
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 135
CADMIUM - ENVIRONMENTAL ASPECTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr S. Dobson,
Institute of Terrestrial Ecology, United Kingdom
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Cadmium : environmental aspects.
(Environmental health criteria ; 135)
1.Cadmium - toxicity 2.Environmental exposure
I.Series
ISBN 92 4 157135 7 (NLM Classification: QV 290)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM - ENVIRONMENTAL ASPECTS
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Physical and chemical properties
2.2. Analytical procedures
2.2.1. Sampling and preparation
2.2.2. Quantitative instrumental methods
3. NATURAL OCCURRENCE AND SOURCES OF ENVIRONMENTAL CONTAMINATION
3.1. Natural occurrence
3.2. Industrial uses
3.3. Sources of environmental cadmium
3.3.1. Sources of atmospheric cadmium
3.3.2. Sources of aquatic cadmium
3.3.3. Sources of terrestrial cadmium
3.4. Environmental transport and distribution
3.4.1. Atmospheric deposition
3.4.2. Transport from water to soil
3.5. Concentrations in various biota
3.5.1. Concentrations in fish
3.5.2. Concentrations in sea-birds
3.5.3. Concentrations in sea mammals
3.6. Concentrations adjacent to highways
3.7. Concentrations from industrial sources
4. KINETICS AND METABOLISM
4.1. Uptake
4.1.1. Uptake from water by aquatic organisms
4.1.1.1 Microorganisms
4.1.1.2 Aquatic molluscs
4.1.1.3 Other aquatic invertebrates
4.1.1.4 Fish
4.1.1.5 Model aquatic ecosystems
4.1.1.6 Uptake from aquatic sediment
4.1.1.7 Uptake from food relative to uptake from
water
4.1.2. Uptake by terrestrial organisms
4.1.2.1 Uptake into plants
4.1.2.2 Terrestrial invertebrates
4.1.2.3 Birds
4.2. Distribution
4.2.1. Aquatic organisms
4.2.2. Terrestrial organisms
4.2.2.1 Terrestrial plants
4.2.2.2 Terrestrial invertebrates
4.3. Elimination
4.4. Bioaccumulation and biomagnification
5. TOXICITY TO MICROORGANISMS
5.1. Aquatic microorganisms
5.1.1. Freshwater microorganisms
5.1.2. Estuarine and marine microorganisms
5.2. Soil and litter microorganisms
6. TOXICITY TO AQUATIC ORGANISMS
6.1. Toxicity to aquatic plants
6.2. Toxicity to aquatic invertebrates
6.2.1. Acute and short-term toxicity
6.2.1.1 Effects of temperature and salinity on
acute toxicity
6.2.1.2 Effect of water hardness
6.2.1.3 Effect of organic materials and sediment
6.2.1.4 Lifestage sensitivity
6.2.1.5 Other factors affecting acute and
short-term toxicity
6.2.2. Long-term toxicity
6.2.3. Reproductive effects
6.2.4. Physiological and biochemical effects
6.2.5. Behavioural effects
6.2.6. Interactions with other chemicals
6.2.7. Tolerance
6.2.8. Model ecosystems
6.3. Toxicity to fish
6.3.1. Acute and short-term toxicity
6.3.2. Reproductive effects and effects on early life
stages
6.3.3. Metabolic, biochemical and physiological effects
6.3.4. Structural effects and malformations
6.3.5. Behavioural effects
6.3.6. Interactions with other chemicals
6.4. Toxicity to amphibia
7. TOXICITY TO TERRESTRIAL ORGANISMS
7.1. Toxicity to terrestrial plants
7.1.1. Toxicity to plants grown hydroponically
7.1.2. Toxicity to plants grown in soil
7.1.3. In vitro physiological studies
7.2. Toxicity to terrestrial invertebrates
7.3. Toxicity to birds
7.3.1. Acute and short-term toxicity
7.3.2. Reproductive effects
7.3.3. Physiological effects
7.3.4. Behavioural effects
7.4. Toxicity to wild small mammals
8. EFFECTS IN THE FIELD
8.1. Tolerance
8.2. Effects close to industrial sources and highways
8.3. Effects on fish
8.4. Effects on sea-birds
9. EVALUATION
9.1. General considerations
9.2. The aquatic environment
9.3. The terrestrial environment
10. RECOMMENDATIONS FOR PROTECTING THE ENVIRONMENT
11. FURTHER RESEARCH
REFERENCES
APPENDIX 1
APPENDIX 2
APPENDIX 3
APPENDIX 4
APPENDIX 5
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM -
ENVIRONMENTAL ASPECTS
Members
Dr L.A. Albert, Consultores Ambientales Asociados, S.C., Xalapa,
Veracruz, Mexico
Dr J.K. Atherton, Toxic Substances Division, Directorate for Air,
Climate and Toxic Substances, Department of the Environment,
London, United Kingdom
Dr R.W. Elias, Trace Metal Biogeochemistry, Environmental Criteria and
Assessment Office, US Environmental Protection Agency, Research
Triangle Park, North Carolina, USA
Dr A.H. El-Sebae, Faculty of Agriculture, Alexandria University,
Alexandria, Egypt
Dr R. Koch, Bayer AG, Leverkusen, Germany
Professor Y. Kodama, Department of Environmental Health, University of
Occupational and Environmental Health, Japan School of Medicine,
Yahata Nishi-ku, Kitakyushu City, Japan
Dr P. Pärt, Department of Zoophysiology, Uppsala University, Uppsala,
Sweden
Dr J.H.M. Temmink, Department of Toxicology, Agricultural University,
Wageningen, The Netherlands ( Chairman)
Secretariat
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, Cambridgeshire,
United Kingdom ( Rapporteur)
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, Cambridgeshire,
United Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR CADMIUM - ENVIRONMENTAL ASPECTS
A WHO Task Group on Environmental Health Criteria for Cadmium -
Environmental Aspects met at the Institute of Terrestrial Ecology
(ITE), Monks Wood, United Kingdom, from 13 to 17 May 1991. Dr M.
Roberts, Director, ITE, welcomed the participants on behalf of the
host institution and Dr M. Gilbert opened the meeting on behalf of the
three cooperating organizations of the IPCS (UNEP/ILO/WHO). The Task
Group reviewed and revised the draft criteria document and made an
evaluation of the risks for the environment from exposure to cadmium.
The first draft of this document was prepared by Dr S. Dobson
(ITE). Dr M. Gilbert and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the technical development and
editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
ALAD delta-aminolevulinic acid dehydratase
DPTA diaminopropanoltetraacetic acid
EDTA ethylenediaminetetraacetic acid
EEC European Economic Community
EIFAC European Inland Fisheries Advisory Commission of FAO
FAO Food and Agriculture Organization of the United Nations
GESAMP Group of Experts on the Scientific Aspects of Marine
Pollution
MATC maximum acceptable toxicant concentration
NOEL no-observed-effect level
NTA nitrilotriacetic acid
NTEL no-toxic-effect level
1. SUMMARY
Cadmium (atomic number 48; relative atomic mass 112.40) is a
metallic element belonging, together with zinc and mercury, to group
IIb of the periodic table. Some cadmium salts, such as the sulfide,
carbonate, and oxide, are practically insoluble in water; these can be
converted to water-soluble salts in nature. The sulfate, nitrate, and
halides are soluble in water. The speciation of cadmium in the
environment is of importance in evaluating the potential hazard.
The average cadmium content of sea water is about 0.1 µg/litre or
less. River water contains dissolved cadmium at concentrations of
between < 1 and 13.5 ng/litre. In remote, uninhabited areas, cadmium
concentrations in air are usually less than 1 ng/m3. In areas not
known to be polluted, the median cadmium concentration in soil has
been reported to be in the range of 0.2 to 0.4 mg/kg. However, much
higher values, up to 160 mg/kg soil, are occasionally found.
Environmental factors affect the uptake and, therefore, the toxic
impact of cadmium on aquatic organisms. Increasing temperature
increases the uptake and toxic impact, whereas increasing salinity or
water hardness decreases them. Freshwater organisms are affected by
cadmium at lower concentrations than marine organisms. The organic
content of the water generally decreases the uptake and toxic effect
by binding cadmium and reducing its availability to organisms.
However, there is evidence that some organic matter may have the
opposite effect.
Cadmium is readily accumulated by many organisms, particularly by
microorganisms and molluscs where the bioconcentration factors are in
the order of thousands. Soil invertebrates also concentrate cadmium
markedly. Most organisms show low to moderate concentration factors of
less than 100. Cadmium is bound to proteins in many tissues. Specific
heavy-metal-binding proteins (metallothioneins) have been isolated
from cadmium-exposed organisms. The concentration of cadmium is
greatest in the kidney, gills, and liver (or their equivalents).
Elimination of the metal from organisms probably occurs principally
via the kidney, although significant amounts can be eliminated via the
shed exoskeleton in crustaceans. In plants, cadmium is concentrated
primarily in the roots and to a lesser extent in the leaves.
Cadmium is toxic to a wide range of microorganisms. However, the
presence of sediment, high concentrations of dissolved salts or
organic matter all reduces the toxic impact. The main effect is on
growth and replication. The most affected of soil microorganisms are
fungi, some species being eliminated after exposure to cadmium in
soil. There is selection for resistant strains after low exposure to
the metal in soil.
The acute toxicity of cadmium to aquatic organisms is variable,
even between closely related species, and is related to the free ionic
concentration of the metal. Cadmium interacts with the calcium
metabolism of animals. In fish it causes hypocalcaemia, probably by
inhibiting calcium uptake from the water. However, high calcium
concentrations in the water protect fish from cadmium uptake by
competing at uptake sites. Zinc increases the toxicity of cadmium to
aquatic invertebrates. Sublethal effects have been reported on the
growth and reproduction of aquatic invertebrates; there are structural
effects on invertebrate gills. There is evidence of the selection of
resistant strains of aquatic invertebrates after exposure to cadmium
in the field. The toxicity is variable in fish, salmonids being
particularly susceptible to cadmium. Sub-lethal effects in fish,
notably malformation of the spine, have been reported. The most
susceptible life-stages are the embryo and early larva, while eggs are
the least susceptible. There is no consistent interaction between
cadmium and zinc in fish. Cadmium is toxic to some amphibian larvae,
although some protection is afforded by sediment in the test vessel.
Cadmium affects the growth of plants in experimental studies,
although no field effects have been reported. The metal is taken up
into plants more readily from nutrient solutions than from soil;
effects have been mainly shown in studies involving culture in
nutrient solutions. Stomatal opening, transpiration, and
photosynthesis have been reported to be affected by cadmium in
nutrient solutions.
Terrestrial invertebrates are relatively insensitive to the toxic
effects of cadmium, probably due to effective sequestration mechanisms
in specific organs.
Terrestrial snails are affected sublethally by cadmium; the main
effect is on food consumption and dormancy, but only at very high dose
levels. Birds are not lethally affected by the metal even at high
dosage, although kidney damage occurs.
Cadmium has been reported in field studies to be responsible for
changes in species composition in populations of microorganisms and
some aquatic invertebrates. Leaf litter decomposition is greatly
reduced by heavy metal pollution, and cadmium has been identified as
the most potent causative agent for this effect.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Physical and chemical properties
Cadmium (atomic number 48; relative atomic mass 112.40) is a
metallic element belonging, together with zinc and mercury, to group
IIb in the periodic table. It is rarely found in a pure state. It is
present in various types of rocks and soils and in water, as well as
in coal and petroleum. Among these natural sources, zinc, lead, and
copper ore are the main sources of cadmium.
Cadmium can form a number of salts. Its mobility in the
environment and effects on the ecosystem depend to a great extent on
the nature of these salts. Since there is no evidence that
organocadmium compounds, where the metal is covalently bound to
carbon, occur in nature, only inorganic cadmium salts will be
discussed. Cadmium may occur bound to proteins and other organic
molecules and form salts with organic acids, but in these forms, it is
regarded as inorganic.
Cadmium has a relatively high vapour pressure. The vapour is
oxidized quickly to produce cadmium oxide in the air. When reactive
gases or vapour, such as carbon dioxide, water vapour, sulfur dioxide,
sulfur trioxide or hydrogen chloride, are present, the vapour reacts
to produce cadmium carbonate, hydroxide, sulfite, sulfate or chloride,
respectively. These salts may be formed in stacks and emitted to the
environment.
Some of the cadmium salts, such as the sulfide, carbonate or
oxide, are practically insoluble in water. However, these can be
converted to water-soluble salts in nature under the influence of
oxygen and acids; the sulfate, nitrate, and halogenates are soluble in
water. The physical and chemical properties of cadmium and its salts
are summarized in Table 1. Equilibrium data for complexes of group IIB
cations, comparing cadmium with zinc and mercury, can be found in
Table 2. A diagrammatic representation of the capacity of soil types
for metals is given in Fig. 1.
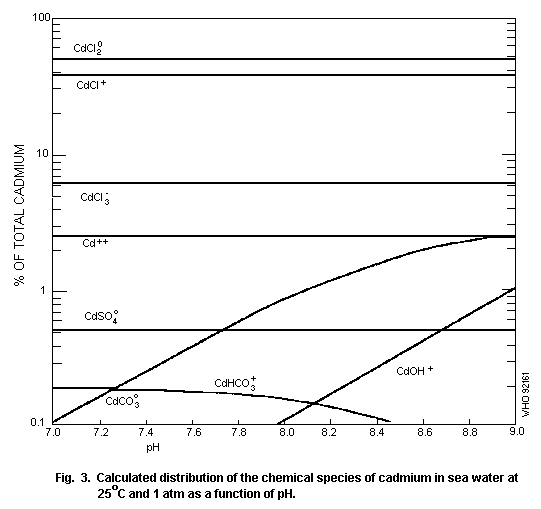
The speciation of cadmium in soil water (Fig. 2) and surface
water (Fig. 3) is important for the evaluation of its potential
hazard.
Most of the cadmium found in mammals, birds, and fish is probably
bound to protein molecules.
Table 1. Physical and chemical properties of cadmium and its salts
Cadmium Cadmium Cadmium Cadmium Cadmium Cadmium Cadmium Cadmium
chloride acetate oxide hydroxide sulfide sulfate sulfite
CAS number 7440-43-9 10108-64-2 543-90-8 1306-19-0 1306-23-6 10124-36-4
Empirical formula Cd CdCl2 C4H6CdO4 CdO Cd(OH)2 CdS CdSO4 CdSO3
Relative atomic or
molecular mass 112.41 183.32 230.50 128.40 146.41 144.46 208.46 192.46
Relative density 8.642 4.047 2.341 6.95 4.79 4.82 4.691
Melting point (°C) 320.9 568 256 < 1426 300 1750 1000 decomposes
(decomposes)
Boiling point (°C) 765 960 decomposes 900-1000
(decomposes)
Water solubility insoluble 1400 very soluble insoluble 0.0026 0.0013 755 slightly soluble
(g/litre) (20 °C) (26 °C) (18 °C) (0 °C)
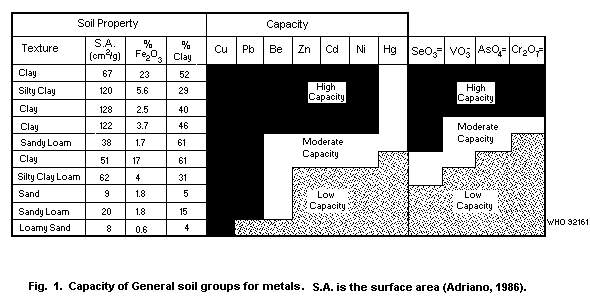
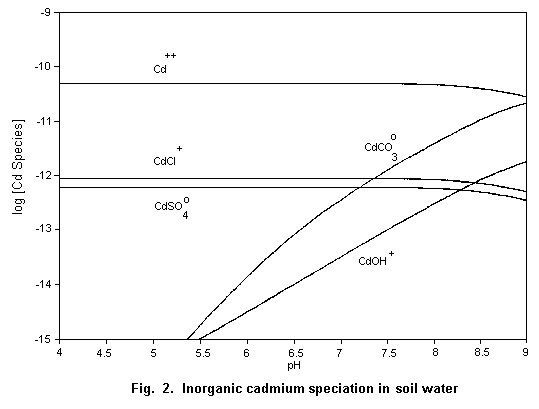 Table 2. Equilibrium data for complexes of group IIB cations a
System Metal log K1 DELTA H1 DELTA S1
(kJ mol-1) (J K-1 mol-1)
zinc 5.0 b 0 b 105
M2+-OH- cadmium 3.9 b 0 79
mercury 10.6 b - -
zinc 0.8 7.5 42
M2+-F- cadmium 0.6 4.2 25
mercury 1.0 c 4.2 c 33 c
zinc - 0.2 5.4 16
M2+-Cl- cadmium 1.5 - 0.4 29
mercury 7.1 - 24.3 54
zinc - 0.6 1.7 - 4
M2+-Br- cadmium 1.7 - 4.2 21
mercury 9.4 - 40.1 46
zinc - 1.5 - -
M2+-I- cadmium 2.1 - 9.2 8
mercury 12.9 c - 75.3 c - 8 c
zinc 5.3 - -
M2+-CN- cadmium 5.6 - 30.5 b 13 b
mercury 18.0 c - 96 b 0 b
zinc 0.7 d - 5.9 d - 4 d
M2+-SCN- cadmium 1.3 d - 9.6 d - 8d
mercury 9.1 d - 49.7 d 8
zinc 1.9 - -
M2+-S2O32- e cadmium 4.7 - 6.3 d 67 d
mercury 29.9 d - -
zinc 2.4 f - 10.9 f 8 f
M2+-NH3 cadmium 2.7 f - 14.6 f 4 f
mercury 8.8 f - -
zinc 4.8 c - 11.3 g 59 g
2+ - cadmium 4.1 d - 8.8 b 50 g
(glycinate)- mercury 10.3 c - -
zinc 16.4 - 20.5 247
M2+-(EDTA)4- cadmium 16.4 - 38.1 184
mercury 21.5 - 79.0 146
a From: Aylett (1979). Data, which refer to first stepwise stability
constant, [ML]/[M][L], unless otherwise stated, are from Sillen
(1964) and Smith & Martell (1974, 1975, 1976); see also Christensen
et al. (1975). All values refer to measurements in water at 25 °C;
the ionic strength is 3 mol/litre unless otherwise stated.
b ionic strength 0
c ionic strength 0.5 mol/litre
d ionic strength 1.0 mol/litre
e Data refer to overall stability constant, ß2 = [ML2]/[M][L]2
f ionic strength 2.0 mol/litre
g ionic strength 0.1 mol/litre
Table 2. Equilibrium data for complexes of group IIB cations a
System Metal log K1 DELTA H1 DELTA S1
(kJ mol-1) (J K-1 mol-1)
zinc 5.0 b 0 b 105
M2+-OH- cadmium 3.9 b 0 79
mercury 10.6 b - -
zinc 0.8 7.5 42
M2+-F- cadmium 0.6 4.2 25
mercury 1.0 c 4.2 c 33 c
zinc - 0.2 5.4 16
M2+-Cl- cadmium 1.5 - 0.4 29
mercury 7.1 - 24.3 54
zinc - 0.6 1.7 - 4
M2+-Br- cadmium 1.7 - 4.2 21
mercury 9.4 - 40.1 46
zinc - 1.5 - -
M2+-I- cadmium 2.1 - 9.2 8
mercury 12.9 c - 75.3 c - 8 c
zinc 5.3 - -
M2+-CN- cadmium 5.6 - 30.5 b 13 b
mercury 18.0 c - 96 b 0 b
zinc 0.7 d - 5.9 d - 4 d
M2+-SCN- cadmium 1.3 d - 9.6 d - 8d
mercury 9.1 d - 49.7 d 8
zinc 1.9 - -
M2+-S2O32- e cadmium 4.7 - 6.3 d 67 d
mercury 29.9 d - -
zinc 2.4 f - 10.9 f 8 f
M2+-NH3 cadmium 2.7 f - 14.6 f 4 f
mercury 8.8 f - -
zinc 4.8 c - 11.3 g 59 g
2+ - cadmium 4.1 d - 8.8 b 50 g
(glycinate)- mercury 10.3 c - -
zinc 16.4 - 20.5 247
M2+-(EDTA)4- cadmium 16.4 - 38.1 184
mercury 21.5 - 79.0 146
a From: Aylett (1979). Data, which refer to first stepwise stability
constant, [ML]/[M][L], unless otherwise stated, are from Sillen
(1964) and Smith & Martell (1974, 1975, 1976); see also Christensen
et al. (1975). All values refer to measurements in water at 25 °C;
the ionic strength is 3 mol/litre unless otherwise stated.
b ionic strength 0
c ionic strength 0.5 mol/litre
d ionic strength 1.0 mol/litre
e Data refer to overall stability constant, ß2 = [ML2]/[M][L]2
f ionic strength 2.0 mol/litre
g ionic strength 0.1 mol/litre
 2.2 Analytical procedures
The following is an outline of the analytical procedure for
cadmium; further information is given in Environmental Health Criteria
134: Cadmium (WHO, 1992).
2.2.1 Sampling and preparation
Only a few nanograms, or even less, of cadmium may be present in
collected samples of air or water, whereas hundreds of micrograms may
be present in small samples of kidney, sewage sludge, and plastics.
Different techniques are, therefore, required for the collection,
preparation, and analysis of the samples.
In general, the techniques available for measuring cadmium in the
environment and biological materials cannot differentiate between
cadmium species. With special separation techniques,
cadmium-containing proteins can be isolated and identified. In most
studies, the concentration or amount of cadmium in water, air, soil,
plants, and other environmental or biological material is determined
as the element.
Standard trace element methods can generally be used for the
collection of samples (LaFleur, 1976; Behne, 1980). During the
handling and storage of samples, particularly liquid samples, special
care must be taken to avoid contamination; coloured materials in
containers, especially plastics and rubber, should be avoided. Glass
and transparent, cadmium-free polyethylene, polypropylene or teflon
containers are usually considered suitable for storing samples. All
containers and glassware should be precleaned in dilute nitric acid
and deionised water. In order to avoid possible adsorption of cadmium
onto the container wall, water samples or standards with low cadmium
concentrations should not be stored for long periods of time.
To prepare samples for analysis, inorganic solid samples (such as
soil or dust samples) are usually dissolved in an acid, e.g., nitric
acid. Organic samples need to be subjected to wet ashing (digested) or
dry ashing. When the cadmium concentration is low, special treatment
is sometimes needed. The procedures for separating cadmium from
interfering compounds and concentrating the samples are very important
steps in obtaining accurate results.
2.2.2 Quantitative instrumental methods
The most commonly used methods, at present, are atomic absorption
spectrometry, electrochemical methods, neutron activation analysis,
atomic emission spectrometry, atomic fluorescence spectrometry and
proton-induced X-ray emissions (PIXE) analysis. Analytical methods for
cadmium have been reviewed by Friberg et al. (1986). Detection limits
of some of the methods are given in Table 3.
Table 3. Analytical procedures a
Method Detection limit Matrix
Atomic absorption 1 to 5 mg/litre water
spectrometry
0.1 mg/kg biological samples
electrothermal a few pg
atomization
Electrochemical method
(potentiometric stripping
analysis) 0.1 mg/litre urine
Neutron activation 0.1 to 1 mg/litre biological
analysis samples/fluids
X-ray atomic 17 mg/kg biological samples
fluorescence
a From: Friberg et al. (1986)
3. NATURAL OCCURRENCE AND SOURCES OF ENVIRONMENTAL CONTAMINATION
3.1 Natural occurrence
A comparison of natural and anthropogenic sources of trace metals
is given in the Appendix 1.
Cadmium is widely distributed in the earth's crust at an average
concentration of about 0.1 mg/kg and is commonly found in association
with zinc. However, higher levels are present in sedimentary rocks:
marine phosphates often contain about 15 mg/kg (GESAMP, 1984).
Weathering and erosion result in the transport by rivers of large
quantities of cadmium to the world's oceans and this represents a
major flux of the global cadmium cycle; an annual gross input of 15
000 tonnes has been estimated (GESAMP, 1987).
In background areas away from ore bodies, surface soil
concentrations of cadmium typically range between 0.1 and 0.4 mg/kg
(Page et al., 1981). The median cadmium concentration in non-volcanic
soil ranges from 0.01 to 1 mg/kg, but in volcanic soil levels of up to
4.5 mg/kg have been found (Korte, 1983).
Volcanic activity is a major natural source of atmospheric
cadmium release. The global annual flux from this source has been
estimated to be 100-500 tonnes (Nriagu, 1979). Deep sea volcanism is
also a source of environmental cadmium release, but the role of this
process in the global cadmium cycle remains to be quantified.
The average cadmium content of sea water is about 0.1 µg/litre or
less (Korte, 1983), while river water (Mississippi, Yangtze, Amazon,
and Orinoco sampled between 1976 and 1982) contains dissolved cadmium
at concentrations of < 1.1-13.5 ng/litre (Shiller & Boyle, 1987).
Cadmium levels of up to 5 mg/kg have been reported in river and lake
sediments and from 0.03 to 1 mg/kg in marine sediments (Korte,1983).
Current measurements of dissolved cadmium in surface waters of
the open oceans give values of < 5 ng/litre. The vertical
distribution of dissolved cadmium in ocean waters is characterized by
a surface depletion and deep water enrichment, which corresponds to
the pattern of nutrient concentrations in these areas (Boyle et al.,
1976). This distribution is considered to result from the absorption
of cadmium by phytoplankton in surface waters and its transport to the
depths, incorporation to biological debris, and subsequent release. In
contrast, cadmium is enriched in the surface waters of areas of
upwelling and this also leads to elevated levels in plankton
unconnected with human activity (Martin & Broenkow, 1975; Boyle et
al., 1976). Oceanic sediments underlying these areas of high
productivity can contain markedly elevated cadmium levels as a result
of inputs associated with biological debris (Simpson, 1981).
In remote, uninhabited areas, cadmium concentrations in air are
usually less than 1 ng/m3 (Korte,1983).
3.2 Industrial uses
The principal applications of cadmium fall into five categories:
protective plating on steel; stabilizers for PVC; pigments in plastics
and glass; electrode material in nickel-cadmium batteries; and as a
component of various alloys (Wilson, 1988).
The relative importance of the major applications has changed
considerably over the last 25 years. The use of cadmium for
electroplating represented in 1960 over half the cadmium consumed
worldwide, but in 1985 its share was less than 25% (Wilson, 1988).
This decline is usually linked to the introduction of stringent
effluent limits from plating works and, more recently, to the
introduction of general restrictions on cadmium consumption in certain
countries. In contrast, the use of cadmium in batteries has shown
considerable growth in recent years from only 8% of the total market
in 1970 to 37% by 1985. The use of cadmium in batteries is
particularly important in Japan and represented over 75% of the total
consumption in 1985 (Wilson, 1988).
Pigments and stabilizers accounted for 22% and 12% of the total
world consumption in 1985. The share of the market by cadmium pigments
remained relatively stable between 1970 and 1985 but the use of the
metal in stabilizers during this period showed a considerable decline,
largely as a result of economic factors. The use of cadmium as a
constituent of alloys is relatively small and has also declined in
importance in recent years, accounting for about 4% of total cadmium
use in 1985 (Wilson, 1988).
3.3 Sources of environmental cadmium
3.3.1 Sources of atmospheric cadmium
Estimates of cadmium emissions to the atmosphere from human and
natural sources have been carried out at the worldwide, regional, and
national level; examples of such inventories are shown in Table 4.
The median global total emission of the metal from human sources
in 1983 was 7570 tonnes (Nriagu & Pacyna, 1988) and represented about
half the total quantity of cadmium produced in the same year. In both
the European Economic Community (EEC) and on a worldwide scale
(Nriagu, 1989), about 10-15% of total airborne cadmium emissions arise
from natural processes, the major source being volcanic action.
Municipal refuse contains cadmium derived from discarded
nickel-cadmium batteries and plastics containing cadmium pigments and
stabilizers. The incineration of refuse is a major source of
atmospheric cadmium release at country, regional, and worldwide level
(Table 4).
Steel production can also be considered as a waste-related
source, as large quantities of cadmium-plated steel scrap are recycled
by this industry. As a result, steel production is responsible for
considerable emissions of atmospheric cadmium.
3.3.2 Sources of aquatic cadmium
Non-ferrous metal mines represent a major source of cadmium
release to the aquatic environment. Contamination can arise from mine
drainage water, waste water from the processing of ores, overflow from
the tailings pond, and rainwater run-off from the general mine area.
The release of these effluents to local watercourses can lead to
extensive contamination downstream of the mining operation. Mines
disused for many years can still be responsible for the continuing
contamination of adjacent watercourses (Johnson & Eaton, 1980).
At the global level, the smelting of non-ferrous metal ores has
been estimated to be the largest human source of cadmium release to
the aquatic environment (Nriagu & Pacyna, 1988). Discharges to fresh
and coastal waters arise from liquid effluents produced by air
pollution control (gas scrubbing) together with the site drainage
waters.
Table 4. Estimates of atmospheric cadmium emissions (tonnes/year) on a national, regional and worldwide basis
Source United EEC b Worldwide c
Kingdom a
Natural sources ND 20 150-2600 d
Non-ferrous metal
production
mining ND ND 0.6-3
zinc and cadmium 20 920-4600
copper 3.7 6 1700-3400
lead 7 39-195
Secondary production ND 2.3-3.6
Production of cadmium-containing
substances ND 3 ND
Iron and steel production 2.3 34 28-284
Fossil fuel combustion
coal 1.9 6 176-882
oil 0.5 41-246
Refuse incineration 5 31 56-1400
Sewage sludge incineration 0.2 2 3-36
Table 4 (contd).
Source United EEC b Worldwide c
Kingdom a
Phosphate fertilizer manufacture ND ND 68-274
Cement manufacture 1 ND 8.9-534
Wood combustion ND ND 60-180
TOTAL EMISSIONS 14 130 3350-14 640
a From: Hutton & Symon (1986); data apply to 1982-1983
b From: Hutton (1983); data apply to 1979-1980 (the EEC consisted,
at that time, of Belgium, Denmark, Federal Republic of Germany,
Italy, Luxembourg, The Netherlands, Republic of Ireland, and the
United Kingdom)
c From: Nriagu & Pacyna (1988); data apply to 1983
d From: Nriagu (1979)
ND Not determined
The manufacture of phosphate fertilizer results in a
redistribution of the cadmium present in the rock phosphates between
the phosphoric acid product and gypsum waste. In many cases, the
gypsum is disposed of by dumping in coastal waters, which leads to
considerable cadmium inputs. Some countries, however, recover the
gypsum for use as a construction material and thus have negligible
cadmium discharges (Hutton, 1982).
The atmospheric fallout of cadmium to fresh and marine waters
represents a major input of cadmium at the global level (Nriagu &
Pacyna, 1988). A GESAMP study of the Mediterranean Sea indicated that
this source is comparable in magnitude to the total river inputs of
cadmium to the region (GESAMP, 1985). Similarly, large cadmium inputs
to the North Sea (110-430 tonnes/year) have also been estimated, based
on the extrapolation from measurements of cadmium deposition along the
coast (van Alst et al., 1983a,b). However, another approach based on
model simulation yielded a modest annual cadmium input of 14 tonnes
(Krell & Roeckner, 1988).
Acidification of soils and lakes may result in enhanced
mobilization of cadmium from soils and sediments and lead to increased
levels in surface and ground waters (WHO Working Group, 1986).
3.3.3 Sources of terrestrial cadmium
Solid wastes are disposed of in landfill sites, resulting in
large cadmium inputs at the national and regional levels when
expressed as total tonnage (Hutton, 1982; Hutton & Symon, 1986).
Sources include the ashes from fossil fuel combustion, waste from
cement manufacture, and the disposal of municipal refuse and sewage
sludge.
Of greater potential environmental significance are the solid
wastes from both non-ferrous metal production and the manufacture of
cadmium-containing articles, as well as the ash residues from refuse
incineration. These three waste materials are characterized by
elevated cadmium levels and as such require disposal to controlled
sites to prevent the contamination of the ground water.
The agricultural application of phosphate fertilizers represents
a direct input of cadmium to arable soils. The cadmium content of
phosphate fertilizers varies widely and depends on the origin of the
rock phosphate. It has been estimated that fertilizers of West African
origin contain 160-255 g cadmium/tonne of phosphorus pentoxide, while
those derived from the southeastern USA contain 35 g/tonne (Hutton,
1982).
The annual rate of cadmium input to arable land from phosphate
fertilizers has been estimated at 5 g/ha for the countries of the EEC
(Hutton 1982). This only represents about 1% of the surface soil
cadmium burden. Despite the relatively small size of this input,
long-term continuous application of phosphate fertilizers has been
shown to cause increased soil cadmium concentrations (Williams &
David, 1973, 1976; Andersson & Hahlin, 1981).
The application of municipal sewage sludge to agricultural soils
as a fertilizer can also be a significant source of cadmium; a value
of 80 g/ha has been estimated for the United Kingdom (Hutton & Symon,
1986). On a national or regional basis, however, these inputs are much
smaller than those from either phosphate fertilizers or atmospheric
deposition (see section 3.4).
Polluted soils can contain cadmium levels of up to 57 mg/kg (dry
weight) resulting from sludge applied to soil and up to 160 mg/kg in
the vicinity of metal-processing industry (Fleischer et al., 1974).
The highest cadmium levels reported appear to be from ancient mining
areas with levels of up to 468 mg/kg.
3.4 Environmental transport and distribution
3.4.1 Atmospheric deposition
Cadmium is removed from the atmosphere by dry deposition and by
precipitation. In rural areas of Scandinavia, annual deposition rates
of 0.4-0.9 g/ha have been measured (Laamanen, 1972; Andersson, 1977).
Similarly, in a rural region of Tennessee, USA, a deposition rate of
0.9 g/ha was observed (Lindberg et al., 1982). Hutton (1982) suggested
that 3 g/ha per year was a representative value for the atmospheric
deposition of cadmium to agricultural soils in rural areas of the EEC.
The corresponding input for these areas from the application of
phosphate fertilizers is 5 g/ha per year (see section 3.3).
Many industrial sources of cadmium possess tall stacks which
bring about the wide dispersion and dilution of particulate emissions.
Nevertheless, cadmium deposition rates around smelter facilities are
often markedly elevated nearest the source and generally decrease
rapidly with distance (Hirata, 1981). Soil cadmium concentrations in
excess of 100 mg/kg are commonly encountered close to long established
smelters (Buchauer, 1972).
Crop plants growing near to atmospheric sources of cadmium may
contain elevated cadmium levels (Carvalho et al., 1986). However, it
is not always possible to distinguish whether the cadmium is derived
directly from surface deposition or originates from root uptake, since
soil levels in such areas are generally higher than normal.
3.4.2 Transport from water to soil
Rivers contaminated with cadmium can contaminate surrounding
land, either through irrigation for agricultural purposes, by the
dumping of dredged sediments, or through flooding (Forstner, 1980;
Sangster et al., 1984). For example, agricultural land adjacent to the
Neckar River, Germany, received dredged sediments to improve the soil,
a practice that produced soil cadmium concentrations in excess of 70
mg/kg (Forstner, 1980).
Much of the cadmium entering fresh waters from industrial sources
is rapidly adsorbed by particulate matter, where it may settle out or
remain suspended, depending on local conditions. This can result in
low concentrations of dissolved cadmium even in rivers that receive
and transport large quantities of the metal (Yamagata & Shigematsu,
1970)
3.5 Concentrations in various biota
Table 5 indicates the levels of cadmium found in various biota
(Eisler, 1985).
Eisler (1985) concluded that there are at least six trends
evident from the abundant residue data available for cadmium.
* Marine organisms generally contain higher cadmium residues than
their freshwater and terrestrial counterparts.
* Cadmium tends to concentrate in the viscera of vertebrates,
especially the liver and kidneys.
* Cadmium concentrations are generally higher in older organisms.
* Higher cadmium residues are generally associated with industrial
and urban sources, although this does not apply to sea birds and
sea mammals.
* Cadmium residues in plants are normally less than 1 mg/kg.
However, plants growing in soil amended with cadmium (e.g., from
sewage sludge) may contain significantly higher levels.
* The species analysed, season of collection, ambient cadmium
levels, and the sex of the organism probably all affect the
residue level.
Table 5. Concentrations of cadmium in biota
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Marine organisms
Algae < 1 to 16
Molluscs soft parts up to 425
kidney up to 547
liver up to 782
digestive gland up to 1163
Crustaceans whole body < 0.4-6.2
Annelids whole body 0.1-3.6
Fish whole body up to 5.2
Birds kidney up to 231
Mammals kidney up to 300
Freshwater organisms
Plants whole plant 0.5-1.8
roots up to 6.7
Molluscs soft parts; fresh weight 0.2-1.4
Annelids whole body; fresh weight 0.5-3.2
Fish whole body; fresh weight 0.01-1.04
Table 5 (contd).
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Terrestrial organisms
Plants whole plant up to 27.1
grain up to 257
Annelids whole body 3-12.6
Birds whole body; fresh weight < 0.05-0.24
kidney; fresh weight up to 7.4
Mammals kidney up to 8.1
3.5.1 Concentrations in fish
May & McKinney (1981) monitored freshwater fish from the USA in
1976 and 1977 and found cadmium concentrations ranging from 0.01 to
1.04 mg/kg (wet weight), the mean being 0.085 mg/kg. This represented
a significant decline from the mean 1972 concentration of 0.112 mg/kg.
The authors pointed out that this decline parallels a decline in
cadmium metal production and consumption over the same period.
Hardisty et al. (1974a) sampled flounder ( Platichthyes flesus)
from the Severn estuary, United Kingdom, and found mean cadmium
concentrations of 3.4-7.3 mg/kg (dry weight). No overall correlation
between cadmium concentration and length or age was observed, although
the largest (27-29 cm) and the oldest („ 5 years) fish gave the
highest mean concentrations. Hardisty et al. (1974b) found a positive
correlation between the cadmium content of a variety of fish species
and the crustacea content of their diet. Lovett et al. (1972) sampled
fish from New York State, USA, and reported mean cadmium
concentrations of < 10-142.7 µg/kg (fresh weight). There was no
relationship between total residues and size, sex or age of lake trout
( Salvelinus namaycush).
3.5.2 Concentrations in sea-birds
Cadmium has been found in a wide variety of birds, and
particularly high levels have been reported in pelagic sea-birds. Much
of the cadmium occurs in the kidney and liver, and relatively little
is transferred to the eggs. A review of the uptake of cadmium and of
the factors that affect it can be found in Scheuhammer (1987).
Interestingly, the concentrations of cadmium in sea-birds are often
higher in areas with little or no contamination from industrial
sources (Bull et al., 1977; Hutton, 1981; Osborn & Nicholson, 1984).
3.5.3 Concentrations in sea mammals
High levels of cadmium have been reported in sea mammals from
areas around the world, which they are assumed to take up from their
diet of fish. Roberts et al. (1976) showed that kidney levels of
cadmium in the common seal off the United Kingdom coast were age
related. Drescher et al. (1977) showed a similar relationship in seals
off the German coast and Hamanaka et al. (1982) in stellar sea lions
off the coast of Japan. Similar trends in dolphins and porpoises have
been reported (Falconer et al., 1983; Honda & Tatsukawa, 1983; Honda
et al., 1986). Muir et al. (1988) sampled white-beaked dolphins
( Lagenorhynchus albirostris) and pilot whales ( Globicephala
melaena) from the coast of Newfoundland, Canada, and reported mean
cadmium levels in kidney (dry weight) of 13.6 mg/kg and 108 mg/kg,
respectively. Cadmium concentrations were age related in pilot whales.
The lower levels found in dolphins were probably related both to
species differences and to the fact that they were all young animals.
3.6 Concentrations adjacent to highways
Muskett & Jones (1980) monitored levels of cadmium adjacent to a
heavily used road. The concentrations in air were highest at a
distance from the road of 0-10 m, and a similar pattern was found in
soil. Cadmium levels in earthworms sampled at known distances from a
highway revealed levels of 12.6 mg/kg (dry weight) within 3 m falling
to 7.1 mg/kg approximately 50 m from the highway. The level in
earthworms from control sites was 3 mg/kg (Gish & Christensen, 1973).
The land snail Cepaea hortensis accumulates cadmium from roadside
verges (Williamson, 1980). The highest concentration of cadmium was
found in the digestive gland (40.3 mg/kg dry weight) and kidney (12.8
mg/kg dry weight). There was little metal in the head and foot, which
make up most of the body tissue. The author showed that age accounted
for 80% of the total variance of soft tissue body burdens. The cadmium
body burdens were found to be effectively immobile, accumulating
progressively with age.
3.7 Concentrations from industrial sources
Burkitt et al. (1972) analysed the cadmium content of ryegrass at
various distances from a zinc smelter and found 50, 10.8, and 1.8
mg/kg dry weight at distances of 0.3, 1.9, and 11.3 km, respectively,
from the smelter.
Teraoka (1989) found that cadmium levels in rice roots were
significantly higher in industrial urban and roadside areas of Japan
compared to sparsely populated areas. The mean level in industrial
areas was 10 mg/kg (dry weight).
Beyer et al. (1985) monitored biota from the vicinity of two zinc
smelters in eastern Pennsylvania, USA. Cadmium concentrations were
highest in carrion insects (25 mg/kg dry weight), followed by fungi
(9.8 mg/kg), leaves (8.1 mg/kg), shrews (7.3 mg/kg), moths (4.9
mg/kg), mice (2.6 mg/kg), songbirds (2.5 mg/kg), and berries (1.2
mg/kg).
Van Hook (1974) sampled soil and earthworms from soil that had
not been disturbed for 30 years and reported mean cadmium levels in
the soils and earthworms of 0.35 and 5.7 mg/kg dry weight,
respectively. Ma et al. (1983) analysed soil and earthworms
( Lumbricus rubellus) at varying distances from a zinc-smelting
plant. Cadmium concentrations ranged from 0.1 to 5.7 mg/kg for the
soil and 20 to 202 mg/kg for the worms, and there was a correlation
between decreasing distance from the smelter and increasing cadmium
levels. Pietz et al. (1984) sampled soil and earthworms ( Aporrectodea
tuberculata) and ( Lumbricus terrestris) from mine soil and
non-mine soil, either amended or not with sewage sludge. Soil and
worms from mine soil gave residues of 0.6 and 3.8 mg/kg dry weight,
respectively, in non-amended soil and 2 and 22 mg/kg in sludge-amended
soil. Residues in soil and worms from non-mined soil were 1 and 12
mg/kg for non-amended and 3.5 and 36 mg/kg for sludge-amended soil,
respectively. The much lower capacity of worms from areas already
contaminated with cadmium to take up the metal suggests some selection
for varieties that control metal uptake. Morgan & Morgan (1988)
sampled earthworms ( Lumbricus rubellus and Dendrodrilus rubidus)
from one uncontaminated site and fifteen metal-contaminated sites (in
the vicinity of disused non-ferrous metalliferous mines) in the United
Kingdom. Cadmium concentrations in the worms ranged from 8 mg/kg (dry
weight) to 1786 mg/kg; they were generally higher than soil levels,
and the total soil cadmium explained 82% to 86% of the variability in
earthworm cadmium concentrations. The authors found some evidence that
cadmium accumulation was suppressed in extremely organic soils.
Martin et al. (1980) reported cadmium levels in a variety of
invertebrates sampled from sites contaminated by airborne cadmium. The
woodlouse was shown to accumulate cadmium principally in the
hepatopancreas.
Van Straalen & van Wensem (1986) analysed 13 species of
arthropods from an area polluted by zinc factory emissions. They found
no effect of body size or trophic level on the cadmium content of the
arthropods.
Roberts & Johnson (1978) sampled invertebrates and their diet
from the area of an abandoned lead-zinc mine in the United Kingdom.
They found cadmium levels higher in herbivorous invertebrates than in
the vegetation on which they fed (but not markedly so). There were
much higher levels of cadmium in carnivorous invertebrates, suggesting
that cadmium might have a capacity for accumulation in food chains.
In contrast to mercury levels, total cadmium body burdens were
higher in sparrows ( Passer domesticus) caught in industrialised
areas of Poland than in those caught in agricultural regions (Pinowska
et al., 1981). Pigeon brain, liver, and kidney sampled in rural,
suburban, and urban areas gave a good indication of the level of
environmental pollution with cadmium (Hutton & Goodman, 1980).
Hunter & Johnson (1982) monitored small mammals near to an
industrial works complex and found that cadmium accumulated
particularly in the liver and kidney. Cadmium levels in the liver
ranged rom 1.5 to 280 mg/kg (dry weight) and in the kidney from 7.4 to
193 mg/kg. Small mammals from unpolluted sites contained liver levels
ranging from 0.5 to 25 mg/kg and kidney levels of 1.5-26 mg/kg. The
insectivorous common shrew ( Sorex areneus) was found to be a more
prominent accumulator of cadmium than omnivorous and herbivorous small
mammals, based on body burden to dietary metal concentration ratios.
Similar results were obtained by Andrews et al. (1984) who monitored
cadmium levels in the herbivorous short-tailed field vole ( Microtus
agrestis) and the insectivorous common shrew ( S. araneus) from a
revegetated metalliferous mine site. Mean cadmium concentrations were
1.84 mg/kg (dry weight) and 52.7 mg/kg for voles and shrews,
respectively, values that were significantly higher than those found
in control sites.
4. KINETICS AND METABOLISM
Appraisal
In aquatic systems, cadmium is most commonly taken up by
organisms directly from water, but may also be ingested with
substantially contaminated food. The free metal ion, Cd2+, is the
form most available to aquatic species. Uptake from water may be
reduced by the concentration of calcium and magnesium salts (water
hardness). Cadmium uptake from sea water may be greatly reduced by
the formation of less available complexes with chloride. Organic
complexes with cadmium can be classified in three groups: those that
are unavailable (e.g., EDTA, NTA, DPTA), those that are available but
less so than the free Cd2+ (e.g., fulvic acids of low relative
molecular mass), and those that form readily available hydrophobic
complexes with cadmium (xanthates and dithiocarbamates).
Organisms in the freshwater environment are contaminated
according to their ability to absorb or adsorb cadmium from the
water, rather than to their position in the food chain. Consequently,
differences in cadmium concentration between species at the same
trophic level are common and there is no evidence for
biomagnification. Conversely, marine organisms take up cadmium
principally from food. The primary source of cadmium in terrestrial
systems is the soil, and uptake follows the typical food chain
pathway, although deposition of cadmium on plant and animal surfaces
can account for some additional contamination at each trophic level.
Variations in uptake and retention occur, and there is some evidence
for biomagnification in carnivores. Organisms that feed on sediment
or detritus may accumulate more cadmium than those in the grazing
food chain. High levels of cadmium have been reported in sea mammals,
pelagic sea-birds, and terrestrial invertebrates.
Within a variety of organisms, cadmium is distributed throughout
most tissues, but tends to accumulate in the roots, gills, livers,
kidneys, hepatopancreas, and exoskeleton. Cadmium in the cell is
often bound to cytoplasmic proteins, a possible detoxifying
mechanism. Elimination probably occurs primarily via the kidney but
also via moulting of the exoskeleton.
There is some evidence of an interaction between cadmium and
other metals, especially calcium and zinc. Cadmium may replace
calcium on the calcium-specific protein calmodulin and is affected by
other physiological processes that regulate the uptake of calcium. In
certain circumstances, zinc increases cadmium retention in the liver
and kidneys of aquatic vertebrates. In terrestrial systems, high soil
zinc levels can reduce cadmium uptake appreciably.
Selection can lead to cadmium-tolerant populations in both the
aquatic and terrestrial environments.
4.1 Uptake
4.1.1 Uptake from water by aquatic organisms
Several studies have shown that the free metal ion, Cd2+, is
the form of cadmium most available to aquatic organisms (Sunda et al.,
1978; Borgmann, 1983; Part et al., 1985; Sprague, 1985).
Inorganic cadmium complexes appear not to be taken up, at least
by fish (Part et al., 1985). This is particularly important in marine
water where cadmium is mainly present in soluble chloride complexes
(Zirino & Yamamoto 1972). It is most probable that chloride
complexation is responsible for the reduced cadmium accumulation and
toxicity in a variety of organisms observed with increasing salinities
(Coombs, 1979).
In the case of organic cadmium complexes, the chemical properties
are of importance with respect to bioavailability. Three categories
can be distinguished. The first comprises cadmium complexes with EDTA,
NTA, and DPTA, which are unavailable to aquatic organisms (Sunda et
al., 1978; Part & Wikmark, 1984). The second consists of complexes
that to some extent contribute to the total metal uptake, i.e. uptake
is higher than predicted from the actual Cd2+ activity, but the
complex is still less available than the free Cd2+ ion. This group
includes fulvic acids of low relative molecular mass (Giesy et al.,
1977; John et al., 1987), the amino acid histidine (Pecon & Powell,
1981), and carboxylic acids like citric acid (Guy & Ross Kean, 1980;
Part & Wikmark, 1984). The third category includes compounds such as
xanthates and dithiocarbamates that form hydrophobic complexes with
heavy metals. These hydrophobic complexes act as metal carriers across
biological membranes and they lead to a greater uptake of cadmium in
aquatic organisms than when the metal is present as the free ion
(Poldoski, 1979; Block & Part, 1986; Gottofrey et al., 1988; Block,
1991). This latter observation is of particular environmental concern
because xanthates are used in the mining industry in the enrichment of
metals from sulfide ores by flotation. Xanthate concentrations of
between 4 and 400 µg/litre have been measured in waters receiving
effluent from metal refineries (enrichment plants) (Waltersson, 1984).
Another water quality parameter affecting cadmium uptake is the
Ca2+ and Mg2+ concentration (hardness) of the water. Increasing
Ca2+ concentration reduces cadmium uptake through fish gills (Part
et al., 1985; Wicklund, 1990), cadmium accumulation (Carroll et al.,
1979), and cadmium toxicity for fish (Calamari et al., 1980). Two
mechanisms can be distinguished for the Ca2+-mediated reduction in
cadmium uptake. The first is an inhibitory effect on uptake into gill
tissue, while the second is related to the adaptive response of the
fish to increased Ca2+ concentrations (Calamari et al., 1980,
Wicklund 1990). Mg2+ also reduces cadmium uptake through fish gills
but at 5 times higher concentrations than Ca2+ (Part et al., 1985).
Cadmium uptake in fish is not strongly pH dependent; uptake in
rainbow trout gills was not affected over the pH range 5-7 (Part et
al., 1985).
Recent data from fish gills indicate that, to some extent, Cd2+
shares uptake mechanisms with Ca2+; these two ions are about the
same size and also form complexes with the same kind of ligands. Thus
Cd2+ can replace Ca2+ in the calcium-specific protein calmodulin
(Flik et al., 1987). In the gills, Cd2+ is assumed to enter the
epithelial cells down its concentration and electrical gradient by
facilitated diffusion through a calcium channel in the apical membrane
(Verbost et al., 1989). Several lines of evidence support this
assumption. Firstly, increasing water Ca2+ concentrations reduce
cadmium uptake. Secondly, cadmium in the water inhibits Ca2+ uptake
in the gills (Verbost et al., 1987; Reid & McDonald, 1988). Thirdly,
La3+, a calcium channel blocker in cell membranes, inhibits both
Ca2+ and Cd2+ uptake in the gills. Fourthly, the hypocalcaemic
hormone stanniocalcin reduces both Ca2+ and Cd2+ uptake in the
gills (Verbost et al., 1989). Stanniocalcin has been shown to close
the apical calcium channel in the gill epithelial cells thereby
reducing Ca2+ uptake from the water (Lafeber et al., 1988). The
hormone is secreted when the fish has a surplus of Ca2+, i.e.
hypercalcaemic. The two-fold effect of Ca2+ on cadmium uptake in
fish discussed previously can be well explained by this model. A
direct competition between Ca2+ and Cd2+ at the apical calcium
channel reduces the uptake of cadmium into the cells, while the
adaptive response in Ca2+-rich water probably involves an increased
stanniocalcin level, which closes the apical calcium/cadmium channel.
The transport mechanism from the epithelial cells to the blood is
unclear. Cadmium is not transported by the high affinity Ca-ATPase in
the basolateral epithelial membrane which transports Ca2+ (Verbost
et al., 1988). The possible involvement of the Na+/Ca2+ exchange
mechanism, where Cd2+ replaces Ca2+, has recently been suggested
as a translocation mechanism to the blood (personal communication to
the IPCS by G. Flik).
Zinc also has been shown to reduce cadmium uptake through the
gills (Wicklund, 1990). Like cadmium, zinc is assumed to enter the
epithelial cell by facilitated diffusion (Spry & Wood, 1989) and,
furthermore, Ca2+ acts antagonistically on zinc uptake.
Taken together, these data suggest that the apical epithelial
membrane of fish gills contains an ion channel shared by cadmium and
calcium, and probably also zinc. The movement of metals through this
channel is controlled both by external factors such as the Ca2+
content of the water and internal factors such as hormones.
Increasing temperature increases the uptake of cadmium from water
(Vernberg et al., 1974; Zaroogian & Cheer, 1976; Denton &
Burdon-Jones, 1981).
4.1.1.1 Microorganisms
In the alga Chlorella pyrenoidosa, uptake of cadmium was
completely blocked by 0.2 mg manganese/litre and inhibited by 2 to 5
mg iron/litre, but calcium, magnesium, molybdenum, copper, zinc, and
cobalt had no effect on uptake (Hart & Scaife, 1977).
Cultures of Chlorella accumulate twice as much cadmium at pH
7.0 as at pH 8.0 when exposed to 0.5 mg cadmium/litre (Hart & Scaife,
1977).
4.1.1.2 Aquatic molluscs
Hardy et al. (1984) found greater uptake of cadmium from sea
water into oysters given an uncontaminated phytoplankton food source
than into those without food. The authors explain their findings on
the basis that the presence of phytoplankton increases the flow of
water through the oysters. Studies on oysters without a food source
may thus underestimate cadmium uptake. Oysters fed phytoplankton
containing cadmium retained only 0.59% of this cadmium; the majority
of the cadmium in molluscs is taken up directly from the water. The
oyster accumulates about twice as much cadmium in summer as in the
winter. This is presumed to reflect the increased flow of water
through the animal at higher temperatures (Zaroogian & Cheer, 1976).
Hardy et al. (1981) showed that clams ( Protothaca staminea)
took up much less cadmium from water in the presence of sediment at
3.6 g/litre. The uptake was only 17% of that measured in sediment-free
water.
Langston & Zhou (1987a,b) found no evidence of cadmium uptake
into the bivalve Macoma balthica involving metallothionein or
metallothionein-like proteins. Accumulation in soft tissues was linear
throughout a 29-day exposure period, whereas uptake onto the shell was
characterized as saturation kinetics. In contrast, the gastropod
Littorina littorea did show induction of specific cadmium-binding
proteins, which contributed to uptake and storage of cadmium.
Watling & Watling (1983) demonstrated uptake of cadmium in a
dose-dependant manner into sandy beach gastropod molluscs in
laboratory experiments. Much of the cadmium (as chloride) accumulated
in the gill. The rate of cadmium uptake was 0.01 mg/kg per day for
Donax serra and 0.16 mg/kg per day for the smaller Bullia
rhodostoma after exposure to cadmium at 20 µg/litre. The freshwater
snail Physa integra took up more cadmium as exposure increased,
concentrations ranging between 1 and 40 µg/litre. The highest
concentration factors were found with the lowest exposure
concentration (Spehar et al., 1978a). Wier & Walter (1976) exposed the
freshwater snail Physa gyrina to 1.3 mg cadmium/litre (as the
chloride) and found an average cadmium uptake rate of 0.55 mg/kg per
hour over 24 h. Heavier snails took up less cadmium, after the same
exposure, than lighter individuals.
4.1.1.3 Other aquatic invertebrates
Rainbow & White (1989) investigated uptake of cadmium and zinc in
three marine crustaceans, Palaemon elegans (Decapoda),
Echinogammarus pirloti (Malacostraca), and Elminius modestus
(Cirripedia) at water concentrations of cadmium between 0.5 and 1000
µg/litre and zinc between 2.5 and 4000 µg/litre. All three crustaceans
accumulated the non-essential cadmium at all dissolved cadmium
concentrations without regulation. Differences between species were
interpreted by the authors in terms of differences in cuticle
permeability and way of life. All three species took up zinc more
rapidly than cadmium; the ratios between molar uptake rates of zinc to
cadmium were 11.4:1, 2.7:1, and 3.7:1 for the three species,
respectively, following an exposure to a molar ratio of 1.7:1.
4.1.1.4 Fish
Cadmium uptake in fish continues for some considerable time in
fish exposed to the metal. The peak of tissue residues may not be
reached for several weeks, particularly after exposure to low
concentrations of the metal (Cearley & Coleman, 1974; Benoit et al.,
1976; Sullivan et al., 1978a).
Douben (1989a) exposed the stone loach Noemacheilus barbatulus
to cadmium in water (as the sulfate) at a concentration of 1 mg/litre
and monitored uptake and loss at different temperatures with fed and
starved fish. The size of the fish affected both uptake and loss of
cadmium, bioconcentration factors decreasing with size. Uptake of
cadmium increased with temperature up to about 16 °C and decreased as
the concentration of cadmium in the water increased. Feeding the fish
increased the rate of uptake of cadmium from the water. The author
concluded that metabolic rate was an important factor in the uptake of
cadmium into the fish and in its subsequent loss.
4.1.1.5 Model aquatic ecosystems
Ferard et al. (1983) investigated the transfer of cadmium through
a model food-chain consisting of an alga, a daphnid, and a fish.
Concentration factors relative to food were low, indicating that
cadmium is mainly taken up directly from water. Daphnids fed algae
containing cadmium at between 4.5 and 570 mg/kg dry weight showed a
maximum concentration factor of 1. Fish fed contaminated daphnids or
algae showed concentration factors of 0.0038 and 0.0018, respectively.
Nimmo et al. (1977) reported low concentration factors, ranging from
0.018 to 0.027, for grass shrimp fed on brine shrimp containing
cadmium at between 27 and 182 mg/kg. Rehwoldt & Karimian-Teherani
(1976) fed zebrafish on food containing cadmium acetate at 10 mg/kg
over a period of 6 months. Maximum residues, in males and females
respectively, were 5.92 and 13.64 mg/kg, the median residue levels
after 6 months of exposure being 5.19 and 12.95 mg/kg (on a dry weight
basis).
4.1.1.6 Uptake from aquatic sediment
Ray et al. (1980b) exposed the ragworm Nereis virens to
sediment to which cadmium chloride had been added. Smaller worms took
up more cadmium relative to body weight than larger worms. The cadmium
was taken up in a dose-related manner and no equilibrium was reached
during the 24-day experiment. The rate of uptake directly from sea
water also increased with exposure concentration over the range of
0.03 to 9.2 mg/litre. For the range of sediment cadmium concentrations
used (1 to 4 mg/kg), the corresponding concentrations in the overlying
sea water were 0.03 to 0.1 mg/litre. Comparing uptake into the
ragworms from water with these concentrations to the uptake from the
spiked sediment produced identical concentrations of cadmium in the
worms. Rate of uptake from sediment was between 16 and 39 times less
than the uptake from the corresponding exposure to cadmium in water.
The authors concluded that all of the uptake of cadmium from sediment
derived from desorbed metal ions in the interstitial water.
4.1.1.7 Uptake from food relative to uptake from water
Fish can take up cadmium from the surrounding water and from
ingested food. The main uptake route in fresh water is from the water
via the gills (Williams & Giesy, 1978). However, the relative
importance of food and water to the body burden depends very much on
the cadmium content of the food organism. In contaminated areas with
an increased cadmium content in food organisms, the relative
importance of food as a cadmium source may increase. In the marine
environment, where cadmium is mainly present in chloride complexes not
available to fish, the relative importance of food as a cadmium source
increases. Consequently food has been shown to be the main cadmium
source in marine fish (Pentreath, 1977; Dallinger et al., 1987).
4.1.2 Uptake by terrestrial organisms
4.1.2.1 Uptake into plants
The uptake of cadmium into plants generally depends upon the
availability of the metal in soil solution. The soil pH and
composition, particularly the nature of soil clays, the organic matter
content, and, obviously, the soil cadmium level, affect this
availability. The relationship between soil cadmium level and plant
uptake is not a simple one because of the wide variety of soil
characteristics that affect the extent of cadmium uptake. Cataldo &
Wildung (1978), Peterson & Alloway (1979), and Page et al. (1981) have
reviewed this subject.
Plants grown in a greenhouse or a container take up more cadmium
than the same plants grown in soil with the same cadmium levels in the
field. This is due to greater root development in a confined volume in
containers and to the fact that all the roots are in contact with
cadmium-contaminated soil. In the field, roots may grow down below the
cadmium-contaminated level (Page & Chang, 1978; De Vries & Tiller,
1978).
Mahler et al. (1978) cultured lettuce and chard on acid or
calcareous soils to which cadmium sulfate had been added at levels up
to 320 mg/kg. For both types of soil there was a dose-related uptake
of cadmium from soil into leaves. The uptake of the metal was much
greater in acid than in calcareous soils, particularly at higher rates
of cadmium application (over 40 mg/kg). At the highest soil
concentration of 320 mg/kg, lettuce leaves contained cadmium at a
concentration of 800 mg/kg and chard leaves 1600 mg/kg when grown in
acid soil. Leaves of lettuce cultured on calcareous soils with cadmium
at 320 mg/kg contained a lower cadmium concentration of 200-300 mg/kg
and chard, similarly cultured, contained 300 mg/kg or less. Bingham et
al. (1980) showed an effect of soil pH on cadmium (as sulfate) uptake
in rice; more metal was incorporated as acidity increased. Chaney et
al. (1975) reported that liming of soil in which soybeans were growing
decreased the concentrations of cadmium in leaves from 33 to 5 mg/kg
dry weight as pH increased from 5.3 to 7.0. Eriksson (1988)
investigated the effect of pH on the uptake of cadmium into perennial
ryegrass ( Lolium perenne) and winter rape ( Brassica napus). The
more soluble fractions of cadmium in soil increased as the pH was
lowered; increasing the pH from 5 to 7 with calcium oxide invariably
reduced the cadmium content of ryegrass plants, but this decrease was
less consistent when the pH was increased from 5 to 6. The cadmium
content of rape plants was markedly higher at pH 4 than pH 5. Adding
more cadmium to the soil increased the amount of cadmium in the plants
in direct proportion to the increased concentration of the metal in
soil over the range 0 to 5 mg/kg. Eriksson (1988) found that soil
organic matter decreased the availability of cadmium to perennial
ryegrass and winter rape grown in pots. Addition of organic material
to sand and clay soils reduced cadmium uptake to a greater extent in
the sand.
When Mitchell & Fretz (1977) cultured seedlings of three species
of tree (red maple, white pine, and Norway spruce) hydroponically or
in soil with added cadmium, the concentration in roots was greater
than that in leaves. Cadmium added to soil was less readily taken up
than cadmium added to nutrient solutions. Similarly, Root et al.
(1975) reported greater cadmium concentration in roots than in shoots
of maize grown hydroponically in a medium containing cadmium chloride.
Harkov et al. (1979) found the highest uptake of cadmium into
hydroponically grown tomatoes in the roots, while stems had lower
cadmium concentrations than leaves.
Lepp et al. (1987) measured high concentrations of cadmium in the
sporophores (fruiting bodies) of the fungus Amanita muscaria growing
in birch woodland. The fungus sporophores contained 29.9 mg/kg dry
weight, compared to a cadmium level of 0.4 mg/kg in the soil on which
they grew. The cadmium was released from the rotting sporophore, after
it had shed its spores, in a form which was readily available to other
plants growing on the woodland soil; this was shown experimentally
with lettuce plants grown in pots. The authors calculated that an
abundant population of sporophores could recycle 1.4% of the total
cadmium load in leaf litter to higher plants over a period of 14 days
(the mean lifespan of the sporophores).
4.1.2.2 Terrestrial invertebrates
Beyer et al. (1982) demonstrated that earthworms concentrated
cadmium from soils amended with sewage sludge containing cadmium
oxide. Cadmium concentrations were as high as 100 mg/kg in worms
exposed to soils containing cadmium at 2 mg/kg, a concentration factor
of 50. Adding calcium carbonate to soils decreased the cadmium uptake
of worms slightly, while high soil zinc levels decreased the cadmium
uptake appreciably. Results were variable with different sludge
treatments. Hartenstein et al. (1980) amended sludge with 10, 50, and
100 mg/kg cadmium (as cadmium sulfate) and added earthworms ( Eisenia
foetida). The worms accumulated 3.9, 2.04, and 1.44 times the
respective sludge levels of cadmium over a period of 5 weeks. In field
trials on non-amended soils containing 12 to 27 mg cadmium/kg, worms
sampled during a 28-week period gave levels of cadmium ranging from 8
to 46 mg/kg.
Terrestrial pulmonate snails retained up to 59% of cadmium
administered in their diet as the chloride (Russell et al., 1981). The
highest retention was after dosing at 25 mg cadmium/kg diet. The
higher the dose (up to 1000 mg/kg diet) the lower the percentage
retention of the metal. Ireland (1981) noted that in the terrestrial
slug Arion ater most of the cadmium was located in the digestive
gland without association with any particular sub-cellular organelles,
and isolated a specific cadmium-binding protein from the animals.
4.1.2.3 Birds
In a study by White & Finley (1978), adult mallard ducks were fed
a diet containing cadmium chloride at levels of 2, 20 or 200 mg/kg and
killed at 30-day intervals. The cadmium content increased with dose
level and time (except in the case of the highest dose where body
burden peaked after 60 days), and the highest concentrations occurred
in the liver and kidney. The highest levels overall occurred after
dosing for 60 days at 200 mg/kg; cadmium concentrations were 109 mg/kg
in the liver and 134 mg/kg in the kidney.
Nicholson & Osborn (1983) dosed starlings ( Sturnus vulgaris)
with cadmium chloride at a concentration of 2 mg/kg body weight, three
times weekly for 6 weeks, and reported a wide range of kidney
concentrations (from < 10 to > 200 mg/kg dry weight).
4.2 Distribution
4.2.1 Aquatic organisms
In higher organisms, cadmium can be bound in several different
tissues, whereas in plants cadmium is bound to the cell wall in roots.
Brooks & Rumsby (1967) measured the cadmium taken up by the
oyster ( Ostrea sinuata) from water containing 115Cd (50 mg/litre).
The soft parts of the oyster contained 100 mg cadmium/kg after 100 h.
Concentrations in tissues were, in decreasing order, 360 mg/kg for
gills, 285 mg/kg for heart, 141 mg/kg for the visceral mass, 83 mg/kg
for the mantle, 53 mg/kg for white muscle, and 25 mg/kg for striated
muscle.
Nimmo et al. (1977) reported that in the pink shrimp the
hepatopancreas took up more cadmium than other tissues. Lower
concentrations were found in the exoskeleton, muscle, and serum.
Short-term exposure of the crab Uca pugilator to cadmium chloride
led to the hepatopancreas and gill concentrations of the metal being
similar after a 24-h exposure to 1 mg cadmium/litre (Vernberg et al.,
1974).
Sangalang & Freeman (1979) determined the cadmium in tissues of
brook trout exposed to the metal (added as the chloride) via the water
or by injection. After water exposure to cadmium chloride at 1
µg/litre, the trout showed greatest uptake of the metal in the gills,
kidney, and liver. The gills and the posterior kidney revealed a
higher metal content than any other tissues. Levels of cadmium in
whole blood and plasma, heart, spleen, testis, stomach, and skin were
higher than control levels after 77 and 93 days of exposure. Smith et
al. (1976) found the greatest accumulation of the metal in the kidney
of catfish exposed to cadmium (as sulfate) in the water. In an
autoradiographic study of cadmium distribution in rainbow trout
exposed to cadmium in water, Tjalve et al. (1986) confirmed the
general picture of cadmium distribution, the metal being found in the
gills, liver, and kidney. However, they also observed heavy labelling
of the olfactory rosette and the olfactory nerve, an observation not
reported earlier. In a detailed study they later showed that cadmium
was transported axonally from the olfactory rosette to the bulbus
olfactorius but not further into the brain (Gottofrey, 1990). The
significance of this observation with respect to the olfactory
responses of fish in cadmium-contaminated environments remains to be
investigated.
The few studies that have been conducted on the subcellular
distribution of cadmium indicate that, while much is located in the
cytosol, a significant proportion can be found in the nucleus and the
mitochondria. Cadmium is bound in the cytosol to proteins of low
relative molecular mass, metallothioneins, and other cadmium-binding
proteins. These proteins are rich in the sulfur-containing amino acid
cysteine but poor in aromatic amino acids.
Metallothioneins have been isolated and characterized in a number
of aquatic and terrestrial organisms. Fish metallothioneins have
received considerable interest in recent years as tools in monitoring
metal pollution in the environment (Hamilton & Mehrle, 1986; Hogstrand
& Haux, 1990a). Simple methods to analyse fish metallothionein have
been developed, including differential pulse polarography (Olson &
Haux, 1986) and radioimmunoassay based on specific antibodies to fish
metallothionein (Hogstrand & Haux, 1990b). Olson & Haux (1986) found
a strong correlation between hepatic metallothionein and cadmium
accumulation in perch collected from cadmium-contaminated water.
4.2.2 Terrestrial organisms
4.2.2.1 Terrestrial plants
Jones & Johnston (1989) analysed cereal grain and herbage from
long-term experimental plots at Rothamsted, United Kingdom, and found
that uptake of cadmium into herbage was greatest where phosphate
fertilizer had been applied. It was also greater from unlimed soils
than from limed soils. However, the authors concluded that there was
little evidence of a long-term (1840-1986) increase in crop cadmium
concentrations.
Byrne et al. (1976) analysed higher fungi from Slovenia,
Yugoslavia, and found levels of cadmium ranging from 0.53 to 39.9
mg/kg dry weight (average 5.0 mg/kg). This is an order of magnitude
higher than in most other plants. Although the fungi were collected
from industrial, urban, and uncontaminated sites, the levels found in
the fungi were not very different between sites. The authors suggested
geological rather than industrial sources for the cadmium in these
soils.
The high uptake by mushrooms and related species is probably due
to a cadmium-binding phosphoglycoprotein, cadmium-myco-phosphatin,
which has been isolated from the mushroom Agaricus macrosporus
(Meisch & Schmitt, 1986).
4.2.2.2 Terrestrial invertebrates
Hopkin & Martin (1985) investigated the storage of cadmium in the
woodlouse Oniscus asellus from heavily contaminated woodland 3 km
downwind from a smelter. The hepatopancreas was found to contain up to
5 g cadmium/kg dry weight without apparent ill effects upon the
organism. Cadmium was reported to be stored intracellularly in the
copper- and sulfur-containing granules of epithelial S cells. In a
later study (Hopkin, 1990) it was found that considerable interspecies
differences exist with regard to storage in the hepatopancreas.
Oniscus asellus stored five times more cadmium than Porcello scaber
under the same conditions. The carnivorous centipede Lithobius
variegatus, when fed on cadmium-contaminated hepatopancreas from
woodlice, accumulated cadmium which was likewise stored in the midgut
(Hopkin & Martin, 1984).
Berger & Dallinger (1989) studied the distribution of cadmium
between several organs of the terrestrial snail Arianta arbustorum
during a 20-day feeding experiment on cadmium-enriched agar. Of the
cadmium in the medium, 54% was taken up, of which 66% was distributed
to the hepatopancreas, leading to a concentration of more than 500
mg/kg dry weight. In other organs (intestine, foot/mantle, gonads),
the cadmium concentration was considerably lower.
In the earthworm Lumbricus rubellus taken from heavy-metal-
polluted soil, more than 70% of the cadmium burden was found in the
posterior alimentary canal (Morgan & Morgan, 1990). This distribution
prevented dissemination of large concentrations of cadmium into other
tissues and, according to the authors, may represent a detoxification
strategy.
4.3 Elimination
Information on loss of cadmium from organisms is relatively
scarce. The information that does exist suggests that this is very
variable, and has been reviewed by Coombs (1979) and Taylor (1983).
Organisms that accumulate cadmium also tend to retain the metal for
long periods. The main excretory route appears to be via the kidney,
except in the case of organisms that moult, where loss from the shed
exoskeleton can be significant.
Robinson & Wells (1975) administered a single oral dose of
cadmium acetate to softshell turtles ( Trionyx spinifer) and killed
and dissected the animals either 48 h or 96 h later. After 48 h, 9.43%
of the total dose was recovered from tissues, while turtles killed
after 96 h had retained 4.02% of the dose. The greatest retention of
cadmium, after both time periods, was in the liver. Cadmium was also
retained in the small intestine for the first 48 h, but the amount had
decreased by 96 h.
Harrison & Klaverkamp (1989) exposed rainbow trout ( Salmo
gairdneri) and lake whitefish (Coregonus clupeaformis) to cadmium in
water, via a continuous-flow system, or the diet, via pelleted food,
for 72 days. The fish were then kept in clean water on a cadmium-free
diet for a further 56 days. In the case of water-exposed fish, the
majority of the cadmium was present in the gill and kidney, but
food-exposed fish retained cadmium principally in the kidney, gut, and
liver. Bioconcentration factors for exposure via the water were 55 for
the trout and 42 for the whitefish, whereas concentration factors from
the food were less than 1 for both species. However, both species
accumulated a greater proportion of the cadmium that was in the food
than that in water (1% as against 0.1%). Equilibrium bioconcentration
factors were estimated to be 161 for trout and 51 for whitefish.
In the same model, the half-times for depuration of accumulated
cadmium ranged from 24 to 63 days. Douben (1989b) investigated the
kinetics of cadmium in freshwater fish (the stone loach Noemacheilus
barbatulus) exposed to cadmium via the diet (tubifex worms
previously contaminated with cadmium by uptake from water). The body
burden of cadmium declined after the period of feeding with
contaminated diet more rapidly in starved than in fed fish. Rate
constants for loss of cadmium appeared to be greater during the
exposure period than after exposure. Both uptake and loss of cadmium
were influenced by the body weight of the fish.
Janssen et al. (1991) investigated uptake and loss of cadmium
from contaminated soil by four species of soil arthropod and developed
kinetic models that gave good predictions of the degree of
accumulation in a variety of species. They also reviewed data on other
soil arthropods (Tables 6 and 7). The kinetics of cadmium in different
arthropods is related to taxonomy and reflects the different
physiological characteristics of the different organisms. Some,
notably isopods and molluscs, take up and retain cadmium in their
tissues with little or no excretion. These species are capable of
holding large quantities of the metal in the hepatopancreas without
apparent ill effect. There is no direct correlation between
assimilation capacity and the capacity to excrete or eliminate
cadmium. Figure 4 illustrates the uptake of cadmium (measured as total
body burden) and its subsequent loss in four species of arthropods.
Elimination half-lives of 53, 8, and 2 days, respectively, have been
reported for Platynothrus peltifer, Orchesella cincta, and
Notiophilus biguttatus; no elimination took place over 130 days in
Neobisium muscorum.
Sawicka-Kapusta et al. (1987) investigated the effect of keeping
the vole Clethrionomys glareolus at different temperatures on the
rate of loss of cadmium from body tissues. Although the different
temperatures (10 °C and 20 °C) affected the metabolic rate of the
voles, there was no difference in the rate of loss of cadmium.
4.4 Bioaccumulation and biomagnification
Bioaccumulation occurs when the concentration in the organism
exceeds the concentration in the nutrient medium and is expressed
quantitatively as a bioconcentration factor. Progressive
bioaccumulation at each trophic level is termed biomagnification.
2.2 Analytical procedures
The following is an outline of the analytical procedure for
cadmium; further information is given in Environmental Health Criteria
134: Cadmium (WHO, 1992).
2.2.1 Sampling and preparation
Only a few nanograms, or even less, of cadmium may be present in
collected samples of air or water, whereas hundreds of micrograms may
be present in small samples of kidney, sewage sludge, and plastics.
Different techniques are, therefore, required for the collection,
preparation, and analysis of the samples.
In general, the techniques available for measuring cadmium in the
environment and biological materials cannot differentiate between
cadmium species. With special separation techniques,
cadmium-containing proteins can be isolated and identified. In most
studies, the concentration or amount of cadmium in water, air, soil,
plants, and other environmental or biological material is determined
as the element.
Standard trace element methods can generally be used for the
collection of samples (LaFleur, 1976; Behne, 1980). During the
handling and storage of samples, particularly liquid samples, special
care must be taken to avoid contamination; coloured materials in
containers, especially plastics and rubber, should be avoided. Glass
and transparent, cadmium-free polyethylene, polypropylene or teflon
containers are usually considered suitable for storing samples. All
containers and glassware should be precleaned in dilute nitric acid
and deionised water. In order to avoid possible adsorption of cadmium
onto the container wall, water samples or standards with low cadmium
concentrations should not be stored for long periods of time.
To prepare samples for analysis, inorganic solid samples (such as
soil or dust samples) are usually dissolved in an acid, e.g., nitric
acid. Organic samples need to be subjected to wet ashing (digested) or
dry ashing. When the cadmium concentration is low, special treatment
is sometimes needed. The procedures for separating cadmium from
interfering compounds and concentrating the samples are very important
steps in obtaining accurate results.
2.2.2 Quantitative instrumental methods
The most commonly used methods, at present, are atomic absorption
spectrometry, electrochemical methods, neutron activation analysis,
atomic emission spectrometry, atomic fluorescence spectrometry and
proton-induced X-ray emissions (PIXE) analysis. Analytical methods for
cadmium have been reviewed by Friberg et al. (1986). Detection limits
of some of the methods are given in Table 3.
Table 3. Analytical procedures a
Method Detection limit Matrix
Atomic absorption 1 to 5 mg/litre water
spectrometry
0.1 mg/kg biological samples
electrothermal a few pg
atomization
Electrochemical method
(potentiometric stripping
analysis) 0.1 mg/litre urine
Neutron activation 0.1 to 1 mg/litre biological
analysis samples/fluids
X-ray atomic 17 mg/kg biological samples
fluorescence
a From: Friberg et al. (1986)
3. NATURAL OCCURRENCE AND SOURCES OF ENVIRONMENTAL CONTAMINATION
3.1 Natural occurrence
A comparison of natural and anthropogenic sources of trace metals
is given in the Appendix 1.
Cadmium is widely distributed in the earth's crust at an average
concentration of about 0.1 mg/kg and is commonly found in association
with zinc. However, higher levels are present in sedimentary rocks:
marine phosphates often contain about 15 mg/kg (GESAMP, 1984).
Weathering and erosion result in the transport by rivers of large
quantities of cadmium to the world's oceans and this represents a
major flux of the global cadmium cycle; an annual gross input of 15
000 tonnes has been estimated (GESAMP, 1987).
In background areas away from ore bodies, surface soil
concentrations of cadmium typically range between 0.1 and 0.4 mg/kg
(Page et al., 1981). The median cadmium concentration in non-volcanic
soil ranges from 0.01 to 1 mg/kg, but in volcanic soil levels of up to
4.5 mg/kg have been found (Korte, 1983).
Volcanic activity is a major natural source of atmospheric
cadmium release. The global annual flux from this source has been
estimated to be 100-500 tonnes (Nriagu, 1979). Deep sea volcanism is
also a source of environmental cadmium release, but the role of this
process in the global cadmium cycle remains to be quantified.
The average cadmium content of sea water is about 0.1 µg/litre or
less (Korte, 1983), while river water (Mississippi, Yangtze, Amazon,
and Orinoco sampled between 1976 and 1982) contains dissolved cadmium
at concentrations of < 1.1-13.5 ng/litre (Shiller & Boyle, 1987).
Cadmium levels of up to 5 mg/kg have been reported in river and lake
sediments and from 0.03 to 1 mg/kg in marine sediments (Korte,1983).
Current measurements of dissolved cadmium in surface waters of
the open oceans give values of < 5 ng/litre. The vertical
distribution of dissolved cadmium in ocean waters is characterized by
a surface depletion and deep water enrichment, which corresponds to
the pattern of nutrient concentrations in these areas (Boyle et al.,
1976). This distribution is considered to result from the absorption
of cadmium by phytoplankton in surface waters and its transport to the
depths, incorporation to biological debris, and subsequent release. In
contrast, cadmium is enriched in the surface waters of areas of
upwelling and this also leads to elevated levels in plankton
unconnected with human activity (Martin & Broenkow, 1975; Boyle et
al., 1976). Oceanic sediments underlying these areas of high
productivity can contain markedly elevated cadmium levels as a result
of inputs associated with biological debris (Simpson, 1981).
In remote, uninhabited areas, cadmium concentrations in air are
usually less than 1 ng/m3 (Korte,1983).
3.2 Industrial uses
The principal applications of cadmium fall into five categories:
protective plating on steel; stabilizers for PVC; pigments in plastics
and glass; electrode material in nickel-cadmium batteries; and as a
component of various alloys (Wilson, 1988).
The relative importance of the major applications has changed
considerably over the last 25 years. The use of cadmium for
electroplating represented in 1960 over half the cadmium consumed
worldwide, but in 1985 its share was less than 25% (Wilson, 1988).
This decline is usually linked to the introduction of stringent
effluent limits from plating works and, more recently, to the
introduction of general restrictions on cadmium consumption in certain
countries. In contrast, the use of cadmium in batteries has shown
considerable growth in recent years from only 8% of the total market
in 1970 to 37% by 1985. The use of cadmium in batteries is
particularly important in Japan and represented over 75% of the total
consumption in 1985 (Wilson, 1988).
Pigments and stabilizers accounted for 22% and 12% of the total
world consumption in 1985. The share of the market by cadmium pigments
remained relatively stable between 1970 and 1985 but the use of the
metal in stabilizers during this period showed a considerable decline,
largely as a result of economic factors. The use of cadmium as a
constituent of alloys is relatively small and has also declined in
importance in recent years, accounting for about 4% of total cadmium
use in 1985 (Wilson, 1988).
3.3 Sources of environmental cadmium
3.3.1 Sources of atmospheric cadmium
Estimates of cadmium emissions to the atmosphere from human and
natural sources have been carried out at the worldwide, regional, and
national level; examples of such inventories are shown in Table 4.
The median global total emission of the metal from human sources
in 1983 was 7570 tonnes (Nriagu & Pacyna, 1988) and represented about
half the total quantity of cadmium produced in the same year. In both
the European Economic Community (EEC) and on a worldwide scale
(Nriagu, 1989), about 10-15% of total airborne cadmium emissions arise
from natural processes, the major source being volcanic action.
Municipal refuse contains cadmium derived from discarded
nickel-cadmium batteries and plastics containing cadmium pigments and
stabilizers. The incineration of refuse is a major source of
atmospheric cadmium release at country, regional, and worldwide level
(Table 4).
Steel production can also be considered as a waste-related
source, as large quantities of cadmium-plated steel scrap are recycled
by this industry. As a result, steel production is responsible for
considerable emissions of atmospheric cadmium.
3.3.2 Sources of aquatic cadmium
Non-ferrous metal mines represent a major source of cadmium
release to the aquatic environment. Contamination can arise from mine
drainage water, waste water from the processing of ores, overflow from
the tailings pond, and rainwater run-off from the general mine area.
The release of these effluents to local watercourses can lead to
extensive contamination downstream of the mining operation. Mines
disused for many years can still be responsible for the continuing
contamination of adjacent watercourses (Johnson & Eaton, 1980).
At the global level, the smelting of non-ferrous metal ores has
been estimated to be the largest human source of cadmium release to
the aquatic environment (Nriagu & Pacyna, 1988). Discharges to fresh
and coastal waters arise from liquid effluents produced by air
pollution control (gas scrubbing) together with the site drainage
waters.
Table 4. Estimates of atmospheric cadmium emissions (tonnes/year) on a national, regional and worldwide basis
Source United EEC b Worldwide c
Kingdom a
Natural sources ND 20 150-2600 d
Non-ferrous metal
production
mining ND ND 0.6-3
zinc and cadmium 20 920-4600
copper 3.7 6 1700-3400
lead 7 39-195
Secondary production ND 2.3-3.6
Production of cadmium-containing
substances ND 3 ND
Iron and steel production 2.3 34 28-284
Fossil fuel combustion
coal 1.9 6 176-882
oil 0.5 41-246
Refuse incineration 5 31 56-1400
Sewage sludge incineration 0.2 2 3-36
Table 4 (contd).
Source United EEC b Worldwide c
Kingdom a
Phosphate fertilizer manufacture ND ND 68-274
Cement manufacture 1 ND 8.9-534
Wood combustion ND ND 60-180
TOTAL EMISSIONS 14 130 3350-14 640
a From: Hutton & Symon (1986); data apply to 1982-1983
b From: Hutton (1983); data apply to 1979-1980 (the EEC consisted,
at that time, of Belgium, Denmark, Federal Republic of Germany,
Italy, Luxembourg, The Netherlands, Republic of Ireland, and the
United Kingdom)
c From: Nriagu & Pacyna (1988); data apply to 1983
d From: Nriagu (1979)
ND Not determined
The manufacture of phosphate fertilizer results in a
redistribution of the cadmium present in the rock phosphates between
the phosphoric acid product and gypsum waste. In many cases, the
gypsum is disposed of by dumping in coastal waters, which leads to
considerable cadmium inputs. Some countries, however, recover the
gypsum for use as a construction material and thus have negligible
cadmium discharges (Hutton, 1982).
The atmospheric fallout of cadmium to fresh and marine waters
represents a major input of cadmium at the global level (Nriagu &
Pacyna, 1988). A GESAMP study of the Mediterranean Sea indicated that
this source is comparable in magnitude to the total river inputs of
cadmium to the region (GESAMP, 1985). Similarly, large cadmium inputs
to the North Sea (110-430 tonnes/year) have also been estimated, based
on the extrapolation from measurements of cadmium deposition along the
coast (van Alst et al., 1983a,b). However, another approach based on
model simulation yielded a modest annual cadmium input of 14 tonnes
(Krell & Roeckner, 1988).
Acidification of soils and lakes may result in enhanced
mobilization of cadmium from soils and sediments and lead to increased
levels in surface and ground waters (WHO Working Group, 1986).
3.3.3 Sources of terrestrial cadmium
Solid wastes are disposed of in landfill sites, resulting in
large cadmium inputs at the national and regional levels when
expressed as total tonnage (Hutton, 1982; Hutton & Symon, 1986).
Sources include the ashes from fossil fuel combustion, waste from
cement manufacture, and the disposal of municipal refuse and sewage
sludge.
Of greater potential environmental significance are the solid
wastes from both non-ferrous metal production and the manufacture of
cadmium-containing articles, as well as the ash residues from refuse
incineration. These three waste materials are characterized by
elevated cadmium levels and as such require disposal to controlled
sites to prevent the contamination of the ground water.
The agricultural application of phosphate fertilizers represents
a direct input of cadmium to arable soils. The cadmium content of
phosphate fertilizers varies widely and depends on the origin of the
rock phosphate. It has been estimated that fertilizers of West African
origin contain 160-255 g cadmium/tonne of phosphorus pentoxide, while
those derived from the southeastern USA contain 35 g/tonne (Hutton,
1982).
The annual rate of cadmium input to arable land from phosphate
fertilizers has been estimated at 5 g/ha for the countries of the EEC
(Hutton 1982). This only represents about 1% of the surface soil
cadmium burden. Despite the relatively small size of this input,
long-term continuous application of phosphate fertilizers has been
shown to cause increased soil cadmium concentrations (Williams &
David, 1973, 1976; Andersson & Hahlin, 1981).
The application of municipal sewage sludge to agricultural soils
as a fertilizer can also be a significant source of cadmium; a value
of 80 g/ha has been estimated for the United Kingdom (Hutton & Symon,
1986). On a national or regional basis, however, these inputs are much
smaller than those from either phosphate fertilizers or atmospheric
deposition (see section 3.4).
Polluted soils can contain cadmium levels of up to 57 mg/kg (dry
weight) resulting from sludge applied to soil and up to 160 mg/kg in
the vicinity of metal-processing industry (Fleischer et al., 1974).
The highest cadmium levels reported appear to be from ancient mining
areas with levels of up to 468 mg/kg.
3.4 Environmental transport and distribution
3.4.1 Atmospheric deposition
Cadmium is removed from the atmosphere by dry deposition and by
precipitation. In rural areas of Scandinavia, annual deposition rates
of 0.4-0.9 g/ha have been measured (Laamanen, 1972; Andersson, 1977).
Similarly, in a rural region of Tennessee, USA, a deposition rate of
0.9 g/ha was observed (Lindberg et al., 1982). Hutton (1982) suggested
that 3 g/ha per year was a representative value for the atmospheric
deposition of cadmium to agricultural soils in rural areas of the EEC.
The corresponding input for these areas from the application of
phosphate fertilizers is 5 g/ha per year (see section 3.3).
Many industrial sources of cadmium possess tall stacks which
bring about the wide dispersion and dilution of particulate emissions.
Nevertheless, cadmium deposition rates around smelter facilities are
often markedly elevated nearest the source and generally decrease
rapidly with distance (Hirata, 1981). Soil cadmium concentrations in
excess of 100 mg/kg are commonly encountered close to long established
smelters (Buchauer, 1972).
Crop plants growing near to atmospheric sources of cadmium may
contain elevated cadmium levels (Carvalho et al., 1986). However, it
is not always possible to distinguish whether the cadmium is derived
directly from surface deposition or originates from root uptake, since
soil levels in such areas are generally higher than normal.
3.4.2 Transport from water to soil
Rivers contaminated with cadmium can contaminate surrounding
land, either through irrigation for agricultural purposes, by the
dumping of dredged sediments, or through flooding (Forstner, 1980;
Sangster et al., 1984). For example, agricultural land adjacent to the
Neckar River, Germany, received dredged sediments to improve the soil,
a practice that produced soil cadmium concentrations in excess of 70
mg/kg (Forstner, 1980).
Much of the cadmium entering fresh waters from industrial sources
is rapidly adsorbed by particulate matter, where it may settle out or
remain suspended, depending on local conditions. This can result in
low concentrations of dissolved cadmium even in rivers that receive
and transport large quantities of the metal (Yamagata & Shigematsu,
1970)
3.5 Concentrations in various biota
Table 5 indicates the levels of cadmium found in various biota
(Eisler, 1985).
Eisler (1985) concluded that there are at least six trends
evident from the abundant residue data available for cadmium.
* Marine organisms generally contain higher cadmium residues than
their freshwater and terrestrial counterparts.
* Cadmium tends to concentrate in the viscera of vertebrates,
especially the liver and kidneys.
* Cadmium concentrations are generally higher in older organisms.
* Higher cadmium residues are generally associated with industrial
and urban sources, although this does not apply to sea birds and
sea mammals.
* Cadmium residues in plants are normally less than 1 mg/kg.
However, plants growing in soil amended with cadmium (e.g., from
sewage sludge) may contain significantly higher levels.
* The species analysed, season of collection, ambient cadmium
levels, and the sex of the organism probably all affect the
residue level.
Table 5. Concentrations of cadmium in biota
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Marine organisms
Algae < 1 to 16
Molluscs soft parts up to 425
kidney up to 547
liver up to 782
digestive gland up to 1163
Crustaceans whole body < 0.4-6.2
Annelids whole body 0.1-3.6
Fish whole body up to 5.2
Birds kidney up to 231
Mammals kidney up to 300
Freshwater organisms
Plants whole plant 0.5-1.8
roots up to 6.7
Molluscs soft parts; fresh weight 0.2-1.4
Annelids whole body; fresh weight 0.5-3.2
Fish whole body; fresh weight 0.01-1.04
Table 5 (contd).
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Terrestrial organisms
Plants whole plant up to 27.1
grain up to 257
Annelids whole body 3-12.6
Birds whole body; fresh weight < 0.05-0.24
kidney; fresh weight up to 7.4
Mammals kidney up to 8.1
3.5.1 Concentrations in fish
May & McKinney (1981) monitored freshwater fish from the USA in
1976 and 1977 and found cadmium concentrations ranging from 0.01 to
1.04 mg/kg (wet weight), the mean being 0.085 mg/kg. This represented
a significant decline from the mean 1972 concentration of 0.112 mg/kg.
The authors pointed out that this decline parallels a decline in
cadmium metal production and consumption over the same period.
Hardisty et al. (1974a) sampled flounder ( Platichthyes flesus)
from the Severn estuary, United Kingdom, and found mean cadmium
concentrations of 3.4-7.3 mg/kg (dry weight). No overall correlation
between cadmium concentration and length or age was observed, although
the largest (27-29 cm) and the oldest („ 5 years) fish gave the
highest mean concentrations. Hardisty et al. (1974b) found a positive
correlation between the cadmium content of a variety of fish species
and the crustacea content of their diet. Lovett et al. (1972) sampled
fish from New York State, USA, and reported mean cadmium
concentrations of < 10-142.7 µg/kg (fresh weight). There was no
relationship between total residues and size, sex or age of lake trout
( Salvelinus namaycush).
3.5.2 Concentrations in sea-birds
Cadmium has been found in a wide variety of birds, and
particularly high levels have been reported in pelagic sea-birds. Much
of the cadmium occurs in the kidney and liver, and relatively little
is transferred to the eggs. A review of the uptake of cadmium and of
the factors that affect it can be found in Scheuhammer (1987).
Interestingly, the concentrations of cadmium in sea-birds are often
higher in areas with little or no contamination from industrial
sources (Bull et al., 1977; Hutton, 1981; Osborn & Nicholson, 1984).
3.5.3 Concentrations in sea mammals
High levels of cadmium have been reported in sea mammals from
areas around the world, which they are assumed to take up from their
diet of fish. Roberts et al. (1976) showed that kidney levels of
cadmium in the common seal off the United Kingdom coast were age
related. Drescher et al. (1977) showed a similar relationship in seals
off the German coast and Hamanaka et al. (1982) in stellar sea lions
off the coast of Japan. Similar trends in dolphins and porpoises have
been reported (Falconer et al., 1983; Honda & Tatsukawa, 1983; Honda
et al., 1986). Muir et al. (1988) sampled white-beaked dolphins
( Lagenorhynchus albirostris) and pilot whales ( Globicephala
melaena) from the coast of Newfoundland, Canada, and reported mean
cadmium levels in kidney (dry weight) of 13.6 mg/kg and 108 mg/kg,
respectively. Cadmium concentrations were age related in pilot whales.
The lower levels found in dolphins were probably related both to
species differences and to the fact that they were all young animals.
3.6 Concentrations adjacent to highways
Muskett & Jones (1980) monitored levels of cadmium adjacent to a
heavily used road. The concentrations in air were highest at a
distance from the road of 0-10 m, and a similar pattern was found in
soil. Cadmium levels in earthworms sampled at known distances from a
highway revealed levels of 12.6 mg/kg (dry weight) within 3 m falling
to 7.1 mg/kg approximately 50 m from the highway. The level in
earthworms from control sites was 3 mg/kg (Gish & Christensen, 1973).
The land snail Cepaea hortensis accumulates cadmium from roadside
verges (Williamson, 1980). The highest concentration of cadmium was
found in the digestive gland (40.3 mg/kg dry weight) and kidney (12.8
mg/kg dry weight). There was little metal in the head and foot, which
make up most of the body tissue. The author showed that age accounted
for 80% of the total variance of soft tissue body burdens. The cadmium
body burdens were found to be effectively immobile, accumulating
progressively with age.
3.7 Concentrations from industrial sources
Burkitt et al. (1972) analysed the cadmium content of ryegrass at
various distances from a zinc smelter and found 50, 10.8, and 1.8
mg/kg dry weight at distances of 0.3, 1.9, and 11.3 km, respectively,
from the smelter.
Teraoka (1989) found that cadmium levels in rice roots were
significantly higher in industrial urban and roadside areas of Japan
compared to sparsely populated areas. The mean level in industrial
areas was 10 mg/kg (dry weight).
Beyer et al. (1985) monitored biota from the vicinity of two zinc
smelters in eastern Pennsylvania, USA. Cadmium concentrations were
highest in carrion insects (25 mg/kg dry weight), followed by fungi
(9.8 mg/kg), leaves (8.1 mg/kg), shrews (7.3 mg/kg), moths (4.9
mg/kg), mice (2.6 mg/kg), songbirds (2.5 mg/kg), and berries (1.2
mg/kg).
Van Hook (1974) sampled soil and earthworms from soil that had
not been disturbed for 30 years and reported mean cadmium levels in
the soils and earthworms of 0.35 and 5.7 mg/kg dry weight,
respectively. Ma et al. (1983) analysed soil and earthworms
( Lumbricus rubellus) at varying distances from a zinc-smelting
plant. Cadmium concentrations ranged from 0.1 to 5.7 mg/kg for the
soil and 20 to 202 mg/kg for the worms, and there was a correlation
between decreasing distance from the smelter and increasing cadmium
levels. Pietz et al. (1984) sampled soil and earthworms ( Aporrectodea
tuberculata) and ( Lumbricus terrestris) from mine soil and
non-mine soil, either amended or not with sewage sludge. Soil and
worms from mine soil gave residues of 0.6 and 3.8 mg/kg dry weight,
respectively, in non-amended soil and 2 and 22 mg/kg in sludge-amended
soil. Residues in soil and worms from non-mined soil were 1 and 12
mg/kg for non-amended and 3.5 and 36 mg/kg for sludge-amended soil,
respectively. The much lower capacity of worms from areas already
contaminated with cadmium to take up the metal suggests some selection
for varieties that control metal uptake. Morgan & Morgan (1988)
sampled earthworms ( Lumbricus rubellus and Dendrodrilus rubidus)
from one uncontaminated site and fifteen metal-contaminated sites (in
the vicinity of disused non-ferrous metalliferous mines) in the United
Kingdom. Cadmium concentrations in the worms ranged from 8 mg/kg (dry
weight) to 1786 mg/kg; they were generally higher than soil levels,
and the total soil cadmium explained 82% to 86% of the variability in
earthworm cadmium concentrations. The authors found some evidence that
cadmium accumulation was suppressed in extremely organic soils.
Martin et al. (1980) reported cadmium levels in a variety of
invertebrates sampled from sites contaminated by airborne cadmium. The
woodlouse was shown to accumulate cadmium principally in the
hepatopancreas.
Van Straalen & van Wensem (1986) analysed 13 species of
arthropods from an area polluted by zinc factory emissions. They found
no effect of body size or trophic level on the cadmium content of the
arthropods.
Roberts & Johnson (1978) sampled invertebrates and their diet
from the area of an abandoned lead-zinc mine in the United Kingdom.
They found cadmium levels higher in herbivorous invertebrates than in
the vegetation on which they fed (but not markedly so). There were
much higher levels of cadmium in carnivorous invertebrates, suggesting
that cadmium might have a capacity for accumulation in food chains.
In contrast to mercury levels, total cadmium body burdens were
higher in sparrows ( Passer domesticus) caught in industrialised
areas of Poland than in those caught in agricultural regions (Pinowska
et al., 1981). Pigeon brain, liver, and kidney sampled in rural,
suburban, and urban areas gave a good indication of the level of
environmental pollution with cadmium (Hutton & Goodman, 1980).
Hunter & Johnson (1982) monitored small mammals near to an
industrial works complex and found that cadmium accumulated
particularly in the liver and kidney. Cadmium levels in the liver
ranged rom 1.5 to 280 mg/kg (dry weight) and in the kidney from 7.4 to
193 mg/kg. Small mammals from unpolluted sites contained liver levels
ranging from 0.5 to 25 mg/kg and kidney levels of 1.5-26 mg/kg. The
insectivorous common shrew ( Sorex areneus) was found to be a more
prominent accumulator of cadmium than omnivorous and herbivorous small
mammals, based on body burden to dietary metal concentration ratios.
Similar results were obtained by Andrews et al. (1984) who monitored
cadmium levels in the herbivorous short-tailed field vole ( Microtus
agrestis) and the insectivorous common shrew ( S. araneus) from a
revegetated metalliferous mine site. Mean cadmium concentrations were
1.84 mg/kg (dry weight) and 52.7 mg/kg for voles and shrews,
respectively, values that were significantly higher than those found
in control sites.
4. KINETICS AND METABOLISM
Appraisal
In aquatic systems, cadmium is most commonly taken up by
organisms directly from water, but may also be ingested with
substantially contaminated food. The free metal ion, Cd2+, is the
form most available to aquatic species. Uptake from water may be
reduced by the concentration of calcium and magnesium salts (water
hardness). Cadmium uptake from sea water may be greatly reduced by
the formation of less available complexes with chloride. Organic
complexes with cadmium can be classified in three groups: those that
are unavailable (e.g., EDTA, NTA, DPTA), those that are available but
less so than the free Cd2+ (e.g., fulvic acids of low relative
molecular mass), and those that form readily available hydrophobic
complexes with cadmium (xanthates and dithiocarbamates).
Organisms in the freshwater environment are contaminated
according to their ability to absorb or adsorb cadmium from the
water, rather than to their position in the food chain. Consequently,
differences in cadmium concentration between species at the same
trophic level are common and there is no evidence for
biomagnification. Conversely, marine organisms take up cadmium
principally from food. The primary source of cadmium in terrestrial
systems is the soil, and uptake follows the typical food chain
pathway, although deposition of cadmium on plant and animal surfaces
can account for some additional contamination at each trophic level.
Variations in uptake and retention occur, and there is some evidence
for biomagnification in carnivores. Organisms that feed on sediment
or detritus may accumulate more cadmium than those in the grazing
food chain. High levels of cadmium have been reported in sea mammals,
pelagic sea-birds, and terrestrial invertebrates.
Within a variety of organisms, cadmium is distributed throughout
most tissues, but tends to accumulate in the roots, gills, livers,
kidneys, hepatopancreas, and exoskeleton. Cadmium in the cell is
often bound to cytoplasmic proteins, a possible detoxifying
mechanism. Elimination probably occurs primarily via the kidney but
also via moulting of the exoskeleton.
There is some evidence of an interaction between cadmium and
other metals, especially calcium and zinc. Cadmium may replace
calcium on the calcium-specific protein calmodulin and is affected by
other physiological processes that regulate the uptake of calcium. In
certain circumstances, zinc increases cadmium retention in the liver
and kidneys of aquatic vertebrates. In terrestrial systems, high soil
zinc levels can reduce cadmium uptake appreciably.
Selection can lead to cadmium-tolerant populations in both the
aquatic and terrestrial environments.
4.1 Uptake
4.1.1 Uptake from water by aquatic organisms
Several studies have shown that the free metal ion, Cd2+, is
the form of cadmium most available to aquatic organisms (Sunda et al.,
1978; Borgmann, 1983; Part et al., 1985; Sprague, 1985).
Inorganic cadmium complexes appear not to be taken up, at least
by fish (Part et al., 1985). This is particularly important in marine
water where cadmium is mainly present in soluble chloride complexes
(Zirino & Yamamoto 1972). It is most probable that chloride
complexation is responsible for the reduced cadmium accumulation and
toxicity in a variety of organisms observed with increasing salinities
(Coombs, 1979).
In the case of organic cadmium complexes, the chemical properties
are of importance with respect to bioavailability. Three categories
can be distinguished. The first comprises cadmium complexes with EDTA,
NTA, and DPTA, which are unavailable to aquatic organisms (Sunda et
al., 1978; Part & Wikmark, 1984). The second consists of complexes
that to some extent contribute to the total metal uptake, i.e. uptake
is higher than predicted from the actual Cd2+ activity, but the
complex is still less available than the free Cd2+ ion. This group
includes fulvic acids of low relative molecular mass (Giesy et al.,
1977; John et al., 1987), the amino acid histidine (Pecon & Powell,
1981), and carboxylic acids like citric acid (Guy & Ross Kean, 1980;
Part & Wikmark, 1984). The third category includes compounds such as
xanthates and dithiocarbamates that form hydrophobic complexes with
heavy metals. These hydrophobic complexes act as metal carriers across
biological membranes and they lead to a greater uptake of cadmium in
aquatic organisms than when the metal is present as the free ion
(Poldoski, 1979; Block & Part, 1986; Gottofrey et al., 1988; Block,
1991). This latter observation is of particular environmental concern
because xanthates are used in the mining industry in the enrichment of
metals from sulfide ores by flotation. Xanthate concentrations of
between 4 and 400 µg/litre have been measured in waters receiving
effluent from metal refineries (enrichment plants) (Waltersson, 1984).
Another water quality parameter affecting cadmium uptake is the
Ca2+ and Mg2+ concentration (hardness) of the water. Increasing
Ca2+ concentration reduces cadmium uptake through fish gills (Part
et al., 1985; Wicklund, 1990), cadmium accumulation (Carroll et al.,
1979), and cadmium toxicity for fish (Calamari et al., 1980). Two
mechanisms can be distinguished for the Ca2+-mediated reduction in
cadmium uptake. The first is an inhibitory effect on uptake into gill
tissue, while the second is related to the adaptive response of the
fish to increased Ca2+ concentrations (Calamari et al., 1980,
Wicklund 1990). Mg2+ also reduces cadmium uptake through fish gills
but at 5 times higher concentrations than Ca2+ (Part et al., 1985).
Cadmium uptake in fish is not strongly pH dependent; uptake in
rainbow trout gills was not affected over the pH range 5-7 (Part et
al., 1985).
Recent data from fish gills indicate that, to some extent, Cd2+
shares uptake mechanisms with Ca2+; these two ions are about the
same size and also form complexes with the same kind of ligands. Thus
Cd2+ can replace Ca2+ in the calcium-specific protein calmodulin
(Flik et al., 1987). In the gills, Cd2+ is assumed to enter the
epithelial cells down its concentration and electrical gradient by
facilitated diffusion through a calcium channel in the apical membrane
(Verbost et al., 1989). Several lines of evidence support this
assumption. Firstly, increasing water Ca2+ concentrations reduce
cadmium uptake. Secondly, cadmium in the water inhibits Ca2+ uptake
in the gills (Verbost et al., 1987; Reid & McDonald, 1988). Thirdly,
La3+, a calcium channel blocker in cell membranes, inhibits both
Ca2+ and Cd2+ uptake in the gills. Fourthly, the hypocalcaemic
hormone stanniocalcin reduces both Ca2+ and Cd2+ uptake in the
gills (Verbost et al., 1989). Stanniocalcin has been shown to close
the apical calcium channel in the gill epithelial cells thereby
reducing Ca2+ uptake from the water (Lafeber et al., 1988). The
hormone is secreted when the fish has a surplus of Ca2+, i.e.
hypercalcaemic. The two-fold effect of Ca2+ on cadmium uptake in
fish discussed previously can be well explained by this model. A
direct competition between Ca2+ and Cd2+ at the apical calcium
channel reduces the uptake of cadmium into the cells, while the
adaptive response in Ca2+-rich water probably involves an increased
stanniocalcin level, which closes the apical calcium/cadmium channel.
The transport mechanism from the epithelial cells to the blood is
unclear. Cadmium is not transported by the high affinity Ca-ATPase in
the basolateral epithelial membrane which transports Ca2+ (Verbost
et al., 1988). The possible involvement of the Na+/Ca2+ exchange
mechanism, where Cd2+ replaces Ca2+, has recently been suggested
as a translocation mechanism to the blood (personal communication to
the IPCS by G. Flik).
Zinc also has been shown to reduce cadmium uptake through the
gills (Wicklund, 1990). Like cadmium, zinc is assumed to enter the
epithelial cell by facilitated diffusion (Spry & Wood, 1989) and,
furthermore, Ca2+ acts antagonistically on zinc uptake.
Taken together, these data suggest that the apical epithelial
membrane of fish gills contains an ion channel shared by cadmium and
calcium, and probably also zinc. The movement of metals through this
channel is controlled both by external factors such as the Ca2+
content of the water and internal factors such as hormones.
Increasing temperature increases the uptake of cadmium from water
(Vernberg et al., 1974; Zaroogian & Cheer, 1976; Denton &
Burdon-Jones, 1981).
4.1.1.1 Microorganisms
In the alga Chlorella pyrenoidosa, uptake of cadmium was
completely blocked by 0.2 mg manganese/litre and inhibited by 2 to 5
mg iron/litre, but calcium, magnesium, molybdenum, copper, zinc, and
cobalt had no effect on uptake (Hart & Scaife, 1977).
Cultures of Chlorella accumulate twice as much cadmium at pH
7.0 as at pH 8.0 when exposed to 0.5 mg cadmium/litre (Hart & Scaife,
1977).
4.1.1.2 Aquatic molluscs
Hardy et al. (1984) found greater uptake of cadmium from sea
water into oysters given an uncontaminated phytoplankton food source
than into those without food. The authors explain their findings on
the basis that the presence of phytoplankton increases the flow of
water through the oysters. Studies on oysters without a food source
may thus underestimate cadmium uptake. Oysters fed phytoplankton
containing cadmium retained only 0.59% of this cadmium; the majority
of the cadmium in molluscs is taken up directly from the water. The
oyster accumulates about twice as much cadmium in summer as in the
winter. This is presumed to reflect the increased flow of water
through the animal at higher temperatures (Zaroogian & Cheer, 1976).
Hardy et al. (1981) showed that clams ( Protothaca staminea)
took up much less cadmium from water in the presence of sediment at
3.6 g/litre. The uptake was only 17% of that measured in sediment-free
water.
Langston & Zhou (1987a,b) found no evidence of cadmium uptake
into the bivalve Macoma balthica involving metallothionein or
metallothionein-like proteins. Accumulation in soft tissues was linear
throughout a 29-day exposure period, whereas uptake onto the shell was
characterized as saturation kinetics. In contrast, the gastropod
Littorina littorea did show induction of specific cadmium-binding
proteins, which contributed to uptake and storage of cadmium.
Watling & Watling (1983) demonstrated uptake of cadmium in a
dose-dependant manner into sandy beach gastropod molluscs in
laboratory experiments. Much of the cadmium (as chloride) accumulated
in the gill. The rate of cadmium uptake was 0.01 mg/kg per day for
Donax serra and 0.16 mg/kg per day for the smaller Bullia
rhodostoma after exposure to cadmium at 20 µg/litre. The freshwater
snail Physa integra took up more cadmium as exposure increased,
concentrations ranging between 1 and 40 µg/litre. The highest
concentration factors were found with the lowest exposure
concentration (Spehar et al., 1978a). Wier & Walter (1976) exposed the
freshwater snail Physa gyrina to 1.3 mg cadmium/litre (as the
chloride) and found an average cadmium uptake rate of 0.55 mg/kg per
hour over 24 h. Heavier snails took up less cadmium, after the same
exposure, than lighter individuals.
4.1.1.3 Other aquatic invertebrates
Rainbow & White (1989) investigated uptake of cadmium and zinc in
three marine crustaceans, Palaemon elegans (Decapoda),
Echinogammarus pirloti (Malacostraca), and Elminius modestus
(Cirripedia) at water concentrations of cadmium between 0.5 and 1000
µg/litre and zinc between 2.5 and 4000 µg/litre. All three crustaceans
accumulated the non-essential cadmium at all dissolved cadmium
concentrations without regulation. Differences between species were
interpreted by the authors in terms of differences in cuticle
permeability and way of life. All three species took up zinc more
rapidly than cadmium; the ratios between molar uptake rates of zinc to
cadmium were 11.4:1, 2.7:1, and 3.7:1 for the three species,
respectively, following an exposure to a molar ratio of 1.7:1.
4.1.1.4 Fish
Cadmium uptake in fish continues for some considerable time in
fish exposed to the metal. The peak of tissue residues may not be
reached for several weeks, particularly after exposure to low
concentrations of the metal (Cearley & Coleman, 1974; Benoit et al.,
1976; Sullivan et al., 1978a).
Douben (1989a) exposed the stone loach Noemacheilus barbatulus
to cadmium in water (as the sulfate) at a concentration of 1 mg/litre
and monitored uptake and loss at different temperatures with fed and
starved fish. The size of the fish affected both uptake and loss of
cadmium, bioconcentration factors decreasing with size. Uptake of
cadmium increased with temperature up to about 16 °C and decreased as
the concentration of cadmium in the water increased. Feeding the fish
increased the rate of uptake of cadmium from the water. The author
concluded that metabolic rate was an important factor in the uptake of
cadmium into the fish and in its subsequent loss.
4.1.1.5 Model aquatic ecosystems
Ferard et al. (1983) investigated the transfer of cadmium through
a model food-chain consisting of an alga, a daphnid, and a fish.
Concentration factors relative to food were low, indicating that
cadmium is mainly taken up directly from water. Daphnids fed algae
containing cadmium at between 4.5 and 570 mg/kg dry weight showed a
maximum concentration factor of 1. Fish fed contaminated daphnids or
algae showed concentration factors of 0.0038 and 0.0018, respectively.
Nimmo et al. (1977) reported low concentration factors, ranging from
0.018 to 0.027, for grass shrimp fed on brine shrimp containing
cadmium at between 27 and 182 mg/kg. Rehwoldt & Karimian-Teherani
(1976) fed zebrafish on food containing cadmium acetate at 10 mg/kg
over a period of 6 months. Maximum residues, in males and females
respectively, were 5.92 and 13.64 mg/kg, the median residue levels
after 6 months of exposure being 5.19 and 12.95 mg/kg (on a dry weight
basis).
4.1.1.6 Uptake from aquatic sediment
Ray et al. (1980b) exposed the ragworm Nereis virens to
sediment to which cadmium chloride had been added. Smaller worms took
up more cadmium relative to body weight than larger worms. The cadmium
was taken up in a dose-related manner and no equilibrium was reached
during the 24-day experiment. The rate of uptake directly from sea
water also increased with exposure concentration over the range of
0.03 to 9.2 mg/litre. For the range of sediment cadmium concentrations
used (1 to 4 mg/kg), the corresponding concentrations in the overlying
sea water were 0.03 to 0.1 mg/litre. Comparing uptake into the
ragworms from water with these concentrations to the uptake from the
spiked sediment produced identical concentrations of cadmium in the
worms. Rate of uptake from sediment was between 16 and 39 times less
than the uptake from the corresponding exposure to cadmium in water.
The authors concluded that all of the uptake of cadmium from sediment
derived from desorbed metal ions in the interstitial water.
4.1.1.7 Uptake from food relative to uptake from water
Fish can take up cadmium from the surrounding water and from
ingested food. The main uptake route in fresh water is from the water
via the gills (Williams & Giesy, 1978). However, the relative
importance of food and water to the body burden depends very much on
the cadmium content of the food organism. In contaminated areas with
an increased cadmium content in food organisms, the relative
importance of food as a cadmium source may increase. In the marine
environment, where cadmium is mainly present in chloride complexes not
available to fish, the relative importance of food as a cadmium source
increases. Consequently food has been shown to be the main cadmium
source in marine fish (Pentreath, 1977; Dallinger et al., 1987).
4.1.2 Uptake by terrestrial organisms
4.1.2.1 Uptake into plants
The uptake of cadmium into plants generally depends upon the
availability of the metal in soil solution. The soil pH and
composition, particularly the nature of soil clays, the organic matter
content, and, obviously, the soil cadmium level, affect this
availability. The relationship between soil cadmium level and plant
uptake is not a simple one because of the wide variety of soil
characteristics that affect the extent of cadmium uptake. Cataldo &
Wildung (1978), Peterson & Alloway (1979), and Page et al. (1981) have
reviewed this subject.
Plants grown in a greenhouse or a container take up more cadmium
than the same plants grown in soil with the same cadmium levels in the
field. This is due to greater root development in a confined volume in
containers and to the fact that all the roots are in contact with
cadmium-contaminated soil. In the field, roots may grow down below the
cadmium-contaminated level (Page & Chang, 1978; De Vries & Tiller,
1978).
Mahler et al. (1978) cultured lettuce and chard on acid or
calcareous soils to which cadmium sulfate had been added at levels up
to 320 mg/kg. For both types of soil there was a dose-related uptake
of cadmium from soil into leaves. The uptake of the metal was much
greater in acid than in calcareous soils, particularly at higher rates
of cadmium application (over 40 mg/kg). At the highest soil
concentration of 320 mg/kg, lettuce leaves contained cadmium at a
concentration of 800 mg/kg and chard leaves 1600 mg/kg when grown in
acid soil. Leaves of lettuce cultured on calcareous soils with cadmium
at 320 mg/kg contained a lower cadmium concentration of 200-300 mg/kg
and chard, similarly cultured, contained 300 mg/kg or less. Bingham et
al. (1980) showed an effect of soil pH on cadmium (as sulfate) uptake
in rice; more metal was incorporated as acidity increased. Chaney et
al. (1975) reported that liming of soil in which soybeans were growing
decreased the concentrations of cadmium in leaves from 33 to 5 mg/kg
dry weight as pH increased from 5.3 to 7.0. Eriksson (1988)
investigated the effect of pH on the uptake of cadmium into perennial
ryegrass ( Lolium perenne) and winter rape ( Brassica napus). The
more soluble fractions of cadmium in soil increased as the pH was
lowered; increasing the pH from 5 to 7 with calcium oxide invariably
reduced the cadmium content of ryegrass plants, but this decrease was
less consistent when the pH was increased from 5 to 6. The cadmium
content of rape plants was markedly higher at pH 4 than pH 5. Adding
more cadmium to the soil increased the amount of cadmium in the plants
in direct proportion to the increased concentration of the metal in
soil over the range 0 to 5 mg/kg. Eriksson (1988) found that soil
organic matter decreased the availability of cadmium to perennial
ryegrass and winter rape grown in pots. Addition of organic material
to sand and clay soils reduced cadmium uptake to a greater extent in
the sand.
When Mitchell & Fretz (1977) cultured seedlings of three species
of tree (red maple, white pine, and Norway spruce) hydroponically or
in soil with added cadmium, the concentration in roots was greater
than that in leaves. Cadmium added to soil was less readily taken up
than cadmium added to nutrient solutions. Similarly, Root et al.
(1975) reported greater cadmium concentration in roots than in shoots
of maize grown hydroponically in a medium containing cadmium chloride.
Harkov et al. (1979) found the highest uptake of cadmium into
hydroponically grown tomatoes in the roots, while stems had lower
cadmium concentrations than leaves.
Lepp et al. (1987) measured high concentrations of cadmium in the
sporophores (fruiting bodies) of the fungus Amanita muscaria growing
in birch woodland. The fungus sporophores contained 29.9 mg/kg dry
weight, compared to a cadmium level of 0.4 mg/kg in the soil on which
they grew. The cadmium was released from the rotting sporophore, after
it had shed its spores, in a form which was readily available to other
plants growing on the woodland soil; this was shown experimentally
with lettuce plants grown in pots. The authors calculated that an
abundant population of sporophores could recycle 1.4% of the total
cadmium load in leaf litter to higher plants over a period of 14 days
(the mean lifespan of the sporophores).
4.1.2.2 Terrestrial invertebrates
Beyer et al. (1982) demonstrated that earthworms concentrated
cadmium from soils amended with sewage sludge containing cadmium
oxide. Cadmium concentrations were as high as 100 mg/kg in worms
exposed to soils containing cadmium at 2 mg/kg, a concentration factor
of 50. Adding calcium carbonate to soils decreased the cadmium uptake
of worms slightly, while high soil zinc levels decreased the cadmium
uptake appreciably. Results were variable with different sludge
treatments. Hartenstein et al. (1980) amended sludge with 10, 50, and
100 mg/kg cadmium (as cadmium sulfate) and added earthworms ( Eisenia
foetida). The worms accumulated 3.9, 2.04, and 1.44 times the
respective sludge levels of cadmium over a period of 5 weeks. In field
trials on non-amended soils containing 12 to 27 mg cadmium/kg, worms
sampled during a 28-week period gave levels of cadmium ranging from 8
to 46 mg/kg.
Terrestrial pulmonate snails retained up to 59% of cadmium
administered in their diet as the chloride (Russell et al., 1981). The
highest retention was after dosing at 25 mg cadmium/kg diet. The
higher the dose (up to 1000 mg/kg diet) the lower the percentage
retention of the metal. Ireland (1981) noted that in the terrestrial
slug Arion ater most of the cadmium was located in the digestive
gland without association with any particular sub-cellular organelles,
and isolated a specific cadmium-binding protein from the animals.
4.1.2.3 Birds
In a study by White & Finley (1978), adult mallard ducks were fed
a diet containing cadmium chloride at levels of 2, 20 or 200 mg/kg and
killed at 30-day intervals. The cadmium content increased with dose
level and time (except in the case of the highest dose where body
burden peaked after 60 days), and the highest concentrations occurred
in the liver and kidney. The highest levels overall occurred after
dosing for 60 days at 200 mg/kg; cadmium concentrations were 109 mg/kg
in the liver and 134 mg/kg in the kidney.
Nicholson & Osborn (1983) dosed starlings ( Sturnus vulgaris)
with cadmium chloride at a concentration of 2 mg/kg body weight, three
times weekly for 6 weeks, and reported a wide range of kidney
concentrations (from < 10 to > 200 mg/kg dry weight).
4.2 Distribution
4.2.1 Aquatic organisms
In higher organisms, cadmium can be bound in several different
tissues, whereas in plants cadmium is bound to the cell wall in roots.
Brooks & Rumsby (1967) measured the cadmium taken up by the
oyster ( Ostrea sinuata) from water containing 115Cd (50 mg/litre).
The soft parts of the oyster contained 100 mg cadmium/kg after 100 h.
Concentrations in tissues were, in decreasing order, 360 mg/kg for
gills, 285 mg/kg for heart, 141 mg/kg for the visceral mass, 83 mg/kg
for the mantle, 53 mg/kg for white muscle, and 25 mg/kg for striated
muscle.
Nimmo et al. (1977) reported that in the pink shrimp the
hepatopancreas took up more cadmium than other tissues. Lower
concentrations were found in the exoskeleton, muscle, and serum.
Short-term exposure of the crab Uca pugilator to cadmium chloride
led to the hepatopancreas and gill concentrations of the metal being
similar after a 24-h exposure to 1 mg cadmium/litre (Vernberg et al.,
1974).
Sangalang & Freeman (1979) determined the cadmium in tissues of
brook trout exposed to the metal (added as the chloride) via the water
or by injection. After water exposure to cadmium chloride at 1
µg/litre, the trout showed greatest uptake of the metal in the gills,
kidney, and liver. The gills and the posterior kidney revealed a
higher metal content than any other tissues. Levels of cadmium in
whole blood and plasma, heart, spleen, testis, stomach, and skin were
higher than control levels after 77 and 93 days of exposure. Smith et
al. (1976) found the greatest accumulation of the metal in the kidney
of catfish exposed to cadmium (as sulfate) in the water. In an
autoradiographic study of cadmium distribution in rainbow trout
exposed to cadmium in water, Tjalve et al. (1986) confirmed the
general picture of cadmium distribution, the metal being found in the
gills, liver, and kidney. However, they also observed heavy labelling
of the olfactory rosette and the olfactory nerve, an observation not
reported earlier. In a detailed study they later showed that cadmium
was transported axonally from the olfactory rosette to the bulbus
olfactorius but not further into the brain (Gottofrey, 1990). The
significance of this observation with respect to the olfactory
responses of fish in cadmium-contaminated environments remains to be
investigated.
The few studies that have been conducted on the subcellular
distribution of cadmium indicate that, while much is located in the
cytosol, a significant proportion can be found in the nucleus and the
mitochondria. Cadmium is bound in the cytosol to proteins of low
relative molecular mass, metallothioneins, and other cadmium-binding
proteins. These proteins are rich in the sulfur-containing amino acid
cysteine but poor in aromatic amino acids.
Metallothioneins have been isolated and characterized in a number
of aquatic and terrestrial organisms. Fish metallothioneins have
received considerable interest in recent years as tools in monitoring
metal pollution in the environment (Hamilton & Mehrle, 1986; Hogstrand
& Haux, 1990a). Simple methods to analyse fish metallothionein have
been developed, including differential pulse polarography (Olson &
Haux, 1986) and radioimmunoassay based on specific antibodies to fish
metallothionein (Hogstrand & Haux, 1990b). Olson & Haux (1986) found
a strong correlation between hepatic metallothionein and cadmium
accumulation in perch collected from cadmium-contaminated water.
4.2.2 Terrestrial organisms
4.2.2.1 Terrestrial plants
Jones & Johnston (1989) analysed cereal grain and herbage from
long-term experimental plots at Rothamsted, United Kingdom, and found
that uptake of cadmium into herbage was greatest where phosphate
fertilizer had been applied. It was also greater from unlimed soils
than from limed soils. However, the authors concluded that there was
little evidence of a long-term (1840-1986) increase in crop cadmium
concentrations.
Byrne et al. (1976) analysed higher fungi from Slovenia,
Yugoslavia, and found levels of cadmium ranging from 0.53 to 39.9
mg/kg dry weight (average 5.0 mg/kg). This is an order of magnitude
higher than in most other plants. Although the fungi were collected
from industrial, urban, and uncontaminated sites, the levels found in
the fungi were not very different between sites. The authors suggested
geological rather than industrial sources for the cadmium in these
soils.
The high uptake by mushrooms and related species is probably due
to a cadmium-binding phosphoglycoprotein, cadmium-myco-phosphatin,
which has been isolated from the mushroom Agaricus macrosporus
(Meisch & Schmitt, 1986).
4.2.2.2 Terrestrial invertebrates
Hopkin & Martin (1985) investigated the storage of cadmium in the
woodlouse Oniscus asellus from heavily contaminated woodland 3 km
downwind from a smelter. The hepatopancreas was found to contain up to
5 g cadmium/kg dry weight without apparent ill effects upon the
organism. Cadmium was reported to be stored intracellularly in the
copper- and sulfur-containing granules of epithelial S cells. In a
later study (Hopkin, 1990) it was found that considerable interspecies
differences exist with regard to storage in the hepatopancreas.
Oniscus asellus stored five times more cadmium than Porcello scaber
under the same conditions. The carnivorous centipede Lithobius
variegatus, when fed on cadmium-contaminated hepatopancreas from
woodlice, accumulated cadmium which was likewise stored in the midgut
(Hopkin & Martin, 1984).
Berger & Dallinger (1989) studied the distribution of cadmium
between several organs of the terrestrial snail Arianta arbustorum
during a 20-day feeding experiment on cadmium-enriched agar. Of the
cadmium in the medium, 54% was taken up, of which 66% was distributed
to the hepatopancreas, leading to a concentration of more than 500
mg/kg dry weight. In other organs (intestine, foot/mantle, gonads),
the cadmium concentration was considerably lower.
In the earthworm Lumbricus rubellus taken from heavy-metal-
polluted soil, more than 70% of the cadmium burden was found in the
posterior alimentary canal (Morgan & Morgan, 1990). This distribution
prevented dissemination of large concentrations of cadmium into other
tissues and, according to the authors, may represent a detoxification
strategy.
4.3 Elimination
Information on loss of cadmium from organisms is relatively
scarce. The information that does exist suggests that this is very
variable, and has been reviewed by Coombs (1979) and Taylor (1983).
Organisms that accumulate cadmium also tend to retain the metal for
long periods. The main excretory route appears to be via the kidney,
except in the case of organisms that moult, where loss from the shed
exoskeleton can be significant.
Robinson & Wells (1975) administered a single oral dose of
cadmium acetate to softshell turtles ( Trionyx spinifer) and killed
and dissected the animals either 48 h or 96 h later. After 48 h, 9.43%
of the total dose was recovered from tissues, while turtles killed
after 96 h had retained 4.02% of the dose. The greatest retention of
cadmium, after both time periods, was in the liver. Cadmium was also
retained in the small intestine for the first 48 h, but the amount had
decreased by 96 h.
Harrison & Klaverkamp (1989) exposed rainbow trout ( Salmo
gairdneri) and lake whitefish (Coregonus clupeaformis) to cadmium in
water, via a continuous-flow system, or the diet, via pelleted food,
for 72 days. The fish were then kept in clean water on a cadmium-free
diet for a further 56 days. In the case of water-exposed fish, the
majority of the cadmium was present in the gill and kidney, but
food-exposed fish retained cadmium principally in the kidney, gut, and
liver. Bioconcentration factors for exposure via the water were 55 for
the trout and 42 for the whitefish, whereas concentration factors from
the food were less than 1 for both species. However, both species
accumulated a greater proportion of the cadmium that was in the food
than that in water (1% as against 0.1%). Equilibrium bioconcentration
factors were estimated to be 161 for trout and 51 for whitefish.
In the same model, the half-times for depuration of accumulated
cadmium ranged from 24 to 63 days. Douben (1989b) investigated the
kinetics of cadmium in freshwater fish (the stone loach Noemacheilus
barbatulus) exposed to cadmium via the diet (tubifex worms
previously contaminated with cadmium by uptake from water). The body
burden of cadmium declined after the period of feeding with
contaminated diet more rapidly in starved than in fed fish. Rate
constants for loss of cadmium appeared to be greater during the
exposure period than after exposure. Both uptake and loss of cadmium
were influenced by the body weight of the fish.
Janssen et al. (1991) investigated uptake and loss of cadmium
from contaminated soil by four species of soil arthropod and developed
kinetic models that gave good predictions of the degree of
accumulation in a variety of species. They also reviewed data on other
soil arthropods (Tables 6 and 7). The kinetics of cadmium in different
arthropods is related to taxonomy and reflects the different
physiological characteristics of the different organisms. Some,
notably isopods and molluscs, take up and retain cadmium in their
tissues with little or no excretion. These species are capable of
holding large quantities of the metal in the hepatopancreas without
apparent ill effect. There is no direct correlation between
assimilation capacity and the capacity to excrete or eliminate
cadmium. Figure 4 illustrates the uptake of cadmium (measured as total
body burden) and its subsequent loss in four species of arthropods.
Elimination half-lives of 53, 8, and 2 days, respectively, have been
reported for Platynothrus peltifer, Orchesella cincta, and
Notiophilus biguttatus; no elimination took place over 130 days in
Neobisium muscorum.
Sawicka-Kapusta et al. (1987) investigated the effect of keeping
the vole Clethrionomys glareolus at different temperatures on the
rate of loss of cadmium from body tissues. Although the different
temperatures (10 °C and 20 °C) affected the metabolic rate of the
voles, there was no difference in the rate of loss of cadmium.
4.4 Bioaccumulation and biomagnification
Bioaccumulation occurs when the concentration in the organism
exceeds the concentration in the nutrient medium and is expressed
quantitatively as a bioconcentration factor. Progressive
bioaccumulation at each trophic level is termed biomagnification.
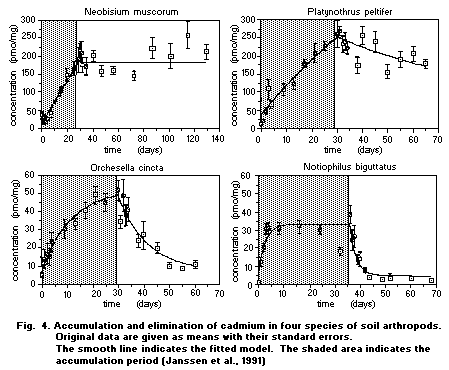 Table 6. Cadmium assimilation efficiencies in different soil invertebrates
Species Food Cadmium concentration Assimilation efficiency Reference
in food (µmol/g) (%)
Snail
Arianta arbusloruma agar 1.48 55-92 Berger & Dallinger (1989) b
Centipede
Lithobius variegatus isopod 1.21-10.2 0-7.2 Hopkin & Martin (1984)
hepatopancreas
Millipede
Clomeris marginata maples leaves 8.2-40.6 Hopkin et al. (1985)
Pseudoscorpion
Neobisium muscorum collembolans 0.20 58.9 Janssen et al. (1991)
Mite
Platynothrus peltifer green algae 0.15 17.2 Janssen et al. (1991)
Insects
Orchesella cincta green algae 0.09 8.3 Van Straalen et al. (1987)
Orchesella cincta green algae 0.15 9.4 Janssen et al. (1991)
Notiophilus biguttatus collembolans 0.23 35.5 Janssen et al. (1991)
a assimilation value for midgut gland
b recalculated from the data
Table 7. Excretion constants (k) for cadmium in different soil invertebrates
Species Taxonomic k Reference
group (day-1)
Helix pomatia snail 0 Dallinger & Wieser (1984) b
Cepaea nemoralis snail 0.007 Williamson (1980) b
Oniscus asellusa isopod 0.002 Hopkin (1989) b
Neobisium muscorum pseudoscorpion 0 Janssen et al. (1991)
Lycosa spp spider 0.007 Van Hook & Yates (1975)
Platynothrus peltifer oribatid mite 0.013 Janssen et al. (1991)
Orchesella cincta collembolan 0.061 Van Straalen et al. (1987)
Orchesella cincta collembolan 0.087 Janssen et al. (1991)
Acheta domesticus cricket 0.090- Van Hook & Yates (1975)
0.110
Notiophilus biguttatus carabid beetle 0.375 Janssen et al. (1991)
a k value for midgut gland or hepatopancreas
b recalculated from the data
Bioconcentration factors (the ratio between the cadmium
concentration in the organism and the concentration in the medium) for
several groups of organisms studied under laboratory conditions are
shown in Table 8. They range from 16 to 130 000 and do not seem to
show any consistent pattern.
Table 8. Bioconcentration of cadmium in laboratory studies
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Freshwater alga 10 10 3000 dw c Ferard et al. (1983)
(Chlorella vulgaris)
Freshwater alga stat 20-22 14 250 4940 daw Cain et al. (1980)
(Scenedesmus obliquus)
Freshwater diatom flow WB 23 10 40 000 Conway (1978)
(Asterionella formosa)
Submerged plant WP 25 30 25 1730 dw Nakada et al. (1979)
(Elodea nuttallii)
Water hyacinth leaves 28 500 16 dw c Kay & Haller (1986)
(Eichhornia crassipes)
American oyster 4.9-5.1 g flow WB 16-20 21 10 116 ww Eisler et al. (1972)
(Crassostrea virginica) 4280 aw
8.1 g flow ST 2.8-22.6 280 5 2376 ww Zaroogian & Cheer (1976)
18 472 dw
Mussel 32-34 mm flow ST 13 166 10 50 802 dw Riisgard et al. (1987)
(Mytilus edulis)
Scallop 6.8-7.7 g flow WB 16-20 21 10 131 ww Eisler et al. (1972)
(Aquipecten irradians) 3970 aw
Bay scallop 0.51-0.73 g flow ST 9.5-16 42 60 20 400 Pesch & Stewart (1980)
(Argopecten irradians)
Crab 2-4 g WB 10 14 37 152 dw Ray et al. (1980a)
(Pandalas montagui)
Grass shrimp 20-33 mm flow WB 9.5-16 42 60 223 Pesch & Stewart (1980)
(Palaemonetes pugio)
Table 8 (contd).
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Lobster 160-169 g flow WB 16-20 21 10 21 ww Eisler et al. (1972)
(Homarus americanus) 10 aw
Mummichog 2.3-2.4 g flow WB 16-20 21 10 15 ww Eisler et al. (1972)
(Fundulus heteroclitus) 200 aw
Fathead minnow flow WB 13.9-15.3 21 49 190 Sullivan et al. (1978a)
(Pimephales promelas)
Red maple leaves 15-27 45 0.5 14 400 dw d Mitchell & Fretz (1977)
(Acer rubrum) roots 15-27 45 0.5 131 800 dw d Mitchell & Fretz (1977)
leaves 15-27 101 2.6 mg/kg 0.76 dw e Mitchell & Fretz (1977)
roots 15-27 101 2.6 mg/kg 12.5 dw e Mitchell & Fretz (1977)
White pine leaves 15-27 66 0.5 3400 dw d Mitchell & Fretz (1977)
(Pinus strobus) roots 15-27 66 0.5 118 400 dw d Mitchell & Fretz (1977) leaves 15-27 36 52.6 mg/kg 1.2 dw e Mitchell & Fretz (1977)
roots 15-27 36 52.6 mg/kg 10.4 dw e Mitchell & Fretz (1977)
a WB = whole body; WP = whole plant; ST = soft tissues
b Chloride salt used unless stated otherwise; bioconcentration factor = concentration in the organism divided by concentration
in the medium; dw = dry weight; ww = wet weight; aw = ash weight; daw = dry ash weight
c Nitrate salt used
d The medium was a cadmium-enriched nutrient solution
e The medium was a cadmium-amended soil mix
Microorganisms generally exhibit a high capacity to take up
cadmium from water and retain the metal in their cells. The highest
bioconcentration factors reported have been for micro-organisms, the
greatest value being 40 000 in a freshwater diatom (Conway, 1978). In
this diatom, 58% of the cadmium was located in the cellular content
with 25% in the organic coating of the frustule and 17% in the
silicaceous frustule. The bioconcentration factor of 3000 for the alga
Chlorella (Ferard et al., 1983) is typical of the value for
microorganisms. Flatau et al. (1988) demonstrated the uptake (it was
not specified whether this referred to absorption or adsorption) of
cadmium from sea water by marine bacteria; the uptake of the metal
increased with its concentration in the water, and the accumulation
rate was a logarithmic function of the dose. Sorption was only
observed with exposure concentrations above 10 µg Cd/litre, suggesting
that a threshold had to be exceeded for cadmium uptake to occur.
Dongmann & Nurnberg (1982) showed that the bioconcentration factor for
a marine diatom, Thalassiosira rotula, decreased with increasing
metal concentration, suggesting a saturation effect. Their reported
concentration factors, which vary between 1000 and 2000, reflect the
reduced sorption of cadmium by marine microorganisms compared with
their freshwater relatives. Hart & Scaife (1977) reported a direct
relationship between the level of cadmium in the medium and sorption
to the alga Chlorella exposed to cadmium concentrations ranging from
0.25 to 1.00 mg/litre.
After water hyacinths had been exposed for 4 weeks to water
containing 0.5 or 1.0 mg cadmium/litre, added as cadmium nitrate, the
leaves had accumulated 8.00 and 17.20 mg/kg, respectively (Kay &
Haller, 1986).
Molluscs concentrate cadmium to a high degree over a period of
time, but uptake is often slow. Oysters showed a concentration factor
of only 149 over a 10-day period (Eisler et al., 1972) but a factor of
2714 after 40 weeks (Zaroogian & Cheer, 1976). Elliott et al. (1985)
examined the accumulation of cadmium, copper, lead, and zinc in the
tissues of the mussel, Mytilus edulis. Under simultaneous exposure
to all four metals, both lead and cadmium were accumulated in direct
proportion to the exposure time, whereas copper and zinc were not.
Accumulation of cadmium was influenced by the presence of other
metals.
Compared with oysters, the related bay scallop shows greater
accumulation of cadmium when exposed to low concentration of the metal
as the chloride over 6 weeks (Pesch & Stewart, 1980). Short-term
exposure of the same scallop to higher concentrations of cadmium
resulted in very much lower concentration factors. Exposure for 96 h
to cadmium (as the chloride) at up to 2.0 mg/litre led to a
bioconcentration factor of around 50 (Nelson et al., 1976).
Bioconcentration factors (from water and food) and
biomagnification factors (from food alone) were calculated for the
freshwater isopod Assellus aquaticus by van Hattum et al. (1989).
Much of the cadmium (added as the chloride) was taken up from the
water (bioconcentration factor 18 000), but there was little uptake
from food (bioconcentration factor 0.08). Direct uptake from water
accounted for between 50 and 98% of the body burden after 30 days of
exposure (based on dry weight measures). Cadmium was readily taken up
by the isopod even at exposure concentrations of 1 µg/litre.
Experiments conducted at two different pHs (5.9 and 7.6) revealed no
significant effect of pH on uptake of cadmium by the isopod.
Wright & Frain (1981b) demonstrated that adult intermoult
amphipods ( Gammarus pulex) accumulated only half as much cadmium
from a solution of 5 mg/litre in the presence of 200 mg calcium/litre
as with 20 mg calcium/litre.
Ramamoorthy & Blumhagen (1984) investigated the uptake of
cadmium, mercury, and zinc by rainbow trout (Salmo gairdneri) in a
model system which simulated the presence of other competing
compartments that would be found in nature. The system consisted of
either a simple sediment/water model or a more complex series of
compartments in dialysis bags of suspended sediment, cation and anion
exchange resins (to represent naturally occurring polyelectrolyte
materials of plant origin), and fish. River water was used as the
fluid transfer medium, and the system was continuously stirred.
Equilibrium with one heavy metal ion did not inhibit the uptake of
other metal ions; cadmium and zinc were taken up after equilibrium
with mercury. The authors calculated approximate partition
coefficients (fish/substrate) to be 2.8 for sediment, 550 for water,
and 2 and 3.6 for the cation and anion exchange resins, respectively.
The problem of expressing changes in concentration between
trophic levels is that the units are not compatible. There is no
significance to a bioconcentration term that expresses a ratio of
cadmium in soil moisture to cadmium in plant tissue, or cadmium in
plant tissue to cadmium in herbivore tissue. Therefore, it is
difficult to assess the impact of cadmium on the environment in terms
of bioconcentration factors. An alternative method is to measure both
cadmium and calcium at each trophic level and express these
measurements as a molar ratio of these two elements. (The molar ratio
should be used to account for the movement by atoms, not grams.)
Differences between trophic levels are calculated as the ratio of the
higher trophic level to the lower. This approach, called
biopurification, recognizes that the flow of the non-nutrient cadmium
through successive trophic levels follows a pathway similar to that of
nutrients such as calcium, and that calcium must pass natural chemical
and physiological barriers, such as membranes and selective enzymes,
that progressively purify the pool of the nutrient calcium relative to
the non-nutrient cadmium. In the case where two similar ecosystems are
compared, and where one is believed to be more contaminated than the
other, the relative degree of contamination can be calculated as the
difference between molar ratios at the same or similar trophic levels.
It is unfortunate that the absence of concentration data on
nutrients such as calcium or, alternatively, zinc, prohibits the
calculation of biopurification factors for any of the studies
discussed in this monograph.
5. TOXICITY TO MICROORGANISMS
Appraisal
Cadmium is toxic to a wide range of microorganisms in culture
(effects of cadmium on microorganisms in the field are discussed in
chapter 8). However, the presence of sediment, organic matter or high
concentrations of dissolved salts reduces the availability of cadmium
to microorganisms and, therefore, reduces the toxic impact.
Freshwater microorganisms in culture are thus affected by cadmium at
lower concentrations than marine species (for example, 50 µg/litre
affects growth in many freshwater species of algae while at least 100
µg/litre, and often 1000 µg/litre, is required to reduce growth in
marine species). Soil microorganisms are partially protected from the
toxic effects of cadmium by the presence of clay.
5.1 Aquatic microorganisms
5.1.1 Freshwater microorganisms
Canton & Slooff (1982) exposed the bacterium Salmonella
typhimurium and the alga Chlorella vulgaris to cadmium in the form
of the chloride, and calculated an 8-h EC50 (growth inhibition) of
10.4 mg/litre for the bacterium and a 96-h EC50 of 3.7 mg/litre for
the alga. No-toxic-effect levels of 0.65 and 1.5 mg/litre were
estimated for the bacterium and alga, respectively. Jana &
Bhattacharya (1988) found significant inhibition of population growth
in the faecal coliform bacterium Escherichia coli during exposure to
cadmium concentrations of 1, 2 or 5 mg cadmium/litre for 7 or 28 days.
Cadmium was the most toxic of the metals tested. Norberg & Molin
(1983) exposed the bacterium Zoogloea ramigera (abundant in sewage
treatment plants) to cadmium chloride concentrations of 1, 3, 5, and
10 mg cadmium/litre for 30 h. A prolonged lag phase and decrease in
growth resulted, the length of the lag phase being proportional to the
concentration of cadmium in the medium. Babich & Stotzky (1977a)
showed that the presence of clay particles protected bacteria from the
toxic effect of cadmium added to culture medium. The degree of
protection was related to the cation exchange capacity of various
clays tested.
Chapman & Dunlop (1981) estimated the 8-h LC50 for the
freshwater protozoan Tetrahymena pyriformis to be less than 1
mg/litre. However, this value increased with increasing water calcium
concentration; at a value of 500 mg calcium/litre, the LC50 was 19
mg/litre. Magnesium also exerted a protective effect against cadmium
when mixed with calcium. Cadmium was consistently more toxic to
Tetrahymena in the presence of magnesium alone. Berk et al. (1985)
calculated a 15-min EC50 (inhibition of ciliate chemotactic
response) for Tetrahymena sp. of 0.35-0.7 mg.
When Skowronski et al. (1988) exposed the green microalga
Stichococcus bacillaris to cadmium chloride concentrations of 45 and
90 µmol/litre for 4 days, growth rate was inhibited by 28% and 45% at
the two respective concentrations. At both exposure levels, dry weight
and chlorophyll a content were reduced in a dose-related manner.
Addition of manganese at concentrations of between 45 and 1800
µmol/litre had a dose-related antagonistic effect on cadmium toxicity.
Bennett (1990) found that the addition of cadmium (1.8 µmol/litre) to
a turbidostat culture of Chlorella pyrenoidosa caused a decrease in
the maximum specific growth rate (toxicity was expressed after a lag
of 5 generations). A gradual decrease in the maximum specific growth
rate was also noted during a 40-day exposure to stepwise increases in
the cadmium concentration (0.96 to 1.68 µmol/litre). The author found
that the addition of manganese (10.4 µmol/litre) had an antagonistic
effect, causing the maximum specific growth rate to increase.
Cadmium is toxic to the growth of the freshwater alga Chlorella
pyrenoidosa (Hart & Scaife, 1977). In cultures maintained at pH 7.0,
doubling times were 11, 21, 22, and 35 h for cadmium concentrations of
0, 0.25, 0.5, and 1.0 mg/litre medium, respectively. At a pH of 8.0,
the effect was somewhat lessened; doubling times were 11, 16, 17, and
25 h for the same range of cadmium doses. There was also a pronounced
effect on carbon dioxide fixation, which was reduced from 0.738 to
0.720, 0.558, and 0.283 µmol HCO3- fixed per hour with cadmium
exposures in the culture medium of 0, 0.246, 0.554, and 1.090
mg/litre, respectively. There was less of an effect on oxygen
evolution over the same dose range. Zinc offered no protection against
cadmium effects.
Wong et al. (1979) exposed four different species of freshwater
algae to cadmium and measured the uptake of 14C-carbonate.
Scenedesmus quadricaudata was the most sensitive species, carbonate
uptake being inhibited by 80% at a cadmium concentration of 20
µg/litre. Chlorella pyrenoidosa showed 70% inhibition of carbonate
uptake at about 100 µg/litre, while Chlorella vulgaris showed only
50% inhibition at about 500 µg/litre. The least sensitive of the four
species tested was Ankistrodesmus falcatus variety acicularis
where an effect on carbonate uptake started only at concentrations
higher than 500 µg/litre. There was no observed effect on the growth
of A. falcatus at cadmium concentrations lower than 5 mg/litre.
Rebhun & Ben-Amotz (1984) demonstrated that the chlorophyll content of
cells of Chlorella stigmatophora was reduced in a dose-dependant
manner across a range of cadmium concentrations of between 1 and 10
mg/litre medium.
Laegreid et al. (1983) studied the effects of cadmium on the alga
Selenastrum capricornutum cultured in the laboratory in water taken
from two lakes at various times throughout the year. The two lake
waters contained different amounts of organic material. The first
lake, a dystrophic bog lake, had a high organic content, while the
second, an eutrophic lake, had a low organic content. In the
dystrophic lake, which had a low pH (4.4), the toxicity of cadmium was
related to the free ionic concentration of the metal, as suggested by
many laboratory experiments. In the eutrophic lake, where there was
less influence from organic material, there was a pronounced seasonal
effect. In the summer, when growth and productivity of the algae were
highest, there was a much greater effect of the metal than predicted.
The toxicity of cadmium, at this time, was far greater than would be
expected even if all of the metal was in the free ionic form and none
was bound to organic compounds. On the basis of their field evidence,
the authors questioned the generally held assumption that organic
binding is the major factor in determining cadmium toxicity to
microorganisms. They considered that the presence of certain organic
compounds of low relative molecular mass could increase cadmium
toxicity. This conclusion is supported by the work of Giesy et al.
(1977), who found that uptake of cadmium into zooplankton could be
increased in the presence of organic compounds of low relative
molecular mass.
Chin & Sina (1978) investigated the cellular basis of cadmium
toxicity in microorganisms using cultures of Physarum polycephalum.
The organism was cultured, in plasmodial form, on the surface of
liquid medium, and replicate discs, cut from the protoplasmic sheet of
the organism, were used for the tests. The discs maintain mitotic
synchrony with each other and, therefore, cadmium could be introduced
at specific points in the cell cycle. The cultures were exposed to
cadmium sulfate (5 x 10-4 mol/litre), which was floated onto the
surface of the culture medium. Exposure to cadmium immediately prior
to early prophase of mitosis extended the normal DNA replication
period from 3 h to 4 h; this was monitored using measurements of
uptake of 3H-thymidine. Two stages of the cell cycle were
particularly susceptible to cadmium. Exposure either at the beginning
of the cycle or 80% of the way through the cycle caused delays in the
completion of mitosis. A 30-min exposure to cadmium at the onset of
early prophase inhibited incorporation of 3H-uridine into RNA for
the following 3 h by 51% and stimulated the incorporation of
3H-thymidine into DNA, for the same period, by 85%. Later in the
cycle, DNA synthesis was inhibited and DNA content was depressed by
12.5%. There was an ultrastructural effect on the nucleoli (less dense
material centrally giving nucleoli in section a "ring" structure),
which was the only structural effect of the metal even after 4 h of
exposure. Accommodation occurred after pre-treatment with sub-toxic
doses of cadmium. Treatment with cadmium at the less sensitive periods
of the cell cycle led to reduced effect at the more sensitive phases;
the organism, in some way, compensated. There was no accommodation by
Physarum after pre-treatment with zinc ions. This result contrasts
with that of a similar study on Escherichia coli where pre-exposure
to zinc reduced the effects of cadmium (Mitra et al., 1975).
5.1.2 Estuarine and marine microorganisms
Chan & Dean (1988) exposed the marine bacterium Pseudo-monas
marina to cadmium sulfate at concentrations of 1 to 25 mg
cadmium/litre. Effects were exposure related and included a lengthened
lag time, reduced growth rate, reduced biomass and oxygen uptake, and
a decrease in the activity of dehydrogenase and alkaline phosphatase.
The IC50 values for inhibition of biomass and of growth rate were 11
and 11.5 mg/litre, respectively. Flatau et al. (1987) progressively
adapted the marine bacterium Pseudomonas sp. to cadmium
concentrations from 1 to 80 mg/litre. Although the length of the lag
phase was not linearly or logarithmically correlated with the cadmium
concentration, it was significantly longer at cadmium concentrations
of 70 mg/litre or more. The growth rate was reduced at 10 mg/litre but
remained constant at cadmium concentrations of 10 to 50 mg/litre; no
growth was observed at 75 or 80 mg/litre. Oxygen consumption was not
different from that of controls at 1 or 5 mg/litre but at
concentrations of 10 mg/litre or more respiratory activity decreased.
Bressan & Brunetti (1988) exposed the marine microalgae
Dunaliella tertiolecta and Isochrysis galbana to cadmium
concentrations of 13.8 and 0.2 mg/litre, respectively, for up to 8
days. Cadmium significantly reduced the population growth, expressed
as the mean number of cells/ml, for both species. The addition of
nitrilotriacetic acid (NTA, a sequestering agent) at ratios of 1:1,
1:2 or 1:3 did not modify the toxic effect of cadmium.
Berk et al. (1985) exposed the marine ciliates Paranophrys sp.
and Miamiensis avidus to cadmium and monitored the inhibition of the
chemotactic response. The 15-min EC50 values were 2-3.1 mg
cadmium/litre and 5.1-7 mg cadmium/litre for the two species,
respectively.
Dongmann & Nurnberg (1982) investigated the effects of cadmium on
the marine diatom Thalassiosira rotula (a chain-forming diatom
native to shallow temperate waters) in petri dish and in batch liquid
cultures. They calculated generation times from the dish cultures and
reported a toxicity threshold for generation time of 30 µmol
cadmium/litre (3.4 mg/litre). At 50 µmol/litre, cadmium increased the
generation time from 24 h to 28 h. Using chain length as a parameter,
the toxicity threshold was 15 µmol/litre (1.7 mg/litre). Cell density
was found to be affected in batch culture. Cell chlorophyll content
and chain length were too variable to show significant effects of the
metal in batch culture.
5.2 Soil and litter microorganisms
Babich & Stotzky (1977b) showed that several species of fungi
tolerated cadmium to a greater degree when grown on cultures amended
with clays than in pure culture medium.
Sato et al. (1986) exposed the soil bacterium Nitrosomonas
europaea both to cadmium concentrations of 0.05, 0.1, and 0.4
mg/litre and to a range of ammonia concentrations (1 to 100 mg/litre
as N). Growth was markedly inhibited at the highest cadmium
concentration, especially when this was coupled with ammonia
concentrations greater than 10 mg/litre. Cadmium toxicity caused a
characteristic growth response. Ammonia oxidation proceeded at a
reduced rate for approximately 3 days and then fell sharply. The
subsequent oxidation proceeded at a diminished but constant rate. At
cadmium concentrations of 0.1 mg/litre or less, the toxic effect of
cadmium could be partially offset by increasing the ammonia
concentration. Prahalad & Seenayya (1988) found that cadmium
concentrations of 3.5 mg/litre inhibited the growth of the bacterium
Alcaligenes faecalis. However, if the nutrient broth was diluted by
one half then growth was inhibited at 2 mg/litre, and with
quarter-strength nutrient broth inhibition occurred at 0.5 mg/litre.
Hartman (1974) reported less species diversity of soil fungi in
areas of high cadmium contamination than in control areas. Samples of
Fusarium oxysporium isolated from contaminated and control soils
showed different tolerances to cadmium. This suggested the development
of some resistance, presumably by selection of more resistant strains.
Bond et al. (1976) incubated coniferous forest soil and litter
(mixed with a pre-prepared homogeneous soil) in microcosm units and
monitored oxygen consumption, carbon dioxide evolution, and bacterial
and fungal populations. With cadmium added to a concentration of 0.01
mg/kg soil and, in the initial stages of incubation, with cadmium at
10 mg/kg soil, there was a stimulation of oxygen consumption,
suggesting an effect of the metal on uncoupling of respiratory
phosphorylation. In the later stages of incubation with cadmium at 10
mg/kg there was a reduction in both oxygen consumption and carbon
dioxide evolution. No effect was seen on numbers of organisms in the
microcosms.
Bewley & Stotzky (1983) investigated the effect of cadmium (100
and 1000 mg/kg soil) on carbon mineralization and on the mycoflora in
glucose-supplemented soils amended with clays (kaolinite or
montmorillonite) at 9%. Cadmium had no significant effect on the
length of the lag period, carbon dioxide evolution or on the amount of
carbon mineralized. When subsequent cadmium additions of 2400 and 4000
mg/kg were made to soils previously treated with 100 or 1000 mg/kg,
the rate of glucose degradation decreased more in the clay-amended
soils than in control soil not amended with clay. Clay protected fungi
from the toxic effect of cadmium at 5000 mg/kg. Fungi from
clay-treated soils were more sensitive to cadmium in the culture media
after they had been isolated from soil pre-treated with cadmium. The
authors interpreted these results as showing a reduction in the
availability of cadmium to organisms in clay-amended soils. The
overall effect would be a prevention of selection of more tolerant
strains. Thus, when the organisms were challenged with high doses of
cadmium, they would have been more susceptible than organisms from
non-amended soil.
Naidu & Reddy (1988) incubated black cotton soil (0.8% organic
carbon; 55% clay) for up to 8 weeks in the presence of cadmium
chloride at concentrations of between 50 and 500 mg cadmium/kg. The
ammoniacal nitrogen (NH4-N) concentration increased for the first
week at all treatment levels and then decreased at concentrations of
50 mg/kg or less. The initial rise in NH4-N levels led to an
increase in nitrate nitrogen (NO3-N) levels, the accumulation of
NO3-N being inversely proportional to the cadmium exposure. The
authors pointed out that at cadmium concentrations of 100 and 500
mg/kg, hydrolysis of urea was significantly poorer than at other
treatment levels, as shown by the lower concentration of NH4-N
observed after 1 week. At all cadmium concentrations there was
significant accumulation of nitrite nitrogen (NO2-N) at every sample
time, compared to control soil, suggesting that cadmium might be toxic
to soil nitrification. At all exposure levels cadmium significantly
depressed both bacterial and fungal populations. Cadmium
concentrations of 10 or 50 mg/kg had no effect on soil actinomycetes,
but both 100 and 500 mg/kg significantly reduced the population. Tyler
et al. (1974) incubated a mull soil for 7 weeks. Cadmium chloride
concentrations of 9 to 18 µmol/g and cadmium acetate concentrations of
9 to 22 µmol/g caused decreases in soil ammonium content and
significantly increased nitrate accumulation.
6. TOXICITY TO AQUATIC ORGANISMS
Appraisal
Cadmium uptake from water by aquatic organisms is extremely
variable and depends on the species and various environmental
conditions such as water hardness (notably the calcium ion
concentration), salinity, temperature, pH, and organic matter content
(see chapter 4).
The majority of chelating agents decrease cadmium uptake but
some, such as dithiocarbamates and xanthates, increase uptake.
As a consequence of the variability in cadmium uptake, the toxic
impact to aquatic organisms also varies across a wide range of
concentrations and is dependent on the species of organism and on the
presence of other metal ions, notably calcium and zinc.
The lowest recorded 96-h LC50 in a flow system is 16 µg
cadmium per litre for the adult shrimp Mysidopsis bahia. A nominal
no-observed-effect level (NOEL) of 0.6 µg cadmium/litre was found for
Daphnia magna, reproductive rate being the most sensitive parameter.
A nomi-nal NOEL has been noted at a similar level (1.7 to 3.4 µg
cadmium per litre) with respect to the reproductive effects on brook
trout.
The available results indicate that the embryonic and larval
stages of aquatic organisms are more sensitive than the adult stage.
Spinal malformations are induced in cadmium-exposed fish. In addition
to causing reproductive effects, cadmium influences the behaviour of
aquatic organisms.
At low concentrations, cadmium inhibits ion transport systems
(10 µg cadmium/litre) and induces metallothionein synthesis (< 1 µg
cadmium/litre) in freshwater fish.
6.1 Toxicity to aquatic plants
Hutchinson & Czyrska (1975) exposed two floating aquatic weeds,
the common duckweed Lemna minor and a floating fern Salvinia
natans, to cadmium concentrations of between 0.01 and 1.0 mg/litre
for up to 3 weeks. Growth was reduced at all concentrations but
especially at 0.05 mg/litre or more. The effect of cadmium on growth
became more marked with time. Loss of green coloration (chlorosis) was
a common symptom of cadmium toxicity, and at concentrations of 0.5 and
1.0 mg/litre Lemna plants died. When the two species were grown in
competition, growth at lower cadmium concentrations (0.01 and 0.05
mg/litre) was greater in Salvinia but less in Lemna than when the
plants were grown alone.
In a study by Nir et al. (1990), water hyacinth plants were
exposed to cadmium concentrations of 0, 0.05, 0.1, 0.4 or 1.0 mg/litre
for 7 days. Concentrations of 0.1 mg/litre or less had no significant
effect on wet or dry biomass gain or on chlorophyll a content.
Concentrations of 0.4 or 1.0 mg/litre significantly reduced both wet
biomass gain and chlorophyll a content but had no significant effect
on dry biomass gain. The chlorophyll a content of leaves decreased
with time in plants exposed to 0.4 mg/litre. After 3 weeks of
exposure, the chlorophyll a levels were 75% lower than in control
plants.
6.2 Toxicity to aquatic invertebrates
Cadmium is moderately to highly toxic to aquatic invertebrates
(see Tables 9 and 10). Its toxic effect is dependent on a variety of
environmental variables. Factors that reduce the free ionic
concentration, e.g., water hardness, salinity, chelating agents, and
high organic content of water, tend to reduce the toxic effect of
cadmium. The presence of zinc increases the toxic effect of cadmium on
invertebrates.
6.2.1 Acute and short-term toxicity
The acute toxicity of cadmium to aquatic invertebrates, as
assessed in laboratory tests, is summarized in Tables 9 and 10. The
most notable features are the variability in cadmium toxicity between
different organisms and the effects of temperature, salinity, and
water hardness. There is considerable variation even amongst closely
related species.
In a study by Canton & Slooff (1982), the water flea Daphnia
magna was exposed to cadmium over a 48-h period. At a water hardness
of 1 mmol/litre there was no mortality at 16 µg cadmium per litre,
while at a water hardness of 2 mmol/litre there was no mortality or
abnormal behaviour at 17 µg/litre.
Clubb et al. (1975b) investigated the toxicity of cadmium to nine
species of aquatic insects, but seven of the species tested were too
insensitive to the effects of the metal for the LC50 to be
determined. The insensitive species were Atherix variegata,
Hexatoma sp., Holorusia sp., Acroneuria pacifica, Arcynopteryx
signata, Pteronarcys californica, and Brachycentrus americanus;
these species represented Dipterans (true flies), Plecoptera
(stoneflies), and Tricoptera (caddis flies).
Table 9. Toxicity of cadmium to marine or estuarine invertebrates
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Purple sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 0.5 (0.4-0.6) m Dinnel et al. (1989)
(Strongylocentrotus
purpuratus)
Green sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 1.8 (1.5-2.2) m Dinnel et al. (1989)
(Strongylocentrotus
droebachiensis)
Sand dollar embryo stat 12.5-13.0 30 8.0-8.1 72 7.4 (5.2-10.8) m Dinnel et al. (1989)
(Dendraster
excentricus)
Starfish 24.5 g stat 20 20 8.0 24 12 n Eisler (1971)
(Asterias forbesi) 24.5 g stat 20 20 8.0 48 1.0 n Eisler (1971)
24.5 g stat 20 20 8.0 96 0.82 n Eisler (1971)
11.2 g stat 20 20 7.8 24 71 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 48 7.1 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
American oyster embryo stat 26 25 7.0-8.5 48 3.8 (2.85-4.48) n Calabrese et al. (1973)
(Crassostrea
virginica)
Mussel stat 18.5 32.9 7.9 96 1.62 (1.19-2.22) m Ahsanullah (1976)
(Mytilus edulis
planulatis)
Blue mussel 4 g stat 20 8.0 24 > 200 n Eisler (1971)
(Mytilus edulis) 4 g stat 20 8.0 48 165 n Eisler (1971)
4 g stat 20 8.0 96 25 n Eisler (1971)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Soft-shell clam 5.2 g stat 20 20 8.0 24 > 20 n Eisler (1971)
(Mya arenaria) 5.2 g stat 20 20 8.0 48 50 n Eisler (1971)
5.2 g stat 20 20 8.0 96 2.2 n Eisler (1971)
4.6 g stat 20 20 7.8 24 32 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 48 2.5 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Bay scallop 20-30 mm stat 20 25 8.0 24 8.2 n Nelson et al. (1976)
(Argopecten 20-30 mm stat 20 25 8.0 48 3.21 n Nelson et al. (1976)
irradians) 20-30 mm stat 20 25 8.0 72 2.18 n Nelson et al. (1976)
20-30 mm stat 20 25 8.0 96 1.48 (0.95-2.31) n Nelson et al. (1976)
Atlantic oyster 0.6 g stat 20 20 8.0 24 158 n Eisler (1971)
drill 0.6 g stat 20 20 8.0 48 28 n Eisler (1971)
(Urosalpinx 0.6 g stat 20 20 8.0 96 6.6 n Eisler (1971)
cinerea)
Eastern mud snail 0.56 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Nassarius 0.56 g stat 20 20 8.0 48 125 n Eisler (1971)
absoletus) 0.56 g stat 20 20 8.0 96 10.5 n Eisler (1971)
Ragworm 8 g stat 20 20 8.0 24 25 n Eisler (1971)
(Nereis virens) 8 g stat 20 20 8.0 48 25 n Eisler (1971)
8 g stat 20 20 8.0 96 11 n Eisler (1971)
7.6 g stat 20 20 7.8 24 56 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 48 9.3 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Copepod nauplius stat 22 10 96 0.06 (0.001-0.2) m Roberts et al. (1982)
(Eurytemora affinis)
Copepod adult stat 22 10 96 0.38 (0.006-1.52) m Roberts et al. (1976)
(Acartia tonsa)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Harpacticoid
copepod adult stat 20-22 3 96 0.43 (0.31-0.55) Bengtsson & Bergstrom (1987)
(Nitocra spinipes) adult stat 20-22 7 96 0.66 (0.53-0.82) Bengtsson & Bergstrom (1987)
adult stat 20-22 15 96 0.78 (0.41-120) Bengtsson & Bergstrom (1987)
Marine amphipod young stat 10 96 3.5 m Wright & Frain (1981a)
(Marinogammarus adult stat 10 96 13.3 m Wright & Frain (1981a)
obtusatus)
Mysid shrimp adult flow 22 20 7.3 96 0.036 (0.022-0.081) m Roberts et al. (1982)
(Neomysis adult flow 22 20 7.8 96 0.02 (0.015-0.027) m Roberts et al. (1982)
americanus)
Mysid shrimp adult stat 22 20 7.3 96 0.017 m Roberts et al. (1982)
(Mysidopsis bahia) adult stat 22 20 7.7 96 0.029 (0.013-0.043) m Roberts et al. (1982)
flow c20-28 15-23 96 0.016 (0.013-0.02) m Nimmo et al. (1978)
Shrimp stat 18.7 32.1 8.0 120 2.3 (1.05-5.06) m Ahsanullah (1976)
(Palaemonetes sp.) stat 18.7 32.1 8.0 168 1.85 (1.32-2.59) m Ahsanullah (1976)
0.38 g flow 16.8 96 6.8 (5.2-9.76) m Ahsanullah (1976)
0.16 g flow 17.8 8.1 96 6.4 (5.73-7.19) m Ahsanullah (1976)
Sandworm 0.37 g stat 18.5 32.7 8.1 168 6.4 (5.82-7.1) m Ahsanullah (1976)
(Neanthes vaali)
Sand shrimp 0.25 g stat 20 20 8.0 24 2.4 n Eisler (1971)
(Crangon 0.25 g stat 20 20 8.0 48 0.5 n Eisler (1971)
septemspinosa) 0.25 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Sand shrimp adult flow 10.2 28.6 7.9 96 2.3 (1.7-5.1) m Dinnel et al. (1989)
(Crangon spp.)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Grass shrimp 0.33 g stat 20 20 8.0 24 43 n Eisler (1971)
(Palaemonetes 0.33 g stat 20 20 8.0 48 3.7 n Eisler (1971)
vulgaris) 0.33 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Shrimp stat 35 96 2.07 (± 0.22) m McClurg (1984)
(Penaeus indicus)
Pink shrimp flow 25 20 96 4.6 m Bahner & Nimmo (1975)
(Penaeus duorarum)
Grapsid crab 1.47 g stat 17.8 32.6 8.1 168 14 (11.2-17.5) m Ahsanullah (1976)
(Paragrapsus 1.08 g stat 17.1 168 16.7 (15.11-18.45) Ahsanullah (1976)
quadridentatus)
Hermit crab 0.47 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Pagurus 0.47 g stat 20 20 8.0 48 3.7 n Eisler (1971)
longicarpus) 0.47 g stat 20 20 8.0 96 0.32 n Eisler (1971)
0.5 g stat 20 20 7.8 24 15 n Eisler & Hennekey (1977)
0.5 g stat 20 20 8.0 96 1.3 n Eisler & Hennekey (1977)
Shore crab 5.9 g stat 20 20 8.0 24 100 n Eisler (1971)
(Carcinus maenus) 5.9 g stat 20 20 8.0 48 16.6 n Eisler (1971)
5.9 g stat 20 20 8.0 96 4.1 n Eisler (1971)
Dungeness crab zoea stat 8.5 30 8.1 96 0.2 (0.1-0.4) m Dinnel et al. (1989)
(Cancer magister)
Squid larva stat 8.6 30 8.1 96 > 10.2 m Dinnel et al. (1989)
(Loligo opalescens)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b organisms exposed to cadmium added as cadmium chloride; m = measured; n = nominal
c intermittent flow-through conditions
Table 10. Toxicity of cadmium to freshwater invertebrates
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Snail adult stat 20-22 6.7 24 7.6 n Wier & Walter (1976)
(Physa adult stat 20-22 6.7 48 4.25 n Wier & Walter (1976)
gyrina) adult stat 20-22 6.7 96 1.37 n Wier & Walter (1976)
adult stat 20-22 6.7 228 0.83 n Wier & Walter (1976)
immature stat 20-22 6.7 48 0.69 n Wier & Walter (1976)
immature stat 20-22 6.7 96 0.41 n Wier & Walter (1976)
Snail flow b 15 7.1-7.7 168 0.114 m Spehar et al. (1978a)
(Physa integra)
Snail 10-12 mm flow 12 128-176 7.7 24 4.4 m Williams et al. (1985)
(Physa 10-12 mm flow 12 128-176 7.7 48 2.1 m Williams et al. (1985)
fontinalis) 10-12 mm flow 12 128-176 7.7 96 0.8 m Williams et al. (1985)
Isopod 8-10 mm flow 12 128-176 7.7 24 13 m Williams et al. (1985)
(Asellus 8-10 mm flow 12 128-176 7.7 48 3.6 m Williams et al. (1985)
aquaticus) 8-10 mm flow 12 128-176 7.7 96 0.6 m Williams et al. (1985)
Scud 10 96 0.12 m Wright & Frain (1981b)
(Gammarus 8-12 mm flow 12 128-176 7.7 24 1.6 m Williams et al. (1985)
pulex) 8-12 mm flow 12 128-176 7.7 48 0.4 m Williams et al. (1985)
8-12 mm flow 12 128-176 7.7 96 0.02 m Williams et al. (1985)
Water flea adult stat 10 0.85 meq/ 7.2 48 0.055 e (0.032-0.095) n Baudouin & Scoppa (1974)
(Daphnia hyalina) litre
Water flea 1 day stat 20 7.6-7.7 24 3 n Kuhn et al. (1989)
(Daphnia < 1 day stat 20-22 110-130 7.8 48 0.04 (0.02-0.07) n Hall et al. (1986)
magna) < 1 day stat 20-22 190-210 7.7 48 0.08 (0.06-0.1) n Hall et al. (1986)
< 1 day stat 18-20 1 mmol/ 48 0.03 m Canton & Slooff (1982)
litre
Table 10 (contd).
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Water flea < 1 day stat 20-22 110-130 7.8 48 0.07 n Hall et al. (1986)
(Daphnia < 1 day stat 20-22 110-130 7.7 48 0.1 (0.07-0.12) n Hall et al. (1986)
ulex) < 1 day stat 19-22 7.7 96 0.047 (0.042-0.052) n Bertram & Hart (1979)
Copepod adult stat 10 0.85 meq/ 7.2 48 3.8 e (2.3-6.3) n Baudouin & Scoppa (1974)
(Cyclops abyssorum) litre
Copepod adult stat 10 0.85 meq/ 7.2 48 0.55 e (0.39-0.77) n Baudouin & Scoppa (1974)
(Eudiaptomus padanus) litre
Crayfish flow 19-21 24-28 6.7-7.0 96 6.1 (4.7-7.9) m Mirenda (1986)
(Orconectes virilis)
Mayfly flow 10 7.8 96 28 m Clubb et al. (1975b)
(Ephemerella grandis
grandis)
Midge 10-12 mm flow 12 128-176 7.7 96 300 m Williams et al. (1985)
(Chironomus riparius)
Stonefly flow 10 7.8 96 18 m Clubb et al. (1975b)
(Pteronarcella badia)
Stonefly 10-15 mm flow 12 128-176 7.7 96 520 m Williams et al. (1985)
(Hydropsyche
angustipennis)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b intermittent flow-through conditions
c hardness expressed as mg CaCO3/litre unless stated otherwise
d organisms exposed to cadmium added as cadmium chloride unless otherwise stated; m = measured concentration; n = nominal concentration
e organism exposed to cadmium as cadmium sulfate
Spehar et al. (1978a) reported a decreased survival of the water
snail Physa integra at a cadmium concentration of 85.5 µg/litre
after 7 days of exposure. After 21 days of exposure, survival was
significantly decreased at 27.5 µg/litre, the next lowest
concentration tested. Some snails exposed to these concentrations
developed a condition in which the animal was extended from the shell
but unable to attach the foot or crawl. A white mucus layer covered
the exposed foot region of some snails and these subsequently died.
Concentrations tested were not high enough to obtain a 4-day LC50
value but the 7-day LC50 of 114 µg/litre was approximately 11 times
higher than the 28-day LC50 value of 10.4 µg/litre.
Mirenda (1986) reported a 14-day LC50 of 0.7 mg cadmium per
litre for the crayfish Orconectes virilis under flow-through
conditions. Pesch & Stewart (1980) estimated the 10-day LC50 for bay
scallops Argopecten irradians to be 0.53 mg/litre in flowing sea
water. The EC50 (for growth) for the same species over 42 days was
0.078 mg/litre. Byssal thread detachment, which precedes death, showed
an EC50 of 0.54 mg/litre of cadmium 8 days into the test and before
there was any appreciable mortality. Robinson et al. (1988) compared
10-day LC50 values for freshly collected and cultured infaunal
amphipods ( Rhepoxynius abronius). Cultured amphipods appeared normal
and survived well (93%) under control toxicity test conditions, but
were more sensitive to cadmium in sediment (10-day LC50 = 4.4 mg/kg)
than were freshly caught amphipods (10-day LC50 = 8.7 mg/kg).
When Winner (1988) exposed Daphnia magna and Ceriodaphnia
dubia to cadmium for 7 days, the most sensitive indicators were mean
body length of primiparous females in D. magna, which was
significantly reduced at 2 µg cadmium/litre, and the total young per
female in C. dubia, significantly reduced at 1 µg/litre.
6.2.1.1 Effects of temperature and salinity on acute toxicity
An increase in toxicity as temperature increases and as salinity
decreases is valid for all organisms that have been tested with these
variables (Tables 9 and 10).
O'Hara (1973) investigated the effects of temperature and
salinity on the toxicity of cadmium to adult male and female fiddler
crabs ( Uca pugilator). Mortality was greatest at high temperatures
and low salinities in tests lasting 240 h. LC50 values varied from
2.9 mg/litre for the lowest salinity (10%) and highest temperature (30
°C) to 47.0 mg/litre for the highest salinity (30%) and lowest
temperature (10 °C). Frank & Robertson (1979) exposed the blue crab
( Callinectes sapidus) to cadmium chloride at salinities of 1, 15,
and 35%. Like O'Hara, they found a decrease in cadmium toxicity with
increase in salinity. For example, 96-h LC50 values were 0.32, 4.7,
and 11.6 mg cadmium/litre for the three salinities, respectively.
Rosenberg & Costlow (1976) reported increased cadmium toxicity during
larval development of two estuarine crab species as salinity decreased
and increased toxicity as temperature increased.
Voyer & Modica (1990) found the same pattern with the mysid
shrimp Mysidopsis bahia. For salinities of 10 and 30%, the 96-h
LC50 values ranged from 15.5 to 28 µg cadmium/litre at a temperature
of 25 °C and from 47 to 84 µg/litre at a temperature of 20 °C. At 30
°C the 96-h LC50 was < 11 µg/litre at both salinities. However,
when Robert & His (1985) exposed embryos and larvae of the Japanese
oyster Crassostrea gigas to cadmium concentrations of up to 50
µg/litre at various salinities (20 to 35%), decreasing the salinity
severely affected the development of the oysters but cadmium had no
effect.
At temperatures higher than 11 °C, the combined effect of
temperature and cadmium caused a heavy stress to the copepod Tisbe
holothuriae so that the effects of salinity were masked
(Verriopoulos & Moraitou-Apostolopoulou, 1981).
6.2.1.2 Effect of water hardness
Using either artificial hard water (hardness: 180 mg CaCO3 per
litre) or dechlorinated tap water (hardness: 60 mg/litre),
Niederlehner et al. (1984) conducted short-term tests on the effects
of cadmium on the freshwater oligochaete Aeolosoma headlyi.
Mortality and population growth/maintenance were assessed over 10 to
14 days. The authors established NOEL values for population growth of
32.0 and 53.6 µg/litre for hard water (two replicate tests), whereas
the NOEL for the softer water was 17.2 µg/litre. The 48-h LC50
values were 4.98 and 1.2 mg/litre, respectively, for hard and softer
water.
6.2.1.3 Effect of organic materials and sediment
When Schuytema et al. (1984) exposed Daphnia magna to cadmium
for a period of 48 h, the mean LC50 value was 39 µg/litre in water
and 91 µg/litre in a water-sediment slurry. Giesy et al. (1977) found
that cadmium was more toxic to water fleas ( Simocephalus serrulatus)
exposed in well water with a low organic content than to those exposed
in pond water with a high organic content. The authors isolated a
series of organic fractions from the pond water by ultrafiltration.
Protection against cadmium toxicity was afforded by fractions of
intermediate relative molecular mass (ranging from approximately 500
to 300 000 daltons). The fraction with a relative molecular mass in
excess of 300 000 daltons marginally increased the toxicity of
cadmium.
Kemp & Swartz (1988) compared the acute toxicity of interstitial
and particle-bound cadmium to the marine infaunal amphipod
Rhepoxynius abronius. The cadmium concentration in interstitial
water was kept constant whereas the sediment cadmium level was varied
by using perfusion through the sediment with peristaltic pumps. The
principal cause of toxicity was found to be cadmium dissolved in
interstitial water, between 70 and 88% of the toxicity being
predictable from interstitial water concentrations.
6.2.1.4 Lifestage sensitivity
When Calabrese et al. (1973) investigated the toxicity of cadmium
to embryos of the American oyster Crassostrea virginica, there was
no mortality at 1 mg/litre and the 48-h LC50 and LC100 values were
3.8 and 6 mg/litre, respectively. Johnson & Gentile (1979) found the
larva of the American lobster Hommarus americanus to be sensitive to
cadmium; the 96-h LC50 in static bioassays was 78 µg/litre. The
mortalities after 96 h at concentrations of 10 and 30 µg/litre were 3%
and 10%, respectively. There is a very steep increase in toxicity of
cadmium to lobster larvae between 24 and 96 h. The 24-h LC50 is
approximately 1 mg/litre; at this concentration the mortality reaches
100% within 48 h.
Verriopoulos & Moraitou-Apostolopoulou (1982) found that the
different life-stages of the copepod Tisbe holothuriae showed
differences in sensitivity to cadmium. One-day-old nauplius larvae of
the copepod were the most sensitive with an LC50 of 0.538 mg/litre,
expressed as ions of cadmium, while 5-day-old nauplii showed an LC50
of 0.645 mg/litre. The value for 10-day-old copepodids (0.906
mg/litre) was not significantly different from that for adult females
(0.916 mg/litre). Females with ovigerous sacs were slightly more
sensitive, with an LC50 of 0.873 mg/litre. When Robinson et al.
(1988) exposed the infaunal phoxocephalid amphipod Rhepoxynius
abronius to sediment contaminated with cadmium, the 10-day LC50
values were 8.2 mg/kg for juveniles and 11.5 mg/kg for adults.
Nebeker et al. (1986) exposed Daphnia magna of different ages
to cadmium for a period of 48 h. Mean EC50 (immobilization) values
ranged from 23 µg/litre for 6 day-old water fleas to 164 µg/litre for
2-day-old Daphnia. Tests on Daphnia of different ages, conducted
in water of different hardness (32 or 76 mg CaCO3 per litre), with
or without feeding and in two different sizes of container, resulted
in a wide range of EC50 values (4 to 307 µg/litre). There was no
consistent effect of any of these variables other than the age of the
test animals. Very young animals were relatively tolerant, with a mean
EC50 value of 109 µg/litre.
McCahon et al. (1989) exposed both cased and uncased 1st, 3rd,
and 4th larval instars of the caddis fly Agapetus fuscipes to
cadmium chloride. The LC50 values ranged from 295 to > 1000 mg
cadmium/litre at 24 h and from 50 to 320 mg/litre at 96 h. First
instar larvae were significantly more sensitive than 3rd or 4th instar
larvae and at all ages cased animals were more resistant than uncased.
6.2.1.5 Other factors affecting acute and short-term toxicity
Chandini (1988; 1989) found that increasing the food source of
the cladocerans Daphnia carinata and Echinisca triserialis greatly
reduced the toxicity of cadmium, expressed as the 48-h or 96-h LC50,
and the effect of cadmium on various other life history parameters,
such as fecundity, growth, and age at first reproduction.
Verriopoulos & Moraitou-Apostolopoulou (1981) exposed adult
females of the copepod Tisbe holothuriae to cadmium and found that
the oxygen concentration in the water was negatively related to
mortality and that population density was positively related after
cadmium exposure. Clubb et al. (1975a) showed that the toxicity of
cadmium to aquatic insects decreased with decreasing dissolved oxygen
levels in the test water.
McCahon et al. (1988) exposed the amphipod Gammarus pulex to
cadmium chloride under static conditions and reported a 96-h LC50 of
0.05 mg cadmium/litre. The acute toxicity of cadmium to G. pulex
parasitized by the acanthocephalan Pomphorhynchus laevis at several
levels of infection was investigated. Toxicity, expressed as LC50,
did not differ significantly between uninfected and infected
amphipods.
Kay & Haller (1986) fed water hyacinth weevils Neochetina
eichhorniae on water hyacinths containing cadmium from previous
exposure to the metal. There was no mortality in weevils fed on leaves
containing up to 17.2 mg cadmium/kg. At this exposure level, the
weevils accumulated a cadmium body burden of 36.67 mg/kg over 20 days.
6.2.2 Long-term toxicity
In a study by Zaroogian & Morrison (1981), adult and larval
oysters of the species Crassostrea virginica were exposed to cadmium
at concentrations of 5 or 15 µg/litre. Some adults were exposed (for
35 or 37 weeks) to cadmium at these two concentrations prior to
spawning, and larvae from these pre-treated adults and from control
treated adults were reared in either control sea water or sea water
containing 5 or 15 µg/litre cadmium. In all there were 11 treatment
combinations. The highest larval mortality occurred when larvae from
parents treated with 15 µg/litre were reared in sea water containing
15 µg/litre for 3 weeks. However, larvae that survived this treatment
grew to lengths not significantly different from controls. The effects
observed with other treatment combinations were only temporary. Growth
and development were slowed but those larvae that survived ultimately
developed normally and to the same size as controls.
When Holcombe et al. (1984) exposed snails, from embryos through
to adult sexual maturity, to cadmium chloride, there was a delayed
hatch, a reduction in percentage hatch and survival, and reduced
growth when compared to controls. Based on these effects, the authors
suggested a maximum acceptable cadmium concentration in water of
between 4 and 8 µg/litre from one test and between 2 and 5 µg/litre
from a replicate test.
Lussier et al. (1985) conducted life-cycle tests, over 35 days,
on the mysid shrimp Mysidopsis bahia. Cadmium affected survival
primarily and no reproductive effects were noted at sublethal
concentrations.
Following a long-term investigation in the laboratory and in the
field, Marshall (1979) suggested a chronic LC10 for the water flea
Daphnia galeata mendotae of 0.15 µg cadmium/litre. Winner (1986)
exposed Daphnia pulex chronically (21 or 42 days) to cadmium, added
as cadmium sulfate, under different water conditions. Increasing the
water hardness from 58 to 116 mg/litre reduced the toxic effect of the
cadmium but a further increase to 230 mg/litre had no further effect.
The most sensitive aspect of the Daphnia life history to cadmium was
the abortion rate of young. Humic acid had no effect on this parameter
in soft or medium-hard water but increased the toxic effect of cadmium
in hard water. Mortality was increased by humic acid (0.75 or 1.50
mg/litre) at all water hardness levels. van Leeuwen et al. (1985)
calculated 14-day and 21-day LC50S for Daphnia magna of 24 and 14
µg cadmium/litre, respectively. No effect on mortality was seen at 3.2
µg/litre. The lowest concentrations producing significant mortality of
young amphipods during 6-week exposures to cadmium were 1 µg/litre for
Hyalella azteca and 3.2 µg/litre for Gammarus fasciatus (Borgmann
et al., 1989b).
6.2.3 Reproductive effects
Mysing-Gubala & Poirrier (1981) conducted laboratory experiments
on the effect of cadmium on the freshwater sponge Ephydatia
fluviatilis. Sponge cuttings were exposed to cadmium concentrations
ranging from 0.001 to 1.0 mg/litre for 1 month. At the end of the
experimental period, the cuttings were classified in four groups
depending on whether the sponge survived, whether it produced asexual
reproductive gemmules, and whether the silicaceous spicules of the
gemmules were normal or malformed. There was some effect of cadmium
even at concentrations as low as 0.001 mg/litre, 17% of the sponge
cuttings showing no gemmule production and 33% showing malformed
spicules. At concentrations of 0.5 and 1.0 mg/litre, all of the sponge
cuttings died.
Lee & Xu (1984) investigated the effects of cadmium at 0.5 and
0.1 mg/litre on the fertilization and development of sea urchin eggs
and the development of Amphioxus. At both cadmium concentrations,
sea urchin development was normal to the gastrulation stage but all
the plutei were abnormal. The effects on Amphioxus development were
different; cleavage of eggs was not affected by cadmium at 0.5 or 0.1
mg/litre but neurulation was. Dinnel et al. (1989) exposed sperm from
various sea urchin species to cadmium chloride for 60 min and assessed
fertilization success; EC50 values ranged from 12 to 26 mg/litre. An
EC50 of 8 mg/litre was calculated for the sand dollar Dendraster
excentricus. Den Besten et al. (1989) exposed the sea star Asterias
rubens to cadmium chloride at a concentration of 25 µg cadmium/litre
for 5 months. No effect on spermatozoa was found, but maturation of
oocytes was delayed and early development of embryos was adversely
affected.
Conrad (1988) studied the effect of cadmium on newly fertilized
eggs of the mud snail Ilyanassa obsoleta. No apparent effect was
observed at a cadmium concentration of 10-6 mol/litre. The minimum
concentrations producing abnormal veliger development and abnormal
late cleavage and stopping early cleavage were 10-5-10-4
mol/litre, 10-3 mol/litre, and 10-3 mol/litre, respectively.
Biesinger & Christensen (1972) estimated a 3-week 16%
reproductive impairment concentration of 0.17 µg cadmium/litre.
In a 21-day reproduction test on Daphnia magna, Kuhn et al.
(1989) determined a nominal NOEL of 0.6 µg Cd2+/litre, reproduction
rate being the most sensitive parameter. A nominal NOEL of 1 µg/litre
was found when daphnids were exposed to the cadmium chloride salt.
Reproduction of Daphnia magna was completely inhibited at
concentrations exceeding 3.2 µg/litre and time-dependant survival and
reproduction were significantly reduced at 1.8 µg/litre. No effects on
reproduction were observed at 1 µg/litre (van Leeuwen et al., 1985).
When Bertram & Hart (1979) exposed the cladoceran Daphnia pulex to
cadmium concentrations of 1 to 30 µg/litre, there was no significant
effect on the number of days required for onset of reproduction or on
its frequency. At 1 µg/litre no significant effect on longevity of
individuals was observed, but at concentrations of 5 µg/litre or more
there was a significant reduction. All cadmium concentrations caused
a reduction in the average brood size, the average number of broods
per adult, and the total number of progeny. The authors also exposed
daphnids to cadmium-contaminated food in the form of the phytoplankton
Chlorella, the cadmium concentration being 0.3-0.6 µg cadmium/litre
of medium. This resulted in a significant reduction in the average
number of broods per adult and the average brood size. However, there
was no significant effect on average longevity of individuals, the
percentage of adults producing broods, the average number of days to
the first brood or the average number of days between broods.
Bengtsson & Bergstrom (1987) exposed newly fertilized female
harpacticoids ( Nitocra spinipes) to cadmium chloride at two
salinities for 13 days. At 3% the fecundity EC50 was 37-46 µg/litre
at a salinity of 3% and 6-15 µg/litre at 15%.
Williams et al. (1987) provided water containing nominal cadmium
chloride concentrations of 0, 0.3, 30, 100 or 300 mg cadmium/litre
cadmium to newly-emerged adults of the midge Chironomus riparius in
which they could lay their eggs. The females preferred to lay their
eggs in water with no cadmium or in the lower concentrations;
significant preferences were recorded. Eggs of Chironomids are laid
within a protective gelatinous matrix. Eggs exposed to cadmium after
complete formation of the matrix in control water were unaffected (the
hatch was 80-100%), whereas those exposed after removal of the matrix
had a reduced hatching rate (60%) at all test concentrations. Eggs
laid directly in water containing cadmium were unaffected by a
concentration of 0.3 mg/litre, but hatching rates were reduced to 45%
at 30 mg/litre, 8% at 100 mg/litre, and 0% at 300 mg/litre. Clearly,
cadmium only affects the unprotected egg, either when it is newly laid
and before its gelatinous protection has developed or when this
gelatinous matrix is removed.
In a flow-through study, Hatakeyama (1987) exposed the chironomid
Polypedilum nubifer, from the egg stage, to cadmium chloride. There
was no effect on total number of adults, emergence success, sex ratio,
total number of egg clusters, oviposition success or hatchability at
10 µg cadmium/litre. There was a significant decrease in the total
number of adults and emergence success at 20 µg/litre or more and in
the total number of egg clusters at 40 µg/litre or more. At 80
µg/litre survival and reproduction were very significantly depressed.
In a separate experiment midges were fed cadmium-contaminated food
(dried yeast) at concentrations of 22, 220, and 1800 mg cadmium/kg.
Emergence success was decreased at 220 and 1800 mg/kg but no other
effects were observed.
6.2.4 Physiological and biochemical effects
Berglind (1986) investigated the effect of cadmium alone and in
combination with other metals on the delta-aminolevulinic acid
dehydratase (ALAD) activity of Daphnia magna. ALAD activity was
enhanced by cadmium alone (at 2 µg/litre but not at 0.2 µg/litre) but
this enhancement was abolished in the presence of zinc at 200
µg/litre.
Vernberg et al. (1977) investigated sub-lethal concentrations of
cadmium and their effects on the adult shrimp Palaemonetes pugio
under static and flow-through conditions. Adult shrimps were exposed
to cadmium at 50 µg/litre. This shrimp is highly tolerant to cadmium
and after exposure to 23 mg/litre the mortality rate was only 10%. The
authors found increased uptake of cadmium into the body of the shrimps
with decreasing salinity. Similarly, at higher salinity levels,
toxicity was lower. In shrimps kept at the lowest salinity level (5%),
where cadmium body burden reached 40 mg/kg, there was inhibition of
moulting. At more moderate cadmium body burdens of 23 mg/kg and 10
mg/kg, observed at salinities of 10% and 20%, respectively, moulting
was stimulated by the presence of cadmium. An investigation of the
effects of cadmium on respiratory rate was inconclusive because of
considerable variation. The authors considered that the flow-through
system more nearly approximated field conditions than the static
system. The relationship between salinity and cadmium uptake was
eliminated in a static system at 15 °C, but was quite clearly present
when the system was flow-through. After an extensive study on the
effects of cadmium on three species of shrimps, Penaeus duorarum,
Palaemonetes pugio, and Palaemonetes vulgaris, Nimmo et al. (1977)
reported sublethal histological effects and blackening and damage to
gill filaments. Placing shrimps with blackened gills in cadmium-free
water resulted in the sloughing-off of blackened portions of the
branchia and the shrimps appeared normal within 14 days. The effect on
the gills occurred with exposure to cadmium concentrations approaching
the LC50 which, with an exposure duration of 96 h, was 0.76 mg/litre
for Palaemonetes vulgaris and 3.5 mg/litre for Penaeus duorarum.
Thurberg et al. (1973) exposed two species of crabs ( arcinus maenus
and Cancer irroratus) to various concentrations of cadmium chloride
for 48 h and at five different salinities. At the end of each exposure
period, tests of blood serum osmolarity and gill tissue oxygen
consumption were performed. Cadmium increased the osmolarity of
Carcinus serum above its normal hyperosmotic state and reduced
oxygen consumption by the gill tissue of both species. Effects on
oxygen consumption were dose related over a range of cadmium
concentrations from 0 to 4 mg/litre.
Cadmium has been shown to cause an increase in oxygen consumption
rates in the mud snail Nassarius obsoletus at concentrations of
between 0.5 and 4.0 mg/litre over a 72-h exposure period (MacInnes &
Thurberg, 1973). A similar increase in oxygen consumption rate was
observed in the marine snail Murex trunculus during chronic exposure
to 0.05 mg cadmium/litre (Dalla Via et al., 1989) and in crabs
( Callinectes similis) exposed to cadmium concentrations of between
2.48 and 10.05 mg/litre for up to 96 h (Ramirez et al., 1989).
6.2.5 Behavioural effects
Olla et al. (1988) monitored the burrowing behaviour of three
polychaete species, Nereis virens, Glycera dibranchiata, and
Nephtys caeca during a 28-day exposure to a sediment concentration
of 40 mg cadmium/kg. Most comparisons of burrowing times and rates
between exposed and unexposed worms were not statistically
significant. Four out of 15 comparisons gave significant results but
these were randomly spread amongst species and exposure periods. The
authors concluded that these results would probably have little
ecological significance. The feeding behaviour of G. dibranchiata
was also monitored but no significant effect of the cadmium treatment
was found.
6.2.6 Interactions with other chemicals
Sunda et al. (1978) carried out experiments in diluted sea water
with various concentrations of the chelating agent nitrilotriacetic
acid (NTA) to determine the relationship between the chemical
speciation of cadmium and the toxicity of the metal to the grass
shrimp Palaemonetes pugio. After 4 days of exposure to a given
concentration of cadmium chloride, shrimp mortality decreased with
increasing salinity and increasing concentration of the chelating
agent. The protective effect of high salinity or NTA was attributable
to complexation of cadmium; mortality was related to the measured free
cadmium ion concentration, which was determined by measuring total
concentration of cadmium and deducting the calculated level of
complexation by either the chloride ion or NTA. The mortality at a
free cadmium ion concentration of approximately 4 x 10-7 mol/litre
was 50%. In a study of the combined effects of zinc and cadmium on the
shrimp Callianassa australiensis by Negilski et al. (1981), there
was interaction between the two metals; in combination they gave
greater mortality than would be expected if there were no interaction.
The authors demonstrated that each metal increased the accumulation of
the other.
6.2.7 Tolerance
Khan et al. (1988) exposed two different populations of grass
shrimp Palaemonetes pugio to cadmium under static conditions and
calculated 96-h LC50 values of 3.28 mg/litre for shrimps from an
industrialized area and 1.83 mg/litre for those from a non-
industrialized area. Pre-exposure of shrimps to 0.05 mg cadmium per
litre caused an increase in the LC50 values to 6.81 and 3.89
mg/litre for the two respective populations.
Moraitou-Apostalopoulou et al. (1982) collected the shrimp
Palaemon elegans from two different areas with different natural
concentrations of cadmium and investigated the sublethal effects of
the metal. They found that cadmium decreased respiration rates in the
shrimp at sublethal concentrations. Shrimps sampled from an area with
a natural cadmium concentration of 0.6 µg/litre were more tolerant to
the metal than were those from an area with 0.1 µg/litre. There was
also a difference in the acute toxicity of cadmium to the two
populations, indicating that tolerance had developed. In an earlier
study (Moraitou-Apostolopoulou et al., 1979), similar development of
tolerance was reported for a copepod Acartia clausi.
In a study of the toxicity of cadmium to the freshwater cyclopoid
copepod Tropocyclops prasinus mexicanus, Lalande & Pinel-Alloul
(1986) sampled animals from three Quebec lakes, one polluted and two
unpolluted with cadmium. The cultures from the two unpolluted lakes
showed lower LC50 values in 48-h tests than the culture from the
polluted lake. However, the polluted lake had a significantly higher
hardness (120 mg calcium carbonate per litre) than the other two lakes
(10 mg/litre).
6.2.8 Model ecosystems
Borgmann et al. (1989a) established a Daphnia-phytoplankton
model ecosystem and exposed it to cadmium sulfate at concentrations of
1, 5 and 15 µg cadmium/litre. At 5 µg/litre the Daphnia population
collapsed after 9 weeks of treatment and chlorophyll levels increased.
At 15 µg/litre the Daphnia population collapsed after 5 weeks, but
chlorophyll levels remained low. There appeared to be no effect on
this model system at 1 µg/litre.
6.3 Toxicity to Fish
The toxicity of cadmium has been studied in a variety of fish
species in both fresh and sea water at various temperatures and
dissolved oxygen concentrations. Generally, increasing dissolved salt
concentration decreases the toxicity, whereas increasing temperature
increases it. An increase in the dissolved oxygen content of the water
decreases the toxicity of cadmium to freshwater fish. Salmonids appear
to be particularly susceptible to the metal. Sublethal effects have
been reported, notably malformation of the spine.
6.3.1 Acute and short-term toxicity
The acute toxicity of cadmium to fish is summarized in Tables 11
and 12. Pickering & Henderson (1966) calculated the LC50 values for
five species of warm-water fish, some of which were tested in both
soft and hard water. There was surprisingly little difference in
fathead minnows ( Pimephales promelas) between the 24-h, 48-h, and
96-h LC50 values, which in soft water, were 1.09, 1.09, and 1.05
mg/litre, respectively. This species was very much more resistant to
cadmium in hard water with 24-h, 48-h, and 96-h LC50 values of 78.1,
72.6, and 72.6 mg/litre, respectively. The hardness for the two types
of water was 20 and 360 mg CaCO3 per litre and the alkalinity 80 and
300 mg/litre. The dissolved oxygen concentration was similar in the
two types of water. However, the pH was 7.5 for soft water and 8.2 for
hard water, this being an area of the pH range where speciation of
cadmium undergoes major change. Bluegill sunfish, goldfish, and
guppies showed a decrease in LC50 with increase in exposure duration
from 24 to 96 h (Table 12), but these were tested only in soft water.
The green sunfish Lepomis cyanellus showed LC50 values in soft
water of 7.84, 3.68, and 2.84 mg/litre with exposure durations of 24
h, 48 h, and 96 h, respectively. This species was also tested in hard
water, where, like the fathead minnow, it showed considerably less
cadmium toxicity. The 24-h LC50 rose from 7.84 in soft water to 88.6
mg/litre in hard water.
Pickering & Gast (1972) determined a maximum acceptable toxicant
concentration (MATC) for the fathead minnow Pimephales promelas of
between 37 and 57 µg cadmium/litre. The experimental concentration of
57 µg/litre decreased survival of the developing embryos, this being
the most sensitive life-stage, but at lower concentrations (between
4.5 and 37 µg/litre) no adverse effect was found on survival, growth
or reproduction. Carroll et al. (1979) investigated the protective
effects of various constituents of hard water on the toxicity of
cadmium to the brook trout ( Salvelinus fontinalis) and concluded
that calcium, added as either the sulfate or carbonate, was the most
significant source of protection. This protective effect was observed
in the absence of significant cadmium precipitation. Magnesium,
sulfate, and sodium ions and the carbonate system provided little or
no protection. Calamari et al. (1980) found an influence of water
hardness on the toxicity of cadmium to Salmo gairdneri; 48-h LC50
values increased from 91 µg/litre, with a hardness of 20 mg/litre in
the test water, to 3700 µg/litre at a water hardness of 320 mg/litre.
The 48-h LC50 of fish acclimatized to a hardness of 320 mg/litre and
then tested at a hardness of 20 mg/litre was about 7 times higher than
that of fish acclimatized and tested in the same soft water. There are
two types of biological effects of hardness on the availability of
cadmium to fish; one of them persists after acclimatization in hard
water.
Table 11. Toxicity of cadmium to marine or estuarine fish
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Striped killifish 0.95 g stat 20 20 8.0 24 125 n Eisler (1971)
(Fundulus majalis) 0.95 g stat 20 20 8.0 48 59 n Eisler (1971)
0.95 g stat 20 20 8.0 96 21 n Eisler (1971)
Mummichog 0.89 g stat 20 20 8.0 24 > 100 n Eisler (1971)
(Fundulus 0.89 g stat 20 20 8.0 48 > 100 n Eisler (1971)
heteroclitus) 0.89 g stat 20 20 8.0 96 55 n Eisler (1971)
1.3 g stat 20 20 7.8 24 220 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 96 22 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 168 22 n Eisler & Hennekey (1977)
1 day stat 20 20 48 16.2 (12.7-21.2) n Middaugh & Dean (1977)
7 days stat 20 20 48 9 (6.4-12.5) n Middaugh & Dean (1977)
14 days stat 20 20 48 32 (24.6-41.6) n Middaugh & Dean (1977)
adult stat 20 20 48 60 (40-90) n Middaugh & Dean (1977)
1 day stat 20 30 48 23 (19.2-27.6) n Middaugh & Dean (1977)
7 days stat 20 30 48 12 (9.2-15.6) n Middaugh & Dean (1977)
14 days stat 20 30 48 7.8 (5.6-10.3) n Middaugh & Dean (1977)
adult stat 20 30 48 43 (33-56) n Middaugh & Dean (1977)
Sheepshead minnow 1.1 g stat 20 20 8.0 24 100 n Eisler (1971)
(Cyprinodon 1.1 g stat 20 20 8.0 48 50 n Eisler (1971)
variegatus) 1.1 g stat 20 20 8.0 96 50 n Eisler (1971)
Coho salmon smolt flow 11.2 28.3 7.9 96 1.5 (1.2-2.4) m Dinnel et al. (1989)
(Oncorhynchus
kisutch)
Yellow-eye mullet 1.49 g flow 18.5 34.5 7.8 58 15.5 (12.2-19.8) m Negilski (1976)
(Aldrichetta 1.15 g flow 18.6 34.8 7.8 120 14.3 (8.4-24.3) m Negilski (1976)
forsteri)
Small-mouthed 1.36 g flow 17.9 34.5 7.9 168 12.7 (8.3-19.4) m Negilski (1976)
hardyhead 1.12 g flow 18.0 34.5 7.8 168 16.6 (13.3-20.7) m Negilski (1976)
(Atherinasoma microstoma)
Table 11 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Atlantic silverside adult stat 20 20 48 13 (9-20) n Middaugh & Dean (1977)
(Menidia menidia) adult stat 20 30 48 12 (8-16) n Middaugh & Dean (1977)
Tidewater silverside larva stat 26 22 96 0.31 (0.25-0.38) n Mayer (1987)
(Menidia peninsulae)
Shiner perch adult flow 13 30.1 7.8 96 11 (5-20) m Dinnel et al. (1989)
(Cymatogaster aggregata)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained)
b organisms exposed to cadmium added as cadmium chloride; m = measured concentration; n = nominal concentration
Table 12. Toxicity of cadmium to freshwater fish
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Chinook salmon juvenile flow 11-13 20-22 7.0-7.3 96 0.001 (± 0.0007) f m Finlayson & Verrue (1982)
(Onchorhynchus
tshawytscha)
Rainbow trout juvenile flow 6.4-8.3 96 0.0066 g m Hale (1977)
(Salmo 5-15 g stat 8.5-10.7 61-65 7.4 48 2.9 m Pascoe et al. (1986)
gairdneri) 5-15 g stat 8.5-10.7 283-317 7.4 48 5.7 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 1.3 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 2.6 m Pascoe et al. (1986)
Fathead minnow adult stat 25 20 7.5 24 1.09 (0.79-2.91) n Pickering & Henderson (1966)
(Pimephales adult stat 25 360 8.2 24 78.1 (57.2-117) n Pickering & Henderson (1966)
promelas) adult stat 25 20 7.5 48 1.09 (0.79-2.91) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 1.05 (0.7-4.43) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 18-22 190-210 7.7 48 0.1 (0.07-0.17) n Hall et al. (1986)
adult stat 18-22 190-210 7.7 96 0.09 (0.07-0.14) n Hall et al. (1986)
Bluegill sunfish adult stat 25 20 7.5 24 4.56 (3.64-6.08) n Pickering & Henderson (1966)
(Lepomis adult stat 25 20 7.5 48 2.76 (2.02-3.46) n Pickering & Henderson (1966)
macrochirus) adult stat 25 20 7.5 96 1.94 (1.33-2.35) n Pickering & Henderson (1966)
Goldfish adult stat 25 20 7.5 24 3.46 (2.85-4.82) n Pickering & Henderson (1966)
(Carassius adult stat 25 20 7.5 48 2.62 (2.04-3.68) n Pickering & Henderson (1966)
auratus) adult stat 25 20 7.5 96 2.34 (1.81-3.16) n Pickering & Henderson (1966)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Guppy adult stat 25 20 7.5 24 3.37 (2.73-4.81) n Pickering & Henderson (1966)
(Poecilia adult stat 25 20 7.5 48 2.31 (1.78-3.11) n Pickering & Henderson (1966)
reticulata) adult stat 25 20 7.5 96 1.27 (0.97-1.71) n Pickering & Henderson (1966)
3-4 weeks flow c 23-25 1 mM 24 10.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 48 5.7 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 72 4.3 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 96 3.8 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 24 33 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 48 20.5 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 72 14.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 96 11.1 m Canton & Slooff (1982)
Green sunfish adult stat 25 20 7.5 24 7.84 (6.13-14.2) n Pickering & Henderson (1966)
(Lepomis adult stat 25 360 8.2 24 88.6 (74-106) n Pickering & Henderson (1966)
cyanellus) adult stat 25 20 7.5 48 3.68 (2.89-4.69) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 71.3 (56.3-92.2) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 2.84 (2.1-3.56) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 66 (51.7-84.4) n Pickering & Henderson (1966)
Golden shiner flow 72.2 7.5 96 2.8 (1.9-4.3) m Hartwell et al. (1989)
(Notemigonus
crysoleucas)
Puntius 2.4 g stat b 23-27 60-70 7.5 24 59.99 (58.5-61.5) n Shivaraj & Patil (1988)
arulius 2.4 g stat b 23-27 60-70 7.5 48 45.7 (43.9-47.5) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 72 41.7 (39.7-43.8) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 96 39 (36.5-41.7) n Shivaraj & Patil (1988)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Killifish 4-5 weeks stat b 23-25 1 mM 48 > 2.8 m Canton & Slooff (1982)
(Oryzias 4-5 weeks stat b 23-25 1 mM 72 0.35 m Canton & Slooff (1982)
latipes) 4-5 weeks stat b 23-25 1 mM 96 0.35 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 24 > 2.6 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 48 1.8 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 72 0.17 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 96 0.13 m Canton & Slooff (1982)
Zebra fish 6 months flow 19-21 1.7 mM 24 7 m Canton & Slooff (1982)
(Brachydanio 6 months flow 19-21 1.7 mM 48 4.2 m Canton & Slooff (1982)
rerio)
a stat = static conditions (water unchanged for duration of test unless stated otherwise); flow = flow-through conditions
(cadmium concentration in water continuously maintained unless stated otherwise)
b static conditions but water renewed every 24 h
c intermittent flow-through conditions
d Hardness was expressed as mg CaCO3/litre unless stated otherwise
e fish were exposed to cadmium added as the chloride unless stated otherwise; m = measured concentration; n = nominal concentration
f cadmium was added as the sulfate
g cadmium was added as the nitrate
In a large scale study of the toxicity of cadmium to the
mummichog Fundulus heteroclitus, Voyer (1975) examined effects of
salinity, pre-exposure to high salinity and different concentrations
of dissolved oxygen on the tolerance of fish to cadmium over 96 h. He
found no significant influence of dissolved oxygen levels between 4.0
mg/litre and saturation regardless of salinity during acclimatization
or during the test. By contrast, Voyer et al. (1975) showed a distinct
effect of dissolved oxygen concentration on toxicity of cadmium to the
same species of fish in fresh water. Median tolerance concentrations
at 96 h ranged upwards from 1.3 to 3.0 mg cadmium/litre with 2.3 and
8.5 mg/litre of dissolved oxygen, respectively. They demonstrated
statistically an independent effect of dissolved oxygen and time
against cadmium toxicity. It should be noted that cadmium is 10 times
more toxic to this species in fresh water than in sea water.
Toxicity of cadmium to both marine (Eisler, 1971) and freshwater
(Roch & Maly, 1979) fish has been shown to be greater at higher
temperatures.
Canton & Slooff (1982) exposed several fish species to cadmium in
short-term toxicity tests. At a water hardness of 1.7 mmol/litre they
found the no-observed-adverse-effect level (NOAEL) for mortality in
the zebra fish ( Brachydanio rerio) to be 2 mg/litre over a 48-h
exposure period. For the killifish ( Oryzias latipes), the 96-h NOAEL
was 0.06 mg/litre for mortality and 0.03 mg/litre for mortality and
abnormal behaviour at a water hardness of 2 mmol/litre. The
corresponding values at a water hardness of 1 mmol/litre were 0.055
and 0.006 mg/litre. For the guppy ( Poecilia reticulata), the 96-h
NOAEL for mortality and abnormal behaviour was 5.2 mg/litre at a water
hardness of 2 mmol/litre and 0.6 mg/litre at 1 mmol/litre. The authors
calculated a 24-h NOAEL for the rainbow trout ( Salmo gairdneri) of
0.01 mg/litre for inhibition of opercular movements.
Abel & Papoutsoglou (1986) studied the toxicity of cadmium to
Cyprinus carpio and Tilapia aurea and reviewed data on other
species of freshwater fish. The found for all species examined that
the median survival time changed little over a wide range of cadmium
concentrations and that a toxic threshold was clear in most studies.
For Tilapia this threshold lay between 0.1 and 0.5 mg cadmium/litre;
negligible mortality was recorded in fish exposed to 0.1 mg/litre for
3 months.
6.3.2 Reproductive effects and effects on early life-stages
Meteyer et al. (1988) exposed sheepshead minnow ( Cyprinodon
variegatus) eggs to cadmium concentrations of between 0.39 and 1020
µg/litre from approximately 4 h after fertilization. Hatching was
delayed by up to 3 days at the highest cadmium concentration. All
treated larvae were shorter than controls, but there was no
dose-related effect of cadmium on growth.
Middaugh & Dean (1977) examined the toxicity of cadmium to
various life-stages of the mummichog Fundulus heteroclitus. In 48-h
tests, eggs were highly resistant to cadmium. The greatest effect (54%
non-emergence) occurred at a cadmium concentration of 32 mg/litre; the
control non-emergence was 17%. Newly-emerged larvae were less
sensitive to cadmium than were 7-day-old larvae. There was an effect
of salinity on the sensitivity of 14-day-old larvae to the metal
(Table 11). Adults were less sensitive to cadmium than were larvae.
Similar results were obtained with the Atlantic silverside ( Menidia
menidia), larvae being the most sensitive life-stage. Weis & Weis
(1977) found no effect of cadmium, at concentrations up to 10
mg/litre, on embryos of Fundulus heteroclitus. Rombough & Garside
(1982) found the most sensitive indicator of cadmium toxicity to early
life-stages of the Atlantic salmon ( Salmo salar) to be inhibition of
growth of alevins, where significant reductions occurred with cadmium
concentrations of 0.47 µg/litre. The LC50 for the interval between
fertilization and viable hatch lay between 300 and 800 µg per litre.
Newly hatched alevins showed a 24-h LC50 of between 1.5 and 2.4
mg/litre. Sensitivity increased sharply in late alevins and
significant mortality was recorded at a concentration of 8.2 µg/litre.
Eaton et al. (1978) exposed embryos and larvae of seven
freshwater fish species to nominal cadmium concentrations of 0, 0.4,
1.2, 3.7, 11.6, 33.3, and 100 mg/litre for periods ranging from 3 to
126 days. Actual concentrations were monitored and used in the
assessment of the results. Results were expressed in terms of
"standing crop", which the authors estimated as the product of the
proportion of fish surviving and their total biomass. The lowest
concentration of cadmium at which the standing crop was significantly
different from controls was approximately 12 µg/litre for the white
sucker, northern pike, smallmouth bass, west coast coho salmon, lake
trout, and brown trout exposed as embryos or larvae for up to 64 days.
The value was lower for brook trout (0.48 µg per litre) after exposure
of larvae/juveniles for 65 days and for Lake Superior coho salmon (3.4
µg/litre) after exposure of larvae/juveniles for 27 days. The highest
cadmium concentration at which standing crop was not significantly
different from controls varied for the seven species between 1.1 and
4.2 µg/litre.
Woodall et al. (1988) exposed rainbow trout ( Salmo gairdneri)
fry to cadmium concentrations of 0, 0.1, 1.0 or 5.0 mg/litre for up to
90 h. In preliminary experiments, they calculated that the 90-h LC50
lay between 0.1 and 1.0 mg/litre. Pre-treatment of trout fry with
cadmium initially had little effect (up to 30 h). However, with
exposure periods of between 45 and 90 h, some protection was induced
by pre-treatment. Benoit et al. (1976) exposed three generations of
brook trout ( Salvelinus fontinalis) to concentrations of total
cadmium varying between 0.06 and 6.4 µg/litre. Significant numbers of
first and second generation adult males died during spawning when
exposed to 3.4 µg/litre. This concentration also significantly
retarded the growth of juvenile second and third generation offspring,
but at a concentration of 1.7 µg/litre these effects were not seen.
In a study by Borgmann & Ralph (1986), white sucker larvae
Catostomus commersoni and young common shiners Notropis cornutus
were exposed to cadmium chloride at concentrations ranging from 6.24
to 200 µg cadmium/litre. The relative growth rate of fish was
significantly reduced at concentrations of 36 µg/litre or more in the
case of suckers and 63 µg/litre or more in the case of shiners.
Cadmium had no effect on the relative feeding rates.
Hatakeyama & Yasuno (1987) studied the chronic effects of cadmium
on the reproduction of the guppy Poecilia reticulata. The fish were
exposed to cadmium via cadmium-accumulated midge larvae used as their
food source. The cumulative numbers of fry produced by guppies fed
midge larvae containing 500, 800 or 1300 mg cadmium/kg for 6 months
decreased to 79%, 65%, and 55% of the controls, respectively. At the
highest dose, the mortality of females was significantly elevated at
6 months, but no such effect was observed with the males.
Michibata (1981) reported a protective effect of water hard-ness
against the effects of cadmium on the eggs of Oryzias latipes.
6.3.3 Metabolic, biochemical and physiological effects
Protective metal-binding proteins (metallothioneins) are induced
by cadmium in fish (chapter 4).
A manifest symptom of cadmium toxicity in freshwater fish is
ionic imbalance with reduced plasma Ca2+, Na+, and CL-. The
probable explanation is that cadmium is a potent inhibitor of
ion-transporting enzymes. Verbost et al. (1988) showed that cadmium
inhibited Ca-ATPase in the cell membranes of fish gut. It probably
does the same in the gills because cadmium exposure has been shown to
inhibit calcium uptake in the gills of adults (Verbost et al., 1987;
Reid & McDonald, 1988) as well as in larvae (Wright et al., 1985).
Similarly, cadmium has been shown to inhibit Na/K-ATPase in fish gills
(Watson & Benson 1987), which, taken with the fact that cadmium
probably also affects the production of ATP in the gills (Dickson et
al., 1982), could explain the reduction of plasma Na+. Huiguang Fu
(1989) studied the role of the hormones prolactin and cortisol in
correcting cadmium-induced impairment of the calcium balance in
tilapia ( Oreochromis mossambicus). Fish were able to recover from
initial hypocalcaemia during a 35-day exposure to a concentration of
10 µg cadmium/litre. This recovery involves prolactin-induced
stimulation of active Ca2+ uptake and reduction of passive Ca2+
efflux, together with cortisol-induced changes in chloride cells and
stimulation of metallothionein synthesis in the liver, kidney, and
gills. The author stated that the capacity to survive prolonged
exposure to 10 µg cadmium/litre through physiological adaptation does
not indicate that this cadmium concentration is acceptable to tilapia.
Adaptation may be achieved at the expense of other essential processes
like growth and reproduction.
Arillo et al. (1984) investigated the effect of cadmium at levels
of 1-10 µg/litre (concentrations above the water quality criteria
value of 0.75 µg/litre proposed for salmonids by EIFAC/FAO) on a wide
variety of biochemical parameters in the rainbow trout (Salmo
gairdneri). The exposure period was 4 months. Only at the highest
test concentration of 10 µg/litre were there any effects on the fish,
and then only on liver aminolevulinic acid dehydratase activity. The
authors concluded that the water quality criterion was realistic.
Dawson et al. (1977) exposed juvenile striped bass ( Morone
saxatilis) to cadmium chloride concentrations of 0.5, 2.5 or 5
µg/litre for 30 to 90 days, and the fish were then allowed to recover
for 30 days in clean, running sea water. There was an inhibition of
gill tissue respiration at 30 and 90 days, which recovered during the
30-day period with clean water. The activities of the various enzymes
measured were not affected. MacInnes et al. (1977) reported reduced
gill tissue oxygen consumption in cunner ( Tautogolabrus adspersus)
exposed to cadmium (0.05 or 0.1 mg/litre) for 30 or 60 days. They also
reported a reduced activity of aspartate aminotransferase and an
increased activity of glucose-6-phosphate dehydrogenase in the liver
of the fish after 30 days of exposure to cadmium. Gill & Pant (1983)
measured levels of various blood and tissue constituents after acute
(24 h) or chronic (90 day) exposure to cadmium. Acute exposure to
12.65 mg/litre led to significant hyperglycaemia and an increase in
liver, kidney, and ovarian cholesterol levels. Chronic exposure to
0.63 or 0.84 mg/litre, by contrast, led to an enduring hypoglycaemia
and diminished levels of cholesterol in tissues. Both acute and
chronic exposure to cadmium caused marked hypocholesterolaemia,
glycogenolysis in the liver and brain and a rise in myocardial
glycogen. Testis cholesterol was depleted after 60 days in both acute
and chronic exposures.
Sastry & Subhadra (1983) exposed the catfish Heteropneustes
fossilis to cadmium in the water at the sublethal concentration of
2.3 µmol/litre for 15 or 30 days. The cadmium caused reduced
absorption of glucose and fructose from the gut, this effect being
more pronounced after 30 days of exposure than after 15 days. Filling
intestinal sacs, in vivo, with cadmium solutions (1 µmol per litre)
reduced absorption of the sugars significantly over 1 h.
Merlini (1978) pre-treated immature sunfish ( Lepomis gibbosus)
with cadmium (0.004 mg/litre) and then fed treated and control fish
with a single ration containing 58Co-labelled vitamin B12. The
fish were subsequently fed non-radioactive food for 31 days before
sacrifice. The author reported that cadmium-treated fish stored
significantly less vitamin B12 in the liver than did controls.
Carrier & Beitinger (1988a) studied the effect of cadmium on the
critical thermal maximum (the temperature at which loss of equilibrium
is coupled with loss of righting response) in the red shiner
( Notropis lutrensis) and fathead minnow ( Pimephales promelas). The
red shiner was exposed to sublethal cadmium concentrations of 4.88,
5.07, and 5.46 mg/litre and the fathead minnow to 0.09, 0.48, and 1.26
mg/litre. Critical thermal maxima were significantly reduced for both
species over a 10-day exposure period. The effect was found to be dose
and time dependant. In the green sunfish ( Lepomis cyanellus) there
was no effect of cadmium concentrations of 2.76, 4.22 or 5.17 mg/litre
on the critical thermal maximum (Carrier & Beitinger, 1988b).
6.3.4 Structural effects and malformations
When Bengtsson et al. (1975) exposed 180 minnows ( Phoxinus
phoxinus) to various concentrations of cadmium ranging from 7.5
µg/litre to 4.8 mg/litre for 70 days, 31 of the 101 fish that survived
developed lesions in the spinal column. Fractured vertebrae occurred
in the caudal end of the abdominal section of the spine or in the
caudal section; 64% of all fractures occurred in the first 7 caudal
vertebrae and 21% occurred in abdominal vertebrae numbers 8 to 14.
Hiraoka & Okuda (1984) cultured medaka ( Oryzias latipes) eggs in a
cadmium solution of 0.01 mg/litre for 1 week and then investigated
abnormalities in the vertebrae of the hatched fry, which were reared
in clean water. There was no damage to the centra of the vertebrae in
newly hatched fry. However, centrum-damaged fish were found in the
first week and the numbers increased rapidly up to the 4th week after
hatch. The cumulative frequency of vertebral-damaged fish was 13% and
14% in the 5th and 6th weeks, respectively, and seemed to remain
constant after this. Muramoto (1981b) reported that fish showing
malformations of the spine after cadmium treatment had significantly
less calcium in the vertebral column than did control fish.
Voyer et al. (1975) found no short-term histopathological effects
of cadmium, but long-term exposure to 28 mg/litre caused necrosis and
sloughing of the mucosa of gills. Tissue damage was also evident in
the nasal passages and buccal cavity. This histopathological effect
was seen between exposure times of 512 and 612 h, but not before.
6.3.5 Behavioural effects
Hartwell et al. (1989) studied the avoidance response of the
golden shiner ( Notemigonus crysoleucas) to cadmium concentrations of
up to 68 µg/litre, but found no significant avoidance.
Sullivan et al. (1978b) subjected fathead minnows ( Pimephales
promelas) to acute (24 h) or subacute (21 days) exposure at
sublethal cadmium concentrations and then placed them in experimental
chambers with largemouth bass ( Micropterus salmoides), a fish which
preys upon them. The minnows displayed altered behaviour patterns,
including abnormal schooling behaviour, and were more vulnerable to
predation than controls. The lowest acute cadmium exposure level that
increased vulnerability was 0.375 mg/litre and the lowest subacute
level 0.025 mg/litre. The authors pointed out that the subacute value
of 0.025 mg/litre was well below reported no-effect levels of cadmium
for fathead minnows established with respect to survival and
reproductive effects. The avoidance threshold for cadmium in water by
the rainbow trout ( Salmo gairdneri) is 50 µg/litre (Black & Birge,
1980), which is 50 times higher than the 96-h LC50 value reported
for this species.
6.3.6 Interactions with other chemicals
Muramoto (1981a) showed that the chelating agents EDTA and DPTA
afforded some protection against the effects of cadmium on carp
( Cyprinus carpio) exposed to 0.05 or 0.1 mg cadmium/litre for 3
months. There were effects on the vertebrae of exposed fish.
Chelating agents that form hydrophobic complexes with heavy
metals increase the bioavailability of the metal to aquatic organisms.
Examples of these are xanthates and dithiocarbamates. Xanthates are
used in the mining industry in the flotation process to refine metal
from sulfide ores. As discussed in section 4.1, these compounds
increase the uptake rate of cadmium through the gills in fish (Block
& Part, 1986; Gottofrey et al., 1988; Block, 1991). Furthermore, they
change the tissue distribution of the metal in such a way that more
cadmium is found in lipid-rich tissues such as nervous tissue (brain)
and adipose tissue than when fish are exposed to the metal alone.
Finlayson & Verrue (1982) reported 96-h LC50 values for
juvenile chinook salmon ( Onchorynchus tshawytscha) ranging from 0.6
to 1.6 µg/litre. They found no synergistic or antagonistic toxic
effects after combining cadmium and zinc in their test system. The
results of tests were additive, the overall LC50 being a simple
combination of individual metal effects at a zinc:cadmium ratio of
1:0.008. Spehar et al. (1978b) exposed the flagfish Jordanella
floridae to cadmium and zinc, both individually and as a mixture, at
concentrations ranging from 4.3 to 8.5 µg cadmium/litre and 73.4 to
139 µg zinc/litre, through one complete life-cycle of the fish. There
was no additive effect of cadmium and zinc at sublethal concentrations
in mixed exposure. Effects on survival showed that the toxicity of
cadmium and zinc mixtures was only slightly, if at all, greater than
the toxicity of zinc alone. Anadu et al. (1989) reported that
pre-exposure of rainbow trout ( Salmo gairdneri) to zinc (100
µg/litre) for 17 days increased the subsequent 120-h LC50 for
cadmium from 1.1 µg/litre to 4.1 µg/litre.
6.4 Toxicity to Amphibia
Francis et al. (1984) exposed eggs of the leopard frog ( Rana
pipiens) to cadmium-enriched sediments during their development and
for 4 days after the larvae had hatched. Measured concentrations of
cadmium ranged from 1.04 to 1074 mg/kg in sediment and from 1.0 to
76.5 mg/litre in water above the sediment. Cadmium concentrations in
the tissues of the tadpoles at the end of the experiment ranged from
0.08 to 12.55 mg/kg. There was no mortality as a result of cadmium
exposure. The LC50 for this species has been reported to be 50
µg/litre for water without sediment by Westerman (1977). Slooff &
Baerselman (1980) determined 48-h LC50 values for the neotenous
larval mexican axolotl ( Ambystoma mexicanum) and larval South
African clawed toad ( Xenopus laevis) of 1.3 mg/litre and 32
mg/litre, respectively, after exposure to cadmium nitrate. Canton &
Slooff (1982) exposed Xenopus laevis to cadmium, added as cadmium
chloride, and obtained 24-h and 48-h LC50 values of 4 and 3.2
mg/litre, respectively. A NOEL of 2.2 mg/litre was reported for both
exposure periods. In a longer-term exposure (100 days), by the same
authors, an LC50 value of 1500 µg/litre was found and the EC50
value for inhibition of larval development was 650 µg/litre. For
mortality and larval development, the NOELs were 30 and 9 µg/litre,
respectively. De Zwart & Sloof (1987) determined a 48-h LC50 of 20.2
mg/litre for tadpoles of the clawed toad. Khangarot & Ray (1987)
established a 96-h LC50 value of 8.18 (6.96-9.53) mg/litre for the
tadpoles of the toad Bufo melanosticus. The test water was obtained
from a well and had a hardness of 185 mg per litre, pH 7.4, and
temperature of 31 °C. Solids were present at a level of 920 mg/litre.
Muino et al. (1990) calculated the 48-h and 96-h LC50 for Bufo
arenarum tadpoles to be 2.52 and 2.08 mg cadmium/litre for the two
exposure periods, respectively, under semi-static conditions. In tests
to study the effect of a sublethal cadmium concentration (1.0 mg
cadmium/litre) on the water balance of the animals, the authors found
that all animals died within a few hours in ion-free media. Tadpoles
exposed in ionic solutions showed mortality of less than 10%
(equivalent to control groups).
Woodall et al. (1988) exposed Xenopus laevis tadpoles to
cadmium concentrations of 0, 50, 80, and 100 mg cadmium/litre for up
to 90 h. In preliminary experiments, they calculated the 90-h LC50
to lie between 80 and 100 mg/litre. The authors found that
pre-treatment of tadpoles with cadmium induced protection, which
decreased with an increase in the subsequent exposure concentration.
Cadmium pre-treatment induced maximum protection to cadmium at a
concentration of 50 mg/litre at both 45 and 90 h.
Perez-Coll et al. (1986) exposed developing Bufo arenarum
embryos to cadmium chloride concentrations of 6 x 10-7 to 1.5 x
10-5 mol Cd2+/litre during gastrulation at 20 °C and 30 °C.
Initial failures at gastrulation resulted mainly in axial
incurvations, microcephaly, hydropsy, and abnormal tail development.
At the higher temperature, high concentrations of cadmium caused a
significant increase in early malformations and at low concentrations
the high temperature prevented alterations.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
Both terrestrial plants and animals accumulate cadmium, but the
rate of accumulation is much higher under experimental conditions,
where cadmium is available in solution, than it is with plants grown
in soil, when part of the cadmium is bound and less available.
Cadmium has adverse effects on hydroponically grown plants at
concentrations in the mg/litre range, whereas plants grown in soil
only show reduced growth in contaminated soils with hundreds of mg
cadmium/kg. Terrestrial invertebrates are relatively insensitive to
cadmium-induced toxic effects, probably due to effective
sequestration mechanisms in specific organs. When toxic effects do
occur, they consist of reduced growth and reproduction.
7.1 Toxicity to terrestrial plants
Cadmium has been shown to have an adverse effect on plant growth
and yield in laboratory experiments. However, plants grown in soil are
generally insensitive to the effects of cadmium except at high doses.
Effects are only seen when cadmium is given in nutrient solutions
rather than in soil, where the cadmium is bound and is therefore less
available to the plants. Cadmium is only available to plants in
solution in soil. There is considerable evidence from field studies
that plants are able to develop tolerance to various heavy metals in
their growth medium. Research into cadmium tolerance has been more
limited than for other metals, but there is some evidence of tolerance
developing.
7.1.1 Toxicity to plants grown hydroponically
Mitchell & Fretz (1977) cultured seedlings of three species of
tree, the white pine ( Pinus strobus), red maple ( Acer rubrum), and
Norway spruce ( Picea abies), in sand. Plants were irrigated with a
nutrient solution containing cadmium at concentrations of 0, 0.5, 1,
2, 4, 8, and 16 µg/litre; white pine seedlings were also treated with
32 and 64 µg/litre. Both roots and foliage were affected by the
cadmium. In the red maple symptoms of cadmium toxicity began with
interveinal chlorosis and stunting of leaves in most cases. As
exposure increased cadmium caused wilting and then death. The first
observed effect of the metal on the pine was inhibition of needle
expansion and, in the case of the spruce, chlorotic tips to new
growth. Tissue accumulation of cadmium correlated well with exposure.
Red maple, white pine, and spruce exhibited foliage effects at leaf
cadmium concentrations of 22.8, 61.3, and 7.5 mg/kg, respectively,
corresponding to nutrient solutions of 8.0, 32.0, and 4.0 µg/litre.
There was an increasing effect on root development with increasing
exposure to cadmium. At the higher doses, there was severe reduction
in the number of roots initiated and stunting of those that grew.
Accumulation of cadmium was greater in roots than in leaves.
Root et al. (1975) grew maize ( Zea mays) in hydroponic
solutions containing cadmium chloride at concentrations ranging from
1 to 40 mg/litre. Uptake of cadmium into the plants increased with
time, and cadmium was present at higher concentrations in roots than
in shoots. Leaf chlorophyll concentration and yield (as dry weight) of
both roots and shoots decreased with increasing cadmium concentration.
As the cadmium concentration in the leaves increased, the
concentration of zinc decreased and the concentration of iron
increased. This gave a linear correlation between cadmium in the leaf
and iron/zinc ratio. Chlorosis resulting from cadmium in the leaves
(seen at a cadmium concentration of 1 mg/litre nutrient solution)
appeared comparable to iron-deficiency chlorosis. However, in this
case, the chlorosis was not due to iron deficiency, as previously
suggested by other workers, but was associated in some way with an
increasing iron/zinc ratio.
Harkov et al. (1979) found no effect on the yield of tomatoes
grown in vermiculite and cultured with a nutrient solution containing
cadmium at concentrations of 0.25 or 0.75 mg/litre. The plants were
more susceptible to damage by ozone, under conditions where ozone
damage would have been slight, after exposure to cadmium. Where ozone
damage was heavy or when conditions were not conducive to ozone
damage, there was no effect of cadmium. When Wong et al. (1988)
exposed pea ( Pisum sativum) seeds to cadmium concentrations of 1, 5,
10, and 20 mg/litre in culture solutions, germination was
significantly reduced at 20 mg/litre and radicle elongation to 1 cm
was significantly reduced at 5 mg/litre and to 2 cm at 1 mg/litre.
Early development (shoot elongation, leaf development, root and shoot
growth) was inhibited in a dose-dependant manner. Slight inhibition
was observed at 1 mg/litre, increased inhibition at 5 mg/litre, and
significant inhibition at 10 and 20 mg/litre.
7.1.2 Toxicity to plants grown in soil
Mitchell & Fretz (1977) showed that the effects on the growth of
red maple, white pine and Norway spruce plants in soil, amended with
cadmium, were similar but less severe, owing to reduced uptake of the
metal, than in the case of the same plants grown hydroponically.
Cadmium only affected current growth of the plants, except where it
was present in excess. Mahler et al. (1978) treated eight soils, the
pH values of which ranged from 4.8 to 7.8, with 1% (by weight) sewage
sludge containing added cadmium sulfate, leading to cadmium
concentrations in the soil ranging from 0.1 to 320 mg/kg. Two plants,
lettuce ( Lactuca sativa variety longifolia) and Swiss chard ( Beta
vulgaris variety cicla), were grown in the soils in pots. The EC50
(yield) for lettuce was 214 and 139 mg/kg soil for acid and calcareous
soils, respectively, whereas the values for chard were 175 and 250
mg/kg for acid and calareous soils. The corresponding tissue
concentrations of cadmium associated with these effects were 470 and
160 mg/kg for lettuce and 714 and 203 mg/kg for chard. Thus, a
markedly lower tissue concentration of cadmium produced 50% yield
reduction on calcareous soils than on acid soils. Alloway et al.
(1990) reported stunted growth and toxic signs on leaves of lettuce,
cabbage, carrot, and radish plants, but only at the highest
concentrations of cadmium tested (which resulted in a cadmium content
of around 20 mg/kg in the upper parts of the plants).
7.1.3 In vitro physiological studies
Bazzaz et al. (1974) demonstrated an effect of cadmium on
transpiration and photosynthesis in excised sunflower heads. The heads
(15 cm diameter) were placed in flasks containing distilled water or
cadmium salt solutions (2, 20, 100 or 200 mg/litre) and transpiration
and photosynthesis were measured at daily intervals over 4 to 5 days.
Cadmium reduced transpiration and photosynthesis at concentrations of
100 or 200 mg/litre. Excised epidermal peels floating on solutions of
cadmium salts showed a log-linear relationship between metal
concentration and stomatal opening. The stomata opened less with
increasing cadmium concentration; this accounted for the effect on
transpiration and, hence, on photosynthesis.
7.2 Toxicity to terrestrial invertebrates
Haight et al. (1982) calculated 24-h, 48-h, and 72-h LC50
values of 36, 15.1, and 5.85 mg cadmium/litre, respectively, for
juvenile free-living nematodes ( Panagrellus silusiae) and 111, 26.3,
and 13.2 mg/litre for adults. Williams & Dusenbery (1990) exposed the
free-living nematode Caenorhabditis elegans to cadmium and found
values of 904, 22, and 1.5 mg/litre, respectively, for the same three
exposure periods. They also calculated a 96-h LC50 of 0.06 mg/litre.
Popham & Webster (1979) found that a 6-h exposure to 3.26 x
10-7 moles of cadmium significantly decreased the fecundity of C.
elegans. A 3.5-day exposure to 10-8 moles caused the same effect.
Nematodes exposed to 4 x 10-6 moles of cadmium never grew to the
same length as controls and resembled worms from starved, overcrowded
cultures. Van Kessel et al. (1989) exposed juvenile C. elegans to
various concentrations of cadmium chloride and found that growth and
subsequent reproduction were significantly reduced at 1 µmol/litre. At
levels of 160 and 320 µmol/litre the nematodes did not reach the adult
stage and, therefore, did not reproduce.
Doelman et al. (1984) exposed the soil nematodes Mesorhabditus
monhystera and Aphelenchus avenae to cadmium via food for up to 22
days and monitored the size of the population. M. monhystera was
exposed, via bacteria and fungi, to concentrations of 0.23, 4.4, and
12.7 mg cadmium/kg, and a significant reduction in the size of the
population was found at all doses. A. avenae was exposed, via fungi
alone, to concentrations of 1, 10, and 25 mg/kg. At 1 mg/kg there was
no effect on the population size, whereas at 10 mg/kg, a reduction was
observed until the final day. A concentration of 25 mg/kg
significantly reduced the size of the population. The authors noted
that exposure via fungi alone gave far more variable results.
In studies by Van Straalen et al. (1989), the collembolan
Orchesella cincta and the oribatid mite Platynothrus peltifer were
exposed to cadmium in the food. The 9-week LC50 values were 1.6 and
7.27 µmol/g and the no-observed-effect levels (NOEL) were 0.042 and
0.026 µmol/g for O. cincta and P. peltifer, respectively. The most
sensitive parameters were female growth for O. cincta and
reproduction in P. peltifer.
Russell et al. (1981) fed subadult garden snails ( Helix aspersa)
on diets containing six different levels of cadmium ranging from 10 to
1000 mg/kg diet over a period of 30 days. There was little mortality
(two animals out of 350 died, one at an exposure level of 50 mg/kg and
the other at 1000 mg/kg) but food consumption declined with each
increase in cadmium dose. Food consumption was strongly depressed at
cadmium doses of 100 mg/kg or more. Relative weight loss was the most
pronounced effect of cadmium treatment; this was dose related and
directly attributable to reduced feeding rates. At doses of 25 mg/kg
or more, shell growth and reproductive activity were depressed while
the incidence of sealing response (the sealing of the operculum with
a disc of mucus and a dormancy reaction) increased markedly. These
three effects were all related to the dietary cadmium concentration.
7.3 Toxicity to birds
7.3.1 Acute and short-term toxicity
The acute and short-term toxicity of cadmium salts to birds in
laboratory studies is summarized in Table 13. Dosing for 5 days,
followed by 3 days of clean diet, resulted in LC50 values generally
in excess of 2000 mg/kg diet. Only the pheasant showed greater
sensitivity to cadmium but, even in this species, the LC50 was close
to 1000 mg/kg diet. All the birds used were between 10 and 14 days
old.
Table 13. Toxicity of cadmium to birds a
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Japanese quail 14 cadmium chloride 2440 (1807-3294) Hill &
(Coturnix coturnix 14 cadmium succinate 2052 (1621-2598) Camardese
japonica) (1986)
Pheasant 10 cadmium chloride 767 (651-898) Hill et al.
(Phasianus 14 cadmium succinate 1411 (1202-1657) (1975)
colchicus)
Bobwhite quail 14 cadmium succinate 1728 (1381-2132) Hill et al.
(Colinus (1975)
virginianus)
Table 13 (contd).
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Mallard duck 10 cadmium chloride > 5000 Hill et al.
(Anas 10 cadmium succinate > 5000 (1975)
platyrhynchos)
a Birds were fed with a dosed diet for 5 days and then a "clean" diet for 3 days.
In a study by Pritzl et al. (1974), 2-week-old leghorn chicken chicks
were dosed with dietary cadmium chloride for 20 days. In the first
experiment, chicks dosed with 700 mg cadmium/kg diet showed an
increase in the weight of the gastrointestinal tract, kidney, and
gizzard expressed as a ratio to the body weight. In a second
experiment, chicks were fed diets containing 400, 600, 800 or 1000 mg
cadmium/kg. Weight gain and food consumption were decreased, relative
to controls, at all dose levels, and at levels higher than 400 mg/kg
the birds lost weight. All the birds fed diets containing 800 or 1000
mg/kg died within 20 days. The LC50 was calculated to be 565 mg/kg
diet.
When Cain et al. (1983) fed 1-day-old mallard ducklings ( Anas
platyrhynchos) a diet containing cadmium chloride at concentrations
of 5, 10 or 20 mg cadmium/kg for 12 weeks, significant effects were
only noted at the highest dose. These included a significant reduction
in packed cell volume and haemoglobin concentrations and a significant
increase in serum glutamic-pyruvic transaminase. Mild to severe kidney
lesions were evident in ducklings fed 20 mg/kg for 12 weeks. Body
weight, liver weight, and femur weight-to-length ratio were unaffected
by the cadmium treatment. No other haematological or histological
effects were found.
7.3.2 Reproductive effects
Lofts & Murton (1967) injected 0.2 ml of a solution of cadmium
chloride (0.04 mol/litre) intramuscularly into wood pigeons ( Columba
palumbus). When the cadmium was given to birds with regressed
testes, which were then stimulated into reproductive condition by long
photoperiods (16 h of light per day), there was a reduction in the
numbers of birds showing full testicular development in the treated
group. Only one out of six birds given cadmium had developed
spermatozoa in the testis by the end of the experiment. The remainder
had not even produced spermatids; two birds had only spermatogonia in
the seminiferous epithelium while the other three had secondary
spermatocytes. Of the six control birds, four had spermatozoa, one had
spermatids, and one primary spermatocytes. Injection of cadmium into
birds late in the season had no effect on the autumnal regression of
the testes. There was no sign of testicular necrosis in the treated
birds. An intratesticular injection of cadmium caused local necrosis
in the testis of feral pigeons. A dietary concentration of 200 mg
cadmium/kg, in the form of cadmium chloride, reduced spermatogenesis
in male mallards and egg production in females, but a lower dose of 20
mg/kg produced no effects (White & Finley, 1978; White et al., 1978).
7.3.3 Physiological effects
Mayack et al. (1981) found that the survival and growth of the
wood duck ( Aix sponsa) were unaffected by a cadmium chloride dietary
level of 100 mg/kg, although some kidney damage was reported.
Nicholson et al. (1983) compared the ultrastructure of the
kidneys of sea-birds contaminated with cadmium in the wild, sea-birds
from uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg per kg, respectively.
Damage was greatest in the proximal tubule of the kidney, and included
cell necrosis, nuclear pyknosis, mitochondrial swelling, and some
tubulorrhexis. The tubulorrhexis would be irreversible. There was some
indication of regeneration, judging from the number of
undifferentiated cells present in the tubule, in both dosed and
naturally contaminated birds. Debris was found in the distal nephron
lumen and there was some damage in the distal tubule and the renal
corpuscles. Necrosis of kidney cells was very rare in control birds or
uncontaminated sea-birds.
7.3.4 Behavioural effects
Heinz et al. (1983) assessed the avoidance response to a visual
fright stimulus of ducklings fed a diet containing cadmium chloride at
a concentration of 4 or 40 mg/kg. The parents of the ducklings had
also been fed this cadmium-containing diet. Ducklings fed 4 mg/kg were
hypersensitive to the fright stimulus, whereas those fed the higher
dose reacted as did controls. The authors could offer no explanation
of why the higher dose had no effect but pointed to similar results
from other materials. They were of the opinion that hypersensitivity
to behavioural signals could be as deleterious to the organism in the
wild as a failure to respond.
7.4 Toxicity to wild small mammals
Shore et al. (1991) fed herbivorous bank voles ( Clethrionomys
glareolus) and granivorous wood mice ( Apodemus sylvaticus) a
pelleted diet contaminated with cadmium chloride and collected urine
and faeces in metabolism cages. Bank voles fed diets containing 10.3
mg/kg for 40 days and then 4.5 mg/kg for 35 days suffered significant
net daily loss of calcium and sodium, and reduced net gain of
potassium and magnesium compared to controls. Assimilation of the
macroelements was not significantly altered in wood mice fed 10.3
mg/kg for 75 days.
8. EFFECTS IN THE FIELD
Appraisal
Tolerance to cadmium has been demonstrated in soil fungi,
plants, aquatic invertebrates, and fish from cadmium-contaminated
sites. Some field evidence suggests that cadmium is responsible for
reduced leaf litter degradation and a failure to recycle nutrients
due to adverse effects on populations, particularly of
microorganisms. However, no studies have identified cadmium as the
sole cause of the effect, since it is always associated with other
metals. Although soil invertebrates in contaminated sites accumulate
cadmium and other metals, there is evidence that most populations are
not affected. A field study has shown that fish from a
cadmium-contaminated river have physiological abnormalities. Kidney
damage has been found in pelagic sea-birds from areas away from
industrial or other anthropogenic sources of cadmium, but there was
no effect on survival or reproduction of populations. In industrially
contaminated areas, kidney damage has been observed in several
species of birds found to contain cadmium plus other metals.
8.1 Tolerance
Tolerance to cadmium has been demonstrated in soil fungi (section
5.2), aquatic invertebrates (section 6.2.7), and plants collected from
sites with high cadmium levels, such as those in the vicinity of
metalliferous mines and smelters.
Coughtrey & Martin (1977) experimentally demonstrated tolerance
of the grass Holcus lanatus to cadmium to be greater in plants
collected from an area subject to high fall-out of cadmium than in
plants collected from a control site. Growth of tolerant plants was
reduced in uncontaminated culture solutions, relative to controls, but
was similar to that of control plants in solutions where cadmium salts
had been added at levels similar to the field exposure. Simon (1977)
reported cadmium tolerance in the grasses Festuca ovina and
Agrostis tennuis. The tolerant grasses were collected from areas
contaminated by mining and aerial fall-out of cadmium.
8.2 Effects close to industrial sources and highways
There have been several reports of effects of heavy metal
deposition on the accumulation rate of leaf litter in deciduous
woodlands. These effects are restricted to areas close to, or down
wind from, smelter sites. The separation of the effects of cadmium
from those of other heavy metals present in the litter is difficult.
A study by Coughtrey et al. (1979) attempted to do this by detailed
analysis of the litter itself and by the use of statistics to separate
effects of different components of the pollution fall-out. Seven areas
of woodland in the vicinity of, or up to 28 km away from, a
lead-zinc-cadmium smelter in Avonmouth, United Kingdom, were studied.
The leaf litter contained lead, zinc, copper, and cadmium (in that
order of concentrations), and metal levels were high in samples taken
from within 3 km of the smelter. Litter from a wood 6.8 km from the
smelter had similar levels to control litter collected 30 km away, but
the prevailing wind would not have carried much of the fall-out in the
direction of this wood. For the four woods within 3 km of the smelter,
cadmium levels ranged from 23 to 98 mg/kg litter (lead levels were
between 721 and 2179 mg/kg, zinc levels between 764 and 2814 mg/kg,
and copper levels between 47 and 135 mg/kg). The weight of litter
accumulated per unit area was markedly greater in the contami-nated
sites than in the uncontaminated ones; litter standing crop ranged
from 7.91 to 13.16 kg/m2 in contaminated and from 0.913 to 3.104
kg/m2 in uncontaminated sites. The litter accumulation correlated
well with levels of all metals but not with the pH of the litter,
which varied between 3.88 and 6.3 over the sites. Partial correlation
analysis showed that cadmium and zinc interrelated; with both cadmium
and zinc, partial correlation coefficients were highly significant
when lead, copper or pH effects were accounted for. However,
correlations were low for lead and copper when cadmium or zinc were
accounted for. Of further interest was an analysis of cadmium and zinc
in leaf litter from various sites. There was an increase in the
smaller particle sizes in contaminated sites relative to
uncontaminated ones. These smaller particles contained a
disproportionate amount of the metals present, particularly in the
case of cadmium. Litter standing crop and cadmium concentrations were
highly correlated; the correlation between cadmium content and
different particle sizes of litter was better for small particles
sizes, and the slope of the regression line between cadmium
concentration and litter weight decreased with increasing particle
size. The authors argued that litter degradation was not affected at
the early stages but only when breakdown had progressed to much
smaller particle sizes. This perhaps supports the view that
microorganisms are inhibited by metals to a greater extent than
invertebrates, which would produce the initial reduction in size of
litter fragments. Taking the figure of 900 g/m2 as the normal leaf
litter level for woodland, the extra accumulation in contaminated
woods represented 25 to 30 years of litter accumulation (the smelter
in question had been operating for 48 years) and, possibly a large
proportion of the total capital of nutrients normally recycled to the
plants.
Other authors have also reported accumulation of leaf litter in
areas contaminated by metals (Tyler, 1972; Strojan, 1978), although
they disagree about the probable cause. Strojan (1978) proposed that
the effect relates to the absence of some groups of invertebrates,
while Jordan & Lechavalier (1975) suggested that the effect is on
microorganisms. There is no direct evidence that invertebrates in leaf
litter are adversely affected by metals, although they do accumulate
all the metals found in litter (Martin et al., 1976; Coughtrey &
Martin, 1976). Both Tyler (1972) and Strojan (1978) argued that the
productivity of woodland may be adversely affected by the failure to
recycle nutrients in areas contaminated by heavy metals. Coughtrey et
al. (1979) considered that the litter is an important sink for heavy
metals, and that the result of litter organisms developing tolerance
to the metals and, therefore, in the long-term increasing the rate of
degradation is unpredictable since metals would be released at the
same time as nutrients.
In a study by van Straalen et al. (1987), the metal excretion
efficiency of the collembolan Orchesella cincta collected from
various contaminated forest soils was monitored. The authors found
that moderate to high soil cadmium contamination of industrial origin
did not evoke increased cadmium excretion. In fact contamination
initiated in this century from a zinc factory caused a significant
decrease in excretion efficiency. Soils that had been contaminated
with cadmium for many years (lead/zinc factory) or to an extreme
degree (lead smelter) were inhabited by Collembola able to increase
metal excretion.
Muskett & Jones (1980) found levels of cadmium to be higher than
normal within 10 m of a road with a heavy traffic, but no effect on
the numbers of invertebrates caught or their species diversity was
observed.
8.3 Effects on fish
Field studies in Sweden showed that perch ( Perca fluviatilis)
from a cadmium-contaminated river (0.1 to 0.2 µg cadmium/litre) had
physiological abnormalities similar to those shown in laboratory
experiments (Sjobeck et al., 1984).
8.4 Effects on sea-birds
The reported effects on the kidney of sea-birds are not always a
result of exposure to cadmium as an industrial pollutant, since the
individuals most affected come from areas where there is no industrial
effluent. This is often, therefore, a response to naturally occurring
cadmium presumed to derive from the oceans. The birds appear to cope
with this damage to the kidney and suffer no effects on survival or
breeding success. No damage resulting from exposure to strictly
anthropogenically derived cadmium appears to have been reported on the
same scale as that from exposure to naturally occurring cadmium.
Nicholson et al. (1983) compared the ultrastructure of the kidneys of
sea-birds contaminated with cadmium in the wild, sea-birds from
uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg/kg, respectively (see
section 7.3.3). Nicholson & Osborn (1983) reported kidney lesions
(described in section 7.3.3) in several different species of sea-bird
caught in contaminated areas, although other pollutant metals such as
mercury were also present in the tissues.
9. EVALUATION
9.1 General considerations
In evaluating the environmental hazard of cadmium, it is
necessary to extrapolate from laboratories studies to ecosystems. This
must be done with extreme caution for a number of reasons.
a) The availability of cadmium to organisms in the environment is
limited by its strong adsorption to environmental components such
as soil, sediment, and organic matter. Organisms in contaminated
areas accumulate high body burdens of cadmium.
b) Environmental variables such as temperature, pH, and the chemical
composition of water or soil have been shown to affect both the
uptake and the toxic impact of cadmium.
c) Available, rather than nominal or total, cadmium is the
determinant in assessing uptake by, and effects on, organisms.
d) There are limited data from controlled experimental studies on
the effects of mixtures of metals. Organisms in the environment
are exposed to mixtures of pollutants. Acid deposition can
release metals, including cadmium, into the environment.
e) Little experimental work has been carried out on species or
communities that are either representative or key components of
natural communities and ecosystems. Studies have not considered
all of the interactions between populations and all of the
environmental factors affecting these populations. As a result,
the impact of cadmium on ecosystems may have been underestimated.
f) Results from laboratory studies based on very sensitive
parameters may be indicative of physiological impacts on
individuals rather than impacts on ecosystems.
9.2 The aquatic environment
Cadmium input to the aquatic environment is through dis-charge of
industrial waste, surface run-off, and deposition. It is strongly
adsorbed onto sediments and soils. The average cadmium content of sea
water is about 0.1 µg/litre or less, while fresh waters contain <
0.01 to 0.06 µg/litre in unpolluted areas. Cadmium levels of up to 5
mg/kg and 0.03 to 1 mg/kg have been reported for freshwater sediments
and marine sediments, respectively.
The rate of uptake and the toxic impact of cadmium on aquatic
organisms is greatly affected by physicochemical factors such as
temperature, ionic concentration, and organic matter content.
Cadmium is translocated by aquatic plants and concentrated in
roots and leaves. It is also taken up and accumulated by various
aquatic animals. The toxicity of cadmium to freshwater organisms
varies considerably depending on the exposure duration, species, and
life-stage. The early life-stages and the reproductive system are the
most vulnerable. Cadmium is, by comparison, one of the most toxic
heavy metals in the freshwater environment. Manifest responses of
certain organisms to cadmium are observed at environmental
concentrations lower than 1 µg/litre.
Cadmium-induced kidney damage has been reported in sea-birds
sampled from the field. However, this damage is present in both
cadmium-polluted areas and areas remote from industrial contamination.
The effect is probably, therefore, due to natural cadmium in certain
species and areas.
9.3 The terrestrial environment
Cadmium is introduced into the terrestrial environment from
mining, non-ferrous metal production, landfill sites and from the
application of sewage sludge, phosphate fertilizers, and manure.
Background concentrations of cadmium are in the range of 0.1 to 0.4
mg/kg soil and can reach 4.5 mg/kg in volcanic soils. Levels up to 160
mg/kg soil have been found close to metal processing sources.
Reduced breakdown of leaf litter and recycling of nutrients has
been attributed to metal pollution in the field. Cadmium appears to be
the most potent metal at inhibiting litter degradation. The effect is
thought to be due largely to reduced populations of microorganisms,
which are responsible for the final stages of litter decomposition.
Plants take up cadmium and can translocate and accumulate it.
However, uptake from soil is limited. Where there is high-level
exposure to cadmium (in the range of hundreds of mg/kg), growth
reduction is the major effect. Plants exposed to cadmium in the field
for long periods can develop tolerance to the metal. There is no
evidence of adverse effects of cadmium on plant populations in the
field.
Terrestrial invertebrates vary considerably in their sensitivity
to cadmium. Some species can take up and store cadmium to levels of up
to 5000 mg/kg body weight without apparent ill effects, while others
show population effects at levels of a few mg/kg soil. Populations of
some terrestrial invertebrates could be adversely affected at levels
of cadmium contamination seen in the field. Isopods and earthworms are
useful biomonitors for cadmium contamination. Invertebrates with high
body burdens may pose a threat to predators.
Kidney damage was found in experimental birds fed 20 mg
cadmium/kg diet for 12 weeks, but not at lower doses. Reproductive
effects have been observed at 200 mg/kg diet. A dose of 4 mg/kg
affected the behaviour of ducklings. No effects of cadmium have been
seen in terrestrial birds sampled from the field, although the cadmium
level in the brain, kidney, and liver of pigeons has proved to be a
good indicator of urban cadmium contamination.
Small mammals accumulate cadmium in the vicinity of mining spoil.
The ionic balance was affected in voles exposed experimentally to a
concentration of 10 mg/kg diet.
Populations of terrestrial organisms may also develop tolerance
to cadmium after long-term exposure.
10. RECOMMENDATIONS FOR PROTECTING THE ENVIRONMENT
To eliminate environmental effects, emissions of cadmium from the
following sources should be reduced as far as is practicable:
* smelters
* incinerators
* sewage sludge applied to the land
* phosphate fertilisers
* cadmium-containing manure
11. FURTHER RESEARCH
a) More study is needed to clarify the effects of cadmium on the
decomposition process of plant debris. Effects on the degree of
nutrient cycling and long-term plant growth and the exact nature
of the inhibition of decomposition require further attention.
b) The adsorption of cadmium to soil and sediment requires further
study and quantification of coefficients. Modelling of binding
and distribution in the environment is needed.
c) Organisms that are particularly sensitive (i.e. indicator
species) or that play a critical role in ecological systems
should be identified and studied with regard to the effects of
cadmium.
d) Studies are needed on the basic mechanisms by which cadmium
interacts with physiological and biochemical processes in
organisms and within individual cells.
There is a need to take certain precautions in studies on
cadmium. Firstly, the speciation of the metal should be considered in
experimental design and procedures and a clear measure of the
available cadmium should be reported. Secondly, studies of the uptake
and movement between trophic levels should include the relationship
between the non-nutrient cadmium and the nutrients calcium and zinc.
REFERENCES
ABEL, P.D. & PAPOUTSOGLOU, S.E. (1986) Lethal toxicity of cadmium to
Cyprinus carpio and Tilapia aurea. Bull. environ. Contam.
Toxicol., 37: 382-386.
ADRIANO, D.C. (1986) Trace elements in the terrestrial environment,
Berlin, Heidelberg, New York, Springer-Verlag, 533 pp.
AHSANULLAH, M. (1976) Acute toxicity of cadmium and zinc to seven
invertebrate species from Western Port, Victoria. Aust. J. mar.
freshwater Res., 27: 187-196.
ALLOWAY, B.J., JACKSON, A.P., & MORGAN, H. (1990) The accumulation of
cadmium by vegetables grown on soils contaminated from a variety of
sources. Sci. total Environ., 91: 223-236.
ANADU, D.I., CHAPMAN, G.A., CURTIS, L.R., & TUBB, R.A. (1989) Effect
of zinc exposure on subsequent acute tolerance to heavy metals in
rainbow trout. Bull. environ. Contam. Toxicol., 43: 329-336.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDREWS, S.M., JOHNSON, M.S., & COOKE, J.A. (1984) Cadmium in small
mammals from grassland established on metalliferous mine waste.
Environ. Pollut., 33A: 153-162.
ARILLO, A., CALAMARI, D., MARGIOCCO, C., MELODIA, F., & MENSI, P.
(1984) Biochemical effects of long-term exposure to cadmium and copper
on rainbow trout ( Salmo gairdneri): validation of water quality
criteria. Ecotoxicol. environ. Saf., 8: 106-117.
AYLETT, B.J. (1979) The chemistry and bioinorganic chemistry of
cadmium. In: Webb, M., ed. The chemistry, biochemistry and biology of
cadmium, Amsterdam, Elsevier/North Holland, pp. 1-43.
BABICH, H. & STOTZKY, G. (1977a) Reductions in the toxicity of cadmium
to microorganisms by clay minerals. Appl. environ. Microbiol., 33:
696-705.
BABICH, H. & STOTZKY, G. (1977b) Effect of cadmium on fungi and on
interactions between fungi and bacteria in soil: influence of clay
minerals and pH. Appl. environ. Microbiol., 33: 1059-1066.
BAHNER, L.H. & NIMMO, D.R. (1975) Methods to assess effects of
combinations of toxicants, salinity and temperature on estuarine
animals. In: Hemphill, D.D., ed. Trace substances in environmental
health - IX, Columbia, University of Missouri, pp. 169-177.
BAUDOUIN, M.F. & SCOPPA, P. (1974) Acute toxicity of various metals to
freshwater zooplankton. Bull. environ. Contam. Toxicol., 12: 745-751.
BAZZAZ, F.A., CARLSON, R.W., & ROLFE, G.L. (1974) The effect of heavy
metals on plants: part 1. Inhibition of gas exchange in sunflower by
Pb, Cd, Ni and Tl. Environ. Pollut., 7A: 241-246.
BEHNE, D. (1980) Problems of sampling and sample preparation for trace
element analysis in the health sciences. In: Bratter, P. & Scramel,
P., ed. Trace elements analytical chemistry in medicine and biology.
Proceedings of the 1st International Workshop, Neuherberg, Germany,
Berlin, Walter de Gruyter, pp. 769-782.
BENGTSSON, B-E. & BERGSTROM, B. (1987) A flowthrough fecundity test
with Nitocra spinipes (Harpacticoidea Crustacea) for aquatic
toxicity. Ecotoxicol. environ. Saf., 14: 260-268.
BENGTSSON, B-E., CARLIN, C.H., LARSSON, A., & SVANBERG, O. (1975)
Vertebral damage in minnows, Phoxinus phoxinus L., exposed to
cadmium. Ambio, 4: 166-168.
BENNETT, W.N. (1990) Measurement of manganese amelioration of cadmium
toxicity in Chlorella pyrenoidosa using turbidostat culture. Arch.
environ. Contam. Toxicol., 19: 118-123.
BENOIT, D.A., LEONARD, E.N., CHRISTENSEN, G.M., & FIANDT, J.T. (1976)
Toxic effects of cadmium on three generations of brook trout
( Salvelinus fontinalis). Trans. Am. Fish. Soc., 105: 550-560.
BERGER, B. & DALLINGER, R. (1989) Accumulation of cadmium and copper
by the terrestrial snail Arianta arbustorum L.: kinetics and
budgets. Oecologia, 79: 60-65.
BERGLIND, R. (1986) Combined and separate effects of cadmium, lead and
zinc on ALA-D activity, growth and hemoglobin content in Daphnia
magna. Environ. Toxicol. Chem., 5: 989-995.
BERK, S.G., GUNDERSON, J.H., & DERK, L.A. (1985) Effects of cadmium
and copper on chemotaxis of marine and freshwater ciliates. Bull.
environ. Contam. Toxicol., 34: 897-903.
BERTRAM, P.E. & HART, B.A. (1979) Longevity and reproduction of
Daphnia pulex (de Geer) exposed to cadmium-contaminated food or
water. Environ. Pollut., 19A: 295-305.
BESTEN, P.J. DEN, HERWIG, H.J., ZANDEE, D.I., & VOOGT, P.A. (1989)
Effects of cadmium and PCBs on reproduction of the sea star Asterias
rubens: Aberrations in the early development. Ecotoxicol. environ.
Saf., 18: 173-180.
BEWLEY, R.J.F. & STOTZKY, G. (1983) Effects of cadmium and zinc on
microbial activity in soil; influence of clay minerals. Part 1: Metals
added individually. Sci. total Environ., 31: 41-55.
BEYER, W.N., CHANEY, R.L., & MULHERN, B.M. (1982) Heavy metal
concentrations in earthworms from soil amended with sewage sludge. J.
environ. Qual., 11: 381-385.
BEYER, W.N., PATTEE, O.H., SILEO, L., HOFFMAN, D.J., & MULHERN, B.M.
(1985) Metal contamination in wildlife living near two zinc smelters.
Environ. Pollut., 38A: 63-86.
BIESINGER, K.E. & CHRISTENSEN, G.M. (1972) Effects of various metals
on survival, growth, reproduction, and metabolism of Daphnia magna.
J. Fish Res. Board Can., 29: 1691-1700.
BINGHAM, F.T., PAGE, A.L., & STRONG, J.E. (1980) Yield and cadmium
content of rice grain in relation to addition rate of cadmium, copper,
nickel and zinc, with sewage sludge and liming. Soil Sci., 130: 32-38.
BLACK, J.A. & BIRGE, W.J. (1989) An avoidance response bioassay for
aquatic pollutants, Lexington, Kentucky, University of Kentucky, Water
Resources Research Institute (Research Report No. 123).
BLOCK, M. (1991) Uptake of cadmium in fish. Effects of xanthates and
diethyldithiocarbamate, University of Uppsala, Sweden, Faculty of
Sciences (Universitatis Uppsaliensis - Comprehensive summaries of
Uppsala dissertations from the Faculty of Science. No. 326).
BLOCK, M. & PART, P. (1986) Increased availability of cadmium to
perfused rainbow trout ( Salmo gairdneri Rich.) gills in the presence
of the complexing agents diethyl dithiocarbamate, ethyl xanthate and
isopropyl xanthate. Aquat. Toxicol., 8: 295-302.
BOND, H., LIGHTHART, B., SHIMABUKU, R., & RUSSELL, L. (1976) Some
effects of cadmium on coniferous forest soil and litter microcosms.
Soil Sci., 121: 278-287.
BORGMANN, U. (1983) Metal speciation and toxicity of free metal ions
to aquatic biota. In: Nriagu, J.O., ed. Aquatic toxicology, New York,
Chichester, John Wiley & Sons, pp. 47-72.
BORGMANN, U. & RALPH, K.M. (1986) Effects of cadmium,
2,4-dichlorophenol, and pentachlorophenol on feeding, growth, and
particle-size-conversion efficiency of white sucker larvae and young
common shiners. Arch. environ. Contam. Toxicol., 15: 473-480.
BORGMANN, U., MILLARD, E.S., & CHARLTON, C.C. (1989a) Effect of
cadmium on a stable, large volume, laboratory ecosystem containing
Daphnia and phytoplankton. Can. J. Fish. aquat. Sci., 46: 399-405.
BORGMANN, U., RALPH, K.M., & NORWOOD, W.P. (1989b) Toxicity test
procedures for Hyalella azteca, and chronic toxicity of cadmium and
pentachlorophenol to H. azteca, Gammarus fasciatus, and Daphnia
magna. Arch. environ. Contam. Toxicol., 18: 756-764.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BRESSAN, M. & BRUNETTI, R. (1988) The effects of nitriloacetic acid,
Cd and Hg on the marine algae Dunaliella tertiolecta and Isochrysis
galbana. Water Res., 22: 553-556.
BROOKS, R.R. & RUMSBY, M.G. (1967) Studies on the uptake of cadmium by
the oyster, Ostrea sinuata (Lamarck). Aust. J. mar. freshwater Res.,
15: 53-61.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BULL, K.R., MURTON, R.K., OSBORN, D., WARD, P., & CHENG, L. (1977)
High levels of cadmium in Atlantic seabirds and sea-skaters. Nature
(Lond.), 269: 507-509.
BURKITT, A., LESTER, P., & NICKLESS, G. (1972) Distribution of heavy
metals in the vicinity of an industrial complex. Nature (Lond.), 238:
327-328.
BYRNE, A.R., RAVNIK, V., & KOSTA, L. (1976) Trace element
concentrations in higher fungi. Sci. total Environ., 6: 65-78.
CAIN, J.R., PASCHAL, D.C., & HAYDEN, C.M. (1980) Toxicity and
bioaccumulation of cadmium in the colonial green alga Scenedesmus
obliquus. Arch. environ. Contam. Toxicol., 9: 9-16.
CAIN, B.W., SILEO, L., FRANSON, J.C., & MOORE, J. (1983) Effects of
dietary cadmium on mallard ducklings. Environ. Res., 32: 286-297.
CALABRESE, A., COLLIER, R.S., NELSON, D.A. & MACINNES, J.R. (1973) The
toxicity of heavy metals to embryos of the American oyster
Crassostrea virginica. Mar. Biol., 18: 162-166.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1980) Influence of water
hardness on cadmium toxicity to Salmo gairdneri Rich. Water Res.,
14: 1421-1426.
CANTON, J.H. & SLOOFF, W. (1982) Toxicity and accumulation studies of
cadmium (Cd2+) with freshwater organisms of different trophic
levels. Ecotoxicol. environ. Saf., 6: 113-128.
CARRIER, R. & BEITINGER, T.L. (1988a) Reduction in thermal tolerance
of Notropis lutrensis and Pimephales promelas exposed to cadmium.
Water Res., 22: 511-515.
CARRIER, R. & BEITINGER, T.L. (1988b) Resistance of temperature
tolerance ability of green sunfish to cadmium exposure. Bull. environ.
Contam. Toxicol., 40: 475-480.
CARROLL, J.J., ELLIS, S.J., & OLIVER, W.S. (1979) Influences of
hardness constituents on the acute toxicity of cadmium to brook trout
( Salvelinus fontinalis). Bull. environ. Contam. Toxicol., 22:
575-581.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living near
a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CATALDO, D.A. & WILDUNG, R.E. (1978) Soil and plant factors
influencing the accumulation of heavy metals by plants. Environ.
Health Perspect., 27: 149-159.
CEARLEY, J.E. & COLEMAN, R.L. (1974) Cadmium toxicity and
bioconcentration in largemouth bass and bluegill. Bull. environ.
Contam. Toxicol., 11: 146-151.
CHAN, K.-Y. & DEAN, A.C.R (1988) Effects of cadmium and lead on
growth, respiration and enzyme activity of the marine bacterium
Pseudomonas marina. Chemosphere, 17: 597-607.
CHANDINI, T. (1988) Changes in food [ Chlorella] levels and the acute
toxicity of cadmium to Daphnia carinata (Daphnidae) and Echinisca
triserialis (Macrothricidae) [Crustacea: Cladocera]. Bull. environ.
Contam. Toxicol., 41: 398-403.
CHANDINI, T. (1989) Survival, growth and reproduction of Daphnia
carinata (Crustacea: Cladocera) exposed to chronic cadmium stress at
different food ( Chlorella) levels. Environ. Pollut., 60: 29-45.
CHANEY, R.L., WHITE, M.C., & SIMON, P.W. (1975) Plant uptake of heavy
metals from sewage sludge applied to land. In: Proceedings of the 2nd
National Conference on Municipal Sludge Management, Rockville,
Information Transfer Inc., pp. 169-178.
CHAPMAN, G. & DUNLOP, S. (1981) Detoxification of zinc and cadmium by
the freshwater protozoan Tetrahymena pyriformis. I. The effect of
water hardness. Environ. Res., 26: 81-86.
CHIN, B. & SINA, J.F. (1978) Cellular bases of cadmium toxicity in
Physarum. In: Hemphill, D.D., ed. Trace substances in environmental
health, Columbia, University of Missouri, pp. 214-219.
CHRISTENSEN, J.J., EATOUGH, D.J., & IZATT, R.M. (1975) In: Handbook of
metal ligand heats and related thermodynamic quantities, 2nd ed.
revis. expand., New York, Basel, Marcel Dekker, Inc., 495 pp.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975a) Synergism between
dissolved oxygen and cadmium toxicity in five species of aquatic
insects. Environ. Res., 9: 285-289.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975b) Acute cadmium
toxicity studies upon nine species of aquatic insects. Environ. Res.,
9: 331-341.
CONRAD, G.W. (1988) Heavy metal effects on cellular shape changes,
cleavage, and larval development of the marine gastropod mollusk,
( Ilyanassa obsoleta Say). Bull. environ. Contam. Toxicol., 41:
79-85.
CONWAY, H.L. (1978) Sorption of arsenic and cadmium and their effects
on growth, micronutrient utilization, and photosynthetic pigment
composition of Asterionella formosa. J. Fish Res. Board. Can., 35:
286-294.
COOMBS, T.L. (1979) Cadmium in aquatic organisms. In: Webb, M., ed.
The chemistry, biochemistry and biology of cadmium, Amsterdam,
Elsevier/North Holland, pp. 93-139.
COUGHTREY, P.J. & MARTIN, M.H. (1976) The distribution of Pb, Zn, Cd
and Cu within the pulmonate mollusc Helix aspersa Muller. Oecologia,
23: 315-322.
COUGHTREY, P.J. & MARTIN, M.H. (1977) Cadmium tolerance of Holcus
lanatus from a site contaminated by aerial fallout. New Phytol., 79:
273-280.
COUGHTREY, P.J., JONES, C.H., MARTIN, M.H., & SHALES, S.W. (1979)
Litter accumulation in woodlands contaminated by Pb, Zn, Cd and Cu.
Oecologia, 39: 51-60.
DALLA VIA, G.J., DALLINGER, R., & CARPENE, E. (1989) Effects of
cadmium on Murex trunculus from the Adriatic Sea. II. Oxygen
consumption and acclimation effects. Arch. environ. Contam. Toxicol.,
18: 562-567.
DALLINGER, R. & WIESER, W. (1984) Patterns of accumulation,
distribution and liberation of Zn, Cu, Cd and Pb in different organs
of the land snail Helix pomatia L. Comp. Biochem. Physiol., 79C:
117-124.
DALLINGER, R., PROSI, F., SEGNER, H., & BACK, H. (1987) Contaminated
food and uptake of heavy metals by fish: a review and proposal for
further research. Oecologia, 73: 91-98.
DAWSON, M.A., GOULD, E., THURBERG, F.P., & CALABRESE, A. (1977)
Physiological response of juvenile striped bass, Morone saxatilis,
to low levels of cadmium and mercury. Chesapeake Sci., 18: 353-359.
DENTON, G.R.W. & BURDON-JONES, C. (1981) Influence of temperature and
salinity on the uptake, distribution and depuration of mercury,
cadmium and lead by the black-lip oyster Saccostrea echinata. Mar.
Biol., 64: 317-326.
DE VRIES, M.P.C. & TILLER, K.G. (1978) Sewage sludge as a soil
amendment with special reference to Cd, Cu, Mn, Ni, Pb and Zn -
comparison of results from experiments conducted inside and outside a
glasshouse. Environ. Pollut., 16A: 231-240.
DE ZWART, D. & SLOOF, W. (1987) Toxicity of mixtures of heavy metals
and petrochemicals to Xenopus laevis. Bull. environ. Contam.
Toxicol., 38: 345-351.
DICKSON, G.W., GIESY, J.P., & BRIESE, L.A. (1982) The effect of
chronic cadmium exposure on phosphodenylate concentrations and
adenylate energy of gills and dorsal muscle of crayfish. Environ.
Toxicol. Chem., 1: 147-156.
DINNEL, P.A., LINK, J.M., STOBER, Q.J., LETOURNEAU, M.W., & ROBERTS,
W.E. (1989) Comparative sensitivity of sea urchin sperm bioassays to
metals and pesticides. Arch. environ. Contam. Toxicol., 18: 748-755.
DOELMAN, P., NIEBOER, G., SCHROOTEN, J., & VISSER, M. (1984)
Antagonistic and synergistic toxic effects of Pb and Cd in a simple
foodchain: Nematodes feeding on bacteria or fungi. Bull. environ.
Contam. Toxicol., 32: 717-723.
DONGMANN, G. & NURNBERG, H.W. (1982) Observations with Thalassiosira
rotula (Meunier) on the toxicity and accumulation of cadmium and
nickel. Ecotoxicol. environ. Saf., 6: 535-544.
DOUBEN, P.E.T. (1989a) Uptake and elimination of waterborne cadmium by
the fish Noemacheilus barbatulus L. (stone loach). Arch. environ.
Contam. Toxicol., 18: 576-586.
DOUBEN, P.E.T. (1989b) Metabolic rate and uptake and loss of cadmium
from food by the fish Noemacheilus barbatulus L. (stone loach).
Environ. Pollut., 59: 177-202.
DRESCHER, H.E., HARMS, U., & HUSCHENBETH. E. (1977) Organochlorines
and heavy metals in the harbour seal Phoca vitulina from the German
North Sea coast. Mar. Biol., 41: 99-106.
EATON, J.G., MCKIM, J.M., & HOLCOMBE, G.W. (1978) Metal toxicity to
embryos and larvae of seven freshwater fish species - I. Cadmium.
Bull. environ. Contam. Toxicol., 19: 95-103.
EISLER, R. (1971) Cadmium poisoning in Fundulus heteroclitus
(Pisces: Cyprinodontidae) and other marine organism. J. Fish Res.
Board Can., 28: 1225-1234.
EISLER, R. (1985) Cadmium hazards to fish, wildlife and invertebrates:
A synoptic review, Washington, DC, US Department of the Interior, Fish
and Wildlife Service (Biological Report No. 85).
EISLER, R. & HENNEKEY, R.J. (1977) Acute toxicities of Cd2+, Cr+6,
Hg2+, Ni2+ and Zn2+ to estuarine macrofauna. Arch. environ.
Contam. Toxicol., 6: 315-323.
EISLER, R., ZAROOGIAN, G.E., & HENNEKEY, R.J. (1972) Cadmium uptake by
marine organisms. J. Fish Res. Board. Can., 29: 1367-1369.
ELLIOT, N.G., SWAIN, R., & RITZ, D.A. (1985) The influence of cyclic
exposure on the accumulation of heavy metals by Mytilus edulis
planulatus (Lamarck). Mar. environ. Res., 15: 17-30.
ERIKSSON, J.E. (1988) The effects of clay, organic matter and time on
adsorption and plant uptake of cadmium added to the soil. Water Air
Soil Pollut., 40: 359-373.
FALCONER, C.R., DAVIES, I.M., & TOPPING, G. (1983) Trace metals in the
common porpoise, Phocoena phocoena. Mar. environ. Res., 8: 119-127.
FERARD, J.F., JOUANY, J.M., TRUHAUT, R., & VASSEUR, P. (1983)
Accumulation of cadmium in a freshwater food chain experimental model.
Ecotoxicol. environ. Saf., 7: 43-52.
FINLAYSON, B.J. & VERRUE, K.M. (1982) Toxicities of copper, zinc, and
cadmium mixtures to juvenile chinook salmon. Trans. Am. Fish. Soc.,
111: 645-650.
FLATAU, G.N., CLEMENT, R.L., GAUTHIER, M.J., & LAUMOND, F.M. (1987)
Adaptation to cadmium in a sensitive marine pseudomonad. Environ.
Technol. Lett, 8: 641-646.
FLATAU, G.N., CLEMENT, R.L., & GAUTHIER, M.J. (1988) Uptake of cadmium
by marine bacteria. Prog. Oceanogr., 21: 181-188.
FLEISCHER, M., SAROFIM, A.F., FASSETT, D.W., HAMMOND, P., SHACKLETTE,
H.T., NISBET, I.C.T., & EPSTEIN, S. (1974) Environmental impact of
cadmium: a review by the Panel on Hazardous Trace Substances. Environ.
Health Perspect., 5: 253-323.
FLIK, G., VAN DE WINKEL, J.G.J., PART, P., WENDELAAR BONGA, S.E., &
LOCK, R.A.C. (1987) Calmodulin mediated cadmium inhibition of
phosphodiesterase activity. Arch. Toxicol., 59: 352-359.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FRANCIS, P.C., BIRGE, W.J., & BLACK, J.A. (1984) Effects of
cadmium-enriched sediment on fish and amphibian embryo-larval stages.
Ecotoxicol. environ. Saf., 8: 378-387.
FRANK, P.M. & ROBERTSON, P.B. (1979) The influence of salinity on
toxicity of cadmium and chromium to the blue crab, Callinectes
sapidus. Bull. environ. Contam. Toxicol., 21: 74-78.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
fourteenth session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WHO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the Mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization (Reports
and Studies No. 31).
GIATTINA, J.D. & GARTON, R.R. (1983) A review of the
preference-avoidance responses of fishes to aquatic contaminants.
Residue Rev., 87: 43-90.
GIESY, J.P., LEVERSEE, G.J., & WILLIAMS, D.R. (1977) Effects of
naturally occurring aquatic organic fractions on cadmium toxicity to
Simocephalus serrulatus (Daphnidae) and Gambusia affinis
(Poeciliidae). Water Res., 11: 1013-1020.
GILL, T.S. & PANT, J.C. (1983) Cadmium toxicity: inducement of changes
in blood and tissue metabolites in fish. Toxicol. Lett., 18: 195-200.
GISH, C.D. & CHRISTENSEN, R.E. (1973) Cadmium, nickel, lead and zinc
in earthworms from roadside soil. Environ. Sci. Technol., 7:
1060-1062.
GOTTOFREY, J. (1990) The disposition of cadmium, nickel, mercury and
methylmercury in fish and effects of lipophilic metal chelation,
Uppsala, Swedish University of Agricultural Sciences, Department of
Pharmacology and Toxicology (Thesis).
GOTTOFREY, J., BJORKLUND, I., & TJALVE, H. (1988) Effects of sodium
isoproylxanthate, potassium amylxanthate and sodium
diethyldithiocarbamate on the uptake and disposition of cadmium in the
brown trout ( Salmo trutta). Aquat. Toxicol., 12: 171-184.
GUY, R.D. & ROSS KEAN, A. (1980) Algae as a chemical speciation
monitor. I. A comparison of algal growth and computer calculated
speciation. Water Res., 14: 891-899.
HAIGHT, M., MUDRY, T., & PASTERNAK, J. (1982) Toxicity of seven heavy
metals on Panagrellus silusiae: The efficacy of the free-living
nematode as an in vivo toxicological bioassay. Nematologica, 28: 1-11.
HALE, J.G. (1977) Toxicity of metal mining wastes. Bull. environ.
Contam. Toxicol., 17: 66-73.
HALL, W.S., PAULSON, R.L., HALL, L.W., & BURTON, D.T. (1986) Acute
toxicity of cadmium and sodium pentachlorophenate to Daphnids and
fish. Bull. environ. Contam. Toxicol., 37: 308-316.
HAMANAKA, T., ITOO, T., & MISHIMA, S. (1982) Age related change and
distribution of cadmium and zinc concentrations in the stellar sea
lion ( Eumetopias jubata) from the coast of Hokkaido, Japan. Mar.
Pollut. Bull., 13: 57-61.
HAMILTON, S.J. & MEHRLE, P.M. (1986) Metallothionein in fish: review
of its importance in assessing stress from metal contaminants. Trans.
Am. Fish. Soc., 115: 596-609.
HARDISTY, M.W., HUGGINS, R.J., KARTAR, S., & SAINSBURY, M. (1974a)
Ecological implications of heavy metal in fish from the Severn
Estuary. Mar. Pollut. Bull., 5: 12-15.
HARDISTY, M.W., KARTAR, S., & SAINSBURY, M. (1974b) Dietary habits and
heavy metal concentrations in fish from the Severn Estuary and Bristol
Channel. Mar. Pollut. Bull., 5: 61-63.
HARDY, J.T., SCHMIDT, R.L., & APTS, C.W. (1981) Marine sediment and
interstitial water: effects on bioavailability of cadmium to gills of
the clam Protothaca staminea. Bull. environ. Contam. Toxicol., 27:
798-805.
HARDY, J.T., SULLIVAN, M.F., CRECELIUS, E.A., & APTS, C.W. (1984)
Transfer of cadmium in phytoplankton-oyster-mouse food chain. Arch.
environ. Contam. Toxicol., 13: 419-425.
HARKOV, R., CLARKE, B., & BRENNAN, E. (1979) Cadmium contamination may
modify response of tomato to atmospheric ozone. J. Air Pollut. Control
Assoc., 29: 1247-1249.
HARRISON, S.E. & KLAVERKAMP, J.F. (1989) Uptake, elimination and
tissue distribution of dietary and aqueous cadmium by rainbow trout
( Salmo gairdneri Richardson) and lake whitefish ( Coregonus
clupeaformis Mitchill). Environ. Toxicol. Chem., 8: 87-97.
HART, B.A. & SCAIFE, B.D. (1977) Toxicity and bioaccumulation of
cadmium in Chlorella pyrenoidosa. Environ. Res., 14: 401-413.
HARTENSTEIN, R., NEUHAUSER, E.F., & COLLIER, J. (1980) Accumulation of
heavy metals in the earthworm Eisenia foetida. J. environ. Qual., 9:
23-26.
HARTMAN, L.M. (1974) A preliminary report: Fungal flora of the soil as
conditioned by varying concentrations of heavy metals. Am. J. Bot.,
61: 23 (Abstract).
HARTWELL, S.I., JIN, J.H., CHERRY, D.S., & CAIRNS, J. (1989) Toxicity
versus avoidance response of golden shiner, Notemigonus crysoleucas,
to five metals. J. Fish Biol., 35: 447-456.
HATAKEYAMA, S. (1987) Chronic effects of Cd on reproduction of
Polypedilum nubifer (Chironomidae) through water and food. Environ.
Pollut., 48: 249-261.
HATAKEYAMA, S. & YASUNO, M. (1987) Chronic effects of Cd on the
reproduction of the guppy ( Poecilia reticulata) through
Cd-accumulated midge larvae ( Chironomus yoshimatsui). Ecotoxicol.
environ. Saf., 14: 191-207.
HEINZ, G.H., HASELTINE, S.D., & SILEO, L. (1983) Altered avoidance
behavior of black ducks fed cadmium. Environ. Toxicol. Chem., 2:
419-421.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
US Department of Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Department of Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
HIRAOKA, Y. & OKUDA, H. (1984) Time course study of occurrence of
anomalies in medaka's centrum by cadmium or fenitrothion emulsion.
Bull. environ. Contam. Toxicol., 32: 693-697.
HIRATA, M. (1981) Annaka: Land polluted mainly by fumes and dust from
a zinc smelter. In: Kitagishi, K. & Gamare, I., ed. Heavy metal
pollution in the soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HOGSTRAND, C. & HAUX, C. (1990a) Metallothionein as an indicator of
heavy metal exposure in two subtropical fish species. J. exp. mar.
Biol. Ecol., 138: 69-84.
HOGSTRAND, C. & HAUX, C. (1990b) A radioimmunoassay for perch ( Perca
fluviatilis) metallothionein. Toxicol. appl. Pharmacol., 103: 56-65.
HOLCOMBE, G.W., PHIPPS, G.L., & MARIER, J.W. (1984) Methods for
conducting snail ( Aplexa hypnorum) embryo through adult exposures.
Effects of cadmium and reduced pH levels. Arch. environ. Contam.
Toxicol., 13: 627-634.
HONDA, K. & TATSUKAWA, R. (1983) Distribution of cadmium and zinc in
tissues and organs, and their age-related changes in striped dolphins,
Stenella coeruleoalba. Arch. environ. Contam. Toxicol., 12: 543-550.
HONDA, K., FUJISE, Y., TATSUKAWA, R., ITANO, K., & MIYAZAKI, N. (1986)
Age-related accumulation of heavy metals in bone of the striped
dolphin, Stenella coeruleoalba. Mar. environ. Res., 20: 143-160.
HOPKIN, S.P. (1989) Ecophysiology of metals in terrestrial
invertebrates, London, Elsevier Applied Science.
HOPKIN, S.P. (1990) Species-specific differences in the net
assimilation of zinc, cadmium, lead, copper and iron by the
terrestrial isopods Oniscus asellus and Porcellio scaber. J. appl.
Ecol., 27: 460-474.
HOPKIN, S.P. & MARTIN, M.H. (1984) Assimilation of zinc, cadmium, lead
and copper by the centipede Lithobius variegatus (Chilopoda). J.
appl. Ecol., 21: 535-546.
HOPKIN, S.P. & MARTIN, M.H. (1985) Transfer of heavy metals from leaf
litter to terrestrial invertebrates. J. Sci. Food Agric., 36: 538-539.
HOPKIN, S.P., WATSON, K., MARTIN, M.H., & MOULD, M.L. (1985) The
assimilation of heavy metals by Lithobius variegatus and Glomeris
marginata (Chilopoda: Diplopoda). Bijdr. Dierkd., 55: 88-94.
HUIGUANG FU (1989) Hormonal responses of fish to cadmium, Nijmegen,
The Netherlands, University of Nijmegen (Ph.D. Thesis).
HUNTER, B.A. & JOHNSON, M.S. (1982) Food chain relationships of copper
and cadmium in contaminated grassland ecosystems. Oikos, 38: 108-117.
HUTCHINSON, T.C. & CZYRSKA, H. (1975) Heavy metal toxicity and
synergism to floating aquatic weeds. Verh. Int. Ver. Limnol., 19 :
2102-2111.
HUTTON, M. (1981) Accumulation of heavy metals and selenium in three
seabird species from the United Kingdom. Environ. Pollut., 26A:
129-145.
HUTTON, M. (1982) Cadmium in the European Community: a prospective
assessment of sources, human exposure and environmental impact,
London, Monitoring and Assessment Research Centre, Chelsea College,
University of London, 99 pp (MARC Report No. 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London, 46
pp (MARC Report No. 29).
HUTTON, M. & GOODMAN, G.T. (1980) Metal contamination of feral pigeons
Columba livia from the London area: Part I - tissue accumulation of
lead, cadmium and zinc. Environ. Pollut., 22A: 207-217.
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead, mercury
and arsenic entering the U.K. environment from human activities. Sci.
total Environ., 57: 129-150.
IRELAND, M.P. (1981) Uptake and distribution of cadmium in the
terrestrial slug Arion ater (L.) Comp. Biochem. Physiol., 68A:
37-41.
JANA, S. & BHATTACHARYA, D.N. (1988) Effect of heavy metals on growth
population of a fecal coliform bacterium Escherichia coli in aquatic
environment. Water Air Soil Pollut., 38: 251-254.
JANSSEN, M.P.M., BRUINS, A., DE VRIES, T.H., & VAN STRAALEN, N.M.
(1991) Comparison of cadmium kinetics in four soil arthropod species.
Arch. environ. Contam. Toxicol., 20: 305-312.
JOHN, J., GJESSING, E.T., GRANDE, M., & SALBU, B. (1987) Influence of
aquatic humus and pH on the uptake and depuration of cadmium by the
Atlantic salmon ( Salmo salar). Sci. total Environ., 62: 253-265.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination through
residual trace metal dispersal from derelict lead-zinc mine. J.
environ. Qual., 9(2): 175-179.
JOHNSON, M.W. & GENTILE, J.H. (1979) Acute toxicity of cadmium, copper
and mercury to larval American lobster Homarus americanus. Bull.
environ. Contam. Toxicol., 22: 258-264.
JONES, K.C. & JOHNSTON, A.E. (1989) Cadmium in cereal grain and
herbage from long-term experimental plots at Rothamsted, UK. Environ.
Pollut., 57: 199-216.
JORDAN, M.J. & LECHAVALIER, M.P. (1975) Effects of zinc-smelter
emissions on forest soil microflora. Can. J. Microbiol., 21:
1855-1865.
KAY, S.H. & HALLER, W.T. (1986) Heavy metal bioaccumulation and
effects on waterhyacinth weevils, Neochetina eichhorniae, feeding on
waterhyacinth, Eichhornia crassipes. Bull. environ. Contam.
Toxicol., 37: 239-245.
KEMP, P.F. & SWARTZ, R.C. (1988) Acute toxicity of interstitial and
particle-bound cadmium to a marine infaunal amphipod. Mar. environ.
Res., 26: 135-153.
KHAN, A.T., WEIS, J.S., & D'ANDREA, L. (1988) Studies of cadmium
tolerance in two populations of grass shrimp, Palaemonetes pugio.
Bull. environ. Contam. Toxicol., 40: 30-34.
KHANGAROT, B.S. & RAY, P.K. (1987) Sensitivity of toad tadpoles, Bufo
melanostictus (Schneider), to heavy metals. Bull. environ. Contam.
Toxicol., 38: 523-527.
KORTE, F. (1983) Ecotoxicology of cadmium: general overview.
Ecotoxicol. environ. Saf., 7: 3-8.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ., 22(2):
375-381.
KUHN, R., PATTARD, M., PERNAK, K-D., & WINTER, A. (1989) Results of
the harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res., 23: 501-510.
LAAMANEN, A. (1972) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAAMANEN, A. (1976) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAEGREID, M., ALSTAD, J., KLAVENESS, D., & SEIP, H.M. (1983) Seasonal
variation of cadmium toxicity toward the alga Selenastrum
capricornutum Printz in two lakes with different humus content.
Environ. Sci. Technol., 17: 357-361.
LAFEBER, F.P.J.G., FLIK, G., WENDELAAR BONGA, S.E., & PERRY, S.F.
(1988) Hypocalcin from Stannius corpuscles inhibits gill calcium
uptake in trout. Am. J. Physiol., 254: R891-R896.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling, sample
handling, analysis, Washington, DC, US Department of Commerce,
National Bureau of Standards, Vols. 1 & 2, 1304 pp.
LALANDE, M. & PINEL-ALLOUL, B. (1986) Acute toxicity of cadmium,
copper, mercury and zinc to Tropocyclops prasinus mexicanus
(Cyclopoida, Copepoda) from three Quebec lakes. Environ. Toxicol.
Chem., 5: 95-102.
LANGSTON, W.J. & ZHOU, M. (1987a) Cadmium accumulation, distribution
and metabolism in the gastropod Littorina littorea: the role of
metal-binding proteins. J. Mar. Biol. Assoc. UK, 67: 585-601.
LANGSTON, W.J. & ZHOU, M. (1987b) Cadmium accumulation, distribution
and elimination in the bivalve Macoma balthica: neither
metallothionein nor metallothionein-like proteins are involved. Mar.
environ. Res., 21: 225-237.
LEE, H.H. & XU, C.H. (1984) Differential response of marine organisms
to certain metal and agrichemical pollutants. Bull. environ. Contam.
Toxicol., 33: 460-467.
LEPP, N.W., HARRISON, S.C.S., & MORRELL, B.G. (1987) A role for
Amanita muscaria L. in the circulation of cadmium and vanadium in a
non-polluted woodland. Environ. Geochem. Health, 9: 61-64.
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Process of
atmospheric deposition of metals and acids to forests. Annual Meeting
of the Air Pollution Control Association, New Orleans, 20 June 1982,
Pittsburgh, Pennsylvania, Air Pollution Control Association, 14 pp
(Conference Proceedings 75).
LOFTS, B. & MURTON, R.K. (1967) The effects of cadmium on the avian
testis. J. Reprod. Fert., 13: 155-164.
LOVETT, R.J., GUTENMANN, W.H., PAKKALA, I.S., YOUNGS, W.D., LISK,
D.J., BURDICK, G.E., & HARRIS, E.J. (1972) A survey of the total
cadmium content of 406 fish from 49 New York State fresh waters. J.
Fish Res. Board Can., 29: 1283-1290.
LUSSIER, S.M., GENTILE, J.H., & WALKER, J. (1985) Acute and chronic
effects of heavy metals and cyanide on Mysidopsis bahia (Crustacea:
Mysidacea). Aquat. Toxicol., 7: 25-35.
MA, W., EDELMAN, Th., VAN BEERSUM, I., & JANS, Th. (1983) Uptake of
cadmium, zinc, lead and copper by earthworms near a zinc-smelting
complex: influence of soil pH and organic matter. Bull. environ.
Contam. Toxicol., 30: 424-427.
MCCAHON, C.P., BROWN, A.F., & PASCOE, D. (1988) The effect of the
acanthocephalan Pomphorhynchus laevis (Muller 1776) on the acute
toxicity of cadmium to its intermediate host, the amphipod Gammarus
pulex (L.). Arch. environ. Contam. Toxicol., 17: 239-243.
MCCAHON, C.P., WHILES, A.J., & PASCOE, D. (1989) The toxicity of
cadmium to different larval instars of the trichopteran larvae
Agapetus fuscipes Curtis and the importance of life cycle
information to the design of toxicity tests. Hydrobiologia, 185:
153-162.
MCCLURG, T.P. (1984) Effects of fluoride, cadmium and mercury on the
estuarine prawn Penaeus indicus. Water S. Afr., 10: 40-45.
MACINNES, J.R. & THURBERG, F.P. (1973) Effects of metals on the
behaviour and oxygen consumption of the mud snail. Mar. Pollut. Bull.,
4: 185-186.
MACINNES, J.R., THURBERG, F.P., GREIG, R.A., & GOULD, E. (1977)
Long-term cadmium stress in the cunner, Tautogolabrus adspersus.
Fish. Bull., 75: 199-203.
MAHLER, R.J., BINGHAM, F.T., & PAGE, A.L. (1978) Cadmium-enriched
sewage sludge application to acid and calcareous soils. Effect on
yield and cadmium uptake by lettuce and chard. J. environ. Qual., 7:
274-281.
MARSHALL, J.S. (1979) Cadmium toxicity to laboratory and field
populations of Daphnia galeata mendotae. Bull. environ. Contam.
Toxicol., 21: 453-457.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, M.H., COUGHTREY, P.J., & YOUNG, E.W. (1976) Observations on
the availability of lead, zinc, cadmium and copper in woodland litter
and the uptake of lead, zinc and cadmium by the woodlouse Oniscus
asellus. Chemosphere, 5: 313-318.
MARTIN, M.H., COUGHTREY, P.J., SHALES, S.W., & LITTLE, P. (1980)
Aspect of airborne cadmium contamination of soils and natural
vegetation. In: Inorganic pollution and agriculture, Her Majesty's
Stationary Office, London, pp. 56-69.
MAY, T.W. & MCKINNEY, G.L. (1981) Cadmium, lead, mercury, arsenic, and
selenium concentrations in freshwater fish, 1976-77 - National
pesticide monitoring program. Pestic. monit. J., 15: 14-38.
MAYACK, L.A., BUSH, P.B., FLETCHER, O.J., PAGE, R.K., & FENDLEY, T.T.
(1981) Tissue residues of dietary cadmium in wood ducks. Arch.
environ. Contam. Toxicol., 10: 637-645.
MAYER, F.L (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MEISCH, H.-U. & SCHMITT, J.A. (1986) Characterization studies on
cadmium-mycophosphatin from the mushroom Agaricus macrosporus.
Environ. Health Perspect., 65: 29-32.
MERLINI, M. (1978) Hepatic storage alteration of vitamin B12 by
cadmium in a freshwater fish. Bull. environ. Contam. Toxicol., 19:
767-771.
METEYER, M.J., WRIGHT, D.A., & MARTIN, F.D. (1988) Effect of cadmium
on early developmental stages of the sheepshead minnow ( Cyprinodon
variegatus). Environ. Toxicol. Chem., 7: 321-328.
MICHIBATA, H. (1981) Effect of water hardness on the toxicity of
cadmium to the egg of the teleost Oryzias latipes. Bull. environ.
Contam. Toxicol., 27: 187-192.
MIDDAUGH, D.P. & DEAN, J.M. (1977) Comparative sensitivity of eggs,
larvae, and adults of the estuarine teleosts, Fundulus heteroclitus
and Menidia menidia to cadmium. Bull. environ. Contam. Toxicol., 17:
645-652.
MIRENDA, R.J. (1986) Toxicity and accumulation of cadmium in the
crayfish, Orconectes virilis (Hagen). Arch. environ. Contam.
Toxicol., 15: 401-407.
MITCHELL, C.D. & FRETZ, T.A. (1977) Cadmium and zinc toxicity in white
pine, red maple and Norway spruce. J. Am. Soc. Hortic. Sci., 102:
81-84.
MITRA, R.S., GRAY, R.H., CHIN, B., & BERNSTEIN, I.A. (1975) Molecular
mechanisms of accommodation in Escherichia coli to toxic levels of
Cd2+. J. Bacteriol., 121: 1180-1188.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & LENTZOU, P. (1979)
Effects of sublethal concentrations of cadmium as possible indicators
of cadmium pollution for two populations of Acartia clausi
(Copepoda) living at two differently polluted areas. Bull. environ.
Contam. Toxicol., 23: 642-649.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & ROGDAKIS, I. (1982)
Evaluation of the stress exerted by a polluted environment to a marine
organism by comparative toxicity tests. Bull. environ. Contam.
Toxicol., 28: 416-423.
MORGAN, J.E. & MORGAN, A.J. (1988) Earthworms as biological monitors
of cadmium, copper, lead and zinc in metalliferous soils. Environ.
Pollut., 54: 123-138.
MORGAN, J.E. & MORGAN, A.J. (1990) The distribution of cadmium,
copper, lead, zinc and calcium in the tissues of the earthworm
Lumbricus rubellus sampled from one uncontaminated and four polluted
soils. Oecologia, 84: 559-566.
MUINO, C.V., FERRARI, L., & SALIBIAN, A. (1990) Protective action of
ions against cadmium toxicity to young Bufo arenarum tadpoles. Bull.
environ. Contam. Toxicol., 45: 313-319.
MUIR, D.C.G., WAGEMANN, R., GRIFT, N.P., NORSTROM, R.J., SIMON, M. &
LIEN, J. (1988) Organochlorine chemical and heavy metal contaminants
in white-beaked dolphins ( Lagenorhynchus albirostris) and pilot
whales ( Globicephala melaena) from the coast of Newfoundland,
Canada. Arch. environ. Contam. Toxicol., 17: 613-629.
MURAMOTO, S. (1981a) Influence of complexans (EDTA, DTPA) on the
toxicity of cadmium to fish at chronic levels. Bull. environ. Contam.
Toxicol., 26: 641-646.
MURAMOTO, S. (1981b) Variations of some elements in cadmium-induced
malformed fish. Bull. environ. Contam. Toxicol., 27: 193-200.
MUSKETT, C.J. & JONES, M.P. (1980) The dispersal of lead, cadmium and
nickel from motor vehicles and effects on roadside invertebrate
macrofauna. Environ. Pollut., 23A: 231-242.
MYSING-GUBALA, M. & POIRRIER, M.A. (1981) The effects of cadmium and
mercury on gemmule formation and gemmosclere morphology in Ephydatia
fluviatilis (Porifera: Spongillidae). Hydrobiologia, 76: 145-148.
NAIDU, C.K. & REDDY, T.K.R. (1988) Effect of cadmium on microorganisms
and microbe-mediated mineralization process in the soil. Bull.
environ. Contam. Toxicol., 41: 657-663.
NAKADA, M., FUKAYA, K., TAKESHITA, S., & WADA, Y. (1979) The
accumulation of heavy metals in the submerged plant ( Elodea
nuttallii). Bull. environ. Contam. Toxicol., 22: 21-27.
NEBEKER, A.V., CAIRNS, M.A., ONJUKKA, S.T., & TITUS, R.H. (1986)
Effect of age on sensitivity of Daphnia magna to cadmium, copper and
cyanazine. Environ. Toxicol. Chem., 5: 527-530.
NEGILSKI, D.S. (1976) Acute toxicity of zinc, cadmium and chromium to
the marine fishes, yellow-eye mullet ( Aldrichetta forsteri C & V)
and small-mouthed hardyhead ( Atherinasoma microstoma Whitley). Aust.
J. mar. freshwater Res., 27: 137-149.
NEGILSKI, D.S., AHSANULLAH, M., & MOBLEY, M.C. (1981) Toxicity of
zinc, cadmium and copper to the shrimp Callianassa australiensis. II
Effects of paired and triad combinations of metals. Mar. Biol., 64:
305-309.
NELSON, D.A., CALABRESE, A., NELSON, B.A., MACINNES, J.R., & WENZLOFF,
D.R. (1976) Biological effects of heavy metals on juvenile bay
scallops, Argopecten irradians, in short-term exposures. Bull.
environ. Contam. Toxicol., 16: 275-282.
NICHOLSON, J.K. & OSBORN, D. (1983) Kidney lesions in pelagic seabirds
with high tissue levels of cadmium and mercury. J. Zool. (Lond.), 200:
99-118.
NICHOLSON, J.K., KENDALL, M.D., & OSBORN, D. (1983) Cadmium and
mercury nephrotoxicity. Nature (Lond.), 304: 633-635.
NIEDERLEHNER, B.R., BUIKEMA, A.L., PITTINGER, C.A., & CAIRNS, J.
(1984) Effects of cadmium on the population growth of a benthic
invertebrate Aeolosoma headleyi (Oligochaeta). Environ. Toxicol.
Chem., 3: 255-262.
NIMMO, D.R., LIGHTNER, D.V., & BAHNER, L.H. (1977) Effects of cadmium
on the shrimps Penaeus duorarum, Palaemonetes pugio and
Palaemonetes vulgaris. In: Vernberg, F.J., Calabrese, A., Thurberg,
F.P., & Vernberg, W.B., ed. Physiological responses of marine biota to
pollutants, New York, London, Academic Press, pp. 131-183.
NIMMO, D.R., RIGBY, R.A., BAHNER, L.H., & SHEPPARD, J.M. (1978) The
acute and chronic effects of cadmium on the estuarine mysid,
Mysidopsis bahia. Bull. environ. Contam. Toxicol., 19: 80-85.
NIR, R., GASITH, A., & PERRY, A.S. (1990) Cadmium uptake and toxicity
to water hyacinth: effect of repeated exposures under controlled
conditions. Bull. environ. Contam. Toxicol., 44: 149-157.
NORBERG, A.B. & MOLIN, N. (1983) Toxicity of cadmium, cobalt, uranium
and zinc to Zoogloea ramigera. Water Res., 17: 1333-1336.
NRIAGU, J.O. (1989) A global assessment of natural sources of
atmospheric trace metals. Nature (Lond.), 338: 47-49.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
O'HARA, J. (1973) The influence of temperature and salinity on the
toxicity of cadmium to the fiddler crab, Uca pugilator. Fish. Bull.,
71: 149-153.
OLLA, B.L., ESTELLE, V.B., SWARTZ, R.C., BRAUN, G., & STUDHOLME, A.L.
(1988) Responses of polychaetes to cadmium-contaminated sediment:
Comparison of uptake and behavior. Environ. Toxicol. Chem., 7:
587-592.
OLSON, P.-E. & HAUX, C. (1986) Increased hepatic metallothionein
content correlates to cadmium accumulation in environmentally exposed
perch ( Perca fluviatilis). Aquat. Toxicol., 9: 231-242.
OSBORN, D. & NICHOLSON, J.K. (1984) Cadmium and mercury in seabirds,
Huntingdon, UK Natural Environment Research Council, Institute of
Terrestrial Ecology, pp. 30-34 (ITE Symposium No. 12).
PAGE, A.L. & CHANG, A.C. (1978) Trace elements impact on plants during
cropland disposal of sewage sludges. In: Proceedings of the 5th
National Conference on Acceptable Sludge Disposal Techniques,
Rockville, Maryland, Information Transfer Inc., pp. 91-96.
PAGE, A.L., BINGHAM, F.T., & CHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, London, Applied
Science, Vol. 1, pp. 77-109.
PART, P. & WIKMARK, G. (1984) The influence of certain complexing
agents (EDTA and citrate) on the uptake of cadmium in perfused rainbow
trout gills. Aquat. Toxicol., 5: 277-289.
PART, P., SVANBERG, O., & KIESSLING, A. (1985) The availability of
cadmium to perfused rainbow trout gills in different water qualities.
Water Res., 19: 427-434.
PASCOE, D., EVANS, S.A., & WOODWORTH, J. (1986) Heavy metal toxicity
to fish and the influence of water hardness. Arch. environ. Contam.
Toxicol., 15: 481-487.
PECON, J. & POWELL, E.N. (1981) Effects of the amino acid histidine on
the uptake of cadmium from the digestive system by the blue crab
Callinectes sapidus. Bull. environ. Contam. Toxicol., 27: 34-41.
PENTREATH, R.J. (1977) The accumulation of cadmium by the plaice
Pleuronectes platessa L. and the thornback ray Raia clavata. J.
exp. mar. Biol. Ecol., 30: 223-232.
PEREZ-COLL, C.S., HERKOVITS, J., & SALIBIAN, A. (1986) Teratogenic
effects of cadmium on Bufo arenarum during gastrulation. Experientia
(Basel), 42: 1174-1176.
PESCH, G.G. & STEWART, N.E. (1980) Cadmium toxicity to three species
of estuarine invertebrates. Mar. environ. Res., 3: 145-156.
PETERSON, P.J. & ALLOWAY, B.J. (1979) Cadmium in soils and vegetation.
In: Webb, M., ed. The chemistry, biochemistry and biology of cadmium,
Amsterdam, Elsevier/North Holland, pp. 45-92.
PICKERING, Q.H. & GAST, M.H. (1972) Acute and chronic toxicity of
cadmium to the fathead minnow Pimephales promelas. J. Fish Res.
Board. Can., 29: 1099-1106.
PICKERING, Q.H. & HENDERSON, C. (1966) The acute toxicity of some
heavy metals to different species of warmwater fishes. Int. J. Air
water Pollut., 10: 453-463.
PIETZ, R.I., PETERSON, J.R., PRATER, J.E., & ZENZ, D.R. (1984) Metal
concentrations in earthworms from sewage sludge-amended soils at a
strip mine reclamation site. J. environ. Qual., 13: 651-654.
PINOWSKA, B., KRASNICKI, K., & PINOWSKI, J. (1981) Estimation of the
degree of contamination of granivorous birds with heavy metals in
agricultural and industrial landscape. Ekol. Polska, 29: 137-149.
POLDOSKI, J.E. (1979) Cadmium bioaccumulation assays. Their
relationships to various ion equilibria in Lake Superior water.
Environ. Sci. Technol., 13: 701-706.
POPHAM, J.D. & WEBSTER, J.M. (1979) Cadmium toxicity in the
free-living nematode, Caenorhabditis elegans. Environ. Res., 20:
183-191.
PRAHALAD, A.K. & SEENAYYA, G. (1988) Bioavailability of zinc and
cadmium and their effect on microbial growth and metal uptake. Bull.
environ. Contam. Toxicol., 41: 921-927.
PRITZL, M.C., LIE, Y.H., KIENHOLZ, E.W., & WHITEMAN, C.E. (1974) The
effect of dietary cadmium on development of young chickens. Poult.
Sci., 53: 2026-2029.
RAINBOW, P.S. & WHITE, S.L. (1989) Comparative strategies of heavy
metal accumulation by crustaceans: zinc, copper and cadmium in a
decapod, an amphipod and a barnacle. Hydrobiologia, 174: 245-262.
RAMAMOORTHY, S. & BLUMHAGEN, K. (1984) Uptake of Zn, Cd and Hg by fish
in the presence of competing compartments. Can. J. Fish aquat. Sci.,
41: 750-756.
RAMIREZ, P., BARRERA, G., & ROSAS, C. (1989) Effects of chromium and
cadmium upon respiration and survival of Callinectes similis. Bull.
environ. Contam. Toxicol., 43: 850-857.
RAY, S., MCLEESE, D.W., WAIWOOD, B.A., & PEZZACK. D. (1980a) The
disposition of cadmium and zinc in Pandalus montagui. Arch. environ.
Contam. Toxicol., 9: 675-681.
RAY, S., MCLEESE, D., & PEZZACK, D. (1980b) Accumulation of cadmium by
Nereis virens. Arch. environ. Contam. Toxicol., 9: 1-8.
REBHUN, S. & BEN-AMOTZ, A. (1984) The distribution of cadmium between
the marine alga Chlorella stigmatophora and sea water medium. Water
Res., 18: 173-178.
REHWOLDT, R. & KARIMIAN-TEHERANI, D. (1976) Uptake and effect of
cadmium on zebrafish. Bull. environ. Contam. Toxicol., 15: 442-446.
REID, S.D. & MCDONALD, D.G. (1988) Effects of cadmium, copper and low
pH on ion fluxes in rainbow trout, Salmo gairdneri. Can. J. Fish
aquat. Sci., 45: 244-253.
RIISGARD, H.U., BJORNESTAD, E., & MOHLENBERG, F. (1987) Accumulation
of cadmium in the mussel Mytilus edulis: Kinetics and importance of
uptake via food and sea water. Mar. Biol., 96: 349-353.
ROBERT, R. & HIS, E. (1985) Combined effects of salinity and cadmium
chloride upon embryos and larvae of the Japanese oyster, Crassostrea
gigas. Mar. environ. Res., 15: 303-312.
ROBERTS, M.H., WARINNER, J.E., CHU-FA TSAI, WRIGHT, D., & CRONIN, L.E.
(1982) Comparison of estuarine species sensitivities to three
toxicants. Arch. environ. Contam. Toxicol., 11: 681-692.
ROBERTS, R.D. & JOHNSON, M.S. (1978) Dispersal of heavy metals from
abandoned mine workings and their transference through terrestrial
food chains. Environ. Pollut., 16: 293-310.
ROBERTS, T.M., HEPPLESTON, P.B., & ROBERTS, R.D. (1976) Distribution
of heavy metals in tissues of the common seal. Mar. Pollut. Bull., 7:
194-196.
ROBINSON, A.M., LAMBERSON, J.O., COLE, F.A., & SWARTZ, R.C. (1988)
Effects of culture conditions on the sensitivity of a phoxocephalid
amphipod, Rhepoxynius abronius, to cadmium in sediment. Environ.
Toxicol. Chem., 7: 953-959.
ROBINSON, K.M. & WELLS, M.R. (1975) Retention of a single oral dose of
cadmium in tissues of the softshell turtle, Trionyx spinifer. Bull.
environ. Contam. Toxicol., 14: 750-752.
ROCH, M. & MALY, E.J. (1979) Relationship of cadmium-induced
hypocalcemia with mortality in rainbow trout ( Salmo gairdneri) and
the influence of temperature on toxicity. J. Fish Res. Board Can., 36:
1297-1303.
ROMBOUGH, P.J. & GARSIDE, E.T. (1982) Cadmium toxicity and
accumulation in aggs and alevins of Atlantic salmon Salmo salar. Can.
J. Zool., 60: 2006-2014.
ROOT, R.A., MILLER, R.J., & KOEPPE, D.E. (1975) Uptake of cadmium -
its toxicity, and effect on the iron ratio in hydroponically grown
corn. J. environ. Qual., 4: 473-476.
ROSENBERG, R. & COSTLOW, J.D. (1976) Synergistic effects of cadmium
and salinity combined with constant and cycling temperatures on the
larval development of two estuarine crab species. Mar. Biol., 38:
219-303.
RUSSELL, L.K., DEHAVEN, J.I., & BOTTS, R.P. (1981) Toxic effects of
cadmium on the garden snail ( Helix aspersa). Bull. environ. Contam.
Toxicol., 26: 634-640.
SANGALANG, G.B. & FREEMAN, H.C. (1979) Tissue uptake of cadmium in
brook trout during chronic sublethal exposure. Arch. environ. Contam.
Toxicol., 8: 77-84.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SASTRY, K.V. & SUBHADRA, K.M. (1983) Cadmium induced alterations in
the intestinal absorption of glucose and fructose in a freshwater
catfish Heteropneustes fossilis. Water Air Soil Pollut., 20:
293-297.
SATO, C., SCHNOOR, J.L., & MCDONALD, D.B. (1986) Characterization of
effects of copper, cadmium and nickel on the growth of Nitrosomonas
europaea. Environ. Toxicol. Chem., 5: 403-416.
SAWICKA-KAPUSTA, K., GORECKI, A., SWIERGOSZ, R., JUSZCZAK, G.,
MIELCZAREK, M., & WOJCIK, B. (1987) Effect of metabolic rate on the
rate of elimination of high and low concentrations of cadmium and lead
in the bank vole. Ekol. Polska, 35: 399-430.
SCHEUHAMMER, A.M. (1987) The chronic toxicity of aluminium, cadmium,
mercury and lead in birds: a review. Environ. Pollut., 46: 263-295.
SCHUYTEMA, G.S., NELSON, P.O., MALUEG, K.W., NEBEKER, A.V., KRAWCZYK,
D.F., RATCLIFF, A.K., & GAKSTATTER, J.H. (1984) Toxicity of cadmium in
water and sediment slurries to Daphnia magna. Environ. Toxicol.
Chem., 3: 293-308.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta, 51:
3273-3277.
SHIVARAJ, K.M. & PATIL, H.S. (1988) Toxicity of cadmium and copper to
a freshwater fish Puntius arulius. Environ. Ecol., 6: 5-8.
SHORE, R.F., MYHILL, D.G., POLWORTH, G., & DUNMOW, M. (1991) The
effects of chronic doses of dietary cadmium on macro-element balance
in wood mice and bank voles. In: Momciloric, B., ed. Proceedings of
the Seventh International Symposium on Trace Elements in Man and
Animals (TEMA-7), Dubrovnik, Yugoslavia, 20-25 May 1990, Zagreb, IMI
(Institute for Medical Research and Occupational Health, University of
Zagreb), pp. 17-18.
SILLEN, L.G. & MARTELL, A.E. (1964) Stability constants of metal-ion
complexes. Section I: Inorganic ligands compiled by L.G. Sillen.
Section II: Organic ligands compiled by A.E. Martell, London, The
Chemical Society, 754 pp (Special Publication No. 17).
SIMON, E. (1977) Cadmium tolerance in populations of Agrostis tenuis
and Festuca ovina. Nature (Lond.), 265: 328-330.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SJOBECK, M.-L., HAUX, C., LARSSON, A., & LITHNER, G. (1984)
Biochemical and hematological studies on perch, Perca fluviatilis,
from the cadmium-contaminated river Eman. Ecotoxicol. environ. Saf.,
8: 303-312.
SKOWRONSKI, T., PAWLIK, B., & JAKUBOWSKI, M. (1988) Reduction of
cadmium toxicity to green microalga Stichococcus bacillaris by
manganese. Bull. environ. Contam. Toxicol., 41: 915-920.
SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the usefulness of the
mexican axolotl ( Ambystoma mexicanum) and the clawed toad ( Xenopus
laevis) in toxicological bioassays. Bull. environ. Contam. Toxicol.,
24: 439-443.
SMITH, B.P., HEJTMANCIK, E., & CAMP, B.J. (1976) Acute effects of
cadmium on Ictalurus punctatus (catfish). Bull. environ. Contam.
Toxicol., 15: 271-277.
SMITH, R.M. & MARTELL, A.E. (1974) In: Critical stability constants,
Vol. 1: Amino acids, New York, Plenum Press, 469 pp.
SMITH, R.M. & MARTELL, A.E. (1975) In: Critical stability constants,
Vol. 2: Amines, New York, Plenum Press, 415 pp.
SMITH, R.M. & MARTELL, A.E. (1976) In: Critical stability constants,
Vol. 3: Other organic ligands, New York, Plenum Press, 495 pp.
SPEHAR, R.L., ANDERSON, R.L., & FIANDT, J.T. (1978a) Toxicity and
bioaccumulation of cadmium and lead in aquatic invertebrates. Environ.
Pollut., 15A: 195-208.
SPEHAR, R.L., LEONARD, E.N., & DEFOE, D.L. (1978b) Chronic effects of
cadmium and zinc mixtures on flagfish ( Jordanella floridae). Trans.
Am. Fish. Soc., 107: 354-360.
SPRAGUE, J. (1985) Factors that modify toxicity. In: Rand, G.M. &
Petrocelli, S.R., ed. Fundamentals of aquatic toxicology, New York,
Hemisphere Publishing Corporation, pp. 124-163.
SPRY, D.J. & WOOD, C.M. (1989) A kinetic method for the measurement of
zinc influx in vivo in the rainbow trout, and the effects of
waterborne calcium on flux rates. J. exp. Biol., 142: 425-446.
STROJAN, C.L. (1978) Forest leaf litter decomposition in the vicinity
of a zinc smelter. Oecologia, 32: 203-212.
SULLIVAN, J.F., MURPHY, B.R., ATCHISON, G.J., & MCINTOSH, A.W. (1978a)
Time dependant cadmium uptake by fathead minnows ( Pimephales
promelas) during field and laboratory exposure. Hydrobiologia, 57:
65-68.
SULLIVAN, J.F., ATCHISON, G.J., KOLAR, D.J., & MCINTOSH, A.W. (1978b)
Changes in the predator-prey behavior of fathead minnows ( Pimephales
promelas) and largemouth bass ( Micropterus salmoides) caused by
cadmium. J. Fish Res. Board. Can., 35: 446-451.
SUNDA, W.G., ENGEL, D.W., & THUOTTE, R.M. (1978) Effect of chemical
speciation on toxicity of cadmium to grass shrimp, Palaemonetes
pugio: Importance of free cadmium ion. Environ. Sci. Technol., 12:
409-413.
TAYLOR, D. (1983) The significance of the accumulation of cadmium by
aquatic organisms. Ecotoxicol. environ. Saf., 7: 33-42.
TERAOKA, H. (1989) Impact of atmospheric trace metals on rice roots.
Arch. environ. Contam. Toxicol., 18: 269-275.
THURBERG, F.P., DAWSON, M.A., & COLLIER, R.S. (1973) Effects of copper
and cadmium on osmoregulation and oxygen consumption in two species of
estuarine crabs. Mar. Biol., 23: 171-175.
TJALVE, H., GOTTOFREY, J., & BJORKLUND, I. (1986) Tissue disposition
of 109Cd2+ in the brown trout ( Salmo trutta) studied by
autoradiography and impulse counting. Toxicol. environ. Chem., 12:
31-45.
TYLER, G. (1972) Heavy metals pollute nature, may reduce productivity.
Ambio, 1: 52-59.
TYLER, G., MORNSJO, B., & NILSSON, B. (1974) Effects of cadmium, lead,
and sodium salts on nitrification in a mull soil. Plant Soil, 40:
237-242.
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North Sea.
I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VAN HATTUM, B., DE VOOGT, P., VAN DEN BOSCH, L., VAN STRAALEN, N.M.,
JOOSSE, E.N.G., & GOVERS, H. (1989) Bioaccumulation of cadmium by the
freshwater isopod Asellus aquaticus (L.) from aqueous and dietary
sources. Environ. Pollut., 62: 129-151.
VAN HOOK, R.I. (1974) Cadmium, lead, and zinc distributions between
earthworms and soils: potentials for biological accumulation. Bull.
environ. Contam. Toxicol., 12: 509-512.
VAN HOOK, R.I. & YATES, A.J. (1975) Transient behavior of cadmium in
a grassland arthropod food chain. Environ. Res., 9: 76-83.
VAN KESSEL, W.H.M., BROCADES ZAALBERG, R.W., & SEINEN, W. (1989)
Testing environmental pollutants on soil organisms: A simple assay to
investigate the toxicity of environmental pollutants on soil
organisms, using CdCl2 and nematodes. Ecotoxicol. environ. Saf., 18:
181-190.
VAN LEEUWEN, C.J., LUTTMER, W.J., & GRIFFIOEN, P.S. (1985) The use of
cohorts and populations in chronic toxicity studies with Daphnia
magna: A cadmium example. Ecotoxicol. environ. Saf., 9: 26-39.
VAN STRAALEN, N.M. & VAN WENSEM, J. (1986) Heavy metal content of
forest litter arthropods as related to body-size and trophic level.
Environ. Pollut., 42A: 209-221.
VAN STRAALEN, N.M., BURGHOUTS, T.B.A., DOORNHOF, M.J., GROOT, G.M.,
JANSSEN, M.P.M., JOOSSE, E.N.G., VAN MEERENDONK, J.H., THEEUWEN,
J.P.J.J., VERHOEF, H.A., & ZOOMER, H.R. (1987) Efficiency of lead and
cadmium excretion in populations of Orchesella cincta (Collembola)
from various contaminated forest soils. J. appl. Ecol., 24: 953-968.
VAN STRAALEN, N.M., SCHOBBEN, J.H.M., & DE GOEDE, R.G.M. (1989)
Population consequences of cadmium toxicity in soil microarthropods.
Ecotoxicol. environ. Saf., 17: 190-204.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1987)
Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am. J.
Physiol., 253: R216-R221.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1988)
Cadmium inhibits plasma membrane calcium transport. J. membrane Biol.,
102: 97-104.
VERBOST, P.M., VAN ROOIJ, J., FLIK, G., LOCK, R.A.C., & WENDELAAR
BONGA, S.E. (1989) The movement of cadmium through freshwater trout
branchial epithelium and its interference with calcium transport. J.
exp. Biol., 145: 185-197.
VERNBERG, W.B., DE COURSEY, P.J., & O'HARA, J. (1974) Multiple
environmental factor effects on physiology and behavior of the fiddler
crab, Uca pugilator. In: Vernberg, F.G. & Vernberg, W.B., ed.
Pollution and physiology of marine organisms, New York, London,
Academic Press, pp. 381-425.
VERNBERG, W.B., DE COURSEY, P.J., KELLY, M., & JOHNS, D.M. (1977)
Effects of sublethal concentrations of cadmium on adult Palaemonetes
pugio under static and flow-through conditions. Bull. environ.
Contam. Toxicol., 17: 16-24.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1981) Effects of some
environmental factors on the toxicity of cadmium to the copepod Tisbe
holothuriae. Arch. Hydrobiol., 91: 287-293.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1982) Differentiation
of the sensitivity to copper and cadmium in different life-stages of
a copepod. Mar. Pollut. Bull., 13: 123-125.
VOYER, R.A. (1975) Effect of dissolved oxygen concentration on the
acute toxicity of cadmium to the mummichog, Fundulus heteroclitus
(L.), at various salinities. Trans. Am. Fish. Soc., 104: 129-134.
VOYER, R.A. & MODICA, G. (1990) Influence of salinity and temperature
on acute toxicity of cadmium to Mysidopsis bahia Molenock. Arch.
environ. Contam. Toxicol., 19: 124-131.
VOYER, R.A., YEVICH, P.P., & BARSZCZ, C.A. (1975) Histological and
toxicological responses of the mummichog, Fundulus heteroclitus
(L.), to combinations of levels of cadmium and dissolved oxygen in a
freshwater. Water Res., 9: 1069-1074.
WALTERSSON, E. (1984) [Effects of enrichment reagents - distribution
and spread of xanthates and xanthate derivatives during enrichment
activities], Stockholm, Swedish Environmental Research Institute
(Report B478) (in Swedish).
WATLING, H.R. & WATLING, R.J. (1983) Sandy beach molluscs as possible
bioindicators of metal pollution. 2. Laboratory studies. Bull.
environ. Contam. Toxicol., 31: 339-343.
WATSON, C.F. & BENSON, W.H. (1987) Comparative activity of gill
ATPases in three freshwater teleosts exposed to cadmium. Ecotoxicol.
Environ. Saf., 14: 252-259.
WEIS, J.S. & WEIS, P. (1977) Effects of heavy metals on development of
the killifish, Fundulus heteroclitus. J. Fish Biol., 11: 49-54.
WESTERMAN, A.G. (1977) Lethal and teratogenic effects of inorganic
mercury and cadmium on embryonic development of Anurans, Lexington,
University of Kentucky (M.S. Thesis).
WHITE, D.H. & FINLEY, M.T. (1978) Uptake and retention of dietary
cadmium in mallard ducks. Environ. Res., 17: 53-59.
WHITE, D.H., FINLEY, M.T., & FERRELL, J.F. (1978) Histopathologic
effects of dietary cadmium on kidneys and testes of mallard ducks. J.
Toxicol. environ. Health, 4: 551-558.
WHO (1992) Environmental Health Criteria 134: Cadmium. Geneva, World
Health Organization, 280 pp.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WICKLUND, A. (1990) Metabolism of cadmium and zinc in fish, University
of Uppsala, Sweden, Faculty of Science (Acta Uiversitatis Uppsaliensis
- Comprehensive summaries of Uppsala dissertations from the Faculty of
Science, No. 250).
WIER, C.F. & WALTER, W.M. (1976) Toxicity of cadmium in the freshwater
snail, Physa gyrina Say. J. environ. Qual., 5: 359-362.
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soil and plants. Aust. J. soil Res., 11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILLIAMS, D.R. & GIESY, J.P. (1978) Relative importance of food and
water sources to cadmium uptake by Gambusia affinis ( Poeciliidae).
Environ. Res., 16: 326-332.
WILLIAMS, K.A., GREEN, D.W.J., & PASCOE, D. (1985) Studies on the
acute toxicity of pollutants to freshwater macroinvertebrates. I.
Cadmium. Arch. Hydrobiol., 102: 461-471.
WILLIAMS, K.A., GREEN, D.W.J., PASCOE, D., & GOWER, D.E. (1987) Effect
of cadmium on oviposition and egg viability in Chironomus riparius
(Diptera: Chironomidae). Bull. environ. Contam. Toxicol., 38: 86-90.
WILLIAMS, P.L. & DUSENBERY, D.B. (1990) Aquatic toxicity testing using
the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem., 9:
1285-1290.
WILLIAMSON, P. (1980) Variables affecting body burdens of lead, zinc
and cadmium in a roadside population of the snail Cepaea hortensis
Muller. Oecologia, 44: 213-220.
WILSON, D.N. (1988) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium Conference,
London, Cadmium Association, pp. 9-16.
WINNER, R.W. (1986) Interactive effects of water hardness and humic
acid on the chronic toxicity of cadmium to Daphnia pulex. Aquat.
Toxicol., 8: 281-293.
WINNER, R.W. (1988) Evaluation of the relative sensitivities of 7-d
Daphnia magna and Ceriodaphnia dubia toxicity tests for cadmium
and sodium pentachlorophenate. Environ. Toxicol. Chem., 7: 153-159.
WONG, P.T.S., BURNISON, G., & CHAU, Y.K. (1979) Cadmium toxicity to
freshwater algae. Bull. environ. Contam. Toxicol., 23: 487-490.
WONG, Y.S., LAM, H.M., DHILLON, E., TAM, N.F.Y., & LEUNG, W.N. (1988)
Physiological effects and uptake of cadmium in Pisum sativum.
Environ. Int., 14: 535-543.
WOODALL, C., MACLEAN, N., & CROSSLEY, F. (1988) Responses of trout fry
( Salmo gairdneri) and Xenopus laevis tadpoles to cadmium and zinc.
Comp. Biochem. Physiol., 89C: 93-99.
WRIGHT, D.A. & FRAIN, J.W. (1981a) Cadmium toxicity in Marinogammarus
obtusatus. Effect of external calcium. Environ. Res., 24: 338-344.
WRIGHT, D.A. & FRAIN, J.W. (1981b) The effect of calcium on cadmium
toxicity in the freshwater amphipod, Gammarus pulex (L.). Arch.
environ. Contam. Toxicol., 10: 321-328.
WRIGHT, D.A., METEYER, M.J., & MARTIN, F.D. (1985) Effect of calcium
on cadmium uptake and toxicity in larvae and juveniles of striped bass
( Morone saxatilis). Bull. environ. Contam. Toxicol., 34: 196-204.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in perspective.
Bull. Inst. Public Health (Tokyo), 24: 18-24.
ZAROOGIAN, G.E. & CHEER, S. (1976) Accumulation of cadmium by the
American oyster, Crassostrea virginica. Nature (Lond.), 261:
408-410.
ZAROOGIAN, G.E. & MORRISON, G. (1981) Effect of cadmium body burdens
in adult Crassostrea virginica on fecundity and viability of larvae.
Bull. environ. Contam. Toxicol., 27: 344-348.
ZIRINO, A. & YAMAMOTO, S. (1972) A pH-dependent model for the chemical
speciation of copper, zinc, cadmium and lead in seawater. Limnol.
Oceanogr., 17(5): 661-671.
Appendix 1. Global emissions of trace metals from natural sources (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Wind-borne soil particles
range 0.3-5.0 0.01-0.4 0.9-15 0-0.01 0.3-7.5 0.01-0.35 3.0-35
median 2.6 0.21 8.0 0.05 3.9 0.18 19
Sea salt spray
range 0.19-3.1 0-0.11 0.23-6.9 0-0.04 0.02-2.8 0-1.1 0.02-0.86
median 1.7 0.06 3.6 0.02 1.4 0.55 0.44
Volcanoes
range 0.15-7.5 0.14-1.5 0.9-18 0.03-2.0 0.54-6.0 0.10-1.8 0.31-19
median 3.8 0.82 9.4 1.0 3.3 0.95 9.6
Forest fires
range 0-0.38 0-0.22 0.1-7.5 0-0.05 0.06-3.8 0-0.52 0.3-15
median 0.19 0.11 3.8 0.02 1.9 0.26 7.6
Biogenic continental particulates
range 0.2-0.5 0-0.83 0.1-5.0 0-0.04 0.02-2.5 0-0.25 0.3-5.0
median 0.26 0.15 2.6 0.02 1.3 1.12 2.6
Biogenic continental volatiles
range 0.03-2.5 0-0.8 0.01-0.62 0.02-1.2 0.01-0.38 0.15-5.0 0.02-5.0
median 1.3 0.04 0.32 0.61 0.20 2.6 2.5
Appendix 1 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Biogenic marine
range 0.16-4.5 0-0.1 0.02-0.75 0.04-1.5 0.02-0.45 0.4-9.0 0.04-6.0
median 2.3 0.05 0.39 0.77 0.24 4.7 3.0
Total emission
range 0.86-23 0.15-2.6 2.3-54 0.1-4.9 0.97-23 0.66-18 4.0-86
median 12 1.3 28 2.5 12 9.3 45
a From: Nriagu (1989)
Appendix 2. Natural and anthropogenic emissions of trace metals to the atmosphere in 1983 (x 1000 tonnes/year) a
Trace metal Anthropogenic source Natural source Total emission Natural/total emissions
Arsenic 19 (12-26) 12 (0.86-23) 31 (13-49) 0.39
Cadmium 7.6 (3.1-12) 1.3 (0.15-2.6) 8.9 (3.2-15) 0.15
Copper 35 (20-51) 28 (2.3-54) 63 (22-105) 0.44
Mercury 3.6 (0.91-6.2) 2.5 (0.10-4.9) 6.1 (1.0-11) 0.41
Lead 332 (289-376) 12 (0.97-23) 344 (290-399) 0.04
Selenium 6.3 (3.0-9.7) 9.3 (0.66-18) 16 (2.5-24) 0.58
Zinc 132 (70-194) 45 (4.0-86) 177 (74-280) 0.34
a From: Nriagu (1989)
Appendix 3. Sources of global emissions of trace elements to the atmosphere in 1983 (tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Coal combustion - electric utilities
232-1550 77-387 930-3100 155-542 775-4650 108-775 1085-7750
Coal combustion - industry and domestic
198-1980 99-495 1390-4950 495-2970 990-9900 792-1980 1485-11 880
Oil combustion - electric utilities
5.8-29 23-174 348-2230 - 232-1740 35-290 174-1280
Oil combustion - industry and domestic
7.2-72 18-72 179-1070 - 716-2150 107-537 358-2506
Pyrometallurgical non-ferrous metal production - mining
40.0-80 0.6-3 160-800 - 1700-3400 18-176 310-620
Pyrometallurgical non-ferrous metal production - Pb production
780-1560 39-195 234-312 7.8-16 11 700-31 200 195-390 195-468
Pyrometallurgical non-ferrous metal production - Cu-Ni production
8500-12 750 1700-3400 14 450-33 600 37-207 11 050-22 100 427-1280 4250-8500
Pyrometallurgical non-ferrous metal production - Zn-Cd production
230-690 920-4600 230-690 - 5520-11 500 92-23 46 000-82 800
Secondary non-ferrous metal production
- 2.3-3.6 55-165 - 90-1440 3.8-19 270-1440
Steel and iron manufacturing
355-2480 28-284 142-2840 - 1065-14 200 0.8-2.2 7100-31 950
Refuse incineration - municipal
154-392 56-1400 980-1960 140-2100 1400-2800 28-70 2800-8400
Refuse incineration - sewage sludge
15-60 3-36 30-180 15-60 240-300 3-30 150-450
Appendix 3 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Phosphate fertilizers
- 68-274 137-685 - 55-274 0.4-1.2 1370-6850
Cement production
178-890 8.9-534 - - 18-14 240 - 1780-17 800
Wood combustion
60-300 60-180 600-1200 60-300 1200-3000 - 1200-6000
Mobile sources (gasoline)
- - - - 248 030 - -
Miscellaneous
1250-2800 - - - 3900-5100 - 1724-4783
Total emissions - range
12 000-25 630 3100-12 040 19 860-50 870 910-6200 288 700-376 000 1810-5780 70 250-193 500
Total emissions - median
18 820 7570 35 370 3560 332 350 3790b 131 880
a From: Nriagu & Pacyna (1988)
b This value applies to particulate selenium only. Since volatile selenium accounts for about
40% of the selenium released, the total selenium emission is estimated to be 6300 tonnes per year.
Appendix 4. Anthropogenic inputs of trace elements to aquatic ecosystems (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
3.6-7.7 0.9-3.6 6.0-15 0.22-1.8 87-113 0.54-1.1 21-58
Other industrial sources
8.4-62.3 1.2-13.4 29-75 0.08-7.0 10-67 9.5-70.9 56-317
Total - range
12-70 2.1-17 35-90 0.3-8.8 97-180 10-72 77-375
Total - median
41 9.4 62 4.6 138 41 226
Atmospheric fallout/total anthropogenic inputs (%)
14 24 17 22 72 2 17
a From: Nriagu & Pacyna (1988)
Appendix 5. Anthropogenic inputs of trace elements to soils (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
8.4-18 2.2-8.4 14-36 0.63-4.3 202-263 1.3-2.6 49-135
Other industrial sources
43.6-94 3.4-29.6 527-1331 0.97-10.7 277-850 4.7-73.4 640-1919
Total input - range b
52-112 5.6-38 541-1367 1.6-15 479-1113 6.0-76 689-2054
Total input - median b
82 22 954 8.3 796 41 1372
Atmospheric fallout/total anthropogenic inputs (%) b
16 24 3 30 29 5 7
a From: Nriagu & Pacyna (1988)
b These data do not include inputs from mine tailings, smelter slags and wastes to land.
RESUME
Le cadmium (numéro atomique 48; masse atomique relative 112,40)
est un élément métallique qui appartient, avec le zinc et le mercure,
au groupe IIb du tableau périodique. Certains sels de cadmium, tels
que le sulfure, le carbonate et l'oxyde sont presque insolubles dans
l'eau; ils peuvent cependant être transformés en sels solubles dans
l'environnement naturel. La formation de différents dérivés du cadmium
dans l'environnement est importante pour l'évaluation du risque.
La teneur moyenne en cadmium de l'eau de mer est d'environ 0,1
µg/litre ou moins. L'eau des cours d'eau contient du cadmium dissous
à des concentrations allant de < 1 à 13,5 ng/litre. Dans les régions
écartées et inhabitées, on observe en général des concentrations de
cadmium dans l'air inférieures à 1 ng/m3. Dans les régions où l'on
ne connaît pas de pollution, la concentration médiane en cadmium dans
le sol se situerait entre 0,2 et 0,4 mg/kg. Toutefois, on rencontre
occasionnellement des valeurs beaucoup plus élevées pouvant aller
jusqu'à 160 mg/kg de terre.
Certains facteurs environnementaux influent sur la fixation, et
par voie de conséquence, sur les effets toxiques du cadmium sur les
organismes aquatiques. Une élévation de température augmente la
fixation et l'effet toxique du cadmium qui sont en revanche réduits
lorsque la salinité de l'eau ou sa dureté augmentent. Les organismes
d'eau douce sont sensibles à des concentrations plus faibles de
cadmium que les organismes marins. Plus la teneur de l'eau en matières
organiques est élevée, plus la fixation et les effets toxiques du
cadmium sont réduits car ces matières se lient au cadmium et en
réduisent la biodisponibilité. Toutefois, on est fondé à croire que
certains dérivés organiques pourraient avoir un effet inverse.
Le cadmium s'accumule facilement dans de nombreux organismes, en
particulier les microorganismes et les mollusques, pour lesquels le
facteur de bioconcentration peut atteindre plusieurs milliers. Les
invertébrés terricoles concentrent égale-ment assez fortement le
cadmium. Pour la plupart des organismes, le facteur de concentration
est faible à modéré, généralement inférieur à 100. Dans de nombreux
tissus, le cadmium est lié aux protéines. On a isolé d'organismes
exposés au cadmium, des pro-téines qui fixent spécifiquement les
métaux lourds (métallothio-néines). Le cadmium se concentre
préférentiellement dans les reins, les branchies et le foie (ou leurs
équivalents). L'élimination du métal s'effectue probablement par le
rein, encore que chez les crustacés, il puisse être éliminé en
quantités notables en passant dans l'exosquelette. Chez les plantes,
le cadmium se concentre principalement dans les racines et à un
moindre degré dans les feuilles.
Le cadmium est toxique pour de nombreux microorganismes.
Toutefois, la présence de sédiments et de fortes concentrations de
sels ou de matières organiques en solution réduit ces effets toxiques.
Ces effets s'exercent principalement sur la croissance et la
réplication. Parmi les microorganismes terricoles, les plus affectés
sont les champignons, dont certaines espèces peuvent être éliminées
lorsque le sol est contaminé par du cadmium. Une faible exposition au
cadmium présent dans le sol peut entraîner une sélection des souches
résistantes. La toxicité du cadmium pour les organismes aquatiques est
variable, même lorsqu'il s'agit d'espèces très proches et elle est
liée à la concentration du métal sous forme ionique. Le cadmium
perturbe le métabolisme du calcium chez l'animal. Chez les poissons,
il provoque une hypocalcémie, probablement en inhibant la fixation du
calcium à partir de l'eau. Toutefois, la présence de fortes
concentrations de calcium dans l'eau protège les poissons par
inhibition compétitive de la fixation de cadmium. Le zinc accroît la
toxicité du cadmium pour les invertébrés aquatiques. On a fait état
d'effets sublétaux sur la croissance et la reproduction d'invertébrés
aquatiques; des effets ont été également observés sur la structure des
branchies d'invertébrés. Le cadmium est plus ou moins toxique pour les
poissons, les salmonidés étant particulièrement sensibles. On a
signalé des effets sublétaux chez les poissons, en particuliers des
malformations de l'épine dorsale. Les stades les plus sensibles sont
les embryons et les jeunes larves, les moins sensibles étant les
oeufs. Chez les poissons, on n'observe pas d'interaction systématique
entre le cadmium et le zinc. Le cadmium est toxique pour certaines
larves d'amphibiens, mais on a constaté que la présence de sédiments
dans les aquariums expérimentaux apportait une certaine protection.
Le cadmium perturbe la croissance des végétaux au laboratoire,
mais aucun effet n'a été observé dans la nature. Les plantes captent
plus facilement le métal lorsqu'il est présent dans les solutions
nutritives que lorsqu'il est dans le sol; les effets observés l'ont
été essentiellement lors d'études portant sur des cultures
hydroponiques. Il semblerait que le cadmium présent dans les solutions
nutritives affecte l'ouverture des stomates, la transpiration et la
photosynthèse.
Les invertébrés terrestres sont relativement insensibles aux
effets toxiques du cadmium, probablement à cause de l'intervention de
mécanismes de séquestration efficaces au niveau des divers organes.
Les gastéropodes terrestres peuvent subir des effets sublétaux,
principalement en ce qui concerne la consommation de nourriture et la
dormance, mais uniquement à des doses très élevées. Même à forte dose,
le cadmium n'entraîne pas la mortalité des oiseaux mais peut provoquer
des lésions rénales.
Des études effectuées sur le terrain ont montré que le cadmium
pouvait entraîner la modification de la proportion relative des
diverses espèces dans les populations de microorganismes et de
certains invertébrés aquatiques. La décomposition des feuilles mortes
est fortement entravée par une forte pollution due aux métaux lourds,
et le cadmium en serait le principal responsable.
RESUMEN
El cadmio (número atómico 48; masa atómica relativa 112,40) es un
elemento metálico que pertenece, junto con el zinc y el mercurio, al
grupo IIb de la tabla periódica. Algunas sales de cadmio, como el
sulfuro, el carbonato y el óxido, son prácticamente insolubles en
agua; pueden convertirse en sales hidrosolubles en el medio natural.
El sulfato, el nitrato y los haluros son hidrosolubles. La especiación
del cadmio en el medio ambiente tiene importancia para evaluar su
potencial de riesgo.
El contenido medio de cadmio en el agua de mar es de alrededor de
0,1 µg/litro o menos. El agua de los ríos contiene cadmio disuelto en
concentraciones que varían entre < 1 y 13,5 ng/litro. En zonas
aisladas y deshabitadas, las concentraciones de cadmio en el aire
suelen ser inferiores a 1 ng/m3. En zonas que se suponen no
contaminadas, se ha comunicado que la concentración mediana de cadmio
en el suelo se encuentra entre 0,2 y 0,4 mg/kg. No obstante, a veces
se encuentran valores mucho más altos, que pueden llegar hasta 160
mg/kg de suelo.
Los factores ambientales influyen en la captación y, por ende, en
los efectos tóxicos del cadmio en los organismos acuáticos. Al
aumentar la temperatura aumentan la captación y los efectos tóxicos,
mientras que el aumento de la salinidad o de la dureza del agua los
hace disminuir. Los organismos de agua dulce sufren los efectos del
cadmio en concentraciones inferiores a las que afectan a los
organismos marinos. La materia orgánica contenida en el agua suele
reducir la captación y los efectos tóxicos fijando el cadmio y
reduciendo su disponibilidad para los seres vivos. Sin embargo, hay
pruebas de que cierto tipo de materia orgánica puede ejercer el efecto
contrario.
El cadmio se acumula fácilmente en numerosos seres vivos,
particularmente microorganismos y moluscos, en los que los factores de
bioconcentración son del orden de varios millares. Los invertebrados
del suelo también concentran este metal en grado considerable. La
mayoría de los organismos presentan factores de concentración bajos o
moderados (inferiores a 100). El cadmio está ligado a proteínas en
numerosos tejidos. En organismos expuestos a ese metal se han aislado
proteínas fijadoras de metales pesados (metalotioneínas). La
concentración de cadmio es más elevada en el riñón, las branquias y el
hígado (o sus equivalentes). La eliminación se produce probablemente
por vía renal, si bien los crustáceos pueden eliminar cantidades
importantes con la muda del exoesqueleto. En las plantas, el cadmio se
concentra principalmente en las raíces y, en menor medida, en las
hojas.
El cadmio tiene efectos tóxicos para muy diversos
microorganismos. No obstante, la presencia de sedimentos y las
concentraciones elevadas de sales disueltas o materia orgánica reducen
esos efectos. Los procesos más afectados son el crecimiento y la
replicación. Los organismos del suelo más vulnerables son los hongos:
algunas especies desaparecen tras la exposición al cadmio en el suelo.
Tras exposiciones reducidas al metal en el suelo, se observa una
selección a favor de las cepas resistentes.
La toxicidad aguda del cadmio para los organismos acuáticos es
variable, incluso entre especies estrechamente emparentadas, y guarda
relación con la concentración de iones libres del metal. El cadmio
interacciona con el metabolismo del calcio en los animales. En los
peces provoca hipocalcemia, probablemente al inhibir la captación de
calcio a partir del agua. No obstante, las concentraciones elevadas de
calcio en el agua los protegen de la ingestión de cadmio por
competencia en los lugares de captación. El zinc aumenta la toxicidad
del cadmio para los invertebrados acuáticos. Se han notificado efectos
subletales en el crecimiento y la reproducción de invertebrados
acuáticos, así como modificaciones estructurales en las branquias de
invertebrados. Hay pruebas de la selección de estirpes resistentes de
invertebrados acuáticos tras la exposición al cadmio sobre el terreno.
La toxicidad es variable en los peces; los salmónidos son
especialmente susceptibles. Se han notificado efectos subletales en
los peces, en particular malformaciones de la espina dorsal. Las fases
biológicas más susceptibles son el embrión y la larva joven; los
huevos son los menos vulnerables. No se ha observado una interacción
homogénea entre el cadmio y el zinc en los peces. El cadmio resulta
tóxico para algunas larvas de anfibios, si bien los sedimentos en el
recipiente de ensayo confieren cierta protección.
El cadmio afecta al crecimiento de las plantas en estudios
experimentales, pero sobre el terreno no se ha observado efecto
alguno. El metal es absorbido por las plantas con más rapidez a partir
de soluciones de nutrientes que a partir del suelo; los efectos se han
observado sobre todo en estudios de cultivo en soluciones de
nutrientes. En éstas se ha notificado que el cadmio influye en la
apertura de los estomas, la transpiración y la fotosíntesis.
Los invertebrados terrestres son relativamente insensibles a los
efectos tóxicos del cadmio, probablemente debido a la existencia de
mecanismos eficaces de captura y fijación en ciertos órganos.
Los gasterópodos terrestres sufren efectos subletales; los
principales procesos afectados son el consumo de alimentos y el
letargo, pero sólo con dosis muy elevadas. El metal no produce efectos
letales en las aves, ni siquiera con dosis elevadas, si bien se
observan lesiones renales.
En estudios de campo se ha comunicado que el cadmio induce
cambios en la composición de especies en las poblaciones de
microorganismos y ciertos invertebrados acuáticos. La descomposición
del mantillo de hojas se ve notablemente reducida por la contaminación
con metales pesados; se ha identificado al cadmio como el principal
causante de este efecto.
Table 6. Cadmium assimilation efficiencies in different soil invertebrates
Species Food Cadmium concentration Assimilation efficiency Reference
in food (µmol/g) (%)
Snail
Arianta arbusloruma agar 1.48 55-92 Berger & Dallinger (1989) b
Centipede
Lithobius variegatus isopod 1.21-10.2 0-7.2 Hopkin & Martin (1984)
hepatopancreas
Millipede
Clomeris marginata maples leaves 8.2-40.6 Hopkin et al. (1985)
Pseudoscorpion
Neobisium muscorum collembolans 0.20 58.9 Janssen et al. (1991)
Mite
Platynothrus peltifer green algae 0.15 17.2 Janssen et al. (1991)
Insects
Orchesella cincta green algae 0.09 8.3 Van Straalen et al. (1987)
Orchesella cincta green algae 0.15 9.4 Janssen et al. (1991)
Notiophilus biguttatus collembolans 0.23 35.5 Janssen et al. (1991)
a assimilation value for midgut gland
b recalculated from the data
Table 7. Excretion constants (k) for cadmium in different soil invertebrates
Species Taxonomic k Reference
group (day-1)
Helix pomatia snail 0 Dallinger & Wieser (1984) b
Cepaea nemoralis snail 0.007 Williamson (1980) b
Oniscus asellusa isopod 0.002 Hopkin (1989) b
Neobisium muscorum pseudoscorpion 0 Janssen et al. (1991)
Lycosa spp spider 0.007 Van Hook & Yates (1975)
Platynothrus peltifer oribatid mite 0.013 Janssen et al. (1991)
Orchesella cincta collembolan 0.061 Van Straalen et al. (1987)
Orchesella cincta collembolan 0.087 Janssen et al. (1991)
Acheta domesticus cricket 0.090- Van Hook & Yates (1975)
0.110
Notiophilus biguttatus carabid beetle 0.375 Janssen et al. (1991)
a k value for midgut gland or hepatopancreas
b recalculated from the data
Bioconcentration factors (the ratio between the cadmium
concentration in the organism and the concentration in the medium) for
several groups of organisms studied under laboratory conditions are
shown in Table 8. They range from 16 to 130 000 and do not seem to
show any consistent pattern.
Table 8. Bioconcentration of cadmium in laboratory studies
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Freshwater alga 10 10 3000 dw c Ferard et al. (1983)
(Chlorella vulgaris)
Freshwater alga stat 20-22 14 250 4940 daw Cain et al. (1980)
(Scenedesmus obliquus)
Freshwater diatom flow WB 23 10 40 000 Conway (1978)
(Asterionella formosa)
Submerged plant WP 25 30 25 1730 dw Nakada et al. (1979)
(Elodea nuttallii)
Water hyacinth leaves 28 500 16 dw c Kay & Haller (1986)
(Eichhornia crassipes)
American oyster 4.9-5.1 g flow WB 16-20 21 10 116 ww Eisler et al. (1972)
(Crassostrea virginica) 4280 aw
8.1 g flow ST 2.8-22.6 280 5 2376 ww Zaroogian & Cheer (1976)
18 472 dw
Mussel 32-34 mm flow ST 13 166 10 50 802 dw Riisgard et al. (1987)
(Mytilus edulis)
Scallop 6.8-7.7 g flow WB 16-20 21 10 131 ww Eisler et al. (1972)
(Aquipecten irradians) 3970 aw
Bay scallop 0.51-0.73 g flow ST 9.5-16 42 60 20 400 Pesch & Stewart (1980)
(Argopecten irradians)
Crab 2-4 g WB 10 14 37 152 dw Ray et al. (1980a)
(Pandalas montagui)
Grass shrimp 20-33 mm flow WB 9.5-16 42 60 223 Pesch & Stewart (1980)
(Palaemonetes pugio)
Table 8 (contd).
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Lobster 160-169 g flow WB 16-20 21 10 21 ww Eisler et al. (1972)
(Homarus americanus) 10 aw
Mummichog 2.3-2.4 g flow WB 16-20 21 10 15 ww Eisler et al. (1972)
(Fundulus heteroclitus) 200 aw
Fathead minnow flow WB 13.9-15.3 21 49 190 Sullivan et al. (1978a)
(Pimephales promelas)
Red maple leaves 15-27 45 0.5 14 400 dw d Mitchell & Fretz (1977)
(Acer rubrum) roots 15-27 45 0.5 131 800 dw d Mitchell & Fretz (1977)
leaves 15-27 101 2.6 mg/kg 0.76 dw e Mitchell & Fretz (1977)
roots 15-27 101 2.6 mg/kg 12.5 dw e Mitchell & Fretz (1977)
White pine leaves 15-27 66 0.5 3400 dw d Mitchell & Fretz (1977)
(Pinus strobus) roots 15-27 66 0.5 118 400 dw d Mitchell & Fretz (1977) leaves 15-27 36 52.6 mg/kg 1.2 dw e Mitchell & Fretz (1977)
roots 15-27 36 52.6 mg/kg 10.4 dw e Mitchell & Fretz (1977)
a WB = whole body; WP = whole plant; ST = soft tissues
b Chloride salt used unless stated otherwise; bioconcentration factor = concentration in the organism divided by concentration
in the medium; dw = dry weight; ww = wet weight; aw = ash weight; daw = dry ash weight
c Nitrate salt used
d The medium was a cadmium-enriched nutrient solution
e The medium was a cadmium-amended soil mix
Microorganisms generally exhibit a high capacity to take up
cadmium from water and retain the metal in their cells. The highest
bioconcentration factors reported have been for micro-organisms, the
greatest value being 40 000 in a freshwater diatom (Conway, 1978). In
this diatom, 58% of the cadmium was located in the cellular content
with 25% in the organic coating of the frustule and 17% in the
silicaceous frustule. The bioconcentration factor of 3000 for the alga
Chlorella (Ferard et al., 1983) is typical of the value for
microorganisms. Flatau et al. (1988) demonstrated the uptake (it was
not specified whether this referred to absorption or adsorption) of
cadmium from sea water by marine bacteria; the uptake of the metal
increased with its concentration in the water, and the accumulation
rate was a logarithmic function of the dose. Sorption was only
observed with exposure concentrations above 10 µg Cd/litre, suggesting
that a threshold had to be exceeded for cadmium uptake to occur.
Dongmann & Nurnberg (1982) showed that the bioconcentration factor for
a marine diatom, Thalassiosira rotula, decreased with increasing
metal concentration, suggesting a saturation effect. Their reported
concentration factors, which vary between 1000 and 2000, reflect the
reduced sorption of cadmium by marine microorganisms compared with
their freshwater relatives. Hart & Scaife (1977) reported a direct
relationship between the level of cadmium in the medium and sorption
to the alga Chlorella exposed to cadmium concentrations ranging from
0.25 to 1.00 mg/litre.
After water hyacinths had been exposed for 4 weeks to water
containing 0.5 or 1.0 mg cadmium/litre, added as cadmium nitrate, the
leaves had accumulated 8.00 and 17.20 mg/kg, respectively (Kay &
Haller, 1986).
Molluscs concentrate cadmium to a high degree over a period of
time, but uptake is often slow. Oysters showed a concentration factor
of only 149 over a 10-day period (Eisler et al., 1972) but a factor of
2714 after 40 weeks (Zaroogian & Cheer, 1976). Elliott et al. (1985)
examined the accumulation of cadmium, copper, lead, and zinc in the
tissues of the mussel, Mytilus edulis. Under simultaneous exposure
to all four metals, both lead and cadmium were accumulated in direct
proportion to the exposure time, whereas copper and zinc were not.
Accumulation of cadmium was influenced by the presence of other
metals.
Compared with oysters, the related bay scallop shows greater
accumulation of cadmium when exposed to low concentration of the metal
as the chloride over 6 weeks (Pesch & Stewart, 1980). Short-term
exposure of the same scallop to higher concentrations of cadmium
resulted in very much lower concentration factors. Exposure for 96 h
to cadmium (as the chloride) at up to 2.0 mg/litre led to a
bioconcentration factor of around 50 (Nelson et al., 1976).
Bioconcentration factors (from water and food) and
biomagnification factors (from food alone) were calculated for the
freshwater isopod Assellus aquaticus by van Hattum et al. (1989).
Much of the cadmium (added as the chloride) was taken up from the
water (bioconcentration factor 18 000), but there was little uptake
from food (bioconcentration factor 0.08). Direct uptake from water
accounted for between 50 and 98% of the body burden after 30 days of
exposure (based on dry weight measures). Cadmium was readily taken up
by the isopod even at exposure concentrations of 1 µg/litre.
Experiments conducted at two different pHs (5.9 and 7.6) revealed no
significant effect of pH on uptake of cadmium by the isopod.
Wright & Frain (1981b) demonstrated that adult intermoult
amphipods ( Gammarus pulex) accumulated only half as much cadmium
from a solution of 5 mg/litre in the presence of 200 mg calcium/litre
as with 20 mg calcium/litre.
Ramamoorthy & Blumhagen (1984) investigated the uptake of
cadmium, mercury, and zinc by rainbow trout (Salmo gairdneri) in a
model system which simulated the presence of other competing
compartments that would be found in nature. The system consisted of
either a simple sediment/water model or a more complex series of
compartments in dialysis bags of suspended sediment, cation and anion
exchange resins (to represent naturally occurring polyelectrolyte
materials of plant origin), and fish. River water was used as the
fluid transfer medium, and the system was continuously stirred.
Equilibrium with one heavy metal ion did not inhibit the uptake of
other metal ions; cadmium and zinc were taken up after equilibrium
with mercury. The authors calculated approximate partition
coefficients (fish/substrate) to be 2.8 for sediment, 550 for water,
and 2 and 3.6 for the cation and anion exchange resins, respectively.
The problem of expressing changes in concentration between
trophic levels is that the units are not compatible. There is no
significance to a bioconcentration term that expresses a ratio of
cadmium in soil moisture to cadmium in plant tissue, or cadmium in
plant tissue to cadmium in herbivore tissue. Therefore, it is
difficult to assess the impact of cadmium on the environment in terms
of bioconcentration factors. An alternative method is to measure both
cadmium and calcium at each trophic level and express these
measurements as a molar ratio of these two elements. (The molar ratio
should be used to account for the movement by atoms, not grams.)
Differences between trophic levels are calculated as the ratio of the
higher trophic level to the lower. This approach, called
biopurification, recognizes that the flow of the non-nutrient cadmium
through successive trophic levels follows a pathway similar to that of
nutrients such as calcium, and that calcium must pass natural chemical
and physiological barriers, such as membranes and selective enzymes,
that progressively purify the pool of the nutrient calcium relative to
the non-nutrient cadmium. In the case where two similar ecosystems are
compared, and where one is believed to be more contaminated than the
other, the relative degree of contamination can be calculated as the
difference between molar ratios at the same or similar trophic levels.
It is unfortunate that the absence of concentration data on
nutrients such as calcium or, alternatively, zinc, prohibits the
calculation of biopurification factors for any of the studies
discussed in this monograph.
5. TOXICITY TO MICROORGANISMS
Appraisal
Cadmium is toxic to a wide range of microorganisms in culture
(effects of cadmium on microorganisms in the field are discussed in
chapter 8). However, the presence of sediment, organic matter or high
concentrations of dissolved salts reduces the availability of cadmium
to microorganisms and, therefore, reduces the toxic impact.
Freshwater microorganisms in culture are thus affected by cadmium at
lower concentrations than marine species (for example, 50 µg/litre
affects growth in many freshwater species of algae while at least 100
µg/litre, and often 1000 µg/litre, is required to reduce growth in
marine species). Soil microorganisms are partially protected from the
toxic effects of cadmium by the presence of clay.
5.1 Aquatic microorganisms
5.1.1 Freshwater microorganisms
Canton & Slooff (1982) exposed the bacterium Salmonella
typhimurium and the alga Chlorella vulgaris to cadmium in the form
of the chloride, and calculated an 8-h EC50 (growth inhibition) of
10.4 mg/litre for the bacterium and a 96-h EC50 of 3.7 mg/litre for
the alga. No-toxic-effect levels of 0.65 and 1.5 mg/litre were
estimated for the bacterium and alga, respectively. Jana &
Bhattacharya (1988) found significant inhibition of population growth
in the faecal coliform bacterium Escherichia coli during exposure to
cadmium concentrations of 1, 2 or 5 mg cadmium/litre for 7 or 28 days.
Cadmium was the most toxic of the metals tested. Norberg & Molin
(1983) exposed the bacterium Zoogloea ramigera (abundant in sewage
treatment plants) to cadmium chloride concentrations of 1, 3, 5, and
10 mg cadmium/litre for 30 h. A prolonged lag phase and decrease in
growth resulted, the length of the lag phase being proportional to the
concentration of cadmium in the medium. Babich & Stotzky (1977a)
showed that the presence of clay particles protected bacteria from the
toxic effect of cadmium added to culture medium. The degree of
protection was related to the cation exchange capacity of various
clays tested.
Chapman & Dunlop (1981) estimated the 8-h LC50 for the
freshwater protozoan Tetrahymena pyriformis to be less than 1
mg/litre. However, this value increased with increasing water calcium
concentration; at a value of 500 mg calcium/litre, the LC50 was 19
mg/litre. Magnesium also exerted a protective effect against cadmium
when mixed with calcium. Cadmium was consistently more toxic to
Tetrahymena in the presence of magnesium alone. Berk et al. (1985)
calculated a 15-min EC50 (inhibition of ciliate chemotactic
response) for Tetrahymena sp. of 0.35-0.7 mg.
When Skowronski et al. (1988) exposed the green microalga
Stichococcus bacillaris to cadmium chloride concentrations of 45 and
90 µmol/litre for 4 days, growth rate was inhibited by 28% and 45% at
the two respective concentrations. At both exposure levels, dry weight
and chlorophyll a content were reduced in a dose-related manner.
Addition of manganese at concentrations of between 45 and 1800
µmol/litre had a dose-related antagonistic effect on cadmium toxicity.
Bennett (1990) found that the addition of cadmium (1.8 µmol/litre) to
a turbidostat culture of Chlorella pyrenoidosa caused a decrease in
the maximum specific growth rate (toxicity was expressed after a lag
of 5 generations). A gradual decrease in the maximum specific growth
rate was also noted during a 40-day exposure to stepwise increases in
the cadmium concentration (0.96 to 1.68 µmol/litre). The author found
that the addition of manganese (10.4 µmol/litre) had an antagonistic
effect, causing the maximum specific growth rate to increase.
Cadmium is toxic to the growth of the freshwater alga Chlorella
pyrenoidosa (Hart & Scaife, 1977). In cultures maintained at pH 7.0,
doubling times were 11, 21, 22, and 35 h for cadmium concentrations of
0, 0.25, 0.5, and 1.0 mg/litre medium, respectively. At a pH of 8.0,
the effect was somewhat lessened; doubling times were 11, 16, 17, and
25 h for the same range of cadmium doses. There was also a pronounced
effect on carbon dioxide fixation, which was reduced from 0.738 to
0.720, 0.558, and 0.283 µmol HCO3- fixed per hour with cadmium
exposures in the culture medium of 0, 0.246, 0.554, and 1.090
mg/litre, respectively. There was less of an effect on oxygen
evolution over the same dose range. Zinc offered no protection against
cadmium effects.
Wong et al. (1979) exposed four different species of freshwater
algae to cadmium and measured the uptake of 14C-carbonate.
Scenedesmus quadricaudata was the most sensitive species, carbonate
uptake being inhibited by 80% at a cadmium concentration of 20
µg/litre. Chlorella pyrenoidosa showed 70% inhibition of carbonate
uptake at about 100 µg/litre, while Chlorella vulgaris showed only
50% inhibition at about 500 µg/litre. The least sensitive of the four
species tested was Ankistrodesmus falcatus variety acicularis
where an effect on carbonate uptake started only at concentrations
higher than 500 µg/litre. There was no observed effect on the growth
of A. falcatus at cadmium concentrations lower than 5 mg/litre.
Rebhun & Ben-Amotz (1984) demonstrated that the chlorophyll content of
cells of Chlorella stigmatophora was reduced in a dose-dependant
manner across a range of cadmium concentrations of between 1 and 10
mg/litre medium.
Laegreid et al. (1983) studied the effects of cadmium on the alga
Selenastrum capricornutum cultured in the laboratory in water taken
from two lakes at various times throughout the year. The two lake
waters contained different amounts of organic material. The first
lake, a dystrophic bog lake, had a high organic content, while the
second, an eutrophic lake, had a low organic content. In the
dystrophic lake, which had a low pH (4.4), the toxicity of cadmium was
related to the free ionic concentration of the metal, as suggested by
many laboratory experiments. In the eutrophic lake, where there was
less influence from organic material, there was a pronounced seasonal
effect. In the summer, when growth and productivity of the algae were
highest, there was a much greater effect of the metal than predicted.
The toxicity of cadmium, at this time, was far greater than would be
expected even if all of the metal was in the free ionic form and none
was bound to organic compounds. On the basis of their field evidence,
the authors questioned the generally held assumption that organic
binding is the major factor in determining cadmium toxicity to
microorganisms. They considered that the presence of certain organic
compounds of low relative molecular mass could increase cadmium
toxicity. This conclusion is supported by the work of Giesy et al.
(1977), who found that uptake of cadmium into zooplankton could be
increased in the presence of organic compounds of low relative
molecular mass.
Chin & Sina (1978) investigated the cellular basis of cadmium
toxicity in microorganisms using cultures of Physarum polycephalum.
The organism was cultured, in plasmodial form, on the surface of
liquid medium, and replicate discs, cut from the protoplasmic sheet of
the organism, were used for the tests. The discs maintain mitotic
synchrony with each other and, therefore, cadmium could be introduced
at specific points in the cell cycle. The cultures were exposed to
cadmium sulfate (5 x 10-4 mol/litre), which was floated onto the
surface of the culture medium. Exposure to cadmium immediately prior
to early prophase of mitosis extended the normal DNA replication
period from 3 h to 4 h; this was monitored using measurements of
uptake of 3H-thymidine. Two stages of the cell cycle were
particularly susceptible to cadmium. Exposure either at the beginning
of the cycle or 80% of the way through the cycle caused delays in the
completion of mitosis. A 30-min exposure to cadmium at the onset of
early prophase inhibited incorporation of 3H-uridine into RNA for
the following 3 h by 51% and stimulated the incorporation of
3H-thymidine into DNA, for the same period, by 85%. Later in the
cycle, DNA synthesis was inhibited and DNA content was depressed by
12.5%. There was an ultrastructural effect on the nucleoli (less dense
material centrally giving nucleoli in section a "ring" structure),
which was the only structural effect of the metal even after 4 h of
exposure. Accommodation occurred after pre-treatment with sub-toxic
doses of cadmium. Treatment with cadmium at the less sensitive periods
of the cell cycle led to reduced effect at the more sensitive phases;
the organism, in some way, compensated. There was no accommodation by
Physarum after pre-treatment with zinc ions. This result contrasts
with that of a similar study on Escherichia coli where pre-exposure
to zinc reduced the effects of cadmium (Mitra et al., 1975).
5.1.2 Estuarine and marine microorganisms
Chan & Dean (1988) exposed the marine bacterium Pseudo-monas
marina to cadmium sulfate at concentrations of 1 to 25 mg
cadmium/litre. Effects were exposure related and included a lengthened
lag time, reduced growth rate, reduced biomass and oxygen uptake, and
a decrease in the activity of dehydrogenase and alkaline phosphatase.
The IC50 values for inhibition of biomass and of growth rate were 11
and 11.5 mg/litre, respectively. Flatau et al. (1987) progressively
adapted the marine bacterium Pseudomonas sp. to cadmium
concentrations from 1 to 80 mg/litre. Although the length of the lag
phase was not linearly or logarithmically correlated with the cadmium
concentration, it was significantly longer at cadmium concentrations
of 70 mg/litre or more. The growth rate was reduced at 10 mg/litre but
remained constant at cadmium concentrations of 10 to 50 mg/litre; no
growth was observed at 75 or 80 mg/litre. Oxygen consumption was not
different from that of controls at 1 or 5 mg/litre but at
concentrations of 10 mg/litre or more respiratory activity decreased.
Bressan & Brunetti (1988) exposed the marine microalgae
Dunaliella tertiolecta and Isochrysis galbana to cadmium
concentrations of 13.8 and 0.2 mg/litre, respectively, for up to 8
days. Cadmium significantly reduced the population growth, expressed
as the mean number of cells/ml, for both species. The addition of
nitrilotriacetic acid (NTA, a sequestering agent) at ratios of 1:1,
1:2 or 1:3 did not modify the toxic effect of cadmium.
Berk et al. (1985) exposed the marine ciliates Paranophrys sp.
and Miamiensis avidus to cadmium and monitored the inhibition of the
chemotactic response. The 15-min EC50 values were 2-3.1 mg
cadmium/litre and 5.1-7 mg cadmium/litre for the two species,
respectively.
Dongmann & Nurnberg (1982) investigated the effects of cadmium on
the marine diatom Thalassiosira rotula (a chain-forming diatom
native to shallow temperate waters) in petri dish and in batch liquid
cultures. They calculated generation times from the dish cultures and
reported a toxicity threshold for generation time of 30 µmol
cadmium/litre (3.4 mg/litre). At 50 µmol/litre, cadmium increased the
generation time from 24 h to 28 h. Using chain length as a parameter,
the toxicity threshold was 15 µmol/litre (1.7 mg/litre). Cell density
was found to be affected in batch culture. Cell chlorophyll content
and chain length were too variable to show significant effects of the
metal in batch culture.
5.2 Soil and litter microorganisms
Babich & Stotzky (1977b) showed that several species of fungi
tolerated cadmium to a greater degree when grown on cultures amended
with clays than in pure culture medium.
Sato et al. (1986) exposed the soil bacterium Nitrosomonas
europaea both to cadmium concentrations of 0.05, 0.1, and 0.4
mg/litre and to a range of ammonia concentrations (1 to 100 mg/litre
as N). Growth was markedly inhibited at the highest cadmium
concentration, especially when this was coupled with ammonia
concentrations greater than 10 mg/litre. Cadmium toxicity caused a
characteristic growth response. Ammonia oxidation proceeded at a
reduced rate for approximately 3 days and then fell sharply. The
subsequent oxidation proceeded at a diminished but constant rate. At
cadmium concentrations of 0.1 mg/litre or less, the toxic effect of
cadmium could be partially offset by increasing the ammonia
concentration. Prahalad & Seenayya (1988) found that cadmium
concentrations of 3.5 mg/litre inhibited the growth of the bacterium
Alcaligenes faecalis. However, if the nutrient broth was diluted by
one half then growth was inhibited at 2 mg/litre, and with
quarter-strength nutrient broth inhibition occurred at 0.5 mg/litre.
Hartman (1974) reported less species diversity of soil fungi in
areas of high cadmium contamination than in control areas. Samples of
Fusarium oxysporium isolated from contaminated and control soils
showed different tolerances to cadmium. This suggested the development
of some resistance, presumably by selection of more resistant strains.
Bond et al. (1976) incubated coniferous forest soil and litter
(mixed with a pre-prepared homogeneous soil) in microcosm units and
monitored oxygen consumption, carbon dioxide evolution, and bacterial
and fungal populations. With cadmium added to a concentration of 0.01
mg/kg soil and, in the initial stages of incubation, with cadmium at
10 mg/kg soil, there was a stimulation of oxygen consumption,
suggesting an effect of the metal on uncoupling of respiratory
phosphorylation. In the later stages of incubation with cadmium at 10
mg/kg there was a reduction in both oxygen consumption and carbon
dioxide evolution. No effect was seen on numbers of organisms in the
microcosms.
Bewley & Stotzky (1983) investigated the effect of cadmium (100
and 1000 mg/kg soil) on carbon mineralization and on the mycoflora in
glucose-supplemented soils amended with clays (kaolinite or
montmorillonite) at 9%. Cadmium had no significant effect on the
length of the lag period, carbon dioxide evolution or on the amount of
carbon mineralized. When subsequent cadmium additions of 2400 and 4000
mg/kg were made to soils previously treated with 100 or 1000 mg/kg,
the rate of glucose degradation decreased more in the clay-amended
soils than in control soil not amended with clay. Clay protected fungi
from the toxic effect of cadmium at 5000 mg/kg. Fungi from
clay-treated soils were more sensitive to cadmium in the culture media
after they had been isolated from soil pre-treated with cadmium. The
authors interpreted these results as showing a reduction in the
availability of cadmium to organisms in clay-amended soils. The
overall effect would be a prevention of selection of more tolerant
strains. Thus, when the organisms were challenged with high doses of
cadmium, they would have been more susceptible than organisms from
non-amended soil.
Naidu & Reddy (1988) incubated black cotton soil (0.8% organic
carbon; 55% clay) for up to 8 weeks in the presence of cadmium
chloride at concentrations of between 50 and 500 mg cadmium/kg. The
ammoniacal nitrogen (NH4-N) concentration increased for the first
week at all treatment levels and then decreased at concentrations of
50 mg/kg or less. The initial rise in NH4-N levels led to an
increase in nitrate nitrogen (NO3-N) levels, the accumulation of
NO3-N being inversely proportional to the cadmium exposure. The
authors pointed out that at cadmium concentrations of 100 and 500
mg/kg, hydrolysis of urea was significantly poorer than at other
treatment levels, as shown by the lower concentration of NH4-N
observed after 1 week. At all cadmium concentrations there was
significant accumulation of nitrite nitrogen (NO2-N) at every sample
time, compared to control soil, suggesting that cadmium might be toxic
to soil nitrification. At all exposure levels cadmium significantly
depressed both bacterial and fungal populations. Cadmium
concentrations of 10 or 50 mg/kg had no effect on soil actinomycetes,
but both 100 and 500 mg/kg significantly reduced the population. Tyler
et al. (1974) incubated a mull soil for 7 weeks. Cadmium chloride
concentrations of 9 to 18 µmol/g and cadmium acetate concentrations of
9 to 22 µmol/g caused decreases in soil ammonium content and
significantly increased nitrate accumulation.
6. TOXICITY TO AQUATIC ORGANISMS
Appraisal
Cadmium uptake from water by aquatic organisms is extremely
variable and depends on the species and various environmental
conditions such as water hardness (notably the calcium ion
concentration), salinity, temperature, pH, and organic matter content
(see chapter 4).
The majority of chelating agents decrease cadmium uptake but
some, such as dithiocarbamates and xanthates, increase uptake.
As a consequence of the variability in cadmium uptake, the toxic
impact to aquatic organisms also varies across a wide range of
concentrations and is dependent on the species of organism and on the
presence of other metal ions, notably calcium and zinc.
The lowest recorded 96-h LC50 in a flow system is 16 µg
cadmium per litre for the adult shrimp Mysidopsis bahia. A nominal
no-observed-effect level (NOEL) of 0.6 µg cadmium/litre was found for
Daphnia magna, reproductive rate being the most sensitive parameter.
A nomi-nal NOEL has been noted at a similar level (1.7 to 3.4 µg
cadmium per litre) with respect to the reproductive effects on brook
trout.
The available results indicate that the embryonic and larval
stages of aquatic organisms are more sensitive than the adult stage.
Spinal malformations are induced in cadmium-exposed fish. In addition
to causing reproductive effects, cadmium influences the behaviour of
aquatic organisms.
At low concentrations, cadmium inhibits ion transport systems
(10 µg cadmium/litre) and induces metallothionein synthesis (< 1 µg
cadmium/litre) in freshwater fish.
6.1 Toxicity to aquatic plants
Hutchinson & Czyrska (1975) exposed two floating aquatic weeds,
the common duckweed Lemna minor and a floating fern Salvinia
natans, to cadmium concentrations of between 0.01 and 1.0 mg/litre
for up to 3 weeks. Growth was reduced at all concentrations but
especially at 0.05 mg/litre or more. The effect of cadmium on growth
became more marked with time. Loss of green coloration (chlorosis) was
a common symptom of cadmium toxicity, and at concentrations of 0.5 and
1.0 mg/litre Lemna plants died. When the two species were grown in
competition, growth at lower cadmium concentrations (0.01 and 0.05
mg/litre) was greater in Salvinia but less in Lemna than when the
plants were grown alone.
In a study by Nir et al. (1990), water hyacinth plants were
exposed to cadmium concentrations of 0, 0.05, 0.1, 0.4 or 1.0 mg/litre
for 7 days. Concentrations of 0.1 mg/litre or less had no significant
effect on wet or dry biomass gain or on chlorophyll a content.
Concentrations of 0.4 or 1.0 mg/litre significantly reduced both wet
biomass gain and chlorophyll a content but had no significant effect
on dry biomass gain. The chlorophyll a content of leaves decreased
with time in plants exposed to 0.4 mg/litre. After 3 weeks of
exposure, the chlorophyll a levels were 75% lower than in control
plants.
6.2 Toxicity to aquatic invertebrates
Cadmium is moderately to highly toxic to aquatic invertebrates
(see Tables 9 and 10). Its toxic effect is dependent on a variety of
environmental variables. Factors that reduce the free ionic
concentration, e.g., water hardness, salinity, chelating agents, and
high organic content of water, tend to reduce the toxic effect of
cadmium. The presence of zinc increases the toxic effect of cadmium on
invertebrates.
6.2.1 Acute and short-term toxicity
The acute toxicity of cadmium to aquatic invertebrates, as
assessed in laboratory tests, is summarized in Tables 9 and 10. The
most notable features are the variability in cadmium toxicity between
different organisms and the effects of temperature, salinity, and
water hardness. There is considerable variation even amongst closely
related species.
In a study by Canton & Slooff (1982), the water flea Daphnia
magna was exposed to cadmium over a 48-h period. At a water hardness
of 1 mmol/litre there was no mortality at 16 µg cadmium per litre,
while at a water hardness of 2 mmol/litre there was no mortality or
abnormal behaviour at 17 µg/litre.
Clubb et al. (1975b) investigated the toxicity of cadmium to nine
species of aquatic insects, but seven of the species tested were too
insensitive to the effects of the metal for the LC50 to be
determined. The insensitive species were Atherix variegata,
Hexatoma sp., Holorusia sp., Acroneuria pacifica, Arcynopteryx
signata, Pteronarcys californica, and Brachycentrus americanus;
these species represented Dipterans (true flies), Plecoptera
(stoneflies), and Tricoptera (caddis flies).
Table 9. Toxicity of cadmium to marine or estuarine invertebrates
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Purple sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 0.5 (0.4-0.6) m Dinnel et al. (1989)
(Strongylocentrotus
purpuratus)
Green sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 1.8 (1.5-2.2) m Dinnel et al. (1989)
(Strongylocentrotus
droebachiensis)
Sand dollar embryo stat 12.5-13.0 30 8.0-8.1 72 7.4 (5.2-10.8) m Dinnel et al. (1989)
(Dendraster
excentricus)
Starfish 24.5 g stat 20 20 8.0 24 12 n Eisler (1971)
(Asterias forbesi) 24.5 g stat 20 20 8.0 48 1.0 n Eisler (1971)
24.5 g stat 20 20 8.0 96 0.82 n Eisler (1971)
11.2 g stat 20 20 7.8 24 71 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 48 7.1 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
American oyster embryo stat 26 25 7.0-8.5 48 3.8 (2.85-4.48) n Calabrese et al. (1973)
(Crassostrea
virginica)
Mussel stat 18.5 32.9 7.9 96 1.62 (1.19-2.22) m Ahsanullah (1976)
(Mytilus edulis
planulatis)
Blue mussel 4 g stat 20 8.0 24 > 200 n Eisler (1971)
(Mytilus edulis) 4 g stat 20 8.0 48 165 n Eisler (1971)
4 g stat 20 8.0 96 25 n Eisler (1971)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Soft-shell clam 5.2 g stat 20 20 8.0 24 > 20 n Eisler (1971)
(Mya arenaria) 5.2 g stat 20 20 8.0 48 50 n Eisler (1971)
5.2 g stat 20 20 8.0 96 2.2 n Eisler (1971)
4.6 g stat 20 20 7.8 24 32 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 48 2.5 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Bay scallop 20-30 mm stat 20 25 8.0 24 8.2 n Nelson et al. (1976)
(Argopecten 20-30 mm stat 20 25 8.0 48 3.21 n Nelson et al. (1976)
irradians) 20-30 mm stat 20 25 8.0 72 2.18 n Nelson et al. (1976)
20-30 mm stat 20 25 8.0 96 1.48 (0.95-2.31) n Nelson et al. (1976)
Atlantic oyster 0.6 g stat 20 20 8.0 24 158 n Eisler (1971)
drill 0.6 g stat 20 20 8.0 48 28 n Eisler (1971)
(Urosalpinx 0.6 g stat 20 20 8.0 96 6.6 n Eisler (1971)
cinerea)
Eastern mud snail 0.56 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Nassarius 0.56 g stat 20 20 8.0 48 125 n Eisler (1971)
absoletus) 0.56 g stat 20 20 8.0 96 10.5 n Eisler (1971)
Ragworm 8 g stat 20 20 8.0 24 25 n Eisler (1971)
(Nereis virens) 8 g stat 20 20 8.0 48 25 n Eisler (1971)
8 g stat 20 20 8.0 96 11 n Eisler (1971)
7.6 g stat 20 20 7.8 24 56 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 48 9.3 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Copepod nauplius stat 22 10 96 0.06 (0.001-0.2) m Roberts et al. (1982)
(Eurytemora affinis)
Copepod adult stat 22 10 96 0.38 (0.006-1.52) m Roberts et al. (1976)
(Acartia tonsa)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Harpacticoid
copepod adult stat 20-22 3 96 0.43 (0.31-0.55) Bengtsson & Bergstrom (1987)
(Nitocra spinipes) adult stat 20-22 7 96 0.66 (0.53-0.82) Bengtsson & Bergstrom (1987)
adult stat 20-22 15 96 0.78 (0.41-120) Bengtsson & Bergstrom (1987)
Marine amphipod young stat 10 96 3.5 m Wright & Frain (1981a)
(Marinogammarus adult stat 10 96 13.3 m Wright & Frain (1981a)
obtusatus)
Mysid shrimp adult flow 22 20 7.3 96 0.036 (0.022-0.081) m Roberts et al. (1982)
(Neomysis adult flow 22 20 7.8 96 0.02 (0.015-0.027) m Roberts et al. (1982)
americanus)
Mysid shrimp adult stat 22 20 7.3 96 0.017 m Roberts et al. (1982)
(Mysidopsis bahia) adult stat 22 20 7.7 96 0.029 (0.013-0.043) m Roberts et al. (1982)
flow c20-28 15-23 96 0.016 (0.013-0.02) m Nimmo et al. (1978)
Shrimp stat 18.7 32.1 8.0 120 2.3 (1.05-5.06) m Ahsanullah (1976)
(Palaemonetes sp.) stat 18.7 32.1 8.0 168 1.85 (1.32-2.59) m Ahsanullah (1976)
0.38 g flow 16.8 96 6.8 (5.2-9.76) m Ahsanullah (1976)
0.16 g flow 17.8 8.1 96 6.4 (5.73-7.19) m Ahsanullah (1976)
Sandworm 0.37 g stat 18.5 32.7 8.1 168 6.4 (5.82-7.1) m Ahsanullah (1976)
(Neanthes vaali)
Sand shrimp 0.25 g stat 20 20 8.0 24 2.4 n Eisler (1971)
(Crangon 0.25 g stat 20 20 8.0 48 0.5 n Eisler (1971)
septemspinosa) 0.25 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Sand shrimp adult flow 10.2 28.6 7.9 96 2.3 (1.7-5.1) m Dinnel et al. (1989)
(Crangon spp.)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Grass shrimp 0.33 g stat 20 20 8.0 24 43 n Eisler (1971)
(Palaemonetes 0.33 g stat 20 20 8.0 48 3.7 n Eisler (1971)
vulgaris) 0.33 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Shrimp stat 35 96 2.07 (± 0.22) m McClurg (1984)
(Penaeus indicus)
Pink shrimp flow 25 20 96 4.6 m Bahner & Nimmo (1975)
(Penaeus duorarum)
Grapsid crab 1.47 g stat 17.8 32.6 8.1 168 14 (11.2-17.5) m Ahsanullah (1976)
(Paragrapsus 1.08 g stat 17.1 168 16.7 (15.11-18.45) Ahsanullah (1976)
quadridentatus)
Hermit crab 0.47 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Pagurus 0.47 g stat 20 20 8.0 48 3.7 n Eisler (1971)
longicarpus) 0.47 g stat 20 20 8.0 96 0.32 n Eisler (1971)
0.5 g stat 20 20 7.8 24 15 n Eisler & Hennekey (1977)
0.5 g stat 20 20 8.0 96 1.3 n Eisler & Hennekey (1977)
Shore crab 5.9 g stat 20 20 8.0 24 100 n Eisler (1971)
(Carcinus maenus) 5.9 g stat 20 20 8.0 48 16.6 n Eisler (1971)
5.9 g stat 20 20 8.0 96 4.1 n Eisler (1971)
Dungeness crab zoea stat 8.5 30 8.1 96 0.2 (0.1-0.4) m Dinnel et al. (1989)
(Cancer magister)
Squid larva stat 8.6 30 8.1 96 > 10.2 m Dinnel et al. (1989)
(Loligo opalescens)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b organisms exposed to cadmium added as cadmium chloride; m = measured; n = nominal
c intermittent flow-through conditions
Table 10. Toxicity of cadmium to freshwater invertebrates
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Snail adult stat 20-22 6.7 24 7.6 n Wier & Walter (1976)
(Physa adult stat 20-22 6.7 48 4.25 n Wier & Walter (1976)
gyrina) adult stat 20-22 6.7 96 1.37 n Wier & Walter (1976)
adult stat 20-22 6.7 228 0.83 n Wier & Walter (1976)
immature stat 20-22 6.7 48 0.69 n Wier & Walter (1976)
immature stat 20-22 6.7 96 0.41 n Wier & Walter (1976)
Snail flow b 15 7.1-7.7 168 0.114 m Spehar et al. (1978a)
(Physa integra)
Snail 10-12 mm flow 12 128-176 7.7 24 4.4 m Williams et al. (1985)
(Physa 10-12 mm flow 12 128-176 7.7 48 2.1 m Williams et al. (1985)
fontinalis) 10-12 mm flow 12 128-176 7.7 96 0.8 m Williams et al. (1985)
Isopod 8-10 mm flow 12 128-176 7.7 24 13 m Williams et al. (1985)
(Asellus 8-10 mm flow 12 128-176 7.7 48 3.6 m Williams et al. (1985)
aquaticus) 8-10 mm flow 12 128-176 7.7 96 0.6 m Williams et al. (1985)
Scud 10 96 0.12 m Wright & Frain (1981b)
(Gammarus 8-12 mm flow 12 128-176 7.7 24 1.6 m Williams et al. (1985)
pulex) 8-12 mm flow 12 128-176 7.7 48 0.4 m Williams et al. (1985)
8-12 mm flow 12 128-176 7.7 96 0.02 m Williams et al. (1985)
Water flea adult stat 10 0.85 meq/ 7.2 48 0.055 e (0.032-0.095) n Baudouin & Scoppa (1974)
(Daphnia hyalina) litre
Water flea 1 day stat 20 7.6-7.7 24 3 n Kuhn et al. (1989)
(Daphnia < 1 day stat 20-22 110-130 7.8 48 0.04 (0.02-0.07) n Hall et al. (1986)
magna) < 1 day stat 20-22 190-210 7.7 48 0.08 (0.06-0.1) n Hall et al. (1986)
< 1 day stat 18-20 1 mmol/ 48 0.03 m Canton & Slooff (1982)
litre
Table 10 (contd).
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Water flea < 1 day stat 20-22 110-130 7.8 48 0.07 n Hall et al. (1986)
(Daphnia < 1 day stat 20-22 110-130 7.7 48 0.1 (0.07-0.12) n Hall et al. (1986)
ulex) < 1 day stat 19-22 7.7 96 0.047 (0.042-0.052) n Bertram & Hart (1979)
Copepod adult stat 10 0.85 meq/ 7.2 48 3.8 e (2.3-6.3) n Baudouin & Scoppa (1974)
(Cyclops abyssorum) litre
Copepod adult stat 10 0.85 meq/ 7.2 48 0.55 e (0.39-0.77) n Baudouin & Scoppa (1974)
(Eudiaptomus padanus) litre
Crayfish flow 19-21 24-28 6.7-7.0 96 6.1 (4.7-7.9) m Mirenda (1986)
(Orconectes virilis)
Mayfly flow 10 7.8 96 28 m Clubb et al. (1975b)
(Ephemerella grandis
grandis)
Midge 10-12 mm flow 12 128-176 7.7 96 300 m Williams et al. (1985)
(Chironomus riparius)
Stonefly flow 10 7.8 96 18 m Clubb et al. (1975b)
(Pteronarcella badia)
Stonefly 10-15 mm flow 12 128-176 7.7 96 520 m Williams et al. (1985)
(Hydropsyche
angustipennis)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b intermittent flow-through conditions
c hardness expressed as mg CaCO3/litre unless stated otherwise
d organisms exposed to cadmium added as cadmium chloride unless otherwise stated; m = measured concentration; n = nominal concentration
e organism exposed to cadmium as cadmium sulfate
Spehar et al. (1978a) reported a decreased survival of the water
snail Physa integra at a cadmium concentration of 85.5 µg/litre
after 7 days of exposure. After 21 days of exposure, survival was
significantly decreased at 27.5 µg/litre, the next lowest
concentration tested. Some snails exposed to these concentrations
developed a condition in which the animal was extended from the shell
but unable to attach the foot or crawl. A white mucus layer covered
the exposed foot region of some snails and these subsequently died.
Concentrations tested were not high enough to obtain a 4-day LC50
value but the 7-day LC50 of 114 µg/litre was approximately 11 times
higher than the 28-day LC50 value of 10.4 µg/litre.
Mirenda (1986) reported a 14-day LC50 of 0.7 mg cadmium per
litre for the crayfish Orconectes virilis under flow-through
conditions. Pesch & Stewart (1980) estimated the 10-day LC50 for bay
scallops Argopecten irradians to be 0.53 mg/litre in flowing sea
water. The EC50 (for growth) for the same species over 42 days was
0.078 mg/litre. Byssal thread detachment, which precedes death, showed
an EC50 of 0.54 mg/litre of cadmium 8 days into the test and before
there was any appreciable mortality. Robinson et al. (1988) compared
10-day LC50 values for freshly collected and cultured infaunal
amphipods ( Rhepoxynius abronius). Cultured amphipods appeared normal
and survived well (93%) under control toxicity test conditions, but
were more sensitive to cadmium in sediment (10-day LC50 = 4.4 mg/kg)
than were freshly caught amphipods (10-day LC50 = 8.7 mg/kg).
When Winner (1988) exposed Daphnia magna and Ceriodaphnia
dubia to cadmium for 7 days, the most sensitive indicators were mean
body length of primiparous females in D. magna, which was
significantly reduced at 2 µg cadmium/litre, and the total young per
female in C. dubia, significantly reduced at 1 µg/litre.
6.2.1.1 Effects of temperature and salinity on acute toxicity
An increase in toxicity as temperature increases and as salinity
decreases is valid for all organisms that have been tested with these
variables (Tables 9 and 10).
O'Hara (1973) investigated the effects of temperature and
salinity on the toxicity of cadmium to adult male and female fiddler
crabs ( Uca pugilator). Mortality was greatest at high temperatures
and low salinities in tests lasting 240 h. LC50 values varied from
2.9 mg/litre for the lowest salinity (10%) and highest temperature (30
°C) to 47.0 mg/litre for the highest salinity (30%) and lowest
temperature (10 °C). Frank & Robertson (1979) exposed the blue crab
( Callinectes sapidus) to cadmium chloride at salinities of 1, 15,
and 35%. Like O'Hara, they found a decrease in cadmium toxicity with
increase in salinity. For example, 96-h LC50 values were 0.32, 4.7,
and 11.6 mg cadmium/litre for the three salinities, respectively.
Rosenberg & Costlow (1976) reported increased cadmium toxicity during
larval development of two estuarine crab species as salinity decreased
and increased toxicity as temperature increased.
Voyer & Modica (1990) found the same pattern with the mysid
shrimp Mysidopsis bahia. For salinities of 10 and 30%, the 96-h
LC50 values ranged from 15.5 to 28 µg cadmium/litre at a temperature
of 25 °C and from 47 to 84 µg/litre at a temperature of 20 °C. At 30
°C the 96-h LC50 was < 11 µg/litre at both salinities. However,
when Robert & His (1985) exposed embryos and larvae of the Japanese
oyster Crassostrea gigas to cadmium concentrations of up to 50
µg/litre at various salinities (20 to 35%), decreasing the salinity
severely affected the development of the oysters but cadmium had no
effect.
At temperatures higher than 11 °C, the combined effect of
temperature and cadmium caused a heavy stress to the copepod Tisbe
holothuriae so that the effects of salinity were masked
(Verriopoulos & Moraitou-Apostolopoulou, 1981).
6.2.1.2 Effect of water hardness
Using either artificial hard water (hardness: 180 mg CaCO3 per
litre) or dechlorinated tap water (hardness: 60 mg/litre),
Niederlehner et al. (1984) conducted short-term tests on the effects
of cadmium on the freshwater oligochaete Aeolosoma headlyi.
Mortality and population growth/maintenance were assessed over 10 to
14 days. The authors established NOEL values for population growth of
32.0 and 53.6 µg/litre for hard water (two replicate tests), whereas
the NOEL for the softer water was 17.2 µg/litre. The 48-h LC50
values were 4.98 and 1.2 mg/litre, respectively, for hard and softer
water.
6.2.1.3 Effect of organic materials and sediment
When Schuytema et al. (1984) exposed Daphnia magna to cadmium
for a period of 48 h, the mean LC50 value was 39 µg/litre in water
and 91 µg/litre in a water-sediment slurry. Giesy et al. (1977) found
that cadmium was more toxic to water fleas ( Simocephalus serrulatus)
exposed in well water with a low organic content than to those exposed
in pond water with a high organic content. The authors isolated a
series of organic fractions from the pond water by ultrafiltration.
Protection against cadmium toxicity was afforded by fractions of
intermediate relative molecular mass (ranging from approximately 500
to 300 000 daltons). The fraction with a relative molecular mass in
excess of 300 000 daltons marginally increased the toxicity of
cadmium.
Kemp & Swartz (1988) compared the acute toxicity of interstitial
and particle-bound cadmium to the marine infaunal amphipod
Rhepoxynius abronius. The cadmium concentration in interstitial
water was kept constant whereas the sediment cadmium level was varied
by using perfusion through the sediment with peristaltic pumps. The
principal cause of toxicity was found to be cadmium dissolved in
interstitial water, between 70 and 88% of the toxicity being
predictable from interstitial water concentrations.
6.2.1.4 Lifestage sensitivity
When Calabrese et al. (1973) investigated the toxicity of cadmium
to embryos of the American oyster Crassostrea virginica, there was
no mortality at 1 mg/litre and the 48-h LC50 and LC100 values were
3.8 and 6 mg/litre, respectively. Johnson & Gentile (1979) found the
larva of the American lobster Hommarus americanus to be sensitive to
cadmium; the 96-h LC50 in static bioassays was 78 µg/litre. The
mortalities after 96 h at concentrations of 10 and 30 µg/litre were 3%
and 10%, respectively. There is a very steep increase in toxicity of
cadmium to lobster larvae between 24 and 96 h. The 24-h LC50 is
approximately 1 mg/litre; at this concentration the mortality reaches
100% within 48 h.
Verriopoulos & Moraitou-Apostolopoulou (1982) found that the
different life-stages of the copepod Tisbe holothuriae showed
differences in sensitivity to cadmium. One-day-old nauplius larvae of
the copepod were the most sensitive with an LC50 of 0.538 mg/litre,
expressed as ions of cadmium, while 5-day-old nauplii showed an LC50
of 0.645 mg/litre. The value for 10-day-old copepodids (0.906
mg/litre) was not significantly different from that for adult females
(0.916 mg/litre). Females with ovigerous sacs were slightly more
sensitive, with an LC50 of 0.873 mg/litre. When Robinson et al.
(1988) exposed the infaunal phoxocephalid amphipod Rhepoxynius
abronius to sediment contaminated with cadmium, the 10-day LC50
values were 8.2 mg/kg for juveniles and 11.5 mg/kg for adults.
Nebeker et al. (1986) exposed Daphnia magna of different ages
to cadmium for a period of 48 h. Mean EC50 (immobilization) values
ranged from 23 µg/litre for 6 day-old water fleas to 164 µg/litre for
2-day-old Daphnia. Tests on Daphnia of different ages, conducted
in water of different hardness (32 or 76 mg CaCO3 per litre), with
or without feeding and in two different sizes of container, resulted
in a wide range of EC50 values (4 to 307 µg/litre). There was no
consistent effect of any of these variables other than the age of the
test animals. Very young animals were relatively tolerant, with a mean
EC50 value of 109 µg/litre.
McCahon et al. (1989) exposed both cased and uncased 1st, 3rd,
and 4th larval instars of the caddis fly Agapetus fuscipes to
cadmium chloride. The LC50 values ranged from 295 to > 1000 mg
cadmium/litre at 24 h and from 50 to 320 mg/litre at 96 h. First
instar larvae were significantly more sensitive than 3rd or 4th instar
larvae and at all ages cased animals were more resistant than uncased.
6.2.1.5 Other factors affecting acute and short-term toxicity
Chandini (1988; 1989) found that increasing the food source of
the cladocerans Daphnia carinata and Echinisca triserialis greatly
reduced the toxicity of cadmium, expressed as the 48-h or 96-h LC50,
and the effect of cadmium on various other life history parameters,
such as fecundity, growth, and age at first reproduction.
Verriopoulos & Moraitou-Apostolopoulou (1981) exposed adult
females of the copepod Tisbe holothuriae to cadmium and found that
the oxygen concentration in the water was negatively related to
mortality and that population density was positively related after
cadmium exposure. Clubb et al. (1975a) showed that the toxicity of
cadmium to aquatic insects decreased with decreasing dissolved oxygen
levels in the test water.
McCahon et al. (1988) exposed the amphipod Gammarus pulex to
cadmium chloride under static conditions and reported a 96-h LC50 of
0.05 mg cadmium/litre. The acute toxicity of cadmium to G. pulex
parasitized by the acanthocephalan Pomphorhynchus laevis at several
levels of infection was investigated. Toxicity, expressed as LC50,
did not differ significantly between uninfected and infected
amphipods.
Kay & Haller (1986) fed water hyacinth weevils Neochetina
eichhorniae on water hyacinths containing cadmium from previous
exposure to the metal. There was no mortality in weevils fed on leaves
containing up to 17.2 mg cadmium/kg. At this exposure level, the
weevils accumulated a cadmium body burden of 36.67 mg/kg over 20 days.
6.2.2 Long-term toxicity
In a study by Zaroogian & Morrison (1981), adult and larval
oysters of the species Crassostrea virginica were exposed to cadmium
at concentrations of 5 or 15 µg/litre. Some adults were exposed (for
35 or 37 weeks) to cadmium at these two concentrations prior to
spawning, and larvae from these pre-treated adults and from control
treated adults were reared in either control sea water or sea water
containing 5 or 15 µg/litre cadmium. In all there were 11 treatment
combinations. The highest larval mortality occurred when larvae from
parents treated with 15 µg/litre were reared in sea water containing
15 µg/litre for 3 weeks. However, larvae that survived this treatment
grew to lengths not significantly different from controls. The effects
observed with other treatment combinations were only temporary. Growth
and development were slowed but those larvae that survived ultimately
developed normally and to the same size as controls.
When Holcombe et al. (1984) exposed snails, from embryos through
to adult sexual maturity, to cadmium chloride, there was a delayed
hatch, a reduction in percentage hatch and survival, and reduced
growth when compared to controls. Based on these effects, the authors
suggested a maximum acceptable cadmium concentration in water of
between 4 and 8 µg/litre from one test and between 2 and 5 µg/litre
from a replicate test.
Lussier et al. (1985) conducted life-cycle tests, over 35 days,
on the mysid shrimp Mysidopsis bahia. Cadmium affected survival
primarily and no reproductive effects were noted at sublethal
concentrations.
Following a long-term investigation in the laboratory and in the
field, Marshall (1979) suggested a chronic LC10 for the water flea
Daphnia galeata mendotae of 0.15 µg cadmium/litre. Winner (1986)
exposed Daphnia pulex chronically (21 or 42 days) to cadmium, added
as cadmium sulfate, under different water conditions. Increasing the
water hardness from 58 to 116 mg/litre reduced the toxic effect of the
cadmium but a further increase to 230 mg/litre had no further effect.
The most sensitive aspect of the Daphnia life history to cadmium was
the abortion rate of young. Humic acid had no effect on this parameter
in soft or medium-hard water but increased the toxic effect of cadmium
in hard water. Mortality was increased by humic acid (0.75 or 1.50
mg/litre) at all water hardness levels. van Leeuwen et al. (1985)
calculated 14-day and 21-day LC50S for Daphnia magna of 24 and 14
µg cadmium/litre, respectively. No effect on mortality was seen at 3.2
µg/litre. The lowest concentrations producing significant mortality of
young amphipods during 6-week exposures to cadmium were 1 µg/litre for
Hyalella azteca and 3.2 µg/litre for Gammarus fasciatus (Borgmann
et al., 1989b).
6.2.3 Reproductive effects
Mysing-Gubala & Poirrier (1981) conducted laboratory experiments
on the effect of cadmium on the freshwater sponge Ephydatia
fluviatilis. Sponge cuttings were exposed to cadmium concentrations
ranging from 0.001 to 1.0 mg/litre for 1 month. At the end of the
experimental period, the cuttings were classified in four groups
depending on whether the sponge survived, whether it produced asexual
reproductive gemmules, and whether the silicaceous spicules of the
gemmules were normal or malformed. There was some effect of cadmium
even at concentrations as low as 0.001 mg/litre, 17% of the sponge
cuttings showing no gemmule production and 33% showing malformed
spicules. At concentrations of 0.5 and 1.0 mg/litre, all of the sponge
cuttings died.
Lee & Xu (1984) investigated the effects of cadmium at 0.5 and
0.1 mg/litre on the fertilization and development of sea urchin eggs
and the development of Amphioxus. At both cadmium concentrations,
sea urchin development was normal to the gastrulation stage but all
the plutei were abnormal. The effects on Amphioxus development were
different; cleavage of eggs was not affected by cadmium at 0.5 or 0.1
mg/litre but neurulation was. Dinnel et al. (1989) exposed sperm from
various sea urchin species to cadmium chloride for 60 min and assessed
fertilization success; EC50 values ranged from 12 to 26 mg/litre. An
EC50 of 8 mg/litre was calculated for the sand dollar Dendraster
excentricus. Den Besten et al. (1989) exposed the sea star Asterias
rubens to cadmium chloride at a concentration of 25 µg cadmium/litre
for 5 months. No effect on spermatozoa was found, but maturation of
oocytes was delayed and early development of embryos was adversely
affected.
Conrad (1988) studied the effect of cadmium on newly fertilized
eggs of the mud snail Ilyanassa obsoleta. No apparent effect was
observed at a cadmium concentration of 10-6 mol/litre. The minimum
concentrations producing abnormal veliger development and abnormal
late cleavage and stopping early cleavage were 10-5-10-4
mol/litre, 10-3 mol/litre, and 10-3 mol/litre, respectively.
Biesinger & Christensen (1972) estimated a 3-week 16%
reproductive impairment concentration of 0.17 µg cadmium/litre.
In a 21-day reproduction test on Daphnia magna, Kuhn et al.
(1989) determined a nominal NOEL of 0.6 µg Cd2+/litre, reproduction
rate being the most sensitive parameter. A nominal NOEL of 1 µg/litre
was found when daphnids were exposed to the cadmium chloride salt.
Reproduction of Daphnia magna was completely inhibited at
concentrations exceeding 3.2 µg/litre and time-dependant survival and
reproduction were significantly reduced at 1.8 µg/litre. No effects on
reproduction were observed at 1 µg/litre (van Leeuwen et al., 1985).
When Bertram & Hart (1979) exposed the cladoceran Daphnia pulex to
cadmium concentrations of 1 to 30 µg/litre, there was no significant
effect on the number of days required for onset of reproduction or on
its frequency. At 1 µg/litre no significant effect on longevity of
individuals was observed, but at concentrations of 5 µg/litre or more
there was a significant reduction. All cadmium concentrations caused
a reduction in the average brood size, the average number of broods
per adult, and the total number of progeny. The authors also exposed
daphnids to cadmium-contaminated food in the form of the phytoplankton
Chlorella, the cadmium concentration being 0.3-0.6 µg cadmium/litre
of medium. This resulted in a significant reduction in the average
number of broods per adult and the average brood size. However, there
was no significant effect on average longevity of individuals, the
percentage of adults producing broods, the average number of days to
the first brood or the average number of days between broods.
Bengtsson & Bergstrom (1987) exposed newly fertilized female
harpacticoids ( Nitocra spinipes) to cadmium chloride at two
salinities for 13 days. At 3% the fecundity EC50 was 37-46 µg/litre
at a salinity of 3% and 6-15 µg/litre at 15%.
Williams et al. (1987) provided water containing nominal cadmium
chloride concentrations of 0, 0.3, 30, 100 or 300 mg cadmium/litre
cadmium to newly-emerged adults of the midge Chironomus riparius in
which they could lay their eggs. The females preferred to lay their
eggs in water with no cadmium or in the lower concentrations;
significant preferences were recorded. Eggs of Chironomids are laid
within a protective gelatinous matrix. Eggs exposed to cadmium after
complete formation of the matrix in control water were unaffected (the
hatch was 80-100%), whereas those exposed after removal of the matrix
had a reduced hatching rate (60%) at all test concentrations. Eggs
laid directly in water containing cadmium were unaffected by a
concentration of 0.3 mg/litre, but hatching rates were reduced to 45%
at 30 mg/litre, 8% at 100 mg/litre, and 0% at 300 mg/litre. Clearly,
cadmium only affects the unprotected egg, either when it is newly laid
and before its gelatinous protection has developed or when this
gelatinous matrix is removed.
In a flow-through study, Hatakeyama (1987) exposed the chironomid
Polypedilum nubifer, from the egg stage, to cadmium chloride. There
was no effect on total number of adults, emergence success, sex ratio,
total number of egg clusters, oviposition success or hatchability at
10 µg cadmium/litre. There was a significant decrease in the total
number of adults and emergence success at 20 µg/litre or more and in
the total number of egg clusters at 40 µg/litre or more. At 80
µg/litre survival and reproduction were very significantly depressed.
In a separate experiment midges were fed cadmium-contaminated food
(dried yeast) at concentrations of 22, 220, and 1800 mg cadmium/kg.
Emergence success was decreased at 220 and 1800 mg/kg but no other
effects were observed.
6.2.4 Physiological and biochemical effects
Berglind (1986) investigated the effect of cadmium alone and in
combination with other metals on the delta-aminolevulinic acid
dehydratase (ALAD) activity of Daphnia magna. ALAD activity was
enhanced by cadmium alone (at 2 µg/litre but not at 0.2 µg/litre) but
this enhancement was abolished in the presence of zinc at 200
µg/litre.
Vernberg et al. (1977) investigated sub-lethal concentrations of
cadmium and their effects on the adult shrimp Palaemonetes pugio
under static and flow-through conditions. Adult shrimps were exposed
to cadmium at 50 µg/litre. This shrimp is highly tolerant to cadmium
and after exposure to 23 mg/litre the mortality rate was only 10%. The
authors found increased uptake of cadmium into the body of the shrimps
with decreasing salinity. Similarly, at higher salinity levels,
toxicity was lower. In shrimps kept at the lowest salinity level (5%),
where cadmium body burden reached 40 mg/kg, there was inhibition of
moulting. At more moderate cadmium body burdens of 23 mg/kg and 10
mg/kg, observed at salinities of 10% and 20%, respectively, moulting
was stimulated by the presence of cadmium. An investigation of the
effects of cadmium on respiratory rate was inconclusive because of
considerable variation. The authors considered that the flow-through
system more nearly approximated field conditions than the static
system. The relationship between salinity and cadmium uptake was
eliminated in a static system at 15 °C, but was quite clearly present
when the system was flow-through. After an extensive study on the
effects of cadmium on three species of shrimps, Penaeus duorarum,
Palaemonetes pugio, and Palaemonetes vulgaris, Nimmo et al. (1977)
reported sublethal histological effects and blackening and damage to
gill filaments. Placing shrimps with blackened gills in cadmium-free
water resulted in the sloughing-off of blackened portions of the
branchia and the shrimps appeared normal within 14 days. The effect on
the gills occurred with exposure to cadmium concentrations approaching
the LC50 which, with an exposure duration of 96 h, was 0.76 mg/litre
for Palaemonetes vulgaris and 3.5 mg/litre for Penaeus duorarum.
Thurberg et al. (1973) exposed two species of crabs ( arcinus maenus
and Cancer irroratus) to various concentrations of cadmium chloride
for 48 h and at five different salinities. At the end of each exposure
period, tests of blood serum osmolarity and gill tissue oxygen
consumption were performed. Cadmium increased the osmolarity of
Carcinus serum above its normal hyperosmotic state and reduced
oxygen consumption by the gill tissue of both species. Effects on
oxygen consumption were dose related over a range of cadmium
concentrations from 0 to 4 mg/litre.
Cadmium has been shown to cause an increase in oxygen consumption
rates in the mud snail Nassarius obsoletus at concentrations of
between 0.5 and 4.0 mg/litre over a 72-h exposure period (MacInnes &
Thurberg, 1973). A similar increase in oxygen consumption rate was
observed in the marine snail Murex trunculus during chronic exposure
to 0.05 mg cadmium/litre (Dalla Via et al., 1989) and in crabs
( Callinectes similis) exposed to cadmium concentrations of between
2.48 and 10.05 mg/litre for up to 96 h (Ramirez et al., 1989).
6.2.5 Behavioural effects
Olla et al. (1988) monitored the burrowing behaviour of three
polychaete species, Nereis virens, Glycera dibranchiata, and
Nephtys caeca during a 28-day exposure to a sediment concentration
of 40 mg cadmium/kg. Most comparisons of burrowing times and rates
between exposed and unexposed worms were not statistically
significant. Four out of 15 comparisons gave significant results but
these were randomly spread amongst species and exposure periods. The
authors concluded that these results would probably have little
ecological significance. The feeding behaviour of G. dibranchiata
was also monitored but no significant effect of the cadmium treatment
was found.
6.2.6 Interactions with other chemicals
Sunda et al. (1978) carried out experiments in diluted sea water
with various concentrations of the chelating agent nitrilotriacetic
acid (NTA) to determine the relationship between the chemical
speciation of cadmium and the toxicity of the metal to the grass
shrimp Palaemonetes pugio. After 4 days of exposure to a given
concentration of cadmium chloride, shrimp mortality decreased with
increasing salinity and increasing concentration of the chelating
agent. The protective effect of high salinity or NTA was attributable
to complexation of cadmium; mortality was related to the measured free
cadmium ion concentration, which was determined by measuring total
concentration of cadmium and deducting the calculated level of
complexation by either the chloride ion or NTA. The mortality at a
free cadmium ion concentration of approximately 4 x 10-7 mol/litre
was 50%. In a study of the combined effects of zinc and cadmium on the
shrimp Callianassa australiensis by Negilski et al. (1981), there
was interaction between the two metals; in combination they gave
greater mortality than would be expected if there were no interaction.
The authors demonstrated that each metal increased the accumulation of
the other.
6.2.7 Tolerance
Khan et al. (1988) exposed two different populations of grass
shrimp Palaemonetes pugio to cadmium under static conditions and
calculated 96-h LC50 values of 3.28 mg/litre for shrimps from an
industrialized area and 1.83 mg/litre for those from a non-
industrialized area. Pre-exposure of shrimps to 0.05 mg cadmium per
litre caused an increase in the LC50 values to 6.81 and 3.89
mg/litre for the two respective populations.
Moraitou-Apostalopoulou et al. (1982) collected the shrimp
Palaemon elegans from two different areas with different natural
concentrations of cadmium and investigated the sublethal effects of
the metal. They found that cadmium decreased respiration rates in the
shrimp at sublethal concentrations. Shrimps sampled from an area with
a natural cadmium concentration of 0.6 µg/litre were more tolerant to
the metal than were those from an area with 0.1 µg/litre. There was
also a difference in the acute toxicity of cadmium to the two
populations, indicating that tolerance had developed. In an earlier
study (Moraitou-Apostolopoulou et al., 1979), similar development of
tolerance was reported for a copepod Acartia clausi.
In a study of the toxicity of cadmium to the freshwater cyclopoid
copepod Tropocyclops prasinus mexicanus, Lalande & Pinel-Alloul
(1986) sampled animals from three Quebec lakes, one polluted and two
unpolluted with cadmium. The cultures from the two unpolluted lakes
showed lower LC50 values in 48-h tests than the culture from the
polluted lake. However, the polluted lake had a significantly higher
hardness (120 mg calcium carbonate per litre) than the other two lakes
(10 mg/litre).
6.2.8 Model ecosystems
Borgmann et al. (1989a) established a Daphnia-phytoplankton
model ecosystem and exposed it to cadmium sulfate at concentrations of
1, 5 and 15 µg cadmium/litre. At 5 µg/litre the Daphnia population
collapsed after 9 weeks of treatment and chlorophyll levels increased.
At 15 µg/litre the Daphnia population collapsed after 5 weeks, but
chlorophyll levels remained low. There appeared to be no effect on
this model system at 1 µg/litre.
6.3 Toxicity to Fish
The toxicity of cadmium has been studied in a variety of fish
species in both fresh and sea water at various temperatures and
dissolved oxygen concentrations. Generally, increasing dissolved salt
concentration decreases the toxicity, whereas increasing temperature
increases it. An increase in the dissolved oxygen content of the water
decreases the toxicity of cadmium to freshwater fish. Salmonids appear
to be particularly susceptible to the metal. Sublethal effects have
been reported, notably malformation of the spine.
6.3.1 Acute and short-term toxicity
The acute toxicity of cadmium to fish is summarized in Tables 11
and 12. Pickering & Henderson (1966) calculated the LC50 values for
five species of warm-water fish, some of which were tested in both
soft and hard water. There was surprisingly little difference in
fathead minnows ( Pimephales promelas) between the 24-h, 48-h, and
96-h LC50 values, which in soft water, were 1.09, 1.09, and 1.05
mg/litre, respectively. This species was very much more resistant to
cadmium in hard water with 24-h, 48-h, and 96-h LC50 values of 78.1,
72.6, and 72.6 mg/litre, respectively. The hardness for the two types
of water was 20 and 360 mg CaCO3 per litre and the alkalinity 80 and
300 mg/litre. The dissolved oxygen concentration was similar in the
two types of water. However, the pH was 7.5 for soft water and 8.2 for
hard water, this being an area of the pH range where speciation of
cadmium undergoes major change. Bluegill sunfish, goldfish, and
guppies showed a decrease in LC50 with increase in exposure duration
from 24 to 96 h (Table 12), but these were tested only in soft water.
The green sunfish Lepomis cyanellus showed LC50 values in soft
water of 7.84, 3.68, and 2.84 mg/litre with exposure durations of 24
h, 48 h, and 96 h, respectively. This species was also tested in hard
water, where, like the fathead minnow, it showed considerably less
cadmium toxicity. The 24-h LC50 rose from 7.84 in soft water to 88.6
mg/litre in hard water.
Pickering & Gast (1972) determined a maximum acceptable toxicant
concentration (MATC) for the fathead minnow Pimephales promelas of
between 37 and 57 µg cadmium/litre. The experimental concentration of
57 µg/litre decreased survival of the developing embryos, this being
the most sensitive life-stage, but at lower concentrations (between
4.5 and 37 µg/litre) no adverse effect was found on survival, growth
or reproduction. Carroll et al. (1979) investigated the protective
effects of various constituents of hard water on the toxicity of
cadmium to the brook trout ( Salvelinus fontinalis) and concluded
that calcium, added as either the sulfate or carbonate, was the most
significant source of protection. This protective effect was observed
in the absence of significant cadmium precipitation. Magnesium,
sulfate, and sodium ions and the carbonate system provided little or
no protection. Calamari et al. (1980) found an influence of water
hardness on the toxicity of cadmium to Salmo gairdneri; 48-h LC50
values increased from 91 µg/litre, with a hardness of 20 mg/litre in
the test water, to 3700 µg/litre at a water hardness of 320 mg/litre.
The 48-h LC50 of fish acclimatized to a hardness of 320 mg/litre and
then tested at a hardness of 20 mg/litre was about 7 times higher than
that of fish acclimatized and tested in the same soft water. There are
two types of biological effects of hardness on the availability of
cadmium to fish; one of them persists after acclimatization in hard
water.
Table 11. Toxicity of cadmium to marine or estuarine fish
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Striped killifish 0.95 g stat 20 20 8.0 24 125 n Eisler (1971)
(Fundulus majalis) 0.95 g stat 20 20 8.0 48 59 n Eisler (1971)
0.95 g stat 20 20 8.0 96 21 n Eisler (1971)
Mummichog 0.89 g stat 20 20 8.0 24 > 100 n Eisler (1971)
(Fundulus 0.89 g stat 20 20 8.0 48 > 100 n Eisler (1971)
heteroclitus) 0.89 g stat 20 20 8.0 96 55 n Eisler (1971)
1.3 g stat 20 20 7.8 24 220 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 96 22 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 168 22 n Eisler & Hennekey (1977)
1 day stat 20 20 48 16.2 (12.7-21.2) n Middaugh & Dean (1977)
7 days stat 20 20 48 9 (6.4-12.5) n Middaugh & Dean (1977)
14 days stat 20 20 48 32 (24.6-41.6) n Middaugh & Dean (1977)
adult stat 20 20 48 60 (40-90) n Middaugh & Dean (1977)
1 day stat 20 30 48 23 (19.2-27.6) n Middaugh & Dean (1977)
7 days stat 20 30 48 12 (9.2-15.6) n Middaugh & Dean (1977)
14 days stat 20 30 48 7.8 (5.6-10.3) n Middaugh & Dean (1977)
adult stat 20 30 48 43 (33-56) n Middaugh & Dean (1977)
Sheepshead minnow 1.1 g stat 20 20 8.0 24 100 n Eisler (1971)
(Cyprinodon 1.1 g stat 20 20 8.0 48 50 n Eisler (1971)
variegatus) 1.1 g stat 20 20 8.0 96 50 n Eisler (1971)
Coho salmon smolt flow 11.2 28.3 7.9 96 1.5 (1.2-2.4) m Dinnel et al. (1989)
(Oncorhynchus
kisutch)
Yellow-eye mullet 1.49 g flow 18.5 34.5 7.8 58 15.5 (12.2-19.8) m Negilski (1976)
(Aldrichetta 1.15 g flow 18.6 34.8 7.8 120 14.3 (8.4-24.3) m Negilski (1976)
forsteri)
Small-mouthed 1.36 g flow 17.9 34.5 7.9 168 12.7 (8.3-19.4) m Negilski (1976)
hardyhead 1.12 g flow 18.0 34.5 7.8 168 16.6 (13.3-20.7) m Negilski (1976)
(Atherinasoma microstoma)
Table 11 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Atlantic silverside adult stat 20 20 48 13 (9-20) n Middaugh & Dean (1977)
(Menidia menidia) adult stat 20 30 48 12 (8-16) n Middaugh & Dean (1977)
Tidewater silverside larva stat 26 22 96 0.31 (0.25-0.38) n Mayer (1987)
(Menidia peninsulae)
Shiner perch adult flow 13 30.1 7.8 96 11 (5-20) m Dinnel et al. (1989)
(Cymatogaster aggregata)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained)
b organisms exposed to cadmium added as cadmium chloride; m = measured concentration; n = nominal concentration
Table 12. Toxicity of cadmium to freshwater fish
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Chinook salmon juvenile flow 11-13 20-22 7.0-7.3 96 0.001 (± 0.0007) f m Finlayson & Verrue (1982)
(Onchorhynchus
tshawytscha)
Rainbow trout juvenile flow 6.4-8.3 96 0.0066 g m Hale (1977)
(Salmo 5-15 g stat 8.5-10.7 61-65 7.4 48 2.9 m Pascoe et al. (1986)
gairdneri) 5-15 g stat 8.5-10.7 283-317 7.4 48 5.7 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 1.3 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 2.6 m Pascoe et al. (1986)
Fathead minnow adult stat 25 20 7.5 24 1.09 (0.79-2.91) n Pickering & Henderson (1966)
(Pimephales adult stat 25 360 8.2 24 78.1 (57.2-117) n Pickering & Henderson (1966)
promelas) adult stat 25 20 7.5 48 1.09 (0.79-2.91) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 1.05 (0.7-4.43) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 18-22 190-210 7.7 48 0.1 (0.07-0.17) n Hall et al. (1986)
adult stat 18-22 190-210 7.7 96 0.09 (0.07-0.14) n Hall et al. (1986)
Bluegill sunfish adult stat 25 20 7.5 24 4.56 (3.64-6.08) n Pickering & Henderson (1966)
(Lepomis adult stat 25 20 7.5 48 2.76 (2.02-3.46) n Pickering & Henderson (1966)
macrochirus) adult stat 25 20 7.5 96 1.94 (1.33-2.35) n Pickering & Henderson (1966)
Goldfish adult stat 25 20 7.5 24 3.46 (2.85-4.82) n Pickering & Henderson (1966)
(Carassius adult stat 25 20 7.5 48 2.62 (2.04-3.68) n Pickering & Henderson (1966)
auratus) adult stat 25 20 7.5 96 2.34 (1.81-3.16) n Pickering & Henderson (1966)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Guppy adult stat 25 20 7.5 24 3.37 (2.73-4.81) n Pickering & Henderson (1966)
(Poecilia adult stat 25 20 7.5 48 2.31 (1.78-3.11) n Pickering & Henderson (1966)
reticulata) adult stat 25 20 7.5 96 1.27 (0.97-1.71) n Pickering & Henderson (1966)
3-4 weeks flow c 23-25 1 mM 24 10.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 48 5.7 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 72 4.3 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 96 3.8 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 24 33 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 48 20.5 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 72 14.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 96 11.1 m Canton & Slooff (1982)
Green sunfish adult stat 25 20 7.5 24 7.84 (6.13-14.2) n Pickering & Henderson (1966)
(Lepomis adult stat 25 360 8.2 24 88.6 (74-106) n Pickering & Henderson (1966)
cyanellus) adult stat 25 20 7.5 48 3.68 (2.89-4.69) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 71.3 (56.3-92.2) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 2.84 (2.1-3.56) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 66 (51.7-84.4) n Pickering & Henderson (1966)
Golden shiner flow 72.2 7.5 96 2.8 (1.9-4.3) m Hartwell et al. (1989)
(Notemigonus
crysoleucas)
Puntius 2.4 g stat b 23-27 60-70 7.5 24 59.99 (58.5-61.5) n Shivaraj & Patil (1988)
arulius 2.4 g stat b 23-27 60-70 7.5 48 45.7 (43.9-47.5) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 72 41.7 (39.7-43.8) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 96 39 (36.5-41.7) n Shivaraj & Patil (1988)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Killifish 4-5 weeks stat b 23-25 1 mM 48 > 2.8 m Canton & Slooff (1982)
(Oryzias 4-5 weeks stat b 23-25 1 mM 72 0.35 m Canton & Slooff (1982)
latipes) 4-5 weeks stat b 23-25 1 mM 96 0.35 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 24 > 2.6 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 48 1.8 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 72 0.17 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 96 0.13 m Canton & Slooff (1982)
Zebra fish 6 months flow 19-21 1.7 mM 24 7 m Canton & Slooff (1982)
(Brachydanio 6 months flow 19-21 1.7 mM 48 4.2 m Canton & Slooff (1982)
rerio)
a stat = static conditions (water unchanged for duration of test unless stated otherwise); flow = flow-through conditions
(cadmium concentration in water continuously maintained unless stated otherwise)
b static conditions but water renewed every 24 h
c intermittent flow-through conditions
d Hardness was expressed as mg CaCO3/litre unless stated otherwise
e fish were exposed to cadmium added as the chloride unless stated otherwise; m = measured concentration; n = nominal concentration
f cadmium was added as the sulfate
g cadmium was added as the nitrate
In a large scale study of the toxicity of cadmium to the
mummichog Fundulus heteroclitus, Voyer (1975) examined effects of
salinity, pre-exposure to high salinity and different concentrations
of dissolved oxygen on the tolerance of fish to cadmium over 96 h. He
found no significant influence of dissolved oxygen levels between 4.0
mg/litre and saturation regardless of salinity during acclimatization
or during the test. By contrast, Voyer et al. (1975) showed a distinct
effect of dissolved oxygen concentration on toxicity of cadmium to the
same species of fish in fresh water. Median tolerance concentrations
at 96 h ranged upwards from 1.3 to 3.0 mg cadmium/litre with 2.3 and
8.5 mg/litre of dissolved oxygen, respectively. They demonstrated
statistically an independent effect of dissolved oxygen and time
against cadmium toxicity. It should be noted that cadmium is 10 times
more toxic to this species in fresh water than in sea water.
Toxicity of cadmium to both marine (Eisler, 1971) and freshwater
(Roch & Maly, 1979) fish has been shown to be greater at higher
temperatures.
Canton & Slooff (1982) exposed several fish species to cadmium in
short-term toxicity tests. At a water hardness of 1.7 mmol/litre they
found the no-observed-adverse-effect level (NOAEL) for mortality in
the zebra fish ( Brachydanio rerio) to be 2 mg/litre over a 48-h
exposure period. For the killifish ( Oryzias latipes), the 96-h NOAEL
was 0.06 mg/litre for mortality and 0.03 mg/litre for mortality and
abnormal behaviour at a water hardness of 2 mmol/litre. The
corresponding values at a water hardness of 1 mmol/litre were 0.055
and 0.006 mg/litre. For the guppy ( Poecilia reticulata), the 96-h
NOAEL for mortality and abnormal behaviour was 5.2 mg/litre at a water
hardness of 2 mmol/litre and 0.6 mg/litre at 1 mmol/litre. The authors
calculated a 24-h NOAEL for the rainbow trout ( Salmo gairdneri) of
0.01 mg/litre for inhibition of opercular movements.
Abel & Papoutsoglou (1986) studied the toxicity of cadmium to
Cyprinus carpio and Tilapia aurea and reviewed data on other
species of freshwater fish. The found for all species examined that
the median survival time changed little over a wide range of cadmium
concentrations and that a toxic threshold was clear in most studies.
For Tilapia this threshold lay between 0.1 and 0.5 mg cadmium/litre;
negligible mortality was recorded in fish exposed to 0.1 mg/litre for
3 months.
6.3.2 Reproductive effects and effects on early life-stages
Meteyer et al. (1988) exposed sheepshead minnow ( Cyprinodon
variegatus) eggs to cadmium concentrations of between 0.39 and 1020
µg/litre from approximately 4 h after fertilization. Hatching was
delayed by up to 3 days at the highest cadmium concentration. All
treated larvae were shorter than controls, but there was no
dose-related effect of cadmium on growth.
Middaugh & Dean (1977) examined the toxicity of cadmium to
various life-stages of the mummichog Fundulus heteroclitus. In 48-h
tests, eggs were highly resistant to cadmium. The greatest effect (54%
non-emergence) occurred at a cadmium concentration of 32 mg/litre; the
control non-emergence was 17%. Newly-emerged larvae were less
sensitive to cadmium than were 7-day-old larvae. There was an effect
of salinity on the sensitivity of 14-day-old larvae to the metal
(Table 11). Adults were less sensitive to cadmium than were larvae.
Similar results were obtained with the Atlantic silverside ( Menidia
menidia), larvae being the most sensitive life-stage. Weis & Weis
(1977) found no effect of cadmium, at concentrations up to 10
mg/litre, on embryos of Fundulus heteroclitus. Rombough & Garside
(1982) found the most sensitive indicator of cadmium toxicity to early
life-stages of the Atlantic salmon ( Salmo salar) to be inhibition of
growth of alevins, where significant reductions occurred with cadmium
concentrations of 0.47 µg/litre. The LC50 for the interval between
fertilization and viable hatch lay between 300 and 800 µg per litre.
Newly hatched alevins showed a 24-h LC50 of between 1.5 and 2.4
mg/litre. Sensitivity increased sharply in late alevins and
significant mortality was recorded at a concentration of 8.2 µg/litre.
Eaton et al. (1978) exposed embryos and larvae of seven
freshwater fish species to nominal cadmium concentrations of 0, 0.4,
1.2, 3.7, 11.6, 33.3, and 100 mg/litre for periods ranging from 3 to
126 days. Actual concentrations were monitored and used in the
assessment of the results. Results were expressed in terms of
"standing crop", which the authors estimated as the product of the
proportion of fish surviving and their total biomass. The lowest
concentration of cadmium at which the standing crop was significantly
different from controls was approximately 12 µg/litre for the white
sucker, northern pike, smallmouth bass, west coast coho salmon, lake
trout, and brown trout exposed as embryos or larvae for up to 64 days.
The value was lower for brook trout (0.48 µg per litre) after exposure
of larvae/juveniles for 65 days and for Lake Superior coho salmon (3.4
µg/litre) after exposure of larvae/juveniles for 27 days. The highest
cadmium concentration at which standing crop was not significantly
different from controls varied for the seven species between 1.1 and
4.2 µg/litre.
Woodall et al. (1988) exposed rainbow trout ( Salmo gairdneri)
fry to cadmium concentrations of 0, 0.1, 1.0 or 5.0 mg/litre for up to
90 h. In preliminary experiments, they calculated that the 90-h LC50
lay between 0.1 and 1.0 mg/litre. Pre-treatment of trout fry with
cadmium initially had little effect (up to 30 h). However, with
exposure periods of between 45 and 90 h, some protection was induced
by pre-treatment. Benoit et al. (1976) exposed three generations of
brook trout ( Salvelinus fontinalis) to concentrations of total
cadmium varying between 0.06 and 6.4 µg/litre. Significant numbers of
first and second generation adult males died during spawning when
exposed to 3.4 µg/litre. This concentration also significantly
retarded the growth of juvenile second and third generation offspring,
but at a concentration of 1.7 µg/litre these effects were not seen.
In a study by Borgmann & Ralph (1986), white sucker larvae
Catostomus commersoni and young common shiners Notropis cornutus
were exposed to cadmium chloride at concentrations ranging from 6.24
to 200 µg cadmium/litre. The relative growth rate of fish was
significantly reduced at concentrations of 36 µg/litre or more in the
case of suckers and 63 µg/litre or more in the case of shiners.
Cadmium had no effect on the relative feeding rates.
Hatakeyama & Yasuno (1987) studied the chronic effects of cadmium
on the reproduction of the guppy Poecilia reticulata. The fish were
exposed to cadmium via cadmium-accumulated midge larvae used as their
food source. The cumulative numbers of fry produced by guppies fed
midge larvae containing 500, 800 or 1300 mg cadmium/kg for 6 months
decreased to 79%, 65%, and 55% of the controls, respectively. At the
highest dose, the mortality of females was significantly elevated at
6 months, but no such effect was observed with the males.
Michibata (1981) reported a protective effect of water hard-ness
against the effects of cadmium on the eggs of Oryzias latipes.
6.3.3 Metabolic, biochemical and physiological effects
Protective metal-binding proteins (metallothioneins) are induced
by cadmium in fish (chapter 4).
A manifest symptom of cadmium toxicity in freshwater fish is
ionic imbalance with reduced plasma Ca2+, Na+, and CL-. The
probable explanation is that cadmium is a potent inhibitor of
ion-transporting enzymes. Verbost et al. (1988) showed that cadmium
inhibited Ca-ATPase in the cell membranes of fish gut. It probably
does the same in the gills because cadmium exposure has been shown to
inhibit calcium uptake in the gills of adults (Verbost et al., 1987;
Reid & McDonald, 1988) as well as in larvae (Wright et al., 1985).
Similarly, cadmium has been shown to inhibit Na/K-ATPase in fish gills
(Watson & Benson 1987), which, taken with the fact that cadmium
probably also affects the production of ATP in the gills (Dickson et
al., 1982), could explain the reduction of plasma Na+. Huiguang Fu
(1989) studied the role of the hormones prolactin and cortisol in
correcting cadmium-induced impairment of the calcium balance in
tilapia ( Oreochromis mossambicus). Fish were able to recover from
initial hypocalcaemia during a 35-day exposure to a concentration of
10 µg cadmium/litre. This recovery involves prolactin-induced
stimulation of active Ca2+ uptake and reduction of passive Ca2+
efflux, together with cortisol-induced changes in chloride cells and
stimulation of metallothionein synthesis in the liver, kidney, and
gills. The author stated that the capacity to survive prolonged
exposure to 10 µg cadmium/litre through physiological adaptation does
not indicate that this cadmium concentration is acceptable to tilapia.
Adaptation may be achieved at the expense of other essential processes
like growth and reproduction.
Arillo et al. (1984) investigated the effect of cadmium at levels
of 1-10 µg/litre (concentrations above the water quality criteria
value of 0.75 µg/litre proposed for salmonids by EIFAC/FAO) on a wide
variety of biochemical parameters in the rainbow trout (Salmo
gairdneri). The exposure period was 4 months. Only at the highest
test concentration of 10 µg/litre were there any effects on the fish,
and then only on liver aminolevulinic acid dehydratase activity. The
authors concluded that the water quality criterion was realistic.
Dawson et al. (1977) exposed juvenile striped bass ( Morone
saxatilis) to cadmium chloride concentrations of 0.5, 2.5 or 5
µg/litre for 30 to 90 days, and the fish were then allowed to recover
for 30 days in clean, running sea water. There was an inhibition of
gill tissue respiration at 30 and 90 days, which recovered during the
30-day period with clean water. The activities of the various enzymes
measured were not affected. MacInnes et al. (1977) reported reduced
gill tissue oxygen consumption in cunner ( Tautogolabrus adspersus)
exposed to cadmium (0.05 or 0.1 mg/litre) for 30 or 60 days. They also
reported a reduced activity of aspartate aminotransferase and an
increased activity of glucose-6-phosphate dehydrogenase in the liver
of the fish after 30 days of exposure to cadmium. Gill & Pant (1983)
measured levels of various blood and tissue constituents after acute
(24 h) or chronic (90 day) exposure to cadmium. Acute exposure to
12.65 mg/litre led to significant hyperglycaemia and an increase in
liver, kidney, and ovarian cholesterol levels. Chronic exposure to
0.63 or 0.84 mg/litre, by contrast, led to an enduring hypoglycaemia
and diminished levels of cholesterol in tissues. Both acute and
chronic exposure to cadmium caused marked hypocholesterolaemia,
glycogenolysis in the liver and brain and a rise in myocardial
glycogen. Testis cholesterol was depleted after 60 days in both acute
and chronic exposures.
Sastry & Subhadra (1983) exposed the catfish Heteropneustes
fossilis to cadmium in the water at the sublethal concentration of
2.3 µmol/litre for 15 or 30 days. The cadmium caused reduced
absorption of glucose and fructose from the gut, this effect being
more pronounced after 30 days of exposure than after 15 days. Filling
intestinal sacs, in vivo, with cadmium solutions (1 µmol per litre)
reduced absorption of the sugars significantly over 1 h.
Merlini (1978) pre-treated immature sunfish ( Lepomis gibbosus)
with cadmium (0.004 mg/litre) and then fed treated and control fish
with a single ration containing 58Co-labelled vitamin B12. The
fish were subsequently fed non-radioactive food for 31 days before
sacrifice. The author reported that cadmium-treated fish stored
significantly less vitamin B12 in the liver than did controls.
Carrier & Beitinger (1988a) studied the effect of cadmium on the
critical thermal maximum (the temperature at which loss of equilibrium
is coupled with loss of righting response) in the red shiner
( Notropis lutrensis) and fathead minnow ( Pimephales promelas). The
red shiner was exposed to sublethal cadmium concentrations of 4.88,
5.07, and 5.46 mg/litre and the fathead minnow to 0.09, 0.48, and 1.26
mg/litre. Critical thermal maxima were significantly reduced for both
species over a 10-day exposure period. The effect was found to be dose
and time dependant. In the green sunfish ( Lepomis cyanellus) there
was no effect of cadmium concentrations of 2.76, 4.22 or 5.17 mg/litre
on the critical thermal maximum (Carrier & Beitinger, 1988b).
6.3.4 Structural effects and malformations
When Bengtsson et al. (1975) exposed 180 minnows ( Phoxinus
phoxinus) to various concentrations of cadmium ranging from 7.5
µg/litre to 4.8 mg/litre for 70 days, 31 of the 101 fish that survived
developed lesions in the spinal column. Fractured vertebrae occurred
in the caudal end of the abdominal section of the spine or in the
caudal section; 64% of all fractures occurred in the first 7 caudal
vertebrae and 21% occurred in abdominal vertebrae numbers 8 to 14.
Hiraoka & Okuda (1984) cultured medaka ( Oryzias latipes) eggs in a
cadmium solution of 0.01 mg/litre for 1 week and then investigated
abnormalities in the vertebrae of the hatched fry, which were reared
in clean water. There was no damage to the centra of the vertebrae in
newly hatched fry. However, centrum-damaged fish were found in the
first week and the numbers increased rapidly up to the 4th week after
hatch. The cumulative frequency of vertebral-damaged fish was 13% and
14% in the 5th and 6th weeks, respectively, and seemed to remain
constant after this. Muramoto (1981b) reported that fish showing
malformations of the spine after cadmium treatment had significantly
less calcium in the vertebral column than did control fish.
Voyer et al. (1975) found no short-term histopathological effects
of cadmium, but long-term exposure to 28 mg/litre caused necrosis and
sloughing of the mucosa of gills. Tissue damage was also evident in
the nasal passages and buccal cavity. This histopathological effect
was seen between exposure times of 512 and 612 h, but not before.
6.3.5 Behavioural effects
Hartwell et al. (1989) studied the avoidance response of the
golden shiner ( Notemigonus crysoleucas) to cadmium concentrations of
up to 68 µg/litre, but found no significant avoidance.
Sullivan et al. (1978b) subjected fathead minnows ( Pimephales
promelas) to acute (24 h) or subacute (21 days) exposure at
sublethal cadmium concentrations and then placed them in experimental
chambers with largemouth bass ( Micropterus salmoides), a fish which
preys upon them. The minnows displayed altered behaviour patterns,
including abnormal schooling behaviour, and were more vulnerable to
predation than controls. The lowest acute cadmium exposure level that
increased vulnerability was 0.375 mg/litre and the lowest subacute
level 0.025 mg/litre. The authors pointed out that the subacute value
of 0.025 mg/litre was well below reported no-effect levels of cadmium
for fathead minnows established with respect to survival and
reproductive effects. The avoidance threshold for cadmium in water by
the rainbow trout ( Salmo gairdneri) is 50 µg/litre (Black & Birge,
1980), which is 50 times higher than the 96-h LC50 value reported
for this species.
6.3.6 Interactions with other chemicals
Muramoto (1981a) showed that the chelating agents EDTA and DPTA
afforded some protection against the effects of cadmium on carp
( Cyprinus carpio) exposed to 0.05 or 0.1 mg cadmium/litre for 3
months. There were effects on the vertebrae of exposed fish.
Chelating agents that form hydrophobic complexes with heavy
metals increase the bioavailability of the metal to aquatic organisms.
Examples of these are xanthates and dithiocarbamates. Xanthates are
used in the mining industry in the flotation process to refine metal
from sulfide ores. As discussed in section 4.1, these compounds
increase the uptake rate of cadmium through the gills in fish (Block
& Part, 1986; Gottofrey et al., 1988; Block, 1991). Furthermore, they
change the tissue distribution of the metal in such a way that more
cadmium is found in lipid-rich tissues such as nervous tissue (brain)
and adipose tissue than when fish are exposed to the metal alone.
Finlayson & Verrue (1982) reported 96-h LC50 values for
juvenile chinook salmon ( Onchorynchus tshawytscha) ranging from 0.6
to 1.6 µg/litre. They found no synergistic or antagonistic toxic
effects after combining cadmium and zinc in their test system. The
results of tests were additive, the overall LC50 being a simple
combination of individual metal effects at a zinc:cadmium ratio of
1:0.008. Spehar et al. (1978b) exposed the flagfish Jordanella
floridae to cadmium and zinc, both individually and as a mixture, at
concentrations ranging from 4.3 to 8.5 µg cadmium/litre and 73.4 to
139 µg zinc/litre, through one complete life-cycle of the fish. There
was no additive effect of cadmium and zinc at sublethal concentrations
in mixed exposure. Effects on survival showed that the toxicity of
cadmium and zinc mixtures was only slightly, if at all, greater than
the toxicity of zinc alone. Anadu et al. (1989) reported that
pre-exposure of rainbow trout ( Salmo gairdneri) to zinc (100
µg/litre) for 17 days increased the subsequent 120-h LC50 for
cadmium from 1.1 µg/litre to 4.1 µg/litre.
6.4 Toxicity to Amphibia
Francis et al. (1984) exposed eggs of the leopard frog ( Rana
pipiens) to cadmium-enriched sediments during their development and
for 4 days after the larvae had hatched. Measured concentrations of
cadmium ranged from 1.04 to 1074 mg/kg in sediment and from 1.0 to
76.5 mg/litre in water above the sediment. Cadmium concentrations in
the tissues of the tadpoles at the end of the experiment ranged from
0.08 to 12.55 mg/kg. There was no mortality as a result of cadmium
exposure. The LC50 for this species has been reported to be 50
µg/litre for water without sediment by Westerman (1977). Slooff &
Baerselman (1980) determined 48-h LC50 values for the neotenous
larval mexican axolotl ( Ambystoma mexicanum) and larval South
African clawed toad ( Xenopus laevis) of 1.3 mg/litre and 32
mg/litre, respectively, after exposure to cadmium nitrate. Canton &
Slooff (1982) exposed Xenopus laevis to cadmium, added as cadmium
chloride, and obtained 24-h and 48-h LC50 values of 4 and 3.2
mg/litre, respectively. A NOEL of 2.2 mg/litre was reported for both
exposure periods. In a longer-term exposure (100 days), by the same
authors, an LC50 value of 1500 µg/litre was found and the EC50
value for inhibition of larval development was 650 µg/litre. For
mortality and larval development, the NOELs were 30 and 9 µg/litre,
respectively. De Zwart & Sloof (1987) determined a 48-h LC50 of 20.2
mg/litre for tadpoles of the clawed toad. Khangarot & Ray (1987)
established a 96-h LC50 value of 8.18 (6.96-9.53) mg/litre for the
tadpoles of the toad Bufo melanosticus. The test water was obtained
from a well and had a hardness of 185 mg per litre, pH 7.4, and
temperature of 31 °C. Solids were present at a level of 920 mg/litre.
Muino et al. (1990) calculated the 48-h and 96-h LC50 for Bufo
arenarum tadpoles to be 2.52 and 2.08 mg cadmium/litre for the two
exposure periods, respectively, under semi-static conditions. In tests
to study the effect of a sublethal cadmium concentration (1.0 mg
cadmium/litre) on the water balance of the animals, the authors found
that all animals died within a few hours in ion-free media. Tadpoles
exposed in ionic solutions showed mortality of less than 10%
(equivalent to control groups).
Woodall et al. (1988) exposed Xenopus laevis tadpoles to
cadmium concentrations of 0, 50, 80, and 100 mg cadmium/litre for up
to 90 h. In preliminary experiments, they calculated the 90-h LC50
to lie between 80 and 100 mg/litre. The authors found that
pre-treatment of tadpoles with cadmium induced protection, which
decreased with an increase in the subsequent exposure concentration.
Cadmium pre-treatment induced maximum protection to cadmium at a
concentration of 50 mg/litre at both 45 and 90 h.
Perez-Coll et al. (1986) exposed developing Bufo arenarum
embryos to cadmium chloride concentrations of 6 x 10-7 to 1.5 x
10-5 mol Cd2+/litre during gastrulation at 20 °C and 30 °C.
Initial failures at gastrulation resulted mainly in axial
incurvations, microcephaly, hydropsy, and abnormal tail development.
At the higher temperature, high concentrations of cadmium caused a
significant increase in early malformations and at low concentrations
the high temperature prevented alterations.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
Both terrestrial plants and animals accumulate cadmium, but the
rate of accumulation is much higher under experimental conditions,
where cadmium is available in solution, than it is with plants grown
in soil, when part of the cadmium is bound and less available.
Cadmium has adverse effects on hydroponically grown plants at
concentrations in the mg/litre range, whereas plants grown in soil
only show reduced growth in contaminated soils with hundreds of mg
cadmium/kg. Terrestrial invertebrates are relatively insensitive to
cadmium-induced toxic effects, probably due to effective
sequestration mechanisms in specific organs. When toxic effects do
occur, they consist of reduced growth and reproduction.
7.1 Toxicity to terrestrial plants
Cadmium has been shown to have an adverse effect on plant growth
and yield in laboratory experiments. However, plants grown in soil are
generally insensitive to the effects of cadmium except at high doses.
Effects are only seen when cadmium is given in nutrient solutions
rather than in soil, where the cadmium is bound and is therefore less
available to the plants. Cadmium is only available to plants in
solution in soil. There is considerable evidence from field studies
that plants are able to develop tolerance to various heavy metals in
their growth medium. Research into cadmium tolerance has been more
limited than for other metals, but there is some evidence of tolerance
developing.
7.1.1 Toxicity to plants grown hydroponically
Mitchell & Fretz (1977) cultured seedlings of three species of
tree, the white pine ( Pinus strobus), red maple ( Acer rubrum), and
Norway spruce ( Picea abies), in sand. Plants were irrigated with a
nutrient solution containing cadmium at concentrations of 0, 0.5, 1,
2, 4, 8, and 16 µg/litre; white pine seedlings were also treated with
32 and 64 µg/litre. Both roots and foliage were affected by the
cadmium. In the red maple symptoms of cadmium toxicity began with
interveinal chlorosis and stunting of leaves in most cases. As
exposure increased cadmium caused wilting and then death. The first
observed effect of the metal on the pine was inhibition of needle
expansion and, in the case of the spruce, chlorotic tips to new
growth. Tissue accumulation of cadmium correlated well with exposure.
Red maple, white pine, and spruce exhibited foliage effects at leaf
cadmium concentrations of 22.8, 61.3, and 7.5 mg/kg, respectively,
corresponding to nutrient solutions of 8.0, 32.0, and 4.0 µg/litre.
There was an increasing effect on root development with increasing
exposure to cadmium. At the higher doses, there was severe reduction
in the number of roots initiated and stunting of those that grew.
Accumulation of cadmium was greater in roots than in leaves.
Root et al. (1975) grew maize ( Zea mays) in hydroponic
solutions containing cadmium chloride at concentrations ranging from
1 to 40 mg/litre. Uptake of cadmium into the plants increased with
time, and cadmium was present at higher concentrations in roots than
in shoots. Leaf chlorophyll concentration and yield (as dry weight) of
both roots and shoots decreased with increasing cadmium concentration.
As the cadmium concentration in the leaves increased, the
concentration of zinc decreased and the concentration of iron
increased. This gave a linear correlation between cadmium in the leaf
and iron/zinc ratio. Chlorosis resulting from cadmium in the leaves
(seen at a cadmium concentration of 1 mg/litre nutrient solution)
appeared comparable to iron-deficiency chlorosis. However, in this
case, the chlorosis was not due to iron deficiency, as previously
suggested by other workers, but was associated in some way with an
increasing iron/zinc ratio.
Harkov et al. (1979) found no effect on the yield of tomatoes
grown in vermiculite and cultured with a nutrient solution containing
cadmium at concentrations of 0.25 or 0.75 mg/litre. The plants were
more susceptible to damage by ozone, under conditions where ozone
damage would have been slight, after exposure to cadmium. Where ozone
damage was heavy or when conditions were not conducive to ozone
damage, there was no effect of cadmium. When Wong et al. (1988)
exposed pea ( Pisum sativum) seeds to cadmium concentrations of 1, 5,
10, and 20 mg/litre in culture solutions, germination was
significantly reduced at 20 mg/litre and radicle elongation to 1 cm
was significantly reduced at 5 mg/litre and to 2 cm at 1 mg/litre.
Early development (shoot elongation, leaf development, root and shoot
growth) was inhibited in a dose-dependant manner. Slight inhibition
was observed at 1 mg/litre, increased inhibition at 5 mg/litre, and
significant inhibition at 10 and 20 mg/litre.
7.1.2 Toxicity to plants grown in soil
Mitchell & Fretz (1977) showed that the effects on the growth of
red maple, white pine and Norway spruce plants in soil, amended with
cadmium, were similar but less severe, owing to reduced uptake of the
metal, than in the case of the same plants grown hydroponically.
Cadmium only affected current growth of the plants, except where it
was present in excess. Mahler et al. (1978) treated eight soils, the
pH values of which ranged from 4.8 to 7.8, with 1% (by weight) sewage
sludge containing added cadmium sulfate, leading to cadmium
concentrations in the soil ranging from 0.1 to 320 mg/kg. Two plants,
lettuce ( Lactuca sativa variety longifolia) and Swiss chard ( Beta
vulgaris variety cicla), were grown in the soils in pots. The EC50
(yield) for lettuce was 214 and 139 mg/kg soil for acid and calcareous
soils, respectively, whereas the values for chard were 175 and 250
mg/kg for acid and calareous soils. The corresponding tissue
concentrations of cadmium associated with these effects were 470 and
160 mg/kg for lettuce and 714 and 203 mg/kg for chard. Thus, a
markedly lower tissue concentration of cadmium produced 50% yield
reduction on calcareous soils than on acid soils. Alloway et al.
(1990) reported stunted growth and toxic signs on leaves of lettuce,
cabbage, carrot, and radish plants, but only at the highest
concentrations of cadmium tested (which resulted in a cadmium content
of around 20 mg/kg in the upper parts of the plants).
7.1.3 In vitro physiological studies
Bazzaz et al. (1974) demonstrated an effect of cadmium on
transpiration and photosynthesis in excised sunflower heads. The heads
(15 cm diameter) were placed in flasks containing distilled water or
cadmium salt solutions (2, 20, 100 or 200 mg/litre) and transpiration
and photosynthesis were measured at daily intervals over 4 to 5 days.
Cadmium reduced transpiration and photosynthesis at concentrations of
100 or 200 mg/litre. Excised epidermal peels floating on solutions of
cadmium salts showed a log-linear relationship between metal
concentration and stomatal opening. The stomata opened less with
increasing cadmium concentration; this accounted for the effect on
transpiration and, hence, on photosynthesis.
7.2 Toxicity to terrestrial invertebrates
Haight et al. (1982) calculated 24-h, 48-h, and 72-h LC50
values of 36, 15.1, and 5.85 mg cadmium/litre, respectively, for
juvenile free-living nematodes ( Panagrellus silusiae) and 111, 26.3,
and 13.2 mg/litre for adults. Williams & Dusenbery (1990) exposed the
free-living nematode Caenorhabditis elegans to cadmium and found
values of 904, 22, and 1.5 mg/litre, respectively, for the same three
exposure periods. They also calculated a 96-h LC50 of 0.06 mg/litre.
Popham & Webster (1979) found that a 6-h exposure to 3.26 x
10-7 moles of cadmium significantly decreased the fecundity of C.
elegans. A 3.5-day exposure to 10-8 moles caused the same effect.
Nematodes exposed to 4 x 10-6 moles of cadmium never grew to the
same length as controls and resembled worms from starved, overcrowded
cultures. Van Kessel et al. (1989) exposed juvenile C. elegans to
various concentrations of cadmium chloride and found that growth and
subsequent reproduction were significantly reduced at 1 µmol/litre. At
levels of 160 and 320 µmol/litre the nematodes did not reach the adult
stage and, therefore, did not reproduce.
Doelman et al. (1984) exposed the soil nematodes Mesorhabditus
monhystera and Aphelenchus avenae to cadmium via food for up to 22
days and monitored the size of the population. M. monhystera was
exposed, via bacteria and fungi, to concentrations of 0.23, 4.4, and
12.7 mg cadmium/kg, and a significant reduction in the size of the
population was found at all doses. A. avenae was exposed, via fungi
alone, to concentrations of 1, 10, and 25 mg/kg. At 1 mg/kg there was
no effect on the population size, whereas at 10 mg/kg, a reduction was
observed until the final day. A concentration of 25 mg/kg
significantly reduced the size of the population. The authors noted
that exposure via fungi alone gave far more variable results.
In studies by Van Straalen et al. (1989), the collembolan
Orchesella cincta and the oribatid mite Platynothrus peltifer were
exposed to cadmium in the food. The 9-week LC50 values were 1.6 and
7.27 µmol/g and the no-observed-effect levels (NOEL) were 0.042 and
0.026 µmol/g for O. cincta and P. peltifer, respectively. The most
sensitive parameters were female growth for O. cincta and
reproduction in P. peltifer.
Russell et al. (1981) fed subadult garden snails ( Helix aspersa)
on diets containing six different levels of cadmium ranging from 10 to
1000 mg/kg diet over a period of 30 days. There was little mortality
(two animals out of 350 died, one at an exposure level of 50 mg/kg and
the other at 1000 mg/kg) but food consumption declined with each
increase in cadmium dose. Food consumption was strongly depressed at
cadmium doses of 100 mg/kg or more. Relative weight loss was the most
pronounced effect of cadmium treatment; this was dose related and
directly attributable to reduced feeding rates. At doses of 25 mg/kg
or more, shell growth and reproductive activity were depressed while
the incidence of sealing response (the sealing of the operculum with
a disc of mucus and a dormancy reaction) increased markedly. These
three effects were all related to the dietary cadmium concentration.
7.3 Toxicity to birds
7.3.1 Acute and short-term toxicity
The acute and short-term toxicity of cadmium salts to birds in
laboratory studies is summarized in Table 13. Dosing for 5 days,
followed by 3 days of clean diet, resulted in LC50 values generally
in excess of 2000 mg/kg diet. Only the pheasant showed greater
sensitivity to cadmium but, even in this species, the LC50 was close
to 1000 mg/kg diet. All the birds used were between 10 and 14 days
old.
Table 13. Toxicity of cadmium to birds a
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Japanese quail 14 cadmium chloride 2440 (1807-3294) Hill &
(Coturnix coturnix 14 cadmium succinate 2052 (1621-2598) Camardese
japonica) (1986)
Pheasant 10 cadmium chloride 767 (651-898) Hill et al.
(Phasianus 14 cadmium succinate 1411 (1202-1657) (1975)
colchicus)
Bobwhite quail 14 cadmium succinate 1728 (1381-2132) Hill et al.
(Colinus (1975)
virginianus)
Table 13 (contd).
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Mallard duck 10 cadmium chloride > 5000 Hill et al.
(Anas 10 cadmium succinate > 5000 (1975)
platyrhynchos)
a Birds were fed with a dosed diet for 5 days and then a "clean" diet for 3 days.
In a study by Pritzl et al. (1974), 2-week-old leghorn chicken chicks
were dosed with dietary cadmium chloride for 20 days. In the first
experiment, chicks dosed with 700 mg cadmium/kg diet showed an
increase in the weight of the gastrointestinal tract, kidney, and
gizzard expressed as a ratio to the body weight. In a second
experiment, chicks were fed diets containing 400, 600, 800 or 1000 mg
cadmium/kg. Weight gain and food consumption were decreased, relative
to controls, at all dose levels, and at levels higher than 400 mg/kg
the birds lost weight. All the birds fed diets containing 800 or 1000
mg/kg died within 20 days. The LC50 was calculated to be 565 mg/kg
diet.
When Cain et al. (1983) fed 1-day-old mallard ducklings ( Anas
platyrhynchos) a diet containing cadmium chloride at concentrations
of 5, 10 or 20 mg cadmium/kg for 12 weeks, significant effects were
only noted at the highest dose. These included a significant reduction
in packed cell volume and haemoglobin concentrations and a significant
increase in serum glutamic-pyruvic transaminase. Mild to severe kidney
lesions were evident in ducklings fed 20 mg/kg for 12 weeks. Body
weight, liver weight, and femur weight-to-length ratio were unaffected
by the cadmium treatment. No other haematological or histological
effects were found.
7.3.2 Reproductive effects
Lofts & Murton (1967) injected 0.2 ml of a solution of cadmium
chloride (0.04 mol/litre) intramuscularly into wood pigeons ( Columba
palumbus). When the cadmium was given to birds with regressed
testes, which were then stimulated into reproductive condition by long
photoperiods (16 h of light per day), there was a reduction in the
numbers of birds showing full testicular development in the treated
group. Only one out of six birds given cadmium had developed
spermatozoa in the testis by the end of the experiment. The remainder
had not even produced spermatids; two birds had only spermatogonia in
the seminiferous epithelium while the other three had secondary
spermatocytes. Of the six control birds, four had spermatozoa, one had
spermatids, and one primary spermatocytes. Injection of cadmium into
birds late in the season had no effect on the autumnal regression of
the testes. There was no sign of testicular necrosis in the treated
birds. An intratesticular injection of cadmium caused local necrosis
in the testis of feral pigeons. A dietary concentration of 200 mg
cadmium/kg, in the form of cadmium chloride, reduced spermatogenesis
in male mallards and egg production in females, but a lower dose of 20
mg/kg produced no effects (White & Finley, 1978; White et al., 1978).
7.3.3 Physiological effects
Mayack et al. (1981) found that the survival and growth of the
wood duck ( Aix sponsa) were unaffected by a cadmium chloride dietary
level of 100 mg/kg, although some kidney damage was reported.
Nicholson et al. (1983) compared the ultrastructure of the
kidneys of sea-birds contaminated with cadmium in the wild, sea-birds
from uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg per kg, respectively.
Damage was greatest in the proximal tubule of the kidney, and included
cell necrosis, nuclear pyknosis, mitochondrial swelling, and some
tubulorrhexis. The tubulorrhexis would be irreversible. There was some
indication of regeneration, judging from the number of
undifferentiated cells present in the tubule, in both dosed and
naturally contaminated birds. Debris was found in the distal nephron
lumen and there was some damage in the distal tubule and the renal
corpuscles. Necrosis of kidney cells was very rare in control birds or
uncontaminated sea-birds.
7.3.4 Behavioural effects
Heinz et al. (1983) assessed the avoidance response to a visual
fright stimulus of ducklings fed a diet containing cadmium chloride at
a concentration of 4 or 40 mg/kg. The parents of the ducklings had
also been fed this cadmium-containing diet. Ducklings fed 4 mg/kg were
hypersensitive to the fright stimulus, whereas those fed the higher
dose reacted as did controls. The authors could offer no explanation
of why the higher dose had no effect but pointed to similar results
from other materials. They were of the opinion that hypersensitivity
to behavioural signals could be as deleterious to the organism in the
wild as a failure to respond.
7.4 Toxicity to wild small mammals
Shore et al. (1991) fed herbivorous bank voles ( Clethrionomys
glareolus) and granivorous wood mice ( Apodemus sylvaticus) a
pelleted diet contaminated with cadmium chloride and collected urine
and faeces in metabolism cages. Bank voles fed diets containing 10.3
mg/kg for 40 days and then 4.5 mg/kg for 35 days suffered significant
net daily loss of calcium and sodium, and reduced net gain of
potassium and magnesium compared to controls. Assimilation of the
macroelements was not significantly altered in wood mice fed 10.3
mg/kg for 75 days.
8. EFFECTS IN THE FIELD
Appraisal
Tolerance to cadmium has been demonstrated in soil fungi,
plants, aquatic invertebrates, and fish from cadmium-contaminated
sites. Some field evidence suggests that cadmium is responsible for
reduced leaf litter degradation and a failure to recycle nutrients
due to adverse effects on populations, particularly of
microorganisms. However, no studies have identified cadmium as the
sole cause of the effect, since it is always associated with other
metals. Although soil invertebrates in contaminated sites accumulate
cadmium and other metals, there is evidence that most populations are
not affected. A field study has shown that fish from a
cadmium-contaminated river have physiological abnormalities. Kidney
damage has been found in pelagic sea-birds from areas away from
industrial or other anthropogenic sources of cadmium, but there was
no effect on survival or reproduction of populations. In industrially
contaminated areas, kidney damage has been observed in several
species of birds found to contain cadmium plus other metals.
8.1 Tolerance
Tolerance to cadmium has been demonstrated in soil fungi (section
5.2), aquatic invertebrates (section 6.2.7), and plants collected from
sites with high cadmium levels, such as those in the vicinity of
metalliferous mines and smelters.
Coughtrey & Martin (1977) experimentally demonstrated tolerance
of the grass Holcus lanatus to cadmium to be greater in plants
collected from an area subject to high fall-out of cadmium than in
plants collected from a control site. Growth of tolerant plants was
reduced in uncontaminated culture solutions, relative to controls, but
was similar to that of control plants in solutions where cadmium salts
had been added at levels similar to the field exposure. Simon (1977)
reported cadmium tolerance in the grasses Festuca ovina and
Agrostis tennuis. The tolerant grasses were collected from areas
contaminated by mining and aerial fall-out of cadmium.
8.2 Effects close to industrial sources and highways
There have been several reports of effects of heavy metal
deposition on the accumulation rate of leaf litter in deciduous
woodlands. These effects are restricted to areas close to, or down
wind from, smelter sites. The separation of the effects of cadmium
from those of other heavy metals present in the litter is difficult.
A study by Coughtrey et al. (1979) attempted to do this by detailed
analysis of the litter itself and by the use of statistics to separate
effects of different components of the pollution fall-out. Seven areas
of woodland in the vicinity of, or up to 28 km away from, a
lead-zinc-cadmium smelter in Avonmouth, United Kingdom, were studied.
The leaf litter contained lead, zinc, copper, and cadmium (in that
order of concentrations), and metal levels were high in samples taken
from within 3 km of the smelter. Litter from a wood 6.8 km from the
smelter had similar levels to control litter collected 30 km away, but
the prevailing wind would not have carried much of the fall-out in the
direction of this wood. For the four woods within 3 km of the smelter,
cadmium levels ranged from 23 to 98 mg/kg litter (lead levels were
between 721 and 2179 mg/kg, zinc levels between 764 and 2814 mg/kg,
and copper levels between 47 and 135 mg/kg). The weight of litter
accumulated per unit area was markedly greater in the contami-nated
sites than in the uncontaminated ones; litter standing crop ranged
from 7.91 to 13.16 kg/m2 in contaminated and from 0.913 to 3.104
kg/m2 in uncontaminated sites. The litter accumulation correlated
well with levels of all metals but not with the pH of the litter,
which varied between 3.88 and 6.3 over the sites. Partial correlation
analysis showed that cadmium and zinc interrelated; with both cadmium
and zinc, partial correlation coefficients were highly significant
when lead, copper or pH effects were accounted for. However,
correlations were low for lead and copper when cadmium or zinc were
accounted for. Of further interest was an analysis of cadmium and zinc
in leaf litter from various sites. There was an increase in the
smaller particle sizes in contaminated sites relative to
uncontaminated ones. These smaller particles contained a
disproportionate amount of the metals present, particularly in the
case of cadmium. Litter standing crop and cadmium concentrations were
highly correlated; the correlation between cadmium content and
different particle sizes of litter was better for small particles
sizes, and the slope of the regression line between cadmium
concentration and litter weight decreased with increasing particle
size. The authors argued that litter degradation was not affected at
the early stages but only when breakdown had progressed to much
smaller particle sizes. This perhaps supports the view that
microorganisms are inhibited by metals to a greater extent than
invertebrates, which would produce the initial reduction in size of
litter fragments. Taking the figure of 900 g/m2 as the normal leaf
litter level for woodland, the extra accumulation in contaminated
woods represented 25 to 30 years of litter accumulation (the smelter
in question had been operating for 48 years) and, possibly a large
proportion of the total capital of nutrients normally recycled to the
plants.
Other authors have also reported accumulation of leaf litter in
areas contaminated by metals (Tyler, 1972; Strojan, 1978), although
they disagree about the probable cause. Strojan (1978) proposed that
the effect relates to the absence of some groups of invertebrates,
while Jordan & Lechavalier (1975) suggested that the effect is on
microorganisms. There is no direct evidence that invertebrates in leaf
litter are adversely affected by metals, although they do accumulate
all the metals found in litter (Martin et al., 1976; Coughtrey &
Martin, 1976). Both Tyler (1972) and Strojan (1978) argued that the
productivity of woodland may be adversely affected by the failure to
recycle nutrients in areas contaminated by heavy metals. Coughtrey et
al. (1979) considered that the litter is an important sink for heavy
metals, and that the result of litter organisms developing tolerance
to the metals and, therefore, in the long-term increasing the rate of
degradation is unpredictable since metals would be released at the
same time as nutrients.
In a study by van Straalen et al. (1987), the metal excretion
efficiency of the collembolan Orchesella cincta collected from
various contaminated forest soils was monitored. The authors found
that moderate to high soil cadmium contamination of industrial origin
did not evoke increased cadmium excretion. In fact contamination
initiated in this century from a zinc factory caused a significant
decrease in excretion efficiency. Soils that had been contaminated
with cadmium for many years (lead/zinc factory) or to an extreme
degree (lead smelter) were inhabited by Collembola able to increase
metal excretion.
Muskett & Jones (1980) found levels of cadmium to be higher than
normal within 10 m of a road with a heavy traffic, but no effect on
the numbers of invertebrates caught or their species diversity was
observed.
8.3 Effects on fish
Field studies in Sweden showed that perch ( Perca fluviatilis)
from a cadmium-contaminated river (0.1 to 0.2 µg cadmium/litre) had
physiological abnormalities similar to those shown in laboratory
experiments (Sjobeck et al., 1984).
8.4 Effects on sea-birds
The reported effects on the kidney of sea-birds are not always a
result of exposure to cadmium as an industrial pollutant, since the
individuals most affected come from areas where there is no industrial
effluent. This is often, therefore, a response to naturally occurring
cadmium presumed to derive from the oceans. The birds appear to cope
with this damage to the kidney and suffer no effects on survival or
breeding success. No damage resulting from exposure to strictly
anthropogenically derived cadmium appears to have been reported on the
same scale as that from exposure to naturally occurring cadmium.
Nicholson et al. (1983) compared the ultrastructure of the kidneys of
sea-birds contaminated with cadmium in the wild, sea-birds from
uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg/kg, respectively (see
section 7.3.3). Nicholson & Osborn (1983) reported kidney lesions
(described in section 7.3.3) in several different species of sea-bird
caught in contaminated areas, although other pollutant metals such as
mercury were also present in the tissues.
9. EVALUATION
9.1 General considerations
In evaluating the environmental hazard of cadmium, it is
necessary to extrapolate from laboratories studies to ecosystems. This
must be done with extreme caution for a number of reasons.
a) The availability of cadmium to organisms in the environment is
limited by its strong adsorption to environmental components such
as soil, sediment, and organic matter. Organisms in contaminated
areas accumulate high body burdens of cadmium.
b) Environmental variables such as temperature, pH, and the chemical
composition of water or soil have been shown to affect both the
uptake and the toxic impact of cadmium.
c) Available, rather than nominal or total, cadmium is the
determinant in assessing uptake by, and effects on, organisms.
d) There are limited data from controlled experimental studies on
the effects of mixtures of metals. Organisms in the environment
are exposed to mixtures of pollutants. Acid deposition can
release metals, including cadmium, into the environment.
e) Little experimental work has been carried out on species or
communities that are either representative or key components of
natural communities and ecosystems. Studies have not considered
all of the interactions between populations and all of the
environmental factors affecting these populations. As a result,
the impact of cadmium on ecosystems may have been underestimated.
f) Results from laboratory studies based on very sensitive
parameters may be indicative of physiological impacts on
individuals rather than impacts on ecosystems.
9.2 The aquatic environment
Cadmium input to the aquatic environment is through dis-charge of
industrial waste, surface run-off, and deposition. It is strongly
adsorbed onto sediments and soils. The average cadmium content of sea
water is about 0.1 µg/litre or less, while fresh waters contain <
0.01 to 0.06 µg/litre in unpolluted areas. Cadmium levels of up to 5
mg/kg and 0.03 to 1 mg/kg have been reported for freshwater sediments
and marine sediments, respectively.
The rate of uptake and the toxic impact of cadmium on aquatic
organisms is greatly affected by physicochemical factors such as
temperature, ionic concentration, and organic matter content.
Cadmium is translocated by aquatic plants and concentrated in
roots and leaves. It is also taken up and accumulated by various
aquatic animals. The toxicity of cadmium to freshwater organisms
varies considerably depending on the exposure duration, species, and
life-stage. The early life-stages and the reproductive system are the
most vulnerable. Cadmium is, by comparison, one of the most toxic
heavy metals in the freshwater environment. Manifest responses of
certain organisms to cadmium are observed at environmental
concentrations lower than 1 µg/litre.
Cadmium-induced kidney damage has been reported in sea-birds
sampled from the field. However, this damage is present in both
cadmium-polluted areas and areas remote from industrial contamination.
The effect is probably, therefore, due to natural cadmium in certain
species and areas.
9.3 The terrestrial environment
Cadmium is introduced into the terrestrial environment from
mining, non-ferrous metal production, landfill sites and from the
application of sewage sludge, phosphate fertilizers, and manure.
Background concentrations of cadmium are in the range of 0.1 to 0.4
mg/kg soil and can reach 4.5 mg/kg in volcanic soils. Levels up to 160
mg/kg soil have been found close to metal processing sources.
Reduced breakdown of leaf litter and recycling of nutrients has
been attributed to metal pollution in the field. Cadmium appears to be
the most potent metal at inhibiting litter degradation. The effect is
thought to be due largely to reduced populations of microorganisms,
which are responsible for the final stages of litter decomposition.
Plants take up cadmium and can translocate and accumulate it.
However, uptake from soil is limited. Where there is high-level
exposure to cadmium (in the range of hundreds of mg/kg), growth
reduction is the major effect. Plants exposed to cadmium in the field
for long periods can develop tolerance to the metal. There is no
evidence of adverse effects of cadmium on plant populations in the
field.
Terrestrial invertebrates vary considerably in their sensitivity
to cadmium. Some species can take up and store cadmium to levels of up
to 5000 mg/kg body weight without apparent ill effects, while others
show population effects at levels of a few mg/kg soil. Populations of
some terrestrial invertebrates could be adversely affected at levels
of cadmium contamination seen in the field. Isopods and earthworms are
useful biomonitors for cadmium contamination. Invertebrates with high
body burdens may pose a threat to predators.
Kidney damage was found in experimental birds fed 20 mg
cadmium/kg diet for 12 weeks, but not at lower doses. Reproductive
effects have been observed at 200 mg/kg diet. A dose of 4 mg/kg
affected the behaviour of ducklings. No effects of cadmium have been
seen in terrestrial birds sampled from the field, although the cadmium
level in the brain, kidney, and liver of pigeons has proved to be a
good indicator of urban cadmium contamination.
Small mammals accumulate cadmium in the vicinity of mining spoil.
The ionic balance was affected in voles exposed experimentally to a
concentration of 10 mg/kg diet.
Populations of terrestrial organisms may also develop tolerance
to cadmium after long-term exposure.
10. RECOMMENDATIONS FOR PROTECTING THE ENVIRONMENT
To eliminate environmental effects, emissions of cadmium from the
following sources should be reduced as far as is practicable:
* smelters
* incinerators
* sewage sludge applied to the land
* phosphate fertilisers
* cadmium-containing manure
11. FURTHER RESEARCH
a) More study is needed to clarify the effects of cadmium on the
decomposition process of plant debris. Effects on the degree of
nutrient cycling and long-term plant growth and the exact nature
of the inhibition of decomposition require further attention.
b) The adsorption of cadmium to soil and sediment requires further
study and quantification of coefficients. Modelling of binding
and distribution in the environment is needed.
c) Organisms that are particularly sensitive (i.e. indicator
species) or that play a critical role in ecological systems
should be identified and studied with regard to the effects of
cadmium.
d) Studies are needed on the basic mechanisms by which cadmium
interacts with physiological and biochemical processes in
organisms and within individual cells.
There is a need to take certain precautions in studies on
cadmium. Firstly, the speciation of the metal should be considered in
experimental design and procedures and a clear measure of the
available cadmium should be reported. Secondly, studies of the uptake
and movement between trophic levels should include the relationship
between the non-nutrient cadmium and the nutrients calcium and zinc.
REFERENCES
ABEL, P.D. & PAPOUTSOGLOU, S.E. (1986) Lethal toxicity of cadmium to
Cyprinus carpio and Tilapia aurea. Bull. environ. Contam.
Toxicol., 37: 382-386.
ADRIANO, D.C. (1986) Trace elements in the terrestrial environment,
Berlin, Heidelberg, New York, Springer-Verlag, 533 pp.
AHSANULLAH, M. (1976) Acute toxicity of cadmium and zinc to seven
invertebrate species from Western Port, Victoria. Aust. J. mar.
freshwater Res., 27: 187-196.
ALLOWAY, B.J., JACKSON, A.P., & MORGAN, H. (1990) The accumulation of
cadmium by vegetables grown on soils contaminated from a variety of
sources. Sci. total Environ., 91: 223-236.
ANADU, D.I., CHAPMAN, G.A., CURTIS, L.R., & TUBB, R.A. (1989) Effect
of zinc exposure on subsequent acute tolerance to heavy metals in
rainbow trout. Bull. environ. Contam. Toxicol., 43: 329-336.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDREWS, S.M., JOHNSON, M.S., & COOKE, J.A. (1984) Cadmium in small
mammals from grassland established on metalliferous mine waste.
Environ. Pollut., 33A: 153-162.
ARILLO, A., CALAMARI, D., MARGIOCCO, C., MELODIA, F., & MENSI, P.
(1984) Biochemical effects of long-term exposure to cadmium and copper
on rainbow trout ( Salmo gairdneri): validation of water quality
criteria. Ecotoxicol. environ. Saf., 8: 106-117.
AYLETT, B.J. (1979) The chemistry and bioinorganic chemistry of
cadmium. In: Webb, M., ed. The chemistry, biochemistry and biology of
cadmium, Amsterdam, Elsevier/North Holland, pp. 1-43.
BABICH, H. & STOTZKY, G. (1977a) Reductions in the toxicity of cadmium
to microorganisms by clay minerals. Appl. environ. Microbiol., 33:
696-705.
BABICH, H. & STOTZKY, G. (1977b) Effect of cadmium on fungi and on
interactions between fungi and bacteria in soil: influence of clay
minerals and pH. Appl. environ. Microbiol., 33: 1059-1066.
BAHNER, L.H. & NIMMO, D.R. (1975) Methods to assess effects of
combinations of toxicants, salinity and temperature on estuarine
animals. In: Hemphill, D.D., ed. Trace substances in environmental
health - IX, Columbia, University of Missouri, pp. 169-177.
BAUDOUIN, M.F. & SCOPPA, P. (1974) Acute toxicity of various metals to
freshwater zooplankton. Bull. environ. Contam. Toxicol., 12: 745-751.
BAZZAZ, F.A., CARLSON, R.W., & ROLFE, G.L. (1974) The effect of heavy
metals on plants: part 1. Inhibition of gas exchange in sunflower by
Pb, Cd, Ni and Tl. Environ. Pollut., 7A: 241-246.
BEHNE, D. (1980) Problems of sampling and sample preparation for trace
element analysis in the health sciences. In: Bratter, P. & Scramel,
P., ed. Trace elements analytical chemistry in medicine and biology.
Proceedings of the 1st International Workshop, Neuherberg, Germany,
Berlin, Walter de Gruyter, pp. 769-782.
BENGTSSON, B-E. & BERGSTROM, B. (1987) A flowthrough fecundity test
with Nitocra spinipes (Harpacticoidea Crustacea) for aquatic
toxicity. Ecotoxicol. environ. Saf., 14: 260-268.
BENGTSSON, B-E., CARLIN, C.H., LARSSON, A., & SVANBERG, O. (1975)
Vertebral damage in minnows, Phoxinus phoxinus L., exposed to
cadmium. Ambio, 4: 166-168.
BENNETT, W.N. (1990) Measurement of manganese amelioration of cadmium
toxicity in Chlorella pyrenoidosa using turbidostat culture. Arch.
environ. Contam. Toxicol., 19: 118-123.
BENOIT, D.A., LEONARD, E.N., CHRISTENSEN, G.M., & FIANDT, J.T. (1976)
Toxic effects of cadmium on three generations of brook trout
( Salvelinus fontinalis). Trans. Am. Fish. Soc., 105: 550-560.
BERGER, B. & DALLINGER, R. (1989) Accumulation of cadmium and copper
by the terrestrial snail Arianta arbustorum L.: kinetics and
budgets. Oecologia, 79: 60-65.
BERGLIND, R. (1986) Combined and separate effects of cadmium, lead and
zinc on ALA-D activity, growth and hemoglobin content in Daphnia
magna. Environ. Toxicol. Chem., 5: 989-995.
BERK, S.G., GUNDERSON, J.H., & DERK, L.A. (1985) Effects of cadmium
and copper on chemotaxis of marine and freshwater ciliates. Bull.
environ. Contam. Toxicol., 34: 897-903.
BERTRAM, P.E. & HART, B.A. (1979) Longevity and reproduction of
Daphnia pulex (de Geer) exposed to cadmium-contaminated food or
water. Environ. Pollut., 19A: 295-305.
BESTEN, P.J. DEN, HERWIG, H.J., ZANDEE, D.I., & VOOGT, P.A. (1989)
Effects of cadmium and PCBs on reproduction of the sea star Asterias
rubens: Aberrations in the early development. Ecotoxicol. environ.
Saf., 18: 173-180.
BEWLEY, R.J.F. & STOTZKY, G. (1983) Effects of cadmium and zinc on
microbial activity in soil; influence of clay minerals. Part 1: Metals
added individually. Sci. total Environ., 31: 41-55.
BEYER, W.N., CHANEY, R.L., & MULHERN, B.M. (1982) Heavy metal
concentrations in earthworms from soil amended with sewage sludge. J.
environ. Qual., 11: 381-385.
BEYER, W.N., PATTEE, O.H., SILEO, L., HOFFMAN, D.J., & MULHERN, B.M.
(1985) Metal contamination in wildlife living near two zinc smelters.
Environ. Pollut., 38A: 63-86.
BIESINGER, K.E. & CHRISTENSEN, G.M. (1972) Effects of various metals
on survival, growth, reproduction, and metabolism of Daphnia magna.
J. Fish Res. Board Can., 29: 1691-1700.
BINGHAM, F.T., PAGE, A.L., & STRONG, J.E. (1980) Yield and cadmium
content of rice grain in relation to addition rate of cadmium, copper,
nickel and zinc, with sewage sludge and liming. Soil Sci., 130: 32-38.
BLACK, J.A. & BIRGE, W.J. (1989) An avoidance response bioassay for
aquatic pollutants, Lexington, Kentucky, University of Kentucky, Water
Resources Research Institute (Research Report No. 123).
BLOCK, M. (1991) Uptake of cadmium in fish. Effects of xanthates and
diethyldithiocarbamate, University of Uppsala, Sweden, Faculty of
Sciences (Universitatis Uppsaliensis - Comprehensive summaries of
Uppsala dissertations from the Faculty of Science. No. 326).
BLOCK, M. & PART, P. (1986) Increased availability of cadmium to
perfused rainbow trout ( Salmo gairdneri Rich.) gills in the presence
of the complexing agents diethyl dithiocarbamate, ethyl xanthate and
isopropyl xanthate. Aquat. Toxicol., 8: 295-302.
BOND, H., LIGHTHART, B., SHIMABUKU, R., & RUSSELL, L. (1976) Some
effects of cadmium on coniferous forest soil and litter microcosms.
Soil Sci., 121: 278-287.
BORGMANN, U. (1983) Metal speciation and toxicity of free metal ions
to aquatic biota. In: Nriagu, J.O., ed. Aquatic toxicology, New York,
Chichester, John Wiley & Sons, pp. 47-72.
BORGMANN, U. & RALPH, K.M. (1986) Effects of cadmium,
2,4-dichlorophenol, and pentachlorophenol on feeding, growth, and
particle-size-conversion efficiency of white sucker larvae and young
common shiners. Arch. environ. Contam. Toxicol., 15: 473-480.
BORGMANN, U., MILLARD, E.S., & CHARLTON, C.C. (1989a) Effect of
cadmium on a stable, large volume, laboratory ecosystem containing
Daphnia and phytoplankton. Can. J. Fish. aquat. Sci., 46: 399-405.
BORGMANN, U., RALPH, K.M., & NORWOOD, W.P. (1989b) Toxicity test
procedures for Hyalella azteca, and chronic toxicity of cadmium and
pentachlorophenol to H. azteca, Gammarus fasciatus, and Daphnia
magna. Arch. environ. Contam. Toxicol., 18: 756-764.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BRESSAN, M. & BRUNETTI, R. (1988) The effects of nitriloacetic acid,
Cd and Hg on the marine algae Dunaliella tertiolecta and Isochrysis
galbana. Water Res., 22: 553-556.
BROOKS, R.R. & RUMSBY, M.G. (1967) Studies on the uptake of cadmium by
the oyster, Ostrea sinuata (Lamarck). Aust. J. mar. freshwater Res.,
15: 53-61.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BULL, K.R., MURTON, R.K., OSBORN, D., WARD, P., & CHENG, L. (1977)
High levels of cadmium in Atlantic seabirds and sea-skaters. Nature
(Lond.), 269: 507-509.
BURKITT, A., LESTER, P., & NICKLESS, G. (1972) Distribution of heavy
metals in the vicinity of an industrial complex. Nature (Lond.), 238:
327-328.
BYRNE, A.R., RAVNIK, V., & KOSTA, L. (1976) Trace element
concentrations in higher fungi. Sci. total Environ., 6: 65-78.
CAIN, J.R., PASCHAL, D.C., & HAYDEN, C.M. (1980) Toxicity and
bioaccumulation of cadmium in the colonial green alga Scenedesmus
obliquus. Arch. environ. Contam. Toxicol., 9: 9-16.
CAIN, B.W., SILEO, L., FRANSON, J.C., & MOORE, J. (1983) Effects of
dietary cadmium on mallard ducklings. Environ. Res., 32: 286-297.
CALABRESE, A., COLLIER, R.S., NELSON, D.A. & MACINNES, J.R. (1973) The
toxicity of heavy metals to embryos of the American oyster
Crassostrea virginica. Mar. Biol., 18: 162-166.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1980) Influence of water
hardness on cadmium toxicity to Salmo gairdneri Rich. Water Res.,
14: 1421-1426.
CANTON, J.H. & SLOOFF, W. (1982) Toxicity and accumulation studies of
cadmium (Cd2+) with freshwater organisms of different trophic
levels. Ecotoxicol. environ. Saf., 6: 113-128.
CARRIER, R. & BEITINGER, T.L. (1988a) Reduction in thermal tolerance
of Notropis lutrensis and Pimephales promelas exposed to cadmium.
Water Res., 22: 511-515.
CARRIER, R. & BEITINGER, T.L. (1988b) Resistance of temperature
tolerance ability of green sunfish to cadmium exposure. Bull. environ.
Contam. Toxicol., 40: 475-480.
CARROLL, J.J., ELLIS, S.J., & OLIVER, W.S. (1979) Influences of
hardness constituents on the acute toxicity of cadmium to brook trout
( Salvelinus fontinalis). Bull. environ. Contam. Toxicol., 22:
575-581.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living near
a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CATALDO, D.A. & WILDUNG, R.E. (1978) Soil and plant factors
influencing the accumulation of heavy metals by plants. Environ.
Health Perspect., 27: 149-159.
CEARLEY, J.E. & COLEMAN, R.L. (1974) Cadmium toxicity and
bioconcentration in largemouth bass and bluegill. Bull. environ.
Contam. Toxicol., 11: 146-151.
CHAN, K.-Y. & DEAN, A.C.R (1988) Effects of cadmium and lead on
growth, respiration and enzyme activity of the marine bacterium
Pseudomonas marina. Chemosphere, 17: 597-607.
CHANDINI, T. (1988) Changes in food [ Chlorella] levels and the acute
toxicity of cadmium to Daphnia carinata (Daphnidae) and Echinisca
triserialis (Macrothricidae) [Crustacea: Cladocera]. Bull. environ.
Contam. Toxicol., 41: 398-403.
CHANDINI, T. (1989) Survival, growth and reproduction of Daphnia
carinata (Crustacea: Cladocera) exposed to chronic cadmium stress at
different food ( Chlorella) levels. Environ. Pollut., 60: 29-45.
CHANEY, R.L., WHITE, M.C., & SIMON, P.W. (1975) Plant uptake of heavy
metals from sewage sludge applied to land. In: Proceedings of the 2nd
National Conference on Municipal Sludge Management, Rockville,
Information Transfer Inc., pp. 169-178.
CHAPMAN, G. & DUNLOP, S. (1981) Detoxification of zinc and cadmium by
the freshwater protozoan Tetrahymena pyriformis. I. The effect of
water hardness. Environ. Res., 26: 81-86.
CHIN, B. & SINA, J.F. (1978) Cellular bases of cadmium toxicity in
Physarum. In: Hemphill, D.D., ed. Trace substances in environmental
health, Columbia, University of Missouri, pp. 214-219.
CHRISTENSEN, J.J., EATOUGH, D.J., & IZATT, R.M. (1975) In: Handbook of
metal ligand heats and related thermodynamic quantities, 2nd ed.
revis. expand., New York, Basel, Marcel Dekker, Inc., 495 pp.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975a) Synergism between
dissolved oxygen and cadmium toxicity in five species of aquatic
insects. Environ. Res., 9: 285-289.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975b) Acute cadmium
toxicity studies upon nine species of aquatic insects. Environ. Res.,
9: 331-341.
CONRAD, G.W. (1988) Heavy metal effects on cellular shape changes,
cleavage, and larval development of the marine gastropod mollusk,
( Ilyanassa obsoleta Say). Bull. environ. Contam. Toxicol., 41:
79-85.
CONWAY, H.L. (1978) Sorption of arsenic and cadmium and their effects
on growth, micronutrient utilization, and photosynthetic pigment
composition of Asterionella formosa. J. Fish Res. Board. Can., 35:
286-294.
COOMBS, T.L. (1979) Cadmium in aquatic organisms. In: Webb, M., ed.
The chemistry, biochemistry and biology of cadmium, Amsterdam,
Elsevier/North Holland, pp. 93-139.
COUGHTREY, P.J. & MARTIN, M.H. (1976) The distribution of Pb, Zn, Cd
and Cu within the pulmonate mollusc Helix aspersa Muller. Oecologia,
23: 315-322.
COUGHTREY, P.J. & MARTIN, M.H. (1977) Cadmium tolerance of Holcus
lanatus from a site contaminated by aerial fallout. New Phytol., 79:
273-280.
COUGHTREY, P.J., JONES, C.H., MARTIN, M.H., & SHALES, S.W. (1979)
Litter accumulation in woodlands contaminated by Pb, Zn, Cd and Cu.
Oecologia, 39: 51-60.
DALLA VIA, G.J., DALLINGER, R., & CARPENE, E. (1989) Effects of
cadmium on Murex trunculus from the Adriatic Sea. II. Oxygen
consumption and acclimation effects. Arch. environ. Contam. Toxicol.,
18: 562-567.
DALLINGER, R. & WIESER, W. (1984) Patterns of accumulation,
distribution and liberation of Zn, Cu, Cd and Pb in different organs
of the land snail Helix pomatia L. Comp. Biochem. Physiol., 79C:
117-124.
DALLINGER, R., PROSI, F., SEGNER, H., & BACK, H. (1987) Contaminated
food and uptake of heavy metals by fish: a review and proposal for
further research. Oecologia, 73: 91-98.
DAWSON, M.A., GOULD, E., THURBERG, F.P., & CALABRESE, A. (1977)
Physiological response of juvenile striped bass, Morone saxatilis,
to low levels of cadmium and mercury. Chesapeake Sci., 18: 353-359.
DENTON, G.R.W. & BURDON-JONES, C. (1981) Influence of temperature and
salinity on the uptake, distribution and depuration of mercury,
cadmium and lead by the black-lip oyster Saccostrea echinata. Mar.
Biol., 64: 317-326.
DE VRIES, M.P.C. & TILLER, K.G. (1978) Sewage sludge as a soil
amendment with special reference to Cd, Cu, Mn, Ni, Pb and Zn -
comparison of results from experiments conducted inside and outside a
glasshouse. Environ. Pollut., 16A: 231-240.
DE ZWART, D. & SLOOF, W. (1987) Toxicity of mixtures of heavy metals
and petrochemicals to Xenopus laevis. Bull. environ. Contam.
Toxicol., 38: 345-351.
DICKSON, G.W., GIESY, J.P., & BRIESE, L.A. (1982) The effect of
chronic cadmium exposure on phosphodenylate concentrations and
adenylate energy of gills and dorsal muscle of crayfish. Environ.
Toxicol. Chem., 1: 147-156.
DINNEL, P.A., LINK, J.M., STOBER, Q.J., LETOURNEAU, M.W., & ROBERTS,
W.E. (1989) Comparative sensitivity of sea urchin sperm bioassays to
metals and pesticides. Arch. environ. Contam. Toxicol., 18: 748-755.
DOELMAN, P., NIEBOER, G., SCHROOTEN, J., & VISSER, M. (1984)
Antagonistic and synergistic toxic effects of Pb and Cd in a simple
foodchain: Nematodes feeding on bacteria or fungi. Bull. environ.
Contam. Toxicol., 32: 717-723.
DONGMANN, G. & NURNBERG, H.W. (1982) Observations with Thalassiosira
rotula (Meunier) on the toxicity and accumulation of cadmium and
nickel. Ecotoxicol. environ. Saf., 6: 535-544.
DOUBEN, P.E.T. (1989a) Uptake and elimination of waterborne cadmium by
the fish Noemacheilus barbatulus L. (stone loach). Arch. environ.
Contam. Toxicol., 18: 576-586.
DOUBEN, P.E.T. (1989b) Metabolic rate and uptake and loss of cadmium
from food by the fish Noemacheilus barbatulus L. (stone loach).
Environ. Pollut., 59: 177-202.
DRESCHER, H.E., HARMS, U., & HUSCHENBETH. E. (1977) Organochlorines
and heavy metals in the harbour seal Phoca vitulina from the German
North Sea coast. Mar. Biol., 41: 99-106.
EATON, J.G., MCKIM, J.M., & HOLCOMBE, G.W. (1978) Metal toxicity to
embryos and larvae of seven freshwater fish species - I. Cadmium.
Bull. environ. Contam. Toxicol., 19: 95-103.
EISLER, R. (1971) Cadmium poisoning in Fundulus heteroclitus
(Pisces: Cyprinodontidae) and other marine organism. J. Fish Res.
Board Can., 28: 1225-1234.
EISLER, R. (1985) Cadmium hazards to fish, wildlife and invertebrates:
A synoptic review, Washington, DC, US Department of the Interior, Fish
and Wildlife Service (Biological Report No. 85).
EISLER, R. & HENNEKEY, R.J. (1977) Acute toxicities of Cd2+, Cr+6,
Hg2+, Ni2+ and Zn2+ to estuarine macrofauna. Arch. environ.
Contam. Toxicol., 6: 315-323.
EISLER, R., ZAROOGIAN, G.E., & HENNEKEY, R.J. (1972) Cadmium uptake by
marine organisms. J. Fish Res. Board. Can., 29: 1367-1369.
ELLIOT, N.G., SWAIN, R., & RITZ, D.A. (1985) The influence of cyclic
exposure on the accumulation of heavy metals by Mytilus edulis
planulatus (Lamarck). Mar. environ. Res., 15: 17-30.
ERIKSSON, J.E. (1988) The effects of clay, organic matter and time on
adsorption and plant uptake of cadmium added to the soil. Water Air
Soil Pollut., 40: 359-373.
FALCONER, C.R., DAVIES, I.M., & TOPPING, G. (1983) Trace metals in the
common porpoise, Phocoena phocoena. Mar. environ. Res., 8: 119-127.
FERARD, J.F., JOUANY, J.M., TRUHAUT, R., & VASSEUR, P. (1983)
Accumulation of cadmium in a freshwater food chain experimental model.
Ecotoxicol. environ. Saf., 7: 43-52.
FINLAYSON, B.J. & VERRUE, K.M. (1982) Toxicities of copper, zinc, and
cadmium mixtures to juvenile chinook salmon. Trans. Am. Fish. Soc.,
111: 645-650.
FLATAU, G.N., CLEMENT, R.L., GAUTHIER, M.J., & LAUMOND, F.M. (1987)
Adaptation to cadmium in a sensitive marine pseudomonad. Environ.
Technol. Lett, 8: 641-646.
FLATAU, G.N., CLEMENT, R.L., & GAUTHIER, M.J. (1988) Uptake of cadmium
by marine bacteria. Prog. Oceanogr., 21: 181-188.
FLEISCHER, M., SAROFIM, A.F., FASSETT, D.W., HAMMOND, P., SHACKLETTE,
H.T., NISBET, I.C.T., & EPSTEIN, S. (1974) Environmental impact of
cadmium: a review by the Panel on Hazardous Trace Substances. Environ.
Health Perspect., 5: 253-323.
FLIK, G., VAN DE WINKEL, J.G.J., PART, P., WENDELAAR BONGA, S.E., &
LOCK, R.A.C. (1987) Calmodulin mediated cadmium inhibition of
phosphodiesterase activity. Arch. Toxicol., 59: 352-359.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FRANCIS, P.C., BIRGE, W.J., & BLACK, J.A. (1984) Effects of
cadmium-enriched sediment on fish and amphibian embryo-larval stages.
Ecotoxicol. environ. Saf., 8: 378-387.
FRANK, P.M. & ROBERTSON, P.B. (1979) The influence of salinity on
toxicity of cadmium and chromium to the blue crab, Callinectes
sapidus. Bull. environ. Contam. Toxicol., 21: 74-78.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
fourteenth session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WHO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the Mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization (Reports
and Studies No. 31).
GIATTINA, J.D. & GARTON, R.R. (1983) A review of the
preference-avoidance responses of fishes to aquatic contaminants.
Residue Rev., 87: 43-90.
GIESY, J.P., LEVERSEE, G.J., & WILLIAMS, D.R. (1977) Effects of
naturally occurring aquatic organic fractions on cadmium toxicity to
Simocephalus serrulatus (Daphnidae) and Gambusia affinis
(Poeciliidae). Water Res., 11: 1013-1020.
GILL, T.S. & PANT, J.C. (1983) Cadmium toxicity: inducement of changes
in blood and tissue metabolites in fish. Toxicol. Lett., 18: 195-200.
GISH, C.D. & CHRISTENSEN, R.E. (1973) Cadmium, nickel, lead and zinc
in earthworms from roadside soil. Environ. Sci. Technol., 7:
1060-1062.
GOTTOFREY, J. (1990) The disposition of cadmium, nickel, mercury and
methylmercury in fish and effects of lipophilic metal chelation,
Uppsala, Swedish University of Agricultural Sciences, Department of
Pharmacology and Toxicology (Thesis).
GOTTOFREY, J., BJORKLUND, I., & TJALVE, H. (1988) Effects of sodium
isoproylxanthate, potassium amylxanthate and sodium
diethyldithiocarbamate on the uptake and disposition of cadmium in the
brown trout ( Salmo trutta). Aquat. Toxicol., 12: 171-184.
GUY, R.D. & ROSS KEAN, A. (1980) Algae as a chemical speciation
monitor. I. A comparison of algal growth and computer calculated
speciation. Water Res., 14: 891-899.
HAIGHT, M., MUDRY, T., & PASTERNAK, J. (1982) Toxicity of seven heavy
metals on Panagrellus silusiae: The efficacy of the free-living
nematode as an in vivo toxicological bioassay. Nematologica, 28: 1-11.
HALE, J.G. (1977) Toxicity of metal mining wastes. Bull. environ.
Contam. Toxicol., 17: 66-73.
HALL, W.S., PAULSON, R.L., HALL, L.W., & BURTON, D.T. (1986) Acute
toxicity of cadmium and sodium pentachlorophenate to Daphnids and
fish. Bull. environ. Contam. Toxicol., 37: 308-316.
HAMANAKA, T., ITOO, T., & MISHIMA, S. (1982) Age related change and
distribution of cadmium and zinc concentrations in the stellar sea
lion ( Eumetopias jubata) from the coast of Hokkaido, Japan. Mar.
Pollut. Bull., 13: 57-61.
HAMILTON, S.J. & MEHRLE, P.M. (1986) Metallothionein in fish: review
of its importance in assessing stress from metal contaminants. Trans.
Am. Fish. Soc., 115: 596-609.
HARDISTY, M.W., HUGGINS, R.J., KARTAR, S., & SAINSBURY, M. (1974a)
Ecological implications of heavy metal in fish from the Severn
Estuary. Mar. Pollut. Bull., 5: 12-15.
HARDISTY, M.W., KARTAR, S., & SAINSBURY, M. (1974b) Dietary habits and
heavy metal concentrations in fish from the Severn Estuary and Bristol
Channel. Mar. Pollut. Bull., 5: 61-63.
HARDY, J.T., SCHMIDT, R.L., & APTS, C.W. (1981) Marine sediment and
interstitial water: effects on bioavailability of cadmium to gills of
the clam Protothaca staminea. Bull. environ. Contam. Toxicol., 27:
798-805.
HARDY, J.T., SULLIVAN, M.F., CRECELIUS, E.A., & APTS, C.W. (1984)
Transfer of cadmium in phytoplankton-oyster-mouse food chain. Arch.
environ. Contam. Toxicol., 13: 419-425.
HARKOV, R., CLARKE, B., & BRENNAN, E. (1979) Cadmium contamination may
modify response of tomato to atmospheric ozone. J. Air Pollut. Control
Assoc., 29: 1247-1249.
HARRISON, S.E. & KLAVERKAMP, J.F. (1989) Uptake, elimination and
tissue distribution of dietary and aqueous cadmium by rainbow trout
( Salmo gairdneri Richardson) and lake whitefish ( Coregonus
clupeaformis Mitchill). Environ. Toxicol. Chem., 8: 87-97.
HART, B.A. & SCAIFE, B.D. (1977) Toxicity and bioaccumulation of
cadmium in Chlorella pyrenoidosa. Environ. Res., 14: 401-413.
HARTENSTEIN, R., NEUHAUSER, E.F., & COLLIER, J. (1980) Accumulation of
heavy metals in the earthworm Eisenia foetida. J. environ. Qual., 9:
23-26.
HARTMAN, L.M. (1974) A preliminary report: Fungal flora of the soil as
conditioned by varying concentrations of heavy metals. Am. J. Bot.,
61: 23 (Abstract).
HARTWELL, S.I., JIN, J.H., CHERRY, D.S., & CAIRNS, J. (1989) Toxicity
versus avoidance response of golden shiner, Notemigonus crysoleucas,
to five metals. J. Fish Biol., 35: 447-456.
HATAKEYAMA, S. (1987) Chronic effects of Cd on reproduction of
Polypedilum nubifer (Chironomidae) through water and food. Environ.
Pollut., 48: 249-261.
HATAKEYAMA, S. & YASUNO, M. (1987) Chronic effects of Cd on the
reproduction of the guppy ( Poecilia reticulata) through
Cd-accumulated midge larvae ( Chironomus yoshimatsui). Ecotoxicol.
environ. Saf., 14: 191-207.
HEINZ, G.H., HASELTINE, S.D., & SILEO, L. (1983) Altered avoidance
behavior of black ducks fed cadmium. Environ. Toxicol. Chem., 2:
419-421.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
US Department of Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Department of Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
HIRAOKA, Y. & OKUDA, H. (1984) Time course study of occurrence of
anomalies in medaka's centrum by cadmium or fenitrothion emulsion.
Bull. environ. Contam. Toxicol., 32: 693-697.
HIRATA, M. (1981) Annaka: Land polluted mainly by fumes and dust from
a zinc smelter. In: Kitagishi, K. & Gamare, I., ed. Heavy metal
pollution in the soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HOGSTRAND, C. & HAUX, C. (1990a) Metallothionein as an indicator of
heavy metal exposure in two subtropical fish species. J. exp. mar.
Biol. Ecol., 138: 69-84.
HOGSTRAND, C. & HAUX, C. (1990b) A radioimmunoassay for perch ( Perca
fluviatilis) metallothionein. Toxicol. appl. Pharmacol., 103: 56-65.
HOLCOMBE, G.W., PHIPPS, G.L., & MARIER, J.W. (1984) Methods for
conducting snail ( Aplexa hypnorum) embryo through adult exposures.
Effects of cadmium and reduced pH levels. Arch. environ. Contam.
Toxicol., 13: 627-634.
HONDA, K. & TATSUKAWA, R. (1983) Distribution of cadmium and zinc in
tissues and organs, and their age-related changes in striped dolphins,
Stenella coeruleoalba. Arch. environ. Contam. Toxicol., 12: 543-550.
HONDA, K., FUJISE, Y., TATSUKAWA, R., ITANO, K., & MIYAZAKI, N. (1986)
Age-related accumulation of heavy metals in bone of the striped
dolphin, Stenella coeruleoalba. Mar. environ. Res., 20: 143-160.
HOPKIN, S.P. (1989) Ecophysiology of metals in terrestrial
invertebrates, London, Elsevier Applied Science.
HOPKIN, S.P. (1990) Species-specific differences in the net
assimilation of zinc, cadmium, lead, copper and iron by the
terrestrial isopods Oniscus asellus and Porcellio scaber. J. appl.
Ecol., 27: 460-474.
HOPKIN, S.P. & MARTIN, M.H. (1984) Assimilation of zinc, cadmium, lead
and copper by the centipede Lithobius variegatus (Chilopoda). J.
appl. Ecol., 21: 535-546.
HOPKIN, S.P. & MARTIN, M.H. (1985) Transfer of heavy metals from leaf
litter to terrestrial invertebrates. J. Sci. Food Agric., 36: 538-539.
HOPKIN, S.P., WATSON, K., MARTIN, M.H., & MOULD, M.L. (1985) The
assimilation of heavy metals by Lithobius variegatus and Glomeris
marginata (Chilopoda: Diplopoda). Bijdr. Dierkd., 55: 88-94.
HUIGUANG FU (1989) Hormonal responses of fish to cadmium, Nijmegen,
The Netherlands, University of Nijmegen (Ph.D. Thesis).
HUNTER, B.A. & JOHNSON, M.S. (1982) Food chain relationships of copper
and cadmium in contaminated grassland ecosystems. Oikos, 38: 108-117.
HUTCHINSON, T.C. & CZYRSKA, H. (1975) Heavy metal toxicity and
synergism to floating aquatic weeds. Verh. Int. Ver. Limnol., 19 :
2102-2111.
HUTTON, M. (1981) Accumulation of heavy metals and selenium in three
seabird species from the United Kingdom. Environ. Pollut., 26A:
129-145.
HUTTON, M. (1982) Cadmium in the European Community: a prospective
assessment of sources, human exposure and environmental impact,
London, Monitoring and Assessment Research Centre, Chelsea College,
University of London, 99 pp (MARC Report No. 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London, 46
pp (MARC Report No. 29).
HUTTON, M. & GOODMAN, G.T. (1980) Metal contamination of feral pigeons
Columba livia from the London area: Part I - tissue accumulation of
lead, cadmium and zinc. Environ. Pollut., 22A: 207-217.
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead, mercury
and arsenic entering the U.K. environment from human activities. Sci.
total Environ., 57: 129-150.
IRELAND, M.P. (1981) Uptake and distribution of cadmium in the
terrestrial slug Arion ater (L.) Comp. Biochem. Physiol., 68A:
37-41.
JANA, S. & BHATTACHARYA, D.N. (1988) Effect of heavy metals on growth
population of a fecal coliform bacterium Escherichia coli in aquatic
environment. Water Air Soil Pollut., 38: 251-254.
JANSSEN, M.P.M., BRUINS, A., DE VRIES, T.H., & VAN STRAALEN, N.M.
(1991) Comparison of cadmium kinetics in four soil arthropod species.
Arch. environ. Contam. Toxicol., 20: 305-312.
JOHN, J., GJESSING, E.T., GRANDE, M., & SALBU, B. (1987) Influence of
aquatic humus and pH on the uptake and depuration of cadmium by the
Atlantic salmon ( Salmo salar). Sci. total Environ., 62: 253-265.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination through
residual trace metal dispersal from derelict lead-zinc mine. J.
environ. Qual., 9(2): 175-179.
JOHNSON, M.W. & GENTILE, J.H. (1979) Acute toxicity of cadmium, copper
and mercury to larval American lobster Homarus americanus. Bull.
environ. Contam. Toxicol., 22: 258-264.
JONES, K.C. & JOHNSTON, A.E. (1989) Cadmium in cereal grain and
herbage from long-term experimental plots at Rothamsted, UK. Environ.
Pollut., 57: 199-216.
JORDAN, M.J. & LECHAVALIER, M.P. (1975) Effects of zinc-smelter
emissions on forest soil microflora. Can. J. Microbiol., 21:
1855-1865.
KAY, S.H. & HALLER, W.T. (1986) Heavy metal bioaccumulation and
effects on waterhyacinth weevils, Neochetina eichhorniae, feeding on
waterhyacinth, Eichhornia crassipes. Bull. environ. Contam.
Toxicol., 37: 239-245.
KEMP, P.F. & SWARTZ, R.C. (1988) Acute toxicity of interstitial and
particle-bound cadmium to a marine infaunal amphipod. Mar. environ.
Res., 26: 135-153.
KHAN, A.T., WEIS, J.S., & D'ANDREA, L. (1988) Studies of cadmium
tolerance in two populations of grass shrimp, Palaemonetes pugio.
Bull. environ. Contam. Toxicol., 40: 30-34.
KHANGAROT, B.S. & RAY, P.K. (1987) Sensitivity of toad tadpoles, Bufo
melanostictus (Schneider), to heavy metals. Bull. environ. Contam.
Toxicol., 38: 523-527.
KORTE, F. (1983) Ecotoxicology of cadmium: general overview.
Ecotoxicol. environ. Saf., 7: 3-8.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ., 22(2):
375-381.
KUHN, R., PATTARD, M., PERNAK, K-D., & WINTER, A. (1989) Results of
the harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res., 23: 501-510.
LAAMANEN, A. (1972) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAAMANEN, A. (1976) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAEGREID, M., ALSTAD, J., KLAVENESS, D., & SEIP, H.M. (1983) Seasonal
variation of cadmium toxicity toward the alga Selenastrum
capricornutum Printz in two lakes with different humus content.
Environ. Sci. Technol., 17: 357-361.
LAFEBER, F.P.J.G., FLIK, G., WENDELAAR BONGA, S.E., & PERRY, S.F.
(1988) Hypocalcin from Stannius corpuscles inhibits gill calcium
uptake in trout. Am. J. Physiol., 254: R891-R896.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling, sample
handling, analysis, Washington, DC, US Department of Commerce,
National Bureau of Standards, Vols. 1 & 2, 1304 pp.
LALANDE, M. & PINEL-ALLOUL, B. (1986) Acute toxicity of cadmium,
copper, mercury and zinc to Tropocyclops prasinus mexicanus
(Cyclopoida, Copepoda) from three Quebec lakes. Environ. Toxicol.
Chem., 5: 95-102.
LANGSTON, W.J. & ZHOU, M. (1987a) Cadmium accumulation, distribution
and metabolism in the gastropod Littorina littorea: the role of
metal-binding proteins. J. Mar. Biol. Assoc. UK, 67: 585-601.
LANGSTON, W.J. & ZHOU, M. (1987b) Cadmium accumulation, distribution
and elimination in the bivalve Macoma balthica: neither
metallothionein nor metallothionein-like proteins are involved. Mar.
environ. Res., 21: 225-237.
LEE, H.H. & XU, C.H. (1984) Differential response of marine organisms
to certain metal and agrichemical pollutants. Bull. environ. Contam.
Toxicol., 33: 460-467.
LEPP, N.W., HARRISON, S.C.S., & MORRELL, B.G. (1987) A role for
Amanita muscaria L. in the circulation of cadmium and vanadium in a
non-polluted woodland. Environ. Geochem. Health, 9: 61-64.
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Process of
atmospheric deposition of metals and acids to forests. Annual Meeting
of the Air Pollution Control Association, New Orleans, 20 June 1982,
Pittsburgh, Pennsylvania, Air Pollution Control Association, 14 pp
(Conference Proceedings 75).
LOFTS, B. & MURTON, R.K. (1967) The effects of cadmium on the avian
testis. J. Reprod. Fert., 13: 155-164.
LOVETT, R.J., GUTENMANN, W.H., PAKKALA, I.S., YOUNGS, W.D., LISK,
D.J., BURDICK, G.E., & HARRIS, E.J. (1972) A survey of the total
cadmium content of 406 fish from 49 New York State fresh waters. J.
Fish Res. Board Can., 29: 1283-1290.
LUSSIER, S.M., GENTILE, J.H., & WALKER, J. (1985) Acute and chronic
effects of heavy metals and cyanide on Mysidopsis bahia (Crustacea:
Mysidacea). Aquat. Toxicol., 7: 25-35.
MA, W., EDELMAN, Th., VAN BEERSUM, I., & JANS, Th. (1983) Uptake of
cadmium, zinc, lead and copper by earthworms near a zinc-smelting
complex: influence of soil pH and organic matter. Bull. environ.
Contam. Toxicol., 30: 424-427.
MCCAHON, C.P., BROWN, A.F., & PASCOE, D. (1988) The effect of the
acanthocephalan Pomphorhynchus laevis (Muller 1776) on the acute
toxicity of cadmium to its intermediate host, the amphipod Gammarus
pulex (L.). Arch. environ. Contam. Toxicol., 17: 239-243.
MCCAHON, C.P., WHILES, A.J., & PASCOE, D. (1989) The toxicity of
cadmium to different larval instars of the trichopteran larvae
Agapetus fuscipes Curtis and the importance of life cycle
information to the design of toxicity tests. Hydrobiologia, 185:
153-162.
MCCLURG, T.P. (1984) Effects of fluoride, cadmium and mercury on the
estuarine prawn Penaeus indicus. Water S. Afr., 10: 40-45.
MACINNES, J.R. & THURBERG, F.P. (1973) Effects of metals on the
behaviour and oxygen consumption of the mud snail. Mar. Pollut. Bull.,
4: 185-186.
MACINNES, J.R., THURBERG, F.P., GREIG, R.A., & GOULD, E. (1977)
Long-term cadmium stress in the cunner, Tautogolabrus adspersus.
Fish. Bull., 75: 199-203.
MAHLER, R.J., BINGHAM, F.T., & PAGE, A.L. (1978) Cadmium-enriched
sewage sludge application to acid and calcareous soils. Effect on
yield and cadmium uptake by lettuce and chard. J. environ. Qual., 7:
274-281.
MARSHALL, J.S. (1979) Cadmium toxicity to laboratory and field
populations of Daphnia galeata mendotae. Bull. environ. Contam.
Toxicol., 21: 453-457.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, M.H., COUGHTREY, P.J., & YOUNG, E.W. (1976) Observations on
the availability of lead, zinc, cadmium and copper in woodland litter
and the uptake of lead, zinc and cadmium by the woodlouse Oniscus
asellus. Chemosphere, 5: 313-318.
MARTIN, M.H., COUGHTREY, P.J., SHALES, S.W., & LITTLE, P. (1980)
Aspect of airborne cadmium contamination of soils and natural
vegetation. In: Inorganic pollution and agriculture, Her Majesty's
Stationary Office, London, pp. 56-69.
MAY, T.W. & MCKINNEY, G.L. (1981) Cadmium, lead, mercury, arsenic, and
selenium concentrations in freshwater fish, 1976-77 - National
pesticide monitoring program. Pestic. monit. J., 15: 14-38.
MAYACK, L.A., BUSH, P.B., FLETCHER, O.J., PAGE, R.K., & FENDLEY, T.T.
(1981) Tissue residues of dietary cadmium in wood ducks. Arch.
environ. Contam. Toxicol., 10: 637-645.
MAYER, F.L (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MEISCH, H.-U. & SCHMITT, J.A. (1986) Characterization studies on
cadmium-mycophosphatin from the mushroom Agaricus macrosporus.
Environ. Health Perspect., 65: 29-32.
MERLINI, M. (1978) Hepatic storage alteration of vitamin B12 by
cadmium in a freshwater fish. Bull. environ. Contam. Toxicol., 19:
767-771.
METEYER, M.J., WRIGHT, D.A., & MARTIN, F.D. (1988) Effect of cadmium
on early developmental stages of the sheepshead minnow ( Cyprinodon
variegatus). Environ. Toxicol. Chem., 7: 321-328.
MICHIBATA, H. (1981) Effect of water hardness on the toxicity of
cadmium to the egg of the teleost Oryzias latipes. Bull. environ.
Contam. Toxicol., 27: 187-192.
MIDDAUGH, D.P. & DEAN, J.M. (1977) Comparative sensitivity of eggs,
larvae, and adults of the estuarine teleosts, Fundulus heteroclitus
and Menidia menidia to cadmium. Bull. environ. Contam. Toxicol., 17:
645-652.
MIRENDA, R.J. (1986) Toxicity and accumulation of cadmium in the
crayfish, Orconectes virilis (Hagen). Arch. environ. Contam.
Toxicol., 15: 401-407.
MITCHELL, C.D. & FRETZ, T.A. (1977) Cadmium and zinc toxicity in white
pine, red maple and Norway spruce. J. Am. Soc. Hortic. Sci., 102:
81-84.
MITRA, R.S., GRAY, R.H., CHIN, B., & BERNSTEIN, I.A. (1975) Molecular
mechanisms of accommodation in Escherichia coli to toxic levels of
Cd2+. J. Bacteriol., 121: 1180-1188.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & LENTZOU, P. (1979)
Effects of sublethal concentrations of cadmium as possible indicators
of cadmium pollution for two populations of Acartia clausi
(Copepoda) living at two differently polluted areas. Bull. environ.
Contam. Toxicol., 23: 642-649.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & ROGDAKIS, I. (1982)
Evaluation of the stress exerted by a polluted environment to a marine
organism by comparative toxicity tests. Bull. environ. Contam.
Toxicol., 28: 416-423.
MORGAN, J.E. & MORGAN, A.J. (1988) Earthworms as biological monitors
of cadmium, copper, lead and zinc in metalliferous soils. Environ.
Pollut., 54: 123-138.
MORGAN, J.E. & MORGAN, A.J. (1990) The distribution of cadmium,
copper, lead, zinc and calcium in the tissues of the earthworm
Lumbricus rubellus sampled from one uncontaminated and four polluted
soils. Oecologia, 84: 559-566.
MUINO, C.V., FERRARI, L., & SALIBIAN, A. (1990) Protective action of
ions against cadmium toxicity to young Bufo arenarum tadpoles. Bull.
environ. Contam. Toxicol., 45: 313-319.
MUIR, D.C.G., WAGEMANN, R., GRIFT, N.P., NORSTROM, R.J., SIMON, M. &
LIEN, J. (1988) Organochlorine chemical and heavy metal contaminants
in white-beaked dolphins ( Lagenorhynchus albirostris) and pilot
whales ( Globicephala melaena) from the coast of Newfoundland,
Canada. Arch. environ. Contam. Toxicol., 17: 613-629.
MURAMOTO, S. (1981a) Influence of complexans (EDTA, DTPA) on the
toxicity of cadmium to fish at chronic levels. Bull. environ. Contam.
Toxicol., 26: 641-646.
MURAMOTO, S. (1981b) Variations of some elements in cadmium-induced
malformed fish. Bull. environ. Contam. Toxicol., 27: 193-200.
MUSKETT, C.J. & JONES, M.P. (1980) The dispersal of lead, cadmium and
nickel from motor vehicles and effects on roadside invertebrate
macrofauna. Environ. Pollut., 23A: 231-242.
MYSING-GUBALA, M. & POIRRIER, M.A. (1981) The effects of cadmium and
mercury on gemmule formation and gemmosclere morphology in Ephydatia
fluviatilis (Porifera: Spongillidae). Hydrobiologia, 76: 145-148.
NAIDU, C.K. & REDDY, T.K.R. (1988) Effect of cadmium on microorganisms
and microbe-mediated mineralization process in the soil. Bull.
environ. Contam. Toxicol., 41: 657-663.
NAKADA, M., FUKAYA, K., TAKESHITA, S., & WADA, Y. (1979) The
accumulation of heavy metals in the submerged plant ( Elodea
nuttallii). Bull. environ. Contam. Toxicol., 22: 21-27.
NEBEKER, A.V., CAIRNS, M.A., ONJUKKA, S.T., & TITUS, R.H. (1986)
Effect of age on sensitivity of Daphnia magna to cadmium, copper and
cyanazine. Environ. Toxicol. Chem., 5: 527-530.
NEGILSKI, D.S. (1976) Acute toxicity of zinc, cadmium and chromium to
the marine fishes, yellow-eye mullet ( Aldrichetta forsteri C & V)
and small-mouthed hardyhead ( Atherinasoma microstoma Whitley). Aust.
J. mar. freshwater Res., 27: 137-149.
NEGILSKI, D.S., AHSANULLAH, M., & MOBLEY, M.C. (1981) Toxicity of
zinc, cadmium and copper to the shrimp Callianassa australiensis. II
Effects of paired and triad combinations of metals. Mar. Biol., 64:
305-309.
NELSON, D.A., CALABRESE, A., NELSON, B.A., MACINNES, J.R., & WENZLOFF,
D.R. (1976) Biological effects of heavy metals on juvenile bay
scallops, Argopecten irradians, in short-term exposures. Bull.
environ. Contam. Toxicol., 16: 275-282.
NICHOLSON, J.K. & OSBORN, D. (1983) Kidney lesions in pelagic seabirds
with high tissue levels of cadmium and mercury. J. Zool. (Lond.), 200:
99-118.
NICHOLSON, J.K., KENDALL, M.D., & OSBORN, D. (1983) Cadmium and
mercury nephrotoxicity. Nature (Lond.), 304: 633-635.
NIEDERLEHNER, B.R., BUIKEMA, A.L., PITTINGER, C.A., & CAIRNS, J.
(1984) Effects of cadmium on the population growth of a benthic
invertebrate Aeolosoma headleyi (Oligochaeta). Environ. Toxicol.
Chem., 3: 255-262.
NIMMO, D.R., LIGHTNER, D.V., & BAHNER, L.H. (1977) Effects of cadmium
on the shrimps Penaeus duorarum, Palaemonetes pugio and
Palaemonetes vulgaris. In: Vernberg, F.J., Calabrese, A., Thurberg,
F.P., & Vernberg, W.B., ed. Physiological responses of marine biota to
pollutants, New York, London, Academic Press, pp. 131-183.
NIMMO, D.R., RIGBY, R.A., BAHNER, L.H., & SHEPPARD, J.M. (1978) The
acute and chronic effects of cadmium on the estuarine mysid,
Mysidopsis bahia. Bull. environ. Contam. Toxicol., 19: 80-85.
NIR, R., GASITH, A., & PERRY, A.S. (1990) Cadmium uptake and toxicity
to water hyacinth: effect of repeated exposures under controlled
conditions. Bull. environ. Contam. Toxicol., 44: 149-157.
NORBERG, A.B. & MOLIN, N. (1983) Toxicity of cadmium, cobalt, uranium
and zinc to Zoogloea ramigera. Water Res., 17: 1333-1336.
NRIAGU, J.O. (1989) A global assessment of natural sources of
atmospheric trace metals. Nature (Lond.), 338: 47-49.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
O'HARA, J. (1973) The influence of temperature and salinity on the
toxicity of cadmium to the fiddler crab, Uca pugilator. Fish. Bull.,
71: 149-153.
OLLA, B.L., ESTELLE, V.B., SWARTZ, R.C., BRAUN, G., & STUDHOLME, A.L.
(1988) Responses of polychaetes to cadmium-contaminated sediment:
Comparison of uptake and behavior. Environ. Toxicol. Chem., 7:
587-592.
OLSON, P.-E. & HAUX, C. (1986) Increased hepatic metallothionein
content correlates to cadmium accumulation in environmentally exposed
perch ( Perca fluviatilis). Aquat. Toxicol., 9: 231-242.
OSBORN, D. & NICHOLSON, J.K. (1984) Cadmium and mercury in seabirds,
Huntingdon, UK Natural Environment Research Council, Institute of
Terrestrial Ecology, pp. 30-34 (ITE Symposium No. 12).
PAGE, A.L. & CHANG, A.C. (1978) Trace elements impact on plants during
cropland disposal of sewage sludges. In: Proceedings of the 5th
National Conference on Acceptable Sludge Disposal Techniques,
Rockville, Maryland, Information Transfer Inc., pp. 91-96.
PAGE, A.L., BINGHAM, F.T., & CHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, London, Applied
Science, Vol. 1, pp. 77-109.
PART, P. & WIKMARK, G. (1984) The influence of certain complexing
agents (EDTA and citrate) on the uptake of cadmium in perfused rainbow
trout gills. Aquat. Toxicol., 5: 277-289.
PART, P., SVANBERG, O., & KIESSLING, A. (1985) The availability of
cadmium to perfused rainbow trout gills in different water qualities.
Water Res., 19: 427-434.
PASCOE, D., EVANS, S.A., & WOODWORTH, J. (1986) Heavy metal toxicity
to fish and the influence of water hardness. Arch. environ. Contam.
Toxicol., 15: 481-487.
PECON, J. & POWELL, E.N. (1981) Effects of the amino acid histidine on
the uptake of cadmium from the digestive system by the blue crab
Callinectes sapidus. Bull. environ. Contam. Toxicol., 27: 34-41.
PENTREATH, R.J. (1977) The accumulation of cadmium by the plaice
Pleuronectes platessa L. and the thornback ray Raia clavata. J.
exp. mar. Biol. Ecol., 30: 223-232.
PEREZ-COLL, C.S., HERKOVITS, J., & SALIBIAN, A. (1986) Teratogenic
effects of cadmium on Bufo arenarum during gastrulation. Experientia
(Basel), 42: 1174-1176.
PESCH, G.G. & STEWART, N.E. (1980) Cadmium toxicity to three species
of estuarine invertebrates. Mar. environ. Res., 3: 145-156.
PETERSON, P.J. & ALLOWAY, B.J. (1979) Cadmium in soils and vegetation.
In: Webb, M., ed. The chemistry, biochemistry and biology of cadmium,
Amsterdam, Elsevier/North Holland, pp. 45-92.
PICKERING, Q.H. & GAST, M.H. (1972) Acute and chronic toxicity of
cadmium to the fathead minnow Pimephales promelas. J. Fish Res.
Board. Can., 29: 1099-1106.
PICKERING, Q.H. & HENDERSON, C. (1966) The acute toxicity of some
heavy metals to different species of warmwater fishes. Int. J. Air
water Pollut., 10: 453-463.
PIETZ, R.I., PETERSON, J.R., PRATER, J.E., & ZENZ, D.R. (1984) Metal
concentrations in earthworms from sewage sludge-amended soils at a
strip mine reclamation site. J. environ. Qual., 13: 651-654.
PINOWSKA, B., KRASNICKI, K., & PINOWSKI, J. (1981) Estimation of the
degree of contamination of granivorous birds with heavy metals in
agricultural and industrial landscape. Ekol. Polska, 29: 137-149.
POLDOSKI, J.E. (1979) Cadmium bioaccumulation assays. Their
relationships to various ion equilibria in Lake Superior water.
Environ. Sci. Technol., 13: 701-706.
POPHAM, J.D. & WEBSTER, J.M. (1979) Cadmium toxicity in the
free-living nematode, Caenorhabditis elegans. Environ. Res., 20:
183-191.
PRAHALAD, A.K. & SEENAYYA, G. (1988) Bioavailability of zinc and
cadmium and their effect on microbial growth and metal uptake. Bull.
environ. Contam. Toxicol., 41: 921-927.
PRITZL, M.C., LIE, Y.H., KIENHOLZ, E.W., & WHITEMAN, C.E. (1974) The
effect of dietary cadmium on development of young chickens. Poult.
Sci., 53: 2026-2029.
RAINBOW, P.S. & WHITE, S.L. (1989) Comparative strategies of heavy
metal accumulation by crustaceans: zinc, copper and cadmium in a
decapod, an amphipod and a barnacle. Hydrobiologia, 174: 245-262.
RAMAMOORTHY, S. & BLUMHAGEN, K. (1984) Uptake of Zn, Cd and Hg by fish
in the presence of competing compartments. Can. J. Fish aquat. Sci.,
41: 750-756.
RAMIREZ, P., BARRERA, G., & ROSAS, C. (1989) Effects of chromium and
cadmium upon respiration and survival of Callinectes similis. Bull.
environ. Contam. Toxicol., 43: 850-857.
RAY, S., MCLEESE, D.W., WAIWOOD, B.A., & PEZZACK. D. (1980a) The
disposition of cadmium and zinc in Pandalus montagui. Arch. environ.
Contam. Toxicol., 9: 675-681.
RAY, S., MCLEESE, D., & PEZZACK, D. (1980b) Accumulation of cadmium by
Nereis virens. Arch. environ. Contam. Toxicol., 9: 1-8.
REBHUN, S. & BEN-AMOTZ, A. (1984) The distribution of cadmium between
the marine alga Chlorella stigmatophora and sea water medium. Water
Res., 18: 173-178.
REHWOLDT, R. & KARIMIAN-TEHERANI, D. (1976) Uptake and effect of
cadmium on zebrafish. Bull. environ. Contam. Toxicol., 15: 442-446.
REID, S.D. & MCDONALD, D.G. (1988) Effects of cadmium, copper and low
pH on ion fluxes in rainbow trout, Salmo gairdneri. Can. J. Fish
aquat. Sci., 45: 244-253.
RIISGARD, H.U., BJORNESTAD, E., & MOHLENBERG, F. (1987) Accumulation
of cadmium in the mussel Mytilus edulis: Kinetics and importance of
uptake via food and sea water. Mar. Biol., 96: 349-353.
ROBERT, R. & HIS, E. (1985) Combined effects of salinity and cadmium
chloride upon embryos and larvae of the Japanese oyster, Crassostrea
gigas. Mar. environ. Res., 15: 303-312.
ROBERTS, M.H., WARINNER, J.E., CHU-FA TSAI, WRIGHT, D., & CRONIN, L.E.
(1982) Comparison of estuarine species sensitivities to three
toxicants. Arch. environ. Contam. Toxicol., 11: 681-692.
ROBERTS, R.D. & JOHNSON, M.S. (1978) Dispersal of heavy metals from
abandoned mine workings and their transference through terrestrial
food chains. Environ. Pollut., 16: 293-310.
ROBERTS, T.M., HEPPLESTON, P.B., & ROBERTS, R.D. (1976) Distribution
of heavy metals in tissues of the common seal. Mar. Pollut. Bull., 7:
194-196.
ROBINSON, A.M., LAMBERSON, J.O., COLE, F.A., & SWARTZ, R.C. (1988)
Effects of culture conditions on the sensitivity of a phoxocephalid
amphipod, Rhepoxynius abronius, to cadmium in sediment. Environ.
Toxicol. Chem., 7: 953-959.
ROBINSON, K.M. & WELLS, M.R. (1975) Retention of a single oral dose of
cadmium in tissues of the softshell turtle, Trionyx spinifer. Bull.
environ. Contam. Toxicol., 14: 750-752.
ROCH, M. & MALY, E.J. (1979) Relationship of cadmium-induced
hypocalcemia with mortality in rainbow trout ( Salmo gairdneri) and
the influence of temperature on toxicity. J. Fish Res. Board Can., 36:
1297-1303.
ROMBOUGH, P.J. & GARSIDE, E.T. (1982) Cadmium toxicity and
accumulation in aggs and alevins of Atlantic salmon Salmo salar. Can.
J. Zool., 60: 2006-2014.
ROOT, R.A., MILLER, R.J., & KOEPPE, D.E. (1975) Uptake of cadmium -
its toxicity, and effect on the iron ratio in hydroponically grown
corn. J. environ. Qual., 4: 473-476.
ROSENBERG, R. & COSTLOW, J.D. (1976) Synergistic effects of cadmium
and salinity combined with constant and cycling temperatures on the
larval development of two estuarine crab species. Mar. Biol., 38:
219-303.
RUSSELL, L.K., DEHAVEN, J.I., & BOTTS, R.P. (1981) Toxic effects of
cadmium on the garden snail ( Helix aspersa). Bull. environ. Contam.
Toxicol., 26: 634-640.
SANGALANG, G.B. & FREEMAN, H.C. (1979) Tissue uptake of cadmium in
brook trout during chronic sublethal exposure. Arch. environ. Contam.
Toxicol., 8: 77-84.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SASTRY, K.V. & SUBHADRA, K.M. (1983) Cadmium induced alterations in
the intestinal absorption of glucose and fructose in a freshwater
catfish Heteropneustes fossilis. Water Air Soil Pollut., 20:
293-297.
SATO, C., SCHNOOR, J.L., & MCDONALD, D.B. (1986) Characterization of
effects of copper, cadmium and nickel on the growth of Nitrosomonas
europaea. Environ. Toxicol. Chem., 5: 403-416.
SAWICKA-KAPUSTA, K., GORECKI, A., SWIERGOSZ, R., JUSZCZAK, G.,
MIELCZAREK, M., & WOJCIK, B. (1987) Effect of metabolic rate on the
rate of elimination of high and low concentrations of cadmium and lead
in the bank vole. Ekol. Polska, 35: 399-430.
SCHEUHAMMER, A.M. (1987) The chronic toxicity of aluminium, cadmium,
mercury and lead in birds: a review. Environ. Pollut., 46: 263-295.
SCHUYTEMA, G.S., NELSON, P.O., MALUEG, K.W., NEBEKER, A.V., KRAWCZYK,
D.F., RATCLIFF, A.K., & GAKSTATTER, J.H. (1984) Toxicity of cadmium in
water and sediment slurries to Daphnia magna. Environ. Toxicol.
Chem., 3: 293-308.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta, 51:
3273-3277.
SHIVARAJ, K.M. & PATIL, H.S. (1988) Toxicity of cadmium and copper to
a freshwater fish Puntius arulius. Environ. Ecol., 6: 5-8.
SHORE, R.F., MYHILL, D.G., POLWORTH, G., & DUNMOW, M. (1991) The
effects of chronic doses of dietary cadmium on macro-element balance
in wood mice and bank voles. In: Momciloric, B., ed. Proceedings of
the Seventh International Symposium on Trace Elements in Man and
Animals (TEMA-7), Dubrovnik, Yugoslavia, 20-25 May 1990, Zagreb, IMI
(Institute for Medical Research and Occupational Health, University of
Zagreb), pp. 17-18.
SILLEN, L.G. & MARTELL, A.E. (1964) Stability constants of metal-ion
complexes. Section I: Inorganic ligands compiled by L.G. Sillen.
Section II: Organic ligands compiled by A.E. Martell, London, The
Chemical Society, 754 pp (Special Publication No. 17).
SIMON, E. (1977) Cadmium tolerance in populations of Agrostis tenuis
and Festuca ovina. Nature (Lond.), 265: 328-330.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SJOBECK, M.-L., HAUX, C., LARSSON, A., & LITHNER, G. (1984)
Biochemical and hematological studies on perch, Perca fluviatilis,
from the cadmium-contaminated river Eman. Ecotoxicol. environ. Saf.,
8: 303-312.
SKOWRONSKI, T., PAWLIK, B., & JAKUBOWSKI, M. (1988) Reduction of
cadmium toxicity to green microalga Stichococcus bacillaris by
manganese. Bull. environ. Contam. Toxicol., 41: 915-920.
SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the usefulness of the
mexican axolotl ( Ambystoma mexicanum) and the clawed toad ( Xenopus
laevis) in toxicological bioassays. Bull. environ. Contam. Toxicol.,
24: 439-443.
SMITH, B.P., HEJTMANCIK, E., & CAMP, B.J. (1976) Acute effects of
cadmium on Ictalurus punctatus (catfish). Bull. environ. Contam.
Toxicol., 15: 271-277.
SMITH, R.M. & MARTELL, A.E. (1974) In: Critical stability constants,
Vol. 1: Amino acids, New York, Plenum Press, 469 pp.
SMITH, R.M. & MARTELL, A.E. (1975) In: Critical stability constants,
Vol. 2: Amines, New York, Plenum Press, 415 pp.
SMITH, R.M. & MARTELL, A.E. (1976) In: Critical stability constants,
Vol. 3: Other organic ligands, New York, Plenum Press, 495 pp.
SPEHAR, R.L., ANDERSON, R.L., & FIANDT, J.T. (1978a) Toxicity and
bioaccumulation of cadmium and lead in aquatic invertebrates. Environ.
Pollut., 15A: 195-208.
SPEHAR, R.L., LEONARD, E.N., & DEFOE, D.L. (1978b) Chronic effects of
cadmium and zinc mixtures on flagfish ( Jordanella floridae). Trans.
Am. Fish. Soc., 107: 354-360.
SPRAGUE, J. (1985) Factors that modify toxicity. In: Rand, G.M. &
Petrocelli, S.R., ed. Fundamentals of aquatic toxicology, New York,
Hemisphere Publishing Corporation, pp. 124-163.
SPRY, D.J. & WOOD, C.M. (1989) A kinetic method for the measurement of
zinc influx in vivo in the rainbow trout, and the effects of
waterborne calcium on flux rates. J. exp. Biol., 142: 425-446.
STROJAN, C.L. (1978) Forest leaf litter decomposition in the vicinity
of a zinc smelter. Oecologia, 32: 203-212.
SULLIVAN, J.F., MURPHY, B.R., ATCHISON, G.J., & MCINTOSH, A.W. (1978a)
Time dependant cadmium uptake by fathead minnows ( Pimephales
promelas) during field and laboratory exposure. Hydrobiologia, 57:
65-68.
SULLIVAN, J.F., ATCHISON, G.J., KOLAR, D.J., & MCINTOSH, A.W. (1978b)
Changes in the predator-prey behavior of fathead minnows ( Pimephales
promelas) and largemouth bass ( Micropterus salmoides) caused by
cadmium. J. Fish Res. Board. Can., 35: 446-451.
SUNDA, W.G., ENGEL, D.W., & THUOTTE, R.M. (1978) Effect of chemical
speciation on toxicity of cadmium to grass shrimp, Palaemonetes
pugio: Importance of free cadmium ion. Environ. Sci. Technol., 12:
409-413.
TAYLOR, D. (1983) The significance of the accumulation of cadmium by
aquatic organisms. Ecotoxicol. environ. Saf., 7: 33-42.
TERAOKA, H. (1989) Impact of atmospheric trace metals on rice roots.
Arch. environ. Contam. Toxicol., 18: 269-275.
THURBERG, F.P., DAWSON, M.A., & COLLIER, R.S. (1973) Effects of copper
and cadmium on osmoregulation and oxygen consumption in two species of
estuarine crabs. Mar. Biol., 23: 171-175.
TJALVE, H., GOTTOFREY, J., & BJORKLUND, I. (1986) Tissue disposition
of 109Cd2+ in the brown trout ( Salmo trutta) studied by
autoradiography and impulse counting. Toxicol. environ. Chem., 12:
31-45.
TYLER, G. (1972) Heavy metals pollute nature, may reduce productivity.
Ambio, 1: 52-59.
TYLER, G., MORNSJO, B., & NILSSON, B. (1974) Effects of cadmium, lead,
and sodium salts on nitrification in a mull soil. Plant Soil, 40:
237-242.
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North Sea.
I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VAN HATTUM, B., DE VOOGT, P., VAN DEN BOSCH, L., VAN STRAALEN, N.M.,
JOOSSE, E.N.G., & GOVERS, H. (1989) Bioaccumulation of cadmium by the
freshwater isopod Asellus aquaticus (L.) from aqueous and dietary
sources. Environ. Pollut., 62: 129-151.
VAN HOOK, R.I. (1974) Cadmium, lead, and zinc distributions between
earthworms and soils: potentials for biological accumulation. Bull.
environ. Contam. Toxicol., 12: 509-512.
VAN HOOK, R.I. & YATES, A.J. (1975) Transient behavior of cadmium in
a grassland arthropod food chain. Environ. Res., 9: 76-83.
VAN KESSEL, W.H.M., BROCADES ZAALBERG, R.W., & SEINEN, W. (1989)
Testing environmental pollutants on soil organisms: A simple assay to
investigate the toxicity of environmental pollutants on soil
organisms, using CdCl2 and nematodes. Ecotoxicol. environ. Saf., 18:
181-190.
VAN LEEUWEN, C.J., LUTTMER, W.J., & GRIFFIOEN, P.S. (1985) The use of
cohorts and populations in chronic toxicity studies with Daphnia
magna: A cadmium example. Ecotoxicol. environ. Saf., 9: 26-39.
VAN STRAALEN, N.M. & VAN WENSEM, J. (1986) Heavy metal content of
forest litter arthropods as related to body-size and trophic level.
Environ. Pollut., 42A: 209-221.
VAN STRAALEN, N.M., BURGHOUTS, T.B.A., DOORNHOF, M.J., GROOT, G.M.,
JANSSEN, M.P.M., JOOSSE, E.N.G., VAN MEERENDONK, J.H., THEEUWEN,
J.P.J.J., VERHOEF, H.A., & ZOOMER, H.R. (1987) Efficiency of lead and
cadmium excretion in populations of Orchesella cincta (Collembola)
from various contaminated forest soils. J. appl. Ecol., 24: 953-968.
VAN STRAALEN, N.M., SCHOBBEN, J.H.M., & DE GOEDE, R.G.M. (1989)
Population consequences of cadmium toxicity in soil microarthropods.
Ecotoxicol. environ. Saf., 17: 190-204.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1987)
Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am. J.
Physiol., 253: R216-R221.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1988)
Cadmium inhibits plasma membrane calcium transport. J. membrane Biol.,
102: 97-104.
VERBOST, P.M., VAN ROOIJ, J., FLIK, G., LOCK, R.A.C., & WENDELAAR
BONGA, S.E. (1989) The movement of cadmium through freshwater trout
branchial epithelium and its interference with calcium transport. J.
exp. Biol., 145: 185-197.
VERNBERG, W.B., DE COURSEY, P.J., & O'HARA, J. (1974) Multiple
environmental factor effects on physiology and behavior of the fiddler
crab, Uca pugilator. In: Vernberg, F.G. & Vernberg, W.B., ed.
Pollution and physiology of marine organisms, New York, London,
Academic Press, pp. 381-425.
VERNBERG, W.B., DE COURSEY, P.J., KELLY, M., & JOHNS, D.M. (1977)
Effects of sublethal concentrations of cadmium on adult Palaemonetes
pugio under static and flow-through conditions. Bull. environ.
Contam. Toxicol., 17: 16-24.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1981) Effects of some
environmental factors on the toxicity of cadmium to the copepod Tisbe
holothuriae. Arch. Hydrobiol., 91: 287-293.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1982) Differentiation
of the sensitivity to copper and cadmium in different life-stages of
a copepod. Mar. Pollut. Bull., 13: 123-125.
VOYER, R.A. (1975) Effect of dissolved oxygen concentration on the
acute toxicity of cadmium to the mummichog, Fundulus heteroclitus
(L.), at various salinities. Trans. Am. Fish. Soc., 104: 129-134.
VOYER, R.A. & MODICA, G. (1990) Influence of salinity and temperature
on acute toxicity of cadmium to Mysidopsis bahia Molenock. Arch.
environ. Contam. Toxicol., 19: 124-131.
VOYER, R.A., YEVICH, P.P., & BARSZCZ, C.A. (1975) Histological and
toxicological responses of the mummichog, Fundulus heteroclitus
(L.), to combinations of levels of cadmium and dissolved oxygen in a
freshwater. Water Res., 9: 1069-1074.
WALTERSSON, E. (1984) [Effects of enrichment reagents - distribution
and spread of xanthates and xanthate derivatives during enrichment
activities], Stockholm, Swedish Environmental Research Institute
(Report B478) (in Swedish).
WATLING, H.R. & WATLING, R.J. (1983) Sandy beach molluscs as possible
bioindicators of metal pollution. 2. Laboratory studies. Bull.
environ. Contam. Toxicol., 31: 339-343.
WATSON, C.F. & BENSON, W.H. (1987) Comparative activity of gill
ATPases in three freshwater teleosts exposed to cadmium. Ecotoxicol.
Environ. Saf., 14: 252-259.
WEIS, J.S. & WEIS, P. (1977) Effects of heavy metals on development of
the killifish, Fundulus heteroclitus. J. Fish Biol., 11: 49-54.
WESTERMAN, A.G. (1977) Lethal and teratogenic effects of inorganic
mercury and cadmium on embryonic development of Anurans, Lexington,
University of Kentucky (M.S. Thesis).
WHITE, D.H. & FINLEY, M.T. (1978) Uptake and retention of dietary
cadmium in mallard ducks. Environ. Res., 17: 53-59.
WHITE, D.H., FINLEY, M.T., & FERRELL, J.F. (1978) Histopathologic
effects of dietary cadmium on kidneys and testes of mallard ducks. J.
Toxicol. environ. Health, 4: 551-558.
WHO (1992) Environmental Health Criteria 134: Cadmium. Geneva, World
Health Organization, 280 pp.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WICKLUND, A. (1990) Metabolism of cadmium and zinc in fish, University
of Uppsala, Sweden, Faculty of Science (Acta Uiversitatis Uppsaliensis
- Comprehensive summaries of Uppsala dissertations from the Faculty of
Science, No. 250).
WIER, C.F. & WALTER, W.M. (1976) Toxicity of cadmium in the freshwater
snail, Physa gyrina Say. J. environ. Qual., 5: 359-362.
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soil and plants. Aust. J. soil Res., 11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILLIAMS, D.R. & GIESY, J.P. (1978) Relative importance of food and
water sources to cadmium uptake by Gambusia affinis ( Poeciliidae).
Environ. Res., 16: 326-332.
WILLIAMS, K.A., GREEN, D.W.J., & PASCOE, D. (1985) Studies on the
acute toxicity of pollutants to freshwater macroinvertebrates. I.
Cadmium. Arch. Hydrobiol., 102: 461-471.
WILLIAMS, K.A., GREEN, D.W.J., PASCOE, D., & GOWER, D.E. (1987) Effect
of cadmium on oviposition and egg viability in Chironomus riparius
(Diptera: Chironomidae). Bull. environ. Contam. Toxicol., 38: 86-90.
WILLIAMS, P.L. & DUSENBERY, D.B. (1990) Aquatic toxicity testing using
the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem., 9:
1285-1290.
WILLIAMSON, P. (1980) Variables affecting body burdens of lead, zinc
and cadmium in a roadside population of the snail Cepaea hortensis
Muller. Oecologia, 44: 213-220.
WILSON, D.N. (1988) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium Conference,
London, Cadmium Association, pp. 9-16.
WINNER, R.W. (1986) Interactive effects of water hardness and humic
acid on the chronic toxicity of cadmium to Daphnia pulex. Aquat.
Toxicol., 8: 281-293.
WINNER, R.W. (1988) Evaluation of the relative sensitivities of 7-d
Daphnia magna and Ceriodaphnia dubia toxicity tests for cadmium
and sodium pentachlorophenate. Environ. Toxicol. Chem., 7: 153-159.
WONG, P.T.S., BURNISON, G., & CHAU, Y.K. (1979) Cadmium toxicity to
freshwater algae. Bull. environ. Contam. Toxicol., 23: 487-490.
WONG, Y.S., LAM, H.M., DHILLON, E., TAM, N.F.Y., & LEUNG, W.N. (1988)
Physiological effects and uptake of cadmium in Pisum sativum.
Environ. Int., 14: 535-543.
WOODALL, C., MACLEAN, N., & CROSSLEY, F. (1988) Responses of trout fry
( Salmo gairdneri) and Xenopus laevis tadpoles to cadmium and zinc.
Comp. Biochem. Physiol., 89C: 93-99.
WRIGHT, D.A. & FRAIN, J.W. (1981a) Cadmium toxicity in Marinogammarus
obtusatus. Effect of external calcium. Environ. Res., 24: 338-344.
WRIGHT, D.A. & FRAIN, J.W. (1981b) The effect of calcium on cadmium
toxicity in the freshwater amphipod, Gammarus pulex (L.). Arch.
environ. Contam. Toxicol., 10: 321-328.
WRIGHT, D.A., METEYER, M.J., & MARTIN, F.D. (1985) Effect of calcium
on cadmium uptake and toxicity in larvae and juveniles of striped bass
( Morone saxatilis). Bull. environ. Contam. Toxicol., 34: 196-204.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in perspective.
Bull. Inst. Public Health (Tokyo), 24: 18-24.
ZAROOGIAN, G.E. & CHEER, S. (1976) Accumulation of cadmium by the
American oyster, Crassostrea virginica. Nature (Lond.), 261:
408-410.
ZAROOGIAN, G.E. & MORRISON, G. (1981) Effect of cadmium body burdens
in adult Crassostrea virginica on fecundity and viability of larvae.
Bull. environ. Contam. Toxicol., 27: 344-348.
ZIRINO, A. & YAMAMOTO, S. (1972) A pH-dependent model for the chemical
speciation of copper, zinc, cadmium and lead in seawater. Limnol.
Oceanogr., 17(5): 661-671.
Appendix 1. Global emissions of trace metals from natural sources (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Wind-borne soil particles
range 0.3-5.0 0.01-0.4 0.9-15 0-0.01 0.3-7.5 0.01-0.35 3.0-35
median 2.6 0.21 8.0 0.05 3.9 0.18 19
Sea salt spray
range 0.19-3.1 0-0.11 0.23-6.9 0-0.04 0.02-2.8 0-1.1 0.02-0.86
median 1.7 0.06 3.6 0.02 1.4 0.55 0.44
Volcanoes
range 0.15-7.5 0.14-1.5 0.9-18 0.03-2.0 0.54-6.0 0.10-1.8 0.31-19
median 3.8 0.82 9.4 1.0 3.3 0.95 9.6
Forest fires
range 0-0.38 0-0.22 0.1-7.5 0-0.05 0.06-3.8 0-0.52 0.3-15
median 0.19 0.11 3.8 0.02 1.9 0.26 7.6
Biogenic continental particulates
range 0.2-0.5 0-0.83 0.1-5.0 0-0.04 0.02-2.5 0-0.25 0.3-5.0
median 0.26 0.15 2.6 0.02 1.3 1.12 2.6
Biogenic continental volatiles
range 0.03-2.5 0-0.8 0.01-0.62 0.02-1.2 0.01-0.38 0.15-5.0 0.02-5.0
median 1.3 0.04 0.32 0.61 0.20 2.6 2.5
Appendix 1 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Biogenic marine
range 0.16-4.5 0-0.1 0.02-0.75 0.04-1.5 0.02-0.45 0.4-9.0 0.04-6.0
median 2.3 0.05 0.39 0.77 0.24 4.7 3.0
Total emission
range 0.86-23 0.15-2.6 2.3-54 0.1-4.9 0.97-23 0.66-18 4.0-86
median 12 1.3 28 2.5 12 9.3 45
a From: Nriagu (1989)
Appendix 2. Natural and anthropogenic emissions of trace metals to the atmosphere in 1983 (x 1000 tonnes/year) a
Trace metal Anthropogenic source Natural source Total emission Natural/total emissions
Arsenic 19 (12-26) 12 (0.86-23) 31 (13-49) 0.39
Cadmium 7.6 (3.1-12) 1.3 (0.15-2.6) 8.9 (3.2-15) 0.15
Copper 35 (20-51) 28 (2.3-54) 63 (22-105) 0.44
Mercury 3.6 (0.91-6.2) 2.5 (0.10-4.9) 6.1 (1.0-11) 0.41
Lead 332 (289-376) 12 (0.97-23) 344 (290-399) 0.04
Selenium 6.3 (3.0-9.7) 9.3 (0.66-18) 16 (2.5-24) 0.58
Zinc 132 (70-194) 45 (4.0-86) 177 (74-280) 0.34
a From: Nriagu (1989)
Appendix 3. Sources of global emissions of trace elements to the atmosphere in 1983 (tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Coal combustion - electric utilities
232-1550 77-387 930-3100 155-542 775-4650 108-775 1085-7750
Coal combustion - industry and domestic
198-1980 99-495 1390-4950 495-2970 990-9900 792-1980 1485-11 880
Oil combustion - electric utilities
5.8-29 23-174 348-2230 - 232-1740 35-290 174-1280
Oil combustion - industry and domestic
7.2-72 18-72 179-1070 - 716-2150 107-537 358-2506
Pyrometallurgical non-ferrous metal production - mining
40.0-80 0.6-3 160-800 - 1700-3400 18-176 310-620
Pyrometallurgical non-ferrous metal production - Pb production
780-1560 39-195 234-312 7.8-16 11 700-31 200 195-390 195-468
Pyrometallurgical non-ferrous metal production - Cu-Ni production
8500-12 750 1700-3400 14 450-33 600 37-207 11 050-22 100 427-1280 4250-8500
Pyrometallurgical non-ferrous metal production - Zn-Cd production
230-690 920-4600 230-690 - 5520-11 500 92-23 46 000-82 800
Secondary non-ferrous metal production
- 2.3-3.6 55-165 - 90-1440 3.8-19 270-1440
Steel and iron manufacturing
355-2480 28-284 142-2840 - 1065-14 200 0.8-2.2 7100-31 950
Refuse incineration - municipal
154-392 56-1400 980-1960 140-2100 1400-2800 28-70 2800-8400
Refuse incineration - sewage sludge
15-60 3-36 30-180 15-60 240-300 3-30 150-450
Appendix 3 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Phosphate fertilizers
- 68-274 137-685 - 55-274 0.4-1.2 1370-6850
Cement production
178-890 8.9-534 - - 18-14 240 - 1780-17 800
Wood combustion
60-300 60-180 600-1200 60-300 1200-3000 - 1200-6000
Mobile sources (gasoline)
- - - - 248 030 - -
Miscellaneous
1250-2800 - - - 3900-5100 - 1724-4783
Total emissions - range
12 000-25 630 3100-12 040 19 860-50 870 910-6200 288 700-376 000 1810-5780 70 250-193 500
Total emissions - median
18 820 7570 35 370 3560 332 350 3790b 131 880
a From: Nriagu & Pacyna (1988)
b This value applies to particulate selenium only. Since volatile selenium accounts for about
40% of the selenium released, the total selenium emission is estimated to be 6300 tonnes per year.
Appendix 4. Anthropogenic inputs of trace elements to aquatic ecosystems (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
3.6-7.7 0.9-3.6 6.0-15 0.22-1.8 87-113 0.54-1.1 21-58
Other industrial sources
8.4-62.3 1.2-13.4 29-75 0.08-7.0 10-67 9.5-70.9 56-317
Total - range
12-70 2.1-17 35-90 0.3-8.8 97-180 10-72 77-375
Total - median
41 9.4 62 4.6 138 41 226
Atmospheric fallout/total anthropogenic inputs (%)
14 24 17 22 72 2 17
a From: Nriagu & Pacyna (1988)
Appendix 5. Anthropogenic inputs of trace elements to soils (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
8.4-18 2.2-8.4 14-36 0.63-4.3 202-263 1.3-2.6 49-135
Other industrial sources
43.6-94 3.4-29.6 527-1331 0.97-10.7 277-850 4.7-73.4 640-1919
Total input - range b
52-112 5.6-38 541-1367 1.6-15 479-1113 6.0-76 689-2054
Total input - median b
82 22 954 8.3 796 41 1372
Atmospheric fallout/total anthropogenic inputs (%) b
16 24 3 30 29 5 7
a From: Nriagu & Pacyna (1988)
b These data do not include inputs from mine tailings, smelter slags and wastes to land.
RESUME
Le cadmium (numéro atomique 48; masse atomique relative 112,40)
est un élément métallique qui appartient, avec le zinc et le mercure,
au groupe IIb du tableau périodique. Certains sels de cadmium, tels
que le sulfure, le carbonate et l'oxyde sont presque insolubles dans
l'eau; ils peuvent cependant être transformés en sels solubles dans
l'environnement naturel. La formation de différents dérivés du cadmium
dans l'environnement est importante pour l'évaluation du risque.
La teneur moyenne en cadmium de l'eau de mer est d'environ 0,1
µg/litre ou moins. L'eau des cours d'eau contient du cadmium dissous
à des concentrations allant de < 1 à 13,5 ng/litre. Dans les régions
écartées et inhabitées, on observe en général des concentrations de
cadmium dans l'air inférieures à 1 ng/m3. Dans les régions où l'on
ne connaît pas de pollution, la concentration médiane en cadmium dans
le sol se situerait entre 0,2 et 0,4 mg/kg. Toutefois, on rencontre
occasionnellement des valeurs beaucoup plus élevées pouvant aller
jusqu'à 160 mg/kg de terre.
Certains facteurs environnementaux influent sur la fixation, et
par voie de conséquence, sur les effets toxiques du cadmium sur les
organismes aquatiques. Une élévation de température augmente la
fixation et l'effet toxique du cadmium qui sont en revanche réduits
lorsque la salinité de l'eau ou sa dureté augmentent. Les organismes
d'eau douce sont sensibles à des concentrations plus faibles de
cadmium que les organismes marins. Plus la teneur de l'eau en matières
organiques est élevée, plus la fixation et les effets toxiques du
cadmium sont réduits car ces matières se lient au cadmium et en
réduisent la biodisponibilité. Toutefois, on est fondé à croire que
certains dérivés organiques pourraient avoir un effet inverse.
Le cadmium s'accumule facilement dans de nombreux organismes, en
particulier les microorganismes et les mollusques, pour lesquels le
facteur de bioconcentration peut atteindre plusieurs milliers. Les
invertébrés terricoles concentrent égale-ment assez fortement le
cadmium. Pour la plupart des organismes, le facteur de concentration
est faible à modéré, généralement inférieur à 100. Dans de nombreux
tissus, le cadmium est lié aux protéines. On a isolé d'organismes
exposés au cadmium, des pro-téines qui fixent spécifiquement les
métaux lourds (métallothio-néines). Le cadmium se concentre
préférentiellement dans les reins, les branchies et le foie (ou leurs
équivalents). L'élimination du métal s'effectue probablement par le
rein, encore que chez les crustacés, il puisse être éliminé en
quantités notables en passant dans l'exosquelette. Chez les plantes,
le cadmium se concentre principalement dans les racines et à un
moindre degré dans les feuilles.
Le cadmium est toxique pour de nombreux microorganismes.
Toutefois, la présence de sédiments et de fortes concentrations de
sels ou de matières organiques en solution réduit ces effets toxiques.
Ces effets s'exercent principalement sur la croissance et la
réplication. Parmi les microorganismes terricoles, les plus affectés
sont les champignons, dont certaines espèces peuvent être éliminées
lorsque le sol est contaminé par du cadmium. Une faible exposition au
cadmium présent dans le sol peut entraîner une sélection des souches
résistantes. La toxicité du cadmium pour les organismes aquatiques est
variable, même lorsqu'il s'agit d'espèces très proches et elle est
liée à la concentration du métal sous forme ionique. Le cadmium
perturbe le métabolisme du calcium chez l'animal. Chez les poissons,
il provoque une hypocalcémie, probablement en inhibant la fixation du
calcium à partir de l'eau. Toutefois, la présence de fortes
concentrations de calcium dans l'eau protège les poissons par
inhibition compétitive de la fixation de cadmium. Le zinc accroît la
toxicité du cadmium pour les invertébrés aquatiques. On a fait état
d'effets sublétaux sur la croissance et la reproduction d'invertébrés
aquatiques; des effets ont été également observés sur la structure des
branchies d'invertébrés. Le cadmium est plus ou moins toxique pour les
poissons, les salmonidés étant particulièrement sensibles. On a
signalé des effets sublétaux chez les poissons, en particuliers des
malformations de l'épine dorsale. Les stades les plus sensibles sont
les embryons et les jeunes larves, les moins sensibles étant les
oeufs. Chez les poissons, on n'observe pas d'interaction systématique
entre le cadmium et le zinc. Le cadmium est toxique pour certaines
larves d'amphibiens, mais on a constaté que la présence de sédiments
dans les aquariums expérimentaux apportait une certaine protection.
Le cadmium perturbe la croissance des végétaux au laboratoire,
mais aucun effet n'a été observé dans la nature. Les plantes captent
plus facilement le métal lorsqu'il est présent dans les solutions
nutritives que lorsqu'il est dans le sol; les effets observés l'ont
été essentiellement lors d'études portant sur des cultures
hydroponiques. Il semblerait que le cadmium présent dans les solutions
nutritives affecte l'ouverture des stomates, la transpiration et la
photosynthèse.
Les invertébrés terrestres sont relativement insensibles aux
effets toxiques du cadmium, probablement à cause de l'intervention de
mécanismes de séquestration efficaces au niveau des divers organes.
Les gastéropodes terrestres peuvent subir des effets sublétaux,
principalement en ce qui concerne la consommation de nourriture et la
dormance, mais uniquement à des doses très élevées. Même à forte dose,
le cadmium n'entraîne pas la mortalité des oiseaux mais peut provoquer
des lésions rénales.
Des études effectuées sur le terrain ont montré que le cadmium
pouvait entraîner la modification de la proportion relative des
diverses espèces dans les populations de microorganismes et de
certains invertébrés aquatiques. La décomposition des feuilles mortes
est fortement entravée par une forte pollution due aux métaux lourds,
et le cadmium en serait le principal responsable.
RESUMEN
El cadmio (número atómico 48; masa atómica relativa 112,40) es un
elemento metálico que pertenece, junto con el zinc y el mercurio, al
grupo IIb de la tabla periódica. Algunas sales de cadmio, como el
sulfuro, el carbonato y el óxido, son prácticamente insolubles en
agua; pueden convertirse en sales hidrosolubles en el medio natural.
El sulfato, el nitrato y los haluros son hidrosolubles. La especiación
del cadmio en el medio ambiente tiene importancia para evaluar su
potencial de riesgo.
El contenido medio de cadmio en el agua de mar es de alrededor de
0,1 µg/litro o menos. El agua de los ríos contiene cadmio disuelto en
concentraciones que varían entre < 1 y 13,5 ng/litro. En zonas
aisladas y deshabitadas, las concentraciones de cadmio en el aire
suelen ser inferiores a 1 ng/m3. En zonas que se suponen no
contaminadas, se ha comunicado que la concentración mediana de cadmio
en el suelo se encuentra entre 0,2 y 0,4 mg/kg. No obstante, a veces
se encuentran valores mucho más altos, que pueden llegar hasta 160
mg/kg de suelo.
Los factores ambientales influyen en la captación y, por ende, en
los efectos tóxicos del cadmio en los organismos acuáticos. Al
aumentar la temperatura aumentan la captación y los efectos tóxicos,
mientras que el aumento de la salinidad o de la dureza del agua los
hace disminuir. Los organismos de agua dulce sufren los efectos del
cadmio en concentraciones inferiores a las que afectan a los
organismos marinos. La materia orgánica contenida en el agua suele
reducir la captación y los efectos tóxicos fijando el cadmio y
reduciendo su disponibilidad para los seres vivos. Sin embargo, hay
pruebas de que cierto tipo de materia orgánica puede ejercer el efecto
contrario.
El cadmio se acumula fácilmente en numerosos seres vivos,
particularmente microorganismos y moluscos, en los que los factores de
bioconcentración son del orden de varios millares. Los invertebrados
del suelo también concentran este metal en grado considerable. La
mayoría de los organismos presentan factores de concentración bajos o
moderados (inferiores a 100). El cadmio está ligado a proteínas en
numerosos tejidos. En organismos expuestos a ese metal se han aislado
proteínas fijadoras de metales pesados (metalotioneínas). La
concentración de cadmio es más elevada en el riñón, las branquias y el
hígado (o sus equivalentes). La eliminación se produce probablemente
por vía renal, si bien los crustáceos pueden eliminar cantidades
importantes con la muda del exoesqueleto. En las plantas, el cadmio se
concentra principalmente en las raíces y, en menor medida, en las
hojas.
El cadmio tiene efectos tóxicos para muy diversos
microorganismos. No obstante, la presencia de sedimentos y las
concentraciones elevadas de sales disueltas o materia orgánica reducen
esos efectos. Los procesos más afectados son el crecimiento y la
replicación. Los organismos del suelo más vulnerables son los hongos:
algunas especies desaparecen tras la exposición al cadmio en el suelo.
Tras exposiciones reducidas al metal en el suelo, se observa una
selección a favor de las cepas resistentes.
La toxicidad aguda del cadmio para los organismos acuáticos es
variable, incluso entre especies estrechamente emparentadas, y guarda
relación con la concentración de iones libres del metal. El cadmio
interacciona con el metabolismo del calcio en los animales. En los
peces provoca hipocalcemia, probablemente al inhibir la captación de
calcio a partir del agua. No obstante, las concentraciones elevadas de
calcio en el agua los protegen de la ingestión de cadmio por
competencia en los lugares de captación. El zinc aumenta la toxicidad
del cadmio para los invertebrados acuáticos. Se han notificado efectos
subletales en el crecimiento y la reproducción de invertebrados
acuáticos, así como modificaciones estructurales en las branquias de
invertebrados. Hay pruebas de la selección de estirpes resistentes de
invertebrados acuáticos tras la exposición al cadmio sobre el terreno.
La toxicidad es variable en los peces; los salmónidos son
especialmente susceptibles. Se han notificado efectos subletales en
los peces, en particular malformaciones de la espina dorsal. Las fases
biológicas más susceptibles son el embrión y la larva joven; los
huevos son los menos vulnerables. No se ha observado una interacción
homogénea entre el cadmio y el zinc en los peces. El cadmio resulta
tóxico para algunas larvas de anfibios, si bien los sedimentos en el
recipiente de ensayo confieren cierta protección.
El cadmio afecta al crecimiento de las plantas en estudios
experimentales, pero sobre el terreno no se ha observado efecto
alguno. El metal es absorbido por las plantas con más rapidez a partir
de soluciones de nutrientes que a partir del suelo; los efectos se han
observado sobre todo en estudios de cultivo en soluciones de
nutrientes. En éstas se ha notificado que el cadmio influye en la
apertura de los estomas, la transpiración y la fotosíntesis.
Los invertebrados terrestres son relativamente insensibles a los
efectos tóxicos del cadmio, probablemente debido a la existencia de
mecanismos eficaces de captura y fijación en ciertos órganos.
Los gasterópodos terrestres sufren efectos subletales; los
principales procesos afectados son el consumo de alimentos y el
letargo, pero sólo con dosis muy elevadas. El metal no produce efectos
letales en las aves, ni siquiera con dosis elevadas, si bien se
observan lesiones renales.
En estudios de campo se ha comunicado que el cadmio induce
cambios en la composición de especies en las poblaciones de
microorganismos y ciertos invertebrados acuáticos. La descomposición
del mantillo de hojas se ve notablemente reducida por la contaminación
con metales pesados; se ha identificado al cadmio como el principal
causante de este efecto.
See Also:
Toxicological Abbreviations

 Table 2. Equilibrium data for complexes of group IIB cations a
System Metal log K1 DELTA H1 DELTA S1
(kJ mol-1) (J K-1 mol-1)
zinc 5.0 b 0 b 105
M2+-OH- cadmium 3.9 b 0 79
mercury 10.6 b - -
zinc 0.8 7.5 42
M2+-F- cadmium 0.6 4.2 25
mercury 1.0 c 4.2 c 33 c
zinc - 0.2 5.4 16
M2+-Cl- cadmium 1.5 - 0.4 29
mercury 7.1 - 24.3 54
zinc - 0.6 1.7 - 4
M2+-Br- cadmium 1.7 - 4.2 21
mercury 9.4 - 40.1 46
zinc - 1.5 - -
M2+-I- cadmium 2.1 - 9.2 8
mercury 12.9 c - 75.3 c - 8 c
zinc 5.3 - -
M2+-CN- cadmium 5.6 - 30.5 b 13 b
mercury 18.0 c - 96 b 0 b
zinc 0.7 d - 5.9 d - 4 d
M2+-SCN- cadmium 1.3 d - 9.6 d - 8d
mercury 9.1 d - 49.7 d 8
zinc 1.9 - -
M2+-S2O32- e cadmium 4.7 - 6.3 d 67 d
mercury 29.9 d - -
zinc 2.4 f - 10.9 f 8 f
M2+-NH3 cadmium 2.7 f - 14.6 f 4 f
mercury 8.8 f - -
zinc 4.8 c - 11.3 g 59 g
2+ - cadmium 4.1 d - 8.8 b 50 g
(glycinate)- mercury 10.3 c - -
zinc 16.4 - 20.5 247
M2+-(EDTA)4- cadmium 16.4 - 38.1 184
mercury 21.5 - 79.0 146
a From: Aylett (1979). Data, which refer to first stepwise stability
constant, [ML]/[M][L], unless otherwise stated, are from Sillen
(1964) and Smith & Martell (1974, 1975, 1976); see also Christensen
et al. (1975). All values refer to measurements in water at 25 °C;
the ionic strength is 3 mol/litre unless otherwise stated.
b ionic strength 0
c ionic strength 0.5 mol/litre
d ionic strength 1.0 mol/litre
e Data refer to overall stability constant, ß2 = [ML2]/[M][L]2
f ionic strength 2.0 mol/litre
g ionic strength 0.1 mol/litre
Table 2. Equilibrium data for complexes of group IIB cations a
System Metal log K1 DELTA H1 DELTA S1
(kJ mol-1) (J K-1 mol-1)
zinc 5.0 b 0 b 105
M2+-OH- cadmium 3.9 b 0 79
mercury 10.6 b - -
zinc 0.8 7.5 42
M2+-F- cadmium 0.6 4.2 25
mercury 1.0 c 4.2 c 33 c
zinc - 0.2 5.4 16
M2+-Cl- cadmium 1.5 - 0.4 29
mercury 7.1 - 24.3 54
zinc - 0.6 1.7 - 4
M2+-Br- cadmium 1.7 - 4.2 21
mercury 9.4 - 40.1 46
zinc - 1.5 - -
M2+-I- cadmium 2.1 - 9.2 8
mercury 12.9 c - 75.3 c - 8 c
zinc 5.3 - -
M2+-CN- cadmium 5.6 - 30.5 b 13 b
mercury 18.0 c - 96 b 0 b
zinc 0.7 d - 5.9 d - 4 d
M2+-SCN- cadmium 1.3 d - 9.6 d - 8d
mercury 9.1 d - 49.7 d 8
zinc 1.9 - -
M2+-S2O32- e cadmium 4.7 - 6.3 d 67 d
mercury 29.9 d - -
zinc 2.4 f - 10.9 f 8 f
M2+-NH3 cadmium 2.7 f - 14.6 f 4 f
mercury 8.8 f - -
zinc 4.8 c - 11.3 g 59 g
2+ - cadmium 4.1 d - 8.8 b 50 g
(glycinate)- mercury 10.3 c - -
zinc 16.4 - 20.5 247
M2+-(EDTA)4- cadmium 16.4 - 38.1 184
mercury 21.5 - 79.0 146
a From: Aylett (1979). Data, which refer to first stepwise stability
constant, [ML]/[M][L], unless otherwise stated, are from Sillen
(1964) and Smith & Martell (1974, 1975, 1976); see also Christensen
et al. (1975). All values refer to measurements in water at 25 °C;
the ionic strength is 3 mol/litre unless otherwise stated.
b ionic strength 0
c ionic strength 0.5 mol/litre
d ionic strength 1.0 mol/litre
e Data refer to overall stability constant, ß2 = [ML2]/[M][L]2
f ionic strength 2.0 mol/litre
g ionic strength 0.1 mol/litre
 2.2 Analytical procedures
The following is an outline of the analytical procedure for
cadmium; further information is given in Environmental Health Criteria
134: Cadmium (WHO, 1992).
2.2.1 Sampling and preparation
Only a few nanograms, or even less, of cadmium may be present in
collected samples of air or water, whereas hundreds of micrograms may
be present in small samples of kidney, sewage sludge, and plastics.
Different techniques are, therefore, required for the collection,
preparation, and analysis of the samples.
In general, the techniques available for measuring cadmium in the
environment and biological materials cannot differentiate between
cadmium species. With special separation techniques,
cadmium-containing proteins can be isolated and identified. In most
studies, the concentration or amount of cadmium in water, air, soil,
plants, and other environmental or biological material is determined
as the element.
Standard trace element methods can generally be used for the
collection of samples (LaFleur, 1976; Behne, 1980). During the
handling and storage of samples, particularly liquid samples, special
care must be taken to avoid contamination; coloured materials in
containers, especially plastics and rubber, should be avoided. Glass
and transparent, cadmium-free polyethylene, polypropylene or teflon
containers are usually considered suitable for storing samples. All
containers and glassware should be precleaned in dilute nitric acid
and deionised water. In order to avoid possible adsorption of cadmium
onto the container wall, water samples or standards with low cadmium
concentrations should not be stored for long periods of time.
To prepare samples for analysis, inorganic solid samples (such as
soil or dust samples) are usually dissolved in an acid, e.g., nitric
acid. Organic samples need to be subjected to wet ashing (digested) or
dry ashing. When the cadmium concentration is low, special treatment
is sometimes needed. The procedures for separating cadmium from
interfering compounds and concentrating the samples are very important
steps in obtaining accurate results.
2.2.2 Quantitative instrumental methods
The most commonly used methods, at present, are atomic absorption
spectrometry, electrochemical methods, neutron activation analysis,
atomic emission spectrometry, atomic fluorescence spectrometry and
proton-induced X-ray emissions (PIXE) analysis. Analytical methods for
cadmium have been reviewed by Friberg et al. (1986). Detection limits
of some of the methods are given in Table 3.
Table 3. Analytical procedures a
Method Detection limit Matrix
Atomic absorption 1 to 5 mg/litre water
spectrometry
0.1 mg/kg biological samples
electrothermal a few pg
atomization
Electrochemical method
(potentiometric stripping
analysis) 0.1 mg/litre urine
Neutron activation 0.1 to 1 mg/litre biological
analysis samples/fluids
X-ray atomic 17 mg/kg biological samples
fluorescence
a From: Friberg et al. (1986)
3. NATURAL OCCURRENCE AND SOURCES OF ENVIRONMENTAL CONTAMINATION
3.1 Natural occurrence
A comparison of natural and anthropogenic sources of trace metals
is given in the Appendix 1.
Cadmium is widely distributed in the earth's crust at an average
concentration of about 0.1 mg/kg and is commonly found in association
with zinc. However, higher levels are present in sedimentary rocks:
marine phosphates often contain about 15 mg/kg (GESAMP, 1984).
Weathering and erosion result in the transport by rivers of large
quantities of cadmium to the world's oceans and this represents a
major flux of the global cadmium cycle; an annual gross input of 15
000 tonnes has been estimated (GESAMP, 1987).
In background areas away from ore bodies, surface soil
concentrations of cadmium typically range between 0.1 and 0.4 mg/kg
(Page et al., 1981). The median cadmium concentration in non-volcanic
soil ranges from 0.01 to 1 mg/kg, but in volcanic soil levels of up to
4.5 mg/kg have been found (Korte, 1983).
Volcanic activity is a major natural source of atmospheric
cadmium release. The global annual flux from this source has been
estimated to be 100-500 tonnes (Nriagu, 1979). Deep sea volcanism is
also a source of environmental cadmium release, but the role of this
process in the global cadmium cycle remains to be quantified.
The average cadmium content of sea water is about 0.1 µg/litre or
less (Korte, 1983), while river water (Mississippi, Yangtze, Amazon,
and Orinoco sampled between 1976 and 1982) contains dissolved cadmium
at concentrations of < 1.1-13.5 ng/litre (Shiller & Boyle, 1987).
Cadmium levels of up to 5 mg/kg have been reported in river and lake
sediments and from 0.03 to 1 mg/kg in marine sediments (Korte,1983).
Current measurements of dissolved cadmium in surface waters of
the open oceans give values of < 5 ng/litre. The vertical
distribution of dissolved cadmium in ocean waters is characterized by
a surface depletion and deep water enrichment, which corresponds to
the pattern of nutrient concentrations in these areas (Boyle et al.,
1976). This distribution is considered to result from the absorption
of cadmium by phytoplankton in surface waters and its transport to the
depths, incorporation to biological debris, and subsequent release. In
contrast, cadmium is enriched in the surface waters of areas of
upwelling and this also leads to elevated levels in plankton
unconnected with human activity (Martin & Broenkow, 1975; Boyle et
al., 1976). Oceanic sediments underlying these areas of high
productivity can contain markedly elevated cadmium levels as a result
of inputs associated with biological debris (Simpson, 1981).
In remote, uninhabited areas, cadmium concentrations in air are
usually less than 1 ng/m3 (Korte,1983).
3.2 Industrial uses
The principal applications of cadmium fall into five categories:
protective plating on steel; stabilizers for PVC; pigments in plastics
and glass; electrode material in nickel-cadmium batteries; and as a
component of various alloys (Wilson, 1988).
The relative importance of the major applications has changed
considerably over the last 25 years. The use of cadmium for
electroplating represented in 1960 over half the cadmium consumed
worldwide, but in 1985 its share was less than 25% (Wilson, 1988).
This decline is usually linked to the introduction of stringent
effluent limits from plating works and, more recently, to the
introduction of general restrictions on cadmium consumption in certain
countries. In contrast, the use of cadmium in batteries has shown
considerable growth in recent years from only 8% of the total market
in 1970 to 37% by 1985. The use of cadmium in batteries is
particularly important in Japan and represented over 75% of the total
consumption in 1985 (Wilson, 1988).
Pigments and stabilizers accounted for 22% and 12% of the total
world consumption in 1985. The share of the market by cadmium pigments
remained relatively stable between 1970 and 1985 but the use of the
metal in stabilizers during this period showed a considerable decline,
largely as a result of economic factors. The use of cadmium as a
constituent of alloys is relatively small and has also declined in
importance in recent years, accounting for about 4% of total cadmium
use in 1985 (Wilson, 1988).
3.3 Sources of environmental cadmium
3.3.1 Sources of atmospheric cadmium
Estimates of cadmium emissions to the atmosphere from human and
natural sources have been carried out at the worldwide, regional, and
national level; examples of such inventories are shown in Table 4.
The median global total emission of the metal from human sources
in 1983 was 7570 tonnes (Nriagu & Pacyna, 1988) and represented about
half the total quantity of cadmium produced in the same year. In both
the European Economic Community (EEC) and on a worldwide scale
(Nriagu, 1989), about 10-15% of total airborne cadmium emissions arise
from natural processes, the major source being volcanic action.
Municipal refuse contains cadmium derived from discarded
nickel-cadmium batteries and plastics containing cadmium pigments and
stabilizers. The incineration of refuse is a major source of
atmospheric cadmium release at country, regional, and worldwide level
(Table 4).
Steel production can also be considered as a waste-related
source, as large quantities of cadmium-plated steel scrap are recycled
by this industry. As a result, steel production is responsible for
considerable emissions of atmospheric cadmium.
3.3.2 Sources of aquatic cadmium
Non-ferrous metal mines represent a major source of cadmium
release to the aquatic environment. Contamination can arise from mine
drainage water, waste water from the processing of ores, overflow from
the tailings pond, and rainwater run-off from the general mine area.
The release of these effluents to local watercourses can lead to
extensive contamination downstream of the mining operation. Mines
disused for many years can still be responsible for the continuing
contamination of adjacent watercourses (Johnson & Eaton, 1980).
At the global level, the smelting of non-ferrous metal ores has
been estimated to be the largest human source of cadmium release to
the aquatic environment (Nriagu & Pacyna, 1988). Discharges to fresh
and coastal waters arise from liquid effluents produced by air
pollution control (gas scrubbing) together with the site drainage
waters.
Table 4. Estimates of atmospheric cadmium emissions (tonnes/year) on a national, regional and worldwide basis
Source United EEC b Worldwide c
Kingdom a
Natural sources ND 20 150-2600 d
Non-ferrous metal
production
mining ND ND 0.6-3
zinc and cadmium 20 920-4600
copper 3.7 6 1700-3400
lead 7 39-195
Secondary production ND 2.3-3.6
Production of cadmium-containing
substances ND 3 ND
Iron and steel production 2.3 34 28-284
Fossil fuel combustion
coal 1.9 6 176-882
oil 0.5 41-246
Refuse incineration 5 31 56-1400
Sewage sludge incineration 0.2 2 3-36
Table 4 (contd).
Source United EEC b Worldwide c
Kingdom a
Phosphate fertilizer manufacture ND ND 68-274
Cement manufacture 1 ND 8.9-534
Wood combustion ND ND 60-180
TOTAL EMISSIONS 14 130 3350-14 640
a From: Hutton & Symon (1986); data apply to 1982-1983
b From: Hutton (1983); data apply to 1979-1980 (the EEC consisted,
at that time, of Belgium, Denmark, Federal Republic of Germany,
Italy, Luxembourg, The Netherlands, Republic of Ireland, and the
United Kingdom)
c From: Nriagu & Pacyna (1988); data apply to 1983
d From: Nriagu (1979)
ND Not determined
The manufacture of phosphate fertilizer results in a
redistribution of the cadmium present in the rock phosphates between
the phosphoric acid product and gypsum waste. In many cases, the
gypsum is disposed of by dumping in coastal waters, which leads to
considerable cadmium inputs. Some countries, however, recover the
gypsum for use as a construction material and thus have negligible
cadmium discharges (Hutton, 1982).
The atmospheric fallout of cadmium to fresh and marine waters
represents a major input of cadmium at the global level (Nriagu &
Pacyna, 1988). A GESAMP study of the Mediterranean Sea indicated that
this source is comparable in magnitude to the total river inputs of
cadmium to the region (GESAMP, 1985). Similarly, large cadmium inputs
to the North Sea (110-430 tonnes/year) have also been estimated, based
on the extrapolation from measurements of cadmium deposition along the
coast (van Alst et al., 1983a,b). However, another approach based on
model simulation yielded a modest annual cadmium input of 14 tonnes
(Krell & Roeckner, 1988).
Acidification of soils and lakes may result in enhanced
mobilization of cadmium from soils and sediments and lead to increased
levels in surface and ground waters (WHO Working Group, 1986).
3.3.3 Sources of terrestrial cadmium
Solid wastes are disposed of in landfill sites, resulting in
large cadmium inputs at the national and regional levels when
expressed as total tonnage (Hutton, 1982; Hutton & Symon, 1986).
Sources include the ashes from fossil fuel combustion, waste from
cement manufacture, and the disposal of municipal refuse and sewage
sludge.
Of greater potential environmental significance are the solid
wastes from both non-ferrous metal production and the manufacture of
cadmium-containing articles, as well as the ash residues from refuse
incineration. These three waste materials are characterized by
elevated cadmium levels and as such require disposal to controlled
sites to prevent the contamination of the ground water.
The agricultural application of phosphate fertilizers represents
a direct input of cadmium to arable soils. The cadmium content of
phosphate fertilizers varies widely and depends on the origin of the
rock phosphate. It has been estimated that fertilizers of West African
origin contain 160-255 g cadmium/tonne of phosphorus pentoxide, while
those derived from the southeastern USA contain 35 g/tonne (Hutton,
1982).
The annual rate of cadmium input to arable land from phosphate
fertilizers has been estimated at 5 g/ha for the countries of the EEC
(Hutton 1982). This only represents about 1% of the surface soil
cadmium burden. Despite the relatively small size of this input,
long-term continuous application of phosphate fertilizers has been
shown to cause increased soil cadmium concentrations (Williams &
David, 1973, 1976; Andersson & Hahlin, 1981).
The application of municipal sewage sludge to agricultural soils
as a fertilizer can also be a significant source of cadmium; a value
of 80 g/ha has been estimated for the United Kingdom (Hutton & Symon,
1986). On a national or regional basis, however, these inputs are much
smaller than those from either phosphate fertilizers or atmospheric
deposition (see section 3.4).
Polluted soils can contain cadmium levels of up to 57 mg/kg (dry
weight) resulting from sludge applied to soil and up to 160 mg/kg in
the vicinity of metal-processing industry (Fleischer et al., 1974).
The highest cadmium levels reported appear to be from ancient mining
areas with levels of up to 468 mg/kg.
3.4 Environmental transport and distribution
3.4.1 Atmospheric deposition
Cadmium is removed from the atmosphere by dry deposition and by
precipitation. In rural areas of Scandinavia, annual deposition rates
of 0.4-0.9 g/ha have been measured (Laamanen, 1972; Andersson, 1977).
Similarly, in a rural region of Tennessee, USA, a deposition rate of
0.9 g/ha was observed (Lindberg et al., 1982). Hutton (1982) suggested
that 3 g/ha per year was a representative value for the atmospheric
deposition of cadmium to agricultural soils in rural areas of the EEC.
The corresponding input for these areas from the application of
phosphate fertilizers is 5 g/ha per year (see section 3.3).
Many industrial sources of cadmium possess tall stacks which
bring about the wide dispersion and dilution of particulate emissions.
Nevertheless, cadmium deposition rates around smelter facilities are
often markedly elevated nearest the source and generally decrease
rapidly with distance (Hirata, 1981). Soil cadmium concentrations in
excess of 100 mg/kg are commonly encountered close to long established
smelters (Buchauer, 1972).
Crop plants growing near to atmospheric sources of cadmium may
contain elevated cadmium levels (Carvalho et al., 1986). However, it
is not always possible to distinguish whether the cadmium is derived
directly from surface deposition or originates from root uptake, since
soil levels in such areas are generally higher than normal.
3.4.2 Transport from water to soil
Rivers contaminated with cadmium can contaminate surrounding
land, either through irrigation for agricultural purposes, by the
dumping of dredged sediments, or through flooding (Forstner, 1980;
Sangster et al., 1984). For example, agricultural land adjacent to the
Neckar River, Germany, received dredged sediments to improve the soil,
a practice that produced soil cadmium concentrations in excess of 70
mg/kg (Forstner, 1980).
Much of the cadmium entering fresh waters from industrial sources
is rapidly adsorbed by particulate matter, where it may settle out or
remain suspended, depending on local conditions. This can result in
low concentrations of dissolved cadmium even in rivers that receive
and transport large quantities of the metal (Yamagata & Shigematsu,
1970)
3.5 Concentrations in various biota
Table 5 indicates the levels of cadmium found in various biota
(Eisler, 1985).
Eisler (1985) concluded that there are at least six trends
evident from the abundant residue data available for cadmium.
* Marine organisms generally contain higher cadmium residues than
their freshwater and terrestrial counterparts.
* Cadmium tends to concentrate in the viscera of vertebrates,
especially the liver and kidneys.
* Cadmium concentrations are generally higher in older organisms.
* Higher cadmium residues are generally associated with industrial
and urban sources, although this does not apply to sea birds and
sea mammals.
* Cadmium residues in plants are normally less than 1 mg/kg.
However, plants growing in soil amended with cadmium (e.g., from
sewage sludge) may contain significantly higher levels.
* The species analysed, season of collection, ambient cadmium
levels, and the sex of the organism probably all affect the
residue level.
Table 5. Concentrations of cadmium in biota
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Marine organisms
Algae < 1 to 16
Molluscs soft parts up to 425
kidney up to 547
liver up to 782
digestive gland up to 1163
Crustaceans whole body < 0.4-6.2
Annelids whole body 0.1-3.6
Fish whole body up to 5.2
Birds kidney up to 231
Mammals kidney up to 300
Freshwater organisms
Plants whole plant 0.5-1.8
roots up to 6.7
Molluscs soft parts; fresh weight 0.2-1.4
Annelids whole body; fresh weight 0.5-3.2
Fish whole body; fresh weight 0.01-1.04
Table 5 (contd).
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Terrestrial organisms
Plants whole plant up to 27.1
grain up to 257
Annelids whole body 3-12.6
Birds whole body; fresh weight < 0.05-0.24
kidney; fresh weight up to 7.4
Mammals kidney up to 8.1
3.5.1 Concentrations in fish
May & McKinney (1981) monitored freshwater fish from the USA in
1976 and 1977 and found cadmium concentrations ranging from 0.01 to
1.04 mg/kg (wet weight), the mean being 0.085 mg/kg. This represented
a significant decline from the mean 1972 concentration of 0.112 mg/kg.
The authors pointed out that this decline parallels a decline in
cadmium metal production and consumption over the same period.
Hardisty et al. (1974a) sampled flounder ( Platichthyes flesus)
from the Severn estuary, United Kingdom, and found mean cadmium
concentrations of 3.4-7.3 mg/kg (dry weight). No overall correlation
between cadmium concentration and length or age was observed, although
the largest (27-29 cm) and the oldest („ 5 years) fish gave the
highest mean concentrations. Hardisty et al. (1974b) found a positive
correlation between the cadmium content of a variety of fish species
and the crustacea content of their diet. Lovett et al. (1972) sampled
fish from New York State, USA, and reported mean cadmium
concentrations of < 10-142.7 µg/kg (fresh weight). There was no
relationship between total residues and size, sex or age of lake trout
( Salvelinus namaycush).
3.5.2 Concentrations in sea-birds
Cadmium has been found in a wide variety of birds, and
particularly high levels have been reported in pelagic sea-birds. Much
of the cadmium occurs in the kidney and liver, and relatively little
is transferred to the eggs. A review of the uptake of cadmium and of
the factors that affect it can be found in Scheuhammer (1987).
Interestingly, the concentrations of cadmium in sea-birds are often
higher in areas with little or no contamination from industrial
sources (Bull et al., 1977; Hutton, 1981; Osborn & Nicholson, 1984).
3.5.3 Concentrations in sea mammals
High levels of cadmium have been reported in sea mammals from
areas around the world, which they are assumed to take up from their
diet of fish. Roberts et al. (1976) showed that kidney levels of
cadmium in the common seal off the United Kingdom coast were age
related. Drescher et al. (1977) showed a similar relationship in seals
off the German coast and Hamanaka et al. (1982) in stellar sea lions
off the coast of Japan. Similar trends in dolphins and porpoises have
been reported (Falconer et al., 1983; Honda & Tatsukawa, 1983; Honda
et al., 1986). Muir et al. (1988) sampled white-beaked dolphins
( Lagenorhynchus albirostris) and pilot whales ( Globicephala
melaena) from the coast of Newfoundland, Canada, and reported mean
cadmium levels in kidney (dry weight) of 13.6 mg/kg and 108 mg/kg,
respectively. Cadmium concentrations were age related in pilot whales.
The lower levels found in dolphins were probably related both to
species differences and to the fact that they were all young animals.
3.6 Concentrations adjacent to highways
Muskett & Jones (1980) monitored levels of cadmium adjacent to a
heavily used road. The concentrations in air were highest at a
distance from the road of 0-10 m, and a similar pattern was found in
soil. Cadmium levels in earthworms sampled at known distances from a
highway revealed levels of 12.6 mg/kg (dry weight) within 3 m falling
to 7.1 mg/kg approximately 50 m from the highway. The level in
earthworms from control sites was 3 mg/kg (Gish & Christensen, 1973).
The land snail Cepaea hortensis accumulates cadmium from roadside
verges (Williamson, 1980). The highest concentration of cadmium was
found in the digestive gland (40.3 mg/kg dry weight) and kidney (12.8
mg/kg dry weight). There was little metal in the head and foot, which
make up most of the body tissue. The author showed that age accounted
for 80% of the total variance of soft tissue body burdens. The cadmium
body burdens were found to be effectively immobile, accumulating
progressively with age.
3.7 Concentrations from industrial sources
Burkitt et al. (1972) analysed the cadmium content of ryegrass at
various distances from a zinc smelter and found 50, 10.8, and 1.8
mg/kg dry weight at distances of 0.3, 1.9, and 11.3 km, respectively,
from the smelter.
Teraoka (1989) found that cadmium levels in rice roots were
significantly higher in industrial urban and roadside areas of Japan
compared to sparsely populated areas. The mean level in industrial
areas was 10 mg/kg (dry weight).
Beyer et al. (1985) monitored biota from the vicinity of two zinc
smelters in eastern Pennsylvania, USA. Cadmium concentrations were
highest in carrion insects (25 mg/kg dry weight), followed by fungi
(9.8 mg/kg), leaves (8.1 mg/kg), shrews (7.3 mg/kg), moths (4.9
mg/kg), mice (2.6 mg/kg), songbirds (2.5 mg/kg), and berries (1.2
mg/kg).
Van Hook (1974) sampled soil and earthworms from soil that had
not been disturbed for 30 years and reported mean cadmium levels in
the soils and earthworms of 0.35 and 5.7 mg/kg dry weight,
respectively. Ma et al. (1983) analysed soil and earthworms
( Lumbricus rubellus) at varying distances from a zinc-smelting
plant. Cadmium concentrations ranged from 0.1 to 5.7 mg/kg for the
soil and 20 to 202 mg/kg for the worms, and there was a correlation
between decreasing distance from the smelter and increasing cadmium
levels. Pietz et al. (1984) sampled soil and earthworms ( Aporrectodea
tuberculata) and ( Lumbricus terrestris) from mine soil and
non-mine soil, either amended or not with sewage sludge. Soil and
worms from mine soil gave residues of 0.6 and 3.8 mg/kg dry weight,
respectively, in non-amended soil and 2 and 22 mg/kg in sludge-amended
soil. Residues in soil and worms from non-mined soil were 1 and 12
mg/kg for non-amended and 3.5 and 36 mg/kg for sludge-amended soil,
respectively. The much lower capacity of worms from areas already
contaminated with cadmium to take up the metal suggests some selection
for varieties that control metal uptake. Morgan & Morgan (1988)
sampled earthworms ( Lumbricus rubellus and Dendrodrilus rubidus)
from one uncontaminated site and fifteen metal-contaminated sites (in
the vicinity of disused non-ferrous metalliferous mines) in the United
Kingdom. Cadmium concentrations in the worms ranged from 8 mg/kg (dry
weight) to 1786 mg/kg; they were generally higher than soil levels,
and the total soil cadmium explained 82% to 86% of the variability in
earthworm cadmium concentrations. The authors found some evidence that
cadmium accumulation was suppressed in extremely organic soils.
Martin et al. (1980) reported cadmium levels in a variety of
invertebrates sampled from sites contaminated by airborne cadmium. The
woodlouse was shown to accumulate cadmium principally in the
hepatopancreas.
Van Straalen & van Wensem (1986) analysed 13 species of
arthropods from an area polluted by zinc factory emissions. They found
no effect of body size or trophic level on the cadmium content of the
arthropods.
Roberts & Johnson (1978) sampled invertebrates and their diet
from the area of an abandoned lead-zinc mine in the United Kingdom.
They found cadmium levels higher in herbivorous invertebrates than in
the vegetation on which they fed (but not markedly so). There were
much higher levels of cadmium in carnivorous invertebrates, suggesting
that cadmium might have a capacity for accumulation in food chains.
In contrast to mercury levels, total cadmium body burdens were
higher in sparrows ( Passer domesticus) caught in industrialised
areas of Poland than in those caught in agricultural regions (Pinowska
et al., 1981). Pigeon brain, liver, and kidney sampled in rural,
suburban, and urban areas gave a good indication of the level of
environmental pollution with cadmium (Hutton & Goodman, 1980).
Hunter & Johnson (1982) monitored small mammals near to an
industrial works complex and found that cadmium accumulated
particularly in the liver and kidney. Cadmium levels in the liver
ranged rom 1.5 to 280 mg/kg (dry weight) and in the kidney from 7.4 to
193 mg/kg. Small mammals from unpolluted sites contained liver levels
ranging from 0.5 to 25 mg/kg and kidney levels of 1.5-26 mg/kg. The
insectivorous common shrew ( Sorex areneus) was found to be a more
prominent accumulator of cadmium than omnivorous and herbivorous small
mammals, based on body burden to dietary metal concentration ratios.
Similar results were obtained by Andrews et al. (1984) who monitored
cadmium levels in the herbivorous short-tailed field vole ( Microtus
agrestis) and the insectivorous common shrew ( S. araneus) from a
revegetated metalliferous mine site. Mean cadmium concentrations were
1.84 mg/kg (dry weight) and 52.7 mg/kg for voles and shrews,
respectively, values that were significantly higher than those found
in control sites.
4. KINETICS AND METABOLISM
Appraisal
In aquatic systems, cadmium is most commonly taken up by
organisms directly from water, but may also be ingested with
substantially contaminated food. The free metal ion, Cd2+, is the
form most available to aquatic species. Uptake from water may be
reduced by the concentration of calcium and magnesium salts (water
hardness). Cadmium uptake from sea water may be greatly reduced by
the formation of less available complexes with chloride. Organic
complexes with cadmium can be classified in three groups: those that
are unavailable (e.g., EDTA, NTA, DPTA), those that are available but
less so than the free Cd2+ (e.g., fulvic acids of low relative
molecular mass), and those that form readily available hydrophobic
complexes with cadmium (xanthates and dithiocarbamates).
Organisms in the freshwater environment are contaminated
according to their ability to absorb or adsorb cadmium from the
water, rather than to their position in the food chain. Consequently,
differences in cadmium concentration between species at the same
trophic level are common and there is no evidence for
biomagnification. Conversely, marine organisms take up cadmium
principally from food. The primary source of cadmium in terrestrial
systems is the soil, and uptake follows the typical food chain
pathway, although deposition of cadmium on plant and animal surfaces
can account for some additional contamination at each trophic level.
Variations in uptake and retention occur, and there is some evidence
for biomagnification in carnivores. Organisms that feed on sediment
or detritus may accumulate more cadmium than those in the grazing
food chain. High levels of cadmium have been reported in sea mammals,
pelagic sea-birds, and terrestrial invertebrates.
Within a variety of organisms, cadmium is distributed throughout
most tissues, but tends to accumulate in the roots, gills, livers,
kidneys, hepatopancreas, and exoskeleton. Cadmium in the cell is
often bound to cytoplasmic proteins, a possible detoxifying
mechanism. Elimination probably occurs primarily via the kidney but
also via moulting of the exoskeleton.
There is some evidence of an interaction between cadmium and
other metals, especially calcium and zinc. Cadmium may replace
calcium on the calcium-specific protein calmodulin and is affected by
other physiological processes that regulate the uptake of calcium. In
certain circumstances, zinc increases cadmium retention in the liver
and kidneys of aquatic vertebrates. In terrestrial systems, high soil
zinc levels can reduce cadmium uptake appreciably.
Selection can lead to cadmium-tolerant populations in both the
aquatic and terrestrial environments.
4.1 Uptake
4.1.1 Uptake from water by aquatic organisms
Several studies have shown that the free metal ion, Cd2+, is
the form of cadmium most available to aquatic organisms (Sunda et al.,
1978; Borgmann, 1983; Part et al., 1985; Sprague, 1985).
Inorganic cadmium complexes appear not to be taken up, at least
by fish (Part et al., 1985). This is particularly important in marine
water where cadmium is mainly present in soluble chloride complexes
(Zirino & Yamamoto 1972). It is most probable that chloride
complexation is responsible for the reduced cadmium accumulation and
toxicity in a variety of organisms observed with increasing salinities
(Coombs, 1979).
In the case of organic cadmium complexes, the chemical properties
are of importance with respect to bioavailability. Three categories
can be distinguished. The first comprises cadmium complexes with EDTA,
NTA, and DPTA, which are unavailable to aquatic organisms (Sunda et
al., 1978; Part & Wikmark, 1984). The second consists of complexes
that to some extent contribute to the total metal uptake, i.e. uptake
is higher than predicted from the actual Cd2+ activity, but the
complex is still less available than the free Cd2+ ion. This group
includes fulvic acids of low relative molecular mass (Giesy et al.,
1977; John et al., 1987), the amino acid histidine (Pecon & Powell,
1981), and carboxylic acids like citric acid (Guy & Ross Kean, 1980;
Part & Wikmark, 1984). The third category includes compounds such as
xanthates and dithiocarbamates that form hydrophobic complexes with
heavy metals. These hydrophobic complexes act as metal carriers across
biological membranes and they lead to a greater uptake of cadmium in
aquatic organisms than when the metal is present as the free ion
(Poldoski, 1979; Block & Part, 1986; Gottofrey et al., 1988; Block,
1991). This latter observation is of particular environmental concern
because xanthates are used in the mining industry in the enrichment of
metals from sulfide ores by flotation. Xanthate concentrations of
between 4 and 400 µg/litre have been measured in waters receiving
effluent from metal refineries (enrichment plants) (Waltersson, 1984).
Another water quality parameter affecting cadmium uptake is the
Ca2+ and Mg2+ concentration (hardness) of the water. Increasing
Ca2+ concentration reduces cadmium uptake through fish gills (Part
et al., 1985; Wicklund, 1990), cadmium accumulation (Carroll et al.,
1979), and cadmium toxicity for fish (Calamari et al., 1980). Two
mechanisms can be distinguished for the Ca2+-mediated reduction in
cadmium uptake. The first is an inhibitory effect on uptake into gill
tissue, while the second is related to the adaptive response of the
fish to increased Ca2+ concentrations (Calamari et al., 1980,
Wicklund 1990). Mg2+ also reduces cadmium uptake through fish gills
but at 5 times higher concentrations than Ca2+ (Part et al., 1985).
Cadmium uptake in fish is not strongly pH dependent; uptake in
rainbow trout gills was not affected over the pH range 5-7 (Part et
al., 1985).
Recent data from fish gills indicate that, to some extent, Cd2+
shares uptake mechanisms with Ca2+; these two ions are about the
same size and also form complexes with the same kind of ligands. Thus
Cd2+ can replace Ca2+ in the calcium-specific protein calmodulin
(Flik et al., 1987). In the gills, Cd2+ is assumed to enter the
epithelial cells down its concentration and electrical gradient by
facilitated diffusion through a calcium channel in the apical membrane
(Verbost et al., 1989). Several lines of evidence support this
assumption. Firstly, increasing water Ca2+ concentrations reduce
cadmium uptake. Secondly, cadmium in the water inhibits Ca2+ uptake
in the gills (Verbost et al., 1987; Reid & McDonald, 1988). Thirdly,
La3+, a calcium channel blocker in cell membranes, inhibits both
Ca2+ and Cd2+ uptake in the gills. Fourthly, the hypocalcaemic
hormone stanniocalcin reduces both Ca2+ and Cd2+ uptake in the
gills (Verbost et al., 1989). Stanniocalcin has been shown to close
the apical calcium channel in the gill epithelial cells thereby
reducing Ca2+ uptake from the water (Lafeber et al., 1988). The
hormone is secreted when the fish has a surplus of Ca2+, i.e.
hypercalcaemic. The two-fold effect of Ca2+ on cadmium uptake in
fish discussed previously can be well explained by this model. A
direct competition between Ca2+ and Cd2+ at the apical calcium
channel reduces the uptake of cadmium into the cells, while the
adaptive response in Ca2+-rich water probably involves an increased
stanniocalcin level, which closes the apical calcium/cadmium channel.
The transport mechanism from the epithelial cells to the blood is
unclear. Cadmium is not transported by the high affinity Ca-ATPase in
the basolateral epithelial membrane which transports Ca2+ (Verbost
et al., 1988). The possible involvement of the Na+/Ca2+ exchange
mechanism, where Cd2+ replaces Ca2+, has recently been suggested
as a translocation mechanism to the blood (personal communication to
the IPCS by G. Flik).
Zinc also has been shown to reduce cadmium uptake through the
gills (Wicklund, 1990). Like cadmium, zinc is assumed to enter the
epithelial cell by facilitated diffusion (Spry & Wood, 1989) and,
furthermore, Ca2+ acts antagonistically on zinc uptake.
Taken together, these data suggest that the apical epithelial
membrane of fish gills contains an ion channel shared by cadmium and
calcium, and probably also zinc. The movement of metals through this
channel is controlled both by external factors such as the Ca2+
content of the water and internal factors such as hormones.
Increasing temperature increases the uptake of cadmium from water
(Vernberg et al., 1974; Zaroogian & Cheer, 1976; Denton &
Burdon-Jones, 1981).
4.1.1.1 Microorganisms
In the alga Chlorella pyrenoidosa, uptake of cadmium was
completely blocked by 0.2 mg manganese/litre and inhibited by 2 to 5
mg iron/litre, but calcium, magnesium, molybdenum, copper, zinc, and
cobalt had no effect on uptake (Hart & Scaife, 1977).
Cultures of Chlorella accumulate twice as much cadmium at pH
7.0 as at pH 8.0 when exposed to 0.5 mg cadmium/litre (Hart & Scaife,
1977).
4.1.1.2 Aquatic molluscs
Hardy et al. (1984) found greater uptake of cadmium from sea
water into oysters given an uncontaminated phytoplankton food source
than into those without food. The authors explain their findings on
the basis that the presence of phytoplankton increases the flow of
water through the oysters. Studies on oysters without a food source
may thus underestimate cadmium uptake. Oysters fed phytoplankton
containing cadmium retained only 0.59% of this cadmium; the majority
of the cadmium in molluscs is taken up directly from the water. The
oyster accumulates about twice as much cadmium in summer as in the
winter. This is presumed to reflect the increased flow of water
through the animal at higher temperatures (Zaroogian & Cheer, 1976).
Hardy et al. (1981) showed that clams ( Protothaca staminea)
took up much less cadmium from water in the presence of sediment at
3.6 g/litre. The uptake was only 17% of that measured in sediment-free
water.
Langston & Zhou (1987a,b) found no evidence of cadmium uptake
into the bivalve Macoma balthica involving metallothionein or
metallothionein-like proteins. Accumulation in soft tissues was linear
throughout a 29-day exposure period, whereas uptake onto the shell was
characterized as saturation kinetics. In contrast, the gastropod
Littorina littorea did show induction of specific cadmium-binding
proteins, which contributed to uptake and storage of cadmium.
Watling & Watling (1983) demonstrated uptake of cadmium in a
dose-dependant manner into sandy beach gastropod molluscs in
laboratory experiments. Much of the cadmium (as chloride) accumulated
in the gill. The rate of cadmium uptake was 0.01 mg/kg per day for
Donax serra and 0.16 mg/kg per day for the smaller Bullia
rhodostoma after exposure to cadmium at 20 µg/litre. The freshwater
snail Physa integra took up more cadmium as exposure increased,
concentrations ranging between 1 and 40 µg/litre. The highest
concentration factors were found with the lowest exposure
concentration (Spehar et al., 1978a). Wier & Walter (1976) exposed the
freshwater snail Physa gyrina to 1.3 mg cadmium/litre (as the
chloride) and found an average cadmium uptake rate of 0.55 mg/kg per
hour over 24 h. Heavier snails took up less cadmium, after the same
exposure, than lighter individuals.
4.1.1.3 Other aquatic invertebrates
Rainbow & White (1989) investigated uptake of cadmium and zinc in
three marine crustaceans, Palaemon elegans (Decapoda),
Echinogammarus pirloti (Malacostraca), and Elminius modestus
(Cirripedia) at water concentrations of cadmium between 0.5 and 1000
µg/litre and zinc between 2.5 and 4000 µg/litre. All three crustaceans
accumulated the non-essential cadmium at all dissolved cadmium
concentrations without regulation. Differences between species were
interpreted by the authors in terms of differences in cuticle
permeability and way of life. All three species took up zinc more
rapidly than cadmium; the ratios between molar uptake rates of zinc to
cadmium were 11.4:1, 2.7:1, and 3.7:1 for the three species,
respectively, following an exposure to a molar ratio of 1.7:1.
4.1.1.4 Fish
Cadmium uptake in fish continues for some considerable time in
fish exposed to the metal. The peak of tissue residues may not be
reached for several weeks, particularly after exposure to low
concentrations of the metal (Cearley & Coleman, 1974; Benoit et al.,
1976; Sullivan et al., 1978a).
Douben (1989a) exposed the stone loach Noemacheilus barbatulus
to cadmium in water (as the sulfate) at a concentration of 1 mg/litre
and monitored uptake and loss at different temperatures with fed and
starved fish. The size of the fish affected both uptake and loss of
cadmium, bioconcentration factors decreasing with size. Uptake of
cadmium increased with temperature up to about 16 °C and decreased as
the concentration of cadmium in the water increased. Feeding the fish
increased the rate of uptake of cadmium from the water. The author
concluded that metabolic rate was an important factor in the uptake of
cadmium into the fish and in its subsequent loss.
4.1.1.5 Model aquatic ecosystems
Ferard et al. (1983) investigated the transfer of cadmium through
a model food-chain consisting of an alga, a daphnid, and a fish.
Concentration factors relative to food were low, indicating that
cadmium is mainly taken up directly from water. Daphnids fed algae
containing cadmium at between 4.5 and 570 mg/kg dry weight showed a
maximum concentration factor of 1. Fish fed contaminated daphnids or
algae showed concentration factors of 0.0038 and 0.0018, respectively.
Nimmo et al. (1977) reported low concentration factors, ranging from
0.018 to 0.027, for grass shrimp fed on brine shrimp containing
cadmium at between 27 and 182 mg/kg. Rehwoldt & Karimian-Teherani
(1976) fed zebrafish on food containing cadmium acetate at 10 mg/kg
over a period of 6 months. Maximum residues, in males and females
respectively, were 5.92 and 13.64 mg/kg, the median residue levels
after 6 months of exposure being 5.19 and 12.95 mg/kg (on a dry weight
basis).
4.1.1.6 Uptake from aquatic sediment
Ray et al. (1980b) exposed the ragworm Nereis virens to
sediment to which cadmium chloride had been added. Smaller worms took
up more cadmium relative to body weight than larger worms. The cadmium
was taken up in a dose-related manner and no equilibrium was reached
during the 24-day experiment. The rate of uptake directly from sea
water also increased with exposure concentration over the range of
0.03 to 9.2 mg/litre. For the range of sediment cadmium concentrations
used (1 to 4 mg/kg), the corresponding concentrations in the overlying
sea water were 0.03 to 0.1 mg/litre. Comparing uptake into the
ragworms from water with these concentrations to the uptake from the
spiked sediment produced identical concentrations of cadmium in the
worms. Rate of uptake from sediment was between 16 and 39 times less
than the uptake from the corresponding exposure to cadmium in water.
The authors concluded that all of the uptake of cadmium from sediment
derived from desorbed metal ions in the interstitial water.
4.1.1.7 Uptake from food relative to uptake from water
Fish can take up cadmium from the surrounding water and from
ingested food. The main uptake route in fresh water is from the water
via the gills (Williams & Giesy, 1978). However, the relative
importance of food and water to the body burden depends very much on
the cadmium content of the food organism. In contaminated areas with
an increased cadmium content in food organisms, the relative
importance of food as a cadmium source may increase. In the marine
environment, where cadmium is mainly present in chloride complexes not
available to fish, the relative importance of food as a cadmium source
increases. Consequently food has been shown to be the main cadmium
source in marine fish (Pentreath, 1977; Dallinger et al., 1987).
4.1.2 Uptake by terrestrial organisms
4.1.2.1 Uptake into plants
The uptake of cadmium into plants generally depends upon the
availability of the metal in soil solution. The soil pH and
composition, particularly the nature of soil clays, the organic matter
content, and, obviously, the soil cadmium level, affect this
availability. The relationship between soil cadmium level and plant
uptake is not a simple one because of the wide variety of soil
characteristics that affect the extent of cadmium uptake. Cataldo &
Wildung (1978), Peterson & Alloway (1979), and Page et al. (1981) have
reviewed this subject.
Plants grown in a greenhouse or a container take up more cadmium
than the same plants grown in soil with the same cadmium levels in the
field. This is due to greater root development in a confined volume in
containers and to the fact that all the roots are in contact with
cadmium-contaminated soil. In the field, roots may grow down below the
cadmium-contaminated level (Page & Chang, 1978; De Vries & Tiller,
1978).
Mahler et al. (1978) cultured lettuce and chard on acid or
calcareous soils to which cadmium sulfate had been added at levels up
to 320 mg/kg. For both types of soil there was a dose-related uptake
of cadmium from soil into leaves. The uptake of the metal was much
greater in acid than in calcareous soils, particularly at higher rates
of cadmium application (over 40 mg/kg). At the highest soil
concentration of 320 mg/kg, lettuce leaves contained cadmium at a
concentration of 800 mg/kg and chard leaves 1600 mg/kg when grown in
acid soil. Leaves of lettuce cultured on calcareous soils with cadmium
at 320 mg/kg contained a lower cadmium concentration of 200-300 mg/kg
and chard, similarly cultured, contained 300 mg/kg or less. Bingham et
al. (1980) showed an effect of soil pH on cadmium (as sulfate) uptake
in rice; more metal was incorporated as acidity increased. Chaney et
al. (1975) reported that liming of soil in which soybeans were growing
decreased the concentrations of cadmium in leaves from 33 to 5 mg/kg
dry weight as pH increased from 5.3 to 7.0. Eriksson (1988)
investigated the effect of pH on the uptake of cadmium into perennial
ryegrass ( Lolium perenne) and winter rape ( Brassica napus). The
more soluble fractions of cadmium in soil increased as the pH was
lowered; increasing the pH from 5 to 7 with calcium oxide invariably
reduced the cadmium content of ryegrass plants, but this decrease was
less consistent when the pH was increased from 5 to 6. The cadmium
content of rape plants was markedly higher at pH 4 than pH 5. Adding
more cadmium to the soil increased the amount of cadmium in the plants
in direct proportion to the increased concentration of the metal in
soil over the range 0 to 5 mg/kg. Eriksson (1988) found that soil
organic matter decreased the availability of cadmium to perennial
ryegrass and winter rape grown in pots. Addition of organic material
to sand and clay soils reduced cadmium uptake to a greater extent in
the sand.
When Mitchell & Fretz (1977) cultured seedlings of three species
of tree (red maple, white pine, and Norway spruce) hydroponically or
in soil with added cadmium, the concentration in roots was greater
than that in leaves. Cadmium added to soil was less readily taken up
than cadmium added to nutrient solutions. Similarly, Root et al.
(1975) reported greater cadmium concentration in roots than in shoots
of maize grown hydroponically in a medium containing cadmium chloride.
Harkov et al. (1979) found the highest uptake of cadmium into
hydroponically grown tomatoes in the roots, while stems had lower
cadmium concentrations than leaves.
Lepp et al. (1987) measured high concentrations of cadmium in the
sporophores (fruiting bodies) of the fungus Amanita muscaria growing
in birch woodland. The fungus sporophores contained 29.9 mg/kg dry
weight, compared to a cadmium level of 0.4 mg/kg in the soil on which
they grew. The cadmium was released from the rotting sporophore, after
it had shed its spores, in a form which was readily available to other
plants growing on the woodland soil; this was shown experimentally
with lettuce plants grown in pots. The authors calculated that an
abundant population of sporophores could recycle 1.4% of the total
cadmium load in leaf litter to higher plants over a period of 14 days
(the mean lifespan of the sporophores).
4.1.2.2 Terrestrial invertebrates
Beyer et al. (1982) demonstrated that earthworms concentrated
cadmium from soils amended with sewage sludge containing cadmium
oxide. Cadmium concentrations were as high as 100 mg/kg in worms
exposed to soils containing cadmium at 2 mg/kg, a concentration factor
of 50. Adding calcium carbonate to soils decreased the cadmium uptake
of worms slightly, while high soil zinc levels decreased the cadmium
uptake appreciably. Results were variable with different sludge
treatments. Hartenstein et al. (1980) amended sludge with 10, 50, and
100 mg/kg cadmium (as cadmium sulfate) and added earthworms ( Eisenia
foetida). The worms accumulated 3.9, 2.04, and 1.44 times the
respective sludge levels of cadmium over a period of 5 weeks. In field
trials on non-amended soils containing 12 to 27 mg cadmium/kg, worms
sampled during a 28-week period gave levels of cadmium ranging from 8
to 46 mg/kg.
Terrestrial pulmonate snails retained up to 59% of cadmium
administered in their diet as the chloride (Russell et al., 1981). The
highest retention was after dosing at 25 mg cadmium/kg diet. The
higher the dose (up to 1000 mg/kg diet) the lower the percentage
retention of the metal. Ireland (1981) noted that in the terrestrial
slug Arion ater most of the cadmium was located in the digestive
gland without association with any particular sub-cellular organelles,
and isolated a specific cadmium-binding protein from the animals.
4.1.2.3 Birds
In a study by White & Finley (1978), adult mallard ducks were fed
a diet containing cadmium chloride at levels of 2, 20 or 200 mg/kg and
killed at 30-day intervals. The cadmium content increased with dose
level and time (except in the case of the highest dose where body
burden peaked after 60 days), and the highest concentrations occurred
in the liver and kidney. The highest levels overall occurred after
dosing for 60 days at 200 mg/kg; cadmium concentrations were 109 mg/kg
in the liver and 134 mg/kg in the kidney.
Nicholson & Osborn (1983) dosed starlings ( Sturnus vulgaris)
with cadmium chloride at a concentration of 2 mg/kg body weight, three
times weekly for 6 weeks, and reported a wide range of kidney
concentrations (from < 10 to > 200 mg/kg dry weight).
4.2 Distribution
4.2.1 Aquatic organisms
In higher organisms, cadmium can be bound in several different
tissues, whereas in plants cadmium is bound to the cell wall in roots.
Brooks & Rumsby (1967) measured the cadmium taken up by the
oyster ( Ostrea sinuata) from water containing 115Cd (50 mg/litre).
The soft parts of the oyster contained 100 mg cadmium/kg after 100 h.
Concentrations in tissues were, in decreasing order, 360 mg/kg for
gills, 285 mg/kg for heart, 141 mg/kg for the visceral mass, 83 mg/kg
for the mantle, 53 mg/kg for white muscle, and 25 mg/kg for striated
muscle.
Nimmo et al. (1977) reported that in the pink shrimp the
hepatopancreas took up more cadmium than other tissues. Lower
concentrations were found in the exoskeleton, muscle, and serum.
Short-term exposure of the crab Uca pugilator to cadmium chloride
led to the hepatopancreas and gill concentrations of the metal being
similar after a 24-h exposure to 1 mg cadmium/litre (Vernberg et al.,
1974).
Sangalang & Freeman (1979) determined the cadmium in tissues of
brook trout exposed to the metal (added as the chloride) via the water
or by injection. After water exposure to cadmium chloride at 1
µg/litre, the trout showed greatest uptake of the metal in the gills,
kidney, and liver. The gills and the posterior kidney revealed a
higher metal content than any other tissues. Levels of cadmium in
whole blood and plasma, heart, spleen, testis, stomach, and skin were
higher than control levels after 77 and 93 days of exposure. Smith et
al. (1976) found the greatest accumulation of the metal in the kidney
of catfish exposed to cadmium (as sulfate) in the water. In an
autoradiographic study of cadmium distribution in rainbow trout
exposed to cadmium in water, Tjalve et al. (1986) confirmed the
general picture of cadmium distribution, the metal being found in the
gills, liver, and kidney. However, they also observed heavy labelling
of the olfactory rosette and the olfactory nerve, an observation not
reported earlier. In a detailed study they later showed that cadmium
was transported axonally from the olfactory rosette to the bulbus
olfactorius but not further into the brain (Gottofrey, 1990). The
significance of this observation with respect to the olfactory
responses of fish in cadmium-contaminated environments remains to be
investigated.
The few studies that have been conducted on the subcellular
distribution of cadmium indicate that, while much is located in the
cytosol, a significant proportion can be found in the nucleus and the
mitochondria. Cadmium is bound in the cytosol to proteins of low
relative molecular mass, metallothioneins, and other cadmium-binding
proteins. These proteins are rich in the sulfur-containing amino acid
cysteine but poor in aromatic amino acids.
Metallothioneins have been isolated and characterized in a number
of aquatic and terrestrial organisms. Fish metallothioneins have
received considerable interest in recent years as tools in monitoring
metal pollution in the environment (Hamilton & Mehrle, 1986; Hogstrand
& Haux, 1990a). Simple methods to analyse fish metallothionein have
been developed, including differential pulse polarography (Olson &
Haux, 1986) and radioimmunoassay based on specific antibodies to fish
metallothionein (Hogstrand & Haux, 1990b). Olson & Haux (1986) found
a strong correlation between hepatic metallothionein and cadmium
accumulation in perch collected from cadmium-contaminated water.
4.2.2 Terrestrial organisms
4.2.2.1 Terrestrial plants
Jones & Johnston (1989) analysed cereal grain and herbage from
long-term experimental plots at Rothamsted, United Kingdom, and found
that uptake of cadmium into herbage was greatest where phosphate
fertilizer had been applied. It was also greater from unlimed soils
than from limed soils. However, the authors concluded that there was
little evidence of a long-term (1840-1986) increase in crop cadmium
concentrations.
Byrne et al. (1976) analysed higher fungi from Slovenia,
Yugoslavia, and found levels of cadmium ranging from 0.53 to 39.9
mg/kg dry weight (average 5.0 mg/kg). This is an order of magnitude
higher than in most other plants. Although the fungi were collected
from industrial, urban, and uncontaminated sites, the levels found in
the fungi were not very different between sites. The authors suggested
geological rather than industrial sources for the cadmium in these
soils.
The high uptake by mushrooms and related species is probably due
to a cadmium-binding phosphoglycoprotein, cadmium-myco-phosphatin,
which has been isolated from the mushroom Agaricus macrosporus
(Meisch & Schmitt, 1986).
4.2.2.2 Terrestrial invertebrates
Hopkin & Martin (1985) investigated the storage of cadmium in the
woodlouse Oniscus asellus from heavily contaminated woodland 3 km
downwind from a smelter. The hepatopancreas was found to contain up to
5 g cadmium/kg dry weight without apparent ill effects upon the
organism. Cadmium was reported to be stored intracellularly in the
copper- and sulfur-containing granules of epithelial S cells. In a
later study (Hopkin, 1990) it was found that considerable interspecies
differences exist with regard to storage in the hepatopancreas.
Oniscus asellus stored five times more cadmium than Porcello scaber
under the same conditions. The carnivorous centipede Lithobius
variegatus, when fed on cadmium-contaminated hepatopancreas from
woodlice, accumulated cadmium which was likewise stored in the midgut
(Hopkin & Martin, 1984).
Berger & Dallinger (1989) studied the distribution of cadmium
between several organs of the terrestrial snail Arianta arbustorum
during a 20-day feeding experiment on cadmium-enriched agar. Of the
cadmium in the medium, 54% was taken up, of which 66% was distributed
to the hepatopancreas, leading to a concentration of more than 500
mg/kg dry weight. In other organs (intestine, foot/mantle, gonads),
the cadmium concentration was considerably lower.
In the earthworm Lumbricus rubellus taken from heavy-metal-
polluted soil, more than 70% of the cadmium burden was found in the
posterior alimentary canal (Morgan & Morgan, 1990). This distribution
prevented dissemination of large concentrations of cadmium into other
tissues and, according to the authors, may represent a detoxification
strategy.
4.3 Elimination
Information on loss of cadmium from organisms is relatively
scarce. The information that does exist suggests that this is very
variable, and has been reviewed by Coombs (1979) and Taylor (1983).
Organisms that accumulate cadmium also tend to retain the metal for
long periods. The main excretory route appears to be via the kidney,
except in the case of organisms that moult, where loss from the shed
exoskeleton can be significant.
Robinson & Wells (1975) administered a single oral dose of
cadmium acetate to softshell turtles ( Trionyx spinifer) and killed
and dissected the animals either 48 h or 96 h later. After 48 h, 9.43%
of the total dose was recovered from tissues, while turtles killed
after 96 h had retained 4.02% of the dose. The greatest retention of
cadmium, after both time periods, was in the liver. Cadmium was also
retained in the small intestine for the first 48 h, but the amount had
decreased by 96 h.
Harrison & Klaverkamp (1989) exposed rainbow trout ( Salmo
gairdneri) and lake whitefish (Coregonus clupeaformis) to cadmium in
water, via a continuous-flow system, or the diet, via pelleted food,
for 72 days. The fish were then kept in clean water on a cadmium-free
diet for a further 56 days. In the case of water-exposed fish, the
majority of the cadmium was present in the gill and kidney, but
food-exposed fish retained cadmium principally in the kidney, gut, and
liver. Bioconcentration factors for exposure via the water were 55 for
the trout and 42 for the whitefish, whereas concentration factors from
the food were less than 1 for both species. However, both species
accumulated a greater proportion of the cadmium that was in the food
than that in water (1% as against 0.1%). Equilibrium bioconcentration
factors were estimated to be 161 for trout and 51 for whitefish.
In the same model, the half-times for depuration of accumulated
cadmium ranged from 24 to 63 days. Douben (1989b) investigated the
kinetics of cadmium in freshwater fish (the stone loach Noemacheilus
barbatulus) exposed to cadmium via the diet (tubifex worms
previously contaminated with cadmium by uptake from water). The body
burden of cadmium declined after the period of feeding with
contaminated diet more rapidly in starved than in fed fish. Rate
constants for loss of cadmium appeared to be greater during the
exposure period than after exposure. Both uptake and loss of cadmium
were influenced by the body weight of the fish.
Janssen et al. (1991) investigated uptake and loss of cadmium
from contaminated soil by four species of soil arthropod and developed
kinetic models that gave good predictions of the degree of
accumulation in a variety of species. They also reviewed data on other
soil arthropods (Tables 6 and 7). The kinetics of cadmium in different
arthropods is related to taxonomy and reflects the different
physiological characteristics of the different organisms. Some,
notably isopods and molluscs, take up and retain cadmium in their
tissues with little or no excretion. These species are capable of
holding large quantities of the metal in the hepatopancreas without
apparent ill effect. There is no direct correlation between
assimilation capacity and the capacity to excrete or eliminate
cadmium. Figure 4 illustrates the uptake of cadmium (measured as total
body burden) and its subsequent loss in four species of arthropods.
Elimination half-lives of 53, 8, and 2 days, respectively, have been
reported for Platynothrus peltifer, Orchesella cincta, and
Notiophilus biguttatus; no elimination took place over 130 days in
Neobisium muscorum.
Sawicka-Kapusta et al. (1987) investigated the effect of keeping
the vole Clethrionomys glareolus at different temperatures on the
rate of loss of cadmium from body tissues. Although the different
temperatures (10 °C and 20 °C) affected the metabolic rate of the
voles, there was no difference in the rate of loss of cadmium.
4.4 Bioaccumulation and biomagnification
Bioaccumulation occurs when the concentration in the organism
exceeds the concentration in the nutrient medium and is expressed
quantitatively as a bioconcentration factor. Progressive
bioaccumulation at each trophic level is termed biomagnification.
2.2 Analytical procedures
The following is an outline of the analytical procedure for
cadmium; further information is given in Environmental Health Criteria
134: Cadmium (WHO, 1992).
2.2.1 Sampling and preparation
Only a few nanograms, or even less, of cadmium may be present in
collected samples of air or water, whereas hundreds of micrograms may
be present in small samples of kidney, sewage sludge, and plastics.
Different techniques are, therefore, required for the collection,
preparation, and analysis of the samples.
In general, the techniques available for measuring cadmium in the
environment and biological materials cannot differentiate between
cadmium species. With special separation techniques,
cadmium-containing proteins can be isolated and identified. In most
studies, the concentration or amount of cadmium in water, air, soil,
plants, and other environmental or biological material is determined
as the element.
Standard trace element methods can generally be used for the
collection of samples (LaFleur, 1976; Behne, 1980). During the
handling and storage of samples, particularly liquid samples, special
care must be taken to avoid contamination; coloured materials in
containers, especially plastics and rubber, should be avoided. Glass
and transparent, cadmium-free polyethylene, polypropylene or teflon
containers are usually considered suitable for storing samples. All
containers and glassware should be precleaned in dilute nitric acid
and deionised water. In order to avoid possible adsorption of cadmium
onto the container wall, water samples or standards with low cadmium
concentrations should not be stored for long periods of time.
To prepare samples for analysis, inorganic solid samples (such as
soil or dust samples) are usually dissolved in an acid, e.g., nitric
acid. Organic samples need to be subjected to wet ashing (digested) or
dry ashing. When the cadmium concentration is low, special treatment
is sometimes needed. The procedures for separating cadmium from
interfering compounds and concentrating the samples are very important
steps in obtaining accurate results.
2.2.2 Quantitative instrumental methods
The most commonly used methods, at present, are atomic absorption
spectrometry, electrochemical methods, neutron activation analysis,
atomic emission spectrometry, atomic fluorescence spectrometry and
proton-induced X-ray emissions (PIXE) analysis. Analytical methods for
cadmium have been reviewed by Friberg et al. (1986). Detection limits
of some of the methods are given in Table 3.
Table 3. Analytical procedures a
Method Detection limit Matrix
Atomic absorption 1 to 5 mg/litre water
spectrometry
0.1 mg/kg biological samples
electrothermal a few pg
atomization
Electrochemical method
(potentiometric stripping
analysis) 0.1 mg/litre urine
Neutron activation 0.1 to 1 mg/litre biological
analysis samples/fluids
X-ray atomic 17 mg/kg biological samples
fluorescence
a From: Friberg et al. (1986)
3. NATURAL OCCURRENCE AND SOURCES OF ENVIRONMENTAL CONTAMINATION
3.1 Natural occurrence
A comparison of natural and anthropogenic sources of trace metals
is given in the Appendix 1.
Cadmium is widely distributed in the earth's crust at an average
concentration of about 0.1 mg/kg and is commonly found in association
with zinc. However, higher levels are present in sedimentary rocks:
marine phosphates often contain about 15 mg/kg (GESAMP, 1984).
Weathering and erosion result in the transport by rivers of large
quantities of cadmium to the world's oceans and this represents a
major flux of the global cadmium cycle; an annual gross input of 15
000 tonnes has been estimated (GESAMP, 1987).
In background areas away from ore bodies, surface soil
concentrations of cadmium typically range between 0.1 and 0.4 mg/kg
(Page et al., 1981). The median cadmium concentration in non-volcanic
soil ranges from 0.01 to 1 mg/kg, but in volcanic soil levels of up to
4.5 mg/kg have been found (Korte, 1983).
Volcanic activity is a major natural source of atmospheric
cadmium release. The global annual flux from this source has been
estimated to be 100-500 tonnes (Nriagu, 1979). Deep sea volcanism is
also a source of environmental cadmium release, but the role of this
process in the global cadmium cycle remains to be quantified.
The average cadmium content of sea water is about 0.1 µg/litre or
less (Korte, 1983), while river water (Mississippi, Yangtze, Amazon,
and Orinoco sampled between 1976 and 1982) contains dissolved cadmium
at concentrations of < 1.1-13.5 ng/litre (Shiller & Boyle, 1987).
Cadmium levels of up to 5 mg/kg have been reported in river and lake
sediments and from 0.03 to 1 mg/kg in marine sediments (Korte,1983).
Current measurements of dissolved cadmium in surface waters of
the open oceans give values of < 5 ng/litre. The vertical
distribution of dissolved cadmium in ocean waters is characterized by
a surface depletion and deep water enrichment, which corresponds to
the pattern of nutrient concentrations in these areas (Boyle et al.,
1976). This distribution is considered to result from the absorption
of cadmium by phytoplankton in surface waters and its transport to the
depths, incorporation to biological debris, and subsequent release. In
contrast, cadmium is enriched in the surface waters of areas of
upwelling and this also leads to elevated levels in plankton
unconnected with human activity (Martin & Broenkow, 1975; Boyle et
al., 1976). Oceanic sediments underlying these areas of high
productivity can contain markedly elevated cadmium levels as a result
of inputs associated with biological debris (Simpson, 1981).
In remote, uninhabited areas, cadmium concentrations in air are
usually less than 1 ng/m3 (Korte,1983).
3.2 Industrial uses
The principal applications of cadmium fall into five categories:
protective plating on steel; stabilizers for PVC; pigments in plastics
and glass; electrode material in nickel-cadmium batteries; and as a
component of various alloys (Wilson, 1988).
The relative importance of the major applications has changed
considerably over the last 25 years. The use of cadmium for
electroplating represented in 1960 over half the cadmium consumed
worldwide, but in 1985 its share was less than 25% (Wilson, 1988).
This decline is usually linked to the introduction of stringent
effluent limits from plating works and, more recently, to the
introduction of general restrictions on cadmium consumption in certain
countries. In contrast, the use of cadmium in batteries has shown
considerable growth in recent years from only 8% of the total market
in 1970 to 37% by 1985. The use of cadmium in batteries is
particularly important in Japan and represented over 75% of the total
consumption in 1985 (Wilson, 1988).
Pigments and stabilizers accounted for 22% and 12% of the total
world consumption in 1985. The share of the market by cadmium pigments
remained relatively stable between 1970 and 1985 but the use of the
metal in stabilizers during this period showed a considerable decline,
largely as a result of economic factors. The use of cadmium as a
constituent of alloys is relatively small and has also declined in
importance in recent years, accounting for about 4% of total cadmium
use in 1985 (Wilson, 1988).
3.3 Sources of environmental cadmium
3.3.1 Sources of atmospheric cadmium
Estimates of cadmium emissions to the atmosphere from human and
natural sources have been carried out at the worldwide, regional, and
national level; examples of such inventories are shown in Table 4.
The median global total emission of the metal from human sources
in 1983 was 7570 tonnes (Nriagu & Pacyna, 1988) and represented about
half the total quantity of cadmium produced in the same year. In both
the European Economic Community (EEC) and on a worldwide scale
(Nriagu, 1989), about 10-15% of total airborne cadmium emissions arise
from natural processes, the major source being volcanic action.
Municipal refuse contains cadmium derived from discarded
nickel-cadmium batteries and plastics containing cadmium pigments and
stabilizers. The incineration of refuse is a major source of
atmospheric cadmium release at country, regional, and worldwide level
(Table 4).
Steel production can also be considered as a waste-related
source, as large quantities of cadmium-plated steel scrap are recycled
by this industry. As a result, steel production is responsible for
considerable emissions of atmospheric cadmium.
3.3.2 Sources of aquatic cadmium
Non-ferrous metal mines represent a major source of cadmium
release to the aquatic environment. Contamination can arise from mine
drainage water, waste water from the processing of ores, overflow from
the tailings pond, and rainwater run-off from the general mine area.
The release of these effluents to local watercourses can lead to
extensive contamination downstream of the mining operation. Mines
disused for many years can still be responsible for the continuing
contamination of adjacent watercourses (Johnson & Eaton, 1980).
At the global level, the smelting of non-ferrous metal ores has
been estimated to be the largest human source of cadmium release to
the aquatic environment (Nriagu & Pacyna, 1988). Discharges to fresh
and coastal waters arise from liquid effluents produced by air
pollution control (gas scrubbing) together with the site drainage
waters.
Table 4. Estimates of atmospheric cadmium emissions (tonnes/year) on a national, regional and worldwide basis
Source United EEC b Worldwide c
Kingdom a
Natural sources ND 20 150-2600 d
Non-ferrous metal
production
mining ND ND 0.6-3
zinc and cadmium 20 920-4600
copper 3.7 6 1700-3400
lead 7 39-195
Secondary production ND 2.3-3.6
Production of cadmium-containing
substances ND 3 ND
Iron and steel production 2.3 34 28-284
Fossil fuel combustion
coal 1.9 6 176-882
oil 0.5 41-246
Refuse incineration 5 31 56-1400
Sewage sludge incineration 0.2 2 3-36
Table 4 (contd).
Source United EEC b Worldwide c
Kingdom a
Phosphate fertilizer manufacture ND ND 68-274
Cement manufacture 1 ND 8.9-534
Wood combustion ND ND 60-180
TOTAL EMISSIONS 14 130 3350-14 640
a From: Hutton & Symon (1986); data apply to 1982-1983
b From: Hutton (1983); data apply to 1979-1980 (the EEC consisted,
at that time, of Belgium, Denmark, Federal Republic of Germany,
Italy, Luxembourg, The Netherlands, Republic of Ireland, and the
United Kingdom)
c From: Nriagu & Pacyna (1988); data apply to 1983
d From: Nriagu (1979)
ND Not determined
The manufacture of phosphate fertilizer results in a
redistribution of the cadmium present in the rock phosphates between
the phosphoric acid product and gypsum waste. In many cases, the
gypsum is disposed of by dumping in coastal waters, which leads to
considerable cadmium inputs. Some countries, however, recover the
gypsum for use as a construction material and thus have negligible
cadmium discharges (Hutton, 1982).
The atmospheric fallout of cadmium to fresh and marine waters
represents a major input of cadmium at the global level (Nriagu &
Pacyna, 1988). A GESAMP study of the Mediterranean Sea indicated that
this source is comparable in magnitude to the total river inputs of
cadmium to the region (GESAMP, 1985). Similarly, large cadmium inputs
to the North Sea (110-430 tonnes/year) have also been estimated, based
on the extrapolation from measurements of cadmium deposition along the
coast (van Alst et al., 1983a,b). However, another approach based on
model simulation yielded a modest annual cadmium input of 14 tonnes
(Krell & Roeckner, 1988).
Acidification of soils and lakes may result in enhanced
mobilization of cadmium from soils and sediments and lead to increased
levels in surface and ground waters (WHO Working Group, 1986).
3.3.3 Sources of terrestrial cadmium
Solid wastes are disposed of in landfill sites, resulting in
large cadmium inputs at the national and regional levels when
expressed as total tonnage (Hutton, 1982; Hutton & Symon, 1986).
Sources include the ashes from fossil fuel combustion, waste from
cement manufacture, and the disposal of municipal refuse and sewage
sludge.
Of greater potential environmental significance are the solid
wastes from both non-ferrous metal production and the manufacture of
cadmium-containing articles, as well as the ash residues from refuse
incineration. These three waste materials are characterized by
elevated cadmium levels and as such require disposal to controlled
sites to prevent the contamination of the ground water.
The agricultural application of phosphate fertilizers represents
a direct input of cadmium to arable soils. The cadmium content of
phosphate fertilizers varies widely and depends on the origin of the
rock phosphate. It has been estimated that fertilizers of West African
origin contain 160-255 g cadmium/tonne of phosphorus pentoxide, while
those derived from the southeastern USA contain 35 g/tonne (Hutton,
1982).
The annual rate of cadmium input to arable land from phosphate
fertilizers has been estimated at 5 g/ha for the countries of the EEC
(Hutton 1982). This only represents about 1% of the surface soil
cadmium burden. Despite the relatively small size of this input,
long-term continuous application of phosphate fertilizers has been
shown to cause increased soil cadmium concentrations (Williams &
David, 1973, 1976; Andersson & Hahlin, 1981).
The application of municipal sewage sludge to agricultural soils
as a fertilizer can also be a significant source of cadmium; a value
of 80 g/ha has been estimated for the United Kingdom (Hutton & Symon,
1986). On a national or regional basis, however, these inputs are much
smaller than those from either phosphate fertilizers or atmospheric
deposition (see section 3.4).
Polluted soils can contain cadmium levels of up to 57 mg/kg (dry
weight) resulting from sludge applied to soil and up to 160 mg/kg in
the vicinity of metal-processing industry (Fleischer et al., 1974).
The highest cadmium levels reported appear to be from ancient mining
areas with levels of up to 468 mg/kg.
3.4 Environmental transport and distribution
3.4.1 Atmospheric deposition
Cadmium is removed from the atmosphere by dry deposition and by
precipitation. In rural areas of Scandinavia, annual deposition rates
of 0.4-0.9 g/ha have been measured (Laamanen, 1972; Andersson, 1977).
Similarly, in a rural region of Tennessee, USA, a deposition rate of
0.9 g/ha was observed (Lindberg et al., 1982). Hutton (1982) suggested
that 3 g/ha per year was a representative value for the atmospheric
deposition of cadmium to agricultural soils in rural areas of the EEC.
The corresponding input for these areas from the application of
phosphate fertilizers is 5 g/ha per year (see section 3.3).
Many industrial sources of cadmium possess tall stacks which
bring about the wide dispersion and dilution of particulate emissions.
Nevertheless, cadmium deposition rates around smelter facilities are
often markedly elevated nearest the source and generally decrease
rapidly with distance (Hirata, 1981). Soil cadmium concentrations in
excess of 100 mg/kg are commonly encountered close to long established
smelters (Buchauer, 1972).
Crop plants growing near to atmospheric sources of cadmium may
contain elevated cadmium levels (Carvalho et al., 1986). However, it
is not always possible to distinguish whether the cadmium is derived
directly from surface deposition or originates from root uptake, since
soil levels in such areas are generally higher than normal.
3.4.2 Transport from water to soil
Rivers contaminated with cadmium can contaminate surrounding
land, either through irrigation for agricultural purposes, by the
dumping of dredged sediments, or through flooding (Forstner, 1980;
Sangster et al., 1984). For example, agricultural land adjacent to the
Neckar River, Germany, received dredged sediments to improve the soil,
a practice that produced soil cadmium concentrations in excess of 70
mg/kg (Forstner, 1980).
Much of the cadmium entering fresh waters from industrial sources
is rapidly adsorbed by particulate matter, where it may settle out or
remain suspended, depending on local conditions. This can result in
low concentrations of dissolved cadmium even in rivers that receive
and transport large quantities of the metal (Yamagata & Shigematsu,
1970)
3.5 Concentrations in various biota
Table 5 indicates the levels of cadmium found in various biota
(Eisler, 1985).
Eisler (1985) concluded that there are at least six trends
evident from the abundant residue data available for cadmium.
* Marine organisms generally contain higher cadmium residues than
their freshwater and terrestrial counterparts.
* Cadmium tends to concentrate in the viscera of vertebrates,
especially the liver and kidneys.
* Cadmium concentrations are generally higher in older organisms.
* Higher cadmium residues are generally associated with industrial
and urban sources, although this does not apply to sea birds and
sea mammals.
* Cadmium residues in plants are normally less than 1 mg/kg.
However, plants growing in soil amended with cadmium (e.g., from
sewage sludge) may contain significantly higher levels.
* The species analysed, season of collection, ambient cadmium
levels, and the sex of the organism probably all affect the
residue level.
Table 5. Concentrations of cadmium in biota
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Marine organisms
Algae < 1 to 16
Molluscs soft parts up to 425
kidney up to 547
liver up to 782
digestive gland up to 1163
Crustaceans whole body < 0.4-6.2
Annelids whole body 0.1-3.6
Fish whole body up to 5.2
Birds kidney up to 231
Mammals kidney up to 300
Freshwater organisms
Plants whole plant 0.5-1.8
roots up to 6.7
Molluscs soft parts; fresh weight 0.2-1.4
Annelids whole body; fresh weight 0.5-3.2
Fish whole body; fresh weight 0.01-1.04
Table 5 (contd).
Organisms Parts of the Cadmium concentration
organisms (mg/kg dry weight)
Terrestrial organisms
Plants whole plant up to 27.1
grain up to 257
Annelids whole body 3-12.6
Birds whole body; fresh weight < 0.05-0.24
kidney; fresh weight up to 7.4
Mammals kidney up to 8.1
3.5.1 Concentrations in fish
May & McKinney (1981) monitored freshwater fish from the USA in
1976 and 1977 and found cadmium concentrations ranging from 0.01 to
1.04 mg/kg (wet weight), the mean being 0.085 mg/kg. This represented
a significant decline from the mean 1972 concentration of 0.112 mg/kg.
The authors pointed out that this decline parallels a decline in
cadmium metal production and consumption over the same period.
Hardisty et al. (1974a) sampled flounder ( Platichthyes flesus)
from the Severn estuary, United Kingdom, and found mean cadmium
concentrations of 3.4-7.3 mg/kg (dry weight). No overall correlation
between cadmium concentration and length or age was observed, although
the largest (27-29 cm) and the oldest („ 5 years) fish gave the
highest mean concentrations. Hardisty et al. (1974b) found a positive
correlation between the cadmium content of a variety of fish species
and the crustacea content of their diet. Lovett et al. (1972) sampled
fish from New York State, USA, and reported mean cadmium
concentrations of < 10-142.7 µg/kg (fresh weight). There was no
relationship between total residues and size, sex or age of lake trout
( Salvelinus namaycush).
3.5.2 Concentrations in sea-birds
Cadmium has been found in a wide variety of birds, and
particularly high levels have been reported in pelagic sea-birds. Much
of the cadmium occurs in the kidney and liver, and relatively little
is transferred to the eggs. A review of the uptake of cadmium and of
the factors that affect it can be found in Scheuhammer (1987).
Interestingly, the concentrations of cadmium in sea-birds are often
higher in areas with little or no contamination from industrial
sources (Bull et al., 1977; Hutton, 1981; Osborn & Nicholson, 1984).
3.5.3 Concentrations in sea mammals
High levels of cadmium have been reported in sea mammals from
areas around the world, which they are assumed to take up from their
diet of fish. Roberts et al. (1976) showed that kidney levels of
cadmium in the common seal off the United Kingdom coast were age
related. Drescher et al. (1977) showed a similar relationship in seals
off the German coast and Hamanaka et al. (1982) in stellar sea lions
off the coast of Japan. Similar trends in dolphins and porpoises have
been reported (Falconer et al., 1983; Honda & Tatsukawa, 1983; Honda
et al., 1986). Muir et al. (1988) sampled white-beaked dolphins
( Lagenorhynchus albirostris) and pilot whales ( Globicephala
melaena) from the coast of Newfoundland, Canada, and reported mean
cadmium levels in kidney (dry weight) of 13.6 mg/kg and 108 mg/kg,
respectively. Cadmium concentrations were age related in pilot whales.
The lower levels found in dolphins were probably related both to
species differences and to the fact that they were all young animals.
3.6 Concentrations adjacent to highways
Muskett & Jones (1980) monitored levels of cadmium adjacent to a
heavily used road. The concentrations in air were highest at a
distance from the road of 0-10 m, and a similar pattern was found in
soil. Cadmium levels in earthworms sampled at known distances from a
highway revealed levels of 12.6 mg/kg (dry weight) within 3 m falling
to 7.1 mg/kg approximately 50 m from the highway. The level in
earthworms from control sites was 3 mg/kg (Gish & Christensen, 1973).
The land snail Cepaea hortensis accumulates cadmium from roadside
verges (Williamson, 1980). The highest concentration of cadmium was
found in the digestive gland (40.3 mg/kg dry weight) and kidney (12.8
mg/kg dry weight). There was little metal in the head and foot, which
make up most of the body tissue. The author showed that age accounted
for 80% of the total variance of soft tissue body burdens. The cadmium
body burdens were found to be effectively immobile, accumulating
progressively with age.
3.7 Concentrations from industrial sources
Burkitt et al. (1972) analysed the cadmium content of ryegrass at
various distances from a zinc smelter and found 50, 10.8, and 1.8
mg/kg dry weight at distances of 0.3, 1.9, and 11.3 km, respectively,
from the smelter.
Teraoka (1989) found that cadmium levels in rice roots were
significantly higher in industrial urban and roadside areas of Japan
compared to sparsely populated areas. The mean level in industrial
areas was 10 mg/kg (dry weight).
Beyer et al. (1985) monitored biota from the vicinity of two zinc
smelters in eastern Pennsylvania, USA. Cadmium concentrations were
highest in carrion insects (25 mg/kg dry weight), followed by fungi
(9.8 mg/kg), leaves (8.1 mg/kg), shrews (7.3 mg/kg), moths (4.9
mg/kg), mice (2.6 mg/kg), songbirds (2.5 mg/kg), and berries (1.2
mg/kg).
Van Hook (1974) sampled soil and earthworms from soil that had
not been disturbed for 30 years and reported mean cadmium levels in
the soils and earthworms of 0.35 and 5.7 mg/kg dry weight,
respectively. Ma et al. (1983) analysed soil and earthworms
( Lumbricus rubellus) at varying distances from a zinc-smelting
plant. Cadmium concentrations ranged from 0.1 to 5.7 mg/kg for the
soil and 20 to 202 mg/kg for the worms, and there was a correlation
between decreasing distance from the smelter and increasing cadmium
levels. Pietz et al. (1984) sampled soil and earthworms ( Aporrectodea
tuberculata) and ( Lumbricus terrestris) from mine soil and
non-mine soil, either amended or not with sewage sludge. Soil and
worms from mine soil gave residues of 0.6 and 3.8 mg/kg dry weight,
respectively, in non-amended soil and 2 and 22 mg/kg in sludge-amended
soil. Residues in soil and worms from non-mined soil were 1 and 12
mg/kg for non-amended and 3.5 and 36 mg/kg for sludge-amended soil,
respectively. The much lower capacity of worms from areas already
contaminated with cadmium to take up the metal suggests some selection
for varieties that control metal uptake. Morgan & Morgan (1988)
sampled earthworms ( Lumbricus rubellus and Dendrodrilus rubidus)
from one uncontaminated site and fifteen metal-contaminated sites (in
the vicinity of disused non-ferrous metalliferous mines) in the United
Kingdom. Cadmium concentrations in the worms ranged from 8 mg/kg (dry
weight) to 1786 mg/kg; they were generally higher than soil levels,
and the total soil cadmium explained 82% to 86% of the variability in
earthworm cadmium concentrations. The authors found some evidence that
cadmium accumulation was suppressed in extremely organic soils.
Martin et al. (1980) reported cadmium levels in a variety of
invertebrates sampled from sites contaminated by airborne cadmium. The
woodlouse was shown to accumulate cadmium principally in the
hepatopancreas.
Van Straalen & van Wensem (1986) analysed 13 species of
arthropods from an area polluted by zinc factory emissions. They found
no effect of body size or trophic level on the cadmium content of the
arthropods.
Roberts & Johnson (1978) sampled invertebrates and their diet
from the area of an abandoned lead-zinc mine in the United Kingdom.
They found cadmium levels higher in herbivorous invertebrates than in
the vegetation on which they fed (but not markedly so). There were
much higher levels of cadmium in carnivorous invertebrates, suggesting
that cadmium might have a capacity for accumulation in food chains.
In contrast to mercury levels, total cadmium body burdens were
higher in sparrows ( Passer domesticus) caught in industrialised
areas of Poland than in those caught in agricultural regions (Pinowska
et al., 1981). Pigeon brain, liver, and kidney sampled in rural,
suburban, and urban areas gave a good indication of the level of
environmental pollution with cadmium (Hutton & Goodman, 1980).
Hunter & Johnson (1982) monitored small mammals near to an
industrial works complex and found that cadmium accumulated
particularly in the liver and kidney. Cadmium levels in the liver
ranged rom 1.5 to 280 mg/kg (dry weight) and in the kidney from 7.4 to
193 mg/kg. Small mammals from unpolluted sites contained liver levels
ranging from 0.5 to 25 mg/kg and kidney levels of 1.5-26 mg/kg. The
insectivorous common shrew ( Sorex areneus) was found to be a more
prominent accumulator of cadmium than omnivorous and herbivorous small
mammals, based on body burden to dietary metal concentration ratios.
Similar results were obtained by Andrews et al. (1984) who monitored
cadmium levels in the herbivorous short-tailed field vole ( Microtus
agrestis) and the insectivorous common shrew ( S. araneus) from a
revegetated metalliferous mine site. Mean cadmium concentrations were
1.84 mg/kg (dry weight) and 52.7 mg/kg for voles and shrews,
respectively, values that were significantly higher than those found
in control sites.
4. KINETICS AND METABOLISM
Appraisal
In aquatic systems, cadmium is most commonly taken up by
organisms directly from water, but may also be ingested with
substantially contaminated food. The free metal ion, Cd2+, is the
form most available to aquatic species. Uptake from water may be
reduced by the concentration of calcium and magnesium salts (water
hardness). Cadmium uptake from sea water may be greatly reduced by
the formation of less available complexes with chloride. Organic
complexes with cadmium can be classified in three groups: those that
are unavailable (e.g., EDTA, NTA, DPTA), those that are available but
less so than the free Cd2+ (e.g., fulvic acids of low relative
molecular mass), and those that form readily available hydrophobic
complexes with cadmium (xanthates and dithiocarbamates).
Organisms in the freshwater environment are contaminated
according to their ability to absorb or adsorb cadmium from the
water, rather than to their position in the food chain. Consequently,
differences in cadmium concentration between species at the same
trophic level are common and there is no evidence for
biomagnification. Conversely, marine organisms take up cadmium
principally from food. The primary source of cadmium in terrestrial
systems is the soil, and uptake follows the typical food chain
pathway, although deposition of cadmium on plant and animal surfaces
can account for some additional contamination at each trophic level.
Variations in uptake and retention occur, and there is some evidence
for biomagnification in carnivores. Organisms that feed on sediment
or detritus may accumulate more cadmium than those in the grazing
food chain. High levels of cadmium have been reported in sea mammals,
pelagic sea-birds, and terrestrial invertebrates.
Within a variety of organisms, cadmium is distributed throughout
most tissues, but tends to accumulate in the roots, gills, livers,
kidneys, hepatopancreas, and exoskeleton. Cadmium in the cell is
often bound to cytoplasmic proteins, a possible detoxifying
mechanism. Elimination probably occurs primarily via the kidney but
also via moulting of the exoskeleton.
There is some evidence of an interaction between cadmium and
other metals, especially calcium and zinc. Cadmium may replace
calcium on the calcium-specific protein calmodulin and is affected by
other physiological processes that regulate the uptake of calcium. In
certain circumstances, zinc increases cadmium retention in the liver
and kidneys of aquatic vertebrates. In terrestrial systems, high soil
zinc levels can reduce cadmium uptake appreciably.
Selection can lead to cadmium-tolerant populations in both the
aquatic and terrestrial environments.
4.1 Uptake
4.1.1 Uptake from water by aquatic organisms
Several studies have shown that the free metal ion, Cd2+, is
the form of cadmium most available to aquatic organisms (Sunda et al.,
1978; Borgmann, 1983; Part et al., 1985; Sprague, 1985).
Inorganic cadmium complexes appear not to be taken up, at least
by fish (Part et al., 1985). This is particularly important in marine
water where cadmium is mainly present in soluble chloride complexes
(Zirino & Yamamoto 1972). It is most probable that chloride
complexation is responsible for the reduced cadmium accumulation and
toxicity in a variety of organisms observed with increasing salinities
(Coombs, 1979).
In the case of organic cadmium complexes, the chemical properties
are of importance with respect to bioavailability. Three categories
can be distinguished. The first comprises cadmium complexes with EDTA,
NTA, and DPTA, which are unavailable to aquatic organisms (Sunda et
al., 1978; Part & Wikmark, 1984). The second consists of complexes
that to some extent contribute to the total metal uptake, i.e. uptake
is higher than predicted from the actual Cd2+ activity, but the
complex is still less available than the free Cd2+ ion. This group
includes fulvic acids of low relative molecular mass (Giesy et al.,
1977; John et al., 1987), the amino acid histidine (Pecon & Powell,
1981), and carboxylic acids like citric acid (Guy & Ross Kean, 1980;
Part & Wikmark, 1984). The third category includes compounds such as
xanthates and dithiocarbamates that form hydrophobic complexes with
heavy metals. These hydrophobic complexes act as metal carriers across
biological membranes and they lead to a greater uptake of cadmium in
aquatic organisms than when the metal is present as the free ion
(Poldoski, 1979; Block & Part, 1986; Gottofrey et al., 1988; Block,
1991). This latter observation is of particular environmental concern
because xanthates are used in the mining industry in the enrichment of
metals from sulfide ores by flotation. Xanthate concentrations of
between 4 and 400 µg/litre have been measured in waters receiving
effluent from metal refineries (enrichment plants) (Waltersson, 1984).
Another water quality parameter affecting cadmium uptake is the
Ca2+ and Mg2+ concentration (hardness) of the water. Increasing
Ca2+ concentration reduces cadmium uptake through fish gills (Part
et al., 1985; Wicklund, 1990), cadmium accumulation (Carroll et al.,
1979), and cadmium toxicity for fish (Calamari et al., 1980). Two
mechanisms can be distinguished for the Ca2+-mediated reduction in
cadmium uptake. The first is an inhibitory effect on uptake into gill
tissue, while the second is related to the adaptive response of the
fish to increased Ca2+ concentrations (Calamari et al., 1980,
Wicklund 1990). Mg2+ also reduces cadmium uptake through fish gills
but at 5 times higher concentrations than Ca2+ (Part et al., 1985).
Cadmium uptake in fish is not strongly pH dependent; uptake in
rainbow trout gills was not affected over the pH range 5-7 (Part et
al., 1985).
Recent data from fish gills indicate that, to some extent, Cd2+
shares uptake mechanisms with Ca2+; these two ions are about the
same size and also form complexes with the same kind of ligands. Thus
Cd2+ can replace Ca2+ in the calcium-specific protein calmodulin
(Flik et al., 1987). In the gills, Cd2+ is assumed to enter the
epithelial cells down its concentration and electrical gradient by
facilitated diffusion through a calcium channel in the apical membrane
(Verbost et al., 1989). Several lines of evidence support this
assumption. Firstly, increasing water Ca2+ concentrations reduce
cadmium uptake. Secondly, cadmium in the water inhibits Ca2+ uptake
in the gills (Verbost et al., 1987; Reid & McDonald, 1988). Thirdly,
La3+, a calcium channel blocker in cell membranes, inhibits both
Ca2+ and Cd2+ uptake in the gills. Fourthly, the hypocalcaemic
hormone stanniocalcin reduces both Ca2+ and Cd2+ uptake in the
gills (Verbost et al., 1989). Stanniocalcin has been shown to close
the apical calcium channel in the gill epithelial cells thereby
reducing Ca2+ uptake from the water (Lafeber et al., 1988). The
hormone is secreted when the fish has a surplus of Ca2+, i.e.
hypercalcaemic. The two-fold effect of Ca2+ on cadmium uptake in
fish discussed previously can be well explained by this model. A
direct competition between Ca2+ and Cd2+ at the apical calcium
channel reduces the uptake of cadmium into the cells, while the
adaptive response in Ca2+-rich water probably involves an increased
stanniocalcin level, which closes the apical calcium/cadmium channel.
The transport mechanism from the epithelial cells to the blood is
unclear. Cadmium is not transported by the high affinity Ca-ATPase in
the basolateral epithelial membrane which transports Ca2+ (Verbost
et al., 1988). The possible involvement of the Na+/Ca2+ exchange
mechanism, where Cd2+ replaces Ca2+, has recently been suggested
as a translocation mechanism to the blood (personal communication to
the IPCS by G. Flik).
Zinc also has been shown to reduce cadmium uptake through the
gills (Wicklund, 1990). Like cadmium, zinc is assumed to enter the
epithelial cell by facilitated diffusion (Spry & Wood, 1989) and,
furthermore, Ca2+ acts antagonistically on zinc uptake.
Taken together, these data suggest that the apical epithelial
membrane of fish gills contains an ion channel shared by cadmium and
calcium, and probably also zinc. The movement of metals through this
channel is controlled both by external factors such as the Ca2+
content of the water and internal factors such as hormones.
Increasing temperature increases the uptake of cadmium from water
(Vernberg et al., 1974; Zaroogian & Cheer, 1976; Denton &
Burdon-Jones, 1981).
4.1.1.1 Microorganisms
In the alga Chlorella pyrenoidosa, uptake of cadmium was
completely blocked by 0.2 mg manganese/litre and inhibited by 2 to 5
mg iron/litre, but calcium, magnesium, molybdenum, copper, zinc, and
cobalt had no effect on uptake (Hart & Scaife, 1977).
Cultures of Chlorella accumulate twice as much cadmium at pH
7.0 as at pH 8.0 when exposed to 0.5 mg cadmium/litre (Hart & Scaife,
1977).
4.1.1.2 Aquatic molluscs
Hardy et al. (1984) found greater uptake of cadmium from sea
water into oysters given an uncontaminated phytoplankton food source
than into those without food. The authors explain their findings on
the basis that the presence of phytoplankton increases the flow of
water through the oysters. Studies on oysters without a food source
may thus underestimate cadmium uptake. Oysters fed phytoplankton
containing cadmium retained only 0.59% of this cadmium; the majority
of the cadmium in molluscs is taken up directly from the water. The
oyster accumulates about twice as much cadmium in summer as in the
winter. This is presumed to reflect the increased flow of water
through the animal at higher temperatures (Zaroogian & Cheer, 1976).
Hardy et al. (1981) showed that clams ( Protothaca staminea)
took up much less cadmium from water in the presence of sediment at
3.6 g/litre. The uptake was only 17% of that measured in sediment-free
water.
Langston & Zhou (1987a,b) found no evidence of cadmium uptake
into the bivalve Macoma balthica involving metallothionein or
metallothionein-like proteins. Accumulation in soft tissues was linear
throughout a 29-day exposure period, whereas uptake onto the shell was
characterized as saturation kinetics. In contrast, the gastropod
Littorina littorea did show induction of specific cadmium-binding
proteins, which contributed to uptake and storage of cadmium.
Watling & Watling (1983) demonstrated uptake of cadmium in a
dose-dependant manner into sandy beach gastropod molluscs in
laboratory experiments. Much of the cadmium (as chloride) accumulated
in the gill. The rate of cadmium uptake was 0.01 mg/kg per day for
Donax serra and 0.16 mg/kg per day for the smaller Bullia
rhodostoma after exposure to cadmium at 20 µg/litre. The freshwater
snail Physa integra took up more cadmium as exposure increased,
concentrations ranging between 1 and 40 µg/litre. The highest
concentration factors were found with the lowest exposure
concentration (Spehar et al., 1978a). Wier & Walter (1976) exposed the
freshwater snail Physa gyrina to 1.3 mg cadmium/litre (as the
chloride) and found an average cadmium uptake rate of 0.55 mg/kg per
hour over 24 h. Heavier snails took up less cadmium, after the same
exposure, than lighter individuals.
4.1.1.3 Other aquatic invertebrates
Rainbow & White (1989) investigated uptake of cadmium and zinc in
three marine crustaceans, Palaemon elegans (Decapoda),
Echinogammarus pirloti (Malacostraca), and Elminius modestus
(Cirripedia) at water concentrations of cadmium between 0.5 and 1000
µg/litre and zinc between 2.5 and 4000 µg/litre. All three crustaceans
accumulated the non-essential cadmium at all dissolved cadmium
concentrations without regulation. Differences between species were
interpreted by the authors in terms of differences in cuticle
permeability and way of life. All three species took up zinc more
rapidly than cadmium; the ratios between molar uptake rates of zinc to
cadmium were 11.4:1, 2.7:1, and 3.7:1 for the three species,
respectively, following an exposure to a molar ratio of 1.7:1.
4.1.1.4 Fish
Cadmium uptake in fish continues for some considerable time in
fish exposed to the metal. The peak of tissue residues may not be
reached for several weeks, particularly after exposure to low
concentrations of the metal (Cearley & Coleman, 1974; Benoit et al.,
1976; Sullivan et al., 1978a).
Douben (1989a) exposed the stone loach Noemacheilus barbatulus
to cadmium in water (as the sulfate) at a concentration of 1 mg/litre
and monitored uptake and loss at different temperatures with fed and
starved fish. The size of the fish affected both uptake and loss of
cadmium, bioconcentration factors decreasing with size. Uptake of
cadmium increased with temperature up to about 16 °C and decreased as
the concentration of cadmium in the water increased. Feeding the fish
increased the rate of uptake of cadmium from the water. The author
concluded that metabolic rate was an important factor in the uptake of
cadmium into the fish and in its subsequent loss.
4.1.1.5 Model aquatic ecosystems
Ferard et al. (1983) investigated the transfer of cadmium through
a model food-chain consisting of an alga, a daphnid, and a fish.
Concentration factors relative to food were low, indicating that
cadmium is mainly taken up directly from water. Daphnids fed algae
containing cadmium at between 4.5 and 570 mg/kg dry weight showed a
maximum concentration factor of 1. Fish fed contaminated daphnids or
algae showed concentration factors of 0.0038 and 0.0018, respectively.
Nimmo et al. (1977) reported low concentration factors, ranging from
0.018 to 0.027, for grass shrimp fed on brine shrimp containing
cadmium at between 27 and 182 mg/kg. Rehwoldt & Karimian-Teherani
(1976) fed zebrafish on food containing cadmium acetate at 10 mg/kg
over a period of 6 months. Maximum residues, in males and females
respectively, were 5.92 and 13.64 mg/kg, the median residue levels
after 6 months of exposure being 5.19 and 12.95 mg/kg (on a dry weight
basis).
4.1.1.6 Uptake from aquatic sediment
Ray et al. (1980b) exposed the ragworm Nereis virens to
sediment to which cadmium chloride had been added. Smaller worms took
up more cadmium relative to body weight than larger worms. The cadmium
was taken up in a dose-related manner and no equilibrium was reached
during the 24-day experiment. The rate of uptake directly from sea
water also increased with exposure concentration over the range of
0.03 to 9.2 mg/litre. For the range of sediment cadmium concentrations
used (1 to 4 mg/kg), the corresponding concentrations in the overlying
sea water were 0.03 to 0.1 mg/litre. Comparing uptake into the
ragworms from water with these concentrations to the uptake from the
spiked sediment produced identical concentrations of cadmium in the
worms. Rate of uptake from sediment was between 16 and 39 times less
than the uptake from the corresponding exposure to cadmium in water.
The authors concluded that all of the uptake of cadmium from sediment
derived from desorbed metal ions in the interstitial water.
4.1.1.7 Uptake from food relative to uptake from water
Fish can take up cadmium from the surrounding water and from
ingested food. The main uptake route in fresh water is from the water
via the gills (Williams & Giesy, 1978). However, the relative
importance of food and water to the body burden depends very much on
the cadmium content of the food organism. In contaminated areas with
an increased cadmium content in food organisms, the relative
importance of food as a cadmium source may increase. In the marine
environment, where cadmium is mainly present in chloride complexes not
available to fish, the relative importance of food as a cadmium source
increases. Consequently food has been shown to be the main cadmium
source in marine fish (Pentreath, 1977; Dallinger et al., 1987).
4.1.2 Uptake by terrestrial organisms
4.1.2.1 Uptake into plants
The uptake of cadmium into plants generally depends upon the
availability of the metal in soil solution. The soil pH and
composition, particularly the nature of soil clays, the organic matter
content, and, obviously, the soil cadmium level, affect this
availability. The relationship between soil cadmium level and plant
uptake is not a simple one because of the wide variety of soil
characteristics that affect the extent of cadmium uptake. Cataldo &
Wildung (1978), Peterson & Alloway (1979), and Page et al. (1981) have
reviewed this subject.
Plants grown in a greenhouse or a container take up more cadmium
than the same plants grown in soil with the same cadmium levels in the
field. This is due to greater root development in a confined volume in
containers and to the fact that all the roots are in contact with
cadmium-contaminated soil. In the field, roots may grow down below the
cadmium-contaminated level (Page & Chang, 1978; De Vries & Tiller,
1978).
Mahler et al. (1978) cultured lettuce and chard on acid or
calcareous soils to which cadmium sulfate had been added at levels up
to 320 mg/kg. For both types of soil there was a dose-related uptake
of cadmium from soil into leaves. The uptake of the metal was much
greater in acid than in calcareous soils, particularly at higher rates
of cadmium application (over 40 mg/kg). At the highest soil
concentration of 320 mg/kg, lettuce leaves contained cadmium at a
concentration of 800 mg/kg and chard leaves 1600 mg/kg when grown in
acid soil. Leaves of lettuce cultured on calcareous soils with cadmium
at 320 mg/kg contained a lower cadmium concentration of 200-300 mg/kg
and chard, similarly cultured, contained 300 mg/kg or less. Bingham et
al. (1980) showed an effect of soil pH on cadmium (as sulfate) uptake
in rice; more metal was incorporated as acidity increased. Chaney et
al. (1975) reported that liming of soil in which soybeans were growing
decreased the concentrations of cadmium in leaves from 33 to 5 mg/kg
dry weight as pH increased from 5.3 to 7.0. Eriksson (1988)
investigated the effect of pH on the uptake of cadmium into perennial
ryegrass ( Lolium perenne) and winter rape ( Brassica napus). The
more soluble fractions of cadmium in soil increased as the pH was
lowered; increasing the pH from 5 to 7 with calcium oxide invariably
reduced the cadmium content of ryegrass plants, but this decrease was
less consistent when the pH was increased from 5 to 6. The cadmium
content of rape plants was markedly higher at pH 4 than pH 5. Adding
more cadmium to the soil increased the amount of cadmium in the plants
in direct proportion to the increased concentration of the metal in
soil over the range 0 to 5 mg/kg. Eriksson (1988) found that soil
organic matter decreased the availability of cadmium to perennial
ryegrass and winter rape grown in pots. Addition of organic material
to sand and clay soils reduced cadmium uptake to a greater extent in
the sand.
When Mitchell & Fretz (1977) cultured seedlings of three species
of tree (red maple, white pine, and Norway spruce) hydroponically or
in soil with added cadmium, the concentration in roots was greater
than that in leaves. Cadmium added to soil was less readily taken up
than cadmium added to nutrient solutions. Similarly, Root et al.
(1975) reported greater cadmium concentration in roots than in shoots
of maize grown hydroponically in a medium containing cadmium chloride.
Harkov et al. (1979) found the highest uptake of cadmium into
hydroponically grown tomatoes in the roots, while stems had lower
cadmium concentrations than leaves.
Lepp et al. (1987) measured high concentrations of cadmium in the
sporophores (fruiting bodies) of the fungus Amanita muscaria growing
in birch woodland. The fungus sporophores contained 29.9 mg/kg dry
weight, compared to a cadmium level of 0.4 mg/kg in the soil on which
they grew. The cadmium was released from the rotting sporophore, after
it had shed its spores, in a form which was readily available to other
plants growing on the woodland soil; this was shown experimentally
with lettuce plants grown in pots. The authors calculated that an
abundant population of sporophores could recycle 1.4% of the total
cadmium load in leaf litter to higher plants over a period of 14 days
(the mean lifespan of the sporophores).
4.1.2.2 Terrestrial invertebrates
Beyer et al. (1982) demonstrated that earthworms concentrated
cadmium from soils amended with sewage sludge containing cadmium
oxide. Cadmium concentrations were as high as 100 mg/kg in worms
exposed to soils containing cadmium at 2 mg/kg, a concentration factor
of 50. Adding calcium carbonate to soils decreased the cadmium uptake
of worms slightly, while high soil zinc levels decreased the cadmium
uptake appreciably. Results were variable with different sludge
treatments. Hartenstein et al. (1980) amended sludge with 10, 50, and
100 mg/kg cadmium (as cadmium sulfate) and added earthworms ( Eisenia
foetida). The worms accumulated 3.9, 2.04, and 1.44 times the
respective sludge levels of cadmium over a period of 5 weeks. In field
trials on non-amended soils containing 12 to 27 mg cadmium/kg, worms
sampled during a 28-week period gave levels of cadmium ranging from 8
to 46 mg/kg.
Terrestrial pulmonate snails retained up to 59% of cadmium
administered in their diet as the chloride (Russell et al., 1981). The
highest retention was after dosing at 25 mg cadmium/kg diet. The
higher the dose (up to 1000 mg/kg diet) the lower the percentage
retention of the metal. Ireland (1981) noted that in the terrestrial
slug Arion ater most of the cadmium was located in the digestive
gland without association with any particular sub-cellular organelles,
and isolated a specific cadmium-binding protein from the animals.
4.1.2.3 Birds
In a study by White & Finley (1978), adult mallard ducks were fed
a diet containing cadmium chloride at levels of 2, 20 or 200 mg/kg and
killed at 30-day intervals. The cadmium content increased with dose
level and time (except in the case of the highest dose where body
burden peaked after 60 days), and the highest concentrations occurred
in the liver and kidney. The highest levels overall occurred after
dosing for 60 days at 200 mg/kg; cadmium concentrations were 109 mg/kg
in the liver and 134 mg/kg in the kidney.
Nicholson & Osborn (1983) dosed starlings ( Sturnus vulgaris)
with cadmium chloride at a concentration of 2 mg/kg body weight, three
times weekly for 6 weeks, and reported a wide range of kidney
concentrations (from < 10 to > 200 mg/kg dry weight).
4.2 Distribution
4.2.1 Aquatic organisms
In higher organisms, cadmium can be bound in several different
tissues, whereas in plants cadmium is bound to the cell wall in roots.
Brooks & Rumsby (1967) measured the cadmium taken up by the
oyster ( Ostrea sinuata) from water containing 115Cd (50 mg/litre).
The soft parts of the oyster contained 100 mg cadmium/kg after 100 h.
Concentrations in tissues were, in decreasing order, 360 mg/kg for
gills, 285 mg/kg for heart, 141 mg/kg for the visceral mass, 83 mg/kg
for the mantle, 53 mg/kg for white muscle, and 25 mg/kg for striated
muscle.
Nimmo et al. (1977) reported that in the pink shrimp the
hepatopancreas took up more cadmium than other tissues. Lower
concentrations were found in the exoskeleton, muscle, and serum.
Short-term exposure of the crab Uca pugilator to cadmium chloride
led to the hepatopancreas and gill concentrations of the metal being
similar after a 24-h exposure to 1 mg cadmium/litre (Vernberg et al.,
1974).
Sangalang & Freeman (1979) determined the cadmium in tissues of
brook trout exposed to the metal (added as the chloride) via the water
or by injection. After water exposure to cadmium chloride at 1
µg/litre, the trout showed greatest uptake of the metal in the gills,
kidney, and liver. The gills and the posterior kidney revealed a
higher metal content than any other tissues. Levels of cadmium in
whole blood and plasma, heart, spleen, testis, stomach, and skin were
higher than control levels after 77 and 93 days of exposure. Smith et
al. (1976) found the greatest accumulation of the metal in the kidney
of catfish exposed to cadmium (as sulfate) in the water. In an
autoradiographic study of cadmium distribution in rainbow trout
exposed to cadmium in water, Tjalve et al. (1986) confirmed the
general picture of cadmium distribution, the metal being found in the
gills, liver, and kidney. However, they also observed heavy labelling
of the olfactory rosette and the olfactory nerve, an observation not
reported earlier. In a detailed study they later showed that cadmium
was transported axonally from the olfactory rosette to the bulbus
olfactorius but not further into the brain (Gottofrey, 1990). The
significance of this observation with respect to the olfactory
responses of fish in cadmium-contaminated environments remains to be
investigated.
The few studies that have been conducted on the subcellular
distribution of cadmium indicate that, while much is located in the
cytosol, a significant proportion can be found in the nucleus and the
mitochondria. Cadmium is bound in the cytosol to proteins of low
relative molecular mass, metallothioneins, and other cadmium-binding
proteins. These proteins are rich in the sulfur-containing amino acid
cysteine but poor in aromatic amino acids.
Metallothioneins have been isolated and characterized in a number
of aquatic and terrestrial organisms. Fish metallothioneins have
received considerable interest in recent years as tools in monitoring
metal pollution in the environment (Hamilton & Mehrle, 1986; Hogstrand
& Haux, 1990a). Simple methods to analyse fish metallothionein have
been developed, including differential pulse polarography (Olson &
Haux, 1986) and radioimmunoassay based on specific antibodies to fish
metallothionein (Hogstrand & Haux, 1990b). Olson & Haux (1986) found
a strong correlation between hepatic metallothionein and cadmium
accumulation in perch collected from cadmium-contaminated water.
4.2.2 Terrestrial organisms
4.2.2.1 Terrestrial plants
Jones & Johnston (1989) analysed cereal grain and herbage from
long-term experimental plots at Rothamsted, United Kingdom, and found
that uptake of cadmium into herbage was greatest where phosphate
fertilizer had been applied. It was also greater from unlimed soils
than from limed soils. However, the authors concluded that there was
little evidence of a long-term (1840-1986) increase in crop cadmium
concentrations.
Byrne et al. (1976) analysed higher fungi from Slovenia,
Yugoslavia, and found levels of cadmium ranging from 0.53 to 39.9
mg/kg dry weight (average 5.0 mg/kg). This is an order of magnitude
higher than in most other plants. Although the fungi were collected
from industrial, urban, and uncontaminated sites, the levels found in
the fungi were not very different between sites. The authors suggested
geological rather than industrial sources for the cadmium in these
soils.
The high uptake by mushrooms and related species is probably due
to a cadmium-binding phosphoglycoprotein, cadmium-myco-phosphatin,
which has been isolated from the mushroom Agaricus macrosporus
(Meisch & Schmitt, 1986).
4.2.2.2 Terrestrial invertebrates
Hopkin & Martin (1985) investigated the storage of cadmium in the
woodlouse Oniscus asellus from heavily contaminated woodland 3 km
downwind from a smelter. The hepatopancreas was found to contain up to
5 g cadmium/kg dry weight without apparent ill effects upon the
organism. Cadmium was reported to be stored intracellularly in the
copper- and sulfur-containing granules of epithelial S cells. In a
later study (Hopkin, 1990) it was found that considerable interspecies
differences exist with regard to storage in the hepatopancreas.
Oniscus asellus stored five times more cadmium than Porcello scaber
under the same conditions. The carnivorous centipede Lithobius
variegatus, when fed on cadmium-contaminated hepatopancreas from
woodlice, accumulated cadmium which was likewise stored in the midgut
(Hopkin & Martin, 1984).
Berger & Dallinger (1989) studied the distribution of cadmium
between several organs of the terrestrial snail Arianta arbustorum
during a 20-day feeding experiment on cadmium-enriched agar. Of the
cadmium in the medium, 54% was taken up, of which 66% was distributed
to the hepatopancreas, leading to a concentration of more than 500
mg/kg dry weight. In other organs (intestine, foot/mantle, gonads),
the cadmium concentration was considerably lower.
In the earthworm Lumbricus rubellus taken from heavy-metal-
polluted soil, more than 70% of the cadmium burden was found in the
posterior alimentary canal (Morgan & Morgan, 1990). This distribution
prevented dissemination of large concentrations of cadmium into other
tissues and, according to the authors, may represent a detoxification
strategy.
4.3 Elimination
Information on loss of cadmium from organisms is relatively
scarce. The information that does exist suggests that this is very
variable, and has been reviewed by Coombs (1979) and Taylor (1983).
Organisms that accumulate cadmium also tend to retain the metal for
long periods. The main excretory route appears to be via the kidney,
except in the case of organisms that moult, where loss from the shed
exoskeleton can be significant.
Robinson & Wells (1975) administered a single oral dose of
cadmium acetate to softshell turtles ( Trionyx spinifer) and killed
and dissected the animals either 48 h or 96 h later. After 48 h, 9.43%
of the total dose was recovered from tissues, while turtles killed
after 96 h had retained 4.02% of the dose. The greatest retention of
cadmium, after both time periods, was in the liver. Cadmium was also
retained in the small intestine for the first 48 h, but the amount had
decreased by 96 h.
Harrison & Klaverkamp (1989) exposed rainbow trout ( Salmo
gairdneri) and lake whitefish (Coregonus clupeaformis) to cadmium in
water, via a continuous-flow system, or the diet, via pelleted food,
for 72 days. The fish were then kept in clean water on a cadmium-free
diet for a further 56 days. In the case of water-exposed fish, the
majority of the cadmium was present in the gill and kidney, but
food-exposed fish retained cadmium principally in the kidney, gut, and
liver. Bioconcentration factors for exposure via the water were 55 for
the trout and 42 for the whitefish, whereas concentration factors from
the food were less than 1 for both species. However, both species
accumulated a greater proportion of the cadmium that was in the food
than that in water (1% as against 0.1%). Equilibrium bioconcentration
factors were estimated to be 161 for trout and 51 for whitefish.
In the same model, the half-times for depuration of accumulated
cadmium ranged from 24 to 63 days. Douben (1989b) investigated the
kinetics of cadmium in freshwater fish (the stone loach Noemacheilus
barbatulus) exposed to cadmium via the diet (tubifex worms
previously contaminated with cadmium by uptake from water). The body
burden of cadmium declined after the period of feeding with
contaminated diet more rapidly in starved than in fed fish. Rate
constants for loss of cadmium appeared to be greater during the
exposure period than after exposure. Both uptake and loss of cadmium
were influenced by the body weight of the fish.
Janssen et al. (1991) investigated uptake and loss of cadmium
from contaminated soil by four species of soil arthropod and developed
kinetic models that gave good predictions of the degree of
accumulation in a variety of species. They also reviewed data on other
soil arthropods (Tables 6 and 7). The kinetics of cadmium in different
arthropods is related to taxonomy and reflects the different
physiological characteristics of the different organisms. Some,
notably isopods and molluscs, take up and retain cadmium in their
tissues with little or no excretion. These species are capable of
holding large quantities of the metal in the hepatopancreas without
apparent ill effect. There is no direct correlation between
assimilation capacity and the capacity to excrete or eliminate
cadmium. Figure 4 illustrates the uptake of cadmium (measured as total
body burden) and its subsequent loss in four species of arthropods.
Elimination half-lives of 53, 8, and 2 days, respectively, have been
reported for Platynothrus peltifer, Orchesella cincta, and
Notiophilus biguttatus; no elimination took place over 130 days in
Neobisium muscorum.
Sawicka-Kapusta et al. (1987) investigated the effect of keeping
the vole Clethrionomys glareolus at different temperatures on the
rate of loss of cadmium from body tissues. Although the different
temperatures (10 °C and 20 °C) affected the metabolic rate of the
voles, there was no difference in the rate of loss of cadmium.
4.4 Bioaccumulation and biomagnification
Bioaccumulation occurs when the concentration in the organism
exceeds the concentration in the nutrient medium and is expressed
quantitatively as a bioconcentration factor. Progressive
bioaccumulation at each trophic level is termed biomagnification.
 Table 6. Cadmium assimilation efficiencies in different soil invertebrates
Species Food Cadmium concentration Assimilation efficiency Reference
in food (µmol/g) (%)
Snail
Arianta arbusloruma agar 1.48 55-92 Berger & Dallinger (1989) b
Centipede
Lithobius variegatus isopod 1.21-10.2 0-7.2 Hopkin & Martin (1984)
hepatopancreas
Millipede
Clomeris marginata maples leaves 8.2-40.6 Hopkin et al. (1985)
Pseudoscorpion
Neobisium muscorum collembolans 0.20 58.9 Janssen et al. (1991)
Mite
Platynothrus peltifer green algae 0.15 17.2 Janssen et al. (1991)
Insects
Orchesella cincta green algae 0.09 8.3 Van Straalen et al. (1987)
Orchesella cincta green algae 0.15 9.4 Janssen et al. (1991)
Notiophilus biguttatus collembolans 0.23 35.5 Janssen et al. (1991)
a assimilation value for midgut gland
b recalculated from the data
Table 7. Excretion constants (k) for cadmium in different soil invertebrates
Species Taxonomic k Reference
group (day-1)
Helix pomatia snail 0 Dallinger & Wieser (1984) b
Cepaea nemoralis snail 0.007 Williamson (1980) b
Oniscus asellusa isopod 0.002 Hopkin (1989) b
Neobisium muscorum pseudoscorpion 0 Janssen et al. (1991)
Lycosa spp spider 0.007 Van Hook & Yates (1975)
Platynothrus peltifer oribatid mite 0.013 Janssen et al. (1991)
Orchesella cincta collembolan 0.061 Van Straalen et al. (1987)
Orchesella cincta collembolan 0.087 Janssen et al. (1991)
Acheta domesticus cricket 0.090- Van Hook & Yates (1975)
0.110
Notiophilus biguttatus carabid beetle 0.375 Janssen et al. (1991)
a k value for midgut gland or hepatopancreas
b recalculated from the data
Bioconcentration factors (the ratio between the cadmium
concentration in the organism and the concentration in the medium) for
several groups of organisms studied under laboratory conditions are
shown in Table 8. They range from 16 to 130 000 and do not seem to
show any consistent pattern.
Table 8. Bioconcentration of cadmium in laboratory studies
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Freshwater alga 10 10 3000 dw c Ferard et al. (1983)
(Chlorella vulgaris)
Freshwater alga stat 20-22 14 250 4940 daw Cain et al. (1980)
(Scenedesmus obliquus)
Freshwater diatom flow WB 23 10 40 000 Conway (1978)
(Asterionella formosa)
Submerged plant WP 25 30 25 1730 dw Nakada et al. (1979)
(Elodea nuttallii)
Water hyacinth leaves 28 500 16 dw c Kay & Haller (1986)
(Eichhornia crassipes)
American oyster 4.9-5.1 g flow WB 16-20 21 10 116 ww Eisler et al. (1972)
(Crassostrea virginica) 4280 aw
8.1 g flow ST 2.8-22.6 280 5 2376 ww Zaroogian & Cheer (1976)
18 472 dw
Mussel 32-34 mm flow ST 13 166 10 50 802 dw Riisgard et al. (1987)
(Mytilus edulis)
Scallop 6.8-7.7 g flow WB 16-20 21 10 131 ww Eisler et al. (1972)
(Aquipecten irradians) 3970 aw
Bay scallop 0.51-0.73 g flow ST 9.5-16 42 60 20 400 Pesch & Stewart (1980)
(Argopecten irradians)
Crab 2-4 g WB 10 14 37 152 dw Ray et al. (1980a)
(Pandalas montagui)
Grass shrimp 20-33 mm flow WB 9.5-16 42 60 223 Pesch & Stewart (1980)
(Palaemonetes pugio)
Table 8 (contd).
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Lobster 160-169 g flow WB 16-20 21 10 21 ww Eisler et al. (1972)
(Homarus americanus) 10 aw
Mummichog 2.3-2.4 g flow WB 16-20 21 10 15 ww Eisler et al. (1972)
(Fundulus heteroclitus) 200 aw
Fathead minnow flow WB 13.9-15.3 21 49 190 Sullivan et al. (1978a)
(Pimephales promelas)
Red maple leaves 15-27 45 0.5 14 400 dw d Mitchell & Fretz (1977)
(Acer rubrum) roots 15-27 45 0.5 131 800 dw d Mitchell & Fretz (1977)
leaves 15-27 101 2.6 mg/kg 0.76 dw e Mitchell & Fretz (1977)
roots 15-27 101 2.6 mg/kg 12.5 dw e Mitchell & Fretz (1977)
White pine leaves 15-27 66 0.5 3400 dw d Mitchell & Fretz (1977)
(Pinus strobus) roots 15-27 66 0.5 118 400 dw d Mitchell & Fretz (1977) leaves 15-27 36 52.6 mg/kg 1.2 dw e Mitchell & Fretz (1977)
roots 15-27 36 52.6 mg/kg 10.4 dw e Mitchell & Fretz (1977)
a WB = whole body; WP = whole plant; ST = soft tissues
b Chloride salt used unless stated otherwise; bioconcentration factor = concentration in the organism divided by concentration
in the medium; dw = dry weight; ww = wet weight; aw = ash weight; daw = dry ash weight
c Nitrate salt used
d The medium was a cadmium-enriched nutrient solution
e The medium was a cadmium-amended soil mix
Microorganisms generally exhibit a high capacity to take up
cadmium from water and retain the metal in their cells. The highest
bioconcentration factors reported have been for micro-organisms, the
greatest value being 40 000 in a freshwater diatom (Conway, 1978). In
this diatom, 58% of the cadmium was located in the cellular content
with 25% in the organic coating of the frustule and 17% in the
silicaceous frustule. The bioconcentration factor of 3000 for the alga
Chlorella (Ferard et al., 1983) is typical of the value for
microorganisms. Flatau et al. (1988) demonstrated the uptake (it was
not specified whether this referred to absorption or adsorption) of
cadmium from sea water by marine bacteria; the uptake of the metal
increased with its concentration in the water, and the accumulation
rate was a logarithmic function of the dose. Sorption was only
observed with exposure concentrations above 10 µg Cd/litre, suggesting
that a threshold had to be exceeded for cadmium uptake to occur.
Dongmann & Nurnberg (1982) showed that the bioconcentration factor for
a marine diatom, Thalassiosira rotula, decreased with increasing
metal concentration, suggesting a saturation effect. Their reported
concentration factors, which vary between 1000 and 2000, reflect the
reduced sorption of cadmium by marine microorganisms compared with
their freshwater relatives. Hart & Scaife (1977) reported a direct
relationship between the level of cadmium in the medium and sorption
to the alga Chlorella exposed to cadmium concentrations ranging from
0.25 to 1.00 mg/litre.
After water hyacinths had been exposed for 4 weeks to water
containing 0.5 or 1.0 mg cadmium/litre, added as cadmium nitrate, the
leaves had accumulated 8.00 and 17.20 mg/kg, respectively (Kay &
Haller, 1986).
Molluscs concentrate cadmium to a high degree over a period of
time, but uptake is often slow. Oysters showed a concentration factor
of only 149 over a 10-day period (Eisler et al., 1972) but a factor of
2714 after 40 weeks (Zaroogian & Cheer, 1976). Elliott et al. (1985)
examined the accumulation of cadmium, copper, lead, and zinc in the
tissues of the mussel, Mytilus edulis. Under simultaneous exposure
to all four metals, both lead and cadmium were accumulated in direct
proportion to the exposure time, whereas copper and zinc were not.
Accumulation of cadmium was influenced by the presence of other
metals.
Compared with oysters, the related bay scallop shows greater
accumulation of cadmium when exposed to low concentration of the metal
as the chloride over 6 weeks (Pesch & Stewart, 1980). Short-term
exposure of the same scallop to higher concentrations of cadmium
resulted in very much lower concentration factors. Exposure for 96 h
to cadmium (as the chloride) at up to 2.0 mg/litre led to a
bioconcentration factor of around 50 (Nelson et al., 1976).
Bioconcentration factors (from water and food) and
biomagnification factors (from food alone) were calculated for the
freshwater isopod Assellus aquaticus by van Hattum et al. (1989).
Much of the cadmium (added as the chloride) was taken up from the
water (bioconcentration factor 18 000), but there was little uptake
from food (bioconcentration factor 0.08). Direct uptake from water
accounted for between 50 and 98% of the body burden after 30 days of
exposure (based on dry weight measures). Cadmium was readily taken up
by the isopod even at exposure concentrations of 1 µg/litre.
Experiments conducted at two different pHs (5.9 and 7.6) revealed no
significant effect of pH on uptake of cadmium by the isopod.
Wright & Frain (1981b) demonstrated that adult intermoult
amphipods ( Gammarus pulex) accumulated only half as much cadmium
from a solution of 5 mg/litre in the presence of 200 mg calcium/litre
as with 20 mg calcium/litre.
Ramamoorthy & Blumhagen (1984) investigated the uptake of
cadmium, mercury, and zinc by rainbow trout (Salmo gairdneri) in a
model system which simulated the presence of other competing
compartments that would be found in nature. The system consisted of
either a simple sediment/water model or a more complex series of
compartments in dialysis bags of suspended sediment, cation and anion
exchange resins (to represent naturally occurring polyelectrolyte
materials of plant origin), and fish. River water was used as the
fluid transfer medium, and the system was continuously stirred.
Equilibrium with one heavy metal ion did not inhibit the uptake of
other metal ions; cadmium and zinc were taken up after equilibrium
with mercury. The authors calculated approximate partition
coefficients (fish/substrate) to be 2.8 for sediment, 550 for water,
and 2 and 3.6 for the cation and anion exchange resins, respectively.
The problem of expressing changes in concentration between
trophic levels is that the units are not compatible. There is no
significance to a bioconcentration term that expresses a ratio of
cadmium in soil moisture to cadmium in plant tissue, or cadmium in
plant tissue to cadmium in herbivore tissue. Therefore, it is
difficult to assess the impact of cadmium on the environment in terms
of bioconcentration factors. An alternative method is to measure both
cadmium and calcium at each trophic level and express these
measurements as a molar ratio of these two elements. (The molar ratio
should be used to account for the movement by atoms, not grams.)
Differences between trophic levels are calculated as the ratio of the
higher trophic level to the lower. This approach, called
biopurification, recognizes that the flow of the non-nutrient cadmium
through successive trophic levels follows a pathway similar to that of
nutrients such as calcium, and that calcium must pass natural chemical
and physiological barriers, such as membranes and selective enzymes,
that progressively purify the pool of the nutrient calcium relative to
the non-nutrient cadmium. In the case where two similar ecosystems are
compared, and where one is believed to be more contaminated than the
other, the relative degree of contamination can be calculated as the
difference between molar ratios at the same or similar trophic levels.
It is unfortunate that the absence of concentration data on
nutrients such as calcium or, alternatively, zinc, prohibits the
calculation of biopurification factors for any of the studies
discussed in this monograph.
5. TOXICITY TO MICROORGANISMS
Appraisal
Cadmium is toxic to a wide range of microorganisms in culture
(effects of cadmium on microorganisms in the field are discussed in
chapter 8). However, the presence of sediment, organic matter or high
concentrations of dissolved salts reduces the availability of cadmium
to microorganisms and, therefore, reduces the toxic impact.
Freshwater microorganisms in culture are thus affected by cadmium at
lower concentrations than marine species (for example, 50 µg/litre
affects growth in many freshwater species of algae while at least 100
µg/litre, and often 1000 µg/litre, is required to reduce growth in
marine species). Soil microorganisms are partially protected from the
toxic effects of cadmium by the presence of clay.
5.1 Aquatic microorganisms
5.1.1 Freshwater microorganisms
Canton & Slooff (1982) exposed the bacterium Salmonella
typhimurium and the alga Chlorella vulgaris to cadmium in the form
of the chloride, and calculated an 8-h EC50 (growth inhibition) of
10.4 mg/litre for the bacterium and a 96-h EC50 of 3.7 mg/litre for
the alga. No-toxic-effect levels of 0.65 and 1.5 mg/litre were
estimated for the bacterium and alga, respectively. Jana &
Bhattacharya (1988) found significant inhibition of population growth
in the faecal coliform bacterium Escherichia coli during exposure to
cadmium concentrations of 1, 2 or 5 mg cadmium/litre for 7 or 28 days.
Cadmium was the most toxic of the metals tested. Norberg & Molin
(1983) exposed the bacterium Zoogloea ramigera (abundant in sewage
treatment plants) to cadmium chloride concentrations of 1, 3, 5, and
10 mg cadmium/litre for 30 h. A prolonged lag phase and decrease in
growth resulted, the length of the lag phase being proportional to the
concentration of cadmium in the medium. Babich & Stotzky (1977a)
showed that the presence of clay particles protected bacteria from the
toxic effect of cadmium added to culture medium. The degree of
protection was related to the cation exchange capacity of various
clays tested.
Chapman & Dunlop (1981) estimated the 8-h LC50 for the
freshwater protozoan Tetrahymena pyriformis to be less than 1
mg/litre. However, this value increased with increasing water calcium
concentration; at a value of 500 mg calcium/litre, the LC50 was 19
mg/litre. Magnesium also exerted a protective effect against cadmium
when mixed with calcium. Cadmium was consistently more toxic to
Tetrahymena in the presence of magnesium alone. Berk et al. (1985)
calculated a 15-min EC50 (inhibition of ciliate chemotactic
response) for Tetrahymena sp. of 0.35-0.7 mg.
When Skowronski et al. (1988) exposed the green microalga
Stichococcus bacillaris to cadmium chloride concentrations of 45 and
90 µmol/litre for 4 days, growth rate was inhibited by 28% and 45% at
the two respective concentrations. At both exposure levels, dry weight
and chlorophyll a content were reduced in a dose-related manner.
Addition of manganese at concentrations of between 45 and 1800
µmol/litre had a dose-related antagonistic effect on cadmium toxicity.
Bennett (1990) found that the addition of cadmium (1.8 µmol/litre) to
a turbidostat culture of Chlorella pyrenoidosa caused a decrease in
the maximum specific growth rate (toxicity was expressed after a lag
of 5 generations). A gradual decrease in the maximum specific growth
rate was also noted during a 40-day exposure to stepwise increases in
the cadmium concentration (0.96 to 1.68 µmol/litre). The author found
that the addition of manganese (10.4 µmol/litre) had an antagonistic
effect, causing the maximum specific growth rate to increase.
Cadmium is toxic to the growth of the freshwater alga Chlorella
pyrenoidosa (Hart & Scaife, 1977). In cultures maintained at pH 7.0,
doubling times were 11, 21, 22, and 35 h for cadmium concentrations of
0, 0.25, 0.5, and 1.0 mg/litre medium, respectively. At a pH of 8.0,
the effect was somewhat lessened; doubling times were 11, 16, 17, and
25 h for the same range of cadmium doses. There was also a pronounced
effect on carbon dioxide fixation, which was reduced from 0.738 to
0.720, 0.558, and 0.283 µmol HCO3- fixed per hour with cadmium
exposures in the culture medium of 0, 0.246, 0.554, and 1.090
mg/litre, respectively. There was less of an effect on oxygen
evolution over the same dose range. Zinc offered no protection against
cadmium effects.
Wong et al. (1979) exposed four different species of freshwater
algae to cadmium and measured the uptake of 14C-carbonate.
Scenedesmus quadricaudata was the most sensitive species, carbonate
uptake being inhibited by 80% at a cadmium concentration of 20
µg/litre. Chlorella pyrenoidosa showed 70% inhibition of carbonate
uptake at about 100 µg/litre, while Chlorella vulgaris showed only
50% inhibition at about 500 µg/litre. The least sensitive of the four
species tested was Ankistrodesmus falcatus variety acicularis
where an effect on carbonate uptake started only at concentrations
higher than 500 µg/litre. There was no observed effect on the growth
of A. falcatus at cadmium concentrations lower than 5 mg/litre.
Rebhun & Ben-Amotz (1984) demonstrated that the chlorophyll content of
cells of Chlorella stigmatophora was reduced in a dose-dependant
manner across a range of cadmium concentrations of between 1 and 10
mg/litre medium.
Laegreid et al. (1983) studied the effects of cadmium on the alga
Selenastrum capricornutum cultured in the laboratory in water taken
from two lakes at various times throughout the year. The two lake
waters contained different amounts of organic material. The first
lake, a dystrophic bog lake, had a high organic content, while the
second, an eutrophic lake, had a low organic content. In the
dystrophic lake, which had a low pH (4.4), the toxicity of cadmium was
related to the free ionic concentration of the metal, as suggested by
many laboratory experiments. In the eutrophic lake, where there was
less influence from organic material, there was a pronounced seasonal
effect. In the summer, when growth and productivity of the algae were
highest, there was a much greater effect of the metal than predicted.
The toxicity of cadmium, at this time, was far greater than would be
expected even if all of the metal was in the free ionic form and none
was bound to organic compounds. On the basis of their field evidence,
the authors questioned the generally held assumption that organic
binding is the major factor in determining cadmium toxicity to
microorganisms. They considered that the presence of certain organic
compounds of low relative molecular mass could increase cadmium
toxicity. This conclusion is supported by the work of Giesy et al.
(1977), who found that uptake of cadmium into zooplankton could be
increased in the presence of organic compounds of low relative
molecular mass.
Chin & Sina (1978) investigated the cellular basis of cadmium
toxicity in microorganisms using cultures of Physarum polycephalum.
The organism was cultured, in plasmodial form, on the surface of
liquid medium, and replicate discs, cut from the protoplasmic sheet of
the organism, were used for the tests. The discs maintain mitotic
synchrony with each other and, therefore, cadmium could be introduced
at specific points in the cell cycle. The cultures were exposed to
cadmium sulfate (5 x 10-4 mol/litre), which was floated onto the
surface of the culture medium. Exposure to cadmium immediately prior
to early prophase of mitosis extended the normal DNA replication
period from 3 h to 4 h; this was monitored using measurements of
uptake of 3H-thymidine. Two stages of the cell cycle were
particularly susceptible to cadmium. Exposure either at the beginning
of the cycle or 80% of the way through the cycle caused delays in the
completion of mitosis. A 30-min exposure to cadmium at the onset of
early prophase inhibited incorporation of 3H-uridine into RNA for
the following 3 h by 51% and stimulated the incorporation of
3H-thymidine into DNA, for the same period, by 85%. Later in the
cycle, DNA synthesis was inhibited and DNA content was depressed by
12.5%. There was an ultrastructural effect on the nucleoli (less dense
material centrally giving nucleoli in section a "ring" structure),
which was the only structural effect of the metal even after 4 h of
exposure. Accommodation occurred after pre-treatment with sub-toxic
doses of cadmium. Treatment with cadmium at the less sensitive periods
of the cell cycle led to reduced effect at the more sensitive phases;
the organism, in some way, compensated. There was no accommodation by
Physarum after pre-treatment with zinc ions. This result contrasts
with that of a similar study on Escherichia coli where pre-exposure
to zinc reduced the effects of cadmium (Mitra et al., 1975).
5.1.2 Estuarine and marine microorganisms
Chan & Dean (1988) exposed the marine bacterium Pseudo-monas
marina to cadmium sulfate at concentrations of 1 to 25 mg
cadmium/litre. Effects were exposure related and included a lengthened
lag time, reduced growth rate, reduced biomass and oxygen uptake, and
a decrease in the activity of dehydrogenase and alkaline phosphatase.
The IC50 values for inhibition of biomass and of growth rate were 11
and 11.5 mg/litre, respectively. Flatau et al. (1987) progressively
adapted the marine bacterium Pseudomonas sp. to cadmium
concentrations from 1 to 80 mg/litre. Although the length of the lag
phase was not linearly or logarithmically correlated with the cadmium
concentration, it was significantly longer at cadmium concentrations
of 70 mg/litre or more. The growth rate was reduced at 10 mg/litre but
remained constant at cadmium concentrations of 10 to 50 mg/litre; no
growth was observed at 75 or 80 mg/litre. Oxygen consumption was not
different from that of controls at 1 or 5 mg/litre but at
concentrations of 10 mg/litre or more respiratory activity decreased.
Bressan & Brunetti (1988) exposed the marine microalgae
Dunaliella tertiolecta and Isochrysis galbana to cadmium
concentrations of 13.8 and 0.2 mg/litre, respectively, for up to 8
days. Cadmium significantly reduced the population growth, expressed
as the mean number of cells/ml, for both species. The addition of
nitrilotriacetic acid (NTA, a sequestering agent) at ratios of 1:1,
1:2 or 1:3 did not modify the toxic effect of cadmium.
Berk et al. (1985) exposed the marine ciliates Paranophrys sp.
and Miamiensis avidus to cadmium and monitored the inhibition of the
chemotactic response. The 15-min EC50 values were 2-3.1 mg
cadmium/litre and 5.1-7 mg cadmium/litre for the two species,
respectively.
Dongmann & Nurnberg (1982) investigated the effects of cadmium on
the marine diatom Thalassiosira rotula (a chain-forming diatom
native to shallow temperate waters) in petri dish and in batch liquid
cultures. They calculated generation times from the dish cultures and
reported a toxicity threshold for generation time of 30 µmol
cadmium/litre (3.4 mg/litre). At 50 µmol/litre, cadmium increased the
generation time from 24 h to 28 h. Using chain length as a parameter,
the toxicity threshold was 15 µmol/litre (1.7 mg/litre). Cell density
was found to be affected in batch culture. Cell chlorophyll content
and chain length were too variable to show significant effects of the
metal in batch culture.
5.2 Soil and litter microorganisms
Babich & Stotzky (1977b) showed that several species of fungi
tolerated cadmium to a greater degree when grown on cultures amended
with clays than in pure culture medium.
Sato et al. (1986) exposed the soil bacterium Nitrosomonas
europaea both to cadmium concentrations of 0.05, 0.1, and 0.4
mg/litre and to a range of ammonia concentrations (1 to 100 mg/litre
as N). Growth was markedly inhibited at the highest cadmium
concentration, especially when this was coupled with ammonia
concentrations greater than 10 mg/litre. Cadmium toxicity caused a
characteristic growth response. Ammonia oxidation proceeded at a
reduced rate for approximately 3 days and then fell sharply. The
subsequent oxidation proceeded at a diminished but constant rate. At
cadmium concentrations of 0.1 mg/litre or less, the toxic effect of
cadmium could be partially offset by increasing the ammonia
concentration. Prahalad & Seenayya (1988) found that cadmium
concentrations of 3.5 mg/litre inhibited the growth of the bacterium
Alcaligenes faecalis. However, if the nutrient broth was diluted by
one half then growth was inhibited at 2 mg/litre, and with
quarter-strength nutrient broth inhibition occurred at 0.5 mg/litre.
Hartman (1974) reported less species diversity of soil fungi in
areas of high cadmium contamination than in control areas. Samples of
Fusarium oxysporium isolated from contaminated and control soils
showed different tolerances to cadmium. This suggested the development
of some resistance, presumably by selection of more resistant strains.
Bond et al. (1976) incubated coniferous forest soil and litter
(mixed with a pre-prepared homogeneous soil) in microcosm units and
monitored oxygen consumption, carbon dioxide evolution, and bacterial
and fungal populations. With cadmium added to a concentration of 0.01
mg/kg soil and, in the initial stages of incubation, with cadmium at
10 mg/kg soil, there was a stimulation of oxygen consumption,
suggesting an effect of the metal on uncoupling of respiratory
phosphorylation. In the later stages of incubation with cadmium at 10
mg/kg there was a reduction in both oxygen consumption and carbon
dioxide evolution. No effect was seen on numbers of organisms in the
microcosms.
Bewley & Stotzky (1983) investigated the effect of cadmium (100
and 1000 mg/kg soil) on carbon mineralization and on the mycoflora in
glucose-supplemented soils amended with clays (kaolinite or
montmorillonite) at 9%. Cadmium had no significant effect on the
length of the lag period, carbon dioxide evolution or on the amount of
carbon mineralized. When subsequent cadmium additions of 2400 and 4000
mg/kg were made to soils previously treated with 100 or 1000 mg/kg,
the rate of glucose degradation decreased more in the clay-amended
soils than in control soil not amended with clay. Clay protected fungi
from the toxic effect of cadmium at 5000 mg/kg. Fungi from
clay-treated soils were more sensitive to cadmium in the culture media
after they had been isolated from soil pre-treated with cadmium. The
authors interpreted these results as showing a reduction in the
availability of cadmium to organisms in clay-amended soils. The
overall effect would be a prevention of selection of more tolerant
strains. Thus, when the organisms were challenged with high doses of
cadmium, they would have been more susceptible than organisms from
non-amended soil.
Naidu & Reddy (1988) incubated black cotton soil (0.8% organic
carbon; 55% clay) for up to 8 weeks in the presence of cadmium
chloride at concentrations of between 50 and 500 mg cadmium/kg. The
ammoniacal nitrogen (NH4-N) concentration increased for the first
week at all treatment levels and then decreased at concentrations of
50 mg/kg or less. The initial rise in NH4-N levels led to an
increase in nitrate nitrogen (NO3-N) levels, the accumulation of
NO3-N being inversely proportional to the cadmium exposure. The
authors pointed out that at cadmium concentrations of 100 and 500
mg/kg, hydrolysis of urea was significantly poorer than at other
treatment levels, as shown by the lower concentration of NH4-N
observed after 1 week. At all cadmium concentrations there was
significant accumulation of nitrite nitrogen (NO2-N) at every sample
time, compared to control soil, suggesting that cadmium might be toxic
to soil nitrification. At all exposure levels cadmium significantly
depressed both bacterial and fungal populations. Cadmium
concentrations of 10 or 50 mg/kg had no effect on soil actinomycetes,
but both 100 and 500 mg/kg significantly reduced the population. Tyler
et al. (1974) incubated a mull soil for 7 weeks. Cadmium chloride
concentrations of 9 to 18 µmol/g and cadmium acetate concentrations of
9 to 22 µmol/g caused decreases in soil ammonium content and
significantly increased nitrate accumulation.
6. TOXICITY TO AQUATIC ORGANISMS
Appraisal
Cadmium uptake from water by aquatic organisms is extremely
variable and depends on the species and various environmental
conditions such as water hardness (notably the calcium ion
concentration), salinity, temperature, pH, and organic matter content
(see chapter 4).
The majority of chelating agents decrease cadmium uptake but
some, such as dithiocarbamates and xanthates, increase uptake.
As a consequence of the variability in cadmium uptake, the toxic
impact to aquatic organisms also varies across a wide range of
concentrations and is dependent on the species of organism and on the
presence of other metal ions, notably calcium and zinc.
The lowest recorded 96-h LC50 in a flow system is 16 µg
cadmium per litre for the adult shrimp Mysidopsis bahia. A nominal
no-observed-effect level (NOEL) of 0.6 µg cadmium/litre was found for
Daphnia magna, reproductive rate being the most sensitive parameter.
A nomi-nal NOEL has been noted at a similar level (1.7 to 3.4 µg
cadmium per litre) with respect to the reproductive effects on brook
trout.
The available results indicate that the embryonic and larval
stages of aquatic organisms are more sensitive than the adult stage.
Spinal malformations are induced in cadmium-exposed fish. In addition
to causing reproductive effects, cadmium influences the behaviour of
aquatic organisms.
At low concentrations, cadmium inhibits ion transport systems
(10 µg cadmium/litre) and induces metallothionein synthesis (< 1 µg
cadmium/litre) in freshwater fish.
6.1 Toxicity to aquatic plants
Hutchinson & Czyrska (1975) exposed two floating aquatic weeds,
the common duckweed Lemna minor and a floating fern Salvinia
natans, to cadmium concentrations of between 0.01 and 1.0 mg/litre
for up to 3 weeks. Growth was reduced at all concentrations but
especially at 0.05 mg/litre or more. The effect of cadmium on growth
became more marked with time. Loss of green coloration (chlorosis) was
a common symptom of cadmium toxicity, and at concentrations of 0.5 and
1.0 mg/litre Lemna plants died. When the two species were grown in
competition, growth at lower cadmium concentrations (0.01 and 0.05
mg/litre) was greater in Salvinia but less in Lemna than when the
plants were grown alone.
In a study by Nir et al. (1990), water hyacinth plants were
exposed to cadmium concentrations of 0, 0.05, 0.1, 0.4 or 1.0 mg/litre
for 7 days. Concentrations of 0.1 mg/litre or less had no significant
effect on wet or dry biomass gain or on chlorophyll a content.
Concentrations of 0.4 or 1.0 mg/litre significantly reduced both wet
biomass gain and chlorophyll a content but had no significant effect
on dry biomass gain. The chlorophyll a content of leaves decreased
with time in plants exposed to 0.4 mg/litre. After 3 weeks of
exposure, the chlorophyll a levels were 75% lower than in control
plants.
6.2 Toxicity to aquatic invertebrates
Cadmium is moderately to highly toxic to aquatic invertebrates
(see Tables 9 and 10). Its toxic effect is dependent on a variety of
environmental variables. Factors that reduce the free ionic
concentration, e.g., water hardness, salinity, chelating agents, and
high organic content of water, tend to reduce the toxic effect of
cadmium. The presence of zinc increases the toxic effect of cadmium on
invertebrates.
6.2.1 Acute and short-term toxicity
The acute toxicity of cadmium to aquatic invertebrates, as
assessed in laboratory tests, is summarized in Tables 9 and 10. The
most notable features are the variability in cadmium toxicity between
different organisms and the effects of temperature, salinity, and
water hardness. There is considerable variation even amongst closely
related species.
In a study by Canton & Slooff (1982), the water flea Daphnia
magna was exposed to cadmium over a 48-h period. At a water hardness
of 1 mmol/litre there was no mortality at 16 µg cadmium per litre,
while at a water hardness of 2 mmol/litre there was no mortality or
abnormal behaviour at 17 µg/litre.
Clubb et al. (1975b) investigated the toxicity of cadmium to nine
species of aquatic insects, but seven of the species tested were too
insensitive to the effects of the metal for the LC50 to be
determined. The insensitive species were Atherix variegata,
Hexatoma sp., Holorusia sp., Acroneuria pacifica, Arcynopteryx
signata, Pteronarcys californica, and Brachycentrus americanus;
these species represented Dipterans (true flies), Plecoptera
(stoneflies), and Tricoptera (caddis flies).
Table 9. Toxicity of cadmium to marine or estuarine invertebrates
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Purple sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 0.5 (0.4-0.6) m Dinnel et al. (1989)
(Strongylocentrotus
purpuratus)
Green sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 1.8 (1.5-2.2) m Dinnel et al. (1989)
(Strongylocentrotus
droebachiensis)
Sand dollar embryo stat 12.5-13.0 30 8.0-8.1 72 7.4 (5.2-10.8) m Dinnel et al. (1989)
(Dendraster
excentricus)
Starfish 24.5 g stat 20 20 8.0 24 12 n Eisler (1971)
(Asterias forbesi) 24.5 g stat 20 20 8.0 48 1.0 n Eisler (1971)
24.5 g stat 20 20 8.0 96 0.82 n Eisler (1971)
11.2 g stat 20 20 7.8 24 71 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 48 7.1 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
American oyster embryo stat 26 25 7.0-8.5 48 3.8 (2.85-4.48) n Calabrese et al. (1973)
(Crassostrea
virginica)
Mussel stat 18.5 32.9 7.9 96 1.62 (1.19-2.22) m Ahsanullah (1976)
(Mytilus edulis
planulatis)
Blue mussel 4 g stat 20 8.0 24 > 200 n Eisler (1971)
(Mytilus edulis) 4 g stat 20 8.0 48 165 n Eisler (1971)
4 g stat 20 8.0 96 25 n Eisler (1971)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Soft-shell clam 5.2 g stat 20 20 8.0 24 > 20 n Eisler (1971)
(Mya arenaria) 5.2 g stat 20 20 8.0 48 50 n Eisler (1971)
5.2 g stat 20 20 8.0 96 2.2 n Eisler (1971)
4.6 g stat 20 20 7.8 24 32 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 48 2.5 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Bay scallop 20-30 mm stat 20 25 8.0 24 8.2 n Nelson et al. (1976)
(Argopecten 20-30 mm stat 20 25 8.0 48 3.21 n Nelson et al. (1976)
irradians) 20-30 mm stat 20 25 8.0 72 2.18 n Nelson et al. (1976)
20-30 mm stat 20 25 8.0 96 1.48 (0.95-2.31) n Nelson et al. (1976)
Atlantic oyster 0.6 g stat 20 20 8.0 24 158 n Eisler (1971)
drill 0.6 g stat 20 20 8.0 48 28 n Eisler (1971)
(Urosalpinx 0.6 g stat 20 20 8.0 96 6.6 n Eisler (1971)
cinerea)
Eastern mud snail 0.56 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Nassarius 0.56 g stat 20 20 8.0 48 125 n Eisler (1971)
absoletus) 0.56 g stat 20 20 8.0 96 10.5 n Eisler (1971)
Ragworm 8 g stat 20 20 8.0 24 25 n Eisler (1971)
(Nereis virens) 8 g stat 20 20 8.0 48 25 n Eisler (1971)
8 g stat 20 20 8.0 96 11 n Eisler (1971)
7.6 g stat 20 20 7.8 24 56 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 48 9.3 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Copepod nauplius stat 22 10 96 0.06 (0.001-0.2) m Roberts et al. (1982)
(Eurytemora affinis)
Copepod adult stat 22 10 96 0.38 (0.006-1.52) m Roberts et al. (1976)
(Acartia tonsa)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Harpacticoid
copepod adult stat 20-22 3 96 0.43 (0.31-0.55) Bengtsson & Bergstrom (1987)
(Nitocra spinipes) adult stat 20-22 7 96 0.66 (0.53-0.82) Bengtsson & Bergstrom (1987)
adult stat 20-22 15 96 0.78 (0.41-120) Bengtsson & Bergstrom (1987)
Marine amphipod young stat 10 96 3.5 m Wright & Frain (1981a)
(Marinogammarus adult stat 10 96 13.3 m Wright & Frain (1981a)
obtusatus)
Mysid shrimp adult flow 22 20 7.3 96 0.036 (0.022-0.081) m Roberts et al. (1982)
(Neomysis adult flow 22 20 7.8 96 0.02 (0.015-0.027) m Roberts et al. (1982)
americanus)
Mysid shrimp adult stat 22 20 7.3 96 0.017 m Roberts et al. (1982)
(Mysidopsis bahia) adult stat 22 20 7.7 96 0.029 (0.013-0.043) m Roberts et al. (1982)
flow c20-28 15-23 96 0.016 (0.013-0.02) m Nimmo et al. (1978)
Shrimp stat 18.7 32.1 8.0 120 2.3 (1.05-5.06) m Ahsanullah (1976)
(Palaemonetes sp.) stat 18.7 32.1 8.0 168 1.85 (1.32-2.59) m Ahsanullah (1976)
0.38 g flow 16.8 96 6.8 (5.2-9.76) m Ahsanullah (1976)
0.16 g flow 17.8 8.1 96 6.4 (5.73-7.19) m Ahsanullah (1976)
Sandworm 0.37 g stat 18.5 32.7 8.1 168 6.4 (5.82-7.1) m Ahsanullah (1976)
(Neanthes vaali)
Sand shrimp 0.25 g stat 20 20 8.0 24 2.4 n Eisler (1971)
(Crangon 0.25 g stat 20 20 8.0 48 0.5 n Eisler (1971)
septemspinosa) 0.25 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Sand shrimp adult flow 10.2 28.6 7.9 96 2.3 (1.7-5.1) m Dinnel et al. (1989)
(Crangon spp.)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Grass shrimp 0.33 g stat 20 20 8.0 24 43 n Eisler (1971)
(Palaemonetes 0.33 g stat 20 20 8.0 48 3.7 n Eisler (1971)
vulgaris) 0.33 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Shrimp stat 35 96 2.07 (± 0.22) m McClurg (1984)
(Penaeus indicus)
Pink shrimp flow 25 20 96 4.6 m Bahner & Nimmo (1975)
(Penaeus duorarum)
Grapsid crab 1.47 g stat 17.8 32.6 8.1 168 14 (11.2-17.5) m Ahsanullah (1976)
(Paragrapsus 1.08 g stat 17.1 168 16.7 (15.11-18.45) Ahsanullah (1976)
quadridentatus)
Hermit crab 0.47 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Pagurus 0.47 g stat 20 20 8.0 48 3.7 n Eisler (1971)
longicarpus) 0.47 g stat 20 20 8.0 96 0.32 n Eisler (1971)
0.5 g stat 20 20 7.8 24 15 n Eisler & Hennekey (1977)
0.5 g stat 20 20 8.0 96 1.3 n Eisler & Hennekey (1977)
Shore crab 5.9 g stat 20 20 8.0 24 100 n Eisler (1971)
(Carcinus maenus) 5.9 g stat 20 20 8.0 48 16.6 n Eisler (1971)
5.9 g stat 20 20 8.0 96 4.1 n Eisler (1971)
Dungeness crab zoea stat 8.5 30 8.1 96 0.2 (0.1-0.4) m Dinnel et al. (1989)
(Cancer magister)
Squid larva stat 8.6 30 8.1 96 > 10.2 m Dinnel et al. (1989)
(Loligo opalescens)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b organisms exposed to cadmium added as cadmium chloride; m = measured; n = nominal
c intermittent flow-through conditions
Table 10. Toxicity of cadmium to freshwater invertebrates
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Snail adult stat 20-22 6.7 24 7.6 n Wier & Walter (1976)
(Physa adult stat 20-22 6.7 48 4.25 n Wier & Walter (1976)
gyrina) adult stat 20-22 6.7 96 1.37 n Wier & Walter (1976)
adult stat 20-22 6.7 228 0.83 n Wier & Walter (1976)
immature stat 20-22 6.7 48 0.69 n Wier & Walter (1976)
immature stat 20-22 6.7 96 0.41 n Wier & Walter (1976)
Snail flow b 15 7.1-7.7 168 0.114 m Spehar et al. (1978a)
(Physa integra)
Snail 10-12 mm flow 12 128-176 7.7 24 4.4 m Williams et al. (1985)
(Physa 10-12 mm flow 12 128-176 7.7 48 2.1 m Williams et al. (1985)
fontinalis) 10-12 mm flow 12 128-176 7.7 96 0.8 m Williams et al. (1985)
Isopod 8-10 mm flow 12 128-176 7.7 24 13 m Williams et al. (1985)
(Asellus 8-10 mm flow 12 128-176 7.7 48 3.6 m Williams et al. (1985)
aquaticus) 8-10 mm flow 12 128-176 7.7 96 0.6 m Williams et al. (1985)
Scud 10 96 0.12 m Wright & Frain (1981b)
(Gammarus 8-12 mm flow 12 128-176 7.7 24 1.6 m Williams et al. (1985)
pulex) 8-12 mm flow 12 128-176 7.7 48 0.4 m Williams et al. (1985)
8-12 mm flow 12 128-176 7.7 96 0.02 m Williams et al. (1985)
Water flea adult stat 10 0.85 meq/ 7.2 48 0.055 e (0.032-0.095) n Baudouin & Scoppa (1974)
(Daphnia hyalina) litre
Water flea 1 day stat 20 7.6-7.7 24 3 n Kuhn et al. (1989)
(Daphnia < 1 day stat 20-22 110-130 7.8 48 0.04 (0.02-0.07) n Hall et al. (1986)
magna) < 1 day stat 20-22 190-210 7.7 48 0.08 (0.06-0.1) n Hall et al. (1986)
< 1 day stat 18-20 1 mmol/ 48 0.03 m Canton & Slooff (1982)
litre
Table 10 (contd).
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Water flea < 1 day stat 20-22 110-130 7.8 48 0.07 n Hall et al. (1986)
(Daphnia < 1 day stat 20-22 110-130 7.7 48 0.1 (0.07-0.12) n Hall et al. (1986)
ulex) < 1 day stat 19-22 7.7 96 0.047 (0.042-0.052) n Bertram & Hart (1979)
Copepod adult stat 10 0.85 meq/ 7.2 48 3.8 e (2.3-6.3) n Baudouin & Scoppa (1974)
(Cyclops abyssorum) litre
Copepod adult stat 10 0.85 meq/ 7.2 48 0.55 e (0.39-0.77) n Baudouin & Scoppa (1974)
(Eudiaptomus padanus) litre
Crayfish flow 19-21 24-28 6.7-7.0 96 6.1 (4.7-7.9) m Mirenda (1986)
(Orconectes virilis)
Mayfly flow 10 7.8 96 28 m Clubb et al. (1975b)
(Ephemerella grandis
grandis)
Midge 10-12 mm flow 12 128-176 7.7 96 300 m Williams et al. (1985)
(Chironomus riparius)
Stonefly flow 10 7.8 96 18 m Clubb et al. (1975b)
(Pteronarcella badia)
Stonefly 10-15 mm flow 12 128-176 7.7 96 520 m Williams et al. (1985)
(Hydropsyche
angustipennis)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b intermittent flow-through conditions
c hardness expressed as mg CaCO3/litre unless stated otherwise
d organisms exposed to cadmium added as cadmium chloride unless otherwise stated; m = measured concentration; n = nominal concentration
e organism exposed to cadmium as cadmium sulfate
Spehar et al. (1978a) reported a decreased survival of the water
snail Physa integra at a cadmium concentration of 85.5 µg/litre
after 7 days of exposure. After 21 days of exposure, survival was
significantly decreased at 27.5 µg/litre, the next lowest
concentration tested. Some snails exposed to these concentrations
developed a condition in which the animal was extended from the shell
but unable to attach the foot or crawl. A white mucus layer covered
the exposed foot region of some snails and these subsequently died.
Concentrations tested were not high enough to obtain a 4-day LC50
value but the 7-day LC50 of 114 µg/litre was approximately 11 times
higher than the 28-day LC50 value of 10.4 µg/litre.
Mirenda (1986) reported a 14-day LC50 of 0.7 mg cadmium per
litre for the crayfish Orconectes virilis under flow-through
conditions. Pesch & Stewart (1980) estimated the 10-day LC50 for bay
scallops Argopecten irradians to be 0.53 mg/litre in flowing sea
water. The EC50 (for growth) for the same species over 42 days was
0.078 mg/litre. Byssal thread detachment, which precedes death, showed
an EC50 of 0.54 mg/litre of cadmium 8 days into the test and before
there was any appreciable mortality. Robinson et al. (1988) compared
10-day LC50 values for freshly collected and cultured infaunal
amphipods ( Rhepoxynius abronius). Cultured amphipods appeared normal
and survived well (93%) under control toxicity test conditions, but
were more sensitive to cadmium in sediment (10-day LC50 = 4.4 mg/kg)
than were freshly caught amphipods (10-day LC50 = 8.7 mg/kg).
When Winner (1988) exposed Daphnia magna and Ceriodaphnia
dubia to cadmium for 7 days, the most sensitive indicators were mean
body length of primiparous females in D. magna, which was
significantly reduced at 2 µg cadmium/litre, and the total young per
female in C. dubia, significantly reduced at 1 µg/litre.
6.2.1.1 Effects of temperature and salinity on acute toxicity
An increase in toxicity as temperature increases and as salinity
decreases is valid for all organisms that have been tested with these
variables (Tables 9 and 10).
O'Hara (1973) investigated the effects of temperature and
salinity on the toxicity of cadmium to adult male and female fiddler
crabs ( Uca pugilator). Mortality was greatest at high temperatures
and low salinities in tests lasting 240 h. LC50 values varied from
2.9 mg/litre for the lowest salinity (10%) and highest temperature (30
°C) to 47.0 mg/litre for the highest salinity (30%) and lowest
temperature (10 °C). Frank & Robertson (1979) exposed the blue crab
( Callinectes sapidus) to cadmium chloride at salinities of 1, 15,
and 35%. Like O'Hara, they found a decrease in cadmium toxicity with
increase in salinity. For example, 96-h LC50 values were 0.32, 4.7,
and 11.6 mg cadmium/litre for the three salinities, respectively.
Rosenberg & Costlow (1976) reported increased cadmium toxicity during
larval development of two estuarine crab species as salinity decreased
and increased toxicity as temperature increased.
Voyer & Modica (1990) found the same pattern with the mysid
shrimp Mysidopsis bahia. For salinities of 10 and 30%, the 96-h
LC50 values ranged from 15.5 to 28 µg cadmium/litre at a temperature
of 25 °C and from 47 to 84 µg/litre at a temperature of 20 °C. At 30
°C the 96-h LC50 was < 11 µg/litre at both salinities. However,
when Robert & His (1985) exposed embryos and larvae of the Japanese
oyster Crassostrea gigas to cadmium concentrations of up to 50
µg/litre at various salinities (20 to 35%), decreasing the salinity
severely affected the development of the oysters but cadmium had no
effect.
At temperatures higher than 11 °C, the combined effect of
temperature and cadmium caused a heavy stress to the copepod Tisbe
holothuriae so that the effects of salinity were masked
(Verriopoulos & Moraitou-Apostolopoulou, 1981).
6.2.1.2 Effect of water hardness
Using either artificial hard water (hardness: 180 mg CaCO3 per
litre) or dechlorinated tap water (hardness: 60 mg/litre),
Niederlehner et al. (1984) conducted short-term tests on the effects
of cadmium on the freshwater oligochaete Aeolosoma headlyi.
Mortality and population growth/maintenance were assessed over 10 to
14 days. The authors established NOEL values for population growth of
32.0 and 53.6 µg/litre for hard water (two replicate tests), whereas
the NOEL for the softer water was 17.2 µg/litre. The 48-h LC50
values were 4.98 and 1.2 mg/litre, respectively, for hard and softer
water.
6.2.1.3 Effect of organic materials and sediment
When Schuytema et al. (1984) exposed Daphnia magna to cadmium
for a period of 48 h, the mean LC50 value was 39 µg/litre in water
and 91 µg/litre in a water-sediment slurry. Giesy et al. (1977) found
that cadmium was more toxic to water fleas ( Simocephalus serrulatus)
exposed in well water with a low organic content than to those exposed
in pond water with a high organic content. The authors isolated a
series of organic fractions from the pond water by ultrafiltration.
Protection against cadmium toxicity was afforded by fractions of
intermediate relative molecular mass (ranging from approximately 500
to 300 000 daltons). The fraction with a relative molecular mass in
excess of 300 000 daltons marginally increased the toxicity of
cadmium.
Kemp & Swartz (1988) compared the acute toxicity of interstitial
and particle-bound cadmium to the marine infaunal amphipod
Rhepoxynius abronius. The cadmium concentration in interstitial
water was kept constant whereas the sediment cadmium level was varied
by using perfusion through the sediment with peristaltic pumps. The
principal cause of toxicity was found to be cadmium dissolved in
interstitial water, between 70 and 88% of the toxicity being
predictable from interstitial water concentrations.
6.2.1.4 Lifestage sensitivity
When Calabrese et al. (1973) investigated the toxicity of cadmium
to embryos of the American oyster Crassostrea virginica, there was
no mortality at 1 mg/litre and the 48-h LC50 and LC100 values were
3.8 and 6 mg/litre, respectively. Johnson & Gentile (1979) found the
larva of the American lobster Hommarus americanus to be sensitive to
cadmium; the 96-h LC50 in static bioassays was 78 µg/litre. The
mortalities after 96 h at concentrations of 10 and 30 µg/litre were 3%
and 10%, respectively. There is a very steep increase in toxicity of
cadmium to lobster larvae between 24 and 96 h. The 24-h LC50 is
approximately 1 mg/litre; at this concentration the mortality reaches
100% within 48 h.
Verriopoulos & Moraitou-Apostolopoulou (1982) found that the
different life-stages of the copepod Tisbe holothuriae showed
differences in sensitivity to cadmium. One-day-old nauplius larvae of
the copepod were the most sensitive with an LC50 of 0.538 mg/litre,
expressed as ions of cadmium, while 5-day-old nauplii showed an LC50
of 0.645 mg/litre. The value for 10-day-old copepodids (0.906
mg/litre) was not significantly different from that for adult females
(0.916 mg/litre). Females with ovigerous sacs were slightly more
sensitive, with an LC50 of 0.873 mg/litre. When Robinson et al.
(1988) exposed the infaunal phoxocephalid amphipod Rhepoxynius
abronius to sediment contaminated with cadmium, the 10-day LC50
values were 8.2 mg/kg for juveniles and 11.5 mg/kg for adults.
Nebeker et al. (1986) exposed Daphnia magna of different ages
to cadmium for a period of 48 h. Mean EC50 (immobilization) values
ranged from 23 µg/litre for 6 day-old water fleas to 164 µg/litre for
2-day-old Daphnia. Tests on Daphnia of different ages, conducted
in water of different hardness (32 or 76 mg CaCO3 per litre), with
or without feeding and in two different sizes of container, resulted
in a wide range of EC50 values (4 to 307 µg/litre). There was no
consistent effect of any of these variables other than the age of the
test animals. Very young animals were relatively tolerant, with a mean
EC50 value of 109 µg/litre.
McCahon et al. (1989) exposed both cased and uncased 1st, 3rd,
and 4th larval instars of the caddis fly Agapetus fuscipes to
cadmium chloride. The LC50 values ranged from 295 to > 1000 mg
cadmium/litre at 24 h and from 50 to 320 mg/litre at 96 h. First
instar larvae were significantly more sensitive than 3rd or 4th instar
larvae and at all ages cased animals were more resistant than uncased.
6.2.1.5 Other factors affecting acute and short-term toxicity
Chandini (1988; 1989) found that increasing the food source of
the cladocerans Daphnia carinata and Echinisca triserialis greatly
reduced the toxicity of cadmium, expressed as the 48-h or 96-h LC50,
and the effect of cadmium on various other life history parameters,
such as fecundity, growth, and age at first reproduction.
Verriopoulos & Moraitou-Apostolopoulou (1981) exposed adult
females of the copepod Tisbe holothuriae to cadmium and found that
the oxygen concentration in the water was negatively related to
mortality and that population density was positively related after
cadmium exposure. Clubb et al. (1975a) showed that the toxicity of
cadmium to aquatic insects decreased with decreasing dissolved oxygen
levels in the test water.
McCahon et al. (1988) exposed the amphipod Gammarus pulex to
cadmium chloride under static conditions and reported a 96-h LC50 of
0.05 mg cadmium/litre. The acute toxicity of cadmium to G. pulex
parasitized by the acanthocephalan Pomphorhynchus laevis at several
levels of infection was investigated. Toxicity, expressed as LC50,
did not differ significantly between uninfected and infected
amphipods.
Kay & Haller (1986) fed water hyacinth weevils Neochetina
eichhorniae on water hyacinths containing cadmium from previous
exposure to the metal. There was no mortality in weevils fed on leaves
containing up to 17.2 mg cadmium/kg. At this exposure level, the
weevils accumulated a cadmium body burden of 36.67 mg/kg over 20 days.
6.2.2 Long-term toxicity
In a study by Zaroogian & Morrison (1981), adult and larval
oysters of the species Crassostrea virginica were exposed to cadmium
at concentrations of 5 or 15 µg/litre. Some adults were exposed (for
35 or 37 weeks) to cadmium at these two concentrations prior to
spawning, and larvae from these pre-treated adults and from control
treated adults were reared in either control sea water or sea water
containing 5 or 15 µg/litre cadmium. In all there were 11 treatment
combinations. The highest larval mortality occurred when larvae from
parents treated with 15 µg/litre were reared in sea water containing
15 µg/litre for 3 weeks. However, larvae that survived this treatment
grew to lengths not significantly different from controls. The effects
observed with other treatment combinations were only temporary. Growth
and development were slowed but those larvae that survived ultimately
developed normally and to the same size as controls.
When Holcombe et al. (1984) exposed snails, from embryos through
to adult sexual maturity, to cadmium chloride, there was a delayed
hatch, a reduction in percentage hatch and survival, and reduced
growth when compared to controls. Based on these effects, the authors
suggested a maximum acceptable cadmium concentration in water of
between 4 and 8 µg/litre from one test and between 2 and 5 µg/litre
from a replicate test.
Lussier et al. (1985) conducted life-cycle tests, over 35 days,
on the mysid shrimp Mysidopsis bahia. Cadmium affected survival
primarily and no reproductive effects were noted at sublethal
concentrations.
Following a long-term investigation in the laboratory and in the
field, Marshall (1979) suggested a chronic LC10 for the water flea
Daphnia galeata mendotae of 0.15 µg cadmium/litre. Winner (1986)
exposed Daphnia pulex chronically (21 or 42 days) to cadmium, added
as cadmium sulfate, under different water conditions. Increasing the
water hardness from 58 to 116 mg/litre reduced the toxic effect of the
cadmium but a further increase to 230 mg/litre had no further effect.
The most sensitive aspect of the Daphnia life history to cadmium was
the abortion rate of young. Humic acid had no effect on this parameter
in soft or medium-hard water but increased the toxic effect of cadmium
in hard water. Mortality was increased by humic acid (0.75 or 1.50
mg/litre) at all water hardness levels. van Leeuwen et al. (1985)
calculated 14-day and 21-day LC50S for Daphnia magna of 24 and 14
µg cadmium/litre, respectively. No effect on mortality was seen at 3.2
µg/litre. The lowest concentrations producing significant mortality of
young amphipods during 6-week exposures to cadmium were 1 µg/litre for
Hyalella azteca and 3.2 µg/litre for Gammarus fasciatus (Borgmann
et al., 1989b).
6.2.3 Reproductive effects
Mysing-Gubala & Poirrier (1981) conducted laboratory experiments
on the effect of cadmium on the freshwater sponge Ephydatia
fluviatilis. Sponge cuttings were exposed to cadmium concentrations
ranging from 0.001 to 1.0 mg/litre for 1 month. At the end of the
experimental period, the cuttings were classified in four groups
depending on whether the sponge survived, whether it produced asexual
reproductive gemmules, and whether the silicaceous spicules of the
gemmules were normal or malformed. There was some effect of cadmium
even at concentrations as low as 0.001 mg/litre, 17% of the sponge
cuttings showing no gemmule production and 33% showing malformed
spicules. At concentrations of 0.5 and 1.0 mg/litre, all of the sponge
cuttings died.
Lee & Xu (1984) investigated the effects of cadmium at 0.5 and
0.1 mg/litre on the fertilization and development of sea urchin eggs
and the development of Amphioxus. At both cadmium concentrations,
sea urchin development was normal to the gastrulation stage but all
the plutei were abnormal. The effects on Amphioxus development were
different; cleavage of eggs was not affected by cadmium at 0.5 or 0.1
mg/litre but neurulation was. Dinnel et al. (1989) exposed sperm from
various sea urchin species to cadmium chloride for 60 min and assessed
fertilization success; EC50 values ranged from 12 to 26 mg/litre. An
EC50 of 8 mg/litre was calculated for the sand dollar Dendraster
excentricus. Den Besten et al. (1989) exposed the sea star Asterias
rubens to cadmium chloride at a concentration of 25 µg cadmium/litre
for 5 months. No effect on spermatozoa was found, but maturation of
oocytes was delayed and early development of embryos was adversely
affected.
Conrad (1988) studied the effect of cadmium on newly fertilized
eggs of the mud snail Ilyanassa obsoleta. No apparent effect was
observed at a cadmium concentration of 10-6 mol/litre. The minimum
concentrations producing abnormal veliger development and abnormal
late cleavage and stopping early cleavage were 10-5-10-4
mol/litre, 10-3 mol/litre, and 10-3 mol/litre, respectively.
Biesinger & Christensen (1972) estimated a 3-week 16%
reproductive impairment concentration of 0.17 µg cadmium/litre.
In a 21-day reproduction test on Daphnia magna, Kuhn et al.
(1989) determined a nominal NOEL of 0.6 µg Cd2+/litre, reproduction
rate being the most sensitive parameter. A nominal NOEL of 1 µg/litre
was found when daphnids were exposed to the cadmium chloride salt.
Reproduction of Daphnia magna was completely inhibited at
concentrations exceeding 3.2 µg/litre and time-dependant survival and
reproduction were significantly reduced at 1.8 µg/litre. No effects on
reproduction were observed at 1 µg/litre (van Leeuwen et al., 1985).
When Bertram & Hart (1979) exposed the cladoceran Daphnia pulex to
cadmium concentrations of 1 to 30 µg/litre, there was no significant
effect on the number of days required for onset of reproduction or on
its frequency. At 1 µg/litre no significant effect on longevity of
individuals was observed, but at concentrations of 5 µg/litre or more
there was a significant reduction. All cadmium concentrations caused
a reduction in the average brood size, the average number of broods
per adult, and the total number of progeny. The authors also exposed
daphnids to cadmium-contaminated food in the form of the phytoplankton
Chlorella, the cadmium concentration being 0.3-0.6 µg cadmium/litre
of medium. This resulted in a significant reduction in the average
number of broods per adult and the average brood size. However, there
was no significant effect on average longevity of individuals, the
percentage of adults producing broods, the average number of days to
the first brood or the average number of days between broods.
Bengtsson & Bergstrom (1987) exposed newly fertilized female
harpacticoids ( Nitocra spinipes) to cadmium chloride at two
salinities for 13 days. At 3% the fecundity EC50 was 37-46 µg/litre
at a salinity of 3% and 6-15 µg/litre at 15%.
Williams et al. (1987) provided water containing nominal cadmium
chloride concentrations of 0, 0.3, 30, 100 or 300 mg cadmium/litre
cadmium to newly-emerged adults of the midge Chironomus riparius in
which they could lay their eggs. The females preferred to lay their
eggs in water with no cadmium or in the lower concentrations;
significant preferences were recorded. Eggs of Chironomids are laid
within a protective gelatinous matrix. Eggs exposed to cadmium after
complete formation of the matrix in control water were unaffected (the
hatch was 80-100%), whereas those exposed after removal of the matrix
had a reduced hatching rate (60%) at all test concentrations. Eggs
laid directly in water containing cadmium were unaffected by a
concentration of 0.3 mg/litre, but hatching rates were reduced to 45%
at 30 mg/litre, 8% at 100 mg/litre, and 0% at 300 mg/litre. Clearly,
cadmium only affects the unprotected egg, either when it is newly laid
and before its gelatinous protection has developed or when this
gelatinous matrix is removed.
In a flow-through study, Hatakeyama (1987) exposed the chironomid
Polypedilum nubifer, from the egg stage, to cadmium chloride. There
was no effect on total number of adults, emergence success, sex ratio,
total number of egg clusters, oviposition success or hatchability at
10 µg cadmium/litre. There was a significant decrease in the total
number of adults and emergence success at 20 µg/litre or more and in
the total number of egg clusters at 40 µg/litre or more. At 80
µg/litre survival and reproduction were very significantly depressed.
In a separate experiment midges were fed cadmium-contaminated food
(dried yeast) at concentrations of 22, 220, and 1800 mg cadmium/kg.
Emergence success was decreased at 220 and 1800 mg/kg but no other
effects were observed.
6.2.4 Physiological and biochemical effects
Berglind (1986) investigated the effect of cadmium alone and in
combination with other metals on the delta-aminolevulinic acid
dehydratase (ALAD) activity of Daphnia magna. ALAD activity was
enhanced by cadmium alone (at 2 µg/litre but not at 0.2 µg/litre) but
this enhancement was abolished in the presence of zinc at 200
µg/litre.
Vernberg et al. (1977) investigated sub-lethal concentrations of
cadmium and their effects on the adult shrimp Palaemonetes pugio
under static and flow-through conditions. Adult shrimps were exposed
to cadmium at 50 µg/litre. This shrimp is highly tolerant to cadmium
and after exposure to 23 mg/litre the mortality rate was only 10%. The
authors found increased uptake of cadmium into the body of the shrimps
with decreasing salinity. Similarly, at higher salinity levels,
toxicity was lower. In shrimps kept at the lowest salinity level (5%),
where cadmium body burden reached 40 mg/kg, there was inhibition of
moulting. At more moderate cadmium body burdens of 23 mg/kg and 10
mg/kg, observed at salinities of 10% and 20%, respectively, moulting
was stimulated by the presence of cadmium. An investigation of the
effects of cadmium on respiratory rate was inconclusive because of
considerable variation. The authors considered that the flow-through
system more nearly approximated field conditions than the static
system. The relationship between salinity and cadmium uptake was
eliminated in a static system at 15 °C, but was quite clearly present
when the system was flow-through. After an extensive study on the
effects of cadmium on three species of shrimps, Penaeus duorarum,
Palaemonetes pugio, and Palaemonetes vulgaris, Nimmo et al. (1977)
reported sublethal histological effects and blackening and damage to
gill filaments. Placing shrimps with blackened gills in cadmium-free
water resulted in the sloughing-off of blackened portions of the
branchia and the shrimps appeared normal within 14 days. The effect on
the gills occurred with exposure to cadmium concentrations approaching
the LC50 which, with an exposure duration of 96 h, was 0.76 mg/litre
for Palaemonetes vulgaris and 3.5 mg/litre for Penaeus duorarum.
Thurberg et al. (1973) exposed two species of crabs ( arcinus maenus
and Cancer irroratus) to various concentrations of cadmium chloride
for 48 h and at five different salinities. At the end of each exposure
period, tests of blood serum osmolarity and gill tissue oxygen
consumption were performed. Cadmium increased the osmolarity of
Carcinus serum above its normal hyperosmotic state and reduced
oxygen consumption by the gill tissue of both species. Effects on
oxygen consumption were dose related over a range of cadmium
concentrations from 0 to 4 mg/litre.
Cadmium has been shown to cause an increase in oxygen consumption
rates in the mud snail Nassarius obsoletus at concentrations of
between 0.5 and 4.0 mg/litre over a 72-h exposure period (MacInnes &
Thurberg, 1973). A similar increase in oxygen consumption rate was
observed in the marine snail Murex trunculus during chronic exposure
to 0.05 mg cadmium/litre (Dalla Via et al., 1989) and in crabs
( Callinectes similis) exposed to cadmium concentrations of between
2.48 and 10.05 mg/litre for up to 96 h (Ramirez et al., 1989).
6.2.5 Behavioural effects
Olla et al. (1988) monitored the burrowing behaviour of three
polychaete species, Nereis virens, Glycera dibranchiata, and
Nephtys caeca during a 28-day exposure to a sediment concentration
of 40 mg cadmium/kg. Most comparisons of burrowing times and rates
between exposed and unexposed worms were not statistically
significant. Four out of 15 comparisons gave significant results but
these were randomly spread amongst species and exposure periods. The
authors concluded that these results would probably have little
ecological significance. The feeding behaviour of G. dibranchiata
was also monitored but no significant effect of the cadmium treatment
was found.
6.2.6 Interactions with other chemicals
Sunda et al. (1978) carried out experiments in diluted sea water
with various concentrations of the chelating agent nitrilotriacetic
acid (NTA) to determine the relationship between the chemical
speciation of cadmium and the toxicity of the metal to the grass
shrimp Palaemonetes pugio. After 4 days of exposure to a given
concentration of cadmium chloride, shrimp mortality decreased with
increasing salinity and increasing concentration of the chelating
agent. The protective effect of high salinity or NTA was attributable
to complexation of cadmium; mortality was related to the measured free
cadmium ion concentration, which was determined by measuring total
concentration of cadmium and deducting the calculated level of
complexation by either the chloride ion or NTA. The mortality at a
free cadmium ion concentration of approximately 4 x 10-7 mol/litre
was 50%. In a study of the combined effects of zinc and cadmium on the
shrimp Callianassa australiensis by Negilski et al. (1981), there
was interaction between the two metals; in combination they gave
greater mortality than would be expected if there were no interaction.
The authors demonstrated that each metal increased the accumulation of
the other.
6.2.7 Tolerance
Khan et al. (1988) exposed two different populations of grass
shrimp Palaemonetes pugio to cadmium under static conditions and
calculated 96-h LC50 values of 3.28 mg/litre for shrimps from an
industrialized area and 1.83 mg/litre for those from a non-
industrialized area. Pre-exposure of shrimps to 0.05 mg cadmium per
litre caused an increase in the LC50 values to 6.81 and 3.89
mg/litre for the two respective populations.
Moraitou-Apostalopoulou et al. (1982) collected the shrimp
Palaemon elegans from two different areas with different natural
concentrations of cadmium and investigated the sublethal effects of
the metal. They found that cadmium decreased respiration rates in the
shrimp at sublethal concentrations. Shrimps sampled from an area with
a natural cadmium concentration of 0.6 µg/litre were more tolerant to
the metal than were those from an area with 0.1 µg/litre. There was
also a difference in the acute toxicity of cadmium to the two
populations, indicating that tolerance had developed. In an earlier
study (Moraitou-Apostolopoulou et al., 1979), similar development of
tolerance was reported for a copepod Acartia clausi.
In a study of the toxicity of cadmium to the freshwater cyclopoid
copepod Tropocyclops prasinus mexicanus, Lalande & Pinel-Alloul
(1986) sampled animals from three Quebec lakes, one polluted and two
unpolluted with cadmium. The cultures from the two unpolluted lakes
showed lower LC50 values in 48-h tests than the culture from the
polluted lake. However, the polluted lake had a significantly higher
hardness (120 mg calcium carbonate per litre) than the other two lakes
(10 mg/litre).
6.2.8 Model ecosystems
Borgmann et al. (1989a) established a Daphnia-phytoplankton
model ecosystem and exposed it to cadmium sulfate at concentrations of
1, 5 and 15 µg cadmium/litre. At 5 µg/litre the Daphnia population
collapsed after 9 weeks of treatment and chlorophyll levels increased.
At 15 µg/litre the Daphnia population collapsed after 5 weeks, but
chlorophyll levels remained low. There appeared to be no effect on
this model system at 1 µg/litre.
6.3 Toxicity to Fish
The toxicity of cadmium has been studied in a variety of fish
species in both fresh and sea water at various temperatures and
dissolved oxygen concentrations. Generally, increasing dissolved salt
concentration decreases the toxicity, whereas increasing temperature
increases it. An increase in the dissolved oxygen content of the water
decreases the toxicity of cadmium to freshwater fish. Salmonids appear
to be particularly susceptible to the metal. Sublethal effects have
been reported, notably malformation of the spine.
6.3.1 Acute and short-term toxicity
The acute toxicity of cadmium to fish is summarized in Tables 11
and 12. Pickering & Henderson (1966) calculated the LC50 values for
five species of warm-water fish, some of which were tested in both
soft and hard water. There was surprisingly little difference in
fathead minnows ( Pimephales promelas) between the 24-h, 48-h, and
96-h LC50 values, which in soft water, were 1.09, 1.09, and 1.05
mg/litre, respectively. This species was very much more resistant to
cadmium in hard water with 24-h, 48-h, and 96-h LC50 values of 78.1,
72.6, and 72.6 mg/litre, respectively. The hardness for the two types
of water was 20 and 360 mg CaCO3 per litre and the alkalinity 80 and
300 mg/litre. The dissolved oxygen concentration was similar in the
two types of water. However, the pH was 7.5 for soft water and 8.2 for
hard water, this being an area of the pH range where speciation of
cadmium undergoes major change. Bluegill sunfish, goldfish, and
guppies showed a decrease in LC50 with increase in exposure duration
from 24 to 96 h (Table 12), but these were tested only in soft water.
The green sunfish Lepomis cyanellus showed LC50 values in soft
water of 7.84, 3.68, and 2.84 mg/litre with exposure durations of 24
h, 48 h, and 96 h, respectively. This species was also tested in hard
water, where, like the fathead minnow, it showed considerably less
cadmium toxicity. The 24-h LC50 rose from 7.84 in soft water to 88.6
mg/litre in hard water.
Pickering & Gast (1972) determined a maximum acceptable toxicant
concentration (MATC) for the fathead minnow Pimephales promelas of
between 37 and 57 µg cadmium/litre. The experimental concentration of
57 µg/litre decreased survival of the developing embryos, this being
the most sensitive life-stage, but at lower concentrations (between
4.5 and 37 µg/litre) no adverse effect was found on survival, growth
or reproduction. Carroll et al. (1979) investigated the protective
effects of various constituents of hard water on the toxicity of
cadmium to the brook trout ( Salvelinus fontinalis) and concluded
that calcium, added as either the sulfate or carbonate, was the most
significant source of protection. This protective effect was observed
in the absence of significant cadmium precipitation. Magnesium,
sulfate, and sodium ions and the carbonate system provided little or
no protection. Calamari et al. (1980) found an influence of water
hardness on the toxicity of cadmium to Salmo gairdneri; 48-h LC50
values increased from 91 µg/litre, with a hardness of 20 mg/litre in
the test water, to 3700 µg/litre at a water hardness of 320 mg/litre.
The 48-h LC50 of fish acclimatized to a hardness of 320 mg/litre and
then tested at a hardness of 20 mg/litre was about 7 times higher than
that of fish acclimatized and tested in the same soft water. There are
two types of biological effects of hardness on the availability of
cadmium to fish; one of them persists after acclimatization in hard
water.
Table 11. Toxicity of cadmium to marine or estuarine fish
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Striped killifish 0.95 g stat 20 20 8.0 24 125 n Eisler (1971)
(Fundulus majalis) 0.95 g stat 20 20 8.0 48 59 n Eisler (1971)
0.95 g stat 20 20 8.0 96 21 n Eisler (1971)
Mummichog 0.89 g stat 20 20 8.0 24 > 100 n Eisler (1971)
(Fundulus 0.89 g stat 20 20 8.0 48 > 100 n Eisler (1971)
heteroclitus) 0.89 g stat 20 20 8.0 96 55 n Eisler (1971)
1.3 g stat 20 20 7.8 24 220 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 96 22 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 168 22 n Eisler & Hennekey (1977)
1 day stat 20 20 48 16.2 (12.7-21.2) n Middaugh & Dean (1977)
7 days stat 20 20 48 9 (6.4-12.5) n Middaugh & Dean (1977)
14 days stat 20 20 48 32 (24.6-41.6) n Middaugh & Dean (1977)
adult stat 20 20 48 60 (40-90) n Middaugh & Dean (1977)
1 day stat 20 30 48 23 (19.2-27.6) n Middaugh & Dean (1977)
7 days stat 20 30 48 12 (9.2-15.6) n Middaugh & Dean (1977)
14 days stat 20 30 48 7.8 (5.6-10.3) n Middaugh & Dean (1977)
adult stat 20 30 48 43 (33-56) n Middaugh & Dean (1977)
Sheepshead minnow 1.1 g stat 20 20 8.0 24 100 n Eisler (1971)
(Cyprinodon 1.1 g stat 20 20 8.0 48 50 n Eisler (1971)
variegatus) 1.1 g stat 20 20 8.0 96 50 n Eisler (1971)
Coho salmon smolt flow 11.2 28.3 7.9 96 1.5 (1.2-2.4) m Dinnel et al. (1989)
(Oncorhynchus
kisutch)
Yellow-eye mullet 1.49 g flow 18.5 34.5 7.8 58 15.5 (12.2-19.8) m Negilski (1976)
(Aldrichetta 1.15 g flow 18.6 34.8 7.8 120 14.3 (8.4-24.3) m Negilski (1976)
forsteri)
Small-mouthed 1.36 g flow 17.9 34.5 7.9 168 12.7 (8.3-19.4) m Negilski (1976)
hardyhead 1.12 g flow 18.0 34.5 7.8 168 16.6 (13.3-20.7) m Negilski (1976)
(Atherinasoma microstoma)
Table 11 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Atlantic silverside adult stat 20 20 48 13 (9-20) n Middaugh & Dean (1977)
(Menidia menidia) adult stat 20 30 48 12 (8-16) n Middaugh & Dean (1977)
Tidewater silverside larva stat 26 22 96 0.31 (0.25-0.38) n Mayer (1987)
(Menidia peninsulae)
Shiner perch adult flow 13 30.1 7.8 96 11 (5-20) m Dinnel et al. (1989)
(Cymatogaster aggregata)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained)
b organisms exposed to cadmium added as cadmium chloride; m = measured concentration; n = nominal concentration
Table 12. Toxicity of cadmium to freshwater fish
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Chinook salmon juvenile flow 11-13 20-22 7.0-7.3 96 0.001 (± 0.0007) f m Finlayson & Verrue (1982)
(Onchorhynchus
tshawytscha)
Rainbow trout juvenile flow 6.4-8.3 96 0.0066 g m Hale (1977)
(Salmo 5-15 g stat 8.5-10.7 61-65 7.4 48 2.9 m Pascoe et al. (1986)
gairdneri) 5-15 g stat 8.5-10.7 283-317 7.4 48 5.7 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 1.3 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 2.6 m Pascoe et al. (1986)
Fathead minnow adult stat 25 20 7.5 24 1.09 (0.79-2.91) n Pickering & Henderson (1966)
(Pimephales adult stat 25 360 8.2 24 78.1 (57.2-117) n Pickering & Henderson (1966)
promelas) adult stat 25 20 7.5 48 1.09 (0.79-2.91) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 1.05 (0.7-4.43) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 18-22 190-210 7.7 48 0.1 (0.07-0.17) n Hall et al. (1986)
adult stat 18-22 190-210 7.7 96 0.09 (0.07-0.14) n Hall et al. (1986)
Bluegill sunfish adult stat 25 20 7.5 24 4.56 (3.64-6.08) n Pickering & Henderson (1966)
(Lepomis adult stat 25 20 7.5 48 2.76 (2.02-3.46) n Pickering & Henderson (1966)
macrochirus) adult stat 25 20 7.5 96 1.94 (1.33-2.35) n Pickering & Henderson (1966)
Goldfish adult stat 25 20 7.5 24 3.46 (2.85-4.82) n Pickering & Henderson (1966)
(Carassius adult stat 25 20 7.5 48 2.62 (2.04-3.68) n Pickering & Henderson (1966)
auratus) adult stat 25 20 7.5 96 2.34 (1.81-3.16) n Pickering & Henderson (1966)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Guppy adult stat 25 20 7.5 24 3.37 (2.73-4.81) n Pickering & Henderson (1966)
(Poecilia adult stat 25 20 7.5 48 2.31 (1.78-3.11) n Pickering & Henderson (1966)
reticulata) adult stat 25 20 7.5 96 1.27 (0.97-1.71) n Pickering & Henderson (1966)
3-4 weeks flow c 23-25 1 mM 24 10.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 48 5.7 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 72 4.3 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 96 3.8 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 24 33 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 48 20.5 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 72 14.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 96 11.1 m Canton & Slooff (1982)
Green sunfish adult stat 25 20 7.5 24 7.84 (6.13-14.2) n Pickering & Henderson (1966)
(Lepomis adult stat 25 360 8.2 24 88.6 (74-106) n Pickering & Henderson (1966)
cyanellus) adult stat 25 20 7.5 48 3.68 (2.89-4.69) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 71.3 (56.3-92.2) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 2.84 (2.1-3.56) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 66 (51.7-84.4) n Pickering & Henderson (1966)
Golden shiner flow 72.2 7.5 96 2.8 (1.9-4.3) m Hartwell et al. (1989)
(Notemigonus
crysoleucas)
Puntius 2.4 g stat b 23-27 60-70 7.5 24 59.99 (58.5-61.5) n Shivaraj & Patil (1988)
arulius 2.4 g stat b 23-27 60-70 7.5 48 45.7 (43.9-47.5) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 72 41.7 (39.7-43.8) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 96 39 (36.5-41.7) n Shivaraj & Patil (1988)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Killifish 4-5 weeks stat b 23-25 1 mM 48 > 2.8 m Canton & Slooff (1982)
(Oryzias 4-5 weeks stat b 23-25 1 mM 72 0.35 m Canton & Slooff (1982)
latipes) 4-5 weeks stat b 23-25 1 mM 96 0.35 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 24 > 2.6 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 48 1.8 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 72 0.17 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 96 0.13 m Canton & Slooff (1982)
Zebra fish 6 months flow 19-21 1.7 mM 24 7 m Canton & Slooff (1982)
(Brachydanio 6 months flow 19-21 1.7 mM 48 4.2 m Canton & Slooff (1982)
rerio)
a stat = static conditions (water unchanged for duration of test unless stated otherwise); flow = flow-through conditions
(cadmium concentration in water continuously maintained unless stated otherwise)
b static conditions but water renewed every 24 h
c intermittent flow-through conditions
d Hardness was expressed as mg CaCO3/litre unless stated otherwise
e fish were exposed to cadmium added as the chloride unless stated otherwise; m = measured concentration; n = nominal concentration
f cadmium was added as the sulfate
g cadmium was added as the nitrate
In a large scale study of the toxicity of cadmium to the
mummichog Fundulus heteroclitus, Voyer (1975) examined effects of
salinity, pre-exposure to high salinity and different concentrations
of dissolved oxygen on the tolerance of fish to cadmium over 96 h. He
found no significant influence of dissolved oxygen levels between 4.0
mg/litre and saturation regardless of salinity during acclimatization
or during the test. By contrast, Voyer et al. (1975) showed a distinct
effect of dissolved oxygen concentration on toxicity of cadmium to the
same species of fish in fresh water. Median tolerance concentrations
at 96 h ranged upwards from 1.3 to 3.0 mg cadmium/litre with 2.3 and
8.5 mg/litre of dissolved oxygen, respectively. They demonstrated
statistically an independent effect of dissolved oxygen and time
against cadmium toxicity. It should be noted that cadmium is 10 times
more toxic to this species in fresh water than in sea water.
Toxicity of cadmium to both marine (Eisler, 1971) and freshwater
(Roch & Maly, 1979) fish has been shown to be greater at higher
temperatures.
Canton & Slooff (1982) exposed several fish species to cadmium in
short-term toxicity tests. At a water hardness of 1.7 mmol/litre they
found the no-observed-adverse-effect level (NOAEL) for mortality in
the zebra fish ( Brachydanio rerio) to be 2 mg/litre over a 48-h
exposure period. For the killifish ( Oryzias latipes), the 96-h NOAEL
was 0.06 mg/litre for mortality and 0.03 mg/litre for mortality and
abnormal behaviour at a water hardness of 2 mmol/litre. The
corresponding values at a water hardness of 1 mmol/litre were 0.055
and 0.006 mg/litre. For the guppy ( Poecilia reticulata), the 96-h
NOAEL for mortality and abnormal behaviour was 5.2 mg/litre at a water
hardness of 2 mmol/litre and 0.6 mg/litre at 1 mmol/litre. The authors
calculated a 24-h NOAEL for the rainbow trout ( Salmo gairdneri) of
0.01 mg/litre for inhibition of opercular movements.
Abel & Papoutsoglou (1986) studied the toxicity of cadmium to
Cyprinus carpio and Tilapia aurea and reviewed data on other
species of freshwater fish. The found for all species examined that
the median survival time changed little over a wide range of cadmium
concentrations and that a toxic threshold was clear in most studies.
For Tilapia this threshold lay between 0.1 and 0.5 mg cadmium/litre;
negligible mortality was recorded in fish exposed to 0.1 mg/litre for
3 months.
6.3.2 Reproductive effects and effects on early life-stages
Meteyer et al. (1988) exposed sheepshead minnow ( Cyprinodon
variegatus) eggs to cadmium concentrations of between 0.39 and 1020
µg/litre from approximately 4 h after fertilization. Hatching was
delayed by up to 3 days at the highest cadmium concentration. All
treated larvae were shorter than controls, but there was no
dose-related effect of cadmium on growth.
Middaugh & Dean (1977) examined the toxicity of cadmium to
various life-stages of the mummichog Fundulus heteroclitus. In 48-h
tests, eggs were highly resistant to cadmium. The greatest effect (54%
non-emergence) occurred at a cadmium concentration of 32 mg/litre; the
control non-emergence was 17%. Newly-emerged larvae were less
sensitive to cadmium than were 7-day-old larvae. There was an effect
of salinity on the sensitivity of 14-day-old larvae to the metal
(Table 11). Adults were less sensitive to cadmium than were larvae.
Similar results were obtained with the Atlantic silverside ( Menidia
menidia), larvae being the most sensitive life-stage. Weis & Weis
(1977) found no effect of cadmium, at concentrations up to 10
mg/litre, on embryos of Fundulus heteroclitus. Rombough & Garside
(1982) found the most sensitive indicator of cadmium toxicity to early
life-stages of the Atlantic salmon ( Salmo salar) to be inhibition of
growth of alevins, where significant reductions occurred with cadmium
concentrations of 0.47 µg/litre. The LC50 for the interval between
fertilization and viable hatch lay between 300 and 800 µg per litre.
Newly hatched alevins showed a 24-h LC50 of between 1.5 and 2.4
mg/litre. Sensitivity increased sharply in late alevins and
significant mortality was recorded at a concentration of 8.2 µg/litre.
Eaton et al. (1978) exposed embryos and larvae of seven
freshwater fish species to nominal cadmium concentrations of 0, 0.4,
1.2, 3.7, 11.6, 33.3, and 100 mg/litre for periods ranging from 3 to
126 days. Actual concentrations were monitored and used in the
assessment of the results. Results were expressed in terms of
"standing crop", which the authors estimated as the product of the
proportion of fish surviving and their total biomass. The lowest
concentration of cadmium at which the standing crop was significantly
different from controls was approximately 12 µg/litre for the white
sucker, northern pike, smallmouth bass, west coast coho salmon, lake
trout, and brown trout exposed as embryos or larvae for up to 64 days.
The value was lower for brook trout (0.48 µg per litre) after exposure
of larvae/juveniles for 65 days and for Lake Superior coho salmon (3.4
µg/litre) after exposure of larvae/juveniles for 27 days. The highest
cadmium concentration at which standing crop was not significantly
different from controls varied for the seven species between 1.1 and
4.2 µg/litre.
Woodall et al. (1988) exposed rainbow trout ( Salmo gairdneri)
fry to cadmium concentrations of 0, 0.1, 1.0 or 5.0 mg/litre for up to
90 h. In preliminary experiments, they calculated that the 90-h LC50
lay between 0.1 and 1.0 mg/litre. Pre-treatment of trout fry with
cadmium initially had little effect (up to 30 h). However, with
exposure periods of between 45 and 90 h, some protection was induced
by pre-treatment. Benoit et al. (1976) exposed three generations of
brook trout ( Salvelinus fontinalis) to concentrations of total
cadmium varying between 0.06 and 6.4 µg/litre. Significant numbers of
first and second generation adult males died during spawning when
exposed to 3.4 µg/litre. This concentration also significantly
retarded the growth of juvenile second and third generation offspring,
but at a concentration of 1.7 µg/litre these effects were not seen.
In a study by Borgmann & Ralph (1986), white sucker larvae
Catostomus commersoni and young common shiners Notropis cornutus
were exposed to cadmium chloride at concentrations ranging from 6.24
to 200 µg cadmium/litre. The relative growth rate of fish was
significantly reduced at concentrations of 36 µg/litre or more in the
case of suckers and 63 µg/litre or more in the case of shiners.
Cadmium had no effect on the relative feeding rates.
Hatakeyama & Yasuno (1987) studied the chronic effects of cadmium
on the reproduction of the guppy Poecilia reticulata. The fish were
exposed to cadmium via cadmium-accumulated midge larvae used as their
food source. The cumulative numbers of fry produced by guppies fed
midge larvae containing 500, 800 or 1300 mg cadmium/kg for 6 months
decreased to 79%, 65%, and 55% of the controls, respectively. At the
highest dose, the mortality of females was significantly elevated at
6 months, but no such effect was observed with the males.
Michibata (1981) reported a protective effect of water hard-ness
against the effects of cadmium on the eggs of Oryzias latipes.
6.3.3 Metabolic, biochemical and physiological effects
Protective metal-binding proteins (metallothioneins) are induced
by cadmium in fish (chapter 4).
A manifest symptom of cadmium toxicity in freshwater fish is
ionic imbalance with reduced plasma Ca2+, Na+, and CL-. The
probable explanation is that cadmium is a potent inhibitor of
ion-transporting enzymes. Verbost et al. (1988) showed that cadmium
inhibited Ca-ATPase in the cell membranes of fish gut. It probably
does the same in the gills because cadmium exposure has been shown to
inhibit calcium uptake in the gills of adults (Verbost et al., 1987;
Reid & McDonald, 1988) as well as in larvae (Wright et al., 1985).
Similarly, cadmium has been shown to inhibit Na/K-ATPase in fish gills
(Watson & Benson 1987), which, taken with the fact that cadmium
probably also affects the production of ATP in the gills (Dickson et
al., 1982), could explain the reduction of plasma Na+. Huiguang Fu
(1989) studied the role of the hormones prolactin and cortisol in
correcting cadmium-induced impairment of the calcium balance in
tilapia ( Oreochromis mossambicus). Fish were able to recover from
initial hypocalcaemia during a 35-day exposure to a concentration of
10 µg cadmium/litre. This recovery involves prolactin-induced
stimulation of active Ca2+ uptake and reduction of passive Ca2+
efflux, together with cortisol-induced changes in chloride cells and
stimulation of metallothionein synthesis in the liver, kidney, and
gills. The author stated that the capacity to survive prolonged
exposure to 10 µg cadmium/litre through physiological adaptation does
not indicate that this cadmium concentration is acceptable to tilapia.
Adaptation may be achieved at the expense of other essential processes
like growth and reproduction.
Arillo et al. (1984) investigated the effect of cadmium at levels
of 1-10 µg/litre (concentrations above the water quality criteria
value of 0.75 µg/litre proposed for salmonids by EIFAC/FAO) on a wide
variety of biochemical parameters in the rainbow trout (Salmo
gairdneri). The exposure period was 4 months. Only at the highest
test concentration of 10 µg/litre were there any effects on the fish,
and then only on liver aminolevulinic acid dehydratase activity. The
authors concluded that the water quality criterion was realistic.
Dawson et al. (1977) exposed juvenile striped bass ( Morone
saxatilis) to cadmium chloride concentrations of 0.5, 2.5 or 5
µg/litre for 30 to 90 days, and the fish were then allowed to recover
for 30 days in clean, running sea water. There was an inhibition of
gill tissue respiration at 30 and 90 days, which recovered during the
30-day period with clean water. The activities of the various enzymes
measured were not affected. MacInnes et al. (1977) reported reduced
gill tissue oxygen consumption in cunner ( Tautogolabrus adspersus)
exposed to cadmium (0.05 or 0.1 mg/litre) for 30 or 60 days. They also
reported a reduced activity of aspartate aminotransferase and an
increased activity of glucose-6-phosphate dehydrogenase in the liver
of the fish after 30 days of exposure to cadmium. Gill & Pant (1983)
measured levels of various blood and tissue constituents after acute
(24 h) or chronic (90 day) exposure to cadmium. Acute exposure to
12.65 mg/litre led to significant hyperglycaemia and an increase in
liver, kidney, and ovarian cholesterol levels. Chronic exposure to
0.63 or 0.84 mg/litre, by contrast, led to an enduring hypoglycaemia
and diminished levels of cholesterol in tissues. Both acute and
chronic exposure to cadmium caused marked hypocholesterolaemia,
glycogenolysis in the liver and brain and a rise in myocardial
glycogen. Testis cholesterol was depleted after 60 days in both acute
and chronic exposures.
Sastry & Subhadra (1983) exposed the catfish Heteropneustes
fossilis to cadmium in the water at the sublethal concentration of
2.3 µmol/litre for 15 or 30 days. The cadmium caused reduced
absorption of glucose and fructose from the gut, this effect being
more pronounced after 30 days of exposure than after 15 days. Filling
intestinal sacs, in vivo, with cadmium solutions (1 µmol per litre)
reduced absorption of the sugars significantly over 1 h.
Merlini (1978) pre-treated immature sunfish ( Lepomis gibbosus)
with cadmium (0.004 mg/litre) and then fed treated and control fish
with a single ration containing 58Co-labelled vitamin B12. The
fish were subsequently fed non-radioactive food for 31 days before
sacrifice. The author reported that cadmium-treated fish stored
significantly less vitamin B12 in the liver than did controls.
Carrier & Beitinger (1988a) studied the effect of cadmium on the
critical thermal maximum (the temperature at which loss of equilibrium
is coupled with loss of righting response) in the red shiner
( Notropis lutrensis) and fathead minnow ( Pimephales promelas). The
red shiner was exposed to sublethal cadmium concentrations of 4.88,
5.07, and 5.46 mg/litre and the fathead minnow to 0.09, 0.48, and 1.26
mg/litre. Critical thermal maxima were significantly reduced for both
species over a 10-day exposure period. The effect was found to be dose
and time dependant. In the green sunfish ( Lepomis cyanellus) there
was no effect of cadmium concentrations of 2.76, 4.22 or 5.17 mg/litre
on the critical thermal maximum (Carrier & Beitinger, 1988b).
6.3.4 Structural effects and malformations
When Bengtsson et al. (1975) exposed 180 minnows ( Phoxinus
phoxinus) to various concentrations of cadmium ranging from 7.5
µg/litre to 4.8 mg/litre for 70 days, 31 of the 101 fish that survived
developed lesions in the spinal column. Fractured vertebrae occurred
in the caudal end of the abdominal section of the spine or in the
caudal section; 64% of all fractures occurred in the first 7 caudal
vertebrae and 21% occurred in abdominal vertebrae numbers 8 to 14.
Hiraoka & Okuda (1984) cultured medaka ( Oryzias latipes) eggs in a
cadmium solution of 0.01 mg/litre for 1 week and then investigated
abnormalities in the vertebrae of the hatched fry, which were reared
in clean water. There was no damage to the centra of the vertebrae in
newly hatched fry. However, centrum-damaged fish were found in the
first week and the numbers increased rapidly up to the 4th week after
hatch. The cumulative frequency of vertebral-damaged fish was 13% and
14% in the 5th and 6th weeks, respectively, and seemed to remain
constant after this. Muramoto (1981b) reported that fish showing
malformations of the spine after cadmium treatment had significantly
less calcium in the vertebral column than did control fish.
Voyer et al. (1975) found no short-term histopathological effects
of cadmium, but long-term exposure to 28 mg/litre caused necrosis and
sloughing of the mucosa of gills. Tissue damage was also evident in
the nasal passages and buccal cavity. This histopathological effect
was seen between exposure times of 512 and 612 h, but not before.
6.3.5 Behavioural effects
Hartwell et al. (1989) studied the avoidance response of the
golden shiner ( Notemigonus crysoleucas) to cadmium concentrations of
up to 68 µg/litre, but found no significant avoidance.
Sullivan et al. (1978b) subjected fathead minnows ( Pimephales
promelas) to acute (24 h) or subacute (21 days) exposure at
sublethal cadmium concentrations and then placed them in experimental
chambers with largemouth bass ( Micropterus salmoides), a fish which
preys upon them. The minnows displayed altered behaviour patterns,
including abnormal schooling behaviour, and were more vulnerable to
predation than controls. The lowest acute cadmium exposure level that
increased vulnerability was 0.375 mg/litre and the lowest subacute
level 0.025 mg/litre. The authors pointed out that the subacute value
of 0.025 mg/litre was well below reported no-effect levels of cadmium
for fathead minnows established with respect to survival and
reproductive effects. The avoidance threshold for cadmium in water by
the rainbow trout ( Salmo gairdneri) is 50 µg/litre (Black & Birge,
1980), which is 50 times higher than the 96-h LC50 value reported
for this species.
6.3.6 Interactions with other chemicals
Muramoto (1981a) showed that the chelating agents EDTA and DPTA
afforded some protection against the effects of cadmium on carp
( Cyprinus carpio) exposed to 0.05 or 0.1 mg cadmium/litre for 3
months. There were effects on the vertebrae of exposed fish.
Chelating agents that form hydrophobic complexes with heavy
metals increase the bioavailability of the metal to aquatic organisms.
Examples of these are xanthates and dithiocarbamates. Xanthates are
used in the mining industry in the flotation process to refine metal
from sulfide ores. As discussed in section 4.1, these compounds
increase the uptake rate of cadmium through the gills in fish (Block
& Part, 1986; Gottofrey et al., 1988; Block, 1991). Furthermore, they
change the tissue distribution of the metal in such a way that more
cadmium is found in lipid-rich tissues such as nervous tissue (brain)
and adipose tissue than when fish are exposed to the metal alone.
Finlayson & Verrue (1982) reported 96-h LC50 values for
juvenile chinook salmon ( Onchorynchus tshawytscha) ranging from 0.6
to 1.6 µg/litre. They found no synergistic or antagonistic toxic
effects after combining cadmium and zinc in their test system. The
results of tests were additive, the overall LC50 being a simple
combination of individual metal effects at a zinc:cadmium ratio of
1:0.008. Spehar et al. (1978b) exposed the flagfish Jordanella
floridae to cadmium and zinc, both individually and as a mixture, at
concentrations ranging from 4.3 to 8.5 µg cadmium/litre and 73.4 to
139 µg zinc/litre, through one complete life-cycle of the fish. There
was no additive effect of cadmium and zinc at sublethal concentrations
in mixed exposure. Effects on survival showed that the toxicity of
cadmium and zinc mixtures was only slightly, if at all, greater than
the toxicity of zinc alone. Anadu et al. (1989) reported that
pre-exposure of rainbow trout ( Salmo gairdneri) to zinc (100
µg/litre) for 17 days increased the subsequent 120-h LC50 for
cadmium from 1.1 µg/litre to 4.1 µg/litre.
6.4 Toxicity to Amphibia
Francis et al. (1984) exposed eggs of the leopard frog ( Rana
pipiens) to cadmium-enriched sediments during their development and
for 4 days after the larvae had hatched. Measured concentrations of
cadmium ranged from 1.04 to 1074 mg/kg in sediment and from 1.0 to
76.5 mg/litre in water above the sediment. Cadmium concentrations in
the tissues of the tadpoles at the end of the experiment ranged from
0.08 to 12.55 mg/kg. There was no mortality as a result of cadmium
exposure. The LC50 for this species has been reported to be 50
µg/litre for water without sediment by Westerman (1977). Slooff &
Baerselman (1980) determined 48-h LC50 values for the neotenous
larval mexican axolotl ( Ambystoma mexicanum) and larval South
African clawed toad ( Xenopus laevis) of 1.3 mg/litre and 32
mg/litre, respectively, after exposure to cadmium nitrate. Canton &
Slooff (1982) exposed Xenopus laevis to cadmium, added as cadmium
chloride, and obtained 24-h and 48-h LC50 values of 4 and 3.2
mg/litre, respectively. A NOEL of 2.2 mg/litre was reported for both
exposure periods. In a longer-term exposure (100 days), by the same
authors, an LC50 value of 1500 µg/litre was found and the EC50
value for inhibition of larval development was 650 µg/litre. For
mortality and larval development, the NOELs were 30 and 9 µg/litre,
respectively. De Zwart & Sloof (1987) determined a 48-h LC50 of 20.2
mg/litre for tadpoles of the clawed toad. Khangarot & Ray (1987)
established a 96-h LC50 value of 8.18 (6.96-9.53) mg/litre for the
tadpoles of the toad Bufo melanosticus. The test water was obtained
from a well and had a hardness of 185 mg per litre, pH 7.4, and
temperature of 31 °C. Solids were present at a level of 920 mg/litre.
Muino et al. (1990) calculated the 48-h and 96-h LC50 for Bufo
arenarum tadpoles to be 2.52 and 2.08 mg cadmium/litre for the two
exposure periods, respectively, under semi-static conditions. In tests
to study the effect of a sublethal cadmium concentration (1.0 mg
cadmium/litre) on the water balance of the animals, the authors found
that all animals died within a few hours in ion-free media. Tadpoles
exposed in ionic solutions showed mortality of less than 10%
(equivalent to control groups).
Woodall et al. (1988) exposed Xenopus laevis tadpoles to
cadmium concentrations of 0, 50, 80, and 100 mg cadmium/litre for up
to 90 h. In preliminary experiments, they calculated the 90-h LC50
to lie between 80 and 100 mg/litre. The authors found that
pre-treatment of tadpoles with cadmium induced protection, which
decreased with an increase in the subsequent exposure concentration.
Cadmium pre-treatment induced maximum protection to cadmium at a
concentration of 50 mg/litre at both 45 and 90 h.
Perez-Coll et al. (1986) exposed developing Bufo arenarum
embryos to cadmium chloride concentrations of 6 x 10-7 to 1.5 x
10-5 mol Cd2+/litre during gastrulation at 20 °C and 30 °C.
Initial failures at gastrulation resulted mainly in axial
incurvations, microcephaly, hydropsy, and abnormal tail development.
At the higher temperature, high concentrations of cadmium caused a
significant increase in early malformations and at low concentrations
the high temperature prevented alterations.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
Both terrestrial plants and animals accumulate cadmium, but the
rate of accumulation is much higher under experimental conditions,
where cadmium is available in solution, than it is with plants grown
in soil, when part of the cadmium is bound and less available.
Cadmium has adverse effects on hydroponically grown plants at
concentrations in the mg/litre range, whereas plants grown in soil
only show reduced growth in contaminated soils with hundreds of mg
cadmium/kg. Terrestrial invertebrates are relatively insensitive to
cadmium-induced toxic effects, probably due to effective
sequestration mechanisms in specific organs. When toxic effects do
occur, they consist of reduced growth and reproduction.
7.1 Toxicity to terrestrial plants
Cadmium has been shown to have an adverse effect on plant growth
and yield in laboratory experiments. However, plants grown in soil are
generally insensitive to the effects of cadmium except at high doses.
Effects are only seen when cadmium is given in nutrient solutions
rather than in soil, where the cadmium is bound and is therefore less
available to the plants. Cadmium is only available to plants in
solution in soil. There is considerable evidence from field studies
that plants are able to develop tolerance to various heavy metals in
their growth medium. Research into cadmium tolerance has been more
limited than for other metals, but there is some evidence of tolerance
developing.
7.1.1 Toxicity to plants grown hydroponically
Mitchell & Fretz (1977) cultured seedlings of three species of
tree, the white pine ( Pinus strobus), red maple ( Acer rubrum), and
Norway spruce ( Picea abies), in sand. Plants were irrigated with a
nutrient solution containing cadmium at concentrations of 0, 0.5, 1,
2, 4, 8, and 16 µg/litre; white pine seedlings were also treated with
32 and 64 µg/litre. Both roots and foliage were affected by the
cadmium. In the red maple symptoms of cadmium toxicity began with
interveinal chlorosis and stunting of leaves in most cases. As
exposure increased cadmium caused wilting and then death. The first
observed effect of the metal on the pine was inhibition of needle
expansion and, in the case of the spruce, chlorotic tips to new
growth. Tissue accumulation of cadmium correlated well with exposure.
Red maple, white pine, and spruce exhibited foliage effects at leaf
cadmium concentrations of 22.8, 61.3, and 7.5 mg/kg, respectively,
corresponding to nutrient solutions of 8.0, 32.0, and 4.0 µg/litre.
There was an increasing effect on root development with increasing
exposure to cadmium. At the higher doses, there was severe reduction
in the number of roots initiated and stunting of those that grew.
Accumulation of cadmium was greater in roots than in leaves.
Root et al. (1975) grew maize ( Zea mays) in hydroponic
solutions containing cadmium chloride at concentrations ranging from
1 to 40 mg/litre. Uptake of cadmium into the plants increased with
time, and cadmium was present at higher concentrations in roots than
in shoots. Leaf chlorophyll concentration and yield (as dry weight) of
both roots and shoots decreased with increasing cadmium concentration.
As the cadmium concentration in the leaves increased, the
concentration of zinc decreased and the concentration of iron
increased. This gave a linear correlation between cadmium in the leaf
and iron/zinc ratio. Chlorosis resulting from cadmium in the leaves
(seen at a cadmium concentration of 1 mg/litre nutrient solution)
appeared comparable to iron-deficiency chlorosis. However, in this
case, the chlorosis was not due to iron deficiency, as previously
suggested by other workers, but was associated in some way with an
increasing iron/zinc ratio.
Harkov et al. (1979) found no effect on the yield of tomatoes
grown in vermiculite and cultured with a nutrient solution containing
cadmium at concentrations of 0.25 or 0.75 mg/litre. The plants were
more susceptible to damage by ozone, under conditions where ozone
damage would have been slight, after exposure to cadmium. Where ozone
damage was heavy or when conditions were not conducive to ozone
damage, there was no effect of cadmium. When Wong et al. (1988)
exposed pea ( Pisum sativum) seeds to cadmium concentrations of 1, 5,
10, and 20 mg/litre in culture solutions, germination was
significantly reduced at 20 mg/litre and radicle elongation to 1 cm
was significantly reduced at 5 mg/litre and to 2 cm at 1 mg/litre.
Early development (shoot elongation, leaf development, root and shoot
growth) was inhibited in a dose-dependant manner. Slight inhibition
was observed at 1 mg/litre, increased inhibition at 5 mg/litre, and
significant inhibition at 10 and 20 mg/litre.
7.1.2 Toxicity to plants grown in soil
Mitchell & Fretz (1977) showed that the effects on the growth of
red maple, white pine and Norway spruce plants in soil, amended with
cadmium, were similar but less severe, owing to reduced uptake of the
metal, than in the case of the same plants grown hydroponically.
Cadmium only affected current growth of the plants, except where it
was present in excess. Mahler et al. (1978) treated eight soils, the
pH values of which ranged from 4.8 to 7.8, with 1% (by weight) sewage
sludge containing added cadmium sulfate, leading to cadmium
concentrations in the soil ranging from 0.1 to 320 mg/kg. Two plants,
lettuce ( Lactuca sativa variety longifolia) and Swiss chard ( Beta
vulgaris variety cicla), were grown in the soils in pots. The EC50
(yield) for lettuce was 214 and 139 mg/kg soil for acid and calcareous
soils, respectively, whereas the values for chard were 175 and 250
mg/kg for acid and calareous soils. The corresponding tissue
concentrations of cadmium associated with these effects were 470 and
160 mg/kg for lettuce and 714 and 203 mg/kg for chard. Thus, a
markedly lower tissue concentration of cadmium produced 50% yield
reduction on calcareous soils than on acid soils. Alloway et al.
(1990) reported stunted growth and toxic signs on leaves of lettuce,
cabbage, carrot, and radish plants, but only at the highest
concentrations of cadmium tested (which resulted in a cadmium content
of around 20 mg/kg in the upper parts of the plants).
7.1.3 In vitro physiological studies
Bazzaz et al. (1974) demonstrated an effect of cadmium on
transpiration and photosynthesis in excised sunflower heads. The heads
(15 cm diameter) were placed in flasks containing distilled water or
cadmium salt solutions (2, 20, 100 or 200 mg/litre) and transpiration
and photosynthesis were measured at daily intervals over 4 to 5 days.
Cadmium reduced transpiration and photosynthesis at concentrations of
100 or 200 mg/litre. Excised epidermal peels floating on solutions of
cadmium salts showed a log-linear relationship between metal
concentration and stomatal opening. The stomata opened less with
increasing cadmium concentration; this accounted for the effect on
transpiration and, hence, on photosynthesis.
7.2 Toxicity to terrestrial invertebrates
Haight et al. (1982) calculated 24-h, 48-h, and 72-h LC50
values of 36, 15.1, and 5.85 mg cadmium/litre, respectively, for
juvenile free-living nematodes ( Panagrellus silusiae) and 111, 26.3,
and 13.2 mg/litre for adults. Williams & Dusenbery (1990) exposed the
free-living nematode Caenorhabditis elegans to cadmium and found
values of 904, 22, and 1.5 mg/litre, respectively, for the same three
exposure periods. They also calculated a 96-h LC50 of 0.06 mg/litre.
Popham & Webster (1979) found that a 6-h exposure to 3.26 x
10-7 moles of cadmium significantly decreased the fecundity of C.
elegans. A 3.5-day exposure to 10-8 moles caused the same effect.
Nematodes exposed to 4 x 10-6 moles of cadmium never grew to the
same length as controls and resembled worms from starved, overcrowded
cultures. Van Kessel et al. (1989) exposed juvenile C. elegans to
various concentrations of cadmium chloride and found that growth and
subsequent reproduction were significantly reduced at 1 µmol/litre. At
levels of 160 and 320 µmol/litre the nematodes did not reach the adult
stage and, therefore, did not reproduce.
Doelman et al. (1984) exposed the soil nematodes Mesorhabditus
monhystera and Aphelenchus avenae to cadmium via food for up to 22
days and monitored the size of the population. M. monhystera was
exposed, via bacteria and fungi, to concentrations of 0.23, 4.4, and
12.7 mg cadmium/kg, and a significant reduction in the size of the
population was found at all doses. A. avenae was exposed, via fungi
alone, to concentrations of 1, 10, and 25 mg/kg. At 1 mg/kg there was
no effect on the population size, whereas at 10 mg/kg, a reduction was
observed until the final day. A concentration of 25 mg/kg
significantly reduced the size of the population. The authors noted
that exposure via fungi alone gave far more variable results.
In studies by Van Straalen et al. (1989), the collembolan
Orchesella cincta and the oribatid mite Platynothrus peltifer were
exposed to cadmium in the food. The 9-week LC50 values were 1.6 and
7.27 µmol/g and the no-observed-effect levels (NOEL) were 0.042 and
0.026 µmol/g for O. cincta and P. peltifer, respectively. The most
sensitive parameters were female growth for O. cincta and
reproduction in P. peltifer.
Russell et al. (1981) fed subadult garden snails ( Helix aspersa)
on diets containing six different levels of cadmium ranging from 10 to
1000 mg/kg diet over a period of 30 days. There was little mortality
(two animals out of 350 died, one at an exposure level of 50 mg/kg and
the other at 1000 mg/kg) but food consumption declined with each
increase in cadmium dose. Food consumption was strongly depressed at
cadmium doses of 100 mg/kg or more. Relative weight loss was the most
pronounced effect of cadmium treatment; this was dose related and
directly attributable to reduced feeding rates. At doses of 25 mg/kg
or more, shell growth and reproductive activity were depressed while
the incidence of sealing response (the sealing of the operculum with
a disc of mucus and a dormancy reaction) increased markedly. These
three effects were all related to the dietary cadmium concentration.
7.3 Toxicity to birds
7.3.1 Acute and short-term toxicity
The acute and short-term toxicity of cadmium salts to birds in
laboratory studies is summarized in Table 13. Dosing for 5 days,
followed by 3 days of clean diet, resulted in LC50 values generally
in excess of 2000 mg/kg diet. Only the pheasant showed greater
sensitivity to cadmium but, even in this species, the LC50 was close
to 1000 mg/kg diet. All the birds used were between 10 and 14 days
old.
Table 13. Toxicity of cadmium to birds a
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Japanese quail 14 cadmium chloride 2440 (1807-3294) Hill &
(Coturnix coturnix 14 cadmium succinate 2052 (1621-2598) Camardese
japonica) (1986)
Pheasant 10 cadmium chloride 767 (651-898) Hill et al.
(Phasianus 14 cadmium succinate 1411 (1202-1657) (1975)
colchicus)
Bobwhite quail 14 cadmium succinate 1728 (1381-2132) Hill et al.
(Colinus (1975)
virginianus)
Table 13 (contd).
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Mallard duck 10 cadmium chloride > 5000 Hill et al.
(Anas 10 cadmium succinate > 5000 (1975)
platyrhynchos)
a Birds were fed with a dosed diet for 5 days and then a "clean" diet for 3 days.
In a study by Pritzl et al. (1974), 2-week-old leghorn chicken chicks
were dosed with dietary cadmium chloride for 20 days. In the first
experiment, chicks dosed with 700 mg cadmium/kg diet showed an
increase in the weight of the gastrointestinal tract, kidney, and
gizzard expressed as a ratio to the body weight. In a second
experiment, chicks were fed diets containing 400, 600, 800 or 1000 mg
cadmium/kg. Weight gain and food consumption were decreased, relative
to controls, at all dose levels, and at levels higher than 400 mg/kg
the birds lost weight. All the birds fed diets containing 800 or 1000
mg/kg died within 20 days. The LC50 was calculated to be 565 mg/kg
diet.
When Cain et al. (1983) fed 1-day-old mallard ducklings ( Anas
platyrhynchos) a diet containing cadmium chloride at concentrations
of 5, 10 or 20 mg cadmium/kg for 12 weeks, significant effects were
only noted at the highest dose. These included a significant reduction
in packed cell volume and haemoglobin concentrations and a significant
increase in serum glutamic-pyruvic transaminase. Mild to severe kidney
lesions were evident in ducklings fed 20 mg/kg for 12 weeks. Body
weight, liver weight, and femur weight-to-length ratio were unaffected
by the cadmium treatment. No other haematological or histological
effects were found.
7.3.2 Reproductive effects
Lofts & Murton (1967) injected 0.2 ml of a solution of cadmium
chloride (0.04 mol/litre) intramuscularly into wood pigeons ( Columba
palumbus). When the cadmium was given to birds with regressed
testes, which were then stimulated into reproductive condition by long
photoperiods (16 h of light per day), there was a reduction in the
numbers of birds showing full testicular development in the treated
group. Only one out of six birds given cadmium had developed
spermatozoa in the testis by the end of the experiment. The remainder
had not even produced spermatids; two birds had only spermatogonia in
the seminiferous epithelium while the other three had secondary
spermatocytes. Of the six control birds, four had spermatozoa, one had
spermatids, and one primary spermatocytes. Injection of cadmium into
birds late in the season had no effect on the autumnal regression of
the testes. There was no sign of testicular necrosis in the treated
birds. An intratesticular injection of cadmium caused local necrosis
in the testis of feral pigeons. A dietary concentration of 200 mg
cadmium/kg, in the form of cadmium chloride, reduced spermatogenesis
in male mallards and egg production in females, but a lower dose of 20
mg/kg produced no effects (White & Finley, 1978; White et al., 1978).
7.3.3 Physiological effects
Mayack et al. (1981) found that the survival and growth of the
wood duck ( Aix sponsa) were unaffected by a cadmium chloride dietary
level of 100 mg/kg, although some kidney damage was reported.
Nicholson et al. (1983) compared the ultrastructure of the
kidneys of sea-birds contaminated with cadmium in the wild, sea-birds
from uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg per kg, respectively.
Damage was greatest in the proximal tubule of the kidney, and included
cell necrosis, nuclear pyknosis, mitochondrial swelling, and some
tubulorrhexis. The tubulorrhexis would be irreversible. There was some
indication of regeneration, judging from the number of
undifferentiated cells present in the tubule, in both dosed and
naturally contaminated birds. Debris was found in the distal nephron
lumen and there was some damage in the distal tubule and the renal
corpuscles. Necrosis of kidney cells was very rare in control birds or
uncontaminated sea-birds.
7.3.4 Behavioural effects
Heinz et al. (1983) assessed the avoidance response to a visual
fright stimulus of ducklings fed a diet containing cadmium chloride at
a concentration of 4 or 40 mg/kg. The parents of the ducklings had
also been fed this cadmium-containing diet. Ducklings fed 4 mg/kg were
hypersensitive to the fright stimulus, whereas those fed the higher
dose reacted as did controls. The authors could offer no explanation
of why the higher dose had no effect but pointed to similar results
from other materials. They were of the opinion that hypersensitivity
to behavioural signals could be as deleterious to the organism in the
wild as a failure to respond.
7.4 Toxicity to wild small mammals
Shore et al. (1991) fed herbivorous bank voles ( Clethrionomys
glareolus) and granivorous wood mice ( Apodemus sylvaticus) a
pelleted diet contaminated with cadmium chloride and collected urine
and faeces in metabolism cages. Bank voles fed diets containing 10.3
mg/kg for 40 days and then 4.5 mg/kg for 35 days suffered significant
net daily loss of calcium and sodium, and reduced net gain of
potassium and magnesium compared to controls. Assimilation of the
macroelements was not significantly altered in wood mice fed 10.3
mg/kg for 75 days.
8. EFFECTS IN THE FIELD
Appraisal
Tolerance to cadmium has been demonstrated in soil fungi,
plants, aquatic invertebrates, and fish from cadmium-contaminated
sites. Some field evidence suggests that cadmium is responsible for
reduced leaf litter degradation and a failure to recycle nutrients
due to adverse effects on populations, particularly of
microorganisms. However, no studies have identified cadmium as the
sole cause of the effect, since it is always associated with other
metals. Although soil invertebrates in contaminated sites accumulate
cadmium and other metals, there is evidence that most populations are
not affected. A field study has shown that fish from a
cadmium-contaminated river have physiological abnormalities. Kidney
damage has been found in pelagic sea-birds from areas away from
industrial or other anthropogenic sources of cadmium, but there was
no effect on survival or reproduction of populations. In industrially
contaminated areas, kidney damage has been observed in several
species of birds found to contain cadmium plus other metals.
8.1 Tolerance
Tolerance to cadmium has been demonstrated in soil fungi (section
5.2), aquatic invertebrates (section 6.2.7), and plants collected from
sites with high cadmium levels, such as those in the vicinity of
metalliferous mines and smelters.
Coughtrey & Martin (1977) experimentally demonstrated tolerance
of the grass Holcus lanatus to cadmium to be greater in plants
collected from an area subject to high fall-out of cadmium than in
plants collected from a control site. Growth of tolerant plants was
reduced in uncontaminated culture solutions, relative to controls, but
was similar to that of control plants in solutions where cadmium salts
had been added at levels similar to the field exposure. Simon (1977)
reported cadmium tolerance in the grasses Festuca ovina and
Agrostis tennuis. The tolerant grasses were collected from areas
contaminated by mining and aerial fall-out of cadmium.
8.2 Effects close to industrial sources and highways
There have been several reports of effects of heavy metal
deposition on the accumulation rate of leaf litter in deciduous
woodlands. These effects are restricted to areas close to, or down
wind from, smelter sites. The separation of the effects of cadmium
from those of other heavy metals present in the litter is difficult.
A study by Coughtrey et al. (1979) attempted to do this by detailed
analysis of the litter itself and by the use of statistics to separate
effects of different components of the pollution fall-out. Seven areas
of woodland in the vicinity of, or up to 28 km away from, a
lead-zinc-cadmium smelter in Avonmouth, United Kingdom, were studied.
The leaf litter contained lead, zinc, copper, and cadmium (in that
order of concentrations), and metal levels were high in samples taken
from within 3 km of the smelter. Litter from a wood 6.8 km from the
smelter had similar levels to control litter collected 30 km away, but
the prevailing wind would not have carried much of the fall-out in the
direction of this wood. For the four woods within 3 km of the smelter,
cadmium levels ranged from 23 to 98 mg/kg litter (lead levels were
between 721 and 2179 mg/kg, zinc levels between 764 and 2814 mg/kg,
and copper levels between 47 and 135 mg/kg). The weight of litter
accumulated per unit area was markedly greater in the contami-nated
sites than in the uncontaminated ones; litter standing crop ranged
from 7.91 to 13.16 kg/m2 in contaminated and from 0.913 to 3.104
kg/m2 in uncontaminated sites. The litter accumulation correlated
well with levels of all metals but not with the pH of the litter,
which varied between 3.88 and 6.3 over the sites. Partial correlation
analysis showed that cadmium and zinc interrelated; with both cadmium
and zinc, partial correlation coefficients were highly significant
when lead, copper or pH effects were accounted for. However,
correlations were low for lead and copper when cadmium or zinc were
accounted for. Of further interest was an analysis of cadmium and zinc
in leaf litter from various sites. There was an increase in the
smaller particle sizes in contaminated sites relative to
uncontaminated ones. These smaller particles contained a
disproportionate amount of the metals present, particularly in the
case of cadmium. Litter standing crop and cadmium concentrations were
highly correlated; the correlation between cadmium content and
different particle sizes of litter was better for small particles
sizes, and the slope of the regression line between cadmium
concentration and litter weight decreased with increasing particle
size. The authors argued that litter degradation was not affected at
the early stages but only when breakdown had progressed to much
smaller particle sizes. This perhaps supports the view that
microorganisms are inhibited by metals to a greater extent than
invertebrates, which would produce the initial reduction in size of
litter fragments. Taking the figure of 900 g/m2 as the normal leaf
litter level for woodland, the extra accumulation in contaminated
woods represented 25 to 30 years of litter accumulation (the smelter
in question had been operating for 48 years) and, possibly a large
proportion of the total capital of nutrients normally recycled to the
plants.
Other authors have also reported accumulation of leaf litter in
areas contaminated by metals (Tyler, 1972; Strojan, 1978), although
they disagree about the probable cause. Strojan (1978) proposed that
the effect relates to the absence of some groups of invertebrates,
while Jordan & Lechavalier (1975) suggested that the effect is on
microorganisms. There is no direct evidence that invertebrates in leaf
litter are adversely affected by metals, although they do accumulate
all the metals found in litter (Martin et al., 1976; Coughtrey &
Martin, 1976). Both Tyler (1972) and Strojan (1978) argued that the
productivity of woodland may be adversely affected by the failure to
recycle nutrients in areas contaminated by heavy metals. Coughtrey et
al. (1979) considered that the litter is an important sink for heavy
metals, and that the result of litter organisms developing tolerance
to the metals and, therefore, in the long-term increasing the rate of
degradation is unpredictable since metals would be released at the
same time as nutrients.
In a study by van Straalen et al. (1987), the metal excretion
efficiency of the collembolan Orchesella cincta collected from
various contaminated forest soils was monitored. The authors found
that moderate to high soil cadmium contamination of industrial origin
did not evoke increased cadmium excretion. In fact contamination
initiated in this century from a zinc factory caused a significant
decrease in excretion efficiency. Soils that had been contaminated
with cadmium for many years (lead/zinc factory) or to an extreme
degree (lead smelter) were inhabited by Collembola able to increase
metal excretion.
Muskett & Jones (1980) found levels of cadmium to be higher than
normal within 10 m of a road with a heavy traffic, but no effect on
the numbers of invertebrates caught or their species diversity was
observed.
8.3 Effects on fish
Field studies in Sweden showed that perch ( Perca fluviatilis)
from a cadmium-contaminated river (0.1 to 0.2 µg cadmium/litre) had
physiological abnormalities similar to those shown in laboratory
experiments (Sjobeck et al., 1984).
8.4 Effects on sea-birds
The reported effects on the kidney of sea-birds are not always a
result of exposure to cadmium as an industrial pollutant, since the
individuals most affected come from areas where there is no industrial
effluent. This is often, therefore, a response to naturally occurring
cadmium presumed to derive from the oceans. The birds appear to cope
with this damage to the kidney and suffer no effects on survival or
breeding success. No damage resulting from exposure to strictly
anthropogenically derived cadmium appears to have been reported on the
same scale as that from exposure to naturally occurring cadmium.
Nicholson et al. (1983) compared the ultrastructure of the kidneys of
sea-birds contaminated with cadmium in the wild, sea-birds from
uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg/kg, respectively (see
section 7.3.3). Nicholson & Osborn (1983) reported kidney lesions
(described in section 7.3.3) in several different species of sea-bird
caught in contaminated areas, although other pollutant metals such as
mercury were also present in the tissues.
9. EVALUATION
9.1 General considerations
In evaluating the environmental hazard of cadmium, it is
necessary to extrapolate from laboratories studies to ecosystems. This
must be done with extreme caution for a number of reasons.
a) The availability of cadmium to organisms in the environment is
limited by its strong adsorption to environmental components such
as soil, sediment, and organic matter. Organisms in contaminated
areas accumulate high body burdens of cadmium.
b) Environmental variables such as temperature, pH, and the chemical
composition of water or soil have been shown to affect both the
uptake and the toxic impact of cadmium.
c) Available, rather than nominal or total, cadmium is the
determinant in assessing uptake by, and effects on, organisms.
d) There are limited data from controlled experimental studies on
the effects of mixtures of metals. Organisms in the environment
are exposed to mixtures of pollutants. Acid deposition can
release metals, including cadmium, into the environment.
e) Little experimental work has been carried out on species or
communities that are either representative or key components of
natural communities and ecosystems. Studies have not considered
all of the interactions between populations and all of the
environmental factors affecting these populations. As a result,
the impact of cadmium on ecosystems may have been underestimated.
f) Results from laboratory studies based on very sensitive
parameters may be indicative of physiological impacts on
individuals rather than impacts on ecosystems.
9.2 The aquatic environment
Cadmium input to the aquatic environment is through dis-charge of
industrial waste, surface run-off, and deposition. It is strongly
adsorbed onto sediments and soils. The average cadmium content of sea
water is about 0.1 µg/litre or less, while fresh waters contain <
0.01 to 0.06 µg/litre in unpolluted areas. Cadmium levels of up to 5
mg/kg and 0.03 to 1 mg/kg have been reported for freshwater sediments
and marine sediments, respectively.
The rate of uptake and the toxic impact of cadmium on aquatic
organisms is greatly affected by physicochemical factors such as
temperature, ionic concentration, and organic matter content.
Cadmium is translocated by aquatic plants and concentrated in
roots and leaves. It is also taken up and accumulated by various
aquatic animals. The toxicity of cadmium to freshwater organisms
varies considerably depending on the exposure duration, species, and
life-stage. The early life-stages and the reproductive system are the
most vulnerable. Cadmium is, by comparison, one of the most toxic
heavy metals in the freshwater environment. Manifest responses of
certain organisms to cadmium are observed at environmental
concentrations lower than 1 µg/litre.
Cadmium-induced kidney damage has been reported in sea-birds
sampled from the field. However, this damage is present in both
cadmium-polluted areas and areas remote from industrial contamination.
The effect is probably, therefore, due to natural cadmium in certain
species and areas.
9.3 The terrestrial environment
Cadmium is introduced into the terrestrial environment from
mining, non-ferrous metal production, landfill sites and from the
application of sewage sludge, phosphate fertilizers, and manure.
Background concentrations of cadmium are in the range of 0.1 to 0.4
mg/kg soil and can reach 4.5 mg/kg in volcanic soils. Levels up to 160
mg/kg soil have been found close to metal processing sources.
Reduced breakdown of leaf litter and recycling of nutrients has
been attributed to metal pollution in the field. Cadmium appears to be
the most potent metal at inhibiting litter degradation. The effect is
thought to be due largely to reduced populations of microorganisms,
which are responsible for the final stages of litter decomposition.
Plants take up cadmium and can translocate and accumulate it.
However, uptake from soil is limited. Where there is high-level
exposure to cadmium (in the range of hundreds of mg/kg), growth
reduction is the major effect. Plants exposed to cadmium in the field
for long periods can develop tolerance to the metal. There is no
evidence of adverse effects of cadmium on plant populations in the
field.
Terrestrial invertebrates vary considerably in their sensitivity
to cadmium. Some species can take up and store cadmium to levels of up
to 5000 mg/kg body weight without apparent ill effects, while others
show population effects at levels of a few mg/kg soil. Populations of
some terrestrial invertebrates could be adversely affected at levels
of cadmium contamination seen in the field. Isopods and earthworms are
useful biomonitors for cadmium contamination. Invertebrates with high
body burdens may pose a threat to predators.
Kidney damage was found in experimental birds fed 20 mg
cadmium/kg diet for 12 weeks, but not at lower doses. Reproductive
effects have been observed at 200 mg/kg diet. A dose of 4 mg/kg
affected the behaviour of ducklings. No effects of cadmium have been
seen in terrestrial birds sampled from the field, although the cadmium
level in the brain, kidney, and liver of pigeons has proved to be a
good indicator of urban cadmium contamination.
Small mammals accumulate cadmium in the vicinity of mining spoil.
The ionic balance was affected in voles exposed experimentally to a
concentration of 10 mg/kg diet.
Populations of terrestrial organisms may also develop tolerance
to cadmium after long-term exposure.
10. RECOMMENDATIONS FOR PROTECTING THE ENVIRONMENT
To eliminate environmental effects, emissions of cadmium from the
following sources should be reduced as far as is practicable:
* smelters
* incinerators
* sewage sludge applied to the land
* phosphate fertilisers
* cadmium-containing manure
11. FURTHER RESEARCH
a) More study is needed to clarify the effects of cadmium on the
decomposition process of plant debris. Effects on the degree of
nutrient cycling and long-term plant growth and the exact nature
of the inhibition of decomposition require further attention.
b) The adsorption of cadmium to soil and sediment requires further
study and quantification of coefficients. Modelling of binding
and distribution in the environment is needed.
c) Organisms that are particularly sensitive (i.e. indicator
species) or that play a critical role in ecological systems
should be identified and studied with regard to the effects of
cadmium.
d) Studies are needed on the basic mechanisms by which cadmium
interacts with physiological and biochemical processes in
organisms and within individual cells.
There is a need to take certain precautions in studies on
cadmium. Firstly, the speciation of the metal should be considered in
experimental design and procedures and a clear measure of the
available cadmium should be reported. Secondly, studies of the uptake
and movement between trophic levels should include the relationship
between the non-nutrient cadmium and the nutrients calcium and zinc.
REFERENCES
ABEL, P.D. & PAPOUTSOGLOU, S.E. (1986) Lethal toxicity of cadmium to
Cyprinus carpio and Tilapia aurea. Bull. environ. Contam.
Toxicol., 37: 382-386.
ADRIANO, D.C. (1986) Trace elements in the terrestrial environment,
Berlin, Heidelberg, New York, Springer-Verlag, 533 pp.
AHSANULLAH, M. (1976) Acute toxicity of cadmium and zinc to seven
invertebrate species from Western Port, Victoria. Aust. J. mar.
freshwater Res., 27: 187-196.
ALLOWAY, B.J., JACKSON, A.P., & MORGAN, H. (1990) The accumulation of
cadmium by vegetables grown on soils contaminated from a variety of
sources. Sci. total Environ., 91: 223-236.
ANADU, D.I., CHAPMAN, G.A., CURTIS, L.R., & TUBB, R.A. (1989) Effect
of zinc exposure on subsequent acute tolerance to heavy metals in
rainbow trout. Bull. environ. Contam. Toxicol., 43: 329-336.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDREWS, S.M., JOHNSON, M.S., & COOKE, J.A. (1984) Cadmium in small
mammals from grassland established on metalliferous mine waste.
Environ. Pollut., 33A: 153-162.
ARILLO, A., CALAMARI, D., MARGIOCCO, C., MELODIA, F., & MENSI, P.
(1984) Biochemical effects of long-term exposure to cadmium and copper
on rainbow trout ( Salmo gairdneri): validation of water quality
criteria. Ecotoxicol. environ. Saf., 8: 106-117.
AYLETT, B.J. (1979) The chemistry and bioinorganic chemistry of
cadmium. In: Webb, M., ed. The chemistry, biochemistry and biology of
cadmium, Amsterdam, Elsevier/North Holland, pp. 1-43.
BABICH, H. & STOTZKY, G. (1977a) Reductions in the toxicity of cadmium
to microorganisms by clay minerals. Appl. environ. Microbiol., 33:
696-705.
BABICH, H. & STOTZKY, G. (1977b) Effect of cadmium on fungi and on
interactions between fungi and bacteria in soil: influence of clay
minerals and pH. Appl. environ. Microbiol., 33: 1059-1066.
BAHNER, L.H. & NIMMO, D.R. (1975) Methods to assess effects of
combinations of toxicants, salinity and temperature on estuarine
animals. In: Hemphill, D.D., ed. Trace substances in environmental
health - IX, Columbia, University of Missouri, pp. 169-177.
BAUDOUIN, M.F. & SCOPPA, P. (1974) Acute toxicity of various metals to
freshwater zooplankton. Bull. environ. Contam. Toxicol., 12: 745-751.
BAZZAZ, F.A., CARLSON, R.W., & ROLFE, G.L. (1974) The effect of heavy
metals on plants: part 1. Inhibition of gas exchange in sunflower by
Pb, Cd, Ni and Tl. Environ. Pollut., 7A: 241-246.
BEHNE, D. (1980) Problems of sampling and sample preparation for trace
element analysis in the health sciences. In: Bratter, P. & Scramel,
P., ed. Trace elements analytical chemistry in medicine and biology.
Proceedings of the 1st International Workshop, Neuherberg, Germany,
Berlin, Walter de Gruyter, pp. 769-782.
BENGTSSON, B-E. & BERGSTROM, B. (1987) A flowthrough fecundity test
with Nitocra spinipes (Harpacticoidea Crustacea) for aquatic
toxicity. Ecotoxicol. environ. Saf., 14: 260-268.
BENGTSSON, B-E., CARLIN, C.H., LARSSON, A., & SVANBERG, O. (1975)
Vertebral damage in minnows, Phoxinus phoxinus L., exposed to
cadmium. Ambio, 4: 166-168.
BENNETT, W.N. (1990) Measurement of manganese amelioration of cadmium
toxicity in Chlorella pyrenoidosa using turbidostat culture. Arch.
environ. Contam. Toxicol., 19: 118-123.
BENOIT, D.A., LEONARD, E.N., CHRISTENSEN, G.M., & FIANDT, J.T. (1976)
Toxic effects of cadmium on three generations of brook trout
( Salvelinus fontinalis). Trans. Am. Fish. Soc., 105: 550-560.
BERGER, B. & DALLINGER, R. (1989) Accumulation of cadmium and copper
by the terrestrial snail Arianta arbustorum L.: kinetics and
budgets. Oecologia, 79: 60-65.
BERGLIND, R. (1986) Combined and separate effects of cadmium, lead and
zinc on ALA-D activity, growth and hemoglobin content in Daphnia
magna. Environ. Toxicol. Chem., 5: 989-995.
BERK, S.G., GUNDERSON, J.H., & DERK, L.A. (1985) Effects of cadmium
and copper on chemotaxis of marine and freshwater ciliates. Bull.
environ. Contam. Toxicol., 34: 897-903.
BERTRAM, P.E. & HART, B.A. (1979) Longevity and reproduction of
Daphnia pulex (de Geer) exposed to cadmium-contaminated food or
water. Environ. Pollut., 19A: 295-305.
BESTEN, P.J. DEN, HERWIG, H.J., ZANDEE, D.I., & VOOGT, P.A. (1989)
Effects of cadmium and PCBs on reproduction of the sea star Asterias
rubens: Aberrations in the early development. Ecotoxicol. environ.
Saf., 18: 173-180.
BEWLEY, R.J.F. & STOTZKY, G. (1983) Effects of cadmium and zinc on
microbial activity in soil; influence of clay minerals. Part 1: Metals
added individually. Sci. total Environ., 31: 41-55.
BEYER, W.N., CHANEY, R.L., & MULHERN, B.M. (1982) Heavy metal
concentrations in earthworms from soil amended with sewage sludge. J.
environ. Qual., 11: 381-385.
BEYER, W.N., PATTEE, O.H., SILEO, L., HOFFMAN, D.J., & MULHERN, B.M.
(1985) Metal contamination in wildlife living near two zinc smelters.
Environ. Pollut., 38A: 63-86.
BIESINGER, K.E. & CHRISTENSEN, G.M. (1972) Effects of various metals
on survival, growth, reproduction, and metabolism of Daphnia magna.
J. Fish Res. Board Can., 29: 1691-1700.
BINGHAM, F.T., PAGE, A.L., & STRONG, J.E. (1980) Yield and cadmium
content of rice grain in relation to addition rate of cadmium, copper,
nickel and zinc, with sewage sludge and liming. Soil Sci., 130: 32-38.
BLACK, J.A. & BIRGE, W.J. (1989) An avoidance response bioassay for
aquatic pollutants, Lexington, Kentucky, University of Kentucky, Water
Resources Research Institute (Research Report No. 123).
BLOCK, M. (1991) Uptake of cadmium in fish. Effects of xanthates and
diethyldithiocarbamate, University of Uppsala, Sweden, Faculty of
Sciences (Universitatis Uppsaliensis - Comprehensive summaries of
Uppsala dissertations from the Faculty of Science. No. 326).
BLOCK, M. & PART, P. (1986) Increased availability of cadmium to
perfused rainbow trout ( Salmo gairdneri Rich.) gills in the presence
of the complexing agents diethyl dithiocarbamate, ethyl xanthate and
isopropyl xanthate. Aquat. Toxicol., 8: 295-302.
BOND, H., LIGHTHART, B., SHIMABUKU, R., & RUSSELL, L. (1976) Some
effects of cadmium on coniferous forest soil and litter microcosms.
Soil Sci., 121: 278-287.
BORGMANN, U. (1983) Metal speciation and toxicity of free metal ions
to aquatic biota. In: Nriagu, J.O., ed. Aquatic toxicology, New York,
Chichester, John Wiley & Sons, pp. 47-72.
BORGMANN, U. & RALPH, K.M. (1986) Effects of cadmium,
2,4-dichlorophenol, and pentachlorophenol on feeding, growth, and
particle-size-conversion efficiency of white sucker larvae and young
common shiners. Arch. environ. Contam. Toxicol., 15: 473-480.
BORGMANN, U., MILLARD, E.S., & CHARLTON, C.C. (1989a) Effect of
cadmium on a stable, large volume, laboratory ecosystem containing
Daphnia and phytoplankton. Can. J. Fish. aquat. Sci., 46: 399-405.
BORGMANN, U., RALPH, K.M., & NORWOOD, W.P. (1989b) Toxicity test
procedures for Hyalella azteca, and chronic toxicity of cadmium and
pentachlorophenol to H. azteca, Gammarus fasciatus, and Daphnia
magna. Arch. environ. Contam. Toxicol., 18: 756-764.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BRESSAN, M. & BRUNETTI, R. (1988) The effects of nitriloacetic acid,
Cd and Hg on the marine algae Dunaliella tertiolecta and Isochrysis
galbana. Water Res., 22: 553-556.
BROOKS, R.R. & RUMSBY, M.G. (1967) Studies on the uptake of cadmium by
the oyster, Ostrea sinuata (Lamarck). Aust. J. mar. freshwater Res.,
15: 53-61.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BULL, K.R., MURTON, R.K., OSBORN, D., WARD, P., & CHENG, L. (1977)
High levels of cadmium in Atlantic seabirds and sea-skaters. Nature
(Lond.), 269: 507-509.
BURKITT, A., LESTER, P., & NICKLESS, G. (1972) Distribution of heavy
metals in the vicinity of an industrial complex. Nature (Lond.), 238:
327-328.
BYRNE, A.R., RAVNIK, V., & KOSTA, L. (1976) Trace element
concentrations in higher fungi. Sci. total Environ., 6: 65-78.
CAIN, J.R., PASCHAL, D.C., & HAYDEN, C.M. (1980) Toxicity and
bioaccumulation of cadmium in the colonial green alga Scenedesmus
obliquus. Arch. environ. Contam. Toxicol., 9: 9-16.
CAIN, B.W., SILEO, L., FRANSON, J.C., & MOORE, J. (1983) Effects of
dietary cadmium on mallard ducklings. Environ. Res., 32: 286-297.
CALABRESE, A., COLLIER, R.S., NELSON, D.A. & MACINNES, J.R. (1973) The
toxicity of heavy metals to embryos of the American oyster
Crassostrea virginica. Mar. Biol., 18: 162-166.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1980) Influence of water
hardness on cadmium toxicity to Salmo gairdneri Rich. Water Res.,
14: 1421-1426.
CANTON, J.H. & SLOOFF, W. (1982) Toxicity and accumulation studies of
cadmium (Cd2+) with freshwater organisms of different trophic
levels. Ecotoxicol. environ. Saf., 6: 113-128.
CARRIER, R. & BEITINGER, T.L. (1988a) Reduction in thermal tolerance
of Notropis lutrensis and Pimephales promelas exposed to cadmium.
Water Res., 22: 511-515.
CARRIER, R. & BEITINGER, T.L. (1988b) Resistance of temperature
tolerance ability of green sunfish to cadmium exposure. Bull. environ.
Contam. Toxicol., 40: 475-480.
CARROLL, J.J., ELLIS, S.J., & OLIVER, W.S. (1979) Influences of
hardness constituents on the acute toxicity of cadmium to brook trout
( Salvelinus fontinalis). Bull. environ. Contam. Toxicol., 22:
575-581.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living near
a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CATALDO, D.A. & WILDUNG, R.E. (1978) Soil and plant factors
influencing the accumulation of heavy metals by plants. Environ.
Health Perspect., 27: 149-159.
CEARLEY, J.E. & COLEMAN, R.L. (1974) Cadmium toxicity and
bioconcentration in largemouth bass and bluegill. Bull. environ.
Contam. Toxicol., 11: 146-151.
CHAN, K.-Y. & DEAN, A.C.R (1988) Effects of cadmium and lead on
growth, respiration and enzyme activity of the marine bacterium
Pseudomonas marina. Chemosphere, 17: 597-607.
CHANDINI, T. (1988) Changes in food [ Chlorella] levels and the acute
toxicity of cadmium to Daphnia carinata (Daphnidae) and Echinisca
triserialis (Macrothricidae) [Crustacea: Cladocera]. Bull. environ.
Contam. Toxicol., 41: 398-403.
CHANDINI, T. (1989) Survival, growth and reproduction of Daphnia
carinata (Crustacea: Cladocera) exposed to chronic cadmium stress at
different food ( Chlorella) levels. Environ. Pollut., 60: 29-45.
CHANEY, R.L., WHITE, M.C., & SIMON, P.W. (1975) Plant uptake of heavy
metals from sewage sludge applied to land. In: Proceedings of the 2nd
National Conference on Municipal Sludge Management, Rockville,
Information Transfer Inc., pp. 169-178.
CHAPMAN, G. & DUNLOP, S. (1981) Detoxification of zinc and cadmium by
the freshwater protozoan Tetrahymena pyriformis. I. The effect of
water hardness. Environ. Res., 26: 81-86.
CHIN, B. & SINA, J.F. (1978) Cellular bases of cadmium toxicity in
Physarum. In: Hemphill, D.D., ed. Trace substances in environmental
health, Columbia, University of Missouri, pp. 214-219.
CHRISTENSEN, J.J., EATOUGH, D.J., & IZATT, R.M. (1975) In: Handbook of
metal ligand heats and related thermodynamic quantities, 2nd ed.
revis. expand., New York, Basel, Marcel Dekker, Inc., 495 pp.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975a) Synergism between
dissolved oxygen and cadmium toxicity in five species of aquatic
insects. Environ. Res., 9: 285-289.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975b) Acute cadmium
toxicity studies upon nine species of aquatic insects. Environ. Res.,
9: 331-341.
CONRAD, G.W. (1988) Heavy metal effects on cellular shape changes,
cleavage, and larval development of the marine gastropod mollusk,
( Ilyanassa obsoleta Say). Bull. environ. Contam. Toxicol., 41:
79-85.
CONWAY, H.L. (1978) Sorption of arsenic and cadmium and their effects
on growth, micronutrient utilization, and photosynthetic pigment
composition of Asterionella formosa. J. Fish Res. Board. Can., 35:
286-294.
COOMBS, T.L. (1979) Cadmium in aquatic organisms. In: Webb, M., ed.
The chemistry, biochemistry and biology of cadmium, Amsterdam,
Elsevier/North Holland, pp. 93-139.
COUGHTREY, P.J. & MARTIN, M.H. (1976) The distribution of Pb, Zn, Cd
and Cu within the pulmonate mollusc Helix aspersa Muller. Oecologia,
23: 315-322.
COUGHTREY, P.J. & MARTIN, M.H. (1977) Cadmium tolerance of Holcus
lanatus from a site contaminated by aerial fallout. New Phytol., 79:
273-280.
COUGHTREY, P.J., JONES, C.H., MARTIN, M.H., & SHALES, S.W. (1979)
Litter accumulation in woodlands contaminated by Pb, Zn, Cd and Cu.
Oecologia, 39: 51-60.
DALLA VIA, G.J., DALLINGER, R., & CARPENE, E. (1989) Effects of
cadmium on Murex trunculus from the Adriatic Sea. II. Oxygen
consumption and acclimation effects. Arch. environ. Contam. Toxicol.,
18: 562-567.
DALLINGER, R. & WIESER, W. (1984) Patterns of accumulation,
distribution and liberation of Zn, Cu, Cd and Pb in different organs
of the land snail Helix pomatia L. Comp. Biochem. Physiol., 79C:
117-124.
DALLINGER, R., PROSI, F., SEGNER, H., & BACK, H. (1987) Contaminated
food and uptake of heavy metals by fish: a review and proposal for
further research. Oecologia, 73: 91-98.
DAWSON, M.A., GOULD, E., THURBERG, F.P., & CALABRESE, A. (1977)
Physiological response of juvenile striped bass, Morone saxatilis,
to low levels of cadmium and mercury. Chesapeake Sci., 18: 353-359.
DENTON, G.R.W. & BURDON-JONES, C. (1981) Influence of temperature and
salinity on the uptake, distribution and depuration of mercury,
cadmium and lead by the black-lip oyster Saccostrea echinata. Mar.
Biol., 64: 317-326.
DE VRIES, M.P.C. & TILLER, K.G. (1978) Sewage sludge as a soil
amendment with special reference to Cd, Cu, Mn, Ni, Pb and Zn -
comparison of results from experiments conducted inside and outside a
glasshouse. Environ. Pollut., 16A: 231-240.
DE ZWART, D. & SLOOF, W. (1987) Toxicity of mixtures of heavy metals
and petrochemicals to Xenopus laevis. Bull. environ. Contam.
Toxicol., 38: 345-351.
DICKSON, G.W., GIESY, J.P., & BRIESE, L.A. (1982) The effect of
chronic cadmium exposure on phosphodenylate concentrations and
adenylate energy of gills and dorsal muscle of crayfish. Environ.
Toxicol. Chem., 1: 147-156.
DINNEL, P.A., LINK, J.M., STOBER, Q.J., LETOURNEAU, M.W., & ROBERTS,
W.E. (1989) Comparative sensitivity of sea urchin sperm bioassays to
metals and pesticides. Arch. environ. Contam. Toxicol., 18: 748-755.
DOELMAN, P., NIEBOER, G., SCHROOTEN, J., & VISSER, M. (1984)
Antagonistic and synergistic toxic effects of Pb and Cd in a simple
foodchain: Nematodes feeding on bacteria or fungi. Bull. environ.
Contam. Toxicol., 32: 717-723.
DONGMANN, G. & NURNBERG, H.W. (1982) Observations with Thalassiosira
rotula (Meunier) on the toxicity and accumulation of cadmium and
nickel. Ecotoxicol. environ. Saf., 6: 535-544.
DOUBEN, P.E.T. (1989a) Uptake and elimination of waterborne cadmium by
the fish Noemacheilus barbatulus L. (stone loach). Arch. environ.
Contam. Toxicol., 18: 576-586.
DOUBEN, P.E.T. (1989b) Metabolic rate and uptake and loss of cadmium
from food by the fish Noemacheilus barbatulus L. (stone loach).
Environ. Pollut., 59: 177-202.
DRESCHER, H.E., HARMS, U., & HUSCHENBETH. E. (1977) Organochlorines
and heavy metals in the harbour seal Phoca vitulina from the German
North Sea coast. Mar. Biol., 41: 99-106.
EATON, J.G., MCKIM, J.M., & HOLCOMBE, G.W. (1978) Metal toxicity to
embryos and larvae of seven freshwater fish species - I. Cadmium.
Bull. environ. Contam. Toxicol., 19: 95-103.
EISLER, R. (1971) Cadmium poisoning in Fundulus heteroclitus
(Pisces: Cyprinodontidae) and other marine organism. J. Fish Res.
Board Can., 28: 1225-1234.
EISLER, R. (1985) Cadmium hazards to fish, wildlife and invertebrates:
A synoptic review, Washington, DC, US Department of the Interior, Fish
and Wildlife Service (Biological Report No. 85).
EISLER, R. & HENNEKEY, R.J. (1977) Acute toxicities of Cd2+, Cr+6,
Hg2+, Ni2+ and Zn2+ to estuarine macrofauna. Arch. environ.
Contam. Toxicol., 6: 315-323.
EISLER, R., ZAROOGIAN, G.E., & HENNEKEY, R.J. (1972) Cadmium uptake by
marine organisms. J. Fish Res. Board. Can., 29: 1367-1369.
ELLIOT, N.G., SWAIN, R., & RITZ, D.A. (1985) The influence of cyclic
exposure on the accumulation of heavy metals by Mytilus edulis
planulatus (Lamarck). Mar. environ. Res., 15: 17-30.
ERIKSSON, J.E. (1988) The effects of clay, organic matter and time on
adsorption and plant uptake of cadmium added to the soil. Water Air
Soil Pollut., 40: 359-373.
FALCONER, C.R., DAVIES, I.M., & TOPPING, G. (1983) Trace metals in the
common porpoise, Phocoena phocoena. Mar. environ. Res., 8: 119-127.
FERARD, J.F., JOUANY, J.M., TRUHAUT, R., & VASSEUR, P. (1983)
Accumulation of cadmium in a freshwater food chain experimental model.
Ecotoxicol. environ. Saf., 7: 43-52.
FINLAYSON, B.J. & VERRUE, K.M. (1982) Toxicities of copper, zinc, and
cadmium mixtures to juvenile chinook salmon. Trans. Am. Fish. Soc.,
111: 645-650.
FLATAU, G.N., CLEMENT, R.L., GAUTHIER, M.J., & LAUMOND, F.M. (1987)
Adaptation to cadmium in a sensitive marine pseudomonad. Environ.
Technol. Lett, 8: 641-646.
FLATAU, G.N., CLEMENT, R.L., & GAUTHIER, M.J. (1988) Uptake of cadmium
by marine bacteria. Prog. Oceanogr., 21: 181-188.
FLEISCHER, M., SAROFIM, A.F., FASSETT, D.W., HAMMOND, P., SHACKLETTE,
H.T., NISBET, I.C.T., & EPSTEIN, S. (1974) Environmental impact of
cadmium: a review by the Panel on Hazardous Trace Substances. Environ.
Health Perspect., 5: 253-323.
FLIK, G., VAN DE WINKEL, J.G.J., PART, P., WENDELAAR BONGA, S.E., &
LOCK, R.A.C. (1987) Calmodulin mediated cadmium inhibition of
phosphodiesterase activity. Arch. Toxicol., 59: 352-359.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FRANCIS, P.C., BIRGE, W.J., & BLACK, J.A. (1984) Effects of
cadmium-enriched sediment on fish and amphibian embryo-larval stages.
Ecotoxicol. environ. Saf., 8: 378-387.
FRANK, P.M. & ROBERTSON, P.B. (1979) The influence of salinity on
toxicity of cadmium and chromium to the blue crab, Callinectes
sapidus. Bull. environ. Contam. Toxicol., 21: 74-78.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
fourteenth session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WHO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the Mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization (Reports
and Studies No. 31).
GIATTINA, J.D. & GARTON, R.R. (1983) A review of the
preference-avoidance responses of fishes to aquatic contaminants.
Residue Rev., 87: 43-90.
GIESY, J.P., LEVERSEE, G.J., & WILLIAMS, D.R. (1977) Effects of
naturally occurring aquatic organic fractions on cadmium toxicity to
Simocephalus serrulatus (Daphnidae) and Gambusia affinis
(Poeciliidae). Water Res., 11: 1013-1020.
GILL, T.S. & PANT, J.C. (1983) Cadmium toxicity: inducement of changes
in blood and tissue metabolites in fish. Toxicol. Lett., 18: 195-200.
GISH, C.D. & CHRISTENSEN, R.E. (1973) Cadmium, nickel, lead and zinc
in earthworms from roadside soil. Environ. Sci. Technol., 7:
1060-1062.
GOTTOFREY, J. (1990) The disposition of cadmium, nickel, mercury and
methylmercury in fish and effects of lipophilic metal chelation,
Uppsala, Swedish University of Agricultural Sciences, Department of
Pharmacology and Toxicology (Thesis).
GOTTOFREY, J., BJORKLUND, I., & TJALVE, H. (1988) Effects of sodium
isoproylxanthate, potassium amylxanthate and sodium
diethyldithiocarbamate on the uptake and disposition of cadmium in the
brown trout ( Salmo trutta). Aquat. Toxicol., 12: 171-184.
GUY, R.D. & ROSS KEAN, A. (1980) Algae as a chemical speciation
monitor. I. A comparison of algal growth and computer calculated
speciation. Water Res., 14: 891-899.
HAIGHT, M., MUDRY, T., & PASTERNAK, J. (1982) Toxicity of seven heavy
metals on Panagrellus silusiae: The efficacy of the free-living
nematode as an in vivo toxicological bioassay. Nematologica, 28: 1-11.
HALE, J.G. (1977) Toxicity of metal mining wastes. Bull. environ.
Contam. Toxicol., 17: 66-73.
HALL, W.S., PAULSON, R.L., HALL, L.W., & BURTON, D.T. (1986) Acute
toxicity of cadmium and sodium pentachlorophenate to Daphnids and
fish. Bull. environ. Contam. Toxicol., 37: 308-316.
HAMANAKA, T., ITOO, T., & MISHIMA, S. (1982) Age related change and
distribution of cadmium and zinc concentrations in the stellar sea
lion ( Eumetopias jubata) from the coast of Hokkaido, Japan. Mar.
Pollut. Bull., 13: 57-61.
HAMILTON, S.J. & MEHRLE, P.M. (1986) Metallothionein in fish: review
of its importance in assessing stress from metal contaminants. Trans.
Am. Fish. Soc., 115: 596-609.
HARDISTY, M.W., HUGGINS, R.J., KARTAR, S., & SAINSBURY, M. (1974a)
Ecological implications of heavy metal in fish from the Severn
Estuary. Mar. Pollut. Bull., 5: 12-15.
HARDISTY, M.W., KARTAR, S., & SAINSBURY, M. (1974b) Dietary habits and
heavy metal concentrations in fish from the Severn Estuary and Bristol
Channel. Mar. Pollut. Bull., 5: 61-63.
HARDY, J.T., SCHMIDT, R.L., & APTS, C.W. (1981) Marine sediment and
interstitial water: effects on bioavailability of cadmium to gills of
the clam Protothaca staminea. Bull. environ. Contam. Toxicol., 27:
798-805.
HARDY, J.T., SULLIVAN, M.F., CRECELIUS, E.A., & APTS, C.W. (1984)
Transfer of cadmium in phytoplankton-oyster-mouse food chain. Arch.
environ. Contam. Toxicol., 13: 419-425.
HARKOV, R., CLARKE, B., & BRENNAN, E. (1979) Cadmium contamination may
modify response of tomato to atmospheric ozone. J. Air Pollut. Control
Assoc., 29: 1247-1249.
HARRISON, S.E. & KLAVERKAMP, J.F. (1989) Uptake, elimination and
tissue distribution of dietary and aqueous cadmium by rainbow trout
( Salmo gairdneri Richardson) and lake whitefish ( Coregonus
clupeaformis Mitchill). Environ. Toxicol. Chem., 8: 87-97.
HART, B.A. & SCAIFE, B.D. (1977) Toxicity and bioaccumulation of
cadmium in Chlorella pyrenoidosa. Environ. Res., 14: 401-413.
HARTENSTEIN, R., NEUHAUSER, E.F., & COLLIER, J. (1980) Accumulation of
heavy metals in the earthworm Eisenia foetida. J. environ. Qual., 9:
23-26.
HARTMAN, L.M. (1974) A preliminary report: Fungal flora of the soil as
conditioned by varying concentrations of heavy metals. Am. J. Bot.,
61: 23 (Abstract).
HARTWELL, S.I., JIN, J.H., CHERRY, D.S., & CAIRNS, J. (1989) Toxicity
versus avoidance response of golden shiner, Notemigonus crysoleucas,
to five metals. J. Fish Biol., 35: 447-456.
HATAKEYAMA, S. (1987) Chronic effects of Cd on reproduction of
Polypedilum nubifer (Chironomidae) through water and food. Environ.
Pollut., 48: 249-261.
HATAKEYAMA, S. & YASUNO, M. (1987) Chronic effects of Cd on the
reproduction of the guppy ( Poecilia reticulata) through
Cd-accumulated midge larvae ( Chironomus yoshimatsui). Ecotoxicol.
environ. Saf., 14: 191-207.
HEINZ, G.H., HASELTINE, S.D., & SILEO, L. (1983) Altered avoidance
behavior of black ducks fed cadmium. Environ. Toxicol. Chem., 2:
419-421.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
US Department of Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Department of Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
HIRAOKA, Y. & OKUDA, H. (1984) Time course study of occurrence of
anomalies in medaka's centrum by cadmium or fenitrothion emulsion.
Bull. environ. Contam. Toxicol., 32: 693-697.
HIRATA, M. (1981) Annaka: Land polluted mainly by fumes and dust from
a zinc smelter. In: Kitagishi, K. & Gamare, I., ed. Heavy metal
pollution in the soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HOGSTRAND, C. & HAUX, C. (1990a) Metallothionein as an indicator of
heavy metal exposure in two subtropical fish species. J. exp. mar.
Biol. Ecol., 138: 69-84.
HOGSTRAND, C. & HAUX, C. (1990b) A radioimmunoassay for perch ( Perca
fluviatilis) metallothionein. Toxicol. appl. Pharmacol., 103: 56-65.
HOLCOMBE, G.W., PHIPPS, G.L., & MARIER, J.W. (1984) Methods for
conducting snail ( Aplexa hypnorum) embryo through adult exposures.
Effects of cadmium and reduced pH levels. Arch. environ. Contam.
Toxicol., 13: 627-634.
HONDA, K. & TATSUKAWA, R. (1983) Distribution of cadmium and zinc in
tissues and organs, and their age-related changes in striped dolphins,
Stenella coeruleoalba. Arch. environ. Contam. Toxicol., 12: 543-550.
HONDA, K., FUJISE, Y., TATSUKAWA, R., ITANO, K., & MIYAZAKI, N. (1986)
Age-related accumulation of heavy metals in bone of the striped
dolphin, Stenella coeruleoalba. Mar. environ. Res., 20: 143-160.
HOPKIN, S.P. (1989) Ecophysiology of metals in terrestrial
invertebrates, London, Elsevier Applied Science.
HOPKIN, S.P. (1990) Species-specific differences in the net
assimilation of zinc, cadmium, lead, copper and iron by the
terrestrial isopods Oniscus asellus and Porcellio scaber. J. appl.
Ecol., 27: 460-474.
HOPKIN, S.P. & MARTIN, M.H. (1984) Assimilation of zinc, cadmium, lead
and copper by the centipede Lithobius variegatus (Chilopoda). J.
appl. Ecol., 21: 535-546.
HOPKIN, S.P. & MARTIN, M.H. (1985) Transfer of heavy metals from leaf
litter to terrestrial invertebrates. J. Sci. Food Agric., 36: 538-539.
HOPKIN, S.P., WATSON, K., MARTIN, M.H., & MOULD, M.L. (1985) The
assimilation of heavy metals by Lithobius variegatus and Glomeris
marginata (Chilopoda: Diplopoda). Bijdr. Dierkd., 55: 88-94.
HUIGUANG FU (1989) Hormonal responses of fish to cadmium, Nijmegen,
The Netherlands, University of Nijmegen (Ph.D. Thesis).
HUNTER, B.A. & JOHNSON, M.S. (1982) Food chain relationships of copper
and cadmium in contaminated grassland ecosystems. Oikos, 38: 108-117.
HUTCHINSON, T.C. & CZYRSKA, H. (1975) Heavy metal toxicity and
synergism to floating aquatic weeds. Verh. Int. Ver. Limnol., 19 :
2102-2111.
HUTTON, M. (1981) Accumulation of heavy metals and selenium in three
seabird species from the United Kingdom. Environ. Pollut., 26A:
129-145.
HUTTON, M. (1982) Cadmium in the European Community: a prospective
assessment of sources, human exposure and environmental impact,
London, Monitoring and Assessment Research Centre, Chelsea College,
University of London, 99 pp (MARC Report No. 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London, 46
pp (MARC Report No. 29).
HUTTON, M. & GOODMAN, G.T. (1980) Metal contamination of feral pigeons
Columba livia from the London area: Part I - tissue accumulation of
lead, cadmium and zinc. Environ. Pollut., 22A: 207-217.
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead, mercury
and arsenic entering the U.K. environment from human activities. Sci.
total Environ., 57: 129-150.
IRELAND, M.P. (1981) Uptake and distribution of cadmium in the
terrestrial slug Arion ater (L.) Comp. Biochem. Physiol., 68A:
37-41.
JANA, S. & BHATTACHARYA, D.N. (1988) Effect of heavy metals on growth
population of a fecal coliform bacterium Escherichia coli in aquatic
environment. Water Air Soil Pollut., 38: 251-254.
JANSSEN, M.P.M., BRUINS, A., DE VRIES, T.H., & VAN STRAALEN, N.M.
(1991) Comparison of cadmium kinetics in four soil arthropod species.
Arch. environ. Contam. Toxicol., 20: 305-312.
JOHN, J., GJESSING, E.T., GRANDE, M., & SALBU, B. (1987) Influence of
aquatic humus and pH on the uptake and depuration of cadmium by the
Atlantic salmon ( Salmo salar). Sci. total Environ., 62: 253-265.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination through
residual trace metal dispersal from derelict lead-zinc mine. J.
environ. Qual., 9(2): 175-179.
JOHNSON, M.W. & GENTILE, J.H. (1979) Acute toxicity of cadmium, copper
and mercury to larval American lobster Homarus americanus. Bull.
environ. Contam. Toxicol., 22: 258-264.
JONES, K.C. & JOHNSTON, A.E. (1989) Cadmium in cereal grain and
herbage from long-term experimental plots at Rothamsted, UK. Environ.
Pollut., 57: 199-216.
JORDAN, M.J. & LECHAVALIER, M.P. (1975) Effects of zinc-smelter
emissions on forest soil microflora. Can. J. Microbiol., 21:
1855-1865.
KAY, S.H. & HALLER, W.T. (1986) Heavy metal bioaccumulation and
effects on waterhyacinth weevils, Neochetina eichhorniae, feeding on
waterhyacinth, Eichhornia crassipes. Bull. environ. Contam.
Toxicol., 37: 239-245.
KEMP, P.F. & SWARTZ, R.C. (1988) Acute toxicity of interstitial and
particle-bound cadmium to a marine infaunal amphipod. Mar. environ.
Res., 26: 135-153.
KHAN, A.T., WEIS, J.S., & D'ANDREA, L. (1988) Studies of cadmium
tolerance in two populations of grass shrimp, Palaemonetes pugio.
Bull. environ. Contam. Toxicol., 40: 30-34.
KHANGAROT, B.S. & RAY, P.K. (1987) Sensitivity of toad tadpoles, Bufo
melanostictus (Schneider), to heavy metals. Bull. environ. Contam.
Toxicol., 38: 523-527.
KORTE, F. (1983) Ecotoxicology of cadmium: general overview.
Ecotoxicol. environ. Saf., 7: 3-8.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ., 22(2):
375-381.
KUHN, R., PATTARD, M., PERNAK, K-D., & WINTER, A. (1989) Results of
the harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res., 23: 501-510.
LAAMANEN, A. (1972) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAAMANEN, A. (1976) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAEGREID, M., ALSTAD, J., KLAVENESS, D., & SEIP, H.M. (1983) Seasonal
variation of cadmium toxicity toward the alga Selenastrum
capricornutum Printz in two lakes with different humus content.
Environ. Sci. Technol., 17: 357-361.
LAFEBER, F.P.J.G., FLIK, G., WENDELAAR BONGA, S.E., & PERRY, S.F.
(1988) Hypocalcin from Stannius corpuscles inhibits gill calcium
uptake in trout. Am. J. Physiol., 254: R891-R896.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling, sample
handling, analysis, Washington, DC, US Department of Commerce,
National Bureau of Standards, Vols. 1 & 2, 1304 pp.
LALANDE, M. & PINEL-ALLOUL, B. (1986) Acute toxicity of cadmium,
copper, mercury and zinc to Tropocyclops prasinus mexicanus
(Cyclopoida, Copepoda) from three Quebec lakes. Environ. Toxicol.
Chem., 5: 95-102.
LANGSTON, W.J. & ZHOU, M. (1987a) Cadmium accumulation, distribution
and metabolism in the gastropod Littorina littorea: the role of
metal-binding proteins. J. Mar. Biol. Assoc. UK, 67: 585-601.
LANGSTON, W.J. & ZHOU, M. (1987b) Cadmium accumulation, distribution
and elimination in the bivalve Macoma balthica: neither
metallothionein nor metallothionein-like proteins are involved. Mar.
environ. Res., 21: 225-237.
LEE, H.H. & XU, C.H. (1984) Differential response of marine organisms
to certain metal and agrichemical pollutants. Bull. environ. Contam.
Toxicol., 33: 460-467.
LEPP, N.W., HARRISON, S.C.S., & MORRELL, B.G. (1987) A role for
Amanita muscaria L. in the circulation of cadmium and vanadium in a
non-polluted woodland. Environ. Geochem. Health, 9: 61-64.
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Process of
atmospheric deposition of metals and acids to forests. Annual Meeting
of the Air Pollution Control Association, New Orleans, 20 June 1982,
Pittsburgh, Pennsylvania, Air Pollution Control Association, 14 pp
(Conference Proceedings 75).
LOFTS, B. & MURTON, R.K. (1967) The effects of cadmium on the avian
testis. J. Reprod. Fert., 13: 155-164.
LOVETT, R.J., GUTENMANN, W.H., PAKKALA, I.S., YOUNGS, W.D., LISK,
D.J., BURDICK, G.E., & HARRIS, E.J. (1972) A survey of the total
cadmium content of 406 fish from 49 New York State fresh waters. J.
Fish Res. Board Can., 29: 1283-1290.
LUSSIER, S.M., GENTILE, J.H., & WALKER, J. (1985) Acute and chronic
effects of heavy metals and cyanide on Mysidopsis bahia (Crustacea:
Mysidacea). Aquat. Toxicol., 7: 25-35.
MA, W., EDELMAN, Th., VAN BEERSUM, I., & JANS, Th. (1983) Uptake of
cadmium, zinc, lead and copper by earthworms near a zinc-smelting
complex: influence of soil pH and organic matter. Bull. environ.
Contam. Toxicol., 30: 424-427.
MCCAHON, C.P., BROWN, A.F., & PASCOE, D. (1988) The effect of the
acanthocephalan Pomphorhynchus laevis (Muller 1776) on the acute
toxicity of cadmium to its intermediate host, the amphipod Gammarus
pulex (L.). Arch. environ. Contam. Toxicol., 17: 239-243.
MCCAHON, C.P., WHILES, A.J., & PASCOE, D. (1989) The toxicity of
cadmium to different larval instars of the trichopteran larvae
Agapetus fuscipes Curtis and the importance of life cycle
information to the design of toxicity tests. Hydrobiologia, 185:
153-162.
MCCLURG, T.P. (1984) Effects of fluoride, cadmium and mercury on the
estuarine prawn Penaeus indicus. Water S. Afr., 10: 40-45.
MACINNES, J.R. & THURBERG, F.P. (1973) Effects of metals on the
behaviour and oxygen consumption of the mud snail. Mar. Pollut. Bull.,
4: 185-186.
MACINNES, J.R., THURBERG, F.P., GREIG, R.A., & GOULD, E. (1977)
Long-term cadmium stress in the cunner, Tautogolabrus adspersus.
Fish. Bull., 75: 199-203.
MAHLER, R.J., BINGHAM, F.T., & PAGE, A.L. (1978) Cadmium-enriched
sewage sludge application to acid and calcareous soils. Effect on
yield and cadmium uptake by lettuce and chard. J. environ. Qual., 7:
274-281.
MARSHALL, J.S. (1979) Cadmium toxicity to laboratory and field
populations of Daphnia galeata mendotae. Bull. environ. Contam.
Toxicol., 21: 453-457.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, M.H., COUGHTREY, P.J., & YOUNG, E.W. (1976) Observations on
the availability of lead, zinc, cadmium and copper in woodland litter
and the uptake of lead, zinc and cadmium by the woodlouse Oniscus
asellus. Chemosphere, 5: 313-318.
MARTIN, M.H., COUGHTREY, P.J., SHALES, S.W., & LITTLE, P. (1980)
Aspect of airborne cadmium contamination of soils and natural
vegetation. In: Inorganic pollution and agriculture, Her Majesty's
Stationary Office, London, pp. 56-69.
MAY, T.W. & MCKINNEY, G.L. (1981) Cadmium, lead, mercury, arsenic, and
selenium concentrations in freshwater fish, 1976-77 - National
pesticide monitoring program. Pestic. monit. J., 15: 14-38.
MAYACK, L.A., BUSH, P.B., FLETCHER, O.J., PAGE, R.K., & FENDLEY, T.T.
(1981) Tissue residues of dietary cadmium in wood ducks. Arch.
environ. Contam. Toxicol., 10: 637-645.
MAYER, F.L (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MEISCH, H.-U. & SCHMITT, J.A. (1986) Characterization studies on
cadmium-mycophosphatin from the mushroom Agaricus macrosporus.
Environ. Health Perspect., 65: 29-32.
MERLINI, M. (1978) Hepatic storage alteration of vitamin B12 by
cadmium in a freshwater fish. Bull. environ. Contam. Toxicol., 19:
767-771.
METEYER, M.J., WRIGHT, D.A., & MARTIN, F.D. (1988) Effect of cadmium
on early developmental stages of the sheepshead minnow ( Cyprinodon
variegatus). Environ. Toxicol. Chem., 7: 321-328.
MICHIBATA, H. (1981) Effect of water hardness on the toxicity of
cadmium to the egg of the teleost Oryzias latipes. Bull. environ.
Contam. Toxicol., 27: 187-192.
MIDDAUGH, D.P. & DEAN, J.M. (1977) Comparative sensitivity of eggs,
larvae, and adults of the estuarine teleosts, Fundulus heteroclitus
and Menidia menidia to cadmium. Bull. environ. Contam. Toxicol., 17:
645-652.
MIRENDA, R.J. (1986) Toxicity and accumulation of cadmium in the
crayfish, Orconectes virilis (Hagen). Arch. environ. Contam.
Toxicol., 15: 401-407.
MITCHELL, C.D. & FRETZ, T.A. (1977) Cadmium and zinc toxicity in white
pine, red maple and Norway spruce. J. Am. Soc. Hortic. Sci., 102:
81-84.
MITRA, R.S., GRAY, R.H., CHIN, B., & BERNSTEIN, I.A. (1975) Molecular
mechanisms of accommodation in Escherichia coli to toxic levels of
Cd2+. J. Bacteriol., 121: 1180-1188.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & LENTZOU, P. (1979)
Effects of sublethal concentrations of cadmium as possible indicators
of cadmium pollution for two populations of Acartia clausi
(Copepoda) living at two differently polluted areas. Bull. environ.
Contam. Toxicol., 23: 642-649.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & ROGDAKIS, I. (1982)
Evaluation of the stress exerted by a polluted environment to a marine
organism by comparative toxicity tests. Bull. environ. Contam.
Toxicol., 28: 416-423.
MORGAN, J.E. & MORGAN, A.J. (1988) Earthworms as biological monitors
of cadmium, copper, lead and zinc in metalliferous soils. Environ.
Pollut., 54: 123-138.
MORGAN, J.E. & MORGAN, A.J. (1990) The distribution of cadmium,
copper, lead, zinc and calcium in the tissues of the earthworm
Lumbricus rubellus sampled from one uncontaminated and four polluted
soils. Oecologia, 84: 559-566.
MUINO, C.V., FERRARI, L., & SALIBIAN, A. (1990) Protective action of
ions against cadmium toxicity to young Bufo arenarum tadpoles. Bull.
environ. Contam. Toxicol., 45: 313-319.
MUIR, D.C.G., WAGEMANN, R., GRIFT, N.P., NORSTROM, R.J., SIMON, M. &
LIEN, J. (1988) Organochlorine chemical and heavy metal contaminants
in white-beaked dolphins ( Lagenorhynchus albirostris) and pilot
whales ( Globicephala melaena) from the coast of Newfoundland,
Canada. Arch. environ. Contam. Toxicol., 17: 613-629.
MURAMOTO, S. (1981a) Influence of complexans (EDTA, DTPA) on the
toxicity of cadmium to fish at chronic levels. Bull. environ. Contam.
Toxicol., 26: 641-646.
MURAMOTO, S. (1981b) Variations of some elements in cadmium-induced
malformed fish. Bull. environ. Contam. Toxicol., 27: 193-200.
MUSKETT, C.J. & JONES, M.P. (1980) The dispersal of lead, cadmium and
nickel from motor vehicles and effects on roadside invertebrate
macrofauna. Environ. Pollut., 23A: 231-242.
MYSING-GUBALA, M. & POIRRIER, M.A. (1981) The effects of cadmium and
mercury on gemmule formation and gemmosclere morphology in Ephydatia
fluviatilis (Porifera: Spongillidae). Hydrobiologia, 76: 145-148.
NAIDU, C.K. & REDDY, T.K.R. (1988) Effect of cadmium on microorganisms
and microbe-mediated mineralization process in the soil. Bull.
environ. Contam. Toxicol., 41: 657-663.
NAKADA, M., FUKAYA, K., TAKESHITA, S., & WADA, Y. (1979) The
accumulation of heavy metals in the submerged plant ( Elodea
nuttallii). Bull. environ. Contam. Toxicol., 22: 21-27.
NEBEKER, A.V., CAIRNS, M.A., ONJUKKA, S.T., & TITUS, R.H. (1986)
Effect of age on sensitivity of Daphnia magna to cadmium, copper and
cyanazine. Environ. Toxicol. Chem., 5: 527-530.
NEGILSKI, D.S. (1976) Acute toxicity of zinc, cadmium and chromium to
the marine fishes, yellow-eye mullet ( Aldrichetta forsteri C & V)
and small-mouthed hardyhead ( Atherinasoma microstoma Whitley). Aust.
J. mar. freshwater Res., 27: 137-149.
NEGILSKI, D.S., AHSANULLAH, M., & MOBLEY, M.C. (1981) Toxicity of
zinc, cadmium and copper to the shrimp Callianassa australiensis. II
Effects of paired and triad combinations of metals. Mar. Biol., 64:
305-309.
NELSON, D.A., CALABRESE, A., NELSON, B.A., MACINNES, J.R., & WENZLOFF,
D.R. (1976) Biological effects of heavy metals on juvenile bay
scallops, Argopecten irradians, in short-term exposures. Bull.
environ. Contam. Toxicol., 16: 275-282.
NICHOLSON, J.K. & OSBORN, D. (1983) Kidney lesions in pelagic seabirds
with high tissue levels of cadmium and mercury. J. Zool. (Lond.), 200:
99-118.
NICHOLSON, J.K., KENDALL, M.D., & OSBORN, D. (1983) Cadmium and
mercury nephrotoxicity. Nature (Lond.), 304: 633-635.
NIEDERLEHNER, B.R., BUIKEMA, A.L., PITTINGER, C.A., & CAIRNS, J.
(1984) Effects of cadmium on the population growth of a benthic
invertebrate Aeolosoma headleyi (Oligochaeta). Environ. Toxicol.
Chem., 3: 255-262.
NIMMO, D.R., LIGHTNER, D.V., & BAHNER, L.H. (1977) Effects of cadmium
on the shrimps Penaeus duorarum, Palaemonetes pugio and
Palaemonetes vulgaris. In: Vernberg, F.J., Calabrese, A., Thurberg,
F.P., & Vernberg, W.B., ed. Physiological responses of marine biota to
pollutants, New York, London, Academic Press, pp. 131-183.
NIMMO, D.R., RIGBY, R.A., BAHNER, L.H., & SHEPPARD, J.M. (1978) The
acute and chronic effects of cadmium on the estuarine mysid,
Mysidopsis bahia. Bull. environ. Contam. Toxicol., 19: 80-85.
NIR, R., GASITH, A., & PERRY, A.S. (1990) Cadmium uptake and toxicity
to water hyacinth: effect of repeated exposures under controlled
conditions. Bull. environ. Contam. Toxicol., 44: 149-157.
NORBERG, A.B. & MOLIN, N. (1983) Toxicity of cadmium, cobalt, uranium
and zinc to Zoogloea ramigera. Water Res., 17: 1333-1336.
NRIAGU, J.O. (1989) A global assessment of natural sources of
atmospheric trace metals. Nature (Lond.), 338: 47-49.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
O'HARA, J. (1973) The influence of temperature and salinity on the
toxicity of cadmium to the fiddler crab, Uca pugilator. Fish. Bull.,
71: 149-153.
OLLA, B.L., ESTELLE, V.B., SWARTZ, R.C., BRAUN, G., & STUDHOLME, A.L.
(1988) Responses of polychaetes to cadmium-contaminated sediment:
Comparison of uptake and behavior. Environ. Toxicol. Chem., 7:
587-592.
OLSON, P.-E. & HAUX, C. (1986) Increased hepatic metallothionein
content correlates to cadmium accumulation in environmentally exposed
perch ( Perca fluviatilis). Aquat. Toxicol., 9: 231-242.
OSBORN, D. & NICHOLSON, J.K. (1984) Cadmium and mercury in seabirds,
Huntingdon, UK Natural Environment Research Council, Institute of
Terrestrial Ecology, pp. 30-34 (ITE Symposium No. 12).
PAGE, A.L. & CHANG, A.C. (1978) Trace elements impact on plants during
cropland disposal of sewage sludges. In: Proceedings of the 5th
National Conference on Acceptable Sludge Disposal Techniques,
Rockville, Maryland, Information Transfer Inc., pp. 91-96.
PAGE, A.L., BINGHAM, F.T., & CHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, London, Applied
Science, Vol. 1, pp. 77-109.
PART, P. & WIKMARK, G. (1984) The influence of certain complexing
agents (EDTA and citrate) on the uptake of cadmium in perfused rainbow
trout gills. Aquat. Toxicol., 5: 277-289.
PART, P., SVANBERG, O., & KIESSLING, A. (1985) The availability of
cadmium to perfused rainbow trout gills in different water qualities.
Water Res., 19: 427-434.
PASCOE, D., EVANS, S.A., & WOODWORTH, J. (1986) Heavy metal toxicity
to fish and the influence of water hardness. Arch. environ. Contam.
Toxicol., 15: 481-487.
PECON, J. & POWELL, E.N. (1981) Effects of the amino acid histidine on
the uptake of cadmium from the digestive system by the blue crab
Callinectes sapidus. Bull. environ. Contam. Toxicol., 27: 34-41.
PENTREATH, R.J. (1977) The accumulation of cadmium by the plaice
Pleuronectes platessa L. and the thornback ray Raia clavata. J.
exp. mar. Biol. Ecol., 30: 223-232.
PEREZ-COLL, C.S., HERKOVITS, J., & SALIBIAN, A. (1986) Teratogenic
effects of cadmium on Bufo arenarum during gastrulation. Experientia
(Basel), 42: 1174-1176.
PESCH, G.G. & STEWART, N.E. (1980) Cadmium toxicity to three species
of estuarine invertebrates. Mar. environ. Res., 3: 145-156.
PETERSON, P.J. & ALLOWAY, B.J. (1979) Cadmium in soils and vegetation.
In: Webb, M., ed. The chemistry, biochemistry and biology of cadmium,
Amsterdam, Elsevier/North Holland, pp. 45-92.
PICKERING, Q.H. & GAST, M.H. (1972) Acute and chronic toxicity of
cadmium to the fathead minnow Pimephales promelas. J. Fish Res.
Board. Can., 29: 1099-1106.
PICKERING, Q.H. & HENDERSON, C. (1966) The acute toxicity of some
heavy metals to different species of warmwater fishes. Int. J. Air
water Pollut., 10: 453-463.
PIETZ, R.I., PETERSON, J.R., PRATER, J.E., & ZENZ, D.R. (1984) Metal
concentrations in earthworms from sewage sludge-amended soils at a
strip mine reclamation site. J. environ. Qual., 13: 651-654.
PINOWSKA, B., KRASNICKI, K., & PINOWSKI, J. (1981) Estimation of the
degree of contamination of granivorous birds with heavy metals in
agricultural and industrial landscape. Ekol. Polska, 29: 137-149.
POLDOSKI, J.E. (1979) Cadmium bioaccumulation assays. Their
relationships to various ion equilibria in Lake Superior water.
Environ. Sci. Technol., 13: 701-706.
POPHAM, J.D. & WEBSTER, J.M. (1979) Cadmium toxicity in the
free-living nematode, Caenorhabditis elegans. Environ. Res., 20:
183-191.
PRAHALAD, A.K. & SEENAYYA, G. (1988) Bioavailability of zinc and
cadmium and their effect on microbial growth and metal uptake. Bull.
environ. Contam. Toxicol., 41: 921-927.
PRITZL, M.C., LIE, Y.H., KIENHOLZ, E.W., & WHITEMAN, C.E. (1974) The
effect of dietary cadmium on development of young chickens. Poult.
Sci., 53: 2026-2029.
RAINBOW, P.S. & WHITE, S.L. (1989) Comparative strategies of heavy
metal accumulation by crustaceans: zinc, copper and cadmium in a
decapod, an amphipod and a barnacle. Hydrobiologia, 174: 245-262.
RAMAMOORTHY, S. & BLUMHAGEN, K. (1984) Uptake of Zn, Cd and Hg by fish
in the presence of competing compartments. Can. J. Fish aquat. Sci.,
41: 750-756.
RAMIREZ, P., BARRERA, G., & ROSAS, C. (1989) Effects of chromium and
cadmium upon respiration and survival of Callinectes similis. Bull.
environ. Contam. Toxicol., 43: 850-857.
RAY, S., MCLEESE, D.W., WAIWOOD, B.A., & PEZZACK. D. (1980a) The
disposition of cadmium and zinc in Pandalus montagui. Arch. environ.
Contam. Toxicol., 9: 675-681.
RAY, S., MCLEESE, D., & PEZZACK, D. (1980b) Accumulation of cadmium by
Nereis virens. Arch. environ. Contam. Toxicol., 9: 1-8.
REBHUN, S. & BEN-AMOTZ, A. (1984) The distribution of cadmium between
the marine alga Chlorella stigmatophora and sea water medium. Water
Res., 18: 173-178.
REHWOLDT, R. & KARIMIAN-TEHERANI, D. (1976) Uptake and effect of
cadmium on zebrafish. Bull. environ. Contam. Toxicol., 15: 442-446.
REID, S.D. & MCDONALD, D.G. (1988) Effects of cadmium, copper and low
pH on ion fluxes in rainbow trout, Salmo gairdneri. Can. J. Fish
aquat. Sci., 45: 244-253.
RIISGARD, H.U., BJORNESTAD, E., & MOHLENBERG, F. (1987) Accumulation
of cadmium in the mussel Mytilus edulis: Kinetics and importance of
uptake via food and sea water. Mar. Biol., 96: 349-353.
ROBERT, R. & HIS, E. (1985) Combined effects of salinity and cadmium
chloride upon embryos and larvae of the Japanese oyster, Crassostrea
gigas. Mar. environ. Res., 15: 303-312.
ROBERTS, M.H., WARINNER, J.E., CHU-FA TSAI, WRIGHT, D., & CRONIN, L.E.
(1982) Comparison of estuarine species sensitivities to three
toxicants. Arch. environ. Contam. Toxicol., 11: 681-692.
ROBERTS, R.D. & JOHNSON, M.S. (1978) Dispersal of heavy metals from
abandoned mine workings and their transference through terrestrial
food chains. Environ. Pollut., 16: 293-310.
ROBERTS, T.M., HEPPLESTON, P.B., & ROBERTS, R.D. (1976) Distribution
of heavy metals in tissues of the common seal. Mar. Pollut. Bull., 7:
194-196.
ROBINSON, A.M., LAMBERSON, J.O., COLE, F.A., & SWARTZ, R.C. (1988)
Effects of culture conditions on the sensitivity of a phoxocephalid
amphipod, Rhepoxynius abronius, to cadmium in sediment. Environ.
Toxicol. Chem., 7: 953-959.
ROBINSON, K.M. & WELLS, M.R. (1975) Retention of a single oral dose of
cadmium in tissues of the softshell turtle, Trionyx spinifer. Bull.
environ. Contam. Toxicol., 14: 750-752.
ROCH, M. & MALY, E.J. (1979) Relationship of cadmium-induced
hypocalcemia with mortality in rainbow trout ( Salmo gairdneri) and
the influence of temperature on toxicity. J. Fish Res. Board Can., 36:
1297-1303.
ROMBOUGH, P.J. & GARSIDE, E.T. (1982) Cadmium toxicity and
accumulation in aggs and alevins of Atlantic salmon Salmo salar. Can.
J. Zool., 60: 2006-2014.
ROOT, R.A., MILLER, R.J., & KOEPPE, D.E. (1975) Uptake of cadmium -
its toxicity, and effect on the iron ratio in hydroponically grown
corn. J. environ. Qual., 4: 473-476.
ROSENBERG, R. & COSTLOW, J.D. (1976) Synergistic effects of cadmium
and salinity combined with constant and cycling temperatures on the
larval development of two estuarine crab species. Mar. Biol., 38:
219-303.
RUSSELL, L.K., DEHAVEN, J.I., & BOTTS, R.P. (1981) Toxic effects of
cadmium on the garden snail ( Helix aspersa). Bull. environ. Contam.
Toxicol., 26: 634-640.
SANGALANG, G.B. & FREEMAN, H.C. (1979) Tissue uptake of cadmium in
brook trout during chronic sublethal exposure. Arch. environ. Contam.
Toxicol., 8: 77-84.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SASTRY, K.V. & SUBHADRA, K.M. (1983) Cadmium induced alterations in
the intestinal absorption of glucose and fructose in a freshwater
catfish Heteropneustes fossilis. Water Air Soil Pollut., 20:
293-297.
SATO, C., SCHNOOR, J.L., & MCDONALD, D.B. (1986) Characterization of
effects of copper, cadmium and nickel on the growth of Nitrosomonas
europaea. Environ. Toxicol. Chem., 5: 403-416.
SAWICKA-KAPUSTA, K., GORECKI, A., SWIERGOSZ, R., JUSZCZAK, G.,
MIELCZAREK, M., & WOJCIK, B. (1987) Effect of metabolic rate on the
rate of elimination of high and low concentrations of cadmium and lead
in the bank vole. Ekol. Polska, 35: 399-430.
SCHEUHAMMER, A.M. (1987) The chronic toxicity of aluminium, cadmium,
mercury and lead in birds: a review. Environ. Pollut., 46: 263-295.
SCHUYTEMA, G.S., NELSON, P.O., MALUEG, K.W., NEBEKER, A.V., KRAWCZYK,
D.F., RATCLIFF, A.K., & GAKSTATTER, J.H. (1984) Toxicity of cadmium in
water and sediment slurries to Daphnia magna. Environ. Toxicol.
Chem., 3: 293-308.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta, 51:
3273-3277.
SHIVARAJ, K.M. & PATIL, H.S. (1988) Toxicity of cadmium and copper to
a freshwater fish Puntius arulius. Environ. Ecol., 6: 5-8.
SHORE, R.F., MYHILL, D.G., POLWORTH, G., & DUNMOW, M. (1991) The
effects of chronic doses of dietary cadmium on macro-element balance
in wood mice and bank voles. In: Momciloric, B., ed. Proceedings of
the Seventh International Symposium on Trace Elements in Man and
Animals (TEMA-7), Dubrovnik, Yugoslavia, 20-25 May 1990, Zagreb, IMI
(Institute for Medical Research and Occupational Health, University of
Zagreb), pp. 17-18.
SILLEN, L.G. & MARTELL, A.E. (1964) Stability constants of metal-ion
complexes. Section I: Inorganic ligands compiled by L.G. Sillen.
Section II: Organic ligands compiled by A.E. Martell, London, The
Chemical Society, 754 pp (Special Publication No. 17).
SIMON, E. (1977) Cadmium tolerance in populations of Agrostis tenuis
and Festuca ovina. Nature (Lond.), 265: 328-330.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SJOBECK, M.-L., HAUX, C., LARSSON, A., & LITHNER, G. (1984)
Biochemical and hematological studies on perch, Perca fluviatilis,
from the cadmium-contaminated river Eman. Ecotoxicol. environ. Saf.,
8: 303-312.
SKOWRONSKI, T., PAWLIK, B., & JAKUBOWSKI, M. (1988) Reduction of
cadmium toxicity to green microalga Stichococcus bacillaris by
manganese. Bull. environ. Contam. Toxicol., 41: 915-920.
SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the usefulness of the
mexican axolotl ( Ambystoma mexicanum) and the clawed toad ( Xenopus
laevis) in toxicological bioassays. Bull. environ. Contam. Toxicol.,
24: 439-443.
SMITH, B.P., HEJTMANCIK, E., & CAMP, B.J. (1976) Acute effects of
cadmium on Ictalurus punctatus (catfish). Bull. environ. Contam.
Toxicol., 15: 271-277.
SMITH, R.M. & MARTELL, A.E. (1974) In: Critical stability constants,
Vol. 1: Amino acids, New York, Plenum Press, 469 pp.
SMITH, R.M. & MARTELL, A.E. (1975) In: Critical stability constants,
Vol. 2: Amines, New York, Plenum Press, 415 pp.
SMITH, R.M. & MARTELL, A.E. (1976) In: Critical stability constants,
Vol. 3: Other organic ligands, New York, Plenum Press, 495 pp.
SPEHAR, R.L., ANDERSON, R.L., & FIANDT, J.T. (1978a) Toxicity and
bioaccumulation of cadmium and lead in aquatic invertebrates. Environ.
Pollut., 15A: 195-208.
SPEHAR, R.L., LEONARD, E.N., & DEFOE, D.L. (1978b) Chronic effects of
cadmium and zinc mixtures on flagfish ( Jordanella floridae). Trans.
Am. Fish. Soc., 107: 354-360.
SPRAGUE, J. (1985) Factors that modify toxicity. In: Rand, G.M. &
Petrocelli, S.R., ed. Fundamentals of aquatic toxicology, New York,
Hemisphere Publishing Corporation, pp. 124-163.
SPRY, D.J. & WOOD, C.M. (1989) A kinetic method for the measurement of
zinc influx in vivo in the rainbow trout, and the effects of
waterborne calcium on flux rates. J. exp. Biol., 142: 425-446.
STROJAN, C.L. (1978) Forest leaf litter decomposition in the vicinity
of a zinc smelter. Oecologia, 32: 203-212.
SULLIVAN, J.F., MURPHY, B.R., ATCHISON, G.J., & MCINTOSH, A.W. (1978a)
Time dependant cadmium uptake by fathead minnows ( Pimephales
promelas) during field and laboratory exposure. Hydrobiologia, 57:
65-68.
SULLIVAN, J.F., ATCHISON, G.J., KOLAR, D.J., & MCINTOSH, A.W. (1978b)
Changes in the predator-prey behavior of fathead minnows ( Pimephales
promelas) and largemouth bass ( Micropterus salmoides) caused by
cadmium. J. Fish Res. Board. Can., 35: 446-451.
SUNDA, W.G., ENGEL, D.W., & THUOTTE, R.M. (1978) Effect of chemical
speciation on toxicity of cadmium to grass shrimp, Palaemonetes
pugio: Importance of free cadmium ion. Environ. Sci. Technol., 12:
409-413.
TAYLOR, D. (1983) The significance of the accumulation of cadmium by
aquatic organisms. Ecotoxicol. environ. Saf., 7: 33-42.
TERAOKA, H. (1989) Impact of atmospheric trace metals on rice roots.
Arch. environ. Contam. Toxicol., 18: 269-275.
THURBERG, F.P., DAWSON, M.A., & COLLIER, R.S. (1973) Effects of copper
and cadmium on osmoregulation and oxygen consumption in two species of
estuarine crabs. Mar. Biol., 23: 171-175.
TJALVE, H., GOTTOFREY, J., & BJORKLUND, I. (1986) Tissue disposition
of 109Cd2+ in the brown trout ( Salmo trutta) studied by
autoradiography and impulse counting. Toxicol. environ. Chem., 12:
31-45.
TYLER, G. (1972) Heavy metals pollute nature, may reduce productivity.
Ambio, 1: 52-59.
TYLER, G., MORNSJO, B., & NILSSON, B. (1974) Effects of cadmium, lead,
and sodium salts on nitrification in a mull soil. Plant Soil, 40:
237-242.
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North Sea.
I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VAN HATTUM, B., DE VOOGT, P., VAN DEN BOSCH, L., VAN STRAALEN, N.M.,
JOOSSE, E.N.G., & GOVERS, H. (1989) Bioaccumulation of cadmium by the
freshwater isopod Asellus aquaticus (L.) from aqueous and dietary
sources. Environ. Pollut., 62: 129-151.
VAN HOOK, R.I. (1974) Cadmium, lead, and zinc distributions between
earthworms and soils: potentials for biological accumulation. Bull.
environ. Contam. Toxicol., 12: 509-512.
VAN HOOK, R.I. & YATES, A.J. (1975) Transient behavior of cadmium in
a grassland arthropod food chain. Environ. Res., 9: 76-83.
VAN KESSEL, W.H.M., BROCADES ZAALBERG, R.W., & SEINEN, W. (1989)
Testing environmental pollutants on soil organisms: A simple assay to
investigate the toxicity of environmental pollutants on soil
organisms, using CdCl2 and nematodes. Ecotoxicol. environ. Saf., 18:
181-190.
VAN LEEUWEN, C.J., LUTTMER, W.J., & GRIFFIOEN, P.S. (1985) The use of
cohorts and populations in chronic toxicity studies with Daphnia
magna: A cadmium example. Ecotoxicol. environ. Saf., 9: 26-39.
VAN STRAALEN, N.M. & VAN WENSEM, J. (1986) Heavy metal content of
forest litter arthropods as related to body-size and trophic level.
Environ. Pollut., 42A: 209-221.
VAN STRAALEN, N.M., BURGHOUTS, T.B.A., DOORNHOF, M.J., GROOT, G.M.,
JANSSEN, M.P.M., JOOSSE, E.N.G., VAN MEERENDONK, J.H., THEEUWEN,
J.P.J.J., VERHOEF, H.A., & ZOOMER, H.R. (1987) Efficiency of lead and
cadmium excretion in populations of Orchesella cincta (Collembola)
from various contaminated forest soils. J. appl. Ecol., 24: 953-968.
VAN STRAALEN, N.M., SCHOBBEN, J.H.M., & DE GOEDE, R.G.M. (1989)
Population consequences of cadmium toxicity in soil microarthropods.
Ecotoxicol. environ. Saf., 17: 190-204.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1987)
Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am. J.
Physiol., 253: R216-R221.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1988)
Cadmium inhibits plasma membrane calcium transport. J. membrane Biol.,
102: 97-104.
VERBOST, P.M., VAN ROOIJ, J., FLIK, G., LOCK, R.A.C., & WENDELAAR
BONGA, S.E. (1989) The movement of cadmium through freshwater trout
branchial epithelium and its interference with calcium transport. J.
exp. Biol., 145: 185-197.
VERNBERG, W.B., DE COURSEY, P.J., & O'HARA, J. (1974) Multiple
environmental factor effects on physiology and behavior of the fiddler
crab, Uca pugilator. In: Vernberg, F.G. & Vernberg, W.B., ed.
Pollution and physiology of marine organisms, New York, London,
Academic Press, pp. 381-425.
VERNBERG, W.B., DE COURSEY, P.J., KELLY, M., & JOHNS, D.M. (1977)
Effects of sublethal concentrations of cadmium on adult Palaemonetes
pugio under static and flow-through conditions. Bull. environ.
Contam. Toxicol., 17: 16-24.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1981) Effects of some
environmental factors on the toxicity of cadmium to the copepod Tisbe
holothuriae. Arch. Hydrobiol., 91: 287-293.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1982) Differentiation
of the sensitivity to copper and cadmium in different life-stages of
a copepod. Mar. Pollut. Bull., 13: 123-125.
VOYER, R.A. (1975) Effect of dissolved oxygen concentration on the
acute toxicity of cadmium to the mummichog, Fundulus heteroclitus
(L.), at various salinities. Trans. Am. Fish. Soc., 104: 129-134.
VOYER, R.A. & MODICA, G. (1990) Influence of salinity and temperature
on acute toxicity of cadmium to Mysidopsis bahia Molenock. Arch.
environ. Contam. Toxicol., 19: 124-131.
VOYER, R.A., YEVICH, P.P., & BARSZCZ, C.A. (1975) Histological and
toxicological responses of the mummichog, Fundulus heteroclitus
(L.), to combinations of levels of cadmium and dissolved oxygen in a
freshwater. Water Res., 9: 1069-1074.
WALTERSSON, E. (1984) [Effects of enrichment reagents - distribution
and spread of xanthates and xanthate derivatives during enrichment
activities], Stockholm, Swedish Environmental Research Institute
(Report B478) (in Swedish).
WATLING, H.R. & WATLING, R.J. (1983) Sandy beach molluscs as possible
bioindicators of metal pollution. 2. Laboratory studies. Bull.
environ. Contam. Toxicol., 31: 339-343.
WATSON, C.F. & BENSON, W.H. (1987) Comparative activity of gill
ATPases in three freshwater teleosts exposed to cadmium. Ecotoxicol.
Environ. Saf., 14: 252-259.
WEIS, J.S. & WEIS, P. (1977) Effects of heavy metals on development of
the killifish, Fundulus heteroclitus. J. Fish Biol., 11: 49-54.
WESTERMAN, A.G. (1977) Lethal and teratogenic effects of inorganic
mercury and cadmium on embryonic development of Anurans, Lexington,
University of Kentucky (M.S. Thesis).
WHITE, D.H. & FINLEY, M.T. (1978) Uptake and retention of dietary
cadmium in mallard ducks. Environ. Res., 17: 53-59.
WHITE, D.H., FINLEY, M.T., & FERRELL, J.F. (1978) Histopathologic
effects of dietary cadmium on kidneys and testes of mallard ducks. J.
Toxicol. environ. Health, 4: 551-558.
WHO (1992) Environmental Health Criteria 134: Cadmium. Geneva, World
Health Organization, 280 pp.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WICKLUND, A. (1990) Metabolism of cadmium and zinc in fish, University
of Uppsala, Sweden, Faculty of Science (Acta Uiversitatis Uppsaliensis
- Comprehensive summaries of Uppsala dissertations from the Faculty of
Science, No. 250).
WIER, C.F. & WALTER, W.M. (1976) Toxicity of cadmium in the freshwater
snail, Physa gyrina Say. J. environ. Qual., 5: 359-362.
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soil and plants. Aust. J. soil Res., 11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILLIAMS, D.R. & GIESY, J.P. (1978) Relative importance of food and
water sources to cadmium uptake by Gambusia affinis ( Poeciliidae).
Environ. Res., 16: 326-332.
WILLIAMS, K.A., GREEN, D.W.J., & PASCOE, D. (1985) Studies on the
acute toxicity of pollutants to freshwater macroinvertebrates. I.
Cadmium. Arch. Hydrobiol., 102: 461-471.
WILLIAMS, K.A., GREEN, D.W.J., PASCOE, D., & GOWER, D.E. (1987) Effect
of cadmium on oviposition and egg viability in Chironomus riparius
(Diptera: Chironomidae). Bull. environ. Contam. Toxicol., 38: 86-90.
WILLIAMS, P.L. & DUSENBERY, D.B. (1990) Aquatic toxicity testing using
the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem., 9:
1285-1290.
WILLIAMSON, P. (1980) Variables affecting body burdens of lead, zinc
and cadmium in a roadside population of the snail Cepaea hortensis
Muller. Oecologia, 44: 213-220.
WILSON, D.N. (1988) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium Conference,
London, Cadmium Association, pp. 9-16.
WINNER, R.W. (1986) Interactive effects of water hardness and humic
acid on the chronic toxicity of cadmium to Daphnia pulex. Aquat.
Toxicol., 8: 281-293.
WINNER, R.W. (1988) Evaluation of the relative sensitivities of 7-d
Daphnia magna and Ceriodaphnia dubia toxicity tests for cadmium
and sodium pentachlorophenate. Environ. Toxicol. Chem., 7: 153-159.
WONG, P.T.S., BURNISON, G., & CHAU, Y.K. (1979) Cadmium toxicity to
freshwater algae. Bull. environ. Contam. Toxicol., 23: 487-490.
WONG, Y.S., LAM, H.M., DHILLON, E., TAM, N.F.Y., & LEUNG, W.N. (1988)
Physiological effects and uptake of cadmium in Pisum sativum.
Environ. Int., 14: 535-543.
WOODALL, C., MACLEAN, N., & CROSSLEY, F. (1988) Responses of trout fry
( Salmo gairdneri) and Xenopus laevis tadpoles to cadmium and zinc.
Comp. Biochem. Physiol., 89C: 93-99.
WRIGHT, D.A. & FRAIN, J.W. (1981a) Cadmium toxicity in Marinogammarus
obtusatus. Effect of external calcium. Environ. Res., 24: 338-344.
WRIGHT, D.A. & FRAIN, J.W. (1981b) The effect of calcium on cadmium
toxicity in the freshwater amphipod, Gammarus pulex (L.). Arch.
environ. Contam. Toxicol., 10: 321-328.
WRIGHT, D.A., METEYER, M.J., & MARTIN, F.D. (1985) Effect of calcium
on cadmium uptake and toxicity in larvae and juveniles of striped bass
( Morone saxatilis). Bull. environ. Contam. Toxicol., 34: 196-204.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in perspective.
Bull. Inst. Public Health (Tokyo), 24: 18-24.
ZAROOGIAN, G.E. & CHEER, S. (1976) Accumulation of cadmium by the
American oyster, Crassostrea virginica. Nature (Lond.), 261:
408-410.
ZAROOGIAN, G.E. & MORRISON, G. (1981) Effect of cadmium body burdens
in adult Crassostrea virginica on fecundity and viability of larvae.
Bull. environ. Contam. Toxicol., 27: 344-348.
ZIRINO, A. & YAMAMOTO, S. (1972) A pH-dependent model for the chemical
speciation of copper, zinc, cadmium and lead in seawater. Limnol.
Oceanogr., 17(5): 661-671.
Appendix 1. Global emissions of trace metals from natural sources (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Wind-borne soil particles
range 0.3-5.0 0.01-0.4 0.9-15 0-0.01 0.3-7.5 0.01-0.35 3.0-35
median 2.6 0.21 8.0 0.05 3.9 0.18 19
Sea salt spray
range 0.19-3.1 0-0.11 0.23-6.9 0-0.04 0.02-2.8 0-1.1 0.02-0.86
median 1.7 0.06 3.6 0.02 1.4 0.55 0.44
Volcanoes
range 0.15-7.5 0.14-1.5 0.9-18 0.03-2.0 0.54-6.0 0.10-1.8 0.31-19
median 3.8 0.82 9.4 1.0 3.3 0.95 9.6
Forest fires
range 0-0.38 0-0.22 0.1-7.5 0-0.05 0.06-3.8 0-0.52 0.3-15
median 0.19 0.11 3.8 0.02 1.9 0.26 7.6
Biogenic continental particulates
range 0.2-0.5 0-0.83 0.1-5.0 0-0.04 0.02-2.5 0-0.25 0.3-5.0
median 0.26 0.15 2.6 0.02 1.3 1.12 2.6
Biogenic continental volatiles
range 0.03-2.5 0-0.8 0.01-0.62 0.02-1.2 0.01-0.38 0.15-5.0 0.02-5.0
median 1.3 0.04 0.32 0.61 0.20 2.6 2.5
Appendix 1 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Biogenic marine
range 0.16-4.5 0-0.1 0.02-0.75 0.04-1.5 0.02-0.45 0.4-9.0 0.04-6.0
median 2.3 0.05 0.39 0.77 0.24 4.7 3.0
Total emission
range 0.86-23 0.15-2.6 2.3-54 0.1-4.9 0.97-23 0.66-18 4.0-86
median 12 1.3 28 2.5 12 9.3 45
a From: Nriagu (1989)
Appendix 2. Natural and anthropogenic emissions of trace metals to the atmosphere in 1983 (x 1000 tonnes/year) a
Trace metal Anthropogenic source Natural source Total emission Natural/total emissions
Arsenic 19 (12-26) 12 (0.86-23) 31 (13-49) 0.39
Cadmium 7.6 (3.1-12) 1.3 (0.15-2.6) 8.9 (3.2-15) 0.15
Copper 35 (20-51) 28 (2.3-54) 63 (22-105) 0.44
Mercury 3.6 (0.91-6.2) 2.5 (0.10-4.9) 6.1 (1.0-11) 0.41
Lead 332 (289-376) 12 (0.97-23) 344 (290-399) 0.04
Selenium 6.3 (3.0-9.7) 9.3 (0.66-18) 16 (2.5-24) 0.58
Zinc 132 (70-194) 45 (4.0-86) 177 (74-280) 0.34
a From: Nriagu (1989)
Appendix 3. Sources of global emissions of trace elements to the atmosphere in 1983 (tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Coal combustion - electric utilities
232-1550 77-387 930-3100 155-542 775-4650 108-775 1085-7750
Coal combustion - industry and domestic
198-1980 99-495 1390-4950 495-2970 990-9900 792-1980 1485-11 880
Oil combustion - electric utilities
5.8-29 23-174 348-2230 - 232-1740 35-290 174-1280
Oil combustion - industry and domestic
7.2-72 18-72 179-1070 - 716-2150 107-537 358-2506
Pyrometallurgical non-ferrous metal production - mining
40.0-80 0.6-3 160-800 - 1700-3400 18-176 310-620
Pyrometallurgical non-ferrous metal production - Pb production
780-1560 39-195 234-312 7.8-16 11 700-31 200 195-390 195-468
Pyrometallurgical non-ferrous metal production - Cu-Ni production
8500-12 750 1700-3400 14 450-33 600 37-207 11 050-22 100 427-1280 4250-8500
Pyrometallurgical non-ferrous metal production - Zn-Cd production
230-690 920-4600 230-690 - 5520-11 500 92-23 46 000-82 800
Secondary non-ferrous metal production
- 2.3-3.6 55-165 - 90-1440 3.8-19 270-1440
Steel and iron manufacturing
355-2480 28-284 142-2840 - 1065-14 200 0.8-2.2 7100-31 950
Refuse incineration - municipal
154-392 56-1400 980-1960 140-2100 1400-2800 28-70 2800-8400
Refuse incineration - sewage sludge
15-60 3-36 30-180 15-60 240-300 3-30 150-450
Appendix 3 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Phosphate fertilizers
- 68-274 137-685 - 55-274 0.4-1.2 1370-6850
Cement production
178-890 8.9-534 - - 18-14 240 - 1780-17 800
Wood combustion
60-300 60-180 600-1200 60-300 1200-3000 - 1200-6000
Mobile sources (gasoline)
- - - - 248 030 - -
Miscellaneous
1250-2800 - - - 3900-5100 - 1724-4783
Total emissions - range
12 000-25 630 3100-12 040 19 860-50 870 910-6200 288 700-376 000 1810-5780 70 250-193 500
Total emissions - median
18 820 7570 35 370 3560 332 350 3790b 131 880
a From: Nriagu & Pacyna (1988)
b This value applies to particulate selenium only. Since volatile selenium accounts for about
40% of the selenium released, the total selenium emission is estimated to be 6300 tonnes per year.
Appendix 4. Anthropogenic inputs of trace elements to aquatic ecosystems (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
3.6-7.7 0.9-3.6 6.0-15 0.22-1.8 87-113 0.54-1.1 21-58
Other industrial sources
8.4-62.3 1.2-13.4 29-75 0.08-7.0 10-67 9.5-70.9 56-317
Total - range
12-70 2.1-17 35-90 0.3-8.8 97-180 10-72 77-375
Total - median
41 9.4 62 4.6 138 41 226
Atmospheric fallout/total anthropogenic inputs (%)
14 24 17 22 72 2 17
a From: Nriagu & Pacyna (1988)
Appendix 5. Anthropogenic inputs of trace elements to soils (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
8.4-18 2.2-8.4 14-36 0.63-4.3 202-263 1.3-2.6 49-135
Other industrial sources
43.6-94 3.4-29.6 527-1331 0.97-10.7 277-850 4.7-73.4 640-1919
Total input - range b
52-112 5.6-38 541-1367 1.6-15 479-1113 6.0-76 689-2054
Total input - median b
82 22 954 8.3 796 41 1372
Atmospheric fallout/total anthropogenic inputs (%) b
16 24 3 30 29 5 7
a From: Nriagu & Pacyna (1988)
b These data do not include inputs from mine tailings, smelter slags and wastes to land.
RESUME
Le cadmium (numéro atomique 48; masse atomique relative 112,40)
est un élément métallique qui appartient, avec le zinc et le mercure,
au groupe IIb du tableau périodique. Certains sels de cadmium, tels
que le sulfure, le carbonate et l'oxyde sont presque insolubles dans
l'eau; ils peuvent cependant être transformés en sels solubles dans
l'environnement naturel. La formation de différents dérivés du cadmium
dans l'environnement est importante pour l'évaluation du risque.
La teneur moyenne en cadmium de l'eau de mer est d'environ 0,1
µg/litre ou moins. L'eau des cours d'eau contient du cadmium dissous
à des concentrations allant de < 1 à 13,5 ng/litre. Dans les régions
écartées et inhabitées, on observe en général des concentrations de
cadmium dans l'air inférieures à 1 ng/m3. Dans les régions où l'on
ne connaît pas de pollution, la concentration médiane en cadmium dans
le sol se situerait entre 0,2 et 0,4 mg/kg. Toutefois, on rencontre
occasionnellement des valeurs beaucoup plus élevées pouvant aller
jusqu'à 160 mg/kg de terre.
Certains facteurs environnementaux influent sur la fixation, et
par voie de conséquence, sur les effets toxiques du cadmium sur les
organismes aquatiques. Une élévation de température augmente la
fixation et l'effet toxique du cadmium qui sont en revanche réduits
lorsque la salinité de l'eau ou sa dureté augmentent. Les organismes
d'eau douce sont sensibles à des concentrations plus faibles de
cadmium que les organismes marins. Plus la teneur de l'eau en matières
organiques est élevée, plus la fixation et les effets toxiques du
cadmium sont réduits car ces matières se lient au cadmium et en
réduisent la biodisponibilité. Toutefois, on est fondé à croire que
certains dérivés organiques pourraient avoir un effet inverse.
Le cadmium s'accumule facilement dans de nombreux organismes, en
particulier les microorganismes et les mollusques, pour lesquels le
facteur de bioconcentration peut atteindre plusieurs milliers. Les
invertébrés terricoles concentrent égale-ment assez fortement le
cadmium. Pour la plupart des organismes, le facteur de concentration
est faible à modéré, généralement inférieur à 100. Dans de nombreux
tissus, le cadmium est lié aux protéines. On a isolé d'organismes
exposés au cadmium, des pro-téines qui fixent spécifiquement les
métaux lourds (métallothio-néines). Le cadmium se concentre
préférentiellement dans les reins, les branchies et le foie (ou leurs
équivalents). L'élimination du métal s'effectue probablement par le
rein, encore que chez les crustacés, il puisse être éliminé en
quantités notables en passant dans l'exosquelette. Chez les plantes,
le cadmium se concentre principalement dans les racines et à un
moindre degré dans les feuilles.
Le cadmium est toxique pour de nombreux microorganismes.
Toutefois, la présence de sédiments et de fortes concentrations de
sels ou de matières organiques en solution réduit ces effets toxiques.
Ces effets s'exercent principalement sur la croissance et la
réplication. Parmi les microorganismes terricoles, les plus affectés
sont les champignons, dont certaines espèces peuvent être éliminées
lorsque le sol est contaminé par du cadmium. Une faible exposition au
cadmium présent dans le sol peut entraîner une sélection des souches
résistantes. La toxicité du cadmium pour les organismes aquatiques est
variable, même lorsqu'il s'agit d'espèces très proches et elle est
liée à la concentration du métal sous forme ionique. Le cadmium
perturbe le métabolisme du calcium chez l'animal. Chez les poissons,
il provoque une hypocalcémie, probablement en inhibant la fixation du
calcium à partir de l'eau. Toutefois, la présence de fortes
concentrations de calcium dans l'eau protège les poissons par
inhibition compétitive de la fixation de cadmium. Le zinc accroît la
toxicité du cadmium pour les invertébrés aquatiques. On a fait état
d'effets sublétaux sur la croissance et la reproduction d'invertébrés
aquatiques; des effets ont été également observés sur la structure des
branchies d'invertébrés. Le cadmium est plus ou moins toxique pour les
poissons, les salmonidés étant particulièrement sensibles. On a
signalé des effets sublétaux chez les poissons, en particuliers des
malformations de l'épine dorsale. Les stades les plus sensibles sont
les embryons et les jeunes larves, les moins sensibles étant les
oeufs. Chez les poissons, on n'observe pas d'interaction systématique
entre le cadmium et le zinc. Le cadmium est toxique pour certaines
larves d'amphibiens, mais on a constaté que la présence de sédiments
dans les aquariums expérimentaux apportait une certaine protection.
Le cadmium perturbe la croissance des végétaux au laboratoire,
mais aucun effet n'a été observé dans la nature. Les plantes captent
plus facilement le métal lorsqu'il est présent dans les solutions
nutritives que lorsqu'il est dans le sol; les effets observés l'ont
été essentiellement lors d'études portant sur des cultures
hydroponiques. Il semblerait que le cadmium présent dans les solutions
nutritives affecte l'ouverture des stomates, la transpiration et la
photosynthèse.
Les invertébrés terrestres sont relativement insensibles aux
effets toxiques du cadmium, probablement à cause de l'intervention de
mécanismes de séquestration efficaces au niveau des divers organes.
Les gastéropodes terrestres peuvent subir des effets sublétaux,
principalement en ce qui concerne la consommation de nourriture et la
dormance, mais uniquement à des doses très élevées. Même à forte dose,
le cadmium n'entraîne pas la mortalité des oiseaux mais peut provoquer
des lésions rénales.
Des études effectuées sur le terrain ont montré que le cadmium
pouvait entraîner la modification de la proportion relative des
diverses espèces dans les populations de microorganismes et de
certains invertébrés aquatiques. La décomposition des feuilles mortes
est fortement entravée par une forte pollution due aux métaux lourds,
et le cadmium en serait le principal responsable.
RESUMEN
El cadmio (número atómico 48; masa atómica relativa 112,40) es un
elemento metálico que pertenece, junto con el zinc y el mercurio, al
grupo IIb de la tabla periódica. Algunas sales de cadmio, como el
sulfuro, el carbonato y el óxido, son prácticamente insolubles en
agua; pueden convertirse en sales hidrosolubles en el medio natural.
El sulfato, el nitrato y los haluros son hidrosolubles. La especiación
del cadmio en el medio ambiente tiene importancia para evaluar su
potencial de riesgo.
El contenido medio de cadmio en el agua de mar es de alrededor de
0,1 µg/litro o menos. El agua de los ríos contiene cadmio disuelto en
concentraciones que varían entre < 1 y 13,5 ng/litro. En zonas
aisladas y deshabitadas, las concentraciones de cadmio en el aire
suelen ser inferiores a 1 ng/m3. En zonas que se suponen no
contaminadas, se ha comunicado que la concentración mediana de cadmio
en el suelo se encuentra entre 0,2 y 0,4 mg/kg. No obstante, a veces
se encuentran valores mucho más altos, que pueden llegar hasta 160
mg/kg de suelo.
Los factores ambientales influyen en la captación y, por ende, en
los efectos tóxicos del cadmio en los organismos acuáticos. Al
aumentar la temperatura aumentan la captación y los efectos tóxicos,
mientras que el aumento de la salinidad o de la dureza del agua los
hace disminuir. Los organismos de agua dulce sufren los efectos del
cadmio en concentraciones inferiores a las que afectan a los
organismos marinos. La materia orgánica contenida en el agua suele
reducir la captación y los efectos tóxicos fijando el cadmio y
reduciendo su disponibilidad para los seres vivos. Sin embargo, hay
pruebas de que cierto tipo de materia orgánica puede ejercer el efecto
contrario.
El cadmio se acumula fácilmente en numerosos seres vivos,
particularmente microorganismos y moluscos, en los que los factores de
bioconcentración son del orden de varios millares. Los invertebrados
del suelo también concentran este metal en grado considerable. La
mayoría de los organismos presentan factores de concentración bajos o
moderados (inferiores a 100). El cadmio está ligado a proteínas en
numerosos tejidos. En organismos expuestos a ese metal se han aislado
proteínas fijadoras de metales pesados (metalotioneínas). La
concentración de cadmio es más elevada en el riñón, las branquias y el
hígado (o sus equivalentes). La eliminación se produce probablemente
por vía renal, si bien los crustáceos pueden eliminar cantidades
importantes con la muda del exoesqueleto. En las plantas, el cadmio se
concentra principalmente en las raíces y, en menor medida, en las
hojas.
El cadmio tiene efectos tóxicos para muy diversos
microorganismos. No obstante, la presencia de sedimentos y las
concentraciones elevadas de sales disueltas o materia orgánica reducen
esos efectos. Los procesos más afectados son el crecimiento y la
replicación. Los organismos del suelo más vulnerables son los hongos:
algunas especies desaparecen tras la exposición al cadmio en el suelo.
Tras exposiciones reducidas al metal en el suelo, se observa una
selección a favor de las cepas resistentes.
La toxicidad aguda del cadmio para los organismos acuáticos es
variable, incluso entre especies estrechamente emparentadas, y guarda
relación con la concentración de iones libres del metal. El cadmio
interacciona con el metabolismo del calcio en los animales. En los
peces provoca hipocalcemia, probablemente al inhibir la captación de
calcio a partir del agua. No obstante, las concentraciones elevadas de
calcio en el agua los protegen de la ingestión de cadmio por
competencia en los lugares de captación. El zinc aumenta la toxicidad
del cadmio para los invertebrados acuáticos. Se han notificado efectos
subletales en el crecimiento y la reproducción de invertebrados
acuáticos, así como modificaciones estructurales en las branquias de
invertebrados. Hay pruebas de la selección de estirpes resistentes de
invertebrados acuáticos tras la exposición al cadmio sobre el terreno.
La toxicidad es variable en los peces; los salmónidos son
especialmente susceptibles. Se han notificado efectos subletales en
los peces, en particular malformaciones de la espina dorsal. Las fases
biológicas más susceptibles son el embrión y la larva joven; los
huevos son los menos vulnerables. No se ha observado una interacción
homogénea entre el cadmio y el zinc en los peces. El cadmio resulta
tóxico para algunas larvas de anfibios, si bien los sedimentos en el
recipiente de ensayo confieren cierta protección.
El cadmio afecta al crecimiento de las plantas en estudios
experimentales, pero sobre el terreno no se ha observado efecto
alguno. El metal es absorbido por las plantas con más rapidez a partir
de soluciones de nutrientes que a partir del suelo; los efectos se han
observado sobre todo en estudios de cultivo en soluciones de
nutrientes. En éstas se ha notificado que el cadmio influye en la
apertura de los estomas, la transpiración y la fotosíntesis.
Los invertebrados terrestres son relativamente insensibles a los
efectos tóxicos del cadmio, probablemente debido a la existencia de
mecanismos eficaces de captura y fijación en ciertos órganos.
Los gasterópodos terrestres sufren efectos subletales; los
principales procesos afectados son el consumo de alimentos y el
letargo, pero sólo con dosis muy elevadas. El metal no produce efectos
letales en las aves, ni siquiera con dosis elevadas, si bien se
observan lesiones renales.
En estudios de campo se ha comunicado que el cadmio induce
cambios en la composición de especies en las poblaciones de
microorganismos y ciertos invertebrados acuáticos. La descomposición
del mantillo de hojas se ve notablemente reducida por la contaminación
con metales pesados; se ha identificado al cadmio como el principal
causante de este efecto.
Table 6. Cadmium assimilation efficiencies in different soil invertebrates
Species Food Cadmium concentration Assimilation efficiency Reference
in food (µmol/g) (%)
Snail
Arianta arbusloruma agar 1.48 55-92 Berger & Dallinger (1989) b
Centipede
Lithobius variegatus isopod 1.21-10.2 0-7.2 Hopkin & Martin (1984)
hepatopancreas
Millipede
Clomeris marginata maples leaves 8.2-40.6 Hopkin et al. (1985)
Pseudoscorpion
Neobisium muscorum collembolans 0.20 58.9 Janssen et al. (1991)
Mite
Platynothrus peltifer green algae 0.15 17.2 Janssen et al. (1991)
Insects
Orchesella cincta green algae 0.09 8.3 Van Straalen et al. (1987)
Orchesella cincta green algae 0.15 9.4 Janssen et al. (1991)
Notiophilus biguttatus collembolans 0.23 35.5 Janssen et al. (1991)
a assimilation value for midgut gland
b recalculated from the data
Table 7. Excretion constants (k) for cadmium in different soil invertebrates
Species Taxonomic k Reference
group (day-1)
Helix pomatia snail 0 Dallinger & Wieser (1984) b
Cepaea nemoralis snail 0.007 Williamson (1980) b
Oniscus asellusa isopod 0.002 Hopkin (1989) b
Neobisium muscorum pseudoscorpion 0 Janssen et al. (1991)
Lycosa spp spider 0.007 Van Hook & Yates (1975)
Platynothrus peltifer oribatid mite 0.013 Janssen et al. (1991)
Orchesella cincta collembolan 0.061 Van Straalen et al. (1987)
Orchesella cincta collembolan 0.087 Janssen et al. (1991)
Acheta domesticus cricket 0.090- Van Hook & Yates (1975)
0.110
Notiophilus biguttatus carabid beetle 0.375 Janssen et al. (1991)
a k value for midgut gland or hepatopancreas
b recalculated from the data
Bioconcentration factors (the ratio between the cadmium
concentration in the organism and the concentration in the medium) for
several groups of organisms studied under laboratory conditions are
shown in Table 8. They range from 16 to 130 000 and do not seem to
show any consistent pattern.
Table 8. Bioconcentration of cadmium in laboratory studies
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Freshwater alga 10 10 3000 dw c Ferard et al. (1983)
(Chlorella vulgaris)
Freshwater alga stat 20-22 14 250 4940 daw Cain et al. (1980)
(Scenedesmus obliquus)
Freshwater diatom flow WB 23 10 40 000 Conway (1978)
(Asterionella formosa)
Submerged plant WP 25 30 25 1730 dw Nakada et al. (1979)
(Elodea nuttallii)
Water hyacinth leaves 28 500 16 dw c Kay & Haller (1986)
(Eichhornia crassipes)
American oyster 4.9-5.1 g flow WB 16-20 21 10 116 ww Eisler et al. (1972)
(Crassostrea virginica) 4280 aw
8.1 g flow ST 2.8-22.6 280 5 2376 ww Zaroogian & Cheer (1976)
18 472 dw
Mussel 32-34 mm flow ST 13 166 10 50 802 dw Riisgard et al. (1987)
(Mytilus edulis)
Scallop 6.8-7.7 g flow WB 16-20 21 10 131 ww Eisler et al. (1972)
(Aquipecten irradians) 3970 aw
Bay scallop 0.51-0.73 g flow ST 9.5-16 42 60 20 400 Pesch & Stewart (1980)
(Argopecten irradians)
Crab 2-4 g WB 10 14 37 152 dw Ray et al. (1980a)
(Pandalas montagui)
Grass shrimp 20-33 mm flow WB 9.5-16 42 60 223 Pesch & Stewart (1980)
(Palaemonetes pugio)
Table 8 (contd).
Organism Size Stat/ Organ a Temperature Duration Exposure Bioconcentration Reference
flow (°C) (days) (µg/litre) factor b
Lobster 160-169 g flow WB 16-20 21 10 21 ww Eisler et al. (1972)
(Homarus americanus) 10 aw
Mummichog 2.3-2.4 g flow WB 16-20 21 10 15 ww Eisler et al. (1972)
(Fundulus heteroclitus) 200 aw
Fathead minnow flow WB 13.9-15.3 21 49 190 Sullivan et al. (1978a)
(Pimephales promelas)
Red maple leaves 15-27 45 0.5 14 400 dw d Mitchell & Fretz (1977)
(Acer rubrum) roots 15-27 45 0.5 131 800 dw d Mitchell & Fretz (1977)
leaves 15-27 101 2.6 mg/kg 0.76 dw e Mitchell & Fretz (1977)
roots 15-27 101 2.6 mg/kg 12.5 dw e Mitchell & Fretz (1977)
White pine leaves 15-27 66 0.5 3400 dw d Mitchell & Fretz (1977)
(Pinus strobus) roots 15-27 66 0.5 118 400 dw d Mitchell & Fretz (1977) leaves 15-27 36 52.6 mg/kg 1.2 dw e Mitchell & Fretz (1977)
roots 15-27 36 52.6 mg/kg 10.4 dw e Mitchell & Fretz (1977)
a WB = whole body; WP = whole plant; ST = soft tissues
b Chloride salt used unless stated otherwise; bioconcentration factor = concentration in the organism divided by concentration
in the medium; dw = dry weight; ww = wet weight; aw = ash weight; daw = dry ash weight
c Nitrate salt used
d The medium was a cadmium-enriched nutrient solution
e The medium was a cadmium-amended soil mix
Microorganisms generally exhibit a high capacity to take up
cadmium from water and retain the metal in their cells. The highest
bioconcentration factors reported have been for micro-organisms, the
greatest value being 40 000 in a freshwater diatom (Conway, 1978). In
this diatom, 58% of the cadmium was located in the cellular content
with 25% in the organic coating of the frustule and 17% in the
silicaceous frustule. The bioconcentration factor of 3000 for the alga
Chlorella (Ferard et al., 1983) is typical of the value for
microorganisms. Flatau et al. (1988) demonstrated the uptake (it was
not specified whether this referred to absorption or adsorption) of
cadmium from sea water by marine bacteria; the uptake of the metal
increased with its concentration in the water, and the accumulation
rate was a logarithmic function of the dose. Sorption was only
observed with exposure concentrations above 10 µg Cd/litre, suggesting
that a threshold had to be exceeded for cadmium uptake to occur.
Dongmann & Nurnberg (1982) showed that the bioconcentration factor for
a marine diatom, Thalassiosira rotula, decreased with increasing
metal concentration, suggesting a saturation effect. Their reported
concentration factors, which vary between 1000 and 2000, reflect the
reduced sorption of cadmium by marine microorganisms compared with
their freshwater relatives. Hart & Scaife (1977) reported a direct
relationship between the level of cadmium in the medium and sorption
to the alga Chlorella exposed to cadmium concentrations ranging from
0.25 to 1.00 mg/litre.
After water hyacinths had been exposed for 4 weeks to water
containing 0.5 or 1.0 mg cadmium/litre, added as cadmium nitrate, the
leaves had accumulated 8.00 and 17.20 mg/kg, respectively (Kay &
Haller, 1986).
Molluscs concentrate cadmium to a high degree over a period of
time, but uptake is often slow. Oysters showed a concentration factor
of only 149 over a 10-day period (Eisler et al., 1972) but a factor of
2714 after 40 weeks (Zaroogian & Cheer, 1976). Elliott et al. (1985)
examined the accumulation of cadmium, copper, lead, and zinc in the
tissues of the mussel, Mytilus edulis. Under simultaneous exposure
to all four metals, both lead and cadmium were accumulated in direct
proportion to the exposure time, whereas copper and zinc were not.
Accumulation of cadmium was influenced by the presence of other
metals.
Compared with oysters, the related bay scallop shows greater
accumulation of cadmium when exposed to low concentration of the metal
as the chloride over 6 weeks (Pesch & Stewart, 1980). Short-term
exposure of the same scallop to higher concentrations of cadmium
resulted in very much lower concentration factors. Exposure for 96 h
to cadmium (as the chloride) at up to 2.0 mg/litre led to a
bioconcentration factor of around 50 (Nelson et al., 1976).
Bioconcentration factors (from water and food) and
biomagnification factors (from food alone) were calculated for the
freshwater isopod Assellus aquaticus by van Hattum et al. (1989).
Much of the cadmium (added as the chloride) was taken up from the
water (bioconcentration factor 18 000), but there was little uptake
from food (bioconcentration factor 0.08). Direct uptake from water
accounted for between 50 and 98% of the body burden after 30 days of
exposure (based on dry weight measures). Cadmium was readily taken up
by the isopod even at exposure concentrations of 1 µg/litre.
Experiments conducted at two different pHs (5.9 and 7.6) revealed no
significant effect of pH on uptake of cadmium by the isopod.
Wright & Frain (1981b) demonstrated that adult intermoult
amphipods ( Gammarus pulex) accumulated only half as much cadmium
from a solution of 5 mg/litre in the presence of 200 mg calcium/litre
as with 20 mg calcium/litre.
Ramamoorthy & Blumhagen (1984) investigated the uptake of
cadmium, mercury, and zinc by rainbow trout (Salmo gairdneri) in a
model system which simulated the presence of other competing
compartments that would be found in nature. The system consisted of
either a simple sediment/water model or a more complex series of
compartments in dialysis bags of suspended sediment, cation and anion
exchange resins (to represent naturally occurring polyelectrolyte
materials of plant origin), and fish. River water was used as the
fluid transfer medium, and the system was continuously stirred.
Equilibrium with one heavy metal ion did not inhibit the uptake of
other metal ions; cadmium and zinc were taken up after equilibrium
with mercury. The authors calculated approximate partition
coefficients (fish/substrate) to be 2.8 for sediment, 550 for water,
and 2 and 3.6 for the cation and anion exchange resins, respectively.
The problem of expressing changes in concentration between
trophic levels is that the units are not compatible. There is no
significance to a bioconcentration term that expresses a ratio of
cadmium in soil moisture to cadmium in plant tissue, or cadmium in
plant tissue to cadmium in herbivore tissue. Therefore, it is
difficult to assess the impact of cadmium on the environment in terms
of bioconcentration factors. An alternative method is to measure both
cadmium and calcium at each trophic level and express these
measurements as a molar ratio of these two elements. (The molar ratio
should be used to account for the movement by atoms, not grams.)
Differences between trophic levels are calculated as the ratio of the
higher trophic level to the lower. This approach, called
biopurification, recognizes that the flow of the non-nutrient cadmium
through successive trophic levels follows a pathway similar to that of
nutrients such as calcium, and that calcium must pass natural chemical
and physiological barriers, such as membranes and selective enzymes,
that progressively purify the pool of the nutrient calcium relative to
the non-nutrient cadmium. In the case where two similar ecosystems are
compared, and where one is believed to be more contaminated than the
other, the relative degree of contamination can be calculated as the
difference between molar ratios at the same or similar trophic levels.
It is unfortunate that the absence of concentration data on
nutrients such as calcium or, alternatively, zinc, prohibits the
calculation of biopurification factors for any of the studies
discussed in this monograph.
5. TOXICITY TO MICROORGANISMS
Appraisal
Cadmium is toxic to a wide range of microorganisms in culture
(effects of cadmium on microorganisms in the field are discussed in
chapter 8). However, the presence of sediment, organic matter or high
concentrations of dissolved salts reduces the availability of cadmium
to microorganisms and, therefore, reduces the toxic impact.
Freshwater microorganisms in culture are thus affected by cadmium at
lower concentrations than marine species (for example, 50 µg/litre
affects growth in many freshwater species of algae while at least 100
µg/litre, and often 1000 µg/litre, is required to reduce growth in
marine species). Soil microorganisms are partially protected from the
toxic effects of cadmium by the presence of clay.
5.1 Aquatic microorganisms
5.1.1 Freshwater microorganisms
Canton & Slooff (1982) exposed the bacterium Salmonella
typhimurium and the alga Chlorella vulgaris to cadmium in the form
of the chloride, and calculated an 8-h EC50 (growth inhibition) of
10.4 mg/litre for the bacterium and a 96-h EC50 of 3.7 mg/litre for
the alga. No-toxic-effect levels of 0.65 and 1.5 mg/litre were
estimated for the bacterium and alga, respectively. Jana &
Bhattacharya (1988) found significant inhibition of population growth
in the faecal coliform bacterium Escherichia coli during exposure to
cadmium concentrations of 1, 2 or 5 mg cadmium/litre for 7 or 28 days.
Cadmium was the most toxic of the metals tested. Norberg & Molin
(1983) exposed the bacterium Zoogloea ramigera (abundant in sewage
treatment plants) to cadmium chloride concentrations of 1, 3, 5, and
10 mg cadmium/litre for 30 h. A prolonged lag phase and decrease in
growth resulted, the length of the lag phase being proportional to the
concentration of cadmium in the medium. Babich & Stotzky (1977a)
showed that the presence of clay particles protected bacteria from the
toxic effect of cadmium added to culture medium. The degree of
protection was related to the cation exchange capacity of various
clays tested.
Chapman & Dunlop (1981) estimated the 8-h LC50 for the
freshwater protozoan Tetrahymena pyriformis to be less than 1
mg/litre. However, this value increased with increasing water calcium
concentration; at a value of 500 mg calcium/litre, the LC50 was 19
mg/litre. Magnesium also exerted a protective effect against cadmium
when mixed with calcium. Cadmium was consistently more toxic to
Tetrahymena in the presence of magnesium alone. Berk et al. (1985)
calculated a 15-min EC50 (inhibition of ciliate chemotactic
response) for Tetrahymena sp. of 0.35-0.7 mg.
When Skowronski et al. (1988) exposed the green microalga
Stichococcus bacillaris to cadmium chloride concentrations of 45 and
90 µmol/litre for 4 days, growth rate was inhibited by 28% and 45% at
the two respective concentrations. At both exposure levels, dry weight
and chlorophyll a content were reduced in a dose-related manner.
Addition of manganese at concentrations of between 45 and 1800
µmol/litre had a dose-related antagonistic effect on cadmium toxicity.
Bennett (1990) found that the addition of cadmium (1.8 µmol/litre) to
a turbidostat culture of Chlorella pyrenoidosa caused a decrease in
the maximum specific growth rate (toxicity was expressed after a lag
of 5 generations). A gradual decrease in the maximum specific growth
rate was also noted during a 40-day exposure to stepwise increases in
the cadmium concentration (0.96 to 1.68 µmol/litre). The author found
that the addition of manganese (10.4 µmol/litre) had an antagonistic
effect, causing the maximum specific growth rate to increase.
Cadmium is toxic to the growth of the freshwater alga Chlorella
pyrenoidosa (Hart & Scaife, 1977). In cultures maintained at pH 7.0,
doubling times were 11, 21, 22, and 35 h for cadmium concentrations of
0, 0.25, 0.5, and 1.0 mg/litre medium, respectively. At a pH of 8.0,
the effect was somewhat lessened; doubling times were 11, 16, 17, and
25 h for the same range of cadmium doses. There was also a pronounced
effect on carbon dioxide fixation, which was reduced from 0.738 to
0.720, 0.558, and 0.283 µmol HCO3- fixed per hour with cadmium
exposures in the culture medium of 0, 0.246, 0.554, and 1.090
mg/litre, respectively. There was less of an effect on oxygen
evolution over the same dose range. Zinc offered no protection against
cadmium effects.
Wong et al. (1979) exposed four different species of freshwater
algae to cadmium and measured the uptake of 14C-carbonate.
Scenedesmus quadricaudata was the most sensitive species, carbonate
uptake being inhibited by 80% at a cadmium concentration of 20
µg/litre. Chlorella pyrenoidosa showed 70% inhibition of carbonate
uptake at about 100 µg/litre, while Chlorella vulgaris showed only
50% inhibition at about 500 µg/litre. The least sensitive of the four
species tested was Ankistrodesmus falcatus variety acicularis
where an effect on carbonate uptake started only at concentrations
higher than 500 µg/litre. There was no observed effect on the growth
of A. falcatus at cadmium concentrations lower than 5 mg/litre.
Rebhun & Ben-Amotz (1984) demonstrated that the chlorophyll content of
cells of Chlorella stigmatophora was reduced in a dose-dependant
manner across a range of cadmium concentrations of between 1 and 10
mg/litre medium.
Laegreid et al. (1983) studied the effects of cadmium on the alga
Selenastrum capricornutum cultured in the laboratory in water taken
from two lakes at various times throughout the year. The two lake
waters contained different amounts of organic material. The first
lake, a dystrophic bog lake, had a high organic content, while the
second, an eutrophic lake, had a low organic content. In the
dystrophic lake, which had a low pH (4.4), the toxicity of cadmium was
related to the free ionic concentration of the metal, as suggested by
many laboratory experiments. In the eutrophic lake, where there was
less influence from organic material, there was a pronounced seasonal
effect. In the summer, when growth and productivity of the algae were
highest, there was a much greater effect of the metal than predicted.
The toxicity of cadmium, at this time, was far greater than would be
expected even if all of the metal was in the free ionic form and none
was bound to organic compounds. On the basis of their field evidence,
the authors questioned the generally held assumption that organic
binding is the major factor in determining cadmium toxicity to
microorganisms. They considered that the presence of certain organic
compounds of low relative molecular mass could increase cadmium
toxicity. This conclusion is supported by the work of Giesy et al.
(1977), who found that uptake of cadmium into zooplankton could be
increased in the presence of organic compounds of low relative
molecular mass.
Chin & Sina (1978) investigated the cellular basis of cadmium
toxicity in microorganisms using cultures of Physarum polycephalum.
The organism was cultured, in plasmodial form, on the surface of
liquid medium, and replicate discs, cut from the protoplasmic sheet of
the organism, were used for the tests. The discs maintain mitotic
synchrony with each other and, therefore, cadmium could be introduced
at specific points in the cell cycle. The cultures were exposed to
cadmium sulfate (5 x 10-4 mol/litre), which was floated onto the
surface of the culture medium. Exposure to cadmium immediately prior
to early prophase of mitosis extended the normal DNA replication
period from 3 h to 4 h; this was monitored using measurements of
uptake of 3H-thymidine. Two stages of the cell cycle were
particularly susceptible to cadmium. Exposure either at the beginning
of the cycle or 80% of the way through the cycle caused delays in the
completion of mitosis. A 30-min exposure to cadmium at the onset of
early prophase inhibited incorporation of 3H-uridine into RNA for
the following 3 h by 51% and stimulated the incorporation of
3H-thymidine into DNA, for the same period, by 85%. Later in the
cycle, DNA synthesis was inhibited and DNA content was depressed by
12.5%. There was an ultrastructural effect on the nucleoli (less dense
material centrally giving nucleoli in section a "ring" structure),
which was the only structural effect of the metal even after 4 h of
exposure. Accommodation occurred after pre-treatment with sub-toxic
doses of cadmium. Treatment with cadmium at the less sensitive periods
of the cell cycle led to reduced effect at the more sensitive phases;
the organism, in some way, compensated. There was no accommodation by
Physarum after pre-treatment with zinc ions. This result contrasts
with that of a similar study on Escherichia coli where pre-exposure
to zinc reduced the effects of cadmium (Mitra et al., 1975).
5.1.2 Estuarine and marine microorganisms
Chan & Dean (1988) exposed the marine bacterium Pseudo-monas
marina to cadmium sulfate at concentrations of 1 to 25 mg
cadmium/litre. Effects were exposure related and included a lengthened
lag time, reduced growth rate, reduced biomass and oxygen uptake, and
a decrease in the activity of dehydrogenase and alkaline phosphatase.
The IC50 values for inhibition of biomass and of growth rate were 11
and 11.5 mg/litre, respectively. Flatau et al. (1987) progressively
adapted the marine bacterium Pseudomonas sp. to cadmium
concentrations from 1 to 80 mg/litre. Although the length of the lag
phase was not linearly or logarithmically correlated with the cadmium
concentration, it was significantly longer at cadmium concentrations
of 70 mg/litre or more. The growth rate was reduced at 10 mg/litre but
remained constant at cadmium concentrations of 10 to 50 mg/litre; no
growth was observed at 75 or 80 mg/litre. Oxygen consumption was not
different from that of controls at 1 or 5 mg/litre but at
concentrations of 10 mg/litre or more respiratory activity decreased.
Bressan & Brunetti (1988) exposed the marine microalgae
Dunaliella tertiolecta and Isochrysis galbana to cadmium
concentrations of 13.8 and 0.2 mg/litre, respectively, for up to 8
days. Cadmium significantly reduced the population growth, expressed
as the mean number of cells/ml, for both species. The addition of
nitrilotriacetic acid (NTA, a sequestering agent) at ratios of 1:1,
1:2 or 1:3 did not modify the toxic effect of cadmium.
Berk et al. (1985) exposed the marine ciliates Paranophrys sp.
and Miamiensis avidus to cadmium and monitored the inhibition of the
chemotactic response. The 15-min EC50 values were 2-3.1 mg
cadmium/litre and 5.1-7 mg cadmium/litre for the two species,
respectively.
Dongmann & Nurnberg (1982) investigated the effects of cadmium on
the marine diatom Thalassiosira rotula (a chain-forming diatom
native to shallow temperate waters) in petri dish and in batch liquid
cultures. They calculated generation times from the dish cultures and
reported a toxicity threshold for generation time of 30 µmol
cadmium/litre (3.4 mg/litre). At 50 µmol/litre, cadmium increased the
generation time from 24 h to 28 h. Using chain length as a parameter,
the toxicity threshold was 15 µmol/litre (1.7 mg/litre). Cell density
was found to be affected in batch culture. Cell chlorophyll content
and chain length were too variable to show significant effects of the
metal in batch culture.
5.2 Soil and litter microorganisms
Babich & Stotzky (1977b) showed that several species of fungi
tolerated cadmium to a greater degree when grown on cultures amended
with clays than in pure culture medium.
Sato et al. (1986) exposed the soil bacterium Nitrosomonas
europaea both to cadmium concentrations of 0.05, 0.1, and 0.4
mg/litre and to a range of ammonia concentrations (1 to 100 mg/litre
as N). Growth was markedly inhibited at the highest cadmium
concentration, especially when this was coupled with ammonia
concentrations greater than 10 mg/litre. Cadmium toxicity caused a
characteristic growth response. Ammonia oxidation proceeded at a
reduced rate for approximately 3 days and then fell sharply. The
subsequent oxidation proceeded at a diminished but constant rate. At
cadmium concentrations of 0.1 mg/litre or less, the toxic effect of
cadmium could be partially offset by increasing the ammonia
concentration. Prahalad & Seenayya (1988) found that cadmium
concentrations of 3.5 mg/litre inhibited the growth of the bacterium
Alcaligenes faecalis. However, if the nutrient broth was diluted by
one half then growth was inhibited at 2 mg/litre, and with
quarter-strength nutrient broth inhibition occurred at 0.5 mg/litre.
Hartman (1974) reported less species diversity of soil fungi in
areas of high cadmium contamination than in control areas. Samples of
Fusarium oxysporium isolated from contaminated and control soils
showed different tolerances to cadmium. This suggested the development
of some resistance, presumably by selection of more resistant strains.
Bond et al. (1976) incubated coniferous forest soil and litter
(mixed with a pre-prepared homogeneous soil) in microcosm units and
monitored oxygen consumption, carbon dioxide evolution, and bacterial
and fungal populations. With cadmium added to a concentration of 0.01
mg/kg soil and, in the initial stages of incubation, with cadmium at
10 mg/kg soil, there was a stimulation of oxygen consumption,
suggesting an effect of the metal on uncoupling of respiratory
phosphorylation. In the later stages of incubation with cadmium at 10
mg/kg there was a reduction in both oxygen consumption and carbon
dioxide evolution. No effect was seen on numbers of organisms in the
microcosms.
Bewley & Stotzky (1983) investigated the effect of cadmium (100
and 1000 mg/kg soil) on carbon mineralization and on the mycoflora in
glucose-supplemented soils amended with clays (kaolinite or
montmorillonite) at 9%. Cadmium had no significant effect on the
length of the lag period, carbon dioxide evolution or on the amount of
carbon mineralized. When subsequent cadmium additions of 2400 and 4000
mg/kg were made to soils previously treated with 100 or 1000 mg/kg,
the rate of glucose degradation decreased more in the clay-amended
soils than in control soil not amended with clay. Clay protected fungi
from the toxic effect of cadmium at 5000 mg/kg. Fungi from
clay-treated soils were more sensitive to cadmium in the culture media
after they had been isolated from soil pre-treated with cadmium. The
authors interpreted these results as showing a reduction in the
availability of cadmium to organisms in clay-amended soils. The
overall effect would be a prevention of selection of more tolerant
strains. Thus, when the organisms were challenged with high doses of
cadmium, they would have been more susceptible than organisms from
non-amended soil.
Naidu & Reddy (1988) incubated black cotton soil (0.8% organic
carbon; 55% clay) for up to 8 weeks in the presence of cadmium
chloride at concentrations of between 50 and 500 mg cadmium/kg. The
ammoniacal nitrogen (NH4-N) concentration increased for the first
week at all treatment levels and then decreased at concentrations of
50 mg/kg or less. The initial rise in NH4-N levels led to an
increase in nitrate nitrogen (NO3-N) levels, the accumulation of
NO3-N being inversely proportional to the cadmium exposure. The
authors pointed out that at cadmium concentrations of 100 and 500
mg/kg, hydrolysis of urea was significantly poorer than at other
treatment levels, as shown by the lower concentration of NH4-N
observed after 1 week. At all cadmium concentrations there was
significant accumulation of nitrite nitrogen (NO2-N) at every sample
time, compared to control soil, suggesting that cadmium might be toxic
to soil nitrification. At all exposure levels cadmium significantly
depressed both bacterial and fungal populations. Cadmium
concentrations of 10 or 50 mg/kg had no effect on soil actinomycetes,
but both 100 and 500 mg/kg significantly reduced the population. Tyler
et al. (1974) incubated a mull soil for 7 weeks. Cadmium chloride
concentrations of 9 to 18 µmol/g and cadmium acetate concentrations of
9 to 22 µmol/g caused decreases in soil ammonium content and
significantly increased nitrate accumulation.
6. TOXICITY TO AQUATIC ORGANISMS
Appraisal
Cadmium uptake from water by aquatic organisms is extremely
variable and depends on the species and various environmental
conditions such as water hardness (notably the calcium ion
concentration), salinity, temperature, pH, and organic matter content
(see chapter 4).
The majority of chelating agents decrease cadmium uptake but
some, such as dithiocarbamates and xanthates, increase uptake.
As a consequence of the variability in cadmium uptake, the toxic
impact to aquatic organisms also varies across a wide range of
concentrations and is dependent on the species of organism and on the
presence of other metal ions, notably calcium and zinc.
The lowest recorded 96-h LC50 in a flow system is 16 µg
cadmium per litre for the adult shrimp Mysidopsis bahia. A nominal
no-observed-effect level (NOEL) of 0.6 µg cadmium/litre was found for
Daphnia magna, reproductive rate being the most sensitive parameter.
A nomi-nal NOEL has been noted at a similar level (1.7 to 3.4 µg
cadmium per litre) with respect to the reproductive effects on brook
trout.
The available results indicate that the embryonic and larval
stages of aquatic organisms are more sensitive than the adult stage.
Spinal malformations are induced in cadmium-exposed fish. In addition
to causing reproductive effects, cadmium influences the behaviour of
aquatic organisms.
At low concentrations, cadmium inhibits ion transport systems
(10 µg cadmium/litre) and induces metallothionein synthesis (< 1 µg
cadmium/litre) in freshwater fish.
6.1 Toxicity to aquatic plants
Hutchinson & Czyrska (1975) exposed two floating aquatic weeds,
the common duckweed Lemna minor and a floating fern Salvinia
natans, to cadmium concentrations of between 0.01 and 1.0 mg/litre
for up to 3 weeks. Growth was reduced at all concentrations but
especially at 0.05 mg/litre or more. The effect of cadmium on growth
became more marked with time. Loss of green coloration (chlorosis) was
a common symptom of cadmium toxicity, and at concentrations of 0.5 and
1.0 mg/litre Lemna plants died. When the two species were grown in
competition, growth at lower cadmium concentrations (0.01 and 0.05
mg/litre) was greater in Salvinia but less in Lemna than when the
plants were grown alone.
In a study by Nir et al. (1990), water hyacinth plants were
exposed to cadmium concentrations of 0, 0.05, 0.1, 0.4 or 1.0 mg/litre
for 7 days. Concentrations of 0.1 mg/litre or less had no significant
effect on wet or dry biomass gain or on chlorophyll a content.
Concentrations of 0.4 or 1.0 mg/litre significantly reduced both wet
biomass gain and chlorophyll a content but had no significant effect
on dry biomass gain. The chlorophyll a content of leaves decreased
with time in plants exposed to 0.4 mg/litre. After 3 weeks of
exposure, the chlorophyll a levels were 75% lower than in control
plants.
6.2 Toxicity to aquatic invertebrates
Cadmium is moderately to highly toxic to aquatic invertebrates
(see Tables 9 and 10). Its toxic effect is dependent on a variety of
environmental variables. Factors that reduce the free ionic
concentration, e.g., water hardness, salinity, chelating agents, and
high organic content of water, tend to reduce the toxic effect of
cadmium. The presence of zinc increases the toxic effect of cadmium on
invertebrates.
6.2.1 Acute and short-term toxicity
The acute toxicity of cadmium to aquatic invertebrates, as
assessed in laboratory tests, is summarized in Tables 9 and 10. The
most notable features are the variability in cadmium toxicity between
different organisms and the effects of temperature, salinity, and
water hardness. There is considerable variation even amongst closely
related species.
In a study by Canton & Slooff (1982), the water flea Daphnia
magna was exposed to cadmium over a 48-h period. At a water hardness
of 1 mmol/litre there was no mortality at 16 µg cadmium per litre,
while at a water hardness of 2 mmol/litre there was no mortality or
abnormal behaviour at 17 µg/litre.
Clubb et al. (1975b) investigated the toxicity of cadmium to nine
species of aquatic insects, but seven of the species tested were too
insensitive to the effects of the metal for the LC50 to be
determined. The insensitive species were Atherix variegata,
Hexatoma sp., Holorusia sp., Acroneuria pacifica, Arcynopteryx
signata, Pteronarcys californica, and Brachycentrus americanus;
these species represented Dipterans (true flies), Plecoptera
(stoneflies), and Tricoptera (caddis flies).
Table 9. Toxicity of cadmium to marine or estuarine invertebrates
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Purple sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 0.5 (0.4-0.6) m Dinnel et al. (1989)
(Strongylocentrotus
purpuratus)
Green sea urchin embryo stat 8.2-8.4 30 7.8-8.1 120 1.8 (1.5-2.2) m Dinnel et al. (1989)
(Strongylocentrotus
droebachiensis)
Sand dollar embryo stat 12.5-13.0 30 8.0-8.1 72 7.4 (5.2-10.8) m Dinnel et al. (1989)
(Dendraster
excentricus)
Starfish 24.5 g stat 20 20 8.0 24 12 n Eisler (1971)
(Asterias forbesi) 24.5 g stat 20 20 8.0 48 1.0 n Eisler (1971)
24.5 g stat 20 20 8.0 96 0.82 n Eisler (1971)
11.2 g stat 20 20 7.8 24 71 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 48 7.1 n Eisler & Hennekey (1977)
11.2 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
American oyster embryo stat 26 25 7.0-8.5 48 3.8 (2.85-4.48) n Calabrese et al. (1973)
(Crassostrea
virginica)
Mussel stat 18.5 32.9 7.9 96 1.62 (1.19-2.22) m Ahsanullah (1976)
(Mytilus edulis
planulatis)
Blue mussel 4 g stat 20 8.0 24 > 200 n Eisler (1971)
(Mytilus edulis) 4 g stat 20 8.0 48 165 n Eisler (1971)
4 g stat 20 8.0 96 25 n Eisler (1971)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Soft-shell clam 5.2 g stat 20 20 8.0 24 > 20 n Eisler (1971)
(Mya arenaria) 5.2 g stat 20 20 8.0 48 50 n Eisler (1971)
5.2 g stat 20 20 8.0 96 2.2 n Eisler (1971)
4.6 g stat 20 20 7.8 24 32 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 48 2.5 n Eisler & Hennekey (1977)
4.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Bay scallop 20-30 mm stat 20 25 8.0 24 8.2 n Nelson et al. (1976)
(Argopecten 20-30 mm stat 20 25 8.0 48 3.21 n Nelson et al. (1976)
irradians) 20-30 mm stat 20 25 8.0 72 2.18 n Nelson et al. (1976)
20-30 mm stat 20 25 8.0 96 1.48 (0.95-2.31) n Nelson et al. (1976)
Atlantic oyster 0.6 g stat 20 20 8.0 24 158 n Eisler (1971)
drill 0.6 g stat 20 20 8.0 48 28 n Eisler (1971)
(Urosalpinx 0.6 g stat 20 20 8.0 96 6.6 n Eisler (1971)
cinerea)
Eastern mud snail 0.56 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Nassarius 0.56 g stat 20 20 8.0 48 125 n Eisler (1971)
absoletus) 0.56 g stat 20 20 8.0 96 10.5 n Eisler (1971)
Ragworm 8 g stat 20 20 8.0 24 25 n Eisler (1971)
(Nereis virens) 8 g stat 20 20 8.0 48 25 n Eisler (1971)
8 g stat 20 20 8.0 96 11 n Eisler (1971)
7.6 g stat 20 20 7.8 24 56 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 48 9.3 n Eisler & Hennekey (1977)
7.6 g stat 20 20 7.8 96 0.7 n Eisler & Hennekey (1977)
Copepod nauplius stat 22 10 96 0.06 (0.001-0.2) m Roberts et al. (1982)
(Eurytemora affinis)
Copepod adult stat 22 10 96 0.38 (0.006-1.52) m Roberts et al. (1976)
(Acartia tonsa)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Harpacticoid
copepod adult stat 20-22 3 96 0.43 (0.31-0.55) Bengtsson & Bergstrom (1987)
(Nitocra spinipes) adult stat 20-22 7 96 0.66 (0.53-0.82) Bengtsson & Bergstrom (1987)
adult stat 20-22 15 96 0.78 (0.41-120) Bengtsson & Bergstrom (1987)
Marine amphipod young stat 10 96 3.5 m Wright & Frain (1981a)
(Marinogammarus adult stat 10 96 13.3 m Wright & Frain (1981a)
obtusatus)
Mysid shrimp adult flow 22 20 7.3 96 0.036 (0.022-0.081) m Roberts et al. (1982)
(Neomysis adult flow 22 20 7.8 96 0.02 (0.015-0.027) m Roberts et al. (1982)
americanus)
Mysid shrimp adult stat 22 20 7.3 96 0.017 m Roberts et al. (1982)
(Mysidopsis bahia) adult stat 22 20 7.7 96 0.029 (0.013-0.043) m Roberts et al. (1982)
flow c20-28 15-23 96 0.016 (0.013-0.02) m Nimmo et al. (1978)
Shrimp stat 18.7 32.1 8.0 120 2.3 (1.05-5.06) m Ahsanullah (1976)
(Palaemonetes sp.) stat 18.7 32.1 8.0 168 1.85 (1.32-2.59) m Ahsanullah (1976)
0.38 g flow 16.8 96 6.8 (5.2-9.76) m Ahsanullah (1976)
0.16 g flow 17.8 8.1 96 6.4 (5.73-7.19) m Ahsanullah (1976)
Sandworm 0.37 g stat 18.5 32.7 8.1 168 6.4 (5.82-7.1) m Ahsanullah (1976)
(Neanthes vaali)
Sand shrimp 0.25 g stat 20 20 8.0 24 2.4 n Eisler (1971)
(Crangon 0.25 g stat 20 20 8.0 48 0.5 n Eisler (1971)
septemspinosa) 0.25 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Sand shrimp adult flow 10.2 28.6 7.9 96 2.3 (1.7-5.1) m Dinnel et al. (1989)
(Crangon spp.)
Table 9 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre)b
Grass shrimp 0.33 g stat 20 20 8.0 24 43 n Eisler (1971)
(Palaemonetes 0.33 g stat 20 20 8.0 48 3.7 n Eisler (1971)
vulgaris) 0.33 g stat 20 20 8.0 96 0.32 n Eisler (1971)
Shrimp stat 35 96 2.07 (± 0.22) m McClurg (1984)
(Penaeus indicus)
Pink shrimp flow 25 20 96 4.6 m Bahner & Nimmo (1975)
(Penaeus duorarum)
Grapsid crab 1.47 g stat 17.8 32.6 8.1 168 14 (11.2-17.5) m Ahsanullah (1976)
(Paragrapsus 1.08 g stat 17.1 168 16.7 (15.11-18.45) Ahsanullah (1976)
quadridentatus)
Hermit crab 0.47 g stat 20 20 8.0 24 > 200 n Eisler (1971)
(Pagurus 0.47 g stat 20 20 8.0 48 3.7 n Eisler (1971)
longicarpus) 0.47 g stat 20 20 8.0 96 0.32 n Eisler (1971)
0.5 g stat 20 20 7.8 24 15 n Eisler & Hennekey (1977)
0.5 g stat 20 20 8.0 96 1.3 n Eisler & Hennekey (1977)
Shore crab 5.9 g stat 20 20 8.0 24 100 n Eisler (1971)
(Carcinus maenus) 5.9 g stat 20 20 8.0 48 16.6 n Eisler (1971)
5.9 g stat 20 20 8.0 96 4.1 n Eisler (1971)
Dungeness crab zoea stat 8.5 30 8.1 96 0.2 (0.1-0.4) m Dinnel et al. (1989)
(Cancer magister)
Squid larva stat 8.6 30 8.1 96 > 10.2 m Dinnel et al. (1989)
(Loligo opalescens)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b organisms exposed to cadmium added as cadmium chloride; m = measured; n = nominal
c intermittent flow-through conditions
Table 10. Toxicity of cadmium to freshwater invertebrates
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Snail adult stat 20-22 6.7 24 7.6 n Wier & Walter (1976)
(Physa adult stat 20-22 6.7 48 4.25 n Wier & Walter (1976)
gyrina) adult stat 20-22 6.7 96 1.37 n Wier & Walter (1976)
adult stat 20-22 6.7 228 0.83 n Wier & Walter (1976)
immature stat 20-22 6.7 48 0.69 n Wier & Walter (1976)
immature stat 20-22 6.7 96 0.41 n Wier & Walter (1976)
Snail flow b 15 7.1-7.7 168 0.114 m Spehar et al. (1978a)
(Physa integra)
Snail 10-12 mm flow 12 128-176 7.7 24 4.4 m Williams et al. (1985)
(Physa 10-12 mm flow 12 128-176 7.7 48 2.1 m Williams et al. (1985)
fontinalis) 10-12 mm flow 12 128-176 7.7 96 0.8 m Williams et al. (1985)
Isopod 8-10 mm flow 12 128-176 7.7 24 13 m Williams et al. (1985)
(Asellus 8-10 mm flow 12 128-176 7.7 48 3.6 m Williams et al. (1985)
aquaticus) 8-10 mm flow 12 128-176 7.7 96 0.6 m Williams et al. (1985)
Scud 10 96 0.12 m Wright & Frain (1981b)
(Gammarus 8-12 mm flow 12 128-176 7.7 24 1.6 m Williams et al. (1985)
pulex) 8-12 mm flow 12 128-176 7.7 48 0.4 m Williams et al. (1985)
8-12 mm flow 12 128-176 7.7 96 0.02 m Williams et al. (1985)
Water flea adult stat 10 0.85 meq/ 7.2 48 0.055 e (0.032-0.095) n Baudouin & Scoppa (1974)
(Daphnia hyalina) litre
Water flea 1 day stat 20 7.6-7.7 24 3 n Kuhn et al. (1989)
(Daphnia < 1 day stat 20-22 110-130 7.8 48 0.04 (0.02-0.07) n Hall et al. (1986)
magna) < 1 day stat 20-22 190-210 7.7 48 0.08 (0.06-0.1) n Hall et al. (1986)
< 1 day stat 18-20 1 mmol/ 48 0.03 m Canton & Slooff (1982)
litre
Table 10 (contd).
Organism Size/ Stat/ Temperature Hardness c pH Duration LC50d Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Water flea < 1 day stat 20-22 110-130 7.8 48 0.07 n Hall et al. (1986)
(Daphnia < 1 day stat 20-22 110-130 7.7 48 0.1 (0.07-0.12) n Hall et al. (1986)
ulex) < 1 day stat 19-22 7.7 96 0.047 (0.042-0.052) n Bertram & Hart (1979)
Copepod adult stat 10 0.85 meq/ 7.2 48 3.8 e (2.3-6.3) n Baudouin & Scoppa (1974)
(Cyclops abyssorum) litre
Copepod adult stat 10 0.85 meq/ 7.2 48 0.55 e (0.39-0.77) n Baudouin & Scoppa (1974)
(Eudiaptomus padanus) litre
Crayfish flow 19-21 24-28 6.7-7.0 96 6.1 (4.7-7.9) m Mirenda (1986)
(Orconectes virilis)
Mayfly flow 10 7.8 96 28 m Clubb et al. (1975b)
(Ephemerella grandis
grandis)
Midge 10-12 mm flow 12 128-176 7.7 96 300 m Williams et al. (1985)
(Chironomus riparius)
Stonefly flow 10 7.8 96 18 m Clubb et al. (1975b)
(Pteronarcella badia)
Stonefly 10-15 mm flow 12 128-176 7.7 96 520 m Williams et al. (1985)
(Hydropsyche
angustipennis)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained) unless stated otherwise
b intermittent flow-through conditions
c hardness expressed as mg CaCO3/litre unless stated otherwise
d organisms exposed to cadmium added as cadmium chloride unless otherwise stated; m = measured concentration; n = nominal concentration
e organism exposed to cadmium as cadmium sulfate
Spehar et al. (1978a) reported a decreased survival of the water
snail Physa integra at a cadmium concentration of 85.5 µg/litre
after 7 days of exposure. After 21 days of exposure, survival was
significantly decreased at 27.5 µg/litre, the next lowest
concentration tested. Some snails exposed to these concentrations
developed a condition in which the animal was extended from the shell
but unable to attach the foot or crawl. A white mucus layer covered
the exposed foot region of some snails and these subsequently died.
Concentrations tested were not high enough to obtain a 4-day LC50
value but the 7-day LC50 of 114 µg/litre was approximately 11 times
higher than the 28-day LC50 value of 10.4 µg/litre.
Mirenda (1986) reported a 14-day LC50 of 0.7 mg cadmium per
litre for the crayfish Orconectes virilis under flow-through
conditions. Pesch & Stewart (1980) estimated the 10-day LC50 for bay
scallops Argopecten irradians to be 0.53 mg/litre in flowing sea
water. The EC50 (for growth) for the same species over 42 days was
0.078 mg/litre. Byssal thread detachment, which precedes death, showed
an EC50 of 0.54 mg/litre of cadmium 8 days into the test and before
there was any appreciable mortality. Robinson et al. (1988) compared
10-day LC50 values for freshly collected and cultured infaunal
amphipods ( Rhepoxynius abronius). Cultured amphipods appeared normal
and survived well (93%) under control toxicity test conditions, but
were more sensitive to cadmium in sediment (10-day LC50 = 4.4 mg/kg)
than were freshly caught amphipods (10-day LC50 = 8.7 mg/kg).
When Winner (1988) exposed Daphnia magna and Ceriodaphnia
dubia to cadmium for 7 days, the most sensitive indicators were mean
body length of primiparous females in D. magna, which was
significantly reduced at 2 µg cadmium/litre, and the total young per
female in C. dubia, significantly reduced at 1 µg/litre.
6.2.1.1 Effects of temperature and salinity on acute toxicity
An increase in toxicity as temperature increases and as salinity
decreases is valid for all organisms that have been tested with these
variables (Tables 9 and 10).
O'Hara (1973) investigated the effects of temperature and
salinity on the toxicity of cadmium to adult male and female fiddler
crabs ( Uca pugilator). Mortality was greatest at high temperatures
and low salinities in tests lasting 240 h. LC50 values varied from
2.9 mg/litre for the lowest salinity (10%) and highest temperature (30
°C) to 47.0 mg/litre for the highest salinity (30%) and lowest
temperature (10 °C). Frank & Robertson (1979) exposed the blue crab
( Callinectes sapidus) to cadmium chloride at salinities of 1, 15,
and 35%. Like O'Hara, they found a decrease in cadmium toxicity with
increase in salinity. For example, 96-h LC50 values were 0.32, 4.7,
and 11.6 mg cadmium/litre for the three salinities, respectively.
Rosenberg & Costlow (1976) reported increased cadmium toxicity during
larval development of two estuarine crab species as salinity decreased
and increased toxicity as temperature increased.
Voyer & Modica (1990) found the same pattern with the mysid
shrimp Mysidopsis bahia. For salinities of 10 and 30%, the 96-h
LC50 values ranged from 15.5 to 28 µg cadmium/litre at a temperature
of 25 °C and from 47 to 84 µg/litre at a temperature of 20 °C. At 30
°C the 96-h LC50 was < 11 µg/litre at both salinities. However,
when Robert & His (1985) exposed embryos and larvae of the Japanese
oyster Crassostrea gigas to cadmium concentrations of up to 50
µg/litre at various salinities (20 to 35%), decreasing the salinity
severely affected the development of the oysters but cadmium had no
effect.
At temperatures higher than 11 °C, the combined effect of
temperature and cadmium caused a heavy stress to the copepod Tisbe
holothuriae so that the effects of salinity were masked
(Verriopoulos & Moraitou-Apostolopoulou, 1981).
6.2.1.2 Effect of water hardness
Using either artificial hard water (hardness: 180 mg CaCO3 per
litre) or dechlorinated tap water (hardness: 60 mg/litre),
Niederlehner et al. (1984) conducted short-term tests on the effects
of cadmium on the freshwater oligochaete Aeolosoma headlyi.
Mortality and population growth/maintenance were assessed over 10 to
14 days. The authors established NOEL values for population growth of
32.0 and 53.6 µg/litre for hard water (two replicate tests), whereas
the NOEL for the softer water was 17.2 µg/litre. The 48-h LC50
values were 4.98 and 1.2 mg/litre, respectively, for hard and softer
water.
6.2.1.3 Effect of organic materials and sediment
When Schuytema et al. (1984) exposed Daphnia magna to cadmium
for a period of 48 h, the mean LC50 value was 39 µg/litre in water
and 91 µg/litre in a water-sediment slurry. Giesy et al. (1977) found
that cadmium was more toxic to water fleas ( Simocephalus serrulatus)
exposed in well water with a low organic content than to those exposed
in pond water with a high organic content. The authors isolated a
series of organic fractions from the pond water by ultrafiltration.
Protection against cadmium toxicity was afforded by fractions of
intermediate relative molecular mass (ranging from approximately 500
to 300 000 daltons). The fraction with a relative molecular mass in
excess of 300 000 daltons marginally increased the toxicity of
cadmium.
Kemp & Swartz (1988) compared the acute toxicity of interstitial
and particle-bound cadmium to the marine infaunal amphipod
Rhepoxynius abronius. The cadmium concentration in interstitial
water was kept constant whereas the sediment cadmium level was varied
by using perfusion through the sediment with peristaltic pumps. The
principal cause of toxicity was found to be cadmium dissolved in
interstitial water, between 70 and 88% of the toxicity being
predictable from interstitial water concentrations.
6.2.1.4 Lifestage sensitivity
When Calabrese et al. (1973) investigated the toxicity of cadmium
to embryos of the American oyster Crassostrea virginica, there was
no mortality at 1 mg/litre and the 48-h LC50 and LC100 values were
3.8 and 6 mg/litre, respectively. Johnson & Gentile (1979) found the
larva of the American lobster Hommarus americanus to be sensitive to
cadmium; the 96-h LC50 in static bioassays was 78 µg/litre. The
mortalities after 96 h at concentrations of 10 and 30 µg/litre were 3%
and 10%, respectively. There is a very steep increase in toxicity of
cadmium to lobster larvae between 24 and 96 h. The 24-h LC50 is
approximately 1 mg/litre; at this concentration the mortality reaches
100% within 48 h.
Verriopoulos & Moraitou-Apostolopoulou (1982) found that the
different life-stages of the copepod Tisbe holothuriae showed
differences in sensitivity to cadmium. One-day-old nauplius larvae of
the copepod were the most sensitive with an LC50 of 0.538 mg/litre,
expressed as ions of cadmium, while 5-day-old nauplii showed an LC50
of 0.645 mg/litre. The value for 10-day-old copepodids (0.906
mg/litre) was not significantly different from that for adult females
(0.916 mg/litre). Females with ovigerous sacs were slightly more
sensitive, with an LC50 of 0.873 mg/litre. When Robinson et al.
(1988) exposed the infaunal phoxocephalid amphipod Rhepoxynius
abronius to sediment contaminated with cadmium, the 10-day LC50
values were 8.2 mg/kg for juveniles and 11.5 mg/kg for adults.
Nebeker et al. (1986) exposed Daphnia magna of different ages
to cadmium for a period of 48 h. Mean EC50 (immobilization) values
ranged from 23 µg/litre for 6 day-old water fleas to 164 µg/litre for
2-day-old Daphnia. Tests on Daphnia of different ages, conducted
in water of different hardness (32 or 76 mg CaCO3 per litre), with
or without feeding and in two different sizes of container, resulted
in a wide range of EC50 values (4 to 307 µg/litre). There was no
consistent effect of any of these variables other than the age of the
test animals. Very young animals were relatively tolerant, with a mean
EC50 value of 109 µg/litre.
McCahon et al. (1989) exposed both cased and uncased 1st, 3rd,
and 4th larval instars of the caddis fly Agapetus fuscipes to
cadmium chloride. The LC50 values ranged from 295 to > 1000 mg
cadmium/litre at 24 h and from 50 to 320 mg/litre at 96 h. First
instar larvae were significantly more sensitive than 3rd or 4th instar
larvae and at all ages cased animals were more resistant than uncased.
6.2.1.5 Other factors affecting acute and short-term toxicity
Chandini (1988; 1989) found that increasing the food source of
the cladocerans Daphnia carinata and Echinisca triserialis greatly
reduced the toxicity of cadmium, expressed as the 48-h or 96-h LC50,
and the effect of cadmium on various other life history parameters,
such as fecundity, growth, and age at first reproduction.
Verriopoulos & Moraitou-Apostolopoulou (1981) exposed adult
females of the copepod Tisbe holothuriae to cadmium and found that
the oxygen concentration in the water was negatively related to
mortality and that population density was positively related after
cadmium exposure. Clubb et al. (1975a) showed that the toxicity of
cadmium to aquatic insects decreased with decreasing dissolved oxygen
levels in the test water.
McCahon et al. (1988) exposed the amphipod Gammarus pulex to
cadmium chloride under static conditions and reported a 96-h LC50 of
0.05 mg cadmium/litre. The acute toxicity of cadmium to G. pulex
parasitized by the acanthocephalan Pomphorhynchus laevis at several
levels of infection was investigated. Toxicity, expressed as LC50,
did not differ significantly between uninfected and infected
amphipods.
Kay & Haller (1986) fed water hyacinth weevils Neochetina
eichhorniae on water hyacinths containing cadmium from previous
exposure to the metal. There was no mortality in weevils fed on leaves
containing up to 17.2 mg cadmium/kg. At this exposure level, the
weevils accumulated a cadmium body burden of 36.67 mg/kg over 20 days.
6.2.2 Long-term toxicity
In a study by Zaroogian & Morrison (1981), adult and larval
oysters of the species Crassostrea virginica were exposed to cadmium
at concentrations of 5 or 15 µg/litre. Some adults were exposed (for
35 or 37 weeks) to cadmium at these two concentrations prior to
spawning, and larvae from these pre-treated adults and from control
treated adults were reared in either control sea water or sea water
containing 5 or 15 µg/litre cadmium. In all there were 11 treatment
combinations. The highest larval mortality occurred when larvae from
parents treated with 15 µg/litre were reared in sea water containing
15 µg/litre for 3 weeks. However, larvae that survived this treatment
grew to lengths not significantly different from controls. The effects
observed with other treatment combinations were only temporary. Growth
and development were slowed but those larvae that survived ultimately
developed normally and to the same size as controls.
When Holcombe et al. (1984) exposed snails, from embryos through
to adult sexual maturity, to cadmium chloride, there was a delayed
hatch, a reduction in percentage hatch and survival, and reduced
growth when compared to controls. Based on these effects, the authors
suggested a maximum acceptable cadmium concentration in water of
between 4 and 8 µg/litre from one test and between 2 and 5 µg/litre
from a replicate test.
Lussier et al. (1985) conducted life-cycle tests, over 35 days,
on the mysid shrimp Mysidopsis bahia. Cadmium affected survival
primarily and no reproductive effects were noted at sublethal
concentrations.
Following a long-term investigation in the laboratory and in the
field, Marshall (1979) suggested a chronic LC10 for the water flea
Daphnia galeata mendotae of 0.15 µg cadmium/litre. Winner (1986)
exposed Daphnia pulex chronically (21 or 42 days) to cadmium, added
as cadmium sulfate, under different water conditions. Increasing the
water hardness from 58 to 116 mg/litre reduced the toxic effect of the
cadmium but a further increase to 230 mg/litre had no further effect.
The most sensitive aspect of the Daphnia life history to cadmium was
the abortion rate of young. Humic acid had no effect on this parameter
in soft or medium-hard water but increased the toxic effect of cadmium
in hard water. Mortality was increased by humic acid (0.75 or 1.50
mg/litre) at all water hardness levels. van Leeuwen et al. (1985)
calculated 14-day and 21-day LC50S for Daphnia magna of 24 and 14
µg cadmium/litre, respectively. No effect on mortality was seen at 3.2
µg/litre. The lowest concentrations producing significant mortality of
young amphipods during 6-week exposures to cadmium were 1 µg/litre for
Hyalella azteca and 3.2 µg/litre for Gammarus fasciatus (Borgmann
et al., 1989b).
6.2.3 Reproductive effects
Mysing-Gubala & Poirrier (1981) conducted laboratory experiments
on the effect of cadmium on the freshwater sponge Ephydatia
fluviatilis. Sponge cuttings were exposed to cadmium concentrations
ranging from 0.001 to 1.0 mg/litre for 1 month. At the end of the
experimental period, the cuttings were classified in four groups
depending on whether the sponge survived, whether it produced asexual
reproductive gemmules, and whether the silicaceous spicules of the
gemmules were normal or malformed. There was some effect of cadmium
even at concentrations as low as 0.001 mg/litre, 17% of the sponge
cuttings showing no gemmule production and 33% showing malformed
spicules. At concentrations of 0.5 and 1.0 mg/litre, all of the sponge
cuttings died.
Lee & Xu (1984) investigated the effects of cadmium at 0.5 and
0.1 mg/litre on the fertilization and development of sea urchin eggs
and the development of Amphioxus. At both cadmium concentrations,
sea urchin development was normal to the gastrulation stage but all
the plutei were abnormal. The effects on Amphioxus development were
different; cleavage of eggs was not affected by cadmium at 0.5 or 0.1
mg/litre but neurulation was. Dinnel et al. (1989) exposed sperm from
various sea urchin species to cadmium chloride for 60 min and assessed
fertilization success; EC50 values ranged from 12 to 26 mg/litre. An
EC50 of 8 mg/litre was calculated for the sand dollar Dendraster
excentricus. Den Besten et al. (1989) exposed the sea star Asterias
rubens to cadmium chloride at a concentration of 25 µg cadmium/litre
for 5 months. No effect on spermatozoa was found, but maturation of
oocytes was delayed and early development of embryos was adversely
affected.
Conrad (1988) studied the effect of cadmium on newly fertilized
eggs of the mud snail Ilyanassa obsoleta. No apparent effect was
observed at a cadmium concentration of 10-6 mol/litre. The minimum
concentrations producing abnormal veliger development and abnormal
late cleavage and stopping early cleavage were 10-5-10-4
mol/litre, 10-3 mol/litre, and 10-3 mol/litre, respectively.
Biesinger & Christensen (1972) estimated a 3-week 16%
reproductive impairment concentration of 0.17 µg cadmium/litre.
In a 21-day reproduction test on Daphnia magna, Kuhn et al.
(1989) determined a nominal NOEL of 0.6 µg Cd2+/litre, reproduction
rate being the most sensitive parameter. A nominal NOEL of 1 µg/litre
was found when daphnids were exposed to the cadmium chloride salt.
Reproduction of Daphnia magna was completely inhibited at
concentrations exceeding 3.2 µg/litre and time-dependant survival and
reproduction were significantly reduced at 1.8 µg/litre. No effects on
reproduction were observed at 1 µg/litre (van Leeuwen et al., 1985).
When Bertram & Hart (1979) exposed the cladoceran Daphnia pulex to
cadmium concentrations of 1 to 30 µg/litre, there was no significant
effect on the number of days required for onset of reproduction or on
its frequency. At 1 µg/litre no significant effect on longevity of
individuals was observed, but at concentrations of 5 µg/litre or more
there was a significant reduction. All cadmium concentrations caused
a reduction in the average brood size, the average number of broods
per adult, and the total number of progeny. The authors also exposed
daphnids to cadmium-contaminated food in the form of the phytoplankton
Chlorella, the cadmium concentration being 0.3-0.6 µg cadmium/litre
of medium. This resulted in a significant reduction in the average
number of broods per adult and the average brood size. However, there
was no significant effect on average longevity of individuals, the
percentage of adults producing broods, the average number of days to
the first brood or the average number of days between broods.
Bengtsson & Bergstrom (1987) exposed newly fertilized female
harpacticoids ( Nitocra spinipes) to cadmium chloride at two
salinities for 13 days. At 3% the fecundity EC50 was 37-46 µg/litre
at a salinity of 3% and 6-15 µg/litre at 15%.
Williams et al. (1987) provided water containing nominal cadmium
chloride concentrations of 0, 0.3, 30, 100 or 300 mg cadmium/litre
cadmium to newly-emerged adults of the midge Chironomus riparius in
which they could lay their eggs. The females preferred to lay their
eggs in water with no cadmium or in the lower concentrations;
significant preferences were recorded. Eggs of Chironomids are laid
within a protective gelatinous matrix. Eggs exposed to cadmium after
complete formation of the matrix in control water were unaffected (the
hatch was 80-100%), whereas those exposed after removal of the matrix
had a reduced hatching rate (60%) at all test concentrations. Eggs
laid directly in water containing cadmium were unaffected by a
concentration of 0.3 mg/litre, but hatching rates were reduced to 45%
at 30 mg/litre, 8% at 100 mg/litre, and 0% at 300 mg/litre. Clearly,
cadmium only affects the unprotected egg, either when it is newly laid
and before its gelatinous protection has developed or when this
gelatinous matrix is removed.
In a flow-through study, Hatakeyama (1987) exposed the chironomid
Polypedilum nubifer, from the egg stage, to cadmium chloride. There
was no effect on total number of adults, emergence success, sex ratio,
total number of egg clusters, oviposition success or hatchability at
10 µg cadmium/litre. There was a significant decrease in the total
number of adults and emergence success at 20 µg/litre or more and in
the total number of egg clusters at 40 µg/litre or more. At 80
µg/litre survival and reproduction were very significantly depressed.
In a separate experiment midges were fed cadmium-contaminated food
(dried yeast) at concentrations of 22, 220, and 1800 mg cadmium/kg.
Emergence success was decreased at 220 and 1800 mg/kg but no other
effects were observed.
6.2.4 Physiological and biochemical effects
Berglind (1986) investigated the effect of cadmium alone and in
combination with other metals on the delta-aminolevulinic acid
dehydratase (ALAD) activity of Daphnia magna. ALAD activity was
enhanced by cadmium alone (at 2 µg/litre but not at 0.2 µg/litre) but
this enhancement was abolished in the presence of zinc at 200
µg/litre.
Vernberg et al. (1977) investigated sub-lethal concentrations of
cadmium and their effects on the adult shrimp Palaemonetes pugio
under static and flow-through conditions. Adult shrimps were exposed
to cadmium at 50 µg/litre. This shrimp is highly tolerant to cadmium
and after exposure to 23 mg/litre the mortality rate was only 10%. The
authors found increased uptake of cadmium into the body of the shrimps
with decreasing salinity. Similarly, at higher salinity levels,
toxicity was lower. In shrimps kept at the lowest salinity level (5%),
where cadmium body burden reached 40 mg/kg, there was inhibition of
moulting. At more moderate cadmium body burdens of 23 mg/kg and 10
mg/kg, observed at salinities of 10% and 20%, respectively, moulting
was stimulated by the presence of cadmium. An investigation of the
effects of cadmium on respiratory rate was inconclusive because of
considerable variation. The authors considered that the flow-through
system more nearly approximated field conditions than the static
system. The relationship between salinity and cadmium uptake was
eliminated in a static system at 15 °C, but was quite clearly present
when the system was flow-through. After an extensive study on the
effects of cadmium on three species of shrimps, Penaeus duorarum,
Palaemonetes pugio, and Palaemonetes vulgaris, Nimmo et al. (1977)
reported sublethal histological effects and blackening and damage to
gill filaments. Placing shrimps with blackened gills in cadmium-free
water resulted in the sloughing-off of blackened portions of the
branchia and the shrimps appeared normal within 14 days. The effect on
the gills occurred with exposure to cadmium concentrations approaching
the LC50 which, with an exposure duration of 96 h, was 0.76 mg/litre
for Palaemonetes vulgaris and 3.5 mg/litre for Penaeus duorarum.
Thurberg et al. (1973) exposed two species of crabs ( arcinus maenus
and Cancer irroratus) to various concentrations of cadmium chloride
for 48 h and at five different salinities. At the end of each exposure
period, tests of blood serum osmolarity and gill tissue oxygen
consumption were performed. Cadmium increased the osmolarity of
Carcinus serum above its normal hyperosmotic state and reduced
oxygen consumption by the gill tissue of both species. Effects on
oxygen consumption were dose related over a range of cadmium
concentrations from 0 to 4 mg/litre.
Cadmium has been shown to cause an increase in oxygen consumption
rates in the mud snail Nassarius obsoletus at concentrations of
between 0.5 and 4.0 mg/litre over a 72-h exposure period (MacInnes &
Thurberg, 1973). A similar increase in oxygen consumption rate was
observed in the marine snail Murex trunculus during chronic exposure
to 0.05 mg cadmium/litre (Dalla Via et al., 1989) and in crabs
( Callinectes similis) exposed to cadmium concentrations of between
2.48 and 10.05 mg/litre for up to 96 h (Ramirez et al., 1989).
6.2.5 Behavioural effects
Olla et al. (1988) monitored the burrowing behaviour of three
polychaete species, Nereis virens, Glycera dibranchiata, and
Nephtys caeca during a 28-day exposure to a sediment concentration
of 40 mg cadmium/kg. Most comparisons of burrowing times and rates
between exposed and unexposed worms were not statistically
significant. Four out of 15 comparisons gave significant results but
these were randomly spread amongst species and exposure periods. The
authors concluded that these results would probably have little
ecological significance. The feeding behaviour of G. dibranchiata
was also monitored but no significant effect of the cadmium treatment
was found.
6.2.6 Interactions with other chemicals
Sunda et al. (1978) carried out experiments in diluted sea water
with various concentrations of the chelating agent nitrilotriacetic
acid (NTA) to determine the relationship between the chemical
speciation of cadmium and the toxicity of the metal to the grass
shrimp Palaemonetes pugio. After 4 days of exposure to a given
concentration of cadmium chloride, shrimp mortality decreased with
increasing salinity and increasing concentration of the chelating
agent. The protective effect of high salinity or NTA was attributable
to complexation of cadmium; mortality was related to the measured free
cadmium ion concentration, which was determined by measuring total
concentration of cadmium and deducting the calculated level of
complexation by either the chloride ion or NTA. The mortality at a
free cadmium ion concentration of approximately 4 x 10-7 mol/litre
was 50%. In a study of the combined effects of zinc and cadmium on the
shrimp Callianassa australiensis by Negilski et al. (1981), there
was interaction between the two metals; in combination they gave
greater mortality than would be expected if there were no interaction.
The authors demonstrated that each metal increased the accumulation of
the other.
6.2.7 Tolerance
Khan et al. (1988) exposed two different populations of grass
shrimp Palaemonetes pugio to cadmium under static conditions and
calculated 96-h LC50 values of 3.28 mg/litre for shrimps from an
industrialized area and 1.83 mg/litre for those from a non-
industrialized area. Pre-exposure of shrimps to 0.05 mg cadmium per
litre caused an increase in the LC50 values to 6.81 and 3.89
mg/litre for the two respective populations.
Moraitou-Apostalopoulou et al. (1982) collected the shrimp
Palaemon elegans from two different areas with different natural
concentrations of cadmium and investigated the sublethal effects of
the metal. They found that cadmium decreased respiration rates in the
shrimp at sublethal concentrations. Shrimps sampled from an area with
a natural cadmium concentration of 0.6 µg/litre were more tolerant to
the metal than were those from an area with 0.1 µg/litre. There was
also a difference in the acute toxicity of cadmium to the two
populations, indicating that tolerance had developed. In an earlier
study (Moraitou-Apostolopoulou et al., 1979), similar development of
tolerance was reported for a copepod Acartia clausi.
In a study of the toxicity of cadmium to the freshwater cyclopoid
copepod Tropocyclops prasinus mexicanus, Lalande & Pinel-Alloul
(1986) sampled animals from three Quebec lakes, one polluted and two
unpolluted with cadmium. The cultures from the two unpolluted lakes
showed lower LC50 values in 48-h tests than the culture from the
polluted lake. However, the polluted lake had a significantly higher
hardness (120 mg calcium carbonate per litre) than the other two lakes
(10 mg/litre).
6.2.8 Model ecosystems
Borgmann et al. (1989a) established a Daphnia-phytoplankton
model ecosystem and exposed it to cadmium sulfate at concentrations of
1, 5 and 15 µg cadmium/litre. At 5 µg/litre the Daphnia population
collapsed after 9 weeks of treatment and chlorophyll levels increased.
At 15 µg/litre the Daphnia population collapsed after 5 weeks, but
chlorophyll levels remained low. There appeared to be no effect on
this model system at 1 µg/litre.
6.3 Toxicity to Fish
The toxicity of cadmium has been studied in a variety of fish
species in both fresh and sea water at various temperatures and
dissolved oxygen concentrations. Generally, increasing dissolved salt
concentration decreases the toxicity, whereas increasing temperature
increases it. An increase in the dissolved oxygen content of the water
decreases the toxicity of cadmium to freshwater fish. Salmonids appear
to be particularly susceptible to the metal. Sublethal effects have
been reported, notably malformation of the spine.
6.3.1 Acute and short-term toxicity
The acute toxicity of cadmium to fish is summarized in Tables 11
and 12. Pickering & Henderson (1966) calculated the LC50 values for
five species of warm-water fish, some of which were tested in both
soft and hard water. There was surprisingly little difference in
fathead minnows ( Pimephales promelas) between the 24-h, 48-h, and
96-h LC50 values, which in soft water, were 1.09, 1.09, and 1.05
mg/litre, respectively. This species was very much more resistant to
cadmium in hard water with 24-h, 48-h, and 96-h LC50 values of 78.1,
72.6, and 72.6 mg/litre, respectively. The hardness for the two types
of water was 20 and 360 mg CaCO3 per litre and the alkalinity 80 and
300 mg/litre. The dissolved oxygen concentration was similar in the
two types of water. However, the pH was 7.5 for soft water and 8.2 for
hard water, this being an area of the pH range where speciation of
cadmium undergoes major change. Bluegill sunfish, goldfish, and
guppies showed a decrease in LC50 with increase in exposure duration
from 24 to 96 h (Table 12), but these were tested only in soft water.
The green sunfish Lepomis cyanellus showed LC50 values in soft
water of 7.84, 3.68, and 2.84 mg/litre with exposure durations of 24
h, 48 h, and 96 h, respectively. This species was also tested in hard
water, where, like the fathead minnow, it showed considerably less
cadmium toxicity. The 24-h LC50 rose from 7.84 in soft water to 88.6
mg/litre in hard water.
Pickering & Gast (1972) determined a maximum acceptable toxicant
concentration (MATC) for the fathead minnow Pimephales promelas of
between 37 and 57 µg cadmium/litre. The experimental concentration of
57 µg/litre decreased survival of the developing embryos, this being
the most sensitive life-stage, but at lower concentrations (between
4.5 and 37 µg/litre) no adverse effect was found on survival, growth
or reproduction. Carroll et al. (1979) investigated the protective
effects of various constituents of hard water on the toxicity of
cadmium to the brook trout ( Salvelinus fontinalis) and concluded
that calcium, added as either the sulfate or carbonate, was the most
significant source of protection. This protective effect was observed
in the absence of significant cadmium precipitation. Magnesium,
sulfate, and sodium ions and the carbonate system provided little or
no protection. Calamari et al. (1980) found an influence of water
hardness on the toxicity of cadmium to Salmo gairdneri; 48-h LC50
values increased from 91 µg/litre, with a hardness of 20 mg/litre in
the test water, to 3700 µg/litre at a water hardness of 320 mg/litre.
The 48-h LC50 of fish acclimatized to a hardness of 320 mg/litre and
then tested at a hardness of 20 mg/litre was about 7 times higher than
that of fish acclimatized and tested in the same soft water. There are
two types of biological effects of hardness on the availability of
cadmium to fish; one of them persists after acclimatization in hard
water.
Table 11. Toxicity of cadmium to marine or estuarine fish
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Striped killifish 0.95 g stat 20 20 8.0 24 125 n Eisler (1971)
(Fundulus majalis) 0.95 g stat 20 20 8.0 48 59 n Eisler (1971)
0.95 g stat 20 20 8.0 96 21 n Eisler (1971)
Mummichog 0.89 g stat 20 20 8.0 24 > 100 n Eisler (1971)
(Fundulus 0.89 g stat 20 20 8.0 48 > 100 n Eisler (1971)
heteroclitus) 0.89 g stat 20 20 8.0 96 55 n Eisler (1971)
1.3 g stat 20 20 7.8 24 220 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 96 22 n Eisler & Hennekey (1977)
1.3 g stat 20 20 7.8 168 22 n Eisler & Hennekey (1977)
1 day stat 20 20 48 16.2 (12.7-21.2) n Middaugh & Dean (1977)
7 days stat 20 20 48 9 (6.4-12.5) n Middaugh & Dean (1977)
14 days stat 20 20 48 32 (24.6-41.6) n Middaugh & Dean (1977)
adult stat 20 20 48 60 (40-90) n Middaugh & Dean (1977)
1 day stat 20 30 48 23 (19.2-27.6) n Middaugh & Dean (1977)
7 days stat 20 30 48 12 (9.2-15.6) n Middaugh & Dean (1977)
14 days stat 20 30 48 7.8 (5.6-10.3) n Middaugh & Dean (1977)
adult stat 20 30 48 43 (33-56) n Middaugh & Dean (1977)
Sheepshead minnow 1.1 g stat 20 20 8.0 24 100 n Eisler (1971)
(Cyprinodon 1.1 g stat 20 20 8.0 48 50 n Eisler (1971)
variegatus) 1.1 g stat 20 20 8.0 96 50 n Eisler (1971)
Coho salmon smolt flow 11.2 28.3 7.9 96 1.5 (1.2-2.4) m Dinnel et al. (1989)
(Oncorhynchus
kisutch)
Yellow-eye mullet 1.49 g flow 18.5 34.5 7.8 58 15.5 (12.2-19.8) m Negilski (1976)
(Aldrichetta 1.15 g flow 18.6 34.8 7.8 120 14.3 (8.4-24.3) m Negilski (1976)
forsteri)
Small-mouthed 1.36 g flow 17.9 34.5 7.9 168 12.7 (8.3-19.4) m Negilski (1976)
hardyhead 1.12 g flow 18.0 34.5 7.8 168 16.6 (13.3-20.7) m Negilski (1976)
(Atherinasoma microstoma)
Table 11 (contd).
Organism Size/ Stat/ Temperature Salinity pH Duration LC50 Reference
age flow a (°C) (%) (h) (mg/litre) b
Atlantic silverside adult stat 20 20 48 13 (9-20) n Middaugh & Dean (1977)
(Menidia menidia) adult stat 20 30 48 12 (8-16) n Middaugh & Dean (1977)
Tidewater silverside larva stat 26 22 96 0.31 (0.25-0.38) n Mayer (1987)
(Menidia peninsulae)
Shiner perch adult flow 13 30.1 7.8 96 11 (5-20) m Dinnel et al. (1989)
(Cymatogaster aggregata)
a stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (cadmium concentration
in water continuously maintained)
b organisms exposed to cadmium added as cadmium chloride; m = measured concentration; n = nominal concentration
Table 12. Toxicity of cadmium to freshwater fish
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Chinook salmon juvenile flow 11-13 20-22 7.0-7.3 96 0.001 (± 0.0007) f m Finlayson & Verrue (1982)
(Onchorhynchus
tshawytscha)
Rainbow trout juvenile flow 6.4-8.3 96 0.0066 g m Hale (1977)
(Salmo 5-15 g stat 8.5-10.7 61-65 7.4 48 2.9 m Pascoe et al. (1986)
gairdneri) 5-15 g stat 8.5-10.7 283-317 7.4 48 5.7 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 1.3 m Pascoe et al. (1986)
5-15 g stat 8.5-10.7 61-65 7.4 96 2.6 m Pascoe et al. (1986)
Fathead minnow adult stat 25 20 7.5 24 1.09 (0.79-2.91) n Pickering & Henderson (1966)
(Pimephales adult stat 25 360 8.2 24 78.1 (57.2-117) n Pickering & Henderson (1966)
promelas) adult stat 25 20 7.5 48 1.09 (0.79-2.91) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 1.05 (0.7-4.43) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 72.6 (52.7-105) n Pickering & Henderson (1966)
adult stat 18-22 190-210 7.7 48 0.1 (0.07-0.17) n Hall et al. (1986)
adult stat 18-22 190-210 7.7 96 0.09 (0.07-0.14) n Hall et al. (1986)
Bluegill sunfish adult stat 25 20 7.5 24 4.56 (3.64-6.08) n Pickering & Henderson (1966)
(Lepomis adult stat 25 20 7.5 48 2.76 (2.02-3.46) n Pickering & Henderson (1966)
macrochirus) adult stat 25 20 7.5 96 1.94 (1.33-2.35) n Pickering & Henderson (1966)
Goldfish adult stat 25 20 7.5 24 3.46 (2.85-4.82) n Pickering & Henderson (1966)
(Carassius adult stat 25 20 7.5 48 2.62 (2.04-3.68) n Pickering & Henderson (1966)
auratus) adult stat 25 20 7.5 96 2.34 (1.81-3.16) n Pickering & Henderson (1966)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Guppy adult stat 25 20 7.5 24 3.37 (2.73-4.81) n Pickering & Henderson (1966)
(Poecilia adult stat 25 20 7.5 48 2.31 (1.78-3.11) n Pickering & Henderson (1966)
reticulata) adult stat 25 20 7.5 96 1.27 (0.97-1.71) n Pickering & Henderson (1966)
3-4 weeks flow c 23-25 1 mM 24 10.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 48 5.7 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 72 4.3 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 1 mM 96 3.8 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 24 33 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 48 20.5 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 72 14.4 m Canton & Slooff (1982)
3-4 weeks flow c 23-25 2 mM 96 11.1 m Canton & Slooff (1982)
Green sunfish adult stat 25 20 7.5 24 7.84 (6.13-14.2) n Pickering & Henderson (1966)
(Lepomis adult stat 25 360 8.2 24 88.6 (74-106) n Pickering & Henderson (1966)
cyanellus) adult stat 25 20 7.5 48 3.68 (2.89-4.69) n Pickering & Henderson (1966)
adult stat 25 360 8.2 48 71.3 (56.3-92.2) n Pickering & Henderson (1966)
adult stat 25 20 7.5 96 2.84 (2.1-3.56) n Pickering & Henderson (1966)
adult stat 25 360 8.2 96 66 (51.7-84.4) n Pickering & Henderson (1966)
Golden shiner flow 72.2 7.5 96 2.8 (1.9-4.3) m Hartwell et al. (1989)
(Notemigonus
crysoleucas)
Puntius 2.4 g stat b 23-27 60-70 7.5 24 59.99 (58.5-61.5) n Shivaraj & Patil (1988)
arulius 2.4 g stat b 23-27 60-70 7.5 48 45.7 (43.9-47.5) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 72 41.7 (39.7-43.8) n Shivaraj & Patil (1988)
2.4 g stat b 23-27 60-70 7.5 96 39 (36.5-41.7) n Shivaraj & Patil (1988)
Table 12 (contd).
Organism Size/ Stat/ Temperature Hardness d pH Duration LC50e Reference
age flow a (°C) (mg/litre) (h) (mg/litre)
Killifish 4-5 weeks stat b 23-25 1 mM 48 > 2.8 m Canton & Slooff (1982)
(Oryzias 4-5 weeks stat b 23-25 1 mM 72 0.35 m Canton & Slooff (1982)
latipes) 4-5 weeks stat b 23-25 1 mM 96 0.35 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 24 > 2.6 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 48 1.8 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 72 0.17 m Canton & Slooff (1982)
4-5 weeks stat b 23-25 2 mM 96 0.13 m Canton & Slooff (1982)
Zebra fish 6 months flow 19-21 1.7 mM 24 7 m Canton & Slooff (1982)
(Brachydanio 6 months flow 19-21 1.7 mM 48 4.2 m Canton & Slooff (1982)
rerio)
a stat = static conditions (water unchanged for duration of test unless stated otherwise); flow = flow-through conditions
(cadmium concentration in water continuously maintained unless stated otherwise)
b static conditions but water renewed every 24 h
c intermittent flow-through conditions
d Hardness was expressed as mg CaCO3/litre unless stated otherwise
e fish were exposed to cadmium added as the chloride unless stated otherwise; m = measured concentration; n = nominal concentration
f cadmium was added as the sulfate
g cadmium was added as the nitrate
In a large scale study of the toxicity of cadmium to the
mummichog Fundulus heteroclitus, Voyer (1975) examined effects of
salinity, pre-exposure to high salinity and different concentrations
of dissolved oxygen on the tolerance of fish to cadmium over 96 h. He
found no significant influence of dissolved oxygen levels between 4.0
mg/litre and saturation regardless of salinity during acclimatization
or during the test. By contrast, Voyer et al. (1975) showed a distinct
effect of dissolved oxygen concentration on toxicity of cadmium to the
same species of fish in fresh water. Median tolerance concentrations
at 96 h ranged upwards from 1.3 to 3.0 mg cadmium/litre with 2.3 and
8.5 mg/litre of dissolved oxygen, respectively. They demonstrated
statistically an independent effect of dissolved oxygen and time
against cadmium toxicity. It should be noted that cadmium is 10 times
more toxic to this species in fresh water than in sea water.
Toxicity of cadmium to both marine (Eisler, 1971) and freshwater
(Roch & Maly, 1979) fish has been shown to be greater at higher
temperatures.
Canton & Slooff (1982) exposed several fish species to cadmium in
short-term toxicity tests. At a water hardness of 1.7 mmol/litre they
found the no-observed-adverse-effect level (NOAEL) for mortality in
the zebra fish ( Brachydanio rerio) to be 2 mg/litre over a 48-h
exposure period. For the killifish ( Oryzias latipes), the 96-h NOAEL
was 0.06 mg/litre for mortality and 0.03 mg/litre for mortality and
abnormal behaviour at a water hardness of 2 mmol/litre. The
corresponding values at a water hardness of 1 mmol/litre were 0.055
and 0.006 mg/litre. For the guppy ( Poecilia reticulata), the 96-h
NOAEL for mortality and abnormal behaviour was 5.2 mg/litre at a water
hardness of 2 mmol/litre and 0.6 mg/litre at 1 mmol/litre. The authors
calculated a 24-h NOAEL for the rainbow trout ( Salmo gairdneri) of
0.01 mg/litre for inhibition of opercular movements.
Abel & Papoutsoglou (1986) studied the toxicity of cadmium to
Cyprinus carpio and Tilapia aurea and reviewed data on other
species of freshwater fish. The found for all species examined that
the median survival time changed little over a wide range of cadmium
concentrations and that a toxic threshold was clear in most studies.
For Tilapia this threshold lay between 0.1 and 0.5 mg cadmium/litre;
negligible mortality was recorded in fish exposed to 0.1 mg/litre for
3 months.
6.3.2 Reproductive effects and effects on early life-stages
Meteyer et al. (1988) exposed sheepshead minnow ( Cyprinodon
variegatus) eggs to cadmium concentrations of between 0.39 and 1020
µg/litre from approximately 4 h after fertilization. Hatching was
delayed by up to 3 days at the highest cadmium concentration. All
treated larvae were shorter than controls, but there was no
dose-related effect of cadmium on growth.
Middaugh & Dean (1977) examined the toxicity of cadmium to
various life-stages of the mummichog Fundulus heteroclitus. In 48-h
tests, eggs were highly resistant to cadmium. The greatest effect (54%
non-emergence) occurred at a cadmium concentration of 32 mg/litre; the
control non-emergence was 17%. Newly-emerged larvae were less
sensitive to cadmium than were 7-day-old larvae. There was an effect
of salinity on the sensitivity of 14-day-old larvae to the metal
(Table 11). Adults were less sensitive to cadmium than were larvae.
Similar results were obtained with the Atlantic silverside ( Menidia
menidia), larvae being the most sensitive life-stage. Weis & Weis
(1977) found no effect of cadmium, at concentrations up to 10
mg/litre, on embryos of Fundulus heteroclitus. Rombough & Garside
(1982) found the most sensitive indicator of cadmium toxicity to early
life-stages of the Atlantic salmon ( Salmo salar) to be inhibition of
growth of alevins, where significant reductions occurred with cadmium
concentrations of 0.47 µg/litre. The LC50 for the interval between
fertilization and viable hatch lay between 300 and 800 µg per litre.
Newly hatched alevins showed a 24-h LC50 of between 1.5 and 2.4
mg/litre. Sensitivity increased sharply in late alevins and
significant mortality was recorded at a concentration of 8.2 µg/litre.
Eaton et al. (1978) exposed embryos and larvae of seven
freshwater fish species to nominal cadmium concentrations of 0, 0.4,
1.2, 3.7, 11.6, 33.3, and 100 mg/litre for periods ranging from 3 to
126 days. Actual concentrations were monitored and used in the
assessment of the results. Results were expressed in terms of
"standing crop", which the authors estimated as the product of the
proportion of fish surviving and their total biomass. The lowest
concentration of cadmium at which the standing crop was significantly
different from controls was approximately 12 µg/litre for the white
sucker, northern pike, smallmouth bass, west coast coho salmon, lake
trout, and brown trout exposed as embryos or larvae for up to 64 days.
The value was lower for brook trout (0.48 µg per litre) after exposure
of larvae/juveniles for 65 days and for Lake Superior coho salmon (3.4
µg/litre) after exposure of larvae/juveniles for 27 days. The highest
cadmium concentration at which standing crop was not significantly
different from controls varied for the seven species between 1.1 and
4.2 µg/litre.
Woodall et al. (1988) exposed rainbow trout ( Salmo gairdneri)
fry to cadmium concentrations of 0, 0.1, 1.0 or 5.0 mg/litre for up to
90 h. In preliminary experiments, they calculated that the 90-h LC50
lay between 0.1 and 1.0 mg/litre. Pre-treatment of trout fry with
cadmium initially had little effect (up to 30 h). However, with
exposure periods of between 45 and 90 h, some protection was induced
by pre-treatment. Benoit et al. (1976) exposed three generations of
brook trout ( Salvelinus fontinalis) to concentrations of total
cadmium varying between 0.06 and 6.4 µg/litre. Significant numbers of
first and second generation adult males died during spawning when
exposed to 3.4 µg/litre. This concentration also significantly
retarded the growth of juvenile second and third generation offspring,
but at a concentration of 1.7 µg/litre these effects were not seen.
In a study by Borgmann & Ralph (1986), white sucker larvae
Catostomus commersoni and young common shiners Notropis cornutus
were exposed to cadmium chloride at concentrations ranging from 6.24
to 200 µg cadmium/litre. The relative growth rate of fish was
significantly reduced at concentrations of 36 µg/litre or more in the
case of suckers and 63 µg/litre or more in the case of shiners.
Cadmium had no effect on the relative feeding rates.
Hatakeyama & Yasuno (1987) studied the chronic effects of cadmium
on the reproduction of the guppy Poecilia reticulata. The fish were
exposed to cadmium via cadmium-accumulated midge larvae used as their
food source. The cumulative numbers of fry produced by guppies fed
midge larvae containing 500, 800 or 1300 mg cadmium/kg for 6 months
decreased to 79%, 65%, and 55% of the controls, respectively. At the
highest dose, the mortality of females was significantly elevated at
6 months, but no such effect was observed with the males.
Michibata (1981) reported a protective effect of water hard-ness
against the effects of cadmium on the eggs of Oryzias latipes.
6.3.3 Metabolic, biochemical and physiological effects
Protective metal-binding proteins (metallothioneins) are induced
by cadmium in fish (chapter 4).
A manifest symptom of cadmium toxicity in freshwater fish is
ionic imbalance with reduced plasma Ca2+, Na+, and CL-. The
probable explanation is that cadmium is a potent inhibitor of
ion-transporting enzymes. Verbost et al. (1988) showed that cadmium
inhibited Ca-ATPase in the cell membranes of fish gut. It probably
does the same in the gills because cadmium exposure has been shown to
inhibit calcium uptake in the gills of adults (Verbost et al., 1987;
Reid & McDonald, 1988) as well as in larvae (Wright et al., 1985).
Similarly, cadmium has been shown to inhibit Na/K-ATPase in fish gills
(Watson & Benson 1987), which, taken with the fact that cadmium
probably also affects the production of ATP in the gills (Dickson et
al., 1982), could explain the reduction of plasma Na+. Huiguang Fu
(1989) studied the role of the hormones prolactin and cortisol in
correcting cadmium-induced impairment of the calcium balance in
tilapia ( Oreochromis mossambicus). Fish were able to recover from
initial hypocalcaemia during a 35-day exposure to a concentration of
10 µg cadmium/litre. This recovery involves prolactin-induced
stimulation of active Ca2+ uptake and reduction of passive Ca2+
efflux, together with cortisol-induced changes in chloride cells and
stimulation of metallothionein synthesis in the liver, kidney, and
gills. The author stated that the capacity to survive prolonged
exposure to 10 µg cadmium/litre through physiological adaptation does
not indicate that this cadmium concentration is acceptable to tilapia.
Adaptation may be achieved at the expense of other essential processes
like growth and reproduction.
Arillo et al. (1984) investigated the effect of cadmium at levels
of 1-10 µg/litre (concentrations above the water quality criteria
value of 0.75 µg/litre proposed for salmonids by EIFAC/FAO) on a wide
variety of biochemical parameters in the rainbow trout (Salmo
gairdneri). The exposure period was 4 months. Only at the highest
test concentration of 10 µg/litre were there any effects on the fish,
and then only on liver aminolevulinic acid dehydratase activity. The
authors concluded that the water quality criterion was realistic.
Dawson et al. (1977) exposed juvenile striped bass ( Morone
saxatilis) to cadmium chloride concentrations of 0.5, 2.5 or 5
µg/litre for 30 to 90 days, and the fish were then allowed to recover
for 30 days in clean, running sea water. There was an inhibition of
gill tissue respiration at 30 and 90 days, which recovered during the
30-day period with clean water. The activities of the various enzymes
measured were not affected. MacInnes et al. (1977) reported reduced
gill tissue oxygen consumption in cunner ( Tautogolabrus adspersus)
exposed to cadmium (0.05 or 0.1 mg/litre) for 30 or 60 days. They also
reported a reduced activity of aspartate aminotransferase and an
increased activity of glucose-6-phosphate dehydrogenase in the liver
of the fish after 30 days of exposure to cadmium. Gill & Pant (1983)
measured levels of various blood and tissue constituents after acute
(24 h) or chronic (90 day) exposure to cadmium. Acute exposure to
12.65 mg/litre led to significant hyperglycaemia and an increase in
liver, kidney, and ovarian cholesterol levels. Chronic exposure to
0.63 or 0.84 mg/litre, by contrast, led to an enduring hypoglycaemia
and diminished levels of cholesterol in tissues. Both acute and
chronic exposure to cadmium caused marked hypocholesterolaemia,
glycogenolysis in the liver and brain and a rise in myocardial
glycogen. Testis cholesterol was depleted after 60 days in both acute
and chronic exposures.
Sastry & Subhadra (1983) exposed the catfish Heteropneustes
fossilis to cadmium in the water at the sublethal concentration of
2.3 µmol/litre for 15 or 30 days. The cadmium caused reduced
absorption of glucose and fructose from the gut, this effect being
more pronounced after 30 days of exposure than after 15 days. Filling
intestinal sacs, in vivo, with cadmium solutions (1 µmol per litre)
reduced absorption of the sugars significantly over 1 h.
Merlini (1978) pre-treated immature sunfish ( Lepomis gibbosus)
with cadmium (0.004 mg/litre) and then fed treated and control fish
with a single ration containing 58Co-labelled vitamin B12. The
fish were subsequently fed non-radioactive food for 31 days before
sacrifice. The author reported that cadmium-treated fish stored
significantly less vitamin B12 in the liver than did controls.
Carrier & Beitinger (1988a) studied the effect of cadmium on the
critical thermal maximum (the temperature at which loss of equilibrium
is coupled with loss of righting response) in the red shiner
( Notropis lutrensis) and fathead minnow ( Pimephales promelas). The
red shiner was exposed to sublethal cadmium concentrations of 4.88,
5.07, and 5.46 mg/litre and the fathead minnow to 0.09, 0.48, and 1.26
mg/litre. Critical thermal maxima were significantly reduced for both
species over a 10-day exposure period. The effect was found to be dose
and time dependant. In the green sunfish ( Lepomis cyanellus) there
was no effect of cadmium concentrations of 2.76, 4.22 or 5.17 mg/litre
on the critical thermal maximum (Carrier & Beitinger, 1988b).
6.3.4 Structural effects and malformations
When Bengtsson et al. (1975) exposed 180 minnows ( Phoxinus
phoxinus) to various concentrations of cadmium ranging from 7.5
µg/litre to 4.8 mg/litre for 70 days, 31 of the 101 fish that survived
developed lesions in the spinal column. Fractured vertebrae occurred
in the caudal end of the abdominal section of the spine or in the
caudal section; 64% of all fractures occurred in the first 7 caudal
vertebrae and 21% occurred in abdominal vertebrae numbers 8 to 14.
Hiraoka & Okuda (1984) cultured medaka ( Oryzias latipes) eggs in a
cadmium solution of 0.01 mg/litre for 1 week and then investigated
abnormalities in the vertebrae of the hatched fry, which were reared
in clean water. There was no damage to the centra of the vertebrae in
newly hatched fry. However, centrum-damaged fish were found in the
first week and the numbers increased rapidly up to the 4th week after
hatch. The cumulative frequency of vertebral-damaged fish was 13% and
14% in the 5th and 6th weeks, respectively, and seemed to remain
constant after this. Muramoto (1981b) reported that fish showing
malformations of the spine after cadmium treatment had significantly
less calcium in the vertebral column than did control fish.
Voyer et al. (1975) found no short-term histopathological effects
of cadmium, but long-term exposure to 28 mg/litre caused necrosis and
sloughing of the mucosa of gills. Tissue damage was also evident in
the nasal passages and buccal cavity. This histopathological effect
was seen between exposure times of 512 and 612 h, but not before.
6.3.5 Behavioural effects
Hartwell et al. (1989) studied the avoidance response of the
golden shiner ( Notemigonus crysoleucas) to cadmium concentrations of
up to 68 µg/litre, but found no significant avoidance.
Sullivan et al. (1978b) subjected fathead minnows ( Pimephales
promelas) to acute (24 h) or subacute (21 days) exposure at
sublethal cadmium concentrations and then placed them in experimental
chambers with largemouth bass ( Micropterus salmoides), a fish which
preys upon them. The minnows displayed altered behaviour patterns,
including abnormal schooling behaviour, and were more vulnerable to
predation than controls. The lowest acute cadmium exposure level that
increased vulnerability was 0.375 mg/litre and the lowest subacute
level 0.025 mg/litre. The authors pointed out that the subacute value
of 0.025 mg/litre was well below reported no-effect levels of cadmium
for fathead minnows established with respect to survival and
reproductive effects. The avoidance threshold for cadmium in water by
the rainbow trout ( Salmo gairdneri) is 50 µg/litre (Black & Birge,
1980), which is 50 times higher than the 96-h LC50 value reported
for this species.
6.3.6 Interactions with other chemicals
Muramoto (1981a) showed that the chelating agents EDTA and DPTA
afforded some protection against the effects of cadmium on carp
( Cyprinus carpio) exposed to 0.05 or 0.1 mg cadmium/litre for 3
months. There were effects on the vertebrae of exposed fish.
Chelating agents that form hydrophobic complexes with heavy
metals increase the bioavailability of the metal to aquatic organisms.
Examples of these are xanthates and dithiocarbamates. Xanthates are
used in the mining industry in the flotation process to refine metal
from sulfide ores. As discussed in section 4.1, these compounds
increase the uptake rate of cadmium through the gills in fish (Block
& Part, 1986; Gottofrey et al., 1988; Block, 1991). Furthermore, they
change the tissue distribution of the metal in such a way that more
cadmium is found in lipid-rich tissues such as nervous tissue (brain)
and adipose tissue than when fish are exposed to the metal alone.
Finlayson & Verrue (1982) reported 96-h LC50 values for
juvenile chinook salmon ( Onchorynchus tshawytscha) ranging from 0.6
to 1.6 µg/litre. They found no synergistic or antagonistic toxic
effects after combining cadmium and zinc in their test system. The
results of tests were additive, the overall LC50 being a simple
combination of individual metal effects at a zinc:cadmium ratio of
1:0.008. Spehar et al. (1978b) exposed the flagfish Jordanella
floridae to cadmium and zinc, both individually and as a mixture, at
concentrations ranging from 4.3 to 8.5 µg cadmium/litre and 73.4 to
139 µg zinc/litre, through one complete life-cycle of the fish. There
was no additive effect of cadmium and zinc at sublethal concentrations
in mixed exposure. Effects on survival showed that the toxicity of
cadmium and zinc mixtures was only slightly, if at all, greater than
the toxicity of zinc alone. Anadu et al. (1989) reported that
pre-exposure of rainbow trout ( Salmo gairdneri) to zinc (100
µg/litre) for 17 days increased the subsequent 120-h LC50 for
cadmium from 1.1 µg/litre to 4.1 µg/litre.
6.4 Toxicity to Amphibia
Francis et al. (1984) exposed eggs of the leopard frog ( Rana
pipiens) to cadmium-enriched sediments during their development and
for 4 days after the larvae had hatched. Measured concentrations of
cadmium ranged from 1.04 to 1074 mg/kg in sediment and from 1.0 to
76.5 mg/litre in water above the sediment. Cadmium concentrations in
the tissues of the tadpoles at the end of the experiment ranged from
0.08 to 12.55 mg/kg. There was no mortality as a result of cadmium
exposure. The LC50 for this species has been reported to be 50
µg/litre for water without sediment by Westerman (1977). Slooff &
Baerselman (1980) determined 48-h LC50 values for the neotenous
larval mexican axolotl ( Ambystoma mexicanum) and larval South
African clawed toad ( Xenopus laevis) of 1.3 mg/litre and 32
mg/litre, respectively, after exposure to cadmium nitrate. Canton &
Slooff (1982) exposed Xenopus laevis to cadmium, added as cadmium
chloride, and obtained 24-h and 48-h LC50 values of 4 and 3.2
mg/litre, respectively. A NOEL of 2.2 mg/litre was reported for both
exposure periods. In a longer-term exposure (100 days), by the same
authors, an LC50 value of 1500 µg/litre was found and the EC50
value for inhibition of larval development was 650 µg/litre. For
mortality and larval development, the NOELs were 30 and 9 µg/litre,
respectively. De Zwart & Sloof (1987) determined a 48-h LC50 of 20.2
mg/litre for tadpoles of the clawed toad. Khangarot & Ray (1987)
established a 96-h LC50 value of 8.18 (6.96-9.53) mg/litre for the
tadpoles of the toad Bufo melanosticus. The test water was obtained
from a well and had a hardness of 185 mg per litre, pH 7.4, and
temperature of 31 °C. Solids were present at a level of 920 mg/litre.
Muino et al. (1990) calculated the 48-h and 96-h LC50 for Bufo
arenarum tadpoles to be 2.52 and 2.08 mg cadmium/litre for the two
exposure periods, respectively, under semi-static conditions. In tests
to study the effect of a sublethal cadmium concentration (1.0 mg
cadmium/litre) on the water balance of the animals, the authors found
that all animals died within a few hours in ion-free media. Tadpoles
exposed in ionic solutions showed mortality of less than 10%
(equivalent to control groups).
Woodall et al. (1988) exposed Xenopus laevis tadpoles to
cadmium concentrations of 0, 50, 80, and 100 mg cadmium/litre for up
to 90 h. In preliminary experiments, they calculated the 90-h LC50
to lie between 80 and 100 mg/litre. The authors found that
pre-treatment of tadpoles with cadmium induced protection, which
decreased with an increase in the subsequent exposure concentration.
Cadmium pre-treatment induced maximum protection to cadmium at a
concentration of 50 mg/litre at both 45 and 90 h.
Perez-Coll et al. (1986) exposed developing Bufo arenarum
embryos to cadmium chloride concentrations of 6 x 10-7 to 1.5 x
10-5 mol Cd2+/litre during gastrulation at 20 °C and 30 °C.
Initial failures at gastrulation resulted mainly in axial
incurvations, microcephaly, hydropsy, and abnormal tail development.
At the higher temperature, high concentrations of cadmium caused a
significant increase in early malformations and at low concentrations
the high temperature prevented alterations.
7. TOXICITY TO TERRESTRIAL ORGANISMS
Appraisal
Both terrestrial plants and animals accumulate cadmium, but the
rate of accumulation is much higher under experimental conditions,
where cadmium is available in solution, than it is with plants grown
in soil, when part of the cadmium is bound and less available.
Cadmium has adverse effects on hydroponically grown plants at
concentrations in the mg/litre range, whereas plants grown in soil
only show reduced growth in contaminated soils with hundreds of mg
cadmium/kg. Terrestrial invertebrates are relatively insensitive to
cadmium-induced toxic effects, probably due to effective
sequestration mechanisms in specific organs. When toxic effects do
occur, they consist of reduced growth and reproduction.
7.1 Toxicity to terrestrial plants
Cadmium has been shown to have an adverse effect on plant growth
and yield in laboratory experiments. However, plants grown in soil are
generally insensitive to the effects of cadmium except at high doses.
Effects are only seen when cadmium is given in nutrient solutions
rather than in soil, where the cadmium is bound and is therefore less
available to the plants. Cadmium is only available to plants in
solution in soil. There is considerable evidence from field studies
that plants are able to develop tolerance to various heavy metals in
their growth medium. Research into cadmium tolerance has been more
limited than for other metals, but there is some evidence of tolerance
developing.
7.1.1 Toxicity to plants grown hydroponically
Mitchell & Fretz (1977) cultured seedlings of three species of
tree, the white pine ( Pinus strobus), red maple ( Acer rubrum), and
Norway spruce ( Picea abies), in sand. Plants were irrigated with a
nutrient solution containing cadmium at concentrations of 0, 0.5, 1,
2, 4, 8, and 16 µg/litre; white pine seedlings were also treated with
32 and 64 µg/litre. Both roots and foliage were affected by the
cadmium. In the red maple symptoms of cadmium toxicity began with
interveinal chlorosis and stunting of leaves in most cases. As
exposure increased cadmium caused wilting and then death. The first
observed effect of the metal on the pine was inhibition of needle
expansion and, in the case of the spruce, chlorotic tips to new
growth. Tissue accumulation of cadmium correlated well with exposure.
Red maple, white pine, and spruce exhibited foliage effects at leaf
cadmium concentrations of 22.8, 61.3, and 7.5 mg/kg, respectively,
corresponding to nutrient solutions of 8.0, 32.0, and 4.0 µg/litre.
There was an increasing effect on root development with increasing
exposure to cadmium. At the higher doses, there was severe reduction
in the number of roots initiated and stunting of those that grew.
Accumulation of cadmium was greater in roots than in leaves.
Root et al. (1975) grew maize ( Zea mays) in hydroponic
solutions containing cadmium chloride at concentrations ranging from
1 to 40 mg/litre. Uptake of cadmium into the plants increased with
time, and cadmium was present at higher concentrations in roots than
in shoots. Leaf chlorophyll concentration and yield (as dry weight) of
both roots and shoots decreased with increasing cadmium concentration.
As the cadmium concentration in the leaves increased, the
concentration of zinc decreased and the concentration of iron
increased. This gave a linear correlation between cadmium in the leaf
and iron/zinc ratio. Chlorosis resulting from cadmium in the leaves
(seen at a cadmium concentration of 1 mg/litre nutrient solution)
appeared comparable to iron-deficiency chlorosis. However, in this
case, the chlorosis was not due to iron deficiency, as previously
suggested by other workers, but was associated in some way with an
increasing iron/zinc ratio.
Harkov et al. (1979) found no effect on the yield of tomatoes
grown in vermiculite and cultured with a nutrient solution containing
cadmium at concentrations of 0.25 or 0.75 mg/litre. The plants were
more susceptible to damage by ozone, under conditions where ozone
damage would have been slight, after exposure to cadmium. Where ozone
damage was heavy or when conditions were not conducive to ozone
damage, there was no effect of cadmium. When Wong et al. (1988)
exposed pea ( Pisum sativum) seeds to cadmium concentrations of 1, 5,
10, and 20 mg/litre in culture solutions, germination was
significantly reduced at 20 mg/litre and radicle elongation to 1 cm
was significantly reduced at 5 mg/litre and to 2 cm at 1 mg/litre.
Early development (shoot elongation, leaf development, root and shoot
growth) was inhibited in a dose-dependant manner. Slight inhibition
was observed at 1 mg/litre, increased inhibition at 5 mg/litre, and
significant inhibition at 10 and 20 mg/litre.
7.1.2 Toxicity to plants grown in soil
Mitchell & Fretz (1977) showed that the effects on the growth of
red maple, white pine and Norway spruce plants in soil, amended with
cadmium, were similar but less severe, owing to reduced uptake of the
metal, than in the case of the same plants grown hydroponically.
Cadmium only affected current growth of the plants, except where it
was present in excess. Mahler et al. (1978) treated eight soils, the
pH values of which ranged from 4.8 to 7.8, with 1% (by weight) sewage
sludge containing added cadmium sulfate, leading to cadmium
concentrations in the soil ranging from 0.1 to 320 mg/kg. Two plants,
lettuce ( Lactuca sativa variety longifolia) and Swiss chard ( Beta
vulgaris variety cicla), were grown in the soils in pots. The EC50
(yield) for lettuce was 214 and 139 mg/kg soil for acid and calcareous
soils, respectively, whereas the values for chard were 175 and 250
mg/kg for acid and calareous soils. The corresponding tissue
concentrations of cadmium associated with these effects were 470 and
160 mg/kg for lettuce and 714 and 203 mg/kg for chard. Thus, a
markedly lower tissue concentration of cadmium produced 50% yield
reduction on calcareous soils than on acid soils. Alloway et al.
(1990) reported stunted growth and toxic signs on leaves of lettuce,
cabbage, carrot, and radish plants, but only at the highest
concentrations of cadmium tested (which resulted in a cadmium content
of around 20 mg/kg in the upper parts of the plants).
7.1.3 In vitro physiological studies
Bazzaz et al. (1974) demonstrated an effect of cadmium on
transpiration and photosynthesis in excised sunflower heads. The heads
(15 cm diameter) were placed in flasks containing distilled water or
cadmium salt solutions (2, 20, 100 or 200 mg/litre) and transpiration
and photosynthesis were measured at daily intervals over 4 to 5 days.
Cadmium reduced transpiration and photosynthesis at concentrations of
100 or 200 mg/litre. Excised epidermal peels floating on solutions of
cadmium salts showed a log-linear relationship between metal
concentration and stomatal opening. The stomata opened less with
increasing cadmium concentration; this accounted for the effect on
transpiration and, hence, on photosynthesis.
7.2 Toxicity to terrestrial invertebrates
Haight et al. (1982) calculated 24-h, 48-h, and 72-h LC50
values of 36, 15.1, and 5.85 mg cadmium/litre, respectively, for
juvenile free-living nematodes ( Panagrellus silusiae) and 111, 26.3,
and 13.2 mg/litre for adults. Williams & Dusenbery (1990) exposed the
free-living nematode Caenorhabditis elegans to cadmium and found
values of 904, 22, and 1.5 mg/litre, respectively, for the same three
exposure periods. They also calculated a 96-h LC50 of 0.06 mg/litre.
Popham & Webster (1979) found that a 6-h exposure to 3.26 x
10-7 moles of cadmium significantly decreased the fecundity of C.
elegans. A 3.5-day exposure to 10-8 moles caused the same effect.
Nematodes exposed to 4 x 10-6 moles of cadmium never grew to the
same length as controls and resembled worms from starved, overcrowded
cultures. Van Kessel et al. (1989) exposed juvenile C. elegans to
various concentrations of cadmium chloride and found that growth and
subsequent reproduction were significantly reduced at 1 µmol/litre. At
levels of 160 and 320 µmol/litre the nematodes did not reach the adult
stage and, therefore, did not reproduce.
Doelman et al. (1984) exposed the soil nematodes Mesorhabditus
monhystera and Aphelenchus avenae to cadmium via food for up to 22
days and monitored the size of the population. M. monhystera was
exposed, via bacteria and fungi, to concentrations of 0.23, 4.4, and
12.7 mg cadmium/kg, and a significant reduction in the size of the
population was found at all doses. A. avenae was exposed, via fungi
alone, to concentrations of 1, 10, and 25 mg/kg. At 1 mg/kg there was
no effect on the population size, whereas at 10 mg/kg, a reduction was
observed until the final day. A concentration of 25 mg/kg
significantly reduced the size of the population. The authors noted
that exposure via fungi alone gave far more variable results.
In studies by Van Straalen et al. (1989), the collembolan
Orchesella cincta and the oribatid mite Platynothrus peltifer were
exposed to cadmium in the food. The 9-week LC50 values were 1.6 and
7.27 µmol/g and the no-observed-effect levels (NOEL) were 0.042 and
0.026 µmol/g for O. cincta and P. peltifer, respectively. The most
sensitive parameters were female growth for O. cincta and
reproduction in P. peltifer.
Russell et al. (1981) fed subadult garden snails ( Helix aspersa)
on diets containing six different levels of cadmium ranging from 10 to
1000 mg/kg diet over a period of 30 days. There was little mortality
(two animals out of 350 died, one at an exposure level of 50 mg/kg and
the other at 1000 mg/kg) but food consumption declined with each
increase in cadmium dose. Food consumption was strongly depressed at
cadmium doses of 100 mg/kg or more. Relative weight loss was the most
pronounced effect of cadmium treatment; this was dose related and
directly attributable to reduced feeding rates. At doses of 25 mg/kg
or more, shell growth and reproductive activity were depressed while
the incidence of sealing response (the sealing of the operculum with
a disc of mucus and a dormancy reaction) increased markedly. These
three effects were all related to the dietary cadmium concentration.
7.3 Toxicity to birds
7.3.1 Acute and short-term toxicity
The acute and short-term toxicity of cadmium salts to birds in
laboratory studies is summarized in Table 13. Dosing for 5 days,
followed by 3 days of clean diet, resulted in LC50 values generally
in excess of 2000 mg/kg diet. Only the pheasant showed greater
sensitivity to cadmium but, even in this species, the LC50 was close
to 1000 mg/kg diet. All the birds used were between 10 and 14 days
old.
Table 13. Toxicity of cadmium to birds a
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Japanese quail 14 cadmium chloride 2440 (1807-3294) Hill &
(Coturnix coturnix 14 cadmium succinate 2052 (1621-2598) Camardese
japonica) (1986)
Pheasant 10 cadmium chloride 767 (651-898) Hill et al.
(Phasianus 14 cadmium succinate 1411 (1202-1657) (1975)
colchicus)
Bobwhite quail 14 cadmium succinate 1728 (1381-2132) Hill et al.
(Colinus (1975)
virginianus)
Table 13 (contd).
Species Age Salt LC50 Reference
(days) (mg/kg diet)
Mallard duck 10 cadmium chloride > 5000 Hill et al.
(Anas 10 cadmium succinate > 5000 (1975)
platyrhynchos)
a Birds were fed with a dosed diet for 5 days and then a "clean" diet for 3 days.
In a study by Pritzl et al. (1974), 2-week-old leghorn chicken chicks
were dosed with dietary cadmium chloride for 20 days. In the first
experiment, chicks dosed with 700 mg cadmium/kg diet showed an
increase in the weight of the gastrointestinal tract, kidney, and
gizzard expressed as a ratio to the body weight. In a second
experiment, chicks were fed diets containing 400, 600, 800 or 1000 mg
cadmium/kg. Weight gain and food consumption were decreased, relative
to controls, at all dose levels, and at levels higher than 400 mg/kg
the birds lost weight. All the birds fed diets containing 800 or 1000
mg/kg died within 20 days. The LC50 was calculated to be 565 mg/kg
diet.
When Cain et al. (1983) fed 1-day-old mallard ducklings ( Anas
platyrhynchos) a diet containing cadmium chloride at concentrations
of 5, 10 or 20 mg cadmium/kg for 12 weeks, significant effects were
only noted at the highest dose. These included a significant reduction
in packed cell volume and haemoglobin concentrations and a significant
increase in serum glutamic-pyruvic transaminase. Mild to severe kidney
lesions were evident in ducklings fed 20 mg/kg for 12 weeks. Body
weight, liver weight, and femur weight-to-length ratio were unaffected
by the cadmium treatment. No other haematological or histological
effects were found.
7.3.2 Reproductive effects
Lofts & Murton (1967) injected 0.2 ml of a solution of cadmium
chloride (0.04 mol/litre) intramuscularly into wood pigeons ( Columba
palumbus). When the cadmium was given to birds with regressed
testes, which were then stimulated into reproductive condition by long
photoperiods (16 h of light per day), there was a reduction in the
numbers of birds showing full testicular development in the treated
group. Only one out of six birds given cadmium had developed
spermatozoa in the testis by the end of the experiment. The remainder
had not even produced spermatids; two birds had only spermatogonia in
the seminiferous epithelium while the other three had secondary
spermatocytes. Of the six control birds, four had spermatozoa, one had
spermatids, and one primary spermatocytes. Injection of cadmium into
birds late in the season had no effect on the autumnal regression of
the testes. There was no sign of testicular necrosis in the treated
birds. An intratesticular injection of cadmium caused local necrosis
in the testis of feral pigeons. A dietary concentration of 200 mg
cadmium/kg, in the form of cadmium chloride, reduced spermatogenesis
in male mallards and egg production in females, but a lower dose of 20
mg/kg produced no effects (White & Finley, 1978; White et al., 1978).
7.3.3 Physiological effects
Mayack et al. (1981) found that the survival and growth of the
wood duck ( Aix sponsa) were unaffected by a cadmium chloride dietary
level of 100 mg/kg, although some kidney damage was reported.
Nicholson et al. (1983) compared the ultrastructure of the
kidneys of sea-birds contaminated with cadmium in the wild, sea-birds
from uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg per kg, respectively.
Damage was greatest in the proximal tubule of the kidney, and included
cell necrosis, nuclear pyknosis, mitochondrial swelling, and some
tubulorrhexis. The tubulorrhexis would be irreversible. There was some
indication of regeneration, judging from the number of
undifferentiated cells present in the tubule, in both dosed and
naturally contaminated birds. Debris was found in the distal nephron
lumen and there was some damage in the distal tubule and the renal
corpuscles. Necrosis of kidney cells was very rare in control birds or
uncontaminated sea-birds.
7.3.4 Behavioural effects
Heinz et al. (1983) assessed the avoidance response to a visual
fright stimulus of ducklings fed a diet containing cadmium chloride at
a concentration of 4 or 40 mg/kg. The parents of the ducklings had
also been fed this cadmium-containing diet. Ducklings fed 4 mg/kg were
hypersensitive to the fright stimulus, whereas those fed the higher
dose reacted as did controls. The authors could offer no explanation
of why the higher dose had no effect but pointed to similar results
from other materials. They were of the opinion that hypersensitivity
to behavioural signals could be as deleterious to the organism in the
wild as a failure to respond.
7.4 Toxicity to wild small mammals
Shore et al. (1991) fed herbivorous bank voles ( Clethrionomys
glareolus) and granivorous wood mice ( Apodemus sylvaticus) a
pelleted diet contaminated with cadmium chloride and collected urine
and faeces in metabolism cages. Bank voles fed diets containing 10.3
mg/kg for 40 days and then 4.5 mg/kg for 35 days suffered significant
net daily loss of calcium and sodium, and reduced net gain of
potassium and magnesium compared to controls. Assimilation of the
macroelements was not significantly altered in wood mice fed 10.3
mg/kg for 75 days.
8. EFFECTS IN THE FIELD
Appraisal
Tolerance to cadmium has been demonstrated in soil fungi,
plants, aquatic invertebrates, and fish from cadmium-contaminated
sites. Some field evidence suggests that cadmium is responsible for
reduced leaf litter degradation and a failure to recycle nutrients
due to adverse effects on populations, particularly of
microorganisms. However, no studies have identified cadmium as the
sole cause of the effect, since it is always associated with other
metals. Although soil invertebrates in contaminated sites accumulate
cadmium and other metals, there is evidence that most populations are
not affected. A field study has shown that fish from a
cadmium-contaminated river have physiological abnormalities. Kidney
damage has been found in pelagic sea-birds from areas away from
industrial or other anthropogenic sources of cadmium, but there was
no effect on survival or reproduction of populations. In industrially
contaminated areas, kidney damage has been observed in several
species of birds found to contain cadmium plus other metals.
8.1 Tolerance
Tolerance to cadmium has been demonstrated in soil fungi (section
5.2), aquatic invertebrates (section 6.2.7), and plants collected from
sites with high cadmium levels, such as those in the vicinity of
metalliferous mines and smelters.
Coughtrey & Martin (1977) experimentally demonstrated tolerance
of the grass Holcus lanatus to cadmium to be greater in plants
collected from an area subject to high fall-out of cadmium than in
plants collected from a control site. Growth of tolerant plants was
reduced in uncontaminated culture solutions, relative to controls, but
was similar to that of control plants in solutions where cadmium salts
had been added at levels similar to the field exposure. Simon (1977)
reported cadmium tolerance in the grasses Festuca ovina and
Agrostis tennuis. The tolerant grasses were collected from areas
contaminated by mining and aerial fall-out of cadmium.
8.2 Effects close to industrial sources and highways
There have been several reports of effects of heavy metal
deposition on the accumulation rate of leaf litter in deciduous
woodlands. These effects are restricted to areas close to, or down
wind from, smelter sites. The separation of the effects of cadmium
from those of other heavy metals present in the litter is difficult.
A study by Coughtrey et al. (1979) attempted to do this by detailed
analysis of the litter itself and by the use of statistics to separate
effects of different components of the pollution fall-out. Seven areas
of woodland in the vicinity of, or up to 28 km away from, a
lead-zinc-cadmium smelter in Avonmouth, United Kingdom, were studied.
The leaf litter contained lead, zinc, copper, and cadmium (in that
order of concentrations), and metal levels were high in samples taken
from within 3 km of the smelter. Litter from a wood 6.8 km from the
smelter had similar levels to control litter collected 30 km away, but
the prevailing wind would not have carried much of the fall-out in the
direction of this wood. For the four woods within 3 km of the smelter,
cadmium levels ranged from 23 to 98 mg/kg litter (lead levels were
between 721 and 2179 mg/kg, zinc levels between 764 and 2814 mg/kg,
and copper levels between 47 and 135 mg/kg). The weight of litter
accumulated per unit area was markedly greater in the contami-nated
sites than in the uncontaminated ones; litter standing crop ranged
from 7.91 to 13.16 kg/m2 in contaminated and from 0.913 to 3.104
kg/m2 in uncontaminated sites. The litter accumulation correlated
well with levels of all metals but not with the pH of the litter,
which varied between 3.88 and 6.3 over the sites. Partial correlation
analysis showed that cadmium and zinc interrelated; with both cadmium
and zinc, partial correlation coefficients were highly significant
when lead, copper or pH effects were accounted for. However,
correlations were low for lead and copper when cadmium or zinc were
accounted for. Of further interest was an analysis of cadmium and zinc
in leaf litter from various sites. There was an increase in the
smaller particle sizes in contaminated sites relative to
uncontaminated ones. These smaller particles contained a
disproportionate amount of the metals present, particularly in the
case of cadmium. Litter standing crop and cadmium concentrations were
highly correlated; the correlation between cadmium content and
different particle sizes of litter was better for small particles
sizes, and the slope of the regression line between cadmium
concentration and litter weight decreased with increasing particle
size. The authors argued that litter degradation was not affected at
the early stages but only when breakdown had progressed to much
smaller particle sizes. This perhaps supports the view that
microorganisms are inhibited by metals to a greater extent than
invertebrates, which would produce the initial reduction in size of
litter fragments. Taking the figure of 900 g/m2 as the normal leaf
litter level for woodland, the extra accumulation in contaminated
woods represented 25 to 30 years of litter accumulation (the smelter
in question had been operating for 48 years) and, possibly a large
proportion of the total capital of nutrients normally recycled to the
plants.
Other authors have also reported accumulation of leaf litter in
areas contaminated by metals (Tyler, 1972; Strojan, 1978), although
they disagree about the probable cause. Strojan (1978) proposed that
the effect relates to the absence of some groups of invertebrates,
while Jordan & Lechavalier (1975) suggested that the effect is on
microorganisms. There is no direct evidence that invertebrates in leaf
litter are adversely affected by metals, although they do accumulate
all the metals found in litter (Martin et al., 1976; Coughtrey &
Martin, 1976). Both Tyler (1972) and Strojan (1978) argued that the
productivity of woodland may be adversely affected by the failure to
recycle nutrients in areas contaminated by heavy metals. Coughtrey et
al. (1979) considered that the litter is an important sink for heavy
metals, and that the result of litter organisms developing tolerance
to the metals and, therefore, in the long-term increasing the rate of
degradation is unpredictable since metals would be released at the
same time as nutrients.
In a study by van Straalen et al. (1987), the metal excretion
efficiency of the collembolan Orchesella cincta collected from
various contaminated forest soils was monitored. The authors found
that moderate to high soil cadmium contamination of industrial origin
did not evoke increased cadmium excretion. In fact contamination
initiated in this century from a zinc factory caused a significant
decrease in excretion efficiency. Soils that had been contaminated
with cadmium for many years (lead/zinc factory) or to an extreme
degree (lead smelter) were inhabited by Collembola able to increase
metal excretion.
Muskett & Jones (1980) found levels of cadmium to be higher than
normal within 10 m of a road with a heavy traffic, but no effect on
the numbers of invertebrates caught or their species diversity was
observed.
8.3 Effects on fish
Field studies in Sweden showed that perch ( Perca fluviatilis)
from a cadmium-contaminated river (0.1 to 0.2 µg cadmium/litre) had
physiological abnormalities similar to those shown in laboratory
experiments (Sjobeck et al., 1984).
8.4 Effects on sea-birds
The reported effects on the kidney of sea-birds are not always a
result of exposure to cadmium as an industrial pollutant, since the
individuals most affected come from areas where there is no industrial
effluent. This is often, therefore, a response to naturally occurring
cadmium presumed to derive from the oceans. The birds appear to cope
with this damage to the kidney and suffer no effects on survival or
breeding success. No damage resulting from exposure to strictly
anthropogenically derived cadmium appears to have been reported on the
same scale as that from exposure to naturally occurring cadmium.
Nicholson et al. (1983) compared the ultrastructure of the kidneys of
sea-birds contaminated with cadmium in the wild, sea-birds from
uncontaminated colonies, starlings dosed with cadmium in the
laboratory, and control starlings. They found damage to kidney cells
to be comparable between wild sea-birds and dosed starlings having
kidney cadmium levels of 60-480 and 95-240 mg/kg, respectively (see
section 7.3.3). Nicholson & Osborn (1983) reported kidney lesions
(described in section 7.3.3) in several different species of sea-bird
caught in contaminated areas, although other pollutant metals such as
mercury were also present in the tissues.
9. EVALUATION
9.1 General considerations
In evaluating the environmental hazard of cadmium, it is
necessary to extrapolate from laboratories studies to ecosystems. This
must be done with extreme caution for a number of reasons.
a) The availability of cadmium to organisms in the environment is
limited by its strong adsorption to environmental components such
as soil, sediment, and organic matter. Organisms in contaminated
areas accumulate high body burdens of cadmium.
b) Environmental variables such as temperature, pH, and the chemical
composition of water or soil have been shown to affect both the
uptake and the toxic impact of cadmium.
c) Available, rather than nominal or total, cadmium is the
determinant in assessing uptake by, and effects on, organisms.
d) There are limited data from controlled experimental studies on
the effects of mixtures of metals. Organisms in the environment
are exposed to mixtures of pollutants. Acid deposition can
release metals, including cadmium, into the environment.
e) Little experimental work has been carried out on species or
communities that are either representative or key components of
natural communities and ecosystems. Studies have not considered
all of the interactions between populations and all of the
environmental factors affecting these populations. As a result,
the impact of cadmium on ecosystems may have been underestimated.
f) Results from laboratory studies based on very sensitive
parameters may be indicative of physiological impacts on
individuals rather than impacts on ecosystems.
9.2 The aquatic environment
Cadmium input to the aquatic environment is through dis-charge of
industrial waste, surface run-off, and deposition. It is strongly
adsorbed onto sediments and soils. The average cadmium content of sea
water is about 0.1 µg/litre or less, while fresh waters contain <
0.01 to 0.06 µg/litre in unpolluted areas. Cadmium levels of up to 5
mg/kg and 0.03 to 1 mg/kg have been reported for freshwater sediments
and marine sediments, respectively.
The rate of uptake and the toxic impact of cadmium on aquatic
organisms is greatly affected by physicochemical factors such as
temperature, ionic concentration, and organic matter content.
Cadmium is translocated by aquatic plants and concentrated in
roots and leaves. It is also taken up and accumulated by various
aquatic animals. The toxicity of cadmium to freshwater organisms
varies considerably depending on the exposure duration, species, and
life-stage. The early life-stages and the reproductive system are the
most vulnerable. Cadmium is, by comparison, one of the most toxic
heavy metals in the freshwater environment. Manifest responses of
certain organisms to cadmium are observed at environmental
concentrations lower than 1 µg/litre.
Cadmium-induced kidney damage has been reported in sea-birds
sampled from the field. However, this damage is present in both
cadmium-polluted areas and areas remote from industrial contamination.
The effect is probably, therefore, due to natural cadmium in certain
species and areas.
9.3 The terrestrial environment
Cadmium is introduced into the terrestrial environment from
mining, non-ferrous metal production, landfill sites and from the
application of sewage sludge, phosphate fertilizers, and manure.
Background concentrations of cadmium are in the range of 0.1 to 0.4
mg/kg soil and can reach 4.5 mg/kg in volcanic soils. Levels up to 160
mg/kg soil have been found close to metal processing sources.
Reduced breakdown of leaf litter and recycling of nutrients has
been attributed to metal pollution in the field. Cadmium appears to be
the most potent metal at inhibiting litter degradation. The effect is
thought to be due largely to reduced populations of microorganisms,
which are responsible for the final stages of litter decomposition.
Plants take up cadmium and can translocate and accumulate it.
However, uptake from soil is limited. Where there is high-level
exposure to cadmium (in the range of hundreds of mg/kg), growth
reduction is the major effect. Plants exposed to cadmium in the field
for long periods can develop tolerance to the metal. There is no
evidence of adverse effects of cadmium on plant populations in the
field.
Terrestrial invertebrates vary considerably in their sensitivity
to cadmium. Some species can take up and store cadmium to levels of up
to 5000 mg/kg body weight without apparent ill effects, while others
show population effects at levels of a few mg/kg soil. Populations of
some terrestrial invertebrates could be adversely affected at levels
of cadmium contamination seen in the field. Isopods and earthworms are
useful biomonitors for cadmium contamination. Invertebrates with high
body burdens may pose a threat to predators.
Kidney damage was found in experimental birds fed 20 mg
cadmium/kg diet for 12 weeks, but not at lower doses. Reproductive
effects have been observed at 200 mg/kg diet. A dose of 4 mg/kg
affected the behaviour of ducklings. No effects of cadmium have been
seen in terrestrial birds sampled from the field, although the cadmium
level in the brain, kidney, and liver of pigeons has proved to be a
good indicator of urban cadmium contamination.
Small mammals accumulate cadmium in the vicinity of mining spoil.
The ionic balance was affected in voles exposed experimentally to a
concentration of 10 mg/kg diet.
Populations of terrestrial organisms may also develop tolerance
to cadmium after long-term exposure.
10. RECOMMENDATIONS FOR PROTECTING THE ENVIRONMENT
To eliminate environmental effects, emissions of cadmium from the
following sources should be reduced as far as is practicable:
* smelters
* incinerators
* sewage sludge applied to the land
* phosphate fertilisers
* cadmium-containing manure
11. FURTHER RESEARCH
a) More study is needed to clarify the effects of cadmium on the
decomposition process of plant debris. Effects on the degree of
nutrient cycling and long-term plant growth and the exact nature
of the inhibition of decomposition require further attention.
b) The adsorption of cadmium to soil and sediment requires further
study and quantification of coefficients. Modelling of binding
and distribution in the environment is needed.
c) Organisms that are particularly sensitive (i.e. indicator
species) or that play a critical role in ecological systems
should be identified and studied with regard to the effects of
cadmium.
d) Studies are needed on the basic mechanisms by which cadmium
interacts with physiological and biochemical processes in
organisms and within individual cells.
There is a need to take certain precautions in studies on
cadmium. Firstly, the speciation of the metal should be considered in
experimental design and procedures and a clear measure of the
available cadmium should be reported. Secondly, studies of the uptake
and movement between trophic levels should include the relationship
between the non-nutrient cadmium and the nutrients calcium and zinc.
REFERENCES
ABEL, P.D. & PAPOUTSOGLOU, S.E. (1986) Lethal toxicity of cadmium to
Cyprinus carpio and Tilapia aurea. Bull. environ. Contam.
Toxicol., 37: 382-386.
ADRIANO, D.C. (1986) Trace elements in the terrestrial environment,
Berlin, Heidelberg, New York, Springer-Verlag, 533 pp.
AHSANULLAH, M. (1976) Acute toxicity of cadmium and zinc to seven
invertebrate species from Western Port, Victoria. Aust. J. mar.
freshwater Res., 27: 187-196.
ALLOWAY, B.J., JACKSON, A.P., & MORGAN, H. (1990) The accumulation of
cadmium by vegetables grown on soils contaminated from a variety of
sources. Sci. total Environ., 91: 223-236.
ANADU, D.I., CHAPMAN, G.A., CURTIS, L.R., & TUBB, R.A. (1989) Effect
of zinc exposure on subsequent acute tolerance to heavy metals in
rainbow trout. Bull. environ. Contam. Toxicol., 43: 329-336.
ANDERSSON, A. (1977) Heavy metals in Swedish soils: on their
retention, distribution and amounts. Swed. J. agric. Res., 7: 7-20.
ANDERSSON, A. & HAHLIN, M. (1981) Cadmium effects from phosphorus
fertilization in field experiments. Swed. J. agric. Res., 11: 3-10.
ANDREWS, S.M., JOHNSON, M.S., & COOKE, J.A. (1984) Cadmium in small
mammals from grassland established on metalliferous mine waste.
Environ. Pollut., 33A: 153-162.
ARILLO, A., CALAMARI, D., MARGIOCCO, C., MELODIA, F., & MENSI, P.
(1984) Biochemical effects of long-term exposure to cadmium and copper
on rainbow trout ( Salmo gairdneri): validation of water quality
criteria. Ecotoxicol. environ. Saf., 8: 106-117.
AYLETT, B.J. (1979) The chemistry and bioinorganic chemistry of
cadmium. In: Webb, M., ed. The chemistry, biochemistry and biology of
cadmium, Amsterdam, Elsevier/North Holland, pp. 1-43.
BABICH, H. & STOTZKY, G. (1977a) Reductions in the toxicity of cadmium
to microorganisms by clay minerals. Appl. environ. Microbiol., 33:
696-705.
BABICH, H. & STOTZKY, G. (1977b) Effect of cadmium on fungi and on
interactions between fungi and bacteria in soil: influence of clay
minerals and pH. Appl. environ. Microbiol., 33: 1059-1066.
BAHNER, L.H. & NIMMO, D.R. (1975) Methods to assess effects of
combinations of toxicants, salinity and temperature on estuarine
animals. In: Hemphill, D.D., ed. Trace substances in environmental
health - IX, Columbia, University of Missouri, pp. 169-177.
BAUDOUIN, M.F. & SCOPPA, P. (1974) Acute toxicity of various metals to
freshwater zooplankton. Bull. environ. Contam. Toxicol., 12: 745-751.
BAZZAZ, F.A., CARLSON, R.W., & ROLFE, G.L. (1974) The effect of heavy
metals on plants: part 1. Inhibition of gas exchange in sunflower by
Pb, Cd, Ni and Tl. Environ. Pollut., 7A: 241-246.
BEHNE, D. (1980) Problems of sampling and sample preparation for trace
element analysis in the health sciences. In: Bratter, P. & Scramel,
P., ed. Trace elements analytical chemistry in medicine and biology.
Proceedings of the 1st International Workshop, Neuherberg, Germany,
Berlin, Walter de Gruyter, pp. 769-782.
BENGTSSON, B-E. & BERGSTROM, B. (1987) A flowthrough fecundity test
with Nitocra spinipes (Harpacticoidea Crustacea) for aquatic
toxicity. Ecotoxicol. environ. Saf., 14: 260-268.
BENGTSSON, B-E., CARLIN, C.H., LARSSON, A., & SVANBERG, O. (1975)
Vertebral damage in minnows, Phoxinus phoxinus L., exposed to
cadmium. Ambio, 4: 166-168.
BENNETT, W.N. (1990) Measurement of manganese amelioration of cadmium
toxicity in Chlorella pyrenoidosa using turbidostat culture. Arch.
environ. Contam. Toxicol., 19: 118-123.
BENOIT, D.A., LEONARD, E.N., CHRISTENSEN, G.M., & FIANDT, J.T. (1976)
Toxic effects of cadmium on three generations of brook trout
( Salvelinus fontinalis). Trans. Am. Fish. Soc., 105: 550-560.
BERGER, B. & DALLINGER, R. (1989) Accumulation of cadmium and copper
by the terrestrial snail Arianta arbustorum L.: kinetics and
budgets. Oecologia, 79: 60-65.
BERGLIND, R. (1986) Combined and separate effects of cadmium, lead and
zinc on ALA-D activity, growth and hemoglobin content in Daphnia
magna. Environ. Toxicol. Chem., 5: 989-995.
BERK, S.G., GUNDERSON, J.H., & DERK, L.A. (1985) Effects of cadmium
and copper on chemotaxis of marine and freshwater ciliates. Bull.
environ. Contam. Toxicol., 34: 897-903.
BERTRAM, P.E. & HART, B.A. (1979) Longevity and reproduction of
Daphnia pulex (de Geer) exposed to cadmium-contaminated food or
water. Environ. Pollut., 19A: 295-305.
BESTEN, P.J. DEN, HERWIG, H.J., ZANDEE, D.I., & VOOGT, P.A. (1989)
Effects of cadmium and PCBs on reproduction of the sea star Asterias
rubens: Aberrations in the early development. Ecotoxicol. environ.
Saf., 18: 173-180.
BEWLEY, R.J.F. & STOTZKY, G. (1983) Effects of cadmium and zinc on
microbial activity in soil; influence of clay minerals. Part 1: Metals
added individually. Sci. total Environ., 31: 41-55.
BEYER, W.N., CHANEY, R.L., & MULHERN, B.M. (1982) Heavy metal
concentrations in earthworms from soil amended with sewage sludge. J.
environ. Qual., 11: 381-385.
BEYER, W.N., PATTEE, O.H., SILEO, L., HOFFMAN, D.J., & MULHERN, B.M.
(1985) Metal contamination in wildlife living near two zinc smelters.
Environ. Pollut., 38A: 63-86.
BIESINGER, K.E. & CHRISTENSEN, G.M. (1972) Effects of various metals
on survival, growth, reproduction, and metabolism of Daphnia magna.
J. Fish Res. Board Can., 29: 1691-1700.
BINGHAM, F.T., PAGE, A.L., & STRONG, J.E. (1980) Yield and cadmium
content of rice grain in relation to addition rate of cadmium, copper,
nickel and zinc, with sewage sludge and liming. Soil Sci., 130: 32-38.
BLACK, J.A. & BIRGE, W.J. (1989) An avoidance response bioassay for
aquatic pollutants, Lexington, Kentucky, University of Kentucky, Water
Resources Research Institute (Research Report No. 123).
BLOCK, M. (1991) Uptake of cadmium in fish. Effects of xanthates and
diethyldithiocarbamate, University of Uppsala, Sweden, Faculty of
Sciences (Universitatis Uppsaliensis - Comprehensive summaries of
Uppsala dissertations from the Faculty of Science. No. 326).
BLOCK, M. & PART, P. (1986) Increased availability of cadmium to
perfused rainbow trout ( Salmo gairdneri Rich.) gills in the presence
of the complexing agents diethyl dithiocarbamate, ethyl xanthate and
isopropyl xanthate. Aquat. Toxicol., 8: 295-302.
BOND, H., LIGHTHART, B., SHIMABUKU, R., & RUSSELL, L. (1976) Some
effects of cadmium on coniferous forest soil and litter microcosms.
Soil Sci., 121: 278-287.
BORGMANN, U. (1983) Metal speciation and toxicity of free metal ions
to aquatic biota. In: Nriagu, J.O., ed. Aquatic toxicology, New York,
Chichester, John Wiley & Sons, pp. 47-72.
BORGMANN, U. & RALPH, K.M. (1986) Effects of cadmium,
2,4-dichlorophenol, and pentachlorophenol on feeding, growth, and
particle-size-conversion efficiency of white sucker larvae and young
common shiners. Arch. environ. Contam. Toxicol., 15: 473-480.
BORGMANN, U., MILLARD, E.S., & CHARLTON, C.C. (1989a) Effect of
cadmium on a stable, large volume, laboratory ecosystem containing
Daphnia and phytoplankton. Can. J. Fish. aquat. Sci., 46: 399-405.
BORGMANN, U., RALPH, K.M., & NORWOOD, W.P. (1989b) Toxicity test
procedures for Hyalella azteca, and chronic toxicity of cadmium and
pentachlorophenol to H. azteca, Gammarus fasciatus, and Daphnia
magna. Arch. environ. Contam. Toxicol., 18: 756-764.
BOYLE, E.A., SCALTER, F., & EDMOND, J.M. (1976) On the marine
geochemistry of cadmium. Nature (Lond.), 263: 42-44.
BRESSAN, M. & BRUNETTI, R. (1988) The effects of nitriloacetic acid,
Cd and Hg on the marine algae Dunaliella tertiolecta and Isochrysis
galbana. Water Res., 22: 553-556.
BROOKS, R.R. & RUMSBY, M.G. (1967) Studies on the uptake of cadmium by
the oyster, Ostrea sinuata (Lamarck). Aust. J. mar. freshwater Res.,
15: 53-61.
BUCHAUER, M.J. (1972) The effects of zinc and cadmium pollution on
forest vegetation. Bull. New Jersey Acad. Sci., 17(2): 42.
BULL, K.R., MURTON, R.K., OSBORN, D., WARD, P., & CHENG, L. (1977)
High levels of cadmium in Atlantic seabirds and sea-skaters. Nature
(Lond.), 269: 507-509.
BURKITT, A., LESTER, P., & NICKLESS, G. (1972) Distribution of heavy
metals in the vicinity of an industrial complex. Nature (Lond.), 238:
327-328.
BYRNE, A.R., RAVNIK, V., & KOSTA, L. (1976) Trace element
concentrations in higher fungi. Sci. total Environ., 6: 65-78.
CAIN, J.R., PASCHAL, D.C., & HAYDEN, C.M. (1980) Toxicity and
bioaccumulation of cadmium in the colonial green alga Scenedesmus
obliquus. Arch. environ. Contam. Toxicol., 9: 9-16.
CAIN, B.W., SILEO, L., FRANSON, J.C., & MOORE, J. (1983) Effects of
dietary cadmium on mallard ducklings. Environ. Res., 32: 286-297.
CALABRESE, A., COLLIER, R.S., NELSON, D.A. & MACINNES, J.R. (1973) The
toxicity of heavy metals to embryos of the American oyster
Crassostrea virginica. Mar. Biol., 18: 162-166.
CALAMARI, D., MARCHETTI, R., & VAILATI, G. (1980) Influence of water
hardness on cadmium toxicity to Salmo gairdneri Rich. Water Res.,
14: 1421-1426.
CANTON, J.H. & SLOOFF, W. (1982) Toxicity and accumulation studies of
cadmium (Cd2+) with freshwater organisms of different trophic
levels. Ecotoxicol. environ. Saf., 6: 113-128.
CARRIER, R. & BEITINGER, T.L. (1988a) Reduction in thermal tolerance
of Notropis lutrensis and Pimephales promelas exposed to cadmium.
Water Res., 22: 511-515.
CARRIER, R. & BEITINGER, T.L. (1988b) Resistance of temperature
tolerance ability of green sunfish to cadmium exposure. Bull. environ.
Contam. Toxicol., 40: 475-480.
CARROLL, J.J., ELLIS, S.J., & OLIVER, W.S. (1979) Influences of
hardness constituents on the acute toxicity of cadmium to brook trout
( Salvelinus fontinalis). Bull. environ. Contam. Toxicol., 22:
575-581.
CARVALHO, F.M., TAVARES, T.M., SILVANY-NETO, A.M., LIMA, M.E.C., &
ALT, F. (1986) Cadmium concentrations in blood of children living near
a lead smelter in Bahia, Brazil. Environ. Res., 40: 437-449.
CATALDO, D.A. & WILDUNG, R.E. (1978) Soil and plant factors
influencing the accumulation of heavy metals by plants. Environ.
Health Perspect., 27: 149-159.
CEARLEY, J.E. & COLEMAN, R.L. (1974) Cadmium toxicity and
bioconcentration in largemouth bass and bluegill. Bull. environ.
Contam. Toxicol., 11: 146-151.
CHAN, K.-Y. & DEAN, A.C.R (1988) Effects of cadmium and lead on
growth, respiration and enzyme activity of the marine bacterium
Pseudomonas marina. Chemosphere, 17: 597-607.
CHANDINI, T. (1988) Changes in food [ Chlorella] levels and the acute
toxicity of cadmium to Daphnia carinata (Daphnidae) and Echinisca
triserialis (Macrothricidae) [Crustacea: Cladocera]. Bull. environ.
Contam. Toxicol., 41: 398-403.
CHANDINI, T. (1989) Survival, growth and reproduction of Daphnia
carinata (Crustacea: Cladocera) exposed to chronic cadmium stress at
different food ( Chlorella) levels. Environ. Pollut., 60: 29-45.
CHANEY, R.L., WHITE, M.C., & SIMON, P.W. (1975) Plant uptake of heavy
metals from sewage sludge applied to land. In: Proceedings of the 2nd
National Conference on Municipal Sludge Management, Rockville,
Information Transfer Inc., pp. 169-178.
CHAPMAN, G. & DUNLOP, S. (1981) Detoxification of zinc and cadmium by
the freshwater protozoan Tetrahymena pyriformis. I. The effect of
water hardness. Environ. Res., 26: 81-86.
CHIN, B. & SINA, J.F. (1978) Cellular bases of cadmium toxicity in
Physarum. In: Hemphill, D.D., ed. Trace substances in environmental
health, Columbia, University of Missouri, pp. 214-219.
CHRISTENSEN, J.J., EATOUGH, D.J., & IZATT, R.M. (1975) In: Handbook of
metal ligand heats and related thermodynamic quantities, 2nd ed.
revis. expand., New York, Basel, Marcel Dekker, Inc., 495 pp.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975a) Synergism between
dissolved oxygen and cadmium toxicity in five species of aquatic
insects. Environ. Res., 9: 285-289.
CLUBB, R.W., GAUFIN, A.R., & LORDS, J.L. (1975b) Acute cadmium
toxicity studies upon nine species of aquatic insects. Environ. Res.,
9: 331-341.
CONRAD, G.W. (1988) Heavy metal effects on cellular shape changes,
cleavage, and larval development of the marine gastropod mollusk,
( Ilyanassa obsoleta Say). Bull. environ. Contam. Toxicol., 41:
79-85.
CONWAY, H.L. (1978) Sorption of arsenic and cadmium and their effects
on growth, micronutrient utilization, and photosynthetic pigment
composition of Asterionella formosa. J. Fish Res. Board. Can., 35:
286-294.
COOMBS, T.L. (1979) Cadmium in aquatic organisms. In: Webb, M., ed.
The chemistry, biochemistry and biology of cadmium, Amsterdam,
Elsevier/North Holland, pp. 93-139.
COUGHTREY, P.J. & MARTIN, M.H. (1976) The distribution of Pb, Zn, Cd
and Cu within the pulmonate mollusc Helix aspersa Muller. Oecologia,
23: 315-322.
COUGHTREY, P.J. & MARTIN, M.H. (1977) Cadmium tolerance of Holcus
lanatus from a site contaminated by aerial fallout. New Phytol., 79:
273-280.
COUGHTREY, P.J., JONES, C.H., MARTIN, M.H., & SHALES, S.W. (1979)
Litter accumulation in woodlands contaminated by Pb, Zn, Cd and Cu.
Oecologia, 39: 51-60.
DALLA VIA, G.J., DALLINGER, R., & CARPENE, E. (1989) Effects of
cadmium on Murex trunculus from the Adriatic Sea. II. Oxygen
consumption and acclimation effects. Arch. environ. Contam. Toxicol.,
18: 562-567.
DALLINGER, R. & WIESER, W. (1984) Patterns of accumulation,
distribution and liberation of Zn, Cu, Cd and Pb in different organs
of the land snail Helix pomatia L. Comp. Biochem. Physiol., 79C:
117-124.
DALLINGER, R., PROSI, F., SEGNER, H., & BACK, H. (1987) Contaminated
food and uptake of heavy metals by fish: a review and proposal for
further research. Oecologia, 73: 91-98.
DAWSON, M.A., GOULD, E., THURBERG, F.P., & CALABRESE, A. (1977)
Physiological response of juvenile striped bass, Morone saxatilis,
to low levels of cadmium and mercury. Chesapeake Sci., 18: 353-359.
DENTON, G.R.W. & BURDON-JONES, C. (1981) Influence of temperature and
salinity on the uptake, distribution and depuration of mercury,
cadmium and lead by the black-lip oyster Saccostrea echinata. Mar.
Biol., 64: 317-326.
DE VRIES, M.P.C. & TILLER, K.G. (1978) Sewage sludge as a soil
amendment with special reference to Cd, Cu, Mn, Ni, Pb and Zn -
comparison of results from experiments conducted inside and outside a
glasshouse. Environ. Pollut., 16A: 231-240.
DE ZWART, D. & SLOOF, W. (1987) Toxicity of mixtures of heavy metals
and petrochemicals to Xenopus laevis. Bull. environ. Contam.
Toxicol., 38: 345-351.
DICKSON, G.W., GIESY, J.P., & BRIESE, L.A. (1982) The effect of
chronic cadmium exposure on phosphodenylate concentrations and
adenylate energy of gills and dorsal muscle of crayfish. Environ.
Toxicol. Chem., 1: 147-156.
DINNEL, P.A., LINK, J.M., STOBER, Q.J., LETOURNEAU, M.W., & ROBERTS,
W.E. (1989) Comparative sensitivity of sea urchin sperm bioassays to
metals and pesticides. Arch. environ. Contam. Toxicol., 18: 748-755.
DOELMAN, P., NIEBOER, G., SCHROOTEN, J., & VISSER, M. (1984)
Antagonistic and synergistic toxic effects of Pb and Cd in a simple
foodchain: Nematodes feeding on bacteria or fungi. Bull. environ.
Contam. Toxicol., 32: 717-723.
DONGMANN, G. & NURNBERG, H.W. (1982) Observations with Thalassiosira
rotula (Meunier) on the toxicity and accumulation of cadmium and
nickel. Ecotoxicol. environ. Saf., 6: 535-544.
DOUBEN, P.E.T. (1989a) Uptake and elimination of waterborne cadmium by
the fish Noemacheilus barbatulus L. (stone loach). Arch. environ.
Contam. Toxicol., 18: 576-586.
DOUBEN, P.E.T. (1989b) Metabolic rate and uptake and loss of cadmium
from food by the fish Noemacheilus barbatulus L. (stone loach).
Environ. Pollut., 59: 177-202.
DRESCHER, H.E., HARMS, U., & HUSCHENBETH. E. (1977) Organochlorines
and heavy metals in the harbour seal Phoca vitulina from the German
North Sea coast. Mar. Biol., 41: 99-106.
EATON, J.G., MCKIM, J.M., & HOLCOMBE, G.W. (1978) Metal toxicity to
embryos and larvae of seven freshwater fish species - I. Cadmium.
Bull. environ. Contam. Toxicol., 19: 95-103.
EISLER, R. (1971) Cadmium poisoning in Fundulus heteroclitus
(Pisces: Cyprinodontidae) and other marine organism. J. Fish Res.
Board Can., 28: 1225-1234.
EISLER, R. (1985) Cadmium hazards to fish, wildlife and invertebrates:
A synoptic review, Washington, DC, US Department of the Interior, Fish
and Wildlife Service (Biological Report No. 85).
EISLER, R. & HENNEKEY, R.J. (1977) Acute toxicities of Cd2+, Cr+6,
Hg2+, Ni2+ and Zn2+ to estuarine macrofauna. Arch. environ.
Contam. Toxicol., 6: 315-323.
EISLER, R., ZAROOGIAN, G.E., & HENNEKEY, R.J. (1972) Cadmium uptake by
marine organisms. J. Fish Res. Board. Can., 29: 1367-1369.
ELLIOT, N.G., SWAIN, R., & RITZ, D.A. (1985) The influence of cyclic
exposure on the accumulation of heavy metals by Mytilus edulis
planulatus (Lamarck). Mar. environ. Res., 15: 17-30.
ERIKSSON, J.E. (1988) The effects of clay, organic matter and time on
adsorption and plant uptake of cadmium added to the soil. Water Air
Soil Pollut., 40: 359-373.
FALCONER, C.R., DAVIES, I.M., & TOPPING, G. (1983) Trace metals in the
common porpoise, Phocoena phocoena. Mar. environ. Res., 8: 119-127.
FERARD, J.F., JOUANY, J.M., TRUHAUT, R., & VASSEUR, P. (1983)
Accumulation of cadmium in a freshwater food chain experimental model.
Ecotoxicol. environ. Saf., 7: 43-52.
FINLAYSON, B.J. & VERRUE, K.M. (1982) Toxicities of copper, zinc, and
cadmium mixtures to juvenile chinook salmon. Trans. Am. Fish. Soc.,
111: 645-650.
FLATAU, G.N., CLEMENT, R.L., GAUTHIER, M.J., & LAUMOND, F.M. (1987)
Adaptation to cadmium in a sensitive marine pseudomonad. Environ.
Technol. Lett, 8: 641-646.
FLATAU, G.N., CLEMENT, R.L., & GAUTHIER, M.J. (1988) Uptake of cadmium
by marine bacteria. Prog. Oceanogr., 21: 181-188.
FLEISCHER, M., SAROFIM, A.F., FASSETT, D.W., HAMMOND, P., SHACKLETTE,
H.T., NISBET, I.C.T., & EPSTEIN, S. (1974) Environmental impact of
cadmium: a review by the Panel on Hazardous Trace Substances. Environ.
Health Perspect., 5: 253-323.
FLIK, G., VAN DE WINKEL, J.G.J., PART, P., WENDELAAR BONGA, S.E., &
LOCK, R.A.C. (1987) Calmodulin mediated cadmium inhibition of
phosphodiesterase activity. Arch. Toxicol., 59: 352-359.
FORSTNER, U. (1980) Cadmium in the environment, Part I. In: Nriagu,
J.O., ed. Cadmium in polluted sediments, New York, Chichester, John
Wiley & Sons, pp. 305-363.
FRANCIS, P.C., BIRGE, W.J., & BLACK, J.A. (1984) Effects of
cadmium-enriched sediment on fish and amphibian embryo-larval stages.
Ecotoxicol. environ. Saf., 8: 378-387.
FRANK, P.M. & ROBERTSON, P.B. (1979) The influence of salinity on
toxicity of cadmium and chromium to the blue crab, Callinectes
sapidus. Bull. environ. Contam. Toxicol., 21: 74-78.
FRIBERG, L., ELINDER, C.G., KJELLSTRÖM, T., & NORDBERG, G.F. (1986)
Cadmium and health, a toxicological and epidemiological appraisal.
Vol. II. Effects and response, Cleveland, Ohio, CRC Press, 303 pp.
GESAMP (1984) IMO/FAO/UNESCO/WMO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Report of the
fourteenth session, Vienna, 26-30 March, 1984, Vienna, International
Atomic Energy Agency (Reports and Studies No. 21).
GESAMP (1985) IMO/FAO/UNESCO/WHO/IAEA/UN/UNEP Joint Group of Experts
on the Scientific Aspects of Marine Pollution: Atmospheric transport
of contaminants into the Mediterranean region, Athens, Geneva, World
Meteorological Association (Reports and Studies No. 26).
GESAMP (1987) IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of
Experts on the Scientific Aspects of Marine Pollution: Report of the
seventeenth session, Rome, Geneva, World Health Organization (Reports
and Studies No. 31).
GIATTINA, J.D. & GARTON, R.R. (1983) A review of the
preference-avoidance responses of fishes to aquatic contaminants.
Residue Rev., 87: 43-90.
GIESY, J.P., LEVERSEE, G.J., & WILLIAMS, D.R. (1977) Effects of
naturally occurring aquatic organic fractions on cadmium toxicity to
Simocephalus serrulatus (Daphnidae) and Gambusia affinis
(Poeciliidae). Water Res., 11: 1013-1020.
GILL, T.S. & PANT, J.C. (1983) Cadmium toxicity: inducement of changes
in blood and tissue metabolites in fish. Toxicol. Lett., 18: 195-200.
GISH, C.D. & CHRISTENSEN, R.E. (1973) Cadmium, nickel, lead and zinc
in earthworms from roadside soil. Environ. Sci. Technol., 7:
1060-1062.
GOTTOFREY, J. (1990) The disposition of cadmium, nickel, mercury and
methylmercury in fish and effects of lipophilic metal chelation,
Uppsala, Swedish University of Agricultural Sciences, Department of
Pharmacology and Toxicology (Thesis).
GOTTOFREY, J., BJORKLUND, I., & TJALVE, H. (1988) Effects of sodium
isoproylxanthate, potassium amylxanthate and sodium
diethyldithiocarbamate on the uptake and disposition of cadmium in the
brown trout ( Salmo trutta). Aquat. Toxicol., 12: 171-184.
GUY, R.D. & ROSS KEAN, A. (1980) Algae as a chemical speciation
monitor. I. A comparison of algal growth and computer calculated
speciation. Water Res., 14: 891-899.
HAIGHT, M., MUDRY, T., & PASTERNAK, J. (1982) Toxicity of seven heavy
metals on Panagrellus silusiae: The efficacy of the free-living
nematode as an in vivo toxicological bioassay. Nematologica, 28: 1-11.
HALE, J.G. (1977) Toxicity of metal mining wastes. Bull. environ.
Contam. Toxicol., 17: 66-73.
HALL, W.S., PAULSON, R.L., HALL, L.W., & BURTON, D.T. (1986) Acute
toxicity of cadmium and sodium pentachlorophenate to Daphnids and
fish. Bull. environ. Contam. Toxicol., 37: 308-316.
HAMANAKA, T., ITOO, T., & MISHIMA, S. (1982) Age related change and
distribution of cadmium and zinc concentrations in the stellar sea
lion ( Eumetopias jubata) from the coast of Hokkaido, Japan. Mar.
Pollut. Bull., 13: 57-61.
HAMILTON, S.J. & MEHRLE, P.M. (1986) Metallothionein in fish: review
of its importance in assessing stress from metal contaminants. Trans.
Am. Fish. Soc., 115: 596-609.
HARDISTY, M.W., HUGGINS, R.J., KARTAR, S., & SAINSBURY, M. (1974a)
Ecological implications of heavy metal in fish from the Severn
Estuary. Mar. Pollut. Bull., 5: 12-15.
HARDISTY, M.W., KARTAR, S., & SAINSBURY, M. (1974b) Dietary habits and
heavy metal concentrations in fish from the Severn Estuary and Bristol
Channel. Mar. Pollut. Bull., 5: 61-63.
HARDY, J.T., SCHMIDT, R.L., & APTS, C.W. (1981) Marine sediment and
interstitial water: effects on bioavailability of cadmium to gills of
the clam Protothaca staminea. Bull. environ. Contam. Toxicol., 27:
798-805.
HARDY, J.T., SULLIVAN, M.F., CRECELIUS, E.A., & APTS, C.W. (1984)
Transfer of cadmium in phytoplankton-oyster-mouse food chain. Arch.
environ. Contam. Toxicol., 13: 419-425.
HARKOV, R., CLARKE, B., & BRENNAN, E. (1979) Cadmium contamination may
modify response of tomato to atmospheric ozone. J. Air Pollut. Control
Assoc., 29: 1247-1249.
HARRISON, S.E. & KLAVERKAMP, J.F. (1989) Uptake, elimination and
tissue distribution of dietary and aqueous cadmium by rainbow trout
( Salmo gairdneri Richardson) and lake whitefish ( Coregonus
clupeaformis Mitchill). Environ. Toxicol. Chem., 8: 87-97.
HART, B.A. & SCAIFE, B.D. (1977) Toxicity and bioaccumulation of
cadmium in Chlorella pyrenoidosa. Environ. Res., 14: 401-413.
HARTENSTEIN, R., NEUHAUSER, E.F., & COLLIER, J. (1980) Accumulation of
heavy metals in the earthworm Eisenia foetida. J. environ. Qual., 9:
23-26.
HARTMAN, L.M. (1974) A preliminary report: Fungal flora of the soil as
conditioned by varying concentrations of heavy metals. Am. J. Bot.,
61: 23 (Abstract).
HARTWELL, S.I., JIN, J.H., CHERRY, D.S., & CAIRNS, J. (1989) Toxicity
versus avoidance response of golden shiner, Notemigonus crysoleucas,
to five metals. J. Fish Biol., 35: 447-456.
HATAKEYAMA, S. (1987) Chronic effects of Cd on reproduction of
Polypedilum nubifer (Chironomidae) through water and food. Environ.
Pollut., 48: 249-261.
HATAKEYAMA, S. & YASUNO, M. (1987) Chronic effects of Cd on the
reproduction of the guppy ( Poecilia reticulata) through
Cd-accumulated midge larvae ( Chironomus yoshimatsui). Ecotoxicol.
environ. Saf., 14: 191-207.
HEINZ, G.H., HASELTINE, S.D., & SILEO, L. (1983) Altered avoidance
behavior of black ducks fed cadmium. Environ. Toxicol. Chem., 2:
419-421.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
US Department of Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington,
DC, US Department of Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
HIRAOKA, Y. & OKUDA, H. (1984) Time course study of occurrence of
anomalies in medaka's centrum by cadmium or fenitrothion emulsion.
Bull. environ. Contam. Toxicol., 32: 693-697.
HIRATA, M. (1981) Annaka: Land polluted mainly by fumes and dust from
a zinc smelter. In: Kitagishi, K. & Gamare, I., ed. Heavy metal
pollution in the soils of Japan, Tokyo, Japan Scientific Societies
Press, pp. 149-163.
HOGSTRAND, C. & HAUX, C. (1990a) Metallothionein as an indicator of
heavy metal exposure in two subtropical fish species. J. exp. mar.
Biol. Ecol., 138: 69-84.
HOGSTRAND, C. & HAUX, C. (1990b) A radioimmunoassay for perch ( Perca
fluviatilis) metallothionein. Toxicol. appl. Pharmacol., 103: 56-65.
HOLCOMBE, G.W., PHIPPS, G.L., & MARIER, J.W. (1984) Methods for
conducting snail ( Aplexa hypnorum) embryo through adult exposures.
Effects of cadmium and reduced pH levels. Arch. environ. Contam.
Toxicol., 13: 627-634.
HONDA, K. & TATSUKAWA, R. (1983) Distribution of cadmium and zinc in
tissues and organs, and their age-related changes in striped dolphins,
Stenella coeruleoalba. Arch. environ. Contam. Toxicol., 12: 543-550.
HONDA, K., FUJISE, Y., TATSUKAWA, R., ITANO, K., & MIYAZAKI, N. (1986)
Age-related accumulation of heavy metals in bone of the striped
dolphin, Stenella coeruleoalba. Mar. environ. Res., 20: 143-160.
HOPKIN, S.P. (1989) Ecophysiology of metals in terrestrial
invertebrates, London, Elsevier Applied Science.
HOPKIN, S.P. (1990) Species-specific differences in the net
assimilation of zinc, cadmium, lead, copper and iron by the
terrestrial isopods Oniscus asellus and Porcellio scaber. J. appl.
Ecol., 27: 460-474.
HOPKIN, S.P. & MARTIN, M.H. (1984) Assimilation of zinc, cadmium, lead
and copper by the centipede Lithobius variegatus (Chilopoda). J.
appl. Ecol., 21: 535-546.
HOPKIN, S.P. & MARTIN, M.H. (1985) Transfer of heavy metals from leaf
litter to terrestrial invertebrates. J. Sci. Food Agric., 36: 538-539.
HOPKIN, S.P., WATSON, K., MARTIN, M.H., & MOULD, M.L. (1985) The
assimilation of heavy metals by Lithobius variegatus and Glomeris
marginata (Chilopoda: Diplopoda). Bijdr. Dierkd., 55: 88-94.
HUIGUANG FU (1989) Hormonal responses of fish to cadmium, Nijmegen,
The Netherlands, University of Nijmegen (Ph.D. Thesis).
HUNTER, B.A. & JOHNSON, M.S. (1982) Food chain relationships of copper
and cadmium in contaminated grassland ecosystems. Oikos, 38: 108-117.
HUTCHINSON, T.C. & CZYRSKA, H. (1975) Heavy metal toxicity and
synergism to floating aquatic weeds. Verh. Int. Ver. Limnol., 19 :
2102-2111.
HUTTON, M. (1981) Accumulation of heavy metals and selenium in three
seabird species from the United Kingdom. Environ. Pollut., 26A:
129-145.
HUTTON, M. (1982) Cadmium in the European Community: a prospective
assessment of sources, human exposure and environmental impact,
London, Monitoring and Assessment Research Centre, Chelsea College,
University of London, 99 pp (MARC Report No. 26).
HUTTON, M. (1983) Evaluation of the relationships between cadmium
exposure and indicators of kidney function, London, Monitoring and
Assessment Research Centre, Chelsea College, University of London, 46
pp (MARC Report No. 29).
HUTTON, M. & GOODMAN, G.T. (1980) Metal contamination of feral pigeons
Columba livia from the London area: Part I - tissue accumulation of
lead, cadmium and zinc. Environ. Pollut., 22A: 207-217.
HUTTON, M. & SYMON, C. (1986) The quantities of cadmium, lead, mercury
and arsenic entering the U.K. environment from human activities. Sci.
total Environ., 57: 129-150.
IRELAND, M.P. (1981) Uptake and distribution of cadmium in the
terrestrial slug Arion ater (L.) Comp. Biochem. Physiol., 68A:
37-41.
JANA, S. & BHATTACHARYA, D.N. (1988) Effect of heavy metals on growth
population of a fecal coliform bacterium Escherichia coli in aquatic
environment. Water Air Soil Pollut., 38: 251-254.
JANSSEN, M.P.M., BRUINS, A., DE VRIES, T.H., & VAN STRAALEN, N.M.
(1991) Comparison of cadmium kinetics in four soil arthropod species.
Arch. environ. Contam. Toxicol., 20: 305-312.
JOHN, J., GJESSING, E.T., GRANDE, M., & SALBU, B. (1987) Influence of
aquatic humus and pH on the uptake and depuration of cadmium by the
Atlantic salmon ( Salmo salar). Sci. total Environ., 62: 253-265.
JOHNSON, M.S. & EATON, J.W. (1980) Environmental contamination through
residual trace metal dispersal from derelict lead-zinc mine. J.
environ. Qual., 9(2): 175-179.
JOHNSON, M.W. & GENTILE, J.H. (1979) Acute toxicity of cadmium, copper
and mercury to larval American lobster Homarus americanus. Bull.
environ. Contam. Toxicol., 22: 258-264.
JONES, K.C. & JOHNSTON, A.E. (1989) Cadmium in cereal grain and
herbage from long-term experimental plots at Rothamsted, UK. Environ.
Pollut., 57: 199-216.
JORDAN, M.J. & LECHAVALIER, M.P. (1975) Effects of zinc-smelter
emissions on forest soil microflora. Can. J. Microbiol., 21:
1855-1865.
KAY, S.H. & HALLER, W.T. (1986) Heavy metal bioaccumulation and
effects on waterhyacinth weevils, Neochetina eichhorniae, feeding on
waterhyacinth, Eichhornia crassipes. Bull. environ. Contam.
Toxicol., 37: 239-245.
KEMP, P.F. & SWARTZ, R.C. (1988) Acute toxicity of interstitial and
particle-bound cadmium to a marine infaunal amphipod. Mar. environ.
Res., 26: 135-153.
KHAN, A.T., WEIS, J.S., & D'ANDREA, L. (1988) Studies of cadmium
tolerance in two populations of grass shrimp, Palaemonetes pugio.
Bull. environ. Contam. Toxicol., 40: 30-34.
KHANGAROT, B.S. & RAY, P.K. (1987) Sensitivity of toad tadpoles, Bufo
melanostictus (Schneider), to heavy metals. Bull. environ. Contam.
Toxicol., 38: 523-527.
KORTE, F. (1983) Ecotoxicology of cadmium: general overview.
Ecotoxicol. environ. Saf., 7: 3-8.
KRELL, U. & ROECKNER, E. (1988) Model simulation of the atmospheric
input of lead and cadmium into the North Sea. Atmos. Environ., 22(2):
375-381.
KUHN, R., PATTARD, M., PERNAK, K-D., & WINTER, A. (1989) Results of
the harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res., 23: 501-510.
LAAMANEN, A. (1972) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAAMANEN, A. (1976) Functions, progress and prospects for an
environmental subarctic base level station. Work environ. Health, 9:
17-25.
LAEGREID, M., ALSTAD, J., KLAVENESS, D., & SEIP, H.M. (1983) Seasonal
variation of cadmium toxicity toward the alga Selenastrum
capricornutum Printz in two lakes with different humus content.
Environ. Sci. Technol., 17: 357-361.
LAFEBER, F.P.J.G., FLIK, G., WENDELAAR BONGA, S.E., & PERRY, S.F.
(1988) Hypocalcin from Stannius corpuscles inhibits gill calcium
uptake in trout. Am. J. Physiol., 254: R891-R896.
LAFLEUR, P.D., ed. (1976) Accuracy in trace analysis: sampling, sample
handling, analysis, Washington, DC, US Department of Commerce,
National Bureau of Standards, Vols. 1 & 2, 1304 pp.
LALANDE, M. & PINEL-ALLOUL, B. (1986) Acute toxicity of cadmium,
copper, mercury and zinc to Tropocyclops prasinus mexicanus
(Cyclopoida, Copepoda) from three Quebec lakes. Environ. Toxicol.
Chem., 5: 95-102.
LANGSTON, W.J. & ZHOU, M. (1987a) Cadmium accumulation, distribution
and metabolism in the gastropod Littorina littorea: the role of
metal-binding proteins. J. Mar. Biol. Assoc. UK, 67: 585-601.
LANGSTON, W.J. & ZHOU, M. (1987b) Cadmium accumulation, distribution
and elimination in the bivalve Macoma balthica: neither
metallothionein nor metallothionein-like proteins are involved. Mar.
environ. Res., 21: 225-237.
LEE, H.H. & XU, C.H. (1984) Differential response of marine organisms
to certain metal and agrichemical pollutants. Bull. environ. Contam.
Toxicol., 33: 460-467.
LEPP, N.W., HARRISON, S.C.S., & MORRELL, B.G. (1987) A role for
Amanita muscaria L. in the circulation of cadmium and vanadium in a
non-polluted woodland. Environ. Geochem. Health, 9: 61-64.
LINDBERG, S.E., TURNER, R.R., & LOVETT, G.M. (1982) Process of
atmospheric deposition of metals and acids to forests. Annual Meeting
of the Air Pollution Control Association, New Orleans, 20 June 1982,
Pittsburgh, Pennsylvania, Air Pollution Control Association, 14 pp
(Conference Proceedings 75).
LOFTS, B. & MURTON, R.K. (1967) The effects of cadmium on the avian
testis. J. Reprod. Fert., 13: 155-164.
LOVETT, R.J., GUTENMANN, W.H., PAKKALA, I.S., YOUNGS, W.D., LISK,
D.J., BURDICK, G.E., & HARRIS, E.J. (1972) A survey of the total
cadmium content of 406 fish from 49 New York State fresh waters. J.
Fish Res. Board Can., 29: 1283-1290.
LUSSIER, S.M., GENTILE, J.H., & WALKER, J. (1985) Acute and chronic
effects of heavy metals and cyanide on Mysidopsis bahia (Crustacea:
Mysidacea). Aquat. Toxicol., 7: 25-35.
MA, W., EDELMAN, Th., VAN BEERSUM, I., & JANS, Th. (1983) Uptake of
cadmium, zinc, lead and copper by earthworms near a zinc-smelting
complex: influence of soil pH and organic matter. Bull. environ.
Contam. Toxicol., 30: 424-427.
MCCAHON, C.P., BROWN, A.F., & PASCOE, D. (1988) The effect of the
acanthocephalan Pomphorhynchus laevis (Muller 1776) on the acute
toxicity of cadmium to its intermediate host, the amphipod Gammarus
pulex (L.). Arch. environ. Contam. Toxicol., 17: 239-243.
MCCAHON, C.P., WHILES, A.J., & PASCOE, D. (1989) The toxicity of
cadmium to different larval instars of the trichopteran larvae
Agapetus fuscipes Curtis and the importance of life cycle
information to the design of toxicity tests. Hydrobiologia, 185:
153-162.
MCCLURG, T.P. (1984) Effects of fluoride, cadmium and mercury on the
estuarine prawn Penaeus indicus. Water S. Afr., 10: 40-45.
MACINNES, J.R. & THURBERG, F.P. (1973) Effects of metals on the
behaviour and oxygen consumption of the mud snail. Mar. Pollut. Bull.,
4: 185-186.
MACINNES, J.R., THURBERG, F.P., GREIG, R.A., & GOULD, E. (1977)
Long-term cadmium stress in the cunner, Tautogolabrus adspersus.
Fish. Bull., 75: 199-203.
MAHLER, R.J., BINGHAM, F.T., & PAGE, A.L. (1978) Cadmium-enriched
sewage sludge application to acid and calcareous soils. Effect on
yield and cadmium uptake by lettuce and chard. J. environ. Qual., 7:
274-281.
MARSHALL, J.S. (1979) Cadmium toxicity to laboratory and field
populations of Daphnia galeata mendotae. Bull. environ. Contam.
Toxicol., 21: 453-457.
MARTIN, J.H. & BROENKOW, W.W. (1975) Cadmium in plankton: elevated
concentrations off Baja California. Science, 190: 884-885.
MARTIN, M.H., COUGHTREY, P.J., & YOUNG, E.W. (1976) Observations on
the availability of lead, zinc, cadmium and copper in woodland litter
and the uptake of lead, zinc and cadmium by the woodlouse Oniscus
asellus. Chemosphere, 5: 313-318.
MARTIN, M.H., COUGHTREY, P.J., SHALES, S.W., & LITTLE, P. (1980)
Aspect of airborne cadmium contamination of soils and natural
vegetation. In: Inorganic pollution and agriculture, Her Majesty's
Stationary Office, London, pp. 56-69.
MAY, T.W. & MCKINNEY, G.L. (1981) Cadmium, lead, mercury, arsenic, and
selenium concentrations in freshwater fish, 1976-77 - National
pesticide monitoring program. Pestic. monit. J., 15: 14-38.
MAYACK, L.A., BUSH, P.B., FLETCHER, O.J., PAGE, R.K., & FENDLEY, T.T.
(1981) Tissue residues of dietary cadmium in wood ducks. Arch.
environ. Contam. Toxicol., 10: 637-645.
MAYER, F.L (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Washington, DC, US Department of Commerce, National
Technical Information Service, 274 pp (NTIS PB87-188686).
MEISCH, H.-U. & SCHMITT, J.A. (1986) Characterization studies on
cadmium-mycophosphatin from the mushroom Agaricus macrosporus.
Environ. Health Perspect., 65: 29-32.
MERLINI, M. (1978) Hepatic storage alteration of vitamin B12 by
cadmium in a freshwater fish. Bull. environ. Contam. Toxicol., 19:
767-771.
METEYER, M.J., WRIGHT, D.A., & MARTIN, F.D. (1988) Effect of cadmium
on early developmental stages of the sheepshead minnow ( Cyprinodon
variegatus). Environ. Toxicol. Chem., 7: 321-328.
MICHIBATA, H. (1981) Effect of water hardness on the toxicity of
cadmium to the egg of the teleost Oryzias latipes. Bull. environ.
Contam. Toxicol., 27: 187-192.
MIDDAUGH, D.P. & DEAN, J.M. (1977) Comparative sensitivity of eggs,
larvae, and adults of the estuarine teleosts, Fundulus heteroclitus
and Menidia menidia to cadmium. Bull. environ. Contam. Toxicol., 17:
645-652.
MIRENDA, R.J. (1986) Toxicity and accumulation of cadmium in the
crayfish, Orconectes virilis (Hagen). Arch. environ. Contam.
Toxicol., 15: 401-407.
MITCHELL, C.D. & FRETZ, T.A. (1977) Cadmium and zinc toxicity in white
pine, red maple and Norway spruce. J. Am. Soc. Hortic. Sci., 102:
81-84.
MITRA, R.S., GRAY, R.H., CHIN, B., & BERNSTEIN, I.A. (1975) Molecular
mechanisms of accommodation in Escherichia coli to toxic levels of
Cd2+. J. Bacteriol., 121: 1180-1188.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & LENTZOU, P. (1979)
Effects of sublethal concentrations of cadmium as possible indicators
of cadmium pollution for two populations of Acartia clausi
(Copepoda) living at two differently polluted areas. Bull. environ.
Contam. Toxicol., 23: 642-649.
MORAITOU-APOSTOLOPOULOU, M., VERRIOPOULOS, G., & ROGDAKIS, I. (1982)
Evaluation of the stress exerted by a polluted environment to a marine
organism by comparative toxicity tests. Bull. environ. Contam.
Toxicol., 28: 416-423.
MORGAN, J.E. & MORGAN, A.J. (1988) Earthworms as biological monitors
of cadmium, copper, lead and zinc in metalliferous soils. Environ.
Pollut., 54: 123-138.
MORGAN, J.E. & MORGAN, A.J. (1990) The distribution of cadmium,
copper, lead, zinc and calcium in the tissues of the earthworm
Lumbricus rubellus sampled from one uncontaminated and four polluted
soils. Oecologia, 84: 559-566.
MUINO, C.V., FERRARI, L., & SALIBIAN, A. (1990) Protective action of
ions against cadmium toxicity to young Bufo arenarum tadpoles. Bull.
environ. Contam. Toxicol., 45: 313-319.
MUIR, D.C.G., WAGEMANN, R., GRIFT, N.P., NORSTROM, R.J., SIMON, M. &
LIEN, J. (1988) Organochlorine chemical and heavy metal contaminants
in white-beaked dolphins ( Lagenorhynchus albirostris) and pilot
whales ( Globicephala melaena) from the coast of Newfoundland,
Canada. Arch. environ. Contam. Toxicol., 17: 613-629.
MURAMOTO, S. (1981a) Influence of complexans (EDTA, DTPA) on the
toxicity of cadmium to fish at chronic levels. Bull. environ. Contam.
Toxicol., 26: 641-646.
MURAMOTO, S. (1981b) Variations of some elements in cadmium-induced
malformed fish. Bull. environ. Contam. Toxicol., 27: 193-200.
MUSKETT, C.J. & JONES, M.P. (1980) The dispersal of lead, cadmium and
nickel from motor vehicles and effects on roadside invertebrate
macrofauna. Environ. Pollut., 23A: 231-242.
MYSING-GUBALA, M. & POIRRIER, M.A. (1981) The effects of cadmium and
mercury on gemmule formation and gemmosclere morphology in Ephydatia
fluviatilis (Porifera: Spongillidae). Hydrobiologia, 76: 145-148.
NAIDU, C.K. & REDDY, T.K.R. (1988) Effect of cadmium on microorganisms
and microbe-mediated mineralization process in the soil. Bull.
environ. Contam. Toxicol., 41: 657-663.
NAKADA, M., FUKAYA, K., TAKESHITA, S., & WADA, Y. (1979) The
accumulation of heavy metals in the submerged plant ( Elodea
nuttallii). Bull. environ. Contam. Toxicol., 22: 21-27.
NEBEKER, A.V., CAIRNS, M.A., ONJUKKA, S.T., & TITUS, R.H. (1986)
Effect of age on sensitivity of Daphnia magna to cadmium, copper and
cyanazine. Environ. Toxicol. Chem., 5: 527-530.
NEGILSKI, D.S. (1976) Acute toxicity of zinc, cadmium and chromium to
the marine fishes, yellow-eye mullet ( Aldrichetta forsteri C & V)
and small-mouthed hardyhead ( Atherinasoma microstoma Whitley). Aust.
J. mar. freshwater Res., 27: 137-149.
NEGILSKI, D.S., AHSANULLAH, M., & MOBLEY, M.C. (1981) Toxicity of
zinc, cadmium and copper to the shrimp Callianassa australiensis. II
Effects of paired and triad combinations of metals. Mar. Biol., 64:
305-309.
NELSON, D.A., CALABRESE, A., NELSON, B.A., MACINNES, J.R., & WENZLOFF,
D.R. (1976) Biological effects of heavy metals on juvenile bay
scallops, Argopecten irradians, in short-term exposures. Bull.
environ. Contam. Toxicol., 16: 275-282.
NICHOLSON, J.K. & OSBORN, D. (1983) Kidney lesions in pelagic seabirds
with high tissue levels of cadmium and mercury. J. Zool. (Lond.), 200:
99-118.
NICHOLSON, J.K., KENDALL, M.D., & OSBORN, D. (1983) Cadmium and
mercury nephrotoxicity. Nature (Lond.), 304: 633-635.
NIEDERLEHNER, B.R., BUIKEMA, A.L., PITTINGER, C.A., & CAIRNS, J.
(1984) Effects of cadmium on the population growth of a benthic
invertebrate Aeolosoma headleyi (Oligochaeta). Environ. Toxicol.
Chem., 3: 255-262.
NIMMO, D.R., LIGHTNER, D.V., & BAHNER, L.H. (1977) Effects of cadmium
on the shrimps Penaeus duorarum, Palaemonetes pugio and
Palaemonetes vulgaris. In: Vernberg, F.J., Calabrese, A., Thurberg,
F.P., & Vernberg, W.B., ed. Physiological responses of marine biota to
pollutants, New York, London, Academic Press, pp. 131-183.
NIMMO, D.R., RIGBY, R.A., BAHNER, L.H., & SHEPPARD, J.M. (1978) The
acute and chronic effects of cadmium on the estuarine mysid,
Mysidopsis bahia. Bull. environ. Contam. Toxicol., 19: 80-85.
NIR, R., GASITH, A., & PERRY, A.S. (1990) Cadmium uptake and toxicity
to water hyacinth: effect of repeated exposures under controlled
conditions. Bull. environ. Contam. Toxicol., 44: 149-157.
NORBERG, A.B. & MOLIN, N. (1983) Toxicity of cadmium, cobalt, uranium
and zinc to Zoogloea ramigera. Water Res., 17: 1333-1336.
NRIAGU, J.O. (1989) A global assessment of natural sources of
atmospheric trace metals. Nature (Lond.), 338: 47-49.
NRIAGU, J.O. & PACYNA, J.M. (1988) Quantitative assessment of
worldwide contamination of air, water and soils by trace metals.
Nature (Lond.), 333: 134-139.
O'HARA, J. (1973) The influence of temperature and salinity on the
toxicity of cadmium to the fiddler crab, Uca pugilator. Fish. Bull.,
71: 149-153.
OLLA, B.L., ESTELLE, V.B., SWARTZ, R.C., BRAUN, G., & STUDHOLME, A.L.
(1988) Responses of polychaetes to cadmium-contaminated sediment:
Comparison of uptake and behavior. Environ. Toxicol. Chem., 7:
587-592.
OLSON, P.-E. & HAUX, C. (1986) Increased hepatic metallothionein
content correlates to cadmium accumulation in environmentally exposed
perch ( Perca fluviatilis). Aquat. Toxicol., 9: 231-242.
OSBORN, D. & NICHOLSON, J.K. (1984) Cadmium and mercury in seabirds,
Huntingdon, UK Natural Environment Research Council, Institute of
Terrestrial Ecology, pp. 30-34 (ITE Symposium No. 12).
PAGE, A.L. & CHANG, A.C. (1978) Trace elements impact on plants during
cropland disposal of sewage sludges. In: Proceedings of the 5th
National Conference on Acceptable Sludge Disposal Techniques,
Rockville, Maryland, Information Transfer Inc., pp. 91-96.
PAGE, A.L., BINGHAM, F.T., & CHANG, A.C. (1981) Cadmium. In: Lepp,
N.W., ed. Effect of heavy metal pollution on plants, London, Applied
Science, Vol. 1, pp. 77-109.
PART, P. & WIKMARK, G. (1984) The influence of certain complexing
agents (EDTA and citrate) on the uptake of cadmium in perfused rainbow
trout gills. Aquat. Toxicol., 5: 277-289.
PART, P., SVANBERG, O., & KIESSLING, A. (1985) The availability of
cadmium to perfused rainbow trout gills in different water qualities.
Water Res., 19: 427-434.
PASCOE, D., EVANS, S.A., & WOODWORTH, J. (1986) Heavy metal toxicity
to fish and the influence of water hardness. Arch. environ. Contam.
Toxicol., 15: 481-487.
PECON, J. & POWELL, E.N. (1981) Effects of the amino acid histidine on
the uptake of cadmium from the digestive system by the blue crab
Callinectes sapidus. Bull. environ. Contam. Toxicol., 27: 34-41.
PENTREATH, R.J. (1977) The accumulation of cadmium by the plaice
Pleuronectes platessa L. and the thornback ray Raia clavata. J.
exp. mar. Biol. Ecol., 30: 223-232.
PEREZ-COLL, C.S., HERKOVITS, J., & SALIBIAN, A. (1986) Teratogenic
effects of cadmium on Bufo arenarum during gastrulation. Experientia
(Basel), 42: 1174-1176.
PESCH, G.G. & STEWART, N.E. (1980) Cadmium toxicity to three species
of estuarine invertebrates. Mar. environ. Res., 3: 145-156.
PETERSON, P.J. & ALLOWAY, B.J. (1979) Cadmium in soils and vegetation.
In: Webb, M., ed. The chemistry, biochemistry and biology of cadmium,
Amsterdam, Elsevier/North Holland, pp. 45-92.
PICKERING, Q.H. & GAST, M.H. (1972) Acute and chronic toxicity of
cadmium to the fathead minnow Pimephales promelas. J. Fish Res.
Board. Can., 29: 1099-1106.
PICKERING, Q.H. & HENDERSON, C. (1966) The acute toxicity of some
heavy metals to different species of warmwater fishes. Int. J. Air
water Pollut., 10: 453-463.
PIETZ, R.I., PETERSON, J.R., PRATER, J.E., & ZENZ, D.R. (1984) Metal
concentrations in earthworms from sewage sludge-amended soils at a
strip mine reclamation site. J. environ. Qual., 13: 651-654.
PINOWSKA, B., KRASNICKI, K., & PINOWSKI, J. (1981) Estimation of the
degree of contamination of granivorous birds with heavy metals in
agricultural and industrial landscape. Ekol. Polska, 29: 137-149.
POLDOSKI, J.E. (1979) Cadmium bioaccumulation assays. Their
relationships to various ion equilibria in Lake Superior water.
Environ. Sci. Technol., 13: 701-706.
POPHAM, J.D. & WEBSTER, J.M. (1979) Cadmium toxicity in the
free-living nematode, Caenorhabditis elegans. Environ. Res., 20:
183-191.
PRAHALAD, A.K. & SEENAYYA, G. (1988) Bioavailability of zinc and
cadmium and their effect on microbial growth and metal uptake. Bull.
environ. Contam. Toxicol., 41: 921-927.
PRITZL, M.C., LIE, Y.H., KIENHOLZ, E.W., & WHITEMAN, C.E. (1974) The
effect of dietary cadmium on development of young chickens. Poult.
Sci., 53: 2026-2029.
RAINBOW, P.S. & WHITE, S.L. (1989) Comparative strategies of heavy
metal accumulation by crustaceans: zinc, copper and cadmium in a
decapod, an amphipod and a barnacle. Hydrobiologia, 174: 245-262.
RAMAMOORTHY, S. & BLUMHAGEN, K. (1984) Uptake of Zn, Cd and Hg by fish
in the presence of competing compartments. Can. J. Fish aquat. Sci.,
41: 750-756.
RAMIREZ, P., BARRERA, G., & ROSAS, C. (1989) Effects of chromium and
cadmium upon respiration and survival of Callinectes similis. Bull.
environ. Contam. Toxicol., 43: 850-857.
RAY, S., MCLEESE, D.W., WAIWOOD, B.A., & PEZZACK. D. (1980a) The
disposition of cadmium and zinc in Pandalus montagui. Arch. environ.
Contam. Toxicol., 9: 675-681.
RAY, S., MCLEESE, D., & PEZZACK, D. (1980b) Accumulation of cadmium by
Nereis virens. Arch. environ. Contam. Toxicol., 9: 1-8.
REBHUN, S. & BEN-AMOTZ, A. (1984) The distribution of cadmium between
the marine alga Chlorella stigmatophora and sea water medium. Water
Res., 18: 173-178.
REHWOLDT, R. & KARIMIAN-TEHERANI, D. (1976) Uptake and effect of
cadmium on zebrafish. Bull. environ. Contam. Toxicol., 15: 442-446.
REID, S.D. & MCDONALD, D.G. (1988) Effects of cadmium, copper and low
pH on ion fluxes in rainbow trout, Salmo gairdneri. Can. J. Fish
aquat. Sci., 45: 244-253.
RIISGARD, H.U., BJORNESTAD, E., & MOHLENBERG, F. (1987) Accumulation
of cadmium in the mussel Mytilus edulis: Kinetics and importance of
uptake via food and sea water. Mar. Biol., 96: 349-353.
ROBERT, R. & HIS, E. (1985) Combined effects of salinity and cadmium
chloride upon embryos and larvae of the Japanese oyster, Crassostrea
gigas. Mar. environ. Res., 15: 303-312.
ROBERTS, M.H., WARINNER, J.E., CHU-FA TSAI, WRIGHT, D., & CRONIN, L.E.
(1982) Comparison of estuarine species sensitivities to three
toxicants. Arch. environ. Contam. Toxicol., 11: 681-692.
ROBERTS, R.D. & JOHNSON, M.S. (1978) Dispersal of heavy metals from
abandoned mine workings and their transference through terrestrial
food chains. Environ. Pollut., 16: 293-310.
ROBERTS, T.M., HEPPLESTON, P.B., & ROBERTS, R.D. (1976) Distribution
of heavy metals in tissues of the common seal. Mar. Pollut. Bull., 7:
194-196.
ROBINSON, A.M., LAMBERSON, J.O., COLE, F.A., & SWARTZ, R.C. (1988)
Effects of culture conditions on the sensitivity of a phoxocephalid
amphipod, Rhepoxynius abronius, to cadmium in sediment. Environ.
Toxicol. Chem., 7: 953-959.
ROBINSON, K.M. & WELLS, M.R. (1975) Retention of a single oral dose of
cadmium in tissues of the softshell turtle, Trionyx spinifer. Bull.
environ. Contam. Toxicol., 14: 750-752.
ROCH, M. & MALY, E.J. (1979) Relationship of cadmium-induced
hypocalcemia with mortality in rainbow trout ( Salmo gairdneri) and
the influence of temperature on toxicity. J. Fish Res. Board Can., 36:
1297-1303.
ROMBOUGH, P.J. & GARSIDE, E.T. (1982) Cadmium toxicity and
accumulation in aggs and alevins of Atlantic salmon Salmo salar. Can.
J. Zool., 60: 2006-2014.
ROOT, R.A., MILLER, R.J., & KOEPPE, D.E. (1975) Uptake of cadmium -
its toxicity, and effect on the iron ratio in hydroponically grown
corn. J. environ. Qual., 4: 473-476.
ROSENBERG, R. & COSTLOW, J.D. (1976) Synergistic effects of cadmium
and salinity combined with constant and cycling temperatures on the
larval development of two estuarine crab species. Mar. Biol., 38:
219-303.
RUSSELL, L.K., DEHAVEN, J.I., & BOTTS, R.P. (1981) Toxic effects of
cadmium on the garden snail ( Helix aspersa). Bull. environ. Contam.
Toxicol., 26: 634-640.
SANGALANG, G.B. & FREEMAN, H.C. (1979) Tissue uptake of cadmium in
brook trout during chronic sublethal exposure. Arch. environ. Contam.
Toxicol., 8: 77-84.
SANGSTER, B., DE GROOT, G., LOEBER, J.G., DERKS, H.J.G.M., KRAJNC,
E.I., & SAVELKOUL, T.J.F. (1984) Urinary excretion of cadmium,
protein, beta-2-microglobulin and glucose in individuals living in a
cadmium-polluted area. Hum. Toxicol., 3: 7-21.
SASTRY, K.V. & SUBHADRA, K.M. (1983) Cadmium induced alterations in
the intestinal absorption of glucose and fructose in a freshwater
catfish Heteropneustes fossilis. Water Air Soil Pollut., 20:
293-297.
SATO, C., SCHNOOR, J.L., & MCDONALD, D.B. (1986) Characterization of
effects of copper, cadmium and nickel on the growth of Nitrosomonas
europaea. Environ. Toxicol. Chem., 5: 403-416.
SAWICKA-KAPUSTA, K., GORECKI, A., SWIERGOSZ, R., JUSZCZAK, G.,
MIELCZAREK, M., & WOJCIK, B. (1987) Effect of metabolic rate on the
rate of elimination of high and low concentrations of cadmium and lead
in the bank vole. Ekol. Polska, 35: 399-430.
SCHEUHAMMER, A.M. (1987) The chronic toxicity of aluminium, cadmium,
mercury and lead in birds: a review. Environ. Pollut., 46: 263-295.
SCHUYTEMA, G.S., NELSON, P.O., MALUEG, K.W., NEBEKER, A.V., KRAWCZYK,
D.F., RATCLIFF, A.K., & GAKSTATTER, J.H. (1984) Toxicity of cadmium in
water and sediment slurries to Daphnia magna. Environ. Toxicol.
Chem., 3: 293-308.
SHILLER, A.M. & BOYLE, E.A. (1987) Variability of dissolved trace
metals in the Mississippi River. Geochim. Cosmochim. Acta, 51:
3273-3277.
SHIVARAJ, K.M. & PATIL, H.S. (1988) Toxicity of cadmium and copper to
a freshwater fish Puntius arulius. Environ. Ecol., 6: 5-8.
SHORE, R.F., MYHILL, D.G., POLWORTH, G., & DUNMOW, M. (1991) The
effects of chronic doses of dietary cadmium on macro-element balance
in wood mice and bank voles. In: Momciloric, B., ed. Proceedings of
the Seventh International Symposium on Trace Elements in Man and
Animals (TEMA-7), Dubrovnik, Yugoslavia, 20-25 May 1990, Zagreb, IMI
(Institute for Medical Research and Occupational Health, University of
Zagreb), pp. 17-18.
SILLEN, L.G. & MARTELL, A.E. (1964) Stability constants of metal-ion
complexes. Section I: Inorganic ligands compiled by L.G. Sillen.
Section II: Organic ligands compiled by A.E. Martell, London, The
Chemical Society, 754 pp (Special Publication No. 17).
SIMON, E. (1977) Cadmium tolerance in populations of Agrostis tenuis
and Festuca ovina. Nature (Lond.), 265: 328-330.
SIMPSON, W.R. (1981) A critical review of cadmium in the marine
environment. Prog. Oceanogr., 10: 1-70.
SJOBECK, M.-L., HAUX, C., LARSSON, A., & LITHNER, G. (1984)
Biochemical and hematological studies on perch, Perca fluviatilis,
from the cadmium-contaminated river Eman. Ecotoxicol. environ. Saf.,
8: 303-312.
SKOWRONSKI, T., PAWLIK, B., & JAKUBOWSKI, M. (1988) Reduction of
cadmium toxicity to green microalga Stichococcus bacillaris by
manganese. Bull. environ. Contam. Toxicol., 41: 915-920.
SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the usefulness of the
mexican axolotl ( Ambystoma mexicanum) and the clawed toad ( Xenopus
laevis) in toxicological bioassays. Bull. environ. Contam. Toxicol.,
24: 439-443.
SMITH, B.P., HEJTMANCIK, E., & CAMP, B.J. (1976) Acute effects of
cadmium on Ictalurus punctatus (catfish). Bull. environ. Contam.
Toxicol., 15: 271-277.
SMITH, R.M. & MARTELL, A.E. (1974) In: Critical stability constants,
Vol. 1: Amino acids, New York, Plenum Press, 469 pp.
SMITH, R.M. & MARTELL, A.E. (1975) In: Critical stability constants,
Vol. 2: Amines, New York, Plenum Press, 415 pp.
SMITH, R.M. & MARTELL, A.E. (1976) In: Critical stability constants,
Vol. 3: Other organic ligands, New York, Plenum Press, 495 pp.
SPEHAR, R.L., ANDERSON, R.L., & FIANDT, J.T. (1978a) Toxicity and
bioaccumulation of cadmium and lead in aquatic invertebrates. Environ.
Pollut., 15A: 195-208.
SPEHAR, R.L., LEONARD, E.N., & DEFOE, D.L. (1978b) Chronic effects of
cadmium and zinc mixtures on flagfish ( Jordanella floridae). Trans.
Am. Fish. Soc., 107: 354-360.
SPRAGUE, J. (1985) Factors that modify toxicity. In: Rand, G.M. &
Petrocelli, S.R., ed. Fundamentals of aquatic toxicology, New York,
Hemisphere Publishing Corporation, pp. 124-163.
SPRY, D.J. & WOOD, C.M. (1989) A kinetic method for the measurement of
zinc influx in vivo in the rainbow trout, and the effects of
waterborne calcium on flux rates. J. exp. Biol., 142: 425-446.
STROJAN, C.L. (1978) Forest leaf litter decomposition in the vicinity
of a zinc smelter. Oecologia, 32: 203-212.
SULLIVAN, J.F., MURPHY, B.R., ATCHISON, G.J., & MCINTOSH, A.W. (1978a)
Time dependant cadmium uptake by fathead minnows ( Pimephales
promelas) during field and laboratory exposure. Hydrobiologia, 57:
65-68.
SULLIVAN, J.F., ATCHISON, G.J., KOLAR, D.J., & MCINTOSH, A.W. (1978b)
Changes in the predator-prey behavior of fathead minnows ( Pimephales
promelas) and largemouth bass ( Micropterus salmoides) caused by
cadmium. J. Fish Res. Board. Can., 35: 446-451.
SUNDA, W.G., ENGEL, D.W., & THUOTTE, R.M. (1978) Effect of chemical
speciation on toxicity of cadmium to grass shrimp, Palaemonetes
pugio: Importance of free cadmium ion. Environ. Sci. Technol., 12:
409-413.
TAYLOR, D. (1983) The significance of the accumulation of cadmium by
aquatic organisms. Ecotoxicol. environ. Saf., 7: 33-42.
TERAOKA, H. (1989) Impact of atmospheric trace metals on rice roots.
Arch. environ. Contam. Toxicol., 18: 269-275.
THURBERG, F.P., DAWSON, M.A., & COLLIER, R.S. (1973) Effects of copper
and cadmium on osmoregulation and oxygen consumption in two species of
estuarine crabs. Mar. Biol., 23: 171-175.
TJALVE, H., GOTTOFREY, J., & BJORKLUND, I. (1986) Tissue disposition
of 109Cd2+ in the brown trout ( Salmo trutta) studied by
autoradiography and impulse counting. Toxicol. environ. Chem., 12:
31-45.
TYLER, G. (1972) Heavy metals pollute nature, may reduce productivity.
Ambio, 1: 52-59.
TYLER, G., MORNSJO, B., & NILSSON, B. (1974) Effects of cadmium, lead,
and sodium salts on nitrification in a mull soil. Plant Soil, 40:
237-242.
VAN AALST, R.M., VAN ARDENNE, R.A.M., DE KRUK, J.F., & LEMS, T.
(1983a) Pollution of the North Sea from the atmosphere, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. C182/152).
VAN AALST, R.M., DUYZER, J.H., & VELDT, C. (1983b) Atmospheric
deposition of lead and cadmium in the southern part of the North Sea.
I. Emission and preliminary model calculations, Apeldoorn, The
Netherlands, Organization for Applied Scientific Research (TNO)
(Report No. R83/222).
VAN HATTUM, B., DE VOOGT, P., VAN DEN BOSCH, L., VAN STRAALEN, N.M.,
JOOSSE, E.N.G., & GOVERS, H. (1989) Bioaccumulation of cadmium by the
freshwater isopod Asellus aquaticus (L.) from aqueous and dietary
sources. Environ. Pollut., 62: 129-151.
VAN HOOK, R.I. (1974) Cadmium, lead, and zinc distributions between
earthworms and soils: potentials for biological accumulation. Bull.
environ. Contam. Toxicol., 12: 509-512.
VAN HOOK, R.I. & YATES, A.J. (1975) Transient behavior of cadmium in
a grassland arthropod food chain. Environ. Res., 9: 76-83.
VAN KESSEL, W.H.M., BROCADES ZAALBERG, R.W., & SEINEN, W. (1989)
Testing environmental pollutants on soil organisms: A simple assay to
investigate the toxicity of environmental pollutants on soil
organisms, using CdCl2 and nematodes. Ecotoxicol. environ. Saf., 18:
181-190.
VAN LEEUWEN, C.J., LUTTMER, W.J., & GRIFFIOEN, P.S. (1985) The use of
cohorts and populations in chronic toxicity studies with Daphnia
magna: A cadmium example. Ecotoxicol. environ. Saf., 9: 26-39.
VAN STRAALEN, N.M. & VAN WENSEM, J. (1986) Heavy metal content of
forest litter arthropods as related to body-size and trophic level.
Environ. Pollut., 42A: 209-221.
VAN STRAALEN, N.M., BURGHOUTS, T.B.A., DOORNHOF, M.J., GROOT, G.M.,
JANSSEN, M.P.M., JOOSSE, E.N.G., VAN MEERENDONK, J.H., THEEUWEN,
J.P.J.J., VERHOEF, H.A., & ZOOMER, H.R. (1987) Efficiency of lead and
cadmium excretion in populations of Orchesella cincta (Collembola)
from various contaminated forest soils. J. appl. Ecol., 24: 953-968.
VAN STRAALEN, N.M., SCHOBBEN, J.H.M., & DE GOEDE, R.G.M. (1989)
Population consequences of cadmium toxicity in soil microarthropods.
Ecotoxicol. environ. Saf., 17: 190-204.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1987)
Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am. J.
Physiol., 253: R216-R221.
VERBOST, P.M., FLIK, G., LOCK, R.A.C., & WENDELAAR BONGA, S.E. (1988)
Cadmium inhibits plasma membrane calcium transport. J. membrane Biol.,
102: 97-104.
VERBOST, P.M., VAN ROOIJ, J., FLIK, G., LOCK, R.A.C., & WENDELAAR
BONGA, S.E. (1989) The movement of cadmium through freshwater trout
branchial epithelium and its interference with calcium transport. J.
exp. Biol., 145: 185-197.
VERNBERG, W.B., DE COURSEY, P.J., & O'HARA, J. (1974) Multiple
environmental factor effects on physiology and behavior of the fiddler
crab, Uca pugilator. In: Vernberg, F.G. & Vernberg, W.B., ed.
Pollution and physiology of marine organisms, New York, London,
Academic Press, pp. 381-425.
VERNBERG, W.B., DE COURSEY, P.J., KELLY, M., & JOHNS, D.M. (1977)
Effects of sublethal concentrations of cadmium on adult Palaemonetes
pugio under static and flow-through conditions. Bull. environ.
Contam. Toxicol., 17: 16-24.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1981) Effects of some
environmental factors on the toxicity of cadmium to the copepod Tisbe
holothuriae. Arch. Hydrobiol., 91: 287-293.
VERRIOPOULOS, G. & MORAITOU-APOSTOLOPOULOU, M. (1982) Differentiation
of the sensitivity to copper and cadmium in different life-stages of
a copepod. Mar. Pollut. Bull., 13: 123-125.
VOYER, R.A. (1975) Effect of dissolved oxygen concentration on the
acute toxicity of cadmium to the mummichog, Fundulus heteroclitus
(L.), at various salinities. Trans. Am. Fish. Soc., 104: 129-134.
VOYER, R.A. & MODICA, G. (1990) Influence of salinity and temperature
on acute toxicity of cadmium to Mysidopsis bahia Molenock. Arch.
environ. Contam. Toxicol., 19: 124-131.
VOYER, R.A., YEVICH, P.P., & BARSZCZ, C.A. (1975) Histological and
toxicological responses of the mummichog, Fundulus heteroclitus
(L.), to combinations of levels of cadmium and dissolved oxygen in a
freshwater. Water Res., 9: 1069-1074.
WALTERSSON, E. (1984) [Effects of enrichment reagents - distribution
and spread of xanthates and xanthate derivatives during enrichment
activities], Stockholm, Swedish Environmental Research Institute
(Report B478) (in Swedish).
WATLING, H.R. & WATLING, R.J. (1983) Sandy beach molluscs as possible
bioindicators of metal pollution. 2. Laboratory studies. Bull.
environ. Contam. Toxicol., 31: 339-343.
WATSON, C.F. & BENSON, W.H. (1987) Comparative activity of gill
ATPases in three freshwater teleosts exposed to cadmium. Ecotoxicol.
Environ. Saf., 14: 252-259.
WEIS, J.S. & WEIS, P. (1977) Effects of heavy metals on development of
the killifish, Fundulus heteroclitus. J. Fish Biol., 11: 49-54.
WESTERMAN, A.G. (1977) Lethal and teratogenic effects of inorganic
mercury and cadmium on embryonic development of Anurans, Lexington,
University of Kentucky (M.S. Thesis).
WHITE, D.H. & FINLEY, M.T. (1978) Uptake and retention of dietary
cadmium in mallard ducks. Environ. Res., 17: 53-59.
WHITE, D.H., FINLEY, M.T., & FERRELL, J.F. (1978) Histopathologic
effects of dietary cadmium on kidneys and testes of mallard ducks. J.
Toxicol. environ. Health, 4: 551-558.
WHO (1992) Environmental Health Criteria 134: Cadmium. Geneva, World
Health Organization, 280 pp.
WHO (1986) Health impact of acidic deposition. Sci. total Environ.,
52: 157-187.
WICKLUND, A. (1990) Metabolism of cadmium and zinc in fish, University
of Uppsala, Sweden, Faculty of Science (Acta Uiversitatis Uppsaliensis
- Comprehensive summaries of Uppsala dissertations from the Faculty of
Science, No. 250).
WIER, C.F. & WALTER, W.M. (1976) Toxicity of cadmium in the freshwater
snail, Physa gyrina Say. J. environ. Qual., 5: 359-362.
WILLIAMS, C.H. & DAVID, D.J. (1973) The effect of superphosphate on
the cadmium content of soil and plants. Aust. J. soil Res., 11: 43-56.
WILLIAMS, C.H. & DAVID, D.J. (1976) The accumulation in soil of
cadmium residues from phosphate for fertilizers and their effect on
the cadmium content of plants. Soil Sci., 121: 86-93.
WILLIAMS, D.R. & GIESY, J.P. (1978) Relative importance of food and
water sources to cadmium uptake by Gambusia affinis ( Poeciliidae).
Environ. Res., 16: 326-332.
WILLIAMS, K.A., GREEN, D.W.J., & PASCOE, D. (1985) Studies on the
acute toxicity of pollutants to freshwater macroinvertebrates. I.
Cadmium. Arch. Hydrobiol., 102: 461-471.
WILLIAMS, K.A., GREEN, D.W.J., PASCOE, D., & GOWER, D.E. (1987) Effect
of cadmium on oviposition and egg viability in Chironomus riparius
(Diptera: Chironomidae). Bull. environ. Contam. Toxicol., 38: 86-90.
WILLIAMS, P.L. & DUSENBERY, D.B. (1990) Aquatic toxicity testing using
the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem., 9:
1285-1290.
WILLIAMSON, P. (1980) Variables affecting body burdens of lead, zinc
and cadmium in a roadside population of the snail Cepaea hortensis
Muller. Oecologia, 44: 213-220.
WILSON, D.N. (1988) Cadmium - market trends and influences. In:
Cadmium 87. Proceedings of the 6th International Cadmium Conference,
London, Cadmium Association, pp. 9-16.
WINNER, R.W. (1986) Interactive effects of water hardness and humic
acid on the chronic toxicity of cadmium to Daphnia pulex. Aquat.
Toxicol., 8: 281-293.
WINNER, R.W. (1988) Evaluation of the relative sensitivities of 7-d
Daphnia magna and Ceriodaphnia dubia toxicity tests for cadmium
and sodium pentachlorophenate. Environ. Toxicol. Chem., 7: 153-159.
WONG, P.T.S., BURNISON, G., & CHAU, Y.K. (1979) Cadmium toxicity to
freshwater algae. Bull. environ. Contam. Toxicol., 23: 487-490.
WONG, Y.S., LAM, H.M., DHILLON, E., TAM, N.F.Y., & LEUNG, W.N. (1988)
Physiological effects and uptake of cadmium in Pisum sativum.
Environ. Int., 14: 535-543.
WOODALL, C., MACLEAN, N., & CROSSLEY, F. (1988) Responses of trout fry
( Salmo gairdneri) and Xenopus laevis tadpoles to cadmium and zinc.
Comp. Biochem. Physiol., 89C: 93-99.
WRIGHT, D.A. & FRAIN, J.W. (1981a) Cadmium toxicity in Marinogammarus
obtusatus. Effect of external calcium. Environ. Res., 24: 338-344.
WRIGHT, D.A. & FRAIN, J.W. (1981b) The effect of calcium on cadmium
toxicity in the freshwater amphipod, Gammarus pulex (L.). Arch.
environ. Contam. Toxicol., 10: 321-328.
WRIGHT, D.A., METEYER, M.J., & MARTIN, F.D. (1985) Effect of calcium
on cadmium uptake and toxicity in larvae and juveniles of striped bass
( Morone saxatilis). Bull. environ. Contam. Toxicol., 34: 196-204.
YAMAGATA, N. & SHIGEMATSU, I. (1970) Cadmium pollution in perspective.
Bull. Inst. Public Health (Tokyo), 24: 18-24.
ZAROOGIAN, G.E. & CHEER, S. (1976) Accumulation of cadmium by the
American oyster, Crassostrea virginica. Nature (Lond.), 261:
408-410.
ZAROOGIAN, G.E. & MORRISON, G. (1981) Effect of cadmium body burdens
in adult Crassostrea virginica on fecundity and viability of larvae.
Bull. environ. Contam. Toxicol., 27: 344-348.
ZIRINO, A. & YAMAMOTO, S. (1972) A pH-dependent model for the chemical
speciation of copper, zinc, cadmium and lead in seawater. Limnol.
Oceanogr., 17(5): 661-671.
Appendix 1. Global emissions of trace metals from natural sources (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Wind-borne soil particles
range 0.3-5.0 0.01-0.4 0.9-15 0-0.01 0.3-7.5 0.01-0.35 3.0-35
median 2.6 0.21 8.0 0.05 3.9 0.18 19
Sea salt spray
range 0.19-3.1 0-0.11 0.23-6.9 0-0.04 0.02-2.8 0-1.1 0.02-0.86
median 1.7 0.06 3.6 0.02 1.4 0.55 0.44
Volcanoes
range 0.15-7.5 0.14-1.5 0.9-18 0.03-2.0 0.54-6.0 0.10-1.8 0.31-19
median 3.8 0.82 9.4 1.0 3.3 0.95 9.6
Forest fires
range 0-0.38 0-0.22 0.1-7.5 0-0.05 0.06-3.8 0-0.52 0.3-15
median 0.19 0.11 3.8 0.02 1.9 0.26 7.6
Biogenic continental particulates
range 0.2-0.5 0-0.83 0.1-5.0 0-0.04 0.02-2.5 0-0.25 0.3-5.0
median 0.26 0.15 2.6 0.02 1.3 1.12 2.6
Biogenic continental volatiles
range 0.03-2.5 0-0.8 0.01-0.62 0.02-1.2 0.01-0.38 0.15-5.0 0.02-5.0
median 1.3 0.04 0.32 0.61 0.20 2.6 2.5
Appendix 1 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Biogenic marine
range 0.16-4.5 0-0.1 0.02-0.75 0.04-1.5 0.02-0.45 0.4-9.0 0.04-6.0
median 2.3 0.05 0.39 0.77 0.24 4.7 3.0
Total emission
range 0.86-23 0.15-2.6 2.3-54 0.1-4.9 0.97-23 0.66-18 4.0-86
median 12 1.3 28 2.5 12 9.3 45
a From: Nriagu (1989)
Appendix 2. Natural and anthropogenic emissions of trace metals to the atmosphere in 1983 (x 1000 tonnes/year) a
Trace metal Anthropogenic source Natural source Total emission Natural/total emissions
Arsenic 19 (12-26) 12 (0.86-23) 31 (13-49) 0.39
Cadmium 7.6 (3.1-12) 1.3 (0.15-2.6) 8.9 (3.2-15) 0.15
Copper 35 (20-51) 28 (2.3-54) 63 (22-105) 0.44
Mercury 3.6 (0.91-6.2) 2.5 (0.10-4.9) 6.1 (1.0-11) 0.41
Lead 332 (289-376) 12 (0.97-23) 344 (290-399) 0.04
Selenium 6.3 (3.0-9.7) 9.3 (0.66-18) 16 (2.5-24) 0.58
Zinc 132 (70-194) 45 (4.0-86) 177 (74-280) 0.34
a From: Nriagu (1989)
Appendix 3. Sources of global emissions of trace elements to the atmosphere in 1983 (tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Coal combustion - electric utilities
232-1550 77-387 930-3100 155-542 775-4650 108-775 1085-7750
Coal combustion - industry and domestic
198-1980 99-495 1390-4950 495-2970 990-9900 792-1980 1485-11 880
Oil combustion - electric utilities
5.8-29 23-174 348-2230 - 232-1740 35-290 174-1280
Oil combustion - industry and domestic
7.2-72 18-72 179-1070 - 716-2150 107-537 358-2506
Pyrometallurgical non-ferrous metal production - mining
40.0-80 0.6-3 160-800 - 1700-3400 18-176 310-620
Pyrometallurgical non-ferrous metal production - Pb production
780-1560 39-195 234-312 7.8-16 11 700-31 200 195-390 195-468
Pyrometallurgical non-ferrous metal production - Cu-Ni production
8500-12 750 1700-3400 14 450-33 600 37-207 11 050-22 100 427-1280 4250-8500
Pyrometallurgical non-ferrous metal production - Zn-Cd production
230-690 920-4600 230-690 - 5520-11 500 92-23 46 000-82 800
Secondary non-ferrous metal production
- 2.3-3.6 55-165 - 90-1440 3.8-19 270-1440
Steel and iron manufacturing
355-2480 28-284 142-2840 - 1065-14 200 0.8-2.2 7100-31 950
Refuse incineration - municipal
154-392 56-1400 980-1960 140-2100 1400-2800 28-70 2800-8400
Refuse incineration - sewage sludge
15-60 3-36 30-180 15-60 240-300 3-30 150-450
Appendix 3 (contd).
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Phosphate fertilizers
- 68-274 137-685 - 55-274 0.4-1.2 1370-6850
Cement production
178-890 8.9-534 - - 18-14 240 - 1780-17 800
Wood combustion
60-300 60-180 600-1200 60-300 1200-3000 - 1200-6000
Mobile sources (gasoline)
- - - - 248 030 - -
Miscellaneous
1250-2800 - - - 3900-5100 - 1724-4783
Total emissions - range
12 000-25 630 3100-12 040 19 860-50 870 910-6200 288 700-376 000 1810-5780 70 250-193 500
Total emissions - median
18 820 7570 35 370 3560 332 350 3790b 131 880
a From: Nriagu & Pacyna (1988)
b This value applies to particulate selenium only. Since volatile selenium accounts for about
40% of the selenium released, the total selenium emission is estimated to be 6300 tonnes per year.
Appendix 4. Anthropogenic inputs of trace elements to aquatic ecosystems (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
3.6-7.7 0.9-3.6 6.0-15 0.22-1.8 87-113 0.54-1.1 21-58
Other industrial sources
8.4-62.3 1.2-13.4 29-75 0.08-7.0 10-67 9.5-70.9 56-317
Total - range
12-70 2.1-17 35-90 0.3-8.8 97-180 10-72 77-375
Total - median
41 9.4 62 4.6 138 41 226
Atmospheric fallout/total anthropogenic inputs (%)
14 24 17 22 72 2 17
a From: Nriagu & Pacyna (1988)
Appendix 5. Anthropogenic inputs of trace elements to soils (x 1000 tonnes/year) a
Arsenic Cadmium Copper Mercury Lead Selenium Zinc
Atmospheric fallout
8.4-18 2.2-8.4 14-36 0.63-4.3 202-263 1.3-2.6 49-135
Other industrial sources
43.6-94 3.4-29.6 527-1331 0.97-10.7 277-850 4.7-73.4 640-1919
Total input - range b
52-112 5.6-38 541-1367 1.6-15 479-1113 6.0-76 689-2054
Total input - median b
82 22 954 8.3 796 41 1372
Atmospheric fallout/total anthropogenic inputs (%) b
16 24 3 30 29 5 7
a From: Nriagu & Pacyna (1988)
b These data do not include inputs from mine tailings, smelter slags and wastes to land.
RESUME
Le cadmium (numéro atomique 48; masse atomique relative 112,40)
est un élément métallique qui appartient, avec le zinc et le mercure,
au groupe IIb du tableau périodique. Certains sels de cadmium, tels
que le sulfure, le carbonate et l'oxyde sont presque insolubles dans
l'eau; ils peuvent cependant être transformés en sels solubles dans
l'environnement naturel. La formation de différents dérivés du cadmium
dans l'environnement est importante pour l'évaluation du risque.
La teneur moyenne en cadmium de l'eau de mer est d'environ 0,1
µg/litre ou moins. L'eau des cours d'eau contient du cadmium dissous
à des concentrations allant de < 1 à 13,5 ng/litre. Dans les régions
écartées et inhabitées, on observe en général des concentrations de
cadmium dans l'air inférieures à 1 ng/m3. Dans les régions où l'on
ne connaît pas de pollution, la concentration médiane en cadmium dans
le sol se situerait entre 0,2 et 0,4 mg/kg. Toutefois, on rencontre
occasionnellement des valeurs beaucoup plus élevées pouvant aller
jusqu'à 160 mg/kg de terre.
Certains facteurs environnementaux influent sur la fixation, et
par voie de conséquence, sur les effets toxiques du cadmium sur les
organismes aquatiques. Une élévation de température augmente la
fixation et l'effet toxique du cadmium qui sont en revanche réduits
lorsque la salinité de l'eau ou sa dureté augmentent. Les organismes
d'eau douce sont sensibles à des concentrations plus faibles de
cadmium que les organismes marins. Plus la teneur de l'eau en matières
organiques est élevée, plus la fixation et les effets toxiques du
cadmium sont réduits car ces matières se lient au cadmium et en
réduisent la biodisponibilité. Toutefois, on est fondé à croire que
certains dérivés organiques pourraient avoir un effet inverse.
Le cadmium s'accumule facilement dans de nombreux organismes, en
particulier les microorganismes et les mollusques, pour lesquels le
facteur de bioconcentration peut atteindre plusieurs milliers. Les
invertébrés terricoles concentrent égale-ment assez fortement le
cadmium. Pour la plupart des organismes, le facteur de concentration
est faible à modéré, généralement inférieur à 100. Dans de nombreux
tissus, le cadmium est lié aux protéines. On a isolé d'organismes
exposés au cadmium, des pro-téines qui fixent spécifiquement les
métaux lourds (métallothio-néines). Le cadmium se concentre
préférentiellement dans les reins, les branchies et le foie (ou leurs
équivalents). L'élimination du métal s'effectue probablement par le
rein, encore que chez les crustacés, il puisse être éliminé en
quantités notables en passant dans l'exosquelette. Chez les plantes,
le cadmium se concentre principalement dans les racines et à un
moindre degré dans les feuilles.
Le cadmium est toxique pour de nombreux microorganismes.
Toutefois, la présence de sédiments et de fortes concentrations de
sels ou de matières organiques en solution réduit ces effets toxiques.
Ces effets s'exercent principalement sur la croissance et la
réplication. Parmi les microorganismes terricoles, les plus affectés
sont les champignons, dont certaines espèces peuvent être éliminées
lorsque le sol est contaminé par du cadmium. Une faible exposition au
cadmium présent dans le sol peut entraîner une sélection des souches
résistantes. La toxicité du cadmium pour les organismes aquatiques est
variable, même lorsqu'il s'agit d'espèces très proches et elle est
liée à la concentration du métal sous forme ionique. Le cadmium
perturbe le métabolisme du calcium chez l'animal. Chez les poissons,
il provoque une hypocalcémie, probablement en inhibant la fixation du
calcium à partir de l'eau. Toutefois, la présence de fortes
concentrations de calcium dans l'eau protège les poissons par
inhibition compétitive de la fixation de cadmium. Le zinc accroît la
toxicité du cadmium pour les invertébrés aquatiques. On a fait état
d'effets sublétaux sur la croissance et la reproduction d'invertébrés
aquatiques; des effets ont été également observés sur la structure des
branchies d'invertébrés. Le cadmium est plus ou moins toxique pour les
poissons, les salmonidés étant particulièrement sensibles. On a
signalé des effets sublétaux chez les poissons, en particuliers des
malformations de l'épine dorsale. Les stades les plus sensibles sont
les embryons et les jeunes larves, les moins sensibles étant les
oeufs. Chez les poissons, on n'observe pas d'interaction systématique
entre le cadmium et le zinc. Le cadmium est toxique pour certaines
larves d'amphibiens, mais on a constaté que la présence de sédiments
dans les aquariums expérimentaux apportait une certaine protection.
Le cadmium perturbe la croissance des végétaux au laboratoire,
mais aucun effet n'a été observé dans la nature. Les plantes captent
plus facilement le métal lorsqu'il est présent dans les solutions
nutritives que lorsqu'il est dans le sol; les effets observés l'ont
été essentiellement lors d'études portant sur des cultures
hydroponiques. Il semblerait que le cadmium présent dans les solutions
nutritives affecte l'ouverture des stomates, la transpiration et la
photosynthèse.
Les invertébrés terrestres sont relativement insensibles aux
effets toxiques du cadmium, probablement à cause de l'intervention de
mécanismes de séquestration efficaces au niveau des divers organes.
Les gastéropodes terrestres peuvent subir des effets sublétaux,
principalement en ce qui concerne la consommation de nourriture et la
dormance, mais uniquement à des doses très élevées. Même à forte dose,
le cadmium n'entraîne pas la mortalité des oiseaux mais peut provoquer
des lésions rénales.
Des études effectuées sur le terrain ont montré que le cadmium
pouvait entraîner la modification de la proportion relative des
diverses espèces dans les populations de microorganismes et de
certains invertébrés aquatiques. La décomposition des feuilles mortes
est fortement entravée par une forte pollution due aux métaux lourds,
et le cadmium en serait le principal responsable.
RESUMEN
El cadmio (número atómico 48; masa atómica relativa 112,40) es un
elemento metálico que pertenece, junto con el zinc y el mercurio, al
grupo IIb de la tabla periódica. Algunas sales de cadmio, como el
sulfuro, el carbonato y el óxido, son prácticamente insolubles en
agua; pueden convertirse en sales hidrosolubles en el medio natural.
El sulfato, el nitrato y los haluros son hidrosolubles. La especiación
del cadmio en el medio ambiente tiene importancia para evaluar su
potencial de riesgo.
El contenido medio de cadmio en el agua de mar es de alrededor de
0,1 µg/litro o menos. El agua de los ríos contiene cadmio disuelto en
concentraciones que varían entre < 1 y 13,5 ng/litro. En zonas
aisladas y deshabitadas, las concentraciones de cadmio en el aire
suelen ser inferiores a 1 ng/m3. En zonas que se suponen no
contaminadas, se ha comunicado que la concentración mediana de cadmio
en el suelo se encuentra entre 0,2 y 0,4 mg/kg. No obstante, a veces
se encuentran valores mucho más altos, que pueden llegar hasta 160
mg/kg de suelo.
Los factores ambientales influyen en la captación y, por ende, en
los efectos tóxicos del cadmio en los organismos acuáticos. Al
aumentar la temperatura aumentan la captación y los efectos tóxicos,
mientras que el aumento de la salinidad o de la dureza del agua los
hace disminuir. Los organismos de agua dulce sufren los efectos del
cadmio en concentraciones inferiores a las que afectan a los
organismos marinos. La materia orgánica contenida en el agua suele
reducir la captación y los efectos tóxicos fijando el cadmio y
reduciendo su disponibilidad para los seres vivos. Sin embargo, hay
pruebas de que cierto tipo de materia orgánica puede ejercer el efecto
contrario.
El cadmio se acumula fácilmente en numerosos seres vivos,
particularmente microorganismos y moluscos, en los que los factores de
bioconcentración son del orden de varios millares. Los invertebrados
del suelo también concentran este metal en grado considerable. La
mayoría de los organismos presentan factores de concentración bajos o
moderados (inferiores a 100). El cadmio está ligado a proteínas en
numerosos tejidos. En organismos expuestos a ese metal se han aislado
proteínas fijadoras de metales pesados (metalotioneínas). La
concentración de cadmio es más elevada en el riñón, las branquias y el
hígado (o sus equivalentes). La eliminación se produce probablemente
por vía renal, si bien los crustáceos pueden eliminar cantidades
importantes con la muda del exoesqueleto. En las plantas, el cadmio se
concentra principalmente en las raíces y, en menor medida, en las
hojas.
El cadmio tiene efectos tóxicos para muy diversos
microorganismos. No obstante, la presencia de sedimentos y las
concentraciones elevadas de sales disueltas o materia orgánica reducen
esos efectos. Los procesos más afectados son el crecimiento y la
replicación. Los organismos del suelo más vulnerables son los hongos:
algunas especies desaparecen tras la exposición al cadmio en el suelo.
Tras exposiciones reducidas al metal en el suelo, se observa una
selección a favor de las cepas resistentes.
La toxicidad aguda del cadmio para los organismos acuáticos es
variable, incluso entre especies estrechamente emparentadas, y guarda
relación con la concentración de iones libres del metal. El cadmio
interacciona con el metabolismo del calcio en los animales. En los
peces provoca hipocalcemia, probablemente al inhibir la captación de
calcio a partir del agua. No obstante, las concentraciones elevadas de
calcio en el agua los protegen de la ingestión de cadmio por
competencia en los lugares de captación. El zinc aumenta la toxicidad
del cadmio para los invertebrados acuáticos. Se han notificado efectos
subletales en el crecimiento y la reproducción de invertebrados
acuáticos, así como modificaciones estructurales en las branquias de
invertebrados. Hay pruebas de la selección de estirpes resistentes de
invertebrados acuáticos tras la exposición al cadmio sobre el terreno.
La toxicidad es variable en los peces; los salmónidos son
especialmente susceptibles. Se han notificado efectos subletales en
los peces, en particular malformaciones de la espina dorsal. Las fases
biológicas más susceptibles son el embrión y la larva joven; los
huevos son los menos vulnerables. No se ha observado una interacción
homogénea entre el cadmio y el zinc en los peces. El cadmio resulta
tóxico para algunas larvas de anfibios, si bien los sedimentos en el
recipiente de ensayo confieren cierta protección.
El cadmio afecta al crecimiento de las plantas en estudios
experimentales, pero sobre el terreno no se ha observado efecto
alguno. El metal es absorbido por las plantas con más rapidez a partir
de soluciones de nutrientes que a partir del suelo; los efectos se han
observado sobre todo en estudios de cultivo en soluciones de
nutrientes. En éstas se ha notificado que el cadmio influye en la
apertura de los estomas, la transpiración y la fotosíntesis.
Los invertebrados terrestres son relativamente insensibles a los
efectos tóxicos del cadmio, probablemente debido a la existencia de
mecanismos eficaces de captura y fijación en ciertos órganos.
Los gasterópodos terrestres sufren efectos subletales; los
principales procesos afectados son el consumo de alimentos y el
letargo, pero sólo con dosis muy elevadas. El metal no produce efectos
letales en las aves, ni siquiera con dosis elevadas, si bien se
observan lesiones renales.
En estudios de campo se ha comunicado que el cadmio induce
cambios en la composición de especies en las poblaciones de
microorganismos y ciertos invertebrados acuáticos. La descomposición
del mantillo de hojas se ve notablemente reducida por la contaminación
con metales pesados; se ha identificado al cadmio como el principal
causante de este efecto.
