al.
acetone 1:1 in soil (1982)
Sewage sludge hexane/ TLC IR, NMR, MS 10 ng/kg no PBBs found Strachan
methanol GC et al.
Soxhlet (1983)
extraction
Plants cut, Florisil GC ECD 0.3 µg/kg no translocation Chou et al.
extracted wet basis in plants (1978)
with hexane/
acetone
Table 13 (contd).
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. MS = Mass spectrometry.
FID = Flame ionization detector. NMR = Nuclear magnetic resonance.
GC = Gas chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatography.
Table 14. Determination of PBBs in food/feeda
Matrix Extraction Clean up Analytical Detection Detection Comment References
method limit
Dairy fat extracted by AOAC GPC, 25% toluene GC ECD 7 µg/kg comparison Fehringer
products (1975) methods in ethyl acetate of methods (1975a)
(methanol/ether)
Florisil/pet ether TLC 0.2 mg/kg
Dry animal finely ground feed Florisil/pet ether GC, TLC ECD 8 µg/kg hexabromo Fehringer
feeds packed into a column 30 µg/kg isomer (1975b)
containing celite, measured
elution with methylene
chloride
Feeds and see Fehringer GC before and ECD 5 µg/kg confirmation Erney
dairy (1975a,b) after UV irradi- of PBB (1975)
products ation to determine residues
background using UV
irradiation
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. TLC = Thin layer chromatography.
GC = Gas chromatography. UV = Ultraviolet.
GPC = Gel permeation chromatography.
Table 15. Determination of PBBs in biological tissues and fluidsa
Matrix Extraction Clean up Detection Detection limit Comment References
a) Serum/blood
Human serum methanol-treated serum, Florisil ECD 5 pg analysis based on HxBB Bekesi et al.
extraction with hexane peak (1978)
Human serum methanol-treated serum, Florisil ECD 0.2 µg/litre Wolff et al.
extraction with hexane/ (1978)
ether
Human/rat serum methanol-treated serum, Florisil ECD 0.2 µg/litre PBB homologues as % HxBB Wolff &
extraction with hexane/ peak Aubrey
ether (1978)
methanol-treated serum, Florisil ECD, MS < 1 mg/ml Wolff et al.
extraction with hexane/ (1979a)
ether
Plasma from multiple extraction with Florisil ECD 0.001 µg/litre recovery 96% Willett
PBB-fed cows mixture of diethyl and pet. et al.
ethers (1978)
Human serum methanol-treated serum; Florisil ECD 0.1 µg/litre interlaboratory comparison Burse et al.
extraction with hexane/ (1980)
ether
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Plasma, white methanol; precipitated Florisil MS-SIM 0.1 µg/mg very exact details with Roboz et al.
cell fraction protein removed; NCI protein review spectra (1980)
erythrocytes extraction with hexane/
ß-lipoprotein ether (1:1)
Human serum methanol; hexane/ silica gel ECD 1 µg/litre Needham
diethylether (1:1) et al.
(1981)
Human serum + methanol precipitated Florisil ECD < 1 µg/litre serum protein precipitated Roboz et al.
protein not removed + MS-NCI with methanol should not (1982)
hexane/diethylether (1:1) be removed from sample
Human serum see Burse et al. (1980) ECD 1 µg/litre Eyster
et al.
(1983)
Blood (in vitro see Roboz et al. (1982) MS- in vitro Roboz et al.
experimental) (PPINCI) (1985a)
Human blood see Roboz et al. (1982) ECD, SIM, 10-35 ng distribution of PBBs Roboz et al.
(model and NCI individual among blood components (1985b)
environmentally serum congener/litre
exposed)
b) Adipose and other tissues
Adipose tissue toluene/ethyl acetate GPC (Bio ECD 0.5 µg/kg major HxBB peak determined Wolff et al.
from exposed (1+3) Beads (1979a)
workers toluene/ethyl
acetate (1+3)
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Various rat Burse et al. (1980) ECD 10 µg/kg comparison of Miceli &
tissues and concentrations of PBBs Marks
serum in various tissues with (1981)
time
Liver and 1) hexane (liver and 1) Florisil ECD, NAA comparison of extraction Fawkes et al.
perirenal adipose) methods (showed PBB (1982)
adipose tissue extraction with hexane
from dosed rats leads to erratic recoveries
and results) increase in
detection limits over
ECD ( 2 pg FireMaster);
2) chloroform: 2) acidic 1 µg/litre or less of
methanol (liver) alumina hexa congener)
3) methylene chloride
chloroform (adipose)
Human adipose 15% diethyl ether Florisil/GPC ECD GPC clean-up tested (85% MacLeod
tissue in hexane MS recovery); MS free of et al.
serious interference (1982)
from 46 to 500 m/z
Adipose tissue 6% diethyl ether Florisil/GPC MS 1-2 µg/kg HxBB peak Lewis &
from general in hexane Sovocool
population (1982)
Human adipose hexane/diethylether silica gel ECD 1 µg/kg Eyster
tissue, placenta, et al.
cord blood, (1983)
biliary fluid,
faeces
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Human postmortem Chromaflex ECD 0.5 µg/kg HxBB peak Miceli
tissue adsorption et al.
column with (1985)
5% silica
gel + sodium
sulfate/
hexane
Adipose tissue hexane solid phase ECD 1-14 ng/kg Florisil Chiang et al.
(bovine), spiked Florisil cartridges cartridges to separate (1987)
for model system fat; 116% recovery
c) Milk
Human milk potassium oxalate, ECD 1 µg/kg Eyster et al.
ethanol/diethyl ether; (1983)
hexane
Human milk potassium oxalate, Bio Beads/ MS (NCI, 1 ng/kg separation of coplanar Krüger (1988)
ethanol/diethyl ether Florisil/ SIM) and planar isomers with
activated charcoal
charcoal
d) Biological samples from the environment
Fish, seal freeze, pulverize, Bio Beads/ MS (NCI, 10 ng/kg Krüger (1988)
pet. ether Florisil/ SIM)
activated
charcoal
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Dolphin fat/ Soxhlet; hexane, GPC; silica MS no data given lowest value given: Kuehl et al.
organ tissue methylene chloride gel 40 µg/kg (1991)
Terrestrial, diethyl ether/hexane hydrolysis MS (NCI) no data given lowest value given: Jansson
freshwater and with 98% 40 ng/kg et al.
marine samples H2SO4/Bio (1991, 1992)
Beads/ silica
gel/activated
charcoal
a Analytical method used was gas chromatography.
Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NCI = Negative ion chemical ionization mass spectrometry.
GPC = Gel permeation chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
MS = Mass spectrometry. SIM = Selected ion monitoring.
NAA = Neutron activation analysis.
Using negative ion chemical ionization mass spectrometry
(MS-NCI), the bromide ions can be used to detect brominated
compounds with high sensitivity and selectivity. However, using this
detection method (or ECD), interference between congeners of PBB and
polybrominated diphenyl ethers is possible.
The 209 possible PBB congeners have a wide range of volatility,
which causes very difficult separation problems (Farrell, 1980). In
earlier studies, gas chromatography (GC) with packed columns, e.g.,
3% OV-1 on 80/100 mesh Chromosorb W(HP) was used (Fehringer,
1975a,b). Capillary columns enable a good separation with lower
brominated biphenyls but do not have any great advantages over
packed columns for more highly substituted biphenyls (Farrell, 1980;
Orti et al., 1983; Robertson et al., 1984b).
The detection method most frequently used is that of pulsed
63Ni electron capture detection (ECD). In general, retention times
and electron capture responses increase with increasing bromination.
This is a sensitive method, but has some shortcomings. ECD is a
group selective detector that responds to halogens and other
electronegative groups. This places stringent requirements on
chromatographic separation. Moreover, ECD responds differently to
different compounds, depending on the molecular structure. The
response or sensitivity of the ECD depends on the position of the
halogen on the biphenyl nucleus as well as the number of halogens.
This necessitates running a standard for each compound to be
determined (Zerezghi et al., 1984). Sweetman & Boettner (1982)
analysed the structure-sensitivity of PBBs using ECD (see Table 12).
Flame ionisation detection (FID) can only be used for the
analysis of standard substances because of its low specificity
(Krüger, 1988).
A microwave-induced plasma emission detector has been used as a
specific method of detection for bromine (Mulligan et al., 1980;
Zerezghi et al., 1984). However, the method is not sensitive enough
for environmental samples.
Some authors have confirmed their results by GC/ECD
determination before, and after, exposure to UVR. The PBBs present
are photolyzed and, in this way, the background values can be
eliminated (Erney, 1975; Trotter, 1977).
Very often, the presence of PBBs is confirmed using mass
spectrometry (MS) together with gas chromatography. The purity of
the sample can be verified by comparison with known standards.
Negative chemical ionization (NCI) mass spectrometry has a
sensitivity comparable with, and somewhat better than, GC/ECD
analysis. The detection level for hexabromobiphenyl standards is
lower by a factor of 20 to 10-35 ng/ml in comparison with GC/ECD
analyses (Roboz et al., 1982). This relatively new method has also
been used to detect polychlorinated and polybrominated dioxins and
furans (see section 4.3).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
PBBs are not known to occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 World production figures
1) United States of America
The commercial production of PBBs in the USA commenced in 1970
(Neufeld et al., 1977). Several US producers of commercial
quantities have been identified (Mumma & Wallace, 1975; Neufeld
et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok, 1980).
In 1976, a US firm had a combined production of about 0.45
million kg of PBBs for export to Europe (Anon., 1977).
A list of suppliers of laboratory quantities (with a maximum
production or importation of about 2 kg/year) is presented by Mumma
& Wallace (1975) and Neufeld et al. (1977).
As a result of the Michigan catastrophe of mid-1973, the sole
US manufacturer of hexabromobiphenyl ceased production in November
1974. It is not clear, whether the production of bromine-based fire
retardants was resumed by another US company in 1978 (Brinkman & de
Kok, 1980). Two other companies continued their production of octa-
and deca-PBB until 1977 (Di Carlo et al., 1978). According to the
German "Umweltbundesamt" (UBA, 1989), decabromobiphenyl was produced
in the USA until 1979.
There are repeated statements that all PBBs manufactured in the
USA since 1975-76 have been exported, mainly to Europe, and that
there is no importation of any PBB mixtures into the USA (Brinkman &
de Kok, 1980).
Relevant production data for the period 1970-76 are presented
in Table 16.
Table 16. Commercial production of polybrominated biphenyls in the USA, 1970-76a
Estimated production in thousand kg
Product 1970 1971 1972 1973 1974 1975 1976 1970-76
Hexabromobiphenyl 9.5 84.2 1011 1770 2221 0 0 5369
Octabromobiphenyl 14.1 14.1 14.6 163 48 77.3 366 702
and decabromobiphenylb
Total PBBs 23.6 98.3 1025 1933 2269 77.3 366 6071
a From: Di Carlo et al. (1978).
b Manufacture was continued in 1977, but production figures are not available.
Hexabrominated biphenyl forms the major part (about
5.4 million kg FireMaster(R) BP-6 plus some 68 300 kg
FireMaster(R) FF-1) of the estimated total production of
6.1 million kg (Neufeld et al., 1977). The remaining 0.7 million kg
are accounted for by the higher brominated biphenyls. In 1976, for
example, 0.35 million kg of decabromobiphenyl and 13 600 kg of
octabromobiphenyl were manufactured (Neufeld et al., 1977). No
production figures are available for 1977 (Di Carlo et al., 1978).
2) Japan
According to IARC (1978), PBBs have never been produced in
Japan, but, up to 1978, some were imported.
3) Europe
a) Germany
A German firm produced a mixture of highly brominated PBBs,
called Bromkal 80-9D until mid-1985, when the activities concerning
bromine-based fire retardants were shifted to the USA. No production
figures are available.
b) France
A French firm manufactures a technical-grade decabromobiphenyl,
sold as Adine 0102, production being a few hundred thousand kg/year
(Atochem, 1988). It is marketed in France, Great Britain, Spain and
the Netherlands (Atochem, 1988; UBA, 1989). More than 200 tonnes
decabromobiphenyl/year were used in the Netherlands for
incorporation into polybutylenterephthalate plastics (UBA, 1989).
c) United Kingdom
Two companies are reported to have marketed or produced
technical-grade decabromobiphenyl in the United Kingdom (Brinkman &
de Kok, 1980). In 1977, the production of PBBs was discontinued
(Neufeld et al., 1977).
No production or sales data are available.
d) Netherlands
No domestic producer has been identified. An Israeli company
with two bromine plants in Holland denied the production of PBBs
(Neufeld et al., 1977). However, the amount of decabromobi phenyl
sold annually in the Netherlands was estimated to be of the order of
91 000 kg (Brinkman & de Kok, 1980).
No information is available on production in other parts of the
world.
3.2.1.2 Manufacturing processes
The process of manufacturing PBBs consists of a Friedel-Crafts
type reaction in which biphenyl is reacted with bromine in the
presence of chloride in an organic solvent, using aluminium
chloride, aluminium bromide, or iron as catalyst (Brinkman & de Kok,
1980). In the Atochem decabromobiphenyl manufacturing process,
biphenyl is directly brominated in a large excess of bromine, used
as reactant and solvent in the presence of a Lewis acid catalyst
(aluminium type). Decabromobiphenyl is further purified by
distillation of the excess bromine in the presence of a brominated
solvent (Atochem, 1992).
3.2.1.3 Loss into the environment during normal production
Data are published only for the USA. The following information
refers to reviews by Neufeld et al. (1977) and Di Carlo et al.
(1978).
Losses of PBBs to the environment at sites of its manufacture
can total 51 kg/1000 kg of product. These losses occur through:
1) Emission into the air
In 1977, the maximum air losses as particulate matter at
production sites were estimated to total 1.1 kg of PBBs/1000 kg
manufactured.
(a) Emission to the air from the vents of the hydrogen bromide
recovery system:
Total emission of FireMaster(R) PB-6 was estimated to amount
to 70 mg/1000 kg produced.
(b) Loss of particulate PBB to the atmosphere during centrifugation
(which was carried out to separate the solid reaction products
from the organic solvent).
A New Jersey permit application by Hexcel Corp. plant (1976)
indicated a loss of less than 0.05% of the product.
(c) Loss of dust from drying and pulverizing PBBs to a fine powder
(dust from this operation was removed by a bag type filter).
In 1974, atmospheric levels of PB-6 in the Michigan Chemical
Corp. bagger area were 16-32 mg/m3 during the bagging operation
and 3 mg/m3 after bagging was completed. Lower levels were
detected in other areas of the plant.
(d) Emission of hexabromobiphenyl as a vapour contaminant in
vapour streams leaving scrubbers or equivalent equipment was
calculated to be less than 25 µg/m3 (1 ppb) at ambient
temperature (Neufeld et al., 1977).
2) Losses in waste waters resulting from the quenching and
washing of the PBBs as they are recovered from the reaction mass:
The losses of PBBs to sewers at manufacturing sites were
estimated, in 1977, to be 4.6 µg/kg of product.
- In 1972, samples of the Michigan Chemical Corp. effluent
discharges were found to contain PBB levels of 98-503 µg/litre
(Hesse, 1975);
- The total quantity of PBBs being discharged to the Pine River
was estimated as 0.11 kg daily.
- Unfiltered water from an industrial storm sewer at the Hexcel
Corp. plant contained 92 µg/litre, mainly as decabromobiphenyl
(hexa-, octa-, and nonabromobiphenyls levels were also
measurable).
- Liquid effluents, diluted by canal water, from the White
Chemical Co. plant showed values of up to 31 µg PBBs/litre.
3) Solid losses to landfills resulting from drying, handling,
shipping and transportation.
An estimate of PBB losses as solid waste to landfills was
50 g/kg of product.
According to a report of the Michigan Chemical Corp., their
solid waste included approximately 5% of the BP-6 produced.
4) Losses to the soil
Soil samples from the bagging and loading areas of the Michigan
Chemical corp. contained PBBs at concentrations of 3500 and
2500 mg/kg, respectively.
Losses of other compounds:
The following typical air contaminants released during PBB-
manufacture were reported: hydrogen chloride, bromine, ethylene
dichloride, aluminium chloride, and biphenyls. The total quantity
emitted was stated to be less than 5.5 kg/day.
3.2.1.4 Methods of transport, accidental release, and disposal of
production wastes
Details of present-day labelling and transport regulations are
given in the Health and Safety Guide for PBBs (WHO, 1993).
In 1973, an accidental release of PBBs occurred in Michigan
("Michigan disaster"), when two products manufactured by the
Michigan Chemical Company were inadvertently confused, i.e.,
250-500 kg (Di Carlo et al., 1978) of FireMaster(R), instead of
NutriMaster(R), a magnesium oxide-based cattle feed supplement,
were added to animal feed and distributed to farms within the state.
The compound is believed to have been FireMaster(R) FF-1
(e.g., Fries, 1985b), even if in some publications the name
FireMaster(R) BP-6 is used (e.g., Neufeld et al., 1977; Di Carlo
et al., 1978). This accidental mix up resulted in widespread
contamination by PBBs (see section 5). As a result of this incident,
the production of FireMaster(R) BP-6 by Michigan Chemical Corp.
was stopped in 1974 (Di Carlo et al., 1978). Chronological reports
or reviews of the PBB disaster are given by Carter (1976), Getty
et al. (1977), Kay (1977), Di Carlo et al. (1978), Damstra et al.
(1982), Zabik (1982), and Fries (1985b).
Details of the disposal of manufacturing waste during present
production are not available. In a report by Neufeld et al. (1977),
solids from manufacturing operations were disposed of in landfills.
Waste waters containing small amounts of PBBs were discharged into
the chemical sewer.
3.2.2 Uses
Commercially manufactured PBBs are processed by industrial
users, primarily as flame retardants in polymeric materials. PBBs
were developed for this major application, because: they are able to
meet the flame-resistance performance requirements, they are
economically feasible, and they have little effect on the
flexibility of the base compounds (Mumma & Wallace, 1975).
The process of application is basically one of physical
blending: the PBBs are not functional additives, and on blending
with the dry solid or liquid polymeric material, provide filter-type
flame retardant action with the chemical release of hydrogen bromide
if ignited (Neufeld et al., 1977).
Neufeld et al. (1977) list 34 applications of PBBs found in
patent and technical literature. The majority are related to the use
of the PBBs as flame retardants in polymeric materials, other claims
include self-extinguishing properties and improved wearability and
machinability. Further potential uses of PBBs are: in the synthesis
of biphenyl esters or in a modified Wurtz-Fittig-synthesis; in
light sensitive compositions to act as colour activators; as
relative molecular mass control agents for polybutadiene; as wood
preservatives; as voltage stabilizing agents in electrical
insulation; as functional fluids, such as dielectric media (Neufeld
et al., 1977). In the USA and Canada, hexabromobiphenyl
(FireMaster(R)) was the principal PBB product. It was used as a
fire retardant in three main commercial products:
acrylonitrile-butadiene-styrene (ABS) plastics; coatings and
lacquers; and polyurethane foam (Neufeld et al., 1977).
The types of ABS plastic products in which FireMaster(R) BP-6
was used are compiled in Table 17.
According to Neufeld et al. (1977), the use of FireMaster(R)
BP-6 as a flame retardant in thermoplastic resins was confined to
products that do not come into contact with food or feed and are not
used in fabrics to which humans are exposed.
Although more than 130 companies in the USA used PBBs prior to
1976 (Di Carlo et al., 1978), only a limited number seems to have
been the major users of PBBs. For example, in 1974, the final year
of US production, Borg Warner Corp. (Parkersburg, W.Va.; using
FireMaster(R) in ABS plastics) and Standard T Chemical Co. (Staten
Island, New York; using FireMaster(R) in fire retardant coatings
for industry) consumed over 50% of the total US yearly production
(Mumma & Wallace, 1975; Jamieson, 1977; Neufeld et al., 1977;
Brinkman & de Kok, 1980).
Of the estimated 2200 tonnes hexabromobiphenyl produced in 1974
(IARC, 1978), about 900 tonnes (Mumma & Wallace, 1975; Neufeld
et al., 1977; IARC, 1978) were used in ABS plastic products and
about 34 000 tonnes (Mumma & Wallace, 1975; Neufeld et al., 1977;
IARC, 1978) in cable coatings.
The exact quantity of FireMaster(R) used in polyurethane foam
for automobile upholstery was not published. The two larger
consumers ceased using hexabromobiphenyl (one of these in 1972)
because PBBs did not decompose in the ultimate incineration of
scrapped automobiles (Neufeld et al., 1977).
No current users of hexabromobiphenyl have been identified
(Neufeld et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok,
1980). As regards octa- and decabromobiphenyl, no commercial use was
reported in the USA during 1970-74 (Neufeld et al., 1977). In
Western Europe, the use of higher brominated PBBs seems to be
dominant. The decabromobiphenyl Adine 0102(R) (in the past
manufactured by Ugine Kuhlmann, at present by Atochem) is used as a
flame retardant for thermoplastics and thermosets (e.g., in
polyesters, epoxy resins, polystyrene, ABS, polyolefines, and PVC),
for elastomers (e.g., in PU-elastomers and india rubber) and for
cellulosics (e.g., chip-board). It is applied frequently in
association with antimony trioxide (Sb2O3) (Atochem, 1984a). Its use
in paints and varnishes has also been reported (Brinkman & de Kok,
1980).
Table 17. Uses of FireMaster(R) BP-6 in ABS plastics in the USAa
Industry Approximate % Examples
of total use
Business machines and 48 Typewriter, calculator and microfilm-reader
industrial equipment housings; business machine housings
Electrical 35 Radio and TV parts, thermostats, shaver and
hand-tool housings
Fabricated products 12 Projector housings, movie equipment cases
Transportation 1 Miscellaneous small automotive parts;
electrical-wire connectors, switch
connectors, speaker grills
Miscellaneous 4 Small parts for electrical applications,
motor housings; components for industrial
equipment
a From: Brinkman & de Kok (1980).
Losses of PBBs to the environment from processing plants are
possible, but little information is available about this.
Although decabromobiphenyl and, possibly, other PBBs are still
produced commercially, alternative chemicals have been introduced to
replace them as flame retardants, in particular polybrominated
biphenyl ethers (oxides) (PBBO), e.g., decabromobiphenyl ether
(Adine 505; Bromkal 82-0 DE; Great Lakes DE-83TM and DE 83RTM),
octabromobiphenyl ether (Bromkal 79-8 DE; Great Lakes DE 79), and
pentabromobiphenyl ether (Bromkal 70-5 DE; Great Lakes DE-71TM:
Atochem, 1984b; Great Lakes Chemical Corp., 1986).
Decabromobiphenyl ether (DBBO) for example, appears to be a
much less toxic material than PBBs. However, DBBO is said to have a
tendency to degrade to lower brominated biphenyl oxides. It is
possible that these lower order compounds may pose environmental
problems similar to those of the lower brominated PBBs (Mumma &
Wallace, 1975). In addition, on pyrolysis, PBBOs produce larger
amounts of dioxins and furans than PBBs and so may themselves have
to be replaced by other compounds.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The commercial PBB-mixtures are solids at room temperature.
Despite their low vapour pressure, air pollution by PBBs can occur
as follows:
PBBs may be released into the atmosphere as vapour or dust
from production and processing plants. Stratton & Whitlock (1979)
found indirect evidence of airborne discharges of PBBs near two out
of three chosen industrial sites in north-eastern New Jersey and
Staten Island, New York, where these materials had been manufactured
or used in product formulations.
Further air contamination may occur during the incineration of
industrial and municipal wastes. Most municipal incinerators are not
very effective in destroying halogenated biphenyls. Like PCBs, PBBs
do not burn readily and incinerating conditions must be carefully
controlled, otherwise these compounds will reenter the environment
in the stack gases (Griffin & Chou, 1981a) or may be transformed to
polybro minated dibenzofurans.
Flameless combustion of the consumer products causes
volatilization of intact PBBs (Benbow & Cullis, 1975).
An appreciable loss of PBBs during the lifetime of PBB-
containing products is unlikely.
Secondary ways of entrance of PBBs into the atmosphere, e.g.,
through evaporation from contaminated soils, are thought to be
negligible, though small losses of PBBs from soil during long-term
(6 months) incubation studies were observed, which were associated
with volatilization rather than sorption or masking (Griffin & Chou,
1981a).
The ability of PBBs to co-distil from landfills or from the
surface layer of water bodies, as reported for PCBs (Kalmaz &
Kalmaz, 1979), has not yet been examined.
By analogy with PCBs, it might be expected that PBBs entering
the atmosphere in the vapour phase would be adsorbed rapidly onto
particles, which would then be deposited by particle sedimentation,
depending on micro- and macrometeorological conditions.
According to Eisenreich et al. (1981) organic compounds having
a vapour pressure > 10-5 kPa should exist almost entirely in
the vapour phase, and those having a vapour pressure > 10-9 kPa
should exist almost entirely in the particulate phase.
PBBs, e.g., hexabromobiphenyl or FireMaster with a vapour
pressure of 6.9 x 10-9 kPa (Jacobs et al., 1976), belong to the
latter. In reality, distribution and atmospheric lifetimes of
organic compounds with a high relative molecular mass depend largely
on the particle concentration and composition in the atmosphere
(Eisenreich et al., 1981). Gas-phase reactions with hydroxyl (OH)
radicals also influence the lifetimes of organic compounds emitted
into the atmosphere. Atkinson et al. (1984) determined rate
constants for the gas-phase reaction of OH radicals with biphenyl
and predicted, from their findings, that the chlorine - and
bromine-substituted biphenyls would have OH radical rate constants
of < 8 x 10-12 cm3/molecule per second at room temperature.
4.1.2 Water
The principal route of entrance of PBBs into aquatic
environments is from industrial waste streams into receiving waters.
Further potential routes (of minor relevance) are atmospheric
deposition and erosion of polluted soils. Groundwater contamination
is possible, if these compounds are leached from landfills (Shah,
1978).
Because compounds like PBB are very poorly soluble, they are
primarily found in sediments of polluted lakes and rivers
(Kimbrough, 1980a). In laboratory experiments, Simmons & Kotz (1982)
determined the "percent adsorption" of PBBs in sediments from sites
at Lake Michigan and the Huron River and concluded from values
ranging from 9 to 32% that the capacity of the sediments for PBB was
small to moderate.
PBBs in water are mainly adsorbed on particulate matter
followed by sedimentation at a rate that depends on several factors,
such as the size and type of the sediment and/or the organic
contents of both the sediment and the overlying water mass. The
relative importance of these parameters is controversial (Simmons &
Kotz, 1982). Laboratory results concerning PCBs (Jensen et al.,
1973) led to the assumption that the kinetics of the sorption
reaction may vary inversely with particle size (because smaller
particles have a larger surface area for surface adsorption).
Leland et al. (1973), Choi & Chen (1976), and Simmons et al. (1980)
have shown that the organic content of the sediment is directly
related to its adsorptive capacity for a specific contaminant.
According to Schwarzenbach & Westall (1981), adsorption of non-polar
compounds is highly correlated with the organic carbon content of
sorbents containing more than 0.1% organic carbon. Simmons & Kotz
(1982) found strong correlations between the adsorptive capacity of
the sediments for PBBs and TOC (total organic carbon) and the %
silt/clay fraction.
Sediments are potential sources as well as sinks for most
chemicals (Simmons & Kotz, 1982). Desorption of a contaminant from
the sediments is favoured where a high concentration of organic
matter exists in the water column (Huang, 1971). The presence of
organic matter may also enhance the partitioning of the contaminant
in the water phase and, thus, facilitate further movement with the
water mass (Hassett & Anderson, 1979). Laboratory studies on the
mode of action that PBBs may take in their movement through the
water column have verified that the total organic content of the
natural water will decrease the adsorption of PBB onto sediment and
therefore keep the PBB in the water phase. For example, comparing
the distilled water versus natural water systems, in river water
with an organic content of 11-12 mg C/litre, the % PBB-adsorption
was decreased by 33-43%; for lake water with an organic content of
3.8 mg/litre, the % PBB-adsorption was reduced by about 12% (Simmons
& Kotz, 1982). Another investigation indicated that the solubilities
of PBBs were directly correlated with the levels of dissolved
organics in the water (Griffin & Chou, 1981a) (see also section
4.1.3).
However, in the natural environment, upon settling out, the
association of the contaminant with the sediment may become the
dominant process in the water/sediment system (Simmons & Kotz,
1982). Transport of PBBs is thought to take place, when mixing or
bioturbation of sediments causes redistribution of the contaminant
in the water column (Simmons & Kotz, 1982), and through transport of
the sediment itself.
4.1.3 Soil
Pollution of soils can originate from point sources such as PBB
plant areas and waste dumps. Very few data are available on the
deposition of PBBs on soil via the atmosphere, sewage sludge from
municipal sewage treatment systems, and the dredging of sludge from
contaminated waters.
Other possible sources are illicit, or improper, disposal of
such chemicals (Kimbrough, 1980a) and incidents. For example, as a
consequence of an incident in 1973, Michigan soils have been
contaminated by manure from PBB-fed animals and by the disposal of
contaminated feed, milk, carcasses, etc. (Getty et al., 1977; Chou
et al., 1978; Damstra et al., 1982; Fries, 1985b). Once PBBs have
been introduced into the soil, they appear to have little tendency
to translocate (Damstra et al., 1982).
The ability of rainfall to carry PBBs through the soil was
tested in a laboratory simulation. Filonow et al. (1976) percolated
water through columns of 4 Michigan soils containing 100 mg
2,2',4,4',5,5'-hexabromobiphenyl/kg. They found a loss of less than
0.6% of the hexabromobiphenyl congener from each soil, even with
leachate quantities equivalent to 20 times the average annual
rainfall in Michigan.
Field investigations also indicated that PBBs were retained in
the top soil. Results of subsequent studies on highly contaminated
farm soils showed that PBBs did not move below the 15 cm level,
except where there was a history of physical mixing of the soil
(Fries, 1985b).
The mobility in soils of a chemical like PBBs will largely be
governed by its solubility in water and its adsorption, or
interaction, with soil particles (Jacobs et al., 1978).
As already mentioned, PBBs have a very low solubility in water.
However, studies with distilled, tap, river, and soil waters showed
that their solubility was markedly influenced by water purity
(Jacobs et al., 1978). Griffin & Chou (1981a) ascertained, under
well defined conditions, the following average solubilities of PBBs:
0.06 µg/litre in distilled water, 0.3 µg/litre in deionized water,
0.5 µg/litre in creek water, 8.9 µg/litre in Du Page leachate, and
16.9 µg/litre in Blackwell leachate.
Hence, PBBs were more than 200 times more soluble in landfill
leachate than in distilled water; the solubilities of PBBs were also
higher in creek water than in distilled water. As shown by the TOC
(total organic carbon) values for the waters, the higher
solubilities of PBBs were directly correlated with the level of
dissolved organic compounds in the waters. The type of dissolved
organic matter may also influence the solubility (Griffin & Chou,
1981a).
PBBs are quite soluble in organic solvents, such as dioxane,
carbon tetrachloride, acetone, and methanol. This could play a major
role in soil environments where leachates from chemical waste
disposal sites are percolating.
The other important factor affecting the migration of PBBs,
i.e., their adsorption by soils, was also studied under laboratory
conditions. The hydrophobic properties of PBBs make them easily
adsorbed from aqueous solutions onto soils. Filonow et al. (1976)
examined the adsorption of purified 2,2',4,4',5,5'-hexabromobi
phenyl (BB153) on four soil types. They found that the adsorption of
2,2',4,4',5,5'-hexabromobiphenyl conformed well to Freundlich
adsorption isotherms, and that 2-19% of the available HBB was
adsorbed. Adsorption of HBB was influenced primarily by the organic
content of the soils. An increase in the organic matter content of
soils enhanced their adsorption capacity.
Neither percentage clay nor pH correlated well with BB153
adsorption. Any effect that the clay contents may have had, was
apparently masked by the effect of the organic contents on
adsorption (Filonow et al., 1976).
Griffin & Chou (1981a,b), using the PBB-mixture FireMaster(R)
BP-6 or 14C-labelled-PBB, also confirmed the strong adsorption of
PBBs on soils and indicated a very high direct correlation between
the total organic carbon content (TOC) of three different soils and
the amounts of PBBs adsorbed. However, they pointed out that, in
soils with a low TOC, the mineral fraction may contribute markedly
to the adsorption capacity.
Furthermore, preferential adsorption of PBB congeners and
isomers was noted, depending on the characteristics of the
adsorbent, e.g., organic content (Griffin & Chou, 1981a), as well as
on the degree and position of bromine substitution (Griffin & Chou,
1980, 1981b).
No measurable adsorption on soils occurred of PBBs from organic
solvents (Griffin & Chou, 1981a).
The results of migration studies were in agreement with the
findings discussed above. The mobility of PBBs in five soils was
measured with several leaching solvents, using a thin-layer
chromatography technique and column leaching studies (Griffin &
Chou, 1981a,b). PBBs remained immobile in the soils when leached
with water or landfill leachate, but were highly mobile when leached
with organic solvents. Mobility was directly proportional to the
solubility in the leaching solvents and inversely proportional to
the soil total organic content.
On the other hand, because PBBs are bound to soil, wherever
contaminated soil moves, whether through wind or water erosion or
animal ingestion and migration, traces of PBBs (if present) can be
expected to be found (Jacobs et al., 1978).
4.1.4 Biota
PBBs are stable and persistent, lipophilic, and only very
slightly soluble in water; they are poorly metabolized, and
therefore accumulate in lipid compartments of biota. Once they have
been released into the environment they will reach the food chain,
where they are concentrated. Fish and wildlife are the most
consistent targets for such contamination, but livestock and humans
may also become contaminated (Kimbrough, 1980a). The precise routes
and transport mechanisms of PBBs travelling through biota have not
been thoroughly investigated as pointed out below.
4.1.4.1 Terrestrial ecosystems
Several studies have been concerned with whether plants in
terrestrial ecosystems would take up, translocate, and introduce
PBBs into the food chain. Jacobs et al. (1976) selected orchard
grass (Dactylus glomerata) as test plants in their greenhouse
studies because of its extensive root mass, and carrots (Daucus
carota), which, according to Iwata et al. (1974), have an
outstanding ability to absorb pesticide residues from the soil. They
did not detect any PBBs in the tops of either species grown in soils
supplied with high levels of PBBs (10 or 100 mg/kg of
FireMaster(R) BP-6). However, they did find traces of PBBs
(20-40 µg/kg) associated with carrot roots. 14C-uptake studies
(autoradiography and GC-analysis) on corn and soybean seedlings
grown in hydroponic solutions and on three root crops (radishes,
carrots, and onions) grown in two different soils, also showed no
translocation of PBBs into plant tops (Chou et al., 1978). In
addition, these authors found that the amount of PBBs associated
with roots depended on plant species and the clay and organic matter
contents of the soil. Roots of carrots contained more PBBs than
those of radish or onion bulbs; all roots had higher levels of PBBs
(50-500 µg/kg tissue) when grown in a high-PBB treatment soil
(100 mg/kg) with lower clay and organic content, than they did
(30-120 ng/g plant tissue) in a soil containing more clay and
organic matter. Furthermore, PBBs seem to be localized on the
surfaces of roots, because a significant portion of 14C-PBBs was
removed, when the roots were dipped in acetone.
Analyses of field samples from plant tissues of corn, alfalfa,
and sudax, grown on Michigan fields with soil PBB levels ranging
from 9 to 371 µg/kg, resulted in no detectable (detection limit:
0.3 µg/kg) PBB (Jacobs et al., 1978). The same was true for washed
radishes from a garden with an estimated PBB concen tration of
500-1000 µg/kg and for corn leaf whorls containing dust from a PBB
contaminated soil (102 µg/kg) (Chou et al., 1978).
However, Stratton & Whitlock (1979), who conducted a field
screening survey near sites of manufacture and use of PBBs, found
high surface contamination of lichens and reeds.
The salt marsh cordgrass (Spartina alterniflora) is reported
to take up, accumulate, and transfer effectively PCB from
contaminated sediments to food chains (Mrozek et al., 1982). No data
are available with regard to PBBs.
So far, except for the surface contamination of roots from
contaminated soils and of foliage via air deposition processes,
plants are generally free of significant amounts of residue. Thus,
vegetation on PBB-contaminated soils is a less likely source of
contamination of animals (Damstra et al., 1982; Fries, 1985a,b).
In contrast, a major route of residue transmission from soils
to animals is the direct ingestion of soil (Fries 1982; 1985a). The
degree of contamination depends on the amount of soil ingested and
the bioavailability of the residues.
Quantitative data on soil ingestion by farm animals are given
by several authors (Healy et al., 1967; Healy, 1968; Fries, 1982;
Fries et al., 1982a,b) and range from 2 to 15% of the intake of dry
matter.
Fries (1985a) determined the bioavailability of soil-borne PBBs
in sheep, under controlled feeding conditions, using diets
containing 5% PBB-contaminated soil, and found 65% PBB absorption
from this diet, which contained 9 µg PBB/kg. Addition of activated
carbon to soil had only little effect on bioavailability of PBB.
The same author recorded PBBs in the fat of beef cows, beef
calves, ewes, and pigs from several farms on which soil-borne PBBs
in confinement areas was the only source of PBBs. It can also be
concluded from these results that the animals consumed soil, and
that soil-borne PBB was bioavailable. As might be expected, pigs
accumulated higher PBB concentrations from a soil environment than
ruminants (Fries, 1985a). Recontamination of soil by animal excreta
(Getty et al., 1977; Fries, 1985a) or carcasses (Shah, 1978) also
occurred.
Recently, PBBs have been detected in European herbivorous
mammals (Swedish reindeers: Jansson et al., 1992; German cows
(milk): Krüger, 1988) (see also sections 5.1.4 and 5.1.6).
Despite the affinity of PBBs for soil, there are no
investigations on the role of the soil fauna in the transfer of
PBBs. Earthworms are of great ecological importance and might be
expected to take up and accumulate PBBs as has been ascertained for
PCBs (Diercxsens et al., 1985) and, thus, introduce them into the
food chain.
4.1.4.2 Aquatic ecosystems
PBBs enter the aquatic food chains via water and food. Bacteria
and plankton play an important role in the accumulation and
translocation of PCBs to higher trophic levels (Kalmaz & Kalmaz,
1979; Lorenz & Neumeier, 1983). According to Falkner & Simonis
(1982), sorption processes probably control uptake and accumulation
of PCBs by phytoplankton, because of its high surface-volume ratio.
These mechanisms could also be valid for PBBs. However, Stratton &
Whitlock (1979) did not find PBBs in algae collected in the vicinity
of industrial sites, where PBB concentrations of sediments ranged
from 20 to 60 µg/kg and where captured fish contained 220-230 µg
PBB/kg (detection limit: not given).
No information on the uptake of PBBs from sediment through
bottom living organisms (e.g., mollusca or oligochaete worms) is
available.
In contrast, several laboratory (Norris et al., 1973; Zitko &
Hutzinger, 1976; Zitko, 1977; Sugiura et al., 1978) and field (Hesse
& Powers, 1978; Stratton & Whitlock, 1979; Jaffe et al., 1985)
studies on fish have been conducted. They confirm PBB uptake from
water and food, with the exception of hepta- and octabromobiphenyl
(Norris et al., 1973; Zitko, 1977), which were not taken up from
water.
Consequently, ingestion of fish is a source of PBB transfer to
mammals and birds. Because of the possible selective accumulation
and metabolism of PBB congeners in prey, it can be expected that
predators will be subjected to a somewhat different PBB congener
composition than that found in the surrounding media (sediment,
water, etc.).
In natural situations, food chains become linked together in
complex food webs, and PBBs are distributed in the corresponding
manner.
PBBs have been detected in other species of wildlife besides
fish, e.g., in ducks living near contaminated waters (Hesse &
Powers, 1978), in a turtle (Stratton & Whitlock, 1979), in the eggs
of waterbirds (Haseltine et al., 1981; Heinz et al., 1983, 1985), in
eagles (Kaiser et al., 1980), and in marine mammals (Jansson et al.,
1987, 1992; Krüger, 1988; Kuehl et al., 1991) (see also section
5.1.6).
4.1.4.3 Accidental contamination of the food chain
A special case of entrance of PBBs into the food chain occurred
accidentally in 1973 in Michigan, when FireMaster(R) FF-1 was
inadvertently substituted for magnesium oxide as a supplement in the
formulation of cattle feed (Damstra et al., 1982). Ten to twenty
bags, 22.8 kg each, of PBBs (Carter, 1976) were mixed into feeds,
that were widely distributed to Michigan farmers.
In addition, feeds not formulated to contain magnesium oxide
also became contaminated (with relatively low concentrations)
because of carryover of PBBs from batch to batch in the mixing
equipment (Dunckel, 1975) and, on farms, through the recycling of
contaminated products (Kay, 1977). Distribution of contaminated
antibiotics, e.g., aureomycin, also contributed to the introduction
of PBBs into farm animals (Di Carlo et al., 1978).
The mixing error was not discovered immediately, and it was
almost a year before analyses indicated that a compound of PBB was
involved in the illness or death of farm animals (Getty et al.,
1977). During this time (IARC, 1978; Zabik, 1982), contaminated
animals and their produce entered the human food supply and the
environment of the state of Michigan. Hundreds of farms were
affected. Altogether, at least 29 800 cattle, 5920 pigs, 1470 sheep,
and 1.5 million chickens had been killed and buried by the end of
1975 (Robertson & Chynoweth, 1975; Carter, 1976), in order to
minimize further human exposure. In addition, at least
785 thousand kg of feed, 8185 kg of cheese, 1197 kg of butter,
15 500 kg of dried milk products, and nearly 5 million eggs were
destroyed (Carter, 1976). The number of animals quarantined or
contaminated below quarantine level was estimated to be several
thousands (Isleib & Whitehead, 1975). Although the Michigan PBB
episode was primarily an incident of feed contamination, it also
resulted in secondary contamination of animals from contaminated
soil (Fries, 1985a).
4.2 Degradation
Compounds like PBBs are very stable to hydrolysis, chemical
oxidation, and thermal decomposition. Degradation by purely abiotic
chemical reactions (excluding photochemical reactions) is therefore
considered an unlikely environmental sink (Pomerantz et al., 1978;
Pearson, 1982).
The persistence of PBBs under actual field conditions is
reported in some publications. Jacobs et al. (1976) detected PBBs in
soils from a field that had received manure from a
FireMaster(R)-contaminated dairy herd 10 months earlier.
Follow-up surveys over a three-year period following the
termination of PBB production showed no significant decline in PBB
levels in sediments from the Pine River (Hesse & Powers, 1978). Soil
samples from the former PBB-manufacturing site in St. Louis,
Michigan, analysed several years (nearly ten years?) after
contamination (during the early 1970s) still contained PBBs.
However, the PBB congener composition differed from that of the
original FireMaster(R) mixture, indicating a partial degradation
of the PBB residue in the soil sample (Hill et al., 1982).
The chemical Inspection and Testing Institute, Japan (1987) has
listed decabromobiphenyl as non-biodegradable.
The most probable degradation mechanisms of PBBs in the
environment, if there is any degradation at all, are
photodecomposition and microbial degradation.
4.2.1 Photolytic degradation
Under laboratory conditions, PBBs were easily degraded by UVR.
The photoreactivity of PBBs has been used to confirm PBB residues
(Erney, 1975; Trotter, 1977). The predominant photochemical reaction
of PBBs in organic solvents was a reductive debromination.
Irradiation of 4-monobromobiphenyl at 300 nm in various polar and
nonpolar solvents led to the formation of biphenyl as the sole
product (Freeman et al., 1991). Earlier studies using lower
brominated PBB congeners (i.e., tetra and lower) reported a
preferential loss of ortho bromines (Bunce et al., 1975; Ruzo
et al., 1976). Irradiation of higher brominated congeners yielded a
series of photoproducts (Table 18), but a stepwise cleavage of
orthobromines did not appear to be preferred to meta or para
debromination (Patterson et al., 1980; Millis & Aust, 1985).
The photoreactivity of 2,2',4,4',5,5'-hexabromobiphenyl, the
main component of FireMaster(R), was consistently found to be
relatively high (Andersson et al., 1975; Ruzo et al., 1976;
Robertson et al., 1983a; Millis & Aust, 1985), and degradation
occurred more rapid than with the hexachloro analogue (Andersson
et al., 1975; Ruzo & Zabik, 1975).
Consistent with the dehalogenation pathway, photodegradation of
the commercial FireMaster(R) mixture led to reduced concentrations
of the more highly substituted PBB congeners (De Kok et al., 1977;
Robertson et al., 1981b, 1983; Epling et al., 1987). Robertson
et al. (1983a) examined changes in the composition of
FireMaster(R) BP-6 during photolysis (300 nm for 2-12 h; solvent:
cyclohexane) by monitoring 25 individual PBB congeners; they also
did not find a preferential loss of ortho bromines. Nevertheless,
the photoproducts of FireMaster(R) did contain increased
concentrations of congeners possessing no ortho bromines (e.g.,
3,4,4'-tri-, 3,3',4,4'-tetra-, 3,3',4,4',5-penta bromobiphenyl).
Moreover, other congeners, known as relatively toxic (e.g.,
2,3',4,4',5-pentabromobiphenyl), were enriched (Robertson et al.,
1983). Biphenyl, the ultimate product of the debromination pathway,
was found only to a small extent after the photolysis of
FireMaster(R) BP-6 (Epling et al., 1987).
Table 18. Photodegradation of higher brominated PBB congeners under laboratory conditions
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,5,5'- 254 nm hexane 43.4a 2,3',4',5-tetra ortho-debromination Millis & Aust
penta (up to 100 (minor product) (1985)
min) 2,2',4,5'-tetra meta-debromination
2,2',5,5'-tetra para-debromination
(major product)
(additional production
of a yellow gum)
2,3',4,4',5- 254 nm hexane 50a 2,3',4',5-tetra para-debromination Millis & Aust
penta (up to 90 3,3',4',4'-tetra ortho-debromination (1985)
min)
2,2',4,4',5,5'- 366 nm methanol not specified lower brominated PBBs degradation (90% after 9 Andersson et al.
hexa (main products) min) more rapid than with (1975)
BB 153 methoxy- PBBs (minor the hexachloro analogue
products)
> 300 nm hexane not specified lower brominated PBBs BB 153 was 24.4 times Ruzo et al.
(0.5-2 h) quaterphenyls (< 5%) more reactive than (1976)
4,4'-dibromobiphenyl
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5,5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
Table 18 (contd).
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5.5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
secondary photoproduct:
3,3',4,4'-tetra
formation of yellow
gum at 25 min
2,2',3,4,4',5,5'- sunlight not not 2,2',4,4',5,5'-hexa meta-debromination Patterson
hepta (390 min) specified specified (major product) et al. (1980)
2,3',4,4',5,5'-hexa ortho-debromination
2,2',3,3',4,4', sunlight not not unidentified hexa- Patterson
5,5'-octa (300 min) specified specified PBB (major product) ortho- and meta- et al. (1980)
2,3',4,4',5,5'-hexa debromination
2,2',3,3',5,5', 300 nm hexane not specified di- to Ruzo et al.
6,6'-octa (0.5-2 h) heptabromobiphenyls, ortho debromination (1976)
e.g., 3,3',5,5'-tetra
a Original PBB concentration = 1.59 mmol/litre.
Technical octabromobiphenyl has been reported to photo degrade
in xylene by reductive debromination with a half-life of 40 h
(Norris et al., 1973).
There were investigations to enhance the photochemical process
aiming at a potential technique for the breakdown and removal of
PBBs from the environment. In laboratory testing, photodegra dation
of PBBs was accelerated in the presence of ethylenediamine and
tertbutylamine (Christensen & Weimer, 1979) and in the presence of
sodium borohydride (Epling et al., 1987).
Epling et al. (1987) obtained high yields of biphenyl during
borohydride enhanced photolysis of FireMaster(R) BP-6 (irradiation
under nitrogen at 254 nm; solvent: 90% acetonitrile/water).
The rates and extent of photolytic reactions of PBBs in the
environment have not been determined in detail. However, the few
field observations available indicate a high persistence of the
original PBBs (Jacobs et al., 1978) or a partial degradation to less
brominated (and often more toxic) photoproducts (Hill et al., 1982).
Jacobs et al. (1978) examined field soil that had received manure
from FireMaster(R)-contaminated cattle, for the first time, 2-3
years earlier. They did not detect any significant changes in the
relative concentrations of the major PBB peaks (Br5, Br6, Br7)
compared with the FireMaster(R) standard. In contrast, soil
samples, obtained from the former FireMaster(R) manufacturing site
in Michigan and analysed several years (approximately 10 years?)
after contamination, contained enhanced concentrations of possible
photodegradation products including 2,3',4,4',5-pentabromobiphenyl,
2,2',4,4',5-pentabromobiphenyl, and two unidentified
tetrabromobiphenyls (Hill et al., 1982).
Considering the diversity of microenvironments, both laboratory
and field data on photo alteration of PBBs are incomplete; there is
a lack of studies on the photochemistry of PBBs in water, or in the
vapour or solid states.
4.2.2 Microbial degradation
In laboratory investigations, mixtures of PBBs appear to be
fairly resistant to microbial degradation. Soil incubation studies
using FireMaster(R) BP-6 (lot no. 6244A) and 14C-PBB
(lot 872-244) showed a little, but not significant, degradation of
the major hexa- and heptabromobiphenyl congeners after 6 months or
1 year; only pentabromobiphenyl was assumed to degrade slowly
(Jacobs et al., 1976, 1978). These results were deduced from
recovery rates of PBBs from soil, 14CO2 production, and the lack of
14C-PBB intermediates.
Soils incubated with photodecomposition products of 14C-hexa
and heptabromobiphenyl caused enhanced, but still minor, degradation
(ca. 3%) as measured by 14CO2 production (Jacobs et al., 1978).
These findings are consistent with observations according to which
degradation of PCBs by bacteria increases with decreasing
chlorination (Kalmaz & Kalmaz, 1979; Fries, 1982).
In further incubation experiments with FireMaster(R) BP-6
(lot no. 6244A) in sterilized and nonsterilized Catlin-soil, Griffin
& Chou (1981a) measured the recoveries of penta-, hexa-, and
heptabromobiphenyls and found that all PBBs persisted for 6 months
with no significant microbial degradation. They observed the same
kind of persistence over a period of 4 weeks in PBB incubations with
mixed cultures of microorganisms (predominantly Alkaligenes
odorans, A. denitrificans, and an unidentified bacterium). This
culture had been isolated previously and was known to degrade
water-soluble PCBs (Clark et al., 1979). No PBB metabolites were
found in the PBB-saturated mineral solution after 4 weeks of
incubation (Griffin & Chou, 1981a).
As with PCBs, the high degree (penta or greater) of halogen
substitution of its major components probably accounts for the lack
of degradation of the FireMaster(R)-mixture (Griffin & Chou,
1981a). Congruently, biodegradation of monobrominated biphenyls has
recently been reported.
A soil isolate, strain S93B1, identified as Pseudomonas
cruciviae, could grow on more than ten biphenyl-related compounds
including o-bromobiphenyl (Takase et al., 1986). O-bromobiphenyl
was converted to o-bromobenzoic acid (Fig. 3) (identified by
IR-spectrum). This is analogous with some PCBs showing chlorinated
benzoates as metabolites (Ballschmiter et al., 1977). In these
experiments, biphenyl-related compounds 0.2-0.5% (w/v) were added as
the sole sources of carbon to the liquid artificial medium.
However, this pathway is also realized under simulated natural
conditions (aquatic environments), as reported by Kong & Sayler
(1983). They used river water as supportive culture medium and
"mixed bacterial cultures" (not identified), which were obtained
from PCB-contaminated river sediments. This mixed bacterial culture
was capable of degrading monohalogenated biphenyls.
Polybrominated benzenes and a possible methylbrominated furan
have also been reported to occur in FireMaster(R) (Brinkman & de
Kok, 1980).
Approximately 20 compounds, other than PBBs, were either
tentatively identified in FireMaster(R) or partially characterized
by Hass et al. (1978).
Polybromodibenzo- p-dioxins and polybromodibenzofurans were
searched for, because of their extreme toxicity and because
chlorinated dibenzofurans had been detected in commercial PCBs
(Nagayama et al., 1976). If present, their concentrations did not
exceed 0.5 mg/kg (Hass et al., 1978, O'Keefe, 1979). Polybromo
dibenzodioxins and polybromodibenzofurans were determined in a
sample of Adine 0102 (decabromobiphenyl). Monobromobenzo difurans
were present at a level of 1 mg/kg (1 ppm), otherwise all other
polybromodibenzodioxins and polybromodibenzofurans were present only
at less than 0.01 mg/kg (Atochem, 1990).
So far, phenoxyphenols and hydroxybiphenyls, which might be
intermediates in the formation of brominated dibenzo- p-dioxins and
brominated dibenzofurans, respectively, have not been identified
(O'Keefe, 1979).
Some impurities in PBBs result from impurities in the original
biphenyl material. According to two major manufacturers, their
biphenyl grade used for bromination contained less than 5 mg/kg and
5000 mg/kg, respectively, of impurities, e.g., toluene, naphthalene,
methylene biphenyl (fluorene), and various methyl biphenyls (Neufeld
et al., 1977).
2.2 Physical and chemical properties
In general, PBBs show an unusual chemical stability and
resistance to breakdown by acids, bases, heat, and reducing and
oxidizing agents (Safe, 1984).
PBBs can be compared chemically to the PCBs. Bromine, however,
is a better leaving group in chemical reactions than chlorine.
Unlike PCBs, the reactivity of PBBs has not been well studied and
documented in the literature (Pomerantz et al., 1978). Like PCBs
their chemical stability is dependent, in part, on the degree of
bromination and the specific substitution patterns (Safe, 1984). All
highly brominated PBB-mixtures are known to degrade rather rapidly
with UV irradiation (Brinkman & de Kok, 1980).
The technical mixtures typically are white, off-white, or beige
powdered solids. Some physical data on commercial PBB mixtures are
given in Table 9. It can be seen that there are discrepancies in the
values for the solubility of commercial PBBs in water (given in
Table 9) as well as those calculated for various PBB congeners
(Table 10). The source and quality of the water is important.
Determinations of water solubility of these very hydrophobic
compounds are also difficult to perform. Adsorption effects on
particles and glass surfaces may influence the results. PBBs were
found to be 200 times more soluble in landfill leachate than in
distilled water (Griffin & Chou, 1981a). In general, it can be said
that PBBs are only slightly soluble in water and that the solubility
decreases with increasing bromination.
For details of thermal decomposition, see section 4.3.2.
2.2.1 Physical and chemical properties of individual congeners
PBBs show a wide range of volatility (Farrell, 1980). Partition
coefficients between water/ n-hexane and water/1-octanol, as well
as aqueous solubilities for some individual PBB congeners are given
in Table 10. Correlations for predicting aqueous solubility and
partition coefficients for PBBs based on molecular structure have
been proposed (Patil, 1991). The solubility of PBBs in n-hexane
decreases rapidly with increasing bromine content (de Kok et al.,
1977).
Data on the melting points and UV absorption of individual PBB
congeners are summarized in Table 11. The main band in these spectra
is caused by pi -> pi* electron transitions, while the k band is
generally attributed to the conjugated biphenyl system with the
contribution of both biphenyl rings. With the k band, the
introduction of bromine atoms in positions meta or para to the
phenyl-phenyl bond induces a shift in kmax towards the visible
region, as is illustrated by 3,3',5,5'-tetra- and 3,3',4,4',5,5'-
hexabromobiphenyl. On the other hand, ortho substitution, which
causes a considerable hindrance for free rotation of the rings and,
thus, a loss in coplanarity, effects a sharp decrease in the
extinction coefficient of the k band (de Kok et al., 1977).
Data on NMR spectra are given by Orti et al. (1983), Robertson
et al. (1984b), and Kubiczak et al. (1989), and on mass spectrometry
(MS) by Erickson et al. (1980), Roboz et al. (1980), Buser (1986),
and Sovocool et al. (1987a,b). The "ortho" effect, observed for PBBs
and PCBs having 2,2'-; 2,2',6- or 2,2',6,6'- halogens can be
combined with GC retention index for isomer specific identifications
by gas chromatography and mass spectrometry (GC/MS) (Sovocool
et al., 1987a).
Table 9. Some physical data on commercial PBB mixturesa
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Melting point (°C) 72 200-250 220-290 380-386b
360-380
385
Lambda max (nm) 219e 225e 224e 227e
Density (g/cm3) at 2.6 - 3.2 3.2
room temperature
Solubility in water 11f 20-30 < 30
(µg/litre) at 25 °C 30f (pure 2,2', 4,4', 5,5'-)
610f
0.06g (deionized)
0.32g (distilled) insoluble
Solubility in organic
solvents (g/kg
solvent) at 28 °C
petroleum ether 20 18 insoluble in common
acetone 60 organic solvents
carbon tetrachloride 300 10e
chloroform 400
benzene 750 81
toluene 970
dioxane 1150
copra oil (37 °C) 0.8
Table 9 (contd).
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Vapour pressure (Pa)
25 °C 0.000007h < 0.000006
90 °C 0.01 (temperature not given)
140 °C 1
220 °C 100
Volatility (% weight loss) < 1% at 250 °C 1-2% at 300 °C < 5% at 341 °C
< 10% at 330 °C < 10% at 363 °C
< 50% at 350 °C < 25% at 388 °C
log Pow < 7 (calculated)d 8.6 (calculated)
Decomposition temperature 300-400 °C 435 °C 435 °C 395 °C
> 400° C
a Mumma & Wallace 1975).
b Norris et al. (1973).
c Kerscher (1979); CFK (1982).
d Atochem (1990).
e Brinkman & de Kok (1980).
f Filonow et al. (1976).
g Griffin & Chou (1981a,b).
h Jacobs et al. (1976).
Table 10. Partition coefficients between water and n-hexane (KHW) and 1-octanol
(Kow) and aqueous solubilities (Sw) for some individual PBB congeners (all
aqueous solubility measurements were carried out by the generator method)
Log KHW Log KOW Sw mol per Sw µg/litred
litre x 10-9 (25 °C)
(25 °C)
2-bromobiphenyl 4.59a
3-bromobiphenyl 4.85a
4-bromobiphenyl 4.96a 2800 650
3,5-dibromobiphenyl 5.78c
4,4'-dibromobiphneyl 5.61 5.72 18.4 5.7
2,4,6-tribromobiphenyl 6.21 6.03 41.1 16
3,4',5-tribromobiphenyl 6.42c
2,2',5,5',tetrabromobiphenyl 6.72 6.50 8.6 4
3,3',5,5'-tetrabromobiphenyl 7.42c
2,2',4,5,5'-pentabromobiphenyl 7.10a 0.8b 0.4
2,2',4,4',6,6'-hexabromobiphenyl 7.52 7.20 0.9 0.56
decabromobiphenyl 8.58a
Values from Gobas et al. (1988) with the exception of:
a From: Doucette & Andren (1987).
b From: Doucette & Andren (1988).
c From: Sugiura et al. (1978).
d calculated.
Table 11. Melting points and UV spectral data for some PBB congenersa
UV conditions: solutions in n-hexane; Beckman Acta CIII spectrometer
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
Biphenyl 71 201 4.66 246 4.26
1 2- e 201 4.51 240 3.90
2 3- e 205 4.60 248 4.21
3 4- e 200 4.67 254 4.38
4 2,2'- 81 198 4.64 220-230 e
9 2,5- e 203 4.49 226 4.38
15 4,4'- 164 201 4.64 261 4.43
20 2,4,6- 65-66 213 4.70 220-230 e
21 2,2',5- 78 200 4.66 235-245 e
26 2,3',5- e 213 4.57 e e
31 2,4',5- 78 205 4.60 245-255 e
49 2,2',4,5'- 84 207 4.66 235-245 e
52 2,2',5,5'- 143 204 4.67 235-240 e
80 3,3',5,5'- 188 220 4.76 255 4.18
114b 2,3,4,4',5- 128 222.6 (54.8) 258 e
137b 2,2',3,4,4',5- 124 223.1 (35.4) e e
141b 2,2',3,4,5,5'- 127 223.4 (191) e e
153 2,2',4,4',5,5'- (159-160)f 216 4.66 e e
156b 2,3,3',4,4',5- 178 224.9 (229) 259 e
159b 2,3,3',4,5,5'- 195 226.1 (61.4) 258 e
167 2,3',4,4',5,5'- (165-166)f e e e e
Table 11 (contd).
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
169 3,3',4,4',5,5'- 248 227 4.76 272 4.34
180b 2,2',3,4,4',5,5'- 166(165-166)f 224.1 (62.4) e e
189b 2,3,3',4,4',5,5'- 219 230.7 (102) 265 e
194b 2,2',3,3',4,4',5,5'- 235(232-233)f 223.7 (51.6) e e
202 2,2',3,3',5,5',6,6'- e 224 4.85 e e
206b 2,2',3,3',4,4',5,5',6- 262(263-264)f,g 225.2 (131) e e
Nona-(unidentified) e 225 5.18 e e
Deca- 378 227 5.11 e e
a Adapted from: de Kok et al. (1977), with the exception of the congeners marked with b.
b Congener data, including melting points are taken from Kubiczak et al. (1989). UV measurements: in n-heptane.
c No. according to Ballschmiter & Zell (1980).
d Melting points from Sundström et al. (1976b) but confirmed by de Kok et al. (1977), unless otherwise stated.
e No data available.
f From: Moore & Aust (1978).
2.3 Conversion factors for PBB in air
1 ppm = 26.1 mg/m3 for hexabromobiphenyl at 20 °C and
101.3 kPa.
1 mg/m3 = 0.038 ppm.
2.4 Analytical methods
Analytical methods for the determination of PBBs, which have
been reviewed by de Kok et al. (1977), Pomerantz et al. (1978), and
Fries (1985b), were adapted from established methods for chlorinated
hydrocarbon insecticides and PCBs (AOAC, 1975). The chronological
development of analytical methods for the detection and
quantification of PBB mixtures and congeners is summarized in
Table 12. In the wake of the Michigan disaster, methods were
described for the analysis of: contaminated feed, milk, and milk
products (Fehringer, 1975a,b); animal blood plasma, faeces, milk,
and bile (Willett et al., 1978) and liver and fat (Fawkes et al.,
1982). The methods were developed using tissue from animals fed with
PBBs of known composition. Needham and coworkers developed a method
to determine PBBs in human blood serum (Burse et al., 1980; Needham
et al., 1981) which was thoroughly tested in several laboratories,
but, even here, only the main components of FireMaster(R) were
determined. Similarly, the investigation by Eyster et al. (1983)
into the levels of PBBs in fat, serum, faeces, milk, and placenta
were not isomer specific. Thus, reported values may not reflect the
hazard of the residue because, for example, some congeners are more
toxic than the prominent 2,2',4,4',5,5'-HBB. Most samples of
biological origin have congener distributions that differ from those
of the original material (Fries, 1985b).
Concentrations of PBBs as low as 10 µg/kg in fatty foods
(Fehringer, 1975a), 3 µg/kg in dry feeds (Fehringer, 1975b) and
1 µg/litre in blood serum (Needham et al., 1981) can be detected and
quantified using routine methods. Coefficients of variation become
large as concentrations approach the limits of sensitivity of the
method; thus, values near the limit must be treated with caution
(Fries, 1985b). PBBs adsorb to glass more tenaciously than other
halogenated hydrocarbons, and are not easy to remove by the usual
cleaning methods (Willett et al., 1978). This can lead to erroneous
values, particularly when concentrations in samples are low and
there is a carry over from samples of high concentration. This
problem can be solved by using disposable glassware (Willett et al.,
1978).
Table 12. Analysis of commercial mixtures and individual PBB congeners: A chronological surveya
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) recrystallization GC FID no data identification of Sundström
BP-6 from ethanol/ given BB 153 and a HpBB as et al.
isopropanol major components (1976a)
PBB congeners no data given GC ECD no data routes of synthesis, Sundström
given melting points, relative et al.
retention times, electron (1976b)
capture responses for
some PBB congeners
Commercial - solubility of HPLC, TLC, MS no data survey of analysis for de Kok
mixtures FR 250 13A PBBs in n-hexane UV, GC given PBBs et al.
(octabromobipheyl) decreases rapidly with 1H- & 13C-NMR (1977)
FireMaster(R) BP-6 increasing bromine
and PBB congeners content; PBBs dissolved
in warm CCl4
Commercial sample hexane GC, 13C-NMR, ECD no data identification of Moore
of 1H-NMR, IR given BB 180 et al.
octabromobiphenyl heptabromobiphenyl (1978)
FireMaster(R) methylene chloride; GC, NMR, MS 0.5 mg/kg contains at least 13 Hass
BP-6 hexane HPLC SIM different PBBs and et al.
bromonaphthalene (no (1978)
bromodibenzofurans or
bromodibenzo-p-dioxins
found)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC, NMR MS no data purification and Moore &
FF-1 or BP-6 and given structural characterization Aust
octabromobiphenyl of 6 further PBB congeners (1978)
FireMaster(R) hexane GC ECD 0.03 ng absolute and relative Domino
FF-1 retention times of the 8 et al.
major constituents using (1980a)
tetrabromobiphenyl
as an internal standard
FireMaster(R) hexane GC MS no data mass spectra of major Domino &
FF-1 given PBBs in FireMaster; mixed Domino
poly-bromo and (1980)
chlorobiphenyls detected
FireMaster(R) GC ECD no data comparison of packed and Farrel
BP-6 given capillary columns; solves (1980)
some problems with lower
brominated biphenyls, but
has no great advantages
over packed columns for
more highly substituted
biphenyls
FireMaster(R) no data given GC PED 2.8 mg comparison with ECD; not Mulligan
BP-6 (cf 1.5 ng quite so sensitive, but et al.
ECD) is selective (1980)
Individual PBB toluene GC MS, SIM < 1 ng mono-deca PBB congeners Erickson
congeners et al.
(1980)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
22 individual hexane GC ECD, micro- 40 pg retention times given Sweetman &
PBBs FireMaster(R) (preceeded coulometric for 23 congeners response Boettner
FF-1 by HPLC) GC-detector increases with degree of (1982)
MS bromination, increased
detection temperature gives
improved sensitivity
FireMaster(R) carbon preparative FID, MS polar and unpolar hexane Needham
FF-1 tetrachloride; HPLC and fractions were also et al.
hexane GC 1H-NMR tested for hyperkeratotic (1982)
activity
FireMaster(R) hexane GC NCI, SIM 0.6 ng evaluation of halogen anion Greaves
BP-6 formation by polybrominated et al.
compounds in NCI-MS; SIM of (1982)
bromine anions has greater
specificity than ECD
FireMaster(R) fractionation by GC ECD, MS no data seven congeners were Dannan
BP-6 preferential given purified et al.
acetone (1982d)
solubilization,
repeated
crystallization,
alumina
adsorption
column
chromatography,
reversed phase
Lipidex-500
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) see Needham et al. preparative FID, MS at least 60 components Orti
FF-1 (1982) HPLC, GC, observed; isolated/ et al.
lot FH 7042 GC 1H-NMR determined structure of (1983)
10 minor components of
FireMaster (most are very
polar, later eluting
fractions)
PBB hexane GC helium 230 pg simultaneous monitoring Eckhoff
(unspecified) plasma of 4 atomic emission et al.
atomic wave-lengths; PBB mentioned (1983)
emission
spectrometric
detection
FireMaster(R) no data given GC MS, ECD identity of over 91% of Robertson
BP-6 1H-NMR PBB components in et al.
FireMaster using 22 (1984b)
individual PBB congeners
as standards; identification
of 7 additional PBBs
including 3 very toxic
coplanar PBBs
FireMaster(R) hexane GC PED, rapid multi-element quantification Zerezghi
BP-6 scanning et al.
plasma (1984)
emission
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC SIM, MS determination of suspected Tondeur
FF-1 toxic impurities et al.
(1984)
PBB photolysis hexane GC FID, ECD, purification of PBB congener Barnhart
mixture MS 2 using charcoal pretreatment et al.
and RPLC (1984)
Benzenes, hexane GC NCI-MS 0.1 pg especially valuable for Buser
biphenyls measuring trace levels in (1986)
dibenzodioxins, biological and environmental
dibenzofurans, samples; must be two Br;
diphenylethers, structural information is
benzofurans, partly lost
phenols
PBB congeners no data given GC MS no data use of 'ortho' effect for Sovocool
given PBB and isomer & Wilson
identification; accurate (1982);
structure assignments Sovocool
without use of multiple GC (1987a)
determinations
Various PBB hexane GC, HPLC FID no data relationship between Höfler
congeners given recorded retention data et al.
from HPLC and GC and (1988)
molecular surface area
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
Nine synthetic products purified GC MS 1 ng synthesis of 2,3,4,5- Kubiczak
PBBs; by alumina/Florisil; substituted PBBs and et al.
FireMaster(R) recrystallization characterization (1989)
FF-1 and BP-6 from methanol or
methylene chloride
Mono- and no data given 1H-NMR, no data no data Anklam
poly-brominated 13C-NMR given given (1989)
biphenyls
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NMR = Nuclear magnetic resonance.
FID = Flame ionization detector. PED = Microwave-induced plasma emission detector.
GC = Gas chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
GPC = Gel permeation chromatography. RPLC = Reverse-phase liquid chromatography.
HPLC = High pressure liquid chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatograpy.
MS = Mass spectrometry. Unitrex = Universal Trace Residue Extractor.
NAA = Neutron activation analysis. UV = Ultraviolet.
NCI = Negative ion chemical ionization mass
spectrometry.
Recovery of PBBs using established methods is in the range of
80-90% (Fries, 1985b). The solvent system that is used for sample
extraction can affect recovery. Poor recoveries were often found
with hexane but the optimal solvent conditions depend on the source
of the medium sample.
For extraction conditions see Table 13 (environmental samples),
Table 14 (food/feed), Table 15 (biological tissues and fluids (a)
serum/blood (b) adipose and other tissues).
In soil, Griffin & Chou (1981a) found that a polar organic
solvent was important and obtained the best results with
hexane/acetone (9:1).
For serum and blood, the standard extraction method given by
Burse et al. (1980) has been used by most workers.
Extraction of PBBs from adipose and other tissues presents
greater problems. PBBs are readily soluble in fat. They can
therefore be extracted with the fat out of the tissue/sample but,
afterwards, an intensive clean-up procedure for PBBs is necessary.
Various methods, such as adsorption chromatography with Florisil,
gel permeation chromatography, Florisil cartridges (Chiang et al.,
1987), and Unitrex (Head & Burse, 1987) have been proposed.
The sample extraction and clean-up techniques for the
determination of PBBs are similar to those used for PCBs (Krüger
et al., 1988; Jansson et al., 1991). The lipids can be removed from
the extract by gel permeation (Krüger, 1988) or by hydrolysis
(Jansson et al., 1991). Usually PBBs and PCBs are separated from
more polar compounds by adsorption chromatography on silica gel or
Florisil. If the coplanar compounds are to be determined, they have
to be isolated from the major compounds in the extract. This can be
done using activated charcoal, which adsorbs the planar molecules
more strongly than the non-planar. Brominated naphthalenes, dioxins,
and furans will also be separated from the major PBB components in
this step. HPLC methods are now being adopted for these separations
and both charcoal and modified silica gel columns are available for
HPLC separations of coplanar compounds.
Table 13. Determination of PBBs in environmental samplesa
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
Soil, grass, benzene/ Florisil GC ECD, FID 0.1 µg/kg BB 153, two PeBB Jacobs
carrots 2-propanol MS dry weight (soil) isomers, three et al.
13C-NMR 10 µg/kg additional HxBB (1976)
wet weight (plant) isomers, two HpBB
isomers detected
Soil leachate benzene/ GC ECD 0.1 µg/kg laboratory experiments Filonow
2-propanol dry weight et al.
(1976)
Soil, plant hexane/ Florisil GC ECD 0.1 µg/kg field and laboratory Jacobs
samples acetone dry weight (soil) experiments; no et al.
TLC ECD 0.3 µg/kg significant (1978)
wet weight (plant) degradation of PBBs
after 1 year
Effluent river hexane/ no data GC ECD 0.1 µg/litre environmental Hesse (1975)
water diethyl given (later 0.01 µg/ samples Hesse &
ether litre) Powers
(1978)
Sediment hexane/ no data GC ECD 100 µg/kg environmental Hesse &
acetone given samples Powers
(1978)
Soil hexane/ no data GC ECD, FID, separation of 30 PBB Stratton &
acetone 9:1 given MS congeners tested Whitlock
optimum conditions (1979)
Table 13 (contd).
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
for extraction of
PBBs from soil;
polar organic solvent
important
98 environmental hexane, Florisil GC MS 0.2 µg/kg analysed for hexa-, Stratton
samples Soxhlet hepta-, octa-, nona-, et al.
(fish, sediment, decabromobiphenyls; (1979)
soils, HxBB in 84% of samples
vegetation)
Soil, sediment, hexane Florisil GC MS (SIM) 0.2 µg/kg congeners detected Griffin &
sludge, Chou (1981a)
vegetation
Soil hexane/ Florisil GC FID, ECD degradation of PBBs Hill et al.
acetone 1:1 in soil (1982)
Sewage sludge hexane/ TLC IR, NMR, MS 10 ng/kg no PBBs found Strachan
methanol GC et al.
Soxhlet (1983)
extraction
Plants cut, Florisil GC ECD 0.3 µg/kg no translocation Chou et al.
extracted wet basis in plants (1978)
with hexane/
acetone
Table 13 (contd).
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. MS = Mass spectrometry.
FID = Flame ionization detector. NMR = Nuclear magnetic resonance.
GC = Gas chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatography.
Table 14. Determination of PBBs in food/feeda
Matrix Extraction Clean up Analytical Detection Detection Comment References
method limit
Dairy fat extracted by AOAC GPC, 25% toluene GC ECD 7 µg/kg comparison Fehringer
products (1975) methods in ethyl acetate of methods (1975a)
(methanol/ether)
Florisil/pet ether TLC 0.2 mg/kg
Dry animal finely ground feed Florisil/pet ether GC, TLC ECD 8 µg/kg hexabromo Fehringer
feeds packed into a column 30 µg/kg isomer (1975b)
containing celite, measured
elution with methylene
chloride
Feeds and see Fehringer GC before and ECD 5 µg/kg confirmation Erney
dairy (1975a,b) after UV irradi- of PBB (1975)
products ation to determine residues
background using UV
irradiation
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. TLC = Thin layer chromatography.
GC = Gas chromatography. UV = Ultraviolet.
GPC = Gel permeation chromatography.
Table 15. Determination of PBBs in biological tissues and fluidsa
Matrix Extraction Clean up Detection Detection limit Comment References
a) Serum/blood
Human serum methanol-treated serum, Florisil ECD 5 pg analysis based on HxBB Bekesi et al.
extraction with hexane peak (1978)
Human serum methanol-treated serum, Florisil ECD 0.2 µg/litre Wolff et al.
extraction with hexane/ (1978)
ether
Human/rat serum methanol-treated serum, Florisil ECD 0.2 µg/litre PBB homologues as % HxBB Wolff &
extraction with hexane/ peak Aubrey
ether (1978)
methanol-treated serum, Florisil ECD, MS < 1 mg/ml Wolff et al.
extraction with hexane/ (1979a)
ether
Plasma from multiple extraction with Florisil ECD 0.001 µg/litre recovery 96% Willett
PBB-fed cows mixture of diethyl and pet. et al.
ethers (1978)
Human serum methanol-treated serum; Florisil ECD 0.1 µg/litre interlaboratory comparison Burse et al.
extraction with hexane/ (1980)
ether
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Plasma, white methanol; precipitated Florisil MS-SIM 0.1 µg/mg very exact details with Roboz et al.
cell fraction protein removed; NCI protein review spectra (1980)
erythrocytes extraction with hexane/
ß-lipoprotein ether (1:1)
Human serum methanol; hexane/ silica gel ECD 1 µg/litre Needham
diethylether (1:1) et al.
(1981)
Human serum + methanol precipitated Florisil ECD < 1 µg/litre serum protein precipitated Roboz et al.
protein not removed + MS-NCI with methanol should not (1982)
hexane/diethylether (1:1) be removed from sample
Human serum see Burse et al. (1980) ECD 1 µg/litre Eyster
et al.
(1983)
Blood (in vitro see Roboz et al. (1982) MS- in vitro Roboz et al.
experimental) (PPINCI) (1985a)
Human blood see Roboz et al. (1982) ECD, SIM, 10-35 ng distribution of PBBs Roboz et al.
(model and NCI individual among blood components (1985b)
environmentally serum congener/litre
exposed)
b) Adipose and other tissues
Adipose tissue toluene/ethyl acetate GPC (Bio ECD 0.5 µg/kg major HxBB peak determined Wolff et al.
from exposed (1+3) Beads (1979a)
workers toluene/ethyl
acetate (1+3)
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Various rat Burse et al. (1980) ECD 10 µg/kg comparison of Miceli &
tissues and concentrations of PBBs Marks
serum in various tissues with (1981)
time
Liver and 1) hexane (liver and 1) Florisil ECD, NAA comparison of extraction Fawkes et al.
perirenal adipose) methods (showed PBB (1982)
adipose tissue extraction with hexane
from dosed rats leads to erratic recoveries
and results) increase in
detection limits over
ECD ( 2 pg FireMaster);
2) chloroform: 2) acidic 1 µg/litre or less of
methanol (liver) alumina hexa congener)
3) methylene chloride
chloroform (adipose)
Human adipose 15% diethyl ether Florisil/GPC ECD GPC clean-up tested (85% MacLeod
tissue in hexane MS recovery); MS free of et al.
serious interference (1982)
from 46 to 500 m/z
Adipose tissue 6% diethyl ether Florisil/GPC MS 1-2 µg/kg HxBB peak Lewis &
from general in hexane Sovocool
population (1982)
Human adipose hexane/diethylether silica gel ECD 1 µg/kg Eyster
tissue, placenta, et al.
cord blood, (1983)
biliary fluid,
faeces
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Human postmortem Chromaflex ECD 0.5 µg/kg HxBB peak Miceli
tissue adsorption et al.
column with (1985)
5% silica
gel + sodium
sulfate/
hexane
Adipose tissue hexane solid phase ECD 1-14 ng/kg Florisil Chiang et al.
(bovine), spiked Florisil cartridges cartridges to separate (1987)
for model system fat; 116% recovery
c) Milk
Human milk potassium oxalate, ECD 1 µg/kg Eyster et al.
ethanol/diethyl ether; (1983)
hexane
Human milk potassium oxalate, Bio Beads/ MS (NCI, 1 ng/kg separation of coplanar Krüger (1988)
ethanol/diethyl ether Florisil/ SIM) and planar isomers with
activated charcoal
charcoal
d) Biological samples from the environment
Fish, seal freeze, pulverize, Bio Beads/ MS (NCI, 10 ng/kg Krüger (1988)
pet. ether Florisil/ SIM)
activated
charcoal
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Dolphin fat/ Soxhlet; hexane, GPC; silica MS no data given lowest value given: Kuehl et al.
organ tissue methylene chloride gel 40 µg/kg (1991)
Terrestrial, diethyl ether/hexane hydrolysis MS (NCI) no data given lowest value given: Jansson
freshwater and with 98% 40 ng/kg et al.
marine samples H2SO4/Bio (1991, 1992)
Beads/ silica
gel/activated
charcoal
a Analytical method used was gas chromatography.
Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NCI = Negative ion chemical ionization mass spectrometry.
GPC = Gel permeation chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
MS = Mass spectrometry. SIM = Selected ion monitoring.
NAA = Neutron activation analysis.
Using negative ion chemical ionization mass spectrometry
(MS-NCI), the bromide ions can be used to detect brominated
compounds with high sensitivity and selectivity. However, using this
detection method (or ECD), interference between congeners of PBB and
polybrominated diphenyl ethers is possible.
The 209 possible PBB congeners have a wide range of volatility,
which causes very difficult separation problems (Farrell, 1980). In
earlier studies, gas chromatography (GC) with packed columns, e.g.,
3% OV-1 on 80/100 mesh Chromosorb W(HP) was used (Fehringer,
1975a,b). Capillary columns enable a good separation with lower
brominated biphenyls but do not have any great advantages over
packed columns for more highly substituted biphenyls (Farrell, 1980;
Orti et al., 1983; Robertson et al., 1984b).
The detection method most frequently used is that of pulsed
63Ni electron capture detection (ECD). In general, retention times
and electron capture responses increase with increasing bromination.
This is a sensitive method, but has some shortcomings. ECD is a
group selective detector that responds to halogens and other
electronegative groups. This places stringent requirements on
chromatographic separation. Moreover, ECD responds differently to
different compounds, depending on the molecular structure. The
response or sensitivity of the ECD depends on the position of the
halogen on the biphenyl nucleus as well as the number of halogens.
This necessitates running a standard for each compound to be
determined (Zerezghi et al., 1984). Sweetman & Boettner (1982)
analysed the structure-sensitivity of PBBs using ECD (see Table 12).
Flame ionisation detection (FID) can only be used for the
analysis of standard substances because of its low specificity
(Krüger, 1988).
A microwave-induced plasma emission detector has been used as a
specific method of detection for bromine (Mulligan et al., 1980;
Zerezghi et al., 1984). However, the method is not sensitive enough
for environmental samples.
Some authors have confirmed their results by GC/ECD
determination before, and after, exposure to UVR. The PBBs present
are photolyzed and, in this way, the background values can be
eliminated (Erney, 1975; Trotter, 1977).
Very often, the presence of PBBs is confirmed using mass
spectrometry (MS) together with gas chromatography. The purity of
the sample can be verified by comparison with known standards.
Negative chemical ionization (NCI) mass spectrometry has a
sensitivity comparable with, and somewhat better than, GC/ECD
analysis. The detection level for hexabromobiphenyl standards is
lower by a factor of 20 to 10-35 ng/ml in comparison with GC/ECD
analyses (Roboz et al., 1982). This relatively new method has also
been used to detect polychlorinated and polybrominated dioxins and
furans (see section 4.3).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
PBBs are not known to occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 World production figures
1) United States of America
The commercial production of PBBs in the USA commenced in 1970
(Neufeld et al., 1977). Several US producers of commercial
quantities have been identified (Mumma & Wallace, 1975; Neufeld
et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok, 1980).
In 1976, a US firm had a combined production of about 0.45
million kg of PBBs for export to Europe (Anon., 1977).
A list of suppliers of laboratory quantities (with a maximum
production or importation of about 2 kg/year) is presented by Mumma
& Wallace (1975) and Neufeld et al. (1977).
As a result of the Michigan catastrophe of mid-1973, the sole
US manufacturer of hexabromobiphenyl ceased production in November
1974. It is not clear, whether the production of bromine-based fire
retardants was resumed by another US company in 1978 (Brinkman & de
Kok, 1980). Two other companies continued their production of octa-
and deca-PBB until 1977 (Di Carlo et al., 1978). According to the
German "Umweltbundesamt" (UBA, 1989), decabromobiphenyl was produced
in the USA until 1979.
There are repeated statements that all PBBs manufactured in the
USA since 1975-76 have been exported, mainly to Europe, and that
there is no importation of any PBB mixtures into the USA (Brinkman &
de Kok, 1980).
Relevant production data for the period 1970-76 are presented
in Table 16.
Table 16. Commercial production of polybrominated biphenyls in the USA, 1970-76a
Estimated production in thousand kg
Product 1970 1971 1972 1973 1974 1975 1976 1970-76
Hexabromobiphenyl 9.5 84.2 1011 1770 2221 0 0 5369
Octabromobiphenyl 14.1 14.1 14.6 163 48 77.3 366 702
and decabromobiphenylb
Total PBBs 23.6 98.3 1025 1933 2269 77.3 366 6071
a From: Di Carlo et al. (1978).
b Manufacture was continued in 1977, but production figures are not available.
Hexabrominated biphenyl forms the major part (about
5.4 million kg FireMaster(R) BP-6 plus some 68 300 kg
FireMaster(R) FF-1) of the estimated total production of
6.1 million kg (Neufeld et al., 1977). The remaining 0.7 million kg
are accounted for by the higher brominated biphenyls. In 1976, for
example, 0.35 million kg of decabromobiphenyl and 13 600 kg of
octabromobiphenyl were manufactured (Neufeld et al., 1977). No
production figures are available for 1977 (Di Carlo et al., 1978).
2) Japan
According to IARC (1978), PBBs have never been produced in
Japan, but, up to 1978, some were imported.
3) Europe
a) Germany
A German firm produced a mixture of highly brominated PBBs,
called Bromkal 80-9D until mid-1985, when the activities concerning
bromine-based fire retardants were shifted to the USA. No production
figures are available.
b) France
A French firm manufactures a technical-grade decabromobiphenyl,
sold as Adine 0102, production being a few hundred thousand kg/year
(Atochem, 1988). It is marketed in France, Great Britain, Spain and
the Netherlands (Atochem, 1988; UBA, 1989). More than 200 tonnes
decabromobiphenyl/year were used in the Netherlands for
incorporation into polybutylenterephthalate plastics (UBA, 1989).
c) United Kingdom
Two companies are reported to have marketed or produced
technical-grade decabromobiphenyl in the United Kingdom (Brinkman &
de Kok, 1980). In 1977, the production of PBBs was discontinued
(Neufeld et al., 1977).
No production or sales data are available.
d) Netherlands
No domestic producer has been identified. An Israeli company
with two bromine plants in Holland denied the production of PBBs
(Neufeld et al., 1977). However, the amount of decabromobi phenyl
sold annually in the Netherlands was estimated to be of the order of
91 000 kg (Brinkman & de Kok, 1980).
No information is available on production in other parts of the
world.
3.2.1.2 Manufacturing processes
The process of manufacturing PBBs consists of a Friedel-Crafts
type reaction in which biphenyl is reacted with bromine in the
presence of chloride in an organic solvent, using aluminium
chloride, aluminium bromide, or iron as catalyst (Brinkman & de Kok,
1980). In the Atochem decabromobiphenyl manufacturing process,
biphenyl is directly brominated in a large excess of bromine, used
as reactant and solvent in the presence of a Lewis acid catalyst
(aluminium type). Decabromobiphenyl is further purified by
distillation of the excess bromine in the presence of a brominated
solvent (Atochem, 1992).
3.2.1.3 Loss into the environment during normal production
Data are published only for the USA. The following information
refers to reviews by Neufeld et al. (1977) and Di Carlo et al.
(1978).
Losses of PBBs to the environment at sites of its manufacture
can total 51 kg/1000 kg of product. These losses occur through:
1) Emission into the air
In 1977, the maximum air losses as particulate matter at
production sites were estimated to total 1.1 kg of PBBs/1000 kg
manufactured.
(a) Emission to the air from the vents of the hydrogen bromide
recovery system:
Total emission of FireMaster(R) PB-6 was estimated to amount
to 70 mg/1000 kg produced.
(b) Loss of particulate PBB to the atmosphere during centrifugation
(which was carried out to separate the solid reaction products
from the organic solvent).
A New Jersey permit application by Hexcel Corp. plant (1976)
indicated a loss of less than 0.05% of the product.
(c) Loss of dust from drying and pulverizing PBBs to a fine powder
(dust from this operation was removed by a bag type filter).
In 1974, atmospheric levels of PB-6 in the Michigan Chemical
Corp. bagger area were 16-32 mg/m3 during the bagging operation
and 3 mg/m3 after bagging was completed. Lower levels were
detected in other areas of the plant.
(d) Emission of hexabromobiphenyl as a vapour contaminant in
vapour streams leaving scrubbers or equivalent equipment was
calculated to be less than 25 µg/m3 (1 ppb) at ambient
temperature (Neufeld et al., 1977).
2) Losses in waste waters resulting from the quenching and
washing of the PBBs as they are recovered from the reaction mass:
The losses of PBBs to sewers at manufacturing sites were
estimated, in 1977, to be 4.6 µg/kg of product.
- In 1972, samples of the Michigan Chemical Corp. effluent
discharges were found to contain PBB levels of 98-503 µg/litre
(Hesse, 1975);
- The total quantity of PBBs being discharged to the Pine River
was estimated as 0.11 kg daily.
- Unfiltered water from an industrial storm sewer at the Hexcel
Corp. plant contained 92 µg/litre, mainly as decabromobiphenyl
(hexa-, octa-, and nonabromobiphenyls levels were also
measurable).
- Liquid effluents, diluted by canal water, from the White
Chemical Co. plant showed values of up to 31 µg PBBs/litre.
3) Solid losses to landfills resulting from drying, handling,
shipping and transportation.
An estimate of PBB losses as solid waste to landfills was
50 g/kg of product.
According to a report of the Michigan Chemical Corp., their
solid waste included approximately 5% of the BP-6 produced.
4) Losses to the soil
Soil samples from the bagging and loading areas of the Michigan
Chemical corp. contained PBBs at concentrations of 3500 and
2500 mg/kg, respectively.
Losses of other compounds:
The following typical air contaminants released during PBB-
manufacture were reported: hydrogen chloride, bromine, ethylene
dichloride, aluminium chloride, and biphenyls. The total quantity
emitted was stated to be less than 5.5 kg/day.
3.2.1.4 Methods of transport, accidental release, and disposal of
production wastes
Details of present-day labelling and transport regulations are
given in the Health and Safety Guide for PBBs (WHO, 1993).
In 1973, an accidental release of PBBs occurred in Michigan
("Michigan disaster"), when two products manufactured by the
Michigan Chemical Company were inadvertently confused, i.e.,
250-500 kg (Di Carlo et al., 1978) of FireMaster(R), instead of
NutriMaster(R), a magnesium oxide-based cattle feed supplement,
were added to animal feed and distributed to farms within the state.
The compound is believed to have been FireMaster(R) FF-1
(e.g., Fries, 1985b), even if in some publications the name
FireMaster(R) BP-6 is used (e.g., Neufeld et al., 1977; Di Carlo
et al., 1978). This accidental mix up resulted in widespread
contamination by PBBs (see section 5). As a result of this incident,
the production of FireMaster(R) BP-6 by Michigan Chemical Corp.
was stopped in 1974 (Di Carlo et al., 1978). Chronological reports
or reviews of the PBB disaster are given by Carter (1976), Getty
et al. (1977), Kay (1977), Di Carlo et al. (1978), Damstra et al.
(1982), Zabik (1982), and Fries (1985b).
Details of the disposal of manufacturing waste during present
production are not available. In a report by Neufeld et al. (1977),
solids from manufacturing operations were disposed of in landfills.
Waste waters containing small amounts of PBBs were discharged into
the chemical sewer.
3.2.2 Uses
Commercially manufactured PBBs are processed by industrial
users, primarily as flame retardants in polymeric materials. PBBs
were developed for this major application, because: they are able to
meet the flame-resistance performance requirements, they are
economically feasible, and they have little effect on the
flexibility of the base compounds (Mumma & Wallace, 1975).
The process of application is basically one of physical
blending: the PBBs are not functional additives, and on blending
with the dry solid or liquid polymeric material, provide filter-type
flame retardant action with the chemical release of hydrogen bromide
if ignited (Neufeld et al., 1977).
Neufeld et al. (1977) list 34 applications of PBBs found in
patent and technical literature. The majority are related to the use
of the PBBs as flame retardants in polymeric materials, other claims
include self-extinguishing properties and improved wearability and
machinability. Further potential uses of PBBs are: in the synthesis
of biphenyl esters or in a modified Wurtz-Fittig-synthesis; in
light sensitive compositions to act as colour activators; as
relative molecular mass control agents for polybutadiene; as wood
preservatives; as voltage stabilizing agents in electrical
insulation; as functional fluids, such as dielectric media (Neufeld
et al., 1977). In the USA and Canada, hexabromobiphenyl
(FireMaster(R)) was the principal PBB product. It was used as a
fire retardant in three main commercial products:
acrylonitrile-butadiene-styrene (ABS) plastics; coatings and
lacquers; and polyurethane foam (Neufeld et al., 1977).
The types of ABS plastic products in which FireMaster(R) BP-6
was used are compiled in Table 17.
According to Neufeld et al. (1977), the use of FireMaster(R)
BP-6 as a flame retardant in thermoplastic resins was confined to
products that do not come into contact with food or feed and are not
used in fabrics to which humans are exposed.
Although more than 130 companies in the USA used PBBs prior to
1976 (Di Carlo et al., 1978), only a limited number seems to have
been the major users of PBBs. For example, in 1974, the final year
of US production, Borg Warner Corp. (Parkersburg, W.Va.; using
FireMaster(R) in ABS plastics) and Standard T Chemical Co. (Staten
Island, New York; using FireMaster(R) in fire retardant coatings
for industry) consumed over 50% of the total US yearly production
(Mumma & Wallace, 1975; Jamieson, 1977; Neufeld et al., 1977;
Brinkman & de Kok, 1980).
Of the estimated 2200 tonnes hexabromobiphenyl produced in 1974
(IARC, 1978), about 900 tonnes (Mumma & Wallace, 1975; Neufeld
et al., 1977; IARC, 1978) were used in ABS plastic products and
about 34 000 tonnes (Mumma & Wallace, 1975; Neufeld et al., 1977;
IARC, 1978) in cable coatings.
The exact quantity of FireMaster(R) used in polyurethane foam
for automobile upholstery was not published. The two larger
consumers ceased using hexabromobiphenyl (one of these in 1972)
because PBBs did not decompose in the ultimate incineration of
scrapped automobiles (Neufeld et al., 1977).
No current users of hexabromobiphenyl have been identified
(Neufeld et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok,
1980). As regards octa- and decabromobiphenyl, no commercial use was
reported in the USA during 1970-74 (Neufeld et al., 1977). In
Western Europe, the use of higher brominated PBBs seems to be
dominant. The decabromobiphenyl Adine 0102(R) (in the past
manufactured by Ugine Kuhlmann, at present by Atochem) is used as a
flame retardant for thermoplastics and thermosets (e.g., in
polyesters, epoxy resins, polystyrene, ABS, polyolefines, and PVC),
for elastomers (e.g., in PU-elastomers and india rubber) and for
cellulosics (e.g., chip-board). It is applied frequently in
association with antimony trioxide (Sb2O3) (Atochem, 1984a). Its use
in paints and varnishes has also been reported (Brinkman & de Kok,
1980).
Table 17. Uses of FireMaster(R) BP-6 in ABS plastics in the USAa
Industry Approximate % Examples
of total use
Business machines and 48 Typewriter, calculator and microfilm-reader
industrial equipment housings; business machine housings
Electrical 35 Radio and TV parts, thermostats, shaver and
hand-tool housings
Fabricated products 12 Projector housings, movie equipment cases
Transportation 1 Miscellaneous small automotive parts;
electrical-wire connectors, switch
connectors, speaker grills
Miscellaneous 4 Small parts for electrical applications,
motor housings; components for industrial
equipment
a From: Brinkman & de Kok (1980).
Losses of PBBs to the environment from processing plants are
possible, but little information is available about this.
Although decabromobiphenyl and, possibly, other PBBs are still
produced commercially, alternative chemicals have been introduced to
replace them as flame retardants, in particular polybrominated
biphenyl ethers (oxides) (PBBO), e.g., decabromobiphenyl ether
(Adine 505; Bromkal 82-0 DE; Great Lakes DE-83TM and DE 83RTM),
octabromobiphenyl ether (Bromkal 79-8 DE; Great Lakes DE 79), and
pentabromobiphenyl ether (Bromkal 70-5 DE; Great Lakes DE-71TM:
Atochem, 1984b; Great Lakes Chemical Corp., 1986).
Decabromobiphenyl ether (DBBO) for example, appears to be a
much less toxic material than PBBs. However, DBBO is said to have a
tendency to degrade to lower brominated biphenyl oxides. It is
possible that these lower order compounds may pose environmental
problems similar to those of the lower brominated PBBs (Mumma &
Wallace, 1975). In addition, on pyrolysis, PBBOs produce larger
amounts of dioxins and furans than PBBs and so may themselves have
to be replaced by other compounds.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The commercial PBB-mixtures are solids at room temperature.
Despite their low vapour pressure, air pollution by PBBs can occur
as follows:
PBBs may be released into the atmosphere as vapour or dust
from production and processing plants. Stratton & Whitlock (1979)
found indirect evidence of airborne discharges of PBBs near two out
of three chosen industrial sites in north-eastern New Jersey and
Staten Island, New York, where these materials had been manufactured
or used in product formulations.
Further air contamination may occur during the incineration of
industrial and municipal wastes. Most municipal incinerators are not
very effective in destroying halogenated biphenyls. Like PCBs, PBBs
do not burn readily and incinerating conditions must be carefully
controlled, otherwise these compounds will reenter the environment
in the stack gases (Griffin & Chou, 1981a) or may be transformed to
polybro minated dibenzofurans.
Flameless combustion of the consumer products causes
volatilization of intact PBBs (Benbow & Cullis, 1975).
An appreciable loss of PBBs during the lifetime of PBB-
containing products is unlikely.
Secondary ways of entrance of PBBs into the atmosphere, e.g.,
through evaporation from contaminated soils, are thought to be
negligible, though small losses of PBBs from soil during long-term
(6 months) incubation studies were observed, which were associated
with volatilization rather than sorption or masking (Griffin & Chou,
1981a).
The ability of PBBs to co-distil from landfills or from the
surface layer of water bodies, as reported for PCBs (Kalmaz &
Kalmaz, 1979), has not yet been examined.
By analogy with PCBs, it might be expected that PBBs entering
the atmosphere in the vapour phase would be adsorbed rapidly onto
particles, which would then be deposited by particle sedimentation,
depending on micro- and macrometeorological conditions.
According to Eisenreich et al. (1981) organic compounds having
a vapour pressure > 10-5 kPa should exist almost entirely in
the vapour phase, and those having a vapour pressure > 10-9 kPa
should exist almost entirely in the particulate phase.
PBBs, e.g., hexabromobiphenyl or FireMaster with a vapour
pressure of 6.9 x 10-9 kPa (Jacobs et al., 1976), belong to the
latter. In reality, distribution and atmospheric lifetimes of
organic compounds with a high relative molecular mass depend largely
on the particle concentration and composition in the atmosphere
(Eisenreich et al., 1981). Gas-phase reactions with hydroxyl (OH)
radicals also influence the lifetimes of organic compounds emitted
into the atmosphere. Atkinson et al. (1984) determined rate
constants for the gas-phase reaction of OH radicals with biphenyl
and predicted, from their findings, that the chlorine - and
bromine-substituted biphenyls would have OH radical rate constants
of < 8 x 10-12 cm3/molecule per second at room temperature.
4.1.2 Water
The principal route of entrance of PBBs into aquatic
environments is from industrial waste streams into receiving waters.
Further potential routes (of minor relevance) are atmospheric
deposition and erosion of polluted soils. Groundwater contamination
is possible, if these compounds are leached from landfills (Shah,
1978).
Because compounds like PBB are very poorly soluble, they are
primarily found in sediments of polluted lakes and rivers
(Kimbrough, 1980a). In laboratory experiments, Simmons & Kotz (1982)
determined the "percent adsorption" of PBBs in sediments from sites
at Lake Michigan and the Huron River and concluded from values
ranging from 9 to 32% that the capacity of the sediments for PBB was
small to moderate.
PBBs in water are mainly adsorbed on particulate matter
followed by sedimentation at a rate that depends on several factors,
such as the size and type of the sediment and/or the organic
contents of both the sediment and the overlying water mass. The
relative importance of these parameters is controversial (Simmons &
Kotz, 1982). Laboratory results concerning PCBs (Jensen et al.,
1973) led to the assumption that the kinetics of the sorption
reaction may vary inversely with particle size (because smaller
particles have a larger surface area for surface adsorption).
Leland et al. (1973), Choi & Chen (1976), and Simmons et al. (1980)
have shown that the organic content of the sediment is directly
related to its adsorptive capacity for a specific contaminant.
According to Schwarzenbach & Westall (1981), adsorption of non-polar
compounds is highly correlated with the organic carbon content of
sorbents containing more than 0.1% organic carbon. Simmons & Kotz
(1982) found strong correlations between the adsorptive capacity of
the sediments for PBBs and TOC (total organic carbon) and the %
silt/clay fraction.
Sediments are potential sources as well as sinks for most
chemicals (Simmons & Kotz, 1982). Desorption of a contaminant from
the sediments is favoured where a high concentration of organic
matter exists in the water column (Huang, 1971). The presence of
organic matter may also enhance the partitioning of the contaminant
in the water phase and, thus, facilitate further movement with the
water mass (Hassett & Anderson, 1979). Laboratory studies on the
mode of action that PBBs may take in their movement through the
water column have verified that the total organic content of the
natural water will decrease the adsorption of PBB onto sediment and
therefore keep the PBB in the water phase. For example, comparing
the distilled water versus natural water systems, in river water
with an organic content of 11-12 mg C/litre, the % PBB-adsorption
was decreased by 33-43%; for lake water with an organic content of
3.8 mg/litre, the % PBB-adsorption was reduced by about 12% (Simmons
& Kotz, 1982). Another investigation indicated that the solubilities
of PBBs were directly correlated with the levels of dissolved
organics in the water (Griffin & Chou, 1981a) (see also section
4.1.3).
However, in the natural environment, upon settling out, the
association of the contaminant with the sediment may become the
dominant process in the water/sediment system (Simmons & Kotz,
1982). Transport of PBBs is thought to take place, when mixing or
bioturbation of sediments causes redistribution of the contaminant
in the water column (Simmons & Kotz, 1982), and through transport of
the sediment itself.
4.1.3 Soil
Pollution of soils can originate from point sources such as PBB
plant areas and waste dumps. Very few data are available on the
deposition of PBBs on soil via the atmosphere, sewage sludge from
municipal sewage treatment systems, and the dredging of sludge from
contaminated waters.
Other possible sources are illicit, or improper, disposal of
such chemicals (Kimbrough, 1980a) and incidents. For example, as a
consequence of an incident in 1973, Michigan soils have been
contaminated by manure from PBB-fed animals and by the disposal of
contaminated feed, milk, carcasses, etc. (Getty et al., 1977; Chou
et al., 1978; Damstra et al., 1982; Fries, 1985b). Once PBBs have
been introduced into the soil, they appear to have little tendency
to translocate (Damstra et al., 1982).
The ability of rainfall to carry PBBs through the soil was
tested in a laboratory simulation. Filonow et al. (1976) percolated
water through columns of 4 Michigan soils containing 100 mg
2,2',4,4',5,5'-hexabromobiphenyl/kg. They found a loss of less than
0.6% of the hexabromobiphenyl congener from each soil, even with
leachate quantities equivalent to 20 times the average annual
rainfall in Michigan.
Field investigations also indicated that PBBs were retained in
the top soil. Results of subsequent studies on highly contaminated
farm soils showed that PBBs did not move below the 15 cm level,
except where there was a history of physical mixing of the soil
(Fries, 1985b).
The mobility in soils of a chemical like PBBs will largely be
governed by its solubility in water and its adsorption, or
interaction, with soil particles (Jacobs et al., 1978).
As already mentioned, PBBs have a very low solubility in water.
However, studies with distilled, tap, river, and soil waters showed
that their solubility was markedly influenced by water purity
(Jacobs et al., 1978). Griffin & Chou (1981a) ascertained, under
well defined conditions, the following average solubilities of PBBs:
0.06 µg/litre in distilled water, 0.3 µg/litre in deionized water,
0.5 µg/litre in creek water, 8.9 µg/litre in Du Page leachate, and
16.9 µg/litre in Blackwell leachate.
Hence, PBBs were more than 200 times more soluble in landfill
leachate than in distilled water; the solubilities of PBBs were also
higher in creek water than in distilled water. As shown by the TOC
(total organic carbon) values for the waters, the higher
solubilities of PBBs were directly correlated with the level of
dissolved organic compounds in the waters. The type of dissolved
organic matter may also influence the solubility (Griffin & Chou,
1981a).
PBBs are quite soluble in organic solvents, such as dioxane,
carbon tetrachloride, acetone, and methanol. This could play a major
role in soil environments where leachates from chemical waste
disposal sites are percolating.
The other important factor affecting the migration of PBBs,
i.e., their adsorption by soils, was also studied under laboratory
conditions. The hydrophobic properties of PBBs make them easily
adsorbed from aqueous solutions onto soils. Filonow et al. (1976)
examined the adsorption of purified 2,2',4,4',5,5'-hexabromobi
phenyl (BB153) on four soil types. They found that the adsorption of
2,2',4,4',5,5'-hexabromobiphenyl conformed well to Freundlich
adsorption isotherms, and that 2-19% of the available HBB was
adsorbed. Adsorption of HBB was influenced primarily by the organic
content of the soils. An increase in the organic matter content of
soils enhanced their adsorption capacity.
Neither percentage clay nor pH correlated well with BB153
adsorption. Any effect that the clay contents may have had, was
apparently masked by the effect of the organic contents on
adsorption (Filonow et al., 1976).
Griffin & Chou (1981a,b), using the PBB-mixture FireMaster(R)
BP-6 or 14C-labelled-PBB, also confirmed the strong adsorption of
PBBs on soils and indicated a very high direct correlation between
the total organic carbon content (TOC) of three different soils and
the amounts of PBBs adsorbed. However, they pointed out that, in
soils with a low TOC, the mineral fraction may contribute markedly
to the adsorption capacity.
Furthermore, preferential adsorption of PBB congeners and
isomers was noted, depending on the characteristics of the
adsorbent, e.g., organic content (Griffin & Chou, 1981a), as well as
on the degree and position of bromine substitution (Griffin & Chou,
1980, 1981b).
No measurable adsorption on soils occurred of PBBs from organic
solvents (Griffin & Chou, 1981a).
The results of migration studies were in agreement with the
findings discussed above. The mobility of PBBs in five soils was
measured with several leaching solvents, using a thin-layer
chromatography technique and column leaching studies (Griffin &
Chou, 1981a,b). PBBs remained immobile in the soils when leached
with water or landfill leachate, but were highly mobile when leached
with organic solvents. Mobility was directly proportional to the
solubility in the leaching solvents and inversely proportional to
the soil total organic content.
On the other hand, because PBBs are bound to soil, wherever
contaminated soil moves, whether through wind or water erosion or
animal ingestion and migration, traces of PBBs (if present) can be
expected to be found (Jacobs et al., 1978).
4.1.4 Biota
PBBs are stable and persistent, lipophilic, and only very
slightly soluble in water; they are poorly metabolized, and
therefore accumulate in lipid compartments of biota. Once they have
been released into the environment they will reach the food chain,
where they are concentrated. Fish and wildlife are the most
consistent targets for such contamination, but livestock and humans
may also become contaminated (Kimbrough, 1980a). The precise routes
and transport mechanisms of PBBs travelling through biota have not
been thoroughly investigated as pointed out below.
4.1.4.1 Terrestrial ecosystems
Several studies have been concerned with whether plants in
terrestrial ecosystems would take up, translocate, and introduce
PBBs into the food chain. Jacobs et al. (1976) selected orchard
grass (Dactylus glomerata) as test plants in their greenhouse
studies because of its extensive root mass, and carrots (Daucus
carota), which, according to Iwata et al. (1974), have an
outstanding ability to absorb pesticide residues from the soil. They
did not detect any PBBs in the tops of either species grown in soils
supplied with high levels of PBBs (10 or 100 mg/kg of
FireMaster(R) BP-6). However, they did find traces of PBBs
(20-40 µg/kg) associated with carrot roots. 14C-uptake studies
(autoradiography and GC-analysis) on corn and soybean seedlings
grown in hydroponic solutions and on three root crops (radishes,
carrots, and onions) grown in two different soils, also showed no
translocation of PBBs into plant tops (Chou et al., 1978). In
addition, these authors found that the amount of PBBs associated
with roots depended on plant species and the clay and organic matter
contents of the soil. Roots of carrots contained more PBBs than
those of radish or onion bulbs; all roots had higher levels of PBBs
(50-500 µg/kg tissue) when grown in a high-PBB treatment soil
(100 mg/kg) with lower clay and organic content, than they did
(30-120 ng/g plant tissue) in a soil containing more clay and
organic matter. Furthermore, PBBs seem to be localized on the
surfaces of roots, because a significant portion of 14C-PBBs was
removed, when the roots were dipped in acetone.
Analyses of field samples from plant tissues of corn, alfalfa,
and sudax, grown on Michigan fields with soil PBB levels ranging
from 9 to 371 µg/kg, resulted in no detectable (detection limit:
0.3 µg/kg) PBB (Jacobs et al., 1978). The same was true for washed
radishes from a garden with an estimated PBB concen tration of
500-1000 µg/kg and for corn leaf whorls containing dust from a PBB
contaminated soil (102 µg/kg) (Chou et al., 1978).
However, Stratton & Whitlock (1979), who conducted a field
screening survey near sites of manufacture and use of PBBs, found
high surface contamination of lichens and reeds.
The salt marsh cordgrass (Spartina alterniflora) is reported
to take up, accumulate, and transfer effectively PCB from
contaminated sediments to food chains (Mrozek et al., 1982). No data
are available with regard to PBBs.
So far, except for the surface contamination of roots from
contaminated soils and of foliage via air deposition processes,
plants are generally free of significant amounts of residue. Thus,
vegetation on PBB-contaminated soils is a less likely source of
contamination of animals (Damstra et al., 1982; Fries, 1985a,b).
In contrast, a major route of residue transmission from soils
to animals is the direct ingestion of soil (Fries 1982; 1985a). The
degree of contamination depends on the amount of soil ingested and
the bioavailability of the residues.
Quantitative data on soil ingestion by farm animals are given
by several authors (Healy et al., 1967; Healy, 1968; Fries, 1982;
Fries et al., 1982a,b) and range from 2 to 15% of the intake of dry
matter.
Fries (1985a) determined the bioavailability of soil-borne PBBs
in sheep, under controlled feeding conditions, using diets
containing 5% PBB-contaminated soil, and found 65% PBB absorption
from this diet, which contained 9 µg PBB/kg. Addition of activated
carbon to soil had only little effect on bioavailability of PBB.
The same author recorded PBBs in the fat of beef cows, beef
calves, ewes, and pigs from several farms on which soil-borne PBBs
in confinement areas was the only source of PBBs. It can also be
concluded from these results that the animals consumed soil, and
that soil-borne PBB was bioavailable. As might be expected, pigs
accumulated higher PBB concentrations from a soil environment than
ruminants (Fries, 1985a). Recontamination of soil by animal excreta
(Getty et al., 1977; Fries, 1985a) or carcasses (Shah, 1978) also
occurred.
Recently, PBBs have been detected in European herbivorous
mammals (Swedish reindeers: Jansson et al., 1992; German cows
(milk): Krüger, 1988) (see also sections 5.1.4 and 5.1.6).
Despite the affinity of PBBs for soil, there are no
investigations on the role of the soil fauna in the transfer of
PBBs. Earthworms are of great ecological importance and might be
expected to take up and accumulate PBBs as has been ascertained for
PCBs (Diercxsens et al., 1985) and, thus, introduce them into the
food chain.
4.1.4.2 Aquatic ecosystems
PBBs enter the aquatic food chains via water and food. Bacteria
and plankton play an important role in the accumulation and
translocation of PCBs to higher trophic levels (Kalmaz & Kalmaz,
1979; Lorenz & Neumeier, 1983). According to Falkner & Simonis
(1982), sorption processes probably control uptake and accumulation
of PCBs by phytoplankton, because of its high surface-volume ratio.
These mechanisms could also be valid for PBBs. However, Stratton &
Whitlock (1979) did not find PBBs in algae collected in the vicinity
of industrial sites, where PBB concentrations of sediments ranged
from 20 to 60 µg/kg and where captured fish contained 220-230 µg
PBB/kg (detection limit: not given).
No information on the uptake of PBBs from sediment through
bottom living organisms (e.g., mollusca or oligochaete worms) is
available.
In contrast, several laboratory (Norris et al., 1973; Zitko &
Hutzinger, 1976; Zitko, 1977; Sugiura et al., 1978) and field (Hesse
& Powers, 1978; Stratton & Whitlock, 1979; Jaffe et al., 1985)
studies on fish have been conducted. They confirm PBB uptake from
water and food, with the exception of hepta- and octabromobiphenyl
(Norris et al., 1973; Zitko, 1977), which were not taken up from
water.
Consequently, ingestion of fish is a source of PBB transfer to
mammals and birds. Because of the possible selective accumulation
and metabolism of PBB congeners in prey, it can be expected that
predators will be subjected to a somewhat different PBB congener
composition than that found in the surrounding media (sediment,
water, etc.).
In natural situations, food chains become linked together in
complex food webs, and PBBs are distributed in the corresponding
manner.
PBBs have been detected in other species of wildlife besides
fish, e.g., in ducks living near contaminated waters (Hesse &
Powers, 1978), in a turtle (Stratton & Whitlock, 1979), in the eggs
of waterbirds (Haseltine et al., 1981; Heinz et al., 1983, 1985), in
eagles (Kaiser et al., 1980), and in marine mammals (Jansson et al.,
1987, 1992; Krüger, 1988; Kuehl et al., 1991) (see also section
5.1.6).
4.1.4.3 Accidental contamination of the food chain
A special case of entrance of PBBs into the food chain occurred
accidentally in 1973 in Michigan, when FireMaster(R) FF-1 was
inadvertently substituted for magnesium oxide as a supplement in the
formulation of cattle feed (Damstra et al., 1982). Ten to twenty
bags, 22.8 kg each, of PBBs (Carter, 1976) were mixed into feeds,
that were widely distributed to Michigan farmers.
In addition, feeds not formulated to contain magnesium oxide
also became contaminated (with relatively low concentrations)
because of carryover of PBBs from batch to batch in the mixing
equipment (Dunckel, 1975) and, on farms, through the recycling of
contaminated products (Kay, 1977). Distribution of contaminated
antibiotics, e.g., aureomycin, also contributed to the introduction
of PBBs into farm animals (Di Carlo et al., 1978).
The mixing error was not discovered immediately, and it was
almost a year before analyses indicated that a compound of PBB was
involved in the illness or death of farm animals (Getty et al.,
1977). During this time (IARC, 1978; Zabik, 1982), contaminated
animals and their produce entered the human food supply and the
environment of the state of Michigan. Hundreds of farms were
affected. Altogether, at least 29 800 cattle, 5920 pigs, 1470 sheep,
and 1.5 million chickens had been killed and buried by the end of
1975 (Robertson & Chynoweth, 1975; Carter, 1976), in order to
minimize further human exposure. In addition, at least
785 thousand kg of feed, 8185 kg of cheese, 1197 kg of butter,
15 500 kg of dried milk products, and nearly 5 million eggs were
destroyed (Carter, 1976). The number of animals quarantined or
contaminated below quarantine level was estimated to be several
thousands (Isleib & Whitehead, 1975). Although the Michigan PBB
episode was primarily an incident of feed contamination, it also
resulted in secondary contamination of animals from contaminated
soil (Fries, 1985a).
4.2 Degradation
Compounds like PBBs are very stable to hydrolysis, chemical
oxidation, and thermal decomposition. Degradation by purely abiotic
chemical reactions (excluding photochemical reactions) is therefore
considered an unlikely environmental sink (Pomerantz et al., 1978;
Pearson, 1982).
The persistence of PBBs under actual field conditions is
reported in some publications. Jacobs et al. (1976) detected PBBs in
soils from a field that had received manure from a
FireMaster(R)-contaminated dairy herd 10 months earlier.
Follow-up surveys over a three-year period following the
termination of PBB production showed no significant decline in PBB
levels in sediments from the Pine River (Hesse & Powers, 1978). Soil
samples from the former PBB-manufacturing site in St. Louis,
Michigan, analysed several years (nearly ten years?) after
contamination (during the early 1970s) still contained PBBs.
However, the PBB congener composition differed from that of the
original FireMaster(R) mixture, indicating a partial degradation
of the PBB residue in the soil sample (Hill et al., 1982).
The chemical Inspection and Testing Institute, Japan (1987) has
listed decabromobiphenyl as non-biodegradable.
The most probable degradation mechanisms of PBBs in the
environment, if there is any degradation at all, are
photodecomposition and microbial degradation.
4.2.1 Photolytic degradation
Under laboratory conditions, PBBs were easily degraded by UVR.
The photoreactivity of PBBs has been used to confirm PBB residues
(Erney, 1975; Trotter, 1977). The predominant photochemical reaction
of PBBs in organic solvents was a reductive debromination.
Irradiation of 4-monobromobiphenyl at 300 nm in various polar and
nonpolar solvents led to the formation of biphenyl as the sole
product (Freeman et al., 1991). Earlier studies using lower
brominated PBB congeners (i.e., tetra and lower) reported a
preferential loss of ortho bromines (Bunce et al., 1975; Ruzo
et al., 1976). Irradiation of higher brominated congeners yielded a
series of photoproducts (Table 18), but a stepwise cleavage of
orthobromines did not appear to be preferred to meta or para
debromination (Patterson et al., 1980; Millis & Aust, 1985).
The photoreactivity of 2,2',4,4',5,5'-hexabromobiphenyl, the
main component of FireMaster(R), was consistently found to be
relatively high (Andersson et al., 1975; Ruzo et al., 1976;
Robertson et al., 1983a; Millis & Aust, 1985), and degradation
occurred more rapid than with the hexachloro analogue (Andersson
et al., 1975; Ruzo & Zabik, 1975).
Consistent with the dehalogenation pathway, photodegradation of
the commercial FireMaster(R) mixture led to reduced concentrations
of the more highly substituted PBB congeners (De Kok et al., 1977;
Robertson et al., 1981b, 1983; Epling et al., 1987). Robertson
et al. (1983a) examined changes in the composition of
FireMaster(R) BP-6 during photolysis (300 nm for 2-12 h; solvent:
cyclohexane) by monitoring 25 individual PBB congeners; they also
did not find a preferential loss of ortho bromines. Nevertheless,
the photoproducts of FireMaster(R) did contain increased
concentrations of congeners possessing no ortho bromines (e.g.,
3,4,4'-tri-, 3,3',4,4'-tetra-, 3,3',4,4',5-penta bromobiphenyl).
Moreover, other congeners, known as relatively toxic (e.g.,
2,3',4,4',5-pentabromobiphenyl), were enriched (Robertson et al.,
1983). Biphenyl, the ultimate product of the debromination pathway,
was found only to a small extent after the photolysis of
FireMaster(R) BP-6 (Epling et al., 1987).
Table 18. Photodegradation of higher brominated PBB congeners under laboratory conditions
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,5,5'- 254 nm hexane 43.4a 2,3',4',5-tetra ortho-debromination Millis & Aust
penta (up to 100 (minor product) (1985)
min) 2,2',4,5'-tetra meta-debromination
2,2',5,5'-tetra para-debromination
(major product)
(additional production
of a yellow gum)
2,3',4,4',5- 254 nm hexane 50a 2,3',4',5-tetra para-debromination Millis & Aust
penta (up to 90 3,3',4',4'-tetra ortho-debromination (1985)
min)
2,2',4,4',5,5'- 366 nm methanol not specified lower brominated PBBs degradation (90% after 9 Andersson et al.
hexa (main products) min) more rapid than with (1975)
BB 153 methoxy- PBBs (minor the hexachloro analogue
products)
> 300 nm hexane not specified lower brominated PBBs BB 153 was 24.4 times Ruzo et al.
(0.5-2 h) quaterphenyls (< 5%) more reactive than (1976)
4,4'-dibromobiphenyl
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5,5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
Table 18 (contd).
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5.5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
secondary photoproduct:
3,3',4,4'-tetra
formation of yellow
gum at 25 min
2,2',3,4,4',5,5'- sunlight not not 2,2',4,4',5,5'-hexa meta-debromination Patterson
hepta (390 min) specified specified (major product) et al. (1980)
2,3',4,4',5,5'-hexa ortho-debromination
2,2',3,3',4,4', sunlight not not unidentified hexa- Patterson
5,5'-octa (300 min) specified specified PBB (major product) ortho- and meta- et al. (1980)
2,3',4,4',5,5'-hexa debromination
2,2',3,3',5,5', 300 nm hexane not specified di- to Ruzo et al.
6,6'-octa (0.5-2 h) heptabromobiphenyls, ortho debromination (1976)
e.g., 3,3',5,5'-tetra
a Original PBB concentration = 1.59 mmol/litre.
Technical octabromobiphenyl has been reported to photo degrade
in xylene by reductive debromination with a half-life of 40 h
(Norris et al., 1973).
There were investigations to enhance the photochemical process
aiming at a potential technique for the breakdown and removal of
PBBs from the environment. In laboratory testing, photodegra dation
of PBBs was accelerated in the presence of ethylenediamine and
tertbutylamine (Christensen & Weimer, 1979) and in the presence of
sodium borohydride (Epling et al., 1987).
Epling et al. (1987) obtained high yields of biphenyl during
borohydride enhanced photolysis of FireMaster(R) BP-6 (irradiation
under nitrogen at 254 nm; solvent: 90% acetonitrile/water).
The rates and extent of photolytic reactions of PBBs in the
environment have not been determined in detail. However, the few
field observations available indicate a high persistence of the
original PBBs (Jacobs et al., 1978) or a partial degradation to less
brominated (and often more toxic) photoproducts (Hill et al., 1982).
Jacobs et al. (1978) examined field soil that had received manure
from FireMaster(R)-contaminated cattle, for the first time, 2-3
years earlier. They did not detect any significant changes in the
relative concentrations of the major PBB peaks (Br5, Br6, Br7)
compared with the FireMaster(R) standard. In contrast, soil
samples, obtained from the former FireMaster(R) manufacturing site
in Michigan and analysed several years (approximately 10 years?)
after contamination, contained enhanced concentrations of possible
photodegradation products including 2,3',4,4',5-pentabromobiphenyl,
2,2',4,4',5-pentabromobiphenyl, and two unidentified
tetrabromobiphenyls (Hill et al., 1982).
Considering the diversity of microenvironments, both laboratory
and field data on photo alteration of PBBs are incomplete; there is
a lack of studies on the photochemistry of PBBs in water, or in the
vapour or solid states.
4.2.2 Microbial degradation
In laboratory investigations, mixtures of PBBs appear to be
fairly resistant to microbial degradation. Soil incubation studies
using FireMaster(R) BP-6 (lot no. 6244A) and 14C-PBB
(lot 872-244) showed a little, but not significant, degradation of
the major hexa- and heptabromobiphenyl congeners after 6 months or
1 year; only pentabromobiphenyl was assumed to degrade slowly
(Jacobs et al., 1976, 1978). These results were deduced from
recovery rates of PBBs from soil, 14CO2 production, and the lack of
14C-PBB intermediates.
Soils incubated with photodecomposition products of 14C-hexa
and heptabromobiphenyl caused enhanced, but still minor, degradation
(ca. 3%) as measured by 14CO2 production (Jacobs et al., 1978).
These findings are consistent with observations according to which
degradation of PCBs by bacteria increases with decreasing
chlorination (Kalmaz & Kalmaz, 1979; Fries, 1982).
In further incubation experiments with FireMaster(R) BP-6
(lot no. 6244A) in sterilized and nonsterilized Catlin-soil, Griffin
& Chou (1981a) measured the recoveries of penta-, hexa-, and
heptabromobiphenyls and found that all PBBs persisted for 6 months
with no significant microbial degradation. They observed the same
kind of persistence over a period of 4 weeks in PBB incubations with
mixed cultures of microorganisms (predominantly Alkaligenes
odorans, A. denitrificans, and an unidentified bacterium). This
culture had been isolated previously and was known to degrade
water-soluble PCBs (Clark et al., 1979). No PBB metabolites were
found in the PBB-saturated mineral solution after 4 weeks of
incubation (Griffin & Chou, 1981a).
As with PCBs, the high degree (penta or greater) of halogen
substitution of its major components probably accounts for the lack
of degradation of the FireMaster(R)-mixture (Griffin & Chou,
1981a). Congruently, biodegradation of monobrominated biphenyls has
recently been reported.
A soil isolate, strain S93B1, identified as Pseudomonas
cruciviae, could grow on more than ten biphenyl-related compounds
including o-bromobiphenyl (Takase et al., 1986). O-bromobiphenyl
was converted to o-bromobenzoic acid (Fig. 3) (identified by
IR-spectrum). This is analogous with some PCBs showing chlorinated
benzoates as metabolites (Ballschmiter et al., 1977). In these
experiments, biphenyl-related compounds 0.2-0.5% (w/v) were added as
the sole sources of carbon to the liquid artificial medium.
However, this pathway is also realized under simulated natural
conditions (aquatic environments), as reported by Kong & Sayler
(1983). They used river water as supportive culture medium and
"mixed bacterial cultures" (not identified), which were obtained
from PCB-contaminated river sediments. This mixed bacterial culture
was capable of degrading monohalogenated biphenyls.
 The degradation rates of 2-, 3-, and 4-bromobiphenyl, at
30 µg/ml, were 2.3, 4.2, and 1.4 µg/ml per day, respectively, and
were comparable with those of monochlorinated biphenyls. Degradation
occurred when the substrates were supplied as the sole carbon source
or when added in combination with glucose. The major metabolite of
4-bromobiphenyl (para) was 4-bromobenzoate, identified by means of
cochromatography with an authentic compound in HPLC. Two bacterial
strains of the genus Pseudomonas, isolated from a lake sediment by
using p-chlorobiphenyl as a sole carbon source, were capable of
degrading 2-, and 4-bromobiphenyl, but they did not degrade
4,4'-dibromobiphenyl (Sugiura, 1992).
In contrast to several reports indicating that chlorobenzoates
are the principal stable metabolites of PCBs (Furukawa & Matsumara,
1976; Furukawa et al., 1979; Yagi & Sudo, 1980; Reichardt et al.,
1981), 4-bromobenzoate as well as 4-chlorobenzoate appeared
transient. For, when tested with the same bacterial consortium,
4-bromobenzoate at 30 mg/kg was readily degraded at the rate of
4 µg/ml per day (Kong & Sayler, 1983). The terminal decomposition
product is assumed to be CO2 (Kong & Sayler, 1983), i.e.,
4-bromobiphenyl can most likely be completely mineralized by this
bacterial culture. Suflita et al. (1982) also observed degradation
of bromobenzoates. They reported the reductive dehalogenation of
halobenzoates, including 2-, 3-, or 4- bromobenzoate by
microorganisms of lake sediment and sewage sludge. Dehalogenation
required strict anaerobic conditions. The primary degradative event
was loss of the aryl halide without the alteration of the aromatic
ring, the end products were CH4 and CO2. The stable bacterial
consortium enriched from sludge consisted of both chemolithotrophic
and heterotrophic methanogens as well as three, unidentified,
non-motile Gram- negative rods.
Recently, there was a brief report on the reductive
debromination of the FireMaster(R) mixture (rate and extent of
debromination reaction not given) by anaerobic microorganisms eluted
from PCB-contaminated river sediments (Quensen et al., 1990;
abstract only).
4.2.3 Degradation by plants and animals
No degradation of PBBs by plants has been recorded. In contrast
to plants, animals can easily absorb PBBs and, though they have been
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs (cf. also section 6.3).
The metabolism of crude FireMaster(R) BP-6 by a pig gave a
monohydroxypentabromobiphenyl (Kohli & Safe, 1976). The faeces of
dogs fed FireMaster BP-6 contained a metabolite identified as
6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl (Gardner et al., 1979).
However, the authors do not exclude microbial metabolism of PBB in
the dog's gut followed by excretion into the faeces. Doses of
2,2',4,4',5,5'-[14C]-hexabromobiphenyl given intravenously or
orally to male rats were not subject to appreciable metabolism
(Matthews et al., 1977). Metabolites were not detected in tissue
extracts. A trace of radioactivity, which may have represented a
PBB-metabolite, was found in bile and faeces, but the quantity was
too small to be isolated and identified.
Some investigations imply that fish may debrominate the more
highly brominated components of PBB-mixtures. Fish (juvenile Salmo
salar), exposed in laboratory studies to FireMaster(R) BP-6 in
water, contained several mono to pentabromobiphenyls that were not
present in BP-6. Several additional pentabromobiphenyls were
detected in fish fed FireMaster(R) BP-6-contaminated food. Fish
fed octabromobiphenyl contaminated food contained unidentified
penta-, hexa-, and heptabromobiphenyls in addition to the
octabromobiphenyls (Zitko, 1977). It was not known whether the
partially debrominated biphenyls were generated by the fish, or by
the associated microflora.
Because the carbon-bromine bond is less stable than the
carbon-chlorine bond, reductive debromination may be a degradative
pathway of bromobiphenyls, and this reaction may have toxicological
consequences not encountered with PCBs (Zitko & Hutzinger, 1976;
Zitko, 1977).
4.2.4 Bioaccumulation
As expected from their high lipophilicity, PBBs show a marked
tendency to accumulate in animals. However, data are available only
on single links of food chains. It has been reported that similar
compounds, e.g., PCBs, which are more widely spread in the
environment, may have bioconcentration factors of 3-4 orders of
magnitude between water and fish, with a further 1-2 orders of
magnitude between whole fish and the fat storage tissues of fish
predators, such as cormorant, heron, and seal (Pearson, 1982).
4.2.4.1 Aquatic organisms
Fish are the only aquatic organisms for which the bioaccumu
lation of PBBs has been investigated intensively. They serve as an
example for the efficiency of such bioaccumulation (Damstra et al.,
1982).
Fathead minnows (Pimephales promelas) caged in a river, where
water levels of PBB (Firemaster(R) BP-6; probably measured as
concentration of the main peak, 2,2',4,4',5,5'-hexabromobiphenyl)
remained consistently at less than 0.1 µg/litre, concentrated these
contaminants in their bodies more than 10 000 fold in two weeks of
exposure (Hesse & Powers, 1978). In laboratory studies, accumulation
coefficients of FireMaster(R) BP-6 and technical octabromobiphenyl
from water (A = concentration in fish, µg/g wet weight/concentration
in water, µg/ml) and from food (B = concentration in fish, µg/g wet
weight/concentration in fish-food, µg/g) were determined.
FireMaster(R) BP-6 reached values of A = 48 (after an exposure of
48 h) and B = 1.0 (in equilibrium) in juvenile Atlantic salmon
(Salmo salar). The main component accumulated was
2,2'4,4',5,5'-hexabromobiphenyl (Zitko, 1977). In contrast,
octabromobiphenyl, as such, was not concentrated from water by
Atlantic salmon (Zitko, 1977) and by rainbow trout (Norris et al.,
1973), but a little uptake (B = 0.023) was observed from suspended
food (Zitko, 1977). Instead of octabromobiphenyl, an unidentified
hexabromobiphenyl was mainly accumulated (A = 1.73; B = 0.114;
Zitko, 1977). In comparison with Aroclor 1254, the accumulation of
FireMaster(R) BP-6 from water was less, but accumulation from food
was a little higher than that of the corresponding PCB mixture (A =
282; B = 0.358; Zitko, 1977).
There are some differences in the accumulation of different
congeners. Zitko & Hutzinger (1976) determined accumulation
coefficients of di-, tri-, and tetrabromobiphenyls in Salmo salar.
The coefficients were calculated on the basis of accumulation from
water after a 48-h exposure, and of the extrapolated equilibrium
levels in fish fed contaminated food. The accumulation coefficients
generally decreased with increasing degree of substitution during
the uptake from water, and increased, when taken up from food. Of
the dibromobiphenyls, the 3,4-isomer accumulated from water much
less than the 2,6- and 2,4-isomer, and did not accumulate from food
(Zitko & Hutzinger, 1976). Sugiura et al. (1978) dealing with the
accumulation of lower substituted halobiphenyls (di-, tri- and
tetra-) in killifish (Oryzias latipes) found that equilibrium
accumulation from water was not reached during a period of 20 days.
Their data, derived from a flow through test using PBB
concentrations of 0.5-50 µg/litre, resulted in bioaccumulation
factors (equilibrium extrapolated) ranging from 340 to 7340. They
also found that accumulation factors were proportional to partition
coefficients (n-octanol/water), when the coefficients were below
106, but not when the coefficients were above 106.
It is obviously important to note the lipid contents of test
animals in bioaccumulation studies. For example, the bioconcen
tration factors of PCB congeners for whole fish tissue were
proportional to the lipid content of the different species, which
can range from 3 to nearly 20% (Sugiura et al., 1979). Gobas et al.
(1989) reported lipid weight-based bioconcentration factors, log
KL ranging from 5.06 to 6.16, for some PBBs (di- to hexa-) in the
guppy (Poecilia reticulata).
4.2.4.2 Terrestrial organisms
Bioaccumulation of PBBs in terrestrial organisms has been
considered only for avian and mammalian species of farm and
laboratory animals. Data were obtained through field observations
(accumulation from soil), evaluation of an accident, and through
controlled feeding studies.
Accumulation of soilborne PBBs has been studied in Michigan
farms that were contaminated accidentally by FireMaster(R) FF-1
(Fries, 1985a). Ratios of PBB concentrations between the fat of farm
animals (cows, sheep, pigs) and soil ranged from 0.10 to 1.86.
Multiparous dairy cows had lower ratios, because of the excretion of
PBB in milk during long-term lactation, and swine had higher ratios,
because they ingest greater amounts of soil than other species
(Fries, 1985a). In another study, PBB (FireMaster(R) FF-1) was
applied to the soil surface for experimental purposes. Sheep grazing
for 180 days on these plots containing 33 mg PBB/m2 (plot 1) and
48 mg/m2 (plot 2) reached average residue levels in body fat of
0.30 and 0.79 µg PBB/g fat (quantified as concen trations of
2,2',4,4',5,5'-hexabromobiphenyl), respectively. Average residue
concentrations in ewes that grazed for 60 days were nearly as great
(Fries & Marrow, 1982). A second trial, conducted 3 years later
after ploughing and reseeding the plots, showed that PBBs were
distributed throughout the top 16 cm of soil with an average
concentration of 0.14 µg 2,2',4,4',5,5'- hexabromobiphenyl/g soil in
plot 2. Sheep grazing here for 136 days had average concentrations
of 0.032 µg PBB/g body fat (Fries & Marrow, 1982).
The accidental ingestion of FireMaster(R) FF-1 by cattle on
Michigan farms, first described by Jackson & Halbert (1974),
resulted in high body burdens of PBBs. There were tissue levels of
2,2',4,4',5,5'-hexabromobiphenyl in the fat of cows of up to
approximately 4000 mg/kg, nearly one year after high exposure
(estimated total dose: 150-400 g of FireMaster(R) FF-1/cow over
14 days). Low exposure from cross contamination produced PBB
concentrations in fat of less than 0.3 µg/g (Fries et al., 1978a,b;
Fries, 1983).
Laboratory data for the accumulation of PBBs from known diets
are given in Table 19 (diets supplemented with FireMaster(R)) and
in Table 20 (diets supplemented with single PBB congeners). PBB
levels in tissues of FireMaster(R)-exposed animals were expressed
as the concentration of the most abundant constituent of the
mixture, namely 2,2',4,4',5,5'-hexabromobiphenyl. Fries et al.
(1976) additionally reported the concentration of a heptabromobi
phenyl component (not the pure isomer). They found 31.4 mg/kg of
this component in the body fat of hens fed diets containing 20 mg
FireMaster(R)/kg feed for 63 days. The fate of minor constituents
of the FireMaster(R) mixture is not evident from the studies
compiled in Table 19.
Generally, accumulation of PBBs in body fat depended on dosage
and duration of exposure. The highest accumulation coefficients
(mg PBB/kg of tissue divided by mg PBB/kg feed) were found in minks
(Table 19). PBB residue levels in the adipose tissue of treated
minks were 60 times the amount in the diet (Aulerich & Ringer,
1979). According to the authors, the high diet-to-fat residue
accumulations in the minks may be due, in part, to the relatively
small subcutaneous fat deposits of the test animals, most of which
were extremely emaciated at the time of death. Technical
octabromobiphenyl was also accumulated from the diet, as shown by
analyses of the bromine contents of the tissues (Norris et al.,
1973; Lee et al., 1975a; Waritz et al., 1977). There was a
dose-related build-up of bromine, predominantly in the fat, as well
as in the liver, of rats fed octabromobiphenyl. For example, after 4
weeks of feeding 1, 10, 100, or 1000 mg octabromobiphenyl/kg feed,
the bromine concentrations in adipose tissue were 2, 12, 120, and
600 times, respectively, greater than those of the controls (Lee
et al., 1975a).
Table 19. Accumulation of PBBs in feeding studies on mammals and birds
a) Feeding of FireMaster(R)
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Rat (male) BP-6 0.1 20.9c 9 days 0.3 1.5 brain: 0.5 lipid Render et al. (1982)
Rat (male) BP-6 1 23.3b 9 days 1.7 8.3 brain: 1.8 lipid
Rat (male) BP-6 1 not specified 2-3 weeks - 2.7 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 1 not specified 30 days 7.8 22.3 thymus: 21 lipid Akoso et al. (1982a)
Rat (male) BP-6 10 22.9b 9 days 27 135 brain: 12.3 lipid Render et al. (1982)
Rat (male) BP-6 10 not specified 30 days 61.6 310 kidney: 147 lipid Akoso et al. (1982a)
Rat (male) BP-6 50 not specified 2-3 week - 341 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 50 26b 10 weeks 864 55 - wet Harris et al. (1978b)
Rat (male) BP-6 100 22b 9 days 251 1213 brain: 103 lipid Render et al. (1982)
Rat (male) BP-6 100 not specified 30 days 1535 2507 thymus:1044 lipid Akoso et al. (1982a)
Rat (male) BP-6 100 27b 10 weeks 3460 107 - wet Harris et al. (1978b)
Rat (male) BP-6 150 26b 10 weeks 3574 295 - wet
Rat (male) BP-6 200 26b 10 weeks 3242 245 - wet
Mouse BP-6 100 not specified 14 days 223 33.2 thymus: 391 wet Corbett et al. (1978a)
(male)
Mouse BP-6 1000 not specified 11 days 39.5 2.5 - wet Corbett et al. (1975)
(male)
Mouse FF-1 5 not specified 3 weeks - 7 thymus: 20 wet Loose et al. (1981)
(male)
Mouse FF-1 5 not specified 6 weeks - 15 thymus: 37.8 wet
(male)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mouse FF-1 167 not specified 3 weeks - 154 thymus: 109 wet
(male)
Mouse FF-1 167 not specified 6 weeks - 623 thymus: wet
(male) 3088
Sheep BP-6 50 1000 30 days 25 12 heart: 4.3 wet Gutenmann & Lisk
(male) (omental (1975)
fat)
42 (renal
fat)
17
(brisket
fat)
Pig BP-6 20 1880c 4 weeks 0.33 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 20 1880c 16 weeks 64 8.5 muscle: 6.6 wet
(back fat)
42.9
(leaf fat)
Pig BP-6 200 1230c 4 weeks 6.7 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 200 1230c 16 weeks 503 17.2 muscle: wet
18.4
(back fat)
459
(leaf fat)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mink FF-1 2.5 not specified 136 days 149 - muscle: 7.3 wet Aulerich & Ringer
6.25 172 days - - brain: 66 wet (1979)
15.6 72-93 986 - muscle: 70 wet
days
Japanese not 10 -b 9 weeks - 48 heart: 78 dry Babish et al. (1975a)
quail specified
(male) 20 -b 9 weeks - 374 kidney: 105 dry
100 -b 9 weeks - 642 kidney: 725 dry
Japanese not 10 -b 9 weeks - 99 heart: 48 dry
quail specified
(female) 20 -b 9 weeks - 225 heart: 50 dry
100 -b 9 weeks - 503 kidney: 428 dry
Chicken BP-6 20 not specified 63 days 79.8 - egg: 20 wet Fries et al. (1976)
(White
leghorn
hens)
BP-6 20 -b 4-8 weeks - - egg: 30 wet Cecil & Bitman (1978)
BP-6 64 -b 4-8 weeks - - egg: 100 wet
not 1 106b 5 weeks 0.6 egg: 1.5 wet Ringer & Polin (1977)
specified
not 125 94c 5 weeks - - egg: 209 wet Ringer & Polin (1977)
specified
not 625 28.4c 5 weeks - 80 - wet
specified
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Chicken FF-1 0.2 99b 5 weeks (-)d (-)d egg: 0.3 wet Polin & Ringer
(White (1978a,b)
leghorn FF-1 1 106b 5 weeks (-)d (-)d egg: 1.5 wet Polin & Ringer
hens) (1978a,b)
FF-1 5 100b 5 weeks (-)d (-)d egg: 7.4 wet Polin & Ringer
(1978a,b)
FF-1 25 99b 5 weeks (-)d (-)d egg: 43.4 wet Polin & Ringer
(1978a,b)
FF-1 125 94c 5 weeks (-)d (-)d egg: 215 wet Polin & Ringer
(1978a,b)
Chicken FF-1 0.1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
(White 0.11
leghorn FF-1 1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
cockerels) 0.87
FF-1 10 -b 28 days - 83.8 - lipid Dharma et al. (1982)
FF-1 100 -b 28 days - 752 - lipid Dharma et al. (1982)
a Measured as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
b Values not significantly different from control values.
c Values significantly different from control values.
d Diagrams only, generally, the ratios of tissue PBB: diet PBB averaged 3:1 for adipose tissue, 0.8:1 for liver,
and 1.5:1 for whole egg.
Table 20. Accumulation of PBBs in feeding studies on mammals and birds
b) Feeding of individual PBB congeners
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,2',4,4',5,5'- rat 0.1 23.8a 9 days 0.2 1.7 brain: 0.3 Render et al. (1982)
Hexabromobiphenyl (male)
1 26.2b 9 days 3.1 11.4 brain: 1.1
rat 1 not 30 days 16 68.6 kidney: 38.7 Akoso et al. (1982a)
(male) specified
rat 10 25.5b 9 days 31.2 181 brain: 11.5 Render et al. (1982)
(male)
rat 10 not 30 days 149 693 kidney: 373 Akoso et al. (1982a)
(male) specified
rat 100 23.2a 9 days 436 2558 brain: 143 Render et al. (1982)
(male)
rat 100 not 30 days 992 6062 thymus: 3841 Akoso et al. (1982a)
(male) specified
chicken 10 -a 28 days - 105 - Dharma et al. (1982)
(male)
62 -a 28 days - 751 -
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,3',4,4',5,5'- rat 1 not 30 days 9.1 23.5 kidney: 13.9 Akoso et al. (1982a)
Hexabromobiphenyl (male) specified
rat 10 not 30 days 69.1 242 thymus: 211 Akoso et al. (1982a)
(male) specified (1982a)
rat 100 not 30 days 648 4340 thymus: 1639 Akoso et al. (1982a)
(male) specified
chicken 4 -a 28 days - 32.5 - Dharma et al. (1982)
(male)
chicken 10 -a 28 days - 132 - Dharma et al. (1982)
(male)
3,3',4,4',5,5'- rat 0.1 23.6a 9 days 0 3.3 brain: 0 Render et al. (1982)
Hexabromobiphenyl (male)
1 24.7a 9 days 0.4 101 brain: 0
rat 1 24.7b 30 days 0.6 125 thymus: 0 Akoso et al. (1982a)
(male)
rat 10 20.2b 9 days 1.9 - brain: 0 Render et al. (1982)
(male)
rat 10 21.3b 30 days 6.9 448 thymus: 20.4 Akoso et al. (1982a)
(male)
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
rat 100 13.7b 9 days 22.5 1098 brain: 0 Render et al. (1982)
(male)
a Values not significantly different from control values.
b Values significantly different from control values.
Data on accumulation of technical decabromobiphenyl have not
been found in the literature.
Isomer specific accumulation has been studied for three
hexabromobiphenyl congeners. The residue levels in rats and chickens
fed with 2,2'4,4',5,5'-; 2,3',4,4',5,5'- or 3,3',4,4',5,5'-
hexabromobiphenyl are listed in Table 20. In many cases, the lowest
concentrations in tissues were found with 3,3',4,4',5,5'-
hexabromobiphenyl and the highest, with
2,2',4,4',5,5'-hexabromobiphenyl.
4.3 Ultimate fate following use
4.3.1 Disposal of PBB-contaminated animals and wastes from the
Michigan disaster
Accidental contamination of livestock feed in 1973 by PBBs led
to the destruction of over 30 000 animals in Michigan. As the
toxicity and other physical and chemical properties of PBBs were at
that time not so well known, the State of Michigan decided to locate
an environmentally safe site for the burial of contaminated
carcasses (Shah, 1978). A site in Kalkaska County was chosen and
test drilled in order to determine the long-range protection for
groundwaters in the area. The Kalkaska disposal site received over
10 000 animal carcasses most of which contained PBB levels above
1 mg/kg fat, and close to 20 000 carcasses with PBB levels ranging
from 0.3 to 1 mg/kg. This animal disposal site contains
approximately 45 kg of PBBs in all buried carcasses (Shah, 1978; see
section 5.1.2.3. for groundwater studies).
The Gratiot County landfill near St. Louis became operational
in late 1970, and it was designed only for general municipal solid
waste disposal. According to the Michigan Chemical Corporation
report to the Environmental Protection Agency, PBB wastes were
disposed of in the landfill between 1971 and 1973. Wastes containing
large amounts of PBBs (60-70%) were received in the landfill before
any information about the toxic effects of PBBs on animals was
publicly known (Shah, 1978).
The Forest Waste Disposal site consists of an 11-acre,
abandoned, municipal and industrial waste landfill and 9 surface
impoundments. It is located in Genesee County, Michigan, and is
surrounded by agricultural land and undeveloped woodlands and
wetlands. Forest Waste Disposal conducted landfill operations from
1972 to 1978. PBB-contaminated feed has recently been found in the
landfill. A decontamination programme has been recommended (Anon.,
1988).
The Michigan Chemical Corporation stated that, in their
opinion, PBBs would eventually undergo oxidative/biological
degradation forming carbon dioxide, water, and bromide ion (Cordle
et al., 1978). However, studies on PBBs in soil indicate that they
may remain in soils for many years, because of their resistance to
degradation (Jacobs et al., 1976).
4.3.2 Thermal decomposition of PBBs
There is little information on the pyrolysis of PBBs. The
products of the thermal decomposition of PBBs depend on the
temperature as well as on the amount of oxygen present.
Norris et al. (1973) constructed a special apparatus to measure
the relative amounts of bromine from octabromobiphenyl converted
during combustion, when these materials were used as additives in
thermoplastic resins. An exact temperature is not given. Hydrogen
bromide and bromine were not detected.
Waritz et al. (1977) carried out experiments to determine the
approximate lethal temperature of hexa- and octobromobiphenyl. The
dense clouds of fumes obtained at 350 °C were lethal to rats whereas
those produced at 290 °C were not. The fumes were not analysed.
Earlier experiments by Benbow & Cullis (1975) on the pyrolysis
of decabromobiphenyl pressed together at 160 °C with polystyrene and
polypropylene, respectively, showed that, during flameless
combustion, decabromobiphenyl appeared to be volatilized virtually
unchanged from the polymer, whereas when the polymer burned, the
decabromobiphenyl was converted quantitatively to hydrogen bromide.
In these early experiments, the analytical methods were not so
refined that it was possible to detect furans and dioxins. O'Keefe
(1978) pyrolyzed samples of FireMaster FF-1 at 380-400 °C in open
glass tubes and in tubes sealed after nitrogen flushing. Analysis by
low resolution direct probe mass spectrometry showed the presence of
tetra- and pentabrominated dibenzofurans in extracts of the open
tube pyrolyzed material and trace levels of tetrabromodibenzofuran
in those from PBB pyrolyzed under nitrogen.
Buser et al. (1978) studied the pyrolysis of FireMaster BP-6
with oxygen in sealed tubes. The flame retardant was completely
destroyed at 700 °C, but, at 600 °C, new compounds were formed, one
of which was probably tetrabromodibenzofuran.
The diversity of possible brominated and mixed brominated
furans and their toxicological implications led to further
refinements in analytical methods (Buser, 1986) and to the demand
for, and synthesis of, suitable standard isomers (Mason et al.,
1987a; Sovocool et al., 1987a; Munslow et al., 1989). There are over
5000 halogenated dibenzodioxins and dibenzofurans containing
chlorine and/or bromine, over 400 of which are 2,3,7,8-substituted
tetra-, penta- and hexahalo congeners suspected to be of high
toxicity (Buser 1987). These mixed congeners are of particular
importance with regard to chemical waste burning (Schäfer &
Ballschmiter, 1986).
Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen have shown that small amounts of bromobenzenes and
lower brominated biphenyls are formed (600-900 °C), but no furans
(Thoma et al., 1987a; Thoma & Hutzinger, 1989).
In contrast, the pyrolysis of FireMaster BP-6 in an open quartz
tube (700-900 °C) in the presence of oxygen yielded over 3 mg/kg
(ppm) of di- to heptabrominated dibenzofurans, though the pyrolysis
of pentabromodiphenyl ethers yielded brominated dibenzofurans at
over 300 times this level (Thoma et al., 1987a). In the presence of
polystyrene and polyethylene, higher levels of brominated
(mona-tetra) dibenzofurans (over 8 and 51 mg/kg (ppm), respectively)
were found (Thoma et al., 1987a). Pyrolysis of FireMaster BP-6 with
PVC at 800 °C yielded mixed bromide/chloride biphenyls, the bromine
atoms being substituted by the chlorine. No ring closure to dioxins
and furans occurred (Thoma et al., 1987b).
Decabromobiphenyl was pyrolyzed for 10 min at 800 °C in a
loosely plugged quartz tube. The pyrolysates were extracted with
toluene and after clean-up, analysed using GC/MS. No brominated
dioxins or dibenzofurans were detected (detection limits
0.2-0.8 µg/g). The clean-up was said to be very difficult because of
the formation of a large number of brominated compounds that were
not dioxins or furans. Debromination of decabromobiphenyl appeared
to be the main reaction, but no details were given (Atochem, 1987).
Zacharewski et al. (1988) pyrolyzed samples of FireMaster(R)
BP-6 in open quartz tubes at 800 °C for 10 min. The resulting
products, mainly tetrabromodibenzofurans (1183 µg/g) but also
tribromo-, pentabromo-, hexabromo-, and heptabromodibenzofurans
(187, 584, 107, and 11 µg/g, respectively), were tested for toxicity
(see section 8.12.3.2). Very little is known about the toxicities of
brominated and brominated/chlorinated dioxins and furans, but they
are estimated to be of the same order as those of PCDD and PCDF
(Mason et al., 1987a; Safe, 1987).
Analysis of actual environmental samples has also been carried
out. Monobromo-polychloro substituted benzenes, biphenyls,
dibenzodioxins, and dibenzofurans have been detected in solid
material collected from a chimney of an industrial waste incinerator
(Schäfer & Ballschmiter, 1986). Brominated dibenzo furans with a
very small amount of mixed brominated/chlorinated compounds were
detected in soot from an accidental fire at a bowling alley (Buser,
1986). Schwind et al. (1988; 1989) analysed samples from a municipal
waste incinerator and detected for the first time a complete series
of tetrahalogenated dibenzofurans (Cl4DF, Br1Cl3DF,
Br2Cl2DF, Br3Cl1DF and Br4DF). It is possible that PBCDD/F
could occur during the incineration of flame retardant-treated
plastic material, which produces PBDD and PBDF. These could react
with PVC via the mixed brominated/chlorinated dioxins and furans to
PCDD and PCDF (Schwind et al., 1988).
As with PCB disposal, the destruction of PCB-contaminated waste
should be carefully controlled. For PCBs, a burning temperature
above 1000 °C for 2 seconds is recommended (WHO/EURO, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Only one report is available on PBB levels in air. It refers to
air samples taken in the vicinity of three PBB-manufacturing or -
processing plants in the USA (Stratton & Whitlock, 1979). Traces of
hexabromobiphenyl (0.06-0.10 ng/m3) were found at two of the three
industrial sites examined.
Further information on PBB levels in ambient air, e.g., near
municipal incinerators, is lacking.
5.1.2 Water and sediments
5.1.2.1 Surface waters
Surface waters have been monitored in the vicinity of PBB-
producing or -processing industrial sites in the USA and in the
vicinity of the Gratiot County landfill (Michigan, USA), which had
received 122 000 kg of wastes containing 60-70% PBBs between 1971
and 1973. The results are summarized in Table 21.
Depending on the sources, the predominant PBB compounds
detected in surface waters were hexabromobiphenyl and
decabromobiphenyl. However, only Stratton & Whitlock (1979)
determined all PBB homologues from Br1 to Br10, the percentage
composition of some of which is given in Table 22.
5.1.2.2 Sediments
Generally, PBBs reach higher concentrations in sediments (Table
23) than in the associated waters (Table 21).
PBB concentrations in sediments of the Pine River were as high
as 77 mg/kg near the Michigan Chemical Corp. plant. The assays
conducted from July 1974 to April 1975, upstream and downstream of
the plant, showed a decline in sediment PBB content to 6.2 mg/kg,
half a mile downstream, and to 0.1 mg/kg, 24 miles or 29 miles
downstream (Hesse & Powers, 1978).
Table 21. PBB levels in surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Pine River 1974 8 HxBB 0.01-3.2 Hesse (1975)
(downstream from
the Michigan
Chemical Co.,
St. Louis)
Tittabawassee 1974 2 HxBB < 0.01
Rivera
Canal (called 1977 3 total PBB not detected-46 Stratton & Whitlock (1979);
Platti Kill) in the (MoBB-DeBB) DeCarlo (1979)
vicinity of White
Chemical Co., Bayonne,
New Jersey
Canal (discharging 1977 1 total PBB < 0.2 Stratton & Whitlock (1979)
into Kill van Kull (HxBB-DeBB)
River) in the vicinity
of Standard T Chemical
Co., Staten Island,
New York
Storm sewer, 1977 5 total PBB < 0.2-210 Stratton & Whitlock (1979);
receiving swamp, etc. (HxBB-DeBB) DeCarlo (1979)
in the vicinity of
Hexcel Fine Organics
Sayreville, New Jersey
Table 21 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Drain waters at 1977 not HxBB 0.1-14 Shah (1978);
or near the margin specified Rosenblatt et al. (1982)
of the Gratiot County
landfill, Michigan
a Note that PBBs were not detected in fish from the Tittabawassee River in 1974 but were detected in 1983 (see Table 30).
Table 22. Percentage of different PBB homologues detected in surface water
samples taken in the vicinity of PBB-producing plantsa
PBB homologues Bayonne, New Jersey Sayreville, New Jersey
(White Chemical Corp.)b (Hexcel Corp.)c
Sample 1 Sample 2 Sample 1 Sample 2
PeBB 1 0 - -
HxBB 4 2 5 < 1
HpBB 2 2 1.6 < 1
OcBB 2 17 1 1
NoBB 15 20 1 2.6
DeBB 76 58 91 96
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Producer of laboratory quantities of various PBBs.
Concentrations measured upstream were all less than the
sensitivity limit of 30 µg/kg with the exception of one sample
collected a quarter of a mile upstream of the plant, which contained
60 µg/kg (Hesse, 1975). This latter analysis was repeated in 1977
(Hesse & Powers, 1978) giving 350 µg/kg (detection limit 100 µg/kg).
Hesse & Powers (1978) compared the PBB levels of Pine River
sediments from the same locations over a period of time after the
termination of FireMaster(R) BP-6 production. The results of the
1976 and 1977 analyses showed that PBB distributions and
concentrations in the sediments had not changed significantly in the
three years after PBB manufacture stopped.
Various PBB homologues were identified again only by Stratton &
Whitlock (1979) who examined aquatic sediments, sludge deposits, and
marsh soils near the sites of manufacture and use of PBBs in New
Jersey and New York (see Table 24).
More recently, sewage sludge has been analysed for PBBs
(Strachan et al., 1983). The authors did not detect any PBBs, PCBs,
or chlorinated hydrocarbon pesticides in the three sludge samples
obtained from sewage treatment plants in three Indiana cities (USA).
However, the detection limit for PCBs and PBBs with the GC-MS system
used was about 10 µg/g, and this apparently is not sensitive enough,
even for PCBs. For example, the amounts of PCBs found in sewage
sludge of German (Lorenz, 1983) and Canadian (Webber et al., 1983)
cities ranged from 1.8 to 2.5 µg/g (dry weight) and from 0.13 to
1.61 µg/g (dry weight), respectively. Values for PBBs have not been
reported in these investigations.
Table 23. PBB levels in sediments and sludge from surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Near Michigan 1974/75 19 HxBB < 30-77 000 Hesse (1975)
Chemical Co. 1974 9 < 100-9200 Hesse & Powers (1978)
Pine River 1976 9 < 100-1200
1977 8 < 100-500
Tittibawassee River 1974 2 tr-16 Hesse (1975)
Near White Chemical 1977 3 total PBB < 10-20 Stratton & Whitlock (1979)
Co., Bayonne, New Jersey: (MoBB-DeBB)
Sediments from Platti
Kill Canal and Kill
van Kall River
Sludge from Platti 1 431 000
Kill Canal
Near Standard T Chemical 1977 1 60
Co., Staten Island, New
York Sediment from Kill
van Kull River (at
discharge site)
Near Hexcel Fine 1977 1 total PBB 4600 Stratton & Whitlock (1979)
Organics, Sayreville, (MoBB-DeBB) DeCarlo (1979)
New Jersey
Marsh Soil
Table 23 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Gratiot County 1977 not HxBB up to 17 000 Shah (1978)
landfill, Michigan specified Rosenblatt et al. (1982)
Associated sediments
of surface drain waters
Table 24. Concentration of PBB-homologues in aquatic sediment, sludge deposit,
or marsh soil samples taken in the vicinity of PBB-producing or -processing
plantsa (µg/kg dry weight)
PBB Bayonne, New Jersey Staten Island, Sayreville,
(White Chem. Corp.)b New Jersey New Jersey
(Standard T (Hexcel
Chem Corp.)c Corp.)d
Sediment Sludge Sedimente Marsh soile
samples:e
1 + 2 3
MoBB - n.d. 540 n.d. n.d.
DiBB - n.d. 2200 n.d. n.d.
TrBB - n.d. 4300 n.d. n.d.
TeBB - n.d. n.d. n.d. n.d.
PeBB - n.d. 590 n.d. n.d.
HxBB n.d. 10 3800 40 30
HpBB n.d. 10 3300 20 n.d.
OcBB n.d. n.d. 3600 n.d. n.d.
NoBB n.d. n.d. 22 500 n.d. 80
DeBB n.d. n.d. 390 000 n.d. 4500
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Major user of FireMasterR BP-6.
d Producer of laboratory quantities of various PBBs.
e n.d. = Not detected (detection limit = < 10 µg/kg).
Surficial sediments from the St. Lawrence River (USA/Canada)
were analysed for HxBB, but the compound was not detected (estimated
detection limit: 1 ng/g) at the ten stations surveyed (Sloterdijk,
1991).
5.1.2.3 Groundwater
Groundwater monitoring data from the Gratiot County landfill
(Michigan, USA) mentioned above, have shown trace levels of PBBs,
even outside the landfill area (see Table 25). However, so far,
domestic drinking-water wells have not shown any traces of PBBs
(Shah, 1978). Groundwater near the disposal site of PBB-
contaminated animals and other products (see section 4.3.1) in
Kalkaska County (Michigan, USA) is reported not to be contaminated
by PBBs (Shah, 1978).
Table 25. PBB levels in groundwater from Gratiot County landfill, 1977a
Site No. of PBB compound PBB
samples examined concentration
range (µg/litre)
Test wells within 4 HxBB 0.5-26
the landfill site
Observation wells 11 0.1-4.4
outside the landfill
area
Domestic not specified not detected
drinkingwater wells
near the landfill
a From: Shah (1978).
5.1.3 Soil
Data on soil pollution by PBBs are available for areas of
manufacture, use, or disposal of PBBs (Table 26), and for soils from
fields, etc. of the PBB-contaminated Michigan farms (Tables 27 and
28).
Concentrations of PBBs in soils from industrial sites were
highest (more than 2000 mg/kg) in areas around the Michigan Chemical
Company (see Table 26). Although such highly contaminated soils
were removed (Hesse & Powers, 1978), Hill et al. (1982) still found,
some years later, PBB levels up to 2130 mg/kg in soils of the former
manufacturing site. Various PBB homologues from Br4 to Br10 were
present in the industrial soil samples (see Table 27).
Table 26. PBB levels in soils near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
Michigan Chemical Co.,
St. Louis, Michigan
bagging area not specified 1 HxBB 3500 mg/kg Hesse (1975);
Hess & Powers (1978)
loading area of not specified 1 HxBB 2500 mg/kg
the plant
"former manufacturing not specified 3 PBB 16-2130 mg/kg Hill et al. (1982)
site" (C12H6Br4-C12H3Br7)
Vicinity of White
Chemical Co.,
Bayonne, New Jersey
150 m east 1977 1 total PBB 4.250 mg/kg Stratton & Whitlock (1979)
150 m west of the 1977 1 (C12H9Br1-C12Br10) 1.135 mg/kg DeCarlo (1979)
plant
not specified not specified PBB 0.75-2.8 mg/kg Di Carlo et al. (1978)
Vicinity of Standard 1977 4 total PBB 10-100 µg/kg Stratton & Whitlock (1979)
T Chemical Co., Staten (MoBB-DeBB)
Island, New York
75 m south west 30
900 m west 10
Table 26 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
1500 m south 10
700 m east (prevailing 100
down-wind direction)
Vicinity of Hexcel 1977 total PBB Stratton & Whitlock (1979);
Fine Organics, (MoBB-DeBB) DeCarlo (1979)
Sayreville, New Jersey
75 m southeast 1 40 µg/kg
Soil in roadside 1 3400 µg/kg
ditch
Gratiot County landfill,
St. Louis, Michigan
Samples inside of the not not "PBB" 12 (16) mg/kg Rosenblatt et al. (1982)
landfill from the specified specified HxBB
uppermost 2.5 cm
(after capping of
the landfill)
Sample somewhat distant "PBB" 61 µg/kg
from the landfill, in (HxBB)
the area of the Michigan
Chemical plant
Table 27. Concentration of PBB homologues detected in soil samples taken in the vicinity of PBB-producing or processing plants
(µg/kg dry weight)
PBB Bayonne, New Jerseya Staten Island, New Yorka Sayreville, New Yorka Michiganb
(White Chemical Corp.)c (Standard T-Chemical Corp.)d (Hexcel Corp.)e (Michigan Chemical Corp.)f
MoBB n.d.g n.d.g n.d.g -
DiBB n.d.g n.d.g n.d.g -
TrBB n.d.g n.d.g n.d.g -
TeBB n.d.g n.d.g n.d.g < 1000-510 000
PeBB n.d.g n.d.-100 n.d.g 4000-60 000
HxBB 15-30 n.d.-10 40-90 12 000-670 000
HpBB 30-110 n.d.-10 n.d.-90 < 1000-190 000
OcBB 90-150 n.d. n.d.-170 -
NoBB 330-2200 n.d. n.d.-440 -
DeBB 530-2100 n.d.-10 n.d.-2600 -
a Data from: Stratton & Whitlock (1979); No. of samples = 2, 4, 2 respectively.
b Data from: Hill et al. (1982); No. of samples = 3.
c Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
d Major user of FireMaster(R) BP-6.
e Producer of laboratory quantities of various PBBs.
f Producer of FireMaster(R) BP-6.
g n.d. = Not detected (detection limit = < 10 µg/kg).
Table 28. Composition and concentration of PBBs in soil samples from former
FireMaster(R) manufacturing plant site (St. Louis, Michigan)a
% Composition (concentration in mg/kg)
FireMaster(R)
Compound Lot #5143 Soil 1 Soil 2 Soil 3
Tetrabromobiphenyls < 0.1 23.9 (510) 11.3 (6) (< 1)
Pentabromobiphenyls
2,2',4,5,5'- 3.9 2.8 (60) 9.4 (5) 12.5 (2)
2,2',4,4',5- < 0.1 5.2 (110) (< 1) (< 1)
2,3',4,4',5- 5.7 27.7 (590) 9.4 (5) 12.5 (2)
Hexabromobiphenyls
2,2',4,4',5,5'- 54.9 24.4 (520) 56.6 (30) 62.5 (10)
2,2',3',4,4',5- 10.3 4.7 (100) 7.5 (4) 6.2 (1)
2,3',4,4',5,5'- 5.0 1.9 (40) 5.7 (3) 6.2 (1)
2,3,3',4,4',5- 2.1 0.5 (10) (< 1) (< 1)
Heptabromobiphenyls
2,2',3,4,4',5,5'- 12.8 5.2 (110) (< 1) (< 1)
2,2',3,3',4,4',5- 1.7 3.8 (80) (< 1) (< 1)
(Total PBBs) (2130) (53) (16)
a Adapted from: Hill et al. (1982).
Hill et al. (1982) identified not only PBB homologues, but also
the isomeric composition of PBBs in the soil samples from the
Michigan Chemical Corp. plant (Table 28). Thus, they provided more
exact analytical data and were able to make an interesting
comparison with the original FireMaster(R) mixture; conclusions
could then be drawn on the environmental fate of PBBs (section 4.2).
According to Shah (1978), test samples of the Gratiot County
landfill showed that, in general, the concentrations of PBBs in the
fill increased with depth and were highest at a depth of 3 to 7.6 m
below the top of the refuse.
As a consequence of the Michigan cattle food mixing error, the
soils of the farms involved have been contaminated by PBBs, mainly
through the faeces of the exposed animals. Fries (1985b) calculated
that about 145 kg of PBBs were distributed in this way, and that
most of this was located on 20-25 farms. (The total number of
quarantined farms was over 500; Robertson & Chynoweth, 1975).
Concentrations of PBBs in soil samples from fields that had
received PBB-contaminated manure were as high as 371 µg/kg (dry
weight), whereas levels in samples from manure piles and from dirt
exercise lots were as high as 2000 µg/kg (Jacobs et al., 1978;
Fries, 1985b).
Soil contamination by PBBs can result in PBB accumulation in
animals, when they have direct access to the contaminated soil.
This is most likely to occur when animals are confined to dirt lots
on which manure-containing PBB has been deposited. Crops grown on
PBB-contaminated soils are not considered an important source of PBB
contamination in animals (Fries & Jacobs, 1986).
Soils from industrial sites have, in general, been more heavily
contaminated than Michigan soils.
5.1.4 Feed and food
5.1.4.1 Feed
Contamination of feed by PBBs has been reported only in
connection with the Michigan PBB incident.
In 1973, about 290 kg (Fries, 1985b) - 1000 kg (IARC, 1978) of
FireMaster(R) FF-1 was inadvertently mixed in cattle feeds and
delivered to Michigan farms.
Three feed preparations appeared initially to be involved in
the Michigan episode with PBB levels as follows:
Feed No. 405, 2.4 mg PBB/kg,
Feed No. 410, 1790 mg PBB/kg,
Feed No. 407, 4300 mg PBB/kg
(Cordle et al., 1978).
A concentration as high as 13 500 mg PBB/kg was also cited
(Kay, 1977; Di Carlo et al., 1978; Damstra et al., 1982). Feed of
one highly contaminated farm (Halbert farm) is reported to have
contained 2900 mg PBB/kg (Fries, 1985b).
In 1974, 68% of 1770 feed samples collected in Michigan
contained PBB residues: 60% in the range of trace to 0.99 mg/kg, and
8% over 1 mg/kg. Resampling in 1975 revealed that 6% of 1208 feed
samples were contaminated and that fewer than 0.16% contained more
than 1 mg PBB/kg. In 1976, only 0.3% of 663 samples analysed were
contaminated: no samples contained more than 0.1 mg/kg (Di Carlo
et al., 1978).
PBB residues were not detected in harvested forages grown on
soils with residue levels as high as 0.3 mg/kg (Fries & Jacobs,
1980).
In 1974 and 1975, low-level feed contamination with PBBs was
detected in Indiana and Illinois, which are neighbours of Michigan
(Di Carlo et al., 1978).
5.1.4.2 Food
Again, almost all the data available on PBB residues in food
are derived from the Michigan cattle food contamination incident in
1973.
The extent to which the general population was exposed depended
on where they obtained their milk, dairy products, and eggs, i.e.,
direct from the contaminated farms or from sources where
contaminated products had been mixed with non-contaminated samples.
Table 29 shows examples of some PBB levels in Michigan foods.
Whereas in 1974 milk from some highly contaminated cows contained
PBB concentrations of up to 900 mg/kg fat (Robertson & Chynoweth,
1975), canned milk samples contained concentrations of up to
1.6 mg/kg fat (Cordle et al., 1978). The most highly contaminated
milk (1 to > 100 mg PBB/kg milk fat) originated from a total of 40
herds with different levels of PBBs at the time of detection (Fries,
1985b). In 1975, PBBs were still detected in milk from some herds
(Kay, 1977).
Data on meat can be derived from Table 33, which shows PBB
levels in Michigan farm animals.
Milk contains far less fat than meat (about 4% versus 30%), and
butterfat contains only 40% of the PBB concentration found in the
animal from which it comes (Fries et al., 1978b; Rosenblatt et al.,
1982). Among the dairy products, PBBs are again concentrated in the
high-fat products (Murata et al., 1977; Zabik et al., 1978).
Table 29. Some examples of PBB levels in food (contaminated as a consequence of
the Michigan PBB incident in 1973)
Product Year of PBB concentration References
sampling (mg/kg)
Milka 1974 2.8-270.5h Cordle et al. (1978)
Milkb 1974 44-900h Robertson & Chynoweth (1975)
Milkc 1974 43-56h Jackson & Halbert (1974)
Milkd 1974 up to 595 Kay (1977); IARC (1978)
Milke 1974 1-> 100h Fries (1985b)
Canned milk 1974 1.15-1.62h Cordle et al. (1978)
Dry skimmed milkf 1974 0.75-1.5 Isleib & Whitehead (1975)
Fluid milk 1974 < 0.02-1.15
processors' products
Butter 1974 1-2h Cordle et al. (1978)
Cheese 1974 1.4-15.0h
Milkg 1975 1-13h Kay (1977); IARC (1978)
Eggs 1974 up to 59.7
a = Collected from individual farms.
b = Collected from 21 cows.
c = Collected from 2 cows (having 174 and 200 mg PBBs/kg in body fat,
respectively).
d = Collected from 22 farms.
e = Collected from 28 herds.
f = From one dairy plant.
g = Collected from 16 herds.
h = On a fat basis.
In May 1974, the US Food and Drug Administration (FDA)
established the following enforcement limits for unavoidable
residues of PBBs in foods: 1 mg/kg in the fat of meat, milk, and
dairy products, 0.3 mg/kg in animal feeds, 0.1 mg/kg in eggs. These
enforcement guidelines were reduced in November 1974 to 0.3 mg/kg in
the fat of meat, milk, and dairy products, and 0.5 mg/kg in eggs and
animal feeds. In February 1977, the FDA rejected a petition to lower
the enforcement guideline level to 0.02 mg/kg for all food products
(IARC, 1978). However, according to Fries (1985b), final
legislation, Act 77, lowered the tolerance to 0.02 mg/kg in the body
fat of all cull dairy cows offered for slaughter. (Unlike the
situation under the previous regulations, the finding of a single
animal with a higher than legal body fat level did not lead to
quarantine and the disposal of the whole herd.) As a result of the
rigid quarantine policy, the food levels of PBBs decreased in
Michigan. In 1975, none of 18 milk samples, 3 out of 14 butter
samples, and none of 13 cheese samples exceeded FDA guidelines
(0.3 mg/kg). Also in 1975, 245 of 2040 meat samples were
contaminated with PBBs: 24 contained more than 0.3 mg/kg. None of
the meat specimens collected in 1976 exceeded FDA guidelines: 96% of
1430 samples were contaminated, but only 1 sample contained more
than 0.6 mg/kg of PBBs. A market basket survey of meat in 1976
revealed detectable PBBs in only 1 out of 102 samples in Michigan
(Di Carlo et al., 1978).
Additional information on PBB findings is presented in several
government reports, which are cited by Di Carlo et al. (1978).
According to these reports 29 170 products had been assayed. In
1974, 14 out of 16 milk samples, 4 out of 34 butter samples and 11
out of 23 cheese samples, collected in Michigan, were found to
exceed FDA guidelines for PBBs. Another survey showed that 24.9% of
272 finished product samples, collected from May to October 1974,
were contaminated with PBBs and that 15.8% contained more than
0.3 mg/kg (Di Carlo et al., 1978).
PBBs were also detected in other states in the USA, for example
in beef in Iowa, duck in Wisconsin, chicken in Alabama, Mississippi,
New York, and Texas, and turkey in Indiana: the levels were
extremely low. During 1975 and 1976, PBBs were found in 9 out of 597
food samples outside of Michigan (Di Carlo et al., 1978).
Food contamination, not derived from the Michigan PBB-
incident, becomes evident, when looking at PBB levels in fish (see
Table 30) some of which are used for human consumption. For example,
skinless fillets of carp from the Pine River, captured in the
vicinity of Michigan Chemical Company, contained 1.33 mg PBBs/kg
(wet weight basis) which is approximately equivalent to 30 mg/kg on
a fat weight basis (Hesse & Powers, 1978). This was obviously
greatly in excess of the US FDA tolerance limit for beef, a
tolerance limit for fish has not been established (Hesse & Powers,
1978).
More recent information on "background" PBB levels in food may
be expected in future via a USA data collection programme
(Foodcontam) initiated by the US Food and Drug Administration which
includes PBBs besides other chemicals (Minyard et al., 1989).
Table 30. PBB levels in fish
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1974 Pine River, downstream Carp skinless not detected-1330 wet HxBB Hesse &
from St. Louis (vicinity (Cyprimus carpio) fillets Powers
of Michigan Chemical Co.) (1978)
White sucker 670
Northern pike 540
Bullhead 450-780
1974 Tittabawassee Carp not detected
River (Cyprimus carpio)
Freshwater drum not detected
(Aplodinotus
grunniens
1976 Pine River, downstream Carp 60-750
from St. Louis (vicinity (Cyprimus carpio)
of Michigan Chemical Co.)
Northern pike 180-230
1976 Pine River, downstream Largemouth skinless not detected-740 wet HxBB Hesse &
from St. Louis (vicinity bass fillets Powers
of Michigan Chemical Co.) (1978)
Smallmouth bass 130
Rockbass 320-700
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1977 Kill van Kull River Killifish whole 220 dry total PBB Stratton &
(vicinity of White Chemical HxBB-DeBB Whitlock
Co., Bayonne, New Jersey); (1979)
Port Johnson
1977 Kill van Kull River Killifish whole 230 dry total PBB
(vicinity of Standarad T HxBB-DeBB
Chemical, Staten Island,
New York); canal at
discharge site
Not Lake Huron Yellow perch 0.3-0.8 Kreis & Rice
specified (Saginaw Bay) (1985)
Saginaw Bay Catfish 21.0
1983 Pine River Hogsucker whole 6000 fat most Jaffe et al.
abundant (1985)
congeners
1983 Chippewa River Carp 5300-15 000
1983 Tittabawassee 140-160
River
1983 Shiawassee River 120
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1983 Flint River 15-32
1983 Saginaw River 80-200
1983 Saginaw Bay 110-1100
Four samples of cow's milk from Germany have been analysed for
PBBs (Krüger, 1988). Three congeners were detected; BB 153
(0.025-0.053 µg/kg milk fat), BB 180 (0.001-0.007 µg/kg) and BB 187
(0.005-0.014 µg/kg). The other 30 congeners covered by the method
were not detected with detection limits ranging from 0.001 to
0.003 µg/kg milk fat (see Table 33).
The processing and cooking of contaminated food have been found
to have some potential for reducing PBB levels. Spray-drying
appeared to reduce the contents of PBBs in whole milk and skim milk
by 30-36% and 61-69%, respectively (Murata et al., 1977; Zabik
et al., 1978). Pressure cooking of chicken pieces also resulted in a
loss of PBBs, however, part of the PBBs lost were found in the drip
(Zabik et al., 1978).
5.1.5 Other products
Antibiotics used for attending farm animals were also found to
be contaminated: Levels of PBBs in aureomycin, which was distributed
by the Michigan Farm Bureau, were as high as 70 mg/kg (Di Carlo
et al., 1978).
5.1.6 Terrestrial and aquatic organisms
5.1.6.1 Aquatic and terrestrial plants
Only few data on PBB contamination of aquatic and terrestrial
plants are available. Stratton & Whitlock (1979) analysed algae
(e.g., filamentous green algae) from surface waters in the vicinity
of White Chemical (Bayonne, New Jersey) and near Standard T Chemical
Company (Staten Island, New York) for PBBs (MoBB through DeBB). The
two samples did not contain detectable levels of PBBs (detection
limit: 10 µg/kg, dry weight). However, bottom sediments taken in the
same location contained hexa- and heptabromobiphenyl.
Surface contamination was observed on terrestrial vegetation in
the vicinity of PBB facilities (up to 92 mg/kg dry weight; Stratton
& Whitlock, 1979). As Chou et al. (1978) reported, the PBB
contamination of field soils in Michigan (USA) did not result in any
detectable surface contamination of field crops.
5.1.6.2 Animals
a) Wildlife
Most earlier data available on PBB contamination of wildlife
refer to freshwater fish (Table 30) and birds (Tables 31 and 32),
primarily waterfowl in the USA. Recent reports refer to PBB
contamination of fish-eating mammals and birds from marine
environments in the USA (Kuehl et al., 1991) and in Europe (Jansson
et al., 1987, 1992; Krüger, 1988). Residues were found also in
terrestrial mammals (Jansson et al., 1992) and in freshwater and
marine fish in Europe (Krüger, 1988; Jansson et al., 1992).
Table 30 gives PBB levels in fish captured for analysis in
industrialized areas of the USA, at various distances from PBB-
containing or -using facilities. PBBs were detected in several fish
species from all rivers or bays examined. The PBB levels ranged up
to a maximum of 1.33 mg/kg wet weight (approximately equivalent to
30 mg/kg on a fat basis) found in carp from the Pine River near
Michigan Chemical Company (Hesse & Powers, 1978).
No apparent change in PBB concentrations was observed in Pine
River fish between 1974 and 1976 (Hesse & Powers, 1978; see also
Table 30). Although Michigan Chemical Co. had terminated PBB
production in 1974, even in 1983, Jaffe et al. (1985) detected PBB
in fish from the Saginaw River system, with highest concentrations
in fish from Pine and Chippewa Rivers (Table 30). While carp from
Tittabawassee River, to which Pine River joins, did not contain any
detectable PBBs in 1974, PBB-residues were detected (approximately
150 µg/kg on a fat basis) in 1983 (Table 30).
Various PBB homologues were examined in killifish (Oryzias
latipes) from Kill van Kull River near White Chemical Co.
(Bayonne, New Jersey). The main component found was NoBB. In the
vicinity of a FireMaster(R)-using facility (Staten Island, New
York), killifish samples contained only HxBB (Stratton & Whitlock,
1979).
PBB contamination has been reported in wild ducks collected
within two miles of the Michigan Chemical Corporation plant (Table
31), in eggs of waterfowl nesting around Green Bay and other areas
of Lake Michigan and on Lake Michigan island (Table 32), and, in
bald eagles found moribund or dead in 13 US states (Table 31).
Approximately one third of bald eagles examined contained PBB
residues (see Table 31).
Concentrations of PBBs in duck samples with skin left on were
considerably higher than those in skinless samples (see Table 31)
indicating that much of the PBBs is associated with the skin or fat
layer between the skin and muscle (Hesse & Powers, 1978).
Table 31. PBB residues in birds (ducks and bald eagles)
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
1974 Pine River Mallard breast tissue 3 0.25 Hesse & Powers
within two miles (skinless) (1978)
downstream from
St. Louis
Wood duck 0.29
Teal 1.8
1976 Mallard breast tissue:
skinless 3c 0.24
with skin 3c 2.00
Wood duck
4c 0.17
4c 2.70
1977 Wood duck breast tissue:
skinless 4c 0.08
with skin 4c 0.23
1977 Teal breast tissue:
skinless 0c (1) not detected
with skin 0c (1) not detected
Table 31 (contd).
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
13 US states Bald eagle found moribund Kaiser et al.
(Haliaeetus or dead; (1980)
leucocephalus)
carcass 10 0.03-0.27 0.07
(32)d
brain 7 0.03-0.17 0.05
a Number of samples containing residues; median is based on this number. Total number of samples in parentheses.
b PBB values were based on the major hexabromobiphenyl peak (BB 153).
c Paired samples.
d Detection limit: 0.02 mg PBBs/kg.
Table 32. PBB residues in eggs of fish-eating and non-fish-eating waterbirds from Green Bay and Lake Michigan (USA)
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Fish eater
1975 Green Bay Little gull 1 n.d. Heinz et al.
(Sensila Wildlife Area) (Larus minutus) (1985)
1977 three Lake Michigan islands Red-breasted merganser 114 0.06 n.d.-0.13 Haseltine et al.
off the tip of Door County, (Mergus serrator) (109) (1981)
Wisconsin
1977 islands in north-western Red-breasted merganser Heinz et al.
Lake Michigan (Mergus serrator): (1983)
eggs from the same nests
randomly selected 49 0.05
unhatched 49 0.04
1977 Lake Michigan Herring gull 9 0.18 0.11-0.25 Heinz et
(Gravel Island) (Larus argentatus) (9) al. (1985)
1977 Green Bay Common tern 10 0.06 0.02-0.22 Heinz et al.
(Lone Tree Island) (Sterna hirundo) (10) (1985)
Green Bay (St. Vital Island) Common tern 2 (2) 0.03 0.03-0.04
(Sterna hirundo)
Green Bay (Portage Point) 2 (2) 0.03 0.02-0.06
Green Bay (Cat Island) Double-crested 4 (3) 0.01 n.d.-0.02
cormorant
(Phalocrocorax
auritus)
Table 32 (contd).
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Lake Michigan (Fish Island) 6 (3) 0.02 n.d.-0.05
Green Bay (Oconto Marsh) Black-crowned night-heron 1 (1) 0.02
(Nycticorax nycticorax)
Green Bay (Oconto Marsh) Green-backed heron 1 n.d.
(Butorides striatus)
Non-fish eater
1977 Three Lake Michigan Mallard 22 n.d. Haseltine
islands off the tip of (Anas platyrhynchos) et al. (1981)
Door County, Wisconsin
1977 Lake Michigan (three Gadwall 4 n.d.
islands off the tip of (Anas strepera)
Door County, Wisconsin)
Black duck (Anas rubripes) 3 n.d.
a Number of collected eggs (in parentheses: number of eggs with quantifiable levels of PBBs).
b PBB values were based on hexabromobiphenyl;
n.d. = No residue of quantifiable level. Level over which quantification was possible: 0.02 mg/kg.
Samples with no detectable residues were calculated in the means as one-half the quantification level.
While the majority of ducks analysed from the Pine River
contained measurable concentrations of PBBs (Table 31), the eggs of
ducks from Lake Michigan islands did not contain detectable PBB
residues (Table 32). In contrast, most eggs of fish-eating
waterbirds from Green Bay and Lake Michigan showed PBB residues
(Table 32). Highest concentrations were detected in herring gull
eggs (0.18 mg/kg wet weight), perhaps reflecting their year round
residence on the Great Lakes (Heinz et al., 1985).
Stratton & Whitlock (1979) analysed a snapping turtle captured
in the vicinity of Hexcel Fine Organics Division (Sayreville, New
Jersey) for hexa- to decabromobiphenyls and found a tissue
concentration of 20 µg hexabromobiphenyl/kg (dry weight).
Di Carlo et al. (1978) reported on PBB contamination of
miscellaneous wildlife, such as deer, rabbits, coyotes, and ravens,
without, however, specifying the sampling locations and the levels
of contamination.
In Europe, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153) was found
in fish from German and Swedish rivers at concentrations ranging
from 0.3 to 0.6 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see
also Tables 33 and 34). A trout sample from a breeding farm
contained much lower levels of PBBs than the fish samples from the
rivers (Krüger, 1988).
A residue of 22 µg BB 153/kg lipid was observed in pooled
samples of osprey specimens found dead in various parts of Sweden
(Jansson et al., 1992; Table 34).
Swedish reindeers (pooled samples) showed BB 153 levels as low
as 0.04 µg/kg lipid (Jansson et al., 1992; Table 34).
PBBs (as a group) were not found in otters (Lutra canadensis)
from a region relatively remote from industrial sites in north
eastern Alberta (Canada) (Somers et al., 1987).
Fish samples (freshwater and marine species) collected in 1983
from an industrial area of Japan (Osaka) did not contain "PBBs" (not
specified) (Watanabe & Tatsukawa, 1990).
Recently, PBBs have been identified in bottlenose dolphins
(Tursiops truncatus) collected during the 1987/88 mass mortality
event along the Atlantic Coast of the USA. All three animals
(females), analysed for PBBs (tetrabromo- to hexabromobiphenyl
congeners) within a subset of the screening programme for
anthropogenic contaminants, contained PBBs at concentrations ranging
from 14 to 20 ng/g lipid (Kuehl et al., 1991).
Table 33. Average concentrations (µg/kg lipid) of PBB congeners in fish, seals, cows, and human milk samples
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 103 0.02 0.12 0.10 < 0.02 < 0.02 not analysed
BB 131 + 142/146 0.30 0.62 0.25 0.03 < 0.02 < 0.01
BB 132 0.33 1.25 0.62 0.15 < 0.02 0.05
BB 135 + 144/151 0.69 4.10 1.48 0.46 < 0.02 0.12
BB 147/135 + 144 0.21 0.31 0.25 < 0.02 < 0.02 < 0.01
BB 148/136 0.10 0.13 0.11 < 0.02 < 0.02 < 0.01
BB 149 0.26 0.45 0.53 < 0.02 < 0.02 < 0.01
BB 153 0.60 2.39 1.31 0.81 0.04 1.03
BB 154/151 0.22 0.54 0.37 < 0.02 < 0.02 0.01
BB 155 0.66 2.64 1.11 0.40 < 0.03 0.05
BB 169 < 0.01 15.16 < 0.01 < 0.01 < 0.01 0.05
BB 176 0.03 < 0.01 0.02 < 0.01 < 0.01 < 0.05
BB 178 0.18 0.87 0.36 0.03 < 0.01 0.09
BB 179 0.08 0.04 0.04 < 0.01 < 0.01 < 0.05
BB 180 0.02 < 0.01 0.02 < 0.01 < 0.04 0.02
BB 181 + 174 0.01 0.01 0.01 < 0.01 < 0.01 < 0.05
BB 184 0.05 0.09 0.03 < 0.01 < 0.01 0.01
BB 185 0.03 < 0.01 < 0.01 < 0.01 < 0.01 < 0.05
BB 186 0.30 0.40 0.16 0.01 < 0.01 0.02
BB 187 + 182 0.03 0.05 0.04 0.03 0.01 0.33
BB 188 0.11 0.28 0.11 < 0.01 < 0.01 0.01
BB 192 0.01 < 0.01 < 0.01 < 0.01 < 0.01 n< 0.05
BB 194 0.07 < 0.02 0.04 < 0.02 < 0.02 < 0.05
BB 197 0.11 0.11 0.08 < 0.02 < 0.02 0.04
BB 198 0.27 0.14 0.12 < 0.02 < 0.02 < 0.05
BB 200 + 204 0.41 0.36 0.24 < 0.02 < 0.02 0.02
BB 201 0.09 < 0.02 0.03 < 0.02 < 0.02 < 0.05
Table 33 (contd).
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 202 0.87 0.42 0.36 < 0.02 < 0.02 0.01
BB 206 0.05 < 0.03 0.04 < 0.03 < 0.03 < 0.01
BB 207 0.06 < 0.03 0.02 < 0.03 < 0.03 < 0.01
BB 208 0.16 0.04 0.04 < 0.03 < 0.03 < 0.01
PBB 6.3 30.5 7.9 1.9 0.05 2.0
From: Krüger (1988).
Table 34. Concentrations (µg/kg lipid) of 2,2',4,4',5,5'-HxBB (BB 153)
in pooled biological samplesa
Species Number of Sampling Concentration
specimens in site of BB 153
the homogenate
Rabbit (Oryctlagus cuniculus) 15 S. Sweden not detected
Moose (Alces alces) 13 not detected
Reindeer (Rangifer tarandus) 31 N. Sweden 0.037
White fish (Coregonus sp.) 35 0.29
Arctic char (Salvelinus alpinus) 15 S. Sweden 0.42
Herring (Clupea harengus) 100 Bothnian Bay 0.092
Herring (Clupea harengus) 60 Baltic Proper 0.16
Herring (Clupea harengus) 100 Skagerrak 0.27
Ringed seal (Pusa hispida) 7 Svalbard 0.42
Grey seal (Halichoerus grypus) 8 Baltic Sea 26
Osprey (Pandion haliaetus) 35 S. Sweden 22
a From: Jansson et al. (1992).
In Europe, PBBs have been detected in seals (Phoca vitulina;
Pusa hispida), guillemots (Uria aalge; U. lomvi), and
white-tailed sea eagles (Haliaeetus albicilla). The concentrations
(estimated by comparison with the technical product FM BP-6) ranged
from 3 to 280 µg/kg lipid (Jansson et al., 1987). The concentrations
of PBBs in comparable samples from the Baltic Ocean were all higher
than concentrations in samples from the Arctic Ocean. The same was
true for polybrominated biphenyl ethers and PCBs (Jansson et al.,
1987).
Concentrations of BB 153 determined in marine fish ranged from
0.2 to 2.4 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see also
Tables 33 and 34). BB 153 levels of 0.4-26 µg/kg lipid were found in
seals (Krüger, 1988; Jansson et al., 1992; see also Tables 33 and
34).
Detailed isomer-specific PBB analyses were carried out by
Krüger (1988) in fish (several species) from the Baltic and North
Seas and from sections of the Lippe and Rur rivers in North
Rhine-Westphalia, Germany. Seal samples from Spitsbergen (Norway)
were also included in this investigation (Table 33). All samples
contained PBBs. The smallest number of PBB congeners was found in
seals (n = 5) from an area remote from industrial sites. The main
components were different hexabrominated isomers with
2,2',4,4',5,5'-hexabromobiphenyl reaching a mean concentration of
0.8 µg/kg fat. The mean concentrations of several PBB congeners and
isomers (penta- to nonabrominated biphenyls) measured in fish
(n = 35) ranged, mostly, between 0.01 and 2 µg/kg fat. The pattern
of PBB congeners found in fish differed in a characteristic manner,
depending on the different capture sites. While relatively high
amounts of nona- and octabromobiphenyls (besides polybrominated
biphenyl ethers) were present in fish from German rivers (n = 17;
several species), hexabrominated biphenyls were predominant in fish
from the North Sea and the Baltic Sea (n = 17; several species). In
all samples from the Baltic Sea (n = 6),
3,3',4,4',5,5'-hexabromobiphenyl was found in relatively high
concentrations (maximum concentration: 36 µg/kg fat), but it was not
detected in samples from the North Sea and from rivers. The
concentrations of the other hexabrominated biphenyls were mostly
higher in fish from the Baltic Sea than in fish from the North Sea.
b) Farm animals
Farm animals in Michigan were contaminated by PBBs, when
FireMaster(R) FF-1 was accidentally mixed with animal feed in
mid-1973 (see section 4.1). The PBB levels resulting from this event
varied greatly with the extent of exposure. Data reported in the
literature are compiled in Table 35. The extent of contami nation
can be seen from the fact that, during the months following the
event, 172 dairy and beef herds (18 000 animals), 32 swine herds
(3500 animals), 16 sheep flocks (1200 animals), and 92 chicken
flocks (1.5 million birds) were destroyed (Isleib & Whitehead, 1975;
Robertson & Chynoweth, 1975; Mercer et al., 1976). In relation to
these great numbers, the portion of highly contaminated animals was
small (e.g., 40 herds of cattle, as can be derived from
contamination values measured in milk (section 5.1.4.2).
5.2 General population exposure
Apart from data collected after the Michigan disaster, there is
only limited information on exposure of the general public. PBBs
have been detected in humans in the vicinity of manufacturing
premises and in a few sites in the USA and Europe, not directly
connected with PBB contamination.
Table 35. PBB levels in farm animals (derived from the Michigan
cattle food contamination incident in 1973)
Year Animal PBB concentrationa References
(Type of sample) (mg/kg)
1974 Poultryb (tissue) 4600 Kay (1977); IARC (1978)
Not specified Cattle (fat) up to 200 Pearson (1982)
Not specified Aborted calves 120-400 Kay (1977)
1974 Cattlec (body fat) 110-2480 Robertson &
Chynoweth (1975);
Mercer et al. (1976)
1974-75 Cattled (fat) 9-4100 Fries et al. (1978b)
1974 Cattleb (tissue) up to 2700 Kay (1977); IARC (1978)
1974 Cattlej (body fat) 174-200 Jackson & Halbert (1974)
March (1975) Dairy cattlee 1-12 Kay (1977)
1975 Cowsf (tissue-fat) not detected-1.69 Isleib & Whitehead (1975)
1975 Steers and heifersg not detected-2.27
(tissue-fat)
1975 Pigsh (tissue-fat) not detected-0.58
1975-76 Cattlei (male and not detected-0.13 Cook et al. (1978a)
female) (eye fat)
Not specified Cattlek (body fat) not detected-3.8 Mercer et al. (1976)
a PBB values were based on 2,2',4,4',5,5'-hexabromobiphenyl.
b From 22 farm premises.
c 21 highly exposed cows.
d 32 cows from one herd heavily contaminated during September/October
1973, and 9 calves borne to these cows in 1974.
e 16 herds of dairy cattle with a history of feed levels from 1 to 14 mg/kg PBB.
f-h Slaughter house survey during a 3-month period (January-April 1975).
f Number of samples: 216; mean-PBB: 0.018 mg/kg.
g Number of samples: 247; mean-PBB: 0.030 mg/kg.
h Number of samples: 213; mean-PBB: 0.017 mg/kg.
i Cattle of 5 affected herds.
j 2 cows of the Halbert farm.
k Cattle of 12 affected herds.
5.2.1 Quantified data on human exposure
5.2.1.1 Worldwide
For most human populations, direct data on exposure to PBBs
from various sources have never been documented. This is true also
for the possible exposure of the general population from the use of
PBB-containing plastic products, and from fumes, generated in the
combustion of these products inadvertently in fires, or from burning
in dumps (Kay, 1977), and, additionally, from sources such as
PBB-containing landfills or PBB-manufacturing and processing
plants.
5.2.1.2 The Michigan Accident
Widespread human exposure resulting from direct contact with
contaminated feed, and, primarily, from the consumption of PBBs in
meat, eggs, and dairy products has been reported from the state of
Michigan, USA (Kay, 1977; Landrigan, 1980; Fries, 1985b; Table 36).
Many Michigan residents were exposed to PBBs between the onset of
contamination in the autumn of 1973 and the establishment of the
quarantine of affected farm animals in the spring of 1974. There was
considerable variation in both lengths and levels of exposure. At
least 2000 families (primarily farmers and their neighbours)
received the heaviest exposure (Meester & McCoy, 1976; IARC, 1978).
Brilliant et al. (1978) concluded from their results of human
milk analyses, conducted in 1976, that about 8 million of the 9.1
million residents of Michigan have detectable body burdens of PBBs.
Further studies (see Table 37) confirmed this widespread
distribution of PBBs.
The amount of PBBs consumed or absorbed by the various groups
in Michigan cannot be determined accurately (Safe, 1984). However,
there have been some trials to estimate the possible exposure to
PBBs of farm families and other people. The estimates were based on
kinetic data and other observations, e.g., time and level of animal
exposure, residue levels in herds at the time of the contamination,
and serum levels of exposed people.
In this way, Fries et al. (1978a) estimated (assumptions: see
Fig. 4), that the total exposure of an individual in a farm family
consuming its own milk was, for example, 9.8 g over the 230-day
period, during which the contamination was undetected. The
cumulative intake over time is shown in Fig. 4. In addition, the
authors concluded that the most highly exposed people consumed from
5 to 15 g PBBs over a 230-day period via milk. The projected intake
of PBB via the meat of cows slaughtered for home consumption would
have exceeded the projected intake from milk.
Table 36. Approximate distribution of PBBs in the Michigan episodea
Item Amount (kg)
Total released 295
Not fed to livestock 45
Fed to livestock 250
Eliminated in faeces 125
Absorbed by animals 125
In human foods before regulation 94
a Modified from: Fries (1985b).
Application of a pharmacokinetic model (Tuey & Matthews, 1980)
to the mean serum concentrations for residents of quarantined farms
resulted in similar values, e.g., about 170 mg mean total exposure
per individual and 11.7 g highest exposure to PBBs (Fries, 1985b;
Brown & Nixon, 1979) supposed a consump tion of 1-20 g of PBB by
families on the most contaminated farms.
The exposure of an individual in the general population would
have a pattern over time as projected above for the farm family
(Fries et al., 1978a). However, the exposure level would have been
much less, because of dilution in the normal marketing channels (the
mixing of milk from a large number of producers; the use of meat of
cull dairy cattle for hamburger and processed meat products). The
calculations of Fries (1985b) indicate that total exposure was about
9-10 mg for an average male with an average adipose content.
However, the individual with the highest PBB serum concentration was
projected to have had a total exposure of about 800-900 mg.
Table 37. Distribution of serum levels of PBBs, Michigan, 1974a
Quarantined farms Non-quarantined farms
Adults Children Adults Children
Serum PBBs Number (%) Number (%) Number % Number %
(µg/litre)
0 3 3.7 - - 21 28.4 - -
2-19 43 52.4 8 28.6 52 70.3 29 96.7
20-90 19 23.2 10 35.7 1 1.4 1 3.3
100-490 11 13.4 3 10.7 0 0 0 0
500-2260 6 7.3 7 25.0 0 0 0 0
Total 82 100.0 28 100.0 74 100.1 30 100.0
a From: Humphrey & Hayner (1975).
The degradation rates of 2-, 3-, and 4-bromobiphenyl, at
30 µg/ml, were 2.3, 4.2, and 1.4 µg/ml per day, respectively, and
were comparable with those of monochlorinated biphenyls. Degradation
occurred when the substrates were supplied as the sole carbon source
or when added in combination with glucose. The major metabolite of
4-bromobiphenyl (para) was 4-bromobenzoate, identified by means of
cochromatography with an authentic compound in HPLC. Two bacterial
strains of the genus Pseudomonas, isolated from a lake sediment by
using p-chlorobiphenyl as a sole carbon source, were capable of
degrading 2-, and 4-bromobiphenyl, but they did not degrade
4,4'-dibromobiphenyl (Sugiura, 1992).
In contrast to several reports indicating that chlorobenzoates
are the principal stable metabolites of PCBs (Furukawa & Matsumara,
1976; Furukawa et al., 1979; Yagi & Sudo, 1980; Reichardt et al.,
1981), 4-bromobenzoate as well as 4-chlorobenzoate appeared
transient. For, when tested with the same bacterial consortium,
4-bromobenzoate at 30 mg/kg was readily degraded at the rate of
4 µg/ml per day (Kong & Sayler, 1983). The terminal decomposition
product is assumed to be CO2 (Kong & Sayler, 1983), i.e.,
4-bromobiphenyl can most likely be completely mineralized by this
bacterial culture. Suflita et al. (1982) also observed degradation
of bromobenzoates. They reported the reductive dehalogenation of
halobenzoates, including 2-, 3-, or 4- bromobenzoate by
microorganisms of lake sediment and sewage sludge. Dehalogenation
required strict anaerobic conditions. The primary degradative event
was loss of the aryl halide without the alteration of the aromatic
ring, the end products were CH4 and CO2. The stable bacterial
consortium enriched from sludge consisted of both chemolithotrophic
and heterotrophic methanogens as well as three, unidentified,
non-motile Gram- negative rods.
Recently, there was a brief report on the reductive
debromination of the FireMaster(R) mixture (rate and extent of
debromination reaction not given) by anaerobic microorganisms eluted
from PCB-contaminated river sediments (Quensen et al., 1990;
abstract only).
4.2.3 Degradation by plants and animals
No degradation of PBBs by plants has been recorded. In contrast
to plants, animals can easily absorb PBBs and, though they have been
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs (cf. also section 6.3).
The metabolism of crude FireMaster(R) BP-6 by a pig gave a
monohydroxypentabromobiphenyl (Kohli & Safe, 1976). The faeces of
dogs fed FireMaster BP-6 contained a metabolite identified as
6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl (Gardner et al., 1979).
However, the authors do not exclude microbial metabolism of PBB in
the dog's gut followed by excretion into the faeces. Doses of
2,2',4,4',5,5'-[14C]-hexabromobiphenyl given intravenously or
orally to male rats were not subject to appreciable metabolism
(Matthews et al., 1977). Metabolites were not detected in tissue
extracts. A trace of radioactivity, which may have represented a
PBB-metabolite, was found in bile and faeces, but the quantity was
too small to be isolated and identified.
Some investigations imply that fish may debrominate the more
highly brominated components of PBB-mixtures. Fish (juvenile Salmo
salar), exposed in laboratory studies to FireMaster(R) BP-6 in
water, contained several mono to pentabromobiphenyls that were not
present in BP-6. Several additional pentabromobiphenyls were
detected in fish fed FireMaster(R) BP-6-contaminated food. Fish
fed octabromobiphenyl contaminated food contained unidentified
penta-, hexa-, and heptabromobiphenyls in addition to the
octabromobiphenyls (Zitko, 1977). It was not known whether the
partially debrominated biphenyls were generated by the fish, or by
the associated microflora.
Because the carbon-bromine bond is less stable than the
carbon-chlorine bond, reductive debromination may be a degradative
pathway of bromobiphenyls, and this reaction may have toxicological
consequences not encountered with PCBs (Zitko & Hutzinger, 1976;
Zitko, 1977).
4.2.4 Bioaccumulation
As expected from their high lipophilicity, PBBs show a marked
tendency to accumulate in animals. However, data are available only
on single links of food chains. It has been reported that similar
compounds, e.g., PCBs, which are more widely spread in the
environment, may have bioconcentration factors of 3-4 orders of
magnitude between water and fish, with a further 1-2 orders of
magnitude between whole fish and the fat storage tissues of fish
predators, such as cormorant, heron, and seal (Pearson, 1982).
4.2.4.1 Aquatic organisms
Fish are the only aquatic organisms for which the bioaccumu
lation of PBBs has been investigated intensively. They serve as an
example for the efficiency of such bioaccumulation (Damstra et al.,
1982).
Fathead minnows (Pimephales promelas) caged in a river, where
water levels of PBB (Firemaster(R) BP-6; probably measured as
concentration of the main peak, 2,2',4,4',5,5'-hexabromobiphenyl)
remained consistently at less than 0.1 µg/litre, concentrated these
contaminants in their bodies more than 10 000 fold in two weeks of
exposure (Hesse & Powers, 1978). In laboratory studies, accumulation
coefficients of FireMaster(R) BP-6 and technical octabromobiphenyl
from water (A = concentration in fish, µg/g wet weight/concentration
in water, µg/ml) and from food (B = concentration in fish, µg/g wet
weight/concentration in fish-food, µg/g) were determined.
FireMaster(R) BP-6 reached values of A = 48 (after an exposure of
48 h) and B = 1.0 (in equilibrium) in juvenile Atlantic salmon
(Salmo salar). The main component accumulated was
2,2'4,4',5,5'-hexabromobiphenyl (Zitko, 1977). In contrast,
octabromobiphenyl, as such, was not concentrated from water by
Atlantic salmon (Zitko, 1977) and by rainbow trout (Norris et al.,
1973), but a little uptake (B = 0.023) was observed from suspended
food (Zitko, 1977). Instead of octabromobiphenyl, an unidentified
hexabromobiphenyl was mainly accumulated (A = 1.73; B = 0.114;
Zitko, 1977). In comparison with Aroclor 1254, the accumulation of
FireMaster(R) BP-6 from water was less, but accumulation from food
was a little higher than that of the corresponding PCB mixture (A =
282; B = 0.358; Zitko, 1977).
There are some differences in the accumulation of different
congeners. Zitko & Hutzinger (1976) determined accumulation
coefficients of di-, tri-, and tetrabromobiphenyls in Salmo salar.
The coefficients were calculated on the basis of accumulation from
water after a 48-h exposure, and of the extrapolated equilibrium
levels in fish fed contaminated food. The accumulation coefficients
generally decreased with increasing degree of substitution during
the uptake from water, and increased, when taken up from food. Of
the dibromobiphenyls, the 3,4-isomer accumulated from water much
less than the 2,6- and 2,4-isomer, and did not accumulate from food
(Zitko & Hutzinger, 1976). Sugiura et al. (1978) dealing with the
accumulation of lower substituted halobiphenyls (di-, tri- and
tetra-) in killifish (Oryzias latipes) found that equilibrium
accumulation from water was not reached during a period of 20 days.
Their data, derived from a flow through test using PBB
concentrations of 0.5-50 µg/litre, resulted in bioaccumulation
factors (equilibrium extrapolated) ranging from 340 to 7340. They
also found that accumulation factors were proportional to partition
coefficients (n-octanol/water), when the coefficients were below
106, but not when the coefficients were above 106.
It is obviously important to note the lipid contents of test
animals in bioaccumulation studies. For example, the bioconcen
tration factors of PCB congeners for whole fish tissue were
proportional to the lipid content of the different species, which
can range from 3 to nearly 20% (Sugiura et al., 1979). Gobas et al.
(1989) reported lipid weight-based bioconcentration factors, log
KL ranging from 5.06 to 6.16, for some PBBs (di- to hexa-) in the
guppy (Poecilia reticulata).
4.2.4.2 Terrestrial organisms
Bioaccumulation of PBBs in terrestrial organisms has been
considered only for avian and mammalian species of farm and
laboratory animals. Data were obtained through field observations
(accumulation from soil), evaluation of an accident, and through
controlled feeding studies.
Accumulation of soilborne PBBs has been studied in Michigan
farms that were contaminated accidentally by FireMaster(R) FF-1
(Fries, 1985a). Ratios of PBB concentrations between the fat of farm
animals (cows, sheep, pigs) and soil ranged from 0.10 to 1.86.
Multiparous dairy cows had lower ratios, because of the excretion of
PBB in milk during long-term lactation, and swine had higher ratios,
because they ingest greater amounts of soil than other species
(Fries, 1985a). In another study, PBB (FireMaster(R) FF-1) was
applied to the soil surface for experimental purposes. Sheep grazing
for 180 days on these plots containing 33 mg PBB/m2 (plot 1) and
48 mg/m2 (plot 2) reached average residue levels in body fat of
0.30 and 0.79 µg PBB/g fat (quantified as concen trations of
2,2',4,4',5,5'-hexabromobiphenyl), respectively. Average residue
concentrations in ewes that grazed for 60 days were nearly as great
(Fries & Marrow, 1982). A second trial, conducted 3 years later
after ploughing and reseeding the plots, showed that PBBs were
distributed throughout the top 16 cm of soil with an average
concentration of 0.14 µg 2,2',4,4',5,5'- hexabromobiphenyl/g soil in
plot 2. Sheep grazing here for 136 days had average concentrations
of 0.032 µg PBB/g body fat (Fries & Marrow, 1982).
The accidental ingestion of FireMaster(R) FF-1 by cattle on
Michigan farms, first described by Jackson & Halbert (1974),
resulted in high body burdens of PBBs. There were tissue levels of
2,2',4,4',5,5'-hexabromobiphenyl in the fat of cows of up to
approximately 4000 mg/kg, nearly one year after high exposure
(estimated total dose: 150-400 g of FireMaster(R) FF-1/cow over
14 days). Low exposure from cross contamination produced PBB
concentrations in fat of less than 0.3 µg/g (Fries et al., 1978a,b;
Fries, 1983).
Laboratory data for the accumulation of PBBs from known diets
are given in Table 19 (diets supplemented with FireMaster(R)) and
in Table 20 (diets supplemented with single PBB congeners). PBB
levels in tissues of FireMaster(R)-exposed animals were expressed
as the concentration of the most abundant constituent of the
mixture, namely 2,2',4,4',5,5'-hexabromobiphenyl. Fries et al.
(1976) additionally reported the concentration of a heptabromobi
phenyl component (not the pure isomer). They found 31.4 mg/kg of
this component in the body fat of hens fed diets containing 20 mg
FireMaster(R)/kg feed for 63 days. The fate of minor constituents
of the FireMaster(R) mixture is not evident from the studies
compiled in Table 19.
Generally, accumulation of PBBs in body fat depended on dosage
and duration of exposure. The highest accumulation coefficients
(mg PBB/kg of tissue divided by mg PBB/kg feed) were found in minks
(Table 19). PBB residue levels in the adipose tissue of treated
minks were 60 times the amount in the diet (Aulerich & Ringer,
1979). According to the authors, the high diet-to-fat residue
accumulations in the minks may be due, in part, to the relatively
small subcutaneous fat deposits of the test animals, most of which
were extremely emaciated at the time of death. Technical
octabromobiphenyl was also accumulated from the diet, as shown by
analyses of the bromine contents of the tissues (Norris et al.,
1973; Lee et al., 1975a; Waritz et al., 1977). There was a
dose-related build-up of bromine, predominantly in the fat, as well
as in the liver, of rats fed octabromobiphenyl. For example, after 4
weeks of feeding 1, 10, 100, or 1000 mg octabromobiphenyl/kg feed,
the bromine concentrations in adipose tissue were 2, 12, 120, and
600 times, respectively, greater than those of the controls (Lee
et al., 1975a).
Table 19. Accumulation of PBBs in feeding studies on mammals and birds
a) Feeding of FireMaster(R)
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Rat (male) BP-6 0.1 20.9c 9 days 0.3 1.5 brain: 0.5 lipid Render et al. (1982)
Rat (male) BP-6 1 23.3b 9 days 1.7 8.3 brain: 1.8 lipid
Rat (male) BP-6 1 not specified 2-3 weeks - 2.7 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 1 not specified 30 days 7.8 22.3 thymus: 21 lipid Akoso et al. (1982a)
Rat (male) BP-6 10 22.9b 9 days 27 135 brain: 12.3 lipid Render et al. (1982)
Rat (male) BP-6 10 not specified 30 days 61.6 310 kidney: 147 lipid Akoso et al. (1982a)
Rat (male) BP-6 50 not specified 2-3 week - 341 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 50 26b 10 weeks 864 55 - wet Harris et al. (1978b)
Rat (male) BP-6 100 22b 9 days 251 1213 brain: 103 lipid Render et al. (1982)
Rat (male) BP-6 100 not specified 30 days 1535 2507 thymus:1044 lipid Akoso et al. (1982a)
Rat (male) BP-6 100 27b 10 weeks 3460 107 - wet Harris et al. (1978b)
Rat (male) BP-6 150 26b 10 weeks 3574 295 - wet
Rat (male) BP-6 200 26b 10 weeks 3242 245 - wet
Mouse BP-6 100 not specified 14 days 223 33.2 thymus: 391 wet Corbett et al. (1978a)
(male)
Mouse BP-6 1000 not specified 11 days 39.5 2.5 - wet Corbett et al. (1975)
(male)
Mouse FF-1 5 not specified 3 weeks - 7 thymus: 20 wet Loose et al. (1981)
(male)
Mouse FF-1 5 not specified 6 weeks - 15 thymus: 37.8 wet
(male)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mouse FF-1 167 not specified 3 weeks - 154 thymus: 109 wet
(male)
Mouse FF-1 167 not specified 6 weeks - 623 thymus: wet
(male) 3088
Sheep BP-6 50 1000 30 days 25 12 heart: 4.3 wet Gutenmann & Lisk
(male) (omental (1975)
fat)
42 (renal
fat)
17
(brisket
fat)
Pig BP-6 20 1880c 4 weeks 0.33 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 20 1880c 16 weeks 64 8.5 muscle: 6.6 wet
(back fat)
42.9
(leaf fat)
Pig BP-6 200 1230c 4 weeks 6.7 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 200 1230c 16 weeks 503 17.2 muscle: wet
18.4
(back fat)
459
(leaf fat)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mink FF-1 2.5 not specified 136 days 149 - muscle: 7.3 wet Aulerich & Ringer
6.25 172 days - - brain: 66 wet (1979)
15.6 72-93 986 - muscle: 70 wet
days
Japanese not 10 -b 9 weeks - 48 heart: 78 dry Babish et al. (1975a)
quail specified
(male) 20 -b 9 weeks - 374 kidney: 105 dry
100 -b 9 weeks - 642 kidney: 725 dry
Japanese not 10 -b 9 weeks - 99 heart: 48 dry
quail specified
(female) 20 -b 9 weeks - 225 heart: 50 dry
100 -b 9 weeks - 503 kidney: 428 dry
Chicken BP-6 20 not specified 63 days 79.8 - egg: 20 wet Fries et al. (1976)
(White
leghorn
hens)
BP-6 20 -b 4-8 weeks - - egg: 30 wet Cecil & Bitman (1978)
BP-6 64 -b 4-8 weeks - - egg: 100 wet
not 1 106b 5 weeks 0.6 egg: 1.5 wet Ringer & Polin (1977)
specified
not 125 94c 5 weeks - - egg: 209 wet Ringer & Polin (1977)
specified
not 625 28.4c 5 weeks - 80 - wet
specified
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Chicken FF-1 0.2 99b 5 weeks (-)d (-)d egg: 0.3 wet Polin & Ringer
(White (1978a,b)
leghorn FF-1 1 106b 5 weeks (-)d (-)d egg: 1.5 wet Polin & Ringer
hens) (1978a,b)
FF-1 5 100b 5 weeks (-)d (-)d egg: 7.4 wet Polin & Ringer
(1978a,b)
FF-1 25 99b 5 weeks (-)d (-)d egg: 43.4 wet Polin & Ringer
(1978a,b)
FF-1 125 94c 5 weeks (-)d (-)d egg: 215 wet Polin & Ringer
(1978a,b)
Chicken FF-1 0.1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
(White 0.11
leghorn FF-1 1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
cockerels) 0.87
FF-1 10 -b 28 days - 83.8 - lipid Dharma et al. (1982)
FF-1 100 -b 28 days - 752 - lipid Dharma et al. (1982)
a Measured as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
b Values not significantly different from control values.
c Values significantly different from control values.
d Diagrams only, generally, the ratios of tissue PBB: diet PBB averaged 3:1 for adipose tissue, 0.8:1 for liver,
and 1.5:1 for whole egg.
Table 20. Accumulation of PBBs in feeding studies on mammals and birds
b) Feeding of individual PBB congeners
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,2',4,4',5,5'- rat 0.1 23.8a 9 days 0.2 1.7 brain: 0.3 Render et al. (1982)
Hexabromobiphenyl (male)
1 26.2b 9 days 3.1 11.4 brain: 1.1
rat 1 not 30 days 16 68.6 kidney: 38.7 Akoso et al. (1982a)
(male) specified
rat 10 25.5b 9 days 31.2 181 brain: 11.5 Render et al. (1982)
(male)
rat 10 not 30 days 149 693 kidney: 373 Akoso et al. (1982a)
(male) specified
rat 100 23.2a 9 days 436 2558 brain: 143 Render et al. (1982)
(male)
rat 100 not 30 days 992 6062 thymus: 3841 Akoso et al. (1982a)
(male) specified
chicken 10 -a 28 days - 105 - Dharma et al. (1982)
(male)
62 -a 28 days - 751 -
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,3',4,4',5,5'- rat 1 not 30 days 9.1 23.5 kidney: 13.9 Akoso et al. (1982a)
Hexabromobiphenyl (male) specified
rat 10 not 30 days 69.1 242 thymus: 211 Akoso et al. (1982a)
(male) specified (1982a)
rat 100 not 30 days 648 4340 thymus: 1639 Akoso et al. (1982a)
(male) specified
chicken 4 -a 28 days - 32.5 - Dharma et al. (1982)
(male)
chicken 10 -a 28 days - 132 - Dharma et al. (1982)
(male)
3,3',4,4',5,5'- rat 0.1 23.6a 9 days 0 3.3 brain: 0 Render et al. (1982)
Hexabromobiphenyl (male)
1 24.7a 9 days 0.4 101 brain: 0
rat 1 24.7b 30 days 0.6 125 thymus: 0 Akoso et al. (1982a)
(male)
rat 10 20.2b 9 days 1.9 - brain: 0 Render et al. (1982)
(male)
rat 10 21.3b 30 days 6.9 448 thymus: 20.4 Akoso et al. (1982a)
(male)
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
rat 100 13.7b 9 days 22.5 1098 brain: 0 Render et al. (1982)
(male)
a Values not significantly different from control values.
b Values significantly different from control values.
Data on accumulation of technical decabromobiphenyl have not
been found in the literature.
Isomer specific accumulation has been studied for three
hexabromobiphenyl congeners. The residue levels in rats and chickens
fed with 2,2'4,4',5,5'-; 2,3',4,4',5,5'- or 3,3',4,4',5,5'-
hexabromobiphenyl are listed in Table 20. In many cases, the lowest
concentrations in tissues were found with 3,3',4,4',5,5'-
hexabromobiphenyl and the highest, with
2,2',4,4',5,5'-hexabromobiphenyl.
4.3 Ultimate fate following use
4.3.1 Disposal of PBB-contaminated animals and wastes from the
Michigan disaster
Accidental contamination of livestock feed in 1973 by PBBs led
to the destruction of over 30 000 animals in Michigan. As the
toxicity and other physical and chemical properties of PBBs were at
that time not so well known, the State of Michigan decided to locate
an environmentally safe site for the burial of contaminated
carcasses (Shah, 1978). A site in Kalkaska County was chosen and
test drilled in order to determine the long-range protection for
groundwaters in the area. The Kalkaska disposal site received over
10 000 animal carcasses most of which contained PBB levels above
1 mg/kg fat, and close to 20 000 carcasses with PBB levels ranging
from 0.3 to 1 mg/kg. This animal disposal site contains
approximately 45 kg of PBBs in all buried carcasses (Shah, 1978; see
section 5.1.2.3. for groundwater studies).
The Gratiot County landfill near St. Louis became operational
in late 1970, and it was designed only for general municipal solid
waste disposal. According to the Michigan Chemical Corporation
report to the Environmental Protection Agency, PBB wastes were
disposed of in the landfill between 1971 and 1973. Wastes containing
large amounts of PBBs (60-70%) were received in the landfill before
any information about the toxic effects of PBBs on animals was
publicly known (Shah, 1978).
The Forest Waste Disposal site consists of an 11-acre,
abandoned, municipal and industrial waste landfill and 9 surface
impoundments. It is located in Genesee County, Michigan, and is
surrounded by agricultural land and undeveloped woodlands and
wetlands. Forest Waste Disposal conducted landfill operations from
1972 to 1978. PBB-contaminated feed has recently been found in the
landfill. A decontamination programme has been recommended (Anon.,
1988).
The Michigan Chemical Corporation stated that, in their
opinion, PBBs would eventually undergo oxidative/biological
degradation forming carbon dioxide, water, and bromide ion (Cordle
et al., 1978). However, studies on PBBs in soil indicate that they
may remain in soils for many years, because of their resistance to
degradation (Jacobs et al., 1976).
4.3.2 Thermal decomposition of PBBs
There is little information on the pyrolysis of PBBs. The
products of the thermal decomposition of PBBs depend on the
temperature as well as on the amount of oxygen present.
Norris et al. (1973) constructed a special apparatus to measure
the relative amounts of bromine from octabromobiphenyl converted
during combustion, when these materials were used as additives in
thermoplastic resins. An exact temperature is not given. Hydrogen
bromide and bromine were not detected.
Waritz et al. (1977) carried out experiments to determine the
approximate lethal temperature of hexa- and octobromobiphenyl. The
dense clouds of fumes obtained at 350 °C were lethal to rats whereas
those produced at 290 °C were not. The fumes were not analysed.
Earlier experiments by Benbow & Cullis (1975) on the pyrolysis
of decabromobiphenyl pressed together at 160 °C with polystyrene and
polypropylene, respectively, showed that, during flameless
combustion, decabromobiphenyl appeared to be volatilized virtually
unchanged from the polymer, whereas when the polymer burned, the
decabromobiphenyl was converted quantitatively to hydrogen bromide.
In these early experiments, the analytical methods were not so
refined that it was possible to detect furans and dioxins. O'Keefe
(1978) pyrolyzed samples of FireMaster FF-1 at 380-400 °C in open
glass tubes and in tubes sealed after nitrogen flushing. Analysis by
low resolution direct probe mass spectrometry showed the presence of
tetra- and pentabrominated dibenzofurans in extracts of the open
tube pyrolyzed material and trace levels of tetrabromodibenzofuran
in those from PBB pyrolyzed under nitrogen.
Buser et al. (1978) studied the pyrolysis of FireMaster BP-6
with oxygen in sealed tubes. The flame retardant was completely
destroyed at 700 °C, but, at 600 °C, new compounds were formed, one
of which was probably tetrabromodibenzofuran.
The diversity of possible brominated and mixed brominated
furans and their toxicological implications led to further
refinements in analytical methods (Buser, 1986) and to the demand
for, and synthesis of, suitable standard isomers (Mason et al.,
1987a; Sovocool et al., 1987a; Munslow et al., 1989). There are over
5000 halogenated dibenzodioxins and dibenzofurans containing
chlorine and/or bromine, over 400 of which are 2,3,7,8-substituted
tetra-, penta- and hexahalo congeners suspected to be of high
toxicity (Buser 1987). These mixed congeners are of particular
importance with regard to chemical waste burning (Schäfer &
Ballschmiter, 1986).
Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen have shown that small amounts of bromobenzenes and
lower brominated biphenyls are formed (600-900 °C), but no furans
(Thoma et al., 1987a; Thoma & Hutzinger, 1989).
In contrast, the pyrolysis of FireMaster BP-6 in an open quartz
tube (700-900 °C) in the presence of oxygen yielded over 3 mg/kg
(ppm) of di- to heptabrominated dibenzofurans, though the pyrolysis
of pentabromodiphenyl ethers yielded brominated dibenzofurans at
over 300 times this level (Thoma et al., 1987a). In the presence of
polystyrene and polyethylene, higher levels of brominated
(mona-tetra) dibenzofurans (over 8 and 51 mg/kg (ppm), respectively)
were found (Thoma et al., 1987a). Pyrolysis of FireMaster BP-6 with
PVC at 800 °C yielded mixed bromide/chloride biphenyls, the bromine
atoms being substituted by the chlorine. No ring closure to dioxins
and furans occurred (Thoma et al., 1987b).
Decabromobiphenyl was pyrolyzed for 10 min at 800 °C in a
loosely plugged quartz tube. The pyrolysates were extracted with
toluene and after clean-up, analysed using GC/MS. No brominated
dioxins or dibenzofurans were detected (detection limits
0.2-0.8 µg/g). The clean-up was said to be very difficult because of
the formation of a large number of brominated compounds that were
not dioxins or furans. Debromination of decabromobiphenyl appeared
to be the main reaction, but no details were given (Atochem, 1987).
Zacharewski et al. (1988) pyrolyzed samples of FireMaster(R)
BP-6 in open quartz tubes at 800 °C for 10 min. The resulting
products, mainly tetrabromodibenzofurans (1183 µg/g) but also
tribromo-, pentabromo-, hexabromo-, and heptabromodibenzofurans
(187, 584, 107, and 11 µg/g, respectively), were tested for toxicity
(see section 8.12.3.2). Very little is known about the toxicities of
brominated and brominated/chlorinated dioxins and furans, but they
are estimated to be of the same order as those of PCDD and PCDF
(Mason et al., 1987a; Safe, 1987).
Analysis of actual environmental samples has also been carried
out. Monobromo-polychloro substituted benzenes, biphenyls,
dibenzodioxins, and dibenzofurans have been detected in solid
material collected from a chimney of an industrial waste incinerator
(Schäfer & Ballschmiter, 1986). Brominated dibenzo furans with a
very small amount of mixed brominated/chlorinated compounds were
detected in soot from an accidental fire at a bowling alley (Buser,
1986). Schwind et al. (1988; 1989) analysed samples from a municipal
waste incinerator and detected for the first time a complete series
of tetrahalogenated dibenzofurans (Cl4DF, Br1Cl3DF,
Br2Cl2DF, Br3Cl1DF and Br4DF). It is possible that PBCDD/F
could occur during the incineration of flame retardant-treated
plastic material, which produces PBDD and PBDF. These could react
with PVC via the mixed brominated/chlorinated dioxins and furans to
PCDD and PCDF (Schwind et al., 1988).
As with PCB disposal, the destruction of PCB-contaminated waste
should be carefully controlled. For PCBs, a burning temperature
above 1000 °C for 2 seconds is recommended (WHO/EURO, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Only one report is available on PBB levels in air. It refers to
air samples taken in the vicinity of three PBB-manufacturing or -
processing plants in the USA (Stratton & Whitlock, 1979). Traces of
hexabromobiphenyl (0.06-0.10 ng/m3) were found at two of the three
industrial sites examined.
Further information on PBB levels in ambient air, e.g., near
municipal incinerators, is lacking.
5.1.2 Water and sediments
5.1.2.1 Surface waters
Surface waters have been monitored in the vicinity of PBB-
producing or -processing industrial sites in the USA and in the
vicinity of the Gratiot County landfill (Michigan, USA), which had
received 122 000 kg of wastes containing 60-70% PBBs between 1971
and 1973. The results are summarized in Table 21.
Depending on the sources, the predominant PBB compounds
detected in surface waters were hexabromobiphenyl and
decabromobiphenyl. However, only Stratton & Whitlock (1979)
determined all PBB homologues from Br1 to Br10, the percentage
composition of some of which is given in Table 22.
5.1.2.2 Sediments
Generally, PBBs reach higher concentrations in sediments (Table
23) than in the associated waters (Table 21).
PBB concentrations in sediments of the Pine River were as high
as 77 mg/kg near the Michigan Chemical Corp. plant. The assays
conducted from July 1974 to April 1975, upstream and downstream of
the plant, showed a decline in sediment PBB content to 6.2 mg/kg,
half a mile downstream, and to 0.1 mg/kg, 24 miles or 29 miles
downstream (Hesse & Powers, 1978).
Table 21. PBB levels in surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Pine River 1974 8 HxBB 0.01-3.2 Hesse (1975)
(downstream from
the Michigan
Chemical Co.,
St. Louis)
Tittabawassee 1974 2 HxBB < 0.01
Rivera
Canal (called 1977 3 total PBB not detected-46 Stratton & Whitlock (1979);
Platti Kill) in the (MoBB-DeBB) DeCarlo (1979)
vicinity of White
Chemical Co., Bayonne,
New Jersey
Canal (discharging 1977 1 total PBB < 0.2 Stratton & Whitlock (1979)
into Kill van Kull (HxBB-DeBB)
River) in the vicinity
of Standard T Chemical
Co., Staten Island,
New York
Storm sewer, 1977 5 total PBB < 0.2-210 Stratton & Whitlock (1979);
receiving swamp, etc. (HxBB-DeBB) DeCarlo (1979)
in the vicinity of
Hexcel Fine Organics
Sayreville, New Jersey
Table 21 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Drain waters at 1977 not HxBB 0.1-14 Shah (1978);
or near the margin specified Rosenblatt et al. (1982)
of the Gratiot County
landfill, Michigan
a Note that PBBs were not detected in fish from the Tittabawassee River in 1974 but were detected in 1983 (see Table 30).
Table 22. Percentage of different PBB homologues detected in surface water
samples taken in the vicinity of PBB-producing plantsa
PBB homologues Bayonne, New Jersey Sayreville, New Jersey
(White Chemical Corp.)b (Hexcel Corp.)c
Sample 1 Sample 2 Sample 1 Sample 2
PeBB 1 0 - -
HxBB 4 2 5 < 1
HpBB 2 2 1.6 < 1
OcBB 2 17 1 1
NoBB 15 20 1 2.6
DeBB 76 58 91 96
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Producer of laboratory quantities of various PBBs.
Concentrations measured upstream were all less than the
sensitivity limit of 30 µg/kg with the exception of one sample
collected a quarter of a mile upstream of the plant, which contained
60 µg/kg (Hesse, 1975). This latter analysis was repeated in 1977
(Hesse & Powers, 1978) giving 350 µg/kg (detection limit 100 µg/kg).
Hesse & Powers (1978) compared the PBB levels of Pine River
sediments from the same locations over a period of time after the
termination of FireMaster(R) BP-6 production. The results of the
1976 and 1977 analyses showed that PBB distributions and
concentrations in the sediments had not changed significantly in the
three years after PBB manufacture stopped.
Various PBB homologues were identified again only by Stratton &
Whitlock (1979) who examined aquatic sediments, sludge deposits, and
marsh soils near the sites of manufacture and use of PBBs in New
Jersey and New York (see Table 24).
More recently, sewage sludge has been analysed for PBBs
(Strachan et al., 1983). The authors did not detect any PBBs, PCBs,
or chlorinated hydrocarbon pesticides in the three sludge samples
obtained from sewage treatment plants in three Indiana cities (USA).
However, the detection limit for PCBs and PBBs with the GC-MS system
used was about 10 µg/g, and this apparently is not sensitive enough,
even for PCBs. For example, the amounts of PCBs found in sewage
sludge of German (Lorenz, 1983) and Canadian (Webber et al., 1983)
cities ranged from 1.8 to 2.5 µg/g (dry weight) and from 0.13 to
1.61 µg/g (dry weight), respectively. Values for PBBs have not been
reported in these investigations.
Table 23. PBB levels in sediments and sludge from surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Near Michigan 1974/75 19 HxBB < 30-77 000 Hesse (1975)
Chemical Co. 1974 9 < 100-9200 Hesse & Powers (1978)
Pine River 1976 9 < 100-1200
1977 8 < 100-500
Tittibawassee River 1974 2 tr-16 Hesse (1975)
Near White Chemical 1977 3 total PBB < 10-20 Stratton & Whitlock (1979)
Co., Bayonne, New Jersey: (MoBB-DeBB)
Sediments from Platti
Kill Canal and Kill
van Kall River
Sludge from Platti 1 431 000
Kill Canal
Near Standard T Chemical 1977 1 60
Co., Staten Island, New
York Sediment from Kill
van Kull River (at
discharge site)
Near Hexcel Fine 1977 1 total PBB 4600 Stratton & Whitlock (1979)
Organics, Sayreville, (MoBB-DeBB) DeCarlo (1979)
New Jersey
Marsh Soil
Table 23 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Gratiot County 1977 not HxBB up to 17 000 Shah (1978)
landfill, Michigan specified Rosenblatt et al. (1982)
Associated sediments
of surface drain waters
Table 24. Concentration of PBB-homologues in aquatic sediment, sludge deposit,
or marsh soil samples taken in the vicinity of PBB-producing or -processing
plantsa (µg/kg dry weight)
PBB Bayonne, New Jersey Staten Island, Sayreville,
(White Chem. Corp.)b New Jersey New Jersey
(Standard T (Hexcel
Chem Corp.)c Corp.)d
Sediment Sludge Sedimente Marsh soile
samples:e
1 + 2 3
MoBB - n.d. 540 n.d. n.d.
DiBB - n.d. 2200 n.d. n.d.
TrBB - n.d. 4300 n.d. n.d.
TeBB - n.d. n.d. n.d. n.d.
PeBB - n.d. 590 n.d. n.d.
HxBB n.d. 10 3800 40 30
HpBB n.d. 10 3300 20 n.d.
OcBB n.d. n.d. 3600 n.d. n.d.
NoBB n.d. n.d. 22 500 n.d. 80
DeBB n.d. n.d. 390 000 n.d. 4500
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Major user of FireMasterR BP-6.
d Producer of laboratory quantities of various PBBs.
e n.d. = Not detected (detection limit = < 10 µg/kg).
Surficial sediments from the St. Lawrence River (USA/Canada)
were analysed for HxBB, but the compound was not detected (estimated
detection limit: 1 ng/g) at the ten stations surveyed (Sloterdijk,
1991).
5.1.2.3 Groundwater
Groundwater monitoring data from the Gratiot County landfill
(Michigan, USA) mentioned above, have shown trace levels of PBBs,
even outside the landfill area (see Table 25). However, so far,
domestic drinking-water wells have not shown any traces of PBBs
(Shah, 1978). Groundwater near the disposal site of PBB-
contaminated animals and other products (see section 4.3.1) in
Kalkaska County (Michigan, USA) is reported not to be contaminated
by PBBs (Shah, 1978).
Table 25. PBB levels in groundwater from Gratiot County landfill, 1977a
Site No. of PBB compound PBB
samples examined concentration
range (µg/litre)
Test wells within 4 HxBB 0.5-26
the landfill site
Observation wells 11 0.1-4.4
outside the landfill
area
Domestic not specified not detected
drinkingwater wells
near the landfill
a From: Shah (1978).
5.1.3 Soil
Data on soil pollution by PBBs are available for areas of
manufacture, use, or disposal of PBBs (Table 26), and for soils from
fields, etc. of the PBB-contaminated Michigan farms (Tables 27 and
28).
Concentrations of PBBs in soils from industrial sites were
highest (more than 2000 mg/kg) in areas around the Michigan Chemical
Company (see Table 26). Although such highly contaminated soils
were removed (Hesse & Powers, 1978), Hill et al. (1982) still found,
some years later, PBB levels up to 2130 mg/kg in soils of the former
manufacturing site. Various PBB homologues from Br4 to Br10 were
present in the industrial soil samples (see Table 27).
Table 26. PBB levels in soils near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
Michigan Chemical Co.,
St. Louis, Michigan
bagging area not specified 1 HxBB 3500 mg/kg Hesse (1975);
Hess & Powers (1978)
loading area of not specified 1 HxBB 2500 mg/kg
the plant
"former manufacturing not specified 3 PBB 16-2130 mg/kg Hill et al. (1982)
site" (C12H6Br4-C12H3Br7)
Vicinity of White
Chemical Co.,
Bayonne, New Jersey
150 m east 1977 1 total PBB 4.250 mg/kg Stratton & Whitlock (1979)
150 m west of the 1977 1 (C12H9Br1-C12Br10) 1.135 mg/kg DeCarlo (1979)
plant
not specified not specified PBB 0.75-2.8 mg/kg Di Carlo et al. (1978)
Vicinity of Standard 1977 4 total PBB 10-100 µg/kg Stratton & Whitlock (1979)
T Chemical Co., Staten (MoBB-DeBB)
Island, New York
75 m south west 30
900 m west 10
Table 26 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
1500 m south 10
700 m east (prevailing 100
down-wind direction)
Vicinity of Hexcel 1977 total PBB Stratton & Whitlock (1979);
Fine Organics, (MoBB-DeBB) DeCarlo (1979)
Sayreville, New Jersey
75 m southeast 1 40 µg/kg
Soil in roadside 1 3400 µg/kg
ditch
Gratiot County landfill,
St. Louis, Michigan
Samples inside of the not not "PBB" 12 (16) mg/kg Rosenblatt et al. (1982)
landfill from the specified specified HxBB
uppermost 2.5 cm
(after capping of
the landfill)
Sample somewhat distant "PBB" 61 µg/kg
from the landfill, in (HxBB)
the area of the Michigan
Chemical plant
Table 27. Concentration of PBB homologues detected in soil samples taken in the vicinity of PBB-producing or processing plants
(µg/kg dry weight)
PBB Bayonne, New Jerseya Staten Island, New Yorka Sayreville, New Yorka Michiganb
(White Chemical Corp.)c (Standard T-Chemical Corp.)d (Hexcel Corp.)e (Michigan Chemical Corp.)f
MoBB n.d.g n.d.g n.d.g -
DiBB n.d.g n.d.g n.d.g -
TrBB n.d.g n.d.g n.d.g -
TeBB n.d.g n.d.g n.d.g < 1000-510 000
PeBB n.d.g n.d.-100 n.d.g 4000-60 000
HxBB 15-30 n.d.-10 40-90 12 000-670 000
HpBB 30-110 n.d.-10 n.d.-90 < 1000-190 000
OcBB 90-150 n.d. n.d.-170 -
NoBB 330-2200 n.d. n.d.-440 -
DeBB 530-2100 n.d.-10 n.d.-2600 -
a Data from: Stratton & Whitlock (1979); No. of samples = 2, 4, 2 respectively.
b Data from: Hill et al. (1982); No. of samples = 3.
c Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
d Major user of FireMaster(R) BP-6.
e Producer of laboratory quantities of various PBBs.
f Producer of FireMaster(R) BP-6.
g n.d. = Not detected (detection limit = < 10 µg/kg).
Table 28. Composition and concentration of PBBs in soil samples from former
FireMaster(R) manufacturing plant site (St. Louis, Michigan)a
% Composition (concentration in mg/kg)
FireMaster(R)
Compound Lot #5143 Soil 1 Soil 2 Soil 3
Tetrabromobiphenyls < 0.1 23.9 (510) 11.3 (6) (< 1)
Pentabromobiphenyls
2,2',4,5,5'- 3.9 2.8 (60) 9.4 (5) 12.5 (2)
2,2',4,4',5- < 0.1 5.2 (110) (< 1) (< 1)
2,3',4,4',5- 5.7 27.7 (590) 9.4 (5) 12.5 (2)
Hexabromobiphenyls
2,2',4,4',5,5'- 54.9 24.4 (520) 56.6 (30) 62.5 (10)
2,2',3',4,4',5- 10.3 4.7 (100) 7.5 (4) 6.2 (1)
2,3',4,4',5,5'- 5.0 1.9 (40) 5.7 (3) 6.2 (1)
2,3,3',4,4',5- 2.1 0.5 (10) (< 1) (< 1)
Heptabromobiphenyls
2,2',3,4,4',5,5'- 12.8 5.2 (110) (< 1) (< 1)
2,2',3,3',4,4',5- 1.7 3.8 (80) (< 1) (< 1)
(Total PBBs) (2130) (53) (16)
a Adapted from: Hill et al. (1982).
Hill et al. (1982) identified not only PBB homologues, but also
the isomeric composition of PBBs in the soil samples from the
Michigan Chemical Corp. plant (Table 28). Thus, they provided more
exact analytical data and were able to make an interesting
comparison with the original FireMaster(R) mixture; conclusions
could then be drawn on the environmental fate of PBBs (section 4.2).
According to Shah (1978), test samples of the Gratiot County
landfill showed that, in general, the concentrations of PBBs in the
fill increased with depth and were highest at a depth of 3 to 7.6 m
below the top of the refuse.
As a consequence of the Michigan cattle food mixing error, the
soils of the farms involved have been contaminated by PBBs, mainly
through the faeces of the exposed animals. Fries (1985b) calculated
that about 145 kg of PBBs were distributed in this way, and that
most of this was located on 20-25 farms. (The total number of
quarantined farms was over 500; Robertson & Chynoweth, 1975).
Concentrations of PBBs in soil samples from fields that had
received PBB-contaminated manure were as high as 371 µg/kg (dry
weight), whereas levels in samples from manure piles and from dirt
exercise lots were as high as 2000 µg/kg (Jacobs et al., 1978;
Fries, 1985b).
Soil contamination by PBBs can result in PBB accumulation in
animals, when they have direct access to the contaminated soil.
This is most likely to occur when animals are confined to dirt lots
on which manure-containing PBB has been deposited. Crops grown on
PBB-contaminated soils are not considered an important source of PBB
contamination in animals (Fries & Jacobs, 1986).
Soils from industrial sites have, in general, been more heavily
contaminated than Michigan soils.
5.1.4 Feed and food
5.1.4.1 Feed
Contamination of feed by PBBs has been reported only in
connection with the Michigan PBB incident.
In 1973, about 290 kg (Fries, 1985b) - 1000 kg (IARC, 1978) of
FireMaster(R) FF-1 was inadvertently mixed in cattle feeds and
delivered to Michigan farms.
Three feed preparations appeared initially to be involved in
the Michigan episode with PBB levels as follows:
Feed No. 405, 2.4 mg PBB/kg,
Feed No. 410, 1790 mg PBB/kg,
Feed No. 407, 4300 mg PBB/kg
(Cordle et al., 1978).
A concentration as high as 13 500 mg PBB/kg was also cited
(Kay, 1977; Di Carlo et al., 1978; Damstra et al., 1982). Feed of
one highly contaminated farm (Halbert farm) is reported to have
contained 2900 mg PBB/kg (Fries, 1985b).
In 1974, 68% of 1770 feed samples collected in Michigan
contained PBB residues: 60% in the range of trace to 0.99 mg/kg, and
8% over 1 mg/kg. Resampling in 1975 revealed that 6% of 1208 feed
samples were contaminated and that fewer than 0.16% contained more
than 1 mg PBB/kg. In 1976, only 0.3% of 663 samples analysed were
contaminated: no samples contained more than 0.1 mg/kg (Di Carlo
et al., 1978).
PBB residues were not detected in harvested forages grown on
soils with residue levels as high as 0.3 mg/kg (Fries & Jacobs,
1980).
In 1974 and 1975, low-level feed contamination with PBBs was
detected in Indiana and Illinois, which are neighbours of Michigan
(Di Carlo et al., 1978).
5.1.4.2 Food
Again, almost all the data available on PBB residues in food
are derived from the Michigan cattle food contamination incident in
1973.
The extent to which the general population was exposed depended
on where they obtained their milk, dairy products, and eggs, i.e.,
direct from the contaminated farms or from sources where
contaminated products had been mixed with non-contaminated samples.
Table 29 shows examples of some PBB levels in Michigan foods.
Whereas in 1974 milk from some highly contaminated cows contained
PBB concentrations of up to 900 mg/kg fat (Robertson & Chynoweth,
1975), canned milk samples contained concentrations of up to
1.6 mg/kg fat (Cordle et al., 1978). The most highly contaminated
milk (1 to > 100 mg PBB/kg milk fat) originated from a total of 40
herds with different levels of PBBs at the time of detection (Fries,
1985b). In 1975, PBBs were still detected in milk from some herds
(Kay, 1977).
Data on meat can be derived from Table 33, which shows PBB
levels in Michigan farm animals.
Milk contains far less fat than meat (about 4% versus 30%), and
butterfat contains only 40% of the PBB concentration found in the
animal from which it comes (Fries et al., 1978b; Rosenblatt et al.,
1982). Among the dairy products, PBBs are again concentrated in the
high-fat products (Murata et al., 1977; Zabik et al., 1978).
Table 29. Some examples of PBB levels in food (contaminated as a consequence of
the Michigan PBB incident in 1973)
Product Year of PBB concentration References
sampling (mg/kg)
Milka 1974 2.8-270.5h Cordle et al. (1978)
Milkb 1974 44-900h Robertson & Chynoweth (1975)
Milkc 1974 43-56h Jackson & Halbert (1974)
Milkd 1974 up to 595 Kay (1977); IARC (1978)
Milke 1974 1-> 100h Fries (1985b)
Canned milk 1974 1.15-1.62h Cordle et al. (1978)
Dry skimmed milkf 1974 0.75-1.5 Isleib & Whitehead (1975)
Fluid milk 1974 < 0.02-1.15
processors' products
Butter 1974 1-2h Cordle et al. (1978)
Cheese 1974 1.4-15.0h
Milkg 1975 1-13h Kay (1977); IARC (1978)
Eggs 1974 up to 59.7
a = Collected from individual farms.
b = Collected from 21 cows.
c = Collected from 2 cows (having 174 and 200 mg PBBs/kg in body fat,
respectively).
d = Collected from 22 farms.
e = Collected from 28 herds.
f = From one dairy plant.
g = Collected from 16 herds.
h = On a fat basis.
In May 1974, the US Food and Drug Administration (FDA)
established the following enforcement limits for unavoidable
residues of PBBs in foods: 1 mg/kg in the fat of meat, milk, and
dairy products, 0.3 mg/kg in animal feeds, 0.1 mg/kg in eggs. These
enforcement guidelines were reduced in November 1974 to 0.3 mg/kg in
the fat of meat, milk, and dairy products, and 0.5 mg/kg in eggs and
animal feeds. In February 1977, the FDA rejected a petition to lower
the enforcement guideline level to 0.02 mg/kg for all food products
(IARC, 1978). However, according to Fries (1985b), final
legislation, Act 77, lowered the tolerance to 0.02 mg/kg in the body
fat of all cull dairy cows offered for slaughter. (Unlike the
situation under the previous regulations, the finding of a single
animal with a higher than legal body fat level did not lead to
quarantine and the disposal of the whole herd.) As a result of the
rigid quarantine policy, the food levels of PBBs decreased in
Michigan. In 1975, none of 18 milk samples, 3 out of 14 butter
samples, and none of 13 cheese samples exceeded FDA guidelines
(0.3 mg/kg). Also in 1975, 245 of 2040 meat samples were
contaminated with PBBs: 24 contained more than 0.3 mg/kg. None of
the meat specimens collected in 1976 exceeded FDA guidelines: 96% of
1430 samples were contaminated, but only 1 sample contained more
than 0.6 mg/kg of PBBs. A market basket survey of meat in 1976
revealed detectable PBBs in only 1 out of 102 samples in Michigan
(Di Carlo et al., 1978).
Additional information on PBB findings is presented in several
government reports, which are cited by Di Carlo et al. (1978).
According to these reports 29 170 products had been assayed. In
1974, 14 out of 16 milk samples, 4 out of 34 butter samples and 11
out of 23 cheese samples, collected in Michigan, were found to
exceed FDA guidelines for PBBs. Another survey showed that 24.9% of
272 finished product samples, collected from May to October 1974,
were contaminated with PBBs and that 15.8% contained more than
0.3 mg/kg (Di Carlo et al., 1978).
PBBs were also detected in other states in the USA, for example
in beef in Iowa, duck in Wisconsin, chicken in Alabama, Mississippi,
New York, and Texas, and turkey in Indiana: the levels were
extremely low. During 1975 and 1976, PBBs were found in 9 out of 597
food samples outside of Michigan (Di Carlo et al., 1978).
Food contamination, not derived from the Michigan PBB-
incident, becomes evident, when looking at PBB levels in fish (see
Table 30) some of which are used for human consumption. For example,
skinless fillets of carp from the Pine River, captured in the
vicinity of Michigan Chemical Company, contained 1.33 mg PBBs/kg
(wet weight basis) which is approximately equivalent to 30 mg/kg on
a fat weight basis (Hesse & Powers, 1978). This was obviously
greatly in excess of the US FDA tolerance limit for beef, a
tolerance limit for fish has not been established (Hesse & Powers,
1978).
More recent information on "background" PBB levels in food may
be expected in future via a USA data collection programme
(Foodcontam) initiated by the US Food and Drug Administration which
includes PBBs besides other chemicals (Minyard et al., 1989).
Table 30. PBB levels in fish
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1974 Pine River, downstream Carp skinless not detected-1330 wet HxBB Hesse &
from St. Louis (vicinity (Cyprimus carpio) fillets Powers
of Michigan Chemical Co.) (1978)
White sucker 670
Northern pike 540
Bullhead 450-780
1974 Tittabawassee Carp not detected
River (Cyprimus carpio)
Freshwater drum not detected
(Aplodinotus
grunniens
1976 Pine River, downstream Carp 60-750
from St. Louis (vicinity (Cyprimus carpio)
of Michigan Chemical Co.)
Northern pike 180-230
1976 Pine River, downstream Largemouth skinless not detected-740 wet HxBB Hesse &
from St. Louis (vicinity bass fillets Powers
of Michigan Chemical Co.) (1978)
Smallmouth bass 130
Rockbass 320-700
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1977 Kill van Kull River Killifish whole 220 dry total PBB Stratton &
(vicinity of White Chemical HxBB-DeBB Whitlock
Co., Bayonne, New Jersey); (1979)
Port Johnson
1977 Kill van Kull River Killifish whole 230 dry total PBB
(vicinity of Standarad T HxBB-DeBB
Chemical, Staten Island,
New York); canal at
discharge site
Not Lake Huron Yellow perch 0.3-0.8 Kreis & Rice
specified (Saginaw Bay) (1985)
Saginaw Bay Catfish 21.0
1983 Pine River Hogsucker whole 6000 fat most Jaffe et al.
abundant (1985)
congeners
1983 Chippewa River Carp 5300-15 000
1983 Tittabawassee 140-160
River
1983 Shiawassee River 120
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1983 Flint River 15-32
1983 Saginaw River 80-200
1983 Saginaw Bay 110-1100
Four samples of cow's milk from Germany have been analysed for
PBBs (Krüger, 1988). Three congeners were detected; BB 153
(0.025-0.053 µg/kg milk fat), BB 180 (0.001-0.007 µg/kg) and BB 187
(0.005-0.014 µg/kg). The other 30 congeners covered by the method
were not detected with detection limits ranging from 0.001 to
0.003 µg/kg milk fat (see Table 33).
The processing and cooking of contaminated food have been found
to have some potential for reducing PBB levels. Spray-drying
appeared to reduce the contents of PBBs in whole milk and skim milk
by 30-36% and 61-69%, respectively (Murata et al., 1977; Zabik
et al., 1978). Pressure cooking of chicken pieces also resulted in a
loss of PBBs, however, part of the PBBs lost were found in the drip
(Zabik et al., 1978).
5.1.5 Other products
Antibiotics used for attending farm animals were also found to
be contaminated: Levels of PBBs in aureomycin, which was distributed
by the Michigan Farm Bureau, were as high as 70 mg/kg (Di Carlo
et al., 1978).
5.1.6 Terrestrial and aquatic organisms
5.1.6.1 Aquatic and terrestrial plants
Only few data on PBB contamination of aquatic and terrestrial
plants are available. Stratton & Whitlock (1979) analysed algae
(e.g., filamentous green algae) from surface waters in the vicinity
of White Chemical (Bayonne, New Jersey) and near Standard T Chemical
Company (Staten Island, New York) for PBBs (MoBB through DeBB). The
two samples did not contain detectable levels of PBBs (detection
limit: 10 µg/kg, dry weight). However, bottom sediments taken in the
same location contained hexa- and heptabromobiphenyl.
Surface contamination was observed on terrestrial vegetation in
the vicinity of PBB facilities (up to 92 mg/kg dry weight; Stratton
& Whitlock, 1979). As Chou et al. (1978) reported, the PBB
contamination of field soils in Michigan (USA) did not result in any
detectable surface contamination of field crops.
5.1.6.2 Animals
a) Wildlife
Most earlier data available on PBB contamination of wildlife
refer to freshwater fish (Table 30) and birds (Tables 31 and 32),
primarily waterfowl in the USA. Recent reports refer to PBB
contamination of fish-eating mammals and birds from marine
environments in the USA (Kuehl et al., 1991) and in Europe (Jansson
et al., 1987, 1992; Krüger, 1988). Residues were found also in
terrestrial mammals (Jansson et al., 1992) and in freshwater and
marine fish in Europe (Krüger, 1988; Jansson et al., 1992).
Table 30 gives PBB levels in fish captured for analysis in
industrialized areas of the USA, at various distances from PBB-
containing or -using facilities. PBBs were detected in several fish
species from all rivers or bays examined. The PBB levels ranged up
to a maximum of 1.33 mg/kg wet weight (approximately equivalent to
30 mg/kg on a fat basis) found in carp from the Pine River near
Michigan Chemical Company (Hesse & Powers, 1978).
No apparent change in PBB concentrations was observed in Pine
River fish between 1974 and 1976 (Hesse & Powers, 1978; see also
Table 30). Although Michigan Chemical Co. had terminated PBB
production in 1974, even in 1983, Jaffe et al. (1985) detected PBB
in fish from the Saginaw River system, with highest concentrations
in fish from Pine and Chippewa Rivers (Table 30). While carp from
Tittabawassee River, to which Pine River joins, did not contain any
detectable PBBs in 1974, PBB-residues were detected (approximately
150 µg/kg on a fat basis) in 1983 (Table 30).
Various PBB homologues were examined in killifish (Oryzias
latipes) from Kill van Kull River near White Chemical Co.
(Bayonne, New Jersey). The main component found was NoBB. In the
vicinity of a FireMaster(R)-using facility (Staten Island, New
York), killifish samples contained only HxBB (Stratton & Whitlock,
1979).
PBB contamination has been reported in wild ducks collected
within two miles of the Michigan Chemical Corporation plant (Table
31), in eggs of waterfowl nesting around Green Bay and other areas
of Lake Michigan and on Lake Michigan island (Table 32), and, in
bald eagles found moribund or dead in 13 US states (Table 31).
Approximately one third of bald eagles examined contained PBB
residues (see Table 31).
Concentrations of PBBs in duck samples with skin left on were
considerably higher than those in skinless samples (see Table 31)
indicating that much of the PBBs is associated with the skin or fat
layer between the skin and muscle (Hesse & Powers, 1978).
Table 31. PBB residues in birds (ducks and bald eagles)
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
1974 Pine River Mallard breast tissue 3 0.25 Hesse & Powers
within two miles (skinless) (1978)
downstream from
St. Louis
Wood duck 0.29
Teal 1.8
1976 Mallard breast tissue:
skinless 3c 0.24
with skin 3c 2.00
Wood duck
4c 0.17
4c 2.70
1977 Wood duck breast tissue:
skinless 4c 0.08
with skin 4c 0.23
1977 Teal breast tissue:
skinless 0c (1) not detected
with skin 0c (1) not detected
Table 31 (contd).
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
13 US states Bald eagle found moribund Kaiser et al.
(Haliaeetus or dead; (1980)
leucocephalus)
carcass 10 0.03-0.27 0.07
(32)d
brain 7 0.03-0.17 0.05
a Number of samples containing residues; median is based on this number. Total number of samples in parentheses.
b PBB values were based on the major hexabromobiphenyl peak (BB 153).
c Paired samples.
d Detection limit: 0.02 mg PBBs/kg.
Table 32. PBB residues in eggs of fish-eating and non-fish-eating waterbirds from Green Bay and Lake Michigan (USA)
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Fish eater
1975 Green Bay Little gull 1 n.d. Heinz et al.
(Sensila Wildlife Area) (Larus minutus) (1985)
1977 three Lake Michigan islands Red-breasted merganser 114 0.06 n.d.-0.13 Haseltine et al.
off the tip of Door County, (Mergus serrator) (109) (1981)
Wisconsin
1977 islands in north-western Red-breasted merganser Heinz et al.
Lake Michigan (Mergus serrator): (1983)
eggs from the same nests
randomly selected 49 0.05
unhatched 49 0.04
1977 Lake Michigan Herring gull 9 0.18 0.11-0.25 Heinz et
(Gravel Island) (Larus argentatus) (9) al. (1985)
1977 Green Bay Common tern 10 0.06 0.02-0.22 Heinz et al.
(Lone Tree Island) (Sterna hirundo) (10) (1985)
Green Bay (St. Vital Island) Common tern 2 (2) 0.03 0.03-0.04
(Sterna hirundo)
Green Bay (Portage Point) 2 (2) 0.03 0.02-0.06
Green Bay (Cat Island) Double-crested 4 (3) 0.01 n.d.-0.02
cormorant
(Phalocrocorax
auritus)
Table 32 (contd).
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Lake Michigan (Fish Island) 6 (3) 0.02 n.d.-0.05
Green Bay (Oconto Marsh) Black-crowned night-heron 1 (1) 0.02
(Nycticorax nycticorax)
Green Bay (Oconto Marsh) Green-backed heron 1 n.d.
(Butorides striatus)
Non-fish eater
1977 Three Lake Michigan Mallard 22 n.d. Haseltine
islands off the tip of (Anas platyrhynchos) et al. (1981)
Door County, Wisconsin
1977 Lake Michigan (three Gadwall 4 n.d.
islands off the tip of (Anas strepera)
Door County, Wisconsin)
Black duck (Anas rubripes) 3 n.d.
a Number of collected eggs (in parentheses: number of eggs with quantifiable levels of PBBs).
b PBB values were based on hexabromobiphenyl;
n.d. = No residue of quantifiable level. Level over which quantification was possible: 0.02 mg/kg.
Samples with no detectable residues were calculated in the means as one-half the quantification level.
While the majority of ducks analysed from the Pine River
contained measurable concentrations of PBBs (Table 31), the eggs of
ducks from Lake Michigan islands did not contain detectable PBB
residues (Table 32). In contrast, most eggs of fish-eating
waterbirds from Green Bay and Lake Michigan showed PBB residues
(Table 32). Highest concentrations were detected in herring gull
eggs (0.18 mg/kg wet weight), perhaps reflecting their year round
residence on the Great Lakes (Heinz et al., 1985).
Stratton & Whitlock (1979) analysed a snapping turtle captured
in the vicinity of Hexcel Fine Organics Division (Sayreville, New
Jersey) for hexa- to decabromobiphenyls and found a tissue
concentration of 20 µg hexabromobiphenyl/kg (dry weight).
Di Carlo et al. (1978) reported on PBB contamination of
miscellaneous wildlife, such as deer, rabbits, coyotes, and ravens,
without, however, specifying the sampling locations and the levels
of contamination.
In Europe, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153) was found
in fish from German and Swedish rivers at concentrations ranging
from 0.3 to 0.6 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see
also Tables 33 and 34). A trout sample from a breeding farm
contained much lower levels of PBBs than the fish samples from the
rivers (Krüger, 1988).
A residue of 22 µg BB 153/kg lipid was observed in pooled
samples of osprey specimens found dead in various parts of Sweden
(Jansson et al., 1992; Table 34).
Swedish reindeers (pooled samples) showed BB 153 levels as low
as 0.04 µg/kg lipid (Jansson et al., 1992; Table 34).
PBBs (as a group) were not found in otters (Lutra canadensis)
from a region relatively remote from industrial sites in north
eastern Alberta (Canada) (Somers et al., 1987).
Fish samples (freshwater and marine species) collected in 1983
from an industrial area of Japan (Osaka) did not contain "PBBs" (not
specified) (Watanabe & Tatsukawa, 1990).
Recently, PBBs have been identified in bottlenose dolphins
(Tursiops truncatus) collected during the 1987/88 mass mortality
event along the Atlantic Coast of the USA. All three animals
(females), analysed for PBBs (tetrabromo- to hexabromobiphenyl
congeners) within a subset of the screening programme for
anthropogenic contaminants, contained PBBs at concentrations ranging
from 14 to 20 ng/g lipid (Kuehl et al., 1991).
Table 33. Average concentrations (µg/kg lipid) of PBB congeners in fish, seals, cows, and human milk samples
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 103 0.02 0.12 0.10 < 0.02 < 0.02 not analysed
BB 131 + 142/146 0.30 0.62 0.25 0.03 < 0.02 < 0.01
BB 132 0.33 1.25 0.62 0.15 < 0.02 0.05
BB 135 + 144/151 0.69 4.10 1.48 0.46 < 0.02 0.12
BB 147/135 + 144 0.21 0.31 0.25 < 0.02 < 0.02 < 0.01
BB 148/136 0.10 0.13 0.11 < 0.02 < 0.02 < 0.01
BB 149 0.26 0.45 0.53 < 0.02 < 0.02 < 0.01
BB 153 0.60 2.39 1.31 0.81 0.04 1.03
BB 154/151 0.22 0.54 0.37 < 0.02 < 0.02 0.01
BB 155 0.66 2.64 1.11 0.40 < 0.03 0.05
BB 169 < 0.01 15.16 < 0.01 < 0.01 < 0.01 0.05
BB 176 0.03 < 0.01 0.02 < 0.01 < 0.01 < 0.05
BB 178 0.18 0.87 0.36 0.03 < 0.01 0.09
BB 179 0.08 0.04 0.04 < 0.01 < 0.01 < 0.05
BB 180 0.02 < 0.01 0.02 < 0.01 < 0.04 0.02
BB 181 + 174 0.01 0.01 0.01 < 0.01 < 0.01 < 0.05
BB 184 0.05 0.09 0.03 < 0.01 < 0.01 0.01
BB 185 0.03 < 0.01 < 0.01 < 0.01 < 0.01 < 0.05
BB 186 0.30 0.40 0.16 0.01 < 0.01 0.02
BB 187 + 182 0.03 0.05 0.04 0.03 0.01 0.33
BB 188 0.11 0.28 0.11 < 0.01 < 0.01 0.01
BB 192 0.01 < 0.01 < 0.01 < 0.01 < 0.01 n< 0.05
BB 194 0.07 < 0.02 0.04 < 0.02 < 0.02 < 0.05
BB 197 0.11 0.11 0.08 < 0.02 < 0.02 0.04
BB 198 0.27 0.14 0.12 < 0.02 < 0.02 < 0.05
BB 200 + 204 0.41 0.36 0.24 < 0.02 < 0.02 0.02
BB 201 0.09 < 0.02 0.03 < 0.02 < 0.02 < 0.05
Table 33 (contd).
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 202 0.87 0.42 0.36 < 0.02 < 0.02 0.01
BB 206 0.05 < 0.03 0.04 < 0.03 < 0.03 < 0.01
BB 207 0.06 < 0.03 0.02 < 0.03 < 0.03 < 0.01
BB 208 0.16 0.04 0.04 < 0.03 < 0.03 < 0.01
PBB 6.3 30.5 7.9 1.9 0.05 2.0
From: Krüger (1988).
Table 34. Concentrations (µg/kg lipid) of 2,2',4,4',5,5'-HxBB (BB 153)
in pooled biological samplesa
Species Number of Sampling Concentration
specimens in site of BB 153
the homogenate
Rabbit (Oryctlagus cuniculus) 15 S. Sweden not detected
Moose (Alces alces) 13 not detected
Reindeer (Rangifer tarandus) 31 N. Sweden 0.037
White fish (Coregonus sp.) 35 0.29
Arctic char (Salvelinus alpinus) 15 S. Sweden 0.42
Herring (Clupea harengus) 100 Bothnian Bay 0.092
Herring (Clupea harengus) 60 Baltic Proper 0.16
Herring (Clupea harengus) 100 Skagerrak 0.27
Ringed seal (Pusa hispida) 7 Svalbard 0.42
Grey seal (Halichoerus grypus) 8 Baltic Sea 26
Osprey (Pandion haliaetus) 35 S. Sweden 22
a From: Jansson et al. (1992).
In Europe, PBBs have been detected in seals (Phoca vitulina;
Pusa hispida), guillemots (Uria aalge; U. lomvi), and
white-tailed sea eagles (Haliaeetus albicilla). The concentrations
(estimated by comparison with the technical product FM BP-6) ranged
from 3 to 280 µg/kg lipid (Jansson et al., 1987). The concentrations
of PBBs in comparable samples from the Baltic Ocean were all higher
than concentrations in samples from the Arctic Ocean. The same was
true for polybrominated biphenyl ethers and PCBs (Jansson et al.,
1987).
Concentrations of BB 153 determined in marine fish ranged from
0.2 to 2.4 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see also
Tables 33 and 34). BB 153 levels of 0.4-26 µg/kg lipid were found in
seals (Krüger, 1988; Jansson et al., 1992; see also Tables 33 and
34).
Detailed isomer-specific PBB analyses were carried out by
Krüger (1988) in fish (several species) from the Baltic and North
Seas and from sections of the Lippe and Rur rivers in North
Rhine-Westphalia, Germany. Seal samples from Spitsbergen (Norway)
were also included in this investigation (Table 33). All samples
contained PBBs. The smallest number of PBB congeners was found in
seals (n = 5) from an area remote from industrial sites. The main
components were different hexabrominated isomers with
2,2',4,4',5,5'-hexabromobiphenyl reaching a mean concentration of
0.8 µg/kg fat. The mean concentrations of several PBB congeners and
isomers (penta- to nonabrominated biphenyls) measured in fish
(n = 35) ranged, mostly, between 0.01 and 2 µg/kg fat. The pattern
of PBB congeners found in fish differed in a characteristic manner,
depending on the different capture sites. While relatively high
amounts of nona- and octabromobiphenyls (besides polybrominated
biphenyl ethers) were present in fish from German rivers (n = 17;
several species), hexabrominated biphenyls were predominant in fish
from the North Sea and the Baltic Sea (n = 17; several species). In
all samples from the Baltic Sea (n = 6),
3,3',4,4',5,5'-hexabromobiphenyl was found in relatively high
concentrations (maximum concentration: 36 µg/kg fat), but it was not
detected in samples from the North Sea and from rivers. The
concentrations of the other hexabrominated biphenyls were mostly
higher in fish from the Baltic Sea than in fish from the North Sea.
b) Farm animals
Farm animals in Michigan were contaminated by PBBs, when
FireMaster(R) FF-1 was accidentally mixed with animal feed in
mid-1973 (see section 4.1). The PBB levels resulting from this event
varied greatly with the extent of exposure. Data reported in the
literature are compiled in Table 35. The extent of contami nation
can be seen from the fact that, during the months following the
event, 172 dairy and beef herds (18 000 animals), 32 swine herds
(3500 animals), 16 sheep flocks (1200 animals), and 92 chicken
flocks (1.5 million birds) were destroyed (Isleib & Whitehead, 1975;
Robertson & Chynoweth, 1975; Mercer et al., 1976). In relation to
these great numbers, the portion of highly contaminated animals was
small (e.g., 40 herds of cattle, as can be derived from
contamination values measured in milk (section 5.1.4.2).
5.2 General population exposure
Apart from data collected after the Michigan disaster, there is
only limited information on exposure of the general public. PBBs
have been detected in humans in the vicinity of manufacturing
premises and in a few sites in the USA and Europe, not directly
connected with PBB contamination.
Table 35. PBB levels in farm animals (derived from the Michigan
cattle food contamination incident in 1973)
Year Animal PBB concentrationa References
(Type of sample) (mg/kg)
1974 Poultryb (tissue) 4600 Kay (1977); IARC (1978)
Not specified Cattle (fat) up to 200 Pearson (1982)
Not specified Aborted calves 120-400 Kay (1977)
1974 Cattlec (body fat) 110-2480 Robertson &
Chynoweth (1975);
Mercer et al. (1976)
1974-75 Cattled (fat) 9-4100 Fries et al. (1978b)
1974 Cattleb (tissue) up to 2700 Kay (1977); IARC (1978)
1974 Cattlej (body fat) 174-200 Jackson & Halbert (1974)
March (1975) Dairy cattlee 1-12 Kay (1977)
1975 Cowsf (tissue-fat) not detected-1.69 Isleib & Whitehead (1975)
1975 Steers and heifersg not detected-2.27
(tissue-fat)
1975 Pigsh (tissue-fat) not detected-0.58
1975-76 Cattlei (male and not detected-0.13 Cook et al. (1978a)
female) (eye fat)
Not specified Cattlek (body fat) not detected-3.8 Mercer et al. (1976)
a PBB values were based on 2,2',4,4',5,5'-hexabromobiphenyl.
b From 22 farm premises.
c 21 highly exposed cows.
d 32 cows from one herd heavily contaminated during September/October
1973, and 9 calves borne to these cows in 1974.
e 16 herds of dairy cattle with a history of feed levels from 1 to 14 mg/kg PBB.
f-h Slaughter house survey during a 3-month period (January-April 1975).
f Number of samples: 216; mean-PBB: 0.018 mg/kg.
g Number of samples: 247; mean-PBB: 0.030 mg/kg.
h Number of samples: 213; mean-PBB: 0.017 mg/kg.
i Cattle of 5 affected herds.
j 2 cows of the Halbert farm.
k Cattle of 12 affected herds.
5.2.1 Quantified data on human exposure
5.2.1.1 Worldwide
For most human populations, direct data on exposure to PBBs
from various sources have never been documented. This is true also
for the possible exposure of the general population from the use of
PBB-containing plastic products, and from fumes, generated in the
combustion of these products inadvertently in fires, or from burning
in dumps (Kay, 1977), and, additionally, from sources such as
PBB-containing landfills or PBB-manufacturing and processing
plants.
5.2.1.2 The Michigan Accident
Widespread human exposure resulting from direct contact with
contaminated feed, and, primarily, from the consumption of PBBs in
meat, eggs, and dairy products has been reported from the state of
Michigan, USA (Kay, 1977; Landrigan, 1980; Fries, 1985b; Table 36).
Many Michigan residents were exposed to PBBs between the onset of
contamination in the autumn of 1973 and the establishment of the
quarantine of affected farm animals in the spring of 1974. There was
considerable variation in both lengths and levels of exposure. At
least 2000 families (primarily farmers and their neighbours)
received the heaviest exposure (Meester & McCoy, 1976; IARC, 1978).
Brilliant et al. (1978) concluded from their results of human
milk analyses, conducted in 1976, that about 8 million of the 9.1
million residents of Michigan have detectable body burdens of PBBs.
Further studies (see Table 37) confirmed this widespread
distribution of PBBs.
The amount of PBBs consumed or absorbed by the various groups
in Michigan cannot be determined accurately (Safe, 1984). However,
there have been some trials to estimate the possible exposure to
PBBs of farm families and other people. The estimates were based on
kinetic data and other observations, e.g., time and level of animal
exposure, residue levels in herds at the time of the contamination,
and serum levels of exposed people.
In this way, Fries et al. (1978a) estimated (assumptions: see
Fig. 4), that the total exposure of an individual in a farm family
consuming its own milk was, for example, 9.8 g over the 230-day
period, during which the contamination was undetected. The
cumulative intake over time is shown in Fig. 4. In addition, the
authors concluded that the most highly exposed people consumed from
5 to 15 g PBBs over a 230-day period via milk. The projected intake
of PBB via the meat of cows slaughtered for home consumption would
have exceeded the projected intake from milk.
Table 36. Approximate distribution of PBBs in the Michigan episodea
Item Amount (kg)
Total released 295
Not fed to livestock 45
Fed to livestock 250
Eliminated in faeces 125
Absorbed by animals 125
In human foods before regulation 94
a Modified from: Fries (1985b).
Application of a pharmacokinetic model (Tuey & Matthews, 1980)
to the mean serum concentrations for residents of quarantined farms
resulted in similar values, e.g., about 170 mg mean total exposure
per individual and 11.7 g highest exposure to PBBs (Fries, 1985b;
Brown & Nixon, 1979) supposed a consump tion of 1-20 g of PBB by
families on the most contaminated farms.
The exposure of an individual in the general population would
have a pattern over time as projected above for the farm family
(Fries et al., 1978a). However, the exposure level would have been
much less, because of dilution in the normal marketing channels (the
mixing of milk from a large number of producers; the use of meat of
cull dairy cattle for hamburger and processed meat products). The
calculations of Fries (1985b) indicate that total exposure was about
9-10 mg for an average male with an average adipose content.
However, the individual with the highest PBB serum concentration was
projected to have had a total exposure of about 800-900 mg.
Table 37. Distribution of serum levels of PBBs, Michigan, 1974a
Quarantined farms Non-quarantined farms
Adults Children Adults Children
Serum PBBs Number (%) Number (%) Number % Number %
(µg/litre)
0 3 3.7 - - 21 28.4 - -
2-19 43 52.4 8 28.6 52 70.3 29 96.7
20-90 19 23.2 10 35.7 1 1.4 1 3.3
100-490 11 13.4 3 10.7 0 0 0 0
500-2260 6 7.3 7 25.0 0 0 0 0
Total 82 100.0 28 100.0 74 100.1 30 100.0
a From: Humphrey & Hayner (1975).
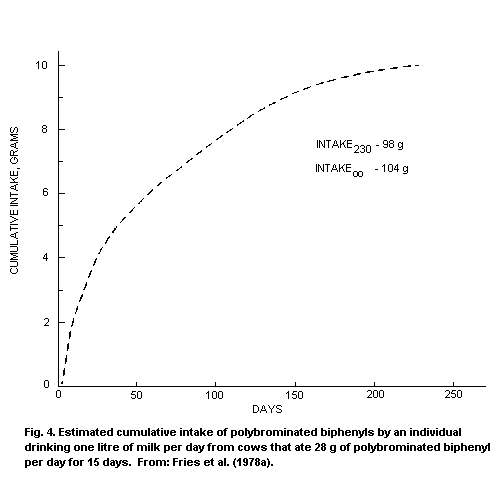 People who bought food primarily from quarantined farms were
thought to have been exposed 10 to 100 times more than the typical
retail store customer (Schwartz & Rae, 1983): ca. 100 mg of PBBs
versus 1-10 mg (Brown & Nixon, 1979).
While many dust and cobweb samples found in the buildings of
some PBB-contaminated farms had very high residue levels, the amount
of PBB residue involved is said not to be sufficient to be an
important contributor to animal residues (Fries & Jacobs, 1980) and,
possibly to human exposure.
5.2.2 Human monitoring methods for PBBs
Usually, suitable human monitoring data, as such, are used to
describe the real exposure to a toxic chemical. As an indicator of
human exposure to PBBs, the presence of PBBs in adipose tissue,
breast-milk, whole blood, serum, red and white blood cells (Bekesi
et al., 1979a,b), and human hair oils (Stratton & Whitlock, 1979)
has been assessed. The most commonly used specimens were serum,
breast-milk, and adipose tissue (see Tables 38-40).
Table 38. Human monitoring data: PBB levels in the Michigan population (USA)
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1974 82 96.3 serum adults from not 14 not Humphrey &
quarantined farms detected- specified Hayner (1975)
2260
28 100 serum children from 2-2260 35 not
quarantined farms specified
5 100 serum lactating females 3-1068
from quarantined
farms
1976 524 serum Michigan farmers 23.7 2.6 0.2 Wolff et al.
µg/litre (1978a);
Lilis et al.
(1978)
283 serum residents on 0.2-> 1000 33.9 3.9
quarantined farms
153 serum residents of 0.2-50 2.9 1.4
non-quarantined
farms
40 serum consumers of 0.3-1000 56.6 4.2
products
from quarantined
farms
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
28 serum consumers of 0-50 3.4 2.2
products from
non-quarantined
farms
consumers and Wolff et al.
residents: (1978)
quarantined farms:
40 serum females < 18 years 28.0 2.3 0.2
µg/litre
102 serum females > 18 years 18.2 2.5
51 serum males < 18 years 67.7 7.3
129 serum males > 18 years 28.2 4.4
consumers and
residents:
non-quarantined
farms:
37 serum females < 18 years 3.1 1.3
57 serum females > 18 years 1.7 0.9
35 serum males < 18 years 4.8 1.7
51 serum males > 18 years 3.1 2.2
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1976 485 serum consumers and Chanda et al.
residents: (1982)
quarantined farms:
27 serum 0-5 years 0.2-64.2 10.15 not
specified
137 6-18 years 0.0-962.4 27.22 not
specified
321 > 18 years 0.2-1778.0 24.42
1976 321 serum consumers and Chanda et al.
residents: (1982)
non-quarantined
farms:
18 0-5 years 0.2-37.4 6.42 not
specified
104 6-18 years 0.0-42.6 3.25 not
specified
177 > 18 years 0.0-94.0 3.03 not
specified
1976 serum Michigan children 3.41 not Barr (1980)
specified
143 females 2.72 not
specified
149 males 4.23 not
specified
33 0-4 years 2.75 not
specified
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
77 5-8 years 5.59 not
specified
81 9-12 years 3.18 not
specified
101 13-16 years 2.65 not
specified
1976-77 3639 serum Michigan residents 0-1900 21.2 3.0 1 µg/litre Landrigan
with various et al.
degrees of exposure (1979);
Landrigan
(1980)
1976-77 1750 serum contaminated farm 0-1900 26.9 4.0 1 µg/litre Landrigan
1114 residents farm 0-659 17.1 3.0 et al.
(1979);
216 product recipients 0-1240 43.0 4.5 Landrigan
(1980)
559 chemical workers and 0-111 3.4 2.0
families volunteers
1976-77 52 serum women at the time not 26.2 2.5 1 Landrigan
of delivery detected- µg/litre et al. (1979)
1150
1977 3683 serum Michigan PBB cohort < 1-3150 23.2 4.1 3 not Kreiss et al.
specified (1982)
1888 males 5.8
1795 females 2.8
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1978 1681 serum Michigan residents Wolff et al.
(1982)
(randomly selected)
1120 68.9 adults 0.2-120.5 1.3 0.6 0.2
µg/litre
461 72.7 children 0.2-37.2 1.8 0.8
232 Upper Peninsula 0.2
Lower Peninsula:
467 Detroit Area 0.5
191 Muskegon Area 1.7
791 remainder of state 0.9
Muskegon County:
54 serum male adults 4.0 2.6 0.2 Wolff et al.
µg/litre (1982)
74 female adults 2.1 1.4
36 male children 4.9 3.4
27 female children 3.8 2.0
1975-80 serum Michigan PBB cohort: Eyster et al.
(1983)
61 pregnant females not 3.5 3 1
detected- µg/litre
1068
56 non-pregnant females not 3.1 2
detected-
873
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
29 male chemical workers 1-1200 25.4 20
83 male farm and other not 5.4 4
workers detected-
1515
1974-75 5 100 milk women from 0.21-92 660 not Humphrey &
quarantined farms specified Hayner (1975)
1976 milk lactating women Brilliant
(fat) (randomly selected et al. (1978)
53 96 from Michigan: not 0.068 0.1 mg/kg
Lower Peninsula detected-
1.2
42 43 Upper Peninsula not
detected-
0.320
1976-77 32 100 milk women at the time of 0.032-93 3.61b 0.225b Landrigan
(fat) delivery (Michigan et al. (1979)
PBB cohort)
1976-78 2986 88.5 milk lactating women not 0.097 0.1 0.06 < 0.05 mg/kg Miller et al.
milk (self-selected) detected-2 (1984)
1975-80 Michigan Eyster et al.
PBB cohort (1983)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
47 milk pregnant females not 0.312b 0.250b 0.001
(fat) detected- mg/kg
92.7
1974-75 15 100 adipose persons from 0.104-175c Humphrey
quarantined farms & Hayner
(1975)
1975-76 53 adipose quarantined farmers 1.965 Meester &
McCoy
(1976)
29 100 non-quarantined 0.516
farmers
9 100 city residents 0.226
116 fat members of farm 0.58-273.0
families
1977 19 subcutaneous children with known Weil et al.
exposure to PBBs (1981);
fat in utero and/or Schwartz &
through breast milk Rae (1983);
Seagull
(1983)
10 100 "high exposure" 0.116- 4.218
20.960
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
9 100 "low exposure" 0.010- 0.050
0.074
1978 844 97.3 adipose Michigan residents < 0.002- 0.4 0.199 0.002 Wolff et al.
36.7 mg/kg (1982)
(randomly selected) 0.015
87 Upper Peninsula
Lower Peninsula:
255 Detroit Area 0.16
84 Muskegon Area 0.50
418 remainder of state 0.24 0.050
1975-80 32 adipose Michigan PBB-cohort: not 0.330 0.540 0.001 Eyster et al.
lipid pregnant females detected- mg/kg (1983)
174
56 non-pregnant females not 0.00057 0.460
detected-
0.619
29 male chemical 0.4-350 5.29 6.0
workers
83 male farm and other 70-350 1.65 1.05
workers healthy male
Not 7 100 adipose volunteers, aged 0.01-2.72 0.001 Schnare
specified lipid 20-30 years mg/kg et al.
(subcutaneous) (1984)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1983 15 100 perirenal autopsy cases from 0.032-1.65 0.475 0.32 0.0005 Miceli
post- the "high" exposure mg/kg et al.
mortem area of Michigan (1985)
adipose (Grand Rapids)
tissue
(wet wt)
a Expressed as the concentration of the major hexabromobiphenyl component (BB 153) in µg/litre serum or
mg/kg milk, or adipose tissue, respectively.
b The sample measuring 93 mg/kg was excluded from statistical analysis.
c Most in the order of 1 mg/kg.
The last one has been preferred for analysis, since depot fat
is the predominant storage site of PBBs (and other persistent
halogenated hydrocarbons), and, therefore, allows an increased
detectability of body burden. For example, had serum PBBs been used
alone as an indicator of exposure in the PBB survey of the general
population of Michigan (Table 38), only 70% of the 839 individuals
would have been considered exposed. When adipose tissue results are
added, an additional 24% indicate exposure, raising the positive
rate to 94%. Even though the limit of detection was an order of
magnitude higher (2.0 µg/litre in adipose tissue vs. 0.2 µg/litre in
serum), the partition ratio of approximately 300:1 made the adipose
limit of detection a more sensitive indicator of exposure (Wolff
et al., 1982; Anderson, 1985).
On the other hand, collection of hair, blood, and breast-milk
samples is simpler and less invasive than adipose tissue biopsy.
Moreover, with some exceptions, significant correlations between
adipose tissue and blood serum or breast-milk PBB levels were found
(section 6.2). Further advantages and limitations of these
techniques are discussed in detail by Anderson (1985) and Fries
(1985b).
It should be noted that levels of PBBs in breast-milk may not
be comparable between studies unless the concentration is adjusted
for fat content, because the fat content of breast-milk varies
widely from woman to woman, and the value increases during feeding
(Rogan et al., 1980).
Although the accurate relationship between observed body levels
of PBBs and individual exposure to PBBs is not clear (Safe, 1984),
PBB concentrations in human tissues can give some idea of the levels
of exposure (Kimbrough, 1980a).
5.2.3 Human monitoring data
In the following subsection, human monitoring data are
presented from contaminated farms in Michigan, from Michigan state,
and from other countries.
Most data available refer to the Michigan PBB incident in
1973-74 (Tables 38 and 39). Because, in this case, the spilling of
PBBs started from farms, the Michigan Department of Public Health
(MDPH) undertook a series of studies on farm families as a high-risk
group in the summer and autumn of 1974.
Serum samples were obtained from 110 persons in the exposed
group (had been working or living in the quarantined farms for six
months or more since the accident) and for 104 persons from the
control group (randomly selected from a list of dairy producers in
the same geographical area, where farms had not been quarantined).
As shown in Tables 37 and 38, serum levels of PBBs were
significantly higher in the people from quarantined farms compared
with those from non-quarantined farms, though low levels were also
observed in the control group (Cordle et al., 1978). In 1976, the
MDPH, together with the Centre for Disease Control, the FDA, NIH,
and EPA, established a cohort of 4545 people in Michigan to be
examined at regular intervals over several decades (Barlow &
Sullivan, 1982) to evaluate the long-term effects of PBB exposure.
Four groups were included (Landrigan, 1980): Quarantined farm
residents, direct recipients of farm produce, chemical workers and
their families, and persons who either volunteered for the study or
who had participated as control subjects in an earlier pilot study
(Humphrey & Hayner, 1975). The first report was published by
Landrigan et al. (1979) followed by several reports on subgroups of
this population (e.g., Kreiss et al., 1982; Eyster et al., 1983).
Study groups of the Mount Sinai School of Medicine have also
conducted comprehensive examinations on similarly categorized
groups, e.g., residents of quarantined farms, residents of
non-quarantined farms, consumers of products directly from
quarantined or non-quarantined farms, and Michigan Chemical Company
workers; residents of the state of Wisconsin were used as a control
group.
It can be seen from Table 38 that nearly 100% of the adipose
samples randomly selected throughout the state had detectable PBB
concentrations. Thus, statewide exposure of Michigan residents to
PBBs can be demonstrated.
Levels of PBBs in serum (Landrigan, 1980; Wolff et al., 1982),
breast-milk (Brilliant et al., 1978; Miller et al., 1984), and
adipose tissue (Wolff et al., 1982) were highest in the area of the
accident (lower peninsula), and lowest in the upper peninsula,
farthest from the source.
Compared with residents of quarantined farms, direct consumers
of products from quarantined farms, and PBB- production workers, the
tissue burdens among the general population of Michigan were 1-3
orders of magnitude lower. Moreover, for example, only 36% of the
general population had serum PBB concentrations greater than
1 µg/litre, compared with 78% among farmers (Anderson et al., 1979;
Wolff et al., 1982).
PBB levels appear to be higher in males than females (Meester &
McCoy, 1976; Landrigan et al., 1979; Landrigan, 1980; Wolff et al.,
1978; 1980; Kreiss et al., 1982; Eyster et al., 1983) and higher in
children (below the age of 10 years) than in adults (Humphrey &
Hayner, 1975; Landrigan et al., 1979; Landrigan, 1980; Barr, 1980;
Wolff et al., 1982).
A later study (Schnare et al., 1984) recorded not only the
concentration of the most abundant congener of the FireMaster(R)-
mixture (2,2',4,4',5,5'-hexabromobiphenyl) but also the concen
trations of other PBB congeners detected in subcutaneous adipose
tissue samples of 7 former participants of the Michigan PBB studies
(Table 39).
Table 39. Range of adipose tissue concentrations of various PBBs in 7 personsa
(mg/kg on a lipid weight basis)b
PBB Rangec (mg/kg)
2,3',4,4',5-penta nd-0.16
2,2',4,4',5,5'-hexa 0.01-2.72
2,2',3,4,4',5'-hexa nd-0.22
2,3',4,4',5,5'-hexa nd-0.09
2,2',3,3',4,4',5-hepta nd-0.26
2,2',3,4,4',5,5'-hepta nd-0.01
a 7 healthy male volunteers, aged 20-30 years, having been exposed to PBBs some
years earlier, as a consequence of the Michigan PBB incident in 1973.
b From: Schnare et al. (1984).
c nd = Not detectable; detection limit = 0.001 mg/kg.
In most cases, PBB concentrations did not appear to be
decreasing significantly over time. Wolff et al. (1979b) did not
find any significant variation in the serum PBB levels of nine dairy
farm residents during 18 month of observation.
Paired serum samples, one collected in 1974 and the other in
1977, were also available for 148 members of the Michigan PBB
cohort. The data indicate that levels were generally stable over the
3-year period with a mean change of 16 µg/litre (Landrigan et al.,
1979). In another study of the Michigan PBB-cohort, the decrements
in median serum levels of PBBs between matched pairs over one -
(1977-78) and two - (1977-79) year intervals were both only 1
µg/litre (Kreiss et al., 1982). No significant change in blood
plasma PBB levels was observed over a 5-month period in 41 residents
of quarantined farms (Humphrey & Hayner, 1975). In contrast, Meester
& McCoy (1976) reported a marked decline over 3 years (1974-76) in
serum levels of PBBs. These authors also found that the average
decrease in PBB concentrations in the fat of 16 individuals was
about 40%, in a period of 6 months. No changes in PBB levels were
seen over an 11-year period (1976-87) in fat samples from a patient
with long-term exposure to PBBs from the early 1970s as a result of
the Michigan PBBs accident. The average fat level of PBBs was
0.8 mg/kg (Sherman, 1991).
In 1981, PBBs were found in 13-21% of serum samples from
4-year-old Michigan children. Their mothers belonged to a group that
was surveyed either with regard to the consumption of Lake Michigan
sport fish (mean PBB level detected in children: 2.4 ng/ml) or with
regard to former exposure to quarantined farm products (mean PBB
level detected in children: 3.0 ng/ml) (Jacobson et al., 1989).
Few human monitoring data are available for the US population
outside of Michigan. They are summarized in Table 40. One study
deals with the population in the vicinity of industrial areas
involved in PBB production or use (Stratton & Whitlock, 1979), the
other with farmers of the state of Wisconsin who were examined as
control group in connection with the Michigan PBB studies (Wolff
et al., 1978).
PBBs were found in all studies, but, because of the limited
data, the significance is unclear. The highest PBB levels were found
in the hair of humans living near PBB industry. Of the nine samples
analysed, five had detectable PBB levels. Both male and female hair
samples contained PBBs (Stratton & Whitlock, 1979).
In contrast to the other surveys, which had regard only to the
major PBBs component (hexabromobiphenyl), Stratton & Whitlock (1979)
identified the different PBB homologues in the extracted oils of the
human hair, collected from barbershops and beauty parlours (Table
41). There were identifiable differences in the composition of PBB
congeners found in hair from the three locations.
The samples with the highest concentrations contained
relatively large amounts of decabromobiphenyl, while the samples
with lower concentrations contained only hexabromobiphenyl (Stratton
& Whitlock, 1979). One sample was different from the others because
it contained dibromobiphenyl (see Table 41). As a result of the
sampling method, it was impossible to ascertain whether the exposure
was related to the workplace or to the ambient environment.
In a report by Lewis & Sovocool (1982), pooled adipose tissue
samples from 202 individuals from nine census regions in the USA
were analysed for HxBB. Although the average concentration was 1-2
µg/kg, it cannot be excluded that this was because of the inclusion
of a few samples with high PBB concentrations.
Table 40. Human monitoring data: PBB levels in the US population (outside of Michigan)
Year No. of % of Specimen PBB PBB Detecion Remarks References
specimens positive tissue group/area concentrationa examined limita
findings range
1977 56 3.6 serum Wisconsin farmers not C12H4Br6 examined as Wolff
a control detected-1.1b et al. (1978)
population
1977 3 33 hair (from males and females, < 100-8100 total PBBs 100 reported as Stratton &
barber- Bayonne New Jersey, µg/kg in oil Whitlock
shops) Vicinity of White (1979)
Chemical
3 100 hair (from males and females, 440-26 600 total PBBs 100 reported as
barber- Staten Island, µg/kg in oil
shops) New York, Vicinity
of Standard T
Chemical Co.
3 33 hair (from males and females, < 100-310 000 total PBBs 100 reported as&
barber- Sayreville New µg/kg in oil
shops) Jersey, Vicinity of
Hexcel Fine
Organics
a (µg/litre or µg/kg).
b PBBs not detected in 54/56 persons. PBBs observed at 1.1 µg/litre in one person, identified as recently
moved from a Michigan farm, and at 0.5 µg/litre in another person.
Table 41. Concentration (range) of different PBB congeners in human hair samples
taken in the vicinity of three industrial sites in the USA, reported as
µg/kg in oila
PBB-congeners Bayonne, New Staten Island, New Sayreville,
Jersey (White York (Standard T New Jersey
Chemical Corp.)b Chemical Comp.)c (Hexcel Corp.)d
MoBB nde nde nde
DiBB nd-8100 nd nd
TrBB nd nd nd
TeBB nd nd nd
PeBB nd nd nd
HxBB nd 440-740 nd-480
HpBB nd nd-890 nd
OcBB nd nd-1100 nd
NoBB nd nd-3600 nd-22 500
DeBB nd nd-20 000 nd-285 000
a Data from: Stratton & Whitlock (1979).
b Having manufactured octa- and decabromobiphenyl along with bromobiphenyl
ethers.
c Major user of FireMaster BP-6.
d Producer of laboratory quantities of various PBBs.
e nd = Not detected (detection limit = 100 µg/kg).
There is very little human monitoring data on PBBs in the
populations of countries other than the USA. Krüger et al. (1988)
reported PBB contamination of breast-milk from European women in a
survey from North Rhine-Westphalia, Germany (Table 33). The milk
samples (n=25) contained a typical pattern of certain PBB congeners.
It included penta- to octabromobiphenyls in concentrations ranging
from 0.002 to 28 µg/kg, based on milk fat. The most abundant
component was 2,2'4,4',5,5'-hexabromobiphenyl (BB 153) followed by a
peak consisting of two heptabromobiphenyl isomers (2,2',3,4',5,5',6-
and 2,2',3,4,4',5,6'-heptabromobiphenyl BB 187 and 182). Differences
in the pattern were only found in the milk given by a Chinese woman
and in that given by a woman having been exposed to several fires in
industry.
Concentrations of BB 153 in human and cow's milk, both
collected from the same region (North Rhine-Westphalia), were
1 µg/kg and 0.03 µg/kg, respectively, measured on a fat basis
(Krüger, 1988).
5.2.4 Subpopulations at special risk
Children are at risk from exposure to PBBs in different ways.
Studies on the Michigan population indicated a significant PBB
transfer to the fetus (Landrigan et al., 1979; Eyster et al., 1983;
Jacobson et al., 1984) and to breast-milk (Brilliant et al., 1978;
Landrigan et al., 1979; Eyster et al., 1983; Jacobson et al., 1984,
1989; Miller et al., 1984). PBB levels to which fetuses and newborn
infants were exposed in the Michigan accident are shown in Table 42.
Because the placenta acts only as a partial barrier to PBBs, a
newborn baby has a body burden, even before breast feeding.
Placental and cord serum levels are much lower than levels in
breast-milk. However, even at low concentrations, intrauterine
exposure may be significant, for several reasons, as pointed out in
detail by Jacobson et al. (1984).
Infants are not only exposed to PBBs through their mothers and
through consumption of contaminated food, but they also through
contact with PBBs from the environment. Young crawling children are
known to ingest accidentally soil or dust to an extent of up to
0.1 g/day.
5.3 Occupational exposure during manufacture, formulation, or use
In general, occupational exposure is to be expected in PBB
manufacturing and processing plants. In Michigan, as a result of the
PBB incident, farmers, and possibly dairymen, elevator, mill
personnel etc. were occupationally exposed (Kay, 1977).
Table 42. PBBb concentrations in maternal serum, adipose lipid, and milk lipid, and in
the cord serum and placenta of Michigan women (Michigan PBB-cohort) at the time of
parturition (1975-80)a
Paired specimen No. Rangec Median Geometric Measure
mean
Maternal serum 61 nd-1068 3 3.5 µg/litre
Placenta nd-370 < 1 - µg/kg
Maternal serum 60 nd-1068 3 3.2 µg/litre
Cord serum nd-104 < 1 - µg/litre
Maternal serum 47 nd-1068 3 3.0 µg/litre
Milk lipid nd-92 667 250 312 µg/kg
Milk lipid 27 52-92 667 384 472 µg/kg
Adipose lipid nd-174 000 522 82 µg/kg
a From: Eyster et al. (1983).
b Concentrations expressed as concentrations of hexabromobiphenyl.
c nd = Not detected (detection limit: 1 µg/litre or kg).
Bialik (1982) reported the following contaminant levels of
decabromobiphenyl measured in 1977 in the manufacturing area of
Hexcel/Fine Organics and Saytech, Inc. (Sayreville; USA):
- Plant air samples: 0.18 and 0.23 mg/m3 8 h TWA (time-weighted
average);
- Wipe tests, unspecified: up to 8 mg/100 cm2; - Wipe tests,
eating Table: 0.1 mg/100 cm2.
At this time, 95% of the plant production consisted of
decabromobiphenyl (18%) and decabromobiphenyl oxide (77%). About
2000 tonnes of decabromobiphenyl were manufactured during 1973-77
(Bialik, 1982).
Employees of chemical plants may be exposed directly to PBBs
(in most cases along with other chemicals) through contact,
inhalation, or ingestion (Wolff et al., 1979a). As an index of
individual exposure, PBB levels in the serum and adipose tissue of
chemical workers have been recorded. The results of several authors
are compiled in Table 43.
Most data refer to the Michigan Chemical Corp., St. Louis
(Michigan), which produced several brominated organic compounds and
manufactured over 5000 tonnes of PBBs, predominantly
hexabromobiphenyl, from 1970 to 1974. Some additional general
exposure from contaminated food can also be included for the workers
of Michigan Chemical.
To summarize, median serum and adipose tissue PBB levels were
higher among chemical workers than among male residents of
quarantined farms.
Non-production workers at the Michigan Chemical plant showed
significantly lower levels than workers involved in PBB production;
for example, median adipose tissue concentrations of PBBs were
2.49 mg/kg and 46.94 mg/kg, respectively.
In another study on workers at a PBB plant in New Jersey, Bahn
et al. (1980b) presented a detailed comparison of serum PBB levels
in various occupational groups. A significantly higher number of PBB
workers had detectable levels of PBBs, compared with other workers
in the study (35.9% compared with 12.2%). Among workers with
detectable PBB levels, the PBB workers had significantly higher
serum levels than workers from neighbourhood industries not using
PBBs.
Although this factory concentrated on manufacturing
decabromobiphenyl and decabromobiphenyl oxide (ether), there was no
positive identification of C12Br10 (Table 44) or C12Br14O
(Bahn et al., 1980b).
No data are available about occupationally exposed women.
Family members of chemical workers have also been found to have
a body burden of PBBs (Landrigan, 1980).
Bekesi et al. (1979b) determined the distribution of PBBs in
the blood compartments of 4 Michigan Chemical plant workers (Table
45) and suggested that the PBB level of the white cell fraction may
be a better indicator for risk potential than the total plasma PBB
concentration.
Table 43. Occupational exposure: PBB levels in chemical workers (USA)
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1975 Michigan workers (8-36 serum 6-85 HxBB Kay (1977)
Chemical Corp. months exposure (7)
(MCC)
(St. Louis,
Michigan)
1976 MCC (St. Louis, employees (55) serum 1.1-1729 123 9.3 HxBB Wolff
Michigan) et al.
(1978,
1979a)
production workers serum 603.9 108.4 HxBB 1 Wolff
(10) et al.
(1979a)
non-production serum 16.5 6.1 HxBB 1
workers (45)
workers (14) serum 1-1530 HxBB < 0.2 Wolff
et al.
(1979b)
1978 workers (14) 1-1363 HxBB < 0.2
(matched pairs)
Table 43 (contd).
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1976-77 MCC (St. Louis, workers and serum not 43.0 4.5 C12H4Br6 1 Landrigan
Michigan) families (216) detected-1240 et al.
(1979)
1975-80 MCC (St. Louis, workers (male) serum 1-2000 (25.4) 20 C12H4Br6 1 Eyster
Michigan) (29) et al.
(1983)
1978 Hexcel/Fine PBB workers serum not MoBB Bahn
Organics and (exposure to PBBs detected-1340 DeBB et al.
Saytech, Inc. (and PBBOs) for at (1980b)
(Sayreville least 6 weeks
New Jersey) between January
1973 and August
1978) (39)
1976 Michigan production workers adipose 5000-581 000 196 490 46 940 HxBB 500 Wolff
(7) tissue et al.
(1979a)
non-production 500-10 000 3880 2490
workers
1975-80 Michigan workers (male) adipose 400-350 000 5290 6000 HxBB 1 Eyster
(29) tissue et al.
(1983)
a In µg/litre or µg/kg.
Table 44. Detectablea serum levels (µg/litre) of PBB homologues in workers
at a plant producing decabromobiphenyl and decabromobiphenyl oxideb
PBB homologue Number of cases Range
C12H9Br 14 0.3-5.5
C12H8Br2 1 6.9
C12H7Br3 1 0.9
C12H6Br4 0 -
C12H5Br5 2 1.6-13.0
C12H4Br6 2 0.4-6.0
C12H3Br7 7 9.0-40.0
C12H2Br8 9 20.0-800.0
C12H Br9 1 500
C12Br10 0 -
Total PBBs 26 0.3-1340
a Excludes cases with "trace", "not confirmed" and "not detectable" levels.
b From: Bahn et al. (1980b).
Despite its significance for toxicological assessment, the
content of minor constituents of FireMaster(R) in the body burden
was rarely investigated. For example, Wolff & Aubrey (1978) examined
other PBB congeners, which are identifiable as peaks by GC/MS
(2 pentabromobiphenyl peaks, and 2 heptabromobiphenyl peaks), in the
serum of Michigan Chemical workers (n = 24) and Michigan dairy
farmers (n = 37), besides the major component (2,2',4,4',5,5'-
hexabromobiphenyl) of FireMaster(R). The relative concentrations,
with respect to the major hexabromobiphenyl peak, of these PBB
components were somewhat different for chemical workers and for
farmers, i.e., the two pentabromobiphenyl values (peak area ratios)
were significantly higher in the serum from chemical workers.
Table 45. Distribution of PBBs in blood compartments of Michigan Chemical Workersa
Polybrominated biphenyls
ng/mg Protein Ratiob
RBC plasma WBC RBC plasma WBC
Michigan Chemical Workers;
not directly involved in 0.07 0.13 3.9 1 : 2 : 56
the production of PBB 0.03 0.23 1.8 1 : 8 : 60
directly involved in 0.67 10.0 57.3 1 : 15 : 86
the production of PBB 0.63 10.2 32.0 1 : 16 : 51
a From: Bekesi et al. (1979b).
b RBC = Red blood cells; WBC = white blood cells.
This variation might be attributed to the different routes
(skin contact, inhalation, direct ingestion versus, primarily,
ingestion of animal foodstuff) and to the different composition
(unchanged versus animal-mediated material) of exposure in chemical
workers versus farmers. Further reasons might be the earlier initial
onset of contamination in workers and slight variations in the
composition (section 2.1.2) of several lots of FireMaster(R) BP-6
(the main product of Michigan Chemical) and FireMaster(R) FF-1,
which caused contamination of livestock feed (Anderson et al.,
1978a; Wolff & Aubrey, 1978; Wolff et al., 1979a).
The change in serum PBB levels over time was investigated in
chemical workers at two facilities. Wolff et al. (1979b) reexamined
serum PBB concentrations (determined as the major hexabromobiphenyl
peak) in 1978 from 14 workers of the Michigan Chemical Corp., who
had also been tested 18 months earlier. They found PBB levels of a
comparable order. In contrast, no subject (n=109) in a study on
chemical workers of Hexcel/Fine Organics and Saytech Inc.
(manufacturing decabromobiphenyl and decabromobiphenyl oxide) showed
any detectable serum level of PBBs (different congeners) in 1981
(Bialik, 1982), which was true, even for the two persons who had
shown high levels of serum PBBs in the previous study of 1978 (Bahn
et al., 1980b; Table 43). However, the results of PBB determination
in the fat of these two cases were positive in 1981. The negative
results of the determination of PBBs in serum were not expected, and
the authors suggested further studies.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Gastrointestinal absorption
Studies have been performed only on the gastrointestinal
absorption of PBBs. Some studies indicate that PBBs are rapidly and
efficiently absorbed, other studies indicate a much lower efficiency
of absorption (see Table 46). No information is available on the
extent of absorption of decabromobiphenyl.
Absorption can be strongly influenced by the vehicle in which
the compound is administered (Birnbaum, 1985). Administration of
hexabromobiphenyl in mineral oil or olive oil solution resulted in
higher absorption than administration in a methyl cellulose
suspension (see Table 46: Rozman et al., 1982). The degree of
halogenation also appeared to influence the absorption of PBB. For
example, less than 10% of 14C-labelled hexabromobiphenyl, but 62%
of a dose of 14C-labelled octabromobiphenyl were eliminated in the
faeces of rats in 24 h, though both compounds had been administered
in corn oil (see Table 46).
The conclusion that more brominated biphenyls are absorbed less
efficiently than less brominated biphenyls can, possibly, be drawn
from other findings. Willett & Durst (1978) observed that, during
feeding of FireMaster(R) BP-6, the relative concentration of
pentabromobiphenyl in the faeces of cows was decreased, and that of
heptabromobiphenyl was elevated compared with the
FireMaster(R)-standard. Similarly, faecal concentrations of
heptabromobiphenyl were enhanced relative to concentrations of
hexabromobiphenyl in the faeces of hens, when FireMaster(R) BP-6
was fed (Fries et al., 1976). However, Polin & Leavitt (1984) found
that the ratio of 3.5 for hexa- to heptabromobiphenyl in the
chemical sample of FireMaster(R) FF-1 shifted to an average ratio
value of 2.5 in the whole carcasses of chickens analysed on days 0,
21, and 42 of withdrawal, inferring a better absorption of hepta-
bromobiphenyl.
Generally, it should be noted that faecal elimination during
the first few days following dosing might be an indicator, but is
not a measure, of lack of absorption, because some absorbed PBB is
eliminated and recycled into the faeces in bile and by diffusion
across intestinal membranes (Rozman et al., 1982; Fries, 1985b).
Table 46. Absorption of PBBs after oral administration
PBB compound Species Vehicle Absorption Methods Comments References
(sex) parametera
[14C-]2,2',4,4',5,5'- rat emulphor-EL 620: 90%, 24 h faeces analysis single dose Matthews
hexabromobiphenyl (male) ethanol: water gut content et al. (1977)
(1 : 1 : 8)
corn oil 90% multiple doses (4)
[14C-] octabromobiphenyl rat (male, corn oil 38%, 24 h faeces analysis single dose Norris et al.
(technical mixture) female) (1973)
[14C-]2,2'4,4',5,5'- rhesus 1% methyl cellulose 40%, 10 days faeces analysis two doses Rozman et al.
hexabromobiphenyl monkey mineral oil 62%, 5 days faeces analysis single dose (1982)
(male) olive oil 66%, 5 days faeces analysis repeated doses (4)
FireMaster(R) BP-6 cow crystalline PBB in 50%, 168 h faeces analysis single dose Willett &
(female) gelatin capsules (7 days) Irving (1976)
calf crystalline PBB in 95%, (9 days) faeces analysis daily feeding
(male) gelatin capsules
a Values based on concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (FireMaster(R) BP-6 sample) or on [14C-]activity.
6.1.1.2 Dermal and inhalation absorption
No quantitative information is available on skin absorption and
intake through inhalation.
6.1.2 Human studies
It is plausible that inhalation and dermal contact are the main
routes of exposure to PBBs for chemical plant workers (Wolff et al.,
1979a), while the main route for Michigan people was the ingestion
of PBBs dissolved in the fat of meat and milk (Di Carlo et al.,
1978). An appropriate model for assessing the latter kind of
absorption is thought to be the rat-corn oil model (Fries, 1985b).
No quantitative data are available on PBB absorption in humans.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Levels in organs and blood
As can be seen from Tables 47, 48, and 49, most studies on the
distribution of PBBs have been conducted with the FireMaster(R)
mixture. A few earlier publications refer to technical
octabromobiphenyl. No experimental data are available on tissue
distribution of decabromobiphenyl. When FireMaster(R) was
administered, the distribution process was studied predominantly as
the distribution of 2,2',4,4',5,5'-hexabromobiphenyl, and, with far
less emphasis, on the distribution of the minor components of the
mixture. Little information is available on the distribution of PBB
congeners, when administered individually.
Investigations on rats, mice, cows, sheep, pigs, and avian
species demonstrated that PBBs were distributed widely throughout
the body tissues in all species. Highest (equilibrium)
concentrations on a wet tissue basis were found in adipose tissues,
consistent with the solubility characteristics of PBBs. Adipose
concentrations are usually an order of magnitude higher than those
of most muscle and organ tissues (see Tables 47, 48, 49, and 50).
Much of the variation in concentrations among tissues can be
accounted for by variations in the fat concentrations in these
tissues (Willett & Durst, 1978; Fries et al., 1978b; Fries, 1985b).
Table 47. Distribution of PBBs in mammals after the administration of a single dose of PBBs
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
FireMaster(R) FF-1 rat oral 1000 10 monthsb adipose tissue (714) > liver (60) > blood Kimbrough et al.
(lot No. 7042) (male) in corn oil (0.94) (1978)
rat 10 monthsb adipose tissue (1202) > liver (37) > blood
(female) (2.9)
FireMaster(R) FF-1 rat oral 80 42 days fat (295) > blood (0.38) Wolff &
(male) in corn oil Selikoff (1979)
FireMaster(R) FF-1 rat oral 500 4 months adipose tissue (1008) > liver (50) > blood Kimbrough et al.
(lot No: 7042) (male) in corn oil (2.1) (1980)
FireMaster(R) FF-1 rat oral 10 24 hb sc fat I (61 500) > sc fat II (38 700) > liver Domino et al.
(lot No: FF-1312-FT) (male) (20 900) > lung (7650) > kidney (7310) > heart (1980b)
(6470) > jejunum (4860) > spleen (3530) >
cerebellum (2990) > grey matter (2850) > white
matter (2750) > testes (2380) > blood (945)
4 weeksb sc fat (19 200) > jejunum (3170) > lung (1240) Domino et al.
> liver (690) > kidney (650) > spleen (520) (1980b)
> heart, testes (both 240) > grey matter
(210) > cerebellum (200) > white matter (170)
> blood (56.9)
FireMaster(R) BP-6 rat intraperitoneal 12 weeks serum (46.80 ng/ml) Miceli &
(male) 10 in corn oil Marks (1981)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
fat (21.90) > adrenal (3.64) > lung (0.98)
> liver (0.59) > pituitary (0.91) > gonad (0.33)
> kidney (0.22) > heart (0.20) > spleen (0.17)
> brain (0.13)
36 weeksb serum (23 ng/ml)
fat (16.62) > adrenal (2.67) > lung (0.51) >
pituitary (0.29) > liver (0.20) > kidney (0.14)
> gonad, brain (both 0.10) > heart (0.08)
> spleen (0.05)
2,2',4,4',5,5'- rat oral 1 in: 1 day muscle (29.9) > adipose (25.5) > skin (17.9) Matthews et al.
[14C]-hexabromobiphenyl (male) Emulphor > liver (9.0) > blood (0.90)c (1977)
EL 600: ethanol:
water (1:1:8)
14C-PBB rat intraperitoneal 28 days ovaries (130) > skin (15.1) > testicles (13.3) McCormack et al.
(male 150 in: peanut > intestine (11.7) > lung (7.3) > liver (4.7) (1979b)
and oil > muscle, heart (1.9) > fat (1.8) > brain (0.9)d
female
pups)
FireMaster(R) BP-6 cow oral 5.95 in: 10 days liver (1.35) > fat (sc: 1.15; perirenal: 1.09; Willett &
(lot No. RP-158) (female) gelatin capsule peri-cardiac: 0.97; intermuscular: 0.81; omental: Irving (1976)
0.78) > brain (pons: 0.27; cortex: 0.08) >
mammary gland (0.25) > kidney (0.12) > heart
(0.11) > lung, muscle (0.08) > ovaries, uterus
(0.06) > plasma, rumen wall (0.04) > bile
(0.02) > synovial fluid (0.01)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
[14C-]octabromobiphenyl rat oral 1 in: 16 days adrenal, adipose, heart, skin > liver, Norris et al.
(technical mixture) (male) corn oil pancreas, spleen (1973)
a Measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity; (in parentheses: values measured - referring
to various measures).
b For additional time points: see original reference.
c Values in average % total PBB dose.
d Values in µg-equivalents/g wet weight.
sc. = Subcutaneous.
Table 48. Studies on the distribution of PBBs in animals following dietary or repeated oral intake
of the FireMaster(R) mixtures or 2,2',4,4',5,5'-[14C]-hexabromobiphenyl
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 fat (330) > mammary gland (318) Rickert
BP-6 (pregnant) feed until day 21 of > kidney (30) > skin (22) > liver, et al. (1978)
gestation lung, brain, heart, small
intestine, placenta, uterus (all
< 5)
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 mammary gland (117) > liver (4) Dent et al.
BP-6 (maternal) feed to 14 days (1977b)
postpartum
FireMaster(R) rat 25 mg/kg day 8 of pregnancy 0 fat (74) > mammary > liver McCormack
BP-6 (maternal) feed to 14 days > kidney > lung (6) et al.
postpartum (1979a)
50 mg/kg 0 fat (483) > mammary > kidney
feed > lung (13)
200 mg/kg 0 fat (966) > mammary > kidney
feed > lung (21)
FireMaster(R) rat 100 mg/kg day 8 of pregnancy 0 fat (813) > liver (54) > mammary McCormack &
BP-6 (maternal) feed to 28 days (43) Hook (1982)
postpartum 14 weeks fat (459) > mammary (225) > liver
(after first (12)
and only
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
litter was
weaned)
> 10 weeks fat (77) > mammary (19) > liver (3)
and after
weaning
their second
litter
2,2'4,4',5,5'- rat 1 mg/kg 4 days 3 days adipose (41.1) > skin > muscle Matthews
hexabromobiphenyl (male) body weight > liver > blood (0.32)b et al. (1977)
per day
FireMaster(R) rat 0.1 mg/kg 9 days 0 liver (1.5) > brain (0.5), Render et al.
BP-6 (Lot 6224A) (male) feed adipose (0.3)c (1982)
1 mg/kg 9 days 0 liver (8.3) > brain (1.8),
feed adipose (1.7)c
10 mg/kg 9 days 0 liver (135) > adipose (27)
feed > brain (12)c
100 mg/kg 9 days 0 liver (1213) > adipose (251)
feed > brain (103)c
FireMaster(R) rat 3 mg/kg 20 days 0 adrenal (93.7) > thyroid (> 20) Allen-
FF-1 (Lot No. (male) body weight > testes (8.7) Rowlands et al.
FF-1312-FT-3) per day (in 1981);
lecithin Castracane
liposomes) et al. (1982)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
20 days 5 months adrenal (481) Castracane
(plus dietary et al. (1982)
restriction)
FireMaster(R) mouse 100 mg/kg 14 days 6 h thymus (391) > fat > liver > brain Corbett et al.
BP-6 feed > pancreas > testicles > spleen (1978a)
(2.7)
14 weeks thymus (50) > adrenals = fat >
liver > testicles > spleen > brain
> pancreas (n.d.)
12 weeks perithymic fat (96) > perirenal
fat > adrenal glands > thymus
gland (5.5)
FireMaster(R) mouse 5 mg/kg 3 weeks 0 thymus (20) > lung, liver > spleen Loose et al.
FF-1 feed > serum (< 0.002) (1981)
(lot No. 7042)
8 weeks 0 thymus (24) > liver, lung > spleen
> serum (0.019)
FireMaster(R) mouse 167 mg/kg 3 weeks 0 thymus (109) > liver > lung > Loose et al.
(lot No. 7042) feed spleen > serum (1.22) (1981)
6 weeks 0 thymus (3088) > liver > spleen
> lung > serum (4.75)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum, (11)
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum (11)
FireMaster(R) cow 50 mg/kg 15 days 15 days renal fat (10) > omental fat Gutenmann &
BP-6 (lactating) feed > brisket fat > liver = thyroid Lisk (1975)
> mammary = chuck muscle > loin
muscle > heart > kidney = brain
> adrenal = spleen (0.4)
FireMaster(R) calf 25 g daily 9 days 0 rumen contents (14257) > feces Willett &
BP-6 (in gelatin > rumen wall > bile > marrow Irving (1976)
(lot 6244 A) capsules) > perirenal fat (441) > kidney
> testes > liver > thymus > heart
> brain, pons > lymph nodes >
tongue > spinal cord > brain,
cortex > plasma > small intestine
> lung > spleen > thyroid >
muscle (25)
FireMaster(R) cow 250 mg daily 60 days 0 fat (25) > liver > glands > nervous Willett &
BP-6 (heifer) (in gelatin > bile > contractiles > plasma Durst (1978)
(lot 6244 A) capsules) > liquids (0.0198)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) cow environmentally ca. 14 days 9 months perirenal fat (380*) > omental fat Fries et al.
FF-1 contaminated > subcutaneous fat > kidney > liver (1978b)
(Michigan PBB > skeletal muscle > cardiac muscle
incident) > lung > brain (10.5*)
ca. 200-400 g 9-12 months perirenal fat (1224*) > omental fat
(killed in >subcutaneous fat > skeletal muscle
extremis) > liver > cardiac muscle > kidney
> lung > brain (57*)
FireMaster(R) calf 10 mg/kg 4 weeks 0 fat (378) > kidney > liver > muscle Robl et al.
FF-1 (female) body weight (1.26) (1978)
per day in
gelatin
capsules
calf 100 mg/kg 6 weeks 0 fat (6080) > kidney > liver
(male) body weight > muscle (34)
per day
cow 1 158 days 182 days brisket fat (4.5) > bone marrow
> stomach fat > tail fat (3.3)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) sheep 50 mg/kg 30 days 0 renal fat (42) > omental fat Gutenmann &
BP-6 feed per day > brisket fat > liver > chuck Lisk (1975)
(complete muscle > loin muscle > heart >
ration) thyroid > brain > adrenal > kidney
= spleen (0.9)
FireMaster(R) pig 20 mg/kg 16 weeks 0 back fat (64) > leaf fat > liver Ku et al.
BP-6 feed > muscle > kidney (0.9) (1978)
200 mg/kg 16 weeks 0 back fat (503) > leaf fat > muscle
feed > liver > kidney (13.5)
FireMaster(R) pig 100 mg/kg during 2nd 0 adipose tissue > liver > kidney > Werner &
BP-6 (lactating feed half gestation > braind Sleight (1981)
sow) 200 mg/kg and during
feed lactation
"PBB" Japanese 20 mg/kg 9 weeks 0 liver (374) > kidney > muscle Babish et al.
(ca 75% quail feed > heart > brain (40)e (1975a)
hexabromobiphenyl) male
female 0 liver (225) > heart > kidney >
> muscle > brain (26)e
FireMaster(R) chicken various 5 weeks 0 adipose tissue (3:1) > whole egg Polin &
FF-1 (White concentrations > liver > muscle (0.008:1)f Ringer
Leghorn (1978a)
hens)
Table 48 (contd)
a Measured as concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (in parentheses: values measured - referring to various measures).
b Values in average % total PBB dose.
c Values on a fat basis.
d Concentrations are listed in Table 53.
e Values in mg/kg dry weight.
f Values as ratios of tissue PBBs: diet PBBs.
* = Geometric mean.
Table 49. Studies on distribution of the following continuous exposure to octabromobiphenyl
Species Exposure Duration of Tissues and organs under study, ranked References
Route, Duration recovery in order of decreasing concentrations
concentration (average bromine content; µg/g wet weight)
Rat in diet Lee et al. (1975a)
(male) 0 mg/kg feed (control) 2 weeks liver (3.4) > fat (1.7) > muscle (1.6)
100 mg/kg feed 2 weeks - liver (83) > fat (73) > muscle (14)
1000 mg/kg feed 2 weeks - fat (333) > liver (319) > muscle (77)
100 mg/kg feed 4 weeks 0,2,6 weeks fat > liver > muscle
100 mg/kg feed 4 weeks 18 weeks fat > muscle > liver
Rat inhalation 23 h/day, 7 days Waritz et al.
(OcBB vapour)a per week, 15 weeks (1977)
0 pg/litre air liver (3) > muscle (1.6) > fat (1.5)
(control)
3.5 pg/litre air - liver (4.2) > fat (3.0) > muscle (1.5)
a OcBB = Octabromobiphenyl, Dow, Lot 102-7-72.
Table 50. Tissue: blood ratios of PBBs estimated in a standard 250-g rata
Compartment Ratio
Liver 17:1
Muscle 5:1
Skin 56.5:1
Adipose 340:1
Intestine tissue 1:1
a From: Tuey & Matthews (1980).
However, even when concentrations are expressed on a fat basis
rather than a wet tissue basis, there are some deviations from
uniform concentrations among tissues (Fries, 1985b). PBB
concentrations, for example, were low in nervous tissue, despite its
high lipid content and often disproportionally high in liver,
considering its relatively low lipid content (see Tables 47, 48, and
49; additional information on fat content percentage, e.g., by
Willett & Irving, 1976; Fries et al., 1978a,b; Kimbrough et al.,
1978; Werner & Sleight, 1981).
The ratios between the PBB concentrations of adipose tissue,
blood, and vital organs are different when animals are not at
equilibrium (see also section 6.5) with respect to dosing regimen or
body condition. Usually, concentrations in liver are very high
compared with those in other tissues, immediately after dosing, and
decline relatively as equilibrium concentrations are established
(e.g., Lee et al., 1975a; Matthews et al., 1977; Miceli & Marks,
1981; Fries, 1985b). Generally, this phenomenon is most pronounced
in tissues that have high blood flow rates relative to tissue mass
(Tuey & Matthews, 1980). As an exception, livers of mice, tested in
a small series, appeared to have relatively concentrated the PBB
with passing time (Corbett et al., 1978a).
On the other hand, body weight changes, pregnancy, parturition,
and lactation can affect the concentration relationships until
equilibrium is reestablished (e.g., Rickert et al., 1978; Willett &
Durst, 1978; McCormack & Hook, 1982).
The route of exposure (oral or intravenous administration) had
no effect on the tissue distribution (blood, liver, muscle, adipose,
skin) of 14C-labelled 2,2',4,4',5,5'-hexabromobiphenyl (dose =
1 mg/kg body weight) in rats (Matthews et al., 1977).
Kimbrough et al. (1980) studied the effects of different diets
and of mineral oil on the HxBB concentration in rats that had
received a single oral dose of FireMaster(R) FF-1 (500 mg/kg body
weight, in corn oil). After 3 months of feeding, GC-analysis of
blood, liver, and adipose tissue showed no statistically significant
differences in PBB concentrations among the differently fed groups,
when concentrations were calculated on a lipid weight basis. On a
wet weight basis, however, the PBB concentrations were significantly
increased in the livers of rats on the experimental diets (Teklad-4%
and -20% fibre) and on mineral oil compared with those of rats on
the basal diet (Purina Chow).
In another study, McCormack et al. (1979a) examined the
consequences of simultaneous exposure to PCBs and PBBs in
(lactating) rats, because human populations that have been exposed
to PBBs are also likely to have been exposed to PCBs. The
extrahepatic tissue (kidney, mammary, lung, fat) concentrations of
PCBs and PBBs were similar, regardless of whether the agents were
administered together or alone. Liver, however, contained lower
concentrations of PBBs after treatment with an equal mixture of PCBs
and PBBs than when PBBs were administered alone. (None of the
tissues had higher concentrations of PBBs than PCBs after
concomitant administration. The reasons for this were not clear).
The distribution of PBB (hexa) among blood compartments
(plasma, red cells) has been studied in rats (Domino et al., 1980b).
It was found that plasma levels of 2,2',4,4',5,5'-hexabromobiphenyl
were generally four times greater than red cell levels.
Matthews et al. (1984) reported that 81% of plasma PBB (hexa)
was associated with the total lipoprotein fraction. In another study
(Kraus & Bernstein, 1986), approximately 65% of all radiolabelled
HxBB incubated with human serum in vitro was recovered in the
lipoprotein fraction. Of the HxBB in the lipoprotein fractions, 40%
was recovered in low-density lipoproteins (LDL), 33% in
very-low-density lipoproteins (VLDL), and 23% in high-density
lipoproteins (HDL). Addition of human lipoprotein to a culture
medium influenced the partition of HxBB between adipocytes and
culture medium (Kraus & Bernstein, 1989).
Some data are available on the tissue distribution of the minor
components of FireMaster(R)-mixture (see also section 6.5). Domino
et al. (1980b) analysed the relative percentages of various PBB
congeners (two penta-, three hexa-, and three heptabromobiphenyls)
in several tissues of rats given FireMaster(R) FF-1. From their
list, it was evident that each of the PBB analogues was found in all
tissues examined (liver, lung, testes, fat; blood, brain) but their
partitioning ratios differed. Distribution has been recorded also
after exposure to single PBB congeners, e.g., tetra-, penta-, and
hexa-isomers (Akoso et al., 1982a; Dharma et al., 1982; Domino
et al., 1982; Render et al., 1982; Millis et al., 1985a). In some
cases, it was not clear whether the differences in partitioning
between congeners were real or were caused by analytical problems
(Render et al., 1982).
6.2.1.2 Transfer to offspring
1) Mammals
Placental transfer
PBBs are capable of passing through the placental barrier into
the developing fetuses. This has been demonstrated in mice (Corbett
et al., 1978a; Welsch & Morgan, 1985), rats (Beaudoin, 1977; Rickert
et al., 1978), guinea-pigs (Ecobichon et al., 1983), minks and
ferrets (Bleavins et al., 1981), cows (Detering et al., 1975; Fries
et al., 1978a,b), and pigs (Werner & Sleight, 1981) by administering
the Fire-Master(R)-mixture or individual PBBs, or by using
technical octabromobiphenyl in rats (Aftosmis et al., 1972a; Waritz
et al., 1977).
The studies compiled in Table 51 are rarely intercomparable.
However, it is obvious that PBBs are readily transferred across
placental membranes, the concentrations among fetal tissues being
highest in the liver. The limited data on the distribution of PBBs
in fetal tissues showed often, but not always (Ecobichon et al.,
1983; Welsch & Morgan, 1985), lower PBB residues in fetal than in
maternal tissues (see Table 51). In cows, the average ratio of PBB
concentrations in fetal or calf tissue to PBB concentrations in dam
tissue was 0.36 : 1 for fat and 0.37 : 1 for blood (Fries et al.,
1978a,b). In contrast, the concentration ratio between the fetal and
maternal liver of mice ranged from 3.5:1 to 10:1 (Welsch & Morgan,
1985).
Species-dependent differences in the amounts of PBBs
transferred have been demonstrated for two mustelids, the mink and
the European ferret. PBB levels in the ferret kit were significantly
greater than those in the mink kit (see Table 51: Bleavins et al.,
1981).
Table 51. Placental transfer: PBB concentrations in the fetus and the mother
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mouse FireMaster(R) BP-6; 39.52 2.51 - 0.53 [HxBB] Corbett
(mg/kg) et al. (1975)
1000 mg/kg diet
on days 7-18 of
pregnancy
0 mg/kg (control) 0.04 0.05
Mouse FireMaster(R) BP-6; 112.74 12.02 - 0.95 - 5.86 - [HxBB] Corbett
(mg/kg) et al.
100 mg/kg diet (1978a)
on days 7-18 of
pregnancy
Mouse 2,2',4,4',5,5'- [HxBB] Welsch &
hexabromobiphenyl (mg/kg) Morgan (1985)
(purity: > 99%)
dietary intake
from day 6-15 of
pregnancy; sacrifice
on day 17
placenta:
100 mg/kg feed 9.08 17.26 3.79 3.06 182.88
300 mg/kg feed 17.30 39.84 8.23 4.56 217.48
500 mg/kg feed 69.13 89.47 24.58 17.64 316.79
750 mg/kg feed 95.36 103.70 54.33 18.64 453.12
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Rat FireMaster(R) BP-6 [HxBB] Beaudoin
(mg/kg) (1977)
single oral dose of
800 mg/kg body
weight (in sesame (pooled
oil) at day 12 of samples
pregnancy; killing: 51 267 13 from 4 rats)
24 h later
48 h later 250 248 6
Rat FireMaster(R) BP-6; 330 4.2 - 1.6 0.2 GI tract: [HxBB] Rickert
0.1 (mg/kg) et al. (1978)
50 mg/kg diet from
day 8-21 of
pregnancy
Rat Octabromobiphenyl Waritz
(technical); et al. (1977)
dietary intake
from day 8-15 of
pregnancy; sacrifice
on day 20;
0 mg/kg (control) 1.43 3.56 4.38 [Br]
100 mg/kg 70.4 16.1 7.62 (mg/kg)
1000 mg/kg 326 79.8 21.2
10 000 mg/kg 590 158 30.1
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mink [14C-]-PBBs (HxBB 0.031 1.622 plasma: 0.002 0 0.005 kidney: % of the Bleavins
and HpBB); iv 0.04 0.003; initial dose et al. (1981)
injection (1 µCi) brain: 0; per g of tissue
in the final intestine: or ml of fluid
trimester of 0.001
gestation; killed
2 h later
Ferret see above 0.124 1.625 plasma: 0.005 0.004 0.013 kidney: see above
0.07 0.010
brain:
0.003
intestine:
0.005
Guinea- FireMaster(R) FF-1; 45 7 kidney: 45 45 kidney: 1 [HxBB] Ecobichon
pig 4.5; (mg/kg) et al. (1983)
single oral dose of lung: 7
50 mg/kg body weight lung: 1.5
at approximately 65
days of gestation;
killed 2 days later
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Pig FireMaster(R) BP-6; Werner &
Sleight (1981)
dietary intake
during 2nd half of
gestation
10 mg/kg feed 0.4 1.0 kidney: [HxBB]
nd; (mg/kg)
brain: nd
100 mg/kg feed 4.9 11.5 kidney:
nd;
brain: nd
200 mg/kg feed 40.3 24.2 kidney: 1.5
brain: 1.8
a HpBB = 2,2',3,4,4',5,5'-heptabromobiphenyl.
b nd = Not detected.
c [HxBB] = Concentration of 2,2',4,4',5,5',-hexabromobiphenyl; [Br] = Concentration of bromide.
Milk transfer
In mammals, the second route of PBB transfer from the mother to
the offspring is nursing. The efficiency of this way has been shown
through determining the PBB content in milk in relation to the body
burden or in relation to exposure levels of contaminated dams, and
through measuring PBB levels in kits that have been exposed to PBBs
only from suckling. The FireMaster(R) mixture was used in all
studies. Mammary transfer of technical octa- or decabromobiphenyl
has not (yet) been assayed.
Most investigations on the PBB contents of milk from
contaminated animals have been conducted on cows (Fries & Marrow,
1975; Willett & Irving, 1976; Robl et al., 1978; Fries et al.,
1978a,b; Willett & Durst, 1978). The ratios of concentrations in
milk fat to body fat in cows no longer receiving PBBs averaged about
0.4 :1 (Willett & Durst, 1978; Fries et al., 1978a,b; see also
Fig. 5). This ratio is much lower than the ratio in humans (see
section 6.2.2).
For other species (guinea-pig, rat, mink, pig) only single data
can be found in the literature. When (lactating) guinea-pigs
received a single oral dose of FireMaster(R) FF-1 (50 mg/kg body
weight) within 6-12 h of parturition, levels of HxBB in breast milk
(and in perirenal adipose tissue) were of the order of 22 µg/g (and
17 µg/g), respectively, 2 days after treatment (Ecobichon et al.,
1983). Rats fed 50 mg FireMaster(R) BP-6/kg in their diet from day
8 of pregnancy until 14 days after delivery showed, on day 14
postpartum, HxBB concentrations of about 51 µg/ml in the milk and
about 483 µg/g wet weight in their body fat (McCormack et al.,
1979a). In the same study, milk transfer of PBBs and PCBs was
compared. Milk usually contained higher concentrations of PCBs than
of PBBs, after simultaneous or separate exposure.
Contrary results were obtained with minks, intraperitoneally
injected with either 3 µCi of 14C-labelled PCB or 3 µCi of 14C-
labelled PBB on the approximate date at which the embryos would have
been implanted (Bleavins et al., 1981). Two weeks postpartum, milk
levels of PBBs were determined to be four times those of PCBs
(0.105% versus 0.025% of the initial maternal dose per gram of
tissue). Werner & Sleight (1981) determined PBB concentrations in
the tissues and milk of sows fed various amounts of FireMaster(R)
BP-6 (Table 53). At the end of lactation (4th week), the adipose
tissue and milk of sows, fed daily with 200 mg PBB/kg feed, had HxBB
concentrations of 194 µg/g tissue (wet weight) and of 22 µg/ml whole
milk, respectively. The authors calculated that, on a body weight
basis, nursing pigs consumed PBBs in concentrations similar to the
concentration given to the sows. Tissue levels of young exposed to
PBBs only via nursing have been determined for rats (Rickert et al.,
1978) and for guinea-pigs (Ecobichon et al., 1983). When pups of
non-treated female rats were nursed by dams fed FireMaster(R) BP-6
(50 mg/kg body weight) on days 1-14 postpartum, hepatic HxBB-
concentrations were on average approximately eight times higher than
those in the dams on day 14 postpartum (Rickert et al., 1978).
People who bought food primarily from quarantined farms were
thought to have been exposed 10 to 100 times more than the typical
retail store customer (Schwartz & Rae, 1983): ca. 100 mg of PBBs
versus 1-10 mg (Brown & Nixon, 1979).
While many dust and cobweb samples found in the buildings of
some PBB-contaminated farms had very high residue levels, the amount
of PBB residue involved is said not to be sufficient to be an
important contributor to animal residues (Fries & Jacobs, 1980) and,
possibly to human exposure.
5.2.2 Human monitoring methods for PBBs
Usually, suitable human monitoring data, as such, are used to
describe the real exposure to a toxic chemical. As an indicator of
human exposure to PBBs, the presence of PBBs in adipose tissue,
breast-milk, whole blood, serum, red and white blood cells (Bekesi
et al., 1979a,b), and human hair oils (Stratton & Whitlock, 1979)
has been assessed. The most commonly used specimens were serum,
breast-milk, and adipose tissue (see Tables 38-40).
Table 38. Human monitoring data: PBB levels in the Michigan population (USA)
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1974 82 96.3 serum adults from not 14 not Humphrey &
quarantined farms detected- specified Hayner (1975)
2260
28 100 serum children from 2-2260 35 not
quarantined farms specified
5 100 serum lactating females 3-1068
from quarantined
farms
1976 524 serum Michigan farmers 23.7 2.6 0.2 Wolff et al.
µg/litre (1978a);
Lilis et al.
(1978)
283 serum residents on 0.2-> 1000 33.9 3.9
quarantined farms
153 serum residents of 0.2-50 2.9 1.4
non-quarantined
farms
40 serum consumers of 0.3-1000 56.6 4.2
products
from quarantined
farms
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
28 serum consumers of 0-50 3.4 2.2
products from
non-quarantined
farms
consumers and Wolff et al.
residents: (1978)
quarantined farms:
40 serum females < 18 years 28.0 2.3 0.2
µg/litre
102 serum females > 18 years 18.2 2.5
51 serum males < 18 years 67.7 7.3
129 serum males > 18 years 28.2 4.4
consumers and
residents:
non-quarantined
farms:
37 serum females < 18 years 3.1 1.3
57 serum females > 18 years 1.7 0.9
35 serum males < 18 years 4.8 1.7
51 serum males > 18 years 3.1 2.2
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1976 485 serum consumers and Chanda et al.
residents: (1982)
quarantined farms:
27 serum 0-5 years 0.2-64.2 10.15 not
specified
137 6-18 years 0.0-962.4 27.22 not
specified
321 > 18 years 0.2-1778.0 24.42
1976 321 serum consumers and Chanda et al.
residents: (1982)
non-quarantined
farms:
18 0-5 years 0.2-37.4 6.42 not
specified
104 6-18 years 0.0-42.6 3.25 not
specified
177 > 18 years 0.0-94.0 3.03 not
specified
1976 serum Michigan children 3.41 not Barr (1980)
specified
143 females 2.72 not
specified
149 males 4.23 not
specified
33 0-4 years 2.75 not
specified
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
77 5-8 years 5.59 not
specified
81 9-12 years 3.18 not
specified
101 13-16 years 2.65 not
specified
1976-77 3639 serum Michigan residents 0-1900 21.2 3.0 1 µg/litre Landrigan
with various et al.
degrees of exposure (1979);
Landrigan
(1980)
1976-77 1750 serum contaminated farm 0-1900 26.9 4.0 1 µg/litre Landrigan
1114 residents farm 0-659 17.1 3.0 et al.
(1979);
216 product recipients 0-1240 43.0 4.5 Landrigan
(1980)
559 chemical workers and 0-111 3.4 2.0
families volunteers
1976-77 52 serum women at the time not 26.2 2.5 1 Landrigan
of delivery detected- µg/litre et al. (1979)
1150
1977 3683 serum Michigan PBB cohort < 1-3150 23.2 4.1 3 not Kreiss et al.
specified (1982)
1888 males 5.8
1795 females 2.8
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1978 1681 serum Michigan residents Wolff et al.
(1982)
(randomly selected)
1120 68.9 adults 0.2-120.5 1.3 0.6 0.2
µg/litre
461 72.7 children 0.2-37.2 1.8 0.8
232 Upper Peninsula 0.2
Lower Peninsula:
467 Detroit Area 0.5
191 Muskegon Area 1.7
791 remainder of state 0.9
Muskegon County:
54 serum male adults 4.0 2.6 0.2 Wolff et al.
µg/litre (1982)
74 female adults 2.1 1.4
36 male children 4.9 3.4
27 female children 3.8 2.0
1975-80 serum Michigan PBB cohort: Eyster et al.
(1983)
61 pregnant females not 3.5 3 1
detected- µg/litre
1068
56 non-pregnant females not 3.1 2
detected-
873
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
29 male chemical workers 1-1200 25.4 20
83 male farm and other not 5.4 4
workers detected-
1515
1974-75 5 100 milk women from 0.21-92 660 not Humphrey &
quarantined farms specified Hayner (1975)
1976 milk lactating women Brilliant
(fat) (randomly selected et al. (1978)
53 96 from Michigan: not 0.068 0.1 mg/kg
Lower Peninsula detected-
1.2
42 43 Upper Peninsula not
detected-
0.320
1976-77 32 100 milk women at the time of 0.032-93 3.61b 0.225b Landrigan
(fat) delivery (Michigan et al. (1979)
PBB cohort)
1976-78 2986 88.5 milk lactating women not 0.097 0.1 0.06 < 0.05 mg/kg Miller et al.
milk (self-selected) detected-2 (1984)
1975-80 Michigan Eyster et al.
PBB cohort (1983)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
47 milk pregnant females not 0.312b 0.250b 0.001
(fat) detected- mg/kg
92.7
1974-75 15 100 adipose persons from 0.104-175c Humphrey
quarantined farms & Hayner
(1975)
1975-76 53 adipose quarantined farmers 1.965 Meester &
McCoy
(1976)
29 100 non-quarantined 0.516
farmers
9 100 city residents 0.226
116 fat members of farm 0.58-273.0
families
1977 19 subcutaneous children with known Weil et al.
exposure to PBBs (1981);
fat in utero and/or Schwartz &
through breast milk Rae (1983);
Seagull
(1983)
10 100 "high exposure" 0.116- 4.218
20.960
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
9 100 "low exposure" 0.010- 0.050
0.074
1978 844 97.3 adipose Michigan residents < 0.002- 0.4 0.199 0.002 Wolff et al.
36.7 mg/kg (1982)
(randomly selected) 0.015
87 Upper Peninsula
Lower Peninsula:
255 Detroit Area 0.16
84 Muskegon Area 0.50
418 remainder of state 0.24 0.050
1975-80 32 adipose Michigan PBB-cohort: not 0.330 0.540 0.001 Eyster et al.
lipid pregnant females detected- mg/kg (1983)
174
56 non-pregnant females not 0.00057 0.460
detected-
0.619
29 male chemical 0.4-350 5.29 6.0
workers
83 male farm and other 70-350 1.65 1.05
workers healthy male
Not 7 100 adipose volunteers, aged 0.01-2.72 0.001 Schnare
specified lipid 20-30 years mg/kg et al.
(subcutaneous) (1984)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1983 15 100 perirenal autopsy cases from 0.032-1.65 0.475 0.32 0.0005 Miceli
post- the "high" exposure mg/kg et al.
mortem area of Michigan (1985)
adipose (Grand Rapids)
tissue
(wet wt)
a Expressed as the concentration of the major hexabromobiphenyl component (BB 153) in µg/litre serum or
mg/kg milk, or adipose tissue, respectively.
b The sample measuring 93 mg/kg was excluded from statistical analysis.
c Most in the order of 1 mg/kg.
The last one has been preferred for analysis, since depot fat
is the predominant storage site of PBBs (and other persistent
halogenated hydrocarbons), and, therefore, allows an increased
detectability of body burden. For example, had serum PBBs been used
alone as an indicator of exposure in the PBB survey of the general
population of Michigan (Table 38), only 70% of the 839 individuals
would have been considered exposed. When adipose tissue results are
added, an additional 24% indicate exposure, raising the positive
rate to 94%. Even though the limit of detection was an order of
magnitude higher (2.0 µg/litre in adipose tissue vs. 0.2 µg/litre in
serum), the partition ratio of approximately 300:1 made the adipose
limit of detection a more sensitive indicator of exposure (Wolff
et al., 1982; Anderson, 1985).
On the other hand, collection of hair, blood, and breast-milk
samples is simpler and less invasive than adipose tissue biopsy.
Moreover, with some exceptions, significant correlations between
adipose tissue and blood serum or breast-milk PBB levels were found
(section 6.2). Further advantages and limitations of these
techniques are discussed in detail by Anderson (1985) and Fries
(1985b).
It should be noted that levels of PBBs in breast-milk may not
be comparable between studies unless the concentration is adjusted
for fat content, because the fat content of breast-milk varies
widely from woman to woman, and the value increases during feeding
(Rogan et al., 1980).
Although the accurate relationship between observed body levels
of PBBs and individual exposure to PBBs is not clear (Safe, 1984),
PBB concentrations in human tissues can give some idea of the levels
of exposure (Kimbrough, 1980a).
5.2.3 Human monitoring data
In the following subsection, human monitoring data are
presented from contaminated farms in Michigan, from Michigan state,
and from other countries.
Most data available refer to the Michigan PBB incident in
1973-74 (Tables 38 and 39). Because, in this case, the spilling of
PBBs started from farms, the Michigan Department of Public Health
(MDPH) undertook a series of studies on farm families as a high-risk
group in the summer and autumn of 1974.
Serum samples were obtained from 110 persons in the exposed
group (had been working or living in the quarantined farms for six
months or more since the accident) and for 104 persons from the
control group (randomly selected from a list of dairy producers in
the same geographical area, where farms had not been quarantined).
As shown in Tables 37 and 38, serum levels of PBBs were
significantly higher in the people from quarantined farms compared
with those from non-quarantined farms, though low levels were also
observed in the control group (Cordle et al., 1978). In 1976, the
MDPH, together with the Centre for Disease Control, the FDA, NIH,
and EPA, established a cohort of 4545 people in Michigan to be
examined at regular intervals over several decades (Barlow &
Sullivan, 1982) to evaluate the long-term effects of PBB exposure.
Four groups were included (Landrigan, 1980): Quarantined farm
residents, direct recipients of farm produce, chemical workers and
their families, and persons who either volunteered for the study or
who had participated as control subjects in an earlier pilot study
(Humphrey & Hayner, 1975). The first report was published by
Landrigan et al. (1979) followed by several reports on subgroups of
this population (e.g., Kreiss et al., 1982; Eyster et al., 1983).
Study groups of the Mount Sinai School of Medicine have also
conducted comprehensive examinations on similarly categorized
groups, e.g., residents of quarantined farms, residents of
non-quarantined farms, consumers of products directly from
quarantined or non-quarantined farms, and Michigan Chemical Company
workers; residents of the state of Wisconsin were used as a control
group.
It can be seen from Table 38 that nearly 100% of the adipose
samples randomly selected throughout the state had detectable PBB
concentrations. Thus, statewide exposure of Michigan residents to
PBBs can be demonstrated.
Levels of PBBs in serum (Landrigan, 1980; Wolff et al., 1982),
breast-milk (Brilliant et al., 1978; Miller et al., 1984), and
adipose tissue (Wolff et al., 1982) were highest in the area of the
accident (lower peninsula), and lowest in the upper peninsula,
farthest from the source.
Compared with residents of quarantined farms, direct consumers
of products from quarantined farms, and PBB- production workers, the
tissue burdens among the general population of Michigan were 1-3
orders of magnitude lower. Moreover, for example, only 36% of the
general population had serum PBB concentrations greater than
1 µg/litre, compared with 78% among farmers (Anderson et al., 1979;
Wolff et al., 1982).
PBB levels appear to be higher in males than females (Meester &
McCoy, 1976; Landrigan et al., 1979; Landrigan, 1980; Wolff et al.,
1978; 1980; Kreiss et al., 1982; Eyster et al., 1983) and higher in
children (below the age of 10 years) than in adults (Humphrey &
Hayner, 1975; Landrigan et al., 1979; Landrigan, 1980; Barr, 1980;
Wolff et al., 1982).
A later study (Schnare et al., 1984) recorded not only the
concentration of the most abundant congener of the FireMaster(R)-
mixture (2,2',4,4',5,5'-hexabromobiphenyl) but also the concen
trations of other PBB congeners detected in subcutaneous adipose
tissue samples of 7 former participants of the Michigan PBB studies
(Table 39).
Table 39. Range of adipose tissue concentrations of various PBBs in 7 personsa
(mg/kg on a lipid weight basis)b
PBB Rangec (mg/kg)
2,3',4,4',5-penta nd-0.16
2,2',4,4',5,5'-hexa 0.01-2.72
2,2',3,4,4',5'-hexa nd-0.22
2,3',4,4',5,5'-hexa nd-0.09
2,2',3,3',4,4',5-hepta nd-0.26
2,2',3,4,4',5,5'-hepta nd-0.01
a 7 healthy male volunteers, aged 20-30 years, having been exposed to PBBs some
years earlier, as a consequence of the Michigan PBB incident in 1973.
b From: Schnare et al. (1984).
c nd = Not detectable; detection limit = 0.001 mg/kg.
In most cases, PBB concentrations did not appear to be
decreasing significantly over time. Wolff et al. (1979b) did not
find any significant variation in the serum PBB levels of nine dairy
farm residents during 18 month of observation.
Paired serum samples, one collected in 1974 and the other in
1977, were also available for 148 members of the Michigan PBB
cohort. The data indicate that levels were generally stable over the
3-year period with a mean change of 16 µg/litre (Landrigan et al.,
1979). In another study of the Michigan PBB-cohort, the decrements
in median serum levels of PBBs between matched pairs over one -
(1977-78) and two - (1977-79) year intervals were both only 1
µg/litre (Kreiss et al., 1982). No significant change in blood
plasma PBB levels was observed over a 5-month period in 41 residents
of quarantined farms (Humphrey & Hayner, 1975). In contrast, Meester
& McCoy (1976) reported a marked decline over 3 years (1974-76) in
serum levels of PBBs. These authors also found that the average
decrease in PBB concentrations in the fat of 16 individuals was
about 40%, in a period of 6 months. No changes in PBB levels were
seen over an 11-year period (1976-87) in fat samples from a patient
with long-term exposure to PBBs from the early 1970s as a result of
the Michigan PBBs accident. The average fat level of PBBs was
0.8 mg/kg (Sherman, 1991).
In 1981, PBBs were found in 13-21% of serum samples from
4-year-old Michigan children. Their mothers belonged to a group that
was surveyed either with regard to the consumption of Lake Michigan
sport fish (mean PBB level detected in children: 2.4 ng/ml) or with
regard to former exposure to quarantined farm products (mean PBB
level detected in children: 3.0 ng/ml) (Jacobson et al., 1989).
Few human monitoring data are available for the US population
outside of Michigan. They are summarized in Table 40. One study
deals with the population in the vicinity of industrial areas
involved in PBB production or use (Stratton & Whitlock, 1979), the
other with farmers of the state of Wisconsin who were examined as
control group in connection with the Michigan PBB studies (Wolff
et al., 1978).
PBBs were found in all studies, but, because of the limited
data, the significance is unclear. The highest PBB levels were found
in the hair of humans living near PBB industry. Of the nine samples
analysed, five had detectable PBB levels. Both male and female hair
samples contained PBBs (Stratton & Whitlock, 1979).
In contrast to the other surveys, which had regard only to the
major PBBs component (hexabromobiphenyl), Stratton & Whitlock (1979)
identified the different PBB homologues in the extracted oils of the
human hair, collected from barbershops and beauty parlours (Table
41). There were identifiable differences in the composition of PBB
congeners found in hair from the three locations.
The samples with the highest concentrations contained
relatively large amounts of decabromobiphenyl, while the samples
with lower concentrations contained only hexabromobiphenyl (Stratton
& Whitlock, 1979). One sample was different from the others because
it contained dibromobiphenyl (see Table 41). As a result of the
sampling method, it was impossible to ascertain whether the exposure
was related to the workplace or to the ambient environment.
In a report by Lewis & Sovocool (1982), pooled adipose tissue
samples from 202 individuals from nine census regions in the USA
were analysed for HxBB. Although the average concentration was 1-2
µg/kg, it cannot be excluded that this was because of the inclusion
of a few samples with high PBB concentrations.
Table 40. Human monitoring data: PBB levels in the US population (outside of Michigan)
Year No. of % of Specimen PBB PBB Detecion Remarks References
specimens positive tissue group/area concentrationa examined limita
findings range
1977 56 3.6 serum Wisconsin farmers not C12H4Br6 examined as Wolff
a control detected-1.1b et al. (1978)
population
1977 3 33 hair (from males and females, < 100-8100 total PBBs 100 reported as Stratton &
barber- Bayonne New Jersey, µg/kg in oil Whitlock
shops) Vicinity of White (1979)
Chemical
3 100 hair (from males and females, 440-26 600 total PBBs 100 reported as
barber- Staten Island, µg/kg in oil
shops) New York, Vicinity
of Standard T
Chemical Co.
3 33 hair (from males and females, < 100-310 000 total PBBs 100 reported as&
barber- Sayreville New µg/kg in oil
shops) Jersey, Vicinity of
Hexcel Fine
Organics
a (µg/litre or µg/kg).
b PBBs not detected in 54/56 persons. PBBs observed at 1.1 µg/litre in one person, identified as recently
moved from a Michigan farm, and at 0.5 µg/litre in another person.
Table 41. Concentration (range) of different PBB congeners in human hair samples
taken in the vicinity of three industrial sites in the USA, reported as
µg/kg in oila
PBB-congeners Bayonne, New Staten Island, New Sayreville,
Jersey (White York (Standard T New Jersey
Chemical Corp.)b Chemical Comp.)c (Hexcel Corp.)d
MoBB nde nde nde
DiBB nd-8100 nd nd
TrBB nd nd nd
TeBB nd nd nd
PeBB nd nd nd
HxBB nd 440-740 nd-480
HpBB nd nd-890 nd
OcBB nd nd-1100 nd
NoBB nd nd-3600 nd-22 500
DeBB nd nd-20 000 nd-285 000
a Data from: Stratton & Whitlock (1979).
b Having manufactured octa- and decabromobiphenyl along with bromobiphenyl
ethers.
c Major user of FireMaster BP-6.
d Producer of laboratory quantities of various PBBs.
e nd = Not detected (detection limit = 100 µg/kg).
There is very little human monitoring data on PBBs in the
populations of countries other than the USA. Krüger et al. (1988)
reported PBB contamination of breast-milk from European women in a
survey from North Rhine-Westphalia, Germany (Table 33). The milk
samples (n=25) contained a typical pattern of certain PBB congeners.
It included penta- to octabromobiphenyls in concentrations ranging
from 0.002 to 28 µg/kg, based on milk fat. The most abundant
component was 2,2'4,4',5,5'-hexabromobiphenyl (BB 153) followed by a
peak consisting of two heptabromobiphenyl isomers (2,2',3,4',5,5',6-
and 2,2',3,4,4',5,6'-heptabromobiphenyl BB 187 and 182). Differences
in the pattern were only found in the milk given by a Chinese woman
and in that given by a woman having been exposed to several fires in
industry.
Concentrations of BB 153 in human and cow's milk, both
collected from the same region (North Rhine-Westphalia), were
1 µg/kg and 0.03 µg/kg, respectively, measured on a fat basis
(Krüger, 1988).
5.2.4 Subpopulations at special risk
Children are at risk from exposure to PBBs in different ways.
Studies on the Michigan population indicated a significant PBB
transfer to the fetus (Landrigan et al., 1979; Eyster et al., 1983;
Jacobson et al., 1984) and to breast-milk (Brilliant et al., 1978;
Landrigan et al., 1979; Eyster et al., 1983; Jacobson et al., 1984,
1989; Miller et al., 1984). PBB levels to which fetuses and newborn
infants were exposed in the Michigan accident are shown in Table 42.
Because the placenta acts only as a partial barrier to PBBs, a
newborn baby has a body burden, even before breast feeding.
Placental and cord serum levels are much lower than levels in
breast-milk. However, even at low concentrations, intrauterine
exposure may be significant, for several reasons, as pointed out in
detail by Jacobson et al. (1984).
Infants are not only exposed to PBBs through their mothers and
through consumption of contaminated food, but they also through
contact with PBBs from the environment. Young crawling children are
known to ingest accidentally soil or dust to an extent of up to
0.1 g/day.
5.3 Occupational exposure during manufacture, formulation, or use
In general, occupational exposure is to be expected in PBB
manufacturing and processing plants. In Michigan, as a result of the
PBB incident, farmers, and possibly dairymen, elevator, mill
personnel etc. were occupationally exposed (Kay, 1977).
Table 42. PBBb concentrations in maternal serum, adipose lipid, and milk lipid, and in
the cord serum and placenta of Michigan women (Michigan PBB-cohort) at the time of
parturition (1975-80)a
Paired specimen No. Rangec Median Geometric Measure
mean
Maternal serum 61 nd-1068 3 3.5 µg/litre
Placenta nd-370 < 1 - µg/kg
Maternal serum 60 nd-1068 3 3.2 µg/litre
Cord serum nd-104 < 1 - µg/litre
Maternal serum 47 nd-1068 3 3.0 µg/litre
Milk lipid nd-92 667 250 312 µg/kg
Milk lipid 27 52-92 667 384 472 µg/kg
Adipose lipid nd-174 000 522 82 µg/kg
a From: Eyster et al. (1983).
b Concentrations expressed as concentrations of hexabromobiphenyl.
c nd = Not detected (detection limit: 1 µg/litre or kg).
Bialik (1982) reported the following contaminant levels of
decabromobiphenyl measured in 1977 in the manufacturing area of
Hexcel/Fine Organics and Saytech, Inc. (Sayreville; USA):
- Plant air samples: 0.18 and 0.23 mg/m3 8 h TWA (time-weighted
average);
- Wipe tests, unspecified: up to 8 mg/100 cm2; - Wipe tests,
eating Table: 0.1 mg/100 cm2.
At this time, 95% of the plant production consisted of
decabromobiphenyl (18%) and decabromobiphenyl oxide (77%). About
2000 tonnes of decabromobiphenyl were manufactured during 1973-77
(Bialik, 1982).
Employees of chemical plants may be exposed directly to PBBs
(in most cases along with other chemicals) through contact,
inhalation, or ingestion (Wolff et al., 1979a). As an index of
individual exposure, PBB levels in the serum and adipose tissue of
chemical workers have been recorded. The results of several authors
are compiled in Table 43.
Most data refer to the Michigan Chemical Corp., St. Louis
(Michigan), which produced several brominated organic compounds and
manufactured over 5000 tonnes of PBBs, predominantly
hexabromobiphenyl, from 1970 to 1974. Some additional general
exposure from contaminated food can also be included for the workers
of Michigan Chemical.
To summarize, median serum and adipose tissue PBB levels were
higher among chemical workers than among male residents of
quarantined farms.
Non-production workers at the Michigan Chemical plant showed
significantly lower levels than workers involved in PBB production;
for example, median adipose tissue concentrations of PBBs were
2.49 mg/kg and 46.94 mg/kg, respectively.
In another study on workers at a PBB plant in New Jersey, Bahn
et al. (1980b) presented a detailed comparison of serum PBB levels
in various occupational groups. A significantly higher number of PBB
workers had detectable levels of PBBs, compared with other workers
in the study (35.9% compared with 12.2%). Among workers with
detectable PBB levels, the PBB workers had significantly higher
serum levels than workers from neighbourhood industries not using
PBBs.
Although this factory concentrated on manufacturing
decabromobiphenyl and decabromobiphenyl oxide (ether), there was no
positive identification of C12Br10 (Table 44) or C12Br14O
(Bahn et al., 1980b).
No data are available about occupationally exposed women.
Family members of chemical workers have also been found to have
a body burden of PBBs (Landrigan, 1980).
Bekesi et al. (1979b) determined the distribution of PBBs in
the blood compartments of 4 Michigan Chemical plant workers (Table
45) and suggested that the PBB level of the white cell fraction may
be a better indicator for risk potential than the total plasma PBB
concentration.
Table 43. Occupational exposure: PBB levels in chemical workers (USA)
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1975 Michigan workers (8-36 serum 6-85 HxBB Kay (1977)
Chemical Corp. months exposure (7)
(MCC)
(St. Louis,
Michigan)
1976 MCC (St. Louis, employees (55) serum 1.1-1729 123 9.3 HxBB Wolff
Michigan) et al.
(1978,
1979a)
production workers serum 603.9 108.4 HxBB 1 Wolff
(10) et al.
(1979a)
non-production serum 16.5 6.1 HxBB 1
workers (45)
workers (14) serum 1-1530 HxBB < 0.2 Wolff
et al.
(1979b)
1978 workers (14) 1-1363 HxBB < 0.2
(matched pairs)
Table 43 (contd).
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1976-77 MCC (St. Louis, workers and serum not 43.0 4.5 C12H4Br6 1 Landrigan
Michigan) families (216) detected-1240 et al.
(1979)
1975-80 MCC (St. Louis, workers (male) serum 1-2000 (25.4) 20 C12H4Br6 1 Eyster
Michigan) (29) et al.
(1983)
1978 Hexcel/Fine PBB workers serum not MoBB Bahn
Organics and (exposure to PBBs detected-1340 DeBB et al.
Saytech, Inc. (and PBBOs) for at (1980b)
(Sayreville least 6 weeks
New Jersey) between January
1973 and August
1978) (39)
1976 Michigan production workers adipose 5000-581 000 196 490 46 940 HxBB 500 Wolff
(7) tissue et al.
(1979a)
non-production 500-10 000 3880 2490
workers
1975-80 Michigan workers (male) adipose 400-350 000 5290 6000 HxBB 1 Eyster
(29) tissue et al.
(1983)
a In µg/litre or µg/kg.
Table 44. Detectablea serum levels (µg/litre) of PBB homologues in workers
at a plant producing decabromobiphenyl and decabromobiphenyl oxideb
PBB homologue Number of cases Range
C12H9Br 14 0.3-5.5
C12H8Br2 1 6.9
C12H7Br3 1 0.9
C12H6Br4 0 -
C12H5Br5 2 1.6-13.0
C12H4Br6 2 0.4-6.0
C12H3Br7 7 9.0-40.0
C12H2Br8 9 20.0-800.0
C12H Br9 1 500
C12Br10 0 -
Total PBBs 26 0.3-1340
a Excludes cases with "trace", "not confirmed" and "not detectable" levels.
b From: Bahn et al. (1980b).
Despite its significance for toxicological assessment, the
content of minor constituents of FireMaster(R) in the body burden
was rarely investigated. For example, Wolff & Aubrey (1978) examined
other PBB congeners, which are identifiable as peaks by GC/MS
(2 pentabromobiphenyl peaks, and 2 heptabromobiphenyl peaks), in the
serum of Michigan Chemical workers (n = 24) and Michigan dairy
farmers (n = 37), besides the major component (2,2',4,4',5,5'-
hexabromobiphenyl) of FireMaster(R). The relative concentrations,
with respect to the major hexabromobiphenyl peak, of these PBB
components were somewhat different for chemical workers and for
farmers, i.e., the two pentabromobiphenyl values (peak area ratios)
were significantly higher in the serum from chemical workers.
Table 45. Distribution of PBBs in blood compartments of Michigan Chemical Workersa
Polybrominated biphenyls
ng/mg Protein Ratiob
RBC plasma WBC RBC plasma WBC
Michigan Chemical Workers;
not directly involved in 0.07 0.13 3.9 1 : 2 : 56
the production of PBB 0.03 0.23 1.8 1 : 8 : 60
directly involved in 0.67 10.0 57.3 1 : 15 : 86
the production of PBB 0.63 10.2 32.0 1 : 16 : 51
a From: Bekesi et al. (1979b).
b RBC = Red blood cells; WBC = white blood cells.
This variation might be attributed to the different routes
(skin contact, inhalation, direct ingestion versus, primarily,
ingestion of animal foodstuff) and to the different composition
(unchanged versus animal-mediated material) of exposure in chemical
workers versus farmers. Further reasons might be the earlier initial
onset of contamination in workers and slight variations in the
composition (section 2.1.2) of several lots of FireMaster(R) BP-6
(the main product of Michigan Chemical) and FireMaster(R) FF-1,
which caused contamination of livestock feed (Anderson et al.,
1978a; Wolff & Aubrey, 1978; Wolff et al., 1979a).
The change in serum PBB levels over time was investigated in
chemical workers at two facilities. Wolff et al. (1979b) reexamined
serum PBB concentrations (determined as the major hexabromobiphenyl
peak) in 1978 from 14 workers of the Michigan Chemical Corp., who
had also been tested 18 months earlier. They found PBB levels of a
comparable order. In contrast, no subject (n=109) in a study on
chemical workers of Hexcel/Fine Organics and Saytech Inc.
(manufacturing decabromobiphenyl and decabromobiphenyl oxide) showed
any detectable serum level of PBBs (different congeners) in 1981
(Bialik, 1982), which was true, even for the two persons who had
shown high levels of serum PBBs in the previous study of 1978 (Bahn
et al., 1980b; Table 43). However, the results of PBB determination
in the fat of these two cases were positive in 1981. The negative
results of the determination of PBBs in serum were not expected, and
the authors suggested further studies.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Gastrointestinal absorption
Studies have been performed only on the gastrointestinal
absorption of PBBs. Some studies indicate that PBBs are rapidly and
efficiently absorbed, other studies indicate a much lower efficiency
of absorption (see Table 46). No information is available on the
extent of absorption of decabromobiphenyl.
Absorption can be strongly influenced by the vehicle in which
the compound is administered (Birnbaum, 1985). Administration of
hexabromobiphenyl in mineral oil or olive oil solution resulted in
higher absorption than administration in a methyl cellulose
suspension (see Table 46: Rozman et al., 1982). The degree of
halogenation also appeared to influence the absorption of PBB. For
example, less than 10% of 14C-labelled hexabromobiphenyl, but 62%
of a dose of 14C-labelled octabromobiphenyl were eliminated in the
faeces of rats in 24 h, though both compounds had been administered
in corn oil (see Table 46).
The conclusion that more brominated biphenyls are absorbed less
efficiently than less brominated biphenyls can, possibly, be drawn
from other findings. Willett & Durst (1978) observed that, during
feeding of FireMaster(R) BP-6, the relative concentration of
pentabromobiphenyl in the faeces of cows was decreased, and that of
heptabromobiphenyl was elevated compared with the
FireMaster(R)-standard. Similarly, faecal concentrations of
heptabromobiphenyl were enhanced relative to concentrations of
hexabromobiphenyl in the faeces of hens, when FireMaster(R) BP-6
was fed (Fries et al., 1976). However, Polin & Leavitt (1984) found
that the ratio of 3.5 for hexa- to heptabromobiphenyl in the
chemical sample of FireMaster(R) FF-1 shifted to an average ratio
value of 2.5 in the whole carcasses of chickens analysed on days 0,
21, and 42 of withdrawal, inferring a better absorption of hepta-
bromobiphenyl.
Generally, it should be noted that faecal elimination during
the first few days following dosing might be an indicator, but is
not a measure, of lack of absorption, because some absorbed PBB is
eliminated and recycled into the faeces in bile and by diffusion
across intestinal membranes (Rozman et al., 1982; Fries, 1985b).
Table 46. Absorption of PBBs after oral administration
PBB compound Species Vehicle Absorption Methods Comments References
(sex) parametera
[14C-]2,2',4,4',5,5'- rat emulphor-EL 620: 90%, 24 h faeces analysis single dose Matthews
hexabromobiphenyl (male) ethanol: water gut content et al. (1977)
(1 : 1 : 8)
corn oil 90% multiple doses (4)
[14C-] octabromobiphenyl rat (male, corn oil 38%, 24 h faeces analysis single dose Norris et al.
(technical mixture) female) (1973)
[14C-]2,2'4,4',5,5'- rhesus 1% methyl cellulose 40%, 10 days faeces analysis two doses Rozman et al.
hexabromobiphenyl monkey mineral oil 62%, 5 days faeces analysis single dose (1982)
(male) olive oil 66%, 5 days faeces analysis repeated doses (4)
FireMaster(R) BP-6 cow crystalline PBB in 50%, 168 h faeces analysis single dose Willett &
(female) gelatin capsules (7 days) Irving (1976)
calf crystalline PBB in 95%, (9 days) faeces analysis daily feeding
(male) gelatin capsules
a Values based on concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (FireMaster(R) BP-6 sample) or on [14C-]activity.
6.1.1.2 Dermal and inhalation absorption
No quantitative information is available on skin absorption and
intake through inhalation.
6.1.2 Human studies
It is plausible that inhalation and dermal contact are the main
routes of exposure to PBBs for chemical plant workers (Wolff et al.,
1979a), while the main route for Michigan people was the ingestion
of PBBs dissolved in the fat of meat and milk (Di Carlo et al.,
1978). An appropriate model for assessing the latter kind of
absorption is thought to be the rat-corn oil model (Fries, 1985b).
No quantitative data are available on PBB absorption in humans.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Levels in organs and blood
As can be seen from Tables 47, 48, and 49, most studies on the
distribution of PBBs have been conducted with the FireMaster(R)
mixture. A few earlier publications refer to technical
octabromobiphenyl. No experimental data are available on tissue
distribution of decabromobiphenyl. When FireMaster(R) was
administered, the distribution process was studied predominantly as
the distribution of 2,2',4,4',5,5'-hexabromobiphenyl, and, with far
less emphasis, on the distribution of the minor components of the
mixture. Little information is available on the distribution of PBB
congeners, when administered individually.
Investigations on rats, mice, cows, sheep, pigs, and avian
species demonstrated that PBBs were distributed widely throughout
the body tissues in all species. Highest (equilibrium)
concentrations on a wet tissue basis were found in adipose tissues,
consistent with the solubility characteristics of PBBs. Adipose
concentrations are usually an order of magnitude higher than those
of most muscle and organ tissues (see Tables 47, 48, 49, and 50).
Much of the variation in concentrations among tissues can be
accounted for by variations in the fat concentrations in these
tissues (Willett & Durst, 1978; Fries et al., 1978b; Fries, 1985b).
Table 47. Distribution of PBBs in mammals after the administration of a single dose of PBBs
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
FireMaster(R) FF-1 rat oral 1000 10 monthsb adipose tissue (714) > liver (60) > blood Kimbrough et al.
(lot No. 7042) (male) in corn oil (0.94) (1978)
rat 10 monthsb adipose tissue (1202) > liver (37) > blood
(female) (2.9)
FireMaster(R) FF-1 rat oral 80 42 days fat (295) > blood (0.38) Wolff &
(male) in corn oil Selikoff (1979)
FireMaster(R) FF-1 rat oral 500 4 months adipose tissue (1008) > liver (50) > blood Kimbrough et al.
(lot No: 7042) (male) in corn oil (2.1) (1980)
FireMaster(R) FF-1 rat oral 10 24 hb sc fat I (61 500) > sc fat II (38 700) > liver Domino et al.
(lot No: FF-1312-FT) (male) (20 900) > lung (7650) > kidney (7310) > heart (1980b)
(6470) > jejunum (4860) > spleen (3530) >
cerebellum (2990) > grey matter (2850) > white
matter (2750) > testes (2380) > blood (945)
4 weeksb sc fat (19 200) > jejunum (3170) > lung (1240) Domino et al.
> liver (690) > kidney (650) > spleen (520) (1980b)
> heart, testes (both 240) > grey matter
(210) > cerebellum (200) > white matter (170)
> blood (56.9)
FireMaster(R) BP-6 rat intraperitoneal 12 weeks serum (46.80 ng/ml) Miceli &
(male) 10 in corn oil Marks (1981)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
fat (21.90) > adrenal (3.64) > lung (0.98)
> liver (0.59) > pituitary (0.91) > gonad (0.33)
> kidney (0.22) > heart (0.20) > spleen (0.17)
> brain (0.13)
36 weeksb serum (23 ng/ml)
fat (16.62) > adrenal (2.67) > lung (0.51) >
pituitary (0.29) > liver (0.20) > kidney (0.14)
> gonad, brain (both 0.10) > heart (0.08)
> spleen (0.05)
2,2',4,4',5,5'- rat oral 1 in: 1 day muscle (29.9) > adipose (25.5) > skin (17.9) Matthews et al.
[14C]-hexabromobiphenyl (male) Emulphor > liver (9.0) > blood (0.90)c (1977)
EL 600: ethanol:
water (1:1:8)
14C-PBB rat intraperitoneal 28 days ovaries (130) > skin (15.1) > testicles (13.3) McCormack et al.
(male 150 in: peanut > intestine (11.7) > lung (7.3) > liver (4.7) (1979b)
and oil > muscle, heart (1.9) > fat (1.8) > brain (0.9)d
female
pups)
FireMaster(R) BP-6 cow oral 5.95 in: 10 days liver (1.35) > fat (sc: 1.15; perirenal: 1.09; Willett &
(lot No. RP-158) (female) gelatin capsule peri-cardiac: 0.97; intermuscular: 0.81; omental: Irving (1976)
0.78) > brain (pons: 0.27; cortex: 0.08) >
mammary gland (0.25) > kidney (0.12) > heart
(0.11) > lung, muscle (0.08) > ovaries, uterus
(0.06) > plasma, rumen wall (0.04) > bile
(0.02) > synovial fluid (0.01)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
[14C-]octabromobiphenyl rat oral 1 in: 16 days adrenal, adipose, heart, skin > liver, Norris et al.
(technical mixture) (male) corn oil pancreas, spleen (1973)
a Measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity; (in parentheses: values measured - referring
to various measures).
b For additional time points: see original reference.
c Values in average % total PBB dose.
d Values in µg-equivalents/g wet weight.
sc. = Subcutaneous.
Table 48. Studies on the distribution of PBBs in animals following dietary or repeated oral intake
of the FireMaster(R) mixtures or 2,2',4,4',5,5'-[14C]-hexabromobiphenyl
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 fat (330) > mammary gland (318) Rickert
BP-6 (pregnant) feed until day 21 of > kidney (30) > skin (22) > liver, et al. (1978)
gestation lung, brain, heart, small
intestine, placenta, uterus (all
< 5)
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 mammary gland (117) > liver (4) Dent et al.
BP-6 (maternal) feed to 14 days (1977b)
postpartum
FireMaster(R) rat 25 mg/kg day 8 of pregnancy 0 fat (74) > mammary > liver McCormack
BP-6 (maternal) feed to 14 days > kidney > lung (6) et al.
postpartum (1979a)
50 mg/kg 0 fat (483) > mammary > kidney
feed > lung (13)
200 mg/kg 0 fat (966) > mammary > kidney
feed > lung (21)
FireMaster(R) rat 100 mg/kg day 8 of pregnancy 0 fat (813) > liver (54) > mammary McCormack &
BP-6 (maternal) feed to 28 days (43) Hook (1982)
postpartum 14 weeks fat (459) > mammary (225) > liver
(after first (12)
and only
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
litter was
weaned)
> 10 weeks fat (77) > mammary (19) > liver (3)
and after
weaning
their second
litter
2,2'4,4',5,5'- rat 1 mg/kg 4 days 3 days adipose (41.1) > skin > muscle Matthews
hexabromobiphenyl (male) body weight > liver > blood (0.32)b et al. (1977)
per day
FireMaster(R) rat 0.1 mg/kg 9 days 0 liver (1.5) > brain (0.5), Render et al.
BP-6 (Lot 6224A) (male) feed adipose (0.3)c (1982)
1 mg/kg 9 days 0 liver (8.3) > brain (1.8),
feed adipose (1.7)c
10 mg/kg 9 days 0 liver (135) > adipose (27)
feed > brain (12)c
100 mg/kg 9 days 0 liver (1213) > adipose (251)
feed > brain (103)c
FireMaster(R) rat 3 mg/kg 20 days 0 adrenal (93.7) > thyroid (> 20) Allen-
FF-1 (Lot No. (male) body weight > testes (8.7) Rowlands et al.
FF-1312-FT-3) per day (in 1981);
lecithin Castracane
liposomes) et al. (1982)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
20 days 5 months adrenal (481) Castracane
(plus dietary et al. (1982)
restriction)
FireMaster(R) mouse 100 mg/kg 14 days 6 h thymus (391) > fat > liver > brain Corbett et al.
BP-6 feed > pancreas > testicles > spleen (1978a)
(2.7)
14 weeks thymus (50) > adrenals = fat >
liver > testicles > spleen > brain
> pancreas (n.d.)
12 weeks perithymic fat (96) > perirenal
fat > adrenal glands > thymus
gland (5.5)
FireMaster(R) mouse 5 mg/kg 3 weeks 0 thymus (20) > lung, liver > spleen Loose et al.
FF-1 feed > serum (< 0.002) (1981)
(lot No. 7042)
8 weeks 0 thymus (24) > liver, lung > spleen
> serum (0.019)
FireMaster(R) mouse 167 mg/kg 3 weeks 0 thymus (109) > liver > lung > Loose et al.
(lot No. 7042) feed spleen > serum (1.22) (1981)
6 weeks 0 thymus (3088) > liver > spleen
> lung > serum (4.75)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum, (11)
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum (11)
FireMaster(R) cow 50 mg/kg 15 days 15 days renal fat (10) > omental fat Gutenmann &
BP-6 (lactating) feed > brisket fat > liver = thyroid Lisk (1975)
> mammary = chuck muscle > loin
muscle > heart > kidney = brain
> adrenal = spleen (0.4)
FireMaster(R) calf 25 g daily 9 days 0 rumen contents (14257) > feces Willett &
BP-6 (in gelatin > rumen wall > bile > marrow Irving (1976)
(lot 6244 A) capsules) > perirenal fat (441) > kidney
> testes > liver > thymus > heart
> brain, pons > lymph nodes >
tongue > spinal cord > brain,
cortex > plasma > small intestine
> lung > spleen > thyroid >
muscle (25)
FireMaster(R) cow 250 mg daily 60 days 0 fat (25) > liver > glands > nervous Willett &
BP-6 (heifer) (in gelatin > bile > contractiles > plasma Durst (1978)
(lot 6244 A) capsules) > liquids (0.0198)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) cow environmentally ca. 14 days 9 months perirenal fat (380*) > omental fat Fries et al.
FF-1 contaminated > subcutaneous fat > kidney > liver (1978b)
(Michigan PBB > skeletal muscle > cardiac muscle
incident) > lung > brain (10.5*)
ca. 200-400 g 9-12 months perirenal fat (1224*) > omental fat
(killed in >subcutaneous fat > skeletal muscle
extremis) > liver > cardiac muscle > kidney
> lung > brain (57*)
FireMaster(R) calf 10 mg/kg 4 weeks 0 fat (378) > kidney > liver > muscle Robl et al.
FF-1 (female) body weight (1.26) (1978)
per day in
gelatin
capsules
calf 100 mg/kg 6 weeks 0 fat (6080) > kidney > liver
(male) body weight > muscle (34)
per day
cow 1 158 days 182 days brisket fat (4.5) > bone marrow
> stomach fat > tail fat (3.3)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) sheep 50 mg/kg 30 days 0 renal fat (42) > omental fat Gutenmann &
BP-6 feed per day > brisket fat > liver > chuck Lisk (1975)
(complete muscle > loin muscle > heart >
ration) thyroid > brain > adrenal > kidney
= spleen (0.9)
FireMaster(R) pig 20 mg/kg 16 weeks 0 back fat (64) > leaf fat > liver Ku et al.
BP-6 feed > muscle > kidney (0.9) (1978)
200 mg/kg 16 weeks 0 back fat (503) > leaf fat > muscle
feed > liver > kidney (13.5)
FireMaster(R) pig 100 mg/kg during 2nd 0 adipose tissue > liver > kidney > Werner &
BP-6 (lactating feed half gestation > braind Sleight (1981)
sow) 200 mg/kg and during
feed lactation
"PBB" Japanese 20 mg/kg 9 weeks 0 liver (374) > kidney > muscle Babish et al.
(ca 75% quail feed > heart > brain (40)e (1975a)
hexabromobiphenyl) male
female 0 liver (225) > heart > kidney >
> muscle > brain (26)e
FireMaster(R) chicken various 5 weeks 0 adipose tissue (3:1) > whole egg Polin &
FF-1 (White concentrations > liver > muscle (0.008:1)f Ringer
Leghorn (1978a)
hens)
Table 48 (contd)
a Measured as concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (in parentheses: values measured - referring to various measures).
b Values in average % total PBB dose.
c Values on a fat basis.
d Concentrations are listed in Table 53.
e Values in mg/kg dry weight.
f Values as ratios of tissue PBBs: diet PBBs.
* = Geometric mean.
Table 49. Studies on distribution of the following continuous exposure to octabromobiphenyl
Species Exposure Duration of Tissues and organs under study, ranked References
Route, Duration recovery in order of decreasing concentrations
concentration (average bromine content; µg/g wet weight)
Rat in diet Lee et al. (1975a)
(male) 0 mg/kg feed (control) 2 weeks liver (3.4) > fat (1.7) > muscle (1.6)
100 mg/kg feed 2 weeks - liver (83) > fat (73) > muscle (14)
1000 mg/kg feed 2 weeks - fat (333) > liver (319) > muscle (77)
100 mg/kg feed 4 weeks 0,2,6 weeks fat > liver > muscle
100 mg/kg feed 4 weeks 18 weeks fat > muscle > liver
Rat inhalation 23 h/day, 7 days Waritz et al.
(OcBB vapour)a per week, 15 weeks (1977)
0 pg/litre air liver (3) > muscle (1.6) > fat (1.5)
(control)
3.5 pg/litre air - liver (4.2) > fat (3.0) > muscle (1.5)
a OcBB = Octabromobiphenyl, Dow, Lot 102-7-72.
Table 50. Tissue: blood ratios of PBBs estimated in a standard 250-g rata
Compartment Ratio
Liver 17:1
Muscle 5:1
Skin 56.5:1
Adipose 340:1
Intestine tissue 1:1
a From: Tuey & Matthews (1980).
However, even when concentrations are expressed on a fat basis
rather than a wet tissue basis, there are some deviations from
uniform concentrations among tissues (Fries, 1985b). PBB
concentrations, for example, were low in nervous tissue, despite its
high lipid content and often disproportionally high in liver,
considering its relatively low lipid content (see Tables 47, 48, and
49; additional information on fat content percentage, e.g., by
Willett & Irving, 1976; Fries et al., 1978a,b; Kimbrough et al.,
1978; Werner & Sleight, 1981).
The ratios between the PBB concentrations of adipose tissue,
blood, and vital organs are different when animals are not at
equilibrium (see also section 6.5) with respect to dosing regimen or
body condition. Usually, concentrations in liver are very high
compared with those in other tissues, immediately after dosing, and
decline relatively as equilibrium concentrations are established
(e.g., Lee et al., 1975a; Matthews et al., 1977; Miceli & Marks,
1981; Fries, 1985b). Generally, this phenomenon is most pronounced
in tissues that have high blood flow rates relative to tissue mass
(Tuey & Matthews, 1980). As an exception, livers of mice, tested in
a small series, appeared to have relatively concentrated the PBB
with passing time (Corbett et al., 1978a).
On the other hand, body weight changes, pregnancy, parturition,
and lactation can affect the concentration relationships until
equilibrium is reestablished (e.g., Rickert et al., 1978; Willett &
Durst, 1978; McCormack & Hook, 1982).
The route of exposure (oral or intravenous administration) had
no effect on the tissue distribution (blood, liver, muscle, adipose,
skin) of 14C-labelled 2,2',4,4',5,5'-hexabromobiphenyl (dose =
1 mg/kg body weight) in rats (Matthews et al., 1977).
Kimbrough et al. (1980) studied the effects of different diets
and of mineral oil on the HxBB concentration in rats that had
received a single oral dose of FireMaster(R) FF-1 (500 mg/kg body
weight, in corn oil). After 3 months of feeding, GC-analysis of
blood, liver, and adipose tissue showed no statistically significant
differences in PBB concentrations among the differently fed groups,
when concentrations were calculated on a lipid weight basis. On a
wet weight basis, however, the PBB concentrations were significantly
increased in the livers of rats on the experimental diets (Teklad-4%
and -20% fibre) and on mineral oil compared with those of rats on
the basal diet (Purina Chow).
In another study, McCormack et al. (1979a) examined the
consequences of simultaneous exposure to PCBs and PBBs in
(lactating) rats, because human populations that have been exposed
to PBBs are also likely to have been exposed to PCBs. The
extrahepatic tissue (kidney, mammary, lung, fat) concentrations of
PCBs and PBBs were similar, regardless of whether the agents were
administered together or alone. Liver, however, contained lower
concentrations of PBBs after treatment with an equal mixture of PCBs
and PBBs than when PBBs were administered alone. (None of the
tissues had higher concentrations of PBBs than PCBs after
concomitant administration. The reasons for this were not clear).
The distribution of PBB (hexa) among blood compartments
(plasma, red cells) has been studied in rats (Domino et al., 1980b).
It was found that plasma levels of 2,2',4,4',5,5'-hexabromobiphenyl
were generally four times greater than red cell levels.
Matthews et al. (1984) reported that 81% of plasma PBB (hexa)
was associated with the total lipoprotein fraction. In another study
(Kraus & Bernstein, 1986), approximately 65% of all radiolabelled
HxBB incubated with human serum in vitro was recovered in the
lipoprotein fraction. Of the HxBB in the lipoprotein fractions, 40%
was recovered in low-density lipoproteins (LDL), 33% in
very-low-density lipoproteins (VLDL), and 23% in high-density
lipoproteins (HDL). Addition of human lipoprotein to a culture
medium influenced the partition of HxBB between adipocytes and
culture medium (Kraus & Bernstein, 1989).
Some data are available on the tissue distribution of the minor
components of FireMaster(R)-mixture (see also section 6.5). Domino
et al. (1980b) analysed the relative percentages of various PBB
congeners (two penta-, three hexa-, and three heptabromobiphenyls)
in several tissues of rats given FireMaster(R) FF-1. From their
list, it was evident that each of the PBB analogues was found in all
tissues examined (liver, lung, testes, fat; blood, brain) but their
partitioning ratios differed. Distribution has been recorded also
after exposure to single PBB congeners, e.g., tetra-, penta-, and
hexa-isomers (Akoso et al., 1982a; Dharma et al., 1982; Domino
et al., 1982; Render et al., 1982; Millis et al., 1985a). In some
cases, it was not clear whether the differences in partitioning
between congeners were real or were caused by analytical problems
(Render et al., 1982).
6.2.1.2 Transfer to offspring
1) Mammals
Placental transfer
PBBs are capable of passing through the placental barrier into
the developing fetuses. This has been demonstrated in mice (Corbett
et al., 1978a; Welsch & Morgan, 1985), rats (Beaudoin, 1977; Rickert
et al., 1978), guinea-pigs (Ecobichon et al., 1983), minks and
ferrets (Bleavins et al., 1981), cows (Detering et al., 1975; Fries
et al., 1978a,b), and pigs (Werner & Sleight, 1981) by administering
the Fire-Master(R)-mixture or individual PBBs, or by using
technical octabromobiphenyl in rats (Aftosmis et al., 1972a; Waritz
et al., 1977).
The studies compiled in Table 51 are rarely intercomparable.
However, it is obvious that PBBs are readily transferred across
placental membranes, the concentrations among fetal tissues being
highest in the liver. The limited data on the distribution of PBBs
in fetal tissues showed often, but not always (Ecobichon et al.,
1983; Welsch & Morgan, 1985), lower PBB residues in fetal than in
maternal tissues (see Table 51). In cows, the average ratio of PBB
concentrations in fetal or calf tissue to PBB concentrations in dam
tissue was 0.36 : 1 for fat and 0.37 : 1 for blood (Fries et al.,
1978a,b). In contrast, the concentration ratio between the fetal and
maternal liver of mice ranged from 3.5:1 to 10:1 (Welsch & Morgan,
1985).
Species-dependent differences in the amounts of PBBs
transferred have been demonstrated for two mustelids, the mink and
the European ferret. PBB levels in the ferret kit were significantly
greater than those in the mink kit (see Table 51: Bleavins et al.,
1981).
Table 51. Placental transfer: PBB concentrations in the fetus and the mother
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mouse FireMaster(R) BP-6; 39.52 2.51 - 0.53 [HxBB] Corbett
(mg/kg) et al. (1975)
1000 mg/kg diet
on days 7-18 of
pregnancy
0 mg/kg (control) 0.04 0.05
Mouse FireMaster(R) BP-6; 112.74 12.02 - 0.95 - 5.86 - [HxBB] Corbett
(mg/kg) et al.
100 mg/kg diet (1978a)
on days 7-18 of
pregnancy
Mouse 2,2',4,4',5,5'- [HxBB] Welsch &
hexabromobiphenyl (mg/kg) Morgan (1985)
(purity: > 99%)
dietary intake
from day 6-15 of
pregnancy; sacrifice
on day 17
placenta:
100 mg/kg feed 9.08 17.26 3.79 3.06 182.88
300 mg/kg feed 17.30 39.84 8.23 4.56 217.48
500 mg/kg feed 69.13 89.47 24.58 17.64 316.79
750 mg/kg feed 95.36 103.70 54.33 18.64 453.12
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Rat FireMaster(R) BP-6 [HxBB] Beaudoin
(mg/kg) (1977)
single oral dose of
800 mg/kg body
weight (in sesame (pooled
oil) at day 12 of samples
pregnancy; killing: 51 267 13 from 4 rats)
24 h later
48 h later 250 248 6
Rat FireMaster(R) BP-6; 330 4.2 - 1.6 0.2 GI tract: [HxBB] Rickert
0.1 (mg/kg) et al. (1978)
50 mg/kg diet from
day 8-21 of
pregnancy
Rat Octabromobiphenyl Waritz
(technical); et al. (1977)
dietary intake
from day 8-15 of
pregnancy; sacrifice
on day 20;
0 mg/kg (control) 1.43 3.56 4.38 [Br]
100 mg/kg 70.4 16.1 7.62 (mg/kg)
1000 mg/kg 326 79.8 21.2
10 000 mg/kg 590 158 30.1
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mink [14C-]-PBBs (HxBB 0.031 1.622 plasma: 0.002 0 0.005 kidney: % of the Bleavins
and HpBB); iv 0.04 0.003; initial dose et al. (1981)
injection (1 µCi) brain: 0; per g of tissue
in the final intestine: or ml of fluid
trimester of 0.001
gestation; killed
2 h later
Ferret see above 0.124 1.625 plasma: 0.005 0.004 0.013 kidney: see above
0.07 0.010
brain:
0.003
intestine:
0.005
Guinea- FireMaster(R) FF-1; 45 7 kidney: 45 45 kidney: 1 [HxBB] Ecobichon
pig 4.5; (mg/kg) et al. (1983)
single oral dose of lung: 7
50 mg/kg body weight lung: 1.5
at approximately 65
days of gestation;
killed 2 days later
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Pig FireMaster(R) BP-6; Werner &
Sleight (1981)
dietary intake
during 2nd half of
gestation
10 mg/kg feed 0.4 1.0 kidney: [HxBB]
nd; (mg/kg)
brain: nd
100 mg/kg feed 4.9 11.5 kidney:
nd;
brain: nd
200 mg/kg feed 40.3 24.2 kidney: 1.5
brain: 1.8
a HpBB = 2,2',3,4,4',5,5'-heptabromobiphenyl.
b nd = Not detected.
c [HxBB] = Concentration of 2,2',4,4',5,5',-hexabromobiphenyl; [Br] = Concentration of bromide.
Milk transfer
In mammals, the second route of PBB transfer from the mother to
the offspring is nursing. The efficiency of this way has been shown
through determining the PBB content in milk in relation to the body
burden or in relation to exposure levels of contaminated dams, and
through measuring PBB levels in kits that have been exposed to PBBs
only from suckling. The FireMaster(R) mixture was used in all
studies. Mammary transfer of technical octa- or decabromobiphenyl
has not (yet) been assayed.
Most investigations on the PBB contents of milk from
contaminated animals have been conducted on cows (Fries & Marrow,
1975; Willett & Irving, 1976; Robl et al., 1978; Fries et al.,
1978a,b; Willett & Durst, 1978). The ratios of concentrations in
milk fat to body fat in cows no longer receiving PBBs averaged about
0.4 :1 (Willett & Durst, 1978; Fries et al., 1978a,b; see also
Fig. 5). This ratio is much lower than the ratio in humans (see
section 6.2.2).
For other species (guinea-pig, rat, mink, pig) only single data
can be found in the literature. When (lactating) guinea-pigs
received a single oral dose of FireMaster(R) FF-1 (50 mg/kg body
weight) within 6-12 h of parturition, levels of HxBB in breast milk
(and in perirenal adipose tissue) were of the order of 22 µg/g (and
17 µg/g), respectively, 2 days after treatment (Ecobichon et al.,
1983). Rats fed 50 mg FireMaster(R) BP-6/kg in their diet from day
8 of pregnancy until 14 days after delivery showed, on day 14
postpartum, HxBB concentrations of about 51 µg/ml in the milk and
about 483 µg/g wet weight in their body fat (McCormack et al.,
1979a). In the same study, milk transfer of PBBs and PCBs was
compared. Milk usually contained higher concentrations of PCBs than
of PBBs, after simultaneous or separate exposure.
Contrary results were obtained with minks, intraperitoneally
injected with either 3 µCi of 14C-labelled PCB or 3 µCi of 14C-
labelled PBB on the approximate date at which the embryos would have
been implanted (Bleavins et al., 1981). Two weeks postpartum, milk
levels of PBBs were determined to be four times those of PCBs
(0.105% versus 0.025% of the initial maternal dose per gram of
tissue). Werner & Sleight (1981) determined PBB concentrations in
the tissues and milk of sows fed various amounts of FireMaster(R)
BP-6 (Table 53). At the end of lactation (4th week), the adipose
tissue and milk of sows, fed daily with 200 mg PBB/kg feed, had HxBB
concentrations of 194 µg/g tissue (wet weight) and of 22 µg/ml whole
milk, respectively. The authors calculated that, on a body weight
basis, nursing pigs consumed PBBs in concentrations similar to the
concentration given to the sows. Tissue levels of young exposed to
PBBs only via nursing have been determined for rats (Rickert et al.,
1978) and for guinea-pigs (Ecobichon et al., 1983). When pups of
non-treated female rats were nursed by dams fed FireMaster(R) BP-6
(50 mg/kg body weight) on days 1-14 postpartum, hepatic HxBB-
concentrations were on average approximately eight times higher than
those in the dams on day 14 postpartum (Rickert et al., 1978).
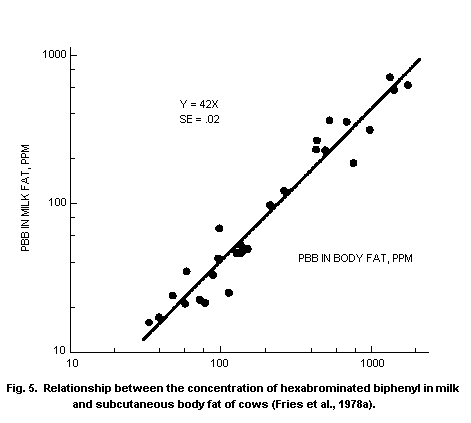 When dams of guinea-pigs received a dose of FireMaster(R)
FF-1 on day 1 after delivery, the concentrations of 2,2',4,4',5,5'-
HxBB in the lungs, livers, kidneys, and fat of the pups were similar
to those of the dams for 4-60 days after treatment (Ecobichon
et al., 1983).
Combined placental and milk transfer
Rickert et al. (1978), Bleavins et al. (1981), and Werner &
Sleight (1981) concluded from their studies on rats, minks, and
pigs, respectively, that milk transfer is far more important than
placental transfer; studies on guinea-pigs (Ecobichon et al., 1983)
did not confirm this observation. However, under less controlled
conditions, perinatal exposure (both placental and milk transfer)
occurs and results in a marked body burden in the offspring, as has
been shown in studies on rats (Table 52 and McDaniel & Lucier, 1979)
and pigs (Table 53). From minks, it has been reported that
14-day-old kits of dams that had received a single intraperitoneal
dose of 14C-PBB at an early stage of pregnancy, contained about 3%
of the initial maternal dose (Bleavins et al., 1981). PBB body
burdens in the offspring of rats were still measurable at 328 days
of age and at the end of their life span (see Table 52).
Moreover, a multigeneration study on rats (McCormack et al.,
1981) demonstrated that administration of PBBs to a single
generation resulted in detectable residues in two subsequent
generations (Table 54). The concentrations of PBBs measured in the
tissues of F1-animals were approximately 5-30 times higher than
those in tissues from F2-animals and approximately 50-1000 times
higher than those in tissues from F3-animals (see Table 54).
2) Birds
In birds, eggs are the medium of PBB transfer to the offspring.
The ratio of egg PBB contents to dietary level has been reported to
be 1 : 1 (Fries et al., 1976) and 1.3 - 1.5 : 1 (Babish et al.,
1975a; Ringer & Polin, 1977; Cecil & Bitman, 1978; Polin & Ringer,
1978a) in chickens (White Leghorn hens) and Japanese quail,
respectively. After 63 days of feeding FireMaster(R) BP-6 in the
diet, the PBB level in body fat of White Leghorn hens was about 4
times the level in eggs (Fries et al., 1976).
6.2.2 Human studies
Studies on the distribution of PBBs in humans refer only to
people having been exposed in a direct or indirect way to the
FireMaster(R)-mixture.
As can be seen from a post-mortem study on people from a "high"
exposure area of the State of Michigan (USA), PBBs are distributed
throughout the entire human body (Table 55). Moreover, it was found
that fat and fat-rich tissue had the highest HxBB concentrations.
Perirenal fat had the highest mean concentration (475 ng/g).
Adrenal, atheromatus aorta, and thymus had mean concentrations of
about half that of perirenal fat; all other tissues had mean
concentrations of only one-tenth or less of that of perirenal fat
(Miceli et al., 1985).
Table 52. Tissue concentrations of PBBs in rats following perinatal exposure to PBBs (FireMaster(R)-mixture BP-6 or FF-1)
Dosing regimen to dams Age of Tissue concentrations of PBBsa (mg/kg wet weight) References
offspring
Offspring Dams
50 mg BP-6/kg diet: day 8 of 14 days liver carcass liver Rickert et al.
gestation through day 14 9.5 149.7 4.0 (1978)
postpartum
100 mg PB-6/kg diet: day 8 of liver kidney fat b McCormack et al.
pregnancy through 28 days 28 days 397 96 1693 (1980)
postpartum 328 days 17 11 387
BP-6 (lot 6244 A) in the diet: 28 days lung liver kidney fat b McCormack et al.
day 8 of pregnancy through (1982a)
28 days postpartum:
10 mg/kg 5 18 8 162
100 mg/kg 32 410 109 1693
200 mg FF-1/kg body weight liver: liver: liver Groce &
(in corn oil; by stomach tube): (female) (male) Kimbrough (1984)
day 7 and 14 of pregnancy 2 months 2.4 (218)c 3.0 (280)c 7.8 (542)c
(weaning at day 21 of age) 2 years 0.8 (107)c 0.6 (58)c
a Concentration expressed as the concentrations of 2,2',4,4',5,5'-hexabromobiphenyl.
b See McCormack & Hook (1982) and Table 48.
c Values calculated on a lipid basis.
Table 53. Mean concentrations of PBBs (mg/kg of tissue, wet weight) in tissues of sows and
4-week-old nursing pigs following perinatal exposure to PBBsa
PBBsb Liver Adipose tissue Kidney Brain
(mg/kg feed) Sows Pigs Sows Pigs Sows Pigsc Sows Pigsc
10 1.0 2.4 15.2 14.8 0.6 nd 0.2 nd
100 45.8 30.2 96.3 96.7 2.3 nd 1.7 nd
200 92.6 41.3 194.2 222.5 3.7 4.1 2.7 4.2
a From: Werner & Sleight (1981).
b FireMaster(R) BP-6 fed to the sows during the second half of gestation and during lactation.
c nd = Not detected.
Table 54. Tissue concentrations of PBBs in several generations of rats following perinatal exposure to PBBsa,b,c
Treatment Liver Kidney Lung Thyroid Testis Ovary Fat
Control < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1
F1-10 17.1 ± 3.6 7.5 ± 0.6 5.1 ± 0.8 4.4 ± 1.0 8.2 ± 5.3 24.0 ± 1.6 161.7 ± 24.2
F2-10 0.4 ± 0.1 0.6 ± 0.2 0.9 ± 0.1 0.5 ± 0.1 0.2 ± 0.1 3.0 ± 0.5 6.7 ± 1.9
F1-100 410.2 ± 40.6 108.6 ± 11.2 32.4 ± 2.0 162.6 ± 20.8 -d -d 1693.2 ± 250.4
F2-100 21.8 ± 3.2 7.2 ± 1.3 8.7 ± 1.0 2.7 ± 0.6 1.8 ± 0.11 6.5 ± 2.6 159.5 ± 16.9
F3-100 0.4 ± 0.1 0.8 ± 0.4 0.6 ± 0.1 0.2 ± 0.1 0.3 ± 0.1 0.6 ± 0.1 4.9 ± 0.8
a From: McCormack et al. (1981).
b Rats were fed 0,10, or 100 mg PBBs (FireMaster(R) BP-6)/kg from day 8 of pregnancy until 28 days postpartum at which
time all offspring (F1-10 and F1-100) were weaned on to a control diet, allowed to mature sexually, and bred with
littermates to produce the F2-generation (F2-10 and F2-100). F2-100 littermates were bred to produce F3-100 animals.
c Values are means in mg 2,2',4,4',5,5'-hexabromobiphenyl/kg wet tissue ± SE for at least three animals at 28 days of age.
d Sample not available.
Most of the distribution studies performed with living subjects
used paired samples of serum, adipose tissue (fat biopsy technique,
e.g., Daum et al., 1978), and breast-milk (see Tables 56, 57 and
58). Other tissues or fluids have rarely been analysed for PBB
content, e.g., there is one report on liver biopsy tissue (300 µg
HxBB/kg), fat (1069 µg/kg), bone marrow (3.5 µg/kg) and synovial
fluid (twice the amount present in serum) of a single person
(Meester & McCoy, 1976).
In some early investigations, serum (plasma, resp.) and adipose
tissue levels (Table 56) or serum (plasma, resp.) and breast-milk
levels (Table 57) did not seem to correlate well with each other.
Later studies, performed when continuing exposure had ceased
(Anderson, 1985), did reveal good correlations between serum and
adipose levels (Table 56) and between breast-milk and serum or
adipose tissue levels (Tables 57 and 58). The PBB concentrations
measured are compiled in section 5.2 and 5.3.
Different groups of the population can differ in their ratios.
For example, lowest adipose to serum ratios were found in lactating
and pregnant females (see Table 56). Eyster et al. (1983) found
statistically different ratios for females and male chemical workers
versus farm workers and other males. The adipose to serum ratio of
340:1, theoretically predicted by Tuey & Matthews (1980) was between
the values reported for the Michigan general population (see Table
56). Factors that appear to affect the partitioning ratios are sex,
pregnancy, occupational status, the total amount of PBBs present,
and whether samples were collected during exposure or during the
recovery phase (Eyster et al., 1983; Anderson, 1985). Generally, the
stability of a ratio would depend on a person being at equilibrium
with respect to PBB intake and fat mobilization (Fries, 1985b).
Transfer of PBBs to offspring occurs via transplacental passage
and via breast-milk (see also section 5.2.4). Breast-milk had levels
of more than 100 times the maternal serum levels (Table 57). The
ratios of 2,2',4,4',5,5'-HxBB concentrations in milk fat to maternal
body fat were found to be in the range of 0.7-0.9 : 1 (Table 58).
Placental tissue and cord or fetal serum levels were 1/6 to 1/10 the
maternal serum levels (see Tables 59 and 60).
According to Jacobson et al. (1984), the higher maternal serum
levels were, in part, reflecting the greater concentration of lipids
found in maternal serum (Table 60). Nevertheless, even when
calculated on a fat basis, PBB concentrations in maternal serum were
still about three times higher than those in cord serum (Jacobson
et al., 1984).
Table 55. PBB postmortem study: autopsied tissue samples from humans in the Grand Rapids area (Michigan, USA)a
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
1 40 - 2 6 2 4 9 2 4 80 8 3 - 3
2 407 118 1 15 4 15 17 2 43 457 10 9 295 9
3 242 247 16 21 18 61 0 63 51 320 42 2 - 80
4 148 294 103 36 32 30 147 73 653 430 84 123 - -
5 43 18 3 4 3 6 39 4 135 134 7 1 - 5
6 98 64 24 126 53 77 104 14 130 110 45 64 29 12
7 35 6 1 52 5 6 5 2 9 32 2 2 0 5
8 868 1011 142 233 61 100 259 93 188 1650 22 69 - -
9 - 75 16 15 6 2 59 6 158 94 11 0 - 12
10 110 285 - 110 95 81 31 62 170 1110 57 312 - 40
11 602 107 0 21 29 38 37 11 148 607 33 15 617 41
12 196 29 11 4 17 31 30 80 92 322 13 4 30 22
13 17 44 9 4 2 3 22 4 20 205 14 2 - 6
14 48 - 5 6 10 5 15 4 17 167 8 4 - 8
15 850 514 13 37 42 67 143 43 146 1390 53 36 - 37
Mean 265 216 27 46 25 35 61 31 131 475 27 43 243 22
SEM 80 77 12 16 7 9 18 9 41 131 6 21 140 6
Range 17-850 6-1011 1-103 2-95 2-100 0-259 2-93 4-653 32-1650 2-84 2-84 0-312 29-617 3-80
Table 55. (contd)
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
Tissue/
renal
fat:
mean
ratio 0.56 0.45 0.06 0.10 0.05 0.07 0.15 0.07 0.28 1.0 0.06 0.09 0.51 0.06
a From: Miceli et al. (1985).
b Measured (by GC) as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl; limit of detection: 0.5 µg/kg.
Table 56. Adipose tissue and serum partitioning ratios of 2,2'4,4',5,5'-hexabromobiphenyl
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Male chemical workers
(No. = 27) 1976 287:1 0.96 Wolff et al. (1979a)
(No. = 22) 1975-80 190-260:1 0.89 Eyster et al. (1983)
Farm residents, etc.
Mixed sexes 1974-75 175:1 Humphrey & Hayner (1975)b
(No. = 13) (range: 61-370:1)
Mixed sexes 1975-76 4752 (± 793):1 Meester & McCoy (1976)
(No. = 116) (range: 27-14 850:1)
Mixed sexes 1976-77 363:1 0.96 Landrigan et al. (1979)
(No. = 132)
Mixed sexes 1976 370:1 0.94 Wolff et al. (1979b)
(No. = 31) 1976-77 300:1
(No. > 31) not specified 320:1
Mixed sexes 1974-77 358:1 0.81d Tuey & Matthews (1980)b,c
(No. = 197)
Pregnant females 1975-80 140-180:1 0.91 Eyster et al. (1983)
(No. = 30)
Non-pregnant females 1975-80 193-230:1 0.84 Eyster et al. (1983)
(No. = 51)
Males (No. 75) 325-329:1 0.95 Eyster et al. (1983)
Lactating women throughout 1976 100:1 0.72 Brilliant et al. (1978)
Michigan (No. = 8)
Table 56 (contd).
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Michigan general population 1978-79 370:1 "high" Selikoff & Anderson (1979)
(No. = 396)
Michigan general population 1978 300:1 0.96 Wolff et al. (1982)
(No. = 588)
a No. = Number of paired samples.
b Use of "plasma" (instead of "serum").
c Values based on data of Gladen & Rogan (1979).
d Spearman correlation coefficient.
Table 57. Breast-milk (fat) and serum, partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired ratios coefficient
samples
1974-75 5 70-132:1 0.78 Humphrey & Hayner (1975)
1976 0.81 Brilliant et al. (1978)
1976-77 21 122:1 Landrigan et al. (1979)
(62-257:1)
1975-80 46 107-119:1 0.95 Eyster et al. (1983)
Not specified 92 0.71a Jacobson et al. (1984)
a Pearson product moment correlation.
Table 58. Adipose tissue and breast-milk (fat) partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired samples ratios coefficient
1976 10 1.07:1 0.88 Brilliant et al. (1978)
1975-80 24 1.1-1.5 :1 0.97 Eyster et al. (1983)
Incidentally, in contrast to PBBs, PCB levels (examined
additionally) were not significantly different in the maternal and
cord serum when compared on a fat basis (Jacobson et al., 1984). PBB
levels measured in children with known exposure to PBB in utero
and/or through breast-milk (see Table 38: Weil et al., 1981) also
indicate significant PBB transfer.
Table 59. Placental transfer of PBB: partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl between fetal and maternal tissues
Year No.a Paired tissues Ratios Correlation Reference
collected coefficient
1976-77 13 maternal serum/ 7.04:1 Landrigan et al.
cord serum (1.5-10.3:1) (1979)
1975-80 58 cord serum/ 0.10-0.14:1 0.88 Eyster et al.
maternal serum (1983)
1975-80 56 placenta/ 0.10-0.17:1 0.85 Eyster et al.
maternal serum (1983)
Not 153 maternal serum/ 0.81b Jacobson et al.
specified cord serum (1984)
Not 107 maternal milk/ 0.39b Jacobson et al.
specified cord serum (1984)
a No. = Number of paired samples.
b Pearson product moment correlation.
Table 60. Mean PBB and lipid levels in cord serum and maternal serum and milka
No. Mean PBBb levels Mean lipid levels
Cord serum 230 0.3 µg/litre 3.24 g/litre
Maternal serum 205 1.7 µg/litre
206 6.181 g/litre
Maternal milk 138 3.6 µg/litre 0.029%
Maternal milk-fat 138 105.1 µg/kg
a From: Jacobson et al. (1984).
b Probably measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
The results of analysis of fat and thymus specimens from 2
infants, taken at autopsy, are shown in Table 61. The ratio of
thymus/fat "hexabromobiphenyl" concentrations, expressed as
percentage, were 13 and 37% (Corbett et al., 1978a).
Table 61. Fat and thymus concentrations of hexabromobiphenyl (HBB) in
3-day-old infants
HxBB concentration (mg/kg)
Fat Thymus
Patient I 0.091 0.012 (13.2%)
Patient II 0.062 0.023 (37.1%)
a From: Corbett et al. (1978a).
The distribution pattern of PBB congeners, other than
2,2'4,4',5'5-hexabromobiphenyl (BB 153), has been studied by Wolff
et al. (1979a). They examined the relative (to BB 153) distribution
of PBB congeners (penta- to heptabromobiphenyls) in the fat and
serum of Michigan chemical workers and farm residents, and found
different partition ratios for different homologues.
The distribution of PBBs among blood compartments was studied
by Bekesi et al. (1979a,b), Greaves et al. (1984), and Roboz et al.
(1980, 1985a,b). In in vitro models (PBBs added to blood), the
distribution of PBBs among plasma, erythrocytes, mononucleocytes
(white cell fraction) and polymorphonucleocytes (white cell
fraction) was found to be 89:9:< 1:< 1 (Roboz et al., 1985a,b).
When, however, the amount of PBBs per cell was considered, there was
an approximately 100-fold excess of PBBs in the white cell fractions
compared with the erythrocyte fraction (Roboz et al., 1985b). In
environmentally contaminated human blood, PBBs were also present in
higher concentrations per mg protein in lymphocytes than in
erythrocytes (Bekesi et al., 1979a,b,; Roboz et al., 1980; see also
Table 45).
It is thought that the relatively large amounts of PBBs
associated with the white blood cells are possibly the cause of the
immunological dysfunctions that result from exposure to PBBs (Roboz
et al., 1985b).
In serum, 20% of the PBBs were not bound to protein. The
remaining 80% were bound to apolipoproteins B and A in a 3:1 ratio
(Greaves et al., 1984). Roboz et al. (1985b) reported a ratio of
4:1, which was close to the ratio (by weight) of the lipid content
of these apolipoproteins. For comparison: the ratio between their
amino acid content was 1.6:1 (Roboz et al., 1985b). There was no
evidence of differential binding of various PBB congeners (penta-,
hexa-, heptabromobiphenyls) to any of the serum fractions (Greaves
et al., 1984; Roboz et al., 1985a,b).
6.3 Metabolic transformation
Indirect evidence for vertebrate metabolism of some PBB
congeners has been obtained by analysis of tissues from
experimentally and environmentally exposed animals and humans (see
section 6.5). While many PBB congeners tended to be persistent,
others were frequently absent or diminished. However, the changes in
the relative abundances can reflect differences in uptake,
distribution, and excretion among the congeners, as well as
differences in the metabolism (Moore et al., 1980).
There are several in vitro and in vivo methods for studying
the metabolism of PBB congeners and mixtures.
6.3.1 In vitro studies
Using in vitro techniques, hepatic microsomes from rats (or
rabbits: Kohli et al., 1978) were incubated with individual PBB
congeners or a PBB mixture, in the presence of nicotinamide adenine
dinucleotide phosphate (reduced) (NADPH) and atmospheric oxygen.
Most of the authors measured rates of disappearance of congeners
(Dannan et al., 1978b; Moore et al., 1980; Parkinson & Safe, 1982;
Millis et al., 1985a,b; Mills et al., 1985). A second approach was
to examine the incubation mixture for metabolites (Kohli et al.,
1978; Purdy & Safe, 1980; Safe et al., 1980; Sparling et al., 1980).
The initial studies (Dannan et al., 1978b; Moore et al., 1980)
revealed that only two of twelve PBB congeners from FireMaster(R)
were metabolized when incubated with microsomes isolated from rats,
pretreated with phenobarbital (PB) or PBB, namely
2,4,5,2',5'-pentabromobiphenyl and 2,3,6,2',4',5'-hexabromobiphenyl.
No metabolism could be observed with control microsomes or
microsomes from 3-methyl cholanthrene (MC)-treated rats. These and
other investigations performed with a number of PBB congeners
(Br1-Br7 = model congeners, FireMaster(R) components,
photolysis products of several hexabromobiphenyls) and with liver
microsomal enzymes induced by either PB or MC are summarized in
Table 62.
The results suggest that the rates of metabolism of PBB
congeners are dependent upon the positions of bromine and the type
of cytochrome induced (P-450:PB-induced; P-448:MC- induced).
The following structure-activity relationships have been
derived: PBB congeners that possessed adjacent non-brominated
carbons meta and para to the biphenyl bridge on at least one
ring were metabolized by PB-induced microsomes (Dannan et al.,
1978b; Moore et al., 1980; Mills et al., 1985). In one case, even
one free para position was reportedly sufficient for PB-induced
metabolism (Moore et al., 1980). Increasing bromination of PBB
congeners did not appear to prevent their metabolism (Moore et al.,
1980; Mills et al., 1985). Significant metabolism by MC- pretreated
microsomes required adjacent ortho and meta positions free of
bromines on at least one ring of lower substituted congeners (up to
Br4). Higher substituted congeners (Br5, Br6) were not
metabolized, though they fulfilled this criterion (Mills et al.,
1985).
In contrast to the studies mentioned above, Purdy & Safe (1980)
found that radiolabelled [3H]-2,2',4,4',5,5'-hexabromobiphenyl
(purity: > 98%) was metabolized in vitro by rat liver microsomal
enzymes. They determined polar, lipophilic metabolites after
incubation with control and PBB-induced microsomes, however, in
quantities that were much smaller than those yielded from
[3H]-4-bromobiphenyl.
Mono- and dihydroxylated derivatives have been identified as
major in vitro metabolism products of lower brominated PBBs (Kohli
et al., 1978; Purdy & Safe, 1980; Safe et al.; 1980, Sparling
et al., 1980; Parkinson & Safe, 1982).
Some PBB congeners may induce their own metabolism (Aust
et al., 1983), and some PBB congeners may influence the metabolism
of other PBB congeners (Purdy & Safe, 1980; Mills et al., 1985).
However, it should be noted that PBB congeners that induce
microsomal enzymes (see also section 8.8.1) are not necessarily
metabolized (Aust et al., 1983).
Some studies were carried out using AHH, specific DNA binding,
and 7-ethoxyresorufin assays. The results showed that PBBs (FM FF-1)
can induce in vitro cytochromes P450 IA (P448) in the primary
cultures of human, and newly-born rat, epidermal keratinocytes, and
of a rat hepatoma cell-line (Yao et al., 1991).
6.3.2 In vivo studies
In vivo metabolism studies included attempts to find and
identify PBB metabolites from intact animals. Hydroxylated
derivatives have been reported most commonly as metabolites of
vertebrates.
Table 62. Metabolism of PBB congeners with rat liver microsomes
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
4-MoBB Yes Yes Parkinson &
(172) (53.1) Safe (1982)
2,2'-DiBB Yes Yes Mills et al. (1985)
(0.33) (201.7)
Yes Moore et al. (1980)
(> 2100) Dannan et al. (1978b)
4,4'-DiBB - No Moore et al. (1980)
(< 0.02) Dannan et al. (1978b)
3,4,4'-TrBB Yes No Mills et al. (1985)
(100.7) (0.02)
2,4,2',4'-TeBB Yes No Mills et al. (1985)
2,4,2',5'-TeBB Yes Yes Mills et al. (1985)
(43.5) (184.6)
Yes Moore et al. (1980)
(24)
2,4,2',6'-TeBB Yes Yes Mills et al. (1985)
2,5,2',5'-TeBB No Yes Mills et al. (1985)
(0.0) (66.2)
Yes Moore et al. (1980)
(27)
2,6,2',6'-TeBB No Yes Mills et al. (1985)
2,3,3',4'-TeBB Yes Yes Mills et al. (1985)
(57.6) (52.8)
2,5,3',4'-TeBB Yes Yes Mills et al. (1985)
(49.7) (47.5)
3,4,3',4'-TeBB Yes No Mills et al. (1985)
(58.8) (0.01)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
3,4,3',5'-TeBB Yes No Mills et al. (1985)
3,5,3',5'-TeBB No No Mills et al. (1985)
(0.06) (0.0)
Yes Moore et al. (1980)
2,4,6,2',4'-PeBB No No Mills et al. (1985)
2,4,5,2',5'-PeBB No Yes Mills et al. (1985)
(0.02) (23.7)
No Dannan et al. Yes Moore et al. (1980)
(1978b) (13) Dannan et al. (1978b)
2,4,6,2',6'-PeBB No Yes Mills et al. (1985)
2,4,5,3',4'-PeBB No No Mills et al. (1985)
(0.05) (0.03)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.06) Dannan et al. (1978b)
3,4,5,3',4'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
3,4,5,3',5'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
2,3,4,2',4',5'-HxBBB No No Mills et al. (1985)
(0.16) (0.0)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
2,3,6,2',4',5'-HxBBB No Dannan et al. Yes Moore et al. (1980)
(1978b) (19) Dannan et al. (1978b)
2,4,5,2',4',5'-HxBBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
2,3,4,5,3',4'-HxBBB No No Mills et al. (1985)
(0.11) (0.0)
No No Dannan et al. (1978b)
2,4,5,3',4',5'-HxBBB No No Dannan et al. (1978b)
3,4,5,3',4',5'-HxBBB No No Mills et al. (1985)
(0.20) 0.0)
2,3,4,5,2',4',5'-HpBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
a Measured as the rate of substrate disappearance (in parentheses: values measured
in pmol/min per mg protein).
b For expediency, a trivial numbering system was used.
c MC microsomes = 3-methylcholanthrene-induced microsomes.
d PB microsomes = phenobarbital-induced microsomes.
As can be seen from Table 63, administration of lower
brominated congeners resulted in low yields of, mainly, mono- or
dihydroxy metabolites. However, most of the studies compiled
suffered from the fact that the purities of the congeners used in
these studies were not determined (Moore et al., 1980). The two
studies with 2,2',4,4',5,5'-HxBB did not provide evidence of
significant metabolism (Table 63).
Various results were obtained after administering commercial
PBB mixtures. No hydroxylated PBBs could be detected in the urine of
cows given single 3-g doses of FireMaster(R) BP-6 (Willett &
Irving, 1976) or in the milk of cows (accidentally contaminated by
FireMaster(R) FF-1) with PBB residues as high as 900 µg/kg, on a
whole milk basis (Gardner et al., 1976). Approximately 1% of the
FireMaster(R) BP-6 administered to a pig was eliminated as an
unidentified pentabromobiphenylol (Kohli & Safe, 1976). It is not
clear whether this metabolite was formed from hexabromobiphenyl (by
reductive debromination followed by hydroxylation) or directly from
pentabromobiphenyl (by hydroxylation).
Table 63. PBB in vivo metabolites reported in the literature
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
Monobromobiphenyls
2-bromobiphenyl rabbit 1% 2'-bromo-4-biphenylol Kohli et al.
(urine) (1978)
traces mono-hycroxybromobiphenyl
rat (>) 2-bromo-4-4'-biphenyldiol Sparling et al.
(1980)
(>) 2-bromo-4-4'-biphenyldiol
(<) 2-bromo-4-biphenylol
3-bromobiphenyl rabbit 4% 3-bromo-4-biphenylol Kohli et al.
(urine) or 5-bromo-2-biphenylol (1978)
< 1% dihydroxybromobiphenyl
rat (>) 3-bromo-4,4'biphenyldiol Sparling et al.
(urine) and an unidentified diol (1980)
(<) monohydroxybromobiphenyls
4-bromobiphenyl rabbit 4% 4'-bromo-4-biphenylol Kohli et al.
(urine) 1.5% 4'-bromo-3,4-biphenyldiol (1978)
rat (>) 4'-bromo-4-biphenylos Sparling et al.
(urine) (<) mono- and dihydroxylated (1980)
species
pig 3% 4'-bromo-4-biphenylol Kohli &
(urine) Safe (1976)
traces monohydroxybromobiphenyl
0.5% 4'-bromo-3-methoxy-
4-biphenylol
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
chicken 12.2% 4'-bromo-4-biphenylol Jones et al.
(excreta) 9.8% 4'-bromo-3,4-biphenyldiol (1979)
(eggs) 0% no metabolites
Dibromobiphenyls
4,4'-dibromobiphenyl rabbit 10% 4,4'-dibromo-3-biphenylol Safe et
(urine) (1976)
1% 3,4'-dibromo-4-biphenylol
2% 4'-bromo-4-biphenylol
pig 5% 4,4'dibromo-3-biphenylol Kohli &
(urine) Safe (1976)
1% 3,4'-dibromo-4-biphenylol
traces dibromomethoxy-
biphenylol
1% 4'-bromo-3-methoxy-
4-biphenylol
traces dibromomethoxybiphenyl
Mixture of di-, fish Salmo dibromobiphenylol Zitko &
tri-, and tetra- salar (whole Hutzinger
bromobiphenyls animal) (1976)
Hexabromobiphenyls
2,2',4,4',5,5'- rat (urine traces "metabolites" Safe et al.
hexabromobiphenyl and faeces) (1978)
(purity: 95%)
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
2,2',4,4',5,5'- rat 0% no metabolites Matthews et al.
hexabromobiphenyl (tissues) (1977)
(purity: 99%)
(bile and traces may be metabolites
faeces) (1-4%)
a (>) and (<) = major and minor components (no further quantitative information).
The faeces of dogs fed FireMaster(R) BP-6 contained a
metabolite identified as 6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl
(Gardner et al., 1979). However, the authors did not exclude
microbial metabolism in the dog's gut, because no
hydroxyhexabromobiphenyl was found in the liver of the dog, though
PBB was present. Matthews et al. (1979) mentioned that dogs were
able to metabolize 2,2',4,4',5,5'-hexachlorinated biphenyl, but were
unable to metabolize the analogous PBB at an appreciable rate.
Some investigations implied that fish may debrominate the more
highly brominated components of PBB mixtures. Juvenile Atlantic
salmon (Salmo salar) experimentally exposed to FireMaster(R)
BP-6, in water or in food, contained several mono- to
pentabromobiphenyls, not present in FireMaster(R) BP-6 (Zitko,
1977). Fish (Salmo salar) fed octabromobiphenyl (Dow
Chemical)-contaminated food contained unidentified penta-, hexa-,
and heptabromobiphenyls in addition to the octabromo biphenyls. It
was not known whether the partially debrominated biphenyls were
generated by the fish, or by the associated microflora (Zitko,
1977). Analyses of fish captured from natural waters may also
indicate the possibility of the debromination reaction in fish,
unless selective accumulation or elimination takes place (Stratton &
Whitlock, 1979). In fish from water mainly contaminated with
decabromobiphenyl, only the nona- and hexa bromobiphenyl congeners
were present. However, in fish from FireMaster(R)
BP-6-contaminated waters, only hexabromobiphenyl was detected.
6.3.3 Metabolic pathway
The most frequently reported route of PBB metabolism was
hydroxylation. However, according to Zitko & Hutzinger (1976), a
reductive debromination may be a degradative pathway of higher
brominated biphenyls, because the carbon-bromine bond is less stable
than, e.g., the carbon-chlorine bond.
The hydroxylation reaction probably proceeds via both arene
oxide intermediates (Safe et al., 1976, 1978, 1980; Kohli et al.,
1978; Moore et al., 1980) and by direct hydroxylation (Kohli et al.,
1978; Sparling et al., 1980; Moore et al., 1980). Schemes of
possible metabolic routes have been published by Matthews (1982) and
Safe (1989). The most important enzyme involved in the oxidation of
xenobiotics, such as PBBs, is aryl hydrocarbon hydroxylase. It is a
highly inducible, haem-containing mono oxygenase belonging to the
family of cytochromes P-450 (e.g., Safe et al., 1980). A (partial)
summary of the AHH-mediated metabolism is given by Safe et al.
(1978).
The formation of covalently bound macromolecular adducts has
been reported. Less than 10% of the metabolites were present in the
urine of rabbits in a bound form, e.g., as glucuronides (Safe
et al., 1976). In vitro metabolism also resulted in macromolecular
conjugates (Kohli et al., 1978; Purdy & Safe, 1980; Safe et al.,
1980; see also section 6.6). In general, the biotransformation of
PBBs is a slow process, but the stereochemistry and molecular size
vary so widely among PBBs that there are great differences in their
metabolic activity.
6.4 Elimination and excretion in expired air, faeces, urine
6.4.1 Animal studies
Elimination of PBBs from the body has been studied using hexa-
and octabromobiphenyl. No information is available on
decabromobiphenyl. Some data found in the literature (see also
section 6.1) are compiled in Table 64 (14C-labelled single doses),
Table 65 (14C-labelled multiple doses), and Table 66 (daily
feeding).
The only study conducted with octabromobiphenyl resulted in a
much higher elimination from rats than was found for HxBB in another
study on rats (Table 64). However, there are also reports on the
rapid elimination of HxBB, e.g., by Willett & Irving (1976), who
found a 50% recovery of HxBB after 168 h in the faeces of two cows
given single intraruminal doses (3 g) of FireMaster(R) BP-6.
However, relatively high concentrations of radioactivity or of HxBB,
found, in some cases, in the faeces during the first few days after
dosing or during daily dosing, may have been due to incomplete
absorption (Tuey & Matthews, 1980). Approximately 60% of the total
dose was also recovered in the faeces of rhesus monkeys (Rozman
et al., 1982: Table 64).
Elimination of PBBs was primarily via the bile and the
intestine into the faeces and, in general, was found to be a slow
process.
Biliary concentrations have been measured in a Rhesus monkey
(Rozman et al., 1982), in rats (Matthews et al., 1977), and in
cattle (Willett & Irving, 1976; Willett & Durst, 1978). In rats,
excretion of HxBB in the bile accounted for 0.68% of the total PBB
dose between 0 and 4 h after intravenous (iv) administration.
Twenty- four hours after an iv dose, 0.032% of the total dose was
excreted in the bile in 1 h, and, 7 or 42 days after dosing
concentrations were too low to quantify (Matthews et al., 1977).
Because of this small amount cleared with the bile, enterohepatic
recirculation of HxBB in rats is not important (Tuey & Matthews,
1980). Concen trations of HxBB in the bile of cattle were two-three
times greater than the concentration in the plasma (Willett &
Irving, 1976; Willett & Durst, 1978).
According to Fries (1985b), excretion of PBB in the urine is
not expected, because of the insolubility of PBBs in water. He
attributed the few instances in which low concentrations of PBBs
were reported to cross-contamination with faeces. On the other hand,
this route of excretion may account for minor or metabolized
biphenyls (Damstra et al., 1982; see also Table 63). However,
excretion of PBB metabolites may be of minor relevance, since the
more abundant PBB congeners are not, or only slightly, metabolized
(see also section 6.3). The amounts of urinary or faecal metabolites
were low. A study on a pig that received a single intraperitoneal
(ip) dose of FireMaster(R) BP-6 (100 mg/kg body weight) reported a
yield of about 1% of pentabromobiphenylol in the urine and faeces,
collected for 7 days (Kohli & Safe, 1976). The level of a hydroxy
metabolite detected in the faeces of dogs fed FireMaster(R) BP-6
was about an order of magnitude less than the PBB levels (Gardner
et al., 1979). Yields of lower brominated urinary PBB metabolites
are compiled in Table 63.
In their study on cows, Willett & Durst (1978) did not find any
direct relationships between the amount of PBBs fed and the
concentration in the faeces. In contrast, Babish et al. (1975a) did
find a linear correlation (r > 0.97) of dietary PBBs with excreta
residues in Japanese quails. In another study on cows, it was also
reported that relationships were approximately constant (Robl
et al., 1978: Table 66).
Table 64. Faecal, urinary, and exhalative elimination of 14C activity as a percentage of a single dose of [14C-]PBB
Species Agent; solvent; dose (mg/kg Time % recovery in: Reference
body weight); route faeces urine expired air
Rhesus monkey (male) [14C-]HxBB in mineral oil; 2, oral 5 days 38 (0.18) - Rozman et al.
(1982)
Rat (male) [14C-]HxBB in Emulphor EL-620: - Matthews et al.
ethanol: water (1:1:8) 24 h 7.9 (1977); Tuey &
1, oral 24 h 0.96 Matthews
1, intravenous 7 days 3.28 < 0.1 (1978, 1980)
1, intravenous 42 days 6.6 not detected
Rat (male and female) [14C-]OcBBa in corn oil; 1, oral 24 h 62 Norris et al.
48 h 69 (1973)
16 days 73 < 1 < 1
Mink (Mustela vison) [14C-]PBBb in propylene glycol Bleavins et al.
(pregnant female) (1 µCi in 0.1 ml) intravenous 2 h - 0.003 - (1981)
Ferret (Mustela 2 h - 0.004
puttorius (furo)
(pregnant female)
Dog [14C-]2,2',4,4',5,5'-hexabromo 25 days 8 Sipes et al.
biphenyl (solvent not specified) (1979)
0.6, intravenous
a OcBB = technical octabromobiphenyl.
b Consisted of 2,2', 4,4', 5,5'-HxBB and 2,2', 3, 4,4', 5,5'-HpBB.
Table 65. Urinary and faecal elimination of HxBB and/or metabolites in male Rhesus monkeys and male rats,
dosed repeatedly with [14C-]HxBB
Species Dose (mg/kg body Days after Urine Faeces Cumulative % Reference
weight) first dose (µg/kg per (µg/kg per recovery of
day) day) total dose
in faeces
Monkey 50 1-10 3.2 5890 ca 60% Rozman et al. (1982)
(No. = 2) in methyl cellulose; 11-17 2.5 5.0
oral on days 1 and 5 203-209 not detectable 3.5
Rat 1 7 ca 14% Matthews et al. (1977)
(No. = 3) in corn oil; oral on
days 1, 2, 3, and 4
Table 66. Elimination of HxBB (2,2',4,4',5,5'-hexabromobiphenyl) in faeces and urine during the feeding of FireMaster(R)-mixtures
Species Intake of FireMaster Sampling Concentration in: Approximate % Reference
(sex) BP-6 or FF-1 time (mg/kg) recovery in
Faeces Urine faeces
Dog BP-6: in corn oil (capsule) last day of 7 Gardner et al.
(female) 1 mg/kg body weight per day dosing (1979)
for 6 weeks
Pig BP-6: in corn oil at week 4 Ku et al. (1978)
20 mg/kg diet 21.3a 0.015
200 mg/kg diet 182.0a 0.07
(ad libitum)
for > 4 weeks
Cow FF-1: in gelatin capsules; Robl et al. (1978)
for 90 days
0.1 mg/kg diet 0.02 mostly 15% of
1.0 mg/kg diet 0.15 n.d.b ingested
10 mg/kg diet 1.5 dose
Calf BP-6:(capsule); 25 g daily total 8045 5% of total Willet & Irving
(male) for 9 days collection dose (1976)
Hen BP-6: 20 mg/kg in the diet weekly 9% of daily Fries et al.
for 63 days dose (1976)
Hen FF-1: not specified not specified 11% of daily Ringer &
dose Polin (1977)
a Faecal samples oven-dried.
b nd = Not detected (detection limit = 0.005 mg/kg).
Following withdrawal of PBBs (FireMaster(R) BP-6) from the
diet of cows, the faecal concentrations of HxBB declined to 1-2% of
faecal levels during dosing (Willett & Durst, 1978) and to less than
5% in hens (Fries et al., 1976). Faecal concentrations were small in
relation to body burden. Cows that received 250 mg FireMaster(R)
BP-6 daily had HxBB concentration ratios in body fat to faeces of
about 750 : 1. Their faeces to plasma ratio was 0.7 :1 (Willett &
Durst, 1978). Cows environmentally contaminated (FireMaster(R)
FF-1) 7-9 months before examination had comparable body fat to
faeces ratios, but a different faeces to blood ratio of 4.2 : 1
(Detering et al., 1975; Cook et al., 1978b; Fries et al., 1978a).
Post-exposure lactating cows eliminated via milk fat three times the
quantity of HxBB cleared in faeces (Willett & Durst, 1978).
There have been some studies on the means to enhance the
elimination of PBBs. PBBs used were: FireMaster(R) FF-1 (Cook
et al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980; Polin
& Leavitt, 1984; Polin et al., 1985) and [14C-]HxBB (Rozman
et al., 1982). The treatments included activated carbon in rats
(McConnell et al., 1980) and cows (Cook et al., 1978b),
cholestyramine in rats (McConnell et al., 1980) and monkeys (Rozman
et al., 1982), colestipol in chickens (Polin & Leavitt, 1984; Polin
et al., 1985), mineral oil in rats (Kimbrough et al., 1980), monkeys
(Rozman et al., 1982), and chickens (Polin et al., 1985), high-fibre
diets in rats (Kimbrough et al., 1980), and phenobarbital in cows
(Cook et al., 1978b). The effects of restricted caloric intake,
alone, or in combination with other treatments, was investigated in
rats (McConnell et al., 1980) and chickens (Polin & Leavitt, 1984;
Polin et al., 1985). The procedures were found not to be (Cook et
al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980), or to
be only partially, effective (Rozman et al., 1982; Polin & Leavitt,
1984; Polin et al., 1985) in reducing the body burden of PBBs
(measured as concentrations of HxBB, total bromine levels, or
14C-activity).
6.4.2 Human studies
The concentrations of PBBs in human bile and faeces represent a
minor proportion of the total body burden, as has been demonstrated
by Eyster et al. (1983), who determined HxBB levels in Michigan farm
and chemical workers in 1975-80. Concen trations of HxBB observed in
the bile and faeces were about 1/2 to 7/10 of the serum levels (on a
whole-weight basis) and were estimated to be approximately 0.5% of
the adipose tissue levels (Table 67).
These findings are consistent with the theoretical predictions
of Tuey & Matthews (1980), who calculated slow rates of faecal
excretion in humans. In addition, these authors showed that the
excretion rate in lean individuals exposed to HxBB would be higher
than those in overweight individuals.
6.5 Retention and turnover
6.5.1 Animal studies
The time course of PBB tissue concentrations has been studied
predominantly in rats, and, to a lesser extent, in cattle, chickens,
and guinea-pigs (Table 68). Incomplete data are available for mice,
pigs, and monkeys. With the exception of 3 older studies (Norris
et al., 1973; Lee et al., 1975a; Waritz et al., 1977), in which the
behaviour of technical octabromobiphenyl in rats was observed, the
majority of investigators used the FireMaster(R)- mixture (BP-6 or
FF-1). Of the individual PBB congeners,
2,2',4,4',5,5'-hexabromobiphenyl (Matthews et al., 1977; Millis
et al., 1985b) and 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985b) were administered.
Table 67. Medians, range, and geometric means of 2,2',4,4',5,5'-
hexabromobiphenyl (HxBB) for paired specimens of serum, faeces, and bile
obtained from farm and chemical workersa
Number Paired Median Rangeb Geometric
specimen mean
51 serum 7 µg/litre 1-1540 µg/litre 14.4 µg/litre
faeces 5 µg/kg nd-862 µg/kg 9.0 µg/kg
20 serum 3.5 µg/litre 1-153 µg/litre 4.2 µg/litre
biliary fluid 2 µg/litre nd-70 µg/litre 2.7 µg/litre
a From: Eyster et al. (1983).
b nd = Not detectable (detection limit: 1 µg/kg or µg/litre).
Complex and varied relationships were found in tissue
concentrations with time after PBB administration (see also Tables
47, 48, and 49).
Table 68. Reported biological half-lifes of PBBs in mammals and birds, after single or repeated exposure
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Rhesus Monkey (male) [14C-]HxBB (50 mg/kg body weight body > 4 years Rozman et al.
on days 1 and 5; oral) 209 days (1982)
Rat (male) [14C-]OcBB (1 mg/kg body weight; faeces < 24 h (1st phase) Norris et al.
single dose; oral) 16 days > 16 days (2nd phase) (1973)
Rat [14C-]HxBB (1 mg/kg body weight; faeces 2 days (1st phase)c Birnbaum
single iv dose) 42 days (1985)
"tissues" ca 24 daysc Ecobichon et al.
(blood, liver (1983)
muscle, skin)
Rat (male) FM BP-6 (1 mg/100 g body weight; serum 23.1 weeks Miceli &
single ip dose; 36 weeks fat 69.3 weeks Marks (1981)
adrenal 43.3 weeks
brain 63.0 weeks (2nd phase)
liver 11.5 weeks (2nd phase)
lung 11.2 weeks
spleen 9.0 weeks
Rat (male) FM FF-1 (10 mg/kg body weight; whole blood 3.27 h (') Domino et al.
single dose; oral) 112 days 33.3 h (ý) (1982)
145 days (')
Guinea-pig (lactating FM FF-1 (50 mg/kg body weight; tissues (fat, ca 22 days Ecobichon
females and pups) single dose; oral) 60 days liver, kidney, et al. (1983)
lung) of both
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Cow FM BP-6 (fed 10 mg/day for milk fat ca 58 days Fries &
60 days) 60 days (of withdrawal) (2nd phase) Marrow (1975)
Cow FM BP-6 (fed 1.13 g/day for milk 10.5 days Gutenmann &
15 days; = 50 mg/kg in the diet) Lisk (1975)
15 days (of withdrawal)
Cow FM FF-1 (environmentally milk fat ca 60 days Fries et al.
contaminated 1 year earlier; (range: 36-301 days) (1978a); Cook
6 months of observation) et al. (1978b)
Cow FM BP-6 (fed 0.25 mg-25 g/day milk fat > 6 monthsd Fries
for various periods) up to (body) (1985b)
3 years of observation
Chicken (White FM FF-1 (fed in the diet) egg 17 days Ringer &
Leghorn hens) 28 days (of withdrawal) Polin (1977)
Chicken (White FM FF-1 (fed 0.2-125 mg/kg egg 17 days Polin &
Leghorn hens) in the diet for 5 weeks) Ringer (1978a)
56 days (of withdrawal)
(fed 1125 and 625 mg/kg in liver 31 days
the diet for 5 weeks)
56 days (of withdrawal)
(fed 5-625 mg/kg in the diet muscle 17 days
for 5 weeks) 56 days
(of withdrawal)
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Chicken (White FM BP-6 (fed 20 mg/kg in the egg 28 days Fries et al.
Leghorn hens) diet for 63 days) 49 days (2nd phase) (1976)
(of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg egg 112 days Cecil &
Leghorn hens) in the diet for 8 weeks) (late phase) Bitman (1978)
266 days (of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg excreta 4-5 dayse Polin &
Leghorn hens) in the diet for 8 weeks) Leavitt (1984)
266 days (of withdrawal)
a HxBB = 2,2',4,4',5,5'-hexabromobiphenyl; OcBB = octobromobiphenyl (technical mixture);
FM BP-6 = FireMaster(R) BP-6; FM FF-1 = FireMaster(R) FF-1.
b Referring to 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity.
c Half-life calculated from data of Matthews et al. (1978); see also Fig. 6.
d Half-life calculated from data of Willett & Durst (1978).
e Half-life calculated from data of Fries et al. (1976).
6.5.1.1 Time trends, retention: 2,2',4,4',5,5'-hexabromobiphenyl
(BB 153)
a) Rat
When rats dosed with 2,2',4,4',5,5'-hexabromobiphenyl (Matthews
et al., 1977; Tuey & Matthews, 1980) or with FireMaster(R) FF-1
(Kimbrough et al., 1978; Domino et al., 1980b; 1982), BB 153
concentrations in the blood were highest immediately after dosing,
but fell rapidly during the first day (as PBBs are taken up from
blood by the liver and muscle tissues, which are highly perfused
tissues). Then, concentrations in the blood, liver, and muscle
declined less quickly (as the dose was redistributed to the adipose
tissue; Tuey & Matthews, 1980; Fries, 1985b), finally decreasing
only very slowly. After a single intravenous dose of BB 153 (1 mg/kg
body weight), adipose tissue contained more than 60% of the total
body burden within four days (Tuey & Matthews, 1980; see Table 50).
BB 153 concentrations in the adipose tissue peaked later than
in other tissues and remained high throughout the period of
observation (6 weeks: Matthews et al., 1977; 16 weeks; Domino
et al., 1980b; 36 weeks: Miceli & Marks, 1981). For example, ratios
between fat and serum levels in rats rose from 221 : 1 to 722 : 1
between 6 and 36 weeks after a single dose exposure (Miceli & Marks,
1981), reflecting the much more rapid clearance of BB 153 from serum
than from fat.
Kinetic models to describe the principal toxicokinetics of BB
153 in the rat were constructed by Tuey & Matthews (1980; a blood
flow-limited physiological compartmental model) and by Domino et al.
(1982). The latter developed a three-compartment model. Tissues
within a compartment showed similar kinetic characteristics, but
concentrations could vary widely. Compartment 1 consisted of whole
blood, spleen, kidney, and heart. Compartment 2 included liver,
lung, cerebral grey and white matter, cerebellum, and testes, and
compartment 3 consisted of subcutaneous fat. Jejunum could not be
classified.
However, results of Miceli & Marks (1981) (PBB levels monitored
over longer periods) do not fit in well with this scheme. For
example, these authors observed typical first-order-elimination
kinetics of BB 153 in the serum of rats, but kinetics of disappear
ance from heart and kidney (and pituitary) do not appear to be first
order, though belonging to the same compartment (according to Domino
et al., 1982). BB 153 concentrations in the brain and liver (both
"compartment 2") declined rapidly during the interval from 6-12
weeks after exposure, but, thereafter, (from week 12 to 36) brain
concentrations fell far more slowly than those of the liver. The
study of Miceli & Marks (1981) showed that the (long-term)
retention, but not always the concentration, of BB 153 in lipid-rich
tissues (brain, adrenal, adipose) was much greater than in most
other tissues (see also Table 68).
Corresponding to the small decline in BB 153 from the tissues
of rats, the elimination rates in the faeces were slow (see Fig. 6).
Less than 7% of the dose was eliminated 42 days after a single iv
dose, most, during the first 3-4 days (Tuey & Matthews, 1980).
When dams of guinea-pigs received a dose of FireMaster(R)
FF-1 on day 1 after delivery, the concentrations of 2,2',4,4',5,5'-
HxBB in the lungs, livers, kidneys, and fat of the pups were similar
to those of the dams for 4-60 days after treatment (Ecobichon
et al., 1983).
Combined placental and milk transfer
Rickert et al. (1978), Bleavins et al. (1981), and Werner &
Sleight (1981) concluded from their studies on rats, minks, and
pigs, respectively, that milk transfer is far more important than
placental transfer; studies on guinea-pigs (Ecobichon et al., 1983)
did not confirm this observation. However, under less controlled
conditions, perinatal exposure (both placental and milk transfer)
occurs and results in a marked body burden in the offspring, as has
been shown in studies on rats (Table 52 and McDaniel & Lucier, 1979)
and pigs (Table 53). From minks, it has been reported that
14-day-old kits of dams that had received a single intraperitoneal
dose of 14C-PBB at an early stage of pregnancy, contained about 3%
of the initial maternal dose (Bleavins et al., 1981). PBB body
burdens in the offspring of rats were still measurable at 328 days
of age and at the end of their life span (see Table 52).
Moreover, a multigeneration study on rats (McCormack et al.,
1981) demonstrated that administration of PBBs to a single
generation resulted in detectable residues in two subsequent
generations (Table 54). The concentrations of PBBs measured in the
tissues of F1-animals were approximately 5-30 times higher than
those in tissues from F2-animals and approximately 50-1000 times
higher than those in tissues from F3-animals (see Table 54).
2) Birds
In birds, eggs are the medium of PBB transfer to the offspring.
The ratio of egg PBB contents to dietary level has been reported to
be 1 : 1 (Fries et al., 1976) and 1.3 - 1.5 : 1 (Babish et al.,
1975a; Ringer & Polin, 1977; Cecil & Bitman, 1978; Polin & Ringer,
1978a) in chickens (White Leghorn hens) and Japanese quail,
respectively. After 63 days of feeding FireMaster(R) BP-6 in the
diet, the PBB level in body fat of White Leghorn hens was about 4
times the level in eggs (Fries et al., 1976).
6.2.2 Human studies
Studies on the distribution of PBBs in humans refer only to
people having been exposed in a direct or indirect way to the
FireMaster(R)-mixture.
As can be seen from a post-mortem study on people from a "high"
exposure area of the State of Michigan (USA), PBBs are distributed
throughout the entire human body (Table 55). Moreover, it was found
that fat and fat-rich tissue had the highest HxBB concentrations.
Perirenal fat had the highest mean concentration (475 ng/g).
Adrenal, atheromatus aorta, and thymus had mean concentrations of
about half that of perirenal fat; all other tissues had mean
concentrations of only one-tenth or less of that of perirenal fat
(Miceli et al., 1985).
Table 52. Tissue concentrations of PBBs in rats following perinatal exposure to PBBs (FireMaster(R)-mixture BP-6 or FF-1)
Dosing regimen to dams Age of Tissue concentrations of PBBsa (mg/kg wet weight) References
offspring
Offspring Dams
50 mg BP-6/kg diet: day 8 of 14 days liver carcass liver Rickert et al.
gestation through day 14 9.5 149.7 4.0 (1978)
postpartum
100 mg PB-6/kg diet: day 8 of liver kidney fat b McCormack et al.
pregnancy through 28 days 28 days 397 96 1693 (1980)
postpartum 328 days 17 11 387
BP-6 (lot 6244 A) in the diet: 28 days lung liver kidney fat b McCormack et al.
day 8 of pregnancy through (1982a)
28 days postpartum:
10 mg/kg 5 18 8 162
100 mg/kg 32 410 109 1693
200 mg FF-1/kg body weight liver: liver: liver Groce &
(in corn oil; by stomach tube): (female) (male) Kimbrough (1984)
day 7 and 14 of pregnancy 2 months 2.4 (218)c 3.0 (280)c 7.8 (542)c
(weaning at day 21 of age) 2 years 0.8 (107)c 0.6 (58)c
a Concentration expressed as the concentrations of 2,2',4,4',5,5'-hexabromobiphenyl.
b See McCormack & Hook (1982) and Table 48.
c Values calculated on a lipid basis.
Table 53. Mean concentrations of PBBs (mg/kg of tissue, wet weight) in tissues of sows and
4-week-old nursing pigs following perinatal exposure to PBBsa
PBBsb Liver Adipose tissue Kidney Brain
(mg/kg feed) Sows Pigs Sows Pigs Sows Pigsc Sows Pigsc
10 1.0 2.4 15.2 14.8 0.6 nd 0.2 nd
100 45.8 30.2 96.3 96.7 2.3 nd 1.7 nd
200 92.6 41.3 194.2 222.5 3.7 4.1 2.7 4.2
a From: Werner & Sleight (1981).
b FireMaster(R) BP-6 fed to the sows during the second half of gestation and during lactation.
c nd = Not detected.
Table 54. Tissue concentrations of PBBs in several generations of rats following perinatal exposure to PBBsa,b,c
Treatment Liver Kidney Lung Thyroid Testis Ovary Fat
Control < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1
F1-10 17.1 ± 3.6 7.5 ± 0.6 5.1 ± 0.8 4.4 ± 1.0 8.2 ± 5.3 24.0 ± 1.6 161.7 ± 24.2
F2-10 0.4 ± 0.1 0.6 ± 0.2 0.9 ± 0.1 0.5 ± 0.1 0.2 ± 0.1 3.0 ± 0.5 6.7 ± 1.9
F1-100 410.2 ± 40.6 108.6 ± 11.2 32.4 ± 2.0 162.6 ± 20.8 -d -d 1693.2 ± 250.4
F2-100 21.8 ± 3.2 7.2 ± 1.3 8.7 ± 1.0 2.7 ± 0.6 1.8 ± 0.11 6.5 ± 2.6 159.5 ± 16.9
F3-100 0.4 ± 0.1 0.8 ± 0.4 0.6 ± 0.1 0.2 ± 0.1 0.3 ± 0.1 0.6 ± 0.1 4.9 ± 0.8
a From: McCormack et al. (1981).
b Rats were fed 0,10, or 100 mg PBBs (FireMaster(R) BP-6)/kg from day 8 of pregnancy until 28 days postpartum at which
time all offspring (F1-10 and F1-100) were weaned on to a control diet, allowed to mature sexually, and bred with
littermates to produce the F2-generation (F2-10 and F2-100). F2-100 littermates were bred to produce F3-100 animals.
c Values are means in mg 2,2',4,4',5,5'-hexabromobiphenyl/kg wet tissue ± SE for at least three animals at 28 days of age.
d Sample not available.
Most of the distribution studies performed with living subjects
used paired samples of serum, adipose tissue (fat biopsy technique,
e.g., Daum et al., 1978), and breast-milk (see Tables 56, 57 and
58). Other tissues or fluids have rarely been analysed for PBB
content, e.g., there is one report on liver biopsy tissue (300 µg
HxBB/kg), fat (1069 µg/kg), bone marrow (3.5 µg/kg) and synovial
fluid (twice the amount present in serum) of a single person
(Meester & McCoy, 1976).
In some early investigations, serum (plasma, resp.) and adipose
tissue levels (Table 56) or serum (plasma, resp.) and breast-milk
levels (Table 57) did not seem to correlate well with each other.
Later studies, performed when continuing exposure had ceased
(Anderson, 1985), did reveal good correlations between serum and
adipose levels (Table 56) and between breast-milk and serum or
adipose tissue levels (Tables 57 and 58). The PBB concentrations
measured are compiled in section 5.2 and 5.3.
Different groups of the population can differ in their ratios.
For example, lowest adipose to serum ratios were found in lactating
and pregnant females (see Table 56). Eyster et al. (1983) found
statistically different ratios for females and male chemical workers
versus farm workers and other males. The adipose to serum ratio of
340:1, theoretically predicted by Tuey & Matthews (1980) was between
the values reported for the Michigan general population (see Table
56). Factors that appear to affect the partitioning ratios are sex,
pregnancy, occupational status, the total amount of PBBs present,
and whether samples were collected during exposure or during the
recovery phase (Eyster et al., 1983; Anderson, 1985). Generally, the
stability of a ratio would depend on a person being at equilibrium
with respect to PBB intake and fat mobilization (Fries, 1985b).
Transfer of PBBs to offspring occurs via transplacental passage
and via breast-milk (see also section 5.2.4). Breast-milk had levels
of more than 100 times the maternal serum levels (Table 57). The
ratios of 2,2',4,4',5,5'-HxBB concentrations in milk fat to maternal
body fat were found to be in the range of 0.7-0.9 : 1 (Table 58).
Placental tissue and cord or fetal serum levels were 1/6 to 1/10 the
maternal serum levels (see Tables 59 and 60).
According to Jacobson et al. (1984), the higher maternal serum
levels were, in part, reflecting the greater concentration of lipids
found in maternal serum (Table 60). Nevertheless, even when
calculated on a fat basis, PBB concentrations in maternal serum were
still about three times higher than those in cord serum (Jacobson
et al., 1984).
Table 55. PBB postmortem study: autopsied tissue samples from humans in the Grand Rapids area (Michigan, USA)a
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
1 40 - 2 6 2 4 9 2 4 80 8 3 - 3
2 407 118 1 15 4 15 17 2 43 457 10 9 295 9
3 242 247 16 21 18 61 0 63 51 320 42 2 - 80
4 148 294 103 36 32 30 147 73 653 430 84 123 - -
5 43 18 3 4 3 6 39 4 135 134 7 1 - 5
6 98 64 24 126 53 77 104 14 130 110 45 64 29 12
7 35 6 1 52 5 6 5 2 9 32 2 2 0 5
8 868 1011 142 233 61 100 259 93 188 1650 22 69 - -
9 - 75 16 15 6 2 59 6 158 94 11 0 - 12
10 110 285 - 110 95 81 31 62 170 1110 57 312 - 40
11 602 107 0 21 29 38 37 11 148 607 33 15 617 41
12 196 29 11 4 17 31 30 80 92 322 13 4 30 22
13 17 44 9 4 2 3 22 4 20 205 14 2 - 6
14 48 - 5 6 10 5 15 4 17 167 8 4 - 8
15 850 514 13 37 42 67 143 43 146 1390 53 36 - 37
Mean 265 216 27 46 25 35 61 31 131 475 27 43 243 22
SEM 80 77 12 16 7 9 18 9 41 131 6 21 140 6
Range 17-850 6-1011 1-103 2-95 2-100 0-259 2-93 4-653 32-1650 2-84 2-84 0-312 29-617 3-80
Table 55. (contd)
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
Tissue/
renal
fat:
mean
ratio 0.56 0.45 0.06 0.10 0.05 0.07 0.15 0.07 0.28 1.0 0.06 0.09 0.51 0.06
a From: Miceli et al. (1985).
b Measured (by GC) as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl; limit of detection: 0.5 µg/kg.
Table 56. Adipose tissue and serum partitioning ratios of 2,2'4,4',5,5'-hexabromobiphenyl
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Male chemical workers
(No. = 27) 1976 287:1 0.96 Wolff et al. (1979a)
(No. = 22) 1975-80 190-260:1 0.89 Eyster et al. (1983)
Farm residents, etc.
Mixed sexes 1974-75 175:1 Humphrey & Hayner (1975)b
(No. = 13) (range: 61-370:1)
Mixed sexes 1975-76 4752 (± 793):1 Meester & McCoy (1976)
(No. = 116) (range: 27-14 850:1)
Mixed sexes 1976-77 363:1 0.96 Landrigan et al. (1979)
(No. = 132)
Mixed sexes 1976 370:1 0.94 Wolff et al. (1979b)
(No. = 31) 1976-77 300:1
(No. > 31) not specified 320:1
Mixed sexes 1974-77 358:1 0.81d Tuey & Matthews (1980)b,c
(No. = 197)
Pregnant females 1975-80 140-180:1 0.91 Eyster et al. (1983)
(No. = 30)
Non-pregnant females 1975-80 193-230:1 0.84 Eyster et al. (1983)
(No. = 51)
Males (No. 75) 325-329:1 0.95 Eyster et al. (1983)
Lactating women throughout 1976 100:1 0.72 Brilliant et al. (1978)
Michigan (No. = 8)
Table 56 (contd).
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Michigan general population 1978-79 370:1 "high" Selikoff & Anderson (1979)
(No. = 396)
Michigan general population 1978 300:1 0.96 Wolff et al. (1982)
(No. = 588)
a No. = Number of paired samples.
b Use of "plasma" (instead of "serum").
c Values based on data of Gladen & Rogan (1979).
d Spearman correlation coefficient.
Table 57. Breast-milk (fat) and serum, partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired ratios coefficient
samples
1974-75 5 70-132:1 0.78 Humphrey & Hayner (1975)
1976 0.81 Brilliant et al. (1978)
1976-77 21 122:1 Landrigan et al. (1979)
(62-257:1)
1975-80 46 107-119:1 0.95 Eyster et al. (1983)
Not specified 92 0.71a Jacobson et al. (1984)
a Pearson product moment correlation.
Table 58. Adipose tissue and breast-milk (fat) partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired samples ratios coefficient
1976 10 1.07:1 0.88 Brilliant et al. (1978)
1975-80 24 1.1-1.5 :1 0.97 Eyster et al. (1983)
Incidentally, in contrast to PBBs, PCB levels (examined
additionally) were not significantly different in the maternal and
cord serum when compared on a fat basis (Jacobson et al., 1984). PBB
levels measured in children with known exposure to PBB in utero
and/or through breast-milk (see Table 38: Weil et al., 1981) also
indicate significant PBB transfer.
Table 59. Placental transfer of PBB: partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl between fetal and maternal tissues
Year No.a Paired tissues Ratios Correlation Reference
collected coefficient
1976-77 13 maternal serum/ 7.04:1 Landrigan et al.
cord serum (1.5-10.3:1) (1979)
1975-80 58 cord serum/ 0.10-0.14:1 0.88 Eyster et al.
maternal serum (1983)
1975-80 56 placenta/ 0.10-0.17:1 0.85 Eyster et al.
maternal serum (1983)
Not 153 maternal serum/ 0.81b Jacobson et al.
specified cord serum (1984)
Not 107 maternal milk/ 0.39b Jacobson et al.
specified cord serum (1984)
a No. = Number of paired samples.
b Pearson product moment correlation.
Table 60. Mean PBB and lipid levels in cord serum and maternal serum and milka
No. Mean PBBb levels Mean lipid levels
Cord serum 230 0.3 µg/litre 3.24 g/litre
Maternal serum 205 1.7 µg/litre
206 6.181 g/litre
Maternal milk 138 3.6 µg/litre 0.029%
Maternal milk-fat 138 105.1 µg/kg
a From: Jacobson et al. (1984).
b Probably measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
The results of analysis of fat and thymus specimens from 2
infants, taken at autopsy, are shown in Table 61. The ratio of
thymus/fat "hexabromobiphenyl" concentrations, expressed as
percentage, were 13 and 37% (Corbett et al., 1978a).
Table 61. Fat and thymus concentrations of hexabromobiphenyl (HBB) in
3-day-old infants
HxBB concentration (mg/kg)
Fat Thymus
Patient I 0.091 0.012 (13.2%)
Patient II 0.062 0.023 (37.1%)
a From: Corbett et al. (1978a).
The distribution pattern of PBB congeners, other than
2,2'4,4',5'5-hexabromobiphenyl (BB 153), has been studied by Wolff
et al. (1979a). They examined the relative (to BB 153) distribution
of PBB congeners (penta- to heptabromobiphenyls) in the fat and
serum of Michigan chemical workers and farm residents, and found
different partition ratios for different homologues.
The distribution of PBBs among blood compartments was studied
by Bekesi et al. (1979a,b), Greaves et al. (1984), and Roboz et al.
(1980, 1985a,b). In in vitro models (PBBs added to blood), the
distribution of PBBs among plasma, erythrocytes, mononucleocytes
(white cell fraction) and polymorphonucleocytes (white cell
fraction) was found to be 89:9:< 1:< 1 (Roboz et al., 1985a,b).
When, however, the amount of PBBs per cell was considered, there was
an approximately 100-fold excess of PBBs in the white cell fractions
compared with the erythrocyte fraction (Roboz et al., 1985b). In
environmentally contaminated human blood, PBBs were also present in
higher concentrations per mg protein in lymphocytes than in
erythrocytes (Bekesi et al., 1979a,b,; Roboz et al., 1980; see also
Table 45).
It is thought that the relatively large amounts of PBBs
associated with the white blood cells are possibly the cause of the
immunological dysfunctions that result from exposure to PBBs (Roboz
et al., 1985b).
In serum, 20% of the PBBs were not bound to protein. The
remaining 80% were bound to apolipoproteins B and A in a 3:1 ratio
(Greaves et al., 1984). Roboz et al. (1985b) reported a ratio of
4:1, which was close to the ratio (by weight) of the lipid content
of these apolipoproteins. For comparison: the ratio between their
amino acid content was 1.6:1 (Roboz et al., 1985b). There was no
evidence of differential binding of various PBB congeners (penta-,
hexa-, heptabromobiphenyls) to any of the serum fractions (Greaves
et al., 1984; Roboz et al., 1985a,b).
6.3 Metabolic transformation
Indirect evidence for vertebrate metabolism of some PBB
congeners has been obtained by analysis of tissues from
experimentally and environmentally exposed animals and humans (see
section 6.5). While many PBB congeners tended to be persistent,
others were frequently absent or diminished. However, the changes in
the relative abundances can reflect differences in uptake,
distribution, and excretion among the congeners, as well as
differences in the metabolism (Moore et al., 1980).
There are several in vitro and in vivo methods for studying
the metabolism of PBB congeners and mixtures.
6.3.1 In vitro studies
Using in vitro techniques, hepatic microsomes from rats (or
rabbits: Kohli et al., 1978) were incubated with individual PBB
congeners or a PBB mixture, in the presence of nicotinamide adenine
dinucleotide phosphate (reduced) (NADPH) and atmospheric oxygen.
Most of the authors measured rates of disappearance of congeners
(Dannan et al., 1978b; Moore et al., 1980; Parkinson & Safe, 1982;
Millis et al., 1985a,b; Mills et al., 1985). A second approach was
to examine the incubation mixture for metabolites (Kohli et al.,
1978; Purdy & Safe, 1980; Safe et al., 1980; Sparling et al., 1980).
The initial studies (Dannan et al., 1978b; Moore et al., 1980)
revealed that only two of twelve PBB congeners from FireMaster(R)
were metabolized when incubated with microsomes isolated from rats,
pretreated with phenobarbital (PB) or PBB, namely
2,4,5,2',5'-pentabromobiphenyl and 2,3,6,2',4',5'-hexabromobiphenyl.
No metabolism could be observed with control microsomes or
microsomes from 3-methyl cholanthrene (MC)-treated rats. These and
other investigations performed with a number of PBB congeners
(Br1-Br7 = model congeners, FireMaster(R) components,
photolysis products of several hexabromobiphenyls) and with liver
microsomal enzymes induced by either PB or MC are summarized in
Table 62.
The results suggest that the rates of metabolism of PBB
congeners are dependent upon the positions of bromine and the type
of cytochrome induced (P-450:PB-induced; P-448:MC- induced).
The following structure-activity relationships have been
derived: PBB congeners that possessed adjacent non-brominated
carbons meta and para to the biphenyl bridge on at least one
ring were metabolized by PB-induced microsomes (Dannan et al.,
1978b; Moore et al., 1980; Mills et al., 1985). In one case, even
one free para position was reportedly sufficient for PB-induced
metabolism (Moore et al., 1980). Increasing bromination of PBB
congeners did not appear to prevent their metabolism (Moore et al.,
1980; Mills et al., 1985). Significant metabolism by MC- pretreated
microsomes required adjacent ortho and meta positions free of
bromines on at least one ring of lower substituted congeners (up to
Br4). Higher substituted congeners (Br5, Br6) were not
metabolized, though they fulfilled this criterion (Mills et al.,
1985).
In contrast to the studies mentioned above, Purdy & Safe (1980)
found that radiolabelled [3H]-2,2',4,4',5,5'-hexabromobiphenyl
(purity: > 98%) was metabolized in vitro by rat liver microsomal
enzymes. They determined polar, lipophilic metabolites after
incubation with control and PBB-induced microsomes, however, in
quantities that were much smaller than those yielded from
[3H]-4-bromobiphenyl.
Mono- and dihydroxylated derivatives have been identified as
major in vitro metabolism products of lower brominated PBBs (Kohli
et al., 1978; Purdy & Safe, 1980; Safe et al.; 1980, Sparling
et al., 1980; Parkinson & Safe, 1982).
Some PBB congeners may induce their own metabolism (Aust
et al., 1983), and some PBB congeners may influence the metabolism
of other PBB congeners (Purdy & Safe, 1980; Mills et al., 1985).
However, it should be noted that PBB congeners that induce
microsomal enzymes (see also section 8.8.1) are not necessarily
metabolized (Aust et al., 1983).
Some studies were carried out using AHH, specific DNA binding,
and 7-ethoxyresorufin assays. The results showed that PBBs (FM FF-1)
can induce in vitro cytochromes P450 IA (P448) in the primary
cultures of human, and newly-born rat, epidermal keratinocytes, and
of a rat hepatoma cell-line (Yao et al., 1991).
6.3.2 In vivo studies
In vivo metabolism studies included attempts to find and
identify PBB metabolites from intact animals. Hydroxylated
derivatives have been reported most commonly as metabolites of
vertebrates.
Table 62. Metabolism of PBB congeners with rat liver microsomes
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
4-MoBB Yes Yes Parkinson &
(172) (53.1) Safe (1982)
2,2'-DiBB Yes Yes Mills et al. (1985)
(0.33) (201.7)
Yes Moore et al. (1980)
(> 2100) Dannan et al. (1978b)
4,4'-DiBB - No Moore et al. (1980)
(< 0.02) Dannan et al. (1978b)
3,4,4'-TrBB Yes No Mills et al. (1985)
(100.7) (0.02)
2,4,2',4'-TeBB Yes No Mills et al. (1985)
2,4,2',5'-TeBB Yes Yes Mills et al. (1985)
(43.5) (184.6)
Yes Moore et al. (1980)
(24)
2,4,2',6'-TeBB Yes Yes Mills et al. (1985)
2,5,2',5'-TeBB No Yes Mills et al. (1985)
(0.0) (66.2)
Yes Moore et al. (1980)
(27)
2,6,2',6'-TeBB No Yes Mills et al. (1985)
2,3,3',4'-TeBB Yes Yes Mills et al. (1985)
(57.6) (52.8)
2,5,3',4'-TeBB Yes Yes Mills et al. (1985)
(49.7) (47.5)
3,4,3',4'-TeBB Yes No Mills et al. (1985)
(58.8) (0.01)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
3,4,3',5'-TeBB Yes No Mills et al. (1985)
3,5,3',5'-TeBB No No Mills et al. (1985)
(0.06) (0.0)
Yes Moore et al. (1980)
2,4,6,2',4'-PeBB No No Mills et al. (1985)
2,4,5,2',5'-PeBB No Yes Mills et al. (1985)
(0.02) (23.7)
No Dannan et al. Yes Moore et al. (1980)
(1978b) (13) Dannan et al. (1978b)
2,4,6,2',6'-PeBB No Yes Mills et al. (1985)
2,4,5,3',4'-PeBB No No Mills et al. (1985)
(0.05) (0.03)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.06) Dannan et al. (1978b)
3,4,5,3',4'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
3,4,5,3',5'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
2,3,4,2',4',5'-HxBBB No No Mills et al. (1985)
(0.16) (0.0)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
2,3,6,2',4',5'-HxBBB No Dannan et al. Yes Moore et al. (1980)
(1978b) (19) Dannan et al. (1978b)
2,4,5,2',4',5'-HxBBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
2,3,4,5,3',4'-HxBBB No No Mills et al. (1985)
(0.11) (0.0)
No No Dannan et al. (1978b)
2,4,5,3',4',5'-HxBBB No No Dannan et al. (1978b)
3,4,5,3',4',5'-HxBBB No No Mills et al. (1985)
(0.20) 0.0)
2,3,4,5,2',4',5'-HpBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
a Measured as the rate of substrate disappearance (in parentheses: values measured
in pmol/min per mg protein).
b For expediency, a trivial numbering system was used.
c MC microsomes = 3-methylcholanthrene-induced microsomes.
d PB microsomes = phenobarbital-induced microsomes.
As can be seen from Table 63, administration of lower
brominated congeners resulted in low yields of, mainly, mono- or
dihydroxy metabolites. However, most of the studies compiled
suffered from the fact that the purities of the congeners used in
these studies were not determined (Moore et al., 1980). The two
studies with 2,2',4,4',5,5'-HxBB did not provide evidence of
significant metabolism (Table 63).
Various results were obtained after administering commercial
PBB mixtures. No hydroxylated PBBs could be detected in the urine of
cows given single 3-g doses of FireMaster(R) BP-6 (Willett &
Irving, 1976) or in the milk of cows (accidentally contaminated by
FireMaster(R) FF-1) with PBB residues as high as 900 µg/kg, on a
whole milk basis (Gardner et al., 1976). Approximately 1% of the
FireMaster(R) BP-6 administered to a pig was eliminated as an
unidentified pentabromobiphenylol (Kohli & Safe, 1976). It is not
clear whether this metabolite was formed from hexabromobiphenyl (by
reductive debromination followed by hydroxylation) or directly from
pentabromobiphenyl (by hydroxylation).
Table 63. PBB in vivo metabolites reported in the literature
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
Monobromobiphenyls
2-bromobiphenyl rabbit 1% 2'-bromo-4-biphenylol Kohli et al.
(urine) (1978)
traces mono-hycroxybromobiphenyl
rat (>) 2-bromo-4-4'-biphenyldiol Sparling et al.
(1980)
(>) 2-bromo-4-4'-biphenyldiol
(<) 2-bromo-4-biphenylol
3-bromobiphenyl rabbit 4% 3-bromo-4-biphenylol Kohli et al.
(urine) or 5-bromo-2-biphenylol (1978)
< 1% dihydroxybromobiphenyl
rat (>) 3-bromo-4,4'biphenyldiol Sparling et al.
(urine) and an unidentified diol (1980)
(<) monohydroxybromobiphenyls
4-bromobiphenyl rabbit 4% 4'-bromo-4-biphenylol Kohli et al.
(urine) 1.5% 4'-bromo-3,4-biphenyldiol (1978)
rat (>) 4'-bromo-4-biphenylos Sparling et al.
(urine) (<) mono- and dihydroxylated (1980)
species
pig 3% 4'-bromo-4-biphenylol Kohli &
(urine) Safe (1976)
traces monohydroxybromobiphenyl
0.5% 4'-bromo-3-methoxy-
4-biphenylol
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
chicken 12.2% 4'-bromo-4-biphenylol Jones et al.
(excreta) 9.8% 4'-bromo-3,4-biphenyldiol (1979)
(eggs) 0% no metabolites
Dibromobiphenyls
4,4'-dibromobiphenyl rabbit 10% 4,4'-dibromo-3-biphenylol Safe et
(urine) (1976)
1% 3,4'-dibromo-4-biphenylol
2% 4'-bromo-4-biphenylol
pig 5% 4,4'dibromo-3-biphenylol Kohli &
(urine) Safe (1976)
1% 3,4'-dibromo-4-biphenylol
traces dibromomethoxy-
biphenylol
1% 4'-bromo-3-methoxy-
4-biphenylol
traces dibromomethoxybiphenyl
Mixture of di-, fish Salmo dibromobiphenylol Zitko &
tri-, and tetra- salar (whole Hutzinger
bromobiphenyls animal) (1976)
Hexabromobiphenyls
2,2',4,4',5,5'- rat (urine traces "metabolites" Safe et al.
hexabromobiphenyl and faeces) (1978)
(purity: 95%)
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
2,2',4,4',5,5'- rat 0% no metabolites Matthews et al.
hexabromobiphenyl (tissues) (1977)
(purity: 99%)
(bile and traces may be metabolites
faeces) (1-4%)
a (>) and (<) = major and minor components (no further quantitative information).
The faeces of dogs fed FireMaster(R) BP-6 contained a
metabolite identified as 6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl
(Gardner et al., 1979). However, the authors did not exclude
microbial metabolism in the dog's gut, because no
hydroxyhexabromobiphenyl was found in the liver of the dog, though
PBB was present. Matthews et al. (1979) mentioned that dogs were
able to metabolize 2,2',4,4',5,5'-hexachlorinated biphenyl, but were
unable to metabolize the analogous PBB at an appreciable rate.
Some investigations implied that fish may debrominate the more
highly brominated components of PBB mixtures. Juvenile Atlantic
salmon (Salmo salar) experimentally exposed to FireMaster(R)
BP-6, in water or in food, contained several mono- to
pentabromobiphenyls, not present in FireMaster(R) BP-6 (Zitko,
1977). Fish (Salmo salar) fed octabromobiphenyl (Dow
Chemical)-contaminated food contained unidentified penta-, hexa-,
and heptabromobiphenyls in addition to the octabromo biphenyls. It
was not known whether the partially debrominated biphenyls were
generated by the fish, or by the associated microflora (Zitko,
1977). Analyses of fish captured from natural waters may also
indicate the possibility of the debromination reaction in fish,
unless selective accumulation or elimination takes place (Stratton &
Whitlock, 1979). In fish from water mainly contaminated with
decabromobiphenyl, only the nona- and hexa bromobiphenyl congeners
were present. However, in fish from FireMaster(R)
BP-6-contaminated waters, only hexabromobiphenyl was detected.
6.3.3 Metabolic pathway
The most frequently reported route of PBB metabolism was
hydroxylation. However, according to Zitko & Hutzinger (1976), a
reductive debromination may be a degradative pathway of higher
brominated biphenyls, because the carbon-bromine bond is less stable
than, e.g., the carbon-chlorine bond.
The hydroxylation reaction probably proceeds via both arene
oxide intermediates (Safe et al., 1976, 1978, 1980; Kohli et al.,
1978; Moore et al., 1980) and by direct hydroxylation (Kohli et al.,
1978; Sparling et al., 1980; Moore et al., 1980). Schemes of
possible metabolic routes have been published by Matthews (1982) and
Safe (1989). The most important enzyme involved in the oxidation of
xenobiotics, such as PBBs, is aryl hydrocarbon hydroxylase. It is a
highly inducible, haem-containing mono oxygenase belonging to the
family of cytochromes P-450 (e.g., Safe et al., 1980). A (partial)
summary of the AHH-mediated metabolism is given by Safe et al.
(1978).
The formation of covalently bound macromolecular adducts has
been reported. Less than 10% of the metabolites were present in the
urine of rabbits in a bound form, e.g., as glucuronides (Safe
et al., 1976). In vitro metabolism also resulted in macromolecular
conjugates (Kohli et al., 1978; Purdy & Safe, 1980; Safe et al.,
1980; see also section 6.6). In general, the biotransformation of
PBBs is a slow process, but the stereochemistry and molecular size
vary so widely among PBBs that there are great differences in their
metabolic activity.
6.4 Elimination and excretion in expired air, faeces, urine
6.4.1 Animal studies
Elimination of PBBs from the body has been studied using hexa-
and octabromobiphenyl. No information is available on
decabromobiphenyl. Some data found in the literature (see also
section 6.1) are compiled in Table 64 (14C-labelled single doses),
Table 65 (14C-labelled multiple doses), and Table 66 (daily
feeding).
The only study conducted with octabromobiphenyl resulted in a
much higher elimination from rats than was found for HxBB in another
study on rats (Table 64). However, there are also reports on the
rapid elimination of HxBB, e.g., by Willett & Irving (1976), who
found a 50% recovery of HxBB after 168 h in the faeces of two cows
given single intraruminal doses (3 g) of FireMaster(R) BP-6.
However, relatively high concentrations of radioactivity or of HxBB,
found, in some cases, in the faeces during the first few days after
dosing or during daily dosing, may have been due to incomplete
absorption (Tuey & Matthews, 1980). Approximately 60% of the total
dose was also recovered in the faeces of rhesus monkeys (Rozman
et al., 1982: Table 64).
Elimination of PBBs was primarily via the bile and the
intestine into the faeces and, in general, was found to be a slow
process.
Biliary concentrations have been measured in a Rhesus monkey
(Rozman et al., 1982), in rats (Matthews et al., 1977), and in
cattle (Willett & Irving, 1976; Willett & Durst, 1978). In rats,
excretion of HxBB in the bile accounted for 0.68% of the total PBB
dose between 0 and 4 h after intravenous (iv) administration.
Twenty- four hours after an iv dose, 0.032% of the total dose was
excreted in the bile in 1 h, and, 7 or 42 days after dosing
concentrations were too low to quantify (Matthews et al., 1977).
Because of this small amount cleared with the bile, enterohepatic
recirculation of HxBB in rats is not important (Tuey & Matthews,
1980). Concen trations of HxBB in the bile of cattle were two-three
times greater than the concentration in the plasma (Willett &
Irving, 1976; Willett & Durst, 1978).
According to Fries (1985b), excretion of PBB in the urine is
not expected, because of the insolubility of PBBs in water. He
attributed the few instances in which low concentrations of PBBs
were reported to cross-contamination with faeces. On the other hand,
this route of excretion may account for minor or metabolized
biphenyls (Damstra et al., 1982; see also Table 63). However,
excretion of PBB metabolites may be of minor relevance, since the
more abundant PBB congeners are not, or only slightly, metabolized
(see also section 6.3). The amounts of urinary or faecal metabolites
were low. A study on a pig that received a single intraperitoneal
(ip) dose of FireMaster(R) BP-6 (100 mg/kg body weight) reported a
yield of about 1% of pentabromobiphenylol in the urine and faeces,
collected for 7 days (Kohli & Safe, 1976). The level of a hydroxy
metabolite detected in the faeces of dogs fed FireMaster(R) BP-6
was about an order of magnitude less than the PBB levels (Gardner
et al., 1979). Yields of lower brominated urinary PBB metabolites
are compiled in Table 63.
In their study on cows, Willett & Durst (1978) did not find any
direct relationships between the amount of PBBs fed and the
concentration in the faeces. In contrast, Babish et al. (1975a) did
find a linear correlation (r > 0.97) of dietary PBBs with excreta
residues in Japanese quails. In another study on cows, it was also
reported that relationships were approximately constant (Robl
et al., 1978: Table 66).
Table 64. Faecal, urinary, and exhalative elimination of 14C activity as a percentage of a single dose of [14C-]PBB
Species Agent; solvent; dose (mg/kg Time % recovery in: Reference
body weight); route faeces urine expired air
Rhesus monkey (male) [14C-]HxBB in mineral oil; 2, oral 5 days 38 (0.18) - Rozman et al.
(1982)
Rat (male) [14C-]HxBB in Emulphor EL-620: - Matthews et al.
ethanol: water (1:1:8) 24 h 7.9 (1977); Tuey &
1, oral 24 h 0.96 Matthews
1, intravenous 7 days 3.28 < 0.1 (1978, 1980)
1, intravenous 42 days 6.6 not detected
Rat (male and female) [14C-]OcBBa in corn oil; 1, oral 24 h 62 Norris et al.
48 h 69 (1973)
16 days 73 < 1 < 1
Mink (Mustela vison) [14C-]PBBb in propylene glycol Bleavins et al.
(pregnant female) (1 µCi in 0.1 ml) intravenous 2 h - 0.003 - (1981)
Ferret (Mustela 2 h - 0.004
puttorius (furo)
(pregnant female)
Dog [14C-]2,2',4,4',5,5'-hexabromo 25 days 8 Sipes et al.
biphenyl (solvent not specified) (1979)
0.6, intravenous
a OcBB = technical octabromobiphenyl.
b Consisted of 2,2', 4,4', 5,5'-HxBB and 2,2', 3, 4,4', 5,5'-HpBB.
Table 65. Urinary and faecal elimination of HxBB and/or metabolites in male Rhesus monkeys and male rats,
dosed repeatedly with [14C-]HxBB
Species Dose (mg/kg body Days after Urine Faeces Cumulative % Reference
weight) first dose (µg/kg per (µg/kg per recovery of
day) day) total dose
in faeces
Monkey 50 1-10 3.2 5890 ca 60% Rozman et al. (1982)
(No. = 2) in methyl cellulose; 11-17 2.5 5.0
oral on days 1 and 5 203-209 not detectable 3.5
Rat 1 7 ca 14% Matthews et al. (1977)
(No. = 3) in corn oil; oral on
days 1, 2, 3, and 4
Table 66. Elimination of HxBB (2,2',4,4',5,5'-hexabromobiphenyl) in faeces and urine during the feeding of FireMaster(R)-mixtures
Species Intake of FireMaster Sampling Concentration in: Approximate % Reference
(sex) BP-6 or FF-1 time (mg/kg) recovery in
Faeces Urine faeces
Dog BP-6: in corn oil (capsule) last day of 7 Gardner et al.
(female) 1 mg/kg body weight per day dosing (1979)
for 6 weeks
Pig BP-6: in corn oil at week 4 Ku et al. (1978)
20 mg/kg diet 21.3a 0.015
200 mg/kg diet 182.0a 0.07
(ad libitum)
for > 4 weeks
Cow FF-1: in gelatin capsules; Robl et al. (1978)
for 90 days
0.1 mg/kg diet 0.02 mostly 15% of
1.0 mg/kg diet 0.15 n.d.b ingested
10 mg/kg diet 1.5 dose
Calf BP-6:(capsule); 25 g daily total 8045 5% of total Willet & Irving
(male) for 9 days collection dose (1976)
Hen BP-6: 20 mg/kg in the diet weekly 9% of daily Fries et al.
for 63 days dose (1976)
Hen FF-1: not specified not specified 11% of daily Ringer &
dose Polin (1977)
a Faecal samples oven-dried.
b nd = Not detected (detection limit = 0.005 mg/kg).
Following withdrawal of PBBs (FireMaster(R) BP-6) from the
diet of cows, the faecal concentrations of HxBB declined to 1-2% of
faecal levels during dosing (Willett & Durst, 1978) and to less than
5% in hens (Fries et al., 1976). Faecal concentrations were small in
relation to body burden. Cows that received 250 mg FireMaster(R)
BP-6 daily had HxBB concentration ratios in body fat to faeces of
about 750 : 1. Their faeces to plasma ratio was 0.7 :1 (Willett &
Durst, 1978). Cows environmentally contaminated (FireMaster(R)
FF-1) 7-9 months before examination had comparable body fat to
faeces ratios, but a different faeces to blood ratio of 4.2 : 1
(Detering et al., 1975; Cook et al., 1978b; Fries et al., 1978a).
Post-exposure lactating cows eliminated via milk fat three times the
quantity of HxBB cleared in faeces (Willett & Durst, 1978).
There have been some studies on the means to enhance the
elimination of PBBs. PBBs used were: FireMaster(R) FF-1 (Cook
et al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980; Polin
& Leavitt, 1984; Polin et al., 1985) and [14C-]HxBB (Rozman
et al., 1982). The treatments included activated carbon in rats
(McConnell et al., 1980) and cows (Cook et al., 1978b),
cholestyramine in rats (McConnell et al., 1980) and monkeys (Rozman
et al., 1982), colestipol in chickens (Polin & Leavitt, 1984; Polin
et al., 1985), mineral oil in rats (Kimbrough et al., 1980), monkeys
(Rozman et al., 1982), and chickens (Polin et al., 1985), high-fibre
diets in rats (Kimbrough et al., 1980), and phenobarbital in cows
(Cook et al., 1978b). The effects of restricted caloric intake,
alone, or in combination with other treatments, was investigated in
rats (McConnell et al., 1980) and chickens (Polin & Leavitt, 1984;
Polin et al., 1985). The procedures were found not to be (Cook et
al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980), or to
be only partially, effective (Rozman et al., 1982; Polin & Leavitt,
1984; Polin et al., 1985) in reducing the body burden of PBBs
(measured as concentrations of HxBB, total bromine levels, or
14C-activity).
6.4.2 Human studies
The concentrations of PBBs in human bile and faeces represent a
minor proportion of the total body burden, as has been demonstrated
by Eyster et al. (1983), who determined HxBB levels in Michigan farm
and chemical workers in 1975-80. Concen trations of HxBB observed in
the bile and faeces were about 1/2 to 7/10 of the serum levels (on a
whole-weight basis) and were estimated to be approximately 0.5% of
the adipose tissue levels (Table 67).
These findings are consistent with the theoretical predictions
of Tuey & Matthews (1980), who calculated slow rates of faecal
excretion in humans. In addition, these authors showed that the
excretion rate in lean individuals exposed to HxBB would be higher
than those in overweight individuals.
6.5 Retention and turnover
6.5.1 Animal studies
The time course of PBB tissue concentrations has been studied
predominantly in rats, and, to a lesser extent, in cattle, chickens,
and guinea-pigs (Table 68). Incomplete data are available for mice,
pigs, and monkeys. With the exception of 3 older studies (Norris
et al., 1973; Lee et al., 1975a; Waritz et al., 1977), in which the
behaviour of technical octabromobiphenyl in rats was observed, the
majority of investigators used the FireMaster(R)- mixture (BP-6 or
FF-1). Of the individual PBB congeners,
2,2',4,4',5,5'-hexabromobiphenyl (Matthews et al., 1977; Millis
et al., 1985b) and 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985b) were administered.
Table 67. Medians, range, and geometric means of 2,2',4,4',5,5'-
hexabromobiphenyl (HxBB) for paired specimens of serum, faeces, and bile
obtained from farm and chemical workersa
Number Paired Median Rangeb Geometric
specimen mean
51 serum 7 µg/litre 1-1540 µg/litre 14.4 µg/litre
faeces 5 µg/kg nd-862 µg/kg 9.0 µg/kg
20 serum 3.5 µg/litre 1-153 µg/litre 4.2 µg/litre
biliary fluid 2 µg/litre nd-70 µg/litre 2.7 µg/litre
a From: Eyster et al. (1983).
b nd = Not detectable (detection limit: 1 µg/kg or µg/litre).
Complex and varied relationships were found in tissue
concentrations with time after PBB administration (see also Tables
47, 48, and 49).
Table 68. Reported biological half-lifes of PBBs in mammals and birds, after single or repeated exposure
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Rhesus Monkey (male) [14C-]HxBB (50 mg/kg body weight body > 4 years Rozman et al.
on days 1 and 5; oral) 209 days (1982)
Rat (male) [14C-]OcBB (1 mg/kg body weight; faeces < 24 h (1st phase) Norris et al.
single dose; oral) 16 days > 16 days (2nd phase) (1973)
Rat [14C-]HxBB (1 mg/kg body weight; faeces 2 days (1st phase)c Birnbaum
single iv dose) 42 days (1985)
"tissues" ca 24 daysc Ecobichon et al.
(blood, liver (1983)
muscle, skin)
Rat (male) FM BP-6 (1 mg/100 g body weight; serum 23.1 weeks Miceli &
single ip dose; 36 weeks fat 69.3 weeks Marks (1981)
adrenal 43.3 weeks
brain 63.0 weeks (2nd phase)
liver 11.5 weeks (2nd phase)
lung 11.2 weeks
spleen 9.0 weeks
Rat (male) FM FF-1 (10 mg/kg body weight; whole blood 3.27 h (') Domino et al.
single dose; oral) 112 days 33.3 h (ý) (1982)
145 days (')
Guinea-pig (lactating FM FF-1 (50 mg/kg body weight; tissues (fat, ca 22 days Ecobichon
females and pups) single dose; oral) 60 days liver, kidney, et al. (1983)
lung) of both
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Cow FM BP-6 (fed 10 mg/day for milk fat ca 58 days Fries &
60 days) 60 days (of withdrawal) (2nd phase) Marrow (1975)
Cow FM BP-6 (fed 1.13 g/day for milk 10.5 days Gutenmann &
15 days; = 50 mg/kg in the diet) Lisk (1975)
15 days (of withdrawal)
Cow FM FF-1 (environmentally milk fat ca 60 days Fries et al.
contaminated 1 year earlier; (range: 36-301 days) (1978a); Cook
6 months of observation) et al. (1978b)
Cow FM BP-6 (fed 0.25 mg-25 g/day milk fat > 6 monthsd Fries
for various periods) up to (body) (1985b)
3 years of observation
Chicken (White FM FF-1 (fed in the diet) egg 17 days Ringer &
Leghorn hens) 28 days (of withdrawal) Polin (1977)
Chicken (White FM FF-1 (fed 0.2-125 mg/kg egg 17 days Polin &
Leghorn hens) in the diet for 5 weeks) Ringer (1978a)
56 days (of withdrawal)
(fed 1125 and 625 mg/kg in liver 31 days
the diet for 5 weeks)
56 days (of withdrawal)
(fed 5-625 mg/kg in the diet muscle 17 days
for 5 weeks) 56 days
(of withdrawal)
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Chicken (White FM BP-6 (fed 20 mg/kg in the egg 28 days Fries et al.
Leghorn hens) diet for 63 days) 49 days (2nd phase) (1976)
(of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg egg 112 days Cecil &
Leghorn hens) in the diet for 8 weeks) (late phase) Bitman (1978)
266 days (of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg excreta 4-5 dayse Polin &
Leghorn hens) in the diet for 8 weeks) Leavitt (1984)
266 days (of withdrawal)
a HxBB = 2,2',4,4',5,5'-hexabromobiphenyl; OcBB = octobromobiphenyl (technical mixture);
FM BP-6 = FireMaster(R) BP-6; FM FF-1 = FireMaster(R) FF-1.
b Referring to 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity.
c Half-life calculated from data of Matthews et al. (1978); see also Fig. 6.
d Half-life calculated from data of Willett & Durst (1978).
e Half-life calculated from data of Fries et al. (1976).
6.5.1.1 Time trends, retention: 2,2',4,4',5,5'-hexabromobiphenyl
(BB 153)
a) Rat
When rats dosed with 2,2',4,4',5,5'-hexabromobiphenyl (Matthews
et al., 1977; Tuey & Matthews, 1980) or with FireMaster(R) FF-1
(Kimbrough et al., 1978; Domino et al., 1980b; 1982), BB 153
concentrations in the blood were highest immediately after dosing,
but fell rapidly during the first day (as PBBs are taken up from
blood by the liver and muscle tissues, which are highly perfused
tissues). Then, concentrations in the blood, liver, and muscle
declined less quickly (as the dose was redistributed to the adipose
tissue; Tuey & Matthews, 1980; Fries, 1985b), finally decreasing
only very slowly. After a single intravenous dose of BB 153 (1 mg/kg
body weight), adipose tissue contained more than 60% of the total
body burden within four days (Tuey & Matthews, 1980; see Table 50).
BB 153 concentrations in the adipose tissue peaked later than
in other tissues and remained high throughout the period of
observation (6 weeks: Matthews et al., 1977; 16 weeks; Domino
et al., 1980b; 36 weeks: Miceli & Marks, 1981). For example, ratios
between fat and serum levels in rats rose from 221 : 1 to 722 : 1
between 6 and 36 weeks after a single dose exposure (Miceli & Marks,
1981), reflecting the much more rapid clearance of BB 153 from serum
than from fat.
Kinetic models to describe the principal toxicokinetics of BB
153 in the rat were constructed by Tuey & Matthews (1980; a blood
flow-limited physiological compartmental model) and by Domino et al.
(1982). The latter developed a three-compartment model. Tissues
within a compartment showed similar kinetic characteristics, but
concentrations could vary widely. Compartment 1 consisted of whole
blood, spleen, kidney, and heart. Compartment 2 included liver,
lung, cerebral grey and white matter, cerebellum, and testes, and
compartment 3 consisted of subcutaneous fat. Jejunum could not be
classified.
However, results of Miceli & Marks (1981) (PBB levels monitored
over longer periods) do not fit in well with this scheme. For
example, these authors observed typical first-order-elimination
kinetics of BB 153 in the serum of rats, but kinetics of disappear
ance from heart and kidney (and pituitary) do not appear to be first
order, though belonging to the same compartment (according to Domino
et al., 1982). BB 153 concentrations in the brain and liver (both
"compartment 2") declined rapidly during the interval from 6-12
weeks after exposure, but, thereafter, (from week 12 to 36) brain
concentrations fell far more slowly than those of the liver. The
study of Miceli & Marks (1981) showed that the (long-term)
retention, but not always the concentration, of BB 153 in lipid-rich
tissues (brain, adrenal, adipose) was much greater than in most
other tissues (see also Table 68).
Corresponding to the small decline in BB 153 from the tissues
of rats, the elimination rates in the faeces were slow (see Fig. 6).
Less than 7% of the dose was eliminated 42 days after a single iv
dose, most, during the first 3-4 days (Tuey & Matthews, 1980).
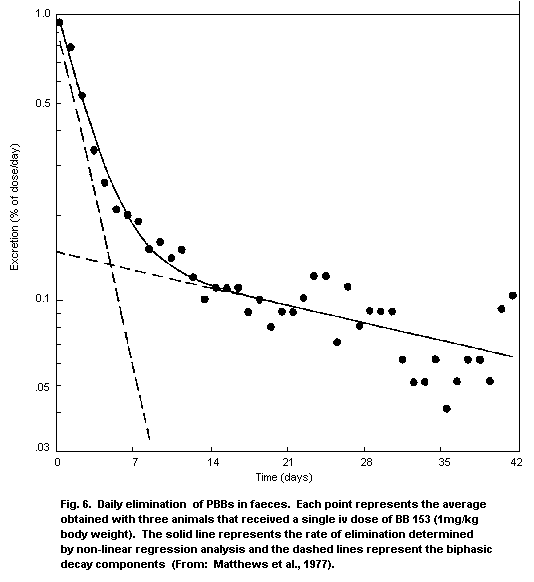 The kinetic models or calculations for BB 153 were based on the
results of studies on adult male rats. In maternal rats the
situation is more complex, and only a little information is
available. For example, BB 153 levels in the liver and fat of
maternal rats decreased during a period from the end of lactation
(and exposure) until 14 weeks later (decline in fat: 50%), but BB
153-concentrations in the mammary glands increased (5 times higher)
during the same period (see also Table 48), indicating
redistribution of BB 153 during the recovery period following
lactation (McCormack & Hook, 1982).
During continued exposure to FireMaster(R) BP-6, BB 153
concentrations in the milk of lactating rats were determined 0, 3,
5, 7, and 14 days following parturition and showed a decline from
180 mg/litre (via 115, 89, 63 mg/litre) to 50 mg/litre (McCormack
et al., 1979a).
The kinetics of BB 153 in growing animals have not been
frequently investigated. The differences in BB 153 levels and organ
weights have been reported only between two ages. McCormack et al.
(1980) showed that at 328 days of age, the concentrations of BB 153
in the liver, kidney, and fat of rats were approximately 5, 10, and
25% of the respective tissue concentrations at weaning (28 days of
age; cessation of exposure). Another long-term study (Groce &
Kimbrough, 1984) compared BB 153 levels in the livers of perinatally
exposed rats at the age of 2 months and 2 years and also found
diminished values (see Table 52).
Comparing BB 153 concentrations in the blood and adipose tissue
of rats after 10 and 14 months recovery resulted in no "true"
decrease (Kimbrough et al., 1978). Other studies also indicated a
long retention of PBBs in the brain (Geller et al., 1979), thyroid,
and liver (Allen-Rowlands et al., 1981), and in the adrenal glands
(Castracane et al., 1982).
b) Other species
The species, the second most often examined, was cattle, but
the kinetic models used for cattle (e.g., Fries et al., 1978a) were
less sophisticated than those described for rats (Fries, 1985b).
In cows given FireMaster(R) BP-6, the BB 153 concentration in
blood plasma was maximal 24 h after exposure (Willett & Irving,
1976; Willett & Durst, 1978). When multiple doses were administered,
plasma concentrations were at equilibrium by 15 days. When dosing
was terminated, concentrations declined approximately 50% in 10 days
and 66% by day 20. Thereafter, plasma residues did not fit a
consistent decline model (Willett & Durst, 1978).
While BB 153 was detectable in plasma within 2-4 h of exposure
(Willett & Durst, 1978), it was detected in the milk of cows 13 h
after exposure (Willett & Irving, 1976). With continued exposure, a
steady state of BB 153 concentrations in milk fat was reached after
20-40 days (Fries & Marrow, 1975; Willett & Durst, 1978; Fries
et al., 1978a; Robl et al., 1978). When the feeding of PBBs was
stopped, concentrations in milk fat declined rapidly for a short
time (Fries & Marrow, 1975). When a new equilibrium was established,
milk fat and body fat concentrations declined in a parallel manner
(Fries, 1985b). An example of the biphasic decline is given in Fig.
7. However, as Fries et al. (1978a) observed, the stage of lactation
influenced the rate of elimination, and, in some cases, BB 153
levels in milk increased shortly after calving.
The kinetic models or calculations for BB 153 were based on the
results of studies on adult male rats. In maternal rats the
situation is more complex, and only a little information is
available. For example, BB 153 levels in the liver and fat of
maternal rats decreased during a period from the end of lactation
(and exposure) until 14 weeks later (decline in fat: 50%), but BB
153-concentrations in the mammary glands increased (5 times higher)
during the same period (see also Table 48), indicating
redistribution of BB 153 during the recovery period following
lactation (McCormack & Hook, 1982).
During continued exposure to FireMaster(R) BP-6, BB 153
concentrations in the milk of lactating rats were determined 0, 3,
5, 7, and 14 days following parturition and showed a decline from
180 mg/litre (via 115, 89, 63 mg/litre) to 50 mg/litre (McCormack
et al., 1979a).
The kinetics of BB 153 in growing animals have not been
frequently investigated. The differences in BB 153 levels and organ
weights have been reported only between two ages. McCormack et al.
(1980) showed that at 328 days of age, the concentrations of BB 153
in the liver, kidney, and fat of rats were approximately 5, 10, and
25% of the respective tissue concentrations at weaning (28 days of
age; cessation of exposure). Another long-term study (Groce &
Kimbrough, 1984) compared BB 153 levels in the livers of perinatally
exposed rats at the age of 2 months and 2 years and also found
diminished values (see Table 52).
Comparing BB 153 concentrations in the blood and adipose tissue
of rats after 10 and 14 months recovery resulted in no "true"
decrease (Kimbrough et al., 1978). Other studies also indicated a
long retention of PBBs in the brain (Geller et al., 1979), thyroid,
and liver (Allen-Rowlands et al., 1981), and in the adrenal glands
(Castracane et al., 1982).
b) Other species
The species, the second most often examined, was cattle, but
the kinetic models used for cattle (e.g., Fries et al., 1978a) were
less sophisticated than those described for rats (Fries, 1985b).
In cows given FireMaster(R) BP-6, the BB 153 concentration in
blood plasma was maximal 24 h after exposure (Willett & Irving,
1976; Willett & Durst, 1978). When multiple doses were administered,
plasma concentrations were at equilibrium by 15 days. When dosing
was terminated, concentrations declined approximately 50% in 10 days
and 66% by day 20. Thereafter, plasma residues did not fit a
consistent decline model (Willett & Durst, 1978).
While BB 153 was detectable in plasma within 2-4 h of exposure
(Willett & Durst, 1978), it was detected in the milk of cows 13 h
after exposure (Willett & Irving, 1976). With continued exposure, a
steady state of BB 153 concentrations in milk fat was reached after
20-40 days (Fries & Marrow, 1975; Willett & Durst, 1978; Fries
et al., 1978a; Robl et al., 1978). When the feeding of PBBs was
stopped, concentrations in milk fat declined rapidly for a short
time (Fries & Marrow, 1975). When a new equilibrium was established,
milk fat and body fat concentrations declined in a parallel manner
(Fries, 1985b). An example of the biphasic decline is given in Fig.
7. However, as Fries et al. (1978a) observed, the stage of lactation
influenced the rate of elimination, and, in some cases, BB 153
levels in milk increased shortly after calving.
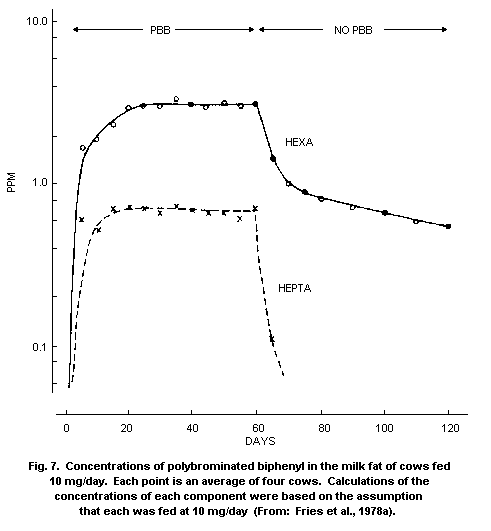 Data from Detering et al. (1975) indicated that BB 153 levels
in cow's milk would decrease from 200-400 mg/kg in fat to 0.3 mg/kg
in 120 weeks.
Information on a time course of PBB levels in the body fat of
cows is limited. According to Willett & Durst (1978), e.g., BB 153
concentrations in the subcutaneous fat of cows had declined by 40%,
20 days after exposure. Two measurements, one at parturition (ca.
150 days later) and one after heavy lactation (ca. 200 days later)
showed an increase in residues. During late lactation, significant
declines occurred.
Probably, a multicompartment system is necessary to describe
the long-term behaviour of PBBs in lactating cows, with special
trends when pregnancy, parturition, etc. occur (Willett & Durst,
1978; Fries et al., 1978a; Fries, 1985b).
The appearance and decline of BB 153 in faeces were reported
for two cows given a single intraruminal dose of FireMaster(R)
BP-6 (Willett & Irving, 1976) and for cows fed BP-6 daily for 60
days (Willett & Durst, 1978). In the first case, BB 153 was detected
12 h after administration, peaked between 20 and 36 h, and declined
to about 2% of the peak concentration during the subsequent 48 h. By
day 8, approximately 50% of the dose was detectable in the faeces
(Willett & Irving, 1976). During daily exposure, BB 153
concentrations in the faeces reached a steady state by day 10. After
withdrawal of the PBBs, the levels of BB 153 declined within 10 days
to approximately 1% of the concen tration present during exposure
(Willett & Durst, 1978). Fries (1985b) inferred low rates of
elimination in faeces, because faecal BB 153 concentrations in
cattle that were no longer being exposed to PBBs were relatively low
(Willett & Durst, 1978; Fries et al., 1978a).
Another domestic species in which the kinetics have been
examined is the chicken. Whole carcass analysis of male White
leghorn chickens showed that, during the 2 weeks that
FireMaster(R) FF-1 was fed at 0.1 or 1.0 mg/kg, the chickens
retained 88 and 69%, respectively, of the FF-1 that was consumed
(Polin & Leavitt, 1984). Withdrawal rates were determined in a
similar study on egg- and meat-type chickens fed diets containing 1
or 10 mg FF-1/kg. Body burdens of BB 153 in chickens, previously fed
10 mg/kg, did not decrease significantly during a withdrawal period
of 42 days (e.g., 3% loss by day 21 of withdrawal). In contrast,
chickens, previously fed 1 mg/kg, eliminated up to 40% of the BB 153
(Polin et al., 1985).
Withdrawal of PBBs from the adipose tissue of laying hens fed
FireMaster(R) FF-1 at different dietary levels (0.2, 1, 5, 25,
125, or 625 mg/kg) has been followed by Polin & Ringer (1978a). BB
153 levels remained unchanged over the 56 days of withdrawal. Lillie
et al. (1975) calculated a 50% reduction after more than 16 weeks.
Withdrawal from other tissues and from eggs was more rapid (Ringer &
Polin, 1977; Polin & Ringer, 1978b).
BB 153 levels in eggs laid by hens fed 20 or 64 mg
FireMaster(R) BP-6/kg diet for 8-9 weeks reached a plateau by the
third or fourth week of feeding. When feeding of BP-6 stopped,
residues decreased in a two-phase rate pattern with a phase of rapid
decline shortly after exposure had ceased and a late phase of slow
decrease (Fries et al., 1976; Cecil & Bitman, 1978). In one study
(Fries et al., 1976), the levels after 49 days were approximately
10% of the values on day 0 of cessation; in the other study by Cecil
& Bitman (1978), detectable amounts were still present 33 weeks
after withdrawal of BP-6 from the diet.
Excreta were analysed from hens fed 20 mg FireMaster(R)
BP-6/kg for 63 days (Fries et al., 1976). After an initial rise and
decline, the BB 153 levels remained fairly constant (at about
2 mg/kg on a wet weight basis) during the feeding of PBBs. After
withdrawal of PBBs, the residues dropped to a negligible level
(< 0.1 mg/kg).
Only some milk data during continued exposure are available for
pigs. Milk of sows having received 10, 100, or 200 mg of
FireMaster(R) BP-6/kg feed during the second half of gestation and
during lactation was monitored until the 4th week. On a fat basis,
concentrations of BB 153 were highest in the colostrum and decreased
slowly during lactation (Werner & Sleight, 1981).
Disappearance of BB 153 from the tissues of lactating
guinea-pigs and of their pups was described by Ecobichon et al.
(1983). The maternal animals had received a single oral dose of
FireMaster(R) FF-1 (50 mg/kg body weight) within 6-12 h of
parturition, and the residue levels of nursing young and the dams
were measured up to 60 days after exposure (at intervals of 2, 4, 7,
14, 28, 42, and 60 days: see Fig. 8). Following the initial 7 days
during which there was obvious inter-tissue transportation and
sequestration in body fat, a gradual, but similar, linear rate of
decline was observed in the livers, kidneys, and lungs of both the
young and their dams. A similar rate of reduction was observed in
the body fat of both pups and dams.
In a study on mice, BB 153 residue levels were measured 6 h
after dietary intake of 100 mg/kg. FireMaster(R) BP-6 (for 14
days) can be compared with those obtained 14 weeks after feeding.
There was a decline in all tissues including fat (see Table 48),
however, to a different extent, resulting in changes in the relative
BB 153-concentrations between tissues (Corbett et al., 1978a).
BB 153 blood levels of rhesus monkeys, dosed with BB 153,
markedly declined over time (day 5: 1.5 mg/kg, day 11: 0.2 mg/kg;
day 25 < accurate measurement levels) (Rozman et al., 1982).
Data from Detering et al. (1975) indicated that BB 153 levels
in cow's milk would decrease from 200-400 mg/kg in fat to 0.3 mg/kg
in 120 weeks.
Information on a time course of PBB levels in the body fat of
cows is limited. According to Willett & Durst (1978), e.g., BB 153
concentrations in the subcutaneous fat of cows had declined by 40%,
20 days after exposure. Two measurements, one at parturition (ca.
150 days later) and one after heavy lactation (ca. 200 days later)
showed an increase in residues. During late lactation, significant
declines occurred.
Probably, a multicompartment system is necessary to describe
the long-term behaviour of PBBs in lactating cows, with special
trends when pregnancy, parturition, etc. occur (Willett & Durst,
1978; Fries et al., 1978a; Fries, 1985b).
The appearance and decline of BB 153 in faeces were reported
for two cows given a single intraruminal dose of FireMaster(R)
BP-6 (Willett & Irving, 1976) and for cows fed BP-6 daily for 60
days (Willett & Durst, 1978). In the first case, BB 153 was detected
12 h after administration, peaked between 20 and 36 h, and declined
to about 2% of the peak concentration during the subsequent 48 h. By
day 8, approximately 50% of the dose was detectable in the faeces
(Willett & Irving, 1976). During daily exposure, BB 153
concentrations in the faeces reached a steady state by day 10. After
withdrawal of the PBBs, the levels of BB 153 declined within 10 days
to approximately 1% of the concen tration present during exposure
(Willett & Durst, 1978). Fries (1985b) inferred low rates of
elimination in faeces, because faecal BB 153 concentrations in
cattle that were no longer being exposed to PBBs were relatively low
(Willett & Durst, 1978; Fries et al., 1978a).
Another domestic species in which the kinetics have been
examined is the chicken. Whole carcass analysis of male White
leghorn chickens showed that, during the 2 weeks that
FireMaster(R) FF-1 was fed at 0.1 or 1.0 mg/kg, the chickens
retained 88 and 69%, respectively, of the FF-1 that was consumed
(Polin & Leavitt, 1984). Withdrawal rates were determined in a
similar study on egg- and meat-type chickens fed diets containing 1
or 10 mg FF-1/kg. Body burdens of BB 153 in chickens, previously fed
10 mg/kg, did not decrease significantly during a withdrawal period
of 42 days (e.g., 3% loss by day 21 of withdrawal). In contrast,
chickens, previously fed 1 mg/kg, eliminated up to 40% of the BB 153
(Polin et al., 1985).
Withdrawal of PBBs from the adipose tissue of laying hens fed
FireMaster(R) FF-1 at different dietary levels (0.2, 1, 5, 25,
125, or 625 mg/kg) has been followed by Polin & Ringer (1978a). BB
153 levels remained unchanged over the 56 days of withdrawal. Lillie
et al. (1975) calculated a 50% reduction after more than 16 weeks.
Withdrawal from other tissues and from eggs was more rapid (Ringer &
Polin, 1977; Polin & Ringer, 1978b).
BB 153 levels in eggs laid by hens fed 20 or 64 mg
FireMaster(R) BP-6/kg diet for 8-9 weeks reached a plateau by the
third or fourth week of feeding. When feeding of BP-6 stopped,
residues decreased in a two-phase rate pattern with a phase of rapid
decline shortly after exposure had ceased and a late phase of slow
decrease (Fries et al., 1976; Cecil & Bitman, 1978). In one study
(Fries et al., 1976), the levels after 49 days were approximately
10% of the values on day 0 of cessation; in the other study by Cecil
& Bitman (1978), detectable amounts were still present 33 weeks
after withdrawal of BP-6 from the diet.
Excreta were analysed from hens fed 20 mg FireMaster(R)
BP-6/kg for 63 days (Fries et al., 1976). After an initial rise and
decline, the BB 153 levels remained fairly constant (at about
2 mg/kg on a wet weight basis) during the feeding of PBBs. After
withdrawal of PBBs, the residues dropped to a negligible level
(< 0.1 mg/kg).
Only some milk data during continued exposure are available for
pigs. Milk of sows having received 10, 100, or 200 mg of
FireMaster(R) BP-6/kg feed during the second half of gestation and
during lactation was monitored until the 4th week. On a fat basis,
concentrations of BB 153 were highest in the colostrum and decreased
slowly during lactation (Werner & Sleight, 1981).
Disappearance of BB 153 from the tissues of lactating
guinea-pigs and of their pups was described by Ecobichon et al.
(1983). The maternal animals had received a single oral dose of
FireMaster(R) FF-1 (50 mg/kg body weight) within 6-12 h of
parturition, and the residue levels of nursing young and the dams
were measured up to 60 days after exposure (at intervals of 2, 4, 7,
14, 28, 42, and 60 days: see Fig. 8). Following the initial 7 days
during which there was obvious inter-tissue transportation and
sequestration in body fat, a gradual, but similar, linear rate of
decline was observed in the livers, kidneys, and lungs of both the
young and their dams. A similar rate of reduction was observed in
the body fat of both pups and dams.
In a study on mice, BB 153 residue levels were measured 6 h
after dietary intake of 100 mg/kg. FireMaster(R) BP-6 (for 14
days) can be compared with those obtained 14 weeks after feeding.
There was a decline in all tissues including fat (see Table 48),
however, to a different extent, resulting in changes in the relative
BB 153-concentrations between tissues (Corbett et al., 1978a).
BB 153 blood levels of rhesus monkeys, dosed with BB 153,
markedly declined over time (day 5: 1.5 mg/kg, day 11: 0.2 mg/kg;
day 25 < accurate measurement levels) (Rozman et al., 1982).
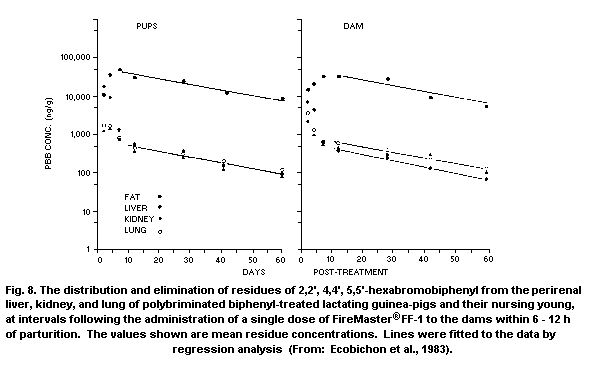 6.5.1.2 Biological half-lives
The biological half-lives of PBBs reported in the literature
for various species have been compiled in Table 68. McDaniel &
Lucier (1979) reported a half-life of BB 153 exceeding the lifetime
of rats.
In some cases the results obtained by different authors are
similar, in other cases, large discrepancies exist. These deviations
were, perhaps, caused by measuring the half-lifes under different
circumstances of exposure and for different lengths of time (Fries,
1985b). By means of simulation studies, Domino et al. (1982)
demonstrated the effects of different amounts of body fat on the
half-life of BB 153 in the rat (see Table 69).
Tuey & Matthews (1980) have simulated the effects of a growing
animal on the kinetics of BB 153 and found that, though
concentrations of BB 153 in fat fell faster than in a stable animal,
the "actual" half-life of the substance was prolonged, because of an
increase in the relative fat proportions.
6.5.1.3 Differences between individual congeners
There are some reports on the differences in turnover and
retention of individual PBB congeners. Mostly, they refer to changes
in the relative abundances of certain FireMaster(R) components,
namely: 2,2',4,5,5'-pentabromobiphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118), 2,2',3,4',5',6-hexa
bromobiphenyl (BB 149), 2,2',4,4',5,5'-hexabromobiphenyl (BB 153),
2,2',3,4,4',5'-hexabromobiphenyl (BB 138), 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167), 2,3,3',4,4',5-hexabromobiphenyl (BB
156), 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), and
2,2',3,3',4,4'5-heptabromobiphenyl (BB 170). To describe such
changes, the GC profile of the original FireMaster(R) mixture was
compared with the GC profile obtained on analysis of tissues from
animals treated with this mixture, by measuring either the area
(e.g., Wolff & Aubrey, 1978; Domino et al., 1980b) or the height
(e.g., McCormack et al., 1982a; Bernert & Groce, 1984; Groce &
Kimbrough, 1984) of the GC peaks and by normalizing the values to BB
153 as 100. A few other authors (Fries & Marrow, 1975; Fries et al.,
1976) based their calculations on the assumption that each component
(BB 153 and BB 180) was fed at the same rate in the diet. According
to Fries (1985b), some differential behaviour may be due to
analytical artefacts, introduced by differential recovery of the
congeners or by adsorption of congeners on the glassware (Willett
et al., 1978), but many changes appear real.
Table 69. Effect of different amounts of body fat on the half-life of
2,2',4,4',5,5'-hexabromobiphenyl in the rata
Amount fat Half-life
(% normal fat) (days)
Emaciated 25 60.5
50 88.8
75 117
90 134
Normal 100 145
110 156
125 173
150 200
175 228
200 256
Obese 250 311
a From: Domino et al. (1982).
The trends reported in the literature include the selective
retention of 5 minor components of the FireMaster(R) mixture in
the tissues of rats, pigs, cows, and chickens (Fries & Marrow, 1975;
Fries et al., 1976, 1978a; Dannan et al., 1978b; Willett & Durst,
1978; Wolff & Aubrey, 1978; Wolff & Selikoff, 1979; Domino et al.,
1980b; McCormack et al., 1980, 1982a; Werner & Sleight, 1981; Groce
& Kimbrough, 1984; Polin & Leavitt, 1984). They can be summarized,
as follows.
With some exceptions, the concentrations of 2,2',4,5,5'-penta
bromobiphenyl (BB 101) and 2,2',3,4,4',5,5'-heptabromobiphenyl (BB
180) relative to BB 153 appeared to be lower in tissues from treated
animals than in those fed FireMaster(R) mixture. The relative
concentrations of 2,3',4,4',5-pentabromobiphenyl (BB 118) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) appeared to be higher or
unchanged in many cases. 2,2',3,4',5',6-Hexabromo biphenyl (BB 149)
was barely, or not, detected in the tissues of any animals.
The results of a multigeneration study on rats also reflected
the differential behaviour of certain PBB congeners (McCormack
et al., 1981). Of the first eight peaks (penta- to
heptabromobiphenyls) in the GC profile of FireMaster(R), all
except peak 3 (BB 149) were detected in the livers of rats in the
F1, F2, and F3 generations (experimental design: see Table
54). For example, the concentration of
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) relative to BB 153 was
higher in the livers of animals in the F1 generation than in the
FireMaster(R) BP-6 standard, but decreased with each subsequent
generation. Although F2-10 and F3-100 animals (10 and 100 mg/kg
treatment, respectively; see Table 54) had similar hepatic
concentrations of BB 153, the relative concentrations of other PBB
congeners, including BB 167 appeared to be lower in the livers from
F3-100 than F2-10 animals (McCormack et al., 1981).
Domino et al. (1980b) found that 2,2',4,5,5'-pentabromobiphenyl
(BB 101) penetrated the brain of rats more rapidly than
2,3',4,4',5-pentabromobiphenyl (BB 118) or any of the higher
relative molecular mass homologues. Fries et al. (1976) reported a
faster clearance of 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180)
(half-time = approximately 20 days) than of BB 153 (half-time =
approximately 28 days) from eggs of hens after feeding stopped.
However, analyses of whole chicken carcasses resulted in unchanged
ratios of BB 153/BB 180 on days 0, 21, and 42 of withdrawal (Polin &
Leavitt, 1984). The authors judged that the dynamics for withdrawal
of these two congeners from tissues of chickens were parallel. The
withdrawal from milk of dairy cows was found to be more rapid for BB
180 than for BB 153 (Fries & Marrow, 1975; Fries et al., 1978a; see
also Fig. 7).
In just one study (Millis et al., 1985b), equimolar doses of
individual PBB congeners were administered to the test animals.
Immature male rats received a single oral dose (21.3 µmol/kg body
weight) of 3,3',4,4',5,5'-hexabromobiphenyl or 3,3',4,4'-tetra
bromobiphenyl and were analysed at various times up to 14 days after
treatment. Adipose tissue and liver concentrations of
3,3',4,4',5,5'-hexabromobiphenyl appeared unchanged over time
whereas the tissue concentrations of 3,3',4,4'-tetrabromobiphenyl
decreased in a biphasic manner.
6.5.1.4 Octabromobiphenyl
Some kinetic data are available for technical octabromo
biphenyl. Bromine levels in the adipose tissue of rats dosed with
octabromobiphenyl did not decrease during a period of 90 days
(Norris et al., 1973) or 18 weeks (Aftosmis et al., 1972a; Waritz
et al., 1977) after cessation of dosing, or, according to Lee et al.
(1975a), levels even increased 18 weeks after exposure. A partial
elimination of bromine was observed from the livers of these rats
after recovery (Norris et al., 1973; Lee et al., 1975a; Waritz
et al., 1977).
The contents of total PBB (octabromobiphenyl plus an
unidentified hexabromobiphenyl) in juvenile Atlantic salmon (Salmo
salar), fed 90 days with octabromobiphenyl (Dow
Chemical)-contaminated food, also remained fairly constant after 28
days of withdrawal (Zitko, 1977).
6.5.2 Human studies
Information on the time trends of BB 153 distribution or
retention in humans is limited. As with animals, BB 153 seems to be
highly persistent in humans. This conclusion has been drawn from
monitoring BB 153 levels over time in both individual persons and
the Michigan population.
Most paired serial samples exist for serum (or plasma) from
farmers, etc. (Humphrey & Hayner, 1975: sampled June/Autumn, 1974;
Landrigan et al., 1979: sampled 1974/77; Wolff et al., 1979b:
sampled 1976/77/78; Kreiss et al., 1982: sampled 1977/78/79;
Sherman, 1991: sampled 1976/80/87) and chemical workers (Wolff
et al., 1979b: sampled 1976/78; Bahn et al., 1980b and Bialik, 1982:
sampled 1978/1981; Lambert et al., 1990) (in parentheses: references
plus year of sampling). The values obtained indicated no, or little,
decrease (see sections 5.2 and 5.3, and Table 38) with the exception
of the results of Bahn et al. (1980b) and Bialik (1982) (see section
5.3). Paired adipose analyses have been reported only by Meester &
McCoy (1976) who found a consider able average decline in HxBB
levels over six months (see also section 5.2). However, one of the
16 persons tested had increasing or unchanged fat levels, while
serum levels slowly dropped. The significance of these observations
and the combined results of Bahn et al. (1980b) and Bialik (1982)
are unclear. A most recent case report (Sherman, 1991) showed that
"PBB" could be identified in the serum and fat of a cancer patient
over an 11-year period (1976-87). Serial testing of 11 breast-milk
samples from one lactating Michigan woman showed that HxBB
concentrations varied between 0.1 and 0.2 mg/kg (expressed on a fat
basis) during a three-month period, without any significant downward
trend (Brilliant et al., 1978).
Comparisons at the population level have been made for serum
(Meester & McCoy, 1976), breast-milk (Miller et al., 1984), and
adipose tissue (Miceli et al., 1985). Generally, the presence of
PBBs in the tissues of Michigan people many years after the spill
(1973) may be an indicator for its persistence (see Tables 38 and
39). Meester & McCoy (1976) found that the average HxBB level in the
serum of farmers during the first six months of 1976 was ten times
lower than that during the last six months of 1975 (0.2 µg/litre
versus 2.0 µg/litre). Miller et al. (1984) concluded from their
analyses of breast-milk from 2986 lactating women during May 1976
and December 1978 that HxBB levels were not declining. Approximately
5 years after the Michigan PBB incident occurred, adipose tissue
from live residents of a "high" exposure area (Muskegon County area)
contained median HxBB levels of 500 µg/kg (Wolff et al., 1982).
Approximately 10 years after exposure, postmortem adipose tissue
contained median HxBB levels of 320 µg/kg (Miceli et al., 1985).
On the basis of the two last values, Miceli et al. (1985)
calculated a half-life of 7.8 years for HxBB (BB 153) in human
adipose tissue. This prediction was close to the body burden
half-time of 6.5 years estimated by Tuey & Matthews (1980) using
pharmacokinetic data obtained from rats.
A median serum half-life of BB 153 of 12 years (range: 4.6-94.7
years) has been determined by comparing previous and more recent
serum BB 153 levels of Michigan residents (Lambert et al., 1990).
As Tuey & Matthews (1980) explained, the half-life may be
longer in growing children or in persons gaining weight. However,
calculating the effects of different amounts of body fat on the
retention of BB 153, these authors also found that adipose tissue
may act as a protective reservoir in mature humans, because the
concentration of BB 153 in the blood (and possibly other more
critical tissues) of obese persons should be significantly less than
those of leaner individuals who received comparable exposures.
There was some evidence for the differential retention of
various PBB congeners in humans, when PBB congeners in serum samples
from Michigan subjects were compared with FireMaster(R) BP-6. The
main congeners assayed by using GC-MS analysis (Wolff & Aubrey,
1978; Wolff et al., 1978) or Negative Chemical Ionisation
Spectrometry (NCIMS) analysis (Roboz et al., 1982; Greaves et al.,
1984) were as follows: 2,2',4,5,5'-pentabromo biphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118),
2,2',3,4',5',6-hexabromobiphenyl (BB 149), 2,2',4,4',5,5'-hexa
bromobiphenyl (BB 153), 2,2',3,4,4',5'-hexabromobiphenyl (BB 138),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). The most obvious
changes refer to the two penta isomers and to the major
heptabromobiphenyl. The 2,2',3,4,4',5,5'-heptabromobiphenyl was
absent (Roboz et al., 1982) or greatly diminished in serum (Wolff &
Aubrey, 1978; Wolff et al., 1979a; Greaves et al., 1984) in relation
to the concentrations of BB 153 (taken as 100%). The relative
amounts of 2,2',4,5,5'-pentabromobiphenyl (BB 101) were also greatly
decreased (e.g., 80%: Greaves et al., 1984) in all samples. However,
a marked decrease in 2,3',4,4',5-pentabromobiphenyl (BB 118)
concentrations was observed only in the serum of farmers, taken
several years after exposure (e.g., 60%: Greaves et al., 1984), but
not in the serum of chemical workers (Wolff & Aubrey, 1978; Wolff
et al., 1979a; Roboz et al., 1982).
6.6 Reaction with body components
6.6.1 Animal studies
When a 14C-PBB mixture (FireMaster(R)) was incubated with
rat liver microsomes, no binding to exogenous DNA was detected, and
only a small amount of radioactivity was covalently bound to
microsomal protein (Dannan et al., 1978b). The formation of low and
high relative molecular mass adducts with PBB metabolites has been
quoted elsewhere (section 6.3).
6.6.2 Human studies
Studies on human serum revealed that lipoproteins are the
predominant protein carriers of PBBs in serum (Greaves et al.,
1983). 80% of the PBBs were bound to apolipoproteins B and A in a 4
: 1 ratio (Greaves et al., 1984; Roboz et al, 1985a). According to
Roboz et al. (1985b), the ratio was 3 : 1. No preferential binding
of PBB congeners (2,2',4,4',5,5'-hexa-; 2,2',4,5,5'-penta-;
2,2',4,5',6-pentabromobiphenyl) was found (Roboz et al., 1985a,b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only few data are available on effects of PBBs on organisms in
the environment. They refer to microorganisms, water fleas,
waterbirds, (rodents), and farm animals.
7.1 Microorganisms
The toxicity of technical decabromobiphenyl (Adine 0102)
against bacteria (Pseudomonas putida M.) was determined by the
cell multiplication inhibition test (according to the ISO TC 147/SC
5/WG 1/N 111 standard, and using 0.1-1% DMSO (dimethylsulf oxide) as
a solvent. An EC10 of 53 mg/litre was found (Atochem, 1990).
7.2 Aquatic organisms
In a short-term test (according to the ISO standard 6341) the
immobilization of Daphnia magna (Crustacea) by technical
decabromobiphenyl (Adine 0102) has been investigated. The following
results were obtained after dissolution of the test material in DMSO
because of its weak solubility in water:
- EC50 (24 h): > 66 mg/litre;
- The maximum concentration resulting in 0% immobilization
24 h): < 2 mg/litre (Atochem, 1990).
7.3 Terrestrial organisms
7.3.1 Wildlife
In 1977 and 1978, Haseltine et al. (1981) and Heinz et al.
(1983) studied red-breasted mergansers (Mergus serrator) nesting
on islands in the northwestern Lake Michigan in order to determine
whether environmental contaminants (organochlorines and metals) were
producing effects on reproduction. Seventeen contaminants, including
PBBs, were measured in randomly chosen eggs from 206 nests under
study. Using a variety of statistical approaches, Heinz et al.
(1983) looked for effects of individual contaminants and
combinations of contaminants on reproductive measurements, such as
nest desertion, failure of eggs to hatch, death of newly hatched
ducklings, percentage hatching success, number of ducklings leaving
the nest, and egg-shell thickness. PBBs and other chemicals were
sometimes negatively correlated with shell thickness or thickness
index, but not consistently so, as found for DDE. However, no
contaminant or combination of contaminants measured seemed to have a
pronounced effect on the aspects of reproduction mentioned above.
The hatching success of the mergansers averaged 81.7% in 1977.
According to Heinz et al. (1983), the hatching success averaged
85.6% in 1978 (Haseltine et al., 1981).
Although it is not valid to compare reproductive success among
different species, it should be noted that dabbling ducks, whose
eggs contained only a fraction of the contaminant burdens (e.g., no
PBBs: Haseltine et al., 1981; see also Table 32), of the redbreasted
mergansers had better hatching success (Heinz et al., 1983).
An interesting observation on rodents living on a
PBB-contaminated farm has been reported by Jackson & Halbert (1974).
They observed that rats and mice were apparently eradicated when
they came into contact with PBB-contaminated cattle feed pellets.
7.3.2 Farm animals
Farm animals in Michigan that ingested feed inadvertently
containing PBB (FireMaster(R) FF-1) in place of magnesium oxide
fell sick. First symptoms were observed in the cattle of several
dairy herds and revealed this so-called "Michigan PBB incident".
7.3.2.1 Cattle
The exact doses of PBBs to which Michigan cattle were exposed
are not known. Fries (1985b) estimated the maximum PBB doses of
individual cattle based on milk or tissue fat concentration at the
time of detection, which ranged from 9 to 24 months after the cattle
had consumed contaminated feed. For example, if PBB were detected 9
months after exposure, PBB concentrations of 0.1 mg/litre of milk
and 0.25 mg/kg tissue would indicate that the cow had received a
total dose of 1 mg/kg of body weight. Increased PBB concentrations
in milk and tissue would indicate a proportionally higher dose. If
detection were delayed for as long as 24 months, the above example
for milk and tissue concen trations would indicate an exposure of
6 mg/kg of body weight.
a) High-level contamination in cattle
Adverse effects of PBBs in lactating cows were first reported
by Jackson & Halbert (1974).
In reconstructing the accident, it was assumed that the cows on
this farm (Halbert farm) consumed PBB-contaminated feed (PBB content
from 3 to 4 g/kg; Isleib & Whitehead, 1975) over 16 days, and
ingested about 20 g PBB/day, initially. The total average exposure
of these cows was estimated to be 250 mg/kg body weight (Fries,
1985b). The contaminated feed was also fed to a group of 6- to
18-month-old calves. The dose might have been about 58 mg/kg body
weight per day when feeding started, and the total doses may have
reached 700 mg/kg body weight over 6 weeks (Fries, 1985b), if feed
was consumed at the same rates as in experimental studies (Durst
et al., 1977).
The clinical signs of toxicity, described by Jackson & Halbert
(1974), were anorexia (50% reduction in feed consumption) and a 40%
decrease in milk production a few weeks after ingestion of the
contaminated feed. Although the supplemented feed was discontinued
within 16 days, milk production was not restored, and the cows
continued to lose weight. An unspecified number of cows had
increased frequency of urination and lacrimation and developed
haematomas, abscesses, abnormal hoof growth, lameness, alopecia,
hyperkeratosis, and cachexia, and several died within 6 months.
Altogether, the death rate of Halbert cows was about 24/400.
The death rate of the 6- to 18-month-old calves (heifers and
bulls) to which the suspected feed was offered, was much higher.
About 50% of the calves died within 6 weeks. After 5 months, only
two of twelve animals were alive, and they had developed
hyperkeratosis over their entire bodies. There were also a variety
of reproductive problems including embryo resorption, abortions,
stillbirths, deaths shortly after births, delayed deliveries, and
enlarged calves. The clinical signs were variable, and, with the
exception of decreased milk production and weight loss, no
particular symptom was predominant in the affected animals (Jackson
& Halbert, 1974; Robertson & Chynoweth, 1975).
Necropsy findings have been reported for some of the 24 mature
cows that died during the 6 months following exposure (Jackson &
Halbert, 1974). Gross lesions observed included somewhat enlarged
livers, haematomas and abscesses in the thoracic and abdominal
cavities, abomasal ulcers, necrotic metritis, suppurative
bronchopneumonia, and pericarditis. As in all observations without
controls, it was difficult to draw definitive conclusions as to
which lesions were caused by PBBs and which were unrelated to PBBs
(Fries, 1985b). Two of the twelve calves in a calf feeding trial had
massive liver abscesses.
Histopathological studies on ten of the cows revealed various
liver and kidney changes. Liver lesions were reported in seven
animals and included fatty changes and amyloidosis. Renal tubular
nephrosis and interstitial nephritis were reported in four of the
cows (Jackson & Halbert, 1974; Getty et al., 1977).
Several clinical signs and pathological changes, reported by
Jackson & Halbert (1974), were also described in cows in controlled
feeding studies (Durst et al., 1977; Durst et al., 1978a,b; Moorhead
et al., 1977, 1978; Robl et al., 1978; Willett et al., 1980; see
also section 8). These included anorexia, dehydration, excessive
lacrimation, emaciation, hyperkeratosis, reproductive difficulties
(fetal death, enlarged calves, and difficulty in calving,
hypospermatogenesis), and renal damage. Conditions described in the
accidentally exposed cows, but not confirmed in the controlled
studies, included haematomas, abscesses, abnormal hoof growth,
extensive hair loss, liver abscesses, necrosis, and metritis (Fries,
1983, 1985b).
The Halbert herd was one of about 12 "highly" contaminated
herds that had PBB concentrations of more than 30 mg/kg in milk fat,
when detected. According to Fries (1985b), it is likely that all of
these herds had some clinical signs of toxicosis. According to the
same author, there was no comprehensive clinical examination of any
herd that had milk concentrations in the range of 1-30 mg/litre, but
it appears that most animals in these herds did not have clinical
signs when the farms were depopulated. A preliminary report (cited
by Getty et al., 1977) indicated that cows that had been exposed to
PBBs 19 months earlier had levels of up to 80 mg/kg in body fat, but
were apparently normal clinically and were producing normal
quantities of milk. Gross or microscopic lesions that could be
attributed to PBB exposure were not found.
b) Low-level contamination in cattle
Residue concentrations in the body fat or milk fat of cows,
classed as having low-level contamination, rarely exceeded 1 mg/kg,
and, for the most part, not even 0.3 mg/kg. There were several
studies on Michigan cattle with such low exposures.
Results of an evaluation of 72 low-level contaminated herds
have been reported by Kay (1977) and Getty et al. (1977).
Production drop and sterility were two consistent signs and were
regarded as interrelated. The retardation of growth of young stock
was very significant, as it was in the Jackson & Halbert study
(1974). Some other findings were not consistent. A mail
questionnaire survey (Getty et al., 1977) showed similar
observations.
Cows (n = 46) from six herds across Michigan, whose body fat
contained a mean concentration of 0.31 mg/kg and a maximum
concentration of 1.8 mg/kg, were compared with a group of cows from
Wisconsin (n = 40) that had not been exposed to PBB. The two groups
were reared together and subjected to the same feeding and
management system. There were no significant differences in the
animals' milk production, body weight, weight gain, breeding and
reproductive performance, incidence of commonly experienced health
problems, calving rate, and the health of their calves. Also no
pattern of gross or histopathological lesions was seen between test
animals and control animals upon necropsy (Wastell et al., 1978).
An epidemiological survey, which compared the health status of
16 herds with low PBB exposure (traces to 1 mg/kg body fat or milk
fat) with the status of 15 herds with no PBB exposure, also
indicated that productivity and general health conditions between
the two groups of herds were similar. Of the biochemical parameters
tested (9 urinalyses, 13 serum chemistry parameters), three resulted
in significantly different values. Serum concen trations of calcium,
glucose, and cholesterol in contaminated herds were significantly
lower than those of the control herds. But the relationship to PBB
exposure was unknown (Mercer et al., 1976).
Instead of specific clinical conditions, Fries (1983) evaluated
the overall performance of exposed herds (residues in tissue or milk
fat generally < 0.3 mg/kg) and of "relatively unexposed" herds
(residues < 0.02 mg/kg) of comparable size, breed, and location by
analysing Dairy Herd Improvement Association records. He found that
no productive or reproductive character istics of the herds were
affected by PBB exposure.
It should be noted that the classification of herds as having a
high or a low level of contamination refers to PBB levels at the
time of detection. Thus, it is sometimes impossible to know whether
there was a history of a short-term, high exposure or a long-term,
low exposure, which may produce different syndromes. For example,
Fries (1985b) pointed out that feed that was contaminated by
cross-contamination in the feed mills was being fed at the time of
detection, in some cases. Under this circum stance, PBB intakes as
low as 0.1 mg/kg of body weight per day could produce milk fat
residues as high as 20 mg/kg (Fries & Marrow, 1975; Fries 1985b).
7.3.2.2 Other farm animals
Although it was cattle feed that was originally involved in the
accidental substitution, all other feeds became involved by cross
contamination, e.g., in the mixing machinery of feed companies that
had been exposed to PBB (Dunckel, 1975). It is likely that other
animals were not exposed to the same high levels as cattle.
(i) Poultry
There are no reports of clinical signs or problems associated
with the accidental contamination of poultry feed. However, some
controlled feeding studies have been published (see section 8).
(ii) Pig
Adverse health effects in pigs, identified as contaminated,
were rarely reported. Only one review (Reggiani & Bruppacher, 1985)
mentioned that abortions occurred in pigs. Two controlled feeding
studies (Ku et al., 1978; Werner & Sleight, 1981) have been
conducted (see section 8).
(iii) Horse, rabbit, goat, sheep
Other species of farm animals, including at least 2 horses, 32
rabbits, 2 goats, and 19 flocks of sheep, were identified as
contaminated and buried at Kalkaska, but details of ill effects were
not recorded (Dunckel, 1975; Getty et al., 1977).
One experimental feeding study on sheep (Gutenmann & Lisk,
1975) is available (see section 8).
7.4 Population and ecosystem effects
No information available.
7.5 Effects on the abiotic environment
No information available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Differences in toxic responses between acute, high-level
exposures and long-term, low-level exposures to halogenated aromatic
compounds are mainly quantitative (McConnell & Moore, 1979).
Moreover, the delayed onset of toxic signs and the persistence of
PBBs in the body, which may cause long-term exposure of the target
organs from a single dose, "tend to blur the usual distinctions that
are made between acute and chronic exposure" (Fries, 1985b). For
these reasons, symptoms after single and short-term exposures are
reviewed together in section 8.2. Another feature of the toxicity
of PBBs and related classes of compounds is the latent period
between the time of exposure and the time of death, which ranged
from several days to weeks (Di Carlo et al., 1978; Safe, 1984;
Hutzinger et al., 1985a; McConnell, 1985). Thus, the classical
LD50 values and other mortality data found are summarized in one
section (8.1).
8.1 Lethality
"Acute" toxicity data (LD50 and LC50 values) of commercial
PBB mixtures have been compiled in Table 70. The LD50 values of
all mixtures show a relatively low order of acute toxicity (LD50
> 1 g/kg body weight) in rats, rabbits and quails, regardless of
the route of administration, and range from > 1 to 21.5 g/kg body
weight. Regarding the LC50 values for minks, this species may be
highly sensitive to PBBs, but a direct comparison is complicated by
differences in the experimental design.
As with TCDD and PCBs (McConnell, 1984; Safe, 1984), the
apparent toxicity of PBBs is higher with multiple-dose rather than
single-dose administration. For example, the single oral LD50 of
FireMaster(R) in rats was quoted to be 21.5 g/kg body weight, but,
if given in small repeated doses, the total lethal dose was
approximately 1-3 g/kg body weight (see Table 70).
Deaths after exposure to PBBs are delayed (Di Carlo et al.,
1978; Tables 71 and 72). Thus, Gupta & Moore (1979) have recommended
that the LD50 of halogenated aromatic hydrocarbons or chemicals
that may have a long-term build-up with delayed toxicity should be
determined more accurately after multiple dosing and an extended
period of observation.
Generally, the susceptibility to the toxic effects of aryl
hydrocarbons is dependent on the sex, age, species, and strain of
the experimental animals (e.g., Safe, 1984; Hutzinger et al.,
1985b). In the case of PBBs, female rats dosed with FireMaster(R)
FF-1 showed a lower LD50 than male rats (Gupta & Moore, 1979;
Table 70).
Table 70. Toxicity of PBB mixtures
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) rat oral LD50 21.5 single dose Di Carlo
et al.
(1978)
FireMaster(R) FF1 rat (Fischer- female oral 90 days LD50 1.43 22 doses Gupta &
(Lot No. 1312 FT) 344/N) (over 30 Moore
(in corn oil) male oral 90 days LD50 3.28 days) 22 (1979)
doses (over
30 days)
FireMaster(R) BP-6 rabbit dermal LD50 5 Aftosmis
et al. (1972b)
FireMaster(R) rabbit male dermal 14 days ALD 5 24 h of Waritz et al.
(Lot 635-71) (New Zealand, exposure (1977)
(in corn oil) albino)
FireMaster(R) FF1 mink male, in feed 313 days LC50 3.95 Ringer et al.
(Mustela vison) female (1981)
FireMaster(R) Bobwhite quail in feed 8 days LC50 428 5 days of Cottrell
(Colinus treated diet et al. (1984)
virginianus)
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) Japanese quail oral not LD50 > 1 Strik
(1973a)
specified
Octabromobiphenyl rat male oral LD50 2 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl rat male oral 7 days ALD > 17 single or Waritz et al.
(Dow Lot 102-7-72) (Sprague-Dawley) repeated dose (1977)
(in acetone: corn
oil = 15:85)
Octabromobiphenyl rabbit dermal LD50 > 10 Aftosmis
et al. (1972b)
Octabromobiphenyl rabbit (New male dermal 14 days ALD > 10 24 h of Waritz et al.
(Dow Lot 102-7-72) Zealand albino) exposure (1977)
Octabromobiphenyl Japanese quail oral LD50 > 12.5 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl Bobwhite quail male, oral 14 days ALD > 12.5 single dose Waritz et al.
(Dow Lot 102-7-72) female (1977)
(in corn oil)
Nonabromobiphenyl mouse (B6C3F1) male, oral 14 days LD50 > 15 single dose Momma (1986)
(Bromkal 80-9D) female
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rat male, oral 14 days LD50 > 5 single dose Millischer
(in corn oil) (Sprague-Dawley female et al.
CFY) dermal 14 days LD50 > 5 single dose (1979)
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rabbit dermal LD50 > 8 c
a ALD = Approximate lethal dose.
b LD50 or ALD in g/kg body weight; LC50 in mg/kg diet (ppm).
c Consumer Product Testing Co. (1977) quoted by Di Carlo et al. (1978).
Table 71. Mortality associated with PBB administration: Commercial mixtures (dosing studies)
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF1 rat male oral 0.03-30 60 days 22 doses no mortality Luster et al.
(No. 1312 FT) over 30 (1978)
(in corn oil)
rat female oral 0.1-10 6 months 122 doses no mortality Luster et al.
(Fisher) over 6 (1980)
months
rat female oral 100 90 days 22 doses 100% 41-53 Gupta & Moore
(Fisher over 30 (1979)
344/N) days
male oral 100 90 days 22 doses 38% 50-73 Gupta & Moore
over 30 days (1979)
female, oral 300 90 days 9-21 doses 100% 14-29 Gupta & Moore
male (1979)
female, oral 1000 90 days 6-10 doses 100% 9-14 Gupta & Moore
male (1979)
FM BP-6 rat inhalation 71 mg not 1 h of no mortality Di Carlo et al.
per specified exposure (1978)
litre
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF-1 mouse female oral 0.03-30 60 days 22 doses no mortality Luster et al.
(Lot No. over 30 (1978)
1312 FT in days
corn oil) mouse female oral 0.1-10 6 months 122 doses no mortality Luster et al.
over 6 months (1980)
FM guinea-pig oral 50 60 days single dose no mortality Ecobichon
(Hartley), mentioned et al. (1983)
albino
FM BP-6 cattle oral 25 60 days daily doses 100% (animals 33-60 Irving et al.
(finely g/dayc became moribund (1976);Durst
ground) and were et al. (1977);
necropsied) Moorhead et al.
(1977, 1978)
FM BP-6 cattle female oral 250 180 days daily doses no mortality Durst et al.
(finely mg/day (1978b)
ground)
cattle female oral 0.3d 340 days daily no mortality Robl et al.
doses over (1978)
158-228
days
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM BP-6 rainbow trout intraperitoneal 150 15 days single dose no mortality Elcombe & Lech
(in corn oil) (Oncorhynchus mentioned (1978)
mykiss)
FM BP-6 brook trout oral 66.3 18 days 3 doses over no mortality Law & Addison
(in dogfish (Salvelinus 5 days mentioned (1981)
oil) fontinalis)
FM FF-1 sheepshead intraperitoneal 15 56 days single dose no mortality James & Little
(Archosargus (1981)
probatocephalus) 50 4-5 days single dose 2/5 (remaining James & Little
3 moribund)
OcBB rat female oral 126- 14 days single dose no mortality Norris et al.
(in corn oil) Sprague- 2000 (1973)
Dawley)
OcBB rat male oral 1000 28 days single dose no mortality Lee et al.
Sprague- (1975b)
Dawley)
OcBB rat male inhalation 0.96 7 days 4 h of no mortality Waritz et al.
(Dow Lot (Sprague- mg/ exposure (1977)
102-7-72) Dawley) litre
air
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
DeBB rat inhalation 200 not 1 h of no mortality Di Carlo
mg/ specified exposure et (1978)
litre
air
DeBB rat male, inhalation 0.05-5 4 weeks 6 h/day; no mortality Millischer
(Sprague- mg/ 5 days/week et al. (1979)
Dawley) litre
air
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b In mg/kg body weight per day, unless otherwise specified.
c Initially equivalent to 67.2 mg/kg body weight per day.
d Equivalent to 10 mg/kg feed.
Table 72. Mortality associated with PBB administration: Commercial mixtures (feeding studies)
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 rat (Sprague- female 4.7-300 2/2 weeks no mortality Dent et al. (1976a)
Dawley) mentioned
FM rat (Sprague- male 1-500 30/30 days no mortality Sleight & Sanger (1976)
Dawley)
FM BP-6 mouse (BALB/c) female 1000 9/9 days 70% Fraker & Aust (1978);
Fraker (1980)
FM BP-6 guinea-pig 50 45/45 7/8 Vos & van Genderen
(Lot No. 182 RP) (1974)
FM guinea-pig 100 30/30 days 4/6 Sleight & Sanger (1976)
guinea-pig 500 15/15 days 100%
FM FF-1 mink male, 1 313/313 days no mortality Aulerich & Ringer (1979)
(Mustela vison) female 2.5 10% 136 days
90% 63-294 days Ringer et al. (1981)
100% 25-93 days
FM pig 20, 200 16/16 weeks no mortality Ku et al. (1978)
mentioned
FM FF-1 rhesus monkey male 25 25/25 weeks 1/1 25 weeks Allen et al. (1978)
(adult) (total: 1 g)
(juvenile) female 300 137/137 days 1/1 137 days Allen et al. (1978)
(total:
6.4 g)
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 chicks (Hubbard male 400 15/15 days 37% Vos & van Genderen
(Lot No. 182 RP) Leghorn) (1974)
FM BP-6 chicken (White 20-200 8/16 weeks no mortality Cecil & Bitman (1978)
Leghorn)
4/4 weeks 60% (control 10%) Cecil & Bitman (1978)
FM FF-1 chicken (White 3125 4/4 weeks 100% Polin & Ringer (1978a)
Leghorn)
FM Japanese quail 10-100 no mortality Babish et al. (1975a)
500-1000 "few" days 100% Babish et al. (1975a)
FM Bobwhite quail 100 5/8 days no mortality Cottrell et al. (1984)
200 5/8 days 1/10 8 days Cottrell et al. (1984)
600 5/8 days 100% (10/10) 5.3 days Cottrell et al. (1984)
(mean)
FM BP-6 Atlantic salmon 100 42/70 days no mortality Zitko (1977)
(Salmo salar) (in excess of
that of
control fish)
OcBB rat (Sprague- male 100-10 000 30/30 days no mortality Norris et al. (1973)
Dawley) mentioned
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
OcBB rat (Sprague- male 1-1000 4/22 weeks no mortality Lee et al. (1975a)
Dawley)
OcBB (Dow Atlantic salmon 100 74/96 days no mortality (in Zitko (1977)
Chemical) (Salmo salar) excess of that of
control fish)
DeBB rat (Sprague- female, 1-2000 90/90 days no mortality Millischer et al.
Dawley) male mentioned (1979)
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
Although the studies, in most cases, are not directly
comparable, species differences in the response to PBBs are
reflected, in part, by the mortality data collated in Tables 71 and
72. For example, minks appear to be more sensitive to the
FireMaster(R) mixture than the other species of animals tested.
Compared to rats, guinea-pigs were far more susceptible to
FireMaster(R) (Table 72).
The few studies performed with commercial octa- and
decabromobiphenyl mixtures did not result in any mortality in rats
and fish (Tables 71 and 72). Of individual PBB congeners, only three
hexa isomers have been tested. Apparently, 3,3',4,4',5,5'-
hexabromobiphenyl is more toxic for rats than
2,2',4,4',5,5'-hexabromobiphenyl (Table 73).
With few exceptions, the cause of death by halogenated aryl
hydrocarbons cannot usually be ascribed to pathology in a given
organ or system as with most toxicants (McConnell & Moore, 1979;
McConnell, 1984). At lethal doses, the affected animals develop, as
a first indication of toxicity, a "wasting syndrome", i.e., a
progressive loss of body weight, which may not be related simply to
decreased food consumption. It is followed by weakness,
debilitation, and finally death (Hutzinger et al., 1985a; McConnell,
1985). Some authors (Allen et al., 1978) use the term "metabolic
death".
The extended course of the disease can be complicated by other
diseases, usually of an infectious etiology (McConnell, 1984). For
instance, parasitism, diarrhoea, and pulmonary infections have been
observed in PBB-contaminated cattle (Jackson & Halbert, 1974;
Willett & Irving, 1976; Moorhead et al., 1978). Many of the pigs
that died during lactation after perinatal exposure to PBB had acute
suppurative pneumonia (Werner & Sleight, 1981). Severely intoxicated
rhesus monkeys may have succumbed finally to gastrointestinal and
respiratory infections (Allen et al., 1978). Male rats that were
given 100 mg FireMaster(R) FF-1/kg body weight per day and died
after 90 days had liver lesions (Gupta & Moore, 1979). Such
overlying disease problems often result in difficulties in
establishing a definitive etiological diagnosis in environmental
exposures (McConnell, 1984).
Table 73. Mortality associated with PBB administration: Individual PBB congeners
PBB Species Sex Exposure Dose Mortality (in percentage References
concentration or No. dead/No. treated)
2,2',4,4',5,5'- rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
mouse (B6C3F1) female in diet for 10 days 750a 2/8 Welsch &
(pregnant) Morgan (1985)
sheepshead intraperitoneal; single 20b no mortality James & Little
(Archosargus or multiple doses; (1981)
probatocephalus observation: 17-40 days
3,3',4,4',5,5'- rat (Sprague-Dawley) male in diet for 20 days 100a 1/2 (the remaining Render et al.
hexabromobiphenyl rat was moribund) (1982)
2,3',4,4',5,5' rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
Mixture of di-, Atlantic salmon in diet for 40 days plus 7.75a (100% mortality) Zitko &
tri-, and (Salmo salar) additional stress at day Hutzinger (1976)
tetrabromobiphenyls 4; observation: 42 days
a In mg/kg feed.
b In mg/kg body weight per day.
8.2 Single and short-term exposures: general signs of toxicity
8.2.1 PBB mixtures
8.2.1.1 Overt clinical signs, food intake, and body weight changes
In many acute and short-term studies, signs of PBB toxicity
include reduction in feed consumption and weight loss or a decreased
weight gain (see Tables 74 and 75). Most of the reports refer to the
FireMaster(R) mixture, which was tested in rats, mice,
guinea-pigs, pigs, cattle and avian species. From these studies with
various protocols, the minimum effective doses of FireMaster(R)
(FM) in the diet or by gavage ranged between 0.3 and 500 mg FM/kg
feed or between 4 and 100 mg FM/kg body weight per day,
respectively. Only a few studies have been performed with technical
octabromobiphenyl and technical decabromobiphenyl; no effects were
observed on feed consumption or body weight of rats (Tables 74 and
75).
Weight loss is not necessarily accompanied by decreased food
intake (e.g., Allen et al., 1978; Lambrecht et al., 1978; Gupta &
Moore, 1979) suggesting that PBBs may cause poor feed utiliz ation.
On the other hand, increased efficiency of feed utilization has been
reported in growing pigs (Ku et al., 1978) and weight loss in hens
was accounted for completely by reductions in feed consumption in
paired feeding studies (Cecil & Bitman, 1978; Ringer, 1978). If the
concentrations of PBBs are high enough, a total refusal of feed will
occur (Babish et al., 1975a; Gutenmann & Lisk, 1975; Ringer & Polin,
1977; Cecil & Bitman, 1978; Polin & Ringer, 1978a; Aulerich &
Ringer, 1979). At death, the loss in body weight can be as great as
30-40% (Allen et al., 1978; Aulerich & Ringer, 1979; Durst et al.,
1978b).
At sublethal doses, decreased food intake and weight loss may
be the only overt signs observed in many species. However, calves
(fed 100 mg FireMaster(R) FF-1/kg body weight per day) also
developed keratitis and lacrimation from day 35, and alopecia from
day 50 (Robl et al; 1978). During a 16-week test period, some pigs
receiving FireMaster(R) BP-6 at 200 mg/kg in the diet had
dermatosis (Ku et al., 1978). Loss of hair (including eye lashes),
dry scaly skin, and periorbital oedema were reported in a juvenile
female rhesus monkey given 25 mg FireMaster(R) FF-1/kg feed for 50
weeks (total dose: approximately 1.5 g), however, the time of onset
of these symptoms was not specified (Allen et al., 1978). A
decreased heart rate (bradycardia) was measured in White Leghorn
cockerels fed 150 mg FireMaster(R) FF-1/kg feed for approximately
9 weeks (Heinemann & Ringer, 1976; Ringer, 1978). Another
characteristic sign in chickens was general oedema (Ringer & Polin,
1977; see also section 8.2.1.3).
Table 74. Effects on feed consumption and changes in body and organ weights after single or short-term exposure to commercial
PBB mixtures (dosing studies)
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 rat male, oral single dose 1000 n.r. no effect liver: increase Kimbrough
(in peanut oil) (Sherman) female t.p.: 2 months et al.
(1978)
M BP-6 rat male oral single dose 500 n.r. -- liver: increase Bernert Jr &
(Lot No. 5143 (Sherman) t.p.: 1-8 weeks Groce (1984)
in corn oil)
FM BP-6 rat female oral daily doses (9) 1 no effect no effect liver: increase; spleen Harris
(in olive oil) (Sprague- during pregnancy (mg/day) kidney, adrenal, ovary et al.
(1978a)
Dawley) t.p.: 13 days gravid uterus, perirenal
(after first dose) fat pads: no effect
FM FF-1 rat male oral multiple doses 3 n.r. no effect thymus spleen: decrease Luster
(Lot No. (Fischer) (22) over 30 days et al.
1312 FT) t.p.: 60 days 30 n.r. reduced thymus, spleen: decrease (1978)
(in corn oil) (after first dose)
FM FF-1 rat male, oral multiple doses 100-1000 reduced reduced liver, spleen: increase Gupta &
(Lot No. (Fischer female (22) over 30 days (day 10) thymus: decrease Moore (1979)
(1312 FT) 344/N) t.p.: up to 90 days
(in corn oil)
male 30 no effect reduced -- Gupta &
(day 15) Moore (1979)
female 30 variable reduced --
(day 10)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
male, 3 -- -- liver: increase (day 15) Gupta et al.
female 30 no effect thymus: decrease (day (1981)
reduced 15); lung, heart, spleen,
(3 week) kidney, adrenal, thyroid,
testis, ovary, uterus,
brain: no effect
FM FF-1 rat male oral multiple doses 1-6 n.r. no effect liver, thyroid: increase Allen-Rowlands
(Lot No. (Sprague- (20) t.p.: 4 weeks adrenal, testis: et al. (1981);
1312 FT) Dawley) (after 1 dose) no effect Castracane
(in lecithin et al. (1982)
liposomes)
FM rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
(in corn oil) single dose et al. (1972b)
t.p.: 7 days
FM BP-6 rat female intraperitoneal n.r. no effect liver: increase Dent et al.
(in peanut oil) (Sprague- single dose 150 (1976b)
Dawley) t.p.: 2 weeks
FM FF-1 rat female intraperitoneal 200-1000 n.r. no effect liver: increase Goldstein
(in corn oil) (Fischer) single dose (µmol/kg et al. (1979)
t.p.: 4 days body
weight)
FM FF-1 rat male intraperitoneal 90
(in (Sprague- single dose
polyethylene Dawley) t.p.: 1 week n.r. n.r. liver: increase Dannan et al.
glycol) (1978a)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
t.p.: 2 weeks n.r. no effect liver: increase; Dannan et al.
no effect spleen, thymus: (1982c);
Millis et al.
(1985a)
FM BP-6 rat male intraperitoneal (days 30 n.r. n.r. liver, spleen: increase Robertson
(Lot 7062) (Wistar) 1 and 3) two doses 75 n.r. n.r. liver: increase; spleen, et al. (1981b)
(in corn oil) t.p.: 6 days thymus: decrease
(after 1 dose)
FM BP-6 rat male intraperitoneal three 0.2 n.r. n.r. liver: increase Ecobichon
(in peanut (Wistar) doses (days 1, 2, 3) mmol/kg et al. (1979)
oil) t.p.: 7 days body
(after 1 dose) weight
FM BP-6 rat male intraperitoneal single 500 n.r. reduced liver: increase; Andres et al.
(in corn oil) (Wistar) or multiple doses (4) thymus, spleen: decrease (1983)
t.p.: 15 days
FM FF-1 mouse female oral multiple doses 30 n.r. no effect spleen: decrease Luster et al.
(Lot No. (B6C3F1) (22) over 30 days thymus: no effect (1978)
1312 FT) t.p.: 60 days
(in corn oil) (after 1 dose)
mouse female oral multiple doses 30 n.r. no effect spleen: increase; Luster et al.
(B6C3F1) (22) from gestational thymus: decrease (1980)
(pregnant) day 0 until litters
were weaned
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 mouse male, oral multiple doses 3-30 no effect variable liver: increase (day 15) Gupta et al.
(Lot No. (B6C3F1/N) female (22) over 30 days lung, heart, spleen, (1981)
FT 1312) t.p.: up to 90 days kidney, adrenal thyroid
(in corn oil) testis, ovary, uterus,
brain: no effect
male 30 no effect reduced thymus: decrease
(only day (only day 30)
30)
female 0.3, 3, no effect increased thymus: no effect
30 (only
day 45)
FM BP-6 mouse female intraperitoneal single 150 n.r. n.r. liver: increase (48, Dent et al.
(in peanut oil) (NMRI) dose t.p.: up to 192 h 96 h), no effect (24, (1977a)
192 h)
FM BP-6 mouse male intraperitoneal 1.50 n.r. no effect liver: increase; Robertson
(in corn oil) (C57BL/6J) single dose (mmol/kg thymus: decrease; et al. (1984c)
t.p.: 5 days body spleen: no effect
weight)
mouse male intraperitoneal 1.50 n.r. no effect liver: increase;
(DBA/2J) single dose (mmol/kg thymus, spleen:
t.p.: 5 days body no effect
weight)
FM FF-1 guinea-pig female oral single dose 50 n.r. no effect "tissues": no effect Ecobichon
(Lot FA (Hartley, t.p.: 2-60 days (liver, kidney, lung, et al. (1983)
7042) (in albino) perirenal fat)
peanut oil) (pregnant)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM BP-6 guinea-pig male intraperitoneal single 50 n.r. -- liver, kidney: no effect Smith
(in peanut oil) Hartley dose t.p.: 120 h et al.
(1986)
hamster male intraperitoneal single 50 n.r. -- liver, kidney: no effect
(golden dose t.p.: 120 h
Syrian)
FM (Lot rabbit male dermal 24 h of 100- n.r. reduced liver: increase Waritz
635-71) (New exposure t.p.: 10 000 (not et al.
Zealand, 14 days significant) (1977)
albino)
FM BP-6 calf male oral daily doses 0.54 no effect reduced -- Willett &
(Lot RP-158) for 44 days mg/day Irving
(in milk) (1976)
FM BP-6 cattle female oral daily doses 25 g reduced reduced liver, kidney, perirenal Irving
(Lot (pregnant) per day (day 4) (day 20) lymph nodes: increase et al.
6244 A) thymus: decrease (1976);
(days 33-66) Willett
& Irving
(1976);
Durst
et al.
(1977);
Moorhead
et al.
(1977)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
250 mg no effect no effect no effect
per day
FM FF-1 calf male, oral daily doses 100 reduced reduced -- Robl et al.
female (day 36) (day 35) (1978)
cattle female oral daily doses 0.3 no effect no effect --
for 158 days
t.p.: 340 days
(after 1 dose)
FM FF-1 dog male, oral daily doses 1 n.r. -- liver: increase Farber et al.
(Beagle) female for 7 weeks (1976)
dog male oral daily doses 4 n.r. reduced -- Farber et al.
(Beagle) (day 30) (1978)
FM Japanese oral daily doses 25-1000 n.r. reduced liver: increase Strik (1973b)
quail for 7 days
FM BP-6 rainbow intraperitoneal n.r. n.r. liver: no effect Elcombe &
(in corn oil) trout single dose t.p.: Lech (1978)
(Oncorhynchus up to 15 days
mykiss)
FM BP-6 brook trout oral multiple (200 mg/kg n.r. reduced liver: no effect Law & Addison
(in dogfish (Salvelinus doses t.p.: body (not (1981)
oil) (fontinalis) 18 days (after weight significant)
1 dose) total dose)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
OcBB (in rat female oral single dose 126-2000 n.r. no effect n.r. Norris et al.
corn oil) (Sprague- t.p.: 14 days (1973, 1975)
(Dawley)
OcBB (in rat male oral single dose 1000 no effect no effect liver: increase (days Lee et al.
corn oil) (Sprague- t.p.: 21 days 2-11), no effect (1975b)
Dawley) (day 21)
oral daily doses (2) 3000 no effect no effect liver: increase (up Lee et al.
t.p.: 28 days (after to day 21), no effect (1975b)
lase dose) (day 28)
OcBB (Dow rat male oral single dose 3400- n.r. -- liver: increase Waritz et al.
Lot 102-7-72) (Sprague- t.p.: 7 days 17 000 (1977)
(in 40-50% Dawley)
acetone: corn
oil = 15:85)
OcBB (in rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
corn oil) single dose t.p.: et al. (1972b)
7 days
rat inhalation 4 h 3.1 n.r. n.r. liver: no effect
exposure for µg/litre
10 days air
OcBB rat male inhalation 4 h 0.96 n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot Sprague- exposure mg/litre significant) (1977)
102-7-72) Dawley) t.p.: 7 days air
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat not inhalation (25 g) n.r. n.r. liver: no effect
(Sprague- specified 23 h/day; (2-15 weeks) kidney,
Dawley) 7 days/week for thyroid: no effect
2-15 weeks (15 weeks)
OcBB rabbit male dermal 24 h 100- n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot (New exposure 10 000 significant) (1977)
102-7-72) Zealand; t.p.: 14 days
(in corn oil) albino)
male dermal 6 h/day; 1 n.r. no effect liver: increase
5 days/week, for
2 weeks t.p.:
18 days (after last
dose)
OcBB bobwhite male, oral single dose 12 500 n.r. n.r. any internal organs:
(Dow Lot quail female t.p.: 14 days no effect
102-7-72)
(in corn oil)
NoBB mouse male, oral single dose up to n.r. n.r. liver: increase Momma (1986)
(B6C3F1) female t.p.: 14 days 15 000
DeBB (in rat male, oral single dose 5000 n.r. no effect liver: no effect Millischer
corn oil) (Sprague- female t.p.: 14 days et al. (1979)
Dawley)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat intraperitoneal 1000 n.r. n.r. liver: no effect Aftosmis
single dose et al. (1972b)
t.p.: 7 days
rat male, dermal 24 h 5000 n.r. no effect liver: no effect Millischer
(Sprague- female exposure t.p.: et al. (1979)
Dawley) 14 days
rat male, inhalation 6 h/day, 0.05-5 no effect no effect liver: increase
(Sprague- female 5 days/week for mg/litre
Dawley) 4 weeks air
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; NoBB = nonabromobiphenyl; DeBB = decabromobiphenyl.
b t.p. = time post-exposure.
c In mg/kg body weight per day, unless otherwise specified.
d n.r. = not recorded.
e Absolute or relative to body weight, respectively.
Table 75. Effects on feed consumption and changes in body and organ weights caused by short-term feeding of commercial PBB mixtures
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- female 75, 300 2/2 weeks no effect no effect liver: increase Dent et al.
Dawley) (1976a)
FM rat (Sprague- male 100 30/30 days no effect no effect liver: increase Sleight &
Dawley) Sanger (1976)
500 30/30 days reduced reduced liver: increase;
kidney: no effect
FM BP-6 rat (Sprague- male 50 3/3 weeks no effect no effect liver: increase Babish &
Dawley) Stoeswand
(1977)
FM BP-6 rat (Sprague- female 50 day 8 of n.r. no effect liver: increase Dent et al.
Dawley) gestation through (1977b)
day 14 post-
partum
FM BP-6 rat male 5 variable no effect no effect liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); kidney: decrease et al. (1977)
(week 3)
rat male 500 variable no effect reduced liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); testis: no effect et al. (1977)
(week 3)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- not 50 (in pre-, post- and n.r. n.r. liver: increase Cagen &
Dawley) specified mother's or perinatal Gibson (1978)
pups weanling's exposure/
diet) of age
15-49 age
days
FM BP-6 rat (Sprague- day 8 of
Dawley) gestation to day
15 postpartum
mothers female 50 n.r. no effect liver: increase; kidney, Dent et al.
mammary: no effect (1978b)
pups male, (50) (in pre- post-, and n.r. no effect liver: increase
female mother's perinatal
diet) exposure
FM BP-6 rat (Sprague- male, (mothers perinatal n.r. reduced liver, spleen: increase Harris et al.
Dawley) female dose: 10 exposure (day 3 of (day 60 of age) (males (1978a)
pups mg/day on age) more affected than
gestation females
days 7-15)
adults males 50-100 10/10 weeks no effect no effect liver: increase Harris et al.
150, 200 10/10 weeks no effect reduced liver: increase; adrenal, (1978b)
spleen, kidney, testes,
seminal vesicles: no
effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
female 600 10/10 weeks reduced reduced n.r.
FM BP-6 rat male 50-500 5/5 weeks n.r. no effect liver: increase Kasza et al.
(Holtzman) (1978a)
FM BP-6 rat (Sprague- male 20 28/28 days no effect no effect liver: increase Chu et al.
Dawley) (1980)
FM BP-6 rat (Sprague- male, 100 (in perinatal n.r. reduced M: ventral Johnston
Dawley) mothers or exposure until (more prostate: decrease et al. (1980)
pups weanlings 9 weeks of age pronounced
diet) in males)
FM BP-6 rat (Sprague- male 1-100 30/30 days no effect no effect liver: increase; thymus, Akoso et al.
Dawley) spleen: no effect (1982a)
male 100 30/30 days no effect no effect thyroid: increase Akoso et al.
(1982b)
FM BP-6 rat (Sprague- male 100 9/9 days no effect no effect liver, thyroid: increase Render et al.
Dawley) kidney: no effect (1982)
FM BP-6 rat (Fisher) male 100 10/10 days no effect no effect liver: increase Raber &
Carter (1986)
FM BP-6 mouse female 1000 11/11 days n.r. -- liver: increase Corbett
(Swiss, et al. (1975)
ICR) male 1000 up to 14/14 n.r. reduced liver: increase (day 4); Corbett
days (day 4) testis: no effect et al.
(1978a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 mouse female 50-200 2/2 weeks no effect no effect liver: increase Cagen et al.
(Swiss, (1977); Cagen
Webster) & Gibson
adult (1978)
pups not 50 (in postnatal -- -- liver: increase Cagen &
specified mothers exposure until Gibson (1978)
diet) 15 days of age
FM mouse not 100 30/30 days n.r. no effect liver: increase; thymus, Fraker & Aust
(BALB/c) specified spleen: decrease (1978)
not 1000 14/14 days n.r. reduced liver: increase; thymus:
specified decrease (nearly athymic)
FM BP-6 mouse female 1, 10 30/30 days n.r. no effect liver, spleen: no effect; Fraker (1980)
(BALB/c) thymus: decrease
female 100 30/30 days n.r. no effect liver: increase; thymus,
spleen: decrease
FM FF-1 mouse male 5 8/8 weeks n.r. no effect liver: increase Loose et al.
(Lot No. (BALB/c (1981)
7042) ByJ) 167 6/6 weeks n.r. no effect liver: increase
167 8/8 weeks n.r. reduced liver: increase
5 3/3 weeks n.r. -- thymus: no effect
5 6-8/6-8 weeks n.r. -- thymus: decrease
167 3-8/3-8 weeks n.r. -- thymus, spleen: decrease
5, 167 3-8/3-8 weeks n.r. -- lung: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mouse male mothers dose: perinatal n.r. no effect spleen: increase; Luster et al.
(Lot No. (B6C3F1) 10 mg/kg exposure thymus: no effect (1980)
1312 FT) pups body weight
per day
(22 doses)
FM BP-6 guinea-pig not 10 45/45 days n.r. no effect liver: increase Vos & van
specified Genderen
(1974)
FM guinea-pig not 1, 10 30/30 days no effect no effect liver: no consistent Sleight &
specified effect Sanger (1976)
100 up to 30 days n.r. reduced liver: increase
(week 3)
500 15/15 days n.r. reduced liver: increase
(week 1)
FM BP-6 pig not 20, 200 16/16 weeks reduced reduced liver, kidney: increase Ku et al.
(growing) specified (1978)
pig not 100 (in perinatal n.r. reduced liver: increase (but no Werner &
(4-week-old) specified mothers exposure (not effect in sows) Sleight
(lactating) diet) significant) (1981)
pig not 100 (in prenatal -- -- thyroid: increase;
(newborn) specified mothers diet) exposure liver: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mink female 1-1.54 several months -- no effect liver, kidney: increase Aulerich &
2.5 several months -- no effect (in animals that died Ringer
(within first during treatment) (1979);
3 months, Ringer et al.
(1981)
later reduced)
6-16 several months (refused) reduced
FM FF-1 Rhesus female 0.3 several months no effect reduced -- Allen et al.
monkey (1978)
(adult)
(infants) not 0.3 (in perinatal -- reduced --
specified mothers exposure;
diet) up to 12
weeks of age
(juvenile) female 300 up to 137 no effect reduced --
days (until a
few days
prior to
death)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM Japanese male, 500, 1000 a few days (refused) Babish et al.
quail female (1975a)
10-100 9/9 weeks no effect no effect liver: increase
FM Bobwhite 100-700 5/8 days reduced n.r. n.r. Cottrell
quail (Colinus et al. (1984)
virginianus)
FM BP-6 chicks male 400 15/15 days n.r. reduced bursa of Fabricius: Vos & van
(Lot No. (Hubbard decrease Genderen
182 RP) Leghorn) 15, 30 63/63 days n.r. reduced bursa of Fabricius, (1974)
spleen decrease
"PBB" pullet 50, 200 4/4 weeks n.r. reduced -- Chang &
(FM) Zindel (1975)
FM chicken male variable, several weeks -- -- liver, thyroid: Ringer & Polin
(White up to 200 increase; comb: (1977)
Leghorn) decrease; testis:
increase (low dose)
decrease (higher dose)
female 125 several weeks reduced -- --
chicks (White 75 several weeks -- reduced --
Leghorn)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 chicken female 20, 64, 8/16 weeks no effect no effect -- Cecil &
(White 200 2/2 weeks reduced reduced -- Bitman (1978)
Leghorn) 640, 2000 2/2 weeks (refused) reduced --
FM FF-1 chicken female 0-25 5/5 weeks no effect -- -- Polin &
(White 125 5/5 weeks reduced -- -- Ringer
Leghorn) 3125 5/5 weeks refused -- -- (1978a)
FM chicks male 75-250 up to 42 reduced reduced liver, thyroid: Ringer (1978)
(White days increase; comb, testes,
Leghorn) spleen, bursa, thymus:
decrease
FM FF-1 cockerels male 10, 100 28/28 days no effect no effect liver: increase; bursa: Dharma et al.
(White decrease; thyroid, (1982)
Leghorn) spleen, testicles,
comb: no effect
OcBB rat 100 28/28 days -- -- liver: increase Aftosmis
et al.
(1972a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
OcBB rat (Sprague- male 100-1000 30/30 days no effect no effect liver: increase; heart, Norris et al.
Dawley) testes, brain: no (1973, 1975)
1000, 10 000 30/30 days effect; kidney:
increase
OcBB rat (Sprague- male 100, 1000 2-4/2-4 weeks no effect no effect liver: increase Lee et al.
Dawley) (1975a)
1000 4/22 weeks no effect no effect liver: increase
OcBB rat male 100, 1000 2-4/2-22 no effect no effect liver: increase Waritz et al.
(Dow Lot (Sprague- weeks (1977)
102-7-72) Dawley)
DeBB rat (Sprague- male, 2000 13/13 weeks no effect no effect liver: increase Millischer
Dawley) female et al. (1979)
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b n.r. = Not recorded.
c Absolute or relative to body weight, resp.
At lethal doses, the "wasting syndrome" (see section 8.1) can
also be accompanied by other symptoms. Rats, which became moribund,
had hunchback posture, sunken eyes, appeared dehydrated, and were
lethargic (Gupta & Moore, 1979). Minks have been reported to show an
unthrifty appearance (Aulerich & Ringer, 1979; Ringer et al., 1981).
Cattle that died later showed many similar clinical signs, but they
also had excessive lacrimation and salivation, diarrhoea and
depressed heart and respiratory rates (Irving et al., 1976; Durst
et al; 1977, 1978b; Moorhead et al., 1977). In two rhesus monkeys,
the time to death was as long as 3-5 months. In addition to body
weight loss, the dead animals exhibited alopecia and oedema,
particularly on the face and eyelids (including loss of eyelashes),
and dry scaly skin (Allen et al., 1978). Observations of intoxicated
Bobwhite quails, prior to death, included asthenia, low carriage, an
unkempt appearance, wing droop, diarrhoea, limited ataxia, and
general lethargy (Cottrell et al., 1984).
8.2.1.2 Haematology and clinical chemistry
a) Haematology
At lethal doses, leukopenia and erythropenia were observed in a
rhesus monkey (Allen et al., 1978), but not in cattle (Moorhead
et al., 1977). Packed cell volume, white blood cell count, and red
blood cell count fell gradually in the monkey (dietary
concentration: 300 mg/kg of feed; total dose: 6.4 g of
FireMaster(R) FF-1), while changes in packed cell volume,
haemoglobin content, total erythrocyte and leukocyte counts, and
differential leukocyte counts were minimal in the cows (dose: 25 g
FireMaster(R) BP-6 per day).
At sublethal doses, haematological parameters of rhesus monkeys
remained within normal limits (Allen et al., 1978). The same was
true for cattle (Moorhead et al., 1977; Robl et al., 1978), with the
exception of a calf dosed with 100 mg FireMaster(R) FF-1/kg body
weight per day, the total leukocyte count of which was elevated
(Robl et al., 1978). Rats given 30 mg FireMaster(R) FF-1/kg body
weight per day for 30 days (22 total doses) showed a significant
decrease in the packed cell volume, haemoglobin concentrations, and
platelet counts at 30 days of exposure (Gupta et al., 1981) and at 6
months after the start of dosing (Gupta & Moore, 1979), but were
normal at 45, 60, or 90 days after the start of dosing (Gupta
et al., 1981). White and red blood cell values were only
occasionally reduced; for example, there was moderate lymphopenia in
female animals at 30 days of exposure (Gupta & Moore, 1979; Gupta
et al., 1981). No significant differences in values for the
erythrocyte count, packed cell volume, haemoglobin, and total and
differential leukocyte counts were found in male rats fed various
levels of FireMaster(R) BP-6 (1-500 mg/kg diet) for 30-60 days
(Sleight & Sanger, 1976; Garthoff et al., 1977; Sleight et al.,
1978); only a possible increase in total white blood cell count was
noted at the 500 mg/kg feed level (Garthoff et al., 1977). A mild
but significant decrease in the packed cell volume and the number of
platelets was observed in mice exposed to 30 mg FireMaster(R)
FF-1/kg body weight per day for 30 days (22 total doses). The
leukocyte values were within normal limits (Gupta et al., 1981).
Growing pigs in a 16-week trial showed significant reductions in
haemoglobin and haematocrit only after 6 weeks of feeding 200 mg
FireMaster(R) BP-6/kg diet (Ku et al., 1978). There was no
appreciable effect on standard haematological values of nursing pigs
and their sows fed FireMaster(R) BP-6 (10-200 mg/kg feed) during
pregnancy and lactation (Werner & Sleight, 1981). White Leghorn
cockerels fed PBB (FireMaster(R) FF-1) at dietary concentrations
of 75 and 150 mg/kg from 3 to 4 days of age until 5-9 weeks showed a
significant decrease in packed cell volume and haemoglobin values
(Heinemann & Ringer, 1976; Ringer, 1978).
Technical octabromobiphenyl caused a significant decrease in
packed cell volume and total red blood cell count in male rats fed
dietary concentrations of 10 000 mg/kg for 30 days, but had no
effect at dietary concentrations of 100 and 1000 mg/kg (Norris
et al., 1973, 1975).
Standard haematological determinations revealed no treatment-
related changes in blood from rats exposed to technical
decabromobiphenyl via diet (1-2000 mg/kg) for 4-12 weeks and via
inhalation (0.005-5 mg/litre, 6 h/day, 5 days/week) for 4 weeks
(Millischer et al., 1979).
b) Clinical chemistry
Clinical chemistry values examined in many studies refer to
serum protein (total protein, specific fractions), serum enzymes,
serum glucose, blood urea nitrogen, serum lipids, serum cholesterol,
and urine.
(i) Serum protein
Decreases in total serum protein, due primarily to a reduction
in the albumin fraction, occurred in severely intoxicated cattle
(Durst et al., 1978a; Schanbacher et al., 1978) and monkeys (Allen
et al., 1978). No consistent effect of dietary PBBs on total serum
protein concentrations or electrophoretic profiles was observed in
pigs fed FireMaster(R) BP-6 at levels of 20 or 200 mg/kg for 16
weeks (Ku et al, 1978). In rats fed 5, 50, or 500 mg FireMaster(R)
BP-6/kg for 3 weeks, total plasma protein was slightly increased by
the highest concentration (Garthoff et al., 1977). A marked increase
(50%) in serum protein was found in rats given 22 oral doses of
FireMaster(R) FF-1 (30 mg/kg body weight per day) over a 30-day
period and observed for some additional weeks. These changes were
primarily associated with increased ß-globulin fractions (Gupta
et al., 1981). Reductions in serum immuno globulin levels
(gamma-globulin fractions) have been reported in mice given oral
doses (30 mg/kg body weight per day) of FireMaster(R) FF-1 (Luster
et al., 1978) or diets containing 167 mg FireMaster(R) FF-1/kg
(Loose et al., 1981).
No treatment-related changes in total serum protein were found
in rats exposed to technical decabromobiphenyl (1-2000 mg/kg feed
for 4-12 weeks; inhalation: 0.005-5 mg/litre; 6 h/day; 5 days/week
for 4 weeks) (Millischer et al., 1979).
(ii) Serum enzymes
Alterations in serum enzymes, most of which are indicative of
liver lesions, have been found in some instances.
Gamma-Glutamyl transpeptidase (gamma-GTP) was elevated by oral
doses of FireMaster(R) FF-1 (30 mg/kg body weight per day) in
female rats and mice of both sexes (Gupta et al., 1981).
No consistent increase in serum glutamic pyruvic transaminase
(SGPT) occurred in rats after dietary (Garthoff et al, 1977;
Matthews et al., 1978) or oral (Gupta et al., 1981) exposure to
FireMaster(R). No changes were found in cows on diets containing
up to 10 mg FireMaster(R) FF-1/kg (Robl et al, 1978). However, a
gradual increase in SGPT activity was observed in lethally
intoxicated rhesus monkeys (Allen et al., 1978). Technical
decabromobiphenyl did not influence SGPT in rats (Millischer et al.,
1979).
Serum glutamic-oxaloacetic transaminase (SGOT) was
significantly increased in cattle at high doses of PBBs (25 g FM
BP-6 per day) (Moorhead et al., 1977; Durst et al., 1978b). It was
unaffected in cattle given lower doses (250 mg FM BP-6/day: Moorhead
et al., 1977; 0.01-10 mg FM FF-1/kg feed: Robl et al., 1978), as
well as in pigs (Ku et al., 1978) or in rats (Sleight & Sanger,
1976; Garthoff et al., 1977; Sleight et al., 1978) fed
FireMaster(R) BP-6 (20 and 200 mg/kg diet or 1-500 mg/kg diet,
respectively). A possible decrease in serum GOT has been reported in
rats exposed to technical decabromobiphenyl via inhalation
(Millischer et al., 1979).
Lactic dehydrogenase (LDH) was within normal ranges in PBB-
contaminated cows without clinical signs of toxicosis (Moorhead
et al., 1977), but it was increased in a group of apparently
intoxicated animals (dose: 25 g FM BP-6/day) (Moorhead et al., 1977;
Durst et al., 1978b). A significant decrease was found in growing
pigs (Ku et al., 1978), but not in nursing pigs and their sows
(Werner & Sleight, 1981), fed FireMaster(R) BP-6.
Electropherograms of LDH isozymes at 60 days, from rats given
FireMaster(R) BP-6 (100 mg/kg feed), showed appreciable changes
(Sleight et al., 1978).
With the exception of calves (Robl et al., 1978) and newborn
pigs (Werner & Sleight, 1981), alkaline phosphatase levels in
animals were unaffected by FireMaster(R), e.g., rats (Sleight
et al., 1978; Gupta et al, 1981), dogs (Farber et al, 1976), and
growing pigs (Ku et al., 1978). Technical decabromobiphenyl also did
not have any effect on alkaline phosphatase levels in rats
(Millischer et al., 1979).
Serum isocitrate dehydrogenase (sICDH) did not show any
discernible rise in dairy cattle dosed with FireMaster(R) BP-6,
until doses were sufficient to cause toxicosis (25 g/day). These
cows showed moderate increases in sICDH (approximately a two-fold
increase). Elevation of sICDH was coincident with fetal trauma.
Non-pregnant cows, equally intoxicated, showed minimal sICDH
elevation (Schanbacher et al., 1987).
Serum creatine phosphokinase levels in growing pigs were
unaffected by 20 or 200 mg FireMaster(R) BP-6/kg feed (Ku et al.
1978).
In male Japanese quails, serum glutamate dehydrogenase levels
were increased by "hexabromobiphenyl" (STRIK, 1973b).
(iii) Serum glucose
A slight decrease in serum glucose was observed in rats and
mice administered 22 total doses of 30 mg FireMaster(R) FF-1/kg
body weight (Gupta et al., 1981) and in rats fed 50 mg
FireMaster(R) BP-6/kg diet for 3 weeks (Garthoff et al., 1977),
but not in rats fed 500 mg/kg diet for the same period (Garthoff
et al., 1977). FireMaster(R) did not produce any effects on
glucose concentrations in either sublethally or lethally intoxicated
cattle (Durst et al., 1978a; Robl et al., 1978), and
decabromobiphenyl did not affect glucose levels in rats (Millischer
et al., 1979).
(iv) Blood urea nitrogen
Values for blood urea nitrogen (BUN) remained in the normal
range in mice (Gupta et al., 1981) and rats (Sleight & Sanger, 1976;
Garthoff et al., 1977; Sleight et al., 1978; Gupta et al., 1981) or
were elevated in rats at some concentrations (Sleight & Sanger,
1976; Garthoff et al., 1977). A significant increase in BUN occurred
in cows (Moorhead et al., 1977; Durst et al., 1978a) and a calf
(Robl et al., 1978) that had received doses of FireMaster(R) high
enough to produce overt signs of toxicosis (25 g FM BP-6 per day
(equivalent to 50 mg/kg body weight per day, and 100 mg FM FF-1/kg
body weight per day, respectively).
(v) Serum lipids
Characteristic alterations in serum phospholipid HPLC profiles,
which were maintained for at least two months after dosing, were
found in rats given a single oral dose of FireMaster(R) BP-6
(500 mg/kg body weight) (Bernert et al., 1985).
(vi) Serum cholesterol
In rats, cholesterol levels appear to be the blood parameter
most sensitive to PBBs. There were dose-related increases in
cholesterol concentrations in short-term (Garthoff et al., 1977;
Spear et al., 1990) and long-term (see 8.4: Bernert et al., 1983;
Gupta et al., 1983a,b) studies, and these increases were significant
at dietary concentrations of FireMaster(R) as low as 5 mg/kg
(Garthoff et al., 1977). No noticeable effects of FireMaster(R) on
cholesterol levels have been reported in cattle (Durst et al.,
1978a) and pigs (Werner & Sleight, 1981). Rhesus monkeys showed a
gradual decrease in serum cholesterol when they were lethally
intoxicated (Allen et al., 1978).
(vii) Urinalysis
Tests of urine for pH, protein, glucose, ketones, bilirubin,
occult blood, and specific gravity showed no significant changes due
to FireMaster(R) in mice (Gupta et al., 1981) and rats (Sleight
et al., 1978; Gupta et al., 1981) or due to technical decabromo
biphenyl in rats (Millischer et al., 1979). Only Sleight & Sanger
(1976) reported higher readings for protein in rats fed
FireMaster(R). Differences in urinary protein patterns between
control rats and rats given FireMaster were detected by means of
two-dimensional electrophoresis (Myrick et al., 1987). The principal
urine changes in cattle lethally intoxicated were decreased specific
gravity and moderate proteinuria (Moorhead et al., 1977; Durst
et al., 1978a).
8.2.1.3 Morphological and histopathological changes
a) Liver
The liver is the site of the most prominent gross morphological
and histopathological changes due to PBBs in many species.
Enlargement of the liver was a characteristic response to
exposure to FireMaster(R), technical octabromobiphenyl, and
technical decabromobiphenyl, and it frequently occurred at
concentrations lower than required to produce body weight changes
(see Tables 74 and 75). Generally, increases in absolute or relative
liver weights were dose and time dependent (e.g., Garthoff et al.,
1977). A notable exception was in cows, which showed decreases in
body weight as well as an increase in liver weights only at lethal
doses (Table 74).
Grossly, the livers of rats were often friable and had a
mottled surface (Sleight & Sanger, 1976; Waritz et al., 1977; Akoso
et al., 1982a; Render et al., 1982; Raber & Carter, 1986). Red
fluorescence of liver (and other tissues) under UVR (366 nm)
indicated excess porphyrin accumulation. In contrast to rats, which
developed porphyria after long-term exposure, the liver of female
mice given 30 mg FM FF-1/kg body weight per day (22 total doses)
became porphyric after 45 days (Gupta et al., 1981).
FireMaster(R) FF-1 given orally at a dose rate of 22.5 mg/kg body
weight per day for 4 days caused centrolobular accumulation of
Oil-red O-staining lipids in the liver of rats (Kohli et al., 1981).
The principal histopathological alterations in rodent species
consisted of extensive swelling and vacuolation of hepatocytes and
proliferation of smooth-surfaced endoplasmic reticulum (SER) (seen
as "foamy cytoplasm" in light microscopy). The vacuoles were filled
with fat indicating excess lipid accumulation. Proliferation of SER
may be a morphological reflection of enhanced enzyme activity
(Sleight & Sanger, 1976; Render et al., 1982). The changes depended
on dose and length of exposure. They have been reported in rats
after dietary intake of FireMaster(R) (Sleight & Sanger, 1976;
Kasza et al., 1978a; Sleight et al., 1978; Hinton et al., 1979;
Akoso et al., 1982a; Render et al., 1982; Raber & Carter, 1986) and
of technical octabromobiphenyl (Norris et al., 1973; Lee et al.,
1975a). The dietary concentrations ranged from 0.1 to 500 mg/kg for
FireMaster(R) and from 100 to 10 000 mg/kg for octabromobiphenyl.
Effects were seen as early as the tenth day of feeding 100 mg FM
BP-6/kg (Raber & Carter, 1986). In contrast, during a 90-day feeding
trial, technical decabromobiphenyl (dietary concentration: 100, 500,
or 2000 mg/kg) caused hepatic damage only at the highest level
(Millischer et al., 1979). Liver changes, as noted above, were
observed also after a single i.p. injection (200-1000 µmol/kg) of
FireMaster(R) (Goldstein et al., 1979), after single oral dosing
(1000 mg/kg body weight) of FireMaster(R) (Kimbrough et al., 1978)
and of octabromobiphenyl (Lee et al., 1975b), and after multiple
oral dosing of FireMaster(R) (22 doses over a 30-day period:
30 mg/kg body weight per day (Gupta & Moore, 1979; Gupta et al.,
1981) and of octabromobiphenyl (two doses: 3000 mg/kg body weight;
Lee et al., 1975b). As soon as 24 h (Kimbrough et al., 1978), 3 days
(Lee et al., 1975b), or 4 days (Goldstein et al., 1979) after
treatment, histological changes could be detected.
Light and electron microscopic changes also reported in the
liver of rats included reduction or disintegration of rough-
surfaced endoplasmic reticulum (RER) (Lee et al., 1975a,b; Gupta
et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), presence
of myelin bodies (cytoplasmic inclusions; membrane whorls) (Lee
et al., 1975a,b; Sleight & Sanger, 1976; Kasza et al., 1978a;
Kimbrough et al., 1978; Sleight et al., 1978; Hinton et al., 1979;
Gupta et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), di-
or multinucleated cells (Kasza et al., 1978a; Kimbrough et al.,
1978; Gupta & Moore, 1979; Hinton et al., 1979), diminution of
glycogen (Millischer et al., 1979; Gupta et al., 1981; Raber &
Carter, 1986) and mitochondria that were swollen and reduced in
number or had degenerated as time passed (Sleight & Sanger, 1976;
Kasza et al., 1978a; Akoso et al., 1982a). There was also necrosis
of hepatocytes (Kimbrough et al., 1978; Gupta & Moore, 1979; Gupta
et al., 1981), and these necrotic foci were infiltrated with
polymorphonuclear cells and lymphocytes (Gupta et al., 1981). With
octabromobiphenyl, myelin configurations developed 7 days after
treatment and subsequently disappeared one week later (Lee et al.,
1975b).
Changes in the hepatocytes were more advanced in the centri
lobular and midzonal regions than in the periportal area of the
liver lobule (Render et al., 1982; Raber & Carter, 1986). Fatty
infiltration in the livers of male rats was much more pronounced
than in those of female rats (Gupta & Moore, 1979).
Male rats given 100 mg FM FF-1/kg body weight per day (22 total
doses) and dying after 90 days had subacute to chronic hepatitis
with marked focal proliferation of bile ducts (Gupta & Moore, 1979).
Changes in the bile canaliculus (proliferation of microvilli)
were also found in mice fed 1000 mg FM BP-6/kg for 4-14 days.
Changes in the hepatocytes of these mice were increase in cell size,
decrease in RER, increase in SER, degeneration of mitochondria,
decrease in glycogen, and increase in size and number of nucleoli
(Corbett et al., 1978a). Fatty infiltration of the cytoplasm was
reported only in another two studies on mice dosed with 30 mg FM
FF-1/kg body weight per day (Gupta et al., 1981) or fed 167 mg FM
BP-6/kg for 6 weeks (Loose et al., 1981). However, in (moribund)
mice fed 167 mg FM BP-6/kg feed for 12 weeks, lipid vacuoles were
not found within the cytoplasm, but, almost exclusively, within the
nucleus (Martino et al., 1981). Hepatocellular necrosis has also
been observed (Loose et al., 1981).
Hepatocytes of guinea-pigs were swollen and had many more large
vacuoles than those of comparably dosed rats (Sleight & Sanger,
1976), even at lower dietary concentrations (< 10 mg FM/kg). Liver
damage was reported to be minimal at dietary levels of 50 mg FM/kg
(Vos & van Genderen, 1974), but severe centrilobular fatty changes
were found at 100 and 500 mg FM BP-6/kg (Sleight & Sanger, 1976).
Livers of rabbits, dermally treated with FireMaster(R) at 5
or 10 g/kg body weight, showed a mottled appearance, necrotic foci,
and were friable. No gross pathological effects were seen in rabbits
dermally exposed to octabromobiphenyl at 10 g/kg body weight (Waritz
et al., 1977).
Pigs fed FM BP-6 (up to 200 mg/kg) showed the following liver
alterations: fatty change, centrolobular necrosis, swollen
hepatocytes, and homogeneous cytoplasm (Werner & Sleight, 1981).
Compared with other species, liver changes observed in cattle
were less dramatic. Only an early stage of centrilobular fatty
degeneration and glycogen depletion were found in the enlarged
livers of lethally (25 g FM BP-6/day) dosed cows. In addition, there
were changes in the gallbladder and bile duct (Mercer et al., 1978;
Moorhead et al., 1978). Single calves exposed to FM FF-1 for 6-12
weeks had slightly enlarged hepatocytes (dose: 1 mg/kg body weight
per day) or necrosis of individual or small foci of hepatocytes
(dose: 100 mg/kg body weight per day) (Robl et al., 1978).
The only consistent histopathological lesion in mink which died
(exposure: 6.25 mg FM FF-1/kg feed), was a fatty infiltration of the
liver (Aulerich & Ringer, 1979).
No hepatocellular damage was found in dogs given oral doses of
FM BP-6 (1 mg/kg body weight per day) for seven weeks (Farber
et al., 1976).
Biopsies of the livers of two rhesus monkeys given a diet
containing 25 mg FM FF-1/kg at 12 weeks revealed enlargement of
hepatocytes and a marked proliferation of SER (Allen et al., 1978).
Liver changes in avian species were similar to those observed
in mammals. White Leghorn cockerels fed a diet of 10 or 100 mg FM
FF-1/kg showed enlargement and vacuolation of hepatocytes, increased
SER, swollen mitochondria, and disruption of mitochondrial cristae.
But, unlike rats, increased SER was not a significant feature
(Dharma et al., 1982).
b) Thymus
The thymus is also an organ sensitive to PBB exposure.
Decreases in thymus weights were observed in rats, mice, and cattle
after oral or intraperitoneal doses (Table 74) and in mice after
feeding (Table 75) of FireMaster(R) mixtures. There were no
reports on thymus weight changes due to exposure to commercial octa-
or decabromobiphenyl (Tables 74 and 75). The weight of the thymus
was reduced as early as 6 (Robertson et al., 1981b) or 15 days
(Gupta et al., 1981; Andres et al., 1983) after rats were given high
doses of FireMaster(R) (Table 74). Frequently, decreases in thymus
weights were accompanied by increases in liver weights (see: Tables
74 and 75). But, in some cases, changes in thymus weights were seen
at doses lower than those required for liver changes (Fraker, 1980),
or vice versa (Akoso et al., 1982a). Rats (Akoso et al., 1982a) and
mice (Fraker & Aust, 1978) both fed FireMaster(R) BP-6 (100 mg/kg
feed) for 30 days differed in their thymic response in that rats
remained unaffected and mice showed decreased thymic weight
(Table 75). In contrast, rats appeared to be more sensitive than
mice when given equal oral doses (30 mg/kg body weight per day for a
period of 30 days) of FireMaster(R) FF-1 (Luster et al., 1978;
Gupta et al., 1981: Table 74). Two strains of inbred mice are known
to differ in their thymic sensitivity to FireMaster(R) (Robertson
et al., 1984c: Table 74).
In some studies, the histological appearance of the thymus of
rats and mice that had survived exposure to FireMaster(R) was only
minimally, or not, affected (Gupta & Moore, 1979; Gupta et al.,
1981; Loose et al., 1981; Akoso et al., 1982a), even when organ
weights were altered. In other studies, a preferential atrophy of
the cortex of the thymus was found in rats and mice similarly
exposed (Luster et al., 1978; Fraker, 1980). At high doses (1000 mg
FM BP-6/kg feed), "surviving" mice (30%) were essentially athymic by
day 14 (Fraker & Aust, 1978). The thymus was markedly involuted also
in moribund rats. The normal architecture of the thymus was
obliterated with marked atrophy and loss of demarcation between the
cortical and medullary regions and disappearance of cortical
thymocytes (Gupta & Moore; 1979). Moderate to marked atrophy of the
thymus was also observed in guinea-pigs (Vos & van Genderen, 1974)
or cattle (Moorhead et al., 1978) that were lethally intoxicated.
The bursa of Fabricius, an analogous organ in avian species,
was also affected by FireMaster(R) (Table 75: Vos & van Genderen,
1974; Ringer, 1978; Dharma et al., 1982). Reductions in weight
occurred at exposures as low as 10 mg/kg feed in cockerels (Dharma
et al., 1982). Histologically, the bursa showed depletion of the
lymphoid cells especially in the medulla (Vos & van Genderen, 1974;
Ringer, 1978; Dharma et al., 1982).
c) Spleen
Changes in spleen weights are given in Tables 74 and 75.
Feeding of FireMaster(R) (100-200 mg/kg equivalent to 10-20 mg/kg
body weight per day) had no effect on the spleen weights of young
rats (Harris et al., 1978b; Akoso et al., 1982a), but resulted in a
decrease in the spleen weights of mice (equivalent to 15-30 mg/kg
body weight per day) (Fraker & Aust, 1978; Fraker, 1980; Loose
et al., 1981). An increase in spleen weights was observed in pups of
both rats (Harris et al., 1978a) and mice (Luster et al., 1980)
perinatally exposed to FireMaster(R). No effects were found in
some oral dosing studies on rats (Harris et al., 1978a; Gupta
et al., 1981); in another study, spleen weights were decreased
(Luster et al., 1978), and in the highest-dosage study reported
spleen weights increased (Gupta & Moore, 1979). On the other hand,
rats that received i.p. injection of FireMaster(R) showed
decreases in spleen weight at the higher doses (Robertson et al.,
1981b; Andres et al., 1983) and increases at the lower dose
(Robertson et al., 1981b). While female mice orally dosed with
FireMaster(R) showed decreased spleen weights (Luster et al.,
1978), pregnant and nursing females comparably dosed exhibited
increased spleen weights (Luster et al., 1980). No effect was found
in mice, 5 days after a single i.p. injection of FireMaster(R),
though the liver and thymus were affected (Robertson et al., 1984c).
Spleen weights of chicks fed FireMaster(R) were reduced (Vos & van
Genderen, 1974; Ringer, 1978) or remained unaffected (Dharma et al.,
1982).
Significant histopathological changes in the spleen have not
been reported, except in rats that were moribund from high doses of
FireMaster(R). In these animals, the splenic lymphatic follicles
were small because of a lack of periarterial lymphoid cells (Gupta &
Moore, 1979).
d) Thyroid
Exposure to FireMaster(R) resulted in an increase in thyroid
weight, if there was any weight change (see Tables 74 and 75).
Increases in thyroid weight have been observed in rats (Sleight
et al., 1978; Allen-Rowlands et al., 1981; Akoso et al., 1982b;
Render et al., 1982), newborn pigs (Werner & Sleight, 1981) and in
chickens (Ringer & Polin, 1977; Ringer, 1978). When no weight change
occurred (rats: Sleight et al., 1978; Gupta et al., 1981; mice:
Gupta et al., 1981; chickens: Dharma et al., 1982), the extent of
exposure was not always less than in the former studies.
Octabromobiphenyl tested in one inhalation study did not have any
effect on thyroid weight in rats (Waritz et al., 1977).
Histological changes in the thyroid gland developed in rats at
dietary concentrations of FireMaster(R) BP-6 as low as 5 mg/kg
(Kasza et al., 1978b). Hyperplasia of follicular cells occurred in
rats (Kasza et al., 1978b; Sleight et al., 1978) and newborn pigs
(Werner & Sleight, 1981). The normal low cuboidal follicular
epithelium was altered to one that had a more columnar appearance
(Kasza et al., 1978b; Sleight et al., 1978). The most prominent
ultrastructural lesions found in rats fed 5-500 mg
FireMaster(R)/kg for 5 weeks were dose-dependent. They included
abnormal lysosomes and colloid droplets in the cytoplasm, vacuolated
mitochondria with disrupted cristae, luminal surfaces with short and
abnormally branched microvilli or devoid of microvilli, and abnormal
cytoplasmic processes into the lumen (Kasza et al., 1978b).
e) Kidney
In most studies using rodents (Sleight & Sanger, 1976; Waritz
et al; 1977; Harris et al., 1978a,b; Gupta et al., 1981; Render
et al., 1982; Ecobichon et al., 1983; Smith et al., 1986), kidney
weights did not change with PBB exposure (Tables 74 and 75).
Increases in kidney weights were caused by FireMaster(R) in minks
(Aulerich & Ringer, 1979) pigs (Ku et al., 1978), and cattle
(Moorhead et al., 1977), and by octabromobiphenyl in rats (Norris
et al., 1973). A single study reported a decrease in kidney weight
in rats fed FireMaster(R) BP-6 (Garthoff et al., 1977).
In cattle, kidneys were severely affected, and doubled in size
in animals that were moribund from high doses of FireMaster(R)
(Moorhead et al., 1977). The kidneys were distended with fluid, and
pale tan to gray in colour. Perirenal lymph nodes were enlarged and
oedematous. The principal histological lesions consisted of
dilatation of collecting ducts and convoluted tubules, and tubular
epithelial degenerative changes (Moorhead et al., 1977). Similar
renal lesions were found in calves treated with different doses of
FireMaster(R) (0.1-100 mg FM FF-1/kg body weight per day, for 2-12
weeks). The severity of renal damage was related to dose level and
length of exposure (Robl et al., 1978). Despite the extensive
morphological damage, effective renal plasma flow rates and
glomerular filtration rates were not affected in cows (Mercer
et al., 1978; Schanbacher et al., 1978).
Ultrastructural analysis of kidneys of rats and mice given a
single i.p. injection of 150 mg "PBB" (not specified)/kg body weight
revealed proliferation of SER and increased numbers of peroxisomes
in the proximal tubule of the rat, 15 days after dosing.
Proliferation of SER was confined to only one segment (S3) of the
proximal tubule. Mice had only marginal increases in SER and no
significant increases in peroxisomes (Rush et al., 1986).
f) Stomach
Biopsies of the stomachs of two rhesus monkeys given
FireMaster(R) FF-1 (25 mg/kg diet) were made at 12 weeks. In the
gastric mucosa, there was evidence of early epithelial hyperplasia
and penetration of the submucosa by glandular epithelium (Allen
et al., 1978).
g) Testicle
Changes in the weights of the testes due to PBB exposure (see
Tables 74 and 75) were not found in rats (Norris et al., 1973;
Garthoff et al., 1977; Harris et al., 1978b; Gupta et al., 1981;
Castracane et al., 1982) and mice (Corbett et al., 1978a; Gupta
et al., 1981). The results of studies on chickens (Ringer & Polin,
1977; Ringer, 1978; Dharma et al., 1982) were inconsistent (see
Table 75).
Histologically, treatment-associated changes (e.g.,
hypospermatogenesis) were observed in the testes of male calves
administered FireMaster(R) FF-1 (0.1-100 mg/kg body weight per
day) for 2-12 weeks (Robl et al., 1978). Chickens fed
FireMaster(R) (50 mg/kg of feed) showed lipid infiltration into
the testicular parenchyma (Ringer & Polin, 1977).
h) Fluid accumulation
The presence of fluid accumulation, i.e., hydropericardium and
ascites, was noted in chickens (Heinemann & Ringer, 1976; Ringer,
1978). This lesion is known as "chick oedema disease" (McConnell,
1980). Oedema were observed also in the skin of rhesus monkeys
(Allen et al., 1978) and in the kidney, perirenal lymph nodes, and
the gastrointestinal tract of cattle (Moorhead et al., 1978) that
were lethally intoxicated.
Occasionally, weight changes in organs other than those
discussed above have been reported (see Tables 74 and 75, but they
appear to be of minor importance.
Some special effects occuring after single or short-term
exposure to PBBs are reviewed in the respective sections.
8.2.2 Individual PBB congeners and comparative studies
A lot of individual PBB congeners have been examined for
prominent general signs of toxicity, such as changes in body and
relative organ weights (see Tables 76 and 77) and for histopatho
logical changes (see Tables 78 and 79). It is evident from these
records that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological
changes in the liver and thymus. Despite variations in experimental
protocols, a tendency can be seen that the most severe effects are
elicited by congeners listed under category I. The relative severity
of damage decreases in categories II and III with the least effects
in the last group. Within a category, the degree of bromination may
also influence toxicity. Categorization of halogenated biphenyls has
been made on a structural basis (Parkinson et al., 1983; Safe,
1984). Category I comprises isomers and congeners lacking
ortho-substituents. They are referred to as coplanar PBBs.
Mono- ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more ortho-bromines) have
been organized into the third category. Structure-activity
relationships are discussed in detail by several authors (e.g.,
Goldstein, 1979; Parkinson et al., 1983; Safe, 1984).
Orders of toxicity derived from comparative studies included
only a limited number of congeners in any given study, but they
agreed with the trends described above. Ecobichon et al. (1979)
evaluated ultrastructural effects of lower and higher brominated
congeners on hepatocytes of rats and showed that the highly
brominated congeners (tetra-, penta-, hexa-, octabromobiphenyls)
were more active than the low bromine-containing congeners (di-,
tribromobiphenyls). Results of comparative studies dealing with
higher brominated isomers and congeners, predominantly constituents
of the FireMaster(R)-mixture, and the mixture itself have been
summarized in Table 80.
In all combinations tested, 3,3',4,4',5,5'-hexabromobiphenyl
(BB 169) was found to be the most toxic PBB. This congener, only a
very minor constituent of FireMaster(R), resembles 2,3,7,8-tetra
chlorodibenzo- p-dioxin (TCDD), the typical and the most toxic
member of the class of polyhalogenated hydrocarbons (e.g., Poland &
Knutson, 1982). Of the major FireMaster(R) constituents,
2,3,3',4,4',5-hexabromobiphenyl (BB 156) appeared to be the most
toxic, followed by 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5-pentabromobiphenyl (BB 118). The main component of the
FireMaster(R)-mixture, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153)
was relatively nontoxic as well as the second most abundant
constituent, 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). Compared
with the mixture itself, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)
was consistently more toxic than FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) less toxic. With
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), different results were
obtained from a dosing study (Dannan et al., 1978a) and two feeding
studies (Akoso et al., 1982a,b; Dharma et al., 1982). The latter
attributed FireMaster(R) a higher toxicity than 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167); 2,2',4,5,5'-pentabromobiphenyl (BB 101)
was less effective in producing adverse effects than
2,3',4,4',5-pentabromobiphenyl (BB 118), which matched
FireMaster(R) in some aspects (see Table 80).
Table 76. Effect of individual PBB congeners on body weight (or body weight gain) and relative organ weights of mice
and rats (dosing studies)
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Categoryd I PBBs ("Coplanar" congeners)
4,4'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon
600 (4-6) (days 1,2,3) (peanut oil) et al. (1979)
t.p.: 7 dayse
3,4,4',tri rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4'-tri rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
300 (3) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,4,4',5-tetra rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4',5-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982)
t.p.: 5 dayse
3,3',4,4'-tetra rat (Sprague- male oral single dose (corn no effect no effect (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4'-tetra rat (Long male intraperitoneal single no effect increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4'-tetra rat (Wistar) male intraperitoneal single reduced increased reduced Andres et al.
150 (4-5) dose (corn oil) (1983);
t.p.: 2 weeks Robertson
et al. (1983b)
3,3',4,4'-tetra rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
4.25g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4'-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4'-tetra mouse male intraperitoneal single no effect increased reduced Robertson et al.
(C57BL/6J dose (corn oil) (1984c)
1500 and DBA/2J) t.p.: 5 days
(10)
3,3',4,4',5-penta rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5-penta rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4',5,5'-hexa rat (Sprague- male oral single dose (corn no effect (increased)f (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
3,3',4,4',5,5'-hexa rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
3.19g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) t.p.: 7 daysd (1979)
Category II PBBs (Monoortho "coplanar" derivatives)
2,3',4,4'-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4'-tetra mouse male intraperitoneal single Robertson et al.
1500 dose (corn oil) (1984c)
t.p.: 5 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
(C57BL/6J) no effect no effect no effect
(5)
(DBA/2J) no effect increased no effect
(5)
2,3',4,4',5-penta rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single reduced increased reduced Dannan et al.
Dawley) (6) dose (polyethylene glycol) (1982c)
164g t.p.: 2 weeks no effect increased no effect Millis et al.
(1985a)
2,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single reduced increased (reduced)f Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1978a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Sprague- male intraperitoneal 2 doses reduced increased reduced Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
3.8-60 (1-4) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,3,3',4,4',5'-hexa rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Category III PBBs (Others)
4-mono rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (day 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2'-di rat (Sprague- male intraperitoneal single -- no effect -- Moore et al.
289g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: 2-22 days
2,2'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,5'-di rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,2'5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,3',4'5-tetra rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
150 (4) (days 1,3) (corn oil) (1980)
t.p.: 5 dayse
2,2',5,5'-tetra rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4,4',6-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,3',4,4',6-penta rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5,5'-penta rat (Long male intraperitoneal single no effect increased no effect
500 Evans) (3) dose (corn oil)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single no effect no effect no effect Dannan et al.
164g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
(6) t.p.: 14 days no effect increased no effect Millis et al.
(1985a)
2,2',3,4,4',5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',4,4',6,6'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa rat (Fischer male, oral 22 doses (over no effect increased no effect Gupta et al.
590g 344/N) (6) female 30 days) (corn oil) (1981)
t.p.: 15, 30, 45, 60,
90 dayse
2,2',4,4',5,5'-hexa rat (Fischer) female intraperitoneal single Goldstein et al.
(4) dose (corn oil) (1979)
t.p.: 4 days
40 no effect no effect --
200, no effect (increased)f --
1000
2,2',4,4',5,5'-hexa rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Moore et al.
144g Dawley) (6) dose (polyethylene glycol) (1978b); Millis
t.p.: 2 weeks et al. (1985a)
2,2',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa mouse male, oral 22 doses (over no effect increased no effect Gupta et al.
590g (B6C3F1/N) female 30 days) (corn oil) (1981)
(6) t.p.: 15, 30, 45, 69,
90 dayse
2,2',3,3',4,4'5-hepta rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
127g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',3,4,4',5,5'-hepta rat (Sprague- male intraperitoneal single -- increased -- Moore et al.
127g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: up to 22 days
2,3,3',4,4',5,6-hepta rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
6 (2) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,2',3,3',4,4',5,5'- rat (not male intraperitoneal single -- increased -- Besaw et al.
octa 115g specified) dose (solvent not (1978)
specified) t.p.: 7 days
Table 76 (contd).
a Total dose in µmol/kg body weight.
b t.p. = Time post-exposure; -- = data not given.
c Only statistically significant changes; organ weight changes relative to body weight.
d Categorization according to Safe (1984); see also text in section 8.2.2.
e After first dose.
f Only absolute data given.
g Calculated from original value given in mg/kg body weight.
Table 77. Effect of various hexabromobiphenyl isomers on feed intake, body weight (or body weight gain)
and (relative) organ weights (feeding studies)
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Categoryb I PBBs
3,3',4,4',5,5'- rat male 10 30 reduced reduced increased reduced spleen: reduced Akoso et
hexa (Sprague- brain: no effect (1982a,b)
Dawley) thyroid:
(6) increased
rat male 1 9 no effect no effect no effect Render
(Sprague- 10 9 reduced reduced increased reduced et al.
Dawley) 100 9 reduced reduced increased reduced (1982)
(6)
(2) 100 20 refused
(day 16)
Category II PBBs
2,3',4,4',5,5'- rat male 1, 10 30 no effect no effect no effect no effect brain: increased Akoso
hexa (Sprague- thyroid: no effect et al.
Dawley) 100 30 no effect no effect increased no effect brain: increased (1982a,b)
(6) thyroid: no effect
cockerel male 4, 10 28 no effect no effect no effect bursa of Dharma
(White Fabricius, et al.
Leghorn) thyroid, spleen, (1982)
(10) testicles, comb:
no effect
Table 77 (contd).
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Category III PBBs
2,2',4,4',5,5'- rat male 1 30 no effect no effect increased no effect brain: increased Akoso
hexa (Sprague- thyroid: no et al.
Dawley) effect (1982a,b)
(6)
(6) 10, 100 30 no effect no effect increased increased brain: increased
thyroid: no
effect
rat male 10 9 increased no effect increased n.r. kidney: no Render
(Sprague- effect et al.
Dawley) 100 9 no effect no effect increased n.r. kidney: no (1982)
(6) effect
mouse female 100, 300 gd 6 no effect no effect increased n.r. Welsch &
(C57 BL), through Morgan
pregnant 15 (1985)
(3) 500, 750 sacrifice no effect reduced increased n.r.
gd 17
1000 reduced reduced increased n.r.
cockerel male 10 28 no effect no effect no effect n.r. bursa of Fabri- Dharma et al.
(White cius, thyroid, (1982)
Leghorn) 62 28 no effect no effect increased n.r. spleen, testicles,
(10) comb, no effect
(both concen-
trations)
Table 77 (contd).
a Only statistically significant changes; organ weight changes relative to body weight except for Render et al.
(1982) giving absolute data.
b Categorization according to Safe (1984); see also text in section 8.2.2.
c gd = Gestation day.
d n.r. = Not recorded.
Table 78. Histopathology in relation to individual PBB congeners (dosing studies)
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
Categorya I PBBs
4,4'-di rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(6) t.p.: 7 daysd (1977)
3,3',4,4'-tetra rat (Sprague- male oral single dose 21.3 + ++ 8 tissues: Millis et al.
Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Sprague- male intraperitoneal single 4.25e + 0 11 tissues: Millis et al.
Dawley) (6) dose t.p.: 2 weeks 0 (1985a)
rat (Wistar) male intraperitoneal single 150 + ++ spleen, Andres et al.
(4) dose t.p.: 2 weeks kidney: 0 (1983); Robertson
et al. (1983b)
3,3',4,4',5,5'- rat (Sprague- male oral single dose 21.3 ++ ++ 8 tissues: Millis et al.
hexa Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
Categorya II PBBs
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) (6) dose t.p.: 2 weeks tissues: 0 (1982c); Millis
et al. (1985a)
2,3',4,4',5,5'- rat (Sprague- male intraperitoneal single 144e ++ n.r. 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,3,3',4,4',5- rat (Sprague- male intraperitoneal single 144e ++ ++ 9 tissues:
hexa Dawley) (4) dose t.p.: 1 week 0
Categorya III PBBs
4-mono rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2'-di rat (Sprague- male intraperitoneal single 289e 0 0 8 tissues: Moore et al.
Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,5'-di rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r.
(4-6) t.p.: 7 daysd
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,2',5,5'-tetra rat (Wistar) male intraperitoneal single 150 + 0 spleen, Robertson et al.
(not specified) dose t.p.: 2 weeks kidney: 0 (1983b)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) dose t.p.: 1-2 weeks tissues: 0 (1982a); Millis
(4-6) et al. (1985a)
2,2',3,4,4',5'- rat (Sprague- male intraperitoneal single 144e + 0 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',4,4',5,5'- rat (Fischer male, oral 22 doses 1052e + 0 16 tissues: Gupta et al.
hexa 344/N) female t.p.: up to 90 daysd 0 0 (1981)
2,2',4,4',5,5'- rat (Fischer) male intraperitoneal single 200-1000 + n.r. n.r. Goldstein et al.
hexa (4) dose t.p.: 4 days (1979)
rat (Sprague- male intraperitoneal single 144e + 0 8-11 Moore et al.
Dawley) dose t.p.: up to 14 days tissues: 0 (1978b); Millis
(3-6) et al. (1985a)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,4',6,6'- rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
hexa (4-6) t.p.: 7 daysd
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,2',3,3',4,4',5- rat (Sprague- male intraperitoneal single 127e + 0 9 tissues: Dannan et al.
hepta Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',3,4,4',5,5'- rat (Sprague- male intraperitoneal single 127e + 0 8 tissues: Moore et al.
hepta Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,2',3,3',4,4', rat (not male intraperitoneal single 115e + 0 several Besaw et al.
5,5'-octa specified) dose t.p.: 7 days tissues: 0 (1978)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
a Categorization according to Safe (1984); see also text in section 8.2.2.
b Total dose in µmol/kg body weight.
c t.p. = Time post-exposure.
d After first dose.
e Calculated from original value given in mg/kg body weight.
f n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Table 79. Histopathology to individual PBB congeners (feeding studies)
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
Categorya I PBBs
3,3',4,4',5,5'- rat (Sprague- male 1 10 + 0 Render et al. (1982)
hexa Dawley) (6) 10 10 ++ ++
100 10 ++ ++ spleen, lymph
nodes: +
rat (Sprague- male 1 30 + 0 thyroid: + Akoso et al.
Dawley) (6) 10 30 ++ ++ thyroid, (1982a,b)
pituitary
gland: +
Categorya II PBBs
2,3',4,4',5,5'- rat (Sprague- male 1, 10, 100 30 + 0 thyroid: +
hexa Dawley) (6)
cockerels male 10 28 + n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: +
(10)
Categorya III PBBs
2,2',4,4',5,5'- rat (Sprague- male 10, 100 10 + 0 18 tissues: 0 Render et al. (1982)
hexa Dawley) (6)
rat (Sprague- male 1, 10, 100 30 + 0 thyroid: + Akoso et al.
Dawley) (6) (1982a,b)
Table 79 (cont'd).
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
cockerel male 4, 10 28 0 n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: 0
(10) 62 28 + n.r. bursa of
Fabricius: +
a Categorization according to Safe (1984); see also text in section 8.2.2.
b n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Dannan et al. (1982b) recombined 9 purified FireMaster(R)
constituents, namely BB 101 (2,2',4,5,5'-penta-,) BB 118
(2,3',4,4',5-penta-), BB 153 (2,2',4,4',5,5'-hexa-), BB 138
(2,2',3,4,4',5'-hexa-), BB 167 (2,3',4,4',5,5'-hexa), BB 156
(2,3,3',4,4',5-hexa-), BB 180 (2,2',3,4,4',5,5'-hepta-), BB 170
(2,2',3,3',4,4',5-hepta-), and BB 194 (2,2',3,3',4,4',5,5'-
octabromobiphenyl), totalling 97% of either FireMaster(R) mixture,
to form a reconstituted FireMaster(R) BP-6-like mixture and
compared some effects of the reconstituted mixture to those of crude
FireMaster(R) mixtures BP-6 and FF-1. Rats were treated with a
single dose (90 mg/kg body weight of either mixture) and sacrificed
one week later. Evaluating changes in body and selected organ
(liver, thymus, spleen) weights, the conclusion was reached that
adverse effects of FireMaster(R) (FF-1 or BP-6) must be due to the
effects of the congeners studied. Moreover, it was found that the
increase in liver weights was greater with FM BP-6 and the
reconstituted mixture than with FM FF-1, consistent with the higher
proportion of minor components (BBs: 118, 138, 167, 156) in FM BP-6
(25% versus 15%).
Differences in toxicity between PBBs were sometimes of a
qualitative kind, but mostly they were quantitative. Some striking
relations will be reviewed in detail according to the parameter
tested.
8.2.2.1 Food intake, overt clinical signs, body weight changes
So far, in the congeners tested, food intake of rats was
reduced only by 3,3'4,4',5,5'-hexabromobiphenyl (Table 77: Akoso
et al., 1982a; Render et al., 1982). The effective dietary
concentrations ranged from 1 to 100 mg/kg. A much higher
concentration of 2,2',4,4',5,5'-hexabromobiphenyl (1000 mg/kg of
feed) was needed to evoke reduction in the food consumption of mice
(Table 77: Welsch & Morgan, 1985).
Rats, moribund from 3,3',4,4',5,5'-hexabromobiphenyl treatment,
had symptoms similar to those observed with FireMaster(R)
toxicosis (see section 8.2.1.1). They became less active, had a
roughened hair coat, developed sunken eyes, and were emaciated
(Render et al., 1982).
3,3',4,4',5,5'-Hexabromobiphenyl significantly decreased body
weight (Render et al., 1982) or body weight gain (Akoso et al.,
1982a) in rats, while the same concentrations of FireMaster(R)
BP-6, 2,2',4,4',5,5'-hexa- and 2,3',4,4',5,5'-hexabromobiphenyl in
the diet had no effects. When rats were injected i.p. with identical
Table 80. Order of toxicity of higher brominated PBB congeners and the FireMaster(R) mixture on the basis of comparative studies
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Dosing studies
Rat body weight gain x x 167 > FM Dannan et al.
(1978a)
Rat liver weight and x x FM > 153 Moore et al.
histopathology (1978b); Goldstein
et al. (1979)
Rat liver weight and x x FM > 180 Moore et al.
histopathology (1979a)
Rat body and organ x x FM > 153 Gupta et al.
(Mouse) weights, histopathology (1981)
Rat body and organ x x 118 > FM Dannan et al.
weights, histopathology (1982c)
Rat body and organ x x x x x 156 > 167 > Dannan et al.
weights, histopathology 138, 170 > (1982a)
101
Rat body and organ x x x 169 > 118, Parkinson et al.
weights 153 (1983)
Rat liver histopathology x x x 118, 153 > Millis et al.
101 (1985a)
Table 80 (cont'd)
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Feeding studies
Rat death, body and organ x x x 169 > FM Sleight et al.
weights, histopathology > 153 (1981)
Rat feed intake, body and x x x x 169 > FM Akoso et al.
organ weights, > 167 > 153 (1982a,b)
histopathology
Rat death, feed intake, x x x 169 > FM, Render et al.
body and organ weights, 153 (1982)
histopathology
Cockerel organ weights, x x x FM > 167 Dharma et al.
histopathology > 153 (1982)
a FM = FireMaster(R) mixture (BP-6 or FF-1); PPB numbering according to Ballschmiter & Zell (1980):
101, 118 = pentabromobiphenyls (2,2',4,5,5'-; 2,3',4,4',5-).
153, 138, 167, 156, 169 = hexabromobiphenyls (2,2',4,4',5,5'-; 2,2',3,4,4',5'-; 2,3',4,4',5,5'-;
2,3,3',4,4',5-; 3,3', 4,4', 5,5'-).
180, 170 = heptabromobiphenyls (2,2',3,4,4',5,5'; 2,2',3,3',4,4'5-).
amounts of several constituents of FireMaster(R) (BBs: 101, 138,
167, 156, 170), only 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 156) depressed body weight gain
(Dannan et al., 1978a, 1982a). The body weight gain of rats treated
with BB 167 was half that of FireMaster(R)-treated animals (Dannan
et al., 1978a). Rats given BB 118 (2,3',4,4',5-hexabromo biphenyl)
also gained less weight per day than those given FireMaster(R)
(Dannan et al., 1982c).
Liver weights in rats were increased by all treatments with
congeners of category I, except for 4,4'-dibromobiphenyl. The lowest
effective dose used was 2 mg/kg body weight (Millis et al., 1985a).
Significant effects were seen as early as 4 days after dosing (e.g.,
Parkinson et al., 1983). Many of the congeners listed under
categories II and III were also capable of increasing liver weights
(see Tables 76 and 77). Generally, all the changes in organ weights
were dose-dependent. Gradual differences between congeners were also
seen. Liver weights were significantly higher in rats given
3,3',4,4',5,5'-hexabromobiphenyl than the liver weights in
3,3',4,4'-tetrabromobiphenyl-treated rats, 6 days after a single
dose (Millis et al., 1985b). In studies comparing the effects of
five FireMaster(R) constituents (BB 101: 2,2',4,5,5'-penta; BB
138: 2,2',3,4,4',5'-hexa; BB 167: 2,3',4,4',5,5'-hexa; BB 156:
2,3,3',4,4',5-hexa; BB 170: 2,2',3,3',4,4',5-hepta), 7 days after
dosing, 2,3,3',4,4',5-hexabromobiphenyl (BB 156) caused the largest
increase in liver weights (60% increase) while 2,2',4,5,5'-
pentabromobiphenyl (BB 101) failed to enlarge this organ. Increases
caused by FireMaster(R) and BB 167 (2,3',4,4',5,5'-hexa) were
similar (Dannan et al., 1978a, 1982a). The increases in liver
weights due to BB 153 (2,2',4,4',5,5'-hexa), the main component of
FireMaster(R), were far less than the increase in response to
FireMaster(R), approximately 33% versus 60-80% (Moore et al.,
1978b; Goldstein et al., 1979).
Nevertheless, the increase was rapid with a 25% increase within
two days of treatment (Moore et al., 1978b). The effect of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), the second most
abundant component of FireMaster(R), was also less than that
caused by the mixture itself (Moore et al., 1979a). When
FireMaster(R) BP-6, 2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), and
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) were added to the diet of
rats for 30 days, BB 153 and BB 169 significantly increased liver
weights at 1 mg/kg, but FireMaster(R) and BB 167 did not. At 100
mg/kg, FireMaster(R) increased liver weight more than BB 153 or BB
167 (Akoso et al., 1982a).
Thymus weights in rodents were decreased by most of the
treatments with congeners of category I. Among congeners in category
II, 2,2',4,4',5-pentabromobiphenyl (BB 118),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), and
2,3,3',4,4',5-hexabromobiphenyl (BB 156) were capable of reducing
thymus weights. Congeners listed under the third category failed to
reduce thymus weights (see Tables 76 and 77). Both 3,3',4,4'-tetra
and 3,3',4,4',5,5'-hexabromobiphenyl caused a significant reduction
in the thymus weights of rats, 3,3',4,4',5,5'-hexabromobiphenyl
being more effective than 3,3',4,4'-tetrabromobiphenyl (Millis
et al., 1985b). There was a 50-60% loss in thymic weight in rats
injected i.p. with BB 167 or BB 156 and sacrificed 7 days later
(Dannan et al., 1978a, 1982a). However, thymus weight: body weight
ratios were not significantly affected when rats were fed BB 167 or
FireMaster(R) BP-6 for 30 days (Akoso et al., 1982a). In the same
study, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) significantly
decreased the ratio, while there was an increase with
2,2',4,4',5,5'-hexabromobiphenyl (BB 153).
In cockerels fed FireMaster(R) FF-1, BB 153, or BB 167, for
28 days, only FireMaster(R) FF-1 reduced relative bursal weights
(Dharma et al., 1982).
When young rats were fed diets containing FireMaster(R) BP-6,
2,2',4,4',5,5'-hexa-, 2,3',4,4',5,5'-hexa-, or 3,3',4,4',5,5'-
hexabromobiphenyl, for 30 days, thyroid weight was increased only by
100 mg FireMaster(R) BP-6/kg feed and by 1 and 10 mg
3,3'4,4',5,5'-hexabromobiphenyl/kg feed (Akoso et al., 1982b).
8.2.2.2 Haematology and clinical chemistry
Haematological and clinical chemistry findings were of minor
importance in comparative studies. Gamma-Glutamyl transpeptidase
(gamma-GTP) was elevated in female rats at high doses of both
FireMaster(R) FF-1 (30 mg/kg body weight per day) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) (16.8 mg/kg body weight
per day) 30 and 60 days after the last dose (Gupta et al., 1981).
8.2.2.3 Morphological and histopathological changes
Rats fed diets containing 100 mg FireMaster(R) BP-6/kg or 10
or 100 mg 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)/kg had friable
yellow livers (Render et al., 1982). The architectural structure of
the lobules was abnormal after feeding 3,3',4,4',5,5'-
hexabromobiphenyl (Akoso et al., 1982a; Millis et al., 1985b), and
bile duct hyperplasia was observed (Render et al., 1982).
With the exception of the lower brominated congeners of
category III, all PBB congeners caused histopathological changes in
the liver (see Tables 78 and 79). The extent of the changes depended
on the dose and the individual congener. The least severe effects
were confined to a slight proliferation of hepatic SER (e.g.,
4,4'-dibromobiphenyl: Ecobichon et al., 1977). More progressive,
general changes were enlargement of hepatocytes and increased
numbers of cytoplasmic lipid vacuoles. Corresponding ultrastructural
lesions consisted mainly of increased SER and lipid vacuolation (see
Tables 78 and 79). Additional changes seen with the more toxic
congeners included myelin body formation (membrane whorls) (BBs:
118, 167, 156, 169), disorganization of RER (BB 169), an increase in
number of binucleated hepatocytes (BB 169), pycnotic nuclei (BB
169), and, occasionally, multifocal areas of necrosis (BB 169)
(Akoso et al., 1980, 1982a; Sleight et al., 1981; Dannan et al.,
1982a,c; Render et al., 1982; Millis et al., 1985b).
The thymus was affected by 3,3',4,4'-tetrabromobiphenyl (Andres
et al., 1983; Millis et al., 1985b), possibly by BB 167 (Dannan
et al., 1978a: "several" tissues: not accurately specified), by BB
156 (Dannan et al., 1982a) and by BB 169 (Sleight et al., 1981;
Akoso et al., 1982a; Render et al., 1982; Millis et al., 1985b).
There was a loss of thymocytes, especially in the cortex, the
demarcation between cortex and medulla was indistinct, and
macrophages were prominent in the remaining portion of the cortex.
One study reported histological alterations in the thyroid of
rats treated with 2,2',4,4',5,5'-hexa- (BB 153), 2,3',4,4',5,5'-hexa
(BB 167), or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169), and in the
pituitary gland with BB 169 (Akoso et al., 1982b). Prominent lesions
of the thyroid were extensive hyperplasia and hypertrophy of
follicular cells and a lack of colloid. The pituitary gland showed
swollen and vacuolated chromophobe cells.
The spleen and lymph nodes of rats given 100 mg 3,3',4,4',5,5'-
hexabromobiphenyl/kg feed for 10 and 20 days had an increased number
of macrophages intermixed with mature lymphocytes. Changes were
similar to those seen in the thymus, but were not as pronounced
(Render et al., 1982).
The main component of FireMaster(R),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), is the congener most
frequently examined and implicated in many comparative studies.
The principal changes seen with BB 153 were vacuolation and
enlargement of hepatocytes with proliferation of SER. They occurred
in dosing studies (Moore et al., 1978b; Ecobichon et al., 1979;
Goldstein et al., 1979; Gupta et al., 1981; Millis et al., 1985a) as
well as in feeding studies (Sleight et al., 1981; Akoso et al.,
1982a; Dharma et al., 1982), and were observed as early as two days
after treatment (Moore et al., 1978b). Generally, the
ultrastructural changes in BB 153-exposed rats were less severe than
those in rats exposed to the FireMaster(R) mixture (e.g.,
Goldstein et al., 1979; Gupta et al., 1981; Akoso et al., 1982a).
Myelin figures and marked disorganization of RER, as seen with the
FireMaster(R) mixture, were not observed with BB 153 in
comparative studies (Gupta et al., 1981; Akoso et al., 1982a).
Moreover, changes caused by FireMaster(R) FF-1 in the livers of
rats persisted, while the livers of rats dosed with BB 153 were
comparable to those of the controls, 60 days after treatment (Gupta
et al., 1981).
The histological appearance of thymuses in rats was not
affected by BB 153 (Tables 78 and 79), but, in cockerels, the
lymphoid cells of the bursa of Fabricius were depleted by 62 mg BB
153/kg feed, by 10 mg FireMaster(R) FF-1/kg feed, and by 10 mg BB
167/kg feed (Dharma et al., 1982). When BB 153, the FireMaster(R)
mixture, BB 167, and BB 169, were fed to rats, proliferation of SER,
decreased RER, and increased fat droplets were seen in hepatocytes,
with all chemicals, but, with BB 169
(3,3',4,4',5,5'-hexabromobiphenyl), proliferation of SER was not as
prominent as with the other three PBBs. In contrast, the RER was
severely altered by BB 169 (Akoso et al., 1982a).
Myelin bodies were observed in rats fed 100 mg BB 169/kg for 20
days (Render et al., 1982) or FireMaster(R) BP-6 for 30 days
(Akoso et al., 1982a), or BB 167 for 60 days (Akoso et al., 1980). A
comparative experimental series testing single doses of six
FireMaster(R) constituents, namely BB 118 (Dannan et al., 1982c;
Millis et al., 1985a), BBs 101, 138, 167, 156, and 170 (Dannan
et al., 1978a, 1982a) found the least severe histological changes
with BB 101, and intermediate effects, which were limited to the
proliferation of SER and cytoplasmic vacuolation in hepatocytes,
with BB 138 and BB 170. The most pronounced changes resulted from
BBs 118, 167, and 156 and consisted of proliferation of SER,
increases in fat vacuoles and myelin figures in hepatocytes (BBs
118, 167, 156) and thymus damage (BB 156). Rats given a single
equimolar dose of 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or
3,3',4,4'-tetrabromobiphenyl showed moderate to severe hepatic
changes 14 days after treatment with BB 169, while the
tetrabromobiphenyl-treated rats showed only mild hepatic changes
(Millis et al., 1985b).
The microscopic hepatic effects of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180) and of
2,2',3,3',4,4',5,5'-octabromobiphenyl (BB 194) were reported to be
similar to those of BB 153 (Besaw et al., 1978; Moore et al., 1979a,
1980).
8.3 Skin and eye irritation, sensitization, dermal lesions,
and acne
Common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions due to the technical
PBB mixtures tested, namely octabromobiphenyl and decabromobiphenyl
(Table 81).
Table 81. Skin and eye irritation or sensitization tests of commercial PBB mixtures
PBBa Species Application Test Observations References
OcBB guinea-pig 50% (w/v) slurry in irritation (intact mild irritation Waritz et al.
(Hartley) propylene glycol shaved dorsal skin) (1977)
(0.05 ml)
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 9 x 50% slurry abraded shaved skin
(topical application)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 4 x 1% (w/v) solution
in dimethyl sulfoxide
(intradermal injection)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB rabbit irritation Norris et al.
(New Zealand) (1973)
dry solid (single and intact shaved skin no response
multiple exposures) abraded shaved skin slight erythematous
and edematous response
Table 81 (contd).
PBBa Species Application Test Observations References
OcBB rabbit moistened with water
(New Zealand) (single exposure) intact shaved skin no response
(repeated exposure) intact shaved skin slight erythematous
response
OcBB rabbit moistened with water abraded shaved skin moderate erythematous
(New Zealand) (single and repeated and slight oedematous
exposures) response
rabbit dry solid eye irritation transient irritation
(New Zealand) of the conjunctival
membranes
OcBB rabbit powder (100 mg) eye irritation no irritating or corneal Waritz et al.
effects; mild (1977)
conjunctival redness and
swelling and a copious
discharge (disappeared
within 4 h)
DeBB rabbit 50% in olive oil irritation mild irritation Millischer et al.
(intact shaved skin) (1979)
DeBB rabbit 50% in olive oil eye irritation no irritating effect
DeBB rabbit powder eye irritation mild irritating
a Commercial PBB mixtures: OcBB = Octabromobiphenyl; DeBB = Decabromobiphenyl.
However, diverse lesions in the skin and skin appendages of
certain animal species, e.g., rhesus monkeys and cattle, occurred
after the ingestion of the FireMaster(R) mixture (Table 82). The
main features were dry scaly skin and hair loss. Hyperkeratosis of
the interfollicular epidermis and of the hair follicle, and atrophy
and squamous metaplasia of the sebaceous glands were observed on
microscopic examination of these lesions. As with related compounds,
comparable epidermal changes have not generally been found in other
laboratory animals, such as guinea-pigs or rats, but they were
similar to those observed in humans following PBB exposure
(section 9) and were described as chloracne (McConnell, 1980;
Kimbrough, 1980b; Poland & Knutson, 1982).
The rabbit ear (inner surface), but not any other part of the
rabbit skin (Crow, 1983), is particularly sensitive to acne-causing
compounds, which was first recognized by Adams et al. (1941). The
reaction is hyperkeratosis. Painting the rabbit ear has become a
standard bioassay to detect hyperkeratotic (acnegenic) activity.
Results of rabbit ear tests obtained with diverse PBBs (technical
octabromobiphenyl, technical decabromobiphenyl FireMaster(R)
mixture, fractions of this mixture, and purified PBB congeners) have
been summarized in Table 83. The FireMaster(R) mixture itself
produced hyperkeratosis, but its main components, BB 153
(2,2',4,4',5,5'-hexa) and BB 180 (2,2',3,4,4',5,5'-hepta), did not.
Fractionation of FireMaster(R) indicated that most activity was
associated with the more polar fraction containing minor compo nents
(Needham et al., 1982). Sunlight irradiation of BB 153 also yielded
products that caused severe hyperkeratosis. It is not clear whether
one or more of the suspected PBBs is responsible for hyperkeratotic
activity (Patterson et al., 1981). The model congener
3,3',4,4',5,5'-hexabromobiphenyl and 3,3'4,4'-tetra bromobiphenyl
were shown to be hyperkeratotic (Table 83).
8.4 Long-term toxicity
Toxic effects of PBBs, observed after long-term exposures, as
well as a long time after exposure had ceased, are summarized in
Tables 84 and 85. Experimental animals tested were rats, mice,
cattle, minks, and rhesus monkeys. The majority of studies refer to
the commercial FireMaster(R) mixture.
The following comments refer mainly to the general signs of
long-term toxicity. Other long-term effects will be reviewed in
detail in the respective sections, e.g., carcinogenicity (section
8.7), reproductive dysfunctions (section 8.5).
Table 82. Dermal lesions observed in cattle, rhesus monkeys, and rabbits after exposure to PBBs
PBBa Species Route Dose (duration) Dermal lesions observed References
FM BP-6 cattle oral 25 g/day (for subcutaneous emphysema and haemorrhage; changes Moorhead et al.
33-60 days) in the eyelids: hyperkeratosis, with accumulations of (1977, 1978)
keratin in hair follicles of the epidermis and squamous
metaplasia with keratin cysts in the tarsal glands
FM FF-1 calf oral 100 mg/kg body keratitis, alopecia, hyperkeratosis involving the Robl et al.
weight (up to head, cervical and dorsal thoracic region (1978)
12 weeks)
oral 0.1, 10, 100 acanthosis, hyperkeratosis and/or dermal infiltrates
mg/kg body of mononuclear cells (dose-dependent severity)
weight (up to
12 weeks)
FM FF-1 rhesus in diet 25 mg/kg feed alopecia, dry scaly skin, loss of eyelashes; Allen et al.
monkey (for 25 weeks) generalized subcutaneous oedema, marked oedema of (1978)
adult total dose: the eyelids; keratinization of hair follicles
(male) approx. 1 g and sebaceous glands
juvenile in diet 25 mg/kg feed moderate loss of hair including eyelashes, dry
(female) (for 50 weeks) scaly skin; periorbital oedema
total dose:
approx. 1.5 g
juvenile in diet 300 mg/kg feed a rather generalized loss of hair, absent eyelashes; Allen et al.
(female) (for 137 days) considerable periorbital congestion and oedema; (1978)
total dose: keratinization of hair follicles
approx. 6.4 g
Table 82 (contd).
PBBa Species Route Dose (duration) Dermal lesions observed References
FM FF-1 rhesus in diet 1.5 mg/kg feed periorbital oedema Allen &
monkey (for over 5 Lambrecht (1978)
months)
total dose:
approx. 75 mg
OcBB rabbit dermal not specified erythema, exfoliation in the ear Norris et al.
(ear) (1 month) (1973)
Various rabbit dermal variable hyperkeratosis in the ear see Table 83
PBBs (ear)
FM = FireMaster(R); OcBB = Technical octabromobiphenyl.
Table 83. Hyperkeratotic activity of commercial PBB mixtures, the fractionateda FireMaster(R) mixture
and purified PBB congeners, derived from the rabbit ear test
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
DeBB (Adine 0102) 200, 2000 (acetone) x Atochem (1990)
OcBB not specified x Norris et al.
(chloroform) (1973)
FM FF-1 (lot FH 7042)
mixture itself 60 (not specified) x Kimbrough
polar fraction not specified (not specified) x (+++) et al.
non-polar fraction not specified (not specified) x (+) (1977)
FM BP-6
mixture itself 6.5 µg/kg body weight x Hass et al.
(benzene-decane, 1:9) (1978)
fraction 1 (non-polar) containing PBBs 6.5 µg/kg body weight x
(benzene-decane, 1:9)
FM FF-1 (lot FH 7042)
mixture itself 100 (toluene) x (++) Needham et al.
less polar fractions 50-210 (toluene) x (1982)
more polar fraction containing minor 185 (toluene) x (+++)
components of the FM
mixture
Compound 4 predominantly BB 153 5 (toluene) x
Compound 8 predominantly BB 180 3.3 (toluene) x
Table 83 (contd).
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
2,2',4,4',5,5'-hexa- 10 (not specified) x Patterson et al.
bromobiphenyl (BB 153) (1981)
(96% pure)
sunlight degradation mixture of BB 153 10 (not specified) x (+++) Patterson et al.
products of BB 153 and other PBBs (1981)
(probably BB 101,
BB 118 and 3,3',4,4'-
tetra-bromobiphenyl)
3,3',4,4',5,5'-hexa- 0.32-9.6 (toluene) x (++) Needham et al.
bromobiphenyl (1982)
3,3',4,4'-tetra- 0.245 and 0.02 x (++)
bromobiphenyl (not specified)
a The mixture was fractioned by different methods (on Florisil: Hass et al., 1978; on alumina:
Kimbrough et al., 1977; by HPLC and GC: Needham et al., 1982).
b Total dose in mg/rabbit ear, unless otherwise specified.
c (+), (++), (+++) = severity of hyperkeratosis.
d DeBB = technical decabromobiphenyl; OcBB = technical octabromobiphenyl; FM = FireMaster(R).
BB 101 = 2,2',4,5,5'-pentabromobiphenyl; BB 118 = 2,3',4,4',5-pentabromobiphenyl;
BB 153 = 2,2',4,4',5,5'-hexabromobiphenyl; BB 180 = 2,2',3,4,4',5,5'-heptabromobiphenyl.
Table 84. Long-term effects observed after exposure to PBBs by gavage
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, single dose 1000 liver: lipid accumulation (more pronounced Kimbrough et al.
(lot FH 7042) (Sherman) female t.p.: 6,10,14 in males); uroporphyrin accumulation (1977, 1978)
(in peanut oil) (5) months (females); histopathological changes;
neo-plastic nodules; increase in relative
weight
FM FF-1 rat male, 22 doses over 30 liver: marked hepatotoxic effects; atypical Gupta &
(lot No. 1312 FT) (Fischer female 30 days; t.p.: nodules Moore (1979)
(in corn oil) 344/N) 6 months after
(9) first dose
FM FF-1 rat female single dose 1000 liver: porphyria; neoplastic nodules; foci Kimbrough et al.
(lot. 7042) (Sherman) t.p.: 23 months or altered areas; carcinoma; adenofibrosis (1981)
(in corn oil) (65)
(16) female single dose 200 liver: neoplastic nodules; altered areas;
t.p.: 22 months multinucleated cells
(30) female 12 doses over 100 liver: neoplastic nodules; foci or altered
3 weeks; t.p.: areas; carcinoma; adenofibrosis
24 months
FM FF-1 rat female 122 doses over 0.1, 1, increased white blood cell and lymphocyte Luster et al.
(lot. FF 1312 FT) (Fischer) 6 months t.p.: 3, 10 counts (0.1-10); decrease in body weight (1980)
(in corn oil) (4-10) 6 months after (3,10); decrease in thymus weight (3,10);
first dose increase in relative spleen weight (3,10);
decrease in adrenal weight (10); immune
alterations (3,10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, 22 doses over 30 decrease in body weight; increase in relative Gupta et al.
(lot 1312 FT) (Fischer female 30 days t.p.: liver weight; decrease in relative thymus (1981)
(in corn oil) 344/N) 120 days after weight (F); increase in serum protein
(3) first dose (ß-globulin fraction); porphyria (F);
pronounced hepatocellular alterations
2,2',4,4',5,5'- rat male 22 doses over 16.8 increase in relative liver weight (M);
hexabromobiphenyl (Fischer female 30 days t.p.: minimal hepatocellular changes
(99% purity 344/N) 120 days after
(in corn oil) first dose
FM FF-1 rat male single dose 500 no significant effects on body and liver Bernert, Jr
(lot 7042) (Sherman) t.p.: 18 months weight; increase in serum cholesterol and et al. (1983)
(in corn oil) total serum phospholipids; enhancement of
hepatic per-oxidation; reduced retinol
levels in serum and liver microsomes;
reduced alpha-tocopherol content in
microsomes (but not in serum)
FM FF-1 rat male, 125 doses over 0.1, 0.3 no effect on food consumption; dose-related Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, decrease in body weight gain; dose-related (1983a)
(in corn oil) 344/N) 6 months after 10 increase in the absolute and relative liver
(10) first dose weights (Female: 0.1-10; Male: 0.3-10);
decrease in thymus weight (0.3-10); increase
in spleen weight (1-10); decrease in Hb and
PCV values, in MCV and MCH values (10);
dose-related increase in serum cholesterol;
decrease in serum thyroid hormone levels;
porphyrin accumulation in liver, bone, teeth
(more pronounced in Female (1-10);
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
dose-related hepatocellular alterations;
increase in white blood cell count (Female:
1-10); increase in serum GGTP (Female: 10);
decrease in serum glucose (Female: 10);
dose-related decrease in serum protein,
primarily due to albumin (Female: 0.1-10);
carcinoma in urinary bladder (Female:10);
histopathological changes in the thyroid
glands and kidneys (Male: 10); decrease in
serum triglyceride (Male: 0.3-10)
FM FF-1 rat male, 125 doses over 0.1,0.3 dose-related body weight reductionso; Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, dose-dependent porphyrogenic effects on (1983b)
344) = up to 29 10 teeth, bones, liver (1-10); enlarged, pale,
(11-40) months after or mottled livers with necrotic foci (1-10);
first dose hepatocellular alterations (1-10);
dose-dependent incidence of liver tumours
and cholangiocarcinoma (higher doses);
dose-dependent decline in survival time
(Male: 1-10); chronic progressive nephropathy
(Male: 1-10); gastric ulcers and hyperplastic
gastropathy (Male: 3,10)
FM FF-1 mouse female 122 doses over 10 increase in body weight; increase in relative Luster et al.
(lot. FF 1312 FT) (B6C3F1) 6 months; t.p.: spleen weight; slight immune alterations (1980)
(in corn oil) (4-10) 6 months after
first dose
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse male, 22 doses over 30 no effect on food consumption; increase in Gupta et al.
(lot 1312 FT) (B6C3F1/N) female 30 days; t.p.: relative liver weight, histopathological (1981)
(in corn oil) (3) 120 days after alterations in liver
first dose
2,2',4,4',5,5'- mouse male, 22 doses over 16.8 no effect on food consumption and body weight
hexabromobiphenyl (B6C3F1/N) female 30 days; t.p.: gain; increase in relative liver weight;
(99% purity) (3) 120 days after minimal hepatocellular alterations
(in corn oil) first dose
FM FF-1 mouse male, 125 doses over 0.1, 0.3, no effect on food consumption; decrease in Gupta et al.
(lot. 1312 FT) (B6C3F1) female 6 months 1, 3, 10 body weight gain (only Male: 10); increase (1983a)
(in corn oil) (10) in body weight (only Female: 10); increase
in absolute and relative liver weight
(Female: 0.3-10; Male: 1-10); increase in
spleen weight (Female: 10); decrease in
uterine weight (Female: 10); haematological
alterations: dose-related increase in red
blood cell count; dose-related decrease in
MCV; decrease in platelet counts (Female:
3,10); leukocytosis (Female: 10); clinical
chemistry changes: increase in serum levels
of GGTP and SGPT (10) and alkaline
phosphatase (10); decrease in serum glucose
(Female: 10); dose-related hepatic porphyria
(more pronounced in Female) pale and
mottled livers (higher doses); dose-related
microscopic changes in the liver (1-10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse 125 doses over 0.1, 0.3, shortened survival time (Male: 10); hepatic Gupta et al.
lot. 1312 FT) (B6C3F1) 6 months; t.p.: 1 ,3, 10 porphyria (Male: 3); slight reduction in body (1983b)
(in corn oil) (8-27) up to 30 weight (Male: 10); enlarged liver; fluid
months after accumulation in peritoneal cavity; liver
first dose carcinoma (10); metastasis to lung (Female:
10); hyperplasia and adenoma of follicular
cells of thyroid
FM BP-6 cattle daily doses 250 no adverse clinicopathological chanages Durst et al.
(in gelatin (6) for 60 days mg/day (1978a)
capsule) t.p.: 80-190
days after
first dose
FM BP-6 cattle daily doses 250 no gross or histopathological signs of Moorhead et al.
(in gelatin (No. not for 60 days mg/day toxicosis (1978)
capsule) specified) t.p.: 220
days after
last dose
FM FF-1 cattle female daily doses 0.3 no effects on milk production, body weights, Robl et al.
(in gelatin (6) for 158 days amount of food consumed; no effects on (1978)
capsule) t.p.: 182 haematological and clinical chemistry and
days after urinalysis values;
last dose (1 cow: overgrowth of hooves from day 129)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM BP-6 cattle (Female) daily doses 250 no effect on milk production, body weight, Durst et al.
(in gelatine (1-4) for 60, 180 mg/day number of infections or general injuries; (1978b)
capsule) 202 days increased frequencies of reproductive Willett et al.
t.p.: up to dysfunctions (1980)
1500 days
after first
dose
a FM = Fire Master.(R)
b No. = number of animals.
c t.p. = time post-exposure.
d In mg/kg body weight per day, unless otherwise specified.
e Hb = Haemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular haemoglobin.
Table 85. Long-term effects of feeding PBBs to young or adult animals
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
"Hexabromobiphenyl" rat female 50 7 months increase in relative weights of liver, Sepkovic &
(Monsanto Co., (Sprague- ovary, and thyroid Byrne (1984)
St. Louis) Dawley) (8)
FM BP-6 rat female 1, 10, 50 5-7 months no effect on food consumption, body Byrne et al.
(Sprague- weight, and relative thyroid weight; (1987)
Dawley) (10) slightly elevated liver weight (10, 50);
primary hypothyroidism
FM BP-6 rat female 1, 5, 10, 5 or more no effect on food consumption and relative Byrne et al.
(Sprague- 50 months liver weight; decrease in relative adrenal (1988)
Dawley) (10) weight; depression of circulating levels
of adrenal cortex hormones
OcBB rat male, 0.01-1 180 days no effect on food consumption and body Norris et al.
(Dow Chemical) (Sprague- female weight (1973)
Dawley) (not
specified)
OcBB rat male 100 4/22 weeks return to normal liver weights Lee et al.
(Dow Chemical) (Sprague- (1975a);
Dawley) (8) 1000 4/22 weeks increased liver weights; Waritz et al.
hepatocellular alterations (1977)
OcBB rat female 50 7 months increase in relative liver weight Sepkovic &
(Monsanto Co., (Sprague- Byrne (1984)
St. Louis) Dawley) (8)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
NoBB mouse male, 100, 300 18 months decrease in body weight (Male, Female); Momma (1986)
(Bromkal 80-9D) female shortened survival time (300 mg/kg; Male);
enlargement of thyroid glands (300 mg/kg;
Female); decrease in triglyceride and
non-esterified fatty acids; reduction of
pentobarbital sleeping time (13 months);
liver carcinoma (Male, Female)
FM FF-1 mink (9) male, 6.25 210 days deaths; decrease in body weight; increase Aulerich &
female (mean) in relative liver and kidney weights; Ringer (1979);
liver histopathology Ringer et al.
(1981)
mink (8) female 1-2.5 9 months 10% reduction in body weight (2.5); Aulerich &
adverse effects on reproduction Ringer (1979);
Ringer et al.
(1981)
FM FF-1 rhesus female 0.3 7 months- weight loss; sterile abscesses (2/7); Allen et al.
monkey (total dose: > 1 year reproductive dysfunctions (1978); Allen
(7) > 25 mg) & Lambrecht
(1978);
Lambrecht
et al. (1978)
rhesus ? 1.5 > 5 months weight loss; periorbital oedema; Allen &
monkey (total dose: immunological alterations Lambrecht
(not approximately (1978)
specified) 75 mg)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
FM FF-1 rhesus male 25 25 weeks death; weight loss; haematological and Allen et al.
monkey (total dose: clinical chemistry changes: decrease in (1978)
(adult) approximately PCV, cholesterol, total serum protein and
(1) 1 g) albumin; increase in serum GPT activity;
epidermal changes:
alopecia, dry scaly skin, loss of eyelashs;
keratinization of hair follicles and
sebaceous glands;
oedema:
generalized subcutaneous oedema and
marked oedema of eyelids; enlarged heart
and liver; hyperplastic gastroenteritis;
severe ulcerative colitis; hypoactive
seminiferous tubules; hyperplasia of bile
duct epithelium
FM FF-1 rhesus female 25 50 weeks no weight gain: loss of hair (including Allen et al.
monkey (total dose: eyelashes); dry scaly skin; periorbital (1978)
(juvenile) approximately oedema; increase in serum GPT activity
(1) 1.5 g)
FM FF-1 rhesus female 300 137 days death; weight loss; haematological and
monkey (total dose: clinical chemistry changes: decrease in
(juvenile) approximately PCV, white blood cell count, red blood cell
6.4 g) count, serum cholesterol, serum protein;
increase in serum GPT activity
epidermal changes:
loss of hair (including eyelashes); atrophy
and squamous metaplasia of the sebaceous
glands; keratinization of hair follicles;
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
periorbital congestion and oedema;
subcutaneous oedema; enlarged
hepatocytes; hyperplastic gastroenteritis;
hyperplasia of the epithelium of the
bladder and of the bile ducts; focal
areas of haemorrhage in the adrenal glands
FM FF-1 rhesus female 1.5 36 weeks weight loss; decrease in serum Lambrecht et al.
monkey (total dose: cholesterol enlarged hepatocytes with (1978)
(adult) 70 mg) moderate fatty infiltration (as indicated
(3) by liver biopsy)
rhesus male, 25 14 weeks weight loss (Male, adult) or lack of
monkey female (total dose: weight gain (Female, juvenile); decrease
(adult approximately in serum cholesterol; hyperplastic
juvenile) 500 mg) gastritis
(2)
a FM = FireMaster; OcBB = technical octabromobiphenyl; NoBB = nonabromobiphenyl.
b No. = number of animals.
c PCV = packed cell volumes.
8.4.1 Rat
8.4.1.1 Overt clinical signs, body weight changes, food intake
In rats, the low-dose, long-term feeding of FireMaster(R)
(Byrne et al., 1987, 1988) or technical octabromobiphenyl (Norris
et al., 1973) had no effect on food consumption and body weight
(Table 85). Nevertheless, the fur of PBB-fed animals was slightly
less dense and slightly coarser than that of control animals (Byrne
et al., 1987). A dose-dependent decline in survival time (Gupta
et al., 1983b), and body weight reductions or depressed rates of
body weight gain as a function of time and dose (Luster et al.,
1980; Gupta et al., 1981, 1983a,b) have been found in rats orally
dosed with FireMaster(R) (Table 84); also there was no significant
difference in food consumption between treated and control animals
(Gupta et al., 1981, 1983a).
8.4.1.2 Haematology and clinical chemistry
Several haematological and clinical chemistry parameters were
altered, some in only one sex (Table 84: Kimbrough et al., 1977;
Luster et al., 1980; Bernert et al., 1983; Gupta et al., 1983a).
Rats of both sexes had decreased haemoglobin and packed cell volume
values (Gupta et al., 1983a), a dose-related increase in serum
cholesterol (Bernert et al., 1983; Gupta et al., 1983a), and a
dose-related hepatic porphyria (Gupta et al., 1983a).
8.4.1.3 Morphological changes
Most of the livers of treated rats from higher dose groups were
pale or slightly yellow and mottled (Gupta et al., 1983a) and
contained white necrotic foci (Gupta et al., 1983b). Porphyrin
accumulation in the liver, bone, and teeth, seen as red fluorescence
under UVR, was more pronounced in treated females compared with
treated males (Kimbrough et al., 1977; Gupta et al., 1983a,b). After
withdrawal of treatment, visual fluorescence declined slightly in
the lower dose groups (1 and 3 mg FM/kg body weight), but remained
the same in the 10 mg FM/kg body weight dose groups (males and
females) during lifetime observation (Gupta et al., 1983a,b).
In long-term studies, an increase in the relative liver weights
of rats exposed to FireMaster(R) or a related mixture was usually
found (Kimbrough et al., 1978; Gupta et al., 1981, 1983a; Sepkovic &
Byrne, 1984; Byrne et al., 1987), but in some instances (Bernert et
al., 1983; Byrne et al., 1988), there was no effect on liver weight
(see Tables 84 and 85). [During a six-month exposure to
FireMaster(R), the increases in absolute and relative liver
weights were dose-related (Gupta et al., 1983a)].
Long-term feeding of commercial octabromobiphenyl (OcBB) also
caused an increase in relative liver weights (Sepkovic & Byrne,
1984; Table 85). Rats fed OcBB for 4 weeks still had increased liver
weights at 18 weeks of recovery or had returned to near normal
limits, depending on the concentration of OcBB in the test diet (Lee
et al, 1975a; Table 85).
The only individual PBB congener tested over the long-term,
namely 2,2',4,4',5,5'-hexabromobiphenyl, caused an increase in the
relative liver weight in male rats only (Gupta et al., 1981; Table
84).
Weight changes following PBB exposure, noted in organs other
than the liver, included a decrease in the thymus (Luster et al.,
1980; Gupta et al., 1981, 1983a), an increase in the spleen (Luster
et al, 1980; Gupta et al., 1983a), a decrease in the adrenal glands
(Luster et al., 1980; Byrne et al., 1988), and an increase in the
thyroid and ovary (Sepkovic & Byrne, 1984) (see Tables 84 and 85).
8.4.1.4 Histopathological changes
Hepatocellular alterations (exclusive of hepatocellular
carcinomas), observed in long-term studies with FireMaster(R) (see
Table 84), consisted mainly of enlarged hepatocytes, fatty
infiltration, proliferation and disorganization of RER, single cell
necrosis, multinucleated cells, microabscesses, atypical foci, and
bile duct proliferation (Kimbrough et al., 1977, 1978, 1981; Gupta
et al., 1981, 1983a,b).
In contrast to FireMaster(R)-treated rats, rats given
2,2',4,4',5,5'- hexabromobiphenyl over 30 days tended to recover
when examined during postexposure periods (Gupta et al., 1981).
Myelin figures, induced by the feeding of technical OcBB,
persisted, together with fatty changes, as late as 18 weeks after
withdrawal of a test diet containing 1000 mg OcBB/kg; however, the
normal morphology of RER was restored independently of the
occurrence of myelin figures, after treatment was discontinued (Lee
et al., 1975a).
Although there was no significant difference in the weights of
the thyroid gland, slight to moderate microscopic changes were
observed, primarily in male rats exposed for 6 months to 10 mg
FireMaster(R)/kg body weight. The thyroid gland had thin, sparse,
or bluish colloid with basophilic stippling and some follicles were
lined with columnar epithelium and contained a few epithelial
papillary projections (Gupta et al., 1983a).
The kidneys of male rats equally treated consistently showed
atrophy of a few glomerular tufts with marked dilatation of Bowman's
capsule, which contained amorphous eosinophilic staining material. A
few glomerular tufts also appeared oedematous (Gupta et al., 1983a).
Male rats, exposed to FireMaster(R) for 6 months (dose levels:
1-10 mg/kg body weight per day) and observed for a lifetime, showed
a higher incidence of chronic progressive nephropathy than the
controls. Their kidneys were characterized by eosinophilic
proteinaceous casts, sclerosis and thickening of glomerular tufts
and Bowman's capsule, mononuclear leukocytic infiltration, and
interstitial fibrosis (Gupta et al., 1983b).
Gastric ulcers and hyperplastic gastropathy of the glandular
portion of the stomach were found with significantly increased
incidence in male rats held under the same conditions (dose levels:
3 or 10 mg FM/kg body weight per day). Microscopic lesions consisted
of hyperplasia of the mucosal epithelium, glandular metaplasia to
goblet cells, hyperchromasia, and increased mitosis (Gupta et al.,
1983b).
8.4.2 Mouse
As far as it has been tested (Table 84), FireMaster(R) has
not produced any significant effects on food consumption in mice of
both sexes (Gupta et al., 1981, 1983a). Interestingly, there were
increases in the body weights of female mice with long-term exposure
to FireMaster(R), while a decrease occurred only in males at the
high dose (Luster et al., 1980; Gupta et al., 1983a,b). The high
dose of FireMaster(R) also shortened the survival time of males
(Gupta et al., 1983b). There were no significant differences in food
consumption and body weight gain in mice treated with
2,2',4,4',5,5'-hexabromobiphenyl (Gupta et al., 1982).
A number of haematological and clinical chemistry changes have
been found in mice exposed for 6 months to FireMaster(R) (Table
84: Gupta et al., 1983a). Hepatic porphyrin levels were increased in
a dose-related manner after a six-month exposure (Gupta et al.,
1983a), but (unlike levels in rats) they tended to decrease
following cessation of exposure (Gupta et al., 1983b).
Organ weight changes noted in long-term studies on mice (Table
84) consisted of an increase in liver (Gupta et al., 1981, 1983a,b)
and spleen (Luster et al., 1980; Gupta et al., 1983a) weights and a
decrease in uterine weights (Gupta et al., 1983a). Most of the
affected mice observed for a lifetime contained serosanguineous
fluid in the peritoneal cavity (Gupta et al., 1983b). The livers of
mice with long-term exposure were pale and contained, primarily in
males, grayish white foci (Gupta et al., 1981, 1983a).
Microscopic alterations were marked swelling of hepatocytes,
foamy or vacuolated cytoplasm with hyaline bodies, focal coagulative
necrosis or scattered single cell necrosis of hepatocytes, and
atypical hepatocellular foci (Gupta et al., 1983a). Hyperplasia was
observed in the follicular cells of thyroid (Gupta et al., 1983b).
Adverse effects (besides liver carcinoma; see section 8.7),
observed after an 18-month exposure of mice to technical
nonabromobiphenyl (Bromkal 80-9D), included decreases in body
weight, enlargement of the thyroid gland, and biochemical
alterations (Momma, 1986; Table 85).
8.4.3 Cattle
Except for a single animal, cattle with long-term exposure to
low doses of FireMaster(R) FF-1 or BP-6, observed for up to 220
days post-treatment (see Table 84) did not show any adverse effects
with regard to food intake, clinical signs, clinicopatho logical
changes, or performance (Durst et al., 1978a,b; Moorhead et al.,
1978; Robl et al., 1978). However, reproductive dysfunction occurred
more frequently (Willett et al., 1980; see also section 8.5).
8.4.4 Mink
Minks were found to be very susceptible to PBB toxicity (Table
85: Aulerich & Ringer, 1979). When fed with FireMaster(R) for
several months, they responded with food rejection, loss of weight,
an unthrifty appearance, and death. Upon necropsy of animals that
died, increases in relative liver and kidney weights and a fatty
infiltration of the liver were evident. A relatively low daily
intake of FireMaster(R) by female mink had an adverse effect on
their reproductive performance (see also section 8.5).
8.4.5 Rhesus monkey
Rhesus monkeys are also among the species more sensitive to
FireMaster(R) (Table 85: Allen et al., 1978; Allen & Lambrecht,
1978; Lambrecht et al., 1978). At exposures of 1.5-300 mg FM/kg
feed, they developed weight loss or a lack of weight gain,
haematological and clinical chemistry changes, loss of hair, skin
lesions, oedema, enlargement of the heart and liver, areas of
haemorrhage in the adrenal glands; reduced spermatogenesis, and
immune incompetence. Microscopic changes in the liver included
enlarged hepatocytes with fatty infiltration. The most severe lesion
was hyperplastic gastroenteritis and the accompanying ulcerations.
Exposures to 25 or 300 mg FM/kg feed also caused death. The female
monkeys fed with lower doses of FireMaster(R) (0.3 mg/kg diet),
for over one year, did not develop any of the signs of intoxication
noted above, except for weight loss. However, they were affected by
reproductive dysfunctions (see also section 8.5).
8.4.6 Pre- and perinatal exposure
Long-term effects following pre- or perinatal exposure to
FireMaster(R) have been recorded in rats and cattle.
Stunted growth, increased mortality rates, and liver tumours
were observed in the offspring of Sherman rats given 200 mg
FireMaster(R) FF-1/kg body weight on days 7 and 14 of pregnancy,
when a total of 50 male and 50 female offspring per group were
followed until they were 2 years old (Groce & Kimbrough, 1984).
Sprague-Dawley rats (No. = 10), perinatally exposed (day 8 of
pregnancy-28 days post partum) to FireMaster(R) BP-6 (100 mg/kg of
feed) and weaned on to a PBB-free diet, had increased relative liver
weights at 28 and 150, but not 328 days of age. Although the liver
was not enlarged 10 months after weaning, hepatic histopathological
alterations, such as cellular swelling, vacuolation, some necrosis,
and eccentric and pyknotic nuclei were observed throughout this
residual phase. Other long-lasting alterations were stimulation of
renal and hepatic microsomal enzymes, and a reduction in the
duration of anaesthesia produced by pentobarbital (McCormack et al.,
1980). In a multigeneration study (McCormack et al., 1981), rats
perinatally exposed to FireMaster(R) BP-6, as described above
(F1-generation), were allowed to mature sexually and bred with
littermates to produce the F2-generation. Even these F2-animals
exhibited increased relative liver weights and histopathological
liver changes at 28 days of age. The light microscopic changes
included vacuolation, focal necrosis, pyknotic nuclei, swelling, and
myelin bodies. However, the severity of lesions was much less
prominent in F2-animals than in F1-animals.
PBBs given to a single generation also produced hepatic and
renal microsomal enzyme stimulation, a reduction in the duration of
anaesthesia elicited by pentobarbital or a large dose of
progesterone, and a decrease in the concentration of vitamin A in
the liver, in both subsequent generations (F1 and F2).
Cows whose dams or granddams had received daily oral doses of
FireMaster(R) BP-6 (up to 250 mg/day) for 60-202 days showed no
general health problems, but they had conception difficulties
(Willett et al., 1982).
8.5 Reproduction, embryotoxicity, and teratogenicity
Effects of PBBs on reproduction (reproductive system, overall
reproductive performance, embryotoxicity, teratogenicity) have been
summarized in Tables 86 and 87. Most of the studies refer to the
FireMaster(R) mixture. Few reports deal with technical
octabromobiphenyl (Aftosmis et al., 1972a; Waritz et al., 1977) or
technical decabromobiphenyl (Millischer et al., 1979), and, among
the individual PBB congeners, only 2,2',4,4',5,5'-hexabromobiphenyl
has been evaluated by a few authors.
8.5.1 PBB mixtures
8.5.1.1 Mammals
a) Reproductive system and performance.
As listed in Tables 74 and 75, administration of
FireMaster(R) by gavage and in the diet had no effect on the
weight of male (Garthoff et al., 1977; Corbett et al., 1978a; Harris
et al., 1978b, Gupta et al., 1981; Castracane et al., 1982) or
female (Gupta et al., 1981) sex organs in rats and/or mice. An
exception was an increase in the uterine weight of mice dosed for 6
months with FireMaster(R) (Gupta et al., 1983a). Testes weights in
rats fed commercial OcBB also remained unaffected (Norris et al.,
1973). However, there was a reduction in the ventral prostate
weight-to-body weight ratio in pubescent male rats (Johnston et al.,
1980; Table 87) and a delay in vaginal opening in female rats after
perinatal exposure to FireMaster(R) (Harris et al., 1978a;
McCormack et al., 1981; Tables 86 and 87). Both findings may suggest
retarded sexual maturation.
Male rats, moribund from multiple doses of FireMaster(R),
exhibited degenerative and hyperplastic changes in the ductus
deferens (hyperplasia and squamous metaplasia with keratinization in
the epithelial lining of the ductus deferens). However, such changes
were not observed in surviving male rats, examined six months after
the treatment. Lesions found in surviving animals were acute
prostatitis (30 mg/kg body weight per day) and the presence of sperm
granuloma in the epididymis (100 mg/kg body weight per day) (Gupta &
Moore, 1979; Table 86). A rhesus monkey, exposed to FireMaster(R)
in the diet, had hypoactive seminiferous tubules at death (Allen
et al., 1978; Table 87). Little work was done on the possible
impairment of spermatogenesis. Young bulls may develop testicular
atrophy and reduced spermatogenesis when exposed to FM (Robl et al.,
1978), but more numerous controlled studies are needed.
The estrous cycle of cows (No. = 8) was not affected during
administration of FireMaster(R) FF-1 (0.3 mg/kg body weight per
day) for 158-228 days and during a recovery period of 112-182 days
(Robl et al., 1978).
The estrous cycle of female rats was increased in length after
perinatal and subsequent dietary exposure to FireMaster(R) BP-6
(Johnston et al., 1980; Table 87) at concentrations of 100 mg/kg
(equivalent to 5 mg/kg body weight per day). Prolonged menstrual
cycles were seen in rhesus monkeys after consuming FireMaster(R)
FF-1 for six months at a concentration of 0.3 mg/kg (equivalent to
0.02 mg/kg body weight per day). This alteration was correlated with
decreased serum progesterone levels (Allen et al., 1978; Lambrecht
et al., 1978; Table 87).
Table 86. Summary of effects of PBBs on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): dosing studies
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral single 400, 800 reductions in fetal and placental Beaudoin
(Wistar) dose one of weight; fetal death (primarily (1977)
(3-15) gd 6-14 800 mg/kg body weight per day at
s.t.: gd 20 day 7-12); malformed (cleft
palate; diaphragmatic hernia)
fetuses (400 mg/kg body weight
per day: 0-11.8%; 800 mg/kg body
weight per day: 0-60%)
FM FF-1 rat (No. oral multiple 100 (total none no effects on number of Ficsor &
not doses (6) gd dose: 600 fetuses, dead implants, Wertz (1976);
specified 6-16 at 2 day mg/kg body fetal malformation Wertz &
(15) intervals weight Ficsor (1978)
s.t.: gd 19
FM BP-6 rat oral multiple 0.25-10 reduced fetal weight and no effects on implantation Harris
(Sprague- doses gd 7-15 mg/day crown-rump length (only number of live fetuses or et al. (1978a)
Dawley) s.t.: gd 20 0.25 mg/day) gross malformations
(6-8)
s.t.: 48, 60 10 mg/day reductions in offspring weight no effects on litter size Harris
days of age (from 3 days of age onwards, and birth weight et al. (1978a)
more pronounced in male pups);
increased postnatal mortality (at
weaning 14.3% versus 1.5% in
control); delay in vaginal opening
of female offspring
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral multiple 8-160 mg reduced implantation rates no malformed fetuses Beaudoin
(Wistar) doses gd 0-14 per animal (80-160 mg); fetal death (1979)
(No. not on alternate (total
specified) days dose)
FM FF-1 rat oral multiple 100 moribund rats: degenerative and Gupta &
(Fischer doses (22) hyperplastic changes in the ducts Moore (1979)
344/N) over 30 days deferens (73 days after
(9 males) s.t.: up to 6 treatment); surviving rats: sperm
months granuloma in the epididymis (6
months after treatment; 1/2e)
30 acute prostatitis (4/9)e
FM FF-1 rat oral multiple 200 reduction in offspring survival Groce &
(Sherman) doses (2) gd 7 rate-to-weaning Kimbrough
(16) and 14 o.t.: (1984)
up to Weaning
(21 days of age)
FM BP-6 rat 1) in vivo- 800 inhibited rate of embryo Fisher (1980);
(Wistar) treatment: oral development in vitro (effects on Beaudoin &
(7) single dose gd 9 axial rotation, heart rate, neural Fisher (1981)
s.t.: gd 10 tube closure, formation of the
2) whole embryo anterior limb buds, somite
culture: 24 h development, and establishment of
visceral yolk sac circulation);
reduction in DNA content;
pericardial oedema
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
DeBB rat oral multiple 10-1000 none no teratogenicity or Millischer
(Sprague- doses gd 6-15 embryotoxicity et al. (1979)
Dawley) s.t.: gd 21
(25)
2,2',4,4' mouse oral multiple 0.3-32 none no fetotoxicity or Lucier et al.
5,5'-hexa- (C.D. - 1) doses gd 10-16 teratogenicity; no effects (1978)
bromobiphenyl (up to 25) s.t.: gd 19 on fertility, gestation,
(purity o.t.: 4, 20 viability, survival, and
> 97% days of age lactation indices
FM BP-6 cow (6) oral multiple 25 g/day abortion (3/6)e or fetal Durst et al.
doses during death (3/6)e (1977, 1978b);
pregnancy for Moorhead
32-60 days et al. (1977)
o.t.: up to 60
days
BM BP-6 cow oral multiple 0.25 and none Durst et al.
(3) doses during 250 mg/day (1978b)
pregnancy for
60 days
(1) for 180 days 250 mg/day stillbirth (due to dystocia)
o.t.: up to
305 days
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 cow (3-5) oral multiple 0.25-250 high incidence of dystocia; a total of 75 calves Willett et al.
(4 dose doses before mg/day increased birth weights of calves; were studied; no effects (1980, 1982)
groups) breeding or stillbirths; increased number of on growth, development,
during late inseminations required for and survival of calves
pregnancy for conception in 1 and 2 generation born alive
60-202 days offspring
o.t.: up to 5.5
years (1-5
parturition;
2 generations)
a FM = FireMaster(R); DeBB = technical decabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, of recorded); o.t. = observation time; s.t. = sacrifice time.
d In mg/kg body weight per day, unless otherwise specified.
e No. affected/no. treated.
Table 87. Summary of PBB effects on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): feeding studies
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 rat (Sprague- gd 7-18 100, 1000 decreasing fetal weight with suggestion of late Corbett
Dawley) s.t.: gd 20 increasing dosage fetal mortality et al.
(6-7) (high dose); no (1975)
teratogenicity
FM BP-6 rat (Sprague- gd 8-9th week 100 reduction in ventral no effect on fetal Johnston
Dawley) (at of age s.t.: prostate weight of male survival rate et al.
least 8) 9th week of age offspring; increased length (1980)
of estrous cycle of female
offspring
FM BP-6 rat (Sprague- gd 8-Weaning 100 reduction in survival no effects on length McCormack
Dawley) s.t.: Weaning rate-to-weaning (87% of of gestation, litter et al.
control value); delay in size, incidence
(8) (28 days of vaginal opening in female of gross external
age) offspring anomalies, pup body
weight at birth
OcBB rat (not gd 6-15 100, 1000, gastroschisis (one fetus abstract only (no Aftosmis
specified) s.t.: gd 20 10 000 at each level); anasarca on number of fetuses et al.
(one fetus at each of the information examined) (1972a)
two highest levels)
OcBB rat gd 6-15 100, 1000, no significant gastroschisis Waritz et al.
(ChR-CD) s.t.: gd 20 10 000 embryotoxicity or (1/259e: 1000 mg/kg: (1977)
(23-27) teratogenicity 1/1/283e
10 000 mg/kg);
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
anasarca (1/259e: 1000
mg/kg; 1/283e: 10 000
mg/kg); no effects on
growth or viability of
embryos
FM BP-6 mouse: gd 7-18 50, 100, dose-related decrease in fetal incidence of Corbett
(Swiss, ICR) s.t.: gd 18 1000 weight with increasing dosage; malformations et al.
(9-12) exencephaly (5/295g: 100, statistically (1975,
1000 mg/kg); cleft palate significant only 1978b)
(5/208g: all levelsg; compared with pooled
hydro-nephrosis (2/87: historical controls
1000 mg/kg)
FM mouse (Swiss- gd 4-16 100, 200 increase in number of dead or (abstract only) Preache
Webster) s.t.: gd 19 resorbed fetuses (200 mg/kg) et al.
(No. not (1976)
specified) gd 8-16 100, 200 decrease in fetal body weight
s.t.: gd 19 (200 mg/kg)
gd 8-16 50, 100 reduction in number of live no effects on Preache
o.t.: up to 6 offspring postnatal mortality or et al.
weeks of age body weight (1976)
days 1-29 pp 50, 100 increased mortality; decreased
o.t.: up to body weight
weaning
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
2,2',4,4'- mouse gd 6-15 100, 300, at 300 mg/kg: 17-182 fetuses were Welsch &
5,5'-hexa- (C57BL) o.t.: gd 17 500, 750, decrease in pregnancy rates of examined Morgan (1985)
bromobiphenyl (2-22) 1000 plug-positive mice;
(purity: at > 300 mg/kg:
> 99%) reduced fetal body weight;
malformed fetuses (cleft
palate combined with a "cystic
brain deviation": 5/166,
3/172, 5/46, 6/17g,
respectively, at 300-1000
mg/kg; minor abnormalities of
brain development); at >
500 mg/kg: reduced gravid
uterine weight
FM BP-6 pig (2-4) 2nd half of 10, 100, 200 increased mortality during no effect on litter Werner &
gestation and lactation size Sleight
lactation o.t.: (1981)
up to weaning
(4 weeks)
FM FF-1 mink (8 for 9 months 1 reduced litter size; reduced both male and female Aulerich &
females; (start before kit weight at birth and at 4 on PBB diet; no Ringer
2 males) breeding) weeks; increased mortality effects on breeding or (1979)
4 weeks) (birth to periods; no gestation
teratogenicity
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 rhesus for over 1 year 0.3 prolonged menstrual cycles; Allen et al.
monkey (start 7 months decreased serum progesterone (1978,
(7) prior to breeding) levels (4/7e after 6 months); 1979); Allen
excessive and prolonged & Lambrecht
implantation bleeding (2/7e); (1978);
abortion (1/7); stillbirth Lambrecht
(1/7e); reduced weight of et al.
infants at birth and at (1978)
weaning (4 months of age)
FM FF-1 rhesus for 25 weeks 25 hypoactive seminiferous tubules Allen et al.
monkey (at the time of death (1978)
(1 male)
FM BP-6 chicken (White for 9 weeks 20 none no effect on Cecil et al.
Leghorn) (35) hatchability (1974)
"PBB" chicken for 8 weeks 5, 10, 20 none no effects on egg Lillie et al.
(White production, egg (1975)
Leghorn) (20) weight, egg shell
thickness, and
fertility; no effects
on hatchability,
embryonal development
and progeny health
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 chicken for 8 weeks 20 increased mortality of progeny no effects on egg Cecil &
(White production, egg Bitman
Leghorn) (10) weight, egg shell (1978)
thickness, fertility,
hatchability, and
embryonic death
for 4-8 weeks 64, 200, reduced egg production or stop
640, 2000 in egg production at 200-2000
mg/kg; decreased hatchability
of fertile eggs; increased
mortality of progeny; reduced
growth rate of progeny;
embryonic deaths; embryonic
abnormalities (subcutaneous
oedema, oedematous cysts,
unabsorbed yolk)
FM FF-1 chicken for 5 weeks 0.2-3125 decline in egg production Polin &
(White (MELf: 30-45 mg/kg); loss of Ringer
Leghorn) (24) egg production (> 625 mg/kg, (1978a,b)
within 2 weeks);
reduced hatchability (MELf:
30-45 mg/kg); dose-related
increase in offspring
mortality (MELf: 30-45 mg/kg;
embryonic oedema
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 chicken (10) for 21 days 80 decline in hatchability; oedema no effect on fertility Polin et al.
of embryos and newly hatched (1979)
chicks
2,2',4,4', chicken (10) for 21 days 52 none no decline in
5,5'-hexa- hatchability; no
bromobiphenyl oedema of chicks and
(purity: and embryos
not
specified)
FM Japanese for 9 weeks 10, 20, 100 at 100 mg/kg: no eggshell thinning Babish
quail (No. reduced egg production (17% et al.
not specified) versus 68% in controls); no (1975a)
hatchability; embryonal deaths
FM FF-1 Japanese for 5 weeks 80 reduced egg production fertility and Polin et al.
quail hatchability not (1982);
(Coturnix significantly Bursian
coturnix different from et al.
japonica) controls (1983)
(36 males,
36 females)
a FM = FireMaster; OcBB = technical octabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, if recorded; exception: Waritz et al. (1977): sperm day = gd 1); pp = postpartum;
o.t. = observed time; s.t. = sacrifice time.
d In mg/kg of feed, unless otherwise specified.
e No. affected/no. treated.
f MEL = Minimum effective level.
g As reported by Corbett et al. (1978a).
The number of inseminations required for conception was
increased in cows whose dams or grand dams had received oral doses
of FireMaster(R) BP-6 (Willett et al., 1982; Table 86).
Most of the studies evaluating the effects of PBBs on
reproductive performance started with treatment during pregnancy
(Tables 86 and 87). In few instances, were females exposed before
mating (Allen et al., 1978; Aulerich & Ringer, 1979; Willett et al.,
1980, 1982), and animals of both sexes were treated in only one
study (Aulerich & Ringer, 1979). Under these conditions,
FireMaster(R) reduced litter size in mice (Preache et al., 1976)
and minks (Aulerich & Ringer, 1979), but not in rats (Harris et al.,
1978a; McCormack et al., 1981) and pigs (Werner & Sleight, 1981).
The adverse effect on litter size was observed in minks given
PBB-contaminated poultry as well as in those fed FireMaster(R),
directly added to the diet (Aulerich & Ringer, 1979). Stillbirths
occurred in cattle (Durst et al., 1978b; Willett et al., 1982) and
rhesus monkeys (Allen et al., 1978; Lambrecht et al., 1978).
Reduced birth weights were reported in rhesus monkeys (Allen et al.,
1978; Lambrecht et al., 1978), minks (Aulerich & Ringer, 1979), and
mice (Corbett et al., 1978b) at intakes of 0.3, 1, or 50-1000 mg
FireMaster(R)/kg feed, respectively (see Table 87). On the other
hand, increased birth weights of calves resulted in a higher
incidence of dystocia in cows (Durst et al., 1978b; Willett et al.,
1980, 1982). The growth of progeny during lactation (indicated by
weight at weaning) was also reduced by FireMaster(R) in rats
(Harris et al., 1978a), mice (Preache et al., 1976), minks (Aulerich
& Ringer, 1979), and rhesus monkeys (Allen et al., 1978; Lambrecht
et al., 1978).
In the study by Lambrecht et al. (1978), 14 female rhesus
monkeys were used as an animal model (7 controls, 7 treated) to
evaluate the toxicological effects of FM FF-1. The substance was
added to the pelleted diet at a concentration of 0.3 mg/kg and 200 g
of this diet was fed per day; after a dosing period of 7 months, the
animals had ingested a total of 10.5 mg FM FF-1. Although the food
consumption during this period was unaffected, there was a loss of
7.4% of the initial body weight in the treated group. The total
exposure is not stated, but, on the basis of information given in
the paper, it appears that the dams had been exposed for a total of
12.5 months at the time of the birth of the infants. After 6 months,
menstrual cycles in 4/7 monkeys were lengthened (controls: 28 days;
treated monkeys: 31 days). The treated animals showed a
corresponding flattening of the progesterone peak. After mating, 5/7
treated monkeys delivered normal-appearing but small infants (455 g
vs 519 g in controls) with reduced weight gain in these infants
during the postnatal period. Two out of 7 treated monkeys aborted a
mummified fetus and a stillborn infant, respectively. All control
animals delivered normal-appearing infants.
The survival rate-to-weaning was adversely affected in the
offspring of rats, mice, pigs, and minks, but not in calves (see
Tables 86 and 87). FireMaster(R) levels producing the effects were
200 mg/kg body weight per day (Groce & Kimbrough, 1984), or
10 mg/day (Harris et al., 1978a), or 100 mg/kg feed (McCormack
et al., 1981) in rats, 100 mg/kg feed in mice (Preache et al., 1976)
and pigs (Werner & Sleight, 1981), and 1 mg/kg feed in minks
(Aulerich & Ringer, 1979).
For neurotoxic effects after perinatal exposure see section
8.11.2.
Ranges of maternal toxic parameters can be derived from Tables
71, 72, 74, and 75.
b) Embryotoxicity and teratogenicity
PBBs were embryotoxic in rats, mice, cattle, and rhesus
monkeys. Rats and cows appeared to be less susceptible than mice and
rhesus monkeys (see Tables 86 and 87).
Treatment of rats with a single high dose of FireMaster(R)
(> 400 mg/kg body weight) on day 6 of pregnancy resulted in 100%
embryo resorption. Generally, the number of fetal resorptions
depended on the dose and the pregnancy stage at treatment. For
example, after day 8 (400 mg/kg body weight dose) or day 12
(800 mg/kg body weight dose), the embryolethal effect of
FireMaster(R) declined abruptly (Beaudoin, 1977). Reductions in
both fetal and placental weights were observed only at high doses
(> 400 mg/kg body weight), and the most susceptible period of
pregnancy was days 11-13 (Beaudoin, 1977).
The long-term administration of lower doses (total dose: 8 or
40 mg FireMaster(R)/animal) during pregnancy (from day 0) markedly
increased embryonic death over that seen following administration of
an equivalent single dose (Beaudoin, 1979). However, long-term
intake of relatively low doses (see Tables 86 and 87) during late
gestation (start: day 7) did not influence fetal survival rate
(Corbett et al., 1975; Harris et al., 1978a; Johnston et al., 1980),
but decreased fetal weight (Corbett et al., 1975; Harris et al.,
1978a). A total dose of 600 mg FireMaster(R)/kg body weight, given
at two-day intervals (start: day 6) had no effects on fetal death or
fetal weight (Wertz & Ficsor, 1978).
No significant effects on the growth or mortality of embryos
were noted after the exposure of rats (Tables 86 and 87) to
technical octabromobiphenyl (Waritz et al., 1977) or technical
decabromobiphenyl (Millischer et al., 1979). Single cases of fetal
oedema have been observed in OcBB-treated rats (Aftosmis et al.,
1972a; Waritz et al., 1977).
The incidence of dead or resorbed fetuses was increased in mice
fed 200 mg FireMaster(R)/kg feed on days 4-16 of pregnancy
(Preache et al., 1976). Feeding several concentrations of FM from
day 7 (Corbett et al., 1975) or day 8 (Preache et al., 1976) of
pregnancy reduced fetal weight (Table 87).
Cattle given 25 g FM/day had 3 abortions and 3 dead fetuses
from 6 treated animals (Durst et al., 1977; Table 86). The fetuses
were oedematous and haemorrhagic, concomitant uterine lesions were
haemorrhage and necrosis of the cotyledons (Moorhead et al., 1977).
Two out of 7 rhesus monkeys fed FM (Table 87) had long
implantation bleedings, one aborted a mummified fetus at 146 days of
gestation and one gave birth to a stillborn infant at 154 days. The
remaining 5 monkeys delivered small infants at 156-165 days of
gestation (normal gestation: 165 days) (Allen et al., 1978;
Lambrecht et al., 1978).
Terata due to PBB exposure have been reported only in rats and
mice (Tables 86 and 87). A single high dose of FireMaster(R)
produced cleft palate and diaphragmatic hernia in rats (Beaudoin,
1977). The majority of malformations were found following
administration of 800 mg/kg body weight on day 11, 12, or 13 of
gestation. No terata were noted in rats after multiple doses of
FireMaster(R) (Table 86) (Harris et al., 1978a; Wertz & Ficsor,
1978; Beaudoin, 1979) and after feeding diets containing up to
1000 mg FireMaster(R)/kg feed (Corbett et al., 1975). In contrast
to rats comparably treated (50-1000 mg FM/kg maternal diet), a
higher incidence of exencephaly and cleft palate was seen in fetal
mice, compared with pooled historical controls, but not compared
with concurrent controls, though neither of these anomalies was seen
in control fetuses (Corbett et al., 1975, 1978b).
It is unclear whether the few cases of fetal gastroschisis,
observed in rats fed technical OcBB (Table 87), are PBB-related or
fortuitous (Aftosmis et al., 1972a; Waritz et al., 1977). No
teratogenicity was found in rats exposed to DeBB (Millischer et al.,
1979; Table 86).
Embryo development was studied in vitro. Maternal rats
received a single, oral dose of 800 mg FM/kg body weight on
gestation day 9, and the embryos were isolated on day 10 (24 h
post-treatment) for cultivation over a 24- or 42-h period (Fisher,
1980; Beaudoin & Fisher, 1981). Teratogenic effects observed (see
Table 86) were not correlated with types of malformations found in
earlier in vitro experiments (Beaudoin, 1977). Some developmental
disturbances tended to be corrected after 42 h of PBB-free culture.
In addition to retarded development, there was a significant
reduction in the DNA contents of cultured embryos (Fisher, 1980).
The survival rate of embryos was not affected during the cultivation
period (Beaudoin & Fisher, 1981).
8.5.1.2 Avian species
Avian reproduction was studied primarily in chickens.
Cockerels fed FM at various levels (10-250 mg/kg feed), for several
weeks, showed inconsistent weight changes in the comb and testes
(see Table 75: Ringer & Polin, 1977; Ringer, 1978; Dharma et al.,
1982). Histologically, lipid infiltration into testicular parenchyma
was noted (Ringer & Polin, 1977). No studies on the reproductive
performance of these birds were made.
Detrimental effects were seen when FireMaster(R) was fed to
hens of both White Leghorn chickens and Japanese quails, the only
two species studied (Table 87). Chickens (Cecil et al., 1974; Lillie
et al., 1975; Cecil & Bitman, 1978; Polin & Ringer, 1978a,; Polin
et al., 1979) appeared to be more sensitive to FireMaster(R) than
the Japanese quails (Babish et al., 1975a; Bursian et al., 1983), on
the basis of egg production and hatchability. Egg production in
chickens was reduced by dietary levels of FireMaster(R) as low as
30-45 mg/kg (Ringer & Polin, 1977), while the lowest effective level
reported for Japanese quails was 80 mg/kg (Bursian et al., 1983).
Chickens stopped laying when concentrations of FireMaster(R) in
diets exceeded 600 mg/kg (Cecil & Bitman, 1978). The hatchability of
eggs was adversely affected by 30-45 mg FireMaster(R)/kg feed in
chickens (Ringer & Polin, 1977) and by 100 mg FireMaster(R)/kg
feed in Japanese quails (Babish et al., 1975a).
Increased mortality (Cecil & Bitman, 1978; Polin & Ringer,
1978a, 1978b) and reduced growth rates (Cecil & Bitman, 1978) were
seen in the offspring of chickens fed FireMaster(R) at low levels
(< 64 mg/kg; Table 87).
Embryonic deaths occurred in chickens (Cecil & Bitman, 1978;
50% of deaths on the last day of incubation) and in Japanese quails
(Babish et al., 1975b; 40% of deaths on the first day or two of
development). The most common abnormality of embryos and newly
hatched chicks after maternal feeding of FireMaster(R) was oedema
(Cecil & Bitman, 1978; Polin & Ringer, 1978b; Polin et al., 1979).
Although there was a reduction in the feed intake of exposed
chickens, the decreased hatchability, higher incidence of
abnormalities, and poorer progeny survival could not be reproduced
by pair-fed control birds (Cecil & Bitman, 1978). At high dietary
levels (> 600 mg FM/kg of feed), the direct effects of
FireMaster(R) on egg production could not be separated from the
effects of reduced food consumption (Cecil & Bitman, 1978).
Adverse effects on the reproduction of chickens could return to
normal after withdrawal of FireMaster(R) from the diet. Time for
recovery depended on the concentration of PBB in the feed (Cecil &
Bitman, 1978; Polin & Ringer, 1978a).
8.5.2 Individual PBB congeners
Individual PBB congeners have not been reported to cause weight
changes in sex organs. Similarly, the testes of rats or cockerels,
did not show any remarkable histological changes after treatment
with 2,2'-dibromobiphenyl, 2,2',3,4,4',5,5'-heptabromobiophenyl,
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), 2,3',4,4',5,5' (BB 167),
or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) (Moore et al., 1979a;
Gupta et al., 1981; Akoso et al; 1982a; Dharma et al., 1982; Render
et al., 1982). An exception was a decrease in the gravid uterine
weight of pregnant mice given more than 500 mg BB 153/kg body weight
(Welsch & Morgan, 1985).
Embryotoxicity and other reproductive parameters have been
studied only with BB 153 mice (Lucier et al., 1978; Welsch & Morgan,
1985) or chickens (Polin et al., 1979). BB 153 did not cause the
deleterious effects (decline in hatchability, oedema) that were
produced by equivalent dietary levels of FireMaster(R) in chickens
(Polin et al., 1979; Table 87). However, BB 153 was capable of
producing cleft palate and cystic lesions in the region of the
cerebellum in mouse embryos (Welsch & Morgan, 1985; Table 87). These
adverse effects were observed at exposure concentrations that also
cause toxicity in dams. Another less complete study (Lucier et al.,
1978; Table 86) did not find developmental toxicity of BB 153 in
mice, possibly because of lower exposure levels, later onset of
exposure, and strain differences.
8.6 Mutagenicity and related end-points
The potential of PBBs for mutagenicity has been tested with
several in vitro and in vivo assays referring to four major test
categories: microbial and mammalian cell mutagenesis; mammalian cell
chromosomal damage (cytogenetic tests; in vitro and in vivo);
mammalian cell transformation in vitro; and DNA damage and repair
(UDS). Results have been summarized in Table 88. With one exception
(Kohli et al., 1978), all studies failed to indicate any
mutagenicity of individual PBB congeners or commercial PBB mixtures.
Haworth et al. (1983) did not confirm the mutagenic effect of
4-bromobiphenyl observed by Kohli et al. (1978), but they used
different Salmonella strains.
However, FireMaster(R) gave positive results in a recently
developed test, the Microscreen assay using lambda prophage
induction in Escherichia coli. This test has been found to be
sensitive in detecting carcinogens which are negative in mutagenesis
assays (Rossman et al., 1991).
8.7 Carcinogenicity
8.7.1 Carcinogenicity in long-term toxicity studies
The carcinogenic effects of PBB in long-term toxicity studies
have been compiled in Table 89. From this table, it is evident that
the principal site of tumours was the liver. The incidence of
hepatocellular carcinoma was significantly increased in both sexes
of mice and rats receiving relatively high oral doses of the
FireMaster(R) mixture.
The results of one of these studies (Gupta et al., 1983b),
carried out by the NCI/NTP (USA), were interpreted as
FireMaster(R) showing carcinogenic effects (Haseman et al., 1984;
Tennant et al., 1986). In this study, a statistically significant
higher incidence of liver tumours was found at multiple doses of
3 mg FM/kg body weight per day (male rats: 21%) and of 10 mg FM/kg
body weight per day (female/male rats: 35/23%; female/male mice:
88/95%). Atypical foci and neoplastic nodules were found at lower
doses (Table 89). Dose-responses were statistically significant (P
< 0.01) in male and female rats with regard to atypical foci,
hepatocellular carcinoma, and cholangiocarcinoma, and, in female
rats, also with regard to neoplastic nodules (Gupta et al., 1983b).
Hepatocellular carcinoma could be produced, even after single dosing
with FireMaster(R) (Kimbrough et al., 1981). Rats given a single
dose of 1000 mg FM/kg or 12 doses of 100 mg FM/kg body weight, by
gavage, developed incidences of carcinomas of 41.4 and 67.8%,
respectively (Kimbrough et al., 1981). Liver tumours were also
observed in female and male rats two years after perinatal exposure
to FireMaster(R) (Groce & Kimbrough, 1984; Table 89). Although
concurrent controls did not show such lesions, the IARC Working
Group noted the lack of statistical significance (IARC, 1986).
Nevertheless, the incidences of 5.9% (females) and 9.6% (males),
respectively, exceeded the spontaneous incidence of carcinomas of
the liver in this particular strain of rat, which is reportedly less
than 1% (Groce & Kimbrough, 1984).
Recently, NTP performed further carcinogenicity studies on
FireMaster(R) to determine the following: (a) the effects of PBBs
in F344/N rats and B6C3F1 mice receiving adult (F1) exposure only
(0, 10, or 30 mg PBB/kg diet); (b) the toxic and carcinogenic
effects of PBBs in rats and mice receiving perinatal (Fo) exposure
only (10 mg/kg in rats and 30 mg/kg in mice); and (c) the effects of
combined perinatal and adult exposure to PBBs (NTP, 1993). In an
adult-only exposure study, both sexes in rats and mice receiving 10
or 30 mg/kg (0.5 or 1.5 mg/kg body weight per day) showed increased
incidences of hepatocellular adenoma/carcinoma, quite similar to
the results of the previous studies (Gupta et al., 1983b).
Table 88. Summary of PBB genetic toxicity test results
Assay Detailsb Metabolic activation PBBa Result Remarks References
Salmonella mutagenesis
S. typhimurium strains: TA 98 ± Aroclor 1254 FM FF-1 - Tennant
TA 100 induced liver - et al. (1986)
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 hexabromobiphenylc - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats and -
TA 1537 and Syrian hamsters
S. typhimurium strains: TA 1535 ± Aroclor DeBB - Millischer
(spot test; Ames) TA 1537 induced rat - et al. (1979)
TA 1538 liver S-9 -
S. typhimurium strain: TA 1538 - DeBB - mice receiving oral
(host-mediated doses of 5, 10, and
assay) 20 g/kg body weight
S. typhimurium strains: TA 98 ± Aroclor 1254 2-bromobiphenyl - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats -
TA 1537 and Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 3-bromobiphenyl -
TA 100 induced liver -
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
S. typhimurium strain: TA 1538 + Aroclor 1254 4-bromobiphenyld + Kohli et al.
induced rat liver S-9 (1978)
none -
S. typhimurium strains: TA 98 ± Aroclor 1254 4-bromobiphenyle - Haworth et al.
TA 100 induced liver (1983)
TA 1535 S-9 from rats and
TA 1537 Syrian hamsters
Mammalian cell mutagenesis
Adult rat liver (ARL) HGPRT locus none FM FF-1 (lot No. - at the highest non-toxic Tong et al.
epithelial cells 1312-FS) dose of 10-3 mol (1983); Williams
et al. (1984)
Human fibroblasts HGPRT locus rat hepatocytes FM FF-1 (lot No. - at the highest non-toxic
1312-FS) dose of 10-4 mol
Chinese hamster HGPRT and Na-K ± Aroclor 1254 induced FM BP-6 - cell survival Kavanagh et al.
V 79 cells ATPase loci rat liver S 15 unaffected (1-40 µg/ml) (1985)
Chinese hamster HGPRT locus ± Aroclor 1254 induced 3,3',4,4'-tetra- - cell survival
V 79 cells rat liver S 15 bromobiphenyl unaffected (1-10 µg/ml)
Chinese hamster HGPRT locus none 2,2',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (20-50 µg/ml)
WB rat liver cells HGPRT locus - 2,2',4,4',5,5'- - growth stimulation
hexabromobiphenyl effects (5-20 µg/ml)
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chinese hamster HGPRT locus none 3,3',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (7-12 µg/ml)
WB rat liver cells HGPRT locus - 3,3',4,4',5,5'- - reduced cell survival
hexabromobiphenyl
Mammalian cell chromosomal
damage in vitro
Chromosome CHO cells ± Aroclor 1254 FM FF-1 - Tennant et al.
aberrations induced rat (1986)
Sister chromatid CHO cells liver S-9 -
exchange
Chromosome CHO cells ± Aroclor 1254 hexabromobiphenyl - Galloway et al.
aberrations induced rat (1987)
liver S-9
Sister chromatid CHO cells ?f
exchange
Mammalian cell chromosomal
damage in vivo
(Cytogenetic effects)
Chromosome bone marrow cells - FM - colchicine-mitosis Ficsor & Wertz
aberrations rat (pregnant) synergism (1976)
six oral doses of
100 mg/kg body
weight
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chromosome bone marrow cells - FM - no colchicine-mitosis Wertz & Ficsor
aberrations mouse (male) synergism; increase in (1978)
single dose of 50 no. of gaps
or 500 mg/kg body
weight
No. of cells in rat (male) in diet for
mitosis 5 weeks: 5-500 mg/kg
feed
Chromosome bone marrow cells; - FM BP-6 - Garthoff et al.
aberrations spermatogonial (1977)
cells
Micronucleus bone marrow cells DeBB - Millischer
mouse (male and et al. (1979)
female) total dose
of 5, 10, or
20 g/kg body
weight at 2 doses
Mammalian cell transformation
in vitro
Transformation mouse Balb/c 3T3 ± non-induced FM FF-1 - Tennant et al.
cells male F 344 rat (1986)
hepatocytes
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
DNA damage and repair
(in vitro)
Unscheduled DNA hepatocyte primary - FM FF-1 - at the highest nontoxic Tong et al.
synthesis (UDS) cultures (HPCs) doses of 10-3 mol (mice, (1983);
from rat, hamster, hamsters) or 10-5 mol Williams et al.
and mouse (rats) (1984)
DNA damage and repair
(in vivo - in vitro)
UDS hepatocyte primary - FM FF-1 - Tennant et al.
cultures (HPCs) (1986)
from treated male
F 344 rats
UDS hepatocyte primary - FM FF-1 - simultaneous measurement Mirsalis et al.
cultures (HPCs) of hepatic cell (1985)
from treated proliferative ability:
B6C3F1 mice (males increased cell
and females) proliferation
a Commercial PBB mixtures: FM = FireMaster(R); DeBB = decabromobiphenyl.
b HGPRT = hypoxanthine-guanine phosphoribosyl transferase.
c CAS No.: 36355-01-8; source: Pfaltz & Bauer.
d Source of PBB: Aldrich & Eastman Chemicals.
e Source of PBB: Pfaltz & Bauer.
f There was a very slight (16%) increase in SCEs without S-9 doses that produced severe cell cycle delay.
Table 89. Summary of carcinogenic effects of PBBs in long-term toxicity studies
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M oral 1000 10 months liver: neoplastic nodules Kimbrough
(lot no. 7042) (Sherman) single dose (preliminary study) (F: 4/5; et al. (1977,
(in peanut oil) (5) 14 months M: 0/5); (F: 3/5; M: 2/5); 1978)
(control: 0/5)
FM FF-1 rat F oral 1000 23 months liver: trabecular carcinoma Kimbrough
(lot no. 7042) (Sherman) single dose (24/58 = 41.4% versus 0% in et al. (1981)
(in corn oil) (65) control) neoplastic nodules
(42/58 = 72.4% versus 0% in
control) foci or altered areas
(57/58 = 98.3% versus 1/53 in
control)
(16) 200 18-22 liver: no carcinoma
months neoplastic nodules
(5/16 = 31.2% versus 0% in
control) altered areas
(8/16 = 50% versus 1/19 in
control)
FM BP-6 rat F oral two total dose: 120 days liver: small numbers of enzyme Rezabek et
(in corn oil) (Sprague- doses over 13 and 130 altered foci (EAF) al. (1987)
Dawley) 24 h mg/kg body
weight
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat M oral 22 30 6 months liver: atypical nodules (2/9) Gupta &
(lot no. 1312 (Fischer doses over (after 1 liver: atypical nodules (1/2) Moore (1979)
FT) (in corn 344/N) 4.5 weeks 100 dose) epididymis: sperm granuloma (1/2)
oil) (9)
FM FF-1 rat F oral 12 100 24 months liver: trabecular carcinoma Kimbrough
(lot no. 7042) (Sherman) doses over (17/28 = 60.7% versus 0% in et al. (1981)
(in corn oil) (30) 4 months control) neoplastic nodules
(24/28 = 85.7% versus 1/25 in
control) foci or altered areas
(23/28 = 82.1% versus 1/25 in
control) adenocarcinoma
(1/28 = 3.6% versus 0% in control)
malignant tumour with metastases
to heart (1/28 = 3.6% versus 0%
in control)
Total malignant liver tumours:
19/28 (67.8% versus 0/25 in
controls)
FM FF-1 rat F, M oral 125 0.1, 0.3, 6 months liver: atypical foci (3/100); Gupta et al.
(lot no. 1312 (Fischer doses over 1, 3, 10 (after 1 urinary bladder: squamous cell (1983a)
FT) (in corn 344/N) 6 months dose) carcinoma (F: 1/51, high dose
oil) (51)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M oral 125 lifetime Gupta et al.
(lot no. 1312 (Fischer doses over (1983b)
FT) (in corn 344/N) 6 months
oil) (total: 320) (sacrifice of
10% animals
alive, 23
months post-
treatment)
F, M 0.1 liver: atypical foci
(M: 3/39 = 8%; F= 0/21)
neoplastic nodules
(M: 0/39; F: 2/21 = 10%)
carcinoma (M: 2/39 = 5%; F: 0/21)
0.3 liver: atypical foci
(M: 12/40 = 30%, P < 0.01; F:
1/21 = 5%) neoplastic nodules
(M: 1/40 = 2%; F: 0/21) carcinoma
(M: 0/40; F: 0/21)
FM FF-1 rat F, M 1 liver: atypical foci Gupta et al.
(lot No. 1312 (Fischer (M: 11/31 = 35%, P < 0.01; F: (1983b)
FT) (in corn 344/N 2/11 = 18%) neoplastic nodules
oil) (total: (M: 4/31 = 13%, P < 0.05; F: 2/11)
320) carcinoma
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
3 liver: atypical foci
(M: 13/33 = 39%, P < 0.01; F:
4/19 = 21%) neoplastic nodules
(M: 4/33 = 12%; F: 5/19 = 26%,
P < 0.01) carcinoma
(M: 7/33 = 21%, P < 0.01; F:
3/19 = 16%)
10 liver: atypical foci
(M: 12/31 = 39%, P < 0.01; F:
8/20 = 40%, P < 0.01 neoplastic
nodules (M: 1/31 = 3% F: 8/20 =
40%, P < 0.01 carcinoma (M:
7/31 = 23%, P < 0.01; F: 7/20 =
35%, P < 0.01; control: 0/33)
cholangiocarcinoma (M: 2/31 = 6 %,
F: 7/20 = 35%, P < 0.01)
FM FF-1 rat F, M perinatal total dose: 2 years liver: neoplastic nodules Groce &
(lot 7042) (Sherman) exposure: 400 mg/kg of age (M: 2/41 = 4,9%, F: 9/51 = 17.6%) Kimbrough
(in corn oil) (41-51) two maternal body trabecular carcinoma (1984)
oral doses weight (M: 4/41 = 9.6%;
on gestation F: 3/51 = 5.9%; controls: 0%)
days 7 and
14
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M adult only 0 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (Fischer exposure (in (M: 36%; F: 6%)
FT) 344/N) diet for oval cell hyperplasia
(50) 24 months) (M: 0%; F: 0%)
hepatocellular adenoma
(M 2%: F: 0%)
hepatocellular carcinoma
(M: 0%; F: 0%)
10 liver: eosinophilic focus (M:
90%, p < 0.01; F: 94%, P < 0.01)
oval cell hyperplasia (M: 22%,
P < 0.01; F: 24%, P < 0.01)
hepatocellular adenoma (M: 20%;
F: 20%) hepatocellular carcinoma
(M: 4%; F: 4%) hepatocellular
adenoma/carcinoma (M: 24%, P
< 0.001; F: 24%, P < 0.001)
30 liver: eosinophilic focus NTP (1993)
(M: 92%, P < 0.01; F: 96%, P
< 0.01) oval cell hyperplasia
(M: 74%, P 0.01; F: 84%, P
< 0.01) hepatocellular adenoma
(M: 76%, P < 0.001; F: 76%, P <
0.001); hepatocellular carcinoma
(M: 38%, P < 0.001; F: 8%)
hepatocellular adenoma/carcinoma
(M: 82%, P < 0.001; F: 78%, P
< 0.001)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M perinatal only 10 24 months liver: eosinophilic focus
(lot FF-1312- (Fischer exposure (M: 58%, P < 0.01; F: 38%, P
FT) 344/N) (female parents < 0.01) hepatocellular adenoma
(50) received (M: 10%; F: 0%)
FM-containing
diet beginning
60 days prior
to breeding and
throughout
gestation,
lactation, and
up to 4 weeks
postweaning)
FM FF-1 rat F, M combined 1:3e 24 months liver: eosinophilic focus NTP (1993)
(lot FF1312- (Fischer perinatal and (M: 80%; F: 38%)
FT) 344/N) adult exposure oval cell hyperplasia (M: 8%;
(50) F: 0%) hepatocellular adenoma
(M: 6%; F: 4%) hepatocelluar
carcinoma (M: 2%; F: 0%)
10:10f 24 months liver: oval cell hyperplasia
(M: 46% versus 22% in 10 mg/kg
adult exposure group, P < 0.01
F: 68% versus 24% in 10 mg/kg
adult exposure group, P < 0.01)
hepatocellular adenoma
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
(M: 32% versus 20% in 10 mg/kg
adult exposure group;
F: 70% versus 20% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 2% versus 4% in 10 mg/kg
adult exposure group;
F: 16% versus 4% in 10 mg/kg
adult exposure group)
hepatocellular adenoma/carcinoma
(M: 32% versus 24% in 10 mg/kg
adult exposure group;
F: 78% versus 24% in 10 mg/kg
adult exposure group, P < 0.001)
10:30g 24 months liver: oval cell hyperplasia NTP (1993)
(M: 82% versus 74% in 30 mg/kg
adult exposure group;
F: 88% versus 84% in 30 mg/kg
adult exposure group)
hepatocellular adenoma
(M: 76% versus 76% in 30 mg/kg
adult exposure group
F: 90% versus 76% in 30 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 46% versus 38% in 30 mg/kg
adult exposure group
F: 44% versus 8% in 30 mg/kg
adult exposure group, P < 0.01)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 mouse F, M oral 125 doses 10 6 months liver: atypical foci Gupta et al.
(lot no. 1312 (B6C3F1) over 6 months (11/100 versus 1/20 in control) (1983a)
FT) (in corn (50) (M: 9/50; F: 2/50)
oil)
FM FF-1 mouse F, M oral 125 doses 0.1, 0.3, lifetime high dose: liver: carcinoma Gupta et al.
(lot no. 1312 (B6C3F1) over 6 months 10 (M: 21/22 = 95% versus 12/25 in (1983b)
FT) (in corn (8-27) (sacrifice of control; P < 0.01; F: 7/8 = 88%
oil) 10% animals versus 0/13 in control; P < 0.01)
alive 24 metastasis to lung
months post- (F: 3/8 = 38% versus 0/13 in
treatment) control; P < 0.05)
FM FF-1 mouse F, M adult only 0 24 months liver: eosinophilic focus (M: 6%; NTP (1993)
(lot FF1312- (B6C3F1) exposure (in F: 2%) bile duct hyperplasia
FT) (50) diet for 24 (M: 0%; F: 0%) hepatocellular
months) ademona (M: 18%; F: 8%)
hepatocellular carcinoma
(M: 16%; F: 2%)
10 liver: eosinophilic focus (M: 33%,
P < 0.01; F: 36%, P < 0.01) bile
duct hyperplasia (M: 8%, < 0.01;
F: 18%, P < 0.01) hepatocellular
adenoma (M: 98%, P < 0.01; F: 78%,
P < 0.01) hepatocellular carcinoma
(M: 61%, P < 0.01; F: 44%, P <
0.01 hepatocellular adenoma/
carcinoma (M: 98%, P < 0.001;
F: 84%, P < 0.001)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
30 liver: eosinophilic focus NTP (1993)
(M: 12%, F: 8%); bile duct
hyperplasia (M: 68%, P 0.01;
F: 81%, P < 0.01) hepatocellular
adenoma (M: 84%, P < 0.001;
F: 96%, P < 0.001); hepatocellular
carcinoma (M: 72%, P < 0.001;
F: 73%, P < 0.001) hepatocellular
adenoma/carcinoma (M: 96%, P <
0.001; F: 98%, P < 0.001)
FM FF-1 mouse F, M perinatal only 30 24 months liver: eosinophilic focus NTP (1993)
(lot FF-1312- (B6C3F1) exposure (M: 40%, P < 0.01; F: 6%, P <
FT) (50) (female parents 0.01) hepatocellular adenoma
received (M: 62%, P < 0.001; F: 38%, P <
FM-containing 0.001) hepatocellular carcinoma
diet beginning (M: 34%, F: 8%) hepatocellular
60 days prior adenoma/carcinoma (M: 80%, P <
to breeding and 0.001; F: 42%, P < 0.001)
throughout
gestation,
lactation, and
up to 4 weeks
postweaning)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 mouse F, M combined 10:10f 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (B6C3F1) perinatal and (M: 8% versus 33% in 10 mg/kg
FT) (50) adult exposure adult exposure group,
F: 32% versus 36% in 10 mg/kg
adult exposure group) bile duct
hyperplasia (M: 0% versus 0% in
10 mg/kg adult exposure group
(F 18% versus 18% in 10 mg/kg)
adult exposure group)
hepatocellular adenoma
(M: 94% versus 98% in 10 mg/kg
adult exposure group;
F: 76% versus 78% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 63% versus 61% in 10 mg/kg
adult exposure group;
F: 52% versus 44% in 10 mg/kg
adult exposure group)
FM FF-1 mouse F, M combined 30:10h 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (B6C3F1) perinatal and (M: 0% versus 33% in 10 mg/kg
FT) (50) adult exposure adult exposure group, P < 0.01;
F: 8% versus 36% in 10 mg/kg
adult exposure group, P < 0.01)
bile duct hyperplasia
(M: 16% versus 0% in 10 mg/kg
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
adult exposure group, P < 0.01;
F: 12% versus 18% in 10 mg/kg
adult exposure group)
hepatocellular adenoma
(M: 96% versus 98% in 10 mg/kg
adult exposure group;
F: 94% versus 78% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 80% versus 61% in 10 mg/kg
adult exposure group;
F: 88% versus 44% in 10 mg/kg
adult exposure group)
hepatocellular adenoma/carcinoma
(M: 96% versus 98% in 10 mg/kg
adult exposure group;
F: 100% versus 84% in 10 mg/kg
adult exposure group)
30:30i 24 months liver: hepatocellular adenoma NTP (1993)
(M: 96% versus 84% in 30 mg/kg
adult exposure group,
F: 87% versus 96% in 30 mg/kg
adult exposure group,
hepatocellular carcinoma
(M: 70% versus 72% in 30 mg/kg
adult exposure group;
F: 62% versus 73% in 30 mg/kg
adult exposure group)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
NoBB mouse F, M in diet for 100 18 months liver: carcinoma: 300/100 mg/kg Momma (1986)
(Bromkal (B6C3F1) 18 months 300 (M: 28%/78%; F: 76%/17% versus
80-9D) (50) 14% (M), 0% (F) in controls)
neoplastic nodules: 300/100 mg/kg
(M: 96%/98%, F: 92%/72% versus
38% (M), 0% (F) in controls)
hepatoblastoma: 300 mg/kg
(M: 2%/12% versus 0% in controls)
2,2',4,4',5,5' rat F in diet for 10 180 days liver: no neoplastic nodules; Jensen &
hexabromobiphenyl (Sprague- for 180 days no EAF Sleight
(BB 153) Dawley) (3) (1986)
100 100 days liver: neoplastic nodules (1/3)
small numbers of EAF
(6) F in diet 10 450 days liver: no nodules, no carcinoma Jensen &
for 140 days Sleight
(1986)
3,3',4,4',5,5' rat (Sprague- F in diet 0.1 450 days liver: no nodules, no carcinoma
hexambromobiphenyl Dawley) for 140 days
(BB 169) (6)
Mixture of BB rat (Sprague- F in diet 10 plus 450 days liver: no nodules, no carcinoma
153 and BB 169 Dawley) for 140 days 0.1 resp.
Table 89 (contd).
a Number per experimental group, unless otherwise specified.
b F = female; M = male.
c In mg/kg body weight per day or in mg/kg feed, unless otherwise specified.
d Including nodules and atypical foci (numbers in parentheses: number affected/number treated); EAF = enzyme altered foci.
e F1 animals born after the perinatal exposure at dietary level of 1 mg/kg PBBs received diets containing 3 mg/kg PBBs up to 2 years.
f F1 animals born after the perinatal exposure at dietary level of 10 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
g F1 animals born after the perinatal exposure at dietary level of 10 mg/kg PBBs received diets containing 30 mg/kg PBBs up to 2 years.
h F1 animals born after the perinatal exposure at dietary level of 30 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
i F1 animals born after the perinatal exposure at dietary level of 30 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
Perinatal-only exposure (through dietary administration of
10 mg PBBs/kg to the dams) had no effect on the incidence of hepatic
neoplasms in female rats, but, in male rats, this exposure was
associated with a marginally increased incidence of hepatocellular
adenomas. Perinatal exposure to 30 mg PBBs/kg showed significantly
increased incidences of hepatocellular adenoma/carcinoma in male and
female mice.
Combined perinatal and adult exposure to PBBs confirmed the
findings of the adult-only exposures in rats and mice. Although
there were no enhancing effects of combined perinatal and adult
exposure, perinatal exposure enhanced the susceptibility to the
induction of liver tumours of adult female rats, exposed to 10 or
30 mg/kg diet. In male and female mice, it was not possible to
assess adequately the enhancing effects on hepatocellular tumours of
combined perinatal and adult exposure, because adult-only exposure
to 10 or 30 mg PBBs/kg resulted in high incidences (84-98%) of
hepatocellular adenoma/carcinoma. However, with increased perinatal
exposure, there were increases in the numbers of mice with
hepatocellular carcinomas and mice with multiple hepatocellular
adenomas, which suggests an enhancement of PBB-related
hepatocellular carcinogenicity associated with perinatal exposure. A
dietary dose of 3 mg/kg (0.15 mg/kg body weight per day) and pre-
and perinatal exposure of the dam to 1 mg/kg (0.05 mg/kg body weight
per day), the lowest dose in this combined perinatal and adult
exposure, did not cause any adverse effects on rats.
Feeding of 10-100 mg FireMaster(R) BP-6 or
2,2',4,4',5,5'-hexa-bromobiphenyl (BB 153)/kg diet or of 0.1 mg
3,3',4,4',5,5'-hexa-bromobiphenyl/kg diet did not cause
hepatocellular carcinoma in rats, but neoplastic nodules were found
at 100 mg FireMaster(R) or BB 153/kg of feed (Jensen et al., 1982;
Jensen & Sleight, 1986). However, the number of animals used was
small, and the observation time less than 2 years.
The incidence of hepatocellular carcinomas was increased in
male and female mice receiving diets containing commercial
nonabromobiphenyl (Bromkal 80-9D) at 100 or 300 mg/kg diet, for 18
months; hepatoblastomas were also seen in males (Momma, 1986; Table
89).
The carcinogenic effects of commercial OcBB and DeBB have not
been studied.
8.7.2 Mechanisms of carcinogenicity
Generally, the development of cancer is described as a
multistage process consisting of initiation and promotion phases
(e.g., Safe, 1984). The question of the mechanism of PBB-induced
carcinogenicity is addressed in several studies.
8.7.2.1 Tumour initiation
As seen in section 8.6, there is no strong evidence for the
mutagenicity or genotoxicity of PBBs, which is a property of known
initiators. Additionally, PBBs (a mixture of almost exclusively BB
153 and BB 180) did not bind to DNA (Dannan et al., 1978b).
FireMaster(R) and BB 153 were also tested as tumour
initiators (and promotors) in a two-stage mouse skin tumorogenesis
assay (Haroz & Aust, 1979). A tumour promoter,
12-0-tetradecanoyl-phorbol-13-acetate (TPA), was applied to the skin
of a mouse of a tumour-susceptible strain (SENCAR). After 14 weeks
of treatment, neither FM nor BB 153 exhibited tumour initiating (or
promoting: see Table 90) activity. However,
3,3',4,4'-tetrabromo-biphenyl, tested in a two-stage rat bioassay
(promotion by phenobarbital), showed some evidence for weak
initiating activity (Dixon et al., 1985, 1988). In contrast to
3,3',4,4'-tetrabromobiphenyl which can be metabolized,
FireMaster(R) has not been tested for initiating activity in this
bioassay, since FireMaster(R) would persist in the tissues of the
animals beyond the initiation phase and throughout the promotion
phase (Rezabek et al., 1987).
8.7.2.2 Tumour promotion
Varied results (Table 90) were obtained with tumour promotion
assays, in which PBBs were tested in combination with known
carcinogens (2-acetylaminofluorene in rats, Schwartz et al., 1980;
partial hepatectomy plus i.p. administration of N-nitrosodiethyl
amine in rats, Jensen et al., 1982, 1983, 1984; Jensen & Sleight,
1986; Rezabek et al., 1987; Dixon et al., 1988; subcutaneous
administration of N-nitrosodiethylamine in hamsters, Wasito &
Sleight, 1989; 7,12-dimethyl-benz [a]-anthracene by skin
application in mice, Berry et al., 1978; Haroz & Aust, 1979;
N-methyl- N-nitro- N-nitroso-guanidine by skin application in
mice, Poland et al., 1982).
On evaluating the development of tumours, hepatocellular
carcinoma was found in rats (Jensen, 1983) and skin papilloma in
mice (Poland et al., 1982). Other studies with FireMaster(R)
resulted in negative findings (Berry et al., 1978; Haroz & Aust,
1979; Schwartz et al., 1980). Only few hepatocellular carcinomas
were present in initiated rats given
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) or
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or a mixture of both
(Jensen et al., 1982). In a mouse skin test, BB 169 was effective in
promoting papillomas, but BB 153 was not (Poland et al., 1982).
Another indicator of tumour-promoting ability in initiated rats
was the counting and measuring of enzyme-altered foci (EAF;
exhibiting gamma-glutamyltranspeptidase activity) and hepatic
nodules, which were presumed to be precursor lesions of
hepatocellular carcinomas (e.g., Sleight, 1985). In these studies
(see Table 90) devised by Pitot et al. (1978), FireMaster(R)
(Jensen et al., 1982, 1984; Jensen & Sleight, 1986; Rezabek et al.,
1987) and its non-toxic major congener BB 153 (Jensen et al., 1982;
Jensen & Sleight, 1986) acted as tumour promoters, FM being more
effective than Bb 153 at dietary concentrations of 10 and 100 mg/kg
(Jensen et al., 1982). Short-term feeding of FM was as effective as
long-term feeding in enhancing the development of enzyme-altered
foci (Jensen et al., 1984). Similarly, an oral 24-h administration
of FM was sufficient to enhance EAFs (Rezabek et al., 1987). An oral
dose of 13 mg FM/kg body weight was found to be close to a possible
no-effect threshold level for the enhancement of EAF (Rezabek
et al., 1987). BB 169 (3,3',4,4',5,5'- hexa) was tested positive
only at a dose (1 mg/kg feed) that was hepatotoxic (Jensen et al.,
1983). It was concluded (e.g., Sleight, 1985) that toxicity and
carcinogenicity are not necessarily related. Synergistic as well as
inhibitory effects on tumour-promoting ability could be elicited by
special combinations of BB 153 and BB 169 (Sleight, 1985; Jensen &
Sleight, 1986).
Preliminary studies with FireMaster(R) BP-6 indicated that
iron overload may enhance the hepatocarcinogenicity of PBBs in
C57BL/10 ScSn male mice (Smith et al., 1990b).
Interestingly, FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl inhibited intercellular
communication in vitro at non-toxic doses, a property of known
tumour promoters (e.g., Sleight, 1985), but
3,3',4,4',5,5'-hexabromobiphenyl did not (Table 95; section 8.9).
Probably, non-toxic congeners, such as BB 153, have a direct
tumour-promoting effect by interfering in normal cell-to-cell
communication, whereas toxic congeners like BB 169 promote tumours
secondarily to hepatic degeneration and necrosis (e.g., Sleight,
1985).
8.7.2.3 PBBs acting as complete carcinogens
The development of tumours, hepatic nodules, or small numbers
of EAF (Table 89) in PBB-exposed rodents that were not
experimentally initiated has been interpreted in two ways: either
PBBs (or some of them) have both initiating and promoting activity
or the observed effects may have resulted from the promotion of
"environmentally initiated" cells. As yet, neither of the two
possibilities can be ruled out (Jensen et al., 1982; Sleight, 1985;
Rezabek et al., 1987).
Table 90. Effects of PBB in tumour promotion assays
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
FM BP-6 rat (Sprague- simultaneous treatment: in diet 50 0 inhibition of 2-FAA induced Schwartz
Dawley) 2-acetylaminofluorene mg/kg for mammary and ear duct et al. (1980)
(F, 8-12) (2-FAA) in diet 57 weeks carcinogenesis; no significant
(300 mg/kg feed) effect on hepatic tumours
(5/12 versus 3/8 in 2-FAA
group)
FM BP-6 rat (Sprague- pretreatment: 70% partial in diet 10 0 liver: neoplastic nodules Jensen et al.
(lot 6224 A) Dawley) hepatectomy plus N-nitro- and 100 mg/kg (6/6) increase in EAF (P < (1982)
(F,6) sodiethylamine (DEN) for 180 days 0.05) (both levels)
(single ip dose of 10
mg/kg body weight)
FM rat (4) pretreatment 70% partial in diet 10 275 days liver: carcinoma (4/4 versus 0 Jensen (1983)
hepatectomy plus for mg/kg in control)
140 days DEN (single ip
dose of 10 mg/kg
body weight)
FM BP-6 rat (Sprague- pretreatment: 70% partial in diet 100 0 liver: increase in EAF Jensen et al.
(lot 6224 A) Dawley) hepatectomy plus DEN mg/kg for (P < 0.05) (1984)
(F,6) (single ip dose of for 15 days
10 mg/kg body weight) 10 mg/kg for 0 liver: increase in EAF
140 days (P < 0.05)
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
FM BP-6 rat (Sprague- pretreatment: two-thirds oral two doses 120 days Rezabek et al.
Dawley) partial hepatectomy plus over 24 h (1987)
(F,6) DEN (single ip dose of total dose:
10 mg/kg body weight) 13 mg/kg and liver: increase in EAF (not
130 mg/kg body significant) liver: increas
weights in EAF (P < 0.05)
FM BP-6 mouse CD1 pretreatment: 7,12-di- dermal 0 skin: no papilloma Berry et al.
(F,30) methylbenz(a)anthracene multiple doses (1978)
(DMBA) (single dermal (twice weekly
dose of 200 nmol) over 30 weeks)
100 µg
FM BP-6 mouse pretreatment. DMBA dermal 0 skin: no tumours Haroz & Aust
(SENCAR) (single dermal multiple doses (1979)
(sex, no.: subcarcinogenic dose) (twice weekly
not over 14 weeks)
specified) dose: not
specified
FM FF-1 mouse pretreatment: N-methyl- dermal 0 skin: papilloma (9/15 = 60%) Poland et al.
hairless N'-nitro-N- multiple doses (1982)
HRS/J nitrosoguanidine (MNNG) (twice weekly
(F,20-26) (single dermal dose of over 20 weeks)
5 µmol) 2 mg, 5 weeks,
then
FM BP-6 hamster pretreatment: DEN (single in diet 100 133 days respiratory tract: increase in Wasito &
(Syrian sc dose of 80 mg/kg mg/kg for number of tracheal papillomas Sleight (1989)
golden) body weight 140 days (P < 0.05)
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
3,3',4,4'- rat (Sprague- pretreatment: 70% partial in diet 0.1, 0 liver: increase in EAF Dixon et al.
tetrabromobiphenyl Dawley) hepatectomy plus DEN 1, 5 mg/kg (significant at the high (1988)
(F, 6) (single ip dose of 10 for 180 days dose)
mg/kg body weight)
3,3',4,4'- rat (Wistar) pretreatment: DEN (oral intraperitoneal until 1 week liver: increase in EAF Buchmann
tetrabromobiphenyl (F,6) dose of 10 mg/kg body 15 µmol/kg and 9 weeks et al. (1991)
weight for 10 days) body weight
once weekly
for 8 weeks
2,2',4,4',5,5'- rat (Sprague- pretreatment: 70% partial in diet 10 0 liver: neoplastic nodules Jensen et al.
hexabromobiphenyl Dawley) hepatectomy plus DEN and 100 mg/kg (3/6) increase in EAF (P (1982)
(BB 153) (F,6) (single ip dose of 10 for 180 days < 0.05) neoplastic nodules
mg/kg body weight) (5/6) increase in EAF (P <
0.05)
in diet 10 0 liver: increase in EAF (P Jensen &
mg/kg for 70 days < 0.05) increase in EAF (P Sleight (1986)
140 days 310 days < 0.05) increase in hepatic
nodules (P < 0.05) carcinoma
(1/10 versus 0 in controls)
mouse pretreatment: DMBA dermal multiple 0 skin: no tumours Haroz &
(SENCAR) (single dermal doses (twice Aust (1979)
(sex, no.: sub-carcinogenic dose) weekly over 14
not weeks) dose:
specified) not specified
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
2,2',4,4',5,5'- mouse pretreatment MNNG dermal 0 skin: no papilloma (0/22) Poland et al.
hexabromobiphenyl hairless (single dermal dose multiple doses (1982)
HRS/J of 5 µmol) (twice weekly
(F,20-26) over 20 weeks)
20 µg
3,3',4,4',5,5'- rat (Sprague- pretreatement: 70% in diet 0.1 0 liver: no effect on EAF Jensen &
hexabromobiphenyl Dawley) partial hepatectomy mg/kg for 70 days no effect on EAF Sleight (1986)
(BB 169) (6) plus DEN (single ip dose 140 days 310 days no significant effect on
of 10 mg/kg body weight) hepatic nodules; carcinoma
(1/11 versus 0 in controls)
3,3',4,4',5,5'- mouse, pretreatment: MNNG dermal multiple 0 skin: papilloma (12/20) Poland et al.
hexabromobiphenyl hairless (single dermal dose doses (twice (1982)
HRS/J of 5 µmol) weekly over 20
(f,20) weeks) 20 µg
Mixture of BB rat (Sprague- pretreatment 70% partial in diet 10 mg 0 liver: synergistic effect Jensen &
153 and Dawley) hepatectomy plus DEN BB 153/kg and 70 days on development of EAF Sleight (1986)
BB 169 (single ip dose of 10 0.1 mg BB 310 days synergistic effect on
mg/kg body weight) 169/kg feed for development of hepatic
140 days nodules; carcinoma (1/11
versus 0 in control)
rat (Sprague- in diet 100 mg 0 liver: inhibitory effect on Jensen et al.
Dawley) BB 153/kg and 1 development of EAF (1983)
F1 6) mg BB 169/kg
feed for 140
days
Table 90 (contd).
a No. = Number per experimental group; F = female.
b Numbers in parentheses signify No. affected/No. treated; EAF = Enzyme altered foci (exhibiting gamma glutamyl
transpeptidase activity);
DEN = N-nitrosodiethylamine; DMBA = 7,12-dimethyl-benz(a)anthracene; 2-FAA = 2-acetylaminofluorene;
MNNG = N-methyl-N'-nitro-N-nitroso-guanidine.
On the basis of the US National Toxicology Program (NTP) data,
possible correlations between carcinogenicity and toxicity in
laboratory rodents (Hoel et al., 1988) and between carcinogenicity
and in vitro genetic toxicity assays (Tennant et al., 1987;
Benigni, 1989; Ashby & Tennant, 1991) have been analysed for a
series of chemicals including the FireMaster(R) mixture. Results
confirm that FireMaster(R) can be classified as a nongenotoxic
(epigenetic) carcinogen (see also Loury et al., 1987; Williams
et al., 1989).
8.8 Biochemical toxicity
8.8.1 Induction of microsomal enzymes
One of the most intensively studied effects of the PBBs is
their induction of mixed function oxidase (MFO) enzymes. Generally,
MFO inducers and MFO systems are classified into two main groups,
typified by phenobarbital (PB) and 3-methylcholanthrene (MC). The
inducing capabilities of commercial PBB mixtures and individual PBB
isomers and congeners have been summarized in Tables 91 and 92,
respectively. The results were obtained from enzymatic, spectral,
electrophoretic, immunochemical, and metabolic studies (see also
section 6.3.1).
8.8.1.1 Commercial PBB mixtures
With one exception dealing with octabromobiphenyl (Ahotupa &
Aitio, 1978), all studies available referred to the FireMaster(R)-
mixture (Table 91).
Consistently, FM was found to be a mixed-type inducer of
hepatic microsomal enzymes in rats. Induction was observed at
intakes as low as 1 mg FM/kg feed for 21 days (Babish & Stoeswand,
1977). The no-effect level of a single ip dose was 8 µmol FM/kg body
weight, corresponding to 4.7 mg FM/kg body weight (Goldstein et al.,
1979). When FM was given for 5 days a week, over 30-50 days, changes
in hepatic enzymes occurred with doses as low as 0.3 mg/kg body
weight per day (Goldstein et al., 1979). The dose of FM BP-6
effecting half maximal AHH (benzo[a]pyrene hydroxylase) induction
was approximately 50 mg/kg body weight (Robertson et al., 1981c).
Pre-, post-, or perinatal exposures were also effective in inducing
microsomal enzymes in rats (Dent et al., 1977b,c, 1978b; Moore
et al., 1978a; McCormack et al., 1978a, 1980, 1981). Nursing pups
were approximately ten times more sensitive to these effects than
the dams. The approximate no-effect level for microsomal enzyme
induction in nursing rats was 0.1 mg FM/kg feed, in the diet of the
adult (Moore et al., 1978a).
Induction could be detected as early as 24 h after an ip
administration of 150 mg FM/kg body weight (Dent, 1978; Dent et al.,
1978a) or after an oral dose of 90 mg/kg body weight (Kluwe & Hook,
1981). Although most of the investigations were short-term, there
was some evidence for persistent stimulation of hepatic microsomal
enzymes. For example, maternal rats fed 100 mg FM/kg feed, during
pregnancy and lactation, had elevated enzyme activity 14 weeks after
weaning their first litter or even after weaning a second litter
(recovery period: 12-16 weeks) (McCormack & Hook, 1982). Perinatal
exposure to FM induced microsomal enzymes in rats at 28, 150, and
328 days of age (McCormack et al., 1980). Feeding of FM during
pregnancy and lactation to F0-animals stimulated microsomal
enzymes, not only in the F1-generation, but also in the
F2-generation (McCormack et al., 1981). Also, persistence of an
increased activity of microsomal enzymes, 120 or 125 days after
cessation of exposure, was noted in rats exposed to FireMaster(R)
BP-6 during hepatocarcinogenesis assays (Jensen et al., 1984;
Rezabek et al., 1987).
FM induced hepatic microsomal enzymes not only in the rat, but
in all other animal species tested, i.e., mouse (Corbett et al.,
1975; Dent et al., 1977a; Ahotupa & Aitio, 1978; Dannan et al.,
1980; Ahmadizadeh et al., 1984; Robertson et al., 1984c), guinea-pig
(Rush et al., 1982; Ecobichon et al., 1983; Smith et al., 1986),
hamster (Rush et al., 1982; Smith et al., 1986), cattle (Schanbacher
et al., 1978), pig (Werner & Sleight, 1981), dog (Farber et al.,
1976), Japanese quail (Babish et al., 1975a,b; Bursian et al, 1983),
and fish (Elcombe & Lech, 1978; Franklin et al., 1981; James &
Little, 1981; Law & Addison, 1981). Unlike the mammalian situation,
induction in several species of fresh- and saltwater fish was only
of the MC-type (Table 91).
Although the activities of microsomal enzymes were highest in
the liver, induction was also found in extrahepatic tissues
including the kidney (Dent et al., 1977c, 1978b; Ahotupa & Aitio,
1978; McCormack et al., 1978a,b, 1979a,b, 1980, 1981; Kluwe & Hook,
1981; Werner & Sleight, 1981; Ahmadizadeh et al., 1984; Rush et al.,
1986; Smith et al., 1986), intestine (Manis & Kim, 1980; Traber
et al., 1988a,b), mammary gland (Dent et al., 1977b,c, 1978b;
McCormack et al., 1979a; McCormack & Hook, 1982) and lung (McCormack
et al., 1979a). However, patterns of induction were organ specific.
No induction was noted in the testes of rats (Kluwe & Hook, 1981)
(see also Table 91).
FM was also able to stimulate aryl hydrocarbon hydroxylase
(AHH) activity in rat hepatoma cell culture (Garthoff et al., 1977).
Commercial OcBB has been tested in only one study on mice and
was found to be an inducer of drug-metabolizing enzymes. However,
OcBB was a less potent inducer than FM (Ahotupa & Aitio, 1978).
Table 91. Induction of microsomal enzymes (MFOs) by commercial PBB mixtures
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM FF-1 (various rat (Fischer F oral 9-22 doses 14-64 liver x PB + MC Goldstein
concentrations) F 344/N) over 30 days et al.
(0.03-30 mg/kg daysb (1979)
body weight/day)
FM FF-1 (lot no. rat M oral single 1-18 days liver x MCd Manis & Kim
FF-1312-FT) (Sprague- dose 1-3 days intestine x MCd (1980)
(50-400 mg/kg Dawley) 4-18 days intestine x
body weight)
(0.1 mg/day) oral 25 doses 5 weeks liver x
over 5 weeks 5 weeks intestine x
FM BP-6 (90 rat (Fischer M oral single 9, 24, 72 liver x PB + MC Kluwe &
mg/kg body 344) dose 216 h kidney x only MC Hook (1981)
weight) testes x
4 doses 7 days liver x PB + MC
over 6 days kidney x only MC
testes x
FM BP-6 (25, rat F intra- single 12, 24, 48, liver x PB + MC Dent et al.
150 mg/kg (Sprague peritoneal dose 192 h (1976b,
body weight) Dawley) 336 h 1978a)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (150 rat (Sprague- not intra- single 28 days kidney x MC McCormack
mg/kg Dawley) specified peritoneal dose et al.
body weight) pups (7 or (1978a)
11 days old)
FM BP-6 rat (Wistar) M intra- 2 doses on 6 days liver x MCd Safe et al.
(100 mg/rat) peritoneal days 1 & 3 (1978)
FM FF-1 (lot no. rat F intra- single 4 days liver x PB + MC Goldstein
FF-1312 FT) (Fischer) peritoneal dose et al.
(varied (1979)
concentrations)
(1.8-1000
µmol/kg body
weight)
FM FF-1 (90 rat M intra- single 2 weeks liver x PB + MC Dannan
mg/kg body (Sprague- peritoneal dose et al.
weight) Dawley) (1982c)
FM BP-6 (1500 rat (Long M intra- single 4 days liver x PB + MC Parkinson
or 750 µmol/kg Evans) peritoneal dose et al.
body weight) (1983);
Haake et al.
(1985)
FM BP-6 (150 rat M intra- single 5, 10, 15 kidney x x MCd Rush et al.
mg/kg body (Sprague- peritoneal dose days (day (day (1986)
weight) Dawley) 5,10) 15)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (6400 rat M intra- 4 doses 4 days liver x PBe Traber
mg/kg body (Sprague- peritoneal over 4 days small et al.
weight; total Dawley) intestine x PBe (1988a,b)
dose)
FM BP-6 rat F in diet 2 weeks 2 weeks liver x PB + MC Dent et al.
(4.6-200 mg/kg (Sprague- (1976a)
of feed) Dawley)
FM BP-6 (50 rat (Sprague- M in diet 5-20 days 5-20 days liver x PB + MC Babish &
mg/kg of feed) Dawley) Stoeswand
(1977)
FM BP-6 (50 rat (Sprague- F in diet gd 8-day 14 day 14 post- liver x at organ Dent et al.
mg/kg of feed) Dawley) postpartum partum kidney x specific (1977b,c)
maternal mammary patterns
gland x
FM BP-6 (5, 50, rat M in diet 2, 3, 5 2, 3, 5 liver x PBe Garthoff
500 mg/kg feed) (Holtzmann) weeks weeks et al.
(1977)
FM BP-6 (100 rat (Sprague- F in diet 3 months 3 months liver x MC + PB McCormack
mg/kg feed) Dawley) kidney x MC et al.
(1978a,b)
FM FF-1 (lot rat (Sprague- F in diet 18 days 18 days liver x PB + MC Moore
7042) (0.1-10 Dawley) postpartum et al.
mg/kg feed) lactating (1978a)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (25-200 rat (Sprague- F in diet gd 8-day 14 day 14 post- lung x MCd McCormack
mg/kg feed) Dawley) postpartum partum kidney x MCd et al.
lactating mammary (1979a)
gland x MCd
liver x MCd
FM BP-6 (100 rat (Sprague- M in diet 30 days 30 days liver x PB + MC Akoso et al.
mg/kg feed) Dawley) (1982a)
FM BP-6 (100 rat F in diet gd 8-day 28 W1 litter, liver x PB + MC McCormack &
mg/kg feed) (Sprague- postpartum 14 weeks mammary Hook (1982)
Dawley) weanling after W1 gland x MC
maternal (6 weeks) and W2
litters
FM BP-6 (50 rat (pups) placental, pre-, post- at birth liver x PB + MC Dent et al.
mg/kg feed in milk or or perinatal or at day (1977c,
maternal diet) both 15 post- 1978b)
partum
FM FF-1 (lot rat milk postnatal day 18 post- liver x PB + MC Moore et al.
7042) (0.1-10 (Sprague- partum (1978a)
mg/kg feed in Dawley)
maternal diet (pups) F1
0.1-10 mg/kg rat (Sprague- F milk and postnatal several liver x PB + MC
feed in maternal Dawley) in diet until mating weeks
diet plus 0.1-1 (lactating: and day 18
mg/kg feed F1) postpartum
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
0.1-10 mg/kg rat (Sprague- placental perinatal day 18 post- liver x PB + MC
feed in maternal Dawley) and milk partum
diet plus 0.1-1 (pups: F2)
mg/kg feed
FM BP-6 (100 rat (Sprague- placental perinatal weanling liver x PB + MC McCormack
mg/kg feed) Dawley) and milk (28 days kidney x MC et al.
of age) (1980)
150 days liver x PB + MC
of age kidney x MC
328 days liver x PB + MC
of age kidney x MC
FM BP-6 (10, rat (Sprague- placental perinatal W1 liver x PB + MC McCormack
100 mg/kg Dawley) and milk (F0: gd 8- kidney x MC et al.
feed) day 28 (1981)
postpartum)
W2 liver x PB + MC
kidney x MC
W3 liver x
kidney x
FM BP-6 mouse F intra- single 24-192 h liver x PB + MC Dent et al.
(150 mg/kg (NMRI) peritoneal dose (1977a)
body weight)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 mouse M intra- single 10 days liver x MCd Ahotupa &
(75 mg/kg (C57) peritoneal dose kidney Aitio (1978)
body weight) lung x
FM BP-6 mouse M intra- 2 doses on 6 days liver x PB + MC Robertson
(500 µmol/kg (C57BL/) peritoneal days 1 & 3 et al.
body weight) 6J) (1984c)
mouse M intra- 2 doses on 6 days liver x PB + (MC)
(DBA/2J) peritoneal days 1 & 3
FM BP-6 mouse M intra- single 5, 10, 15 kidney x Rush et al.
(150 mg/kg (ICR) peritoneal dose days (1986)
body weight)
FM BP-6 (1000 mouse F in diet 11 days 11 days liver x not Corbett
mg/kg feed) (Swiss/ICR) specified et al.
(1975)
FM (100 mg/kg mouse M in diet 28 days 28 days liver x MCd Ahmadizadeh
feed) (C57/6J) kidney x MCd et al.
(1984)
mouse M in diet 28 days 28 days liver x MCd
(DBA/2J) kidney x
FM BP-6 guinea- M intra- single 4 days liver x MCd Rush et al.
(50 mg/kg pig peritoneal dose kidney x MCd (1982);
body weight) (Hartley) Smith et al.
(1986)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM FF-1 (lot FA- guinea- placental prenatal 2 days liver x Ecobichon
7042) (maternal, pig (from gd 65) et al.
single oral (Hartley) milk postnatal 2-60 days liver x x (1983)
dose of 50 mg/kg pups (from 6-12 h of age (2,4, (14,
body weight) after 7,28 60,90
parturition) days days
of of
age) age
FM BP-6 hamster M intra- single 4 days liver x MCd Rush et al.
(50 mg/kg (Golden peritoneal dose kidney x (1982);
body weight) Syrian) Smith et al.
(1986)
FM BP-6 (lot cattle F oral 90-180 90-180 liver x Schanbacher
6244 A) days/daily days et al.
(250 mg/day) dose) (1978)
FM BP-6 pig F in diet 2nd half of gestation and liver x Werner &
(10-200 mg/kg (sow) lactation kidney x Sleight
feed) (pups) placental prenatal at birth liver x (1981)
kidney x
placental perinatal 4 weeks liver x
and milk of age kidney x
FM BP-6 (1 Beagle dog M,F oral 7 weeks 7 weeks liver x Farber
mg/kg body et al.
weight per day) (1976)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM (10-1000 Japanese M,F in diet 9 weeks 9 weeks liver x PBe Babish
mg/kg feed) quail et al.
(1975b)
FM FF-1 Japanese M,F in diet 5 weeks 5 weeks liver x PB + MC Polin et al.
(40, 80 mg/kg quail (1982);
feed) (Coturnix Bursian
coturnix et al.
japonica) (1983)
(3 genetic
lines)
FM BP-6 brook trout oral 18 days 18 days liver x MC Law &
(200 mg/kg (Salvelinus (multiple Addison
body weight) fontinalis) doses) (1981)
FM BP-6 rainbow intra- single up to liver x MC Elcombe &
(150 mg/kg trout peritoneal dose 2 weeks Lech (1978)
(Oncorhynchus
mykiss)
FM BP-6 rainbow parenteral single 5 days liver x MC Franklin
(150, 500 mg/kg trout dose et al.
body weight) (O. mykiss) (1981)
FM FF-1 sheepshead intra- single up to liver x MCd James &
(15 mg/kg minnow peritoneal dose 56 days Little
body weight) (Archosargus (1981);
probatocephalus) James & Bend
(1982)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
OcBB (FR 25013 mouse M intra- single 10 days liver x MCd Ahotupa &
A, Dow Chemical) (C57) peritoneal dose kidney x Aitio (1978)
(75 mg/kg lung x
body weight)
a Commercial PBB mixtures; FM = FireMaster; OcBB = octabromobiphenyl.
b Dosing: 5 days per week for 30 days; examination points: 14 days (9 doses); 31 days (22 doses); 46 and 64 days (22 doses
plus a 15-day and a 33-day recovery period, respectively.
c After first dose.
d Only MC-typical parameters recorded.
e Only PB-typical parameters recorded.
Abbreviations:
gd = gestation day; MFO = mixed function oxidase; MC = 3-methylcholanthrene; PB = phenobarbital.
Table 92. Induction of hepatic microsomal enzymes (MFOs) by PBB congeners
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
4-mono- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1979)
2,2'-di- rat (Sprague- M intra- single 2-22 x Moore et al.
(90 mg/kg body Dawley) peritoneal dose days (1979a)
weight)
(600) rat M intra- 3 doses 7 days x Ecobichon
(Wistar) peritoneal et al. (1979)
2,5'-di- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
4,4'-di- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1977,
1979)
(300) rat M intra- 2 doses 6 days x PB Robertson
(Wistar) peritoneal et al. (1982b)
2,2',5-tri- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1979)
2,3',5-tri- rat M intra- 3 doses 7 days x Ecobichon et al.
(600) (Wistar) peritoneal (1979)
2,4,6-tri- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,4',5-tri- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
3,4,4'-tri- rat M intra- single 4 days x MC Parkinson et al.
(250) (Long Evans) peritoneal dose (1983)
(300) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',5,5'-tetra- rat M intra- single dose 2 weeks x PB Robertson et al.
(150) (Wistar) peritoneal (1983b)
(500) rat (Long M intra- single 4 days x PB Parkinson et al.
Evans) peritoneal dose (1983)
(600) rat M intra- 3 doses 7 days x Ecobichon et al.
(Wistar) peritoneal (1979)
2,3',4,4'-tetra- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
(250) Evans) peritoneal dose (1983)
(1500) mouse M intra- 2 doses 6 days x Robertson et al.
(C57BL/6J) peritoneal (1984c)
(1500) mouse M intra- 2 doses 6 days x
(DBA/2J) peritoneal
2,3',4',5-tetra- rat M intra- 2 doses 6 days x PB Robertson et al.
(150) (Wistar) peritoneal (1980)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,4,4',6-tetra- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
(500) Evans) peritoneal dose (1983)
3,3',4,4'-tetra- rat (Sprague- M oral single 1-10 x MC Millis et al.
(21.3) Dawley) dose days (1985b)
(250) rat (Long M intra- single 4 days x MC Parkinson et al.
Evans) peritoneal dose (1983)
(2 mg/kg body rat (Sprague- M intra- single 2 weeks x MC Millis et al.
weight) Dawley) peritoneal dose (1985a)
(10, 60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b); Andres et
al. (1983)
(750) mouse M intra- 2 doses 6 days x MC Robertson et al.
(C57BL/6J) peritoneal (1984c)
(1500) mouse M intra- 2 doses 6 days x MC
(DBA/2J) peritoneal
3,3',5,5'-tetra- rat M intra- 3 doses 7 days x Ecobichon et al.
(600) (Wistar) peritoneal (1979)
(not specified) chicken via shell single 28 h x Poland & Glover
embryo into the dose (1977)
air sac
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
3,4,4',5-tetra- rat (Long M intra- single 4 days x MC Parkinson et al.
(250) Evans) peritoneal dose (1983)
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',4,5,5'- rat (Long M intra- single 4 days x PB Parkinson et al.
penta- Evans) peritoneal dose (1983)
(BB 101) (500)
(90 mg/kg body rat (Sprague- M intra- single 7-14 x PB Dannan et al.
weight) Dawley) peritoneal dose days (1982a); Millis
et al. (1985a)
2,2',4,5',6- rat M intra- 3 doses 7 days x Ecobichon et al.
penta- (600) (Wistar) peritoneal (1979)
2,3',4,4',5- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
penta- Evans) peritoneal dose (1983)
(BB 118) (250)
(90 mg/kg body rat (Sprague- M intra- single 2 weeks x PB + MC Dannan et al.
weight) Dawley) peritoneal dose (1982c); Millis
et al. (1985a)
(30, 150) rat (Wistar) M intra- 2 doses 6 days x PB + MC Robertson et al.
peritoneal (1980)
(500) mouse M intra- 2 doses 6 days x PB + MC Robertson et al.
(C57BL/6J) peritoneal (1984c)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(500) mouse M intra- 2 doses 6 days x
(DBA/2J) peritoneal
2,3',4,4',6- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
penta- (500) Evans) peritoneal dose (1983)
3,3',4,4',5- rat (Long M intra- single 4 days x MC
penta- (100) Evans) peritoneal dose
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',3,4,4',5'- rat (Sprague- M intra- single 7 days x PB + MC Dannan et al.
hexa- Dawley) peritoneal dose (1982a)
(BB 138) (90
mg/kg body
weight)
2,2',4,4',5,5'- rat F gavage multiple 60 days x PB Goldstein et al.
hexa- (BB 153) (F 344/N) doses (over (1979)
(16.8 mg/kg 30 days)
body weight)
(40-1000) rat F intra- single 4 days x PB
(Fischer) peritoneal dose
(500) rat (Long M intra- single 4 days x PB Parkinson et al.
Evans) peritoneal dose (1983); Haake et
al. (1985)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(90 mg/kg body rat (Sprague- M intra- single 1-14 x PB Moore et al.
weight) Dawley) peritoneal dose days (1978b); Millis
et al. (1985a)
(30 mg/kg body rat M intra- single 72 h x PB Lubet et al.
weight) (F 344) peritoneal dose (1990)
(600) rat M intra- 3 doses 7 days x PB Ecobichon et al.
(Wistar) peritoneal (1979)
(100 mg/kg feed) rat (Sprague- M in diet 30 days 30 days x PB Akoso et al.
Dawley) (1982a)
(10, 100 mg/kg rat (Sprague- M in diet 9 days 10 days x PB Render et al.
feed) Dawley) (1982)
(150 mg/kg body rainbow trout parenteral single 5 days x Franklin et al.
weight) (Oncorhynchus dose (1981)
mykiss)
2,2',4,4',5,5'- sheepshead intra- single 17 days x James & Little
hexa- (20 mg/kg minnow peritoneal dose (1981); James &
body weight) (Archosargus Bend (1982)
probatocephalus)
(60, 100 mg/kg intra- multiple 28-40 x
body weight) peritoneal doses days
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,2',4,4',6,6'- rat M intra- 3 doses 7 days x Ecobichon et al.
hexa- (600) (Wistar) peritoneal (1979)
2,3,3',4,4',5- rat (Sprague- M intra- single 7 days x (PB) + MC Dannan et al.
hexa- (BB 156) Dawley) peritoneal dose (1982a)
(90 mg/kg body
weight)
(3.75-60) rat M intra- 2 doses 6 days x (PB) + MC Robertson et al.
(Wistar) peritoneal (1981a)
2,3,3',4,4',5'- rat (Long M intra- single 4 days x Parkinson et al.
hexa- (100) Evans) peritoneal dose (1983)
2,3',4,4',5,5'- rat M intra- single 7 days x PB + MC Dannan et al.
hexa- (BB 167) (Sprague- peritoneal dose (1978a)
(90 mg/kg body Dawley)
weight)
(100 mg/kg feed) rat (Sprague- M in diet 30 days 30 days x PB + MC Akoso et al.
Dawley) (1982a)
2,3',4,4',5',6- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
hexa- (BB 168) Evans) peritoneal dose (1983)
(250)
3,3',4,4',5,5',- rat (Sprague- M oral single 1-14 days x MC Millis et al.
hexa- (21.3) Dawley) dose (1985b)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(100) rat (Long M intra- single 4 days x MC Parkinson et al.
Evans) peritoneal dose (1983)
(30 mg/kg body rat M intra- single 72 h x MC Lubet et al.
weight) (F 344) peritoneal dose (1990)
(2, 30 mg/kg rat (Sprague- M intra- single 1-2 x MC Dannan et al.
body weight) Dawley) peritoneal dose weeks (1982c); Millis
et al. (1985a)
30 mg/kg body rat M intra- single 72 h x MC Lubet et al.
weight (F344) peritoneal dose (1990)
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
(600) rat M intra- 3 doses 7 days x Ecobichon et al.
(Wistar) peritoneal (1979)
(1, 10 mg/kg rat (Sprague- M in diet 30 days 30 days x MC Akoso et al.
feed) Dawley) (1982a)
(10, 100 mg/kg rat (Sprague- M in diet 9 days 10 days x MC Render et al.
feed) Dawley) (1982)
(not specified) chick via shell single 24 h x MC Poland & Glover
embryo into the dose (1977)
air sac
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(150 mg/kg body rainbow trout parenteral single 5 days x MC Franklin et al.
weight) (Oncorhynchus dose (1981)
mykiss)
2,2',3,3',4,4',5- rat (Sprague- M intra- single 7 days x PB (+MC?) Dannan et al.
hepta- (BB 170) Dawley) peritoneal dose (1982a)
2,2',3,4,4',5,5'- rat M intra- single 2-22 days x PB Moore et al.
hepta- (BB 180) (Sprague- peritoneal dose (1979a)
(90 mg/kg body Dawley)
weight)
(150 mg/kg body rainbow trout parenteral single 5 days x Franklin et al.
weight) (O. mykiss) dose (1981)
2,3,3',4,4',5,6- rat M intra- 2 doses 6 days x Robertson et al.
hepta- (6) (Wistar) peritoneal (1981a)
2,2',3,3',4,4', rat M intra- single 7 days x PB Besaw et al.
5,5'-octa- (BB (not speci- peritoneal dose (1978)
194) (90 mg/kg
body weight)
(600) rat M intra- 3 doses 7 days x PB Ecobichon et al.
(Wistar) peritoneal (1979)
Table 92 (contd).
a Total dose in µmol/kg body weight, unless otherwise specified.
b After first dose.
c MFO = Mixed function oxidase; PB = phenobarbital; MC = 3-methylcholanthrene.
d Only noted when categorized into PB- or MC- type by the authors themselves.
The FM mixture was fractionated and reconstituted in order to
test whether PBBs or contaminants (e.g., brominated dibenzofurans or
dibenzodioxins, or brominated naphthalenes) were responsible for the
inductive effects. The results indicated that most effects
associated with the mixture were due to the brominated biphenyls
(Safe et al., 1978; Robertson et al., 1981b; Dannan et al., 1982b).
8.8.1.2 Individual PBB congeners
The capability of individual PBB congeners to induce hepatic
microsomal enzymes in vivo is shown in Table 92. There was a broad
range of responses, depending on the congener or species/strain
tested. In addition to qualitative differences, the extent of the
induction also differed between congeners (e.g., Ecobichon et al.,
1979; Safe et al., 1981; Parkinson et al., 1983 and other references
from Table 92). As far as they have been tested, in vitro enzyme
induction assays using rat hepatoma H-4-II-E cells in culture, have
confirmed the results of the in vivo studies (Andres et al., 1983;
Bandiera et al., 1982, 1983).
The most abundant components of the FM mixture, BB 153
(2,2',4,4',5,5'-hexa-) and BB 180 (2,2',3,4,4',5,5'-hepta-), were
strict PB-type inducers in rats. Congeners 118 (2,3',4,4',5-penta-),
138 (2,2',3,4,4',5'-hexa-), and 167 (2,3',4,4',5,5'-hexa-) showed a
mixed PB- and MC-type induction, and 156 (2,3,3',4,4',5-hexa-) was
described (Robertson et al., 1981a; Dannan et al., 1982a) to be a
very potent MC-type inducer (see Table 8.8/2).
3,3',4,4'-tetrabromobiphenyl and the model congener
3,3',4,4',5,5'-hexabromobiphenyl were pure MC-type inducers in rats
(Table 92), 3,3',4,4'-tetrabromobiphenyl being less effective than
3,3',4,4',5,5'-hexabromobiphenyl (Millis et al., 1985a,b). The
ED50 value of 3,3',4,4',5,5'-hexabromobiphenyl determined in the
chicken embryo was approximately 90 nmol/kg body weight (= 3.4 nmol
per egg) (Poland & Glover, 1977). On the other hand,
3,3',4,4'-tetrabromobiphenyl was at least 50 times more potent as an
inducer of AHH activity than the commercial PBB mixture: ED50
values (measured in rats) were 1-2 µmol/kg body weight for
3,3',4,4'-tetrabromobiphenyl and 75-80 µmol/kg body weight for FM
BP-6 (Robertson et al., 1982).
Congeners that elicited PB-type induction in rats (e.g., BB 153
and BB 180) failed to do so in fish, whereas MC-type inducers were
effective in both rats and fish (Table 92).
Correlations between the structure and microsomal enzyme-
inducing activity (and toxicity) of individual PBB congeners have
been demonstrated in several studies and reviews (e.g., Goldstein,
1980; Moore et al., 1980; Aust et al., 1981; McKinney & Singh, 1981;
Robertson et al., 1982b; Andres et al., 1983; Dannan et al., 1983;
Parkinson et al., 1983; Safe, 1984; Safe et al., 1985).
When 3,3',4,4',5,5'-hexabromobiphenyl was used to induce
cytochrome P-450 in rats (at 10 µmol/kg body weight), it was found
to be selectively associated with cytochrome P-450d (Voorman & Aust,
1987).
8.8.2 Endocrine interactions
PBBs have been demonstrated to interact with the endocrine
system.
8.8.2.1 Thyroid hormones
Rats (No. = 10), given oral doses of FM FF-1 (Lot No. 1312 FT)
over 6 months, showed dose-related decreases in serum thyroxine
(T4) and triiodothyronine (T3). Significant decreases in T4
were seen at doses as low as 0.3 mg PBB/kg body weight per day in
males, and 1 mg/kg body weight per day in females. The reduction in
T3 was somewhat less and only significant at high doses (3-10
mg/kg body weight per day) in females (Gupta et al., 1983a). A time-
and dose-dependent reduction in plasma T4 levels was also found in
male rats (n = 8-11) administered FM FF-1, by gavage, for 10 or 20
days at 1, 3, or 6 mg/kg body weight per day (Allen-Rowlands et al.,
1981). In addition, the reduced plasma T4 levels were correlated
with an increase in thyroid stimulating hormone (TSH) at 20 days. At
6 mg/kg body weight per day, the thyroid uptake of iodine was
increased, but the incorporation of iodine in monoiodotyrosine was
decreased. While short-term feeding (7 months) of female rats with
commercial hexabromobiophenyl at dietary concentrations of
1-50 mg/kg also resulted in an decrease in serum T3 and T4
levels (Sepkovic & Byrne, 1984; Byrne et al., 1987), feeding of
technical octabromobiphenyl had no effect on serum T3 levels
(Sepkovic & Byrne, 1984). "PBB" (not specified) caused not only
reduced serum T4 levels and elevated thyrotropin levels in rats,
but also produced goitres (Bastomsky, 1986).
Single ip injections of 3,3',4,4',5,5'-hexabromobiphenyl in
juvenile male rats (20 and 40 mg/kg body weight) caused a
significant decrease in serum T4 concentrations, while serum T3
levels did not change significantly during the 28-day observation
period. The decrease in serum T4 concentrations was dose-dependent
(Spear et al., 1990).
Serum concentrations of T3 and T4 were decreased in swine
and their newborn piglets at 200 mg FM BP-6/kg feed. After nursing
for 4 weeks, piglets born to sows fed 100 mg FM BP-6/kg feed also
showed significant reductions in T3 and T4 (Werner & Sleight,
1981).
8.8.2.2 Sex hormones
Hepatic microsomes, prepared from rats exposed to FM BP-6
(100 mg/kg of feed) from day 8 of gestation until they were killed
at 4-21 weeks of age, showed an increased metabolism of progesterone
(Arneric et al., 1980), testosterone (Newton et al., 1980, 1982a),
and of the estrogens estradiol, estrone, and ethynylestradiol
(Bonhaus et al., 1981). In contrast to the increased hydroxylation
reaction, reduction of testosterone was inhibited by pretreatment
with PBBs (Newton et al., 1982a). The metabolism of exogenously
administered and labelled steroid hormones including progesterone,
testosterone, and estradiol, was also enhanced in vivo in rats
following perinatal exposure (gestation day 8-28 days postpartum) to
10 or 100 mg FM BP-6/kg feed, as shown by diminished steroid action
and reduced radio activity in serum and target organs (McCormack
et al., 1979c). In contrast, endogenously produced concentrations of
luteinizing hormone, prolactin, or corticosterone were not affected
in rats treated with 100 mg FM BP-6/kg feed from gestation day 8
until the ninth week of age (Johnston et al., 1980). Rats dosed with
1, 3, or 6 mg FM FF-1/kg body weight per day for 20 days also did
not show any alterations in plasma corticosterone or testosterone
levels, but, at 6 mg/kg body weight per day, there was a significant
reduction in plasma prolactin levels (Castracane et al., 1982).
Long-term, low-dose treatment with FM BP-6 (1, 10, or 50 m/kg feed
for 5-7 months) caused cumulative and dose-dependent decreases in
the serum corticosterone levels of female rats, as well as
reductions in the circulating levels of dehydroepiandrosterone and
dehydroepiandrosterone sulfate (Byrne et al., 1988). Alterations in
the urinary metabolic profile in the corticosteroid region of the
profile were observed after exposure (not specified) of rats to PBBs
(not specified) (Vrbanac, 1984).
Plasma corticosterone concentrations in female mice (BALB/c)
fed 100 mg FM BP-6 for 24 or 30 days were only modestly elevated
(Fraker, 1980).
Endogenous concentrations of progesterone and estradiol
determined in cows (No. = 2) administered toxic doses of FM BP-6
(25 g/day for 39 or 50 days) were in the range normally expected
(Willett et al., 1983a). The clearance rate of radiolabelled
progesterone and estradiol from the blood of both cows was decreased
(Willett et al., 1983a); elimination of radiolabel from these
hormones in the urine and faeces also declined (Sprosty et al.,
1979; Willett et al., 1983b).
Ingestion of FM FF-1 by adult, female rhesus monkeys at
concentrations of 0.3 mg/kg feed for 7 months (total dose:
approximately 10 mg PBB) caused prolonged menstrual cycles and
decreased concentrations of serum progesterone (Allen et al., 1978;
Lambrecht et al., 1978; for details in study design see section
8.5).
8.8.2.3 Prostaglandins
The effect of PBBs on prostaglandin has been examined in an
in vitro study using rat liver microsomes. A single ip injection
of FM BP-6 (100 mg/kg body weight) in male rats resulted in an
elevated metabolism of prostaglandin E1, 7 days after dosing
(Theoharides & Kupfer, 1981).
8.8.3 Interaction with drugs and toxicants
Several studies have demonstrated that PBBs have the ability to
alter the biological activity of a variety of drugs and toxicants.
In part, it may depend on the capability of the PBBs to induce
microsomal enzymes involved in the activation or deactivation of
xenobiotics. A summary of interactions, reported after the treatment
of animals with a combination of PBBs and drugs or toxicants, is
listed in Table 93 according to enhanced or miscellaneous effects.
The majority of reports are limited to the FireMaster(R) mixture.
One study (Halvorson et al., 1985) included several congeners, and
it was found that the results of interaction (formation of aflatoxin
metabolites) varied according to congener.
The toxicities of carbon tetrachloride, bromobenzene,
chloroform, trichloroethylene, and trichloroethane have been
enhanced by FM in rodents (Roes et al., 1977; Kluwe et al., 1978,
1979, 1982; Ahmadizadeh et al., 1984). Mirex-type compounds
aggravated the histological damage due to PBBs in rats (Chu et al.,
1980). Pretreatment with FM increased mortality during anaesthesia
elicited by pentobarbital in Japanese quails (Cecil et al., 1975).
On the other hand, a reduction in the toxicity of some toxicants has
also been observed, e.g., a reduction in ouabain lethality (Cagen &
Gibson, 1977a). Some other interactions are also recorded in Table
93.
8.8.4 Effects on vitamin A storage
Like related compounds, PBBs cause profound alterations in
vitamin A homeostasis. Significant reductions in hepatic vitamin A
stores have been seen in rats after exposure to individual PBB
congeners and the FireMaster(R) mixture (Table 94).
2,2',4,4',5,5'- Hexabromobiphenyl was less potent in reducing
vitamin A levels in the liver than the FM mixture and two other
hexa-isomers (Table 94).
Rats (Sherman, adult, male) given a single oral dose of 500 mg
FM FF-1/kg body weight had lower levels of retinol in the serum and
in liver microsomes compared with control animals, after an 18-month
recovery period (Bernert et al., 1983).
Table 93. Interactions of PBBs with drugs and toxicants
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Enhancement of toxicity
Rat (Sprague- FM BP-6 (20 mg/kg in diet mirex and related aggravation of histological changes due to Chu et al.
Dawley) M feed for 28 days) compounds (Kepone, PBB (additive rather than potentiative) (1980)
photomirex)
Rat (Sprague- FM BP-6 (100 mg/kg in diet carbon tetrachloride increase in CCl4-induced lethality and 48-h Kluwe et al.
Dawley) M feed for 20 days) (CCl4) (single ip dose growth retardation; increase in severity of (1982)
of 0.03-2 ml/kg body liver damage and renal tubular functional
weight)b impairment
Mouse (NMRI) FM BP-6 (single intraperitoneal bromobenzene (single decrease in bromobenzene LT50c Roes et al.
F dose of 150 mg/kg ip dose of 3150 mg/kg (1977)
body weight) body weight)b
Mouse FM BP-6 (1, 20, in diet chloroform (CHCl3) increase in CHCl3-induced lethality (96-h Kluwe et al.
(ICR) M 25, or 100 mg/kg (single ip dose of LD50; at 100 mg PBB/kg feed, P < 0.05); (1978)
feed for 14-28 various higher susceptibility to CHCl3-induced
days) concentrations)b renal and hepatic damage
Mouse (C57 FM (100 mg/kg in diet chloroform (CHCl3) enhancement of CHCl3 hepatoxicity (both Ahmadizadeh
BL/6J) and feed for 28 days) (single ip doses of strains); enhancement of nephrotoxicity et al. (1984)
(DBA/2J) M 0.025-0.25 ml/kg (only in C57 strain)
body weight)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Mouse FM BP-6 (1, 20, in diet carbon tetrachloride increase in CCl4-induced lethality (96-h Kluwe et al.
(ICR) M 25, or 100 mg/kg (CCl4) (single ip LD50; at 20 and 100 mg PBB/kg feed, (1978, 1979)
feed for 14-28 dose of various P < 0.05); higher susceptibility to
days) concentrations)b CCl4-induced renal and hepatic damage
(e.g.: decrease in renal PAH accumulation
after 0.125 ml CCl4/kg body weight)
Mouse FM BP-6 (1, 20, in diet trichloroethylene (TRI) potentiation of TRI-induced renal
(ICR) M 25, or 100 mg/kg (single ip dose of dysfunction (decrease in renal PAH
feed for 14-28 1 ml/kg body weight)b accumulation)
days)
FM BP-6 (1, 20, in diet 1,1,2-trichloroethane potentiation of TRI-induced renal
25, or 100 mg/kg (TCE) (single ip dose dysfunction (decrease in renal PAH
feed for 14-28 of 0.15 ml/kg body accumulation)
days) weight)b
Japanese FM BP-6 (single gavage pentobarbital (single increased mortality during anaesthesia Cecil et al.
quail dose of 100 mg/kg im dose of 50 (M) or when the pentobarbital was administered (1975)
body weight) 60 (F) mg/kg body 2 h after PBB dosing; reduction in
weight) pentobarbital sleeping times 48 h
after PBB dosing
FM BP-6 (300 mg/kg in diet pentobarbital (single reduction in pentobarbital sleeping
feed for 3 days) im dose of 50 (M) or times
60 (F) mg/kg body
weight)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Miscellaneous effects
Rat (Sprague- FM BP-6 (single intraperitoneal N-methylnicotinamide no effect on uptake of NMN Evers et al.
Dawley) F dose of 130-165 (NMN) (incubation of (1977)
mg/kg body renal cortical slices
weight) with 6 x 10-6 mol/litre
[14C]-NMN)
p-aminohippuric acid elevated uptake of PAH (significant
(PAH) (incubation of only at 32 days after PBB dosing)
renal cortical slices
with 7.4 x 10-5 mol
per litre PAH)
Rat (Sprague- FM BP-6 (direct in vitro N-methylnicotinamide no effect on uptake of NMN Evers et al.
Dawley) F exposure of renal (NMN) (incubation with (1977)
cortical slices 6 x 10-6 mol/litre
from untreated [14]-NMN)
animals to animals
to 1 x 10-3
mol/litre or 1 x
10-6 mol/litre)
in vitro p-aminohippuric acid no effect on uptake of PAH
(PAH) (incubation with
7.4 x 10-5 mol/litre
PAH)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Rat (Sprague- FM BP-6 (50 and pre- and/or ouabain (single iv enhanced hepatic uptake and transport Cagen & Gibson
Dawley) 100 mg/kg feed postnatal dose of 1 mg/kg body of ouabain from plasma into bile in (1977a,b,
in mother's diet) weight)b 15-day-old rats 1978); Cagen
et al. (1977)
postnatal ouabain (single ip elevated 24-h LD50 values in Cagen & Gibson
doses)b 15-day-old rats (1977a)
Rat (Sprague- FM BP-6 (single intraperitoneal ouabain (in vitro decrease in steady-state concentration Eaton &
Dawley) dose of 200 mg/kg 150 µmol in cell of ouabain; tendency to increased Klaassen
body weight; suspension) rate of efflux (1979)
isolation of
hepatocytes: 3
days after dosing)
procaine amide increased rate of efflux
ethobromide (PAEB)
Rat (Sprague- FM BP-6 (100 mg/kg in diet sulfobromophtalein lower plasma concentrations of BSP; Cagen & Gibson
Dawley) feed for 2 weeks) (BSP) (single iv dose increased biliary excretion of BSP and (1978)
of 120 mg/kg body conjugated BSP
weight)b
Rat (Fischer FM BP-6 (100 mg/kg in diet cephaloridine (single decrease in cephaloridine nephrotoxicity Kuo & Hook
344) M, F feed for 10 days) ip dose of 1000-2000 (1982)
mg/kg body weight)b
Rat (Fischer FM BP-6 (doses of gavage p-aminophenol (PAP) marked reduction in nephrotoxicity Newton et al.
344) M 90 mg/kg body (100 or 200 mg/kg produced by PAP (1982b)
weight per day body weight)b
for 2 days)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Rat (Fischer FM BP-6 (doses of gavage N-acetyl-p- accelerated excretion of mercapturic Newton et al.
344) 90 mg/kg body aminophenol (APAP) acid; enhanced ability of APAP to (1982c)
(isolated weight per day (3 x 10-8 mol/litre deplete glutathione
perfused for 2 days) in the perfusate)b
kidney)
Rat (Sprague- FM BP-6 (single intraperitoneal aflatoxin B1 (64 nmol increase in in vitro metabolism of Shepherd et al.
Dawley) M dose of 575 mg/kg per 5 ml incubation aflatoxin B1 (to aflatoxin M1) (1984)
(isolated body weight on mixture)
liver day 1; isolation
microsomes) of microsomes
on day 4)
Rat (Wistar) several PBBs (2 intraperitoneal aflatoxin B1 (64 nmol Halvorson
M (isolated doses of 150 per 5 ml incubation et al. (1985)
liver µmol/kg body mixture)
microsomes) weight on days 1
and 3; isolation
of microsomes on
day 6):
FM BP-6 increase in in vitro metabolism of
aflatoxin B1 (to aflatoxin M1)
2,2',4,5,5'-penta- increase in in vitro metabolism of
bromobiphenyl aflatoxin B1 (to aflatoxin Q1)
3,3',4,4'-tetra- increase in in vitro metabolism of
bromobiphenyl aflatoxin B1 (to aflatoxin M1)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
2,3,4,4'5-penta- increase in in vitro metabolism of
bromobphenyl aflatoxin B1 to aflatoxin M1);
decrease in metabolism to aflatoxin Q1
Mouse (Swiss- FM BP-6 (100, 200 in diet ouabain (single iv lower plasma ouabain concentrations Cagen et al.
Webster) F mg/kg feed for dose of 0.1 mg/kg no enhanced capacity for ouabain (1977); Cagen
2 weeks) body weight)b excretion & Gibson
(1978)
ouabain (diverse no effect on ouabain 24-h LD50 values Cagen et al.
single doses) (1977)
Mouse (Swiss- FM BP-6 (50 mg/kg pre- and/or ouabain (single iv enhanced disappearance of ouabain from Cagen & Gibson
Webster) feed in mother's postnatal dose of 1 mg/kg the plasma; increase in ouabain excretion (1977a,b,
diet) body weight)b in 15-day-old mice 1978)
ouabain (ip dose) no effect on ouabain 24-h LD50 values Cagen & Gibson
(1977a,b)
Mouse (Swiss- FM BP-6 (100, 150, in diet indocyanine green enhanced initial disappearance of Cagen et al.
Webster) 200 mg/kg feed (ICG) (single iv dose ICG from plasma (correlated with (1977)
for 2 weeks) of 40 mg/kg body higher hepatic ICG content)
weight)b
Mouse FM BP-6 (100 mg/kg in diet ethylene dibromide decrease in renal and hepatic NPS Kluwe et al.
(ICR) M feed for 18 days) (EDB) (single ip dose (non-protein sulfhydryl)-depleting (1981)
of 100 mg/kg body effects of EDP
weight)b
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
(diverse single no significant effect on LD50 (220 mg/kg Kluwe et al.
ip doses) body weight versus 250 mg/kg in control) (1981)
Mouse FM BP-6 (100 mg/kg in diet 1,2-dibromo-3-chloro- decrease in renal and hepatic NPS Kluwe et al.
(ICR) M feed for 18 days) propane (DBCP) (single (non-protein sulfhydryl)-depleting (1981)
ip dose of 100 mg/kg effects of DBCP
body weight)b
Mouse FM BP-6 (single not chloroform (CHCl3) no significant effect on GPT or OCT Plaa &
M dose of 500 mg/kg specified (single oral dose of activity Hewitt (1982)
body weight) 0.1 ml/kg body
weight)b
a F = female; M = male.
b Administration after PBB treatment.
c LT50 = median time to death.
d GPT = Glutamic-pyruvic transaminase; OCT = ornithine carbamyl transferase; PAH = p-aminohippurate.
Rats (Sprague-Dawley, female) treated with FM BP-6 (100 mg/kg
diet for up to 140 days) had lower hepatic vitamin A and higher
kidney vitamin A levels than controls, but showed an increase in
serum retinol (Jensen & Zile, 1988).
Short-term feeding of 3,3',4,4',5,5'-hexabromobiphenyl (1 mg/kg
diet for 140 days) to rats (Sprague-Dawley, female) caused a severe
decrease (approximately 20-fold) in hepatic retinol and retinyl
esters and a 6-7-fold increase in retinol and retinyl esters in the
kidneys, while serum concentrations of retinol were unaffected by
PBB feeding (Jensen et al., 1987). Examination of liver enzymes in
these rats revealed reductions of 50% and 63% of acetyl-CoA-retinol
acetyltransferase and retinyl palmitate hydrolase, respectively
(Jensen et al., 1987). Consistent with the previous studies, there
was an increased accumulation of retinol and retinyl esters in the
kidneys of rats given a single oral dose of
3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight) as well as a
decrease in liver retinyl ester pools (Zile et al., 1989).
In studies using radioactive retinyl acetate, a two-fold
increase in the elimination of vitamin A metabolites in the urine
and faeces was observed in rats (Sprague-Dawley, male) 12 h-8 days
after a single anorectic, oral dose of
3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight). The
potential effect of the PBB on the absorption of vitamin A was
excluded by the experimental design in this study (Cullum & Zile,
1985). Similarly, long-term, dietary administration of
3,3',4,4',5,5'-hexabromobiphenyl (1 mg/kg diet for 140 days)
resulted in a greatly increased faecal and urinary elimination of
radioactivity, when rats (Sprague-Dawley, female) were given a
physiological dose of [11-3H]retinyl acetate (Jensen et al.,
1987).
Changes in vitamin A metabolite patterns were found in tissues
(liver, kidney, small intestinal mucosa) of rats after a single oral
dose of 3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight),
which produced a shift of vitamin A metabolism towards the more
polar forms of vitamin A, such as retinoic acid, its oxidation and
conjugation products (retinoyl glucuronide), and polar retinoid
metabolites (Zile et al., 1989).
The influence of the PBB on the vitamin A balance was long
lasting. As seen in Table 94, in a multigeneration study, FM BP-6
produced a decrease in hepatic vitamin A concentration, even in the
second generation of offspring (28 days of age) of rats when PBB was
fed (100 mg/kg diet) only during the first pregnancy (day 8) until
28 days post partum. At this time, all offspring (F1) were weaned
onto a control diet, allowed to mature sexually, and bred with
litter-mates to produce the F2-generation (McCormack et al.,
1981).
Table 94. Reduction of hepatic vitamin A content in the rat by various PBBs (single congeners and commercial mixtures)
Compound Strain/sex Dose and route of Exposure period/ Reduction in hepatic References
administration observation/period vitamin A
concentration (%)
2,2',4,4',5,5'- Sprague-Dawley 100 mg/kg diet 30 days 28 Akoso et al.
hexabromobiphenyl (male) (1982a)
2,3',4,4',5,5'- Sprague-Dawley 100 mg/kg diet 30 days 57
hexabromobiphenyl (male)
3,3',4,4',5,5'- Sprague-Dawley 10 mg/kg diet 30 days 59
hexabromobiphenyl (male)
3,3',4,4',5,5'- Sprague-Dawley 1 mg/kg diet 140 days 95 Jensen et al.
hexabromobiphenyl (female) (1987)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg diet 30 days 72 Akoso et al.
(male) (1982a)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg diet 140 days 92 Jensen & Zile
(female) (1988)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg in perinatal: day 8 of pregnancy approximately McCormack et al.
(female) mother's diet until 4, 8, or 14 weeks of 50 (1982b)
age
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg in F0: perinatal: day 8 of 57 McCormack et al.
(female) mother's diet pregnancy until 28 days (1981)
post-partum/F1 (28 days of
age)
Table 94 (contd).
Compound Strain/sex Dose and route of Exposure period/ Reduction in hepatic References
administration observation/period vitamin A
concentration (%)
F0: perinatal: day 8 of 28
pregnancy until 28 days
post-partum/F2 (28 days of
age)
a F0 = parental generation; F1 = first filial generation; F2 = second filial generation.
The influence of dietary levels of vitamin A on the toxicity of
PBB has been studied. Vitamin A supplementation (up to 30 000 IU)
provided only partial protection against decreases in body weight
gain and thymic weight and against hyperplasia of the common bile
duct in rats intoxicated with 100 mg FM BP-6/kg diet (Darjono
et al., 1983). High dietary levels of vitamin A (200 000 IU) also
had some inhibitory effect on carcinogenesis, i.e., on the promotion
of hepatic-altered foci by 3,3',4,4',5,5'- hexabromobiphenyl in
initiated rats (Rezabek et al., 1989).
The mechanism by which PBBs influence vitamin A homeosta sis is
not fully understood. Conflicting results may be because of
analytical problems and biological variables. However, according to
Zile et al. (1989), it is very likely that polyhalogenated aromatic
hydrocarbons affect specific enzymes involved in the regulation of
vitamin A storage and in the vitamin A metabolic pathway. Others
have suggested that PCBs and related compounds may interfere with
Vitamin A transport in the serum by inhibiting the formation of the
serum transport protein complex carrying retinol and thyroxin
(Brouwer & van den Berg, 1986).
8.8.5 Porphyria
Hexabromobiphenyl is able to produce chemical porphyria in
Japanese quail (Strik, 1973b), rats, and mice (Gupta et al., 1983a;
Hill, 1985). Following studies on adult Japanese quail, which were
orally dosed with gelatin capsules or fed diet containing up to
1000 mg/kg body weight FireMaster(R) BP-6, Strik (1978) stated
that PBB porphyria was preceded by liver and kidney damage.
Accumulation of porphyrins in the liver is not solely due to an
increase in delta-amino-levulinic acid synthetase activity, but
rather to drug enzyme induction and the formation of a reactive
intermediate that causes centrilobular liver damage and ultimately
porphyria. The hepatic mitochondria decrease in number and are
damaged (elevated serum glutamic acid dehydrogenase); the
profilerated endoplasmic reticulum, including the haemoprotein
P-450, is no longer capable of normal activity; uroporphyrinogen
decarboxylase activity is reduced to zero; renal porphyrins
accumulate and are excreted in the liver and via the bile.
In tests with chick-embryo liver cell cultures, Debets et al.
(1980) showed that pretreatment with inducers of the drug-
metabolizing enzyme system markedly stimulated the accumulation of
porphyrins, after exposure to FireMaster(R) BP-6. Inhibition of
hepatic drug metabolism or the addition of compounds, known to trap
electrophiles or radicals, protected against the porphyrinogenic
action of FireMaster(R) BP-6 in vitro.
In the same test system, a DeBB solution of 10 µg/ml medium did
not show any porphyrogenic potential with, or without, pretreatment
with ß-naphthoflavone (3 µg/ml medium) for 20 h (Koster et al.,
1980). Gupta et al. (1983a) found dose-related, elevated hepatic
prophyrin levels in Fischer 344/N rats and B6C3F1 mice, orally
dosed with 0.1-10.0 mg FireMaster(R) BP-6/kg body weight (5
steps), primarily in females.
Hill (1985) studied the urinary porphyrin pattern by ion-pair
chromatography in female Sherman rats given 0 or 1 g PBB/kg body
weight. The route of administration was not stated. Chronic hepatic
porphyria became evident about 55-60 days after dosing, as indicated
by the large increase in uroporphyrin and heptacarboxylporphyrin and
the corresponding ratios of these porphyrins to coproporphyrin.
Between 85 and 100 days, chronic hepatic porphyria developed into
its most severe form (porphyria cutanea tarda).
Summarizing all reported facts on porphyrin metabolism, Hill
(1985) pointed out that chronic hepatic porphyria is characterized
as a membrane disease in which there is damage to the membranes of
the cell walls of the hepatocytes and organelles (i.e., mito
chondria, endoplasmic reticulum) within the liver cell. The cause of
damage is unknown. There is some evidence that PBBs produce changes
in lipid metabolism, which in turn alters membrane structure
(Bernert et al., 1983). This change in membrane structure may cause
a change in membrane permeability (damage) so that porphyrins are
excreted in the bile capillaries and the intercellular substance. In
addition to these changes, PBBs, or their metabolites, cause the
induction of delta-aminolevulinic acid synthetase and the inhibition
of the enzyme uroporphyrinogen decarboxylase. The changes in enzyme
activities produce a build-up of uroporphyrinogen and
heptacarboxylporphyrinogen, and these precursors are excreted in the
urine, where they are easily oxidized to uroporphyrin and
heptacarboxylporphyrin.
A marked sex difference in the development of uroporphyria
occurred after administration to 10-week-old F344/N rats of 0.005%
FireMaster(R) BP-6 added to the diet. Thus, the propensity of
female rats to develop uroporphyria appeared to be a general
response to this class of halogenated chemicals. Liver to body
weight ratio, liver porphyrins, and the activity of a
uroporphyrinogen decarboxylase inhibitor were significantly greater
in both sexes compared with appropriate controls. Comparing treated
males and females, the liver to body weight ratio and
uroporphyrinogen decarboxylase inhibitor activity were significantly
greater in males whereas the liver porphyrin content was greater in
females. Levels of total cytochrome P-450 and pentoxyresorufin and
benzyloxyresorufin dealkylase activities (associated with cytochrome
P450IIB1) were greater in microsomes from control, and PBB-treated
male rats compared with females. In contrast, ethoxyresorufin
deethylase activity (associated with cytochrome P450 IA1) was
significantly greater in females (Smith et al., 1990a).
Iron potentiated the development of uroporphyria after oral
exposure to FireMaster(R) BP-6 for 2 months (dose not stated) in 7
to 10-week-old, male Ah-responsive C57Bl/10ScSn mice (Smith et al.,
1990b).
8.8.6 Miscellaneous effects
Alterations in liver microsomal membrane lipid composition,
increased peroxidation activities, and an increase in membrane
fluidity were found after a single oral dose of 500 mg
FireMaster(R) BP-6/kg body weight during short- and long-term
observations (Bernert et al., 1983; Bernert & Groce, 1984).
PBBs were found to inhibit rabbit muscle glycogen phosphorylase
(1,4-alpha-D-glucan: orthophosphate alpha-D-glucosyltransferase, EC
2.4.1.1), an enzyme that catalyzes the breakdown of glycogen to
glucose 1-phosphate. The enzyme exists in two metabolically
significant forms, phosphorylase a and b.
2,2',4,4',5,5'-Hexabromobiphenyl and FireMaster(R) BP-6 were
strong inhibitors of phosphorylase b (92% and 88% inhibition at
60 µmol/litre, respectively; Ki = 15 µmol/litre), while exhibiting
little or no inhibition of phosphorylase a (Mead et al., 1982).
The activity of the enzyme uridine 5'-diphosphoglucuronyl-
transferase (UDP-GT), responsible for the conjugation of a wide
variety of substrates, was significantly increased in microsomes
prepared from rats ip injected with 3,3',4'4',5,5'-hexabromobiphenyl
at 20 mg/kg body weight (Spear et al., 1990).
The influence of FireMaster(R) BP-6 on glutathione peroxidase
activity, which is present in most mammalian species, was studied in
the rat (liver cytosol). This enzyme is represented, on the one
hand, by a selenium-containing enzyme, glutathione peroxidase (E.C.
1.11.1.9), which is able to reduce hydrogen peroxide to water and
organic hydroperoxides to the corresponding hydroxy compounds, and,
on the other hand, by certain isozymes of glutathione transferase
(E.C. 2.5.1.18). The activity of the selenium-dependent glutathione
peroxidase was decreased to about 50% of control values on day 16
following the ip administration of FireMaster(R) BP-6 (500 mg/kg
body weight). Inversely, there was a potent induction of glutathione
transferases during the 16-day period (Schramm et al., 1985).
Adenylate cyclase [ATP pyrophosphatase lyase (cyclizing), EC
4.6.1.1] catalyzes the conversion of adenosine triphosphate (ATP) to
adenosine 3',5'-cyclic monophosphate (cyclic AMP), which acts as a
central regulator of several diverse cell activities. In preliminary
experiments, the in vitro effect of FireMaster(R) BP-6 on
adenylate cyclase activity in the plasma membranes of rat lung
alveoli was determined. At concentrations of 10 µg/ml, the
FireMaster(R) mixture stimulated the basal adenylate cyclase
activity of plasma membranes 2- to 2.5-fold (Sidhu & Michelakis,
1978). The authors discuss the possible immunological relevance of
this observation.
8.9 Effects on intercellular communication
PBBs show a significant epigenetic activity. The
FireMaster(R) mixture and some of its major components were found
to be capable of inhibiting intercellular communication, measured by
metabolic cooperation between HGPRT+ and HGPRT- cells in culture
(Table 95). This inhibition occurred at non-cytotoxic
concentrations. In contrast to FireMaster(R) and to its major
constituents BB 153 and BB 180, 3,3',4,4',5,5'-hexabromobiphenyl and
3,3',4,4'-tetrabromobiphenyl did not interrupt cell-cell
communication at noncytotoxic concentrations. However, both
congeners were markedly cytotoxic (Table 95). Congeners with
intermediate toxicity such as 2,3'4'4',5-PeBB (BB 118),
3,3',4,4',5'- PeBB (BB 127), and 2,3',4,4',5,5' HxBB (BB 167), were
also shown to interfere with cell-cell communication at noncytotoxic
concentrations. Both the cytotoxicity and the metabolic
cooperation-inhibiting properties of PBB congeners seem to be
related to their structure, i.e., presence or lack of ortho-
substitution (Tsushimoto et al., 1982; Kavanagh et al., 1987; see
also section 8.7.2.2).
Recently, the ability of FireMaster(R) BP-6 to inhibit gap
junction-mediated intercellular communication has been confirmed by
the FRAP assay, using cultured rat liver epithelial cells (Rezabek
et al., 1988). This assay, which has been described by Wade et al.
(1986), evaluated the inhibition of fluorescence redistribution
after photobleaching (FRAP), which occurs between cells loaded with
a fluorescent dye.
Results obtained by a similar new method, the scrape-
loading/dye-transfer (SL/DT) technique (El-Fouly et al., 1987)
confirmed the inhibitory potency of 2,2',4,4',5,5'-hexabromobiphenyl
(Evans et al., 1988; Table 95).
Several different cell types (human fibroblasts, rat kidney
epithelial cells, rat liver oval cells, rat Leydig cells, rat glial
cells, mouse keratinocytes) differ in their response to chemicals
that alter gap junctional intercellular communication. After
exposure to FireMaster(R) BP-6 (20 µg/ml), intercellular
communication was inhibited to different extents in three (WB rat
liver oval cells, RG-1 rat glial cells, JB-6 mouse keratinocytes)
out of the six cell types tested, rat liver oval cell being the most
sensitive cell (Bombick, 1990).
Table 95. Inhibition of intercellular communication by PBBs: results of in vitro assays testing metabolic cooperation
between 6-thioguanine-sensitive (HGPRT+) and- resistant (HGPRT-) cells or testing dye transfera
PBBa Cells in culture Inhibition Remark References
Yes No
FM BP-6 Chinese hamster x non-lethal range of the Trosko et al. (1981)
V79 lung cells chemical
FM FF-1 rat liver cells x no cytotoxicity mentioned Williams et al. (1984)
FM BP-6 human teratocarcinoma x only slight effect on Kavanagh et al. (1987)
cells cell survival
FM BP-6 rat liver epithelial cells x at non-toxic FM concentration Rezabek et al. (1988)
(WB-F 344)
3,3',4,4',-tetrabromobiphenyl human teratocarcinoma x moderately cytotoxic Kavanagh et al. (1987)
(BB 77) cells
2,3',4,4',5- Chinese hamster x inhibition before Tsushimoto et al.
pentabromobiphenyl (BB 118) V79 cells cytotoxicity occurs (1982)
3,3'4,5,5'-pentabromobiphenyl Chinese hamster x slight inhibition Tsushimoto et al.
(BB 127) V79 cells before cytotoxicity (1982)
2,2',4,4',5,5'- Chinese hamster x relatively nontoxic
hexabromobiphenyl (BB 153) V79 cells
rat liver epithelial x x at non-cytotoxic Evans et al.
cells (WB-F344) concentrations (1988)
Table 95 (contd).
PBBa Cells in culture Inhibition Remark References
Yes No
human teratocarcinoma x only slight effect Kavanagh et al.
cells on cell survival (1987)
2,3',4,4',5,5'- Chinese hamster x inhibition before Tsushimoto et al.
hexabromobiphenyl (BB 167) V79 cells cytotoxicity occurs (1982)
3,3',4,4',5,5'- Chinese hamster x highly cytotoxic Tsushimoto et al.
hexabromobiphenyl (BB 169) V79 cells (1982)
human teratocarcinoma x highly cytotoxic Kavanagh et al.
cells (1987)
2,2',3,4,4',5,5'- Chinese hamster x relatively non-cytotoxic Tsushimoto et al.
heptabromobiphenyl (BB 180) V79 cells (1982)
2,2',3,3',4,4',5,5'- Chinese hamster x relatively non-cytotoxic
octabromobiphenyl V79 cells
(BB 194)
a HGPRT = Hypoxanthine-guanine phosphoribosyl transferase locus.
b Purity of PBB congeners tested: > 99%; FM = FireMaster.
8.10 Immunotoxicity
The effects of PBBs (commercial mixtures and individual
congeners) on the weight and histology of the thymus, bursa of
Fabricius, and spleen have been reviewed in sections 8.2 (single and
short-term exposures: 8.2.1.3, commercial mixtures; 8.2.2,
congeners) and 8.4 (long-term exposures). In summary, atrophy of
thymus was a frequent observation following PBB exposure.
Other indicators of a suppressed immune function have been
compiled in Table 96. These data refer only to the FireMaster(R)-
mixture, because information on OcBB, DeBB, or individual PBB
congeners (with the exception of 3,3',4,4'-tetrabromobiphenyl) is
lacking.
In addition to the thymus or spleen, other lymphoid tissues
were affected by PBB, e.g., bone marrow and the lymph nodes of dogs
(Farber et al., 1978; Table 96).
Serum immunoglobulin levels in mice were changed following
short-or long-term exposure to FireMaster(R) (Luster et al., 1978,
1980; Loose et al., 1981). Suppression of antibody response to sheep
erythrocytes (or bovine gamma globulin) was reported after the
short-term exposure of mice (Fraker & Aust, 1978; Luster et al.,
1978; Fraker, 1980; Loose et al., 1981) and after a six-month
exposure of rats (Luster et al., 1980). Conditions of study are
summarized in Table 96.
Interestingly, mice showed no response in the 6-month study
(Luster et al., 1980). A decrease in antibody titres to tetanus
toxoid was observed in guinea-pigs (Vos & van Genderen, 1974; Table
96).
Although mortality rates following infection with Listeria
monocytogenes were not affected by FireMaster(R) exposure in
mice exposed long-term, an increased susceptibility to infection
with Listeria was suggested, because a decrease in time to death
occurred (Luster et al., 1980; Table 96). No effects on mean
survival time were observed in mice fed 5 or 167 mg/kg feed for 3 or
6 weeks and then challenged by Plasmodium berghei (murine malaria)
infection (Mudzinski et al., 1979; Loose et al., 1981).
An increased susceptibility to endotoxin was found in mice
after short-term (Mudzinski et al., 1979; Loose et al., 1981) or
perinatal (Luster et al., 1980) exposure to FireMaster(R) (Table
96). Mice with long-term exposure did not show endotoxin sensitivity
(Luster et al., 1980). Of the individual PBB congeners, 3,3'4,4'-
tetrabromobiphenyl was found to increase sensitivity to endotoxin
(lipopolysaccharide from Escherichia coli) 1-2 days after
administration (single intraperitoneal dose of 150 µmol/kg body
weight) to rats (Shedlofsky et al., 1991).
Table 96. Immunotoxicity of FireMaster(R)a
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 rat gavage 22 doses of 0.03, 30 days depression of T-cell responsiveness to Luster
(lot FF 1312 FT) (Fischer) 0.3, 3, or 30 mg/kg mitogens (PHA: overall dose response: et al.
(M) body weight P < 0.01; 3 and 30 mg/kg per day: (1978)
P < 0.05; Con A: overall dose response:
P < 0.1; 30 mg/kg per day; P < 0.05)
FM FF-1 rat gavage 122 doses of 0.1, 6 months depression of both B-(10 mg/kg per day) Luster
(lot FF 1312 FT) (Fischer) 0.3, 1, 3, or 10 and T-(1,3, or 10 mg/kg per day) cell et al.
(F) mg/kg body weight mitogenic (PHA, Con A, PWM) and (1980)
allo-geneic responses (P < 0.05 or 0.01);
decreased antibody responses to bovine
globulin (10 mg/kg per day; P < 0.1);
suppressed delayed hypersensitivity
reactions (3 and 10 mg/kg per day;
P < 0.05)
FM FF-1 mouse gavage 22 doses of 0.03, 30 days depression of T- and B-cell responsiveness Luster
(lot FF 1312 FT) (B6C3F1) 0.3, 3, or 30 mg/kg to mitogens PHA, Con A, and LPS (overall et al.
(F) body weight dose response: P < 0.01; 3 mg/kg per day: (1978)
PHA, Con A and 30 mg/kg per day: PHA,
Con A, LPS: P < 0.10 or 0.01); decreased
antibody responses to SRBC (30 mg/kg per
day; 27% reduction): decrease in serum IgM
and IgG2 levels (30 mg/kg per day;
P < 0.01 and 0.10, respectively)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 mouse gavage 122 doses of 0.1, 6 months enhanced number of bone marrow colony Luster
(lot FF 1312 FT) (B6C3F1) 0.3, 1, 3, or 10 forming units (only F at 1 and 10 mg/kg et al.
(F,M) mg/kg body weight body weight per day; P < 0.01); decrease (1980)
per day in serum IgG, IgM, and IgA levels (10 mg/kg
body weight per day P < 0.01 or 0.05);
increase in serum IgG (1 mg/kg body weight
per day; P < 0.01) and IgA (0.3 mg/kg body
weight per day; P < 0.1); depression of B-
and T-cell responisiveness to mitogens
(PHA, Con A, LPS) at 10 mg/kg body weight
per day (P < 0.05): increased
susceptibility to infection with Listeria
mono-cytogenes (10 mg/kg body weight
per day)
FM FF-1 mouse perinatal maternal doses of gestation enhanced number of bone marrow colony Luster
(lot FF-1312 FT) (B6C3F1 0.3, 1, 3, or 10 day 0 until forming units (F: 1 mg/kg body weight (1980)
(F,M) mg/kg body weight weaning (on per day; P < 0.05): increased
per day alternate susceptibility to endotoxin (LPS,
day) E. coli) (marginal dose response
P = 0.06)
FM BP-6 mouse in diet 1, 10, 100 mg/kg 30 days reduced antibody responses to SRBC Fraker &
(BALB/c) feed (10, 100 mg/kg: P < 0.001) Aust (1978)
1000 mg/kg feed 14 days survivors incapable of mounting an Fraker
antibody-mediated response to SRBC (1980)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 mouse in diet 167 mg/kg feed 3 or 6 increase in endotoxin (LPS; Loose
(lot No. 7042) (BALB/cByJ) weeks Salmonella typhosa) sensitivity (P < 0.05); et al.
(M) reduced primary antibody reaction to (1981)
SRBC (only at 3 weeks)
5 mg/kg feed 3 or 6 reduced serum IgM levels (at 3 and 6
weeks weeks; (P < 0.05)
FM BP-6 guinea-pig in diet 10, 50 mg/kg feed 45 days reduction in antibody titres to tetanus Vos & van
(F) toxoid (P < 0.025) P < 0.05); reduced serum Genderen
IgG levels (at 6 weeks, P < 0.05) reduced (1974)
serum IgM levels (at 3 and 6 weeks;
P < 0.05)
FM BP-6 pig in diet 100, 200 mg/kg last half decreased responses of peripheral blood Howard
(sows) feed of lymphocytes to mitogen (PHA, PWM) et al.
gestation stimulation (200 mg/kg; P < 0.05) (1980)
and 4
weeks of
lactation
(12 weeks)
FM BP-6 pig perinatal 100, 200 mg/kg last half of normal mitogen (PHA, PWM) responses at
(piglets) feed gestation birth; decreased mitogen responses at
and 4 weeks 4 weeks of age (PWM = 200 mg/kg;
of lactation P < 0.002)
(12 weeks)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM BP-6 dog gavage 0.06-4 mg/kg 61 days degenerating lymphocytes in blood smears Farber
body weight (all levels); depletion of lymphocytes in et al.
per day the lymph nodes, (4 mg/kg); reduced (1978)
erythro-poiesis in bone marrow (4 mg/kg);
reduction in IgG-containing lymphocytes in
popliteal lymph nodes (4 mg/kg)
a Exclusive of effects on the weight and histology of thymus and spleen, which are noted in another section.
b FM = FireMaster(R).
c M = Male; F = Female.
d Con A = Concanavalin A; LPS = bacterial lipolysaccharide; PHA = phytohemagglutinin; PWM = pokeweed mitogen;
SRBC = sheep red blood cells.
FireMaster(R) depressed lymphoproliferative (B- and/or
T-cell) responsiveness to mitogens in rats (Luster et al., 1978,
1980), mice (Luster et al., 1978, 1980), and pigs (Howard et al.,
1980). Response to mitogens was variable among the species tested
(Table 96), e.g., rats exposed long-term were more sensitive (dose
required: < 10 mg/kg body weight per day) than mice (dose required
= 10 mg/kg body weight per day) in the same study (Luster et al.,
1980).
Delayed hypersensitivity reactions were depressed in adult rats
at higher doses of PBBs (Luster et al., 1980; Table 96), but they
were comparable to controls in mice, exposed perinatally and
long-term (Luster et al., 1980).
Some haematological parameters of immunological interest, e.g.,
changes in peripheral lymphocyte and leukocyte counts are reported
in sections 8.2.1.2. and 8.4.
In summary, there was a wide spectrum of immunotoxic effects of
FireMaster(R) in different animal species, but immune
dearrangement was often recorded at doses that produced other signs
of toxicity.
8.11 Neurotoxicity
Behavioural and neurological parameters have been examined in
rodents and rhesus monkeys treated with the commercial
FireMaster(R) mixture or with its main component, 2,2',4,4',5,5'-
hexabromobiphenyl (BB 153).
8.11.1 Exposure of adult animals
Adult rats (Fischer 344/N) and mice (B6C3F1) of both
sexes were exposed to oral doses of 0.03-30 mg FM FF-1/kg body
weight per day or 0.168-16.8 mg 2,2',4,4',5,5'-hexabromobiphenyl per
kg body weight per day (5 days per week) for a total of 22 doses.
Neurobehavioural toxicity was assessed at the end of the 30-day
dosing regimen and also 30 days after cessation of dosing (Tilson
et al., 1978; Tilson & Cabe, 1978, 1979). Additionally, rats having
received 130 oral doses of 3 or 10 mg FM FF-1/kg body weight per day
(5 days per week) were tested after 6 months of dosing (Tilson &
Cabe, 1979). Mainly at the higher doses, FM FF-1, and, to a much
lesser extent, BB 153 led to neuromuscular dysfunction, such as
decreased motor activity, depressed neuromuscular reflexes, and
impaired forelimb grip strength. Rats were generally more affected
than mice. Visual placement responses were also decreased in male
rats and mice by FM FF-1 and BB 153. Hypothermia (decreased rectal
temperature) was caused by FM FF-1 in mice. In general, rats tended
to remain the same or get worse during the 30 days of no dosing,
while mice tended to improve. In another study (Geller et al.,
1979), the influence of PBB on cognitive function was evaluated.
Male rats (Sprague-Dawley) received "hexabrominated biphenyl" (not
specified) at 1 mg/kg body weight per day (5 days per week) for a
total of 20 doses during a one-month period and were then trained
for a simple auditory discrimination task. PBB-treated animals did
not significantly differ from controls with respect to accuracy on
the discrimination task. However, throughout 24 weeks of
discrimination training, PBB rats made many more extra responses and
showed longer response times (response latencies), thereby reducing
their efficiency. Upon completion of the behavioural study, a group
of rats, dosed concurrently with the behavioural animals, was
sacrificed immediately after the end of dosing, and the brains were
used to prepare both intact synaptosomes and synaptic plasma
membranes (Gause et al., 1979). Both calcium binding to synaptic
plasma membranes and calcium uptake by intact synaptosomes was
significantly reduced in the brains of rats administered 1 mg PBB/kg
body weight per day. From these results, the authors derived that
both spontaneous and evoked transmitter release could be reduced
during PBB treatment.
8.11.2 Perinatal exposure
Functional impairment of offspring after perinatal exposure to
PBBs has been observed at PBB concentrations not causing overt
maternal toxicity. Locomotor activity was decreased in the offspring
of Swiss-Webster-mice fed FireMaster(R) (100 mg/kg feed) during
lactation (postnatal days 1-29). Increased mortality and decreased
body weight were also seen in the pups (Preache et al., 1976). Prior
to breeding, female rats (Sprague-Dawley) were dosed orally for 20
days (5 days/week) with FireMaster(R) FF-1 (0.5 or 5 mg/kg body
weight), and the male offspring were used for measurements of motor
activity (Gause et al., 1984), pain threshold (Gause et al., 1984),
operant behaviour, and response to central nervous system-active
drugs (Gause et al., 1984; Geller et al., 1985). The first two of
these four measurements started from weaning and resulted (over a 6
week period) in higher levels of activity and in changes in the pain
threshold in animals exposed to PBB. Both of the last tests were
conducted with the adult (> 75 days of age) offspring: There were
no detectable effects of PBB on the acquisition or performance of
the operant discrimination task; however, the pharmacological
challenge showed that F1 males from PBB-treated dams were less
sensitive to both phenobarbital and d-amphetamine than F1 males
from control dams. In another study, pregnant rats (Sprague-Dawley)
received oral doses of 0.2 or 2 mg FireMaster(R) BP-6/kg body
weight per day from day 6 of gestation through day 24 postpartum
(Henck & Rech, 1986). Several signs of neurobehavioural toxicity
were found in male and female offspring of dams given 2 mg
FireMaster(R) BP-6/kg per day, a dose that produced tissue levels
within the range of those measured in highly exposed Michigan
people. There were significant effects on the acquisition of
foreword locomotion, cliff avoidance, cage emergence, and open field
activity (Henck, 1986). At 6 months of age, the offspring were
tested for a series of operant responses of increasing difficulty.
It was found that the learning of various operant behavioural
patterns was impaired in a relatively subtle manner, and that both
sexes can differ in their responses (Henck, 1986; Henck & Rech,
1986).
8.12 Factors modifying toxicity, toxicity of metabolites
8.12.1 Contaminants affecting toxicity
8.12.1.1 Polybrominated naphthalenes (PBNs)
PBNs have been identified as contaminants of the commercial
FireMaster(R) mixture (at concentrations of the order of
200 mg/kg: see section 2.1.2). In structure, they resemble other
classes of halogenated aromatic hydrocarbons, such as
polychlorinated naphthalenes, polyhalogenated biphenyls,
dibenzodioxins, and dibenzofurans, and may elicit similar
qualitative effects (e.g., Kimbrough, 1980a,b). A summary of
biologic and toxicological responses reported on PBNs is given in
Tables 97 and 98. It shows that some PBNs are potent toxicants and
teratogens. PBNs were teratogenic in mice at dose levels below those
capable of producing overt maternal toxicity (Miller & Birnbaum,
1986). Compared with FireMaster(R) (consult also previous
sections), PBN mixtures were much more potent in causing adverse
effects. For example, a PBN mixture was at least 10 times more
effective than FM BP-6 in producing maximal induction of aryl
hydrocarbon hydroxylase (30 µmol/kg body weight versus 300 µmol/kg;
Robertson et al., 1981a, 1984a). Although PBNs are present only at
low levels in the FireMaster(R) mixture, some authors (Robertson
et al., 1984a; Miller & Birnbaum, 1986) believe that they may
contribute to the toxicity of FireMaster(R).
8.12.1.2 Mixed polybromo-chlorobiphenyls
At present, only a monochloropentabromobiphenyl has been
identified as a trace impurity in FireMaster(R) FF-1 (see section
2.1.2). No information is available on its toxicological properties.
However, there is one study by Andres et al. (1983) in which the
biological and toxic effects are compared of a series of laterally
substituted 3,3',4,4'-tetrahalobiphenyls containing the following
variable molecular Cl/Br ratios: Br4, Br3Cl, Br2Cl2 (two
isomers), BrCl3, and Cl4. Parameters examined included: growth
rate, effects on the thymus, and hepatic microsomal enzyme induction
in male Wistar rats, as well as enzyme induction in rat hepatoma
cells in culture and relative binding affinities to the rat
cytosolic receptor protein. Data obtained demonstrated that the
activity of these (mixed) halogenated biphenyls was enhanced with
increasing bromine (and decreasing chlorine) substitution.
Table 97. LD50 values of several polybrominated naphthalenes (PBN)
in guinea-piga,b
PBN LD50
(µg/kg body weight)
2,3,6,7-tetrabromonaphthalene 242
1,2,4,6,7-pentabromonaphthalene 200
1,2,3,4,6,7-hexabromonaphthalene 361
1,2,3,5,6,7-hexabromonaphthalene > 3610
a From: McKinney & McConnell (1982).
b Hartley strain guinea-pigs given a single oral dose (gavage) and
observed for 30 days.
8.12.2 Toxicity of metabolites
No experimental data are available on the toxicity of PBB
metabolites.
8.12.3 Toxicity of photolysis and pyrolysis products
8.12.3.1 Photolysis products
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-hexabromobiphenyl, showed that the photolysis
products were more toxic than the original PBB. The parameters of
toxicity compared were liver and thymus weight changes, liver
histology, hepatic microsomal enzyme induction, and binding affinity
to the cytosolic receptor in rats, as well as development of
hyperkeratosis in the rabbit ear (Table 99). Probably, one or more
of the lower brominated PBBs formed by photolysis (see section
4.2.1) are responsible for the increased potency. It is believed
that the increased potency of irradiated
2,2',4,4',5,5'-hexabromobiphe nyl is due mostly to
2,3',4,4',5-pentabromobiphenyl and, because of metabolism, to a
lesser extent to 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985a). The enhanced toxicity of the photolysed FireMaster(R)
mixture may be explained similarly by increased concentrations of
2,3',4,4',5-pentabromobiphenyl and of congeners containing no ortho
bromines (Robertson et al., 1983a).
Table 98. Summary of reported biological alterations and toxic effects of polybrominated naphthalenes
Species Sexa PBNb Dosage regimenc Observed effects Reference
Pathological features
Rat M 1,2,3,4,6,7-HBN oral doses of 5 mg/kg body centrilobular hepatic accumulation of lipid Kohli et al.
weight per day for 4 days (1981)
Mouse F synthetic HBN oral doses of 0.5, 1.0, 2.5, decrease in body weight (at 7.5 and 10.0 Miller &
(C57 BL/6N) mixture (mainly 5.0, 7.5, or 10.0 mg/kg mg/kg); increase in relative liver weight Birnbaum
(pregnant) 1,2,3,4,6,7-HBN body weight per day on (at all levels); wasting, listlessness, (1986)
and 2,3,4,5,6,7- gd 6-15; s.t.: gd 18 vaginal bleeding, death (at 5-10 mg/kg)
HBN)
Rat F 2,3,6,7-TBN (not 2 daily ip doses of 0.2 increased liver weight; histological Goldstein
(Fischer) identified in mmol/kg body weight per liver changes et al. (1979)
FireMaster) day; s.t.: 3 days after
last dose
Rat M 3 synthetic single ip dose of 0.3 decrease in body weight gain; enlarged Robertson
(Wistar) PBN mixtures mmol/kg body weight livers; decreased thymuses; histological et al. (1984a)
on day 1; s.t.: day 15 changes in liver and thymus;
Hepatic microsomal enzyme induction
Rat F 2,3,6,7-TBN (not 2 ip doses of 0.2 mmol MC-type induction: approximate Goldstein
(Fischer) identified in per kg body weight per ED50 = 40 µmol/kg (18 mg/kg) et al. (1979)
FireMaster(R)) day; s.t.: 3 days (approximately 10-fold more potent than
after last dose FM FF-1)
Table 98 (contd).
Species Sexa PBNb Dosage regimenc Observed effects Reference
Rat M 3 synthetic ip doses of 15 or 150 MC-type induction Robertson
(Wistar) PBN mixtures µmol/kg body weight per (ED100 = at most 30 µmol/kg) et al. (1984a)
(5-6 bromines day on days 1 and 3;
per naphthalene) s.t.: day 6
Fetal toxicity
Mouse - synthetic HBN maternal oral doses of dose-related increases in fetal mortality Miller &
(C57 BL/6N) mixture 0.5-10 mg/kg body weight (at 5-10 mg/kg); dose-related increase in Birnbaum
per day on gd 6-15; incidence of various teratogenic effects (1986); Miller
s.t.: gd 18 (all dose levels): et al. (1985)
- kidney lesions (100% of fetuses at
1 mg/kg)d
- reduction in size of thymus and spleen
- cleft palate (4.8% of fetuses at 1 mg/kg;
98.6% of fetuses at 2.5 mg/kg)
- subcutaneous edema
- sternebral anomalies
- delayed cranial ossification
a F = female; M = male.
b PBN = polybrominated naphthalene(s); HBN = hexabrominated naphthalene(s); TBN = tetrabrominated naphthalene(s).
c gd = gestation days; s.t. = sacrifice time; ip = intraperitoneal.
d Estimated NOEL (no-observed-effect level) = 0.1-0.25 mg/kg per day.
8.12.3.2 Pyrolysis products
Recently, the toxicity of the pyrolyzed FireMaster(R) mixture
has been determined in vitro by measurements of EC50 values for
the induction of aryl hydrocarbon hydroxylase (AHH) and
ethoxyresorufin O-deethylase (EROD) in rat hepatoma H-4-II E
cells, and, in vivo by measurements of ED50 values for hepatic
microsomal AHH and EROD induction, body weight loss, and thymic
atrophy in immature male Wistar rats (Zacharewski et al., 1988).
FireMaster(R) BP-6 was pyrolyzed at 800 °C, and the residue was
extracted with toluene. Solvents used for the application of the
test material to the cell cultures and for ip injection of the
animals were DMSO and corn oil, respectively. Both the in vitro
and in vivo dose-response effects were compared with the relative
activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and expressed
as the concentrations of "2,3,7,8-TCDD equivalents".
The calculated in vitro and in vivo "2,3,7,8-TCDD equivalents"
(µg/g sample) for the six bioassays ranged between 480 and 1680 µ/g
(Table 100).
Several polybrominated dibenzofurans (PBDFs) have been
identified in the highly complex combustion mixture of
FireMaster(R) (see section 4.3.2). However, toxicity tests have
been conducted for only one PBDF congener, namely 2,3,7,8-dibenzo
furan (Moore et al., 1979b).
Generally, PBDFs may have a higher toxicity than the chloro
analogues (Poland & Knutson, 1982).
The toxicity of pyrolyzed technical octabromobiphenyl (Dow, Lot
102-7-72) has been studied in a less sophisticated manner than that
of FireMaster(R). Rats were exposed, via inhalation, to OcBB,
which had been heated at 290 °C (4 h/day, for up to 10 days) and
examined for liver damage. Atmospheric concentrations of
> 2.5 µg/litre (as Br) of brominated 290 °C pyrolysis products caused
liver enlargement; microscopic abnormalities in the liver were not
detected (Aftosmis et al., 1972b; Waritz et al., 1977).
8.13 Mechanism of toxicity including carcinogenicity
PBB and related halogenated compounds are known to elicit a
large number of different effects in animal species. However, the
underlying mechanisms of toxicity are unknown. It is likely that
several molecular mechanisms may operate (e.g., Silberhorn et al.,
1990).
Table 99. Summary of comparative toxicology data of photolysed PBBs
PBB Irradiation Parametera Effects of References
(solvent during (species) PBB irradiated PBB
irradiation)
FM BP-6 300 nm thymus weight dose-related decreases dose-related decreases; Robertson
(lot 7062) (cyclohexane) (rat, immature) significant at 75 mg/kg decreases significant at et al.
body weight 25 mg/kg body weight (1981b,c)
AHH-induction ED50 = 50 mg/kg ED50 = 9 mg/kg
(rat, immature) body weight body weight
displacement of EC50 = 300 µmol EC50 = 2 µmol Robertson
[3H] TCDD from the et al. (1981b)
Ah receptor
(rat, immature)
2,2',4,4',5,5'- sunlight hyperkeratosis no hyperkeratosis severe hyperkeratosis Patterson
hexabromobiphenyl (hexane) (rabbit ear) et al. (1981)
(BB 153)
254 nm hepatic microsomal PB-type mixed type Millis et al.
(hexane) enzyme induction (PB and MC) (1985a)
(rat, outbred)
liver weight increase increase (more than
(rat, outbred) with BB 153)
Table 99. (cont'd).
PBB Irradiation Parametera Effects of References
(solvent during (species) PBB irradiated PBB
irradiation)
liver histology hepatocyte moderate to severe
(rat, outbred) enlargement hepatocyte enlargement
a AHH = benzo[a]pyrene hydroxylase; TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
b MC = 3-methylcholanthrene; PB = phenobarbital.
Table 100. FireMaster(R) BP-6 pyrolysate: in vitro and in vivo determination
of "2,3,7,8-TCDD equivalents"a
Bioassayb FireMaster(R) BP-6 pyrolysatec:
Sample "2,3,7,8-TCDD equivalents"d
(µg/g)
AHH induction (in vitro) 1400
EROD induction (in vitro) 480
AHH induction (in vivo) 540
EROD induction (in vivo) 520
Body weight loss (in vivo) 760
Thymic atrophy (in vivo) 1680
a From: Zacharewski et al. (1988).
b AHH = aryl hydrocarbon hydroxylase; EROD = ethoxyresorufin O-deethylase.
c Pyrolysis temperature: 800 °C.
d 2,3,7,8-TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
As has been discussed in a number of comparative studies and
reviews, there is good evidence relating the effects of PBB
congeners that are MC inducers to a receptor-mediated model of
toxicity (Poland & Glover, 1977, 1980; Poland et al., 1979;
Goldstein, 1980; Moore et al., 1980; McKinney & Singh, 1981;
Parkinson & Safe, 1981; Bandiera et al., 1982, 1983; McKinney &
McConnell, 1982; Nebert et al., 1982; Poland & Knutson, 1982;
Robertson et al., 1982b, 1984c,d; Safe et al., 1982, 1985; Aust
et al., 1983; Dannan et al., 1983; Lai, 1984; Safe, 1984). The
particular PBB-induced toxic syndrome of wasting, and other effects
that are common to related halogenated compounds isosteromeric to
TCDD, are related to an interaction with the cytosolic Ah - or TCDD
- receptor protein. The Ah receptor is believed to be a member of
the steroid/retinoid/thyroid hormone nuclear receptor superfamily,
however, no endogenous ligands have been detected so far for the Ah
receptor (Nebert et al., 1990; Poellinger et al., 1992). Activation
of the Ah receptor, translocation into the nucleus, and binding to
responsive elements on DNA are complex processes involving heat
shock protein 90 and several other unknown, or less well described,
steps (Poellinger et al., 1992). This activation of the Ah receptor
leads by less well understood mechanisms to altered expression of a
number of different genes (among these at least 6 drug- metabolizing
enzymes) resulting in a pleiotypic response (Nebert et al., 1990).
Furthermore, TCDD, apparently via activation of the Ah receptor, has
an antiestrogenic effect by down-regulation of the nuclear estrogen
receptor and affects the epidermal growth factor (EGF)
receptor-binding (DeVito et al., 1991; Safe et al., 1991). The most
active TCDD-like PBBs are those lacking ortho bromine substitution,
being coplanar and approximate isostereomers to TCDD. It should be
noted that the interaction between ligands and the Ah receptor, as
well as the action on different genes and species differences, are
only partly understood.
Under certain conditions, TCDD can elicit programmed cell death
(apoptosis) in freshly isolated thymocytes from young rats (McConkey
et al., 1988). This has been suggested as an important mechanism for
the effects of TCDD on cells in the thymus. It is not known whether
this effect is mediated via the receptor or whether it can occur
with coplanar PBBs. For tissues in general, apoptosis is believed to
be important for differentiation, normal cell turnover,
hormone-dependent atrophy, and tumour promotion (Nebert et al.,
1990).
PBBs also have a number of other effects at the molecular and
biochemical levels, e.g., increased cell proliferation in the B633F1
mouse liver (Mirsalis et al., 1985, Table 88; Loury et al., 1987;
Mirsalis et al., 1989; Mirsalis & Steinmetz, 1990), and effects on
membranes (Bernert & Groce, 1984) or membrane-mediated processes in
rats (Shukla & Albro, 1987). PBBs substituted at the ortho
positions cause PB-type microsomal induction. The mechanism of this
type of enzyme induction is unknown. This group of PBBs also causes
inhibition of intercellular communication (Trosko et al., 1981;
Tsushimoto et al., 1982; Williams et al., 1984; Kavanagh et al.,
1987; Rezabek et al., 1988), which has been suggested to be
important for the promotion phase in the carcinogenic process.
PBB microsomal enzyme induction, as well as the induction of
other drug metabolizing enzymes, may lead to a number of secondary
events with enhanced metabolic conversions of both xenobiotica and
endogenous compounds, such as steroid hormones. Furthermore, a high
level of P450 enzymes could lead to the generation of oxygen
radicals. A relationship between the induction of cytochrome P-450
enzymes (see section 8.8.1) and liver tumour promoting activity has
been noted for a number of chemicals including PBBs (e.g., Lubet
et al., 1989; Beebe et al., 1991; Buchmann et al., 1991).
Some of the toxic effects of PBBs could also be mediated via
changes in the metabolism of vitamin A (retinol compounds and
retinoic acid), which is important for cellular growth and
differentiation. However, the mechanism of the effects of PBBs on
vitamin A metabolism is still unknown.
9. EFFECTS ON HUMANS
The human health effects of PBBs have been reviewed by Safe
(1984), Reggiani & Bruppacher (1985), Fries (1985b), IARC (1986),
Kimbrough (1987), Anderson (1989), Silberhorn et al. (1990), and
Waldron (1990).
9.1 General population exposure
9.1.1 Acute toxicity-poisoning incidents
Most information on the effects of PBBs on humans was obtained
as the result of a poisoning incident in Michigan, USA, 1973, when
several hundred kg PBBs were introduced into cattle feed through a
labelling accident. Widespread human exposure in this area resulted
from direct contact with contaminated feed and from the consumption
of PBBs in meat, eggs, and dairy products. During an interval of
more than 9 months between the accident, the identification of its
cause, the beginning of statewide testing and the establishment of
quarantines, commercially marketed products entered the Michigan
food chain.
For exposure data see sections 5.2 and 5.3.
There was no instance of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures (Fries,
1985b).
9.1.2 Epidemiological studies
In the epidemiological studies reviewed below, efforts have
been made to evaluate the relationship between PBB exposure and a
large number of adverse effects, including behavioural effects and
subjective complaints. However, most studies suffer from major
failures in design introducing confounders that make it difficult,
or impossible, to draw conclusions regarding the relationship
between PBB exposure and possible health effects.
A number of studies had no comparison groups. In other reports,
small groups of patients were selected because of existing illness
and not because their PBB body burdens were particularly elevated.
Some of the outcomes measured, such as urinary porphyrin levels,
liver function tests, and immunological tests, did not show any
clinically relevant changes or were not positively correlated with
PBB body burdens. The clinical relevance of some of the tests is
also not known, at present, because no reference values exist. Most
of these reports dealt with cross sectional studies of heterogeneous
groups of people. No detailed reports exist on long-term, follow-up
studies.
9.1.2.1 Studies conducted by the Michigan Department of Public
Health (MDPH studies)
The health status of 165 persons living on PBB-quarantined
farms was compared with that of 133 persons living on unexposed
farms in the same area. Although a variety of symptoms were reported
by both groups, no consistent pattern of differences between the
groups was observed. Physical examinations did not show any
abnormalities of the heart, liver, spleen, or nervous system that
could be related to PBB exposure. There were no differences between
groups in urine analysis and blood counts (Humphrey & Hayner, 1975).
A cohort study of Michigan residents (4545 persons), exposed to
PBBs, was conducted to examine, among other things, whether there
was an increased incidence of acute or subacute illness in relation
to PBB exposure, 4 years after the accident. Six groups with various
levels of potential exposure were included in the cohort. The groups
were quarantined farm residents, farm product recipients, chemical
workers and their families, pilot study control participants,
self-referred individuals who resided on farms that were
contaminated with low amounts of PBBs, and self-referred individuals
who had no direct connection with contaminated farm premises. Mean
and median serum concen trations of PBBs were much higher in the
first three groups than in the latter three. The prevalence of
selected symptoms by group was examined. Symptoms generally were
most prevalent in the two self-selected groups and were least
prevalent in the group composed of chemical workers and their
families. An evaluation of dose-response relationships was
undertaken by dividing the cohort into seven segments on the basis
of serum PBB levels. No positive associations were found between
serum concentrations of PBB and reported symptom frequencies.
Symptom-prevalence rates (excluding volunteers) were slightly higher
in persons with no detectable PBBs in serum than in those with
measurable quantities. Relationships between symptom-prevalence
rates and serum PBB levels were also examined within each enrolment
group, and no positive trends were found; in all groups, including
chemical workers and quarantined farm residents, the highest
prevalence rates occurred in persons with the lowest serum PBB
levels (Landrigan et al., 1979).
9.1.2.2 Studies conducted by the Environmental Science Laboratory,
Mount Sinai School of Medicine, New York (ESL studies)
Anderson et al. (1978b, 1979) reported on a study of PBB-
exposed farmers and residents in Michigan and a control group of
unexposed Wisconsin dairy farmers, who were examined in November
1976 and March 1977, respectively. The results were given on the
basis of the examination of 933 exposed persons and 229 controls in
the 1978 report, and of 993 exposed subjects and 228 controls in the
1979 report.
The study included four groups: families chosen randomly from
Michigan farms, consumers of produce bought directly from
participating farms, self-selected Michigan families, and Wisconsin
dairy farmers not exposed to PBBs. All subjects completed
comprehensive questionnaires on medical histories and 43 symptoms,
and they were subjected to physical examination and certain
laboratory tests. Statistical analysis of the prevalence of symptoms
at the time of examination or during the preceding year in the
Michigan and Wisconsin populations studied, found the Michigan group
to have a significantly higher prevalence of skin, neurological, and
musculoskeletal symptoms. The increase was seen among the younger
age groups of 16-35 years and 36-55 years. Michigan females had a
higher prevalence of neurological symptoms than Michigan males
(Anderson et al., 1978b).
No statistically significant, positive correlations were found
between serum PBB values and any individual current symptom. Liver
function tests showed that serum glutamic pyruvic transaminase
(SGPT), serum glutamic oxaloacetic transaminase (SGOT), and lactate
dehydrogenase (LDH) elevations were significantly more prevalent in
the Michigan group (chi square test). No differences were seen for
alkaline phosphatase. Within the Michigan group, males had a higher
prevalence of SGPT and LDH increases than females, and comparing
males in both groups, Michigan males had a significantly higher
prevalence of SGPT and SGOT increases than Wisconsin males (Anderson
et al., 1979).
The comparison of findings among residents on Michigan dairy
farms (quarantined and non-quarantined farms) and corresponding
consumers of produce purchased from these farms (cross-sectional
clinical survey of 1029 persons) gave the following results:
prevalence of symptoms (dermatological, neurological,
musculoskeletal, and gastrointestinal) in consumers of farm products
from quarantined farms was similar to that found in farmers on
quarantined farms; the prevalence was lower in consumers of products
from non-quarantined farms. The prevalence of liver function
abnormalities (increase in alkaline phosphatase, SGOT, SGPT, and
LDH) was similar in dairy farmers and consumers. The distribution,
mean and median values of serum PBB levels in consumers were found
to be similar to those in dairy farmers (Lilis et al., 1978).
Although some results of the ESL studies were at times
interpreted differently from the results of the MDPH studies, there
was one area of consistent agreement. Neither set of studies
demonstrated a positive dose-response relationship between PBB
concentrations in serum or adipose tissue and the prevalence of
symptoms or abnormal clinical measurements (Fries, 1985b).
9.1.3 Special studies
9.1.3.1 Examination of subjects with complaints
In the original cohort (Landrigan et al., 1979) a subset of
individuals (19 males and 4 females), predominantly from the
quarantined farms, was identified who had a number of disabling
health complaints.
This group was systematically evaluated at a hospital together
with a group of 28 PBB workers selected randomly from a pool of 100
people with previous occupational PBB exposure. The physical
examination of this group of exposed farmers demonstrated a
relatively high prevalence of hepatomegaly. Eight patients (35%)
showed evidence of liver enlargement, defined as greater than 11 cm
of vertical height in the midclavicular line. Liver scanning
confirmed the presence of hepatomegaly in four of these individuals
(17%), and two of them had a history of substantial alcohol intake.
Ten individuals (43%) had skin lesions, but these were common
dermatological problems, such as superficial mycoses and actinic
keratosis, which are not uncommon in the general population and in
people working outdoors. Biochemical and haematological testing
revealed few abnormalities, and electro myograms, nerve conduction
velocities, endocrine studies, and lymphocyte transformation studies
did not provide any objective findings that correlated with
subjective complaints. Psychiatric evaluation revealed a high
prevalence of depression (78%) among the farmers. Multiple tests of
intelligence, memory, and functional ability did not demonstrate
abnormalities. There was no relation ship between fat PBB levels and
physical or laboratory findings (Stross et al., 1981). The present
study did not use epidemiological methods to evaluate the
relationship between health effects and exposure to PBB and should,
thus, be regarded as a report on cases in a group with known, but
unquantified, PBB exposure.
9.1.3.2 Cutaneous effects
Three years after the Michigan PBB incident, the cutaneous
effects were examined in a multidisciplinary study of the farming
population (498 persons). Results were divided into two categories:
subjective findings or symptoms of which subjects complained, and
objective findings or signs, which were detected on physical
examination. The results of the subjective findings were: 32% of
adult residents and consumers associated with quarantined and
non-quarantined farms complained of the development of unexplained
cutaneous itching in a 3-year period from the PBB contamination
episode to the time of the examination, compared with 22% reported
by the control group. There was also a higher proportion of dryness
reported; 32% of the quarantined and non-quarantined farm adults
compared with 24% of the control group. Similarly, 17% of the farm
study groups complained of the development of unexplained peeling
and scaling during the same period compared with an incidence of 9%
in the control group. The prevalence of erythema in the combined
farm study groups was 12% compared with a prevalence of 5% in the
controls. Increased unexplained nail growth was reported by 8% of
the quarantined and non-quarantined adults compared with none in the
controls. Increased abnormal sweating was a complaint of 22% of the
quarantined and non-quarantined group compared with 13% in the
controls. Unexplained hair loss was a complaint of 12% of the
quarantined and non-quarantined group compared with 5% in the
control group. All exposed groups had a significantly increased
prevalence of skin symptoms compared with the comparison group. Of
the symptoms elicited in a physician interview, rash was the most
frequently reported. Among the farmers, rash was reported by 14%
compared with 9% in the control group.
Objective findings, determined by examination, were diffuse
unexplained alopecia in 4% of the combined quarantined and
non-quarantined farm groups, compared with none in controls (P <
0.005) (Chanda et al., 1982).
Because of major flaws in the study design and biases
introduced during the selection processes in the cohort and a poor
response rate, no causal association with PBBs could be deduced from
this study.
9.1.3.3 Effects on liver function
The serum activities of SGOT, SGPT, LDH, and alkaline
phosphatase were measured in 614 Michigan adults exposed to PBB and
141 unexposed Wisconsin adults. The Michigan groups had a higher
prevalence of elevated SGOT (P < 0.005) and SGPT (P < 0.005). A
clear sex difference was observed. Michigan men had a higher
prevalence of elevated SGPT (P < 0.005) and LDH (P < 0.005) than
Michigan women, and a higher prevalence of elevated SGOT than
Wisconsin men (P < 0.005) and SGPT (P < 0.01). On the basis of
serum PBB analyses, no obvious relationship was observed between PBB
values and liver function tests (Anderson et al., 1978d).
9.1.3.4 Porphyria
In 1977, urine samples were collected from members of farm
families in Michigan, who had ingested PBB-contaminated meat and
dairy products, beginning in 1973, and from members of farm families
in Wisconsin with no known exposure to PBB (control group).
The total porphyrin excretion of both groups was below
200 µg/litre. Therefore, at this level, this parameter cannot be
used as an indication of exposure of humans to PBB.
Out of a group of 142 persons, at least 47% were found with
secondary coproporphyrinuria or with chronic hepatic porphyria Type
A, demonstrating an abnormal porphyrin pattern. The incidence of
this indicator of liver malfunction was higher in the PBB-exposed
group than in the controls (6%). The study was limited to an
assessment of liver damage as manifested by porphyrin excretion
(Strik et al., 1979).
9.1.3.5 Effects on spermatogenesis
Analysis of semen from 52 PBB-exposed men compared with
analysis of semen from a control group of 52 men not exposed to PBB
revealed no differences in the distribution of sperm counts,
motility, or morphology (Rosenman et al., 1979).
9.1.3.6 Paediatric aspects
In 1976, the paediatric aspects of PBB exposure were studied in
Michigan children, using Wisconsin children as a control group
(Barr, 1978, 1980).
Examination of the data from 292 Michigan farm children showed
that the prevalence of symptoms was related to the quarantine status
of the farm and to the method of invitation into the study. Serum
PBB levels were related to the quarantine status of the farm, but
not to the method of invitation into the study. No significant
effects of age or sex were found on the prevalence of symptoms or
serum PBB levels, except that the teenage (13-16 years of age) males
had somewhat higher PBB levels. Despite the frequent reporting of
symptoms of ill health, physical examination failed to reveal any
objective alterations that could be attributed to PBBs. The most
striking finding was a statistically significant negative
correlation between the prevalence of symptoms and the serum PBB
levels.
The effects of PBBs on 33 children born between September 1,
1973, and December 31, 1975, were evaluated in September, 1977.
These children, born to families who lived on quarantined farms,
were compared with 20 children who had not been exposed to PBBs. The
birthdate interval was selected to obtain children who were exposed
in utero or in early infancy, or both, the two time periods when
damage to developing tissues and organ systems should have been
maximal. The results of these studies failed to identify any effects
on physical growth, physical examination, or neurological
assessment, though the parents indicated by historical review that
the exposed children had more illnesses, especially respiratory,
than the control children. There were some indications of an inverse
relationship between fat PBB levels and performance on selected
developmental tests (Weil et al., 1981).
Seagull (1983) studied 19 of these children between the ages of
2 years 5 months and 3 years 11 months using five tests of the
McCarthy Scales of Children's Abilities and concluded that four of
the five tests had significant (P < 0.05) correlations with PBB
exposure, i.e., the higher the PBB levels in the adipose tissue, the
lower the child's developmental abilities.
Schwartz & Rae (1983) later studied these same children between
the ages of 4 years 1 month and 6 years 1 month (N = 18 because one
family refused to participate in the follow-up study) with the
entire battery of McCarthy Scales of Children's Abilities, plus the
Wechsler Preschool and Primary Scale of Intelligence, and concluded
that no significant (P > 0,05) differences existed.
These different conclusions from studies on the same children
were summarized by Nebert et al. (1983) and commented on from
statistical, clinical paediatric, and toxicological points of view.
The authors stated that different approaches to the analysis of
the data were used and that, in one case, the ability tests of only
five children were selected, because of time limitations in the
study situation.
Comparison of fetal death rates among residents of Michigan's
Lower Peninsula counties with a high percentage of quarantined farms
and among residents of Upper Peninsula counties with no quarantined
farms revealed no important differences in rates or trends after the
contamination. Since counts of early spontaneous abortions were
lacking, a complete assessment of the possible impact on
reproductive outcome could not be made (Humble & Speizer, 1984).
9.1.3.7 Neurological and neuropsychiatric aspects
Neurological symptoms were the earliest and most prominent
symptoms recorded in Michigan farm residents exposed to PBBs
compared with an unexposed control farm population in Wisconsin. The
prevalence and incidence of neurological symptoms were analysed in
over 620 adults from Michigan and 153 from Wisconsin. Subsamples of
both groups were examined in objective performance tests used for
the assessment of neuropsychological dysfunction. In Michigan
(particularly among males), those who exhibited the most marked
symptoms tended to show diminished performance. Low indices of
performance were also significantly correlated with intake of
home-produced foodstuffs, particularly during the years 1972-74 and
store-bought products during the years 1975-76. Between 1972 and
1976, the Michigan farm residents studied made significant changes
in their consumption patterns of products suspected to be
contaminated with PBBs compared with those of Wisconsin farm
residents. Serum PBB levels were not found to be significantly
higher in Michigan males and females exhibiting the most prominent
neurological symptoms. Serum PBB levels were negatively correlated
with performance test scores, particularly in males in older age
groups (Valciukas et al., 1978, 1979).
Twenty-one persons exposed to PBBs were compared with hospital
volunteers on a battery of tests measuring memory, motor strength
and coordination, cortical-sensory perception, personality, and
higher cognitive functioning. Patients exposed to PBBs were selected
for the study only if they had persistent medical complaints. The
adipose PBB levels were not correlated with performance on any test
in the battery. The two groups did differ on the Minnesota
Multiphasic Personality Inventory, suggesting an adjustment reaction
with depressive symptoms and somatizing defences. Persons exposed to
PBBs were also impaired compared with control subjects in tests of
prose recall, short-term memory, concentration, and cognitive
flexibility. However, these differences vanished when group
differences on education and personality were statistically held
constant. The selective admission criteria for the study limited the
possibility of generalizing these findings (Brown & Nixon, 1979).
Forty-six persons (37 men and 9 women) with known exposure to
PBBs were examined in a study designed to evaluate neurobehavioural
complaints (Stross et al., 1979). These people complained of a
serious deterioration in their health status and were unable to
engage in their previous occupations. Comprehen sive medical
investigations were carried out including neurological studies and
psychological evaluation. Electromyograms were abnormal in six
patients (13%) with no consistent or diagnostic findings. Nerve
conduction studies were abnormal in 19 patients (41%), with slowing
in sensory nerve latencies the predominant finding. The abnormal
values averaged 4.7 milliseconds compared with the normal value of
< 3.9 milliseconds. There was an excellent correlation between
patients with objective findings on neurological examination and
abnormal nerve conduction studies (r value not stated). There was no
relationship between the presence of these abnormalities and serum
or fat PBB levels.
Despite the fact that an extensive battery of tests was
administered in the psychological evaluation, few objective
abnormalities were documented. The most common findings were those
of somatic preoccupation, irritability, and mild depression. The
tests of motor function were normal, while the tests of sensory
modalities showed minor differences that were not outside normal
limits. Most had IQs of between 100 and 140 with no differences
between estimated and observed levels. Although most patients
complained of memory difficulties, no objective deterioration in
memory could be elicited.
The results of the psychiatric interviews showed that 31
patients (67%) were depressed. No evidence of endogenous depression
was noted, and it was the opinion of the psychiatrists involved that
the findings were characteristic of reactive depression.
9.1.3.8 Lymphocyte and immune function
The immunotoxicology of PBBs has been reviewed by Amos (1986)
and Steele et al. (1989).
Bekesi et al. (1983b) summarized the findings of lymphocyte and
immunological function studies, conducted in 1976 on 45 adult
Michigan dairy farm residents who had consumed PBB-contaminated food
products for periods ranging from three month to three years. Test
comparisons were made with a group of 46 dairy farm residents in
central Wisconsin who had not been exposed to PBB-contaminated food
and to a group of 76 healthy subjects from the New York Metropolitan
area (Bekesi et al., 1978, 1979a,b).
Marked changes in various immunological parameters were noted
among the Michigan dairy farm residents compared with both the
Wisconsin and New York control populations. The peripheral blood
lymphocytes of only 27 of the 45 Michigan subjects exhibited a
normal response to the T-cell mitogens phytohaemagglutinin and
concanavalin A, to the alloantigens in the mixed leukocyte culture
reaction, and to the B-cell mitogen (pokeweed mitogen). In the
remaining 18 subjects, the lymphocytes showed an impaired functional
response to all mitogens and alloantigens.
The lymphocytes of all 45 PBB-exposed study subjects showed a
reduced proliferative T-cell response in mixed leukocyte cultures.
The group of 18 individuals with decreased T-cell function had
values measuring one-third to one-quarter of those obtained from the
normal controls. The number of viable cells in the various
subpopulations of peripheral blood lymphocytes were measured
according to their ability to form stable rosettes with sheep
erythrocytes in the case of the T-cells, while the B-lymphocytes
were quantified by either direct immunofluorescence or by sheep
erythrocytes sensitized with antibody and complement. The 27
Michigan farm residents with normal lymphocyte functions also
exhibited the normal distribution of T- and B-lymphocytes. Eighteen
of the 45 subjects with lymphocyte dysfunction showed significantly
reduced populations of T-cells. Despite the marked changes in the
characteristic cell surface markers detected in the peripheral blood
lymphocytes of the PBB-exposed Michigan farm residents, the marker
for monocytes, determined by peroxidase staining or latex digestion,
did not differ from that of either control group. Thus, the most
significant deviation from the control samples was a marked increase
in lymphocytes without detectable surface markers.
Five years later, Bekesi et al. (1983a,b) examined the same
individuals and the data strongly suggested a persistent PBB-induced
immune suppression. The findings were characterized by a decrease in
the percentage and absolute number of T-lymphocytes, with a
concomitant increase in the occurrence of lymphocytes without
detectable membrane surface markers, and, in as many as 30% of the
subjects retested, a reduction in the T-cell function.
Silva et al. (1979) assessed T- and B-lymphocyte numbers and
lymphocyte transformation to 3 mitogens (phytohaemagglutinin,
concanavalin A, and pokeweed mitogen) in 41 persons with a high
exposure to PBBs (mean serum level 787 µg/litre, range
529-2560 µg/litre) and 57 persons with a low exposure (mean serum
level 3 µg/litre, range 1-11 µg/litre). In contrast to the findings
of Bekesi et al. (1978) there were no significant differences in the
percentages of T- and B-lymphocytes among persons who experienced
high or low PBB exposure or in control groups. Similarly, no
significant depression of lymphocyte mitogenic responsiveness were
found in those who experienced high or low PBB exposure compared
with controls. No correlation was found between serum PBB levels and
lymphocyte numbers or function.
In a comprehensive immunotoxicological study, 336 adult
Michigan farm residents, 117 general consumers (for comparison), and
75 dairy farm residents in Wisconsin, who had not eaten
PBB-contaminated food, were examined, as were 79 healthy subjects in
New York City. Abnormalities in the Michigan groups included:
hypergammaglobulinaemia, exaggerated hypersensitive response to
streptococci, significant decreases in absolute numbers and
percentages of T- and B-lymphocytes, and increased numbers of
lymphocytes with no detectable surface markers ("null cells").
Significant reduction of in vitro immune function was noted in
20-25% of the Michigan farm residents who had eaten food containing
PBBs. The decreased immune function detected among the PBB-exposed
farm residents tended to affect families as a unit and was
independent of the age or sex of exposed individuals,
contraindicating the possibility of genetic predisposition (Bekesi
et al., 1987).
Lipson (1987) evaluated the effects of PBBs on the function and
on the synthesis of immunoglobulins by peripheral blood lymphocytes.
Concentrations of PBBs as low as 0.001 µg/105 cells decreased
lymphocyte response to pokeweed mitogen: higher concentrations of
PBBs stimulated the in vitro synthesis and release of
immunoglobulins. PBBs had no effect on the quantity of
E-rosette-forming cells, the total T- or B-cells, or the ratio of
helper to suppressor T-cell subpopulations. Enhanced release of IgG
was identified in lymphocyte cultures obtained from blood specimens
of PBB-exposed Michigan farmers. The data from this study suggest
that PBBs had exerted an adverse effect on cell function, but had
produced a non-specific activation of B lymphocytes.
9.1.3.9 Carcinogenic embryonic antigen plasma levels
Carcinogenic embryonic antigen (CEA) titres were determined for
611 Michigan farmers exposed to PBBs and for a control unexposed
population of 138 Wisconsin farmers. The overall prevalence of
elevated CEA titres was slightly higher in the Michigan study group,
but the difference was not statistically significant. Serum PBB
concentrations appeared to be positively correlated with CEA titres.
The authors discussed the possibility that the effect of PBBs may be
additive to that of other factors that are known to result in an
increased prevalence of elevated CEA titres (Anderson et al.,
1978c).
The possibility of long-term effects of PBBs, such as cancer,
cannot be ruled out. The induction of liver tumours in rodents is a
matter of concern (Fries, 1985b).
9.1.3.10 Biochemical effects
Two hundred and sixty-two residents with a geometric mean serum
PBB level of 19.9 µg/litre submitted at least one blood sample for
clinical chemistry tests of 9 parameters during 4 different years.
No consistent significant correlation with serum PBB levels was
shown for any parameter (Kreiss et al., 1982). The authors suggested
that tests with greater sensitivity and specificity for hepatic
microsomal enzyme induction should be developed for future
evaluations.
Lambert et al. (1987) were the first to study the effects of
PBB exposure on the human cytochrome P-450 system, as determined by
the caffeine breath test (CBT), in healthy non-smoking adults from
rural Michigan with, and without, detectable serum PBB levels
(concentration not stated) and in prepubescent children with known
perinatal exposure to PBBs. The results were compared with the CBT
results obtained from unexposed urban adult non-smokers and
age-matched children. The unexposed and PBB-exposed children had
similar CBT data. The adult groups were not significantly different
from each other, except for the rural adults with detectable PBB
levels who had significantly higher CBT values than the unexposed
urban adults.
Lambert et al. (1990) conducted a field biochemical
epidemiology study using the Michigan cohort consisting of 51 rural
residents exposed to PBBs. The CBT and CMR (caffeine urinary
metabolite ratio) were elevated in the subjects exposed to PBBs
compared with the values obtained from urban non-smokers and were
similar to those found in adults who smoked. A gender effect was
seen in the PBB-exposed subjects, the median CBT and CMR values of
the females being lower than the values of the males. There was a
correlation between the CBT and the serum HxBB values (r2 = 0.2,
p = 0.01) but not between CMR and serum HxBB values.
PBBs induce hepatic cytochrome P-450IA2 enzyme activity in the
human adult, but not in the child. Lambert et al. (1991) there fore
investigated, in a prospective longitudinal study of 14 male and 15
female children exposed transplacentally and transmammil lary to
PBBs in 1973-75, whether PBB exposure altered the normal decrease in
P-450IA2 activity that occurs during puberty. P-450IA2 activity was
monitored via the CBT every 2 years, beginning in 1985, and compared
with the P-450IA2 activity in gender- and Tanner stage-matched
children not exposed to PBBs. Unlike the adult, PBBs did not alter
P-450IA2 activity in the child or in the mid-pubescent adolescent
(Wilcoxon rank, P > 0.05).
9.2 Occupational exposure
9.2.1 Epidemiological studies
Anderson et al. (1978a) investigated the health status of 55
employees (52 men and 3 women) of the Michigan Chemical Corporation,
which manufactured PBBs from 1970 to 1974, in addition to a variety
of other halogenated fire retardant chemicals. Ten of those examined
had formerly worked directly in the PBB production area, the other
45 persons worked in other departments in the plant. For these 55
workers, the route and quality of occupational exposure were
probably different from those of farmers, since they could have been
directly exposed to PBBs. The results were compared with those from
a group of male farm residents and consumers from Michigan.
The prevalence of chest and skin symptoms among chemical
workers as a group was significantly greater than among farmers.
Significantly fewer symptoms were reported in the musculoskeletal
category. The PBB department workers experienced symptoms in the
skin category significantly more frequently than non-PBB workers.
Blood chemistry results were similar for workers and farmers.
However, both groups exhibited a significantly higher prevalence of
elevated liver function tests (SGOT, SGPT) than a control population
of unexposed farmers. Considering only workers with more than five
years in the plant, a significantly higher prevalence of elevated
CEA titres was present compared with the farmers.
9.2.2 Clinical studies
The only abnormality noted during physical examination in a
group of PBB-exposed chemical workers consisting of 24 males and 4
females whose ages ranged from 23 to 62 years with a mean of 40
years, was the presence of hepatomegaly in two patients (7%), both
of whom heavily indulged in alcoholic beverages, and abnormal skin
examination in four patients (14%). In numerous biochemical tests,
only minor elevations of serum uric acid, serum iron, and serum
cholesterol were found in 20% of the chemical workers.
Elevation of triglyceride levels was noted in 50% of the
chemical workers, with a mean of 185 mg % (SD ± 50 mg %), with an
upper limit of normal of 150 mg %. No abnormalities in lymphocyte
number or function could be determined, and there was no
relationship between PBB levels and physical or laboratory
abnormalities (Stross et al., 1981).
9.2.3 Special studies
9.2.3.1 Cutaneous effects
Halogen acne was observed on physical examination in 13%
of 53 Michigan chemical workers exposed to PBBs compared with none
in unexposed controls (P < 0.001) (Chanda et al., 1982).
9.2.3.2 Memory performance
Twenty-five chemical workers, who manufactured PBBs, were given
objective tests of learning and memory. Although this group had high
concentrations of PBBs in adipose tissue, mean scores on all memory
tests were normal. The PBB concentration was not correlated with
memory performance; the most contaminated workers showed no evidence
of memory dysfunction (Brown et al., 1981).
9.2.3.3 Thyroid effects
Thyroid function was investigated in a cohort of 35 male
workers, selected from 86 identified workers exposed for at least 6
weeks, manufacturing decabromobiphenyl, decabromobiphenyl ether, and
bromine. The study revealed four cases of primary hypothyroidism
(11.4%), but none in 89 control subjects. The bromine compounds were
the only common exposure. A significantly higher number of the
exposed workers had detectable serum levels of DeBB
(0.5-1340 ng/ml), but not DeBBO (Bahn et al., 1980a,b).
Bialik (1982) investigated thyroid dysfunction in workers
exposed for at least 240 h to decabromobiphenyl and
decabromobiphenyl oxide over a 4-year period. The average period of
employment was 3.9 years, mean age, 34.7 years. Medical
questionnaires, physical examinations, and laboratory tests were
conducted. For exposure data see section 5.3. Thyroid nodules were
seen in 3 out of 18 workers exposed for 3 years or longer.
No detectable PBBs were found in the serum.
9.2.3.4 Reproductive effects
Bialik (1982) also studied reproductive effects in the same PBB
workers.
A significant correlation was seen between length of employment
and concentrations of follicle stimulating hormone (FSH). An
abnormal FSH value was found in only one worker. A testicular cyst
was found in one exposed worker, and epididymal nodules in two
others. No testicular or epididymal nodules were seen among
controls. No definite statement could be made concerning adverse
effects on the prevalence of testicular and epididymal nodules,
because of their prevalence in the general population.
9.2.3.5 Lymphocyte function
Decreased lymphocyte function occurred in four out of ten
Michigan chemical workers. The decrease was related to higher plasma
levels of PBBs (40-1200 µg/litre) (Bekesi et al., 1979b).
9.2.3.6 Mortality
A historical prospective mortality study was conducted by Wong
et al. (1984) on 3579 white male workers employed between 1935 and
1976 at chemical plants with potential exposures to brominated
compounds. Because of the lack of quantitative data, potential
exposures of workers to PBBs were categorized as "routine" and
"non-routine". None of 91 individuals of the cohort potentially
exposed to PBBs on a routine basis died during the study period.
Among the 237 "non-routinely" exposed, two deaths were
observed, though 6.36 were expected. One was due to cancer of the
large intestine, the other was coded as arteriosclerotic heart
disease.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (1986, 1987)
evaluated the polybrominated biphenyls and concluded that there is
sufficient evidence for the carcinogenicity to experimental animals
of a commercial preparation of PBBs (FireMaster FF-1, various lots),
composed primarily of hexabromobiphenyl with smaller amounts of
penta- and heptabrominated isomers. There is considered to be
inadequate evidence for their carcinogenicity in humans. Commercial
mixtures of PBBs were thus classified into Group 28, possibly
carcinogenic to humans.
The European Community added PBBs to the chemicals banned or
severely restricted to certain uses owing to their effects on human
health and the environment (CEC, 1988).
REFERENCES
Adams EA, Irish DD, Spencer HC, & Rove VK (1941) The response of
rabbit skin to compounds reported to have caused acneform
dermatitis. Ind Med Surg, 2: 1-4.
Aftosmis JG, Culik R, Lee KP, Sherman H, & Waritz EI (1972a)
Toxicology of brominated biphenyls. I. Oral toxicity and
embryotoxicity. Toxicol Appl Pharmacol, 22: 316.
Aftosmis JG, Dashiell OL, Griffith FD, Hornberger CS, McDonnell MM,
Sherman H, Tayfun FO, & Waritz RS (1972b) Toxicology of brominated
biphenyls. II. Skin, eye, and inhalation toxicity and an acute test
method for evaluating hepatotoxicity and accumulation in body fat.
Toxicol Appl Pharmacol, 22: 316-317.
Ahmadizaden M, Kuo C-H, Echt R, & Hook JB (1984) Effect of
polybrominated biphenyls, beta-naphthoflavone and phenobarbital on
arylhydrocarbon hydroxylase activities and chloroform-induced
nephrotoxicity and hepatotoxicity in male C57BL/6J and DBA/2J mice.
Toxicology, 31: 343-352.
Ahotupa M & Aitio A (1978) Effect of polybrominated biphenyls on
drug metabolizing enzymes in different tissues of C57 mice. Toxicol,
11: 309-314.
Akoso BT, Sleight SD, Aust SD, & Nachreiner R (1980) Comparative
study of the toxicopathology of purified congeners of polybrominated
biphenyls in rats. In: Abstracts of papers presented at the
Nineteenth Annual Meeting of the Society of Toxicology, Washington,
9-13 March 1980. Washington, DC, Society of Toxicology (Abstract
A/103).
Akoso BT, Sleight SD, Aust SD, & Stowe HD (1982a) Pathologic effects
of purified polybrominated biphenyl congeners in rats. J Am Coll
Toxicol, 1: 1-21.
Akoso BT, Sleight SD, Nachreiner RF, & Aust SD (1982b) Effects of
purified polybrominated biphenyl congeners on the thyroid and
pituitary glands in rats. J Am Coll Toxicol, 1: 23-36.
Allen JR & Lambrecht L (1978) Responses of rhesus monkeys to PBBs.
Toxicol Appl Pharmacol, 45: 340-341.
Allen JR, Lambrecht LK, & Barsotti DA (1978) Effects of
polybrominated biphenyls in nonhuman primates. J Am Vet Med Assoc,
173: 1485-1489.
Allen JR, Barsotti DA, Lambrecht LK, & Van Miller JP (1979)
Reproductive effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann NY Acad Sci, 320: 419-425.
Allen-Rowlands CF, Castracane VD, Hamilton MG, & Seifter J (1981)
Effect of polybrominated biphenyls (PBB) on the pituitary-thyroid
axis of the rat. Proc Soc Exp Biol Med, 166: 506-514.
Amos HE (1986) Inadvertent immunosuppression as a health hazard. Br
J Clin Pract, 40: 336-340.
Anderson HA (1985) Utilization of adipose tissue biopsy in
characterizing human halogenated hydrocarbon exposure. Environ
Health Perspect, 60: 127-131.
Anderson HA (1989) General population exposure to environmental
concentrations of halogenated biphenyls. In: Kimbrough RD & Jensen
AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products, 2nd ed. Amsterdam, New York,
Oxford, Elsevier/North-Holland Biomedical Press, pp 333-339.
Anderson HA, Wolff MS, Fischbein A, & Selikoff IJ (1978a)
Investigation of the health status of Michigan Chemical Corporation
employees. Environ Health Perspect, 23: 187-191.
Anderson HA, Lilis R, Selikoff IJ, Rosenman KD, Valciukas JA, &
Freedman S (1978b) Unanticipated prevalence of symptoms among dairy
farmers in Michigan and Wisconsin. Environ Health Perspect, 23:
217-226.
Anderson HA, Rosenman KD, & Snyder J (1978c) Carcinoembryonic
antigen (CEA) plasma levels in Michigan and Wisconsin dairy farmers.
Environ Health Perspect, 23: 193-197.
Anderson HA, Holstein EC, Daum SM, Sarkozi L, & Selikoff IJ (1978d)
Liver function tests among Michigan and Wisconsin dairy farmers.
Environ Health Perspect, 23: 333-339.
Anderson HA, Wolff MS, Lilis R, Holstein EC, Valciukas JA, Anderson
KE, Petrocci M, Sarkozi L, & Selikoff IJ (1979) Symptoms and
clinical abnormalities following ingestion of
polybrominated-biphenyl-contaminated food products. Ann NY Acad Sci,
320: 684-702.
Andersson K, Norström A, Rappe C, Rasmuson B, & Swahlin H (1975)
Photochemical degradation of PCB, PBB and other flame retardants.
Environ Qual Saf, 3(Suppl): 798-802.
Andres J, Lambert I, Robertson L, Bandiera S, Sawyer T, Lovering S,
& Safe S (1983) The comparative biologic and toxic potencies of
polychlorinated biphenyls and polybrominated biphenyls. Toxicol Appl
Pharmacol, 70: 204-215.
Anklam E (1989) 1H and 13C NMR studies on mono- and
polybrominated biphenyls. Magn Res Chem, 27: 503-506.
Anon (1977) PBB residue found near three plants in New York, Jersey.
Chem Market Report, 211(26): 4.
Anon (1988) Superfund record of decision: Forest waste disposal, MI
(Second remedial action). Washington, DC, US Environmental
Protection Agency, Office of Emergency and Remedial Response, 141 pp
(EPA/ROD/RO5-88/062).
AOAC (1975) In: Horwitz W, Senzel A, Reynolds H, & Park DL ed.
Official methods of analysis of the Association of Official
Analytical Chemists. Washington, DC, Association of the Official
Analytical Chemists, pp 518-531.
Arneric SP, McCormack KM, Braselton WE Jr, & Hook JB (1980) Altered
metabolism of progesterone by hepatic microsomes from rats following
dietary exposure to polybrominated biphenyls. Toxicol Appl
Pharmacol, 54: 187-196.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity and mutagenicity for 301
chemicals tested by the US NTP (National Toxicology Program). Mutat
Res, 257: 229-306.
Atkinson R, Aschmann SM, & Pitts JS Jr (1984) Kinetics of the
reactions of naphthalene and biphenyl with OH radicals and with O3
at 294 ± 1 K. Environ Sci Technol, 18: 110-113.
ATOCHEM (1984a) Adine 0102(R). Levallois-Perret, France, ATOCHEM,
Centre d'Application de Levallois (Product information No. 126).
ATOCHEM (1984b) Adine 505(R). Levallois-Perret, France, ATOCHEM,
Centre d'Application de Levallois (Product information No. 128).
ATOCHEM (1987) Decabromobiphenyl (Adine 0102): Pyrolisis testing.
Paris La Défense, France, ATOCHEM (Unpublished report).
ATOCHEM (1990) Decabromobiphenyl (Adine 0102). Paris La Défense,
France, ATOCHEM (Unpublished report).
Aulerich RJ & Ringer RK (1979) Toxic effects of dietary
polybrominated biphenyls on mink. Arch Environ Contam Toxicol, 8:
487-498.
Aust SD, Dannan GA, Sleight SD, Fraker PJ, Ringer RK, & Polin D
(1981) Toxicology of polybrominated biphenyls. In: Khan MAQ &
Stanton RH ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects. Oxford, New York, Pergamon Press, pp 73-96.
Aust SD, Trosko JE, & Sleight SD (1983) Toxicity and carcinogenicity
of polybrominated biphenyls. In: Proceedings of the 13th Conference
on Environmental Toxicology, Dayton, Ohio, 16-18 November 1982.
Dayton, Ohio, University of California, pp 270-286
(AFAMRL-TR-82-101).
Babish JG & Stoewsand GS (1977) Polybrominated biphenyls: inducers
of hepatic microsomal enzymes and type A cytochrome P450 in the
rat. J Toxicol Environ Health, 3: 673-682.
Babish JG, Gutenmann WH, & Stoewsand G (1975a) Polybrominated
biphenyls: tissue distribution and effect on hepatic microsomal
enzymes in Japanese quail. J Agric Food Chem, 23: 879-882.
Babish JG, Gutenmann WH, Lisk DJ, & Stoewsand G (1975b) Liver
changes and tissue residues of animals fed hexabromobiphenyl. In:
Abstracts of papers presented at the 59th Annual Meeting of the
Federation of the American Societies for Experimental Biology,
Atlanta City, New Jersey, 13-18 April 1975 (Abstract 157).
Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann
L, & Utiger RD (1980a) Hypothyroidism in workers exposed to
polybrominated biphenyls. New Engl J Med, 302: 31-33.
Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann
L, & Utiger RD (1980b) Health assessment of occupational exposure to
polybrominated biphenyl (PBB) and polybrominated biphenyloxide
(PBBO). Washington, DC, US Environmental Protection Agency
(EPA-560/6-80-001).
Ballschmiter K & Zell M (1980) Analysis of polychlorinated biphenyls
(PCB) by glass capillary gas chromatography. Fresenius Z Anal Chem,
302: 20-31.
Ballschmiter K, Unglert CH, & Neu HJ (1977) [Degradation of
chlorinated aromates: microbiological degradation of polychlorinated
biphenyls (PCB). III: Chlorinated benzoic acid as metabolite of
PCB.] Chemosphere, 1: 51-56 (in German).
Bandiera S, Sawyer TW, Campbell MA, Robertson L, & Safe S (1982)
Halogenated biphenyls as AHH inducers: effects of different halogen
substituents. Life Sci, 31: 517-525.
Bandiera S, Sawyer TW, Campbell MA, Fujita T, & Safe S (1983)
Competitive binding to the cytosolic
2,3,7,8-tetrachlorodibenzo-p-dioxin receptor. Effects of structure
on the affinities of substituted halogenated biphenyls-A QSAR
analysis. Biochem Pharmacol, 32: 3803-3813.
Barlow SM & Sullivan FM (1982) Reproductive hazards of industrial
chemicals. New York, London, San Francisco, Academic Press, pp
437-454.
Barnhart ER, Hill RH, Alexander LR, Orti DL, Groce DF, Patterson DG,
& Head SL (1984) Purification of polybrominated biphenyl congener 2.
Bull Environ Contam Toxicol, 33: 20-25.
Barr M Jr (1978) Pediatric health aspects of PBBs. Environ Health
Perspect, 23: 291-294.
Barr M Jr (1980) Pediatric aspects of the Michigan polybrominated
biphenyl contamination. Environ Res, 21: 255-274.
Bastomsky CH (1986) Polyhalogenated aromatic hydrocarbons and
thyroid function. Presented at the 191st American Chemical Society
National Meeting, New York, 13-18 April 1986. Washington, DC,
American Chemical Society (Abstract No. 23).
Beaudoin AR (1977) Teratogenicity of polybrominated biphenyls in
rats. Environ Res, 14: 81-86.
Beaudoin AR (1979) Embryotoxicity of polybrominated biphenyls. In:
Persaud TVN ed. Advances in the study of birth defects. Volume 2 -
Teratological testing. Baltimore, Maryland, University Park Press,
pp 211-222.
Beaudoin AR & Fisher DL (1981) An in vivo/in vitro evaluation of
teratogenic action. Teratology, 23: 57-61.
Beebe LE, Fox SD, Issaq HJ, & Anderson LM (1991) Biological and
biochemical effects of retained polyhalogenated hydrocarbons.
Environ Toxicol Chem, 10: 757-763.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Anderson HA, Roboz JP, Roboz J, Fischbein A, Selikoff IJ,
& Holland JF (1979a) Immunologic dysfunction among PBB-exposed
Michigan dairy farmers. Ann NY Acad Sci, 320: 717-728.
Bekesi JG, Roboz J, Anderson HA, Roboz JP, Roboz J, Fischbein A,
Selikoff IJ, & Holland JF (1979b) Impaired immune function and
identification of polybrominated biphenyls (PBB) in blood
compartments of exposed Michigan dairy farmers and chemical workers.
Drug Chem Toxicol, 2: 179-191.
Bekesi JG, Roboz JP, Solomon S, Fischbein A, Roboz J, & Selikoff IJ
(1983a) Persistent immune dysfunction in Michigan dairy farm
residents exposed to polybrominated biphenyls. Adv Immunopharmacol,
2: 33-39.
Bekesi JG, Roboz JP, Solomon S, Fischbein A, & Selikoff IJ (1983b)
Altered immune function in Michigan residents exposed to
polybrominated biphenyls. In: Gibson GG, Hibbard R, & Parke DV ed.
Immunotoxicology. New York, London, San Francisco, Academic Press,
pp 181-191.
Bekesi JG, Roboz J, Fischbein A, & Mason P (1987) Immunotoxicology:
Environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the state of Michigan. Cancer Detect
Prev, 1(Suppl):29-37.
Benbow AW & Cullis CF (1975) The formation of toxic products during
the combustion of halogen-containing polymers. Eur Polym J, 11:
723-727.
Benighi R (1989) Analysis of the National Toxicology Program data on
in vitro genetic toxicity tests using multivariate statistical
methods. Mutagenesis, 4: 412-419.
Bernert JT Jr & Groce DF (1984) Acute response of rat liver
microsomal lipids, lipid peroxidation, and membrane anisotropy to a
single oral dose of polybrominated biphenyls. J Toxicol Environ
Health, 13: 673-678.
Bernert JT Jr, Groce DF, & Kimbrough RD (1983) Long-term effects of
a single oral dose of polybrominated biphenyls on serum and liver
lipids in rats. Toxicol Appl Pharmacol, 68: 424-433.
Bernert JT Jr, Meredith NK, Akins JR, & Hannon WH (1985)
Determination of serum phospholipid metabolic profiles by
high-performance liquid chromatography. J Liq Chromatogr, 8:
1573-1592.
Berry DL, Digiovanni J, Juchau MR, Bracken WM, Gleason GL, & Slaga
TJ (1978) Lack of tumor-promoting ability of certain environmental
chemicals in a two-stage mouse skin tumorigenesis assay. Res Commun
Chem Pathol Pharmacol, 20: 101-108.
Besaw LC, Moore RW, Dannan GA, & Aust SD (1978) Effect of
2,2',3,3',4,4',5,5'-octabromobiphenyl on microsomal drug
metabolizing enzymes. Pharmacologist, 20: 251.
Bialik O (1982) Endocrine function of workers exposed to PBB and
PBBO: Terminal progress report (Prepared for the National Institute
of Occupational Safety and Health, Cincinnati). Springfield,
Virginia, National Technical Information Service (NTIS) (PB
84-238377).
Birnbaum LS (1985) The role of structure in the disposition of
halogenated aromatic xenobiotics. Environ Health Perspect, 61:
11-20.
Birnbaum LS & McKinney JD (1985) A persistent hexabromonaphthalene
isomer is 2,3,4,5,6,7-hexabromonaphthalene. J Toxicol Environ
Health, 16: 219-228.
Birnbaum LS, Darcey DJ, & McKinney JD (1983) Hexabromonaphthalene
contaminants of polybrominated biphenyls: chemical composition and
disposition in the rat. J Toxicol Environ Health, 12: 555-573.
Birnbaum LS, Morrissey RE, & Harris MW (1991) Teratogenic effects of
2,3,7,8-tetrabromodibenzo-p-dioxin and three polybrominated
dibenzofurans in C57BL/6N mice. Toxicol Appl Pharmacol, 197:
141-152.
Bleavins MR, Aulerich RJ, & Ringer RK (1981) Placental and mammary
transfer of polychlorinated and polybrominated biphenyls in the mink
and ferret. In: Lamb DW & Kenaga EE ed. Avian and mammalian wildlife
toxicology: Second Conference. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 121-131 (ASTM Special
Technical Publication 757).
Bombick DW (1990) Gap junctional communication in various cell types
after chemical exposure. J Mol Cell Toxicol, 3: 27-39.
Bonhaus DW, McCormack KM, Braselton WE Jr, & Hook JB (1981) Effect
of polybrominated biphenyls on hepatic microsomal metabolism of
estrogens and uterotropic action of administered estrogen in rats. J
Toxicol Environ Health, 8: 141-150.
Brilliant LB, Van Amburg G, Isbister J, Humphrey H, Wilcox K, Eyster
J, Bloomer AW, & Price H (1978) Breast-milk monitoring to measure
Michigan's contamination with polybrominated biphenyls. Lancet, 2:
643-646.
Brinkman UATh & De Kok A (1980) Production, properties usage. In:
Kimbrough RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 1-40.
Brouwer A & Van den Berg KJ (1986) Binding of a metabolite of
3,4,3',4'-tetrachloro biphenyl to transthyretin reduces serum
vitamin A transport by inhibiting the formation of the protein
complex carrying both retinol and thyroxin. Toxicol Appl Pharmacol,
85: 301-312.
Brown GG & Nixon R (1979) Exposure to polybrominated biphenyls, some
effects on personality and cognitive functioning. J Am Med Assoc,
242: 523-527.
Brown GG, Preisman RC, Anderson MD, Nixon RK, Isbister JL, & Price
HA (1981) Memory performance of chemical workers exposed to
polybrominated biphenyls. Science, 212: 1413-1415.
Buchmann A, Ziegler S, Wolf A, Robertson LW, Durham SK, & Schwarz M
(1991) Effects of polychlorinated biphenyls in rat liver:
correlation between primary subcellular effects and promoting
activity. Toxicol Appl Pharmacol, 111: 454-468.
Bunce NJ, Safe S, & Ruzo LO (1975) Photochemistry of bromobiphenyls:
steric effects and electron transfer. J Chem Soc Perkin Trans, 1:
1607-1610.
Burse VW, Needham LL, Liddle JA, Bayse DD, & Price HA (1980)
Interlaboratory comparison for results of analyses for
polybrominated biphenyls in human serum. J Anal Toxicol, 4: 22-26.
Bursian SJ, Polin D, Olson BA, Shull LR, Marks HL, & Siegel HS
(1983) Microsomal enzyme induction, egg production, and reproduction
in three lines of Japanese quail fed polybrominated biphenyls. J
Toxicol Environ Health, 12: 291-307.
Buser HR (1986) Selective detection of brominated aromatic compounds
using gas chromatography/negative chemical ionization mass
spectrometry. Anal Chem, 58: 2913-2919.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants.
Chemosphere, 16: 713-732.
Buser HR, Bosshardt HP, & Rappe C (1978) Formation of
polychlorinated dibenzofurans (PCDFs) from the pyrolysis of PCBs.
Chemosphere, 1: 109-119.
Byrne JJ, Carbone JP, & Hanson EA (1987) Hypothyroidism and
abnormalities in the kinetics of thyroid hormone metabolism in rats
treated chronically with polychlorinated biphenyl and polybrominated
biphenyl. Endocrinology, 121: 520-527.
Byrne JJ, Carbone JP, & Pepe MG (1988) Suppression of serum adrenal
cortex hormones by chronic low-dose polychlorobiphenyl or
polybromobiphenyl treatments. Arch Environ Contam Toxicol, 17:
47-53.
Cagen SZ & Gibson JE (1977a) Ouabain lethality as a measure of
biliary function in developing mice and rats and effect of
polybrominated biphenyls. Toxicol Appl Pharmacol, 40: 327-334.
Cagen SZ & Gibson JE (1977b) Stimulation of biliary function
following polybrominated biphenyls. Abstract: 6th Annual Meeting.
Toxicol Appl Pharmacol, 41: 147.
Cagen SZ & Gibson JE (1978) Effect of polybrominated biphenyls on
hepatic excretory function in rats and mice. Environ Health
Perspect, 23: 233-239.
Cagen SZ, Preache MM, & Gibson JE (1977) Enhanced disappearance of
drugs from plasma following polybrominated biphenyls. Toxicol Appl
Pharmacol, 40: 317-325.
Carter LJ (1976) Michigan's PBB incident: chemical mix-up leads to
disaster. Science, 192: 240-243.
Castracane VD, Allen-Rowlands CF, Hamilton MG, & Seifter J (1982)
The effect of polybrominated biphenyl on testes, adrenal and
pituitary function in the rat. Proc Soc Exp Biol Med, 169: 343-347.
CEC (Commission of the European Communities) (1988) [Council
Regulation No. 1734/88 of 16 June 1988, concerning export from and
import into the Community of certain dangerous chemicals.] Off J Eur
Communities, L 155: 2-6 (in German).
Cecil HC & Bitman J (1978) Toxicity of polybrominated biphenyl and
its effects on reproduction of White Leghorn hens. Poult Sci, 57:
1027-1036.
Cecil HC, Bitman J, Lillie RJ, Fries GF, & Verrett J (1974)
Embryotoxic and teratogenic effects in unhatched fertile eggs from
hens fed polychlorinated biphenyls (PCBs). Bull Environ Contam
Toxicol, 11: 489-495.
Cecil HC, Harris SJ, & Bitman J (1975) Effects of polychlorinated
biphenyls and terphenyls and polybrominated biphenyls on
pentobarbital sleeping times of Japanese quail. Arch Environ Contam
Toxicol, 3: 183-192.
CFK (1982) [Potassiumbromide fire protection equipment: Information
sheet.] Köln, Chemische Fabrik Kalk GmbH (in German).
Chanda JJ, Anderson HA, Glamb RW, Lomatch DL, Wolff MS, Voorhees JJ,
& Selikoff IJ (1982) Cutaneous effects of exposure to polybrominated
biphenyls (PBBs): the Michigan PBB incident. Environ Res, 29:
97-108.
Chang TS & Zindel HC (1975) Toxic effect of polybrominated biphenyl
on young poultry. Poult Sci, 54: 1743-1744.
Chiang TCH, Liao W, & Williams LR (1987) Use of solid phase florisil
cartridges to separate fat from semivolatile organic compounds in
adipose tissue. J Assoc Off Anal Chem, 70: 100-102.
Choi WW & Chen KY (1976) Associations of chlorinated hydrocarbons
with fine particles and humic substances in nearshore surficial
sediments. Environ Sci Technol, 10: 782-786.
Chou SF, Jacobs LW, Penner D, & Tiedje JM (1978) Absence of plant
uptake and translocation of polybrominated biphenyls (PBBs). Environ
Health Perspect, 23: 9-12.
Christensen DC & Weimer WC (1979) Enhanced photodegradation of
persistent halogenated organic materials. In: Bell JM ed.
Proceedings of the 34th Industrial Waste Conference, Purdue
University, Lafayette, Indiana, 8-10 May 1979. Ann Arbor, Michigan,
Ann Arbor Science Publishers, Inc., pp 160-167.
Chu I, Villeneuve DC, Becking GC, Iverson F, Ritter L, Valli VE, &
Reynolds LM (1980) Short-term study of the combined effects of
mirex, photomirex, and kepone with halogenated biphenyls in rats. J
Toxicol Environ Health, 6: 421-432.
Clark RR, Chian ES, & Griffin RA (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl Environ
Microbiol, 4: 680-685.
Cook H, Helland DR, Van der Weele BH, & de Jong RJ (1978a)
Histotoxic effects of polybrominated biphenyls in Michigan dairy
cattle. Environ Res, 15: 82-89.
Cook RM, Prewitt LR, & Fries GF (1978b) Effects of activated carbon,
phenobarbital, and vitamins A, D, and E on polybrominated biphenyl
excretion in cows. J Dairy Sci, 61: 414-419.
Corbett TH, Beaudoin AR, Cornell RG, Anver MR, Schumacher R, Endres
J, & Szwabowska M (1975) Toxicity of polybrominated biphenyls
(FireMaster BP-6) in rodents. Environ Res, 10: 390-396.
Corbett TH, Simmons JL, Kawanishi H, & Endres JL (1978a) EM changes
and other toxic effects of FireMaster BP-6 (polybrominated
biphenyls) in the mouse. Environ Health Perspect, 23: 275-281.
Corbett TH, Simmons JL, & Endres J (1978b) Teratogenicity and tissue
distribution studies of polybrominated biphenyls (FireMaster BP-6)
in rodents. Teratology, 17: 37A.
Cordle F, Corneliussen P, Jelinek C, Hackley B, Lehmann R,
McLaughlin J, Rhoden R, & Shapiro R (1978) Human exposure to
polychlorinated biphenyls and polybrominated biphenyls. Environ
Health Perspect, 24: 157-172.
Cottrell WO, Ringer RK, & Babish JG (1984) Acute toxicity of dietary
polybrominated biphenyls in bobwhite quail. Bull Environ Contam
Toxicol, 33: 308-312.
Crow KD (1983) Chloracne (Halogen Acne). In: Marzulli FN & Maibach
HI ed. Determatotoxicology, 2nd ed. Washington, DC, Hemisphere
Publishing Company, pp 461-481.
Cullum A & Zile MH (1985) Acute polybrominated biphenyl toxicosis
alters vitamin A homeostasis and enhances degradation of vitamin A.
Toxicol Appl Pharmacol, 81: 177-181.
Damstra T, Jurgelski W, Posner HS, Vouk VB, Bernheim NJ, Guthrie J,
Luster M, & Falk HL (1982) Toxicity of polybrominated biphenyls
(PBBs) in domestic and laboratory animals. Environ Health Perspect,
44: 175-188.
Dannan GA, Moore RW, Besaw LC, & Aust SD (1978a)
2,4,5,3',4',5'-hexabromobiphenyl is both a 3-methylcholanthrene- and
a phenobarbital-type inducer of microsomal drug metabolizing
enzymes. Biochem Biophys Res Commun, 85: 450-458.
Dannan GA, Moore RW, & Aust SD (1978b) Studies on the microsomal
metabolism and binding of polybrominated biphenyls (PBBs). Environ
Health Perspect, 23: 51-61.
Dannan GA, Sleight SD, & Aust SD (1980) Toxicity and enzyme
induction effects of polybrominated biphenyls. Pharmacologist, 22:
158.
Dannan GA, Sleight SD, & Aust SD (1982a) Toxicity and microsomal
enzyme induction effects of several polybrominated biphenyls of
FireMaster. Fundam Appl Toxicol, 2: 313-321.
Dannan GA, Mileski GJ, & Aust SD (1982b) Reconstitution of some
biochemical and toxicological effects of commercial mixture of
polybrominated biphenyls. Fundam Appl Toxicol, 2: 322-326.
Dannan GA, Sleight SD, Fraker PJ, Krehbiel JD, & Aust SD (1982c)
Liver microsomal enzyme induction and toxicity studies with
2,4,5,3',4'-pentabromobiphenyl. Toxicol Appl Pharmacol, 64: 187-203.
Dannan GA, Mileski GJ, & Aust SD (1982d) Purification of
polybrominated biphenyl congeners. J Toxicol Environ Health, 9:
423-438.
Dannan GA, Guengerich FP, Kaminsky LS, & Aust SD (1983) Regulation
of cytochrome P-450: Immunochemical quantitation of 8 isozymes in
liver microsomes of rats treated with polybrominated biphenyl
congeners. J Biol Chem, 258: 1282-1288.
Darjono, Sleight SD, Stowe HD, & Aust SD (1983) Vitamin A status,
polybrominated biphenyl (PBB) toxicosis, and common bile duct
hyperplasia in rats. Toxicol Appl Pharmacol, 71: 184-193.
Daum SM, Knittle J, Rosenman K, Ron WN, & Holstein EC (1978) A
simple technique for fat biopsy of PBB-exposed individuals. Environ
Health Perspect, 23: 183-185.
Debets FMH, Hamers WJHMB, & Strik JJTWA (1980) Metabolism as a
prerequisite for the porphyrinogenic action of polyhalogenated
aromatics, with special reference to hexachlorobenzene and
polybrominated biphenyls (FireMaster BP-6). Int J Biochem, 12:
1019-1025.
DeCarlo VJ (1979) Studies on brominated chemicals in the
environment. Ann NY Acad Sci, 320: 678-681.
De Kok JJ, De Kok A, Brinkman UAT, & Kok RM (1977) Analysis of
polybrominated biphenyls. J Chromatogr, 142: 367-383.
Dent JG (1978) Characteristics of cytochrome P-450 and mixed
function oxidase enzymes following treatment with PBBs. Environ
Health Perspect, 23: 301-307.
Dent JG, Netter KF, & Gibson JE (1976a) Effects of chronic
administration of polybrominated biphenyls on parameters associated
with hepatic drug metabolism. Res Commun Chem Pathol Pharmacol, 13:
75-82.
Dent JG, Netter KF, & Gibson JE (1976b) The induction of hepatic
microsomal metabolism in rats following acute administration of a
mixture of polybrominated biphenyls. Toxicol Appl Pharmacol, 38:
237-249.
Dent JG, Roes U, Netter KJ, & Gibson E (1977a) Stimulation of
hepatic microsomal metabolism in mice by a mixture of polybrominated
biphenyls. J Toxicol Environ Health, 3: 651-661.
Dent JG, Cagen SZ, McCormack KM, Rickert DE, & Gibson JE (1977b)
Liver and mammary arylhydrocarbon hydroxylase and epoxide hydratase
in lactating rats fed polybrominated biphenyls. Life Sci, 20:
2075-2080.
Dent JG, Cagen SZ, McCormack KM, Rickert DE, & Gibson JG (1977c)
Microsomal enzyme induction in maternal liver, kidney, mammary
glands and neonatal liver following polybrominated biphenyls. Fed
Proc, 36: 1009.
Dent JG, Elcombe CR, Netter KJ, & Gibson JE (1978a) Rat hepatic
microsomal cytochrome(s) P-450 induced by polybrominated biphenyls.
Drug Metab Dispos, 6: 96-101.
Dent JG, McCormack KM, Rickert DE, Cagen SZ, Melrose P, & Gibson JE
(1978b) Mixed function oxidase activities in lactating rats and
their offspring following dietary exposure to polybrominated
biphenyls. Toxicol Appl Pharmacol, 46: 727-735.
Detering CN, Prewitt RL, Cook RM, & Fries GF (1975) Placental
transfer of polybrominated biphenyl by Holstein cows. J Dairy Sci,
58: 764-765.
Devito M, Umbreit TH, Thomas T, & Gallo MA (1991) An analogy between
the actions of the Ah receptor and the estrogen receptor for use in
the biological basis for risk assessment of dioxin. In: Gallo MA,
Scheuplein RJ, & van der Heijden KE ed. Biological basis for risk
assessment of dioxins and related compounds. Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory Press, pp 367-377 (Banbury
Report No. 35).
Dharmo DN, Sleight SD, Ringer RK, & Aust SD (1982) Pathologic
effects of 2,2',4,4',5,5'- and 2,3',4,4',5,5'-hexabromobiphenyl in
White Leghorn cockerels. Avian Dis, 26: 542-552.
Di Carlo FJ, Seifter J, & DeCarlo VJ (1978) Assessment of the
hazards of polybrominated biphenyls. Environ Health Perspect, 23:
351-365.
Diercxsens P, DeWeck D, Borsinger N, Rosset B, & Tarradellas J
(1985) Earthworm contamination by PCBs and heavy metals.
Chemosphere, 14: 511-522.
Dixon D, Sleight SD, Aust SD, Jensen RK, & Rezabek MS (1985)
Assessment of 3,4,3',4'-tetrabromobiphenyl (3,4-TBB) as a promotor
or initiator of experimental hepatocarcinogenesis in rats.
Toxicologist, 5: 12.
Dixon D, Sleight SD, Aust SD, & Rezabek MS (1988) Tumor-promoting,
initiating, and hepatotoxic effects of 3,4,3',4'-tetrabromobiphenyl
(34-TBB) in rats. J Am Coll Toxicol, 7: 687-697.
Domino EF & Domino SE (1980) Gas chromatographic-mass spectrometric
analysis of polybrominated biphenyl constituents of FireMaster FF-1.
J Chromatogr, 197: 258-262.
Domino EF, Wright DD, & Domino SE (1980a) GC-EC analysis of
polybrominated biphenyl constituents of FireMaster FF-1 using
tetrabromobiphenyl as an internal standard. J Anal Toxicol, 4:
299-304.
Domino EF, Fivenson DP, & Domino SE (1980b) Differential tissue
distribution of various polybrominated biphenyls of FireMaster FF-1
in male rats. Drug Metab Dispos, 8: 332-336.
Domino LE, Domino SE, & Domino EF (1982) Toxicokinetics of
2,2',4,4',5,5'-hexabromo biphenyl in the rat. J Toxicol Environ
Health, 9: 815-833.
Doucette WJ & Andren AW (1987) Correlation of octanol/water
partition coefficients and total molecular surface area for highly
hydrophobic aromatic compounds. Environ Sci Technol, 21: 821-824.
Doucette WJ & Andren AW (1988) Aqueous solubility of selected
biphenyl, furan, and dioxin congeners. Chemosphere, 17: 243-252.
Dunckel AE (1975) An updating on the polybrominated biphenyl
disaster in Michigan. J Am Vet Med Assoc, 167: 838-841.
Durst HI, Willett LB, Brumm CJ, & Mercer HD (1977) Effects of
polybrominated biphenyls on health and performance of pregnant
Holstein heifers. J Dairy Sci, 60: 1294-1300.
Durst HI, Willett LB, Brumm CJ, & Schanbacher FL (1978a) Changes in
blood and urine composition from feeding polybrominated biphenyls to
pregnant Holstein heifers. J Dairy Sci, 61: 197-205.
Durst HI, Willett LB, Schanbacher FL, & Moorhead PD (1978b) Effects
of PBBs on cattle. I. Clinical evaluations and clinical chemistry.
Environ Health Perspect, 23: 83-89.
Eaton DL & Klaassen CD (1979) Effects of
2,3,7,8-tetrachlorodibenzo-p-dioxin, kepone, and polybrominated
biphenyls on transport systems in isolated rat hepatocytes. Toxicol
Appl Pharmacol, 51: 137-144.
Eckhoff MA, Ridgway TH, & Caruso JA (1983) Polychromator system for
multielement determination by gas chromatography with helium plasma
atomic emission spectrometric detection. Anal Chem, 55: 1004-1009.
Ecobichon DJ, Hansell MM, & Safe S (1977) Halogen substituents at
the 4- and 4'-positions of biphenyl: influence on hepatic function
in the rat. Toxicol Appl Pharmacol, 42: 359-366.
Ecobichon DJ, Hansell MM, & Safe S (1979) Isomerically pure
bromobiphenyl congeners and hepatic mono-oxygenase activities in the
rat: influence of position and degree of bromination. Toxicol Appl
Pharmacol, 47: 341-352.
Ecobichon DJ, Hidvegi S, Comeau AM, & Cameron PH (1983)
Transplacental and milk transfer of polybrominated biphenyls to
perinatal guinea-pigs from treated dams. Toxicology, 28: 51-63.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Elcombe CR & Lech JJ (1978) Induction of monooxygenation in rainbow
trout by polybrominated biphenyls: a comparative study. Environ
Health Perspect, 23: 309-314.
El-Fouly MH, Trosko JE, & Chang CC (1987) Scrape-loading and dye
transfer: a rapid and simple technique to study gap junctional
intercellular communication. Exp Cell Res, 168: 422-430.
Epling GA, McVicar W, & Kumar A (1987) Accelerated debromination of
biphenyls by photolysis with sodium borohydride. Chemosphere, 16:
1013-1020.
Erickson MD, Kelner L, Bursey JT, Rosenthal D, Zweidinger RA, &
Pellizzari ED (1980) Method for analysis of polybrominated biphenyls
by gas chromatography mass spectrometry. Biomed Mass Spectrom, 7:
99-104.
Erney DR (1975) Confirmation of polybrominated biphenyl residues in
feeds and dairy products, using an ultraviolet
irradiation-gas-liquid chromatographic technique. J Assoc Off Anal
Chem, 58: 1202-1205.
Evans MG, El-Fouly MH, Trosko JE, & Sleight SD (1988) Anchored cell
analysis/sorting coupled with the scrape-loading/dye-transfer
technique to quantify inhibition of gap-junctional intercellular
communication in WB-F344 cells by 2,2',4,4',5,5'-hexabromo biphenyl.
J Toxicol Environ Health, 24: 261-271.
Evers WD, Hook JB, & Bond JT (1977) Effect of polybrominated
biphenyls on renal tubular transport of organic ions. J Toxicol
Environ Health, 3: 759-767.
Eyster JT, Humphrey HEB, & Kimbrough RD (1983) Partitioning of
polybrominated biphenyls in (PBBs) in serum, adipose tissue, breast
milk, placenta, cord blood, biliary fluid, and feces. Arch Environ
Health, 38: 47-53.
Falkner R & Simonis W (1982) [Polychlorinated biphenyls (PCBs) in
the aquatic environment (uptake and accumulation by organisms -
problems related on the passage through the food pyramid.] Arch
Hydrobiol Beih, 17: 1-74 (in German).
Farber TM, Balazs T, Marks E, & Cerra F (1976) The influence of
microsomal induction on serum alkaline phosphate activity in dogs.
Fed Proc, 35: A943.
Farber T, Kasza L, Giovetti A, Carter C, Earl F, & Balazs T (1978)
Effect of polybrominated biphenyls (FireMaster BP-6) on the
immunologic system of the beagle dog. Toxicol Appl Pharmacol, 45:
343.
Farrell TJ (1980) Glass capillary gas chromatography of chlorinated
dibenzofurans, chlorinated anisoles, and brominated biphenyls. J
Chromatogr Sci, 18: 10-17.
Fawkes J, Albro PW, Walters DB, & McKinney JD (1982) Comparison of
extraction methods for determination of polybrominated biphenyl
residues in animal tissue. Anal Chem, 54: 1866-1871.
Fehringer NV (1975a) Determination of polybrominated biphenyl
residues in dairy products. J Assoc Off Anal Chem, 58: 978-982.
Fehringer NV (1975b) Determination of polybrominated biphenyl
residues in dry animal feeds. J Assoc Off Anal Chem, 58: 1206-1210.
Ficsor G & Wertz GF (1976) Polybrominated biphenyl non-teratogenic,
c-mitosis synergist in rat. Mutat Res, 38: 388.
Filonow AB, Jacobs LW, & Mortland MM (1976) Fate of polybrominated
biphenyls (PBB's) in soils. Retention of hexabromobiphenyl in four
Michigan soils. J Agric Food Chem, 24: 1201-1204.
Fisher DL (1980) Effect of polybrominated biphenyls on the
accumulation of DNA, RNA, and protein in cultured rat embryos
following maternal administration. Environ Res, 23: 334-340.
Fraker PJ (1980) The antibody-mediated and delayed type
hypersensitivity response of mice exposed to polybrominated
biphenyls. Toxicol Appl Pharmacol, 53: 1-7.
Fraker PJ & Aust S (1978) The effect of polybrominated biphenyls on
the immune response of Balb/c mice. In: Asher JM ed. Inadvertent
modifications of the immune response: The effects of foods, drugs
and environmental contaminants. Proceeding of the Fourth FDA Science
Symposium, US Naval Academy, 28-30 August 1978. Rockville, Maryland,
Food and Drug Administration, Office of Health Affairs, pp 270-271
(HHS Publication No. (FDA) 80-1074).
Franklin RB, Vodicnik MJ, Elcombe CR, & Lech JJ (1981) Alterations
in hepatic mixed-function oxidase activity of rainbow trout after
acute treatment with polybrominated biphenyl isomers and FireMaster
BP-6. J Toxicol Environ Health, 7: 817-827.
Freeman PK, Jang J-S, & Ramnath N (1991) The photochemistry of
polyhaloarenes. 10. The photochemistry of 4-bromobiphenyl. J Org
Chem, 56: 6072-6079.
Fries GF (1982) Potential polychlorinated biphenyl residues in
animal products from application of contaminated sewage sludge to
land. J Environ Qual, 11: 14-20.
Fries GF (1983) Effect of low exposure to polybrominated biphenyl on
production and other indicators of dairy herd performance. J Dairy
Sci, 66: 1303-1311.
Fries GF (1985a) Bioavailability of soil-borne polybrominated
biphenyls ingested by farm animals. J Toxicol Environ Health, 16:
565-579.
Fries GF (1985b) The PBB episode in Michigan: an overall appraisal.
CRC Crit Rev Toxicol, 16: 105-156.
Fries GF & Jacobs LW (1980) Residual polybrominated biphenyl
contamination: locations, amounts and significance on dairy farms. J
Dairy Sci, 64: 114.
Fries GF & Jacobs LW (1986) Evaluation of residual polybrominated
biphenyl contamination present on Michigan farms in 1978. East
Lansing, Michigan State University, Agricultural Experiment Station,
15 pp (Research Report Farm Science-477).
Fries GF & Marrow GS (1975) Excretion of polybrominated biphenyls
into the milk of cows. J Dairy Sci, 58: 947-951.
Fries GF & Marrow GS (1982) Residues in the fat of ewes grazing on
soil contaminated with halogenated hydrocarbons. J Anim Sci, 55:
1118-1124.
Fries GF, Cecil HC, Bitman J, & Lillie RJ (1976) Retention and
excretion of polybrominated biphenyls by hens. Bull Environ Contam
Toxicol, 15: 278-282.
Fries GF, Marrow GS, & Cook RM (1978a) Distribution and kinetics of
PBB residues in cattle. Environ Health Perspect, 23: 43-50.
Fries GF, Cook RM, & Prewitt LR (1978b) Distribution of
polybrominated biphenyl residues in the tissues of environmentally
contaminated dairy cows. J Dairy Sci, 61: 420-425.
Fries GF, Marrow GS, & Snow PA (1982a) Soil ingestion by swine as a
route of contaminant exposure. Environ Toxicol Chem, 1: 201-204.
Fries GF, Marrow GS, & Snow PA (1982b) Soil ingestion by dairy
cattle. J Dairy Sci, 65: 611-618.
Furukawa K & Matsumura F (1976) Microbial metabolism of
polychlorinated biphenyls. Studies on the relative degradability of
polychlorinated biphenyl components by Alkaligenes sp. J Agric
Food Chem, 24: 251-256.
Furukawa K, Tomizuka N, & Kamibayashi A (1979) Effect of chlorine
substitution on the bacterial metabolism of various polychlorinated
biphenyls. Appl Environ Microbiol, 38: 301-310.
Galloway SM, Armstrong JJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10: 1-175.
Gardner AM, Righter HF, & Roach AG (1976) Excretion of hydroxylated
polychlorinated biphenyl metabolites in cows' milk. J Assoc Off Anal
Chem, 59: 273-277.
Gardner AM, Warren VL, Chen JYT, & Mazzola EP (1979) A metabolite of
poly brominated biphenyls: its identification and decomposition to a
brominated dibenzofuran in the gas chromatograph mass spectrometer.
J Agric Food Chem, 27: 116-119.
Garthoff LH, Friedman L, Farber TM, Locke KK, Sobotka TJ, Green S,
Hurley NE, Peters EL, Story GE, Moreland FM, Graham CH, Keys JE,
Taylor MJ, Scalera JV, Rothlein JE, Marks EM, Cerra FE, Rodi SB, &
Sporn EM (1977) Biochemical and cytogenetic effects in rats caused
by short-term ingestion of Aroclor 1254 or FireMaster BP6. J Toxicol
Environ Health, 3: 769-796.
Gause EM, Ross DH, Hamilton MG, Leal BZ, Seifter J, & Geller I
(1979) Correlation of systemic and biochemical effects of PBB with
behavioral effects. Neurobehav Toxicol, 1: 269-274.
Gause EM, Leal BZ, Hartmann RJ, & Geller I (1984) Influence of
maternal PBB body burden on the response to drugs. Proc West
Pharmacol Soc, 27: 501-506.
Geller I, Gause E, Hartmann RJ, & Seifter J (1979) Use of
discrimination behavior for the evaluation of toxicants. Neurobehav
Toxicol, 1: 9-13. Geller I, Gause EM, Leal BZ, Hartmann RJ, &
Seifter J (1985) Behavioral effects of drugs as a function of
maternal polybrominated biphenyl body burden. Toxicol Lett, 24:
229-234.
Getty SM, Rickert DE, & Trapp AL (1977) Polybrominated biphenyl
(PBB) toxicosis: an environmental accident. CRC Crit Rev Environ
Control, 7(4): 309-323.
Gladen B & Rogan W (1979) Misclassification and the design of
environmental studies. Am J Epidemiol, 109: 607-619.
Gobas FA, Lahittete JM, Garofalo G, Shiu WY, & MacKay D (1988) A
novel method for measuring membrane-water partition coefficients of
hydrophobic organic chemicals: comparison with 1-octanol-water
partitioning. J Pharmacol Sci, 77: 265-272.
Gobas FA, Clark KE, Shiu WY, & MacKay D (1989) Bioconcentration of
polybrominated benzenes and biphenyls and related superhydrophobic
chemicals in fish: role of bioavailability and elimination into the
feces. Environ Toxicol Chem, 8: 231-245.
Goldstein JA (1979) The structure-activity relationships of
halogenated biphenyls as enzyme inducers. Ann NY Acad Sci, 320:
164-178.
Goldstein JA (1980) Structure-activity relationships for the
biochemical effects and the relationship to toxicity. In: Kimbrough
RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 151-178.
Goldstein JA, Linko PC, Levy LA, McKinney JD, Gupta BN, & Moore JA
(1979) A comparison of a commercial polybrominated biphenyl mixture
2,4,5,2',4',5'-hexabromo biphenyl and 2,3,6,7-tetrabromonaphthalene
as inducers of liver microsomal drug-metabolizing enzymes. Biochem
Pharmacol, 28: 2947-2956.
Great Lakes Chemical Corp. (1986) Product information: Great Lakes
Chemical flame retardants. Effective flame retardant performance for
a variety of polymer systems. West Lafayette, Indiana, Great Lakes
Chemical Corp., 6 pp.
Greaves J, Bekesi JG, & Roboz J (1982) Halogen anion formation by
polybrominated compounds in negative chemical ionization mass
spectrometry. Biomed Mass Spectrom, 9: 406-410.
Greaves J, Bekesi JG, Holland JF, & Roboz J (1983) Studies on the
protein binding of hexabromobiphenyl (HxBB) in 14C-HxBB spiked and
environmentally contaminated serum. In: The Thirty-First Annual
Conference on Mass Spectrometry and Allied Topics, Boston, 8-13 May
1983. East Lansing, Michigan, American Society for Mass
Spectrometry, pp 475-476.
Greaves J, Roboz J, Holland JF, & Bekesi JG (1984) Determination of
the binding of polybrominated biphenyls to serum proteins by
negative chemical ionization mass spectrometry. Chemosphere, 13:
651-656.
Griffin RA & Chou SFJ (1980) Attenuation of polybrominated biphenyls
and hexachlorobenzene by earth materials. Urbana, Illinois,
Institute of Natural Resources, State Geological Survey Division, 53
pp (Environmental Geology Note No. 87).
Griffin RA & Chou SFJ (1981a) Attenuation of polybrominated
biphenyls and hexachlorobenzene by earth materials. Washington, DC,
US Environmental Protection Agency (EPA-600/2-81-186).
Griffin RA & Chou SFJ (1981b) Movement of PCBs and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Groce DF & Kimbrough RD (1984) Stunted growth, increase mortality,
and liver tumors in offspring of polybrominated biphenyl dosed
Sherman rats. J Toxicol Environ Health, 14: 695-706.
Gupta BN & Moore JA (1979) Toxicologic assessments of a commercial
polybrominated biphenyl mixture in the rat. Am J Vet Res, 40:
1458-1468.
Gupta BN, McConnell EE, Harris MW, & Moore JA (1981) Polybrominated
biphenyl toxicosis in the rat and mouse. Toxicol Appl Pharmacol, 57:
99-118.
Gupta BN, McConnell EE, Goldstein JA, Harris MW, & Moore JA (1983a)
Effects of a polybrominated biphenyl mixtures in the rat and mouse.
I. Six-month exposure. Toxicol Appl Pharmacol, 68: 1-18.
Gupta BN, McConnell EE, Moore JA, & Haseman JK (1983b) Effects of
polybrominated biphenyl mixture in the rat and mouse. 2. Lifetime
study. Toxicol Appl Pharmacol, 68: 19-35.
Gutenmann WH & Lisk DJ (1975) Tissue storage and excretion in milk
of polybrominated biphenyls in ruminants. J Agric Food Chem, 23:
1005-1007.
Haake JM, Merrill JC, & Safe S (1985) The in vitro metabolism of
benzo[a]pyrene by polychlorinated and polybrominated biphenyl
induced rat hepatic microsomal monooxygenases. Can J Physiol
Pharmacol, 63: 1096-1100.
Halvorson MR, Phillips TD, Safe SH, & Robertson LW (1985) Metabolism
of aflatoxin B1 by rat hepatic microsomes induced by
polyhalogenated biphenyl congeners. Appl Environ Microbiol, 49:
882-886.
Haroz RK & Aust SD (1979) Assessment of tumor initiating and
promoting activity of a mixture of polybrominated biphenyls
(FireMaster BP-6), and certain purified isomers of PBB. Toxicol Appl
Pharmacol, 48: A158 (Abstract 317).
Harris SJ, Cecil HC, & Bitman J (1978a) Embryotoxic effects of
polybrominated biphenyls (PBB) in rats. Environ Health Perspect, 23:
295-300.
Harris SJ, Cecil HC, & Bitman J (1978b) Effects of feeding of
polybrominated biphenyl flame retardant (FireMaster BP-6) to male
rats. Bull Environ Contam Toxicol, 19: 692-696.
Haseltine SD, Heinz GH, Reichel WL, & Moore JF (1981) Organochlorine
and metal residues in eggs of waterfowl nesting on islands in Lake
Michigan off Door County, Wisconsin, USA. Pestic Monit J, 15: 90-97.
Haseman JK, Crawford DD, Huff JE, Boormann GA, & McConnell EE (1984)
Results from 86 2-year carcinogenicity studies conducted by the
National Toxicology Program. J Toxicol Environ Health, 14: 621-640.
Hass JR, McConnell EE, & Harvan DJ (1978) Chemical and toxicologic
evaluation of FireMaster BP-6. J Agric Food Chem, 26: 94-99.
Hassett JP & Anderson MA (1979) Association of hydrophobic compounds
with dissolved organic matter in aquatic systems. Environ Sci
Technol, 13: 1526-1529.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Head SL & Burse VW (1987) Organochlorine recovery from small adipose
samples with the universal trace residue extractor (Unitrex). Bull
Environ Contam Toxicol, 39: 848-856.
Healy WB (1968) Ingestion of soil by dairy cows. NZ J Agric Res, 11:
487-499.
Healy WB, Cutress TW, & Michie C (1967) Wear of sheep's teeth. IV.
Reduction of soil ingestion and tooth wear by supplementary feeding.
NZ J Agric Res, 10: 201-209.
Heinemann FW & Ringer RK (1976) Cardiovascular effects of a
halogenated hydrocarbon, polybrominated biphenyl. Fed Proc, 35: 399.
Heinz GH, Haseltine SD, Reichel WL, & Hensler GL (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ Pollut, A32: 211-232.
Heinz GH, Erdmann TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henck JW (1986) Neurobehavioral toxicity of perinatally administered
polybrominated biphenyls. Diss Abstr Int, B46: 2214-2215.
Henck JW & Rech RH (1986) Effect of perinatal polybrominated
biphenyl exposure on acquisition and performance of an autoshaping
paradigm. Neurotoxicology, 7: 651-663.
Hesse JL (1975) Water pollution aspects of PBB production: results
of surveys in the pine river in the vicinity of St. Louis, Michigan.
In: Proceedings of the Second National Conference on Complete Water
Reuse: Water's Interface with Energy, Air and Solids, Chicago, 4-8
May 1975. New York, American Institute of Chemical Engineers, pp
1190-1199.
Hesse JL & Powers RA (1978) Polybrominated biphenyl (PBB)
contamination of the Pine River, Gratiot, and Midland Counties,
Michigan. Environ Health Perspect, 23: 19-25.
Hill RH Jr (1985) Effects of polyhalogenated aromatic compounds on
porphyrin metabolism. Environ Health Perspect, 60: 139-143.
Hill RH, Patterson DG, Orti DL, Holler JS, Needham LL, Sirmans SL, &
Liddle JA (1982) Evidence of degradation of polybrominated biphenyls
in soil samples from Michigan. J Environ Sci Health, B17: 19-33.
Hinton DE, Trump BF, Kasza L, Weinberger MA, Friedman L, & Garthoff
LH (1979) Comparative toxicity of polychlorinated biphenyl (PCB) and
polybrominated biphenyl (PBB) in the liver of Holtzman rats: light
and electron microscopic alterations. In: Animals as monitors of
environmental pollutants. Washington, DC, National Academy of
Sciences, pp 382-383.
Hoel DG, Hasemann JK, Hogan MD, Huff J, & McConnell EE (1988) The
impact of toxicity on carcinogenicity studies: implications for risk
assessment. Carcinogenesis, 9: 2045-2052.
Höfler F, Melzer H, Möckel HJ, Robertson LW, & Anklam E (1988)
Relationship between liquid and gas chromatographic retention
behavior and calculated molecular surface area of selected
polyhalogenated biphenyls. J Agric Food Chem, 36: 961-965.
Howard SK, Werner PR, & Sleight SD (1980) Polybrominated biphenyl
toxicosis in swine: effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol, 55:
146-153.
Huang IC (1971) Effect of selected factors on pesticide sorption and
desorption in the aquatic system. J Water Pollut Control Fed, 43:
1739-1748.
Humble CG & Speizer FE (1984) Polybrominated biphenyls and fetal
mortality in Michigan. Am J Public Health, 74: 1130-1132.
Humphrey HEB & Hayner NS (1975) Polybrominated biphenyls: an
agricultural incident and its consequences. II. An epidemiological
investigation of human exposure. Trace Subst Environ Health, 9:
57-63.
Hutzinger O, Berg MVD, Olie K, Opperhuizen A, & Safe S (1985a)
Dioxins and furans in the environment: evaluating toxicological risk
from different sources by multi-criteria analysis. In: Kamrin MA &
Rodgers PW ed. Dioxins in the environment: Symposium, East Lansing,
Michigan, 6-9 December 1983. New York, Hemisphere Publishing
Company, pp 9-32.
Hutzinger O, Blumich MJ, Berg MVD, & Olie K (1985b) Sources and fate
of PCDDs and PCDFs: an overview. Chemosphere, 14: 581-600.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer, 140 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Volume 18).
IARC (1986) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer, 434 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: an updating of
IARC Monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 434 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Irving HA, Willett LB, Brumm CJ, & Mercer HD (1976) The effects of
polybrominated biphenyls on growth, feed intake, and health of
pregnant heifers. Am Dairy Sci Assoc Annu Meet, 71: 79.
Isbister JL (1977) PBBs in human health. Clin Med, 84: 22-24.
Isleib DR & Whitehead GL (1975) Polybrominated biphenyls: an
agricultural incident and its consequences. I. The agricultural
effects of exposure. Trace Subst Environ Health, 9: 47-55.
Iwata Y, Gunther FA, & Westlake WE (1974) Uptake of a PCB (Aroclor
1254) from soil by carrots under field conditions. Bull Environ
Contam Toxicol, 11: 523-528.
Jackson TF & Halbert FL (1974) A toxic syndrome associated with the
feeding of polybrominated biphenyl-contaminated protein concentrate
to dairy cattle. J Am Vet Med Assoc, 165: 436-439.
Jacobs LW, Chou SF, & Tiedje JM (1976) Fate of polybrominated
biphenyls (PBB's) in soils. Persistence and plant uptake. J Agric
Food Chem, 24: 1198-1201.
Jacobs LW, Chou SF, & Tiedje JM (1978) Field concentrations and
persistence of polybrominated biphenyls in soils and solubility of
PBB in natural waters. Environ Health Perspect, 23: 1-8.
Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, & Dowler JK (1984)
The transfer of polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs) across the human placenta and into maternal milk.
Am J Public Health, 74: 378-379.
Jacobson JL, Humphrey HEB, Jacobson SW, Schantz SL, Mullin MD, &
Welch R (1989) Determinants of polychlorinated biphenyls (PCBs),
polybrominated biphenyls (PBBs), and dichlorodiphenyl
trichloroethane (DDT) levels in the sera of young children. Am J
Public Health, 79: 1401-1404.
Jaffe R, Stemmler EA, Eitzer BD, & Hites RA (1985) Anthropogenic,
polyhalogenated, organic compounds in sedentary fish from Lake Huron
and Lake Superior tributaries and embayments. J Great Lakes Res, 11:
156-162.
James, MO & Bend JR (1982) Effect of polynuclear aromatic
hydrocarbons and polyhalogenated biphenyls on hepatic mixed-function
oxidase activity in marine fish. In: Richards NL & Jackson BL ed.
Proceedings of the Symposium on Carcinogenic Polynuclear Aromatic
Hydrocarbons in the Marine Environment, Pensacola Beach, Florida,
14-18 August 1978. Gulf Breeze, Florida, US Environmental Protection
Agency, pp 172-190 (EPA-600/9-83-013).
James MO & Little PJ (1981) Polyhalogenated biphenyls and
phenobarbital: evaluation as inducers of drug metabolizing enzymes
in the sheepshead, Archosargus probatocephalus. Chem-Biol
Interact, 36: 229-248.
Jamieson JWS (1977) Polybrominated biphenyls in the environment.
Ottawa, Environmental Protection Service (EPS-3E[-77-18]).
Jansson B, Asplund L, & Olsson M (1987) Brominated flame retardants
- ubiquitous environmental pollutants? Chemosphere, 16: 2343-2349.
Jansson B, Andersson R, Asplund L, Bergman A, Litzen K, Nylund K,
Reutergardh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in
biological samples from the environment. Environ Toxicol Chem,
12(7): 1163-1174.
Jensen RK (1983) Pathologic effects and hepatic tumor promoting
ability of FireMaster BP-6, 3,3',4,4',5,5'-hexabromobiphenyl and
2,2',4,4',5,5'-hexabromobiphenyl in the rat. East Lansing, Michigan,
Michigan State University (Ph.D. Thesis).
Jensen RK & Sleight SD (1986) Sequential study on the synergistic
effects of 2,2',4,4',5,5'-hexabromobiphenyl on hepatic tumor
promotion. Carcinogenesis, 7: 1771-1774.
Jensen RK & Zile MH (1988) Effect of dietary retinoic acid on
circulatory vitamin A homeostasis in polybrominated biphenyl-treated
rats. J Nutr, 118: 416-419.
Jensen S, Renberg L, & Vaz R (1973) Problems in the quantification
of PCB in biological material. In: Ahnland E & Akerblom A ed. PCB
Conference II, Stockholm, 14 December 1972. Solna, Sweden, National
Environment Protection Board, pp 7-13.
Jensen RK, Sleight SD, Goodman JI, Aust SD, & Trosko JE (1982)
Polybrominated biphenyls as promoters in experimental
hepatocarcinogenesis in rats. Carcinogenesis, 3: 1183-1186.
Jensen RK, Sleight SD, & Aust SD (1983) Hepatic tumor promotion by
congeners of polybrominated biphenyls. Toxicologist, 3: 33
(Abstract).
Jensen RK, Sleight SD, & Aust SD (1984) Effect of varying the length
of exposure to polybrominated biphenyls on the development of
gamma-glutamyl transpeptidase enzyme-altered foci. Carcinogenesis,
5: 63-66.
Jensen RK, Cullum ME, Deyo J, & Zile MH (1987) Vitamin A metabolism
in rats chronically treated with 3,3',4,4',5,5'-hexabromobiphenyl.
Biochim Biophys Acta, 926: 310-320.
Johnston CA, Demarest KT, McCormack KM, Hook JB, & Moore KE (1980)
Endocrinological, neurochemical, and anabolic effects of
polybrominated biphenyls in male and female rats. Toxicol Appl
Pharmacol, 56: 240-247.
Jones DH, Kohli J, & Safe S (1979) Avian metabolism of halogenated
biphenyls. Xenobiotica, 9: 733-736.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Krynitsky AJ, Lamont
TG, Mulhern BM, Prouty RM, Stafford CJ, & Swineford DM (1980)
Organochlorine pesticide, PCB, and PBB residues and necropsy data
for bald eagles from 29 states - 1975-77. Pestic Monit J, 13:
145-149.
Kalmaz EV & Kalmaz GD (1979) Transport, distribution and toxic
effects of polychlorinated biphenyls in ecosystems: review. Ecol
Model, 6: 223-251.
Kasza L, Weinberger MA, Hinton DE, Trump BF, Patel C, Friedman L, &
Garthoff LH (1978a) Comparative toxicity of polychlorinated biphenyl
and polybrominated biphenyl in the rat liver: light and electron
microscopic alterations after subacute dietary exposure. J Environ
Pathol Toxicol, 1: 241-257.
Kasza L, Collins WT, Capen CC, Garthoff LH, & Friedman L (1978b)
Comparative toxicity of polychlorinated biphenyl and polybrominated
biphenyl in the rat thyroid gland: light and electron icroscoic
alterations after subacute dietary exposure. J Environ Pathol
Toxicol, 1: 587-599.
Kavanagh TJ, Rubinstein C, Liu PL, Chang C-C, Trosko JE, & Sleight
SD (1985) Failure to induce mutations in Chinese hamster V79 cells
and WB rat liver cells by the 2,2',4,4',5,5'-hexabromobiphenyl,
3,3',4,4',5,5'-hexabromobiphenyl, and 3,3',4,4'-tetra bromobiphenyl.
Toxicol Appl Pharmacol, 79: 91-98.
Kavanagh TJ, Chang C-C, & Trosko JE (1987) Effect of various
polybrominated biphenyls on cell-cell communication in cultured
human teratocarcinoma cells. Fundam Appl Toxicol, 8: 127-131.
Kay K (1977) Polybrominated biphenyls (PBB) environmental
contamination in Michigan, 1973-1976. Environ Res, 13: 74-93.
Kerscher U (1979) [Flame retardants containing bromine for the fire
protecting of plastics.] Kunstst J, 1: 6-16 (in German).
Kimbrough RD (1980a) Environmental pollution of air, water and soil.
In: Kimbrough RD ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products. Amsterdam,
Oxford, New York, Elsevier/North-Holland Biomedical Press, pp 77-80.
Kimbrough RD (1980b) Occupational exposure. In: Kimbrough RD ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and
related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 373-397.
Kimbrough RD ed. (1980c) Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products. Amsterdam,
Oxford, New York, Elsevier/North-Holland Biomedical Press.
Kimbrough RD (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls. Annu Rev Pharmacol
Toxicol, 27: 87-111.
Kimbrough RD, Burse VW, Liddle JA, & Fries GF (1977) Toxicity of
polybrominated biphenyl. Lancet, 2: 602-603.
Kimbrough RD, Burse VW, & Liddle JA (1978) Persistent liver lesions
in rats after a single oral dose of polybrominated biphenyls
(FireMaster FF-1) and concomitant PBB tissue levels. Environ Health
Perspect, 23: 265-273.
Kimbrough RD, Korver MP, Burse VW, & Groce DF (1980) The effect of
different diets or mineral oil on liver pathology and polybrominated
biphenyl concentration in tissues. Toxicol Appl Pharmacol, 52:
442-453.
Kimbrough RD, Groce DF, Korver MP, & Burse VM (1981) Induction of
liver tumors in female Sherman strain rats by polybrominated
biphenyls. J Natl Cancer Inst, 66: 535-542.
Kluwe WM & Hook JB (1981) Comparative induction of xenobiotic
metabolism in rodent kidney, testis and liver by commercial mixtures
of polybrominated biphenyls and polychlorinated biphenyls,
phenobarbital and 3-methylcholanthrene: absolute and temporal
effects. Toxicology, 20: 259-273.
Kluwe WM, McCormack KM, & Hook JB (1978) Potention of hepatic and
renal toxicity of various compounds by prior exposure to
polybrominated biphenyls. Environ Health Perspect, 23: 241-246.
Kluwe WM, Hermann CL, & Hook JB (1979) Effects of dietary
polychlorinated biphenyls and polybrominated biphenyls on the renal
and hepatic toxicities of several chlorinated hydrocarbon solvents
in mice. J Toxicol Environ Health, 5: 605-615.
Kluwe WM, McNish R, Smithson K, & Hook JB (1981) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane, tris
(2,3-dibromopropyl) phosphate and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and nontarget organs.
Biochem Pharmacol, 30: 2265-2272.
Kluwe WM, Hook JB, & Bernstein J (1982) Synergistic toxicity of
carbon tetrachloride and several aromatic organohalide compounds.
Toxicology, 23: 321-336.
Kohli J & Safe S (1976) The metabolism of brominated aromatic
compounds. Chemosphere, 6: 433-437.
Kohli J, Wyndham C, Smylie M, & Safe S (1978) Metabolism of
bromobiphenyls. Biochem Pharmacol, 27: 1245-1249.
Kohli KK, Gupta BN, Albro PW, & McKinney JD (1981) Effects of
inducers of drug metabolism enzymes on triglyceride and phospholipid
biosynthesis. Chem-Biol Interact, 36: 117-121.
Kong H-L & Sayler GS (1983) Degradation and total mineralization of
monohalogenated biphenyls in natural sediment and mixed bacterial
culture. Appl Environ Microbiol, 46: 666-672.
Koster P, Debets FMH, & Strik JJTWA (1980) Porphyrinogenic action of
fire retardants. Bull Environ Contam Toxicol, 25: 313-315.
Kraus AL & Bernstein IA (1986) Influence of adipocyte triglyceride
on the partition of 2,2',4,4',5,5'-hexabromobiphenyl between 3T3L1
adipocytes and surrounding pseudoblood. J Toxicol Environ Health,
19: 541-554.
Kraus AL & Bernstein IA (1989) Human lipoprotein influence on the
partition of 2,2',4,4',5,5'-hexabromobiphenyl between 3T3L1
adipocytes and culture medium. J Toxicol Environ Health, 26:
157-174.
Kreis RG & Rice CP (1985) Status of organic contaminants in Lake
Huron: atmosphere, water, algae, fish, herring gull eggs, and
sediment. Chicago, Illinois, US Environmental Protection Agency,
Great Lakes National Program Office (Special Report No. 114).
Kreiss K, Roberts C, & Humphrey HEB (1982) Serial PBB levels, and
clinical chemistries in Michigan's PBB cohort. Arch Environ Health,
37: 141-147.
Krüger C (1988) [Polybrominated biphenyls and polybrominated
biphenyl ether-detection and quantitation in selected foods.]
Münster, University of Münster (Thesis) (in German).
Krüger C, Fürst P, & Gröbel W (1988) [Detection and determination of
polybrominated biphenyls in human milk.] Dtsch Lebensm Rundsch, 84:
273-276 (in German).
Ku PK, Hogberg MG, Trapp AL, Brady PS, & Miller ER (1978)
Polybrominated biphenyl (PBB in the growing pig diet). Environ
Health Perspect, 23: 13-18.
Kubiczak GA, Oesch F, Borlakoglu JT, Kunz H, & Robertson LW (1989) A
unique approach to the synthesis of 2,3,4,5,-substituted
polybrominated biphenyls: quantitation in FireMaster FF-1 and
FireMaster BP-6. J Agric Food Chem, 37: 1160-1164.
Kuehl DW, Haebler R, & Potter C (1991) Chemical residues in dolphins
from the U.S. Atlantic coast including atlantic bottlenose obtained
during the 1987/88 mass mortality. Chemosphere, 22: 1071-1084.
Kuo CH & Hook JB (1982) Effects of drug-metabolizing enzyme inducers
on cephaloridine toxicity in Fischer 344 rats. Toxicology, 24:
293-303.
Lai DY (1984) Halogenated benzenes, naphthalenes, biphenyls and
terphenyls in the environment: their carcinogenic, mutagenic and
teratogenic potential and toxic effects. J Environ Sci Health, C2:
135-184.
Lambert GH, Humphrey H, & Schoeller D (1987) The effects of
polybrominated biphenyls (PBB) on the human cytochrome P-450 system
(P-450). Pedriatr Res, 21: 258 (Abstract).
Lambert GH, Schoeller DA, Humphrey HEB, Kotake AN, Lietz H, Campbell
M, Kalow W, Spielberg SP, & Budd M (1990) The caffeine breath test
and caffeine urinary metabolite ratios in the Michigan cohort
exposed to polybrominated biphenyls. A preliminary study. Environ
Health Perspect, 89: 175-181.
Lambert GH, Schoeller DA, Lietz HW, Doyle CM, & Humphrey H (1991)
Effects of polybrominated biphenyls (PBBs) on the maturation of
hepatic cytochrome P450IA2 activity during puberty. Pediatr Res, 29:
62A.
Lambrecht LK, Barsotti DA, & Allen JR (1978) Response of nonhuman
primates to a polybrominated biphenyl mixture. Environ Health
Perspect, 23: 139-145.
Landrigan PJ (1980) General population exposure to environmental
concentrations of halogenated biphenyls. In: Kimbrough RD ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and
related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 267-286.
Landrigan PJ, Wilcox KRJ, Silva JJ, Humphrey HEB, Kauffman C, &
Heath CW (1979) Cohort study of Michigan residents exposed to
polybrominated biphenyls: epidemiologic and immunologic findings.
Ann NY Acad Sci, 320: 284-294.
Law FCP & Addison RF (1981) Response of trout hepatic mixed-function
oxidases to experimental feeding of 10 known or possible chlorinated
environmental contaminants. Bull Environ Contam Toxicol, 27:
605-609.
Lee KP, Herbert RR, Sherman H, Aftosmis JG, & Waritz RS (1975a)
Bromine tissue residue and hepatotoxic effects of octabromobiphenyl
in rats. Toxicol Appl Pharmacol, 34: 115-127.
Lee KP, Herbert RR, Sherman H, Aftosmis JG, & Waritz RS (1975b)
Octabromobiphenyl-induced ultrastructural changes in rat liver. Arch
Environ Health, 30: 465-471.
Leland HV, Bruce WN, & Shimp NF (1973) Chlorinated hydrocarbon
insecticides in sediments of southern lake Michigan. Environ Sci
Technol, 7: 833-838.
Lewis RG & Sovocool GW (1982) Identification of polybrominated
biphenyls in the adipose tissues of the general population of the
USA. J Anal Toxicol, 6: 196-198.
Lilis R, Anderson HA, Valciukas JA, Freedman S, & Selikoff IJ (1978)
Comparison of findings among residents on Michigan dairy farms and
consumers of produce purchased from these farms. Environ Health
Perspect, 23: 105-109.
Lillie RJ, Cecil HC, Bitman J, Fries GF, & Verrett J (1975) Toxicity
of certain polychlorinated and polybrominated biphenyls on
reproductive efficiency of caged chickens. Poult Sci, 54: 1550-1555.
Lipson SM (1987) Effect of polybrominated biphenyls on the growth
and maturation of human peripheral blood lymphocytes. Clin Immunol
Immunophathol, 43: 65-72.
Loose LD, Mudzinski SP, & Silkworth JB (1981) Influence of dietary
polybrominated biphenyl on antibody and host defense responses in
mice. Toxicol Appl Pharmacol, 59: 25-39.
Lorenz H (1983) [PCB-contents in different matrixes, their behavior
in the food chain and possibilities of reducing the contents.] In:
Lorenz H & Neumeier G ed. [Polychlorinated biphenyls (PCB).] Münich,
MMV Medizin Verlag, pp 81-91 (BGA Report No. 4/83) (in German).
Lorenz H & Neumeier G ed. (1983) [Polychlorinated biphenyls (PCB).]
Münich, MMV Medizin Verlag (BGA Report No. 4/83) (in German).
Loury DJ, Goldsworthy TL, & Butterworth BE (1987) The value of
measuring cell replication as a predictive index of tissue-specific
tumorigenic potential. In: Nongenotoxic mechanisms in
carcinogenesis. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 119-136 (Banbury Report No. 25).
Lubet RA, Nims RW, Ward JM, Rice JM, & Diwan BA (1989) Induction of
cytochrome P450b and its relationship to liver tumor promotion. J Am
Coll Toxicol, 8: 259-268.
Lubet RA, Guengerich FP, & Nims RW (1990) The induction of
alkoxyresorufin metabolism: a potential indicator of environmental
contamination. Arch Environ Contam Toxicol, 19: 157-163.
Lucier GW, Davis GJ, & McLachlan JA (1978) Transplacental toxicology
of the polychlorinated and polybrominated biphenyls. In:
Developmental toxicology of energy related pollutants. Proceedings
of the 17th Annual Hanford Biology Symposium, Richland, 17-19
October 1977. Washington, DC, US Department of Energy, Technical
Information Center, pp 188-203.
Luster MI, Faith RE, & Moore JA (1978) Effects of polybrominated
biphenyls (PBB) on immune response in rodents. Environ Health
Perspect, 23: 227-232.
Luster MI, Boorman GA, Harris MW, & Moore JA (1980) Laboratory
studies on polybrominated biphenyl-induced immune alterations
following low-level chronic or pre/postnatal exposure. Int J
Immunopharmacol, 2: 69-80.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature chymocytes by
CA2+-mediated endonuclease activation. Science, 242: 256-259.
McConnell EE (1980) Acute and chronic toxicity, carcinogenesis,
reproduction, teratogenesis and mutagenesis in animals. In:
Kimbrough RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 109-150.
McConnell EE (1984) Clinicopathologic concepts of dibenzo-p-dioxin
intoxication. In: Poland A & Kimbrough R ed. Biological mechanism of
dioxin action. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 27-37 (Banbury Report No. 18).
McConnell EE (1985) Comparative toxicity of PCBs and related
compounds in various species of animals. Environ Health Perspect,
60: 29-33.
McConnell EE & Moore JA (1979) Toxicopathology characteristics of
the halogenated aromatics. Ann NY Acad Sci, 320: 138-150.
McConnell EE, Harris MW, & Moore JA (1980) Studies on the use of
activated charcoal and cholestyramine for reducing the body burden
of polybrominated biphenyls. Drug Chem Toxicol, 3: 277-292.
McCormack KM & Hook JB (1982) Effects of lactation and nursing on
tissue concentrations of polybrominated biphenyls and on microsomal
enzyme activity in mammary gland and liver in maternal rats. Environ
Res, 27: 110-117.
McCormack KM, Kluwe WM, Sanger VL, & Hook JB (1978a) Effects of
polybrominated biphenyls on kidney function and activity of renal
microsomal enzymes. Environ Health Perspect, 23: 153-157.
McCormack KM, Kluwe WM, Rickert DE, Sanger VL, & Hook JB (1978b)
Renal and hepatic microsomal enzyme stimulation and renal function
following three months of dietary exposure to polybrominated
biphenyls. Toxicol Appl Pharmacol, 44: 539-553.
McCormack KM, Melrose P, Rickert DE, Dent JG, Gibson JE, & Hook JB
(1979a) Concomitant dietary exposure to polychlorinated biphenyls
and polybrominated biphenyls: tissue distribution and
arylhydrocarbon hydroxylase activity in lactating rats. Toxicol Appl
Pharmacol, 47: 95-104.
McCormack KM, Cagen SZ, Rickert DE, Gibson JE, & Dent JG (1979b)
Stimulation of hepatic and renal mixed-function oxidase in
developing rats by polybrominated biphenyls. Drug Metab Dispos, 7:
252-259.
McCormack KM, Arneric SP, & Hook JB (1979c) Action of exogenously
administered steroid hormones following perinatal exposure to
polybrominated biphenyls. J Toxicol Environ Health, 5: 1085-1094.
McCormack KM, Braselton WEJR, Sanger VL, & Hook JB (1980) Residual
effects of polybrominated biphenyls following perinatal exposure in
rats. Toxicol Appl Pharmacol, 53: 108-115.
McCormack KM, Lepper LF, Wilson DM, & Hook JB (1981) Biochemical and
physiological sequelae to perinatal exposure to polybrominated
biphenyls: a multigeneration study in rats. Toxicol Appl Pharmacol,
59: 300-313.
McCormack KM, Roth RA, Wallace KB, Ross LM, & Hook JB (1982a)
Nonrespiratory metabolic function and morphology of lung following
exposure to polybrominated biphenyls in rats. J Toxicol Environ
Health, 9: 27-39. McCormack KM, Stickney JL, Bonhaus DW, & Hook JB
(1982b) Cardiac and hepatic effects of pre- and postnatal exposure
to polybrominated biphenyls in rats. J Toxicol Environ Health, 9:
13-26.
McDaniel OS & Lucier GW (1979) Perinatal tissue distribution of
persistent organohalogens. Teratology, 19: 38A.
McKinney J & McConnell E (1982) Structural specificity and the
dioxin receptor. In: Hutzinger O, Frei RW, Merian E, & Pocchiari F
ed. Chlorinated dioxins and related compounds impact on the
environment. Oxford, New York, Pergamon Press, pp 367-381.
McKinney JD & Singh P (1981) Structure-activity relationships in
halogenated biphenyls: Unifying hypothesis for structural
specificity. Chem-Biol Interact, 33: 271-284.
MacLeod KE, Hanisch RC, & Lewis RG (1982) Evaluation of gel
permeation chromatography for clean up of human adipose tissue
samples for GC/MS analysis of pesticides and other chemicals. J Anal
Toxicol, 6: 38-40.
Manis J & Kim G (1980) Polybrominated biphenyl: acute and chronic
effect on iron absorption and benzo(a)pyrene hydroxylase. Toxicol
Appl Pharmacol, 54: 41-47.
Martino LJ, Wilson-Martino NA, & Benitz KF (1981) The presence of
intranuclear lipid inclusions in hepatocytes of mice after chronic
ingestion of polybrominated biphenyl. Arch Toxicol, 47: 155-158.
Mason G, Denomme MA, Safe L, & Safe S (1987a) Polybrominated and
chlorinated dibenzo-p-dioxins: synthesis biologic and toxic effects
and structure-activity relationships. Chemosphere, 16: 1729-1731.
Mason G, Zacharewski MA, Denomme MA, Safe L, & Safe S (1987b)
Polybrominated dibenzo-p-dioxins and related compounds: quantitative
in vivo and in vitro structure-activity relationships.
Toxicology, 44: 245-255.
Matthews HB (1982) Aryl halides. In: Jakoby WB, Bend JR, & Caldwell
J ed. Metabolic basis of detoxication. New York, London, San
Francisco, Academic Press, pp 51-68.
Matthews HB, Kato S, Morales NM, & Tuey DB (1977) Distribution and
excretion of 2,4,5,2',4',5'-hexabromobiphenyl, the major component
of FireMaster BP-6. J Toxicol Environ Health, 3: 599-605.
Matthews H, Fries G, Gardner A, Garthoff L, Goldstein J, Ku Y, &
Moore J (1978) Metabolism and biochemical toxicity of PCBs and PBBs.
Environ Health Perspect, 24: 147-155.
Matthews HB, Surles JR, Carver JG, & Anderson MW (1984) Halogenated
biphenyl transport by blood components. Fundam Appl Toxicol, 4:
420-428.
Mead RC, Hart MH, & Gable W (1982) Inhibition of purified rabbit
muscle phosphorylase a and phosphorylase b by polychlorinated
biphenyls, polychlorinated biphenylols and polybrominated biphenyls.
Biochim Biophys Acta, 701: 173-179.
Meester WD & McCoy DJ (1976) Human toxicology of polybrominated
biphenyls. In: Management of the poisoned patient. Princeton, New
Jersey, Science Press, pp 32-61.
Mercer HD, Teske RH, Condon RJ, Furr A, Meerdink G, Buck W, & Fries
G (1976) Herd health status of animals exposed to polybrominated
biphenyls. J Toxicol Environ Health, 2: 335-349.
Mercer HD, Willett LB, Schandbacher FL, Moorhead PD, & Powers TE
(1978) Use of the double-isotope, single-injection method for
estimating renal function in normal and polybrominated
biphenyl-exposed dairy cows. Am J Vet Res, 39: 1262-1268.
Miceli JN & Marks BH (1981) Tissue distribution and elimination
kinetics of polybrominated biphenyls from rat tissue. Toxicol Lett,
9: 315-320.
Miceli JN, Nolan DC, Marks B, & Hariharan M (1985) Persistence of
polybrominated biphenyls (PBB) in human post-mortem tissue. Environ
Health Perspect, 60: 399-403.
Miller CP & Birnbaum LS (1986) Teratologic evaluation of
hexabrominated naphthalenes in C57BL/6N mice. Fundam Appl Toxicol,
7: 398-405.
Miller FD, Brilliant LB, & Copeland R (1984) Polybrominated
biphenyls in lactating Michigan women: persistence in the
population. Bull Environ Contam Toxicol, 32: 125-133.
Miller CP, Harris MW, & Birnbaum LS (1985) Teratogenicity of
hexabrominated naphthalene, a toxic contaminant of polybrominated
biphenyls, in C57B1/6N mice. Toxicologist, 5: 122.
Millis CD & Aust SD (1985) Characterization of the photolysis of
2,4,5,2'4',5'-hexabromobiphenyl. Fundam Appl Toxicol, 5: 546-554.
Millis CD, Mills R, Sleight SD, & Aust SD (1985a) Photolysis
products of 2,4,5,2',4',5'-hexabromobiphenyl: hepatic microsomal
enzyme induction and toxicity in Sprague-Dawley rats. Fundam Appl
Toxicol, 5: 555-567.
Millis CD, Mills RA, Sleight SD, & Aust SD (1985b) Toxicity of
3,4,5,3',4',5'-hexabrominated biphenyl and 3,4,3',4'-tetrabrominated
biphenyl. Toxicol Appl Pharmacol, 78: 88-95.
Millischer R, Girault F, Heywood R, Clarke G, Hossack D, & Clair M
(1979) Décabromobiphényle: Etude toxicologique. Toxicol Eur Res, 2:
155-161.
Mills RA, Millis CD, Dannan GA, Guengerich FP, & Aust SD (1985)
Studies on the structure-activity relationships for the metabolism
of polybrominated biphenyls by rat liver microsomes. Toxicol Appl
Pharmacol, 78: 96-104.
Minyard JP Jr, Roberts WE, & Cobb WY (1989) State programs for
pesticide residues in foods. J Assoc Off Anal Chem, 72: 525-533.
Mirsalis JC & Steinmetz KL (1990) The role of hyperplasia in liver
carcinogenesis. In: Mouse liver carcinogenesis: mechanisms and
species comparisons. New York, Alan R. Liss, Inc., pp 149-161.
Mirsalis JC, Tyson CK, Loh EN, Steinmetz KL, Bakke JP, Hamilton CM,
Spak DK, & Spalding JW (1985) Induction of hepatic cell
proliferation and unscheduled DNA synthesis in mouse hepatocytes
following in vivo treatment. Carcinogenesis, 6: 1521-1524.
Mirsalis JC, Tyson CK, Steinmetz KL, Loh EK, Hamilton CM, Bakke JP,
& Spalding JW (1989) Measurement of unscheduled DNA synthesis and
s-phase synthesis in rodent hepatocytes following in vivo
treatment: testing of 24 compounds. Environ Mol Mutagen, 14:
155-164.
Momma J (1986) [Studies on the carcinogenicity and chronic toxicity
of nonabromobiphenyl (NBB) in mice in comparison with those of
polychlorinated biphenyl (PCB).] Jpn Pharmacol Ther, 14: 11-33 (in
Japanese).
Moore RW & Aust SD (1978) Purification and structural
characterization of polybrominated biphenyl congeners. Biochem
Biophys Res Commun, 84: 936-942.
Moore RW, Dannan GA, & Aust SD (1978a) Induction of drug
metabolizing enzymes in polybrominated biphenyl-fed lactating rats
and their pups. Environ Health Perspect, 23: 159-165.
Moore RW, Sleight SD, & Aust SD (1978b) Induction of liver
microsomal drug-metabolizing enzymes by
2,2',4,4',5,5'-hexabromobiphenyl. Toxicol Appl Pharmacol, 44:
309-321.
Moore RW, O'Connor JV, & Aust SD (1978c) Identification of a major
component of polybrominated biphenyls as
2,2'3,4,4',5,5'-heptabromobiphenyl. Bull Environ Contam Toxicol, 20:
478-483.
Moore RW, Sleight SD, & Aust SD (1979a) Effects of
2,2'-dibromobiphenyl and 2,2',3,4,4',5,5'-heptabromobiphenyl on
liver microsomal drug metabolizing enzymes. Toxicol Appl Pharmacol,
48: 73-86.
Moore JA, McConnell EE, Dalgard DW, & Harris MW (1979b) Comparative
toxicity of three halogenated dibenzofurans in guinea pigs, mice,
and rhesus monkeys. Ann NY Acad Sci, 320: 151-163.
Moore RW, Dannan GA, & Aust SD (1980) Structure-function
relationships for the pharmacological and toxicological effects and
metabolism of polybrominated biphenyl congeners. In: Bhatnagar RS
ed. Molecular basis of environmental toxicity. Ann Arbor, Michigan,
Ann Arbor Science Publishers, Inc., pp 173-212.
Moorhead PD, Willett LB, Brumm CJ, & Mercer HD (1977) Pathology of
experimentally induced polybrominated biphenyl toxicosis in pregnant
heifers. J Am Vet Med Assoc, 170: 307-313.
Moorhead PD, Willett LB, & Schanbacher FL (1978) Effects of PBB on
cattle. II. Gross pathology and histopathology. Environ Health
Perspect, 23: 111-118.
Mrozek E Jr, Seneca ED, & Hobbs LL (1982) Polychlorinated biphenyl
uptake and translation by Spartina alterniflora loisel. Water Air
Soil Pollut, 17: 3-15.
Mudzinski S, Silkworth JB, Wilson NM, & Loose LD (1979) Influence of
polybrominated biphenyl on immunological and host defense
parameters. Toxicol Appl Pharmacol, 49: A87.
Mulligan KJ, Caruso JA, & Fricke FL (1980) Determination of
polybrominated biphenyl and related compounds by gas-liquid
chromatography with a plasma emission detector. Analyst, 105:
1060-1067.
Mumma CE & Wallace DD (1975) Survey of industrial processing data.
Task II: Pollution potential of polybrominated biphenyls.
Washington, DC, US Environmental Protection Agency
(EPA-560/3-75-004).
Munslow WD, Donnelly JR, Mitchum RK, & Sovocool GW (1989) Synthesis
of polyhalogenated dibenzo-p-dioxins. Chemosphere, 18: 225-233.
Murata T, Zabik ME, & Zabik M (1977) Polybrominated biphenyls in raw
milk and processed dairy products. J Dairy Sci, 60: 516-520.
Myrick JE, Robinson MK, Hubert IL, Smith SJ, & Hannon WH (1987)
Effects of polybrominated biphenyls upon rat urinary protein
patterns as detected by two-dimensional electrophoresis. Arch
Environ Contam Toxicol, 16: 579-585.
Nagayama J, Kuratsune M, & Masuda Y (1976) Determination of
chlorinated dibenzofurants in kanechlors and "Yusho oil". Bull
Environ Contam Toxicol, 15: 9.
Nebert DW, Negishi M, Lang MA, Hjelmeland LM, & Eisen HJ (1982) The
Ah locus, a multigene family necessary for survival in a
chemically adverse environment: comparison with immune system. Adv
Genet, 21: 1-52.
Nebert DM, Elashoff JD, & Wilcox KR (1983) Possible effect of
neonatal polybrominated biphenyl exposure on the developmental
abilities of children. Am J Public Health, 73: 286-289.
Nebert DW, Peterson DD, & Fornace AJ Jr (1990) Cellular responses to
oxidative stress: the [Ah] gene battery as a paradigm. Environ
Health Perspect, 88: 13-25.
Needham LL, Burse YW, & Price HA (1981) Temperature-programmed gas
chromatographic determination of polychlorinated and polybrominated
biphenyls in serum. J Assoc Off Anal Chem, 64: 1131-1137.
Needham LL, Hill RHJR, Orti DL, Patterson DG, Kimbrough RD, Groce
DF, & Liddle JA (1982) Investigation of hyperkeratotic activity of
polybrominated biphenyls in FireMaster FF-1. J Toxicol Environ
Health, 9: 877-888.
Neufeld ML, Sittenfield M, & Wolk KF (1977) Market input/output
studies. Task IV: Polybrominated biphenyls. Washington, DC, US
Environmental Protection Agency (EPA-560/6-77-017).
Newton JF, Braselton WE Jr, Lepper LF, McCormack KM, & Hook JB
(1980) Effects of polybrominated biphenyls (PBBs) on the microsomal
metabolism of testosterone. Pharmacologist, 22: 173.
Newton JF, Braselton WE Jr, Lepper LF, McCormack KM, & Hook JB
(1982a) Effects of polybrominated biphenyls on metabolism of
testosterone by rat hepatic microsomes. Toxicol Appl Pharmacol, 63:
142-149.
Newton JF, Kou C-H, Gemborys MW, Mudge GH, & Hook JB (1982b)
Nephrotoxicity of p-aminophenol, a metabolite of acetaminophen, in
the Fischer 344 rat. Toxicol Appl Pharmacol, 65: 336-344.
Newton JF, Braselton WE Jr, Kuo C-H, Kluwe WM, Gemborys MW, Mudge
GH, & Hook JB (1982c) Metabolism of acetaminophen by the isolated
perfused kidney. J Pharmacol Exp Ther, 221: 76-79.
Norris JM, Ehrmantraut JW, Gibbson CL, Kociba RJ, Schwetz BA, Rose
JQ, Humiston CG, Jewett GL, Crummett WB, Gehring PJ, Tirsell JB, &
Brosier JS (1973) Toxicological and environmental factors involved
in the selection of decarbromodiphenyl oxide as a fire retardant
chemical. Appl Polym Symp, 22: 195-219.
Norris JM, Kociba RJ, Schwetz BA, Rose JQ, Humiston CG, Jewett GL,
Gehring PJ, & Mailhes JB (1975) Toxicology of octabromobiphenyl and
decabromodiphenyl oxide. Environ Health Perspect, 11: 153-161.
Norström A, Anderson K, & Rappe C (1976) Major components of some
brominated aromatics used as flame retardants. Chemosphere, 4:
255-261.
NTP (1993) Toxicology and carcinogenesis studies of polybrominated
biphenyls (FireMaster FF-1(R)) (CAS No. 67774-32-7) in F344/N rats
and B6C3F1 mice (Feed studies). Research Triangle Park, North
Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP TR 398; NIH Publication No. 92-2853).
O'Keefe PW (1978) Formation of brominated dibenzofurans from
pyrolysis of the polybrominated biphenyl fire retardant, FireMaster
FF-1. Environ Health Perspect, 23: 347-350.
O'Keefe PW (1979) Trace contaminants in a polybrominated biphenyl
fire retardant and a search for these compounds in environmental
samples. Bull Environ Contam Toxicol, 22: 420-425.
Orti DL, Hill RH Jr, Patterson DG, Needham LL, Kimbrough RD, Alley
CC, & Lee HCJ (1983) Structure elucidation of some minor components
of the polybromobiphenyl mixture, FireMaster. Arch Environ Contam
Toxicol, 12: 603-614.
Parkinson A & Safe S (1981) Aryl hydrocarbon hydroxylase induction
and its relationship to the toxicity of halogenated aryl
hydrocarbons. Toxicol Environ Chem Rev, 4: 1-46.
Parkinson A & Safe S (1982) Cytochrome P-450-mediated metabolism of
biphenyl and the 4-halobiphenyls. Biochem Pharmacol, 31: 1849-1856.
Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, &
Levin W (1983) Immunochemical quantitation of cytochrome P-450
isozymes and epoxide hydrolase in liver microsomes from
polychlorinated or polybrominated biphenyl-treated rats. A study of
structure-activity relationships. J Biol Chem, 258: 5967-5976.
Patil GS (1991) Correlation of aqueous solubility and octanol-water
partition coefficient based on molecular structure. Chemosphere, 22:
723-738.
Patterson DG, Yert LW, Orti DL, & Holler JS (1980) Sunlight
photodegradation of purified polybrominated biphenyl (PBB) congeners
studied by GCMS. Paper presented at the 28th Annual Conference on
Mass Spectrometry and Allied Topics, New York, 25-30 May 1980. Salt
Lake City, Utah, American Society for Mass Spectrometry.
Patterson DG, Hill RH, Needham LL, Orti DL, Kimbrough RD, & Liddle
JA (1981) Hyperkeratosis induced by sunlight degradation products of
the major polybrominated biphenyl in FireMaster. Science, 213:
901-902.
Pearson CR (1982) Halogenated aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry. 3. Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 89-116.
Pitot HC, Barsness L, Goldsworthy T, & Kitagawa T (1978) Biochemical
characterization of stages of heptacarcinogenesis after a single
dose of diethylnitrosamine. Nature (Lond), 271: 456-458.
Plaa GL & Hewitt WR (1982) Methodological approaches for interaction
studies: potentiation of haloalkane-induced hepatotoxicity. In:
Stich HF, Leung HW, & Roberts JR ed. Workshop on the Combined
Effects of Xenobiotics, Ottawa, 22-23 June 1981. Ottawa, National
Research Council of Canada, pp 67-96 (Publication NRCC No. 18978).
Poellinger L, Göttlicher M, & Gustafsson J-A (1992) The dioxin and
peroxisome proliferator-activated receptors: nuclear receptors in
search of endogenous ligands. Trends Pharmacol Sci, 13: 241-245.
Poland A & Glover E (1977) Chlorinated biphenyl induction of aryl
hydrocarbon hydroxylase activity: a study of the structure-activity
relationship. Mol Pharmacol, 13: 924-938.
Poland A & Glover E (1980) 2,3,7,8-Tetrachlorodibenzo-p-dioxin:
segregation of toxicity with the Ah locus. Mol Pharmacol, 17: 86-94.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p-dioxin and
related halogenated aromatic hydrocarbons: examination of the
mechanism of toxicity. Ann Res Toxicol, 22: 517-554.
Poland A, Greenlee WF, & Kende AS (1979) Studies on the mechanism of
action of the chlorinated dibenzo-p-dioxins and related compounds.
Ann NY Acad Sci, 320: 214-230.
Poland A, Palen D, & Glover E (1982) Tumor promotion by TCDD in skin
of HRS/J hairless mice. Nature (Lond), 300: 271-273.
Polin D & Leavitt RA (1984) Colestipol and energy restriction as an
approach to hasten removal of PBBs from chickens. J Toxicol Environ
Health, 13: 659-671.
Polin D & Ringer RK (1978a) PBB fed to adult female chickens: its
effect on egg production, reproduction, viability of offspring, and
residues in tissues and eggs. Environ Health Perspect, 23: 283-290.
Polin D & Ringer RK (1978b) Polybrominated biphenyls in chicken eggs
vs. hatchability. Proc Soc Biol Med, 159: 131-135.
Polin D, Ringer RK, & Aust SD (1979) Effect of congeners of
polybrominated biphenyls on hatchability of chicken eggs. I.
2,2',4,4',5,5'-hexabromobiphenyl vs. PBB. Proc Soc Exp Biol Med,
161: 44-47.
Polin D, Bursian S, & Shull L (1982) Microsomal enzyme induction and
reproduction in three lines of Japanese quail fed polybrominated
biphenyls. Toxicologist, 2: 40.
Polin D, Lehning E, Pullen D, Bursian S, & Leavitt R (1985)
Procedures to enhance withdrawal of xenobiotics from chickens. J
Toxicol Environ Health, 16: 243-254.
Pomerantz I, Burke J, Firestone D, McKinney J, Roach J, & Trotter W
(1978) Chemistry of PCBs and PBBs. Environ Health Perspect, 24:
133-146.
Preache MM, Cagen SZ, & Gibson JE (1976) Perinatal toxicity in mice
following maternal dietary exposure to polybrominated biphenyls.
Toxicol Appl Pharmacol, 37: 171.
Purdy R & Safe S (1980) The in vitro metabolism of
2,2'4,4',5,5'-hexabromobiphenyl. J Environ Pathol Toxicol, 4:
277-284.
Quensen JF III, Morris PJ, & Boyd SA (1990) Dehalogenation of PCBs
and PBBs by anaerobic microorganisms from sediments. In: Abstracts
of the 90th Annual Meeting of the American Society for Microbiology,
Anaheim, California, 13-17 May 1990. Washington, DC, American
Society for Microbiology, p 295.
Raber BT & Carter JW (1986) Localization of ultrastructural
alterations induced in rat liver by dietary polybromobiphenyls
(FireMaster BP-6). Arch Environ Contam Toxicol, 15: 725-732.
Reggiani G & Bruppacher R (1985) Symptoms, signs and findings in
humans exposed to PCBs and their derivatives. Environ Health
Perspect, 60: 225-232.
Reichardt PB, Chadwick BL, Cole MA, Robertson BR, & Button DK (1981)
Kinetic study of the biodegradation of biphenyl and its
monochlorinated analogues by a mixed marine microbial community.
Environ Sci Technol, 15: 75-79.
Render JA, Aust SD, & Sleight SD (1982) Acute pathologic effects of
3,3',4,4',5,5'-hexabromobiphenyl in rats: comparison of its effects
with FireMaster BP-6 and 2,2',4,4',5,5'-hexabromobiphenyl. Toxicol
Appl Pharmacol, 62: 428-444.
Rezabek MS, Sleight SD, Jensen RK, Aust SD, & Dixon D (1987)
Short-term oral administration of polybrominated biphenyls enhances
the development of hepatic enzyme-altered foci in initiated rats. J
Toxicol Environ Health, 20: 347-356.
Rezabek MS, Trosko JE, Jone C, & Sleight SD (1988) Effects of
hepatic tumor promoters phenobarbital and polybrominated biphenyls
on intercellular communication between rat liver epithelial cells.
In Vitro Toxicol, 2: 45-58.
Rezabek MS, Sleight SD, Jensen RK, & Aust SD (1989) Effects of
dietary retinyl acetate on the promotion of hepatic enzyme-altered
foci by polybrominated biphenyls in initiated rats. Food Chem
Toxicol, 27: 539-544.
Rickert DE, Dent JG, Cagen SZ, McCormack KM, Melrose P, & Gibson JE
(1978) Distribution of polybrominated biphenyls after dietary
exposure in pregnant and lactating rats and their offspring. Environ
Health Perspect, 23: 63-66.
Ringer RK (1978) PBB fed to immature chickens: its effect on organ
weights and function and on the cardiovascular system. Environ
Health Perspect, 23: 247-255.
Ringer RK & Polin D (1977) The biological effects of polybrominated
biphenyls in avian species. Fed Proc, 36: 1894-1898.
Ringer RK, Aulerich RJ, & Bleavins MR (1981) Biological effects of
PCBs and PBBs on mink and ferrets - a review. In: Khan MAQ & Stanton
RH ed. Toxicology of halogenated hydrocarbons: Health and ecological
effects. Oxford, New York, Pergamon Press, p 329.
Robertson LW & Chynoweth DP (1975) Another halogenated hydrocarbon.
Environment, 17: 25-27.
Robertson LW, Parkinson A, & Safe S (1980) Induction of both
cytochromes P-450 and P-448 by 2,3',4,4',5-pentabromobiphenyl, a
component of FireMaster. Biochem Biophys Res Commun, 92: 175-182.
Robertson LW, Parkinson A, Bandiera S, & Safe S (1981a) Potent
induction of rat liver microsomal, drug-metabolizing enzymes by
2,3,3',4,4',5-hexabromobiphenyl, a component of FireMaster.
Chem-Biol Interact, 35: 13-24.
Robertson LW, Parkinson A, Chittim B, Bandiera S, Sawyer TW, & Safe
S (1981b) Aryl hydrocarbon hydroxylase (AHH) induction by
polybrominated biphenyls (PBBs): enhancement by photolysis.
Toxicology, 22: 103-114.
Robertson LW, Parkinson A, Bandiera S, Chittim R, & Safe S (1981c)
Enhanced biologic potency of photolyzed PBBs. Toxicologist, 1: 126.
Robertson LW, Parkinson A, Campbell MA, & Safe S (1982a)
Polybrominated biphenyls as aryl hydrocarbon hydroxylase inducers:
structure-activity correlations. Chem-Biol Interact, 42: 53-66.
Robertson LW, Parkinson A, & Safe S (1982b) Effects of structure on
the activity of polybrominated biphenyls as microsomal enzyme
inducers. 73rd Annual Meeting of the American Oil Chemists' Society,
Toronto, Ontario, Canada, May 2-6. J Am Oil Chem Soc, 59: 286A.
Robertson LW, Chittim B, Safe SH, Mullin MD, & Pochini CM (1983a)
Photodecomposition of a commercial polybrominated biphenyl fire
retardant: high resolution gas chromatographic analysis. J Agric
Food Chem, 31: 454-457.
Robertson LW, Andres JL, Safe SH, & Lovering SL (1983b) Toxicity of
3,3',4,4'- and 2,2',5,5'-tetrabromobiphenyl: correlation of activity
with aryl hydrocarbon hydroxylase induction and lack of protection
by antioxidants. J Toxicol Environ Health, 11: 81-91.
Robertson LW, Thompson K, & Parkinson A (1984a) Synthesis,
characterization and biologic effects of polybrominated
naphthalenes. Arch Toxicol, 55: 127-131.
Robertson LW, Safe SH, Parkinson A, Pellizzari E, Pochini C, &
Mullin MD (1984b) Synthesis and identification of highly toxic
polybrominated biphenyls in the fire retardant FireMaster BP-6. J
Agric Food Chem, 32: 1107-1111.
Robertson LW, Parkinson A, Bandiera S, Lambert I, Merrill J, & Safe
SH (1984c) PCBs and PBBs: biologic and toxic effects on C57BL/6J and
DBA/J inbred mice. Toxicology, 31: 191-206.
Robl MG, Jenkins DH, Wingender RJ, & Gordon DE (1978) Toxicity and
residue studies in dairy animals with FireMaster FF-1
(polybrominated biphenyls). Environ Health Perspect, 23: 91-97.
Roboz J, Suzuki RK, Bekesi JG, Holland JF, Roseman K, & Selikoff IJ
(1980) Mass spectrometric identification and quantification of
polybrominated biphenyls in blood compartments of exposed Michigan
chemical workers. J Environ Pathol Toxicol, 3: 363-378.
Roboz J, Greaves J, Holland JF, & Bekesi JG (1982) Determination of
polybrominated biphenyls in serum by negative chemical ionization
mass spectrometry. Anal Chem, 54: 1104-1108.
Roboz J, Greaves J, McCamish M, Holland JF, & Bekesi G (1985a) An
in vitro model for the binding of polybrominated biphenyls in
environmentally contaminated blood. Arch Environ Contam Toxicol, 14:
137-142.
Roboz J, Greaves J, & Bekesi JG (1985b) Polybrominated biphenyls in
model and environmentally contaminated human blood: protein binding
and immunotoxicological studies. Environ Health Perspect, 60:
107-113.
Roes U, Dent JG, Netter KJ, & Gibson JE (1977) Effect of
polybrominated biphenyls on bromobenzene lethality in mice. J
Toxicol Environ Health, 3: 663-671.
Rogan WJ, Bagniewska A, & Damstra T (1980) Pollutants in breast
milk. New Engl J Med, 302: 1450-1453.
Rosenblatt DH, Kainz RJ, & Fries GF (1982) Options and
recommendations for a polybromobiphenyl control strategy at the
Gratiot County, Michigan, Landfill. Frederick, Maryland, US Army
Medical Research and Development Command, Fort Detrick, 40 pp
(Technical Report No. 8204).
Rosenman KD, Anderson HA, Selikoff IJ, Wolff MS, & Holstein E (1979)
Spermatogenesis in men exposed to polybrominated biphenyl (PBB).
Fertil Steril, 32: 209-213.
Rossman TG, Molina M, Meyer L, Boone P, Klein CB, Wang Z, Li F, Lin
WC, & Kinney PL (1991) Performance of 133 compounds in the lambda
prophage induction endpoint of the microscreen assay and a
comparison with S. typhimurium mutagenicity and rodent
carcinogenicity assays. Mutat Res, 260: 349-367.
Rozman KK, Rozman TA, Williams J, & Greim HA (1982) Effect of
mineral oil and/or cholestyramine in the diet on biliary and
intestinal elimination of 2,4,5,2',4',5'-hexabromobiphenyl in the
rhesus monkey. J Toxicol Environ Health, 9: 611-618.
Rush GF, Smith JH, & Hook JB (1982) Induction of hepatic and renal
mixed function oxidases in the hamster and guinea-pig. 66th Annual
Meeting of the Federation of American Societies for Experimental
Biology, New Orleans, LA, USA, 15-23 April 1982. Fed Proc, 41(5):
1638 (Abstract 7999).
Rush GF, Pratt IS, Lock EA, & Hook JB (1986) Induction of renal
mixed function oxidases in the rat and mouse: correlation with
ultrastructural changes in the proximal tubule. Fundam Appl Toxicol,
6: 307-316.
Ruzo LO & Zabik MJ (1975) Polyhalogenated biphenyls: photolysis of
hexabromo- and hexachlorobiphenyls in methanol solution. Bull
Environ Contam Toxicol, 13: 181-182.
Ruzo LO, Sundstrom G, Hutzinger O, & Safe S (1976) Photodegradation
of polybromobiphenyls (PBB). J Agric Food Chem, 24: 1062-1065.
Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs): biochemistry, toxicology, and mechanism of action.
CRC Crit Rev Toxicol, 13: 319-395.
Safe S (1987) Determination of 2,3,7,8,-TCDD toxic equivalent
factors (TEFs): support for the use of the in vitro AHH induction
assay. Chemosphere, 16: 791-802.
Safe S (1989) Polyhalogenated aromatics: uptake, disposition and
metabolism. In: Kimbrough RD & Jensen AA ed. Halogenated biphenyls,
terphenyls, naphthalenes, dibenzodioxins and related products.
Amsterdam, Oxford, New York, Elsevier/North-Holland Biomedical
Press, pp 131-159.
Safe S, Jones D, & Hutzinger O (1976) Metabolism of
4,4'-dihalogenobiphenyls. J Chem Soc Perkin Trans, I/4: 357-359.
Safe S, Kohli J, & Crawford A (1978) FireMaster BP-6: fractionation,
metabolic and enzyme induction studies. Environ Health Perspect, 23:
147-152.
Safe S, Wyndham C, Parkinson A, Purdy R, & Crawford A (1980)
Halogenated biphenyl metabolism. In: Afghan BK & Mackay D ed.
Hydrocarbons and halogenated hydrocarbons in the aquatic
environment. New York, London, Plenum Press, pp 537-544.
Safe S, Robertson L, Parkinson A, Shilling M, Cockerline R, &
Campbell MA (1981) Polybrominated biphenyls, polychlorinated
naphthalenes and polychlorinated terphenyls as microsomal enzyme
inducers. In: Khan MAQ & Stanton RH ed. Toxicology of halogenated
hydrocarbons: Health and ecological effects. Oxford, New York,
Pergamon Press, pp 97-105.
Safe S, Robertson LW, Safe L, Parkinson A, Bandiera T, Sawyer T, &
Campbell MA (1982) Halogenated biphenyls: molecular toxicology. Can
J Physiol Pharmacol, 60: 1057-1064.
Safe S, Bandiera S, Sawyer T, Zmudzka B, Mason G, Romkes M, Denomme
MA, Sparling J, Okey AB, & Fujita T (1985) Effects of structure on
binding to the 2,3,7,8-TCDD receptor protein and AHH
induction-halogenated biphenyls. Environ Health Perspect, 61: 21-33.
Safe S, Harris M, Biegel L, & Zacharewski T (1991) Mechanism of
action of TCDD as an antiestrogen in transformed human breast cancer
and rodent cell lines. In: Gallo MA, Scheuplein RJ, & van der
Heijden KE ed. Biological basis for risk assessment of dioxins and
related compounds. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 367-377 (Banbury Report No. 35).
Sax NI (1979) Dangerous properties of industrial materials, 5th ed.
New York, Van Nostrand Reinhold Company.
Schäfer W & Ballschmiter K (1986) Monobromo-polychloro-derivatives
of benzene, biphenyl, dibenzofurane and dibenzodioxine formed in
chemical-waste burning. Chemosphere, 15: 755-763.
Schanbacher FL, Willett LB, Moorhead PD, & Mercer HD (1978) Effects
of PBBs on cattle. III. Target organ modification as shown by renal
function and liver biochemistry. Environ Health Perspect, 23:
119-127.
Schanbacher FL, Willett LB, Moorhead PD, & Durst HI (1987) Serum
isocitrate dehydrogenase during polybrominated biphenyl toxicosis in
dairy cattle. J Anim Sci, 64: 467-473.
Schnare DW, Ben M, & Shields MG (1984) Body burden reductions of
polychlorinated biphenyls, polybrominated biphenyls and chlorinated
pesticides in human subjects. Ambio, 13: 378-380.
Schramm H, Robertson LW, & Oesch F (1985) Differential regulation of
hepatic glutathione transferase and glutathione peroxidase
activities in the rat. Biochem Pharmacol, 34: 3735-3739.
Schwartz EM & Rae WA (1983) Effect of polybrominated biphenyls (PBB)
on developmental abilities in young children. Am J Public Health,
73: 227-281.
Schwartz EL, Kluwe WM, Sleight SD, Hook JB, & Goodman JI (1980)
Inhibition of N-2-fluorenylacetamide-induced mammary tumorigenesis
in rats by dietary polybrominated biphenyls. J Natl Cancer Inst, 64:
63-67.
Schwarzenbach R & Westall J (1981) Transport of nonpolar organic
compounds from surface water to groundwater. Laboratory sorption
studies. Environ Sci Technol, 15: 1360-1367.
Schwind KH, Hosseinpour J, & Thoma H (1988) Brominated/chlorinated
dibenzo-p-dioxins and dibenzofurans. Part 1: Brominated/chlorinated
and brominated dibenzo-p-dioxins and dibenzofurans in fly ash from a
municipal waste incinerator. Chemosphere, 17: 1875-1884.
Schwind KH, Hosseinpour J, & Thoma H (1989) [Brominated/chlorinated
dioxins and furans from incineration of domestic rubbish.] Z
Umweltchem Ökotox, 1: 24 (in German).
Seagull EAW (1983) Developmental abilities of children exposed to
polybrominated biphenyls (PBB). Am J Public Health, 73: 281-285.
Selikoff IJ & Anderson HA (1979) A survey of the general population
of Michigan for health effects of PBB exposure (Contract final
report). Lansing, Michigan, Michigan Department of Public Health.
Sepkovic DW & Byrne JJ (1984) Kinetic parameters of
L-[125I]triiodothyronine degradation in rats pretreated with
polyhalogenated biphenyls. Food Chem Toxicol, 22: 743-747.
Shah BP (1978) Environmental consideration for the disposal of
PBB-contaminated animals and wastes. Environ Health Perspect, 23:
27-35.
Shedlofsky SI, Hoglen NC, Rodman LE, Honchel R, Robinson FR, Swim
AT, McClain CJ, & Robertson LW (1991) 3,3',4,4'-Tetrabromobiphenyl
sensitizes rats to the hepatotoxic effects of endotoxin by a
mechanism that involves more than tumor necrosis factor. Hepatology,
14: 1201-1208.
Shepherd EC, Phillips TD, Irvin TR, Safe SH, & Robertson LW (1984)
Aflatoxin B1 metabolism in the rat: polyhalogenated biphenyl
enhanced conversion to aflatoxin M1. Xenobiotica, 14: 741-750.
Sherman JD (1991) Polybrominated biphenyl exposure and human cancer:
report of a case and public health implications. Toxicol Ind Health,
7: 197-205.
Shukla RR & Albro PW (1987) In vitro modulation of protein kinase
C activity by environmental chemical pollutants. Biochem Biophys Res
Commun, 142: 567-572.
Sidhu KS & Michelakis AM (1978) Effect of polybrominated biphenyls
on adenylate cyclase activity in rat lung alveoli. Environ Health
Perspect, 23: 329-331.
Silberhorn EM, Glauert HP, & Robertson LW (1990) Carcinogenicity of
polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol, 20:
439-496.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW
Jr, Wilcox KR Jr, Van Amburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls. J
Reticuloendothel Soc, 26: 341-347.
Simmons MS & Kotz KT (1982) Association studies of polybrominated
biphenyls in aquatic systems. Bull Environ Contam Toxicol, 29:
58-63.
Simmons MS, Bialosky DI, & Rossmann R (1980) Polychlorinated
biphenyl contamination in surficial sediments of northeastern Lake
Michigan. J Great Lakes Res, 6: 167-171.
Sipes IG, McKelvie DH, & Collins R (1979) Excretion of
hexachlorobiphenyls and 2,4,5,2',4',5'-hexabromobiphenyl in the dog.
Toxicol Appl Pharmacol, 48: A155.
Sleight S (1985) Effects of PCBs and related compounds on
hepatocarcinogenesis in rats and mice. Environ Health Perspect, 60:
35-39.
Sleight SD & Sanger VL (1976) Pathologic features of polybrominated
biphenyl toxicosis in the rat and guinea pig. J Am Vet Med Assoc,
169: 1231-1235.
Sleight SD, Mangkoewidjojo S, Akoso BT, & Sanger VL (1978)
Polybrominated biphenyl toxicosis in rats fed an iodine-deficient,
iodine-adequate, or iodine-excess diet. Environ Health Perspect, 23:
341-346.
Sleight SD, Render JA, Akoso BT, Aust SD, & Nachreiner R (1981)
Comparative toxipathology of FireMaster BP-6,
2,2',4,4',5,5'hexabromobiphenyl (HBB) and 3,3',4,4',5,5'-HBB after
10 and 30 days of dietary administration to rats. Toxicologist, 1:
12.
Sloterdijk HH (1991) Mercury and organochlorinated hydrocarbons in
surficial sediments of the St. Lawrence River (Lake St. Francis).
Water Pollut Res J Can, 26: 41-60.
Smith JH, Rush GF, & Hook JB (1986) Induction of renal and hepatic
mixed function oxidases in the hamster and guinea-pig. Toxicology,
38: 209-218.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM
(1990a) Sex-linked hepatic uropophyria and the induction of
cytochromes P450IA in rats caused by hexachlorobenzene and
polyhalogenated biphenyls. Biochem Pharmacol, 40(9): 2059-2068.
Smith AG, Francis JE, & Carthew P (1990b) Iron as a synergist for
hepatocellular carcinoma induced by polychlorinated biphenyls in
Ah-responsive C57BL/10ScSn mice. Carcinogenesis, 11: 437-444.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sovocool GW & Wilson NK (1982) Differentiation of brominated
biphenyls by carbon-13 nuclear magnetic resonance and gas
chromatography/mass spectrometry. J Org Chem, 47: 4032-4037.
Sovocool GW, Mitchum RK, & Donnelly JR (1987a) Use of the `ortho
effect' for chlorinated biphenyl and brominated biphenyl isomer
identification. Biomed Environ Mass Spectrom, 14: 579-582.
Sovocool GW, Munslow WD, Donnelly JR, & Mitchum RK (1987b)
Electrophilic bromination of dibenzofuran. Chemosphere, 16: 221-224.
Sparling J, Fung D, & Safe S (1980) Bromo- and chlorobiphenyl
metabolism: gas chromatographic mass spectrometric identification of
urinary metabolites and the effects of structure on their rates of
excretion. Biomed Mass Spectrom, 7: 13-19.
Spear PA, Higueret P, & Garcin H (1990) Increased thyroxine turnover
after 3,3',4,4',5,5'- hexabromobiphenyl injection and lack of effect
on peripheral triiodothyronine production. Can J Physiol Pharmacol,
68: 1079-1084.
Sprosty AL, Willett LB, Schanbacher FL, & Moorhead PD (1979) The
effect of polybrominated biphenyl on the metabolism and clearance of
steroids. J Dairy Sci, 62: 90.
Steele RW, Williams LW, & Beck SA (1989) Immunotoxicology. Ann
Allergy, 63: 168-174.
Strachan SD, Nelson DW, & Sommers LE (1983) Sewage sludge components
extractable with nonaqueous solvents. J Environ Qual, 12: 69-74.
Stratton CL & Whitlock SA (1979) A survey of polybrominated
biphenyls (PBBs) near sites of manufacture and use in Northwestern
New Jersey. Washington, DC, US Environmental Protection Agency (EPA
560/13-79-002).
Stratton CL, Mousa JJ, & Bursey JT (1979) Analysis for
polybrominated biphenyls (PBBs) in environmental samples.
Washington, DC, US Environmental Protection Agency (EPA
560/13-79-001).
Strik JJTWA (1973a) Toxicity of hexachlorobenzene (HCB) and
hexabromobiphenyl (HBB). Meded Fac Landbouwwet Rijksuniv Gent, 38:
709-716.
Strik JJTWA (1973b) Chemical porphyria in Japanese quail. Enzyme,
16: 211-223.
Strik JJTWA (1978) Toxicity of PBBs with special reference to
porphyrinogenic action and spectral interaction with hepatic
cytochrome P-450. Environ Health Perspect, 23: 167-175.
Strik JJTWA, Doss M, Schraa G, Robertson LW, Von Tiepermann R, &
Harmsen EGM (1979) Coproporphyrinuria and chronic hepatic porphyria
type A found in farm families from Michigan (USA) exposed to
polybrominated biphenyls (PBB). In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Oxford, New York, Elsevier-
North Holland Biomedical Press, pp 29-53.
Stross JK, Nixon RK, & Anderson MD (1979) Neuropsychiatric findings
in patients exposed to polybrominated biphenyls. Ann NY Acad Sci,
320: 368-372.
Stross JK, Smokler IA, Isbister J, & Wilcox KR (1981) Human health
effects of exposure to polybrominated biphenyls. Toxicol Appl
Pharmacol, 58: 145-150.
Suflita JM, Horowitz A, Shelton D, & Tiedje JM (1982)
Dehalogenation: a novel pathway for the anaerobic biodegradation of
haloaromatic compounds. Science, 218: 1115-1117.
Sugiura K (1992) Microbial degradation of polychlorinated biphenyls
in aquatic environments. Chemosphere, 24: 881-890.
Sugiura K, Ito N, Matsumoto N, Mihara Y, Murata K, Tsukakoshi Y, &
Goto M (1978) Accumulation of polychlorinated biphenyls and
polybrominated biphenyls in fish: limitation of "correlation between
partition coefficients and accumulation factors". Chemosphere, 9:
731-736.
Sugiura K, Washino T, Hattori M, Sato E, & Goto M (1979)
Accumulation of organochlorine compounds in fishes. Difference of
accumulation factors by fishes. Chemosphere, 6: 359-364.
Sundström G, Hutzinger 0, & Safe S (1976a) Identification of
2,2',4,4',5,5'-hexabromobiphenyl as the major component of flame
retardant FireMaster BP-6. Chemosphere, 1: 11-14.
Sundström G, Hutzinger O, Safe S, & Zitko V (1976b) The synthesis
and gas chromatographic properties of bromobiphenyls. Sci Total
Environ, 6: 15-29.
Sweetman JA & Boettner EA (1982) Analysis of polybrominated biphenyl
by gas chromatography with electron-capture detection. J Chromatogr,
236: 127-136.
Takase I, Omori T, & Minoda Y (1986) Microbial degradation products
from biphenyl- related compounds. Agric Biol Chem, 50: 681-686.
Tennant RW, Stasiewicz S, & Spalding JW (1986) Comparison of
multiple parameters of rodent carcinogenicity and in vitro genetic
toxicity. Environ Mutagen, 8: 205-227.
Tennant RW, Margolin BH, Shelby MD, Zeiger E, Haseman JK, Spalding
J, Caspary W, Resnick M, Stasiewicz S, Anderson B, & Minor R (1987)
Prediction of chemical carcinogenicity in rodents from
in vitro genetic toxicity assays. Science, 236: 933-941.
Theoharides AD & Kupfer D (1981) Effects of polyhalogenated
biphenyls on the metabolism of prostaglandin E1 and xenobiotics by
hepatic mono-oxygenases in the rat. Drug Metab Dispos, 9: 580-581.
Thoma H & Hutzinger O (1989) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemosphere, 18:
1047-1050.
Thoma H, Hauschulz G, Knorr E, & Hutzinger O (1987a) Polybrominated
dibenzofurans (PBDF) and dibenzodioxins (PBDD) from the pyrolysis of
neat brominated diphenylethers, biphenyls and plastic mixtures of
these compounds. Chemosphere, 16: 277-285.
Thoma H, Hauschulz G, & Hutzinger O (1987b) PVC-induced
chlorine-bromine exchange in the pyrolysis of polybrominated
diphenyl ethers, -biphenyls, -dibenzodioxins and dibenzofurans.
Chemosphere, 16: 297-307.
Tilson HA & Cabe PA (1978) Behavioral toxicologic effects of
polybrominated biphenyl compounds in rodents. Toxicol Appl
Pharmacol, 45: 334.
Tilson HA & Cabe PA (1979) Studies on the neurobehavioral effects of
polybrominated biphenyls in rats. Ann NY Acad Sci, 320: 325-336.
Tilson HA, Cabe PA, & Mitchell CL (1978) Behavioral and neurological
toxicity of polybrominated biphenyls in rats and mice. Environ
Health Perspect, 23: 257-263.
Tondeur Y, Hass JR, Harvan DJ, Albro PW, & McKinney JD (1984)
Determination of suspected toxic impurities in FireMaster FF-1 and
FireMaster BP-6 by high-resolution gas
chromatography-high-resolution mass spectrometry. J Agric Food Chem,
32: 406-410.
Tong C, Telang S, & Williams GM (1983) The lack of genotoxicity of
polybrominated biphenyls (PBB) in the adult liver cell culture
systems. Environ Mutagen, 5: 416.
Traber PG, Chianale J, Florence R, Kim K, Wojcik E, & Gumucio JJ
(1988a) Expression of cytochrome P450b and P450e genes in small
intestinal mucosa of rats following treatment with phenobarbital,
polyhalogenated biphenyls, and organochlorine pesticides. J Biol
Chem, 263: 9449-9455.
Traber PG, Chianale J, Florence R, Kim K, Wojcik E, & Gumucio JJ
(1988b) Expression of cytochrome P450b and P450e genes in small
intestine of rats following treatment with phenobarbital (PB),
polyhalogenated biphenyls and organochlorine pesticides. Clin Res,
36: 402A.
Trosko JE, Dawson B, & Chang CC (1981) PBB inhibits metabolic
cooperation in Chinese hamster cells in vitro: its potential as a
tumor promoter. Environ Health Perspect, 37: 179-182.
Trotter WJ (1977) Confirming low levels of hexabromobiphenyl by
gas-liquid chromatography of photolysis products. Bull Environ
Contam Toxicol, 18: 726-733.
Tsushimoto G, Trosko JE, Chang C, & Aust SD (1982) Inhibition of
metabolic cooperation in Chinese hamster V79 cells in culture by
various polybrominated biphenyl (PBB) congeners. Carcinogenesis, 3:
181-185.
Tuey DB & Matthews HB (1978) Pharmacokinetics of hexabromobiphenyl
disposition in the rat. Toxicol Appl Pharmacol, 45: 337 (Abstract
No. 275).
Tuey DB & Matthews HB (1980) Distribution and excretion of
2,2',4,4',5,5'- hexabromobiphenyl in rats and man: pharmacokinetic
model predictions. Toxicol Appl Pharmacol, 53: 420-431.
UBA (Federal Environment Agency) (1989) [State of facts.
Polybrominated dibenzodioxins (PBDD) - Polybrominated dibenzofurans
(PBDF). Second supplement: Polybrominated biphenyls.] In:
[Polybrominated dibenzodioxins and dibenzofurans (PBDD/PBDF) from
brominated flame retardants. Report of the working group on
brominated flame retardants to the Environment Minister Conference.]
Bonn, Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, pp 95-97 (in German).
Valciukas JA, Lilis R, Wolff MS, & Anderson HA (1978) Comparative
neurobehavioral study of a polybrominated biphenyl-exposed
population in Michigan and nonexposed group in Wisconsin. Environ
Health Perspect, 23: 199-210.
Valciukas JA, Lilis R, Anderson HA, Wolff MS, & Petrocci M (1979)
The neurotoxicity of polybrominated biphenyls: results of a medical
field survey. Ann NY Acad Sci, 320: 337-367.
Voorman R & Aust SD (1987) Specific binding of polyhalogenated
aromatic hydrocarbon inducers of cytochrome P-450d to the cytochrome
and inhibition of its estradiol 2- hydroxylase activity. Toxicol
Appl Pharmacol, 90: 69-78.
Vos JG & Van Genderen H (1974) Toxicological aspects of
immunosuppression. In: Deichmann WB ed. Pesticides and the
environment: A continuing controversy, 2nd ed. New York,
Intercontinental Medical Book Corp., pp 527-545.
Vrbanac JJ Jr (1984) Effects of exposure to polybrominated biphenyls
on urinary steroid metabolic profiles in man and rats as determined
by capillary GC and GC/MS/DS. Diss Abstr Int, B45(4): 1169.
Wade MH, Trosko JE, & Schindler M (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
Waldron HA (1990) The health effects of environmental chemicals. In:
Harrison RM ed. Pollution: causes, effects, and control, 2nd ed.
Cambridge, United Kingdom, Royal Society of Chemistry, pp 261-276.
Waritz RS, Aftosmis JG, Culik R, Dashiell OL, Faunce MM, Griffith
FD, Hornberger CS, Lee KP, Sherman H, & Tayfun FO (1977)
Toxicological evaluations of some brominated biphenyls. Am Ind Hyg
Assoc J, 38: 307-320.
Wasito & Sleight SD (1989) Promoting effect of polybrominated
biphenyls on tracheal papillomas in Syrian golden hamsters. J
Toxicol Environ Health, 27: 173-187.
Wastell ME, Moody DL, & Plog JF Jr (1978) Effects of polybrominated
biphenyl on milk production, reproduction, and health problems in
Holstein cows. Environ Health Perspect, 23: 99-103.
Watanabe I & Tatsukawa R (1990) Anthropogenic brominated aromatics
in the Japanese environment. In: Freij L ed. Proceedings on the
Workshop on Brominated Aromatic Flame Retardants, Skoloster, Sweden,
24-26 October 1989. Solna, Sweden, National Chemicals Inspectorate,
pp 63-71.
Webber MD, Monteith HD, & Corneau DGM (1983) Assessment of heavy
metals and PCBs at sludge application sites. J Water Pollut Control
Fed, 55: 187-195.
Weil WB, Spencer M, Benjamin D, & Seagull E (1981) The effect of
polybrominated biphenyl on infants and young children. J Pediatr,
98: 47-51.
Welsch F & Morgan KT (1985) Placental transfer and developmental
toxicity of 2,2',4,4',5,5'-hexabromobiphenyl in B6C3F1 mice. Toxicol
Appl Pharmacol, 81: 431-442.
Werner PR & Sleight SD (1981) Toxicosis in sows and their pigs
caused by feeding rations containing polybrominated biphenyls to
sows during pregnancy and lactation. Am J Vet Res, 42: 183-188.
Wertz GF & Ficsor G (1978) Cytogenetic and teratogenic test of
polybrominated biphenyls in rodents. Environ Health Perspect, 23:
129-132.
WHO (1987) In: Rantanen JH, Silano V, Tarkowski S, & Yrjänheikki E
ed. PCB's, PCDD's and PCDF's, prevention and control of accidental
and environmental exposures. Copenhagen, World Health Organization,
Regional Office for Europe.
Willett LB & Durst HI (1978) Effects of PBBs on cattle. IV.
Distribution and clearance of components of FireMaster BP-6. Environ
Health Perspect, 23: 67-74.
Willett LB & Irving HA (1976) Distribution and clearance of
polybrominated biphenyls in cows and calves. J Dairy Sci, 59:
1429-1439.
Willett LB, Brumm CJ, & Williams CL (1978) Method for extraction,
isolation and detection of free polybrominated biphenyls (PBBs) from
plasma, feces, milk, and bile using disposable glassware. J Agric
Food Chem, 26: 122-125.
Willett LB, Schanbacher FL, Durst HI, & Moorhead PD (1980) Long-term
performance and health of cows experimentally exposed to
polybrominated biphenyls. J Dairy Sci, 63: 2090-2102.
Willett LB, Durst HI, Liu T-TY, Schanbacher FL, & Moorhead PD (1982)
Performance and health of offspring of cows experimentally exposed
to polybrominated biphenyls. J Dairy Sci, 65: 81-91.
Willett LB, Liu TT, Durst HI, & Schanbacher FL (1983a) Effects of
polybrominated biphenyls on the concentration and clearance of
steroids from blood. J Anim Sci, 56: 1134-1144.
Willett LB, Schanbacher FL, & Moorhead PD (1983b) Effects of
polybrominated biphenyls on the excretion of steroids. J Anim Sci,
56: 1145-1152.
Williams GM, Tong C, & Telang S (1984) Polybrominated biphenyls are
nongenotoxic and produce an epigenetic membrane effect in cultured
liver cells. Environ Res, 34: 310-320.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutat Res, 221: 263-286.
Wolff MS & Aubrey B (1978) PBB homologs in sera of Michigan dairy
farmers and Michigan chemical workers. Environ Health Perspect, 23:
211-215.
Wolff MS & Selikoff IJ (1979) Variation of polybrominated biphenyl
homolog peaks in blood of rats following treatment with FireMaster
FF-1. Bull Environ Contam Toxicol, 21: 771-774.
Wolff MS, Aubrey B, Camper F, & Haymes N (1978) Relation of DDE and
PBB serum levels in farm residents, consumers, and Michigan chemical
corporation employees. Environ Health Perspect, 23: 177-181.
Wolff MS, Anderson HA, Camper F, Ninaido MN, Daum SM, Haymes N, &
Selikoff J (1979a) Analysis of adipose tissue and serum from PBB
(polybrominated biphenyl)- exposed workers. J Environ Pathol
Toxicol, 2: 1397-1411.
Wolff MS, Anderson HA, Rosenman KD, & Selikoff IJ (1979b)
Equilibrium of polybrominated biphenyl (PBB) residues in serum and
fat of Michigan residents. Bull Environ Contam Toxicol, 21: 775-781.
Wolff MS, Anderson HA, Irving J, & Selikoff IJ (1982) Human tissue
burdens of halogenated aromatic chemicals in Michigan. J Am Med
Assoc, 247: 2112-2116.
Wong O, Brocker W, Davis HV, & Nagle GS (1984) Mortality of workers
potentially exposed to organic and inorganic brominated chemicals,
DBCP, TRIS, PBB, and DDT. Br J Ind Med, 41: 15-24.
Yagi O & Sudo R (1980) Degradation of polychlorinated biphenyls by
microorganisms. J Water Pollut Control Fed, 52: 1035-1043.
Yao P, Wang Y, Guo J, & Bernstein IA (1991) The in vitro
inducibility of cytochromes P448 by polybrominated biphenyls and
1,2-benzanthracene. In: 13th Asian Conference on Occupational Health
and the 3rd Conference of South-East Asian Ergonomics Society,
Bangkok, 25-27 November. Bangkok, Bangkok Medical Publisher, p 37
(Abstract A-23).
Zabik ME (1982) The polybrominated biphenyl episode in Michigan,
USA. J Am Oil Chem Soc, 59: 279A.
Zabik ME, Johnson TM, & Smith S (1978) Effects of processing and
cooking on PBB residues. Environ Health Perspect, 23: 37-41.
Zacharewski T, Harris M, Safe S, Thoma H, & Hutzinger O (1988)
Applications of the in vitro aryl hydrocarbon hydroxylase
induction assay for determining "2,3,7,8-
tetrachlorodibenzo-p-dioxin equivalents": pyrolyzed brominated flame
retardants. Toxicology, 51: 177-189.
Zerezghi M, Mulligan KJ, & Caruso JA (1984) Application of a rapid
scanning plasma emission detector and gas chromatography for
multi-element quantification of halogenated hydrocarbons. J
Chromatogr Sci, 22: 348-352.
Zile MH, Bank PA, & Roltsch IA (1989) Alterations in vitamin A
metabolism by polyhalogenated aromatic hydrocarbons. Z Ernähr.wiss,
28: 93-102.
Zitko V (1977) The accumulation of polybrominated biphenyls by fish.
Bull Environ Contam Toxicol, 17: 285-292.
Zitko V & Hutzinger O (1976) Uptake of chloro- and bromobiphenyls,
hexachloro- and hexabromobenzene by fish. Bull Environ Contam
Toxicol, 16: 665-673.
ABBREVIATIONS USED IN THE MONOGRAPH
1. PBB nomenclature
PBB = polybrominated biphenyl
MoBB = monobromobiphenyl
DiBB = dibromobiphenyl
TrBB = tribromobiphenyl
TeBB = tetrabromobiphenyl
PeBB = pentabromobiphenyl
HxBB = hexabromobiphenyl
OcBB = octabromobiphenyl
NoBB = nonabromobiphenyl
DeBB = decabromobiphenyl
FM = FireMaster(R)
PBB congener numbering system used (Table 3).
2. Enzyme nomenclature
Designation of the cytochrome P-450-dependent mixed function
monooxygenase (P-450) system, as relevant for PBBs:
Systematic nomenclaturea Trivial names
PB (phenobarbital)-inducible
P-450 II P-450
P-450 II B 1 P-450b
P-450 II B 2 P-450e
MC (3-methylcholanthrene)-inducible
P-450 I P-448
P-450 I A 1 P-450c
P-450 I A 2 P-450d
a From: Nebert et al. (1987, 1989).
3. Other compounds
AHH aryl hydrocarbon hydroxylase
AP alkaline phosphatase
BUN blood urea nitrogen
DBBO decabromobiphenyloxide
DMBA 7,12-dimethyl-benz (a)anthracene
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
GTP glutamyl transpeptidase
LDH lactic dehydrogenase
MFO mixed function oxidase
MNNG N-methyl- N'-nitro- N-nitroso-guanidine
PBCDD polybrominated/chlorinated dibenzo- p-dioxin
PBCDF polybrominated/chlorinated dibenzofuran
PBDD polybrominated dibenzo- p-dioxin
PBDF polybrominated dibenzofuran
PCDD polychlorinated dibenzo- p-dioxin
PCDF polychlorinated dibenzofuran
PEG polyethylene glycol
TPA 12- O-tetradecanoylphorbol-13-acetate
4. Other abbreviations
ALD approximate lethal dose
F female
GLC gas liquid chromatography
HPLC high pressure liquid chromatography
ip intraperitoneal
IPCS International Programme on Chemical Safety
LC50 lethal concentration, median
LD50 lethal dose, median
M male
NCI National Cancer Institute (USA)
No. number of animals
NTP National Toxicology Program (USA)
RER rough-surfaced endoplasmic reticulum
SER smooth-surfaced endoplasmic reticulum
t.p. time post-exposure
UV ultraviolet
WHO World Health Organization
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
On désigne par biphényles polybromés ou polybromobiphényles
(PBB) un groupe d'hydrocarbures halogénés obtenus par substitution
des hydrogènes du noyau biphényle par le brome. On ne les connaît
pas à l'état naturel. Leur formule brute est
C12H(10-x-y)Br(x+y) dans laquelle x et y peuvent prendre
toutes les valeurs de 1 à 5. Il y a théoriquement 209 homologues
possibles. On n'en a synthétisé et caractérisé que quelques-uns. Les
PBB produits dans un but commercial consistent essentiellement en
hexa-, octa-, nona- et décabromobiphényles, mais ils contiennent
également d'autres homologues. Ce sont des retardateurs de flammes
de type additif et, en mélange avec les polymères ou solides secs,
ils exercent une action retardatrice sur les flammes par filtrage,
avec libération de bromure d'hydrogène en cas d'inflammation.
Les PBB sont préparés au moyen d'une réaction du type Friedel
et Crafts, par action du brome sur le biphényle avec ou sans solvant
organique et en présence de chlorure d'aluminium, de bromure
d'aluminium, de fer, etc, comme catalyseur.
La plupart des recherches ont été consacrées au FireMaster(R)
BP-6 et au FF1 qui étaient impliqués dans la catastrophe du Michigan
où ce composé a été ajouté par inadvertance à de la nourriture pour
animaux à la place d'oxyde de magnésium. L'intoxication des animaux
qui s'en est suivie a abouti à la perte de milliers de bovins, porcs
et moutons et de millions de poulets.
La composition du FireMaster(R) varie d'un lot à l'autre mais
il est principalement composé de 2,2',4,4',5,5'-hexabromobiphényle
(60 à 80%) et de 2,2',3,4,4',5,5'-heptabromobiphényle (12 à 25%) à
côté d'homologues inférieurs dus à une bromation incomplète. Parmi
les constituants mineurs du FireMaster(R) on a également observé
la présence de bromochlorobiphényles et de polybromo naphtalènes. Le
FireMaster FF-1 (poudre blanche) est du FireMaster BP-6 (paillettes
brunes) auquel on a ajouté 2% de silicate de calcium comme
antiagglomérant.
Les PBB sont des solides dont la faible volatilité décroît à
mesure qu'augmente le nombre d'atomes de brome. Ils sont
pratiquement insolubles dans l'eau, solubles dans les graisses et
légèrement à fortement solubles dans divers solvants organiques; la
solubilité diminue également à mesure qu'augmente le nombre d'atomes
de brome. Ces composés sont relativement stables et chimiquement
inertes, encore que des mélanges de PBB fortement substitués
subissent une photodégradation avec débromation réductrice sous
l'action du rayonnement ultraviolet.
Les produits de la décomposition thermique expérimentale des
PBB dépendent de la température, de la quantité d'oxygène présente
et d'un certain nombre d'autres facteurs. L'étude de la pyrolyse du
FireMaster BP-6 en l'absence d'oxygène (600 à 900 °C) a montré qu'il
se forme des bromobenzènes et des bromobiphényles inférieurs mais
pas de polybromofuranes. En revanche, la pyrolyse en présence
d'oxygène (700 à 900 °C) conduit à la formation de
bromodibenzofuranes bi- à hepta substitués. Des quantités plus
importantes ont été trouvées en présence de polystyrène et de
polyéthylène. La pyrolyse du FireMaster BP-6 en présence de PVC à
800 °C a fourni un mélange de bromochlorobiphényles. On ignore
qu'elle est la nature des produits d'incinération des matériaux
contenant des PBB. On manque également de données sur la toxicité
des dioxines et des furanes bromés et chlorobromés, mais on estime
qu'elle doit être à peu près du même ordre que celle des dioxines et
des furanes chlorés.
Après la catastrophe du Michigan, la principale technique
d'analyse qu'on a utilisée pour la surveillance biologique des PBB
dans des échantillons de tissus et de liquides biologiques ou
provenant de l'environnement, était la chromatographie en phase
gazeuse avec détection par capture d'électrons. Le dosage des
différents homologues peut s'effectuer par chromatographie en phase
gazeuse en tube capillaire avec détection par spectrométrie de masse
à sélectivité ionique. En raison du nombre élevé d'homologues
possibles, les recherches sont gênées par le manque d'étalons de
synthèse convenables. On utilise, pour extraire les PBB des
échantillons biologiques, des méthodes du type de celles qu'on
applique aux pesticides. Les PBB sont extraits avec la fraction
lipidique puis purifiés.
Le fait que l'on ait récemment découvert des homologues des PBB
dans des échantillons biologiques prélevés dans l'environne ment
général n'implique pas forcément que la concentration de ces
produits y soit en augmentation. La mise au point de techniques
d'analyse plus sensibles comme la spectrométrie de masse avec
production d'ions négatifs par ionisation chimique, pourrait en être
la cause. Il est donc urgent de procéder à des études
rétrospectives. L'amélioration des méthodes de purification devrait
permettre d'effectuer le dosage spécifique des homologues
coplanaires toxiques car des données sont également nécessaires à
leur sujet.
1.2 Sources d'exposition humaine et environnementale
La production commerciale du FireMaster(R) a commencé aux
Etats-Unis en 1970. Elle a été arrêtée après la catastrophe du
Michigan (novembre 1974). On estime qu'entre 1970 et 1976 les
Etats-Unis ont produit 6000 tonnes de PBB (produits du commerce). La
production de l'octabromobiphényle et du décabromobiphényle s'est
poursuivie aux Etats-Unis jusqu'en 1979. En outre, l'Allemagne a
produit jusqu'à la moitié de 1985 un mélange de PBB fortement
bromés, le Bromkal 80-9 D. La France produit actuellement du
décabromobiphényle (Adine 0102) de qualité technique. Autant qu'on
sache, c'est le seul PBB qui soit actuellement produit.
Les PBB ont fait leur apparition sur le marché comme
retardateurs de flamme au début des années 1970. Avant novembre
1974, l'hexabromobiphényle était le principal PBB produit dans le
commerce aux Etats-Unis; il était incorporé aux résines ABS
(acrylonitrile-butadiène-styrène) dans la proportion de 10% et
utilisé principalement pour la fabrication de divers accessoires
d'automobiles, les peintures et les vernis ainsi que dans la mousse
de polyuréthanne. Les autres retardateurs de flamme à base de PBB
ont des applications similaires.
Des PBB peuvent passer dans l'environnement au cours du
processus normal de production à la faveur d'émissions dans l'air,
dans les eaux usées, ou encore par libération dans le sol et dans
les décharges, mais les concentrations correspondantes se sont
révélées faibles en général.
Ces produits peuvent également passer dans l'environnement lors
du transport et de la manipulation ou par suite d'accidents comme
cela s'est produit dans le Michigan.
Il y a également risque de pénétration dans l'environnement
lors de l'incinération de produits contenant des PBB ou lors
d'incendies au cours desquels il se forme d'autres substances
toxiques comme des polybromodibenzofuranes ou des dérivés mixtes
bromochlorés.
La majeure partie du volume totale de ces produits finit de
toute façon par se retrouver dans l'environnement soit tels quels,
soit sous forme de produits de décomposition.
1.3 Transport, distribution et transformation dans l'environnement
Il n'est pas démontré que les PBB puissent être transportés à
longue distance dans l'atmosphère, mais la présence de ces composés
chez les phoques de l'Arctique indique qu'ils sont largement
disséminés sur la planète.
Les principales voies de pénétration des PBB dans l'environne
ment aquatique sont, d'une part la pollution des eaux réceptrices,
les décharges de déchets industriels et, d'autre part le lessivage
de dépotoirs de déchets industriels, ou encore, l'érosion de sols
pollués. Les PBB sont pratiquement insolubles dans l'eau et on les
retrouve principalement dans les sédiments des lacs et des rivières
pollués.
La pollution du sol peut trouver son origine dans des sources
polluantes ponctuelles comme par exemple des unités de production de
PBB ou des dépotoirs. Une fois qu'ils ont pénétré dans le sol, les
PBB ne semblent pas être facilement mobilisables. On a constaté
qu'ils étaient 200 fois plus solubles dans le produit de lessivage
d'une décharge que dans l'eau distillée; cela pourrait entraîner une
plus large distribution dans l'environnement. Du fait de leurs
propriétés hydrophobes, les PBB sont facilement adsorbés sur le sol
à partir des solutions aqueuses. On a observé que les différents
homologues étaient adsorbés préférentiellement en fonction des
caractéristiques du sol (par exemple la teneur en matières
organiques) ainsi que de la position et du nombre des atomes de
brome.
Les PBB sont stables et persistants; ils sont lipophiles et ne
sont en outre que légèrement solubles dans l'eau; certains
homologues sont peu métabolisés et s'accumulent dans la fraction
lipidique des organismes vivants. Une fois libérés dans
l'environnement, ils peuvent entrer dans la chaîne alimentaire où
ils subissent une concentration.
On a décelé des PBB dans les poissons de diverses régions. Les
PBB peuvent pénétrer dans l'organisme des mammifères et des oiseaux
par suite de l'ingestion de ces poissons.
Il est improbable que les PBB subissent une dégradation
chimique purement abiotique (à l'exclusion d'une photodécompo
sition). On a fait état d'une persistance des PBB sur le terrain.
Des échantillons de sol prélevés sur l'emplacement d'une ancienne
unité de production de PBB ont été analysés plusieurs années après
l'accident du Michigan; ils contenaient encore des PBB mais la
proportion des divers homologues était différente, ce qui indique
qu'il y avait eu décomposition partielle des résidus de PBB dans les
échantillons en question.
Au laboratoire, les PBB sont facilement décomposés par le
rayonnement ultraviolet. La photodécomposition d'un mélange
commercial (FireMaster(R)) a provoqué une diminution de la
concentration des homologues les plus substitués. La vitesse et le
degré de photolyse des PBB dans l'environnement n'ont pas été
déterminées avec précision, encore que l'observation sur le terrain
montre que les PBB de départ sont très tenaces, avec dégradation
partielle des homologues les moins bromés.
D'après les études en laboratoire, les mélanges de PBB semblent
assez résistants à la dégradation microbienne.
On ne connaît pas d'exemple de fixation ou de décomposition des
PBB par les végétaux. Par contre, les PBB sont facilement absorbés
par l'organisme animal et bien qu'ils se soient révélés très
persistants chez les animaux, on a tout de même retrouvé de petites
quantités de métabolites. Ces métabolites consistaient
principalement en dérivés hydroxylés et, dans certains cas, il y
avait des traces de PBB partiellement débromés. Aucune étude sur les
métabolites soufrés analogues à ceux des PCB n'a été publiée.
La bioaccumulation des PBB a été étudiée dans les poissons. En
ce qui concerne les animaux terrestres, on l'a étudiée chez
différentes espèces d'oiseaux et de mammifères. Les données ont été
fournies par des observations sur le terrain, par l'étude des
conséquences de la catastrophe du Michigan et par des études
d'alimentation contrôlée. En général, on a constaté que
l'accumulation des PBB dans les graisses de l'organisme était liée à
la dose et à la durée d'exposition.
La bioaccumulation des différents homologues des PBB augmente
avec le degré de bromation, au moins jusqu'aux dérivés tétrabromés.
On peut penser que les homologues plus substitués s'accumulent dans
une proportion encore plus importante. Toutefois, on ne dispose
d'aucun renseignement sur le décabromobiphényle; il est possible
qu'il soit peu absorbé.
On a signalé la présence de dibenzofuranes bromés et de PBB
partiellement débromés comme produits de la décomposition thermique
des PBB. La formation de ces composés dépend de plusieurs variables
(par exemple, température, oxygène).
1.4 Concentrations dans l'environnement et exposition humaine
On ne dispose que d'un seul rapport sur la concentration des
PBB dans l'atmosphère. Il s'agit d'une étude au cours de laquelle on
a mesuré la concentration de ces composés au voisinage des trois
unités de production et de traitement des PBB aux Etats-Unis
d'Amérique.
La concentration des PBB dans les eaux de surface du même
secteur ainsi que dans la décharge du Comté de Gratiot (Michigan,
Etats-Unis d'Amérique) qui avait reçu entre 1971 et 1973 plus de 100
000 kg de déchets contenant 60 à 70% de PBB, a fait l'objet d'une
surveillance.
Les données relatives à la surveillance des eaux souterraines
au voisinage de la décharge du Comté de Gratiot ont révélé la
présence de traces de PBB, même au-delà du voisinage immédiat de la
décharge; toutefois aucun de ces composés n'a été décelé dans les
puits d'eau potable du secteur.
On dispose de données sur la pollution tellurique par les PBB
dans les secteurs où des PBB ont été ou sont produits, utilisés ou
rejetés et également sur la pollution tellurique des terrains
agricoles du Michigan contaminés par ces composés.
Lors de la catastrophe du Michigan, du FireMaster(R) a été
ajouté par inadvertance à de la nourriture pour animaux. Il a fallu
presque une année pour qu'on s'aperçoive de cette erreur et pour que
les analyses révèlent que les PBB étaient en cause. Pendant cette
période (de l'été 1973 à mai 1974), des animaux et des produits
animaux contaminés ont été utilisés pour l'alimentation humaine et
ont pénétré dans l'environnement de l'Etat du Michigan. Des
centaines d'exploitations agricoles ont été affectées; il a fallu
abattre et enterrer des milliers d'animaux et détruire des milliers
de tonnes de produits agricoles.
La plupart des données concernant la contamination de la faune
sauvage par les PBB concernent des poissons et des oiseaux des
Etats-Unis d'Amérique et d'Europe (essentiellement la sauvagine
vivant à proximité des sites industriels) ainsi que des mammifères
marins.
Selon des rapports récents sur la contamination de poissons, de
mammifères terrestres ou marins et d'oiseaux aux Etats-Unis
d'Amérique et en Europe, ces composés seraient très disséminés. La
composition en homologues dans les échantillons de poissons est très
différentes de celle qu'on trouve dans des produits commerciaux.
Pour les principaux, ils pourraient dans beaucoup de cas résulter
d'une débromation photochimique du décabromobiphényle (BB 209), mais
cela n'a pas été confirmé.
On a observé une exposition professionnelle chez des employés
d'usines chimiques aux Etats-Unis d'Amérique, ainsi que chez des
ouvriers agricoles, à la suite de l'accident du Michigan. Les taux
médians de PBB dans le sérum et les tissus adipeux étaient plus
élevés chez les employés de l'industrie chimique. On ne dispose pas
de renseignements en provenance d'autres pays ou d'autres
entreprises sur l'exposition professionnelle lors de la fabrication,
de la formulation et de l'utilisation commerciale de ces produits.
On ne dispose pas, pour la plupart des populations humaines,
d'une documentation qui fournisse des données de première main sur
l'exposition aux PBB de diverses origines. Dans le Michigan, on a
observé de très nombreux cas d'exposition humaine résultant d'un
contact direct avec de la nourriture pour animaux contaminée et pour
l'essentiel, de la consommation de viande, d'oeufs et de produits
laitiers qui avaient également été contaminés par des PBB. Au moins
2000 familles (principalement des exploitants agricoles et leurs
voisins) ont été fortement contaminées. En Allemagne, on a récemment
décelé des PBB dans du lait de vache et du lait humain.
La composition en homologues de ces échantillons diffère de
celle que l'on trouve dans le poisson. La concentration relative du
BB 153 est plus élevée dans le lait humain que dans le poisson.
Les voies d'exposition de la population générale aux PBB sont
mal connues. Pour autant que l'on sache, ils ne sont pas présents à
fortes concentrations dans l'air ambiant ni dans l'eau. Plus
importants à cet égard sont probablement les produits alimentaires
riches en lipides tirés d'eaux contaminées. On ne possède aucun
renseignement sur le niveau d'exposition dans l'air intérieur ni sur
l'exposition par voie percutanée par suite d'un contact avec des
retardateurs de flammes à base de PBB.
La composition en homologues observée dans le lait humain
prélevé en Allemagne rappelait celle que l'on trouvait dans le lait
de vaches de la même région, mais à concentrations sensiblement plus
élevées.
Pour évaluer l'apport journalier de PBB par l'intermédiaire de
la nourriture dans la population générale, on ne dispose que de très
peu de données. Si l'on suppose que le poisson contient 20 µg de
PBB/kg de matières grasses et 5% de matières grasses et qu'une
personne de 60 kg consomme 100 g de poisson par jour, on arrive à
une ingestion journalière de 0,002 µg/kg de poids corporel. Avec une
concentration de PBB de 0,05 µg/kg de matières grasses dans le lait
(4% de matières grasses) et une consommation de lait de
500 ml/jour, la même personne en ingèrera quotidiennement environ
0,00002 µg/kg de poids corporel.
Un nourrisson de 6 kg consommant 800 ml de lait humain (3,5% de
matières grasses) par jour ingèrera 0,01 µg de PBB/kg de poids
corporel, si le lait contient de 2 µg de PBB/kg de matières grasses.
1.5 Cinétique et métabolisme
La résorption des PBB dans les voies digestives varie selon le
degré de bromation, les composés les moins bromés étant les plus
facilement résorbés.
Les données relatives à la résorption du DeBB et de l'OcBB/NoBB
sont insuffisantes.
On retrouve des PBB dans l'ensemble du règne animal et des
populations humaines, les concentrations d'équilibre les plus
élevées étant observées dans les tissus adipeux. Les concentrations
sont relativement élevées également dans le foie, particulièrement
en ce qui concerne les homologues les plus toxiques qui semblent se
concentrer dans cet organe. Le coefficient de partage des différents
homologues varie d'un tissu à l'autre. En général, on note une
tendance marquée à la bioaccumulation. Chez les mammifères, la
transmission des PBB à la descendance se produit par la voie
transplacentaire et par le lait maternel. On a constaté la présence
de 2,2',4,4',5,5'-hexabromobiphényles dans du lait humain à des
concentrations 100 fois plus élevées que dans le sérum maternel.
Lors d'une étude portant sur plusieurs générations de rats,
l'administration de PBB à une seule génération a entraîné la
présence de résidus décelables dans plus de deux des générations
suivantes. Chez les oiseaux, la teneur en PBB de l'organisme
maternel entraîne également la présence de résidus dans les oeufs.
Des nombreux homologues des PBB persistent dans les systèmes
biologiques. Ainsi, les constituants les plus abondants du
FireMaster(R), de même que l'octabromobiphényle et le décabromo
biphényle, n'ont pas paru être métabolisés ou excrétés dans une
proportion sensible. Les études métaboliques in vitro montrent que
l'on peut établir des relations structure-activité dans le cas du
métabolisme de PBB. Les PBB pourraient être métabolisés par les
microsomes induits par le phénobarbital à la condition de posséder
des atomes de carbone adjacents non bromés, en méta et en para
du pont biphényle, sur au moins un des cycles. La métabolisation des
homologues inférieurs par les microsomes induits par le
3-méthylcholanthrène nécessite la présence d'atomes de carbone non
bromés en ortho et méta du pont biphényle sur au moins un des
cycles; en outre, un degré important de bromation empêche la
métabolisation. Chez les vertébrés, les principaux produits de
métabolisation in vitro et in vivo des homologues inférieurs
sont des dérivés hydroxylés. Le rendement métabolique observé est
relativement faible. La réaction d'hydroxylation d'effectue
probablement par l'intermédiaire d'un oxyde d'arène ou par
hydroxylation directe.
L'homme, le rat, le singe rhésus, le porc, la vache et le
poulet éliminent les PBB, principalement dans leurs matières
fécales. Dans la plupart des cas, il semble que la vitesse
d'excrétion soit faible. Les concentrations de
2,2',4,4',5,5'-hexabromobiphényles observées dans la bile et les
matières fécales de sujets humains étaient égales à environ 50-70%
des taux sériques et à environ 0,5% des taux dans les tissus
adipeux. Les traitements administrés en vue d'améliorer
l'élimination des PBB chez l'animal ou l'homme n'ont guère eu de
succès. Le lait constitue une autre voie d'élimination des PBB.
Après administration de PBB à des rats et à d'autres animaux,
on a constaté que les relations entre la concentration tissulaire
des PBB et le temps étaient complexes et variables. Ces relations
ont pu être établies en utilisant divers modèles comportementaux. On
a calculé que la demi-vie d'élimination du 2,2',4,4',5,5'-hexa
bromobiphényles à partir des tissus adipeux du rat était d'environ
69 semaines. Dans le cas des singes rhésus, on a trouvé une demi-vie
de plus de quatre ans. Chez l'homme, on estime que la demi-vie
moyenne se situe dans le cas de ce composé entre 8 et 12 ans. Dans
la littérature, on trouve des valeurs allant de 5 à 95 ans. On
constate quelques différences dans la rétention et le "turnover" des
divers homologues. Les résultats fournis par l'analyse du sérum des
agriculteurs et des travailleurs de l'industrie chimique en vue de
doser le 2,3',4,4',5-pentabromobiphényle étaient incohérents. Cette
incohérence est probablement due à la diversité des sources
d'exposition. Les ouvriers étaient exposés à la totalité des
constituants du FireMaster(R) alors que la population du Michigan
n'avait consommé que de la viande et du lait contaminés contenant
des mélanges de PBB différents par suite de la métabolisation des
produits initiaux par les animaux de boucherie. Après administration
d'octobromobiphényle à des rats, on n'a pas noté de diminution des
taux de brome dans les tissus adipeux. On ne dispose d'aucune donnée
sur la rétention du décabromobiphényle.
L'organisme humain a davantage tendance à retenir certains
homologues des PBB que celui des animaux de laboratoire. C'est un
facteur à prendre en considération lorsqu'on évalue le danger pour
la santé humaine que représentent ces composés.
En conclusion, toutes les données disponibles indiquent que les
PBB ont une forte tendance à s'accumuler et à persister dans les
organismes vivants. Ils sont peu métabolisés et leur demi-vie chez
l'homme est de 8 à 12 ans ou davantage.
1.6 Effets sur les êtres vivants dans leur milieu naturel
On ne dispose que de quelques données au sujet des effets que
les PBB exercent sur les êtres vivants dans leur milieu naturel.
Elles portent sur les microorganismes, les puces d'eau, les oiseaux
aquatiques et les animaux d'élevage.
Les oiseaux aquatiques qui nichent sur les îles du nord-ouest
du lac Michigan ont été étudiés afin de voir si les polluants du
milieu étaient susceptibles d'affecter leur reproduction. On a ainsi
procédé au dosage de 17 polluants et notamment de PBB, dont aucun
n'a paru avoir d'effets notables sur la reproduction.
Les animaux d'élevage qui avaient ingéré une nourriture à
laquelle avait été ajouté par inadvertance du FireMaster(R) FF-1 à
la place d'oxyde de magnésium, sont tombés malades. Dans la première
ferme fortement contaminée que l'on ait observée, l'exposition
moyenne estimative des vaches était égale à 250 mg/kg de poids
corporel. Les signes cliniques d'intoxication consistaient dans une
réduction de 50% de la consommation de nourriture (anorexie) et une
diminution de 40% de la production laitière, dans les semaines
suivant l'ingestion des aliments contaminés. Bien qu'en l'espace de
16 jours on ait cessé de donner aux animaux la nourriture en
question, la production laitière n'est pas revenue à sa valeur
normale. Certaines vaches ont présenté une pollakiurie, un
larmoiement, avec en outre des hématomes, des abcès, une croissance
anormale des sabots, une boiterie, une alopécie, une hyperkératose
et une cachexie; plusieurs animaux sont morts dans les six mois
suivant l'exposition. Au total, la mortalité dans cette exploitation
a été de 24/400. Chez les veaux âgés de six à 18 mois, elle était
beaucoup plus élevée. Environ 50% d'entre eux sont morts dans les
six semaines avec seulement deux survivants sur 12 au bout de cinq
mois. Ces animaux étaient atteints d'hyperkératose sur l'ensemble du
corps. On a également noté divers problèmes affectant la
reproduction.
Les résultats des autopsies sont connus pour certaines des
vaches qui étaient mortes dans les six mois suivants l'exposition.
L'étude histopathologique a révélé la présence d'altérations
variables au niveau du foie et des reins.
Plusieurs des signes cliniques et des altérations
anatomopathologiques indiqués ci-dessus ont été confirmés par la
suite par des études d'alimentation contrôlée (anorexie,
déshydratation, hyperlarmoiement, émaciation, hyperkératose,
problèmes de reproduction, modification d'un certain nombre des
paramètres biochimiques, lésions rénales).
Chez les troupeaux faiblement contaminés, on a noté une chute
de la production et des cas de stérilité. Ces résultats contrastent
avec ceux des études contrôlées, qui n'ont pas révélé des
différences sensibles entre les troupeaux faiblement contaminés et
les troupeaux témoins.
A l'origine, la substitution accidentelle concernait des
aliments pour bovins, mais d'autres types de nourriture animale ont
subi une contamination croisée, notamment par l'intermédiaire du
matériel servant à la préparation de ces aliments. Il est probable
que l'exposition qui s'en est suivie n'a pas été aussi intense que
dans le cas des bovins. La contamination d'autres animaux a été
signalée (volailles, porcs, chevaux, lapins, chèvres et moutons) et
ces animaux ont été abattus; toutefois, les troubles dont ils
auraient pu souffrir n'ont pas été précisés.
On ne dispose d'aucun renseignement au sujet des effets des PBB
sur l'écosystème.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Les valeurs de la DL50 pour les mélanges du commerce corre
spondent à une toxicité aiguë relativement faible (DL50 > 1 g/kg
de poids corporel) pour le rat, le lapin et la caille, après
administration par voie orale ou percutanée. Une fois la dose de PBB
administrée, il y a d'ailleurs un certain délai avant l'apparition
des manifestations toxiques et la mort. C'est la dose totale
administrée qui détermine l'ampleur de l'intoxication, qu'elle soit
donnée en une seule fois ou qu'elle soit fractionnée et administrée
sur une courte période (jusqu'à 50 jours). La toxicité des PBB s'est
révélée plus importante après des doses multiples qu'après une dose
unique. L'exposition aux PBB n'entraîne pas immédiatement la mort.
Les quelques études effectuées sur des mélanges commerciaux
d'octo- et de décabromobiphényles n'ont pas provoqué de mortalité
chez les rats ni les poissons. En ce qui concerne les différents
homologues des PBB, on n'a étudié que trois hexaisomères, le
3,3',4,4',5,5'-HxBB et le 2,3',4,4',5,5'-HxBB étant plus toxiques
pour le rat que le 2,2',4,4',5,5'-HxBB. Sur la base des données
disponibles qui restent limitées, l'OcBB et le DeBB se révèlent
moins toxiques et sont moins bien résorbés que les mélanges de PBB.
De nombreuses études destinées à faire ressortir les effets
aigus et les effets à court terme ont montré que, parmi les signes
d'intoxication par les PBB (la plupart du temps, du FireMaster),
figurait une réduction de la consommation de nourriture. Aux doses
mortelles, on ne peut pas attribuer la mort à des lésions
anatomopathologiques affectant un organe déterminé mais plutôt à un
syndrome "cachectique" qui se développe chez l'animal et constitue
le premier signe d'intoxication. Au moment de la mort, la perte de
poids peut atteindre 30 à 40%. Les quelques études consacrées à
l'OcBB et au DeBB techniques n'ont pas révélé d'effets de ce genre.
C'est essentiellement au niveau du foie que l'on observe les
altérations morphologiques et histopathologiques imputables à
l'exposition aux PBB. Ainsi l'hypertrophie du foie s'observe
fréquemment à des doses plus faibles que celles qui entraînent une
perte de poids. Chez les rongeurs, les principales altérations
histopathologiques pourraient être un gonflement et une
vacuolisation généralisée des hépatocytes, la prolifération du
réticulum endoplasmique agranulaire et la nécrose des cellules
individuelles. La gravité des lésions dépend de la dose et de la
composition du mélange administré.
On a observé une diminution du poids du thymus chez des rats,
des souris et des bovins après absorption de FireMaster(R), mais
non d'OcBB ou de DeBB.
On a fait état d'une augmentation du poids de la thyroïde et de
modifications histologiques au niveau de cette glande chez le rat à
des concentrations faibles.
Il est évident que les différents homologues des PBB ne
présentent pas le même type de toxicité. Les isomères et les
homologues les plus toxiques provoquent une réduction du poids du
thymus ou du corps et déterminent des altérations histologiques
marquées au niveau du foie et du thymus. On a classé les byphényles
halogénés en fonction de leur structure. La catégorie 1 comporte les
isomères et les homologues qui n'ont pas de substi tuants en ortho
(PBB coplanaires). Les dérivés monosubstitués en ortho
constituent la deuxième catégorie. Les autres PBB (principalement
ceux qui comportent deux bromes ou davantage en ortho) sont
classés dans la troisième catégorie. Les homologues de la catégorie
1 ont tendance à provoquer les effets les plus graves alors que ceux
de la deuxième et de la troisième catégorie entraînent des effets
toxicologiques qui vont diminuant. A l'intérieur d'une même
catégorie, le degré de bromation peut également avoir une influence
sur la toxicité.
Sur toutes les combinaisons étudiées, c'est le
3,3',4,4',5,5'-HxBB qui s'est révélé le plus toxique. Cet homologue
est présent à faibles concentrations dans le FireMaster(R). Parmi
les principaux constituants du FireMaster(R), c'est le
2,3,3',4,4',5-HxBB qui s'est révélé le plus toxique devant le
2,3',4,4',5,5'-HxBB et le 2,3',4,4',5-PeBB, dans cet ordre. Le
principal constituant du FireMaster(R), le 2,2',4,4'5,5'-HxBB
s'est révélé relativement non toxique, de même que le
2,2',3,4,4',5,5'-HpBB, qui vient en seconde position par ordre de
concentration.
On ne connaît pas très bien la toxicité des mélanges d'OcBB et
de DeBB techniques eu égard à leur teneur en divers homologues (et
autres contaminants éventuels).
Les tests habituels d'irritation cutanée et oculaire de même
que les tests de sensibilisation qui ont été effectués sur des
mélanges de PBB techniques (OcBB et DeBB) n'ont révélé aucune
réaction ou du moins, seulement des réactions légères. Toutefois on
a relevé une hyperkératose et une alopécie chez les bovins exposés
et des lésions rappelant la chloracné ont été observés chez les
singes rhésus après ingestion de FireMaster(R). Le FireMaster(R)
a produit une hyperkératose de la surface interne de l'oreille chez
le lapin, mais ses deux principaux constituants (le
2,2',4,4',5,5'-HxBB et le 2,2',3,4,4',5,5'-HpBB) ne produisaient pas
cet effet. En fraction nant le FireMaster(R), on a constaté que
l'essentiel de son activité était le fait des fractions les plus
polaires contenant des constituants mineurs. En traitant des lapins
avec de l'HxBB exposé à la lumière solaire, on a constaté
l'apparition d'une hyperkératose grave au niveau de l'oreille.
L'administration de faibles doses d'OcBB technique pendant une
longue période à des rats n'a pas affecté leur consommation de
nourriture ni leur poids corporel, mais on a constaté chez les rats
qui avaient reçu pendant sept mois une dose de 2,5 mg/kg de poids
corporel, une augmentation du poids relatif du foie. L'adminis
tration pendant une longue durée à des rats de FireMaster(R) mêlé
à leur nourriture à la dose de 10 ng/kg de poids corporel pendant
six mois, est restée sans effet sur leur consommation de nourriture.
En revanche à la dose de 1 mg/kg de poids corporel administrée sur
une période de six mois, on constatait une modification du poids du
foie. Chez les rattes qui recevaient 0,3 mg/kg de poids corporel de
FireMaster(R), on constatait une réduction du poids du thymus. Des
altérations histopathologiques ont également été observées. Des
études d'alimentation contrôlées poursuivies pendant une longue
période sur des bovins exposés à de faibles doses de
FireMaster(R), n'ont pas fait ressortir d'effets nocifs, à en
juger par la prise de nourriture, les signes cliniques, les
modifications clinicopathologiques ou le rendement du bétail. Les
visons, les cobayes et les singes se sont révélés plus sensibles à
l'intoxication par les PBB.
On a observé chez le rat des effets à long terme attribuables à
la rétention des PBB après administration de fortes doses de
FireMaster(R) au cours de la période prénatale ou périnatale.
Les effets délétères les plus fréquemment observés sur la
reproduction consistaient en une résorption du foetus et une moindre
viabilité de la progéniture. Chez les visons, on observait encore
certains effets à la concentration de 1 mg/kg de nourriture. Une
réduction de la viabilité de la progéniture a été observée chez des
singes rhésus après 12,5 mois d'exposition au FireMaster(R) (dose:
0,3 mg/kg de nourriture). Les singes ont reçu une dose quotidienne
de ce mélange, égale à 0,01 mg/kg de poids corporel et la dose
totale était de 3,8 mg/kg de poids corporel. Il n'a pas été possible
d'évaluer les études de reproduction ni les études
neurocomportementales effectuées sur des singes et des rats à
faibles doses, car les publications en question n'étaient pas
suffisamment explicites quant au protocole expérimental des essais.
Chez les rongeurs, on a observé un faible pouvoir tératogène à des
doses élevées, susceptibles d'être toxiques pour les mères.
Les PBB perturbent les fonctions endocrines. Chez des rats et
des porcs on a observé une réduction des taux sériques de thyroxine
et de triiodothyronine qui était liée à la dose. Les PBB
perturberaient également, dans la plupart des cas, les taux
d'hormones stéroïdiennes. L'ampleur des effets dépend de l'espèce
ainsi que de la dose et de la durée de l'administration.
Les PBB ont également produit une porphyrie chez des rats et
des souris mâles à des doses quotidiennes ne dépassant pas
0,3 mg/kg de poids corporel. La dose quotidienne maximale sans
effets était de 0,1 mg/kg de poids corporel. On constatait une
influence marquée des PBB sur l'accumulation de vitamine A ainsi que
des effets sur la métabolisme intermédiaire.
Après exposition aux PBB, on observe fréquemment une atrophie
du thymus et on a constaté que d'autres tissus lymphoïdes étaient
également affectés. On a également mis en évidence d'autres
indicateurs témoignant d'une dépression des fonctions immunitaires
par le FireMaster(R). On manque de données concernant l'OcBB, le
NoBB, le DeBB ou les différents homologues des PBB.
Un des effets des PBB qui ait été le plus intensivement étudié
est l'induction des oxydases à fonction mixte. De fait, on a
systématiquement constaté que le FireMaster(R) se comportait comme
un inducteur de type mixte des enzymes microsomiennes du foie chez
le rat et chez toutes les autres espèces étudiées. Cette induction a
été également observée dans d'autres tissus, mais dans une moindre
mesure. L'aptitude à induire les enzymes microso miennes hépatiques
varie d'un homologue à l'autre. On a mis en évidence des
corrélations entre la structure et l'aptitude à induire les enzymes
microsomiennes.
Plusieurs études ont montré que les PBB étaient capables de
modifier l'activité biologique de divers médicaments et substances
toxiques. Cela s'explique peut-être en partie par le fait que les
PBB sont capables d'induire les enzymes microsomiennes qui
interviennent dans l'activation ou la désactivation des substances
xénobiotiques.
Le FireMaster(R) et certains de ces principaux constituants
se sont révélés capables d'inhiber la communication intercellulaire
in vitro. Cette inhibition s'est produite à des concentrations non
cytotoxiques. Cette cytotoxicité, de même que la capacité d'inhiber
la coopération métabolique paraît liée à la structure et plus
précisément à la présence ou à l'absence de substitution en ortho.
Les épreuves in vitro et in vivo (mutagénèse des cellules
microbiennes et mammaliennes, altération des chromosomes de cellules
mammaliennes, transformation des cellules mammaliennes, lésion et
réparation de l'ADN) n'ont pu mettre en évidence de mutagénicité ou
de génotoxicité imputables aux divers homologues des PBB ou aux
mélanges qui sont vendus dans le commerce.
Les études de toxicité à long terme ont montré que le foie
était la principale cible des effets cancérogènes du PBB. Chez des
souris et des rats, mâles et femelles, qui recevaient du
FireMaster(R) par voie orale, on a noté une augmentation sensible
de l'incidence des carcinomes hépatocellulaires. Des effets
cancérogènes sur le foie ont également été observés chez des souris
qui avaient reçu pendant 18 mois une alimentation contenant une dose
totale de 100 mg/kg ou davantage de Bromkal 80-9D
(nonabromobiphényle technique) en doses quotidiennes de 5 mg/kg de
poids corporel. La dose quotidienne de PBB la plus faible qui ait
produit des tumeurs (pour la plupart, des adénomes) chez des
rongeurs, était de 0,5 mg/kg de poids corporel pendant deux ans. Les
rats qui en avaient reçu quotidiennement 0,15 mg/kg de poids
corporel, en plus de ce qu'il leur avait été administré pendant la
période prénatale et périnatale, n'ont pas présenté le moindre effet
indésirable. La pouvoir cancérogène de l'octabromobiphényle et du
décabromobiphényle techniques n'a pas été étudié.
Ni le FireMaster BP-6 ni le 2,2',4,4',5,5'-hexabromobiphényle
ne se sont comportés comme des initiateurs tumoraux (en utilisant le
TPA comme promoteur) ou comme des promoteurs tumoraux (en utilisant
la DMBA comme initiateur) lors d'épreuves bio logiques sur
l'épiderme de souris. Toutefois, en utilisant le même modèle
(épiderme de souris) et avec du DMBA ou de la MNNG comme initiateur,
le FM FF-1 et le 3,3',4,4'5,5'-hexabromo biphényle mais non pas le
2,2',4,4'5,5'-hexabromobiphényle, ont présenté une activité
tumoro-promotrice. Lors d'une épreuve biologique sur foie de rat
effectuée en deux temps, et avec du phénobarbital comme promoteur,
on a constaté que le 3,3',4,4'- tétrabromobiphényle se comportait
comme un initiateur faible. Avec ce même modèle animal, en présence
de diéthylnitrosamine et après hépatectomie partielle, on a constaté
que le FM, le 3,3',4,4'-tétrabromobiphényle et le
2,2',4,4',5,5'-hexabromobi phényle, mais non pas le
3,3',4,4',5,5'-hexabromobiphényle, se comportaient comme des
promoteurs tumoraux.
Les résultats des études sur la communication cellulaire, les
résultats négatifs fournis par les études de génotoxicité et de
mutagénicité, ainsi que ceux des épreuves de promotion tumorale,
montrent que les mélanges de PBB et les divers homologues étudiés
provoquent l'apparition de cancers par un mécanisme épigéné tique.
On ne dispose d'aucun renseignement sur l'octa-, le nona-, le
décabromobiphényles techniques.
Le mode d'action qui est à la base des nombreuses manifes
tations de la toxicité des PBB et des composés apparentés reste
inconnu. Toutefois, certains des effets observés, comme le syndrome
cachectique, l'atrophie du thymus et l'hépatotoxicité, les
manifestations dermatologiques et les effets délétères sur la
fonction de reproduction peuvent être attribués à une interaction
avec les récepteurs Ah ou TCDD, interaction qui entraîne une
modification de l'expression d'un certain nombre de gènes.
L'interaction avec ces récepteurs varie selon les divers homologues,
les homologues coplanaires étant les plus actifs.
Nombre des effets des PBB s'observent après une exposition de
longue durée. Cela s'explique peut-être par le fait que certains
homologues s'accumulent fortement et que l'organisme ne les
métabolise et ne les élimine que difficilement. Il s'en suit une
accumulation de ces composés dans l'organisme qui finit par
submerger les mécanismes de compensation et entraîne des effets
délétères.
Certains contaminants connus du FireMaster(R), en
l'occurrence des polybromonaphtalènes (PBN) sont fortement toxiques
et tératogènes. Bien qu'ils ne soient présents qu'en petites
quantités dans le FireMaster(R), il n'est pas exclu qu'ils
contribuent à sa toxicité.
Les études portant sur le FireMaster(R) et son principal
constituant le 2,2',4,4'5,5'-HxBB, ont montré que leurs produits de
photolyse étaient plus toxiques que les composés initiaux. Les
produits du pyrolyse du FM provoquent l'induction des oxydases à
fonction mixte, une perte de poids et une atrophie du thymus. On a
également observé que les produits de pyrolyse de l'OcBB technique
produisaient une hypertrophie du foie.
1.8 Effets sur l'homme
On ne connaît aucun exemple d'intoxication aiguë par les PBB
chez l'homme auquel on puisse comparer les effets potentiels à
faibles doses résultant de l'accident survenu dans le Michigan aux
Etats-Unis d'Amérique en 1973. Les principales études
épidémiologiques ont été menées par le Michigan Department of Public
Health (MDPH) et l'Environmental Science Laboratory de la Mount
Sinaï School of Medicine, New York (ESL).
On estime que les personnes les plus fortement contaminées
avaient consommé 5 à 15 g de PBB sur une période de 230 jours par
l'intermédiaire du lait. Il est possible que la consommation de
viande ait constitué une source de contamination supplémentaire.
Chez certains des agriculteurs et chez la plupart des membres de la
population générale du Michigan, le niveau d'exposition était
beaucoup plus faible, la dose totale étant de 9 à 10 mg. Il est
possible cependant que certaines personnes aient reçu une dose
totale d'environ 800 à 900 mg. (Une dose totale de 9 mg correspond à
0,15 mg/kg de poids corporel et une dose de 900 mg, à 15 mg/kg de
poids corporel pour un adulte moyen de 60 kg; pour un enfant, la
dose par kg/poids corporel serait plus élevée).
En 1974, la première étude du MDPH a consisté à comparer l'état
de santé des personnes travaillant dans les fermes mises en
quarantaine, à celui du personnel des fermes de la même région qui
n'étaient pas frappées par cette mesure. Dans les deux groupes, on a
constaté divers symptômes, mais sans pouvoir dégager de différences.
Aucune anomalie inhabituelle n'a été constatée, qu'il s'agisse du
coeur, du foie, de la rate, du système nerveux, des résultats de
l'analyse d'urine et de la NFS, non plus qu'en ce qui concerne tous
les autres paramètres médicaux examinés. Une étude ultérieure très
complète menée par le MDPH et portant sur des groupes soumis à une
exposition de degré variable, n'a pas permis de mettre en évidence
de corrélation positive entre les taux sériques de PBB et la
fréquence des symptômes ou des affections observés. L'ESL a étudié
environ 990 personnes vivant sur des exploitations agricoles, 55
travailleurs de l'industrie chimique et un groupe de producteurs de
lait du Wisconsin qui ont fait office de témoins. Les symptômes
étaient plus fréquent chez les exploitants du Michigan que chez ceux
du Wisconsin. C'est dans le cas des symptômes neurologiques et
musculo-squelettiques, au sens large, que les différences étaient
les plus importantes. De même les taux sériques de certaines enzymes
hépatiques et de l'antigène carcino-embryonnaire étaient plus
fréquemment élevés chez les fermiers du Michigan que chez ceux du
Wisconsin. La prévalence des symptômes respiratoires et cutanés
était plus forte chez les travailleurs de l'industrie chimique avec
également des symptômes musculo-squelettiques moins fréquents que
chez les agriculteurs. Les résultats des travaux de l'ESL n'ont pas
toujours été interprétés de la même manière que ceux d'autres études
comparables, mais tous sont d'accord sur un point. Aucune de ces
séries d'études n'a mis en évidence de corrélation dose-réponse
positive entre les taux de PBB dans le sérum ou les tissus adipeux
et la prévalence des symptômes ou des anomalies cliniques. Un
certain nombre d'aspects cliniques ont été étudiés de manière plus
intensive par des méthodes spéciales. En particulier, l'examen des
aspects neurologiques au moyen de tests objectifs de performance a
révélé, dans une étude tout du moins, l'existence d'une corrélation
négative entre les taux sériques de PBB et les résultats des tests,
en particulier chez les hommes d'âge mûr. Quant aux autres études,
elles n'ont pas mis en évidence de relation entre la concentration
des PBB dans le sérum ou les tissus adipeux et les résultats d'une
batterie de tests portant sur la mémoire, la force musculaire, la
coordination, la perception cortico-sensorielle, la personnalité,
les fonctions cognitives supérieures et d'autres fonctions. Les
aspects pédiatriques de l'exposition aux PBB ont été étudiés dans
les familles examinées par l'ESL. Bien que de nombreux symptômes
aient été signalés, l'examen médical n'a pas révélé d'anomalie
objective attribuable à l'exposition au PBB. Des opinions
différentes se sont exprimées à propos des effets
neurospychologiques plus subtils observés dans la descendance de ces
personnes et les résultats des études portant sur la capacité de
développement sont également controversés. Cela vaut aussi pour
l'étude des lymphocytes et de la fonction immunitaire. C'est ainsi
que selon plusieurs auteurs, il n'y avait pas de différences entre
les groupes à fort et faible taux sérique de PBB pour ce qui
concerne le nombre de lymphocytes et les fonctions lymphocytaires,
alors que d'autres ont constaté une réduction sensible des
sous-populations de lymphocytes T et de lymphocytes B chez environ
40% des personnes exposées dans le Michigan, par rapport aux groupes
non exposés, avec en outre une altération de la fonction
lymphocytaire, à savoir une diminution de la réponse aux mitogènes.
Dans les études épidémiologiques qui ont été passées en revue,
on s'est efforcé d'évaluer la relation entre l'exposition aux PBB et
un grand nombre d'effets sur le comportement et de symptômes
subjectifs. Cependant, la plupart des ces études présentent de
graves insuffisances au niveau de la conception et en particulier,
elles laissent subsister des facteurs de confusion qui rendent
difficile, voire impossible, toute conclusion sur la relation
éventuelle entre l'exposition aux PBB et d'éventuels effets sur la
santé. On ne dispose pas d'un recul suffisant pour pouvoir évaluer
les effets cancérogènes éventuels de ces composés.
On a identifié deux petits groupes de travailleurs exposés de
par leur profession à un mélange de PBB ou à du DeBB et du DBBO. Des
lésions rappelant une chloracné ont été observées chez 13% des
travailleurs exposés au mélange de PBB, alors que ceux qui avaient
été exposés au DeBB ne présentaient pas de telles lésions. Toutefois
la prévalence de l'hypothyroïdie était plus élevée dans ce groupe.
1.9 Evaluation globale de la toxicité et de la cancérogénicité
La seule étude toxicologique qui ait été effectuée à vie sur
des animaux d'expérience a été menée récemment dans le cadre du
Programme national de toxicologie (National Toxicology Programme,
NTP) sur des rats et des souris. La dose la plus faible étudiée qui
produisait encore des effets cancérogènes était égale à 0,5 mg/kg de
poids corporel et par jour (formation de tumeurs hépatiques chez les
rongeurs). D'autres études du même genre ont mis en évidence un
effet cancérogène à la dose quotidienne de 3 mg/kg de poids
corporel, sur une durée de six mois. L'étude de six mois montre
qu'une exposition pendant une durée inférieure à la vie normale de
l'animal à des doses voisines, entraîne également des effets
délétères analogues. Il est possible qu'à plus faibles doses, les
PBB exercent des effets sur la reproduction des primates
sous-hominiens et des visons.
En outre, l'étude de deux ans effectuée dans le cadre du NTP a
montré qu'une dose quotidienne de 0,15 mg/kg de poids corporel avec
exposition prénatale et périnatale de la mère à une dose quotidienne
de 0,05 mg/kg de poids corporel, ne produisait aucun effet nocif.
Par conséquent, la dose totale absorbée quotidienne ment à partir de
la nourriture, de l'eau, de l'air et du sol devrait être inférieure
à 0,15 µg/kg de poids corporel, si l'on extrapole les résultats
obtenus concernant la dose sans effet nocif observable lors d'une
étude de cancérogénicité positive, en appliquant à cet effet un
coefficient d'incertitude (coefficient de sécurité) de 1000, puisque
ces composés induisent probablement des cancers par un mécanisme
épigénétique.
On estime que la dose totale reçue par le sous groupe de
population du Michigan se situait entre 0,15 et 15 mg/kg de poids
corporel sur une période de 230 jours. Si on rapporte cette dose à
la durée moyenne de vie normale d'un être humain, cela
correspondrait, pour cette population, à une dose quotidienne de 0,6
ng à 6 µg/kg de poids corporel.
On estime que pour les adultes de la population générale,
l'apport quotidien total de PBB par kg de poids corporel à partir
des sources répertoriées, est de l'ordre de 2 ng; il est de 10 ng
pour les nourrissons nourris au sein. Il convient de noter que ces
estimations reposent sur des données régionales très limitées.
Ces calculs reposent sur l'hypothèse que la concentration des
PBB n'atteindra pas un état stationnaire au cours de l'existence et
que l'on peut substituer une exposition forte sur une courte durée à
une exposition faible sur une longue durée, étant donné que ces
composés sont très mal métabolisés et excrétés.
On ne dispose pas de données suffisantes pour l'OcBB, le NoBB
et le DeBB pour calculer quel serait l'apport quotidien total
maximal ne produisant pas d'effets indésirables.
2. Conclusions
La plupart des homologues des PBB qui entrent dans la
composition des retardateurs de flammes vendus dans le commerce,
sont lipophiles, persistants et s'accumulent dans les biotes. Ces
composé subissent une bioamplification le long des réseaux
trophiques et constituent une menace, en particulier pour les
organismes qui se trouvent en fin de réseau. En outre, certains
constituants des PBB sont les précurseurs de dibenzofuranes
polybromés toxiques qui se forment lors de la combustion.
Outre les émissions qui se produisent au cours de la
fabrication et de l'utilisation des PBB, ceux-ci pénètrent dans
l'environnement du fait de la très large utilisation des
retardateurs de flammes dont ils sont les constituants. Une part
très importante des PBB produits finit par passer dans
l'environnement du fait de la très grande stabilité de ces composés.
On trouve également des PBB dans des échantillons prélevés dans
l'environnement et sur des sujets humains, en des lieux éloignés des
endroits où l'on sait que ces composés sont produits. La
composition en homologues des PBB dans les échantillons provenant de
l'environnement ne correspond pas à celle que l'on trouve dans les
produits techniques, ce qui indique qu'il y a transformation dans le
milieu, peut-être à la suite d'une débromation photochimique.
On dispose actuellement des très peu de données sur l'ampleur
de l'exposition de la population générale aux PBB. Toutefois, dans
les quelques cas où on a procédé à des mesures, on a pu mettre en
évidence des traces de PBB. Actuellement, cette exposition ne cause
pas d'inquiétude, mais il faudrait éviter que ces composés ne
continuent à s'accumuler. D'après les observations faites sur
l'homme à la suite de l'accident du Michigan, il semblerait que les
personnes contaminées aient été exposées à des doses de plusieurs
ordres de grandeur supérieures à celles que l'on observe dans la
population générale. On n'a pas observé dans la population du
Michigan d'effets concluants qui puissent être attribués à
l'exposition aux PBB, encore que la période de suivi ne soit pas
suffisante pour permettre à d'éventuels cancers de se manifester.
Etant donné que les taux de PBB dans les tissus adipeux et le sérum
restent élevés dans la population du Michigan, il y a poursuite de
l'exposition interne. En revanche, on a bien observé des effets
toxiques chez les bovins de cette région. On explique cette
discordance par le fait que les bovins avaient été davantage
exposés.
L'exposition professionnelle n'a été étudiée que dans deux
unités de production des Etats-Unis d'Amérique. Il semble que chez
les travailleurs employés à la production des PBB, il puisse y avoir
apparition de lésions rappelant une chloracné; quant aux
travailleurs exposés au DeBB, ils peuvent présenter une
hypothyroïdie. Aucune enquête n'a été menée chez les travailleurs
qui confectionnent des produits commerciaux à base de deca-, d'octa-
ou de nona-bromobiphényles.
Les PBB sont extrêmement persistants chez les organismes
vivants et ils peuvent conduire à une intoxication chronique et à
des cancers. Bien que la toxicité aiguë soit faible, on constate
l'apparition de cancers à des doses quotidiennes de 0,5 mg/kg de
poids corporel avec une dose sans effet observable de 0,15 mg/kg de
poids corporel et par jour. On a observé un certain nombre d'effets
toxiques chroniques chez les animaux de laboratoire à des doses
quotidiennes de l'ordre de 1 mg/kg de poids corporel, administrées
pendant de longues périodes.
3. Recommandations
3.1 Généralités
Le Groupe de travail estime qu'il faut éviter à l'homme et à
l'environnement d'être exposés aux PBB en raison de la forte
persistance et de la forte bioaccumulation de ces composés ainsi que
des effets nocifs qu'ils peuvent provoquer en cas d'exposition de
longue durée à de faibles doses. Aussi convient-il de ne plus
utiliser de PBB dans des produits du commerce.
Comme les données dont on dispose sur la toxicité du DeBB et de
l'OcBB sont limitées, qu'ils sont extrêmement persistants et
susceptibles d'être dégradés dans l'environnement et qu'en outre,
leur combustion entraîne la formation de dérivés encore plus
toxiques, ils ne doivent pas être utilisés dans le commerce, du
moins tant qu'on aura pas démontré que cet usage est sans danger.
La cohorte du Michigan est toujours en observation et il est
nécessaire que les données obtenues soient publiées.
3.2 Recherches futures
Il convient de développer la surveillance des PBB chez l'homme
et dans l'environnement, et en particulier sur les lieux de travail,
qu'il s'agisse de la fabrication proprement dite des PBB ou de leur
utilisation; cette surveillance devra porter sur chaque homologue en
particulier et englober également l'OcBB, le NoBB et le DeBB. Ces
composés doivent figurer dans les programmes de surveillance des
dérivés halogénés actuellement en cours. On devra notamment
continuer à suivre la tendance des concentrations de PBB dans
l'environnement et leur distribution géographique. On procédera
également à un relevé des décharges où des PBB sont susceptibles de
passer dans l'environnement.
Il faudrait procéder à des expériences de thermolyse simulant
les conditions d'un incendie accidentel ou de l'incinération de
déchets municipaux. Des travaux complémentaires devront également
être consacrés à l'étude du mécanisme de la toxicité et de la
cancérogénicité des PBB et des composés apparentés. Les PBB peuvent
être utilisés comme modèles pour ces recherches. Tous ces travaux
devront utiliser des homologues purifiés.
Les effets des PBB sur la reproduction restent mal connus.
Aussi serait-il souhaitable d'effectuer des études de longue durée
bien conçues, concernant l'effet des faibles doses sur la
reproduction, en utilisant une espèce vulnérable.
Il importe également d'obtenir davantage de renseignements sur
la biodisponibilité et la toxicocinétique de l'OcBB/NoBB, du DeBB et
d'un certain nombre d'homologues.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
Los bifenilos polibromados o polibromobifenilos (PBB) son un
grupo de hidrocarburos halogenados formados por sustitución del
hidrógeno del bifenilo por bromo. No se conoce ningún PBB de origen
natural. Estas moléculas responden a la fórmula
C12H(10-x-y)Br(x+y), donde x e y están comprendidos entre 1 y
5. Por consiguiente, teóricamente son posibles 209 formas
moleculares, pero sólo se han sintetizado individualmente y
caracterizado un reducido número. Los PBB fabricados para uso
comercial consisten principalmente en hexa-, octa-, nona-, y
decabromobifenilos, pero contienen también otros productos de la
misma familia. Son pirorretardantes que se emplean como aditivos, y
que mezclados con material polimérico líquido o sólido seco le
confieren propiedades pirorretardantes de tipo filtrante; en caso de
ignición se produce una liberación química de ácido bromhídrico.
La fabricación de los PBB se basa en una reacción de
Friedel-Crafts entre el bifenilo y el bromo, en presencia de un
disolvente orgánico en ocasiones, y de un catalizador que puede ser
cloruro de aluminio, bromuro de aluminio o hierro.
La mayor parte de las investigaciones realizadas se refieren a
los productos FireMaster BP-6 y FF-1, responsables de la catástrofe
que se produjo en Michigan cuando, inadvertidamente, fueron
agregados al pienso de los animales en lugar del óxido de magnesio
que correspondía. La consiguiente contaminación acarreó la muerte de
miles de cabezas de ganado vacuno, porcino y ovino, así como de
millones de pollos.
La composición de la mezcla FireMaster(R) varía de un lote a
otro, pero sus principales componentes son el 2,2',4,4',5,5'-
hexabromobifenilo (60-80%) y el 2,2',3,4,4',5,5'-heptabromobifenilo
(12-25%), junto con los cuales se hallan también otros compuestos
menos bromados que son el resultado de una reacción de bromación
incompleta. Se han detectado también bromoclorobifenilos y
naftalenos polibromados como componentes minoritarios de
FireMaster(R). FireMaster FF-1 (polvo blanco) se obtiene a partir
de FireMaster BP-6 (escamas pardas), por adición, como agente
antiaglutinante, de silicato de calcio al 2%.
Los PBB son sólidos de baja volatilidad; ésta disminuye al
aumentar el número de átomos de bromo. Son prácticamente insolubles
en agua, solubles en grasas, y entre poco y muy solubles en diversos
disolventes orgánicos; la solubilidad también disminuye al aumentar
el número de átomos de bromo. Son compuestos relativamente estables
y químicamente inertes, pero las mezclas de PBB muy bromados se
fotodegradan y sufren una debromación reductiva al ser expuestas a
la radiación ultravioleta.
Los productos de la descomposición térmica experimental de los
PBB dependen de la temperatura, de la cantidad de oxígeno presente y
de otros varios factores. Las investigaciones realizadas sobre la
pirólisis de FireMaster BP-6 en ausencia de oxígeno (600-900 °C) han
demostrado que se forman bromobencenos y bifenilos menos bromados,
pero no así furanos polibromados. Por el contrario, la pirólisis en
presencia de oxígeno (700-900 °C) generó una cierta cantidad de di-
a heptabromodibenzofuranos. En presencia de poliestireno y
polietileno se hallaron niveles más altos. La pirólisis de
FireMaster BP-6 en presencia de PVC a 800 °C dio lugar a una mezcla
de bromoclorobifenilos. No se dispone de información sobre la
naturaleza de los productos de incineración de los materiales que
contienen PBB. Poco se sabe acerca de la toxicidad de las dioxinas y
furanos bromados y bromados/clorados, pero se estima que debe ser de
aproximadamente la misma magnitud que la de las dioxinas y furanos
clorados.
La principal técnica analítica empleada para el control
biológico de los PBB en muestras del medio y en tejidos y líquidos
biológicos tras la catástrofe de Michigan fue la cromatografía de
gases con detector de captura de electrones. Los diversos productos
de la familia pueden determinarse individualmente mediante cromato
grafía de gases capilar, y aún es posible conseguir una detección
más específica si se emplea la espectrometría de masas de control de
determinados iones. Como el número de posibles miembros de esta
familia de productos es muy elevado, las investigaciones se ven
dificultadas por la falta de patrones sintéticos adecuados. Los
métodos empleados para extraer PBB de muestras biológicas se han
venido basando en los usados con los plaguicidas. Los PBB son
extraídos con la grasa, y purificados a continuación.
El hallazgo reciente de PBB en muestras biológicas de fondo no
significa necesariamente que su concentración esté aumentando en el
medio: ello podría deberse a la aparición de técnicas analíticas más
sensibles, como la espectrometría de masas de ionización química de
iones negativos. De ahí la necesidad de realizar cuanto antes
estudios retrospectivos. Los métodos mejorados de purificación
exhaustiva permiten realizar análisis específicos de los PBB
coplanares tóxicos, datos que son igualmente necesarios.
1.2 Fuentes de exposición humana y ambiental
La producción comercial de FireMaster(R) comenzó en los
Estados Unidos en 1970, pero se interrumpió tras la catástrofe de
Michigan (noviembre de 1974). La producción estimada de PBB en los
Estados Unidos entre 1970 y 1976 fue de 6000 toneladas (cantidades
comerciales); hasta 1979 se siguió produciendo en el país
octabromobifenilo y decabromobifenilo. En Alemania se produjo hasta
mediados de 1985 una mezcla de PBB altamente bromados conocida como
Bromkal 80-9 D. Actualmente se produce en Francia decabromobifenilo
(Adine 0102) de calidad técnica. Al parecer, esos son los únicos PBB
que se siguen produciendo hoy en día.
Los PBB se introdujeron a principios de los años setenta como
pirorretardantes. Hasta noviembre de 1974 el PBB más importante
comercialmente en los Estados Unidos era el hexabromobifenilo,
producto que se incorporaba a los plásticos (10% de contenido de
PBB), de acrilonitrilo-butadieno-estireno (ABS), material usado
principalmente en la fabricación de pequeños utensilios y
componentes de automóvil, revestimientos, barnices y espuma de
poliuretano. Los otros PBB pirorretardantes tienen aplicaciones
similares.
Durante el proceso normal de producción pueden tener lugar
pérdidas de PBB en el medio ambiente, por emisión a la atmósfera o
por su incorporación a aguas residuales, suelos o vertederos,
pérdidas que sin embargo, según se ha observado, son por lo general
de escasa importancia.
Estos compuestos pueden llegar también al medio durante su
transporte y manipulación, así como de manera accidental, como
ocurrió en Michigan.
Existe también la posibilidad de que pasen al medio de resultas
de la incineración de materiales que contienen PBB, o a causa de
fuegos accidentales, formándose en estos casos otros productos
tóxicos, como polibromodibenzofuranos o derivados mixtos de bromo y
cloro.
La mayor parte de los compuestos así formados acaban
difundiéndose a la larga al medio, como tales o en forma de
productos de degradación.
1.3 Transporte, distribución y transformación en el medio ambiente
No hay pruebas de que los PBB se propaguen por la atmósfera a
grandes distancias, pero la presencia de estos compuestos en
muestras de focas del Artico pone de manifiesto una amplia
distribución geográfica.
Las principales vías conocidas de llegada de los PBB al medio
acuático son los vertidos de desechos industriales y los lixiviados
de lugares de vertimiento industrial que alcanzan las aguas, así
como la erosión de suelos contaminados. Los PBB son casi insolubles
en agua y se hallan sobre todo en los sedimentos de lagos y ríos
contaminados.
Los focos de contaminación del suelo pueden ser las fábricas o
los depósitos de residuos de PBB. Los PBB que llegan a penetrar en
el suelo no se desplazan fácilmente. Se ha observado que los PBB son
200 veces más solubles en el lixiviado de un vertedero que en el
agua destilada, lo que puede significar una mayor propagación en el
medio ambiente. Debido a sus propiedades hidrofóbicas, cuando están
en solución acuosa estos productos son fácilmente adsorbidos por los
suelos. Se observó una adsorción preferencial de determinados PBB en
función de las características del suelo (por ejemplo de su
contenido orgánico) y del número y posición de los radicales de
bromo.
Los PBB son estables y persistentes, lipofílicos, y sólo
ligeramente solubles en agua; algunos de los compuestos de esta
familia apenas son metabolizados y se acumulan en los compartimentos
lipídicos de la biota. Una vez liberados en el medio ambiente,
pueden alcanzar la cadena alimentaria y concentrarse en ella.
Se han detectado PBB en el pescado capturado en varias
regiones. La ingestión de pescado es una vía de transmisión de PBB a
los mamíferos y las aves.
Se considera improbable que los PBB se degraden mediante
reacciones químicas puramente abióticas (excluidas las reacciones
fotoquímicas). Se ha notificado la persistencia de PBB en el
terreno. Al cabo de varios años del accidente de Michigan, el
análisis de muestras del suelo de un antiguo centro de fabricación
de PBB reveló la presencia, aún, de ese tipo de productos, aunque el
perfil de los PBB era distinto, debido a la degradación parcial
sufrida por los residuos en la muestra de suelo.
En condiciones de laboratorio los PBB son degradados fácilmente
por la radiación ultravioleta. La fotodegradación de la mezcla
comercial FireMaster(R) se refleja en una menor concentración de
los PBB que presentan más sustituyentes. No se han determinado con
exactitud ni la velocidad ni la magnitud de las reacciones
fotolíticas que sufren los PBB en el medio, pero las observaciones
realizadas sobre el terreno muestran una elevada persistencia de los
PBB originales, o bien una degradación parcial a formas menos
bromadas.
En las investigaciones de laboratorio las mezclas de PBB
parecen bastante resistentes a la degradación microbiana.
No se ha descrito ningún fenómeno de captación o degradación de
PBB por las plantas. En cambio, los PBB son fácilmente absorbidos
por los animales, en los que se ha observado que son muy
persistentes, aun cuando se han detectado pequeñas cantidades de
metabolitos. Los principales productos metabólicos eran
hidroxiderivados, y en algunos casos se hallaron indicios de la
existencia de PBB parcialmente debromados. No se ha descrito
investigación alguna sobre posibles metabolitos sulfurados análogos
a los de los PCB.
Se ha investigado la bioacumulación de PBB en el pescado, así
como la que se produce en animales terrestres, en este caso mediante
el estudio de especies de mamíferos y aves. Los datos obtenidos
proceden de observaciones sobre el terreno, de la evaluación de la
catástrofe de Michigan, y de estudios controlados de alimentación de
los animales. Por lo general se observó que la acumulación de PBB en
la grasa corporal dependía de la dosis y de la duración de la
exposición.
Al analizar individualmente los PBB, se observa que su
bioacumulación aumenta con el grado de bromación, al menos hasta los
tetrabromobifenilos. Cabe suponer que los productos más bromados de
la familia se acumulan aún en mayor medida. No obstante, no se
dispone de información sobre el decabromo bifenilo, cuya absorción
es posiblemente escasa.
Se ha notificado la generación de dibenzofuranos bromados o PBB
parcialmente debromados como productos de la descomposición térmica
de los PBB. Su aparición depende de varios factores, como por
ejemplo la temperatura, el oxígeno, etc.
1.4 Niveles ambientales y exposición humana
Tan sólo se dispone de los resultados de un estudio sobre los
niveles de PBB en la atmósfera. En dicho estudio se determinaron las
concentraciones de esos productos en las proximidades de tres
plantas de fabricación o procesamiento de PBB de los Estados Unidos.
Se analizaron también los niveles alcanzados en las aguas
superficiales en esas mismas inmediaciones y en el vertedero del
distrito de Gratiot/Michigan (EE.UU.), al que entre 1971 y 1973
fueron a parar más de 100 000 kg de desechos, constituidos en un
60-70% por PBB.
El análisis de las aguas subterráneas del vertedero del
distrito de Gratiot reveló la presencia de cantidades ínfimas de PBB
incluso fuera de la zona del vertedero, pero no se detectaron PBB en
los pozos de agua de bebida del entorno.
Se dispone de datos sobre la contaminación del suelo por PBB en
zonas de fabricación, empleo o evacuación de PBB, así como en los
suelos de los campos de las granjas de Michigan contaminadas por
PBB.
La catástrofe de Michigan sobrevino porque, por inadvertencia,
se añadió FireMaster(R) al pienso destinado a los animales. No fue
sino al cabo de casi un año cuando se descubrió el error de mezcla,
y los análisis efectuados mostraron que el origen del problema eran
los PBB. Durante ese periodo (verano de 1973 a mayo de 1974), los
animales contaminados y sus productos se difundieron entre los
suministros de alimentos para el hombre y en el medio en el estado
de Michigan. Centenares de granjas se vieron afectadas, y miles de
animales tuvieron que ser sacrificados y enterrados, al igual que
hubo que enterrar miles de toneladas de productos agrícolas.
La mayor parte de los datos disponibles sobre la contaminación
de la fauna por PBB se refieren a peces y aves de los Estados Unidos
y Europa, sobre todo aves acuáticas, de las inmediaciones de centros
industriales, y mamíferos marinos.
Los últimos estudios sobre la contaminación por PBB de peces,
mamíferos terrestres y marinos y aves de los Estados Unidos y Europa
muestran una amplia distribución de esos compuestos. El perfil de
los PBB hallados en las muestras de pescado es muy distinto del
hallado en los productos comerciales. Muchos de los picos más
importantes podrían ser el resultado de la debromación fotoquímica
del decabromobifenilo (BB 209), hipótesis no confirmada.
Tras el accidente de Michigan se observaron casos de exposición
profesional entre los empleados de fábricas de la industria química
de los Estados Unidos, así como entre los trabajadores agrícolas.
Los niveles medianos de PBB en suero y tejido adiposo eran mayores
entre los trabajadores de la industria química. No se dispone de
información de otros países o compañías sobre la exposición
profesional asociada a la fabricación, formulación y usos
comerciales de esos productos.
Respecto a la mayoría de las poblaciones humanas, no se han
notificado datos directos sobre la exposición a PBB a partir de
diversas fuentes. En relación con el caso de Michigan (Estados
Unidos) se ha notificado la exposición humana masiva resultante del
contacto directo con pienso contaminado y, sobre todo, del consumo
de carne, huevos y productos lácteos que contenían PBB. Al menos
2000 familias (principalmente agricultores y sus vecinos) se vieron
expuestas a muy altos niveles. Recientemente se han detectado PBB en
muestras de leche de vaca y leche humana en Alemania.
El perfil de los PBB de estas muestras difiere del hallado en
el pescado. Así, la concentración relativa de BB 153 es mayor en la
leche humana que en el pescado.
Las vías de exposición de la población general a los PBB no se
conocen con precisión. A tenor de los conocimientos actuales, el
aire y el agua ambientales no contienen niveles elevados. Los
alimentos ricos en lípidos, sobre todo los procedentes de aguas
contaminadas, son probablemente muy importantes a ese respecto. No
se dispone de información sobre los niveles de exposición en el aire
de espacios interiores ni sobre la exposición cutánea a materiales
con PBB pirorretardantes.
El perfil de los PBB detectados en la leche humana analizada en
Alemania era parecido al hallado en la leche de vaca de la misma
región, pero los niveles detectados en las muestras humanas eran
considerablemente mayores.
Son muy pocos los datos disponibles para fundamentar el cálculo
de la ingesta diaria de PBB a través de los alimentos por parte de
la población general. Si suponemos que el pescado contiene 20 µg
PBB/kg de lípido y un 5% de lípidos y que una persona de 60 kg
consume 100 g de pescado al día, la ingesta resultante es de 0,002
µg/kg de peso corporal al día. Para esa misma persona, una
concentración de PBB de 0,05 µg/kg de lípido en la leche (4% de
lípidos) y un consumo de leche de 500 ml/día determinarían una
ingesta de PBB de aproximadamente 0,00002 µg/kg de peso corporal al
día.
Si suponemos que la leche materna contiene 2 µg PBB/kg de
lípido, un lactante de 6 kg que consuma 800 ml de esa leche (3,5% de
lípidos) al día ingerirá 0,01 µg PBB/kg de peso corporal al día.
1.5 Cinética y metabolismo
La absorción gastrointestinal de los PBB varía según el grado
de bromación; así, los compuestos menos bromados se absorben más
fácilmente.
La información disponible sobre la absorción de DeBB y
OcBB/NoBB es insuficiente.
Los PBB están distribuidos en todas las especies animales y en
el hombre y alcanzan su mayor concentración de equilibrio en el
tejido adiposo. También se han hallado niveles relativamente altos
en el hígado, sobre todo de los PBB más tóxicos, que al
parecertienden a concentrarse en ese órgano. Los coeficientes de
reparto de los diversos PBB entre varios tejidos parecen diferir.
Por lo general se observa una marcada tendencia a la bioacumulación.
En los mamíferos la transferencia de PBB a la descendencia se
produce a través de la placenta y de la leche. Se observó que la
leche humana contenía niveles de 2,2',4,4',5,5'-hexabromobifenilo
más de 100 veces superiores a los niveles séricos maternos. En un
estudio realizado sobre varias generaciones de ratas, tras
administrar PBB a una de las generaciones se observaron residuos
detectables en más de dos generaciones sucesivas. Los huevos de
especies aviares también se vieron afectados por el contenido
corporal materno de PBB.
Muchos PBB tienden a persistir en los sistemas biológicos. No
se obtuvieron indicios de un metabolismo o excreción importantes de
los componentes más abundantes en la mezcla FireMaster(R) ni del
octa- o decabromobifenilo. Los estudios metabólicos in vitro
mostraron que hay relaciones estructura-actividad que explican el
metabolismo de los PBB. Los microsomas inducidos por FB
(fenobarbital) sólo metabolizaban los PBB que poseían carbonos
adyacentes no bromados, meta y para respecto al puente bifenilo en
al menos uno de los anillos. La metabolización por microsomas
inducidos por MC (3-metilcolantreno) estaba condicionada por la
presencia de posiciones adyacentes orto y meta no bromadas en al
menos un anillo del PBB, que debía tener pocos sustituyentes, ya que
una mayor bromación parecía impedir el metabolismo. Se ha observado
que, en los vertebrados, los derivados hidroxilados son unos de los
principales productos del metabolismo, in vitro e
in vivo, de los bifenilos menos bromados; la intensidad de la
transformación metabólica fue relativamente baja. La hidroxilación
se produce probablemente tanto mediante la generación previa de
óxidos de hidrocarburos aromáticos como de forma directa.
El hombre, la rata, el rhesus, el cerdo, la vaca y la gallina
eliminan los PBB fundamentalmente por las heces. En la mayoría de
los casos la velocidad de excreción parece ser baja. Las
concentraciones de 2,2',4,4',5,5'-hexabromobifenilo observadas en la
bilis y las heces humanas equivalían aproximadamente a entre 1/2 y
7/10 de los niveles séricos y a aproximadamente el 0,5% de los
niveles observados en el tejido adiposo. Los tratamientos aplicados
para facilitar la eliminación de los PBB por los animales o el
hombre fueron de escasa o nula eficacia. Otra forma de eliminación
es la excreción a través de la leche.
Tras la administración de PBB a ratas y otros animales, las
concentraciones tisulares del producto evolucionaron con el tiempo
de forma compleja y diversa. Esa evolución se ha descrito mediante
modelos de varios compartimentos. Se calculó una semivida de
aproximadamente 69 semanas para la eliminación del
2,2',4,4',5,5'-hexabromobifenilo de la grasa corporal de la rata. En
el rhesus se observó una semivida de más de cuatro años. En el
hombre se calcula que la semivida de ese mismo compuesto oscila como
promedio entre 8 y 12 años; pero, según lo publicado, ese margen
podría estar comprendido entre 5 y 95 años. Hay algunas diferencias
de retención y de recambio entre los distintos PBB. Los resultados
de los análisis del suero de agricultores y trabajadores de la
industria química por lo que se refiere al
2,3',4,4',5-pentabromobifenilo fueron incongruentes, probablemente
porque las fuentes de exposición eran distintas. Los trabajadores de
la industria estaban expuestos a todos los componentes de la mezcla
FireMaster(R), mientras que la población de Michigan estuvo
expuesta a carne y leche contaminadas por otra combinación de PBB,
debido a los cambios metabólicos sufridos por los compuestos en los
animales de trabajo. En un estudio realizado en ratas, los niveles
de bromo del tejido adiposo no disminuyeron cuando se administró
octabromobifenilo de calidad técnica. No se dispone de información
sobre la retención del decabromobifenilo.
El hombre presenta quizá una mayor tendencia a retener
determinados PBB que la observada en los animales de
experimentación. Este factor debería tenerse en cuenta a la hora de
evaluar los riesgos que entrañan esos productos químicos para la
salud humana.
En resumen, todos los datos disponibles indican que los PBB
presentan una marcada tendencia a la bioacumulación y la
persistencia. Se metabolizan lentamente, y sus semividas en el
hombre son del orden de al menos 8 a 12 años.
1.6 Efectos en los seres vivos del medio ambiente
Los pocos datos de que se dispone sobre los efectos de los PBB
en los seres vivos del medio ambiente se refieren a micro
organismos, pulgas de agua, aves acuáticas y animales de trabajo.
Se realizó un estudio sobre las aves acuáticas que anidaban en
las islas del noroeste del lago Michigan para averiguar si los
contaminantes del medio tenían alguna influencia en su reproduc
ción. Se determinaron los niveles de 17 contaminantes, incluidos
diversos PBB, pero al parecer ninguno tenía efectos pronunciados
sobre la reproducción.
El ganado de labor que ingirió el pienso que por error contenía
FireMaster(R) FF-1 en lugar de óxido de magnesio enfermó. El valor
promedio estimado de la exposición sufrida por las vacas de la
primera explotación en que se observó una alta contaminación fue de
250 mg/kg de peso corporal. Los signos clínicos de toxicidad
consistieron en una reducción del 50% del consumo de pienso
(anorexia) y una disminución del 40% de la producción de leche,
manifestaciones observadas algunas semanas después de la ingestión
del pienso contaminado. Aunque la administración del pienso
suplementado se interrrumpió a los 16 días, la producción de leche
no se reanudó. Algunas de las vacas presentaron una mayor frecuencia
miccional y lacrimación y desarrollaron hematomas, abscesos,
crecimiento anormal de las pezuñas, cojera, alopecia,
hiperqueratosis y caquexia; varias murieron menos de seis meses
después de la exposición. Globalmente, la tasa de mortalidad en esa
explotación fue de 24/400. Sin embargo, entre los terneros de 6 a 18
meses la tasa de mortalidad fue mucho más alta: aproximadamente un
50% murieron al cabo de menos de seis semanas, y sólo dos de 12
sobrevivieron más de cinco meses. Los animales desarrollaron
hiperqueratosis por todo el cuerpo. Se observaron también diversos
problemas relacionados con la reproducción.
Se han notificado los resultados de las autopsias de algunas de
las vacas adultas que murieron durante los primeros seis meses tras
la exposición. Los estudios histopatológicos revelaron alteraciones
de diverso tipo del hígado y los riñones.
Varios de los signos clínicos y cambios patológicos señalados
anteriormente fueron confirmados más tarde mediante estudios
controlados de administración de pienso (anorexia, deshidratación,
lacrimación excesiva, emaciación, hiperqueratosis, problemas de
reproducción, ciertos cambios de los parámetros químicos clínicos y
lesiones renales).
Se notificó una caída de la producción y la aparición de
esterilidad en las manadas afectadas por un bajo nivel de
contaminación. Esto contrasta con los resultados de unos estudios
controlados en los que no se halló ninguna diferencia significativa
entre los rebaños sometidos a una contaminación baja y los rebaños
testigo.
Aunque el error que provocó el accidente afectó originalmente
al pienso del ganado vacuno, el pienso de otros animales también se
vio afectado por la contaminación cruzada que se produjo, por
ejemplo, en los mezcladores de las compañías productoras del pienso.
Probablemente la exposición no llegó a ser tan alta como la del
ganado vacuno. Se notificó también la contaminación de otros
animales que fueron igualmente sacrificados (aves de corral, cerdos,
caballos, conejos, cabras y ovejas), pero sin concretar las
manifestaciones de la enfermedad.
No se dispone de información sobre los efectos de los PBB en el
ecosistema.
1.7 Efectos en los animales de experimentación y en los sistemas
de prueba in vitro
Las DL50 de las mezclas comerciales administradas por vía
oral o cutánea muestran un nivel relativamente bajo de toxicidad
aguda (DL50 > 1 g/kg de peso corporal) en la rata, el conejo y la
codorniz. En estos casos la muerte y las manifestaciones agudas de
toxicidad aparecieron con mayor retraso tras la administración de
PBB. La dosis total administrada determinó el grado de toxicidad, ya
se tratase de una dosis única, ya de dosis repetidas durante breves
periodos (hasta 50 días). La toxicidad de los PBB fue mayor cuando
se administraron varias dosis que cuando se administró una sola
dosis. Se observa un efecto dilatorio sobre la mortalidad tras la
exposición a los PBB.
Los escasos estudios realizados con mezclas comerciales de
octa- y decabromobifenilo no revelaron ninguna influencia en la
mortalidad de ratas y peces. Por lo que se refiere al análisis
individual de los PBB, sólo se han analizado tres hexaisómeros:
3,3',4,4',5,5'-HxBB, 2,2',4,4',5,5'-HxBB y 2,3',4,4',5,5'-HxBB, el
último de los cuales es más tóxico para la rata que el anterior. A
juzgar por los datos limitados de que se dispone, el OcBB y el DeBB
parecen menos tóxicos que las mezclas de PBB y son peor absorbidos.
En numerosos estudios sobre los efectos agudos y a corto plazo,
entre los signos de toxicidad por PBB (sobre todo por FireMaster) se
ha observado una disminución del consumo de pienso. A dosis letales,
la muerte no se puede atribuir a la alteración patológica de un
determinado órgano sino más bien a un «síndrome de emaciación» que
desarrollan los animales como primera manifes tación de toxicidad.
En el momento de la muerte la pérdida de peso puede ser de hasta un
30-40%. Los pocos estudios realizados con OcBB y DeBB de calidad
técnica no revelaron ningún efecto de ese tipo.
Los cambios morfológicos e histopatológicos causados por la
exposición a los PBB afectan sobre todo al hígado. El aumento de
tamaño de este órgano se produce con frecuencia a dosis inferiores a
las requeridas para inducir cambios en el peso corporal. En las
especies roedoras las alteraciones histopatológicas consisten
principalmente en la hinchazón y vacuolación masivas de los
hepatocitos, la proliferación del retículo endoplasmático liso y una
necrosis de células aisladas. La gravedad de las lesiones depende de
la dosis y del tipo de PBB administrados.
Se observó una disminución del peso del timo en la rata, el
ratón y el ganado vacuno tras la exposición a FireMaster(R), pero
no así a OcBB o DeBB.
En algunas publicaciones se menciona un aumento del peso de la
glándula tiroides y cambios histológicos en el tiroides de la rata,
efectos observados a bajas concentraciones.
Está demostrado que los distintos PBB difieren en cuanto al
perfil de toxicidad. Los PBB más tóxicos provocan una disminución
del peso del timo y/o del organismo y causan cambios histológicos
pronunciados en el hígado y el timo. Se ha propuesto una
clasificación de los bifenilos halogenados basada en criterios
estructurales. La clase 1 abarca los productos de la familia (con
sus distintos isómeros) que carecen de sustituyentes en posición
orto (PBB coplanares). La segunda clase abarca los monoderivados con
sustituyente en posición orto. Los otros PBB (principalmente los que
poseen dos o más orto-bromos) pertenecen a la tercera clase. Los PBB
de la clase 1 son los que suelen tener efectos más graves, mientras
que los productos de la segunda y la tercera clases presentan una
toxicidad decreciente. Dentro de cada clase la toxicidad depende
también del grado de bromación.
En todas las combinaciones analizadas, el PBB más tóxico
resultó ser el 3,3',4,4',5,5'-HxBB. Este compuesto está presente a
baja concentración en la mezcla FireMaster(R). De los principales
componentes de ésta, el más tóxico fue el 2,3,3',4,4',5-HxBB,
seguido del 2,3',4,4',5,5'-HxBB y el 2,3',4,4',5-PeBB. El principal
componente de la mezcla FireMaster, el 2,2',4,4',5,5'-HxBB, era
relativamente atóxico, al igual que el 2,2',3,4,4',5,5'-HpBB,
segundo componente más abundante.
La influencia del contenido de los diversos PBB (y de otros
posibles contaminantes) sobre la toxicidad de las mezclas de calidad
técnica de OcBB y DeBB no se conoce con tanto detalle.
Las pruebas habituales de irritación cutánea y ocular y de
sensibilización efectuadas con las mezclas PBB de calidad técnica
analizadas (OcBB y DeBB) fueron negativas, o a lo sumo revelaron una
reacción moderada. No obstante, se observaron hiperqueratosis y
pérdida de pelaje en el ganado vacuno, y lesiones parecidas al
cloracne en el rhesus, provocadas en los animales por la ingestión
de FireMaster(R). Esta mezcla provocó hiperqueratosis en la
superficie interna de la oreja del conejo, cosa que no ocurrió con
sus principales componentes (2,2',4,4',5,5'-HxBB y
2,2',3,4,4',5,5'-HpBB). El fraccionamiento de la mezcla
FireMaster(R) mostró que la mayor parte de la actividad
correspondía a las fracciones más polares, que contenían componentes
minoritarios. La aplicación de HxBB irradiado con luz solar provocó
una grave hiperqueratosis en la oreja del conejo.
En trabajos realizados en la rata, la administración prolongada
de dosis bajas de OcBB de calidad técnica no influyó ni en el
consumo de pienso ni en el peso corporal, pero se observó un aumento
del peso relativo del hígado de las ratas expuestas a dosis de 2,5
mg/kg de peso corporal durante 7 meses. La administración prolongada
de FireMaster(R) a ratas a dosis de 10 mg/kg de peso corporal
durante 6 meses no influyó en el consumo de alimento. La
administración durante 6 meses de dosis de 1 mg/kg de peso corporal
afectó al peso del hígado. El peso del timo disminuyó en las ratas
hembras a las que se administraron 0,3 mg/kg de peso corporal. Se
observaron también cambios histopatológicos. Los estudios
prolongados y controlados de administración de pienso a ganado
vacuno expuesto a dosis bajas de FireMaster(R) no pusieron de
manifiesto ningún efecto adverso por lo que se refiere a ingesta de
alimentos, signos clínicos, cambios clinicopatológicos o desarrollo
físico. El visón, el cobayo y el mono parecen más susceptibles a la
toxicidad por PBB.
Se han estudiado los efectos a largo plazo provocados en la
rata por los PBB retenidos tras la exposición pre- o perinatal a
dosis altas de FireMaster(R).
Los efectos adversos más frecuentes sobre la reproducción
fueron los embarazos malogrados o perdidos y la disminución de la
viabilidad de la descendencia. Se observaron aún ciertos efectos en
el visón a concentraciones de 1 mg/kg de alimento. Se observó
también una disminución de la viabilidad de la descendencia de
rhesus expuestos durante 12,5 meses a FireMaster(R) (0,3 mg/kg de
alimento). Los monos recibieron una dosis diaria de 0,01 mg/kg de
peso corporal y una dosis total de 3,8 mg/kg de peso corporal. Los
resultados de los estudios sobre reproducción y neurología del
comportamiento realizados con monos y ratas expuestos a dosis bajas
no pudieron ser evaluados debido a que la información aportada en
los artículos publicados acerca del diseño de los experimentos era
insuficiente. Se observó un débil efecto teratógeno en experimentos
realizados con roedores, a dosis elevadas que podrían haber causado
una cierta toxicidad en la madre.
Los PBB interaccionan con el sistema endocrino. En la rata y el
cerdo se observaron disminuciones dosis-dependientes de la tiroxina
y la triyodotironina séricas. También se ha notificado que los PBB
alteran los niveles de las hormonas esteroides en la mayoría de los
casos. La intensidad del efecto depende de la especie, así como de
la dosis y la duración del tratamiento.
Los PBB provocan porfiria en la rata y en el ratón macho a
dosis de sólo 0,3 mg/kg de peso corporal al día. El nivel sin efecto
fue de 0,1 mg/kg de peso corporal al día. Se observó una pronunciada
influencia de los PBB sobre la acumulación de vitamina A, así como
efectos sobre el metabolismo intermediario.
Una observación frecuente tras la exposición a PBB fue la
atrofia del timo, y se han observado también efectos en otros
tejidos linfoides. Se ha demostrado asimismo la existencia de otros
signos de depresión de la función inmunitaria en respuesta a la
mezcla FireMaster(R). No hay datos disponibles sobre los OcBB,
NoBB y DeBB ni sobre PBB particulares.
Uno de los efectos más estudiados de los PBB es la inducción
que provocan de las enzimas de actividad oxidasa de función mixta
(MFO). Como era de esperar, se descubrió que FireMaster(R) era un
inductor de tipo mixto de las enzimas microsómicas hepáticas tanto
en la rata como en todas las demás especies animales estudiadas.
Este fenómeno de inducción se observó también en menor medida en
otros tejidos. La capacidad de inducción de las enzimas microsómicas
hepáticas variaba de un PBB a otro; no obstante, se han descubierto
correlaciones entre su estructura y la actividad de inducción de las
enzimas microsómicas.
Varios estudios han puesto de manifesto que los PBB pueden
modificar la actividad biológica de diversos fármacos y sustancias
tóxicas. Esto se debe quizá en parte a la capacidad de los PBB para
inducir las enzimas microsómicas implicadas en la activación o
desactivación de los productos xenobióticos.
Se observó que FireMaster(R) y algunos de sus principales
componentes podían inhibir la comunicación intercelular in vitro;
esta inhibición se produce a concentraciones no citotóxicas. Tanto
la citotoxicidad como las propiedades de inhibición de la
cooperación metabólica parecen guardar relación con la estructura de
los PBB, concretamente con la presencia o ausencia de sustituyentes
en posición orto.
Los ensayos realizados in vitro e in vivo (mutagénesis de
células microbianas y de mamífero, lesiones cromosómicas de células
de mamífero, transformación de células de mamífero, lesión y
reparación del ADN) no han revelado ningún tipo de mutagenicidad o
genotoxicidad causadas por PBB particulares o por mezclas
comerciales de los mismos.
Los estudios de toxicidad prolongados han revelado que el
principal órgano de manifestación de los efectos carcinógenos de los
PBB es el hígado. La incidencia de carcinoma hepatocelular aumentó
significativamente en las ratas y los ratones machos y hembras a los
que se administró la mezcla FireMaster(R) por vía oral. Se han
notificado efectos carcinógenos en el hígado de ratones sometidos a
dietas que contenían Bromkal 80-9D (nonabromobifenilo de calidad
técnica) a dosis de 100 mg/kg (5 mg/kg de peso corporal al día) o
más durante 18 meses. La dosis más baja de PBB que produjo tumores
(fundamentalmente adenomas) en los roedores fue de 0,5 mg/kg de peso
corporal al día durante 2 años. Las ratas que recibieron 0,15 mg/kg
de peso corporal al día además de la exposición pre- y perinatal no
sufrieron ningún efecto adverso. No se ha estudiado la
carcinogenicidad del octabromobifenilo y el decabromobifenilo de
calidad técnica.
En un bioensayo realizado sobre la piel del ratón no se observó
ninguna actividad de iniciación tumoral (usando
12-O-tetradecanoilforbol-13-acetato (TPA) como agente activador) o
activación tumoral (usando 7,12-dimetil-benz(a)antraceno (DMBA) como
iniciador) por parte de FireMaster BP-6 o del 2,2',4,4',5,5'-
hexabromobifenilo. Sin embargo, en otros modelos basados en la piel
del ratón (en los que se empleó DMBA o DN-metil- N'-nitro-
N-nitroso-guamidina (MNNG) como iniciadores), FM FF-1 y el
3,3',4,4',5,5'-hexabromobifenilo, pero no así el 2,2',4,4',5,5'-
hexabromobifenilo, tuvieron efecto como activadores tumorales. En un
bioensayo de dos fases realizado con hígado de rata usando
fenobarbital como activador, el 3,3',4,4'-tetrabromobifenilo mostró
una débil actividad iniciadora. En el modelo de dos fases utilizado
con el hígado de rata, con empleo de dietilnitrosamina y
hepatectomía parcial, la mezcla FM, el 3,3',4,4'-tetrabromobifenilo
y el 2,2',4,4',5,5'-hexabromobifenilo, pero no así el
3,3',4,4',5,5'-hexabromobifenilo, tuvieron efecto como activadores
tumorales.
Los resultados de los estudios sobre la comunicación celular,
los resultados negativos de los estudios de genotoxicidad y
mutagenicidad y los resultados de los ensayos de activación tumoral
indican que las mezclas y los miembros de la familia estudiados
provocan cáncer por mecanismos epigenéticos. No se dispone de
información sobre los octa-, nona-, o decabromo bifenilos de calidad
técnica.
Se desconocen los mecanismos de acción subyacentes a las
numerosas manifestaciones de toxicidad de los PBB y de otros
compuestos relacionados. No obstante, algunos de los efectos, como
el síndrome de emaciación, la atrofia del timo, la hepatotoxicidad,
los trastornos cutáneos y la toxicidad sobre el sistema reproductor
podrían guardar relación con la interacción con el llamado receptor
Ah- o TCDD, que alteraría la expresión de una serie de genes. Los
distintos PBB difieren en lo que respecta a esa interacción con el
receptor, y en ese sentido los coplanares son más activos.
Muchos de los efectos de los PBB se observan sólo después de
una exposición prolongada. La razón podría ser la marcada
acumulación de algunos de ellos y la escasa capacidad del organismo
para metabolizarlos y eliminarlos. Ello determina la progresiva
concentración del producto en el organismo; los mecanismos de
compensación se ven desbordados y aparecen los efectos adversos.
Algunos naftalenos polibromados (PBN), que se sabe son
contaminantes de FireMaster(R), tienen potentes efectos tóxicos y
teratógenos. Las concentraciones de PBN presentes en esa mezcla son
bajas, pero es posible que contribuyan a su toxicidad.
Los estudios realizados sobre la mezcla FireMaster(R) y su
principal componente, el 2,2',4,4',5,5'-HxBB, mostraron que los
productos de fotólisis eran más tóxicos que el PBB original. Los
productos de la pirólisis de FM causaron la inducción del sistema
enzimático MFO, pérdida de peso corporal y atrofia tímica. Los
productos de pirólisis del OcBB de calidad técnica provocaron un
aumento del tamaño del hígado.
1.8 Efectos en el hombre
No había ningún ejemplo de toxicosis aguda por PBB en el hombre
con el que poder comparar los efectos potenciales de las bajas
exposiciones que siguieron a la intoxicación accidental acaecida en
Michigan (Estados Unidos) en 1973. Los principales estudios
epidemiológicos fueron realizados por el Departamento de Salud
Pública de Michigan (MDPH) y el Laboratorio de Ciencias del Medio
Ambiente (ESL) de la Facultad de Medicina Mount Sinai de Nueva York.
Se calculó que las personas más expuestas habían consumido
entre 5 y 15 g de PBB durante un periodo de 230 días a través de la
leche. Podría haberse producido una cierta exposición adicional a
través de la carne. La exposición de algunos de los agricultores y
de la mayoría de la población general de Michigan fue mucho menor:
9-10 mg de exposición total. No obstante, algunas de estas últimas
personas podrían haber recibido una dosis total de aproximadamente
800-900 mg. (Una dosis total de 9 mg corresponde a 0,15 mg/kg de
peso corporal, y 900 mg, a 15 mg/kg de peso corporal, cifras
referidas a un adulto de 60 kg de peso como valor promedio; la
dosis/kg de peso corporal sería mayor en los niños).
En 1974, en el primer estudio llevado a cabo por el MDPH se
procedió a comparar el estado de salud de las personas de las
granjas sometidas a cuarentena con el de las personas de las
explotaciones no sometidas a cuarentena de la misma zona. Los dos
grupos declararon diversos síntomas, pero no se observó ninguna
diferencia entre ambos. No se detectaron alteraciones inhabituales
del corazón, hígado, bazo, sistema nervioso, orina, sangre o
cualquiera de los otros parámetros médicos examinados. En un estudio
completo llevado a cabo más adelante por el MDPH, que abarcaba
grupos sometidos a distintos niveles de exposición, no se observó
relación alguna entre las concentraciones séricas de PBB y la
incidencia de los síntomas o enfermedades notificados. En los
estudios del ESL se examinó aproximadamente a 990 residentes de las
explotaciones agrícolas, a 55 trabajadores de la industria química y
a un grupo de trabajadores de la industria láctea que fueron
utilizados como testigos. La incidencia de síntomas entre los
agricultores de Michigan fue mayor que la hallada entre los
agricultores de Wisconsin. Las mayores diferencias fueron las
observadas dentro del amplio apartado de síntomas neurológicos y
musculoesqueléticos. En los agricultores de Michigan se halló una
mayor prevalencia de aumentos de las concentraciones séricas de
algunos antígenos carcinoembrionarios y enzimas hepáticas que en los
agricultores de Wisconsin. Los trabajadores de la industria química
presentaron una mayor prevalencia de síntomas torácicos y cutáneos y
una menor prevalencia de síntomas musculoesqueléticos que los
agricultores. Aunque la interpretación de los resultados de los
estudios del ESL divergió en ocasiones de la de otros resultados de
estudios comparables, hubo un aspecto en que los datos coincidieron:
ninguno de los estudios puso de manifiesto una relación dosis-
respuesta positiva entre los niveles de PBB en el suero o el tejido
adiposo y la prevalencia de síntomas o trastornos clínicos. Se
investigaron varios aspectos clínicos mediante estudios especiales
más detallados. En uno de ellos, el examen del sistema neurológoco
mediante pruebas funcionales objetivas reveló una correlación
negativa entre los niveles séricos de PBB y los resultados de las
pruebas funcionales, sobre todo entre los varones de los grupos de
edad avanzada. Los otros estudios no mostraron ninguna relación
entre las concentraciones de PBB en el suero o la grasa y la
actividad funcional determinada mediante una batería de pruebas en
las que se analizaron la memoria, fuerza motora, coordinación,
percepción corticosensorial, personalidad, funciones cognitivas
superiores y otro tipo de funciones. Se examinaron los aspectos
pediátricos de la exposición a PBB en algunas de las familias
estudiadas por el ESL. Se notificaron numerosos síntomas, pero la
exploración física no reveló ningún trastorno objetivo que pudiera
atribuirse a los PBB. Hubo opiniones discrepantes acerca de los
efectos neuropsicológicos en la descendencia, más sutiles, y los
resultados de las investigaciones sobre los signos de desarrollo de
las capacidades siguen siendo objeto de polémica. Lo mismo ocurre
con la investigación sobre los linfocitos y la función inmunitaria.
Algunos autores no hallaron ninguna diferencia numérica o funcional
entre los linfocitos de los grupos que presentaban niveles altos y
bajos de PBB en el suero, mientras que otros autores detectaron una
disminución significativa de las subpoblaciones de linfocitos T y B
en aproximadamente un 40% del grupo expuesto de Michigan en
comparación con los grupos no expuestos, así como trastornos de la
función linfocitaria, concretamente una menor respuesta a los
mitógenos.
El análisis de los estudios epidemiológicos realizados muestra
que se ha procurado evaluar la relación entre la exposición a PBB y
un elevado número de efectos adversos, incluidos efectos sobre la
conducta y trastornos subjetivos. No obstante, la mayoría de esos
estudios adolecen de fallos graves de diseño pues introducen
imprecisiones que hacen difícil, por no decir imposible, extraer
conclusiones acerca de la relación entre la exposición a PBB y los
posibles efectos sobre la salud. Además, el seguimiento no se ha
prolongado lo necesario para poder evaluar los posibles efectos
carcinógenos.
Se identificó a dos pequeños grupos de trabajadores que habían
sufrido exposición ocupacional a una mezcla de PBB o a DeBB y
decabromobifenilóxido. Se observaron lesiones parecidas al cloracne
en el 13% de los trabajadores expuestos a la mezcla de PBB, lesiones
ausentes en cambio en los trabajadores expuestos al DeBB. No
obstante, en este último grupo se observó una prevalencia
significativamente mayor de hipotiroidismo.
1.9 Evaluación global de la toxicidad y carcinogenicidad
El único estudio realizado con una mezcla de PBB y prolongado
durante todo el ciclo de vida fue el bioensayo llevado a cabo hace
poco con ratas y ratones en el marco del Programa Nacional de
Toxicología (NTP) de los Estados Unidos. La dosis más baja con
efectos carcinógenos fue de 0,5 mg/kg de peso corporal al día
(tumores hepáticos en roedores). En otros estudios realizados al
efecto se observó una respuesta carcinógena con 3 mg/kg de peso
corporal al día administrados durante 6 meses. El estudio prolongado
durante 6 meses demuestra que una exposición a dosis análogas
limitada a parte del ciclo de vida tiene también efectos adversos
similares. A dosis inferiores se observan efectos sobre el sistema
reproductor en los primates y el visón.
Además, en el estudio llevado a cabo durante 2 años por el NTP
con ratas, una dosis diaria de 0,15 mg/kg de peso corporal al día y
la exposición prenatal y perinatal de la madre a 0,05 mg/kg de peso
corporal al día no provocó ningún efecto adverso. Así pues, la
ingesta diaria total a partir de los alimentos, el agua, el aire y
el suelo debería ser inferior a 0,15 µg/kg de peso corporal al día,
cifra extrapolada del NOAEL (nivel sin efectos adversos observados)
observado en un estudio de carcinogenicidad que arrojó resultados
positivos, usando un factor de incertidumbre (seguridad) de 1000,
dado que estos compuestos probablemente producen cáncer por un
mecanismo epigenético.
Se calculó que la dosis total recibida por la subpoblación de
Michigan había sido de entre 0,15 y 15 mg/kg de peso corporal
durante un periodo de 230 días. Para esa población, la división de
esas dosis por el valor promedio de la duración de la vida del ser
humano arrojaría una cifra equivalente a una dosis diaria de entre 6
ng y 0,6 µg/kg de peso corporal al día.
Se ha calculado una ingesta total de 2 ng PBB/kg de peso
corporal al día, de fuentes conocidas, para los adultos de la
población general, y de 10 ng/kg de peso corporal al día para los
lactantes alimentados con leche materna. No debe olvidarse que estos
cálculos están basados en datos de carácter muy limitado y regional.
Estos cálculos se basan en el supuesto de que durante el ciclo
de vida los PBB no alcanzan un estado estacionario, y de que una
exposición alta y breve equivale a una exposición baja y prolongada,
toda vez que estos compuestos son metabolizados y excretados con
suma lentitud.
La información disponible sobre los OcBB, NoBB, y DeBB no es
suficiente para poder calcular una ingesta diaria total carente de
efectos adversos.
2. Conclusiones
La mayoría de los PBB presentes en los pirorretardantes
comerciales son lipofílicos, persistentes y bioacumulables. Estos
compuestos tienden a concentrarse en las tramas alimentarias del
medio y suponen una amenaza, especialmente para los organismos que
ocupan los niveles superiores de esas tramas. Además, en caso de
combustión, algunos PBB generan dibenzofuranos polibromados tóxicos.
Aparte de las emisiones que se producen durante su fabricación
y empleo, los PBB pasan al medio como consecuencia del uso
generalizado de pirorretardantes. Debido a la gran estabilidad de
los PBB, una parte considerable de ellos alcanza en un momento u
otro el medio.
También se detectan PBB en muestras ambientales o humanas de
lugares alejados de los focos conocidos. El perfil de PBB de las
muestras del medio no se corresponde con el hallado en los productos
de uso industrial, lo que indica que éstos sufren transformaciones
en el medio ambiente, posiblemente como resultado de una debromación
fotoquímica.
Se dispone actualmente de muy poca información sobre el grado
de exposición de la población general a los PBB. No obstante, en los
pocos casos en que se determinaron sus niveles se descubrieron
cantidades ínfimas. En la actualidad, ese nivel de exposición no
resulta preocupante, pero deberá evitarse que prosiga la
acumulación. Los datos sobre las personas afectadas en Michigan
indican que en este caso las exposiciones fueron varios órdenes de
magnitud superiores a la de la población general. No se han
observado efectos concluyentes sobre la salud atribuibles a la
exposición a PBB en la población de Michigan, pero el periodo de
seguimiento no ha sido lo suficientemente dilatado para descartar la
aparición de cáncer. Los niveles de PBB en el tejido adiposo y el
suero de la población de Michigan siguen siendo altos, por lo que la
exposición interna continúa. Por el contrario, sí se observó la
aparición de toxicidad en el ganado vacuno de Michigan. Esta
discrepancia se explica por el diferente grado de exposición del
ganado.
Sólo se han estudiado casos de exposición profesional en dos
fábricas de los Estados Unidos. Parece que los trabajadores que
producen PBB pueden desarrollar lesiones parecidas al cloracne, y
los expuestos a DeBB, hipotiroidismo. No se han realizado estudios
sobre los trabajadores que incorporan deca- u octa-/nona-
bromobifenilo en productos comerciales.
Los PBB persisten durante mucho tiempo en los organismos vivos,
y se ha demostrado que producen toxicidad crónica y cáncer en los
animales. Así, se indujo cáncer a una dosis de 0,5 mg/kg de peso
corporal al día, pese a la baja toxicidad aguda asociada a esa
dosis, y el nivel sin efectos observados fue de 0,15 mg/kg de peso
corporal al día. Se han observado diversos efectos tóxicos crónicos
en animales de experimentación tras la exposición prolongada a dosis
de aproximadamente 1 mg/kg de peso corporal al día.
3. Recomendaciones
3.1 Recomendaciones generales
El Grupo Especial de Trabajo considera que el hombre y el medio
ambiente no deben verse expuestos a los PBB, habida cuenta de su
elevada persistencia y bioacumulación y de los efectos adversos que
pueden aparecer tras la exposición prolongada a muy bajos niveles.
Por consiguiente, deberá interrumpirse todo uso comercial de los
PBB.
Respecto al DeBB y el OcBB, teniendo en cuenta los escasos
datos sobre su toxicidad, su extrema persistencia y su posible
degradación en el medio ambiente, así como la mayor toxicidad de los
compuestos persistentes generados durante la combustión, no deberán
ser empleados comercialmente, a menos que se demuestre su inocuidad.
Es sabido que se siguen formulando observaciones sobre la
cohorte de Michigan, información que convendría fuese publicada.
3.2 Futuras investigaciones
La futura vigilancia de los PBB en el hombre y en el medio,
incluida la vigilancia en el lugar de trabajo en las industrias de
fabricación o uso de esos productos, deberá ampliarse, deberá
centrarse en PBB específicos, y deberá incluir los OcBB/NoBB y DeBB.
Es preciso incorporar también estos compuestos en los programas en
curso de vigilancia de otros productos halogenados. La evolución
temporal y la distribución geográfica de los niveles de PBB en el
medio ambiente deberán seguir siendo objeto de vigilancia. Habrá que
controlar también la liberación de PBB en el medio a partir de los
lugares de evacuación de desechos.
Deberán efectuarse experimentos de termólisis mediante la
simulación de fuegos accidentales y la incineración municipal.
Conviene realizar nuevas investigaciones sobre los mecanismos de
toxicidad y carcinogenicidad de los PBB y de otros compuestos
relacionados. Los PBB podrían servir como modelo para el estudio de
esos mecanismos, y en las investigaciones al efecto deberán
emplearse PBB purificados.
Los efectos de los PBB sobre la reproducción no están bien
dilucidados. Por consiguiente, deberán realizarse estudios bien
diseñados y prolongados sobre los efectos de las dosis bajas en la
reproducción, usando para ello una especie vulnerable.
Es preciso disponer también de más información sobre la
biodisponibilidad y toxicocinética de los OcBB/NoBB y DeBB, así como
de determinados productos de la misma familia.
6.5.1.2 Biological half-lives
The biological half-lives of PBBs reported in the literature
for various species have been compiled in Table 68. McDaniel &
Lucier (1979) reported a half-life of BB 153 exceeding the lifetime
of rats.
In some cases the results obtained by different authors are
similar, in other cases, large discrepancies exist. These deviations
were, perhaps, caused by measuring the half-lifes under different
circumstances of exposure and for different lengths of time (Fries,
1985b). By means of simulation studies, Domino et al. (1982)
demonstrated the effects of different amounts of body fat on the
half-life of BB 153 in the rat (see Table 69).
Tuey & Matthews (1980) have simulated the effects of a growing
animal on the kinetics of BB 153 and found that, though
concentrations of BB 153 in fat fell faster than in a stable animal,
the "actual" half-life of the substance was prolonged, because of an
increase in the relative fat proportions.
6.5.1.3 Differences between individual congeners
There are some reports on the differences in turnover and
retention of individual PBB congeners. Mostly, they refer to changes
in the relative abundances of certain FireMaster(R) components,
namely: 2,2',4,5,5'-pentabromobiphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118), 2,2',3,4',5',6-hexa
bromobiphenyl (BB 149), 2,2',4,4',5,5'-hexabromobiphenyl (BB 153),
2,2',3,4,4',5'-hexabromobiphenyl (BB 138), 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167), 2,3,3',4,4',5-hexabromobiphenyl (BB
156), 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), and
2,2',3,3',4,4'5-heptabromobiphenyl (BB 170). To describe such
changes, the GC profile of the original FireMaster(R) mixture was
compared with the GC profile obtained on analysis of tissues from
animals treated with this mixture, by measuring either the area
(e.g., Wolff & Aubrey, 1978; Domino et al., 1980b) or the height
(e.g., McCormack et al., 1982a; Bernert & Groce, 1984; Groce &
Kimbrough, 1984) of the GC peaks and by normalizing the values to BB
153 as 100. A few other authors (Fries & Marrow, 1975; Fries et al.,
1976) based their calculations on the assumption that each component
(BB 153 and BB 180) was fed at the same rate in the diet. According
to Fries (1985b), some differential behaviour may be due to
analytical artefacts, introduced by differential recovery of the
congeners or by adsorption of congeners on the glassware (Willett
et al., 1978), but many changes appear real.
Table 69. Effect of different amounts of body fat on the half-life of
2,2',4,4',5,5'-hexabromobiphenyl in the rata
Amount fat Half-life
(% normal fat) (days)
Emaciated 25 60.5
50 88.8
75 117
90 134
Normal 100 145
110 156
125 173
150 200
175 228
200 256
Obese 250 311
a From: Domino et al. (1982).
The trends reported in the literature include the selective
retention of 5 minor components of the FireMaster(R) mixture in
the tissues of rats, pigs, cows, and chickens (Fries & Marrow, 1975;
Fries et al., 1976, 1978a; Dannan et al., 1978b; Willett & Durst,
1978; Wolff & Aubrey, 1978; Wolff & Selikoff, 1979; Domino et al.,
1980b; McCormack et al., 1980, 1982a; Werner & Sleight, 1981; Groce
& Kimbrough, 1984; Polin & Leavitt, 1984). They can be summarized,
as follows.
With some exceptions, the concentrations of 2,2',4,5,5'-penta
bromobiphenyl (BB 101) and 2,2',3,4,4',5,5'-heptabromobiphenyl (BB
180) relative to BB 153 appeared to be lower in tissues from treated
animals than in those fed FireMaster(R) mixture. The relative
concentrations of 2,3',4,4',5-pentabromobiphenyl (BB 118) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) appeared to be higher or
unchanged in many cases. 2,2',3,4',5',6-Hexabromo biphenyl (BB 149)
was barely, or not, detected in the tissues of any animals.
The results of a multigeneration study on rats also reflected
the differential behaviour of certain PBB congeners (McCormack
et al., 1981). Of the first eight peaks (penta- to
heptabromobiphenyls) in the GC profile of FireMaster(R), all
except peak 3 (BB 149) were detected in the livers of rats in the
F1, F2, and F3 generations (experimental design: see Table
54). For example, the concentration of
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) relative to BB 153 was
higher in the livers of animals in the F1 generation than in the
FireMaster(R) BP-6 standard, but decreased with each subsequent
generation. Although F2-10 and F3-100 animals (10 and 100 mg/kg
treatment, respectively; see Table 54) had similar hepatic
concentrations of BB 153, the relative concentrations of other PBB
congeners, including BB 167 appeared to be lower in the livers from
F3-100 than F2-10 animals (McCormack et al., 1981).
Domino et al. (1980b) found that 2,2',4,5,5'-pentabromobiphenyl
(BB 101) penetrated the brain of rats more rapidly than
2,3',4,4',5-pentabromobiphenyl (BB 118) or any of the higher
relative molecular mass homologues. Fries et al. (1976) reported a
faster clearance of 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180)
(half-time = approximately 20 days) than of BB 153 (half-time =
approximately 28 days) from eggs of hens after feeding stopped.
However, analyses of whole chicken carcasses resulted in unchanged
ratios of BB 153/BB 180 on days 0, 21, and 42 of withdrawal (Polin &
Leavitt, 1984). The authors judged that the dynamics for withdrawal
of these two congeners from tissues of chickens were parallel. The
withdrawal from milk of dairy cows was found to be more rapid for BB
180 than for BB 153 (Fries & Marrow, 1975; Fries et al., 1978a; see
also Fig. 7).
In just one study (Millis et al., 1985b), equimolar doses of
individual PBB congeners were administered to the test animals.
Immature male rats received a single oral dose (21.3 µmol/kg body
weight) of 3,3',4,4',5,5'-hexabromobiphenyl or 3,3',4,4'-tetra
bromobiphenyl and were analysed at various times up to 14 days after
treatment. Adipose tissue and liver concentrations of
3,3',4,4',5,5'-hexabromobiphenyl appeared unchanged over time
whereas the tissue concentrations of 3,3',4,4'-tetrabromobiphenyl
decreased in a biphasic manner.
6.5.1.4 Octabromobiphenyl
Some kinetic data are available for technical octabromo
biphenyl. Bromine levels in the adipose tissue of rats dosed with
octabromobiphenyl did not decrease during a period of 90 days
(Norris et al., 1973) or 18 weeks (Aftosmis et al., 1972a; Waritz
et al., 1977) after cessation of dosing, or, according to Lee et al.
(1975a), levels even increased 18 weeks after exposure. A partial
elimination of bromine was observed from the livers of these rats
after recovery (Norris et al., 1973; Lee et al., 1975a; Waritz
et al., 1977).
The contents of total PBB (octabromobiphenyl plus an
unidentified hexabromobiphenyl) in juvenile Atlantic salmon (Salmo
salar), fed 90 days with octabromobiphenyl (Dow
Chemical)-contaminated food, also remained fairly constant after 28
days of withdrawal (Zitko, 1977).
6.5.2 Human studies
Information on the time trends of BB 153 distribution or
retention in humans is limited. As with animals, BB 153 seems to be
highly persistent in humans. This conclusion has been drawn from
monitoring BB 153 levels over time in both individual persons and
the Michigan population.
Most paired serial samples exist for serum (or plasma) from
farmers, etc. (Humphrey & Hayner, 1975: sampled June/Autumn, 1974;
Landrigan et al., 1979: sampled 1974/77; Wolff et al., 1979b:
sampled 1976/77/78; Kreiss et al., 1982: sampled 1977/78/79;
Sherman, 1991: sampled 1976/80/87) and chemical workers (Wolff
et al., 1979b: sampled 1976/78; Bahn et al., 1980b and Bialik, 1982:
sampled 1978/1981; Lambert et al., 1990) (in parentheses: references
plus year of sampling). The values obtained indicated no, or little,
decrease (see sections 5.2 and 5.3, and Table 38) with the exception
of the results of Bahn et al. (1980b) and Bialik (1982) (see section
5.3). Paired adipose analyses have been reported only by Meester &
McCoy (1976) who found a consider able average decline in HxBB
levels over six months (see also section 5.2). However, one of the
16 persons tested had increasing or unchanged fat levels, while
serum levels slowly dropped. The significance of these observations
and the combined results of Bahn et al. (1980b) and Bialik (1982)
are unclear. A most recent case report (Sherman, 1991) showed that
"PBB" could be identified in the serum and fat of a cancer patient
over an 11-year period (1976-87). Serial testing of 11 breast-milk
samples from one lactating Michigan woman showed that HxBB
concentrations varied between 0.1 and 0.2 mg/kg (expressed on a fat
basis) during a three-month period, without any significant downward
trend (Brilliant et al., 1978).
Comparisons at the population level have been made for serum
(Meester & McCoy, 1976), breast-milk (Miller et al., 1984), and
adipose tissue (Miceli et al., 1985). Generally, the presence of
PBBs in the tissues of Michigan people many years after the spill
(1973) may be an indicator for its persistence (see Tables 38 and
39). Meester & McCoy (1976) found that the average HxBB level in the
serum of farmers during the first six months of 1976 was ten times
lower than that during the last six months of 1975 (0.2 µg/litre
versus 2.0 µg/litre). Miller et al. (1984) concluded from their
analyses of breast-milk from 2986 lactating women during May 1976
and December 1978 that HxBB levels were not declining. Approximately
5 years after the Michigan PBB incident occurred, adipose tissue
from live residents of a "high" exposure area (Muskegon County area)
contained median HxBB levels of 500 µg/kg (Wolff et al., 1982).
Approximately 10 years after exposure, postmortem adipose tissue
contained median HxBB levels of 320 µg/kg (Miceli et al., 1985).
On the basis of the two last values, Miceli et al. (1985)
calculated a half-life of 7.8 years for HxBB (BB 153) in human
adipose tissue. This prediction was close to the body burden
half-time of 6.5 years estimated by Tuey & Matthews (1980) using
pharmacokinetic data obtained from rats.
A median serum half-life of BB 153 of 12 years (range: 4.6-94.7
years) has been determined by comparing previous and more recent
serum BB 153 levels of Michigan residents (Lambert et al., 1990).
As Tuey & Matthews (1980) explained, the half-life may be
longer in growing children or in persons gaining weight. However,
calculating the effects of different amounts of body fat on the
retention of BB 153, these authors also found that adipose tissue
may act as a protective reservoir in mature humans, because the
concentration of BB 153 in the blood (and possibly other more
critical tissues) of obese persons should be significantly less than
those of leaner individuals who received comparable exposures.
There was some evidence for the differential retention of
various PBB congeners in humans, when PBB congeners in serum samples
from Michigan subjects were compared with FireMaster(R) BP-6. The
main congeners assayed by using GC-MS analysis (Wolff & Aubrey,
1978; Wolff et al., 1978) or Negative Chemical Ionisation
Spectrometry (NCIMS) analysis (Roboz et al., 1982; Greaves et al.,
1984) were as follows: 2,2',4,5,5'-pentabromo biphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118),
2,2',3,4',5',6-hexabromobiphenyl (BB 149), 2,2',4,4',5,5'-hexa
bromobiphenyl (BB 153), 2,2',3,4,4',5'-hexabromobiphenyl (BB 138),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). The most obvious
changes refer to the two penta isomers and to the major
heptabromobiphenyl. The 2,2',3,4,4',5,5'-heptabromobiphenyl was
absent (Roboz et al., 1982) or greatly diminished in serum (Wolff &
Aubrey, 1978; Wolff et al., 1979a; Greaves et al., 1984) in relation
to the concentrations of BB 153 (taken as 100%). The relative
amounts of 2,2',4,5,5'-pentabromobiphenyl (BB 101) were also greatly
decreased (e.g., 80%: Greaves et al., 1984) in all samples. However,
a marked decrease in 2,3',4,4',5-pentabromobiphenyl (BB 118)
concentrations was observed only in the serum of farmers, taken
several years after exposure (e.g., 60%: Greaves et al., 1984), but
not in the serum of chemical workers (Wolff & Aubrey, 1978; Wolff
et al., 1979a; Roboz et al., 1982).
6.6 Reaction with body components
6.6.1 Animal studies
When a 14C-PBB mixture (FireMaster(R)) was incubated with
rat liver microsomes, no binding to exogenous DNA was detected, and
only a small amount of radioactivity was covalently bound to
microsomal protein (Dannan et al., 1978b). The formation of low and
high relative molecular mass adducts with PBB metabolites has been
quoted elsewhere (section 6.3).
6.6.2 Human studies
Studies on human serum revealed that lipoproteins are the
predominant protein carriers of PBBs in serum (Greaves et al.,
1983). 80% of the PBBs were bound to apolipoproteins B and A in a 4
: 1 ratio (Greaves et al., 1984; Roboz et al, 1985a). According to
Roboz et al. (1985b), the ratio was 3 : 1. No preferential binding
of PBB congeners (2,2',4,4',5,5'-hexa-; 2,2',4,5,5'-penta-;
2,2',4,5',6-pentabromobiphenyl) was found (Roboz et al., 1985a,b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only few data are available on effects of PBBs on organisms in
the environment. They refer to microorganisms, water fleas,
waterbirds, (rodents), and farm animals.
7.1 Microorganisms
The toxicity of technical decabromobiphenyl (Adine 0102)
against bacteria (Pseudomonas putida M.) was determined by the
cell multiplication inhibition test (according to the ISO TC 147/SC
5/WG 1/N 111 standard, and using 0.1-1% DMSO (dimethylsulf oxide) as
a solvent. An EC10 of 53 mg/litre was found (Atochem, 1990).
7.2 Aquatic organisms
In a short-term test (according to the ISO standard 6341) the
immobilization of Daphnia magna (Crustacea) by technical
decabromobiphenyl (Adine 0102) has been investigated. The following
results were obtained after dissolution of the test material in DMSO
because of its weak solubility in water:
- EC50 (24 h): > 66 mg/litre;
- The maximum concentration resulting in 0% immobilization
24 h): < 2 mg/litre (Atochem, 1990).
7.3 Terrestrial organisms
7.3.1 Wildlife
In 1977 and 1978, Haseltine et al. (1981) and Heinz et al.
(1983) studied red-breasted mergansers (Mergus serrator) nesting
on islands in the northwestern Lake Michigan in order to determine
whether environmental contaminants (organochlorines and metals) were
producing effects on reproduction. Seventeen contaminants, including
PBBs, were measured in randomly chosen eggs from 206 nests under
study. Using a variety of statistical approaches, Heinz et al.
(1983) looked for effects of individual contaminants and
combinations of contaminants on reproductive measurements, such as
nest desertion, failure of eggs to hatch, death of newly hatched
ducklings, percentage hatching success, number of ducklings leaving
the nest, and egg-shell thickness. PBBs and other chemicals were
sometimes negatively correlated with shell thickness or thickness
index, but not consistently so, as found for DDE. However, no
contaminant or combination of contaminants measured seemed to have a
pronounced effect on the aspects of reproduction mentioned above.
The hatching success of the mergansers averaged 81.7% in 1977.
According to Heinz et al. (1983), the hatching success averaged
85.6% in 1978 (Haseltine et al., 1981).
Although it is not valid to compare reproductive success among
different species, it should be noted that dabbling ducks, whose
eggs contained only a fraction of the contaminant burdens (e.g., no
PBBs: Haseltine et al., 1981; see also Table 32), of the redbreasted
mergansers had better hatching success (Heinz et al., 1983).
An interesting observation on rodents living on a
PBB-contaminated farm has been reported by Jackson & Halbert (1974).
They observed that rats and mice were apparently eradicated when
they came into contact with PBB-contaminated cattle feed pellets.
7.3.2 Farm animals
Farm animals in Michigan that ingested feed inadvertently
containing PBB (FireMaster(R) FF-1) in place of magnesium oxide
fell sick. First symptoms were observed in the cattle of several
dairy herds and revealed this so-called "Michigan PBB incident".
7.3.2.1 Cattle
The exact doses of PBBs to which Michigan cattle were exposed
are not known. Fries (1985b) estimated the maximum PBB doses of
individual cattle based on milk or tissue fat concentration at the
time of detection, which ranged from 9 to 24 months after the cattle
had consumed contaminated feed. For example, if PBB were detected 9
months after exposure, PBB concentrations of 0.1 mg/litre of milk
and 0.25 mg/kg tissue would indicate that the cow had received a
total dose of 1 mg/kg of body weight. Increased PBB concentrations
in milk and tissue would indicate a proportionally higher dose. If
detection were delayed for as long as 24 months, the above example
for milk and tissue concen trations would indicate an exposure of
6 mg/kg of body weight.
a) High-level contamination in cattle
Adverse effects of PBBs in lactating cows were first reported
by Jackson & Halbert (1974).
In reconstructing the accident, it was assumed that the cows on
this farm (Halbert farm) consumed PBB-contaminated feed (PBB content
from 3 to 4 g/kg; Isleib & Whitehead, 1975) over 16 days, and
ingested about 20 g PBB/day, initially. The total average exposure
of these cows was estimated to be 250 mg/kg body weight (Fries,
1985b). The contaminated feed was also fed to a group of 6- to
18-month-old calves. The dose might have been about 58 mg/kg body
weight per day when feeding started, and the total doses may have
reached 700 mg/kg body weight over 6 weeks (Fries, 1985b), if feed
was consumed at the same rates as in experimental studies (Durst
et al., 1977).
The clinical signs of toxicity, described by Jackson & Halbert
(1974), were anorexia (50% reduction in feed consumption) and a 40%
decrease in milk production a few weeks after ingestion of the
contaminated feed. Although the supplemented feed was discontinued
within 16 days, milk production was not restored, and the cows
continued to lose weight. An unspecified number of cows had
increased frequency of urination and lacrimation and developed
haematomas, abscesses, abnormal hoof growth, lameness, alopecia,
hyperkeratosis, and cachexia, and several died within 6 months.
Altogether, the death rate of Halbert cows was about 24/400.
The death rate of the 6- to 18-month-old calves (heifers and
bulls) to which the suspected feed was offered, was much higher.
About 50% of the calves died within 6 weeks. After 5 months, only
two of twelve animals were alive, and they had developed
hyperkeratosis over their entire bodies. There were also a variety
of reproductive problems including embryo resorption, abortions,
stillbirths, deaths shortly after births, delayed deliveries, and
enlarged calves. The clinical signs were variable, and, with the
exception of decreased milk production and weight loss, no
particular symptom was predominant in the affected animals (Jackson
& Halbert, 1974; Robertson & Chynoweth, 1975).
Necropsy findings have been reported for some of the 24 mature
cows that died during the 6 months following exposure (Jackson &
Halbert, 1974). Gross lesions observed included somewhat enlarged
livers, haematomas and abscesses in the thoracic and abdominal
cavities, abomasal ulcers, necrotic metritis, suppurative
bronchopneumonia, and pericarditis. As in all observations without
controls, it was difficult to draw definitive conclusions as to
which lesions were caused by PBBs and which were unrelated to PBBs
(Fries, 1985b). Two of the twelve calves in a calf feeding trial had
massive liver abscesses.
Histopathological studies on ten of the cows revealed various
liver and kidney changes. Liver lesions were reported in seven
animals and included fatty changes and amyloidosis. Renal tubular
nephrosis and interstitial nephritis were reported in four of the
cows (Jackson & Halbert, 1974; Getty et al., 1977).
Several clinical signs and pathological changes, reported by
Jackson & Halbert (1974), were also described in cows in controlled
feeding studies (Durst et al., 1977; Durst et al., 1978a,b; Moorhead
et al., 1977, 1978; Robl et al., 1978; Willett et al., 1980; see
also section 8). These included anorexia, dehydration, excessive
lacrimation, emaciation, hyperkeratosis, reproductive difficulties
(fetal death, enlarged calves, and difficulty in calving,
hypospermatogenesis), and renal damage. Conditions described in the
accidentally exposed cows, but not confirmed in the controlled
studies, included haematomas, abscesses, abnormal hoof growth,
extensive hair loss, liver abscesses, necrosis, and metritis (Fries,
1983, 1985b).
The Halbert herd was one of about 12 "highly" contaminated
herds that had PBB concentrations of more than 30 mg/kg in milk fat,
when detected. According to Fries (1985b), it is likely that all of
these herds had some clinical signs of toxicosis. According to the
same author, there was no comprehensive clinical examination of any
herd that had milk concentrations in the range of 1-30 mg/litre, but
it appears that most animals in these herds did not have clinical
signs when the farms were depopulated. A preliminary report (cited
by Getty et al., 1977) indicated that cows that had been exposed to
PBBs 19 months earlier had levels of up to 80 mg/kg in body fat, but
were apparently normal clinically and were producing normal
quantities of milk. Gross or microscopic lesions that could be
attributed to PBB exposure were not found.
b) Low-level contamination in cattle
Residue concentrations in the body fat or milk fat of cows,
classed as having low-level contamination, rarely exceeded 1 mg/kg,
and, for the most part, not even 0.3 mg/kg. There were several
studies on Michigan cattle with such low exposures.
Results of an evaluation of 72 low-level contaminated herds
have been reported by Kay (1977) and Getty et al. (1977).
Production drop and sterility were two consistent signs and were
regarded as interrelated. The retardation of growth of young stock
was very significant, as it was in the Jackson & Halbert study
(1974). Some other findings were not consistent. A mail
questionnaire survey (Getty et al., 1977) showed similar
observations.
Cows (n = 46) from six herds across Michigan, whose body fat
contained a mean concentration of 0.31 mg/kg and a maximum
concentration of 1.8 mg/kg, were compared with a group of cows from
Wisconsin (n = 40) that had not been exposed to PBB. The two groups
were reared together and subjected to the same feeding and
management system. There were no significant differences in the
animals' milk production, body weight, weight gain, breeding and
reproductive performance, incidence of commonly experienced health
problems, calving rate, and the health of their calves. Also no
pattern of gross or histopathological lesions was seen between test
animals and control animals upon necropsy (Wastell et al., 1978).
An epidemiological survey, which compared the health status of
16 herds with low PBB exposure (traces to 1 mg/kg body fat or milk
fat) with the status of 15 herds with no PBB exposure, also
indicated that productivity and general health conditions between
the two groups of herds were similar. Of the biochemical parameters
tested (9 urinalyses, 13 serum chemistry parameters), three resulted
in significantly different values. Serum concen trations of calcium,
glucose, and cholesterol in contaminated herds were significantly
lower than those of the control herds. But the relationship to PBB
exposure was unknown (Mercer et al., 1976).
Instead of specific clinical conditions, Fries (1983) evaluated
the overall performance of exposed herds (residues in tissue or milk
fat generally < 0.3 mg/kg) and of "relatively unexposed" herds
(residues < 0.02 mg/kg) of comparable size, breed, and location by
analysing Dairy Herd Improvement Association records. He found that
no productive or reproductive character istics of the herds were
affected by PBB exposure.
It should be noted that the classification of herds as having a
high or a low level of contamination refers to PBB levels at the
time of detection. Thus, it is sometimes impossible to know whether
there was a history of a short-term, high exposure or a long-term,
low exposure, which may produce different syndromes. For example,
Fries (1985b) pointed out that feed that was contaminated by
cross-contamination in the feed mills was being fed at the time of
detection, in some cases. Under this circum stance, PBB intakes as
low as 0.1 mg/kg of body weight per day could produce milk fat
residues as high as 20 mg/kg (Fries & Marrow, 1975; Fries 1985b).
7.3.2.2 Other farm animals
Although it was cattle feed that was originally involved in the
accidental substitution, all other feeds became involved by cross
contamination, e.g., in the mixing machinery of feed companies that
had been exposed to PBB (Dunckel, 1975). It is likely that other
animals were not exposed to the same high levels as cattle.
(i) Poultry
There are no reports of clinical signs or problems associated
with the accidental contamination of poultry feed. However, some
controlled feeding studies have been published (see section 8).
(ii) Pig
Adverse health effects in pigs, identified as contaminated,
were rarely reported. Only one review (Reggiani & Bruppacher, 1985)
mentioned that abortions occurred in pigs. Two controlled feeding
studies (Ku et al., 1978; Werner & Sleight, 1981) have been
conducted (see section 8).
(iii) Horse, rabbit, goat, sheep
Other species of farm animals, including at least 2 horses, 32
rabbits, 2 goats, and 19 flocks of sheep, were identified as
contaminated and buried at Kalkaska, but details of ill effects were
not recorded (Dunckel, 1975; Getty et al., 1977).
One experimental feeding study on sheep (Gutenmann & Lisk,
1975) is available (see section 8).
7.4 Population and ecosystem effects
No information available.
7.5 Effects on the abiotic environment
No information available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Differences in toxic responses between acute, high-level
exposures and long-term, low-level exposures to halogenated aromatic
compounds are mainly quantitative (McConnell & Moore, 1979).
Moreover, the delayed onset of toxic signs and the persistence of
PBBs in the body, which may cause long-term exposure of the target
organs from a single dose, "tend to blur the usual distinctions that
are made between acute and chronic exposure" (Fries, 1985b). For
these reasons, symptoms after single and short-term exposures are
reviewed together in section 8.2. Another feature of the toxicity
of PBBs and related classes of compounds is the latent period
between the time of exposure and the time of death, which ranged
from several days to weeks (Di Carlo et al., 1978; Safe, 1984;
Hutzinger et al., 1985a; McConnell, 1985). Thus, the classical
LD50 values and other mortality data found are summarized in one
section (8.1).
8.1 Lethality
"Acute" toxicity data (LD50 and LC50 values) of commercial
PBB mixtures have been compiled in Table 70. The LD50 values of
all mixtures show a relatively low order of acute toxicity (LD50
> 1 g/kg body weight) in rats, rabbits and quails, regardless of
the route of administration, and range from > 1 to 21.5 g/kg body
weight. Regarding the LC50 values for minks, this species may be
highly sensitive to PBBs, but a direct comparison is complicated by
differences in the experimental design.
As with TCDD and PCBs (McConnell, 1984; Safe, 1984), the
apparent toxicity of PBBs is higher with multiple-dose rather than
single-dose administration. For example, the single oral LD50 of
FireMaster(R) in rats was quoted to be 21.5 g/kg body weight, but,
if given in small repeated doses, the total lethal dose was
approximately 1-3 g/kg body weight (see Table 70).
Deaths after exposure to PBBs are delayed (Di Carlo et al.,
1978; Tables 71 and 72). Thus, Gupta & Moore (1979) have recommended
that the LD50 of halogenated aromatic hydrocarbons or chemicals
that may have a long-term build-up with delayed toxicity should be
determined more accurately after multiple dosing and an extended
period of observation.
Generally, the susceptibility to the toxic effects of aryl
hydrocarbons is dependent on the sex, age, species, and strain of
the experimental animals (e.g., Safe, 1984; Hutzinger et al.,
1985b). In the case of PBBs, female rats dosed with FireMaster(R)
FF-1 showed a lower LD50 than male rats (Gupta & Moore, 1979;
Table 70).
Table 70. Toxicity of PBB mixtures
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) rat oral LD50 21.5 single dose Di Carlo
et al.
(1978)
FireMaster(R) FF1 rat (Fischer- female oral 90 days LD50 1.43 22 doses Gupta &
(Lot No. 1312 FT) 344/N) (over 30 Moore
(in corn oil) male oral 90 days LD50 3.28 days) 22 (1979)
doses (over
30 days)
FireMaster(R) BP-6 rabbit dermal LD50 5 Aftosmis
et al. (1972b)
FireMaster(R) rabbit male dermal 14 days ALD 5 24 h of Waritz et al.
(Lot 635-71) (New Zealand, exposure (1977)
(in corn oil) albino)
FireMaster(R) FF1 mink male, in feed 313 days LC50 3.95 Ringer et al.
(Mustela vison) female (1981)
FireMaster(R) Bobwhite quail in feed 8 days LC50 428 5 days of Cottrell
(Colinus treated diet et al. (1984)
virginianus)
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) Japanese quail oral not LD50 > 1 Strik
(1973a)
specified
Octabromobiphenyl rat male oral LD50 2 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl rat male oral 7 days ALD > 17 single or Waritz et al.
(Dow Lot 102-7-72) (Sprague-Dawley) repeated dose (1977)
(in acetone: corn
oil = 15:85)
Octabromobiphenyl rabbit dermal LD50 > 10 Aftosmis
et al. (1972b)
Octabromobiphenyl rabbit (New male dermal 14 days ALD > 10 24 h of Waritz et al.
(Dow Lot 102-7-72) Zealand albino) exposure (1977)
Octabromobiphenyl Japanese quail oral LD50 > 12.5 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl Bobwhite quail male, oral 14 days ALD > 12.5 single dose Waritz et al.
(Dow Lot 102-7-72) female (1977)
(in corn oil)
Nonabromobiphenyl mouse (B6C3F1) male, oral 14 days LD50 > 15 single dose Momma (1986)
(Bromkal 80-9D) female
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rat male, oral 14 days LD50 > 5 single dose Millischer
(in corn oil) (Sprague-Dawley female et al.
CFY) dermal 14 days LD50 > 5 single dose (1979)
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rabbit dermal LD50 > 8 c
a ALD = Approximate lethal dose.
b LD50 or ALD in g/kg body weight; LC50 in mg/kg diet (ppm).
c Consumer Product Testing Co. (1977) quoted by Di Carlo et al. (1978).
Table 71. Mortality associated with PBB administration: Commercial mixtures (dosing studies)
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF1 rat male oral 0.03-30 60 days 22 doses no mortality Luster et al.
(No. 1312 FT) over 30 (1978)
(in corn oil)
rat female oral 0.1-10 6 months 122 doses no mortality Luster et al.
(Fisher) over 6 (1980)
months
rat female oral 100 90 days 22 doses 100% 41-53 Gupta & Moore
(Fisher over 30 (1979)
344/N) days
male oral 100 90 days 22 doses 38% 50-73 Gupta & Moore
over 30 days (1979)
female, oral 300 90 days 9-21 doses 100% 14-29 Gupta & Moore
male (1979)
female, oral 1000 90 days 6-10 doses 100% 9-14 Gupta & Moore
male (1979)
FM BP-6 rat inhalation 71 mg not 1 h of no mortality Di Carlo et al.
per specified exposure (1978)
litre
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF-1 mouse female oral 0.03-30 60 days 22 doses no mortality Luster et al.
(Lot No. over 30 (1978)
1312 FT in days
corn oil) mouse female oral 0.1-10 6 months 122 doses no mortality Luster et al.
over 6 months (1980)
FM guinea-pig oral 50 60 days single dose no mortality Ecobichon
(Hartley), mentioned et al. (1983)
albino
FM BP-6 cattle oral 25 60 days daily doses 100% (animals 33-60 Irving et al.
(finely g/dayc became moribund (1976);Durst
ground) and were et al. (1977);
necropsied) Moorhead et al.
(1977, 1978)
FM BP-6 cattle female oral 250 180 days daily doses no mortality Durst et al.
(finely mg/day (1978b)
ground)
cattle female oral 0.3d 340 days daily no mortality Robl et al.
doses over (1978)
158-228
days
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM BP-6 rainbow trout intraperitoneal 150 15 days single dose no mortality Elcombe & Lech
(in corn oil) (Oncorhynchus mentioned (1978)
mykiss)
FM BP-6 brook trout oral 66.3 18 days 3 doses over no mortality Law & Addison
(in dogfish (Salvelinus 5 days mentioned (1981)
oil) fontinalis)
FM FF-1 sheepshead intraperitoneal 15 56 days single dose no mortality James & Little
(Archosargus (1981)
probatocephalus) 50 4-5 days single dose 2/5 (remaining James & Little
3 moribund)
OcBB rat female oral 126- 14 days single dose no mortality Norris et al.
(in corn oil) Sprague- 2000 (1973)
Dawley)
OcBB rat male oral 1000 28 days single dose no mortality Lee et al.
Sprague- (1975b)
Dawley)
OcBB rat male inhalation 0.96 7 days 4 h of no mortality Waritz et al.
(Dow Lot (Sprague- mg/ exposure (1977)
102-7-72) Dawley) litre
air
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
DeBB rat inhalation 200 not 1 h of no mortality Di Carlo
mg/ specified exposure et (1978)
litre
air
DeBB rat male, inhalation 0.05-5 4 weeks 6 h/day; no mortality Millischer
(Sprague- mg/ 5 days/week et al. (1979)
Dawley) litre
air
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b In mg/kg body weight per day, unless otherwise specified.
c Initially equivalent to 67.2 mg/kg body weight per day.
d Equivalent to 10 mg/kg feed.
Table 72. Mortality associated with PBB administration: Commercial mixtures (feeding studies)
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 rat (Sprague- female 4.7-300 2/2 weeks no mortality Dent et al. (1976a)
Dawley) mentioned
FM rat (Sprague- male 1-500 30/30 days no mortality Sleight & Sanger (1976)
Dawley)
FM BP-6 mouse (BALB/c) female 1000 9/9 days 70% Fraker & Aust (1978);
Fraker (1980)
FM BP-6 guinea-pig 50 45/45 7/8 Vos & van Genderen
(Lot No. 182 RP) (1974)
FM guinea-pig 100 30/30 days 4/6 Sleight & Sanger (1976)
guinea-pig 500 15/15 days 100%
FM FF-1 mink male, 1 313/313 days no mortality Aulerich & Ringer (1979)
(Mustela vison) female 2.5 10% 136 days
90% 63-294 days Ringer et al. (1981)
100% 25-93 days
FM pig 20, 200 16/16 weeks no mortality Ku et al. (1978)
mentioned
FM FF-1 rhesus monkey male 25 25/25 weeks 1/1 25 weeks Allen et al. (1978)
(adult) (total: 1 g)
(juvenile) female 300 137/137 days 1/1 137 days Allen et al. (1978)
(total:
6.4 g)
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 chicks (Hubbard male 400 15/15 days 37% Vos & van Genderen
(Lot No. 182 RP) Leghorn) (1974)
FM BP-6 chicken (White 20-200 8/16 weeks no mortality Cecil & Bitman (1978)
Leghorn)
4/4 weeks 60% (control 10%) Cecil & Bitman (1978)
FM FF-1 chicken (White 3125 4/4 weeks 100% Polin & Ringer (1978a)
Leghorn)
FM Japanese quail 10-100 no mortality Babish et al. (1975a)
500-1000 "few" days 100% Babish et al. (1975a)
FM Bobwhite quail 100 5/8 days no mortality Cottrell et al. (1984)
200 5/8 days 1/10 8 days Cottrell et al. (1984)
600 5/8 days 100% (10/10) 5.3 days Cottrell et al. (1984)
(mean)
FM BP-6 Atlantic salmon 100 42/70 days no mortality Zitko (1977)
(Salmo salar) (in excess of
that of
control fish)
OcBB rat (Sprague- male 100-10 000 30/30 days no mortality Norris et al. (1973)
Dawley) mentioned
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
OcBB rat (Sprague- male 1-1000 4/22 weeks no mortality Lee et al. (1975a)
Dawley)
OcBB (Dow Atlantic salmon 100 74/96 days no mortality (in Zitko (1977)
Chemical) (Salmo salar) excess of that of
control fish)
DeBB rat (Sprague- female, 1-2000 90/90 days no mortality Millischer et al.
Dawley) male mentioned (1979)
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
Although the studies, in most cases, are not directly
comparable, species differences in the response to PBBs are
reflected, in part, by the mortality data collated in Tables 71 and
72. For example, minks appear to be more sensitive to the
FireMaster(R) mixture than the other species of animals tested.
Compared to rats, guinea-pigs were far more susceptible to
FireMaster(R) (Table 72).
The few studies performed with commercial octa- and
decabromobiphenyl mixtures did not result in any mortality in rats
and fish (Tables 71 and 72). Of individual PBB congeners, only three
hexa isomers have been tested. Apparently, 3,3',4,4',5,5'-
hexabromobiphenyl is more toxic for rats than
2,2',4,4',5,5'-hexabromobiphenyl (Table 73).
With few exceptions, the cause of death by halogenated aryl
hydrocarbons cannot usually be ascribed to pathology in a given
organ or system as with most toxicants (McConnell & Moore, 1979;
McConnell, 1984). At lethal doses, the affected animals develop, as
a first indication of toxicity, a "wasting syndrome", i.e., a
progressive loss of body weight, which may not be related simply to
decreased food consumption. It is followed by weakness,
debilitation, and finally death (Hutzinger et al., 1985a; McConnell,
1985). Some authors (Allen et al., 1978) use the term "metabolic
death".
The extended course of the disease can be complicated by other
diseases, usually of an infectious etiology (McConnell, 1984). For
instance, parasitism, diarrhoea, and pulmonary infections have been
observed in PBB-contaminated cattle (Jackson & Halbert, 1974;
Willett & Irving, 1976; Moorhead et al., 1978). Many of the pigs
that died during lactation after perinatal exposure to PBB had acute
suppurative pneumonia (Werner & Sleight, 1981). Severely intoxicated
rhesus monkeys may have succumbed finally to gastrointestinal and
respiratory infections (Allen et al., 1978). Male rats that were
given 100 mg FireMaster(R) FF-1/kg body weight per day and died
after 90 days had liver lesions (Gupta & Moore, 1979). Such
overlying disease problems often result in difficulties in
establishing a definitive etiological diagnosis in environmental
exposures (McConnell, 1984).
Table 73. Mortality associated with PBB administration: Individual PBB congeners
PBB Species Sex Exposure Dose Mortality (in percentage References
concentration or No. dead/No. treated)
2,2',4,4',5,5'- rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
mouse (B6C3F1) female in diet for 10 days 750a 2/8 Welsch &
(pregnant) Morgan (1985)
sheepshead intraperitoneal; single 20b no mortality James & Little
(Archosargus or multiple doses; (1981)
probatocephalus observation: 17-40 days
3,3',4,4',5,5'- rat (Sprague-Dawley) male in diet for 20 days 100a 1/2 (the remaining Render et al.
hexabromobiphenyl rat was moribund) (1982)
2,3',4,4',5,5' rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
Mixture of di-, Atlantic salmon in diet for 40 days plus 7.75a (100% mortality) Zitko &
tri-, and (Salmo salar) additional stress at day Hutzinger (1976)
tetrabromobiphenyls 4; observation: 42 days
a In mg/kg feed.
b In mg/kg body weight per day.
8.2 Single and short-term exposures: general signs of toxicity
8.2.1 PBB mixtures
8.2.1.1 Overt clinical signs, food intake, and body weight changes
In many acute and short-term studies, signs of PBB toxicity
include reduction in feed consumption and weight loss or a decreased
weight gain (see Tables 74 and 75). Most of the reports refer to the
FireMaster(R) mixture, which was tested in rats, mice,
guinea-pigs, pigs, cattle and avian species. From these studies with
various protocols, the minimum effective doses of FireMaster(R)
(FM) in the diet or by gavage ranged between 0.3 and 500 mg FM/kg
feed or between 4 and 100 mg FM/kg body weight per day,
respectively. Only a few studies have been performed with technical
octabromobiphenyl and technical decabromobiphenyl; no effects were
observed on feed consumption or body weight of rats (Tables 74 and
75).
Weight loss is not necessarily accompanied by decreased food
intake (e.g., Allen et al., 1978; Lambrecht et al., 1978; Gupta &
Moore, 1979) suggesting that PBBs may cause poor feed utiliz ation.
On the other hand, increased efficiency of feed utilization has been
reported in growing pigs (Ku et al., 1978) and weight loss in hens
was accounted for completely by reductions in feed consumption in
paired feeding studies (Cecil & Bitman, 1978; Ringer, 1978). If the
concentrations of PBBs are high enough, a total refusal of feed will
occur (Babish et al., 1975a; Gutenmann & Lisk, 1975; Ringer & Polin,
1977; Cecil & Bitman, 1978; Polin & Ringer, 1978a; Aulerich &
Ringer, 1979). At death, the loss in body weight can be as great as
30-40% (Allen et al., 1978; Aulerich & Ringer, 1979; Durst et al.,
1978b).
At sublethal doses, decreased food intake and weight loss may
be the only overt signs observed in many species. However, calves
(fed 100 mg FireMaster(R) FF-1/kg body weight per day) also
developed keratitis and lacrimation from day 35, and alopecia from
day 50 (Robl et al; 1978). During a 16-week test period, some pigs
receiving FireMaster(R) BP-6 at 200 mg/kg in the diet had
dermatosis (Ku et al., 1978). Loss of hair (including eye lashes),
dry scaly skin, and periorbital oedema were reported in a juvenile
female rhesus monkey given 25 mg FireMaster(R) FF-1/kg feed for 50
weeks (total dose: approximately 1.5 g), however, the time of onset
of these symptoms was not specified (Allen et al., 1978). A
decreased heart rate (bradycardia) was measured in White Leghorn
cockerels fed 150 mg FireMaster(R) FF-1/kg feed for approximately
9 weeks (Heinemann & Ringer, 1976; Ringer, 1978). Another
characteristic sign in chickens was general oedema (Ringer & Polin,
1977; see also section 8.2.1.3).
Table 74. Effects on feed consumption and changes in body and organ weights after single or short-term exposure to commercial
PBB mixtures (dosing studies)
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 rat male, oral single dose 1000 n.r. no effect liver: increase Kimbrough
(in peanut oil) (Sherman) female t.p.: 2 months et al.
(1978)
M BP-6 rat male oral single dose 500 n.r. -- liver: increase Bernert Jr &
(Lot No. 5143 (Sherman) t.p.: 1-8 weeks Groce (1984)
in corn oil)
FM BP-6 rat female oral daily doses (9) 1 no effect no effect liver: increase; spleen Harris
(in olive oil) (Sprague- during pregnancy (mg/day) kidney, adrenal, ovary et al.
(1978a)
Dawley) t.p.: 13 days gravid uterus, perirenal
(after first dose) fat pads: no effect
FM FF-1 rat male oral multiple doses 3 n.r. no effect thymus spleen: decrease Luster
(Lot No. (Fischer) (22) over 30 days et al.
1312 FT) t.p.: 60 days 30 n.r. reduced thymus, spleen: decrease (1978)
(in corn oil) (after first dose)
FM FF-1 rat male, oral multiple doses 100-1000 reduced reduced liver, spleen: increase Gupta &
(Lot No. (Fischer female (22) over 30 days (day 10) thymus: decrease Moore (1979)
(1312 FT) 344/N) t.p.: up to 90 days
(in corn oil)
male 30 no effect reduced -- Gupta &
(day 15) Moore (1979)
female 30 variable reduced --
(day 10)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
male, 3 -- -- liver: increase (day 15) Gupta et al.
female 30 no effect thymus: decrease (day (1981)
reduced 15); lung, heart, spleen,
(3 week) kidney, adrenal, thyroid,
testis, ovary, uterus,
brain: no effect
FM FF-1 rat male oral multiple doses 1-6 n.r. no effect liver, thyroid: increase Allen-Rowlands
(Lot No. (Sprague- (20) t.p.: 4 weeks adrenal, testis: et al. (1981);
1312 FT) Dawley) (after 1 dose) no effect Castracane
(in lecithin et al. (1982)
liposomes)
FM rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
(in corn oil) single dose et al. (1972b)
t.p.: 7 days
FM BP-6 rat female intraperitoneal n.r. no effect liver: increase Dent et al.
(in peanut oil) (Sprague- single dose 150 (1976b)
Dawley) t.p.: 2 weeks
FM FF-1 rat female intraperitoneal 200-1000 n.r. no effect liver: increase Goldstein
(in corn oil) (Fischer) single dose (µmol/kg et al. (1979)
t.p.: 4 days body
weight)
FM FF-1 rat male intraperitoneal 90
(in (Sprague- single dose
polyethylene Dawley) t.p.: 1 week n.r. n.r. liver: increase Dannan et al.
glycol) (1978a)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
t.p.: 2 weeks n.r. no effect liver: increase; Dannan et al.
no effect spleen, thymus: (1982c);
Millis et al.
(1985a)
FM BP-6 rat male intraperitoneal (days 30 n.r. n.r. liver, spleen: increase Robertson
(Lot 7062) (Wistar) 1 and 3) two doses 75 n.r. n.r. liver: increase; spleen, et al. (1981b)
(in corn oil) t.p.: 6 days thymus: decrease
(after 1 dose)
FM BP-6 rat male intraperitoneal three 0.2 n.r. n.r. liver: increase Ecobichon
(in peanut (Wistar) doses (days 1, 2, 3) mmol/kg et al. (1979)
oil) t.p.: 7 days body
(after 1 dose) weight
FM BP-6 rat male intraperitoneal single 500 n.r. reduced liver: increase; Andres et al.
(in corn oil) (Wistar) or multiple doses (4) thymus, spleen: decrease (1983)
t.p.: 15 days
FM FF-1 mouse female oral multiple doses 30 n.r. no effect spleen: decrease Luster et al.
(Lot No. (B6C3F1) (22) over 30 days thymus: no effect (1978)
1312 FT) t.p.: 60 days
(in corn oil) (after 1 dose)
mouse female oral multiple doses 30 n.r. no effect spleen: increase; Luster et al.
(B6C3F1) (22) from gestational thymus: decrease (1980)
(pregnant) day 0 until litters
were weaned
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 mouse male, oral multiple doses 3-30 no effect variable liver: increase (day 15) Gupta et al.
(Lot No. (B6C3F1/N) female (22) over 30 days lung, heart, spleen, (1981)
FT 1312) t.p.: up to 90 days kidney, adrenal thyroid
(in corn oil) testis, ovary, uterus,
brain: no effect
male 30 no effect reduced thymus: decrease
(only day (only day 30)
30)
female 0.3, 3, no effect increased thymus: no effect
30 (only
day 45)
FM BP-6 mouse female intraperitoneal single 150 n.r. n.r. liver: increase (48, Dent et al.
(in peanut oil) (NMRI) dose t.p.: up to 192 h 96 h), no effect (24, (1977a)
192 h)
FM BP-6 mouse male intraperitoneal 1.50 n.r. no effect liver: increase; Robertson
(in corn oil) (C57BL/6J) single dose (mmol/kg thymus: decrease; et al. (1984c)
t.p.: 5 days body spleen: no effect
weight)
mouse male intraperitoneal 1.50 n.r. no effect liver: increase;
(DBA/2J) single dose (mmol/kg thymus, spleen:
t.p.: 5 days body no effect
weight)
FM FF-1 guinea-pig female oral single dose 50 n.r. no effect "tissues": no effect Ecobichon
(Lot FA (Hartley, t.p.: 2-60 days (liver, kidney, lung, et al. (1983)
7042) (in albino) perirenal fat)
peanut oil) (pregnant)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM BP-6 guinea-pig male intraperitoneal single 50 n.r. -- liver, kidney: no effect Smith
(in peanut oil) Hartley dose t.p.: 120 h et al.
(1986)
hamster male intraperitoneal single 50 n.r. -- liver, kidney: no effect
(golden dose t.p.: 120 h
Syrian)
FM (Lot rabbit male dermal 24 h of 100- n.r. reduced liver: increase Waritz
635-71) (New exposure t.p.: 10 000 (not et al.
Zealand, 14 days significant) (1977)
albino)
FM BP-6 calf male oral daily doses 0.54 no effect reduced -- Willett &
(Lot RP-158) for 44 days mg/day Irving
(in milk) (1976)
FM BP-6 cattle female oral daily doses 25 g reduced reduced liver, kidney, perirenal Irving
(Lot (pregnant) per day (day 4) (day 20) lymph nodes: increase et al.
6244 A) thymus: decrease (1976);
(days 33-66) Willett
& Irving
(1976);
Durst
et al.
(1977);
Moorhead
et al.
(1977)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
250 mg no effect no effect no effect
per day
FM FF-1 calf male, oral daily doses 100 reduced reduced -- Robl et al.
female (day 36) (day 35) (1978)
cattle female oral daily doses 0.3 no effect no effect --
for 158 days
t.p.: 340 days
(after 1 dose)
FM FF-1 dog male, oral daily doses 1 n.r. -- liver: increase Farber et al.
(Beagle) female for 7 weeks (1976)
dog male oral daily doses 4 n.r. reduced -- Farber et al.
(Beagle) (day 30) (1978)
FM Japanese oral daily doses 25-1000 n.r. reduced liver: increase Strik (1973b)
quail for 7 days
FM BP-6 rainbow intraperitoneal n.r. n.r. liver: no effect Elcombe &
(in corn oil) trout single dose t.p.: Lech (1978)
(Oncorhynchus up to 15 days
mykiss)
FM BP-6 brook trout oral multiple (200 mg/kg n.r. reduced liver: no effect Law & Addison
(in dogfish (Salvelinus doses t.p.: body (not (1981)
oil) (fontinalis) 18 days (after weight significant)
1 dose) total dose)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
OcBB (in rat female oral single dose 126-2000 n.r. no effect n.r. Norris et al.
corn oil) (Sprague- t.p.: 14 days (1973, 1975)
(Dawley)
OcBB (in rat male oral single dose 1000 no effect no effect liver: increase (days Lee et al.
corn oil) (Sprague- t.p.: 21 days 2-11), no effect (1975b)
Dawley) (day 21)
oral daily doses (2) 3000 no effect no effect liver: increase (up Lee et al.
t.p.: 28 days (after to day 21), no effect (1975b)
lase dose) (day 28)
OcBB (Dow rat male oral single dose 3400- n.r. -- liver: increase Waritz et al.
Lot 102-7-72) (Sprague- t.p.: 7 days 17 000 (1977)
(in 40-50% Dawley)
acetone: corn
oil = 15:85)
OcBB (in rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
corn oil) single dose t.p.: et al. (1972b)
7 days
rat inhalation 4 h 3.1 n.r. n.r. liver: no effect
exposure for µg/litre
10 days air
OcBB rat male inhalation 4 h 0.96 n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot Sprague- exposure mg/litre significant) (1977)
102-7-72) Dawley) t.p.: 7 days air
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat not inhalation (25 g) n.r. n.r. liver: no effect
(Sprague- specified 23 h/day; (2-15 weeks) kidney,
Dawley) 7 days/week for thyroid: no effect
2-15 weeks (15 weeks)
OcBB rabbit male dermal 24 h 100- n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot (New exposure 10 000 significant) (1977)
102-7-72) Zealand; t.p.: 14 days
(in corn oil) albino)
male dermal 6 h/day; 1 n.r. no effect liver: increase
5 days/week, for
2 weeks t.p.:
18 days (after last
dose)
OcBB bobwhite male, oral single dose 12 500 n.r. n.r. any internal organs:
(Dow Lot quail female t.p.: 14 days no effect
102-7-72)
(in corn oil)
NoBB mouse male, oral single dose up to n.r. n.r. liver: increase Momma (1986)
(B6C3F1) female t.p.: 14 days 15 000
DeBB (in rat male, oral single dose 5000 n.r. no effect liver: no effect Millischer
corn oil) (Sprague- female t.p.: 14 days et al. (1979)
Dawley)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat intraperitoneal 1000 n.r. n.r. liver: no effect Aftosmis
single dose et al. (1972b)
t.p.: 7 days
rat male, dermal 24 h 5000 n.r. no effect liver: no effect Millischer
(Sprague- female exposure t.p.: et al. (1979)
Dawley) 14 days
rat male, inhalation 6 h/day, 0.05-5 no effect no effect liver: increase
(Sprague- female 5 days/week for mg/litre
Dawley) 4 weeks air
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; NoBB = nonabromobiphenyl; DeBB = decabromobiphenyl.
b t.p. = time post-exposure.
c In mg/kg body weight per day, unless otherwise specified.
d n.r. = not recorded.
e Absolute or relative to body weight, respectively.
Table 75. Effects on feed consumption and changes in body and organ weights caused by short-term feeding of commercial PBB mixtures
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- female 75, 300 2/2 weeks no effect no effect liver: increase Dent et al.
Dawley) (1976a)
FM rat (Sprague- male 100 30/30 days no effect no effect liver: increase Sleight &
Dawley) Sanger (1976)
500 30/30 days reduced reduced liver: increase;
kidney: no effect
FM BP-6 rat (Sprague- male 50 3/3 weeks no effect no effect liver: increase Babish &
Dawley) Stoeswand
(1977)
FM BP-6 rat (Sprague- female 50 day 8 of n.r. no effect liver: increase Dent et al.
Dawley) gestation through (1977b)
day 14 post-
partum
FM BP-6 rat male 5 variable no effect no effect liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); kidney: decrease et al. (1977)
(week 3)
rat male 500 variable no effect reduced liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); testis: no effect et al. (1977)
(week 3)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- not 50 (in pre-, post- and n.r. n.r. liver: increase Cagen &
Dawley) specified mother's or perinatal Gibson (1978)
pups weanling's exposure/
diet) of age
15-49 age
days
FM BP-6 rat (Sprague- day 8 of
Dawley) gestation to day
15 postpartum
mothers female 50 n.r. no effect liver: increase; kidney, Dent et al.
mammary: no effect (1978b)
pups male, (50) (in pre- post-, and n.r. no effect liver: increase
female mother's perinatal
diet) exposure
FM BP-6 rat (Sprague- male, (mothers perinatal n.r. reduced liver, spleen: increase Harris et al.
Dawley) female dose: 10 exposure (day 3 of (day 60 of age) (males (1978a)
pups mg/day on age) more affected than
gestation females
days 7-15)
adults males 50-100 10/10 weeks no effect no effect liver: increase Harris et al.
150, 200 10/10 weeks no effect reduced liver: increase; adrenal, (1978b)
spleen, kidney, testes,
seminal vesicles: no
effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
female 600 10/10 weeks reduced reduced n.r.
FM BP-6 rat male 50-500 5/5 weeks n.r. no effect liver: increase Kasza et al.
(Holtzman) (1978a)
FM BP-6 rat (Sprague- male 20 28/28 days no effect no effect liver: increase Chu et al.
Dawley) (1980)
FM BP-6 rat (Sprague- male, 100 (in perinatal n.r. reduced M: ventral Johnston
Dawley) mothers or exposure until (more prostate: decrease et al. (1980)
pups weanlings 9 weeks of age pronounced
diet) in males)
FM BP-6 rat (Sprague- male 1-100 30/30 days no effect no effect liver: increase; thymus, Akoso et al.
Dawley) spleen: no effect (1982a)
male 100 30/30 days no effect no effect thyroid: increase Akoso et al.
(1982b)
FM BP-6 rat (Sprague- male 100 9/9 days no effect no effect liver, thyroid: increase Render et al.
Dawley) kidney: no effect (1982)
FM BP-6 rat (Fisher) male 100 10/10 days no effect no effect liver: increase Raber &
Carter (1986)
FM BP-6 mouse female 1000 11/11 days n.r. -- liver: increase Corbett
(Swiss, et al. (1975)
ICR) male 1000 up to 14/14 n.r. reduced liver: increase (day 4); Corbett
days (day 4) testis: no effect et al.
(1978a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 mouse female 50-200 2/2 weeks no effect no effect liver: increase Cagen et al.
(Swiss, (1977); Cagen
Webster) & Gibson
adult (1978)
pups not 50 (in postnatal -- -- liver: increase Cagen &
specified mothers exposure until Gibson (1978)
diet) 15 days of age
FM mouse not 100 30/30 days n.r. no effect liver: increase; thymus, Fraker & Aust
(BALB/c) specified spleen: decrease (1978)
not 1000 14/14 days n.r. reduced liver: increase; thymus:
specified decrease (nearly athymic)
FM BP-6 mouse female 1, 10 30/30 days n.r. no effect liver, spleen: no effect; Fraker (1980)
(BALB/c) thymus: decrease
female 100 30/30 days n.r. no effect liver: increase; thymus,
spleen: decrease
FM FF-1 mouse male 5 8/8 weeks n.r. no effect liver: increase Loose et al.
(Lot No. (BALB/c (1981)
7042) ByJ) 167 6/6 weeks n.r. no effect liver: increase
167 8/8 weeks n.r. reduced liver: increase
5 3/3 weeks n.r. -- thymus: no effect
5 6-8/6-8 weeks n.r. -- thymus: decrease
167 3-8/3-8 weeks n.r. -- thymus, spleen: decrease
5, 167 3-8/3-8 weeks n.r. -- lung: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mouse male mothers dose: perinatal n.r. no effect spleen: increase; Luster et al.
(Lot No. (B6C3F1) 10 mg/kg exposure thymus: no effect (1980)
1312 FT) pups body weight
per day
(22 doses)
FM BP-6 guinea-pig not 10 45/45 days n.r. no effect liver: increase Vos & van
specified Genderen
(1974)
FM guinea-pig not 1, 10 30/30 days no effect no effect liver: no consistent Sleight &
specified effect Sanger (1976)
100 up to 30 days n.r. reduced liver: increase
(week 3)
500 15/15 days n.r. reduced liver: increase
(week 1)
FM BP-6 pig not 20, 200 16/16 weeks reduced reduced liver, kidney: increase Ku et al.
(growing) specified (1978)
pig not 100 (in perinatal n.r. reduced liver: increase (but no Werner &
(4-week-old) specified mothers exposure (not effect in sows) Sleight
(lactating) diet) significant) (1981)
pig not 100 (in prenatal -- -- thyroid: increase;
(newborn) specified mothers diet) exposure liver: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mink female 1-1.54 several months -- no effect liver, kidney: increase Aulerich &
2.5 several months -- no effect (in animals that died Ringer
(within first during treatment) (1979);
3 months, Ringer et al.
(1981)
later reduced)
6-16 several months (refused) reduced
FM FF-1 Rhesus female 0.3 several months no effect reduced -- Allen et al.
monkey (1978)
(adult)
(infants) not 0.3 (in perinatal -- reduced --
specified mothers exposure;
diet) up to 12
weeks of age
(juvenile) female 300 up to 137 no effect reduced --
days (until a
few days
prior to
death)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM Japanese male, 500, 1000 a few days (refused) Babish et al.
quail female (1975a)
10-100 9/9 weeks no effect no effect liver: increase
FM Bobwhite 100-700 5/8 days reduced n.r. n.r. Cottrell
quail (Colinus et al. (1984)
virginianus)
FM BP-6 chicks male 400 15/15 days n.r. reduced bursa of Fabricius: Vos & van
(Lot No. (Hubbard decrease Genderen
182 RP) Leghorn) 15, 30 63/63 days n.r. reduced bursa of Fabricius, (1974)
spleen decrease
"PBB" pullet 50, 200 4/4 weeks n.r. reduced -- Chang &
(FM) Zindel (1975)
FM chicken male variable, several weeks -- -- liver, thyroid: Ringer & Polin
(White up to 200 increase; comb: (1977)
Leghorn) decrease; testis:
increase (low dose)
decrease (higher dose)
female 125 several weeks reduced -- --
chicks (White 75 several weeks -- reduced --
Leghorn)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 chicken female 20, 64, 8/16 weeks no effect no effect -- Cecil &
(White 200 2/2 weeks reduced reduced -- Bitman (1978)
Leghorn) 640, 2000 2/2 weeks (refused) reduced --
FM FF-1 chicken female 0-25 5/5 weeks no effect -- -- Polin &
(White 125 5/5 weeks reduced -- -- Ringer
Leghorn) 3125 5/5 weeks refused -- -- (1978a)
FM chicks male 75-250 up to 42 reduced reduced liver, thyroid: Ringer (1978)
(White days increase; comb, testes,
Leghorn) spleen, bursa, thymus:
decrease
FM FF-1 cockerels male 10, 100 28/28 days no effect no effect liver: increase; bursa: Dharma et al.
(White decrease; thyroid, (1982)
Leghorn) spleen, testicles,
comb: no effect
OcBB rat 100 28/28 days -- -- liver: increase Aftosmis
et al.
(1972a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
OcBB rat (Sprague- male 100-1000 30/30 days no effect no effect liver: increase; heart, Norris et al.
Dawley) testes, brain: no (1973, 1975)
1000, 10 000 30/30 days effect; kidney:
increase
OcBB rat (Sprague- male 100, 1000 2-4/2-4 weeks no effect no effect liver: increase Lee et al.
Dawley) (1975a)
1000 4/22 weeks no effect no effect liver: increase
OcBB rat male 100, 1000 2-4/2-22 no effect no effect liver: increase Waritz et al.
(Dow Lot (Sprague- weeks (1977)
102-7-72) Dawley)
DeBB rat (Sprague- male, 2000 13/13 weeks no effect no effect liver: increase Millischer
Dawley) female et al. (1979)
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b n.r. = Not recorded.
c Absolute or relative to body weight, resp.
At lethal doses, the "wasting syndrome" (see section 8.1) can
also be accompanied by other symptoms. Rats, which became moribund,
had hunchback posture, sunken eyes, appeared dehydrated, and were
lethargic (Gupta & Moore, 1979). Minks have been reported to show an
unthrifty appearance (Aulerich & Ringer, 1979; Ringer et al., 1981).
Cattle that died later showed many similar clinical signs, but they
also had excessive lacrimation and salivation, diarrhoea and
depressed heart and respiratory rates (Irving et al., 1976; Durst
et al; 1977, 1978b; Moorhead et al., 1977). In two rhesus monkeys,
the time to death was as long as 3-5 months. In addition to body
weight loss, the dead animals exhibited alopecia and oedema,
particularly on the face and eyelids (including loss of eyelashes),
and dry scaly skin (Allen et al., 1978). Observations of intoxicated
Bobwhite quails, prior to death, included asthenia, low carriage, an
unkempt appearance, wing droop, diarrhoea, limited ataxia, and
general lethargy (Cottrell et al., 1984).
8.2.1.2 Haematology and clinical chemistry
a) Haematology
At lethal doses, leukopenia and erythropenia were observed in a
rhesus monkey (Allen et al., 1978), but not in cattle (Moorhead
et al., 1977). Packed cell volume, white blood cell count, and red
blood cell count fell gradually in the monkey (dietary
concentration: 300 mg/kg of feed; total dose: 6.4 g of
FireMaster(R) FF-1), while changes in packed cell volume,
haemoglobin content, total erythrocyte and leukocyte counts, and
differential leukocyte counts were minimal in the cows (dose: 25 g
FireMaster(R) BP-6 per day).
At sublethal doses, haematological parameters of rhesus monkeys
remained within normal limits (Allen et al., 1978). The same was
true for cattle (Moorhead et al., 1977; Robl et al., 1978), with the
exception of a calf dosed with 100 mg FireMaster(R) FF-1/kg body
weight per day, the total leukocyte count of which was elevated
(Robl et al., 1978). Rats given 30 mg FireMaster(R) FF-1/kg body
weight per day for 30 days (22 total doses) showed a significant
decrease in the packed cell volume, haemoglobin concentrations, and
platelet counts at 30 days of exposure (Gupta et al., 1981) and at 6
months after the start of dosing (Gupta & Moore, 1979), but were
normal at 45, 60, or 90 days after the start of dosing (Gupta
et al., 1981). White and red blood cell values were only
occasionally reduced; for example, there was moderate lymphopenia in
female animals at 30 days of exposure (Gupta & Moore, 1979; Gupta
et al., 1981). No significant differences in values for the
erythrocyte count, packed cell volume, haemoglobin, and total and
differential leukocyte counts were found in male rats fed various
levels of FireMaster(R) BP-6 (1-500 mg/kg diet) for 30-60 days
(Sleight & Sanger, 1976; Garthoff et al., 1977; Sleight et al.,
1978); only a possible increase in total white blood cell count was
noted at the 500 mg/kg feed level (Garthoff et al., 1977). A mild
but significant decrease in the packed cell volume and the number of
platelets was observed in mice exposed to 30 mg FireMaster(R)
FF-1/kg body weight per day for 30 days (22 total doses). The
leukocyte values were within normal limits (Gupta et al., 1981).
Growing pigs in a 16-week trial showed significant reductions in
haemoglobin and haematocrit only after 6 weeks of feeding 200 mg
FireMaster(R) BP-6/kg diet (Ku et al., 1978). There was no
appreciable effect on standard haematological values of nursing pigs
and their sows fed FireMaster(R) BP-6 (10-200 mg/kg feed) during
pregnancy and lactation (Werner & Sleight, 1981). White Leghorn
cockerels fed PBB (FireMaster(R) FF-1) at dietary concentrations
of 75 and 150 mg/kg from 3 to 4 days of age until 5-9 weeks showed a
significant decrease in packed cell volume and haemoglobin values
(Heinemann & Ringer, 1976; Ringer, 1978).
Technical octabromobiphenyl caused a significant decrease in
packed cell volume and total red blood cell count in male rats fed
dietary concentrations of 10 000 mg/kg for 30 days, but had no
effect at dietary concentrations of 100 and 1000 mg/kg (Norris
et al., 1973, 1975).
Standard haematological determinations revealed no treatment-
related changes in blood from rats exposed to technical
decabromobiphenyl via diet (1-2000 mg/kg) for 4-12 weeks and via
inhalation (0.005-5 mg/litre, 6 h/day, 5 days/week) for 4 weeks
(Millischer et al., 1979).
b) Clinical chemistry
Clinical chemistry values examined in many studies refer to
serum protein (total protein, specific fractions), serum enzymes,
serum glucose, blood urea nitrogen, serum lipids, serum cholesterol,
and urine.
(i) Serum protein
Decreases in total serum protein, due primarily to a reduction
in the albumin fraction, occurred in severely intoxicated cattle
(Durst et al., 1978a; Schanbacher et al., 1978) and monkeys (Allen
et al., 1978). No consistent effect of dietary PBBs on total serum
protein concentrations or electrophoretic profiles was observed in
pigs fed FireMaster(R) BP-6 at levels of 20 or 200 mg/kg for 16
weeks (Ku et al, 1978). In rats fed 5, 50, or 500 mg FireMaster(R)
BP-6/kg for 3 weeks, total plasma protein was slightly increased by
the highest concentration (Garthoff et al., 1977). A marked increase
(50%) in serum protein was found in rats given 22 oral doses of
FireMaster(R) FF-1 (30 mg/kg body weight per day) over a 30-day
period and observed for some additional weeks. These changes were
primarily associated with increased ß-globulin fractions (Gupta
et al., 1981). Reductions in serum immuno globulin levels
(gamma-globulin fractions) have been reported in mice given oral
doses (30 mg/kg body weight per day) of FireMaster(R) FF-1 (Luster
et al., 1978) or diets containing 167 mg FireMaster(R) FF-1/kg
(Loose et al., 1981).
No treatment-related changes in total serum protein were found
in rats exposed to technical decabromobiphenyl (1-2000 mg/kg feed
for 4-12 weeks; inhalation: 0.005-5 mg/litre; 6 h/day; 5 days/week
for 4 weeks) (Millischer et al., 1979).
(ii) Serum enzymes
Alterations in serum enzymes, most of which are indicative of
liver lesions, have been found in some instances.
Gamma-Glutamyl transpeptidase (gamma-GTP) was elevated by oral
doses of FireMaster(R) FF-1 (30 mg/kg body weight per day) in
female rats and mice of both sexes (Gupta et al., 1981).
No consistent increase in serum glutamic pyruvic transaminase
(SGPT) occurred in rats after dietary (Garthoff et al, 1977;
Matthews et al., 1978) or oral (Gupta et al., 1981) exposure to
FireMaster(R). No changes were found in cows on diets containing
up to 10 mg FireMaster(R) FF-1/kg (Robl et al, 1978). However, a
gradual increase in SGPT activity was observed in lethally
intoxicated rhesus monkeys (Allen et al., 1978). Technical
decabromobiphenyl did not influence SGPT in rats (Millischer et al.,
1979).
Serum glutamic-oxaloacetic transaminase (SGOT) was
significantly increased in cattle at high doses of PBBs (25 g FM
BP-6 per day) (Moorhead et al., 1977; Durst et al., 1978b). It was
unaffected in cattle given lower doses (250 mg FM BP-6/day: Moorhead
et al., 1977; 0.01-10 mg FM FF-1/kg feed: Robl et al., 1978), as
well as in pigs (Ku et al., 1978) or in rats (Sleight & Sanger,
1976; Garthoff et al., 1977; Sleight et al., 1978) fed
FireMaster(R) BP-6 (20 and 200 mg/kg diet or 1-500 mg/kg diet,
respectively). A possible decrease in serum GOT has been reported in
rats exposed to technical decabromobiphenyl via inhalation
(Millischer et al., 1979).
Lactic dehydrogenase (LDH) was within normal ranges in PBB-
contaminated cows without clinical signs of toxicosis (Moorhead
et al., 1977), but it was increased in a group of apparently
intoxicated animals (dose: 25 g FM BP-6/day) (Moorhead et al., 1977;
Durst et al., 1978b). A significant decrease was found in growing
pigs (Ku et al., 1978), but not in nursing pigs and their sows
(Werner & Sleight, 1981), fed FireMaster(R) BP-6.
Electropherograms of LDH isozymes at 60 days, from rats given
FireMaster(R) BP-6 (100 mg/kg feed), showed appreciable changes
(Sleight et al., 1978).
With the exception of calves (Robl et al., 1978) and newborn
pigs (Werner & Sleight, 1981), alkaline phosphatase levels in
animals were unaffected by FireMaster(R), e.g., rats (Sleight
et al., 1978; Gupta et al, 1981), dogs (Farber et al, 1976), and
growing pigs (Ku et al., 1978). Technical decabromobiphenyl also did
not have any effect on alkaline phosphatase levels in rats
(Millischer et al., 1979).
Serum isocitrate dehydrogenase (sICDH) did not show any
discernible rise in dairy cattle dosed with FireMaster(R) BP-6,
until doses were sufficient to cause toxicosis (25 g/day). These
cows showed moderate increases in sICDH (approximately a two-fold
increase). Elevation of sICDH was coincident with fetal trauma.
Non-pregnant cows, equally intoxicated, showed minimal sICDH
elevation (Schanbacher et al., 1987).
Serum creatine phosphokinase levels in growing pigs were
unaffected by 20 or 200 mg FireMaster(R) BP-6/kg feed (Ku et al.
1978).
In male Japanese quails, serum glutamate dehydrogenase levels
were increased by "hexabromobiphenyl" (STRIK, 1973b).
(iii) Serum glucose
A slight decrease in serum glucose was observed in rats and
mice administered 22 total doses of 30 mg FireMaster(R) FF-1/kg
body weight (Gupta et al., 1981) and in rats fed 50 mg
FireMaster(R) BP-6/kg diet for 3 weeks (Garthoff et al., 1977),
but not in rats fed 500 mg/kg diet for the same period (Garthoff
et al., 1977). FireMaster(R) did not produce any effects on
glucose concentrations in either sublethally or lethally intoxicated
cattle (Durst et al., 1978a; Robl et al., 1978), and
decabromobiphenyl did not affect glucose levels in rats (Millischer
et al., 1979).
(iv) Blood urea nitrogen
Values for blood urea nitrogen (BUN) remained in the normal
range in mice (Gupta et al., 1981) and rats (Sleight & Sanger, 1976;
Garthoff et al., 1977; Sleight et al., 1978; Gupta et al., 1981) or
were elevated in rats at some concentrations (Sleight & Sanger,
1976; Garthoff et al., 1977). A significant increase in BUN occurred
in cows (Moorhead et al., 1977; Durst et al., 1978a) and a calf
(Robl et al., 1978) that had received doses of FireMaster(R) high
enough to produce overt signs of toxicosis (25 g FM BP-6 per day
(equivalent to 50 mg/kg body weight per day, and 100 mg FM FF-1/kg
body weight per day, respectively).
(v) Serum lipids
Characteristic alterations in serum phospholipid HPLC profiles,
which were maintained for at least two months after dosing, were
found in rats given a single oral dose of FireMaster(R) BP-6
(500 mg/kg body weight) (Bernert et al., 1985).
(vi) Serum cholesterol
In rats, cholesterol levels appear to be the blood parameter
most sensitive to PBBs. There were dose-related increases in
cholesterol concentrations in short-term (Garthoff et al., 1977;
Spear et al., 1990) and long-term (see 8.4: Bernert et al., 1983;
Gupta et al., 1983a,b) studies, and these increases were significant
at dietary concentrations of FireMaster(R) as low as 5 mg/kg
(Garthoff et al., 1977). No noticeable effects of FireMaster(R) on
cholesterol levels have been reported in cattle (Durst et al.,
1978a) and pigs (Werner & Sleight, 1981). Rhesus monkeys showed a
gradual decrease in serum cholesterol when they were lethally
intoxicated (Allen et al., 1978).
(vii) Urinalysis
Tests of urine for pH, protein, glucose, ketones, bilirubin,
occult blood, and specific gravity showed no significant changes due
to FireMaster(R) in mice (Gupta et al., 1981) and rats (Sleight
et al., 1978; Gupta et al., 1981) or due to technical decabromo
biphenyl in rats (Millischer et al., 1979). Only Sleight & Sanger
(1976) reported higher readings for protein in rats fed
FireMaster(R). Differences in urinary protein patterns between
control rats and rats given FireMaster were detected by means of
two-dimensional electrophoresis (Myrick et al., 1987). The principal
urine changes in cattle lethally intoxicated were decreased specific
gravity and moderate proteinuria (Moorhead et al., 1977; Durst
et al., 1978a).
8.2.1.3 Morphological and histopathological changes
a) Liver
The liver is the site of the most prominent gross morphological
and histopathological changes due to PBBs in many species.
Enlargement of the liver was a characteristic response to
exposure to FireMaster(R), technical octabromobiphenyl, and
technical decabromobiphenyl, and it frequently occurred at
concentrations lower than required to produce body weight changes
(see Tables 74 and 75). Generally, increases in absolute or relative
liver weights were dose and time dependent (e.g., Garthoff et al.,
1977). A notable exception was in cows, which showed decreases in
body weight as well as an increase in liver weights only at lethal
doses (Table 74).
Grossly, the livers of rats were often friable and had a
mottled surface (Sleight & Sanger, 1976; Waritz et al., 1977; Akoso
et al., 1982a; Render et al., 1982; Raber & Carter, 1986). Red
fluorescence of liver (and other tissues) under UVR (366 nm)
indicated excess porphyrin accumulation. In contrast to rats, which
developed porphyria after long-term exposure, the liver of female
mice given 30 mg FM FF-1/kg body weight per day (22 total doses)
became porphyric after 45 days (Gupta et al., 1981).
FireMaster(R) FF-1 given orally at a dose rate of 22.5 mg/kg body
weight per day for 4 days caused centrolobular accumulation of
Oil-red O-staining lipids in the liver of rats (Kohli et al., 1981).
The principal histopathological alterations in rodent species
consisted of extensive swelling and vacuolation of hepatocytes and
proliferation of smooth-surfaced endoplasmic reticulum (SER) (seen
as "foamy cytoplasm" in light microscopy). The vacuoles were filled
with fat indicating excess lipid accumulation. Proliferation of SER
may be a morphological reflection of enhanced enzyme activity
(Sleight & Sanger, 1976; Render et al., 1982). The changes depended
on dose and length of exposure. They have been reported in rats
after dietary intake of FireMaster(R) (Sleight & Sanger, 1976;
Kasza et al., 1978a; Sleight et al., 1978; Hinton et al., 1979;
Akoso et al., 1982a; Render et al., 1982; Raber & Carter, 1986) and
of technical octabromobiphenyl (Norris et al., 1973; Lee et al.,
1975a). The dietary concentrations ranged from 0.1 to 500 mg/kg for
FireMaster(R) and from 100 to 10 000 mg/kg for octabromobiphenyl.
Effects were seen as early as the tenth day of feeding 100 mg FM
BP-6/kg (Raber & Carter, 1986). In contrast, during a 90-day feeding
trial, technical decabromobiphenyl (dietary concentration: 100, 500,
or 2000 mg/kg) caused hepatic damage only at the highest level
(Millischer et al., 1979). Liver changes, as noted above, were
observed also after a single i.p. injection (200-1000 µmol/kg) of
FireMaster(R) (Goldstein et al., 1979), after single oral dosing
(1000 mg/kg body weight) of FireMaster(R) (Kimbrough et al., 1978)
and of octabromobiphenyl (Lee et al., 1975b), and after multiple
oral dosing of FireMaster(R) (22 doses over a 30-day period:
30 mg/kg body weight per day (Gupta & Moore, 1979; Gupta et al.,
1981) and of octabromobiphenyl (two doses: 3000 mg/kg body weight;
Lee et al., 1975b). As soon as 24 h (Kimbrough et al., 1978), 3 days
(Lee et al., 1975b), or 4 days (Goldstein et al., 1979) after
treatment, histological changes could be detected.
Light and electron microscopic changes also reported in the
liver of rats included reduction or disintegration of rough-
surfaced endoplasmic reticulum (RER) (Lee et al., 1975a,b; Gupta
et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), presence
of myelin bodies (cytoplasmic inclusions; membrane whorls) (Lee
et al., 1975a,b; Sleight & Sanger, 1976; Kasza et al., 1978a;
Kimbrough et al., 1978; Sleight et al., 1978; Hinton et al., 1979;
Gupta et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), di-
or multinucleated cells (Kasza et al., 1978a; Kimbrough et al.,
1978; Gupta & Moore, 1979; Hinton et al., 1979), diminution of
glycogen (Millischer et al., 1979; Gupta et al., 1981; Raber &
Carter, 1986) and mitochondria that were swollen and reduced in
number or had degenerated as time passed (Sleight & Sanger, 1976;
Kasza et al., 1978a; Akoso et al., 1982a). There was also necrosis
of hepatocytes (Kimbrough et al., 1978; Gupta & Moore, 1979; Gupta
et al., 1981), and these necrotic foci were infiltrated with
polymorphonuclear cells and lymphocytes (Gupta et al., 1981). With
octabromobiphenyl, myelin configurations developed 7 days after
treatment and subsequently disappeared one week later (Lee et al.,
1975b).
Changes in the hepatocytes were more advanced in the centri
lobular and midzonal regions than in the periportal area of the
liver lobule (Render et al., 1982; Raber & Carter, 1986). Fatty
infiltration in the livers of male rats was much more pronounced
than in those of female rats (Gupta & Moore, 1979).
Male rats given 100 mg FM FF-1/kg body weight per day (22 total
doses) and dying after 90 days had subacute to chronic hepatitis
with marked focal proliferation of bile ducts (Gupta & Moore, 1979).
Changes in the bile canaliculus (proliferation of microvilli)
were also found in mice fed 1000 mg FM BP-6/kg for 4-14 days.
Changes in the hepatocytes of these mice were increase in cell size,
decrease in RER, increase in SER, degeneration of mitochondria,
decrease in glycogen, and increase in size and number of nucleoli
(Corbett et al., 1978a). Fatty infiltration of the cytoplasm was
reported only in another two studies on mice dosed with 30 mg FM
FF-1/kg body weight per day (Gupta et al., 1981) or fed 167 mg FM
BP-6/kg for 6 weeks (Loose et al., 1981). However, in (moribund)
mice fed 167 mg FM BP-6/kg feed for 12 weeks, lipid vacuoles were
not found within the cytoplasm, but, almost exclusively, within the
nucleus (Martino et al., 1981). Hepatocellular necrosis has also
been observed (Loose et al., 1981).
Hepatocytes of guinea-pigs were swollen and had many more large
vacuoles than those of comparably dosed rats (Sleight & Sanger,
1976), even at lower dietary concentrations (< 10 mg FM/kg). Liver
damage was reported to be minimal at dietary levels of 50 mg FM/kg
(Vos & van Genderen, 1974), but severe centrilobular fatty changes
were found at 100 and 500 mg FM BP-6/kg (Sleight & Sanger, 1976).
Livers of rabbits, dermally treated with FireMaster(R) at 5
or 10 g/kg body weight, showed a mottled appearance, necrotic foci,
and were friable. No gross pathological effects were seen in rabbits
dermally exposed to octabromobiphenyl at 10 g/kg body weight (Waritz
et al., 1977).
Pigs fed FM BP-6 (up to 200 mg/kg) showed the following liver
alterations: fatty change, centrolobular necrosis, swollen
hepatocytes, and homogeneous cytoplasm (Werner & Sleight, 1981).
Compared with other species, liver changes observed in cattle
were less dramatic. Only an early stage of centrilobular fatty
degeneration and glycogen depletion were found in the enlarged
livers of lethally (25 g FM BP-6/day) dosed cows. In addition, there
were changes in the gallbladder and bile duct (Mercer et al., 1978;
Moorhead et al., 1978). Single calves exposed to FM FF-1 for 6-12
weeks had slightly enlarged hepatocytes (dose: 1 mg/kg body weight
per day) or necrosis of individual or small foci of hepatocytes
(dose: 100 mg/kg body weight per day) (Robl et al., 1978).
The only consistent histopathological lesion in mink which died
(exposure: 6.25 mg FM FF-1/kg feed), was a fatty infiltration of the
liver (Aulerich & Ringer, 1979).
No hepatocellular damage was found in dogs given oral doses of
FM BP-6 (1 mg/kg body weight per day) for seven weeks (Farber
et al., 1976).
Biopsies of the livers of two rhesus monkeys given a diet
containing 25 mg FM FF-1/kg at 12 weeks revealed enlargement of
hepatocytes and a marked proliferation of SER (Allen et al., 1978).
Liver changes in avian species were similar to those observed
in mammals. White Leghorn cockerels fed a diet of 10 or 100 mg FM
FF-1/kg showed enlargement and vacuolation of hepatocytes, increased
SER, swollen mitochondria, and disruption of mitochondrial cristae.
But, unlike rats, increased SER was not a significant feature
(Dharma et al., 1982).
b) Thymus
The thymus is also an organ sensitive to PBB exposure.
Decreases in thymus weights were observed in rats, mice, and cattle
after oral or intraperitoneal doses (Table 74) and in mice after
feeding (Table 75) of FireMaster(R) mixtures. There were no
reports on thymus weight changes due to exposure to commercial octa-
or decabromobiphenyl (Tables 74 and 75). The weight of the thymus
was reduced as early as 6 (Robertson et al., 1981b) or 15 days
(Gupta et al., 1981; Andres et al., 1983) after rats were given high
doses of FireMaster(R) (Table 74). Frequently, decreases in thymus
weights were accompanied by increases in liver weights (see: Tables
74 and 75). But, in some cases, changes in thymus weights were seen
at doses lower than those required for liver changes (Fraker, 1980),
or vice versa (Akoso et al., 1982a). Rats (Akoso et al., 1982a) and
mice (Fraker & Aust, 1978) both fed FireMaster(R) BP-6 (100 mg/kg
feed) for 30 days differed in their thymic response in that rats
remained unaffected and mice showed decreased thymic weight
(Table 75). In contrast, rats appeared to be more sensitive than
mice when given equal oral doses (30 mg/kg body weight per day for a
period of 30 days) of FireMaster(R) FF-1 (Luster et al., 1978;
Gupta et al., 1981: Table 74). Two strains of inbred mice are known
to differ in their thymic sensitivity to FireMaster(R) (Robertson
et al., 1984c: Table 74).
In some studies, the histological appearance of the thymus of
rats and mice that had survived exposure to FireMaster(R) was only
minimally, or not, affected (Gupta & Moore, 1979; Gupta et al.,
1981; Loose et al., 1981; Akoso et al., 1982a), even when organ
weights were altered. In other studies, a preferential atrophy of
the cortex of the thymus was found in rats and mice similarly
exposed (Luster et al., 1978; Fraker, 1980). At high doses (1000 mg
FM BP-6/kg feed), "surviving" mice (30%) were essentially athymic by
day 14 (Fraker & Aust, 1978). The thymus was markedly involuted also
in moribund rats. The normal architecture of the thymus was
obliterated with marked atrophy and loss of demarcation between the
cortical and medullary regions and disappearance of cortical
thymocytes (Gupta & Moore; 1979). Moderate to marked atrophy of the
thymus was also observed in guinea-pigs (Vos & van Genderen, 1974)
or cattle (Moorhead et al., 1978) that were lethally intoxicated.
The bursa of Fabricius, an analogous organ in avian species,
was also affected by FireMaster(R) (Table 75: Vos & van Genderen,
1974; Ringer, 1978; Dharma et al., 1982). Reductions in weight
occurred at exposures as low as 10 mg/kg feed in cockerels (Dharma
et al., 1982). Histologically, the bursa showed depletion of the
lymphoid cells especially in the medulla (Vos & van Genderen, 1974;
Ringer, 1978; Dharma et al., 1982).
c) Spleen
Changes in spleen weights are given in Tables 74 and 75.
Feeding of FireMaster(R) (100-200 mg/kg equivalent to 10-20 mg/kg
body weight per day) had no effect on the spleen weights of young
rats (Harris et al., 1978b; Akoso et al., 1982a), but resulted in a
decrease in the spleen weights of mice (equivalent to 15-30 mg/kg
body weight per day) (Fraker & Aust, 1978; Fraker, 1980; Loose
et al., 1981). An increase in spleen weights was observed in pups of
both rats (Harris et al., 1978a) and mice (Luster et al., 1980)
perinatally exposed to FireMaster(R). No effects were found in
some oral dosing studies on rats (Harris et al., 1978a; Gupta
et al., 1981); in another study, spleen weights were decreased
(Luster et al., 1978), and in the highest-dosage study reported
spleen weights increased (Gupta & Moore, 1979). On the other hand,
rats that received i.p. injection of FireMaster(R) showed
decreases in spleen weight at the higher doses (Robertson et al.,
1981b; Andres et al., 1983) and increases at the lower dose
(Robertson et al., 1981b). While female mice orally dosed with
FireMaster(R) showed decreased spleen weights (Luster et al.,
1978), pregnant and nursing females comparably dosed exhibited
increased spleen weights (Luster et al., 1980). No effect was found
in mice, 5 days after a single i.p. injection of FireMaster(R),
though the liver and thymus were affected (Robertson et al., 1984c).
Spleen weights of chicks fed FireMaster(R) were reduced (Vos & van
Genderen, 1974; Ringer, 1978) or remained unaffected (Dharma et al.,
1982).
Significant histopathological changes in the spleen have not
been reported, except in rats that were moribund from high doses of
FireMaster(R). In these animals, the splenic lymphatic follicles
were small because of a lack of periarterial lymphoid cells (Gupta &
Moore, 1979).
d) Thyroid
Exposure to FireMaster(R) resulted in an increase in thyroid
weight, if there was any weight change (see Tables 74 and 75).
Increases in thyroid weight have been observed in rats (Sleight
et al., 1978; Allen-Rowlands et al., 1981; Akoso et al., 1982b;
Render et al., 1982), newborn pigs (Werner & Sleight, 1981) and in
chickens (Ringer & Polin, 1977; Ringer, 1978). When no weight change
occurred (rats: Sleight et al., 1978; Gupta et al., 1981; mice:
Gupta et al., 1981; chickens: Dharma et al., 1982), the extent of
exposure was not always less than in the former studies.
Octabromobiphenyl tested in one inhalation study did not have any
effect on thyroid weight in rats (Waritz et al., 1977).
Histological changes in the thyroid gland developed in rats at
dietary concentrations of FireMaster(R) BP-6 as low as 5 mg/kg
(Kasza et al., 1978b). Hyperplasia of follicular cells occurred in
rats (Kasza et al., 1978b; Sleight et al., 1978) and newborn pigs
(Werner & Sleight, 1981). The normal low cuboidal follicular
epithelium was altered to one that had a more columnar appearance
(Kasza et al., 1978b; Sleight et al., 1978). The most prominent
ultrastructural lesions found in rats fed 5-500 mg
FireMaster(R)/kg for 5 weeks were dose-dependent. They included
abnormal lysosomes and colloid droplets in the cytoplasm, vacuolated
mitochondria with disrupted cristae, luminal surfaces with short and
abnormally branched microvilli or devoid of microvilli, and abnormal
cytoplasmic processes into the lumen (Kasza et al., 1978b).
e) Kidney
In most studies using rodents (Sleight & Sanger, 1976; Waritz
et al; 1977; Harris et al., 1978a,b; Gupta et al., 1981; Render
et al., 1982; Ecobichon et al., 1983; Smith et al., 1986), kidney
weights did not change with PBB exposure (Tables 74 and 75).
Increases in kidney weights were caused by FireMaster(R) in minks
(Aulerich & Ringer, 1979) pigs (Ku et al., 1978), and cattle
(Moorhead et al., 1977), and by octabromobiphenyl in rats (Norris
et al., 1973). A single study reported a decrease in kidney weight
in rats fed FireMaster(R) BP-6 (Garthoff et al., 1977).
In cattle, kidneys were severely affected, and doubled in size
in animals that were moribund from high doses of FireMaster(R)
(Moorhead et al., 1977). The kidneys were distended with fluid, and
pale tan to gray in colour. Perirenal lymph nodes were enlarged and
oedematous. The principal histological lesions consisted of
dilatation of collecting ducts and convoluted tubules, and tubular
epithelial degenerative changes (Moorhead et al., 1977). Similar
renal lesions were found in calves treated with different doses of
FireMaster(R) (0.1-100 mg FM FF-1/kg body weight per day, for 2-12
weeks). The severity of renal damage was related to dose level and
length of exposure (Robl et al., 1978). Despite the extensive
morphological damage, effective renal plasma flow rates and
glomerular filtration rates were not affected in cows (Mercer
et al., 1978; Schanbacher et al., 1978).
Ultrastructural analysis of kidneys of rats and mice given a
single i.p. injection of 150 mg "PBB" (not specified)/kg body weight
revealed proliferation of SER and increased numbers of peroxisomes
in the proximal tubule of the rat, 15 days after dosing.
Proliferation of SER was confined to only one segment (S3) of the
proximal tubule. Mice had only marginal increases in SER and no
significant increases in peroxisomes (Rush et al., 1986).
f) Stomach
Biopsies of the stomachs of two rhesus monkeys given
FireMaster(R) FF-1 (25 mg/kg diet) were made at 12 weeks. In the
gastric mucosa, there was evidence of early epithelial hyperplasia
and penetration of the submucosa by glandular epithelium (Allen
et al., 1978).
g) Testicle
Changes in the weights of the testes due to PBB exposure (see
Tables 74 and 75) were not found in rats (Norris et al., 1973;
Garthoff et al., 1977; Harris et al., 1978b; Gupta et al., 1981;
Castracane et al., 1982) and mice (Corbett et al., 1978a; Gupta
et al., 1981). The results of studies on chickens (Ringer & Polin,
1977; Ringer, 1978; Dharma et al., 1982) were inconsistent (see
Table 75).
Histologically, treatment-associated changes (e.g.,
hypospermatogenesis) were observed in the testes of male calves
administered FireMaster(R) FF-1 (0.1-100 mg/kg body weight per
day) for 2-12 weeks (Robl et al., 1978). Chickens fed
FireMaster(R) (50 mg/kg of feed) showed lipid infiltration into
the testicular parenchyma (Ringer & Polin, 1977).
h) Fluid accumulation
The presence of fluid accumulation, i.e., hydropericardium and
ascites, was noted in chickens (Heinemann & Ringer, 1976; Ringer,
1978). This lesion is known as "chick oedema disease" (McConnell,
1980). Oedema were observed also in the skin of rhesus monkeys
(Allen et al., 1978) and in the kidney, perirenal lymph nodes, and
the gastrointestinal tract of cattle (Moorhead et al., 1978) that
were lethally intoxicated.
Occasionally, weight changes in organs other than those
discussed above have been reported (see Tables 74 and 75, but they
appear to be of minor importance.
Some special effects occuring after single or short-term
exposure to PBBs are reviewed in the respective sections.
8.2.2 Individual PBB congeners and comparative studies
A lot of individual PBB congeners have been examined for
prominent general signs of toxicity, such as changes in body and
relative organ weights (see Tables 76 and 77) and for histopatho
logical changes (see Tables 78 and 79). It is evident from these
records that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological
changes in the liver and thymus. Despite variations in experimental
protocols, a tendency can be seen that the most severe effects are
elicited by congeners listed under category I. The relative severity
of damage decreases in categories II and III with the least effects
in the last group. Within a category, the degree of bromination may
also influence toxicity. Categorization of halogenated biphenyls has
been made on a structural basis (Parkinson et al., 1983; Safe,
1984). Category I comprises isomers and congeners lacking
ortho-substituents. They are referred to as coplanar PBBs.
Mono- ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more ortho-bromines) have
been organized into the third category. Structure-activity
relationships are discussed in detail by several authors (e.g.,
Goldstein, 1979; Parkinson et al., 1983; Safe, 1984).
Orders of toxicity derived from comparative studies included
only a limited number of congeners in any given study, but they
agreed with the trends described above. Ecobichon et al. (1979)
evaluated ultrastructural effects of lower and higher brominated
congeners on hepatocytes of rats and showed that the highly
brominated congeners (tetra-, penta-, hexa-, octabromobiphenyls)
were more active than the low bromine-containing congeners (di-,
tribromobiphenyls). Results of comparative studies dealing with
higher brominated isomers and congeners, predominantly constituents
of the FireMaster(R)-mixture, and the mixture itself have been
summarized in Table 80.
In all combinations tested, 3,3',4,4',5,5'-hexabromobiphenyl
(BB 169) was found to be the most toxic PBB. This congener, only a
very minor constituent of FireMaster(R), resembles 2,3,7,8-tetra
chlorodibenzo- p-dioxin (TCDD), the typical and the most toxic
member of the class of polyhalogenated hydrocarbons (e.g., Poland &
Knutson, 1982). Of the major FireMaster(R) constituents,
2,3,3',4,4',5-hexabromobiphenyl (BB 156) appeared to be the most
toxic, followed by 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5-pentabromobiphenyl (BB 118). The main component of the
FireMaster(R)-mixture, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153)
was relatively nontoxic as well as the second most abundant
constituent, 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). Compared
with the mixture itself, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)
was consistently more toxic than FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) less toxic. With
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), different results were
obtained from a dosing study (Dannan et al., 1978a) and two feeding
studies (Akoso et al., 1982a,b; Dharma et al., 1982). The latter
attributed FireMaster(R) a higher toxicity than 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167); 2,2',4,5,5'-pentabromobiphenyl (BB 101)
was less effective in producing adverse effects than
2,3',4,4',5-pentabromobiphenyl (BB 118), which matched
FireMaster(R) in some aspects (see Table 80).
Table 76. Effect of individual PBB congeners on body weight (or body weight gain) and relative organ weights of mice
and rats (dosing studies)
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Categoryd I PBBs ("Coplanar" congeners)
4,4'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon
600 (4-6) (days 1,2,3) (peanut oil) et al. (1979)
t.p.: 7 dayse
3,4,4',tri rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4'-tri rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
300 (3) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,4,4',5-tetra rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4',5-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982)
t.p.: 5 dayse
3,3',4,4'-tetra rat (Sprague- male oral single dose (corn no effect no effect (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4'-tetra rat (Long male intraperitoneal single no effect increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4'-tetra rat (Wistar) male intraperitoneal single reduced increased reduced Andres et al.
150 (4-5) dose (corn oil) (1983);
t.p.: 2 weeks Robertson
et al. (1983b)
3,3',4,4'-tetra rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
4.25g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4'-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4'-tetra mouse male intraperitoneal single no effect increased reduced Robertson et al.
(C57BL/6J dose (corn oil) (1984c)
1500 and DBA/2J) t.p.: 5 days
(10)
3,3',4,4',5-penta rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5-penta rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4',5,5'-hexa rat (Sprague- male oral single dose (corn no effect (increased)f (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
3,3',4,4',5,5'-hexa rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
3.19g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) t.p.: 7 daysd (1979)
Category II PBBs (Monoortho "coplanar" derivatives)
2,3',4,4'-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4'-tetra mouse male intraperitoneal single Robertson et al.
1500 dose (corn oil) (1984c)
t.p.: 5 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
(C57BL/6J) no effect no effect no effect
(5)
(DBA/2J) no effect increased no effect
(5)
2,3',4,4',5-penta rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single reduced increased reduced Dannan et al.
Dawley) (6) dose (polyethylene glycol) (1982c)
164g t.p.: 2 weeks no effect increased no effect Millis et al.
(1985a)
2,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single reduced increased (reduced)f Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1978a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Sprague- male intraperitoneal 2 doses reduced increased reduced Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
3.8-60 (1-4) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,3,3',4,4',5'-hexa rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Category III PBBs (Others)
4-mono rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (day 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2'-di rat (Sprague- male intraperitoneal single -- no effect -- Moore et al.
289g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: 2-22 days
2,2'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,5'-di rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,2'5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,3',4'5-tetra rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
150 (4) (days 1,3) (corn oil) (1980)
t.p.: 5 dayse
2,2',5,5'-tetra rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4,4',6-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,3',4,4',6-penta rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5,5'-penta rat (Long male intraperitoneal single no effect increased no effect
500 Evans) (3) dose (corn oil)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single no effect no effect no effect Dannan et al.
164g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
(6) t.p.: 14 days no effect increased no effect Millis et al.
(1985a)
2,2',3,4,4',5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',4,4',6,6'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa rat (Fischer male, oral 22 doses (over no effect increased no effect Gupta et al.
590g 344/N) (6) female 30 days) (corn oil) (1981)
t.p.: 15, 30, 45, 60,
90 dayse
2,2',4,4',5,5'-hexa rat (Fischer) female intraperitoneal single Goldstein et al.
(4) dose (corn oil) (1979)
t.p.: 4 days
40 no effect no effect --
200, no effect (increased)f --
1000
2,2',4,4',5,5'-hexa rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Moore et al.
144g Dawley) (6) dose (polyethylene glycol) (1978b); Millis
t.p.: 2 weeks et al. (1985a)
2,2',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa mouse male, oral 22 doses (over no effect increased no effect Gupta et al.
590g (B6C3F1/N) female 30 days) (corn oil) (1981)
(6) t.p.: 15, 30, 45, 69,
90 dayse
2,2',3,3',4,4'5-hepta rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
127g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',3,4,4',5,5'-hepta rat (Sprague- male intraperitoneal single -- increased -- Moore et al.
127g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: up to 22 days
2,3,3',4,4',5,6-hepta rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
6 (2) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,2',3,3',4,4',5,5'- rat (not male intraperitoneal single -- increased -- Besaw et al.
octa 115g specified) dose (solvent not (1978)
specified) t.p.: 7 days
Table 76 (contd).
a Total dose in µmol/kg body weight.
b t.p. = Time post-exposure; -- = data not given.
c Only statistically significant changes; organ weight changes relative to body weight.
d Categorization according to Safe (1984); see also text in section 8.2.2.
e After first dose.
f Only absolute data given.
g Calculated from original value given in mg/kg body weight.
Table 77. Effect of various hexabromobiphenyl isomers on feed intake, body weight (or body weight gain)
and (relative) organ weights (feeding studies)
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Categoryb I PBBs
3,3',4,4',5,5'- rat male 10 30 reduced reduced increased reduced spleen: reduced Akoso et
hexa (Sprague- brain: no effect (1982a,b)
Dawley) thyroid:
(6) increased
rat male 1 9 no effect no effect no effect Render
(Sprague- 10 9 reduced reduced increased reduced et al.
Dawley) 100 9 reduced reduced increased reduced (1982)
(6)
(2) 100 20 refused
(day 16)
Category II PBBs
2,3',4,4',5,5'- rat male 1, 10 30 no effect no effect no effect no effect brain: increased Akoso
hexa (Sprague- thyroid: no effect et al.
Dawley) 100 30 no effect no effect increased no effect brain: increased (1982a,b)
(6) thyroid: no effect
cockerel male 4, 10 28 no effect no effect no effect bursa of Dharma
(White Fabricius, et al.
Leghorn) thyroid, spleen, (1982)
(10) testicles, comb:
no effect
Table 77 (contd).
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Category III PBBs
2,2',4,4',5,5'- rat male 1 30 no effect no effect increased no effect brain: increased Akoso
hexa (Sprague- thyroid: no et al.
Dawley) effect (1982a,b)
(6)
(6) 10, 100 30 no effect no effect increased increased brain: increased
thyroid: no
effect
rat male 10 9 increased no effect increased n.r. kidney: no Render
(Sprague- effect et al.
Dawley) 100 9 no effect no effect increased n.r. kidney: no (1982)
(6) effect
mouse female 100, 300 gd 6 no effect no effect increased n.r. Welsch &
(C57 BL), through Morgan
pregnant 15 (1985)
(3) 500, 750 sacrifice no effect reduced increased n.r.
gd 17
1000 reduced reduced increased n.r.
cockerel male 10 28 no effect no effect no effect n.r. bursa of Fabri- Dharma et al.
(White cius, thyroid, (1982)
Leghorn) 62 28 no effect no effect increased n.r. spleen, testicles,
(10) comb, no effect
(both concen-
trations)
Table 77 (contd).
a Only statistically significant changes; organ weight changes relative to body weight except for Render et al.
(1982) giving absolute data.
b Categorization according to Safe (1984); see also text in section 8.2.2.
c gd = Gestation day.
d n.r. = Not recorded.
Table 78. Histopathology in relation to individual PBB congeners (dosing studies)
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
Categorya I PBBs
4,4'-di rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(6) t.p.: 7 daysd (1977)
3,3',4,4'-tetra rat (Sprague- male oral single dose 21.3 + ++ 8 tissues: Millis et al.
Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Sprague- male intraperitoneal single 4.25e + 0 11 tissues: Millis et al.
Dawley) (6) dose t.p.: 2 weeks 0 (1985a)
rat (Wistar) male intraperitoneal single 150 + ++ spleen, Andres et al.
(4) dose t.p.: 2 weeks kidney: 0 (1983); Robertson
et al. (1983b)
3,3',4,4',5,5'- rat (Sprague- male oral single dose 21.3 ++ ++ 8 tissues: Millis et al.
hexa Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
Categorya II PBBs
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) (6) dose t.p.: 2 weeks tissues: 0 (1982c); Millis
et al. (1985a)
2,3',4,4',5,5'- rat (Sprague- male intraperitoneal single 144e ++ n.r. 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,3,3',4,4',5- rat (Sprague- male intraperitoneal single 144e ++ ++ 9 tissues:
hexa Dawley) (4) dose t.p.: 1 week 0
Categorya III PBBs
4-mono rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2'-di rat (Sprague- male intraperitoneal single 289e 0 0 8 tissues: Moore et al.
Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,5'-di rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r.
(4-6) t.p.: 7 daysd
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,2',5,5'-tetra rat (Wistar) male intraperitoneal single 150 + 0 spleen, Robertson et al.
(not specified) dose t.p.: 2 weeks kidney: 0 (1983b)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) dose t.p.: 1-2 weeks tissues: 0 (1982a); Millis
(4-6) et al. (1985a)
2,2',3,4,4',5'- rat (Sprague- male intraperitoneal single 144e + 0 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',4,4',5,5'- rat (Fischer male, oral 22 doses 1052e + 0 16 tissues: Gupta et al.
hexa 344/N) female t.p.: up to 90 daysd 0 0 (1981)
2,2',4,4',5,5'- rat (Fischer) male intraperitoneal single 200-1000 + n.r. n.r. Goldstein et al.
hexa (4) dose t.p.: 4 days (1979)
rat (Sprague- male intraperitoneal single 144e + 0 8-11 Moore et al.
Dawley) dose t.p.: up to 14 days tissues: 0 (1978b); Millis
(3-6) et al. (1985a)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,4',6,6'- rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
hexa (4-6) t.p.: 7 daysd
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,2',3,3',4,4',5- rat (Sprague- male intraperitoneal single 127e + 0 9 tissues: Dannan et al.
hepta Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',3,4,4',5,5'- rat (Sprague- male intraperitoneal single 127e + 0 8 tissues: Moore et al.
hepta Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,2',3,3',4,4', rat (not male intraperitoneal single 115e + 0 several Besaw et al.
5,5'-octa specified) dose t.p.: 7 days tissues: 0 (1978)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
a Categorization according to Safe (1984); see also text in section 8.2.2.
b Total dose in µmol/kg body weight.
c t.p. = Time post-exposure.
d After first dose.
e Calculated from original value given in mg/kg body weight.
f n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Table 79. Histopathology to individual PBB congeners (feeding studies)
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
Categorya I PBBs
3,3',4,4',5,5'- rat (Sprague- male 1 10 + 0 Render et al. (1982)
hexa Dawley) (6) 10 10 ++ ++
100 10 ++ ++ spleen, lymph
nodes: +
rat (Sprague- male 1 30 + 0 thyroid: + Akoso et al.
Dawley) (6) 10 30 ++ ++ thyroid, (1982a,b)
pituitary
gland: +
Categorya II PBBs
2,3',4,4',5,5'- rat (Sprague- male 1, 10, 100 30 + 0 thyroid: +
hexa Dawley) (6)
cockerels male 10 28 + n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: +
(10)
Categorya III PBBs
2,2',4,4',5,5'- rat (Sprague- male 10, 100 10 + 0 18 tissues: 0 Render et al. (1982)
hexa Dawley) (6)
rat (Sprague- male 1, 10, 100 30 + 0 thyroid: + Akoso et al.
Dawley) (6) (1982a,b)
Table 79 (cont'd).
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
cockerel male 4, 10 28 0 n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: 0
(10) 62 28 + n.r. bursa of
Fabricius: +
a Categorization according to Safe (1984); see also text in section 8.2.2.
b n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Dannan et al. (1982b) recombined 9 purified FireMaster(R)
constituents, namely BB 101 (2,2',4,5,5'-penta-,) BB 118
(2,3',4,4',5-penta-), BB 153 (2,2',4,4',5,5'-hexa-), BB 138
(2,2',3,4,4',5'-hexa-), BB 167 (2,3',4,4',5,5'-hexa), BB 156
(2,3,3',4,4',5-hexa-), BB 180 (2,2',3,4,4',5,5'-hepta-), BB 170
(2,2',3,3',4,4',5-hepta-), and BB 194 (2,2',3,3',4,4',5,5'-
octabromobiphenyl), totalling 97% of either FireMaster(R) mixture,
to form a reconstituted FireMaster(R) BP-6-like mixture and
compared some effects of the reconstituted mixture to those of crude
FireMaster(R) mixtures BP-6 and FF-1. Rats were treated with a
single dose (90 mg/kg body weight of either mixture) and sacrificed
one week later. Evaluating changes in body and selected organ
(liver, thymus, spleen) weights, the conclusion was reached that
adverse effects of FireMaster(R) (FF-1 or BP-6) must be due to the
effects of the congeners studied. Moreover, it was found that the
increase in liver weights was greater with FM BP-6 and the
reconstituted mixture than with FM FF-1, consistent with the higher
proportion of minor components (BBs: 118, 138, 167, 156) in FM BP-6
(25% versus 15%).
Differences in toxicity between PBBs were sometimes of a
qualitative kind, but mostly they were quantitative. Some striking
relations will be reviewed in detail according to the parameter
tested.
8.2.2.1 Food intake, overt clinical signs, body weight changes
So far, in the congeners tested, food intake of rats was
reduced only by 3,3'4,4',5,5'-hexabromobiphenyl (Table 77: Akoso
et al., 1982a; Render et al., 1982). The effective dietary
concentrations ranged from 1 to 100 mg/kg. A much higher
concentration of 2,2',4,4',5,5'-hexabromobiphenyl (1000 mg/kg of
feed) was needed to evoke reduction in the food consumption of mice
(Table 77: Welsch & Morgan, 1985).
Rats, moribund from 3,3',4,4',5,5'-hexabromobiphenyl treatment,
had symptoms similar to those observed with FireMaster(R)
toxicosis (see section 8.2.1.1). They became less active, had a
roughened hair coat, developed sunken eyes, and were emaciated
(Render et al., 1982).
3,3',4,4',5,5'-Hexabromobiphenyl significantly decreased body
weight (Render et al., 1982) or body weight gain (Akoso et al.,
1982a) in rats, while the same concentrations of FireMaster(R)
BP-6, 2,2',4,4',5,5'-hexa- and 2,3',4,4',5,5'-hexabromobiphenyl in
the diet had no effects. When rats were injected i.p. with identical
Table 80. Order of toxicity of higher brominated PBB congeners and the FireMaster(R) mixture on the basis of comparative studies
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Dosing studies
Rat body weight gain x x 167 > FM Dannan et al.
(1978a)
Rat liver weight and x x FM > 153 Moore et al.
histopathology (1978b); Goldstein
et al. (1979)
Rat liver weight and x x FM > 180 Moore et al.
histopathology (1979a)
Rat body and organ x x FM > 153 Gupta et al.
(Mouse) weights, histopathology (1981)
Rat body and organ x x 118 > FM Dannan et al.
weights, histopathology (1982c)
Rat body and organ x x x x x 156 > 167 > Dannan et al.
weights, histopathology 138, 170 > (1982a)
101
Rat body and organ x x x 169 > 118, Parkinson et al.
weights 153 (1983)
Rat liver histopathology x x x 118, 153 > Millis et al.
101 (1985a)
Table 80 (cont'd)
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Feeding studies
Rat death, body and organ x x x 169 > FM Sleight et al.
weights, histopathology > 153 (1981)
Rat feed intake, body and x x x x 169 > FM Akoso et al.
organ weights, > 167 > 153 (1982a,b)
histopathology
Rat death, feed intake, x x x 169 > FM, Render et al.
body and organ weights, 153 (1982)
histopathology
Cockerel organ weights, x x x FM > 167 Dharma et al.
histopathology > 153 (1982)
a FM = FireMaster(R) mixture (BP-6 or FF-1); PPB numbering according to Ballschmiter & Zell (1980):
101, 118 = pentabromobiphenyls (2,2',4,5,5'-; 2,3',4,4',5-).
153, 138, 167, 156, 169 = hexabromobiphenyls (2,2',4,4',5,5'-; 2,2',3,4,4',5'-; 2,3',4,4',5,5'-;
2,3,3',4,4',5-; 3,3', 4,4', 5,5'-).
180, 170 = heptabromobiphenyls (2,2',3,4,4',5,5'; 2,2',3,3',4,4'5-).
amounts of several constituents of FireMaster(R) (BBs: 101, 138,
167, 156, 170), only 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 156) depressed body weight gain
(Dannan et al., 1978a, 1982a). The body weight gain of rats treated
with BB 167 was half that of FireMaster(R)-treated animals (Dannan
et al., 1978a). Rats given BB 118 (2,3',4,4',5-hexabromo biphenyl)
also gained less weight per day than those given FireMaster(R)
(Dannan et al., 1982c).
Liver weights in rats were increased by all treatments with
congeners of category I, except for 4,4'-dibromobiphenyl. The lowest
effective dose used was 2 mg/kg body weight (Millis et al., 1985a).
Significant effects were seen as early as 4 days after dosing (e.g.,
Parkinson et al., 1983). Many of the congeners listed under
categories II and III were also capable of increasing liver weights
(see Tables 76 and 77). Generally, all the changes in organ weights
were dose-dependent. Gradual differences between congeners were also
seen. Liver weights were significantly higher in rats given
3,3',4,4',5,5'-hexabromobiphenyl than the liver weights in
3,3',4,4'-tetrabromobiphenyl-treated rats, 6 days after a single
dose (Millis et al., 1985b). In studies comparing the effects of
five FireMaster(R) constituents (BB 101: 2,2',4,5,5'-penta; BB
138: 2,2',3,4,4',5'-hexa; BB 167: 2,3',4,4',5,5'-hexa; BB 156:
2,3,3',4,4',5-hexa; BB 170: 2,2',3,3',4,4',5-hepta), 7 days after
dosing, 2,3,3',4,4',5-hexabromobiphenyl (BB 156) caused the largest
increase in liver weights (60% increase) while 2,2',4,5,5'-
pentabromobiphenyl (BB 101) failed to enlarge this organ. Increases
caused by FireMaster(R) and BB 167 (2,3',4,4',5,5'-hexa) were
similar (Dannan et al., 1978a, 1982a). The increases in liver
weights due to BB 153 (2,2',4,4',5,5'-hexa), the main component of
FireMaster(R), were far less than the increase in response to
FireMaster(R), approximately 33% versus 60-80% (Moore et al.,
1978b; Goldstein et al., 1979).
Nevertheless, the increase was rapid with a 25% increase within
two days of treatment (Moore et al., 1978b). The effect of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), the second most
abundant component of FireMaster(R), was also less than that
caused by the mixture itself (Moore et al., 1979a). When
FireMaster(R) BP-6, 2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), and
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) were added to the diet of
rats for 30 days, BB 153 and BB 169 significantly increased liver
weights at 1 mg/kg, but FireMaster(R) and BB 167 did not. At 100
mg/kg, FireMaster(R) increased liver weight more than BB 153 or BB
167 (Akoso et al., 1982a).
Thymus weights in rodents were decreased by most of the
treatments with congeners of category I. Among congeners in category
II, 2,2',4,4',5-pentabromobiphenyl (BB 118),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), and
2,3,3',4,4',5-hexabromobiphenyl (BB 156) were capable of reducing
thymus weights. Congeners listed under the third category failed to
reduce thymus weights (see Tables 76 and 77). Both 3,3',4,4'-tetra
and 3,3',4,4',5,5'-hexabromobiphenyl caused a significant reduction
in the thymus weights of rats, 3,3',4,4',5,5'-hexabromobiphenyl
being more effective than 3,3',4,4'-tetrabromobiphenyl (Millis
et al., 1985b). There was a 50-60% loss in thymic weight in rats
injected i.p. with BB 167 or BB 156 and sacrificed 7 days later
(Dannan et al., 1978a, 1982a). However, thymus weight: body weight
ratios were not significantly affected when rats were fed BB 167 or
FireMaster(R) BP-6 for 30 days (Akoso et al., 1982a). In the same
study, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) significantly
decreased the ratio, while there was an increase with
2,2',4,4',5,5'-hexabromobiphenyl (BB 153).
In cockerels fed FireMaster(R) FF-1, BB 153, or BB 167, for
28 days, only FireMaster(R) FF-1 reduced relative bursal weights
(Dharma et al., 1982).
When young rats were fed diets containing FireMaster(R) BP-6,
2,2',4,4',5,5'-hexa-, 2,3',4,4',5,5'-hexa-, or 3,3',4,4',5,5'-
hexabromobiphenyl, for 30 days, thyroid weight was increased only by
100 mg FireMaster(R) BP-6/kg feed and by 1 and 10 mg
3,3'4,4',5,5'-hexabromobiphenyl/kg feed (Akoso et al., 1982b).
8.2.2.2 Haematology and clinical chemistry
Haematological and clinical chemistry findings were of minor
importance in comparative studies. Gamma-Glutamyl transpeptidase
(gamma-GTP) was elevated in female rats at high doses of both
FireMaster(R) FF-1 (30 mg/kg body weight per day) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) (16.8 mg/kg body weight
per day) 30 and 60 days after the last dose (Gupta et al., 1981).
8.2.2.3 Morphological and histopathological changes
Rats fed diets containing 100 mg FireMaster(R) BP-6/kg or 10
or 100 mg 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)/kg had friable
yellow livers (Render et al., 1982). The architectural structure of
the lobules was abnormal after feeding 3,3',4,4',5,5'-
hexabromobiphenyl (Akoso et al., 1982a; Millis et al., 1985b), and
bile duct hyperplasia was observed (Render et al., 1982).
With the exception of the lower brominated congeners of
category III, all PBB congeners caused histopathological changes in
the liver (see Tables 78 and 79). The extent of the changes depended
on the dose and the individual congener. The least severe effects
were confined to a slight proliferation of hepatic SER (e.g.,
4,4'-dibromobiphenyl: Ecobichon et al., 1977). More progressive,
general changes were enlargement of hepatocytes and increased
numbers of cytoplasmic lipid vacuoles. Corresponding ultrastructural
lesions consisted mainly of increased SER and lipid vacuolation (see
Tables 78 and 79). Additional changes seen with the more toxic
congeners included myelin body formation (membrane whorls) (BBs:
118, 167, 156, 169), disorganization of RER (BB 169), an increase in
number of binucleated hepatocytes (BB 169), pycnotic nuclei (BB
169), and, occasionally, multifocal areas of necrosis (BB 169)
(Akoso et al., 1980, 1982a; Sleight et al., 1981; Dannan et al.,
1982a,c; Render et al., 1982; Millis et al., 1985b).
The thymus was affected by 3,3',4,4'-tetrabromobiphenyl (Andres
et al., 1983; Millis et al., 1985b), possibly by BB 167 (Dannan
et al., 1978a: "several" tissues: not accurately specified), by BB
156 (Dannan et al., 1982a) and by BB 169 (Sleight et al., 1981;
Akoso et al., 1982a; Render et al., 1982; Millis et al., 1985b).
There was a loss of thymocytes, especially in the cortex, the
demarcation between cortex and medulla was indistinct, and
macrophages were prominent in the remaining portion of the cortex.
One study reported histological alterations in the thyroid of
rats treated with 2,2',4,4',5,5'-hexa- (BB 153), 2,3',4,4',5,5'-hexa
(BB 167), or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169), and in the
pituitary gland with BB 169 (Akoso et al., 1982b). Prominent lesions
of the thyroid were extensive hyperplasia and hypertrophy of
follicular cells and a lack of colloid. The pituitary gland showed
swollen and vacuolated chromophobe cells.
The spleen and lymph nodes of rats given 100 mg 3,3',4,4',5,5'-
hexabromobiphenyl/kg feed for 10 and 20 days had an increased number
of macrophages intermixed with mature lymphocytes. Changes were
similar to those seen in the thymus, but were not as pronounced
(Render et al., 1982).
The main component of FireMaster(R),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), is the congener most
frequently examined and implicated in many comparative studies.
The principal changes seen with BB 153 were vacuolation and
enlargement of hepatocytes with proliferation of SER. They occurred
in dosing studies (Moore et al., 1978b; Ecobichon et al., 1979;
Goldstein et al., 1979; Gupta et al., 1981; Millis et al., 1985a) as
well as in feeding studies (Sleight et al., 1981; Akoso et al.,
1982a; Dharma et al., 1982), and were observed as early as two days
after treatment (Moore et al., 1978b). Generally, the
ultrastructural changes in BB 153-exposed rats were less severe than
those in rats exposed to the FireMaster(R) mixture (e.g.,
Goldstein et al., 1979; Gupta et al., 1981; Akoso et al., 1982a).
Myelin figures and marked disorganization of RER, as seen with the
FireMaster(R) mixture, were not observed with BB 153 in
comparative studies (Gupta et al., 1981; Akoso et al., 1982a).
Moreover, changes caused by FireMaster(R) FF-1 in the livers of
rats persisted, while the livers of rats dosed with BB 153 were
comparable to those of the controls, 60 days after treatment (Gupta
et al., 1981).
The histological appearance of thymuses in rats was not
affected by BB 153 (Tables 78 and 79), but, in cockerels, the
lymphoid cells of the bursa of Fabricius were depleted by 62 mg BB
153/kg feed, by 10 mg FireMaster(R) FF-1/kg feed, and by 10 mg BB
167/kg feed (Dharma et al., 1982). When BB 153, the FireMaster(R)
mixture, BB 167, and BB 169, were fed to rats, proliferation of SER,
decreased RER, and increased fat droplets were seen in hepatocytes,
with all chemicals, but, with BB 169
(3,3',4,4',5,5'-hexabromobiphenyl), proliferation of SER was not as
prominent as with the other three PBBs. In contrast, the RER was
severely altered by BB 169 (Akoso et al., 1982a).
Myelin bodies were observed in rats fed 100 mg BB 169/kg for 20
days (Render et al., 1982) or FireMaster(R) BP-6 for 30 days
(Akoso et al., 1982a), or BB 167 for 60 days (Akoso et al., 1980). A
comparative experimental series testing single doses of six
FireMaster(R) constituents, namely BB 118 (Dannan et al., 1982c;
Millis et al., 1985a), BBs 101, 138, 167, 156, and 170 (Dannan
et al., 1978a, 1982a) found the least severe histological changes
with BB 101, and intermediate effects, which were limited to the
proliferation of SER and cytoplasmic vacuolation in hepatocytes,
with BB 138 and BB 170. The most pronounced changes resulted from
BBs 118, 167, and 156 and consisted of proliferation of SER,
increases in fat vacuoles and myelin figures in hepatocytes (BBs
118, 167, 156) and thymus damage (BB 156). Rats given a single
equimolar dose of 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or
3,3',4,4'-tetrabromobiphenyl showed moderate to severe hepatic
changes 14 days after treatment with BB 169, while the
tetrabromobiphenyl-treated rats showed only mild hepatic changes
(Millis et al., 1985b).
The microscopic hepatic effects of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180) and of
2,2',3,3',4,4',5,5'-octabromobiphenyl (BB 194) were reported to be
similar to those of BB 153 (Besaw et al., 1978; Moore et al., 1979a,
1980).
8.3 Skin and eye irritation, sensitization, dermal lesions,
and acne
Common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions due to the technical
PBB mixtures tested, namely octabromobiphenyl and decabromobiphenyl
(Table 81).
Table 81. Skin and eye irritation or sensitization tests of commercial PBB mixtures
PBBa Species Application Test Observations References
OcBB guinea-pig 50% (w/v) slurry in irritation (intact mild irritation Waritz et al.
(Hartley) propylene glycol shaved dorsal skin) (1977)
(0.05 ml)
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 9 x 50% slurry abraded shaved skin
(topical application)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 4 x 1% (w/v) solution
in dimethyl sulfoxide
(intradermal injection)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB rabbit irritation Norris et al.
(New Zealand) (1973)
dry solid (single and intact shaved skin no response
multiple exposures) abraded shaved skin slight erythematous
and edematous response
Table 81 (contd).
PBBa Species Application Test Observations References
OcBB rabbit moistened with water
(New Zealand) (single exposure) intact shaved skin no response
(repeated exposure) intact shaved skin slight erythematous
response
OcBB rabbit moistened with water abraded shaved skin moderate erythematous
(New Zealand) (single and repeated and slight oedematous
exposures) response
rabbit dry solid eye irritation transient irritation
(New Zealand) of the conjunctival
membranes
OcBB rabbit powder (100 mg) eye irritation no irritating or corneal Waritz et al.
effects; mild (1977)
conjunctival redness and
swelling and a copious
discharge (disappeared
within 4 h)
DeBB rabbit 50% in olive oil irritation mild irritation Millischer et al.
(intact shaved skin) (1979)
DeBB rabbit 50% in olive oil eye irritation no irritating effect
DeBB rabbit powder eye irritation mild irritating
a Commercial PBB mixtures: OcBB = Octabromobiphenyl; DeBB = Decabromobiphenyl.
However, diverse lesions in the skin and skin appendages of
certain animal species, e.g., rhesus monkeys and cattle, occurred
after the ingestion of the FireMaster(R) mixture (Table 82). The
main features were dry scaly skin and hair loss. Hyperkeratosis of
the interfollicular epidermis and of the hair follicle, and atrophy
and squamous metaplasia of the sebaceous glands were observed on
microscopic examination of these lesions. As with related compounds,
comparable epidermal changes have not generally been found in other
laboratory animals, such as guinea-pigs or rats, but they were
similar to those observed in humans following PBB exposure
(section 9) and were described as chloracne (McConnell, 1980;
Kimbrough, 1980b; Poland & Knutson, 1982).
The rabbit ear (inner surface), but not any other part of the
rabbit skin (Crow, 1983), is particularly sensitive to acne-causing
compounds, which was first recognized by Adams et al. (1941). The
reaction is hyperkeratosis. Painting the rabbit ear has become a
standard bioassay to detect hyperkeratotic (acnegenic) activity.
Results of rabbit ear tests obtained with diverse PBBs (technical
octabromobiphenyl, technical decabromobiphenyl FireMaster(R)
mixture, fractions of this mixture, and purified PBB congeners) have
been summarized in Table 83. The FireMaster(R) mixture itself
produced hyperkeratosis, but its main components, BB 153
(2,2',4,4',5,5'-hexa) and BB 180 (2,2',3,4,4',5,5'-hepta), did not.
Fractionation of FireMaster(R) indicated that most activity was
associated with the more polar fraction containing minor compo nents
(Needham et al., 1982). Sunlight irradiation of BB 153 also yielded
products that caused severe hyperkeratosis. It is not clear whether
one or more of the suspected PBBs is responsible for hyperkeratotic
activity (Patterson et al., 1981). The model congener
3,3',4,4',5,5'-hexabromobiphenyl and 3,3'4,4'-tetra bromobiphenyl
were shown to be hyperkeratotic (Table 83).
8.4 Long-term toxicity
Toxic effects of PBBs, observed after long-term exposures, as
well as a long time after exposure had ceased, are summarized in
Tables 84 and 85. Experimental animals tested were rats, mice,
cattle, minks, and rhesus monkeys. The majority of studies refer to
the commercial FireMaster(R) mixture.
The following comments refer mainly to the general signs of
long-term toxicity. Other long-term effects will be reviewed in
detail in the respective sections, e.g., carcinogenicity (section
8.7), reproductive dysfunctions (section 8.5).
Table 82. Dermal lesions observed in cattle, rhesus monkeys, and rabbits after exposure to PBBs
PBBa Species Route Dose (duration) Dermal lesions observed References
FM BP-6 cattle oral 25 g/day (for subcutaneous emphysema and haemorrhage; changes Moorhead et al.
33-60 days) in the eyelids: hyperkeratosis, with accumulations of (1977, 1978)
keratin in hair follicles of the epidermis and squamous
metaplasia with keratin cysts in the tarsal glands
FM FF-1 calf oral 100 mg/kg body keratitis, alopecia, hyperkeratosis involving the Robl et al.
weight (up to head, cervical and dorsal thoracic region (1978)
12 weeks)
oral 0.1, 10, 100 acanthosis, hyperkeratosis and/or dermal infiltrates
mg/kg body of mononuclear cells (dose-dependent severity)
weight (up to
12 weeks)
FM FF-1 rhesus in diet 25 mg/kg feed alopecia, dry scaly skin, loss of eyelashes; Allen et al.
monkey (for 25 weeks) generalized subcutaneous oedema, marked oedema of (1978)
adult total dose: the eyelids; keratinization of hair follicles
(male) approx. 1 g and sebaceous glands
juvenile in diet 25 mg/kg feed moderate loss of hair including eyelashes, dry
(female) (for 50 weeks) scaly skin; periorbital oedema
total dose:
approx. 1.5 g
juvenile in diet 300 mg/kg feed a rather generalized loss of hair, absent eyelashes; Allen et al.
(female) (for 137 days) considerable periorbital congestion and oedema; (1978)
total dose: keratinization of hair follicles
approx. 6.4 g
Table 82 (contd).
PBBa Species Route Dose (duration) Dermal lesions observed References
FM FF-1 rhesus in diet 1.5 mg/kg feed periorbital oedema Allen &
monkey (for over 5 Lambrecht (1978)
months)
total dose:
approx. 75 mg
OcBB rabbit dermal not specified erythema, exfoliation in the ear Norris et al.
(ear) (1 month) (1973)
Various rabbit dermal variable hyperkeratosis in the ear see Table 83
PBBs (ear)
FM = FireMaster(R); OcBB = Technical octabromobiphenyl.
Table 83. Hyperkeratotic activity of commercial PBB mixtures, the fractionateda FireMaster(R) mixture
and purified PBB congeners, derived from the rabbit ear test
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
DeBB (Adine 0102) 200, 2000 (acetone) x Atochem (1990)
OcBB not specified x Norris et al.
(chloroform) (1973)
FM FF-1 (lot FH 7042)
mixture itself 60 (not specified) x Kimbrough
polar fraction not specified (not specified) x (+++) et al.
non-polar fraction not specified (not specified) x (+) (1977)
FM BP-6
mixture itself 6.5 µg/kg body weight x Hass et al.
(benzene-decane, 1:9) (1978)
fraction 1 (non-polar) containing PBBs 6.5 µg/kg body weight x
(benzene-decane, 1:9)
FM FF-1 (lot FH 7042)
mixture itself 100 (toluene) x (++) Needham et al.
less polar fractions 50-210 (toluene) x (1982)
more polar fraction containing minor 185 (toluene) x (+++)
components of the FM
mixture
Compound 4 predominantly BB 153 5 (toluene) x
Compound 8 predominantly BB 180 3.3 (toluene) x
Table 83 (contd).
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
2,2',4,4',5,5'-hexa- 10 (not specified) x Patterson et al.
bromobiphenyl (BB 153) (1981)
(96% pure)
sunlight degradation mixture of BB 153 10 (not specified) x (+++) Patterson et al.
products of BB 153 and other PBBs (1981)
(probably BB 101,
BB 118 and 3,3',4,4'-
tetra-bromobiphenyl)
3,3',4,4',5,5'-hexa- 0.32-9.6 (toluene) x (++) Needham et al.
bromobiphenyl (1982)
3,3',4,4'-tetra- 0.245 and 0.02 x (++)
bromobiphenyl (not specified)
a The mixture was fractioned by different methods (on Florisil: Hass et al., 1978; on alumina:
Kimbrough et al., 1977; by HPLC and GC: Needham et al., 1982).
b Total dose in mg/rabbit ear, unless otherwise specified.
c (+), (++), (+++) = severity of hyperkeratosis.
d DeBB = technical decabromobiphenyl; OcBB = technical octabromobiphenyl; FM = FireMaster(R).
BB 101 = 2,2',4,5,5'-pentabromobiphenyl; BB 118 = 2,3',4,4',5-pentabromobiphenyl;
BB 153 = 2,2',4,4',5,5'-hexabromobiphenyl; BB 180 = 2,2',3,4,4',5,5'-heptabromobiphenyl.
Table 84. Long-term effects observed after exposure to PBBs by gavage
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, single dose 1000 liver: lipid accumulation (more pronounced Kimbrough et al.
(lot FH 7042) (Sherman) female t.p.: 6,10,14 in males); uroporphyrin accumulation (1977, 1978)
(in peanut oil) (5) months (females); histopathological changes;
neo-plastic nodules; increase in relative
weight
FM FF-1 rat male, 22 doses over 30 liver: marked hepatotoxic effects; atypical Gupta &
(lot No. 1312 FT) (Fischer female 30 days; t.p.: nodules Moore (1979)
(in corn oil) 344/N) 6 months after
(9) first dose
FM FF-1 rat female single dose 1000 liver: porphyria; neoplastic nodules; foci Kimbrough et al.
(lot. 7042) (Sherman) t.p.: 23 months or altered areas; carcinoma; adenofibrosis (1981)
(in corn oil) (65)
(16) female single dose 200 liver: neoplastic nodules; altered areas;
t.p.: 22 months multinucleated cells
(30) female 12 doses over 100 liver: neoplastic nodules; foci or altered
3 weeks; t.p.: areas; carcinoma; adenofibrosis
24 months
FM FF-1 rat female 122 doses over 0.1, 1, increased white blood cell and lymphocyte Luster et al.
(lot. FF 1312 FT) (Fischer) 6 months t.p.: 3, 10 counts (0.1-10); decrease in body weight (1980)
(in corn oil) (4-10) 6 months after (3,10); decrease in thymus weight (3,10);
first dose increase in relative spleen weight (3,10);
decrease in adrenal weight (10); immune
alterations (3,10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, 22 doses over 30 decrease in body weight; increase in relative Gupta et al.
(lot 1312 FT) (Fischer female 30 days t.p.: liver weight; decrease in relative thymus (1981)
(in corn oil) 344/N) 120 days after weight (F); increase in serum protein
(3) first dose (ß-globulin fraction); porphyria (F);
pronounced hepatocellular alterations
2,2',4,4',5,5'- rat male 22 doses over 16.8 increase in relative liver weight (M);
hexabromobiphenyl (Fischer female 30 days t.p.: minimal hepatocellular changes
(99% purity 344/N) 120 days after
(in corn oil) first dose
FM FF-1 rat male single dose 500 no significant effects on body and liver Bernert, Jr
(lot 7042) (Sherman) t.p.: 18 months weight; increase in serum cholesterol and et al. (1983)
(in corn oil) total serum phospholipids; enhancement of
hepatic per-oxidation; reduced retinol
levels in serum and liver microsomes;
reduced alpha-tocopherol content in
microsomes (but not in serum)
FM FF-1 rat male, 125 doses over 0.1, 0.3 no effect on food consumption; dose-related Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, decrease in body weight gain; dose-related (1983a)
(in corn oil) 344/N) 6 months after 10 increase in the absolute and relative liver
(10) first dose weights (Female: 0.1-10; Male: 0.3-10);
decrease in thymus weight (0.3-10); increase
in spleen weight (1-10); decrease in Hb and
PCV values, in MCV and MCH values (10);
dose-related increase in serum cholesterol;
decrease in serum thyroid hormone levels;
porphyrin accumulation in liver, bone, teeth
(more pronounced in Female (1-10);
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
dose-related hepatocellular alterations;
increase in white blood cell count (Female:
1-10); increase in serum GGTP (Female: 10);
decrease in serum glucose (Female: 10);
dose-related decrease in serum protein,
primarily due to albumin (Female: 0.1-10);
carcinoma in urinary bladder (Female:10);
histopathological changes in the thyroid
glands and kidneys (Male: 10); decrease in
serum triglyceride (Male: 0.3-10)
FM FF-1 rat male, 125 doses over 0.1,0.3 dose-related body weight reductionso; Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, dose-dependent porphyrogenic effects on (1983b)
344) = up to 29 10 teeth, bones, liver (1-10); enlarged, pale,
(11-40) months after or mottled livers with necrotic foci (1-10);
first dose hepatocellular alterations (1-10);
dose-dependent incidence of liver tumours
and cholangiocarcinoma (higher doses);
dose-dependent decline in survival time
(Male: 1-10); chronic progressive nephropathy
(Male: 1-10); gastric ulcers and hyperplastic
gastropathy (Male: 3,10)
FM FF-1 mouse female 122 doses over 10 increase in body weight; increase in relative Luster et al.
(lot. FF 1312 FT) (B6C3F1) 6 months; t.p.: spleen weight; slight immune alterations (1980)
(in corn oil) (4-10) 6 months after
first dose
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse male, 22 doses over 30 no effect on food consumption; increase in Gupta et al.
(lot 1312 FT) (B6C3F1/N) female 30 days; t.p.: relative liver weight, histopathological (1981)
(in corn oil) (3) 120 days after alterations in liver
first dose
2,2',4,4',5,5'- mouse male, 22 doses over 16.8 no effect on food consumption and body weight
hexabromobiphenyl (B6C3F1/N) female 30 days; t.p.: gain; increase in relative liver weight;
(99% purity) (3) 120 days after minimal hepatocellular alterations
(in corn oil) first dose
FM FF-1 mouse male, 125 doses over 0.1, 0.3, no effect on food consumption; decrease in Gupta et al.
(lot. 1312 FT) (B6C3F1) female 6 months 1, 3, 10 body weight gain (only Male: 10); increase (1983a)
(in corn oil) (10) in body weight (only Female: 10); increase
in absolute and relative liver weight
(Female: 0.3-10; Male: 1-10); increase in
spleen weight (Female: 10); decrease in
uterine weight (Female: 10); haematological
alterations: dose-related increase in red
blood cell count; dose-related decrease in
MCV; decrease in platelet counts (Female:
3,10); leukocytosis (Female: 10); clinical
chemistry changes: increase in serum levels
of GGTP and SGPT (10) and alkaline
phosphatase (10); decrease in serum glucose
(Female: 10); dose-related hepatic porphyria
(more pronounced in Female) pale and
mottled livers (higher doses); dose-related
microscopic changes in the liver (1-10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse 125 doses over 0.1, 0.3, shortened survival time (Male: 10); hepatic Gupta et al.
lot. 1312 FT) (B6C3F1) 6 months; t.p.: 1 ,3, 10 porphyria (Male: 3); slight reduction in body (1983b)
(in corn oil) (8-27) up to 30 weight (Male: 10); enlarged liver; fluid
months after accumulation in peritoneal cavity; liver
first dose carcinoma (10); metastasis to lung (Female:
10); hyperplasia and adenoma of follicular
cells of thyroid
FM BP-6 cattle daily doses 250 no adverse clinicopathological chanages Durst et al.
(in gelatin (6) for 60 days mg/day (1978a)
capsule) t.p.: 80-190
days after
first dose
FM BP-6 cattle daily doses 250 no gross or histopathological signs of Moorhead et al.
(in gelatin (No. not for 60 days mg/day toxicosis (1978)
capsule) specified) t.p.: 220
days after
last dose
FM FF-1 cattle female daily doses 0.3 no effects on milk production, body weights, Robl et al.
(in gelatin (6) for 158 days amount of food consumed; no effects on (1978)
capsule) t.p.: 182 haematological and clinical chemistry and
days after urinalysis values;
last dose (1 cow: overgrowth of hooves from day 129)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM BP-6 cattle (Female) daily doses 250 no effect on milk production, body weight, Durst et al.
(in gelatine (1-4) for 60, 180 mg/day number of infections or general injuries; (1978b)
capsule) 202 days increased frequencies of reproductive Willett et al.
t.p.: up to dysfunctions (1980)
1500 days
after first
dose
a FM = Fire Master.(R)
b No. = number of animals.
c t.p. = time post-exposure.
d In mg/kg body weight per day, unless otherwise specified.
e Hb = Haemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular haemoglobin.
Table 85. Long-term effects of feeding PBBs to young or adult animals
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
"Hexabromobiphenyl" rat female 50 7 months increase in relative weights of liver, Sepkovic &
(Monsanto Co., (Sprague- ovary, and thyroid Byrne (1984)
St. Louis) Dawley) (8)
FM BP-6 rat female 1, 10, 50 5-7 months no effect on food consumption, body Byrne et al.
(Sprague- weight, and relative thyroid weight; (1987)
Dawley) (10) slightly elevated liver weight (10, 50);
primary hypothyroidism
FM BP-6 rat female 1, 5, 10, 5 or more no effect on food consumption and relative Byrne et al.
(Sprague- 50 months liver weight; decrease in relative adrenal (1988)
Dawley) (10) weight; depression of circulating levels
of adrenal cortex hormones
OcBB rat male, 0.01-1 180 days no effect on food consumption and body Norris et al.
(Dow Chemical) (Sprague- female weight (1973)
Dawley) (not
specified)
OcBB rat male 100 4/22 weeks return to normal liver weights Lee et al.
(Dow Chemical) (Sprague- (1975a);
Dawley) (8) 1000 4/22 weeks increased liver weights; Waritz et al.
hepatocellular alterations (1977)
OcBB rat female 50 7 months increase in relative liver weight Sepkovic &
(Monsanto Co., (Sprague- Byrne (1984)
St. Louis) Dawley) (8)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
NoBB mouse male, 100, 300 18 months decrease in body weight (Male, Female); Momma (1986)
(Bromkal 80-9D) female shortened survival time (300 mg/kg; Male);
enlargement of thyroid glands (300 mg/kg;
Female); decrease in triglyceride and
non-esterified fatty acids; reduction of
pentobarbital sleeping time (13 months);
liver carcinoma (Male, Female)
FM FF-1 mink (9) male, 6.25 210 days deaths; decrease in body weight; increase Aulerich &
female (mean) in relative liver and kidney weights; Ringer (1979);
liver histopathology Ringer et al.
(1981)
mink (8) female 1-2.5 9 months 10% reduction in body weight (2.5); Aulerich &
adverse effects on reproduction Ringer (1979);
Ringer et al.
(1981)
FM FF-1 rhesus female 0.3 7 months- weight loss; sterile abscesses (2/7); Allen et al.
monkey (total dose: > 1 year reproductive dysfunctions (1978); Allen
(7) > 25 mg) & Lambrecht
(1978);
Lambrecht
et al. (1978)
rhesus ? 1.5 > 5 months weight loss; periorbital oedema; Allen &
monkey (total dose: immunological alterations Lambrecht
(not approximately (1978)
specified) 75 mg)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
FM FF-1 rhesus male 25 25 weeks death; weight loss; haematological and Allen et al.
monkey (total dose: clinical chemistry changes: decrease in (1978)
(adult) approximately PCV, cholesterol, total serum protein and
(1) 1 g) albumin; increase in serum GPT activity;
epidermal changes:
alopecia, dry scaly skin, loss of eyelashs;
keratinization of hair follicles and
sebaceous glands;
oedema:
generalized subcutaneous oedema and
marked oedema of eyelids; enlarged heart
and liver; hyperplastic gastroenteritis;
severe ulcerative colitis; hypoactive
seminiferous tubules; hyperplasia of bile
duct epithelium
FM FF-1 rhesus female 25 50 weeks no weight gain: loss of hair (including Allen et al.
monkey (total dose: eyelashes); dry scaly skin; periorbital (1978)
(juvenile) approximately oedema; increase in serum GPT activity
(1) 1.5 g)
FM FF-1 rhesus female 300 137 days death; weight loss; haematological and
monkey (total dose: clinical chemistry changes: decrease in
(juvenile) approximately PCV, white blood cell count, red blood cell
6.4 g) count, serum cholesterol, serum protein;
increase in serum GPT activity
epidermal changes:
loss of hair (including eyelashes); atrophy
and squamous metaplasia of the sebaceous
glands; keratinization of hair follicles;
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
periorbital congestion and oedema;
subcutaneous oedema; enlarged
hepatocytes; hyperplastic gastroenteritis;
hyperplasia of the epithelium of the
bladder and of the bile ducts; focal
areas of haemorrhage in the adrenal glands
FM FF-1 rhesus female 1.5 36 weeks weight loss; decrease in serum Lambrecht et al.
monkey (total dose: cholesterol enlarged hepatocytes with (1978)
(adult) 70 mg) moderate fatty infiltration (as indicated
(3) by liver biopsy)
rhesus male, 25 14 weeks weight loss (Male, adult) or lack of
monkey female (total dose: weight gain (Female, juvenile); decrease
(adult approximately in serum cholesterol; hyperplastic
juvenile) 500 mg) gastritis
(2)
a FM = FireMaster; OcBB = technical octabromobiphenyl; NoBB = nonabromobiphenyl.
b No. = number of animals.
c PCV = packed cell volumes.
8.4.1 Rat
8.4.1.1 Overt clinical signs, body weight changes, food intake
In rats, the low-dose, long-term feeding of FireMaster(R)
(Byrne et al., 1987, 1988) or technical octabromobiphenyl (Norris
et al., 1973) had no effect on food consumption and body weight
(Table 85). Nevertheless, the fur of PBB-fed animals was slightly
less dense and slightly coarser than that of control animals (Byrne
et al., 1987). A dose-dependent decline in survival time (Gupta
et al., 1983b), and body weight reductions or depressed rates of
body weight gain as a function of time and dose (Luster et al.,
1980; Gupta et al., 1981, 1983a,b) have been found in rats orally
dosed with FireMaster(R) (Table 84); also there was no significant
difference in food consumption between treated and control animals
(Gupta et al., 1981, 1983a).
8.4.1.2 Haematology and clinical chemistry
Several haematological and clinical chemistry parameters were
altered, some in only one sex (Table 84: Kimbrough et al., 1977;
Luster et al., 1980; Bernert et al., 1983; Gupta et al., 1983a).
Rats of both sexes had decreased haemoglobin and packed cell volume
values (Gupta et al., 1983a), a dose-related increase in serum
cholesterol (Bernert et al., 1983; Gupta et al., 1983a), and a
dose-related hepatic porphyria (Gupta et al., 1983a).
8.4.1.3 Morphological changes
Most of the livers of treated rats from higher dose groups were
pale or slightly yellow and mottled (Gupta et al., 1983a) and
contained white necrotic foci (Gupta et al., 1983b). Porphyrin
accumulation in the liver, bone, and teeth, seen as red fluorescence
under UVR, was more pronounced in treated females compared with
treated males (Kimbrough et al., 1977; Gupta et al., 1983a,b). After
withdrawal of treatment, visual fluorescence declined slightly in
the lower dose groups (1 and 3 mg FM/kg body weight), but remained
the same in the 10 mg FM/kg body weight dose groups (males and
females) during lifetime observation (Gupta et al., 1983a,b).
In long-term studies, an increase in the relative liver weights
of rats exposed to FireMaster(R) or a related mixture was usually
found (Kimbrough et al., 1978; Gupta et al., 1981, 1983a; Sepkovic &
Byrne, 1984; Byrne et al., 1987), but in some instances (Bernert et
al., 1983; Byrne et al., 1988), there was no effect on liver weight
(see Tables 84 and 85). [During a six-month exposure to
FireMaster(R), the increases in absolute and relative liver
weights were dose-related (Gupta et al., 1983a)].
Long-term feeding of commercial octabromobiphenyl (OcBB) also
caused an increase in relative liver weights (Sepkovic & Byrne,
1984; Table 85). Rats fed OcBB for 4 weeks still had increased liver
weights at 18 weeks of recovery or had returned to near normal
limits, depending on the concentration of OcBB in the test diet (Lee
et al, 1975a; Table 85).
The only individual PBB congener tested over the long-term,
namely 2,2',4,4',5,5'-hexabromobiphenyl, caused an increase in the
relative liver weight in male rats only (Gupta et al., 1981; Table
84).
Weight changes following PBB exposure, noted in organs other
than the liver, included a decrease in the thymus (Luster et al.,
1980; Gupta et al., 1981, 1983a), an increase in the spleen (Luster
et al, 1980; Gupta et al., 1983a), a decrease in the adrenal glands
(Luster et al., 1980; Byrne et al., 1988), and an increase in the
thyroid and ovary (Sepkovic & Byrne, 1984) (see Tables 84 and 85).
8.4.1.4 Histopathological changes
Hepatocellular alterations (exclusive of hepatocellular
carcinomas), observed in long-term studies with FireMaster(R) (see
Table 84), consisted mainly of enlarged hepatocytes, fatty
infiltration, proliferation and disorganization of RER, single cell
necrosis, multinucleated cells, microabscesses, atypical foci, and
bile duct proliferation (Kimbrough et al., 1977, 1978, 1981; Gupta
et al., 1981, 1983a,b).
In contrast to FireMaster(R)-treated rats, rats given
2,2',4,4',5,5'- hexabromobiphenyl over 30 days tended to recover
when examined during postexposure periods (Gupta et al., 1981).
Myelin figures, induced by the feeding of technical OcBB,
persisted, together with fatty changes, as late as 18 weeks after
withdrawal of a test diet containing 1000 mg OcBB/kg; however, the
normal morphology of RER was restored independently of the
occurrence of myelin figures, after treatment was discontinued (Lee
et al., 1975a).
Although there was no significant difference in the weights of
the thyroid gland, slight to moderate microscopic changes were
observed, primarily in male rats exposed for 6 months to 10 mg
FireMaster(R)/kg body weight. The thyroid gland had thin, sparse,
or bluish colloid with basophilic stippling and some follicles were
lined with columnar epithelium and contained a few epithelial
papillary projections (Gupta et al., 1983a).
The kidneys of male rats equally treated consistently showed
atrophy of a few glomerular tufts with marked dilatation of Bowman's
capsule, which contained amorphous eosinophilic staining material. A
few glomerular tufts also appeared oedematous (Gupta et al., 1983a).
Male rats, exposed to FireMaster(R) for 6 months (dose levels:
1-10 mg/kg body weight per day) and observed for a lifetime, showed
a higher incidence of chronic progressive nephropathy than the
controls. Their kidneys were characterized by eosinophilic
proteinaceous casts, sclerosis and thickening of glomerular tufts
and Bowman's capsule, mononuclear leukocytic infiltration, and
interstitial fibrosis (Gupta et al., 1983b).
Gastric ulcers and hyperplastic gastropathy of the glandular
portion of the stomach were found with significantly increased
incidence in male rats held under the same conditions (dose levels:
3 or 10 mg FM/kg body weight per day). Microscopic lesions consisted
of hyperplasia of the mucosal epithelium, glandular metaplasia to
goblet cells, hyperchromasia, and increased mitosis (Gupta et al.,
1983b).
8.4.2 Mouse
As far as it has been tested (Table 84), FireMaster(R) has
not produced any significant effects on food consumption in mice of
both sexes (Gupta et al., 1981, 1983a). Interestingly, there were
increases in the body weights of female mice with long-term exposure
to FireMaster(R), while a decrease occurred only in males at the
high dose (Luster et al., 1980; Gupta et al., 1983a,b). The high
dose of FireMaster(R) also shortened the survival time of males
(Gupta et al., 1983b). There were no significant differences in food
consumption and body weight gain in mice treated with
2,2',4,4',5,5'-hexabromobiphenyl (Gupta et al., 1982).
A number of haematological and clinical chemistry changes have
been found in mice exposed for 6 months to FireMaster(R) (Table
84: Gupta et al., 1983a). Hepatic porphyrin levels were increased in
a dose-related manner after a six-month exposure (Gupta et al.,
1983a), but (unlike levels in rats) they tended to decrease
following cessation of exposure (Gupta et al., 1983b).
Organ weight changes noted in long-term studies on mice (Table
84) consisted of an increase in liver (Gupta et al., 1981, 1983a,b)
and spleen (Luster et al., 1980; Gupta et al., 1983a) weights and a
decrease in uterine weights (Gupta et al., 1983a). Most of the
affected mice observed for a lifetime contained serosanguineous
fluid in the peritoneal cavity (Gupta et al., 1983b). The livers of
mice with long-term exposure were pale and contained, primarily in
males, grayish white foci (Gupta et al., 1981, 1983a).
Microscopic alterations were marked swelling of hepatocytes,
foamy or vacuolated cytoplasm with hyaline bodies, focal coagulative
necrosis or scattered single cell necrosis of hepatocytes, and
atypical hepatocellular foci (Gupta et al., 1983a). Hyperplasia was
observed in the follicular cells of thyroid (Gupta et al., 1983b).
Adverse effects (besides liver carcinoma; see section 8.7),
observed after an 18-month exposure of mice to technical
nonabromobiphenyl (Bromkal 80-9D), included decreases in body
weight, enlargement of the thyroid gland, and biochemical
alterations (Momma, 1986; Table 85).
8.4.3 Cattle
Except for a single animal, cattle with long-term exposure to
low doses of FireMaster(R) FF-1 or BP-6, observed for up to 220
days post-treatment (see Table 84) did not show any adverse effects
with regard to food intake, clinical signs, clinicopatho logical
changes, or performance (Durst et al., 1978a,b; Moorhead et al.,
1978; Robl et al., 1978). However, reproductive dysfunction occurred
more frequently (Willett et al., 1980; see also section 8.5).
8.4.4 Mink
Minks were found to be very susceptible to PBB toxicity (Table
85: Aulerich & Ringer, 1979). When fed with FireMaster(R) for
several months, they responded with food rejection, loss of weight,
an unthrifty appearance, and death. Upon necropsy of animals that
died, increases in relative liver and kidney weights and a fatty
infiltration of the liver were evident. A relatively low daily
intake of FireMaster(R) by female mink had an adverse effect on
their reproductive performance (see also section 8.5).
8.4.5 Rhesus monkey
Rhesus monkeys are also among the species more sensitive to
FireMaster(R) (Table 85: Allen et al., 1978; Allen & Lambrecht,
1978; Lambrecht et al., 1978). At exposures of 1.5-300 mg FM/kg
feed, they developed weight loss or a lack of weight gain,
haematological and clinical chemistry changes, loss of hair, skin
lesions, oedema, enlargement of the heart and liver, areas of
haemorrhage in the adrenal glands; reduced spermatogenesis, and
immune incompetence. Microscopic changes in the liver included
enlarged hepatocytes with fatty infiltration. The most severe lesion
was hyperplastic gastroenteritis and the accompanying ulcerations.
Exposures to 25 or 300 mg FM/kg feed also caused death. The female
monkeys fed with lower doses of FireMaster(R) (0.3 mg/kg diet),
for over one year, did not develop any of the signs of intoxication
noted above, except for weight loss. However, they were affected by
reproductive dysfunctions (see also section 8.5).
8.4.6 Pre- and perinatal exposure
Long-term effects following pre- or perinatal exposure to
FireMaster(R) have been recorded in rats and cattle.
Stunted growth, increased mortality rates, and liver tumours
were observed in the offspring of Sherman rats given 200 mg
FireMaster(R) FF-1/kg body weight on days 7 and 14 of pregnancy,
when a total of 50 male and 50 female offspring per group were
followed until they were 2 years old (Groce & Kimbrough, 1984).
Sprague-Dawley rats (No. = 10), perinatally exposed (day 8 of
pregnancy-28 days post partum) to FireMaster(R) BP-6 (100 mg/kg of
feed) and weaned on to a PBB-free diet, had increased relative liver
weights at 28 and 150, but not 328 days of age. Although the liver
was not enlarged 10 months after weaning, hepatic histopathological
alterations, such as cellular swelling, vacuolation, some necrosis,
and eccentric and pyknotic nuclei were observed throughout this
residual phase. Other long-lasting alterations were stimulation of
renal and hepatic microsomal enzymes, and a reduction in the
duration of anaesthesia produced by pentobarbital (McCormack et al.,
1980). In a multigeneration study (McCormack et al., 1981), rats
perinatally exposed to FireMaster(R) BP-6, as described above
(F1-generation), were allowed to mature sexually and bred with
littermates to produce the F2-generation. Even these F2-animals
exhibited increased relative liver weights and histopathological
liver changes at 28 days of age. The light microscopic changes
included vacuolation, focal necrosis, pyknotic nuclei, swelling, and
myelin bodies. However, the severity of lesions was much less
prominent in F2-animals than in F1-animals.
PBBs given to a single generation also produced hepatic and
renal microsomal enzyme stimulation, a reduction in the duration of
anaesthesia elicited by pentobarbital or a large dose of
progesterone, and a decrease in the concentration of vitamin A in
the liver, in both subsequent generations (F1 and F2).
Cows whose dams or granddams had received daily oral doses of
FireMaster(R) BP-6 (up to 250 mg/day) for 60-202 days showed no
general health problems, but they had conception difficulties
(Willett et al., 1982).
8.5 Reproduction, embryotoxicity, and teratogenicity
Effects of PBBs on reproduction (reproductive system, overall
reproductive performance, embryotoxicity, teratogenicity) have been
summarized in Tables 86 and 87. Most of the studies refer to the
FireMaster(R) mixture. Few reports deal with technical
octabromobiphenyl (Aftosmis et al., 1972a; Waritz et al., 1977) or
technical decabromobiphenyl (Millischer et al., 1979), and, among
the individual PBB congeners, only 2,2',4,4',5,5'-hexabromobiphenyl
has been evaluated by a few authors.
8.5.1 PBB mixtures
8.5.1.1 Mammals
a) Reproductive system and performance.
As listed in Tables 74 and 75, administration of
FireMaster(R) by gavage and in the diet had no effect on the
weight of male (Garthoff et al., 1977; Corbett et al., 1978a; Harris
et al., 1978b, Gupta et al., 1981; Castracane et al., 1982) or
female (Gupta et al., 1981) sex organs in rats and/or mice. An
exception was an increase in the uterine weight of mice dosed for 6
months with FireMaster(R) (Gupta et al., 1983a). Testes weights in
rats fed commercial OcBB also remained unaffected (Norris et al.,
1973). However, there was a reduction in the ventral prostate
weight-to-body weight ratio in pubescent male rats (Johnston et al.,
1980; Table 87) and a delay in vaginal opening in female rats after
perinatal exposure to FireMaster(R) (Harris et al., 1978a;
McCormack et al., 1981; Tables 86 and 87). Both findings may suggest
retarded sexual maturation.
Male rats, moribund from multiple doses of FireMaster(R),
exhibited degenerative and hyperplastic changes in the ductus
deferens (hyperplasia and squamous metaplasia with keratinization in
the epithelial lining of the ductus deferens). However, such changes
were not observed in surviving male rats, examined six months after
the treatment. Lesions found in surviving animals were acute
prostatitis (30 mg/kg body weight per day) and the presence of sperm
granuloma in the epididymis (100 mg/kg body weight per day) (Gupta &
Moore, 1979; Table 86). A rhesus monkey, exposed to FireMaster(R)
in the diet, had hypoactive seminiferous tubules at death (Allen
et al., 1978; Table 87). Little work was done on the possible
impairment of spermatogenesis. Young bulls may develop testicular
atrophy and reduced spermatogenesis when exposed to FM (Robl et al.,
1978), but more numerous controlled studies are needed.
The estrous cycle of cows (No. = 8) was not affected during
administration of FireMaster(R) FF-1 (0.3 mg/kg body weight per
day) for 158-228 days and during a recovery period of 112-182 days
(Robl et al., 1978).
The estrous cycle of female rats was increased in length after
perinatal and subsequent dietary exposure to FireMaster(R) BP-6
(Johnston et al., 1980; Table 87) at concentrations of 100 mg/kg
(equivalent to 5 mg/kg body weight per day). Prolonged menstrual
cycles were seen in rhesus monkeys after consuming FireMaster(R)
FF-1 for six months at a concentration of 0.3 mg/kg (equivalent to
0.02 mg/kg body weight per day). This alteration was correlated with
decreased serum progesterone levels (Allen et al., 1978; Lambrecht
et al., 1978; Table 87).
Table 86. Summary of effects of PBBs on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): dosing studies
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral single 400, 800 reductions in fetal and placental Beaudoin
(Wistar) dose one of weight; fetal death (primarily (1977)
(3-15) gd 6-14 800 mg/kg body weight per day at
s.t.: gd 20 day 7-12); malformed (cleft
palate; diaphragmatic hernia)
fetuses (400 mg/kg body weight
per day: 0-11.8%; 800 mg/kg body
weight per day: 0-60%)
FM FF-1 rat (No. oral multiple 100 (total none no effects on number of Ficsor &
not doses (6) gd dose: 600 fetuses, dead implants, Wertz (1976);
specified 6-16 at 2 day mg/kg body fetal malformation Wertz &
(15) intervals weight Ficsor (1978)
s.t.: gd 19
FM BP-6 rat oral multiple 0.25-10 reduced fetal weight and no effects on implantation Harris
(Sprague- doses gd 7-15 mg/day crown-rump length (only number of live fetuses or et al. (1978a)
Dawley) s.t.: gd 20 0.25 mg/day) gross malformations
(6-8)
s.t.: 48, 60 10 mg/day reductions in offspring weight no effects on litter size Harris
days of age (from 3 days of age onwards, and birth weight et al. (1978a)
more pronounced in male pups);
increased postnatal mortality (at
weaning 14.3% versus 1.5% in
control); delay in vaginal opening
of female offspring
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral multiple 8-160 mg reduced implantation rates no malformed fetuses Beaudoin
(Wistar) doses gd 0-14 per animal (80-160 mg); fetal death (1979)
(No. not on alternate (total
specified) days dose)
FM FF-1 rat oral multiple 100 moribund rats: degenerative and Gupta &
(Fischer doses (22) hyperplastic changes in the ducts Moore (1979)
344/N) over 30 days deferens (73 days after
(9 males) s.t.: up to 6 treatment); surviving rats: sperm
months granuloma in the epididymis (6
months after treatment; 1/2e)
30 acute prostatitis (4/9)e
FM FF-1 rat oral multiple 200 reduction in offspring survival Groce &
(Sherman) doses (2) gd 7 rate-to-weaning Kimbrough
(16) and 14 o.t.: (1984)
up to Weaning
(21 days of age)
FM BP-6 rat 1) in vivo- 800 inhibited rate of embryo Fisher (1980);
(Wistar) treatment: oral development in vitro (effects on Beaudoin &
(7) single dose gd 9 axial rotation, heart rate, neural Fisher (1981)
s.t.: gd 10 tube closure, formation of the
2) whole embryo anterior limb buds, somite
culture: 24 h development, and establishment of
visceral yolk sac circulation);
reduction in DNA content;
pericardial oedema
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
DeBB rat oral multiple 10-1000 none no teratogenicity or Millischer
(Sprague- doses gd 6-15 embryotoxicity et al. (1979)
Dawley) s.t.: gd 21
(25)
2,2',4,4' mouse oral multiple 0.3-32 none no fetotoxicity or Lucier et al.
5,5'-hexa- (C.D. - 1) doses gd 10-16 teratogenicity; no effects (1978)
bromobiphenyl (up to 25) s.t.: gd 19 on fertility, gestation,
(purity o.t.: 4, 20 viability, survival, and
> 97% days of age lactation indices
FM BP-6 cow (6) oral multiple 25 g/day abortion (3/6)e or fetal Durst et al.
doses during death (3/6)e (1977, 1978b);
pregnancy for Moorhead
32-60 days et al. (1977)
o.t.: up to 60
days
BM BP-6 cow oral multiple 0.25 and none Durst et al.
(3) doses during 250 mg/day (1978b)
pregnancy for
60 days
(1) for 180 days 250 mg/day stillbirth (due to dystocia)
o.t.: up to
305 days
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 cow (3-5) oral multiple 0.25-250 high incidence of dystocia; a total of 75 calves Willett et al.
(4 dose doses before mg/day increased birth weights of calves; were studied; no effects (1980, 1982)
groups) breeding or stillbirths; increased number of on growth, development,
during late inseminations required for and survival of calves
pregnancy for conception in 1 and 2 generation born alive
60-202 days offspring
o.t.: up to 5.5
years (1-5
parturition;
2 generations)
a FM = FireMaster(R); DeBB = technical decabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, of recorded); o.t. = observation time; s.t. = sacrifice time.
d In mg/kg body weight per day, unless otherwise specified.
e No. affected/no. treated.
Table 87. Summary of PBB effects on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): feeding studies
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 rat (Sprague- gd 7-18 100, 1000 decreasing fetal weight with suggestion of late Corbett
Dawley) s.t.: gd 20 increasing dosage fetal mortality et al.
(6-7) (high dose); no (1975)
teratogenicity
FM BP-6 rat (Sprague- gd 8-9th week 100 reduction in ventral no effect on fetal Johnston
Dawley) (at of age s.t.: prostate weight of male survival rate et al.
least 8) 9th week of age offspring; increased length (1980)
of estrous cycle of female
offspring
FM BP-6 rat (Sprague- gd 8-Weaning 100 reduction in survival no effects on length McCormack
Dawley) s.t.: Weaning rate-to-weaning (87% of of gestation, litter et al.
control value); delay in size, incidence
(8) (28 days of vaginal opening in female of gross external
age) offspring anomalies, pup body
weight at birth
OcBB rat (not gd 6-15 100, 1000, gastroschisis (one fetus abstract only (no Aftosmis
specified) s.t.: gd 20 10 000 at each level); anasarca on number of fetuses et al.
(one fetus at each of the information examined) (1972a)
two highest levels)
OcBB rat gd 6-15 100, 1000, no significant gastroschisis Waritz et al.
(ChR-CD) s.t.: gd 20 10 000 embryotoxicity or (1/259e: 1000 mg/kg: (1977)
(23-27) teratogenicity 1/1/283e
10 000 mg/kg);
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
anasarca (1/259e: 1000
mg/kg; 1/283e: 10 000
mg/kg); no effects on
growth or viability of
embryos
FM BP-6 mouse: gd 7-18 50, 100, dose-related decrease in fetal incidence of Corbett
(Swiss, ICR) s.t.: gd 18 1000 weight with increasing dosage; malformations et al.
(9-12) exencephaly (5/295g: 100, statistically (1975,
1000 mg/kg); cleft palate significant only 1978b)
(5/208g: all levelsg; compared with pooled
hydro-nephrosis (2/87: historical controls
1000 mg/kg)
FM mouse (Swiss- gd 4-16 100, 200 increase in number of dead or (abstract only) Preache
Webster) s.t.: gd 19 resorbed fetuses (200 mg/kg) et al.
(No. not (1976)
specified) gd 8-16 100, 200 decrease in fetal body weight
s.t.: gd 19 (200 mg/kg)
gd 8-16 50, 100 reduction in number of live no effects on Preache
o.t.: up to 6 offspring postnatal mortality or et al.
weeks of age body weight (1976)
days 1-29 pp 50, 100 increased mortality; decreased
o.t.: up to body weight
weaning
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
2,2',4,4'- mouse gd 6-15 100, 300, at 300 mg/kg: 17-182 fetuses were Welsch &
5,5'-hexa- (C57BL) o.t.: gd 17 500, 750, decrease in pregnancy rates of examined Morgan (1985)
bromobiphenyl (2-22) 1000 plug-positive mice;
(purity: at > 300 mg/kg:
> 99%) reduced fetal body weight;
malformed fetuses (cleft
palate combined with a "cystic
brain deviation": 5/166,
3/172, 5/46, 6/17g,
respectively, at 300-1000
mg/kg; minor abnormalities of
brain development); at >
500 mg/kg: reduced gravid
uterine weight
FM BP-6 pig (2-4) 2nd half of 10, 100, 200 increased mortality during no effect on litter Werner &
gestation and lactation size Sleight
lactation o.t.: (1981)
up to weaning
(4 weeks)
FM FF-1 mink (8 for 9 months 1 reduced litter size; reduced both male and female Aulerich &
females; (start before kit weight at birth and at 4 on PBB diet; no Ringer
2 males) breeding) weeks; increased mortality effects on breeding or (1979)
4 weeks) (birth to periods; no gestation
teratogenicity
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 rhesus for over 1 year 0.3 prolonged menstrual cycles; Allen et al.
monkey (start 7 months decreased serum progesterone (1978,
(7) prior to breeding) levels (4/7e after 6 months); 1979); Allen
excessive and prolonged & Lambrecht
implantation bleeding (2/7e); (1978);
abortion (1/7); stillbirth Lambrecht
(1/7e); reduced weight of et al.
infants at birth and at (1978)
weaning (4 months of age)
FM FF-1 rhesus for 25 weeks 25 hypoactive seminiferous tubules Allen et al.
monkey (at the time of death (1978)
(1 male)
FM BP-6 chicken (White for 9 weeks 20 none no effect on Cecil et al.
Leghorn) (35) hatchability (1974)
"PBB" chicken for 8 weeks 5, 10, 20 none no effects on egg Lillie et al.
(White production, egg (1975)
Leghorn) (20) weight, egg shell
thickness, and
fertility; no effects
on hatchability,
embryonal development
and progeny health
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 chicken for 8 weeks 20 increased mortality of progeny no effects on egg Cecil &
(White production, egg Bitman
Leghorn) (10) weight, egg shell (1978)
thickness, fertility,
hatchability, and
embryonic death
for 4-8 weeks 64, 200, reduced egg production or stop
640, 2000 in egg production at 200-2000
mg/kg; decreased hatchability
of fertile eggs; increased
mortality of progeny; reduced
growth rate of progeny;
embryonic deaths; embryonic
abnormalities (subcutaneous
oedema, oedematous cysts,
unabsorbed yolk)
FM FF-1 chicken for 5 weeks 0.2-3125 decline in egg production Polin &
(White (MELf: 30-45 mg/kg); loss of Ringer
Leghorn) (24) egg production (> 625 mg/kg, (1978a,b)
within 2 weeks);
reduced hatchability (MELf:
30-45 mg/kg); dose-related
increase in offspring
mortality (MELf: 30-45 mg/kg;
embryonic oedema
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 chicken (10) for 21 days 80 decline in hatchability; oedema no effect on fertility Polin et al.
of embryos and newly hatched (1979)
chicks
2,2',4,4', chicken (10) for 21 days 52 none no decline in
5,5'-hexa- hatchability; no
bromobiphenyl oedema of chicks and
(purity: and embryos
not
specified)
FM Japanese for 9 weeks 10, 20, 100 at 100 mg/kg: no eggshell thinning Babish
quail (No. reduced egg production (17% et al.
not specified) versus 68% in controls); no (1975a)
hatchability; embryonal deaths
FM FF-1 Japanese for 5 weeks 80 reduced egg production fertility and Polin et al.
quail hatchability not (1982);
(Coturnix significantly Bursian
coturnix different from et al.
japonica) controls (1983)
(36 males,
36 females)
a FM = FireMaster; OcBB = technical octabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, if recorded; exception: Waritz et al. (1977): sperm day = gd 1); pp = postpartum;
o.t. = observed time; s.t. = sacrifice time.
d In mg/kg of feed, unless otherwise specified.
e No. affected/no. treated.
f MEL = Minimum effective level.
g As reported by Corbett et al. (1978a).
The number of inseminations required for conception was
increased in cows whose dams or grand dams had received oral doses
of FireMaster(R) BP-6 (Willett et al., 1982; Table 86).
Most of the studies evaluating the effects of PBBs on
reproductive performance started with treatment during pregnancy
(Tables 86 and 87). In few instances, were females exposed before
mating (Allen et al., 1978; Aulerich & Ringer, 1979; Willett et al.,
1980, 1982), and animals of both sexes were treated in only one
study (Aulerich & Ringer, 1979). Under these conditions,
FireMaster(R) reduced litter size in mice (Preache et al., 1976)
and minks (Aulerich & Ringer, 1979), but not in rats (Harris et al.,
1978a; McCormack et al., 1981) and pigs (Werner & Sleight, 1981).
The adverse effect on litter size was observed in minks given
PBB-contaminated poultry as well as in those fed FireMaster(R),
directly added to the diet (Aulerich & Ringer, 1979). Stillbirths
occurred in cattle (Durst et al., 1978b; Willett et al., 1982) and
rhesus monkeys (Allen et al., 1978; Lambrecht et al., 1978).
Reduced birth weights were reported in rhesus monkeys (Allen et al.,
1978; Lambrecht et al., 1978), minks (Aulerich & Ringer, 1979), and
mice (Corbett et al., 1978b) at intakes of 0.3, 1, or 50-1000 mg
FireMaster(R)/kg feed, respectively (see Table 87). On the other
hand, increased birth weights of calves resulted in a higher
incidence of dystocia in cows (Durst et al., 1978b; Willett et al.,
1980, 1982). The growth of progeny during lactation (indicated by
weight at weaning) was also reduced by FireMaster(R) in rats
(Harris et al., 1978a), mice (Preache et al., 1976), minks (Aulerich
& Ringer, 1979), and rhesus monkeys (Allen et al., 1978; Lambrecht
et al., 1978).
In the study by Lambrecht et al. (1978), 14 female rhesus
monkeys were used as an animal model (7 controls, 7 treated) to
evaluate the toxicological effects of FM FF-1. The substance was
added to the pelleted diet at a concentration of 0.3 mg/kg and 200 g
of this diet was fed per day; after a dosing period of 7 months, the
animals had ingested a total of 10.5 mg FM FF-1. Although the food
consumption during this period was unaffected, there was a loss of
7.4% of the initial body weight in the treated group. The total
exposure is not stated, but, on the basis of information given in
the paper, it appears that the dams had been exposed for a total of
12.5 months at the time of the birth of the infants. After 6 months,
menstrual cycles in 4/7 monkeys were lengthened (controls: 28 days;
treated monkeys: 31 days). The treated animals showed a
corresponding flattening of the progesterone peak. After mating, 5/7
treated monkeys delivered normal-appearing but small infants (455 g
vs 519 g in controls) with reduced weight gain in these infants
during the postnatal period. Two out of 7 treated monkeys aborted a
mummified fetus and a stillborn infant, respectively. All control
animals delivered normal-appearing infants.
The survival rate-to-weaning was adversely affected in the
offspring of rats, mice, pigs, and minks, but not in calves (see
Tables 86 and 87). FireMaster(R) levels producing the effects were
200 mg/kg body weight per day (Groce & Kimbrough, 1984), or
10 mg/day (Harris et al., 1978a), or 100 mg/kg feed (McCormack
et al., 1981) in rats, 100 mg/kg feed in mice (Preache et al., 1976)
and pigs (Werner & Sleight, 1981), and 1 mg/kg feed in minks
(Aulerich & Ringer, 1979).
For neurotoxic effects after perinatal exposure see section
8.11.2.
Ranges of maternal toxic parameters can be derived from Tables
71, 72, 74, and 75.
b) Embryotoxicity and teratogenicity
PBBs were embryotoxic in rats, mice, cattle, and rhesus
monkeys. Rats and cows appeared to be less susceptible than mice and
rhesus monkeys (see Tables 86 and 87).
Treatment of rats with a single high dose of FireMaster(R)
(> 400 mg/kg body weight) on day 6 of pregnancy resulted in 100%
embryo resorption. Generally, the number of fetal resorptions
depended on the dose and the pregnancy stage at treatment. For
example, after day 8 (400 mg/kg body weight dose) or day 12
(800 mg/kg body weight dose), the embryolethal effect of
FireMaster(R) declined abruptly (Beaudoin, 1977). Reductions in
both fetal and placental weights were observed only at high doses
(> 400 mg/kg body weight), and the most susceptible period of
pregnancy was days 11-13 (Beaudoin, 1977).
The long-term administration of lower doses (total dose: 8 or
40 mg FireMaster(R)/animal) during pregnancy (from day 0) markedly
increased embryonic death over that seen following administration of
an equivalent single dose (Beaudoin, 1979). However, long-term
intake of relatively low doses (see Tables 86 and 87) during late
gestation (start: day 7) did not influence fetal survival rate
(Corbett et al., 1975; Harris et al., 1978a; Johnston et al., 1980),
but decreased fetal weight (Corbett et al., 1975; Harris et al.,
1978a). A total dose of 600 mg FireMaster(R)/kg body weight, given
at two-day intervals (start: day 6) had no effects on fetal death or
fetal weight (Wertz & Ficsor, 1978).
No significant effects on the growth or mortality of embryos
were noted after the exposure of rats (Tables 86 and 87) to
technical octabromobiphenyl (Waritz et al., 1977) or technical
decabromobiphenyl (Millischer et al., 1979). Single cases of fetal
oedema have been observed in OcBB-treated rats (Aftosmis et al.,
1972a; Waritz et al., 1977).
The incidence of dead or resorbed fetuses was increased in mice
fed 200 mg FireMaster(R)/kg feed on days 4-16 of pregnancy
(Preache et al., 1976). Feeding several concentrations of FM from
day 7 (Corbett et al., 1975) or day 8 (Preache et al., 1976) of
pregnancy reduced fetal weight (Table 87).
Cattle given 25 g FM/day had 3 abortions and 3 dead fetuses
from 6 treated animals (Durst et al., 1977; Table 86). The fetuses
were oedematous and haemorrhagic, concomitant uterine lesions were
haemorrhage and necrosis of the cotyledons (Moorhead et al., 1977).
Two out of 7 rhesus monkeys fed FM (Table 87) had long
implantation bleedings, one aborted a mummified fetus at 146 days of
gestation and one gave birth to a stillborn infant at 154 days. The
remaining 5 monkeys delivered small infants at 156-165 days of
gestation (normal gestation: 165 days) (Allen et al., 1978;
Lambrecht et al., 1978).
Terata due to PBB exposure have been reported only in rats and
mice (Tables 86 and 87). A single high dose of FireMaster(R)
produced cleft palate and diaphragmatic hernia in rats (Beaudoin,
1977). The majority of malformations were found following
administration of 800 mg/kg body weight on day 11, 12, or 13 of
gestation. No terata were noted in rats after multiple doses of
FireMaster(R) (Table 86) (Harris et al., 1978a; Wertz & Ficsor,
1978; Beaudoin, 1979) and after feeding diets containing up to
1000 mg FireMaster(R)/kg feed (Corbett et al., 1975). In contrast
to rats comparably treated (50-1000 mg FM/kg maternal diet), a
higher incidence of exencephaly and cleft palate was seen in fetal
mice, compared with pooled historical controls, but not compared
with concurrent controls, though neither of these anomalies was seen
in control fetuses (Corbett et al., 1975, 1978b).
It is unclear whether the few cases of fetal gastroschisis,
observed in rats fed technical OcBB (Table 87), are PBB-related or
fortuitous (Aftosmis et al., 1972a; Waritz et al., 1977). No
teratogenicity was found in rats exposed to DeBB (Millischer et al.,
1979; Table 86).
Embryo development was studied in vitro. Maternal rats
received a single, oral dose of 800 mg FM/kg body weight on
gestation day 9, and the embryos were isolated on day 10 (24 h
post-treatment) for cultivation over a 24- or 42-h period (Fisher,
1980; Beaudoin & Fisher, 1981). Teratogenic effects observed (see
Table 86) were not correlated with types of malformations found in
earlier in vitro experiments (Beaudoin, 1977). Some developmental
disturbances tended to be corrected after 42 h of PBB-free culture.
In addition to retarded development, there was a significant
reduction in the DNA contents of cultured embryos (Fisher, 1980).
The survival rate of embryos was not affected during the cultivation
period (Beaudoin & Fisher, 1981).
8.5.1.2 Avian species
Avian reproduction was studied primarily in chickens.
Cockerels fed FM at various levels (10-250 mg/kg feed), for several
weeks, showed inconsistent weight changes in the comb and testes
(see Table 75: Ringer & Polin, 1977; Ringer, 1978; Dharma et al.,
1982). Histologically, lipid infiltration into testicular parenchyma
was noted (Ringer & Polin, 1977). No studies on the reproductive
performance of these birds were made.
Detrimental effects were seen when FireMaster(R) was fed to
hens of both White Leghorn chickens and Japanese quails, the only
two species studied (Table 87). Chickens (Cecil et al., 1974; Lillie
et al., 1975; Cecil & Bitman, 1978; Polin & Ringer, 1978a,; Polin
et al., 1979) appeared to be more sensitive to FireMaster(R) than
the Japanese quails (Babish et al., 1975a; Bursian et al., 1983), on
the basis of egg production and hatchability. Egg production in
chickens was reduced by dietary levels of FireMaster(R) as low as
30-45 mg/kg (Ringer & Polin, 1977), while the lowest effective level
reported for Japanese quails was 80 mg/kg (Bursian et al., 1983).
Chickens stopped laying when concentrations of FireMaster(R) in
diets exceeded 600 mg/kg (Cecil & Bitman, 1978). The hatchability of
eggs was adversely affected by 30-45 mg FireMaster(R)/kg feed in
chickens (Ringer & Polin, 1977) and by 100 mg FireMaster(R)/kg
feed in Japanese quails (Babish et al., 1975a).
Increased mortality (Cecil & Bitman, 1978; Polin & Ringer,
1978a, 1978b) and reduced growth rates (Cecil & Bitman, 1978) were
seen in the offspring of chickens fed FireMaster(R) at low levels
(< 64 mg/kg; Table 87).
Embryonic deaths occurred in chickens (Cecil & Bitman, 1978;
50% of deaths on the last day of incubation) and in Japanese quails
(Babish et al., 1975b; 40% of deaths on the first day or two of
development). The most common abnormality of embryos and newly
hatched chicks after maternal feeding of FireMaster(R) was oedema
(Cecil & Bitman, 1978; Polin & Ringer, 1978b; Polin et al., 1979).
Although there was a reduction in the feed intake of exposed
chickens, the decreased hatchability, higher incidence of
abnormalities, and poorer progeny survival could not be reproduced
by pair-fed control birds (Cecil & Bitman, 1978). At high dietary
levels (> 600 mg FM/kg of feed), the direct effects of
FireMaster(R) on egg production could not be separated from the
effects of reduced food consumption (Cecil & Bitman, 1978).
Adverse effects on the reproduction of chickens could return to
normal after withdrawal of FireMaster(R) from the diet. Time for
recovery depended on the concentration of PBB in the feed (Cecil &
Bitman, 1978; Polin & Ringer, 1978a).
8.5.2 Individual PBB congeners
Individual PBB congeners have not been reported to cause weight
changes in sex organs. Similarly, the testes of rats or cockerels,
did not show any remarkable histological changes after treatment
with 2,2'-dibromobiphenyl, 2,2',3,4,4',5,5'-heptabromobiophenyl,
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), 2,3',4,4',5,5' (BB 167),
or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) (Moore et al., 1979a;
Gupta et al., 1981; Akoso et al; 1982a; Dharma et al., 1982; Render
et al., 1982). An exception was a decrease in the gravid uterine
weight of pregnant mice given more than 500 mg BB 153/kg body weight
(Welsch & Morgan, 1985).
Embryotoxicity and other reproductive parameters have been
studied only with BB 153 mice (Lucier et al., 1978; Welsch & Morgan,
1985) or chickens (Polin et al., 1979). BB 153 did not cause the
deleterious effects (decline in hatchability, oedema) that were
produced by equivalent dietary levels of FireMaster(R) in chickens
(Polin et al., 1979; Table 87). However, BB 153 was capable of
producing cleft palate and cystic lesions in the region of the
cerebellum in mouse embryos (Welsch & Morgan, 1985; Table 87). These
adverse effects were observed at exposure concentrations that also
cause toxicity in dams. Another less complete study (Lucier et al.,
1978; Table 86) did not find developmental toxicity of BB 153 in
mice, possibly because of lower exposure levels, later onset of
exposure, and strain differences.
8.6 Mutagenicity and related end-points
The potential of PBBs for mutagenicity has been tested with
several in vitro and in vivo assays referring to four major test
categories: microbial and mammalian cell mutagenesis; mammalian cell
chromosomal damage (cytogenetic tests; in vitro and in vivo);
mammalian cell transformation in vitro; and DNA damage and repair
(UDS). Results have been summarized in Table 88. With one exception
(Kohli et al., 1978), all studies failed to indicate any
mutagenicity of individual PBB congeners or commercial PBB mixtures.
Haworth et al. (1983) did not confirm the mutagenic effect of
4-bromobiphenyl observed by Kohli et al. (1978), but they used
different Salmonella strains.
However, FireMaster(R) gave positive results in a recently
developed test, the Microscreen assay using lambda prophage
induction in Escherichia coli. This test has been found to be
sensitive in detecting carcinogens which are negative in mutagenesis
assays (Rossman et al., 1991).
8.7 Carcinogenicity
8.7.1 Carcinogenicity in long-term toxicity studies
The carcinogenic effects of PBB in long-term toxicity studies
have been compiled in Table 89. From this table, it is evident that
the principal site of tumours was the liver. The incidence of
hepatocellular carcinoma was significantly increased in both sexes
of mice and rats receiving relatively high oral doses of the
FireMaster(R) mixture.
The results of one of these studies (Gupta et al., 1983b),
carried out by the NCI/NTP (USA), were interpreted as
FireMaster(R) showing carcinogenic effects (Haseman et al., 1984;
Tennant et al., 1986). In this study, a statistically significant
higher incidence of liver tumours was found at multiple doses of
3 mg FM/kg body weight per day (male rats: 21%) and of 10 mg FM/kg
body weight per day (female/male rats: 35/23%; female/male mice:
88/95%). Atypical foci and neoplastic nodules were found at lower
doses (Table 89). Dose-responses were statistically significant (P
< 0.01) in male and female rats with regard to atypical foci,
hepatocellular carcinoma, and cholangiocarcinoma, and, in female
rats, also with regard to neoplastic nodules (Gupta et al., 1983b).
Hepatocellular carcinoma could be produced, even after single dosing
with FireMaster(R) (Kimbrough et al., 1981). Rats given a single
dose of 1000 mg FM/kg or 12 doses of 100 mg FM/kg body weight, by
gavage, developed incidences of carcinomas of 41.4 and 67.8%,
respectively (Kimbrough et al., 1981). Liver tumours were also
observed in female and male rats two years after perinatal exposure
to FireMaster(R) (Groce & Kimbrough, 1984; Table 89). Although
concurrent controls did not show such lesions, the IARC Working
Group noted the lack of statistical significance (IARC, 1986).
Nevertheless, the incidences of 5.9% (females) and 9.6% (males),
respectively, exceeded the spontaneous incidence of carcinomas of
the liver in this particular strain of rat, which is reportedly less
than 1% (Groce & Kimbrough, 1984).
Recently, NTP performed further carcinogenicity studies on
FireMaster(R) to determine the following: (a) the effects of PBBs
in F344/N rats and B6C3F1 mice receiving adult (F1) exposure only
(0, 10, or 30 mg PBB/kg diet); (b) the toxic and carcinogenic
effects of PBBs in rats and mice receiving perinatal (Fo) exposure
only (10 mg/kg in rats and 30 mg/kg in mice); and (c) the effects of
combined perinatal and adult exposure to PBBs (NTP, 1993). In an
adult-only exposure study, both sexes in rats and mice receiving 10
or 30 mg/kg (0.5 or 1.5 mg/kg body weight per day) showed increased
incidences of hepatocellular adenoma/carcinoma, quite similar to
the results of the previous studies (Gupta et al., 1983b).
Table 88. Summary of PBB genetic toxicity test results
Assay Detailsb Metabolic activation PBBa Result Remarks References
Salmonella mutagenesis
S. typhimurium strains: TA 98 ± Aroclor 1254 FM FF-1 - Tennant
TA 100 induced liver - et al. (1986)
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 hexabromobiphenylc - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats and -
TA 1537 and Syrian hamsters
S. typhimurium strains: TA 1535 ± Aroclor DeBB - Millischer
(spot test; Ames) TA 1537 induced rat - et al. (1979)
TA 1538 liver S-9 -
S. typhimurium strain: TA 1538 - DeBB - mice receiving oral
(host-mediated doses of 5, 10, and
assay) 20 g/kg body weight
S. typhimurium strains: TA 98 ± Aroclor 1254 2-bromobiphenyl - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats -
TA 1537 and Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 3-bromobiphenyl -
TA 100 induced liver -
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
S. typhimurium strain: TA 1538 + Aroclor 1254 4-bromobiphenyld + Kohli et al.
induced rat liver S-9 (1978)
none -
S. typhimurium strains: TA 98 ± Aroclor 1254 4-bromobiphenyle - Haworth et al.
TA 100 induced liver (1983)
TA 1535 S-9 from rats and
TA 1537 Syrian hamsters
Mammalian cell mutagenesis
Adult rat liver (ARL) HGPRT locus none FM FF-1 (lot No. - at the highest non-toxic Tong et al.
epithelial cells 1312-FS) dose of 10-3 mol (1983); Williams
et al. (1984)
Human fibroblasts HGPRT locus rat hepatocytes FM FF-1 (lot No. - at the highest non-toxic
1312-FS) dose of 10-4 mol
Chinese hamster HGPRT and Na-K ± Aroclor 1254 induced FM BP-6 - cell survival Kavanagh et al.
V 79 cells ATPase loci rat liver S 15 unaffected (1-40 µg/ml) (1985)
Chinese hamster HGPRT locus ± Aroclor 1254 induced 3,3',4,4'-tetra- - cell survival
V 79 cells rat liver S 15 bromobiphenyl unaffected (1-10 µg/ml)
Chinese hamster HGPRT locus none 2,2',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (20-50 µg/ml)
WB rat liver cells HGPRT locus - 2,2',4,4',5,5'- - growth stimulation
hexabromobiphenyl effects (5-20 µg/ml)
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chinese hamster HGPRT locus none 3,3',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (7-12 µg/ml)
WB rat liver cells HGPRT locus - 3,3',4,4',5,5'- - reduced cell survival
hexabromobiphenyl
Mammalian cell chromosomal
damage in vitro
Chromosome CHO cells ± Aroclor 1254 FM FF-1 - Tennant et al.
aberrations induced rat (1986)
Sister chromatid CHO cells liver S-9 -
exchange
Chromosome CHO cells ± Aroclor 1254 hexabromobiphenyl - Galloway et al.
aberrations induced rat (1987)
liver S-9
Sister chromatid CHO cells ?f
exchange
Mammalian cell chromosomal
damage in vivo
(Cytogenetic effects)
Chromosome bone marrow cells - FM - colchicine-mitosis Ficsor & Wertz
aberrations rat (pregnant) synergism (1976)
six oral doses of
100 mg/kg body
weight
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chromosome bone marrow cells - FM - no colchicine-mitosis Wertz & Ficsor
aberrations mouse (male) synergism; increase in (1978)
single dose of 50 no. of gaps
or 500 mg/kg body
weight
No. of cells in rat (male) in diet for
mitosis 5 weeks: 5-500 mg/kg
feed
Chromosome bone marrow cells; - FM BP-6 - Garthoff et al.
aberrations spermatogonial (1977)
cells
Micronucleus bone marrow cells DeBB - Millischer
mouse (male and et al. (1979)
female) total dose
of 5, 10, or
20 g/kg body
weight at 2 doses
Mammalian cell transformation
in vitro
Transformation mouse Balb/c 3T3 ± non-induced FM FF-1 - Tennant et al.
cells male F 344 rat (1986)
hepatocytes
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
DNA damage and repair
(in vitro)
Unscheduled DNA hepatocyte primary - FM FF-1 - at the highest nontoxic Tong et al.
synthesis (UDS) cultures (HPCs) doses of 10-3 mol (mice, (1983);
from rat, hamster, hamsters) or 10-5 mol Williams et al.
and mouse (rats) (1984)
DNA damage and repair
(in vivo - in vitro)
UDS hepatocyte primary - FM FF-1 - Tennant et al.
cultures (HPCs) (1986)
from treated male
F 344 rats
UDS hepatocyte primary - FM FF-1 - simultaneous measurement Mirsalis et al.
cultures (HPCs) of hepatic cell (1985)
from treated proliferative ability:
B6C3F1 mice (males increased cell
and females) proliferation
a Commercial PBB mixtures: FM = FireMaster(R); DeBB = decabromobiphenyl.
b HGPRT = hypoxanthine-guanine phosphoribosyl transferase.
c CAS No.: 36355-01-8; source: Pfaltz & Bauer.
d Source of PBB: Aldrich & Eastman Chemicals.
e Source of PBB: Pfaltz & Bauer.
f There was a very slight (16%) increase in SCEs without S-9 doses that produced severe cell cycle delay.
Table 89. Summary of carcinogenic effects of PBBs in long-term toxicity studies
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M oral 1000 10 months liver: neoplastic nodules Kimbrough
(lot no. 7042) (Sherman) single dose (preliminary study) (F: 4/5; et al. (1977,
(in peanut oil) (5) 14 months M: 0/5); (F: 3/5; M: 2/5); 1978)
(control: 0/5)
FM FF-1 rat F oral 1000 23 months liver: trabecular carcinoma Kimbrough
(lot no. 7042) (Sherman) single dose (24/58 = 41.4% versus 0% in et al. (1981)
(in corn oil) (65) control) neoplastic nodules
(42/58 = 72.4% versus 0% in
control) foci or altered areas
(57/58 = 98.3% versus 1/53 in
control)
(16) 200 18-22 liver: no carcinoma
months neoplastic nodules
(5/16 = 31.2% versus 0% in
control) altered areas
(8/16 = 50% versus 1/19 in
control)
FM BP-6 rat F oral two total dose: 120 days liver: small numbers of enzyme Rezabek et
(in corn oil) (Sprague- doses over 13 and 130 altered foci (EAF) al. (1987)
Dawley) 24 h mg/kg body
weight
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat M oral 22 30 6 months liver: atypical nodules (2/9) Gupta &
(lot no. 1312 (Fischer doses over (after 1 liver: atypical nodules (1/2) Moore (1979)
FT) (in corn 344/N) 4.5 weeks 100 dose) epididymis: sperm granuloma (1/2)
oil) (9)
FM FF-1 rat F oral 12 100 24 months liver: trabecular carcinoma Kimbrough
(lot no. 7042) (Sherman) doses over (17/28 = 60.7% versus 0% in et al. (1981)
(in corn oil) (30) 4 months control) neoplastic nodules
(24/28 = 85.7% versus 1/25 in
control) foci or altered areas
(23/28 = 82.1% versus 1/25 in
control) adenocarcinoma
(1/28 = 3.6% versus 0% in control)
malignant tumour with metastases
to heart (1/28 = 3.6% versus 0%
in control)
Total malignant liver tumours:
19/28 (67.8% versus 0/25 in
controls)
FM FF-1 rat F, M oral 125 0.1, 0.3, 6 months liver: atypical foci (3/100); Gupta et al.
(lot no. 1312 (Fischer doses over 1, 3, 10 (after 1 urinary bladder: squamous cell (1983a)
FT) (in corn 344/N) 6 months dose) carcinoma (F: 1/51, high dose
oil) (51)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M oral 125 lifetime Gupta et al.
(lot no. 1312 (Fischer doses over (1983b)
FT) (in corn 344/N) 6 months
oil) (total: 320) (sacrifice of
10% animals
alive, 23
months post-
treatment)
F, M 0.1 liver: atypical foci
(M: 3/39 = 8%; F= 0/21)
neoplastic nodules
(M: 0/39; F: 2/21 = 10%)
carcinoma (M: 2/39 = 5%; F: 0/21)
0.3 liver: atypical foci
(M: 12/40 = 30%, P < 0.01; F:
1/21 = 5%) neoplastic nodules
(M: 1/40 = 2%; F: 0/21) carcinoma
(M: 0/40; F: 0/21)
FM FF-1 rat F, M 1 liver: atypical foci Gupta et al.
(lot No. 1312 (Fischer (M: 11/31 = 35%, P < 0.01; F: (1983b)
FT) (in corn 344/N 2/11 = 18%) neoplastic nodules
oil) (total: (M: 4/31 = 13%, P < 0.05; F: 2/11)
320) carcinoma
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
3 liver: atypical foci
(M: 13/33 = 39%, P < 0.01; F:
4/19 = 21%) neoplastic nodules
(M: 4/33 = 12%; F: 5/19 = 26%,
P < 0.01) carcinoma
(M: 7/33 = 21%, P < 0.01; F:
3/19 = 16%)
10 liver: atypical foci
(M: 12/31 = 39%, P < 0.01; F:
8/20 = 40%, P < 0.01 neoplastic
nodules (M: 1/31 = 3% F: 8/20 =
40%, P < 0.01 carcinoma (M:
7/31 = 23%, P < 0.01; F: 7/20 =
35%, P < 0.01; control: 0/33)
cholangiocarcinoma (M: 2/31 = 6 %,
F: 7/20 = 35%, P < 0.01)
FM FF-1 rat F, M perinatal total dose: 2 years liver: neoplastic nodules Groce &
(lot 7042) (Sherman) exposure: 400 mg/kg of age (M: 2/41 = 4,9%, F: 9/51 = 17.6%) Kimbrough
(in corn oil) (41-51) two maternal body trabecular carcinoma (1984)
oral doses weight (M: 4/41 = 9.6%;
on gestation F: 3/51 = 5.9%; controls: 0%)
days 7 and
14
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M adult only 0 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (Fischer exposure (in (M: 36%; F: 6%)
FT) 344/N) diet for oval cell hyperplasia
(50) 24 months) (M: 0%; F: 0%)
hepatocellular adenoma
(M 2%: F: 0%)
hepatocellular carcinoma
(M: 0%; F: 0%)
10 liver: eosinophilic focus (M:
90%, p < 0.01; F: 94%, P < 0.01)
oval cell hyperplasia (M: 22%,
P < 0.01; F: 24%, P < 0.01)
hepatocellular adenoma (M: 20%;
F: 20%) hepatocellular carcinoma
(M: 4%; F: 4%) hepatocellular
adenoma/carcinoma (M: 24%, P
< 0.001; F: 24%, P < 0.001)
30 liver: eosinophilic focus NTP (1993)
(M: 92%, P < 0.01; F: 96%, P
< 0.01) oval cell hyperplasia
(M: 74%, P 0.01; F: 84%, P
< 0.01) hepatocellular adenoma
(M: 76%, P < 0.001; F: 76%, P <
0.001); hepatocellular carcinoma
(M: 38%, P < 0.001; F: 8%)
hepatocellular adenoma/carcinoma
(M: 82%, P < 0.001; F: 78%, P
< 0.001)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 rat F, M perinatal only 10 24 months liver: eosinophilic focus
(lot FF-1312- (Fischer exposure (M: 58%, P < 0.01; F: 38%, P
FT) 344/N) (female parents < 0.01) hepatocellular adenoma
(50) received (M: 10%; F: 0%)
FM-containing
diet beginning
60 days prior
to breeding and
throughout
gestation,
lactation, and
up to 4 weeks
postweaning)
FM FF-1 rat F, M combined 1:3e 24 months liver: eosinophilic focus NTP (1993)
(lot FF1312- (Fischer perinatal and (M: 80%; F: 38%)
FT) 344/N) adult exposure oval cell hyperplasia (M: 8%;
(50) F: 0%) hepatocellular adenoma
(M: 6%; F: 4%) hepatocelluar
carcinoma (M: 2%; F: 0%)
10:10f 24 months liver: oval cell hyperplasia
(M: 46% versus 22% in 10 mg/kg
adult exposure group, P < 0.01
F: 68% versus 24% in 10 mg/kg
adult exposure group, P < 0.01)
hepatocellular adenoma
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
(M: 32% versus 20% in 10 mg/kg
adult exposure group;
F: 70% versus 20% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 2% versus 4% in 10 mg/kg
adult exposure group;
F: 16% versus 4% in 10 mg/kg
adult exposure group)
hepatocellular adenoma/carcinoma
(M: 32% versus 24% in 10 mg/kg
adult exposure group;
F: 78% versus 24% in 10 mg/kg
adult exposure group, P < 0.001)
10:30g 24 months liver: oval cell hyperplasia NTP (1993)
(M: 82% versus 74% in 30 mg/kg
adult exposure group;
F: 88% versus 84% in 30 mg/kg
adult exposure group)
hepatocellular adenoma
(M: 76% versus 76% in 30 mg/kg
adult exposure group
F: 90% versus 76% in 30 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 46% versus 38% in 30 mg/kg
adult exposure group
F: 44% versus 8% in 30 mg/kg
adult exposure group, P < 0.01)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 mouse F, M oral 125 doses 10 6 months liver: atypical foci Gupta et al.
(lot no. 1312 (B6C3F1) over 6 months (11/100 versus 1/20 in control) (1983a)
FT) (in corn (50) (M: 9/50; F: 2/50)
oil)
FM FF-1 mouse F, M oral 125 doses 0.1, 0.3, lifetime high dose: liver: carcinoma Gupta et al.
(lot no. 1312 (B6C3F1) over 6 months 10 (M: 21/22 = 95% versus 12/25 in (1983b)
FT) (in corn (8-27) (sacrifice of control; P < 0.01; F: 7/8 = 88%
oil) 10% animals versus 0/13 in control; P < 0.01)
alive 24 metastasis to lung
months post- (F: 3/8 = 38% versus 0/13 in
treatment) control; P < 0.05)
FM FF-1 mouse F, M adult only 0 24 months liver: eosinophilic focus (M: 6%; NTP (1993)
(lot FF1312- (B6C3F1) exposure (in F: 2%) bile duct hyperplasia
FT) (50) diet for 24 (M: 0%; F: 0%) hepatocellular
months) ademona (M: 18%; F: 8%)
hepatocellular carcinoma
(M: 16%; F: 2%)
10 liver: eosinophilic focus (M: 33%,
P < 0.01; F: 36%, P < 0.01) bile
duct hyperplasia (M: 8%, < 0.01;
F: 18%, P < 0.01) hepatocellular
adenoma (M: 98%, P < 0.01; F: 78%,
P < 0.01) hepatocellular carcinoma
(M: 61%, P < 0.01; F: 44%, P <
0.01 hepatocellular adenoma/
carcinoma (M: 98%, P < 0.001;
F: 84%, P < 0.001)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
30 liver: eosinophilic focus NTP (1993)
(M: 12%, F: 8%); bile duct
hyperplasia (M: 68%, P 0.01;
F: 81%, P < 0.01) hepatocellular
adenoma (M: 84%, P < 0.001;
F: 96%, P < 0.001); hepatocellular
carcinoma (M: 72%, P < 0.001;
F: 73%, P < 0.001) hepatocellular
adenoma/carcinoma (M: 96%, P <
0.001; F: 98%, P < 0.001)
FM FF-1 mouse F, M perinatal only 30 24 months liver: eosinophilic focus NTP (1993)
(lot FF-1312- (B6C3F1) exposure (M: 40%, P < 0.01; F: 6%, P <
FT) (50) (female parents 0.01) hepatocellular adenoma
received (M: 62%, P < 0.001; F: 38%, P <
FM-containing 0.001) hepatocellular carcinoma
diet beginning (M: 34%, F: 8%) hepatocellular
60 days prior adenoma/carcinoma (M: 80%, P <
to breeding and 0.001; F: 42%, P < 0.001)
throughout
gestation,
lactation, and
up to 4 weeks
postweaning)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
FM FF-1 mouse F, M combined 10:10f 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (B6C3F1) perinatal and (M: 8% versus 33% in 10 mg/kg
FT) (50) adult exposure adult exposure group,
F: 32% versus 36% in 10 mg/kg
adult exposure group) bile duct
hyperplasia (M: 0% versus 0% in
10 mg/kg adult exposure group
(F 18% versus 18% in 10 mg/kg)
adult exposure group)
hepatocellular adenoma
(M: 94% versus 98% in 10 mg/kg
adult exposure group;
F: 76% versus 78% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 63% versus 61% in 10 mg/kg
adult exposure group;
F: 52% versus 44% in 10 mg/kg
adult exposure group)
FM FF-1 mouse F, M combined 30:10h 24 months liver: eosinophilic focus NTP (1993)
(lot FF 1312- (B6C3F1) perinatal and (M: 0% versus 33% in 10 mg/kg
FT) (50) adult exposure adult exposure group, P < 0.01;
F: 8% versus 36% in 10 mg/kg
adult exposure group, P < 0.01)
bile duct hyperplasia
(M: 16% versus 0% in 10 mg/kg
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
adult exposure group, P < 0.01;
F: 12% versus 18% in 10 mg/kg
adult exposure group)
hepatocellular adenoma
(M: 96% versus 98% in 10 mg/kg
adult exposure group;
F: 94% versus 78% in 10 mg/kg
adult exposure group)
hepatocellular carcinoma
(M: 80% versus 61% in 10 mg/kg
adult exposure group;
F: 88% versus 44% in 10 mg/kg
adult exposure group)
hepatocellular adenoma/carcinoma
(M: 96% versus 98% in 10 mg/kg
adult exposure group;
F: 100% versus 84% in 10 mg/kg
adult exposure group)
30:30i 24 months liver: hepatocellular adenoma NTP (1993)
(M: 96% versus 84% in 30 mg/kg
adult exposure group,
F: 87% versus 96% in 30 mg/kg
adult exposure group,
hepatocellular carcinoma
(M: 70% versus 72% in 30 mg/kg
adult exposure group;
F: 62% versus 73% in 30 mg/kg
adult exposure group)
Table 89 (contd).
PBB Species Sexb Treatment Dose/ Observation Site or type of tumourd References
(strain) concentrationc period
(No.a)
NoBB mouse F, M in diet for 100 18 months liver: carcinoma: 300/100 mg/kg Momma (1986)
(Bromkal (B6C3F1) 18 months 300 (M: 28%/78%; F: 76%/17% versus
80-9D) (50) 14% (M), 0% (F) in controls)
neoplastic nodules: 300/100 mg/kg
(M: 96%/98%, F: 92%/72% versus
38% (M), 0% (F) in controls)
hepatoblastoma: 300 mg/kg
(M: 2%/12% versus 0% in controls)
2,2',4,4',5,5' rat F in diet for 10 180 days liver: no neoplastic nodules; Jensen &
hexabromobiphenyl (Sprague- for 180 days no EAF Sleight
(BB 153) Dawley) (3) (1986)
100 100 days liver: neoplastic nodules (1/3)
small numbers of EAF
(6) F in diet 10 450 days liver: no nodules, no carcinoma Jensen &
for 140 days Sleight
(1986)
3,3',4,4',5,5' rat (Sprague- F in diet 0.1 450 days liver: no nodules, no carcinoma
hexambromobiphenyl Dawley) for 140 days
(BB 169) (6)
Mixture of BB rat (Sprague- F in diet 10 plus 450 days liver: no nodules, no carcinoma
153 and BB 169 Dawley) for 140 days 0.1 resp.
Table 89 (contd).
a Number per experimental group, unless otherwise specified.
b F = female; M = male.
c In mg/kg body weight per day or in mg/kg feed, unless otherwise specified.
d Including nodules and atypical foci (numbers in parentheses: number affected/number treated); EAF = enzyme altered foci.
e F1 animals born after the perinatal exposure at dietary level of 1 mg/kg PBBs received diets containing 3 mg/kg PBBs up to 2 years.
f F1 animals born after the perinatal exposure at dietary level of 10 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
g F1 animals born after the perinatal exposure at dietary level of 10 mg/kg PBBs received diets containing 30 mg/kg PBBs up to 2 years.
h F1 animals born after the perinatal exposure at dietary level of 30 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
i F1 animals born after the perinatal exposure at dietary level of 30 mg/kg PBBs received diets containing 10 mg/kg PBBs up to 2 years.
Perinatal-only exposure (through dietary administration of
10 mg PBBs/kg to the dams) had no effect on the incidence of hepatic
neoplasms in female rats, but, in male rats, this exposure was
associated with a marginally increased incidence of hepatocellular
adenomas. Perinatal exposure to 30 mg PBBs/kg showed significantly
increased incidences of hepatocellular adenoma/carcinoma in male and
female mice.
Combined perinatal and adult exposure to PBBs confirmed the
findings of the adult-only exposures in rats and mice. Although
there were no enhancing effects of combined perinatal and adult
exposure, perinatal exposure enhanced the susceptibility to the
induction of liver tumours of adult female rats, exposed to 10 or
30 mg/kg diet. In male and female mice, it was not possible to
assess adequately the enhancing effects on hepatocellular tumours of
combined perinatal and adult exposure, because adult-only exposure
to 10 or 30 mg PBBs/kg resulted in high incidences (84-98%) of
hepatocellular adenoma/carcinoma. However, with increased perinatal
exposure, there were increases in the numbers of mice with
hepatocellular carcinomas and mice with multiple hepatocellular
adenomas, which suggests an enhancement of PBB-related
hepatocellular carcinogenicity associated with perinatal exposure. A
dietary dose of 3 mg/kg (0.15 mg/kg body weight per day) and pre-
and perinatal exposure of the dam to 1 mg/kg (0.05 mg/kg body weight
per day), the lowest dose in this combined perinatal and adult
exposure, did not cause any adverse effects on rats.
Feeding of 10-100 mg FireMaster(R) BP-6 or
2,2',4,4',5,5'-hexa-bromobiphenyl (BB 153)/kg diet or of 0.1 mg
3,3',4,4',5,5'-hexa-bromobiphenyl/kg diet did not cause
hepatocellular carcinoma in rats, but neoplastic nodules were found
at 100 mg FireMaster(R) or BB 153/kg of feed (Jensen et al., 1982;
Jensen & Sleight, 1986). However, the number of animals used was
small, and the observation time less than 2 years.
The incidence of hepatocellular carcinomas was increased in
male and female mice receiving diets containing commercial
nonabromobiphenyl (Bromkal 80-9D) at 100 or 300 mg/kg diet, for 18
months; hepatoblastomas were also seen in males (Momma, 1986; Table
89).
The carcinogenic effects of commercial OcBB and DeBB have not
been studied.
8.7.2 Mechanisms of carcinogenicity
Generally, the development of cancer is described as a
multistage process consisting of initiation and promotion phases
(e.g., Safe, 1984). The question of the mechanism of PBB-induced
carcinogenicity is addressed in several studies.
8.7.2.1 Tumour initiation
As seen in section 8.6, there is no strong evidence for the
mutagenicity or genotoxicity of PBBs, which is a property of known
initiators. Additionally, PBBs (a mixture of almost exclusively BB
153 and BB 180) did not bind to DNA (Dannan et al., 1978b).
FireMaster(R) and BB 153 were also tested as tumour
initiators (and promotors) in a two-stage mouse skin tumorogenesis
assay (Haroz & Aust, 1979). A tumour promoter,
12-0-tetradecanoyl-phorbol-13-acetate (TPA), was applied to the skin
of a mouse of a tumour-susceptible strain (SENCAR). After 14 weeks
of treatment, neither FM nor BB 153 exhibited tumour initiating (or
promoting: see Table 90) activity. However,
3,3',4,4'-tetrabromo-biphenyl, tested in a two-stage rat bioassay
(promotion by phenobarbital), showed some evidence for weak
initiating activity (Dixon et al., 1985, 1988). In contrast to
3,3',4,4'-tetrabromobiphenyl which can be metabolized,
FireMaster(R) has not been tested for initiating activity in this
bioassay, since FireMaster(R) would persist in the tissues of the
animals beyond the initiation phase and throughout the promotion
phase (Rezabek et al., 1987).
8.7.2.2 Tumour promotion
Varied results (Table 90) were obtained with tumour promotion
assays, in which PBBs were tested in combination with known
carcinogens (2-acetylaminofluorene in rats, Schwartz et al., 1980;
partial hepatectomy plus i.p. administration of N-nitrosodiethyl
amine in rats, Jensen et al., 1982, 1983, 1984; Jensen & Sleight,
1986; Rezabek et al., 1987; Dixon et al., 1988; subcutaneous
administration of N-nitrosodiethylamine in hamsters, Wasito &
Sleight, 1989; 7,12-dimethyl-benz [a]-anthracene by skin
application in mice, Berry et al., 1978; Haroz & Aust, 1979;
N-methyl- N-nitro- N-nitroso-guanidine by skin application in
mice, Poland et al., 1982).
On evaluating the development of tumours, hepatocellular
carcinoma was found in rats (Jensen, 1983) and skin papilloma in
mice (Poland et al., 1982). Other studies with FireMaster(R)
resulted in negative findings (Berry et al., 1978; Haroz & Aust,
1979; Schwartz et al., 1980). Only few hepatocellular carcinomas
were present in initiated rats given
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) or
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or a mixture of both
(Jensen et al., 1982). In a mouse skin test, BB 169 was effective in
promoting papillomas, but BB 153 was not (Poland et al., 1982).
Another indicator of tumour-promoting ability in initiated rats
was the counting and measuring of enzyme-altered foci (EAF;
exhibiting gamma-glutamyltranspeptidase activity) and hepatic
nodules, which were presumed to be precursor lesions of
hepatocellular carcinomas (e.g., Sleight, 1985). In these studies
(see Table 90) devised by Pitot et al. (1978), FireMaster(R)
(Jensen et al., 1982, 1984; Jensen & Sleight, 1986; Rezabek et al.,
1987) and its non-toxic major congener BB 153 (Jensen et al., 1982;
Jensen & Sleight, 1986) acted as tumour promoters, FM being more
effective than Bb 153 at dietary concentrations of 10 and 100 mg/kg
(Jensen et al., 1982). Short-term feeding of FM was as effective as
long-term feeding in enhancing the development of enzyme-altered
foci (Jensen et al., 1984). Similarly, an oral 24-h administration
of FM was sufficient to enhance EAFs (Rezabek et al., 1987). An oral
dose of 13 mg FM/kg body weight was found to be close to a possible
no-effect threshold level for the enhancement of EAF (Rezabek
et al., 1987). BB 169 (3,3',4,4',5,5'- hexa) was tested positive
only at a dose (1 mg/kg feed) that was hepatotoxic (Jensen et al.,
1983). It was concluded (e.g., Sleight, 1985) that toxicity and
carcinogenicity are not necessarily related. Synergistic as well as
inhibitory effects on tumour-promoting ability could be elicited by
special combinations of BB 153 and BB 169 (Sleight, 1985; Jensen &
Sleight, 1986).
Preliminary studies with FireMaster(R) BP-6 indicated that
iron overload may enhance the hepatocarcinogenicity of PBBs in
C57BL/10 ScSn male mice (Smith et al., 1990b).
Interestingly, FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl inhibited intercellular
communication in vitro at non-toxic doses, a property of known
tumour promoters (e.g., Sleight, 1985), but
3,3',4,4',5,5'-hexabromobiphenyl did not (Table 95; section 8.9).
Probably, non-toxic congeners, such as BB 153, have a direct
tumour-promoting effect by interfering in normal cell-to-cell
communication, whereas toxic congeners like BB 169 promote tumours
secondarily to hepatic degeneration and necrosis (e.g., Sleight,
1985).
8.7.2.3 PBBs acting as complete carcinogens
The development of tumours, hepatic nodules, or small numbers
of EAF (Table 89) in PBB-exposed rodents that were not
experimentally initiated has been interpreted in two ways: either
PBBs (or some of them) have both initiating and promoting activity
or the observed effects may have resulted from the promotion of
"environmentally initiated" cells. As yet, neither of the two
possibilities can be ruled out (Jensen et al., 1982; Sleight, 1985;
Rezabek et al., 1987).
Table 90. Effects of PBB in tumour promotion assays
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
FM BP-6 rat (Sprague- simultaneous treatment: in diet 50 0 inhibition of 2-FAA induced Schwartz
Dawley) 2-acetylaminofluorene mg/kg for mammary and ear duct et al. (1980)
(F, 8-12) (2-FAA) in diet 57 weeks carcinogenesis; no significant
(300 mg/kg feed) effect on hepatic tumours
(5/12 versus 3/8 in 2-FAA
group)
FM BP-6 rat (Sprague- pretreatment: 70% partial in diet 10 0 liver: neoplastic nodules Jensen et al.
(lot 6224 A) Dawley) hepatectomy plus N-nitro- and 100 mg/kg (6/6) increase in EAF (P < (1982)
(F,6) sodiethylamine (DEN) for 180 days 0.05) (both levels)
(single ip dose of 10
mg/kg body weight)
FM rat (4) pretreatment 70% partial in diet 10 275 days liver: carcinoma (4/4 versus 0 Jensen (1983)
hepatectomy plus for mg/kg in control)
140 days DEN (single ip
dose of 10 mg/kg
body weight)
FM BP-6 rat (Sprague- pretreatment: 70% partial in diet 100 0 liver: increase in EAF Jensen et al.
(lot 6224 A) Dawley) hepatectomy plus DEN mg/kg for (P < 0.05) (1984)
(F,6) (single ip dose of for 15 days
10 mg/kg body weight) 10 mg/kg for 0 liver: increase in EAF
140 days (P < 0.05)
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
FM BP-6 rat (Sprague- pretreatment: two-thirds oral two doses 120 days Rezabek et al.
Dawley) partial hepatectomy plus over 24 h (1987)
(F,6) DEN (single ip dose of total dose:
10 mg/kg body weight) 13 mg/kg and liver: increase in EAF (not
130 mg/kg body significant) liver: increas
weights in EAF (P < 0.05)
FM BP-6 mouse CD1 pretreatment: 7,12-di- dermal 0 skin: no papilloma Berry et al.
(F,30) methylbenz(a)anthracene multiple doses (1978)
(DMBA) (single dermal (twice weekly
dose of 200 nmol) over 30 weeks)
100 µg
FM BP-6 mouse pretreatment. DMBA dermal 0 skin: no tumours Haroz & Aust
(SENCAR) (single dermal multiple doses (1979)
(sex, no.: subcarcinogenic dose) (twice weekly
not over 14 weeks)
specified) dose: not
specified
FM FF-1 mouse pretreatment: N-methyl- dermal 0 skin: papilloma (9/15 = 60%) Poland et al.
hairless N'-nitro-N- multiple doses (1982)
HRS/J nitrosoguanidine (MNNG) (twice weekly
(F,20-26) (single dermal dose of over 20 weeks)
5 µmol) 2 mg, 5 weeks,
then
FM BP-6 hamster pretreatment: DEN (single in diet 100 133 days respiratory tract: increase in Wasito &
(Syrian sc dose of 80 mg/kg mg/kg for number of tracheal papillomas Sleight (1989)
golden) body weight 140 days (P < 0.05)
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
3,3',4,4'- rat (Sprague- pretreatment: 70% partial in diet 0.1, 0 liver: increase in EAF Dixon et al.
tetrabromobiphenyl Dawley) hepatectomy plus DEN 1, 5 mg/kg (significant at the high (1988)
(F, 6) (single ip dose of 10 for 180 days dose)
mg/kg body weight)
3,3',4,4'- rat (Wistar) pretreatment: DEN (oral intraperitoneal until 1 week liver: increase in EAF Buchmann
tetrabromobiphenyl (F,6) dose of 10 mg/kg body 15 µmol/kg and 9 weeks et al. (1991)
weight for 10 days) body weight
once weekly
for 8 weeks
2,2',4,4',5,5'- rat (Sprague- pretreatment: 70% partial in diet 10 0 liver: neoplastic nodules Jensen et al.
hexabromobiphenyl Dawley) hepatectomy plus DEN and 100 mg/kg (3/6) increase in EAF (P (1982)
(BB 153) (F,6) (single ip dose of 10 for 180 days < 0.05) neoplastic nodules
mg/kg body weight) (5/6) increase in EAF (P <
0.05)
in diet 10 0 liver: increase in EAF (P Jensen &
mg/kg for 70 days < 0.05) increase in EAF (P Sleight (1986)
140 days 310 days < 0.05) increase in hepatic
nodules (P < 0.05) carcinoma
(1/10 versus 0 in controls)
mouse pretreatment: DMBA dermal multiple 0 skin: no tumours Haroz &
(SENCAR) (single dermal doses (twice Aust (1979)
(sex, no.: sub-carcinogenic dose) weekly over 14
not weeks) dose:
specified) not specified
Table 90 (contd).
PBB Species Initiation of PBB Observation Effectsb References
(strain) carcinogenesis treatment period after
(No.)a PBB treatment
2,2',4,4',5,5'- mouse pretreatment MNNG dermal 0 skin: no papilloma (0/22) Poland et al.
hexabromobiphenyl hairless (single dermal dose multiple doses (1982)
HRS/J of 5 µmol) (twice weekly
(F,20-26) over 20 weeks)
20 µg
3,3',4,4',5,5'- rat (Sprague- pretreatement: 70% in diet 0.1 0 liver: no effect on EAF Jensen &
hexabromobiphenyl Dawley) partial hepatectomy mg/kg for 70 days no effect on EAF Sleight (1986)
(BB 169) (6) plus DEN (single ip dose 140 days 310 days no significant effect on
of 10 mg/kg body weight) hepatic nodules; carcinoma
(1/11 versus 0 in controls)
3,3',4,4',5,5'- mouse, pretreatment: MNNG dermal multiple 0 skin: papilloma (12/20) Poland et al.
hexabromobiphenyl hairless (single dermal dose doses (twice (1982)
HRS/J of 5 µmol) weekly over 20
(f,20) weeks) 20 µg
Mixture of BB rat (Sprague- pretreatment 70% partial in diet 10 mg 0 liver: synergistic effect Jensen &
153 and Dawley) hepatectomy plus DEN BB 153/kg and 70 days on development of EAF Sleight (1986)
BB 169 (single ip dose of 10 0.1 mg BB 310 days synergistic effect on
mg/kg body weight) 169/kg feed for development of hepatic
140 days nodules; carcinoma (1/11
versus 0 in control)
rat (Sprague- in diet 100 mg 0 liver: inhibitory effect on Jensen et al.
Dawley) BB 153/kg and 1 development of EAF (1983)
F1 6) mg BB 169/kg
feed for 140
days
Table 90 (contd).
a No. = Number per experimental group; F = female.
b Numbers in parentheses signify No. affected/No. treated; EAF = Enzyme altered foci (exhibiting gamma glutamyl
transpeptidase activity);
DEN = N-nitrosodiethylamine; DMBA = 7,12-dimethyl-benz(a)anthracene; 2-FAA = 2-acetylaminofluorene;
MNNG = N-methyl-N'-nitro-N-nitroso-guanidine.
On the basis of the US National Toxicology Program (NTP) data,
possible correlations between carcinogenicity and toxicity in
laboratory rodents (Hoel et al., 1988) and between carcinogenicity
and in vitro genetic toxicity assays (Tennant et al., 1987;
Benigni, 1989; Ashby & Tennant, 1991) have been analysed for a
series of chemicals including the FireMaster(R) mixture. Results
confirm that FireMaster(R) can be classified as a nongenotoxic
(epigenetic) carcinogen (see also Loury et al., 1987; Williams
et al., 1989).
8.8 Biochemical toxicity
8.8.1 Induction of microsomal enzymes
One of the most intensively studied effects of the PBBs is
their induction of mixed function oxidase (MFO) enzymes. Generally,
MFO inducers and MFO systems are classified into two main groups,
typified by phenobarbital (PB) and 3-methylcholanthrene (MC). The
inducing capabilities of commercial PBB mixtures and individual PBB
isomers and congeners have been summarized in Tables 91 and 92,
respectively. The results were obtained from enzymatic, spectral,
electrophoretic, immunochemical, and metabolic studies (see also
section 6.3.1).
8.8.1.1 Commercial PBB mixtures
With one exception dealing with octabromobiphenyl (Ahotupa &
Aitio, 1978), all studies available referred to the FireMaster(R)-
mixture (Table 91).
Consistently, FM was found to be a mixed-type inducer of
hepatic microsomal enzymes in rats. Induction was observed at
intakes as low as 1 mg FM/kg feed for 21 days (Babish & Stoeswand,
1977). The no-effect level of a single ip dose was 8 µmol FM/kg body
weight, corresponding to 4.7 mg FM/kg body weight (Goldstein et al.,
1979). When FM was given for 5 days a week, over 30-50 days, changes
in hepatic enzymes occurred with doses as low as 0.3 mg/kg body
weight per day (Goldstein et al., 1979). The dose of FM BP-6
effecting half maximal AHH (benzo[a]pyrene hydroxylase) induction
was approximately 50 mg/kg body weight (Robertson et al., 1981c).
Pre-, post-, or perinatal exposures were also effective in inducing
microsomal enzymes in rats (Dent et al., 1977b,c, 1978b; Moore
et al., 1978a; McCormack et al., 1978a, 1980, 1981). Nursing pups
were approximately ten times more sensitive to these effects than
the dams. The approximate no-effect level for microsomal enzyme
induction in nursing rats was 0.1 mg FM/kg feed, in the diet of the
adult (Moore et al., 1978a).
Induction could be detected as early as 24 h after an ip
administration of 150 mg FM/kg body weight (Dent, 1978; Dent et al.,
1978a) or after an oral dose of 90 mg/kg body weight (Kluwe & Hook,
1981). Although most of the investigations were short-term, there
was some evidence for persistent stimulation of hepatic microsomal
enzymes. For example, maternal rats fed 100 mg FM/kg feed, during
pregnancy and lactation, had elevated enzyme activity 14 weeks after
weaning their first litter or even after weaning a second litter
(recovery period: 12-16 weeks) (McCormack & Hook, 1982). Perinatal
exposure to FM induced microsomal enzymes in rats at 28, 150, and
328 days of age (McCormack et al., 1980). Feeding of FM during
pregnancy and lactation to F0-animals stimulated microsomal
enzymes, not only in the F1-generation, but also in the
F2-generation (McCormack et al., 1981). Also, persistence of an
increased activity of microsomal enzymes, 120 or 125 days after
cessation of exposure, was noted in rats exposed to FireMaster(R)
BP-6 during hepatocarcinogenesis assays (Jensen et al., 1984;
Rezabek et al., 1987).
FM induced hepatic microsomal enzymes not only in the rat, but
in all other animal species tested, i.e., mouse (Corbett et al.,
1975; Dent et al., 1977a; Ahotupa & Aitio, 1978; Dannan et al.,
1980; Ahmadizadeh et al., 1984; Robertson et al., 1984c), guinea-pig
(Rush et al., 1982; Ecobichon et al., 1983; Smith et al., 1986),
hamster (Rush et al., 1982; Smith et al., 1986), cattle (Schanbacher
et al., 1978), pig (Werner & Sleight, 1981), dog (Farber et al.,
1976), Japanese quail (Babish et al., 1975a,b; Bursian et al, 1983),
and fish (Elcombe & Lech, 1978; Franklin et al., 1981; James &
Little, 1981; Law & Addison, 1981). Unlike the mammalian situation,
induction in several species of fresh- and saltwater fish was only
of the MC-type (Table 91).
Although the activities of microsomal enzymes were highest in
the liver, induction was also found in extrahepatic tissues
including the kidney (Dent et al., 1977c, 1978b; Ahotupa & Aitio,
1978; McCormack et al., 1978a,b, 1979a,b, 1980, 1981; Kluwe & Hook,
1981; Werner & Sleight, 1981; Ahmadizadeh et al., 1984; Rush et al.,
1986; Smith et al., 1986), intestine (Manis & Kim, 1980; Traber
et al., 1988a,b), mammary gland (Dent et al., 1977b,c, 1978b;
McCormack et al., 1979a; McCormack & Hook, 1982) and lung (McCormack
et al., 1979a). However, patterns of induction were organ specific.
No induction was noted in the testes of rats (Kluwe & Hook, 1981)
(see also Table 91).
FM was also able to stimulate aryl hydrocarbon hydroxylase
(AHH) activity in rat hepatoma cell culture (Garthoff et al., 1977).
Commercial OcBB has been tested in only one study on mice and
was found to be an inducer of drug-metabolizing enzymes. However,
OcBB was a less potent inducer than FM (Ahotupa & Aitio, 1978).
Table 91. Induction of microsomal enzymes (MFOs) by commercial PBB mixtures
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM FF-1 (various rat (Fischer F oral 9-22 doses 14-64 liver x PB + MC Goldstein
concentrations) F 344/N) over 30 days et al.
(0.03-30 mg/kg daysb (1979)
body weight/day)
FM FF-1 (lot no. rat M oral single 1-18 days liver x MCd Manis & Kim
FF-1312-FT) (Sprague- dose 1-3 days intestine x MCd (1980)
(50-400 mg/kg Dawley) 4-18 days intestine x
body weight)
(0.1 mg/day) oral 25 doses 5 weeks liver x
over 5 weeks 5 weeks intestine x
FM BP-6 (90 rat (Fischer M oral single 9, 24, 72 liver x PB + MC Kluwe &
mg/kg body 344) dose 216 h kidney x only MC Hook (1981)
weight) testes x
4 doses 7 days liver x PB + MC
over 6 days kidney x only MC
testes x
FM BP-6 (25, rat F intra- single 12, 24, 48, liver x PB + MC Dent et al.
150 mg/kg (Sprague peritoneal dose 192 h (1976b,
body weight) Dawley) 336 h 1978a)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (150 rat (Sprague- not intra- single 28 days kidney x MC McCormack
mg/kg Dawley) specified peritoneal dose et al.
body weight) pups (7 or (1978a)
11 days old)
FM BP-6 rat (Wistar) M intra- 2 doses on 6 days liver x MCd Safe et al.
(100 mg/rat) peritoneal days 1 & 3 (1978)
FM FF-1 (lot no. rat F intra- single 4 days liver x PB + MC Goldstein
FF-1312 FT) (Fischer) peritoneal dose et al.
(varied (1979)
concentrations)
(1.8-1000
µmol/kg body
weight)
FM FF-1 (90 rat M intra- single 2 weeks liver x PB + MC Dannan
mg/kg body (Sprague- peritoneal dose et al.
weight) Dawley) (1982c)
FM BP-6 (1500 rat (Long M intra- single 4 days liver x PB + MC Parkinson
or 750 µmol/kg Evans) peritoneal dose et al.
body weight) (1983);
Haake et al.
(1985)
FM BP-6 (150 rat M intra- single 5, 10, 15 kidney x x MCd Rush et al.
mg/kg body (Sprague- peritoneal dose days (day (day (1986)
weight) Dawley) 5,10) 15)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (6400 rat M intra- 4 doses 4 days liver x PBe Traber
mg/kg body (Sprague- peritoneal over 4 days small et al.
weight; total Dawley) intestine x PBe (1988a,b)
dose)
FM BP-6 rat F in diet 2 weeks 2 weeks liver x PB + MC Dent et al.
(4.6-200 mg/kg (Sprague- (1976a)
of feed) Dawley)
FM BP-6 (50 rat (Sprague- M in diet 5-20 days 5-20 days liver x PB + MC Babish &
mg/kg of feed) Dawley) Stoeswand
(1977)
FM BP-6 (50 rat (Sprague- F in diet gd 8-day 14 day 14 post- liver x at organ Dent et al.
mg/kg of feed) Dawley) postpartum partum kidney x specific (1977b,c)
maternal mammary patterns
gland x
FM BP-6 (5, 50, rat M in diet 2, 3, 5 2, 3, 5 liver x PBe Garthoff
500 mg/kg feed) (Holtzmann) weeks weeks et al.
(1977)
FM BP-6 (100 rat (Sprague- F in diet 3 months 3 months liver x MC + PB McCormack
mg/kg feed) Dawley) kidney x MC et al.
(1978a,b)
FM FF-1 (lot rat (Sprague- F in diet 18 days 18 days liver x PB + MC Moore
7042) (0.1-10 Dawley) postpartum et al.
mg/kg feed) lactating (1978a)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 (25-200 rat (Sprague- F in diet gd 8-day 14 day 14 post- lung x MCd McCormack
mg/kg feed) Dawley) postpartum partum kidney x MCd et al.
lactating mammary (1979a)
gland x MCd
liver x MCd
FM BP-6 (100 rat (Sprague- M in diet 30 days 30 days liver x PB + MC Akoso et al.
mg/kg feed) Dawley) (1982a)
FM BP-6 (100 rat F in diet gd 8-day 28 W1 litter, liver x PB + MC McCormack &
mg/kg feed) (Sprague- postpartum 14 weeks mammary Hook (1982)
Dawley) weanling after W1 gland x MC
maternal (6 weeks) and W2
litters
FM BP-6 (50 rat (pups) placental, pre-, post- at birth liver x PB + MC Dent et al.
mg/kg feed in milk or or perinatal or at day (1977c,
maternal diet) both 15 post- 1978b)
partum
FM FF-1 (lot rat milk postnatal day 18 post- liver x PB + MC Moore et al.
7042) (0.1-10 (Sprague- partum (1978a)
mg/kg feed in Dawley)
maternal diet (pups) F1
0.1-10 mg/kg rat (Sprague- F milk and postnatal several liver x PB + MC
feed in maternal Dawley) in diet until mating weeks
diet plus 0.1-1 (lactating: and day 18
mg/kg feed F1) postpartum
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
0.1-10 mg/kg rat (Sprague- placental perinatal day 18 post- liver x PB + MC
feed in maternal Dawley) and milk partum
diet plus 0.1-1 (pups: F2)
mg/kg feed
FM BP-6 (100 rat (Sprague- placental perinatal weanling liver x PB + MC McCormack
mg/kg feed) Dawley) and milk (28 days kidney x MC et al.
of age) (1980)
150 days liver x PB + MC
of age kidney x MC
328 days liver x PB + MC
of age kidney x MC
FM BP-6 (10, rat (Sprague- placental perinatal W1 liver x PB + MC McCormack
100 mg/kg Dawley) and milk (F0: gd 8- kidney x MC et al.
feed) day 28 (1981)
postpartum)
W2 liver x PB + MC
kidney x MC
W3 liver x
kidney x
FM BP-6 mouse F intra- single 24-192 h liver x PB + MC Dent et al.
(150 mg/kg (NMRI) peritoneal dose (1977a)
body weight)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM BP-6 mouse M intra- single 10 days liver x MCd Ahotupa &
(75 mg/kg (C57) peritoneal dose kidney Aitio (1978)
body weight) lung x
FM BP-6 mouse M intra- 2 doses on 6 days liver x PB + MC Robertson
(500 µmol/kg (C57BL/) peritoneal days 1 & 3 et al.
body weight) 6J) (1984c)
mouse M intra- 2 doses on 6 days liver x PB + (MC)
(DBA/2J) peritoneal days 1 & 3
FM BP-6 mouse M intra- single 5, 10, 15 kidney x Rush et al.
(150 mg/kg (ICR) peritoneal dose days (1986)
body weight)
FM BP-6 (1000 mouse F in diet 11 days 11 days liver x not Corbett
mg/kg feed) (Swiss/ICR) specified et al.
(1975)
FM (100 mg/kg mouse M in diet 28 days 28 days liver x MCd Ahmadizadeh
feed) (C57/6J) kidney x MCd et al.
(1984)
mouse M in diet 28 days 28 days liver x MCd
(DBA/2J) kidney x
FM BP-6 guinea- M intra- single 4 days liver x MCd Rush et al.
(50 mg/kg pig peritoneal dose kidney x MCd (1982);
body weight) (Hartley) Smith et al.
(1986)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM FF-1 (lot FA- guinea- placental prenatal 2 days liver x Ecobichon
7042) (maternal, pig (from gd 65) et al.
single oral (Hartley) milk postnatal 2-60 days liver x x (1983)
dose of 50 mg/kg pups (from 6-12 h of age (2,4, (14,
body weight) after 7,28 60,90
parturition) days days
of of
age) age
FM BP-6 hamster M intra- single 4 days liver x MCd Rush et al.
(50 mg/kg (Golden peritoneal dose kidney x (1982);
body weight) Syrian) Smith et al.
(1986)
FM BP-6 (lot cattle F oral 90-180 90-180 liver x Schanbacher
6244 A) days/daily days et al.
(250 mg/day) dose) (1978)
FM BP-6 pig F in diet 2nd half of gestation and liver x Werner &
(10-200 mg/kg (sow) lactation kidney x Sleight
feed) (pups) placental prenatal at birth liver x (1981)
kidney x
placental perinatal 4 weeks liver x
and milk of age kidney x
FM BP-6 (1 Beagle dog M,F oral 7 weeks 7 weeks liver x Farber
mg/kg body et al.
weight per day) (1976)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
FM (10-1000 Japanese M,F in diet 9 weeks 9 weeks liver x PBe Babish
mg/kg feed) quail et al.
(1975b)
FM FF-1 Japanese M,F in diet 5 weeks 5 weeks liver x PB + MC Polin et al.
(40, 80 mg/kg quail (1982);
feed) (Coturnix Bursian
coturnix et al.
japonica) (1983)
(3 genetic
lines)
FM BP-6 brook trout oral 18 days 18 days liver x MC Law &
(200 mg/kg (Salvelinus (multiple Addison
body weight) fontinalis) doses) (1981)
FM BP-6 rainbow intra- single up to liver x MC Elcombe &
(150 mg/kg trout peritoneal dose 2 weeks Lech (1978)
(Oncorhynchus
mykiss)
FM BP-6 rainbow parenteral single 5 days liver x MC Franklin
(150, 500 mg/kg trout dose et al.
body weight) (O. mykiss) (1981)
FM FF-1 sheepshead intra- single up to liver x MCd James &
(15 mg/kg minnow peritoneal dose 56 days Little
body weight) (Archosargus (1981);
probatocephalus) James & Bend
(1982)
Table 91 (contd).
PBBa Species Sex Route Period of MFO induction References
(Dose/ (strain) Exposure Observationc Tissue Induction Type
concentration) yes no
OcBB (FR 25013 mouse M intra- single 10 days liver x MCd Ahotupa &
A, Dow Chemical) (C57) peritoneal dose kidney x Aitio (1978)
(75 mg/kg lung x
body weight)
a Commercial PBB mixtures; FM = FireMaster; OcBB = octabromobiphenyl.
b Dosing: 5 days per week for 30 days; examination points: 14 days (9 doses); 31 days (22 doses); 46 and 64 days (22 doses
plus a 15-day and a 33-day recovery period, respectively.
c After first dose.
d Only MC-typical parameters recorded.
e Only PB-typical parameters recorded.
Abbreviations:
gd = gestation day; MFO = mixed function oxidase; MC = 3-methylcholanthrene; PB = phenobarbital.
Table 92. Induction of hepatic microsomal enzymes (MFOs) by PBB congeners
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
4-mono- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1979)
2,2'-di- rat (Sprague- M intra- single 2-22 x Moore et al.
(90 mg/kg body Dawley) peritoneal dose days (1979a)
weight)
(600) rat M intra- 3 doses 7 days x Ecobichon
(Wistar) peritoneal et al. (1979)
2,5'-di- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
4,4'-di- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1977,
1979)
(300) rat M intra- 2 doses 6 days x PB Robertson
(Wistar) peritoneal et al. (1982b)
2,2',5-tri- rat M intra- 3 doses 7 days x Ecobichon
(600) (Wistar) peritoneal et al. (1979)
2,3',5-tri- rat M intra- 3 doses 7 days x Ecobichon et al.
(600) (Wistar) peritoneal (1979)
2,4,6-tri- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,4',5-tri- rat M intra- 3 doses 7 days x
(600) (Wistar) peritoneal
3,4,4'-tri- rat M intra- single 4 days x MC Parkinson et al.
(250) (Long Evans) peritoneal dose (1983)
(300) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',5,5'-tetra- rat M intra- single dose 2 weeks x PB Robertson et al.
(150) (Wistar) peritoneal (1983b)
(500) rat (Long M intra- single 4 days x PB Parkinson et al.
Evans) peritoneal dose (1983)
(600) rat M intra- 3 doses 7 days x Ecobichon et al.
(Wistar) peritoneal (1979)
2,3',4,4'-tetra- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
(250) Evans) peritoneal dose (1983)
(1500) mouse M intra- 2 doses 6 days x Robertson et al.
(C57BL/6J) peritoneal (1984c)
(1500) mouse M intra- 2 doses 6 days x
(DBA/2J) peritoneal
2,3',4',5-tetra- rat M intra- 2 doses 6 days x PB Robertson et al.
(150) (Wistar) peritoneal (1980)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,4,4',6-tetra- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
(500) Evans) peritoneal dose (1983)
3,3',4,4'-tetra- rat (Sprague- M oral single 1-10 x MC Millis et al.
(21.3) Dawley) dose days (1985b)
(250) rat (Long M intra- single 4 days x MC Parkinson et al.
Evans) peritoneal dose (1983)
(2 mg/kg body rat (Sprague- M intra- single 2 weeks x MC Millis et al.
weight) Dawley) peritoneal dose (1985a)
(10, 60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b); Andres et
al. (1983)
(750) mouse M intra- 2 doses 6 days x MC Robertson et al.
(C57BL/6J) peritoneal (1984c)
(1500) mouse M intra- 2 doses 6 days x MC
(DBA/2J) peritoneal
3,3',5,5'-tetra- rat M intra- 3 doses 7 days x Ecobichon et al.
(600) (Wistar) peritoneal (1979)
(not specified) chicken via shell single 28 h x Poland & Glover
embryo into the dose (1977)
air sac
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
3,4,4',5-tetra- rat (Long M intra- single 4 days x MC Parkinson et al.
(250) Evans) peritoneal dose (1983)
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',4,5,5'- rat (Long M intra- single 4 days x PB Parkinson et al.
penta- Evans) peritoneal dose (1983)
(BB 101) (500)
(90 mg/kg body rat (Sprague- M intra- single 7-14 x PB Dannan et al.
weight) Dawley) peritoneal dose days (1982a); Millis
et al. (1985a)
2,2',4,5',6- rat M intra- 3 doses 7 days x Ecobichon et al.
penta- (600) (Wistar) peritoneal (1979)
2,3',4,4',5- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
penta- Evans) peritoneal dose (1983)
(BB 118) (250)
(90 mg/kg body rat (Sprague- M intra- single 2 weeks x PB + MC Dannan et al.
weight) Dawley) peritoneal dose (1982c); Millis
et al. (1985a)
(30, 150) rat (Wistar) M intra- 2 doses 6 days x PB + MC Robertson et al.
peritoneal (1980)
(500) mouse M intra- 2 doses 6 days x PB + MC Robertson et al.
(C57BL/6J) peritoneal (1984c)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(500) mouse M intra- 2 doses 6 days x
(DBA/2J) peritoneal
2,3',4,4',6- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
penta- (500) Evans) peritoneal dose (1983)
3,3',4,4',5- rat (Long M intra- single 4 days x MC
penta- (100) Evans) peritoneal dose
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
2,2',3,4,4',5'- rat (Sprague- M intra- single 7 days x PB + MC Dannan et al.
hexa- Dawley) peritoneal dose (1982a)
(BB 138) (90
mg/kg body
weight)
2,2',4,4',5,5'- rat F gavage multiple 60 days x PB Goldstein et al.
hexa- (BB 153) (F 344/N) doses (over (1979)
(16.8 mg/kg 30 days)
body weight)
(40-1000) rat F intra- single 4 days x PB
(Fischer) peritoneal dose
(500) rat (Long M intra- single 4 days x PB Parkinson et al.
Evans) peritoneal dose (1983); Haake et
al. (1985)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(90 mg/kg body rat (Sprague- M intra- single 1-14 x PB Moore et al.
weight) Dawley) peritoneal dose days (1978b); Millis
et al. (1985a)
(30 mg/kg body rat M intra- single 72 h x PB Lubet et al.
weight) (F 344) peritoneal dose (1990)
(600) rat M intra- 3 doses 7 days x PB Ecobichon et al.
(Wistar) peritoneal (1979)
(100 mg/kg feed) rat (Sprague- M in diet 30 days 30 days x PB Akoso et al.
Dawley) (1982a)
(10, 100 mg/kg rat (Sprague- M in diet 9 days 10 days x PB Render et al.
feed) Dawley) (1982)
(150 mg/kg body rainbow trout parenteral single 5 days x Franklin et al.
weight) (Oncorhynchus dose (1981)
mykiss)
2,2',4,4',5,5'- sheepshead intra- single 17 days x James & Little
hexa- (20 mg/kg minnow peritoneal dose (1981); James &
body weight) (Archosargus Bend (1982)
probatocephalus)
(60, 100 mg/kg intra- multiple 28-40 x
body weight) peritoneal doses days
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
2,2',4,4',6,6'- rat M intra- 3 doses 7 days x Ecobichon et al.
hexa- (600) (Wistar) peritoneal (1979)
2,3,3',4,4',5- rat (Sprague- M intra- single 7 days x (PB) + MC Dannan et al.
hexa- (BB 156) Dawley) peritoneal dose (1982a)
(90 mg/kg body
weight)
(3.75-60) rat M intra- 2 doses 6 days x (PB) + MC Robertson et al.
(Wistar) peritoneal (1981a)
2,3,3',4,4',5'- rat (Long M intra- single 4 days x Parkinson et al.
hexa- (100) Evans) peritoneal dose (1983)
2,3',4,4',5,5'- rat M intra- single 7 days x PB + MC Dannan et al.
hexa- (BB 167) (Sprague- peritoneal dose (1978a)
(90 mg/kg body Dawley)
weight)
(100 mg/kg feed) rat (Sprague- M in diet 30 days 30 days x PB + MC Akoso et al.
Dawley) (1982a)
2,3',4,4',5',6- rat (Long M intra- single 4 days x PB + MC Parkinson et al.
hexa- (BB 168) Evans) peritoneal dose (1983)
(250)
3,3',4,4',5,5',- rat (Sprague- M oral single 1-14 days x MC Millis et al.
hexa- (21.3) Dawley) dose (1985b)
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(100) rat (Long M intra- single 4 days x MC Parkinson et al.
Evans) peritoneal dose (1983)
(30 mg/kg body rat M intra- single 72 h x MC Lubet et al.
weight) (F 344) peritoneal dose (1990)
(2, 30 mg/kg rat (Sprague- M intra- single 1-2 x MC Dannan et al.
body weight) Dawley) peritoneal dose weeks (1982c); Millis
et al. (1985a)
30 mg/kg body rat M intra- single 72 h x MC Lubet et al.
weight (F344) peritoneal dose (1990)
(60) rat M intra- 2 doses 6 days x MC Robertson et al.
(Wistar) peritoneal (1982b)
(600) rat M intra- 3 doses 7 days x Ecobichon et al.
(Wistar) peritoneal (1979)
(1, 10 mg/kg rat (Sprague- M in diet 30 days 30 days x MC Akoso et al.
feed) Dawley) (1982a)
(10, 100 mg/kg rat (Sprague- M in diet 9 days 10 days x MC Render et al.
feed) Dawley) (1982)
(not specified) chick via shell single 24 h x MC Poland & Glover
embryo into the dose (1977)
air sac
Table 92 (contd).
PBBa Species Sex Route Period of MFOc induction References
(Dose/ (strain) No. of doses Observationb Induction Typed
concentration) yes no
(150 mg/kg body rainbow trout parenteral single 5 days x MC Franklin et al.
weight) (Oncorhynchus dose (1981)
mykiss)
2,2',3,3',4,4',5- rat (Sprague- M intra- single 7 days x PB (+MC?) Dannan et al.
hepta- (BB 170) Dawley) peritoneal dose (1982a)
2,2',3,4,4',5,5'- rat M intra- single 2-22 days x PB Moore et al.
hepta- (BB 180) (Sprague- peritoneal dose (1979a)
(90 mg/kg body Dawley)
weight)
(150 mg/kg body rainbow trout parenteral single 5 days x Franklin et al.
weight) (O. mykiss) dose (1981)
2,3,3',4,4',5,6- rat M intra- 2 doses 6 days x Robertson et al.
hepta- (6) (Wistar) peritoneal (1981a)
2,2',3,3',4,4', rat M intra- single 7 days x PB Besaw et al.
5,5'-octa- (BB (not speci- peritoneal dose (1978)
194) (90 mg/kg
body weight)
(600) rat M intra- 3 doses 7 days x PB Ecobichon et al.
(Wistar) peritoneal (1979)
Table 92 (contd).
a Total dose in µmol/kg body weight, unless otherwise specified.
b After first dose.
c MFO = Mixed function oxidase; PB = phenobarbital; MC = 3-methylcholanthrene.
d Only noted when categorized into PB- or MC- type by the authors themselves.
The FM mixture was fractionated and reconstituted in order to
test whether PBBs or contaminants (e.g., brominated dibenzofurans or
dibenzodioxins, or brominated naphthalenes) were responsible for the
inductive effects. The results indicated that most effects
associated with the mixture were due to the brominated biphenyls
(Safe et al., 1978; Robertson et al., 1981b; Dannan et al., 1982b).
8.8.1.2 Individual PBB congeners
The capability of individual PBB congeners to induce hepatic
microsomal enzymes in vivo is shown in Table 92. There was a broad
range of responses, depending on the congener or species/strain
tested. In addition to qualitative differences, the extent of the
induction also differed between congeners (e.g., Ecobichon et al.,
1979; Safe et al., 1981; Parkinson et al., 1983 and other references
from Table 92). As far as they have been tested, in vitro enzyme
induction assays using rat hepatoma H-4-II-E cells in culture, have
confirmed the results of the in vivo studies (Andres et al., 1983;
Bandiera et al., 1982, 1983).
The most abundant components of the FM mixture, BB 153
(2,2',4,4',5,5'-hexa-) and BB 180 (2,2',3,4,4',5,5'-hepta-), were
strict PB-type inducers in rats. Congeners 118 (2,3',4,4',5-penta-),
138 (2,2',3,4,4',5'-hexa-), and 167 (2,3',4,4',5,5'-hexa-) showed a
mixed PB- and MC-type induction, and 156 (2,3,3',4,4',5-hexa-) was
described (Robertson et al., 1981a; Dannan et al., 1982a) to be a
very potent MC-type inducer (see Table 8.8/2).
3,3',4,4'-tetrabromobiphenyl and the model congener
3,3',4,4',5,5'-hexabromobiphenyl were pure MC-type inducers in rats
(Table 92), 3,3',4,4'-tetrabromobiphenyl being less effective than
3,3',4,4',5,5'-hexabromobiphenyl (Millis et al., 1985a,b). The
ED50 value of 3,3',4,4',5,5'-hexabromobiphenyl determined in the
chicken embryo was approximately 90 nmol/kg body weight (= 3.4 nmol
per egg) (Poland & Glover, 1977). On the other hand,
3,3',4,4'-tetrabromobiphenyl was at least 50 times more potent as an
inducer of AHH activity than the commercial PBB mixture: ED50
values (measured in rats) were 1-2 µmol/kg body weight for
3,3',4,4'-tetrabromobiphenyl and 75-80 µmol/kg body weight for FM
BP-6 (Robertson et al., 1982).
Congeners that elicited PB-type induction in rats (e.g., BB 153
and BB 180) failed to do so in fish, whereas MC-type inducers were
effective in both rats and fish (Table 92).
Correlations between the structure and microsomal enzyme-
inducing activity (and toxicity) of individual PBB congeners have
been demonstrated in several studies and reviews (e.g., Goldstein,
1980; Moore et al., 1980; Aust et al., 1981; McKinney & Singh, 1981;
Robertson et al., 1982b; Andres et al., 1983; Dannan et al., 1983;
Parkinson et al., 1983; Safe, 1984; Safe et al., 1985).
When 3,3',4,4',5,5'-hexabromobiphenyl was used to induce
cytochrome P-450 in rats (at 10 µmol/kg body weight), it was found
to be selectively associated with cytochrome P-450d (Voorman & Aust,
1987).
8.8.2 Endocrine interactions
PBBs have been demonstrated to interact with the endocrine
system.
8.8.2.1 Thyroid hormones
Rats (No. = 10), given oral doses of FM FF-1 (Lot No. 1312 FT)
over 6 months, showed dose-related decreases in serum thyroxine
(T4) and triiodothyronine (T3). Significant decreases in T4
were seen at doses as low as 0.3 mg PBB/kg body weight per day in
males, and 1 mg/kg body weight per day in females. The reduction in
T3 was somewhat less and only significant at high doses (3-10
mg/kg body weight per day) in females (Gupta et al., 1983a). A time-
and dose-dependent reduction in plasma T4 levels was also found in
male rats (n = 8-11) administered FM FF-1, by gavage, for 10 or 20
days at 1, 3, or 6 mg/kg body weight per day (Allen-Rowlands et al.,
1981). In addition, the reduced plasma T4 levels were correlated
with an increase in thyroid stimulating hormone (TSH) at 20 days. At
6 mg/kg body weight per day, the thyroid uptake of iodine was
increased, but the incorporation of iodine in monoiodotyrosine was
decreased. While short-term feeding (7 months) of female rats with
commercial hexabromobiophenyl at dietary concentrations of
1-50 mg/kg also resulted in an decrease in serum T3 and T4
levels (Sepkovic & Byrne, 1984; Byrne et al., 1987), feeding of
technical octabromobiphenyl had no effect on serum T3 levels
(Sepkovic & Byrne, 1984). "PBB" (not specified) caused not only
reduced serum T4 levels and elevated thyrotropin levels in rats,
but also produced goitres (Bastomsky, 1986).
Single ip injections of 3,3',4,4',5,5'-hexabromobiphenyl in
juvenile male rats (20 and 40 mg/kg body weight) caused a
significant decrease in serum T4 concentrations, while serum T3
levels did not change significantly during the 28-day observation
period. The decrease in serum T4 concentrations was dose-dependent
(Spear et al., 1990).
Serum concentrations of T3 and T4 were decreased in swine
and their newborn piglets at 200 mg FM BP-6/kg feed. After nursing
for 4 weeks, piglets born to sows fed 100 mg FM BP-6/kg feed also
showed significant reductions in T3 and T4 (Werner & Sleight,
1981).
8.8.2.2 Sex hormones
Hepatic microsomes, prepared from rats exposed to FM BP-6
(100 mg/kg of feed) from day 8 of gestation until they were killed
at 4-21 weeks of age, showed an increased metabolism of progesterone
(Arneric et al., 1980), testosterone (Newton et al., 1980, 1982a),
and of the estrogens estradiol, estrone, and ethynylestradiol
(Bonhaus et al., 1981). In contrast to the increased hydroxylation
reaction, reduction of testosterone was inhibited by pretreatment
with PBBs (Newton et al., 1982a). The metabolism of exogenously
administered and labelled steroid hormones including progesterone,
testosterone, and estradiol, was also enhanced in vivo in rats
following perinatal exposure (gestation day 8-28 days postpartum) to
10 or 100 mg FM BP-6/kg feed, as shown by diminished steroid action
and reduced radio activity in serum and target organs (McCormack
et al., 1979c). In contrast, endogenously produced concentrations of
luteinizing hormone, prolactin, or corticosterone were not affected
in rats treated with 100 mg FM BP-6/kg feed from gestation day 8
until the ninth week of age (Johnston et al., 1980). Rats dosed with
1, 3, or 6 mg FM FF-1/kg body weight per day for 20 days also did
not show any alterations in plasma corticosterone or testosterone
levels, but, at 6 mg/kg body weight per day, there was a significant
reduction in plasma prolactin levels (Castracane et al., 1982).
Long-term, low-dose treatment with FM BP-6 (1, 10, or 50 m/kg feed
for 5-7 months) caused cumulative and dose-dependent decreases in
the serum corticosterone levels of female rats, as well as
reductions in the circulating levels of dehydroepiandrosterone and
dehydroepiandrosterone sulfate (Byrne et al., 1988). Alterations in
the urinary metabolic profile in the corticosteroid region of the
profile were observed after exposure (not specified) of rats to PBBs
(not specified) (Vrbanac, 1984).
Plasma corticosterone concentrations in female mice (BALB/c)
fed 100 mg FM BP-6 for 24 or 30 days were only modestly elevated
(Fraker, 1980).
Endogenous concentrations of progesterone and estradiol
determined in cows (No. = 2) administered toxic doses of FM BP-6
(25 g/day for 39 or 50 days) were in the range normally expected
(Willett et al., 1983a). The clearance rate of radiolabelled
progesterone and estradiol from the blood of both cows was decreased
(Willett et al., 1983a); elimination of radiolabel from these
hormones in the urine and faeces also declined (Sprosty et al.,
1979; Willett et al., 1983b).
Ingestion of FM FF-1 by adult, female rhesus monkeys at
concentrations of 0.3 mg/kg feed for 7 months (total dose:
approximately 10 mg PBB) caused prolonged menstrual cycles and
decreased concentrations of serum progesterone (Allen et al., 1978;
Lambrecht et al., 1978; for details in study design see section
8.5).
8.8.2.3 Prostaglandins
The effect of PBBs on prostaglandin has been examined in an
in vitro study using rat liver microsomes. A single ip injection
of FM BP-6 (100 mg/kg body weight) in male rats resulted in an
elevated metabolism of prostaglandin E1, 7 days after dosing
(Theoharides & Kupfer, 1981).
8.8.3 Interaction with drugs and toxicants
Several studies have demonstrated that PBBs have the ability to
alter the biological activity of a variety of drugs and toxicants.
In part, it may depend on the capability of the PBBs to induce
microsomal enzymes involved in the activation or deactivation of
xenobiotics. A summary of interactions, reported after the treatment
of animals with a combination of PBBs and drugs or toxicants, is
listed in Table 93 according to enhanced or miscellaneous effects.
The majority of reports are limited to the FireMaster(R) mixture.
One study (Halvorson et al., 1985) included several congeners, and
it was found that the results of interaction (formation of aflatoxin
metabolites) varied according to congener.
The toxicities of carbon tetrachloride, bromobenzene,
chloroform, trichloroethylene, and trichloroethane have been
enhanced by FM in rodents (Roes et al., 1977; Kluwe et al., 1978,
1979, 1982; Ahmadizadeh et al., 1984). Mirex-type compounds
aggravated the histological damage due to PBBs in rats (Chu et al.,
1980). Pretreatment with FM increased mortality during anaesthesia
elicited by pentobarbital in Japanese quails (Cecil et al., 1975).
On the other hand, a reduction in the toxicity of some toxicants has
also been observed, e.g., a reduction in ouabain lethality (Cagen &
Gibson, 1977a). Some other interactions are also recorded in Table
93.
8.8.4 Effects on vitamin A storage
Like related compounds, PBBs cause profound alterations in
vitamin A homeostasis. Significant reductions in hepatic vitamin A
stores have been seen in rats after exposure to individual PBB
congeners and the FireMaster(R) mixture (Table 94).
2,2',4,4',5,5'- Hexabromobiphenyl was less potent in reducing
vitamin A levels in the liver than the FM mixture and two other
hexa-isomers (Table 94).
Rats (Sherman, adult, male) given a single oral dose of 500 mg
FM FF-1/kg body weight had lower levels of retinol in the serum and
in liver microsomes compared with control animals, after an 18-month
recovery period (Bernert et al., 1983).
Table 93. Interactions of PBBs with drugs and toxicants
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Enhancement of toxicity
Rat (Sprague- FM BP-6 (20 mg/kg in diet mirex and related aggravation of histological changes due to Chu et al.
Dawley) M feed for 28 days) compounds (Kepone, PBB (additive rather than potentiative) (1980)
photomirex)
Rat (Sprague- FM BP-6 (100 mg/kg in diet carbon tetrachloride increase in CCl4-induced lethality and 48-h Kluwe et al.
Dawley) M feed for 20 days) (CCl4) (single ip dose growth retardation; increase in severity of (1982)
of 0.03-2 ml/kg body liver damage and renal tubular functional
weight)b impairment
Mouse (NMRI) FM BP-6 (single intraperitoneal bromobenzene (single decrease in bromobenzene LT50c Roes et al.
F dose of 150 mg/kg ip dose of 3150 mg/kg (1977)
body weight) body weight)b
Mouse FM BP-6 (1, 20, in diet chloroform (CHCl3) increase in CHCl3-induced lethality (96-h Kluwe et al.
(ICR) M 25, or 100 mg/kg (single ip dose of LD50; at 100 mg PBB/kg feed, P < 0.05); (1978)
feed for 14-28 various higher susceptibility to CHCl3-induced
days) concentrations)b renal and hepatic damage
Mouse (C57 FM (100 mg/kg in diet chloroform (CHCl3) enhancement of CHCl3 hepatoxicity (both Ahmadizadeh
BL/6J) and feed for 28 days) (single ip doses of strains); enhancement of nephrotoxicity et al. (1984)
(DBA/2J) M 0.025-0.25 ml/kg (only in C57 strain)
body weight)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Mouse FM BP-6 (1, 20, in diet carbon tetrachloride increase in CCl4-induced lethality (96-h Kluwe et al.
(ICR) M 25, or 100 mg/kg (CCl4) (single ip LD50; at 20 and 100 mg PBB/kg feed, (1978, 1979)
feed for 14-28 dose of various P < 0.05); higher susceptibility to
days) concentrations)b CCl4-induced renal and hepatic damage
(e.g.: decrease in renal PAH accumulation
after 0.125 ml CCl4/kg body weight)
Mouse FM BP-6 (1, 20, in diet trichloroethylene (TRI) potentiation of TRI-induced renal
(ICR) M 25, or 100 mg/kg (single ip dose of dysfunction (decrease in renal PAH
feed for 14-28 1 ml/kg body weight)b accumulation)
days)
FM BP-6 (1, 20, in diet 1,1,2-trichloroethane potentiation of TRI-induced renal
25, or 100 mg/kg (TCE) (single ip dose dysfunction (decrease in renal PAH
feed for 14-28 of 0.15 ml/kg body accumulation)
days) weight)b
Japanese FM BP-6 (single gavage pentobarbital (single increased mortality during anaesthesia Cecil et al.
quail dose of 100 mg/kg im dose of 50 (M) or when the pentobarbital was administered (1975)
body weight) 60 (F) mg/kg body 2 h after PBB dosing; reduction in
weight) pentobarbital sleeping times 48 h
after PBB dosing
FM BP-6 (300 mg/kg in diet pentobarbital (single reduction in pentobarbital sleeping
feed for 3 days) im dose of 50 (M) or times
60 (F) mg/kg body
weight)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Miscellaneous effects
Rat (Sprague- FM BP-6 (single intraperitoneal N-methylnicotinamide no effect on uptake of NMN Evers et al.
Dawley) F dose of 130-165 (NMN) (incubation of (1977)
mg/kg body renal cortical slices
weight) with 6 x 10-6 mol/litre
[14C]-NMN)
p-aminohippuric acid elevated uptake of PAH (significant
(PAH) (incubation of only at 32 days after PBB dosing)
renal cortical slices
with 7.4 x 10-5 mol
per litre PAH)
Rat (Sprague- FM BP-6 (direct in vitro N-methylnicotinamide no effect on uptake of NMN Evers et al.
Dawley) F exposure of renal (NMN) (incubation with (1977)
cortical slices 6 x 10-6 mol/litre
from untreated [14]-NMN)
animals to animals
to 1 x 10-3
mol/litre or 1 x
10-6 mol/litre)
in vitro p-aminohippuric acid no effect on uptake of PAH
(PAH) (incubation with
7.4 x 10-5 mol/litre
PAH)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Rat (Sprague- FM BP-6 (50 and pre- and/or ouabain (single iv enhanced hepatic uptake and transport Cagen & Gibson
Dawley) 100 mg/kg feed postnatal dose of 1 mg/kg body of ouabain from plasma into bile in (1977a,b,
in mother's diet) weight)b 15-day-old rats 1978); Cagen
et al. (1977)
postnatal ouabain (single ip elevated 24-h LD50 values in Cagen & Gibson
doses)b 15-day-old rats (1977a)
Rat (Sprague- FM BP-6 (single intraperitoneal ouabain (in vitro decrease in steady-state concentration Eaton &
Dawley) dose of 200 mg/kg 150 µmol in cell of ouabain; tendency to increased Klaassen
body weight; suspension) rate of efflux (1979)
isolation of
hepatocytes: 3
days after dosing)
procaine amide increased rate of efflux
ethobromide (PAEB)
Rat (Sprague- FM BP-6 (100 mg/kg in diet sulfobromophtalein lower plasma concentrations of BSP; Cagen & Gibson
Dawley) feed for 2 weeks) (BSP) (single iv dose increased biliary excretion of BSP and (1978)
of 120 mg/kg body conjugated BSP
weight)b
Rat (Fischer FM BP-6 (100 mg/kg in diet cephaloridine (single decrease in cephaloridine nephrotoxicity Kuo & Hook
344) M, F feed for 10 days) ip dose of 1000-2000 (1982)
mg/kg body weight)b
Rat (Fischer FM BP-6 (doses of gavage p-aminophenol (PAP) marked reduction in nephrotoxicity Newton et al.
344) M 90 mg/kg body (100 or 200 mg/kg produced by PAP (1982b)
weight per day body weight)b
for 2 days)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
Rat (Fischer FM BP-6 (doses of gavage N-acetyl-p- accelerated excretion of mercapturic Newton et al.
344) 90 mg/kg body aminophenol (APAP) acid; enhanced ability of APAP to (1982c)
(isolated weight per day (3 x 10-8 mol/litre deplete glutathione
perfused for 2 days) in the perfusate)b
kidney)
Rat (Sprague- FM BP-6 (single intraperitoneal aflatoxin B1 (64 nmol increase in in vitro metabolism of Shepherd et al.
Dawley) M dose of 575 mg/kg per 5 ml incubation aflatoxin B1 (to aflatoxin M1) (1984)
(isolated body weight on mixture)
liver day 1; isolation
microsomes) of microsomes
on day 4)
Rat (Wistar) several PBBs (2 intraperitoneal aflatoxin B1 (64 nmol Halvorson
M (isolated doses of 150 per 5 ml incubation et al. (1985)
liver µmol/kg body mixture)
microsomes) weight on days 1
and 3; isolation
of microsomes on
day 6):
FM BP-6 increase in in vitro metabolism of
aflatoxin B1 (to aflatoxin M1)
2,2',4,5,5'-penta- increase in in vitro metabolism of
bromobiphenyl aflatoxin B1 (to aflatoxin Q1)
3,3',4,4'-tetra- increase in in vitro metabolism of
bromobiphenyl aflatoxin B1 (to aflatoxin M1)
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
2,3,4,4'5-penta- increase in in vitro metabolism of
bromobphenyl aflatoxin B1 to aflatoxin M1);
decrease in metabolism to aflatoxin Q1
Mouse (Swiss- FM BP-6 (100, 200 in diet ouabain (single iv lower plasma ouabain concentrations Cagen et al.
Webster) F mg/kg feed for dose of 0.1 mg/kg no enhanced capacity for ouabain (1977); Cagen
2 weeks) body weight)b excretion & Gibson
(1978)
ouabain (diverse no effect on ouabain 24-h LD50 values Cagen et al.
single doses) (1977)
Mouse (Swiss- FM BP-6 (50 mg/kg pre- and/or ouabain (single iv enhanced disappearance of ouabain from Cagen & Gibson
Webster) feed in mother's postnatal dose of 1 mg/kg the plasma; increase in ouabain excretion (1977a,b,
diet) body weight)b in 15-day-old mice 1978)
ouabain (ip dose) no effect on ouabain 24-h LD50 values Cagen & Gibson
(1977a,b)
Mouse (Swiss- FM BP-6 (100, 150, in diet indocyanine green enhanced initial disappearance of Cagen et al.
Webster) 200 mg/kg feed (ICG) (single iv dose ICG from plasma (correlated with (1977)
for 2 weeks) of 40 mg/kg body higher hepatic ICG content)
weight)b
Mouse FM BP-6 (100 mg/kg in diet ethylene dibromide decrease in renal and hepatic NPS Kluwe et al.
(ICR) M feed for 18 days) (EDB) (single ip dose (non-protein sulfhydryl)-depleting (1981)
of 100 mg/kg body effects of EDP
weight)b
Table 93 (contd).
Species PBB (dosage Route of PBB Drug/toxicant Observed effects after References
and sexa regimen) administration (dose) combined treatmentd
(diverse single no significant effect on LD50 (220 mg/kg Kluwe et al.
ip doses) body weight versus 250 mg/kg in control) (1981)
Mouse FM BP-6 (100 mg/kg in diet 1,2-dibromo-3-chloro- decrease in renal and hepatic NPS Kluwe et al.
(ICR) M feed for 18 days) propane (DBCP) (single (non-protein sulfhydryl)-depleting (1981)
ip dose of 100 mg/kg effects of DBCP
body weight)b
Mouse FM BP-6 (single not chloroform (CHCl3) no significant effect on GPT or OCT Plaa &
M dose of 500 mg/kg specified (single oral dose of activity Hewitt (1982)
body weight) 0.1 ml/kg body
weight)b
a F = female; M = male.
b Administration after PBB treatment.
c LT50 = median time to death.
d GPT = Glutamic-pyruvic transaminase; OCT = ornithine carbamyl transferase; PAH = p-aminohippurate.
Rats (Sprague-Dawley, female) treated with FM BP-6 (100 mg/kg
diet for up to 140 days) had lower hepatic vitamin A and higher
kidney vitamin A levels than controls, but showed an increase in
serum retinol (Jensen & Zile, 1988).
Short-term feeding of 3,3',4,4',5,5'-hexabromobiphenyl (1 mg/kg
diet for 140 days) to rats (Sprague-Dawley, female) caused a severe
decrease (approximately 20-fold) in hepatic retinol and retinyl
esters and a 6-7-fold increase in retinol and retinyl esters in the
kidneys, while serum concentrations of retinol were unaffected by
PBB feeding (Jensen et al., 1987). Examination of liver enzymes in
these rats revealed reductions of 50% and 63% of acetyl-CoA-retinol
acetyltransferase and retinyl palmitate hydrolase, respectively
(Jensen et al., 1987). Consistent with the previous studies, there
was an increased accumulation of retinol and retinyl esters in the
kidneys of rats given a single oral dose of
3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight) as well as a
decrease in liver retinyl ester pools (Zile et al., 1989).
In studies using radioactive retinyl acetate, a two-fold
increase in the elimination of vitamin A metabolites in the urine
and faeces was observed in rats (Sprague-Dawley, male) 12 h-8 days
after a single anorectic, oral dose of
3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight). The
potential effect of the PBB on the absorption of vitamin A was
excluded by the experimental design in this study (Cullum & Zile,
1985). Similarly, long-term, dietary administration of
3,3',4,4',5,5'-hexabromobiphenyl (1 mg/kg diet for 140 days)
resulted in a greatly increased faecal and urinary elimination of
radioactivity, when rats (Sprague-Dawley, female) were given a
physiological dose of [11-3H]retinyl acetate (Jensen et al.,
1987).
Changes in vitamin A metabolite patterns were found in tissues
(liver, kidney, small intestinal mucosa) of rats after a single oral
dose of 3,3',4,4',5,5'-hexabromobiphenyl (2 mg/kg body weight),
which produced a shift of vitamin A metabolism towards the more
polar forms of vitamin A, such as retinoic acid, its oxidation and
conjugation products (retinoyl glucuronide), and polar retinoid
metabolites (Zile et al., 1989).
The influence of the PBB on the vitamin A balance was long
lasting. As seen in Table 94, in a multigeneration study, FM BP-6
produced a decrease in hepatic vitamin A concentration, even in the
second generation of offspring (28 days of age) of rats when PBB was
fed (100 mg/kg diet) only during the first pregnancy (day 8) until
28 days post partum. At this time, all offspring (F1) were weaned
onto a control diet, allowed to mature sexually, and bred with
litter-mates to produce the F2-generation (McCormack et al.,
1981).
Table 94. Reduction of hepatic vitamin A content in the rat by various PBBs (single congeners and commercial mixtures)
Compound Strain/sex Dose and route of Exposure period/ Reduction in hepatic References
administration observation/period vitamin A
concentration (%)
2,2',4,4',5,5'- Sprague-Dawley 100 mg/kg diet 30 days 28 Akoso et al.
hexabromobiphenyl (male) (1982a)
2,3',4,4',5,5'- Sprague-Dawley 100 mg/kg diet 30 days 57
hexabromobiphenyl (male)
3,3',4,4',5,5'- Sprague-Dawley 10 mg/kg diet 30 days 59
hexabromobiphenyl (male)
3,3',4,4',5,5'- Sprague-Dawley 1 mg/kg diet 140 days 95 Jensen et al.
hexabromobiphenyl (female) (1987)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg diet 30 days 72 Akoso et al.
(male) (1982a)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg diet 140 days 92 Jensen & Zile
(female) (1988)
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg in perinatal: day 8 of pregnancy approximately McCormack et al.
(female) mother's diet until 4, 8, or 14 weeks of 50 (1982b)
age
FireMaster(R) BP-6 Sprague-Dawley 100 mg/kg in F0: perinatal: day 8 of 57 McCormack et al.
(female) mother's diet pregnancy until 28 days (1981)
post-partum/F1 (28 days of
age)
Table 94 (contd).
Compound Strain/sex Dose and route of Exposure period/ Reduction in hepatic References
administration observation/period vitamin A
concentration (%)
F0: perinatal: day 8 of 28
pregnancy until 28 days
post-partum/F2 (28 days of
age)
a F0 = parental generation; F1 = first filial generation; F2 = second filial generation.
The influence of dietary levels of vitamin A on the toxicity of
PBB has been studied. Vitamin A supplementation (up to 30 000 IU)
provided only partial protection against decreases in body weight
gain and thymic weight and against hyperplasia of the common bile
duct in rats intoxicated with 100 mg FM BP-6/kg diet (Darjono
et al., 1983). High dietary levels of vitamin A (200 000 IU) also
had some inhibitory effect on carcinogenesis, i.e., on the promotion
of hepatic-altered foci by 3,3',4,4',5,5'- hexabromobiphenyl in
initiated rats (Rezabek et al., 1989).
The mechanism by which PBBs influence vitamin A homeosta sis is
not fully understood. Conflicting results may be because of
analytical problems and biological variables. However, according to
Zile et al. (1989), it is very likely that polyhalogenated aromatic
hydrocarbons affect specific enzymes involved in the regulation of
vitamin A storage and in the vitamin A metabolic pathway. Others
have suggested that PCBs and related compounds may interfere with
Vitamin A transport in the serum by inhibiting the formation of the
serum transport protein complex carrying retinol and thyroxin
(Brouwer & van den Berg, 1986).
8.8.5 Porphyria
Hexabromobiphenyl is able to produce chemical porphyria in
Japanese quail (Strik, 1973b), rats, and mice (Gupta et al., 1983a;
Hill, 1985). Following studies on adult Japanese quail, which were
orally dosed with gelatin capsules or fed diet containing up to
1000 mg/kg body weight FireMaster(R) BP-6, Strik (1978) stated
that PBB porphyria was preceded by liver and kidney damage.
Accumulation of porphyrins in the liver is not solely due to an
increase in delta-amino-levulinic acid synthetase activity, but
rather to drug enzyme induction and the formation of a reactive
intermediate that causes centrilobular liver damage and ultimately
porphyria. The hepatic mitochondria decrease in number and are
damaged (elevated serum glutamic acid dehydrogenase); the
profilerated endoplasmic reticulum, including the haemoprotein
P-450, is no longer capable of normal activity; uroporphyrinogen
decarboxylase activity is reduced to zero; renal porphyrins
accumulate and are excreted in the liver and via the bile.
In tests with chick-embryo liver cell cultures, Debets et al.
(1980) showed that pretreatment with inducers of the drug-
metabolizing enzyme system markedly stimulated the accumulation of
porphyrins, after exposure to FireMaster(R) BP-6. Inhibition of
hepatic drug metabolism or the addition of compounds, known to trap
electrophiles or radicals, protected against the porphyrinogenic
action of FireMaster(R) BP-6 in vitro.
In the same test system, a DeBB solution of 10 µg/ml medium did
not show any porphyrogenic potential with, or without, pretreatment
with ß-naphthoflavone (3 µg/ml medium) for 20 h (Koster et al.,
1980). Gupta et al. (1983a) found dose-related, elevated hepatic
prophyrin levels in Fischer 344/N rats and B6C3F1 mice, orally
dosed with 0.1-10.0 mg FireMaster(R) BP-6/kg body weight (5
steps), primarily in females.
Hill (1985) studied the urinary porphyrin pattern by ion-pair
chromatography in female Sherman rats given 0 or 1 g PBB/kg body
weight. The route of administration was not stated. Chronic hepatic
porphyria became evident about 55-60 days after dosing, as indicated
by the large increase in uroporphyrin and heptacarboxylporphyrin and
the corresponding ratios of these porphyrins to coproporphyrin.
Between 85 and 100 days, chronic hepatic porphyria developed into
its most severe form (porphyria cutanea tarda).
Summarizing all reported facts on porphyrin metabolism, Hill
(1985) pointed out that chronic hepatic porphyria is characterized
as a membrane disease in which there is damage to the membranes of
the cell walls of the hepatocytes and organelles (i.e., mito
chondria, endoplasmic reticulum) within the liver cell. The cause of
damage is unknown. There is some evidence that PBBs produce changes
in lipid metabolism, which in turn alters membrane structure
(Bernert et al., 1983). This change in membrane structure may cause
a change in membrane permeability (damage) so that porphyrins are
excreted in the bile capillaries and the intercellular substance. In
addition to these changes, PBBs, or their metabolites, cause the
induction of delta-aminolevulinic acid synthetase and the inhibition
of the enzyme uroporphyrinogen decarboxylase. The changes in enzyme
activities produce a build-up of uroporphyrinogen and
heptacarboxylporphyrinogen, and these precursors are excreted in the
urine, where they are easily oxidized to uroporphyrin and
heptacarboxylporphyrin.
A marked sex difference in the development of uroporphyria
occurred after administration to 10-week-old F344/N rats of 0.005%
FireMaster(R) BP-6 added to the diet. Thus, the propensity of
female rats to develop uroporphyria appeared to be a general
response to this class of halogenated chemicals. Liver to body
weight ratio, liver porphyrins, and the activity of a
uroporphyrinogen decarboxylase inhibitor were significantly greater
in both sexes compared with appropriate controls. Comparing treated
males and females, the liver to body weight ratio and
uroporphyrinogen decarboxylase inhibitor activity were significantly
greater in males whereas the liver porphyrin content was greater in
females. Levels of total cytochrome P-450 and pentoxyresorufin and
benzyloxyresorufin dealkylase activities (associated with cytochrome
P450IIB1) were greater in microsomes from control, and PBB-treated
male rats compared with females. In contrast, ethoxyresorufin
deethylase activity (associated with cytochrome P450 IA1) was
significantly greater in females (Smith et al., 1990a).
Iron potentiated the development of uroporphyria after oral
exposure to FireMaster(R) BP-6 for 2 months (dose not stated) in 7
to 10-week-old, male Ah-responsive C57Bl/10ScSn mice (Smith et al.,
1990b).
8.8.6 Miscellaneous effects
Alterations in liver microsomal membrane lipid composition,
increased peroxidation activities, and an increase in membrane
fluidity were found after a single oral dose of 500 mg
FireMaster(R) BP-6/kg body weight during short- and long-term
observations (Bernert et al., 1983; Bernert & Groce, 1984).
PBBs were found to inhibit rabbit muscle glycogen phosphorylase
(1,4-alpha-D-glucan: orthophosphate alpha-D-glucosyltransferase, EC
2.4.1.1), an enzyme that catalyzes the breakdown of glycogen to
glucose 1-phosphate. The enzyme exists in two metabolically
significant forms, phosphorylase a and b.
2,2',4,4',5,5'-Hexabromobiphenyl and FireMaster(R) BP-6 were
strong inhibitors of phosphorylase b (92% and 88% inhibition at
60 µmol/litre, respectively; Ki = 15 µmol/litre), while exhibiting
little or no inhibition of phosphorylase a (Mead et al., 1982).
The activity of the enzyme uridine 5'-diphosphoglucuronyl-
transferase (UDP-GT), responsible for the conjugation of a wide
variety of substrates, was significantly increased in microsomes
prepared from rats ip injected with 3,3',4'4',5,5'-hexabromobiphenyl
at 20 mg/kg body weight (Spear et al., 1990).
The influence of FireMaster(R) BP-6 on glutathione peroxidase
activity, which is present in most mammalian species, was studied in
the rat (liver cytosol). This enzyme is represented, on the one
hand, by a selenium-containing enzyme, glutathione peroxidase (E.C.
1.11.1.9), which is able to reduce hydrogen peroxide to water and
organic hydroperoxides to the corresponding hydroxy compounds, and,
on the other hand, by certain isozymes of glutathione transferase
(E.C. 2.5.1.18). The activity of the selenium-dependent glutathione
peroxidase was decreased to about 50% of control values on day 16
following the ip administration of FireMaster(R) BP-6 (500 mg/kg
body weight). Inversely, there was a potent induction of glutathione
transferases during the 16-day period (Schramm et al., 1985).
Adenylate cyclase [ATP pyrophosphatase lyase (cyclizing), EC
4.6.1.1] catalyzes the conversion of adenosine triphosphate (ATP) to
adenosine 3',5'-cyclic monophosphate (cyclic AMP), which acts as a
central regulator of several diverse cell activities. In preliminary
experiments, the in vitro effect of FireMaster(R) BP-6 on
adenylate cyclase activity in the plasma membranes of rat lung
alveoli was determined. At concentrations of 10 µg/ml, the
FireMaster(R) mixture stimulated the basal adenylate cyclase
activity of plasma membranes 2- to 2.5-fold (Sidhu & Michelakis,
1978). The authors discuss the possible immunological relevance of
this observation.
8.9 Effects on intercellular communication
PBBs show a significant epigenetic activity. The
FireMaster(R) mixture and some of its major components were found
to be capable of inhibiting intercellular communication, measured by
metabolic cooperation between HGPRT+ and HGPRT- cells in culture
(Table 95). This inhibition occurred at non-cytotoxic
concentrations. In contrast to FireMaster(R) and to its major
constituents BB 153 and BB 180, 3,3',4,4',5,5'-hexabromobiphenyl and
3,3',4,4'-tetrabromobiphenyl did not interrupt cell-cell
communication at noncytotoxic concentrations. However, both
congeners were markedly cytotoxic (Table 95). Congeners with
intermediate toxicity such as 2,3'4'4',5-PeBB (BB 118),
3,3',4,4',5'- PeBB (BB 127), and 2,3',4,4',5,5' HxBB (BB 167), were
also shown to interfere with cell-cell communication at noncytotoxic
concentrations. Both the cytotoxicity and the metabolic
cooperation-inhibiting properties of PBB congeners seem to be
related to their structure, i.e., presence or lack of ortho-
substitution (Tsushimoto et al., 1982; Kavanagh et al., 1987; see
also section 8.7.2.2).
Recently, the ability of FireMaster(R) BP-6 to inhibit gap
junction-mediated intercellular communication has been confirmed by
the FRAP assay, using cultured rat liver epithelial cells (Rezabek
et al., 1988). This assay, which has been described by Wade et al.
(1986), evaluated the inhibition of fluorescence redistribution
after photobleaching (FRAP), which occurs between cells loaded with
a fluorescent dye.
Results obtained by a similar new method, the scrape-
loading/dye-transfer (SL/DT) technique (El-Fouly et al., 1987)
confirmed the inhibitory potency of 2,2',4,4',5,5'-hexabromobiphenyl
(Evans et al., 1988; Table 95).
Several different cell types (human fibroblasts, rat kidney
epithelial cells, rat liver oval cells, rat Leydig cells, rat glial
cells, mouse keratinocytes) differ in their response to chemicals
that alter gap junctional intercellular communication. After
exposure to FireMaster(R) BP-6 (20 µg/ml), intercellular
communication was inhibited to different extents in three (WB rat
liver oval cells, RG-1 rat glial cells, JB-6 mouse keratinocytes)
out of the six cell types tested, rat liver oval cell being the most
sensitive cell (Bombick, 1990).
Table 95. Inhibition of intercellular communication by PBBs: results of in vitro assays testing metabolic cooperation
between 6-thioguanine-sensitive (HGPRT+) and- resistant (HGPRT-) cells or testing dye transfera
PBBa Cells in culture Inhibition Remark References
Yes No
FM BP-6 Chinese hamster x non-lethal range of the Trosko et al. (1981)
V79 lung cells chemical
FM FF-1 rat liver cells x no cytotoxicity mentioned Williams et al. (1984)
FM BP-6 human teratocarcinoma x only slight effect on Kavanagh et al. (1987)
cells cell survival
FM BP-6 rat liver epithelial cells x at non-toxic FM concentration Rezabek et al. (1988)
(WB-F 344)
3,3',4,4',-tetrabromobiphenyl human teratocarcinoma x moderately cytotoxic Kavanagh et al. (1987)
(BB 77) cells
2,3',4,4',5- Chinese hamster x inhibition before Tsushimoto et al.
pentabromobiphenyl (BB 118) V79 cells cytotoxicity occurs (1982)
3,3'4,5,5'-pentabromobiphenyl Chinese hamster x slight inhibition Tsushimoto et al.
(BB 127) V79 cells before cytotoxicity (1982)
2,2',4,4',5,5'- Chinese hamster x relatively nontoxic
hexabromobiphenyl (BB 153) V79 cells
rat liver epithelial x x at non-cytotoxic Evans et al.
cells (WB-F344) concentrations (1988)
Table 95 (contd).
PBBa Cells in culture Inhibition Remark References
Yes No
human teratocarcinoma x only slight effect Kavanagh et al.
cells on cell survival (1987)
2,3',4,4',5,5'- Chinese hamster x inhibition before Tsushimoto et al.
hexabromobiphenyl (BB 167) V79 cells cytotoxicity occurs (1982)
3,3',4,4',5,5'- Chinese hamster x highly cytotoxic Tsushimoto et al.
hexabromobiphenyl (BB 169) V79 cells (1982)
human teratocarcinoma x highly cytotoxic Kavanagh et al.
cells (1987)
2,2',3,4,4',5,5'- Chinese hamster x relatively non-cytotoxic Tsushimoto et al.
heptabromobiphenyl (BB 180) V79 cells (1982)
2,2',3,3',4,4',5,5'- Chinese hamster x relatively non-cytotoxic
octabromobiphenyl V79 cells
(BB 194)
a HGPRT = Hypoxanthine-guanine phosphoribosyl transferase locus.
b Purity of PBB congeners tested: > 99%; FM = FireMaster.
8.10 Immunotoxicity
The effects of PBBs (commercial mixtures and individual
congeners) on the weight and histology of the thymus, bursa of
Fabricius, and spleen have been reviewed in sections 8.2 (single and
short-term exposures: 8.2.1.3, commercial mixtures; 8.2.2,
congeners) and 8.4 (long-term exposures). In summary, atrophy of
thymus was a frequent observation following PBB exposure.
Other indicators of a suppressed immune function have been
compiled in Table 96. These data refer only to the FireMaster(R)-
mixture, because information on OcBB, DeBB, or individual PBB
congeners (with the exception of 3,3',4,4'-tetrabromobiphenyl) is
lacking.
In addition to the thymus or spleen, other lymphoid tissues
were affected by PBB, e.g., bone marrow and the lymph nodes of dogs
(Farber et al., 1978; Table 96).
Serum immunoglobulin levels in mice were changed following
short-or long-term exposure to FireMaster(R) (Luster et al., 1978,
1980; Loose et al., 1981). Suppression of antibody response to sheep
erythrocytes (or bovine gamma globulin) was reported after the
short-term exposure of mice (Fraker & Aust, 1978; Luster et al.,
1978; Fraker, 1980; Loose et al., 1981) and after a six-month
exposure of rats (Luster et al., 1980). Conditions of study are
summarized in Table 96.
Interestingly, mice showed no response in the 6-month study
(Luster et al., 1980). A decrease in antibody titres to tetanus
toxoid was observed in guinea-pigs (Vos & van Genderen, 1974; Table
96).
Although mortality rates following infection with Listeria
monocytogenes were not affected by FireMaster(R) exposure in
mice exposed long-term, an increased susceptibility to infection
with Listeria was suggested, because a decrease in time to death
occurred (Luster et al., 1980; Table 96). No effects on mean
survival time were observed in mice fed 5 or 167 mg/kg feed for 3 or
6 weeks and then challenged by Plasmodium berghei (murine malaria)
infection (Mudzinski et al., 1979; Loose et al., 1981).
An increased susceptibility to endotoxin was found in mice
after short-term (Mudzinski et al., 1979; Loose et al., 1981) or
perinatal (Luster et al., 1980) exposure to FireMaster(R) (Table
96). Mice with long-term exposure did not show endotoxin sensitivity
(Luster et al., 1980). Of the individual PBB congeners, 3,3'4,4'-
tetrabromobiphenyl was found to increase sensitivity to endotoxin
(lipopolysaccharide from Escherichia coli) 1-2 days after
administration (single intraperitoneal dose of 150 µmol/kg body
weight) to rats (Shedlofsky et al., 1991).
Table 96. Immunotoxicity of FireMaster(R)a
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 rat gavage 22 doses of 0.03, 30 days depression of T-cell responsiveness to Luster
(lot FF 1312 FT) (Fischer) 0.3, 3, or 30 mg/kg mitogens (PHA: overall dose response: et al.
(M) body weight P < 0.01; 3 and 30 mg/kg per day: (1978)
P < 0.05; Con A: overall dose response:
P < 0.1; 30 mg/kg per day; P < 0.05)
FM FF-1 rat gavage 122 doses of 0.1, 6 months depression of both B-(10 mg/kg per day) Luster
(lot FF 1312 FT) (Fischer) 0.3, 1, 3, or 10 and T-(1,3, or 10 mg/kg per day) cell et al.
(F) mg/kg body weight mitogenic (PHA, Con A, PWM) and (1980)
allo-geneic responses (P < 0.05 or 0.01);
decreased antibody responses to bovine
globulin (10 mg/kg per day; P < 0.1);
suppressed delayed hypersensitivity
reactions (3 and 10 mg/kg per day;
P < 0.05)
FM FF-1 mouse gavage 22 doses of 0.03, 30 days depression of T- and B-cell responsiveness Luster
(lot FF 1312 FT) (B6C3F1) 0.3, 3, or 30 mg/kg to mitogens PHA, Con A, and LPS (overall et al.
(F) body weight dose response: P < 0.01; 3 mg/kg per day: (1978)
PHA, Con A and 30 mg/kg per day: PHA,
Con A, LPS: P < 0.10 or 0.01); decreased
antibody responses to SRBC (30 mg/kg per
day; 27% reduction): decrease in serum IgM
and IgG2 levels (30 mg/kg per day;
P < 0.01 and 0.10, respectively)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 mouse gavage 122 doses of 0.1, 6 months enhanced number of bone marrow colony Luster
(lot FF 1312 FT) (B6C3F1) 0.3, 1, 3, or 10 forming units (only F at 1 and 10 mg/kg et al.
(F,M) mg/kg body weight body weight per day; P < 0.01); decrease (1980)
per day in serum IgG, IgM, and IgA levels (10 mg/kg
body weight per day P < 0.01 or 0.05);
increase in serum IgG (1 mg/kg body weight
per day; P < 0.01) and IgA (0.3 mg/kg body
weight per day; P < 0.1); depression of B-
and T-cell responisiveness to mitogens
(PHA, Con A, LPS) at 10 mg/kg body weight
per day (P < 0.05): increased
susceptibility to infection with Listeria
mono-cytogenes (10 mg/kg body weight
per day)
FM FF-1 mouse perinatal maternal doses of gestation enhanced number of bone marrow colony Luster
(lot FF-1312 FT) (B6C3F1 0.3, 1, 3, or 10 day 0 until forming units (F: 1 mg/kg body weight (1980)
(F,M) mg/kg body weight weaning (on per day; P < 0.05): increased
per day alternate susceptibility to endotoxin (LPS,
day) E. coli) (marginal dose response
P = 0.06)
FM BP-6 mouse in diet 1, 10, 100 mg/kg 30 days reduced antibody responses to SRBC Fraker &
(BALB/c) feed (10, 100 mg/kg: P < 0.001) Aust (1978)
1000 mg/kg feed 14 days survivors incapable of mounting an Fraker
antibody-mediated response to SRBC (1980)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM FF-1 mouse in diet 167 mg/kg feed 3 or 6 increase in endotoxin (LPS; Loose
(lot No. 7042) (BALB/cByJ) weeks Salmonella typhosa) sensitivity (P < 0.05); et al.
(M) reduced primary antibody reaction to (1981)
SRBC (only at 3 weeks)
5 mg/kg feed 3 or 6 reduced serum IgM levels (at 3 and 6
weeks weeks; (P < 0.05)
FM BP-6 guinea-pig in diet 10, 50 mg/kg feed 45 days reduction in antibody titres to tetanus Vos & van
(F) toxoid (P < 0.025) P < 0.05); reduced serum Genderen
IgG levels (at 6 weeks, P < 0.05) reduced (1974)
serum IgM levels (at 3 and 6 weeks;
P < 0.05)
FM BP-6 pig in diet 100, 200 mg/kg last half decreased responses of peripheral blood Howard
(sows) feed of lymphocytes to mitogen (PHA, PWM) et al.
gestation stimulation (200 mg/kg; P < 0.05) (1980)
and 4
weeks of
lactation
(12 weeks)
FM BP-6 pig perinatal 100, 200 mg/kg last half of normal mitogen (PHA, PWM) responses at
(piglets) feed gestation birth; decreased mitogen responses at
and 4 weeks 4 weeks of age (PWM = 200 mg/kg;
of lactation P < 0.002)
(12 weeks)
Table 96 (cont'd).
PBBb Species Route Dosing Period of Observed effectsd Reference
(strain/ exposure
sex)c
FM BP-6 dog gavage 0.06-4 mg/kg 61 days degenerating lymphocytes in blood smears Farber
body weight (all levels); depletion of lymphocytes in et al.
per day the lymph nodes, (4 mg/kg); reduced (1978)
erythro-poiesis in bone marrow (4 mg/kg);
reduction in IgG-containing lymphocytes in
popliteal lymph nodes (4 mg/kg)
a Exclusive of effects on the weight and histology of thymus and spleen, which are noted in another section.
b FM = FireMaster(R).
c M = Male; F = Female.
d Con A = Concanavalin A; LPS = bacterial lipolysaccharide; PHA = phytohemagglutinin; PWM = pokeweed mitogen;
SRBC = sheep red blood cells.
FireMaster(R) depressed lymphoproliferative (B- and/or
T-cell) responsiveness to mitogens in rats (Luster et al., 1978,
1980), mice (Luster et al., 1978, 1980), and pigs (Howard et al.,
1980). Response to mitogens was variable among the species tested
(Table 96), e.g., rats exposed long-term were more sensitive (dose
required: < 10 mg/kg body weight per day) than mice (dose required
= 10 mg/kg body weight per day) in the same study (Luster et al.,
1980).
Delayed hypersensitivity reactions were depressed in adult rats
at higher doses of PBBs (Luster et al., 1980; Table 96), but they
were comparable to controls in mice, exposed perinatally and
long-term (Luster et al., 1980).
Some haematological parameters of immunological interest, e.g.,
changes in peripheral lymphocyte and leukocyte counts are reported
in sections 8.2.1.2. and 8.4.
In summary, there was a wide spectrum of immunotoxic effects of
FireMaster(R) in different animal species, but immune
dearrangement was often recorded at doses that produced other signs
of toxicity.
8.11 Neurotoxicity
Behavioural and neurological parameters have been examined in
rodents and rhesus monkeys treated with the commercial
FireMaster(R) mixture or with its main component, 2,2',4,4',5,5'-
hexabromobiphenyl (BB 153).
8.11.1 Exposure of adult animals
Adult rats (Fischer 344/N) and mice (B6C3F1) of both
sexes were exposed to oral doses of 0.03-30 mg FM FF-1/kg body
weight per day or 0.168-16.8 mg 2,2',4,4',5,5'-hexabromobiphenyl per
kg body weight per day (5 days per week) for a total of 22 doses.
Neurobehavioural toxicity was assessed at the end of the 30-day
dosing regimen and also 30 days after cessation of dosing (Tilson
et al., 1978; Tilson & Cabe, 1978, 1979). Additionally, rats having
received 130 oral doses of 3 or 10 mg FM FF-1/kg body weight per day
(5 days per week) were tested after 6 months of dosing (Tilson &
Cabe, 1979). Mainly at the higher doses, FM FF-1, and, to a much
lesser extent, BB 153 led to neuromuscular dysfunction, such as
decreased motor activity, depressed neuromuscular reflexes, and
impaired forelimb grip strength. Rats were generally more affected
than mice. Visual placement responses were also decreased in male
rats and mice by FM FF-1 and BB 153. Hypothermia (decreased rectal
temperature) was caused by FM FF-1 in mice. In general, rats tended
to remain the same or get worse during the 30 days of no dosing,
while mice tended to improve. In another study (Geller et al.,
1979), the influence of PBB on cognitive function was evaluated.
Male rats (Sprague-Dawley) received "hexabrominated biphenyl" (not
specified) at 1 mg/kg body weight per day (5 days per week) for a
total of 20 doses during a one-month period and were then trained
for a simple auditory discrimination task. PBB-treated animals did
not significantly differ from controls with respect to accuracy on
the discrimination task. However, throughout 24 weeks of
discrimination training, PBB rats made many more extra responses and
showed longer response times (response latencies), thereby reducing
their efficiency. Upon completion of the behavioural study, a group
of rats, dosed concurrently with the behavioural animals, was
sacrificed immediately after the end of dosing, and the brains were
used to prepare both intact synaptosomes and synaptic plasma
membranes (Gause et al., 1979). Both calcium binding to synaptic
plasma membranes and calcium uptake by intact synaptosomes was
significantly reduced in the brains of rats administered 1 mg PBB/kg
body weight per day. From these results, the authors derived that
both spontaneous and evoked transmitter release could be reduced
during PBB treatment.
8.11.2 Perinatal exposure
Functional impairment of offspring after perinatal exposure to
PBBs has been observed at PBB concentrations not causing overt
maternal toxicity. Locomotor activity was decreased in the offspring
of Swiss-Webster-mice fed FireMaster(R) (100 mg/kg feed) during
lactation (postnatal days 1-29). Increased mortality and decreased
body weight were also seen in the pups (Preache et al., 1976). Prior
to breeding, female rats (Sprague-Dawley) were dosed orally for 20
days (5 days/week) with FireMaster(R) FF-1 (0.5 or 5 mg/kg body
weight), and the male offspring were used for measurements of motor
activity (Gause et al., 1984), pain threshold (Gause et al., 1984),
operant behaviour, and response to central nervous system-active
drugs (Gause et al., 1984; Geller et al., 1985). The first two of
these four measurements started from weaning and resulted (over a 6
week period) in higher levels of activity and in changes in the pain
threshold in animals exposed to PBB. Both of the last tests were
conducted with the adult (> 75 days of age) offspring: There were
no detectable effects of PBB on the acquisition or performance of
the operant discrimination task; however, the pharmacological
challenge showed that F1 males from PBB-treated dams were less
sensitive to both phenobarbital and d-amphetamine than F1 males
from control dams. In another study, pregnant rats (Sprague-Dawley)
received oral doses of 0.2 or 2 mg FireMaster(R) BP-6/kg body
weight per day from day 6 of gestation through day 24 postpartum
(Henck & Rech, 1986). Several signs of neurobehavioural toxicity
were found in male and female offspring of dams given 2 mg
FireMaster(R) BP-6/kg per day, a dose that produced tissue levels
within the range of those measured in highly exposed Michigan
people. There were significant effects on the acquisition of
foreword locomotion, cliff avoidance, cage emergence, and open field
activity (Henck, 1986). At 6 months of age, the offspring were
tested for a series of operant responses of increasing difficulty.
It was found that the learning of various operant behavioural
patterns was impaired in a relatively subtle manner, and that both
sexes can differ in their responses (Henck, 1986; Henck & Rech,
1986).
8.12 Factors modifying toxicity, toxicity of metabolites
8.12.1 Contaminants affecting toxicity
8.12.1.1 Polybrominated naphthalenes (PBNs)
PBNs have been identified as contaminants of the commercial
FireMaster(R) mixture (at concentrations of the order of
200 mg/kg: see section 2.1.2). In structure, they resemble other
classes of halogenated aromatic hydrocarbons, such as
polychlorinated naphthalenes, polyhalogenated biphenyls,
dibenzodioxins, and dibenzofurans, and may elicit similar
qualitative effects (e.g., Kimbrough, 1980a,b). A summary of
biologic and toxicological responses reported on PBNs is given in
Tables 97 and 98. It shows that some PBNs are potent toxicants and
teratogens. PBNs were teratogenic in mice at dose levels below those
capable of producing overt maternal toxicity (Miller & Birnbaum,
1986). Compared with FireMaster(R) (consult also previous
sections), PBN mixtures were much more potent in causing adverse
effects. For example, a PBN mixture was at least 10 times more
effective than FM BP-6 in producing maximal induction of aryl
hydrocarbon hydroxylase (30 µmol/kg body weight versus 300 µmol/kg;
Robertson et al., 1981a, 1984a). Although PBNs are present only at
low levels in the FireMaster(R) mixture, some authors (Robertson
et al., 1984a; Miller & Birnbaum, 1986) believe that they may
contribute to the toxicity of FireMaster(R).
8.12.1.2 Mixed polybromo-chlorobiphenyls
At present, only a monochloropentabromobiphenyl has been
identified as a trace impurity in FireMaster(R) FF-1 (see section
2.1.2). No information is available on its toxicological properties.
However, there is one study by Andres et al. (1983) in which the
biological and toxic effects are compared of a series of laterally
substituted 3,3',4,4'-tetrahalobiphenyls containing the following
variable molecular Cl/Br ratios: Br4, Br3Cl, Br2Cl2 (two
isomers), BrCl3, and Cl4. Parameters examined included: growth
rate, effects on the thymus, and hepatic microsomal enzyme induction
in male Wistar rats, as well as enzyme induction in rat hepatoma
cells in culture and relative binding affinities to the rat
cytosolic receptor protein. Data obtained demonstrated that the
activity of these (mixed) halogenated biphenyls was enhanced with
increasing bromine (and decreasing chlorine) substitution.
Table 97. LD50 values of several polybrominated naphthalenes (PBN)
in guinea-piga,b
PBN LD50
(µg/kg body weight)
2,3,6,7-tetrabromonaphthalene 242
1,2,4,6,7-pentabromonaphthalene 200
1,2,3,4,6,7-hexabromonaphthalene 361
1,2,3,5,6,7-hexabromonaphthalene > 3610
a From: McKinney & McConnell (1982).
b Hartley strain guinea-pigs given a single oral dose (gavage) and
observed for 30 days.
8.12.2 Toxicity of metabolites
No experimental data are available on the toxicity of PBB
metabolites.
8.12.3 Toxicity of photolysis and pyrolysis products
8.12.3.1 Photolysis products
Studies of the FireMaster(R) mixture and its main component,
2,2',4,4',5,5'-hexabromobiphenyl, showed that the photolysis
products were more toxic than the original PBB. The parameters of
toxicity compared were liver and thymus weight changes, liver
histology, hepatic microsomal enzyme induction, and binding affinity
to the cytosolic receptor in rats, as well as development of
hyperkeratosis in the rabbit ear (Table 99). Probably, one or more
of the lower brominated PBBs formed by photolysis (see section
4.2.1) are responsible for the increased potency. It is believed
that the increased potency of irradiated
2,2',4,4',5,5'-hexabromobiphe nyl is due mostly to
2,3',4,4',5-pentabromobiphenyl and, because of metabolism, to a
lesser extent to 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985a). The enhanced toxicity of the photolysed FireMaster(R)
mixture may be explained similarly by increased concentrations of
2,3',4,4',5-pentabromobiphenyl and of congeners containing no ortho
bromines (Robertson et al., 1983a).
Table 98. Summary of reported biological alterations and toxic effects of polybrominated naphthalenes
Species Sexa PBNb Dosage regimenc Observed effects Reference
Pathological features
Rat M 1,2,3,4,6,7-HBN oral doses of 5 mg/kg body centrilobular hepatic accumulation of lipid Kohli et al.
weight per day for 4 days (1981)
Mouse F synthetic HBN oral doses of 0.5, 1.0, 2.5, decrease in body weight (at 7.5 and 10.0 Miller &
(C57 BL/6N) mixture (mainly 5.0, 7.5, or 10.0 mg/kg mg/kg); increase in relative liver weight Birnbaum
(pregnant) 1,2,3,4,6,7-HBN body weight per day on (at all levels); wasting, listlessness, (1986)
and 2,3,4,5,6,7- gd 6-15; s.t.: gd 18 vaginal bleeding, death (at 5-10 mg/kg)
HBN)
Rat F 2,3,6,7-TBN (not 2 daily ip doses of 0.2 increased liver weight; histological Goldstein
(Fischer) identified in mmol/kg body weight per liver changes et al. (1979)
FireMaster) day; s.t.: 3 days after
last dose
Rat M 3 synthetic single ip dose of 0.3 decrease in body weight gain; enlarged Robertson
(Wistar) PBN mixtures mmol/kg body weight livers; decreased thymuses; histological et al. (1984a)
on day 1; s.t.: day 15 changes in liver and thymus;
Hepatic microsomal enzyme induction
Rat F 2,3,6,7-TBN (not 2 ip doses of 0.2 mmol MC-type induction: approximate Goldstein
(Fischer) identified in per kg body weight per ED50 = 40 µmol/kg (18 mg/kg) et al. (1979)
FireMaster(R)) day; s.t.: 3 days (approximately 10-fold more potent than
after last dose FM FF-1)
Table 98 (contd).
Species Sexa PBNb Dosage regimenc Observed effects Reference
Rat M 3 synthetic ip doses of 15 or 150 MC-type induction Robertson
(Wistar) PBN mixtures µmol/kg body weight per (ED100 = at most 30 µmol/kg) et al. (1984a)
(5-6 bromines day on days 1 and 3;
per naphthalene) s.t.: day 6
Fetal toxicity
Mouse - synthetic HBN maternal oral doses of dose-related increases in fetal mortality Miller &
(C57 BL/6N) mixture 0.5-10 mg/kg body weight (at 5-10 mg/kg); dose-related increase in Birnbaum
per day on gd 6-15; incidence of various teratogenic effects (1986); Miller
s.t.: gd 18 (all dose levels): et al. (1985)
- kidney lesions (100% of fetuses at
1 mg/kg)d
- reduction in size of thymus and spleen
- cleft palate (4.8% of fetuses at 1 mg/kg;
98.6% of fetuses at 2.5 mg/kg)
- subcutaneous edema
- sternebral anomalies
- delayed cranial ossification
a F = female; M = male.
b PBN = polybrominated naphthalene(s); HBN = hexabrominated naphthalene(s); TBN = tetrabrominated naphthalene(s).
c gd = gestation days; s.t. = sacrifice time; ip = intraperitoneal.
d Estimated NOEL (no-observed-effect level) = 0.1-0.25 mg/kg per day.
8.12.3.2 Pyrolysis products
Recently, the toxicity of the pyrolyzed FireMaster(R) mixture
has been determined in vitro by measurements of EC50 values for
the induction of aryl hydrocarbon hydroxylase (AHH) and
ethoxyresorufin O-deethylase (EROD) in rat hepatoma H-4-II E
cells, and, in vivo by measurements of ED50 values for hepatic
microsomal AHH and EROD induction, body weight loss, and thymic
atrophy in immature male Wistar rats (Zacharewski et al., 1988).
FireMaster(R) BP-6 was pyrolyzed at 800 °C, and the residue was
extracted with toluene. Solvents used for the application of the
test material to the cell cultures and for ip injection of the
animals were DMSO and corn oil, respectively. Both the in vitro
and in vivo dose-response effects were compared with the relative
activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and expressed
as the concentrations of "2,3,7,8-TCDD equivalents".
The calculated in vitro and in vivo "2,3,7,8-TCDD equivalents"
(µg/g sample) for the six bioassays ranged between 480 and 1680 µ/g
(Table 100).
Several polybrominated dibenzofurans (PBDFs) have been
identified in the highly complex combustion mixture of
FireMaster(R) (see section 4.3.2). However, toxicity tests have
been conducted for only one PBDF congener, namely 2,3,7,8-dibenzo
furan (Moore et al., 1979b).
Generally, PBDFs may have a higher toxicity than the chloro
analogues (Poland & Knutson, 1982).
The toxicity of pyrolyzed technical octabromobiphenyl (Dow, Lot
102-7-72) has been studied in a less sophisticated manner than that
of FireMaster(R). Rats were exposed, via inhalation, to OcBB,
which had been heated at 290 °C (4 h/day, for up to 10 days) and
examined for liver damage. Atmospheric concentrations of
> 2.5 µg/litre (as Br) of brominated 290 °C pyrolysis products caused
liver enlargement; microscopic abnormalities in the liver were not
detected (Aftosmis et al., 1972b; Waritz et al., 1977).
8.13 Mechanism of toxicity including carcinogenicity
PBB and related halogenated compounds are known to elicit a
large number of different effects in animal species. However, the
underlying mechanisms of toxicity are unknown. It is likely that
several molecular mechanisms may operate (e.g., Silberhorn et al.,
1990).
Table 99. Summary of comparative toxicology data of photolysed PBBs
PBB Irradiation Parametera Effects of References
(solvent during (species) PBB irradiated PBB
irradiation)
FM BP-6 300 nm thymus weight dose-related decreases dose-related decreases; Robertson
(lot 7062) (cyclohexane) (rat, immature) significant at 75 mg/kg decreases significant at et al.
body weight 25 mg/kg body weight (1981b,c)
AHH-induction ED50 = 50 mg/kg ED50 = 9 mg/kg
(rat, immature) body weight body weight
displacement of EC50 = 300 µmol EC50 = 2 µmol Robertson
[3H] TCDD from the et al. (1981b)
Ah receptor
(rat, immature)
2,2',4,4',5,5'- sunlight hyperkeratosis no hyperkeratosis severe hyperkeratosis Patterson
hexabromobiphenyl (hexane) (rabbit ear) et al. (1981)
(BB 153)
254 nm hepatic microsomal PB-type mixed type Millis et al.
(hexane) enzyme induction (PB and MC) (1985a)
(rat, outbred)
liver weight increase increase (more than
(rat, outbred) with BB 153)
Table 99. (cont'd).
PBB Irradiation Parametera Effects of References
(solvent during (species) PBB irradiated PBB
irradiation)
liver histology hepatocyte moderate to severe
(rat, outbred) enlargement hepatocyte enlargement
a AHH = benzo[a]pyrene hydroxylase; TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
b MC = 3-methylcholanthrene; PB = phenobarbital.
Table 100. FireMaster(R) BP-6 pyrolysate: in vitro and in vivo determination
of "2,3,7,8-TCDD equivalents"a
Bioassayb FireMaster(R) BP-6 pyrolysatec:
Sample "2,3,7,8-TCDD equivalents"d
(µg/g)
AHH induction (in vitro) 1400
EROD induction (in vitro) 480
AHH induction (in vivo) 540
EROD induction (in vivo) 520
Body weight loss (in vivo) 760
Thymic atrophy (in vivo) 1680
a From: Zacharewski et al. (1988).
b AHH = aryl hydrocarbon hydroxylase; EROD = ethoxyresorufin O-deethylase.
c Pyrolysis temperature: 800 °C.
d 2,3,7,8-TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
As has been discussed in a number of comparative studies and
reviews, there is good evidence relating the effects of PBB
congeners that are MC inducers to a receptor-mediated model of
toxicity (Poland & Glover, 1977, 1980; Poland et al., 1979;
Goldstein, 1980; Moore et al., 1980; McKinney & Singh, 1981;
Parkinson & Safe, 1981; Bandiera et al., 1982, 1983; McKinney &
McConnell, 1982; Nebert et al., 1982; Poland & Knutson, 1982;
Robertson et al., 1982b, 1984c,d; Safe et al., 1982, 1985; Aust
et al., 1983; Dannan et al., 1983; Lai, 1984; Safe, 1984). The
particular PBB-induced toxic syndrome of wasting, and other effects
that are common to related halogenated compounds isosteromeric to
TCDD, are related to an interaction with the cytosolic Ah - or TCDD
- receptor protein. The Ah receptor is believed to be a member of
the steroid/retinoid/thyroid hormone nuclear receptor superfamily,
however, no endogenous ligands have been detected so far for the Ah
receptor (Nebert et al., 1990; Poellinger et al., 1992). Activation
of the Ah receptor, translocation into the nucleus, and binding to
responsive elements on DNA are complex processes involving heat
shock protein 90 and several other unknown, or less well described,
steps (Poellinger et al., 1992). This activation of the Ah receptor
leads by less well understood mechanisms to altered expression of a
number of different genes (among these at least 6 drug- metabolizing
enzymes) resulting in a pleiotypic response (Nebert et al., 1990).
Furthermore, TCDD, apparently via activation of the Ah receptor, has
an antiestrogenic effect by down-regulation of the nuclear estrogen
receptor and affects the epidermal growth factor (EGF)
receptor-binding (DeVito et al., 1991; Safe et al., 1991). The most
active TCDD-like PBBs are those lacking ortho bromine substitution,
being coplanar and approximate isostereomers to TCDD. It should be
noted that the interaction between ligands and the Ah receptor, as
well as the action on different genes and species differences, are
only partly understood.
Under certain conditions, TCDD can elicit programmed cell death
(apoptosis) in freshly isolated thymocytes from young rats (McConkey
et al., 1988). This has been suggested as an important mechanism for
the effects of TCDD on cells in the thymus. It is not known whether
this effect is mediated via the receptor or whether it can occur
with coplanar PBBs. For tissues in general, apoptosis is believed to
be important for differentiation, normal cell turnover,
hormone-dependent atrophy, and tumour promotion (Nebert et al.,
1990).
PBBs also have a number of other effects at the molecular and
biochemical levels, e.g., increased cell proliferation in the B633F1
mouse liver (Mirsalis et al., 1985, Table 88; Loury et al., 1987;
Mirsalis et al., 1989; Mirsalis & Steinmetz, 1990), and effects on
membranes (Bernert & Groce, 1984) or membrane-mediated processes in
rats (Shukla & Albro, 1987). PBBs substituted at the ortho
positions cause PB-type microsomal induction. The mechanism of this
type of enzyme induction is unknown. This group of PBBs also causes
inhibition of intercellular communication (Trosko et al., 1981;
Tsushimoto et al., 1982; Williams et al., 1984; Kavanagh et al.,
1987; Rezabek et al., 1988), which has been suggested to be
important for the promotion phase in the carcinogenic process.
PBB microsomal enzyme induction, as well as the induction of
other drug metabolizing enzymes, may lead to a number of secondary
events with enhanced metabolic conversions of both xenobiotica and
endogenous compounds, such as steroid hormones. Furthermore, a high
level of P450 enzymes could lead to the generation of oxygen
radicals. A relationship between the induction of cytochrome P-450
enzymes (see section 8.8.1) and liver tumour promoting activity has
been noted for a number of chemicals including PBBs (e.g., Lubet
et al., 1989; Beebe et al., 1991; Buchmann et al., 1991).
Some of the toxic effects of PBBs could also be mediated via
changes in the metabolism of vitamin A (retinol compounds and
retinoic acid), which is important for cellular growth and
differentiation. However, the mechanism of the effects of PBBs on
vitamin A metabolism is still unknown.
9. EFFECTS ON HUMANS
The human health effects of PBBs have been reviewed by Safe
(1984), Reggiani & Bruppacher (1985), Fries (1985b), IARC (1986),
Kimbrough (1987), Anderson (1989), Silberhorn et al. (1990), and
Waldron (1990).
9.1 General population exposure
9.1.1 Acute toxicity-poisoning incidents
Most information on the effects of PBBs on humans was obtained
as the result of a poisoning incident in Michigan, USA, 1973, when
several hundred kg PBBs were introduced into cattle feed through a
labelling accident. Widespread human exposure in this area resulted
from direct contact with contaminated feed and from the consumption
of PBBs in meat, eggs, and dairy products. During an interval of
more than 9 months between the accident, the identification of its
cause, the beginning of statewide testing and the establishment of
quarantines, commercially marketed products entered the Michigan
food chain.
For exposure data see sections 5.2 and 5.3.
There was no instance of acute PBB toxicosis in humans with
which to compare the potential effects at lower exposures (Fries,
1985b).
9.1.2 Epidemiological studies
In the epidemiological studies reviewed below, efforts have
been made to evaluate the relationship between PBB exposure and a
large number of adverse effects, including behavioural effects and
subjective complaints. However, most studies suffer from major
failures in design introducing confounders that make it difficult,
or impossible, to draw conclusions regarding the relationship
between PBB exposure and possible health effects.
A number of studies had no comparison groups. In other reports,
small groups of patients were selected because of existing illness
and not because their PBB body burdens were particularly elevated.
Some of the outcomes measured, such as urinary porphyrin levels,
liver function tests, and immunological tests, did not show any
clinically relevant changes or were not positively correlated with
PBB body burdens. The clinical relevance of some of the tests is
also not known, at present, because no reference values exist. Most
of these reports dealt with cross sectional studies of heterogeneous
groups of people. No detailed reports exist on long-term, follow-up
studies.
9.1.2.1 Studies conducted by the Michigan Department of Public
Health (MDPH studies)
The health status of 165 persons living on PBB-quarantined
farms was compared with that of 133 persons living on unexposed
farms in the same area. Although a variety of symptoms were reported
by both groups, no consistent pattern of differences between the
groups was observed. Physical examinations did not show any
abnormalities of the heart, liver, spleen, or nervous system that
could be related to PBB exposure. There were no differences between
groups in urine analysis and blood counts (Humphrey & Hayner, 1975).
A cohort study of Michigan residents (4545 persons), exposed to
PBBs, was conducted to examine, among other things, whether there
was an increased incidence of acute or subacute illness in relation
to PBB exposure, 4 years after the accident. Six groups with various
levels of potential exposure were included in the cohort. The groups
were quarantined farm residents, farm product recipients, chemical
workers and their families, pilot study control participants,
self-referred individuals who resided on farms that were
contaminated with low amounts of PBBs, and self-referred individuals
who had no direct connection with contaminated farm premises. Mean
and median serum concen trations of PBBs were much higher in the
first three groups than in the latter three. The prevalence of
selected symptoms by group was examined. Symptoms generally were
most prevalent in the two self-selected groups and were least
prevalent in the group composed of chemical workers and their
families. An evaluation of dose-response relationships was
undertaken by dividing the cohort into seven segments on the basis
of serum PBB levels. No positive associations were found between
serum concentrations of PBB and reported symptom frequencies.
Symptom-prevalence rates (excluding volunteers) were slightly higher
in persons with no detectable PBBs in serum than in those with
measurable quantities. Relationships between symptom-prevalence
rates and serum PBB levels were also examined within each enrolment
group, and no positive trends were found; in all groups, including
chemical workers and quarantined farm residents, the highest
prevalence rates occurred in persons with the lowest serum PBB
levels (Landrigan et al., 1979).
9.1.2.2 Studies conducted by the Environmental Science Laboratory,
Mount Sinai School of Medicine, New York (ESL studies)
Anderson et al. (1978b, 1979) reported on a study of PBB-
exposed farmers and residents in Michigan and a control group of
unexposed Wisconsin dairy farmers, who were examined in November
1976 and March 1977, respectively. The results were given on the
basis of the examination of 933 exposed persons and 229 controls in
the 1978 report, and of 993 exposed subjects and 228 controls in the
1979 report.
The study included four groups: families chosen randomly from
Michigan farms, consumers of produce bought directly from
participating farms, self-selected Michigan families, and Wisconsin
dairy farmers not exposed to PBBs. All subjects completed
comprehensive questionnaires on medical histories and 43 symptoms,
and they were subjected to physical examination and certain
laboratory tests. Statistical analysis of the prevalence of symptoms
at the time of examination or during the preceding year in the
Michigan and Wisconsin populations studied, found the Michigan group
to have a significantly higher prevalence of skin, neurological, and
musculoskeletal symptoms. The increase was seen among the younger
age groups of 16-35 years and 36-55 years. Michigan females had a
higher prevalence of neurological symptoms than Michigan males
(Anderson et al., 1978b).
No statistically significant, positive correlations were found
between serum PBB values and any individual current symptom. Liver
function tests showed that serum glutamic pyruvic transaminase
(SGPT), serum glutamic oxaloacetic transaminase (SGOT), and lactate
dehydrogenase (LDH) elevations were significantly more prevalent in
the Michigan group (chi square test). No differences were seen for
alkaline phosphatase. Within the Michigan group, males had a higher
prevalence of SGPT and LDH increases than females, and comparing
males in both groups, Michigan males had a significantly higher
prevalence of SGPT and SGOT increases than Wisconsin males (Anderson
et al., 1979).
The comparison of findings among residents on Michigan dairy
farms (quarantined and non-quarantined farms) and corresponding
consumers of produce purchased from these farms (cross-sectional
clinical survey of 1029 persons) gave the following results:
prevalence of symptoms (dermatological, neurological,
musculoskeletal, and gastrointestinal) in consumers of farm products
from quarantined farms was similar to that found in farmers on
quarantined farms; the prevalence was lower in consumers of products
from non-quarantined farms. The prevalence of liver function
abnormalities (increase in alkaline phosphatase, SGOT, SGPT, and
LDH) was similar in dairy farmers and consumers. The distribution,
mean and median values of serum PBB levels in consumers were found
to be similar to those in dairy farmers (Lilis et al., 1978).
Although some results of the ESL studies were at times
interpreted differently from the results of the MDPH studies, there
was one area of consistent agreement. Neither set of studies
demonstrated a positive dose-response relationship between PBB
concentrations in serum or adipose tissue and the prevalence of
symptoms or abnormal clinical measurements (Fries, 1985b).
9.1.3 Special studies
9.1.3.1 Examination of subjects with complaints
In the original cohort (Landrigan et al., 1979) a subset of
individuals (19 males and 4 females), predominantly from the
quarantined farms, was identified who had a number of disabling
health complaints.
This group was systematically evaluated at a hospital together
with a group of 28 PBB workers selected randomly from a pool of 100
people with previous occupational PBB exposure. The physical
examination of this group of exposed farmers demonstrated a
relatively high prevalence of hepatomegaly. Eight patients (35%)
showed evidence of liver enlargement, defined as greater than 11 cm
of vertical height in the midclavicular line. Liver scanning
confirmed the presence of hepatomegaly in four of these individuals
(17%), and two of them had a history of substantial alcohol intake.
Ten individuals (43%) had skin lesions, but these were common
dermatological problems, such as superficial mycoses and actinic
keratosis, which are not uncommon in the general population and in
people working outdoors. Biochemical and haematological testing
revealed few abnormalities, and electro myograms, nerve conduction
velocities, endocrine studies, and lymphocyte transformation studies
did not provide any objective findings that correlated with
subjective complaints. Psychiatric evaluation revealed a high
prevalence of depression (78%) among the farmers. Multiple tests of
intelligence, memory, and functional ability did not demonstrate
abnormalities. There was no relation ship between fat PBB levels and
physical or laboratory findings (Stross et al., 1981). The present
study did not use epidemiological methods to evaluate the
relationship between health effects and exposure to PBB and should,
thus, be regarded as a report on cases in a group with known, but
unquantified, PBB exposure.
9.1.3.2 Cutaneous effects
Three years after the Michigan PBB incident, the cutaneous
effects were examined in a multidisciplinary study of the farming
population (498 persons). Results were divided into two categories:
subjective findings or symptoms of which subjects complained, and
objective findings or signs, which were detected on physical
examination. The results of the subjective findings were: 32% of
adult residents and consumers associated with quarantined and
non-quarantined farms complained of the development of unexplained
cutaneous itching in a 3-year period from the PBB contamination
episode to the time of the examination, compared with 22% reported
by the control group. There was also a higher proportion of dryness
reported; 32% of the quarantined and non-quarantined farm adults
compared with 24% of the control group. Similarly, 17% of the farm
study groups complained of the development of unexplained peeling
and scaling during the same period compared with an incidence of 9%
in the control group. The prevalence of erythema in the combined
farm study groups was 12% compared with a prevalence of 5% in the
controls. Increased unexplained nail growth was reported by 8% of
the quarantined and non-quarantined adults compared with none in the
controls. Increased abnormal sweating was a complaint of 22% of the
quarantined and non-quarantined group compared with 13% in the
controls. Unexplained hair loss was a complaint of 12% of the
quarantined and non-quarantined group compared with 5% in the
control group. All exposed groups had a significantly increased
prevalence of skin symptoms compared with the comparison group. Of
the symptoms elicited in a physician interview, rash was the most
frequently reported. Among the farmers, rash was reported by 14%
compared with 9% in the control group.
Objective findings, determined by examination, were diffuse
unexplained alopecia in 4% of the combined quarantined and
non-quarantined farm groups, compared with none in controls (P <
0.005) (Chanda et al., 1982).
Because of major flaws in the study design and biases
introduced during the selection processes in the cohort and a poor
response rate, no causal association with PBBs could be deduced from
this study.
9.1.3.3 Effects on liver function
The serum activities of SGOT, SGPT, LDH, and alkaline
phosphatase were measured in 614 Michigan adults exposed to PBB and
141 unexposed Wisconsin adults. The Michigan groups had a higher
prevalence of elevated SGOT (P < 0.005) and SGPT (P < 0.005). A
clear sex difference was observed. Michigan men had a higher
prevalence of elevated SGPT (P < 0.005) and LDH (P < 0.005) than
Michigan women, and a higher prevalence of elevated SGOT than
Wisconsin men (P < 0.005) and SGPT (P < 0.01). On the basis of
serum PBB analyses, no obvious relationship was observed between PBB
values and liver function tests (Anderson et al., 1978d).
9.1.3.4 Porphyria
In 1977, urine samples were collected from members of farm
families in Michigan, who had ingested PBB-contaminated meat and
dairy products, beginning in 1973, and from members of farm families
in Wisconsin with no known exposure to PBB (control group).
The total porphyrin excretion of both groups was below
200 µg/litre. Therefore, at this level, this parameter cannot be
used as an indication of exposure of humans to PBB.
Out of a group of 142 persons, at least 47% were found with
secondary coproporphyrinuria or with chronic hepatic porphyria Type
A, demonstrating an abnormal porphyrin pattern. The incidence of
this indicator of liver malfunction was higher in the PBB-exposed
group than in the controls (6%). The study was limited to an
assessment of liver damage as manifested by porphyrin excretion
(Strik et al., 1979).
9.1.3.5 Effects on spermatogenesis
Analysis of semen from 52 PBB-exposed men compared with
analysis of semen from a control group of 52 men not exposed to PBB
revealed no differences in the distribution of sperm counts,
motility, or morphology (Rosenman et al., 1979).
9.1.3.6 Paediatric aspects
In 1976, the paediatric aspects of PBB exposure were studied in
Michigan children, using Wisconsin children as a control group
(Barr, 1978, 1980).
Examination of the data from 292 Michigan farm children showed
that the prevalence of symptoms was related to the quarantine status
of the farm and to the method of invitation into the study. Serum
PBB levels were related to the quarantine status of the farm, but
not to the method of invitation into the study. No significant
effects of age or sex were found on the prevalence of symptoms or
serum PBB levels, except that the teenage (13-16 years of age) males
had somewhat higher PBB levels. Despite the frequent reporting of
symptoms of ill health, physical examination failed to reveal any
objective alterations that could be attributed to PBBs. The most
striking finding was a statistically significant negative
correlation between the prevalence of symptoms and the serum PBB
levels.
The effects of PBBs on 33 children born between September 1,
1973, and December 31, 1975, were evaluated in September, 1977.
These children, born to families who lived on quarantined farms,
were compared with 20 children who had not been exposed to PBBs. The
birthdate interval was selected to obtain children who were exposed
in utero or in early infancy, or both, the two time periods when
damage to developing tissues and organ systems should have been
maximal. The results of these studies failed to identify any effects
on physical growth, physical examination, or neurological
assessment, though the parents indicated by historical review that
the exposed children had more illnesses, especially respiratory,
than the control children. There were some indications of an inverse
relationship between fat PBB levels and performance on selected
developmental tests (Weil et al., 1981).
Seagull (1983) studied 19 of these children between the ages of
2 years 5 months and 3 years 11 months using five tests of the
McCarthy Scales of Children's Abilities and concluded that four of
the five tests had significant (P < 0.05) correlations with PBB
exposure, i.e., the higher the PBB levels in the adipose tissue, the
lower the child's developmental abilities.
Schwartz & Rae (1983) later studied these same children between
the ages of 4 years 1 month and 6 years 1 month (N = 18 because one
family refused to participate in the follow-up study) with the
entire battery of McCarthy Scales of Children's Abilities, plus the
Wechsler Preschool and Primary Scale of Intelligence, and concluded
that no significant (P > 0,05) differences existed.
These different conclusions from studies on the same children
were summarized by Nebert et al. (1983) and commented on from
statistical, clinical paediatric, and toxicological points of view.
The authors stated that different approaches to the analysis of
the data were used and that, in one case, the ability tests of only
five children were selected, because of time limitations in the
study situation.
Comparison of fetal death rates among residents of Michigan's
Lower Peninsula counties with a high percentage of quarantined farms
and among residents of Upper Peninsula counties with no quarantined
farms revealed no important differences in rates or trends after the
contamination. Since counts of early spontaneous abortions were
lacking, a complete assessment of the possible impact on
reproductive outcome could not be made (Humble & Speizer, 1984).
9.1.3.7 Neurological and neuropsychiatric aspects
Neurological symptoms were the earliest and most prominent
symptoms recorded in Michigan farm residents exposed to PBBs
compared with an unexposed control farm population in Wisconsin. The
prevalence and incidence of neurological symptoms were analysed in
over 620 adults from Michigan and 153 from Wisconsin. Subsamples of
both groups were examined in objective performance tests used for
the assessment of neuropsychological dysfunction. In Michigan
(particularly among males), those who exhibited the most marked
symptoms tended to show diminished performance. Low indices of
performance were also significantly correlated with intake of
home-produced foodstuffs, particularly during the years 1972-74 and
store-bought products during the years 1975-76. Between 1972 and
1976, the Michigan farm residents studied made significant changes
in their consumption patterns of products suspected to be
contaminated with PBBs compared with those of Wisconsin farm
residents. Serum PBB levels were not found to be significantly
higher in Michigan males and females exhibiting the most prominent
neurological symptoms. Serum PBB levels were negatively correlated
with performance test scores, particularly in males in older age
groups (Valciukas et al., 1978, 1979).
Twenty-one persons exposed to PBBs were compared with hospital
volunteers on a battery of tests measuring memory, motor strength
and coordination, cortical-sensory perception, personality, and
higher cognitive functioning. Patients exposed to PBBs were selected
for the study only if they had persistent medical complaints. The
adipose PBB levels were not correlated with performance on any test
in the battery. The two groups did differ on the Minnesota
Multiphasic Personality Inventory, suggesting an adjustment reaction
with depressive symptoms and somatizing defences. Persons exposed to
PBBs were also impaired compared with control subjects in tests of
prose recall, short-term memory, concentration, and cognitive
flexibility. However, these differences vanished when group
differences on education and personality were statistically held
constant. The selective admission criteria for the study limited the
possibility of generalizing these findings (Brown & Nixon, 1979).
Forty-six persons (37 men and 9 women) with known exposure to
PBBs were examined in a study designed to evaluate neurobehavioural
complaints (Stross et al., 1979). These people complained of a
serious deterioration in their health status and were unable to
engage in their previous occupations. Comprehen sive medical
investigations were carried out including neurological studies and
psychological evaluation. Electromyograms were abnormal in six
patients (13%) with no consistent or diagnostic findings. Nerve
conduction studies were abnormal in 19 patients (41%), with slowing
in sensory nerve latencies the predominant finding. The abnormal
values averaged 4.7 milliseconds compared with the normal value of
< 3.9 milliseconds. There was an excellent correlation between
patients with objective findings on neurological examination and
abnormal nerve conduction studies (r value not stated). There was no
relationship between the presence of these abnormalities and serum
or fat PBB levels.
Despite the fact that an extensive battery of tests was
administered in the psychological evaluation, few objective
abnormalities were documented. The most common findings were those
of somatic preoccupation, irritability, and mild depression. The
tests of motor function were normal, while the tests of sensory
modalities showed minor differences that were not outside normal
limits. Most had IQs of between 100 and 140 with no differences
between estimated and observed levels. Although most patients
complained of memory difficulties, no objective deterioration in
memory could be elicited.
The results of the psychiatric interviews showed that 31
patients (67%) were depressed. No evidence of endogenous depression
was noted, and it was the opinion of the psychiatrists involved that
the findings were characteristic of reactive depression.
9.1.3.8 Lymphocyte and immune function
The immunotoxicology of PBBs has been reviewed by Amos (1986)
and Steele et al. (1989).
Bekesi et al. (1983b) summarized the findings of lymphocyte and
immunological function studies, conducted in 1976 on 45 adult
Michigan dairy farm residents who had consumed PBB-contaminated food
products for periods ranging from three month to three years. Test
comparisons were made with a group of 46 dairy farm residents in
central Wisconsin who had not been exposed to PBB-contaminated food
and to a group of 76 healthy subjects from the New York Metropolitan
area (Bekesi et al., 1978, 1979a,b).
Marked changes in various immunological parameters were noted
among the Michigan dairy farm residents compared with both the
Wisconsin and New York control populations. The peripheral blood
lymphocytes of only 27 of the 45 Michigan subjects exhibited a
normal response to the T-cell mitogens phytohaemagglutinin and
concanavalin A, to the alloantigens in the mixed leukocyte culture
reaction, and to the B-cell mitogen (pokeweed mitogen). In the
remaining 18 subjects, the lymphocytes showed an impaired functional
response to all mitogens and alloantigens.
The lymphocytes of all 45 PBB-exposed study subjects showed a
reduced proliferative T-cell response in mixed leukocyte cultures.
The group of 18 individuals with decreased T-cell function had
values measuring one-third to one-quarter of those obtained from the
normal controls. The number of viable cells in the various
subpopulations of peripheral blood lymphocytes were measured
according to their ability to form stable rosettes with sheep
erythrocytes in the case of the T-cells, while the B-lymphocytes
were quantified by either direct immunofluorescence or by sheep
erythrocytes sensitized with antibody and complement. The 27
Michigan farm residents with normal lymphocyte functions also
exhibited the normal distribution of T- and B-lymphocytes. Eighteen
of the 45 subjects with lymphocyte dysfunction showed significantly
reduced populations of T-cells. Despite the marked changes in the
characteristic cell surface markers detected in the peripheral blood
lymphocytes of the PBB-exposed Michigan farm residents, the marker
for monocytes, determined by peroxidase staining or latex digestion,
did not differ from that of either control group. Thus, the most
significant deviation from the control samples was a marked increase
in lymphocytes without detectable surface markers.
Five years later, Bekesi et al. (1983a,b) examined the same
individuals and the data strongly suggested a persistent PBB-induced
immune suppression. The findings were characterized by a decrease in
the percentage and absolute number of T-lymphocytes, with a
concomitant increase in the occurrence of lymphocytes without
detectable membrane surface markers, and, in as many as 30% of the
subjects retested, a reduction in the T-cell function.
Silva et al. (1979) assessed T- and B-lymphocyte numbers and
lymphocyte transformation to 3 mitogens (phytohaemagglutinin,
concanavalin A, and pokeweed mitogen) in 41 persons with a high
exposure to PBBs (mean serum level 787 µg/litre, range
529-2560 µg/litre) and 57 persons with a low exposure (mean serum
level 3 µg/litre, range 1-11 µg/litre). In contrast to the findings
of Bekesi et al. (1978) there were no significant differences in the
percentages of T- and B-lymphocytes among persons who experienced
high or low PBB exposure or in control groups. Similarly, no
significant depression of lymphocyte mitogenic responsiveness were
found in those who experienced high or low PBB exposure compared
with controls. No correlation was found between serum PBB levels and
lymphocyte numbers or function.
In a comprehensive immunotoxicological study, 336 adult
Michigan farm residents, 117 general consumers (for comparison), and
75 dairy farm residents in Wisconsin, who had not eaten
PBB-contaminated food, were examined, as were 79 healthy subjects in
New York City. Abnormalities in the Michigan groups included:
hypergammaglobulinaemia, exaggerated hypersensitive response to
streptococci, significant decreases in absolute numbers and
percentages of T- and B-lymphocytes, and increased numbers of
lymphocytes with no detectable surface markers ("null cells").
Significant reduction of in vitro immune function was noted in
20-25% of the Michigan farm residents who had eaten food containing
PBBs. The decreased immune function detected among the PBB-exposed
farm residents tended to affect families as a unit and was
independent of the age or sex of exposed individuals,
contraindicating the possibility of genetic predisposition (Bekesi
et al., 1987).
Lipson (1987) evaluated the effects of PBBs on the function and
on the synthesis of immunoglobulins by peripheral blood lymphocytes.
Concentrations of PBBs as low as 0.001 µg/105 cells decreased
lymphocyte response to pokeweed mitogen: higher concentrations of
PBBs stimulated the in vitro synthesis and release of
immunoglobulins. PBBs had no effect on the quantity of
E-rosette-forming cells, the total T- or B-cells, or the ratio of
helper to suppressor T-cell subpopulations. Enhanced release of IgG
was identified in lymphocyte cultures obtained from blood specimens
of PBB-exposed Michigan farmers. The data from this study suggest
that PBBs had exerted an adverse effect on cell function, but had
produced a non-specific activation of B lymphocytes.
9.1.3.9 Carcinogenic embryonic antigen plasma levels
Carcinogenic embryonic antigen (CEA) titres were determined for
611 Michigan farmers exposed to PBBs and for a control unexposed
population of 138 Wisconsin farmers. The overall prevalence of
elevated CEA titres was slightly higher in the Michigan study group,
but the difference was not statistically significant. Serum PBB
concentrations appeared to be positively correlated with CEA titres.
The authors discussed the possibility that the effect of PBBs may be
additive to that of other factors that are known to result in an
increased prevalence of elevated CEA titres (Anderson et al.,
1978c).
The possibility of long-term effects of PBBs, such as cancer,
cannot be ruled out. The induction of liver tumours in rodents is a
matter of concern (Fries, 1985b).
9.1.3.10 Biochemical effects
Two hundred and sixty-two residents with a geometric mean serum
PBB level of 19.9 µg/litre submitted at least one blood sample for
clinical chemistry tests of 9 parameters during 4 different years.
No consistent significant correlation with serum PBB levels was
shown for any parameter (Kreiss et al., 1982). The authors suggested
that tests with greater sensitivity and specificity for hepatic
microsomal enzyme induction should be developed for future
evaluations.
Lambert et al. (1987) were the first to study the effects of
PBB exposure on the human cytochrome P-450 system, as determined by
the caffeine breath test (CBT), in healthy non-smoking adults from
rural Michigan with, and without, detectable serum PBB levels
(concentration not stated) and in prepubescent children with known
perinatal exposure to PBBs. The results were compared with the CBT
results obtained from unexposed urban adult non-smokers and
age-matched children. The unexposed and PBB-exposed children had
similar CBT data. The adult groups were not significantly different
from each other, except for the rural adults with detectable PBB
levels who had significantly higher CBT values than the unexposed
urban adults.
Lambert et al. (1990) conducted a field biochemical
epidemiology study using the Michigan cohort consisting of 51 rural
residents exposed to PBBs. The CBT and CMR (caffeine urinary
metabolite ratio) were elevated in the subjects exposed to PBBs
compared with the values obtained from urban non-smokers and were
similar to those found in adults who smoked. A gender effect was
seen in the PBB-exposed subjects, the median CBT and CMR values of
the females being lower than the values of the males. There was a
correlation between the CBT and the serum HxBB values (r2 = 0.2,
p = 0.01) but not between CMR and serum HxBB values.
PBBs induce hepatic cytochrome P-450IA2 enzyme activity in the
human adult, but not in the child. Lambert et al. (1991) there fore
investigated, in a prospective longitudinal study of 14 male and 15
female children exposed transplacentally and transmammil lary to
PBBs in 1973-75, whether PBB exposure altered the normal decrease in
P-450IA2 activity that occurs during puberty. P-450IA2 activity was
monitored via the CBT every 2 years, beginning in 1985, and compared
with the P-450IA2 activity in gender- and Tanner stage-matched
children not exposed to PBBs. Unlike the adult, PBBs did not alter
P-450IA2 activity in the child or in the mid-pubescent adolescent
(Wilcoxon rank, P > 0.05).
9.2 Occupational exposure
9.2.1 Epidemiological studies
Anderson et al. (1978a) investigated the health status of 55
employees (52 men and 3 women) of the Michigan Chemical Corporation,
which manufactured PBBs from 1970 to 1974, in addition to a variety
of other halogenated fire retardant chemicals. Ten of those examined
had formerly worked directly in the PBB production area, the other
45 persons worked in other departments in the plant. For these 55
workers, the route and quality of occupational exposure were
probably different from those of farmers, since they could have been
directly exposed to PBBs. The results were compared with those from
a group of male farm residents and consumers from Michigan.
The prevalence of chest and skin symptoms among chemical
workers as a group was significantly greater than among farmers.
Significantly fewer symptoms were reported in the musculoskeletal
category. The PBB department workers experienced symptoms in the
skin category significantly more frequently than non-PBB workers.
Blood chemistry results were similar for workers and farmers.
However, both groups exhibited a significantly higher prevalence of
elevated liver function tests (SGOT, SGPT) than a control population
of unexposed farmers. Considering only workers with more than five
years in the plant, a significantly higher prevalence of elevated
CEA titres was present compared with the farmers.
9.2.2 Clinical studies
The only abnormality noted during physical examination in a
group of PBB-exposed chemical workers consisting of 24 males and 4
females whose ages ranged from 23 to 62 years with a mean of 40
years, was the presence of hepatomegaly in two patients (7%), both
of whom heavily indulged in alcoholic beverages, and abnormal skin
examination in four patients (14%). In numerous biochemical tests,
only minor elevations of serum uric acid, serum iron, and serum
cholesterol were found in 20% of the chemical workers.
Elevation of triglyceride levels was noted in 50% of the
chemical workers, with a mean of 185 mg % (SD ± 50 mg %), with an
upper limit of normal of 150 mg %. No abnormalities in lymphocyte
number or function could be determined, and there was no
relationship between PBB levels and physical or laboratory
abnormalities (Stross et al., 1981).
9.2.3 Special studies
9.2.3.1 Cutaneous effects
Halogen acne was observed on physical examination in 13%
of 53 Michigan chemical workers exposed to PBBs compared with none
in unexposed controls (P < 0.001) (Chanda et al., 1982).
9.2.3.2 Memory performance
Twenty-five chemical workers, who manufactured PBBs, were given
objective tests of learning and memory. Although this group had high
concentrations of PBBs in adipose tissue, mean scores on all memory
tests were normal. The PBB concentration was not correlated with
memory performance; the most contaminated workers showed no evidence
of memory dysfunction (Brown et al., 1981).
9.2.3.3 Thyroid effects
Thyroid function was investigated in a cohort of 35 male
workers, selected from 86 identified workers exposed for at least 6
weeks, manufacturing decabromobiphenyl, decabromobiphenyl ether, and
bromine. The study revealed four cases of primary hypothyroidism
(11.4%), but none in 89 control subjects. The bromine compounds were
the only common exposure. A significantly higher number of the
exposed workers had detectable serum levels of DeBB
(0.5-1340 ng/ml), but not DeBBO (Bahn et al., 1980a,b).
Bialik (1982) investigated thyroid dysfunction in workers
exposed for at least 240 h to decabromobiphenyl and
decabromobiphenyl oxide over a 4-year period. The average period of
employment was 3.9 years, mean age, 34.7 years. Medical
questionnaires, physical examinations, and laboratory tests were
conducted. For exposure data see section 5.3. Thyroid nodules were
seen in 3 out of 18 workers exposed for 3 years or longer.
No detectable PBBs were found in the serum.
9.2.3.4 Reproductive effects
Bialik (1982) also studied reproductive effects in the same PBB
workers.
A significant correlation was seen between length of employment
and concentrations of follicle stimulating hormone (FSH). An
abnormal FSH value was found in only one worker. A testicular cyst
was found in one exposed worker, and epididymal nodules in two
others. No testicular or epididymal nodules were seen among
controls. No definite statement could be made concerning adverse
effects on the prevalence of testicular and epididymal nodules,
because of their prevalence in the general population.
9.2.3.5 Lymphocyte function
Decreased lymphocyte function occurred in four out of ten
Michigan chemical workers. The decrease was related to higher plasma
levels of PBBs (40-1200 µg/litre) (Bekesi et al., 1979b).
9.2.3.6 Mortality
A historical prospective mortality study was conducted by Wong
et al. (1984) on 3579 white male workers employed between 1935 and
1976 at chemical plants with potential exposures to brominated
compounds. Because of the lack of quantitative data, potential
exposures of workers to PBBs were categorized as "routine" and
"non-routine". None of 91 individuals of the cohort potentially
exposed to PBBs on a routine basis died during the study period.
Among the 237 "non-routinely" exposed, two deaths were
observed, though 6.36 were expected. One was due to cancer of the
large intestine, the other was coded as arteriosclerotic heart
disease.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (1986, 1987)
evaluated the polybrominated biphenyls and concluded that there is
sufficient evidence for the carcinogenicity to experimental animals
of a commercial preparation of PBBs (FireMaster FF-1, various lots),
composed primarily of hexabromobiphenyl with smaller amounts of
penta- and heptabrominated isomers. There is considered to be
inadequate evidence for their carcinogenicity in humans. Commercial
mixtures of PBBs were thus classified into Group 28, possibly
carcinogenic to humans.
The European Community added PBBs to the chemicals banned or
severely restricted to certain uses owing to their effects on human
health and the environment (CEC, 1988).
REFERENCES
Adams EA, Irish DD, Spencer HC, & Rove VK (1941) The response of
rabbit skin to compounds reported to have caused acneform
dermatitis. Ind Med Surg, 2: 1-4.
Aftosmis JG, Culik R, Lee KP, Sherman H, & Waritz EI (1972a)
Toxicology of brominated biphenyls. I. Oral toxicity and
embryotoxicity. Toxicol Appl Pharmacol, 22: 316.
Aftosmis JG, Dashiell OL, Griffith FD, Hornberger CS, McDonnell MM,
Sherman H, Tayfun FO, & Waritz RS (1972b) Toxicology of brominated
biphenyls. II. Skin, eye, and inhalation toxicity and an acute test
method for evaluating hepatotoxicity and accumulation in body fat.
Toxicol Appl Pharmacol, 22: 316-317.
Ahmadizaden M, Kuo C-H, Echt R, & Hook JB (1984) Effect of
polybrominated biphenyls, beta-naphthoflavone and phenobarbital on
arylhydrocarbon hydroxylase activities and chloroform-induced
nephrotoxicity and hepatotoxicity in male C57BL/6J and DBA/2J mice.
Toxicology, 31: 343-352.
Ahotupa M & Aitio A (1978) Effect of polybrominated biphenyls on
drug metabolizing enzymes in different tissues of C57 mice. Toxicol,
11: 309-314.
Akoso BT, Sleight SD, Aust SD, & Nachreiner R (1980) Comparative
study of the toxicopathology of purified congeners of polybrominated
biphenyls in rats. In: Abstracts of papers presented at the
Nineteenth Annual Meeting of the Society of Toxicology, Washington,
9-13 March 1980. Washington, DC, Society of Toxicology (Abstract
A/103).
Akoso BT, Sleight SD, Aust SD, & Stowe HD (1982a) Pathologic effects
of purified polybrominated biphenyl congeners in rats. J Am Coll
Toxicol, 1: 1-21.
Akoso BT, Sleight SD, Nachreiner RF, & Aust SD (1982b) Effects of
purified polybrominated biphenyl congeners on the thyroid and
pituitary glands in rats. J Am Coll Toxicol, 1: 23-36.
Allen JR & Lambrecht L (1978) Responses of rhesus monkeys to PBBs.
Toxicol Appl Pharmacol, 45: 340-341.
Allen JR, Lambrecht LK, & Barsotti DA (1978) Effects of
polybrominated biphenyls in nonhuman primates. J Am Vet Med Assoc,
173: 1485-1489.
Allen JR, Barsotti DA, Lambrecht LK, & Van Miller JP (1979)
Reproductive effects of halogenated aromatic hydrocarbons on
nonhuman primates. Ann NY Acad Sci, 320: 419-425.
Allen-Rowlands CF, Castracane VD, Hamilton MG, & Seifter J (1981)
Effect of polybrominated biphenyls (PBB) on the pituitary-thyroid
axis of the rat. Proc Soc Exp Biol Med, 166: 506-514.
Amos HE (1986) Inadvertent immunosuppression as a health hazard. Br
J Clin Pract, 40: 336-340.
Anderson HA (1985) Utilization of adipose tissue biopsy in
characterizing human halogenated hydrocarbon exposure. Environ
Health Perspect, 60: 127-131.
Anderson HA (1989) General population exposure to environmental
concentrations of halogenated biphenyls. In: Kimbrough RD & Jensen
AA ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products, 2nd ed. Amsterdam, New York,
Oxford, Elsevier/North-Holland Biomedical Press, pp 333-339.
Anderson HA, Wolff MS, Fischbein A, & Selikoff IJ (1978a)
Investigation of the health status of Michigan Chemical Corporation
employees. Environ Health Perspect, 23: 187-191.
Anderson HA, Lilis R, Selikoff IJ, Rosenman KD, Valciukas JA, &
Freedman S (1978b) Unanticipated prevalence of symptoms among dairy
farmers in Michigan and Wisconsin. Environ Health Perspect, 23:
217-226.
Anderson HA, Rosenman KD, & Snyder J (1978c) Carcinoembryonic
antigen (CEA) plasma levels in Michigan and Wisconsin dairy farmers.
Environ Health Perspect, 23: 193-197.
Anderson HA, Holstein EC, Daum SM, Sarkozi L, & Selikoff IJ (1978d)
Liver function tests among Michigan and Wisconsin dairy farmers.
Environ Health Perspect, 23: 333-339.
Anderson HA, Wolff MS, Lilis R, Holstein EC, Valciukas JA, Anderson
KE, Petrocci M, Sarkozi L, & Selikoff IJ (1979) Symptoms and
clinical abnormalities following ingestion of
polybrominated-biphenyl-contaminated food products. Ann NY Acad Sci,
320: 684-702.
Andersson K, Norström A, Rappe C, Rasmuson B, & Swahlin H (1975)
Photochemical degradation of PCB, PBB and other flame retardants.
Environ Qual Saf, 3(Suppl): 798-802.
Andres J, Lambert I, Robertson L, Bandiera S, Sawyer T, Lovering S,
& Safe S (1983) The comparative biologic and toxic potencies of
polychlorinated biphenyls and polybrominated biphenyls. Toxicol Appl
Pharmacol, 70: 204-215.
Anklam E (1989) 1H and 13C NMR studies on mono- and
polybrominated biphenyls. Magn Res Chem, 27: 503-506.
Anon (1977) PBB residue found near three plants in New York, Jersey.
Chem Market Report, 211(26): 4.
Anon (1988) Superfund record of decision: Forest waste disposal, MI
(Second remedial action). Washington, DC, US Environmental
Protection Agency, Office of Emergency and Remedial Response, 141 pp
(EPA/ROD/RO5-88/062).
AOAC (1975) In: Horwitz W, Senzel A, Reynolds H, & Park DL ed.
Official methods of analysis of the Association of Official
Analytical Chemists. Washington, DC, Association of the Official
Analytical Chemists, pp 518-531.
Arneric SP, McCormack KM, Braselton WE Jr, & Hook JB (1980) Altered
metabolism of progesterone by hepatic microsomes from rats following
dietary exposure to polybrominated biphenyls. Toxicol Appl
Pharmacol, 54: 187-196.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity and mutagenicity for 301
chemicals tested by the US NTP (National Toxicology Program). Mutat
Res, 257: 229-306.
Atkinson R, Aschmann SM, & Pitts JS Jr (1984) Kinetics of the
reactions of naphthalene and biphenyl with OH radicals and with O3
at 294 ± 1 K. Environ Sci Technol, 18: 110-113.
ATOCHEM (1984a) Adine 0102(R). Levallois-Perret, France, ATOCHEM,
Centre d'Application de Levallois (Product information No. 126).
ATOCHEM (1984b) Adine 505(R). Levallois-Perret, France, ATOCHEM,
Centre d'Application de Levallois (Product information No. 128).
ATOCHEM (1987) Decabromobiphenyl (Adine 0102): Pyrolisis testing.
Paris La Défense, France, ATOCHEM (Unpublished report).
ATOCHEM (1990) Decabromobiphenyl (Adine 0102). Paris La Défense,
France, ATOCHEM (Unpublished report).
Aulerich RJ & Ringer RK (1979) Toxic effects of dietary
polybrominated biphenyls on mink. Arch Environ Contam Toxicol, 8:
487-498.
Aust SD, Dannan GA, Sleight SD, Fraker PJ, Ringer RK, & Polin D
(1981) Toxicology of polybrominated biphenyls. In: Khan MAQ &
Stanton RH ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects. Oxford, New York, Pergamon Press, pp 73-96.
Aust SD, Trosko JE, & Sleight SD (1983) Toxicity and carcinogenicity
of polybrominated biphenyls. In: Proceedings of the 13th Conference
on Environmental Toxicology, Dayton, Ohio, 16-18 November 1982.
Dayton, Ohio, University of California, pp 270-286
(AFAMRL-TR-82-101).
Babish JG & Stoewsand GS (1977) Polybrominated biphenyls: inducers
of hepatic microsomal enzymes and type A cytochrome P450 in the
rat. J Toxicol Environ Health, 3: 673-682.
Babish JG, Gutenmann WH, & Stoewsand G (1975a) Polybrominated
biphenyls: tissue distribution and effect on hepatic microsomal
enzymes in Japanese quail. J Agric Food Chem, 23: 879-882.
Babish JG, Gutenmann WH, Lisk DJ, & Stoewsand G (1975b) Liver
changes and tissue residues of animals fed hexabromobiphenyl. In:
Abstracts of papers presented at the 59th Annual Meeting of the
Federation of the American Societies for Experimental Biology,
Atlanta City, New Jersey, 13-18 April 1975 (Abstract 157).
Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann
L, & Utiger RD (1980a) Hypothyroidism in workers exposed to
polybrominated biphenyls. New Engl J Med, 302: 31-33.
Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann
L, & Utiger RD (1980b) Health assessment of occupational exposure to
polybrominated biphenyl (PBB) and polybrominated biphenyloxide
(PBBO). Washington, DC, US Environmental Protection Agency
(EPA-560/6-80-001).
Ballschmiter K & Zell M (1980) Analysis of polychlorinated biphenyls
(PCB) by glass capillary gas chromatography. Fresenius Z Anal Chem,
302: 20-31.
Ballschmiter K, Unglert CH, & Neu HJ (1977) [Degradation of
chlorinated aromates: microbiological degradation of polychlorinated
biphenyls (PCB). III: Chlorinated benzoic acid as metabolite of
PCB.] Chemosphere, 1: 51-56 (in German).
Bandiera S, Sawyer TW, Campbell MA, Robertson L, & Safe S (1982)
Halogenated biphenyls as AHH inducers: effects of different halogen
substituents. Life Sci, 31: 517-525.
Bandiera S, Sawyer TW, Campbell MA, Fujita T, & Safe S (1983)
Competitive binding to the cytosolic
2,3,7,8-tetrachlorodibenzo-p-dioxin receptor. Effects of structure
on the affinities of substituted halogenated biphenyls-A QSAR
analysis. Biochem Pharmacol, 32: 3803-3813.
Barlow SM & Sullivan FM (1982) Reproductive hazards of industrial
chemicals. New York, London, San Francisco, Academic Press, pp
437-454.
Barnhart ER, Hill RH, Alexander LR, Orti DL, Groce DF, Patterson DG,
& Head SL (1984) Purification of polybrominated biphenyl congener 2.
Bull Environ Contam Toxicol, 33: 20-25.
Barr M Jr (1978) Pediatric health aspects of PBBs. Environ Health
Perspect, 23: 291-294.
Barr M Jr (1980) Pediatric aspects of the Michigan polybrominated
biphenyl contamination. Environ Res, 21: 255-274.
Bastomsky CH (1986) Polyhalogenated aromatic hydrocarbons and
thyroid function. Presented at the 191st American Chemical Society
National Meeting, New York, 13-18 April 1986. Washington, DC,
American Chemical Society (Abstract No. 23).
Beaudoin AR (1977) Teratogenicity of polybrominated biphenyls in
rats. Environ Res, 14: 81-86.
Beaudoin AR (1979) Embryotoxicity of polybrominated biphenyls. In:
Persaud TVN ed. Advances in the study of birth defects. Volume 2 -
Teratological testing. Baltimore, Maryland, University Park Press,
pp 211-222.
Beaudoin AR & Fisher DL (1981) An in vivo/in vitro evaluation of
teratogenic action. Teratology, 23: 57-61.
Beebe LE, Fox SD, Issaq HJ, & Anderson LM (1991) Biological and
biochemical effects of retained polyhalogenated hydrocarbons.
Environ Toxicol Chem, 10: 757-763.
Bekesi JG, Holland JF, Anderson HA, Fischbein AS, Rom W, Wolff MS, &
Selikoff IJ (1978) Lymphocyte function of Michigan dairy farmers
exposed to polybrominated biphenyls. Science, 199: 1207-1209.
Bekesi JG, Anderson HA, Roboz JP, Roboz J, Fischbein A, Selikoff IJ,
& Holland JF (1979a) Immunologic dysfunction among PBB-exposed
Michigan dairy farmers. Ann NY Acad Sci, 320: 717-728.
Bekesi JG, Roboz J, Anderson HA, Roboz JP, Roboz J, Fischbein A,
Selikoff IJ, & Holland JF (1979b) Impaired immune function and
identification of polybrominated biphenyls (PBB) in blood
compartments of exposed Michigan dairy farmers and chemical workers.
Drug Chem Toxicol, 2: 179-191.
Bekesi JG, Roboz JP, Solomon S, Fischbein A, Roboz J, & Selikoff IJ
(1983a) Persistent immune dysfunction in Michigan dairy farm
residents exposed to polybrominated biphenyls. Adv Immunopharmacol,
2: 33-39.
Bekesi JG, Roboz JP, Solomon S, Fischbein A, & Selikoff IJ (1983b)
Altered immune function in Michigan residents exposed to
polybrominated biphenyls. In: Gibson GG, Hibbard R, & Parke DV ed.
Immunotoxicology. New York, London, San Francisco, Academic Press,
pp 181-191.
Bekesi JG, Roboz J, Fischbein A, & Mason P (1987) Immunotoxicology:
Environmental contamination by polybrominated biphenyls and immune
dysfunction among residents of the state of Michigan. Cancer Detect
Prev, 1(Suppl):29-37.
Benbow AW & Cullis CF (1975) The formation of toxic products during
the combustion of halogen-containing polymers. Eur Polym J, 11:
723-727.
Benighi R (1989) Analysis of the National Toxicology Program data on
in vitro genetic toxicity tests using multivariate statistical
methods. Mutagenesis, 4: 412-419.
Bernert JT Jr & Groce DF (1984) Acute response of rat liver
microsomal lipids, lipid peroxidation, and membrane anisotropy to a
single oral dose of polybrominated biphenyls. J Toxicol Environ
Health, 13: 673-678.
Bernert JT Jr, Groce DF, & Kimbrough RD (1983) Long-term effects of
a single oral dose of polybrominated biphenyls on serum and liver
lipids in rats. Toxicol Appl Pharmacol, 68: 424-433.
Bernert JT Jr, Meredith NK, Akins JR, & Hannon WH (1985)
Determination of serum phospholipid metabolic profiles by
high-performance liquid chromatography. J Liq Chromatogr, 8:
1573-1592.
Berry DL, Digiovanni J, Juchau MR, Bracken WM, Gleason GL, & Slaga
TJ (1978) Lack of tumor-promoting ability of certain environmental
chemicals in a two-stage mouse skin tumorigenesis assay. Res Commun
Chem Pathol Pharmacol, 20: 101-108.
Besaw LC, Moore RW, Dannan GA, & Aust SD (1978) Effect of
2,2',3,3',4,4',5,5'-octabromobiphenyl on microsomal drug
metabolizing enzymes. Pharmacologist, 20: 251.
Bialik O (1982) Endocrine function of workers exposed to PBB and
PBBO: Terminal progress report (Prepared for the National Institute
of Occupational Safety and Health, Cincinnati). Springfield,
Virginia, National Technical Information Service (NTIS) (PB
84-238377).
Birnbaum LS (1985) The role of structure in the disposition of
halogenated aromatic xenobiotics. Environ Health Perspect, 61:
11-20.
Birnbaum LS & McKinney JD (1985) A persistent hexabromonaphthalene
isomer is 2,3,4,5,6,7-hexabromonaphthalene. J Toxicol Environ
Health, 16: 219-228.
Birnbaum LS, Darcey DJ, & McKinney JD (1983) Hexabromonaphthalene
contaminants of polybrominated biphenyls: chemical composition and
disposition in the rat. J Toxicol Environ Health, 12: 555-573.
Birnbaum LS, Morrissey RE, & Harris MW (1991) Teratogenic effects of
2,3,7,8-tetrabromodibenzo-p-dioxin and three polybrominated
dibenzofurans in C57BL/6N mice. Toxicol Appl Pharmacol, 197:
141-152.
Bleavins MR, Aulerich RJ, & Ringer RK (1981) Placental and mammary
transfer of polychlorinated and polybrominated biphenyls in the mink
and ferret. In: Lamb DW & Kenaga EE ed. Avian and mammalian wildlife
toxicology: Second Conference. Philadelphia, Pennsylvania, American
Society for Testing and Materials, pp 121-131 (ASTM Special
Technical Publication 757).
Bombick DW (1990) Gap junctional communication in various cell types
after chemical exposure. J Mol Cell Toxicol, 3: 27-39.
Bonhaus DW, McCormack KM, Braselton WE Jr, & Hook JB (1981) Effect
of polybrominated biphenyls on hepatic microsomal metabolism of
estrogens and uterotropic action of administered estrogen in rats. J
Toxicol Environ Health, 8: 141-150.
Brilliant LB, Van Amburg G, Isbister J, Humphrey H, Wilcox K, Eyster
J, Bloomer AW, & Price H (1978) Breast-milk monitoring to measure
Michigan's contamination with polybrominated biphenyls. Lancet, 2:
643-646.
Brinkman UATh & De Kok A (1980) Production, properties usage. In:
Kimbrough RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 1-40.
Brouwer A & Van den Berg KJ (1986) Binding of a metabolite of
3,4,3',4'-tetrachloro biphenyl to transthyretin reduces serum
vitamin A transport by inhibiting the formation of the protein
complex carrying both retinol and thyroxin. Toxicol Appl Pharmacol,
85: 301-312.
Brown GG & Nixon R (1979) Exposure to polybrominated biphenyls, some
effects on personality and cognitive functioning. J Am Med Assoc,
242: 523-527.
Brown GG, Preisman RC, Anderson MD, Nixon RK, Isbister JL, & Price
HA (1981) Memory performance of chemical workers exposed to
polybrominated biphenyls. Science, 212: 1413-1415.
Buchmann A, Ziegler S, Wolf A, Robertson LW, Durham SK, & Schwarz M
(1991) Effects of polychlorinated biphenyls in rat liver:
correlation between primary subcellular effects and promoting
activity. Toxicol Appl Pharmacol, 111: 454-468.
Bunce NJ, Safe S, & Ruzo LO (1975) Photochemistry of bromobiphenyls:
steric effects and electron transfer. J Chem Soc Perkin Trans, 1:
1607-1610.
Burse VW, Needham LL, Liddle JA, Bayse DD, & Price HA (1980)
Interlaboratory comparison for results of analyses for
polybrominated biphenyls in human serum. J Anal Toxicol, 4: 22-26.
Bursian SJ, Polin D, Olson BA, Shull LR, Marks HL, & Siegel HS
(1983) Microsomal enzyme induction, egg production, and reproduction
in three lines of Japanese quail fed polybrominated biphenyls. J
Toxicol Environ Health, 12: 291-307.
Buser HR (1986) Selective detection of brominated aromatic compounds
using gas chromatography/negative chemical ionization mass
spectrometry. Anal Chem, 58: 2913-2919.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants.
Chemosphere, 16: 713-732.
Buser HR, Bosshardt HP, & Rappe C (1978) Formation of
polychlorinated dibenzofurans (PCDFs) from the pyrolysis of PCBs.
Chemosphere, 1: 109-119.
Byrne JJ, Carbone JP, & Hanson EA (1987) Hypothyroidism and
abnormalities in the kinetics of thyroid hormone metabolism in rats
treated chronically with polychlorinated biphenyl and polybrominated
biphenyl. Endocrinology, 121: 520-527.
Byrne JJ, Carbone JP, & Pepe MG (1988) Suppression of serum adrenal
cortex hormones by chronic low-dose polychlorobiphenyl or
polybromobiphenyl treatments. Arch Environ Contam Toxicol, 17:
47-53.
Cagen SZ & Gibson JE (1977a) Ouabain lethality as a measure of
biliary function in developing mice and rats and effect of
polybrominated biphenyls. Toxicol Appl Pharmacol, 40: 327-334.
Cagen SZ & Gibson JE (1977b) Stimulation of biliary function
following polybrominated biphenyls. Abstract: 6th Annual Meeting.
Toxicol Appl Pharmacol, 41: 147.
Cagen SZ & Gibson JE (1978) Effect of polybrominated biphenyls on
hepatic excretory function in rats and mice. Environ Health
Perspect, 23: 233-239.
Cagen SZ, Preache MM, & Gibson JE (1977) Enhanced disappearance of
drugs from plasma following polybrominated biphenyls. Toxicol Appl
Pharmacol, 40: 317-325.
Carter LJ (1976) Michigan's PBB incident: chemical mix-up leads to
disaster. Science, 192: 240-243.
Castracane VD, Allen-Rowlands CF, Hamilton MG, & Seifter J (1982)
The effect of polybrominated biphenyl on testes, adrenal and
pituitary function in the rat. Proc Soc Exp Biol Med, 169: 343-347.
CEC (Commission of the European Communities) (1988) [Council
Regulation No. 1734/88 of 16 June 1988, concerning export from and
import into the Community of certain dangerous chemicals.] Off J Eur
Communities, L 155: 2-6 (in German).
Cecil HC & Bitman J (1978) Toxicity of polybrominated biphenyl and
its effects on reproduction of White Leghorn hens. Poult Sci, 57:
1027-1036.
Cecil HC, Bitman J, Lillie RJ, Fries GF, & Verrett J (1974)
Embryotoxic and teratogenic effects in unhatched fertile eggs from
hens fed polychlorinated biphenyls (PCBs). Bull Environ Contam
Toxicol, 11: 489-495.
Cecil HC, Harris SJ, & Bitman J (1975) Effects of polychlorinated
biphenyls and terphenyls and polybrominated biphenyls on
pentobarbital sleeping times of Japanese quail. Arch Environ Contam
Toxicol, 3: 183-192.
CFK (1982) [Potassiumbromide fire protection equipment: Information
sheet.] Köln, Chemische Fabrik Kalk GmbH (in German).
Chanda JJ, Anderson HA, Glamb RW, Lomatch DL, Wolff MS, Voorhees JJ,
& Selikoff IJ (1982) Cutaneous effects of exposure to polybrominated
biphenyls (PBBs): the Michigan PBB incident. Environ Res, 29:
97-108.
Chang TS & Zindel HC (1975) Toxic effect of polybrominated biphenyl
on young poultry. Poult Sci, 54: 1743-1744.
Chiang TCH, Liao W, & Williams LR (1987) Use of solid phase florisil
cartridges to separate fat from semivolatile organic compounds in
adipose tissue. J Assoc Off Anal Chem, 70: 100-102.
Choi WW & Chen KY (1976) Associations of chlorinated hydrocarbons
with fine particles and humic substances in nearshore surficial
sediments. Environ Sci Technol, 10: 782-786.
Chou SF, Jacobs LW, Penner D, & Tiedje JM (1978) Absence of plant
uptake and translocation of polybrominated biphenyls (PBBs). Environ
Health Perspect, 23: 9-12.
Christensen DC & Weimer WC (1979) Enhanced photodegradation of
persistent halogenated organic materials. In: Bell JM ed.
Proceedings of the 34th Industrial Waste Conference, Purdue
University, Lafayette, Indiana, 8-10 May 1979. Ann Arbor, Michigan,
Ann Arbor Science Publishers, Inc., pp 160-167.
Chu I, Villeneuve DC, Becking GC, Iverson F, Ritter L, Valli VE, &
Reynolds LM (1980) Short-term study of the combined effects of
mirex, photomirex, and kepone with halogenated biphenyls in rats. J
Toxicol Environ Health, 6: 421-432.
Clark RR, Chian ES, & Griffin RA (1979) Degradation of
polychlorinated biphenyls by mixed microbial cultures. Appl Environ
Microbiol, 4: 680-685.
Cook H, Helland DR, Van der Weele BH, & de Jong RJ (1978a)
Histotoxic effects of polybrominated biphenyls in Michigan dairy
cattle. Environ Res, 15: 82-89.
Cook RM, Prewitt LR, & Fries GF (1978b) Effects of activated carbon,
phenobarbital, and vitamins A, D, and E on polybrominated biphenyl
excretion in cows. J Dairy Sci, 61: 414-419.
Corbett TH, Beaudoin AR, Cornell RG, Anver MR, Schumacher R, Endres
J, & Szwabowska M (1975) Toxicity of polybrominated biphenyls
(FireMaster BP-6) in rodents. Environ Res, 10: 390-396.
Corbett TH, Simmons JL, Kawanishi H, & Endres JL (1978a) EM changes
and other toxic effects of FireMaster BP-6 (polybrominated
biphenyls) in the mouse. Environ Health Perspect, 23: 275-281.
Corbett TH, Simmons JL, & Endres J (1978b) Teratogenicity and tissue
distribution studies of polybrominated biphenyls (FireMaster BP-6)
in rodents. Teratology, 17: 37A.
Cordle F, Corneliussen P, Jelinek C, Hackley B, Lehmann R,
McLaughlin J, Rhoden R, & Shapiro R (1978) Human exposure to
polychlorinated biphenyls and polybrominated biphenyls. Environ
Health Perspect, 24: 157-172.
Cottrell WO, Ringer RK, & Babish JG (1984) Acute toxicity of dietary
polybrominated biphenyls in bobwhite quail. Bull Environ Contam
Toxicol, 33: 308-312.
Crow KD (1983) Chloracne (Halogen Acne). In: Marzulli FN & Maibach
HI ed. Determatotoxicology, 2nd ed. Washington, DC, Hemisphere
Publishing Company, pp 461-481.
Cullum A & Zile MH (1985) Acute polybrominated biphenyl toxicosis
alters vitamin A homeostasis and enhances degradation of vitamin A.
Toxicol Appl Pharmacol, 81: 177-181.
Damstra T, Jurgelski W, Posner HS, Vouk VB, Bernheim NJ, Guthrie J,
Luster M, & Falk HL (1982) Toxicity of polybrominated biphenyls
(PBBs) in domestic and laboratory animals. Environ Health Perspect,
44: 175-188.
Dannan GA, Moore RW, Besaw LC, & Aust SD (1978a)
2,4,5,3',4',5'-hexabromobiphenyl is both a 3-methylcholanthrene- and
a phenobarbital-type inducer of microsomal drug metabolizing
enzymes. Biochem Biophys Res Commun, 85: 450-458.
Dannan GA, Moore RW, & Aust SD (1978b) Studies on the microsomal
metabolism and binding of polybrominated biphenyls (PBBs). Environ
Health Perspect, 23: 51-61.
Dannan GA, Sleight SD, & Aust SD (1980) Toxicity and enzyme
induction effects of polybrominated biphenyls. Pharmacologist, 22:
158.
Dannan GA, Sleight SD, & Aust SD (1982a) Toxicity and microsomal
enzyme induction effects of several polybrominated biphenyls of
FireMaster. Fundam Appl Toxicol, 2: 313-321.
Dannan GA, Mileski GJ, & Aust SD (1982b) Reconstitution of some
biochemical and toxicological effects of commercial mixture of
polybrominated biphenyls. Fundam Appl Toxicol, 2: 322-326.
Dannan GA, Sleight SD, Fraker PJ, Krehbiel JD, & Aust SD (1982c)
Liver microsomal enzyme induction and toxicity studies with
2,4,5,3',4'-pentabromobiphenyl. Toxicol Appl Pharmacol, 64: 187-203.
Dannan GA, Mileski GJ, & Aust SD (1982d) Purification of
polybrominated biphenyl congeners. J Toxicol Environ Health, 9:
423-438.
Dannan GA, Guengerich FP, Kaminsky LS, & Aust SD (1983) Regulation
of cytochrome P-450: Immunochemical quantitation of 8 isozymes in
liver microsomes of rats treated with polybrominated biphenyl
congeners. J Biol Chem, 258: 1282-1288.
Darjono, Sleight SD, Stowe HD, & Aust SD (1983) Vitamin A status,
polybrominated biphenyl (PBB) toxicosis, and common bile duct
hyperplasia in rats. Toxicol Appl Pharmacol, 71: 184-193.
Daum SM, Knittle J, Rosenman K, Ron WN, & Holstein EC (1978) A
simple technique for fat biopsy of PBB-exposed individuals. Environ
Health Perspect, 23: 183-185.
Debets FMH, Hamers WJHMB, & Strik JJTWA (1980) Metabolism as a
prerequisite for the porphyrinogenic action of polyhalogenated
aromatics, with special reference to hexachlorobenzene and
polybrominated biphenyls (FireMaster BP-6). Int J Biochem, 12:
1019-1025.
DeCarlo VJ (1979) Studies on brominated chemicals in the
environment. Ann NY Acad Sci, 320: 678-681.
De Kok JJ, De Kok A, Brinkman UAT, & Kok RM (1977) Analysis of
polybrominated biphenyls. J Chromatogr, 142: 367-383.
Dent JG (1978) Characteristics of cytochrome P-450 and mixed
function oxidase enzymes following treatment with PBBs. Environ
Health Perspect, 23: 301-307.
Dent JG, Netter KF, & Gibson JE (1976a) Effects of chronic
administration of polybrominated biphenyls on parameters associated
with hepatic drug metabolism. Res Commun Chem Pathol Pharmacol, 13:
75-82.
Dent JG, Netter KF, & Gibson JE (1976b) The induction of hepatic
microsomal metabolism in rats following acute administration of a
mixture of polybrominated biphenyls. Toxicol Appl Pharmacol, 38:
237-249.
Dent JG, Roes U, Netter KJ, & Gibson E (1977a) Stimulation of
hepatic microsomal metabolism in mice by a mixture of polybrominated
biphenyls. J Toxicol Environ Health, 3: 651-661.
Dent JG, Cagen SZ, McCormack KM, Rickert DE, & Gibson JE (1977b)
Liver and mammary arylhydrocarbon hydroxylase and epoxide hydratase
in lactating rats fed polybrominated biphenyls. Life Sci, 20:
2075-2080.
Dent JG, Cagen SZ, McCormack KM, Rickert DE, & Gibson JG (1977c)
Microsomal enzyme induction in maternal liver, kidney, mammary
glands and neonatal liver following polybrominated biphenyls. Fed
Proc, 36: 1009.
Dent JG, Elcombe CR, Netter KJ, & Gibson JE (1978a) Rat hepatic
microsomal cytochrome(s) P-450 induced by polybrominated biphenyls.
Drug Metab Dispos, 6: 96-101.
Dent JG, McCormack KM, Rickert DE, Cagen SZ, Melrose P, & Gibson JE
(1978b) Mixed function oxidase activities in lactating rats and
their offspring following dietary exposure to polybrominated
biphenyls. Toxicol Appl Pharmacol, 46: 727-735.
Detering CN, Prewitt RL, Cook RM, & Fries GF (1975) Placental
transfer of polybrominated biphenyl by Holstein cows. J Dairy Sci,
58: 764-765.
Devito M, Umbreit TH, Thomas T, & Gallo MA (1991) An analogy between
the actions of the Ah receptor and the estrogen receptor for use in
the biological basis for risk assessment of dioxin. In: Gallo MA,
Scheuplein RJ, & van der Heijden KE ed. Biological basis for risk
assessment of dioxins and related compounds. Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory Press, pp 367-377 (Banbury
Report No. 35).
Dharmo DN, Sleight SD, Ringer RK, & Aust SD (1982) Pathologic
effects of 2,2',4,4',5,5'- and 2,3',4,4',5,5'-hexabromobiphenyl in
White Leghorn cockerels. Avian Dis, 26: 542-552.
Di Carlo FJ, Seifter J, & DeCarlo VJ (1978) Assessment of the
hazards of polybrominated biphenyls. Environ Health Perspect, 23:
351-365.
Diercxsens P, DeWeck D, Borsinger N, Rosset B, & Tarradellas J
(1985) Earthworm contamination by PCBs and heavy metals.
Chemosphere, 14: 511-522.
Dixon D, Sleight SD, Aust SD, Jensen RK, & Rezabek MS (1985)
Assessment of 3,4,3',4'-tetrabromobiphenyl (3,4-TBB) as a promotor
or initiator of experimental hepatocarcinogenesis in rats.
Toxicologist, 5: 12.
Dixon D, Sleight SD, Aust SD, & Rezabek MS (1988) Tumor-promoting,
initiating, and hepatotoxic effects of 3,4,3',4'-tetrabromobiphenyl
(34-TBB) in rats. J Am Coll Toxicol, 7: 687-697.
Domino EF & Domino SE (1980) Gas chromatographic-mass spectrometric
analysis of polybrominated biphenyl constituents of FireMaster FF-1.
J Chromatogr, 197: 258-262.
Domino EF, Wright DD, & Domino SE (1980a) GC-EC analysis of
polybrominated biphenyl constituents of FireMaster FF-1 using
tetrabromobiphenyl as an internal standard. J Anal Toxicol, 4:
299-304.
Domino EF, Fivenson DP, & Domino SE (1980b) Differential tissue
distribution of various polybrominated biphenyls of FireMaster FF-1
in male rats. Drug Metab Dispos, 8: 332-336.
Domino LE, Domino SE, & Domino EF (1982) Toxicokinetics of
2,2',4,4',5,5'-hexabromo biphenyl in the rat. J Toxicol Environ
Health, 9: 815-833.
Doucette WJ & Andren AW (1987) Correlation of octanol/water
partition coefficients and total molecular surface area for highly
hydrophobic aromatic compounds. Environ Sci Technol, 21: 821-824.
Doucette WJ & Andren AW (1988) Aqueous solubility of selected
biphenyl, furan, and dioxin congeners. Chemosphere, 17: 243-252.
Dunckel AE (1975) An updating on the polybrominated biphenyl
disaster in Michigan. J Am Vet Med Assoc, 167: 838-841.
Durst HI, Willett LB, Brumm CJ, & Mercer HD (1977) Effects of
polybrominated biphenyls on health and performance of pregnant
Holstein heifers. J Dairy Sci, 60: 1294-1300.
Durst HI, Willett LB, Brumm CJ, & Schanbacher FL (1978a) Changes in
blood and urine composition from feeding polybrominated biphenyls to
pregnant Holstein heifers. J Dairy Sci, 61: 197-205.
Durst HI, Willett LB, Schanbacher FL, & Moorhead PD (1978b) Effects
of PBBs on cattle. I. Clinical evaluations and clinical chemistry.
Environ Health Perspect, 23: 83-89.
Eaton DL & Klaassen CD (1979) Effects of
2,3,7,8-tetrachlorodibenzo-p-dioxin, kepone, and polybrominated
biphenyls on transport systems in isolated rat hepatocytes. Toxicol
Appl Pharmacol, 51: 137-144.
Eckhoff MA, Ridgway TH, & Caruso JA (1983) Polychromator system for
multielement determination by gas chromatography with helium plasma
atomic emission spectrometric detection. Anal Chem, 55: 1004-1009.
Ecobichon DJ, Hansell MM, & Safe S (1977) Halogen substituents at
the 4- and 4'-positions of biphenyl: influence on hepatic function
in the rat. Toxicol Appl Pharmacol, 42: 359-366.
Ecobichon DJ, Hansell MM, & Safe S (1979) Isomerically pure
bromobiphenyl congeners and hepatic mono-oxygenase activities in the
rat: influence of position and degree of bromination. Toxicol Appl
Pharmacol, 47: 341-352.
Ecobichon DJ, Hidvegi S, Comeau AM, & Cameron PH (1983)
Transplacental and milk transfer of polybrominated biphenyls to
perinatal guinea-pigs from treated dams. Toxicology, 28: 51-63.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Elcombe CR & Lech JJ (1978) Induction of monooxygenation in rainbow
trout by polybrominated biphenyls: a comparative study. Environ
Health Perspect, 23: 309-314.
El-Fouly MH, Trosko JE, & Chang CC (1987) Scrape-loading and dye
transfer: a rapid and simple technique to study gap junctional
intercellular communication. Exp Cell Res, 168: 422-430.
Epling GA, McVicar W, & Kumar A (1987) Accelerated debromination of
biphenyls by photolysis with sodium borohydride. Chemosphere, 16:
1013-1020.
Erickson MD, Kelner L, Bursey JT, Rosenthal D, Zweidinger RA, &
Pellizzari ED (1980) Method for analysis of polybrominated biphenyls
by gas chromatography mass spectrometry. Biomed Mass Spectrom, 7:
99-104.
Erney DR (1975) Confirmation of polybrominated biphenyl residues in
feeds and dairy products, using an ultraviolet
irradiation-gas-liquid chromatographic technique. J Assoc Off Anal
Chem, 58: 1202-1205.
Evans MG, El-Fouly MH, Trosko JE, & Sleight SD (1988) Anchored cell
analysis/sorting coupled with the scrape-loading/dye-transfer
technique to quantify inhibition of gap-junctional intercellular
communication in WB-F344 cells by 2,2',4,4',5,5'-hexabromo biphenyl.
J Toxicol Environ Health, 24: 261-271.
Evers WD, Hook JB, & Bond JT (1977) Effect of polybrominated
biphenyls on renal tubular transport of organic ions. J Toxicol
Environ Health, 3: 759-767.
Eyster JT, Humphrey HEB, & Kimbrough RD (1983) Partitioning of
polybrominated biphenyls in (PBBs) in serum, adipose tissue, breast
milk, placenta, cord blood, biliary fluid, and feces. Arch Environ
Health, 38: 47-53.
Falkner R & Simonis W (1982) [Polychlorinated biphenyls (PCBs) in
the aquatic environment (uptake and accumulation by organisms -
problems related on the passage through the food pyramid.] Arch
Hydrobiol Beih, 17: 1-74 (in German).
Farber TM, Balazs T, Marks E, & Cerra F (1976) The influence of
microsomal induction on serum alkaline phosphate activity in dogs.
Fed Proc, 35: A943.
Farber T, Kasza L, Giovetti A, Carter C, Earl F, & Balazs T (1978)
Effect of polybrominated biphenyls (FireMaster BP-6) on the
immunologic system of the beagle dog. Toxicol Appl Pharmacol, 45:
343.
Farrell TJ (1980) Glass capillary gas chromatography of chlorinated
dibenzofurans, chlorinated anisoles, and brominated biphenyls. J
Chromatogr Sci, 18: 10-17.
Fawkes J, Albro PW, Walters DB, & McKinney JD (1982) Comparison of
extraction methods for determination of polybrominated biphenyl
residues in animal tissue. Anal Chem, 54: 1866-1871.
Fehringer NV (1975a) Determination of polybrominated biphenyl
residues in dairy products. J Assoc Off Anal Chem, 58: 978-982.
Fehringer NV (1975b) Determination of polybrominated biphenyl
residues in dry animal feeds. J Assoc Off Anal Chem, 58: 1206-1210.
Ficsor G & Wertz GF (1976) Polybrominated biphenyl non-teratogenic,
c-mitosis synergist in rat. Mutat Res, 38: 388.
Filonow AB, Jacobs LW, & Mortland MM (1976) Fate of polybrominated
biphenyls (PBB's) in soils. Retention of hexabromobiphenyl in four
Michigan soils. J Agric Food Chem, 24: 1201-1204.
Fisher DL (1980) Effect of polybrominated biphenyls on the
accumulation of DNA, RNA, and protein in cultured rat embryos
following maternal administration. Environ Res, 23: 334-340.
Fraker PJ (1980) The antibody-mediated and delayed type
hypersensitivity response of mice exposed to polybrominated
biphenyls. Toxicol Appl Pharmacol, 53: 1-7.
Fraker PJ & Aust S (1978) The effect of polybrominated biphenyls on
the immune response of Balb/c mice. In: Asher JM ed. Inadvertent
modifications of the immune response: The effects of foods, drugs
and environmental contaminants. Proceeding of the Fourth FDA Science
Symposium, US Naval Academy, 28-30 August 1978. Rockville, Maryland,
Food and Drug Administration, Office of Health Affairs, pp 270-271
(HHS Publication No. (FDA) 80-1074).
Franklin RB, Vodicnik MJ, Elcombe CR, & Lech JJ (1981) Alterations
in hepatic mixed-function oxidase activity of rainbow trout after
acute treatment with polybrominated biphenyl isomers and FireMaster
BP-6. J Toxicol Environ Health, 7: 817-827.
Freeman PK, Jang J-S, & Ramnath N (1991) The photochemistry of
polyhaloarenes. 10. The photochemistry of 4-bromobiphenyl. J Org
Chem, 56: 6072-6079.
Fries GF (1982) Potential polychlorinated biphenyl residues in
animal products from application of contaminated sewage sludge to
land. J Environ Qual, 11: 14-20.
Fries GF (1983) Effect of low exposure to polybrominated biphenyl on
production and other indicators of dairy herd performance. J Dairy
Sci, 66: 1303-1311.
Fries GF (1985a) Bioavailability of soil-borne polybrominated
biphenyls ingested by farm animals. J Toxicol Environ Health, 16:
565-579.
Fries GF (1985b) The PBB episode in Michigan: an overall appraisal.
CRC Crit Rev Toxicol, 16: 105-156.
Fries GF & Jacobs LW (1980) Residual polybrominated biphenyl
contamination: locations, amounts and significance on dairy farms. J
Dairy Sci, 64: 114.
Fries GF & Jacobs LW (1986) Evaluation of residual polybrominated
biphenyl contamination present on Michigan farms in 1978. East
Lansing, Michigan State University, Agricultural Experiment Station,
15 pp (Research Report Farm Science-477).
Fries GF & Marrow GS (1975) Excretion of polybrominated biphenyls
into the milk of cows. J Dairy Sci, 58: 947-951.
Fries GF & Marrow GS (1982) Residues in the fat of ewes grazing on
soil contaminated with halogenated hydrocarbons. J Anim Sci, 55:
1118-1124.
Fries GF, Cecil HC, Bitman J, & Lillie RJ (1976) Retention and
excretion of polybrominated biphenyls by hens. Bull Environ Contam
Toxicol, 15: 278-282.
Fries GF, Marrow GS, & Cook RM (1978a) Distribution and kinetics of
PBB residues in cattle. Environ Health Perspect, 23: 43-50.
Fries GF, Cook RM, & Prewitt LR (1978b) Distribution of
polybrominated biphenyl residues in the tissues of environmentally
contaminated dairy cows. J Dairy Sci, 61: 420-425.
Fries GF, Marrow GS, & Snow PA (1982a) Soil ingestion by swine as a
route of contaminant exposure. Environ Toxicol Chem, 1: 201-204.
Fries GF, Marrow GS, & Snow PA (1982b) Soil ingestion by dairy
cattle. J Dairy Sci, 65: 611-618.
Furukawa K & Matsumura F (1976) Microbial metabolism of
polychlorinated biphenyls. Studies on the relative degradability of
polychlorinated biphenyl components by Alkaligenes sp. J Agric
Food Chem, 24: 251-256.
Furukawa K, Tomizuka N, & Kamibayashi A (1979) Effect of chlorine
substitution on the bacterial metabolism of various polychlorinated
biphenyls. Appl Environ Microbiol, 38: 301-310.
Galloway SM, Armstrong JJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, & Zeiger E (1987) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster ovary cells: evaluations of
108 chemicals. Environ Mol Mutagen, 10: 1-175.
Gardner AM, Righter HF, & Roach AG (1976) Excretion of hydroxylated
polychlorinated biphenyl metabolites in cows' milk. J Assoc Off Anal
Chem, 59: 273-277.
Gardner AM, Warren VL, Chen JYT, & Mazzola EP (1979) A metabolite of
poly brominated biphenyls: its identification and decomposition to a
brominated dibenzofuran in the gas chromatograph mass spectrometer.
J Agric Food Chem, 27: 116-119.
Garthoff LH, Friedman L, Farber TM, Locke KK, Sobotka TJ, Green S,
Hurley NE, Peters EL, Story GE, Moreland FM, Graham CH, Keys JE,
Taylor MJ, Scalera JV, Rothlein JE, Marks EM, Cerra FE, Rodi SB, &
Sporn EM (1977) Biochemical and cytogenetic effects in rats caused
by short-term ingestion of Aroclor 1254 or FireMaster BP6. J Toxicol
Environ Health, 3: 769-796.
Gause EM, Ross DH, Hamilton MG, Leal BZ, Seifter J, & Geller I
(1979) Correlation of systemic and biochemical effects of PBB with
behavioral effects. Neurobehav Toxicol, 1: 269-274.
Gause EM, Leal BZ, Hartmann RJ, & Geller I (1984) Influence of
maternal PBB body burden on the response to drugs. Proc West
Pharmacol Soc, 27: 501-506.
Geller I, Gause E, Hartmann RJ, & Seifter J (1979) Use of
discrimination behavior for the evaluation of toxicants. Neurobehav
Toxicol, 1: 9-13. Geller I, Gause EM, Leal BZ, Hartmann RJ, &
Seifter J (1985) Behavioral effects of drugs as a function of
maternal polybrominated biphenyl body burden. Toxicol Lett, 24:
229-234.
Getty SM, Rickert DE, & Trapp AL (1977) Polybrominated biphenyl
(PBB) toxicosis: an environmental accident. CRC Crit Rev Environ
Control, 7(4): 309-323.
Gladen B & Rogan W (1979) Misclassification and the design of
environmental studies. Am J Epidemiol, 109: 607-619.
Gobas FA, Lahittete JM, Garofalo G, Shiu WY, & MacKay D (1988) A
novel method for measuring membrane-water partition coefficients of
hydrophobic organic chemicals: comparison with 1-octanol-water
partitioning. J Pharmacol Sci, 77: 265-272.
Gobas FA, Clark KE, Shiu WY, & MacKay D (1989) Bioconcentration of
polybrominated benzenes and biphenyls and related superhydrophobic
chemicals in fish: role of bioavailability and elimination into the
feces. Environ Toxicol Chem, 8: 231-245.
Goldstein JA (1979) The structure-activity relationships of
halogenated biphenyls as enzyme inducers. Ann NY Acad Sci, 320:
164-178.
Goldstein JA (1980) Structure-activity relationships for the
biochemical effects and the relationship to toxicity. In: Kimbrough
RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 151-178.
Goldstein JA, Linko PC, Levy LA, McKinney JD, Gupta BN, & Moore JA
(1979) A comparison of a commercial polybrominated biphenyl mixture
2,4,5,2',4',5'-hexabromo biphenyl and 2,3,6,7-tetrabromonaphthalene
as inducers of liver microsomal drug-metabolizing enzymes. Biochem
Pharmacol, 28: 2947-2956.
Great Lakes Chemical Corp. (1986) Product information: Great Lakes
Chemical flame retardants. Effective flame retardant performance for
a variety of polymer systems. West Lafayette, Indiana, Great Lakes
Chemical Corp., 6 pp.
Greaves J, Bekesi JG, & Roboz J (1982) Halogen anion formation by
polybrominated compounds in negative chemical ionization mass
spectrometry. Biomed Mass Spectrom, 9: 406-410.
Greaves J, Bekesi JG, Holland JF, & Roboz J (1983) Studies on the
protein binding of hexabromobiphenyl (HxBB) in 14C-HxBB spiked and
environmentally contaminated serum. In: The Thirty-First Annual
Conference on Mass Spectrometry and Allied Topics, Boston, 8-13 May
1983. East Lansing, Michigan, American Society for Mass
Spectrometry, pp 475-476.
Greaves J, Roboz J, Holland JF, & Bekesi JG (1984) Determination of
the binding of polybrominated biphenyls to serum proteins by
negative chemical ionization mass spectrometry. Chemosphere, 13:
651-656.
Griffin RA & Chou SFJ (1980) Attenuation of polybrominated biphenyls
and hexachlorobenzene by earth materials. Urbana, Illinois,
Institute of Natural Resources, State Geological Survey Division, 53
pp (Environmental Geology Note No. 87).
Griffin RA & Chou SFJ (1981a) Attenuation of polybrominated
biphenyls and hexachlorobenzene by earth materials. Washington, DC,
US Environmental Protection Agency (EPA-600/2-81-186).
Griffin RA & Chou SFJ (1981b) Movement of PCBs and other persistent
compounds through soil. Water Sci Technol, 13: 1153-1163.
Groce DF & Kimbrough RD (1984) Stunted growth, increase mortality,
and liver tumors in offspring of polybrominated biphenyl dosed
Sherman rats. J Toxicol Environ Health, 14: 695-706.
Gupta BN & Moore JA (1979) Toxicologic assessments of a commercial
polybrominated biphenyl mixture in the rat. Am J Vet Res, 40:
1458-1468.
Gupta BN, McConnell EE, Harris MW, & Moore JA (1981) Polybrominated
biphenyl toxicosis in the rat and mouse. Toxicol Appl Pharmacol, 57:
99-118.
Gupta BN, McConnell EE, Goldstein JA, Harris MW, & Moore JA (1983a)
Effects of a polybrominated biphenyl mixtures in the rat and mouse.
I. Six-month exposure. Toxicol Appl Pharmacol, 68: 1-18.
Gupta BN, McConnell EE, Moore JA, & Haseman JK (1983b) Effects of
polybrominated biphenyl mixture in the rat and mouse. 2. Lifetime
study. Toxicol Appl Pharmacol, 68: 19-35.
Gutenmann WH & Lisk DJ (1975) Tissue storage and excretion in milk
of polybrominated biphenyls in ruminants. J Agric Food Chem, 23:
1005-1007.
Haake JM, Merrill JC, & Safe S (1985) The in vitro metabolism of
benzo[a]pyrene by polychlorinated and polybrominated biphenyl
induced rat hepatic microsomal monooxygenases. Can J Physiol
Pharmacol, 63: 1096-1100.
Halvorson MR, Phillips TD, Safe SH, & Robertson LW (1985) Metabolism
of aflatoxin B1 by rat hepatic microsomes induced by
polyhalogenated biphenyl congeners. Appl Environ Microbiol, 49:
882-886.
Haroz RK & Aust SD (1979) Assessment of tumor initiating and
promoting activity of a mixture of polybrominated biphenyls
(FireMaster BP-6), and certain purified isomers of PBB. Toxicol Appl
Pharmacol, 48: A158 (Abstract 317).
Harris SJ, Cecil HC, & Bitman J (1978a) Embryotoxic effects of
polybrominated biphenyls (PBB) in rats. Environ Health Perspect, 23:
295-300.
Harris SJ, Cecil HC, & Bitman J (1978b) Effects of feeding of
polybrominated biphenyl flame retardant (FireMaster BP-6) to male
rats. Bull Environ Contam Toxicol, 19: 692-696.
Haseltine SD, Heinz GH, Reichel WL, & Moore JF (1981) Organochlorine
and metal residues in eggs of waterfowl nesting on islands in Lake
Michigan off Door County, Wisconsin, USA. Pestic Monit J, 15: 90-97.
Haseman JK, Crawford DD, Huff JE, Boormann GA, & McConnell EE (1984)
Results from 86 2-year carcinogenicity studies conducted by the
National Toxicology Program. J Toxicol Environ Health, 14: 621-640.
Hass JR, McConnell EE, & Harvan DJ (1978) Chemical and toxicologic
evaluation of FireMaster BP-6. J Agric Food Chem, 26: 94-99.
Hassett JP & Anderson MA (1979) Association of hydrophobic compounds
with dissolved organic matter in aquatic systems. Environ Sci
Technol, 13: 1526-1529.
Haworth S, Lawlor T, Mortelmans K, Speck W, & Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ
Mutagen, 1(Suppl): 3-142.
Head SL & Burse VW (1987) Organochlorine recovery from small adipose
samples with the universal trace residue extractor (Unitrex). Bull
Environ Contam Toxicol, 39: 848-856.
Healy WB (1968) Ingestion of soil by dairy cows. NZ J Agric Res, 11:
487-499.
Healy WB, Cutress TW, & Michie C (1967) Wear of sheep's teeth. IV.
Reduction of soil ingestion and tooth wear by supplementary feeding.
NZ J Agric Res, 10: 201-209.
Heinemann FW & Ringer RK (1976) Cardiovascular effects of a
halogenated hydrocarbon, polybrominated biphenyl. Fed Proc, 35: 399.
Heinz GH, Haseltine SD, Reichel WL, & Hensler GL (1983)
Relationships of environmental contaminants to reproductive success
in red-breasted mergansers Mergus serrator from Lake Michigan.
Environ Pollut, A32: 211-232.
Heinz GH, Erdmann TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henck JW (1986) Neurobehavioral toxicity of perinatally administered
polybrominated biphenyls. Diss Abstr Int, B46: 2214-2215.
Henck JW & Rech RH (1986) Effect of perinatal polybrominated
biphenyl exposure on acquisition and performance of an autoshaping
paradigm. Neurotoxicology, 7: 651-663.
Hesse JL (1975) Water pollution aspects of PBB production: results
of surveys in the pine river in the vicinity of St. Louis, Michigan.
In: Proceedings of the Second National Conference on Complete Water
Reuse: Water's Interface with Energy, Air and Solids, Chicago, 4-8
May 1975. New York, American Institute of Chemical Engineers, pp
1190-1199.
Hesse JL & Powers RA (1978) Polybrominated biphenyl (PBB)
contamination of the Pine River, Gratiot, and Midland Counties,
Michigan. Environ Health Perspect, 23: 19-25.
Hill RH Jr (1985) Effects of polyhalogenated aromatic compounds on
porphyrin metabolism. Environ Health Perspect, 60: 139-143.
Hill RH, Patterson DG, Orti DL, Holler JS, Needham LL, Sirmans SL, &
Liddle JA (1982) Evidence of degradation of polybrominated biphenyls
in soil samples from Michigan. J Environ Sci Health, B17: 19-33.
Hinton DE, Trump BF, Kasza L, Weinberger MA, Friedman L, & Garthoff
LH (1979) Comparative toxicity of polychlorinated biphenyl (PCB) and
polybrominated biphenyl (PBB) in the liver of Holtzman rats: light
and electron microscopic alterations. In: Animals as monitors of
environmental pollutants. Washington, DC, National Academy of
Sciences, pp 382-383.
Hoel DG, Hasemann JK, Hogan MD, Huff J, & McConnell EE (1988) The
impact of toxicity on carcinogenicity studies: implications for risk
assessment. Carcinogenesis, 9: 2045-2052.
Höfler F, Melzer H, Möckel HJ, Robertson LW, & Anklam E (1988)
Relationship between liquid and gas chromatographic retention
behavior and calculated molecular surface area of selected
polyhalogenated biphenyls. J Agric Food Chem, 36: 961-965.
Howard SK, Werner PR, & Sleight SD (1980) Polybrominated biphenyl
toxicosis in swine: effects on some aspects of the immune system in
lactating sows and their offspring. Toxicol Appl Pharmacol, 55:
146-153.
Huang IC (1971) Effect of selected factors on pesticide sorption and
desorption in the aquatic system. J Water Pollut Control Fed, 43:
1739-1748.
Humble CG & Speizer FE (1984) Polybrominated biphenyls and fetal
mortality in Michigan. Am J Public Health, 74: 1130-1132.
Humphrey HEB & Hayner NS (1975) Polybrominated biphenyls: an
agricultural incident and its consequences. II. An epidemiological
investigation of human exposure. Trace Subst Environ Health, 9:
57-63.
Hutzinger O, Berg MVD, Olie K, Opperhuizen A, & Safe S (1985a)
Dioxins and furans in the environment: evaluating toxicological risk
from different sources by multi-criteria analysis. In: Kamrin MA &
Rodgers PW ed. Dioxins in the environment: Symposium, East Lansing,
Michigan, 6-9 December 1983. New York, Hemisphere Publishing
Company, pp 9-32.
Hutzinger O, Blumich MJ, Berg MVD, & Olie K (1985b) Sources and fate
of PCDDs and PCDFs: an overview. Chemosphere, 14: 581-600.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer, 140 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Volume 18).
IARC (1986) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer, 434 pp (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals
to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: an updating of
IARC Monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 434 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Irving HA, Willett LB, Brumm CJ, & Mercer HD (1976) The effects of
polybrominated biphenyls on growth, feed intake, and health of
pregnant heifers. Am Dairy Sci Assoc Annu Meet, 71: 79.
Isbister JL (1977) PBBs in human health. Clin Med, 84: 22-24.
Isleib DR & Whitehead GL (1975) Polybrominated biphenyls: an
agricultural incident and its consequences. I. The agricultural
effects of exposure. Trace Subst Environ Health, 9: 47-55.
Iwata Y, Gunther FA, & Westlake WE (1974) Uptake of a PCB (Aroclor
1254) from soil by carrots under field conditions. Bull Environ
Contam Toxicol, 11: 523-528.
Jackson TF & Halbert FL (1974) A toxic syndrome associated with the
feeding of polybrominated biphenyl-contaminated protein concentrate
to dairy cattle. J Am Vet Med Assoc, 165: 436-439.
Jacobs LW, Chou SF, & Tiedje JM (1976) Fate of polybrominated
biphenyls (PBB's) in soils. Persistence and plant uptake. J Agric
Food Chem, 24: 1198-1201.
Jacobs LW, Chou SF, & Tiedje JM (1978) Field concentrations and
persistence of polybrominated biphenyls in soils and solubility of
PBB in natural waters. Environ Health Perspect, 23: 1-8.
Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, & Dowler JK (1984)
The transfer of polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs) across the human placenta and into maternal milk.
Am J Public Health, 74: 378-379.
Jacobson JL, Humphrey HEB, Jacobson SW, Schantz SL, Mullin MD, &
Welch R (1989) Determinants of polychlorinated biphenyls (PCBs),
polybrominated biphenyls (PBBs), and dichlorodiphenyl
trichloroethane (DDT) levels in the sera of young children. Am J
Public Health, 79: 1401-1404.
Jaffe R, Stemmler EA, Eitzer BD, & Hites RA (1985) Anthropogenic,
polyhalogenated, organic compounds in sedentary fish from Lake Huron
and Lake Superior tributaries and embayments. J Great Lakes Res, 11:
156-162.
James, MO & Bend JR (1982) Effect of polynuclear aromatic
hydrocarbons and polyhalogenated biphenyls on hepatic mixed-function
oxidase activity in marine fish. In: Richards NL & Jackson BL ed.
Proceedings of the Symposium on Carcinogenic Polynuclear Aromatic
Hydrocarbons in the Marine Environment, Pensacola Beach, Florida,
14-18 August 1978. Gulf Breeze, Florida, US Environmental Protection
Agency, pp 172-190 (EPA-600/9-83-013).
James MO & Little PJ (1981) Polyhalogenated biphenyls and
phenobarbital: evaluation as inducers of drug metabolizing enzymes
in the sheepshead, Archosargus probatocephalus. Chem-Biol
Interact, 36: 229-248.
Jamieson JWS (1977) Polybrominated biphenyls in the environment.
Ottawa, Environmental Protection Service (EPS-3E[-77-18]).
Jansson B, Asplund L, & Olsson M (1987) Brominated flame retardants
- ubiquitous environmental pollutants? Chemosphere, 16: 2343-2349.
Jansson B, Andersson R, Asplund L, Bergman A, Litzen K, Nylund K,
Reutergardh L, Sellström U, Uvemo U-B, Wahlberg C, & Wideqvist U
(1991) Multiresidue method for the gas-chromatographic analysis of
some polychlorinated and polybrominated pollutants in biological
samples. Fresenius J Anal Chem, 340: 439-445.
Jansson B, Andersson R, Asplund L, Litzen K, Nylund K, Sellström U,
Uvemo U-B, Wahlberg C, Wideqvist U, Odsjö T, & Olsson M (1993)
Chlorinated and brominated persistent organic compounds in
biological samples from the environment. Environ Toxicol Chem,
12(7): 1163-1174.
Jensen RK (1983) Pathologic effects and hepatic tumor promoting
ability of FireMaster BP-6, 3,3',4,4',5,5'-hexabromobiphenyl and
2,2',4,4',5,5'-hexabromobiphenyl in the rat. East Lansing, Michigan,
Michigan State University (Ph.D. Thesis).
Jensen RK & Sleight SD (1986) Sequential study on the synergistic
effects of 2,2',4,4',5,5'-hexabromobiphenyl on hepatic tumor
promotion. Carcinogenesis, 7: 1771-1774.
Jensen RK & Zile MH (1988) Effect of dietary retinoic acid on
circulatory vitamin A homeostasis in polybrominated biphenyl-treated
rats. J Nutr, 118: 416-419.
Jensen S, Renberg L, & Vaz R (1973) Problems in the quantification
of PCB in biological material. In: Ahnland E & Akerblom A ed. PCB
Conference II, Stockholm, 14 December 1972. Solna, Sweden, National
Environment Protection Board, pp 7-13.
Jensen RK, Sleight SD, Goodman JI, Aust SD, & Trosko JE (1982)
Polybrominated biphenyls as promoters in experimental
hepatocarcinogenesis in rats. Carcinogenesis, 3: 1183-1186.
Jensen RK, Sleight SD, & Aust SD (1983) Hepatic tumor promotion by
congeners of polybrominated biphenyls. Toxicologist, 3: 33
(Abstract).
Jensen RK, Sleight SD, & Aust SD (1984) Effect of varying the length
of exposure to polybrominated biphenyls on the development of
gamma-glutamyl transpeptidase enzyme-altered foci. Carcinogenesis,
5: 63-66.
Jensen RK, Cullum ME, Deyo J, & Zile MH (1987) Vitamin A metabolism
in rats chronically treated with 3,3',4,4',5,5'-hexabromobiphenyl.
Biochim Biophys Acta, 926: 310-320.
Johnston CA, Demarest KT, McCormack KM, Hook JB, & Moore KE (1980)
Endocrinological, neurochemical, and anabolic effects of
polybrominated biphenyls in male and female rats. Toxicol Appl
Pharmacol, 56: 240-247.
Jones DH, Kohli J, & Safe S (1979) Avian metabolism of halogenated
biphenyls. Xenobiotica, 9: 733-736.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Krynitsky AJ, Lamont
TG, Mulhern BM, Prouty RM, Stafford CJ, & Swineford DM (1980)
Organochlorine pesticide, PCB, and PBB residues and necropsy data
for bald eagles from 29 states - 1975-77. Pestic Monit J, 13:
145-149.
Kalmaz EV & Kalmaz GD (1979) Transport, distribution and toxic
effects of polychlorinated biphenyls in ecosystems: review. Ecol
Model, 6: 223-251.
Kasza L, Weinberger MA, Hinton DE, Trump BF, Patel C, Friedman L, &
Garthoff LH (1978a) Comparative toxicity of polychlorinated biphenyl
and polybrominated biphenyl in the rat liver: light and electron
microscopic alterations after subacute dietary exposure. J Environ
Pathol Toxicol, 1: 241-257.
Kasza L, Collins WT, Capen CC, Garthoff LH, & Friedman L (1978b)
Comparative toxicity of polychlorinated biphenyl and polybrominated
biphenyl in the rat thyroid gland: light and electron icroscoic
alterations after subacute dietary exposure. J Environ Pathol
Toxicol, 1: 587-599.
Kavanagh TJ, Rubinstein C, Liu PL, Chang C-C, Trosko JE, & Sleight
SD (1985) Failure to induce mutations in Chinese hamster V79 cells
and WB rat liver cells by the 2,2',4,4',5,5'-hexabromobiphenyl,
3,3',4,4',5,5'-hexabromobiphenyl, and 3,3',4,4'-tetra bromobiphenyl.
Toxicol Appl Pharmacol, 79: 91-98.
Kavanagh TJ, Chang C-C, & Trosko JE (1987) Effect of various
polybrominated biphenyls on cell-cell communication in cultured
human teratocarcinoma cells. Fundam Appl Toxicol, 8: 127-131.
Kay K (1977) Polybrominated biphenyls (PBB) environmental
contamination in Michigan, 1973-1976. Environ Res, 13: 74-93.
Kerscher U (1979) [Flame retardants containing bromine for the fire
protecting of plastics.] Kunstst J, 1: 6-16 (in German).
Kimbrough RD (1980a) Environmental pollution of air, water and soil.
In: Kimbrough RD ed. Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products. Amsterdam,
Oxford, New York, Elsevier/North-Holland Biomedical Press, pp 77-80.
Kimbrough RD (1980b) Occupational exposure. In: Kimbrough RD ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and
related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 373-397.
Kimbrough RD ed. (1980c) Halogenated biphenyls, terphenyls,
naphthalenes, dibenzodioxins and related products. Amsterdam,
Oxford, New York, Elsevier/North-Holland Biomedical Press.
Kimbrough RD (1987) Human health effects of polychlorinated
biphenyls (PCBs) and polybrominated biphenyls. Annu Rev Pharmacol
Toxicol, 27: 87-111.
Kimbrough RD, Burse VW, Liddle JA, & Fries GF (1977) Toxicity of
polybrominated biphenyl. Lancet, 2: 602-603.
Kimbrough RD, Burse VW, & Liddle JA (1978) Persistent liver lesions
in rats after a single oral dose of polybrominated biphenyls
(FireMaster FF-1) and concomitant PBB tissue levels. Environ Health
Perspect, 23: 265-273.
Kimbrough RD, Korver MP, Burse VW, & Groce DF (1980) The effect of
different diets or mineral oil on liver pathology and polybrominated
biphenyl concentration in tissues. Toxicol Appl Pharmacol, 52:
442-453.
Kimbrough RD, Groce DF, Korver MP, & Burse VM (1981) Induction of
liver tumors in female Sherman strain rats by polybrominated
biphenyls. J Natl Cancer Inst, 66: 535-542.
Kluwe WM & Hook JB (1981) Comparative induction of xenobiotic
metabolism in rodent kidney, testis and liver by commercial mixtures
of polybrominated biphenyls and polychlorinated biphenyls,
phenobarbital and 3-methylcholanthrene: absolute and temporal
effects. Toxicology, 20: 259-273.
Kluwe WM, McCormack KM, & Hook JB (1978) Potention of hepatic and
renal toxicity of various compounds by prior exposure to
polybrominated biphenyls. Environ Health Perspect, 23: 241-246.
Kluwe WM, Hermann CL, & Hook JB (1979) Effects of dietary
polychlorinated biphenyls and polybrominated biphenyls on the renal
and hepatic toxicities of several chlorinated hydrocarbon solvents
in mice. J Toxicol Environ Health, 5: 605-615.
Kluwe WM, McNish R, Smithson K, & Hook JB (1981) Depletion by
1,2-dibromoethane, 1,2-dibromo-3-chloropropane, tris
(2,3-dibromopropyl) phosphate and hexachloro-1,3-butadiene of
reduced nonprotein sulfhydryl groups in target and nontarget organs.
Biochem Pharmacol, 30: 2265-2272.
Kluwe WM, Hook JB, & Bernstein J (1982) Synergistic toxicity of
carbon tetrachloride and several aromatic organohalide compounds.
Toxicology, 23: 321-336.
Kohli J & Safe S (1976) The metabolism of brominated aromatic
compounds. Chemosphere, 6: 433-437.
Kohli J, Wyndham C, Smylie M, & Safe S (1978) Metabolism of
bromobiphenyls. Biochem Pharmacol, 27: 1245-1249.
Kohli KK, Gupta BN, Albro PW, & McKinney JD (1981) Effects of
inducers of drug metabolism enzymes on triglyceride and phospholipid
biosynthesis. Chem-Biol Interact, 36: 117-121.
Kong H-L & Sayler GS (1983) Degradation and total mineralization of
monohalogenated biphenyls in natural sediment and mixed bacterial
culture. Appl Environ Microbiol, 46: 666-672.
Koster P, Debets FMH, & Strik JJTWA (1980) Porphyrinogenic action of
fire retardants. Bull Environ Contam Toxicol, 25: 313-315.
Kraus AL & Bernstein IA (1986) Influence of adipocyte triglyceride
on the partition of 2,2',4,4',5,5'-hexabromobiphenyl between 3T3L1
adipocytes and surrounding pseudoblood. J Toxicol Environ Health,
19: 541-554.
Kraus AL & Bernstein IA (1989) Human lipoprotein influence on the
partition of 2,2',4,4',5,5'-hexabromobiphenyl between 3T3L1
adipocytes and culture medium. J Toxicol Environ Health, 26:
157-174.
Kreis RG & Rice CP (1985) Status of organic contaminants in Lake
Huron: atmosphere, water, algae, fish, herring gull eggs, and
sediment. Chicago, Illinois, US Environmental Protection Agency,
Great Lakes National Program Office (Special Report No. 114).
Kreiss K, Roberts C, & Humphrey HEB (1982) Serial PBB levels, and
clinical chemistries in Michigan's PBB cohort. Arch Environ Health,
37: 141-147.
Krüger C (1988) [Polybrominated biphenyls and polybrominated
biphenyl ether-detection and quantitation in selected foods.]
Münster, University of Münster (Thesis) (in German).
Krüger C, Fürst P, & Gröbel W (1988) [Detection and determination of
polybrominated biphenyls in human milk.] Dtsch Lebensm Rundsch, 84:
273-276 (in German).
Ku PK, Hogberg MG, Trapp AL, Brady PS, & Miller ER (1978)
Polybrominated biphenyl (PBB in the growing pig diet). Environ
Health Perspect, 23: 13-18.
Kubiczak GA, Oesch F, Borlakoglu JT, Kunz H, & Robertson LW (1989) A
unique approach to the synthesis of 2,3,4,5,-substituted
polybrominated biphenyls: quantitation in FireMaster FF-1 and
FireMaster BP-6. J Agric Food Chem, 37: 1160-1164.
Kuehl DW, Haebler R, & Potter C (1991) Chemical residues in dolphins
from the U.S. Atlantic coast including atlantic bottlenose obtained
during the 1987/88 mass mortality. Chemosphere, 22: 1071-1084.
Kuo CH & Hook JB (1982) Effects of drug-metabolizing enzyme inducers
on cephaloridine toxicity in Fischer 344 rats. Toxicology, 24:
293-303.
Lai DY (1984) Halogenated benzenes, naphthalenes, biphenyls and
terphenyls in the environment: their carcinogenic, mutagenic and
teratogenic potential and toxic effects. J Environ Sci Health, C2:
135-184.
Lambert GH, Humphrey H, & Schoeller D (1987) The effects of
polybrominated biphenyls (PBB) on the human cytochrome P-450 system
(P-450). Pedriatr Res, 21: 258 (Abstract).
Lambert GH, Schoeller DA, Humphrey HEB, Kotake AN, Lietz H, Campbell
M, Kalow W, Spielberg SP, & Budd M (1990) The caffeine breath test
and caffeine urinary metabolite ratios in the Michigan cohort
exposed to polybrominated biphenyls. A preliminary study. Environ
Health Perspect, 89: 175-181.
Lambert GH, Schoeller DA, Lietz HW, Doyle CM, & Humphrey H (1991)
Effects of polybrominated biphenyls (PBBs) on the maturation of
hepatic cytochrome P450IA2 activity during puberty. Pediatr Res, 29:
62A.
Lambrecht LK, Barsotti DA, & Allen JR (1978) Response of nonhuman
primates to a polybrominated biphenyl mixture. Environ Health
Perspect, 23: 139-145.
Landrigan PJ (1980) General population exposure to environmental
concentrations of halogenated biphenyls. In: Kimbrough RD ed.
Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and
related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 267-286.
Landrigan PJ, Wilcox KRJ, Silva JJ, Humphrey HEB, Kauffman C, &
Heath CW (1979) Cohort study of Michigan residents exposed to
polybrominated biphenyls: epidemiologic and immunologic findings.
Ann NY Acad Sci, 320: 284-294.
Law FCP & Addison RF (1981) Response of trout hepatic mixed-function
oxidases to experimental feeding of 10 known or possible chlorinated
environmental contaminants. Bull Environ Contam Toxicol, 27:
605-609.
Lee KP, Herbert RR, Sherman H, Aftosmis JG, & Waritz RS (1975a)
Bromine tissue residue and hepatotoxic effects of octabromobiphenyl
in rats. Toxicol Appl Pharmacol, 34: 115-127.
Lee KP, Herbert RR, Sherman H, Aftosmis JG, & Waritz RS (1975b)
Octabromobiphenyl-induced ultrastructural changes in rat liver. Arch
Environ Health, 30: 465-471.
Leland HV, Bruce WN, & Shimp NF (1973) Chlorinated hydrocarbon
insecticides in sediments of southern lake Michigan. Environ Sci
Technol, 7: 833-838.
Lewis RG & Sovocool GW (1982) Identification of polybrominated
biphenyls in the adipose tissues of the general population of the
USA. J Anal Toxicol, 6: 196-198.
Lilis R, Anderson HA, Valciukas JA, Freedman S, & Selikoff IJ (1978)
Comparison of findings among residents on Michigan dairy farms and
consumers of produce purchased from these farms. Environ Health
Perspect, 23: 105-109.
Lillie RJ, Cecil HC, Bitman J, Fries GF, & Verrett J (1975) Toxicity
of certain polychlorinated and polybrominated biphenyls on
reproductive efficiency of caged chickens. Poult Sci, 54: 1550-1555.
Lipson SM (1987) Effect of polybrominated biphenyls on the growth
and maturation of human peripheral blood lymphocytes. Clin Immunol
Immunophathol, 43: 65-72.
Loose LD, Mudzinski SP, & Silkworth JB (1981) Influence of dietary
polybrominated biphenyl on antibody and host defense responses in
mice. Toxicol Appl Pharmacol, 59: 25-39.
Lorenz H (1983) [PCB-contents in different matrixes, their behavior
in the food chain and possibilities of reducing the contents.] In:
Lorenz H & Neumeier G ed. [Polychlorinated biphenyls (PCB).] Münich,
MMV Medizin Verlag, pp 81-91 (BGA Report No. 4/83) (in German).
Lorenz H & Neumeier G ed. (1983) [Polychlorinated biphenyls (PCB).]
Münich, MMV Medizin Verlag (BGA Report No. 4/83) (in German).
Loury DJ, Goldsworthy TL, & Butterworth BE (1987) The value of
measuring cell replication as a predictive index of tissue-specific
tumorigenic potential. In: Nongenotoxic mechanisms in
carcinogenesis. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 119-136 (Banbury Report No. 25).
Lubet RA, Nims RW, Ward JM, Rice JM, & Diwan BA (1989) Induction of
cytochrome P450b and its relationship to liver tumor promotion. J Am
Coll Toxicol, 8: 259-268.
Lubet RA, Guengerich FP, & Nims RW (1990) The induction of
alkoxyresorufin metabolism: a potential indicator of environmental
contamination. Arch Environ Contam Toxicol, 19: 157-163.
Lucier GW, Davis GJ, & McLachlan JA (1978) Transplacental toxicology
of the polychlorinated and polybrominated biphenyls. In:
Developmental toxicology of energy related pollutants. Proceedings
of the 17th Annual Hanford Biology Symposium, Richland, 17-19
October 1977. Washington, DC, US Department of Energy, Technical
Information Center, pp 188-203.
Luster MI, Faith RE, & Moore JA (1978) Effects of polybrominated
biphenyls (PBB) on immune response in rodents. Environ Health
Perspect, 23: 227-232.
Luster MI, Boorman GA, Harris MW, & Moore JA (1980) Laboratory
studies on polybrominated biphenyl-induced immune alterations
following low-level chronic or pre/postnatal exposure. Int J
Immunopharmacol, 2: 69-80.
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, & Orrenius S (1988)
2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature chymocytes by
CA2+-mediated endonuclease activation. Science, 242: 256-259.
McConnell EE (1980) Acute and chronic toxicity, carcinogenesis,
reproduction, teratogenesis and mutagenesis in animals. In:
Kimbrough RD ed. Halogenated biphenyls, terphenyls, naphthalenes,
dibenzodioxins and related products. Amsterdam, Oxford, New York,
Elsevier/North-Holland Biomedical Press, pp 109-150.
McConnell EE (1984) Clinicopathologic concepts of dibenzo-p-dioxin
intoxication. In: Poland A & Kimbrough R ed. Biological mechanism of
dioxin action. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 27-37 (Banbury Report No. 18).
McConnell EE (1985) Comparative toxicity of PCBs and related
compounds in various species of animals. Environ Health Perspect,
60: 29-33.
McConnell EE & Moore JA (1979) Toxicopathology characteristics of
the halogenated aromatics. Ann NY Acad Sci, 320: 138-150.
McConnell EE, Harris MW, & Moore JA (1980) Studies on the use of
activated charcoal and cholestyramine for reducing the body burden
of polybrominated biphenyls. Drug Chem Toxicol, 3: 277-292.
McCormack KM & Hook JB (1982) Effects of lactation and nursing on
tissue concentrations of polybrominated biphenyls and on microsomal
enzyme activity in mammary gland and liver in maternal rats. Environ
Res, 27: 110-117.
McCormack KM, Kluwe WM, Sanger VL, & Hook JB (1978a) Effects of
polybrominated biphenyls on kidney function and activity of renal
microsomal enzymes. Environ Health Perspect, 23: 153-157.
McCormack KM, Kluwe WM, Rickert DE, Sanger VL, & Hook JB (1978b)
Renal and hepatic microsomal enzyme stimulation and renal function
following three months of dietary exposure to polybrominated
biphenyls. Toxicol Appl Pharmacol, 44: 539-553.
McCormack KM, Melrose P, Rickert DE, Dent JG, Gibson JE, & Hook JB
(1979a) Concomitant dietary exposure to polychlorinated biphenyls
and polybrominated biphenyls: tissue distribution and
arylhydrocarbon hydroxylase activity in lactating rats. Toxicol Appl
Pharmacol, 47: 95-104.
McCormack KM, Cagen SZ, Rickert DE, Gibson JE, & Dent JG (1979b)
Stimulation of hepatic and renal mixed-function oxidase in
developing rats by polybrominated biphenyls. Drug Metab Dispos, 7:
252-259.
McCormack KM, Arneric SP, & Hook JB (1979c) Action of exogenously
administered steroid hormones following perinatal exposure to
polybrominated biphenyls. J Toxicol Environ Health, 5: 1085-1094.
McCormack KM, Braselton WEJR, Sanger VL, & Hook JB (1980) Residual
effects of polybrominated biphenyls following perinatal exposure in
rats. Toxicol Appl Pharmacol, 53: 108-115.
McCormack KM, Lepper LF, Wilson DM, & Hook JB (1981) Biochemical and
physiological sequelae to perinatal exposure to polybrominated
biphenyls: a multigeneration study in rats. Toxicol Appl Pharmacol,
59: 300-313.
McCormack KM, Roth RA, Wallace KB, Ross LM, & Hook JB (1982a)
Nonrespiratory metabolic function and morphology of lung following
exposure to polybrominated biphenyls in rats. J Toxicol Environ
Health, 9: 27-39. McCormack KM, Stickney JL, Bonhaus DW, & Hook JB
(1982b) Cardiac and hepatic effects of pre- and postnatal exposure
to polybrominated biphenyls in rats. J Toxicol Environ Health, 9:
13-26.
McDaniel OS & Lucier GW (1979) Perinatal tissue distribution of
persistent organohalogens. Teratology, 19: 38A.
McKinney J & McConnell E (1982) Structural specificity and the
dioxin receptor. In: Hutzinger O, Frei RW, Merian E, & Pocchiari F
ed. Chlorinated dioxins and related compounds impact on the
environment. Oxford, New York, Pergamon Press, pp 367-381.
McKinney JD & Singh P (1981) Structure-activity relationships in
halogenated biphenyls: Unifying hypothesis for structural
specificity. Chem-Biol Interact, 33: 271-284.
MacLeod KE, Hanisch RC, & Lewis RG (1982) Evaluation of gel
permeation chromatography for clean up of human adipose tissue
samples for GC/MS analysis of pesticides and other chemicals. J Anal
Toxicol, 6: 38-40.
Manis J & Kim G (1980) Polybrominated biphenyl: acute and chronic
effect on iron absorption and benzo(a)pyrene hydroxylase. Toxicol
Appl Pharmacol, 54: 41-47.
Martino LJ, Wilson-Martino NA, & Benitz KF (1981) The presence of
intranuclear lipid inclusions in hepatocytes of mice after chronic
ingestion of polybrominated biphenyl. Arch Toxicol, 47: 155-158.
Mason G, Denomme MA, Safe L, & Safe S (1987a) Polybrominated and
chlorinated dibenzo-p-dioxins: synthesis biologic and toxic effects
and structure-activity relationships. Chemosphere, 16: 1729-1731.
Mason G, Zacharewski MA, Denomme MA, Safe L, & Safe S (1987b)
Polybrominated dibenzo-p-dioxins and related compounds: quantitative
in vivo and in vitro structure-activity relationships.
Toxicology, 44: 245-255.
Matthews HB (1982) Aryl halides. In: Jakoby WB, Bend JR, & Caldwell
J ed. Metabolic basis of detoxication. New York, London, San
Francisco, Academic Press, pp 51-68.
Matthews HB, Kato S, Morales NM, & Tuey DB (1977) Distribution and
excretion of 2,4,5,2',4',5'-hexabromobiphenyl, the major component
of FireMaster BP-6. J Toxicol Environ Health, 3: 599-605.
Matthews H, Fries G, Gardner A, Garthoff L, Goldstein J, Ku Y, &
Moore J (1978) Metabolism and biochemical toxicity of PCBs and PBBs.
Environ Health Perspect, 24: 147-155.
Matthews HB, Surles JR, Carver JG, & Anderson MW (1984) Halogenated
biphenyl transport by blood components. Fundam Appl Toxicol, 4:
420-428.
Mead RC, Hart MH, & Gable W (1982) Inhibition of purified rabbit
muscle phosphorylase a and phosphorylase b by polychlorinated
biphenyls, polychlorinated biphenylols and polybrominated biphenyls.
Biochim Biophys Acta, 701: 173-179.
Meester WD & McCoy DJ (1976) Human toxicology of polybrominated
biphenyls. In: Management of the poisoned patient. Princeton, New
Jersey, Science Press, pp 32-61.
Mercer HD, Teske RH, Condon RJ, Furr A, Meerdink G, Buck W, & Fries
G (1976) Herd health status of animals exposed to polybrominated
biphenyls. J Toxicol Environ Health, 2: 335-349.
Mercer HD, Willett LB, Schandbacher FL, Moorhead PD, & Powers TE
(1978) Use of the double-isotope, single-injection method for
estimating renal function in normal and polybrominated
biphenyl-exposed dairy cows. Am J Vet Res, 39: 1262-1268.
Miceli JN & Marks BH (1981) Tissue distribution and elimination
kinetics of polybrominated biphenyls from rat tissue. Toxicol Lett,
9: 315-320.
Miceli JN, Nolan DC, Marks B, & Hariharan M (1985) Persistence of
polybrominated biphenyls (PBB) in human post-mortem tissue. Environ
Health Perspect, 60: 399-403.
Miller CP & Birnbaum LS (1986) Teratologic evaluation of
hexabrominated naphthalenes in C57BL/6N mice. Fundam Appl Toxicol,
7: 398-405.
Miller FD, Brilliant LB, & Copeland R (1984) Polybrominated
biphenyls in lactating Michigan women: persistence in the
population. Bull Environ Contam Toxicol, 32: 125-133.
Miller CP, Harris MW, & Birnbaum LS (1985) Teratogenicity of
hexabrominated naphthalene, a toxic contaminant of polybrominated
biphenyls, in C57B1/6N mice. Toxicologist, 5: 122.
Millis CD & Aust SD (1985) Characterization of the photolysis of
2,4,5,2'4',5'-hexabromobiphenyl. Fundam Appl Toxicol, 5: 546-554.
Millis CD, Mills R, Sleight SD, & Aust SD (1985a) Photolysis
products of 2,4,5,2',4',5'-hexabromobiphenyl: hepatic microsomal
enzyme induction and toxicity in Sprague-Dawley rats. Fundam Appl
Toxicol, 5: 555-567.
Millis CD, Mills RA, Sleight SD, & Aust SD (1985b) Toxicity of
3,4,5,3',4',5'-hexabrominated biphenyl and 3,4,3',4'-tetrabrominated
biphenyl. Toxicol Appl Pharmacol, 78: 88-95.
Millischer R, Girault F, Heywood R, Clarke G, Hossack D, & Clair M
(1979) Décabromobiphényle: Etude toxicologique. Toxicol Eur Res, 2:
155-161.
Mills RA, Millis CD, Dannan GA, Guengerich FP, & Aust SD (1985)
Studies on the structure-activity relationships for the metabolism
of polybrominated biphenyls by rat liver microsomes. Toxicol Appl
Pharmacol, 78: 96-104.
Minyard JP Jr, Roberts WE, & Cobb WY (1989) State programs for
pesticide residues in foods. J Assoc Off Anal Chem, 72: 525-533.
Mirsalis JC & Steinmetz KL (1990) The role of hyperplasia in liver
carcinogenesis. In: Mouse liver carcinogenesis: mechanisms and
species comparisons. New York, Alan R. Liss, Inc., pp 149-161.
Mirsalis JC, Tyson CK, Loh EN, Steinmetz KL, Bakke JP, Hamilton CM,
Spak DK, & Spalding JW (1985) Induction of hepatic cell
proliferation and unscheduled DNA synthesis in mouse hepatocytes
following in vivo treatment. Carcinogenesis, 6: 1521-1524.
Mirsalis JC, Tyson CK, Steinmetz KL, Loh EK, Hamilton CM, Bakke JP,
& Spalding JW (1989) Measurement of unscheduled DNA synthesis and
s-phase synthesis in rodent hepatocytes following in vivo
treatment: testing of 24 compounds. Environ Mol Mutagen, 14:
155-164.
Momma J (1986) [Studies on the carcinogenicity and chronic toxicity
of nonabromobiphenyl (NBB) in mice in comparison with those of
polychlorinated biphenyl (PCB).] Jpn Pharmacol Ther, 14: 11-33 (in
Japanese).
Moore RW & Aust SD (1978) Purification and structural
characterization of polybrominated biphenyl congeners. Biochem
Biophys Res Commun, 84: 936-942.
Moore RW, Dannan GA, & Aust SD (1978a) Induction of drug
metabolizing enzymes in polybrominated biphenyl-fed lactating rats
and their pups. Environ Health Perspect, 23: 159-165.
Moore RW, Sleight SD, & Aust SD (1978b) Induction of liver
microsomal drug-metabolizing enzymes by
2,2',4,4',5,5'-hexabromobiphenyl. Toxicol Appl Pharmacol, 44:
309-321.
Moore RW, O'Connor JV, & Aust SD (1978c) Identification of a major
component of polybrominated biphenyls as
2,2'3,4,4',5,5'-heptabromobiphenyl. Bull Environ Contam Toxicol, 20:
478-483.
Moore RW, Sleight SD, & Aust SD (1979a) Effects of
2,2'-dibromobiphenyl and 2,2',3,4,4',5,5'-heptabromobiphenyl on
liver microsomal drug metabolizing enzymes. Toxicol Appl Pharmacol,
48: 73-86.
Moore JA, McConnell EE, Dalgard DW, & Harris MW (1979b) Comparative
toxicity of three halogenated dibenzofurans in guinea pigs, mice,
and rhesus monkeys. Ann NY Acad Sci, 320: 151-163.
Moore RW, Dannan GA, & Aust SD (1980) Structure-function
relationships for the pharmacological and toxicological effects and
metabolism of polybrominated biphenyl congeners. In: Bhatnagar RS
ed. Molecular basis of environmental toxicity. Ann Arbor, Michigan,
Ann Arbor Science Publishers, Inc., pp 173-212.
Moorhead PD, Willett LB, Brumm CJ, & Mercer HD (1977) Pathology of
experimentally induced polybrominated biphenyl toxicosis in pregnant
heifers. J Am Vet Med Assoc, 170: 307-313.
Moorhead PD, Willett LB, & Schanbacher FL (1978) Effects of PBB on
cattle. II. Gross pathology and histopathology. Environ Health
Perspect, 23: 111-118.
Mrozek E Jr, Seneca ED, & Hobbs LL (1982) Polychlorinated biphenyl
uptake and translation by Spartina alterniflora loisel. Water Air
Soil Pollut, 17: 3-15.
Mudzinski S, Silkworth JB, Wilson NM, & Loose LD (1979) Influence of
polybrominated biphenyl on immunological and host defense
parameters. Toxicol Appl Pharmacol, 49: A87.
Mulligan KJ, Caruso JA, & Fricke FL (1980) Determination of
polybrominated biphenyl and related compounds by gas-liquid
chromatography with a plasma emission detector. Analyst, 105:
1060-1067.
Mumma CE & Wallace DD (1975) Survey of industrial processing data.
Task II: Pollution potential of polybrominated biphenyls.
Washington, DC, US Environmental Protection Agency
(EPA-560/3-75-004).
Munslow WD, Donnelly JR, Mitchum RK, & Sovocool GW (1989) Synthesis
of polyhalogenated dibenzo-p-dioxins. Chemosphere, 18: 225-233.
Murata T, Zabik ME, & Zabik M (1977) Polybrominated biphenyls in raw
milk and processed dairy products. J Dairy Sci, 60: 516-520.
Myrick JE, Robinson MK, Hubert IL, Smith SJ, & Hannon WH (1987)
Effects of polybrominated biphenyls upon rat urinary protein
patterns as detected by two-dimensional electrophoresis. Arch
Environ Contam Toxicol, 16: 579-585.
Nagayama J, Kuratsune M, & Masuda Y (1976) Determination of
chlorinated dibenzofurants in kanechlors and "Yusho oil". Bull
Environ Contam Toxicol, 15: 9.
Nebert DW, Negishi M, Lang MA, Hjelmeland LM, & Eisen HJ (1982) The
Ah locus, a multigene family necessary for survival in a
chemically adverse environment: comparison with immune system. Adv
Genet, 21: 1-52.
Nebert DM, Elashoff JD, & Wilcox KR (1983) Possible effect of
neonatal polybrominated biphenyl exposure on the developmental
abilities of children. Am J Public Health, 73: 286-289.
Nebert DW, Peterson DD, & Fornace AJ Jr (1990) Cellular responses to
oxidative stress: the [Ah] gene battery as a paradigm. Environ
Health Perspect, 88: 13-25.
Needham LL, Burse YW, & Price HA (1981) Temperature-programmed gas
chromatographic determination of polychlorinated and polybrominated
biphenyls in serum. J Assoc Off Anal Chem, 64: 1131-1137.
Needham LL, Hill RHJR, Orti DL, Patterson DG, Kimbrough RD, Groce
DF, & Liddle JA (1982) Investigation of hyperkeratotic activity of
polybrominated biphenyls in FireMaster FF-1. J Toxicol Environ
Health, 9: 877-888.
Neufeld ML, Sittenfield M, & Wolk KF (1977) Market input/output
studies. Task IV: Polybrominated biphenyls. Washington, DC, US
Environmental Protection Agency (EPA-560/6-77-017).
Newton JF, Braselton WE Jr, Lepper LF, McCormack KM, & Hook JB
(1980) Effects of polybrominated biphenyls (PBBs) on the microsomal
metabolism of testosterone. Pharmacologist, 22: 173.
Newton JF, Braselton WE Jr, Lepper LF, McCormack KM, & Hook JB
(1982a) Effects of polybrominated biphenyls on metabolism of
testosterone by rat hepatic microsomes. Toxicol Appl Pharmacol, 63:
142-149.
Newton JF, Kou C-H, Gemborys MW, Mudge GH, & Hook JB (1982b)
Nephrotoxicity of p-aminophenol, a metabolite of acetaminophen, in
the Fischer 344 rat. Toxicol Appl Pharmacol, 65: 336-344.
Newton JF, Braselton WE Jr, Kuo C-H, Kluwe WM, Gemborys MW, Mudge
GH, & Hook JB (1982c) Metabolism of acetaminophen by the isolated
perfused kidney. J Pharmacol Exp Ther, 221: 76-79.
Norris JM, Ehrmantraut JW, Gibbson CL, Kociba RJ, Schwetz BA, Rose
JQ, Humiston CG, Jewett GL, Crummett WB, Gehring PJ, Tirsell JB, &
Brosier JS (1973) Toxicological and environmental factors involved
in the selection of decarbromodiphenyl oxide as a fire retardant
chemical. Appl Polym Symp, 22: 195-219.
Norris JM, Kociba RJ, Schwetz BA, Rose JQ, Humiston CG, Jewett GL,
Gehring PJ, & Mailhes JB (1975) Toxicology of octabromobiphenyl and
decabromodiphenyl oxide. Environ Health Perspect, 11: 153-161.
Norström A, Anderson K, & Rappe C (1976) Major components of some
brominated aromatics used as flame retardants. Chemosphere, 4:
255-261.
NTP (1993) Toxicology and carcinogenesis studies of polybrominated
biphenyls (FireMaster FF-1(R)) (CAS No. 67774-32-7) in F344/N rats
and B6C3F1 mice (Feed studies). Research Triangle Park, North
Carolina, US Department of Health and Human Services, National
Toxicology Program (NTP TR 398; NIH Publication No. 92-2853).
O'Keefe PW (1978) Formation of brominated dibenzofurans from
pyrolysis of the polybrominated biphenyl fire retardant, FireMaster
FF-1. Environ Health Perspect, 23: 347-350.
O'Keefe PW (1979) Trace contaminants in a polybrominated biphenyl
fire retardant and a search for these compounds in environmental
samples. Bull Environ Contam Toxicol, 22: 420-425.
Orti DL, Hill RH Jr, Patterson DG, Needham LL, Kimbrough RD, Alley
CC, & Lee HCJ (1983) Structure elucidation of some minor components
of the polybromobiphenyl mixture, FireMaster. Arch Environ Contam
Toxicol, 12: 603-614.
Parkinson A & Safe S (1981) Aryl hydrocarbon hydroxylase induction
and its relationship to the toxicity of halogenated aryl
hydrocarbons. Toxicol Environ Chem Rev, 4: 1-46.
Parkinson A & Safe S (1982) Cytochrome P-450-mediated metabolism of
biphenyl and the 4-halobiphenyls. Biochem Pharmacol, 31: 1849-1856.
Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, &
Levin W (1983) Immunochemical quantitation of cytochrome P-450
isozymes and epoxide hydrolase in liver microsomes from
polychlorinated or polybrominated biphenyl-treated rats. A study of
structure-activity relationships. J Biol Chem, 258: 5967-5976.
Patil GS (1991) Correlation of aqueous solubility and octanol-water
partition coefficient based on molecular structure. Chemosphere, 22:
723-738.
Patterson DG, Yert LW, Orti DL, & Holler JS (1980) Sunlight
photodegradation of purified polybrominated biphenyl (PBB) congeners
studied by GCMS. Paper presented at the 28th Annual Conference on
Mass Spectrometry and Allied Topics, New York, 25-30 May 1980. Salt
Lake City, Utah, American Society for Mass Spectrometry.
Patterson DG, Hill RH, Needham LL, Orti DL, Kimbrough RD, & Liddle
JA (1981) Hyperkeratosis induced by sunlight degradation products of
the major polybrominated biphenyl in FireMaster. Science, 213:
901-902.
Pearson CR (1982) Halogenated aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry. 3. Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 89-116.
Pitot HC, Barsness L, Goldsworthy T, & Kitagawa T (1978) Biochemical
characterization of stages of heptacarcinogenesis after a single
dose of diethylnitrosamine. Nature (Lond), 271: 456-458.
Plaa GL & Hewitt WR (1982) Methodological approaches for interaction
studies: potentiation of haloalkane-induced hepatotoxicity. In:
Stich HF, Leung HW, & Roberts JR ed. Workshop on the Combined
Effects of Xenobiotics, Ottawa, 22-23 June 1981. Ottawa, National
Research Council of Canada, pp 67-96 (Publication NRCC No. 18978).
Poellinger L, Göttlicher M, & Gustafsson J-A (1992) The dioxin and
peroxisome proliferator-activated receptors: nuclear receptors in
search of endogenous ligands. Trends Pharmacol Sci, 13: 241-245.
Poland A & Glover E (1977) Chlorinated biphenyl induction of aryl
hydrocarbon hydroxylase activity: a study of the structure-activity
relationship. Mol Pharmacol, 13: 924-938.
Poland A & Glover E (1980) 2,3,7,8-Tetrachlorodibenzo-p-dioxin:
segregation of toxicity with the Ah locus. Mol Pharmacol, 17: 86-94.
Poland A & Knutson JC (1982) 2,3,7,8-Tetrachlorodibenzo-p-dioxin and
related halogenated aromatic hydrocarbons: examination of the
mechanism of toxicity. Ann Res Toxicol, 22: 517-554.
Poland A, Greenlee WF, & Kende AS (1979) Studies on the mechanism of
action of the chlorinated dibenzo-p-dioxins and related compounds.
Ann NY Acad Sci, 320: 214-230.
Poland A, Palen D, & Glover E (1982) Tumor promotion by TCDD in skin
of HRS/J hairless mice. Nature (Lond), 300: 271-273.
Polin D & Leavitt RA (1984) Colestipol and energy restriction as an
approach to hasten removal of PBBs from chickens. J Toxicol Environ
Health, 13: 659-671.
Polin D & Ringer RK (1978a) PBB fed to adult female chickens: its
effect on egg production, reproduction, viability of offspring, and
residues in tissues and eggs. Environ Health Perspect, 23: 283-290.
Polin D & Ringer RK (1978b) Polybrominated biphenyls in chicken eggs
vs. hatchability. Proc Soc Biol Med, 159: 131-135.
Polin D, Ringer RK, & Aust SD (1979) Effect of congeners of
polybrominated biphenyls on hatchability of chicken eggs. I.
2,2',4,4',5,5'-hexabromobiphenyl vs. PBB. Proc Soc Exp Biol Med,
161: 44-47.
Polin D, Bursian S, & Shull L (1982) Microsomal enzyme induction and
reproduction in three lines of Japanese quail fed polybrominated
biphenyls. Toxicologist, 2: 40.
Polin D, Lehning E, Pullen D, Bursian S, & Leavitt R (1985)
Procedures to enhance withdrawal of xenobiotics from chickens. J
Toxicol Environ Health, 16: 243-254.
Pomerantz I, Burke J, Firestone D, McKinney J, Roach J, & Trotter W
(1978) Chemistry of PCBs and PBBs. Environ Health Perspect, 24:
133-146.
Preache MM, Cagen SZ, & Gibson JE (1976) Perinatal toxicity in mice
following maternal dietary exposure to polybrominated biphenyls.
Toxicol Appl Pharmacol, 37: 171.
Purdy R & Safe S (1980) The in vitro metabolism of
2,2'4,4',5,5'-hexabromobiphenyl. J Environ Pathol Toxicol, 4:
277-284.
Quensen JF III, Morris PJ, & Boyd SA (1990) Dehalogenation of PCBs
and PBBs by anaerobic microorganisms from sediments. In: Abstracts
of the 90th Annual Meeting of the American Society for Microbiology,
Anaheim, California, 13-17 May 1990. Washington, DC, American
Society for Microbiology, p 295.
Raber BT & Carter JW (1986) Localization of ultrastructural
alterations induced in rat liver by dietary polybromobiphenyls
(FireMaster BP-6). Arch Environ Contam Toxicol, 15: 725-732.
Reggiani G & Bruppacher R (1985) Symptoms, signs and findings in
humans exposed to PCBs and their derivatives. Environ Health
Perspect, 60: 225-232.
Reichardt PB, Chadwick BL, Cole MA, Robertson BR, & Button DK (1981)
Kinetic study of the biodegradation of biphenyl and its
monochlorinated analogues by a mixed marine microbial community.
Environ Sci Technol, 15: 75-79.
Render JA, Aust SD, & Sleight SD (1982) Acute pathologic effects of
3,3',4,4',5,5'-hexabromobiphenyl in rats: comparison of its effects
with FireMaster BP-6 and 2,2',4,4',5,5'-hexabromobiphenyl. Toxicol
Appl Pharmacol, 62: 428-444.
Rezabek MS, Sleight SD, Jensen RK, Aust SD, & Dixon D (1987)
Short-term oral administration of polybrominated biphenyls enhances
the development of hepatic enzyme-altered foci in initiated rats. J
Toxicol Environ Health, 20: 347-356.
Rezabek MS, Trosko JE, Jone C, & Sleight SD (1988) Effects of
hepatic tumor promoters phenobarbital and polybrominated biphenyls
on intercellular communication between rat liver epithelial cells.
In Vitro Toxicol, 2: 45-58.
Rezabek MS, Sleight SD, Jensen RK, & Aust SD (1989) Effects of
dietary retinyl acetate on the promotion of hepatic enzyme-altered
foci by polybrominated biphenyls in initiated rats. Food Chem
Toxicol, 27: 539-544.
Rickert DE, Dent JG, Cagen SZ, McCormack KM, Melrose P, & Gibson JE
(1978) Distribution of polybrominated biphenyls after dietary
exposure in pregnant and lactating rats and their offspring. Environ
Health Perspect, 23: 63-66.
Ringer RK (1978) PBB fed to immature chickens: its effect on organ
weights and function and on the cardiovascular system. Environ
Health Perspect, 23: 247-255.
Ringer RK & Polin D (1977) The biological effects of polybrominated
biphenyls in avian species. Fed Proc, 36: 1894-1898.
Ringer RK, Aulerich RJ, & Bleavins MR (1981) Biological effects of
PCBs and PBBs on mink and ferrets - a review. In: Khan MAQ & Stanton
RH ed. Toxicology of halogenated hydrocarbons: Health and ecological
effects. Oxford, New York, Pergamon Press, p 329.
Robertson LW & Chynoweth DP (1975) Another halogenated hydrocarbon.
Environment, 17: 25-27.
Robertson LW, Parkinson A, & Safe S (1980) Induction of both
cytochromes P-450 and P-448 by 2,3',4,4',5-pentabromobiphenyl, a
component of FireMaster. Biochem Biophys Res Commun, 92: 175-182.
Robertson LW, Parkinson A, Bandiera S, & Safe S (1981a) Potent
induction of rat liver microsomal, drug-metabolizing enzymes by
2,3,3',4,4',5-hexabromobiphenyl, a component of FireMaster.
Chem-Biol Interact, 35: 13-24.
Robertson LW, Parkinson A, Chittim B, Bandiera S, Sawyer TW, & Safe
S (1981b) Aryl hydrocarbon hydroxylase (AHH) induction by
polybrominated biphenyls (PBBs): enhancement by photolysis.
Toxicology, 22: 103-114.
Robertson LW, Parkinson A, Bandiera S, Chittim R, & Safe S (1981c)
Enhanced biologic potency of photolyzed PBBs. Toxicologist, 1: 126.
Robertson LW, Parkinson A, Campbell MA, & Safe S (1982a)
Polybrominated biphenyls as aryl hydrocarbon hydroxylase inducers:
structure-activity correlations. Chem-Biol Interact, 42: 53-66.
Robertson LW, Parkinson A, & Safe S (1982b) Effects of structure on
the activity of polybrominated biphenyls as microsomal enzyme
inducers. 73rd Annual Meeting of the American Oil Chemists' Society,
Toronto, Ontario, Canada, May 2-6. J Am Oil Chem Soc, 59: 286A.
Robertson LW, Chittim B, Safe SH, Mullin MD, & Pochini CM (1983a)
Photodecomposition of a commercial polybrominated biphenyl fire
retardant: high resolution gas chromatographic analysis. J Agric
Food Chem, 31: 454-457.
Robertson LW, Andres JL, Safe SH, & Lovering SL (1983b) Toxicity of
3,3',4,4'- and 2,2',5,5'-tetrabromobiphenyl: correlation of activity
with aryl hydrocarbon hydroxylase induction and lack of protection
by antioxidants. J Toxicol Environ Health, 11: 81-91.
Robertson LW, Thompson K, & Parkinson A (1984a) Synthesis,
characterization and biologic effects of polybrominated
naphthalenes. Arch Toxicol, 55: 127-131.
Robertson LW, Safe SH, Parkinson A, Pellizzari E, Pochini C, &
Mullin MD (1984b) Synthesis and identification of highly toxic
polybrominated biphenyls in the fire retardant FireMaster BP-6. J
Agric Food Chem, 32: 1107-1111.
Robertson LW, Parkinson A, Bandiera S, Lambert I, Merrill J, & Safe
SH (1984c) PCBs and PBBs: biologic and toxic effects on C57BL/6J and
DBA/J inbred mice. Toxicology, 31: 191-206.
Robl MG, Jenkins DH, Wingender RJ, & Gordon DE (1978) Toxicity and
residue studies in dairy animals with FireMaster FF-1
(polybrominated biphenyls). Environ Health Perspect, 23: 91-97.
Roboz J, Suzuki RK, Bekesi JG, Holland JF, Roseman K, & Selikoff IJ
(1980) Mass spectrometric identification and quantification of
polybrominated biphenyls in blood compartments of exposed Michigan
chemical workers. J Environ Pathol Toxicol, 3: 363-378.
Roboz J, Greaves J, Holland JF, & Bekesi JG (1982) Determination of
polybrominated biphenyls in serum by negative chemical ionization
mass spectrometry. Anal Chem, 54: 1104-1108.
Roboz J, Greaves J, McCamish M, Holland JF, & Bekesi G (1985a) An
in vitro model for the binding of polybrominated biphenyls in
environmentally contaminated blood. Arch Environ Contam Toxicol, 14:
137-142.
Roboz J, Greaves J, & Bekesi JG (1985b) Polybrominated biphenyls in
model and environmentally contaminated human blood: protein binding
and immunotoxicological studies. Environ Health Perspect, 60:
107-113.
Roes U, Dent JG, Netter KJ, & Gibson JE (1977) Effect of
polybrominated biphenyls on bromobenzene lethality in mice. J
Toxicol Environ Health, 3: 663-671.
Rogan WJ, Bagniewska A, & Damstra T (1980) Pollutants in breast
milk. New Engl J Med, 302: 1450-1453.
Rosenblatt DH, Kainz RJ, & Fries GF (1982) Options and
recommendations for a polybromobiphenyl control strategy at the
Gratiot County, Michigan, Landfill. Frederick, Maryland, US Army
Medical Research and Development Command, Fort Detrick, 40 pp
(Technical Report No. 8204).
Rosenman KD, Anderson HA, Selikoff IJ, Wolff MS, & Holstein E (1979)
Spermatogenesis in men exposed to polybrominated biphenyl (PBB).
Fertil Steril, 32: 209-213.
Rossman TG, Molina M, Meyer L, Boone P, Klein CB, Wang Z, Li F, Lin
WC, & Kinney PL (1991) Performance of 133 compounds in the lambda
prophage induction endpoint of the microscreen assay and a
comparison with S. typhimurium mutagenicity and rodent
carcinogenicity assays. Mutat Res, 260: 349-367.
Rozman KK, Rozman TA, Williams J, & Greim HA (1982) Effect of
mineral oil and/or cholestyramine in the diet on biliary and
intestinal elimination of 2,4,5,2',4',5'-hexabromobiphenyl in the
rhesus monkey. J Toxicol Environ Health, 9: 611-618.
Rush GF, Smith JH, & Hook JB (1982) Induction of hepatic and renal
mixed function oxidases in the hamster and guinea-pig. 66th Annual
Meeting of the Federation of American Societies for Experimental
Biology, New Orleans, LA, USA, 15-23 April 1982. Fed Proc, 41(5):
1638 (Abstract 7999).
Rush GF, Pratt IS, Lock EA, & Hook JB (1986) Induction of renal
mixed function oxidases in the rat and mouse: correlation with
ultrastructural changes in the proximal tubule. Fundam Appl Toxicol,
6: 307-316.
Ruzo LO & Zabik MJ (1975) Polyhalogenated biphenyls: photolysis of
hexabromo- and hexachlorobiphenyls in methanol solution. Bull
Environ Contam Toxicol, 13: 181-182.
Ruzo LO, Sundstrom G, Hutzinger O, & Safe S (1976) Photodegradation
of polybromobiphenyls (PBB). J Agric Food Chem, 24: 1062-1065.
Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated
biphenyls (PBBs): biochemistry, toxicology, and mechanism of action.
CRC Crit Rev Toxicol, 13: 319-395.
Safe S (1987) Determination of 2,3,7,8,-TCDD toxic equivalent
factors (TEFs): support for the use of the in vitro AHH induction
assay. Chemosphere, 16: 791-802.
Safe S (1989) Polyhalogenated aromatics: uptake, disposition and
metabolism. In: Kimbrough RD & Jensen AA ed. Halogenated biphenyls,
terphenyls, naphthalenes, dibenzodioxins and related products.
Amsterdam, Oxford, New York, Elsevier/North-Holland Biomedical
Press, pp 131-159.
Safe S, Jones D, & Hutzinger O (1976) Metabolism of
4,4'-dihalogenobiphenyls. J Chem Soc Perkin Trans, I/4: 357-359.
Safe S, Kohli J, & Crawford A (1978) FireMaster BP-6: fractionation,
metabolic and enzyme induction studies. Environ Health Perspect, 23:
147-152.
Safe S, Wyndham C, Parkinson A, Purdy R, & Crawford A (1980)
Halogenated biphenyl metabolism. In: Afghan BK & Mackay D ed.
Hydrocarbons and halogenated hydrocarbons in the aquatic
environment. New York, London, Plenum Press, pp 537-544.
Safe S, Robertson L, Parkinson A, Shilling M, Cockerline R, &
Campbell MA (1981) Polybrominated biphenyls, polychlorinated
naphthalenes and polychlorinated terphenyls as microsomal enzyme
inducers. In: Khan MAQ & Stanton RH ed. Toxicology of halogenated
hydrocarbons: Health and ecological effects. Oxford, New York,
Pergamon Press, pp 97-105.
Safe S, Robertson LW, Safe L, Parkinson A, Bandiera T, Sawyer T, &
Campbell MA (1982) Halogenated biphenyls: molecular toxicology. Can
J Physiol Pharmacol, 60: 1057-1064.
Safe S, Bandiera S, Sawyer T, Zmudzka B, Mason G, Romkes M, Denomme
MA, Sparling J, Okey AB, & Fujita T (1985) Effects of structure on
binding to the 2,3,7,8-TCDD receptor protein and AHH
induction-halogenated biphenyls. Environ Health Perspect, 61: 21-33.
Safe S, Harris M, Biegel L, & Zacharewski T (1991) Mechanism of
action of TCDD as an antiestrogen in transformed human breast cancer
and rodent cell lines. In: Gallo MA, Scheuplein RJ, & van der
Heijden KE ed. Biological basis for risk assessment of dioxins and
related compounds. Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory Press, pp 367-377 (Banbury Report No. 35).
Sax NI (1979) Dangerous properties of industrial materials, 5th ed.
New York, Van Nostrand Reinhold Company.
Schäfer W & Ballschmiter K (1986) Monobromo-polychloro-derivatives
of benzene, biphenyl, dibenzofurane and dibenzodioxine formed in
chemical-waste burning. Chemosphere, 15: 755-763.
Schanbacher FL, Willett LB, Moorhead PD, & Mercer HD (1978) Effects
of PBBs on cattle. III. Target organ modification as shown by renal
function and liver biochemistry. Environ Health Perspect, 23:
119-127.
Schanbacher FL, Willett LB, Moorhead PD, & Durst HI (1987) Serum
isocitrate dehydrogenase during polybrominated biphenyl toxicosis in
dairy cattle. J Anim Sci, 64: 467-473.
Schnare DW, Ben M, & Shields MG (1984) Body burden reductions of
polychlorinated biphenyls, polybrominated biphenyls and chlorinated
pesticides in human subjects. Ambio, 13: 378-380.
Schramm H, Robertson LW, & Oesch F (1985) Differential regulation of
hepatic glutathione transferase and glutathione peroxidase
activities in the rat. Biochem Pharmacol, 34: 3735-3739.
Schwartz EM & Rae WA (1983) Effect of polybrominated biphenyls (PBB)
on developmental abilities in young children. Am J Public Health,
73: 227-281.
Schwartz EL, Kluwe WM, Sleight SD, Hook JB, & Goodman JI (1980)
Inhibition of N-2-fluorenylacetamide-induced mammary tumorigenesis
in rats by dietary polybrominated biphenyls. J Natl Cancer Inst, 64:
63-67.
Schwarzenbach R & Westall J (1981) Transport of nonpolar organic
compounds from surface water to groundwater. Laboratory sorption
studies. Environ Sci Technol, 15: 1360-1367.
Schwind KH, Hosseinpour J, & Thoma H (1988) Brominated/chlorinated
dibenzo-p-dioxins and dibenzofurans. Part 1: Brominated/chlorinated
and brominated dibenzo-p-dioxins and dibenzofurans in fly ash from a
municipal waste incinerator. Chemosphere, 17: 1875-1884.
Schwind KH, Hosseinpour J, & Thoma H (1989) [Brominated/chlorinated
dioxins and furans from incineration of domestic rubbish.] Z
Umweltchem Ökotox, 1: 24 (in German).
Seagull EAW (1983) Developmental abilities of children exposed to
polybrominated biphenyls (PBB). Am J Public Health, 73: 281-285.
Selikoff IJ & Anderson HA (1979) A survey of the general population
of Michigan for health effects of PBB exposure (Contract final
report). Lansing, Michigan, Michigan Department of Public Health.
Sepkovic DW & Byrne JJ (1984) Kinetic parameters of
L-[125I]triiodothyronine degradation in rats pretreated with
polyhalogenated biphenyls. Food Chem Toxicol, 22: 743-747.
Shah BP (1978) Environmental consideration for the disposal of
PBB-contaminated animals and wastes. Environ Health Perspect, 23:
27-35.
Shedlofsky SI, Hoglen NC, Rodman LE, Honchel R, Robinson FR, Swim
AT, McClain CJ, & Robertson LW (1991) 3,3',4,4'-Tetrabromobiphenyl
sensitizes rats to the hepatotoxic effects of endotoxin by a
mechanism that involves more than tumor necrosis factor. Hepatology,
14: 1201-1208.
Shepherd EC, Phillips TD, Irvin TR, Safe SH, & Robertson LW (1984)
Aflatoxin B1 metabolism in the rat: polyhalogenated biphenyl
enhanced conversion to aflatoxin M1. Xenobiotica, 14: 741-750.
Sherman JD (1991) Polybrominated biphenyl exposure and human cancer:
report of a case and public health implications. Toxicol Ind Health,
7: 197-205.
Shukla RR & Albro PW (1987) In vitro modulation of protein kinase
C activity by environmental chemical pollutants. Biochem Biophys Res
Commun, 142: 567-572.
Sidhu KS & Michelakis AM (1978) Effect of polybrominated biphenyls
on adenylate cyclase activity in rat lung alveoli. Environ Health
Perspect, 23: 329-331.
Silberhorn EM, Glauert HP, & Robertson LW (1990) Carcinogenicity of
polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol, 20:
439-496.
Silva J, Kauffman CA, Simon DG, Landrigan PJ, Humphrey HEB, Heath CW
Jr, Wilcox KR Jr, Van Amburg G, Kaslow RA, Ringel A, & Hoff K (1979)
Lymphocyte function in humans exposed to polybrominated biphenyls. J
Reticuloendothel Soc, 26: 341-347.
Simmons MS & Kotz KT (1982) Association studies of polybrominated
biphenyls in aquatic systems. Bull Environ Contam Toxicol, 29:
58-63.
Simmons MS, Bialosky DI, & Rossmann R (1980) Polychlorinated
biphenyl contamination in surficial sediments of northeastern Lake
Michigan. J Great Lakes Res, 6: 167-171.
Sipes IG, McKelvie DH, & Collins R (1979) Excretion of
hexachlorobiphenyls and 2,4,5,2',4',5'-hexabromobiphenyl in the dog.
Toxicol Appl Pharmacol, 48: A155.
Sleight S (1985) Effects of PCBs and related compounds on
hepatocarcinogenesis in rats and mice. Environ Health Perspect, 60:
35-39.
Sleight SD & Sanger VL (1976) Pathologic features of polybrominated
biphenyl toxicosis in the rat and guinea pig. J Am Vet Med Assoc,
169: 1231-1235.
Sleight SD, Mangkoewidjojo S, Akoso BT, & Sanger VL (1978)
Polybrominated biphenyl toxicosis in rats fed an iodine-deficient,
iodine-adequate, or iodine-excess diet. Environ Health Perspect, 23:
341-346.
Sleight SD, Render JA, Akoso BT, Aust SD, & Nachreiner R (1981)
Comparative toxipathology of FireMaster BP-6,
2,2',4,4',5,5'hexabromobiphenyl (HBB) and 3,3',4,4',5,5'-HBB after
10 and 30 days of dietary administration to rats. Toxicologist, 1:
12.
Sloterdijk HH (1991) Mercury and organochlorinated hydrocarbons in
surficial sediments of the St. Lawrence River (Lake St. Francis).
Water Pollut Res J Can, 26: 41-60.
Smith JH, Rush GF, & Hook JB (1986) Induction of renal and hepatic
mixed function oxidases in the hamster and guinea-pig. Toxicology,
38: 209-218.
Smith AG, Francis JE, Green JA, Greig JB, Wolf CR, & Manson MM
(1990a) Sex-linked hepatic uropophyria and the induction of
cytochromes P450IA in rats caused by hexachlorobenzene and
polyhalogenated biphenyls. Biochem Pharmacol, 40(9): 2059-2068.
Smith AG, Francis JE, & Carthew P (1990b) Iron as a synergist for
hepatocellular carcinoma induced by polychlorinated biphenyls in
Ah-responsive C57BL/10ScSn mice. Carcinogenesis, 11: 437-444.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
Northeastern Alberta otters. Bull Environ Contam Toxicol, 39:
783-790.
Sovocool GW & Wilson NK (1982) Differentiation of brominated
biphenyls by carbon-13 nuclear magnetic resonance and gas
chromatography/mass spectrometry. J Org Chem, 47: 4032-4037.
Sovocool GW, Mitchum RK, & Donnelly JR (1987a) Use of the `ortho
effect' for chlorinated biphenyl and brominated biphenyl isomer
identification. Biomed Environ Mass Spectrom, 14: 579-582.
Sovocool GW, Munslow WD, Donnelly JR, & Mitchum RK (1987b)
Electrophilic bromination of dibenzofuran. Chemosphere, 16: 221-224.
Sparling J, Fung D, & Safe S (1980) Bromo- and chlorobiphenyl
metabolism: gas chromatographic mass spectrometric identification of
urinary metabolites and the effects of structure on their rates of
excretion. Biomed Mass Spectrom, 7: 13-19.
Spear PA, Higueret P, & Garcin H (1990) Increased thyroxine turnover
after 3,3',4,4',5,5'- hexabromobiphenyl injection and lack of effect
on peripheral triiodothyronine production. Can J Physiol Pharmacol,
68: 1079-1084.
Sprosty AL, Willett LB, Schanbacher FL, & Moorhead PD (1979) The
effect of polybrominated biphenyl on the metabolism and clearance of
steroids. J Dairy Sci, 62: 90.
Steele RW, Williams LW, & Beck SA (1989) Immunotoxicology. Ann
Allergy, 63: 168-174.
Strachan SD, Nelson DW, & Sommers LE (1983) Sewage sludge components
extractable with nonaqueous solvents. J Environ Qual, 12: 69-74.
Stratton CL & Whitlock SA (1979) A survey of polybrominated
biphenyls (PBBs) near sites of manufacture and use in Northwestern
New Jersey. Washington, DC, US Environmental Protection Agency (EPA
560/13-79-002).
Stratton CL, Mousa JJ, & Bursey JT (1979) Analysis for
polybrominated biphenyls (PBBs) in environmental samples.
Washington, DC, US Environmental Protection Agency (EPA
560/13-79-001).
Strik JJTWA (1973a) Toxicity of hexachlorobenzene (HCB) and
hexabromobiphenyl (HBB). Meded Fac Landbouwwet Rijksuniv Gent, 38:
709-716.
Strik JJTWA (1973b) Chemical porphyria in Japanese quail. Enzyme,
16: 211-223.
Strik JJTWA (1978) Toxicity of PBBs with special reference to
porphyrinogenic action and spectral interaction with hepatic
cytochrome P-450. Environ Health Perspect, 23: 167-175.
Strik JJTWA, Doss M, Schraa G, Robertson LW, Von Tiepermann R, &
Harmsen EGM (1979) Coproporphyrinuria and chronic hepatic porphyria
type A found in farm families from Michigan (USA) exposed to
polybrominated biphenyls (PBB). In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Oxford, New York, Elsevier-
North Holland Biomedical Press, pp 29-53.
Stross JK, Nixon RK, & Anderson MD (1979) Neuropsychiatric findings
in patients exposed to polybrominated biphenyls. Ann NY Acad Sci,
320: 368-372.
Stross JK, Smokler IA, Isbister J, & Wilcox KR (1981) Human health
effects of exposure to polybrominated biphenyls. Toxicol Appl
Pharmacol, 58: 145-150.
Suflita JM, Horowitz A, Shelton D, & Tiedje JM (1982)
Dehalogenation: a novel pathway for the anaerobic biodegradation of
haloaromatic compounds. Science, 218: 1115-1117.
Sugiura K (1992) Microbial degradation of polychlorinated biphenyls
in aquatic environments. Chemosphere, 24: 881-890.
Sugiura K, Ito N, Matsumoto N, Mihara Y, Murata K, Tsukakoshi Y, &
Goto M (1978) Accumulation of polychlorinated biphenyls and
polybrominated biphenyls in fish: limitation of "correlation between
partition coefficients and accumulation factors". Chemosphere, 9:
731-736.
Sugiura K, Washino T, Hattori M, Sato E, & Goto M (1979)
Accumulation of organochlorine compounds in fishes. Difference of
accumulation factors by fishes. Chemosphere, 6: 359-364.
Sundström G, Hutzinger 0, & Safe S (1976a) Identification of
2,2',4,4',5,5'-hexabromobiphenyl as the major component of flame
retardant FireMaster BP-6. Chemosphere, 1: 11-14.
Sundström G, Hutzinger O, Safe S, & Zitko V (1976b) The synthesis
and gas chromatographic properties of bromobiphenyls. Sci Total
Environ, 6: 15-29.
Sweetman JA & Boettner EA (1982) Analysis of polybrominated biphenyl
by gas chromatography with electron-capture detection. J Chromatogr,
236: 127-136.
Takase I, Omori T, & Minoda Y (1986) Microbial degradation products
from biphenyl- related compounds. Agric Biol Chem, 50: 681-686.
Tennant RW, Stasiewicz S, & Spalding JW (1986) Comparison of
multiple parameters of rodent carcinogenicity and in vitro genetic
toxicity. Environ Mutagen, 8: 205-227.
Tennant RW, Margolin BH, Shelby MD, Zeiger E, Haseman JK, Spalding
J, Caspary W, Resnick M, Stasiewicz S, Anderson B, & Minor R (1987)
Prediction of chemical carcinogenicity in rodents from
in vitro genetic toxicity assays. Science, 236: 933-941.
Theoharides AD & Kupfer D (1981) Effects of polyhalogenated
biphenyls on the metabolism of prostaglandin E1 and xenobiotics by
hepatic mono-oxygenases in the rat. Drug Metab Dispos, 9: 580-581.
Thoma H & Hutzinger O (1989) Pyrolysis and GC/MS-analysis of
brominated flame retardants in on-line operation. Chemosphere, 18:
1047-1050.
Thoma H, Hauschulz G, Knorr E, & Hutzinger O (1987a) Polybrominated
dibenzofurans (PBDF) and dibenzodioxins (PBDD) from the pyrolysis of
neat brominated diphenylethers, biphenyls and plastic mixtures of
these compounds. Chemosphere, 16: 277-285.
Thoma H, Hauschulz G, & Hutzinger O (1987b) PVC-induced
chlorine-bromine exchange in the pyrolysis of polybrominated
diphenyl ethers, -biphenyls, -dibenzodioxins and dibenzofurans.
Chemosphere, 16: 297-307.
Tilson HA & Cabe PA (1978) Behavioral toxicologic effects of
polybrominated biphenyl compounds in rodents. Toxicol Appl
Pharmacol, 45: 334.
Tilson HA & Cabe PA (1979) Studies on the neurobehavioral effects of
polybrominated biphenyls in rats. Ann NY Acad Sci, 320: 325-336.
Tilson HA, Cabe PA, & Mitchell CL (1978) Behavioral and neurological
toxicity of polybrominated biphenyls in rats and mice. Environ
Health Perspect, 23: 257-263.
Tondeur Y, Hass JR, Harvan DJ, Albro PW, & McKinney JD (1984)
Determination of suspected toxic impurities in FireMaster FF-1 and
FireMaster BP-6 by high-resolution gas
chromatography-high-resolution mass spectrometry. J Agric Food Chem,
32: 406-410.
Tong C, Telang S, & Williams GM (1983) The lack of genotoxicity of
polybrominated biphenyls (PBB) in the adult liver cell culture
systems. Environ Mutagen, 5: 416.
Traber PG, Chianale J, Florence R, Kim K, Wojcik E, & Gumucio JJ
(1988a) Expression of cytochrome P450b and P450e genes in small
intestinal mucosa of rats following treatment with phenobarbital,
polyhalogenated biphenyls, and organochlorine pesticides. J Biol
Chem, 263: 9449-9455.
Traber PG, Chianale J, Florence R, Kim K, Wojcik E, & Gumucio JJ
(1988b) Expression of cytochrome P450b and P450e genes in small
intestine of rats following treatment with phenobarbital (PB),
polyhalogenated biphenyls and organochlorine pesticides. Clin Res,
36: 402A.
Trosko JE, Dawson B, & Chang CC (1981) PBB inhibits metabolic
cooperation in Chinese hamster cells in vitro: its potential as a
tumor promoter. Environ Health Perspect, 37: 179-182.
Trotter WJ (1977) Confirming low levels of hexabromobiphenyl by
gas-liquid chromatography of photolysis products. Bull Environ
Contam Toxicol, 18: 726-733.
Tsushimoto G, Trosko JE, Chang C, & Aust SD (1982) Inhibition of
metabolic cooperation in Chinese hamster V79 cells in culture by
various polybrominated biphenyl (PBB) congeners. Carcinogenesis, 3:
181-185.
Tuey DB & Matthews HB (1978) Pharmacokinetics of hexabromobiphenyl
disposition in the rat. Toxicol Appl Pharmacol, 45: 337 (Abstract
No. 275).
Tuey DB & Matthews HB (1980) Distribution and excretion of
2,2',4,4',5,5'- hexabromobiphenyl in rats and man: pharmacokinetic
model predictions. Toxicol Appl Pharmacol, 53: 420-431.
UBA (Federal Environment Agency) (1989) [State of facts.
Polybrominated dibenzodioxins (PBDD) - Polybrominated dibenzofurans
(PBDF). Second supplement: Polybrominated biphenyls.] In:
[Polybrominated dibenzodioxins and dibenzofurans (PBDD/PBDF) from
brominated flame retardants. Report of the working group on
brominated flame retardants to the Environment Minister Conference.]
Bonn, Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, pp 95-97 (in German).
Valciukas JA, Lilis R, Wolff MS, & Anderson HA (1978) Comparative
neurobehavioral study of a polybrominated biphenyl-exposed
population in Michigan and nonexposed group in Wisconsin. Environ
Health Perspect, 23: 199-210.
Valciukas JA, Lilis R, Anderson HA, Wolff MS, & Petrocci M (1979)
The neurotoxicity of polybrominated biphenyls: results of a medical
field survey. Ann NY Acad Sci, 320: 337-367.
Voorman R & Aust SD (1987) Specific binding of polyhalogenated
aromatic hydrocarbon inducers of cytochrome P-450d to the cytochrome
and inhibition of its estradiol 2- hydroxylase activity. Toxicol
Appl Pharmacol, 90: 69-78.
Vos JG & Van Genderen H (1974) Toxicological aspects of
immunosuppression. In: Deichmann WB ed. Pesticides and the
environment: A continuing controversy, 2nd ed. New York,
Intercontinental Medical Book Corp., pp 527-545.
Vrbanac JJ Jr (1984) Effects of exposure to polybrominated biphenyls
on urinary steroid metabolic profiles in man and rats as determined
by capillary GC and GC/MS/DS. Diss Abstr Int, B45(4): 1169.
Wade MH, Trosko JE, & Schindler M (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
Waldron HA (1990) The health effects of environmental chemicals. In:
Harrison RM ed. Pollution: causes, effects, and control, 2nd ed.
Cambridge, United Kingdom, Royal Society of Chemistry, pp 261-276.
Waritz RS, Aftosmis JG, Culik R, Dashiell OL, Faunce MM, Griffith
FD, Hornberger CS, Lee KP, Sherman H, & Tayfun FO (1977)
Toxicological evaluations of some brominated biphenyls. Am Ind Hyg
Assoc J, 38: 307-320.
Wasito & Sleight SD (1989) Promoting effect of polybrominated
biphenyls on tracheal papillomas in Syrian golden hamsters. J
Toxicol Environ Health, 27: 173-187.
Wastell ME, Moody DL, & Plog JF Jr (1978) Effects of polybrominated
biphenyl on milk production, reproduction, and health problems in
Holstein cows. Environ Health Perspect, 23: 99-103.
Watanabe I & Tatsukawa R (1990) Anthropogenic brominated aromatics
in the Japanese environment. In: Freij L ed. Proceedings on the
Workshop on Brominated Aromatic Flame Retardants, Skoloster, Sweden,
24-26 October 1989. Solna, Sweden, National Chemicals Inspectorate,
pp 63-71.
Webber MD, Monteith HD, & Corneau DGM (1983) Assessment of heavy
metals and PCBs at sludge application sites. J Water Pollut Control
Fed, 55: 187-195.
Weil WB, Spencer M, Benjamin D, & Seagull E (1981) The effect of
polybrominated biphenyl on infants and young children. J Pediatr,
98: 47-51.
Welsch F & Morgan KT (1985) Placental transfer and developmental
toxicity of 2,2',4,4',5,5'-hexabromobiphenyl in B6C3F1 mice. Toxicol
Appl Pharmacol, 81: 431-442.
Werner PR & Sleight SD (1981) Toxicosis in sows and their pigs
caused by feeding rations containing polybrominated biphenyls to
sows during pregnancy and lactation. Am J Vet Res, 42: 183-188.
Wertz GF & Ficsor G (1978) Cytogenetic and teratogenic test of
polybrominated biphenyls in rodents. Environ Health Perspect, 23:
129-132.
WHO (1987) In: Rantanen JH, Silano V, Tarkowski S, & Yrjänheikki E
ed. PCB's, PCDD's and PCDF's, prevention and control of accidental
and environmental exposures. Copenhagen, World Health Organization,
Regional Office for Europe.
Willett LB & Durst HI (1978) Effects of PBBs on cattle. IV.
Distribution and clearance of components of FireMaster BP-6. Environ
Health Perspect, 23: 67-74.
Willett LB & Irving HA (1976) Distribution and clearance of
polybrominated biphenyls in cows and calves. J Dairy Sci, 59:
1429-1439.
Willett LB, Brumm CJ, & Williams CL (1978) Method for extraction,
isolation and detection of free polybrominated biphenyls (PBBs) from
plasma, feces, milk, and bile using disposable glassware. J Agric
Food Chem, 26: 122-125.
Willett LB, Schanbacher FL, Durst HI, & Moorhead PD (1980) Long-term
performance and health of cows experimentally exposed to
polybrominated biphenyls. J Dairy Sci, 63: 2090-2102.
Willett LB, Durst HI, Liu T-TY, Schanbacher FL, & Moorhead PD (1982)
Performance and health of offspring of cows experimentally exposed
to polybrominated biphenyls. J Dairy Sci, 65: 81-91.
Willett LB, Liu TT, Durst HI, & Schanbacher FL (1983a) Effects of
polybrominated biphenyls on the concentration and clearance of
steroids from blood. J Anim Sci, 56: 1134-1144.
Willett LB, Schanbacher FL, & Moorhead PD (1983b) Effects of
polybrominated biphenyls on the excretion of steroids. J Anim Sci,
56: 1145-1152.
Williams GM, Tong C, & Telang S (1984) Polybrominated biphenyls are
nongenotoxic and produce an epigenetic membrane effect in cultured
liver cells. Environ Res, 34: 310-320.
Williams GM, Mori H, & McQueen CA (1989) Structure-activity
relationships in the rat hepatocyte DNA-repair test for 300
chemicals. Mutat Res, 221: 263-286.
Wolff MS & Aubrey B (1978) PBB homologs in sera of Michigan dairy
farmers and Michigan chemical workers. Environ Health Perspect, 23:
211-215.
Wolff MS & Selikoff IJ (1979) Variation of polybrominated biphenyl
homolog peaks in blood of rats following treatment with FireMaster
FF-1. Bull Environ Contam Toxicol, 21: 771-774.
Wolff MS, Aubrey B, Camper F, & Haymes N (1978) Relation of DDE and
PBB serum levels in farm residents, consumers, and Michigan chemical
corporation employees. Environ Health Perspect, 23: 177-181.
Wolff MS, Anderson HA, Camper F, Ninaido MN, Daum SM, Haymes N, &
Selikoff J (1979a) Analysis of adipose tissue and serum from PBB
(polybrominated biphenyl)- exposed workers. J Environ Pathol
Toxicol, 2: 1397-1411.
Wolff MS, Anderson HA, Rosenman KD, & Selikoff IJ (1979b)
Equilibrium of polybrominated biphenyl (PBB) residues in serum and
fat of Michigan residents. Bull Environ Contam Toxicol, 21: 775-781.
Wolff MS, Anderson HA, Irving J, & Selikoff IJ (1982) Human tissue
burdens of halogenated aromatic chemicals in Michigan. J Am Med
Assoc, 247: 2112-2116.
Wong O, Brocker W, Davis HV, & Nagle GS (1984) Mortality of workers
potentially exposed to organic and inorganic brominated chemicals,
DBCP, TRIS, PBB, and DDT. Br J Ind Med, 41: 15-24.
Yagi O & Sudo R (1980) Degradation of polychlorinated biphenyls by
microorganisms. J Water Pollut Control Fed, 52: 1035-1043.
Yao P, Wang Y, Guo J, & Bernstein IA (1991) The in vitro
inducibility of cytochromes P448 by polybrominated biphenyls and
1,2-benzanthracene. In: 13th Asian Conference on Occupational Health
and the 3rd Conference of South-East Asian Ergonomics Society,
Bangkok, 25-27 November. Bangkok, Bangkok Medical Publisher, p 37
(Abstract A-23).
Zabik ME (1982) The polybrominated biphenyl episode in Michigan,
USA. J Am Oil Chem Soc, 59: 279A.
Zabik ME, Johnson TM, & Smith S (1978) Effects of processing and
cooking on PBB residues. Environ Health Perspect, 23: 37-41.
Zacharewski T, Harris M, Safe S, Thoma H, & Hutzinger O (1988)
Applications of the in vitro aryl hydrocarbon hydroxylase
induction assay for determining "2,3,7,8-
tetrachlorodibenzo-p-dioxin equivalents": pyrolyzed brominated flame
retardants. Toxicology, 51: 177-189.
Zerezghi M, Mulligan KJ, & Caruso JA (1984) Application of a rapid
scanning plasma emission detector and gas chromatography for
multi-element quantification of halogenated hydrocarbons. J
Chromatogr Sci, 22: 348-352.
Zile MH, Bank PA, & Roltsch IA (1989) Alterations in vitamin A
metabolism by polyhalogenated aromatic hydrocarbons. Z Ernähr.wiss,
28: 93-102.
Zitko V (1977) The accumulation of polybrominated biphenyls by fish.
Bull Environ Contam Toxicol, 17: 285-292.
Zitko V & Hutzinger O (1976) Uptake of chloro- and bromobiphenyls,
hexachloro- and hexabromobenzene by fish. Bull Environ Contam
Toxicol, 16: 665-673.
ABBREVIATIONS USED IN THE MONOGRAPH
1. PBB nomenclature
PBB = polybrominated biphenyl
MoBB = monobromobiphenyl
DiBB = dibromobiphenyl
TrBB = tribromobiphenyl
TeBB = tetrabromobiphenyl
PeBB = pentabromobiphenyl
HxBB = hexabromobiphenyl
OcBB = octabromobiphenyl
NoBB = nonabromobiphenyl
DeBB = decabromobiphenyl
FM = FireMaster(R)
PBB congener numbering system used (Table 3).
2. Enzyme nomenclature
Designation of the cytochrome P-450-dependent mixed function
monooxygenase (P-450) system, as relevant for PBBs:
Systematic nomenclaturea Trivial names
PB (phenobarbital)-inducible
P-450 II P-450
P-450 II B 1 P-450b
P-450 II B 2 P-450e
MC (3-methylcholanthrene)-inducible
P-450 I P-448
P-450 I A 1 P-450c
P-450 I A 2 P-450d
a From: Nebert et al. (1987, 1989).
3. Other compounds
AHH aryl hydrocarbon hydroxylase
AP alkaline phosphatase
BUN blood urea nitrogen
DBBO decabromobiphenyloxide
DMBA 7,12-dimethyl-benz (a)anthracene
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
GTP glutamyl transpeptidase
LDH lactic dehydrogenase
MFO mixed function oxidase
MNNG N-methyl- N'-nitro- N-nitroso-guanidine
PBCDD polybrominated/chlorinated dibenzo- p-dioxin
PBCDF polybrominated/chlorinated dibenzofuran
PBDD polybrominated dibenzo- p-dioxin
PBDF polybrominated dibenzofuran
PCDD polychlorinated dibenzo- p-dioxin
PCDF polychlorinated dibenzofuran
PEG polyethylene glycol
TPA 12- O-tetradecanoylphorbol-13-acetate
4. Other abbreviations
ALD approximate lethal dose
F female
GLC gas liquid chromatography
HPLC high pressure liquid chromatography
ip intraperitoneal
IPCS International Programme on Chemical Safety
LC50 lethal concentration, median
LD50 lethal dose, median
M male
NCI National Cancer Institute (USA)
No. number of animals
NTP National Toxicology Program (USA)
RER rough-surfaced endoplasmic reticulum
SER smooth-surfaced endoplasmic reticulum
t.p. time post-exposure
UV ultraviolet
WHO World Health Organization
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés physiques et chimiques, méthodes
d'analyse
On désigne par biphényles polybromés ou polybromobiphényles
(PBB) un groupe d'hydrocarbures halogénés obtenus par substitution
des hydrogènes du noyau biphényle par le brome. On ne les connaît
pas à l'état naturel. Leur formule brute est
C12H(10-x-y)Br(x+y) dans laquelle x et y peuvent prendre
toutes les valeurs de 1 à 5. Il y a théoriquement 209 homologues
possibles. On n'en a synthétisé et caractérisé que quelques-uns. Les
PBB produits dans un but commercial consistent essentiellement en
hexa-, octa-, nona- et décabromobiphényles, mais ils contiennent
également d'autres homologues. Ce sont des retardateurs de flammes
de type additif et, en mélange avec les polymères ou solides secs,
ils exercent une action retardatrice sur les flammes par filtrage,
avec libération de bromure d'hydrogène en cas d'inflammation.
Les PBB sont préparés au moyen d'une réaction du type Friedel
et Crafts, par action du brome sur le biphényle avec ou sans solvant
organique et en présence de chlorure d'aluminium, de bromure
d'aluminium, de fer, etc, comme catalyseur.
La plupart des recherches ont été consacrées au FireMaster(R)
BP-6 et au FF1 qui étaient impliqués dans la catastrophe du Michigan
où ce composé a été ajouté par inadvertance à de la nourriture pour
animaux à la place d'oxyde de magnésium. L'intoxication des animaux
qui s'en est suivie a abouti à la perte de milliers de bovins, porcs
et moutons et de millions de poulets.
La composition du FireMaster(R) varie d'un lot à l'autre mais
il est principalement composé de 2,2',4,4',5,5'-hexabromobiphényle
(60 à 80%) et de 2,2',3,4,4',5,5'-heptabromobiphényle (12 à 25%) à
côté d'homologues inférieurs dus à une bromation incomplète. Parmi
les constituants mineurs du FireMaster(R) on a également observé
la présence de bromochlorobiphényles et de polybromo naphtalènes. Le
FireMaster FF-1 (poudre blanche) est du FireMaster BP-6 (paillettes
brunes) auquel on a ajouté 2% de silicate de calcium comme
antiagglomérant.
Les PBB sont des solides dont la faible volatilité décroît à
mesure qu'augmente le nombre d'atomes de brome. Ils sont
pratiquement insolubles dans l'eau, solubles dans les graisses et
légèrement à fortement solubles dans divers solvants organiques; la
solubilité diminue également à mesure qu'augmente le nombre d'atomes
de brome. Ces composés sont relativement stables et chimiquement
inertes, encore que des mélanges de PBB fortement substitués
subissent une photodégradation avec débromation réductrice sous
l'action du rayonnement ultraviolet.
Les produits de la décomposition thermique expérimentale des
PBB dépendent de la température, de la quantité d'oxygène présente
et d'un certain nombre d'autres facteurs. L'étude de la pyrolyse du
FireMaster BP-6 en l'absence d'oxygène (600 à 900 °C) a montré qu'il
se forme des bromobenzènes et des bromobiphényles inférieurs mais
pas de polybromofuranes. En revanche, la pyrolyse en présence
d'oxygène (700 à 900 °C) conduit à la formation de
bromodibenzofuranes bi- à hepta substitués. Des quantités plus
importantes ont été trouvées en présence de polystyrène et de
polyéthylène. La pyrolyse du FireMaster BP-6 en présence de PVC à
800 °C a fourni un mélange de bromochlorobiphényles. On ignore
qu'elle est la nature des produits d'incinération des matériaux
contenant des PBB. On manque également de données sur la toxicité
des dioxines et des furanes bromés et chlorobromés, mais on estime
qu'elle doit être à peu près du même ordre que celle des dioxines et
des furanes chlorés.
Après la catastrophe du Michigan, la principale technique
d'analyse qu'on a utilisée pour la surveillance biologique des PBB
dans des échantillons de tissus et de liquides biologiques ou
provenant de l'environnement, était la chromatographie en phase
gazeuse avec détection par capture d'électrons. Le dosage des
différents homologues peut s'effectuer par chromatographie en phase
gazeuse en tube capillaire avec détection par spectrométrie de masse
à sélectivité ionique. En raison du nombre élevé d'homologues
possibles, les recherches sont gênées par le manque d'étalons de
synthèse convenables. On utilise, pour extraire les PBB des
échantillons biologiques, des méthodes du type de celles qu'on
applique aux pesticides. Les PBB sont extraits avec la fraction
lipidique puis purifiés.
Le fait que l'on ait récemment découvert des homologues des PBB
dans des échantillons biologiques prélevés dans l'environne ment
général n'implique pas forcément que la concentration de ces
produits y soit en augmentation. La mise au point de techniques
d'analyse plus sensibles comme la spectrométrie de masse avec
production d'ions négatifs par ionisation chimique, pourrait en être
la cause. Il est donc urgent de procéder à des études
rétrospectives. L'amélioration des méthodes de purification devrait
permettre d'effectuer le dosage spécifique des homologues
coplanaires toxiques car des données sont également nécessaires à
leur sujet.
1.2 Sources d'exposition humaine et environnementale
La production commerciale du FireMaster(R) a commencé aux
Etats-Unis en 1970. Elle a été arrêtée après la catastrophe du
Michigan (novembre 1974). On estime qu'entre 1970 et 1976 les
Etats-Unis ont produit 6000 tonnes de PBB (produits du commerce). La
production de l'octabromobiphényle et du décabromobiphényle s'est
poursuivie aux Etats-Unis jusqu'en 1979. En outre, l'Allemagne a
produit jusqu'à la moitié de 1985 un mélange de PBB fortement
bromés, le Bromkal 80-9 D. La France produit actuellement du
décabromobiphényle (Adine 0102) de qualité technique. Autant qu'on
sache, c'est le seul PBB qui soit actuellement produit.
Les PBB ont fait leur apparition sur le marché comme
retardateurs de flamme au début des années 1970. Avant novembre
1974, l'hexabromobiphényle était le principal PBB produit dans le
commerce aux Etats-Unis; il était incorporé aux résines ABS
(acrylonitrile-butadiène-styrène) dans la proportion de 10% et
utilisé principalement pour la fabrication de divers accessoires
d'automobiles, les peintures et les vernis ainsi que dans la mousse
de polyuréthanne. Les autres retardateurs de flamme à base de PBB
ont des applications similaires.
Des PBB peuvent passer dans l'environnement au cours du
processus normal de production à la faveur d'émissions dans l'air,
dans les eaux usées, ou encore par libération dans le sol et dans
les décharges, mais les concentrations correspondantes se sont
révélées faibles en général.
Ces produits peuvent également passer dans l'environnement lors
du transport et de la manipulation ou par suite d'accidents comme
cela s'est produit dans le Michigan.
Il y a également risque de pénétration dans l'environnement
lors de l'incinération de produits contenant des PBB ou lors
d'incendies au cours desquels il se forme d'autres substances
toxiques comme des polybromodibenzofuranes ou des dérivés mixtes
bromochlorés.
La majeure partie du volume totale de ces produits finit de
toute façon par se retrouver dans l'environnement soit tels quels,
soit sous forme de produits de décomposition.
1.3 Transport, distribution et transformation dans l'environnement
Il n'est pas démontré que les PBB puissent être transportés à
longue distance dans l'atmosphère, mais la présence de ces composés
chez les phoques de l'Arctique indique qu'ils sont largement
disséminés sur la planète.
Les principales voies de pénétration des PBB dans l'environne
ment aquatique sont, d'une part la pollution des eaux réceptrices,
les décharges de déchets industriels et, d'autre part le lessivage
de dépotoirs de déchets industriels, ou encore, l'érosion de sols
pollués. Les PBB sont pratiquement insolubles dans l'eau et on les
retrouve principalement dans les sédiments des lacs et des rivières
pollués.
La pollution du sol peut trouver son origine dans des sources
polluantes ponctuelles comme par exemple des unités de production de
PBB ou des dépotoirs. Une fois qu'ils ont pénétré dans le sol, les
PBB ne semblent pas être facilement mobilisables. On a constaté
qu'ils étaient 200 fois plus solubles dans le produit de lessivage
d'une décharge que dans l'eau distillée; cela pourrait entraîner une
plus large distribution dans l'environnement. Du fait de leurs
propriétés hydrophobes, les PBB sont facilement adsorbés sur le sol
à partir des solutions aqueuses. On a observé que les différents
homologues étaient adsorbés préférentiellement en fonction des
caractéristiques du sol (par exemple la teneur en matières
organiques) ainsi que de la position et du nombre des atomes de
brome.
Les PBB sont stables et persistants; ils sont lipophiles et ne
sont en outre que légèrement solubles dans l'eau; certains
homologues sont peu métabolisés et s'accumulent dans la fraction
lipidique des organismes vivants. Une fois libérés dans
l'environnement, ils peuvent entrer dans la chaîne alimentaire où
ils subissent une concentration.
On a décelé des PBB dans les poissons de diverses régions. Les
PBB peuvent pénétrer dans l'organisme des mammifères et des oiseaux
par suite de l'ingestion de ces poissons.
Il est improbable que les PBB subissent une dégradation
chimique purement abiotique (à l'exclusion d'une photodécompo
sition). On a fait état d'une persistance des PBB sur le terrain.
Des échantillons de sol prélevés sur l'emplacement d'une ancienne
unité de production de PBB ont été analysés plusieurs années après
l'accident du Michigan; ils contenaient encore des PBB mais la
proportion des divers homologues était différente, ce qui indique
qu'il y avait eu décomposition partielle des résidus de PBB dans les
échantillons en question.
Au laboratoire, les PBB sont facilement décomposés par le
rayonnement ultraviolet. La photodécomposition d'un mélange
commercial (FireMaster(R)) a provoqué une diminution de la
concentration des homologues les plus substitués. La vitesse et le
degré de photolyse des PBB dans l'environnement n'ont pas été
déterminées avec précision, encore que l'observation sur le terrain
montre que les PBB de départ sont très tenaces, avec dégradation
partielle des homologues les moins bromés.
D'après les études en laboratoire, les mélanges de PBB semblent
assez résistants à la dégradation microbienne.
On ne connaît pas d'exemple de fixation ou de décomposition des
PBB par les végétaux. Par contre, les PBB sont facilement absorbés
par l'organisme animal et bien qu'ils se soient révélés très
persistants chez les animaux, on a tout de même retrouvé de petites
quantités de métabolites. Ces métabolites consistaient
principalement en dérivés hydroxylés et, dans certains cas, il y
avait des traces de PBB partiellement débromés. Aucune étude sur les
métabolites soufrés analogues à ceux des PCB n'a été publiée.
La bioaccumulation des PBB a été étudiée dans les poissons. En
ce qui concerne les animaux terrestres, on l'a étudiée chez
différentes espèces d'oiseaux et de mammifères. Les données ont été
fournies par des observations sur le terrain, par l'étude des
conséquences de la catastrophe du Michigan et par des études
d'alimentation contrôlée. En général, on a constaté que
l'accumulation des PBB dans les graisses de l'organisme était liée à
la dose et à la durée d'exposition.
La bioaccumulation des différents homologues des PBB augmente
avec le degré de bromation, au moins jusqu'aux dérivés tétrabromés.
On peut penser que les homologues plus substitués s'accumulent dans
une proportion encore plus importante. Toutefois, on ne dispose
d'aucun renseignement sur le décabromobiphényle; il est possible
qu'il soit peu absorbé.
On a signalé la présence de dibenzofuranes bromés et de PBB
partiellement débromés comme produits de la décomposition thermique
des PBB. La formation de ces composés dépend de plusieurs variables
(par exemple, température, oxygène).
1.4 Concentrations dans l'environnement et exposition humaine
On ne dispose que d'un seul rapport sur la concentration des
PBB dans l'atmosphère. Il s'agit d'une étude au cours de laquelle on
a mesuré la concentration de ces composés au voisinage des trois
unités de production et de traitement des PBB aux Etats-Unis
d'Amérique.
La concentration des PBB dans les eaux de surface du même
secteur ainsi que dans la décharge du Comté de Gratiot (Michigan,
Etats-Unis d'Amérique) qui avait reçu entre 1971 et 1973 plus de 100
000 kg de déchets contenant 60 à 70% de PBB, a fait l'objet d'une
surveillance.
Les données relatives à la surveillance des eaux souterraines
au voisinage de la décharge du Comté de Gratiot ont révélé la
présence de traces de PBB, même au-delà du voisinage immédiat de la
décharge; toutefois aucun de ces composés n'a été décelé dans les
puits d'eau potable du secteur.
On dispose de données sur la pollution tellurique par les PBB
dans les secteurs où des PBB ont été ou sont produits, utilisés ou
rejetés et également sur la pollution tellurique des terrains
agricoles du Michigan contaminés par ces composés.
Lors de la catastrophe du Michigan, du FireMaster(R) a été
ajouté par inadvertance à de la nourriture pour animaux. Il a fallu
presque une année pour qu'on s'aperçoive de cette erreur et pour que
les analyses révèlent que les PBB étaient en cause. Pendant cette
période (de l'été 1973 à mai 1974), des animaux et des produits
animaux contaminés ont été utilisés pour l'alimentation humaine et
ont pénétré dans l'environnement de l'Etat du Michigan. Des
centaines d'exploitations agricoles ont été affectées; il a fallu
abattre et enterrer des milliers d'animaux et détruire des milliers
de tonnes de produits agricoles.
La plupart des données concernant la contamination de la faune
sauvage par les PBB concernent des poissons et des oiseaux des
Etats-Unis d'Amérique et d'Europe (essentiellement la sauvagine
vivant à proximité des sites industriels) ainsi que des mammifères
marins.
Selon des rapports récents sur la contamination de poissons, de
mammifères terrestres ou marins et d'oiseaux aux Etats-Unis
d'Amérique et en Europe, ces composés seraient très disséminés. La
composition en homologues dans les échantillons de poissons est très
différentes de celle qu'on trouve dans des produits commerciaux.
Pour les principaux, ils pourraient dans beaucoup de cas résulter
d'une débromation photochimique du décabromobiphényle (BB 209), mais
cela n'a pas été confirmé.
On a observé une exposition professionnelle chez des employés
d'usines chimiques aux Etats-Unis d'Amérique, ainsi que chez des
ouvriers agricoles, à la suite de l'accident du Michigan. Les taux
médians de PBB dans le sérum et les tissus adipeux étaient plus
élevés chez les employés de l'industrie chimique. On ne dispose pas
de renseignements en provenance d'autres pays ou d'autres
entreprises sur l'exposition professionnelle lors de la fabrication,
de la formulation et de l'utilisation commerciale de ces produits.
On ne dispose pas, pour la plupart des populations humaines,
d'une documentation qui fournisse des données de première main sur
l'exposition aux PBB de diverses origines. Dans le Michigan, on a
observé de très nombreux cas d'exposition humaine résultant d'un
contact direct avec de la nourriture pour animaux contaminée et pour
l'essentiel, de la consommation de viande, d'oeufs et de produits
laitiers qui avaient également été contaminés par des PBB. Au moins
2000 familles (principalement des exploitants agricoles et leurs
voisins) ont été fortement contaminées. En Allemagne, on a récemment
décelé des PBB dans du lait de vache et du lait humain.
La composition en homologues de ces échantillons diffère de
celle que l'on trouve dans le poisson. La concentration relative du
BB 153 est plus élevée dans le lait humain que dans le poisson.
Les voies d'exposition de la population générale aux PBB sont
mal connues. Pour autant que l'on sache, ils ne sont pas présents à
fortes concentrations dans l'air ambiant ni dans l'eau. Plus
importants à cet égard sont probablement les produits alimentaires
riches en lipides tirés d'eaux contaminées. On ne possède aucun
renseignement sur le niveau d'exposition dans l'air intérieur ni sur
l'exposition par voie percutanée par suite d'un contact avec des
retardateurs de flammes à base de PBB.
La composition en homologues observée dans le lait humain
prélevé en Allemagne rappelait celle que l'on trouvait dans le lait
de vaches de la même région, mais à concentrations sensiblement plus
élevées.
Pour évaluer l'apport journalier de PBB par l'intermédiaire de
la nourriture dans la population générale, on ne dispose que de très
peu de données. Si l'on suppose que le poisson contient 20 µg de
PBB/kg de matières grasses et 5% de matières grasses et qu'une
personne de 60 kg consomme 100 g de poisson par jour, on arrive à
une ingestion journalière de 0,002 µg/kg de poids corporel. Avec une
concentration de PBB de 0,05 µg/kg de matières grasses dans le lait
(4% de matières grasses) et une consommation de lait de
500 ml/jour, la même personne en ingèrera quotidiennement environ
0,00002 µg/kg de poids corporel.
Un nourrisson de 6 kg consommant 800 ml de lait humain (3,5% de
matières grasses) par jour ingèrera 0,01 µg de PBB/kg de poids
corporel, si le lait contient de 2 µg de PBB/kg de matières grasses.
1.5 Cinétique et métabolisme
La résorption des PBB dans les voies digestives varie selon le
degré de bromation, les composés les moins bromés étant les plus
facilement résorbés.
Les données relatives à la résorption du DeBB et de l'OcBB/NoBB
sont insuffisantes.
On retrouve des PBB dans l'ensemble du règne animal et des
populations humaines, les concentrations d'équilibre les plus
élevées étant observées dans les tissus adipeux. Les concentrations
sont relativement élevées également dans le foie, particulièrement
en ce qui concerne les homologues les plus toxiques qui semblent se
concentrer dans cet organe. Le coefficient de partage des différents
homologues varie d'un tissu à l'autre. En général, on note une
tendance marquée à la bioaccumulation. Chez les mammifères, la
transmission des PBB à la descendance se produit par la voie
transplacentaire et par le lait maternel. On a constaté la présence
de 2,2',4,4',5,5'-hexabromobiphényles dans du lait humain à des
concentrations 100 fois plus élevées que dans le sérum maternel.
Lors d'une étude portant sur plusieurs générations de rats,
l'administration de PBB à une seule génération a entraîné la
présence de résidus décelables dans plus de deux des générations
suivantes. Chez les oiseaux, la teneur en PBB de l'organisme
maternel entraîne également la présence de résidus dans les oeufs.
Des nombreux homologues des PBB persistent dans les systèmes
biologiques. Ainsi, les constituants les plus abondants du
FireMaster(R), de même que l'octabromobiphényle et le décabromo
biphényle, n'ont pas paru être métabolisés ou excrétés dans une
proportion sensible. Les études métaboliques in vitro montrent que
l'on peut établir des relations structure-activité dans le cas du
métabolisme de PBB. Les PBB pourraient être métabolisés par les
microsomes induits par le phénobarbital à la condition de posséder
des atomes de carbone adjacents non bromés, en méta et en para
du pont biphényle, sur au moins un des cycles. La métabolisation des
homologues inférieurs par les microsomes induits par le
3-méthylcholanthrène nécessite la présence d'atomes de carbone non
bromés en ortho et méta du pont biphényle sur au moins un des
cycles; en outre, un degré important de bromation empêche la
métabolisation. Chez les vertébrés, les principaux produits de
métabolisation in vitro et in vivo des homologues inférieurs
sont des dérivés hydroxylés. Le rendement métabolique observé est
relativement faible. La réaction d'hydroxylation d'effectue
probablement par l'intermédiaire d'un oxyde d'arène ou par
hydroxylation directe.
L'homme, le rat, le singe rhésus, le porc, la vache et le
poulet éliminent les PBB, principalement dans leurs matières
fécales. Dans la plupart des cas, il semble que la vitesse
d'excrétion soit faible. Les concentrations de
2,2',4,4',5,5'-hexabromobiphényles observées dans la bile et les
matières fécales de sujets humains étaient égales à environ 50-70%
des taux sériques et à environ 0,5% des taux dans les tissus
adipeux. Les traitements administrés en vue d'améliorer
l'élimination des PBB chez l'animal ou l'homme n'ont guère eu de
succès. Le lait constitue une autre voie d'élimination des PBB.
Après administration de PBB à des rats et à d'autres animaux,
on a constaté que les relations entre la concentration tissulaire
des PBB et le temps étaient complexes et variables. Ces relations
ont pu être établies en utilisant divers modèles comportementaux. On
a calculé que la demi-vie d'élimination du 2,2',4,4',5,5'-hexa
bromobiphényles à partir des tissus adipeux du rat était d'environ
69 semaines. Dans le cas des singes rhésus, on a trouvé une demi-vie
de plus de quatre ans. Chez l'homme, on estime que la demi-vie
moyenne se situe dans le cas de ce composé entre 8 et 12 ans. Dans
la littérature, on trouve des valeurs allant de 5 à 95 ans. On
constate quelques différences dans la rétention et le "turnover" des
divers homologues. Les résultats fournis par l'analyse du sérum des
agriculteurs et des travailleurs de l'industrie chimique en vue de
doser le 2,3',4,4',5-pentabromobiphényle étaient incohérents. Cette
incohérence est probablement due à la diversité des sources
d'exposition. Les ouvriers étaient exposés à la totalité des
constituants du FireMaster(R) alors que la population du Michigan
n'avait consommé que de la viande et du lait contaminés contenant
des mélanges de PBB différents par suite de la métabolisation des
produits initiaux par les animaux de boucherie. Après administration
d'octobromobiphényle à des rats, on n'a pas noté de diminution des
taux de brome dans les tissus adipeux. On ne dispose d'aucune donnée
sur la rétention du décabromobiphényle.
L'organisme humain a davantage tendance à retenir certains
homologues des PBB que celui des animaux de laboratoire. C'est un
facteur à prendre en considération lorsqu'on évalue le danger pour
la santé humaine que représentent ces composés.
En conclusion, toutes les données disponibles indiquent que les
PBB ont une forte tendance à s'accumuler et à persister dans les
organismes vivants. Ils sont peu métabolisés et leur demi-vie chez
l'homme est de 8 à 12 ans ou davantage.
1.6 Effets sur les êtres vivants dans leur milieu naturel
On ne dispose que de quelques données au sujet des effets que
les PBB exercent sur les êtres vivants dans leur milieu naturel.
Elles portent sur les microorganismes, les puces d'eau, les oiseaux
aquatiques et les animaux d'élevage.
Les oiseaux aquatiques qui nichent sur les îles du nord-ouest
du lac Michigan ont été étudiés afin de voir si les polluants du
milieu étaient susceptibles d'affecter leur reproduction. On a ainsi
procédé au dosage de 17 polluants et notamment de PBB, dont aucun
n'a paru avoir d'effets notables sur la reproduction.
Les animaux d'élevage qui avaient ingéré une nourriture à
laquelle avait été ajouté par inadvertance du FireMaster(R) FF-1 à
la place d'oxyde de magnésium, sont tombés malades. Dans la première
ferme fortement contaminée que l'on ait observée, l'exposition
moyenne estimative des vaches était égale à 250 mg/kg de poids
corporel. Les signes cliniques d'intoxication consistaient dans une
réduction de 50% de la consommation de nourriture (anorexie) et une
diminution de 40% de la production laitière, dans les semaines
suivant l'ingestion des aliments contaminés. Bien qu'en l'espace de
16 jours on ait cessé de donner aux animaux la nourriture en
question, la production laitière n'est pas revenue à sa valeur
normale. Certaines vaches ont présenté une pollakiurie, un
larmoiement, avec en outre des hématomes, des abcès, une croissance
anormale des sabots, une boiterie, une alopécie, une hyperkératose
et une cachexie; plusieurs animaux sont morts dans les six mois
suivant l'exposition. Au total, la mortalité dans cette exploitation
a été de 24/400. Chez les veaux âgés de six à 18 mois, elle était
beaucoup plus élevée. Environ 50% d'entre eux sont morts dans les
six semaines avec seulement deux survivants sur 12 au bout de cinq
mois. Ces animaux étaient atteints d'hyperkératose sur l'ensemble du
corps. On a également noté divers problèmes affectant la
reproduction.
Les résultats des autopsies sont connus pour certaines des
vaches qui étaient mortes dans les six mois suivants l'exposition.
L'étude histopathologique a révélé la présence d'altérations
variables au niveau du foie et des reins.
Plusieurs des signes cliniques et des altérations
anatomopathologiques indiqués ci-dessus ont été confirmés par la
suite par des études d'alimentation contrôlée (anorexie,
déshydratation, hyperlarmoiement, émaciation, hyperkératose,
problèmes de reproduction, modification d'un certain nombre des
paramètres biochimiques, lésions rénales).
Chez les troupeaux faiblement contaminés, on a noté une chute
de la production et des cas de stérilité. Ces résultats contrastent
avec ceux des études contrôlées, qui n'ont pas révélé des
différences sensibles entre les troupeaux faiblement contaminés et
les troupeaux témoins.
A l'origine, la substitution accidentelle concernait des
aliments pour bovins, mais d'autres types de nourriture animale ont
subi une contamination croisée, notamment par l'intermédiaire du
matériel servant à la préparation de ces aliments. Il est probable
que l'exposition qui s'en est suivie n'a pas été aussi intense que
dans le cas des bovins. La contamination d'autres animaux a été
signalée (volailles, porcs, chevaux, lapins, chèvres et moutons) et
ces animaux ont été abattus; toutefois, les troubles dont ils
auraient pu souffrir n'ont pas été précisés.
On ne dispose d'aucun renseignement au sujet des effets des PBB
sur l'écosystème.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Les valeurs de la DL50 pour les mélanges du commerce corre
spondent à une toxicité aiguë relativement faible (DL50 > 1 g/kg
de poids corporel) pour le rat, le lapin et la caille, après
administration par voie orale ou percutanée. Une fois la dose de PBB
administrée, il y a d'ailleurs un certain délai avant l'apparition
des manifestations toxiques et la mort. C'est la dose totale
administrée qui détermine l'ampleur de l'intoxication, qu'elle soit
donnée en une seule fois ou qu'elle soit fractionnée et administrée
sur une courte période (jusqu'à 50 jours). La toxicité des PBB s'est
révélée plus importante après des doses multiples qu'après une dose
unique. L'exposition aux PBB n'entraîne pas immédiatement la mort.
Les quelques études effectuées sur des mélanges commerciaux
d'octo- et de décabromobiphényles n'ont pas provoqué de mortalité
chez les rats ni les poissons. En ce qui concerne les différents
homologues des PBB, on n'a étudié que trois hexaisomères, le
3,3',4,4',5,5'-HxBB et le 2,3',4,4',5,5'-HxBB étant plus toxiques
pour le rat que le 2,2',4,4',5,5'-HxBB. Sur la base des données
disponibles qui restent limitées, l'OcBB et le DeBB se révèlent
moins toxiques et sont moins bien résorbés que les mélanges de PBB.
De nombreuses études destinées à faire ressortir les effets
aigus et les effets à court terme ont montré que, parmi les signes
d'intoxication par les PBB (la plupart du temps, du FireMaster),
figurait une réduction de la consommation de nourriture. Aux doses
mortelles, on ne peut pas attribuer la mort à des lésions
anatomopathologiques affectant un organe déterminé mais plutôt à un
syndrome "cachectique" qui se développe chez l'animal et constitue
le premier signe d'intoxication. Au moment de la mort, la perte de
poids peut atteindre 30 à 40%. Les quelques études consacrées à
l'OcBB et au DeBB techniques n'ont pas révélé d'effets de ce genre.
C'est essentiellement au niveau du foie que l'on observe les
altérations morphologiques et histopathologiques imputables à
l'exposition aux PBB. Ainsi l'hypertrophie du foie s'observe
fréquemment à des doses plus faibles que celles qui entraînent une
perte de poids. Chez les rongeurs, les principales altérations
histopathologiques pourraient être un gonflement et une
vacuolisation généralisée des hépatocytes, la prolifération du
réticulum endoplasmique agranulaire et la nécrose des cellules
individuelles. La gravité des lésions dépend de la dose et de la
composition du mélange administré.
On a observé une diminution du poids du thymus chez des rats,
des souris et des bovins après absorption de FireMaster(R), mais
non d'OcBB ou de DeBB.
On a fait état d'une augmentation du poids de la thyroïde et de
modifications histologiques au niveau de cette glande chez le rat à
des concentrations faibles.
Il est évident que les différents homologues des PBB ne
présentent pas le même type de toxicité. Les isomères et les
homologues les plus toxiques provoquent une réduction du poids du
thymus ou du corps et déterminent des altérations histologiques
marquées au niveau du foie et du thymus. On a classé les byphényles
halogénés en fonction de leur structure. La catégorie 1 comporte les
isomères et les homologues qui n'ont pas de substi tuants en ortho
(PBB coplanaires). Les dérivés monosubstitués en ortho
constituent la deuxième catégorie. Les autres PBB (principalement
ceux qui comportent deux bromes ou davantage en ortho) sont
classés dans la troisième catégorie. Les homologues de la catégorie
1 ont tendance à provoquer les effets les plus graves alors que ceux
de la deuxième et de la troisième catégorie entraînent des effets
toxicologiques qui vont diminuant. A l'intérieur d'une même
catégorie, le degré de bromation peut également avoir une influence
sur la toxicité.
Sur toutes les combinaisons étudiées, c'est le
3,3',4,4',5,5'-HxBB qui s'est révélé le plus toxique. Cet homologue
est présent à faibles concentrations dans le FireMaster(R). Parmi
les principaux constituants du FireMaster(R), c'est le
2,3,3',4,4',5-HxBB qui s'est révélé le plus toxique devant le
2,3',4,4',5,5'-HxBB et le 2,3',4,4',5-PeBB, dans cet ordre. Le
principal constituant du FireMaster(R), le 2,2',4,4'5,5'-HxBB
s'est révélé relativement non toxique, de même que le
2,2',3,4,4',5,5'-HpBB, qui vient en seconde position par ordre de
concentration.
On ne connaît pas très bien la toxicité des mélanges d'OcBB et
de DeBB techniques eu égard à leur teneur en divers homologues (et
autres contaminants éventuels).
Les tests habituels d'irritation cutanée et oculaire de même
que les tests de sensibilisation qui ont été effectués sur des
mélanges de PBB techniques (OcBB et DeBB) n'ont révélé aucune
réaction ou du moins, seulement des réactions légères. Toutefois on
a relevé une hyperkératose et une alopécie chez les bovins exposés
et des lésions rappelant la chloracné ont été observés chez les
singes rhésus après ingestion de FireMaster(R). Le FireMaster(R)
a produit une hyperkératose de la surface interne de l'oreille chez
le lapin, mais ses deux principaux constituants (le
2,2',4,4',5,5'-HxBB et le 2,2',3,4,4',5,5'-HpBB) ne produisaient pas
cet effet. En fraction nant le FireMaster(R), on a constaté que
l'essentiel de son activité était le fait des fractions les plus
polaires contenant des constituants mineurs. En traitant des lapins
avec de l'HxBB exposé à la lumière solaire, on a constaté
l'apparition d'une hyperkératose grave au niveau de l'oreille.
L'administration de faibles doses d'OcBB technique pendant une
longue période à des rats n'a pas affecté leur consommation de
nourriture ni leur poids corporel, mais on a constaté chez les rats
qui avaient reçu pendant sept mois une dose de 2,5 mg/kg de poids
corporel, une augmentation du poids relatif du foie. L'adminis
tration pendant une longue durée à des rats de FireMaster(R) mêlé
à leur nourriture à la dose de 10 ng/kg de poids corporel pendant
six mois, est restée sans effet sur leur consommation de nourriture.
En revanche à la dose de 1 mg/kg de poids corporel administrée sur
une période de six mois, on constatait une modification du poids du
foie. Chez les rattes qui recevaient 0,3 mg/kg de poids corporel de
FireMaster(R), on constatait une réduction du poids du thymus. Des
altérations histopathologiques ont également été observées. Des
études d'alimentation contrôlées poursuivies pendant une longue
période sur des bovins exposés à de faibles doses de
FireMaster(R), n'ont pas fait ressortir d'effets nocifs, à en
juger par la prise de nourriture, les signes cliniques, les
modifications clinicopathologiques ou le rendement du bétail. Les
visons, les cobayes et les singes se sont révélés plus sensibles à
l'intoxication par les PBB.
On a observé chez le rat des effets à long terme attribuables à
la rétention des PBB après administration de fortes doses de
FireMaster(R) au cours de la période prénatale ou périnatale.
Les effets délétères les plus fréquemment observés sur la
reproduction consistaient en une résorption du foetus et une moindre
viabilité de la progéniture. Chez les visons, on observait encore
certains effets à la concentration de 1 mg/kg de nourriture. Une
réduction de la viabilité de la progéniture a été observée chez des
singes rhésus après 12,5 mois d'exposition au FireMaster(R) (dose:
0,3 mg/kg de nourriture). Les singes ont reçu une dose quotidienne
de ce mélange, égale à 0,01 mg/kg de poids corporel et la dose
totale était de 3,8 mg/kg de poids corporel. Il n'a pas été possible
d'évaluer les études de reproduction ni les études
neurocomportementales effectuées sur des singes et des rats à
faibles doses, car les publications en question n'étaient pas
suffisamment explicites quant au protocole expérimental des essais.
Chez les rongeurs, on a observé un faible pouvoir tératogène à des
doses élevées, susceptibles d'être toxiques pour les mères.
Les PBB perturbent les fonctions endocrines. Chez des rats et
des porcs on a observé une réduction des taux sériques de thyroxine
et de triiodothyronine qui était liée à la dose. Les PBB
perturberaient également, dans la plupart des cas, les taux
d'hormones stéroïdiennes. L'ampleur des effets dépend de l'espèce
ainsi que de la dose et de la durée de l'administration.
Les PBB ont également produit une porphyrie chez des rats et
des souris mâles à des doses quotidiennes ne dépassant pas
0,3 mg/kg de poids corporel. La dose quotidienne maximale sans
effets était de 0,1 mg/kg de poids corporel. On constatait une
influence marquée des PBB sur l'accumulation de vitamine A ainsi que
des effets sur la métabolisme intermédiaire.
Après exposition aux PBB, on observe fréquemment une atrophie
du thymus et on a constaté que d'autres tissus lymphoïdes étaient
également affectés. On a également mis en évidence d'autres
indicateurs témoignant d'une dépression des fonctions immunitaires
par le FireMaster(R). On manque de données concernant l'OcBB, le
NoBB, le DeBB ou les différents homologues des PBB.
Un des effets des PBB qui ait été le plus intensivement étudié
est l'induction des oxydases à fonction mixte. De fait, on a
systématiquement constaté que le FireMaster(R) se comportait comme
un inducteur de type mixte des enzymes microsomiennes du foie chez
le rat et chez toutes les autres espèces étudiées. Cette induction a
été également observée dans d'autres tissus, mais dans une moindre
mesure. L'aptitude à induire les enzymes microso miennes hépatiques
varie d'un homologue à l'autre. On a mis en évidence des
corrélations entre la structure et l'aptitude à induire les enzymes
microsomiennes.
Plusieurs études ont montré que les PBB étaient capables de
modifier l'activité biologique de divers médicaments et substances
toxiques. Cela s'explique peut-être en partie par le fait que les
PBB sont capables d'induire les enzymes microsomiennes qui
interviennent dans l'activation ou la désactivation des substances
xénobiotiques.
Le FireMaster(R) et certains de ces principaux constituants
se sont révélés capables d'inhiber la communication intercellulaire
in vitro. Cette inhibition s'est produite à des concentrations non
cytotoxiques. Cette cytotoxicité, de même que la capacité d'inhiber
la coopération métabolique paraît liée à la structure et plus
précisément à la présence ou à l'absence de substitution en ortho.
Les épreuves in vitro et in vivo (mutagénèse des cellules
microbiennes et mammaliennes, altération des chromosomes de cellules
mammaliennes, transformation des cellules mammaliennes, lésion et
réparation de l'ADN) n'ont pu mettre en évidence de mutagénicité ou
de génotoxicité imputables aux divers homologues des PBB ou aux
mélanges qui sont vendus dans le commerce.
Les études de toxicité à long terme ont montré que le foie
était la principale cible des effets cancérogènes du PBB. Chez des
souris et des rats, mâles et femelles, qui recevaient du
FireMaster(R) par voie orale, on a noté une augmentation sensible
de l'incidence des carcinomes hépatocellulaires. Des effets
cancérogènes sur le foie ont également été observés chez des souris
qui avaient reçu pendant 18 mois une alimentation contenant une dose
totale de 100 mg/kg ou davantage de Bromkal 80-9D
(nonabromobiphényle technique) en doses quotidiennes de 5 mg/kg de
poids corporel. La dose quotidienne de PBB la plus faible qui ait
produit des tumeurs (pour la plupart, des adénomes) chez des
rongeurs, était de 0,5 mg/kg de poids corporel pendant deux ans. Les
rats qui en avaient reçu quotidiennement 0,15 mg/kg de poids
corporel, en plus de ce qu'il leur avait été administré pendant la
période prénatale et périnatale, n'ont pas présenté le moindre effet
indésirable. La pouvoir cancérogène de l'octabromobiphényle et du
décabromobiphényle techniques n'a pas été étudié.
Ni le FireMaster BP-6 ni le 2,2',4,4',5,5'-hexabromobiphényle
ne se sont comportés comme des initiateurs tumoraux (en utilisant le
TPA comme promoteur) ou comme des promoteurs tumoraux (en utilisant
la DMBA comme initiateur) lors d'épreuves bio logiques sur
l'épiderme de souris. Toutefois, en utilisant le même modèle
(épiderme de souris) et avec du DMBA ou de la MNNG comme initiateur,
le FM FF-1 et le 3,3',4,4'5,5'-hexabromo biphényle mais non pas le
2,2',4,4'5,5'-hexabromobiphényle, ont présenté une activité
tumoro-promotrice. Lors d'une épreuve biologique sur foie de rat
effectuée en deux temps, et avec du phénobarbital comme promoteur,
on a constaté que le 3,3',4,4'- tétrabromobiphényle se comportait
comme un initiateur faible. Avec ce même modèle animal, en présence
de diéthylnitrosamine et après hépatectomie partielle, on a constaté
que le FM, le 3,3',4,4'-tétrabromobiphényle et le
2,2',4,4',5,5'-hexabromobi phényle, mais non pas le
3,3',4,4',5,5'-hexabromobiphényle, se comportaient comme des
promoteurs tumoraux.
Les résultats des études sur la communication cellulaire, les
résultats négatifs fournis par les études de génotoxicité et de
mutagénicité, ainsi que ceux des épreuves de promotion tumorale,
montrent que les mélanges de PBB et les divers homologues étudiés
provoquent l'apparition de cancers par un mécanisme épigéné tique.
On ne dispose d'aucun renseignement sur l'octa-, le nona-, le
décabromobiphényles techniques.
Le mode d'action qui est à la base des nombreuses manifes
tations de la toxicité des PBB et des composés apparentés reste
inconnu. Toutefois, certains des effets observés, comme le syndrome
cachectique, l'atrophie du thymus et l'hépatotoxicité, les
manifestations dermatologiques et les effets délétères sur la
fonction de reproduction peuvent être attribués à une interaction
avec les récepteurs Ah ou TCDD, interaction qui entraîne une
modification de l'expression d'un certain nombre de gènes.
L'interaction avec ces récepteurs varie selon les divers homologues,
les homologues coplanaires étant les plus actifs.
Nombre des effets des PBB s'observent après une exposition de
longue durée. Cela s'explique peut-être par le fait que certains
homologues s'accumulent fortement et que l'organisme ne les
métabolise et ne les élimine que difficilement. Il s'en suit une
accumulation de ces composés dans l'organisme qui finit par
submerger les mécanismes de compensation et entraîne des effets
délétères.
Certains contaminants connus du FireMaster(R), en
l'occurrence des polybromonaphtalènes (PBN) sont fortement toxiques
et tératogènes. Bien qu'ils ne soient présents qu'en petites
quantités dans le FireMaster(R), il n'est pas exclu qu'ils
contribuent à sa toxicité.
Les études portant sur le FireMaster(R) et son principal
constituant le 2,2',4,4'5,5'-HxBB, ont montré que leurs produits de
photolyse étaient plus toxiques que les composés initiaux. Les
produits du pyrolyse du FM provoquent l'induction des oxydases à
fonction mixte, une perte de poids et une atrophie du thymus. On a
également observé que les produits de pyrolyse de l'OcBB technique
produisaient une hypertrophie du foie.
1.8 Effets sur l'homme
On ne connaît aucun exemple d'intoxication aiguë par les PBB
chez l'homme auquel on puisse comparer les effets potentiels à
faibles doses résultant de l'accident survenu dans le Michigan aux
Etats-Unis d'Amérique en 1973. Les principales études
épidémiologiques ont été menées par le Michigan Department of Public
Health (MDPH) et l'Environmental Science Laboratory de la Mount
Sinaï School of Medicine, New York (ESL).
On estime que les personnes les plus fortement contaminées
avaient consommé 5 à 15 g de PBB sur une période de 230 jours par
l'intermédiaire du lait. Il est possible que la consommation de
viande ait constitué une source de contamination supplémentaire.
Chez certains des agriculteurs et chez la plupart des membres de la
population générale du Michigan, le niveau d'exposition était
beaucoup plus faible, la dose totale étant de 9 à 10 mg. Il est
possible cependant que certaines personnes aient reçu une dose
totale d'environ 800 à 900 mg. (Une dose totale de 9 mg correspond à
0,15 mg/kg de poids corporel et une dose de 900 mg, à 15 mg/kg de
poids corporel pour un adulte moyen de 60 kg; pour un enfant, la
dose par kg/poids corporel serait plus élevée).
En 1974, la première étude du MDPH a consisté à comparer l'état
de santé des personnes travaillant dans les fermes mises en
quarantaine, à celui du personnel des fermes de la même région qui
n'étaient pas frappées par cette mesure. Dans les deux groupes, on a
constaté divers symptômes, mais sans pouvoir dégager de différences.
Aucune anomalie inhabituelle n'a été constatée, qu'il s'agisse du
coeur, du foie, de la rate, du système nerveux, des résultats de
l'analyse d'urine et de la NFS, non plus qu'en ce qui concerne tous
les autres paramètres médicaux examinés. Une étude ultérieure très
complète menée par le MDPH et portant sur des groupes soumis à une
exposition de degré variable, n'a pas permis de mettre en évidence
de corrélation positive entre les taux sériques de PBB et la
fréquence des symptômes ou des affections observés. L'ESL a étudié
environ 990 personnes vivant sur des exploitations agricoles, 55
travailleurs de l'industrie chimique et un groupe de producteurs de
lait du Wisconsin qui ont fait office de témoins. Les symptômes
étaient plus fréquent chez les exploitants du Michigan que chez ceux
du Wisconsin. C'est dans le cas des symptômes neurologiques et
musculo-squelettiques, au sens large, que les différences étaient
les plus importantes. De même les taux sériques de certaines enzymes
hépatiques et de l'antigène carcino-embryonnaire étaient plus
fréquemment élevés chez les fermiers du Michigan que chez ceux du
Wisconsin. La prévalence des symptômes respiratoires et cutanés
était plus forte chez les travailleurs de l'industrie chimique avec
également des symptômes musculo-squelettiques moins fréquents que
chez les agriculteurs. Les résultats des travaux de l'ESL n'ont pas
toujours été interprétés de la même manière que ceux d'autres études
comparables, mais tous sont d'accord sur un point. Aucune de ces
séries d'études n'a mis en évidence de corrélation dose-réponse
positive entre les taux de PBB dans le sérum ou les tissus adipeux
et la prévalence des symptômes ou des anomalies cliniques. Un
certain nombre d'aspects cliniques ont été étudiés de manière plus
intensive par des méthodes spéciales. En particulier, l'examen des
aspects neurologiques au moyen de tests objectifs de performance a
révélé, dans une étude tout du moins, l'existence d'une corrélation
négative entre les taux sériques de PBB et les résultats des tests,
en particulier chez les hommes d'âge mûr. Quant aux autres études,
elles n'ont pas mis en évidence de relation entre la concentration
des PBB dans le sérum ou les tissus adipeux et les résultats d'une
batterie de tests portant sur la mémoire, la force musculaire, la
coordination, la perception cortico-sensorielle, la personnalité,
les fonctions cognitives supérieures et d'autres fonctions. Les
aspects pédiatriques de l'exposition aux PBB ont été étudiés dans
les familles examinées par l'ESL. Bien que de nombreux symptômes
aient été signalés, l'examen médical n'a pas révélé d'anomalie
objective attribuable à l'exposition au PBB. Des opinions
différentes se sont exprimées à propos des effets
neurospychologiques plus subtils observés dans la descendance de ces
personnes et les résultats des études portant sur la capacité de
développement sont également controversés. Cela vaut aussi pour
l'étude des lymphocytes et de la fonction immunitaire. C'est ainsi
que selon plusieurs auteurs, il n'y avait pas de différences entre
les groupes à fort et faible taux sérique de PBB pour ce qui
concerne le nombre de lymphocytes et les fonctions lymphocytaires,
alors que d'autres ont constaté une réduction sensible des
sous-populations de lymphocytes T et de lymphocytes B chez environ
40% des personnes exposées dans le Michigan, par rapport aux groupes
non exposés, avec en outre une altération de la fonction
lymphocytaire, à savoir une diminution de la réponse aux mitogènes.
Dans les études épidémiologiques qui ont été passées en revue,
on s'est efforcé d'évaluer la relation entre l'exposition aux PBB et
un grand nombre d'effets sur le comportement et de symptômes
subjectifs. Cependant, la plupart des ces études présentent de
graves insuffisances au niveau de la conception et en particulier,
elles laissent subsister des facteurs de confusion qui rendent
difficile, voire impossible, toute conclusion sur la relation
éventuelle entre l'exposition aux PBB et d'éventuels effets sur la
santé. On ne dispose pas d'un recul suffisant pour pouvoir évaluer
les effets cancérogènes éventuels de ces composés.
On a identifié deux petits groupes de travailleurs exposés de
par leur profession à un mélange de PBB ou à du DeBB et du DBBO. Des
lésions rappelant une chloracné ont été observées chez 13% des
travailleurs exposés au mélange de PBB, alors que ceux qui avaient
été exposés au DeBB ne présentaient pas de telles lésions. Toutefois
la prévalence de l'hypothyroïdie était plus élevée dans ce groupe.
1.9 Evaluation globale de la toxicité et de la cancérogénicité
La seule étude toxicologique qui ait été effectuée à vie sur
des animaux d'expérience a été menée récemment dans le cadre du
Programme national de toxicologie (National Toxicology Programme,
NTP) sur des rats et des souris. La dose la plus faible étudiée qui
produisait encore des effets cancérogènes était égale à 0,5 mg/kg de
poids corporel et par jour (formation de tumeurs hépatiques chez les
rongeurs). D'autres études du même genre ont mis en évidence un
effet cancérogène à la dose quotidienne de 3 mg/kg de poids
corporel, sur une durée de six mois. L'étude de six mois montre
qu'une exposition pendant une durée inférieure à la vie normale de
l'animal à des doses voisines, entraîne également des effets
délétères analogues. Il est possible qu'à plus faibles doses, les
PBB exercent des effets sur la reproduction des primates
sous-hominiens et des visons.
En outre, l'étude de deux ans effectuée dans le cadre du NTP a
montré qu'une dose quotidienne de 0,15 mg/kg de poids corporel avec
exposition prénatale et périnatale de la mère à une dose quotidienne
de 0,05 mg/kg de poids corporel, ne produisait aucun effet nocif.
Par conséquent, la dose totale absorbée quotidienne ment à partir de
la nourriture, de l'eau, de l'air et du sol devrait être inférieure
à 0,15 µg/kg de poids corporel, si l'on extrapole les résultats
obtenus concernant la dose sans effet nocif observable lors d'une
étude de cancérogénicité positive, en appliquant à cet effet un
coefficient d'incertitude (coefficient de sécurité) de 1000, puisque
ces composés induisent probablement des cancers par un mécanisme
épigénétique.
On estime que la dose totale reçue par le sous groupe de
population du Michigan se situait entre 0,15 et 15 mg/kg de poids
corporel sur une période de 230 jours. Si on rapporte cette dose à
la durée moyenne de vie normale d'un être humain, cela
correspondrait, pour cette population, à une dose quotidienne de 0,6
ng à 6 µg/kg de poids corporel.
On estime que pour les adultes de la population générale,
l'apport quotidien total de PBB par kg de poids corporel à partir
des sources répertoriées, est de l'ordre de 2 ng; il est de 10 ng
pour les nourrissons nourris au sein. Il convient de noter que ces
estimations reposent sur des données régionales très limitées.
Ces calculs reposent sur l'hypothèse que la concentration des
PBB n'atteindra pas un état stationnaire au cours de l'existence et
que l'on peut substituer une exposition forte sur une courte durée à
une exposition faible sur une longue durée, étant donné que ces
composés sont très mal métabolisés et excrétés.
On ne dispose pas de données suffisantes pour l'OcBB, le NoBB
et le DeBB pour calculer quel serait l'apport quotidien total
maximal ne produisant pas d'effets indésirables.
2. Conclusions
La plupart des homologues des PBB qui entrent dans la
composition des retardateurs de flammes vendus dans le commerce,
sont lipophiles, persistants et s'accumulent dans les biotes. Ces
composé subissent une bioamplification le long des réseaux
trophiques et constituent une menace, en particulier pour les
organismes qui se trouvent en fin de réseau. En outre, certains
constituants des PBB sont les précurseurs de dibenzofuranes
polybromés toxiques qui se forment lors de la combustion.
Outre les émissions qui se produisent au cours de la
fabrication et de l'utilisation des PBB, ceux-ci pénètrent dans
l'environnement du fait de la très large utilisation des
retardateurs de flammes dont ils sont les constituants. Une part
très importante des PBB produits finit par passer dans
l'environnement du fait de la très grande stabilité de ces composés.
On trouve également des PBB dans des échantillons prélevés dans
l'environnement et sur des sujets humains, en des lieux éloignés des
endroits où l'on sait que ces composés sont produits. La
composition en homologues des PBB dans les échantillons provenant de
l'environnement ne correspond pas à celle que l'on trouve dans les
produits techniques, ce qui indique qu'il y a transformation dans le
milieu, peut-être à la suite d'une débromation photochimique.
On dispose actuellement des très peu de données sur l'ampleur
de l'exposition de la population générale aux PBB. Toutefois, dans
les quelques cas où on a procédé à des mesures, on a pu mettre en
évidence des traces de PBB. Actuellement, cette exposition ne cause
pas d'inquiétude, mais il faudrait éviter que ces composés ne
continuent à s'accumuler. D'après les observations faites sur
l'homme à la suite de l'accident du Michigan, il semblerait que les
personnes contaminées aient été exposées à des doses de plusieurs
ordres de grandeur supérieures à celles que l'on observe dans la
population générale. On n'a pas observé dans la population du
Michigan d'effets concluants qui puissent être attribués à
l'exposition aux PBB, encore que la période de suivi ne soit pas
suffisante pour permettre à d'éventuels cancers de se manifester.
Etant donné que les taux de PBB dans les tissus adipeux et le sérum
restent élevés dans la population du Michigan, il y a poursuite de
l'exposition interne. En revanche, on a bien observé des effets
toxiques chez les bovins de cette région. On explique cette
discordance par le fait que les bovins avaient été davantage
exposés.
L'exposition professionnelle n'a été étudiée que dans deux
unités de production des Etats-Unis d'Amérique. Il semble que chez
les travailleurs employés à la production des PBB, il puisse y avoir
apparition de lésions rappelant une chloracné; quant aux
travailleurs exposés au DeBB, ils peuvent présenter une
hypothyroïdie. Aucune enquête n'a été menée chez les travailleurs
qui confectionnent des produits commerciaux à base de deca-, d'octa-
ou de nona-bromobiphényles.
Les PBB sont extrêmement persistants chez les organismes
vivants et ils peuvent conduire à une intoxication chronique et à
des cancers. Bien que la toxicité aiguë soit faible, on constate
l'apparition de cancers à des doses quotidiennes de 0,5 mg/kg de
poids corporel avec une dose sans effet observable de 0,15 mg/kg de
poids corporel et par jour. On a observé un certain nombre d'effets
toxiques chroniques chez les animaux de laboratoire à des doses
quotidiennes de l'ordre de 1 mg/kg de poids corporel, administrées
pendant de longues périodes.
3. Recommandations
3.1 Généralités
Le Groupe de travail estime qu'il faut éviter à l'homme et à
l'environnement d'être exposés aux PBB en raison de la forte
persistance et de la forte bioaccumulation de ces composés ainsi que
des effets nocifs qu'ils peuvent provoquer en cas d'exposition de
longue durée à de faibles doses. Aussi convient-il de ne plus
utiliser de PBB dans des produits du commerce.
Comme les données dont on dispose sur la toxicité du DeBB et de
l'OcBB sont limitées, qu'ils sont extrêmement persistants et
susceptibles d'être dégradés dans l'environnement et qu'en outre,
leur combustion entraîne la formation de dérivés encore plus
toxiques, ils ne doivent pas être utilisés dans le commerce, du
moins tant qu'on aura pas démontré que cet usage est sans danger.
La cohorte du Michigan est toujours en observation et il est
nécessaire que les données obtenues soient publiées.
3.2 Recherches futures
Il convient de développer la surveillance des PBB chez l'homme
et dans l'environnement, et en particulier sur les lieux de travail,
qu'il s'agisse de la fabrication proprement dite des PBB ou de leur
utilisation; cette surveillance devra porter sur chaque homologue en
particulier et englober également l'OcBB, le NoBB et le DeBB. Ces
composés doivent figurer dans les programmes de surveillance des
dérivés halogénés actuellement en cours. On devra notamment
continuer à suivre la tendance des concentrations de PBB dans
l'environnement et leur distribution géographique. On procédera
également à un relevé des décharges où des PBB sont susceptibles de
passer dans l'environnement.
Il faudrait procéder à des expériences de thermolyse simulant
les conditions d'un incendie accidentel ou de l'incinération de
déchets municipaux. Des travaux complémentaires devront également
être consacrés à l'étude du mécanisme de la toxicité et de la
cancérogénicité des PBB et des composés apparentés. Les PBB peuvent
être utilisés comme modèles pour ces recherches. Tous ces travaux
devront utiliser des homologues purifiés.
Les effets des PBB sur la reproduction restent mal connus.
Aussi serait-il souhaitable d'effectuer des études de longue durée
bien conçues, concernant l'effet des faibles doses sur la
reproduction, en utilisant une espèce vulnérable.
Il importe également d'obtenir davantage de renseignements sur
la biodisponibilité et la toxicocinétique de l'OcBB/NoBB, du DeBB et
d'un certain nombre d'homologues.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
Los bifenilos polibromados o polibromobifenilos (PBB) son un
grupo de hidrocarburos halogenados formados por sustitución del
hidrógeno del bifenilo por bromo. No se conoce ningún PBB de origen
natural. Estas moléculas responden a la fórmula
C12H(10-x-y)Br(x+y), donde x e y están comprendidos entre 1 y
5. Por consiguiente, teóricamente son posibles 209 formas
moleculares, pero sólo se han sintetizado individualmente y
caracterizado un reducido número. Los PBB fabricados para uso
comercial consisten principalmente en hexa-, octa-, nona-, y
decabromobifenilos, pero contienen también otros productos de la
misma familia. Son pirorretardantes que se emplean como aditivos, y
que mezclados con material polimérico líquido o sólido seco le
confieren propiedades pirorretardantes de tipo filtrante; en caso de
ignición se produce una liberación química de ácido bromhídrico.
La fabricación de los PBB se basa en una reacción de
Friedel-Crafts entre el bifenilo y el bromo, en presencia de un
disolvente orgánico en ocasiones, y de un catalizador que puede ser
cloruro de aluminio, bromuro de aluminio o hierro.
La mayor parte de las investigaciones realizadas se refieren a
los productos FireMaster BP-6 y FF-1, responsables de la catástrofe
que se produjo en Michigan cuando, inadvertidamente, fueron
agregados al pienso de los animales en lugar del óxido de magnesio
que correspondía. La consiguiente contaminación acarreó la muerte de
miles de cabezas de ganado vacuno, porcino y ovino, así como de
millones de pollos.
La composición de la mezcla FireMaster(R) varía de un lote a
otro, pero sus principales componentes son el 2,2',4,4',5,5'-
hexabromobifenilo (60-80%) y el 2,2',3,4,4',5,5'-heptabromobifenilo
(12-25%), junto con los cuales se hallan también otros compuestos
menos bromados que son el resultado de una reacción de bromación
incompleta. Se han detectado también bromoclorobifenilos y
naftalenos polibromados como componentes minoritarios de
FireMaster(R). FireMaster FF-1 (polvo blanco) se obtiene a partir
de FireMaster BP-6 (escamas pardas), por adición, como agente
antiaglutinante, de silicato de calcio al 2%.
Los PBB son sólidos de baja volatilidad; ésta disminuye al
aumentar el número de átomos de bromo. Son prácticamente insolubles
en agua, solubles en grasas, y entre poco y muy solubles en diversos
disolventes orgánicos; la solubilidad también disminuye al aumentar
el número de átomos de bromo. Son compuestos relativamente estables
y químicamente inertes, pero las mezclas de PBB muy bromados se
fotodegradan y sufren una debromación reductiva al ser expuestas a
la radiación ultravioleta.
Los productos de la descomposición térmica experimental de los
PBB dependen de la temperatura, de la cantidad de oxígeno presente y
de otros varios factores. Las investigaciones realizadas sobre la
pirólisis de FireMaster BP-6 en ausencia de oxígeno (600-900 °C) han
demostrado que se forman bromobencenos y bifenilos menos bromados,
pero no así furanos polibromados. Por el contrario, la pirólisis en
presencia de oxígeno (700-900 °C) generó una cierta cantidad de di-
a heptabromodibenzofuranos. En presencia de poliestireno y
polietileno se hallaron niveles más altos. La pirólisis de
FireMaster BP-6 en presencia de PVC a 800 °C dio lugar a una mezcla
de bromoclorobifenilos. No se dispone de información sobre la
naturaleza de los productos de incineración de los materiales que
contienen PBB. Poco se sabe acerca de la toxicidad de las dioxinas y
furanos bromados y bromados/clorados, pero se estima que debe ser de
aproximadamente la misma magnitud que la de las dioxinas y furanos
clorados.
La principal técnica analítica empleada para el control
biológico de los PBB en muestras del medio y en tejidos y líquidos
biológicos tras la catástrofe de Michigan fue la cromatografía de
gases con detector de captura de electrones. Los diversos productos
de la familia pueden determinarse individualmente mediante cromato
grafía de gases capilar, y aún es posible conseguir una detección
más específica si se emplea la espectrometría de masas de control de
determinados iones. Como el número de posibles miembros de esta
familia de productos es muy elevado, las investigaciones se ven
dificultadas por la falta de patrones sintéticos adecuados. Los
métodos empleados para extraer PBB de muestras biológicas se han
venido basando en los usados con los plaguicidas. Los PBB son
extraídos con la grasa, y purificados a continuación.
El hallazgo reciente de PBB en muestras biológicas de fondo no
significa necesariamente que su concentración esté aumentando en el
medio: ello podría deberse a la aparición de técnicas analíticas más
sensibles, como la espectrometría de masas de ionización química de
iones negativos. De ahí la necesidad de realizar cuanto antes
estudios retrospectivos. Los métodos mejorados de purificación
exhaustiva permiten realizar análisis específicos de los PBB
coplanares tóxicos, datos que son igualmente necesarios.
1.2 Fuentes de exposición humana y ambiental
La producción comercial de FireMaster(R) comenzó en los
Estados Unidos en 1970, pero se interrumpió tras la catástrofe de
Michigan (noviembre de 1974). La producción estimada de PBB en los
Estados Unidos entre 1970 y 1976 fue de 6000 toneladas (cantidades
comerciales); hasta 1979 se siguió produciendo en el país
octabromobifenilo y decabromobifenilo. En Alemania se produjo hasta
mediados de 1985 una mezcla de PBB altamente bromados conocida como
Bromkal 80-9 D. Actualmente se produce en Francia decabromobifenilo
(Adine 0102) de calidad técnica. Al parecer, esos son los únicos PBB
que se siguen produciendo hoy en día.
Los PBB se introdujeron a principios de los años setenta como
pirorretardantes. Hasta noviembre de 1974 el PBB más importante
comercialmente en los Estados Unidos era el hexabromobifenilo,
producto que se incorporaba a los plásticos (10% de contenido de
PBB), de acrilonitrilo-butadieno-estireno (ABS), material usado
principalmente en la fabricación de pequeños utensilios y
componentes de automóvil, revestimientos, barnices y espuma de
poliuretano. Los otros PBB pirorretardantes tienen aplicaciones
similares.
Durante el proceso normal de producción pueden tener lugar
pérdidas de PBB en el medio ambiente, por emisión a la atmósfera o
por su incorporación a aguas residuales, suelos o vertederos,
pérdidas que sin embargo, según se ha observado, son por lo general
de escasa importancia.
Estos compuestos pueden llegar también al medio durante su
transporte y manipulación, así como de manera accidental, como
ocurrió en Michigan.
Existe también la posibilidad de que pasen al medio de resultas
de la incineración de materiales que contienen PBB, o a causa de
fuegos accidentales, formándose en estos casos otros productos
tóxicos, como polibromodibenzofuranos o derivados mixtos de bromo y
cloro.
La mayor parte de los compuestos así formados acaban
difundiéndose a la larga al medio, como tales o en forma de
productos de degradación.
1.3 Transporte, distribución y transformación en el medio ambiente
No hay pruebas de que los PBB se propaguen por la atmósfera a
grandes distancias, pero la presencia de estos compuestos en
muestras de focas del Artico pone de manifiesto una amplia
distribución geográfica.
Las principales vías conocidas de llegada de los PBB al medio
acuático son los vertidos de desechos industriales y los lixiviados
de lugares de vertimiento industrial que alcanzan las aguas, así
como la erosión de suelos contaminados. Los PBB son casi insolubles
en agua y se hallan sobre todo en los sedimentos de lagos y ríos
contaminados.
Los focos de contaminación del suelo pueden ser las fábricas o
los depósitos de residuos de PBB. Los PBB que llegan a penetrar en
el suelo no se desplazan fácilmente. Se ha observado que los PBB son
200 veces más solubles en el lixiviado de un vertedero que en el
agua destilada, lo que puede significar una mayor propagación en el
medio ambiente. Debido a sus propiedades hidrofóbicas, cuando están
en solución acuosa estos productos son fácilmente adsorbidos por los
suelos. Se observó una adsorción preferencial de determinados PBB en
función de las características del suelo (por ejemplo de su
contenido orgánico) y del número y posición de los radicales de
bromo.
Los PBB son estables y persistentes, lipofílicos, y sólo
ligeramente solubles en agua; algunos de los compuestos de esta
familia apenas son metabolizados y se acumulan en los compartimentos
lipídicos de la biota. Una vez liberados en el medio ambiente,
pueden alcanzar la cadena alimentaria y concentrarse en ella.
Se han detectado PBB en el pescado capturado en varias
regiones. La ingestión de pescado es una vía de transmisión de PBB a
los mamíferos y las aves.
Se considera improbable que los PBB se degraden mediante
reacciones químicas puramente abióticas (excluidas las reacciones
fotoquímicas). Se ha notificado la persistencia de PBB en el
terreno. Al cabo de varios años del accidente de Michigan, el
análisis de muestras del suelo de un antiguo centro de fabricación
de PBB reveló la presencia, aún, de ese tipo de productos, aunque el
perfil de los PBB era distinto, debido a la degradación parcial
sufrida por los residuos en la muestra de suelo.
En condiciones de laboratorio los PBB son degradados fácilmente
por la radiación ultravioleta. La fotodegradación de la mezcla
comercial FireMaster(R) se refleja en una menor concentración de
los PBB que presentan más sustituyentes. No se han determinado con
exactitud ni la velocidad ni la magnitud de las reacciones
fotolíticas que sufren los PBB en el medio, pero las observaciones
realizadas sobre el terreno muestran una elevada persistencia de los
PBB originales, o bien una degradación parcial a formas menos
bromadas.
En las investigaciones de laboratorio las mezclas de PBB
parecen bastante resistentes a la degradación microbiana.
No se ha descrito ningún fenómeno de captación o degradación de
PBB por las plantas. En cambio, los PBB son fácilmente absorbidos
por los animales, en los que se ha observado que son muy
persistentes, aun cuando se han detectado pequeñas cantidades de
metabolitos. Los principales productos metabólicos eran
hidroxiderivados, y en algunos casos se hallaron indicios de la
existencia de PBB parcialmente debromados. No se ha descrito
investigación alguna sobre posibles metabolitos sulfurados análogos
a los de los PCB.
Se ha investigado la bioacumulación de PBB en el pescado, así
como la que se produce en animales terrestres, en este caso mediante
el estudio de especies de mamíferos y aves. Los datos obtenidos
proceden de observaciones sobre el terreno, de la evaluación de la
catástrofe de Michigan, y de estudios controlados de alimentación de
los animales. Por lo general se observó que la acumulación de PBB en
la grasa corporal dependía de la dosis y de la duración de la
exposición.
Al analizar individualmente los PBB, se observa que su
bioacumulación aumenta con el grado de bromación, al menos hasta los
tetrabromobifenilos. Cabe suponer que los productos más bromados de
la familia se acumulan aún en mayor medida. No obstante, no se
dispone de información sobre el decabromo bifenilo, cuya absorción
es posiblemente escasa.
Se ha notificado la generación de dibenzofuranos bromados o PBB
parcialmente debromados como productos de la descomposición térmica
de los PBB. Su aparición depende de varios factores, como por
ejemplo la temperatura, el oxígeno, etc.
1.4 Niveles ambientales y exposición humana
Tan sólo se dispone de los resultados de un estudio sobre los
niveles de PBB en la atmósfera. En dicho estudio se determinaron las
concentraciones de esos productos en las proximidades de tres
plantas de fabricación o procesamiento de PBB de los Estados Unidos.
Se analizaron también los niveles alcanzados en las aguas
superficiales en esas mismas inmediaciones y en el vertedero del
distrito de Gratiot/Michigan (EE.UU.), al que entre 1971 y 1973
fueron a parar más de 100 000 kg de desechos, constituidos en un
60-70% por PBB.
El análisis de las aguas subterráneas del vertedero del
distrito de Gratiot reveló la presencia de cantidades ínfimas de PBB
incluso fuera de la zona del vertedero, pero no se detectaron PBB en
los pozos de agua de bebida del entorno.
Se dispone de datos sobre la contaminación del suelo por PBB en
zonas de fabricación, empleo o evacuación de PBB, así como en los
suelos de los campos de las granjas de Michigan contaminadas por
PBB.
La catástrofe de Michigan sobrevino porque, por inadvertencia,
se añadió FireMaster(R) al pienso destinado a los animales. No fue
sino al cabo de casi un año cuando se descubrió el error de mezcla,
y los análisis efectuados mostraron que el origen del problema eran
los PBB. Durante ese periodo (verano de 1973 a mayo de 1974), los
animales contaminados y sus productos se difundieron entre los
suministros de alimentos para el hombre y en el medio en el estado
de Michigan. Centenares de granjas se vieron afectadas, y miles de
animales tuvieron que ser sacrificados y enterrados, al igual que
hubo que enterrar miles de toneladas de productos agrícolas.
La mayor parte de los datos disponibles sobre la contaminación
de la fauna por PBB se refieren a peces y aves de los Estados Unidos
y Europa, sobre todo aves acuáticas, de las inmediaciones de centros
industriales, y mamíferos marinos.
Los últimos estudios sobre la contaminación por PBB de peces,
mamíferos terrestres y marinos y aves de los Estados Unidos y Europa
muestran una amplia distribución de esos compuestos. El perfil de
los PBB hallados en las muestras de pescado es muy distinto del
hallado en los productos comerciales. Muchos de los picos más
importantes podrían ser el resultado de la debromación fotoquímica
del decabromobifenilo (BB 209), hipótesis no confirmada.
Tras el accidente de Michigan se observaron casos de exposición
profesional entre los empleados de fábricas de la industria química
de los Estados Unidos, así como entre los trabajadores agrícolas.
Los niveles medianos de PBB en suero y tejido adiposo eran mayores
entre los trabajadores de la industria química. No se dispone de
información de otros países o compañías sobre la exposición
profesional asociada a la fabricación, formulación y usos
comerciales de esos productos.
Respecto a la mayoría de las poblaciones humanas, no se han
notificado datos directos sobre la exposición a PBB a partir de
diversas fuentes. En relación con el caso de Michigan (Estados
Unidos) se ha notificado la exposición humana masiva resultante del
contacto directo con pienso contaminado y, sobre todo, del consumo
de carne, huevos y productos lácteos que contenían PBB. Al menos
2000 familias (principalmente agricultores y sus vecinos) se vieron
expuestas a muy altos niveles. Recientemente se han detectado PBB en
muestras de leche de vaca y leche humana en Alemania.
El perfil de los PBB de estas muestras difiere del hallado en
el pescado. Así, la concentración relativa de BB 153 es mayor en la
leche humana que en el pescado.
Las vías de exposición de la población general a los PBB no se
conocen con precisión. A tenor de los conocimientos actuales, el
aire y el agua ambientales no contienen niveles elevados. Los
alimentos ricos en lípidos, sobre todo los procedentes de aguas
contaminadas, son probablemente muy importantes a ese respecto. No
se dispone de información sobre los niveles de exposición en el aire
de espacios interiores ni sobre la exposición cutánea a materiales
con PBB pirorretardantes.
El perfil de los PBB detectados en la leche humana analizada en
Alemania era parecido al hallado en la leche de vaca de la misma
región, pero los niveles detectados en las muestras humanas eran
considerablemente mayores.
Son muy pocos los datos disponibles para fundamentar el cálculo
de la ingesta diaria de PBB a través de los alimentos por parte de
la población general. Si suponemos que el pescado contiene 20 µg
PBB/kg de lípido y un 5% de lípidos y que una persona de 60 kg
consume 100 g de pescado al día, la ingesta resultante es de 0,002
µg/kg de peso corporal al día. Para esa misma persona, una
concentración de PBB de 0,05 µg/kg de lípido en la leche (4% de
lípidos) y un consumo de leche de 500 ml/día determinarían una
ingesta de PBB de aproximadamente 0,00002 µg/kg de peso corporal al
día.
Si suponemos que la leche materna contiene 2 µg PBB/kg de
lípido, un lactante de 6 kg que consuma 800 ml de esa leche (3,5% de
lípidos) al día ingerirá 0,01 µg PBB/kg de peso corporal al día.
1.5 Cinética y metabolismo
La absorción gastrointestinal de los PBB varía según el grado
de bromación; así, los compuestos menos bromados se absorben más
fácilmente.
La información disponible sobre la absorción de DeBB y
OcBB/NoBB es insuficiente.
Los PBB están distribuidos en todas las especies animales y en
el hombre y alcanzan su mayor concentración de equilibrio en el
tejido adiposo. También se han hallado niveles relativamente altos
en el hígado, sobre todo de los PBB más tóxicos, que al
parecertienden a concentrarse en ese órgano. Los coeficientes de
reparto de los diversos PBB entre varios tejidos parecen diferir.
Por lo general se observa una marcada tendencia a la bioacumulación.
En los mamíferos la transferencia de PBB a la descendencia se
produce a través de la placenta y de la leche. Se observó que la
leche humana contenía niveles de 2,2',4,4',5,5'-hexabromobifenilo
más de 100 veces superiores a los niveles séricos maternos. En un
estudio realizado sobre varias generaciones de ratas, tras
administrar PBB a una de las generaciones se observaron residuos
detectables en más de dos generaciones sucesivas. Los huevos de
especies aviares también se vieron afectados por el contenido
corporal materno de PBB.
Muchos PBB tienden a persistir en los sistemas biológicos. No
se obtuvieron indicios de un metabolismo o excreción importantes de
los componentes más abundantes en la mezcla FireMaster(R) ni del
octa- o decabromobifenilo. Los estudios metabólicos in vitro
mostraron que hay relaciones estructura-actividad que explican el
metabolismo de los PBB. Los microsomas inducidos por FB
(fenobarbital) sólo metabolizaban los PBB que poseían carbonos
adyacentes no bromados, meta y para respecto al puente bifenilo en
al menos uno de los anillos. La metabolización por microsomas
inducidos por MC (3-metilcolantreno) estaba condicionada por la
presencia de posiciones adyacentes orto y meta no bromadas en al
menos un anillo del PBB, que debía tener pocos sustituyentes, ya que
una mayor bromación parecía impedir el metabolismo. Se ha observado
que, en los vertebrados, los derivados hidroxilados son unos de los
principales productos del metabolismo, in vitro e
in vivo, de los bifenilos menos bromados; la intensidad de la
transformación metabólica fue relativamente baja. La hidroxilación
se produce probablemente tanto mediante la generación previa de
óxidos de hidrocarburos aromáticos como de forma directa.
El hombre, la rata, el rhesus, el cerdo, la vaca y la gallina
eliminan los PBB fundamentalmente por las heces. En la mayoría de
los casos la velocidad de excreción parece ser baja. Las
concentraciones de 2,2',4,4',5,5'-hexabromobifenilo observadas en la
bilis y las heces humanas equivalían aproximadamente a entre 1/2 y
7/10 de los niveles séricos y a aproximadamente el 0,5% de los
niveles observados en el tejido adiposo. Los tratamientos aplicados
para facilitar la eliminación de los PBB por los animales o el
hombre fueron de escasa o nula eficacia. Otra forma de eliminación
es la excreción a través de la leche.
Tras la administración de PBB a ratas y otros animales, las
concentraciones tisulares del producto evolucionaron con el tiempo
de forma compleja y diversa. Esa evolución se ha descrito mediante
modelos de varios compartimentos. Se calculó una semivida de
aproximadamente 69 semanas para la eliminación del
2,2',4,4',5,5'-hexabromobifenilo de la grasa corporal de la rata. En
el rhesus se observó una semivida de más de cuatro años. En el
hombre se calcula que la semivida de ese mismo compuesto oscila como
promedio entre 8 y 12 años; pero, según lo publicado, ese margen
podría estar comprendido entre 5 y 95 años. Hay algunas diferencias
de retención y de recambio entre los distintos PBB. Los resultados
de los análisis del suero de agricultores y trabajadores de la
industria química por lo que se refiere al
2,3',4,4',5-pentabromobifenilo fueron incongruentes, probablemente
porque las fuentes de exposición eran distintas. Los trabajadores de
la industria estaban expuestos a todos los componentes de la mezcla
FireMaster(R), mientras que la población de Michigan estuvo
expuesta a carne y leche contaminadas por otra combinación de PBB,
debido a los cambios metabólicos sufridos por los compuestos en los
animales de trabajo. En un estudio realizado en ratas, los niveles
de bromo del tejido adiposo no disminuyeron cuando se administró
octabromobifenilo de calidad técnica. No se dispone de información
sobre la retención del decabromobifenilo.
El hombre presenta quizá una mayor tendencia a retener
determinados PBB que la observada en los animales de
experimentación. Este factor debería tenerse en cuenta a la hora de
evaluar los riesgos que entrañan esos productos químicos para la
salud humana.
En resumen, todos los datos disponibles indican que los PBB
presentan una marcada tendencia a la bioacumulación y la
persistencia. Se metabolizan lentamente, y sus semividas en el
hombre son del orden de al menos 8 a 12 años.
1.6 Efectos en los seres vivos del medio ambiente
Los pocos datos de que se dispone sobre los efectos de los PBB
en los seres vivos del medio ambiente se refieren a micro
organismos, pulgas de agua, aves acuáticas y animales de trabajo.
Se realizó un estudio sobre las aves acuáticas que anidaban en
las islas del noroeste del lago Michigan para averiguar si los
contaminantes del medio tenían alguna influencia en su reproduc
ción. Se determinaron los niveles de 17 contaminantes, incluidos
diversos PBB, pero al parecer ninguno tenía efectos pronunciados
sobre la reproducción.
El ganado de labor que ingirió el pienso que por error contenía
FireMaster(R) FF-1 en lugar de óxido de magnesio enfermó. El valor
promedio estimado de la exposición sufrida por las vacas de la
primera explotación en que se observó una alta contaminación fue de
250 mg/kg de peso corporal. Los signos clínicos de toxicidad
consistieron en una reducción del 50% del consumo de pienso
(anorexia) y una disminución del 40% de la producción de leche,
manifestaciones observadas algunas semanas después de la ingestión
del pienso contaminado. Aunque la administración del pienso
suplementado se interrrumpió a los 16 días, la producción de leche
no se reanudó. Algunas de las vacas presentaron una mayor frecuencia
miccional y lacrimación y desarrollaron hematomas, abscesos,
crecimiento anormal de las pezuñas, cojera, alopecia,
hiperqueratosis y caquexia; varias murieron menos de seis meses
después de la exposición. Globalmente, la tasa de mortalidad en esa
explotación fue de 24/400. Sin embargo, entre los terneros de 6 a 18
meses la tasa de mortalidad fue mucho más alta: aproximadamente un
50% murieron al cabo de menos de seis semanas, y sólo dos de 12
sobrevivieron más de cinco meses. Los animales desarrollaron
hiperqueratosis por todo el cuerpo. Se observaron también diversos
problemas relacionados con la reproducción.
Se han notificado los resultados de las autopsias de algunas de
las vacas adultas que murieron durante los primeros seis meses tras
la exposición. Los estudios histopatológicos revelaron alteraciones
de diverso tipo del hígado y los riñones.
Varios de los signos clínicos y cambios patológicos señalados
anteriormente fueron confirmados más tarde mediante estudios
controlados de administración de pienso (anorexia, deshidratación,
lacrimación excesiva, emaciación, hiperqueratosis, problemas de
reproducción, ciertos cambios de los parámetros químicos clínicos y
lesiones renales).
Se notificó una caída de la producción y la aparición de
esterilidad en las manadas afectadas por un bajo nivel de
contaminación. Esto contrasta con los resultados de unos estudios
controlados en los que no se halló ninguna diferencia significativa
entre los rebaños sometidos a una contaminación baja y los rebaños
testigo.
Aunque el error que provocó el accidente afectó originalmente
al pienso del ganado vacuno, el pienso de otros animales también se
vio afectado por la contaminación cruzada que se produjo, por
ejemplo, en los mezcladores de las compañías productoras del pienso.
Probablemente la exposición no llegó a ser tan alta como la del
ganado vacuno. Se notificó también la contaminación de otros
animales que fueron igualmente sacrificados (aves de corral, cerdos,
caballos, conejos, cabras y ovejas), pero sin concretar las
manifestaciones de la enfermedad.
No se dispone de información sobre los efectos de los PBB en el
ecosistema.
1.7 Efectos en los animales de experimentación y en los sistemas
de prueba in vitro
Las DL50 de las mezclas comerciales administradas por vía
oral o cutánea muestran un nivel relativamente bajo de toxicidad
aguda (DL50 > 1 g/kg de peso corporal) en la rata, el conejo y la
codorniz. En estos casos la muerte y las manifestaciones agudas de
toxicidad aparecieron con mayor retraso tras la administración de
PBB. La dosis total administrada determinó el grado de toxicidad, ya
se tratase de una dosis única, ya de dosis repetidas durante breves
periodos (hasta 50 días). La toxicidad de los PBB fue mayor cuando
se administraron varias dosis que cuando se administró una sola
dosis. Se observa un efecto dilatorio sobre la mortalidad tras la
exposición a los PBB.
Los escasos estudios realizados con mezclas comerciales de
octa- y decabromobifenilo no revelaron ninguna influencia en la
mortalidad de ratas y peces. Por lo que se refiere al análisis
individual de los PBB, sólo se han analizado tres hexaisómeros:
3,3',4,4',5,5'-HxBB, 2,2',4,4',5,5'-HxBB y 2,3',4,4',5,5'-HxBB, el
último de los cuales es más tóxico para la rata que el anterior. A
juzgar por los datos limitados de que se dispone, el OcBB y el DeBB
parecen menos tóxicos que las mezclas de PBB y son peor absorbidos.
En numerosos estudios sobre los efectos agudos y a corto plazo,
entre los signos de toxicidad por PBB (sobre todo por FireMaster) se
ha observado una disminución del consumo de pienso. A dosis letales,
la muerte no se puede atribuir a la alteración patológica de un
determinado órgano sino más bien a un «síndrome de emaciación» que
desarrollan los animales como primera manifes tación de toxicidad.
En el momento de la muerte la pérdida de peso puede ser de hasta un
30-40%. Los pocos estudios realizados con OcBB y DeBB de calidad
técnica no revelaron ningún efecto de ese tipo.
Los cambios morfológicos e histopatológicos causados por la
exposición a los PBB afectan sobre todo al hígado. El aumento de
tamaño de este órgano se produce con frecuencia a dosis inferiores a
las requeridas para inducir cambios en el peso corporal. En las
especies roedoras las alteraciones histopatológicas consisten
principalmente en la hinchazón y vacuolación masivas de los
hepatocitos, la proliferación del retículo endoplasmático liso y una
necrosis de células aisladas. La gravedad de las lesiones depende de
la dosis y del tipo de PBB administrados.
Se observó una disminución del peso del timo en la rata, el
ratón y el ganado vacuno tras la exposición a FireMaster(R), pero
no así a OcBB o DeBB.
En algunas publicaciones se menciona un aumento del peso de la
glándula tiroides y cambios histológicos en el tiroides de la rata,
efectos observados a bajas concentraciones.
Está demostrado que los distintos PBB difieren en cuanto al
perfil de toxicidad. Los PBB más tóxicos provocan una disminución
del peso del timo y/o del organismo y causan cambios histológicos
pronunciados en el hígado y el timo. Se ha propuesto una
clasificación de los bifenilos halogenados basada en criterios
estructurales. La clase 1 abarca los productos de la familia (con
sus distintos isómeros) que carecen de sustituyentes en posición
orto (PBB coplanares). La segunda clase abarca los monoderivados con
sustituyente en posición orto. Los otros PBB (principalmente los que
poseen dos o más orto-bromos) pertenecen a la tercera clase. Los PBB
de la clase 1 son los que suelen tener efectos más graves, mientras
que los productos de la segunda y la tercera clases presentan una
toxicidad decreciente. Dentro de cada clase la toxicidad depende
también del grado de bromación.
En todas las combinaciones analizadas, el PBB más tóxico
resultó ser el 3,3',4,4',5,5'-HxBB. Este compuesto está presente a
baja concentración en la mezcla FireMaster(R). De los principales
componentes de ésta, el más tóxico fue el 2,3,3',4,4',5-HxBB,
seguido del 2,3',4,4',5,5'-HxBB y el 2,3',4,4',5-PeBB. El principal
componente de la mezcla FireMaster, el 2,2',4,4',5,5'-HxBB, era
relativamente atóxico, al igual que el 2,2',3,4,4',5,5'-HpBB,
segundo componente más abundante.
La influencia del contenido de los diversos PBB (y de otros
posibles contaminantes) sobre la toxicidad de las mezclas de calidad
técnica de OcBB y DeBB no se conoce con tanto detalle.
Las pruebas habituales de irritación cutánea y ocular y de
sensibilización efectuadas con las mezclas PBB de calidad técnica
analizadas (OcBB y DeBB) fueron negativas, o a lo sumo revelaron una
reacción moderada. No obstante, se observaron hiperqueratosis y
pérdida de pelaje en el ganado vacuno, y lesiones parecidas al
cloracne en el rhesus, provocadas en los animales por la ingestión
de FireMaster(R). Esta mezcla provocó hiperqueratosis en la
superficie interna de la oreja del conejo, cosa que no ocurrió con
sus principales componentes (2,2',4,4',5,5'-HxBB y
2,2',3,4,4',5,5'-HpBB). El fraccionamiento de la mezcla
FireMaster(R) mostró que la mayor parte de la actividad
correspondía a las fracciones más polares, que contenían componentes
minoritarios. La aplicación de HxBB irradiado con luz solar provocó
una grave hiperqueratosis en la oreja del conejo.
En trabajos realizados en la rata, la administración prolongada
de dosis bajas de OcBB de calidad técnica no influyó ni en el
consumo de pienso ni en el peso corporal, pero se observó un aumento
del peso relativo del hígado de las ratas expuestas a dosis de 2,5
mg/kg de peso corporal durante 7 meses. La administración prolongada
de FireMaster(R) a ratas a dosis de 10 mg/kg de peso corporal
durante 6 meses no influyó en el consumo de alimento. La
administración durante 6 meses de dosis de 1 mg/kg de peso corporal
afectó al peso del hígado. El peso del timo disminuyó en las ratas
hembras a las que se administraron 0,3 mg/kg de peso corporal. Se
observaron también cambios histopatológicos. Los estudios
prolongados y controlados de administración de pienso a ganado
vacuno expuesto a dosis bajas de FireMaster(R) no pusieron de
manifiesto ningún efecto adverso por lo que se refiere a ingesta de
alimentos, signos clínicos, cambios clinicopatológicos o desarrollo
físico. El visón, el cobayo y el mono parecen más susceptibles a la
toxicidad por PBB.
Se han estudiado los efectos a largo plazo provocados en la
rata por los PBB retenidos tras la exposición pre- o perinatal a
dosis altas de FireMaster(R).
Los efectos adversos más frecuentes sobre la reproducción
fueron los embarazos malogrados o perdidos y la disminución de la
viabilidad de la descendencia. Se observaron aún ciertos efectos en
el visón a concentraciones de 1 mg/kg de alimento. Se observó
también una disminución de la viabilidad de la descendencia de
rhesus expuestos durante 12,5 meses a FireMaster(R) (0,3 mg/kg de
alimento). Los monos recibieron una dosis diaria de 0,01 mg/kg de
peso corporal y una dosis total de 3,8 mg/kg de peso corporal. Los
resultados de los estudios sobre reproducción y neurología del
comportamiento realizados con monos y ratas expuestos a dosis bajas
no pudieron ser evaluados debido a que la información aportada en
los artículos publicados acerca del diseño de los experimentos era
insuficiente. Se observó un débil efecto teratógeno en experimentos
realizados con roedores, a dosis elevadas que podrían haber causado
una cierta toxicidad en la madre.
Los PBB interaccionan con el sistema endocrino. En la rata y el
cerdo se observaron disminuciones dosis-dependientes de la tiroxina
y la triyodotironina séricas. También se ha notificado que los PBB
alteran los niveles de las hormonas esteroides en la mayoría de los
casos. La intensidad del efecto depende de la especie, así como de
la dosis y la duración del tratamiento.
Los PBB provocan porfiria en la rata y en el ratón macho a
dosis de sólo 0,3 mg/kg de peso corporal al día. El nivel sin efecto
fue de 0,1 mg/kg de peso corporal al día. Se observó una pronunciada
influencia de los PBB sobre la acumulación de vitamina A, así como
efectos sobre el metabolismo intermediario.
Una observación frecuente tras la exposición a PBB fue la
atrofia del timo, y se han observado también efectos en otros
tejidos linfoides. Se ha demostrado asimismo la existencia de otros
signos de depresión de la función inmunitaria en respuesta a la
mezcla FireMaster(R). No hay datos disponibles sobre los OcBB,
NoBB y DeBB ni sobre PBB particulares.
Uno de los efectos más estudiados de los PBB es la inducción
que provocan de las enzimas de actividad oxidasa de función mixta
(MFO). Como era de esperar, se descubrió que FireMaster(R) era un
inductor de tipo mixto de las enzimas microsómicas hepáticas tanto
en la rata como en todas las demás especies animales estudiadas.
Este fenómeno de inducción se observó también en menor medida en
otros tejidos. La capacidad de inducción de las enzimas microsómicas
hepáticas variaba de un PBB a otro; no obstante, se han descubierto
correlaciones entre su estructura y la actividad de inducción de las
enzimas microsómicas.
Varios estudios han puesto de manifesto que los PBB pueden
modificar la actividad biológica de diversos fármacos y sustancias
tóxicas. Esto se debe quizá en parte a la capacidad de los PBB para
inducir las enzimas microsómicas implicadas en la activación o
desactivación de los productos xenobióticos.
Se observó que FireMaster(R) y algunos de sus principales
componentes podían inhibir la comunicación intercelular in vitro;
esta inhibición se produce a concentraciones no citotóxicas. Tanto
la citotoxicidad como las propiedades de inhibición de la
cooperación metabólica parecen guardar relación con la estructura de
los PBB, concretamente con la presencia o ausencia de sustituyentes
en posición orto.
Los ensayos realizados in vitro e in vivo (mutagénesis de
células microbianas y de mamífero, lesiones cromosómicas de células
de mamífero, transformación de células de mamífero, lesión y
reparación del ADN) no han revelado ningún tipo de mutagenicidad o
genotoxicidad causadas por PBB particulares o por mezclas
comerciales de los mismos.
Los estudios de toxicidad prolongados han revelado que el
principal órgano de manifestación de los efectos carcinógenos de los
PBB es el hígado. La incidencia de carcinoma hepatocelular aumentó
significativamente en las ratas y los ratones machos y hembras a los
que se administró la mezcla FireMaster(R) por vía oral. Se han
notificado efectos carcinógenos en el hígado de ratones sometidos a
dietas que contenían Bromkal 80-9D (nonabromobifenilo de calidad
técnica) a dosis de 100 mg/kg (5 mg/kg de peso corporal al día) o
más durante 18 meses. La dosis más baja de PBB que produjo tumores
(fundamentalmente adenomas) en los roedores fue de 0,5 mg/kg de peso
corporal al día durante 2 años. Las ratas que recibieron 0,15 mg/kg
de peso corporal al día además de la exposición pre- y perinatal no
sufrieron ningún efecto adverso. No se ha estudiado la
carcinogenicidad del octabromobifenilo y el decabromobifenilo de
calidad técnica.
En un bioensayo realizado sobre la piel del ratón no se observó
ninguna actividad de iniciación tumoral (usando
12-O-tetradecanoilforbol-13-acetato (TPA) como agente activador) o
activación tumoral (usando 7,12-dimetil-benz(a)antraceno (DMBA) como
iniciador) por parte de FireMaster BP-6 o del 2,2',4,4',5,5'-
hexabromobifenilo. Sin embargo, en otros modelos basados en la piel
del ratón (en los que se empleó DMBA o DN-metil- N'-nitro-
N-nitroso-guamidina (MNNG) como iniciadores), FM FF-1 y el
3,3',4,4',5,5'-hexabromobifenilo, pero no así el 2,2',4,4',5,5'-
hexabromobifenilo, tuvieron efecto como activadores tumorales. En un
bioensayo de dos fases realizado con hígado de rata usando
fenobarbital como activador, el 3,3',4,4'-tetrabromobifenilo mostró
una débil actividad iniciadora. En el modelo de dos fases utilizado
con el hígado de rata, con empleo de dietilnitrosamina y
hepatectomía parcial, la mezcla FM, el 3,3',4,4'-tetrabromobifenilo
y el 2,2',4,4',5,5'-hexabromobifenilo, pero no así el
3,3',4,4',5,5'-hexabromobifenilo, tuvieron efecto como activadores
tumorales.
Los resultados de los estudios sobre la comunicación celular,
los resultados negativos de los estudios de genotoxicidad y
mutagenicidad y los resultados de los ensayos de activación tumoral
indican que las mezclas y los miembros de la familia estudiados
provocan cáncer por mecanismos epigenéticos. No se dispone de
información sobre los octa-, nona-, o decabromo bifenilos de calidad
técnica.
Se desconocen los mecanismos de acción subyacentes a las
numerosas manifestaciones de toxicidad de los PBB y de otros
compuestos relacionados. No obstante, algunos de los efectos, como
el síndrome de emaciación, la atrofia del timo, la hepatotoxicidad,
los trastornos cutáneos y la toxicidad sobre el sistema reproductor
podrían guardar relación con la interacción con el llamado receptor
Ah- o TCDD, que alteraría la expresión de una serie de genes. Los
distintos PBB difieren en lo que respecta a esa interacción con el
receptor, y en ese sentido los coplanares son más activos.
Muchos de los efectos de los PBB se observan sólo después de
una exposición prolongada. La razón podría ser la marcada
acumulación de algunos de ellos y la escasa capacidad del organismo
para metabolizarlos y eliminarlos. Ello determina la progresiva
concentración del producto en el organismo; los mecanismos de
compensación se ven desbordados y aparecen los efectos adversos.
Algunos naftalenos polibromados (PBN), que se sabe son
contaminantes de FireMaster(R), tienen potentes efectos tóxicos y
teratógenos. Las concentraciones de PBN presentes en esa mezcla son
bajas, pero es posible que contribuyan a su toxicidad.
Los estudios realizados sobre la mezcla FireMaster(R) y su
principal componente, el 2,2',4,4',5,5'-HxBB, mostraron que los
productos de fotólisis eran más tóxicos que el PBB original. Los
productos de la pirólisis de FM causaron la inducción del sistema
enzimático MFO, pérdida de peso corporal y atrofia tímica. Los
productos de pirólisis del OcBB de calidad técnica provocaron un
aumento del tamaño del hígado.
1.8 Efectos en el hombre
No había ningún ejemplo de toxicosis aguda por PBB en el hombre
con el que poder comparar los efectos potenciales de las bajas
exposiciones que siguieron a la intoxicación accidental acaecida en
Michigan (Estados Unidos) en 1973. Los principales estudios
epidemiológicos fueron realizados por el Departamento de Salud
Pública de Michigan (MDPH) y el Laboratorio de Ciencias del Medio
Ambiente (ESL) de la Facultad de Medicina Mount Sinai de Nueva York.
Se calculó que las personas más expuestas habían consumido
entre 5 y 15 g de PBB durante un periodo de 230 días a través de la
leche. Podría haberse producido una cierta exposición adicional a
través de la carne. La exposición de algunos de los agricultores y
de la mayoría de la población general de Michigan fue mucho menor:
9-10 mg de exposición total. No obstante, algunas de estas últimas
personas podrían haber recibido una dosis total de aproximadamente
800-900 mg. (Una dosis total de 9 mg corresponde a 0,15 mg/kg de
peso corporal, y 900 mg, a 15 mg/kg de peso corporal, cifras
referidas a un adulto de 60 kg de peso como valor promedio; la
dosis/kg de peso corporal sería mayor en los niños).
En 1974, en el primer estudio llevado a cabo por el MDPH se
procedió a comparar el estado de salud de las personas de las
granjas sometidas a cuarentena con el de las personas de las
explotaciones no sometidas a cuarentena de la misma zona. Los dos
grupos declararon diversos síntomas, pero no se observó ninguna
diferencia entre ambos. No se detectaron alteraciones inhabituales
del corazón, hígado, bazo, sistema nervioso, orina, sangre o
cualquiera de los otros parámetros médicos examinados. En un estudio
completo llevado a cabo más adelante por el MDPH, que abarcaba
grupos sometidos a distintos niveles de exposición, no se observó
relación alguna entre las concentraciones séricas de PBB y la
incidencia de los síntomas o enfermedades notificados. En los
estudios del ESL se examinó aproximadamente a 990 residentes de las
explotaciones agrícolas, a 55 trabajadores de la industria química y
a un grupo de trabajadores de la industria láctea que fueron
utilizados como testigos. La incidencia de síntomas entre los
agricultores de Michigan fue mayor que la hallada entre los
agricultores de Wisconsin. Las mayores diferencias fueron las
observadas dentro del amplio apartado de síntomas neurológicos y
musculoesqueléticos. En los agricultores de Michigan se halló una
mayor prevalencia de aumentos de las concentraciones séricas de
algunos antígenos carcinoembrionarios y enzimas hepáticas que en los
agricultores de Wisconsin. Los trabajadores de la industria química
presentaron una mayor prevalencia de síntomas torácicos y cutáneos y
una menor prevalencia de síntomas musculoesqueléticos que los
agricultores. Aunque la interpretación de los resultados de los
estudios del ESL divergió en ocasiones de la de otros resultados de
estudios comparables, hubo un aspecto en que los datos coincidieron:
ninguno de los estudios puso de manifiesto una relación dosis-
respuesta positiva entre los niveles de PBB en el suero o el tejido
adiposo y la prevalencia de síntomas o trastornos clínicos. Se
investigaron varios aspectos clínicos mediante estudios especiales
más detallados. En uno de ellos, el examen del sistema neurológoco
mediante pruebas funcionales objetivas reveló una correlación
negativa entre los niveles séricos de PBB y los resultados de las
pruebas funcionales, sobre todo entre los varones de los grupos de
edad avanzada. Los otros estudios no mostraron ninguna relación
entre las concentraciones de PBB en el suero o la grasa y la
actividad funcional determinada mediante una batería de pruebas en
las que se analizaron la memoria, fuerza motora, coordinación,
percepción corticosensorial, personalidad, funciones cognitivas
superiores y otro tipo de funciones. Se examinaron los aspectos
pediátricos de la exposición a PBB en algunas de las familias
estudiadas por el ESL. Se notificaron numerosos síntomas, pero la
exploración física no reveló ningún trastorno objetivo que pudiera
atribuirse a los PBB. Hubo opiniones discrepantes acerca de los
efectos neuropsicológicos en la descendencia, más sutiles, y los
resultados de las investigaciones sobre los signos de desarrollo de
las capacidades siguen siendo objeto de polémica. Lo mismo ocurre
con la investigación sobre los linfocitos y la función inmunitaria.
Algunos autores no hallaron ninguna diferencia numérica o funcional
entre los linfocitos de los grupos que presentaban niveles altos y
bajos de PBB en el suero, mientras que otros autores detectaron una
disminución significativa de las subpoblaciones de linfocitos T y B
en aproximadamente un 40% del grupo expuesto de Michigan en
comparación con los grupos no expuestos, así como trastornos de la
función linfocitaria, concretamente una menor respuesta a los
mitógenos.
El análisis de los estudios epidemiológicos realizados muestra
que se ha procurado evaluar la relación entre la exposición a PBB y
un elevado número de efectos adversos, incluidos efectos sobre la
conducta y trastornos subjetivos. No obstante, la mayoría de esos
estudios adolecen de fallos graves de diseño pues introducen
imprecisiones que hacen difícil, por no decir imposible, extraer
conclusiones acerca de la relación entre la exposición a PBB y los
posibles efectos sobre la salud. Además, el seguimiento no se ha
prolongado lo necesario para poder evaluar los posibles efectos
carcinógenos.
Se identificó a dos pequeños grupos de trabajadores que habían
sufrido exposición ocupacional a una mezcla de PBB o a DeBB y
decabromobifenilóxido. Se observaron lesiones parecidas al cloracne
en el 13% de los trabajadores expuestos a la mezcla de PBB, lesiones
ausentes en cambio en los trabajadores expuestos al DeBB. No
obstante, en este último grupo se observó una prevalencia
significativamente mayor de hipotiroidismo.
1.9 Evaluación global de la toxicidad y carcinogenicidad
El único estudio realizado con una mezcla de PBB y prolongado
durante todo el ciclo de vida fue el bioensayo llevado a cabo hace
poco con ratas y ratones en el marco del Programa Nacional de
Toxicología (NTP) de los Estados Unidos. La dosis más baja con
efectos carcinógenos fue de 0,5 mg/kg de peso corporal al día
(tumores hepáticos en roedores). En otros estudios realizados al
efecto se observó una respuesta carcinógena con 3 mg/kg de peso
corporal al día administrados durante 6 meses. El estudio prolongado
durante 6 meses demuestra que una exposición a dosis análogas
limitada a parte del ciclo de vida tiene también efectos adversos
similares. A dosis inferiores se observan efectos sobre el sistema
reproductor en los primates y el visón.
Además, en el estudio llevado a cabo durante 2 años por el NTP
con ratas, una dosis diaria de 0,15 mg/kg de peso corporal al día y
la exposición prenatal y perinatal de la madre a 0,05 mg/kg de peso
corporal al día no provocó ningún efecto adverso. Así pues, la
ingesta diaria total a partir de los alimentos, el agua, el aire y
el suelo debería ser inferior a 0,15 µg/kg de peso corporal al día,
cifra extrapolada del NOAEL (nivel sin efectos adversos observados)
observado en un estudio de carcinogenicidad que arrojó resultados
positivos, usando un factor de incertidumbre (seguridad) de 1000,
dado que estos compuestos probablemente producen cáncer por un
mecanismo epigenético.
Se calculó que la dosis total recibida por la subpoblación de
Michigan había sido de entre 0,15 y 15 mg/kg de peso corporal
durante un periodo de 230 días. Para esa población, la división de
esas dosis por el valor promedio de la duración de la vida del ser
humano arrojaría una cifra equivalente a una dosis diaria de entre 6
ng y 0,6 µg/kg de peso corporal al día.
Se ha calculado una ingesta total de 2 ng PBB/kg de peso
corporal al día, de fuentes conocidas, para los adultos de la
población general, y de 10 ng/kg de peso corporal al día para los
lactantes alimentados con leche materna. No debe olvidarse que estos
cálculos están basados en datos de carácter muy limitado y regional.
Estos cálculos se basan en el supuesto de que durante el ciclo
de vida los PBB no alcanzan un estado estacionario, y de que una
exposición alta y breve equivale a una exposición baja y prolongada,
toda vez que estos compuestos son metabolizados y excretados con
suma lentitud.
La información disponible sobre los OcBB, NoBB, y DeBB no es
suficiente para poder calcular una ingesta diaria total carente de
efectos adversos.
2. Conclusiones
La mayoría de los PBB presentes en los pirorretardantes
comerciales son lipofílicos, persistentes y bioacumulables. Estos
compuestos tienden a concentrarse en las tramas alimentarias del
medio y suponen una amenaza, especialmente para los organismos que
ocupan los niveles superiores de esas tramas. Además, en caso de
combustión, algunos PBB generan dibenzofuranos polibromados tóxicos.
Aparte de las emisiones que se producen durante su fabricación
y empleo, los PBB pasan al medio como consecuencia del uso
generalizado de pirorretardantes. Debido a la gran estabilidad de
los PBB, una parte considerable de ellos alcanza en un momento u
otro el medio.
También se detectan PBB en muestras ambientales o humanas de
lugares alejados de los focos conocidos. El perfil de PBB de las
muestras del medio no se corresponde con el hallado en los productos
de uso industrial, lo que indica que éstos sufren transformaciones
en el medio ambiente, posiblemente como resultado de una debromación
fotoquímica.
Se dispone actualmente de muy poca información sobre el grado
de exposición de la población general a los PBB. No obstante, en los
pocos casos en que se determinaron sus niveles se descubrieron
cantidades ínfimas. En la actualidad, ese nivel de exposición no
resulta preocupante, pero deberá evitarse que prosiga la
acumulación. Los datos sobre las personas afectadas en Michigan
indican que en este caso las exposiciones fueron varios órdenes de
magnitud superiores a la de la población general. No se han
observado efectos concluyentes sobre la salud atribuibles a la
exposición a PBB en la población de Michigan, pero el periodo de
seguimiento no ha sido lo suficientemente dilatado para descartar la
aparición de cáncer. Los niveles de PBB en el tejido adiposo y el
suero de la población de Michigan siguen siendo altos, por lo que la
exposición interna continúa. Por el contrario, sí se observó la
aparición de toxicidad en el ganado vacuno de Michigan. Esta
discrepancia se explica por el diferente grado de exposición del
ganado.
Sólo se han estudiado casos de exposición profesional en dos
fábricas de los Estados Unidos. Parece que los trabajadores que
producen PBB pueden desarrollar lesiones parecidas al cloracne, y
los expuestos a DeBB, hipotiroidismo. No se han realizado estudios
sobre los trabajadores que incorporan deca- u octa-/nona-
bromobifenilo en productos comerciales.
Los PBB persisten durante mucho tiempo en los organismos vivos,
y se ha demostrado que producen toxicidad crónica y cáncer en los
animales. Así, se indujo cáncer a una dosis de 0,5 mg/kg de peso
corporal al día, pese a la baja toxicidad aguda asociada a esa
dosis, y el nivel sin efectos observados fue de 0,15 mg/kg de peso
corporal al día. Se han observado diversos efectos tóxicos crónicos
en animales de experimentación tras la exposición prolongada a dosis
de aproximadamente 1 mg/kg de peso corporal al día.
3. Recomendaciones
3.1 Recomendaciones generales
El Grupo Especial de Trabajo considera que el hombre y el medio
ambiente no deben verse expuestos a los PBB, habida cuenta de su
elevada persistencia y bioacumulación y de los efectos adversos que
pueden aparecer tras la exposición prolongada a muy bajos niveles.
Por consiguiente, deberá interrumpirse todo uso comercial de los
PBB.
Respecto al DeBB y el OcBB, teniendo en cuenta los escasos
datos sobre su toxicidad, su extrema persistencia y su posible
degradación en el medio ambiente, así como la mayor toxicidad de los
compuestos persistentes generados durante la combustión, no deberán
ser empleados comercialmente, a menos que se demuestre su inocuidad.
Es sabido que se siguen formulando observaciones sobre la
cohorte de Michigan, información que convendría fuese publicada.
3.2 Futuras investigaciones
La futura vigilancia de los PBB en el hombre y en el medio,
incluida la vigilancia en el lugar de trabajo en las industrias de
fabricación o uso de esos productos, deberá ampliarse, deberá
centrarse en PBB específicos, y deberá incluir los OcBB/NoBB y DeBB.
Es preciso incorporar también estos compuestos en los programas en
curso de vigilancia de otros productos halogenados. La evolución
temporal y la distribución geográfica de los niveles de PBB en el
medio ambiente deberán seguir siendo objeto de vigilancia. Habrá que
controlar también la liberación de PBB en el medio a partir de los
lugares de evacuación de desechos.
Deberán efectuarse experimentos de termólisis mediante la
simulación de fuegos accidentales y la incineración municipal.
Conviene realizar nuevas investigaciones sobre los mecanismos de
toxicidad y carcinogenicidad de los PBB y de otros compuestos
relacionados. Los PBB podrían servir como modelo para el estudio de
esos mecanismos, y en las investigaciones al efecto deberán
emplearse PBB purificados.
Los efectos de los PBB sobre la reproducción no están bien
dilucidados. Por consiguiente, deberán realizarse estudios bien
diseñados y prolongados sobre los efectos de las dosis bajas en la
reproducción, usando para ello una especie vulnerable.
Es preciso disponer también de más información sobre la
biodisponibilidad y toxicocinética de los OcBB/NoBB y DeBB, así como
de determinados productos de la misma familia.
 Molecular (empirical) formulae for PBB components of different
degrees of substitution and their relative molecular masses are
given in Table 1.
Theoretically, there can be 209 different forms (congeners) of
a brominated biphenyl, depending on the number and position of the
bromine (see Table 2).
At present, 101 individual PBB congeners are listed in the
Chemical Abstracts Service (CAS) registry. Because bromobiphe nyls
are produced commercially by the bromination of biphenyl, the
existence of any of the 209 congeners is possible in any commercial
mixture (Aust et al., 1983). Some PBBs exist primarily as
metabolites or accumulation or degradation products of the original
mixture. With increasing advance in analysis techniques, the number
of actually identified PBB compounds is growing.
Table 1. PBBs: molecular formula and relative molecular mass
PBB Formula Relative
molecular mass
Monobromobiphenyl C12H9Br 232.9
Dibromobiphenyl C12H8Br2 311.8
Tribromobiphenyl C12H7Br3 390.7
Tetrabromobiphenyl C12H6Br4 469.6
Pentabromobiphenyl C12H5Br5 548.5
Hexabromobiphenyl C12H4Br6 627.4
Heptabromobiphenyl C12H3Br7 706.3
Octabromobiphenyl C12H2Br8 785.2
Nonabromobiphenyl C12HBr9 864.1
Decabromobiphenyl C12Br10 943.0
Table 2. Multiplicity of PBB isomers and congenersa
Number of
Br Substituent 1 2 3 4 5 6 7 8 9 10
Number of
Isomers 3 12 24 42 46 42 24 12 3 1
a Modified from: Safe (1984).
The synthesis of pure congeners for use as standards is a
prerequisite for advances in chemical analysis, as well as research
into the toxicological and biological effects of PBBs. Some routes
for the synthesis of PBB congeners have been described by Sundström
et al. (1976b), Robertson et al. (1980, 1982a, 1984a), Höfler et al.
(1988), and Kubiczak et al. (1989).
Table 3 gives a list of all 209 possible congeners and their
CAS numbers, if already designated. The CAS names are designated as
follows:
1,1'-Biphenyl, .......... bromo-
e.g., 1,1'-Biphenyl, 2,2',4,4',5,5'-hexabromo- or
2,2',4,4',5,5'-hexabromo-1,1'-biphenyl (BB-153).
2.1.2 Technical products
2.1.2.1 Major trade names
The PBBs produced for commercial use include mixtures mainly
containing hexa-, octa-/nona-, and decabromobiphenyls. Data on past
and present trade names and manufacturers are summarized in Table 4
(for further details see section 3.2.1).
2.1.2.2 Composition of the technical products
Commercial PBB products are mixtures of various brominated
biphenyls. Several structural isomers of each of these brominated
compounds are possible and may be present in the product. All
mixtures are relatively highly brominated, with bromine contents
ranging from about 76% for hexabromobiphenyls to 81-85% for octa- to
decabromobiphenyl mixtures (Brinkman & de Kok, 1980).
Data on the composition of PBB mixtures are given in Table 5.
As shown in Table 5, the analytical results concerning the various
products are rather divergent. It indicates that the exact
composition of the mixtures varies between batches, and also within
each batch according to the sampling and analytical method. It can
be seen that samples of "octabromobiphenyl" often contained a larger
proportion of nona- than of octa-substituted PBBs. In this
monograph, these compounds are also referred to as "octa/nona"
bromobiphenyls.
Information on the isomeric composition of the octa- to deca-
mixtures is scarce. In an analysis of Bromkal 80, three isomers of
octabromobiphenyl were found to be present at 14, 16, and 42%
(Norström et al., 1976). A comparison of the isomeric composition of
an "octabromobiphenyl"-mixture with the FireMaster(R)- mixture has
been given by Moore & Aust (1978). De Kok et al. (1977) analysed
various "octabromobiphenyl"-mixtures and Bromkal 80-9D and discussed
the structures of isomers. Furthermore, two isomeric octa- and
three hexa-bromobiphenyls of a commercial decabromobiphenyl mixture
(RFR) have been reported (de Kok et al., 1977).
Table 3. Systematic numbering of PBB compounds and their CAS numbers
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
Monobromobiphenyls (
Molecular (empirical) formulae for PBB components of different
degrees of substitution and their relative molecular masses are
given in Table 1.
Theoretically, there can be 209 different forms (congeners) of
a brominated biphenyl, depending on the number and position of the
bromine (see Table 2).
At present, 101 individual PBB congeners are listed in the
Chemical Abstracts Service (CAS) registry. Because bromobiphe nyls
are produced commercially by the bromination of biphenyl, the
existence of any of the 209 congeners is possible in any commercial
mixture (Aust et al., 1983). Some PBBs exist primarily as
metabolites or accumulation or degradation products of the original
mixture. With increasing advance in analysis techniques, the number
of actually identified PBB compounds is growing.
Table 1. PBBs: molecular formula and relative molecular mass
PBB Formula Relative
molecular mass
Monobromobiphenyl C12H9Br 232.9
Dibromobiphenyl C12H8Br2 311.8
Tribromobiphenyl C12H7Br3 390.7
Tetrabromobiphenyl C12H6Br4 469.6
Pentabromobiphenyl C12H5Br5 548.5
Hexabromobiphenyl C12H4Br6 627.4
Heptabromobiphenyl C12H3Br7 706.3
Octabromobiphenyl C12H2Br8 785.2
Nonabromobiphenyl C12HBr9 864.1
Decabromobiphenyl C12Br10 943.0
Table 2. Multiplicity of PBB isomers and congenersa
Number of
Br Substituent 1 2 3 4 5 6 7 8 9 10
Number of
Isomers 3 12 24 42 46 42 24 12 3 1
a Modified from: Safe (1984).
The synthesis of pure congeners for use as standards is a
prerequisite for advances in chemical analysis, as well as research
into the toxicological and biological effects of PBBs. Some routes
for the synthesis of PBB congeners have been described by Sundström
et al. (1976b), Robertson et al. (1980, 1982a, 1984a), Höfler et al.
(1988), and Kubiczak et al. (1989).
Table 3 gives a list of all 209 possible congeners and their
CAS numbers, if already designated. The CAS names are designated as
follows:
1,1'-Biphenyl, .......... bromo-
e.g., 1,1'-Biphenyl, 2,2',4,4',5,5'-hexabromo- or
2,2',4,4',5,5'-hexabromo-1,1'-biphenyl (BB-153).
2.1.2 Technical products
2.1.2.1 Major trade names
The PBBs produced for commercial use include mixtures mainly
containing hexa-, octa-/nona-, and decabromobiphenyls. Data on past
and present trade names and manufacturers are summarized in Table 4
(for further details see section 3.2.1).
2.1.2.2 Composition of the technical products
Commercial PBB products are mixtures of various brominated
biphenyls. Several structural isomers of each of these brominated
compounds are possible and may be present in the product. All
mixtures are relatively highly brominated, with bromine contents
ranging from about 76% for hexabromobiphenyls to 81-85% for octa- to
decabromobiphenyl mixtures (Brinkman & de Kok, 1980).
Data on the composition of PBB mixtures are given in Table 5.
As shown in Table 5, the analytical results concerning the various
products are rather divergent. It indicates that the exact
composition of the mixtures varies between batches, and also within
each batch according to the sampling and analytical method. It can
be seen that samples of "octabromobiphenyl" often contained a larger
proportion of nona- than of octa-substituted PBBs. In this
monograph, these compounds are also referred to as "octa/nona"
bromobiphenyls.
Information on the isomeric composition of the octa- to deca-
mixtures is scarce. In an analysis of Bromkal 80, three isomers of
octabromobiphenyl were found to be present at 14, 16, and 42%
(Norström et al., 1976). A comparison of the isomeric composition of
an "octabromobiphenyl"-mixture with the FireMaster(R)- mixture has
been given by Moore & Aust (1978). De Kok et al. (1977) analysed
various "octabromobiphenyl"-mixtures and Bromkal 80-9D and discussed
the structures of isomers. Furthermore, two isomeric octa- and
three hexa-bromobiphenyls of a commercial decabromobiphenyl mixture
(RFR) have been reported (de Kok et al., 1977).
Table 3. Systematic numbering of PBB compounds and their CAS numbers
BB-No.a Structure CAS No. BB-No.a Structure CAS No.
Monobromobiphenyls ( Polybrominated benzenes and a possible methylbrominated furan
have also been reported to occur in FireMaster(R) (Brinkman & de
Kok, 1980).
Approximately 20 compounds, other than PBBs, were either
tentatively identified in FireMaster(R) or partially characterized
by Hass et al. (1978).
Polybromodibenzo- p-dioxins and polybromodibenzofurans were
searched for, because of their extreme toxicity and because
chlorinated dibenzofurans had been detected in commercial PCBs
(Nagayama et al., 1976). If present, their concentrations did not
exceed 0.5 mg/kg (Hass et al., 1978, O'Keefe, 1979). Polybromo
dibenzodioxins and polybromodibenzofurans were determined in a
sample of Adine 0102 (decabromobiphenyl). Monobromobenzo difurans
were present at a level of 1 mg/kg (1 ppm), otherwise all other
polybromodibenzodioxins and polybromodibenzofurans were present only
at less than 0.01 mg/kg (Atochem, 1990).
So far, phenoxyphenols and hydroxybiphenyls, which might be
intermediates in the formation of brominated dibenzo- p-dioxins and
brominated dibenzofurans, respectively, have not been identified
(O'Keefe, 1979).
Some impurities in PBBs result from impurities in the original
biphenyl material. According to two major manufacturers, their
biphenyl grade used for bromination contained less than 5 mg/kg and
5000 mg/kg, respectively, of impurities, e.g., toluene, naphthalene,
methylene biphenyl (fluorene), and various methyl biphenyls (Neufeld
et al., 1977).
2.2 Physical and chemical properties
In general, PBBs show an unusual chemical stability and
resistance to breakdown by acids, bases, heat, and reducing and
oxidizing agents (Safe, 1984).
PBBs can be compared chemically to the PCBs. Bromine, however,
is a better leaving group in chemical reactions than chlorine.
Unlike PCBs, the reactivity of PBBs has not been well studied and
documented in the literature (Pomerantz et al., 1978). Like PCBs
their chemical stability is dependent, in part, on the degree of
bromination and the specific substitution patterns (Safe, 1984). All
highly brominated PBB-mixtures are known to degrade rather rapidly
with UV irradiation (Brinkman & de Kok, 1980).
The technical mixtures typically are white, off-white, or beige
powdered solids. Some physical data on commercial PBB mixtures are
given in Table 9. It can be seen that there are discrepancies in the
values for the solubility of commercial PBBs in water (given in
Table 9) as well as those calculated for various PBB congeners
(Table 10). The source and quality of the water is important.
Determinations of water solubility of these very hydrophobic
compounds are also difficult to perform. Adsorption effects on
particles and glass surfaces may influence the results. PBBs were
found to be 200 times more soluble in landfill leachate than in
distilled water (Griffin & Chou, 1981a). In general, it can be said
that PBBs are only slightly soluble in water and that the solubility
decreases with increasing bromination.
For details of thermal decomposition, see section 4.3.2.
2.2.1 Physical and chemical properties of individual congeners
PBBs show a wide range of volatility (Farrell, 1980). Partition
coefficients between water/ n-hexane and water/1-octanol, as well
as aqueous solubilities for some individual PBB congeners are given
in Table 10. Correlations for predicting aqueous solubility and
partition coefficients for PBBs based on molecular structure have
been proposed (Patil, 1991). The solubility of PBBs in n-hexane
decreases rapidly with increasing bromine content (de Kok et al.,
1977).
Data on the melting points and UV absorption of individual PBB
congeners are summarized in Table 11. The main band in these spectra
is caused by pi -> pi* electron transitions, while the k band is
generally attributed to the conjugated biphenyl system with the
contribution of both biphenyl rings. With the k band, the
introduction of bromine atoms in positions meta or para to the
phenyl-phenyl bond induces a shift in kmax towards the visible
region, as is illustrated by 3,3',5,5'-tetra- and 3,3',4,4',5,5'-
hexabromobiphenyl. On the other hand, ortho substitution, which
causes a considerable hindrance for free rotation of the rings and,
thus, a loss in coplanarity, effects a sharp decrease in the
extinction coefficient of the k band (de Kok et al., 1977).
Data on NMR spectra are given by Orti et al. (1983), Robertson
et al. (1984b), and Kubiczak et al. (1989), and on mass spectrometry
(MS) by Erickson et al. (1980), Roboz et al. (1980), Buser (1986),
and Sovocool et al. (1987a,b). The "ortho" effect, observed for PBBs
and PCBs having 2,2'-; 2,2',6- or 2,2',6,6'- halogens can be
combined with GC retention index for isomer specific identifications
by gas chromatography and mass spectrometry (GC/MS) (Sovocool
et al., 1987a).
Table 9. Some physical data on commercial PBB mixturesa
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Melting point (°C) 72 200-250 220-290 380-386b
360-380
385
Lambda max (nm) 219e 225e 224e 227e
Density (g/cm3) at 2.6 - 3.2 3.2
room temperature
Solubility in water 11f 20-30 < 30
(µg/litre) at 25 °C 30f (pure 2,2', 4,4', 5,5'-)
610f
0.06g (deionized)
0.32g (distilled) insoluble
Solubility in organic
solvents (g/kg
solvent) at 28 °C
petroleum ether 20 18 insoluble in common
acetone 60 organic solvents
carbon tetrachloride 300 10e
chloroform 400
benzene 750 81
toluene 970
dioxane 1150
copra oil (37 °C) 0.8
Table 9 (contd).
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Vapour pressure (Pa)
25 °C 0.000007h < 0.000006
90 °C 0.01 (temperature not given)
140 °C 1
220 °C 100
Volatility (% weight loss) < 1% at 250 °C 1-2% at 300 °C < 5% at 341 °C
< 10% at 330 °C < 10% at 363 °C
< 50% at 350 °C < 25% at 388 °C
log Pow < 7 (calculated)d 8.6 (calculated)
Decomposition temperature 300-400 °C 435 °C 435 °C 395 °C
> 400° C
a Mumma & Wallace 1975).
b Norris et al. (1973).
c Kerscher (1979); CFK (1982).
d Atochem (1990).
e Brinkman & de Kok (1980).
f Filonow et al. (1976).
g Griffin & Chou (1981a,b).
h Jacobs et al. (1976).
Table 10. Partition coefficients between water and n-hexane (KHW) and 1-octanol
(Kow) and aqueous solubilities (Sw) for some individual PBB congeners (all
aqueous solubility measurements were carried out by the generator method)
Log KHW Log KOW Sw mol per Sw µg/litred
litre x 10-9 (25 °C)
(25 °C)
2-bromobiphenyl 4.59a
3-bromobiphenyl 4.85a
4-bromobiphenyl 4.96a 2800 650
3,5-dibromobiphenyl 5.78c
4,4'-dibromobiphneyl 5.61 5.72 18.4 5.7
2,4,6-tribromobiphenyl 6.21 6.03 41.1 16
3,4',5-tribromobiphenyl 6.42c
2,2',5,5',tetrabromobiphenyl 6.72 6.50 8.6 4
3,3',5,5'-tetrabromobiphenyl 7.42c
2,2',4,5,5'-pentabromobiphenyl 7.10a 0.8b 0.4
2,2',4,4',6,6'-hexabromobiphenyl 7.52 7.20 0.9 0.56
decabromobiphenyl 8.58a
Values from Gobas et al. (1988) with the exception of:
a From: Doucette & Andren (1987).
b From: Doucette & Andren (1988).
c From: Sugiura et al. (1978).
d calculated.
Table 11. Melting points and UV spectral data for some PBB congenersa
UV conditions: solutions in n-hexane; Beckman Acta CIII spectrometer
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
Biphenyl 71 201 4.66 246 4.26
1 2- e 201 4.51 240 3.90
2 3- e 205 4.60 248 4.21
3 4- e 200 4.67 254 4.38
4 2,2'- 81 198 4.64 220-230 e
9 2,5- e 203 4.49 226 4.38
15 4,4'- 164 201 4.64 261 4.43
20 2,4,6- 65-66 213 4.70 220-230 e
21 2,2',5- 78 200 4.66 235-245 e
26 2,3',5- e 213 4.57 e e
31 2,4',5- 78 205 4.60 245-255 e
49 2,2',4,5'- 84 207 4.66 235-245 e
52 2,2',5,5'- 143 204 4.67 235-240 e
80 3,3',5,5'- 188 220 4.76 255 4.18
114b 2,3,4,4',5- 128 222.6 (54.8) 258 e
137b 2,2',3,4,4',5- 124 223.1 (35.4) e e
141b 2,2',3,4,5,5'- 127 223.4 (191) e e
153 2,2',4,4',5,5'- (159-160)f 216 4.66 e e
156b 2,3,3',4,4',5- 178 224.9 (229) 259 e
159b 2,3,3',4,5,5'- 195 226.1 (61.4) 258 e
167 2,3',4,4',5,5'- (165-166)f e e e e
Table 11 (contd).
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
169 3,3',4,4',5,5'- 248 227 4.76 272 4.34
180b 2,2',3,4,4',5,5'- 166(165-166)f 224.1 (62.4) e e
189b 2,3,3',4,4',5,5'- 219 230.7 (102) 265 e
194b 2,2',3,3',4,4',5,5'- 235(232-233)f 223.7 (51.6) e e
202 2,2',3,3',5,5',6,6'- e 224 4.85 e e
206b 2,2',3,3',4,4',5,5',6- 262(263-264)f,g 225.2 (131) e e
Nona-(unidentified) e 225 5.18 e e
Deca- 378 227 5.11 e e
a Adapted from: de Kok et al. (1977), with the exception of the congeners marked with b.
b Congener data, including melting points are taken from Kubiczak et al. (1989). UV measurements: in n-heptane.
c No. according to Ballschmiter & Zell (1980).
d Melting points from Sundström et al. (1976b) but confirmed by de Kok et al. (1977), unless otherwise stated.
e No data available.
f From: Moore & Aust (1978).
2.3 Conversion factors for PBB in air
1 ppm = 26.1 mg/m3 for hexabromobiphenyl at 20 °C and
101.3 kPa.
1 mg/m3 = 0.038 ppm.
2.4 Analytical methods
Analytical methods for the determination of PBBs, which have
been reviewed by de Kok et al. (1977), Pomerantz et al. (1978), and
Fries (1985b), were adapted from established methods for chlorinated
hydrocarbon insecticides and PCBs (AOAC, 1975). The chronological
development of analytical methods for the detection and
quantification of PBB mixtures and congeners is summarized in
Table 12. In the wake of the Michigan disaster, methods were
described for the analysis of: contaminated feed, milk, and milk
products (Fehringer, 1975a,b); animal blood plasma, faeces, milk,
and bile (Willett et al., 1978) and liver and fat (Fawkes et al.,
1982). The methods were developed using tissue from animals fed with
PBBs of known composition. Needham and coworkers developed a method
to determine PBBs in human blood serum (Burse et al., 1980; Needham
et al., 1981) which was thoroughly tested in several laboratories,
but, even here, only the main components of FireMaster(R) were
determined. Similarly, the investigation by Eyster et al. (1983)
into the levels of PBBs in fat, serum, faeces, milk, and placenta
were not isomer specific. Thus, reported values may not reflect the
hazard of the residue because, for example, some congeners are more
toxic than the prominent 2,2',4,4',5,5'-HBB. Most samples of
biological origin have congener distributions that differ from those
of the original material (Fries, 1985b).
Concentrations of PBBs as low as 10 µg/kg in fatty foods
(Fehringer, 1975a), 3 µg/kg in dry feeds (Fehringer, 1975b) and
1 µg/litre in blood serum (Needham et al., 1981) can be detected and
quantified using routine methods. Coefficients of variation become
large as concentrations approach the limits of sensitivity of the
method; thus, values near the limit must be treated with caution
(Fries, 1985b). PBBs adsorb to glass more tenaciously than other
halogenated hydrocarbons, and are not easy to remove by the usual
cleaning methods (Willett et al., 1978). This can lead to erroneous
values, particularly when concentrations in samples are low and
there is a carry over from samples of high concentration. This
problem can be solved by using disposable glassware (Willett et al.,
1978).
Table 12. Analysis of commercial mixtures and individual PBB congeners: A chronological surveya
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) recrystallization GC FID no data identification of Sundström
BP-6 from ethanol/ given BB 153 and a HpBB as et al.
isopropanol major components (1976a)
PBB congeners no data given GC ECD no data routes of synthesis, Sundström
given melting points, relative et al.
retention times, electron (1976b)
capture responses for
some PBB congeners
Commercial - solubility of HPLC, TLC, MS no data survey of analysis for de Kok
mixtures FR 250 13A PBBs in n-hexane UV, GC given PBBs et al.
(octabromobipheyl) decreases rapidly with 1H- & 13C-NMR (1977)
FireMaster(R) BP-6 increasing bromine
and PBB congeners content; PBBs dissolved
in warm CCl4
Commercial sample hexane GC, 13C-NMR, ECD no data identification of Moore
of 1H-NMR, IR given BB 180 et al.
octabromobiphenyl heptabromobiphenyl (1978)
FireMaster(R) methylene chloride; GC, NMR, MS 0.5 mg/kg contains at least 13 Hass
BP-6 hexane HPLC SIM different PBBs and et al.
bromonaphthalene (no (1978)
bromodibenzofurans or
bromodibenzo-p-dioxins
found)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC, NMR MS no data purification and Moore &
FF-1 or BP-6 and given structural characterization Aust
octabromobiphenyl of 6 further PBB congeners (1978)
FireMaster(R) hexane GC ECD 0.03 ng absolute and relative Domino
FF-1 retention times of the 8 et al.
major constituents using (1980a)
tetrabromobiphenyl
as an internal standard
FireMaster(R) hexane GC MS no data mass spectra of major Domino &
FF-1 given PBBs in FireMaster; mixed Domino
poly-bromo and (1980)
chlorobiphenyls detected
FireMaster(R) GC ECD no data comparison of packed and Farrel
BP-6 given capillary columns; solves (1980)
some problems with lower
brominated biphenyls, but
has no great advantages
over packed columns for
more highly substituted
biphenyls
FireMaster(R) no data given GC PED 2.8 mg comparison with ECD; not Mulligan
BP-6 (cf 1.5 ng quite so sensitive, but et al.
ECD) is selective (1980)
Individual PBB toluene GC MS, SIM < 1 ng mono-deca PBB congeners Erickson
congeners et al.
(1980)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
22 individual hexane GC ECD, micro- 40 pg retention times given Sweetman &
PBBs FireMaster(R) (preceeded coulometric for 23 congeners response Boettner
FF-1 by HPLC) GC-detector increases with degree of (1982)
MS bromination, increased
detection temperature gives
improved sensitivity
FireMaster(R) carbon preparative FID, MS polar and unpolar hexane Needham
FF-1 tetrachloride; HPLC and fractions were also et al.
hexane GC 1H-NMR tested for hyperkeratotic (1982)
activity
FireMaster(R) hexane GC NCI, SIM 0.6 ng evaluation of halogen anion Greaves
BP-6 formation by polybrominated et al.
compounds in NCI-MS; SIM of (1982)
bromine anions has greater
specificity than ECD
FireMaster(R) fractionation by GC ECD, MS no data seven congeners were Dannan
BP-6 preferential given purified et al.
acetone (1982d)
solubilization,
repeated
crystallization,
alumina
adsorption
column
chromatography,
reversed phase
Lipidex-500
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) see Needham et al. preparative FID, MS at least 60 components Orti
FF-1 (1982) HPLC, GC, observed; isolated/ et al.
lot FH 7042 GC 1H-NMR determined structure of (1983)
10 minor components of
FireMaster (most are very
polar, later eluting
fractions)
PBB hexane GC helium 230 pg simultaneous monitoring Eckhoff
(unspecified) plasma of 4 atomic emission et al.
atomic wave-lengths; PBB mentioned (1983)
emission
spectrometric
detection
FireMaster(R) no data given GC MS, ECD identity of over 91% of Robertson
BP-6 1H-NMR PBB components in et al.
FireMaster using 22 (1984b)
individual PBB congeners
as standards; identification
of 7 additional PBBs
including 3 very toxic
coplanar PBBs
FireMaster(R) hexane GC PED, rapid multi-element quantification Zerezghi
BP-6 scanning et al.
plasma (1984)
emission
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC SIM, MS determination of suspected Tondeur
FF-1 toxic impurities et al.
(1984)
PBB photolysis hexane GC FID, ECD, purification of PBB congener Barnhart
mixture MS 2 using charcoal pretreatment et al.
and RPLC (1984)
Benzenes, hexane GC NCI-MS 0.1 pg especially valuable for Buser
biphenyls measuring trace levels in (1986)
dibenzodioxins, biological and environmental
dibenzofurans, samples; must be two Br;
diphenylethers, structural information is
benzofurans, partly lost
phenols
PBB congeners no data given GC MS no data use of 'ortho' effect for Sovocool
given PBB and isomer & Wilson
identification; accurate (1982);
structure assignments Sovocool
without use of multiple GC (1987a)
determinations
Various PBB hexane GC, HPLC FID no data relationship between Höfler
congeners given recorded retention data et al.
from HPLC and GC and (1988)
molecular surface area
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
Nine synthetic products purified GC MS 1 ng synthesis of 2,3,4,5- Kubiczak
PBBs; by alumina/Florisil; substituted PBBs and et al.
FireMaster(R) recrystallization characterization (1989)
FF-1 and BP-6 from methanol or
methylene chloride
Mono- and no data given 1H-NMR, no data no data Anklam
poly-brominated 13C-NMR given given (1989)
biphenyls
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NMR = Nuclear magnetic resonance.
FID = Flame ionization detector. PED = Microwave-induced plasma emission detector.
GC = Gas chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
GPC = Gel permeation chromatography. RPLC = Reverse-phase liquid chromatography.
HPLC = High pressure liquid chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatograpy.
MS = Mass spectrometry. Unitrex = Universal Trace Residue Extractor.
NAA = Neutron activation analysis. UV = Ultraviolet.
NCI = Negative ion chemical ionization mass
spectrometry.
Recovery of PBBs using established methods is in the range of
80-90% (Fries, 1985b). The solvent system that is used for sample
extraction can affect recovery. Poor recoveries were often found
with hexane but the optimal solvent conditions depend on the source
of the medium sample.
For extraction conditions see Table 13 (environmental samples),
Table 14 (food/feed), Table 15 (biological tissues and fluids (a)
serum/blood (b) adipose and other tissues).
In soil, Griffin & Chou (1981a) found that a polar organic
solvent was important and obtained the best results with
hexane/acetone (9:1).
For serum and blood, the standard extraction method given by
Burse et al. (1980) has been used by most workers.
Extraction of PBBs from adipose and other tissues presents
greater problems. PBBs are readily soluble in fat. They can
therefore be extracted with the fat out of the tissue/sample but,
afterwards, an intensive clean-up procedure for PBBs is necessary.
Various methods, such as adsorption chromatography with Florisil,
gel permeation chromatography, Florisil cartridges (Chiang et al.,
1987), and Unitrex (Head & Burse, 1987) have been proposed.
The sample extraction and clean-up techniques for the
determination of PBBs are similar to those used for PCBs (Krüger
et al., 1988; Jansson et al., 1991). The lipids can be removed from
the extract by gel permeation (Krüger, 1988) or by hydrolysis
(Jansson et al., 1991). Usually PBBs and PCBs are separated from
more polar compounds by adsorption chromatography on silica gel or
Florisil. If the coplanar compounds are to be determined, they have
to be isolated from the major compounds in the extract. This can be
done using activated charcoal, which adsorbs the planar molecules
more strongly than the non-planar. Brominated naphthalenes, dioxins,
and furans will also be separated from the major PBB components in
this step. HPLC methods are now being adopted for these separations
and both charcoal and modified silica gel columns are available for
HPLC separations of coplanar compounds.
Table 13. Determination of PBBs in environmental samplesa
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
Soil, grass, benzene/ Florisil GC ECD, FID 0.1 µg/kg BB 153, two PeBB Jacobs
carrots 2-propanol MS dry weight (soil) isomers, three et al.
13C-NMR 10 µg/kg additional HxBB (1976)
wet weight (plant) isomers, two HpBB
isomers detected
Soil leachate benzene/ GC ECD 0.1 µg/kg laboratory experiments Filonow
2-propanol dry weight et al.
(1976)
Soil, plant hexane/ Florisil GC ECD 0.1 µg/kg field and laboratory Jacobs
samples acetone dry weight (soil) experiments; no et al.
TLC ECD 0.3 µg/kg significant (1978)
wet weight (plant) degradation of PBBs
after 1 year
Effluent river hexane/ no data GC ECD 0.1 µg/litre environmental Hesse (1975)
water diethyl given (later 0.01 µg/ samples Hesse &
ether litre) Powers
(1978)
Sediment hexane/ no data GC ECD 100 µg/kg environmental Hesse &
acetone given samples Powers
(1978)
Soil hexane/ no data GC ECD, FID, separation of 30 PBB Stratton &
acetone 9:1 given MS congeners tested Whitlock
optimum conditions (1979)
Table 13 (contd).
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
for extraction of
PBBs from soil;
polar organic solvent
important
98 environmental hexane, Florisil GC MS 0.2 µg/kg analysed for hexa-, Stratton
samples Soxhlet hepta-, octa-, nona-, et al.
(fish, sediment, decabromobiphenyls; (1979)
soils, HxBB in 84% of samples
vegetation)
Soil, sediment, hexane Florisil GC MS (SIM) 0.2 µg/kg congeners detected Griffin &
sludge, Chou (1981a)
vegetation
Soil hexane/ Florisil GC FID, ECD degradation of PBBs Hill et al.
acetone 1:1 in soil (1982)
Sewage sludge hexane/ TLC IR, NMR, MS 10 ng/kg no PBBs found Strachan
methanol GC et al.
Soxhlet (1983)
extraction
Plants cut, Florisil GC ECD 0.3 µg/kg no translocation Chou et al.
extracted wet basis in plants (1978)
with hexane/
acetone
Table 13 (contd).
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. MS = Mass spectrometry.
FID = Flame ionization detector. NMR = Nuclear magnetic resonance.
GC = Gas chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatography.
Table 14. Determination of PBBs in food/feeda
Matrix Extraction Clean up Analytical Detection Detection Comment References
method limit
Dairy fat extracted by AOAC GPC, 25% toluene GC ECD 7 µg/kg comparison Fehringer
products (1975) methods in ethyl acetate of methods (1975a)
(methanol/ether)
Florisil/pet ether TLC 0.2 mg/kg
Dry animal finely ground feed Florisil/pet ether GC, TLC ECD 8 µg/kg hexabromo Fehringer
feeds packed into a column 30 µg/kg isomer (1975b)
containing celite, measured
elution with methylene
chloride
Feeds and see Fehringer GC before and ECD 5 µg/kg confirmation Erney
dairy (1975a,b) after UV irradi- of PBB (1975)
products ation to determine residues
background using UV
irradiation
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. TLC = Thin layer chromatography.
GC = Gas chromatography. UV = Ultraviolet.
GPC = Gel permeation chromatography.
Table 15. Determination of PBBs in biological tissues and fluidsa
Matrix Extraction Clean up Detection Detection limit Comment References
a) Serum/blood
Human serum methanol-treated serum, Florisil ECD 5 pg analysis based on HxBB Bekesi et al.
extraction with hexane peak (1978)
Human serum methanol-treated serum, Florisil ECD 0.2 µg/litre Wolff et al.
extraction with hexane/ (1978)
ether
Human/rat serum methanol-treated serum, Florisil ECD 0.2 µg/litre PBB homologues as % HxBB Wolff &
extraction with hexane/ peak Aubrey
ether (1978)
methanol-treated serum, Florisil ECD, MS < 1 mg/ml Wolff et al.
extraction with hexane/ (1979a)
ether
Plasma from multiple extraction with Florisil ECD 0.001 µg/litre recovery 96% Willett
PBB-fed cows mixture of diethyl and pet. et al.
ethers (1978)
Human serum methanol-treated serum; Florisil ECD 0.1 µg/litre interlaboratory comparison Burse et al.
extraction with hexane/ (1980)
ether
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Plasma, white methanol; precipitated Florisil MS-SIM 0.1 µg/mg very exact details with Roboz et al.
cell fraction protein removed; NCI protein review spectra (1980)
erythrocytes extraction with hexane/
ß-lipoprotein ether (1:1)
Human serum methanol; hexane/ silica gel ECD 1 µg/litre Needham
diethylether (1:1) et al.
(1981)
Human serum + methanol precipitated Florisil ECD < 1 µg/litre serum protein precipitated Roboz et al.
protein not removed + MS-NCI with methanol should not (1982)
hexane/diethylether (1:1) be removed from sample
Human serum see Burse et al. (1980) ECD 1 µg/litre Eyster
et al.
(1983)
Blood (in vitro see Roboz et al. (1982) MS- in vitro Roboz et al.
experimental) (PPINCI) (1985a)
Human blood see Roboz et al. (1982) ECD, SIM, 10-35 ng distribution of PBBs Roboz et al.
(model and NCI individual among blood components (1985b)
environmentally serum congener/litre
exposed)
b) Adipose and other tissues
Adipose tissue toluene/ethyl acetate GPC (Bio ECD 0.5 µg/kg major HxBB peak determined Wolff et al.
from exposed (1+3) Beads (1979a)
workers toluene/ethyl
acetate (1+3)
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Various rat Burse et al. (1980) ECD 10 µg/kg comparison of Miceli &
tissues and concentrations of PBBs Marks
serum in various tissues with (1981)
time
Liver and 1) hexane (liver and 1) Florisil ECD, NAA comparison of extraction Fawkes et al.
perirenal adipose) methods (showed PBB (1982)
adipose tissue extraction with hexane
from dosed rats leads to erratic recoveries
and results) increase in
detection limits over
ECD ( 2 pg FireMaster);
2) chloroform: 2) acidic 1 µg/litre or less of
methanol (liver) alumina hexa congener)
3) methylene chloride
chloroform (adipose)
Human adipose 15% diethyl ether Florisil/GPC ECD GPC clean-up tested (85% MacLeod
tissue in hexane MS recovery); MS free of et al.
serious interference (1982)
from 46 to 500 m/z
Adipose tissue 6% diethyl ether Florisil/GPC MS 1-2 µg/kg HxBB peak Lewis &
from general in hexane Sovocool
population (1982)
Human adipose hexane/diethylether silica gel ECD 1 µg/kg Eyster
tissue, placenta, et al.
cord blood, (1983)
biliary fluid,
faeces
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Human postmortem Chromaflex ECD 0.5 µg/kg HxBB peak Miceli
tissue adsorption et al.
column with (1985)
5% silica
gel + sodium
sulfate/
hexane
Adipose tissue hexane solid phase ECD 1-14 ng/kg Florisil Chiang et al.
(bovine), spiked Florisil cartridges cartridges to separate (1987)
for model system fat; 116% recovery
c) Milk
Human milk potassium oxalate, ECD 1 µg/kg Eyster et al.
ethanol/diethyl ether; (1983)
hexane
Human milk potassium oxalate, Bio Beads/ MS (NCI, 1 ng/kg separation of coplanar Krüger (1988)
ethanol/diethyl ether Florisil/ SIM) and planar isomers with
activated charcoal
charcoal
d) Biological samples from the environment
Fish, seal freeze, pulverize, Bio Beads/ MS (NCI, 10 ng/kg Krüger (1988)
pet. ether Florisil/ SIM)
activated
charcoal
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Dolphin fat/ Soxhlet; hexane, GPC; silica MS no data given lowest value given: Kuehl et al.
organ tissue methylene chloride gel 40 µg/kg (1991)
Terrestrial, diethyl ether/hexane hydrolysis MS (NCI) no data given lowest value given: Jansson
freshwater and with 98% 40 ng/kg et al.
marine samples H2SO4/Bio (1991, 1992)
Beads/ silica
gel/activated
charcoal
a Analytical method used was gas chromatography.
Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NCI = Negative ion chemical ionization mass spectrometry.
GPC = Gel permeation chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
MS = Mass spectrometry. SIM = Selected ion monitoring.
NAA = Neutron activation analysis.
Using negative ion chemical ionization mass spectrometry
(MS-NCI), the bromide ions can be used to detect brominated
compounds with high sensitivity and selectivity. However, using this
detection method (or ECD), interference between congeners of PBB and
polybrominated diphenyl ethers is possible.
The 209 possible PBB congeners have a wide range of volatility,
which causes very difficult separation problems (Farrell, 1980). In
earlier studies, gas chromatography (GC) with packed columns, e.g.,
3% OV-1 on 80/100 mesh Chromosorb W(HP) was used (Fehringer,
1975a,b). Capillary columns enable a good separation with lower
brominated biphenyls but do not have any great advantages over
packed columns for more highly substituted biphenyls (Farrell, 1980;
Orti et al., 1983; Robertson et al., 1984b).
The detection method most frequently used is that of pulsed
63Ni electron capture detection (ECD). In general, retention times
and electron capture responses increase with increasing bromination.
This is a sensitive method, but has some shortcomings. ECD is a
group selective detector that responds to halogens and other
electronegative groups. This places stringent requirements on
chromatographic separation. Moreover, ECD responds differently to
different compounds, depending on the molecular structure. The
response or sensitivity of the ECD depends on the position of the
halogen on the biphenyl nucleus as well as the number of halogens.
This necessitates running a standard for each compound to be
determined (Zerezghi et al., 1984). Sweetman & Boettner (1982)
analysed the structure-sensitivity of PBBs using ECD (see Table 12).
Flame ionisation detection (FID) can only be used for the
analysis of standard substances because of its low specificity
(Krüger, 1988).
A microwave-induced plasma emission detector has been used as a
specific method of detection for bromine (Mulligan et al., 1980;
Zerezghi et al., 1984). However, the method is not sensitive enough
for environmental samples.
Some authors have confirmed their results by GC/ECD
determination before, and after, exposure to UVR. The PBBs present
are photolyzed and, in this way, the background values can be
eliminated (Erney, 1975; Trotter, 1977).
Very often, the presence of PBBs is confirmed using mass
spectrometry (MS) together with gas chromatography. The purity of
the sample can be verified by comparison with known standards.
Negative chemical ionization (NCI) mass spectrometry has a
sensitivity comparable with, and somewhat better than, GC/ECD
analysis. The detection level for hexabromobiphenyl standards is
lower by a factor of 20 to 10-35 ng/ml in comparison with GC/ECD
analyses (Roboz et al., 1982). This relatively new method has also
been used to detect polychlorinated and polybrominated dioxins and
furans (see section 4.3).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
PBBs are not known to occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 World production figures
1) United States of America
The commercial production of PBBs in the USA commenced in 1970
(Neufeld et al., 1977). Several US producers of commercial
quantities have been identified (Mumma & Wallace, 1975; Neufeld
et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok, 1980).
In 1976, a US firm had a combined production of about 0.45
million kg of PBBs for export to Europe (Anon., 1977).
A list of suppliers of laboratory quantities (with a maximum
production or importation of about 2 kg/year) is presented by Mumma
& Wallace (1975) and Neufeld et al. (1977).
As a result of the Michigan catastrophe of mid-1973, the sole
US manufacturer of hexabromobiphenyl ceased production in November
1974. It is not clear, whether the production of bromine-based fire
retardants was resumed by another US company in 1978 (Brinkman & de
Kok, 1980). Two other companies continued their production of octa-
and deca-PBB until 1977 (Di Carlo et al., 1978). According to the
German "Umweltbundesamt" (UBA, 1989), decabromobiphenyl was produced
in the USA until 1979.
There are repeated statements that all PBBs manufactured in the
USA since 1975-76 have been exported, mainly to Europe, and that
there is no importation of any PBB mixtures into the USA (Brinkman &
de Kok, 1980).
Relevant production data for the period 1970-76 are presented
in Table 16.
Table 16. Commercial production of polybrominated biphenyls in the USA, 1970-76a
Estimated production in thousand kg
Product 1970 1971 1972 1973 1974 1975 1976 1970-76
Hexabromobiphenyl 9.5 84.2 1011 1770 2221 0 0 5369
Octabromobiphenyl 14.1 14.1 14.6 163 48 77.3 366 702
and decabromobiphenylb
Total PBBs 23.6 98.3 1025 1933 2269 77.3 366 6071
a From: Di Carlo et al. (1978).
b Manufacture was continued in 1977, but production figures are not available.
Hexabrominated biphenyl forms the major part (about
5.4 million kg FireMaster(R) BP-6 plus some 68 300 kg
FireMaster(R) FF-1) of the estimated total production of
6.1 million kg (Neufeld et al., 1977). The remaining 0.7 million kg
are accounted for by the higher brominated biphenyls. In 1976, for
example, 0.35 million kg of decabromobiphenyl and 13 600 kg of
octabromobiphenyl were manufactured (Neufeld et al., 1977). No
production figures are available for 1977 (Di Carlo et al., 1978).
2) Japan
According to IARC (1978), PBBs have never been produced in
Japan, but, up to 1978, some were imported.
3) Europe
a) Germany
A German firm produced a mixture of highly brominated PBBs,
called Bromkal 80-9D until mid-1985, when the activities concerning
bromine-based fire retardants were shifted to the USA. No production
figures are available.
b) France
A French firm manufactures a technical-grade decabromobiphenyl,
sold as Adine 0102, production being a few hundred thousand kg/year
(Atochem, 1988). It is marketed in France, Great Britain, Spain and
the Netherlands (Atochem, 1988; UBA, 1989). More than 200 tonnes
decabromobiphenyl/year were used in the Netherlands for
incorporation into polybutylenterephthalate plastics (UBA, 1989).
c) United Kingdom
Two companies are reported to have marketed or produced
technical-grade decabromobiphenyl in the United Kingdom (Brinkman &
de Kok, 1980). In 1977, the production of PBBs was discontinued
(Neufeld et al., 1977).
No production or sales data are available.
d) Netherlands
No domestic producer has been identified. An Israeli company
with two bromine plants in Holland denied the production of PBBs
(Neufeld et al., 1977). However, the amount of decabromobi phenyl
sold annually in the Netherlands was estimated to be of the order of
91 000 kg (Brinkman & de Kok, 1980).
No information is available on production in other parts of the
world.
3.2.1.2 Manufacturing processes
The process of manufacturing PBBs consists of a Friedel-Crafts
type reaction in which biphenyl is reacted with bromine in the
presence of chloride in an organic solvent, using aluminium
chloride, aluminium bromide, or iron as catalyst (Brinkman & de Kok,
1980). In the Atochem decabromobiphenyl manufacturing process,
biphenyl is directly brominated in a large excess of bromine, used
as reactant and solvent in the presence of a Lewis acid catalyst
(aluminium type). Decabromobiphenyl is further purified by
distillation of the excess bromine in the presence of a brominated
solvent (Atochem, 1992).
3.2.1.3 Loss into the environment during normal production
Data are published only for the USA. The following information
refers to reviews by Neufeld et al. (1977) and Di Carlo et al.
(1978).
Losses of PBBs to the environment at sites of its manufacture
can total 51 kg/1000 kg of product. These losses occur through:
1) Emission into the air
In 1977, the maximum air losses as particulate matter at
production sites were estimated to total 1.1 kg of PBBs/1000 kg
manufactured.
(a) Emission to the air from the vents of the hydrogen bromide
recovery system:
Total emission of FireMaster(R) PB-6 was estimated to amount
to 70 mg/1000 kg produced.
(b) Loss of particulate PBB to the atmosphere during centrifugation
(which was carried out to separate the solid reaction products
from the organic solvent).
A New Jersey permit application by Hexcel Corp. plant (1976)
indicated a loss of less than 0.05% of the product.
(c) Loss of dust from drying and pulverizing PBBs to a fine powder
(dust from this operation was removed by a bag type filter).
In 1974, atmospheric levels of PB-6 in the Michigan Chemical
Corp. bagger area were 16-32 mg/m3 during the bagging operation
and 3 mg/m3 after bagging was completed. Lower levels were
detected in other areas of the plant.
(d) Emission of hexabromobiphenyl as a vapour contaminant in
vapour streams leaving scrubbers or equivalent equipment was
calculated to be less than 25 µg/m3 (1 ppb) at ambient
temperature (Neufeld et al., 1977).
2) Losses in waste waters resulting from the quenching and
washing of the PBBs as they are recovered from the reaction mass:
The losses of PBBs to sewers at manufacturing sites were
estimated, in 1977, to be 4.6 µg/kg of product.
- In 1972, samples of the Michigan Chemical Corp. effluent
discharges were found to contain PBB levels of 98-503 µg/litre
(Hesse, 1975);
- The total quantity of PBBs being discharged to the Pine River
was estimated as 0.11 kg daily.
- Unfiltered water from an industrial storm sewer at the Hexcel
Corp. plant contained 92 µg/litre, mainly as decabromobiphenyl
(hexa-, octa-, and nonabromobiphenyls levels were also
measurable).
- Liquid effluents, diluted by canal water, from the White
Chemical Co. plant showed values of up to 31 µg PBBs/litre.
3) Solid losses to landfills resulting from drying, handling,
shipping and transportation.
An estimate of PBB losses as solid waste to landfills was
50 g/kg of product.
According to a report of the Michigan Chemical Corp., their
solid waste included approximately 5% of the BP-6 produced.
4) Losses to the soil
Soil samples from the bagging and loading areas of the Michigan
Chemical corp. contained PBBs at concentrations of 3500 and
2500 mg/kg, respectively.
Losses of other compounds:
The following typical air contaminants released during PBB-
manufacture were reported: hydrogen chloride, bromine, ethylene
dichloride, aluminium chloride, and biphenyls. The total quantity
emitted was stated to be less than 5.5 kg/day.
3.2.1.4 Methods of transport, accidental release, and disposal of
production wastes
Details of present-day labelling and transport regulations are
given in the Health and Safety Guide for PBBs (WHO, 1993).
In 1973, an accidental release of PBBs occurred in Michigan
("Michigan disaster"), when two products manufactured by the
Michigan Chemical Company were inadvertently confused, i.e.,
250-500 kg (Di Carlo et al., 1978) of FireMaster(R), instead of
NutriMaster(R), a magnesium oxide-based cattle feed supplement,
were added to animal feed and distributed to farms within the state.
The compound is believed to have been FireMaster(R) FF-1
(e.g., Fries, 1985b), even if in some publications the name
FireMaster(R) BP-6 is used (e.g., Neufeld et al., 1977; Di Carlo
et al., 1978). This accidental mix up resulted in widespread
contamination by PBBs (see section 5). As a result of this incident,
the production of FireMaster(R) BP-6 by Michigan Chemical Corp.
was stopped in 1974 (Di Carlo et al., 1978). Chronological reports
or reviews of the PBB disaster are given by Carter (1976), Getty
et al. (1977), Kay (1977), Di Carlo et al. (1978), Damstra et al.
(1982), Zabik (1982), and Fries (1985b).
Details of the disposal of manufacturing waste during present
production are not available. In a report by Neufeld et al. (1977),
solids from manufacturing operations were disposed of in landfills.
Waste waters containing small amounts of PBBs were discharged into
the chemical sewer.
3.2.2 Uses
Commercially manufactured PBBs are processed by industrial
users, primarily as flame retardants in polymeric materials. PBBs
were developed for this major application, because: they are able to
meet the flame-resistance performance requirements, they are
economically feasible, and they have little effect on the
flexibility of the base compounds (Mumma & Wallace, 1975).
The process of application is basically one of physical
blending: the PBBs are not functional additives, and on blending
with the dry solid or liquid polymeric material, provide filter-type
flame retardant action with the chemical release of hydrogen bromide
if ignited (Neufeld et al., 1977).
Neufeld et al. (1977) list 34 applications of PBBs found in
patent and technical literature. The majority are related to the use
of the PBBs as flame retardants in polymeric materials, other claims
include self-extinguishing properties and improved wearability and
machinability. Further potential uses of PBBs are: in the synthesis
of biphenyl esters or in a modified Wurtz-Fittig-synthesis; in
light sensitive compositions to act as colour activators; as
relative molecular mass control agents for polybutadiene; as wood
preservatives; as voltage stabilizing agents in electrical
insulation; as functional fluids, such as dielectric media (Neufeld
et al., 1977). In the USA and Canada, hexabromobiphenyl
(FireMaster(R)) was the principal PBB product. It was used as a
fire retardant in three main commercial products:
acrylonitrile-butadiene-styrene (ABS) plastics; coatings and
lacquers; and polyurethane foam (Neufeld et al., 1977).
The types of ABS plastic products in which FireMaster(R) BP-6
was used are compiled in Table 17.
According to Neufeld et al. (1977), the use of FireMaster(R)
BP-6 as a flame retardant in thermoplastic resins was confined to
products that do not come into contact with food or feed and are not
used in fabrics to which humans are exposed.
Although more than 130 companies in the USA used PBBs prior to
1976 (Di Carlo et al., 1978), only a limited number seems to have
been the major users of PBBs. For example, in 1974, the final year
of US production, Borg Warner Corp. (Parkersburg, W.Va.; using
FireMaster(R) in ABS plastics) and Standard T Chemical Co. (Staten
Island, New York; using FireMaster(R) in fire retardant coatings
for industry) consumed over 50% of the total US yearly production
(Mumma & Wallace, 1975; Jamieson, 1977; Neufeld et al., 1977;
Brinkman & de Kok, 1980).
Of the estimated 2200 tonnes hexabromobiphenyl produced in 1974
(IARC, 1978), about 900 tonnes (Mumma & Wallace, 1975; Neufeld
et al., 1977; IARC, 1978) were used in ABS plastic products and
about 34 000 tonnes (Mumma & Wallace, 1975; Neufeld et al., 1977;
IARC, 1978) in cable coatings.
The exact quantity of FireMaster(R) used in polyurethane foam
for automobile upholstery was not published. The two larger
consumers ceased using hexabromobiphenyl (one of these in 1972)
because PBBs did not decompose in the ultimate incineration of
scrapped automobiles (Neufeld et al., 1977).
No current users of hexabromobiphenyl have been identified
(Neufeld et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok,
1980). As regards octa- and decabromobiphenyl, no commercial use was
reported in the USA during 1970-74 (Neufeld et al., 1977). In
Western Europe, the use of higher brominated PBBs seems to be
dominant. The decabromobiphenyl Adine 0102(R) (in the past
manufactured by Ugine Kuhlmann, at present by Atochem) is used as a
flame retardant for thermoplastics and thermosets (e.g., in
polyesters, epoxy resins, polystyrene, ABS, polyolefines, and PVC),
for elastomers (e.g., in PU-elastomers and india rubber) and for
cellulosics (e.g., chip-board). It is applied frequently in
association with antimony trioxide (Sb2O3) (Atochem, 1984a). Its use
in paints and varnishes has also been reported (Brinkman & de Kok,
1980).
Table 17. Uses of FireMaster(R) BP-6 in ABS plastics in the USAa
Industry Approximate % Examples
of total use
Business machines and 48 Typewriter, calculator and microfilm-reader
industrial equipment housings; business machine housings
Electrical 35 Radio and TV parts, thermostats, shaver and
hand-tool housings
Fabricated products 12 Projector housings, movie equipment cases
Transportation 1 Miscellaneous small automotive parts;
electrical-wire connectors, switch
connectors, speaker grills
Miscellaneous 4 Small parts for electrical applications,
motor housings; components for industrial
equipment
a From: Brinkman & de Kok (1980).
Losses of PBBs to the environment from processing plants are
possible, but little information is available about this.
Although decabromobiphenyl and, possibly, other PBBs are still
produced commercially, alternative chemicals have been introduced to
replace them as flame retardants, in particular polybrominated
biphenyl ethers (oxides) (PBBO), e.g., decabromobiphenyl ether
(Adine 505; Bromkal 82-0 DE; Great Lakes DE-83TM and DE 83RTM),
octabromobiphenyl ether (Bromkal 79-8 DE; Great Lakes DE 79), and
pentabromobiphenyl ether (Bromkal 70-5 DE; Great Lakes DE-71TM:
Atochem, 1984b; Great Lakes Chemical Corp., 1986).
Decabromobiphenyl ether (DBBO) for example, appears to be a
much less toxic material than PBBs. However, DBBO is said to have a
tendency to degrade to lower brominated biphenyl oxides. It is
possible that these lower order compounds may pose environmental
problems similar to those of the lower brominated PBBs (Mumma &
Wallace, 1975). In addition, on pyrolysis, PBBOs produce larger
amounts of dioxins and furans than PBBs and so may themselves have
to be replaced by other compounds.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The commercial PBB-mixtures are solids at room temperature.
Despite their low vapour pressure, air pollution by PBBs can occur
as follows:
PBBs may be released into the atmosphere as vapour or dust
from production and processing plants. Stratton & Whitlock (1979)
found indirect evidence of airborne discharges of PBBs near two out
of three chosen industrial sites in north-eastern New Jersey and
Staten Island, New York, where these materials had been manufactured
or used in product formulations.
Further air contamination may occur during the incineration of
industrial and municipal wastes. Most municipal incinerators are not
very effective in destroying halogenated biphenyls. Like PCBs, PBBs
do not burn readily and incinerating conditions must be carefully
controlled, otherwise these compounds will reenter the environment
in the stack gases (Griffin & Chou, 1981a) or may be transformed to
polybro minated dibenzofurans.
Flameless combustion of the consumer products causes
volatilization of intact PBBs (Benbow & Cullis, 1975).
An appreciable loss of PBBs during the lifetime of PBB-
containing products is unlikely.
Secondary ways of entrance of PBBs into the atmosphere, e.g.,
through evaporation from contaminated soils, are thought to be
negligible, though small losses of PBBs from soil during long-term
(6 months) incubation studies were observed, which were associated
with volatilization rather than sorption or masking (Griffin & Chou,
1981a).
The ability of PBBs to co-distil from landfills or from the
surface layer of water bodies, as reported for PCBs (Kalmaz &
Kalmaz, 1979), has not yet been examined.
By analogy with PCBs, it might be expected that PBBs entering
the atmosphere in the vapour phase would be adsorbed rapidly onto
particles, which would then be deposited by particle sedimentation,
depending on micro- and macrometeorological conditions.
According to Eisenreich et al. (1981) organic compounds having
a vapour pressure > 10-5 kPa should exist almost entirely in
the vapour phase, and those having a vapour pressure > 10-9 kPa
should exist almost entirely in the particulate phase.
PBBs, e.g., hexabromobiphenyl or FireMaster with a vapour
pressure of 6.9 x 10-9 kPa (Jacobs et al., 1976), belong to the
latter. In reality, distribution and atmospheric lifetimes of
organic compounds with a high relative molecular mass depend largely
on the particle concentration and composition in the atmosphere
(Eisenreich et al., 1981). Gas-phase reactions with hydroxyl (OH)
radicals also influence the lifetimes of organic compounds emitted
into the atmosphere. Atkinson et al. (1984) determined rate
constants for the gas-phase reaction of OH radicals with biphenyl
and predicted, from their findings, that the chlorine - and
bromine-substituted biphenyls would have OH radical rate constants
of < 8 x 10-12 cm3/molecule per second at room temperature.
4.1.2 Water
The principal route of entrance of PBBs into aquatic
environments is from industrial waste streams into receiving waters.
Further potential routes (of minor relevance) are atmospheric
deposition and erosion of polluted soils. Groundwater contamination
is possible, if these compounds are leached from landfills (Shah,
1978).
Because compounds like PBB are very poorly soluble, they are
primarily found in sediments of polluted lakes and rivers
(Kimbrough, 1980a). In laboratory experiments, Simmons & Kotz (1982)
determined the "percent adsorption" of PBBs in sediments from sites
at Lake Michigan and the Huron River and concluded from values
ranging from 9 to 32% that the capacity of the sediments for PBB was
small to moderate.
PBBs in water are mainly adsorbed on particulate matter
followed by sedimentation at a rate that depends on several factors,
such as the size and type of the sediment and/or the organic
contents of both the sediment and the overlying water mass. The
relative importance of these parameters is controversial (Simmons &
Kotz, 1982). Laboratory results concerning PCBs (Jensen et al.,
1973) led to the assumption that the kinetics of the sorption
reaction may vary inversely with particle size (because smaller
particles have a larger surface area for surface adsorption).
Leland et al. (1973), Choi & Chen (1976), and Simmons et al. (1980)
have shown that the organic content of the sediment is directly
related to its adsorptive capacity for a specific contaminant.
According to Schwarzenbach & Westall (1981), adsorption of non-polar
compounds is highly correlated with the organic carbon content of
sorbents containing more than 0.1% organic carbon. Simmons & Kotz
(1982) found strong correlations between the adsorptive capacity of
the sediments for PBBs and TOC (total organic carbon) and the %
silt/clay fraction.
Sediments are potential sources as well as sinks for most
chemicals (Simmons & Kotz, 1982). Desorption of a contaminant from
the sediments is favoured where a high concentration of organic
matter exists in the water column (Huang, 1971). The presence of
organic matter may also enhance the partitioning of the contaminant
in the water phase and, thus, facilitate further movement with the
water mass (Hassett & Anderson, 1979). Laboratory studies on the
mode of action that PBBs may take in their movement through the
water column have verified that the total organic content of the
natural water will decrease the adsorption of PBB onto sediment and
therefore keep the PBB in the water phase. For example, comparing
the distilled water versus natural water systems, in river water
with an organic content of 11-12 mg C/litre, the % PBB-adsorption
was decreased by 33-43%; for lake water with an organic content of
3.8 mg/litre, the % PBB-adsorption was reduced by about 12% (Simmons
& Kotz, 1982). Another investigation indicated that the solubilities
of PBBs were directly correlated with the levels of dissolved
organics in the water (Griffin & Chou, 1981a) (see also section
4.1.3).
However, in the natural environment, upon settling out, the
association of the contaminant with the sediment may become the
dominant process in the water/sediment system (Simmons & Kotz,
1982). Transport of PBBs is thought to take place, when mixing or
bioturbation of sediments causes redistribution of the contaminant
in the water column (Simmons & Kotz, 1982), and through transport of
the sediment itself.
4.1.3 Soil
Pollution of soils can originate from point sources such as PBB
plant areas and waste dumps. Very few data are available on the
deposition of PBBs on soil via the atmosphere, sewage sludge from
municipal sewage treatment systems, and the dredging of sludge from
contaminated waters.
Other possible sources are illicit, or improper, disposal of
such chemicals (Kimbrough, 1980a) and incidents. For example, as a
consequence of an incident in 1973, Michigan soils have been
contaminated by manure from PBB-fed animals and by the disposal of
contaminated feed, milk, carcasses, etc. (Getty et al., 1977; Chou
et al., 1978; Damstra et al., 1982; Fries, 1985b). Once PBBs have
been introduced into the soil, they appear to have little tendency
to translocate (Damstra et al., 1982).
The ability of rainfall to carry PBBs through the soil was
tested in a laboratory simulation. Filonow et al. (1976) percolated
water through columns of 4 Michigan soils containing 100 mg
2,2',4,4',5,5'-hexabromobiphenyl/kg. They found a loss of less than
0.6% of the hexabromobiphenyl congener from each soil, even with
leachate quantities equivalent to 20 times the average annual
rainfall in Michigan.
Field investigations also indicated that PBBs were retained in
the top soil. Results of subsequent studies on highly contaminated
farm soils showed that PBBs did not move below the 15 cm level,
except where there was a history of physical mixing of the soil
(Fries, 1985b).
The mobility in soils of a chemical like PBBs will largely be
governed by its solubility in water and its adsorption, or
interaction, with soil particles (Jacobs et al., 1978).
As already mentioned, PBBs have a very low solubility in water.
However, studies with distilled, tap, river, and soil waters showed
that their solubility was markedly influenced by water purity
(Jacobs et al., 1978). Griffin & Chou (1981a) ascertained, under
well defined conditions, the following average solubilities of PBBs:
0.06 µg/litre in distilled water, 0.3 µg/litre in deionized water,
0.5 µg/litre in creek water, 8.9 µg/litre in Du Page leachate, and
16.9 µg/litre in Blackwell leachate.
Hence, PBBs were more than 200 times more soluble in landfill
leachate than in distilled water; the solubilities of PBBs were also
higher in creek water than in distilled water. As shown by the TOC
(total organic carbon) values for the waters, the higher
solubilities of PBBs were directly correlated with the level of
dissolved organic compounds in the waters. The type of dissolved
organic matter may also influence the solubility (Griffin & Chou,
1981a).
PBBs are quite soluble in organic solvents, such as dioxane,
carbon tetrachloride, acetone, and methanol. This could play a major
role in soil environments where leachates from chemical waste
disposal sites are percolating.
The other important factor affecting the migration of PBBs,
i.e., their adsorption by soils, was also studied under laboratory
conditions. The hydrophobic properties of PBBs make them easily
adsorbed from aqueous solutions onto soils. Filonow et al. (1976)
examined the adsorption of purified 2,2',4,4',5,5'-hexabromobi
phenyl (BB153) on four soil types. They found that the adsorption of
2,2',4,4',5,5'-hexabromobiphenyl conformed well to Freundlich
adsorption isotherms, and that 2-19% of the available HBB was
adsorbed. Adsorption of HBB was influenced primarily by the organic
content of the soils. An increase in the organic matter content of
soils enhanced their adsorption capacity.
Neither percentage clay nor pH correlated well with BB153
adsorption. Any effect that the clay contents may have had, was
apparently masked by the effect of the organic contents on
adsorption (Filonow et al., 1976).
Griffin & Chou (1981a,b), using the PBB-mixture FireMaster(R)
BP-6 or 14C-labelled-PBB, also confirmed the strong adsorption of
PBBs on soils and indicated a very high direct correlation between
the total organic carbon content (TOC) of three different soils and
the amounts of PBBs adsorbed. However, they pointed out that, in
soils with a low TOC, the mineral fraction may contribute markedly
to the adsorption capacity.
Furthermore, preferential adsorption of PBB congeners and
isomers was noted, depending on the characteristics of the
adsorbent, e.g., organic content (Griffin & Chou, 1981a), as well as
on the degree and position of bromine substitution (Griffin & Chou,
1980, 1981b).
No measurable adsorption on soils occurred of PBBs from organic
solvents (Griffin & Chou, 1981a).
The results of migration studies were in agreement with the
findings discussed above. The mobility of PBBs in five soils was
measured with several leaching solvents, using a thin-layer
chromatography technique and column leaching studies (Griffin &
Chou, 1981a,b). PBBs remained immobile in the soils when leached
with water or landfill leachate, but were highly mobile when leached
with organic solvents. Mobility was directly proportional to the
solubility in the leaching solvents and inversely proportional to
the soil total organic content.
On the other hand, because PBBs are bound to soil, wherever
contaminated soil moves, whether through wind or water erosion or
animal ingestion and migration, traces of PBBs (if present) can be
expected to be found (Jacobs et al., 1978).
4.1.4 Biota
PBBs are stable and persistent, lipophilic, and only very
slightly soluble in water; they are poorly metabolized, and
therefore accumulate in lipid compartments of biota. Once they have
been released into the environment they will reach the food chain,
where they are concentrated. Fish and wildlife are the most
consistent targets for such contamination, but livestock and humans
may also become contaminated (Kimbrough, 1980a). The precise routes
and transport mechanisms of PBBs travelling through biota have not
been thoroughly investigated as pointed out below.
4.1.4.1 Terrestrial ecosystems
Several studies have been concerned with whether plants in
terrestrial ecosystems would take up, translocate, and introduce
PBBs into the food chain. Jacobs et al. (1976) selected orchard
grass (Dactylus glomerata) as test plants in their greenhouse
studies because of its extensive root mass, and carrots (Daucus
carota), which, according to Iwata et al. (1974), have an
outstanding ability to absorb pesticide residues from the soil. They
did not detect any PBBs in the tops of either species grown in soils
supplied with high levels of PBBs (10 or 100 mg/kg of
FireMaster(R) BP-6). However, they did find traces of PBBs
(20-40 µg/kg) associated with carrot roots. 14C-uptake studies
(autoradiography and GC-analysis) on corn and soybean seedlings
grown in hydroponic solutions and on three root crops (radishes,
carrots, and onions) grown in two different soils, also showed no
translocation of PBBs into plant tops (Chou et al., 1978). In
addition, these authors found that the amount of PBBs associated
with roots depended on plant species and the clay and organic matter
contents of the soil. Roots of carrots contained more PBBs than
those of radish or onion bulbs; all roots had higher levels of PBBs
(50-500 µg/kg tissue) when grown in a high-PBB treatment soil
(100 mg/kg) with lower clay and organic content, than they did
(30-120 ng/g plant tissue) in a soil containing more clay and
organic matter. Furthermore, PBBs seem to be localized on the
surfaces of roots, because a significant portion of 14C-PBBs was
removed, when the roots were dipped in acetone.
Analyses of field samples from plant tissues of corn, alfalfa,
and sudax, grown on Michigan fields with soil PBB levels ranging
from 9 to 371 µg/kg, resulted in no detectable (detection limit:
0.3 µg/kg) PBB (Jacobs et al., 1978). The same was true for washed
radishes from a garden with an estimated PBB concen tration of
500-1000 µg/kg and for corn leaf whorls containing dust from a PBB
contaminated soil (102 µg/kg) (Chou et al., 1978).
However, Stratton & Whitlock (1979), who conducted a field
screening survey near sites of manufacture and use of PBBs, found
high surface contamination of lichens and reeds.
The salt marsh cordgrass (Spartina alterniflora) is reported
to take up, accumulate, and transfer effectively PCB from
contaminated sediments to food chains (Mrozek et al., 1982). No data
are available with regard to PBBs.
So far, except for the surface contamination of roots from
contaminated soils and of foliage via air deposition processes,
plants are generally free of significant amounts of residue. Thus,
vegetation on PBB-contaminated soils is a less likely source of
contamination of animals (Damstra et al., 1982; Fries, 1985a,b).
In contrast, a major route of residue transmission from soils
to animals is the direct ingestion of soil (Fries 1982; 1985a). The
degree of contamination depends on the amount of soil ingested and
the bioavailability of the residues.
Quantitative data on soil ingestion by farm animals are given
by several authors (Healy et al., 1967; Healy, 1968; Fries, 1982;
Fries et al., 1982a,b) and range from 2 to 15% of the intake of dry
matter.
Fries (1985a) determined the bioavailability of soil-borne PBBs
in sheep, under controlled feeding conditions, using diets
containing 5% PBB-contaminated soil, and found 65% PBB absorption
from this diet, which contained 9 µg PBB/kg. Addition of activated
carbon to soil had only little effect on bioavailability of PBB.
The same author recorded PBBs in the fat of beef cows, beef
calves, ewes, and pigs from several farms on which soil-borne PBBs
in confinement areas was the only source of PBBs. It can also be
concluded from these results that the animals consumed soil, and
that soil-borne PBB was bioavailable. As might be expected, pigs
accumulated higher PBB concentrations from a soil environment than
ruminants (Fries, 1985a). Recontamination of soil by animal excreta
(Getty et al., 1977; Fries, 1985a) or carcasses (Shah, 1978) also
occurred.
Recently, PBBs have been detected in European herbivorous
mammals (Swedish reindeers: Jansson et al., 1992; German cows
(milk): Krüger, 1988) (see also sections 5.1.4 and 5.1.6).
Despite the affinity of PBBs for soil, there are no
investigations on the role of the soil fauna in the transfer of
PBBs. Earthworms are of great ecological importance and might be
expected to take up and accumulate PBBs as has been ascertained for
PCBs (Diercxsens et al., 1985) and, thus, introduce them into the
food chain.
4.1.4.2 Aquatic ecosystems
PBBs enter the aquatic food chains via water and food. Bacteria
and plankton play an important role in the accumulation and
translocation of PCBs to higher trophic levels (Kalmaz & Kalmaz,
1979; Lorenz & Neumeier, 1983). According to Falkner & Simonis
(1982), sorption processes probably control uptake and accumulation
of PCBs by phytoplankton, because of its high surface-volume ratio.
These mechanisms could also be valid for PBBs. However, Stratton &
Whitlock (1979) did not find PBBs in algae collected in the vicinity
of industrial sites, where PBB concentrations of sediments ranged
from 20 to 60 µg/kg and where captured fish contained 220-230 µg
PBB/kg (detection limit: not given).
No information on the uptake of PBBs from sediment through
bottom living organisms (e.g., mollusca or oligochaete worms) is
available.
In contrast, several laboratory (Norris et al., 1973; Zitko &
Hutzinger, 1976; Zitko, 1977; Sugiura et al., 1978) and field (Hesse
& Powers, 1978; Stratton & Whitlock, 1979; Jaffe et al., 1985)
studies on fish have been conducted. They confirm PBB uptake from
water and food, with the exception of hepta- and octabromobiphenyl
(Norris et al., 1973; Zitko, 1977), which were not taken up from
water.
Consequently, ingestion of fish is a source of PBB transfer to
mammals and birds. Because of the possible selective accumulation
and metabolism of PBB congeners in prey, it can be expected that
predators will be subjected to a somewhat different PBB congener
composition than that found in the surrounding media (sediment,
water, etc.).
In natural situations, food chains become linked together in
complex food webs, and PBBs are distributed in the corresponding
manner.
PBBs have been detected in other species of wildlife besides
fish, e.g., in ducks living near contaminated waters (Hesse &
Powers, 1978), in a turtle (Stratton & Whitlock, 1979), in the eggs
of waterbirds (Haseltine et al., 1981; Heinz et al., 1983, 1985), in
eagles (Kaiser et al., 1980), and in marine mammals (Jansson et al.,
1987, 1992; Krüger, 1988; Kuehl et al., 1991) (see also section
5.1.6).
4.1.4.3 Accidental contamination of the food chain
A special case of entrance of PBBs into the food chain occurred
accidentally in 1973 in Michigan, when FireMaster(R) FF-1 was
inadvertently substituted for magnesium oxide as a supplement in the
formulation of cattle feed (Damstra et al., 1982). Ten to twenty
bags, 22.8 kg each, of PBBs (Carter, 1976) were mixed into feeds,
that were widely distributed to Michigan farmers.
In addition, feeds not formulated to contain magnesium oxide
also became contaminated (with relatively low concentrations)
because of carryover of PBBs from batch to batch in the mixing
equipment (Dunckel, 1975) and, on farms, through the recycling of
contaminated products (Kay, 1977). Distribution of contaminated
antibiotics, e.g., aureomycin, also contributed to the introduction
of PBBs into farm animals (Di Carlo et al., 1978).
The mixing error was not discovered immediately, and it was
almost a year before analyses indicated that a compound of PBB was
involved in the illness or death of farm animals (Getty et al.,
1977). During this time (IARC, 1978; Zabik, 1982), contaminated
animals and their produce entered the human food supply and the
environment of the state of Michigan. Hundreds of farms were
affected. Altogether, at least 29 800 cattle, 5920 pigs, 1470 sheep,
and 1.5 million chickens had been killed and buried by the end of
1975 (Robertson & Chynoweth, 1975; Carter, 1976), in order to
minimize further human exposure. In addition, at least
785 thousand kg of feed, 8185 kg of cheese, 1197 kg of butter,
15 500 kg of dried milk products, and nearly 5 million eggs were
destroyed (Carter, 1976). The number of animals quarantined or
contaminated below quarantine level was estimated to be several
thousands (Isleib & Whitehead, 1975). Although the Michigan PBB
episode was primarily an incident of feed contamination, it also
resulted in secondary contamination of animals from contaminated
soil (Fries, 1985a).
4.2 Degradation
Compounds like PBBs are very stable to hydrolysis, chemical
oxidation, and thermal decomposition. Degradation by purely abiotic
chemical reactions (excluding photochemical reactions) is therefore
considered an unlikely environmental sink (Pomerantz et al., 1978;
Pearson, 1982).
The persistence of PBBs under actual field conditions is
reported in some publications. Jacobs et al. (1976) detected PBBs in
soils from a field that had received manure from a
FireMaster(R)-contaminated dairy herd 10 months earlier.
Follow-up surveys over a three-year period following the
termination of PBB production showed no significant decline in PBB
levels in sediments from the Pine River (Hesse & Powers, 1978). Soil
samples from the former PBB-manufacturing site in St. Louis,
Michigan, analysed several years (nearly ten years?) after
contamination (during the early 1970s) still contained PBBs.
However, the PBB congener composition differed from that of the
original FireMaster(R) mixture, indicating a partial degradation
of the PBB residue in the soil sample (Hill et al., 1982).
The chemical Inspection and Testing Institute, Japan (1987) has
listed decabromobiphenyl as non-biodegradable.
The most probable degradation mechanisms of PBBs in the
environment, if there is any degradation at all, are
photodecomposition and microbial degradation.
4.2.1 Photolytic degradation
Under laboratory conditions, PBBs were easily degraded by UVR.
The photoreactivity of PBBs has been used to confirm PBB residues
(Erney, 1975; Trotter, 1977). The predominant photochemical reaction
of PBBs in organic solvents was a reductive debromination.
Irradiation of 4-monobromobiphenyl at 300 nm in various polar and
nonpolar solvents led to the formation of biphenyl as the sole
product (Freeman et al., 1991). Earlier studies using lower
brominated PBB congeners (i.e., tetra and lower) reported a
preferential loss of ortho bromines (Bunce et al., 1975; Ruzo
et al., 1976). Irradiation of higher brominated congeners yielded a
series of photoproducts (Table 18), but a stepwise cleavage of
orthobromines did not appear to be preferred to meta or para
debromination (Patterson et al., 1980; Millis & Aust, 1985).
The photoreactivity of 2,2',4,4',5,5'-hexabromobiphenyl, the
main component of FireMaster(R), was consistently found to be
relatively high (Andersson et al., 1975; Ruzo et al., 1976;
Robertson et al., 1983a; Millis & Aust, 1985), and degradation
occurred more rapid than with the hexachloro analogue (Andersson
et al., 1975; Ruzo & Zabik, 1975).
Consistent with the dehalogenation pathway, photodegradation of
the commercial FireMaster(R) mixture led to reduced concentrations
of the more highly substituted PBB congeners (De Kok et al., 1977;
Robertson et al., 1981b, 1983; Epling et al., 1987). Robertson
et al. (1983a) examined changes in the composition of
FireMaster(R) BP-6 during photolysis (300 nm for 2-12 h; solvent:
cyclohexane) by monitoring 25 individual PBB congeners; they also
did not find a preferential loss of ortho bromines. Nevertheless,
the photoproducts of FireMaster(R) did contain increased
concentrations of congeners possessing no ortho bromines (e.g.,
3,4,4'-tri-, 3,3',4,4'-tetra-, 3,3',4,4',5-penta bromobiphenyl).
Moreover, other congeners, known as relatively toxic (e.g.,
2,3',4,4',5-pentabromobiphenyl), were enriched (Robertson et al.,
1983). Biphenyl, the ultimate product of the debromination pathway,
was found only to a small extent after the photolysis of
FireMaster(R) BP-6 (Epling et al., 1987).
Table 18. Photodegradation of higher brominated PBB congeners under laboratory conditions
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,5,5'- 254 nm hexane 43.4a 2,3',4',5-tetra ortho-debromination Millis & Aust
penta (up to 100 (minor product) (1985)
min) 2,2',4,5'-tetra meta-debromination
2,2',5,5'-tetra para-debromination
(major product)
(additional production
of a yellow gum)
2,3',4,4',5- 254 nm hexane 50a 2,3',4',5-tetra para-debromination Millis & Aust
penta (up to 90 3,3',4',4'-tetra ortho-debromination (1985)
min)
2,2',4,4',5,5'- 366 nm methanol not specified lower brominated PBBs degradation (90% after 9 Andersson et al.
hexa (main products) min) more rapid than with (1975)
BB 153 methoxy- PBBs (minor the hexachloro analogue
products)
> 300 nm hexane not specified lower brominated PBBs BB 153 was 24.4 times Ruzo et al.
(0.5-2 h) quaterphenyls (< 5%) more reactive than (1976)
4,4'-dibromobiphenyl
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5,5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
Table 18 (contd).
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5.5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
secondary photoproduct:
3,3',4,4'-tetra
formation of yellow
gum at 25 min
2,2',3,4,4',5,5'- sunlight not not 2,2',4,4',5,5'-hexa meta-debromination Patterson
hepta (390 min) specified specified (major product) et al. (1980)
2,3',4,4',5,5'-hexa ortho-debromination
2,2',3,3',4,4', sunlight not not unidentified hexa- Patterson
5,5'-octa (300 min) specified specified PBB (major product) ortho- and meta- et al. (1980)
2,3',4,4',5,5'-hexa debromination
2,2',3,3',5,5', 300 nm hexane not specified di- to Ruzo et al.
6,6'-octa (0.5-2 h) heptabromobiphenyls, ortho debromination (1976)
e.g., 3,3',5,5'-tetra
a Original PBB concentration = 1.59 mmol/litre.
Technical octabromobiphenyl has been reported to photo degrade
in xylene by reductive debromination with a half-life of 40 h
(Norris et al., 1973).
There were investigations to enhance the photochemical process
aiming at a potential technique for the breakdown and removal of
PBBs from the environment. In laboratory testing, photodegra dation
of PBBs was accelerated in the presence of ethylenediamine and
tertbutylamine (Christensen & Weimer, 1979) and in the presence of
sodium borohydride (Epling et al., 1987).
Epling et al. (1987) obtained high yields of biphenyl during
borohydride enhanced photolysis of FireMaster(R) BP-6 (irradiation
under nitrogen at 254 nm; solvent: 90% acetonitrile/water).
The rates and extent of photolytic reactions of PBBs in the
environment have not been determined in detail. However, the few
field observations available indicate a high persistence of the
original PBBs (Jacobs et al., 1978) or a partial degradation to less
brominated (and often more toxic) photoproducts (Hill et al., 1982).
Jacobs et al. (1978) examined field soil that had received manure
from FireMaster(R)-contaminated cattle, for the first time, 2-3
years earlier. They did not detect any significant changes in the
relative concentrations of the major PBB peaks (Br5, Br6, Br7)
compared with the FireMaster(R) standard. In contrast, soil
samples, obtained from the former FireMaster(R) manufacturing site
in Michigan and analysed several years (approximately 10 years?)
after contamination, contained enhanced concentrations of possible
photodegradation products including 2,3',4,4',5-pentabromobiphenyl,
2,2',4,4',5-pentabromobiphenyl, and two unidentified
tetrabromobiphenyls (Hill et al., 1982).
Considering the diversity of microenvironments, both laboratory
and field data on photo alteration of PBBs are incomplete; there is
a lack of studies on the photochemistry of PBBs in water, or in the
vapour or solid states.
4.2.2 Microbial degradation
In laboratory investigations, mixtures of PBBs appear to be
fairly resistant to microbial degradation. Soil incubation studies
using FireMaster(R) BP-6 (lot no. 6244A) and 14C-PBB
(lot 872-244) showed a little, but not significant, degradation of
the major hexa- and heptabromobiphenyl congeners after 6 months or
1 year; only pentabromobiphenyl was assumed to degrade slowly
(Jacobs et al., 1976, 1978). These results were deduced from
recovery rates of PBBs from soil, 14CO2 production, and the lack of
14C-PBB intermediates.
Soils incubated with photodecomposition products of 14C-hexa
and heptabromobiphenyl caused enhanced, but still minor, degradation
(ca. 3%) as measured by 14CO2 production (Jacobs et al., 1978).
These findings are consistent with observations according to which
degradation of PCBs by bacteria increases with decreasing
chlorination (Kalmaz & Kalmaz, 1979; Fries, 1982).
In further incubation experiments with FireMaster(R) BP-6
(lot no. 6244A) in sterilized and nonsterilized Catlin-soil, Griffin
& Chou (1981a) measured the recoveries of penta-, hexa-, and
heptabromobiphenyls and found that all PBBs persisted for 6 months
with no significant microbial degradation. They observed the same
kind of persistence over a period of 4 weeks in PBB incubations with
mixed cultures of microorganisms (predominantly Alkaligenes
odorans, A. denitrificans, and an unidentified bacterium). This
culture had been isolated previously and was known to degrade
water-soluble PCBs (Clark et al., 1979). No PBB metabolites were
found in the PBB-saturated mineral solution after 4 weeks of
incubation (Griffin & Chou, 1981a).
As with PCBs, the high degree (penta or greater) of halogen
substitution of its major components probably accounts for the lack
of degradation of the FireMaster(R)-mixture (Griffin & Chou,
1981a). Congruently, biodegradation of monobrominated biphenyls has
recently been reported.
A soil isolate, strain S93B1, identified as Pseudomonas
cruciviae, could grow on more than ten biphenyl-related compounds
including o-bromobiphenyl (Takase et al., 1986). O-bromobiphenyl
was converted to o-bromobenzoic acid (Fig. 3) (identified by
IR-spectrum). This is analogous with some PCBs showing chlorinated
benzoates as metabolites (Ballschmiter et al., 1977). In these
experiments, biphenyl-related compounds 0.2-0.5% (w/v) were added as
the sole sources of carbon to the liquid artificial medium.
However, this pathway is also realized under simulated natural
conditions (aquatic environments), as reported by Kong & Sayler
(1983). They used river water as supportive culture medium and
"mixed bacterial cultures" (not identified), which were obtained
from PCB-contaminated river sediments. This mixed bacterial culture
was capable of degrading monohalogenated biphenyls.
Polybrominated benzenes and a possible methylbrominated furan
have also been reported to occur in FireMaster(R) (Brinkman & de
Kok, 1980).
Approximately 20 compounds, other than PBBs, were either
tentatively identified in FireMaster(R) or partially characterized
by Hass et al. (1978).
Polybromodibenzo- p-dioxins and polybromodibenzofurans were
searched for, because of their extreme toxicity and because
chlorinated dibenzofurans had been detected in commercial PCBs
(Nagayama et al., 1976). If present, their concentrations did not
exceed 0.5 mg/kg (Hass et al., 1978, O'Keefe, 1979). Polybromo
dibenzodioxins and polybromodibenzofurans were determined in a
sample of Adine 0102 (decabromobiphenyl). Monobromobenzo difurans
were present at a level of 1 mg/kg (1 ppm), otherwise all other
polybromodibenzodioxins and polybromodibenzofurans were present only
at less than 0.01 mg/kg (Atochem, 1990).
So far, phenoxyphenols and hydroxybiphenyls, which might be
intermediates in the formation of brominated dibenzo- p-dioxins and
brominated dibenzofurans, respectively, have not been identified
(O'Keefe, 1979).
Some impurities in PBBs result from impurities in the original
biphenyl material. According to two major manufacturers, their
biphenyl grade used for bromination contained less than 5 mg/kg and
5000 mg/kg, respectively, of impurities, e.g., toluene, naphthalene,
methylene biphenyl (fluorene), and various methyl biphenyls (Neufeld
et al., 1977).
2.2 Physical and chemical properties
In general, PBBs show an unusual chemical stability and
resistance to breakdown by acids, bases, heat, and reducing and
oxidizing agents (Safe, 1984).
PBBs can be compared chemically to the PCBs. Bromine, however,
is a better leaving group in chemical reactions than chlorine.
Unlike PCBs, the reactivity of PBBs has not been well studied and
documented in the literature (Pomerantz et al., 1978). Like PCBs
their chemical stability is dependent, in part, on the degree of
bromination and the specific substitution patterns (Safe, 1984). All
highly brominated PBB-mixtures are known to degrade rather rapidly
with UV irradiation (Brinkman & de Kok, 1980).
The technical mixtures typically are white, off-white, or beige
powdered solids. Some physical data on commercial PBB mixtures are
given in Table 9. It can be seen that there are discrepancies in the
values for the solubility of commercial PBBs in water (given in
Table 9) as well as those calculated for various PBB congeners
(Table 10). The source and quality of the water is important.
Determinations of water solubility of these very hydrophobic
compounds are also difficult to perform. Adsorption effects on
particles and glass surfaces may influence the results. PBBs were
found to be 200 times more soluble in landfill leachate than in
distilled water (Griffin & Chou, 1981a). In general, it can be said
that PBBs are only slightly soluble in water and that the solubility
decreases with increasing bromination.
For details of thermal decomposition, see section 4.3.2.
2.2.1 Physical and chemical properties of individual congeners
PBBs show a wide range of volatility (Farrell, 1980). Partition
coefficients between water/ n-hexane and water/1-octanol, as well
as aqueous solubilities for some individual PBB congeners are given
in Table 10. Correlations for predicting aqueous solubility and
partition coefficients for PBBs based on molecular structure have
been proposed (Patil, 1991). The solubility of PBBs in n-hexane
decreases rapidly with increasing bromine content (de Kok et al.,
1977).
Data on the melting points and UV absorption of individual PBB
congeners are summarized in Table 11. The main band in these spectra
is caused by pi -> pi* electron transitions, while the k band is
generally attributed to the conjugated biphenyl system with the
contribution of both biphenyl rings. With the k band, the
introduction of bromine atoms in positions meta or para to the
phenyl-phenyl bond induces a shift in kmax towards the visible
region, as is illustrated by 3,3',5,5'-tetra- and 3,3',4,4',5,5'-
hexabromobiphenyl. On the other hand, ortho substitution, which
causes a considerable hindrance for free rotation of the rings and,
thus, a loss in coplanarity, effects a sharp decrease in the
extinction coefficient of the k band (de Kok et al., 1977).
Data on NMR spectra are given by Orti et al. (1983), Robertson
et al. (1984b), and Kubiczak et al. (1989), and on mass spectrometry
(MS) by Erickson et al. (1980), Roboz et al. (1980), Buser (1986),
and Sovocool et al. (1987a,b). The "ortho" effect, observed for PBBs
and PCBs having 2,2'-; 2,2',6- or 2,2',6,6'- halogens can be
combined with GC retention index for isomer specific identifications
by gas chromatography and mass spectrometry (GC/MS) (Sovocool
et al., 1987a).
Table 9. Some physical data on commercial PBB mixturesa
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Melting point (°C) 72 200-250 220-290 380-386b
360-380
385
Lambda max (nm) 219e 225e 224e 227e
Density (g/cm3) at 2.6 - 3.2 3.2
room temperature
Solubility in water 11f 20-30 < 30
(µg/litre) at 25 °C 30f (pure 2,2', 4,4', 5,5'-)
610f
0.06g (deionized)
0.32g (distilled) insoluble
Solubility in organic
solvents (g/kg
solvent) at 28 °C
petroleum ether 20 18 insoluble in common
acetone 60 organic solvents
carbon tetrachloride 300 10e
chloroform 400
benzene 750 81
toluene 970
dioxane 1150
copra oil (37 °C) 0.8
Table 9 (contd).
"Hexabromobiphenyl" "Octabromobiphenyl" "Nonabromobiphenyl" "Decabromobiphenyl"
(Firemaster BP-6) (Dow XN 1902) (Bromkal 80-9D)c (Adine 0102)d
Vapour pressure (Pa)
25 °C 0.000007h < 0.000006
90 °C 0.01 (temperature not given)
140 °C 1
220 °C 100
Volatility (% weight loss) < 1% at 250 °C 1-2% at 300 °C < 5% at 341 °C
< 10% at 330 °C < 10% at 363 °C
< 50% at 350 °C < 25% at 388 °C
log Pow < 7 (calculated)d 8.6 (calculated)
Decomposition temperature 300-400 °C 435 °C 435 °C 395 °C
> 400° C
a Mumma & Wallace 1975).
b Norris et al. (1973).
c Kerscher (1979); CFK (1982).
d Atochem (1990).
e Brinkman & de Kok (1980).
f Filonow et al. (1976).
g Griffin & Chou (1981a,b).
h Jacobs et al. (1976).
Table 10. Partition coefficients between water and n-hexane (KHW) and 1-octanol
(Kow) and aqueous solubilities (Sw) for some individual PBB congeners (all
aqueous solubility measurements were carried out by the generator method)
Log KHW Log KOW Sw mol per Sw µg/litred
litre x 10-9 (25 °C)
(25 °C)
2-bromobiphenyl 4.59a
3-bromobiphenyl 4.85a
4-bromobiphenyl 4.96a 2800 650
3,5-dibromobiphenyl 5.78c
4,4'-dibromobiphneyl 5.61 5.72 18.4 5.7
2,4,6-tribromobiphenyl 6.21 6.03 41.1 16
3,4',5-tribromobiphenyl 6.42c
2,2',5,5',tetrabromobiphenyl 6.72 6.50 8.6 4
3,3',5,5'-tetrabromobiphenyl 7.42c
2,2',4,5,5'-pentabromobiphenyl 7.10a 0.8b 0.4
2,2',4,4',6,6'-hexabromobiphenyl 7.52 7.20 0.9 0.56
decabromobiphenyl 8.58a
Values from Gobas et al. (1988) with the exception of:
a From: Doucette & Andren (1987).
b From: Doucette & Andren (1988).
c From: Sugiura et al. (1978).
d calculated.
Table 11. Melting points and UV spectral data for some PBB congenersa
UV conditions: solutions in n-hexane; Beckman Acta CIII spectrometer
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
Biphenyl 71 201 4.66 246 4.26
1 2- e 201 4.51 240 3.90
2 3- e 205 4.60 248 4.21
3 4- e 200 4.67 254 4.38
4 2,2'- 81 198 4.64 220-230 e
9 2,5- e 203 4.49 226 4.38
15 4,4'- 164 201 4.64 261 4.43
20 2,4,6- 65-66 213 4.70 220-230 e
21 2,2',5- 78 200 4.66 235-245 e
26 2,3',5- e 213 4.57 e e
31 2,4',5- 78 205 4.60 245-255 e
49 2,2',4,5'- 84 207 4.66 235-245 e
52 2,2',5,5'- 143 204 4.67 235-240 e
80 3,3',5,5'- 188 220 4.76 255 4.18
114b 2,3,4,4',5- 128 222.6 (54.8) 258 e
137b 2,2',3,4,4',5- 124 223.1 (35.4) e e
141b 2,2',3,4,5,5'- 127 223.4 (191) e e
153 2,2',4,4',5,5'- (159-160)f 216 4.66 e e
156b 2,3,3',4,4',5- 178 224.9 (229) 259 e
159b 2,3,3',4,5,5'- 195 226.1 (61.4) 258 e
167 2,3',4,4',5,5'- (165-166)f e e e e
Table 11 (contd).
No.c PBB-isomer Melting point Main band k band
(°C)d lambda Log lambda Log
maximum epsilon maximum epsilon
(nm) (1.mol-1.cm-1) (nm) (1.mol-1.cm-1)
169 3,3',4,4',5,5'- 248 227 4.76 272 4.34
180b 2,2',3,4,4',5,5'- 166(165-166)f 224.1 (62.4) e e
189b 2,3,3',4,4',5,5'- 219 230.7 (102) 265 e
194b 2,2',3,3',4,4',5,5'- 235(232-233)f 223.7 (51.6) e e
202 2,2',3,3',5,5',6,6'- e 224 4.85 e e
206b 2,2',3,3',4,4',5,5',6- 262(263-264)f,g 225.2 (131) e e
Nona-(unidentified) e 225 5.18 e e
Deca- 378 227 5.11 e e
a Adapted from: de Kok et al. (1977), with the exception of the congeners marked with b.
b Congener data, including melting points are taken from Kubiczak et al. (1989). UV measurements: in n-heptane.
c No. according to Ballschmiter & Zell (1980).
d Melting points from Sundström et al. (1976b) but confirmed by de Kok et al. (1977), unless otherwise stated.
e No data available.
f From: Moore & Aust (1978).
2.3 Conversion factors for PBB in air
1 ppm = 26.1 mg/m3 for hexabromobiphenyl at 20 °C and
101.3 kPa.
1 mg/m3 = 0.038 ppm.
2.4 Analytical methods
Analytical methods for the determination of PBBs, which have
been reviewed by de Kok et al. (1977), Pomerantz et al. (1978), and
Fries (1985b), were adapted from established methods for chlorinated
hydrocarbon insecticides and PCBs (AOAC, 1975). The chronological
development of analytical methods for the detection and
quantification of PBB mixtures and congeners is summarized in
Table 12. In the wake of the Michigan disaster, methods were
described for the analysis of: contaminated feed, milk, and milk
products (Fehringer, 1975a,b); animal blood plasma, faeces, milk,
and bile (Willett et al., 1978) and liver and fat (Fawkes et al.,
1982). The methods were developed using tissue from animals fed with
PBBs of known composition. Needham and coworkers developed a method
to determine PBBs in human blood serum (Burse et al., 1980; Needham
et al., 1981) which was thoroughly tested in several laboratories,
but, even here, only the main components of FireMaster(R) were
determined. Similarly, the investigation by Eyster et al. (1983)
into the levels of PBBs in fat, serum, faeces, milk, and placenta
were not isomer specific. Thus, reported values may not reflect the
hazard of the residue because, for example, some congeners are more
toxic than the prominent 2,2',4,4',5,5'-HBB. Most samples of
biological origin have congener distributions that differ from those
of the original material (Fries, 1985b).
Concentrations of PBBs as low as 10 µg/kg in fatty foods
(Fehringer, 1975a), 3 µg/kg in dry feeds (Fehringer, 1975b) and
1 µg/litre in blood serum (Needham et al., 1981) can be detected and
quantified using routine methods. Coefficients of variation become
large as concentrations approach the limits of sensitivity of the
method; thus, values near the limit must be treated with caution
(Fries, 1985b). PBBs adsorb to glass more tenaciously than other
halogenated hydrocarbons, and are not easy to remove by the usual
cleaning methods (Willett et al., 1978). This can lead to erroneous
values, particularly when concentrations in samples are low and
there is a carry over from samples of high concentration. This
problem can be solved by using disposable glassware (Willett et al.,
1978).
Table 12. Analysis of commercial mixtures and individual PBB congeners: A chronological surveya
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) recrystallization GC FID no data identification of Sundström
BP-6 from ethanol/ given BB 153 and a HpBB as et al.
isopropanol major components (1976a)
PBB congeners no data given GC ECD no data routes of synthesis, Sundström
given melting points, relative et al.
retention times, electron (1976b)
capture responses for
some PBB congeners
Commercial - solubility of HPLC, TLC, MS no data survey of analysis for de Kok
mixtures FR 250 13A PBBs in n-hexane UV, GC given PBBs et al.
(octabromobipheyl) decreases rapidly with 1H- & 13C-NMR (1977)
FireMaster(R) BP-6 increasing bromine
and PBB congeners content; PBBs dissolved
in warm CCl4
Commercial sample hexane GC, 13C-NMR, ECD no data identification of Moore
of 1H-NMR, IR given BB 180 et al.
octabromobiphenyl heptabromobiphenyl (1978)
FireMaster(R) methylene chloride; GC, NMR, MS 0.5 mg/kg contains at least 13 Hass
BP-6 hexane HPLC SIM different PBBs and et al.
bromonaphthalene (no (1978)
bromodibenzofurans or
bromodibenzo-p-dioxins
found)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC, NMR MS no data purification and Moore &
FF-1 or BP-6 and given structural characterization Aust
octabromobiphenyl of 6 further PBB congeners (1978)
FireMaster(R) hexane GC ECD 0.03 ng absolute and relative Domino
FF-1 retention times of the 8 et al.
major constituents using (1980a)
tetrabromobiphenyl
as an internal standard
FireMaster(R) hexane GC MS no data mass spectra of major Domino &
FF-1 given PBBs in FireMaster; mixed Domino
poly-bromo and (1980)
chlorobiphenyls detected
FireMaster(R) GC ECD no data comparison of packed and Farrel
BP-6 given capillary columns; solves (1980)
some problems with lower
brominated biphenyls, but
has no great advantages
over packed columns for
more highly substituted
biphenyls
FireMaster(R) no data given GC PED 2.8 mg comparison with ECD; not Mulligan
BP-6 (cf 1.5 ng quite so sensitive, but et al.
ECD) is selective (1980)
Individual PBB toluene GC MS, SIM < 1 ng mono-deca PBB congeners Erickson
congeners et al.
(1980)
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
22 individual hexane GC ECD, micro- 40 pg retention times given Sweetman &
PBBs FireMaster(R) (preceeded coulometric for 23 congeners response Boettner
FF-1 by HPLC) GC-detector increases with degree of (1982)
MS bromination, increased
detection temperature gives
improved sensitivity
FireMaster(R) carbon preparative FID, MS polar and unpolar hexane Needham
FF-1 tetrachloride; HPLC and fractions were also et al.
hexane GC 1H-NMR tested for hyperkeratotic (1982)
activity
FireMaster(R) hexane GC NCI, SIM 0.6 ng evaluation of halogen anion Greaves
BP-6 formation by polybrominated et al.
compounds in NCI-MS; SIM of (1982)
bromine anions has greater
specificity than ECD
FireMaster(R) fractionation by GC ECD, MS no data seven congeners were Dannan
BP-6 preferential given purified et al.
acetone (1982d)
solubilization,
repeated
crystallization,
alumina
adsorption
column
chromatography,
reversed phase
Lipidex-500
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) see Needham et al. preparative FID, MS at least 60 components Orti
FF-1 (1982) HPLC, GC, observed; isolated/ et al.
lot FH 7042 GC 1H-NMR determined structure of (1983)
10 minor components of
FireMaster (most are very
polar, later eluting
fractions)
PBB hexane GC helium 230 pg simultaneous monitoring Eckhoff
(unspecified) plasma of 4 atomic emission et al.
atomic wave-lengths; PBB mentioned (1983)
emission
spectrometric
detection
FireMaster(R) no data given GC MS, ECD identity of over 91% of Robertson
BP-6 1H-NMR PBB components in et al.
FireMaster using 22 (1984b)
individual PBB congeners
as standards; identification
of 7 additional PBBs
including 3 very toxic
coplanar PBBs
FireMaster(R) hexane GC PED, rapid multi-element quantification Zerezghi
BP-6 scanning et al.
plasma (1984)
emission
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
FireMaster(R) hexane GC SIM, MS determination of suspected Tondeur
FF-1 toxic impurities et al.
(1984)
PBB photolysis hexane GC FID, ECD, purification of PBB congener Barnhart
mixture MS 2 using charcoal pretreatment et al.
and RPLC (1984)
Benzenes, hexane GC NCI-MS 0.1 pg especially valuable for Buser
biphenyls measuring trace levels in (1986)
dibenzodioxins, biological and environmental
dibenzofurans, samples; must be two Br;
diphenylethers, structural information is
benzofurans, partly lost
phenols
PBB congeners no data given GC MS no data use of 'ortho' effect for Sovocool
given PBB and isomer & Wilson
identification; accurate (1982);
structure assignments Sovocool
without use of multiple GC (1987a)
determinations
Various PBB hexane GC, HPLC FID no data relationship between Höfler
congeners given recorded retention data et al.
from HPLC and GC and (1988)
molecular surface area
Table 12 (contd).
Sample Solvent Analytical method Detection Detection Comment Reference
limit
Nine synthetic products purified GC MS 1 ng synthesis of 2,3,4,5- Kubiczak
PBBs; by alumina/Florisil; substituted PBBs and et al.
FireMaster(R) recrystallization characterization (1989)
FF-1 and BP-6 from methanol or
methylene chloride
Mono- and no data given 1H-NMR, no data no data Anklam
poly-brominated 13C-NMR given given (1989)
biphenyls
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NMR = Nuclear magnetic resonance.
FID = Flame ionization detector. PED = Microwave-induced plasma emission detector.
GC = Gas chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
GPC = Gel permeation chromatography. RPLC = Reverse-phase liquid chromatography.
HPLC = High pressure liquid chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatograpy.
MS = Mass spectrometry. Unitrex = Universal Trace Residue Extractor.
NAA = Neutron activation analysis. UV = Ultraviolet.
NCI = Negative ion chemical ionization mass
spectrometry.
Recovery of PBBs using established methods is in the range of
80-90% (Fries, 1985b). The solvent system that is used for sample
extraction can affect recovery. Poor recoveries were often found
with hexane but the optimal solvent conditions depend on the source
of the medium sample.
For extraction conditions see Table 13 (environmental samples),
Table 14 (food/feed), Table 15 (biological tissues and fluids (a)
serum/blood (b) adipose and other tissues).
In soil, Griffin & Chou (1981a) found that a polar organic
solvent was important and obtained the best results with
hexane/acetone (9:1).
For serum and blood, the standard extraction method given by
Burse et al. (1980) has been used by most workers.
Extraction of PBBs from adipose and other tissues presents
greater problems. PBBs are readily soluble in fat. They can
therefore be extracted with the fat out of the tissue/sample but,
afterwards, an intensive clean-up procedure for PBBs is necessary.
Various methods, such as adsorption chromatography with Florisil,
gel permeation chromatography, Florisil cartridges (Chiang et al.,
1987), and Unitrex (Head & Burse, 1987) have been proposed.
The sample extraction and clean-up techniques for the
determination of PBBs are similar to those used for PCBs (Krüger
et al., 1988; Jansson et al., 1991). The lipids can be removed from
the extract by gel permeation (Krüger, 1988) or by hydrolysis
(Jansson et al., 1991). Usually PBBs and PCBs are separated from
more polar compounds by adsorption chromatography on silica gel or
Florisil. If the coplanar compounds are to be determined, they have
to be isolated from the major compounds in the extract. This can be
done using activated charcoal, which adsorbs the planar molecules
more strongly than the non-planar. Brominated naphthalenes, dioxins,
and furans will also be separated from the major PBB components in
this step. HPLC methods are now being adopted for these separations
and both charcoal and modified silica gel columns are available for
HPLC separations of coplanar compounds.
Table 13. Determination of PBBs in environmental samplesa
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
Soil, grass, benzene/ Florisil GC ECD, FID 0.1 µg/kg BB 153, two PeBB Jacobs
carrots 2-propanol MS dry weight (soil) isomers, three et al.
13C-NMR 10 µg/kg additional HxBB (1976)
wet weight (plant) isomers, two HpBB
isomers detected
Soil leachate benzene/ GC ECD 0.1 µg/kg laboratory experiments Filonow
2-propanol dry weight et al.
(1976)
Soil, plant hexane/ Florisil GC ECD 0.1 µg/kg field and laboratory Jacobs
samples acetone dry weight (soil) experiments; no et al.
TLC ECD 0.3 µg/kg significant (1978)
wet weight (plant) degradation of PBBs
after 1 year
Effluent river hexane/ no data GC ECD 0.1 µg/litre environmental Hesse (1975)
water diethyl given (later 0.01 µg/ samples Hesse &
ether litre) Powers
(1978)
Sediment hexane/ no data GC ECD 100 µg/kg environmental Hesse &
acetone given samples Powers
(1978)
Soil hexane/ no data GC ECD, FID, separation of 30 PBB Stratton &
acetone 9:1 given MS congeners tested Whitlock
optimum conditions (1979)
Table 13 (contd).
Matrix Extraction Clean up Analytical Detection Detection limit Comment References
method
for extraction of
PBBs from soil;
polar organic solvent
important
98 environmental hexane, Florisil GC MS 0.2 µg/kg analysed for hexa-, Stratton
samples Soxhlet hepta-, octa-, nona-, et al.
(fish, sediment, decabromobiphenyls; (1979)
soils, HxBB in 84% of samples
vegetation)
Soil, sediment, hexane Florisil GC MS (SIM) 0.2 µg/kg congeners detected Griffin &
sludge, Chou (1981a)
vegetation
Soil hexane/ Florisil GC FID, ECD degradation of PBBs Hill et al.
acetone 1:1 in soil (1982)
Sewage sludge hexane/ TLC IR, NMR, MS 10 ng/kg no PBBs found Strachan
methanol GC et al.
Soxhlet (1983)
extraction
Plants cut, Florisil GC ECD 0.3 µg/kg no translocation Chou et al.
extracted wet basis in plants (1978)
with hexane/
acetone
Table 13 (contd).
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. MS = Mass spectrometry.
FID = Flame ionization detector. NMR = Nuclear magnetic resonance.
GC = Gas chromatography. SIM = Selected ion monitoring.
IR = Infrared radiation. TLC = Thin layer chromatography.
Table 14. Determination of PBBs in food/feeda
Matrix Extraction Clean up Analytical Detection Detection Comment References
method limit
Dairy fat extracted by AOAC GPC, 25% toluene GC ECD 7 µg/kg comparison Fehringer
products (1975) methods in ethyl acetate of methods (1975a)
(methanol/ether)
Florisil/pet ether TLC 0.2 mg/kg
Dry animal finely ground feed Florisil/pet ether GC, TLC ECD 8 µg/kg hexabromo Fehringer
feeds packed into a column 30 µg/kg isomer (1975b)
containing celite, measured
elution with methylene
chloride
Feeds and see Fehringer GC before and ECD 5 µg/kg confirmation Erney
dairy (1975a,b) after UV irradi- of PBB (1975)
products ation to determine residues
background using UV
irradiation
a Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. TLC = Thin layer chromatography.
GC = Gas chromatography. UV = Ultraviolet.
GPC = Gel permeation chromatography.
Table 15. Determination of PBBs in biological tissues and fluidsa
Matrix Extraction Clean up Detection Detection limit Comment References
a) Serum/blood
Human serum methanol-treated serum, Florisil ECD 5 pg analysis based on HxBB Bekesi et al.
extraction with hexane peak (1978)
Human serum methanol-treated serum, Florisil ECD 0.2 µg/litre Wolff et al.
extraction with hexane/ (1978)
ether
Human/rat serum methanol-treated serum, Florisil ECD 0.2 µg/litre PBB homologues as % HxBB Wolff &
extraction with hexane/ peak Aubrey
ether (1978)
methanol-treated serum, Florisil ECD, MS < 1 mg/ml Wolff et al.
extraction with hexane/ (1979a)
ether
Plasma from multiple extraction with Florisil ECD 0.001 µg/litre recovery 96% Willett
PBB-fed cows mixture of diethyl and pet. et al.
ethers (1978)
Human serum methanol-treated serum; Florisil ECD 0.1 µg/litre interlaboratory comparison Burse et al.
extraction with hexane/ (1980)
ether
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Plasma, white methanol; precipitated Florisil MS-SIM 0.1 µg/mg very exact details with Roboz et al.
cell fraction protein removed; NCI protein review spectra (1980)
erythrocytes extraction with hexane/
ß-lipoprotein ether (1:1)
Human serum methanol; hexane/ silica gel ECD 1 µg/litre Needham
diethylether (1:1) et al.
(1981)
Human serum + methanol precipitated Florisil ECD < 1 µg/litre serum protein precipitated Roboz et al.
protein not removed + MS-NCI with methanol should not (1982)
hexane/diethylether (1:1) be removed from sample
Human serum see Burse et al. (1980) ECD 1 µg/litre Eyster
et al.
(1983)
Blood (in vitro see Roboz et al. (1982) MS- in vitro Roboz et al.
experimental) (PPINCI) (1985a)
Human blood see Roboz et al. (1982) ECD, SIM, 10-35 ng distribution of PBBs Roboz et al.
(model and NCI individual among blood components (1985b)
environmentally serum congener/litre
exposed)
b) Adipose and other tissues
Adipose tissue toluene/ethyl acetate GPC (Bio ECD 0.5 µg/kg major HxBB peak determined Wolff et al.
from exposed (1+3) Beads (1979a)
workers toluene/ethyl
acetate (1+3)
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Various rat Burse et al. (1980) ECD 10 µg/kg comparison of Miceli &
tissues and concentrations of PBBs Marks
serum in various tissues with (1981)
time
Liver and 1) hexane (liver and 1) Florisil ECD, NAA comparison of extraction Fawkes et al.
perirenal adipose) methods (showed PBB (1982)
adipose tissue extraction with hexane
from dosed rats leads to erratic recoveries
and results) increase in
detection limits over
ECD ( 2 pg FireMaster);
2) chloroform: 2) acidic 1 µg/litre or less of
methanol (liver) alumina hexa congener)
3) methylene chloride
chloroform (adipose)
Human adipose 15% diethyl ether Florisil/GPC ECD GPC clean-up tested (85% MacLeod
tissue in hexane MS recovery); MS free of et al.
serious interference (1982)
from 46 to 500 m/z
Adipose tissue 6% diethyl ether Florisil/GPC MS 1-2 µg/kg HxBB peak Lewis &
from general in hexane Sovocool
population (1982)
Human adipose hexane/diethylether silica gel ECD 1 µg/kg Eyster
tissue, placenta, et al.
cord blood, (1983)
biliary fluid,
faeces
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Human postmortem Chromaflex ECD 0.5 µg/kg HxBB peak Miceli
tissue adsorption et al.
column with (1985)
5% silica
gel + sodium
sulfate/
hexane
Adipose tissue hexane solid phase ECD 1-14 ng/kg Florisil Chiang et al.
(bovine), spiked Florisil cartridges cartridges to separate (1987)
for model system fat; 116% recovery
c) Milk
Human milk potassium oxalate, ECD 1 µg/kg Eyster et al.
ethanol/diethyl ether; (1983)
hexane
Human milk potassium oxalate, Bio Beads/ MS (NCI, 1 ng/kg separation of coplanar Krüger (1988)
ethanol/diethyl ether Florisil/ SIM) and planar isomers with
activated charcoal
charcoal
d) Biological samples from the environment
Fish, seal freeze, pulverize, Bio Beads/ MS (NCI, 10 ng/kg Krüger (1988)
pet. ether Florisil/ SIM)
activated
charcoal
Table 15 (contd).
Matrix Extraction Clean up Detection Detection limit Comment References
Dolphin fat/ Soxhlet; hexane, GPC; silica MS no data given lowest value given: Kuehl et al.
organ tissue methylene chloride gel 40 µg/kg (1991)
Terrestrial, diethyl ether/hexane hydrolysis MS (NCI) no data given lowest value given: Jansson
freshwater and with 98% 40 ng/kg et al.
marine samples H2SO4/Bio (1991, 1992)
Beads/ silica
gel/activated
charcoal
a Analytical method used was gas chromatography.
Abbreviations used:
ECD = Pulsed 63Ni electron capture detector. NCI = Negative ion chemical ionization mass spectrometry.
GPC = Gel permeation chromatography. PPINICI = Pulsed positive ion-negative ion chemical ionization.
MS = Mass spectrometry. SIM = Selected ion monitoring.
NAA = Neutron activation analysis.
Using negative ion chemical ionization mass spectrometry
(MS-NCI), the bromide ions can be used to detect brominated
compounds with high sensitivity and selectivity. However, using this
detection method (or ECD), interference between congeners of PBB and
polybrominated diphenyl ethers is possible.
The 209 possible PBB congeners have a wide range of volatility,
which causes very difficult separation problems (Farrell, 1980). In
earlier studies, gas chromatography (GC) with packed columns, e.g.,
3% OV-1 on 80/100 mesh Chromosorb W(HP) was used (Fehringer,
1975a,b). Capillary columns enable a good separation with lower
brominated biphenyls but do not have any great advantages over
packed columns for more highly substituted biphenyls (Farrell, 1980;
Orti et al., 1983; Robertson et al., 1984b).
The detection method most frequently used is that of pulsed
63Ni electron capture detection (ECD). In general, retention times
and electron capture responses increase with increasing bromination.
This is a sensitive method, but has some shortcomings. ECD is a
group selective detector that responds to halogens and other
electronegative groups. This places stringent requirements on
chromatographic separation. Moreover, ECD responds differently to
different compounds, depending on the molecular structure. The
response or sensitivity of the ECD depends on the position of the
halogen on the biphenyl nucleus as well as the number of halogens.
This necessitates running a standard for each compound to be
determined (Zerezghi et al., 1984). Sweetman & Boettner (1982)
analysed the structure-sensitivity of PBBs using ECD (see Table 12).
Flame ionisation detection (FID) can only be used for the
analysis of standard substances because of its low specificity
(Krüger, 1988).
A microwave-induced plasma emission detector has been used as a
specific method of detection for bromine (Mulligan et al., 1980;
Zerezghi et al., 1984). However, the method is not sensitive enough
for environmental samples.
Some authors have confirmed their results by GC/ECD
determination before, and after, exposure to UVR. The PBBs present
are photolyzed and, in this way, the background values can be
eliminated (Erney, 1975; Trotter, 1977).
Very often, the presence of PBBs is confirmed using mass
spectrometry (MS) together with gas chromatography. The purity of
the sample can be verified by comparison with known standards.
Negative chemical ionization (NCI) mass spectrometry has a
sensitivity comparable with, and somewhat better than, GC/ECD
analysis. The detection level for hexabromobiphenyl standards is
lower by a factor of 20 to 10-35 ng/ml in comparison with GC/ECD
analyses (Roboz et al., 1982). This relatively new method has also
been used to detect polychlorinated and polybrominated dioxins and
furans (see section 4.3).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
PBBs are not known to occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 World production figures
1) United States of America
The commercial production of PBBs in the USA commenced in 1970
(Neufeld et al., 1977). Several US producers of commercial
quantities have been identified (Mumma & Wallace, 1975; Neufeld
et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok, 1980).
In 1976, a US firm had a combined production of about 0.45
million kg of PBBs for export to Europe (Anon., 1977).
A list of suppliers of laboratory quantities (with a maximum
production or importation of about 2 kg/year) is presented by Mumma
& Wallace (1975) and Neufeld et al. (1977).
As a result of the Michigan catastrophe of mid-1973, the sole
US manufacturer of hexabromobiphenyl ceased production in November
1974. It is not clear, whether the production of bromine-based fire
retardants was resumed by another US company in 1978 (Brinkman & de
Kok, 1980). Two other companies continued their production of octa-
and deca-PBB until 1977 (Di Carlo et al., 1978). According to the
German "Umweltbundesamt" (UBA, 1989), decabromobiphenyl was produced
in the USA until 1979.
There are repeated statements that all PBBs manufactured in the
USA since 1975-76 have been exported, mainly to Europe, and that
there is no importation of any PBB mixtures into the USA (Brinkman &
de Kok, 1980).
Relevant production data for the period 1970-76 are presented
in Table 16.
Table 16. Commercial production of polybrominated biphenyls in the USA, 1970-76a
Estimated production in thousand kg
Product 1970 1971 1972 1973 1974 1975 1976 1970-76
Hexabromobiphenyl 9.5 84.2 1011 1770 2221 0 0 5369
Octabromobiphenyl 14.1 14.1 14.6 163 48 77.3 366 702
and decabromobiphenylb
Total PBBs 23.6 98.3 1025 1933 2269 77.3 366 6071
a From: Di Carlo et al. (1978).
b Manufacture was continued in 1977, but production figures are not available.
Hexabrominated biphenyl forms the major part (about
5.4 million kg FireMaster(R) BP-6 plus some 68 300 kg
FireMaster(R) FF-1) of the estimated total production of
6.1 million kg (Neufeld et al., 1977). The remaining 0.7 million kg
are accounted for by the higher brominated biphenyls. In 1976, for
example, 0.35 million kg of decabromobiphenyl and 13 600 kg of
octabromobiphenyl were manufactured (Neufeld et al., 1977). No
production figures are available for 1977 (Di Carlo et al., 1978).
2) Japan
According to IARC (1978), PBBs have never been produced in
Japan, but, up to 1978, some were imported.
3) Europe
a) Germany
A German firm produced a mixture of highly brominated PBBs,
called Bromkal 80-9D until mid-1985, when the activities concerning
bromine-based fire retardants were shifted to the USA. No production
figures are available.
b) France
A French firm manufactures a technical-grade decabromobiphenyl,
sold as Adine 0102, production being a few hundred thousand kg/year
(Atochem, 1988). It is marketed in France, Great Britain, Spain and
the Netherlands (Atochem, 1988; UBA, 1989). More than 200 tonnes
decabromobiphenyl/year were used in the Netherlands for
incorporation into polybutylenterephthalate plastics (UBA, 1989).
c) United Kingdom
Two companies are reported to have marketed or produced
technical-grade decabromobiphenyl in the United Kingdom (Brinkman &
de Kok, 1980). In 1977, the production of PBBs was discontinued
(Neufeld et al., 1977).
No production or sales data are available.
d) Netherlands
No domestic producer has been identified. An Israeli company
with two bromine plants in Holland denied the production of PBBs
(Neufeld et al., 1977). However, the amount of decabromobi phenyl
sold annually in the Netherlands was estimated to be of the order of
91 000 kg (Brinkman & de Kok, 1980).
No information is available on production in other parts of the
world.
3.2.1.2 Manufacturing processes
The process of manufacturing PBBs consists of a Friedel-Crafts
type reaction in which biphenyl is reacted with bromine in the
presence of chloride in an organic solvent, using aluminium
chloride, aluminium bromide, or iron as catalyst (Brinkman & de Kok,
1980). In the Atochem decabromobiphenyl manufacturing process,
biphenyl is directly brominated in a large excess of bromine, used
as reactant and solvent in the presence of a Lewis acid catalyst
(aluminium type). Decabromobiphenyl is further purified by
distillation of the excess bromine in the presence of a brominated
solvent (Atochem, 1992).
3.2.1.3 Loss into the environment during normal production
Data are published only for the USA. The following information
refers to reviews by Neufeld et al. (1977) and Di Carlo et al.
(1978).
Losses of PBBs to the environment at sites of its manufacture
can total 51 kg/1000 kg of product. These losses occur through:
1) Emission into the air
In 1977, the maximum air losses as particulate matter at
production sites were estimated to total 1.1 kg of PBBs/1000 kg
manufactured.
(a) Emission to the air from the vents of the hydrogen bromide
recovery system:
Total emission of FireMaster(R) PB-6 was estimated to amount
to 70 mg/1000 kg produced.
(b) Loss of particulate PBB to the atmosphere during centrifugation
(which was carried out to separate the solid reaction products
from the organic solvent).
A New Jersey permit application by Hexcel Corp. plant (1976)
indicated a loss of less than 0.05% of the product.
(c) Loss of dust from drying and pulverizing PBBs to a fine powder
(dust from this operation was removed by a bag type filter).
In 1974, atmospheric levels of PB-6 in the Michigan Chemical
Corp. bagger area were 16-32 mg/m3 during the bagging operation
and 3 mg/m3 after bagging was completed. Lower levels were
detected in other areas of the plant.
(d) Emission of hexabromobiphenyl as a vapour contaminant in
vapour streams leaving scrubbers or equivalent equipment was
calculated to be less than 25 µg/m3 (1 ppb) at ambient
temperature (Neufeld et al., 1977).
2) Losses in waste waters resulting from the quenching and
washing of the PBBs as they are recovered from the reaction mass:
The losses of PBBs to sewers at manufacturing sites were
estimated, in 1977, to be 4.6 µg/kg of product.
- In 1972, samples of the Michigan Chemical Corp. effluent
discharges were found to contain PBB levels of 98-503 µg/litre
(Hesse, 1975);
- The total quantity of PBBs being discharged to the Pine River
was estimated as 0.11 kg daily.
- Unfiltered water from an industrial storm sewer at the Hexcel
Corp. plant contained 92 µg/litre, mainly as decabromobiphenyl
(hexa-, octa-, and nonabromobiphenyls levels were also
measurable).
- Liquid effluents, diluted by canal water, from the White
Chemical Co. plant showed values of up to 31 µg PBBs/litre.
3) Solid losses to landfills resulting from drying, handling,
shipping and transportation.
An estimate of PBB losses as solid waste to landfills was
50 g/kg of product.
According to a report of the Michigan Chemical Corp., their
solid waste included approximately 5% of the BP-6 produced.
4) Losses to the soil
Soil samples from the bagging and loading areas of the Michigan
Chemical corp. contained PBBs at concentrations of 3500 and
2500 mg/kg, respectively.
Losses of other compounds:
The following typical air contaminants released during PBB-
manufacture were reported: hydrogen chloride, bromine, ethylene
dichloride, aluminium chloride, and biphenyls. The total quantity
emitted was stated to be less than 5.5 kg/day.
3.2.1.4 Methods of transport, accidental release, and disposal of
production wastes
Details of present-day labelling and transport regulations are
given in the Health and Safety Guide for PBBs (WHO, 1993).
In 1973, an accidental release of PBBs occurred in Michigan
("Michigan disaster"), when two products manufactured by the
Michigan Chemical Company were inadvertently confused, i.e.,
250-500 kg (Di Carlo et al., 1978) of FireMaster(R), instead of
NutriMaster(R), a magnesium oxide-based cattle feed supplement,
were added to animal feed and distributed to farms within the state.
The compound is believed to have been FireMaster(R) FF-1
(e.g., Fries, 1985b), even if in some publications the name
FireMaster(R) BP-6 is used (e.g., Neufeld et al., 1977; Di Carlo
et al., 1978). This accidental mix up resulted in widespread
contamination by PBBs (see section 5). As a result of this incident,
the production of FireMaster(R) BP-6 by Michigan Chemical Corp.
was stopped in 1974 (Di Carlo et al., 1978). Chronological reports
or reviews of the PBB disaster are given by Carter (1976), Getty
et al. (1977), Kay (1977), Di Carlo et al. (1978), Damstra et al.
(1982), Zabik (1982), and Fries (1985b).
Details of the disposal of manufacturing waste during present
production are not available. In a report by Neufeld et al. (1977),
solids from manufacturing operations were disposed of in landfills.
Waste waters containing small amounts of PBBs were discharged into
the chemical sewer.
3.2.2 Uses
Commercially manufactured PBBs are processed by industrial
users, primarily as flame retardants in polymeric materials. PBBs
were developed for this major application, because: they are able to
meet the flame-resistance performance requirements, they are
economically feasible, and they have little effect on the
flexibility of the base compounds (Mumma & Wallace, 1975).
The process of application is basically one of physical
blending: the PBBs are not functional additives, and on blending
with the dry solid or liquid polymeric material, provide filter-type
flame retardant action with the chemical release of hydrogen bromide
if ignited (Neufeld et al., 1977).
Neufeld et al. (1977) list 34 applications of PBBs found in
patent and technical literature. The majority are related to the use
of the PBBs as flame retardants in polymeric materials, other claims
include self-extinguishing properties and improved wearability and
machinability. Further potential uses of PBBs are: in the synthesis
of biphenyl esters or in a modified Wurtz-Fittig-synthesis; in
light sensitive compositions to act as colour activators; as
relative molecular mass control agents for polybutadiene; as wood
preservatives; as voltage stabilizing agents in electrical
insulation; as functional fluids, such as dielectric media (Neufeld
et al., 1977). In the USA and Canada, hexabromobiphenyl
(FireMaster(R)) was the principal PBB product. It was used as a
fire retardant in three main commercial products:
acrylonitrile-butadiene-styrene (ABS) plastics; coatings and
lacquers; and polyurethane foam (Neufeld et al., 1977).
The types of ABS plastic products in which FireMaster(R) BP-6
was used are compiled in Table 17.
According to Neufeld et al. (1977), the use of FireMaster(R)
BP-6 as a flame retardant in thermoplastic resins was confined to
products that do not come into contact with food or feed and are not
used in fabrics to which humans are exposed.
Although more than 130 companies in the USA used PBBs prior to
1976 (Di Carlo et al., 1978), only a limited number seems to have
been the major users of PBBs. For example, in 1974, the final year
of US production, Borg Warner Corp. (Parkersburg, W.Va.; using
FireMaster(R) in ABS plastics) and Standard T Chemical Co. (Staten
Island, New York; using FireMaster(R) in fire retardant coatings
for industry) consumed over 50% of the total US yearly production
(Mumma & Wallace, 1975; Jamieson, 1977; Neufeld et al., 1977;
Brinkman & de Kok, 1980).
Of the estimated 2200 tonnes hexabromobiphenyl produced in 1974
(IARC, 1978), about 900 tonnes (Mumma & Wallace, 1975; Neufeld
et al., 1977; IARC, 1978) were used in ABS plastic products and
about 34 000 tonnes (Mumma & Wallace, 1975; Neufeld et al., 1977;
IARC, 1978) in cable coatings.
The exact quantity of FireMaster(R) used in polyurethane foam
for automobile upholstery was not published. The two larger
consumers ceased using hexabromobiphenyl (one of these in 1972)
because PBBs did not decompose in the ultimate incineration of
scrapped automobiles (Neufeld et al., 1977).
No current users of hexabromobiphenyl have been identified
(Neufeld et al., 1977; Di Carlo et al., 1978; Brinkman & de Kok,
1980). As regards octa- and decabromobiphenyl, no commercial use was
reported in the USA during 1970-74 (Neufeld et al., 1977). In
Western Europe, the use of higher brominated PBBs seems to be
dominant. The decabromobiphenyl Adine 0102(R) (in the past
manufactured by Ugine Kuhlmann, at present by Atochem) is used as a
flame retardant for thermoplastics and thermosets (e.g., in
polyesters, epoxy resins, polystyrene, ABS, polyolefines, and PVC),
for elastomers (e.g., in PU-elastomers and india rubber) and for
cellulosics (e.g., chip-board). It is applied frequently in
association with antimony trioxide (Sb2O3) (Atochem, 1984a). Its use
in paints and varnishes has also been reported (Brinkman & de Kok,
1980).
Table 17. Uses of FireMaster(R) BP-6 in ABS plastics in the USAa
Industry Approximate % Examples
of total use
Business machines and 48 Typewriter, calculator and microfilm-reader
industrial equipment housings; business machine housings
Electrical 35 Radio and TV parts, thermostats, shaver and
hand-tool housings
Fabricated products 12 Projector housings, movie equipment cases
Transportation 1 Miscellaneous small automotive parts;
electrical-wire connectors, switch
connectors, speaker grills
Miscellaneous 4 Small parts for electrical applications,
motor housings; components for industrial
equipment
a From: Brinkman & de Kok (1980).
Losses of PBBs to the environment from processing plants are
possible, but little information is available about this.
Although decabromobiphenyl and, possibly, other PBBs are still
produced commercially, alternative chemicals have been introduced to
replace them as flame retardants, in particular polybrominated
biphenyl ethers (oxides) (PBBO), e.g., decabromobiphenyl ether
(Adine 505; Bromkal 82-0 DE; Great Lakes DE-83TM and DE 83RTM),
octabromobiphenyl ether (Bromkal 79-8 DE; Great Lakes DE 79), and
pentabromobiphenyl ether (Bromkal 70-5 DE; Great Lakes DE-71TM:
Atochem, 1984b; Great Lakes Chemical Corp., 1986).
Decabromobiphenyl ether (DBBO) for example, appears to be a
much less toxic material than PBBs. However, DBBO is said to have a
tendency to degrade to lower brominated biphenyl oxides. It is
possible that these lower order compounds may pose environmental
problems similar to those of the lower brominated PBBs (Mumma &
Wallace, 1975). In addition, on pyrolysis, PBBOs produce larger
amounts of dioxins and furans than PBBs and so may themselves have
to be replaced by other compounds.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
The commercial PBB-mixtures are solids at room temperature.
Despite their low vapour pressure, air pollution by PBBs can occur
as follows:
PBBs may be released into the atmosphere as vapour or dust
from production and processing plants. Stratton & Whitlock (1979)
found indirect evidence of airborne discharges of PBBs near two out
of three chosen industrial sites in north-eastern New Jersey and
Staten Island, New York, where these materials had been manufactured
or used in product formulations.
Further air contamination may occur during the incineration of
industrial and municipal wastes. Most municipal incinerators are not
very effective in destroying halogenated biphenyls. Like PCBs, PBBs
do not burn readily and incinerating conditions must be carefully
controlled, otherwise these compounds will reenter the environment
in the stack gases (Griffin & Chou, 1981a) or may be transformed to
polybro minated dibenzofurans.
Flameless combustion of the consumer products causes
volatilization of intact PBBs (Benbow & Cullis, 1975).
An appreciable loss of PBBs during the lifetime of PBB-
containing products is unlikely.
Secondary ways of entrance of PBBs into the atmosphere, e.g.,
through evaporation from contaminated soils, are thought to be
negligible, though small losses of PBBs from soil during long-term
(6 months) incubation studies were observed, which were associated
with volatilization rather than sorption or masking (Griffin & Chou,
1981a).
The ability of PBBs to co-distil from landfills or from the
surface layer of water bodies, as reported for PCBs (Kalmaz &
Kalmaz, 1979), has not yet been examined.
By analogy with PCBs, it might be expected that PBBs entering
the atmosphere in the vapour phase would be adsorbed rapidly onto
particles, which would then be deposited by particle sedimentation,
depending on micro- and macrometeorological conditions.
According to Eisenreich et al. (1981) organic compounds having
a vapour pressure > 10-5 kPa should exist almost entirely in
the vapour phase, and those having a vapour pressure > 10-9 kPa
should exist almost entirely in the particulate phase.
PBBs, e.g., hexabromobiphenyl or FireMaster with a vapour
pressure of 6.9 x 10-9 kPa (Jacobs et al., 1976), belong to the
latter. In reality, distribution and atmospheric lifetimes of
organic compounds with a high relative molecular mass depend largely
on the particle concentration and composition in the atmosphere
(Eisenreich et al., 1981). Gas-phase reactions with hydroxyl (OH)
radicals also influence the lifetimes of organic compounds emitted
into the atmosphere. Atkinson et al. (1984) determined rate
constants for the gas-phase reaction of OH radicals with biphenyl
and predicted, from their findings, that the chlorine - and
bromine-substituted biphenyls would have OH radical rate constants
of < 8 x 10-12 cm3/molecule per second at room temperature.
4.1.2 Water
The principal route of entrance of PBBs into aquatic
environments is from industrial waste streams into receiving waters.
Further potential routes (of minor relevance) are atmospheric
deposition and erosion of polluted soils. Groundwater contamination
is possible, if these compounds are leached from landfills (Shah,
1978).
Because compounds like PBB are very poorly soluble, they are
primarily found in sediments of polluted lakes and rivers
(Kimbrough, 1980a). In laboratory experiments, Simmons & Kotz (1982)
determined the "percent adsorption" of PBBs in sediments from sites
at Lake Michigan and the Huron River and concluded from values
ranging from 9 to 32% that the capacity of the sediments for PBB was
small to moderate.
PBBs in water are mainly adsorbed on particulate matter
followed by sedimentation at a rate that depends on several factors,
such as the size and type of the sediment and/or the organic
contents of both the sediment and the overlying water mass. The
relative importance of these parameters is controversial (Simmons &
Kotz, 1982). Laboratory results concerning PCBs (Jensen et al.,
1973) led to the assumption that the kinetics of the sorption
reaction may vary inversely with particle size (because smaller
particles have a larger surface area for surface adsorption).
Leland et al. (1973), Choi & Chen (1976), and Simmons et al. (1980)
have shown that the organic content of the sediment is directly
related to its adsorptive capacity for a specific contaminant.
According to Schwarzenbach & Westall (1981), adsorption of non-polar
compounds is highly correlated with the organic carbon content of
sorbents containing more than 0.1% organic carbon. Simmons & Kotz
(1982) found strong correlations between the adsorptive capacity of
the sediments for PBBs and TOC (total organic carbon) and the %
silt/clay fraction.
Sediments are potential sources as well as sinks for most
chemicals (Simmons & Kotz, 1982). Desorption of a contaminant from
the sediments is favoured where a high concentration of organic
matter exists in the water column (Huang, 1971). The presence of
organic matter may also enhance the partitioning of the contaminant
in the water phase and, thus, facilitate further movement with the
water mass (Hassett & Anderson, 1979). Laboratory studies on the
mode of action that PBBs may take in their movement through the
water column have verified that the total organic content of the
natural water will decrease the adsorption of PBB onto sediment and
therefore keep the PBB in the water phase. For example, comparing
the distilled water versus natural water systems, in river water
with an organic content of 11-12 mg C/litre, the % PBB-adsorption
was decreased by 33-43%; for lake water with an organic content of
3.8 mg/litre, the % PBB-adsorption was reduced by about 12% (Simmons
& Kotz, 1982). Another investigation indicated that the solubilities
of PBBs were directly correlated with the levels of dissolved
organics in the water (Griffin & Chou, 1981a) (see also section
4.1.3).
However, in the natural environment, upon settling out, the
association of the contaminant with the sediment may become the
dominant process in the water/sediment system (Simmons & Kotz,
1982). Transport of PBBs is thought to take place, when mixing or
bioturbation of sediments causes redistribution of the contaminant
in the water column (Simmons & Kotz, 1982), and through transport of
the sediment itself.
4.1.3 Soil
Pollution of soils can originate from point sources such as PBB
plant areas and waste dumps. Very few data are available on the
deposition of PBBs on soil via the atmosphere, sewage sludge from
municipal sewage treatment systems, and the dredging of sludge from
contaminated waters.
Other possible sources are illicit, or improper, disposal of
such chemicals (Kimbrough, 1980a) and incidents. For example, as a
consequence of an incident in 1973, Michigan soils have been
contaminated by manure from PBB-fed animals and by the disposal of
contaminated feed, milk, carcasses, etc. (Getty et al., 1977; Chou
et al., 1978; Damstra et al., 1982; Fries, 1985b). Once PBBs have
been introduced into the soil, they appear to have little tendency
to translocate (Damstra et al., 1982).
The ability of rainfall to carry PBBs through the soil was
tested in a laboratory simulation. Filonow et al. (1976) percolated
water through columns of 4 Michigan soils containing 100 mg
2,2',4,4',5,5'-hexabromobiphenyl/kg. They found a loss of less than
0.6% of the hexabromobiphenyl congener from each soil, even with
leachate quantities equivalent to 20 times the average annual
rainfall in Michigan.
Field investigations also indicated that PBBs were retained in
the top soil. Results of subsequent studies on highly contaminated
farm soils showed that PBBs did not move below the 15 cm level,
except where there was a history of physical mixing of the soil
(Fries, 1985b).
The mobility in soils of a chemical like PBBs will largely be
governed by its solubility in water and its adsorption, or
interaction, with soil particles (Jacobs et al., 1978).
As already mentioned, PBBs have a very low solubility in water.
However, studies with distilled, tap, river, and soil waters showed
that their solubility was markedly influenced by water purity
(Jacobs et al., 1978). Griffin & Chou (1981a) ascertained, under
well defined conditions, the following average solubilities of PBBs:
0.06 µg/litre in distilled water, 0.3 µg/litre in deionized water,
0.5 µg/litre in creek water, 8.9 µg/litre in Du Page leachate, and
16.9 µg/litre in Blackwell leachate.
Hence, PBBs were more than 200 times more soluble in landfill
leachate than in distilled water; the solubilities of PBBs were also
higher in creek water than in distilled water. As shown by the TOC
(total organic carbon) values for the waters, the higher
solubilities of PBBs were directly correlated with the level of
dissolved organic compounds in the waters. The type of dissolved
organic matter may also influence the solubility (Griffin & Chou,
1981a).
PBBs are quite soluble in organic solvents, such as dioxane,
carbon tetrachloride, acetone, and methanol. This could play a major
role in soil environments where leachates from chemical waste
disposal sites are percolating.
The other important factor affecting the migration of PBBs,
i.e., their adsorption by soils, was also studied under laboratory
conditions. The hydrophobic properties of PBBs make them easily
adsorbed from aqueous solutions onto soils. Filonow et al. (1976)
examined the adsorption of purified 2,2',4,4',5,5'-hexabromobi
phenyl (BB153) on four soil types. They found that the adsorption of
2,2',4,4',5,5'-hexabromobiphenyl conformed well to Freundlich
adsorption isotherms, and that 2-19% of the available HBB was
adsorbed. Adsorption of HBB was influenced primarily by the organic
content of the soils. An increase in the organic matter content of
soils enhanced their adsorption capacity.
Neither percentage clay nor pH correlated well with BB153
adsorption. Any effect that the clay contents may have had, was
apparently masked by the effect of the organic contents on
adsorption (Filonow et al., 1976).
Griffin & Chou (1981a,b), using the PBB-mixture FireMaster(R)
BP-6 or 14C-labelled-PBB, also confirmed the strong adsorption of
PBBs on soils and indicated a very high direct correlation between
the total organic carbon content (TOC) of three different soils and
the amounts of PBBs adsorbed. However, they pointed out that, in
soils with a low TOC, the mineral fraction may contribute markedly
to the adsorption capacity.
Furthermore, preferential adsorption of PBB congeners and
isomers was noted, depending on the characteristics of the
adsorbent, e.g., organic content (Griffin & Chou, 1981a), as well as
on the degree and position of bromine substitution (Griffin & Chou,
1980, 1981b).
No measurable adsorption on soils occurred of PBBs from organic
solvents (Griffin & Chou, 1981a).
The results of migration studies were in agreement with the
findings discussed above. The mobility of PBBs in five soils was
measured with several leaching solvents, using a thin-layer
chromatography technique and column leaching studies (Griffin &
Chou, 1981a,b). PBBs remained immobile in the soils when leached
with water or landfill leachate, but were highly mobile when leached
with organic solvents. Mobility was directly proportional to the
solubility in the leaching solvents and inversely proportional to
the soil total organic content.
On the other hand, because PBBs are bound to soil, wherever
contaminated soil moves, whether through wind or water erosion or
animal ingestion and migration, traces of PBBs (if present) can be
expected to be found (Jacobs et al., 1978).
4.1.4 Biota
PBBs are stable and persistent, lipophilic, and only very
slightly soluble in water; they are poorly metabolized, and
therefore accumulate in lipid compartments of biota. Once they have
been released into the environment they will reach the food chain,
where they are concentrated. Fish and wildlife are the most
consistent targets for such contamination, but livestock and humans
may also become contaminated (Kimbrough, 1980a). The precise routes
and transport mechanisms of PBBs travelling through biota have not
been thoroughly investigated as pointed out below.
4.1.4.1 Terrestrial ecosystems
Several studies have been concerned with whether plants in
terrestrial ecosystems would take up, translocate, and introduce
PBBs into the food chain. Jacobs et al. (1976) selected orchard
grass (Dactylus glomerata) as test plants in their greenhouse
studies because of its extensive root mass, and carrots (Daucus
carota), which, according to Iwata et al. (1974), have an
outstanding ability to absorb pesticide residues from the soil. They
did not detect any PBBs in the tops of either species grown in soils
supplied with high levels of PBBs (10 or 100 mg/kg of
FireMaster(R) BP-6). However, they did find traces of PBBs
(20-40 µg/kg) associated with carrot roots. 14C-uptake studies
(autoradiography and GC-analysis) on corn and soybean seedlings
grown in hydroponic solutions and on three root crops (radishes,
carrots, and onions) grown in two different soils, also showed no
translocation of PBBs into plant tops (Chou et al., 1978). In
addition, these authors found that the amount of PBBs associated
with roots depended on plant species and the clay and organic matter
contents of the soil. Roots of carrots contained more PBBs than
those of radish or onion bulbs; all roots had higher levels of PBBs
(50-500 µg/kg tissue) when grown in a high-PBB treatment soil
(100 mg/kg) with lower clay and organic content, than they did
(30-120 ng/g plant tissue) in a soil containing more clay and
organic matter. Furthermore, PBBs seem to be localized on the
surfaces of roots, because a significant portion of 14C-PBBs was
removed, when the roots were dipped in acetone.
Analyses of field samples from plant tissues of corn, alfalfa,
and sudax, grown on Michigan fields with soil PBB levels ranging
from 9 to 371 µg/kg, resulted in no detectable (detection limit:
0.3 µg/kg) PBB (Jacobs et al., 1978). The same was true for washed
radishes from a garden with an estimated PBB concen tration of
500-1000 µg/kg and for corn leaf whorls containing dust from a PBB
contaminated soil (102 µg/kg) (Chou et al., 1978).
However, Stratton & Whitlock (1979), who conducted a field
screening survey near sites of manufacture and use of PBBs, found
high surface contamination of lichens and reeds.
The salt marsh cordgrass (Spartina alterniflora) is reported
to take up, accumulate, and transfer effectively PCB from
contaminated sediments to food chains (Mrozek et al., 1982). No data
are available with regard to PBBs.
So far, except for the surface contamination of roots from
contaminated soils and of foliage via air deposition processes,
plants are generally free of significant amounts of residue. Thus,
vegetation on PBB-contaminated soils is a less likely source of
contamination of animals (Damstra et al., 1982; Fries, 1985a,b).
In contrast, a major route of residue transmission from soils
to animals is the direct ingestion of soil (Fries 1982; 1985a). The
degree of contamination depends on the amount of soil ingested and
the bioavailability of the residues.
Quantitative data on soil ingestion by farm animals are given
by several authors (Healy et al., 1967; Healy, 1968; Fries, 1982;
Fries et al., 1982a,b) and range from 2 to 15% of the intake of dry
matter.
Fries (1985a) determined the bioavailability of soil-borne PBBs
in sheep, under controlled feeding conditions, using diets
containing 5% PBB-contaminated soil, and found 65% PBB absorption
from this diet, which contained 9 µg PBB/kg. Addition of activated
carbon to soil had only little effect on bioavailability of PBB.
The same author recorded PBBs in the fat of beef cows, beef
calves, ewes, and pigs from several farms on which soil-borne PBBs
in confinement areas was the only source of PBBs. It can also be
concluded from these results that the animals consumed soil, and
that soil-borne PBB was bioavailable. As might be expected, pigs
accumulated higher PBB concentrations from a soil environment than
ruminants (Fries, 1985a). Recontamination of soil by animal excreta
(Getty et al., 1977; Fries, 1985a) or carcasses (Shah, 1978) also
occurred.
Recently, PBBs have been detected in European herbivorous
mammals (Swedish reindeers: Jansson et al., 1992; German cows
(milk): Krüger, 1988) (see also sections 5.1.4 and 5.1.6).
Despite the affinity of PBBs for soil, there are no
investigations on the role of the soil fauna in the transfer of
PBBs. Earthworms are of great ecological importance and might be
expected to take up and accumulate PBBs as has been ascertained for
PCBs (Diercxsens et al., 1985) and, thus, introduce them into the
food chain.
4.1.4.2 Aquatic ecosystems
PBBs enter the aquatic food chains via water and food. Bacteria
and plankton play an important role in the accumulation and
translocation of PCBs to higher trophic levels (Kalmaz & Kalmaz,
1979; Lorenz & Neumeier, 1983). According to Falkner & Simonis
(1982), sorption processes probably control uptake and accumulation
of PCBs by phytoplankton, because of its high surface-volume ratio.
These mechanisms could also be valid for PBBs. However, Stratton &
Whitlock (1979) did not find PBBs in algae collected in the vicinity
of industrial sites, where PBB concentrations of sediments ranged
from 20 to 60 µg/kg and where captured fish contained 220-230 µg
PBB/kg (detection limit: not given).
No information on the uptake of PBBs from sediment through
bottom living organisms (e.g., mollusca or oligochaete worms) is
available.
In contrast, several laboratory (Norris et al., 1973; Zitko &
Hutzinger, 1976; Zitko, 1977; Sugiura et al., 1978) and field (Hesse
& Powers, 1978; Stratton & Whitlock, 1979; Jaffe et al., 1985)
studies on fish have been conducted. They confirm PBB uptake from
water and food, with the exception of hepta- and octabromobiphenyl
(Norris et al., 1973; Zitko, 1977), which were not taken up from
water.
Consequently, ingestion of fish is a source of PBB transfer to
mammals and birds. Because of the possible selective accumulation
and metabolism of PBB congeners in prey, it can be expected that
predators will be subjected to a somewhat different PBB congener
composition than that found in the surrounding media (sediment,
water, etc.).
In natural situations, food chains become linked together in
complex food webs, and PBBs are distributed in the corresponding
manner.
PBBs have been detected in other species of wildlife besides
fish, e.g., in ducks living near contaminated waters (Hesse &
Powers, 1978), in a turtle (Stratton & Whitlock, 1979), in the eggs
of waterbirds (Haseltine et al., 1981; Heinz et al., 1983, 1985), in
eagles (Kaiser et al., 1980), and in marine mammals (Jansson et al.,
1987, 1992; Krüger, 1988; Kuehl et al., 1991) (see also section
5.1.6).
4.1.4.3 Accidental contamination of the food chain
A special case of entrance of PBBs into the food chain occurred
accidentally in 1973 in Michigan, when FireMaster(R) FF-1 was
inadvertently substituted for magnesium oxide as a supplement in the
formulation of cattle feed (Damstra et al., 1982). Ten to twenty
bags, 22.8 kg each, of PBBs (Carter, 1976) were mixed into feeds,
that were widely distributed to Michigan farmers.
In addition, feeds not formulated to contain magnesium oxide
also became contaminated (with relatively low concentrations)
because of carryover of PBBs from batch to batch in the mixing
equipment (Dunckel, 1975) and, on farms, through the recycling of
contaminated products (Kay, 1977). Distribution of contaminated
antibiotics, e.g., aureomycin, also contributed to the introduction
of PBBs into farm animals (Di Carlo et al., 1978).
The mixing error was not discovered immediately, and it was
almost a year before analyses indicated that a compound of PBB was
involved in the illness or death of farm animals (Getty et al.,
1977). During this time (IARC, 1978; Zabik, 1982), contaminated
animals and their produce entered the human food supply and the
environment of the state of Michigan. Hundreds of farms were
affected. Altogether, at least 29 800 cattle, 5920 pigs, 1470 sheep,
and 1.5 million chickens had been killed and buried by the end of
1975 (Robertson & Chynoweth, 1975; Carter, 1976), in order to
minimize further human exposure. In addition, at least
785 thousand kg of feed, 8185 kg of cheese, 1197 kg of butter,
15 500 kg of dried milk products, and nearly 5 million eggs were
destroyed (Carter, 1976). The number of animals quarantined or
contaminated below quarantine level was estimated to be several
thousands (Isleib & Whitehead, 1975). Although the Michigan PBB
episode was primarily an incident of feed contamination, it also
resulted in secondary contamination of animals from contaminated
soil (Fries, 1985a).
4.2 Degradation
Compounds like PBBs are very stable to hydrolysis, chemical
oxidation, and thermal decomposition. Degradation by purely abiotic
chemical reactions (excluding photochemical reactions) is therefore
considered an unlikely environmental sink (Pomerantz et al., 1978;
Pearson, 1982).
The persistence of PBBs under actual field conditions is
reported in some publications. Jacobs et al. (1976) detected PBBs in
soils from a field that had received manure from a
FireMaster(R)-contaminated dairy herd 10 months earlier.
Follow-up surveys over a three-year period following the
termination of PBB production showed no significant decline in PBB
levels in sediments from the Pine River (Hesse & Powers, 1978). Soil
samples from the former PBB-manufacturing site in St. Louis,
Michigan, analysed several years (nearly ten years?) after
contamination (during the early 1970s) still contained PBBs.
However, the PBB congener composition differed from that of the
original FireMaster(R) mixture, indicating a partial degradation
of the PBB residue in the soil sample (Hill et al., 1982).
The chemical Inspection and Testing Institute, Japan (1987) has
listed decabromobiphenyl as non-biodegradable.
The most probable degradation mechanisms of PBBs in the
environment, if there is any degradation at all, are
photodecomposition and microbial degradation.
4.2.1 Photolytic degradation
Under laboratory conditions, PBBs were easily degraded by UVR.
The photoreactivity of PBBs has been used to confirm PBB residues
(Erney, 1975; Trotter, 1977). The predominant photochemical reaction
of PBBs in organic solvents was a reductive debromination.
Irradiation of 4-monobromobiphenyl at 300 nm in various polar and
nonpolar solvents led to the formation of biphenyl as the sole
product (Freeman et al., 1991). Earlier studies using lower
brominated PBB congeners (i.e., tetra and lower) reported a
preferential loss of ortho bromines (Bunce et al., 1975; Ruzo
et al., 1976). Irradiation of higher brominated congeners yielded a
series of photoproducts (Table 18), but a stepwise cleavage of
orthobromines did not appear to be preferred to meta or para
debromination (Patterson et al., 1980; Millis & Aust, 1985).
The photoreactivity of 2,2',4,4',5,5'-hexabromobiphenyl, the
main component of FireMaster(R), was consistently found to be
relatively high (Andersson et al., 1975; Ruzo et al., 1976;
Robertson et al., 1983a; Millis & Aust, 1985), and degradation
occurred more rapid than with the hexachloro analogue (Andersson
et al., 1975; Ruzo & Zabik, 1975).
Consistent with the dehalogenation pathway, photodegradation of
the commercial FireMaster(R) mixture led to reduced concentrations
of the more highly substituted PBB congeners (De Kok et al., 1977;
Robertson et al., 1981b, 1983; Epling et al., 1987). Robertson
et al. (1983a) examined changes in the composition of
FireMaster(R) BP-6 during photolysis (300 nm for 2-12 h; solvent:
cyclohexane) by monitoring 25 individual PBB congeners; they also
did not find a preferential loss of ortho bromines. Nevertheless,
the photoproducts of FireMaster(R) did contain increased
concentrations of congeners possessing no ortho bromines (e.g.,
3,4,4'-tri-, 3,3',4,4'-tetra-, 3,3',4,4',5-penta bromobiphenyl).
Moreover, other congeners, known as relatively toxic (e.g.,
2,3',4,4',5-pentabromobiphenyl), were enriched (Robertson et al.,
1983). Biphenyl, the ultimate product of the debromination pathway,
was found only to a small extent after the photolysis of
FireMaster(R) BP-6 (Epling et al., 1987).
Table 18. Photodegradation of higher brominated PBB congeners under laboratory conditions
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,5,5'- 254 nm hexane 43.4a 2,3',4',5-tetra ortho-debromination Millis & Aust
penta (up to 100 (minor product) (1985)
min) 2,2',4,5'-tetra meta-debromination
2,2',5,5'-tetra para-debromination
(major product)
(additional production
of a yellow gum)
2,3',4,4',5- 254 nm hexane 50a 2,3',4',5-tetra para-debromination Millis & Aust
penta (up to 90 3,3',4',4'-tetra ortho-debromination (1985)
min)
2,2',4,4',5,5'- 366 nm methanol not specified lower brominated PBBs degradation (90% after 9 Andersson et al.
hexa (main products) min) more rapid than with (1975)
BB 153 methoxy- PBBs (minor the hexachloro analogue
products)
> 300 nm hexane not specified lower brominated PBBs BB 153 was 24.4 times Ruzo et al.
(0.5-2 h) quaterphenyls (< 5%) more reactive than (1976)
4,4'-dibromobiphenyl
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5,5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
Table 18 (contd).
PBB Irradiation Solvent Initial rate Primary products Remarks References
(duration) of photolysis of photolysis
(nmol/min) identified
2,2',4,4',5,5'- 254 nm hexane 53a 2,2',4,5.5'-penta para-debromination Millis & Aust
hexa (up to 100 (major product) (1985)
min) 2,3',4,4',5-penta ortho-debromination
2,2',4,4',5-penta meta-debromination
secondary photoproduct:
3,3',4,4'-tetra
formation of yellow
gum at 25 min
2,2',3,4,4',5,5'- sunlight not not 2,2',4,4',5,5'-hexa meta-debromination Patterson
hepta (390 min) specified specified (major product) et al. (1980)
2,3',4,4',5,5'-hexa ortho-debromination
2,2',3,3',4,4', sunlight not not unidentified hexa- Patterson
5,5'-octa (300 min) specified specified PBB (major product) ortho- and meta- et al. (1980)
2,3',4,4',5,5'-hexa debromination
2,2',3,3',5,5', 300 nm hexane not specified di- to Ruzo et al.
6,6'-octa (0.5-2 h) heptabromobiphenyls, ortho debromination (1976)
e.g., 3,3',5,5'-tetra
a Original PBB concentration = 1.59 mmol/litre.
Technical octabromobiphenyl has been reported to photo degrade
in xylene by reductive debromination with a half-life of 40 h
(Norris et al., 1973).
There were investigations to enhance the photochemical process
aiming at a potential technique for the breakdown and removal of
PBBs from the environment. In laboratory testing, photodegra dation
of PBBs was accelerated in the presence of ethylenediamine and
tertbutylamine (Christensen & Weimer, 1979) and in the presence of
sodium borohydride (Epling et al., 1987).
Epling et al. (1987) obtained high yields of biphenyl during
borohydride enhanced photolysis of FireMaster(R) BP-6 (irradiation
under nitrogen at 254 nm; solvent: 90% acetonitrile/water).
The rates and extent of photolytic reactions of PBBs in the
environment have not been determined in detail. However, the few
field observations available indicate a high persistence of the
original PBBs (Jacobs et al., 1978) or a partial degradation to less
brominated (and often more toxic) photoproducts (Hill et al., 1982).
Jacobs et al. (1978) examined field soil that had received manure
from FireMaster(R)-contaminated cattle, for the first time, 2-3
years earlier. They did not detect any significant changes in the
relative concentrations of the major PBB peaks (Br5, Br6, Br7)
compared with the FireMaster(R) standard. In contrast, soil
samples, obtained from the former FireMaster(R) manufacturing site
in Michigan and analysed several years (approximately 10 years?)
after contamination, contained enhanced concentrations of possible
photodegradation products including 2,3',4,4',5-pentabromobiphenyl,
2,2',4,4',5-pentabromobiphenyl, and two unidentified
tetrabromobiphenyls (Hill et al., 1982).
Considering the diversity of microenvironments, both laboratory
and field data on photo alteration of PBBs are incomplete; there is
a lack of studies on the photochemistry of PBBs in water, or in the
vapour or solid states.
4.2.2 Microbial degradation
In laboratory investigations, mixtures of PBBs appear to be
fairly resistant to microbial degradation. Soil incubation studies
using FireMaster(R) BP-6 (lot no. 6244A) and 14C-PBB
(lot 872-244) showed a little, but not significant, degradation of
the major hexa- and heptabromobiphenyl congeners after 6 months or
1 year; only pentabromobiphenyl was assumed to degrade slowly
(Jacobs et al., 1976, 1978). These results were deduced from
recovery rates of PBBs from soil, 14CO2 production, and the lack of
14C-PBB intermediates.
Soils incubated with photodecomposition products of 14C-hexa
and heptabromobiphenyl caused enhanced, but still minor, degradation
(ca. 3%) as measured by 14CO2 production (Jacobs et al., 1978).
These findings are consistent with observations according to which
degradation of PCBs by bacteria increases with decreasing
chlorination (Kalmaz & Kalmaz, 1979; Fries, 1982).
In further incubation experiments with FireMaster(R) BP-6
(lot no. 6244A) in sterilized and nonsterilized Catlin-soil, Griffin
& Chou (1981a) measured the recoveries of penta-, hexa-, and
heptabromobiphenyls and found that all PBBs persisted for 6 months
with no significant microbial degradation. They observed the same
kind of persistence over a period of 4 weeks in PBB incubations with
mixed cultures of microorganisms (predominantly Alkaligenes
odorans, A. denitrificans, and an unidentified bacterium). This
culture had been isolated previously and was known to degrade
water-soluble PCBs (Clark et al., 1979). No PBB metabolites were
found in the PBB-saturated mineral solution after 4 weeks of
incubation (Griffin & Chou, 1981a).
As with PCBs, the high degree (penta or greater) of halogen
substitution of its major components probably accounts for the lack
of degradation of the FireMaster(R)-mixture (Griffin & Chou,
1981a). Congruently, biodegradation of monobrominated biphenyls has
recently been reported.
A soil isolate, strain S93B1, identified as Pseudomonas
cruciviae, could grow on more than ten biphenyl-related compounds
including o-bromobiphenyl (Takase et al., 1986). O-bromobiphenyl
was converted to o-bromobenzoic acid (Fig. 3) (identified by
IR-spectrum). This is analogous with some PCBs showing chlorinated
benzoates as metabolites (Ballschmiter et al., 1977). In these
experiments, biphenyl-related compounds 0.2-0.5% (w/v) were added as
the sole sources of carbon to the liquid artificial medium.
However, this pathway is also realized under simulated natural
conditions (aquatic environments), as reported by Kong & Sayler
(1983). They used river water as supportive culture medium and
"mixed bacterial cultures" (not identified), which were obtained
from PCB-contaminated river sediments. This mixed bacterial culture
was capable of degrading monohalogenated biphenyls.
 The degradation rates of 2-, 3-, and 4-bromobiphenyl, at
30 µg/ml, were 2.3, 4.2, and 1.4 µg/ml per day, respectively, and
were comparable with those of monochlorinated biphenyls. Degradation
occurred when the substrates were supplied as the sole carbon source
or when added in combination with glucose. The major metabolite of
4-bromobiphenyl (para) was 4-bromobenzoate, identified by means of
cochromatography with an authentic compound in HPLC. Two bacterial
strains of the genus Pseudomonas, isolated from a lake sediment by
using p-chlorobiphenyl as a sole carbon source, were capable of
degrading 2-, and 4-bromobiphenyl, but they did not degrade
4,4'-dibromobiphenyl (Sugiura, 1992).
In contrast to several reports indicating that chlorobenzoates
are the principal stable metabolites of PCBs (Furukawa & Matsumara,
1976; Furukawa et al., 1979; Yagi & Sudo, 1980; Reichardt et al.,
1981), 4-bromobenzoate as well as 4-chlorobenzoate appeared
transient. For, when tested with the same bacterial consortium,
4-bromobenzoate at 30 mg/kg was readily degraded at the rate of
4 µg/ml per day (Kong & Sayler, 1983). The terminal decomposition
product is assumed to be CO2 (Kong & Sayler, 1983), i.e.,
4-bromobiphenyl can most likely be completely mineralized by this
bacterial culture. Suflita et al. (1982) also observed degradation
of bromobenzoates. They reported the reductive dehalogenation of
halobenzoates, including 2-, 3-, or 4- bromobenzoate by
microorganisms of lake sediment and sewage sludge. Dehalogenation
required strict anaerobic conditions. The primary degradative event
was loss of the aryl halide without the alteration of the aromatic
ring, the end products were CH4 and CO2. The stable bacterial
consortium enriched from sludge consisted of both chemolithotrophic
and heterotrophic methanogens as well as three, unidentified,
non-motile Gram- negative rods.
Recently, there was a brief report on the reductive
debromination of the FireMaster(R) mixture (rate and extent of
debromination reaction not given) by anaerobic microorganisms eluted
from PCB-contaminated river sediments (Quensen et al., 1990;
abstract only).
4.2.3 Degradation by plants and animals
No degradation of PBBs by plants has been recorded. In contrast
to plants, animals can easily absorb PBBs and, though they have been
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs (cf. also section 6.3).
The metabolism of crude FireMaster(R) BP-6 by a pig gave a
monohydroxypentabromobiphenyl (Kohli & Safe, 1976). The faeces of
dogs fed FireMaster BP-6 contained a metabolite identified as
6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl (Gardner et al., 1979).
However, the authors do not exclude microbial metabolism of PBB in
the dog's gut followed by excretion into the faeces. Doses of
2,2',4,4',5,5'-[14C]-hexabromobiphenyl given intravenously or
orally to male rats were not subject to appreciable metabolism
(Matthews et al., 1977). Metabolites were not detected in tissue
extracts. A trace of radioactivity, which may have represented a
PBB-metabolite, was found in bile and faeces, but the quantity was
too small to be isolated and identified.
Some investigations imply that fish may debrominate the more
highly brominated components of PBB-mixtures. Fish (juvenile Salmo
salar), exposed in laboratory studies to FireMaster(R) BP-6 in
water, contained several mono to pentabromobiphenyls that were not
present in BP-6. Several additional pentabromobiphenyls were
detected in fish fed FireMaster(R) BP-6-contaminated food. Fish
fed octabromobiphenyl contaminated food contained unidentified
penta-, hexa-, and heptabromobiphenyls in addition to the
octabromobiphenyls (Zitko, 1977). It was not known whether the
partially debrominated biphenyls were generated by the fish, or by
the associated microflora.
Because the carbon-bromine bond is less stable than the
carbon-chlorine bond, reductive debromination may be a degradative
pathway of bromobiphenyls, and this reaction may have toxicological
consequences not encountered with PCBs (Zitko & Hutzinger, 1976;
Zitko, 1977).
4.2.4 Bioaccumulation
As expected from their high lipophilicity, PBBs show a marked
tendency to accumulate in animals. However, data are available only
on single links of food chains. It has been reported that similar
compounds, e.g., PCBs, which are more widely spread in the
environment, may have bioconcentration factors of 3-4 orders of
magnitude between water and fish, with a further 1-2 orders of
magnitude between whole fish and the fat storage tissues of fish
predators, such as cormorant, heron, and seal (Pearson, 1982).
4.2.4.1 Aquatic organisms
Fish are the only aquatic organisms for which the bioaccumu
lation of PBBs has been investigated intensively. They serve as an
example for the efficiency of such bioaccumulation (Damstra et al.,
1982).
Fathead minnows (Pimephales promelas) caged in a river, where
water levels of PBB (Firemaster(R) BP-6; probably measured as
concentration of the main peak, 2,2',4,4',5,5'-hexabromobiphenyl)
remained consistently at less than 0.1 µg/litre, concentrated these
contaminants in their bodies more than 10 000 fold in two weeks of
exposure (Hesse & Powers, 1978). In laboratory studies, accumulation
coefficients of FireMaster(R) BP-6 and technical octabromobiphenyl
from water (A = concentration in fish, µg/g wet weight/concentration
in water, µg/ml) and from food (B = concentration in fish, µg/g wet
weight/concentration in fish-food, µg/g) were determined.
FireMaster(R) BP-6 reached values of A = 48 (after an exposure of
48 h) and B = 1.0 (in equilibrium) in juvenile Atlantic salmon
(Salmo salar). The main component accumulated was
2,2'4,4',5,5'-hexabromobiphenyl (Zitko, 1977). In contrast,
octabromobiphenyl, as such, was not concentrated from water by
Atlantic salmon (Zitko, 1977) and by rainbow trout (Norris et al.,
1973), but a little uptake (B = 0.023) was observed from suspended
food (Zitko, 1977). Instead of octabromobiphenyl, an unidentified
hexabromobiphenyl was mainly accumulated (A = 1.73; B = 0.114;
Zitko, 1977). In comparison with Aroclor 1254, the accumulation of
FireMaster(R) BP-6 from water was less, but accumulation from food
was a little higher than that of the corresponding PCB mixture (A =
282; B = 0.358; Zitko, 1977).
There are some differences in the accumulation of different
congeners. Zitko & Hutzinger (1976) determined accumulation
coefficients of di-, tri-, and tetrabromobiphenyls in Salmo salar.
The coefficients were calculated on the basis of accumulation from
water after a 48-h exposure, and of the extrapolated equilibrium
levels in fish fed contaminated food. The accumulation coefficients
generally decreased with increasing degree of substitution during
the uptake from water, and increased, when taken up from food. Of
the dibromobiphenyls, the 3,4-isomer accumulated from water much
less than the 2,6- and 2,4-isomer, and did not accumulate from food
(Zitko & Hutzinger, 1976). Sugiura et al. (1978) dealing with the
accumulation of lower substituted halobiphenyls (di-, tri- and
tetra-) in killifish (Oryzias latipes) found that equilibrium
accumulation from water was not reached during a period of 20 days.
Their data, derived from a flow through test using PBB
concentrations of 0.5-50 µg/litre, resulted in bioaccumulation
factors (equilibrium extrapolated) ranging from 340 to 7340. They
also found that accumulation factors were proportional to partition
coefficients (n-octanol/water), when the coefficients were below
106, but not when the coefficients were above 106.
It is obviously important to note the lipid contents of test
animals in bioaccumulation studies. For example, the bioconcen
tration factors of PCB congeners for whole fish tissue were
proportional to the lipid content of the different species, which
can range from 3 to nearly 20% (Sugiura et al., 1979). Gobas et al.
(1989) reported lipid weight-based bioconcentration factors, log
KL ranging from 5.06 to 6.16, for some PBBs (di- to hexa-) in the
guppy (Poecilia reticulata).
4.2.4.2 Terrestrial organisms
Bioaccumulation of PBBs in terrestrial organisms has been
considered only for avian and mammalian species of farm and
laboratory animals. Data were obtained through field observations
(accumulation from soil), evaluation of an accident, and through
controlled feeding studies.
Accumulation of soilborne PBBs has been studied in Michigan
farms that were contaminated accidentally by FireMaster(R) FF-1
(Fries, 1985a). Ratios of PBB concentrations between the fat of farm
animals (cows, sheep, pigs) and soil ranged from 0.10 to 1.86.
Multiparous dairy cows had lower ratios, because of the excretion of
PBB in milk during long-term lactation, and swine had higher ratios,
because they ingest greater amounts of soil than other species
(Fries, 1985a). In another study, PBB (FireMaster(R) FF-1) was
applied to the soil surface for experimental purposes. Sheep grazing
for 180 days on these plots containing 33 mg PBB/m2 (plot 1) and
48 mg/m2 (plot 2) reached average residue levels in body fat of
0.30 and 0.79 µg PBB/g fat (quantified as concen trations of
2,2',4,4',5,5'-hexabromobiphenyl), respectively. Average residue
concentrations in ewes that grazed for 60 days were nearly as great
(Fries & Marrow, 1982). A second trial, conducted 3 years later
after ploughing and reseeding the plots, showed that PBBs were
distributed throughout the top 16 cm of soil with an average
concentration of 0.14 µg 2,2',4,4',5,5'- hexabromobiphenyl/g soil in
plot 2. Sheep grazing here for 136 days had average concentrations
of 0.032 µg PBB/g body fat (Fries & Marrow, 1982).
The accidental ingestion of FireMaster(R) FF-1 by cattle on
Michigan farms, first described by Jackson & Halbert (1974),
resulted in high body burdens of PBBs. There were tissue levels of
2,2',4,4',5,5'-hexabromobiphenyl in the fat of cows of up to
approximately 4000 mg/kg, nearly one year after high exposure
(estimated total dose: 150-400 g of FireMaster(R) FF-1/cow over
14 days). Low exposure from cross contamination produced PBB
concentrations in fat of less than 0.3 µg/g (Fries et al., 1978a,b;
Fries, 1983).
Laboratory data for the accumulation of PBBs from known diets
are given in Table 19 (diets supplemented with FireMaster(R)) and
in Table 20 (diets supplemented with single PBB congeners). PBB
levels in tissues of FireMaster(R)-exposed animals were expressed
as the concentration of the most abundant constituent of the
mixture, namely 2,2',4,4',5,5'-hexabromobiphenyl. Fries et al.
(1976) additionally reported the concentration of a heptabromobi
phenyl component (not the pure isomer). They found 31.4 mg/kg of
this component in the body fat of hens fed diets containing 20 mg
FireMaster(R)/kg feed for 63 days. The fate of minor constituents
of the FireMaster(R) mixture is not evident from the studies
compiled in Table 19.
Generally, accumulation of PBBs in body fat depended on dosage
and duration of exposure. The highest accumulation coefficients
(mg PBB/kg of tissue divided by mg PBB/kg feed) were found in minks
(Table 19). PBB residue levels in the adipose tissue of treated
minks were 60 times the amount in the diet (Aulerich & Ringer,
1979). According to the authors, the high diet-to-fat residue
accumulations in the minks may be due, in part, to the relatively
small subcutaneous fat deposits of the test animals, most of which
were extremely emaciated at the time of death. Technical
octabromobiphenyl was also accumulated from the diet, as shown by
analyses of the bromine contents of the tissues (Norris et al.,
1973; Lee et al., 1975a; Waritz et al., 1977). There was a
dose-related build-up of bromine, predominantly in the fat, as well
as in the liver, of rats fed octabromobiphenyl. For example, after 4
weeks of feeding 1, 10, 100, or 1000 mg octabromobiphenyl/kg feed,
the bromine concentrations in adipose tissue were 2, 12, 120, and
600 times, respectively, greater than those of the controls (Lee
et al., 1975a).
Table 19. Accumulation of PBBs in feeding studies on mammals and birds
a) Feeding of FireMaster(R)
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Rat (male) BP-6 0.1 20.9c 9 days 0.3 1.5 brain: 0.5 lipid Render et al. (1982)
Rat (male) BP-6 1 23.3b 9 days 1.7 8.3 brain: 1.8 lipid
Rat (male) BP-6 1 not specified 2-3 weeks - 2.7 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 1 not specified 30 days 7.8 22.3 thymus: 21 lipid Akoso et al. (1982a)
Rat (male) BP-6 10 22.9b 9 days 27 135 brain: 12.3 lipid Render et al. (1982)
Rat (male) BP-6 10 not specified 30 days 61.6 310 kidney: 147 lipid Akoso et al. (1982a)
Rat (male) BP-6 50 not specified 2-3 week - 341 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 50 26b 10 weeks 864 55 - wet Harris et al. (1978b)
Rat (male) BP-6 100 22b 9 days 251 1213 brain: 103 lipid Render et al. (1982)
Rat (male) BP-6 100 not specified 30 days 1535 2507 thymus:1044 lipid Akoso et al. (1982a)
Rat (male) BP-6 100 27b 10 weeks 3460 107 - wet Harris et al. (1978b)
Rat (male) BP-6 150 26b 10 weeks 3574 295 - wet
Rat (male) BP-6 200 26b 10 weeks 3242 245 - wet
Mouse BP-6 100 not specified 14 days 223 33.2 thymus: 391 wet Corbett et al. (1978a)
(male)
Mouse BP-6 1000 not specified 11 days 39.5 2.5 - wet Corbett et al. (1975)
(male)
Mouse FF-1 5 not specified 3 weeks - 7 thymus: 20 wet Loose et al. (1981)
(male)
Mouse FF-1 5 not specified 6 weeks - 15 thymus: 37.8 wet
(male)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mouse FF-1 167 not specified 3 weeks - 154 thymus: 109 wet
(male)
Mouse FF-1 167 not specified 6 weeks - 623 thymus: wet
(male) 3088
Sheep BP-6 50 1000 30 days 25 12 heart: 4.3 wet Gutenmann & Lisk
(male) (omental (1975)
fat)
42 (renal
fat)
17
(brisket
fat)
Pig BP-6 20 1880c 4 weeks 0.33 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 20 1880c 16 weeks 64 8.5 muscle: 6.6 wet
(back fat)
42.9
(leaf fat)
Pig BP-6 200 1230c 4 weeks 6.7 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 200 1230c 16 weeks 503 17.2 muscle: wet
18.4
(back fat)
459
(leaf fat)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mink FF-1 2.5 not specified 136 days 149 - muscle: 7.3 wet Aulerich & Ringer
6.25 172 days - - brain: 66 wet (1979)
15.6 72-93 986 - muscle: 70 wet
days
Japanese not 10 -b 9 weeks - 48 heart: 78 dry Babish et al. (1975a)
quail specified
(male) 20 -b 9 weeks - 374 kidney: 105 dry
100 -b 9 weeks - 642 kidney: 725 dry
Japanese not 10 -b 9 weeks - 99 heart: 48 dry
quail specified
(female) 20 -b 9 weeks - 225 heart: 50 dry
100 -b 9 weeks - 503 kidney: 428 dry
Chicken BP-6 20 not specified 63 days 79.8 - egg: 20 wet Fries et al. (1976)
(White
leghorn
hens)
BP-6 20 -b 4-8 weeks - - egg: 30 wet Cecil & Bitman (1978)
BP-6 64 -b 4-8 weeks - - egg: 100 wet
not 1 106b 5 weeks 0.6 egg: 1.5 wet Ringer & Polin (1977)
specified
not 125 94c 5 weeks - - egg: 209 wet Ringer & Polin (1977)
specified
not 625 28.4c 5 weeks - 80 - wet
specified
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Chicken FF-1 0.2 99b 5 weeks (-)d (-)d egg: 0.3 wet Polin & Ringer
(White (1978a,b)
leghorn FF-1 1 106b 5 weeks (-)d (-)d egg: 1.5 wet Polin & Ringer
hens) (1978a,b)
FF-1 5 100b 5 weeks (-)d (-)d egg: 7.4 wet Polin & Ringer
(1978a,b)
FF-1 25 99b 5 weeks (-)d (-)d egg: 43.4 wet Polin & Ringer
(1978a,b)
FF-1 125 94c 5 weeks (-)d (-)d egg: 215 wet Polin & Ringer
(1978a,b)
Chicken FF-1 0.1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
(White 0.11
leghorn FF-1 1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
cockerels) 0.87
FF-1 10 -b 28 days - 83.8 - lipid Dharma et al. (1982)
FF-1 100 -b 28 days - 752 - lipid Dharma et al. (1982)
a Measured as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
b Values not significantly different from control values.
c Values significantly different from control values.
d Diagrams only, generally, the ratios of tissue PBB: diet PBB averaged 3:1 for adipose tissue, 0.8:1 for liver,
and 1.5:1 for whole egg.
Table 20. Accumulation of PBBs in feeding studies on mammals and birds
b) Feeding of individual PBB congeners
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,2',4,4',5,5'- rat 0.1 23.8a 9 days 0.2 1.7 brain: 0.3 Render et al. (1982)
Hexabromobiphenyl (male)
1 26.2b 9 days 3.1 11.4 brain: 1.1
rat 1 not 30 days 16 68.6 kidney: 38.7 Akoso et al. (1982a)
(male) specified
rat 10 25.5b 9 days 31.2 181 brain: 11.5 Render et al. (1982)
(male)
rat 10 not 30 days 149 693 kidney: 373 Akoso et al. (1982a)
(male) specified
rat 100 23.2a 9 days 436 2558 brain: 143 Render et al. (1982)
(male)
rat 100 not 30 days 992 6062 thymus: 3841 Akoso et al. (1982a)
(male) specified
chicken 10 -a 28 days - 105 - Dharma et al. (1982)
(male)
62 -a 28 days - 751 -
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,3',4,4',5,5'- rat 1 not 30 days 9.1 23.5 kidney: 13.9 Akoso et al. (1982a)
Hexabromobiphenyl (male) specified
rat 10 not 30 days 69.1 242 thymus: 211 Akoso et al. (1982a)
(male) specified (1982a)
rat 100 not 30 days 648 4340 thymus: 1639 Akoso et al. (1982a)
(male) specified
chicken 4 -a 28 days - 32.5 - Dharma et al. (1982)
(male)
chicken 10 -a 28 days - 132 - Dharma et al. (1982)
(male)
3,3',4,4',5,5'- rat 0.1 23.6a 9 days 0 3.3 brain: 0 Render et al. (1982)
Hexabromobiphenyl (male)
1 24.7a 9 days 0.4 101 brain: 0
rat 1 24.7b 30 days 0.6 125 thymus: 0 Akoso et al. (1982a)
(male)
rat 10 20.2b 9 days 1.9 - brain: 0 Render et al. (1982)
(male)
rat 10 21.3b 30 days 6.9 448 thymus: 20.4 Akoso et al. (1982a)
(male)
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
rat 100 13.7b 9 days 22.5 1098 brain: 0 Render et al. (1982)
(male)
a Values not significantly different from control values.
b Values significantly different from control values.
Data on accumulation of technical decabromobiphenyl have not
been found in the literature.
Isomer specific accumulation has been studied for three
hexabromobiphenyl congeners. The residue levels in rats and chickens
fed with 2,2'4,4',5,5'-; 2,3',4,4',5,5'- or 3,3',4,4',5,5'-
hexabromobiphenyl are listed in Table 20. In many cases, the lowest
concentrations in tissues were found with 3,3',4,4',5,5'-
hexabromobiphenyl and the highest, with
2,2',4,4',5,5'-hexabromobiphenyl.
4.3 Ultimate fate following use
4.3.1 Disposal of PBB-contaminated animals and wastes from the
Michigan disaster
Accidental contamination of livestock feed in 1973 by PBBs led
to the destruction of over 30 000 animals in Michigan. As the
toxicity and other physical and chemical properties of PBBs were at
that time not so well known, the State of Michigan decided to locate
an environmentally safe site for the burial of contaminated
carcasses (Shah, 1978). A site in Kalkaska County was chosen and
test drilled in order to determine the long-range protection for
groundwaters in the area. The Kalkaska disposal site received over
10 000 animal carcasses most of which contained PBB levels above
1 mg/kg fat, and close to 20 000 carcasses with PBB levels ranging
from 0.3 to 1 mg/kg. This animal disposal site contains
approximately 45 kg of PBBs in all buried carcasses (Shah, 1978; see
section 5.1.2.3. for groundwater studies).
The Gratiot County landfill near St. Louis became operational
in late 1970, and it was designed only for general municipal solid
waste disposal. According to the Michigan Chemical Corporation
report to the Environmental Protection Agency, PBB wastes were
disposed of in the landfill between 1971 and 1973. Wastes containing
large amounts of PBBs (60-70%) were received in the landfill before
any information about the toxic effects of PBBs on animals was
publicly known (Shah, 1978).
The Forest Waste Disposal site consists of an 11-acre,
abandoned, municipal and industrial waste landfill and 9 surface
impoundments. It is located in Genesee County, Michigan, and is
surrounded by agricultural land and undeveloped woodlands and
wetlands. Forest Waste Disposal conducted landfill operations from
1972 to 1978. PBB-contaminated feed has recently been found in the
landfill. A decontamination programme has been recommended (Anon.,
1988).
The Michigan Chemical Corporation stated that, in their
opinion, PBBs would eventually undergo oxidative/biological
degradation forming carbon dioxide, water, and bromide ion (Cordle
et al., 1978). However, studies on PBBs in soil indicate that they
may remain in soils for many years, because of their resistance to
degradation (Jacobs et al., 1976).
4.3.2 Thermal decomposition of PBBs
There is little information on the pyrolysis of PBBs. The
products of the thermal decomposition of PBBs depend on the
temperature as well as on the amount of oxygen present.
Norris et al. (1973) constructed a special apparatus to measure
the relative amounts of bromine from octabromobiphenyl converted
during combustion, when these materials were used as additives in
thermoplastic resins. An exact temperature is not given. Hydrogen
bromide and bromine were not detected.
Waritz et al. (1977) carried out experiments to determine the
approximate lethal temperature of hexa- and octobromobiphenyl. The
dense clouds of fumes obtained at 350 °C were lethal to rats whereas
those produced at 290 °C were not. The fumes were not analysed.
Earlier experiments by Benbow & Cullis (1975) on the pyrolysis
of decabromobiphenyl pressed together at 160 °C with polystyrene and
polypropylene, respectively, showed that, during flameless
combustion, decabromobiphenyl appeared to be volatilized virtually
unchanged from the polymer, whereas when the polymer burned, the
decabromobiphenyl was converted quantitatively to hydrogen bromide.
In these early experiments, the analytical methods were not so
refined that it was possible to detect furans and dioxins. O'Keefe
(1978) pyrolyzed samples of FireMaster FF-1 at 380-400 °C in open
glass tubes and in tubes sealed after nitrogen flushing. Analysis by
low resolution direct probe mass spectrometry showed the presence of
tetra- and pentabrominated dibenzofurans in extracts of the open
tube pyrolyzed material and trace levels of tetrabromodibenzofuran
in those from PBB pyrolyzed under nitrogen.
Buser et al. (1978) studied the pyrolysis of FireMaster BP-6
with oxygen in sealed tubes. The flame retardant was completely
destroyed at 700 °C, but, at 600 °C, new compounds were formed, one
of which was probably tetrabromodibenzofuran.
The diversity of possible brominated and mixed brominated
furans and their toxicological implications led to further
refinements in analytical methods (Buser, 1986) and to the demand
for, and synthesis of, suitable standard isomers (Mason et al.,
1987a; Sovocool et al., 1987a; Munslow et al., 1989). There are over
5000 halogenated dibenzodioxins and dibenzofurans containing
chlorine and/or bromine, over 400 of which are 2,3,7,8-substituted
tetra-, penta- and hexahalo congeners suspected to be of high
toxicity (Buser 1987). These mixed congeners are of particular
importance with regard to chemical waste burning (Schäfer &
Ballschmiter, 1986).
Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen have shown that small amounts of bromobenzenes and
lower brominated biphenyls are formed (600-900 °C), but no furans
(Thoma et al., 1987a; Thoma & Hutzinger, 1989).
In contrast, the pyrolysis of FireMaster BP-6 in an open quartz
tube (700-900 °C) in the presence of oxygen yielded over 3 mg/kg
(ppm) of di- to heptabrominated dibenzofurans, though the pyrolysis
of pentabromodiphenyl ethers yielded brominated dibenzofurans at
over 300 times this level (Thoma et al., 1987a). In the presence of
polystyrene and polyethylene, higher levels of brominated
(mona-tetra) dibenzofurans (over 8 and 51 mg/kg (ppm), respectively)
were found (Thoma et al., 1987a). Pyrolysis of FireMaster BP-6 with
PVC at 800 °C yielded mixed bromide/chloride biphenyls, the bromine
atoms being substituted by the chlorine. No ring closure to dioxins
and furans occurred (Thoma et al., 1987b).
Decabromobiphenyl was pyrolyzed for 10 min at 800 °C in a
loosely plugged quartz tube. The pyrolysates were extracted with
toluene and after clean-up, analysed using GC/MS. No brominated
dioxins or dibenzofurans were detected (detection limits
0.2-0.8 µg/g). The clean-up was said to be very difficult because of
the formation of a large number of brominated compounds that were
not dioxins or furans. Debromination of decabromobiphenyl appeared
to be the main reaction, but no details were given (Atochem, 1987).
Zacharewski et al. (1988) pyrolyzed samples of FireMaster(R)
BP-6 in open quartz tubes at 800 °C for 10 min. The resulting
products, mainly tetrabromodibenzofurans (1183 µg/g) but also
tribromo-, pentabromo-, hexabromo-, and heptabromodibenzofurans
(187, 584, 107, and 11 µg/g, respectively), were tested for toxicity
(see section 8.12.3.2). Very little is known about the toxicities of
brominated and brominated/chlorinated dioxins and furans, but they
are estimated to be of the same order as those of PCDD and PCDF
(Mason et al., 1987a; Safe, 1987).
Analysis of actual environmental samples has also been carried
out. Monobromo-polychloro substituted benzenes, biphenyls,
dibenzodioxins, and dibenzofurans have been detected in solid
material collected from a chimney of an industrial waste incinerator
(Schäfer & Ballschmiter, 1986). Brominated dibenzo furans with a
very small amount of mixed brominated/chlorinated compounds were
detected in soot from an accidental fire at a bowling alley (Buser,
1986). Schwind et al. (1988; 1989) analysed samples from a municipal
waste incinerator and detected for the first time a complete series
of tetrahalogenated dibenzofurans (Cl4DF, Br1Cl3DF,
Br2Cl2DF, Br3Cl1DF and Br4DF). It is possible that PBCDD/F
could occur during the incineration of flame retardant-treated
plastic material, which produces PBDD and PBDF. These could react
with PVC via the mixed brominated/chlorinated dioxins and furans to
PCDD and PCDF (Schwind et al., 1988).
As with PCB disposal, the destruction of PCB-contaminated waste
should be carefully controlled. For PCBs, a burning temperature
above 1000 °C for 2 seconds is recommended (WHO/EURO, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Only one report is available on PBB levels in air. It refers to
air samples taken in the vicinity of three PBB-manufacturing or -
processing plants in the USA (Stratton & Whitlock, 1979). Traces of
hexabromobiphenyl (0.06-0.10 ng/m3) were found at two of the three
industrial sites examined.
Further information on PBB levels in ambient air, e.g., near
municipal incinerators, is lacking.
5.1.2 Water and sediments
5.1.2.1 Surface waters
Surface waters have been monitored in the vicinity of PBB-
producing or -processing industrial sites in the USA and in the
vicinity of the Gratiot County landfill (Michigan, USA), which had
received 122 000 kg of wastes containing 60-70% PBBs between 1971
and 1973. The results are summarized in Table 21.
Depending on the sources, the predominant PBB compounds
detected in surface waters were hexabromobiphenyl and
decabromobiphenyl. However, only Stratton & Whitlock (1979)
determined all PBB homologues from Br1 to Br10, the percentage
composition of some of which is given in Table 22.
5.1.2.2 Sediments
Generally, PBBs reach higher concentrations in sediments (Table
23) than in the associated waters (Table 21).
PBB concentrations in sediments of the Pine River were as high
as 77 mg/kg near the Michigan Chemical Corp. plant. The assays
conducted from July 1974 to April 1975, upstream and downstream of
the plant, showed a decline in sediment PBB content to 6.2 mg/kg,
half a mile downstream, and to 0.1 mg/kg, 24 miles or 29 miles
downstream (Hesse & Powers, 1978).
Table 21. PBB levels in surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Pine River 1974 8 HxBB 0.01-3.2 Hesse (1975)
(downstream from
the Michigan
Chemical Co.,
St. Louis)
Tittabawassee 1974 2 HxBB < 0.01
Rivera
Canal (called 1977 3 total PBB not detected-46 Stratton & Whitlock (1979);
Platti Kill) in the (MoBB-DeBB) DeCarlo (1979)
vicinity of White
Chemical Co., Bayonne,
New Jersey
Canal (discharging 1977 1 total PBB < 0.2 Stratton & Whitlock (1979)
into Kill van Kull (HxBB-DeBB)
River) in the vicinity
of Standard T Chemical
Co., Staten Island,
New York
Storm sewer, 1977 5 total PBB < 0.2-210 Stratton & Whitlock (1979);
receiving swamp, etc. (HxBB-DeBB) DeCarlo (1979)
in the vicinity of
Hexcel Fine Organics
Sayreville, New Jersey
Table 21 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Drain waters at 1977 not HxBB 0.1-14 Shah (1978);
or near the margin specified Rosenblatt et al. (1982)
of the Gratiot County
landfill, Michigan
a Note that PBBs were not detected in fish from the Tittabawassee River in 1974 but were detected in 1983 (see Table 30).
Table 22. Percentage of different PBB homologues detected in surface water
samples taken in the vicinity of PBB-producing plantsa
PBB homologues Bayonne, New Jersey Sayreville, New Jersey
(White Chemical Corp.)b (Hexcel Corp.)c
Sample 1 Sample 2 Sample 1 Sample 2
PeBB 1 0 - -
HxBB 4 2 5 < 1
HpBB 2 2 1.6 < 1
OcBB 2 17 1 1
NoBB 15 20 1 2.6
DeBB 76 58 91 96
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Producer of laboratory quantities of various PBBs.
Concentrations measured upstream were all less than the
sensitivity limit of 30 µg/kg with the exception of one sample
collected a quarter of a mile upstream of the plant, which contained
60 µg/kg (Hesse, 1975). This latter analysis was repeated in 1977
(Hesse & Powers, 1978) giving 350 µg/kg (detection limit 100 µg/kg).
Hesse & Powers (1978) compared the PBB levels of Pine River
sediments from the same locations over a period of time after the
termination of FireMaster(R) BP-6 production. The results of the
1976 and 1977 analyses showed that PBB distributions and
concentrations in the sediments had not changed significantly in the
three years after PBB manufacture stopped.
Various PBB homologues were identified again only by Stratton &
Whitlock (1979) who examined aquatic sediments, sludge deposits, and
marsh soils near the sites of manufacture and use of PBBs in New
Jersey and New York (see Table 24).
More recently, sewage sludge has been analysed for PBBs
(Strachan et al., 1983). The authors did not detect any PBBs, PCBs,
or chlorinated hydrocarbon pesticides in the three sludge samples
obtained from sewage treatment plants in three Indiana cities (USA).
However, the detection limit for PCBs and PBBs with the GC-MS system
used was about 10 µg/g, and this apparently is not sensitive enough,
even for PCBs. For example, the amounts of PCBs found in sewage
sludge of German (Lorenz, 1983) and Canadian (Webber et al., 1983)
cities ranged from 1.8 to 2.5 µg/g (dry weight) and from 0.13 to
1.61 µg/g (dry weight), respectively. Values for PBBs have not been
reported in these investigations.
Table 23. PBB levels in sediments and sludge from surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Near Michigan 1974/75 19 HxBB < 30-77 000 Hesse (1975)
Chemical Co. 1974 9 < 100-9200 Hesse & Powers (1978)
Pine River 1976 9 < 100-1200
1977 8 < 100-500
Tittibawassee River 1974 2 tr-16 Hesse (1975)
Near White Chemical 1977 3 total PBB < 10-20 Stratton & Whitlock (1979)
Co., Bayonne, New Jersey: (MoBB-DeBB)
Sediments from Platti
Kill Canal and Kill
van Kall River
Sludge from Platti 1 431 000
Kill Canal
Near Standard T Chemical 1977 1 60
Co., Staten Island, New
York Sediment from Kill
van Kull River (at
discharge site)
Near Hexcel Fine 1977 1 total PBB 4600 Stratton & Whitlock (1979)
Organics, Sayreville, (MoBB-DeBB) DeCarlo (1979)
New Jersey
Marsh Soil
Table 23 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Gratiot County 1977 not HxBB up to 17 000 Shah (1978)
landfill, Michigan specified Rosenblatt et al. (1982)
Associated sediments
of surface drain waters
Table 24. Concentration of PBB-homologues in aquatic sediment, sludge deposit,
or marsh soil samples taken in the vicinity of PBB-producing or -processing
plantsa (µg/kg dry weight)
PBB Bayonne, New Jersey Staten Island, Sayreville,
(White Chem. Corp.)b New Jersey New Jersey
(Standard T (Hexcel
Chem Corp.)c Corp.)d
Sediment Sludge Sedimente Marsh soile
samples:e
1 + 2 3
MoBB - n.d. 540 n.d. n.d.
DiBB - n.d. 2200 n.d. n.d.
TrBB - n.d. 4300 n.d. n.d.
TeBB - n.d. n.d. n.d. n.d.
PeBB - n.d. 590 n.d. n.d.
HxBB n.d. 10 3800 40 30
HpBB n.d. 10 3300 20 n.d.
OcBB n.d. n.d. 3600 n.d. n.d.
NoBB n.d. n.d. 22 500 n.d. 80
DeBB n.d. n.d. 390 000 n.d. 4500
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Major user of FireMasterR BP-6.
d Producer of laboratory quantities of various PBBs.
e n.d. = Not detected (detection limit = < 10 µg/kg).
Surficial sediments from the St. Lawrence River (USA/Canada)
were analysed for HxBB, but the compound was not detected (estimated
detection limit: 1 ng/g) at the ten stations surveyed (Sloterdijk,
1991).
5.1.2.3 Groundwater
Groundwater monitoring data from the Gratiot County landfill
(Michigan, USA) mentioned above, have shown trace levels of PBBs,
even outside the landfill area (see Table 25). However, so far,
domestic drinking-water wells have not shown any traces of PBBs
(Shah, 1978). Groundwater near the disposal site of PBB-
contaminated animals and other products (see section 4.3.1) in
Kalkaska County (Michigan, USA) is reported not to be contaminated
by PBBs (Shah, 1978).
Table 25. PBB levels in groundwater from Gratiot County landfill, 1977a
Site No. of PBB compound PBB
samples examined concentration
range (µg/litre)
Test wells within 4 HxBB 0.5-26
the landfill site
Observation wells 11 0.1-4.4
outside the landfill
area
Domestic not specified not detected
drinkingwater wells
near the landfill
a From: Shah (1978).
5.1.3 Soil
Data on soil pollution by PBBs are available for areas of
manufacture, use, or disposal of PBBs (Table 26), and for soils from
fields, etc. of the PBB-contaminated Michigan farms (Tables 27 and
28).
Concentrations of PBBs in soils from industrial sites were
highest (more than 2000 mg/kg) in areas around the Michigan Chemical
Company (see Table 26). Although such highly contaminated soils
were removed (Hesse & Powers, 1978), Hill et al. (1982) still found,
some years later, PBB levels up to 2130 mg/kg in soils of the former
manufacturing site. Various PBB homologues from Br4 to Br10 were
present in the industrial soil samples (see Table 27).
Table 26. PBB levels in soils near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
Michigan Chemical Co.,
St. Louis, Michigan
bagging area not specified 1 HxBB 3500 mg/kg Hesse (1975);
Hess & Powers (1978)
loading area of not specified 1 HxBB 2500 mg/kg
the plant
"former manufacturing not specified 3 PBB 16-2130 mg/kg Hill et al. (1982)
site" (C12H6Br4-C12H3Br7)
Vicinity of White
Chemical Co.,
Bayonne, New Jersey
150 m east 1977 1 total PBB 4.250 mg/kg Stratton & Whitlock (1979)
150 m west of the 1977 1 (C12H9Br1-C12Br10) 1.135 mg/kg DeCarlo (1979)
plant
not specified not specified PBB 0.75-2.8 mg/kg Di Carlo et al. (1978)
Vicinity of Standard 1977 4 total PBB 10-100 µg/kg Stratton & Whitlock (1979)
T Chemical Co., Staten (MoBB-DeBB)
Island, New York
75 m south west 30
900 m west 10
Table 26 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
1500 m south 10
700 m east (prevailing 100
down-wind direction)
Vicinity of Hexcel 1977 total PBB Stratton & Whitlock (1979);
Fine Organics, (MoBB-DeBB) DeCarlo (1979)
Sayreville, New Jersey
75 m southeast 1 40 µg/kg
Soil in roadside 1 3400 µg/kg
ditch
Gratiot County landfill,
St. Louis, Michigan
Samples inside of the not not "PBB" 12 (16) mg/kg Rosenblatt et al. (1982)
landfill from the specified specified HxBB
uppermost 2.5 cm
(after capping of
the landfill)
Sample somewhat distant "PBB" 61 µg/kg
from the landfill, in (HxBB)
the area of the Michigan
Chemical plant
Table 27. Concentration of PBB homologues detected in soil samples taken in the vicinity of PBB-producing or processing plants
(µg/kg dry weight)
PBB Bayonne, New Jerseya Staten Island, New Yorka Sayreville, New Yorka Michiganb
(White Chemical Corp.)c (Standard T-Chemical Corp.)d (Hexcel Corp.)e (Michigan Chemical Corp.)f
MoBB n.d.g n.d.g n.d.g -
DiBB n.d.g n.d.g n.d.g -
TrBB n.d.g n.d.g n.d.g -
TeBB n.d.g n.d.g n.d.g < 1000-510 000
PeBB n.d.g n.d.-100 n.d.g 4000-60 000
HxBB 15-30 n.d.-10 40-90 12 000-670 000
HpBB 30-110 n.d.-10 n.d.-90 < 1000-190 000
OcBB 90-150 n.d. n.d.-170 -
NoBB 330-2200 n.d. n.d.-440 -
DeBB 530-2100 n.d.-10 n.d.-2600 -
a Data from: Stratton & Whitlock (1979); No. of samples = 2, 4, 2 respectively.
b Data from: Hill et al. (1982); No. of samples = 3.
c Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
d Major user of FireMaster(R) BP-6.
e Producer of laboratory quantities of various PBBs.
f Producer of FireMaster(R) BP-6.
g n.d. = Not detected (detection limit = < 10 µg/kg).
Table 28. Composition and concentration of PBBs in soil samples from former
FireMaster(R) manufacturing plant site (St. Louis, Michigan)a
% Composition (concentration in mg/kg)
FireMaster(R)
Compound Lot #5143 Soil 1 Soil 2 Soil 3
Tetrabromobiphenyls < 0.1 23.9 (510) 11.3 (6) (< 1)
Pentabromobiphenyls
2,2',4,5,5'- 3.9 2.8 (60) 9.4 (5) 12.5 (2)
2,2',4,4',5- < 0.1 5.2 (110) (< 1) (< 1)
2,3',4,4',5- 5.7 27.7 (590) 9.4 (5) 12.5 (2)
Hexabromobiphenyls
2,2',4,4',5,5'- 54.9 24.4 (520) 56.6 (30) 62.5 (10)
2,2',3',4,4',5- 10.3 4.7 (100) 7.5 (4) 6.2 (1)
2,3',4,4',5,5'- 5.0 1.9 (40) 5.7 (3) 6.2 (1)
2,3,3',4,4',5- 2.1 0.5 (10) (< 1) (< 1)
Heptabromobiphenyls
2,2',3,4,4',5,5'- 12.8 5.2 (110) (< 1) (< 1)
2,2',3,3',4,4',5- 1.7 3.8 (80) (< 1) (< 1)
(Total PBBs) (2130) (53) (16)
a Adapted from: Hill et al. (1982).
Hill et al. (1982) identified not only PBB homologues, but also
the isomeric composition of PBBs in the soil samples from the
Michigan Chemical Corp. plant (Table 28). Thus, they provided more
exact analytical data and were able to make an interesting
comparison with the original FireMaster(R) mixture; conclusions
could then be drawn on the environmental fate of PBBs (section 4.2).
According to Shah (1978), test samples of the Gratiot County
landfill showed that, in general, the concentrations of PBBs in the
fill increased with depth and were highest at a depth of 3 to 7.6 m
below the top of the refuse.
As a consequence of the Michigan cattle food mixing error, the
soils of the farms involved have been contaminated by PBBs, mainly
through the faeces of the exposed animals. Fries (1985b) calculated
that about 145 kg of PBBs were distributed in this way, and that
most of this was located on 20-25 farms. (The total number of
quarantined farms was over 500; Robertson & Chynoweth, 1975).
Concentrations of PBBs in soil samples from fields that had
received PBB-contaminated manure were as high as 371 µg/kg (dry
weight), whereas levels in samples from manure piles and from dirt
exercise lots were as high as 2000 µg/kg (Jacobs et al., 1978;
Fries, 1985b).
Soil contamination by PBBs can result in PBB accumulation in
animals, when they have direct access to the contaminated soil.
This is most likely to occur when animals are confined to dirt lots
on which manure-containing PBB has been deposited. Crops grown on
PBB-contaminated soils are not considered an important source of PBB
contamination in animals (Fries & Jacobs, 1986).
Soils from industrial sites have, in general, been more heavily
contaminated than Michigan soils.
5.1.4 Feed and food
5.1.4.1 Feed
Contamination of feed by PBBs has been reported only in
connection with the Michigan PBB incident.
In 1973, about 290 kg (Fries, 1985b) - 1000 kg (IARC, 1978) of
FireMaster(R) FF-1 was inadvertently mixed in cattle feeds and
delivered to Michigan farms.
Three feed preparations appeared initially to be involved in
the Michigan episode with PBB levels as follows:
Feed No. 405, 2.4 mg PBB/kg,
Feed No. 410, 1790 mg PBB/kg,
Feed No. 407, 4300 mg PBB/kg
(Cordle et al., 1978).
A concentration as high as 13 500 mg PBB/kg was also cited
(Kay, 1977; Di Carlo et al., 1978; Damstra et al., 1982). Feed of
one highly contaminated farm (Halbert farm) is reported to have
contained 2900 mg PBB/kg (Fries, 1985b).
In 1974, 68% of 1770 feed samples collected in Michigan
contained PBB residues: 60% in the range of trace to 0.99 mg/kg, and
8% over 1 mg/kg. Resampling in 1975 revealed that 6% of 1208 feed
samples were contaminated and that fewer than 0.16% contained more
than 1 mg PBB/kg. In 1976, only 0.3% of 663 samples analysed were
contaminated: no samples contained more than 0.1 mg/kg (Di Carlo
et al., 1978).
PBB residues were not detected in harvested forages grown on
soils with residue levels as high as 0.3 mg/kg (Fries & Jacobs,
1980).
In 1974 and 1975, low-level feed contamination with PBBs was
detected in Indiana and Illinois, which are neighbours of Michigan
(Di Carlo et al., 1978).
5.1.4.2 Food
Again, almost all the data available on PBB residues in food
are derived from the Michigan cattle food contamination incident in
1973.
The extent to which the general population was exposed depended
on where they obtained their milk, dairy products, and eggs, i.e.,
direct from the contaminated farms or from sources where
contaminated products had been mixed with non-contaminated samples.
Table 29 shows examples of some PBB levels in Michigan foods.
Whereas in 1974 milk from some highly contaminated cows contained
PBB concentrations of up to 900 mg/kg fat (Robertson & Chynoweth,
1975), canned milk samples contained concentrations of up to
1.6 mg/kg fat (Cordle et al., 1978). The most highly contaminated
milk (1 to > 100 mg PBB/kg milk fat) originated from a total of 40
herds with different levels of PBBs at the time of detection (Fries,
1985b). In 1975, PBBs were still detected in milk from some herds
(Kay, 1977).
Data on meat can be derived from Table 33, which shows PBB
levels in Michigan farm animals.
Milk contains far less fat than meat (about 4% versus 30%), and
butterfat contains only 40% of the PBB concentration found in the
animal from which it comes (Fries et al., 1978b; Rosenblatt et al.,
1982). Among the dairy products, PBBs are again concentrated in the
high-fat products (Murata et al., 1977; Zabik et al., 1978).
Table 29. Some examples of PBB levels in food (contaminated as a consequence of
the Michigan PBB incident in 1973)
Product Year of PBB concentration References
sampling (mg/kg)
Milka 1974 2.8-270.5h Cordle et al. (1978)
Milkb 1974 44-900h Robertson & Chynoweth (1975)
Milkc 1974 43-56h Jackson & Halbert (1974)
Milkd 1974 up to 595 Kay (1977); IARC (1978)
Milke 1974 1-> 100h Fries (1985b)
Canned milk 1974 1.15-1.62h Cordle et al. (1978)
Dry skimmed milkf 1974 0.75-1.5 Isleib & Whitehead (1975)
Fluid milk 1974 < 0.02-1.15
processors' products
Butter 1974 1-2h Cordle et al. (1978)
Cheese 1974 1.4-15.0h
Milkg 1975 1-13h Kay (1977); IARC (1978)
Eggs 1974 up to 59.7
a = Collected from individual farms.
b = Collected from 21 cows.
c = Collected from 2 cows (having 174 and 200 mg PBBs/kg in body fat,
respectively).
d = Collected from 22 farms.
e = Collected from 28 herds.
f = From one dairy plant.
g = Collected from 16 herds.
h = On a fat basis.
In May 1974, the US Food and Drug Administration (FDA)
established the following enforcement limits for unavoidable
residues of PBBs in foods: 1 mg/kg in the fat of meat, milk, and
dairy products, 0.3 mg/kg in animal feeds, 0.1 mg/kg in eggs. These
enforcement guidelines were reduced in November 1974 to 0.3 mg/kg in
the fat of meat, milk, and dairy products, and 0.5 mg/kg in eggs and
animal feeds. In February 1977, the FDA rejected a petition to lower
the enforcement guideline level to 0.02 mg/kg for all food products
(IARC, 1978). However, according to Fries (1985b), final
legislation, Act 77, lowered the tolerance to 0.02 mg/kg in the body
fat of all cull dairy cows offered for slaughter. (Unlike the
situation under the previous regulations, the finding of a single
animal with a higher than legal body fat level did not lead to
quarantine and the disposal of the whole herd.) As a result of the
rigid quarantine policy, the food levels of PBBs decreased in
Michigan. In 1975, none of 18 milk samples, 3 out of 14 butter
samples, and none of 13 cheese samples exceeded FDA guidelines
(0.3 mg/kg). Also in 1975, 245 of 2040 meat samples were
contaminated with PBBs: 24 contained more than 0.3 mg/kg. None of
the meat specimens collected in 1976 exceeded FDA guidelines: 96% of
1430 samples were contaminated, but only 1 sample contained more
than 0.6 mg/kg of PBBs. A market basket survey of meat in 1976
revealed detectable PBBs in only 1 out of 102 samples in Michigan
(Di Carlo et al., 1978).
Additional information on PBB findings is presented in several
government reports, which are cited by Di Carlo et al. (1978).
According to these reports 29 170 products had been assayed. In
1974, 14 out of 16 milk samples, 4 out of 34 butter samples and 11
out of 23 cheese samples, collected in Michigan, were found to
exceed FDA guidelines for PBBs. Another survey showed that 24.9% of
272 finished product samples, collected from May to October 1974,
were contaminated with PBBs and that 15.8% contained more than
0.3 mg/kg (Di Carlo et al., 1978).
PBBs were also detected in other states in the USA, for example
in beef in Iowa, duck in Wisconsin, chicken in Alabama, Mississippi,
New York, and Texas, and turkey in Indiana: the levels were
extremely low. During 1975 and 1976, PBBs were found in 9 out of 597
food samples outside of Michigan (Di Carlo et al., 1978).
Food contamination, not derived from the Michigan PBB-
incident, becomes evident, when looking at PBB levels in fish (see
Table 30) some of which are used for human consumption. For example,
skinless fillets of carp from the Pine River, captured in the
vicinity of Michigan Chemical Company, contained 1.33 mg PBBs/kg
(wet weight basis) which is approximately equivalent to 30 mg/kg on
a fat weight basis (Hesse & Powers, 1978). This was obviously
greatly in excess of the US FDA tolerance limit for beef, a
tolerance limit for fish has not been established (Hesse & Powers,
1978).
More recent information on "background" PBB levels in food may
be expected in future via a USA data collection programme
(Foodcontam) initiated by the US Food and Drug Administration which
includes PBBs besides other chemicals (Minyard et al., 1989).
Table 30. PBB levels in fish
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1974 Pine River, downstream Carp skinless not detected-1330 wet HxBB Hesse &
from St. Louis (vicinity (Cyprimus carpio) fillets Powers
of Michigan Chemical Co.) (1978)
White sucker 670
Northern pike 540
Bullhead 450-780
1974 Tittabawassee Carp not detected
River (Cyprimus carpio)
Freshwater drum not detected
(Aplodinotus
grunniens
1976 Pine River, downstream Carp 60-750
from St. Louis (vicinity (Cyprimus carpio)
of Michigan Chemical Co.)
Northern pike 180-230
1976 Pine River, downstream Largemouth skinless not detected-740 wet HxBB Hesse &
from St. Louis (vicinity bass fillets Powers
of Michigan Chemical Co.) (1978)
Smallmouth bass 130
Rockbass 320-700
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1977 Kill van Kull River Killifish whole 220 dry total PBB Stratton &
(vicinity of White Chemical HxBB-DeBB Whitlock
Co., Bayonne, New Jersey); (1979)
Port Johnson
1977 Kill van Kull River Killifish whole 230 dry total PBB
(vicinity of Standarad T HxBB-DeBB
Chemical, Staten Island,
New York); canal at
discharge site
Not Lake Huron Yellow perch 0.3-0.8 Kreis & Rice
specified (Saginaw Bay) (1985)
Saginaw Bay Catfish 21.0
1983 Pine River Hogsucker whole 6000 fat most Jaffe et al.
abundant (1985)
congeners
1983 Chippewa River Carp 5300-15 000
1983 Tittabawassee 140-160
River
1983 Shiawassee River 120
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1983 Flint River 15-32
1983 Saginaw River 80-200
1983 Saginaw Bay 110-1100
Four samples of cow's milk from Germany have been analysed for
PBBs (Krüger, 1988). Three congeners were detected; BB 153
(0.025-0.053 µg/kg milk fat), BB 180 (0.001-0.007 µg/kg) and BB 187
(0.005-0.014 µg/kg). The other 30 congeners covered by the method
were not detected with detection limits ranging from 0.001 to
0.003 µg/kg milk fat (see Table 33).
The processing and cooking of contaminated food have been found
to have some potential for reducing PBB levels. Spray-drying
appeared to reduce the contents of PBBs in whole milk and skim milk
by 30-36% and 61-69%, respectively (Murata et al., 1977; Zabik
et al., 1978). Pressure cooking of chicken pieces also resulted in a
loss of PBBs, however, part of the PBBs lost were found in the drip
(Zabik et al., 1978).
5.1.5 Other products
Antibiotics used for attending farm animals were also found to
be contaminated: Levels of PBBs in aureomycin, which was distributed
by the Michigan Farm Bureau, were as high as 70 mg/kg (Di Carlo
et al., 1978).
5.1.6 Terrestrial and aquatic organisms
5.1.6.1 Aquatic and terrestrial plants
Only few data on PBB contamination of aquatic and terrestrial
plants are available. Stratton & Whitlock (1979) analysed algae
(e.g., filamentous green algae) from surface waters in the vicinity
of White Chemical (Bayonne, New Jersey) and near Standard T Chemical
Company (Staten Island, New York) for PBBs (MoBB through DeBB). The
two samples did not contain detectable levels of PBBs (detection
limit: 10 µg/kg, dry weight). However, bottom sediments taken in the
same location contained hexa- and heptabromobiphenyl.
Surface contamination was observed on terrestrial vegetation in
the vicinity of PBB facilities (up to 92 mg/kg dry weight; Stratton
& Whitlock, 1979). As Chou et al. (1978) reported, the PBB
contamination of field soils in Michigan (USA) did not result in any
detectable surface contamination of field crops.
5.1.6.2 Animals
a) Wildlife
Most earlier data available on PBB contamination of wildlife
refer to freshwater fish (Table 30) and birds (Tables 31 and 32),
primarily waterfowl in the USA. Recent reports refer to PBB
contamination of fish-eating mammals and birds from marine
environments in the USA (Kuehl et al., 1991) and in Europe (Jansson
et al., 1987, 1992; Krüger, 1988). Residues were found also in
terrestrial mammals (Jansson et al., 1992) and in freshwater and
marine fish in Europe (Krüger, 1988; Jansson et al., 1992).
Table 30 gives PBB levels in fish captured for analysis in
industrialized areas of the USA, at various distances from PBB-
containing or -using facilities. PBBs were detected in several fish
species from all rivers or bays examined. The PBB levels ranged up
to a maximum of 1.33 mg/kg wet weight (approximately equivalent to
30 mg/kg on a fat basis) found in carp from the Pine River near
Michigan Chemical Company (Hesse & Powers, 1978).
No apparent change in PBB concentrations was observed in Pine
River fish between 1974 and 1976 (Hesse & Powers, 1978; see also
Table 30). Although Michigan Chemical Co. had terminated PBB
production in 1974, even in 1983, Jaffe et al. (1985) detected PBB
in fish from the Saginaw River system, with highest concentrations
in fish from Pine and Chippewa Rivers (Table 30). While carp from
Tittabawassee River, to which Pine River joins, did not contain any
detectable PBBs in 1974, PBB-residues were detected (approximately
150 µg/kg on a fat basis) in 1983 (Table 30).
Various PBB homologues were examined in killifish (Oryzias
latipes) from Kill van Kull River near White Chemical Co.
(Bayonne, New Jersey). The main component found was NoBB. In the
vicinity of a FireMaster(R)-using facility (Staten Island, New
York), killifish samples contained only HxBB (Stratton & Whitlock,
1979).
PBB contamination has been reported in wild ducks collected
within two miles of the Michigan Chemical Corporation plant (Table
31), in eggs of waterfowl nesting around Green Bay and other areas
of Lake Michigan and on Lake Michigan island (Table 32), and, in
bald eagles found moribund or dead in 13 US states (Table 31).
Approximately one third of bald eagles examined contained PBB
residues (see Table 31).
Concentrations of PBBs in duck samples with skin left on were
considerably higher than those in skinless samples (see Table 31)
indicating that much of the PBBs is associated with the skin or fat
layer between the skin and muscle (Hesse & Powers, 1978).
Table 31. PBB residues in birds (ducks and bald eagles)
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
1974 Pine River Mallard breast tissue 3 0.25 Hesse & Powers
within two miles (skinless) (1978)
downstream from
St. Louis
Wood duck 0.29
Teal 1.8
1976 Mallard breast tissue:
skinless 3c 0.24
with skin 3c 2.00
Wood duck
4c 0.17
4c 2.70
1977 Wood duck breast tissue:
skinless 4c 0.08
with skin 4c 0.23
1977 Teal breast tissue:
skinless 0c (1) not detected
with skin 0c (1) not detected
Table 31 (contd).
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
13 US states Bald eagle found moribund Kaiser et al.
(Haliaeetus or dead; (1980)
leucocephalus)
carcass 10 0.03-0.27 0.07
(32)d
brain 7 0.03-0.17 0.05
a Number of samples containing residues; median is based on this number. Total number of samples in parentheses.
b PBB values were based on the major hexabromobiphenyl peak (BB 153).
c Paired samples.
d Detection limit: 0.02 mg PBBs/kg.
Table 32. PBB residues in eggs of fish-eating and non-fish-eating waterbirds from Green Bay and Lake Michigan (USA)
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Fish eater
1975 Green Bay Little gull 1 n.d. Heinz et al.
(Sensila Wildlife Area) (Larus minutus) (1985)
1977 three Lake Michigan islands Red-breasted merganser 114 0.06 n.d.-0.13 Haseltine et al.
off the tip of Door County, (Mergus serrator) (109) (1981)
Wisconsin
1977 islands in north-western Red-breasted merganser Heinz et al.
Lake Michigan (Mergus serrator): (1983)
eggs from the same nests
randomly selected 49 0.05
unhatched 49 0.04
1977 Lake Michigan Herring gull 9 0.18 0.11-0.25 Heinz et
(Gravel Island) (Larus argentatus) (9) al. (1985)
1977 Green Bay Common tern 10 0.06 0.02-0.22 Heinz et al.
(Lone Tree Island) (Sterna hirundo) (10) (1985)
Green Bay (St. Vital Island) Common tern 2 (2) 0.03 0.03-0.04
(Sterna hirundo)
Green Bay (Portage Point) 2 (2) 0.03 0.02-0.06
Green Bay (Cat Island) Double-crested 4 (3) 0.01 n.d.-0.02
cormorant
(Phalocrocorax
auritus)
Table 32 (contd).
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Lake Michigan (Fish Island) 6 (3) 0.02 n.d.-0.05
Green Bay (Oconto Marsh) Black-crowned night-heron 1 (1) 0.02
(Nycticorax nycticorax)
Green Bay (Oconto Marsh) Green-backed heron 1 n.d.
(Butorides striatus)
Non-fish eater
1977 Three Lake Michigan Mallard 22 n.d. Haseltine
islands off the tip of (Anas platyrhynchos) et al. (1981)
Door County, Wisconsin
1977 Lake Michigan (three Gadwall 4 n.d.
islands off the tip of (Anas strepera)
Door County, Wisconsin)
Black duck (Anas rubripes) 3 n.d.
a Number of collected eggs (in parentheses: number of eggs with quantifiable levels of PBBs).
b PBB values were based on hexabromobiphenyl;
n.d. = No residue of quantifiable level. Level over which quantification was possible: 0.02 mg/kg.
Samples with no detectable residues were calculated in the means as one-half the quantification level.
While the majority of ducks analysed from the Pine River
contained measurable concentrations of PBBs (Table 31), the eggs of
ducks from Lake Michigan islands did not contain detectable PBB
residues (Table 32). In contrast, most eggs of fish-eating
waterbirds from Green Bay and Lake Michigan showed PBB residues
(Table 32). Highest concentrations were detected in herring gull
eggs (0.18 mg/kg wet weight), perhaps reflecting their year round
residence on the Great Lakes (Heinz et al., 1985).
Stratton & Whitlock (1979) analysed a snapping turtle captured
in the vicinity of Hexcel Fine Organics Division (Sayreville, New
Jersey) for hexa- to decabromobiphenyls and found a tissue
concentration of 20 µg hexabromobiphenyl/kg (dry weight).
Di Carlo et al. (1978) reported on PBB contamination of
miscellaneous wildlife, such as deer, rabbits, coyotes, and ravens,
without, however, specifying the sampling locations and the levels
of contamination.
In Europe, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153) was found
in fish from German and Swedish rivers at concentrations ranging
from 0.3 to 0.6 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see
also Tables 33 and 34). A trout sample from a breeding farm
contained much lower levels of PBBs than the fish samples from the
rivers (Krüger, 1988).
A residue of 22 µg BB 153/kg lipid was observed in pooled
samples of osprey specimens found dead in various parts of Sweden
(Jansson et al., 1992; Table 34).
Swedish reindeers (pooled samples) showed BB 153 levels as low
as 0.04 µg/kg lipid (Jansson et al., 1992; Table 34).
PBBs (as a group) were not found in otters (Lutra canadensis)
from a region relatively remote from industrial sites in north
eastern Alberta (Canada) (Somers et al., 1987).
Fish samples (freshwater and marine species) collected in 1983
from an industrial area of Japan (Osaka) did not contain "PBBs" (not
specified) (Watanabe & Tatsukawa, 1990).
Recently, PBBs have been identified in bottlenose dolphins
(Tursiops truncatus) collected during the 1987/88 mass mortality
event along the Atlantic Coast of the USA. All three animals
(females), analysed for PBBs (tetrabromo- to hexabromobiphenyl
congeners) within a subset of the screening programme for
anthropogenic contaminants, contained PBBs at concentrations ranging
from 14 to 20 ng/g lipid (Kuehl et al., 1991).
Table 33. Average concentrations (µg/kg lipid) of PBB congeners in fish, seals, cows, and human milk samples
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 103 0.02 0.12 0.10 < 0.02 < 0.02 not analysed
BB 131 + 142/146 0.30 0.62 0.25 0.03 < 0.02 < 0.01
BB 132 0.33 1.25 0.62 0.15 < 0.02 0.05
BB 135 + 144/151 0.69 4.10 1.48 0.46 < 0.02 0.12
BB 147/135 + 144 0.21 0.31 0.25 < 0.02 < 0.02 < 0.01
BB 148/136 0.10 0.13 0.11 < 0.02 < 0.02 < 0.01
BB 149 0.26 0.45 0.53 < 0.02 < 0.02 < 0.01
BB 153 0.60 2.39 1.31 0.81 0.04 1.03
BB 154/151 0.22 0.54 0.37 < 0.02 < 0.02 0.01
BB 155 0.66 2.64 1.11 0.40 < 0.03 0.05
BB 169 < 0.01 15.16 < 0.01 < 0.01 < 0.01 0.05
BB 176 0.03 < 0.01 0.02 < 0.01 < 0.01 < 0.05
BB 178 0.18 0.87 0.36 0.03 < 0.01 0.09
BB 179 0.08 0.04 0.04 < 0.01 < 0.01 < 0.05
BB 180 0.02 < 0.01 0.02 < 0.01 < 0.04 0.02
BB 181 + 174 0.01 0.01 0.01 < 0.01 < 0.01 < 0.05
BB 184 0.05 0.09 0.03 < 0.01 < 0.01 0.01
BB 185 0.03 < 0.01 < 0.01 < 0.01 < 0.01 < 0.05
BB 186 0.30 0.40 0.16 0.01 < 0.01 0.02
BB 187 + 182 0.03 0.05 0.04 0.03 0.01 0.33
BB 188 0.11 0.28 0.11 < 0.01 < 0.01 0.01
BB 192 0.01 < 0.01 < 0.01 < 0.01 < 0.01 n< 0.05
BB 194 0.07 < 0.02 0.04 < 0.02 < 0.02 < 0.05
BB 197 0.11 0.11 0.08 < 0.02 < 0.02 0.04
BB 198 0.27 0.14 0.12 < 0.02 < 0.02 < 0.05
BB 200 + 204 0.41 0.36 0.24 < 0.02 < 0.02 0.02
BB 201 0.09 < 0.02 0.03 < 0.02 < 0.02 < 0.05
Table 33 (contd).
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 202 0.87 0.42 0.36 < 0.02 < 0.02 0.01
BB 206 0.05 < 0.03 0.04 < 0.03 < 0.03 < 0.01
BB 207 0.06 < 0.03 0.02 < 0.03 < 0.03 < 0.01
BB 208 0.16 0.04 0.04 < 0.03 < 0.03 < 0.01
PBB 6.3 30.5 7.9 1.9 0.05 2.0
From: Krüger (1988).
Table 34. Concentrations (µg/kg lipid) of 2,2',4,4',5,5'-HxBB (BB 153)
in pooled biological samplesa
Species Number of Sampling Concentration
specimens in site of BB 153
the homogenate
Rabbit (Oryctlagus cuniculus) 15 S. Sweden not detected
Moose (Alces alces) 13 not detected
Reindeer (Rangifer tarandus) 31 N. Sweden 0.037
White fish (Coregonus sp.) 35 0.29
Arctic char (Salvelinus alpinus) 15 S. Sweden 0.42
Herring (Clupea harengus) 100 Bothnian Bay 0.092
Herring (Clupea harengus) 60 Baltic Proper 0.16
Herring (Clupea harengus) 100 Skagerrak 0.27
Ringed seal (Pusa hispida) 7 Svalbard 0.42
Grey seal (Halichoerus grypus) 8 Baltic Sea 26
Osprey (Pandion haliaetus) 35 S. Sweden 22
a From: Jansson et al. (1992).
In Europe, PBBs have been detected in seals (Phoca vitulina;
Pusa hispida), guillemots (Uria aalge; U. lomvi), and
white-tailed sea eagles (Haliaeetus albicilla). The concentrations
(estimated by comparison with the technical product FM BP-6) ranged
from 3 to 280 µg/kg lipid (Jansson et al., 1987). The concentrations
of PBBs in comparable samples from the Baltic Ocean were all higher
than concentrations in samples from the Arctic Ocean. The same was
true for polybrominated biphenyl ethers and PCBs (Jansson et al.,
1987).
Concentrations of BB 153 determined in marine fish ranged from
0.2 to 2.4 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see also
Tables 33 and 34). BB 153 levels of 0.4-26 µg/kg lipid were found in
seals (Krüger, 1988; Jansson et al., 1992; see also Tables 33 and
34).
Detailed isomer-specific PBB analyses were carried out by
Krüger (1988) in fish (several species) from the Baltic and North
Seas and from sections of the Lippe and Rur rivers in North
Rhine-Westphalia, Germany. Seal samples from Spitsbergen (Norway)
were also included in this investigation (Table 33). All samples
contained PBBs. The smallest number of PBB congeners was found in
seals (n = 5) from an area remote from industrial sites. The main
components were different hexabrominated isomers with
2,2',4,4',5,5'-hexabromobiphenyl reaching a mean concentration of
0.8 µg/kg fat. The mean concentrations of several PBB congeners and
isomers (penta- to nonabrominated biphenyls) measured in fish
(n = 35) ranged, mostly, between 0.01 and 2 µg/kg fat. The pattern
of PBB congeners found in fish differed in a characteristic manner,
depending on the different capture sites. While relatively high
amounts of nona- and octabromobiphenyls (besides polybrominated
biphenyl ethers) were present in fish from German rivers (n = 17;
several species), hexabrominated biphenyls were predominant in fish
from the North Sea and the Baltic Sea (n = 17; several species). In
all samples from the Baltic Sea (n = 6),
3,3',4,4',5,5'-hexabromobiphenyl was found in relatively high
concentrations (maximum concentration: 36 µg/kg fat), but it was not
detected in samples from the North Sea and from rivers. The
concentrations of the other hexabrominated biphenyls were mostly
higher in fish from the Baltic Sea than in fish from the North Sea.
b) Farm animals
Farm animals in Michigan were contaminated by PBBs, when
FireMaster(R) FF-1 was accidentally mixed with animal feed in
mid-1973 (see section 4.1). The PBB levels resulting from this event
varied greatly with the extent of exposure. Data reported in the
literature are compiled in Table 35. The extent of contami nation
can be seen from the fact that, during the months following the
event, 172 dairy and beef herds (18 000 animals), 32 swine herds
(3500 animals), 16 sheep flocks (1200 animals), and 92 chicken
flocks (1.5 million birds) were destroyed (Isleib & Whitehead, 1975;
Robertson & Chynoweth, 1975; Mercer et al., 1976). In relation to
these great numbers, the portion of highly contaminated animals was
small (e.g., 40 herds of cattle, as can be derived from
contamination values measured in milk (section 5.1.4.2).
5.2 General population exposure
Apart from data collected after the Michigan disaster, there is
only limited information on exposure of the general public. PBBs
have been detected in humans in the vicinity of manufacturing
premises and in a few sites in the USA and Europe, not directly
connected with PBB contamination.
Table 35. PBB levels in farm animals (derived from the Michigan
cattle food contamination incident in 1973)
Year Animal PBB concentrationa References
(Type of sample) (mg/kg)
1974 Poultryb (tissue) 4600 Kay (1977); IARC (1978)
Not specified Cattle (fat) up to 200 Pearson (1982)
Not specified Aborted calves 120-400 Kay (1977)
1974 Cattlec (body fat) 110-2480 Robertson &
Chynoweth (1975);
Mercer et al. (1976)
1974-75 Cattled (fat) 9-4100 Fries et al. (1978b)
1974 Cattleb (tissue) up to 2700 Kay (1977); IARC (1978)
1974 Cattlej (body fat) 174-200 Jackson & Halbert (1974)
March (1975) Dairy cattlee 1-12 Kay (1977)
1975 Cowsf (tissue-fat) not detected-1.69 Isleib & Whitehead (1975)
1975 Steers and heifersg not detected-2.27
(tissue-fat)
1975 Pigsh (tissue-fat) not detected-0.58
1975-76 Cattlei (male and not detected-0.13 Cook et al. (1978a)
female) (eye fat)
Not specified Cattlek (body fat) not detected-3.8 Mercer et al. (1976)
a PBB values were based on 2,2',4,4',5,5'-hexabromobiphenyl.
b From 22 farm premises.
c 21 highly exposed cows.
d 32 cows from one herd heavily contaminated during September/October
1973, and 9 calves borne to these cows in 1974.
e 16 herds of dairy cattle with a history of feed levels from 1 to 14 mg/kg PBB.
f-h Slaughter house survey during a 3-month period (January-April 1975).
f Number of samples: 216; mean-PBB: 0.018 mg/kg.
g Number of samples: 247; mean-PBB: 0.030 mg/kg.
h Number of samples: 213; mean-PBB: 0.017 mg/kg.
i Cattle of 5 affected herds.
j 2 cows of the Halbert farm.
k Cattle of 12 affected herds.
5.2.1 Quantified data on human exposure
5.2.1.1 Worldwide
For most human populations, direct data on exposure to PBBs
from various sources have never been documented. This is true also
for the possible exposure of the general population from the use of
PBB-containing plastic products, and from fumes, generated in the
combustion of these products inadvertently in fires, or from burning
in dumps (Kay, 1977), and, additionally, from sources such as
PBB-containing landfills or PBB-manufacturing and processing
plants.
5.2.1.2 The Michigan Accident
Widespread human exposure resulting from direct contact with
contaminated feed, and, primarily, from the consumption of PBBs in
meat, eggs, and dairy products has been reported from the state of
Michigan, USA (Kay, 1977; Landrigan, 1980; Fries, 1985b; Table 36).
Many Michigan residents were exposed to PBBs between the onset of
contamination in the autumn of 1973 and the establishment of the
quarantine of affected farm animals in the spring of 1974. There was
considerable variation in both lengths and levels of exposure. At
least 2000 families (primarily farmers and their neighbours)
received the heaviest exposure (Meester & McCoy, 1976; IARC, 1978).
Brilliant et al. (1978) concluded from their results of human
milk analyses, conducted in 1976, that about 8 million of the 9.1
million residents of Michigan have detectable body burdens of PBBs.
Further studies (see Table 37) confirmed this widespread
distribution of PBBs.
The amount of PBBs consumed or absorbed by the various groups
in Michigan cannot be determined accurately (Safe, 1984). However,
there have been some trials to estimate the possible exposure to
PBBs of farm families and other people. The estimates were based on
kinetic data and other observations, e.g., time and level of animal
exposure, residue levels in herds at the time of the contamination,
and serum levels of exposed people.
In this way, Fries et al. (1978a) estimated (assumptions: see
Fig. 4), that the total exposure of an individual in a farm family
consuming its own milk was, for example, 9.8 g over the 230-day
period, during which the contamination was undetected. The
cumulative intake over time is shown in Fig. 4. In addition, the
authors concluded that the most highly exposed people consumed from
5 to 15 g PBBs over a 230-day period via milk. The projected intake
of PBB via the meat of cows slaughtered for home consumption would
have exceeded the projected intake from milk.
Table 36. Approximate distribution of PBBs in the Michigan episodea
Item Amount (kg)
Total released 295
Not fed to livestock 45
Fed to livestock 250
Eliminated in faeces 125
Absorbed by animals 125
In human foods before regulation 94
a Modified from: Fries (1985b).
Application of a pharmacokinetic model (Tuey & Matthews, 1980)
to the mean serum concentrations for residents of quarantined farms
resulted in similar values, e.g., about 170 mg mean total exposure
per individual and 11.7 g highest exposure to PBBs (Fries, 1985b;
Brown & Nixon, 1979) supposed a consump tion of 1-20 g of PBB by
families on the most contaminated farms.
The exposure of an individual in the general population would
have a pattern over time as projected above for the farm family
(Fries et al., 1978a). However, the exposure level would have been
much less, because of dilution in the normal marketing channels (the
mixing of milk from a large number of producers; the use of meat of
cull dairy cattle for hamburger and processed meat products). The
calculations of Fries (1985b) indicate that total exposure was about
9-10 mg for an average male with an average adipose content.
However, the individual with the highest PBB serum concentration was
projected to have had a total exposure of about 800-900 mg.
Table 37. Distribution of serum levels of PBBs, Michigan, 1974a
Quarantined farms Non-quarantined farms
Adults Children Adults Children
Serum PBBs Number (%) Number (%) Number % Number %
(µg/litre)
0 3 3.7 - - 21 28.4 - -
2-19 43 52.4 8 28.6 52 70.3 29 96.7
20-90 19 23.2 10 35.7 1 1.4 1 3.3
100-490 11 13.4 3 10.7 0 0 0 0
500-2260 6 7.3 7 25.0 0 0 0 0
Total 82 100.0 28 100.0 74 100.1 30 100.0
a From: Humphrey & Hayner (1975).
The degradation rates of 2-, 3-, and 4-bromobiphenyl, at
30 µg/ml, were 2.3, 4.2, and 1.4 µg/ml per day, respectively, and
were comparable with those of monochlorinated biphenyls. Degradation
occurred when the substrates were supplied as the sole carbon source
or when added in combination with glucose. The major metabolite of
4-bromobiphenyl (para) was 4-bromobenzoate, identified by means of
cochromatography with an authentic compound in HPLC. Two bacterial
strains of the genus Pseudomonas, isolated from a lake sediment by
using p-chlorobiphenyl as a sole carbon source, were capable of
degrading 2-, and 4-bromobiphenyl, but they did not degrade
4,4'-dibromobiphenyl (Sugiura, 1992).
In contrast to several reports indicating that chlorobenzoates
are the principal stable metabolites of PCBs (Furukawa & Matsumara,
1976; Furukawa et al., 1979; Yagi & Sudo, 1980; Reichardt et al.,
1981), 4-bromobenzoate as well as 4-chlorobenzoate appeared
transient. For, when tested with the same bacterial consortium,
4-bromobenzoate at 30 mg/kg was readily degraded at the rate of
4 µg/ml per day (Kong & Sayler, 1983). The terminal decomposition
product is assumed to be CO2 (Kong & Sayler, 1983), i.e.,
4-bromobiphenyl can most likely be completely mineralized by this
bacterial culture. Suflita et al. (1982) also observed degradation
of bromobenzoates. They reported the reductive dehalogenation of
halobenzoates, including 2-, 3-, or 4- bromobenzoate by
microorganisms of lake sediment and sewage sludge. Dehalogenation
required strict anaerobic conditions. The primary degradative event
was loss of the aryl halide without the alteration of the aromatic
ring, the end products were CH4 and CO2. The stable bacterial
consortium enriched from sludge consisted of both chemolithotrophic
and heterotrophic methanogens as well as three, unidentified,
non-motile Gram- negative rods.
Recently, there was a brief report on the reductive
debromination of the FireMaster(R) mixture (rate and extent of
debromination reaction not given) by anaerobic microorganisms eluted
from PCB-contaminated river sediments (Quensen et al., 1990;
abstract only).
4.2.3 Degradation by plants and animals
No degradation of PBBs by plants has been recorded. In contrast
to plants, animals can easily absorb PBBs and, though they have been
found to be very persistent in animals, small amounts of PBB
metabolites have been detected. The main metabolic products were
hydroxy-derivatives and, in some cases, there was evidence of
partially debrominated PBBs (cf. also section 6.3).
The metabolism of crude FireMaster(R) BP-6 by a pig gave a
monohydroxypentabromobiphenyl (Kohli & Safe, 1976). The faeces of
dogs fed FireMaster BP-6 contained a metabolite identified as
6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl (Gardner et al., 1979).
However, the authors do not exclude microbial metabolism of PBB in
the dog's gut followed by excretion into the faeces. Doses of
2,2',4,4',5,5'-[14C]-hexabromobiphenyl given intravenously or
orally to male rats were not subject to appreciable metabolism
(Matthews et al., 1977). Metabolites were not detected in tissue
extracts. A trace of radioactivity, which may have represented a
PBB-metabolite, was found in bile and faeces, but the quantity was
too small to be isolated and identified.
Some investigations imply that fish may debrominate the more
highly brominated components of PBB-mixtures. Fish (juvenile Salmo
salar), exposed in laboratory studies to FireMaster(R) BP-6 in
water, contained several mono to pentabromobiphenyls that were not
present in BP-6. Several additional pentabromobiphenyls were
detected in fish fed FireMaster(R) BP-6-contaminated food. Fish
fed octabromobiphenyl contaminated food contained unidentified
penta-, hexa-, and heptabromobiphenyls in addition to the
octabromobiphenyls (Zitko, 1977). It was not known whether the
partially debrominated biphenyls were generated by the fish, or by
the associated microflora.
Because the carbon-bromine bond is less stable than the
carbon-chlorine bond, reductive debromination may be a degradative
pathway of bromobiphenyls, and this reaction may have toxicological
consequences not encountered with PCBs (Zitko & Hutzinger, 1976;
Zitko, 1977).
4.2.4 Bioaccumulation
As expected from their high lipophilicity, PBBs show a marked
tendency to accumulate in animals. However, data are available only
on single links of food chains. It has been reported that similar
compounds, e.g., PCBs, which are more widely spread in the
environment, may have bioconcentration factors of 3-4 orders of
magnitude between water and fish, with a further 1-2 orders of
magnitude between whole fish and the fat storage tissues of fish
predators, such as cormorant, heron, and seal (Pearson, 1982).
4.2.4.1 Aquatic organisms
Fish are the only aquatic organisms for which the bioaccumu
lation of PBBs has been investigated intensively. They serve as an
example for the efficiency of such bioaccumulation (Damstra et al.,
1982).
Fathead minnows (Pimephales promelas) caged in a river, where
water levels of PBB (Firemaster(R) BP-6; probably measured as
concentration of the main peak, 2,2',4,4',5,5'-hexabromobiphenyl)
remained consistently at less than 0.1 µg/litre, concentrated these
contaminants in their bodies more than 10 000 fold in two weeks of
exposure (Hesse & Powers, 1978). In laboratory studies, accumulation
coefficients of FireMaster(R) BP-6 and technical octabromobiphenyl
from water (A = concentration in fish, µg/g wet weight/concentration
in water, µg/ml) and from food (B = concentration in fish, µg/g wet
weight/concentration in fish-food, µg/g) were determined.
FireMaster(R) BP-6 reached values of A = 48 (after an exposure of
48 h) and B = 1.0 (in equilibrium) in juvenile Atlantic salmon
(Salmo salar). The main component accumulated was
2,2'4,4',5,5'-hexabromobiphenyl (Zitko, 1977). In contrast,
octabromobiphenyl, as such, was not concentrated from water by
Atlantic salmon (Zitko, 1977) and by rainbow trout (Norris et al.,
1973), but a little uptake (B = 0.023) was observed from suspended
food (Zitko, 1977). Instead of octabromobiphenyl, an unidentified
hexabromobiphenyl was mainly accumulated (A = 1.73; B = 0.114;
Zitko, 1977). In comparison with Aroclor 1254, the accumulation of
FireMaster(R) BP-6 from water was less, but accumulation from food
was a little higher than that of the corresponding PCB mixture (A =
282; B = 0.358; Zitko, 1977).
There are some differences in the accumulation of different
congeners. Zitko & Hutzinger (1976) determined accumulation
coefficients of di-, tri-, and tetrabromobiphenyls in Salmo salar.
The coefficients were calculated on the basis of accumulation from
water after a 48-h exposure, and of the extrapolated equilibrium
levels in fish fed contaminated food. The accumulation coefficients
generally decreased with increasing degree of substitution during
the uptake from water, and increased, when taken up from food. Of
the dibromobiphenyls, the 3,4-isomer accumulated from water much
less than the 2,6- and 2,4-isomer, and did not accumulate from food
(Zitko & Hutzinger, 1976). Sugiura et al. (1978) dealing with the
accumulation of lower substituted halobiphenyls (di-, tri- and
tetra-) in killifish (Oryzias latipes) found that equilibrium
accumulation from water was not reached during a period of 20 days.
Their data, derived from a flow through test using PBB
concentrations of 0.5-50 µg/litre, resulted in bioaccumulation
factors (equilibrium extrapolated) ranging from 340 to 7340. They
also found that accumulation factors were proportional to partition
coefficients (n-octanol/water), when the coefficients were below
106, but not when the coefficients were above 106.
It is obviously important to note the lipid contents of test
animals in bioaccumulation studies. For example, the bioconcen
tration factors of PCB congeners for whole fish tissue were
proportional to the lipid content of the different species, which
can range from 3 to nearly 20% (Sugiura et al., 1979). Gobas et al.
(1989) reported lipid weight-based bioconcentration factors, log
KL ranging from 5.06 to 6.16, for some PBBs (di- to hexa-) in the
guppy (Poecilia reticulata).
4.2.4.2 Terrestrial organisms
Bioaccumulation of PBBs in terrestrial organisms has been
considered only for avian and mammalian species of farm and
laboratory animals. Data were obtained through field observations
(accumulation from soil), evaluation of an accident, and through
controlled feeding studies.
Accumulation of soilborne PBBs has been studied in Michigan
farms that were contaminated accidentally by FireMaster(R) FF-1
(Fries, 1985a). Ratios of PBB concentrations between the fat of farm
animals (cows, sheep, pigs) and soil ranged from 0.10 to 1.86.
Multiparous dairy cows had lower ratios, because of the excretion of
PBB in milk during long-term lactation, and swine had higher ratios,
because they ingest greater amounts of soil than other species
(Fries, 1985a). In another study, PBB (FireMaster(R) FF-1) was
applied to the soil surface for experimental purposes. Sheep grazing
for 180 days on these plots containing 33 mg PBB/m2 (plot 1) and
48 mg/m2 (plot 2) reached average residue levels in body fat of
0.30 and 0.79 µg PBB/g fat (quantified as concen trations of
2,2',4,4',5,5'-hexabromobiphenyl), respectively. Average residue
concentrations in ewes that grazed for 60 days were nearly as great
(Fries & Marrow, 1982). A second trial, conducted 3 years later
after ploughing and reseeding the plots, showed that PBBs were
distributed throughout the top 16 cm of soil with an average
concentration of 0.14 µg 2,2',4,4',5,5'- hexabromobiphenyl/g soil in
plot 2. Sheep grazing here for 136 days had average concentrations
of 0.032 µg PBB/g body fat (Fries & Marrow, 1982).
The accidental ingestion of FireMaster(R) FF-1 by cattle on
Michigan farms, first described by Jackson & Halbert (1974),
resulted in high body burdens of PBBs. There were tissue levels of
2,2',4,4',5,5'-hexabromobiphenyl in the fat of cows of up to
approximately 4000 mg/kg, nearly one year after high exposure
(estimated total dose: 150-400 g of FireMaster(R) FF-1/cow over
14 days). Low exposure from cross contamination produced PBB
concentrations in fat of less than 0.3 µg/g (Fries et al., 1978a,b;
Fries, 1983).
Laboratory data for the accumulation of PBBs from known diets
are given in Table 19 (diets supplemented with FireMaster(R)) and
in Table 20 (diets supplemented with single PBB congeners). PBB
levels in tissues of FireMaster(R)-exposed animals were expressed
as the concentration of the most abundant constituent of the
mixture, namely 2,2',4,4',5,5'-hexabromobiphenyl. Fries et al.
(1976) additionally reported the concentration of a heptabromobi
phenyl component (not the pure isomer). They found 31.4 mg/kg of
this component in the body fat of hens fed diets containing 20 mg
FireMaster(R)/kg feed for 63 days. The fate of minor constituents
of the FireMaster(R) mixture is not evident from the studies
compiled in Table 19.
Generally, accumulation of PBBs in body fat depended on dosage
and duration of exposure. The highest accumulation coefficients
(mg PBB/kg of tissue divided by mg PBB/kg feed) were found in minks
(Table 19). PBB residue levels in the adipose tissue of treated
minks were 60 times the amount in the diet (Aulerich & Ringer,
1979). According to the authors, the high diet-to-fat residue
accumulations in the minks may be due, in part, to the relatively
small subcutaneous fat deposits of the test animals, most of which
were extremely emaciated at the time of death. Technical
octabromobiphenyl was also accumulated from the diet, as shown by
analyses of the bromine contents of the tissues (Norris et al.,
1973; Lee et al., 1975a; Waritz et al., 1977). There was a
dose-related build-up of bromine, predominantly in the fat, as well
as in the liver, of rats fed octabromobiphenyl. For example, after 4
weeks of feeding 1, 10, 100, or 1000 mg octabromobiphenyl/kg feed,
the bromine concentrations in adipose tissue were 2, 12, 120, and
600 times, respectively, greater than those of the controls (Lee
et al., 1975a).
Table 19. Accumulation of PBBs in feeding studies on mammals and birds
a) Feeding of FireMaster(R)
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Rat (male) BP-6 0.1 20.9c 9 days 0.3 1.5 brain: 0.5 lipid Render et al. (1982)
Rat (male) BP-6 1 23.3b 9 days 1.7 8.3 brain: 1.8 lipid
Rat (male) BP-6 1 not specified 2-3 weeks - 2.7 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 1 not specified 30 days 7.8 22.3 thymus: 21 lipid Akoso et al. (1982a)
Rat (male) BP-6 10 22.9b 9 days 27 135 brain: 12.3 lipid Render et al. (1982)
Rat (male) BP-6 10 not specified 30 days 61.6 310 kidney: 147 lipid Akoso et al. (1982a)
Rat (male) BP-6 50 not specified 2-3 week - 341 - dry Babish & Stoewsand
(1977)
Rat (male) BP-6 50 26b 10 weeks 864 55 - wet Harris et al. (1978b)
Rat (male) BP-6 100 22b 9 days 251 1213 brain: 103 lipid Render et al. (1982)
Rat (male) BP-6 100 not specified 30 days 1535 2507 thymus:1044 lipid Akoso et al. (1982a)
Rat (male) BP-6 100 27b 10 weeks 3460 107 - wet Harris et al. (1978b)
Rat (male) BP-6 150 26b 10 weeks 3574 295 - wet
Rat (male) BP-6 200 26b 10 weeks 3242 245 - wet
Mouse BP-6 100 not specified 14 days 223 33.2 thymus: 391 wet Corbett et al. (1978a)
(male)
Mouse BP-6 1000 not specified 11 days 39.5 2.5 - wet Corbett et al. (1975)
(male)
Mouse FF-1 5 not specified 3 weeks - 7 thymus: 20 wet Loose et al. (1981)
(male)
Mouse FF-1 5 not specified 6 weeks - 15 thymus: 37.8 wet
(male)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mouse FF-1 167 not specified 3 weeks - 154 thymus: 109 wet
(male)
Mouse FF-1 167 not specified 6 weeks - 623 thymus: wet
(male) 3088
Sheep BP-6 50 1000 30 days 25 12 heart: 4.3 wet Gutenmann & Lisk
(male) (omental (1975)
fat)
42 (renal
fat)
17
(brisket
fat)
Pig BP-6 20 1880c 4 weeks 0.33 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 20 1880c 16 weeks 64 8.5 muscle: 6.6 wet
(back fat)
42.9
(leaf fat)
Pig BP-6 200 1230c 4 weeks 6.7 - - wet Ku et al. (1978)
(back fat)
Pig BP-6 200 1230c 16 weeks 503 17.2 muscle: wet
18.4
(back fat)
459
(leaf fat)
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Mink FF-1 2.5 not specified 136 days 149 - muscle: 7.3 wet Aulerich & Ringer
6.25 172 days - - brain: 66 wet (1979)
15.6 72-93 986 - muscle: 70 wet
days
Japanese not 10 -b 9 weeks - 48 heart: 78 dry Babish et al. (1975a)
quail specified
(male) 20 -b 9 weeks - 374 kidney: 105 dry
100 -b 9 weeks - 642 kidney: 725 dry
Japanese not 10 -b 9 weeks - 99 heart: 48 dry
quail specified
(female) 20 -b 9 weeks - 225 heart: 50 dry
100 -b 9 weeks - 503 kidney: 428 dry
Chicken BP-6 20 not specified 63 days 79.8 - egg: 20 wet Fries et al. (1976)
(White
leghorn
hens)
BP-6 20 -b 4-8 weeks - - egg: 30 wet Cecil & Bitman (1978)
BP-6 64 -b 4-8 weeks - - egg: 100 wet
not 1 106b 5 weeks 0.6 egg: 1.5 wet Ringer & Polin (1977)
specified
not 125 94c 5 weeks - - egg: 209 wet Ringer & Polin (1977)
specified
not 625 28.4c 5 weeks - 80 - wet
specified
Table 19 (contd).
Species FireMaster(R) Dietary Feed intake Feeding Residue level (mg/kg)a Weight References
concentration (g/day) period basis
(mg/kg)
Adipose Liver Others
tissue
Chicken FF-1 0.2 99b 5 weeks (-)d (-)d egg: 0.3 wet Polin & Ringer
(White (1978a,b)
leghorn FF-1 1 106b 5 weeks (-)d (-)d egg: 1.5 wet Polin & Ringer
hens) (1978a,b)
FF-1 5 100b 5 weeks (-)d (-)d egg: 7.4 wet Polin & Ringer
(1978a,b)
FF-1 25 99b 5 weeks (-)d (-)d egg: 43.4 wet Polin & Ringer
(1978a,b)
FF-1 125 94c 5 weeks (-)d (-)d egg: 215 wet Polin & Ringer
(1978a,b)
Chicken FF-1 0.1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
(White 0.11
leghorn FF-1 1 35 2 weeks - - carcass: wet Polin & Leavitt (1984)
cockerels) 0.87
FF-1 10 -b 28 days - 83.8 - lipid Dharma et al. (1982)
FF-1 100 -b 28 days - 752 - lipid Dharma et al. (1982)
a Measured as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
b Values not significantly different from control values.
c Values significantly different from control values.
d Diagrams only, generally, the ratios of tissue PBB: diet PBB averaged 3:1 for adipose tissue, 0.8:1 for liver,
and 1.5:1 for whole egg.
Table 20. Accumulation of PBBs in feeding studies on mammals and birds
b) Feeding of individual PBB congeners
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,2',4,4',5,5'- rat 0.1 23.8a 9 days 0.2 1.7 brain: 0.3 Render et al. (1982)
Hexabromobiphenyl (male)
1 26.2b 9 days 3.1 11.4 brain: 1.1
rat 1 not 30 days 16 68.6 kidney: 38.7 Akoso et al. (1982a)
(male) specified
rat 10 25.5b 9 days 31.2 181 brain: 11.5 Render et al. (1982)
(male)
rat 10 not 30 days 149 693 kidney: 373 Akoso et al. (1982a)
(male) specified
rat 100 23.2a 9 days 436 2558 brain: 143 Render et al. (1982)
(male)
rat 100 not 30 days 992 6062 thymus: 3841 Akoso et al. (1982a)
(male) specified
chicken 10 -a 28 days - 105 - Dharma et al. (1982)
(male)
62 -a 28 days - 751 -
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
2,3',4,4',5,5'- rat 1 not 30 days 9.1 23.5 kidney: 13.9 Akoso et al. (1982a)
Hexabromobiphenyl (male) specified
rat 10 not 30 days 69.1 242 thymus: 211 Akoso et al. (1982a)
(male) specified (1982a)
rat 100 not 30 days 648 4340 thymus: 1639 Akoso et al. (1982a)
(male) specified
chicken 4 -a 28 days - 32.5 - Dharma et al. (1982)
(male)
chicken 10 -a 28 days - 132 - Dharma et al. (1982)
(male)
3,3',4,4',5,5'- rat 0.1 23.6a 9 days 0 3.3 brain: 0 Render et al. (1982)
Hexabromobiphenyl (male)
1 24.7a 9 days 0.4 101 brain: 0
rat 1 24.7b 30 days 0.6 125 thymus: 0 Akoso et al. (1982a)
(male)
rat 10 20.2b 9 days 1.9 - brain: 0 Render et al. (1982)
(male)
rat 10 21.3b 30 days 6.9 448 thymus: 20.4 Akoso et al. (1982a)
(male)
Table 20 (contd).
Species Species Dietary Feed intake Feeding Residue level (mg/kg lipid) References
(sex) concentration (g/day) period
(mg/kg) Adipose Liver Others
tissue
rat 100 13.7b 9 days 22.5 1098 brain: 0 Render et al. (1982)
(male)
a Values not significantly different from control values.
b Values significantly different from control values.
Data on accumulation of technical decabromobiphenyl have not
been found in the literature.
Isomer specific accumulation has been studied for three
hexabromobiphenyl congeners. The residue levels in rats and chickens
fed with 2,2'4,4',5,5'-; 2,3',4,4',5,5'- or 3,3',4,4',5,5'-
hexabromobiphenyl are listed in Table 20. In many cases, the lowest
concentrations in tissues were found with 3,3',4,4',5,5'-
hexabromobiphenyl and the highest, with
2,2',4,4',5,5'-hexabromobiphenyl.
4.3 Ultimate fate following use
4.3.1 Disposal of PBB-contaminated animals and wastes from the
Michigan disaster
Accidental contamination of livestock feed in 1973 by PBBs led
to the destruction of over 30 000 animals in Michigan. As the
toxicity and other physical and chemical properties of PBBs were at
that time not so well known, the State of Michigan decided to locate
an environmentally safe site for the burial of contaminated
carcasses (Shah, 1978). A site in Kalkaska County was chosen and
test drilled in order to determine the long-range protection for
groundwaters in the area. The Kalkaska disposal site received over
10 000 animal carcasses most of which contained PBB levels above
1 mg/kg fat, and close to 20 000 carcasses with PBB levels ranging
from 0.3 to 1 mg/kg. This animal disposal site contains
approximately 45 kg of PBBs in all buried carcasses (Shah, 1978; see
section 5.1.2.3. for groundwater studies).
The Gratiot County landfill near St. Louis became operational
in late 1970, and it was designed only for general municipal solid
waste disposal. According to the Michigan Chemical Corporation
report to the Environmental Protection Agency, PBB wastes were
disposed of in the landfill between 1971 and 1973. Wastes containing
large amounts of PBBs (60-70%) were received in the landfill before
any information about the toxic effects of PBBs on animals was
publicly known (Shah, 1978).
The Forest Waste Disposal site consists of an 11-acre,
abandoned, municipal and industrial waste landfill and 9 surface
impoundments. It is located in Genesee County, Michigan, and is
surrounded by agricultural land and undeveloped woodlands and
wetlands. Forest Waste Disposal conducted landfill operations from
1972 to 1978. PBB-contaminated feed has recently been found in the
landfill. A decontamination programme has been recommended (Anon.,
1988).
The Michigan Chemical Corporation stated that, in their
opinion, PBBs would eventually undergo oxidative/biological
degradation forming carbon dioxide, water, and bromide ion (Cordle
et al., 1978). However, studies on PBBs in soil indicate that they
may remain in soils for many years, because of their resistance to
degradation (Jacobs et al., 1976).
4.3.2 Thermal decomposition of PBBs
There is little information on the pyrolysis of PBBs. The
products of the thermal decomposition of PBBs depend on the
temperature as well as on the amount of oxygen present.
Norris et al. (1973) constructed a special apparatus to measure
the relative amounts of bromine from octabromobiphenyl converted
during combustion, when these materials were used as additives in
thermoplastic resins. An exact temperature is not given. Hydrogen
bromide and bromine were not detected.
Waritz et al. (1977) carried out experiments to determine the
approximate lethal temperature of hexa- and octobromobiphenyl. The
dense clouds of fumes obtained at 350 °C were lethal to rats whereas
those produced at 290 °C were not. The fumes were not analysed.
Earlier experiments by Benbow & Cullis (1975) on the pyrolysis
of decabromobiphenyl pressed together at 160 °C with polystyrene and
polypropylene, respectively, showed that, during flameless
combustion, decabromobiphenyl appeared to be volatilized virtually
unchanged from the polymer, whereas when the polymer burned, the
decabromobiphenyl was converted quantitatively to hydrogen bromide.
In these early experiments, the analytical methods were not so
refined that it was possible to detect furans and dioxins. O'Keefe
(1978) pyrolyzed samples of FireMaster FF-1 at 380-400 °C in open
glass tubes and in tubes sealed after nitrogen flushing. Analysis by
low resolution direct probe mass spectrometry showed the presence of
tetra- and pentabrominated dibenzofurans in extracts of the open
tube pyrolyzed material and trace levels of tetrabromodibenzofuran
in those from PBB pyrolyzed under nitrogen.
Buser et al. (1978) studied the pyrolysis of FireMaster BP-6
with oxygen in sealed tubes. The flame retardant was completely
destroyed at 700 °C, but, at 600 °C, new compounds were formed, one
of which was probably tetrabromodibenzofuran.
The diversity of possible brominated and mixed brominated
furans and their toxicological implications led to further
refinements in analytical methods (Buser, 1986) and to the demand
for, and synthesis of, suitable standard isomers (Mason et al.,
1987a; Sovocool et al., 1987a; Munslow et al., 1989). There are over
5000 halogenated dibenzodioxins and dibenzofurans containing
chlorine and/or bromine, over 400 of which are 2,3,7,8-substituted
tetra-, penta- and hexahalo congeners suspected to be of high
toxicity (Buser 1987). These mixed congeners are of particular
importance with regard to chemical waste burning (Schäfer &
Ballschmiter, 1986).
Investigations into the pyrolysis of FireMaster BP-6 in the
absence of oxygen have shown that small amounts of bromobenzenes and
lower brominated biphenyls are formed (600-900 °C), but no furans
(Thoma et al., 1987a; Thoma & Hutzinger, 1989).
In contrast, the pyrolysis of FireMaster BP-6 in an open quartz
tube (700-900 °C) in the presence of oxygen yielded over 3 mg/kg
(ppm) of di- to heptabrominated dibenzofurans, though the pyrolysis
of pentabromodiphenyl ethers yielded brominated dibenzofurans at
over 300 times this level (Thoma et al., 1987a). In the presence of
polystyrene and polyethylene, higher levels of brominated
(mona-tetra) dibenzofurans (over 8 and 51 mg/kg (ppm), respectively)
were found (Thoma et al., 1987a). Pyrolysis of FireMaster BP-6 with
PVC at 800 °C yielded mixed bromide/chloride biphenyls, the bromine
atoms being substituted by the chlorine. No ring closure to dioxins
and furans occurred (Thoma et al., 1987b).
Decabromobiphenyl was pyrolyzed for 10 min at 800 °C in a
loosely plugged quartz tube. The pyrolysates were extracted with
toluene and after clean-up, analysed using GC/MS. No brominated
dioxins or dibenzofurans were detected (detection limits
0.2-0.8 µg/g). The clean-up was said to be very difficult because of
the formation of a large number of brominated compounds that were
not dioxins or furans. Debromination of decabromobiphenyl appeared
to be the main reaction, but no details were given (Atochem, 1987).
Zacharewski et al. (1988) pyrolyzed samples of FireMaster(R)
BP-6 in open quartz tubes at 800 °C for 10 min. The resulting
products, mainly tetrabromodibenzofurans (1183 µg/g) but also
tribromo-, pentabromo-, hexabromo-, and heptabromodibenzofurans
(187, 584, 107, and 11 µg/g, respectively), were tested for toxicity
(see section 8.12.3.2). Very little is known about the toxicities of
brominated and brominated/chlorinated dioxins and furans, but they
are estimated to be of the same order as those of PCDD and PCDF
(Mason et al., 1987a; Safe, 1987).
Analysis of actual environmental samples has also been carried
out. Monobromo-polychloro substituted benzenes, biphenyls,
dibenzodioxins, and dibenzofurans have been detected in solid
material collected from a chimney of an industrial waste incinerator
(Schäfer & Ballschmiter, 1986). Brominated dibenzo furans with a
very small amount of mixed brominated/chlorinated compounds were
detected in soot from an accidental fire at a bowling alley (Buser,
1986). Schwind et al. (1988; 1989) analysed samples from a municipal
waste incinerator and detected for the first time a complete series
of tetrahalogenated dibenzofurans (Cl4DF, Br1Cl3DF,
Br2Cl2DF, Br3Cl1DF and Br4DF). It is possible that PBCDD/F
could occur during the incineration of flame retardant-treated
plastic material, which produces PBDD and PBDF. These could react
with PVC via the mixed brominated/chlorinated dioxins and furans to
PCDD and PCDF (Schwind et al., 1988).
As with PCB disposal, the destruction of PCB-contaminated waste
should be carefully controlled. For PCBs, a burning temperature
above 1000 °C for 2 seconds is recommended (WHO/EURO, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Only one report is available on PBB levels in air. It refers to
air samples taken in the vicinity of three PBB-manufacturing or -
processing plants in the USA (Stratton & Whitlock, 1979). Traces of
hexabromobiphenyl (0.06-0.10 ng/m3) were found at two of the three
industrial sites examined.
Further information on PBB levels in ambient air, e.g., near
municipal incinerators, is lacking.
5.1.2 Water and sediments
5.1.2.1 Surface waters
Surface waters have been monitored in the vicinity of PBB-
producing or -processing industrial sites in the USA and in the
vicinity of the Gratiot County landfill (Michigan, USA), which had
received 122 000 kg of wastes containing 60-70% PBBs between 1971
and 1973. The results are summarized in Table 21.
Depending on the sources, the predominant PBB compounds
detected in surface waters were hexabromobiphenyl and
decabromobiphenyl. However, only Stratton & Whitlock (1979)
determined all PBB homologues from Br1 to Br10, the percentage
composition of some of which is given in Table 22.
5.1.2.2 Sediments
Generally, PBBs reach higher concentrations in sediments (Table
23) than in the associated waters (Table 21).
PBB concentrations in sediments of the Pine River were as high
as 77 mg/kg near the Michigan Chemical Corp. plant. The assays
conducted from July 1974 to April 1975, upstream and downstream of
the plant, showed a decline in sediment PBB content to 6.2 mg/kg,
half a mile downstream, and to 0.1 mg/kg, 24 miles or 29 miles
downstream (Hesse & Powers, 1978).
Table 21. PBB levels in surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Pine River 1974 8 HxBB 0.01-3.2 Hesse (1975)
(downstream from
the Michigan
Chemical Co.,
St. Louis)
Tittabawassee 1974 2 HxBB < 0.01
Rivera
Canal (called 1977 3 total PBB not detected-46 Stratton & Whitlock (1979);
Platti Kill) in the (MoBB-DeBB) DeCarlo (1979)
vicinity of White
Chemical Co., Bayonne,
New Jersey
Canal (discharging 1977 1 total PBB < 0.2 Stratton & Whitlock (1979)
into Kill van Kull (HxBB-DeBB)
River) in the vicinity
of Standard T Chemical
Co., Staten Island,
New York
Storm sewer, 1977 5 total PBB < 0.2-210 Stratton & Whitlock (1979);
receiving swamp, etc. (HxBB-DeBB) DeCarlo (1979)
in the vicinity of
Hexcel Fine Organics
Sayreville, New Jersey
Table 21 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/litre)
Drain waters at 1977 not HxBB 0.1-14 Shah (1978);
or near the margin specified Rosenblatt et al. (1982)
of the Gratiot County
landfill, Michigan
a Note that PBBs were not detected in fish from the Tittabawassee River in 1974 but were detected in 1983 (see Table 30).
Table 22. Percentage of different PBB homologues detected in surface water
samples taken in the vicinity of PBB-producing plantsa
PBB homologues Bayonne, New Jersey Sayreville, New Jersey
(White Chemical Corp.)b (Hexcel Corp.)c
Sample 1 Sample 2 Sample 1 Sample 2
PeBB 1 0 - -
HxBB 4 2 5 < 1
HpBB 2 2 1.6 < 1
OcBB 2 17 1 1
NoBB 15 20 1 2.6
DeBB 76 58 91 96
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Producer of laboratory quantities of various PBBs.
Concentrations measured upstream were all less than the
sensitivity limit of 30 µg/kg with the exception of one sample
collected a quarter of a mile upstream of the plant, which contained
60 µg/kg (Hesse, 1975). This latter analysis was repeated in 1977
(Hesse & Powers, 1978) giving 350 µg/kg (detection limit 100 µg/kg).
Hesse & Powers (1978) compared the PBB levels of Pine River
sediments from the same locations over a period of time after the
termination of FireMaster(R) BP-6 production. The results of the
1976 and 1977 analyses showed that PBB distributions and
concentrations in the sediments had not changed significantly in the
three years after PBB manufacture stopped.
Various PBB homologues were identified again only by Stratton &
Whitlock (1979) who examined aquatic sediments, sludge deposits, and
marsh soils near the sites of manufacture and use of PBBs in New
Jersey and New York (see Table 24).
More recently, sewage sludge has been analysed for PBBs
(Strachan et al., 1983). The authors did not detect any PBBs, PCBs,
or chlorinated hydrocarbon pesticides in the three sludge samples
obtained from sewage treatment plants in three Indiana cities (USA).
However, the detection limit for PCBs and PBBs with the GC-MS system
used was about 10 µg/g, and this apparently is not sensitive enough,
even for PCBs. For example, the amounts of PCBs found in sewage
sludge of German (Lorenz, 1983) and Canadian (Webber et al., 1983)
cities ranged from 1.8 to 2.5 µg/g (dry weight) and from 0.13 to
1.61 µg/g (dry weight), respectively. Values for PBBs have not been
reported in these investigations.
Table 23. PBB levels in sediments and sludge from surface waters near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Near Michigan 1974/75 19 HxBB < 30-77 000 Hesse (1975)
Chemical Co. 1974 9 < 100-9200 Hesse & Powers (1978)
Pine River 1976 9 < 100-1200
1977 8 < 100-500
Tittibawassee River 1974 2 tr-16 Hesse (1975)
Near White Chemical 1977 3 total PBB < 10-20 Stratton & Whitlock (1979)
Co., Bayonne, New Jersey: (MoBB-DeBB)
Sediments from Platti
Kill Canal and Kill
van Kall River
Sludge from Platti 1 431 000
Kill Canal
Near Standard T Chemical 1977 1 60
Co., Staten Island, New
York Sediment from Kill
van Kull River (at
discharge site)
Near Hexcel Fine 1977 1 total PBB 4600 Stratton & Whitlock (1979)
Organics, Sayreville, (MoBB-DeBB) DeCarlo (1979)
New Jersey
Marsh Soil
Table 23 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (µg/kg dry weight)
Gratiot County 1977 not HxBB up to 17 000 Shah (1978)
landfill, Michigan specified Rosenblatt et al. (1982)
Associated sediments
of surface drain waters
Table 24. Concentration of PBB-homologues in aquatic sediment, sludge deposit,
or marsh soil samples taken in the vicinity of PBB-producing or -processing
plantsa (µg/kg dry weight)
PBB Bayonne, New Jersey Staten Island, Sayreville,
(White Chem. Corp.)b New Jersey New Jersey
(Standard T (Hexcel
Chem Corp.)c Corp.)d
Sediment Sludge Sedimente Marsh soile
samples:e
1 + 2 3
MoBB - n.d. 540 n.d. n.d.
DiBB - n.d. 2200 n.d. n.d.
TrBB - n.d. 4300 n.d. n.d.
TeBB - n.d. n.d. n.d. n.d.
PeBB - n.d. 590 n.d. n.d.
HxBB n.d. 10 3800 40 30
HpBB n.d. 10 3300 20 n.d.
OcBB n.d. n.d. 3600 n.d. n.d.
NoBB n.d. n.d. 22 500 n.d. 80
DeBB n.d. n.d. 390 000 n.d. 4500
a From: Stratton & Whitlock (1979).
b Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
c Major user of FireMasterR BP-6.
d Producer of laboratory quantities of various PBBs.
e n.d. = Not detected (detection limit = < 10 µg/kg).
Surficial sediments from the St. Lawrence River (USA/Canada)
were analysed for HxBB, but the compound was not detected (estimated
detection limit: 1 ng/g) at the ten stations surveyed (Sloterdijk,
1991).
5.1.2.3 Groundwater
Groundwater monitoring data from the Gratiot County landfill
(Michigan, USA) mentioned above, have shown trace levels of PBBs,
even outside the landfill area (see Table 25). However, so far,
domestic drinking-water wells have not shown any traces of PBBs
(Shah, 1978). Groundwater near the disposal site of PBB-
contaminated animals and other products (see section 4.3.1) in
Kalkaska County (Michigan, USA) is reported not to be contaminated
by PBBs (Shah, 1978).
Table 25. PBB levels in groundwater from Gratiot County landfill, 1977a
Site No. of PBB compound PBB
samples examined concentration
range (µg/litre)
Test wells within 4 HxBB 0.5-26
the landfill site
Observation wells 11 0.1-4.4
outside the landfill
area
Domestic not specified not detected
drinkingwater wells
near the landfill
a From: Shah (1978).
5.1.3 Soil
Data on soil pollution by PBBs are available for areas of
manufacture, use, or disposal of PBBs (Table 26), and for soils from
fields, etc. of the PBB-contaminated Michigan farms (Tables 27 and
28).
Concentrations of PBBs in soils from industrial sites were
highest (more than 2000 mg/kg) in areas around the Michigan Chemical
Company (see Table 26). Although such highly contaminated soils
were removed (Hesse & Powers, 1978), Hill et al. (1982) still found,
some years later, PBB levels up to 2130 mg/kg in soils of the former
manufacturing site. Various PBB homologues from Br4 to Br10 were
present in the industrial soil samples (see Table 27).
Table 26. PBB levels in soils near sites of manufacture, use, or disposal in the USA
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
Michigan Chemical Co.,
St. Louis, Michigan
bagging area not specified 1 HxBB 3500 mg/kg Hesse (1975);
Hess & Powers (1978)
loading area of not specified 1 HxBB 2500 mg/kg
the plant
"former manufacturing not specified 3 PBB 16-2130 mg/kg Hill et al. (1982)
site" (C12H6Br4-C12H3Br7)
Vicinity of White
Chemical Co.,
Bayonne, New Jersey
150 m east 1977 1 total PBB 4.250 mg/kg Stratton & Whitlock (1979)
150 m west of the 1977 1 (C12H9Br1-C12Br10) 1.135 mg/kg DeCarlo (1979)
plant
not specified not specified PBB 0.75-2.8 mg/kg Di Carlo et al. (1978)
Vicinity of Standard 1977 4 total PBB 10-100 µg/kg Stratton & Whitlock (1979)
T Chemical Co., Staten (MoBB-DeBB)
Island, New York
75 m south west 30
900 m west 10
Table 26 (contd).
Site Date of sampling No. of PBB compound PBB concentration References
samples examined (range) dry weight
1500 m south 10
700 m east (prevailing 100
down-wind direction)
Vicinity of Hexcel 1977 total PBB Stratton & Whitlock (1979);
Fine Organics, (MoBB-DeBB) DeCarlo (1979)
Sayreville, New Jersey
75 m southeast 1 40 µg/kg
Soil in roadside 1 3400 µg/kg
ditch
Gratiot County landfill,
St. Louis, Michigan
Samples inside of the not not "PBB" 12 (16) mg/kg Rosenblatt et al. (1982)
landfill from the specified specified HxBB
uppermost 2.5 cm
(after capping of
the landfill)
Sample somewhat distant "PBB" 61 µg/kg
from the landfill, in (HxBB)
the area of the Michigan
Chemical plant
Table 27. Concentration of PBB homologues detected in soil samples taken in the vicinity of PBB-producing or processing plants
(µg/kg dry weight)
PBB Bayonne, New Jerseya Staten Island, New Yorka Sayreville, New Yorka Michiganb
(White Chemical Corp.)c (Standard T-Chemical Corp.)d (Hexcel Corp.)e (Michigan Chemical Corp.)f
MoBB n.d.g n.d.g n.d.g -
DiBB n.d.g n.d.g n.d.g -
TrBB n.d.g n.d.g n.d.g -
TeBB n.d.g n.d.g n.d.g < 1000-510 000
PeBB n.d.g n.d.-100 n.d.g 4000-60 000
HxBB 15-30 n.d.-10 40-90 12 000-670 000
HpBB 30-110 n.d.-10 n.d.-90 < 1000-190 000
OcBB 90-150 n.d. n.d.-170 -
NoBB 330-2200 n.d. n.d.-440 -
DeBB 530-2100 n.d.-10 n.d.-2600 -
a Data from: Stratton & Whitlock (1979); No. of samples = 2, 4, 2 respectively.
b Data from: Hill et al. (1982); No. of samples = 3.
c Producer of octa- and decabromobiphenyl along with bromobiphenyl ethers.
d Major user of FireMaster(R) BP-6.
e Producer of laboratory quantities of various PBBs.
f Producer of FireMaster(R) BP-6.
g n.d. = Not detected (detection limit = < 10 µg/kg).
Table 28. Composition and concentration of PBBs in soil samples from former
FireMaster(R) manufacturing plant site (St. Louis, Michigan)a
% Composition (concentration in mg/kg)
FireMaster(R)
Compound Lot #5143 Soil 1 Soil 2 Soil 3
Tetrabromobiphenyls < 0.1 23.9 (510) 11.3 (6) (< 1)
Pentabromobiphenyls
2,2',4,5,5'- 3.9 2.8 (60) 9.4 (5) 12.5 (2)
2,2',4,4',5- < 0.1 5.2 (110) (< 1) (< 1)
2,3',4,4',5- 5.7 27.7 (590) 9.4 (5) 12.5 (2)
Hexabromobiphenyls
2,2',4,4',5,5'- 54.9 24.4 (520) 56.6 (30) 62.5 (10)
2,2',3',4,4',5- 10.3 4.7 (100) 7.5 (4) 6.2 (1)
2,3',4,4',5,5'- 5.0 1.9 (40) 5.7 (3) 6.2 (1)
2,3,3',4,4',5- 2.1 0.5 (10) (< 1) (< 1)
Heptabromobiphenyls
2,2',3,4,4',5,5'- 12.8 5.2 (110) (< 1) (< 1)
2,2',3,3',4,4',5- 1.7 3.8 (80) (< 1) (< 1)
(Total PBBs) (2130) (53) (16)
a Adapted from: Hill et al. (1982).
Hill et al. (1982) identified not only PBB homologues, but also
the isomeric composition of PBBs in the soil samples from the
Michigan Chemical Corp. plant (Table 28). Thus, they provided more
exact analytical data and were able to make an interesting
comparison with the original FireMaster(R) mixture; conclusions
could then be drawn on the environmental fate of PBBs (section 4.2).
According to Shah (1978), test samples of the Gratiot County
landfill showed that, in general, the concentrations of PBBs in the
fill increased with depth and were highest at a depth of 3 to 7.6 m
below the top of the refuse.
As a consequence of the Michigan cattle food mixing error, the
soils of the farms involved have been contaminated by PBBs, mainly
through the faeces of the exposed animals. Fries (1985b) calculated
that about 145 kg of PBBs were distributed in this way, and that
most of this was located on 20-25 farms. (The total number of
quarantined farms was over 500; Robertson & Chynoweth, 1975).
Concentrations of PBBs in soil samples from fields that had
received PBB-contaminated manure were as high as 371 µg/kg (dry
weight), whereas levels in samples from manure piles and from dirt
exercise lots were as high as 2000 µg/kg (Jacobs et al., 1978;
Fries, 1985b).
Soil contamination by PBBs can result in PBB accumulation in
animals, when they have direct access to the contaminated soil.
This is most likely to occur when animals are confined to dirt lots
on which manure-containing PBB has been deposited. Crops grown on
PBB-contaminated soils are not considered an important source of PBB
contamination in animals (Fries & Jacobs, 1986).
Soils from industrial sites have, in general, been more heavily
contaminated than Michigan soils.
5.1.4 Feed and food
5.1.4.1 Feed
Contamination of feed by PBBs has been reported only in
connection with the Michigan PBB incident.
In 1973, about 290 kg (Fries, 1985b) - 1000 kg (IARC, 1978) of
FireMaster(R) FF-1 was inadvertently mixed in cattle feeds and
delivered to Michigan farms.
Three feed preparations appeared initially to be involved in
the Michigan episode with PBB levels as follows:
Feed No. 405, 2.4 mg PBB/kg,
Feed No. 410, 1790 mg PBB/kg,
Feed No. 407, 4300 mg PBB/kg
(Cordle et al., 1978).
A concentration as high as 13 500 mg PBB/kg was also cited
(Kay, 1977; Di Carlo et al., 1978; Damstra et al., 1982). Feed of
one highly contaminated farm (Halbert farm) is reported to have
contained 2900 mg PBB/kg (Fries, 1985b).
In 1974, 68% of 1770 feed samples collected in Michigan
contained PBB residues: 60% in the range of trace to 0.99 mg/kg, and
8% over 1 mg/kg. Resampling in 1975 revealed that 6% of 1208 feed
samples were contaminated and that fewer than 0.16% contained more
than 1 mg PBB/kg. In 1976, only 0.3% of 663 samples analysed were
contaminated: no samples contained more than 0.1 mg/kg (Di Carlo
et al., 1978).
PBB residues were not detected in harvested forages grown on
soils with residue levels as high as 0.3 mg/kg (Fries & Jacobs,
1980).
In 1974 and 1975, low-level feed contamination with PBBs was
detected in Indiana and Illinois, which are neighbours of Michigan
(Di Carlo et al., 1978).
5.1.4.2 Food
Again, almost all the data available on PBB residues in food
are derived from the Michigan cattle food contamination incident in
1973.
The extent to which the general population was exposed depended
on where they obtained their milk, dairy products, and eggs, i.e.,
direct from the contaminated farms or from sources where
contaminated products had been mixed with non-contaminated samples.
Table 29 shows examples of some PBB levels in Michigan foods.
Whereas in 1974 milk from some highly contaminated cows contained
PBB concentrations of up to 900 mg/kg fat (Robertson & Chynoweth,
1975), canned milk samples contained concentrations of up to
1.6 mg/kg fat (Cordle et al., 1978). The most highly contaminated
milk (1 to > 100 mg PBB/kg milk fat) originated from a total of 40
herds with different levels of PBBs at the time of detection (Fries,
1985b). In 1975, PBBs were still detected in milk from some herds
(Kay, 1977).
Data on meat can be derived from Table 33, which shows PBB
levels in Michigan farm animals.
Milk contains far less fat than meat (about 4% versus 30%), and
butterfat contains only 40% of the PBB concentration found in the
animal from which it comes (Fries et al., 1978b; Rosenblatt et al.,
1982). Among the dairy products, PBBs are again concentrated in the
high-fat products (Murata et al., 1977; Zabik et al., 1978).
Table 29. Some examples of PBB levels in food (contaminated as a consequence of
the Michigan PBB incident in 1973)
Product Year of PBB concentration References
sampling (mg/kg)
Milka 1974 2.8-270.5h Cordle et al. (1978)
Milkb 1974 44-900h Robertson & Chynoweth (1975)
Milkc 1974 43-56h Jackson & Halbert (1974)
Milkd 1974 up to 595 Kay (1977); IARC (1978)
Milke 1974 1-> 100h Fries (1985b)
Canned milk 1974 1.15-1.62h Cordle et al. (1978)
Dry skimmed milkf 1974 0.75-1.5 Isleib & Whitehead (1975)
Fluid milk 1974 < 0.02-1.15
processors' products
Butter 1974 1-2h Cordle et al. (1978)
Cheese 1974 1.4-15.0h
Milkg 1975 1-13h Kay (1977); IARC (1978)
Eggs 1974 up to 59.7
a = Collected from individual farms.
b = Collected from 21 cows.
c = Collected from 2 cows (having 174 and 200 mg PBBs/kg in body fat,
respectively).
d = Collected from 22 farms.
e = Collected from 28 herds.
f = From one dairy plant.
g = Collected from 16 herds.
h = On a fat basis.
In May 1974, the US Food and Drug Administration (FDA)
established the following enforcement limits for unavoidable
residues of PBBs in foods: 1 mg/kg in the fat of meat, milk, and
dairy products, 0.3 mg/kg in animal feeds, 0.1 mg/kg in eggs. These
enforcement guidelines were reduced in November 1974 to 0.3 mg/kg in
the fat of meat, milk, and dairy products, and 0.5 mg/kg in eggs and
animal feeds. In February 1977, the FDA rejected a petition to lower
the enforcement guideline level to 0.02 mg/kg for all food products
(IARC, 1978). However, according to Fries (1985b), final
legislation, Act 77, lowered the tolerance to 0.02 mg/kg in the body
fat of all cull dairy cows offered for slaughter. (Unlike the
situation under the previous regulations, the finding of a single
animal with a higher than legal body fat level did not lead to
quarantine and the disposal of the whole herd.) As a result of the
rigid quarantine policy, the food levels of PBBs decreased in
Michigan. In 1975, none of 18 milk samples, 3 out of 14 butter
samples, and none of 13 cheese samples exceeded FDA guidelines
(0.3 mg/kg). Also in 1975, 245 of 2040 meat samples were
contaminated with PBBs: 24 contained more than 0.3 mg/kg. None of
the meat specimens collected in 1976 exceeded FDA guidelines: 96% of
1430 samples were contaminated, but only 1 sample contained more
than 0.6 mg/kg of PBBs. A market basket survey of meat in 1976
revealed detectable PBBs in only 1 out of 102 samples in Michigan
(Di Carlo et al., 1978).
Additional information on PBB findings is presented in several
government reports, which are cited by Di Carlo et al. (1978).
According to these reports 29 170 products had been assayed. In
1974, 14 out of 16 milk samples, 4 out of 34 butter samples and 11
out of 23 cheese samples, collected in Michigan, were found to
exceed FDA guidelines for PBBs. Another survey showed that 24.9% of
272 finished product samples, collected from May to October 1974,
were contaminated with PBBs and that 15.8% contained more than
0.3 mg/kg (Di Carlo et al., 1978).
PBBs were also detected in other states in the USA, for example
in beef in Iowa, duck in Wisconsin, chicken in Alabama, Mississippi,
New York, and Texas, and turkey in Indiana: the levels were
extremely low. During 1975 and 1976, PBBs were found in 9 out of 597
food samples outside of Michigan (Di Carlo et al., 1978).
Food contamination, not derived from the Michigan PBB-
incident, becomes evident, when looking at PBB levels in fish (see
Table 30) some of which are used for human consumption. For example,
skinless fillets of carp from the Pine River, captured in the
vicinity of Michigan Chemical Company, contained 1.33 mg PBBs/kg
(wet weight basis) which is approximately equivalent to 30 mg/kg on
a fat weight basis (Hesse & Powers, 1978). This was obviously
greatly in excess of the US FDA tolerance limit for beef, a
tolerance limit for fish has not been established (Hesse & Powers,
1978).
More recent information on "background" PBB levels in food may
be expected in future via a USA data collection programme
(Foodcontam) initiated by the US Food and Drug Administration which
includes PBBs besides other chemicals (Minyard et al., 1989).
Table 30. PBB levels in fish
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1974 Pine River, downstream Carp skinless not detected-1330 wet HxBB Hesse &
from St. Louis (vicinity (Cyprimus carpio) fillets Powers
of Michigan Chemical Co.) (1978)
White sucker 670
Northern pike 540
Bullhead 450-780
1974 Tittabawassee Carp not detected
River (Cyprimus carpio)
Freshwater drum not detected
(Aplodinotus
grunniens
1976 Pine River, downstream Carp 60-750
from St. Louis (vicinity (Cyprimus carpio)
of Michigan Chemical Co.)
Northern pike 180-230
1976 Pine River, downstream Largemouth skinless not detected-740 wet HxBB Hesse &
from St. Louis (vicinity bass fillets Powers
of Michigan Chemical Co.) (1978)
Smallmouth bass 130
Rockbass 320-700
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1977 Kill van Kull River Killifish whole 220 dry total PBB Stratton &
(vicinity of White Chemical HxBB-DeBB Whitlock
Co., Bayonne, New Jersey); (1979)
Port Johnson
1977 Kill van Kull River Killifish whole 230 dry total PBB
(vicinity of Standarad T HxBB-DeBB
Chemical, Staten Island,
New York); canal at
discharge site
Not Lake Huron Yellow perch 0.3-0.8 Kreis & Rice
specified (Saginaw Bay) (1985)
Saginaw Bay Catfish 21.0
1983 Pine River Hogsucker whole 6000 fat most Jaffe et al.
abundant (1985)
congeners
1983 Chippewa River Carp 5300-15 000
1983 Tittabawassee 140-160
River
1983 Shiawassee River 120
Table 30 (contd).
Year Region Species Type of PBB concentration Weight PBB References
sample (µg/kg) basis examined
1983 Flint River 15-32
1983 Saginaw River 80-200
1983 Saginaw Bay 110-1100
Four samples of cow's milk from Germany have been analysed for
PBBs (Krüger, 1988). Three congeners were detected; BB 153
(0.025-0.053 µg/kg milk fat), BB 180 (0.001-0.007 µg/kg) and BB 187
(0.005-0.014 µg/kg). The other 30 congeners covered by the method
were not detected with detection limits ranging from 0.001 to
0.003 µg/kg milk fat (see Table 33).
The processing and cooking of contaminated food have been found
to have some potential for reducing PBB levels. Spray-drying
appeared to reduce the contents of PBBs in whole milk and skim milk
by 30-36% and 61-69%, respectively (Murata et al., 1977; Zabik
et al., 1978). Pressure cooking of chicken pieces also resulted in a
loss of PBBs, however, part of the PBBs lost were found in the drip
(Zabik et al., 1978).
5.1.5 Other products
Antibiotics used for attending farm animals were also found to
be contaminated: Levels of PBBs in aureomycin, which was distributed
by the Michigan Farm Bureau, were as high as 70 mg/kg (Di Carlo
et al., 1978).
5.1.6 Terrestrial and aquatic organisms
5.1.6.1 Aquatic and terrestrial plants
Only few data on PBB contamination of aquatic and terrestrial
plants are available. Stratton & Whitlock (1979) analysed algae
(e.g., filamentous green algae) from surface waters in the vicinity
of White Chemical (Bayonne, New Jersey) and near Standard T Chemical
Company (Staten Island, New York) for PBBs (MoBB through DeBB). The
two samples did not contain detectable levels of PBBs (detection
limit: 10 µg/kg, dry weight). However, bottom sediments taken in the
same location contained hexa- and heptabromobiphenyl.
Surface contamination was observed on terrestrial vegetation in
the vicinity of PBB facilities (up to 92 mg/kg dry weight; Stratton
& Whitlock, 1979). As Chou et al. (1978) reported, the PBB
contamination of field soils in Michigan (USA) did not result in any
detectable surface contamination of field crops.
5.1.6.2 Animals
a) Wildlife
Most earlier data available on PBB contamination of wildlife
refer to freshwater fish (Table 30) and birds (Tables 31 and 32),
primarily waterfowl in the USA. Recent reports refer to PBB
contamination of fish-eating mammals and birds from marine
environments in the USA (Kuehl et al., 1991) and in Europe (Jansson
et al., 1987, 1992; Krüger, 1988). Residues were found also in
terrestrial mammals (Jansson et al., 1992) and in freshwater and
marine fish in Europe (Krüger, 1988; Jansson et al., 1992).
Table 30 gives PBB levels in fish captured for analysis in
industrialized areas of the USA, at various distances from PBB-
containing or -using facilities. PBBs were detected in several fish
species from all rivers or bays examined. The PBB levels ranged up
to a maximum of 1.33 mg/kg wet weight (approximately equivalent to
30 mg/kg on a fat basis) found in carp from the Pine River near
Michigan Chemical Company (Hesse & Powers, 1978).
No apparent change in PBB concentrations was observed in Pine
River fish between 1974 and 1976 (Hesse & Powers, 1978; see also
Table 30). Although Michigan Chemical Co. had terminated PBB
production in 1974, even in 1983, Jaffe et al. (1985) detected PBB
in fish from the Saginaw River system, with highest concentrations
in fish from Pine and Chippewa Rivers (Table 30). While carp from
Tittabawassee River, to which Pine River joins, did not contain any
detectable PBBs in 1974, PBB-residues were detected (approximately
150 µg/kg on a fat basis) in 1983 (Table 30).
Various PBB homologues were examined in killifish (Oryzias
latipes) from Kill van Kull River near White Chemical Co.
(Bayonne, New Jersey). The main component found was NoBB. In the
vicinity of a FireMaster(R)-using facility (Staten Island, New
York), killifish samples contained only HxBB (Stratton & Whitlock,
1979).
PBB contamination has been reported in wild ducks collected
within two miles of the Michigan Chemical Corporation plant (Table
31), in eggs of waterfowl nesting around Green Bay and other areas
of Lake Michigan and on Lake Michigan island (Table 32), and, in
bald eagles found moribund or dead in 13 US states (Table 31).
Approximately one third of bald eagles examined contained PBB
residues (see Table 31).
Concentrations of PBBs in duck samples with skin left on were
considerably higher than those in skinless samples (see Table 31)
indicating that much of the PBBs is associated with the skin or fat
layer between the skin and muscle (Hesse & Powers, 1978).
Table 31. PBB residues in birds (ducks and bald eagles)
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
1974 Pine River Mallard breast tissue 3 0.25 Hesse & Powers
within two miles (skinless) (1978)
downstream from
St. Louis
Wood duck 0.29
Teal 1.8
1976 Mallard breast tissue:
skinless 3c 0.24
with skin 3c 2.00
Wood duck
4c 0.17
4c 2.70
1977 Wood duck breast tissue:
skinless 4c 0.08
with skin 4c 0.23
1977 Teal breast tissue:
skinless 0c (1) not detected
with skin 0c (1) not detected
Table 31 (contd).
Year Region Species Type of sample No. of PBB concentrationb (mg/kg wet weight) References
samplesa mean range median
13 US states Bald eagle found moribund Kaiser et al.
(Haliaeetus or dead; (1980)
leucocephalus)
carcass 10 0.03-0.27 0.07
(32)d
brain 7 0.03-0.17 0.05
a Number of samples containing residues; median is based on this number. Total number of samples in parentheses.
b PBB values were based on the major hexabromobiphenyl peak (BB 153).
c Paired samples.
d Detection limit: 0.02 mg PBBs/kg.
Table 32. PBB residues in eggs of fish-eating and non-fish-eating waterbirds from Green Bay and Lake Michigan (USA)
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Fish eater
1975 Green Bay Little gull 1 n.d. Heinz et al.
(Sensila Wildlife Area) (Larus minutus) (1985)
1977 three Lake Michigan islands Red-breasted merganser 114 0.06 n.d.-0.13 Haseltine et al.
off the tip of Door County, (Mergus serrator) (109) (1981)
Wisconsin
1977 islands in north-western Red-breasted merganser Heinz et al.
Lake Michigan (Mergus serrator): (1983)
eggs from the same nests
randomly selected 49 0.05
unhatched 49 0.04
1977 Lake Michigan Herring gull 9 0.18 0.11-0.25 Heinz et
(Gravel Island) (Larus argentatus) (9) al. (1985)
1977 Green Bay Common tern 10 0.06 0.02-0.22 Heinz et al.
(Lone Tree Island) (Sterna hirundo) (10) (1985)
Green Bay (St. Vital Island) Common tern 2 (2) 0.03 0.03-0.04
(Sterna hirundo)
Green Bay (Portage Point) 2 (2) 0.03 0.02-0.06
Green Bay (Cat Island) Double-crested 4 (3) 0.01 n.d.-0.02
cormorant
(Phalocrocorax
auritus)
Table 32 (contd).
PBB concentrationb
Year Collection site Species No. of (mg/kg wet weight) References
eggsa geometric mean range
Lake Michigan (Fish Island) 6 (3) 0.02 n.d.-0.05
Green Bay (Oconto Marsh) Black-crowned night-heron 1 (1) 0.02
(Nycticorax nycticorax)
Green Bay (Oconto Marsh) Green-backed heron 1 n.d.
(Butorides striatus)
Non-fish eater
1977 Three Lake Michigan Mallard 22 n.d. Haseltine
islands off the tip of (Anas platyrhynchos) et al. (1981)
Door County, Wisconsin
1977 Lake Michigan (three Gadwall 4 n.d.
islands off the tip of (Anas strepera)
Door County, Wisconsin)
Black duck (Anas rubripes) 3 n.d.
a Number of collected eggs (in parentheses: number of eggs with quantifiable levels of PBBs).
b PBB values were based on hexabromobiphenyl;
n.d. = No residue of quantifiable level. Level over which quantification was possible: 0.02 mg/kg.
Samples with no detectable residues were calculated in the means as one-half the quantification level.
While the majority of ducks analysed from the Pine River
contained measurable concentrations of PBBs (Table 31), the eggs of
ducks from Lake Michigan islands did not contain detectable PBB
residues (Table 32). In contrast, most eggs of fish-eating
waterbirds from Green Bay and Lake Michigan showed PBB residues
(Table 32). Highest concentrations were detected in herring gull
eggs (0.18 mg/kg wet weight), perhaps reflecting their year round
residence on the Great Lakes (Heinz et al., 1985).
Stratton & Whitlock (1979) analysed a snapping turtle captured
in the vicinity of Hexcel Fine Organics Division (Sayreville, New
Jersey) for hexa- to decabromobiphenyls and found a tissue
concentration of 20 µg hexabromobiphenyl/kg (dry weight).
Di Carlo et al. (1978) reported on PBB contamination of
miscellaneous wildlife, such as deer, rabbits, coyotes, and ravens,
without, however, specifying the sampling locations and the levels
of contamination.
In Europe, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153) was found
in fish from German and Swedish rivers at concentrations ranging
from 0.3 to 0.6 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see
also Tables 33 and 34). A trout sample from a breeding farm
contained much lower levels of PBBs than the fish samples from the
rivers (Krüger, 1988).
A residue of 22 µg BB 153/kg lipid was observed in pooled
samples of osprey specimens found dead in various parts of Sweden
(Jansson et al., 1992; Table 34).
Swedish reindeers (pooled samples) showed BB 153 levels as low
as 0.04 µg/kg lipid (Jansson et al., 1992; Table 34).
PBBs (as a group) were not found in otters (Lutra canadensis)
from a region relatively remote from industrial sites in north
eastern Alberta (Canada) (Somers et al., 1987).
Fish samples (freshwater and marine species) collected in 1983
from an industrial area of Japan (Osaka) did not contain "PBBs" (not
specified) (Watanabe & Tatsukawa, 1990).
Recently, PBBs have been identified in bottlenose dolphins
(Tursiops truncatus) collected during the 1987/88 mass mortality
event along the Atlantic Coast of the USA. All three animals
(females), analysed for PBBs (tetrabromo- to hexabromobiphenyl
congeners) within a subset of the screening programme for
anthropogenic contaminants, contained PBBs at concentrations ranging
from 14 to 20 ng/g lipid (Kuehl et al., 1991).
Table 33. Average concentrations (µg/kg lipid) of PBB congeners in fish, seals, cows, and human milk samples
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 103 0.02 0.12 0.10 < 0.02 < 0.02 not analysed
BB 131 + 142/146 0.30 0.62 0.25 0.03 < 0.02 < 0.01
BB 132 0.33 1.25 0.62 0.15 < 0.02 0.05
BB 135 + 144/151 0.69 4.10 1.48 0.46 < 0.02 0.12
BB 147/135 + 144 0.21 0.31 0.25 < 0.02 < 0.02 < 0.01
BB 148/136 0.10 0.13 0.11 < 0.02 < 0.02 < 0.01
BB 149 0.26 0.45 0.53 < 0.02 < 0.02 < 0.01
BB 153 0.60 2.39 1.31 0.81 0.04 1.03
BB 154/151 0.22 0.54 0.37 < 0.02 < 0.02 0.01
BB 155 0.66 2.64 1.11 0.40 < 0.03 0.05
BB 169 < 0.01 15.16 < 0.01 < 0.01 < 0.01 0.05
BB 176 0.03 < 0.01 0.02 < 0.01 < 0.01 < 0.05
BB 178 0.18 0.87 0.36 0.03 < 0.01 0.09
BB 179 0.08 0.04 0.04 < 0.01 < 0.01 < 0.05
BB 180 0.02 < 0.01 0.02 < 0.01 < 0.04 0.02
BB 181 + 174 0.01 0.01 0.01 < 0.01 < 0.01 < 0.05
BB 184 0.05 0.09 0.03 < 0.01 < 0.01 0.01
BB 185 0.03 < 0.01 < 0.01 < 0.01 < 0.01 < 0.05
BB 186 0.30 0.40 0.16 0.01 < 0.01 0.02
BB 187 + 182 0.03 0.05 0.04 0.03 0.01 0.33
BB 188 0.11 0.28 0.11 < 0.01 < 0.01 0.01
BB 192 0.01 < 0.01 < 0.01 < 0.01 < 0.01 n< 0.05
BB 194 0.07 < 0.02 0.04 < 0.02 < 0.02 < 0.05
BB 197 0.11 0.11 0.08 < 0.02 < 0.02 0.04
BB 198 0.27 0.14 0.12 < 0.02 < 0.02 < 0.05
BB 200 + 204 0.41 0.36 0.24 < 0.02 < 0.02 0.02
BB 201 0.09 < 0.02 0.03 < 0.02 < 0.02 < 0.05
Table 33 (contd).
Congener River fish Baltic fish North Sea Spitbergen Cow's milk Human milk
(Germany) fish seal (Germany) (Germany)
(No. = 17) (No. = 6) (No. = 11) (No. = 5) (No. = 4) (No. = 25)
BB 202 0.87 0.42 0.36 < 0.02 < 0.02 0.01
BB 206 0.05 < 0.03 0.04 < 0.03 < 0.03 < 0.01
BB 207 0.06 < 0.03 0.02 < 0.03 < 0.03 < 0.01
BB 208 0.16 0.04 0.04 < 0.03 < 0.03 < 0.01
PBB 6.3 30.5 7.9 1.9 0.05 2.0
From: Krüger (1988).
Table 34. Concentrations (µg/kg lipid) of 2,2',4,4',5,5'-HxBB (BB 153)
in pooled biological samplesa
Species Number of Sampling Concentration
specimens in site of BB 153
the homogenate
Rabbit (Oryctlagus cuniculus) 15 S. Sweden not detected
Moose (Alces alces) 13 not detected
Reindeer (Rangifer tarandus) 31 N. Sweden 0.037
White fish (Coregonus sp.) 35 0.29
Arctic char (Salvelinus alpinus) 15 S. Sweden 0.42
Herring (Clupea harengus) 100 Bothnian Bay 0.092
Herring (Clupea harengus) 60 Baltic Proper 0.16
Herring (Clupea harengus) 100 Skagerrak 0.27
Ringed seal (Pusa hispida) 7 Svalbard 0.42
Grey seal (Halichoerus grypus) 8 Baltic Sea 26
Osprey (Pandion haliaetus) 35 S. Sweden 22
a From: Jansson et al. (1992).
In Europe, PBBs have been detected in seals (Phoca vitulina;
Pusa hispida), guillemots (Uria aalge; U. lomvi), and
white-tailed sea eagles (Haliaeetus albicilla). The concentrations
(estimated by comparison with the technical product FM BP-6) ranged
from 3 to 280 µg/kg lipid (Jansson et al., 1987). The concentrations
of PBBs in comparable samples from the Baltic Ocean were all higher
than concentrations in samples from the Arctic Ocean. The same was
true for polybrominated biphenyl ethers and PCBs (Jansson et al.,
1987).
Concentrations of BB 153 determined in marine fish ranged from
0.2 to 2.4 µg/kg lipid (Krüger, 1988; Jansson et al., 1992; see also
Tables 33 and 34). BB 153 levels of 0.4-26 µg/kg lipid were found in
seals (Krüger, 1988; Jansson et al., 1992; see also Tables 33 and
34).
Detailed isomer-specific PBB analyses were carried out by
Krüger (1988) in fish (several species) from the Baltic and North
Seas and from sections of the Lippe and Rur rivers in North
Rhine-Westphalia, Germany. Seal samples from Spitsbergen (Norway)
were also included in this investigation (Table 33). All samples
contained PBBs. The smallest number of PBB congeners was found in
seals (n = 5) from an area remote from industrial sites. The main
components were different hexabrominated isomers with
2,2',4,4',5,5'-hexabromobiphenyl reaching a mean concentration of
0.8 µg/kg fat. The mean concentrations of several PBB congeners and
isomers (penta- to nonabrominated biphenyls) measured in fish
(n = 35) ranged, mostly, between 0.01 and 2 µg/kg fat. The pattern
of PBB congeners found in fish differed in a characteristic manner,
depending on the different capture sites. While relatively high
amounts of nona- and octabromobiphenyls (besides polybrominated
biphenyl ethers) were present in fish from German rivers (n = 17;
several species), hexabrominated biphenyls were predominant in fish
from the North Sea and the Baltic Sea (n = 17; several species). In
all samples from the Baltic Sea (n = 6),
3,3',4,4',5,5'-hexabromobiphenyl was found in relatively high
concentrations (maximum concentration: 36 µg/kg fat), but it was not
detected in samples from the North Sea and from rivers. The
concentrations of the other hexabrominated biphenyls were mostly
higher in fish from the Baltic Sea than in fish from the North Sea.
b) Farm animals
Farm animals in Michigan were contaminated by PBBs, when
FireMaster(R) FF-1 was accidentally mixed with animal feed in
mid-1973 (see section 4.1). The PBB levels resulting from this event
varied greatly with the extent of exposure. Data reported in the
literature are compiled in Table 35. The extent of contami nation
can be seen from the fact that, during the months following the
event, 172 dairy and beef herds (18 000 animals), 32 swine herds
(3500 animals), 16 sheep flocks (1200 animals), and 92 chicken
flocks (1.5 million birds) were destroyed (Isleib & Whitehead, 1975;
Robertson & Chynoweth, 1975; Mercer et al., 1976). In relation to
these great numbers, the portion of highly contaminated animals was
small (e.g., 40 herds of cattle, as can be derived from
contamination values measured in milk (section 5.1.4.2).
5.2 General population exposure
Apart from data collected after the Michigan disaster, there is
only limited information on exposure of the general public. PBBs
have been detected in humans in the vicinity of manufacturing
premises and in a few sites in the USA and Europe, not directly
connected with PBB contamination.
Table 35. PBB levels in farm animals (derived from the Michigan
cattle food contamination incident in 1973)
Year Animal PBB concentrationa References
(Type of sample) (mg/kg)
1974 Poultryb (tissue) 4600 Kay (1977); IARC (1978)
Not specified Cattle (fat) up to 200 Pearson (1982)
Not specified Aborted calves 120-400 Kay (1977)
1974 Cattlec (body fat) 110-2480 Robertson &
Chynoweth (1975);
Mercer et al. (1976)
1974-75 Cattled (fat) 9-4100 Fries et al. (1978b)
1974 Cattleb (tissue) up to 2700 Kay (1977); IARC (1978)
1974 Cattlej (body fat) 174-200 Jackson & Halbert (1974)
March (1975) Dairy cattlee 1-12 Kay (1977)
1975 Cowsf (tissue-fat) not detected-1.69 Isleib & Whitehead (1975)
1975 Steers and heifersg not detected-2.27
(tissue-fat)
1975 Pigsh (tissue-fat) not detected-0.58
1975-76 Cattlei (male and not detected-0.13 Cook et al. (1978a)
female) (eye fat)
Not specified Cattlek (body fat) not detected-3.8 Mercer et al. (1976)
a PBB values were based on 2,2',4,4',5,5'-hexabromobiphenyl.
b From 22 farm premises.
c 21 highly exposed cows.
d 32 cows from one herd heavily contaminated during September/October
1973, and 9 calves borne to these cows in 1974.
e 16 herds of dairy cattle with a history of feed levels from 1 to 14 mg/kg PBB.
f-h Slaughter house survey during a 3-month period (January-April 1975).
f Number of samples: 216; mean-PBB: 0.018 mg/kg.
g Number of samples: 247; mean-PBB: 0.030 mg/kg.
h Number of samples: 213; mean-PBB: 0.017 mg/kg.
i Cattle of 5 affected herds.
j 2 cows of the Halbert farm.
k Cattle of 12 affected herds.
5.2.1 Quantified data on human exposure
5.2.1.1 Worldwide
For most human populations, direct data on exposure to PBBs
from various sources have never been documented. This is true also
for the possible exposure of the general population from the use of
PBB-containing plastic products, and from fumes, generated in the
combustion of these products inadvertently in fires, or from burning
in dumps (Kay, 1977), and, additionally, from sources such as
PBB-containing landfills or PBB-manufacturing and processing
plants.
5.2.1.2 The Michigan Accident
Widespread human exposure resulting from direct contact with
contaminated feed, and, primarily, from the consumption of PBBs in
meat, eggs, and dairy products has been reported from the state of
Michigan, USA (Kay, 1977; Landrigan, 1980; Fries, 1985b; Table 36).
Many Michigan residents were exposed to PBBs between the onset of
contamination in the autumn of 1973 and the establishment of the
quarantine of affected farm animals in the spring of 1974. There was
considerable variation in both lengths and levels of exposure. At
least 2000 families (primarily farmers and their neighbours)
received the heaviest exposure (Meester & McCoy, 1976; IARC, 1978).
Brilliant et al. (1978) concluded from their results of human
milk analyses, conducted in 1976, that about 8 million of the 9.1
million residents of Michigan have detectable body burdens of PBBs.
Further studies (see Table 37) confirmed this widespread
distribution of PBBs.
The amount of PBBs consumed or absorbed by the various groups
in Michigan cannot be determined accurately (Safe, 1984). However,
there have been some trials to estimate the possible exposure to
PBBs of farm families and other people. The estimates were based on
kinetic data and other observations, e.g., time and level of animal
exposure, residue levels in herds at the time of the contamination,
and serum levels of exposed people.
In this way, Fries et al. (1978a) estimated (assumptions: see
Fig. 4), that the total exposure of an individual in a farm family
consuming its own milk was, for example, 9.8 g over the 230-day
period, during which the contamination was undetected. The
cumulative intake over time is shown in Fig. 4. In addition, the
authors concluded that the most highly exposed people consumed from
5 to 15 g PBBs over a 230-day period via milk. The projected intake
of PBB via the meat of cows slaughtered for home consumption would
have exceeded the projected intake from milk.
Table 36. Approximate distribution of PBBs in the Michigan episodea
Item Amount (kg)
Total released 295
Not fed to livestock 45
Fed to livestock 250
Eliminated in faeces 125
Absorbed by animals 125
In human foods before regulation 94
a Modified from: Fries (1985b).
Application of a pharmacokinetic model (Tuey & Matthews, 1980)
to the mean serum concentrations for residents of quarantined farms
resulted in similar values, e.g., about 170 mg mean total exposure
per individual and 11.7 g highest exposure to PBBs (Fries, 1985b;
Brown & Nixon, 1979) supposed a consump tion of 1-20 g of PBB by
families on the most contaminated farms.
The exposure of an individual in the general population would
have a pattern over time as projected above for the farm family
(Fries et al., 1978a). However, the exposure level would have been
much less, because of dilution in the normal marketing channels (the
mixing of milk from a large number of producers; the use of meat of
cull dairy cattle for hamburger and processed meat products). The
calculations of Fries (1985b) indicate that total exposure was about
9-10 mg for an average male with an average adipose content.
However, the individual with the highest PBB serum concentration was
projected to have had a total exposure of about 800-900 mg.
Table 37. Distribution of serum levels of PBBs, Michigan, 1974a
Quarantined farms Non-quarantined farms
Adults Children Adults Children
Serum PBBs Number (%) Number (%) Number % Number %
(µg/litre)
0 3 3.7 - - 21 28.4 - -
2-19 43 52.4 8 28.6 52 70.3 29 96.7
20-90 19 23.2 10 35.7 1 1.4 1 3.3
100-490 11 13.4 3 10.7 0 0 0 0
500-2260 6 7.3 7 25.0 0 0 0 0
Total 82 100.0 28 100.0 74 100.1 30 100.0
a From: Humphrey & Hayner (1975).
 People who bought food primarily from quarantined farms were
thought to have been exposed 10 to 100 times more than the typical
retail store customer (Schwartz & Rae, 1983): ca. 100 mg of PBBs
versus 1-10 mg (Brown & Nixon, 1979).
While many dust and cobweb samples found in the buildings of
some PBB-contaminated farms had very high residue levels, the amount
of PBB residue involved is said not to be sufficient to be an
important contributor to animal residues (Fries & Jacobs, 1980) and,
possibly to human exposure.
5.2.2 Human monitoring methods for PBBs
Usually, suitable human monitoring data, as such, are used to
describe the real exposure to a toxic chemical. As an indicator of
human exposure to PBBs, the presence of PBBs in adipose tissue,
breast-milk, whole blood, serum, red and white blood cells (Bekesi
et al., 1979a,b), and human hair oils (Stratton & Whitlock, 1979)
has been assessed. The most commonly used specimens were serum,
breast-milk, and adipose tissue (see Tables 38-40).
Table 38. Human monitoring data: PBB levels in the Michigan population (USA)
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1974 82 96.3 serum adults from not 14 not Humphrey &
quarantined farms detected- specified Hayner (1975)
2260
28 100 serum children from 2-2260 35 not
quarantined farms specified
5 100 serum lactating females 3-1068
from quarantined
farms
1976 524 serum Michigan farmers 23.7 2.6 0.2 Wolff et al.
µg/litre (1978a);
Lilis et al.
(1978)
283 serum residents on 0.2-> 1000 33.9 3.9
quarantined farms
153 serum residents of 0.2-50 2.9 1.4
non-quarantined
farms
40 serum consumers of 0.3-1000 56.6 4.2
products
from quarantined
farms
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
28 serum consumers of 0-50 3.4 2.2
products from
non-quarantined
farms
consumers and Wolff et al.
residents: (1978)
quarantined farms:
40 serum females < 18 years 28.0 2.3 0.2
µg/litre
102 serum females > 18 years 18.2 2.5
51 serum males < 18 years 67.7 7.3
129 serum males > 18 years 28.2 4.4
consumers and
residents:
non-quarantined
farms:
37 serum females < 18 years 3.1 1.3
57 serum females > 18 years 1.7 0.9
35 serum males < 18 years 4.8 1.7
51 serum males > 18 years 3.1 2.2
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1976 485 serum consumers and Chanda et al.
residents: (1982)
quarantined farms:
27 serum 0-5 years 0.2-64.2 10.15 not
specified
137 6-18 years 0.0-962.4 27.22 not
specified
321 > 18 years 0.2-1778.0 24.42
1976 321 serum consumers and Chanda et al.
residents: (1982)
non-quarantined
farms:
18 0-5 years 0.2-37.4 6.42 not
specified
104 6-18 years 0.0-42.6 3.25 not
specified
177 > 18 years 0.0-94.0 3.03 not
specified
1976 serum Michigan children 3.41 not Barr (1980)
specified
143 females 2.72 not
specified
149 males 4.23 not
specified
33 0-4 years 2.75 not
specified
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
77 5-8 years 5.59 not
specified
81 9-12 years 3.18 not
specified
101 13-16 years 2.65 not
specified
1976-77 3639 serum Michigan residents 0-1900 21.2 3.0 1 µg/litre Landrigan
with various et al.
degrees of exposure (1979);
Landrigan
(1980)
1976-77 1750 serum contaminated farm 0-1900 26.9 4.0 1 µg/litre Landrigan
1114 residents farm 0-659 17.1 3.0 et al.
(1979);
216 product recipients 0-1240 43.0 4.5 Landrigan
(1980)
559 chemical workers and 0-111 3.4 2.0
families volunteers
1976-77 52 serum women at the time not 26.2 2.5 1 Landrigan
of delivery detected- µg/litre et al. (1979)
1150
1977 3683 serum Michigan PBB cohort < 1-3150 23.2 4.1 3 not Kreiss et al.
specified (1982)
1888 males 5.8
1795 females 2.8
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1978 1681 serum Michigan residents Wolff et al.
(1982)
(randomly selected)
1120 68.9 adults 0.2-120.5 1.3 0.6 0.2
µg/litre
461 72.7 children 0.2-37.2 1.8 0.8
232 Upper Peninsula 0.2
Lower Peninsula:
467 Detroit Area 0.5
191 Muskegon Area 1.7
791 remainder of state 0.9
Muskegon County:
54 serum male adults 4.0 2.6 0.2 Wolff et al.
µg/litre (1982)
74 female adults 2.1 1.4
36 male children 4.9 3.4
27 female children 3.8 2.0
1975-80 serum Michigan PBB cohort: Eyster et al.
(1983)
61 pregnant females not 3.5 3 1
detected- µg/litre
1068
56 non-pregnant females not 3.1 2
detected-
873
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
29 male chemical workers 1-1200 25.4 20
83 male farm and other not 5.4 4
workers detected-
1515
1974-75 5 100 milk women from 0.21-92 660 not Humphrey &
quarantined farms specified Hayner (1975)
1976 milk lactating women Brilliant
(fat) (randomly selected et al. (1978)
53 96 from Michigan: not 0.068 0.1 mg/kg
Lower Peninsula detected-
1.2
42 43 Upper Peninsula not
detected-
0.320
1976-77 32 100 milk women at the time of 0.032-93 3.61b 0.225b Landrigan
(fat) delivery (Michigan et al. (1979)
PBB cohort)
1976-78 2986 88.5 milk lactating women not 0.097 0.1 0.06 < 0.05 mg/kg Miller et al.
milk (self-selected) detected-2 (1984)
1975-80 Michigan Eyster et al.
PBB cohort (1983)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
47 milk pregnant females not 0.312b 0.250b 0.001
(fat) detected- mg/kg
92.7
1974-75 15 100 adipose persons from 0.104-175c Humphrey
quarantined farms & Hayner
(1975)
1975-76 53 adipose quarantined farmers 1.965 Meester &
McCoy
(1976)
29 100 non-quarantined 0.516
farmers
9 100 city residents 0.226
116 fat members of farm 0.58-273.0
families
1977 19 subcutaneous children with known Weil et al.
exposure to PBBs (1981);
fat in utero and/or Schwartz &
through breast milk Rae (1983);
Seagull
(1983)
10 100 "high exposure" 0.116- 4.218
20.960
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
9 100 "low exposure" 0.010- 0.050
0.074
1978 844 97.3 adipose Michigan residents < 0.002- 0.4 0.199 0.002 Wolff et al.
36.7 mg/kg (1982)
(randomly selected) 0.015
87 Upper Peninsula
Lower Peninsula:
255 Detroit Area 0.16
84 Muskegon Area 0.50
418 remainder of state 0.24 0.050
1975-80 32 adipose Michigan PBB-cohort: not 0.330 0.540 0.001 Eyster et al.
lipid pregnant females detected- mg/kg (1983)
174
56 non-pregnant females not 0.00057 0.460
detected-
0.619
29 male chemical 0.4-350 5.29 6.0
workers
83 male farm and other 70-350 1.65 1.05
workers healthy male
Not 7 100 adipose volunteers, aged 0.01-2.72 0.001 Schnare
specified lipid 20-30 years mg/kg et al.
(subcutaneous) (1984)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1983 15 100 perirenal autopsy cases from 0.032-1.65 0.475 0.32 0.0005 Miceli
post- the "high" exposure mg/kg et al.
mortem area of Michigan (1985)
adipose (Grand Rapids)
tissue
(wet wt)
a Expressed as the concentration of the major hexabromobiphenyl component (BB 153) in µg/litre serum or
mg/kg milk, or adipose tissue, respectively.
b The sample measuring 93 mg/kg was excluded from statistical analysis.
c Most in the order of 1 mg/kg.
The last one has been preferred for analysis, since depot fat
is the predominant storage site of PBBs (and other persistent
halogenated hydrocarbons), and, therefore, allows an increased
detectability of body burden. For example, had serum PBBs been used
alone as an indicator of exposure in the PBB survey of the general
population of Michigan (Table 38), only 70% of the 839 individuals
would have been considered exposed. When adipose tissue results are
added, an additional 24% indicate exposure, raising the positive
rate to 94%. Even though the limit of detection was an order of
magnitude higher (2.0 µg/litre in adipose tissue vs. 0.2 µg/litre in
serum), the partition ratio of approximately 300:1 made the adipose
limit of detection a more sensitive indicator of exposure (Wolff
et al., 1982; Anderson, 1985).
On the other hand, collection of hair, blood, and breast-milk
samples is simpler and less invasive than adipose tissue biopsy.
Moreover, with some exceptions, significant correlations between
adipose tissue and blood serum or breast-milk PBB levels were found
(section 6.2). Further advantages and limitations of these
techniques are discussed in detail by Anderson (1985) and Fries
(1985b).
It should be noted that levels of PBBs in breast-milk may not
be comparable between studies unless the concentration is adjusted
for fat content, because the fat content of breast-milk varies
widely from woman to woman, and the value increases during feeding
(Rogan et al., 1980).
Although the accurate relationship between observed body levels
of PBBs and individual exposure to PBBs is not clear (Safe, 1984),
PBB concentrations in human tissues can give some idea of the levels
of exposure (Kimbrough, 1980a).
5.2.3 Human monitoring data
In the following subsection, human monitoring data are
presented from contaminated farms in Michigan, from Michigan state,
and from other countries.
Most data available refer to the Michigan PBB incident in
1973-74 (Tables 38 and 39). Because, in this case, the spilling of
PBBs started from farms, the Michigan Department of Public Health
(MDPH) undertook a series of studies on farm families as a high-risk
group in the summer and autumn of 1974.
Serum samples were obtained from 110 persons in the exposed
group (had been working or living in the quarantined farms for six
months or more since the accident) and for 104 persons from the
control group (randomly selected from a list of dairy producers in
the same geographical area, where farms had not been quarantined).
As shown in Tables 37 and 38, serum levels of PBBs were
significantly higher in the people from quarantined farms compared
with those from non-quarantined farms, though low levels were also
observed in the control group (Cordle et al., 1978). In 1976, the
MDPH, together with the Centre for Disease Control, the FDA, NIH,
and EPA, established a cohort of 4545 people in Michigan to be
examined at regular intervals over several decades (Barlow &
Sullivan, 1982) to evaluate the long-term effects of PBB exposure.
Four groups were included (Landrigan, 1980): Quarantined farm
residents, direct recipients of farm produce, chemical workers and
their families, and persons who either volunteered for the study or
who had participated as control subjects in an earlier pilot study
(Humphrey & Hayner, 1975). The first report was published by
Landrigan et al. (1979) followed by several reports on subgroups of
this population (e.g., Kreiss et al., 1982; Eyster et al., 1983).
Study groups of the Mount Sinai School of Medicine have also
conducted comprehensive examinations on similarly categorized
groups, e.g., residents of quarantined farms, residents of
non-quarantined farms, consumers of products directly from
quarantined or non-quarantined farms, and Michigan Chemical Company
workers; residents of the state of Wisconsin were used as a control
group.
It can be seen from Table 38 that nearly 100% of the adipose
samples randomly selected throughout the state had detectable PBB
concentrations. Thus, statewide exposure of Michigan residents to
PBBs can be demonstrated.
Levels of PBBs in serum (Landrigan, 1980; Wolff et al., 1982),
breast-milk (Brilliant et al., 1978; Miller et al., 1984), and
adipose tissue (Wolff et al., 1982) were highest in the area of the
accident (lower peninsula), and lowest in the upper peninsula,
farthest from the source.
Compared with residents of quarantined farms, direct consumers
of products from quarantined farms, and PBB- production workers, the
tissue burdens among the general population of Michigan were 1-3
orders of magnitude lower. Moreover, for example, only 36% of the
general population had serum PBB concentrations greater than
1 µg/litre, compared with 78% among farmers (Anderson et al., 1979;
Wolff et al., 1982).
PBB levels appear to be higher in males than females (Meester &
McCoy, 1976; Landrigan et al., 1979; Landrigan, 1980; Wolff et al.,
1978; 1980; Kreiss et al., 1982; Eyster et al., 1983) and higher in
children (below the age of 10 years) than in adults (Humphrey &
Hayner, 1975; Landrigan et al., 1979; Landrigan, 1980; Barr, 1980;
Wolff et al., 1982).
A later study (Schnare et al., 1984) recorded not only the
concentration of the most abundant congener of the FireMaster(R)-
mixture (2,2',4,4',5,5'-hexabromobiphenyl) but also the concen
trations of other PBB congeners detected in subcutaneous adipose
tissue samples of 7 former participants of the Michigan PBB studies
(Table 39).
Table 39. Range of adipose tissue concentrations of various PBBs in 7 personsa
(mg/kg on a lipid weight basis)b
PBB Rangec (mg/kg)
2,3',4,4',5-penta nd-0.16
2,2',4,4',5,5'-hexa 0.01-2.72
2,2',3,4,4',5'-hexa nd-0.22
2,3',4,4',5,5'-hexa nd-0.09
2,2',3,3',4,4',5-hepta nd-0.26
2,2',3,4,4',5,5'-hepta nd-0.01
a 7 healthy male volunteers, aged 20-30 years, having been exposed to PBBs some
years earlier, as a consequence of the Michigan PBB incident in 1973.
b From: Schnare et al. (1984).
c nd = Not detectable; detection limit = 0.001 mg/kg.
In most cases, PBB concentrations did not appear to be
decreasing significantly over time. Wolff et al. (1979b) did not
find any significant variation in the serum PBB levels of nine dairy
farm residents during 18 month of observation.
Paired serum samples, one collected in 1974 and the other in
1977, were also available for 148 members of the Michigan PBB
cohort. The data indicate that levels were generally stable over the
3-year period with a mean change of 16 µg/litre (Landrigan et al.,
1979). In another study of the Michigan PBB-cohort, the decrements
in median serum levels of PBBs between matched pairs over one -
(1977-78) and two - (1977-79) year intervals were both only 1
µg/litre (Kreiss et al., 1982). No significant change in blood
plasma PBB levels was observed over a 5-month period in 41 residents
of quarantined farms (Humphrey & Hayner, 1975). In contrast, Meester
& McCoy (1976) reported a marked decline over 3 years (1974-76) in
serum levels of PBBs. These authors also found that the average
decrease in PBB concentrations in the fat of 16 individuals was
about 40%, in a period of 6 months. No changes in PBB levels were
seen over an 11-year period (1976-87) in fat samples from a patient
with long-term exposure to PBBs from the early 1970s as a result of
the Michigan PBBs accident. The average fat level of PBBs was
0.8 mg/kg (Sherman, 1991).
In 1981, PBBs were found in 13-21% of serum samples from
4-year-old Michigan children. Their mothers belonged to a group that
was surveyed either with regard to the consumption of Lake Michigan
sport fish (mean PBB level detected in children: 2.4 ng/ml) or with
regard to former exposure to quarantined farm products (mean PBB
level detected in children: 3.0 ng/ml) (Jacobson et al., 1989).
Few human monitoring data are available for the US population
outside of Michigan. They are summarized in Table 40. One study
deals with the population in the vicinity of industrial areas
involved in PBB production or use (Stratton & Whitlock, 1979), the
other with farmers of the state of Wisconsin who were examined as
control group in connection with the Michigan PBB studies (Wolff
et al., 1978).
PBBs were found in all studies, but, because of the limited
data, the significance is unclear. The highest PBB levels were found
in the hair of humans living near PBB industry. Of the nine samples
analysed, five had detectable PBB levels. Both male and female hair
samples contained PBBs (Stratton & Whitlock, 1979).
In contrast to the other surveys, which had regard only to the
major PBBs component (hexabromobiphenyl), Stratton & Whitlock (1979)
identified the different PBB homologues in the extracted oils of the
human hair, collected from barbershops and beauty parlours (Table
41). There were identifiable differences in the composition of PBB
congeners found in hair from the three locations.
The samples with the highest concentrations contained
relatively large amounts of decabromobiphenyl, while the samples
with lower concentrations contained only hexabromobiphenyl (Stratton
& Whitlock, 1979). One sample was different from the others because
it contained dibromobiphenyl (see Table 41). As a result of the
sampling method, it was impossible to ascertain whether the exposure
was related to the workplace or to the ambient environment.
In a report by Lewis & Sovocool (1982), pooled adipose tissue
samples from 202 individuals from nine census regions in the USA
were analysed for HxBB. Although the average concentration was 1-2
µg/kg, it cannot be excluded that this was because of the inclusion
of a few samples with high PBB concentrations.
Table 40. Human monitoring data: PBB levels in the US population (outside of Michigan)
Year No. of % of Specimen PBB PBB Detecion Remarks References
specimens positive tissue group/area concentrationa examined limita
findings range
1977 56 3.6 serum Wisconsin farmers not C12H4Br6 examined as Wolff
a control detected-1.1b et al. (1978)
population
1977 3 33 hair (from males and females, < 100-8100 total PBBs 100 reported as Stratton &
barber- Bayonne New Jersey, µg/kg in oil Whitlock
shops) Vicinity of White (1979)
Chemical
3 100 hair (from males and females, 440-26 600 total PBBs 100 reported as
barber- Staten Island, µg/kg in oil
shops) New York, Vicinity
of Standard T
Chemical Co.
3 33 hair (from males and females, < 100-310 000 total PBBs 100 reported as&
barber- Sayreville New µg/kg in oil
shops) Jersey, Vicinity of
Hexcel Fine
Organics
a (µg/litre or µg/kg).
b PBBs not detected in 54/56 persons. PBBs observed at 1.1 µg/litre in one person, identified as recently
moved from a Michigan farm, and at 0.5 µg/litre in another person.
Table 41. Concentration (range) of different PBB congeners in human hair samples
taken in the vicinity of three industrial sites in the USA, reported as
µg/kg in oila
PBB-congeners Bayonne, New Staten Island, New Sayreville,
Jersey (White York (Standard T New Jersey
Chemical Corp.)b Chemical Comp.)c (Hexcel Corp.)d
MoBB nde nde nde
DiBB nd-8100 nd nd
TrBB nd nd nd
TeBB nd nd nd
PeBB nd nd nd
HxBB nd 440-740 nd-480
HpBB nd nd-890 nd
OcBB nd nd-1100 nd
NoBB nd nd-3600 nd-22 500
DeBB nd nd-20 000 nd-285 000
a Data from: Stratton & Whitlock (1979).
b Having manufactured octa- and decabromobiphenyl along with bromobiphenyl
ethers.
c Major user of FireMaster BP-6.
d Producer of laboratory quantities of various PBBs.
e nd = Not detected (detection limit = 100 µg/kg).
There is very little human monitoring data on PBBs in the
populations of countries other than the USA. Krüger et al. (1988)
reported PBB contamination of breast-milk from European women in a
survey from North Rhine-Westphalia, Germany (Table 33). The milk
samples (n=25) contained a typical pattern of certain PBB congeners.
It included penta- to octabromobiphenyls in concentrations ranging
from 0.002 to 28 µg/kg, based on milk fat. The most abundant
component was 2,2'4,4',5,5'-hexabromobiphenyl (BB 153) followed by a
peak consisting of two heptabromobiphenyl isomers (2,2',3,4',5,5',6-
and 2,2',3,4,4',5,6'-heptabromobiphenyl BB 187 and 182). Differences
in the pattern were only found in the milk given by a Chinese woman
and in that given by a woman having been exposed to several fires in
industry.
Concentrations of BB 153 in human and cow's milk, both
collected from the same region (North Rhine-Westphalia), were
1 µg/kg and 0.03 µg/kg, respectively, measured on a fat basis
(Krüger, 1988).
5.2.4 Subpopulations at special risk
Children are at risk from exposure to PBBs in different ways.
Studies on the Michigan population indicated a significant PBB
transfer to the fetus (Landrigan et al., 1979; Eyster et al., 1983;
Jacobson et al., 1984) and to breast-milk (Brilliant et al., 1978;
Landrigan et al., 1979; Eyster et al., 1983; Jacobson et al., 1984,
1989; Miller et al., 1984). PBB levels to which fetuses and newborn
infants were exposed in the Michigan accident are shown in Table 42.
Because the placenta acts only as a partial barrier to PBBs, a
newborn baby has a body burden, even before breast feeding.
Placental and cord serum levels are much lower than levels in
breast-milk. However, even at low concentrations, intrauterine
exposure may be significant, for several reasons, as pointed out in
detail by Jacobson et al. (1984).
Infants are not only exposed to PBBs through their mothers and
through consumption of contaminated food, but they also through
contact with PBBs from the environment. Young crawling children are
known to ingest accidentally soil or dust to an extent of up to
0.1 g/day.
5.3 Occupational exposure during manufacture, formulation, or use
In general, occupational exposure is to be expected in PBB
manufacturing and processing plants. In Michigan, as a result of the
PBB incident, farmers, and possibly dairymen, elevator, mill
personnel etc. were occupationally exposed (Kay, 1977).
Table 42. PBBb concentrations in maternal serum, adipose lipid, and milk lipid, and in
the cord serum and placenta of Michigan women (Michigan PBB-cohort) at the time of
parturition (1975-80)a
Paired specimen No. Rangec Median Geometric Measure
mean
Maternal serum 61 nd-1068 3 3.5 µg/litre
Placenta nd-370 < 1 - µg/kg
Maternal serum 60 nd-1068 3 3.2 µg/litre
Cord serum nd-104 < 1 - µg/litre
Maternal serum 47 nd-1068 3 3.0 µg/litre
Milk lipid nd-92 667 250 312 µg/kg
Milk lipid 27 52-92 667 384 472 µg/kg
Adipose lipid nd-174 000 522 82 µg/kg
a From: Eyster et al. (1983).
b Concentrations expressed as concentrations of hexabromobiphenyl.
c nd = Not detected (detection limit: 1 µg/litre or kg).
Bialik (1982) reported the following contaminant levels of
decabromobiphenyl measured in 1977 in the manufacturing area of
Hexcel/Fine Organics and Saytech, Inc. (Sayreville; USA):
- Plant air samples: 0.18 and 0.23 mg/m3 8 h TWA (time-weighted
average);
- Wipe tests, unspecified: up to 8 mg/100 cm2; - Wipe tests,
eating Table: 0.1 mg/100 cm2.
At this time, 95% of the plant production consisted of
decabromobiphenyl (18%) and decabromobiphenyl oxide (77%). About
2000 tonnes of decabromobiphenyl were manufactured during 1973-77
(Bialik, 1982).
Employees of chemical plants may be exposed directly to PBBs
(in most cases along with other chemicals) through contact,
inhalation, or ingestion (Wolff et al., 1979a). As an index of
individual exposure, PBB levels in the serum and adipose tissue of
chemical workers have been recorded. The results of several authors
are compiled in Table 43.
Most data refer to the Michigan Chemical Corp., St. Louis
(Michigan), which produced several brominated organic compounds and
manufactured over 5000 tonnes of PBBs, predominantly
hexabromobiphenyl, from 1970 to 1974. Some additional general
exposure from contaminated food can also be included for the workers
of Michigan Chemical.
To summarize, median serum and adipose tissue PBB levels were
higher among chemical workers than among male residents of
quarantined farms.
Non-production workers at the Michigan Chemical plant showed
significantly lower levels than workers involved in PBB production;
for example, median adipose tissue concentrations of PBBs were
2.49 mg/kg and 46.94 mg/kg, respectively.
In another study on workers at a PBB plant in New Jersey, Bahn
et al. (1980b) presented a detailed comparison of serum PBB levels
in various occupational groups. A significantly higher number of PBB
workers had detectable levels of PBBs, compared with other workers
in the study (35.9% compared with 12.2%). Among workers with
detectable PBB levels, the PBB workers had significantly higher
serum levels than workers from neighbourhood industries not using
PBBs.
Although this factory concentrated on manufacturing
decabromobiphenyl and decabromobiphenyl oxide (ether), there was no
positive identification of C12Br10 (Table 44) or C12Br14O
(Bahn et al., 1980b).
No data are available about occupationally exposed women.
Family members of chemical workers have also been found to have
a body burden of PBBs (Landrigan, 1980).
Bekesi et al. (1979b) determined the distribution of PBBs in
the blood compartments of 4 Michigan Chemical plant workers (Table
45) and suggested that the PBB level of the white cell fraction may
be a better indicator for risk potential than the total plasma PBB
concentration.
Table 43. Occupational exposure: PBB levels in chemical workers (USA)
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1975 Michigan workers (8-36 serum 6-85 HxBB Kay (1977)
Chemical Corp. months exposure (7)
(MCC)
(St. Louis,
Michigan)
1976 MCC (St. Louis, employees (55) serum 1.1-1729 123 9.3 HxBB Wolff
Michigan) et al.
(1978,
1979a)
production workers serum 603.9 108.4 HxBB 1 Wolff
(10) et al.
(1979a)
non-production serum 16.5 6.1 HxBB 1
workers (45)
workers (14) serum 1-1530 HxBB < 0.2 Wolff
et al.
(1979b)
1978 workers (14) 1-1363 HxBB < 0.2
(matched pairs)
Table 43 (contd).
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1976-77 MCC (St. Louis, workers and serum not 43.0 4.5 C12H4Br6 1 Landrigan
Michigan) families (216) detected-1240 et al.
(1979)
1975-80 MCC (St. Louis, workers (male) serum 1-2000 (25.4) 20 C12H4Br6 1 Eyster
Michigan) (29) et al.
(1983)
1978 Hexcel/Fine PBB workers serum not MoBB Bahn
Organics and (exposure to PBBs detected-1340 DeBB et al.
Saytech, Inc. (and PBBOs) for at (1980b)
(Sayreville least 6 weeks
New Jersey) between January
1973 and August
1978) (39)
1976 Michigan production workers adipose 5000-581 000 196 490 46 940 HxBB 500 Wolff
(7) tissue et al.
(1979a)
non-production 500-10 000 3880 2490
workers
1975-80 Michigan workers (male) adipose 400-350 000 5290 6000 HxBB 1 Eyster
(29) tissue et al.
(1983)
a In µg/litre or µg/kg.
Table 44. Detectablea serum levels (µg/litre) of PBB homologues in workers
at a plant producing decabromobiphenyl and decabromobiphenyl oxideb
PBB homologue Number of cases Range
C12H9Br 14 0.3-5.5
C12H8Br2 1 6.9
C12H7Br3 1 0.9
C12H6Br4 0 -
C12H5Br5 2 1.6-13.0
C12H4Br6 2 0.4-6.0
C12H3Br7 7 9.0-40.0
C12H2Br8 9 20.0-800.0
C12H Br9 1 500
C12Br10 0 -
Total PBBs 26 0.3-1340
a Excludes cases with "trace", "not confirmed" and "not detectable" levels.
b From: Bahn et al. (1980b).
Despite its significance for toxicological assessment, the
content of minor constituents of FireMaster(R) in the body burden
was rarely investigated. For example, Wolff & Aubrey (1978) examined
other PBB congeners, which are identifiable as peaks by GC/MS
(2 pentabromobiphenyl peaks, and 2 heptabromobiphenyl peaks), in the
serum of Michigan Chemical workers (n = 24) and Michigan dairy
farmers (n = 37), besides the major component (2,2',4,4',5,5'-
hexabromobiphenyl) of FireMaster(R). The relative concentrations,
with respect to the major hexabromobiphenyl peak, of these PBB
components were somewhat different for chemical workers and for
farmers, i.e., the two pentabromobiphenyl values (peak area ratios)
were significantly higher in the serum from chemical workers.
Table 45. Distribution of PBBs in blood compartments of Michigan Chemical Workersa
Polybrominated biphenyls
ng/mg Protein Ratiob
RBC plasma WBC RBC plasma WBC
Michigan Chemical Workers;
not directly involved in 0.07 0.13 3.9 1 : 2 : 56
the production of PBB 0.03 0.23 1.8 1 : 8 : 60
directly involved in 0.67 10.0 57.3 1 : 15 : 86
the production of PBB 0.63 10.2 32.0 1 : 16 : 51
a From: Bekesi et al. (1979b).
b RBC = Red blood cells; WBC = white blood cells.
This variation might be attributed to the different routes
(skin contact, inhalation, direct ingestion versus, primarily,
ingestion of animal foodstuff) and to the different composition
(unchanged versus animal-mediated material) of exposure in chemical
workers versus farmers. Further reasons might be the earlier initial
onset of contamination in workers and slight variations in the
composition (section 2.1.2) of several lots of FireMaster(R) BP-6
(the main product of Michigan Chemical) and FireMaster(R) FF-1,
which caused contamination of livestock feed (Anderson et al.,
1978a; Wolff & Aubrey, 1978; Wolff et al., 1979a).
The change in serum PBB levels over time was investigated in
chemical workers at two facilities. Wolff et al. (1979b) reexamined
serum PBB concentrations (determined as the major hexabromobiphenyl
peak) in 1978 from 14 workers of the Michigan Chemical Corp., who
had also been tested 18 months earlier. They found PBB levels of a
comparable order. In contrast, no subject (n=109) in a study on
chemical workers of Hexcel/Fine Organics and Saytech Inc.
(manufacturing decabromobiphenyl and decabromobiphenyl oxide) showed
any detectable serum level of PBBs (different congeners) in 1981
(Bialik, 1982), which was true, even for the two persons who had
shown high levels of serum PBBs in the previous study of 1978 (Bahn
et al., 1980b; Table 43). However, the results of PBB determination
in the fat of these two cases were positive in 1981. The negative
results of the determination of PBBs in serum were not expected, and
the authors suggested further studies.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Gastrointestinal absorption
Studies have been performed only on the gastrointestinal
absorption of PBBs. Some studies indicate that PBBs are rapidly and
efficiently absorbed, other studies indicate a much lower efficiency
of absorption (see Table 46). No information is available on the
extent of absorption of decabromobiphenyl.
Absorption can be strongly influenced by the vehicle in which
the compound is administered (Birnbaum, 1985). Administration of
hexabromobiphenyl in mineral oil or olive oil solution resulted in
higher absorption than administration in a methyl cellulose
suspension (see Table 46: Rozman et al., 1982). The degree of
halogenation also appeared to influence the absorption of PBB. For
example, less than 10% of 14C-labelled hexabromobiphenyl, but 62%
of a dose of 14C-labelled octabromobiphenyl were eliminated in the
faeces of rats in 24 h, though both compounds had been administered
in corn oil (see Table 46).
The conclusion that more brominated biphenyls are absorbed less
efficiently than less brominated biphenyls can, possibly, be drawn
from other findings. Willett & Durst (1978) observed that, during
feeding of FireMaster(R) BP-6, the relative concentration of
pentabromobiphenyl in the faeces of cows was decreased, and that of
heptabromobiphenyl was elevated compared with the
FireMaster(R)-standard. Similarly, faecal concentrations of
heptabromobiphenyl were enhanced relative to concentrations of
hexabromobiphenyl in the faeces of hens, when FireMaster(R) BP-6
was fed (Fries et al., 1976). However, Polin & Leavitt (1984) found
that the ratio of 3.5 for hexa- to heptabromobiphenyl in the
chemical sample of FireMaster(R) FF-1 shifted to an average ratio
value of 2.5 in the whole carcasses of chickens analysed on days 0,
21, and 42 of withdrawal, inferring a better absorption of hepta-
bromobiphenyl.
Generally, it should be noted that faecal elimination during
the first few days following dosing might be an indicator, but is
not a measure, of lack of absorption, because some absorbed PBB is
eliminated and recycled into the faeces in bile and by diffusion
across intestinal membranes (Rozman et al., 1982; Fries, 1985b).
Table 46. Absorption of PBBs after oral administration
PBB compound Species Vehicle Absorption Methods Comments References
(sex) parametera
[14C-]2,2',4,4',5,5'- rat emulphor-EL 620: 90%, 24 h faeces analysis single dose Matthews
hexabromobiphenyl (male) ethanol: water gut content et al. (1977)
(1 : 1 : 8)
corn oil 90% multiple doses (4)
[14C-] octabromobiphenyl rat (male, corn oil 38%, 24 h faeces analysis single dose Norris et al.
(technical mixture) female) (1973)
[14C-]2,2'4,4',5,5'- rhesus 1% methyl cellulose 40%, 10 days faeces analysis two doses Rozman et al.
hexabromobiphenyl monkey mineral oil 62%, 5 days faeces analysis single dose (1982)
(male) olive oil 66%, 5 days faeces analysis repeated doses (4)
FireMaster(R) BP-6 cow crystalline PBB in 50%, 168 h faeces analysis single dose Willett &
(female) gelatin capsules (7 days) Irving (1976)
calf crystalline PBB in 95%, (9 days) faeces analysis daily feeding
(male) gelatin capsules
a Values based on concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (FireMaster(R) BP-6 sample) or on [14C-]activity.
6.1.1.2 Dermal and inhalation absorption
No quantitative information is available on skin absorption and
intake through inhalation.
6.1.2 Human studies
It is plausible that inhalation and dermal contact are the main
routes of exposure to PBBs for chemical plant workers (Wolff et al.,
1979a), while the main route for Michigan people was the ingestion
of PBBs dissolved in the fat of meat and milk (Di Carlo et al.,
1978). An appropriate model for assessing the latter kind of
absorption is thought to be the rat-corn oil model (Fries, 1985b).
No quantitative data are available on PBB absorption in humans.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Levels in organs and blood
As can be seen from Tables 47, 48, and 49, most studies on the
distribution of PBBs have been conducted with the FireMaster(R)
mixture. A few earlier publications refer to technical
octabromobiphenyl. No experimental data are available on tissue
distribution of decabromobiphenyl. When FireMaster(R) was
administered, the distribution process was studied predominantly as
the distribution of 2,2',4,4',5,5'-hexabromobiphenyl, and, with far
less emphasis, on the distribution of the minor components of the
mixture. Little information is available on the distribution of PBB
congeners, when administered individually.
Investigations on rats, mice, cows, sheep, pigs, and avian
species demonstrated that PBBs were distributed widely throughout
the body tissues in all species. Highest (equilibrium)
concentrations on a wet tissue basis were found in adipose tissues,
consistent with the solubility characteristics of PBBs. Adipose
concentrations are usually an order of magnitude higher than those
of most muscle and organ tissues (see Tables 47, 48, 49, and 50).
Much of the variation in concentrations among tissues can be
accounted for by variations in the fat concentrations in these
tissues (Willett & Durst, 1978; Fries et al., 1978b; Fries, 1985b).
Table 47. Distribution of PBBs in mammals after the administration of a single dose of PBBs
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
FireMaster(R) FF-1 rat oral 1000 10 monthsb adipose tissue (714) > liver (60) > blood Kimbrough et al.
(lot No. 7042) (male) in corn oil (0.94) (1978)
rat 10 monthsb adipose tissue (1202) > liver (37) > blood
(female) (2.9)
FireMaster(R) FF-1 rat oral 80 42 days fat (295) > blood (0.38) Wolff &
(male) in corn oil Selikoff (1979)
FireMaster(R) FF-1 rat oral 500 4 months adipose tissue (1008) > liver (50) > blood Kimbrough et al.
(lot No: 7042) (male) in corn oil (2.1) (1980)
FireMaster(R) FF-1 rat oral 10 24 hb sc fat I (61 500) > sc fat II (38 700) > liver Domino et al.
(lot No: FF-1312-FT) (male) (20 900) > lung (7650) > kidney (7310) > heart (1980b)
(6470) > jejunum (4860) > spleen (3530) >
cerebellum (2990) > grey matter (2850) > white
matter (2750) > testes (2380) > blood (945)
4 weeksb sc fat (19 200) > jejunum (3170) > lung (1240) Domino et al.
> liver (690) > kidney (650) > spleen (520) (1980b)
> heart, testes (both 240) > grey matter
(210) > cerebellum (200) > white matter (170)
> blood (56.9)
FireMaster(R) BP-6 rat intraperitoneal 12 weeks serum (46.80 ng/ml) Miceli &
(male) 10 in corn oil Marks (1981)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
fat (21.90) > adrenal (3.64) > lung (0.98)
> liver (0.59) > pituitary (0.91) > gonad (0.33)
> kidney (0.22) > heart (0.20) > spleen (0.17)
> brain (0.13)
36 weeksb serum (23 ng/ml)
fat (16.62) > adrenal (2.67) > lung (0.51) >
pituitary (0.29) > liver (0.20) > kidney (0.14)
> gonad, brain (both 0.10) > heart (0.08)
> spleen (0.05)
2,2',4,4',5,5'- rat oral 1 in: 1 day muscle (29.9) > adipose (25.5) > skin (17.9) Matthews et al.
[14C]-hexabromobiphenyl (male) Emulphor > liver (9.0) > blood (0.90)c (1977)
EL 600: ethanol:
water (1:1:8)
14C-PBB rat intraperitoneal 28 days ovaries (130) > skin (15.1) > testicles (13.3) McCormack et al.
(male 150 in: peanut > intestine (11.7) > lung (7.3) > liver (4.7) (1979b)
and oil > muscle, heart (1.9) > fat (1.8) > brain (0.9)d
female
pups)
FireMaster(R) BP-6 cow oral 5.95 in: 10 days liver (1.35) > fat (sc: 1.15; perirenal: 1.09; Willett &
(lot No. RP-158) (female) gelatin capsule peri-cardiac: 0.97; intermuscular: 0.81; omental: Irving (1976)
0.78) > brain (pons: 0.27; cortex: 0.08) >
mammary gland (0.25) > kidney (0.12) > heart
(0.11) > lung, muscle (0.08) > ovaries, uterus
(0.06) > plasma, rumen wall (0.04) > bile
(0.02) > synovial fluid (0.01)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
[14C-]octabromobiphenyl rat oral 1 in: 16 days adrenal, adipose, heart, skin > liver, Norris et al.
(technical mixture) (male) corn oil pancreas, spleen (1973)
a Measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity; (in parentheses: values measured - referring
to various measures).
b For additional time points: see original reference.
c Values in average % total PBB dose.
d Values in µg-equivalents/g wet weight.
sc. = Subcutaneous.
Table 48. Studies on the distribution of PBBs in animals following dietary or repeated oral intake
of the FireMaster(R) mixtures or 2,2',4,4',5,5'-[14C]-hexabromobiphenyl
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 fat (330) > mammary gland (318) Rickert
BP-6 (pregnant) feed until day 21 of > kidney (30) > skin (22) > liver, et al. (1978)
gestation lung, brain, heart, small
intestine, placenta, uterus (all
< 5)
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 mammary gland (117) > liver (4) Dent et al.
BP-6 (maternal) feed to 14 days (1977b)
postpartum
FireMaster(R) rat 25 mg/kg day 8 of pregnancy 0 fat (74) > mammary > liver McCormack
BP-6 (maternal) feed to 14 days > kidney > lung (6) et al.
postpartum (1979a)
50 mg/kg 0 fat (483) > mammary > kidney
feed > lung (13)
200 mg/kg 0 fat (966) > mammary > kidney
feed > lung (21)
FireMaster(R) rat 100 mg/kg day 8 of pregnancy 0 fat (813) > liver (54) > mammary McCormack &
BP-6 (maternal) feed to 28 days (43) Hook (1982)
postpartum 14 weeks fat (459) > mammary (225) > liver
(after first (12)
and only
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
litter was
weaned)
> 10 weeks fat (77) > mammary (19) > liver (3)
and after
weaning
their second
litter
2,2'4,4',5,5'- rat 1 mg/kg 4 days 3 days adipose (41.1) > skin > muscle Matthews
hexabromobiphenyl (male) body weight > liver > blood (0.32)b et al. (1977)
per day
FireMaster(R) rat 0.1 mg/kg 9 days 0 liver (1.5) > brain (0.5), Render et al.
BP-6 (Lot 6224A) (male) feed adipose (0.3)c (1982)
1 mg/kg 9 days 0 liver (8.3) > brain (1.8),
feed adipose (1.7)c
10 mg/kg 9 days 0 liver (135) > adipose (27)
feed > brain (12)c
100 mg/kg 9 days 0 liver (1213) > adipose (251)
feed > brain (103)c
FireMaster(R) rat 3 mg/kg 20 days 0 adrenal (93.7) > thyroid (> 20) Allen-
FF-1 (Lot No. (male) body weight > testes (8.7) Rowlands et al.
FF-1312-FT-3) per day (in 1981);
lecithin Castracane
liposomes) et al. (1982)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
20 days 5 months adrenal (481) Castracane
(plus dietary et al. (1982)
restriction)
FireMaster(R) mouse 100 mg/kg 14 days 6 h thymus (391) > fat > liver > brain Corbett et al.
BP-6 feed > pancreas > testicles > spleen (1978a)
(2.7)
14 weeks thymus (50) > adrenals = fat >
liver > testicles > spleen > brain
> pancreas (n.d.)
12 weeks perithymic fat (96) > perirenal
fat > adrenal glands > thymus
gland (5.5)
FireMaster(R) mouse 5 mg/kg 3 weeks 0 thymus (20) > lung, liver > spleen Loose et al.
FF-1 feed > serum (< 0.002) (1981)
(lot No. 7042)
8 weeks 0 thymus (24) > liver, lung > spleen
> serum (0.019)
FireMaster(R) mouse 167 mg/kg 3 weeks 0 thymus (109) > liver > lung > Loose et al.
(lot No. 7042) feed spleen > serum (1.22) (1981)
6 weeks 0 thymus (3088) > liver > spleen
> lung > serum (4.75)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum, (11)
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum (11)
FireMaster(R) cow 50 mg/kg 15 days 15 days renal fat (10) > omental fat Gutenmann &
BP-6 (lactating) feed > brisket fat > liver = thyroid Lisk (1975)
> mammary = chuck muscle > loin
muscle > heart > kidney = brain
> adrenal = spleen (0.4)
FireMaster(R) calf 25 g daily 9 days 0 rumen contents (14257) > feces Willett &
BP-6 (in gelatin > rumen wall > bile > marrow Irving (1976)
(lot 6244 A) capsules) > perirenal fat (441) > kidney
> testes > liver > thymus > heart
> brain, pons > lymph nodes >
tongue > spinal cord > brain,
cortex > plasma > small intestine
> lung > spleen > thyroid >
muscle (25)
FireMaster(R) cow 250 mg daily 60 days 0 fat (25) > liver > glands > nervous Willett &
BP-6 (heifer) (in gelatin > bile > contractiles > plasma Durst (1978)
(lot 6244 A) capsules) > liquids (0.0198)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) cow environmentally ca. 14 days 9 months perirenal fat (380*) > omental fat Fries et al.
FF-1 contaminated > subcutaneous fat > kidney > liver (1978b)
(Michigan PBB > skeletal muscle > cardiac muscle
incident) > lung > brain (10.5*)
ca. 200-400 g 9-12 months perirenal fat (1224*) > omental fat
(killed in >subcutaneous fat > skeletal muscle
extremis) > liver > cardiac muscle > kidney
> lung > brain (57*)
FireMaster(R) calf 10 mg/kg 4 weeks 0 fat (378) > kidney > liver > muscle Robl et al.
FF-1 (female) body weight (1.26) (1978)
per day in
gelatin
capsules
calf 100 mg/kg 6 weeks 0 fat (6080) > kidney > liver
(male) body weight > muscle (34)
per day
cow 1 158 days 182 days brisket fat (4.5) > bone marrow
> stomach fat > tail fat (3.3)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) sheep 50 mg/kg 30 days 0 renal fat (42) > omental fat Gutenmann &
BP-6 feed per day > brisket fat > liver > chuck Lisk (1975)
(complete muscle > loin muscle > heart >
ration) thyroid > brain > adrenal > kidney
= spleen (0.9)
FireMaster(R) pig 20 mg/kg 16 weeks 0 back fat (64) > leaf fat > liver Ku et al.
BP-6 feed > muscle > kidney (0.9) (1978)
200 mg/kg 16 weeks 0 back fat (503) > leaf fat > muscle
feed > liver > kidney (13.5)
FireMaster(R) pig 100 mg/kg during 2nd 0 adipose tissue > liver > kidney > Werner &
BP-6 (lactating feed half gestation > braind Sleight (1981)
sow) 200 mg/kg and during
feed lactation
"PBB" Japanese 20 mg/kg 9 weeks 0 liver (374) > kidney > muscle Babish et al.
(ca 75% quail feed > heart > brain (40)e (1975a)
hexabromobiphenyl) male
female 0 liver (225) > heart > kidney >
> muscle > brain (26)e
FireMaster(R) chicken various 5 weeks 0 adipose tissue (3:1) > whole egg Polin &
FF-1 (White concentrations > liver > muscle (0.008:1)f Ringer
Leghorn (1978a)
hens)
Table 48 (contd)
a Measured as concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (in parentheses: values measured - referring to various measures).
b Values in average % total PBB dose.
c Values on a fat basis.
d Concentrations are listed in Table 53.
e Values in mg/kg dry weight.
f Values as ratios of tissue PBBs: diet PBBs.
* = Geometric mean.
Table 49. Studies on distribution of the following continuous exposure to octabromobiphenyl
Species Exposure Duration of Tissues and organs under study, ranked References
Route, Duration recovery in order of decreasing concentrations
concentration (average bromine content; µg/g wet weight)
Rat in diet Lee et al. (1975a)
(male) 0 mg/kg feed (control) 2 weeks liver (3.4) > fat (1.7) > muscle (1.6)
100 mg/kg feed 2 weeks - liver (83) > fat (73) > muscle (14)
1000 mg/kg feed 2 weeks - fat (333) > liver (319) > muscle (77)
100 mg/kg feed 4 weeks 0,2,6 weeks fat > liver > muscle
100 mg/kg feed 4 weeks 18 weeks fat > muscle > liver
Rat inhalation 23 h/day, 7 days Waritz et al.
(OcBB vapour)a per week, 15 weeks (1977)
0 pg/litre air liver (3) > muscle (1.6) > fat (1.5)
(control)
3.5 pg/litre air - liver (4.2) > fat (3.0) > muscle (1.5)
a OcBB = Octabromobiphenyl, Dow, Lot 102-7-72.
Table 50. Tissue: blood ratios of PBBs estimated in a standard 250-g rata
Compartment Ratio
Liver 17:1
Muscle 5:1
Skin 56.5:1
Adipose 340:1
Intestine tissue 1:1
a From: Tuey & Matthews (1980).
However, even when concentrations are expressed on a fat basis
rather than a wet tissue basis, there are some deviations from
uniform concentrations among tissues (Fries, 1985b). PBB
concentrations, for example, were low in nervous tissue, despite its
high lipid content and often disproportionally high in liver,
considering its relatively low lipid content (see Tables 47, 48, and
49; additional information on fat content percentage, e.g., by
Willett & Irving, 1976; Fries et al., 1978a,b; Kimbrough et al.,
1978; Werner & Sleight, 1981).
The ratios between the PBB concentrations of adipose tissue,
blood, and vital organs are different when animals are not at
equilibrium (see also section 6.5) with respect to dosing regimen or
body condition. Usually, concentrations in liver are very high
compared with those in other tissues, immediately after dosing, and
decline relatively as equilibrium concentrations are established
(e.g., Lee et al., 1975a; Matthews et al., 1977; Miceli & Marks,
1981; Fries, 1985b). Generally, this phenomenon is most pronounced
in tissues that have high blood flow rates relative to tissue mass
(Tuey & Matthews, 1980). As an exception, livers of mice, tested in
a small series, appeared to have relatively concentrated the PBB
with passing time (Corbett et al., 1978a).
On the other hand, body weight changes, pregnancy, parturition,
and lactation can affect the concentration relationships until
equilibrium is reestablished (e.g., Rickert et al., 1978; Willett &
Durst, 1978; McCormack & Hook, 1982).
The route of exposure (oral or intravenous administration) had
no effect on the tissue distribution (blood, liver, muscle, adipose,
skin) of 14C-labelled 2,2',4,4',5,5'-hexabromobiphenyl (dose =
1 mg/kg body weight) in rats (Matthews et al., 1977).
Kimbrough et al. (1980) studied the effects of different diets
and of mineral oil on the HxBB concentration in rats that had
received a single oral dose of FireMaster(R) FF-1 (500 mg/kg body
weight, in corn oil). After 3 months of feeding, GC-analysis of
blood, liver, and adipose tissue showed no statistically significant
differences in PBB concentrations among the differently fed groups,
when concentrations were calculated on a lipid weight basis. On a
wet weight basis, however, the PBB concentrations were significantly
increased in the livers of rats on the experimental diets (Teklad-4%
and -20% fibre) and on mineral oil compared with those of rats on
the basal diet (Purina Chow).
In another study, McCormack et al. (1979a) examined the
consequences of simultaneous exposure to PCBs and PBBs in
(lactating) rats, because human populations that have been exposed
to PBBs are also likely to have been exposed to PCBs. The
extrahepatic tissue (kidney, mammary, lung, fat) concentrations of
PCBs and PBBs were similar, regardless of whether the agents were
administered together or alone. Liver, however, contained lower
concentrations of PBBs after treatment with an equal mixture of PCBs
and PBBs than when PBBs were administered alone. (None of the
tissues had higher concentrations of PBBs than PCBs after
concomitant administration. The reasons for this were not clear).
The distribution of PBB (hexa) among blood compartments
(plasma, red cells) has been studied in rats (Domino et al., 1980b).
It was found that plasma levels of 2,2',4,4',5,5'-hexabromobiphenyl
were generally four times greater than red cell levels.
Matthews et al. (1984) reported that 81% of plasma PBB (hexa)
was associated with the total lipoprotein fraction. In another study
(Kraus & Bernstein, 1986), approximately 65% of all radiolabelled
HxBB incubated with human serum in vitro was recovered in the
lipoprotein fraction. Of the HxBB in the lipoprotein fractions, 40%
was recovered in low-density lipoproteins (LDL), 33% in
very-low-density lipoproteins (VLDL), and 23% in high-density
lipoproteins (HDL). Addition of human lipoprotein to a culture
medium influenced the partition of HxBB between adipocytes and
culture medium (Kraus & Bernstein, 1989).
Some data are available on the tissue distribution of the minor
components of FireMaster(R)-mixture (see also section 6.5). Domino
et al. (1980b) analysed the relative percentages of various PBB
congeners (two penta-, three hexa-, and three heptabromobiphenyls)
in several tissues of rats given FireMaster(R) FF-1. From their
list, it was evident that each of the PBB analogues was found in all
tissues examined (liver, lung, testes, fat; blood, brain) but their
partitioning ratios differed. Distribution has been recorded also
after exposure to single PBB congeners, e.g., tetra-, penta-, and
hexa-isomers (Akoso et al., 1982a; Dharma et al., 1982; Domino
et al., 1982; Render et al., 1982; Millis et al., 1985a). In some
cases, it was not clear whether the differences in partitioning
between congeners were real or were caused by analytical problems
(Render et al., 1982).
6.2.1.2 Transfer to offspring
1) Mammals
Placental transfer
PBBs are capable of passing through the placental barrier into
the developing fetuses. This has been demonstrated in mice (Corbett
et al., 1978a; Welsch & Morgan, 1985), rats (Beaudoin, 1977; Rickert
et al., 1978), guinea-pigs (Ecobichon et al., 1983), minks and
ferrets (Bleavins et al., 1981), cows (Detering et al., 1975; Fries
et al., 1978a,b), and pigs (Werner & Sleight, 1981) by administering
the Fire-Master(R)-mixture or individual PBBs, or by using
technical octabromobiphenyl in rats (Aftosmis et al., 1972a; Waritz
et al., 1977).
The studies compiled in Table 51 are rarely intercomparable.
However, it is obvious that PBBs are readily transferred across
placental membranes, the concentrations among fetal tissues being
highest in the liver. The limited data on the distribution of PBBs
in fetal tissues showed often, but not always (Ecobichon et al.,
1983; Welsch & Morgan, 1985), lower PBB residues in fetal than in
maternal tissues (see Table 51). In cows, the average ratio of PBB
concentrations in fetal or calf tissue to PBB concentrations in dam
tissue was 0.36 : 1 for fat and 0.37 : 1 for blood (Fries et al.,
1978a,b). In contrast, the concentration ratio between the fetal and
maternal liver of mice ranged from 3.5:1 to 10:1 (Welsch & Morgan,
1985).
Species-dependent differences in the amounts of PBBs
transferred have been demonstrated for two mustelids, the mink and
the European ferret. PBB levels in the ferret kit were significantly
greater than those in the mink kit (see Table 51: Bleavins et al.,
1981).
Table 51. Placental transfer: PBB concentrations in the fetus and the mother
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mouse FireMaster(R) BP-6; 39.52 2.51 - 0.53 [HxBB] Corbett
(mg/kg) et al. (1975)
1000 mg/kg diet
on days 7-18 of
pregnancy
0 mg/kg (control) 0.04 0.05
Mouse FireMaster(R) BP-6; 112.74 12.02 - 0.95 - 5.86 - [HxBB] Corbett
(mg/kg) et al.
100 mg/kg diet (1978a)
on days 7-18 of
pregnancy
Mouse 2,2',4,4',5,5'- [HxBB] Welsch &
hexabromobiphenyl (mg/kg) Morgan (1985)
(purity: > 99%)
dietary intake
from day 6-15 of
pregnancy; sacrifice
on day 17
placenta:
100 mg/kg feed 9.08 17.26 3.79 3.06 182.88
300 mg/kg feed 17.30 39.84 8.23 4.56 217.48
500 mg/kg feed 69.13 89.47 24.58 17.64 316.79
750 mg/kg feed 95.36 103.70 54.33 18.64 453.12
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Rat FireMaster(R) BP-6 [HxBB] Beaudoin
(mg/kg) (1977)
single oral dose of
800 mg/kg body
weight (in sesame (pooled
oil) at day 12 of samples
pregnancy; killing: 51 267 13 from 4 rats)
24 h later
48 h later 250 248 6
Rat FireMaster(R) BP-6; 330 4.2 - 1.6 0.2 GI tract: [HxBB] Rickert
0.1 (mg/kg) et al. (1978)
50 mg/kg diet from
day 8-21 of
pregnancy
Rat Octabromobiphenyl Waritz
(technical); et al. (1977)
dietary intake
from day 8-15 of
pregnancy; sacrifice
on day 20;
0 mg/kg (control) 1.43 3.56 4.38 [Br]
100 mg/kg 70.4 16.1 7.62 (mg/kg)
1000 mg/kg 326 79.8 21.2
10 000 mg/kg 590 158 30.1
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mink [14C-]-PBBs (HxBB 0.031 1.622 plasma: 0.002 0 0.005 kidney: % of the Bleavins
and HpBB); iv 0.04 0.003; initial dose et al. (1981)
injection (1 µCi) brain: 0; per g of tissue
in the final intestine: or ml of fluid
trimester of 0.001
gestation; killed
2 h later
Ferret see above 0.124 1.625 plasma: 0.005 0.004 0.013 kidney: see above
0.07 0.010
brain:
0.003
intestine:
0.005
Guinea- FireMaster(R) FF-1; 45 7 kidney: 45 45 kidney: 1 [HxBB] Ecobichon
pig 4.5; (mg/kg) et al. (1983)
single oral dose of lung: 7
50 mg/kg body weight lung: 1.5
at approximately 65
days of gestation;
killed 2 days later
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Pig FireMaster(R) BP-6; Werner &
Sleight (1981)
dietary intake
during 2nd half of
gestation
10 mg/kg feed 0.4 1.0 kidney: [HxBB]
nd; (mg/kg)
brain: nd
100 mg/kg feed 4.9 11.5 kidney:
nd;
brain: nd
200 mg/kg feed 40.3 24.2 kidney: 1.5
brain: 1.8
a HpBB = 2,2',3,4,4',5,5'-heptabromobiphenyl.
b nd = Not detected.
c [HxBB] = Concentration of 2,2',4,4',5,5',-hexabromobiphenyl; [Br] = Concentration of bromide.
Milk transfer
In mammals, the second route of PBB transfer from the mother to
the offspring is nursing. The efficiency of this way has been shown
through determining the PBB content in milk in relation to the body
burden or in relation to exposure levels of contaminated dams, and
through measuring PBB levels in kits that have been exposed to PBBs
only from suckling. The FireMaster(R) mixture was used in all
studies. Mammary transfer of technical octa- or decabromobiphenyl
has not (yet) been assayed.
Most investigations on the PBB contents of milk from
contaminated animals have been conducted on cows (Fries & Marrow,
1975; Willett & Irving, 1976; Robl et al., 1978; Fries et al.,
1978a,b; Willett & Durst, 1978). The ratios of concentrations in
milk fat to body fat in cows no longer receiving PBBs averaged about
0.4 :1 (Willett & Durst, 1978; Fries et al., 1978a,b; see also
Fig. 5). This ratio is much lower than the ratio in humans (see
section 6.2.2).
For other species (guinea-pig, rat, mink, pig) only single data
can be found in the literature. When (lactating) guinea-pigs
received a single oral dose of FireMaster(R) FF-1 (50 mg/kg body
weight) within 6-12 h of parturition, levels of HxBB in breast milk
(and in perirenal adipose tissue) were of the order of 22 µg/g (and
17 µg/g), respectively, 2 days after treatment (Ecobichon et al.,
1983). Rats fed 50 mg FireMaster(R) BP-6/kg in their diet from day
8 of pregnancy until 14 days after delivery showed, on day 14
postpartum, HxBB concentrations of about 51 µg/ml in the milk and
about 483 µg/g wet weight in their body fat (McCormack et al.,
1979a). In the same study, milk transfer of PBBs and PCBs was
compared. Milk usually contained higher concentrations of PCBs than
of PBBs, after simultaneous or separate exposure.
Contrary results were obtained with minks, intraperitoneally
injected with either 3 µCi of 14C-labelled PCB or 3 µCi of 14C-
labelled PBB on the approximate date at which the embryos would have
been implanted (Bleavins et al., 1981). Two weeks postpartum, milk
levels of PBBs were determined to be four times those of PCBs
(0.105% versus 0.025% of the initial maternal dose per gram of
tissue). Werner & Sleight (1981) determined PBB concentrations in
the tissues and milk of sows fed various amounts of FireMaster(R)
BP-6 (Table 53). At the end of lactation (4th week), the adipose
tissue and milk of sows, fed daily with 200 mg PBB/kg feed, had HxBB
concentrations of 194 µg/g tissue (wet weight) and of 22 µg/ml whole
milk, respectively. The authors calculated that, on a body weight
basis, nursing pigs consumed PBBs in concentrations similar to the
concentration given to the sows. Tissue levels of young exposed to
PBBs only via nursing have been determined for rats (Rickert et al.,
1978) and for guinea-pigs (Ecobichon et al., 1983). When pups of
non-treated female rats were nursed by dams fed FireMaster(R) BP-6
(50 mg/kg body weight) on days 1-14 postpartum, hepatic HxBB-
concentrations were on average approximately eight times higher than
those in the dams on day 14 postpartum (Rickert et al., 1978).
People who bought food primarily from quarantined farms were
thought to have been exposed 10 to 100 times more than the typical
retail store customer (Schwartz & Rae, 1983): ca. 100 mg of PBBs
versus 1-10 mg (Brown & Nixon, 1979).
While many dust and cobweb samples found in the buildings of
some PBB-contaminated farms had very high residue levels, the amount
of PBB residue involved is said not to be sufficient to be an
important contributor to animal residues (Fries & Jacobs, 1980) and,
possibly to human exposure.
5.2.2 Human monitoring methods for PBBs
Usually, suitable human monitoring data, as such, are used to
describe the real exposure to a toxic chemical. As an indicator of
human exposure to PBBs, the presence of PBBs in adipose tissue,
breast-milk, whole blood, serum, red and white blood cells (Bekesi
et al., 1979a,b), and human hair oils (Stratton & Whitlock, 1979)
has been assessed. The most commonly used specimens were serum,
breast-milk, and adipose tissue (see Tables 38-40).
Table 38. Human monitoring data: PBB levels in the Michigan population (USA)
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1974 82 96.3 serum adults from not 14 not Humphrey &
quarantined farms detected- specified Hayner (1975)
2260
28 100 serum children from 2-2260 35 not
quarantined farms specified
5 100 serum lactating females 3-1068
from quarantined
farms
1976 524 serum Michigan farmers 23.7 2.6 0.2 Wolff et al.
µg/litre (1978a);
Lilis et al.
(1978)
283 serum residents on 0.2-> 1000 33.9 3.9
quarantined farms
153 serum residents of 0.2-50 2.9 1.4
non-quarantined
farms
40 serum consumers of 0.3-1000 56.6 4.2
products
from quarantined
farms
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
28 serum consumers of 0-50 3.4 2.2
products from
non-quarantined
farms
consumers and Wolff et al.
residents: (1978)
quarantined farms:
40 serum females < 18 years 28.0 2.3 0.2
µg/litre
102 serum females > 18 years 18.2 2.5
51 serum males < 18 years 67.7 7.3
129 serum males > 18 years 28.2 4.4
consumers and
residents:
non-quarantined
farms:
37 serum females < 18 years 3.1 1.3
57 serum females > 18 years 1.7 0.9
35 serum males < 18 years 4.8 1.7
51 serum males > 18 years 3.1 2.2
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1976 485 serum consumers and Chanda et al.
residents: (1982)
quarantined farms:
27 serum 0-5 years 0.2-64.2 10.15 not
specified
137 6-18 years 0.0-962.4 27.22 not
specified
321 > 18 years 0.2-1778.0 24.42
1976 321 serum consumers and Chanda et al.
residents: (1982)
non-quarantined
farms:
18 0-5 years 0.2-37.4 6.42 not
specified
104 6-18 years 0.0-42.6 3.25 not
specified
177 > 18 years 0.0-94.0 3.03 not
specified
1976 serum Michigan children 3.41 not Barr (1980)
specified
143 females 2.72 not
specified
149 males 4.23 not
specified
33 0-4 years 2.75 not
specified
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
77 5-8 years 5.59 not
specified
81 9-12 years 3.18 not
specified
101 13-16 years 2.65 not
specified
1976-77 3639 serum Michigan residents 0-1900 21.2 3.0 1 µg/litre Landrigan
with various et al.
degrees of exposure (1979);
Landrigan
(1980)
1976-77 1750 serum contaminated farm 0-1900 26.9 4.0 1 µg/litre Landrigan
1114 residents farm 0-659 17.1 3.0 et al.
(1979);
216 product recipients 0-1240 43.0 4.5 Landrigan
(1980)
559 chemical workers and 0-111 3.4 2.0
families volunteers
1976-77 52 serum women at the time not 26.2 2.5 1 Landrigan
of delivery detected- µg/litre et al. (1979)
1150
1977 3683 serum Michigan PBB cohort < 1-3150 23.2 4.1 3 not Kreiss et al.
specified (1982)
1888 males 5.8
1795 females 2.8
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1978 1681 serum Michigan residents Wolff et al.
(1982)
(randomly selected)
1120 68.9 adults 0.2-120.5 1.3 0.6 0.2
µg/litre
461 72.7 children 0.2-37.2 1.8 0.8
232 Upper Peninsula 0.2
Lower Peninsula:
467 Detroit Area 0.5
191 Muskegon Area 1.7
791 remainder of state 0.9
Muskegon County:
54 serum male adults 4.0 2.6 0.2 Wolff et al.
µg/litre (1982)
74 female adults 2.1 1.4
36 male children 4.9 3.4
27 female children 3.8 2.0
1975-80 serum Michigan PBB cohort: Eyster et al.
(1983)
61 pregnant females not 3.5 3 1
detected- µg/litre
1068
56 non-pregnant females not 3.1 2
detected-
873
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
29 male chemical workers 1-1200 25.4 20
83 male farm and other not 5.4 4
workers detected-
1515
1974-75 5 100 milk women from 0.21-92 660 not Humphrey &
quarantined farms specified Hayner (1975)
1976 milk lactating women Brilliant
(fat) (randomly selected et al. (1978)
53 96 from Michigan: not 0.068 0.1 mg/kg
Lower Peninsula detected-
1.2
42 43 Upper Peninsula not
detected-
0.320
1976-77 32 100 milk women at the time of 0.032-93 3.61b 0.225b Landrigan
(fat) delivery (Michigan et al. (1979)
PBB cohort)
1976-78 2986 88.5 milk lactating women not 0.097 0.1 0.06 < 0.05 mg/kg Miller et al.
milk (self-selected) detected-2 (1984)
1975-80 Michigan Eyster et al.
PBB cohort (1983)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
47 milk pregnant females not 0.312b 0.250b 0.001
(fat) detected- mg/kg
92.7
1974-75 15 100 adipose persons from 0.104-175c Humphrey
quarantined farms & Hayner
(1975)
1975-76 53 adipose quarantined farmers 1.965 Meester &
McCoy
(1976)
29 100 non-quarantined 0.516
farmers
9 100 city residents 0.226
116 fat members of farm 0.58-273.0
families
1977 19 subcutaneous children with known Weil et al.
exposure to PBBs (1981);
fat in utero and/or Schwartz &
through breast milk Rae (1983);
Seagull
(1983)
10 100 "high exposure" 0.116- 4.218
20.960
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
9 100 "low exposure" 0.010- 0.050
0.074
1978 844 97.3 adipose Michigan residents < 0.002- 0.4 0.199 0.002 Wolff et al.
36.7 mg/kg (1982)
(randomly selected) 0.015
87 Upper Peninsula
Lower Peninsula:
255 Detroit Area 0.16
84 Muskegon Area 0.50
418 remainder of state 0.24 0.050
1975-80 32 adipose Michigan PBB-cohort: not 0.330 0.540 0.001 Eyster et al.
lipid pregnant females detected- mg/kg (1983)
174
56 non-pregnant females not 0.00057 0.460
detected-
0.619
29 male chemical 0.4-350 5.29 6.0
workers
83 male farm and other 70-350 1.65 1.05
workers healthy male
Not 7 100 adipose volunteers, aged 0.01-2.72 0.001 Schnare
specified lipid 20-30 years mg/kg et al.
(subcutaneous) (1984)
Table 38 (contd).
Year Number Positive Specimen PBB-concentrationa Detection References
of findings (tissue, Group Range Arithmetic Geometric Median limit
specimens (%) etc.) mean mean
1983 15 100 perirenal autopsy cases from 0.032-1.65 0.475 0.32 0.0005 Miceli
post- the "high" exposure mg/kg et al.
mortem area of Michigan (1985)
adipose (Grand Rapids)
tissue
(wet wt)
a Expressed as the concentration of the major hexabromobiphenyl component (BB 153) in µg/litre serum or
mg/kg milk, or adipose tissue, respectively.
b The sample measuring 93 mg/kg was excluded from statistical analysis.
c Most in the order of 1 mg/kg.
The last one has been preferred for analysis, since depot fat
is the predominant storage site of PBBs (and other persistent
halogenated hydrocarbons), and, therefore, allows an increased
detectability of body burden. For example, had serum PBBs been used
alone as an indicator of exposure in the PBB survey of the general
population of Michigan (Table 38), only 70% of the 839 individuals
would have been considered exposed. When adipose tissue results are
added, an additional 24% indicate exposure, raising the positive
rate to 94%. Even though the limit of detection was an order of
magnitude higher (2.0 µg/litre in adipose tissue vs. 0.2 µg/litre in
serum), the partition ratio of approximately 300:1 made the adipose
limit of detection a more sensitive indicator of exposure (Wolff
et al., 1982; Anderson, 1985).
On the other hand, collection of hair, blood, and breast-milk
samples is simpler and less invasive than adipose tissue biopsy.
Moreover, with some exceptions, significant correlations between
adipose tissue and blood serum or breast-milk PBB levels were found
(section 6.2). Further advantages and limitations of these
techniques are discussed in detail by Anderson (1985) and Fries
(1985b).
It should be noted that levels of PBBs in breast-milk may not
be comparable between studies unless the concentration is adjusted
for fat content, because the fat content of breast-milk varies
widely from woman to woman, and the value increases during feeding
(Rogan et al., 1980).
Although the accurate relationship between observed body levels
of PBBs and individual exposure to PBBs is not clear (Safe, 1984),
PBB concentrations in human tissues can give some idea of the levels
of exposure (Kimbrough, 1980a).
5.2.3 Human monitoring data
In the following subsection, human monitoring data are
presented from contaminated farms in Michigan, from Michigan state,
and from other countries.
Most data available refer to the Michigan PBB incident in
1973-74 (Tables 38 and 39). Because, in this case, the spilling of
PBBs started from farms, the Michigan Department of Public Health
(MDPH) undertook a series of studies on farm families as a high-risk
group in the summer and autumn of 1974.
Serum samples were obtained from 110 persons in the exposed
group (had been working or living in the quarantined farms for six
months or more since the accident) and for 104 persons from the
control group (randomly selected from a list of dairy producers in
the same geographical area, where farms had not been quarantined).
As shown in Tables 37 and 38, serum levels of PBBs were
significantly higher in the people from quarantined farms compared
with those from non-quarantined farms, though low levels were also
observed in the control group (Cordle et al., 1978). In 1976, the
MDPH, together with the Centre for Disease Control, the FDA, NIH,
and EPA, established a cohort of 4545 people in Michigan to be
examined at regular intervals over several decades (Barlow &
Sullivan, 1982) to evaluate the long-term effects of PBB exposure.
Four groups were included (Landrigan, 1980): Quarantined farm
residents, direct recipients of farm produce, chemical workers and
their families, and persons who either volunteered for the study or
who had participated as control subjects in an earlier pilot study
(Humphrey & Hayner, 1975). The first report was published by
Landrigan et al. (1979) followed by several reports on subgroups of
this population (e.g., Kreiss et al., 1982; Eyster et al., 1983).
Study groups of the Mount Sinai School of Medicine have also
conducted comprehensive examinations on similarly categorized
groups, e.g., residents of quarantined farms, residents of
non-quarantined farms, consumers of products directly from
quarantined or non-quarantined farms, and Michigan Chemical Company
workers; residents of the state of Wisconsin were used as a control
group.
It can be seen from Table 38 that nearly 100% of the adipose
samples randomly selected throughout the state had detectable PBB
concentrations. Thus, statewide exposure of Michigan residents to
PBBs can be demonstrated.
Levels of PBBs in serum (Landrigan, 1980; Wolff et al., 1982),
breast-milk (Brilliant et al., 1978; Miller et al., 1984), and
adipose tissue (Wolff et al., 1982) were highest in the area of the
accident (lower peninsula), and lowest in the upper peninsula,
farthest from the source.
Compared with residents of quarantined farms, direct consumers
of products from quarantined farms, and PBB- production workers, the
tissue burdens among the general population of Michigan were 1-3
orders of magnitude lower. Moreover, for example, only 36% of the
general population had serum PBB concentrations greater than
1 µg/litre, compared with 78% among farmers (Anderson et al., 1979;
Wolff et al., 1982).
PBB levels appear to be higher in males than females (Meester &
McCoy, 1976; Landrigan et al., 1979; Landrigan, 1980; Wolff et al.,
1978; 1980; Kreiss et al., 1982; Eyster et al., 1983) and higher in
children (below the age of 10 years) than in adults (Humphrey &
Hayner, 1975; Landrigan et al., 1979; Landrigan, 1980; Barr, 1980;
Wolff et al., 1982).
A later study (Schnare et al., 1984) recorded not only the
concentration of the most abundant congener of the FireMaster(R)-
mixture (2,2',4,4',5,5'-hexabromobiphenyl) but also the concen
trations of other PBB congeners detected in subcutaneous adipose
tissue samples of 7 former participants of the Michigan PBB studies
(Table 39).
Table 39. Range of adipose tissue concentrations of various PBBs in 7 personsa
(mg/kg on a lipid weight basis)b
PBB Rangec (mg/kg)
2,3',4,4',5-penta nd-0.16
2,2',4,4',5,5'-hexa 0.01-2.72
2,2',3,4,4',5'-hexa nd-0.22
2,3',4,4',5,5'-hexa nd-0.09
2,2',3,3',4,4',5-hepta nd-0.26
2,2',3,4,4',5,5'-hepta nd-0.01
a 7 healthy male volunteers, aged 20-30 years, having been exposed to PBBs some
years earlier, as a consequence of the Michigan PBB incident in 1973.
b From: Schnare et al. (1984).
c nd = Not detectable; detection limit = 0.001 mg/kg.
In most cases, PBB concentrations did not appear to be
decreasing significantly over time. Wolff et al. (1979b) did not
find any significant variation in the serum PBB levels of nine dairy
farm residents during 18 month of observation.
Paired serum samples, one collected in 1974 and the other in
1977, were also available for 148 members of the Michigan PBB
cohort. The data indicate that levels were generally stable over the
3-year period with a mean change of 16 µg/litre (Landrigan et al.,
1979). In another study of the Michigan PBB-cohort, the decrements
in median serum levels of PBBs between matched pairs over one -
(1977-78) and two - (1977-79) year intervals were both only 1
µg/litre (Kreiss et al., 1982). No significant change in blood
plasma PBB levels was observed over a 5-month period in 41 residents
of quarantined farms (Humphrey & Hayner, 1975). In contrast, Meester
& McCoy (1976) reported a marked decline over 3 years (1974-76) in
serum levels of PBBs. These authors also found that the average
decrease in PBB concentrations in the fat of 16 individuals was
about 40%, in a period of 6 months. No changes in PBB levels were
seen over an 11-year period (1976-87) in fat samples from a patient
with long-term exposure to PBBs from the early 1970s as a result of
the Michigan PBBs accident. The average fat level of PBBs was
0.8 mg/kg (Sherman, 1991).
In 1981, PBBs were found in 13-21% of serum samples from
4-year-old Michigan children. Their mothers belonged to a group that
was surveyed either with regard to the consumption of Lake Michigan
sport fish (mean PBB level detected in children: 2.4 ng/ml) or with
regard to former exposure to quarantined farm products (mean PBB
level detected in children: 3.0 ng/ml) (Jacobson et al., 1989).
Few human monitoring data are available for the US population
outside of Michigan. They are summarized in Table 40. One study
deals with the population in the vicinity of industrial areas
involved in PBB production or use (Stratton & Whitlock, 1979), the
other with farmers of the state of Wisconsin who were examined as
control group in connection with the Michigan PBB studies (Wolff
et al., 1978).
PBBs were found in all studies, but, because of the limited
data, the significance is unclear. The highest PBB levels were found
in the hair of humans living near PBB industry. Of the nine samples
analysed, five had detectable PBB levels. Both male and female hair
samples contained PBBs (Stratton & Whitlock, 1979).
In contrast to the other surveys, which had regard only to the
major PBBs component (hexabromobiphenyl), Stratton & Whitlock (1979)
identified the different PBB homologues in the extracted oils of the
human hair, collected from barbershops and beauty parlours (Table
41). There were identifiable differences in the composition of PBB
congeners found in hair from the three locations.
The samples with the highest concentrations contained
relatively large amounts of decabromobiphenyl, while the samples
with lower concentrations contained only hexabromobiphenyl (Stratton
& Whitlock, 1979). One sample was different from the others because
it contained dibromobiphenyl (see Table 41). As a result of the
sampling method, it was impossible to ascertain whether the exposure
was related to the workplace or to the ambient environment.
In a report by Lewis & Sovocool (1982), pooled adipose tissue
samples from 202 individuals from nine census regions in the USA
were analysed for HxBB. Although the average concentration was 1-2
µg/kg, it cannot be excluded that this was because of the inclusion
of a few samples with high PBB concentrations.
Table 40. Human monitoring data: PBB levels in the US population (outside of Michigan)
Year No. of % of Specimen PBB PBB Detecion Remarks References
specimens positive tissue group/area concentrationa examined limita
findings range
1977 56 3.6 serum Wisconsin farmers not C12H4Br6 examined as Wolff
a control detected-1.1b et al. (1978)
population
1977 3 33 hair (from males and females, < 100-8100 total PBBs 100 reported as Stratton &
barber- Bayonne New Jersey, µg/kg in oil Whitlock
shops) Vicinity of White (1979)
Chemical
3 100 hair (from males and females, 440-26 600 total PBBs 100 reported as
barber- Staten Island, µg/kg in oil
shops) New York, Vicinity
of Standard T
Chemical Co.
3 33 hair (from males and females, < 100-310 000 total PBBs 100 reported as&
barber- Sayreville New µg/kg in oil
shops) Jersey, Vicinity of
Hexcel Fine
Organics
a (µg/litre or µg/kg).
b PBBs not detected in 54/56 persons. PBBs observed at 1.1 µg/litre in one person, identified as recently
moved from a Michigan farm, and at 0.5 µg/litre in another person.
Table 41. Concentration (range) of different PBB congeners in human hair samples
taken in the vicinity of three industrial sites in the USA, reported as
µg/kg in oila
PBB-congeners Bayonne, New Staten Island, New Sayreville,
Jersey (White York (Standard T New Jersey
Chemical Corp.)b Chemical Comp.)c (Hexcel Corp.)d
MoBB nde nde nde
DiBB nd-8100 nd nd
TrBB nd nd nd
TeBB nd nd nd
PeBB nd nd nd
HxBB nd 440-740 nd-480
HpBB nd nd-890 nd
OcBB nd nd-1100 nd
NoBB nd nd-3600 nd-22 500
DeBB nd nd-20 000 nd-285 000
a Data from: Stratton & Whitlock (1979).
b Having manufactured octa- and decabromobiphenyl along with bromobiphenyl
ethers.
c Major user of FireMaster BP-6.
d Producer of laboratory quantities of various PBBs.
e nd = Not detected (detection limit = 100 µg/kg).
There is very little human monitoring data on PBBs in the
populations of countries other than the USA. Krüger et al. (1988)
reported PBB contamination of breast-milk from European women in a
survey from North Rhine-Westphalia, Germany (Table 33). The milk
samples (n=25) contained a typical pattern of certain PBB congeners.
It included penta- to octabromobiphenyls in concentrations ranging
from 0.002 to 28 µg/kg, based on milk fat. The most abundant
component was 2,2'4,4',5,5'-hexabromobiphenyl (BB 153) followed by a
peak consisting of two heptabromobiphenyl isomers (2,2',3,4',5,5',6-
and 2,2',3,4,4',5,6'-heptabromobiphenyl BB 187 and 182). Differences
in the pattern were only found in the milk given by a Chinese woman
and in that given by a woman having been exposed to several fires in
industry.
Concentrations of BB 153 in human and cow's milk, both
collected from the same region (North Rhine-Westphalia), were
1 µg/kg and 0.03 µg/kg, respectively, measured on a fat basis
(Krüger, 1988).
5.2.4 Subpopulations at special risk
Children are at risk from exposure to PBBs in different ways.
Studies on the Michigan population indicated a significant PBB
transfer to the fetus (Landrigan et al., 1979; Eyster et al., 1983;
Jacobson et al., 1984) and to breast-milk (Brilliant et al., 1978;
Landrigan et al., 1979; Eyster et al., 1983; Jacobson et al., 1984,
1989; Miller et al., 1984). PBB levels to which fetuses and newborn
infants were exposed in the Michigan accident are shown in Table 42.
Because the placenta acts only as a partial barrier to PBBs, a
newborn baby has a body burden, even before breast feeding.
Placental and cord serum levels are much lower than levels in
breast-milk. However, even at low concentrations, intrauterine
exposure may be significant, for several reasons, as pointed out in
detail by Jacobson et al. (1984).
Infants are not only exposed to PBBs through their mothers and
through consumption of contaminated food, but they also through
contact with PBBs from the environment. Young crawling children are
known to ingest accidentally soil or dust to an extent of up to
0.1 g/day.
5.3 Occupational exposure during manufacture, formulation, or use
In general, occupational exposure is to be expected in PBB
manufacturing and processing plants. In Michigan, as a result of the
PBB incident, farmers, and possibly dairymen, elevator, mill
personnel etc. were occupationally exposed (Kay, 1977).
Table 42. PBBb concentrations in maternal serum, adipose lipid, and milk lipid, and in
the cord serum and placenta of Michigan women (Michigan PBB-cohort) at the time of
parturition (1975-80)a
Paired specimen No. Rangec Median Geometric Measure
mean
Maternal serum 61 nd-1068 3 3.5 µg/litre
Placenta nd-370 < 1 - µg/kg
Maternal serum 60 nd-1068 3 3.2 µg/litre
Cord serum nd-104 < 1 - µg/litre
Maternal serum 47 nd-1068 3 3.0 µg/litre
Milk lipid nd-92 667 250 312 µg/kg
Milk lipid 27 52-92 667 384 472 µg/kg
Adipose lipid nd-174 000 522 82 µg/kg
a From: Eyster et al. (1983).
b Concentrations expressed as concentrations of hexabromobiphenyl.
c nd = Not detected (detection limit: 1 µg/litre or kg).
Bialik (1982) reported the following contaminant levels of
decabromobiphenyl measured in 1977 in the manufacturing area of
Hexcel/Fine Organics and Saytech, Inc. (Sayreville; USA):
- Plant air samples: 0.18 and 0.23 mg/m3 8 h TWA (time-weighted
average);
- Wipe tests, unspecified: up to 8 mg/100 cm2; - Wipe tests,
eating Table: 0.1 mg/100 cm2.
At this time, 95% of the plant production consisted of
decabromobiphenyl (18%) and decabromobiphenyl oxide (77%). About
2000 tonnes of decabromobiphenyl were manufactured during 1973-77
(Bialik, 1982).
Employees of chemical plants may be exposed directly to PBBs
(in most cases along with other chemicals) through contact,
inhalation, or ingestion (Wolff et al., 1979a). As an index of
individual exposure, PBB levels in the serum and adipose tissue of
chemical workers have been recorded. The results of several authors
are compiled in Table 43.
Most data refer to the Michigan Chemical Corp., St. Louis
(Michigan), which produced several brominated organic compounds and
manufactured over 5000 tonnes of PBBs, predominantly
hexabromobiphenyl, from 1970 to 1974. Some additional general
exposure from contaminated food can also be included for the workers
of Michigan Chemical.
To summarize, median serum and adipose tissue PBB levels were
higher among chemical workers than among male residents of
quarantined farms.
Non-production workers at the Michigan Chemical plant showed
significantly lower levels than workers involved in PBB production;
for example, median adipose tissue concentrations of PBBs were
2.49 mg/kg and 46.94 mg/kg, respectively.
In another study on workers at a PBB plant in New Jersey, Bahn
et al. (1980b) presented a detailed comparison of serum PBB levels
in various occupational groups. A significantly higher number of PBB
workers had detectable levels of PBBs, compared with other workers
in the study (35.9% compared with 12.2%). Among workers with
detectable PBB levels, the PBB workers had significantly higher
serum levels than workers from neighbourhood industries not using
PBBs.
Although this factory concentrated on manufacturing
decabromobiphenyl and decabromobiphenyl oxide (ether), there was no
positive identification of C12Br10 (Table 44) or C12Br14O
(Bahn et al., 1980b).
No data are available about occupationally exposed women.
Family members of chemical workers have also been found to have
a body burden of PBBs (Landrigan, 1980).
Bekesi et al. (1979b) determined the distribution of PBBs in
the blood compartments of 4 Michigan Chemical plant workers (Table
45) and suggested that the PBB level of the white cell fraction may
be a better indicator for risk potential than the total plasma PBB
concentration.
Table 43. Occupational exposure: PBB levels in chemical workers (USA)
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1975 Michigan workers (8-36 serum 6-85 HxBB Kay (1977)
Chemical Corp. months exposure (7)
(MCC)
(St. Louis,
Michigan)
1976 MCC (St. Louis, employees (55) serum 1.1-1729 123 9.3 HxBB Wolff
Michigan) et al.
(1978,
1979a)
production workers serum 603.9 108.4 HxBB 1 Wolff
(10) et al.
(1979a)
non-production serum 16.5 6.1 HxBB 1
workers (45)
workers (14) serum 1-1530 HxBB < 0.2 Wolff
et al.
(1979b)
1978 workers (14) 1-1363 HxBB < 0.2
(matched pairs)
Table 43 (contd).
Year Plant Group Sample PBB concentrationa PBB Detection References
(number) range Mean Median examined limita
(geometric
mean)
1976-77 MCC (St. Louis, workers and serum not 43.0 4.5 C12H4Br6 1 Landrigan
Michigan) families (216) detected-1240 et al.
(1979)
1975-80 MCC (St. Louis, workers (male) serum 1-2000 (25.4) 20 C12H4Br6 1 Eyster
Michigan) (29) et al.
(1983)
1978 Hexcel/Fine PBB workers serum not MoBB Bahn
Organics and (exposure to PBBs detected-1340 DeBB et al.
Saytech, Inc. (and PBBOs) for at (1980b)
(Sayreville least 6 weeks
New Jersey) between January
1973 and August
1978) (39)
1976 Michigan production workers adipose 5000-581 000 196 490 46 940 HxBB 500 Wolff
(7) tissue et al.
(1979a)
non-production 500-10 000 3880 2490
workers
1975-80 Michigan workers (male) adipose 400-350 000 5290 6000 HxBB 1 Eyster
(29) tissue et al.
(1983)
a In µg/litre or µg/kg.
Table 44. Detectablea serum levels (µg/litre) of PBB homologues in workers
at a plant producing decabromobiphenyl and decabromobiphenyl oxideb
PBB homologue Number of cases Range
C12H9Br 14 0.3-5.5
C12H8Br2 1 6.9
C12H7Br3 1 0.9
C12H6Br4 0 -
C12H5Br5 2 1.6-13.0
C12H4Br6 2 0.4-6.0
C12H3Br7 7 9.0-40.0
C12H2Br8 9 20.0-800.0
C12H Br9 1 500
C12Br10 0 -
Total PBBs 26 0.3-1340
a Excludes cases with "trace", "not confirmed" and "not detectable" levels.
b From: Bahn et al. (1980b).
Despite its significance for toxicological assessment, the
content of minor constituents of FireMaster(R) in the body burden
was rarely investigated. For example, Wolff & Aubrey (1978) examined
other PBB congeners, which are identifiable as peaks by GC/MS
(2 pentabromobiphenyl peaks, and 2 heptabromobiphenyl peaks), in the
serum of Michigan Chemical workers (n = 24) and Michigan dairy
farmers (n = 37), besides the major component (2,2',4,4',5,5'-
hexabromobiphenyl) of FireMaster(R). The relative concentrations,
with respect to the major hexabromobiphenyl peak, of these PBB
components were somewhat different for chemical workers and for
farmers, i.e., the two pentabromobiphenyl values (peak area ratios)
were significantly higher in the serum from chemical workers.
Table 45. Distribution of PBBs in blood compartments of Michigan Chemical Workersa
Polybrominated biphenyls
ng/mg Protein Ratiob
RBC plasma WBC RBC plasma WBC
Michigan Chemical Workers;
not directly involved in 0.07 0.13 3.9 1 : 2 : 56
the production of PBB 0.03 0.23 1.8 1 : 8 : 60
directly involved in 0.67 10.0 57.3 1 : 15 : 86
the production of PBB 0.63 10.2 32.0 1 : 16 : 51
a From: Bekesi et al. (1979b).
b RBC = Red blood cells; WBC = white blood cells.
This variation might be attributed to the different routes
(skin contact, inhalation, direct ingestion versus, primarily,
ingestion of animal foodstuff) and to the different composition
(unchanged versus animal-mediated material) of exposure in chemical
workers versus farmers. Further reasons might be the earlier initial
onset of contamination in workers and slight variations in the
composition (section 2.1.2) of several lots of FireMaster(R) BP-6
(the main product of Michigan Chemical) and FireMaster(R) FF-1,
which caused contamination of livestock feed (Anderson et al.,
1978a; Wolff & Aubrey, 1978; Wolff et al., 1979a).
The change in serum PBB levels over time was investigated in
chemical workers at two facilities. Wolff et al. (1979b) reexamined
serum PBB concentrations (determined as the major hexabromobiphenyl
peak) in 1978 from 14 workers of the Michigan Chemical Corp., who
had also been tested 18 months earlier. They found PBB levels of a
comparable order. In contrast, no subject (n=109) in a study on
chemical workers of Hexcel/Fine Organics and Saytech Inc.
(manufacturing decabromobiphenyl and decabromobiphenyl oxide) showed
any detectable serum level of PBBs (different congeners) in 1981
(Bialik, 1982), which was true, even for the two persons who had
shown high levels of serum PBBs in the previous study of 1978 (Bahn
et al., 1980b; Table 43). However, the results of PBB determination
in the fat of these two cases were positive in 1981. The negative
results of the determination of PBBs in serum were not expected, and
the authors suggested further studies.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Gastrointestinal absorption
Studies have been performed only on the gastrointestinal
absorption of PBBs. Some studies indicate that PBBs are rapidly and
efficiently absorbed, other studies indicate a much lower efficiency
of absorption (see Table 46). No information is available on the
extent of absorption of decabromobiphenyl.
Absorption can be strongly influenced by the vehicle in which
the compound is administered (Birnbaum, 1985). Administration of
hexabromobiphenyl in mineral oil or olive oil solution resulted in
higher absorption than administration in a methyl cellulose
suspension (see Table 46: Rozman et al., 1982). The degree of
halogenation also appeared to influence the absorption of PBB. For
example, less than 10% of 14C-labelled hexabromobiphenyl, but 62%
of a dose of 14C-labelled octabromobiphenyl were eliminated in the
faeces of rats in 24 h, though both compounds had been administered
in corn oil (see Table 46).
The conclusion that more brominated biphenyls are absorbed less
efficiently than less brominated biphenyls can, possibly, be drawn
from other findings. Willett & Durst (1978) observed that, during
feeding of FireMaster(R) BP-6, the relative concentration of
pentabromobiphenyl in the faeces of cows was decreased, and that of
heptabromobiphenyl was elevated compared with the
FireMaster(R)-standard. Similarly, faecal concentrations of
heptabromobiphenyl were enhanced relative to concentrations of
hexabromobiphenyl in the faeces of hens, when FireMaster(R) BP-6
was fed (Fries et al., 1976). However, Polin & Leavitt (1984) found
that the ratio of 3.5 for hexa- to heptabromobiphenyl in the
chemical sample of FireMaster(R) FF-1 shifted to an average ratio
value of 2.5 in the whole carcasses of chickens analysed on days 0,
21, and 42 of withdrawal, inferring a better absorption of hepta-
bromobiphenyl.
Generally, it should be noted that faecal elimination during
the first few days following dosing might be an indicator, but is
not a measure, of lack of absorption, because some absorbed PBB is
eliminated and recycled into the faeces in bile and by diffusion
across intestinal membranes (Rozman et al., 1982; Fries, 1985b).
Table 46. Absorption of PBBs after oral administration
PBB compound Species Vehicle Absorption Methods Comments References
(sex) parametera
[14C-]2,2',4,4',5,5'- rat emulphor-EL 620: 90%, 24 h faeces analysis single dose Matthews
hexabromobiphenyl (male) ethanol: water gut content et al. (1977)
(1 : 1 : 8)
corn oil 90% multiple doses (4)
[14C-] octabromobiphenyl rat (male, corn oil 38%, 24 h faeces analysis single dose Norris et al.
(technical mixture) female) (1973)
[14C-]2,2'4,4',5,5'- rhesus 1% methyl cellulose 40%, 10 days faeces analysis two doses Rozman et al.
hexabromobiphenyl monkey mineral oil 62%, 5 days faeces analysis single dose (1982)
(male) olive oil 66%, 5 days faeces analysis repeated doses (4)
FireMaster(R) BP-6 cow crystalline PBB in 50%, 168 h faeces analysis single dose Willett &
(female) gelatin capsules (7 days) Irving (1976)
calf crystalline PBB in 95%, (9 days) faeces analysis daily feeding
(male) gelatin capsules
a Values based on concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (FireMaster(R) BP-6 sample) or on [14C-]activity.
6.1.1.2 Dermal and inhalation absorption
No quantitative information is available on skin absorption and
intake through inhalation.
6.1.2 Human studies
It is plausible that inhalation and dermal contact are the main
routes of exposure to PBBs for chemical plant workers (Wolff et al.,
1979a), while the main route for Michigan people was the ingestion
of PBBs dissolved in the fat of meat and milk (Di Carlo et al.,
1978). An appropriate model for assessing the latter kind of
absorption is thought to be the rat-corn oil model (Fries, 1985b).
No quantitative data are available on PBB absorption in humans.
6.2 Distribution
6.2.1 Animal studies
6.2.1.1 Levels in organs and blood
As can be seen from Tables 47, 48, and 49, most studies on the
distribution of PBBs have been conducted with the FireMaster(R)
mixture. A few earlier publications refer to technical
octabromobiphenyl. No experimental data are available on tissue
distribution of decabromobiphenyl. When FireMaster(R) was
administered, the distribution process was studied predominantly as
the distribution of 2,2',4,4',5,5'-hexabromobiphenyl, and, with far
less emphasis, on the distribution of the minor components of the
mixture. Little information is available on the distribution of PBB
congeners, when administered individually.
Investigations on rats, mice, cows, sheep, pigs, and avian
species demonstrated that PBBs were distributed widely throughout
the body tissues in all species. Highest (equilibrium)
concentrations on a wet tissue basis were found in adipose tissues,
consistent with the solubility characteristics of PBBs. Adipose
concentrations are usually an order of magnitude higher than those
of most muscle and organ tissues (see Tables 47, 48, 49, and 50).
Much of the variation in concentrations among tissues can be
accounted for by variations in the fat concentrations in these
tissues (Willett & Durst, 1978; Fries et al., 1978b; Fries, 1985b).
Table 47. Distribution of PBBs in mammals after the administration of a single dose of PBBs
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
FireMaster(R) FF-1 rat oral 1000 10 monthsb adipose tissue (714) > liver (60) > blood Kimbrough et al.
(lot No. 7042) (male) in corn oil (0.94) (1978)
rat 10 monthsb adipose tissue (1202) > liver (37) > blood
(female) (2.9)
FireMaster(R) FF-1 rat oral 80 42 days fat (295) > blood (0.38) Wolff &
(male) in corn oil Selikoff (1979)
FireMaster(R) FF-1 rat oral 500 4 months adipose tissue (1008) > liver (50) > blood Kimbrough et al.
(lot No: 7042) (male) in corn oil (2.1) (1980)
FireMaster(R) FF-1 rat oral 10 24 hb sc fat I (61 500) > sc fat II (38 700) > liver Domino et al.
(lot No: FF-1312-FT) (male) (20 900) > lung (7650) > kidney (7310) > heart (1980b)
(6470) > jejunum (4860) > spleen (3530) >
cerebellum (2990) > grey matter (2850) > white
matter (2750) > testes (2380) > blood (945)
4 weeksb sc fat (19 200) > jejunum (3170) > lung (1240) Domino et al.
> liver (690) > kidney (650) > spleen (520) (1980b)
> heart, testes (both 240) > grey matter
(210) > cerebellum (200) > white matter (170)
> blood (56.9)
FireMaster(R) BP-6 rat intraperitoneal 12 weeks serum (46.80 ng/ml) Miceli &
(male) 10 in corn oil Marks (1981)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
fat (21.90) > adrenal (3.64) > lung (0.98)
> liver (0.59) > pituitary (0.91) > gonad (0.33)
> kidney (0.22) > heart (0.20) > spleen (0.17)
> brain (0.13)
36 weeksb serum (23 ng/ml)
fat (16.62) > adrenal (2.67) > lung (0.51) >
pituitary (0.29) > liver (0.20) > kidney (0.14)
> gonad, brain (both 0.10) > heart (0.08)
> spleen (0.05)
2,2',4,4',5,5'- rat oral 1 in: 1 day muscle (29.9) > adipose (25.5) > skin (17.9) Matthews et al.
[14C]-hexabromobiphenyl (male) Emulphor > liver (9.0) > blood (0.90)c (1977)
EL 600: ethanol:
water (1:1:8)
14C-PBB rat intraperitoneal 28 days ovaries (130) > skin (15.1) > testicles (13.3) McCormack et al.
(male 150 in: peanut > intestine (11.7) > lung (7.3) > liver (4.7) (1979b)
and oil > muscle, heart (1.9) > fat (1.8) > brain (0.9)d
female
pups)
FireMaster(R) BP-6 cow oral 5.95 in: 10 days liver (1.35) > fat (sc: 1.15; perirenal: 1.09; Willett &
(lot No. RP-158) (female) gelatin capsule peri-cardiac: 0.97; intermuscular: 0.81; omental: Irving (1976)
0.78) > brain (pons: 0.27; cortex: 0.08) >
mammary gland (0.25) > kidney (0.12) > heart
(0.11) > lung, muscle (0.08) > ovaries, uterus
(0.06) > plasma, rumen wall (0.04) > bile
(0.02) > synovial fluid (0.01)
Table 47 (contd).
PBB Species Administration Time after Tissues, organs, under study - ranked in References
(sex) (dose in mg/kg dosing order of decreasing PBB concentrationsa
body weight) (mg/kg or mg/litre, unless otherwise specified)
[14C-]octabromobiphenyl rat oral 1 in: 16 days adrenal, adipose, heart, skin > liver, Norris et al.
(technical mixture) (male) corn oil pancreas, spleen (1973)
a Measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity; (in parentheses: values measured - referring
to various measures).
b For additional time points: see original reference.
c Values in average % total PBB dose.
d Values in µg-equivalents/g wet weight.
sc. = Subcutaneous.
Table 48. Studies on the distribution of PBBs in animals following dietary or repeated oral intake
of the FireMaster(R) mixtures or 2,2',4,4',5,5'-[14C]-hexabromobiphenyl
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 fat (330) > mammary gland (318) Rickert
BP-6 (pregnant) feed until day 21 of > kidney (30) > skin (22) > liver, et al. (1978)
gestation lung, brain, heart, small
intestine, placenta, uterus (all
< 5)
FireMaster(R) rat 50 mg/kg day 8 of gestation 0 mammary gland (117) > liver (4) Dent et al.
BP-6 (maternal) feed to 14 days (1977b)
postpartum
FireMaster(R) rat 25 mg/kg day 8 of pregnancy 0 fat (74) > mammary > liver McCormack
BP-6 (maternal) feed to 14 days > kidney > lung (6) et al.
postpartum (1979a)
50 mg/kg 0 fat (483) > mammary > kidney
feed > lung (13)
200 mg/kg 0 fat (966) > mammary > kidney
feed > lung (21)
FireMaster(R) rat 100 mg/kg day 8 of pregnancy 0 fat (813) > liver (54) > mammary McCormack &
BP-6 (maternal) feed to 28 days (43) Hook (1982)
postpartum 14 weeks fat (459) > mammary (225) > liver
(after first (12)
and only
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
litter was
weaned)
> 10 weeks fat (77) > mammary (19) > liver (3)
and after
weaning
their second
litter
2,2'4,4',5,5'- rat 1 mg/kg 4 days 3 days adipose (41.1) > skin > muscle Matthews
hexabromobiphenyl (male) body weight > liver > blood (0.32)b et al. (1977)
per day
FireMaster(R) rat 0.1 mg/kg 9 days 0 liver (1.5) > brain (0.5), Render et al.
BP-6 (Lot 6224A) (male) feed adipose (0.3)c (1982)
1 mg/kg 9 days 0 liver (8.3) > brain (1.8),
feed adipose (1.7)c
10 mg/kg 9 days 0 liver (135) > adipose (27)
feed > brain (12)c
100 mg/kg 9 days 0 liver (1213) > adipose (251)
feed > brain (103)c
FireMaster(R) rat 3 mg/kg 20 days 0 adrenal (93.7) > thyroid (> 20) Allen-
FF-1 (Lot No. (male) body weight > testes (8.7) Rowlands et al.
FF-1312-FT-3) per day (in 1981);
lecithin Castracane
liposomes) et al. (1982)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
20 days 5 months adrenal (481) Castracane
(plus dietary et al. (1982)
restriction)
FireMaster(R) mouse 100 mg/kg 14 days 6 h thymus (391) > fat > liver > brain Corbett et al.
BP-6 feed > pancreas > testicles > spleen (1978a)
(2.7)
14 weeks thymus (50) > adrenals = fat >
liver > testicles > spleen > brain
> pancreas (n.d.)
12 weeks perithymic fat (96) > perirenal
fat > adrenal glands > thymus
gland (5.5)
FireMaster(R) mouse 5 mg/kg 3 weeks 0 thymus (20) > lung, liver > spleen Loose et al.
FF-1 feed > serum (< 0.002) (1981)
(lot No. 7042)
8 weeks 0 thymus (24) > liver, lung > spleen
> serum (0.019)
FireMaster(R) mouse 167 mg/kg 3 weeks 0 thymus (109) > liver > lung > Loose et al.
(lot No. 7042) feed spleen > serum (1.22) (1981)
6 weeks 0 thymus (3088) > liver > spleen
> lung > serum (4.75)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum, (11)
8 weeks 0 thymus (2426) > liver > lung
> spleen > serum (11)
FireMaster(R) cow 50 mg/kg 15 days 15 days renal fat (10) > omental fat Gutenmann &
BP-6 (lactating) feed > brisket fat > liver = thyroid Lisk (1975)
> mammary = chuck muscle > loin
muscle > heart > kidney = brain
> adrenal = spleen (0.4)
FireMaster(R) calf 25 g daily 9 days 0 rumen contents (14257) > feces Willett &
BP-6 (in gelatin > rumen wall > bile > marrow Irving (1976)
(lot 6244 A) capsules) > perirenal fat (441) > kidney
> testes > liver > thymus > heart
> brain, pons > lymph nodes >
tongue > spinal cord > brain,
cortex > plasma > small intestine
> lung > spleen > thyroid >
muscle (25)
FireMaster(R) cow 250 mg daily 60 days 0 fat (25) > liver > glands > nervous Willett &
BP-6 (heifer) (in gelatin > bile > contractiles > plasma Durst (1978)
(lot 6244 A) capsules) > liquids (0.0198)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) cow environmentally ca. 14 days 9 months perirenal fat (380*) > omental fat Fries et al.
FF-1 contaminated > subcutaneous fat > kidney > liver (1978b)
(Michigan PBB > skeletal muscle > cardiac muscle
incident) > lung > brain (10.5*)
ca. 200-400 g 9-12 months perirenal fat (1224*) > omental fat
(killed in >subcutaneous fat > skeletal muscle
extremis) > liver > cardiac muscle > kidney
> lung > brain (57*)
FireMaster(R) calf 10 mg/kg 4 weeks 0 fat (378) > kidney > liver > muscle Robl et al.
FF-1 (female) body weight (1.26) (1978)
per day in
gelatin
capsules
calf 100 mg/kg 6 weeks 0 fat (6080) > kidney > liver
(male) body weight > muscle (34)
per day
cow 1 158 days 182 days brisket fat (4.5) > bone marrow
> stomach fat > tail fat (3.3)
Table 48 (contd).
PBB Species Exposure Tissues, organs, under study - References
Duration of ranked in order of decreasing
recovery PBB concentrationsa (mg/kg or
Dietary Duration mg/litre wet weight, unless
concentration otherwise specified)
or dose
FireMaster(R) sheep 50 mg/kg 30 days 0 renal fat (42) > omental fat Gutenmann &
BP-6 feed per day > brisket fat > liver > chuck Lisk (1975)
(complete muscle > loin muscle > heart >
ration) thyroid > brain > adrenal > kidney
= spleen (0.9)
FireMaster(R) pig 20 mg/kg 16 weeks 0 back fat (64) > leaf fat > liver Ku et al.
BP-6 feed > muscle > kidney (0.9) (1978)
200 mg/kg 16 weeks 0 back fat (503) > leaf fat > muscle
feed > liver > kidney (13.5)
FireMaster(R) pig 100 mg/kg during 2nd 0 adipose tissue > liver > kidney > Werner &
BP-6 (lactating feed half gestation > braind Sleight (1981)
sow) 200 mg/kg and during
feed lactation
"PBB" Japanese 20 mg/kg 9 weeks 0 liver (374) > kidney > muscle Babish et al.
(ca 75% quail feed > heart > brain (40)e (1975a)
hexabromobiphenyl) male
female 0 liver (225) > heart > kidney >
> muscle > brain (26)e
FireMaster(R) chicken various 5 weeks 0 adipose tissue (3:1) > whole egg Polin &
FF-1 (White concentrations > liver > muscle (0.008:1)f Ringer
Leghorn (1978a)
hens)
Table 48 (contd)
a Measured as concentrations of 2,2',4,4',5,5'-hexabromobiphenyl (in parentheses: values measured - referring to various measures).
b Values in average % total PBB dose.
c Values on a fat basis.
d Concentrations are listed in Table 53.
e Values in mg/kg dry weight.
f Values as ratios of tissue PBBs: diet PBBs.
* = Geometric mean.
Table 49. Studies on distribution of the following continuous exposure to octabromobiphenyl
Species Exposure Duration of Tissues and organs under study, ranked References
Route, Duration recovery in order of decreasing concentrations
concentration (average bromine content; µg/g wet weight)
Rat in diet Lee et al. (1975a)
(male) 0 mg/kg feed (control) 2 weeks liver (3.4) > fat (1.7) > muscle (1.6)
100 mg/kg feed 2 weeks - liver (83) > fat (73) > muscle (14)
1000 mg/kg feed 2 weeks - fat (333) > liver (319) > muscle (77)
100 mg/kg feed 4 weeks 0,2,6 weeks fat > liver > muscle
100 mg/kg feed 4 weeks 18 weeks fat > muscle > liver
Rat inhalation 23 h/day, 7 days Waritz et al.
(OcBB vapour)a per week, 15 weeks (1977)
0 pg/litre air liver (3) > muscle (1.6) > fat (1.5)
(control)
3.5 pg/litre air - liver (4.2) > fat (3.0) > muscle (1.5)
a OcBB = Octabromobiphenyl, Dow, Lot 102-7-72.
Table 50. Tissue: blood ratios of PBBs estimated in a standard 250-g rata
Compartment Ratio
Liver 17:1
Muscle 5:1
Skin 56.5:1
Adipose 340:1
Intestine tissue 1:1
a From: Tuey & Matthews (1980).
However, even when concentrations are expressed on a fat basis
rather than a wet tissue basis, there are some deviations from
uniform concentrations among tissues (Fries, 1985b). PBB
concentrations, for example, were low in nervous tissue, despite its
high lipid content and often disproportionally high in liver,
considering its relatively low lipid content (see Tables 47, 48, and
49; additional information on fat content percentage, e.g., by
Willett & Irving, 1976; Fries et al., 1978a,b; Kimbrough et al.,
1978; Werner & Sleight, 1981).
The ratios between the PBB concentrations of adipose tissue,
blood, and vital organs are different when animals are not at
equilibrium (see also section 6.5) with respect to dosing regimen or
body condition. Usually, concentrations in liver are very high
compared with those in other tissues, immediately after dosing, and
decline relatively as equilibrium concentrations are established
(e.g., Lee et al., 1975a; Matthews et al., 1977; Miceli & Marks,
1981; Fries, 1985b). Generally, this phenomenon is most pronounced
in tissues that have high blood flow rates relative to tissue mass
(Tuey & Matthews, 1980). As an exception, livers of mice, tested in
a small series, appeared to have relatively concentrated the PBB
with passing time (Corbett et al., 1978a).
On the other hand, body weight changes, pregnancy, parturition,
and lactation can affect the concentration relationships until
equilibrium is reestablished (e.g., Rickert et al., 1978; Willett &
Durst, 1978; McCormack & Hook, 1982).
The route of exposure (oral or intravenous administration) had
no effect on the tissue distribution (blood, liver, muscle, adipose,
skin) of 14C-labelled 2,2',4,4',5,5'-hexabromobiphenyl (dose =
1 mg/kg body weight) in rats (Matthews et al., 1977).
Kimbrough et al. (1980) studied the effects of different diets
and of mineral oil on the HxBB concentration in rats that had
received a single oral dose of FireMaster(R) FF-1 (500 mg/kg body
weight, in corn oil). After 3 months of feeding, GC-analysis of
blood, liver, and adipose tissue showed no statistically significant
differences in PBB concentrations among the differently fed groups,
when concentrations were calculated on a lipid weight basis. On a
wet weight basis, however, the PBB concentrations were significantly
increased in the livers of rats on the experimental diets (Teklad-4%
and -20% fibre) and on mineral oil compared with those of rats on
the basal diet (Purina Chow).
In another study, McCormack et al. (1979a) examined the
consequences of simultaneous exposure to PCBs and PBBs in
(lactating) rats, because human populations that have been exposed
to PBBs are also likely to have been exposed to PCBs. The
extrahepatic tissue (kidney, mammary, lung, fat) concentrations of
PCBs and PBBs were similar, regardless of whether the agents were
administered together or alone. Liver, however, contained lower
concentrations of PBBs after treatment with an equal mixture of PCBs
and PBBs than when PBBs were administered alone. (None of the
tissues had higher concentrations of PBBs than PCBs after
concomitant administration. The reasons for this were not clear).
The distribution of PBB (hexa) among blood compartments
(plasma, red cells) has been studied in rats (Domino et al., 1980b).
It was found that plasma levels of 2,2',4,4',5,5'-hexabromobiphenyl
were generally four times greater than red cell levels.
Matthews et al. (1984) reported that 81% of plasma PBB (hexa)
was associated with the total lipoprotein fraction. In another study
(Kraus & Bernstein, 1986), approximately 65% of all radiolabelled
HxBB incubated with human serum in vitro was recovered in the
lipoprotein fraction. Of the HxBB in the lipoprotein fractions, 40%
was recovered in low-density lipoproteins (LDL), 33% in
very-low-density lipoproteins (VLDL), and 23% in high-density
lipoproteins (HDL). Addition of human lipoprotein to a culture
medium influenced the partition of HxBB between adipocytes and
culture medium (Kraus & Bernstein, 1989).
Some data are available on the tissue distribution of the minor
components of FireMaster(R)-mixture (see also section 6.5). Domino
et al. (1980b) analysed the relative percentages of various PBB
congeners (two penta-, three hexa-, and three heptabromobiphenyls)
in several tissues of rats given FireMaster(R) FF-1. From their
list, it was evident that each of the PBB analogues was found in all
tissues examined (liver, lung, testes, fat; blood, brain) but their
partitioning ratios differed. Distribution has been recorded also
after exposure to single PBB congeners, e.g., tetra-, penta-, and
hexa-isomers (Akoso et al., 1982a; Dharma et al., 1982; Domino
et al., 1982; Render et al., 1982; Millis et al., 1985a). In some
cases, it was not clear whether the differences in partitioning
between congeners were real or were caused by analytical problems
(Render et al., 1982).
6.2.1.2 Transfer to offspring
1) Mammals
Placental transfer
PBBs are capable of passing through the placental barrier into
the developing fetuses. This has been demonstrated in mice (Corbett
et al., 1978a; Welsch & Morgan, 1985), rats (Beaudoin, 1977; Rickert
et al., 1978), guinea-pigs (Ecobichon et al., 1983), minks and
ferrets (Bleavins et al., 1981), cows (Detering et al., 1975; Fries
et al., 1978a,b), and pigs (Werner & Sleight, 1981) by administering
the Fire-Master(R)-mixture or individual PBBs, or by using
technical octabromobiphenyl in rats (Aftosmis et al., 1972a; Waritz
et al., 1977).
The studies compiled in Table 51 are rarely intercomparable.
However, it is obvious that PBBs are readily transferred across
placental membranes, the concentrations among fetal tissues being
highest in the liver. The limited data on the distribution of PBBs
in fetal tissues showed often, but not always (Ecobichon et al.,
1983; Welsch & Morgan, 1985), lower PBB residues in fetal than in
maternal tissues (see Table 51). In cows, the average ratio of PBB
concentrations in fetal or calf tissue to PBB concentrations in dam
tissue was 0.36 : 1 for fat and 0.37 : 1 for blood (Fries et al.,
1978a,b). In contrast, the concentration ratio between the fetal and
maternal liver of mice ranged from 3.5:1 to 10:1 (Welsch & Morgan,
1985).
Species-dependent differences in the amounts of PBBs
transferred have been demonstrated for two mustelids, the mink and
the European ferret. PBB levels in the ferret kit were significantly
greater than those in the mink kit (see Table 51: Bleavins et al.,
1981).
Table 51. Placental transfer: PBB concentrations in the fetus and the mother
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mouse FireMaster(R) BP-6; 39.52 2.51 - 0.53 [HxBB] Corbett
(mg/kg) et al. (1975)
1000 mg/kg diet
on days 7-18 of
pregnancy
0 mg/kg (control) 0.04 0.05
Mouse FireMaster(R) BP-6; 112.74 12.02 - 0.95 - 5.86 - [HxBB] Corbett
(mg/kg) et al.
100 mg/kg diet (1978a)
on days 7-18 of
pregnancy
Mouse 2,2',4,4',5,5'- [HxBB] Welsch &
hexabromobiphenyl (mg/kg) Morgan (1985)
(purity: > 99%)
dietary intake
from day 6-15 of
pregnancy; sacrifice
on day 17
placenta:
100 mg/kg feed 9.08 17.26 3.79 3.06 182.88
300 mg/kg feed 17.30 39.84 8.23 4.56 217.48
500 mg/kg feed 69.13 89.47 24.58 17.64 316.79
750 mg/kg feed 95.36 103.70 54.33 18.64 453.12
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Rat FireMaster(R) BP-6 [HxBB] Beaudoin
(mg/kg) (1977)
single oral dose of
800 mg/kg body
weight (in sesame (pooled
oil) at day 12 of samples
pregnancy; killing: 51 267 13 from 4 rats)
24 h later
48 h later 250 248 6
Rat FireMaster(R) BP-6; 330 4.2 - 1.6 0.2 GI tract: [HxBB] Rickert
0.1 (mg/kg) et al. (1978)
50 mg/kg diet from
day 8-21 of
pregnancy
Rat Octabromobiphenyl Waritz
(technical); et al. (1977)
dietary intake
from day 8-15 of
pregnancy; sacrifice
on day 20;
0 mg/kg (control) 1.43 3.56 4.38 [Br]
100 mg/kg 70.4 16.1 7.62 (mg/kg)
1000 mg/kg 326 79.8 21.2
10 000 mg/kg 590 158 30.1
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Mink [14C-]-PBBs (HxBB 0.031 1.622 plasma: 0.002 0 0.005 kidney: % of the Bleavins
and HpBB); iv 0.04 0.003; initial dose et al. (1981)
injection (1 µCi) brain: 0; per g of tissue
in the final intestine: or ml of fluid
trimester of 0.001
gestation; killed
2 h later
Ferret see above 0.124 1.625 plasma: 0.005 0.004 0.013 kidney: see above
0.07 0.010
brain:
0.003
intestine:
0.005
Guinea- FireMaster(R) FF-1; 45 7 kidney: 45 45 kidney: 1 [HxBB] Ecobichon
pig 4.5; (mg/kg) et al. (1983)
single oral dose of lung: 7
50 mg/kg body weight lung: 1.5
at approximately 65
days of gestation;
killed 2 days later
Table 51 (contd).
PBB concentrations
Species Dosing regimena Maternal Whole Fetalb Concentration References
adipose liver others fetus adipose liver others expressed as:c
Pig FireMaster(R) BP-6; Werner &
Sleight (1981)
dietary intake
during 2nd half of
gestation
10 mg/kg feed 0.4 1.0 kidney: [HxBB]
nd; (mg/kg)
brain: nd
100 mg/kg feed 4.9 11.5 kidney:
nd;
brain: nd
200 mg/kg feed 40.3 24.2 kidney: 1.5
brain: 1.8
a HpBB = 2,2',3,4,4',5,5'-heptabromobiphenyl.
b nd = Not detected.
c [HxBB] = Concentration of 2,2',4,4',5,5',-hexabromobiphenyl; [Br] = Concentration of bromide.
Milk transfer
In mammals, the second route of PBB transfer from the mother to
the offspring is nursing. The efficiency of this way has been shown
through determining the PBB content in milk in relation to the body
burden or in relation to exposure levels of contaminated dams, and
through measuring PBB levels in kits that have been exposed to PBBs
only from suckling. The FireMaster(R) mixture was used in all
studies. Mammary transfer of technical octa- or decabromobiphenyl
has not (yet) been assayed.
Most investigations on the PBB contents of milk from
contaminated animals have been conducted on cows (Fries & Marrow,
1975; Willett & Irving, 1976; Robl et al., 1978; Fries et al.,
1978a,b; Willett & Durst, 1978). The ratios of concentrations in
milk fat to body fat in cows no longer receiving PBBs averaged about
0.4 :1 (Willett & Durst, 1978; Fries et al., 1978a,b; see also
Fig. 5). This ratio is much lower than the ratio in humans (see
section 6.2.2).
For other species (guinea-pig, rat, mink, pig) only single data
can be found in the literature. When (lactating) guinea-pigs
received a single oral dose of FireMaster(R) FF-1 (50 mg/kg body
weight) within 6-12 h of parturition, levels of HxBB in breast milk
(and in perirenal adipose tissue) were of the order of 22 µg/g (and
17 µg/g), respectively, 2 days after treatment (Ecobichon et al.,
1983). Rats fed 50 mg FireMaster(R) BP-6/kg in their diet from day
8 of pregnancy until 14 days after delivery showed, on day 14
postpartum, HxBB concentrations of about 51 µg/ml in the milk and
about 483 µg/g wet weight in their body fat (McCormack et al.,
1979a). In the same study, milk transfer of PBBs and PCBs was
compared. Milk usually contained higher concentrations of PCBs than
of PBBs, after simultaneous or separate exposure.
Contrary results were obtained with minks, intraperitoneally
injected with either 3 µCi of 14C-labelled PCB or 3 µCi of 14C-
labelled PBB on the approximate date at which the embryos would have
been implanted (Bleavins et al., 1981). Two weeks postpartum, milk
levels of PBBs were determined to be four times those of PCBs
(0.105% versus 0.025% of the initial maternal dose per gram of
tissue). Werner & Sleight (1981) determined PBB concentrations in
the tissues and milk of sows fed various amounts of FireMaster(R)
BP-6 (Table 53). At the end of lactation (4th week), the adipose
tissue and milk of sows, fed daily with 200 mg PBB/kg feed, had HxBB
concentrations of 194 µg/g tissue (wet weight) and of 22 µg/ml whole
milk, respectively. The authors calculated that, on a body weight
basis, nursing pigs consumed PBBs in concentrations similar to the
concentration given to the sows. Tissue levels of young exposed to
PBBs only via nursing have been determined for rats (Rickert et al.,
1978) and for guinea-pigs (Ecobichon et al., 1983). When pups of
non-treated female rats were nursed by dams fed FireMaster(R) BP-6
(50 mg/kg body weight) on days 1-14 postpartum, hepatic HxBB-
concentrations were on average approximately eight times higher than
those in the dams on day 14 postpartum (Rickert et al., 1978).
 When dams of guinea-pigs received a dose of FireMaster(R)
FF-1 on day 1 after delivery, the concentrations of 2,2',4,4',5,5'-
HxBB in the lungs, livers, kidneys, and fat of the pups were similar
to those of the dams for 4-60 days after treatment (Ecobichon
et al., 1983).
Combined placental and milk transfer
Rickert et al. (1978), Bleavins et al. (1981), and Werner &
Sleight (1981) concluded from their studies on rats, minks, and
pigs, respectively, that milk transfer is far more important than
placental transfer; studies on guinea-pigs (Ecobichon et al., 1983)
did not confirm this observation. However, under less controlled
conditions, perinatal exposure (both placental and milk transfer)
occurs and results in a marked body burden in the offspring, as has
been shown in studies on rats (Table 52 and McDaniel & Lucier, 1979)
and pigs (Table 53). From minks, it has been reported that
14-day-old kits of dams that had received a single intraperitoneal
dose of 14C-PBB at an early stage of pregnancy, contained about 3%
of the initial maternal dose (Bleavins et al., 1981). PBB body
burdens in the offspring of rats were still measurable at 328 days
of age and at the end of their life span (see Table 52).
Moreover, a multigeneration study on rats (McCormack et al.,
1981) demonstrated that administration of PBBs to a single
generation resulted in detectable residues in two subsequent
generations (Table 54). The concentrations of PBBs measured in the
tissues of F1-animals were approximately 5-30 times higher than
those in tissues from F2-animals and approximately 50-1000 times
higher than those in tissues from F3-animals (see Table 54).
2) Birds
In birds, eggs are the medium of PBB transfer to the offspring.
The ratio of egg PBB contents to dietary level has been reported to
be 1 : 1 (Fries et al., 1976) and 1.3 - 1.5 : 1 (Babish et al.,
1975a; Ringer & Polin, 1977; Cecil & Bitman, 1978; Polin & Ringer,
1978a) in chickens (White Leghorn hens) and Japanese quail,
respectively. After 63 days of feeding FireMaster(R) BP-6 in the
diet, the PBB level in body fat of White Leghorn hens was about 4
times the level in eggs (Fries et al., 1976).
6.2.2 Human studies
Studies on the distribution of PBBs in humans refer only to
people having been exposed in a direct or indirect way to the
FireMaster(R)-mixture.
As can be seen from a post-mortem study on people from a "high"
exposure area of the State of Michigan (USA), PBBs are distributed
throughout the entire human body (Table 55). Moreover, it was found
that fat and fat-rich tissue had the highest HxBB concentrations.
Perirenal fat had the highest mean concentration (475 ng/g).
Adrenal, atheromatus aorta, and thymus had mean concentrations of
about half that of perirenal fat; all other tissues had mean
concentrations of only one-tenth or less of that of perirenal fat
(Miceli et al., 1985).
Table 52. Tissue concentrations of PBBs in rats following perinatal exposure to PBBs (FireMaster(R)-mixture BP-6 or FF-1)
Dosing regimen to dams Age of Tissue concentrations of PBBsa (mg/kg wet weight) References
offspring
Offspring Dams
50 mg BP-6/kg diet: day 8 of 14 days liver carcass liver Rickert et al.
gestation through day 14 9.5 149.7 4.0 (1978)
postpartum
100 mg PB-6/kg diet: day 8 of liver kidney fat b McCormack et al.
pregnancy through 28 days 28 days 397 96 1693 (1980)
postpartum 328 days 17 11 387
BP-6 (lot 6244 A) in the diet: 28 days lung liver kidney fat b McCormack et al.
day 8 of pregnancy through (1982a)
28 days postpartum:
10 mg/kg 5 18 8 162
100 mg/kg 32 410 109 1693
200 mg FF-1/kg body weight liver: liver: liver Groce &
(in corn oil; by stomach tube): (female) (male) Kimbrough (1984)
day 7 and 14 of pregnancy 2 months 2.4 (218)c 3.0 (280)c 7.8 (542)c
(weaning at day 21 of age) 2 years 0.8 (107)c 0.6 (58)c
a Concentration expressed as the concentrations of 2,2',4,4',5,5'-hexabromobiphenyl.
b See McCormack & Hook (1982) and Table 48.
c Values calculated on a lipid basis.
Table 53. Mean concentrations of PBBs (mg/kg of tissue, wet weight) in tissues of sows and
4-week-old nursing pigs following perinatal exposure to PBBsa
PBBsb Liver Adipose tissue Kidney Brain
(mg/kg feed) Sows Pigs Sows Pigs Sows Pigsc Sows Pigsc
10 1.0 2.4 15.2 14.8 0.6 nd 0.2 nd
100 45.8 30.2 96.3 96.7 2.3 nd 1.7 nd
200 92.6 41.3 194.2 222.5 3.7 4.1 2.7 4.2
a From: Werner & Sleight (1981).
b FireMaster(R) BP-6 fed to the sows during the second half of gestation and during lactation.
c nd = Not detected.
Table 54. Tissue concentrations of PBBs in several generations of rats following perinatal exposure to PBBsa,b,c
Treatment Liver Kidney Lung Thyroid Testis Ovary Fat
Control < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1
F1-10 17.1 ± 3.6 7.5 ± 0.6 5.1 ± 0.8 4.4 ± 1.0 8.2 ± 5.3 24.0 ± 1.6 161.7 ± 24.2
F2-10 0.4 ± 0.1 0.6 ± 0.2 0.9 ± 0.1 0.5 ± 0.1 0.2 ± 0.1 3.0 ± 0.5 6.7 ± 1.9
F1-100 410.2 ± 40.6 108.6 ± 11.2 32.4 ± 2.0 162.6 ± 20.8 -d -d 1693.2 ± 250.4
F2-100 21.8 ± 3.2 7.2 ± 1.3 8.7 ± 1.0 2.7 ± 0.6 1.8 ± 0.11 6.5 ± 2.6 159.5 ± 16.9
F3-100 0.4 ± 0.1 0.8 ± 0.4 0.6 ± 0.1 0.2 ± 0.1 0.3 ± 0.1 0.6 ± 0.1 4.9 ± 0.8
a From: McCormack et al. (1981).
b Rats were fed 0,10, or 100 mg PBBs (FireMaster(R) BP-6)/kg from day 8 of pregnancy until 28 days postpartum at which
time all offspring (F1-10 and F1-100) were weaned on to a control diet, allowed to mature sexually, and bred with
littermates to produce the F2-generation (F2-10 and F2-100). F2-100 littermates were bred to produce F3-100 animals.
c Values are means in mg 2,2',4,4',5,5'-hexabromobiphenyl/kg wet tissue ± SE for at least three animals at 28 days of age.
d Sample not available.
Most of the distribution studies performed with living subjects
used paired samples of serum, adipose tissue (fat biopsy technique,
e.g., Daum et al., 1978), and breast-milk (see Tables 56, 57 and
58). Other tissues or fluids have rarely been analysed for PBB
content, e.g., there is one report on liver biopsy tissue (300 µg
HxBB/kg), fat (1069 µg/kg), bone marrow (3.5 µg/kg) and synovial
fluid (twice the amount present in serum) of a single person
(Meester & McCoy, 1976).
In some early investigations, serum (plasma, resp.) and adipose
tissue levels (Table 56) or serum (plasma, resp.) and breast-milk
levels (Table 57) did not seem to correlate well with each other.
Later studies, performed when continuing exposure had ceased
(Anderson, 1985), did reveal good correlations between serum and
adipose levels (Table 56) and between breast-milk and serum or
adipose tissue levels (Tables 57 and 58). The PBB concentrations
measured are compiled in section 5.2 and 5.3.
Different groups of the population can differ in their ratios.
For example, lowest adipose to serum ratios were found in lactating
and pregnant females (see Table 56). Eyster et al. (1983) found
statistically different ratios for females and male chemical workers
versus farm workers and other males. The adipose to serum ratio of
340:1, theoretically predicted by Tuey & Matthews (1980) was between
the values reported for the Michigan general population (see Table
56). Factors that appear to affect the partitioning ratios are sex,
pregnancy, occupational status, the total amount of PBBs present,
and whether samples were collected during exposure or during the
recovery phase (Eyster et al., 1983; Anderson, 1985). Generally, the
stability of a ratio would depend on a person being at equilibrium
with respect to PBB intake and fat mobilization (Fries, 1985b).
Transfer of PBBs to offspring occurs via transplacental passage
and via breast-milk (see also section 5.2.4). Breast-milk had levels
of more than 100 times the maternal serum levels (Table 57). The
ratios of 2,2',4,4',5,5'-HxBB concentrations in milk fat to maternal
body fat were found to be in the range of 0.7-0.9 : 1 (Table 58).
Placental tissue and cord or fetal serum levels were 1/6 to 1/10 the
maternal serum levels (see Tables 59 and 60).
According to Jacobson et al. (1984), the higher maternal serum
levels were, in part, reflecting the greater concentration of lipids
found in maternal serum (Table 60). Nevertheless, even when
calculated on a fat basis, PBB concentrations in maternal serum were
still about three times higher than those in cord serum (Jacobson
et al., 1984).
Table 55. PBB postmortem study: autopsied tissue samples from humans in the Grand Rapids area (Michigan, USA)a
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
1 40 - 2 6 2 4 9 2 4 80 8 3 - 3
2 407 118 1 15 4 15 17 2 43 457 10 9 295 9
3 242 247 16 21 18 61 0 63 51 320 42 2 - 80
4 148 294 103 36 32 30 147 73 653 430 84 123 - -
5 43 18 3 4 3 6 39 4 135 134 7 1 - 5
6 98 64 24 126 53 77 104 14 130 110 45 64 29 12
7 35 6 1 52 5 6 5 2 9 32 2 2 0 5
8 868 1011 142 233 61 100 259 93 188 1650 22 69 - -
9 - 75 16 15 6 2 59 6 158 94 11 0 - 12
10 110 285 - 110 95 81 31 62 170 1110 57 312 - 40
11 602 107 0 21 29 38 37 11 148 607 33 15 617 41
12 196 29 11 4 17 31 30 80 92 322 13 4 30 22
13 17 44 9 4 2 3 22 4 20 205 14 2 - 6
14 48 - 5 6 10 5 15 4 17 167 8 4 - 8
15 850 514 13 37 42 67 143 43 146 1390 53 36 - 37
Mean 265 216 27 46 25 35 61 31 131 475 27 43 243 22
SEM 80 77 12 16 7 9 18 9 41 131 6 21 140 6
Range 17-850 6-1011 1-103 2-95 2-100 0-259 2-93 4-653 32-1650 2-84 2-84 0-312 29-617 3-80
Table 55. (contd)
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
Tissue/
renal
fat:
mean
ratio 0.56 0.45 0.06 0.10 0.05 0.07 0.15 0.07 0.28 1.0 0.06 0.09 0.51 0.06
a From: Miceli et al. (1985).
b Measured (by GC) as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl; limit of detection: 0.5 µg/kg.
Table 56. Adipose tissue and serum partitioning ratios of 2,2'4,4',5,5'-hexabromobiphenyl
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Male chemical workers
(No. = 27) 1976 287:1 0.96 Wolff et al. (1979a)
(No. = 22) 1975-80 190-260:1 0.89 Eyster et al. (1983)
Farm residents, etc.
Mixed sexes 1974-75 175:1 Humphrey & Hayner (1975)b
(No. = 13) (range: 61-370:1)
Mixed sexes 1975-76 4752 (± 793):1 Meester & McCoy (1976)
(No. = 116) (range: 27-14 850:1)
Mixed sexes 1976-77 363:1 0.96 Landrigan et al. (1979)
(No. = 132)
Mixed sexes 1976 370:1 0.94 Wolff et al. (1979b)
(No. = 31) 1976-77 300:1
(No. > 31) not specified 320:1
Mixed sexes 1974-77 358:1 0.81d Tuey & Matthews (1980)b,c
(No. = 197)
Pregnant females 1975-80 140-180:1 0.91 Eyster et al. (1983)
(No. = 30)
Non-pregnant females 1975-80 193-230:1 0.84 Eyster et al. (1983)
(No. = 51)
Males (No. 75) 325-329:1 0.95 Eyster et al. (1983)
Lactating women throughout 1976 100:1 0.72 Brilliant et al. (1978)
Michigan (No. = 8)
Table 56 (contd).
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Michigan general population 1978-79 370:1 "high" Selikoff & Anderson (1979)
(No. = 396)
Michigan general population 1978 300:1 0.96 Wolff et al. (1982)
(No. = 588)
a No. = Number of paired samples.
b Use of "plasma" (instead of "serum").
c Values based on data of Gladen & Rogan (1979).
d Spearman correlation coefficient.
Table 57. Breast-milk (fat) and serum, partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired ratios coefficient
samples
1974-75 5 70-132:1 0.78 Humphrey & Hayner (1975)
1976 0.81 Brilliant et al. (1978)
1976-77 21 122:1 Landrigan et al. (1979)
(62-257:1)
1975-80 46 107-119:1 0.95 Eyster et al. (1983)
Not specified 92 0.71a Jacobson et al. (1984)
a Pearson product moment correlation.
Table 58. Adipose tissue and breast-milk (fat) partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired samples ratios coefficient
1976 10 1.07:1 0.88 Brilliant et al. (1978)
1975-80 24 1.1-1.5 :1 0.97 Eyster et al. (1983)
Incidentally, in contrast to PBBs, PCB levels (examined
additionally) were not significantly different in the maternal and
cord serum when compared on a fat basis (Jacobson et al., 1984). PBB
levels measured in children with known exposure to PBB in utero
and/or through breast-milk (see Table 38: Weil et al., 1981) also
indicate significant PBB transfer.
Table 59. Placental transfer of PBB: partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl between fetal and maternal tissues
Year No.a Paired tissues Ratios Correlation Reference
collected coefficient
1976-77 13 maternal serum/ 7.04:1 Landrigan et al.
cord serum (1.5-10.3:1) (1979)
1975-80 58 cord serum/ 0.10-0.14:1 0.88 Eyster et al.
maternal serum (1983)
1975-80 56 placenta/ 0.10-0.17:1 0.85 Eyster et al.
maternal serum (1983)
Not 153 maternal serum/ 0.81b Jacobson et al.
specified cord serum (1984)
Not 107 maternal milk/ 0.39b Jacobson et al.
specified cord serum (1984)
a No. = Number of paired samples.
b Pearson product moment correlation.
Table 60. Mean PBB and lipid levels in cord serum and maternal serum and milka
No. Mean PBBb levels Mean lipid levels
Cord serum 230 0.3 µg/litre 3.24 g/litre
Maternal serum 205 1.7 µg/litre
206 6.181 g/litre
Maternal milk 138 3.6 µg/litre 0.029%
Maternal milk-fat 138 105.1 µg/kg
a From: Jacobson et al. (1984).
b Probably measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
The results of analysis of fat and thymus specimens from 2
infants, taken at autopsy, are shown in Table 61. The ratio of
thymus/fat "hexabromobiphenyl" concentrations, expressed as
percentage, were 13 and 37% (Corbett et al., 1978a).
Table 61. Fat and thymus concentrations of hexabromobiphenyl (HBB) in
3-day-old infants
HxBB concentration (mg/kg)
Fat Thymus
Patient I 0.091 0.012 (13.2%)
Patient II 0.062 0.023 (37.1%)
a From: Corbett et al. (1978a).
The distribution pattern of PBB congeners, other than
2,2'4,4',5'5-hexabromobiphenyl (BB 153), has been studied by Wolff
et al. (1979a). They examined the relative (to BB 153) distribution
of PBB congeners (penta- to heptabromobiphenyls) in the fat and
serum of Michigan chemical workers and farm residents, and found
different partition ratios for different homologues.
The distribution of PBBs among blood compartments was studied
by Bekesi et al. (1979a,b), Greaves et al. (1984), and Roboz et al.
(1980, 1985a,b). In in vitro models (PBBs added to blood), the
distribution of PBBs among plasma, erythrocytes, mononucleocytes
(white cell fraction) and polymorphonucleocytes (white cell
fraction) was found to be 89:9:< 1:< 1 (Roboz et al., 1985a,b).
When, however, the amount of PBBs per cell was considered, there was
an approximately 100-fold excess of PBBs in the white cell fractions
compared with the erythrocyte fraction (Roboz et al., 1985b). In
environmentally contaminated human blood, PBBs were also present in
higher concentrations per mg protein in lymphocytes than in
erythrocytes (Bekesi et al., 1979a,b,; Roboz et al., 1980; see also
Table 45).
It is thought that the relatively large amounts of PBBs
associated with the white blood cells are possibly the cause of the
immunological dysfunctions that result from exposure to PBBs (Roboz
et al., 1985b).
In serum, 20% of the PBBs were not bound to protein. The
remaining 80% were bound to apolipoproteins B and A in a 3:1 ratio
(Greaves et al., 1984). Roboz et al. (1985b) reported a ratio of
4:1, which was close to the ratio (by weight) of the lipid content
of these apolipoproteins. For comparison: the ratio between their
amino acid content was 1.6:1 (Roboz et al., 1985b). There was no
evidence of differential binding of various PBB congeners (penta-,
hexa-, heptabromobiphenyls) to any of the serum fractions (Greaves
et al., 1984; Roboz et al., 1985a,b).
6.3 Metabolic transformation
Indirect evidence for vertebrate metabolism of some PBB
congeners has been obtained by analysis of tissues from
experimentally and environmentally exposed animals and humans (see
section 6.5). While many PBB congeners tended to be persistent,
others were frequently absent or diminished. However, the changes in
the relative abundances can reflect differences in uptake,
distribution, and excretion among the congeners, as well as
differences in the metabolism (Moore et al., 1980).
There are several in vitro and in vivo methods for studying
the metabolism of PBB congeners and mixtures.
6.3.1 In vitro studies
Using in vitro techniques, hepatic microsomes from rats (or
rabbits: Kohli et al., 1978) were incubated with individual PBB
congeners or a PBB mixture, in the presence of nicotinamide adenine
dinucleotide phosphate (reduced) (NADPH) and atmospheric oxygen.
Most of the authors measured rates of disappearance of congeners
(Dannan et al., 1978b; Moore et al., 1980; Parkinson & Safe, 1982;
Millis et al., 1985a,b; Mills et al., 1985). A second approach was
to examine the incubation mixture for metabolites (Kohli et al.,
1978; Purdy & Safe, 1980; Safe et al., 1980; Sparling et al., 1980).
The initial studies (Dannan et al., 1978b; Moore et al., 1980)
revealed that only two of twelve PBB congeners from FireMaster(R)
were metabolized when incubated with microsomes isolated from rats,
pretreated with phenobarbital (PB) or PBB, namely
2,4,5,2',5'-pentabromobiphenyl and 2,3,6,2',4',5'-hexabromobiphenyl.
No metabolism could be observed with control microsomes or
microsomes from 3-methyl cholanthrene (MC)-treated rats. These and
other investigations performed with a number of PBB congeners
(Br1-Br7 = model congeners, FireMaster(R) components,
photolysis products of several hexabromobiphenyls) and with liver
microsomal enzymes induced by either PB or MC are summarized in
Table 62.
The results suggest that the rates of metabolism of PBB
congeners are dependent upon the positions of bromine and the type
of cytochrome induced (P-450:PB-induced; P-448:MC- induced).
The following structure-activity relationships have been
derived: PBB congeners that possessed adjacent non-brominated
carbons meta and para to the biphenyl bridge on at least one
ring were metabolized by PB-induced microsomes (Dannan et al.,
1978b; Moore et al., 1980; Mills et al., 1985). In one case, even
one free para position was reportedly sufficient for PB-induced
metabolism (Moore et al., 1980). Increasing bromination of PBB
congeners did not appear to prevent their metabolism (Moore et al.,
1980; Mills et al., 1985). Significant metabolism by MC- pretreated
microsomes required adjacent ortho and meta positions free of
bromines on at least one ring of lower substituted congeners (up to
Br4). Higher substituted congeners (Br5, Br6) were not
metabolized, though they fulfilled this criterion (Mills et al.,
1985).
In contrast to the studies mentioned above, Purdy & Safe (1980)
found that radiolabelled [3H]-2,2',4,4',5,5'-hexabromobiphenyl
(purity: > 98%) was metabolized in vitro by rat liver microsomal
enzymes. They determined polar, lipophilic metabolites after
incubation with control and PBB-induced microsomes, however, in
quantities that were much smaller than those yielded from
[3H]-4-bromobiphenyl.
Mono- and dihydroxylated derivatives have been identified as
major in vitro metabolism products of lower brominated PBBs (Kohli
et al., 1978; Purdy & Safe, 1980; Safe et al.; 1980, Sparling
et al., 1980; Parkinson & Safe, 1982).
Some PBB congeners may induce their own metabolism (Aust
et al., 1983), and some PBB congeners may influence the metabolism
of other PBB congeners (Purdy & Safe, 1980; Mills et al., 1985).
However, it should be noted that PBB congeners that induce
microsomal enzymes (see also section 8.8.1) are not necessarily
metabolized (Aust et al., 1983).
Some studies were carried out using AHH, specific DNA binding,
and 7-ethoxyresorufin assays. The results showed that PBBs (FM FF-1)
can induce in vitro cytochromes P450 IA (P448) in the primary
cultures of human, and newly-born rat, epidermal keratinocytes, and
of a rat hepatoma cell-line (Yao et al., 1991).
6.3.2 In vivo studies
In vivo metabolism studies included attempts to find and
identify PBB metabolites from intact animals. Hydroxylated
derivatives have been reported most commonly as metabolites of
vertebrates.
Table 62. Metabolism of PBB congeners with rat liver microsomes
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
4-MoBB Yes Yes Parkinson &
(172) (53.1) Safe (1982)
2,2'-DiBB Yes Yes Mills et al. (1985)
(0.33) (201.7)
Yes Moore et al. (1980)
(> 2100) Dannan et al. (1978b)
4,4'-DiBB - No Moore et al. (1980)
(< 0.02) Dannan et al. (1978b)
3,4,4'-TrBB Yes No Mills et al. (1985)
(100.7) (0.02)
2,4,2',4'-TeBB Yes No Mills et al. (1985)
2,4,2',5'-TeBB Yes Yes Mills et al. (1985)
(43.5) (184.6)
Yes Moore et al. (1980)
(24)
2,4,2',6'-TeBB Yes Yes Mills et al. (1985)
2,5,2',5'-TeBB No Yes Mills et al. (1985)
(0.0) (66.2)
Yes Moore et al. (1980)
(27)
2,6,2',6'-TeBB No Yes Mills et al. (1985)
2,3,3',4'-TeBB Yes Yes Mills et al. (1985)
(57.6) (52.8)
2,5,3',4'-TeBB Yes Yes Mills et al. (1985)
(49.7) (47.5)
3,4,3',4'-TeBB Yes No Mills et al. (1985)
(58.8) (0.01)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
3,4,3',5'-TeBB Yes No Mills et al. (1985)
3,5,3',5'-TeBB No No Mills et al. (1985)
(0.06) (0.0)
Yes Moore et al. (1980)
2,4,6,2',4'-PeBB No No Mills et al. (1985)
2,4,5,2',5'-PeBB No Yes Mills et al. (1985)
(0.02) (23.7)
No Dannan et al. Yes Moore et al. (1980)
(1978b) (13) Dannan et al. (1978b)
2,4,6,2',6'-PeBB No Yes Mills et al. (1985)
2,4,5,3',4'-PeBB No No Mills et al. (1985)
(0.05) (0.03)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.06) Dannan et al. (1978b)
3,4,5,3',4'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
3,4,5,3',5'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
2,3,4,2',4',5'-HxBBB No No Mills et al. (1985)
(0.16) (0.0)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
2,3,6,2',4',5'-HxBBB No Dannan et al. Yes Moore et al. (1980)
(1978b) (19) Dannan et al. (1978b)
2,4,5,2',4',5'-HxBBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
2,3,4,5,3',4'-HxBBB No No Mills et al. (1985)
(0.11) (0.0)
No No Dannan et al. (1978b)
2,4,5,3',4',5'-HxBBB No No Dannan et al. (1978b)
3,4,5,3',4',5'-HxBBB No No Mills et al. (1985)
(0.20) 0.0)
2,3,4,5,2',4',5'-HpBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
a Measured as the rate of substrate disappearance (in parentheses: values measured
in pmol/min per mg protein).
b For expediency, a trivial numbering system was used.
c MC microsomes = 3-methylcholanthrene-induced microsomes.
d PB microsomes = phenobarbital-induced microsomes.
As can be seen from Table 63, administration of lower
brominated congeners resulted in low yields of, mainly, mono- or
dihydroxy metabolites. However, most of the studies compiled
suffered from the fact that the purities of the congeners used in
these studies were not determined (Moore et al., 1980). The two
studies with 2,2',4,4',5,5'-HxBB did not provide evidence of
significant metabolism (Table 63).
Various results were obtained after administering commercial
PBB mixtures. No hydroxylated PBBs could be detected in the urine of
cows given single 3-g doses of FireMaster(R) BP-6 (Willett &
Irving, 1976) or in the milk of cows (accidentally contaminated by
FireMaster(R) FF-1) with PBB residues as high as 900 µg/kg, on a
whole milk basis (Gardner et al., 1976). Approximately 1% of the
FireMaster(R) BP-6 administered to a pig was eliminated as an
unidentified pentabromobiphenylol (Kohli & Safe, 1976). It is not
clear whether this metabolite was formed from hexabromobiphenyl (by
reductive debromination followed by hydroxylation) or directly from
pentabromobiphenyl (by hydroxylation).
Table 63. PBB in vivo metabolites reported in the literature
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
Monobromobiphenyls
2-bromobiphenyl rabbit 1% 2'-bromo-4-biphenylol Kohli et al.
(urine) (1978)
traces mono-hycroxybromobiphenyl
rat (>) 2-bromo-4-4'-biphenyldiol Sparling et al.
(1980)
(>) 2-bromo-4-4'-biphenyldiol
(<) 2-bromo-4-biphenylol
3-bromobiphenyl rabbit 4% 3-bromo-4-biphenylol Kohli et al.
(urine) or 5-bromo-2-biphenylol (1978)
< 1% dihydroxybromobiphenyl
rat (>) 3-bromo-4,4'biphenyldiol Sparling et al.
(urine) and an unidentified diol (1980)
(<) monohydroxybromobiphenyls
4-bromobiphenyl rabbit 4% 4'-bromo-4-biphenylol Kohli et al.
(urine) 1.5% 4'-bromo-3,4-biphenyldiol (1978)
rat (>) 4'-bromo-4-biphenylos Sparling et al.
(urine) (<) mono- and dihydroxylated (1980)
species
pig 3% 4'-bromo-4-biphenylol Kohli &
(urine) Safe (1976)
traces monohydroxybromobiphenyl
0.5% 4'-bromo-3-methoxy-
4-biphenylol
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
chicken 12.2% 4'-bromo-4-biphenylol Jones et al.
(excreta) 9.8% 4'-bromo-3,4-biphenyldiol (1979)
(eggs) 0% no metabolites
Dibromobiphenyls
4,4'-dibromobiphenyl rabbit 10% 4,4'-dibromo-3-biphenylol Safe et
(urine) (1976)
1% 3,4'-dibromo-4-biphenylol
2% 4'-bromo-4-biphenylol
pig 5% 4,4'dibromo-3-biphenylol Kohli &
(urine) Safe (1976)
1% 3,4'-dibromo-4-biphenylol
traces dibromomethoxy-
biphenylol
1% 4'-bromo-3-methoxy-
4-biphenylol
traces dibromomethoxybiphenyl
Mixture of di-, fish Salmo dibromobiphenylol Zitko &
tri-, and tetra- salar (whole Hutzinger
bromobiphenyls animal) (1976)
Hexabromobiphenyls
2,2',4,4',5,5'- rat (urine traces "metabolites" Safe et al.
hexabromobiphenyl and faeces) (1978)
(purity: 95%)
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
2,2',4,4',5,5'- rat 0% no metabolites Matthews et al.
hexabromobiphenyl (tissues) (1977)
(purity: 99%)
(bile and traces may be metabolites
faeces) (1-4%)
a (>) and (<) = major and minor components (no further quantitative information).
The faeces of dogs fed FireMaster(R) BP-6 contained a
metabolite identified as 6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl
(Gardner et al., 1979). However, the authors did not exclude
microbial metabolism in the dog's gut, because no
hydroxyhexabromobiphenyl was found in the liver of the dog, though
PBB was present. Matthews et al. (1979) mentioned that dogs were
able to metabolize 2,2',4,4',5,5'-hexachlorinated biphenyl, but were
unable to metabolize the analogous PBB at an appreciable rate.
Some investigations implied that fish may debrominate the more
highly brominated components of PBB mixtures. Juvenile Atlantic
salmon (Salmo salar) experimentally exposed to FireMaster(R)
BP-6, in water or in food, contained several mono- to
pentabromobiphenyls, not present in FireMaster(R) BP-6 (Zitko,
1977). Fish (Salmo salar) fed octabromobiphenyl (Dow
Chemical)-contaminated food contained unidentified penta-, hexa-,
and heptabromobiphenyls in addition to the octabromo biphenyls. It
was not known whether the partially debrominated biphenyls were
generated by the fish, or by the associated microflora (Zitko,
1977). Analyses of fish captured from natural waters may also
indicate the possibility of the debromination reaction in fish,
unless selective accumulation or elimination takes place (Stratton &
Whitlock, 1979). In fish from water mainly contaminated with
decabromobiphenyl, only the nona- and hexa bromobiphenyl congeners
were present. However, in fish from FireMaster(R)
BP-6-contaminated waters, only hexabromobiphenyl was detected.
6.3.3 Metabolic pathway
The most frequently reported route of PBB metabolism was
hydroxylation. However, according to Zitko & Hutzinger (1976), a
reductive debromination may be a degradative pathway of higher
brominated biphenyls, because the carbon-bromine bond is less stable
than, e.g., the carbon-chlorine bond.
The hydroxylation reaction probably proceeds via both arene
oxide intermediates (Safe et al., 1976, 1978, 1980; Kohli et al.,
1978; Moore et al., 1980) and by direct hydroxylation (Kohli et al.,
1978; Sparling et al., 1980; Moore et al., 1980). Schemes of
possible metabolic routes have been published by Matthews (1982) and
Safe (1989). The most important enzyme involved in the oxidation of
xenobiotics, such as PBBs, is aryl hydrocarbon hydroxylase. It is a
highly inducible, haem-containing mono oxygenase belonging to the
family of cytochromes P-450 (e.g., Safe et al., 1980). A (partial)
summary of the AHH-mediated metabolism is given by Safe et al.
(1978).
The formation of covalently bound macromolecular adducts has
been reported. Less than 10% of the metabolites were present in the
urine of rabbits in a bound form, e.g., as glucuronides (Safe
et al., 1976). In vitro metabolism also resulted in macromolecular
conjugates (Kohli et al., 1978; Purdy & Safe, 1980; Safe et al.,
1980; see also section 6.6). In general, the biotransformation of
PBBs is a slow process, but the stereochemistry and molecular size
vary so widely among PBBs that there are great differences in their
metabolic activity.
6.4 Elimination and excretion in expired air, faeces, urine
6.4.1 Animal studies
Elimination of PBBs from the body has been studied using hexa-
and octabromobiphenyl. No information is available on
decabromobiphenyl. Some data found in the literature (see also
section 6.1) are compiled in Table 64 (14C-labelled single doses),
Table 65 (14C-labelled multiple doses), and Table 66 (daily
feeding).
The only study conducted with octabromobiphenyl resulted in a
much higher elimination from rats than was found for HxBB in another
study on rats (Table 64). However, there are also reports on the
rapid elimination of HxBB, e.g., by Willett & Irving (1976), who
found a 50% recovery of HxBB after 168 h in the faeces of two cows
given single intraruminal doses (3 g) of FireMaster(R) BP-6.
However, relatively high concentrations of radioactivity or of HxBB,
found, in some cases, in the faeces during the first few days after
dosing or during daily dosing, may have been due to incomplete
absorption (Tuey & Matthews, 1980). Approximately 60% of the total
dose was also recovered in the faeces of rhesus monkeys (Rozman
et al., 1982: Table 64).
Elimination of PBBs was primarily via the bile and the
intestine into the faeces and, in general, was found to be a slow
process.
Biliary concentrations have been measured in a Rhesus monkey
(Rozman et al., 1982), in rats (Matthews et al., 1977), and in
cattle (Willett & Irving, 1976; Willett & Durst, 1978). In rats,
excretion of HxBB in the bile accounted for 0.68% of the total PBB
dose between 0 and 4 h after intravenous (iv) administration.
Twenty- four hours after an iv dose, 0.032% of the total dose was
excreted in the bile in 1 h, and, 7 or 42 days after dosing
concentrations were too low to quantify (Matthews et al., 1977).
Because of this small amount cleared with the bile, enterohepatic
recirculation of HxBB in rats is not important (Tuey & Matthews,
1980). Concen trations of HxBB in the bile of cattle were two-three
times greater than the concentration in the plasma (Willett &
Irving, 1976; Willett & Durst, 1978).
According to Fries (1985b), excretion of PBB in the urine is
not expected, because of the insolubility of PBBs in water. He
attributed the few instances in which low concentrations of PBBs
were reported to cross-contamination with faeces. On the other hand,
this route of excretion may account for minor or metabolized
biphenyls (Damstra et al., 1982; see also Table 63). However,
excretion of PBB metabolites may be of minor relevance, since the
more abundant PBB congeners are not, or only slightly, metabolized
(see also section 6.3). The amounts of urinary or faecal metabolites
were low. A study on a pig that received a single intraperitoneal
(ip) dose of FireMaster(R) BP-6 (100 mg/kg body weight) reported a
yield of about 1% of pentabromobiphenylol in the urine and faeces,
collected for 7 days (Kohli & Safe, 1976). The level of a hydroxy
metabolite detected in the faeces of dogs fed FireMaster(R) BP-6
was about an order of magnitude less than the PBB levels (Gardner
et al., 1979). Yields of lower brominated urinary PBB metabolites
are compiled in Table 63.
In their study on cows, Willett & Durst (1978) did not find any
direct relationships between the amount of PBBs fed and the
concentration in the faeces. In contrast, Babish et al. (1975a) did
find a linear correlation (r > 0.97) of dietary PBBs with excreta
residues in Japanese quails. In another study on cows, it was also
reported that relationships were approximately constant (Robl
et al., 1978: Table 66).
Table 64. Faecal, urinary, and exhalative elimination of 14C activity as a percentage of a single dose of [14C-]PBB
Species Agent; solvent; dose (mg/kg Time % recovery in: Reference
body weight); route faeces urine expired air
Rhesus monkey (male) [14C-]HxBB in mineral oil; 2, oral 5 days 38 (0.18) - Rozman et al.
(1982)
Rat (male) [14C-]HxBB in Emulphor EL-620: - Matthews et al.
ethanol: water (1:1:8) 24 h 7.9 (1977); Tuey &
1, oral 24 h 0.96 Matthews
1, intravenous 7 days 3.28 < 0.1 (1978, 1980)
1, intravenous 42 days 6.6 not detected
Rat (male and female) [14C-]OcBBa in corn oil; 1, oral 24 h 62 Norris et al.
48 h 69 (1973)
16 days 73 < 1 < 1
Mink (Mustela vison) [14C-]PBBb in propylene glycol Bleavins et al.
(pregnant female) (1 µCi in 0.1 ml) intravenous 2 h - 0.003 - (1981)
Ferret (Mustela 2 h - 0.004
puttorius (furo)
(pregnant female)
Dog [14C-]2,2',4,4',5,5'-hexabromo 25 days 8 Sipes et al.
biphenyl (solvent not specified) (1979)
0.6, intravenous
a OcBB = technical octabromobiphenyl.
b Consisted of 2,2', 4,4', 5,5'-HxBB and 2,2', 3, 4,4', 5,5'-HpBB.
Table 65. Urinary and faecal elimination of HxBB and/or metabolites in male Rhesus monkeys and male rats,
dosed repeatedly with [14C-]HxBB
Species Dose (mg/kg body Days after Urine Faeces Cumulative % Reference
weight) first dose (µg/kg per (µg/kg per recovery of
day) day) total dose
in faeces
Monkey 50 1-10 3.2 5890 ca 60% Rozman et al. (1982)
(No. = 2) in methyl cellulose; 11-17 2.5 5.0
oral on days 1 and 5 203-209 not detectable 3.5
Rat 1 7 ca 14% Matthews et al. (1977)
(No. = 3) in corn oil; oral on
days 1, 2, 3, and 4
Table 66. Elimination of HxBB (2,2',4,4',5,5'-hexabromobiphenyl) in faeces and urine during the feeding of FireMaster(R)-mixtures
Species Intake of FireMaster Sampling Concentration in: Approximate % Reference
(sex) BP-6 or FF-1 time (mg/kg) recovery in
Faeces Urine faeces
Dog BP-6: in corn oil (capsule) last day of 7 Gardner et al.
(female) 1 mg/kg body weight per day dosing (1979)
for 6 weeks
Pig BP-6: in corn oil at week 4 Ku et al. (1978)
20 mg/kg diet 21.3a 0.015
200 mg/kg diet 182.0a 0.07
(ad libitum)
for > 4 weeks
Cow FF-1: in gelatin capsules; Robl et al. (1978)
for 90 days
0.1 mg/kg diet 0.02 mostly 15% of
1.0 mg/kg diet 0.15 n.d.b ingested
10 mg/kg diet 1.5 dose
Calf BP-6:(capsule); 25 g daily total 8045 5% of total Willet & Irving
(male) for 9 days collection dose (1976)
Hen BP-6: 20 mg/kg in the diet weekly 9% of daily Fries et al.
for 63 days dose (1976)
Hen FF-1: not specified not specified 11% of daily Ringer &
dose Polin (1977)
a Faecal samples oven-dried.
b nd = Not detected (detection limit = 0.005 mg/kg).
Following withdrawal of PBBs (FireMaster(R) BP-6) from the
diet of cows, the faecal concentrations of HxBB declined to 1-2% of
faecal levels during dosing (Willett & Durst, 1978) and to less than
5% in hens (Fries et al., 1976). Faecal concentrations were small in
relation to body burden. Cows that received 250 mg FireMaster(R)
BP-6 daily had HxBB concentration ratios in body fat to faeces of
about 750 : 1. Their faeces to plasma ratio was 0.7 :1 (Willett &
Durst, 1978). Cows environmentally contaminated (FireMaster(R)
FF-1) 7-9 months before examination had comparable body fat to
faeces ratios, but a different faeces to blood ratio of 4.2 : 1
(Detering et al., 1975; Cook et al., 1978b; Fries et al., 1978a).
Post-exposure lactating cows eliminated via milk fat three times the
quantity of HxBB cleared in faeces (Willett & Durst, 1978).
There have been some studies on the means to enhance the
elimination of PBBs. PBBs used were: FireMaster(R) FF-1 (Cook
et al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980; Polin
& Leavitt, 1984; Polin et al., 1985) and [14C-]HxBB (Rozman
et al., 1982). The treatments included activated carbon in rats
(McConnell et al., 1980) and cows (Cook et al., 1978b),
cholestyramine in rats (McConnell et al., 1980) and monkeys (Rozman
et al., 1982), colestipol in chickens (Polin & Leavitt, 1984; Polin
et al., 1985), mineral oil in rats (Kimbrough et al., 1980), monkeys
(Rozman et al., 1982), and chickens (Polin et al., 1985), high-fibre
diets in rats (Kimbrough et al., 1980), and phenobarbital in cows
(Cook et al., 1978b). The effects of restricted caloric intake,
alone, or in combination with other treatments, was investigated in
rats (McConnell et al., 1980) and chickens (Polin & Leavitt, 1984;
Polin et al., 1985). The procedures were found not to be (Cook et
al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980), or to
be only partially, effective (Rozman et al., 1982; Polin & Leavitt,
1984; Polin et al., 1985) in reducing the body burden of PBBs
(measured as concentrations of HxBB, total bromine levels, or
14C-activity).
6.4.2 Human studies
The concentrations of PBBs in human bile and faeces represent a
minor proportion of the total body burden, as has been demonstrated
by Eyster et al. (1983), who determined HxBB levels in Michigan farm
and chemical workers in 1975-80. Concen trations of HxBB observed in
the bile and faeces were about 1/2 to 7/10 of the serum levels (on a
whole-weight basis) and were estimated to be approximately 0.5% of
the adipose tissue levels (Table 67).
These findings are consistent with the theoretical predictions
of Tuey & Matthews (1980), who calculated slow rates of faecal
excretion in humans. In addition, these authors showed that the
excretion rate in lean individuals exposed to HxBB would be higher
than those in overweight individuals.
6.5 Retention and turnover
6.5.1 Animal studies
The time course of PBB tissue concentrations has been studied
predominantly in rats, and, to a lesser extent, in cattle, chickens,
and guinea-pigs (Table 68). Incomplete data are available for mice,
pigs, and monkeys. With the exception of 3 older studies (Norris
et al., 1973; Lee et al., 1975a; Waritz et al., 1977), in which the
behaviour of technical octabromobiphenyl in rats was observed, the
majority of investigators used the FireMaster(R)- mixture (BP-6 or
FF-1). Of the individual PBB congeners,
2,2',4,4',5,5'-hexabromobiphenyl (Matthews et al., 1977; Millis
et al., 1985b) and 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985b) were administered.
Table 67. Medians, range, and geometric means of 2,2',4,4',5,5'-
hexabromobiphenyl (HxBB) for paired specimens of serum, faeces, and bile
obtained from farm and chemical workersa
Number Paired Median Rangeb Geometric
specimen mean
51 serum 7 µg/litre 1-1540 µg/litre 14.4 µg/litre
faeces 5 µg/kg nd-862 µg/kg 9.0 µg/kg
20 serum 3.5 µg/litre 1-153 µg/litre 4.2 µg/litre
biliary fluid 2 µg/litre nd-70 µg/litre 2.7 µg/litre
a From: Eyster et al. (1983).
b nd = Not detectable (detection limit: 1 µg/kg or µg/litre).
Complex and varied relationships were found in tissue
concentrations with time after PBB administration (see also Tables
47, 48, and 49).
Table 68. Reported biological half-lifes of PBBs in mammals and birds, after single or repeated exposure
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Rhesus Monkey (male) [14C-]HxBB (50 mg/kg body weight body > 4 years Rozman et al.
on days 1 and 5; oral) 209 days (1982)
Rat (male) [14C-]OcBB (1 mg/kg body weight; faeces < 24 h (1st phase) Norris et al.
single dose; oral) 16 days > 16 days (2nd phase) (1973)
Rat [14C-]HxBB (1 mg/kg body weight; faeces 2 days (1st phase)c Birnbaum
single iv dose) 42 days (1985)
"tissues" ca 24 daysc Ecobichon et al.
(blood, liver (1983)
muscle, skin)
Rat (male) FM BP-6 (1 mg/100 g body weight; serum 23.1 weeks Miceli &
single ip dose; 36 weeks fat 69.3 weeks Marks (1981)
adrenal 43.3 weeks
brain 63.0 weeks (2nd phase)
liver 11.5 weeks (2nd phase)
lung 11.2 weeks
spleen 9.0 weeks
Rat (male) FM FF-1 (10 mg/kg body weight; whole blood 3.27 h (') Domino et al.
single dose; oral) 112 days 33.3 h (ý) (1982)
145 days (')
Guinea-pig (lactating FM FF-1 (50 mg/kg body weight; tissues (fat, ca 22 days Ecobichon
females and pups) single dose; oral) 60 days liver, kidney, et al. (1983)
lung) of both
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Cow FM BP-6 (fed 10 mg/day for milk fat ca 58 days Fries &
60 days) 60 days (of withdrawal) (2nd phase) Marrow (1975)
Cow FM BP-6 (fed 1.13 g/day for milk 10.5 days Gutenmann &
15 days; = 50 mg/kg in the diet) Lisk (1975)
15 days (of withdrawal)
Cow FM FF-1 (environmentally milk fat ca 60 days Fries et al.
contaminated 1 year earlier; (range: 36-301 days) (1978a); Cook
6 months of observation) et al. (1978b)
Cow FM BP-6 (fed 0.25 mg-25 g/day milk fat > 6 monthsd Fries
for various periods) up to (body) (1985b)
3 years of observation
Chicken (White FM FF-1 (fed in the diet) egg 17 days Ringer &
Leghorn hens) 28 days (of withdrawal) Polin (1977)
Chicken (White FM FF-1 (fed 0.2-125 mg/kg egg 17 days Polin &
Leghorn hens) in the diet for 5 weeks) Ringer (1978a)
56 days (of withdrawal)
(fed 1125 and 625 mg/kg in liver 31 days
the diet for 5 weeks)
56 days (of withdrawal)
(fed 5-625 mg/kg in the diet muscle 17 days
for 5 weeks) 56 days
(of withdrawal)
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Chicken (White FM BP-6 (fed 20 mg/kg in the egg 28 days Fries et al.
Leghorn hens) diet for 63 days) 49 days (2nd phase) (1976)
(of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg egg 112 days Cecil &
Leghorn hens) in the diet for 8 weeks) (late phase) Bitman (1978)
266 days (of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg excreta 4-5 dayse Polin &
Leghorn hens) in the diet for 8 weeks) Leavitt (1984)
266 days (of withdrawal)
a HxBB = 2,2',4,4',5,5'-hexabromobiphenyl; OcBB = octobromobiphenyl (technical mixture);
FM BP-6 = FireMaster(R) BP-6; FM FF-1 = FireMaster(R) FF-1.
b Referring to 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity.
c Half-life calculated from data of Matthews et al. (1978); see also Fig. 6.
d Half-life calculated from data of Willett & Durst (1978).
e Half-life calculated from data of Fries et al. (1976).
6.5.1.1 Time trends, retention: 2,2',4,4',5,5'-hexabromobiphenyl
(BB 153)
a) Rat
When rats dosed with 2,2',4,4',5,5'-hexabromobiphenyl (Matthews
et al., 1977; Tuey & Matthews, 1980) or with FireMaster(R) FF-1
(Kimbrough et al., 1978; Domino et al., 1980b; 1982), BB 153
concentrations in the blood were highest immediately after dosing,
but fell rapidly during the first day (as PBBs are taken up from
blood by the liver and muscle tissues, which are highly perfused
tissues). Then, concentrations in the blood, liver, and muscle
declined less quickly (as the dose was redistributed to the adipose
tissue; Tuey & Matthews, 1980; Fries, 1985b), finally decreasing
only very slowly. After a single intravenous dose of BB 153 (1 mg/kg
body weight), adipose tissue contained more than 60% of the total
body burden within four days (Tuey & Matthews, 1980; see Table 50).
BB 153 concentrations in the adipose tissue peaked later than
in other tissues and remained high throughout the period of
observation (6 weeks: Matthews et al., 1977; 16 weeks; Domino
et al., 1980b; 36 weeks: Miceli & Marks, 1981). For example, ratios
between fat and serum levels in rats rose from 221 : 1 to 722 : 1
between 6 and 36 weeks after a single dose exposure (Miceli & Marks,
1981), reflecting the much more rapid clearance of BB 153 from serum
than from fat.
Kinetic models to describe the principal toxicokinetics of BB
153 in the rat were constructed by Tuey & Matthews (1980; a blood
flow-limited physiological compartmental model) and by Domino et al.
(1982). The latter developed a three-compartment model. Tissues
within a compartment showed similar kinetic characteristics, but
concentrations could vary widely. Compartment 1 consisted of whole
blood, spleen, kidney, and heart. Compartment 2 included liver,
lung, cerebral grey and white matter, cerebellum, and testes, and
compartment 3 consisted of subcutaneous fat. Jejunum could not be
classified.
However, results of Miceli & Marks (1981) (PBB levels monitored
over longer periods) do not fit in well with this scheme. For
example, these authors observed typical first-order-elimination
kinetics of BB 153 in the serum of rats, but kinetics of disappear
ance from heart and kidney (and pituitary) do not appear to be first
order, though belonging to the same compartment (according to Domino
et al., 1982). BB 153 concentrations in the brain and liver (both
"compartment 2") declined rapidly during the interval from 6-12
weeks after exposure, but, thereafter, (from week 12 to 36) brain
concentrations fell far more slowly than those of the liver. The
study of Miceli & Marks (1981) showed that the (long-term)
retention, but not always the concentration, of BB 153 in lipid-rich
tissues (brain, adrenal, adipose) was much greater than in most
other tissues (see also Table 68).
Corresponding to the small decline in BB 153 from the tissues
of rats, the elimination rates in the faeces were slow (see Fig. 6).
Less than 7% of the dose was eliminated 42 days after a single iv
dose, most, during the first 3-4 days (Tuey & Matthews, 1980).
When dams of guinea-pigs received a dose of FireMaster(R)
FF-1 on day 1 after delivery, the concentrations of 2,2',4,4',5,5'-
HxBB in the lungs, livers, kidneys, and fat of the pups were similar
to those of the dams for 4-60 days after treatment (Ecobichon
et al., 1983).
Combined placental and milk transfer
Rickert et al. (1978), Bleavins et al. (1981), and Werner &
Sleight (1981) concluded from their studies on rats, minks, and
pigs, respectively, that milk transfer is far more important than
placental transfer; studies on guinea-pigs (Ecobichon et al., 1983)
did not confirm this observation. However, under less controlled
conditions, perinatal exposure (both placental and milk transfer)
occurs and results in a marked body burden in the offspring, as has
been shown in studies on rats (Table 52 and McDaniel & Lucier, 1979)
and pigs (Table 53). From minks, it has been reported that
14-day-old kits of dams that had received a single intraperitoneal
dose of 14C-PBB at an early stage of pregnancy, contained about 3%
of the initial maternal dose (Bleavins et al., 1981). PBB body
burdens in the offspring of rats were still measurable at 328 days
of age and at the end of their life span (see Table 52).
Moreover, a multigeneration study on rats (McCormack et al.,
1981) demonstrated that administration of PBBs to a single
generation resulted in detectable residues in two subsequent
generations (Table 54). The concentrations of PBBs measured in the
tissues of F1-animals were approximately 5-30 times higher than
those in tissues from F2-animals and approximately 50-1000 times
higher than those in tissues from F3-animals (see Table 54).
2) Birds
In birds, eggs are the medium of PBB transfer to the offspring.
The ratio of egg PBB contents to dietary level has been reported to
be 1 : 1 (Fries et al., 1976) and 1.3 - 1.5 : 1 (Babish et al.,
1975a; Ringer & Polin, 1977; Cecil & Bitman, 1978; Polin & Ringer,
1978a) in chickens (White Leghorn hens) and Japanese quail,
respectively. After 63 days of feeding FireMaster(R) BP-6 in the
diet, the PBB level in body fat of White Leghorn hens was about 4
times the level in eggs (Fries et al., 1976).
6.2.2 Human studies
Studies on the distribution of PBBs in humans refer only to
people having been exposed in a direct or indirect way to the
FireMaster(R)-mixture.
As can be seen from a post-mortem study on people from a "high"
exposure area of the State of Michigan (USA), PBBs are distributed
throughout the entire human body (Table 55). Moreover, it was found
that fat and fat-rich tissue had the highest HxBB concentrations.
Perirenal fat had the highest mean concentration (475 ng/g).
Adrenal, atheromatus aorta, and thymus had mean concentrations of
about half that of perirenal fat; all other tissues had mean
concentrations of only one-tenth or less of that of perirenal fat
(Miceli et al., 1985).
Table 52. Tissue concentrations of PBBs in rats following perinatal exposure to PBBs (FireMaster(R)-mixture BP-6 or FF-1)
Dosing regimen to dams Age of Tissue concentrations of PBBsa (mg/kg wet weight) References
offspring
Offspring Dams
50 mg BP-6/kg diet: day 8 of 14 days liver carcass liver Rickert et al.
gestation through day 14 9.5 149.7 4.0 (1978)
postpartum
100 mg PB-6/kg diet: day 8 of liver kidney fat b McCormack et al.
pregnancy through 28 days 28 days 397 96 1693 (1980)
postpartum 328 days 17 11 387
BP-6 (lot 6244 A) in the diet: 28 days lung liver kidney fat b McCormack et al.
day 8 of pregnancy through (1982a)
28 days postpartum:
10 mg/kg 5 18 8 162
100 mg/kg 32 410 109 1693
200 mg FF-1/kg body weight liver: liver: liver Groce &
(in corn oil; by stomach tube): (female) (male) Kimbrough (1984)
day 7 and 14 of pregnancy 2 months 2.4 (218)c 3.0 (280)c 7.8 (542)c
(weaning at day 21 of age) 2 years 0.8 (107)c 0.6 (58)c
a Concentration expressed as the concentrations of 2,2',4,4',5,5'-hexabromobiphenyl.
b See McCormack & Hook (1982) and Table 48.
c Values calculated on a lipid basis.
Table 53. Mean concentrations of PBBs (mg/kg of tissue, wet weight) in tissues of sows and
4-week-old nursing pigs following perinatal exposure to PBBsa
PBBsb Liver Adipose tissue Kidney Brain
(mg/kg feed) Sows Pigs Sows Pigs Sows Pigsc Sows Pigsc
10 1.0 2.4 15.2 14.8 0.6 nd 0.2 nd
100 45.8 30.2 96.3 96.7 2.3 nd 1.7 nd
200 92.6 41.3 194.2 222.5 3.7 4.1 2.7 4.2
a From: Werner & Sleight (1981).
b FireMaster(R) BP-6 fed to the sows during the second half of gestation and during lactation.
c nd = Not detected.
Table 54. Tissue concentrations of PBBs in several generations of rats following perinatal exposure to PBBsa,b,c
Treatment Liver Kidney Lung Thyroid Testis Ovary Fat
Control < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1 < 0.1
F1-10 17.1 ± 3.6 7.5 ± 0.6 5.1 ± 0.8 4.4 ± 1.0 8.2 ± 5.3 24.0 ± 1.6 161.7 ± 24.2
F2-10 0.4 ± 0.1 0.6 ± 0.2 0.9 ± 0.1 0.5 ± 0.1 0.2 ± 0.1 3.0 ± 0.5 6.7 ± 1.9
F1-100 410.2 ± 40.6 108.6 ± 11.2 32.4 ± 2.0 162.6 ± 20.8 -d -d 1693.2 ± 250.4
F2-100 21.8 ± 3.2 7.2 ± 1.3 8.7 ± 1.0 2.7 ± 0.6 1.8 ± 0.11 6.5 ± 2.6 159.5 ± 16.9
F3-100 0.4 ± 0.1 0.8 ± 0.4 0.6 ± 0.1 0.2 ± 0.1 0.3 ± 0.1 0.6 ± 0.1 4.9 ± 0.8
a From: McCormack et al. (1981).
b Rats were fed 0,10, or 100 mg PBBs (FireMaster(R) BP-6)/kg from day 8 of pregnancy until 28 days postpartum at which
time all offspring (F1-10 and F1-100) were weaned on to a control diet, allowed to mature sexually, and bred with
littermates to produce the F2-generation (F2-10 and F2-100). F2-100 littermates were bred to produce F3-100 animals.
c Values are means in mg 2,2',4,4',5,5'-hexabromobiphenyl/kg wet tissue ± SE for at least three animals at 28 days of age.
d Sample not available.
Most of the distribution studies performed with living subjects
used paired samples of serum, adipose tissue (fat biopsy technique,
e.g., Daum et al., 1978), and breast-milk (see Tables 56, 57 and
58). Other tissues or fluids have rarely been analysed for PBB
content, e.g., there is one report on liver biopsy tissue (300 µg
HxBB/kg), fat (1069 µg/kg), bone marrow (3.5 µg/kg) and synovial
fluid (twice the amount present in serum) of a single person
(Meester & McCoy, 1976).
In some early investigations, serum (plasma, resp.) and adipose
tissue levels (Table 56) or serum (plasma, resp.) and breast-milk
levels (Table 57) did not seem to correlate well with each other.
Later studies, performed when continuing exposure had ceased
(Anderson, 1985), did reveal good correlations between serum and
adipose levels (Table 56) and between breast-milk and serum or
adipose tissue levels (Tables 57 and 58). The PBB concentrations
measured are compiled in section 5.2 and 5.3.
Different groups of the population can differ in their ratios.
For example, lowest adipose to serum ratios were found in lactating
and pregnant females (see Table 56). Eyster et al. (1983) found
statistically different ratios for females and male chemical workers
versus farm workers and other males. The adipose to serum ratio of
340:1, theoretically predicted by Tuey & Matthews (1980) was between
the values reported for the Michigan general population (see Table
56). Factors that appear to affect the partitioning ratios are sex,
pregnancy, occupational status, the total amount of PBBs present,
and whether samples were collected during exposure or during the
recovery phase (Eyster et al., 1983; Anderson, 1985). Generally, the
stability of a ratio would depend on a person being at equilibrium
with respect to PBB intake and fat mobilization (Fries, 1985b).
Transfer of PBBs to offspring occurs via transplacental passage
and via breast-milk (see also section 5.2.4). Breast-milk had levels
of more than 100 times the maternal serum levels (Table 57). The
ratios of 2,2',4,4',5,5'-HxBB concentrations in milk fat to maternal
body fat were found to be in the range of 0.7-0.9 : 1 (Table 58).
Placental tissue and cord or fetal serum levels were 1/6 to 1/10 the
maternal serum levels (see Tables 59 and 60).
According to Jacobson et al. (1984), the higher maternal serum
levels were, in part, reflecting the greater concentration of lipids
found in maternal serum (Table 60). Nevertheless, even when
calculated on a fat basis, PBB concentrations in maternal serum were
still about three times higher than those in cord serum (Jacobson
et al., 1984).
Table 55. PBB postmortem study: autopsied tissue samples from humans in the Grand Rapids area (Michigan, USA)a
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
1 40 - 2 6 2 4 9 2 4 80 8 3 - 3
2 407 118 1 15 4 15 17 2 43 457 10 9 295 9
3 242 247 16 21 18 61 0 63 51 320 42 2 - 80
4 148 294 103 36 32 30 147 73 653 430 84 123 - -
5 43 18 3 4 3 6 39 4 135 134 7 1 - 5
6 98 64 24 126 53 77 104 14 130 110 45 64 29 12
7 35 6 1 52 5 6 5 2 9 32 2 2 0 5
8 868 1011 142 233 61 100 259 93 188 1650 22 69 - -
9 - 75 16 15 6 2 59 6 158 94 11 0 - 12
10 110 285 - 110 95 81 31 62 170 1110 57 312 - 40
11 602 107 0 21 29 38 37 11 148 607 33 15 617 41
12 196 29 11 4 17 31 30 80 92 322 13 4 30 22
13 17 44 9 4 2 3 22 4 20 205 14 2 - 6
14 48 - 5 6 10 5 15 4 17 167 8 4 - 8
15 850 514 13 37 42 67 143 43 146 1390 53 36 - 37
Mean 265 216 27 46 25 35 61 31 131 475 27 43 243 22
SEM 80 77 12 16 7 9 18 9 41 131 6 21 140 6
Range 17-850 6-1011 1-103 2-95 2-100 0-259 2-93 4-653 32-1650 2-84 2-84 0-312 29-617 3-80
Table 55. (contd)
PBBsb in tissue (µg/kg wet weight)
Subject Heart Kidney Renal Skeletal
No. Adrenal Aorta Brain I. vent. Cortex Medulla Liver Lung Pancreas fat muscle Spleen Thymus Thyroid
Tissue/
renal
fat:
mean
ratio 0.56 0.45 0.06 0.10 0.05 0.07 0.15 0.07 0.28 1.0 0.06 0.09 0.51 0.06
a From: Miceli et al. (1985).
b Measured (by GC) as the concentration of 2,2',4,4',5,5'-hexabromobiphenyl; limit of detection: 0.5 µg/kg.
Table 56. Adipose tissue and serum partitioning ratios of 2,2'4,4',5,5'-hexabromobiphenyl
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Male chemical workers
(No. = 27) 1976 287:1 0.96 Wolff et al. (1979a)
(No. = 22) 1975-80 190-260:1 0.89 Eyster et al. (1983)
Farm residents, etc.
Mixed sexes 1974-75 175:1 Humphrey & Hayner (1975)b
(No. = 13) (range: 61-370:1)
Mixed sexes 1975-76 4752 (± 793):1 Meester & McCoy (1976)
(No. = 116) (range: 27-14 850:1)
Mixed sexes 1976-77 363:1 0.96 Landrigan et al. (1979)
(No. = 132)
Mixed sexes 1976 370:1 0.94 Wolff et al. (1979b)
(No. = 31) 1976-77 300:1
(No. > 31) not specified 320:1
Mixed sexes 1974-77 358:1 0.81d Tuey & Matthews (1980)b,c
(No. = 197)
Pregnant females 1975-80 140-180:1 0.91 Eyster et al. (1983)
(No. = 30)
Non-pregnant females 1975-80 193-230:1 0.84 Eyster et al. (1983)
(No. = 51)
Males (No. 75) 325-329:1 0.95 Eyster et al. (1983)
Lactating women throughout 1976 100:1 0.72 Brilliant et al. (1978)
Michigan (No. = 8)
Table 56 (contd).
Exposed group collecteda Year Partition coefficient ratios Correlation Reference
Michigan general population 1978-79 370:1 "high" Selikoff & Anderson (1979)
(No. = 396)
Michigan general population 1978 300:1 0.96 Wolff et al. (1982)
(No. = 588)
a No. = Number of paired samples.
b Use of "plasma" (instead of "serum").
c Values based on data of Gladen & Rogan (1979).
d Spearman correlation coefficient.
Table 57. Breast-milk (fat) and serum, partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired ratios coefficient
samples
1974-75 5 70-132:1 0.78 Humphrey & Hayner (1975)
1976 0.81 Brilliant et al. (1978)
1976-77 21 122:1 Landrigan et al. (1979)
(62-257:1)
1975-80 46 107-119:1 0.95 Eyster et al. (1983)
Not specified 92 0.71a Jacobson et al. (1984)
a Pearson product moment correlation.
Table 58. Adipose tissue and breast-milk (fat) partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl in Michigan women
Year Number of Partition Correlation Reference
collected paired samples ratios coefficient
1976 10 1.07:1 0.88 Brilliant et al. (1978)
1975-80 24 1.1-1.5 :1 0.97 Eyster et al. (1983)
Incidentally, in contrast to PBBs, PCB levels (examined
additionally) were not significantly different in the maternal and
cord serum when compared on a fat basis (Jacobson et al., 1984). PBB
levels measured in children with known exposure to PBB in utero
and/or through breast-milk (see Table 38: Weil et al., 1981) also
indicate significant PBB transfer.
Table 59. Placental transfer of PBB: partitioning ratios of
2,2',4,4',5,5'-hexabromobiphenyl between fetal and maternal tissues
Year No.a Paired tissues Ratios Correlation Reference
collected coefficient
1976-77 13 maternal serum/ 7.04:1 Landrigan et al.
cord serum (1.5-10.3:1) (1979)
1975-80 58 cord serum/ 0.10-0.14:1 0.88 Eyster et al.
maternal serum (1983)
1975-80 56 placenta/ 0.10-0.17:1 0.85 Eyster et al.
maternal serum (1983)
Not 153 maternal serum/ 0.81b Jacobson et al.
specified cord serum (1984)
Not 107 maternal milk/ 0.39b Jacobson et al.
specified cord serum (1984)
a No. = Number of paired samples.
b Pearson product moment correlation.
Table 60. Mean PBB and lipid levels in cord serum and maternal serum and milka
No. Mean PBBb levels Mean lipid levels
Cord serum 230 0.3 µg/litre 3.24 g/litre
Maternal serum 205 1.7 µg/litre
206 6.181 g/litre
Maternal milk 138 3.6 µg/litre 0.029%
Maternal milk-fat 138 105.1 µg/kg
a From: Jacobson et al. (1984).
b Probably measured as concentration of 2,2',4,4',5,5'-hexabromobiphenyl.
The results of analysis of fat and thymus specimens from 2
infants, taken at autopsy, are shown in Table 61. The ratio of
thymus/fat "hexabromobiphenyl" concentrations, expressed as
percentage, were 13 and 37% (Corbett et al., 1978a).
Table 61. Fat and thymus concentrations of hexabromobiphenyl (HBB) in
3-day-old infants
HxBB concentration (mg/kg)
Fat Thymus
Patient I 0.091 0.012 (13.2%)
Patient II 0.062 0.023 (37.1%)
a From: Corbett et al. (1978a).
The distribution pattern of PBB congeners, other than
2,2'4,4',5'5-hexabromobiphenyl (BB 153), has been studied by Wolff
et al. (1979a). They examined the relative (to BB 153) distribution
of PBB congeners (penta- to heptabromobiphenyls) in the fat and
serum of Michigan chemical workers and farm residents, and found
different partition ratios for different homologues.
The distribution of PBBs among blood compartments was studied
by Bekesi et al. (1979a,b), Greaves et al. (1984), and Roboz et al.
(1980, 1985a,b). In in vitro models (PBBs added to blood), the
distribution of PBBs among plasma, erythrocytes, mononucleocytes
(white cell fraction) and polymorphonucleocytes (white cell
fraction) was found to be 89:9:< 1:< 1 (Roboz et al., 1985a,b).
When, however, the amount of PBBs per cell was considered, there was
an approximately 100-fold excess of PBBs in the white cell fractions
compared with the erythrocyte fraction (Roboz et al., 1985b). In
environmentally contaminated human blood, PBBs were also present in
higher concentrations per mg protein in lymphocytes than in
erythrocytes (Bekesi et al., 1979a,b,; Roboz et al., 1980; see also
Table 45).
It is thought that the relatively large amounts of PBBs
associated with the white blood cells are possibly the cause of the
immunological dysfunctions that result from exposure to PBBs (Roboz
et al., 1985b).
In serum, 20% of the PBBs were not bound to protein. The
remaining 80% were bound to apolipoproteins B and A in a 3:1 ratio
(Greaves et al., 1984). Roboz et al. (1985b) reported a ratio of
4:1, which was close to the ratio (by weight) of the lipid content
of these apolipoproteins. For comparison: the ratio between their
amino acid content was 1.6:1 (Roboz et al., 1985b). There was no
evidence of differential binding of various PBB congeners (penta-,
hexa-, heptabromobiphenyls) to any of the serum fractions (Greaves
et al., 1984; Roboz et al., 1985a,b).
6.3 Metabolic transformation
Indirect evidence for vertebrate metabolism of some PBB
congeners has been obtained by analysis of tissues from
experimentally and environmentally exposed animals and humans (see
section 6.5). While many PBB congeners tended to be persistent,
others were frequently absent or diminished. However, the changes in
the relative abundances can reflect differences in uptake,
distribution, and excretion among the congeners, as well as
differences in the metabolism (Moore et al., 1980).
There are several in vitro and in vivo methods for studying
the metabolism of PBB congeners and mixtures.
6.3.1 In vitro studies
Using in vitro techniques, hepatic microsomes from rats (or
rabbits: Kohli et al., 1978) were incubated with individual PBB
congeners or a PBB mixture, in the presence of nicotinamide adenine
dinucleotide phosphate (reduced) (NADPH) and atmospheric oxygen.
Most of the authors measured rates of disappearance of congeners
(Dannan et al., 1978b; Moore et al., 1980; Parkinson & Safe, 1982;
Millis et al., 1985a,b; Mills et al., 1985). A second approach was
to examine the incubation mixture for metabolites (Kohli et al.,
1978; Purdy & Safe, 1980; Safe et al., 1980; Sparling et al., 1980).
The initial studies (Dannan et al., 1978b; Moore et al., 1980)
revealed that only two of twelve PBB congeners from FireMaster(R)
were metabolized when incubated with microsomes isolated from rats,
pretreated with phenobarbital (PB) or PBB, namely
2,4,5,2',5'-pentabromobiphenyl and 2,3,6,2',4',5'-hexabromobiphenyl.
No metabolism could be observed with control microsomes or
microsomes from 3-methyl cholanthrene (MC)-treated rats. These and
other investigations performed with a number of PBB congeners
(Br1-Br7 = model congeners, FireMaster(R) components,
photolysis products of several hexabromobiphenyls) and with liver
microsomal enzymes induced by either PB or MC are summarized in
Table 62.
The results suggest that the rates of metabolism of PBB
congeners are dependent upon the positions of bromine and the type
of cytochrome induced (P-450:PB-induced; P-448:MC- induced).
The following structure-activity relationships have been
derived: PBB congeners that possessed adjacent non-brominated
carbons meta and para to the biphenyl bridge on at least one
ring were metabolized by PB-induced microsomes (Dannan et al.,
1978b; Moore et al., 1980; Mills et al., 1985). In one case, even
one free para position was reportedly sufficient for PB-induced
metabolism (Moore et al., 1980). Increasing bromination of PBB
congeners did not appear to prevent their metabolism (Moore et al.,
1980; Mills et al., 1985). Significant metabolism by MC- pretreated
microsomes required adjacent ortho and meta positions free of
bromines on at least one ring of lower substituted congeners (up to
Br4). Higher substituted congeners (Br5, Br6) were not
metabolized, though they fulfilled this criterion (Mills et al.,
1985).
In contrast to the studies mentioned above, Purdy & Safe (1980)
found that radiolabelled [3H]-2,2',4,4',5,5'-hexabromobiphenyl
(purity: > 98%) was metabolized in vitro by rat liver microsomal
enzymes. They determined polar, lipophilic metabolites after
incubation with control and PBB-induced microsomes, however, in
quantities that were much smaller than those yielded from
[3H]-4-bromobiphenyl.
Mono- and dihydroxylated derivatives have been identified as
major in vitro metabolism products of lower brominated PBBs (Kohli
et al., 1978; Purdy & Safe, 1980; Safe et al.; 1980, Sparling
et al., 1980; Parkinson & Safe, 1982).
Some PBB congeners may induce their own metabolism (Aust
et al., 1983), and some PBB congeners may influence the metabolism
of other PBB congeners (Purdy & Safe, 1980; Mills et al., 1985).
However, it should be noted that PBB congeners that induce
microsomal enzymes (see also section 8.8.1) are not necessarily
metabolized (Aust et al., 1983).
Some studies were carried out using AHH, specific DNA binding,
and 7-ethoxyresorufin assays. The results showed that PBBs (FM FF-1)
can induce in vitro cytochromes P450 IA (P448) in the primary
cultures of human, and newly-born rat, epidermal keratinocytes, and
of a rat hepatoma cell-line (Yao et al., 1991).
6.3.2 In vivo studies
In vivo metabolism studies included attempts to find and
identify PBB metabolites from intact animals. Hydroxylated
derivatives have been reported most commonly as metabolites of
vertebrates.
Table 62. Metabolism of PBB congeners with rat liver microsomes
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
4-MoBB Yes Yes Parkinson &
(172) (53.1) Safe (1982)
2,2'-DiBB Yes Yes Mills et al. (1985)
(0.33) (201.7)
Yes Moore et al. (1980)
(> 2100) Dannan et al. (1978b)
4,4'-DiBB - No Moore et al. (1980)
(< 0.02) Dannan et al. (1978b)
3,4,4'-TrBB Yes No Mills et al. (1985)
(100.7) (0.02)
2,4,2',4'-TeBB Yes No Mills et al. (1985)
2,4,2',5'-TeBB Yes Yes Mills et al. (1985)
(43.5) (184.6)
Yes Moore et al. (1980)
(24)
2,4,2',6'-TeBB Yes Yes Mills et al. (1985)
2,5,2',5'-TeBB No Yes Mills et al. (1985)
(0.0) (66.2)
Yes Moore et al. (1980)
(27)
2,6,2',6'-TeBB No Yes Mills et al. (1985)
2,3,3',4'-TeBB Yes Yes Mills et al. (1985)
(57.6) (52.8)
2,5,3',4'-TeBB Yes Yes Mills et al. (1985)
(49.7) (47.5)
3,4,3',4'-TeBB Yes No Mills et al. (1985)
(58.8) (0.01)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
3,4,3',5'-TeBB Yes No Mills et al. (1985)
3,5,3',5'-TeBB No No Mills et al. (1985)
(0.06) (0.0)
Yes Moore et al. (1980)
2,4,6,2',4'-PeBB No No Mills et al. (1985)
2,4,5,2',5'-PeBB No Yes Mills et al. (1985)
(0.02) (23.7)
No Dannan et al. Yes Moore et al. (1980)
(1978b) (13) Dannan et al. (1978b)
2,4,6,2',6'-PeBB No Yes Mills et al. (1985)
2,4,5,3',4'-PeBB No No Mills et al. (1985)
(0.05) (0.03)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.06) Dannan et al. (1978b)
3,4,5,3',4'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
3,4,5,3',5'-PeBB No Dannan et al. No Mills et al. (1985)
(1978b)
2,3,4,2',4',5'-HxBBB No No Mills et al. (1985)
(0.16) (0.0)
No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
2,3,6,2',4',5'-HxBBB No Dannan et al. Yes Moore et al. (1980)
(1978b) (19) Dannan et al. (1978b)
2,4,5,2',4',5'-HxBBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
Table 62 (contd).
Evidence for metabolisma
PBB congenerb MC Reference PB Reference
microsomesc microsomesd
2,3,4,5,3',4'-HxBBB No No Mills et al. (1985)
(0.11) (0.0)
No No Dannan et al. (1978b)
2,4,5,3',4',5'-HxBBB No No Dannan et al. (1978b)
3,4,5,3',4',5'-HxBBB No No Mills et al. (1985)
(0.20) 0.0)
2,3,4,5,2',4',5'-HpBB No Dannan et al. No Moore et al. (1980)
(1978b) (< 0.3) Dannan et al. (1978b)
a Measured as the rate of substrate disappearance (in parentheses: values measured
in pmol/min per mg protein).
b For expediency, a trivial numbering system was used.
c MC microsomes = 3-methylcholanthrene-induced microsomes.
d PB microsomes = phenobarbital-induced microsomes.
As can be seen from Table 63, administration of lower
brominated congeners resulted in low yields of, mainly, mono- or
dihydroxy metabolites. However, most of the studies compiled
suffered from the fact that the purities of the congeners used in
these studies were not determined (Moore et al., 1980). The two
studies with 2,2',4,4',5,5'-HxBB did not provide evidence of
significant metabolism (Table 63).
Various results were obtained after administering commercial
PBB mixtures. No hydroxylated PBBs could be detected in the urine of
cows given single 3-g doses of FireMaster(R) BP-6 (Willett &
Irving, 1976) or in the milk of cows (accidentally contaminated by
FireMaster(R) FF-1) with PBB residues as high as 900 µg/kg, on a
whole milk basis (Gardner et al., 1976). Approximately 1% of the
FireMaster(R) BP-6 administered to a pig was eliminated as an
unidentified pentabromobiphenylol (Kohli & Safe, 1976). It is not
clear whether this metabolite was formed from hexabromobiphenyl (by
reductive debromination followed by hydroxylation) or directly from
pentabromobiphenyl (by hydroxylation).
Table 63. PBB in vivo metabolites reported in the literature
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
Monobromobiphenyls
2-bromobiphenyl rabbit 1% 2'-bromo-4-biphenylol Kohli et al.
(urine) (1978)
traces mono-hycroxybromobiphenyl
rat (>) 2-bromo-4-4'-biphenyldiol Sparling et al.
(1980)
(>) 2-bromo-4-4'-biphenyldiol
(<) 2-bromo-4-biphenylol
3-bromobiphenyl rabbit 4% 3-bromo-4-biphenylol Kohli et al.
(urine) or 5-bromo-2-biphenylol (1978)
< 1% dihydroxybromobiphenyl
rat (>) 3-bromo-4,4'biphenyldiol Sparling et al.
(urine) and an unidentified diol (1980)
(<) monohydroxybromobiphenyls
4-bromobiphenyl rabbit 4% 4'-bromo-4-biphenylol Kohli et al.
(urine) 1.5% 4'-bromo-3,4-biphenyldiol (1978)
rat (>) 4'-bromo-4-biphenylos Sparling et al.
(urine) (<) mono- and dihydroxylated (1980)
species
pig 3% 4'-bromo-4-biphenylol Kohli &
(urine) Safe (1976)
traces monohydroxybromobiphenyl
0.5% 4'-bromo-3-methoxy-
4-biphenylol
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
chicken 12.2% 4'-bromo-4-biphenylol Jones et al.
(excreta) 9.8% 4'-bromo-3,4-biphenyldiol (1979)
(eggs) 0% no metabolites
Dibromobiphenyls
4,4'-dibromobiphenyl rabbit 10% 4,4'-dibromo-3-biphenylol Safe et
(urine) (1976)
1% 3,4'-dibromo-4-biphenylol
2% 4'-bromo-4-biphenylol
pig 5% 4,4'dibromo-3-biphenylol Kohli &
(urine) Safe (1976)
1% 3,4'-dibromo-4-biphenylol
traces dibromomethoxy-
biphenylol
1% 4'-bromo-3-methoxy-
4-biphenylol
traces dibromomethoxybiphenyl
Mixture of di-, fish Salmo dibromobiphenylol Zitko &
tri-, and tetra- salar (whole Hutzinger
bromobiphenyls animal) (1976)
Hexabromobiphenyls
2,2',4,4',5,5'- rat (urine traces "metabolites" Safe et al.
hexabromobiphenyl and faeces) (1978)
(purity: 95%)
Table 63 (contd).
Parent compound Species Yielda Reported References
(tissue, metabolites
etc.)
2,2',4,4',5,5'- rat 0% no metabolites Matthews et al.
hexabromobiphenyl (tissues) (1977)
(purity: 99%)
(bile and traces may be metabolites
faeces) (1-4%)
a (>) and (<) = major and minor components (no further quantitative information).
The faeces of dogs fed FireMaster(R) BP-6 contained a
metabolite identified as 6-hydroxy-2,2',4,4',5,5'-hexabromobiphenyl
(Gardner et al., 1979). However, the authors did not exclude
microbial metabolism in the dog's gut, because no
hydroxyhexabromobiphenyl was found in the liver of the dog, though
PBB was present. Matthews et al. (1979) mentioned that dogs were
able to metabolize 2,2',4,4',5,5'-hexachlorinated biphenyl, but were
unable to metabolize the analogous PBB at an appreciable rate.
Some investigations implied that fish may debrominate the more
highly brominated components of PBB mixtures. Juvenile Atlantic
salmon (Salmo salar) experimentally exposed to FireMaster(R)
BP-6, in water or in food, contained several mono- to
pentabromobiphenyls, not present in FireMaster(R) BP-6 (Zitko,
1977). Fish (Salmo salar) fed octabromobiphenyl (Dow
Chemical)-contaminated food contained unidentified penta-, hexa-,
and heptabromobiphenyls in addition to the octabromo biphenyls. It
was not known whether the partially debrominated biphenyls were
generated by the fish, or by the associated microflora (Zitko,
1977). Analyses of fish captured from natural waters may also
indicate the possibility of the debromination reaction in fish,
unless selective accumulation or elimination takes place (Stratton &
Whitlock, 1979). In fish from water mainly contaminated with
decabromobiphenyl, only the nona- and hexa bromobiphenyl congeners
were present. However, in fish from FireMaster(R)
BP-6-contaminated waters, only hexabromobiphenyl was detected.
6.3.3 Metabolic pathway
The most frequently reported route of PBB metabolism was
hydroxylation. However, according to Zitko & Hutzinger (1976), a
reductive debromination may be a degradative pathway of higher
brominated biphenyls, because the carbon-bromine bond is less stable
than, e.g., the carbon-chlorine bond.
The hydroxylation reaction probably proceeds via both arene
oxide intermediates (Safe et al., 1976, 1978, 1980; Kohli et al.,
1978; Moore et al., 1980) and by direct hydroxylation (Kohli et al.,
1978; Sparling et al., 1980; Moore et al., 1980). Schemes of
possible metabolic routes have been published by Matthews (1982) and
Safe (1989). The most important enzyme involved in the oxidation of
xenobiotics, such as PBBs, is aryl hydrocarbon hydroxylase. It is a
highly inducible, haem-containing mono oxygenase belonging to the
family of cytochromes P-450 (e.g., Safe et al., 1980). A (partial)
summary of the AHH-mediated metabolism is given by Safe et al.
(1978).
The formation of covalently bound macromolecular adducts has
been reported. Less than 10% of the metabolites were present in the
urine of rabbits in a bound form, e.g., as glucuronides (Safe
et al., 1976). In vitro metabolism also resulted in macromolecular
conjugates (Kohli et al., 1978; Purdy & Safe, 1980; Safe et al.,
1980; see also section 6.6). In general, the biotransformation of
PBBs is a slow process, but the stereochemistry and molecular size
vary so widely among PBBs that there are great differences in their
metabolic activity.
6.4 Elimination and excretion in expired air, faeces, urine
6.4.1 Animal studies
Elimination of PBBs from the body has been studied using hexa-
and octabromobiphenyl. No information is available on
decabromobiphenyl. Some data found in the literature (see also
section 6.1) are compiled in Table 64 (14C-labelled single doses),
Table 65 (14C-labelled multiple doses), and Table 66 (daily
feeding).
The only study conducted with octabromobiphenyl resulted in a
much higher elimination from rats than was found for HxBB in another
study on rats (Table 64). However, there are also reports on the
rapid elimination of HxBB, e.g., by Willett & Irving (1976), who
found a 50% recovery of HxBB after 168 h in the faeces of two cows
given single intraruminal doses (3 g) of FireMaster(R) BP-6.
However, relatively high concentrations of radioactivity or of HxBB,
found, in some cases, in the faeces during the first few days after
dosing or during daily dosing, may have been due to incomplete
absorption (Tuey & Matthews, 1980). Approximately 60% of the total
dose was also recovered in the faeces of rhesus monkeys (Rozman
et al., 1982: Table 64).
Elimination of PBBs was primarily via the bile and the
intestine into the faeces and, in general, was found to be a slow
process.
Biliary concentrations have been measured in a Rhesus monkey
(Rozman et al., 1982), in rats (Matthews et al., 1977), and in
cattle (Willett & Irving, 1976; Willett & Durst, 1978). In rats,
excretion of HxBB in the bile accounted for 0.68% of the total PBB
dose between 0 and 4 h after intravenous (iv) administration.
Twenty- four hours after an iv dose, 0.032% of the total dose was
excreted in the bile in 1 h, and, 7 or 42 days after dosing
concentrations were too low to quantify (Matthews et al., 1977).
Because of this small amount cleared with the bile, enterohepatic
recirculation of HxBB in rats is not important (Tuey & Matthews,
1980). Concen trations of HxBB in the bile of cattle were two-three
times greater than the concentration in the plasma (Willett &
Irving, 1976; Willett & Durst, 1978).
According to Fries (1985b), excretion of PBB in the urine is
not expected, because of the insolubility of PBBs in water. He
attributed the few instances in which low concentrations of PBBs
were reported to cross-contamination with faeces. On the other hand,
this route of excretion may account for minor or metabolized
biphenyls (Damstra et al., 1982; see also Table 63). However,
excretion of PBB metabolites may be of minor relevance, since the
more abundant PBB congeners are not, or only slightly, metabolized
(see also section 6.3). The amounts of urinary or faecal metabolites
were low. A study on a pig that received a single intraperitoneal
(ip) dose of FireMaster(R) BP-6 (100 mg/kg body weight) reported a
yield of about 1% of pentabromobiphenylol in the urine and faeces,
collected for 7 days (Kohli & Safe, 1976). The level of a hydroxy
metabolite detected in the faeces of dogs fed FireMaster(R) BP-6
was about an order of magnitude less than the PBB levels (Gardner
et al., 1979). Yields of lower brominated urinary PBB metabolites
are compiled in Table 63.
In their study on cows, Willett & Durst (1978) did not find any
direct relationships between the amount of PBBs fed and the
concentration in the faeces. In contrast, Babish et al. (1975a) did
find a linear correlation (r > 0.97) of dietary PBBs with excreta
residues in Japanese quails. In another study on cows, it was also
reported that relationships were approximately constant (Robl
et al., 1978: Table 66).
Table 64. Faecal, urinary, and exhalative elimination of 14C activity as a percentage of a single dose of [14C-]PBB
Species Agent; solvent; dose (mg/kg Time % recovery in: Reference
body weight); route faeces urine expired air
Rhesus monkey (male) [14C-]HxBB in mineral oil; 2, oral 5 days 38 (0.18) - Rozman et al.
(1982)
Rat (male) [14C-]HxBB in Emulphor EL-620: - Matthews et al.
ethanol: water (1:1:8) 24 h 7.9 (1977); Tuey &
1, oral 24 h 0.96 Matthews
1, intravenous 7 days 3.28 < 0.1 (1978, 1980)
1, intravenous 42 days 6.6 not detected
Rat (male and female) [14C-]OcBBa in corn oil; 1, oral 24 h 62 Norris et al.
48 h 69 (1973)
16 days 73 < 1 < 1
Mink (Mustela vison) [14C-]PBBb in propylene glycol Bleavins et al.
(pregnant female) (1 µCi in 0.1 ml) intravenous 2 h - 0.003 - (1981)
Ferret (Mustela 2 h - 0.004
puttorius (furo)
(pregnant female)
Dog [14C-]2,2',4,4',5,5'-hexabromo 25 days 8 Sipes et al.
biphenyl (solvent not specified) (1979)
0.6, intravenous
a OcBB = technical octabromobiphenyl.
b Consisted of 2,2', 4,4', 5,5'-HxBB and 2,2', 3, 4,4', 5,5'-HpBB.
Table 65. Urinary and faecal elimination of HxBB and/or metabolites in male Rhesus monkeys and male rats,
dosed repeatedly with [14C-]HxBB
Species Dose (mg/kg body Days after Urine Faeces Cumulative % Reference
weight) first dose (µg/kg per (µg/kg per recovery of
day) day) total dose
in faeces
Monkey 50 1-10 3.2 5890 ca 60% Rozman et al. (1982)
(No. = 2) in methyl cellulose; 11-17 2.5 5.0
oral on days 1 and 5 203-209 not detectable 3.5
Rat 1 7 ca 14% Matthews et al. (1977)
(No. = 3) in corn oil; oral on
days 1, 2, 3, and 4
Table 66. Elimination of HxBB (2,2',4,4',5,5'-hexabromobiphenyl) in faeces and urine during the feeding of FireMaster(R)-mixtures
Species Intake of FireMaster Sampling Concentration in: Approximate % Reference
(sex) BP-6 or FF-1 time (mg/kg) recovery in
Faeces Urine faeces
Dog BP-6: in corn oil (capsule) last day of 7 Gardner et al.
(female) 1 mg/kg body weight per day dosing (1979)
for 6 weeks
Pig BP-6: in corn oil at week 4 Ku et al. (1978)
20 mg/kg diet 21.3a 0.015
200 mg/kg diet 182.0a 0.07
(ad libitum)
for > 4 weeks
Cow FF-1: in gelatin capsules; Robl et al. (1978)
for 90 days
0.1 mg/kg diet 0.02 mostly 15% of
1.0 mg/kg diet 0.15 n.d.b ingested
10 mg/kg diet 1.5 dose
Calf BP-6:(capsule); 25 g daily total 8045 5% of total Willet & Irving
(male) for 9 days collection dose (1976)
Hen BP-6: 20 mg/kg in the diet weekly 9% of daily Fries et al.
for 63 days dose (1976)
Hen FF-1: not specified not specified 11% of daily Ringer &
dose Polin (1977)
a Faecal samples oven-dried.
b nd = Not detected (detection limit = 0.005 mg/kg).
Following withdrawal of PBBs (FireMaster(R) BP-6) from the
diet of cows, the faecal concentrations of HxBB declined to 1-2% of
faecal levels during dosing (Willett & Durst, 1978) and to less than
5% in hens (Fries et al., 1976). Faecal concentrations were small in
relation to body burden. Cows that received 250 mg FireMaster(R)
BP-6 daily had HxBB concentration ratios in body fat to faeces of
about 750 : 1. Their faeces to plasma ratio was 0.7 :1 (Willett &
Durst, 1978). Cows environmentally contaminated (FireMaster(R)
FF-1) 7-9 months before examination had comparable body fat to
faeces ratios, but a different faeces to blood ratio of 4.2 : 1
(Detering et al., 1975; Cook et al., 1978b; Fries et al., 1978a).
Post-exposure lactating cows eliminated via milk fat three times the
quantity of HxBB cleared in faeces (Willett & Durst, 1978).
There have been some studies on the means to enhance the
elimination of PBBs. PBBs used were: FireMaster(R) FF-1 (Cook
et al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980; Polin
& Leavitt, 1984; Polin et al., 1985) and [14C-]HxBB (Rozman
et al., 1982). The treatments included activated carbon in rats
(McConnell et al., 1980) and cows (Cook et al., 1978b),
cholestyramine in rats (McConnell et al., 1980) and monkeys (Rozman
et al., 1982), colestipol in chickens (Polin & Leavitt, 1984; Polin
et al., 1985), mineral oil in rats (Kimbrough et al., 1980), monkeys
(Rozman et al., 1982), and chickens (Polin et al., 1985), high-fibre
diets in rats (Kimbrough et al., 1980), and phenobarbital in cows
(Cook et al., 1978b). The effects of restricted caloric intake,
alone, or in combination with other treatments, was investigated in
rats (McConnell et al., 1980) and chickens (Polin & Leavitt, 1984;
Polin et al., 1985). The procedures were found not to be (Cook et
al., 1978b; Kimbrough et al., 1980; McConnell et al., 1980), or to
be only partially, effective (Rozman et al., 1982; Polin & Leavitt,
1984; Polin et al., 1985) in reducing the body burden of PBBs
(measured as concentrations of HxBB, total bromine levels, or
14C-activity).
6.4.2 Human studies
The concentrations of PBBs in human bile and faeces represent a
minor proportion of the total body burden, as has been demonstrated
by Eyster et al. (1983), who determined HxBB levels in Michigan farm
and chemical workers in 1975-80. Concen trations of HxBB observed in
the bile and faeces were about 1/2 to 7/10 of the serum levels (on a
whole-weight basis) and were estimated to be approximately 0.5% of
the adipose tissue levels (Table 67).
These findings are consistent with the theoretical predictions
of Tuey & Matthews (1980), who calculated slow rates of faecal
excretion in humans. In addition, these authors showed that the
excretion rate in lean individuals exposed to HxBB would be higher
than those in overweight individuals.
6.5 Retention and turnover
6.5.1 Animal studies
The time course of PBB tissue concentrations has been studied
predominantly in rats, and, to a lesser extent, in cattle, chickens,
and guinea-pigs (Table 68). Incomplete data are available for mice,
pigs, and monkeys. With the exception of 3 older studies (Norris
et al., 1973; Lee et al., 1975a; Waritz et al., 1977), in which the
behaviour of technical octabromobiphenyl in rats was observed, the
majority of investigators used the FireMaster(R)- mixture (BP-6 or
FF-1). Of the individual PBB congeners,
2,2',4,4',5,5'-hexabromobiphenyl (Matthews et al., 1977; Millis
et al., 1985b) and 3,3',4,4'-tetrabromobiphenyl (Millis et al.,
1985b) were administered.
Table 67. Medians, range, and geometric means of 2,2',4,4',5,5'-
hexabromobiphenyl (HxBB) for paired specimens of serum, faeces, and bile
obtained from farm and chemical workersa
Number Paired Median Rangeb Geometric
specimen mean
51 serum 7 µg/litre 1-1540 µg/litre 14.4 µg/litre
faeces 5 µg/kg nd-862 µg/kg 9.0 µg/kg
20 serum 3.5 µg/litre 1-153 µg/litre 4.2 µg/litre
biliary fluid 2 µg/litre nd-70 µg/litre 2.7 µg/litre
a From: Eyster et al. (1983).
b nd = Not detectable (detection limit: 1 µg/kg or µg/litre).
Complex and varied relationships were found in tissue
concentrations with time after PBB administration (see also Tables
47, 48, and 49).
Table 68. Reported biological half-lifes of PBBs in mammals and birds, after single or repeated exposure
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Rhesus Monkey (male) [14C-]HxBB (50 mg/kg body weight body > 4 years Rozman et al.
on days 1 and 5; oral) 209 days (1982)
Rat (male) [14C-]OcBB (1 mg/kg body weight; faeces < 24 h (1st phase) Norris et al.
single dose; oral) 16 days > 16 days (2nd phase) (1973)
Rat [14C-]HxBB (1 mg/kg body weight; faeces 2 days (1st phase)c Birnbaum
single iv dose) 42 days (1985)
"tissues" ca 24 daysc Ecobichon et al.
(blood, liver (1983)
muscle, skin)
Rat (male) FM BP-6 (1 mg/100 g body weight; serum 23.1 weeks Miceli &
single ip dose; 36 weeks fat 69.3 weeks Marks (1981)
adrenal 43.3 weeks
brain 63.0 weeks (2nd phase)
liver 11.5 weeks (2nd phase)
lung 11.2 weeks
spleen 9.0 weeks
Rat (male) FM FF-1 (10 mg/kg body weight; whole blood 3.27 h (') Domino et al.
single dose; oral) 112 days 33.3 h (ý) (1982)
145 days (')
Guinea-pig (lactating FM FF-1 (50 mg/kg body weight; tissues (fat, ca 22 days Ecobichon
females and pups) single dose; oral) 60 days liver, kidney, et al. (1983)
lung) of both
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Cow FM BP-6 (fed 10 mg/day for milk fat ca 58 days Fries &
60 days) 60 days (of withdrawal) (2nd phase) Marrow (1975)
Cow FM BP-6 (fed 1.13 g/day for milk 10.5 days Gutenmann &
15 days; = 50 mg/kg in the diet) Lisk (1975)
15 days (of withdrawal)
Cow FM FF-1 (environmentally milk fat ca 60 days Fries et al.
contaminated 1 year earlier; (range: 36-301 days) (1978a); Cook
6 months of observation) et al. (1978b)
Cow FM BP-6 (fed 0.25 mg-25 g/day milk fat > 6 monthsd Fries
for various periods) up to (body) (1985b)
3 years of observation
Chicken (White FM FF-1 (fed in the diet) egg 17 days Ringer &
Leghorn hens) 28 days (of withdrawal) Polin (1977)
Chicken (White FM FF-1 (fed 0.2-125 mg/kg egg 17 days Polin &
Leghorn hens) in the diet for 5 weeks) Ringer (1978a)
56 days (of withdrawal)
(fed 1125 and 625 mg/kg in liver 31 days
the diet for 5 weeks)
56 days (of withdrawal)
(fed 5-625 mg/kg in the diet muscle 17 days
for 5 weeks) 56 days
(of withdrawal)
Table 68 (contd).
Species PBBa (Dosing regimen) Elimination Calculated half-lifeb References
(sex) Observation period from: (Kinetic phases)
Chicken (White FM BP-6 (fed 20 mg/kg in the egg 28 days Fries et al.
Leghorn hens) diet for 63 days) 49 days (2nd phase) (1976)
(of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg egg 112 days Cecil &
Leghorn hens) in the diet for 8 weeks) (late phase) Bitman (1978)
266 days (of withdrawal)
Chicken (White FM BP-6 (fed 20 and 64 mg/kg excreta 4-5 dayse Polin &
Leghorn hens) in the diet for 8 weeks) Leavitt (1984)
266 days (of withdrawal)
a HxBB = 2,2',4,4',5,5'-hexabromobiphenyl; OcBB = octobromobiphenyl (technical mixture);
FM BP-6 = FireMaster(R) BP-6; FM FF-1 = FireMaster(R) FF-1.
b Referring to 2,2',4,4',5,5'-hexabromobiphenyl or [14C-]activity.
c Half-life calculated from data of Matthews et al. (1978); see also Fig. 6.
d Half-life calculated from data of Willett & Durst (1978).
e Half-life calculated from data of Fries et al. (1976).
6.5.1.1 Time trends, retention: 2,2',4,4',5,5'-hexabromobiphenyl
(BB 153)
a) Rat
When rats dosed with 2,2',4,4',5,5'-hexabromobiphenyl (Matthews
et al., 1977; Tuey & Matthews, 1980) or with FireMaster(R) FF-1
(Kimbrough et al., 1978; Domino et al., 1980b; 1982), BB 153
concentrations in the blood were highest immediately after dosing,
but fell rapidly during the first day (as PBBs are taken up from
blood by the liver and muscle tissues, which are highly perfused
tissues). Then, concentrations in the blood, liver, and muscle
declined less quickly (as the dose was redistributed to the adipose
tissue; Tuey & Matthews, 1980; Fries, 1985b), finally decreasing
only very slowly. After a single intravenous dose of BB 153 (1 mg/kg
body weight), adipose tissue contained more than 60% of the total
body burden within four days (Tuey & Matthews, 1980; see Table 50).
BB 153 concentrations in the adipose tissue peaked later than
in other tissues and remained high throughout the period of
observation (6 weeks: Matthews et al., 1977; 16 weeks; Domino
et al., 1980b; 36 weeks: Miceli & Marks, 1981). For example, ratios
between fat and serum levels in rats rose from 221 : 1 to 722 : 1
between 6 and 36 weeks after a single dose exposure (Miceli & Marks,
1981), reflecting the much more rapid clearance of BB 153 from serum
than from fat.
Kinetic models to describe the principal toxicokinetics of BB
153 in the rat were constructed by Tuey & Matthews (1980; a blood
flow-limited physiological compartmental model) and by Domino et al.
(1982). The latter developed a three-compartment model. Tissues
within a compartment showed similar kinetic characteristics, but
concentrations could vary widely. Compartment 1 consisted of whole
blood, spleen, kidney, and heart. Compartment 2 included liver,
lung, cerebral grey and white matter, cerebellum, and testes, and
compartment 3 consisted of subcutaneous fat. Jejunum could not be
classified.
However, results of Miceli & Marks (1981) (PBB levels monitored
over longer periods) do not fit in well with this scheme. For
example, these authors observed typical first-order-elimination
kinetics of BB 153 in the serum of rats, but kinetics of disappear
ance from heart and kidney (and pituitary) do not appear to be first
order, though belonging to the same compartment (according to Domino
et al., 1982). BB 153 concentrations in the brain and liver (both
"compartment 2") declined rapidly during the interval from 6-12
weeks after exposure, but, thereafter, (from week 12 to 36) brain
concentrations fell far more slowly than those of the liver. The
study of Miceli & Marks (1981) showed that the (long-term)
retention, but not always the concentration, of BB 153 in lipid-rich
tissues (brain, adrenal, adipose) was much greater than in most
other tissues (see also Table 68).
Corresponding to the small decline in BB 153 from the tissues
of rats, the elimination rates in the faeces were slow (see Fig. 6).
Less than 7% of the dose was eliminated 42 days after a single iv
dose, most, during the first 3-4 days (Tuey & Matthews, 1980).
 The kinetic models or calculations for BB 153 were based on the
results of studies on adult male rats. In maternal rats the
situation is more complex, and only a little information is
available. For example, BB 153 levels in the liver and fat of
maternal rats decreased during a period from the end of lactation
(and exposure) until 14 weeks later (decline in fat: 50%), but BB
153-concentrations in the mammary glands increased (5 times higher)
during the same period (see also Table 48), indicating
redistribution of BB 153 during the recovery period following
lactation (McCormack & Hook, 1982).
During continued exposure to FireMaster(R) BP-6, BB 153
concentrations in the milk of lactating rats were determined 0, 3,
5, 7, and 14 days following parturition and showed a decline from
180 mg/litre (via 115, 89, 63 mg/litre) to 50 mg/litre (McCormack
et al., 1979a).
The kinetics of BB 153 in growing animals have not been
frequently investigated. The differences in BB 153 levels and organ
weights have been reported only between two ages. McCormack et al.
(1980) showed that at 328 days of age, the concentrations of BB 153
in the liver, kidney, and fat of rats were approximately 5, 10, and
25% of the respective tissue concentrations at weaning (28 days of
age; cessation of exposure). Another long-term study (Groce &
Kimbrough, 1984) compared BB 153 levels in the livers of perinatally
exposed rats at the age of 2 months and 2 years and also found
diminished values (see Table 52).
Comparing BB 153 concentrations in the blood and adipose tissue
of rats after 10 and 14 months recovery resulted in no "true"
decrease (Kimbrough et al., 1978). Other studies also indicated a
long retention of PBBs in the brain (Geller et al., 1979), thyroid,
and liver (Allen-Rowlands et al., 1981), and in the adrenal glands
(Castracane et al., 1982).
b) Other species
The species, the second most often examined, was cattle, but
the kinetic models used for cattle (e.g., Fries et al., 1978a) were
less sophisticated than those described for rats (Fries, 1985b).
In cows given FireMaster(R) BP-6, the BB 153 concentration in
blood plasma was maximal 24 h after exposure (Willett & Irving,
1976; Willett & Durst, 1978). When multiple doses were administered,
plasma concentrations were at equilibrium by 15 days. When dosing
was terminated, concentrations declined approximately 50% in 10 days
and 66% by day 20. Thereafter, plasma residues did not fit a
consistent decline model (Willett & Durst, 1978).
While BB 153 was detectable in plasma within 2-4 h of exposure
(Willett & Durst, 1978), it was detected in the milk of cows 13 h
after exposure (Willett & Irving, 1976). With continued exposure, a
steady state of BB 153 concentrations in milk fat was reached after
20-40 days (Fries & Marrow, 1975; Willett & Durst, 1978; Fries
et al., 1978a; Robl et al., 1978). When the feeding of PBBs was
stopped, concentrations in milk fat declined rapidly for a short
time (Fries & Marrow, 1975). When a new equilibrium was established,
milk fat and body fat concentrations declined in a parallel manner
(Fries, 1985b). An example of the biphasic decline is given in Fig.
7. However, as Fries et al. (1978a) observed, the stage of lactation
influenced the rate of elimination, and, in some cases, BB 153
levels in milk increased shortly after calving.
The kinetic models or calculations for BB 153 were based on the
results of studies on adult male rats. In maternal rats the
situation is more complex, and only a little information is
available. For example, BB 153 levels in the liver and fat of
maternal rats decreased during a period from the end of lactation
(and exposure) until 14 weeks later (decline in fat: 50%), but BB
153-concentrations in the mammary glands increased (5 times higher)
during the same period (see also Table 48), indicating
redistribution of BB 153 during the recovery period following
lactation (McCormack & Hook, 1982).
During continued exposure to FireMaster(R) BP-6, BB 153
concentrations in the milk of lactating rats were determined 0, 3,
5, 7, and 14 days following parturition and showed a decline from
180 mg/litre (via 115, 89, 63 mg/litre) to 50 mg/litre (McCormack
et al., 1979a).
The kinetics of BB 153 in growing animals have not been
frequently investigated. The differences in BB 153 levels and organ
weights have been reported only between two ages. McCormack et al.
(1980) showed that at 328 days of age, the concentrations of BB 153
in the liver, kidney, and fat of rats were approximately 5, 10, and
25% of the respective tissue concentrations at weaning (28 days of
age; cessation of exposure). Another long-term study (Groce &
Kimbrough, 1984) compared BB 153 levels in the livers of perinatally
exposed rats at the age of 2 months and 2 years and also found
diminished values (see Table 52).
Comparing BB 153 concentrations in the blood and adipose tissue
of rats after 10 and 14 months recovery resulted in no "true"
decrease (Kimbrough et al., 1978). Other studies also indicated a
long retention of PBBs in the brain (Geller et al., 1979), thyroid,
and liver (Allen-Rowlands et al., 1981), and in the adrenal glands
(Castracane et al., 1982).
b) Other species
The species, the second most often examined, was cattle, but
the kinetic models used for cattle (e.g., Fries et al., 1978a) were
less sophisticated than those described for rats (Fries, 1985b).
In cows given FireMaster(R) BP-6, the BB 153 concentration in
blood plasma was maximal 24 h after exposure (Willett & Irving,
1976; Willett & Durst, 1978). When multiple doses were administered,
plasma concentrations were at equilibrium by 15 days. When dosing
was terminated, concentrations declined approximately 50% in 10 days
and 66% by day 20. Thereafter, plasma residues did not fit a
consistent decline model (Willett & Durst, 1978).
While BB 153 was detectable in plasma within 2-4 h of exposure
(Willett & Durst, 1978), it was detected in the milk of cows 13 h
after exposure (Willett & Irving, 1976). With continued exposure, a
steady state of BB 153 concentrations in milk fat was reached after
20-40 days (Fries & Marrow, 1975; Willett & Durst, 1978; Fries
et al., 1978a; Robl et al., 1978). When the feeding of PBBs was
stopped, concentrations in milk fat declined rapidly for a short
time (Fries & Marrow, 1975). When a new equilibrium was established,
milk fat and body fat concentrations declined in a parallel manner
(Fries, 1985b). An example of the biphasic decline is given in Fig.
7. However, as Fries et al. (1978a) observed, the stage of lactation
influenced the rate of elimination, and, in some cases, BB 153
levels in milk increased shortly after calving.
 Data from Detering et al. (1975) indicated that BB 153 levels
in cow's milk would decrease from 200-400 mg/kg in fat to 0.3 mg/kg
in 120 weeks.
Information on a time course of PBB levels in the body fat of
cows is limited. According to Willett & Durst (1978), e.g., BB 153
concentrations in the subcutaneous fat of cows had declined by 40%,
20 days after exposure. Two measurements, one at parturition (ca.
150 days later) and one after heavy lactation (ca. 200 days later)
showed an increase in residues. During late lactation, significant
declines occurred.
Probably, a multicompartment system is necessary to describe
the long-term behaviour of PBBs in lactating cows, with special
trends when pregnancy, parturition, etc. occur (Willett & Durst,
1978; Fries et al., 1978a; Fries, 1985b).
The appearance and decline of BB 153 in faeces were reported
for two cows given a single intraruminal dose of FireMaster(R)
BP-6 (Willett & Irving, 1976) and for cows fed BP-6 daily for 60
days (Willett & Durst, 1978). In the first case, BB 153 was detected
12 h after administration, peaked between 20 and 36 h, and declined
to about 2% of the peak concentration during the subsequent 48 h. By
day 8, approximately 50% of the dose was detectable in the faeces
(Willett & Irving, 1976). During daily exposure, BB 153
concentrations in the faeces reached a steady state by day 10. After
withdrawal of the PBBs, the levels of BB 153 declined within 10 days
to approximately 1% of the concen tration present during exposure
(Willett & Durst, 1978). Fries (1985b) inferred low rates of
elimination in faeces, because faecal BB 153 concentrations in
cattle that were no longer being exposed to PBBs were relatively low
(Willett & Durst, 1978; Fries et al., 1978a).
Another domestic species in which the kinetics have been
examined is the chicken. Whole carcass analysis of male White
leghorn chickens showed that, during the 2 weeks that
FireMaster(R) FF-1 was fed at 0.1 or 1.0 mg/kg, the chickens
retained 88 and 69%, respectively, of the FF-1 that was consumed
(Polin & Leavitt, 1984). Withdrawal rates were determined in a
similar study on egg- and meat-type chickens fed diets containing 1
or 10 mg FF-1/kg. Body burdens of BB 153 in chickens, previously fed
10 mg/kg, did not decrease significantly during a withdrawal period
of 42 days (e.g., 3% loss by day 21 of withdrawal). In contrast,
chickens, previously fed 1 mg/kg, eliminated up to 40% of the BB 153
(Polin et al., 1985).
Withdrawal of PBBs from the adipose tissue of laying hens fed
FireMaster(R) FF-1 at different dietary levels (0.2, 1, 5, 25,
125, or 625 mg/kg) has been followed by Polin & Ringer (1978a). BB
153 levels remained unchanged over the 56 days of withdrawal. Lillie
et al. (1975) calculated a 50% reduction after more than 16 weeks.
Withdrawal from other tissues and from eggs was more rapid (Ringer &
Polin, 1977; Polin & Ringer, 1978b).
BB 153 levels in eggs laid by hens fed 20 or 64 mg
FireMaster(R) BP-6/kg diet for 8-9 weeks reached a plateau by the
third or fourth week of feeding. When feeding of BP-6 stopped,
residues decreased in a two-phase rate pattern with a phase of rapid
decline shortly after exposure had ceased and a late phase of slow
decrease (Fries et al., 1976; Cecil & Bitman, 1978). In one study
(Fries et al., 1976), the levels after 49 days were approximately
10% of the values on day 0 of cessation; in the other study by Cecil
& Bitman (1978), detectable amounts were still present 33 weeks
after withdrawal of BP-6 from the diet.
Excreta were analysed from hens fed 20 mg FireMaster(R)
BP-6/kg for 63 days (Fries et al., 1976). After an initial rise and
decline, the BB 153 levels remained fairly constant (at about
2 mg/kg on a wet weight basis) during the feeding of PBBs. After
withdrawal of PBBs, the residues dropped to a negligible level
(< 0.1 mg/kg).
Only some milk data during continued exposure are available for
pigs. Milk of sows having received 10, 100, or 200 mg of
FireMaster(R) BP-6/kg feed during the second half of gestation and
during lactation was monitored until the 4th week. On a fat basis,
concentrations of BB 153 were highest in the colostrum and decreased
slowly during lactation (Werner & Sleight, 1981).
Disappearance of BB 153 from the tissues of lactating
guinea-pigs and of their pups was described by Ecobichon et al.
(1983). The maternal animals had received a single oral dose of
FireMaster(R) FF-1 (50 mg/kg body weight) within 6-12 h of
parturition, and the residue levels of nursing young and the dams
were measured up to 60 days after exposure (at intervals of 2, 4, 7,
14, 28, 42, and 60 days: see Fig. 8). Following the initial 7 days
during which there was obvious inter-tissue transportation and
sequestration in body fat, a gradual, but similar, linear rate of
decline was observed in the livers, kidneys, and lungs of both the
young and their dams. A similar rate of reduction was observed in
the body fat of both pups and dams.
In a study on mice, BB 153 residue levels were measured 6 h
after dietary intake of 100 mg/kg. FireMaster(R) BP-6 (for 14
days) can be compared with those obtained 14 weeks after feeding.
There was a decline in all tissues including fat (see Table 48),
however, to a different extent, resulting in changes in the relative
BB 153-concentrations between tissues (Corbett et al., 1978a).
BB 153 blood levels of rhesus monkeys, dosed with BB 153,
markedly declined over time (day 5: 1.5 mg/kg, day 11: 0.2 mg/kg;
day 25 < accurate measurement levels) (Rozman et al., 1982).
Data from Detering et al. (1975) indicated that BB 153 levels
in cow's milk would decrease from 200-400 mg/kg in fat to 0.3 mg/kg
in 120 weeks.
Information on a time course of PBB levels in the body fat of
cows is limited. According to Willett & Durst (1978), e.g., BB 153
concentrations in the subcutaneous fat of cows had declined by 40%,
20 days after exposure. Two measurements, one at parturition (ca.
150 days later) and one after heavy lactation (ca. 200 days later)
showed an increase in residues. During late lactation, significant
declines occurred.
Probably, a multicompartment system is necessary to describe
the long-term behaviour of PBBs in lactating cows, with special
trends when pregnancy, parturition, etc. occur (Willett & Durst,
1978; Fries et al., 1978a; Fries, 1985b).
The appearance and decline of BB 153 in faeces were reported
for two cows given a single intraruminal dose of FireMaster(R)
BP-6 (Willett & Irving, 1976) and for cows fed BP-6 daily for 60
days (Willett & Durst, 1978). In the first case, BB 153 was detected
12 h after administration, peaked between 20 and 36 h, and declined
to about 2% of the peak concentration during the subsequent 48 h. By
day 8, approximately 50% of the dose was detectable in the faeces
(Willett & Irving, 1976). During daily exposure, BB 153
concentrations in the faeces reached a steady state by day 10. After
withdrawal of the PBBs, the levels of BB 153 declined within 10 days
to approximately 1% of the concen tration present during exposure
(Willett & Durst, 1978). Fries (1985b) inferred low rates of
elimination in faeces, because faecal BB 153 concentrations in
cattle that were no longer being exposed to PBBs were relatively low
(Willett & Durst, 1978; Fries et al., 1978a).
Another domestic species in which the kinetics have been
examined is the chicken. Whole carcass analysis of male White
leghorn chickens showed that, during the 2 weeks that
FireMaster(R) FF-1 was fed at 0.1 or 1.0 mg/kg, the chickens
retained 88 and 69%, respectively, of the FF-1 that was consumed
(Polin & Leavitt, 1984). Withdrawal rates were determined in a
similar study on egg- and meat-type chickens fed diets containing 1
or 10 mg FF-1/kg. Body burdens of BB 153 in chickens, previously fed
10 mg/kg, did not decrease significantly during a withdrawal period
of 42 days (e.g., 3% loss by day 21 of withdrawal). In contrast,
chickens, previously fed 1 mg/kg, eliminated up to 40% of the BB 153
(Polin et al., 1985).
Withdrawal of PBBs from the adipose tissue of laying hens fed
FireMaster(R) FF-1 at different dietary levels (0.2, 1, 5, 25,
125, or 625 mg/kg) has been followed by Polin & Ringer (1978a). BB
153 levels remained unchanged over the 56 days of withdrawal. Lillie
et al. (1975) calculated a 50% reduction after more than 16 weeks.
Withdrawal from other tissues and from eggs was more rapid (Ringer &
Polin, 1977; Polin & Ringer, 1978b).
BB 153 levels in eggs laid by hens fed 20 or 64 mg
FireMaster(R) BP-6/kg diet for 8-9 weeks reached a plateau by the
third or fourth week of feeding. When feeding of BP-6 stopped,
residues decreased in a two-phase rate pattern with a phase of rapid
decline shortly after exposure had ceased and a late phase of slow
decrease (Fries et al., 1976; Cecil & Bitman, 1978). In one study
(Fries et al., 1976), the levels after 49 days were approximately
10% of the values on day 0 of cessation; in the other study by Cecil
& Bitman (1978), detectable amounts were still present 33 weeks
after withdrawal of BP-6 from the diet.
Excreta were analysed from hens fed 20 mg FireMaster(R)
BP-6/kg for 63 days (Fries et al., 1976). After an initial rise and
decline, the BB 153 levels remained fairly constant (at about
2 mg/kg on a wet weight basis) during the feeding of PBBs. After
withdrawal of PBBs, the residues dropped to a negligible level
(< 0.1 mg/kg).
Only some milk data during continued exposure are available for
pigs. Milk of sows having received 10, 100, or 200 mg of
FireMaster(R) BP-6/kg feed during the second half of gestation and
during lactation was monitored until the 4th week. On a fat basis,
concentrations of BB 153 were highest in the colostrum and decreased
slowly during lactation (Werner & Sleight, 1981).
Disappearance of BB 153 from the tissues of lactating
guinea-pigs and of their pups was described by Ecobichon et al.
(1983). The maternal animals had received a single oral dose of
FireMaster(R) FF-1 (50 mg/kg body weight) within 6-12 h of
parturition, and the residue levels of nursing young and the dams
were measured up to 60 days after exposure (at intervals of 2, 4, 7,
14, 28, 42, and 60 days: see Fig. 8). Following the initial 7 days
during which there was obvious inter-tissue transportation and
sequestration in body fat, a gradual, but similar, linear rate of
decline was observed in the livers, kidneys, and lungs of both the
young and their dams. A similar rate of reduction was observed in
the body fat of both pups and dams.
In a study on mice, BB 153 residue levels were measured 6 h
after dietary intake of 100 mg/kg. FireMaster(R) BP-6 (for 14
days) can be compared with those obtained 14 weeks after feeding.
There was a decline in all tissues including fat (see Table 48),
however, to a different extent, resulting in changes in the relative
BB 153-concentrations between tissues (Corbett et al., 1978a).
BB 153 blood levels of rhesus monkeys, dosed with BB 153,
markedly declined over time (day 5: 1.5 mg/kg, day 11: 0.2 mg/kg;
day 25 < accurate measurement levels) (Rozman et al., 1982).
 6.5.1.2 Biological half-lives
The biological half-lives of PBBs reported in the literature
for various species have been compiled in Table 68. McDaniel &
Lucier (1979) reported a half-life of BB 153 exceeding the lifetime
of rats.
In some cases the results obtained by different authors are
similar, in other cases, large discrepancies exist. These deviations
were, perhaps, caused by measuring the half-lifes under different
circumstances of exposure and for different lengths of time (Fries,
1985b). By means of simulation studies, Domino et al. (1982)
demonstrated the effects of different amounts of body fat on the
half-life of BB 153 in the rat (see Table 69).
Tuey & Matthews (1980) have simulated the effects of a growing
animal on the kinetics of BB 153 and found that, though
concentrations of BB 153 in fat fell faster than in a stable animal,
the "actual" half-life of the substance was prolonged, because of an
increase in the relative fat proportions.
6.5.1.3 Differences between individual congeners
There are some reports on the differences in turnover and
retention of individual PBB congeners. Mostly, they refer to changes
in the relative abundances of certain FireMaster(R) components,
namely: 2,2',4,5,5'-pentabromobiphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118), 2,2',3,4',5',6-hexa
bromobiphenyl (BB 149), 2,2',4,4',5,5'-hexabromobiphenyl (BB 153),
2,2',3,4,4',5'-hexabromobiphenyl (BB 138), 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167), 2,3,3',4,4',5-hexabromobiphenyl (BB
156), 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), and
2,2',3,3',4,4'5-heptabromobiphenyl (BB 170). To describe such
changes, the GC profile of the original FireMaster(R) mixture was
compared with the GC profile obtained on analysis of tissues from
animals treated with this mixture, by measuring either the area
(e.g., Wolff & Aubrey, 1978; Domino et al., 1980b) or the height
(e.g., McCormack et al., 1982a; Bernert & Groce, 1984; Groce &
Kimbrough, 1984) of the GC peaks and by normalizing the values to BB
153 as 100. A few other authors (Fries & Marrow, 1975; Fries et al.,
1976) based their calculations on the assumption that each component
(BB 153 and BB 180) was fed at the same rate in the diet. According
to Fries (1985b), some differential behaviour may be due to
analytical artefacts, introduced by differential recovery of the
congeners or by adsorption of congeners on the glassware (Willett
et al., 1978), but many changes appear real.
Table 69. Effect of different amounts of body fat on the half-life of
2,2',4,4',5,5'-hexabromobiphenyl in the rata
Amount fat Half-life
(% normal fat) (days)
Emaciated 25 60.5
50 88.8
75 117
90 134
Normal 100 145
110 156
125 173
150 200
175 228
200 256
Obese 250 311
a From: Domino et al. (1982).
The trends reported in the literature include the selective
retention of 5 minor components of the FireMaster(R) mixture in
the tissues of rats, pigs, cows, and chickens (Fries & Marrow, 1975;
Fries et al., 1976, 1978a; Dannan et al., 1978b; Willett & Durst,
1978; Wolff & Aubrey, 1978; Wolff & Selikoff, 1979; Domino et al.,
1980b; McCormack et al., 1980, 1982a; Werner & Sleight, 1981; Groce
& Kimbrough, 1984; Polin & Leavitt, 1984). They can be summarized,
as follows.
With some exceptions, the concentrations of 2,2',4,5,5'-penta
bromobiphenyl (BB 101) and 2,2',3,4,4',5,5'-heptabromobiphenyl (BB
180) relative to BB 153 appeared to be lower in tissues from treated
animals than in those fed FireMaster(R) mixture. The relative
concentrations of 2,3',4,4',5-pentabromobiphenyl (BB 118) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) appeared to be higher or
unchanged in many cases. 2,2',3,4',5',6-Hexabromo biphenyl (BB 149)
was barely, or not, detected in the tissues of any animals.
The results of a multigeneration study on rats also reflected
the differential behaviour of certain PBB congeners (McCormack
et al., 1981). Of the first eight peaks (penta- to
heptabromobiphenyls) in the GC profile of FireMaster(R), all
except peak 3 (BB 149) were detected in the livers of rats in the
F1, F2, and F3 generations (experimental design: see Table
54). For example, the concentration of
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) relative to BB 153 was
higher in the livers of animals in the F1 generation than in the
FireMaster(R) BP-6 standard, but decreased with each subsequent
generation. Although F2-10 and F3-100 animals (10 and 100 mg/kg
treatment, respectively; see Table 54) had similar hepatic
concentrations of BB 153, the relative concentrations of other PBB
congeners, including BB 167 appeared to be lower in the livers from
F3-100 than F2-10 animals (McCormack et al., 1981).
Domino et al. (1980b) found that 2,2',4,5,5'-pentabromobiphenyl
(BB 101) penetrated the brain of rats more rapidly than
2,3',4,4',5-pentabromobiphenyl (BB 118) or any of the higher
relative molecular mass homologues. Fries et al. (1976) reported a
faster clearance of 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180)
(half-time = approximately 20 days) than of BB 153 (half-time =
approximately 28 days) from eggs of hens after feeding stopped.
However, analyses of whole chicken carcasses resulted in unchanged
ratios of BB 153/BB 180 on days 0, 21, and 42 of withdrawal (Polin &
Leavitt, 1984). The authors judged that the dynamics for withdrawal
of these two congeners from tissues of chickens were parallel. The
withdrawal from milk of dairy cows was found to be more rapid for BB
180 than for BB 153 (Fries & Marrow, 1975; Fries et al., 1978a; see
also Fig. 7).
In just one study (Millis et al., 1985b), equimolar doses of
individual PBB congeners were administered to the test animals.
Immature male rats received a single oral dose (21.3 µmol/kg body
weight) of 3,3',4,4',5,5'-hexabromobiphenyl or 3,3',4,4'-tetra
bromobiphenyl and were analysed at various times up to 14 days after
treatment. Adipose tissue and liver concentrations of
3,3',4,4',5,5'-hexabromobiphenyl appeared unchanged over time
whereas the tissue concentrations of 3,3',4,4'-tetrabromobiphenyl
decreased in a biphasic manner.
6.5.1.4 Octabromobiphenyl
Some kinetic data are available for technical octabromo
biphenyl. Bromine levels in the adipose tissue of rats dosed with
octabromobiphenyl did not decrease during a period of 90 days
(Norris et al., 1973) or 18 weeks (Aftosmis et al., 1972a; Waritz
et al., 1977) after cessation of dosing, or, according to Lee et al.
(1975a), levels even increased 18 weeks after exposure. A partial
elimination of bromine was observed from the livers of these rats
after recovery (Norris et al., 1973; Lee et al., 1975a; Waritz
et al., 1977).
The contents of total PBB (octabromobiphenyl plus an
unidentified hexabromobiphenyl) in juvenile Atlantic salmon (Salmo
salar), fed 90 days with octabromobiphenyl (Dow
Chemical)-contaminated food, also remained fairly constant after 28
days of withdrawal (Zitko, 1977).
6.5.2 Human studies
Information on the time trends of BB 153 distribution or
retention in humans is limited. As with animals, BB 153 seems to be
highly persistent in humans. This conclusion has been drawn from
monitoring BB 153 levels over time in both individual persons and
the Michigan population.
Most paired serial samples exist for serum (or plasma) from
farmers, etc. (Humphrey & Hayner, 1975: sampled June/Autumn, 1974;
Landrigan et al., 1979: sampled 1974/77; Wolff et al., 1979b:
sampled 1976/77/78; Kreiss et al., 1982: sampled 1977/78/79;
Sherman, 1991: sampled 1976/80/87) and chemical workers (Wolff
et al., 1979b: sampled 1976/78; Bahn et al., 1980b and Bialik, 1982:
sampled 1978/1981; Lambert et al., 1990) (in parentheses: references
plus year of sampling). The values obtained indicated no, or little,
decrease (see sections 5.2 and 5.3, and Table 38) with the exception
of the results of Bahn et al. (1980b) and Bialik (1982) (see section
5.3). Paired adipose analyses have been reported only by Meester &
McCoy (1976) who found a consider able average decline in HxBB
levels over six months (see also section 5.2). However, one of the
16 persons tested had increasing or unchanged fat levels, while
serum levels slowly dropped. The significance of these observations
and the combined results of Bahn et al. (1980b) and Bialik (1982)
are unclear. A most recent case report (Sherman, 1991) showed that
"PBB" could be identified in the serum and fat of a cancer patient
over an 11-year period (1976-87). Serial testing of 11 breast-milk
samples from one lactating Michigan woman showed that HxBB
concentrations varied between 0.1 and 0.2 mg/kg (expressed on a fat
basis) during a three-month period, without any significant downward
trend (Brilliant et al., 1978).
Comparisons at the population level have been made for serum
(Meester & McCoy, 1976), breast-milk (Miller et al., 1984), and
adipose tissue (Miceli et al., 1985). Generally, the presence of
PBBs in the tissues of Michigan people many years after the spill
(1973) may be an indicator for its persistence (see Tables 38 and
39). Meester & McCoy (1976) found that the average HxBB level in the
serum of farmers during the first six months of 1976 was ten times
lower than that during the last six months of 1975 (0.2 µg/litre
versus 2.0 µg/litre). Miller et al. (1984) concluded from their
analyses of breast-milk from 2986 lactating women during May 1976
and December 1978 that HxBB levels were not declining. Approximately
5 years after the Michigan PBB incident occurred, adipose tissue
from live residents of a "high" exposure area (Muskegon County area)
contained median HxBB levels of 500 µg/kg (Wolff et al., 1982).
Approximately 10 years after exposure, postmortem adipose tissue
contained median HxBB levels of 320 µg/kg (Miceli et al., 1985).
On the basis of the two last values, Miceli et al. (1985)
calculated a half-life of 7.8 years for HxBB (BB 153) in human
adipose tissue. This prediction was close to the body burden
half-time of 6.5 years estimated by Tuey & Matthews (1980) using
pharmacokinetic data obtained from rats.
A median serum half-life of BB 153 of 12 years (range: 4.6-94.7
years) has been determined by comparing previous and more recent
serum BB 153 levels of Michigan residents (Lambert et al., 1990).
As Tuey & Matthews (1980) explained, the half-life may be
longer in growing children or in persons gaining weight. However,
calculating the effects of different amounts of body fat on the
retention of BB 153, these authors also found that adipose tissue
may act as a protective reservoir in mature humans, because the
concentration of BB 153 in the blood (and possibly other more
critical tissues) of obese persons should be significantly less than
those of leaner individuals who received comparable exposures.
There was some evidence for the differential retention of
various PBB congeners in humans, when PBB congeners in serum samples
from Michigan subjects were compared with FireMaster(R) BP-6. The
main congeners assayed by using GC-MS analysis (Wolff & Aubrey,
1978; Wolff et al., 1978) or Negative Chemical Ionisation
Spectrometry (NCIMS) analysis (Roboz et al., 1982; Greaves et al.,
1984) were as follows: 2,2',4,5,5'-pentabromo biphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118),
2,2',3,4',5',6-hexabromobiphenyl (BB 149), 2,2',4,4',5,5'-hexa
bromobiphenyl (BB 153), 2,2',3,4,4',5'-hexabromobiphenyl (BB 138),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). The most obvious
changes refer to the two penta isomers and to the major
heptabromobiphenyl. The 2,2',3,4,4',5,5'-heptabromobiphenyl was
absent (Roboz et al., 1982) or greatly diminished in serum (Wolff &
Aubrey, 1978; Wolff et al., 1979a; Greaves et al., 1984) in relation
to the concentrations of BB 153 (taken as 100%). The relative
amounts of 2,2',4,5,5'-pentabromobiphenyl (BB 101) were also greatly
decreased (e.g., 80%: Greaves et al., 1984) in all samples. However,
a marked decrease in 2,3',4,4',5-pentabromobiphenyl (BB 118)
concentrations was observed only in the serum of farmers, taken
several years after exposure (e.g., 60%: Greaves et al., 1984), but
not in the serum of chemical workers (Wolff & Aubrey, 1978; Wolff
et al., 1979a; Roboz et al., 1982).
6.6 Reaction with body components
6.6.1 Animal studies
When a 14C-PBB mixture (FireMaster(R)) was incubated with
rat liver microsomes, no binding to exogenous DNA was detected, and
only a small amount of radioactivity was covalently bound to
microsomal protein (Dannan et al., 1978b). The formation of low and
high relative molecular mass adducts with PBB metabolites has been
quoted elsewhere (section 6.3).
6.6.2 Human studies
Studies on human serum revealed that lipoproteins are the
predominant protein carriers of PBBs in serum (Greaves et al.,
1983). 80% of the PBBs were bound to apolipoproteins B and A in a 4
: 1 ratio (Greaves et al., 1984; Roboz et al, 1985a). According to
Roboz et al. (1985b), the ratio was 3 : 1. No preferential binding
of PBB congeners (2,2',4,4',5,5'-hexa-; 2,2',4,5,5'-penta-;
2,2',4,5',6-pentabromobiphenyl) was found (Roboz et al., 1985a,b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only few data are available on effects of PBBs on organisms in
the environment. They refer to microorganisms, water fleas,
waterbirds, (rodents), and farm animals.
7.1 Microorganisms
The toxicity of technical decabromobiphenyl (Adine 0102)
against bacteria (Pseudomonas putida M.) was determined by the
cell multiplication inhibition test (according to the ISO TC 147/SC
5/WG 1/N 111 standard, and using 0.1-1% DMSO (dimethylsulf oxide) as
a solvent. An EC10 of 53 mg/litre was found (Atochem, 1990).
7.2 Aquatic organisms
In a short-term test (according to the ISO standard 6341) the
immobilization of Daphnia magna (Crustacea) by technical
decabromobiphenyl (Adine 0102) has been investigated. The following
results were obtained after dissolution of the test material in DMSO
because of its weak solubility in water:
- EC50 (24 h): > 66 mg/litre;
- The maximum concentration resulting in 0% immobilization
24 h): < 2 mg/litre (Atochem, 1990).
7.3 Terrestrial organisms
7.3.1 Wildlife
In 1977 and 1978, Haseltine et al. (1981) and Heinz et al.
(1983) studied red-breasted mergansers (Mergus serrator) nesting
on islands in the northwestern Lake Michigan in order to determine
whether environmental contaminants (organochlorines and metals) were
producing effects on reproduction. Seventeen contaminants, including
PBBs, were measured in randomly chosen eggs from 206 nests under
study. Using a variety of statistical approaches, Heinz et al.
(1983) looked for effects of individual contaminants and
combinations of contaminants on reproductive measurements, such as
nest desertion, failure of eggs to hatch, death of newly hatched
ducklings, percentage hatching success, number of ducklings leaving
the nest, and egg-shell thickness. PBBs and other chemicals were
sometimes negatively correlated with shell thickness or thickness
index, but not consistently so, as found for DDE. However, no
contaminant or combination of contaminants measured seemed to have a
pronounced effect on the aspects of reproduction mentioned above.
The hatching success of the mergansers averaged 81.7% in 1977.
According to Heinz et al. (1983), the hatching success averaged
85.6% in 1978 (Haseltine et al., 1981).
Although it is not valid to compare reproductive success among
different species, it should be noted that dabbling ducks, whose
eggs contained only a fraction of the contaminant burdens (e.g., no
PBBs: Haseltine et al., 1981; see also Table 32), of the redbreasted
mergansers had better hatching success (Heinz et al., 1983).
An interesting observation on rodents living on a
PBB-contaminated farm has been reported by Jackson & Halbert (1974).
They observed that rats and mice were apparently eradicated when
they came into contact with PBB-contaminated cattle feed pellets.
7.3.2 Farm animals
Farm animals in Michigan that ingested feed inadvertently
containing PBB (FireMaster(R) FF-1) in place of magnesium oxide
fell sick. First symptoms were observed in the cattle of several
dairy herds and revealed this so-called "Michigan PBB incident".
7.3.2.1 Cattle
The exact doses of PBBs to which Michigan cattle were exposed
are not known. Fries (1985b) estimated the maximum PBB doses of
individual cattle based on milk or tissue fat concentration at the
time of detection, which ranged from 9 to 24 months after the cattle
had consumed contaminated feed. For example, if PBB were detected 9
months after exposure, PBB concentrations of 0.1 mg/litre of milk
and 0.25 mg/kg tissue would indicate that the cow had received a
total dose of 1 mg/kg of body weight. Increased PBB concentrations
in milk and tissue would indicate a proportionally higher dose. If
detection were delayed for as long as 24 months, the above example
for milk and tissue concen trations would indicate an exposure of
6 mg/kg of body weight.
a) High-level contamination in cattle
Adverse effects of PBBs in lactating cows were first reported
by Jackson & Halbert (1974).
In reconstructing the accident, it was assumed that the cows on
this farm (Halbert farm) consumed PBB-contaminated feed (PBB content
from 3 to 4 g/kg; Isleib & Whitehead, 1975) over 16 days, and
ingested about 20 g PBB/day, initially. The total average exposure
of these cows was estimated to be 250 mg/kg body weight (Fries,
1985b). The contaminated feed was also fed to a group of 6- to
18-month-old calves. The dose might have been about 58 mg/kg body
weight per day when feeding started, and the total doses may have
reached 700 mg/kg body weight over 6 weeks (Fries, 1985b), if feed
was consumed at the same rates as in experimental studies (Durst
et al., 1977).
The clinical signs of toxicity, described by Jackson & Halbert
(1974), were anorexia (50% reduction in feed consumption) and a 40%
decrease in milk production a few weeks after ingestion of the
contaminated feed. Although the supplemented feed was discontinued
within 16 days, milk production was not restored, and the cows
continued to lose weight. An unspecified number of cows had
increased frequency of urination and lacrimation and developed
haematomas, abscesses, abnormal hoof growth, lameness, alopecia,
hyperkeratosis, and cachexia, and several died within 6 months.
Altogether, the death rate of Halbert cows was about 24/400.
The death rate of the 6- to 18-month-old calves (heifers and
bulls) to which the suspected feed was offered, was much higher.
About 50% of the calves died within 6 weeks. After 5 months, only
two of twelve animals were alive, and they had developed
hyperkeratosis over their entire bodies. There were also a variety
of reproductive problems including embryo resorption, abortions,
stillbirths, deaths shortly after births, delayed deliveries, and
enlarged calves. The clinical signs were variable, and, with the
exception of decreased milk production and weight loss, no
particular symptom was predominant in the affected animals (Jackson
& Halbert, 1974; Robertson & Chynoweth, 1975).
Necropsy findings have been reported for some of the 24 mature
cows that died during the 6 months following exposure (Jackson &
Halbert, 1974). Gross lesions observed included somewhat enlarged
livers, haematomas and abscesses in the thoracic and abdominal
cavities, abomasal ulcers, necrotic metritis, suppurative
bronchopneumonia, and pericarditis. As in all observations without
controls, it was difficult to draw definitive conclusions as to
which lesions were caused by PBBs and which were unrelated to PBBs
(Fries, 1985b). Two of the twelve calves in a calf feeding trial had
massive liver abscesses.
Histopathological studies on ten of the cows revealed various
liver and kidney changes. Liver lesions were reported in seven
animals and included fatty changes and amyloidosis. Renal tubular
nephrosis and interstitial nephritis were reported in four of the
cows (Jackson & Halbert, 1974; Getty et al., 1977).
Several clinical signs and pathological changes, reported by
Jackson & Halbert (1974), were also described in cows in controlled
feeding studies (Durst et al., 1977; Durst et al., 1978a,b; Moorhead
et al., 1977, 1978; Robl et al., 1978; Willett et al., 1980; see
also section 8). These included anorexia, dehydration, excessive
lacrimation, emaciation, hyperkeratosis, reproductive difficulties
(fetal death, enlarged calves, and difficulty in calving,
hypospermatogenesis), and renal damage. Conditions described in the
accidentally exposed cows, but not confirmed in the controlled
studies, included haematomas, abscesses, abnormal hoof growth,
extensive hair loss, liver abscesses, necrosis, and metritis (Fries,
1983, 1985b).
The Halbert herd was one of about 12 "highly" contaminated
herds that had PBB concentrations of more than 30 mg/kg in milk fat,
when detected. According to Fries (1985b), it is likely that all of
these herds had some clinical signs of toxicosis. According to the
same author, there was no comprehensive clinical examination of any
herd that had milk concentrations in the range of 1-30 mg/litre, but
it appears that most animals in these herds did not have clinical
signs when the farms were depopulated. A preliminary report (cited
by Getty et al., 1977) indicated that cows that had been exposed to
PBBs 19 months earlier had levels of up to 80 mg/kg in body fat, but
were apparently normal clinically and were producing normal
quantities of milk. Gross or microscopic lesions that could be
attributed to PBB exposure were not found.
b) Low-level contamination in cattle
Residue concentrations in the body fat or milk fat of cows,
classed as having low-level contamination, rarely exceeded 1 mg/kg,
and, for the most part, not even 0.3 mg/kg. There were several
studies on Michigan cattle with such low exposures.
Results of an evaluation of 72 low-level contaminated herds
have been reported by Kay (1977) and Getty et al. (1977).
Production drop and sterility were two consistent signs and were
regarded as interrelated. The retardation of growth of young stock
was very significant, as it was in the Jackson & Halbert study
(1974). Some other findings were not consistent. A mail
questionnaire survey (Getty et al., 1977) showed similar
observations.
Cows (n = 46) from six herds across Michigan, whose body fat
contained a mean concentration of 0.31 mg/kg and a maximum
concentration of 1.8 mg/kg, were compared with a group of cows from
Wisconsin (n = 40) that had not been exposed to PBB. The two groups
were reared together and subjected to the same feeding and
management system. There were no significant differences in the
animals' milk production, body weight, weight gain, breeding and
reproductive performance, incidence of commonly experienced health
problems, calving rate, and the health of their calves. Also no
pattern of gross or histopathological lesions was seen between test
animals and control animals upon necropsy (Wastell et al., 1978).
An epidemiological survey, which compared the health status of
16 herds with low PBB exposure (traces to 1 mg/kg body fat or milk
fat) with the status of 15 herds with no PBB exposure, also
indicated that productivity and general health conditions between
the two groups of herds were similar. Of the biochemical parameters
tested (9 urinalyses, 13 serum chemistry parameters), three resulted
in significantly different values. Serum concen trations of calcium,
glucose, and cholesterol in contaminated herds were significantly
lower than those of the control herds. But the relationship to PBB
exposure was unknown (Mercer et al., 1976).
Instead of specific clinical conditions, Fries (1983) evaluated
the overall performance of exposed herds (residues in tissue or milk
fat generally < 0.3 mg/kg) and of "relatively unexposed" herds
(residues < 0.02 mg/kg) of comparable size, breed, and location by
analysing Dairy Herd Improvement Association records. He found that
no productive or reproductive character istics of the herds were
affected by PBB exposure.
It should be noted that the classification of herds as having a
high or a low level of contamination refers to PBB levels at the
time of detection. Thus, it is sometimes impossible to know whether
there was a history of a short-term, high exposure or a long-term,
low exposure, which may produce different syndromes. For example,
Fries (1985b) pointed out that feed that was contaminated by
cross-contamination in the feed mills was being fed at the time of
detection, in some cases. Under this circum stance, PBB intakes as
low as 0.1 mg/kg of body weight per day could produce milk fat
residues as high as 20 mg/kg (Fries & Marrow, 1975; Fries 1985b).
7.3.2.2 Other farm animals
Although it was cattle feed that was originally involved in the
accidental substitution, all other feeds became involved by cross
contamination, e.g., in the mixing machinery of feed companies that
had been exposed to PBB (Dunckel, 1975). It is likely that other
animals were not exposed to the same high levels as cattle.
(i) Poultry
There are no reports of clinical signs or problems associated
with the accidental contamination of poultry feed. However, some
controlled feeding studies have been published (see section 8).
(ii) Pig
Adverse health effects in pigs, identified as contaminated,
were rarely reported. Only one review (Reggiani & Bruppacher, 1985)
mentioned that abortions occurred in pigs. Two controlled feeding
studies (Ku et al., 1978; Werner & Sleight, 1981) have been
conducted (see section 8).
(iii) Horse, rabbit, goat, sheep
Other species of farm animals, including at least 2 horses, 32
rabbits, 2 goats, and 19 flocks of sheep, were identified as
contaminated and buried at Kalkaska, but details of ill effects were
not recorded (Dunckel, 1975; Getty et al., 1977).
One experimental feeding study on sheep (Gutenmann & Lisk,
1975) is available (see section 8).
7.4 Population and ecosystem effects
No information available.
7.5 Effects on the abiotic environment
No information available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Differences in toxic responses between acute, high-level
exposures and long-term, low-level exposures to halogenated aromatic
compounds are mainly quantitative (McConnell & Moore, 1979).
Moreover, the delayed onset of toxic signs and the persistence of
PBBs in the body, which may cause long-term exposure of the target
organs from a single dose, "tend to blur the usual distinctions that
are made between acute and chronic exposure" (Fries, 1985b). For
these reasons, symptoms after single and short-term exposures are
reviewed together in section 8.2. Another feature of the toxicity
of PBBs and related classes of compounds is the latent period
between the time of exposure and the time of death, which ranged
from several days to weeks (Di Carlo et al., 1978; Safe, 1984;
Hutzinger et al., 1985a; McConnell, 1985). Thus, the classical
LD50 values and other mortality data found are summarized in one
section (8.1).
8.1 Lethality
"Acute" toxicity data (LD50 and LC50 values) of commercial
PBB mixtures have been compiled in Table 70. The LD50 values of
all mixtures show a relatively low order of acute toxicity (LD50
> 1 g/kg body weight) in rats, rabbits and quails, regardless of
the route of administration, and range from > 1 to 21.5 g/kg body
weight. Regarding the LC50 values for minks, this species may be
highly sensitive to PBBs, but a direct comparison is complicated by
differences in the experimental design.
As with TCDD and PCBs (McConnell, 1984; Safe, 1984), the
apparent toxicity of PBBs is higher with multiple-dose rather than
single-dose administration. For example, the single oral LD50 of
FireMaster(R) in rats was quoted to be 21.5 g/kg body weight, but,
if given in small repeated doses, the total lethal dose was
approximately 1-3 g/kg body weight (see Table 70).
Deaths after exposure to PBBs are delayed (Di Carlo et al.,
1978; Tables 71 and 72). Thus, Gupta & Moore (1979) have recommended
that the LD50 of halogenated aromatic hydrocarbons or chemicals
that may have a long-term build-up with delayed toxicity should be
determined more accurately after multiple dosing and an extended
period of observation.
Generally, the susceptibility to the toxic effects of aryl
hydrocarbons is dependent on the sex, age, species, and strain of
the experimental animals (e.g., Safe, 1984; Hutzinger et al.,
1985b). In the case of PBBs, female rats dosed with FireMaster(R)
FF-1 showed a lower LD50 than male rats (Gupta & Moore, 1979;
Table 70).
Table 70. Toxicity of PBB mixtures
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) rat oral LD50 21.5 single dose Di Carlo
et al.
(1978)
FireMaster(R) FF1 rat (Fischer- female oral 90 days LD50 1.43 22 doses Gupta &
(Lot No. 1312 FT) 344/N) (over 30 Moore
(in corn oil) male oral 90 days LD50 3.28 days) 22 (1979)
doses (over
30 days)
FireMaster(R) BP-6 rabbit dermal LD50 5 Aftosmis
et al. (1972b)
FireMaster(R) rabbit male dermal 14 days ALD 5 24 h of Waritz et al.
(Lot 635-71) (New Zealand, exposure (1977)
(in corn oil) albino)
FireMaster(R) FF1 mink male, in feed 313 days LC50 3.95 Ringer et al.
(Mustela vison) female (1981)
FireMaster(R) Bobwhite quail in feed 8 days LC50 428 5 days of Cottrell
(Colinus treated diet et al. (1984)
virginianus)
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) Japanese quail oral not LD50 > 1 Strik
(1973a)
specified
Octabromobiphenyl rat male oral LD50 2 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl rat male oral 7 days ALD > 17 single or Waritz et al.
(Dow Lot 102-7-72) (Sprague-Dawley) repeated dose (1977)
(in acetone: corn
oil = 15:85)
Octabromobiphenyl rabbit dermal LD50 > 10 Aftosmis
et al. (1972b)
Octabromobiphenyl rabbit (New male dermal 14 days ALD > 10 24 h of Waritz et al.
(Dow Lot 102-7-72) Zealand albino) exposure (1977)
Octabromobiphenyl Japanese quail oral LD50 > 12.5 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl Bobwhite quail male, oral 14 days ALD > 12.5 single dose Waritz et al.
(Dow Lot 102-7-72) female (1977)
(in corn oil)
Nonabromobiphenyl mouse (B6C3F1) male, oral 14 days LD50 > 15 single dose Momma (1986)
(Bromkal 80-9D) female
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rat male, oral 14 days LD50 > 5 single dose Millischer
(in corn oil) (Sprague-Dawley female et al.
CFY) dermal 14 days LD50 > 5 single dose (1979)
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rabbit dermal LD50 > 8 c
a ALD = Approximate lethal dose.
b LD50 or ALD in g/kg body weight; LC50 in mg/kg diet (ppm).
c Consumer Product Testing Co. (1977) quoted by Di Carlo et al. (1978).
Table 71. Mortality associated with PBB administration: Commercial mixtures (dosing studies)
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF1 rat male oral 0.03-30 60 days 22 doses no mortality Luster et al.
(No. 1312 FT) over 30 (1978)
(in corn oil)
rat female oral 0.1-10 6 months 122 doses no mortality Luster et al.
(Fisher) over 6 (1980)
months
rat female oral 100 90 days 22 doses 100% 41-53 Gupta & Moore
(Fisher over 30 (1979)
344/N) days
male oral 100 90 days 22 doses 38% 50-73 Gupta & Moore
over 30 days (1979)
female, oral 300 90 days 9-21 doses 100% 14-29 Gupta & Moore
male (1979)
female, oral 1000 90 days 6-10 doses 100% 9-14 Gupta & Moore
male (1979)
FM BP-6 rat inhalation 71 mg not 1 h of no mortality Di Carlo et al.
per specified exposure (1978)
litre
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF-1 mouse female oral 0.03-30 60 days 22 doses no mortality Luster et al.
(Lot No. over 30 (1978)
1312 FT in days
corn oil) mouse female oral 0.1-10 6 months 122 doses no mortality Luster et al.
over 6 months (1980)
FM guinea-pig oral 50 60 days single dose no mortality Ecobichon
(Hartley), mentioned et al. (1983)
albino
FM BP-6 cattle oral 25 60 days daily doses 100% (animals 33-60 Irving et al.
(finely g/dayc became moribund (1976);Durst
ground) and were et al. (1977);
necropsied) Moorhead et al.
(1977, 1978)
FM BP-6 cattle female oral 250 180 days daily doses no mortality Durst et al.
(finely mg/day (1978b)
ground)
cattle female oral 0.3d 340 days daily no mortality Robl et al.
doses over (1978)
158-228
days
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM BP-6 rainbow trout intraperitoneal 150 15 days single dose no mortality Elcombe & Lech
(in corn oil) (Oncorhynchus mentioned (1978)
mykiss)
FM BP-6 brook trout oral 66.3 18 days 3 doses over no mortality Law & Addison
(in dogfish (Salvelinus 5 days mentioned (1981)
oil) fontinalis)
FM FF-1 sheepshead intraperitoneal 15 56 days single dose no mortality James & Little
(Archosargus (1981)
probatocephalus) 50 4-5 days single dose 2/5 (remaining James & Little
3 moribund)
OcBB rat female oral 126- 14 days single dose no mortality Norris et al.
(in corn oil) Sprague- 2000 (1973)
Dawley)
OcBB rat male oral 1000 28 days single dose no mortality Lee et al.
Sprague- (1975b)
Dawley)
OcBB rat male inhalation 0.96 7 days 4 h of no mortality Waritz et al.
(Dow Lot (Sprague- mg/ exposure (1977)
102-7-72) Dawley) litre
air
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
DeBB rat inhalation 200 not 1 h of no mortality Di Carlo
mg/ specified exposure et (1978)
litre
air
DeBB rat male, inhalation 0.05-5 4 weeks 6 h/day; no mortality Millischer
(Sprague- mg/ 5 days/week et al. (1979)
Dawley) litre
air
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b In mg/kg body weight per day, unless otherwise specified.
c Initially equivalent to 67.2 mg/kg body weight per day.
d Equivalent to 10 mg/kg feed.
Table 72. Mortality associated with PBB administration: Commercial mixtures (feeding studies)
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 rat (Sprague- female 4.7-300 2/2 weeks no mortality Dent et al. (1976a)
Dawley) mentioned
FM rat (Sprague- male 1-500 30/30 days no mortality Sleight & Sanger (1976)
Dawley)
FM BP-6 mouse (BALB/c) female 1000 9/9 days 70% Fraker & Aust (1978);
Fraker (1980)
FM BP-6 guinea-pig 50 45/45 7/8 Vos & van Genderen
(Lot No. 182 RP) (1974)
FM guinea-pig 100 30/30 days 4/6 Sleight & Sanger (1976)
guinea-pig 500 15/15 days 100%
FM FF-1 mink male, 1 313/313 days no mortality Aulerich & Ringer (1979)
(Mustela vison) female 2.5 10% 136 days
90% 63-294 days Ringer et al. (1981)
100% 25-93 days
FM pig 20, 200 16/16 weeks no mortality Ku et al. (1978)
mentioned
FM FF-1 rhesus monkey male 25 25/25 weeks 1/1 25 weeks Allen et al. (1978)
(adult) (total: 1 g)
(juvenile) female 300 137/137 days 1/1 137 days Allen et al. (1978)
(total:
6.4 g)
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 chicks (Hubbard male 400 15/15 days 37% Vos & van Genderen
(Lot No. 182 RP) Leghorn) (1974)
FM BP-6 chicken (White 20-200 8/16 weeks no mortality Cecil & Bitman (1978)
Leghorn)
4/4 weeks 60% (control 10%) Cecil & Bitman (1978)
FM FF-1 chicken (White 3125 4/4 weeks 100% Polin & Ringer (1978a)
Leghorn)
FM Japanese quail 10-100 no mortality Babish et al. (1975a)
500-1000 "few" days 100% Babish et al. (1975a)
FM Bobwhite quail 100 5/8 days no mortality Cottrell et al. (1984)
200 5/8 days 1/10 8 days Cottrell et al. (1984)
600 5/8 days 100% (10/10) 5.3 days Cottrell et al. (1984)
(mean)
FM BP-6 Atlantic salmon 100 42/70 days no mortality Zitko (1977)
(Salmo salar) (in excess of
that of
control fish)
OcBB rat (Sprague- male 100-10 000 30/30 days no mortality Norris et al. (1973)
Dawley) mentioned
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
OcBB rat (Sprague- male 1-1000 4/22 weeks no mortality Lee et al. (1975a)
Dawley)
OcBB (Dow Atlantic salmon 100 74/96 days no mortality (in Zitko (1977)
Chemical) (Salmo salar) excess of that of
control fish)
DeBB rat (Sprague- female, 1-2000 90/90 days no mortality Millischer et al.
Dawley) male mentioned (1979)
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
Although the studies, in most cases, are not directly
comparable, species differences in the response to PBBs are
reflected, in part, by the mortality data collated in Tables 71 and
72. For example, minks appear to be more sensitive to the
FireMaster(R) mixture than the other species of animals tested.
Compared to rats, guinea-pigs were far more susceptible to
FireMaster(R) (Table 72).
The few studies performed with commercial octa- and
decabromobiphenyl mixtures did not result in any mortality in rats
and fish (Tables 71 and 72). Of individual PBB congeners, only three
hexa isomers have been tested. Apparently, 3,3',4,4',5,5'-
hexabromobiphenyl is more toxic for rats than
2,2',4,4',5,5'-hexabromobiphenyl (Table 73).
With few exceptions, the cause of death by halogenated aryl
hydrocarbons cannot usually be ascribed to pathology in a given
organ or system as with most toxicants (McConnell & Moore, 1979;
McConnell, 1984). At lethal doses, the affected animals develop, as
a first indication of toxicity, a "wasting syndrome", i.e., a
progressive loss of body weight, which may not be related simply to
decreased food consumption. It is followed by weakness,
debilitation, and finally death (Hutzinger et al., 1985a; McConnell,
1985). Some authors (Allen et al., 1978) use the term "metabolic
death".
The extended course of the disease can be complicated by other
diseases, usually of an infectious etiology (McConnell, 1984). For
instance, parasitism, diarrhoea, and pulmonary infections have been
observed in PBB-contaminated cattle (Jackson & Halbert, 1974;
Willett & Irving, 1976; Moorhead et al., 1978). Many of the pigs
that died during lactation after perinatal exposure to PBB had acute
suppurative pneumonia (Werner & Sleight, 1981). Severely intoxicated
rhesus monkeys may have succumbed finally to gastrointestinal and
respiratory infections (Allen et al., 1978). Male rats that were
given 100 mg FireMaster(R) FF-1/kg body weight per day and died
after 90 days had liver lesions (Gupta & Moore, 1979). Such
overlying disease problems often result in difficulties in
establishing a definitive etiological diagnosis in environmental
exposures (McConnell, 1984).
Table 73. Mortality associated with PBB administration: Individual PBB congeners
PBB Species Sex Exposure Dose Mortality (in percentage References
concentration or No. dead/No. treated)
2,2',4,4',5,5'- rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
mouse (B6C3F1) female in diet for 10 days 750a 2/8 Welsch &
(pregnant) Morgan (1985)
sheepshead intraperitoneal; single 20b no mortality James & Little
(Archosargus or multiple doses; (1981)
probatocephalus observation: 17-40 days
3,3',4,4',5,5'- rat (Sprague-Dawley) male in diet for 20 days 100a 1/2 (the remaining Render et al.
hexabromobiphenyl rat was moribund) (1982)
2,3',4,4',5,5' rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
Mixture of di-, Atlantic salmon in diet for 40 days plus 7.75a (100% mortality) Zitko &
tri-, and (Salmo salar) additional stress at day Hutzinger (1976)
tetrabromobiphenyls 4; observation: 42 days
a In mg/kg feed.
b In mg/kg body weight per day.
8.2 Single and short-term exposures: general signs of toxicity
8.2.1 PBB mixtures
8.2.1.1 Overt clinical signs, food intake, and body weight changes
In many acute and short-term studies, signs of PBB toxicity
include reduction in feed consumption and weight loss or a decreased
weight gain (see Tables 74 and 75). Most of the reports refer to the
FireMaster(R) mixture, which was tested in rats, mice,
guinea-pigs, pigs, cattle and avian species. From these studies with
various protocols, the minimum effective doses of FireMaster(R)
(FM) in the diet or by gavage ranged between 0.3 and 500 mg FM/kg
feed or between 4 and 100 mg FM/kg body weight per day,
respectively. Only a few studies have been performed with technical
octabromobiphenyl and technical decabromobiphenyl; no effects were
observed on feed consumption or body weight of rats (Tables 74 and
75).
Weight loss is not necessarily accompanied by decreased food
intake (e.g., Allen et al., 1978; Lambrecht et al., 1978; Gupta &
Moore, 1979) suggesting that PBBs may cause poor feed utiliz ation.
On the other hand, increased efficiency of feed utilization has been
reported in growing pigs (Ku et al., 1978) and weight loss in hens
was accounted for completely by reductions in feed consumption in
paired feeding studies (Cecil & Bitman, 1978; Ringer, 1978). If the
concentrations of PBBs are high enough, a total refusal of feed will
occur (Babish et al., 1975a; Gutenmann & Lisk, 1975; Ringer & Polin,
1977; Cecil & Bitman, 1978; Polin & Ringer, 1978a; Aulerich &
Ringer, 1979). At death, the loss in body weight can be as great as
30-40% (Allen et al., 1978; Aulerich & Ringer, 1979; Durst et al.,
1978b).
At sublethal doses, decreased food intake and weight loss may
be the only overt signs observed in many species. However, calves
(fed 100 mg FireMaster(R) FF-1/kg body weight per day) also
developed keratitis and lacrimation from day 35, and alopecia from
day 50 (Robl et al; 1978). During a 16-week test period, some pigs
receiving FireMaster(R) BP-6 at 200 mg/kg in the diet had
dermatosis (Ku et al., 1978). Loss of hair (including eye lashes),
dry scaly skin, and periorbital oedema were reported in a juvenile
female rhesus monkey given 25 mg FireMaster(R) FF-1/kg feed for 50
weeks (total dose: approximately 1.5 g), however, the time of onset
of these symptoms was not specified (Allen et al., 1978). A
decreased heart rate (bradycardia) was measured in White Leghorn
cockerels fed 150 mg FireMaster(R) FF-1/kg feed for approximately
9 weeks (Heinemann & Ringer, 1976; Ringer, 1978). Another
characteristic sign in chickens was general oedema (Ringer & Polin,
1977; see also section 8.2.1.3).
Table 74. Effects on feed consumption and changes in body and organ weights after single or short-term exposure to commercial
PBB mixtures (dosing studies)
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 rat male, oral single dose 1000 n.r. no effect liver: increase Kimbrough
(in peanut oil) (Sherman) female t.p.: 2 months et al.
(1978)
M BP-6 rat male oral single dose 500 n.r. -- liver: increase Bernert Jr &
(Lot No. 5143 (Sherman) t.p.: 1-8 weeks Groce (1984)
in corn oil)
FM BP-6 rat female oral daily doses (9) 1 no effect no effect liver: increase; spleen Harris
(in olive oil) (Sprague- during pregnancy (mg/day) kidney, adrenal, ovary et al.
(1978a)
Dawley) t.p.: 13 days gravid uterus, perirenal
(after first dose) fat pads: no effect
FM FF-1 rat male oral multiple doses 3 n.r. no effect thymus spleen: decrease Luster
(Lot No. (Fischer) (22) over 30 days et al.
1312 FT) t.p.: 60 days 30 n.r. reduced thymus, spleen: decrease (1978)
(in corn oil) (after first dose)
FM FF-1 rat male, oral multiple doses 100-1000 reduced reduced liver, spleen: increase Gupta &
(Lot No. (Fischer female (22) over 30 days (day 10) thymus: decrease Moore (1979)
(1312 FT) 344/N) t.p.: up to 90 days
(in corn oil)
male 30 no effect reduced -- Gupta &
(day 15) Moore (1979)
female 30 variable reduced --
(day 10)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
male, 3 -- -- liver: increase (day 15) Gupta et al.
female 30 no effect thymus: decrease (day (1981)
reduced 15); lung, heart, spleen,
(3 week) kidney, adrenal, thyroid,
testis, ovary, uterus,
brain: no effect
FM FF-1 rat male oral multiple doses 1-6 n.r. no effect liver, thyroid: increase Allen-Rowlands
(Lot No. (Sprague- (20) t.p.: 4 weeks adrenal, testis: et al. (1981);
1312 FT) Dawley) (after 1 dose) no effect Castracane
(in lecithin et al. (1982)
liposomes)
FM rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
(in corn oil) single dose et al. (1972b)
t.p.: 7 days
FM BP-6 rat female intraperitoneal n.r. no effect liver: increase Dent et al.
(in peanut oil) (Sprague- single dose 150 (1976b)
Dawley) t.p.: 2 weeks
FM FF-1 rat female intraperitoneal 200-1000 n.r. no effect liver: increase Goldstein
(in corn oil) (Fischer) single dose (µmol/kg et al. (1979)
t.p.: 4 days body
weight)
FM FF-1 rat male intraperitoneal 90
(in (Sprague- single dose
polyethylene Dawley) t.p.: 1 week n.r. n.r. liver: increase Dannan et al.
glycol) (1978a)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
t.p.: 2 weeks n.r. no effect liver: increase; Dannan et al.
no effect spleen, thymus: (1982c);
Millis et al.
(1985a)
FM BP-6 rat male intraperitoneal (days 30 n.r. n.r. liver, spleen: increase Robertson
(Lot 7062) (Wistar) 1 and 3) two doses 75 n.r. n.r. liver: increase; spleen, et al. (1981b)
(in corn oil) t.p.: 6 days thymus: decrease
(after 1 dose)
FM BP-6 rat male intraperitoneal three 0.2 n.r. n.r. liver: increase Ecobichon
(in peanut (Wistar) doses (days 1, 2, 3) mmol/kg et al. (1979)
oil) t.p.: 7 days body
(after 1 dose) weight
FM BP-6 rat male intraperitoneal single 500 n.r. reduced liver: increase; Andres et al.
(in corn oil) (Wistar) or multiple doses (4) thymus, spleen: decrease (1983)
t.p.: 15 days
FM FF-1 mouse female oral multiple doses 30 n.r. no effect spleen: decrease Luster et al.
(Lot No. (B6C3F1) (22) over 30 days thymus: no effect (1978)
1312 FT) t.p.: 60 days
(in corn oil) (after 1 dose)
mouse female oral multiple doses 30 n.r. no effect spleen: increase; Luster et al.
(B6C3F1) (22) from gestational thymus: decrease (1980)
(pregnant) day 0 until litters
were weaned
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 mouse male, oral multiple doses 3-30 no effect variable liver: increase (day 15) Gupta et al.
(Lot No. (B6C3F1/N) female (22) over 30 days lung, heart, spleen, (1981)
FT 1312) t.p.: up to 90 days kidney, adrenal thyroid
(in corn oil) testis, ovary, uterus,
brain: no effect
male 30 no effect reduced thymus: decrease
(only day (only day 30)
30)
female 0.3, 3, no effect increased thymus: no effect
30 (only
day 45)
FM BP-6 mouse female intraperitoneal single 150 n.r. n.r. liver: increase (48, Dent et al.
(in peanut oil) (NMRI) dose t.p.: up to 192 h 96 h), no effect (24, (1977a)
192 h)
FM BP-6 mouse male intraperitoneal 1.50 n.r. no effect liver: increase; Robertson
(in corn oil) (C57BL/6J) single dose (mmol/kg thymus: decrease; et al. (1984c)
t.p.: 5 days body spleen: no effect
weight)
mouse male intraperitoneal 1.50 n.r. no effect liver: increase;
(DBA/2J) single dose (mmol/kg thymus, spleen:
t.p.: 5 days body no effect
weight)
FM FF-1 guinea-pig female oral single dose 50 n.r. no effect "tissues": no effect Ecobichon
(Lot FA (Hartley, t.p.: 2-60 days (liver, kidney, lung, et al. (1983)
7042) (in albino) perirenal fat)
peanut oil) (pregnant)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM BP-6 guinea-pig male intraperitoneal single 50 n.r. -- liver, kidney: no effect Smith
(in peanut oil) Hartley dose t.p.: 120 h et al.
(1986)
hamster male intraperitoneal single 50 n.r. -- liver, kidney: no effect
(golden dose t.p.: 120 h
Syrian)
FM (Lot rabbit male dermal 24 h of 100- n.r. reduced liver: increase Waritz
635-71) (New exposure t.p.: 10 000 (not et al.
Zealand, 14 days significant) (1977)
albino)
FM BP-6 calf male oral daily doses 0.54 no effect reduced -- Willett &
(Lot RP-158) for 44 days mg/day Irving
(in milk) (1976)
FM BP-6 cattle female oral daily doses 25 g reduced reduced liver, kidney, perirenal Irving
(Lot (pregnant) per day (day 4) (day 20) lymph nodes: increase et al.
6244 A) thymus: decrease (1976);
(days 33-66) Willett
& Irving
(1976);
Durst
et al.
(1977);
Moorhead
et al.
(1977)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
250 mg no effect no effect no effect
per day
FM FF-1 calf male, oral daily doses 100 reduced reduced -- Robl et al.
female (day 36) (day 35) (1978)
cattle female oral daily doses 0.3 no effect no effect --
for 158 days
t.p.: 340 days
(after 1 dose)
FM FF-1 dog male, oral daily doses 1 n.r. -- liver: increase Farber et al.
(Beagle) female for 7 weeks (1976)
dog male oral daily doses 4 n.r. reduced -- Farber et al.
(Beagle) (day 30) (1978)
FM Japanese oral daily doses 25-1000 n.r. reduced liver: increase Strik (1973b)
quail for 7 days
FM BP-6 rainbow intraperitoneal n.r. n.r. liver: no effect Elcombe &
(in corn oil) trout single dose t.p.: Lech (1978)
(Oncorhynchus up to 15 days
mykiss)
FM BP-6 brook trout oral multiple (200 mg/kg n.r. reduced liver: no effect Law & Addison
(in dogfish (Salvelinus doses t.p.: body (not (1981)
oil) (fontinalis) 18 days (after weight significant)
1 dose) total dose)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
OcBB (in rat female oral single dose 126-2000 n.r. no effect n.r. Norris et al.
corn oil) (Sprague- t.p.: 14 days (1973, 1975)
(Dawley)
OcBB (in rat male oral single dose 1000 no effect no effect liver: increase (days Lee et al.
corn oil) (Sprague- t.p.: 21 days 2-11), no effect (1975b)
Dawley) (day 21)
oral daily doses (2) 3000 no effect no effect liver: increase (up Lee et al.
t.p.: 28 days (after to day 21), no effect (1975b)
lase dose) (day 28)
OcBB (Dow rat male oral single dose 3400- n.r. -- liver: increase Waritz et al.
Lot 102-7-72) (Sprague- t.p.: 7 days 17 000 (1977)
(in 40-50% Dawley)
acetone: corn
oil = 15:85)
OcBB (in rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
corn oil) single dose t.p.: et al. (1972b)
7 days
rat inhalation 4 h 3.1 n.r. n.r. liver: no effect
exposure for µg/litre
10 days air
OcBB rat male inhalation 4 h 0.96 n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot Sprague- exposure mg/litre significant) (1977)
102-7-72) Dawley) t.p.: 7 days air
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat not inhalation (25 g) n.r. n.r. liver: no effect
(Sprague- specified 23 h/day; (2-15 weeks) kidney,
Dawley) 7 days/week for thyroid: no effect
2-15 weeks (15 weeks)
OcBB rabbit male dermal 24 h 100- n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot (New exposure 10 000 significant) (1977)
102-7-72) Zealand; t.p.: 14 days
(in corn oil) albino)
male dermal 6 h/day; 1 n.r. no effect liver: increase
5 days/week, for
2 weeks t.p.:
18 days (after last
dose)
OcBB bobwhite male, oral single dose 12 500 n.r. n.r. any internal organs:
(Dow Lot quail female t.p.: 14 days no effect
102-7-72)
(in corn oil)
NoBB mouse male, oral single dose up to n.r. n.r. liver: increase Momma (1986)
(B6C3F1) female t.p.: 14 days 15 000
DeBB (in rat male, oral single dose 5000 n.r. no effect liver: no effect Millischer
corn oil) (Sprague- female t.p.: 14 days et al. (1979)
Dawley)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat intraperitoneal 1000 n.r. n.r. liver: no effect Aftosmis
single dose et al. (1972b)
t.p.: 7 days
rat male, dermal 24 h 5000 n.r. no effect liver: no effect Millischer
(Sprague- female exposure t.p.: et al. (1979)
Dawley) 14 days
rat male, inhalation 6 h/day, 0.05-5 no effect no effect liver: increase
(Sprague- female 5 days/week for mg/litre
Dawley) 4 weeks air
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; NoBB = nonabromobiphenyl; DeBB = decabromobiphenyl.
b t.p. = time post-exposure.
c In mg/kg body weight per day, unless otherwise specified.
d n.r. = not recorded.
e Absolute or relative to body weight, respectively.
Table 75. Effects on feed consumption and changes in body and organ weights caused by short-term feeding of commercial PBB mixtures
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- female 75, 300 2/2 weeks no effect no effect liver: increase Dent et al.
Dawley) (1976a)
FM rat (Sprague- male 100 30/30 days no effect no effect liver: increase Sleight &
Dawley) Sanger (1976)
500 30/30 days reduced reduced liver: increase;
kidney: no effect
FM BP-6 rat (Sprague- male 50 3/3 weeks no effect no effect liver: increase Babish &
Dawley) Stoeswand
(1977)
FM BP-6 rat (Sprague- female 50 day 8 of n.r. no effect liver: increase Dent et al.
Dawley) gestation through (1977b)
day 14 post-
partum
FM BP-6 rat male 5 variable no effect no effect liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); kidney: decrease et al. (1977)
(week 3)
rat male 500 variable no effect reduced liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); testis: no effect et al. (1977)
(week 3)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- not 50 (in pre-, post- and n.r. n.r. liver: increase Cagen &
Dawley) specified mother's or perinatal Gibson (1978)
pups weanling's exposure/
diet) of age
15-49 age
days
FM BP-6 rat (Sprague- day 8 of
Dawley) gestation to day
15 postpartum
mothers female 50 n.r. no effect liver: increase; kidney, Dent et al.
mammary: no effect (1978b)
pups male, (50) (in pre- post-, and n.r. no effect liver: increase
female mother's perinatal
diet) exposure
FM BP-6 rat (Sprague- male, (mothers perinatal n.r. reduced liver, spleen: increase Harris et al.
Dawley) female dose: 10 exposure (day 3 of (day 60 of age) (males (1978a)
pups mg/day on age) more affected than
gestation females
days 7-15)
adults males 50-100 10/10 weeks no effect no effect liver: increase Harris et al.
150, 200 10/10 weeks no effect reduced liver: increase; adrenal, (1978b)
spleen, kidney, testes,
seminal vesicles: no
effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
female 600 10/10 weeks reduced reduced n.r.
FM BP-6 rat male 50-500 5/5 weeks n.r. no effect liver: increase Kasza et al.
(Holtzman) (1978a)
FM BP-6 rat (Sprague- male 20 28/28 days no effect no effect liver: increase Chu et al.
Dawley) (1980)
FM BP-6 rat (Sprague- male, 100 (in perinatal n.r. reduced M: ventral Johnston
Dawley) mothers or exposure until (more prostate: decrease et al. (1980)
pups weanlings 9 weeks of age pronounced
diet) in males)
FM BP-6 rat (Sprague- male 1-100 30/30 days no effect no effect liver: increase; thymus, Akoso et al.
Dawley) spleen: no effect (1982a)
male 100 30/30 days no effect no effect thyroid: increase Akoso et al.
(1982b)
FM BP-6 rat (Sprague- male 100 9/9 days no effect no effect liver, thyroid: increase Render et al.
Dawley) kidney: no effect (1982)
FM BP-6 rat (Fisher) male 100 10/10 days no effect no effect liver: increase Raber &
Carter (1986)
FM BP-6 mouse female 1000 11/11 days n.r. -- liver: increase Corbett
(Swiss, et al. (1975)
ICR) male 1000 up to 14/14 n.r. reduced liver: increase (day 4); Corbett
days (day 4) testis: no effect et al.
(1978a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 mouse female 50-200 2/2 weeks no effect no effect liver: increase Cagen et al.
(Swiss, (1977); Cagen
Webster) & Gibson
adult (1978)
pups not 50 (in postnatal -- -- liver: increase Cagen &
specified mothers exposure until Gibson (1978)
diet) 15 days of age
FM mouse not 100 30/30 days n.r. no effect liver: increase; thymus, Fraker & Aust
(BALB/c) specified spleen: decrease (1978)
not 1000 14/14 days n.r. reduced liver: increase; thymus:
specified decrease (nearly athymic)
FM BP-6 mouse female 1, 10 30/30 days n.r. no effect liver, spleen: no effect; Fraker (1980)
(BALB/c) thymus: decrease
female 100 30/30 days n.r. no effect liver: increase; thymus,
spleen: decrease
FM FF-1 mouse male 5 8/8 weeks n.r. no effect liver: increase Loose et al.
(Lot No. (BALB/c (1981)
7042) ByJ) 167 6/6 weeks n.r. no effect liver: increase
167 8/8 weeks n.r. reduced liver: increase
5 3/3 weeks n.r. -- thymus: no effect
5 6-8/6-8 weeks n.r. -- thymus: decrease
167 3-8/3-8 weeks n.r. -- thymus, spleen: decrease
5, 167 3-8/3-8 weeks n.r. -- lung: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mouse male mothers dose: perinatal n.r. no effect spleen: increase; Luster et al.
(Lot No. (B6C3F1) 10 mg/kg exposure thymus: no effect (1980)
1312 FT) pups body weight
per day
(22 doses)
FM BP-6 guinea-pig not 10 45/45 days n.r. no effect liver: increase Vos & van
specified Genderen
(1974)
FM guinea-pig not 1, 10 30/30 days no effect no effect liver: no consistent Sleight &
specified effect Sanger (1976)
100 up to 30 days n.r. reduced liver: increase
(week 3)
500 15/15 days n.r. reduced liver: increase
(week 1)
FM BP-6 pig not 20, 200 16/16 weeks reduced reduced liver, kidney: increase Ku et al.
(growing) specified (1978)
pig not 100 (in perinatal n.r. reduced liver: increase (but no Werner &
(4-week-old) specified mothers exposure (not effect in sows) Sleight
(lactating) diet) significant) (1981)
pig not 100 (in prenatal -- -- thyroid: increase;
(newborn) specified mothers diet) exposure liver: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mink female 1-1.54 several months -- no effect liver, kidney: increase Aulerich &
2.5 several months -- no effect (in animals that died Ringer
(within first during treatment) (1979);
3 months, Ringer et al.
(1981)
later reduced)
6-16 several months (refused) reduced
FM FF-1 Rhesus female 0.3 several months no effect reduced -- Allen et al.
monkey (1978)
(adult)
(infants) not 0.3 (in perinatal -- reduced --
specified mothers exposure;
diet) up to 12
weeks of age
(juvenile) female 300 up to 137 no effect reduced --
days (until a
few days
prior to
death)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM Japanese male, 500, 1000 a few days (refused) Babish et al.
quail female (1975a)
10-100 9/9 weeks no effect no effect liver: increase
FM Bobwhite 100-700 5/8 days reduced n.r. n.r. Cottrell
quail (Colinus et al. (1984)
virginianus)
FM BP-6 chicks male 400 15/15 days n.r. reduced bursa of Fabricius: Vos & van
(Lot No. (Hubbard decrease Genderen
182 RP) Leghorn) 15, 30 63/63 days n.r. reduced bursa of Fabricius, (1974)
spleen decrease
"PBB" pullet 50, 200 4/4 weeks n.r. reduced -- Chang &
(FM) Zindel (1975)
FM chicken male variable, several weeks -- -- liver, thyroid: Ringer & Polin
(White up to 200 increase; comb: (1977)
Leghorn) decrease; testis:
increase (low dose)
decrease (higher dose)
female 125 several weeks reduced -- --
chicks (White 75 several weeks -- reduced --
Leghorn)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 chicken female 20, 64, 8/16 weeks no effect no effect -- Cecil &
(White 200 2/2 weeks reduced reduced -- Bitman (1978)
Leghorn) 640, 2000 2/2 weeks (refused) reduced --
FM FF-1 chicken female 0-25 5/5 weeks no effect -- -- Polin &
(White 125 5/5 weeks reduced -- -- Ringer
Leghorn) 3125 5/5 weeks refused -- -- (1978a)
FM chicks male 75-250 up to 42 reduced reduced liver, thyroid: Ringer (1978)
(White days increase; comb, testes,
Leghorn) spleen, bursa, thymus:
decrease
FM FF-1 cockerels male 10, 100 28/28 days no effect no effect liver: increase; bursa: Dharma et al.
(White decrease; thyroid, (1982)
Leghorn) spleen, testicles,
comb: no effect
OcBB rat 100 28/28 days -- -- liver: increase Aftosmis
et al.
(1972a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
OcBB rat (Sprague- male 100-1000 30/30 days no effect no effect liver: increase; heart, Norris et al.
Dawley) testes, brain: no (1973, 1975)
1000, 10 000 30/30 days effect; kidney:
increase
OcBB rat (Sprague- male 100, 1000 2-4/2-4 weeks no effect no effect liver: increase Lee et al.
Dawley) (1975a)
1000 4/22 weeks no effect no effect liver: increase
OcBB rat male 100, 1000 2-4/2-22 no effect no effect liver: increase Waritz et al.
(Dow Lot (Sprague- weeks (1977)
102-7-72) Dawley)
DeBB rat (Sprague- male, 2000 13/13 weeks no effect no effect liver: increase Millischer
Dawley) female et al. (1979)
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b n.r. = Not recorded.
c Absolute or relative to body weight, resp.
At lethal doses, the "wasting syndrome" (see section 8.1) can
also be accompanied by other symptoms. Rats, which became moribund,
had hunchback posture, sunken eyes, appeared dehydrated, and were
lethargic (Gupta & Moore, 1979). Minks have been reported to show an
unthrifty appearance (Aulerich & Ringer, 1979; Ringer et al., 1981).
Cattle that died later showed many similar clinical signs, but they
also had excessive lacrimation and salivation, diarrhoea and
depressed heart and respiratory rates (Irving et al., 1976; Durst
et al; 1977, 1978b; Moorhead et al., 1977). In two rhesus monkeys,
the time to death was as long as 3-5 months. In addition to body
weight loss, the dead animals exhibited alopecia and oedema,
particularly on the face and eyelids (including loss of eyelashes),
and dry scaly skin (Allen et al., 1978). Observations of intoxicated
Bobwhite quails, prior to death, included asthenia, low carriage, an
unkempt appearance, wing droop, diarrhoea, limited ataxia, and
general lethargy (Cottrell et al., 1984).
8.2.1.2 Haematology and clinical chemistry
a) Haematology
At lethal doses, leukopenia and erythropenia were observed in a
rhesus monkey (Allen et al., 1978), but not in cattle (Moorhead
et al., 1977). Packed cell volume, white blood cell count, and red
blood cell count fell gradually in the monkey (dietary
concentration: 300 mg/kg of feed; total dose: 6.4 g of
FireMaster(R) FF-1), while changes in packed cell volume,
haemoglobin content, total erythrocyte and leukocyte counts, and
differential leukocyte counts were minimal in the cows (dose: 25 g
FireMaster(R) BP-6 per day).
At sublethal doses, haematological parameters of rhesus monkeys
remained within normal limits (Allen et al., 1978). The same was
true for cattle (Moorhead et al., 1977; Robl et al., 1978), with the
exception of a calf dosed with 100 mg FireMaster(R) FF-1/kg body
weight per day, the total leukocyte count of which was elevated
(Robl et al., 1978). Rats given 30 mg FireMaster(R) FF-1/kg body
weight per day for 30 days (22 total doses) showed a significant
decrease in the packed cell volume, haemoglobin concentrations, and
platelet counts at 30 days of exposure (Gupta et al., 1981) and at 6
months after the start of dosing (Gupta & Moore, 1979), but were
normal at 45, 60, or 90 days after the start of dosing (Gupta
et al., 1981). White and red blood cell values were only
occasionally reduced; for example, there was moderate lymphopenia in
female animals at 30 days of exposure (Gupta & Moore, 1979; Gupta
et al., 1981). No significant differences in values for the
erythrocyte count, packed cell volume, haemoglobin, and total and
differential leukocyte counts were found in male rats fed various
levels of FireMaster(R) BP-6 (1-500 mg/kg diet) for 30-60 days
(Sleight & Sanger, 1976; Garthoff et al., 1977; Sleight et al.,
1978); only a possible increase in total white blood cell count was
noted at the 500 mg/kg feed level (Garthoff et al., 1977). A mild
but significant decrease in the packed cell volume and the number of
platelets was observed in mice exposed to 30 mg FireMaster(R)
FF-1/kg body weight per day for 30 days (22 total doses). The
leukocyte values were within normal limits (Gupta et al., 1981).
Growing pigs in a 16-week trial showed significant reductions in
haemoglobin and haematocrit only after 6 weeks of feeding 200 mg
FireMaster(R) BP-6/kg diet (Ku et al., 1978). There was no
appreciable effect on standard haematological values of nursing pigs
and their sows fed FireMaster(R) BP-6 (10-200 mg/kg feed) during
pregnancy and lactation (Werner & Sleight, 1981). White Leghorn
cockerels fed PBB (FireMaster(R) FF-1) at dietary concentrations
of 75 and 150 mg/kg from 3 to 4 days of age until 5-9 weeks showed a
significant decrease in packed cell volume and haemoglobin values
(Heinemann & Ringer, 1976; Ringer, 1978).
Technical octabromobiphenyl caused a significant decrease in
packed cell volume and total red blood cell count in male rats fed
dietary concentrations of 10 000 mg/kg for 30 days, but had no
effect at dietary concentrations of 100 and 1000 mg/kg (Norris
et al., 1973, 1975).
Standard haematological determinations revealed no treatment-
related changes in blood from rats exposed to technical
decabromobiphenyl via diet (1-2000 mg/kg) for 4-12 weeks and via
inhalation (0.005-5 mg/litre, 6 h/day, 5 days/week) for 4 weeks
(Millischer et al., 1979).
b) Clinical chemistry
Clinical chemistry values examined in many studies refer to
serum protein (total protein, specific fractions), serum enzymes,
serum glucose, blood urea nitrogen, serum lipids, serum cholesterol,
and urine.
(i) Serum protein
Decreases in total serum protein, due primarily to a reduction
in the albumin fraction, occurred in severely intoxicated cattle
(Durst et al., 1978a; Schanbacher et al., 1978) and monkeys (Allen
et al., 1978). No consistent effect of dietary PBBs on total serum
protein concentrations or electrophoretic profiles was observed in
pigs fed FireMaster(R) BP-6 at levels of 20 or 200 mg/kg for 16
weeks (Ku et al, 1978). In rats fed 5, 50, or 500 mg FireMaster(R)
BP-6/kg for 3 weeks, total plasma protein was slightly increased by
the highest concentration (Garthoff et al., 1977). A marked increase
(50%) in serum protein was found in rats given 22 oral doses of
FireMaster(R) FF-1 (30 mg/kg body weight per day) over a 30-day
period and observed for some additional weeks. These changes were
primarily associated with increased ß-globulin fractions (Gupta
et al., 1981). Reductions in serum immuno globulin levels
(gamma-globulin fractions) have been reported in mice given oral
doses (30 mg/kg body weight per day) of FireMaster(R) FF-1 (Luster
et al., 1978) or diets containing 167 mg FireMaster(R) FF-1/kg
(Loose et al., 1981).
No treatment-related changes in total serum protein were found
in rats exposed to technical decabromobiphenyl (1-2000 mg/kg feed
for 4-12 weeks; inhalation: 0.005-5 mg/litre; 6 h/day; 5 days/week
for 4 weeks) (Millischer et al., 1979).
(ii) Serum enzymes
Alterations in serum enzymes, most of which are indicative of
liver lesions, have been found in some instances.
Gamma-Glutamyl transpeptidase (gamma-GTP) was elevated by oral
doses of FireMaster(R) FF-1 (30 mg/kg body weight per day) in
female rats and mice of both sexes (Gupta et al., 1981).
No consistent increase in serum glutamic pyruvic transaminase
(SGPT) occurred in rats after dietary (Garthoff et al, 1977;
Matthews et al., 1978) or oral (Gupta et al., 1981) exposure to
FireMaster(R). No changes were found in cows on diets containing
up to 10 mg FireMaster(R) FF-1/kg (Robl et al, 1978). However, a
gradual increase in SGPT activity was observed in lethally
intoxicated rhesus monkeys (Allen et al., 1978). Technical
decabromobiphenyl did not influence SGPT in rats (Millischer et al.,
1979).
Serum glutamic-oxaloacetic transaminase (SGOT) was
significantly increased in cattle at high doses of PBBs (25 g FM
BP-6 per day) (Moorhead et al., 1977; Durst et al., 1978b). It was
unaffected in cattle given lower doses (250 mg FM BP-6/day: Moorhead
et al., 1977; 0.01-10 mg FM FF-1/kg feed: Robl et al., 1978), as
well as in pigs (Ku et al., 1978) or in rats (Sleight & Sanger,
1976; Garthoff et al., 1977; Sleight et al., 1978) fed
FireMaster(R) BP-6 (20 and 200 mg/kg diet or 1-500 mg/kg diet,
respectively). A possible decrease in serum GOT has been reported in
rats exposed to technical decabromobiphenyl via inhalation
(Millischer et al., 1979).
Lactic dehydrogenase (LDH) was within normal ranges in PBB-
contaminated cows without clinical signs of toxicosis (Moorhead
et al., 1977), but it was increased in a group of apparently
intoxicated animals (dose: 25 g FM BP-6/day) (Moorhead et al., 1977;
Durst et al., 1978b). A significant decrease was found in growing
pigs (Ku et al., 1978), but not in nursing pigs and their sows
(Werner & Sleight, 1981), fed FireMaster(R) BP-6.
Electropherograms of LDH isozymes at 60 days, from rats given
FireMaster(R) BP-6 (100 mg/kg feed), showed appreciable changes
(Sleight et al., 1978).
With the exception of calves (Robl et al., 1978) and newborn
pigs (Werner & Sleight, 1981), alkaline phosphatase levels in
animals were unaffected by FireMaster(R), e.g., rats (Sleight
et al., 1978; Gupta et al, 1981), dogs (Farber et al, 1976), and
growing pigs (Ku et al., 1978). Technical decabromobiphenyl also did
not have any effect on alkaline phosphatase levels in rats
(Millischer et al., 1979).
Serum isocitrate dehydrogenase (sICDH) did not show any
discernible rise in dairy cattle dosed with FireMaster(R) BP-6,
until doses were sufficient to cause toxicosis (25 g/day). These
cows showed moderate increases in sICDH (approximately a two-fold
increase). Elevation of sICDH was coincident with fetal trauma.
Non-pregnant cows, equally intoxicated, showed minimal sICDH
elevation (Schanbacher et al., 1987).
Serum creatine phosphokinase levels in growing pigs were
unaffected by 20 or 200 mg FireMaster(R) BP-6/kg feed (Ku et al.
1978).
In male Japanese quails, serum glutamate dehydrogenase levels
were increased by "hexabromobiphenyl" (STRIK, 1973b).
(iii) Serum glucose
A slight decrease in serum glucose was observed in rats and
mice administered 22 total doses of 30 mg FireMaster(R) FF-1/kg
body weight (Gupta et al., 1981) and in rats fed 50 mg
FireMaster(R) BP-6/kg diet for 3 weeks (Garthoff et al., 1977),
but not in rats fed 500 mg/kg diet for the same period (Garthoff
et al., 1977). FireMaster(R) did not produce any effects on
glucose concentrations in either sublethally or lethally intoxicated
cattle (Durst et al., 1978a; Robl et al., 1978), and
decabromobiphenyl did not affect glucose levels in rats (Millischer
et al., 1979).
(iv) Blood urea nitrogen
Values for blood urea nitrogen (BUN) remained in the normal
range in mice (Gupta et al., 1981) and rats (Sleight & Sanger, 1976;
Garthoff et al., 1977; Sleight et al., 1978; Gupta et al., 1981) or
were elevated in rats at some concentrations (Sleight & Sanger,
1976; Garthoff et al., 1977). A significant increase in BUN occurred
in cows (Moorhead et al., 1977; Durst et al., 1978a) and a calf
(Robl et al., 1978) that had received doses of FireMaster(R) high
enough to produce overt signs of toxicosis (25 g FM BP-6 per day
(equivalent to 50 mg/kg body weight per day, and 100 mg FM FF-1/kg
body weight per day, respectively).
(v) Serum lipids
Characteristic alterations in serum phospholipid HPLC profiles,
which were maintained for at least two months after dosing, were
found in rats given a single oral dose of FireMaster(R) BP-6
(500 mg/kg body weight) (Bernert et al., 1985).
(vi) Serum cholesterol
In rats, cholesterol levels appear to be the blood parameter
most sensitive to PBBs. There were dose-related increases in
cholesterol concentrations in short-term (Garthoff et al., 1977;
Spear et al., 1990) and long-term (see 8.4: Bernert et al., 1983;
Gupta et al., 1983a,b) studies, and these increases were significant
at dietary concentrations of FireMaster(R) as low as 5 mg/kg
(Garthoff et al., 1977). No noticeable effects of FireMaster(R) on
cholesterol levels have been reported in cattle (Durst et al.,
1978a) and pigs (Werner & Sleight, 1981). Rhesus monkeys showed a
gradual decrease in serum cholesterol when they were lethally
intoxicated (Allen et al., 1978).
(vii) Urinalysis
Tests of urine for pH, protein, glucose, ketones, bilirubin,
occult blood, and specific gravity showed no significant changes due
to FireMaster(R) in mice (Gupta et al., 1981) and rats (Sleight
et al., 1978; Gupta et al., 1981) or due to technical decabromo
biphenyl in rats (Millischer et al., 1979). Only Sleight & Sanger
(1976) reported higher readings for protein in rats fed
FireMaster(R). Differences in urinary protein patterns between
control rats and rats given FireMaster were detected by means of
two-dimensional electrophoresis (Myrick et al., 1987). The principal
urine changes in cattle lethally intoxicated were decreased specific
gravity and moderate proteinuria (Moorhead et al., 1977; Durst
et al., 1978a).
8.2.1.3 Morphological and histopathological changes
a) Liver
The liver is the site of the most prominent gross morphological
and histopathological changes due to PBBs in many species.
Enlargement of the liver was a characteristic response to
exposure to FireMaster(R), technical octabromobiphenyl, and
technical decabromobiphenyl, and it frequently occurred at
concentrations lower than required to produce body weight changes
(see Tables 74 and 75). Generally, increases in absolute or relative
liver weights were dose and time dependent (e.g., Garthoff et al.,
1977). A notable exception was in cows, which showed decreases in
body weight as well as an increase in liver weights only at lethal
doses (Table 74).
Grossly, the livers of rats were often friable and had a
mottled surface (Sleight & Sanger, 1976; Waritz et al., 1977; Akoso
et al., 1982a; Render et al., 1982; Raber & Carter, 1986). Red
fluorescence of liver (and other tissues) under UVR (366 nm)
indicated excess porphyrin accumulation. In contrast to rats, which
developed porphyria after long-term exposure, the liver of female
mice given 30 mg FM FF-1/kg body weight per day (22 total doses)
became porphyric after 45 days (Gupta et al., 1981).
FireMaster(R) FF-1 given orally at a dose rate of 22.5 mg/kg body
weight per day for 4 days caused centrolobular accumulation of
Oil-red O-staining lipids in the liver of rats (Kohli et al., 1981).
The principal histopathological alterations in rodent species
consisted of extensive swelling and vacuolation of hepatocytes and
proliferation of smooth-surfaced endoplasmic reticulum (SER) (seen
as "foamy cytoplasm" in light microscopy). The vacuoles were filled
with fat indicating excess lipid accumulation. Proliferation of SER
may be a morphological reflection of enhanced enzyme activity
(Sleight & Sanger, 1976; Render et al., 1982). The changes depended
on dose and length of exposure. They have been reported in rats
after dietary intake of FireMaster(R) (Sleight & Sanger, 1976;
Kasza et al., 1978a; Sleight et al., 1978; Hinton et al., 1979;
Akoso et al., 1982a; Render et al., 1982; Raber & Carter, 1986) and
of technical octabromobiphenyl (Norris et al., 1973; Lee et al.,
1975a). The dietary concentrations ranged from 0.1 to 500 mg/kg for
FireMaster(R) and from 100 to 10 000 mg/kg for octabromobiphenyl.
Effects were seen as early as the tenth day of feeding 100 mg FM
BP-6/kg (Raber & Carter, 1986). In contrast, during a 90-day feeding
trial, technical decabromobiphenyl (dietary concentration: 100, 500,
or 2000 mg/kg) caused hepatic damage only at the highest level
(Millischer et al., 1979). Liver changes, as noted above, were
observed also after a single i.p. injection (200-1000 µmol/kg) of
FireMaster(R) (Goldstein et al., 1979), after single oral dosing
(1000 mg/kg body weight) of FireMaster(R) (Kimbrough et al., 1978)
and of octabromobiphenyl (Lee et al., 1975b), and after multiple
oral dosing of FireMaster(R) (22 doses over a 30-day period:
30 mg/kg body weight per day (Gupta & Moore, 1979; Gupta et al.,
1981) and of octabromobiphenyl (two doses: 3000 mg/kg body weight;
Lee et al., 1975b). As soon as 24 h (Kimbrough et al., 1978), 3 days
(Lee et al., 1975b), or 4 days (Goldstein et al., 1979) after
treatment, histological changes could be detected.
Light and electron microscopic changes also reported in the
liver of rats included reduction or disintegration of rough-
surfaced endoplasmic reticulum (RER) (Lee et al., 1975a,b; Gupta
et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), presence
of myelin bodies (cytoplasmic inclusions; membrane whorls) (Lee
et al., 1975a,b; Sleight & Sanger, 1976; Kasza et al., 1978a;
Kimbrough et al., 1978; Sleight et al., 1978; Hinton et al., 1979;
Gupta et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), di-
or multinucleated cells (Kasza et al., 1978a; Kimbrough et al.,
1978; Gupta & Moore, 1979; Hinton et al., 1979), diminution of
glycogen (Millischer et al., 1979; Gupta et al., 1981; Raber &
Carter, 1986) and mitochondria that were swollen and reduced in
number or had degenerated as time passed (Sleight & Sanger, 1976;
Kasza et al., 1978a; Akoso et al., 1982a). There was also necrosis
of hepatocytes (Kimbrough et al., 1978; Gupta & Moore, 1979; Gupta
et al., 1981), and these necrotic foci were infiltrated with
polymorphonuclear cells and lymphocytes (Gupta et al., 1981). With
octabromobiphenyl, myelin configurations developed 7 days after
treatment and subsequently disappeared one week later (Lee et al.,
1975b).
Changes in the hepatocytes were more advanced in the centri
lobular and midzonal regions than in the periportal area of the
liver lobule (Render et al., 1982; Raber & Carter, 1986). Fatty
infiltration in the livers of male rats was much more pronounced
than in those of female rats (Gupta & Moore, 1979).
Male rats given 100 mg FM FF-1/kg body weight per day (22 total
doses) and dying after 90 days had subacute to chronic hepatitis
with marked focal proliferation of bile ducts (Gupta & Moore, 1979).
Changes in the bile canaliculus (proliferation of microvilli)
were also found in mice fed 1000 mg FM BP-6/kg for 4-14 days.
Changes in the hepatocytes of these mice were increase in cell size,
decrease in RER, increase in SER, degeneration of mitochondria,
decrease in glycogen, and increase in size and number of nucleoli
(Corbett et al., 1978a). Fatty infiltration of the cytoplasm was
reported only in another two studies on mice dosed with 30 mg FM
FF-1/kg body weight per day (Gupta et al., 1981) or fed 167 mg FM
BP-6/kg for 6 weeks (Loose et al., 1981). However, in (moribund)
mice fed 167 mg FM BP-6/kg feed for 12 weeks, lipid vacuoles were
not found within the cytoplasm, but, almost exclusively, within the
nucleus (Martino et al., 1981). Hepatocellular necrosis has also
been observed (Loose et al., 1981).
Hepatocytes of guinea-pigs were swollen and had many more large
vacuoles than those of comparably dosed rats (Sleight & Sanger,
1976), even at lower dietary concentrations (< 10 mg FM/kg). Liver
damage was reported to be minimal at dietary levels of 50 mg FM/kg
(Vos & van Genderen, 1974), but severe centrilobular fatty changes
were found at 100 and 500 mg FM BP-6/kg (Sleight & Sanger, 1976).
Livers of rabbits, dermally treated with FireMaster(R) at 5
or 10 g/kg body weight, showed a mottled appearance, necrotic foci,
and were friable. No gross pathological effects were seen in rabbits
dermally exposed to octabromobiphenyl at 10 g/kg body weight (Waritz
et al., 1977).
Pigs fed FM BP-6 (up to 200 mg/kg) showed the following liver
alterations: fatty change, centrolobular necrosis, swollen
hepatocytes, and homogeneous cytoplasm (Werner & Sleight, 1981).
Compared with other species, liver changes observed in cattle
were less dramatic. Only an early stage of centrilobular fatty
degeneration and glycogen depletion were found in the enlarged
livers of lethally (25 g FM BP-6/day) dosed cows. In addition, there
were changes in the gallbladder and bile duct (Mercer et al., 1978;
Moorhead et al., 1978). Single calves exposed to FM FF-1 for 6-12
weeks had slightly enlarged hepatocytes (dose: 1 mg/kg body weight
per day) or necrosis of individual or small foci of hepatocytes
(dose: 100 mg/kg body weight per day) (Robl et al., 1978).
The only consistent histopathological lesion in mink which died
(exposure: 6.25 mg FM FF-1/kg feed), was a fatty infiltration of the
liver (Aulerich & Ringer, 1979).
No hepatocellular damage was found in dogs given oral doses of
FM BP-6 (1 mg/kg body weight per day) for seven weeks (Farber
et al., 1976).
Biopsies of the livers of two rhesus monkeys given a diet
containing 25 mg FM FF-1/kg at 12 weeks revealed enlargement of
hepatocytes and a marked proliferation of SER (Allen et al., 1978).
Liver changes in avian species were similar to those observed
in mammals. White Leghorn cockerels fed a diet of 10 or 100 mg FM
FF-1/kg showed enlargement and vacuolation of hepatocytes, increased
SER, swollen mitochondria, and disruption of mitochondrial cristae.
But, unlike rats, increased SER was not a significant feature
(Dharma et al., 1982).
b) Thymus
The thymus is also an organ sensitive to PBB exposure.
Decreases in thymus weights were observed in rats, mice, and cattle
after oral or intraperitoneal doses (Table 74) and in mice after
feeding (Table 75) of FireMaster(R) mixtures. There were no
reports on thymus weight changes due to exposure to commercial octa-
or decabromobiphenyl (Tables 74 and 75). The weight of the thymus
was reduced as early as 6 (Robertson et al., 1981b) or 15 days
(Gupta et al., 1981; Andres et al., 1983) after rats were given high
doses of FireMaster(R) (Table 74). Frequently, decreases in thymus
weights were accompanied by increases in liver weights (see: Tables
74 and 75). But, in some cases, changes in thymus weights were seen
at doses lower than those required for liver changes (Fraker, 1980),
or vice versa (Akoso et al., 1982a). Rats (Akoso et al., 1982a) and
mice (Fraker & Aust, 1978) both fed FireMaster(R) BP-6 (100 mg/kg
feed) for 30 days differed in their thymic response in that rats
remained unaffected and mice showed decreased thymic weight
(Table 75). In contrast, rats appeared to be more sensitive than
mice when given equal oral doses (30 mg/kg body weight per day for a
period of 30 days) of FireMaster(R) FF-1 (Luster et al., 1978;
Gupta et al., 1981: Table 74). Two strains of inbred mice are known
to differ in their thymic sensitivity to FireMaster(R) (Robertson
et al., 1984c: Table 74).
In some studies, the histological appearance of the thymus of
rats and mice that had survived exposure to FireMaster(R) was only
minimally, or not, affected (Gupta & Moore, 1979; Gupta et al.,
1981; Loose et al., 1981; Akoso et al., 1982a), even when organ
weights were altered. In other studies, a preferential atrophy of
the cortex of the thymus was found in rats and mice similarly
exposed (Luster et al., 1978; Fraker, 1980). At high doses (1000 mg
FM BP-6/kg feed), "surviving" mice (30%) were essentially athymic by
day 14 (Fraker & Aust, 1978). The thymus was markedly involuted also
in moribund rats. The normal architecture of the thymus was
obliterated with marked atrophy and loss of demarcation between the
cortical and medullary regions and disappearance of cortical
thymocytes (Gupta & Moore; 1979). Moderate to marked atrophy of the
thymus was also observed in guinea-pigs (Vos & van Genderen, 1974)
or cattle (Moorhead et al., 1978) that were lethally intoxicated.
The bursa of Fabricius, an analogous organ in avian species,
was also affected by FireMaster(R) (Table 75: Vos & van Genderen,
1974; Ringer, 1978; Dharma et al., 1982). Reductions in weight
occurred at exposures as low as 10 mg/kg feed in cockerels (Dharma
et al., 1982). Histologically, the bursa showed depletion of the
lymphoid cells especially in the medulla (Vos & van Genderen, 1974;
Ringer, 1978; Dharma et al., 1982).
c) Spleen
Changes in spleen weights are given in Tables 74 and 75.
Feeding of FireMaster(R) (100-200 mg/kg equivalent to 10-20 mg/kg
body weight per day) had no effect on the spleen weights of young
rats (Harris et al., 1978b; Akoso et al., 1982a), but resulted in a
decrease in the spleen weights of mice (equivalent to 15-30 mg/kg
body weight per day) (Fraker & Aust, 1978; Fraker, 1980; Loose
et al., 1981). An increase in spleen weights was observed in pups of
both rats (Harris et al., 1978a) and mice (Luster et al., 1980)
perinatally exposed to FireMaster(R). No effects were found in
some oral dosing studies on rats (Harris et al., 1978a; Gupta
et al., 1981); in another study, spleen weights were decreased
(Luster et al., 1978), and in the highest-dosage study reported
spleen weights increased (Gupta & Moore, 1979). On the other hand,
rats that received i.p. injection of FireMaster(R) showed
decreases in spleen weight at the higher doses (Robertson et al.,
1981b; Andres et al., 1983) and increases at the lower dose
(Robertson et al., 1981b). While female mice orally dosed with
FireMaster(R) showed decreased spleen weights (Luster et al.,
1978), pregnant and nursing females comparably dosed exhibited
increased spleen weights (Luster et al., 1980). No effect was found
in mice, 5 days after a single i.p. injection of FireMaster(R),
though the liver and thymus were affected (Robertson et al., 1984c).
Spleen weights of chicks fed FireMaster(R) were reduced (Vos & van
Genderen, 1974; Ringer, 1978) or remained unaffected (Dharma et al.,
1982).
Significant histopathological changes in the spleen have not
been reported, except in rats that were moribund from high doses of
FireMaster(R). In these animals, the splenic lymphatic follicles
were small because of a lack of periarterial lymphoid cells (Gupta &
Moore, 1979).
d) Thyroid
Exposure to FireMaster(R) resulted in an increase in thyroid
weight, if there was any weight change (see Tables 74 and 75).
Increases in thyroid weight have been observed in rats (Sleight
et al., 1978; Allen-Rowlands et al., 1981; Akoso et al., 1982b;
Render et al., 1982), newborn pigs (Werner & Sleight, 1981) and in
chickens (Ringer & Polin, 1977; Ringer, 1978). When no weight change
occurred (rats: Sleight et al., 1978; Gupta et al., 1981; mice:
Gupta et al., 1981; chickens: Dharma et al., 1982), the extent of
exposure was not always less than in the former studies.
Octabromobiphenyl tested in one inhalation study did not have any
effect on thyroid weight in rats (Waritz et al., 1977).
Histological changes in the thyroid gland developed in rats at
dietary concentrations of FireMaster(R) BP-6 as low as 5 mg/kg
(Kasza et al., 1978b). Hyperplasia of follicular cells occurred in
rats (Kasza et al., 1978b; Sleight et al., 1978) and newborn pigs
(Werner & Sleight, 1981). The normal low cuboidal follicular
epithelium was altered to one that had a more columnar appearance
(Kasza et al., 1978b; Sleight et al., 1978). The most prominent
ultrastructural lesions found in rats fed 5-500 mg
FireMaster(R)/kg for 5 weeks were dose-dependent. They included
abnormal lysosomes and colloid droplets in the cytoplasm, vacuolated
mitochondria with disrupted cristae, luminal surfaces with short and
abnormally branched microvilli or devoid of microvilli, and abnormal
cytoplasmic processes into the lumen (Kasza et al., 1978b).
e) Kidney
In most studies using rodents (Sleight & Sanger, 1976; Waritz
et al; 1977; Harris et al., 1978a,b; Gupta et al., 1981; Render
et al., 1982; Ecobichon et al., 1983; Smith et al., 1986), kidney
weights did not change with PBB exposure (Tables 74 and 75).
Increases in kidney weights were caused by FireMaster(R) in minks
(Aulerich & Ringer, 1979) pigs (Ku et al., 1978), and cattle
(Moorhead et al., 1977), and by octabromobiphenyl in rats (Norris
et al., 1973). A single study reported a decrease in kidney weight
in rats fed FireMaster(R) BP-6 (Garthoff et al., 1977).
In cattle, kidneys were severely affected, and doubled in size
in animals that were moribund from high doses of FireMaster(R)
(Moorhead et al., 1977). The kidneys were distended with fluid, and
pale tan to gray in colour. Perirenal lymph nodes were enlarged and
oedematous. The principal histological lesions consisted of
dilatation of collecting ducts and convoluted tubules, and tubular
epithelial degenerative changes (Moorhead et al., 1977). Similar
renal lesions were found in calves treated with different doses of
FireMaster(R) (0.1-100 mg FM FF-1/kg body weight per day, for 2-12
weeks). The severity of renal damage was related to dose level and
length of exposure (Robl et al., 1978). Despite the extensive
morphological damage, effective renal plasma flow rates and
glomerular filtration rates were not affected in cows (Mercer
et al., 1978; Schanbacher et al., 1978).
Ultrastructural analysis of kidneys of rats and mice given a
single i.p. injection of 150 mg "PBB" (not specified)/kg body weight
revealed proliferation of SER and increased numbers of peroxisomes
in the proximal tubule of the rat, 15 days after dosing.
Proliferation of SER was confined to only one segment (S3) of the
proximal tubule. Mice had only marginal increases in SER and no
significant increases in peroxisomes (Rush et al., 1986).
f) Stomach
Biopsies of the stomachs of two rhesus monkeys given
FireMaster(R) FF-1 (25 mg/kg diet) were made at 12 weeks. In the
gastric mucosa, there was evidence of early epithelial hyperplasia
and penetration of the submucosa by glandular epithelium (Allen
et al., 1978).
g) Testicle
Changes in the weights of the testes due to PBB exposure (see
Tables 74 and 75) were not found in rats (Norris et al., 1973;
Garthoff et al., 1977; Harris et al., 1978b; Gupta et al., 1981;
Castracane et al., 1982) and mice (Corbett et al., 1978a; Gupta
et al., 1981). The results of studies on chickens (Ringer & Polin,
1977; Ringer, 1978; Dharma et al., 1982) were inconsistent (see
Table 75).
Histologically, treatment-associated changes (e.g.,
hypospermatogenesis) were observed in the testes of male calves
administered FireMaster(R) FF-1 (0.1-100 mg/kg body weight per
day) for 2-12 weeks (Robl et al., 1978). Chickens fed
FireMaster(R) (50 mg/kg of feed) showed lipid infiltration into
the testicular parenchyma (Ringer & Polin, 1977).
h) Fluid accumulation
The presence of fluid accumulation, i.e., hydropericardium and
ascites, was noted in chickens (Heinemann & Ringer, 1976; Ringer,
1978). This lesion is known as "chick oedema disease" (McConnell,
1980). Oedema were observed also in the skin of rhesus monkeys
(Allen et al., 1978) and in the kidney, perirenal lymph nodes, and
the gastrointestinal tract of cattle (Moorhead et al., 1978) that
were lethally intoxicated.
Occasionally, weight changes in organs other than those
discussed above have been reported (see Tables 74 and 75, but they
appear to be of minor importance.
Some special effects occuring after single or short-term
exposure to PBBs are reviewed in the respective sections.
8.2.2 Individual PBB congeners and comparative studies
A lot of individual PBB congeners have been examined for
prominent general signs of toxicity, such as changes in body and
relative organ weights (see Tables 76 and 77) and for histopatho
logical changes (see Tables 78 and 79). It is evident from these
records that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological
changes in the liver and thymus. Despite variations in experimental
protocols, a tendency can be seen that the most severe effects are
elicited by congeners listed under category I. The relative severity
of damage decreases in categories II and III with the least effects
in the last group. Within a category, the degree of bromination may
also influence toxicity. Categorization of halogenated biphenyls has
been made on a structural basis (Parkinson et al., 1983; Safe,
1984). Category I comprises isomers and congeners lacking
ortho-substituents. They are referred to as coplanar PBBs.
Mono- ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more ortho-bromines) have
been organized into the third category. Structure-activity
relationships are discussed in detail by several authors (e.g.,
Goldstein, 1979; Parkinson et al., 1983; Safe, 1984).
Orders of toxicity derived from comparative studies included
only a limited number of congeners in any given study, but they
agreed with the trends described above. Ecobichon et al. (1979)
evaluated ultrastructural effects of lower and higher brominated
congeners on hepatocytes of rats and showed that the highly
brominated congeners (tetra-, penta-, hexa-, octabromobiphenyls)
were more active than the low bromine-containing congeners (di-,
tribromobiphenyls). Results of comparative studies dealing with
higher brominated isomers and congeners, predominantly constituents
of the FireMaster(R)-mixture, and the mixture itself have been
summarized in Table 80.
In all combinations tested, 3,3',4,4',5,5'-hexabromobiphenyl
(BB 169) was found to be the most toxic PBB. This congener, only a
very minor constituent of FireMaster(R), resembles 2,3,7,8-tetra
chlorodibenzo- p-dioxin (TCDD), the typical and the most toxic
member of the class of polyhalogenated hydrocarbons (e.g., Poland &
Knutson, 1982). Of the major FireMaster(R) constituents,
2,3,3',4,4',5-hexabromobiphenyl (BB 156) appeared to be the most
toxic, followed by 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5-pentabromobiphenyl (BB 118). The main component of the
FireMaster(R)-mixture, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153)
was relatively nontoxic as well as the second most abundant
constituent, 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). Compared
with the mixture itself, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)
was consistently more toxic than FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) less toxic. With
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), different results were
obtained from a dosing study (Dannan et al., 1978a) and two feeding
studies (Akoso et al., 1982a,b; Dharma et al., 1982). The latter
attributed FireMaster(R) a higher toxicity than 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167); 2,2',4,5,5'-pentabromobiphenyl (BB 101)
was less effective in producing adverse effects than
2,3',4,4',5-pentabromobiphenyl (BB 118), which matched
FireMaster(R) in some aspects (see Table 80).
Table 76. Effect of individual PBB congeners on body weight (or body weight gain) and relative organ weights of mice
and rats (dosing studies)
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Categoryd I PBBs ("Coplanar" congeners)
4,4'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon
600 (4-6) (days 1,2,3) (peanut oil) et al. (1979)
t.p.: 7 dayse
3,4,4',tri rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4'-tri rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
300 (3) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,4,4',5-tetra rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4',5-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982)
t.p.: 5 dayse
3,3',4,4'-tetra rat (Sprague- male oral single dose (corn no effect no effect (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4'-tetra rat (Long male intraperitoneal single no effect increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4'-tetra rat (Wistar) male intraperitoneal single reduced increased reduced Andres et al.
150 (4-5) dose (corn oil) (1983);
t.p.: 2 weeks Robertson
et al. (1983b)
3,3',4,4'-tetra rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
4.25g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4'-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4'-tetra mouse male intraperitoneal single no effect increased reduced Robertson et al.
(C57BL/6J dose (corn oil) (1984c)
1500 and DBA/2J) t.p.: 5 days
(10)
3,3',4,4',5-penta rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5-penta rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4',5,5'-hexa rat (Sprague- male oral single dose (corn no effect (increased)f (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
3,3',4,4',5,5'-hexa rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
3.19g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) t.p.: 7 daysd (1979)
Category II PBBs (Monoortho "coplanar" derivatives)
2,3',4,4'-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4'-tetra mouse male intraperitoneal single Robertson et al.
1500 dose (corn oil) (1984c)
t.p.: 5 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
(C57BL/6J) no effect no effect no effect
(5)
(DBA/2J) no effect increased no effect
(5)
2,3',4,4',5-penta rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single reduced increased reduced Dannan et al.
Dawley) (6) dose (polyethylene glycol) (1982c)
164g t.p.: 2 weeks no effect increased no effect Millis et al.
(1985a)
2,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single reduced increased (reduced)f Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1978a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Sprague- male intraperitoneal 2 doses reduced increased reduced Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
3.8-60 (1-4) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,3,3',4,4',5'-hexa rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Category III PBBs (Others)
4-mono rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (day 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2'-di rat (Sprague- male intraperitoneal single -- no effect -- Moore et al.
289g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: 2-22 days
2,2'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,5'-di rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,2'5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,3',4'5-tetra rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
150 (4) (days 1,3) (corn oil) (1980)
t.p.: 5 dayse
2,2',5,5'-tetra rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4,4',6-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,3',4,4',6-penta rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5,5'-penta rat (Long male intraperitoneal single no effect increased no effect
500 Evans) (3) dose (corn oil)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single no effect no effect no effect Dannan et al.
164g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
(6) t.p.: 14 days no effect increased no effect Millis et al.
(1985a)
2,2',3,4,4',5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',4,4',6,6'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa rat (Fischer male, oral 22 doses (over no effect increased no effect Gupta et al.
590g 344/N) (6) female 30 days) (corn oil) (1981)
t.p.: 15, 30, 45, 60,
90 dayse
2,2',4,4',5,5'-hexa rat (Fischer) female intraperitoneal single Goldstein et al.
(4) dose (corn oil) (1979)
t.p.: 4 days
40 no effect no effect --
200, no effect (increased)f --
1000
2,2',4,4',5,5'-hexa rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Moore et al.
144g Dawley) (6) dose (polyethylene glycol) (1978b); Millis
t.p.: 2 weeks et al. (1985a)
2,2',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa mouse male, oral 22 doses (over no effect increased no effect Gupta et al.
590g (B6C3F1/N) female 30 days) (corn oil) (1981)
(6) t.p.: 15, 30, 45, 69,
90 dayse
2,2',3,3',4,4'5-hepta rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
127g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',3,4,4',5,5'-hepta rat (Sprague- male intraperitoneal single -- increased -- Moore et al.
127g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: up to 22 days
2,3,3',4,4',5,6-hepta rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
6 (2) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,2',3,3',4,4',5,5'- rat (not male intraperitoneal single -- increased -- Besaw et al.
octa 115g specified) dose (solvent not (1978)
specified) t.p.: 7 days
Table 76 (contd).
a Total dose in µmol/kg body weight.
b t.p. = Time post-exposure; -- = data not given.
c Only statistically significant changes; organ weight changes relative to body weight.
d Categorization according to Safe (1984); see also text in section 8.2.2.
e After first dose.
f Only absolute data given.
g Calculated from original value given in mg/kg body weight.
Table 77. Effect of various hexabromobiphenyl isomers on feed intake, body weight (or body weight gain)
and (relative) organ weights (feeding studies)
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Categoryb I PBBs
3,3',4,4',5,5'- rat male 10 30 reduced reduced increased reduced spleen: reduced Akoso et
hexa (Sprague- brain: no effect (1982a,b)
Dawley) thyroid:
(6) increased
rat male 1 9 no effect no effect no effect Render
(Sprague- 10 9 reduced reduced increased reduced et al.
Dawley) 100 9 reduced reduced increased reduced (1982)
(6)
(2) 100 20 refused
(day 16)
Category II PBBs
2,3',4,4',5,5'- rat male 1, 10 30 no effect no effect no effect no effect brain: increased Akoso
hexa (Sprague- thyroid: no effect et al.
Dawley) 100 30 no effect no effect increased no effect brain: increased (1982a,b)
(6) thyroid: no effect
cockerel male 4, 10 28 no effect no effect no effect bursa of Dharma
(White Fabricius, et al.
Leghorn) thyroid, spleen, (1982)
(10) testicles, comb:
no effect
Table 77 (contd).
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Category III PBBs
2,2',4,4',5,5'- rat male 1 30 no effect no effect increased no effect brain: increased Akoso
hexa (Sprague- thyroid: no et al.
Dawley) effect (1982a,b)
(6)
(6) 10, 100 30 no effect no effect increased increased brain: increased
thyroid: no
effect
rat male 10 9 increased no effect increased n.r. kidney: no Render
(Sprague- effect et al.
Dawley) 100 9 no effect no effect increased n.r. kidney: no (1982)
(6) effect
mouse female 100, 300 gd 6 no effect no effect increased n.r. Welsch &
(C57 BL), through Morgan
pregnant 15 (1985)
(3) 500, 750 sacrifice no effect reduced increased n.r.
gd 17
1000 reduced reduced increased n.r.
cockerel male 10 28 no effect no effect no effect n.r. bursa of Fabri- Dharma et al.
(White cius, thyroid, (1982)
Leghorn) 62 28 no effect no effect increased n.r. spleen, testicles,
(10) comb, no effect
(both concen-
trations)
Table 77 (contd).
a Only statistically significant changes; organ weight changes relative to body weight except for Render et al.
(1982) giving absolute data.
b Categorization according to Safe (1984); see also text in section 8.2.2.
c gd = Gestation day.
d n.r. = Not recorded.
Table 78. Histopathology in relation to individual PBB congeners (dosing studies)
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
Categorya I PBBs
4,4'-di rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(6) t.p.: 7 daysd (1977)
3,3',4,4'-tetra rat (Sprague- male oral single dose 21.3 + ++ 8 tissues: Millis et al.
Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Sprague- male intraperitoneal single 4.25e + 0 11 tissues: Millis et al.
Dawley) (6) dose t.p.: 2 weeks 0 (1985a)
rat (Wistar) male intraperitoneal single 150 + ++ spleen, Andres et al.
(4) dose t.p.: 2 weeks kidney: 0 (1983); Robertson
et al. (1983b)
3,3',4,4',5,5'- rat (Sprague- male oral single dose 21.3 ++ ++ 8 tissues: Millis et al.
hexa Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
Categorya II PBBs
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) (6) dose t.p.: 2 weeks tissues: 0 (1982c); Millis
et al. (1985a)
2,3',4,4',5,5'- rat (Sprague- male intraperitoneal single 144e ++ n.r. 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,3,3',4,4',5- rat (Sprague- male intraperitoneal single 144e ++ ++ 9 tissues:
hexa Dawley) (4) dose t.p.: 1 week 0
Categorya III PBBs
4-mono rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2'-di rat (Sprague- male intraperitoneal single 289e 0 0 8 tissues: Moore et al.
Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,5'-di rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r.
(4-6) t.p.: 7 daysd
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,2',5,5'-tetra rat (Wistar) male intraperitoneal single 150 + 0 spleen, Robertson et al.
(not specified) dose t.p.: 2 weeks kidney: 0 (1983b)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) dose t.p.: 1-2 weeks tissues: 0 (1982a); Millis
(4-6) et al. (1985a)
2,2',3,4,4',5'- rat (Sprague- male intraperitoneal single 144e + 0 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',4,4',5,5'- rat (Fischer male, oral 22 doses 1052e + 0 16 tissues: Gupta et al.
hexa 344/N) female t.p.: up to 90 daysd 0 0 (1981)
2,2',4,4',5,5'- rat (Fischer) male intraperitoneal single 200-1000 + n.r. n.r. Goldstein et al.
hexa (4) dose t.p.: 4 days (1979)
rat (Sprague- male intraperitoneal single 144e + 0 8-11 Moore et al.
Dawley) dose t.p.: up to 14 days tissues: 0 (1978b); Millis
(3-6) et al. (1985a)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,4',6,6'- rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
hexa (4-6) t.p.: 7 daysd
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,2',3,3',4,4',5- rat (Sprague- male intraperitoneal single 127e + 0 9 tissues: Dannan et al.
hepta Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',3,4,4',5,5'- rat (Sprague- male intraperitoneal single 127e + 0 8 tissues: Moore et al.
hepta Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,2',3,3',4,4', rat (not male intraperitoneal single 115e + 0 several Besaw et al.
5,5'-octa specified) dose t.p.: 7 days tissues: 0 (1978)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
a Categorization according to Safe (1984); see also text in section 8.2.2.
b Total dose in µmol/kg body weight.
c t.p. = Time post-exposure.
d After first dose.
e Calculated from original value given in mg/kg body weight.
f n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Table 79. Histopathology to individual PBB congeners (feeding studies)
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
Categorya I PBBs
3,3',4,4',5,5'- rat (Sprague- male 1 10 + 0 Render et al. (1982)
hexa Dawley) (6) 10 10 ++ ++
100 10 ++ ++ spleen, lymph
nodes: +
rat (Sprague- male 1 30 + 0 thyroid: + Akoso et al.
Dawley) (6) 10 30 ++ ++ thyroid, (1982a,b)
pituitary
gland: +
Categorya II PBBs
2,3',4,4',5,5'- rat (Sprague- male 1, 10, 100 30 + 0 thyroid: +
hexa Dawley) (6)
cockerels male 10 28 + n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: +
(10)
Categorya III PBBs
2,2',4,4',5,5'- rat (Sprague- male 10, 100 10 + 0 18 tissues: 0 Render et al. (1982)
hexa Dawley) (6)
rat (Sprague- male 1, 10, 100 30 + 0 thyroid: + Akoso et al.
Dawley) (6) (1982a,b)
Table 79 (cont'd).
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
cockerel male 4, 10 28 0 n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: 0
(10) 62 28 + n.r. bursa of
Fabricius: +
a Categorization according to Safe (1984); see also text in section 8.2.2.
b n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Dannan et al. (1982b) recombined 9 purified FireMaster(R)
constituents, namely BB 101 (2,2',4,5,5'-penta-,) BB 118
(2,3',4,4',5-penta-), BB 153 (2,2',4,4',5,5'-hexa-), BB 138
(2,2',3,4,4',5'-hexa-), BB 167 (2,3',4,4',5,5'-hexa), BB 156
(2,3,3',4,4',5-hexa-), BB 180 (2,2',3,4,4',5,5'-hepta-), BB 170
(2,2',3,3',4,4',5-hepta-), and BB 194 (2,2',3,3',4,4',5,5'-
octabromobiphenyl), totalling 97% of either FireMaster(R) mixture,
to form a reconstituted FireMaster(R) BP-6-like mixture and
compared some effects of the reconstituted mixture to those of crude
FireMaster(R) mixtures BP-6 and FF-1. Rats were treated with a
single dose (90 mg/kg body weight of either mixture) and sacrificed
one week later. Evaluating changes in body and selected organ
(liver, thymus, spleen) weights, the conclusion was reached that
adverse effects of FireMaster(R) (FF-1 or BP-6) must be due to the
effects of the congeners studied. Moreover, it was found that the
increase in liver weights was greater with FM BP-6 and the
reconstituted mixture than with FM FF-1, consistent with the higher
proportion of minor components (BBs: 118, 138, 167, 156) in FM BP-6
(25% versus 15%).
Differences in toxicity between PBBs were sometimes of a
qualitative kind, but mostly they were quantitative. Some striking
relations will be reviewed in detail according to the parameter
tested.
8.2.2.1 Food intake, overt clinical signs, body weight changes
So far, in the congeners tested, food intake of rats was
reduced only by 3,3'4,4',5,5'-hexabromobiphenyl (Table 77: Akoso
et al., 1982a; Render et al., 1982). The effective dietary
concentrations ranged from 1 to 100 mg/kg. A much higher
concentration of 2,2',4,4',5,5'-hexabromobiphenyl (1000 mg/kg of
feed) was needed to evoke reduction in the food consumption of mice
(Table 77: Welsch & Morgan, 1985).
Rats, moribund from 3,3',4,4',5,5'-hexabromobiphenyl treatment,
had symptoms similar to those observed with FireMaster(R)
toxicosis (see section 8.2.1.1). They became less active, had a
roughened hair coat, developed sunken eyes, and were emaciated
(Render et al., 1982).
3,3',4,4',5,5'-Hexabromobiphenyl significantly decreased body
weight (Render et al., 1982) or body weight gain (Akoso et al.,
1982a) in rats, while the same concentrations of FireMaster(R)
BP-6, 2,2',4,4',5,5'-hexa- and 2,3',4,4',5,5'-hexabromobiphenyl in
the diet had no effects. When rats were injected i.p. with identical
Table 80. Order of toxicity of higher brominated PBB congeners and the FireMaster(R) mixture on the basis of comparative studies
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Dosing studies
Rat body weight gain x x 167 > FM Dannan et al.
(1978a)
Rat liver weight and x x FM > 153 Moore et al.
histopathology (1978b); Goldstein
et al. (1979)
Rat liver weight and x x FM > 180 Moore et al.
histopathology (1979a)
Rat body and organ x x FM > 153 Gupta et al.
(Mouse) weights, histopathology (1981)
Rat body and organ x x 118 > FM Dannan et al.
weights, histopathology (1982c)
Rat body and organ x x x x x 156 > 167 > Dannan et al.
weights, histopathology 138, 170 > (1982a)
101
Rat body and organ x x x 169 > 118, Parkinson et al.
weights 153 (1983)
Rat liver histopathology x x x 118, 153 > Millis et al.
101 (1985a)
Table 80 (cont'd)
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Feeding studies
Rat death, body and organ x x x 169 > FM Sleight et al.
weights, histopathology > 153 (1981)
Rat feed intake, body and x x x x 169 > FM Akoso et al.
organ weights, > 167 > 153 (1982a,b)
histopathology
Rat death, feed intake, x x x 169 > FM, Render et al.
body and organ weights, 153 (1982)
histopathology
Cockerel organ weights, x x x FM > 167 Dharma et al.
histopathology > 153 (1982)
a FM = FireMaster(R) mixture (BP-6 or FF-1); PPB numbering according to Ballschmiter & Zell (1980):
101, 118 = pentabromobiphenyls (2,2',4,5,5'-; 2,3',4,4',5-).
153, 138, 167, 156, 169 = hexabromobiphenyls (2,2',4,4',5,5'-; 2,2',3,4,4',5'-; 2,3',4,4',5,5'-;
2,3,3',4,4',5-; 3,3', 4,4', 5,5'-).
180, 170 = heptabromobiphenyls (2,2',3,4,4',5,5'; 2,2',3,3',4,4'5-).
amounts of several constituents of FireMaster(R) (BBs: 101, 138,
167, 156, 170), only 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 156) depressed body weight gain
(Dannan et al., 1978a, 1982a). The body weight gain of rats treated
with BB 167 was half that of FireMaster(R)-treated animals (Dannan
et al., 1978a). Rats given BB 118 (2,3',4,4',5-hexabromo biphenyl)
also gained less weight per day than those given FireMaster(R)
(Dannan et al., 1982c).
Liver weights in rats were increased by all treatments with
congeners of category I, except for 4,4'-dibromobiphenyl. The lowest
effective dose used was 2 mg/kg body weight (Millis et al., 1985a).
Significant effects were seen as early as 4 days after dosing (e.g.,
Parkinson et al., 1983). Many of the congeners listed under
categories II and III were also capable of increasing liver weights
(see Tables 76 and 77). Generally, all the changes in organ weights
were dose-dependent. Gradual differences between congeners were also
seen. Liver weights were significantly higher in rats given
3,3',4,4',5,5'-hexabromobiphenyl than the liver weights in
3,3',4,4'-tetrabromobiphenyl-treated rats, 6 days after a single
dose (Millis et al., 1985b). In studies comparing the effects of
five FireMaster(R) constituents (BB 101: 2,2',4,5,5'-penta; BB
138: 2,2',3,4,4',5'-hexa; BB 167: 2,3',4,4',5,5'-hexa; BB 156:
2,3,3',4,4',5-hexa; BB 170: 2,2',3,3',4,4',5-hepta), 7 days after
dosing, 2,3,3',4,4',5-hexabromobiphenyl (BB 156) caused the largest
increase in liver weights (60% increase) while 2,2',4,5,5'-
pentabromobiphenyl (BB 101) failed to enlarge this organ. Increases
caused by FireMaster(R) and BB 167 (2,3',4,4',5,5'-hexa) were
similar (Dannan et al., 1978a, 1982a). The increases in liver
weights due to BB 153 (2,2',4,4',5,5'-hexa), the main component of
FireMaster(R), were far less than the increase in response to
FireMaster(R), approximately 33% versus 60-80% (Moore et al.,
1978b; Goldstein et al., 1979).
Nevertheless, the increase was rapid with a 25% increase within
two days of treatment (Moore et al., 1978b). The effect of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), the second most
abundant component of FireMaster(R), was also less than that
caused by the mixture itself (Moore et al., 1979a). When
FireMaster(R) BP-6, 2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), and
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) were added to the diet of
rats for 30 days, BB 153 and BB 169 significantly increased liver
weights at 1 mg/kg, but FireMaster(R) and BB 167 did not. At 100
mg/kg, FireMaster(R) increased liver weight more than BB 153 or BB
167 (Akoso et al., 1982a).
Thymus weights in rodents were decreased by most of the
treatments with congeners of category I. Among congeners in category
II, 2,2',4,4',5-pentabromobiphenyl (BB 118),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), and
2,3,3',4,4',5-hexabromobiphenyl (BB 156) were capable of reducing
thymus weights. Congeners listed under the third category failed to
reduce thymus weights (see Tables 76 and 77). Both 3,3',4,4'-tetra
and 3,3',4,4',5,5'-hexabromobiphenyl caused a significant reduction
in the thymus weights of rats, 3,3',4,4',5,5'-hexabromobiphenyl
being more effective than 3,3',4,4'-tetrabromobiphenyl (Millis
et al., 1985b). There was a 50-60% loss in thymic weight in rats
injected i.p. with BB 167 or BB 156 and sacrificed 7 days later
(Dannan et al., 1978a, 1982a). However, thymus weight: body weight
ratios were not significantly affected when rats were fed BB 167 or
FireMaster(R) BP-6 for 30 days (Akoso et al., 1982a). In the same
study, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) significantly
decreased the ratio, while there was an increase with
2,2',4,4',5,5'-hexabromobiphenyl (BB 153).
In cockerels fed FireMaster(R) FF-1, BB 153, or BB 167, for
28 days, only FireMaster(R) FF-1 reduced relative bursal weights
(Dharma et al., 1982).
When young rats were fed diets containing FireMaster(R) BP-6,
2,2',4,4',5,5'-hexa-, 2,3',4,4',5,5'-hexa-, or 3,3',4,4',5,5'-
hexabromobiphenyl, for 30 days, thyroid weight was increased only by
100 mg FireMaster(R) BP-6/kg feed and by 1 and 10 mg
3,3'4,4',5,5'-hexabromobiphenyl/kg feed (Akoso et al., 1982b).
8.2.2.2 Haematology and clinical chemistry
Haematological and clinical chemistry findings were of minor
importance in comparative studies. Gamma-Glutamyl transpeptidase
(gamma-GTP) was elevated in female rats at high doses of both
FireMaster(R) FF-1 (30 mg/kg body weight per day) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) (16.8 mg/kg body weight
per day) 30 and 60 days after the last dose (Gupta et al., 1981).
8.2.2.3 Morphological and histopathological changes
Rats fed diets containing 100 mg FireMaster(R) BP-6/kg or 10
or 100 mg 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)/kg had friable
yellow livers (Render et al., 1982). The architectural structure of
the lobules was abnormal after feeding 3,3',4,4',5,5'-
hexabromobiphenyl (Akoso et al., 1982a; Millis et al., 1985b), and
bile duct hyperplasia was observed (Render et al., 1982).
With the exception of the lower brominated congeners of
category III, all PBB congeners caused histopathological changes in
the liver (see Tables 78 and 79). The extent of the changes depended
on the dose and the individual congener. The least severe effects
were confined to a slight proliferation of hepatic SER (e.g.,
4,4'-dibromobiphenyl: Ecobichon et al., 1977). More progressive,
general changes were enlargement of hepatocytes and increased
numbers of cytoplasmic lipid vacuoles. Corresponding ultrastructural
lesions consisted mainly of increased SER and lipid vacuolation (see
Tables 78 and 79). Additional changes seen with the more toxic
congeners included myelin body formation (membrane whorls) (BBs:
118, 167, 156, 169), disorganization of RER (BB 169), an increase in
number of binucleated hepatocytes (BB 169), pycnotic nuclei (BB
169), and, occasionally, multifocal areas of necrosis (BB 169)
(Akoso et al., 1980, 1982a; Sleight et al., 1981; Dannan et al.,
1982a,c; Render et al., 1982; Millis et al., 1985b).
The thymus was affected by 3,3',4,4'-tetrabromobiphenyl (Andres
et al., 1983; Millis et al., 1985b), possibly by BB 167 (Dannan
et al., 1978a: "several" tissues: not accurately specified), by BB
156 (Dannan et al., 1982a) and by BB 169 (Sleight et al., 1981;
Akoso et al., 1982a; Render et al., 1982; Millis et al., 1985b).
There was a loss of thymocytes, especially in the cortex, the
demarcation between cortex and medulla was indistinct, and
macrophages were prominent in the remaining portion of the cortex.
One study reported histological alterations in the thyroid of
rats treated with 2,2',4,4',5,5'-hexa- (BB 153), 2,3',4,4',5,5'-hexa
(BB 167), or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169), and in the
pituitary gland with BB 169 (Akoso et al., 1982b). Prominent lesions
of the thyroid were extensive hyperplasia and hypertrophy of
follicular cells and a lack of colloid. The pituitary gland showed
swollen and vacuolated chromophobe cells.
The spleen and lymph nodes of rats given 100 mg 3,3',4,4',5,5'-
hexabromobiphenyl/kg feed for 10 and 20 days had an increased number
of macrophages intermixed with mature lymphocytes. Changes were
similar to those seen in the thymus, but were not as pronounced
(Render et al., 1982).
The main component of FireMaster(R),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), is the congener most
frequently examined and implicated in many comparative studies.
The principal changes seen with BB 153 were vacuolation and
enlargement of hepatocytes with proliferation of SER. They occurred
in dosing studies (Moore et al., 1978b; Ecobichon et al., 1979;
Goldstein et al., 1979; Gupta et al., 1981; Millis et al., 1985a) as
well as in feeding studies (Sleight et al., 1981; Akoso et al.,
1982a; Dharma et al., 1982), and were observed as early as two days
after treatment (Moore et al., 1978b). Generally, the
ultrastructural changes in BB 153-exposed rats were less severe than
those in rats exposed to the FireMaster(R) mixture (e.g.,
Goldstein et al., 1979; Gupta et al., 1981; Akoso et al., 1982a).
Myelin figures and marked disorganization of RER, as seen with the
FireMaster(R) mixture, were not observed with BB 153 in
comparative studies (Gupta et al., 1981; Akoso et al., 1982a).
Moreover, changes caused by FireMaster(R) FF-1 in the livers of
rats persisted, while the livers of rats dosed with BB 153 were
comparable to those of the controls, 60 days after treatment (Gupta
et al., 1981).
The histological appearance of thymuses in rats was not
affected by BB 153 (Tables 78 and 79), but, in cockerels, the
lymphoid cells of the bursa of Fabricius were depleted by 62 mg BB
153/kg feed, by 10 mg FireMaster(R) FF-1/kg feed, and by 10 mg BB
167/kg feed (Dharma et al., 1982). When BB 153, the FireMaster(R)
mixture, BB 167, and BB 169, were fed to rats, proliferation of SER,
decreased RER, and increased fat droplets were seen in hepatocytes,
with all chemicals, but, with BB 169
(3,3',4,4',5,5'-hexabromobiphenyl), proliferation of SER was not as
prominent as with the other three PBBs. In contrast, the RER was
severely altered by BB 169 (Akoso et al., 1982a).
Myelin bodies were observed in rats fed 100 mg BB 169/kg for 20
days (Render et al., 1982) or FireMaster(R) BP-6 for 30 days
(Akoso et al., 1982a), or BB 167 for 60 days (Akoso et al., 1980). A
comparative experimental series testing single doses of six
FireMaster(R) constituents, namely BB 118 (Dannan et al., 1982c;
Millis et al., 1985a), BBs 101, 138, 167, 156, and 170 (Dannan
et al., 1978a, 1982a) found the least severe histological changes
with BB 101, and intermediate effects, which were limited to the
proliferation of SER and cytoplasmic vacuolation in hepatocytes,
with BB 138 and BB 170. The most pronounced changes resulted from
BBs 118, 167, and 156 and consisted of proliferation of SER,
increases in fat vacuoles and myelin figures in hepatocytes (BBs
118, 167, 156) and thymus damage (BB 156). Rats given a single
equimolar dose of 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or
3,3',4,4'-tetrabromobiphenyl showed moderate to severe hepatic
changes 14 days after treatment with BB 169, while the
tetrabromobiphenyl-treated rats showed only mild hepatic changes
(Millis et al., 1985b).
The microscopic hepatic effects of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180) and of
2,2',3,3',4,4',5,5'-octabromobiphenyl (BB 194) were reported to be
similar to those of BB 153 (Besaw et al., 1978; Moore et al., 1979a,
1980).
8.3 Skin and eye irritation, sensitization, dermal lesions,
and acne
Common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions due to the technical
PBB mixtures tested, namely octabromobiphenyl and decabromobiphenyl
(Table 81).
Table 81. Skin and eye irritation or sensitization tests of commercial PBB mixtures
PBBa Species Application Test Observations References
OcBB guinea-pig 50% (w/v) slurry in irritation (intact mild irritation Waritz et al.
(Hartley) propylene glycol shaved dorsal skin) (1977)
(0.05 ml)
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 9 x 50% slurry abraded shaved skin
(topical application)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 4 x 1% (w/v) solution
in dimethyl sulfoxide
(intradermal injection)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB rabbit irritation Norris et al.
(New Zealand) (1973)
dry solid (single and intact shaved skin no response
multiple exposures) abraded shaved skin slight erythematous
and edematous response
Table 81 (contd).
PBBa Species Application Test Observations References
OcBB rabbit moistened with water
(New Zealand) (single exposure) intact shaved skin no response
(repeated exposure) intact shaved skin slight erythematous
response
OcBB rabbit moistened with water abraded shaved skin moderate erythematous
(New Zealand) (single and repeated and slight oedematous
exposures) response
rabbit dry solid eye irritation transient irritation
(New Zealand) of the conjunctival
membranes
OcBB rabbit powder (100 mg) eye irritation no irritating or corneal Waritz et al.
effects; mild (1977)
conjunctival redness and
swelling and a copious
discharge (disappeared
within 4 h)
DeBB rabbit 50% in olive oil irritation mild irritation Millischer et al.
(intact shaved skin) (1979)
DeBB rabbit 50% in olive oil eye irritation no irritating effect
DeBB rabbit powder eye irritation mild irritating
a Commercial PBB mixtures: OcBB = Octabromobiphenyl; DeBB = Decabromobiphenyl.
However, diverse lesions in the skin and skin appendages of
certain animal species, e.g., rhesus monkeys and cattle, occurred
after the ingestion of the FireMaster(R) mixture (Table 82). The
main features were dry scaly skin and hair loss. Hyperkeratosis of
the interfollicular epidermis and of the hair follicle, and atrophy
and squamous metaplasia of the sebaceous glands were observed on
microscopic examination of these lesions. As with related compounds,
comparable epidermal changes have not generally been found in other
laboratory animals, such as guinea-pigs or rats, but they were
similar to those observed in humans following PBB exposure
(section 9) and were described as chloracne (McConnell, 1980;
Kimbrough, 1980b; Poland & Knutson, 1982).
The rabbit ear (inner surface), but not any other part of the
rabbit skin (Crow, 1983), is particularly sensitive to acne-causing
compounds, which was first recognized by Adams et al. (1941). The
reaction is hyperkeratosis. Painting the rabbit ear has become a
standard bioassay to detect hyperkeratotic (acnegenic) activity.
Results of rabbit ear tests obtained with diverse PBBs (technical
octabromobiphenyl, technical decabromobiphenyl FireMaster(R)
mixture, fractions of this mixture, and purified PBB congeners) have
been summarized in Table 83. The FireMaster(R) mixture itself
produced hyperkeratosis, but its main components, BB 153
(2,2',4,4',5,5'-hexa) and BB 180 (2,2',3,4,4',5,5'-hepta), did not.
Fractionation of FireMaster(R) indicated that most activity was
associated with the more polar fraction containing minor compo nents
(Needham et al., 1982). Sunlight irradiation of BB 153 also yielded
products that caused severe hyperkeratosis. It is not clear whether
one or more of the suspected PBBs is responsible for hyperkeratotic
activity (Patterson et al., 1981). The model congener
3,3',4,4',5,5'-hexabromobiphenyl and 3,3'4,4'-tetra bromobiphenyl
were shown to be hyperkeratotic (Table 83).
8.4 Long-term toxicity
Toxic effects of PBBs, observed after long-term exposures, as
well as a long time after exposure had ceased, are summarized in
Tables 84 and 85. Experimental animals tested were rats, mice,
cattle, minks, and rhesus monkeys. The majority of studies refer to
the commercial FireMaster(R) mixture.
The following comments refer mainly to the general signs of
long-term toxicity. Other long-term effects will be reviewed in
detail in the respective sections, e.g., carcinogenicity (section
8.7), reproductive dysfunctions (section 8.5).
Table 82. Dermal lesions observed in cattle, rhesus monkeys, and rabbits after exposure to PBBs
PBBa Species Route Dose (duration) Dermal lesions observed References
FM BP-6 cattle oral 25 g/day (for subcutaneous emphysema and haemorrhage; changes Moorhead et al.
33-60 days) in the eyelids: hyperkeratosis, with accumulations of (1977, 1978)
keratin in hair follicles of the epidermis and squamous
metaplasia with keratin cysts in the tarsal glands
FM FF-1 calf oral 100 mg/kg body keratitis, alopecia, hyperkeratosis involving the Robl et al.
weight (up to head, cervical and dorsal thoracic region (1978)
12 weeks)
oral 0.1, 10, 100 acanthosis, hyperkeratosis and/or dermal infiltrates
mg/kg body of mononuclear cells (dose-dependent severity)
weight (up to
12 weeks)
FM FF-1 rhesus in diet 25 mg/kg feed alopecia, dry scaly skin, loss of eyelashes; Allen et al.
monkey (for 25 weeks) generalized subcutaneous oedema, marked oedema of (1978)
adult total dose: the eyelids; keratinization of hair follicles
(male) approx. 1 g and sebaceous glands
juvenile in diet 25 mg/kg feed moderate loss of hair including eyelashes, dry
(female) (for 50 weeks) scaly skin; periorbital oedema
total dose:
approx. 1.5 g
juvenile in diet 300 mg/kg feed a rather generalized loss of hair, absent eyelashes; Allen et al.
(female) (for 137 days) considerable periorbital congestion and oedema; (1978)
total dose: keratinization of hair follicles
approx. 6.4 g
Table 82 (contd).
PBBa Species Route Dose (duration) Dermal lesions observed References
FM FF-1 rhesus in diet 1.5 mg/kg feed periorbital oedema Allen &
monkey (for over 5 Lambrecht (1978)
months)
total dose:
approx. 75 mg
OcBB rabbit dermal not specified erythema, exfoliation in the ear Norris et al.
(ear) (1 month) (1973)
Various rabbit dermal variable hyperkeratosis in the ear see Table 83
PBBs (ear)
FM = FireMaster(R); OcBB = Technical octabromobiphenyl.
Table 83. Hyperkeratotic activity of commercial PBB mixtures, the fractionateda FireMaster(R) mixture
and purified PBB congeners, derived from the rabbit ear test
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
DeBB (Adine 0102) 200, 2000 (acetone) x Atochem (1990)
OcBB not specified x Norris et al.
(chloroform) (1973)
FM FF-1 (lot FH 7042)
mixture itself 60 (not specified) x Kimbrough
polar fraction not specified (not specified) x (+++) et al.
non-polar fraction not specified (not specified) x (+) (1977)
FM BP-6
mixture itself 6.5 µg/kg body weight x Hass et al.
(benzene-decane, 1:9) (1978)
fraction 1 (non-polar) containing PBBs 6.5 µg/kg body weight x
(benzene-decane, 1:9)
FM FF-1 (lot FH 7042)
mixture itself 100 (toluene) x (++) Needham et al.
less polar fractions 50-210 (toluene) x (1982)
more polar fraction containing minor 185 (toluene) x (+++)
components of the FM
mixture
Compound 4 predominantly BB 153 5 (toluene) x
Compound 8 predominantly BB 180 3.3 (toluene) x
Table 83 (contd).
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
2,2',4,4',5,5'-hexa- 10 (not specified) x Patterson et al.
bromobiphenyl (BB 153) (1981)
(96% pure)
sunlight degradation mixture of BB 153 10 (not specified) x (+++) Patterson et al.
products of BB 153 and other PBBs (1981)
(probably BB 101,
BB 118 and 3,3',4,4'-
tetra-bromobiphenyl)
3,3',4,4',5,5'-hexa- 0.32-9.6 (toluene) x (++) Needham et al.
bromobiphenyl (1982)
3,3',4,4'-tetra- 0.245 and 0.02 x (++)
bromobiphenyl (not specified)
a The mixture was fractioned by different methods (on Florisil: Hass et al., 1978; on alumina:
Kimbrough et al., 1977; by HPLC and GC: Needham et al., 1982).
b Total dose in mg/rabbit ear, unless otherwise specified.
c (+), (++), (+++) = severity of hyperkeratosis.
d DeBB = technical decabromobiphenyl; OcBB = technical octabromobiphenyl; FM = FireMaster(R).
BB 101 = 2,2',4,5,5'-pentabromobiphenyl; BB 118 = 2,3',4,4',5-pentabromobiphenyl;
BB 153 = 2,2',4,4',5,5'-hexabromobiphenyl; BB 180 = 2,2',3,4,4',5,5'-heptabromobiphenyl.
Table 84. Long-term effects observed after exposure to PBBs by gavage
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, single dose 1000 liver: lipid accumulation (more pronounced Kimbrough et al.
(lot FH 7042) (Sherman) female t.p.: 6,10,14 in males); uroporphyrin accumulation (1977, 1978)
(in peanut oil) (5) months (females); histopathological changes;
neo-plastic nodules; increase in relative
weight
FM FF-1 rat male, 22 doses over 30 liver: marked hepatotoxic effects; atypical Gupta &
(lot No. 1312 FT) (Fischer female 30 days; t.p.: nodules Moore (1979)
(in corn oil) 344/N) 6 months after
(9) first dose
FM FF-1 rat female single dose 1000 liver: porphyria; neoplastic nodules; foci Kimbrough et al.
(lot. 7042) (Sherman) t.p.: 23 months or altered areas; carcinoma; adenofibrosis (1981)
(in corn oil) (65)
(16) female single dose 200 liver: neoplastic nodules; altered areas;
t.p.: 22 months multinucleated cells
(30) female 12 doses over 100 liver: neoplastic nodules; foci or altered
3 weeks; t.p.: areas; carcinoma; adenofibrosis
24 months
FM FF-1 rat female 122 doses over 0.1, 1, increased white blood cell and lymphocyte Luster et al.
(lot. FF 1312 FT) (Fischer) 6 months t.p.: 3, 10 counts (0.1-10); decrease in body weight (1980)
(in corn oil) (4-10) 6 months after (3,10); decrease in thymus weight (3,10);
first dose increase in relative spleen weight (3,10);
decrease in adrenal weight (10); immune
alterations (3,10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, 22 doses over 30 decrease in body weight; increase in relative Gupta et al.
(lot 1312 FT) (Fischer female 30 days t.p.: liver weight; decrease in relative thymus (1981)
(in corn oil) 344/N) 120 days after weight (F); increase in serum protein
(3) first dose (ß-globulin fraction); porphyria (F);
pronounced hepatocellular alterations
2,2',4,4',5,5'- rat male 22 doses over 16.8 increase in relative liver weight (M);
hexabromobiphenyl (Fischer female 30 days t.p.: minimal hepatocellular changes
(99% purity 344/N) 120 days after
(in corn oil) first dose
FM FF-1 rat male single dose 500 no significant effects on body and liver Bernert, Jr
(lot 7042) (Sherman) t.p.: 18 months weight; increase in serum cholesterol and et al. (1983)
(in corn oil) total serum phospholipids; enhancement of
hepatic per-oxidation; reduced retinol
levels in serum and liver microsomes;
reduced alpha-tocopherol content in
microsomes (but not in serum)
FM FF-1 rat male, 125 doses over 0.1, 0.3 no effect on food consumption; dose-related Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, decrease in body weight gain; dose-related (1983a)
(in corn oil) 344/N) 6 months after 10 increase in the absolute and relative liver
(10) first dose weights (Female: 0.1-10; Male: 0.3-10);
decrease in thymus weight (0.3-10); increase
in spleen weight (1-10); decrease in Hb and
PCV values, in MCV and MCH values (10);
dose-related increase in serum cholesterol;
decrease in serum thyroid hormone levels;
porphyrin accumulation in liver, bone, teeth
(more pronounced in Female (1-10);
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
dose-related hepatocellular alterations;
increase in white blood cell count (Female:
1-10); increase in serum GGTP (Female: 10);
decrease in serum glucose (Female: 10);
dose-related decrease in serum protein,
primarily due to albumin (Female: 0.1-10);
carcinoma in urinary bladder (Female:10);
histopathological changes in the thyroid
glands and kidneys (Male: 10); decrease in
serum triglyceride (Male: 0.3-10)
FM FF-1 rat male, 125 doses over 0.1,0.3 dose-related body weight reductionso; Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, dose-dependent porphyrogenic effects on (1983b)
344) = up to 29 10 teeth, bones, liver (1-10); enlarged, pale,
(11-40) months after or mottled livers with necrotic foci (1-10);
first dose hepatocellular alterations (1-10);
dose-dependent incidence of liver tumours
and cholangiocarcinoma (higher doses);
dose-dependent decline in survival time
(Male: 1-10); chronic progressive nephropathy
(Male: 1-10); gastric ulcers and hyperplastic
gastropathy (Male: 3,10)
FM FF-1 mouse female 122 doses over 10 increase in body weight; increase in relative Luster et al.
(lot. FF 1312 FT) (B6C3F1) 6 months; t.p.: spleen weight; slight immune alterations (1980)
(in corn oil) (4-10) 6 months after
first dose
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse male, 22 doses over 30 no effect on food consumption; increase in Gupta et al.
(lot 1312 FT) (B6C3F1/N) female 30 days; t.p.: relative liver weight, histopathological (1981)
(in corn oil) (3) 120 days after alterations in liver
first dose
2,2',4,4',5,5'- mouse male, 22 doses over 16.8 no effect on food consumption and body weight
hexabromobiphenyl (B6C3F1/N) female 30 days; t.p.: gain; increase in relative liver weight;
(99% purity) (3) 120 days after minimal hepatocellular alterations
(in corn oil) first dose
FM FF-1 mouse male, 125 doses over 0.1, 0.3, no effect on food consumption; decrease in Gupta et al.
(lot. 1312 FT) (B6C3F1) female 6 months 1, 3, 10 body weight gain (only Male: 10); increase (1983a)
(in corn oil) (10) in body weight (only Female: 10); increase
in absolute and relative liver weight
(Female: 0.3-10; Male: 1-10); increase in
spleen weight (Female: 10); decrease in
uterine weight (Female: 10); haematological
alterations: dose-related increase in red
blood cell count; dose-related decrease in
MCV; decrease in platelet counts (Female:
3,10); leukocytosis (Female: 10); clinical
chemistry changes: increase in serum levels
of GGTP and SGPT (10) and alkaline
phosphatase (10); decrease in serum glucose
(Female: 10); dose-related hepatic porphyria
(more pronounced in Female) pale and
mottled livers (higher doses); dose-related
microscopic changes in the liver (1-10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse 125 doses over 0.1, 0.3, shortened survival time (Male: 10); hepatic Gupta et al.
lot. 1312 FT) (B6C3F1) 6 months; t.p.: 1 ,3, 10 porphyria (Male: 3); slight reduction in body (1983b)
(in corn oil) (8-27) up to 30 weight (Male: 10); enlarged liver; fluid
months after accumulation in peritoneal cavity; liver
first dose carcinoma (10); metastasis to lung (Female:
10); hyperplasia and adenoma of follicular
cells of thyroid
FM BP-6 cattle daily doses 250 no adverse clinicopathological chanages Durst et al.
(in gelatin (6) for 60 days mg/day (1978a)
capsule) t.p.: 80-190
days after
first dose
FM BP-6 cattle daily doses 250 no gross or histopathological signs of Moorhead et al.
(in gelatin (No. not for 60 days mg/day toxicosis (1978)
capsule) specified) t.p.: 220
days after
last dose
FM FF-1 cattle female daily doses 0.3 no effects on milk production, body weights, Robl et al.
(in gelatin (6) for 158 days amount of food consumed; no effects on (1978)
capsule) t.p.: 182 haematological and clinical chemistry and
days after urinalysis values;
last dose (1 cow: overgrowth of hooves from day 129)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM BP-6 cattle (Female) daily doses 250 no effect on milk production, body weight, Durst et al.
(in gelatine (1-4) for 60, 180 mg/day number of infections or general injuries; (1978b)
capsule) 202 days increased frequencies of reproductive Willett et al.
t.p.: up to dysfunctions (1980)
1500 days
after first
dose
a FM = Fire Master.(R)
b No. = number of animals.
c t.p. = time post-exposure.
d In mg/kg body weight per day, unless otherwise specified.
e Hb = Haemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular haemoglobin.
Table 85. Long-term effects of feeding PBBs to young or adult animals
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
"Hexabromobiphenyl" rat female 50 7 months increase in relative weights of liver, Sepkovic &
(Monsanto Co., (Sprague- ovary, and thyroid Byrne (1984)
St. Louis) Dawley) (8)
FM BP-6 rat female 1, 10, 50 5-7 months no effect on food consumption, body Byrne et al.
(Sprague- weight, and relative thyroid weight; (1987)
Dawley) (10) slightly elevated liver weight (10, 50);
primary hypothyroidism
FM BP-6 rat female 1, 5, 10, 5 or more no effect on food consumption and relative Byrne et al.
(Sprague- 50 months liver weight; decrease in relative adrenal (1988)
Dawley) (10) weight; depression of circulating levels
of adrenal cortex hormones
OcBB rat male, 0.01-1 180 days no effect on food consumption and body Norris et al.
(Dow Chemical) (Sprague- female weight (1973)
Dawley) (not
specified)
OcBB rat male 100 4/22 weeks return to normal liver weights Lee et al.
(Dow Chemical) (Sprague- (1975a);
Dawley) (8) 1000 4/22 weeks increased liver weights; Waritz et al.
hepatocellular alterations (1977)
OcBB rat female 50 7 months increase in relative liver weight Sepkovic &
(Monsanto Co., (Sprague- Byrne (1984)
St. Louis) Dawley) (8)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
NoBB mouse male, 100, 300 18 months decrease in body weight (Male, Female); Momma (1986)
(Bromkal 80-9D) female shortened survival time (300 mg/kg; Male);
enlargement of thyroid glands (300 mg/kg;
Female); decrease in triglyceride and
non-esterified fatty acids; reduction of
pentobarbital sleeping time (13 months);
liver carcinoma (Male, Female)
FM FF-1 mink (9) male, 6.25 210 days deaths; decrease in body weight; increase Aulerich &
female (mean) in relative liver and kidney weights; Ringer (1979);
liver histopathology Ringer et al.
(1981)
mink (8) female 1-2.5 9 months 10% reduction in body weight (2.5); Aulerich &
adverse effects on reproduction Ringer (1979);
Ringer et al.
(1981)
FM FF-1 rhesus female 0.3 7 months- weight loss; sterile abscesses (2/7); Allen et al.
monkey (total dose: > 1 year reproductive dysfunctions (1978); Allen
(7) > 25 mg) & Lambrecht
(1978);
Lambrecht
et al. (1978)
rhesus ? 1.5 > 5 months weight loss; periorbital oedema; Allen &
monkey (total dose: immunological alterations Lambrecht
(not approximately (1978)
specified) 75 mg)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
FM FF-1 rhesus male 25 25 weeks death; weight loss; haematological and Allen et al.
monkey (total dose: clinical chemistry changes: decrease in (1978)
(adult) approximately PCV, cholesterol, total serum protein and
(1) 1 g) albumin; increase in serum GPT activity;
epidermal changes:
alopecia, dry scaly skin, loss of eyelashs;
keratinization of hair follicles and
sebaceous glands;
oedema:
generalized subcutaneous oedema and
marked oedema of eyelids; enlarged heart
and liver; hyperplastic gastroenteritis;
severe ulcerative colitis; hypoactive
seminiferous tubules; hyperplasia of bile
duct epithelium
FM FF-1 rhesus female 25 50 weeks no weight gain: loss of hair (including Allen et al.
monkey (total dose: eyelashes); dry scaly skin; periorbital (1978)
(juvenile) approximately oedema; increase in serum GPT activity
(1) 1.5 g)
FM FF-1 rhesus female 300 137 days death; weight loss; haematological and
monkey (total dose: clinical chemistry changes: decrease in
(juvenile) approximately PCV, white blood cell count, red blood cell
6.4 g) count, serum cholesterol, serum protein;
increase in serum GPT activity
epidermal changes:
loss of hair (including eyelashes); atrophy
and squamous metaplasia of the sebaceous
glands; keratinization of hair follicles;
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
periorbital congestion and oedema;
subcutaneous oedema; enlarged
hepatocytes; hyperplastic gastroenteritis;
hyperplasia of the epithelium of the
bladder and of the bile ducts; focal
areas of haemorrhage in the adrenal glands
FM FF-1 rhesus female 1.5 36 weeks weight loss; decrease in serum Lambrecht et al.
monkey (total dose: cholesterol enlarged hepatocytes with (1978)
(adult) 70 mg) moderate fatty infiltration (as indicated
(3) by liver biopsy)
rhesus male, 25 14 weeks weight loss (Male, adult) or lack of
monkey female (total dose: weight gain (Female, juvenile); decrease
(adult approximately in serum cholesterol; hyperplastic
juvenile) 500 mg) gastritis
(2)
a FM = FireMaster; OcBB = technical octabromobiphenyl; NoBB = nonabromobiphenyl.
b No. = number of animals.
c PCV = packed cell volumes.
8.4.1 Rat
8.4.1.1 Overt clinical signs, body weight changes, food intake
In rats, the low-dose, long-term feeding of FireMaster(R)
(Byrne et al., 1987, 1988) or technical octabromobiphenyl (Norris
et al., 1973) had no effect on food consumption and body weight
(Table 85). Nevertheless, the fur of PBB-fed animals was slightly
less dense and slightly coarser than that of control animals (Byrne
et al., 1987). A dose-dependent decline in survival time (Gupta
et al., 1983b), and body weight reductions or depressed rates of
body weight gain as a function of time and dose (Luster et al.,
1980; Gupta et al., 1981, 1983a,b) have been found in rats orally
dosed with FireMaster(R) (Table 84); also there was no significant
difference in food consumption between treated and control animals
(Gupta et al., 1981, 1983a).
8.4.1.2 Haematology and clinical chemistry
Several haematological and clinical chemistry parameters were
altered, some in only one sex (Table 84: Kimbrough et al., 1977;
Luster et al., 1980; Bernert et al., 1983; Gupta et al., 1983a).
Rats of both sexes had decreased haemoglobin and packed cell volume
values (Gupta et al., 1983a), a dose-related increase in serum
cholesterol (Bernert et al., 1983; Gupta et al., 1983a), and a
dose-related hepatic porphyria (Gupta et al., 1983a).
8.4.1.3 Morphological changes
Most of the livers of treated rats from higher dose groups were
pale or slightly yellow and mottled (Gupta et al., 1983a) and
contained white necrotic foci (Gupta et al., 1983b). Porphyrin
accumulation in the liver, bone, and teeth, seen as red fluorescence
under UVR, was more pronounced in treated females compared with
treated males (Kimbrough et al., 1977; Gupta et al., 1983a,b). After
withdrawal of treatment, visual fluorescence declined slightly in
the lower dose groups (1 and 3 mg FM/kg body weight), but remained
the same in the 10 mg FM/kg body weight dose groups (males and
females) during lifetime observation (Gupta et al., 1983a,b).
In long-term studies, an increase in the relative liver weights
of rats exposed to FireMaster(R) or a related mixture was usually
found (Kimbrough et al., 1978; Gupta et al., 1981, 1983a; Sepkovic &
Byrne, 1984; Byrne et al., 1987), but in some instances (Bernert et
al., 1983; Byrne et al., 1988), there was no effect on liver weight
(see Tables 84 and 85). [During a six-month exposure to
FireMaster(R), the increases in absolute and relative liver
weights were dose-related (Gupta et al., 1983a)].
Long-term feeding of commercial octabromobiphenyl (OcBB) also
caused an increase in relative liver weights (Sepkovic & Byrne,
1984; Table 85). Rats fed OcBB for 4 weeks still had increased liver
weights at 18 weeks of recovery or had returned to near normal
limits, depending on the concentration of OcBB in the test diet (Lee
et al, 1975a; Table 85).
The only individual PBB congener tested over the long-term,
namely 2,2',4,4',5,5'-hexabromobiphenyl, caused an increase in the
relative liver weight in male rats only (Gupta et al., 1981; Table
84).
Weight changes following PBB exposure, noted in organs other
than the liver, included a decrease in the thymus (Luster et al.,
1980; Gupta et al., 1981, 1983a), an increase in the spleen (Luster
et al, 1980; Gupta et al., 1983a), a decrease in the adrenal glands
(Luster et al., 1980; Byrne et al., 1988), and an increase in the
thyroid and ovary (Sepkovic & Byrne, 1984) (see Tables 84 and 85).
8.4.1.4 Histopathological changes
Hepatocellular alterations (exclusive of hepatocellular
carcinomas), observed in long-term studies with FireMaster(R) (see
Table 84), consisted mainly of enlarged hepatocytes, fatty
infiltration, proliferation and disorganization of RER, single cell
necrosis, multinucleated cells, microabscesses, atypical foci, and
bile duct proliferation (Kimbrough et al., 1977, 1978, 1981; Gupta
et al., 1981, 1983a,b).
In contrast to FireMaster(R)-treated rats, rats given
2,2',4,4',5,5'- hexabromobiphenyl over 30 days tended to recover
when examined during postexposure periods (Gupta et al., 1981).
Myelin figures, induced by the feeding of technical OcBB,
persisted, together with fatty changes, as late as 18 weeks after
withdrawal of a test diet containing 1000 mg OcBB/kg; however, the
normal morphology of RER was restored independently of the
occurrence of myelin figures, after treatment was discontinued (Lee
et al., 1975a).
Although there was no significant difference in the weights of
the thyroid gland, slight to moderate microscopic changes were
observed, primarily in male rats exposed for 6 months to 10 mg
FireMaster(R)/kg body weight. The thyroid gland had thin, sparse,
or bluish colloid with basophilic stippling and some follicles were
lined with columnar epithelium and contained a few epithelial
papillary projections (Gupta et al., 1983a).
The kidneys of male rats equally treated consistently showed
atrophy of a few glomerular tufts with marked dilatation of Bowman's
capsule, which contained amorphous eosinophilic staining material. A
few glomerular tufts also appeared oedematous (Gupta et al., 1983a).
Male rats, exposed to FireMaster(R) for 6 months (dose levels:
1-10 mg/kg body weight per day) and observed for a lifetime, showed
a higher incidence of chronic progressive nephropathy than the
controls. Their kidneys were characterized by eosinophilic
proteinaceous casts, sclerosis and thickening of glomerular tufts
and Bowman's capsule, mononuclear leukocytic infiltration, and
interstitial fibrosis (Gupta et al., 1983b).
Gastric ulcers and hyperplastic gastropathy of the glandular
portion of the stomach were found with significantly increased
incidence in male rats held under the same conditions (dose levels:
3 or 10 mg FM/kg body weight per day). Microscopic lesions consisted
of hyperplasia of the mucosal epithelium, glandular metaplasia to
goblet cells, hyperchromasia, and increased mitosis (Gupta et al.,
1983b).
8.4.2 Mouse
As far as it has been tested (Table 84), FireMaster(R) has
not produced any significant effects on food consumption in mice of
both sexes (Gupta et al., 1981, 1983a). Interestingly, there were
increases in the body weights of female mice with long-term exposure
to FireMaster(R), while a decrease occurred only in males at the
high dose (Luster et al., 1980; Gupta et al., 1983a,b). The high
dose of FireMaster(R) also shortened the survival time of males
(Gupta et al., 1983b). There were no significant differences in food
consumption and body weight gain in mice treated with
2,2',4,4',5,5'-hexabromobiphenyl (Gupta et al., 1982).
A number of haematological and clinical chemistry changes have
been found in mice exposed for 6 months to FireMaster(R) (Table
84: Gupta et al., 1983a). Hepatic porphyrin levels were increased in
a dose-related manner after a six-month exposure (Gupta et al.,
1983a), but (unlike levels in rats) they tended to decrease
following cessation of exposure (Gupta et al., 1983b).
Organ weight changes noted in long-term studies on mice (Table
84) consisted of an increase in liver (Gupta et al., 1981, 1983a,b)
and spleen (Luster et al., 1980; Gupta et al., 1983a) weights and a
decrease in uterine weights (Gupta et al., 1983a). Most of the
affected mice observed for a lifetime contained serosanguineous
fluid in the peritoneal cavity (Gupta et al., 1983b). The livers of
mice with long-term exposure were pale and contained, primarily in
males, grayish white foci (Gupta et al., 1981, 1983a).
Microscopic alterations were marked swelling of hepatocytes,
foamy or vacuolated cytoplasm with hyaline bodies, focal coagulative
necrosis or scattered single cell necrosis of hepatocytes, and
atypical hepatocellular foci (Gupta et al., 1983a). Hyperplasia was
observed in the follicular cells of thyroid (Gupta et al., 1983b).
Adverse effects (besides liver carcinoma; see section 8.7),
observed after an 18-month exposure of mice to technical
nonabromobiphenyl (Bromkal 80-9D), included decreases in body
weight, enlargement of the thyroid gland, and biochemical
alterations (Momma, 1986; Table 85).
8.4.3 Cattle
Except for a single animal, cattle with long-term exposure to
low doses of FireMaster(R) FF-1 or BP-6, observed for up to 220
days post-treatment (see Table 84) did not show any adverse effects
with regard to food intake, clinical signs, clinicopatho logical
changes, or performance (Durst et al., 1978a,b; Moorhead et al.,
1978; Robl et al., 1978). However, reproductive dysfunction occurred
more frequently (Willett et al., 1980; see also section 8.5).
8.4.4 Mink
Minks were found to be very susceptible to PBB toxicity (Table
85: Aulerich & Ringer, 1979). When fed with FireMaster(R) for
several months, they responded with food rejection, loss of weight,
an unthrifty appearance, and death. Upon necropsy of animals that
died, increases in relative liver and kidney weights and a fatty
infiltration of the liver were evident. A relatively low daily
intake of FireMaster(R) by female mink had an adverse effect on
their reproductive performance (see also section 8.5).
8.4.5 Rhesus monkey
Rhesus monkeys are also among the species more sensitive to
FireMaster(R) (Table 85: Allen et al., 1978; Allen & Lambrecht,
1978; Lambrecht et al., 1978). At exposures of 1.5-300 mg FM/kg
feed, they developed weight loss or a lack of weight gain,
haematological and clinical chemistry changes, loss of hair, skin
lesions, oedema, enlargement of the heart and liver, areas of
haemorrhage in the adrenal glands; reduced spermatogenesis, and
immune incompetence. Microscopic changes in the liver included
enlarged hepatocytes with fatty infiltration. The most severe lesion
was hyperplastic gastroenteritis and the accompanying ulcerations.
Exposures to 25 or 300 mg FM/kg feed also caused death. The female
monkeys fed with lower doses of FireMaster(R) (0.3 mg/kg diet),
for over one year, did not develop any of the signs of intoxication
noted above, except for weight loss. However, they were affected by
reproductive dysfunctions (see also section 8.5).
8.4.6 Pre- and perinatal exposure
Long-term effects following pre- or perinatal exposure to
FireMaster(R) have been recorded in rats and cattle.
Stunted growth, increased mortality rates, and liver tumours
were observed in the offspring of Sherman rats given 200 mg
FireMaster(R) FF-1/kg body weight on days 7 and 14 of pregnancy,
when a total of 50 male and 50 female offspring per group were
followed until they were 2 years old (Groce & Kimbrough, 1984).
Sprague-Dawley rats (No. = 10), perinatally exposed (day 8 of
pregnancy-28 days post partum) to FireMaster(R) BP-6 (100 mg/kg of
feed) and weaned on to a PBB-free diet, had increased relative liver
weights at 28 and 150, but not 328 days of age. Although the liver
was not enlarged 10 months after weaning, hepatic histopathological
alterations, such as cellular swelling, vacuolation, some necrosis,
and eccentric and pyknotic nuclei were observed throughout this
residual phase. Other long-lasting alterations were stimulation of
renal and hepatic microsomal enzymes, and a reduction in the
duration of anaesthesia produced by pentobarbital (McCormack et al.,
1980). In a multigeneration study (McCormack et al., 1981), rats
perinatally exposed to FireMaster(R) BP-6, as described above
(F1-generation), were allowed to mature sexually and bred with
littermates to produce the F2-generation. Even these F2-animals
exhibited increased relative liver weights and histopathological
liver changes at 28 days of age. The light microscopic changes
included vacuolation, focal necrosis, pyknotic nuclei, swelling, and
myelin bodies. However, the severity of lesions was much less
prominent in F2-animals than in F1-animals.
PBBs given to a single generation also produced hepatic and
renal microsomal enzyme stimulation, a reduction in the duration of
anaesthesia elicited by pentobarbital or a large dose of
progesterone, and a decrease in the concentration of vitamin A in
the liver, in both subsequent generations (F1 and F2).
Cows whose dams or granddams had received daily oral doses of
FireMaster(R) BP-6 (up to 250 mg/day) for 60-202 days showed no
general health problems, but they had conception difficulties
(Willett et al., 1982).
8.5 Reproduction, embryotoxicity, and teratogenicity
Effects of PBBs on reproduction (reproductive system, overall
reproductive performance, embryotoxicity, teratogenicity) have been
summarized in Tables 86 and 87. Most of the studies refer to the
FireMaster(R) mixture. Few reports deal with technical
octabromobiphenyl (Aftosmis et al., 1972a; Waritz et al., 1977) or
technical decabromobiphenyl (Millischer et al., 1979), and, among
the individual PBB congeners, only 2,2',4,4',5,5'-hexabromobiphenyl
has been evaluated by a few authors.
8.5.1 PBB mixtures
8.5.1.1 Mammals
a) Reproductive system and performance.
As listed in Tables 74 and 75, administration of
FireMaster(R) by gavage and in the diet had no effect on the
weight of male (Garthoff et al., 1977; Corbett et al., 1978a; Harris
et al., 1978b, Gupta et al., 1981; Castracane et al., 1982) or
female (Gupta et al., 1981) sex organs in rats and/or mice. An
exception was an increase in the uterine weight of mice dosed for 6
months with FireMaster(R) (Gupta et al., 1983a). Testes weights in
rats fed commercial OcBB also remained unaffected (Norris et al.,
1973). However, there was a reduction in the ventral prostate
weight-to-body weight ratio in pubescent male rats (Johnston et al.,
1980; Table 87) and a delay in vaginal opening in female rats after
perinatal exposure to FireMaster(R) (Harris et al., 1978a;
McCormack et al., 1981; Tables 86 and 87). Both findings may suggest
retarded sexual maturation.
Male rats, moribund from multiple doses of FireMaster(R),
exhibited degenerative and hyperplastic changes in the ductus
deferens (hyperplasia and squamous metaplasia with keratinization in
the epithelial lining of the ductus deferens). However, such changes
were not observed in surviving male rats, examined six months after
the treatment. Lesions found in surviving animals were acute
prostatitis (30 mg/kg body weight per day) and the presence of sperm
granuloma in the epididymis (100 mg/kg body weight per day) (Gupta &
Moore, 1979; Table 86). A rhesus monkey, exposed to FireMaster(R)
in the diet, had hypoactive seminiferous tubules at death (Allen
et al., 1978; Table 87). Little work was done on the possible
impairment of spermatogenesis. Young bulls may develop testicular
atrophy and reduced spermatogenesis when exposed to FM (Robl et al.,
1978), but more numerous controlled studies are needed.
The estrous cycle of cows (No. = 8) was not affected during
administration of FireMaster(R) FF-1 (0.3 mg/kg body weight per
day) for 158-228 days and during a recovery period of 112-182 days
(Robl et al., 1978).
The estrous cycle of female rats was increased in length after
perinatal and subsequent dietary exposure to FireMaster(R) BP-6
(Johnston et al., 1980; Table 87) at concentrations of 100 mg/kg
(equivalent to 5 mg/kg body weight per day). Prolonged menstrual
cycles were seen in rhesus monkeys after consuming FireMaster(R)
FF-1 for six months at a concentration of 0.3 mg/kg (equivalent to
0.02 mg/kg body weight per day). This alteration was correlated with
decreased serum progesterone levels (Allen et al., 1978; Lambrecht
et al., 1978; Table 87).
Table 86. Summary of effects of PBBs on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): dosing studies
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral single 400, 800 reductions in fetal and placental Beaudoin
(Wistar) dose one of weight; fetal death (primarily (1977)
(3-15) gd 6-14 800 mg/kg body weight per day at
s.t.: gd 20 day 7-12); malformed (cleft
palate; diaphragmatic hernia)
fetuses (400 mg/kg body weight
per day: 0-11.8%; 800 mg/kg body
weight per day: 0-60%)
FM FF-1 rat (No. oral multiple 100 (total none no effects on number of Ficsor &
not doses (6) gd dose: 600 fetuses, dead implants, Wertz (1976);
specified 6-16 at 2 day mg/kg body fetal malformation Wertz &
(15) intervals weight Ficsor (1978)
s.t.: gd 19
FM BP-6 rat oral multiple 0.25-10 reduced fetal weight and no effects on implantation Harris
(Sprague- doses gd 7-15 mg/day crown-rump length (only number of live fetuses or et al. (1978a)
Dawley) s.t.: gd 20 0.25 mg/day) gross malformations
(6-8)
s.t.: 48, 60 10 mg/day reductions in offspring weight no effects on litter size Harris
days of age (from 3 days of age onwards, and birth weight et al. (1978a)
more pronounced in male pups);
increased postnatal mortality (at
weaning 14.3% versus 1.5% in
control); delay in vaginal opening
of female offspring
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral multiple 8-160 mg reduced implantation rates no malformed fetuses Beaudoin
(Wistar) doses gd 0-14 per animal (80-160 mg); fetal death (1979)
(No. not on alternate (total
specified) days dose)
FM FF-1 rat oral multiple 100 moribund rats: degenerative and Gupta &
(Fischer doses (22) hyperplastic changes in the ducts Moore (1979)
344/N) over 30 days deferens (73 days after
(9 males) s.t.: up to 6 treatment); surviving rats: sperm
months granuloma in the epididymis (6
months after treatment; 1/2e)
30 acute prostatitis (4/9)e
FM FF-1 rat oral multiple 200 reduction in offspring survival Groce &
(Sherman) doses (2) gd 7 rate-to-weaning Kimbrough
(16) and 14 o.t.: (1984)
up to Weaning
(21 days of age)
FM BP-6 rat 1) in vivo- 800 inhibited rate of embryo Fisher (1980);
(Wistar) treatment: oral development in vitro (effects on Beaudoin &
(7) single dose gd 9 axial rotation, heart rate, neural Fisher (1981)
s.t.: gd 10 tube closure, formation of the
2) whole embryo anterior limb buds, somite
culture: 24 h development, and establishment of
visceral yolk sac circulation);
reduction in DNA content;
pericardial oedema
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
DeBB rat oral multiple 10-1000 none no teratogenicity or Millischer
(Sprague- doses gd 6-15 embryotoxicity et al. (1979)
Dawley) s.t.: gd 21
(25)
2,2',4,4' mouse oral multiple 0.3-32 none no fetotoxicity or Lucier et al.
5,5'-hexa- (C.D. - 1) doses gd 10-16 teratogenicity; no effects (1978)
bromobiphenyl (up to 25) s.t.: gd 19 on fertility, gestation,
(purity o.t.: 4, 20 viability, survival, and
> 97% days of age lactation indices
FM BP-6 cow (6) oral multiple 25 g/day abortion (3/6)e or fetal Durst et al.
doses during death (3/6)e (1977, 1978b);
pregnancy for Moorhead
32-60 days et al. (1977)
o.t.: up to 60
days
BM BP-6 cow oral multiple 0.25 and none Durst et al.
(3) doses during 250 mg/day (1978b)
pregnancy for
60 days
(1) for 180 days 250 mg/day stillbirth (due to dystocia)
o.t.: up to
305 days
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 cow (3-5) oral multiple 0.25-250 high incidence of dystocia; a total of 75 calves Willett et al.
(4 dose doses before mg/day increased birth weights of calves; were studied; no effects (1980, 1982)
groups) breeding or stillbirths; increased number of on growth, development,
during late inseminations required for and survival of calves
pregnancy for conception in 1 and 2 generation born alive
60-202 days offspring
o.t.: up to 5.5
years (1-5
parturition;
2 generations)
a FM = FireMaster(R); DeBB = technical decabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, of recorded); o.t. = observation time; s.t. = sacrifice time.
d In mg/kg body weight per day, unless otherwise specified.
e No. affected/no. treated.
Table 87. Summary of PBB effects on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): feeding studies
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 rat (Sprague- gd 7-18 100, 1000 decreasing fetal weight with suggestion of late Corbett
Dawley) s.t.: gd 20 increasing dosage fetal mortality et al.
(6-7) (high dose); no (1975)
teratogenicity
FM BP-6 rat (Sprague- gd 8-9th week 100 reduction in ventral no effect on fetal Johnston
Dawley) (at of age s.t.: prostate weight of male survival rate et al.
least 8) 9th week of age offspring; increased length (1980)
of estrous cycle of female
offspring
FM BP-6 rat (Sprague- gd 8-Weaning 100 reduction in survival no effects on length McCormack
Dawley) s.t.: Weaning rate-to-weaning (87% of of gestation, litter et al.
control value); delay in size, incidence
(8) (28 days of vaginal opening in female of gross external
age) offspring anomalies, pup body
weight at birth
OcBB rat (not gd 6-15 100, 1000, gastroschisis (one fetus abstract only (no Aftosmis
specified) s.t.: gd 20 10 000 at each level); anasarca on number of fetuses et al.
(one fetus at each of the information examined) (1972a)
two highest levels)
OcBB rat gd 6-15 100, 1000, no significant gastroschisis Waritz et al.
(ChR-CD) s.t.: gd 20 10 000 embryotoxicity or (1/259e: 1000 mg/kg: (1977)
(23-27) teratogenicity 1/1/283e
10 000 mg/kg);
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
anasarca (1/259e: 1000
mg/kg; 1/283e: 10 000
mg/kg); no effects on
growth or viability of
embryos
FM BP-6 mouse: gd 7-18 50, 100, dose-related decrease in fetal incidence of Corbett
(Swiss, ICR) s.t.: gd 18 1000 weight with increasing dosage; malformations et al.
(9-12) exencephaly (5/295g: 100, statistically (1975,
1000 mg/kg); cleft palate significant only 1978b)
(5/208g: all levelsg; compared with pooled
hydro-nephrosis (2/87: historical controls
1000 mg/kg)
FM mouse (Swiss- gd 4-16 100, 200 increase in number of dead or (abstract only) Preache
Webster) s.t.: gd 19 resorbed fetuses (200 mg/kg) et al.
(No. not (1976)
specified) gd 8-16 100, 200 decrease in fetal body weight
s.t.: gd 19 (200 mg/kg)
gd 8-16 50, 100 reduction in number of live no effects on Preache
o.t.: up to 6 offspring postnatal mortality or et al.
weeks of age body weight (1976)
days 1-29 pp 50, 100 increased mortality; decreased
o.t.: up to body weight
weaning
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
2,2',4,4'- mouse gd 6-15 100, 300, at 300 mg/kg: 17-182 fetuses were Welsch &
5,5'-hexa- (C57BL) o.t.: gd 17 500, 750, decrease in pregnancy rates of examined Morgan (1985)
bromobiphenyl (2-22) 1000 plug-positive mice;
(purity: at > 300 mg/kg:
> 99%) reduced fetal body weight;
malformed fetuses (cleft
palate combined with a "cystic
brain deviation": 5/166,
3/172, 5/46, 6/17g,
respectively, at 300-1000
mg/kg; minor abnormalities of
brain development); at >
500 mg/kg: reduced gravid
uterine weight
FM BP-6 pig (2-4) 2nd half of 10, 100, 200 increased mortality during no effect on litter Werner &
gestation and lactation size Sleight
lactation o.t.: (1981)
up to weaning
(4 weeks)
FM FF-1 mink (8 for 9 months 1 reduced litter size; reduced both male and female Aulerich &
females; (start before kit weight at birth and at 4 on PBB diet; no Ringer
2 males) breeding) weeks; increased mortality effects on breeding or (1979)
4 weeks) (birth to periods; no gestation
teratogenicity
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 rhesus for over 1 year 0.3 prolonged menstrual cycles; Allen et al.
monkey (start 7 months decreased serum progesterone (1978,
(7) prior to breeding) levels (4/7e after 6 months); 1979); Allen
excessive and prolonged & Lambrecht
implantation bleeding (2/7e); (1978);
abortion (1/7); stillbirth Lambrecht
(1/7e); reduced weight of et al.
infants at birth and at (1978)
weaning (4 months of age)
FM FF-1 rhesus for 25 weeks 25 hypoactive seminiferous tubules Allen et al.
monkey (at the time of death (1978)
(1 male)
FM BP-6 chicken (White for 9 weeks 20 none no effect on Cecil et al.
Leghorn) (35) hatchability (1974)
"PBB" chicken for 8 weeks 5, 10, 20 none no effects on egg Lillie et al.
(White production, egg (1975)
Leghorn) (20) weight, egg shell
thickness, and
fertility; no effects
on hatchability,
embryonal development
and progeny health
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 chicken for 8 weeks 20 increased mortality of progeny no effects on egg Cecil &
(White production, egg Bitman
Leghorn) (10) weight, egg shell (1978)
thickness, fertility,
hatchability, and
embryonic death
for 4-8 weeks 64, 200, reduced egg production or stop
640, 2000 in egg production at 200-2000
mg/kg; decreased hatchability
of fertile eggs; increased
mortality of progeny; reduced
growth rate of progeny;
embryonic deaths; embryonic
abnormalities (subcutaneous
oedema, oedematous cysts,
unabsorbed yolk)
FM FF-1 chicken for 5 weeks 0.2-3125 decline in egg production Polin &
(White (MELf: 30-45 mg/kg); loss of Ringer
Leghorn) (24) egg production (> 625 mg/kg, (1978a,b)
within 2 weeks);
reduced hatchability (MELf:
30-45 mg/kg); dose-related
increase in offspring
mortality (MELf: 30-45 mg/kg;
embryonic oedema
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 chicken (10) for 21 days 80 decline in hatchability; oedema no effect on fertility Polin et al.
of embryos and newly hatched (1979)
chicks
2,2',4,4', chicken (10) for 21 days 52 none no decline in
5,5'-hexa- hatchability; no
bromobiphenyl oedema of chicks and
(purity: and embryos
not
specified)
FM Japanese for 9 weeks 10, 20, 100 at 100 mg/kg: no eggshell thinning Babish
quail (No. reduced egg production (17% et al.
not specified) versus 68% in controls); no (1975a)
hatchability; embryonal deaths
FM FF-1 Japanese for 5 weeks 80 reduced egg production fertility and Polin et al.
quail hatchability not (1982);
(Coturnix significantly Bursian
coturnix different from et al.
japonica) controls (1983)
(36 males,
36 females)
a FM = FireMaster; OcBB = technical octabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, if recorded; exception: Waritz et al. (1977): sperm day = gd 1); pp = postpartum;
o.t. = observed time; s.t. = sacrifice time.
d In mg/kg of feed, unless otherwise specified.
e No. affected/no. treated.
f MEL = Minimum effective level.
g As reported by Corbett et al. (1978a).
The number of inseminations required for conception was
increased in cows whose dams or grand dams had received oral doses
of FireMaster(R) BP-6 (Willett et al., 1982; Table 86).
Most of the studies evaluating the effects of PBBs on
reproductive performance started with treatment during pregnancy
(Tables 86 and 87). In few instances, were females exposed before
mating (Allen et al., 1978; Aulerich & Ringer, 1979; Willett et al.,
1980, 1982), and animals of both sexes were treated in only one
study (Aulerich & Ringer, 1979). Under these conditions,
FireMaster(R) reduced litter size in mice (Preache et al., 1976)
and minks (Aulerich & Ringer, 1979), but not in rats (Harris et al.,
1978a; McCormack et al., 1981) and pigs (Werner & Sleight, 1981).
The adverse effect on litter size was observed in minks given
PBB-contaminated poultry as well as in those fed FireMaster(R),
directly added to the diet (Aulerich & Ringer, 1979). Stillbirths
occurred in cattle (Durst et al., 1978b; Willett et al., 1982) and
rhesus monkeys (Allen et al., 1978; Lambrecht et al., 1978).
Reduced birth weights were reported in rhesus monkeys (Allen et al.,
1978; Lambrecht et al., 1978), minks (Aulerich & Ringer, 1979), and
mice (Corbett et al., 1978b) at intakes of 0.3, 1, or 50-1000 mg
FireMaster(R)/kg feed, respectively (see Table 87). On the other
hand, increased birth weights of calves resulted in a higher
incidence of dystocia in cows (Durst et al., 1978b; Willett et al.,
1980, 1982). The growth of progeny during lactation (indicated by
weight at weaning) was also reduced by FireMaster(R) in rats
(Harris et al., 1978a), mice (Preache et al., 1976), minks (Aulerich
& Ringer, 1979), and rhesus monkeys (Allen et al., 1978; Lambrecht
et al., 1978).
In the study by Lambrecht et al. (1978), 14 female rhesus
monkeys were used as an animal model (7 controls, 7 treated) to
evaluate the toxicological effects of FM FF-1. The substance was
added to the pelleted diet at a concentration of 0.3 mg/kg and 200 g
of this diet was fed per day; after a dosing period of 7 months, the
animals had ingested a total of 10.5 mg FM FF-1. Although the food
consumption during this period was unaffected, there was a loss of
7.4% of the initial body weight in the treated group. The total
exposure is not stated, but, on the basis of information given in
the paper, it appears that the dams had been exposed for a total of
12.5 months at the time of the birth of the infants. After 6 months,
menstrual cycles in 4/7 monkeys were lengthened (controls: 28 days;
treated monkeys: 31 days). The treated animals showed a
corresponding flattening of the progesterone peak. After mating, 5/7
treated monkeys delivered normal-appearing but small infants (455 g
vs 519 g in controls) with reduced weight gain in these infants
during the postnatal period. Two out of 7 treated monkeys aborted a
mummified fetus and a stillborn infant, respectively. All control
animals delivered normal-appearing infants.
The survival rate-to-weaning was adversely affected in the
offspring of rats, mice, pigs, and minks, but not in calves (see
Tables 86 and 87). FireMaster(R) levels producing the effects were
200 mg/kg body weight per day (Groce & Kimbrough, 1984), or
10 mg/day (Harris et al., 1978a), or 100 mg/kg feed (McCormack
et al., 1981) in rats, 100 mg/kg feed in mice (Preache et al., 1976)
and pigs (Werner & Sleight, 1981), and 1 mg/kg feed in minks
(Aulerich & Ringer, 1979).
For neurotoxic effects after perinatal exposure see section
8.11.2.
Ranges of maternal toxic parameters can be derived from Tables
71, 72, 74, and 75.
b) Embryotoxicity and teratogenicity
PBBs were embryotoxic in rats, mice, cattle, and rhesus
monkeys. Rats and cows appeared to be less susceptible than mice and
rhesus monkeys (see Tables 86 and 87).
Treatment of rats with a single high dose of FireMaster(R)
(> 400 mg/kg body weight) on day 6 of pregnancy resulted in 100%
embryo resorption. Generally, the number of fetal resorptions
depended on the dose and the pregnancy stage at treatment. For
example, after day 8 (400 mg/kg body weight dose) or day 12
(800 mg/kg body weight dose), the embryolethal effect of
FireMaster(R) declined abruptly (Beaudoin, 1977). Reductions in
both fetal and placental weights were observed only at high doses
(> 400 mg/kg body weight), and the most susceptible period of
pregnancy was days 11-13 (Beaudoin, 1977).
The long-term administration of lower doses (total dose: 8 or
40 mg FireMaster(R)/animal) during pregnancy (from day 0) markedly
increased embryonic death over that seen following administration of
an equivalent single dose (Beaudoin, 1979). However, long-term
intake of relatively low doses (see Tables 86 and 87) during late
gestation (start: day 7) did not influence fetal survival rate
(Corbett et al., 1975; Harris et al., 1978a; Johnston et al., 1980),
but decreased fetal weight (Corbett et al., 1975; Harris et al.,
1978a). A total dose of 600 mg FireMaster(R)/kg body weight, given
at two-day intervals (start: day 6) had no effects on fetal death or
fetal weight (Wertz & Ficsor, 1978).
No significant effects on the growth or mortality of embryos
were noted after the exposure of rats (Tables 86 and 87) to
technical octabromobiphenyl (Waritz et al., 1977) or technical
decabromobiphenyl (Millischer et al., 1979). Single cases of fetal
oedema have been observed in OcBB-treated rats (Aftosmis et al.,
1972a; Waritz et al., 1977).
The incidence of dead or resorbed fetuses was increased in mice
fed 200 mg FireMaster(R)/kg feed on days 4-16 of pregnancy
(Preache et al., 1976). Feeding several concentrations of FM from
day 7 (Corbett et al., 1975) or day 8 (Preache et al., 1976) of
pregnancy reduced fetal weight (Table 87).
Cattle given 25 g FM/day had 3 abortions and 3 dead fetuses
from 6 treated animals (Durst et al., 1977; Table 86). The fetuses
were oedematous and haemorrhagic, concomitant uterine lesions were
haemorrhage and necrosis of the cotyledons (Moorhead et al., 1977).
Two out of 7 rhesus monkeys fed FM (Table 87) had long
implantation bleedings, one aborted a mummified fetus at 146 days of
gestation and one gave birth to a stillborn infant at 154 days. The
remaining 5 monkeys delivered small infants at 156-165 days of
gestation (normal gestation: 165 days) (Allen et al., 1978;
Lambrecht et al., 1978).
Terata due to PBB exposure have been reported only in rats and
mice (Tables 86 and 87). A single high dose of FireMaster(R)
produced cleft palate and diaphragmatic hernia in rats (Beaudoin,
1977). The majority of malformations were found following
administration of 800 mg/kg body weight on day 11, 12, or 13 of
gestation. No terata were noted in rats after multiple doses of
FireMaster(R) (Table 86) (Harris et al., 1978a; Wertz & Ficsor,
1978; Beaudoin, 1979) and after feeding diets containing up to
1000 mg FireMaster(R)/kg feed (Corbett et al., 1975). In contrast
to rats comparably treated (50-1000 mg FM/kg maternal diet), a
higher incidence of exencephaly and cleft palate was seen in fetal
mice, compared with pooled historical controls, but not compared
with concurrent controls, though neither of these anomalies was seen
in control fetuses (Corbett et al., 1975, 1978b).
It is unclear whether the few cases of fetal gastroschisis,
observed in rats fed technical OcBB (Table 87), are PBB-related or
fortuitous (Aftosmis et al., 1972a; Waritz et al., 1977). No
teratogenicity was found in rats exposed to DeBB (Millischer et al.,
1979; Table 86).
Embryo development was studied in vitro. Maternal rats
received a single, oral dose of 800 mg FM/kg body weight on
gestation day 9, and the embryos were isolated on day 10 (24 h
post-treatment) for cultivation over a 24- or 42-h period (Fisher,
1980; Beaudoin & Fisher, 1981). Teratogenic effects observed (see
Table 86) were not correlated with types of malformations found in
earlier in vitro experiments (Beaudoin, 1977). Some developmental
disturbances tended to be corrected after 42 h of PBB-free culture.
In addition to retarded development, there was a significant
reduction in the DNA contents of cultured embryos (Fisher, 1980).
The survival rate of embryos was not affected during the cultivation
period (Beaudoin & Fisher, 1981).
8.5.1.2 Avian species
Avian reproduction was studied primarily in chickens.
Cockerels fed FM at various levels (10-250 mg/kg feed), for several
weeks, showed inconsistent weight changes in the comb and testes
(see Table 75: Ringer & Polin, 1977; Ringer, 1978; Dharma et al.,
1982). Histologically, lipid infiltration into testicular parenchyma
was noted (Ringer & Polin, 1977). No studies on the reproductive
performance of these birds were made.
Detrimental effects were seen when FireMaster(R) was fed to
hens of both White Leghorn chickens and Japanese quails, the only
two species studied (Table 87). Chickens (Cecil et al., 1974; Lillie
et al., 1975; Cecil & Bitman, 1978; Polin & Ringer, 1978a,; Polin
et al., 1979) appeared to be more sensitive to FireMaster(R) than
the Japanese quails (Babish et al., 1975a; Bursian et al., 1983), on
the basis of egg production and hatchability. Egg production in
chickens was reduced by dietary levels of FireMaster(R) as low as
30-45 mg/kg (Ringer & Polin, 1977), while the lowest effective level
reported for Japanese quails was 80 mg/kg (Bursian et al., 1983).
Chickens stopped laying when concentrations of FireMaster(R) in
diets exceeded 600 mg/kg (Cecil & Bitman, 1978). The hatchability of
eggs was adversely affected by 30-45 mg FireMaster(R)/kg feed in
chickens (Ringer & Polin, 1977) and by 100 mg FireMaster(R)/kg
feed in Japanese quails (Babish et al., 1975a).
Increased mortality (Cecil & Bitman, 1978; Polin & Ringer,
1978a, 1978b) and reduced growth rates (Cecil & Bitman, 1978) were
seen in the offspring of chickens fed FireMaster(R) at low levels
(< 64 mg/kg; Table 87).
Embryonic deaths occurred in chickens (Cecil & Bitman, 1978;
50% of deaths on the last day of incubation) and in Japanese quails
(Babish et al., 1975b; 40% of deaths on the first day or two of
development). The most common abnormality of embryos and newly
hatched chicks after maternal feeding of FireMaster(R) was oedema
(Cecil & Bitman, 1978; Polin & Ringer, 1978b; Polin et al., 1979).
Although there was a reduction in the feed intake of exposed
chickens, the decreased hatchability, higher incidence of
abnormalities, and poorer progeny survival could not be reproduced
by pair-fed control birds (Cecil & Bitman, 1978). At high dietary
levels (> 600 mg FM/kg of feed), the direct effects of
FireMaster(R) on egg production could not be separated from the
effects of reduced food consumption (Cecil & Bitman, 1978).
Adverse effects on the reproduction of chickens could return to
normal after withdrawal of FireMaster(R) from the diet. Time for
recovery depended on the concentration of PBB in the feed (Cecil &
Bitman, 1978; Polin & Ringer, 1978a).
8.5.2 Individual PBB congeners
Individual PBB congeners have not been reported to cause weight
changes in sex organs. Similarly, the testes of rats or cockerels,
did not show any remarkable histological changes after treatment
with 2,2'-dibromobiphenyl, 2,2',3,4,4',5,5'-heptabromobiophenyl,
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), 2,3',4,4',5,5' (BB 167),
or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) (Moore et al., 1979a;
Gupta et al., 1981; Akoso et al; 1982a; Dharma et al., 1982; Render
et al., 1982). An exception was a decrease in the gravid uterine
weight of pregnant mice given more than 500 mg BB 153/kg body weight
(Welsch & Morgan, 1985).
Embryotoxicity and other reproductive parameters have been
studied only with BB 153 mice (Lucier et al., 1978; Welsch & Morgan,
1985) or chickens (Polin et al., 1979). BB 153 did not cause the
deleterious effects (decline in hatchability, oedema) that were
produced by equivalent dietary levels of FireMaster(R) in chickens
(Polin et al., 1979; Table 87). However, BB 153 was capable of
producing cleft palate and cystic lesions in the region of the
cerebellum in mouse embryos (Welsch & Morgan, 1985; Table 87). These
adverse effects were observed at exposure concentrations that also
cause toxicity in dams. Another less complete study (Lucier et al.,
1978; Table 86) did not find developmental toxicity of BB 153 in
mice, possibly because of lower exposure levels, later onset of
exposure, and strain differences.
8.6 Mutagenicity and related end-points
The potential of PBBs for mutagenicity has been tested with
several in vitro and in vivo assays referring to four major test
categories: microbial and mammalian cell mutagenesis; mammalian cell
chromosomal damage (cytogenetic tests; in vitro and in vivo);
mammalian cell transformation in vitro; and DNA damage and repair
(UDS). Results have been summarized in Table 88. With one exception
(Kohli et al., 1978), all studies failed to indicate any
mutagenicity of individual PBB congeners or commercial PBB mixtures.
Haworth et al. (1983) did not confirm the mutagenic effect of
4-bromobiphenyl observed by Kohli et al. (1978), but they used
different Salmonella strains.
However, FireMaster(R) gave positive results in a recently
developed test, the Microscreen assay using lambda prophage
induction in Escherichia coli. This test has been found to be
sensitive in detecting carcinogens which are negative in mutagenesis
assays (Rossman et al., 1991).
8.7 Carcinogenicity
8.7.1 Carcinogenicity in long-term toxicity studies
The carcinogenic effects of PBB in long-term toxicity studies
have been compiled in Table 89. From this table, it is evident that
the principal site of tumours was the liver. The incidence of
hepatocellular carcinoma was significantly increased in both sexes
of mice and rats receiving relatively high oral doses of the
FireMaster(R) mixture.
The results of one of these studies (Gupta et al., 1983b),
carried out by the NCI/NTP (USA), were interpreted as
FireMaster(R) showing carcinogenic effects (Haseman et al., 1984;
Tennant et al., 1986). In this study, a statistically significant
higher incidence of liver tumours was found at multiple doses of
3 mg FM/kg body weight per day (male rats: 21%) and of 10 mg FM/kg
body weight per day (female/male rats: 35/23%; female/male mice:
88/95%). Atypical foci and neoplastic nodules were found at lower
doses (Table 89). Dose-responses were statistically significant (P
< 0.01) in male and female rats with regard to atypical foci,
hepatocellular carcinoma, and cholangiocarcinoma, and, in female
rats, also with regard to neoplastic nodules (Gupta et al., 1983b).
Hepatocellular carcinoma could be produced, even after single dosing
with FireMaster(R) (Kimbrough et al., 1981). Rats given a single
dose of 1000 mg FM/kg or 12 doses of 100 mg FM/kg body weight, by
gavage, developed incidences of carcinomas of 41.4 and 67.8%,
respectively (Kimbrough et al., 1981). Liver tumours were also
observed in female and male rats two years after perinatal exposure
to FireMaster(R) (Groce & Kimbrough, 1984; Table 89). Although
concurrent controls did not show such lesions, the IARC Working
Group noted the lack of statistical significance (IARC, 1986).
Nevertheless, the incidences of 5.9% (females) and 9.6% (males),
respectively, exceeded the spontaneous incidence of carcinomas of
the liver in this particular strain of rat, which is reportedly less
than 1% (Groce & Kimbrough, 1984).
Recently, NTP performed further carcinogenicity studies on
FireMaster(R) to determine the following: (a) the effects of PBBs
in F344/N rats and B6C3F1 mice receiving adult (F1) exposure only
(0, 10, or 30 mg PBB/kg diet); (b) the toxic and carcinogenic
effects of PBBs in rats and mice receiving perinatal (Fo) exposure
only (10 mg/kg in rats and 30 mg/kg in mice); and (c) the effects of
combined perinatal and adult exposure to PBBs (NTP, 1993). In an
adult-only exposure study, both sexes in rats and mice receiving 10
or 30 mg/kg (0.5 or 1.5 mg/kg body weight per day) showed increased
incidences of hepatocellular adenoma/carcinoma, quite similar to
the results of the previous studies (Gupta et al., 1983b).
Table 88. Summary of PBB genetic toxicity test results
Assay Detailsb Metabolic activation PBBa Result Remarks References
Salmonella mutagenesis
S. typhimurium strains: TA 98 ± Aroclor 1254 FM FF-1 - Tennant
TA 100 induced liver - et al. (1986)
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 hexabromobiphenylc - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats and -
TA 1537 and Syrian hamsters
S. typhimurium strains: TA 1535 ± Aroclor DeBB - Millischer
(spot test; Ames) TA 1537 induced rat - et al. (1979)
TA 1538 liver S-9 -
S. typhimurium strain: TA 1538 - DeBB - mice receiving oral
(host-mediated doses of 5, 10, and
assay) 20 g/kg body weight
S. typhimurium strains: TA 98 ± Aroclor 1254 2-bromobiphenyl - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats -
TA 1537 and Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 3-bromobiphenyl -
TA 100 induced liver -
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
S. typhimurium strain: TA 1538 + Aroclor 1254 4-bromobiphenyld + Kohli et al.
induced rat liver S-9 (1978)
none -
S. typhimurium strains: TA 98 ± Aroclor 1254 4-bromobiphenyle - Haworth et al.
TA 100 induced liver (1983)
TA 1535 S-9 from rats and
TA 1537 Syrian hamsters
Mammalian cell mutagenesis
Adult rat liver (ARL) HGPRT locus none FM FF-1 (lot No. - at the highest non-toxic Tong et al.
epithelial cells 1312-FS) dose of 10-3 mol (1983); Williams
et al. (1984)
Human fibroblasts HGPRT locus rat hepatocytes FM FF-1 (lot No. - at the highest non-toxic
1312-FS) dose of 10-4 mol
Chinese hamster HGPRT and Na-K ± Aroclor 1254 induced FM BP-6 - cell survival Kavanagh et al.
V 79 cells ATPase loci rat liver S 15 unaffected (1-40 µg/ml) (1985)
Chinese hamster HGPRT locus ± Aroclor 1254 induced 3,3',4,4'-tetra- - cell survival
V 79 cells rat liver S 15 bromobiphenyl unaffected (1-10 µg/ml)
Chinese hamster HGPRT locus none 2,2',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (20-50 µg/ml)
WB rat liver cells HGPRT locus - 2,2',4,4',5,5'- - growth stimulation
hexabromobiphenyl effects (5-20 µg/ml)
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chinese hamster HGPRT locus none 3,3',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (7-12 µg/ml)
WB rat liver cells HGPRT locus - 3,3',4,4',5,5'- - reduced cell survival
hexabromobiphenyl
Mammalian cell chromosomal
damage in vitro
Chromosome CHO cells ± Aroclor 1254 FM FF-1 - Tennant et al.
aberrations induced rat (1986)
Sister chromatid CHO cells liver S-9 -
exchange
Chromosome CHO cells ± Aroclor 1254 hexabromobiphenyl - Galloway et al.
aberrations induced rat (1987)
liver S-9
Sister chromatid CHO cells ?f
exchange
Mammalian cell chromosomal
damage in vivo
(Cytogenetic effects)
Chromosome bone marrow cells - FM - colchicine-mitosis Ficsor & Wertz
aberrations rat (pregnant) synergism (1976)
six oral doses of
100 mg/kg body
weight
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chromosome bone marrow cells - FM - no colchicine-mitosis Wertz & Ficsor
aberrations mouse (male) synergism; increase in (1978)
single dose of 50 no. of gaps
or 500 mg/kg body
weight
No. of cells in rat (male) in diet for
mitosis 5 weeks: 5-500 mg/kg
feed
Chromosome bone marrow cells; - FM BP-6 - Garthoff et al.
aberrations spermatogonial (1977)
cells
Micronucleus bone marrow cells DeBB - Millischer
mouse (male and et al. (1979)
female) total dose
of 5, 10, or
20 g/kg body
weight at 2 doses
Mammalian cell transformation
in vitro
Transformation mouse Balb/c 3T3 ± non-induced FM FF-1 - Tennant et al.
cells male F 344 rat (1986)
hepatocytes
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
DNA damage and repair
(in vitro)
Unscheduled DNA hepatocyte primary - FM FF-1 - at the highest nontoxic Tong et al.
synthesis (UDS) cultures (HPCs) doses of 10-3 mol (mice, (1983);
from rat, hamster, hamsters) or 10-5 mol Williams et al.
and mouse (rats) (1984)
DNA damage and repair
(in vivo - in vitro)
UDS hepatocyte primary - FM FF-1 - Tennant et al.
cultures (HPCs) (1986)
from treated male
F 344 rats
UDS hepatocyte primary - FM FF-1 - simultaneous measurement Mirsalis et al.
cultures (HPCs) of hepatic cell (1985)
from treated proliferative ability:
B6C3F1 mice (males increased cell
and females) proliferation
a Commercial PBB mixtures: FM = FireMaster(R); DeBB = decabromobiphenyl.
b HGPRT = hypoxanthine-guanine phosphoribosyl transferase.
c CAS No.:
6.5.1.2 Biological half-lives
The biological half-lives of PBBs reported in the literature
for various species have been compiled in Table 68. McDaniel &
Lucier (1979) reported a half-life of BB 153 exceeding the lifetime
of rats.
In some cases the results obtained by different authors are
similar, in other cases, large discrepancies exist. These deviations
were, perhaps, caused by measuring the half-lifes under different
circumstances of exposure and for different lengths of time (Fries,
1985b). By means of simulation studies, Domino et al. (1982)
demonstrated the effects of different amounts of body fat on the
half-life of BB 153 in the rat (see Table 69).
Tuey & Matthews (1980) have simulated the effects of a growing
animal on the kinetics of BB 153 and found that, though
concentrations of BB 153 in fat fell faster than in a stable animal,
the "actual" half-life of the substance was prolonged, because of an
increase in the relative fat proportions.
6.5.1.3 Differences between individual congeners
There are some reports on the differences in turnover and
retention of individual PBB congeners. Mostly, they refer to changes
in the relative abundances of certain FireMaster(R) components,
namely: 2,2',4,5,5'-pentabromobiphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118), 2,2',3,4',5',6-hexa
bromobiphenyl (BB 149), 2,2',4,4',5,5'-hexabromobiphenyl (BB 153),
2,2',3,4,4',5'-hexabromobiphenyl (BB 138), 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167), 2,3,3',4,4',5-hexabromobiphenyl (BB
156), 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), and
2,2',3,3',4,4'5-heptabromobiphenyl (BB 170). To describe such
changes, the GC profile of the original FireMaster(R) mixture was
compared with the GC profile obtained on analysis of tissues from
animals treated with this mixture, by measuring either the area
(e.g., Wolff & Aubrey, 1978; Domino et al., 1980b) or the height
(e.g., McCormack et al., 1982a; Bernert & Groce, 1984; Groce &
Kimbrough, 1984) of the GC peaks and by normalizing the values to BB
153 as 100. A few other authors (Fries & Marrow, 1975; Fries et al.,
1976) based their calculations on the assumption that each component
(BB 153 and BB 180) was fed at the same rate in the diet. According
to Fries (1985b), some differential behaviour may be due to
analytical artefacts, introduced by differential recovery of the
congeners or by adsorption of congeners on the glassware (Willett
et al., 1978), but many changes appear real.
Table 69. Effect of different amounts of body fat on the half-life of
2,2',4,4',5,5'-hexabromobiphenyl in the rata
Amount fat Half-life
(% normal fat) (days)
Emaciated 25 60.5
50 88.8
75 117
90 134
Normal 100 145
110 156
125 173
150 200
175 228
200 256
Obese 250 311
a From: Domino et al. (1982).
The trends reported in the literature include the selective
retention of 5 minor components of the FireMaster(R) mixture in
the tissues of rats, pigs, cows, and chickens (Fries & Marrow, 1975;
Fries et al., 1976, 1978a; Dannan et al., 1978b; Willett & Durst,
1978; Wolff & Aubrey, 1978; Wolff & Selikoff, 1979; Domino et al.,
1980b; McCormack et al., 1980, 1982a; Werner & Sleight, 1981; Groce
& Kimbrough, 1984; Polin & Leavitt, 1984). They can be summarized,
as follows.
With some exceptions, the concentrations of 2,2',4,5,5'-penta
bromobiphenyl (BB 101) and 2,2',3,4,4',5,5'-heptabromobiphenyl (BB
180) relative to BB 153 appeared to be lower in tissues from treated
animals than in those fed FireMaster(R) mixture. The relative
concentrations of 2,3',4,4',5-pentabromobiphenyl (BB 118) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) appeared to be higher or
unchanged in many cases. 2,2',3,4',5',6-Hexabromo biphenyl (BB 149)
was barely, or not, detected in the tissues of any animals.
The results of a multigeneration study on rats also reflected
the differential behaviour of certain PBB congeners (McCormack
et al., 1981). Of the first eight peaks (penta- to
heptabromobiphenyls) in the GC profile of FireMaster(R), all
except peak 3 (BB 149) were detected in the livers of rats in the
F1, F2, and F3 generations (experimental design: see Table
54). For example, the concentration of
2,3',4,4',5,5'-hexabromobiphenyl (BB 167) relative to BB 153 was
higher in the livers of animals in the F1 generation than in the
FireMaster(R) BP-6 standard, but decreased with each subsequent
generation. Although F2-10 and F3-100 animals (10 and 100 mg/kg
treatment, respectively; see Table 54) had similar hepatic
concentrations of BB 153, the relative concentrations of other PBB
congeners, including BB 167 appeared to be lower in the livers from
F3-100 than F2-10 animals (McCormack et al., 1981).
Domino et al. (1980b) found that 2,2',4,5,5'-pentabromobiphenyl
(BB 101) penetrated the brain of rats more rapidly than
2,3',4,4',5-pentabromobiphenyl (BB 118) or any of the higher
relative molecular mass homologues. Fries et al. (1976) reported a
faster clearance of 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180)
(half-time = approximately 20 days) than of BB 153 (half-time =
approximately 28 days) from eggs of hens after feeding stopped.
However, analyses of whole chicken carcasses resulted in unchanged
ratios of BB 153/BB 180 on days 0, 21, and 42 of withdrawal (Polin &
Leavitt, 1984). The authors judged that the dynamics for withdrawal
of these two congeners from tissues of chickens were parallel. The
withdrawal from milk of dairy cows was found to be more rapid for BB
180 than for BB 153 (Fries & Marrow, 1975; Fries et al., 1978a; see
also Fig. 7).
In just one study (Millis et al., 1985b), equimolar doses of
individual PBB congeners were administered to the test animals.
Immature male rats received a single oral dose (21.3 µmol/kg body
weight) of 3,3',4,4',5,5'-hexabromobiphenyl or 3,3',4,4'-tetra
bromobiphenyl and were analysed at various times up to 14 days after
treatment. Adipose tissue and liver concentrations of
3,3',4,4',5,5'-hexabromobiphenyl appeared unchanged over time
whereas the tissue concentrations of 3,3',4,4'-tetrabromobiphenyl
decreased in a biphasic manner.
6.5.1.4 Octabromobiphenyl
Some kinetic data are available for technical octabromo
biphenyl. Bromine levels in the adipose tissue of rats dosed with
octabromobiphenyl did not decrease during a period of 90 days
(Norris et al., 1973) or 18 weeks (Aftosmis et al., 1972a; Waritz
et al., 1977) after cessation of dosing, or, according to Lee et al.
(1975a), levels even increased 18 weeks after exposure. A partial
elimination of bromine was observed from the livers of these rats
after recovery (Norris et al., 1973; Lee et al., 1975a; Waritz
et al., 1977).
The contents of total PBB (octabromobiphenyl plus an
unidentified hexabromobiphenyl) in juvenile Atlantic salmon (Salmo
salar), fed 90 days with octabromobiphenyl (Dow
Chemical)-contaminated food, also remained fairly constant after 28
days of withdrawal (Zitko, 1977).
6.5.2 Human studies
Information on the time trends of BB 153 distribution or
retention in humans is limited. As with animals, BB 153 seems to be
highly persistent in humans. This conclusion has been drawn from
monitoring BB 153 levels over time in both individual persons and
the Michigan population.
Most paired serial samples exist for serum (or plasma) from
farmers, etc. (Humphrey & Hayner, 1975: sampled June/Autumn, 1974;
Landrigan et al., 1979: sampled 1974/77; Wolff et al., 1979b:
sampled 1976/77/78; Kreiss et al., 1982: sampled 1977/78/79;
Sherman, 1991: sampled 1976/80/87) and chemical workers (Wolff
et al., 1979b: sampled 1976/78; Bahn et al., 1980b and Bialik, 1982:
sampled 1978/1981; Lambert et al., 1990) (in parentheses: references
plus year of sampling). The values obtained indicated no, or little,
decrease (see sections 5.2 and 5.3, and Table 38) with the exception
of the results of Bahn et al. (1980b) and Bialik (1982) (see section
5.3). Paired adipose analyses have been reported only by Meester &
McCoy (1976) who found a consider able average decline in HxBB
levels over six months (see also section 5.2). However, one of the
16 persons tested had increasing or unchanged fat levels, while
serum levels slowly dropped. The significance of these observations
and the combined results of Bahn et al. (1980b) and Bialik (1982)
are unclear. A most recent case report (Sherman, 1991) showed that
"PBB" could be identified in the serum and fat of a cancer patient
over an 11-year period (1976-87). Serial testing of 11 breast-milk
samples from one lactating Michigan woman showed that HxBB
concentrations varied between 0.1 and 0.2 mg/kg (expressed on a fat
basis) during a three-month period, without any significant downward
trend (Brilliant et al., 1978).
Comparisons at the population level have been made for serum
(Meester & McCoy, 1976), breast-milk (Miller et al., 1984), and
adipose tissue (Miceli et al., 1985). Generally, the presence of
PBBs in the tissues of Michigan people many years after the spill
(1973) may be an indicator for its persistence (see Tables 38 and
39). Meester & McCoy (1976) found that the average HxBB level in the
serum of farmers during the first six months of 1976 was ten times
lower than that during the last six months of 1975 (0.2 µg/litre
versus 2.0 µg/litre). Miller et al. (1984) concluded from their
analyses of breast-milk from 2986 lactating women during May 1976
and December 1978 that HxBB levels were not declining. Approximately
5 years after the Michigan PBB incident occurred, adipose tissue
from live residents of a "high" exposure area (Muskegon County area)
contained median HxBB levels of 500 µg/kg (Wolff et al., 1982).
Approximately 10 years after exposure, postmortem adipose tissue
contained median HxBB levels of 320 µg/kg (Miceli et al., 1985).
On the basis of the two last values, Miceli et al. (1985)
calculated a half-life of 7.8 years for HxBB (BB 153) in human
adipose tissue. This prediction was close to the body burden
half-time of 6.5 years estimated by Tuey & Matthews (1980) using
pharmacokinetic data obtained from rats.
A median serum half-life of BB 153 of 12 years (range: 4.6-94.7
years) has been determined by comparing previous and more recent
serum BB 153 levels of Michigan residents (Lambert et al., 1990).
As Tuey & Matthews (1980) explained, the half-life may be
longer in growing children or in persons gaining weight. However,
calculating the effects of different amounts of body fat on the
retention of BB 153, these authors also found that adipose tissue
may act as a protective reservoir in mature humans, because the
concentration of BB 153 in the blood (and possibly other more
critical tissues) of obese persons should be significantly less than
those of leaner individuals who received comparable exposures.
There was some evidence for the differential retention of
various PBB congeners in humans, when PBB congeners in serum samples
from Michigan subjects were compared with FireMaster(R) BP-6. The
main congeners assayed by using GC-MS analysis (Wolff & Aubrey,
1978; Wolff et al., 1978) or Negative Chemical Ionisation
Spectrometry (NCIMS) analysis (Roboz et al., 1982; Greaves et al.,
1984) were as follows: 2,2',4,5,5'-pentabromo biphenyl (BB 101),
2,3',4,4',5-pentabromobiphenyl (BB 118),
2,2',3,4',5',6-hexabromobiphenyl (BB 149), 2,2',4,4',5,5'-hexa
bromobiphenyl (BB 153), 2,2',3,4,4',5'-hexabromobiphenyl (BB 138),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). The most obvious
changes refer to the two penta isomers and to the major
heptabromobiphenyl. The 2,2',3,4,4',5,5'-heptabromobiphenyl was
absent (Roboz et al., 1982) or greatly diminished in serum (Wolff &
Aubrey, 1978; Wolff et al., 1979a; Greaves et al., 1984) in relation
to the concentrations of BB 153 (taken as 100%). The relative
amounts of 2,2',4,5,5'-pentabromobiphenyl (BB 101) were also greatly
decreased (e.g., 80%: Greaves et al., 1984) in all samples. However,
a marked decrease in 2,3',4,4',5-pentabromobiphenyl (BB 118)
concentrations was observed only in the serum of farmers, taken
several years after exposure (e.g., 60%: Greaves et al., 1984), but
not in the serum of chemical workers (Wolff & Aubrey, 1978; Wolff
et al., 1979a; Roboz et al., 1982).
6.6 Reaction with body components
6.6.1 Animal studies
When a 14C-PBB mixture (FireMaster(R)) was incubated with
rat liver microsomes, no binding to exogenous DNA was detected, and
only a small amount of radioactivity was covalently bound to
microsomal protein (Dannan et al., 1978b). The formation of low and
high relative molecular mass adducts with PBB metabolites has been
quoted elsewhere (section 6.3).
6.6.2 Human studies
Studies on human serum revealed that lipoproteins are the
predominant protein carriers of PBBs in serum (Greaves et al.,
1983). 80% of the PBBs were bound to apolipoproteins B and A in a 4
: 1 ratio (Greaves et al., 1984; Roboz et al, 1985a). According to
Roboz et al. (1985b), the ratio was 3 : 1. No preferential binding
of PBB congeners (2,2',4,4',5,5'-hexa-; 2,2',4,5,5'-penta-;
2,2',4,5',6-pentabromobiphenyl) was found (Roboz et al., 1985a,b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only few data are available on effects of PBBs on organisms in
the environment. They refer to microorganisms, water fleas,
waterbirds, (rodents), and farm animals.
7.1 Microorganisms
The toxicity of technical decabromobiphenyl (Adine 0102)
against bacteria (Pseudomonas putida M.) was determined by the
cell multiplication inhibition test (according to the ISO TC 147/SC
5/WG 1/N 111 standard, and using 0.1-1% DMSO (dimethylsulf oxide) as
a solvent. An EC10 of 53 mg/litre was found (Atochem, 1990).
7.2 Aquatic organisms
In a short-term test (according to the ISO standard 6341) the
immobilization of Daphnia magna (Crustacea) by technical
decabromobiphenyl (Adine 0102) has been investigated. The following
results were obtained after dissolution of the test material in DMSO
because of its weak solubility in water:
- EC50 (24 h): > 66 mg/litre;
- The maximum concentration resulting in 0% immobilization
24 h): < 2 mg/litre (Atochem, 1990).
7.3 Terrestrial organisms
7.3.1 Wildlife
In 1977 and 1978, Haseltine et al. (1981) and Heinz et al.
(1983) studied red-breasted mergansers (Mergus serrator) nesting
on islands in the northwestern Lake Michigan in order to determine
whether environmental contaminants (organochlorines and metals) were
producing effects on reproduction. Seventeen contaminants, including
PBBs, were measured in randomly chosen eggs from 206 nests under
study. Using a variety of statistical approaches, Heinz et al.
(1983) looked for effects of individual contaminants and
combinations of contaminants on reproductive measurements, such as
nest desertion, failure of eggs to hatch, death of newly hatched
ducklings, percentage hatching success, number of ducklings leaving
the nest, and egg-shell thickness. PBBs and other chemicals were
sometimes negatively correlated with shell thickness or thickness
index, but not consistently so, as found for DDE. However, no
contaminant or combination of contaminants measured seemed to have a
pronounced effect on the aspects of reproduction mentioned above.
The hatching success of the mergansers averaged 81.7% in 1977.
According to Heinz et al. (1983), the hatching success averaged
85.6% in 1978 (Haseltine et al., 1981).
Although it is not valid to compare reproductive success among
different species, it should be noted that dabbling ducks, whose
eggs contained only a fraction of the contaminant burdens (e.g., no
PBBs: Haseltine et al., 1981; see also Table 32), of the redbreasted
mergansers had better hatching success (Heinz et al., 1983).
An interesting observation on rodents living on a
PBB-contaminated farm has been reported by Jackson & Halbert (1974).
They observed that rats and mice were apparently eradicated when
they came into contact with PBB-contaminated cattle feed pellets.
7.3.2 Farm animals
Farm animals in Michigan that ingested feed inadvertently
containing PBB (FireMaster(R) FF-1) in place of magnesium oxide
fell sick. First symptoms were observed in the cattle of several
dairy herds and revealed this so-called "Michigan PBB incident".
7.3.2.1 Cattle
The exact doses of PBBs to which Michigan cattle were exposed
are not known. Fries (1985b) estimated the maximum PBB doses of
individual cattle based on milk or tissue fat concentration at the
time of detection, which ranged from 9 to 24 months after the cattle
had consumed contaminated feed. For example, if PBB were detected 9
months after exposure, PBB concentrations of 0.1 mg/litre of milk
and 0.25 mg/kg tissue would indicate that the cow had received a
total dose of 1 mg/kg of body weight. Increased PBB concentrations
in milk and tissue would indicate a proportionally higher dose. If
detection were delayed for as long as 24 months, the above example
for milk and tissue concen trations would indicate an exposure of
6 mg/kg of body weight.
a) High-level contamination in cattle
Adverse effects of PBBs in lactating cows were first reported
by Jackson & Halbert (1974).
In reconstructing the accident, it was assumed that the cows on
this farm (Halbert farm) consumed PBB-contaminated feed (PBB content
from 3 to 4 g/kg; Isleib & Whitehead, 1975) over 16 days, and
ingested about 20 g PBB/day, initially. The total average exposure
of these cows was estimated to be 250 mg/kg body weight (Fries,
1985b). The contaminated feed was also fed to a group of 6- to
18-month-old calves. The dose might have been about 58 mg/kg body
weight per day when feeding started, and the total doses may have
reached 700 mg/kg body weight over 6 weeks (Fries, 1985b), if feed
was consumed at the same rates as in experimental studies (Durst
et al., 1977).
The clinical signs of toxicity, described by Jackson & Halbert
(1974), were anorexia (50% reduction in feed consumption) and a 40%
decrease in milk production a few weeks after ingestion of the
contaminated feed. Although the supplemented feed was discontinued
within 16 days, milk production was not restored, and the cows
continued to lose weight. An unspecified number of cows had
increased frequency of urination and lacrimation and developed
haematomas, abscesses, abnormal hoof growth, lameness, alopecia,
hyperkeratosis, and cachexia, and several died within 6 months.
Altogether, the death rate of Halbert cows was about 24/400.
The death rate of the 6- to 18-month-old calves (heifers and
bulls) to which the suspected feed was offered, was much higher.
About 50% of the calves died within 6 weeks. After 5 months, only
two of twelve animals were alive, and they had developed
hyperkeratosis over their entire bodies. There were also a variety
of reproductive problems including embryo resorption, abortions,
stillbirths, deaths shortly after births, delayed deliveries, and
enlarged calves. The clinical signs were variable, and, with the
exception of decreased milk production and weight loss, no
particular symptom was predominant in the affected animals (Jackson
& Halbert, 1974; Robertson & Chynoweth, 1975).
Necropsy findings have been reported for some of the 24 mature
cows that died during the 6 months following exposure (Jackson &
Halbert, 1974). Gross lesions observed included somewhat enlarged
livers, haematomas and abscesses in the thoracic and abdominal
cavities, abomasal ulcers, necrotic metritis, suppurative
bronchopneumonia, and pericarditis. As in all observations without
controls, it was difficult to draw definitive conclusions as to
which lesions were caused by PBBs and which were unrelated to PBBs
(Fries, 1985b). Two of the twelve calves in a calf feeding trial had
massive liver abscesses.
Histopathological studies on ten of the cows revealed various
liver and kidney changes. Liver lesions were reported in seven
animals and included fatty changes and amyloidosis. Renal tubular
nephrosis and interstitial nephritis were reported in four of the
cows (Jackson & Halbert, 1974; Getty et al., 1977).
Several clinical signs and pathological changes, reported by
Jackson & Halbert (1974), were also described in cows in controlled
feeding studies (Durst et al., 1977; Durst et al., 1978a,b; Moorhead
et al., 1977, 1978; Robl et al., 1978; Willett et al., 1980; see
also section 8). These included anorexia, dehydration, excessive
lacrimation, emaciation, hyperkeratosis, reproductive difficulties
(fetal death, enlarged calves, and difficulty in calving,
hypospermatogenesis), and renal damage. Conditions described in the
accidentally exposed cows, but not confirmed in the controlled
studies, included haematomas, abscesses, abnormal hoof growth,
extensive hair loss, liver abscesses, necrosis, and metritis (Fries,
1983, 1985b).
The Halbert herd was one of about 12 "highly" contaminated
herds that had PBB concentrations of more than 30 mg/kg in milk fat,
when detected. According to Fries (1985b), it is likely that all of
these herds had some clinical signs of toxicosis. According to the
same author, there was no comprehensive clinical examination of any
herd that had milk concentrations in the range of 1-30 mg/litre, but
it appears that most animals in these herds did not have clinical
signs when the farms were depopulated. A preliminary report (cited
by Getty et al., 1977) indicated that cows that had been exposed to
PBBs 19 months earlier had levels of up to 80 mg/kg in body fat, but
were apparently normal clinically and were producing normal
quantities of milk. Gross or microscopic lesions that could be
attributed to PBB exposure were not found.
b) Low-level contamination in cattle
Residue concentrations in the body fat or milk fat of cows,
classed as having low-level contamination, rarely exceeded 1 mg/kg,
and, for the most part, not even 0.3 mg/kg. There were several
studies on Michigan cattle with such low exposures.
Results of an evaluation of 72 low-level contaminated herds
have been reported by Kay (1977) and Getty et al. (1977).
Production drop and sterility were two consistent signs and were
regarded as interrelated. The retardation of growth of young stock
was very significant, as it was in the Jackson & Halbert study
(1974). Some other findings were not consistent. A mail
questionnaire survey (Getty et al., 1977) showed similar
observations.
Cows (n = 46) from six herds across Michigan, whose body fat
contained a mean concentration of 0.31 mg/kg and a maximum
concentration of 1.8 mg/kg, were compared with a group of cows from
Wisconsin (n = 40) that had not been exposed to PBB. The two groups
were reared together and subjected to the same feeding and
management system. There were no significant differences in the
animals' milk production, body weight, weight gain, breeding and
reproductive performance, incidence of commonly experienced health
problems, calving rate, and the health of their calves. Also no
pattern of gross or histopathological lesions was seen between test
animals and control animals upon necropsy (Wastell et al., 1978).
An epidemiological survey, which compared the health status of
16 herds with low PBB exposure (traces to 1 mg/kg body fat or milk
fat) with the status of 15 herds with no PBB exposure, also
indicated that productivity and general health conditions between
the two groups of herds were similar. Of the biochemical parameters
tested (9 urinalyses, 13 serum chemistry parameters), three resulted
in significantly different values. Serum concen trations of calcium,
glucose, and cholesterol in contaminated herds were significantly
lower than those of the control herds. But the relationship to PBB
exposure was unknown (Mercer et al., 1976).
Instead of specific clinical conditions, Fries (1983) evaluated
the overall performance of exposed herds (residues in tissue or milk
fat generally < 0.3 mg/kg) and of "relatively unexposed" herds
(residues < 0.02 mg/kg) of comparable size, breed, and location by
analysing Dairy Herd Improvement Association records. He found that
no productive or reproductive character istics of the herds were
affected by PBB exposure.
It should be noted that the classification of herds as having a
high or a low level of contamination refers to PBB levels at the
time of detection. Thus, it is sometimes impossible to know whether
there was a history of a short-term, high exposure or a long-term,
low exposure, which may produce different syndromes. For example,
Fries (1985b) pointed out that feed that was contaminated by
cross-contamination in the feed mills was being fed at the time of
detection, in some cases. Under this circum stance, PBB intakes as
low as 0.1 mg/kg of body weight per day could produce milk fat
residues as high as 20 mg/kg (Fries & Marrow, 1975; Fries 1985b).
7.3.2.2 Other farm animals
Although it was cattle feed that was originally involved in the
accidental substitution, all other feeds became involved by cross
contamination, e.g., in the mixing machinery of feed companies that
had been exposed to PBB (Dunckel, 1975). It is likely that other
animals were not exposed to the same high levels as cattle.
(i) Poultry
There are no reports of clinical signs or problems associated
with the accidental contamination of poultry feed. However, some
controlled feeding studies have been published (see section 8).
(ii) Pig
Adverse health effects in pigs, identified as contaminated,
were rarely reported. Only one review (Reggiani & Bruppacher, 1985)
mentioned that abortions occurred in pigs. Two controlled feeding
studies (Ku et al., 1978; Werner & Sleight, 1981) have been
conducted (see section 8).
(iii) Horse, rabbit, goat, sheep
Other species of farm animals, including at least 2 horses, 32
rabbits, 2 goats, and 19 flocks of sheep, were identified as
contaminated and buried at Kalkaska, but details of ill effects were
not recorded (Dunckel, 1975; Getty et al., 1977).
One experimental feeding study on sheep (Gutenmann & Lisk,
1975) is available (see section 8).
7.4 Population and ecosystem effects
No information available.
7.5 Effects on the abiotic environment
No information available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Differences in toxic responses between acute, high-level
exposures and long-term, low-level exposures to halogenated aromatic
compounds are mainly quantitative (McConnell & Moore, 1979).
Moreover, the delayed onset of toxic signs and the persistence of
PBBs in the body, which may cause long-term exposure of the target
organs from a single dose, "tend to blur the usual distinctions that
are made between acute and chronic exposure" (Fries, 1985b). For
these reasons, symptoms after single and short-term exposures are
reviewed together in section 8.2. Another feature of the toxicity
of PBBs and related classes of compounds is the latent period
between the time of exposure and the time of death, which ranged
from several days to weeks (Di Carlo et al., 1978; Safe, 1984;
Hutzinger et al., 1985a; McConnell, 1985). Thus, the classical
LD50 values and other mortality data found are summarized in one
section (8.1).
8.1 Lethality
"Acute" toxicity data (LD50 and LC50 values) of commercial
PBB mixtures have been compiled in Table 70. The LD50 values of
all mixtures show a relatively low order of acute toxicity (LD50
> 1 g/kg body weight) in rats, rabbits and quails, regardless of
the route of administration, and range from > 1 to 21.5 g/kg body
weight. Regarding the LC50 values for minks, this species may be
highly sensitive to PBBs, but a direct comparison is complicated by
differences in the experimental design.
As with TCDD and PCBs (McConnell, 1984; Safe, 1984), the
apparent toxicity of PBBs is higher with multiple-dose rather than
single-dose administration. For example, the single oral LD50 of
FireMaster(R) in rats was quoted to be 21.5 g/kg body weight, but,
if given in small repeated doses, the total lethal dose was
approximately 1-3 g/kg body weight (see Table 70).
Deaths after exposure to PBBs are delayed (Di Carlo et al.,
1978; Tables 71 and 72). Thus, Gupta & Moore (1979) have recommended
that the LD50 of halogenated aromatic hydrocarbons or chemicals
that may have a long-term build-up with delayed toxicity should be
determined more accurately after multiple dosing and an extended
period of observation.
Generally, the susceptibility to the toxic effects of aryl
hydrocarbons is dependent on the sex, age, species, and strain of
the experimental animals (e.g., Safe, 1984; Hutzinger et al.,
1985b). In the case of PBBs, female rats dosed with FireMaster(R)
FF-1 showed a lower LD50 than male rats (Gupta & Moore, 1979;
Table 70).
Table 70. Toxicity of PBB mixtures
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) rat oral LD50 21.5 single dose Di Carlo
et al.
(1978)
FireMaster(R) FF1 rat (Fischer- female oral 90 days LD50 1.43 22 doses Gupta &
(Lot No. 1312 FT) 344/N) (over 30 Moore
(in corn oil) male oral 90 days LD50 3.28 days) 22 (1979)
doses (over
30 days)
FireMaster(R) BP-6 rabbit dermal LD50 5 Aftosmis
et al. (1972b)
FireMaster(R) rabbit male dermal 14 days ALD 5 24 h of Waritz et al.
(Lot 635-71) (New Zealand, exposure (1977)
(in corn oil) albino)
FireMaster(R) FF1 mink male, in feed 313 days LC50 3.95 Ringer et al.
(Mustela vison) female (1981)
FireMaster(R) Bobwhite quail in feed 8 days LC50 428 5 days of Cottrell
(Colinus treated diet et al. (1984)
virginianus)
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
FireMaster(R) Japanese quail oral not LD50 > 1 Strik
(1973a)
specified
Octabromobiphenyl rat male oral LD50 2 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl rat male oral 7 days ALD > 17 single or Waritz et al.
(Dow Lot 102-7-72) (Sprague-Dawley) repeated dose (1977)
(in acetone: corn
oil = 15:85)
Octabromobiphenyl rabbit dermal LD50 > 10 Aftosmis
et al. (1972b)
Octabromobiphenyl rabbit (New male dermal 14 days ALD > 10 24 h of Waritz et al.
(Dow Lot 102-7-72) Zealand albino) exposure (1977)
Octabromobiphenyl Japanese quail oral LD50 > 12.5 single dose Aftosmis
et al. (1972a)
Octabromobiphenyl Bobwhite quail male, oral 14 days ALD > 12.5 single dose Waritz et al.
(Dow Lot 102-7-72) female (1977)
(in corn oil)
Nonabromobiphenyl mouse (B6C3F1) male, oral 14 days LD50 > 15 single dose Momma (1986)
(Bromkal 80-9D) female
Table 70 (contd).
PBB Species Sex Route Observation Parametera Dose/ Details References
(Strain) period concentrationb
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rat male, oral 14 days LD50 > 5 single dose Millischer
(in corn oil) (Sprague-Dawley female et al.
CFY) dermal 14 days LD50 > 5 single dose (1979)
Decabromobiphenyl rat oral LD50 > 20 single dose c
Decabromobiphenyl rabbit dermal LD50 > 8 c
a ALD = Approximate lethal dose.
b LD50 or ALD in g/kg body weight; LC50 in mg/kg diet (ppm).
c Consumer Product Testing Co. (1977) quoted by Di Carlo et al. (1978).
Table 71. Mortality associated with PBB administration: Commercial mixtures (dosing studies)
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF1 rat male oral 0.03-30 60 days 22 doses no mortality Luster et al.
(No. 1312 FT) over 30 (1978)
(in corn oil)
rat female oral 0.1-10 6 months 122 doses no mortality Luster et al.
(Fisher) over 6 (1980)
months
rat female oral 100 90 days 22 doses 100% 41-53 Gupta & Moore
(Fisher over 30 (1979)
344/N) days
male oral 100 90 days 22 doses 38% 50-73 Gupta & Moore
over 30 days (1979)
female, oral 300 90 days 9-21 doses 100% 14-29 Gupta & Moore
male (1979)
female, oral 1000 90 days 6-10 doses 100% 9-14 Gupta & Moore
male (1979)
FM BP-6 rat inhalation 71 mg not 1 h of no mortality Di Carlo et al.
per specified exposure (1978)
litre
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM FF-1 mouse female oral 0.03-30 60 days 22 doses no mortality Luster et al.
(Lot No. over 30 (1978)
1312 FT in days
corn oil) mouse female oral 0.1-10 6 months 122 doses no mortality Luster et al.
over 6 months (1980)
FM guinea-pig oral 50 60 days single dose no mortality Ecobichon
(Hartley), mentioned et al. (1983)
albino
FM BP-6 cattle oral 25 60 days daily doses 100% (animals 33-60 Irving et al.
(finely g/dayc became moribund (1976);Durst
ground) and were et al. (1977);
necropsied) Moorhead et al.
(1977, 1978)
FM BP-6 cattle female oral 250 180 days daily doses no mortality Durst et al.
(finely mg/day (1978b)
ground)
cattle female oral 0.3d 340 days daily no mortality Robl et al.
doses over (1978)
158-228
days
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
FM BP-6 rainbow trout intraperitoneal 150 15 days single dose no mortality Elcombe & Lech
(in corn oil) (Oncorhynchus mentioned (1978)
mykiss)
FM BP-6 brook trout oral 66.3 18 days 3 doses over no mortality Law & Addison
(in dogfish (Salvelinus 5 days mentioned (1981)
oil) fontinalis)
FM FF-1 sheepshead intraperitoneal 15 56 days single dose no mortality James & Little
(Archosargus (1981)
probatocephalus) 50 4-5 days single dose 2/5 (remaining James & Little
3 moribund)
OcBB rat female oral 126- 14 days single dose no mortality Norris et al.
(in corn oil) Sprague- 2000 (1973)
Dawley)
OcBB rat male oral 1000 28 days single dose no mortality Lee et al.
Sprague- (1975b)
Dawley)
OcBB rat male inhalation 0.96 7 days 4 h of no mortality Waritz et al.
(Dow Lot (Sprague- mg/ exposure (1977)
102-7-72) Dawley) litre
air
Table 71 (contd).
PBBa Species Sex Route Doseb Observation Details Mortality (in Time to References
(No.) period percentage or death
No. dead/No. (days)
treated)
DeBB rat inhalation 200 not 1 h of no mortality Di Carlo
mg/ specified exposure et (1978)
litre
air
DeBB rat male, inhalation 0.05-5 4 weeks 6 h/day; no mortality Millischer
(Sprague- mg/ 5 days/week et al. (1979)
Dawley) litre
air
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b In mg/kg body weight per day, unless otherwise specified.
c Initially equivalent to 67.2 mg/kg body weight per day.
d Equivalent to 10 mg/kg feed.
Table 72. Mortality associated with PBB administration: Commercial mixtures (feeding studies)
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 rat (Sprague- female 4.7-300 2/2 weeks no mortality Dent et al. (1976a)
Dawley) mentioned
FM rat (Sprague- male 1-500 30/30 days no mortality Sleight & Sanger (1976)
Dawley)
FM BP-6 mouse (BALB/c) female 1000 9/9 days 70% Fraker & Aust (1978);
Fraker (1980)
FM BP-6 guinea-pig 50 45/45 7/8 Vos & van Genderen
(Lot No. 182 RP) (1974)
FM guinea-pig 100 30/30 days 4/6 Sleight & Sanger (1976)
guinea-pig 500 15/15 days 100%
FM FF-1 mink male, 1 313/313 days no mortality Aulerich & Ringer (1979)
(Mustela vison) female 2.5 10% 136 days
90% 63-294 days Ringer et al. (1981)
100% 25-93 days
FM pig 20, 200 16/16 weeks no mortality Ku et al. (1978)
mentioned
FM FF-1 rhesus monkey male 25 25/25 weeks 1/1 25 weeks Allen et al. (1978)
(adult) (total: 1 g)
(juvenile) female 300 137/137 days 1/1 137 days Allen et al. (1978)
(total:
6.4 g)
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
FM BP-6 chicks (Hubbard male 400 15/15 days 37% Vos & van Genderen
(Lot No. 182 RP) Leghorn) (1974)
FM BP-6 chicken (White 20-200 8/16 weeks no mortality Cecil & Bitman (1978)
Leghorn)
4/4 weeks 60% (control 10%) Cecil & Bitman (1978)
FM FF-1 chicken (White 3125 4/4 weeks 100% Polin & Ringer (1978a)
Leghorn)
FM Japanese quail 10-100 no mortality Babish et al. (1975a)
500-1000 "few" days 100% Babish et al. (1975a)
FM Bobwhite quail 100 5/8 days no mortality Cottrell et al. (1984)
200 5/8 days 1/10 8 days Cottrell et al. (1984)
600 5/8 days 100% (10/10) 5.3 days Cottrell et al. (1984)
(mean)
FM BP-6 Atlantic salmon 100 42/70 days no mortality Zitko (1977)
(Salmo salar) (in excess of
that of
control fish)
OcBB rat (Sprague- male 100-10 000 30/30 days no mortality Norris et al. (1973)
Dawley) mentioned
Table 72 (contd).
PBBa Species Sex Dietary Period of Mortality (in Time to References
concentration treatment percentage or No. death
(mg/kg feed) observation dead/No. treated)
OcBB rat (Sprague- male 1-1000 4/22 weeks no mortality Lee et al. (1975a)
Dawley)
OcBB (Dow Atlantic salmon 100 74/96 days no mortality (in Zitko (1977)
Chemical) (Salmo salar) excess of that of
control fish)
DeBB rat (Sprague- female, 1-2000 90/90 days no mortality Millischer et al.
Dawley) male mentioned (1979)
a Commercial mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
Although the studies, in most cases, are not directly
comparable, species differences in the response to PBBs are
reflected, in part, by the mortality data collated in Tables 71 and
72. For example, minks appear to be more sensitive to the
FireMaster(R) mixture than the other species of animals tested.
Compared to rats, guinea-pigs were far more susceptible to
FireMaster(R) (Table 72).
The few studies performed with commercial octa- and
decabromobiphenyl mixtures did not result in any mortality in rats
and fish (Tables 71 and 72). Of individual PBB congeners, only three
hexa isomers have been tested. Apparently, 3,3',4,4',5,5'-
hexabromobiphenyl is more toxic for rats than
2,2',4,4',5,5'-hexabromobiphenyl (Table 73).
With few exceptions, the cause of death by halogenated aryl
hydrocarbons cannot usually be ascribed to pathology in a given
organ or system as with most toxicants (McConnell & Moore, 1979;
McConnell, 1984). At lethal doses, the affected animals develop, as
a first indication of toxicity, a "wasting syndrome", i.e., a
progressive loss of body weight, which may not be related simply to
decreased food consumption. It is followed by weakness,
debilitation, and finally death (Hutzinger et al., 1985a; McConnell,
1985). Some authors (Allen et al., 1978) use the term "metabolic
death".
The extended course of the disease can be complicated by other
diseases, usually of an infectious etiology (McConnell, 1984). For
instance, parasitism, diarrhoea, and pulmonary infections have been
observed in PBB-contaminated cattle (Jackson & Halbert, 1974;
Willett & Irving, 1976; Moorhead et al., 1978). Many of the pigs
that died during lactation after perinatal exposure to PBB had acute
suppurative pneumonia (Werner & Sleight, 1981). Severely intoxicated
rhesus monkeys may have succumbed finally to gastrointestinal and
respiratory infections (Allen et al., 1978). Male rats that were
given 100 mg FireMaster(R) FF-1/kg body weight per day and died
after 90 days had liver lesions (Gupta & Moore, 1979). Such
overlying disease problems often result in difficulties in
establishing a definitive etiological diagnosis in environmental
exposures (McConnell, 1984).
Table 73. Mortality associated with PBB administration: Individual PBB congeners
PBB Species Sex Exposure Dose Mortality (in percentage References
concentration or No. dead/No. treated)
2,2',4,4',5,5'- rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
mouse (B6C3F1) female in diet for 10 days 750a 2/8 Welsch &
(pregnant) Morgan (1985)
sheepshead intraperitoneal; single 20b no mortality James & Little
(Archosargus or multiple doses; (1981)
probatocephalus observation: 17-40 days
3,3',4,4',5,5'- rat (Sprague-Dawley) male in diet for 20 days 100a 1/2 (the remaining Render et al.
hexabromobiphenyl rat was moribund) (1982)
2,3',4,4',5,5' rat (Sprague-Dawley) male in diet for 30 days 100a no mortality Akoso et al.
hexabromobiphenyl (1982a)
Mixture of di-, Atlantic salmon in diet for 40 days plus 7.75a (100% mortality) Zitko &
tri-, and (Salmo salar) additional stress at day Hutzinger (1976)
tetrabromobiphenyls 4; observation: 42 days
a In mg/kg feed.
b In mg/kg body weight per day.
8.2 Single and short-term exposures: general signs of toxicity
8.2.1 PBB mixtures
8.2.1.1 Overt clinical signs, food intake, and body weight changes
In many acute and short-term studies, signs of PBB toxicity
include reduction in feed consumption and weight loss or a decreased
weight gain (see Tables 74 and 75). Most of the reports refer to the
FireMaster(R) mixture, which was tested in rats, mice,
guinea-pigs, pigs, cattle and avian species. From these studies with
various protocols, the minimum effective doses of FireMaster(R)
(FM) in the diet or by gavage ranged between 0.3 and 500 mg FM/kg
feed or between 4 and 100 mg FM/kg body weight per day,
respectively. Only a few studies have been performed with technical
octabromobiphenyl and technical decabromobiphenyl; no effects were
observed on feed consumption or body weight of rats (Tables 74 and
75).
Weight loss is not necessarily accompanied by decreased food
intake (e.g., Allen et al., 1978; Lambrecht et al., 1978; Gupta &
Moore, 1979) suggesting that PBBs may cause poor feed utiliz ation.
On the other hand, increased efficiency of feed utilization has been
reported in growing pigs (Ku et al., 1978) and weight loss in hens
was accounted for completely by reductions in feed consumption in
paired feeding studies (Cecil & Bitman, 1978; Ringer, 1978). If the
concentrations of PBBs are high enough, a total refusal of feed will
occur (Babish et al., 1975a; Gutenmann & Lisk, 1975; Ringer & Polin,
1977; Cecil & Bitman, 1978; Polin & Ringer, 1978a; Aulerich &
Ringer, 1979). At death, the loss in body weight can be as great as
30-40% (Allen et al., 1978; Aulerich & Ringer, 1979; Durst et al.,
1978b).
At sublethal doses, decreased food intake and weight loss may
be the only overt signs observed in many species. However, calves
(fed 100 mg FireMaster(R) FF-1/kg body weight per day) also
developed keratitis and lacrimation from day 35, and alopecia from
day 50 (Robl et al; 1978). During a 16-week test period, some pigs
receiving FireMaster(R) BP-6 at 200 mg/kg in the diet had
dermatosis (Ku et al., 1978). Loss of hair (including eye lashes),
dry scaly skin, and periorbital oedema were reported in a juvenile
female rhesus monkey given 25 mg FireMaster(R) FF-1/kg feed for 50
weeks (total dose: approximately 1.5 g), however, the time of onset
of these symptoms was not specified (Allen et al., 1978). A
decreased heart rate (bradycardia) was measured in White Leghorn
cockerels fed 150 mg FireMaster(R) FF-1/kg feed for approximately
9 weeks (Heinemann & Ringer, 1976; Ringer, 1978). Another
characteristic sign in chickens was general oedema (Ringer & Polin,
1977; see also section 8.2.1.3).
Table 74. Effects on feed consumption and changes in body and organ weights after single or short-term exposure to commercial
PBB mixtures (dosing studies)
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 rat male, oral single dose 1000 n.r. no effect liver: increase Kimbrough
(in peanut oil) (Sherman) female t.p.: 2 months et al.
(1978)
M BP-6 rat male oral single dose 500 n.r. -- liver: increase Bernert Jr &
(Lot No. 5143 (Sherman) t.p.: 1-8 weeks Groce (1984)
in corn oil)
FM BP-6 rat female oral daily doses (9) 1 no effect no effect liver: increase; spleen Harris
(in olive oil) (Sprague- during pregnancy (mg/day) kidney, adrenal, ovary et al.
(1978a)
Dawley) t.p.: 13 days gravid uterus, perirenal
(after first dose) fat pads: no effect
FM FF-1 rat male oral multiple doses 3 n.r. no effect thymus spleen: decrease Luster
(Lot No. (Fischer) (22) over 30 days et al.
1312 FT) t.p.: 60 days 30 n.r. reduced thymus, spleen: decrease (1978)
(in corn oil) (after first dose)
FM FF-1 rat male, oral multiple doses 100-1000 reduced reduced liver, spleen: increase Gupta &
(Lot No. (Fischer female (22) over 30 days (day 10) thymus: decrease Moore (1979)
(1312 FT) 344/N) t.p.: up to 90 days
(in corn oil)
male 30 no effect reduced -- Gupta &
(day 15) Moore (1979)
female 30 variable reduced --
(day 10)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
male, 3 -- -- liver: increase (day 15) Gupta et al.
female 30 no effect thymus: decrease (day (1981)
reduced 15); lung, heart, spleen,
(3 week) kidney, adrenal, thyroid,
testis, ovary, uterus,
brain: no effect
FM FF-1 rat male oral multiple doses 1-6 n.r. no effect liver, thyroid: increase Allen-Rowlands
(Lot No. (Sprague- (20) t.p.: 4 weeks adrenal, testis: et al. (1981);
1312 FT) Dawley) (after 1 dose) no effect Castracane
(in lecithin et al. (1982)
liposomes)
FM rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
(in corn oil) single dose et al. (1972b)
t.p.: 7 days
FM BP-6 rat female intraperitoneal n.r. no effect liver: increase Dent et al.
(in peanut oil) (Sprague- single dose 150 (1976b)
Dawley) t.p.: 2 weeks
FM FF-1 rat female intraperitoneal 200-1000 n.r. no effect liver: increase Goldstein
(in corn oil) (Fischer) single dose (µmol/kg et al. (1979)
t.p.: 4 days body
weight)
FM FF-1 rat male intraperitoneal 90
(in (Sprague- single dose
polyethylene Dawley) t.p.: 1 week n.r. n.r. liver: increase Dannan et al.
glycol) (1978a)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
t.p.: 2 weeks n.r. no effect liver: increase; Dannan et al.
no effect spleen, thymus: (1982c);
Millis et al.
(1985a)
FM BP-6 rat male intraperitoneal (days 30 n.r. n.r. liver, spleen: increase Robertson
(Lot 7062) (Wistar) 1 and 3) two doses 75 n.r. n.r. liver: increase; spleen, et al. (1981b)
(in corn oil) t.p.: 6 days thymus: decrease
(after 1 dose)
FM BP-6 rat male intraperitoneal three 0.2 n.r. n.r. liver: increase Ecobichon
(in peanut (Wistar) doses (days 1, 2, 3) mmol/kg et al. (1979)
oil) t.p.: 7 days body
(after 1 dose) weight
FM BP-6 rat male intraperitoneal single 500 n.r. reduced liver: increase; Andres et al.
(in corn oil) (Wistar) or multiple doses (4) thymus, spleen: decrease (1983)
t.p.: 15 days
FM FF-1 mouse female oral multiple doses 30 n.r. no effect spleen: decrease Luster et al.
(Lot No. (B6C3F1) (22) over 30 days thymus: no effect (1978)
1312 FT) t.p.: 60 days
(in corn oil) (after 1 dose)
mouse female oral multiple doses 30 n.r. no effect spleen: increase; Luster et al.
(B6C3F1) (22) from gestational thymus: decrease (1980)
(pregnant) day 0 until litters
were weaned
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM FF-1 mouse male, oral multiple doses 3-30 no effect variable liver: increase (day 15) Gupta et al.
(Lot No. (B6C3F1/N) female (22) over 30 days lung, heart, spleen, (1981)
FT 1312) t.p.: up to 90 days kidney, adrenal thyroid
(in corn oil) testis, ovary, uterus,
brain: no effect
male 30 no effect reduced thymus: decrease
(only day (only day 30)
30)
female 0.3, 3, no effect increased thymus: no effect
30 (only
day 45)
FM BP-6 mouse female intraperitoneal single 150 n.r. n.r. liver: increase (48, Dent et al.
(in peanut oil) (NMRI) dose t.p.: up to 192 h 96 h), no effect (24, (1977a)
192 h)
FM BP-6 mouse male intraperitoneal 1.50 n.r. no effect liver: increase; Robertson
(in corn oil) (C57BL/6J) single dose (mmol/kg thymus: decrease; et al. (1984c)
t.p.: 5 days body spleen: no effect
weight)
mouse male intraperitoneal 1.50 n.r. no effect liver: increase;
(DBA/2J) single dose (mmol/kg thymus, spleen:
t.p.: 5 days body no effect
weight)
FM FF-1 guinea-pig female oral single dose 50 n.r. no effect "tissues": no effect Ecobichon
(Lot FA (Hartley, t.p.: 2-60 days (liver, kidney, lung, et al. (1983)
7042) (in albino) perirenal fat)
peanut oil) (pregnant)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
FM BP-6 guinea-pig male intraperitoneal single 50 n.r. -- liver, kidney: no effect Smith
(in peanut oil) Hartley dose t.p.: 120 h et al.
(1986)
hamster male intraperitoneal single 50 n.r. -- liver, kidney: no effect
(golden dose t.p.: 120 h
Syrian)
FM (Lot rabbit male dermal 24 h of 100- n.r. reduced liver: increase Waritz
635-71) (New exposure t.p.: 10 000 (not et al.
Zealand, 14 days significant) (1977)
albino)
FM BP-6 calf male oral daily doses 0.54 no effect reduced -- Willett &
(Lot RP-158) for 44 days mg/day Irving
(in milk) (1976)
FM BP-6 cattle female oral daily doses 25 g reduced reduced liver, kidney, perirenal Irving
(Lot (pregnant) per day (day 4) (day 20) lymph nodes: increase et al.
6244 A) thymus: decrease (1976);
(days 33-66) Willett
& Irving
(1976);
Durst
et al.
(1977);
Moorhead
et al.
(1977)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
250 mg no effect no effect no effect
per day
FM FF-1 calf male, oral daily doses 100 reduced reduced -- Robl et al.
female (day 36) (day 35) (1978)
cattle female oral daily doses 0.3 no effect no effect --
for 158 days
t.p.: 340 days
(after 1 dose)
FM FF-1 dog male, oral daily doses 1 n.r. -- liver: increase Farber et al.
(Beagle) female for 7 weeks (1976)
dog male oral daily doses 4 n.r. reduced -- Farber et al.
(Beagle) (day 30) (1978)
FM Japanese oral daily doses 25-1000 n.r. reduced liver: increase Strik (1973b)
quail for 7 days
FM BP-6 rainbow intraperitoneal n.r. n.r. liver: no effect Elcombe &
(in corn oil) trout single dose t.p.: Lech (1978)
(Oncorhynchus up to 15 days
mykiss)
FM BP-6 brook trout oral multiple (200 mg/kg n.r. reduced liver: no effect Law & Addison
(in dogfish (Salvelinus doses t.p.: body (not (1981)
oil) (fontinalis) 18 days (after weight significant)
1 dose) total dose)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
OcBB (in rat female oral single dose 126-2000 n.r. no effect n.r. Norris et al.
corn oil) (Sprague- t.p.: 14 days (1973, 1975)
(Dawley)
OcBB (in rat male oral single dose 1000 no effect no effect liver: increase (days Lee et al.
corn oil) (Sprague- t.p.: 21 days 2-11), no effect (1975b)
Dawley) (day 21)
oral daily doses (2) 3000 no effect no effect liver: increase (up Lee et al.
t.p.: 28 days (after to day 21), no effect (1975b)
lase dose) (day 28)
OcBB (Dow rat male oral single dose 3400- n.r. -- liver: increase Waritz et al.
Lot 102-7-72) (Sprague- t.p.: 7 days 17 000 (1977)
(in 40-50% Dawley)
acetone: corn
oil = 15:85)
OcBB (in rat intraperitoneal 1000 n.r. n.r. liver: increase Aftosmis
corn oil) single dose t.p.: et al. (1972b)
7 days
rat inhalation 4 h 3.1 n.r. n.r. liver: no effect
exposure for µg/litre
10 days air
OcBB rat male inhalation 4 h 0.96 n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot Sprague- exposure mg/litre significant) (1977)
102-7-72) Dawley) t.p.: 7 days air
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat not inhalation (25 g) n.r. n.r. liver: no effect
(Sprague- specified 23 h/day; (2-15 weeks) kidney,
Dawley) 7 days/week for thyroid: no effect
2-15 weeks (15 weeks)
OcBB rabbit male dermal 24 h 100- n.r. n.r. liver: increase (not Waritz et al.
(Dow Lot (New exposure 10 000 significant) (1977)
102-7-72) Zealand; t.p.: 14 days
(in corn oil) albino)
male dermal 6 h/day; 1 n.r. no effect liver: increase
5 days/week, for
2 weeks t.p.:
18 days (after last
dose)
OcBB bobwhite male, oral single dose 12 500 n.r. n.r. any internal organs:
(Dow Lot quail female t.p.: 14 days no effect
102-7-72)
(in corn oil)
NoBB mouse male, oral single dose up to n.r. n.r. liver: increase Momma (1986)
(B6C3F1) female t.p.: 14 days 15 000
DeBB (in rat male, oral single dose 5000 n.r. no effect liver: no effect Millischer
corn oil) (Sprague- female t.p.: 14 days et al. (1979)
Dawley)
Table 74 (contd).
PBBa Species Sex Exposureb Dosec Feed Weight (gain) changes References
(carrier) (strain) intaked Bodyd Organe
rat intraperitoneal 1000 n.r. n.r. liver: no effect Aftosmis
single dose et al. (1972b)
t.p.: 7 days
rat male, dermal 24 h 5000 n.r. no effect liver: no effect Millischer
(Sprague- female exposure t.p.: et al. (1979)
Dawley) 14 days
rat male, inhalation 6 h/day, 0.05-5 no effect no effect liver: increase
(Sprague- female 5 days/week for mg/litre
Dawley) 4 weeks air
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; NoBB = nonabromobiphenyl; DeBB = decabromobiphenyl.
b t.p. = time post-exposure.
c In mg/kg body weight per day, unless otherwise specified.
d n.r. = not recorded.
e Absolute or relative to body weight, respectively.
Table 75. Effects on feed consumption and changes in body and organ weights caused by short-term feeding of commercial PBB mixtures
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- female 75, 300 2/2 weeks no effect no effect liver: increase Dent et al.
Dawley) (1976a)
FM rat (Sprague- male 100 30/30 days no effect no effect liver: increase Sleight &
Dawley) Sanger (1976)
500 30/30 days reduced reduced liver: increase;
kidney: no effect
FM BP-6 rat (Sprague- male 50 3/3 weeks no effect no effect liver: increase Babish &
Dawley) Stoeswand
(1977)
FM BP-6 rat (Sprague- female 50 day 8 of n.r. no effect liver: increase Dent et al.
Dawley) gestation through (1977b)
day 14 post-
partum
FM BP-6 rat male 5 variable no effect no effect liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); kidney: decrease et al. (1977)
(week 3)
rat male 500 variable no effect reduced liver: increase (weeks Garthoff
(Holtzman) (day 20) 2-5); testis: no effect et al. (1977)
(week 3)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 rat (Sprague- not 50 (in pre-, post- and n.r. n.r. liver: increase Cagen &
Dawley) specified mother's or perinatal Gibson (1978)
pups weanling's exposure/
diet) of age
15-49 age
days
FM BP-6 rat (Sprague- day 8 of
Dawley) gestation to day
15 postpartum
mothers female 50 n.r. no effect liver: increase; kidney, Dent et al.
mammary: no effect (1978b)
pups male, (50) (in pre- post-, and n.r. no effect liver: increase
female mother's perinatal
diet) exposure
FM BP-6 rat (Sprague- male, (mothers perinatal n.r. reduced liver, spleen: increase Harris et al.
Dawley) female dose: 10 exposure (day 3 of (day 60 of age) (males (1978a)
pups mg/day on age) more affected than
gestation females
days 7-15)
adults males 50-100 10/10 weeks no effect no effect liver: increase Harris et al.
150, 200 10/10 weeks no effect reduced liver: increase; adrenal, (1978b)
spleen, kidney, testes,
seminal vesicles: no
effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
female 600 10/10 weeks reduced reduced n.r.
FM BP-6 rat male 50-500 5/5 weeks n.r. no effect liver: increase Kasza et al.
(Holtzman) (1978a)
FM BP-6 rat (Sprague- male 20 28/28 days no effect no effect liver: increase Chu et al.
Dawley) (1980)
FM BP-6 rat (Sprague- male, 100 (in perinatal n.r. reduced M: ventral Johnston
Dawley) mothers or exposure until (more prostate: decrease et al. (1980)
pups weanlings 9 weeks of age pronounced
diet) in males)
FM BP-6 rat (Sprague- male 1-100 30/30 days no effect no effect liver: increase; thymus, Akoso et al.
Dawley) spleen: no effect (1982a)
male 100 30/30 days no effect no effect thyroid: increase Akoso et al.
(1982b)
FM BP-6 rat (Sprague- male 100 9/9 days no effect no effect liver, thyroid: increase Render et al.
Dawley) kidney: no effect (1982)
FM BP-6 rat (Fisher) male 100 10/10 days no effect no effect liver: increase Raber &
Carter (1986)
FM BP-6 mouse female 1000 11/11 days n.r. -- liver: increase Corbett
(Swiss, et al. (1975)
ICR) male 1000 up to 14/14 n.r. reduced liver: increase (day 4); Corbett
days (day 4) testis: no effect et al.
(1978a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 mouse female 50-200 2/2 weeks no effect no effect liver: increase Cagen et al.
(Swiss, (1977); Cagen
Webster) & Gibson
adult (1978)
pups not 50 (in postnatal -- -- liver: increase Cagen &
specified mothers exposure until Gibson (1978)
diet) 15 days of age
FM mouse not 100 30/30 days n.r. no effect liver: increase; thymus, Fraker & Aust
(BALB/c) specified spleen: decrease (1978)
not 1000 14/14 days n.r. reduced liver: increase; thymus:
specified decrease (nearly athymic)
FM BP-6 mouse female 1, 10 30/30 days n.r. no effect liver, spleen: no effect; Fraker (1980)
(BALB/c) thymus: decrease
female 100 30/30 days n.r. no effect liver: increase; thymus,
spleen: decrease
FM FF-1 mouse male 5 8/8 weeks n.r. no effect liver: increase Loose et al.
(Lot No. (BALB/c (1981)
7042) ByJ) 167 6/6 weeks n.r. no effect liver: increase
167 8/8 weeks n.r. reduced liver: increase
5 3/3 weeks n.r. -- thymus: no effect
5 6-8/6-8 weeks n.r. -- thymus: decrease
167 3-8/3-8 weeks n.r. -- thymus, spleen: decrease
5, 167 3-8/3-8 weeks n.r. -- lung: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mouse male mothers dose: perinatal n.r. no effect spleen: increase; Luster et al.
(Lot No. (B6C3F1) 10 mg/kg exposure thymus: no effect (1980)
1312 FT) pups body weight
per day
(22 doses)
FM BP-6 guinea-pig not 10 45/45 days n.r. no effect liver: increase Vos & van
specified Genderen
(1974)
FM guinea-pig not 1, 10 30/30 days no effect no effect liver: no consistent Sleight &
specified effect Sanger (1976)
100 up to 30 days n.r. reduced liver: increase
(week 3)
500 15/15 days n.r. reduced liver: increase
(week 1)
FM BP-6 pig not 20, 200 16/16 weeks reduced reduced liver, kidney: increase Ku et al.
(growing) specified (1978)
pig not 100 (in perinatal n.r. reduced liver: increase (but no Werner &
(4-week-old) specified mothers exposure (not effect in sows) Sleight
(lactating) diet) significant) (1981)
pig not 100 (in prenatal -- -- thyroid: increase;
(newborn) specified mothers diet) exposure liver: no effect
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM FF-1 mink female 1-1.54 several months -- no effect liver, kidney: increase Aulerich &
2.5 several months -- no effect (in animals that died Ringer
(within first during treatment) (1979);
3 months, Ringer et al.
(1981)
later reduced)
6-16 several months (refused) reduced
FM FF-1 Rhesus female 0.3 several months no effect reduced -- Allen et al.
monkey (1978)
(adult)
(infants) not 0.3 (in perinatal -- reduced --
specified mothers exposure;
diet) up to 12
weeks of age
(juvenile) female 300 up to 137 no effect reduced --
days (until a
few days
prior to
death)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM Japanese male, 500, 1000 a few days (refused) Babish et al.
quail female (1975a)
10-100 9/9 weeks no effect no effect liver: increase
FM Bobwhite 100-700 5/8 days reduced n.r. n.r. Cottrell
quail (Colinus et al. (1984)
virginianus)
FM BP-6 chicks male 400 15/15 days n.r. reduced bursa of Fabricius: Vos & van
(Lot No. (Hubbard decrease Genderen
182 RP) Leghorn) 15, 30 63/63 days n.r. reduced bursa of Fabricius, (1974)
spleen decrease
"PBB" pullet 50, 200 4/4 weeks n.r. reduced -- Chang &
(FM) Zindel (1975)
FM chicken male variable, several weeks -- -- liver, thyroid: Ringer & Polin
(White up to 200 increase; comb: (1977)
Leghorn) decrease; testis:
increase (low dose)
decrease (higher dose)
female 125 several weeks reduced -- --
chicks (White 75 several weeks -- reduced --
Leghorn)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
FM BP-6 chicken female 20, 64, 8/16 weeks no effect no effect -- Cecil &
(White 200 2/2 weeks reduced reduced -- Bitman (1978)
Leghorn) 640, 2000 2/2 weeks (refused) reduced --
FM FF-1 chicken female 0-25 5/5 weeks no effect -- -- Polin &
(White 125 5/5 weeks reduced -- -- Ringer
Leghorn) 3125 5/5 weeks refused -- -- (1978a)
FM chicks male 75-250 up to 42 reduced reduced liver, thyroid: Ringer (1978)
(White days increase; comb, testes,
Leghorn) spleen, bursa, thymus:
decrease
FM FF-1 cockerels male 10, 100 28/28 days no effect no effect liver: increase; bursa: Dharma et al.
(White decrease; thyroid, (1982)
Leghorn) spleen, testicles,
comb: no effect
OcBB rat 100 28/28 days -- -- liver: increase Aftosmis
et al.
(1972a)
Table 75 (contd).
PBBa Species Sex Dietary Period of Feed Weight (gain) changes References
(strain) concentration treatment/ intakeb Bodyb Organsc
(mg/kg feed) observation
OcBB rat (Sprague- male 100-1000 30/30 days no effect no effect liver: increase; heart, Norris et al.
Dawley) testes, brain: no (1973, 1975)
1000, 10 000 30/30 days effect; kidney:
increase
OcBB rat (Sprague- male 100, 1000 2-4/2-4 weeks no effect no effect liver: increase Lee et al.
Dawley) (1975a)
1000 4/22 weeks no effect no effect liver: increase
OcBB rat male 100, 1000 2-4/2-22 no effect no effect liver: increase Waritz et al.
(Dow Lot (Sprague- weeks (1977)
102-7-72) Dawley)
DeBB rat (Sprague- male, 2000 13/13 weeks no effect no effect liver: increase Millischer
Dawley) female et al. (1979)
a Commercial PBB mixtures: FM = FireMaster(R); OcBB = octabromobiphenyl; DeBB = decabromobiphenyl.
b n.r. = Not recorded.
c Absolute or relative to body weight, resp.
At lethal doses, the "wasting syndrome" (see section 8.1) can
also be accompanied by other symptoms. Rats, which became moribund,
had hunchback posture, sunken eyes, appeared dehydrated, and were
lethargic (Gupta & Moore, 1979). Minks have been reported to show an
unthrifty appearance (Aulerich & Ringer, 1979; Ringer et al., 1981).
Cattle that died later showed many similar clinical signs, but they
also had excessive lacrimation and salivation, diarrhoea and
depressed heart and respiratory rates (Irving et al., 1976; Durst
et al; 1977, 1978b; Moorhead et al., 1977). In two rhesus monkeys,
the time to death was as long as 3-5 months. In addition to body
weight loss, the dead animals exhibited alopecia and oedema,
particularly on the face and eyelids (including loss of eyelashes),
and dry scaly skin (Allen et al., 1978). Observations of intoxicated
Bobwhite quails, prior to death, included asthenia, low carriage, an
unkempt appearance, wing droop, diarrhoea, limited ataxia, and
general lethargy (Cottrell et al., 1984).
8.2.1.2 Haematology and clinical chemistry
a) Haematology
At lethal doses, leukopenia and erythropenia were observed in a
rhesus monkey (Allen et al., 1978), but not in cattle (Moorhead
et al., 1977). Packed cell volume, white blood cell count, and red
blood cell count fell gradually in the monkey (dietary
concentration: 300 mg/kg of feed; total dose: 6.4 g of
FireMaster(R) FF-1), while changes in packed cell volume,
haemoglobin content, total erythrocyte and leukocyte counts, and
differential leukocyte counts were minimal in the cows (dose: 25 g
FireMaster(R) BP-6 per day).
At sublethal doses, haematological parameters of rhesus monkeys
remained within normal limits (Allen et al., 1978). The same was
true for cattle (Moorhead et al., 1977; Robl et al., 1978), with the
exception of a calf dosed with 100 mg FireMaster(R) FF-1/kg body
weight per day, the total leukocyte count of which was elevated
(Robl et al., 1978). Rats given 30 mg FireMaster(R) FF-1/kg body
weight per day for 30 days (22 total doses) showed a significant
decrease in the packed cell volume, haemoglobin concentrations, and
platelet counts at 30 days of exposure (Gupta et al., 1981) and at 6
months after the start of dosing (Gupta & Moore, 1979), but were
normal at 45, 60, or 90 days after the start of dosing (Gupta
et al., 1981). White and red blood cell values were only
occasionally reduced; for example, there was moderate lymphopenia in
female animals at 30 days of exposure (Gupta & Moore, 1979; Gupta
et al., 1981). No significant differences in values for the
erythrocyte count, packed cell volume, haemoglobin, and total and
differential leukocyte counts were found in male rats fed various
levels of FireMaster(R) BP-6 (1-500 mg/kg diet) for 30-60 days
(Sleight & Sanger, 1976; Garthoff et al., 1977; Sleight et al.,
1978); only a possible increase in total white blood cell count was
noted at the 500 mg/kg feed level (Garthoff et al., 1977). A mild
but significant decrease in the packed cell volume and the number of
platelets was observed in mice exposed to 30 mg FireMaster(R)
FF-1/kg body weight per day for 30 days (22 total doses). The
leukocyte values were within normal limits (Gupta et al., 1981).
Growing pigs in a 16-week trial showed significant reductions in
haemoglobin and haematocrit only after 6 weeks of feeding 200 mg
FireMaster(R) BP-6/kg diet (Ku et al., 1978). There was no
appreciable effect on standard haematological values of nursing pigs
and their sows fed FireMaster(R) BP-6 (10-200 mg/kg feed) during
pregnancy and lactation (Werner & Sleight, 1981). White Leghorn
cockerels fed PBB (FireMaster(R) FF-1) at dietary concentrations
of 75 and 150 mg/kg from 3 to 4 days of age until 5-9 weeks showed a
significant decrease in packed cell volume and haemoglobin values
(Heinemann & Ringer, 1976; Ringer, 1978).
Technical octabromobiphenyl caused a significant decrease in
packed cell volume and total red blood cell count in male rats fed
dietary concentrations of 10 000 mg/kg for 30 days, but had no
effect at dietary concentrations of 100 and 1000 mg/kg (Norris
et al., 1973, 1975).
Standard haematological determinations revealed no treatment-
related changes in blood from rats exposed to technical
decabromobiphenyl via diet (1-2000 mg/kg) for 4-12 weeks and via
inhalation (0.005-5 mg/litre, 6 h/day, 5 days/week) for 4 weeks
(Millischer et al., 1979).
b) Clinical chemistry
Clinical chemistry values examined in many studies refer to
serum protein (total protein, specific fractions), serum enzymes,
serum glucose, blood urea nitrogen, serum lipids, serum cholesterol,
and urine.
(i) Serum protein
Decreases in total serum protein, due primarily to a reduction
in the albumin fraction, occurred in severely intoxicated cattle
(Durst et al., 1978a; Schanbacher et al., 1978) and monkeys (Allen
et al., 1978). No consistent effect of dietary PBBs on total serum
protein concentrations or electrophoretic profiles was observed in
pigs fed FireMaster(R) BP-6 at levels of 20 or 200 mg/kg for 16
weeks (Ku et al, 1978). In rats fed 5, 50, or 500 mg FireMaster(R)
BP-6/kg for 3 weeks, total plasma protein was slightly increased by
the highest concentration (Garthoff et al., 1977). A marked increase
(50%) in serum protein was found in rats given 22 oral doses of
FireMaster(R) FF-1 (30 mg/kg body weight per day) over a 30-day
period and observed for some additional weeks. These changes were
primarily associated with increased ß-globulin fractions (Gupta
et al., 1981). Reductions in serum immuno globulin levels
(gamma-globulin fractions) have been reported in mice given oral
doses (30 mg/kg body weight per day) of FireMaster(R) FF-1 (Luster
et al., 1978) or diets containing 167 mg FireMaster(R) FF-1/kg
(Loose et al., 1981).
No treatment-related changes in total serum protein were found
in rats exposed to technical decabromobiphenyl (1-2000 mg/kg feed
for 4-12 weeks; inhalation: 0.005-5 mg/litre; 6 h/day; 5 days/week
for 4 weeks) (Millischer et al., 1979).
(ii) Serum enzymes
Alterations in serum enzymes, most of which are indicative of
liver lesions, have been found in some instances.
Gamma-Glutamyl transpeptidase (gamma-GTP) was elevated by oral
doses of FireMaster(R) FF-1 (30 mg/kg body weight per day) in
female rats and mice of both sexes (Gupta et al., 1981).
No consistent increase in serum glutamic pyruvic transaminase
(SGPT) occurred in rats after dietary (Garthoff et al, 1977;
Matthews et al., 1978) or oral (Gupta et al., 1981) exposure to
FireMaster(R). No changes were found in cows on diets containing
up to 10 mg FireMaster(R) FF-1/kg (Robl et al, 1978). However, a
gradual increase in SGPT activity was observed in lethally
intoxicated rhesus monkeys (Allen et al., 1978). Technical
decabromobiphenyl did not influence SGPT in rats (Millischer et al.,
1979).
Serum glutamic-oxaloacetic transaminase (SGOT) was
significantly increased in cattle at high doses of PBBs (25 g FM
BP-6 per day) (Moorhead et al., 1977; Durst et al., 1978b). It was
unaffected in cattle given lower doses (250 mg FM BP-6/day: Moorhead
et al., 1977; 0.01-10 mg FM FF-1/kg feed: Robl et al., 1978), as
well as in pigs (Ku et al., 1978) or in rats (Sleight & Sanger,
1976; Garthoff et al., 1977; Sleight et al., 1978) fed
FireMaster(R) BP-6 (20 and 200 mg/kg diet or 1-500 mg/kg diet,
respectively). A possible decrease in serum GOT has been reported in
rats exposed to technical decabromobiphenyl via inhalation
(Millischer et al., 1979).
Lactic dehydrogenase (LDH) was within normal ranges in PBB-
contaminated cows without clinical signs of toxicosis (Moorhead
et al., 1977), but it was increased in a group of apparently
intoxicated animals (dose: 25 g FM BP-6/day) (Moorhead et al., 1977;
Durst et al., 1978b). A significant decrease was found in growing
pigs (Ku et al., 1978), but not in nursing pigs and their sows
(Werner & Sleight, 1981), fed FireMaster(R) BP-6.
Electropherograms of LDH isozymes at 60 days, from rats given
FireMaster(R) BP-6 (100 mg/kg feed), showed appreciable changes
(Sleight et al., 1978).
With the exception of calves (Robl et al., 1978) and newborn
pigs (Werner & Sleight, 1981), alkaline phosphatase levels in
animals were unaffected by FireMaster(R), e.g., rats (Sleight
et al., 1978; Gupta et al, 1981), dogs (Farber et al, 1976), and
growing pigs (Ku et al., 1978). Technical decabromobiphenyl also did
not have any effect on alkaline phosphatase levels in rats
(Millischer et al., 1979).
Serum isocitrate dehydrogenase (sICDH) did not show any
discernible rise in dairy cattle dosed with FireMaster(R) BP-6,
until doses were sufficient to cause toxicosis (25 g/day). These
cows showed moderate increases in sICDH (approximately a two-fold
increase). Elevation of sICDH was coincident with fetal trauma.
Non-pregnant cows, equally intoxicated, showed minimal sICDH
elevation (Schanbacher et al., 1987).
Serum creatine phosphokinase levels in growing pigs were
unaffected by 20 or 200 mg FireMaster(R) BP-6/kg feed (Ku et al.
1978).
In male Japanese quails, serum glutamate dehydrogenase levels
were increased by "hexabromobiphenyl" (STRIK, 1973b).
(iii) Serum glucose
A slight decrease in serum glucose was observed in rats and
mice administered 22 total doses of 30 mg FireMaster(R) FF-1/kg
body weight (Gupta et al., 1981) and in rats fed 50 mg
FireMaster(R) BP-6/kg diet for 3 weeks (Garthoff et al., 1977),
but not in rats fed 500 mg/kg diet for the same period (Garthoff
et al., 1977). FireMaster(R) did not produce any effects on
glucose concentrations in either sublethally or lethally intoxicated
cattle (Durst et al., 1978a; Robl et al., 1978), and
decabromobiphenyl did not affect glucose levels in rats (Millischer
et al., 1979).
(iv) Blood urea nitrogen
Values for blood urea nitrogen (BUN) remained in the normal
range in mice (Gupta et al., 1981) and rats (Sleight & Sanger, 1976;
Garthoff et al., 1977; Sleight et al., 1978; Gupta et al., 1981) or
were elevated in rats at some concentrations (Sleight & Sanger,
1976; Garthoff et al., 1977). A significant increase in BUN occurred
in cows (Moorhead et al., 1977; Durst et al., 1978a) and a calf
(Robl et al., 1978) that had received doses of FireMaster(R) high
enough to produce overt signs of toxicosis (25 g FM BP-6 per day
(equivalent to 50 mg/kg body weight per day, and 100 mg FM FF-1/kg
body weight per day, respectively).
(v) Serum lipids
Characteristic alterations in serum phospholipid HPLC profiles,
which were maintained for at least two months after dosing, were
found in rats given a single oral dose of FireMaster(R) BP-6
(500 mg/kg body weight) (Bernert et al., 1985).
(vi) Serum cholesterol
In rats, cholesterol levels appear to be the blood parameter
most sensitive to PBBs. There were dose-related increases in
cholesterol concentrations in short-term (Garthoff et al., 1977;
Spear et al., 1990) and long-term (see 8.4: Bernert et al., 1983;
Gupta et al., 1983a,b) studies, and these increases were significant
at dietary concentrations of FireMaster(R) as low as 5 mg/kg
(Garthoff et al., 1977). No noticeable effects of FireMaster(R) on
cholesterol levels have been reported in cattle (Durst et al.,
1978a) and pigs (Werner & Sleight, 1981). Rhesus monkeys showed a
gradual decrease in serum cholesterol when they were lethally
intoxicated (Allen et al., 1978).
(vii) Urinalysis
Tests of urine for pH, protein, glucose, ketones, bilirubin,
occult blood, and specific gravity showed no significant changes due
to FireMaster(R) in mice (Gupta et al., 1981) and rats (Sleight
et al., 1978; Gupta et al., 1981) or due to technical decabromo
biphenyl in rats (Millischer et al., 1979). Only Sleight & Sanger
(1976) reported higher readings for protein in rats fed
FireMaster(R). Differences in urinary protein patterns between
control rats and rats given FireMaster were detected by means of
two-dimensional electrophoresis (Myrick et al., 1987). The principal
urine changes in cattle lethally intoxicated were decreased specific
gravity and moderate proteinuria (Moorhead et al., 1977; Durst
et al., 1978a).
8.2.1.3 Morphological and histopathological changes
a) Liver
The liver is the site of the most prominent gross morphological
and histopathological changes due to PBBs in many species.
Enlargement of the liver was a characteristic response to
exposure to FireMaster(R), technical octabromobiphenyl, and
technical decabromobiphenyl, and it frequently occurred at
concentrations lower than required to produce body weight changes
(see Tables 74 and 75). Generally, increases in absolute or relative
liver weights were dose and time dependent (e.g., Garthoff et al.,
1977). A notable exception was in cows, which showed decreases in
body weight as well as an increase in liver weights only at lethal
doses (Table 74).
Grossly, the livers of rats were often friable and had a
mottled surface (Sleight & Sanger, 1976; Waritz et al., 1977; Akoso
et al., 1982a; Render et al., 1982; Raber & Carter, 1986). Red
fluorescence of liver (and other tissues) under UVR (366 nm)
indicated excess porphyrin accumulation. In contrast to rats, which
developed porphyria after long-term exposure, the liver of female
mice given 30 mg FM FF-1/kg body weight per day (22 total doses)
became porphyric after 45 days (Gupta et al., 1981).
FireMaster(R) FF-1 given orally at a dose rate of 22.5 mg/kg body
weight per day for 4 days caused centrolobular accumulation of
Oil-red O-staining lipids in the liver of rats (Kohli et al., 1981).
The principal histopathological alterations in rodent species
consisted of extensive swelling and vacuolation of hepatocytes and
proliferation of smooth-surfaced endoplasmic reticulum (SER) (seen
as "foamy cytoplasm" in light microscopy). The vacuoles were filled
with fat indicating excess lipid accumulation. Proliferation of SER
may be a morphological reflection of enhanced enzyme activity
(Sleight & Sanger, 1976; Render et al., 1982). The changes depended
on dose and length of exposure. They have been reported in rats
after dietary intake of FireMaster(R) (Sleight & Sanger, 1976;
Kasza et al., 1978a; Sleight et al., 1978; Hinton et al., 1979;
Akoso et al., 1982a; Render et al., 1982; Raber & Carter, 1986) and
of technical octabromobiphenyl (Norris et al., 1973; Lee et al.,
1975a). The dietary concentrations ranged from 0.1 to 500 mg/kg for
FireMaster(R) and from 100 to 10 000 mg/kg for octabromobiphenyl.
Effects were seen as early as the tenth day of feeding 100 mg FM
BP-6/kg (Raber & Carter, 1986). In contrast, during a 90-day feeding
trial, technical decabromobiphenyl (dietary concentration: 100, 500,
or 2000 mg/kg) caused hepatic damage only at the highest level
(Millischer et al., 1979). Liver changes, as noted above, were
observed also after a single i.p. injection (200-1000 µmol/kg) of
FireMaster(R) (Goldstein et al., 1979), after single oral dosing
(1000 mg/kg body weight) of FireMaster(R) (Kimbrough et al., 1978)
and of octabromobiphenyl (Lee et al., 1975b), and after multiple
oral dosing of FireMaster(R) (22 doses over a 30-day period:
30 mg/kg body weight per day (Gupta & Moore, 1979; Gupta et al.,
1981) and of octabromobiphenyl (two doses: 3000 mg/kg body weight;
Lee et al., 1975b). As soon as 24 h (Kimbrough et al., 1978), 3 days
(Lee et al., 1975b), or 4 days (Goldstein et al., 1979) after
treatment, histological changes could be detected.
Light and electron microscopic changes also reported in the
liver of rats included reduction or disintegration of rough-
surfaced endoplasmic reticulum (RER) (Lee et al., 1975a,b; Gupta
et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), presence
of myelin bodies (cytoplasmic inclusions; membrane whorls) (Lee
et al., 1975a,b; Sleight & Sanger, 1976; Kasza et al., 1978a;
Kimbrough et al., 1978; Sleight et al., 1978; Hinton et al., 1979;
Gupta et al., 1981; Akoso et al., 1982a; Raber & Carter, 1986), di-
or multinucleated cells (Kasza et al., 1978a; Kimbrough et al.,
1978; Gupta & Moore, 1979; Hinton et al., 1979), diminution of
glycogen (Millischer et al., 1979; Gupta et al., 1981; Raber &
Carter, 1986) and mitochondria that were swollen and reduced in
number or had degenerated as time passed (Sleight & Sanger, 1976;
Kasza et al., 1978a; Akoso et al., 1982a). There was also necrosis
of hepatocytes (Kimbrough et al., 1978; Gupta & Moore, 1979; Gupta
et al., 1981), and these necrotic foci were infiltrated with
polymorphonuclear cells and lymphocytes (Gupta et al., 1981). With
octabromobiphenyl, myelin configurations developed 7 days after
treatment and subsequently disappeared one week later (Lee et al.,
1975b).
Changes in the hepatocytes were more advanced in the centri
lobular and midzonal regions than in the periportal area of the
liver lobule (Render et al., 1982; Raber & Carter, 1986). Fatty
infiltration in the livers of male rats was much more pronounced
than in those of female rats (Gupta & Moore, 1979).
Male rats given 100 mg FM FF-1/kg body weight per day (22 total
doses) and dying after 90 days had subacute to chronic hepatitis
with marked focal proliferation of bile ducts (Gupta & Moore, 1979).
Changes in the bile canaliculus (proliferation of microvilli)
were also found in mice fed 1000 mg FM BP-6/kg for 4-14 days.
Changes in the hepatocytes of these mice were increase in cell size,
decrease in RER, increase in SER, degeneration of mitochondria,
decrease in glycogen, and increase in size and number of nucleoli
(Corbett et al., 1978a). Fatty infiltration of the cytoplasm was
reported only in another two studies on mice dosed with 30 mg FM
FF-1/kg body weight per day (Gupta et al., 1981) or fed 167 mg FM
BP-6/kg for 6 weeks (Loose et al., 1981). However, in (moribund)
mice fed 167 mg FM BP-6/kg feed for 12 weeks, lipid vacuoles were
not found within the cytoplasm, but, almost exclusively, within the
nucleus (Martino et al., 1981). Hepatocellular necrosis has also
been observed (Loose et al., 1981).
Hepatocytes of guinea-pigs were swollen and had many more large
vacuoles than those of comparably dosed rats (Sleight & Sanger,
1976), even at lower dietary concentrations (< 10 mg FM/kg). Liver
damage was reported to be minimal at dietary levels of 50 mg FM/kg
(Vos & van Genderen, 1974), but severe centrilobular fatty changes
were found at 100 and 500 mg FM BP-6/kg (Sleight & Sanger, 1976).
Livers of rabbits, dermally treated with FireMaster(R) at 5
or 10 g/kg body weight, showed a mottled appearance, necrotic foci,
and were friable. No gross pathological effects were seen in rabbits
dermally exposed to octabromobiphenyl at 10 g/kg body weight (Waritz
et al., 1977).
Pigs fed FM BP-6 (up to 200 mg/kg) showed the following liver
alterations: fatty change, centrolobular necrosis, swollen
hepatocytes, and homogeneous cytoplasm (Werner & Sleight, 1981).
Compared with other species, liver changes observed in cattle
were less dramatic. Only an early stage of centrilobular fatty
degeneration and glycogen depletion were found in the enlarged
livers of lethally (25 g FM BP-6/day) dosed cows. In addition, there
were changes in the gallbladder and bile duct (Mercer et al., 1978;
Moorhead et al., 1978). Single calves exposed to FM FF-1 for 6-12
weeks had slightly enlarged hepatocytes (dose: 1 mg/kg body weight
per day) or necrosis of individual or small foci of hepatocytes
(dose: 100 mg/kg body weight per day) (Robl et al., 1978).
The only consistent histopathological lesion in mink which died
(exposure: 6.25 mg FM FF-1/kg feed), was a fatty infiltration of the
liver (Aulerich & Ringer, 1979).
No hepatocellular damage was found in dogs given oral doses of
FM BP-6 (1 mg/kg body weight per day) for seven weeks (Farber
et al., 1976).
Biopsies of the livers of two rhesus monkeys given a diet
containing 25 mg FM FF-1/kg at 12 weeks revealed enlargement of
hepatocytes and a marked proliferation of SER (Allen et al., 1978).
Liver changes in avian species were similar to those observed
in mammals. White Leghorn cockerels fed a diet of 10 or 100 mg FM
FF-1/kg showed enlargement and vacuolation of hepatocytes, increased
SER, swollen mitochondria, and disruption of mitochondrial cristae.
But, unlike rats, increased SER was not a significant feature
(Dharma et al., 1982).
b) Thymus
The thymus is also an organ sensitive to PBB exposure.
Decreases in thymus weights were observed in rats, mice, and cattle
after oral or intraperitoneal doses (Table 74) and in mice after
feeding (Table 75) of FireMaster(R) mixtures. There were no
reports on thymus weight changes due to exposure to commercial octa-
or decabromobiphenyl (Tables 74 and 75). The weight of the thymus
was reduced as early as 6 (Robertson et al., 1981b) or 15 days
(Gupta et al., 1981; Andres et al., 1983) after rats were given high
doses of FireMaster(R) (Table 74). Frequently, decreases in thymus
weights were accompanied by increases in liver weights (see: Tables
74 and 75). But, in some cases, changes in thymus weights were seen
at doses lower than those required for liver changes (Fraker, 1980),
or vice versa (Akoso et al., 1982a). Rats (Akoso et al., 1982a) and
mice (Fraker & Aust, 1978) both fed FireMaster(R) BP-6 (100 mg/kg
feed) for 30 days differed in their thymic response in that rats
remained unaffected and mice showed decreased thymic weight
(Table 75). In contrast, rats appeared to be more sensitive than
mice when given equal oral doses (30 mg/kg body weight per day for a
period of 30 days) of FireMaster(R) FF-1 (Luster et al., 1978;
Gupta et al., 1981: Table 74). Two strains of inbred mice are known
to differ in their thymic sensitivity to FireMaster(R) (Robertson
et al., 1984c: Table 74).
In some studies, the histological appearance of the thymus of
rats and mice that had survived exposure to FireMaster(R) was only
minimally, or not, affected (Gupta & Moore, 1979; Gupta et al.,
1981; Loose et al., 1981; Akoso et al., 1982a), even when organ
weights were altered. In other studies, a preferential atrophy of
the cortex of the thymus was found in rats and mice similarly
exposed (Luster et al., 1978; Fraker, 1980). At high doses (1000 mg
FM BP-6/kg feed), "surviving" mice (30%) were essentially athymic by
day 14 (Fraker & Aust, 1978). The thymus was markedly involuted also
in moribund rats. The normal architecture of the thymus was
obliterated with marked atrophy and loss of demarcation between the
cortical and medullary regions and disappearance of cortical
thymocytes (Gupta & Moore; 1979). Moderate to marked atrophy of the
thymus was also observed in guinea-pigs (Vos & van Genderen, 1974)
or cattle (Moorhead et al., 1978) that were lethally intoxicated.
The bursa of Fabricius, an analogous organ in avian species,
was also affected by FireMaster(R) (Table 75: Vos & van Genderen,
1974; Ringer, 1978; Dharma et al., 1982). Reductions in weight
occurred at exposures as low as 10 mg/kg feed in cockerels (Dharma
et al., 1982). Histologically, the bursa showed depletion of the
lymphoid cells especially in the medulla (Vos & van Genderen, 1974;
Ringer, 1978; Dharma et al., 1982).
c) Spleen
Changes in spleen weights are given in Tables 74 and 75.
Feeding of FireMaster(R) (100-200 mg/kg equivalent to 10-20 mg/kg
body weight per day) had no effect on the spleen weights of young
rats (Harris et al., 1978b; Akoso et al., 1982a), but resulted in a
decrease in the spleen weights of mice (equivalent to 15-30 mg/kg
body weight per day) (Fraker & Aust, 1978; Fraker, 1980; Loose
et al., 1981). An increase in spleen weights was observed in pups of
both rats (Harris et al., 1978a) and mice (Luster et al., 1980)
perinatally exposed to FireMaster(R). No effects were found in
some oral dosing studies on rats (Harris et al., 1978a; Gupta
et al., 1981); in another study, spleen weights were decreased
(Luster et al., 1978), and in the highest-dosage study reported
spleen weights increased (Gupta & Moore, 1979). On the other hand,
rats that received i.p. injection of FireMaster(R) showed
decreases in spleen weight at the higher doses (Robertson et al.,
1981b; Andres et al., 1983) and increases at the lower dose
(Robertson et al., 1981b). While female mice orally dosed with
FireMaster(R) showed decreased spleen weights (Luster et al.,
1978), pregnant and nursing females comparably dosed exhibited
increased spleen weights (Luster et al., 1980). No effect was found
in mice, 5 days after a single i.p. injection of FireMaster(R),
though the liver and thymus were affected (Robertson et al., 1984c).
Spleen weights of chicks fed FireMaster(R) were reduced (Vos & van
Genderen, 1974; Ringer, 1978) or remained unaffected (Dharma et al.,
1982).
Significant histopathological changes in the spleen have not
been reported, except in rats that were moribund from high doses of
FireMaster(R). In these animals, the splenic lymphatic follicles
were small because of a lack of periarterial lymphoid cells (Gupta &
Moore, 1979).
d) Thyroid
Exposure to FireMaster(R) resulted in an increase in thyroid
weight, if there was any weight change (see Tables 74 and 75).
Increases in thyroid weight have been observed in rats (Sleight
et al., 1978; Allen-Rowlands et al., 1981; Akoso et al., 1982b;
Render et al., 1982), newborn pigs (Werner & Sleight, 1981) and in
chickens (Ringer & Polin, 1977; Ringer, 1978). When no weight change
occurred (rats: Sleight et al., 1978; Gupta et al., 1981; mice:
Gupta et al., 1981; chickens: Dharma et al., 1982), the extent of
exposure was not always less than in the former studies.
Octabromobiphenyl tested in one inhalation study did not have any
effect on thyroid weight in rats (Waritz et al., 1977).
Histological changes in the thyroid gland developed in rats at
dietary concentrations of FireMaster(R) BP-6 as low as 5 mg/kg
(Kasza et al., 1978b). Hyperplasia of follicular cells occurred in
rats (Kasza et al., 1978b; Sleight et al., 1978) and newborn pigs
(Werner & Sleight, 1981). The normal low cuboidal follicular
epithelium was altered to one that had a more columnar appearance
(Kasza et al., 1978b; Sleight et al., 1978). The most prominent
ultrastructural lesions found in rats fed 5-500 mg
FireMaster(R)/kg for 5 weeks were dose-dependent. They included
abnormal lysosomes and colloid droplets in the cytoplasm, vacuolated
mitochondria with disrupted cristae, luminal surfaces with short and
abnormally branched microvilli or devoid of microvilli, and abnormal
cytoplasmic processes into the lumen (Kasza et al., 1978b).
e) Kidney
In most studies using rodents (Sleight & Sanger, 1976; Waritz
et al; 1977; Harris et al., 1978a,b; Gupta et al., 1981; Render
et al., 1982; Ecobichon et al., 1983; Smith et al., 1986), kidney
weights did not change with PBB exposure (Tables 74 and 75).
Increases in kidney weights were caused by FireMaster(R) in minks
(Aulerich & Ringer, 1979) pigs (Ku et al., 1978), and cattle
(Moorhead et al., 1977), and by octabromobiphenyl in rats (Norris
et al., 1973). A single study reported a decrease in kidney weight
in rats fed FireMaster(R) BP-6 (Garthoff et al., 1977).
In cattle, kidneys were severely affected, and doubled in size
in animals that were moribund from high doses of FireMaster(R)
(Moorhead et al., 1977). The kidneys were distended with fluid, and
pale tan to gray in colour. Perirenal lymph nodes were enlarged and
oedematous. The principal histological lesions consisted of
dilatation of collecting ducts and convoluted tubules, and tubular
epithelial degenerative changes (Moorhead et al., 1977). Similar
renal lesions were found in calves treated with different doses of
FireMaster(R) (0.1-100 mg FM FF-1/kg body weight per day, for 2-12
weeks). The severity of renal damage was related to dose level and
length of exposure (Robl et al., 1978). Despite the extensive
morphological damage, effective renal plasma flow rates and
glomerular filtration rates were not affected in cows (Mercer
et al., 1978; Schanbacher et al., 1978).
Ultrastructural analysis of kidneys of rats and mice given a
single i.p. injection of 150 mg "PBB" (not specified)/kg body weight
revealed proliferation of SER and increased numbers of peroxisomes
in the proximal tubule of the rat, 15 days after dosing.
Proliferation of SER was confined to only one segment (S3) of the
proximal tubule. Mice had only marginal increases in SER and no
significant increases in peroxisomes (Rush et al., 1986).
f) Stomach
Biopsies of the stomachs of two rhesus monkeys given
FireMaster(R) FF-1 (25 mg/kg diet) were made at 12 weeks. In the
gastric mucosa, there was evidence of early epithelial hyperplasia
and penetration of the submucosa by glandular epithelium (Allen
et al., 1978).
g) Testicle
Changes in the weights of the testes due to PBB exposure (see
Tables 74 and 75) were not found in rats (Norris et al., 1973;
Garthoff et al., 1977; Harris et al., 1978b; Gupta et al., 1981;
Castracane et al., 1982) and mice (Corbett et al., 1978a; Gupta
et al., 1981). The results of studies on chickens (Ringer & Polin,
1977; Ringer, 1978; Dharma et al., 1982) were inconsistent (see
Table 75).
Histologically, treatment-associated changes (e.g.,
hypospermatogenesis) were observed in the testes of male calves
administered FireMaster(R) FF-1 (0.1-100 mg/kg body weight per
day) for 2-12 weeks (Robl et al., 1978). Chickens fed
FireMaster(R) (50 mg/kg of feed) showed lipid infiltration into
the testicular parenchyma (Ringer & Polin, 1977).
h) Fluid accumulation
The presence of fluid accumulation, i.e., hydropericardium and
ascites, was noted in chickens (Heinemann & Ringer, 1976; Ringer,
1978). This lesion is known as "chick oedema disease" (McConnell,
1980). Oedema were observed also in the skin of rhesus monkeys
(Allen et al., 1978) and in the kidney, perirenal lymph nodes, and
the gastrointestinal tract of cattle (Moorhead et al., 1978) that
were lethally intoxicated.
Occasionally, weight changes in organs other than those
discussed above have been reported (see Tables 74 and 75, but they
appear to be of minor importance.
Some special effects occuring after single or short-term
exposure to PBBs are reviewed in the respective sections.
8.2.2 Individual PBB congeners and comparative studies
A lot of individual PBB congeners have been examined for
prominent general signs of toxicity, such as changes in body and
relative organ weights (see Tables 76 and 77) and for histopatho
logical changes (see Tables 78 and 79). It is evident from these
records that individual PBB congeners differ in their pattern of
toxicity. The more toxic isomers and congeners cause a decrease in
thymus and/or body weight and produce pronounced histological
changes in the liver and thymus. Despite variations in experimental
protocols, a tendency can be seen that the most severe effects are
elicited by congeners listed under category I. The relative severity
of damage decreases in categories II and III with the least effects
in the last group. Within a category, the degree of bromination may
also influence toxicity. Categorization of halogenated biphenyls has
been made on a structural basis (Parkinson et al., 1983; Safe,
1984). Category I comprises isomers and congeners lacking
ortho-substituents. They are referred to as coplanar PBBs.
Mono- ortho-substituted derivatives constitute the second category.
Other PBBs (mainly those with two or more ortho-bromines) have
been organized into the third category. Structure-activity
relationships are discussed in detail by several authors (e.g.,
Goldstein, 1979; Parkinson et al., 1983; Safe, 1984).
Orders of toxicity derived from comparative studies included
only a limited number of congeners in any given study, but they
agreed with the trends described above. Ecobichon et al. (1979)
evaluated ultrastructural effects of lower and higher brominated
congeners on hepatocytes of rats and showed that the highly
brominated congeners (tetra-, penta-, hexa-, octabromobiphenyls)
were more active than the low bromine-containing congeners (di-,
tribromobiphenyls). Results of comparative studies dealing with
higher brominated isomers and congeners, predominantly constituents
of the FireMaster(R)-mixture, and the mixture itself have been
summarized in Table 80.
In all combinations tested, 3,3',4,4',5,5'-hexabromobiphenyl
(BB 169) was found to be the most toxic PBB. This congener, only a
very minor constituent of FireMaster(R), resembles 2,3,7,8-tetra
chlorodibenzo- p-dioxin (TCDD), the typical and the most toxic
member of the class of polyhalogenated hydrocarbons (e.g., Poland &
Knutson, 1982). Of the major FireMaster(R) constituents,
2,3,3',4,4',5-hexabromobiphenyl (BB 156) appeared to be the most
toxic, followed by 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5-pentabromobiphenyl (BB 118). The main component of the
FireMaster(R)-mixture, 2,2',4,4',5,5'-hexabromobiphenyl (BB 153)
was relatively nontoxic as well as the second most abundant
constituent, 2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180). Compared
with the mixture itself, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)
was consistently more toxic than FireMaster(R) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) less toxic. With
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), different results were
obtained from a dosing study (Dannan et al., 1978a) and two feeding
studies (Akoso et al., 1982a,b; Dharma et al., 1982). The latter
attributed FireMaster(R) a higher toxicity than 2,3',4,4',5,5'-
hexabromobiphenyl (BB 167); 2,2',4,5,5'-pentabromobiphenyl (BB 101)
was less effective in producing adverse effects than
2,3',4,4',5-pentabromobiphenyl (BB 118), which matched
FireMaster(R) in some aspects (see Table 80).
Table 76. Effect of individual PBB congeners on body weight (or body weight gain) and relative organ weights of mice
and rats (dosing studies)
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Categoryd I PBBs ("Coplanar" congeners)
4,4'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon
600 (4-6) (days 1,2,3) (peanut oil) et al. (1979)
t.p.: 7 dayse
3,4,4',tri rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4'-tri rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
300 (3) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,4,4',5-tetra rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,4,4',5-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982)
t.p.: 5 dayse
3,3',4,4'-tetra rat (Sprague- male oral single dose (corn no effect no effect (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4'-tetra rat (Long male intraperitoneal single no effect increased reduced Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4'-tetra rat (Wistar) male intraperitoneal single reduced increased reduced Andres et al.
150 (4-5) dose (corn oil) (1983);
t.p.: 2 weeks Robertson
et al. (1983b)
3,3',4,4'-tetra rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
4.25g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4'-tetra rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4'-tetra mouse male intraperitoneal single no effect increased reduced Robertson et al.
(C57BL/6J dose (corn oil) (1984c)
1500 and DBA/2J) t.p.: 5 days
(10)
3,3',4,4',5-penta rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5-penta rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',4,4',5,5'-hexa rat (Sprague- male oral single dose (corn no effect (increased)f (reduced)f Millis et al.
21.3 Dawley) (3) oil) t.p.: up to 14 days (1985b)
3,3',4,4',5,5'-hexa rat (Long male intraperitoneal single reduced increased reduced Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
3,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Millis et al.
3.19g Dawley) (6) dose (polyethylene glycol) (1985a)
t.p.: 2 weeks
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 2 doses -- increased reduced Robertson et al.
60 (4) (days 1,3) (corn oil) (1982b)
t.p.: 5 dayse
3,3',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) t.p.: 7 daysd (1979)
Category II PBBs (Monoortho "coplanar" derivatives)
2,3',4,4'-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4'-tetra mouse male intraperitoneal single Robertson et al.
1500 dose (corn oil) (1984c)
t.p.: 5 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
(C57BL/6J) no effect no effect no effect
(5)
(DBA/2J) no effect increased no effect
(5)
2,3',4,4',5-penta rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
250 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single reduced increased reduced Dannan et al.
Dawley) (6) dose (polyethylene glycol) (1982c)
164g t.p.: 2 weeks no effect increased no effect Millis et al.
(1985a)
2,3',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single reduced increased (reduced)f Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1978a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Sprague- male intraperitoneal 2 doses reduced increased reduced Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,3,3',4,4',5-hexa rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
3.8-60 (1-4) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,3,3',4,4',5'-hexa rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
100 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
Category III PBBs (Others)
4-mono rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (day 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2'-di rat (Sprague- male intraperitoneal single -- no effect -- Moore et al.
289g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: 2-22 days
2,2'-di rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,5'-di rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,2'5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (days 1,2,3) (peanut oil)
t.p.: 7 dayse
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses -- no effect --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses -- no effect -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased --
600 (4-6) (peanut oil) t.p.: 7 dayse
2,3',4'5-tetra rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
150 (4) (days 1,3) (corn oil) (1980)
t.p.: 5 dayse
2,2',5,5'-tetra rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (peanut oil) t.p.: 7 dayse (1979)
2,4,4',6-tetra rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,3',4,4',6-penta rat (Long male intraperitoneal single no effect no effect no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
2,2',4,5,5'-penta rat (Long male intraperitoneal single no effect increased no effect
500 Evans) (3) dose (corn oil)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single no effect no effect no effect Dannan et al.
164g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
(6) t.p.: 14 days no effect increased no effect Millis et al.
(1985a)
2,2',3,4,4',5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
144g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',4,4',6,6'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa rat (Fischer male, oral 22 doses (over no effect increased no effect Gupta et al.
590g 344/N) (6) female 30 days) (corn oil) (1981)
t.p.: 15, 30, 45, 60,
90 dayse
2,2',4,4',5,5'-hexa rat (Fischer) female intraperitoneal single Goldstein et al.
(4) dose (corn oil) (1979)
t.p.: 4 days
40 no effect no effect --
200, no effect (increased)f --
1000
2,2',4,4',5,5'-hexa rat (Long male intraperitoneal single no effect increased no effect Parkinson et al.
500 Evans) (3) dose (corn oil) (1983)
t.p.: 4 days
Table 76 (contd).
PBB congener Dosea Species Sex Exposureb Weight changesc in: References
(strain) (solvent)
(No.) Body Liver Thymus
2,2',4,4',5,5'-hexa rat (Sprague- male intraperitoneal single no effect increased no effect Moore et al.
144g Dawley) (6) dose (polyethylene glycol) (1978b); Millis
t.p.: 2 weeks et al. (1985a)
2,2',4,4',5,5'-hexa rat (Wistar) male intraperitoneal 3 doses -- increased -- Ecobichon et al.
600 (4-6) (days 1,2,3) (peanut oil) (1979)
t.p.: 7 dayse
2,2',4,4',5,5'-hexa mouse male, oral 22 doses (over no effect increased no effect Gupta et al.
590g (B6C3F1/N) female 30 days) (corn oil) (1981)
(6) t.p.: 15, 30, 45, 69,
90 dayse
2,2',3,3',4,4'5-hepta rat (Sprague- male intraperitoneal single no effect increased no effect Dannan et al.
127g Dawley) (4) dose (polyethylene glycol) (1982a)
t.p.: 7 days
2,2',3,4,4',5,5'-hepta rat (Sprague- male intraperitoneal single -- increased -- Moore et al.
127g Dawley) (3) dose (polyethylene glycol) (1979a)
t.p.: up to 22 days
2,3,3',4,4',5,6-hepta rat (Wistar) male intraperitoneal 2 doses -- no effect -- Robertson et al.
6 (2) (days 1,3) (corn oil) (1981a)
t.p.: 5 dayse
2,2',3,3',4,4',5,5'- rat (not male intraperitoneal single -- increased -- Besaw et al.
octa 115g specified) dose (solvent not (1978)
specified) t.p.: 7 days
Table 76 (contd).
a Total dose in µmol/kg body weight.
b t.p. = Time post-exposure; -- = data not given.
c Only statistically significant changes; organ weight changes relative to body weight.
d Categorization according to Safe (1984); see also text in section 8.2.2.
e After first dose.
f Only absolute data given.
g Calculated from original value given in mg/kg body weight.
Table 77. Effect of various hexabromobiphenyl isomers on feed intake, body weight (or body weight gain)
and (relative) organ weights (feeding studies)
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Categoryb I PBBs
3,3',4,4',5,5'- rat male 10 30 reduced reduced increased reduced spleen: reduced Akoso et
hexa (Sprague- brain: no effect (1982a,b)
Dawley) thyroid:
(6) increased
rat male 1 9 no effect no effect no effect Render
(Sprague- 10 9 reduced reduced increased reduced et al.
Dawley) 100 9 reduced reduced increased reduced (1982)
(6)
(2) 100 20 refused
(day 16)
Category II PBBs
2,3',4,4',5,5'- rat male 1, 10 30 no effect no effect no effect no effect brain: increased Akoso
hexa (Sprague- thyroid: no effect et al.
Dawley) 100 30 no effect no effect increased no effect brain: increased (1982a,b)
(6) thyroid: no effect
cockerel male 4, 10 28 no effect no effect no effect bursa of Dharma
(White Fabricius, et al.
Leghorn) thyroid, spleen, (1982)
(10) testicles, comb:
no effect
Table 77 (contd).
PBB congener Species Sex Dietary Feeding Food Effects References
(strain) concentration period intake Weight changesa
(No.) (mg/kg feed) (days)c Body Liver Thymus Other organs
Category III PBBs
2,2',4,4',5,5'- rat male 1 30 no effect no effect increased no effect brain: increased Akoso
hexa (Sprague- thyroid: no et al.
Dawley) effect (1982a,b)
(6)
(6) 10, 100 30 no effect no effect increased increased brain: increased
thyroid: no
effect
rat male 10 9 increased no effect increased n.r. kidney: no Render
(Sprague- effect et al.
Dawley) 100 9 no effect no effect increased n.r. kidney: no (1982)
(6) effect
mouse female 100, 300 gd 6 no effect no effect increased n.r. Welsch &
(C57 BL), through Morgan
pregnant 15 (1985)
(3) 500, 750 sacrifice no effect reduced increased n.r.
gd 17
1000 reduced reduced increased n.r.
cockerel male 10 28 no effect no effect no effect n.r. bursa of Fabri- Dharma et al.
(White cius, thyroid, (1982)
Leghorn) 62 28 no effect no effect increased n.r. spleen, testicles,
(10) comb, no effect
(both concen-
trations)
Table 77 (contd).
a Only statistically significant changes; organ weight changes relative to body weight except for Render et al.
(1982) giving absolute data.
b Categorization according to Safe (1984); see also text in section 8.2.2.
c gd = Gestation day.
d n.r. = Not recorded.
Table 78. Histopathology in relation to individual PBB congeners (dosing studies)
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
Categorya I PBBs
4,4'-di rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(6) t.p.: 7 daysd (1977)
3,3',4,4'-tetra rat (Sprague- male oral single dose 21.3 + ++ 8 tissues: Millis et al.
Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Sprague- male intraperitoneal single 4.25e + 0 11 tissues: Millis et al.
Dawley) (6) dose t.p.: 2 weeks 0 (1985a)
rat (Wistar) male intraperitoneal single 150 + ++ spleen, Andres et al.
(4) dose t.p.: 2 weeks kidney: 0 (1983); Robertson
et al. (1983b)
3,3',4,4',5,5'- rat (Sprague- male oral single dose 21.3 ++ ++ 8 tissues: Millis et al.
hexa Dawley) (3) t.p.: up to 14 days 0 (1985b)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
Categorya II PBBs
2,3',4,4',5-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) (6) dose t.p.: 2 weeks tissues: 0 (1982c); Millis
et al. (1985a)
2,3',4,4',5,5'- rat (Sprague- male intraperitoneal single 144e ++ n.r. 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,3,3',4,4',5- rat (Sprague- male intraperitoneal single 144e ++ ++ 9 tissues:
hexa Dawley) (4) dose t.p.: 1 week 0
Categorya III PBBs
4-mono rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2'-di rat (Sprague- male intraperitoneal single 289e 0 0 8 tissues: Moore et al.
Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,5'-di rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,3',5-tri rat (Wistar) male intraperitoneal 3 doses 600 0 n.r. n.r.
(4-6) t.p.: 7 daysd
2,4,6-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,4',5-tri rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
3,3',5,5'-tetra rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
(4-6) t.p.: 7 daysd
2,2',5,5'-tetra rat (Wistar) male intraperitoneal single 150 + 0 spleen, Robertson et al.
(not specified) dose t.p.: 2 weeks kidney: 0 (1983b)
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5',6-penta rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,5,5'-penta rat (Sprague- male intraperitoneal single 164e + 0 9-11 Dannan et al.
Dawley) dose t.p.: 1-2 weeks tissues: 0 (1982a); Millis
(4-6) et al. (1985a)
2,2',3,4,4',5'- rat (Sprague- male intraperitoneal single 144e + 0 9 tissues: Dannan et al.
hexa Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',4,4',5,5'- rat (Fischer male, oral 22 doses 1052e + 0 16 tissues: Gupta et al.
hexa 344/N) female t.p.: up to 90 daysd 0 0 (1981)
2,2',4,4',5,5'- rat (Fischer) male intraperitoneal single 200-1000 + n.r. n.r. Goldstein et al.
hexa (4) dose t.p.: 4 days (1979)
rat (Sprague- male intraperitoneal single 144e + 0 8-11 Moore et al.
Dawley) dose t.p.: up to 14 days tissues: 0 (1978b); Millis
(3-6) et al. (1985a)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
2,2',4,4',6,6'- rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r.
hexa (4-6) t.p.: 7 daysd
Table 78 (contd).
PBB congener Species Sex Exposurec Doseb Histopathological effectsf References
(strain) (No.) Liver Thymus Other organs
2,2',3,3',4,4',5- rat (Sprague- male intraperitoneal single 127e + 0 9 tissues: Dannan et al.
hepta Dawley) (4) dose t.p.: 1 week 0 (1982a)
2,2',3,4,4',5,5'- rat (Sprague- male intraperitoneal single 127e + 0 8 tissues: Moore et al.
hepta Dawley) (3) dose t.p.: up to 22 days 0 (1979a)
2,2',3,3',4,4', rat (not male intraperitoneal single 115e + 0 several Besaw et al.
5,5'-octa specified) dose t.p.: 7 days tissues: 0 (1978)
rat (Wistar) male intraperitoneal 3 doses 600 + n.r. n.r. Ecobichon et al.
(4-6) t.p.: 7 daysd (1979)
a Categorization according to Safe (1984); see also text in section 8.2.2.
b Total dose in µmol/kg body weight.
c t.p. = Time post-exposure.
d After first dose.
e Calculated from original value given in mg/kg body weight.
f n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Table 79. Histopathology to individual PBB congeners (feeding studies)
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
Categorya I PBBs
3,3',4,4',5,5'- rat (Sprague- male 1 10 + 0 Render et al. (1982)
hexa Dawley) (6) 10 10 ++ ++
100 10 ++ ++ spleen, lymph
nodes: +
rat (Sprague- male 1 30 + 0 thyroid: + Akoso et al.
Dawley) (6) 10 30 ++ ++ thyroid, (1982a,b)
pituitary
gland: +
Categorya II PBBs
2,3',4,4',5,5'- rat (Sprague- male 1, 10, 100 30 + 0 thyroid: +
hexa Dawley) (6)
cockerels male 10 28 + n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: +
(10)
Categorya III PBBs
2,2',4,4',5,5'- rat (Sprague- male 10, 100 10 + 0 18 tissues: 0 Render et al. (1982)
hexa Dawley) (6)
rat (Sprague- male 1, 10, 100 30 + 0 thyroid: + Akoso et al.
Dawley) (6) (1982a,b)
Table 79 (cont'd).
PBB congener Species Sex Dietary Feeding Histopathological effectsb References
(strain) concentration period
(No.) (mg/kg feed) (days) Liver Thymus Other organs
cockerel male 4, 10 28 0 n.r. bursa of Dharma et al. (1982)
(White Leghorn) Fabricius: 0
(10) 62 28 + n.r. bursa of
Fabricius: +
a Categorization according to Safe (1984); see also text in section 8.2.2.
b n.r. = Not recorded; 0 = Lesion not observed; + = Lesion observed (number of "+" denote severity).
Dannan et al. (1982b) recombined 9 purified FireMaster(R)
constituents, namely BB 101 (2,2',4,5,5'-penta-,) BB 118
(2,3',4,4',5-penta-), BB 153 (2,2',4,4',5,5'-hexa-), BB 138
(2,2',3,4,4',5'-hexa-), BB 167 (2,3',4,4',5,5'-hexa), BB 156
(2,3,3',4,4',5-hexa-), BB 180 (2,2',3,4,4',5,5'-hepta-), BB 170
(2,2',3,3',4,4',5-hepta-), and BB 194 (2,2',3,3',4,4',5,5'-
octabromobiphenyl), totalling 97% of either FireMaster(R) mixture,
to form a reconstituted FireMaster(R) BP-6-like mixture and
compared some effects of the reconstituted mixture to those of crude
FireMaster(R) mixtures BP-6 and FF-1. Rats were treated with a
single dose (90 mg/kg body weight of either mixture) and sacrificed
one week later. Evaluating changes in body and selected organ
(liver, thymus, spleen) weights, the conclusion was reached that
adverse effects of FireMaster(R) (FF-1 or BP-6) must be due to the
effects of the congeners studied. Moreover, it was found that the
increase in liver weights was greater with FM BP-6 and the
reconstituted mixture than with FM FF-1, consistent with the higher
proportion of minor components (BBs: 118, 138, 167, 156) in FM BP-6
(25% versus 15%).
Differences in toxicity between PBBs were sometimes of a
qualitative kind, but mostly they were quantitative. Some striking
relations will be reviewed in detail according to the parameter
tested.
8.2.2.1 Food intake, overt clinical signs, body weight changes
So far, in the congeners tested, food intake of rats was
reduced only by 3,3'4,4',5,5'-hexabromobiphenyl (Table 77: Akoso
et al., 1982a; Render et al., 1982). The effective dietary
concentrations ranged from 1 to 100 mg/kg. A much higher
concentration of 2,2',4,4',5,5'-hexabromobiphenyl (1000 mg/kg of
feed) was needed to evoke reduction in the food consumption of mice
(Table 77: Welsch & Morgan, 1985).
Rats, moribund from 3,3',4,4',5,5'-hexabromobiphenyl treatment,
had symptoms similar to those observed with FireMaster(R)
toxicosis (see section 8.2.1.1). They became less active, had a
roughened hair coat, developed sunken eyes, and were emaciated
(Render et al., 1982).
3,3',4,4',5,5'-Hexabromobiphenyl significantly decreased body
weight (Render et al., 1982) or body weight gain (Akoso et al.,
1982a) in rats, while the same concentrations of FireMaster(R)
BP-6, 2,2',4,4',5,5'-hexa- and 2,3',4,4',5,5'-hexabromobiphenyl in
the diet had no effects. When rats were injected i.p. with identical
Table 80. Order of toxicity of higher brominated PBB congeners and the FireMaster(R) mixture on the basis of comparative studies
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Dosing studies
Rat body weight gain x x 167 > FM Dannan et al.
(1978a)
Rat liver weight and x x FM > 153 Moore et al.
histopathology (1978b); Goldstein
et al. (1979)
Rat liver weight and x x FM > 180 Moore et al.
histopathology (1979a)
Rat body and organ x x FM > 153 Gupta et al.
(Mouse) weights, histopathology (1981)
Rat body and organ x x 118 > FM Dannan et al.
weights, histopathology (1982c)
Rat body and organ x x x x x 156 > 167 > Dannan et al.
weights, histopathology 138, 170 > (1982a)
101
Rat body and organ x x x 169 > 118, Parkinson et al.
weights 153 (1983)
Rat liver histopathology x x x 118, 153 > Millis et al.
101 (1985a)
Table 80 (cont'd)
Species Parameter tested PBBs testeda Order of References
FM 101 118 153 138 167 156 180 170 169 toxicity
Feeding studies
Rat death, body and organ x x x 169 > FM Sleight et al.
weights, histopathology > 153 (1981)
Rat feed intake, body and x x x x 169 > FM Akoso et al.
organ weights, > 167 > 153 (1982a,b)
histopathology
Rat death, feed intake, x x x 169 > FM, Render et al.
body and organ weights, 153 (1982)
histopathology
Cockerel organ weights, x x x FM > 167 Dharma et al.
histopathology > 153 (1982)
a FM = FireMaster(R) mixture (BP-6 or FF-1); PPB numbering according to Ballschmiter & Zell (1980):
101, 118 = pentabromobiphenyls (2,2',4,5,5'-; 2,3',4,4',5-).
153, 138, 167, 156, 169 = hexabromobiphenyls (2,2',4,4',5,5'-; 2,2',3,4,4',5'-; 2,3',4,4',5,5'-;
2,3,3',4,4',5-; 3,3', 4,4', 5,5'-).
180, 170 = heptabromobiphenyls (2,2',3,4,4',5,5'; 2,2',3,3',4,4'5-).
amounts of several constituents of FireMaster(R) (BBs: 101, 138,
167, 156, 170), only 2,3',4,4',5,5'-hexabromobiphenyl (BB 167) and
2,3',4,4',5,5'-hexabromobiphenyl (BB 156) depressed body weight gain
(Dannan et al., 1978a, 1982a). The body weight gain of rats treated
with BB 167 was half that of FireMaster(R)-treated animals (Dannan
et al., 1978a). Rats given BB 118 (2,3',4,4',5-hexabromo biphenyl)
also gained less weight per day than those given FireMaster(R)
(Dannan et al., 1982c).
Liver weights in rats were increased by all treatments with
congeners of category I, except for 4,4'-dibromobiphenyl. The lowest
effective dose used was 2 mg/kg body weight (Millis et al., 1985a).
Significant effects were seen as early as 4 days after dosing (e.g.,
Parkinson et al., 1983). Many of the congeners listed under
categories II and III were also capable of increasing liver weights
(see Tables 76 and 77). Generally, all the changes in organ weights
were dose-dependent. Gradual differences between congeners were also
seen. Liver weights were significantly higher in rats given
3,3',4,4',5,5'-hexabromobiphenyl than the liver weights in
3,3',4,4'-tetrabromobiphenyl-treated rats, 6 days after a single
dose (Millis et al., 1985b). In studies comparing the effects of
five FireMaster(R) constituents (BB 101: 2,2',4,5,5'-penta; BB
138: 2,2',3,4,4',5'-hexa; BB 167: 2,3',4,4',5,5'-hexa; BB 156:
2,3,3',4,4',5-hexa; BB 170: 2,2',3,3',4,4',5-hepta), 7 days after
dosing, 2,3,3',4,4',5-hexabromobiphenyl (BB 156) caused the largest
increase in liver weights (60% increase) while 2,2',4,5,5'-
pentabromobiphenyl (BB 101) failed to enlarge this organ. Increases
caused by FireMaster(R) and BB 167 (2,3',4,4',5,5'-hexa) were
similar (Dannan et al., 1978a, 1982a). The increases in liver
weights due to BB 153 (2,2',4,4',5,5'-hexa), the main component of
FireMaster(R), were far less than the increase in response to
FireMaster(R), approximately 33% versus 60-80% (Moore et al.,
1978b; Goldstein et al., 1979).
Nevertheless, the increase was rapid with a 25% increase within
two days of treatment (Moore et al., 1978b). The effect of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180), the second most
abundant component of FireMaster(R), was also less than that
caused by the mixture itself (Moore et al., 1979a). When
FireMaster(R) BP-6, 2,3',4,4',5,5'-hexabromobiphenyl (BB 167),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), and
3,3',4,4',5,5'-hexabromobiphenyl (BB 169) were added to the diet of
rats for 30 days, BB 153 and BB 169 significantly increased liver
weights at 1 mg/kg, but FireMaster(R) and BB 167 did not. At 100
mg/kg, FireMaster(R) increased liver weight more than BB 153 or BB
167 (Akoso et al., 1982a).
Thymus weights in rodents were decreased by most of the
treatments with congeners of category I. Among congeners in category
II, 2,2',4,4',5-pentabromobiphenyl (BB 118),
2,3',4,4',5,5'-hexabromobiphenyl (BB 167), and
2,3,3',4,4',5-hexabromobiphenyl (BB 156) were capable of reducing
thymus weights. Congeners listed under the third category failed to
reduce thymus weights (see Tables 76 and 77). Both 3,3',4,4'-tetra
and 3,3',4,4',5,5'-hexabromobiphenyl caused a significant reduction
in the thymus weights of rats, 3,3',4,4',5,5'-hexabromobiphenyl
being more effective than 3,3',4,4'-tetrabromobiphenyl (Millis
et al., 1985b). There was a 50-60% loss in thymic weight in rats
injected i.p. with BB 167 or BB 156 and sacrificed 7 days later
(Dannan et al., 1978a, 1982a). However, thymus weight: body weight
ratios were not significantly affected when rats were fed BB 167 or
FireMaster(R) BP-6 for 30 days (Akoso et al., 1982a). In the same
study, 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) significantly
decreased the ratio, while there was an increase with
2,2',4,4',5,5'-hexabromobiphenyl (BB 153).
In cockerels fed FireMaster(R) FF-1, BB 153, or BB 167, for
28 days, only FireMaster(R) FF-1 reduced relative bursal weights
(Dharma et al., 1982).
When young rats were fed diets containing FireMaster(R) BP-6,
2,2',4,4',5,5'-hexa-, 2,3',4,4',5,5'-hexa-, or 3,3',4,4',5,5'-
hexabromobiphenyl, for 30 days, thyroid weight was increased only by
100 mg FireMaster(R) BP-6/kg feed and by 1 and 10 mg
3,3'4,4',5,5'-hexabromobiphenyl/kg feed (Akoso et al., 1982b).
8.2.2.2 Haematology and clinical chemistry
Haematological and clinical chemistry findings were of minor
importance in comparative studies. Gamma-Glutamyl transpeptidase
(gamma-GTP) was elevated in female rats at high doses of both
FireMaster(R) FF-1 (30 mg/kg body weight per day) and
2,2',4,4',5,5'-hexabromobiphenyl (BB 153) (16.8 mg/kg body weight
per day) 30 and 60 days after the last dose (Gupta et al., 1981).
8.2.2.3 Morphological and histopathological changes
Rats fed diets containing 100 mg FireMaster(R) BP-6/kg or 10
or 100 mg 3,3',4,4',5,5'-hexabromobiphenyl (BB 169)/kg had friable
yellow livers (Render et al., 1982). The architectural structure of
the lobules was abnormal after feeding 3,3',4,4',5,5'-
hexabromobiphenyl (Akoso et al., 1982a; Millis et al., 1985b), and
bile duct hyperplasia was observed (Render et al., 1982).
With the exception of the lower brominated congeners of
category III, all PBB congeners caused histopathological changes in
the liver (see Tables 78 and 79). The extent of the changes depended
on the dose and the individual congener. The least severe effects
were confined to a slight proliferation of hepatic SER (e.g.,
4,4'-dibromobiphenyl: Ecobichon et al., 1977). More progressive,
general changes were enlargement of hepatocytes and increased
numbers of cytoplasmic lipid vacuoles. Corresponding ultrastructural
lesions consisted mainly of increased SER and lipid vacuolation (see
Tables 78 and 79). Additional changes seen with the more toxic
congeners included myelin body formation (membrane whorls) (BBs:
118, 167, 156, 169), disorganization of RER (BB 169), an increase in
number of binucleated hepatocytes (BB 169), pycnotic nuclei (BB
169), and, occasionally, multifocal areas of necrosis (BB 169)
(Akoso et al., 1980, 1982a; Sleight et al., 1981; Dannan et al.,
1982a,c; Render et al., 1982; Millis et al., 1985b).
The thymus was affected by 3,3',4,4'-tetrabromobiphenyl (Andres
et al., 1983; Millis et al., 1985b), possibly by BB 167 (Dannan
et al., 1978a: "several" tissues: not accurately specified), by BB
156 (Dannan et al., 1982a) and by BB 169 (Sleight et al., 1981;
Akoso et al., 1982a; Render et al., 1982; Millis et al., 1985b).
There was a loss of thymocytes, especially in the cortex, the
demarcation between cortex and medulla was indistinct, and
macrophages were prominent in the remaining portion of the cortex.
One study reported histological alterations in the thyroid of
rats treated with 2,2',4,4',5,5'-hexa- (BB 153), 2,3',4,4',5,5'-hexa
(BB 167), or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169), and in the
pituitary gland with BB 169 (Akoso et al., 1982b). Prominent lesions
of the thyroid were extensive hyperplasia and hypertrophy of
follicular cells and a lack of colloid. The pituitary gland showed
swollen and vacuolated chromophobe cells.
The spleen and lymph nodes of rats given 100 mg 3,3',4,4',5,5'-
hexabromobiphenyl/kg feed for 10 and 20 days had an increased number
of macrophages intermixed with mature lymphocytes. Changes were
similar to those seen in the thymus, but were not as pronounced
(Render et al., 1982).
The main component of FireMaster(R),
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), is the congener most
frequently examined and implicated in many comparative studies.
The principal changes seen with BB 153 were vacuolation and
enlargement of hepatocytes with proliferation of SER. They occurred
in dosing studies (Moore et al., 1978b; Ecobichon et al., 1979;
Goldstein et al., 1979; Gupta et al., 1981; Millis et al., 1985a) as
well as in feeding studies (Sleight et al., 1981; Akoso et al.,
1982a; Dharma et al., 1982), and were observed as early as two days
after treatment (Moore et al., 1978b). Generally, the
ultrastructural changes in BB 153-exposed rats were less severe than
those in rats exposed to the FireMaster(R) mixture (e.g.,
Goldstein et al., 1979; Gupta et al., 1981; Akoso et al., 1982a).
Myelin figures and marked disorganization of RER, as seen with the
FireMaster(R) mixture, were not observed with BB 153 in
comparative studies (Gupta et al., 1981; Akoso et al., 1982a).
Moreover, changes caused by FireMaster(R) FF-1 in the livers of
rats persisted, while the livers of rats dosed with BB 153 were
comparable to those of the controls, 60 days after treatment (Gupta
et al., 1981).
The histological appearance of thymuses in rats was not
affected by BB 153 (Tables 78 and 79), but, in cockerels, the
lymphoid cells of the bursa of Fabricius were depleted by 62 mg BB
153/kg feed, by 10 mg FireMaster(R) FF-1/kg feed, and by 10 mg BB
167/kg feed (Dharma et al., 1982). When BB 153, the FireMaster(R)
mixture, BB 167, and BB 169, were fed to rats, proliferation of SER,
decreased RER, and increased fat droplets were seen in hepatocytes,
with all chemicals, but, with BB 169
(3,3',4,4',5,5'-hexabromobiphenyl), proliferation of SER was not as
prominent as with the other three PBBs. In contrast, the RER was
severely altered by BB 169 (Akoso et al., 1982a).
Myelin bodies were observed in rats fed 100 mg BB 169/kg for 20
days (Render et al., 1982) or FireMaster(R) BP-6 for 30 days
(Akoso et al., 1982a), or BB 167 for 60 days (Akoso et al., 1980). A
comparative experimental series testing single doses of six
FireMaster(R) constituents, namely BB 118 (Dannan et al., 1982c;
Millis et al., 1985a), BBs 101, 138, 167, 156, and 170 (Dannan
et al., 1978a, 1982a) found the least severe histological changes
with BB 101, and intermediate effects, which were limited to the
proliferation of SER and cytoplasmic vacuolation in hepatocytes,
with BB 138 and BB 170. The most pronounced changes resulted from
BBs 118, 167, and 156 and consisted of proliferation of SER,
increases in fat vacuoles and myelin figures in hepatocytes (BBs
118, 167, 156) and thymus damage (BB 156). Rats given a single
equimolar dose of 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) or
3,3',4,4'-tetrabromobiphenyl showed moderate to severe hepatic
changes 14 days after treatment with BB 169, while the
tetrabromobiphenyl-treated rats showed only mild hepatic changes
(Millis et al., 1985b).
The microscopic hepatic effects of
2,2',3,4,4',5,5'-heptabromobiphenyl (BB 180) and of
2,2',3,3',4,4',5,5'-octabromobiphenyl (BB 194) were reported to be
similar to those of BB 153 (Besaw et al., 1978; Moore et al., 1979a,
1980).
8.3 Skin and eye irritation, sensitization, dermal lesions,
and acne
Common skin and eye irritation tests, as well as sensitization
tests, resulted in no, or only mild, reactions due to the technical
PBB mixtures tested, namely octabromobiphenyl and decabromobiphenyl
(Table 81).
Table 81. Skin and eye irritation or sensitization tests of commercial PBB mixtures
PBBa Species Application Test Observations References
OcBB guinea-pig 50% (w/v) slurry in irritation (intact mild irritation Waritz et al.
(Hartley) propylene glycol shaved dorsal skin) (1977)
(0.05 ml)
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 9 x 50% slurry abraded shaved skin
(topical application)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB guinea-pig sensitization no sensitization
(Hartley)
1) 1 x 50% (w/v) slurry intact shaved skin
in propylene glycol
2) 4 x 1% (w/v) solution
in dimethyl sulfoxide
(intradermal injection)
3) 2 weeks later: abraded shaved skin
1 x 50% slurry
OcBB rabbit irritation Norris et al.
(New Zealand) (1973)
dry solid (single and intact shaved skin no response
multiple exposures) abraded shaved skin slight erythematous
and edematous response
Table 81 (contd).
PBBa Species Application Test Observations References
OcBB rabbit moistened with water
(New Zealand) (single exposure) intact shaved skin no response
(repeated exposure) intact shaved skin slight erythematous
response
OcBB rabbit moistened with water abraded shaved skin moderate erythematous
(New Zealand) (single and repeated and slight oedematous
exposures) response
rabbit dry solid eye irritation transient irritation
(New Zealand) of the conjunctival
membranes
OcBB rabbit powder (100 mg) eye irritation no irritating or corneal Waritz et al.
effects; mild (1977)
conjunctival redness and
swelling and a copious
discharge (disappeared
within 4 h)
DeBB rabbit 50% in olive oil irritation mild irritation Millischer et al.
(intact shaved skin) (1979)
DeBB rabbit 50% in olive oil eye irritation no irritating effect
DeBB rabbit powder eye irritation mild irritating
a Commercial PBB mixtures: OcBB = Octabromobiphenyl; DeBB = Decabromobiphenyl.
However, diverse lesions in the skin and skin appendages of
certain animal species, e.g., rhesus monkeys and cattle, occurred
after the ingestion of the FireMaster(R) mixture (Table 82). The
main features were dry scaly skin and hair loss. Hyperkeratosis of
the interfollicular epidermis and of the hair follicle, and atrophy
and squamous metaplasia of the sebaceous glands were observed on
microscopic examination of these lesions. As with related compounds,
comparable epidermal changes have not generally been found in other
laboratory animals, such as guinea-pigs or rats, but they were
similar to those observed in humans following PBB exposure
(section 9) and were described as chloracne (McConnell, 1980;
Kimbrough, 1980b; Poland & Knutson, 1982).
The rabbit ear (inner surface), but not any other part of the
rabbit skin (Crow, 1983), is particularly sensitive to acne-causing
compounds, which was first recognized by Adams et al. (1941). The
reaction is hyperkeratosis. Painting the rabbit ear has become a
standard bioassay to detect hyperkeratotic (acnegenic) activity.
Results of rabbit ear tests obtained with diverse PBBs (technical
octabromobiphenyl, technical decabromobiphenyl FireMaster(R)
mixture, fractions of this mixture, and purified PBB congeners) have
been summarized in Table 83. The FireMaster(R) mixture itself
produced hyperkeratosis, but its main components, BB 153
(2,2',4,4',5,5'-hexa) and BB 180 (2,2',3,4,4',5,5'-hepta), did not.
Fractionation of FireMaster(R) indicated that most activity was
associated with the more polar fraction containing minor compo nents
(Needham et al., 1982). Sunlight irradiation of BB 153 also yielded
products that caused severe hyperkeratosis. It is not clear whether
one or more of the suspected PBBs is responsible for hyperkeratotic
activity (Patterson et al., 1981). The model congener
3,3',4,4',5,5'-hexabromobiphenyl and 3,3'4,4'-tetra bromobiphenyl
were shown to be hyperkeratotic (Table 83).
8.4 Long-term toxicity
Toxic effects of PBBs, observed after long-term exposures, as
well as a long time after exposure had ceased, are summarized in
Tables 84 and 85. Experimental animals tested were rats, mice,
cattle, minks, and rhesus monkeys. The majority of studies refer to
the commercial FireMaster(R) mixture.
The following comments refer mainly to the general signs of
long-term toxicity. Other long-term effects will be reviewed in
detail in the respective sections, e.g., carcinogenicity (section
8.7), reproductive dysfunctions (section 8.5).
Table 82. Dermal lesions observed in cattle, rhesus monkeys, and rabbits after exposure to PBBs
PBBa Species Route Dose (duration) Dermal lesions observed References
FM BP-6 cattle oral 25 g/day (for subcutaneous emphysema and haemorrhage; changes Moorhead et al.
33-60 days) in the eyelids: hyperkeratosis, with accumulations of (1977, 1978)
keratin in hair follicles of the epidermis and squamous
metaplasia with keratin cysts in the tarsal glands
FM FF-1 calf oral 100 mg/kg body keratitis, alopecia, hyperkeratosis involving the Robl et al.
weight (up to head, cervical and dorsal thoracic region (1978)
12 weeks)
oral 0.1, 10, 100 acanthosis, hyperkeratosis and/or dermal infiltrates
mg/kg body of mononuclear cells (dose-dependent severity)
weight (up to
12 weeks)
FM FF-1 rhesus in diet 25 mg/kg feed alopecia, dry scaly skin, loss of eyelashes; Allen et al.
monkey (for 25 weeks) generalized subcutaneous oedema, marked oedema of (1978)
adult total dose: the eyelids; keratinization of hair follicles
(male) approx. 1 g and sebaceous glands
juvenile in diet 25 mg/kg feed moderate loss of hair including eyelashes, dry
(female) (for 50 weeks) scaly skin; periorbital oedema
total dose:
approx. 1.5 g
juvenile in diet 300 mg/kg feed a rather generalized loss of hair, absent eyelashes; Allen et al.
(female) (for 137 days) considerable periorbital congestion and oedema; (1978)
total dose: keratinization of hair follicles
approx. 6.4 g
Table 82 (contd).
PBBa Species Route Dose (duration) Dermal lesions observed References
FM FF-1 rhesus in diet 1.5 mg/kg feed periorbital oedema Allen &
monkey (for over 5 Lambrecht (1978)
months)
total dose:
approx. 75 mg
OcBB rabbit dermal not specified erythema, exfoliation in the ear Norris et al.
(ear) (1 month) (1973)
Various rabbit dermal variable hyperkeratosis in the ear see Table 83
PBBs (ear)
FM = FireMaster(R); OcBB = Technical octabromobiphenyl.
Table 83. Hyperkeratotic activity of commercial PBB mixtures, the fractionateda FireMaster(R) mixture
and purified PBB congeners, derived from the rabbit ear test
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
DeBB (Adine 0102) 200, 2000 (acetone) x Atochem (1990)
OcBB not specified x Norris et al.
(chloroform) (1973)
FM FF-1 (lot FH 7042)
mixture itself 60 (not specified) x Kimbrough
polar fraction not specified (not specified) x (+++) et al.
non-polar fraction not specified (not specified) x (+) (1977)
FM BP-6
mixture itself 6.5 µg/kg body weight x Hass et al.
(benzene-decane, 1:9) (1978)
fraction 1 (non-polar) containing PBBs 6.5 µg/kg body weight x
(benzene-decane, 1:9)
FM FF-1 (lot FH 7042)
mixture itself 100 (toluene) x (++) Needham et al.
less polar fractions 50-210 (toluene) x (1982)
more polar fraction containing minor 185 (toluene) x (+++)
components of the FM
mixture
Compound 4 predominantly BB 153 5 (toluene) x
Compound 8 predominantly BB 180 3.3 (toluene) x
Table 83 (contd).
PBBd Comments Doseb (solvent) Hyperkeratosisc References
not observed observed
2,2',4,4',5,5'-hexa- 10 (not specified) x Patterson et al.
bromobiphenyl (BB 153) (1981)
(96% pure)
sunlight degradation mixture of BB 153 10 (not specified) x (+++) Patterson et al.
products of BB 153 and other PBBs (1981)
(probably BB 101,
BB 118 and 3,3',4,4'-
tetra-bromobiphenyl)
3,3',4,4',5,5'-hexa- 0.32-9.6 (toluene) x (++) Needham et al.
bromobiphenyl (1982)
3,3',4,4'-tetra- 0.245 and 0.02 x (++)
bromobiphenyl (not specified)
a The mixture was fractioned by different methods (on Florisil: Hass et al., 1978; on alumina:
Kimbrough et al., 1977; by HPLC and GC: Needham et al., 1982).
b Total dose in mg/rabbit ear, unless otherwise specified.
c (+), (++), (+++) = severity of hyperkeratosis.
d DeBB = technical decabromobiphenyl; OcBB = technical octabromobiphenyl; FM = FireMaster(R).
BB 101 = 2,2',4,5,5'-pentabromobiphenyl; BB 118 = 2,3',4,4',5-pentabromobiphenyl;
BB 153 = 2,2',4,4',5,5'-hexabromobiphenyl; BB 180 = 2,2',3,4,4',5,5'-heptabromobiphenyl.
Table 84. Long-term effects observed after exposure to PBBs by gavage
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, single dose 1000 liver: lipid accumulation (more pronounced Kimbrough et al.
(lot FH 7042) (Sherman) female t.p.: 6,10,14 in males); uroporphyrin accumulation (1977, 1978)
(in peanut oil) (5) months (females); histopathological changes;
neo-plastic nodules; increase in relative
weight
FM FF-1 rat male, 22 doses over 30 liver: marked hepatotoxic effects; atypical Gupta &
(lot No. 1312 FT) (Fischer female 30 days; t.p.: nodules Moore (1979)
(in corn oil) 344/N) 6 months after
(9) first dose
FM FF-1 rat female single dose 1000 liver: porphyria; neoplastic nodules; foci Kimbrough et al.
(lot. 7042) (Sherman) t.p.: 23 months or altered areas; carcinoma; adenofibrosis (1981)
(in corn oil) (65)
(16) female single dose 200 liver: neoplastic nodules; altered areas;
t.p.: 22 months multinucleated cells
(30) female 12 doses over 100 liver: neoplastic nodules; foci or altered
3 weeks; t.p.: areas; carcinoma; adenofibrosis
24 months
FM FF-1 rat female 122 doses over 0.1, 1, increased white blood cell and lymphocyte Luster et al.
(lot. FF 1312 FT) (Fischer) 6 months t.p.: 3, 10 counts (0.1-10); decrease in body weight (1980)
(in corn oil) (4-10) 6 months after (3,10); decrease in thymus weight (3,10);
first dose increase in relative spleen weight (3,10);
decrease in adrenal weight (10); immune
alterations (3,10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 rat male, 22 doses over 30 decrease in body weight; increase in relative Gupta et al.
(lot 1312 FT) (Fischer female 30 days t.p.: liver weight; decrease in relative thymus (1981)
(in corn oil) 344/N) 120 days after weight (F); increase in serum protein
(3) first dose (ß-globulin fraction); porphyria (F);
pronounced hepatocellular alterations
2,2',4,4',5,5'- rat male 22 doses over 16.8 increase in relative liver weight (M);
hexabromobiphenyl (Fischer female 30 days t.p.: minimal hepatocellular changes
(99% purity 344/N) 120 days after
(in corn oil) first dose
FM FF-1 rat male single dose 500 no significant effects on body and liver Bernert, Jr
(lot 7042) (Sherman) t.p.: 18 months weight; increase in serum cholesterol and et al. (1983)
(in corn oil) total serum phospholipids; enhancement of
hepatic per-oxidation; reduced retinol
levels in serum and liver microsomes;
reduced alpha-tocopherol content in
microsomes (but not in serum)
FM FF-1 rat male, 125 doses over 0.1, 0.3 no effect on food consumption; dose-related Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, decrease in body weight gain; dose-related (1983a)
(in corn oil) 344/N) 6 months after 10 increase in the absolute and relative liver
(10) first dose weights (Female: 0.1-10; Male: 0.3-10);
decrease in thymus weight (0.3-10); increase
in spleen weight (1-10); decrease in Hb and
PCV values, in MCV and MCH values (10);
dose-related increase in serum cholesterol;
decrease in serum thyroid hormone levels;
porphyrin accumulation in liver, bone, teeth
(more pronounced in Female (1-10);
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
dose-related hepatocellular alterations;
increase in white blood cell count (Female:
1-10); increase in serum GGTP (Female: 10);
decrease in serum glucose (Female: 10);
dose-related decrease in serum protein,
primarily due to albumin (Female: 0.1-10);
carcinoma in urinary bladder (Female:10);
histopathological changes in the thyroid
glands and kidneys (Male: 10); decrease in
serum triglyceride (Male: 0.3-10)
FM FF-1 rat male, 125 doses over 0.1,0.3 dose-related body weight reductionso; Gupta et al.
(lot. 1312 FT) (Fischer female 6 months; t.p.: 1, 3, dose-dependent porphyrogenic effects on (1983b)
344) = up to 29 10 teeth, bones, liver (1-10); enlarged, pale,
(11-40) months after or mottled livers with necrotic foci (1-10);
first dose hepatocellular alterations (1-10);
dose-dependent incidence of liver tumours
and cholangiocarcinoma (higher doses);
dose-dependent decline in survival time
(Male: 1-10); chronic progressive nephropathy
(Male: 1-10); gastric ulcers and hyperplastic
gastropathy (Male: 3,10)
FM FF-1 mouse female 122 doses over 10 increase in body weight; increase in relative Luster et al.
(lot. FF 1312 FT) (B6C3F1) 6 months; t.p.: spleen weight; slight immune alterations (1980)
(in corn oil) (4-10) 6 months after
first dose
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse male, 22 doses over 30 no effect on food consumption; increase in Gupta et al.
(lot 1312 FT) (B6C3F1/N) female 30 days; t.p.: relative liver weight, histopathological (1981)
(in corn oil) (3) 120 days after alterations in liver
first dose
2,2',4,4',5,5'- mouse male, 22 doses over 16.8 no effect on food consumption and body weight
hexabromobiphenyl (B6C3F1/N) female 30 days; t.p.: gain; increase in relative liver weight;
(99% purity) (3) 120 days after minimal hepatocellular alterations
(in corn oil) first dose
FM FF-1 mouse male, 125 doses over 0.1, 0.3, no effect on food consumption; decrease in Gupta et al.
(lot. 1312 FT) (B6C3F1) female 6 months 1, 3, 10 body weight gain (only Male: 10); increase (1983a)
(in corn oil) (10) in body weight (only Female: 10); increase
in absolute and relative liver weight
(Female: 0.3-10; Male: 1-10); increase in
spleen weight (Female: 10); decrease in
uterine weight (Female: 10); haematological
alterations: dose-related increase in red
blood cell count; dose-related decrease in
MCV; decrease in platelet counts (Female:
3,10); leukocytosis (Female: 10); clinical
chemistry changes: increase in serum levels
of GGTP and SGPT (10) and alkaline
phosphatase (10); decrease in serum glucose
(Female: 10); dose-related hepatic porphyria
(more pronounced in Female) pale and
mottled livers (higher doses); dose-related
microscopic changes in the liver (1-10)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM FF-1 mouse 125 doses over 0.1, 0.3, shortened survival time (Male: 10); hepatic Gupta et al.
lot. 1312 FT) (B6C3F1) 6 months; t.p.: 1 ,3, 10 porphyria (Male: 3); slight reduction in body (1983b)
(in corn oil) (8-27) up to 30 weight (Male: 10); enlarged liver; fluid
months after accumulation in peritoneal cavity; liver
first dose carcinoma (10); metastasis to lung (Female:
10); hyperplasia and adenoma of follicular
cells of thyroid
FM BP-6 cattle daily doses 250 no adverse clinicopathological chanages Durst et al.
(in gelatin (6) for 60 days mg/day (1978a)
capsule) t.p.: 80-190
days after
first dose
FM BP-6 cattle daily doses 250 no gross or histopathological signs of Moorhead et al.
(in gelatin (No. not for 60 days mg/day toxicosis (1978)
capsule) specified) t.p.: 220
days after
last dose
FM FF-1 cattle female daily doses 0.3 no effects on milk production, body weights, Robl et al.
(in gelatin (6) for 158 days amount of food consumed; no effects on (1978)
capsule) t.p.: 182 haematological and clinical chemistry and
days after urinalysis values;
last dose (1 cow: overgrowth of hooves from day 129)
Table 84 (contd).
PBBa Species Sex Dosage regimenc Dosed Observed effectse References
(strain)
(No.)b
FM BP-6 cattle (Female) daily doses 250 no effect on milk production, body weight, Durst et al.
(in gelatine (1-4) for 60, 180 mg/day number of infections or general injuries; (1978b)
capsule) 202 days increased frequencies of reproductive Willett et al.
t.p.: up to dysfunctions (1980)
1500 days
after first
dose
a FM = Fire Master.(R)
b No. = number of animals.
c t.p. = time post-exposure.
d In mg/kg body weight per day, unless otherwise specified.
e Hb = Haemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; MCH = mean corpuscular haemoglobin.
Table 85. Long-term effects of feeding PBBs to young or adult animals
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
"Hexabromobiphenyl" rat female 50 7 months increase in relative weights of liver, Sepkovic &
(Monsanto Co., (Sprague- ovary, and thyroid Byrne (1984)
St. Louis) Dawley) (8)
FM BP-6 rat female 1, 10, 50 5-7 months no effect on food consumption, body Byrne et al.
(Sprague- weight, and relative thyroid weight; (1987)
Dawley) (10) slightly elevated liver weight (10, 50);
primary hypothyroidism
FM BP-6 rat female 1, 5, 10, 5 or more no effect on food consumption and relative Byrne et al.
(Sprague- 50 months liver weight; decrease in relative adrenal (1988)
Dawley) (10) weight; depression of circulating levels
of adrenal cortex hormones
OcBB rat male, 0.01-1 180 days no effect on food consumption and body Norris et al.
(Dow Chemical) (Sprague- female weight (1973)
Dawley) (not
specified)
OcBB rat male 100 4/22 weeks return to normal liver weights Lee et al.
(Dow Chemical) (Sprague- (1975a);
Dawley) (8) 1000 4/22 weeks increased liver weights; Waritz et al.
hepatocellular alterations (1977)
OcBB rat female 50 7 months increase in relative liver weight Sepkovic &
(Monsanto Co., (Sprague- Byrne (1984)
St. Louis) Dawley) (8)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
NoBB mouse male, 100, 300 18 months decrease in body weight (Male, Female); Momma (1986)
(Bromkal 80-9D) female shortened survival time (300 mg/kg; Male);
enlargement of thyroid glands (300 mg/kg;
Female); decrease in triglyceride and
non-esterified fatty acids; reduction of
pentobarbital sleeping time (13 months);
liver carcinoma (Male, Female)
FM FF-1 mink (9) male, 6.25 210 days deaths; decrease in body weight; increase Aulerich &
female (mean) in relative liver and kidney weights; Ringer (1979);
liver histopathology Ringer et al.
(1981)
mink (8) female 1-2.5 9 months 10% reduction in body weight (2.5); Aulerich &
adverse effects on reproduction Ringer (1979);
Ringer et al.
(1981)
FM FF-1 rhesus female 0.3 7 months- weight loss; sterile abscesses (2/7); Allen et al.
monkey (total dose: > 1 year reproductive dysfunctions (1978); Allen
(7) > 25 mg) & Lambrecht
(1978);
Lambrecht
et al. (1978)
rhesus ? 1.5 > 5 months weight loss; periorbital oedema; Allen &
monkey (total dose: immunological alterations Lambrecht
(not approximately (1978)
specified) 75 mg)
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
FM FF-1 rhesus male 25 25 weeks death; weight loss; haematological and Allen et al.
monkey (total dose: clinical chemistry changes: decrease in (1978)
(adult) approximately PCV, cholesterol, total serum protein and
(1) 1 g) albumin; increase in serum GPT activity;
epidermal changes:
alopecia, dry scaly skin, loss of eyelashs;
keratinization of hair follicles and
sebaceous glands;
oedema:
generalized subcutaneous oedema and
marked oedema of eyelids; enlarged heart
and liver; hyperplastic gastroenteritis;
severe ulcerative colitis; hypoactive
seminiferous tubules; hyperplasia of bile
duct epithelium
FM FF-1 rhesus female 25 50 weeks no weight gain: loss of hair (including Allen et al.
monkey (total dose: eyelashes); dry scaly skin; periorbital (1978)
(juvenile) approximately oedema; increase in serum GPT activity
(1) 1.5 g)
FM FF-1 rhesus female 300 137 days death; weight loss; haematological and
monkey (total dose: clinical chemistry changes: decrease in
(juvenile) approximately PCV, white blood cell count, red blood cell
6.4 g) count, serum cholesterol, serum protein;
increase in serum GPT activity
epidermal changes:
loss of hair (including eyelashes); atrophy
and squamous metaplasia of the sebaceous
glands; keratinization of hair follicles;
Table 85 (contd).
PBBa Species Sex Dietary Feeding/ Observed effectsc References
(strain) concentration observation
(No.)b (mg/kg feed) period
periorbital congestion and oedema;
subcutaneous oedema; enlarged
hepatocytes; hyperplastic gastroenteritis;
hyperplasia of the epithelium of the
bladder and of the bile ducts; focal
areas of haemorrhage in the adrenal glands
FM FF-1 rhesus female 1.5 36 weeks weight loss; decrease in serum Lambrecht et al.
monkey (total dose: cholesterol enlarged hepatocytes with (1978)
(adult) 70 mg) moderate fatty infiltration (as indicated
(3) by liver biopsy)
rhesus male, 25 14 weeks weight loss (Male, adult) or lack of
monkey female (total dose: weight gain (Female, juvenile); decrease
(adult approximately in serum cholesterol; hyperplastic
juvenile) 500 mg) gastritis
(2)
a FM = FireMaster; OcBB = technical octabromobiphenyl; NoBB = nonabromobiphenyl.
b No. = number of animals.
c PCV = packed cell volumes.
8.4.1 Rat
8.4.1.1 Overt clinical signs, body weight changes, food intake
In rats, the low-dose, long-term feeding of FireMaster(R)
(Byrne et al., 1987, 1988) or technical octabromobiphenyl (Norris
et al., 1973) had no effect on food consumption and body weight
(Table 85). Nevertheless, the fur of PBB-fed animals was slightly
less dense and slightly coarser than that of control animals (Byrne
et al., 1987). A dose-dependent decline in survival time (Gupta
et al., 1983b), and body weight reductions or depressed rates of
body weight gain as a function of time and dose (Luster et al.,
1980; Gupta et al., 1981, 1983a,b) have been found in rats orally
dosed with FireMaster(R) (Table 84); also there was no significant
difference in food consumption between treated and control animals
(Gupta et al., 1981, 1983a).
8.4.1.2 Haematology and clinical chemistry
Several haematological and clinical chemistry parameters were
altered, some in only one sex (Table 84: Kimbrough et al., 1977;
Luster et al., 1980; Bernert et al., 1983; Gupta et al., 1983a).
Rats of both sexes had decreased haemoglobin and packed cell volume
values (Gupta et al., 1983a), a dose-related increase in serum
cholesterol (Bernert et al., 1983; Gupta et al., 1983a), and a
dose-related hepatic porphyria (Gupta et al., 1983a).
8.4.1.3 Morphological changes
Most of the livers of treated rats from higher dose groups were
pale or slightly yellow and mottled (Gupta et al., 1983a) and
contained white necrotic foci (Gupta et al., 1983b). Porphyrin
accumulation in the liver, bone, and teeth, seen as red fluorescence
under UVR, was more pronounced in treated females compared with
treated males (Kimbrough et al., 1977; Gupta et al., 1983a,b). After
withdrawal of treatment, visual fluorescence declined slightly in
the lower dose groups (1 and 3 mg FM/kg body weight), but remained
the same in the 10 mg FM/kg body weight dose groups (males and
females) during lifetime observation (Gupta et al., 1983a,b).
In long-term studies, an increase in the relative liver weights
of rats exposed to FireMaster(R) or a related mixture was usually
found (Kimbrough et al., 1978; Gupta et al., 1981, 1983a; Sepkovic &
Byrne, 1984; Byrne et al., 1987), but in some instances (Bernert et
al., 1983; Byrne et al., 1988), there was no effect on liver weight
(see Tables 84 and 85). [During a six-month exposure to
FireMaster(R), the increases in absolute and relative liver
weights were dose-related (Gupta et al., 1983a)].
Long-term feeding of commercial octabromobiphenyl (OcBB) also
caused an increase in relative liver weights (Sepkovic & Byrne,
1984; Table 85). Rats fed OcBB for 4 weeks still had increased liver
weights at 18 weeks of recovery or had returned to near normal
limits, depending on the concentration of OcBB in the test diet (Lee
et al, 1975a; Table 85).
The only individual PBB congener tested over the long-term,
namely 2,2',4,4',5,5'-hexabromobiphenyl, caused an increase in the
relative liver weight in male rats only (Gupta et al., 1981; Table
84).
Weight changes following PBB exposure, noted in organs other
than the liver, included a decrease in the thymus (Luster et al.,
1980; Gupta et al., 1981, 1983a), an increase in the spleen (Luster
et al, 1980; Gupta et al., 1983a), a decrease in the adrenal glands
(Luster et al., 1980; Byrne et al., 1988), and an increase in the
thyroid and ovary (Sepkovic & Byrne, 1984) (see Tables 84 and 85).
8.4.1.4 Histopathological changes
Hepatocellular alterations (exclusive of hepatocellular
carcinomas), observed in long-term studies with FireMaster(R) (see
Table 84), consisted mainly of enlarged hepatocytes, fatty
infiltration, proliferation and disorganization of RER, single cell
necrosis, multinucleated cells, microabscesses, atypical foci, and
bile duct proliferation (Kimbrough et al., 1977, 1978, 1981; Gupta
et al., 1981, 1983a,b).
In contrast to FireMaster(R)-treated rats, rats given
2,2',4,4',5,5'- hexabromobiphenyl over 30 days tended to recover
when examined during postexposure periods (Gupta et al., 1981).
Myelin figures, induced by the feeding of technical OcBB,
persisted, together with fatty changes, as late as 18 weeks after
withdrawal of a test diet containing 1000 mg OcBB/kg; however, the
normal morphology of RER was restored independently of the
occurrence of myelin figures, after treatment was discontinued (Lee
et al., 1975a).
Although there was no significant difference in the weights of
the thyroid gland, slight to moderate microscopic changes were
observed, primarily in male rats exposed for 6 months to 10 mg
FireMaster(R)/kg body weight. The thyroid gland had thin, sparse,
or bluish colloid with basophilic stippling and some follicles were
lined with columnar epithelium and contained a few epithelial
papillary projections (Gupta et al., 1983a).
The kidneys of male rats equally treated consistently showed
atrophy of a few glomerular tufts with marked dilatation of Bowman's
capsule, which contained amorphous eosinophilic staining material. A
few glomerular tufts also appeared oedematous (Gupta et al., 1983a).
Male rats, exposed to FireMaster(R) for 6 months (dose levels:
1-10 mg/kg body weight per day) and observed for a lifetime, showed
a higher incidence of chronic progressive nephropathy than the
controls. Their kidneys were characterized by eosinophilic
proteinaceous casts, sclerosis and thickening of glomerular tufts
and Bowman's capsule, mononuclear leukocytic infiltration, and
interstitial fibrosis (Gupta et al., 1983b).
Gastric ulcers and hyperplastic gastropathy of the glandular
portion of the stomach were found with significantly increased
incidence in male rats held under the same conditions (dose levels:
3 or 10 mg FM/kg body weight per day). Microscopic lesions consisted
of hyperplasia of the mucosal epithelium, glandular metaplasia to
goblet cells, hyperchromasia, and increased mitosis (Gupta et al.,
1983b).
8.4.2 Mouse
As far as it has been tested (Table 84), FireMaster(R) has
not produced any significant effects on food consumption in mice of
both sexes (Gupta et al., 1981, 1983a). Interestingly, there were
increases in the body weights of female mice with long-term exposure
to FireMaster(R), while a decrease occurred only in males at the
high dose (Luster et al., 1980; Gupta et al., 1983a,b). The high
dose of FireMaster(R) also shortened the survival time of males
(Gupta et al., 1983b). There were no significant differences in food
consumption and body weight gain in mice treated with
2,2',4,4',5,5'-hexabromobiphenyl (Gupta et al., 1982).
A number of haematological and clinical chemistry changes have
been found in mice exposed for 6 months to FireMaster(R) (Table
84: Gupta et al., 1983a). Hepatic porphyrin levels were increased in
a dose-related manner after a six-month exposure (Gupta et al.,
1983a), but (unlike levels in rats) they tended to decrease
following cessation of exposure (Gupta et al., 1983b).
Organ weight changes noted in long-term studies on mice (Table
84) consisted of an increase in liver (Gupta et al., 1981, 1983a,b)
and spleen (Luster et al., 1980; Gupta et al., 1983a) weights and a
decrease in uterine weights (Gupta et al., 1983a). Most of the
affected mice observed for a lifetime contained serosanguineous
fluid in the peritoneal cavity (Gupta et al., 1983b). The livers of
mice with long-term exposure were pale and contained, primarily in
males, grayish white foci (Gupta et al., 1981, 1983a).
Microscopic alterations were marked swelling of hepatocytes,
foamy or vacuolated cytoplasm with hyaline bodies, focal coagulative
necrosis or scattered single cell necrosis of hepatocytes, and
atypical hepatocellular foci (Gupta et al., 1983a). Hyperplasia was
observed in the follicular cells of thyroid (Gupta et al., 1983b).
Adverse effects (besides liver carcinoma; see section 8.7),
observed after an 18-month exposure of mice to technical
nonabromobiphenyl (Bromkal 80-9D), included decreases in body
weight, enlargement of the thyroid gland, and biochemical
alterations (Momma, 1986; Table 85).
8.4.3 Cattle
Except for a single animal, cattle with long-term exposure to
low doses of FireMaster(R) FF-1 or BP-6, observed for up to 220
days post-treatment (see Table 84) did not show any adverse effects
with regard to food intake, clinical signs, clinicopatho logical
changes, or performance (Durst et al., 1978a,b; Moorhead et al.,
1978; Robl et al., 1978). However, reproductive dysfunction occurred
more frequently (Willett et al., 1980; see also section 8.5).
8.4.4 Mink
Minks were found to be very susceptible to PBB toxicity (Table
85: Aulerich & Ringer, 1979). When fed with FireMaster(R) for
several months, they responded with food rejection, loss of weight,
an unthrifty appearance, and death. Upon necropsy of animals that
died, increases in relative liver and kidney weights and a fatty
infiltration of the liver were evident. A relatively low daily
intake of FireMaster(R) by female mink had an adverse effect on
their reproductive performance (see also section 8.5).
8.4.5 Rhesus monkey
Rhesus monkeys are also among the species more sensitive to
FireMaster(R) (Table 85: Allen et al., 1978; Allen & Lambrecht,
1978; Lambrecht et al., 1978). At exposures of 1.5-300 mg FM/kg
feed, they developed weight loss or a lack of weight gain,
haematological and clinical chemistry changes, loss of hair, skin
lesions, oedema, enlargement of the heart and liver, areas of
haemorrhage in the adrenal glands; reduced spermatogenesis, and
immune incompetence. Microscopic changes in the liver included
enlarged hepatocytes with fatty infiltration. The most severe lesion
was hyperplastic gastroenteritis and the accompanying ulcerations.
Exposures to 25 or 300 mg FM/kg feed also caused death. The female
monkeys fed with lower doses of FireMaster(R) (0.3 mg/kg diet),
for over one year, did not develop any of the signs of intoxication
noted above, except for weight loss. However, they were affected by
reproductive dysfunctions (see also section 8.5).
8.4.6 Pre- and perinatal exposure
Long-term effects following pre- or perinatal exposure to
FireMaster(R) have been recorded in rats and cattle.
Stunted growth, increased mortality rates, and liver tumours
were observed in the offspring of Sherman rats given 200 mg
FireMaster(R) FF-1/kg body weight on days 7 and 14 of pregnancy,
when a total of 50 male and 50 female offspring per group were
followed until they were 2 years old (Groce & Kimbrough, 1984).
Sprague-Dawley rats (No. = 10), perinatally exposed (day 8 of
pregnancy-28 days post partum) to FireMaster(R) BP-6 (100 mg/kg of
feed) and weaned on to a PBB-free diet, had increased relative liver
weights at 28 and 150, but not 328 days of age. Although the liver
was not enlarged 10 months after weaning, hepatic histopathological
alterations, such as cellular swelling, vacuolation, some necrosis,
and eccentric and pyknotic nuclei were observed throughout this
residual phase. Other long-lasting alterations were stimulation of
renal and hepatic microsomal enzymes, and a reduction in the
duration of anaesthesia produced by pentobarbital (McCormack et al.,
1980). In a multigeneration study (McCormack et al., 1981), rats
perinatally exposed to FireMaster(R) BP-6, as described above
(F1-generation), were allowed to mature sexually and bred with
littermates to produce the F2-generation. Even these F2-animals
exhibited increased relative liver weights and histopathological
liver changes at 28 days of age. The light microscopic changes
included vacuolation, focal necrosis, pyknotic nuclei, swelling, and
myelin bodies. However, the severity of lesions was much less
prominent in F2-animals than in F1-animals.
PBBs given to a single generation also produced hepatic and
renal microsomal enzyme stimulation, a reduction in the duration of
anaesthesia elicited by pentobarbital or a large dose of
progesterone, and a decrease in the concentration of vitamin A in
the liver, in both subsequent generations (F1 and F2).
Cows whose dams or granddams had received daily oral doses of
FireMaster(R) BP-6 (up to 250 mg/day) for 60-202 days showed no
general health problems, but they had conception difficulties
(Willett et al., 1982).
8.5 Reproduction, embryotoxicity, and teratogenicity
Effects of PBBs on reproduction (reproductive system, overall
reproductive performance, embryotoxicity, teratogenicity) have been
summarized in Tables 86 and 87. Most of the studies refer to the
FireMaster(R) mixture. Few reports deal with technical
octabromobiphenyl (Aftosmis et al., 1972a; Waritz et al., 1977) or
technical decabromobiphenyl (Millischer et al., 1979), and, among
the individual PBB congeners, only 2,2',4,4',5,5'-hexabromobiphenyl
has been evaluated by a few authors.
8.5.1 PBB mixtures
8.5.1.1 Mammals
a) Reproductive system and performance.
As listed in Tables 74 and 75, administration of
FireMaster(R) by gavage and in the diet had no effect on the
weight of male (Garthoff et al., 1977; Corbett et al., 1978a; Harris
et al., 1978b, Gupta et al., 1981; Castracane et al., 1982) or
female (Gupta et al., 1981) sex organs in rats and/or mice. An
exception was an increase in the uterine weight of mice dosed for 6
months with FireMaster(R) (Gupta et al., 1983a). Testes weights in
rats fed commercial OcBB also remained unaffected (Norris et al.,
1973). However, there was a reduction in the ventral prostate
weight-to-body weight ratio in pubescent male rats (Johnston et al.,
1980; Table 87) and a delay in vaginal opening in female rats after
perinatal exposure to FireMaster(R) (Harris et al., 1978a;
McCormack et al., 1981; Tables 86 and 87). Both findings may suggest
retarded sexual maturation.
Male rats, moribund from multiple doses of FireMaster(R),
exhibited degenerative and hyperplastic changes in the ductus
deferens (hyperplasia and squamous metaplasia with keratinization in
the epithelial lining of the ductus deferens). However, such changes
were not observed in surviving male rats, examined six months after
the treatment. Lesions found in surviving animals were acute
prostatitis (30 mg/kg body weight per day) and the presence of sperm
granuloma in the epididymis (100 mg/kg body weight per day) (Gupta &
Moore, 1979; Table 86). A rhesus monkey, exposed to FireMaster(R)
in the diet, had hypoactive seminiferous tubules at death (Allen
et al., 1978; Table 87). Little work was done on the possible
impairment of spermatogenesis. Young bulls may develop testicular
atrophy and reduced spermatogenesis when exposed to FM (Robl et al.,
1978), but more numerous controlled studies are needed.
The estrous cycle of cows (No. = 8) was not affected during
administration of FireMaster(R) FF-1 (0.3 mg/kg body weight per
day) for 158-228 days and during a recovery period of 112-182 days
(Robl et al., 1978).
The estrous cycle of female rats was increased in length after
perinatal and subsequent dietary exposure to FireMaster(R) BP-6
(Johnston et al., 1980; Table 87) at concentrations of 100 mg/kg
(equivalent to 5 mg/kg body weight per day). Prolonged menstrual
cycles were seen in rhesus monkeys after consuming FireMaster(R)
FF-1 for six months at a concentration of 0.3 mg/kg (equivalent to
0.02 mg/kg body weight per day). This alteration was correlated with
decreased serum progesterone levels (Allen et al., 1978; Lambrecht
et al., 1978; Table 87).
Table 86. Summary of effects of PBBs on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): dosing studies
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral single 400, 800 reductions in fetal and placental Beaudoin
(Wistar) dose one of weight; fetal death (primarily (1977)
(3-15) gd 6-14 800 mg/kg body weight per day at
s.t.: gd 20 day 7-12); malformed (cleft
palate; diaphragmatic hernia)
fetuses (400 mg/kg body weight
per day: 0-11.8%; 800 mg/kg body
weight per day: 0-60%)
FM FF-1 rat (No. oral multiple 100 (total none no effects on number of Ficsor &
not doses (6) gd dose: 600 fetuses, dead implants, Wertz (1976);
specified 6-16 at 2 day mg/kg body fetal malformation Wertz &
(15) intervals weight Ficsor (1978)
s.t.: gd 19
FM BP-6 rat oral multiple 0.25-10 reduced fetal weight and no effects on implantation Harris
(Sprague- doses gd 7-15 mg/day crown-rump length (only number of live fetuses or et al. (1978a)
Dawley) s.t.: gd 20 0.25 mg/day) gross malformations
(6-8)
s.t.: 48, 60 10 mg/day reductions in offspring weight no effects on litter size Harris
days of age (from 3 days of age onwards, and birth weight et al. (1978a)
more pronounced in male pups);
increased postnatal mortality (at
weaning 14.3% versus 1.5% in
control); delay in vaginal opening
of female offspring
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 rat oral multiple 8-160 mg reduced implantation rates no malformed fetuses Beaudoin
(Wistar) doses gd 0-14 per animal (80-160 mg); fetal death (1979)
(No. not on alternate (total
specified) days dose)
FM FF-1 rat oral multiple 100 moribund rats: degenerative and Gupta &
(Fischer doses (22) hyperplastic changes in the ducts Moore (1979)
344/N) over 30 days deferens (73 days after
(9 males) s.t.: up to 6 treatment); surviving rats: sperm
months granuloma in the epididymis (6
months after treatment; 1/2e)
30 acute prostatitis (4/9)e
FM FF-1 rat oral multiple 200 reduction in offspring survival Groce &
(Sherman) doses (2) gd 7 rate-to-weaning Kimbrough
(16) and 14 o.t.: (1984)
up to Weaning
(21 days of age)
FM BP-6 rat 1) in vivo- 800 inhibited rate of embryo Fisher (1980);
(Wistar) treatment: oral development in vitro (effects on Beaudoin &
(7) single dose gd 9 axial rotation, heart rate, neural Fisher (1981)
s.t.: gd 10 tube closure, formation of the
2) whole embryo anterior limb buds, somite
culture: 24 h development, and establishment of
visceral yolk sac circulation);
reduction in DNA content;
pericardial oedema
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
DeBB rat oral multiple 10-1000 none no teratogenicity or Millischer
(Sprague- doses gd 6-15 embryotoxicity et al. (1979)
Dawley) s.t.: gd 21
(25)
2,2',4,4' mouse oral multiple 0.3-32 none no fetotoxicity or Lucier et al.
5,5'-hexa- (C.D. - 1) doses gd 10-16 teratogenicity; no effects (1978)
bromobiphenyl (up to 25) s.t.: gd 19 on fertility, gestation,
(purity o.t.: 4, 20 viability, survival, and
> 97% days of age lactation indices
FM BP-6 cow (6) oral multiple 25 g/day abortion (3/6)e or fetal Durst et al.
doses during death (3/6)e (1977, 1978b);
pregnancy for Moorhead
32-60 days et al. (1977)
o.t.: up to 60
days
BM BP-6 cow oral multiple 0.25 and none Durst et al.
(3) doses during 250 mg/day (1978b)
pregnancy for
60 days
(1) for 180 days 250 mg/day stillbirth (due to dystocia)
o.t.: up to
305 days
Table 86 (contd).
PBB Species Exposurec Dosed Major effects Remarks References
(No.)b
FM BP-6 cow (3-5) oral multiple 0.25-250 high incidence of dystocia; a total of 75 calves Willett et al.
(4 dose doses before mg/day increased birth weights of calves; were studied; no effects (1980, 1982)
groups) breeding or stillbirths; increased number of on growth, development,
during late inseminations required for and survival of calves
pregnancy for conception in 1 and 2 generation born alive
60-202 days offspring
o.t.: up to 5.5
years (1-5
parturition;
2 generations)
a FM = FireMaster(R); DeBB = technical decabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, of recorded); o.t. = observation time; s.t. = sacrifice time.
d In mg/kg body weight per day, unless otherwise specified.
e No. affected/no. treated.
Table 87. Summary of PBB effects on reproduction (reproductive system, overall reproductive performance,
embryotoxicity, teratogenicity): feeding studies
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 rat (Sprague- gd 7-18 100, 1000 decreasing fetal weight with suggestion of late Corbett
Dawley) s.t.: gd 20 increasing dosage fetal mortality et al.
(6-7) (high dose); no (1975)
teratogenicity
FM BP-6 rat (Sprague- gd 8-9th week 100 reduction in ventral no effect on fetal Johnston
Dawley) (at of age s.t.: prostate weight of male survival rate et al.
least 8) 9th week of age offspring; increased length (1980)
of estrous cycle of female
offspring
FM BP-6 rat (Sprague- gd 8-Weaning 100 reduction in survival no effects on length McCormack
Dawley) s.t.: Weaning rate-to-weaning (87% of of gestation, litter et al.
control value); delay in size, incidence
(8) (28 days of vaginal opening in female of gross external
age) offspring anomalies, pup body
weight at birth
OcBB rat (not gd 6-15 100, 1000, gastroschisis (one fetus abstract only (no Aftosmis
specified) s.t.: gd 20 10 000 at each level); anasarca on number of fetuses et al.
(one fetus at each of the information examined) (1972a)
two highest levels)
OcBB rat gd 6-15 100, 1000, no significant gastroschisis Waritz et al.
(ChR-CD) s.t.: gd 20 10 000 embryotoxicity or (1/259e: 1000 mg/kg: (1977)
(23-27) teratogenicity 1/1/283e
10 000 mg/kg);
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
anasarca (1/259e: 1000
mg/kg; 1/283e: 10 000
mg/kg); no effects on
growth or viability of
embryos
FM BP-6 mouse: gd 7-18 50, 100, dose-related decrease in fetal incidence of Corbett
(Swiss, ICR) s.t.: gd 18 1000 weight with increasing dosage; malformations et al.
(9-12) exencephaly (5/295g: 100, statistically (1975,
1000 mg/kg); cleft palate significant only 1978b)
(5/208g: all levelsg; compared with pooled
hydro-nephrosis (2/87: historical controls
1000 mg/kg)
FM mouse (Swiss- gd 4-16 100, 200 increase in number of dead or (abstract only) Preache
Webster) s.t.: gd 19 resorbed fetuses (200 mg/kg) et al.
(No. not (1976)
specified) gd 8-16 100, 200 decrease in fetal body weight
s.t.: gd 19 (200 mg/kg)
gd 8-16 50, 100 reduction in number of live no effects on Preache
o.t.: up to 6 offspring postnatal mortality or et al.
weeks of age body weight (1976)
days 1-29 pp 50, 100 increased mortality; decreased
o.t.: up to body weight
weaning
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
2,2',4,4'- mouse gd 6-15 100, 300, at 300 mg/kg: 17-182 fetuses were Welsch &
5,5'-hexa- (C57BL) o.t.: gd 17 500, 750, decrease in pregnancy rates of examined Morgan (1985)
bromobiphenyl (2-22) 1000 plug-positive mice;
(purity: at > 300 mg/kg:
> 99%) reduced fetal body weight;
malformed fetuses (cleft
palate combined with a "cystic
brain deviation": 5/166,
3/172, 5/46, 6/17g,
respectively, at 300-1000
mg/kg; minor abnormalities of
brain development); at >
500 mg/kg: reduced gravid
uterine weight
FM BP-6 pig (2-4) 2nd half of 10, 100, 200 increased mortality during no effect on litter Werner &
gestation and lactation size Sleight
lactation o.t.: (1981)
up to weaning
(4 weeks)
FM FF-1 mink (8 for 9 months 1 reduced litter size; reduced both male and female Aulerich &
females; (start before kit weight at birth and at 4 on PBB diet; no Ringer
2 males) breeding) weeks; increased mortality effects on breeding or (1979)
4 weeks) (birth to periods; no gestation
teratogenicity
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 rhesus for over 1 year 0.3 prolonged menstrual cycles; Allen et al.
monkey (start 7 months decreased serum progesterone (1978,
(7) prior to breeding) levels (4/7e after 6 months); 1979); Allen
excessive and prolonged & Lambrecht
implantation bleeding (2/7e); (1978);
abortion (1/7); stillbirth Lambrecht
(1/7e); reduced weight of et al.
infants at birth and at (1978)
weaning (4 months of age)
FM FF-1 rhesus for 25 weeks 25 hypoactive seminiferous tubules Allen et al.
monkey (at the time of death (1978)
(1 male)
FM BP-6 chicken (White for 9 weeks 20 none no effect on Cecil et al.
Leghorn) (35) hatchability (1974)
"PBB" chicken for 8 weeks 5, 10, 20 none no effects on egg Lillie et al.
(White production, egg (1975)
Leghorn) (20) weight, egg shell
thickness, and
fertility; no effects
on hatchability,
embryonal development
and progeny health
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM BP-6 chicken for 8 weeks 20 increased mortality of progeny no effects on egg Cecil &
(White production, egg Bitman
Leghorn) (10) weight, egg shell (1978)
thickness, fertility,
hatchability, and
embryonic death
for 4-8 weeks 64, 200, reduced egg production or stop
640, 2000 in egg production at 200-2000
mg/kg; decreased hatchability
of fertile eggs; increased
mortality of progeny; reduced
growth rate of progeny;
embryonic deaths; embryonic
abnormalities (subcutaneous
oedema, oedematous cysts,
unabsorbed yolk)
FM FF-1 chicken for 5 weeks 0.2-3125 decline in egg production Polin &
(White (MELf: 30-45 mg/kg); loss of Ringer
Leghorn) (24) egg production (> 625 mg/kg, (1978a,b)
within 2 weeks);
reduced hatchability (MELf:
30-45 mg/kg); dose-related
increase in offspring
mortality (MELf: 30-45 mg/kg;
embryonic oedema
Table 87 (contd).
PBBa Species Treatmentc Dietary Major effects Remarks References
(No.)b concentrationd
FM FF-1 chicken (10) for 21 days 80 decline in hatchability; oedema no effect on fertility Polin et al.
of embryos and newly hatched (1979)
chicks
2,2',4,4', chicken (10) for 21 days 52 none no decline in
5,5'-hexa- hatchability; no
bromobiphenyl oedema of chicks and
(purity: and embryos
not
specified)
FM Japanese for 9 weeks 10, 20, 100 at 100 mg/kg: no eggshell thinning Babish
quail (No. reduced egg production (17% et al.
not specified) versus 68% in controls); no (1975a)
hatchability; embryonal deaths
FM FF-1 Japanese for 5 weeks 80 reduced egg production fertility and Polin et al.
quail hatchability not (1982);
(Coturnix significantly Bursian
coturnix different from et al.
japonica) controls (1983)
(36 males,
36 females)
a FM = FireMaster; OcBB = technical octabromobiphenyl.
b No. = Number of females, unless otherwise specified.
c gd = Gestation day (in rodents sperm day = gd 0, if recorded; exception: Waritz et al. (1977): sperm day = gd 1); pp = postpartum;
o.t. = observed time; s.t. = sacrifice time.
d In mg/kg of feed, unless otherwise specified.
e No. affected/no. treated.
f MEL = Minimum effective level.
g As reported by Corbett et al. (1978a).
The number of inseminations required for conception was
increased in cows whose dams or grand dams had received oral doses
of FireMaster(R) BP-6 (Willett et al., 1982; Table 86).
Most of the studies evaluating the effects of PBBs on
reproductive performance started with treatment during pregnancy
(Tables 86 and 87). In few instances, were females exposed before
mating (Allen et al., 1978; Aulerich & Ringer, 1979; Willett et al.,
1980, 1982), and animals of both sexes were treated in only one
study (Aulerich & Ringer, 1979). Under these conditions,
FireMaster(R) reduced litter size in mice (Preache et al., 1976)
and minks (Aulerich & Ringer, 1979), but not in rats (Harris et al.,
1978a; McCormack et al., 1981) and pigs (Werner & Sleight, 1981).
The adverse effect on litter size was observed in minks given
PBB-contaminated poultry as well as in those fed FireMaster(R),
directly added to the diet (Aulerich & Ringer, 1979). Stillbirths
occurred in cattle (Durst et al., 1978b; Willett et al., 1982) and
rhesus monkeys (Allen et al., 1978; Lambrecht et al., 1978).
Reduced birth weights were reported in rhesus monkeys (Allen et al.,
1978; Lambrecht et al., 1978), minks (Aulerich & Ringer, 1979), and
mice (Corbett et al., 1978b) at intakes of 0.3, 1, or 50-1000 mg
FireMaster(R)/kg feed, respectively (see Table 87). On the other
hand, increased birth weights of calves resulted in a higher
incidence of dystocia in cows (Durst et al., 1978b; Willett et al.,
1980, 1982). The growth of progeny during lactation (indicated by
weight at weaning) was also reduced by FireMaster(R) in rats
(Harris et al., 1978a), mice (Preache et al., 1976), minks (Aulerich
& Ringer, 1979), and rhesus monkeys (Allen et al., 1978; Lambrecht
et al., 1978).
In the study by Lambrecht et al. (1978), 14 female rhesus
monkeys were used as an animal model (7 controls, 7 treated) to
evaluate the toxicological effects of FM FF-1. The substance was
added to the pelleted diet at a concentration of 0.3 mg/kg and 200 g
of this diet was fed per day; after a dosing period of 7 months, the
animals had ingested a total of 10.5 mg FM FF-1. Although the food
consumption during this period was unaffected, there was a loss of
7.4% of the initial body weight in the treated group. The total
exposure is not stated, but, on the basis of information given in
the paper, it appears that the dams had been exposed for a total of
12.5 months at the time of the birth of the infants. After 6 months,
menstrual cycles in 4/7 monkeys were lengthened (controls: 28 days;
treated monkeys: 31 days). The treated animals showed a
corresponding flattening of the progesterone peak. After mating, 5/7
treated monkeys delivered normal-appearing but small infants (455 g
vs 519 g in controls) with reduced weight gain in these infants
during the postnatal period. Two out of 7 treated monkeys aborted a
mummified fetus and a stillborn infant, respectively. All control
animals delivered normal-appearing infants.
The survival rate-to-weaning was adversely affected in the
offspring of rats, mice, pigs, and minks, but not in calves (see
Tables 86 and 87). FireMaster(R) levels producing the effects were
200 mg/kg body weight per day (Groce & Kimbrough, 1984), or
10 mg/day (Harris et al., 1978a), or 100 mg/kg feed (McCormack
et al., 1981) in rats, 100 mg/kg feed in mice (Preache et al., 1976)
and pigs (Werner & Sleight, 1981), and 1 mg/kg feed in minks
(Aulerich & Ringer, 1979).
For neurotoxic effects after perinatal exposure see section
8.11.2.
Ranges of maternal toxic parameters can be derived from Tables
71, 72, 74, and 75.
b) Embryotoxicity and teratogenicity
PBBs were embryotoxic in rats, mice, cattle, and rhesus
monkeys. Rats and cows appeared to be less susceptible than mice and
rhesus monkeys (see Tables 86 and 87).
Treatment of rats with a single high dose of FireMaster(R)
(> 400 mg/kg body weight) on day 6 of pregnancy resulted in 100%
embryo resorption. Generally, the number of fetal resorptions
depended on the dose and the pregnancy stage at treatment. For
example, after day 8 (400 mg/kg body weight dose) or day 12
(800 mg/kg body weight dose), the embryolethal effect of
FireMaster(R) declined abruptly (Beaudoin, 1977). Reductions in
both fetal and placental weights were observed only at high doses
(> 400 mg/kg body weight), and the most susceptible period of
pregnancy was days 11-13 (Beaudoin, 1977).
The long-term administration of lower doses (total dose: 8 or
40 mg FireMaster(R)/animal) during pregnancy (from day 0) markedly
increased embryonic death over that seen following administration of
an equivalent single dose (Beaudoin, 1979). However, long-term
intake of relatively low doses (see Tables 86 and 87) during late
gestation (start: day 7) did not influence fetal survival rate
(Corbett et al., 1975; Harris et al., 1978a; Johnston et al., 1980),
but decreased fetal weight (Corbett et al., 1975; Harris et al.,
1978a). A total dose of 600 mg FireMaster(R)/kg body weight, given
at two-day intervals (start: day 6) had no effects on fetal death or
fetal weight (Wertz & Ficsor, 1978).
No significant effects on the growth or mortality of embryos
were noted after the exposure of rats (Tables 86 and 87) to
technical octabromobiphenyl (Waritz et al., 1977) or technical
decabromobiphenyl (Millischer et al., 1979). Single cases of fetal
oedema have been observed in OcBB-treated rats (Aftosmis et al.,
1972a; Waritz et al., 1977).
The incidence of dead or resorbed fetuses was increased in mice
fed 200 mg FireMaster(R)/kg feed on days 4-16 of pregnancy
(Preache et al., 1976). Feeding several concentrations of FM from
day 7 (Corbett et al., 1975) or day 8 (Preache et al., 1976) of
pregnancy reduced fetal weight (Table 87).
Cattle given 25 g FM/day had 3 abortions and 3 dead fetuses
from 6 treated animals (Durst et al., 1977; Table 86). The fetuses
were oedematous and haemorrhagic, concomitant uterine lesions were
haemorrhage and necrosis of the cotyledons (Moorhead et al., 1977).
Two out of 7 rhesus monkeys fed FM (Table 87) had long
implantation bleedings, one aborted a mummified fetus at 146 days of
gestation and one gave birth to a stillborn infant at 154 days. The
remaining 5 monkeys delivered small infants at 156-165 days of
gestation (normal gestation: 165 days) (Allen et al., 1978;
Lambrecht et al., 1978).
Terata due to PBB exposure have been reported only in rats and
mice (Tables 86 and 87). A single high dose of FireMaster(R)
produced cleft palate and diaphragmatic hernia in rats (Beaudoin,
1977). The majority of malformations were found following
administration of 800 mg/kg body weight on day 11, 12, or 13 of
gestation. No terata were noted in rats after multiple doses of
FireMaster(R) (Table 86) (Harris et al., 1978a; Wertz & Ficsor,
1978; Beaudoin, 1979) and after feeding diets containing up to
1000 mg FireMaster(R)/kg feed (Corbett et al., 1975). In contrast
to rats comparably treated (50-1000 mg FM/kg maternal diet), a
higher incidence of exencephaly and cleft palate was seen in fetal
mice, compared with pooled historical controls, but not compared
with concurrent controls, though neither of these anomalies was seen
in control fetuses (Corbett et al., 1975, 1978b).
It is unclear whether the few cases of fetal gastroschisis,
observed in rats fed technical OcBB (Table 87), are PBB-related or
fortuitous (Aftosmis et al., 1972a; Waritz et al., 1977). No
teratogenicity was found in rats exposed to DeBB (Millischer et al.,
1979; Table 86).
Embryo development was studied in vitro. Maternal rats
received a single, oral dose of 800 mg FM/kg body weight on
gestation day 9, and the embryos were isolated on day 10 (24 h
post-treatment) for cultivation over a 24- or 42-h period (Fisher,
1980; Beaudoin & Fisher, 1981). Teratogenic effects observed (see
Table 86) were not correlated with types of malformations found in
earlier in vitro experiments (Beaudoin, 1977). Some developmental
disturbances tended to be corrected after 42 h of PBB-free culture.
In addition to retarded development, there was a significant
reduction in the DNA contents of cultured embryos (Fisher, 1980).
The survival rate of embryos was not affected during the cultivation
period (Beaudoin & Fisher, 1981).
8.5.1.2 Avian species
Avian reproduction was studied primarily in chickens.
Cockerels fed FM at various levels (10-250 mg/kg feed), for several
weeks, showed inconsistent weight changes in the comb and testes
(see Table 75: Ringer & Polin, 1977; Ringer, 1978; Dharma et al.,
1982). Histologically, lipid infiltration into testicular parenchyma
was noted (Ringer & Polin, 1977). No studies on the reproductive
performance of these birds were made.
Detrimental effects were seen when FireMaster(R) was fed to
hens of both White Leghorn chickens and Japanese quails, the only
two species studied (Table 87). Chickens (Cecil et al., 1974; Lillie
et al., 1975; Cecil & Bitman, 1978; Polin & Ringer, 1978a,; Polin
et al., 1979) appeared to be more sensitive to FireMaster(R) than
the Japanese quails (Babish et al., 1975a; Bursian et al., 1983), on
the basis of egg production and hatchability. Egg production in
chickens was reduced by dietary levels of FireMaster(R) as low as
30-45 mg/kg (Ringer & Polin, 1977), while the lowest effective level
reported for Japanese quails was 80 mg/kg (Bursian et al., 1983).
Chickens stopped laying when concentrations of FireMaster(R) in
diets exceeded 600 mg/kg (Cecil & Bitman, 1978). The hatchability of
eggs was adversely affected by 30-45 mg FireMaster(R)/kg feed in
chickens (Ringer & Polin, 1977) and by 100 mg FireMaster(R)/kg
feed in Japanese quails (Babish et al., 1975a).
Increased mortality (Cecil & Bitman, 1978; Polin & Ringer,
1978a, 1978b) and reduced growth rates (Cecil & Bitman, 1978) were
seen in the offspring of chickens fed FireMaster(R) at low levels
(< 64 mg/kg; Table 87).
Embryonic deaths occurred in chickens (Cecil & Bitman, 1978;
50% of deaths on the last day of incubation) and in Japanese quails
(Babish et al., 1975b; 40% of deaths on the first day or two of
development). The most common abnormality of embryos and newly
hatched chicks after maternal feeding of FireMaster(R) was oedema
(Cecil & Bitman, 1978; Polin & Ringer, 1978b; Polin et al., 1979).
Although there was a reduction in the feed intake of exposed
chickens, the decreased hatchability, higher incidence of
abnormalities, and poorer progeny survival could not be reproduced
by pair-fed control birds (Cecil & Bitman, 1978). At high dietary
levels (> 600 mg FM/kg of feed), the direct effects of
FireMaster(R) on egg production could not be separated from the
effects of reduced food consumption (Cecil & Bitman, 1978).
Adverse effects on the reproduction of chickens could return to
normal after withdrawal of FireMaster(R) from the diet. Time for
recovery depended on the concentration of PBB in the feed (Cecil &
Bitman, 1978; Polin & Ringer, 1978a).
8.5.2 Individual PBB congeners
Individual PBB congeners have not been reported to cause weight
changes in sex organs. Similarly, the testes of rats or cockerels,
did not show any remarkable histological changes after treatment
with 2,2'-dibromobiphenyl, 2,2',3,4,4',5,5'-heptabromobiophenyl,
2,2',4,4',5,5'-hexabromobiphenyl (BB 153), 2,3',4,4',5,5' (BB 167),
or 3,3',4,4',5,5'-hexabromobiphenyl (BB 169) (Moore et al., 1979a;
Gupta et al., 1981; Akoso et al; 1982a; Dharma et al., 1982; Render
et al., 1982). An exception was a decrease in the gravid uterine
weight of pregnant mice given more than 500 mg BB 153/kg body weight
(Welsch & Morgan, 1985).
Embryotoxicity and other reproductive parameters have been
studied only with BB 153 mice (Lucier et al., 1978; Welsch & Morgan,
1985) or chickens (Polin et al., 1979). BB 153 did not cause the
deleterious effects (decline in hatchability, oedema) that were
produced by equivalent dietary levels of FireMaster(R) in chickens
(Polin et al., 1979; Table 87). However, BB 153 was capable of
producing cleft palate and cystic lesions in the region of the
cerebellum in mouse embryos (Welsch & Morgan, 1985; Table 87). These
adverse effects were observed at exposure concentrations that also
cause toxicity in dams. Another less complete study (Lucier et al.,
1978; Table 86) did not find developmental toxicity of BB 153 in
mice, possibly because of lower exposure levels, later onset of
exposure, and strain differences.
8.6 Mutagenicity and related end-points
The potential of PBBs for mutagenicity has been tested with
several in vitro and in vivo assays referring to four major test
categories: microbial and mammalian cell mutagenesis; mammalian cell
chromosomal damage (cytogenetic tests; in vitro and in vivo);
mammalian cell transformation in vitro; and DNA damage and repair
(UDS). Results have been summarized in Table 88. With one exception
(Kohli et al., 1978), all studies failed to indicate any
mutagenicity of individual PBB congeners or commercial PBB mixtures.
Haworth et al. (1983) did not confirm the mutagenic effect of
4-bromobiphenyl observed by Kohli et al. (1978), but they used
different Salmonella strains.
However, FireMaster(R) gave positive results in a recently
developed test, the Microscreen assay using lambda prophage
induction in Escherichia coli. This test has been found to be
sensitive in detecting carcinogens which are negative in mutagenesis
assays (Rossman et al., 1991).
8.7 Carcinogenicity
8.7.1 Carcinogenicity in long-term toxicity studies
The carcinogenic effects of PBB in long-term toxicity studies
have been compiled in Table 89. From this table, it is evident that
the principal site of tumours was the liver. The incidence of
hepatocellular carcinoma was significantly increased in both sexes
of mice and rats receiving relatively high oral doses of the
FireMaster(R) mixture.
The results of one of these studies (Gupta et al., 1983b),
carried out by the NCI/NTP (USA), were interpreted as
FireMaster(R) showing carcinogenic effects (Haseman et al., 1984;
Tennant et al., 1986). In this study, a statistically significant
higher incidence of liver tumours was found at multiple doses of
3 mg FM/kg body weight per day (male rats: 21%) and of 10 mg FM/kg
body weight per day (female/male rats: 35/23%; female/male mice:
88/95%). Atypical foci and neoplastic nodules were found at lower
doses (Table 89). Dose-responses were statistically significant (P
< 0.01) in male and female rats with regard to atypical foci,
hepatocellular carcinoma, and cholangiocarcinoma, and, in female
rats, also with regard to neoplastic nodules (Gupta et al., 1983b).
Hepatocellular carcinoma could be produced, even after single dosing
with FireMaster(R) (Kimbrough et al., 1981). Rats given a single
dose of 1000 mg FM/kg or 12 doses of 100 mg FM/kg body weight, by
gavage, developed incidences of carcinomas of 41.4 and 67.8%,
respectively (Kimbrough et al., 1981). Liver tumours were also
observed in female and male rats two years after perinatal exposure
to FireMaster(R) (Groce & Kimbrough, 1984; Table 89). Although
concurrent controls did not show such lesions, the IARC Working
Group noted the lack of statistical significance (IARC, 1986).
Nevertheless, the incidences of 5.9% (females) and 9.6% (males),
respectively, exceeded the spontaneous incidence of carcinomas of
the liver in this particular strain of rat, which is reportedly less
than 1% (Groce & Kimbrough, 1984).
Recently, NTP performed further carcinogenicity studies on
FireMaster(R) to determine the following: (a) the effects of PBBs
in F344/N rats and B6C3F1 mice receiving adult (F1) exposure only
(0, 10, or 30 mg PBB/kg diet); (b) the toxic and carcinogenic
effects of PBBs in rats and mice receiving perinatal (Fo) exposure
only (10 mg/kg in rats and 30 mg/kg in mice); and (c) the effects of
combined perinatal and adult exposure to PBBs (NTP, 1993). In an
adult-only exposure study, both sexes in rats and mice receiving 10
or 30 mg/kg (0.5 or 1.5 mg/kg body weight per day) showed increased
incidences of hepatocellular adenoma/carcinoma, quite similar to
the results of the previous studies (Gupta et al., 1983b).
Table 88. Summary of PBB genetic toxicity test results
Assay Detailsb Metabolic activation PBBa Result Remarks References
Salmonella mutagenesis
S. typhimurium strains: TA 98 ± Aroclor 1254 FM FF-1 - Tennant
TA 100 induced liver - et al. (1986)
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 hexabromobiphenylc - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats and -
TA 1537 and Syrian hamsters
S. typhimurium strains: TA 1535 ± Aroclor DeBB - Millischer
(spot test; Ames) TA 1537 induced rat - et al. (1979)
TA 1538 liver S-9 -
S. typhimurium strain: TA 1538 - DeBB - mice receiving oral
(host-mediated doses of 5, 10, and
assay) 20 g/kg body weight
S. typhimurium strains: TA 98 ± Aroclor 1254 2-bromobiphenyl - Haworth et al.
TA 100 induced liver - (1983)
TA 1535 S-9 from rats -
TA 1537 and Syrian hamsters -
S. typhimurium strains: TA 98 ± Aroclor 1254 3-bromobiphenyl -
TA 100 induced liver -
TA 1535 S-9 from rats and -
TA 1537 Syrian hamsters -
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
S. typhimurium strain: TA 1538 + Aroclor 1254 4-bromobiphenyld + Kohli et al.
induced rat liver S-9 (1978)
none -
S. typhimurium strains: TA 98 ± Aroclor 1254 4-bromobiphenyle - Haworth et al.
TA 100 induced liver (1983)
TA 1535 S-9 from rats and
TA 1537 Syrian hamsters
Mammalian cell mutagenesis
Adult rat liver (ARL) HGPRT locus none FM FF-1 (lot No. - at the highest non-toxic Tong et al.
epithelial cells 1312-FS) dose of 10-3 mol (1983); Williams
et al. (1984)
Human fibroblasts HGPRT locus rat hepatocytes FM FF-1 (lot No. - at the highest non-toxic
1312-FS) dose of 10-4 mol
Chinese hamster HGPRT and Na-K ± Aroclor 1254 induced FM BP-6 - cell survival Kavanagh et al.
V 79 cells ATPase loci rat liver S 15 unaffected (1-40 µg/ml) (1985)
Chinese hamster HGPRT locus ± Aroclor 1254 induced 3,3',4,4'-tetra- - cell survival
V 79 cells rat liver S 15 bromobiphenyl unaffected (1-10 µg/ml)
Chinese hamster HGPRT locus none 2,2',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (20-50 µg/ml)
WB rat liver cells HGPRT locus - 2,2',4,4',5,5'- - growth stimulation
hexabromobiphenyl effects (5-20 µg/ml)
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chinese hamster HGPRT locus none 3,3',4,4',5,5'- - reduced cell survival
V 79 cells hexabromobiphenyl (7-12 µg/ml)
WB rat liver cells HGPRT locus - 3,3',4,4',5,5'- - reduced cell survival
hexabromobiphenyl
Mammalian cell chromosomal
damage in vitro
Chromosome CHO cells ± Aroclor 1254 FM FF-1 - Tennant et al.
aberrations induced rat (1986)
Sister chromatid CHO cells liver S-9 -
exchange
Chromosome CHO cells ± Aroclor 1254 hexabromobiphenyl - Galloway et al.
aberrations induced rat (1987)
liver S-9
Sister chromatid CHO cells ?f
exchange
Mammalian cell chromosomal
damage in vivo
(Cytogenetic effects)
Chromosome bone marrow cells - FM - colchicine-mitosis Ficsor & Wertz
aberrations rat (pregnant) synergism (1976)
six oral doses of
100 mg/kg body
weight
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
Chromosome bone marrow cells - FM - no colchicine-mitosis Wertz & Ficsor
aberrations mouse (male) synergism; increase in (1978)
single dose of 50 no. of gaps
or 500 mg/kg body
weight
No. of cells in rat (male) in diet for
mitosis 5 weeks: 5-500 mg/kg
feed
Chromosome bone marrow cells; - FM BP-6 - Garthoff et al.
aberrations spermatogonial (1977)
cells
Micronucleus bone marrow cells DeBB - Millischer
mouse (male and et al. (1979)
female) total dose
of 5, 10, or
20 g/kg body
weight at 2 doses
Mammalian cell transformation
in vitro
Transformation mouse Balb/c 3T3 ± non-induced FM FF-1 - Tennant et al.
cells male F 344 rat (1986)
hepatocytes
Table 88 (contd).
Assay Detailsb Metabolic activation PBBa Result Remarks References
DNA damage and repair
(in vitro)
Unscheduled DNA hepatocyte primary - FM FF-1 - at the highest nontoxic Tong et al.
synthesis (UDS) cultures (HPCs) doses of 10-3 mol (mice, (1983);
from rat, hamster, hamsters) or 10-5 mol Williams et al.
and mouse (rats) (1984)
DNA damage and repair
(in vivo - in vitro)
UDS hepatocyte primary - FM FF-1 - Tennant et al.
cultures (HPCs) (1986)
from treated male
F 344 rats
UDS hepatocyte primary - FM FF-1 - simultaneous measurement Mirsalis et al.
cultures (HPCs) of hepatic cell (1985)
from treated proliferative ability:
B6C3F1 mice (males increased cell
and females) proliferation
a Commercial PBB mixtures: FM = FireMaster(R); DeBB = decabromobiphenyl.
b HGPRT = hypoxanthine-guanine phosphoribosyl transferase.
c CAS No.: 