
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 130
ENDRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr G. T. van Esch, Bilthoven,
Netherlands, and Dr E. A. H. van Heemstra-Lequin,
Laren, Netherlands.
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Endrin.
(Environmental health criteria ; 130)
1.Endrin - toxicity 2.Environmental exposure
I.Series
ISBN 92 4 157130 6 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
1. SUMMARY AND EVALUATION; CONCLUSIONS; RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Exposure
1.1.2 Uptake, metabolism, and excretion
1.1.3 Effects on organisms in the environment
1.1.4 Effects on experimental animals and in vitro
1.1.5 Effects on human beings
1.2 Conclusions
1.3 Recommendations
2 IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES,
ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.2.1 Production levels and processes, uses
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
4.1.2 Water
4.1.3 Soil
4.1.4 Soil-plants
4.2 Abiotic degradation
4.3 Biotransformation
4.3.1 Biodegradation
4.3.2 Bioaccumulation and biomagnification
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Soil, sediments, and sewage sludge
5.1.2.1 Soil
5.1.2.2 Sediments
5.1.2.3 Sewage sludge
5.1.3 Water
5.1.3.1 Surface water
5.1.3.2 Rain and snow
5.1.3.3 Drinking-water
5.1.3.4 Groundwater
5.1.4 Organisms in the environement
5.1.4.1 Birds
5.1.4.2 Fish and shellfish
5.1.4.3 Mixed species
5.1.5 Other food and feed
5.1.5.1 Cereals
5.1.5.2 Fruit and vegetables
5.1.5.3 Meat, poultry, and chicken eggs
5.1.5.4 Milk and milk products
5.1.5.5 Fat and oils
5.1.5.6 Animal feed
5.1.6 Miscellaneous products
5.2 Exposure of the general population
5.2.1 Total-diet studies
5.2.2 Levels in human tissues
5.2.2.1 Adipose tissue
5.2.2.2 Organs
5.2.2.3 Blood
5.2.2.4 Breast milk
5.2.2.5 Appraisal of exposure of the general
population
5.3 Occupational exposure during manufacture, formulation,
and use
5.3.1 Manufacture and formulation
5.3.2 Application
5.3.3 Appraisal of occupational exposure
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Laboratory animals
6.1.1.1 Oral administration
6.1.1.2 Intravenous administration
6.1.2 Domestic animals
6.1.3 Human beings
6.1.4 Systems in vitro
6.2 Biotransformation
6.2.1 Experimental animals
6.2.2 Human beings
6.2.3 Microorganisms
6.2.4 Plants
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.2 Aquatic organisms
7.2.1 Invertebrates
7.2.2 Fish
7.2.2.1 Acute toxicity
7.2.2.2 Short-termtoxicity
7.2.2.3 Studies of resistance
7.2.2.4 Interaction with other chemicals
7.2.2.5 Special studies
7.2.3 Amphibia
7.3 Terrestrial organisms
7.3.1 Honey bees
7.3.2 Birds
7.3.2.1 Acute toxicity
7.3.2.2 Short-term toxicity
7.3.2.3 Studies of reproduction
7.3.2.4 Interaction with other chemicals
7.3.2.5 Special studies
7.3.2.6 Behavioural studies
7.3.3 Mammals
7.3.3.1 Toxicity
7.3.3.2 Studies of resistance
7.4 Effects in the field
7.5 Appraisal of effects on organisms in the environment
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO
8.1 Acute toxicity of technical-grade endrin
8.1.1 Oral administration
8.1.2 Dermal administration
8.1.3 Parenteral administration
8.1.4 Toxicity of metabolites and isomers
8.1.4.1 Mammalian metabolites
8.1.4.2 Isomers
8.1.5 Acute toxicity of formulated material
8.1.5.1 Oral and dermal administration
8.1.5.2 Inhalation
8.2 Short-term exposure
8.2.1 Oral administration
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.1.3 Rabbit
8.2.1.4 Dog
8.2.1.5 Domestic animals
8.2.2 Inhalation
8.2.3 Dermal administration
8.3 Skin irritation
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Mouse
8.4.1.2 Rat
8.4.2 Embryotoxicity and teratogenicity
8.4.2.1 Mouse
8.4.2.2 Rat
8.4.2.3 Hamster
8.4.2.4 Perinatal behavioural development
8.4.3 Appraisal of reproductive effects
8.5 Mutagenicity and related end-points
8.5.1 Effects on microorganisms
8.5.2 Point mutations in mammalian cells
8.5.3 Dominant lethal mutations
8.5.4 Chromosomal and cytogenetic effects
8.5.5 Host-mediated effects
8.5.6 Sister chromatid exchange
8.5.7 Effects in Drosophila melanogaster
8.5.8 Effects on DNA
8.5.9 Appraisal of mutagenicity and related end-points
8.6 Long-term exposure
8.7 Carcinogenicity
8.7.1 Oral administration
8.7.1.1 Mouse
8.7.1.2 Rat
8.7.1.3 Tumour promotion
8.7.2 Appraisal of carcinogenicity
8.8 Special studies
8.8.1 Nervous system
8.8.1.1 Electrophysiological studies
8.8.1.2 Histopathological studies
8.8.1.3 Neurotransmitter systems
8.8.1.4 Appraisal of effects on the nervous
system
8.8.2 Cardiovascular system
8.8.3 Effects on liver enzymes
8.8.3.1 Mouse
8.8.3.2 Rat
8.8.3.3 Guinea-pig
8.8.3.4 In-vitro studies
8.8.4 Miscellaneous studies
8.8.5 Factors that influence toxicity
8.8.5.1 Nutrition
8.8.5.2 Potentiation
9. EFFECTS ON HUMAN BEINGS
9.1 Exposure of the general population
9.1.1 Acute toxicity
9.1.2 Poisoning incidents
9.2 Occupational exposure
9.2.1 Factory workers
9.2.2 Dose-response relationships
9.2.3 Exposures to mixtures
9.2.4 Appraisal of effects of occupational exposures
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX I Chemical names of endrin and its metabolites
ANNEX II Medical treatment of endrin poisoning
ANNEX III Management of major status epilepticus in adults
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA
FOR ENDRIN
Members
Dr L.A. Albert, Consultores Ambientales Asociados, Xalapa, Veracruz,
Mexico
Dr V. Benes, Department of Toxicology and Reference Laboratory,
Institute of Hygiene and Epidemiology, Prague, Czechoslovakia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Dr G.J. van Esch, Bilthoven, Netherlands (Rapporteur)
Dr E.A.H. van Heemstra-Lequin, Laren, Netherlands (Rapporteur)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr Yu.I. Kundiev, Research Institute of Labour Hygiene and
Occupational Diseases, Kiev, Ukraine (Vice-Chairman)
Dr Y. Osman, Ministry of Health, Riyadh, Saudi Arabia
Dr H. Spencer, United States Environmental Protection Agency,
Washington DC, USA (Chairman)
Dr C. Winder, National Institute of Occupational Health and Safety,
Forest Lodge, New South Wales, Australia
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Ms B. Labarthe, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr T.K. Ng, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the Criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400 or
7985850).
* * *
The proprietary information contained in this monograph cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route, and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR ENDRIN
A WHO Task Group on Environmental Health Criteria for Endrin and
Isobenzan met at the World Health Organization, Geneva, from 23 to 27
July 1990. Dr K.W. Jager, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of IPCS, and the three IPCS cooperating
organizations (UNEP, ILO, WHO). The Group reviewed and revised the
draft Criteria monographs and Health and Safety Guides and made an
evaluation of the risks to human health and the environment from
exposure to endrin and isobenzan.
The first drafts of these monographs were prepared in cooperation
between Dr E.A.H. van Heemstra-Lequin and Dr G.J. van Esch of the
Netherlands. Dr van Esch prepared the second drafts, incorporating the
comments received following circulation of the first drafts to the
IPCS contact points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monographs, and Mrs E. Heseltine, St
Léon-sur-Vézère, France, for the editing.
The fact that Shell Oil Co. made available to IPCS and the Task
Group proprietary toxicological information on their products is
gratefully acknowledged. This allowed the Task Group to base their
evaluation on more complete data.
The effort of all who helped in the preparation and finalization
of the monographs is gratefully acknowledged.
* * *
Partial financial support for the publication of this Criteria
monograph was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA, a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY AND EVALUATION; CONCLUSIONS; RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Exposure
Endrin is an organochlorine insecticide which has been used since
the 1950s against a wide range of agricultural pests, mostly on cotton
but also on rice, sugar-cane, maize, and other crops. It is also used
as a rodenticide. It is available commercially as dusts, granules,
pastes, and an emulsifiable concentrate.
Endrin enters the air mainly by volatilization and aerial drift.
In general, volatilization takes place after application to soils and
crops and depends on many factors, such as the organic matter and
moisture content of the soil, humidity, air flow, and the surface area
of plants.
The most important route of contamination of surface water is
run-off from soil. Contamination from precipitation in the form of
snow or rain is negligible. Local contamination of the environment may
occur from industrial effluents and careless application practices.
The major source of endrin in soil is from direct application to
soil and crops. Endrin can be retained, transported, or degraded in
soil, depending on a number of factors. The greatest retention occurs
in soils with a high content of organic matter. The persistence of
endrin is highly dependent upon local conditions; its half-life in
soil can range up to 12 years. Volatilization and photodecomposition
are the primary factors in the disappearance of endrin from soil
surfaces. Under the influence of sunlight (ultraviolet light), the
isomer delta-ketoendrin is formed. In intense summer sun, about 50% of
endrin was isomerized to this ketoendrin within 7 days. Microbial
transformation (in fungi and bacteria) takes place, especially under
anaerobic conditions, to give the same product.
Aquatic invertebrates and fish take up endrin rapidly from water,
but exposed fish transferred to uncontaminated water lose the
pesticide rapidly. Bioconcentration factors of 14-18 000 have been
recorded after continuous exposure. Soil invertebrates may also take
up endrin readily.
The occasional presence of low levels of endrin in air and in
surface and drinking-water in agricultural areas is of little
significance from the point of view of public health. The only
exposure that may be relevant is dietary intake. In general, however,
the reported intake levels are far below the acceptable daily intake
of 0.0002 mg/kg body weight established in 1970 (FAO/WHO, 1971).
1.1.2 Uptake, metabolism, and excretion
Unlike dieldrin, its stereoisomer, endrin is metabolized rapidly
by animals, and very little is accumulated in fat in comparison with
compounds of similar chemical structure.
Both uptake and excretion after oral administration are rapid in
rats, and its biological half-life is 1-6 days, depending on the dose
level. A steady state, at which the excreted amount equals the daily
intake, is reached after 6 days. A sex difference is observed, in that
males excrete endrin and metabolites via the bile much faster than
females, resulting in less accumulation in male adipose tissue. Rats
excrete this compound mainly in the faeces as endrin,
anti-12-hydroxyendrin, and a hydroxylated endrin derivative within
the first 24 h (70-75%); a third metabolite, 12-ketoendrin,
accumulates in tissues. Rabbits excrete 50% of the metabolites of
endrin in urine, whereas in rats only 2% are excreted by this route;
only unchanged endrin is found in the faeces of rabbits.
Cows administered endrin at 0.1 mg/kg of diet for 21 days
excreted up to 65% as metabolites in urine, 20% in faeces, partly as
unchanged endrin, and 3% in milk, also mainly as endrin. These cows
had residue levels of 0.003-0.006 mg/litre in milk, 0.001-0.002 mg/kg
in meat, and 0.02-0.1 mg/kg in fat.
Laying hens fed endrin showed residue levels (depending on the
doses given) of up to 0.1 mg/kg in meat, 1 mg/kg in fat, 0.1-0.2 mg/kg
in eggs (yolk), 0.4 mg/kg in kidney, and 0.5 mg/kg in liver. Except in
liver and kidney, the residues found were mainly unchanged endrin.
About 50% of the administered endrin was excreted in faeces, mainly as
metabolites.
In human beings, rats, rabbits, cows, and hens, the major
biotransformed metabolite of endrin is anti-12-hydroxyendrin,
together with its sulfate and glucuronide conjugates. Four other
metabolites were found but in only minor quantities. Mainly unchanged
endrin is found in body tissues and milk. After this pesticide was
applied to plants, unchanged endrin and two hydrophilic transformation
products were identified.
1.1.3 Effects on organisms in the environment
The effect of endrin on soil bacteria and fungi is minimal. Dose
levels of 10-1000 mg/kg of soil had no effect on decomposition of
organic matter, denitrification, or generation of methane. Endrin is
very toxic to fish, aquatic invertebrates, and phytoplankton: the 96-h
LC50 values are mostly below 1.0 µg/litre. The lowest observed
adverse effect level in a life cycle test on the mysid shrimp,
Mysidopsis bahia, was established at 30 ng/litre.
The reported tests on the acute toxicity of endrin in aquatic
organisms were conducted in aquaria without sediment; the presence of
sediment would be expected to attenuate the effect of endrin. Heavily
contaminated sediment had little effect on species living in open
water, suggesting that sediment-bound endrin has low bioavailability.
Tests have not been conducted on aquatic animals living in sediment.
The LD50 for terrestrial mammals and birds is in the order of
1.0-10.0 mg/kg body weight. Mallard ducks fed up to 3.0 mg/kg body
weight for 12 weeks showed no effect on egg production, fertility, or
hatchability.
Certain species of aquatic invertebrates, fish, and small mammals
have been reported to be resistant to the toxicity of endrin, and
exposure to several different organochlorine pesticides led to
selection of strains resistant to endrin.
Fish kills were observed in areas of agricultural run-off and
industrial discharge; and declining populations of brown pelicans (in
Louisiana, USA) and of sandwich terns (in the Netherlands) have been
attributed to exposure to endrin in combination with other halogenated
chemicals.
1.1.4 Effects on experimental animals and in vitro
Endrin is a highly toxic pesticide, the signs of intoxication
being neurotoxic. The oral LD50 of technical-grade endrin for
laboratory animals is in the range of 3-43 mg/kg body weight; the
dermal LD50 for rats is 5-20 mg/kg body weight. No substantial
difference in acute oral or dermal toxicity was found between
technical-grade and formulated (emulsifiable concentrate and wettable
powder) products.
Short-term experiments for oral toxicity have been carried out
using mice, rats, rabbits, dogs, and domestic animals. In mice and
rats, the maximum tolerated doses for 6 weeks were 5 and 15 mg/kg diet
(equivalent to 0.7 mg/kg body weight), respectively. Rats survived a
16-week exposure to 1 mg/kg diet (equivalent to 0.05 mg/kg body
weight); rabbits died after receiving repeated doses of 1 mg/kg body
weight. In dogs, a dose of 1 mg/kg of diet (approximately equivalent
to 0.025 mg/kg body weight), given over 2 years, was without effect.
The neurological basis of the observed signs of intoxication is
inhibition of gamma-aminobutyric acid (GABA) function at low doses.
Like other chlorinated hydrocarbon insecticides, endrin also affects
the liver, and stimulation of enzyme systems involved in the
metabolism of other chemicals is evident, as shown by, for instance,
decreased hexobarbital sleeping time in mice.
Doses of 75-150 mg/kg applied dermally as a dry powder for 2 h
daily caused convulsions and death in rabbits but did not result in
skin irritation. Production of systemic toxicity without irritation at
the site of contact is noteworthy.
Long-term studies of toxicity and carcinogenicity have been
performed in mice and rats. No carcinogenic effect was found, but
these studies had shortcomings, including poor survival of the
animals. The no-observed-effect level for toxicity in a two-year study
in rats was 1 mg/kg of diet (equivalent to about 0.05 mg/kg body
weight). Tumour promoting effects were not demonstrated when endrin
was tested in combination with subminimal quantities of chemicals
known to be carcinogenic to animals. The Task Group concluded that the
data are insufficient to indicate that endrin is a carcinogenic hazard
to humans.
Endrin was found to be nonmutagenic in several studies.
In most studies, it was not teratogenic to mice, rats, or
hamsters, even at doses that caused maternal or fetotoxicity. The
no-observed-adverse-effect level was 0.5 mg/kg body weight in mice and
rats and 0.75 mg/kg body weight in hamsters. Endrin did not induce
reproductive effects in rats over three generations when given at a
dose of 2 mg/kg of diet (about 0.1 mg/kg body weight).
A number of the metabolites of endrin have similar or higher
acute toxicities than the parent compound. The transformation product,
delta-ketoendrin, is less toxic than endrin, but 12-ketoendrin is
considered to be the most toxic metabolite of endrin in mammals, with
an oral LD50 in rats of 0.8-1.1 mg/kg body weight.
1.1.5 Effects on human beings
Several episodes of fatal and non-fatal accidental and suicidal
poisoning have occurred. Cases of acute non-fatal intoxication due to
accidental over-exposure were observed in workers in an endrin
manufacturing plant. The oral dose that causes death has been
estimated to be approximately 10 mg/kg body weight; the single oral
dose that causes convulsions was estimated to be 0.25-1.0 mg/kg body
weight.
The primary site of action of endrin is the central nervous
system. Exposure of humans to a toxic dose may lead within a few hours
to such signs and symptoms of intoxication as excitability and
convulsions, and death may follow within 2-12 h after exposure if
appropriate treatment is not administered immediately. Recovery from
non-fatal poisoning is rapid and complete.
Endrin does not accumulate in the human body to any significant
degree. No long-term adverse effects were reported in 232
occupationally exposed workers (length of exposure, 4-27 years) under
medical supervision (observation time, 4-29 years). The only effect
observed was indirect evidence of a reversible stimulation of drug
metabolizing enzymes.
Endrin was detected in virtually none of a large number of
samples of adipose tissue, blood, and breast milk analysed in many
countries. The Task Group attributed the absence of endrin in human
samples to the low exposure of the general population to this
pesticide and to its rapid metabolism.
Endrin was detected in blood (at up to 450 µg/litre) and in
adipose tissue (at 89.5 mg/kg) in cases of fatal accidental poisoning.
No endrin was found in workers under normal circumstances. The
threshold level of endrin in blood, below which no sign or symptom of
intoxication occurs, has been estimated to be 50-100 µg/litre. The
half-life of endrin in blood may be in the order of 24 h.
1.2 Conclusions
Endrin is an insecticide with high acute toxicity. It may cause
severe poisoning in cases of over-exposure caused by careless handling
during its manufacture and use or by consumption of contaminated food.
The general public is exposed to endrin mainly as its residues in
food; however, the reported intake of endrin is generally far below
the acceptable daily intake established by FAO/WHO. Such exposures
should not constitute a health hazard to the general population. When
good work practices, hygiene measures, and safety precautions are
enforced, endrin is unlikely to present a hazard to exposed workers.
It is clear that uncontrolled discharges of endrin during its
manufacture, formulation, and use can result in acute environmental
problems associated with its high toxicity. The effects on wildlife of
its agricultural use are less clear, although fish and fish-eating
birds are at risk from surface run-off. Declines in the populations of
some avian species have been associated with the presence of high
levels of residues of various organochlorines in the tissues of adults
and in eggs. Endrin has been measured in some of these species;
however, it is very difficult to separate the effects of the different
organochlorines present.
1.3 Recommendations
1. Endrin should not be used unless it is indispensable and
only when no less toxic alternative is available.
2. For the health and welfare of workers and the general
population, the handling and application of endrin should be
entrusted only to competently supervised, well-trained operators
who will follow adequate safety measures and apply endrin
according to good agricultural practices.
3. The manufacture, formulation, agricultural use, and disposal
of endrin should be managed carefully to minimize contamination
of the environment, particularly surface water.
4. People exposed regularly to endrin should undergo periodic
health evaluations.
5. Epidemiological studies of exposed worker populations should
be continued.
6. In countries where endrin is still used, food should be
monitored for endrin residues.
7. If the use of endrin continues, more information should be
obtained on the presence, ultimate fate, and toxicity of
12-ketoendrin and delta-ketoendrin.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
CAS chemical name: (1a-alpha,2ß,2aß,3-alpha,6-alpha,6aß,
7ß,7a-alpha)-3,4,5,6,9.9-hexachloro
1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-
dimethanonaphth[2,3-b]oxirene
(9CI-CAS)
Former CAS chemical name: 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,5,6,7,8,8a-octahydro-1,4-
endo,endo-5,8-dimethanonaphthalene
IUPAC chemical name: 1 R,4 S,4a S,5 S,6 S,7 R,8 R,8a
R)-1,2,3,4,10,10-hexachloro-
1,4,4a,5,6,7,8,8a-octahydro-6,7-
epoxy-1,4:5,8- dimethanonaphthalene
Chemical structure:
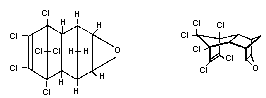 Endrin is the endo,endo stereoisomer of dieldrin
Empirical formula: C12H8Cl6O
Relative molecular mass: 380.93
Common name: Endrin
CAS registry number: 72-20-8
RTECS registry number: I01575000
Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157,
ENT17251, OMS 197, and Mendrin
Trade name: Endrin
Purity: Not less than 92%. Impurities include
dieldrin (0.42%), aldrin (0.03%),
isodrin (0.73%), endrin half-cage
ketone (1.57%), endrin aldehyde
(0.05%), and heptachloronorbornene
(0.09%) (Donoso et al., 1979).
2.2 Physical and chemical properties
Table 1. Physical and chemical properties of endrin
Physical state Crystalline solid
Colour White to light-tan
Odour Mild chemical
Melting-point 226-230 °C
(decomposes at above 245 °C)
Flash-point None (dry powder is non-flammable,
but commercial solutions contain
inflammable liquids with flash-points
as low as 27 °C)
Explosion limits Non-explosive
Specific gravity
(density) 1.64 g/ml at 20 °C
Vapour pressure 2.7 x 10-7 mmHg at 25 °C
(36 µPa at 25 °C)
Solubility in water Practically insoluble
(0.23 mg/litre at 25 °C)
Solubility in organic Sparingly soluble in alcohol and
solvents petroleum hydrocarbons; moderately
soluble in aliphatic hydrocarbons;
and quite soluble in solvents such
as acetone, benzene, carbon
tetrachloride, and xylene
Log P octanol/water 5.34
partition coefficient
Stability: Technical-grade endrin is stable in storage at ambient
temperatures. Endrin is stable in formulations with
basic reagents, alkaline oxidizing agents, emulsifiers,
wetting agents, and solvents. It isomerizes under the
influence of ultraviolet light. It reacts with
concentrated mineral acids, acid catalysts, acid
oxidizing agents and active metals. When mixed with
certain catalytically active carriers, endrin tends to
decompose; however, most active dust carriers can be
deactivated by the addition of hexamethylenetetramine
and form stable mixtures with endrin. When heated to
above 200 °C, endrin undergoes molecular rearrangements
to form delta-ketoendrin, a compound that is less active
as an insectide (IARC, 1974; Donoso et al., 1979).
2.3 Conversion factors
1 ppm = 16 mg/m3 at 20 °C
1 mg/m3 = 0.063 ppm at 20 °C
2.4 Analytical methods
Most of the analytical procedures used since the early 1960s have
been based on the following steps:
(i) extraction using a suitable solvent;
(ii) clean-up by liquid/liquid partition followed by column
chromatography;
(iii) further separation from co-extractives by gas
chromatography (GC); and
(iv) quantification using an electron-capture, coulometric, or
Hall electrolytic detector
General procedures based on these steps are not specific for
endrin; therefore, its identity must be confirmed in environmental
samples. This can be achieved by chemical derivatization and mass
spectrometry (Chau & Cochrane, 1969, 1971; Belisle et al., 1972; Chau,
1974; Safe & Hutzinger, 1979).
Roos et al. (1987) used size exclusion chromatography to clean-up
pesticides after extraction with ethyl acetate from fish oils, animal
fat, cereals, vegetables, fruit, and liver. The recoveries of endrin
were 90-95%, at a limit of detection of 0.02 mg/kg. This method was
found to be adequate for screening and requires only 15% of the amount
of solvents normally used.
Gübeli & Clerc (1988) described a relatively simple gas-liquid
chromatography method for the detection and approximate quantification
of chlorinated pesticides in ethanolic extracts of medicinal plants
(tinctures). The method was based on extraction with hexane and
capillary GC/63Ni-electron-capture detection. The limit of detection
for endrin was 0.005 mg/kg with a recovery of 77.5%.
Suzuki et al. (1974) separated many pesticides from extracts of
crops and soil into different groups by column chromatography prior to
thin-layer chromatography to obtain systematic identification and
determination. Silica gel was used for the column chromatography and
for the thin-layer plates; glass columns packed with different
absorbents were used for GC separation. Determination was done using
electron-capture detection with a 63Ni source.
To improve the separation by heat of 28 organochlorine
insecticides, including endrin, using gas-liquid chromatography with
electron capture detection, Suzuki & Morimoto (1986) tested three
chemically bonded, fused silica capillary columns. The column prepared
with OV-17 performed best. The method was used with minimal clean-up
and gave good results in the analysis of extracts of several soil
samples, avoiding the disadvantages of low resolution of peaks in
packed columns, handling of glass capillary columns and the high cost
of GC-mass spectrometry systems.
Kiang & Grob (1986) developed a screening procedure for the
determination of 49 pollutants of high priority, including endrin, in
soil or sludge. Methylene chloride at two pH values was used in the
extraction procedure, which was followed by capillary GC. No clean-up
procedure was carried out. Separation and identification were
performed with a GC-mass spectrometry system involving a 30-m fused
silica column; a 60-m column was used for quantification. Recovery of
endrin from soil in the base-neutral extract was 92 ± 14% from 2.04
mg/kg but only 70 ± 8% from 20.4 mg/kg.
Japenga et al. (1987) described a rapid clean-up procedure for
the simultaneous determination of groups of micropollutants in
sediment. The samples were pretreated with acid, mixed with silica,
and extracted on a Soxhlet column with a mixture of benzene and
hexane. Humic substances and elemental sulfur were removed by passing
the extract through a chromatographic column containing basic alumina
on which sodium sulfite and sodium hydroxide were absorbed. After
silica fractionation, the concentrations of polycyclic aromatic
hydrocarbons, polychlorinated biphenyls, and chlorinated pesticides
were determined by GC. The recovery of endrin was reported to
fluctuate between 93 and 103%.
The efficiency of clean-up with sulfuric acid and confirmation
with potassium hydroxide-ethanol hydrolysis was studied for 22
organochlorine pesticides and polychlorinated biphenyls in water
samples (Hernandez et al., 1987); analysis was by GC/electron-capture
detection, and the pesticides were extracted by partition with 15%
diethyl ether in hexane. After clean-up with sulfuric acid, only 4.9%
of the endrin was recovered; however, with the potassium
hydroxide-ethanol treatment, 97-100% was recovered, depending on the
endrin concentration and the length of treatment.
Method 8080 of the US Environmental Protection Agency (EPA)
(Manual, SW-846) was evaluated in a single laboratory study by Lopez-
Avila et al. (1988). Since the Florisil clean-up procedure recommended
does not separate organochlorine pesticides from polychlorinated
biphenyls, GC analysis on a packed column may result in false
identifications; therefore, silica gel was substituted for Florisil,
a capillary glass column was used instead of the packed column, and a
procedure to remove elemental sulfur incorporated. Detection limits
for liquid matrices ranged from 0.02 to 0.09 µg/litre for
organochlorine pesticides; for solid matrices, a range of 1-6 µg/kg
was found. The recovery of endrin in liquid waste was up to 102% at a
spiked concentration of 1.0 µg, but for a sandy loam soil it varied
from 47 to 74%.
Donahue et al. (1988) compared two techniques for quantifying
environmental contaminants in human serum: peak area matching and
linear regression. No statistically significant difference was seen in
the results obtained by these two methods when the concentration of
chlorinated pesticides was > 0.5 µg/litre.
The sampling and determination of endrin in air were described in
detail by NIOSH (1989).
A method for determining residues of the metabolite
anti-12-hydroxy-endrin, present as the ß-glucuronide, in urine was
described by Baldwin & Hutson (1980). Following oxidation with sodium
metaperiodate and hydrolysis with a mild base, the metabolite is
determined by gas-liquid chromatography with electron-capture
detection.
Polychlorinated biphenyls and 21 chlorinated pesticides,
including endrin, were analysed in samples of water, soil, and
sediment in six laboratories using uniform calibration solutions,
analytical methods, and special software operating on minicomputers to
control the operation of the mass spectrometer. The results obtained
for solid samples with four combinations of methods for extraction and
clean-up were compared; although no combination was optimal for all
samples, shaker and sonicator extraction, both with Florisil clean-up,
gave the best results. Several factors that affected the quality of
the results were identified, including errors in computation and
transcription and inadequate review of data (Alford-Stevens et al.,
1988).
Seventeen laboratories participated in an international
comparison of analyses for organochlorine compounds (Holden, 1970).
The results for endrin, summarized in Table 2, were more variable than
those for other insecticides. In an inter-laboratory collaborative
study reported by a Committee of the Ministry of Agriculture,
Fisheries, and Food of the United Kingdom (Anon., 1979) for the
determination of endrin in pork fat (fortified to 0.019 mg/kg), the
mean recovery in 11 laboratories was 84%, but the range was 5-131%.
Table 2. Results for endrin of an inter-laboratory study of
the analysis of organochlorine compounds (Holden, 1970)
Type of No. of laboratories Mean concentration Standard Coefficient Range
sample with results for (mg/litre or mg/kg) deviation of variation
endrin (%)
Solution 17 5.929b 1.01 17.1 4.9-8.2
in hexanea
Cod liver 14 0.02 - - NDc-0.20d
oil
Chicken 16 0.136 0.073 54 0.07-0.3e
egg
Sprat 14 0.132 0.039 29 0.09f-0.21
a Containing endrin and five other organochlorine insecticides
b True (nominal, fortified) value, 7.05 mg/litre
c Twelve laboratories reported no detectable residue
d Value reported to be suspect
e Excluding one laboratory that reported suspected presence of endrin
f Excluding one laboratory that reported a 'trace' of endrin
Table 3. Methods for the analysis of endrin
Sample type Extraction Clean-up Detection and Recovery Limit of Reference
quantificationa (%) detection
Adipose tissue Acetone:hexane Fractionation by Capillary column > 100 Lebel & Williams
(15:85 v/v) gel permeation gas chromatography (1986)
chromatography with columns of different
methylene polarity, GC-MS
chloride-cyclo-hexane
Florisil column
Air Hexylene glycol/ Florisil GLC/ECD 77 0.1 ng/m3 Stanley et al.
Greenburg Smith column (1971)
impinger; alumina
column
Air Toluene - GLC/ECD (63Ni) - 0.02µg/ NIOSH (1989)
sample
Water Hexane:ethyl ether - GLC/ECD 65-97 0.002 Lichtenberg
µg/litre et al. (1970)
Soil/sediment Acetone:hexane Alumina GLC/ECD 83 0.1 µg/kg Goerlitz & Law
column (1974)
Soil/sediment Acetone:petroleum Alumina GLC/ECD 90 0.01 mg/kg Wegman & Hofstee
ether column (1982)
Soil/sediment Hexane Alumina/ GLC/ECD - 0.01 mg/kg McIntyne & Lester
silver nitrate + (1984)
silica gel
column
Table 3 (contd)
Sample type Extraction Clean-up Detection and Recovery Limit of Reference
quantificationa (%) detection
Crops Hexane:isopropanol; Carbon absorption GLC/ECD 93-107 - Kathpal & Dewan
or acetonitrile (Nuchar C-190N) (1975)
Fatty foods, Hexane:acetone Alumina GLC/ECD 68-100 5-10 µg/kg Telling et al.
vegetable oils, column (1977)
fish oils
Viscera Diethyl ether Celite GLC/ECD 72-92 - Kurhekar et al.
column (1975)
Birds' brain Petroleum ether: Florisil GLC/ECD 70 0.05 mg/kg Ludke (1976)
samples ethyl ether column
Cows' milk Diethyl ether: Silica gel GLC/ECD 90 0.0001 mg/kg Baldwin et al.
hexane + ether (1976)
Hens' eggs Hexane:acetone Silica gel GLC/ECD 76 0.05 mg/kg Baldwin et al.
(yolks) (1976)
Crops, soil, Hydrocarbon Florisil:Celite + Reduction with 75-100 1 mg/kg Terriere (1964)
milk, animal solvent magnesia column, metallic sodium;
tissues (Skellysolve after alkaline phenyl azide
B) + isopropanol hydrolysis, if colorimetry
appropriate
-a GC-MS, gas chromatography-mass spectometry; GLC-ECD, gas-liquid chromatography-electron capture detection
Thier & Stijve (1986) reported a comparative study among 53
laboratories in Switzerland on the analysis of a vegetable fat spiked
with 13 organochlorine and five organophosphorus compounds. Endrin was
present at a concentration of 0.08 mg/kg and was identified by 77% of
the laboratories.
Some of the methods that are used for the analysis of endrin are
summarized in Table 3; the estimates given of the accuracy of the
procedures and the limits of detection refer to the specific
investigations and are not absolute values. The percentage recoveries
are an indication of the accuracy of the methods; the precision of
individual method is of interest particularly in regard to
inter-laboratory comparisons.
The many publications on specific procedures are reviewed in the
Codex Alimentarius Commission publication Recommendations for Methods
of Analysis of Pesticide Residues, CAC/PR8-1986 (FAO/WHO, 1986a).
That review lists 14 individual publications; it also lists the
following compendia of methods, which may be consulted.
-- Official Methods of Analysis of the Association of Official
Analytical Chemists, 14th Edition, 1984
-- Pesticide Analytical Manual, Washington DC, Food and Drug
Administration
-- Manual on Analytical Methods for Pesticide Residues in Foods,
Ottawa, Health Protection Branch, Health and Welfare Canada, 1985
-- Methodensammlung zur Rückstandsanalytik von
Pflanzenschutzmitteln (Methods for Analysing Residues of Plant
Protection Agents), Weinheim, Verlag Chemie GmbH, 1984
-- Chemistry Laboratory Guidebook, Washington DC, US Department
of Agriculture
Whatever procedure is adopted should be carried out following the
requirements of the Codex Alimentarius Commission publication Codex
Guidelines on Good Laboratory Practice in Pesticide Residue Analysis,
CAC/PR7-1984 (FAO/WHO, 1984).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Endrin does not occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes, uses
Endrin is a foliar insecticide which acts against a wide range of
agricultural pests at doses of the active material of 0.2-0.5 kg/ha.
It has a broad spectrum of control and is particularly effective
against Lepidoptera. It is used mainly on cotton but also against
pests of rice, sugar cane, maize, and other crops. It is also used as
a rodenticide (IARC, 1974). An endrin emulsion of 2% killed 40% of
Achatina fulica snails, an agricultural pest, in India (Singh,
1988).
A general indication of the possible uses of endrin can be
derived from the maximal residue limits recommended by FAO/WHO (1986b;
see section 10).
3.2.1.1 World production figures
Endrin was developed by J. Hyman & Co. and licensed to be
manufactured by Shell International Chemical Co. and Velsicol Chemical
Co. in 1950 (Thompson, 1976). It was made in the USA by Shell and
Velsicol and in the Netherlands by Shell. Its use has been banned in
many countries and severely restricted in others (Donoso et al., 1979;
Gips, 1987; Pearce, 1987). Shell discontinued manufacture of endrin in
1982; it is still manufactured in Mexico.
Whetstone (1964) estimated that 2.3-4.5 million kg of endrin were
sold in the USA in 1962. Imports of endrin into Japan in 1970 were 72
000 kg. The annual quantities of endrin that were used in paddy rice
production in Bali over the period 1963-72 varied from 171 to 10 700
kg (Machbub et al., 1988). After 1972, endrin was no longer used.
3.2.1.2 Manufacturing process
Endrin is produced by condensing vinyl chloride with
hexachloro-cyclopentadiene, dehydrochlorinating the adduct, and
subsequent reaction with cyclopentadiene to form isodrin, which is
epoxidized by peracetic or perbenzoic acid (Whetstone, 1964). The
intermediate isodrin can be manufactured via 1,2,3,4,7,7-
hexachloronorbornadiene (US EPA, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Endrin can enter the air by volatilization, evaporation, and
aerial drift during application, and as a vapour from manufacturing
and formulating plants. Most studies showed rapid volatilization
following application to soils and crops, the extent of vaporization
depending upon a large number of factors, including soil organic
matter, moisture content, air humidity, air flow, and surface area of
plants (Donoso et al., 1979).
4.1.2 Water
Endrin can reach surface water by several routes, including
effluents and waste disposal from endrin manufacturing and formulating
plants and careless aerial application, but by far the most important
route of contamination is surface run-off from soil and crops. Run-off
is affected by numerous, complex factors, such as intensity of
precipitation, irrigation practices, soil permeability, topographic
relief, organic content of the soil, and the degree of vegetative
cover. Soils of low permeability and low organic content allow copious
run-off after heavy precipitation (Donoso et al., 1979). Contamination
of surface water by industrial effluents and careless practices and
disposal (such as washing of drums and spray equipment in streams)
results in regional effects.
In 1961, studies were conducted in the Bayou Yokely basin in
Louisiana, USA, where 3300 acres (1335 ha) of sugar-cane were treated
with nearly 2000 lb (907 kg) of endrin between June and August. Of 18
water samples taken between April and November, six contained endrin
at levels of 0.001-0.36 µg/litre, with an average of 0.1 µg/litre. In
1964, the area was treated with 1200 lb (544 kg) of endrin, and the
pattern of residues was the same. The mean residue levels in samples
taken in September were 0.44 µg/litre in grab samples and 0.53
µg/litre in carbon adsorption samples; after three months, the average
levels were 0.03 and 0.04 µg/litre, respectively. Sediment samples
contained 165 µg/kg; after three months, this level had decreased to
70 µg/kg (Lauer et al., 1966).
Another, less important source of water contamination is run-off
from endrin-coated seeds. Marston et al. (1969) found that although
approximately 11% of the initial amount was washed off by water under
laboratory conditions, in field conditions the loss was smaller. The
total amount detected in the watershed 6 days after aerial application
of endrin-coated seed was 0.12% of the applied dose. The highest
concentration found in the water was 0.07 µg/litre.
A third possible source of contamination is fall-out by
precipitation in the form of rain and snow, but the measured levels
are negligible (see section 5.1.3.2).
4.1.3 Soil
The major source of endrin in soil is from direct application to
soil and crops. The amount of endrin that reaches the soil depends on
the type of crop and the method of application. The fate of endrin in
soil determines the degree to which the rest of the environment (water
and atmosphere) is contaminated. In soil, endrin can be retained,
transported, or degraded, depending on a large number of interrelated
factors (Donoso et al., 1979). When endrin was applied to tall, dense
crops such as tobacco, no residue appeared in the soil; when it was
applied to soil, the amount that remained depended on the retentive
ability of the soil. Although endrin has strong absorptive properties
in soils such as clay and sandy loam, limited residues were found. Far
greater retention was found in soils with a high organic content, in
which it was adsorbed quickly and was difficult to remove. The degree
to which endrin was retained in the soil depended not only on the soil
type but on numerous other factors such as volatilization, leaching,
wind erosion, surface run-off, and crop uptake (Harris et al., 1966).
In general, the persistence of endrin is highly dependent upon local
conditions, and residue levels can range from traces to milligrams per
kilogram. Its half-life in soil can be as long as 12 years (Donoso et
al., 1979).
The factors that affect the degree to which endrin is retained in
soil (Donoso et al., 1979) can be generalized as follows:
(a) Endrin appears to be less persistent if it is applied to the
soil surface or to crops rather than being mixed into the
soil.
(b) Volatilization and photodecomposition are the primary routes
for the disappearance of endrin from soil surfaces.
(c) Microbial degradation of endrin occurs anaerobically and is
accelerated by conditions such as flooding and soil depth.
(d) Soil cultivation and crop rotation accelerate the
dissipation of endrin.
(e) When the percentage of organic matter is high, as in muck -
soils, the persistence of eldrin is greater. In sandy soils,
volatilization is high and persistence is low.
4.1.4 Soil-plants
River and basin sediment was brought on land near Rotterdam, the
Netherlands, after dredging. Once the sediment had settled for several
years, the land was used for agriculture. Some of the sediment came
from a basin near a pesticide manufacturing plant and was contaminated
with many organochlorine hydrocarbons, including the pesticides
hexachlorobenzene, aldrin, dieldrin, and endrin. The mean
concentration of endrin in the sediment of the basin near the plant
(expressed in mg/kg on a dry weight basis) was 0.48 (range, 0.01-2.6)
in 1976 and 0.59 (< 0.01-3.6) in 1977. In crops, the concentration of
endrin ranged from none detected to 0.06 mg/kg of product; in carrots,
however, levels up to 0.73 mg/kg were found (Wegman et al., 1981).
4.2 Abiotic degradation
When endrin was heated to above 200 °C, as can occur during
gas-liquid chromatography at 230 °C, the molecule was isomerized to a
ketone, delta-ketoendrin (1, Fig. 1) and an aldehyde (3). A minor
product of the thermal rearrangement was an isomeric alcohol (4).
Endrin is also transformed to delta-ketoendrin (1) under
acid-catalysed conditions (Phillips et al., 1962).
Irradiation with ultraviolet light for 48 h also results in
rearrangement to this ketone (37%) and, to a much lesser extent, to
the aldehyde (9%) (Rosen et al., 1966; Plimmer, 1972; Mukerjee, 1985).
Endrin underwent a photolytic reaction in hexane and in cyclohexane
after irradiation at 253.7 and 300 nm, resulting in a half-cage
ketone, pentachloro photoproduct (2), in 80% yield. This photolytic
product has also been identified in the field and was found to be
highly resistant to oxidation and reduction (Plimmer, 1972; Zabik et
al., 1971; Mukerjee, 1985). When an acetone solution of endrin was
irradiated with light from a mercury lamp in a quartz cell for 24 h,
three metabolites were formed by the loss of one chlorine atom from
the initially produced delta-ketoendrin; one of these was compound 2
(Dureja et al., 1987).
Endrin has been reported to isomerize to delta-ketoendrin during
5 years' storage in the dark at room temperature (Plimmer, 1972.
In sunlight, mainly the ketone is formed (Soto & Deichmann, 1967;
Rosen, 1972); approximately 50% isomerization to the ketone took place
within 7 ± 2 days with exposure to intense summer sun (Burton &
Pollard, 1974).
The photochemical products are important as terminal residues:
delta-ketoendrin was found on cotton plants and on cabbage and apple
leaves after application of endrin (Plimmer, 1971; Mukerjee, 1985).
4.3 Biotransformation
The mechanisms by which endrin is removed from the environment
include photodecomposition and bacterial degradation. These factors
and their effects on the persistence of endrin have been reviewed by
the US Environmental Protection Agency (Donoso et al., 1979).
Endrin is the endo,endo stereoisomer of dieldrin
Empirical formula: C12H8Cl6O
Relative molecular mass: 380.93
Common name: Endrin
CAS registry number: 72-20-8
RTECS registry number: I01575000
Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157,
ENT17251, OMS 197, and Mendrin
Trade name: Endrin
Purity: Not less than 92%. Impurities include
dieldrin (0.42%), aldrin (0.03%),
isodrin (0.73%), endrin half-cage
ketone (1.57%), endrin aldehyde
(0.05%), and heptachloronorbornene
(0.09%) (Donoso et al., 1979).
2.2 Physical and chemical properties
Table 1. Physical and chemical properties of endrin
Physical state Crystalline solid
Colour White to light-tan
Odour Mild chemical
Melting-point 226-230 °C
(decomposes at above 245 °C)
Flash-point None (dry powder is non-flammable,
but commercial solutions contain
inflammable liquids with flash-points
as low as 27 °C)
Explosion limits Non-explosive
Specific gravity
(density) 1.64 g/ml at 20 °C
Vapour pressure 2.7 x 10-7 mmHg at 25 °C
(36 µPa at 25 °C)
Solubility in water Practically insoluble
(0.23 mg/litre at 25 °C)
Solubility in organic Sparingly soluble in alcohol and
solvents petroleum hydrocarbons; moderately
soluble in aliphatic hydrocarbons;
and quite soluble in solvents such
as acetone, benzene, carbon
tetrachloride, and xylene
Log P octanol/water 5.34
partition coefficient
Stability: Technical-grade endrin is stable in storage at ambient
temperatures. Endrin is stable in formulations with
basic reagents, alkaline oxidizing agents, emulsifiers,
wetting agents, and solvents. It isomerizes under the
influence of ultraviolet light. It reacts with
concentrated mineral acids, acid catalysts, acid
oxidizing agents and active metals. When mixed with
certain catalytically active carriers, endrin tends to
decompose; however, most active dust carriers can be
deactivated by the addition of hexamethylenetetramine
and form stable mixtures with endrin. When heated to
above 200 °C, endrin undergoes molecular rearrangements
to form delta-ketoendrin, a compound that is less active
as an insectide (IARC, 1974; Donoso et al., 1979).
2.3 Conversion factors
1 ppm = 16 mg/m3 at 20 °C
1 mg/m3 = 0.063 ppm at 20 °C
2.4 Analytical methods
Most of the analytical procedures used since the early 1960s have
been based on the following steps:
(i) extraction using a suitable solvent;
(ii) clean-up by liquid/liquid partition followed by column
chromatography;
(iii) further separation from co-extractives by gas
chromatography (GC); and
(iv) quantification using an electron-capture, coulometric, or
Hall electrolytic detector
General procedures based on these steps are not specific for
endrin; therefore, its identity must be confirmed in environmental
samples. This can be achieved by chemical derivatization and mass
spectrometry (Chau & Cochrane, 1969, 1971; Belisle et al., 1972; Chau,
1974; Safe & Hutzinger, 1979).
Roos et al. (1987) used size exclusion chromatography to clean-up
pesticides after extraction with ethyl acetate from fish oils, animal
fat, cereals, vegetables, fruit, and liver. The recoveries of endrin
were 90-95%, at a limit of detection of 0.02 mg/kg. This method was
found to be adequate for screening and requires only 15% of the amount
of solvents normally used.
Gübeli & Clerc (1988) described a relatively simple gas-liquid
chromatography method for the detection and approximate quantification
of chlorinated pesticides in ethanolic extracts of medicinal plants
(tinctures). The method was based on extraction with hexane and
capillary GC/63Ni-electron-capture detection. The limit of detection
for endrin was 0.005 mg/kg with a recovery of 77.5%.
Suzuki et al. (1974) separated many pesticides from extracts of
crops and soil into different groups by column chromatography prior to
thin-layer chromatography to obtain systematic identification and
determination. Silica gel was used for the column chromatography and
for the thin-layer plates; glass columns packed with different
absorbents were used for GC separation. Determination was done using
electron-capture detection with a 63Ni source.
To improve the separation by heat of 28 organochlorine
insecticides, including endrin, using gas-liquid chromatography with
electron capture detection, Suzuki & Morimoto (1986) tested three
chemically bonded, fused silica capillary columns. The column prepared
with OV-17 performed best. The method was used with minimal clean-up
and gave good results in the analysis of extracts of several soil
samples, avoiding the disadvantages of low resolution of peaks in
packed columns, handling of glass capillary columns and the high cost
of GC-mass spectrometry systems.
Kiang & Grob (1986) developed a screening procedure for the
determination of 49 pollutants of high priority, including endrin, in
soil or sludge. Methylene chloride at two pH values was used in the
extraction procedure, which was followed by capillary GC. No clean-up
procedure was carried out. Separation and identification were
performed with a GC-mass spectrometry system involving a 30-m fused
silica column; a 60-m column was used for quantification. Recovery of
endrin from soil in the base-neutral extract was 92 ± 14% from 2.04
mg/kg but only 70 ± 8% from 20.4 mg/kg.
Japenga et al. (1987) described a rapid clean-up procedure for
the simultaneous determination of groups of micropollutants in
sediment. The samples were pretreated with acid, mixed with silica,
and extracted on a Soxhlet column with a mixture of benzene and
hexane. Humic substances and elemental sulfur were removed by passing
the extract through a chromatographic column containing basic alumina
on which sodium sulfite and sodium hydroxide were absorbed. After
silica fractionation, the concentrations of polycyclic aromatic
hydrocarbons, polychlorinated biphenyls, and chlorinated pesticides
were determined by GC. The recovery of endrin was reported to
fluctuate between 93 and 103%.
The efficiency of clean-up with sulfuric acid and confirmation
with potassium hydroxide-ethanol hydrolysis was studied for 22
organochlorine pesticides and polychlorinated biphenyls in water
samples (Hernandez et al., 1987); analysis was by GC/electron-capture
detection, and the pesticides were extracted by partition with 15%
diethyl ether in hexane. After clean-up with sulfuric acid, only 4.9%
of the endrin was recovered; however, with the potassium
hydroxide-ethanol treatment, 97-100% was recovered, depending on the
endrin concentration and the length of treatment.
Method 8080 of the US Environmental Protection Agency (EPA)
(Manual, SW-846) was evaluated in a single laboratory study by Lopez-
Avila et al. (1988). Since the Florisil clean-up procedure recommended
does not separate organochlorine pesticides from polychlorinated
biphenyls, GC analysis on a packed column may result in false
identifications; therefore, silica gel was substituted for Florisil,
a capillary glass column was used instead of the packed column, and a
procedure to remove elemental sulfur incorporated. Detection limits
for liquid matrices ranged from 0.02 to 0.09 µg/litre for
organochlorine pesticides; for solid matrices, a range of 1-6 µg/kg
was found. The recovery of endrin in liquid waste was up to 102% at a
spiked concentration of 1.0 µg, but for a sandy loam soil it varied
from 47 to 74%.
Donahue et al. (1988) compared two techniques for quantifying
environmental contaminants in human serum: peak area matching and
linear regression. No statistically significant difference was seen in
the results obtained by these two methods when the concentration of
chlorinated pesticides was > 0.5 µg/litre.
The sampling and determination of endrin in air were described in
detail by NIOSH (1989).
A method for determining residues of the metabolite
anti-12-hydroxy-endrin, present as the ß-glucuronide, in urine was
described by Baldwin & Hutson (1980). Following oxidation with sodium
metaperiodate and hydrolysis with a mild base, the metabolite is
determined by gas-liquid chromatography with electron-capture
detection.
Polychlorinated biphenyls and 21 chlorinated pesticides,
including endrin, were analysed in samples of water, soil, and
sediment in six laboratories using uniform calibration solutions,
analytical methods, and special software operating on minicomputers to
control the operation of the mass spectrometer. The results obtained
for solid samples with four combinations of methods for extraction and
clean-up were compared; although no combination was optimal for all
samples, shaker and sonicator extraction, both with Florisil clean-up,
gave the best results. Several factors that affected the quality of
the results were identified, including errors in computation and
transcription and inadequate review of data (Alford-Stevens et al.,
1988).
Seventeen laboratories participated in an international
comparison of analyses for organochlorine compounds (Holden, 1970).
The results for endrin, summarized in Table 2, were more variable than
those for other insecticides. In an inter-laboratory collaborative
study reported by a Committee of the Ministry of Agriculture,
Fisheries, and Food of the United Kingdom (Anon., 1979) for the
determination of endrin in pork fat (fortified to 0.019 mg/kg), the
mean recovery in 11 laboratories was 84%, but the range was 5-131%.
Table 2. Results for endrin of an inter-laboratory study of
the analysis of organochlorine compounds (Holden, 1970)
Type of No. of laboratories Mean concentration Standard Coefficient Range
sample with results for (mg/litre or mg/kg) deviation of variation
endrin (%)
Solution 17 5.929b 1.01 17.1 4.9-8.2
in hexanea
Cod liver 14 0.02 - - NDc-0.20d
oil
Chicken 16 0.136 0.073 54 0.07-0.3e
egg
Sprat 14 0.132 0.039 29 0.09f-0.21
a Containing endrin and five other organochlorine insecticides
b True (nominal, fortified) value, 7.05 mg/litre
c Twelve laboratories reported no detectable residue
d Value reported to be suspect
e Excluding one laboratory that reported suspected presence of endrin
f Excluding one laboratory that reported a 'trace' of endrin
Table 3. Methods for the analysis of endrin
Sample type Extraction Clean-up Detection and Recovery Limit of Reference
quantificationa (%) detection
Adipose tissue Acetone:hexane Fractionation by Capillary column > 100 Lebel & Williams
(15:85 v/v) gel permeation gas chromatography (1986)
chromatography with columns of different
methylene polarity, GC-MS
chloride-cyclo-hexane
Florisil column
Air Hexylene glycol/ Florisil GLC/ECD 77 0.1 ng/m3 Stanley et al.
Greenburg Smith column (1971)
impinger; alumina
column
Air Toluene - GLC/ECD (63Ni) - 0.02µg/ NIOSH (1989)
sample
Water Hexane:ethyl ether - GLC/ECD 65-97 0.002 Lichtenberg
µg/litre et al. (1970)
Soil/sediment Acetone:hexane Alumina GLC/ECD 83 0.1 µg/kg Goerlitz & Law
column (1974)
Soil/sediment Acetone:petroleum Alumina GLC/ECD 90 0.01 mg/kg Wegman & Hofstee
ether column (1982)
Soil/sediment Hexane Alumina/ GLC/ECD - 0.01 mg/kg McIntyne & Lester
silver nitrate + (1984)
silica gel
column
Table 3 (contd)
Sample type Extraction Clean-up Detection and Recovery Limit of Reference
quantificationa (%) detection
Crops Hexane:isopropanol; Carbon absorption GLC/ECD 93-107 - Kathpal & Dewan
or acetonitrile (Nuchar C-190N) (1975)
Fatty foods, Hexane:acetone Alumina GLC/ECD 68-100 5-10 µg/kg Telling et al.
vegetable oils, column (1977)
fish oils
Viscera Diethyl ether Celite GLC/ECD 72-92 - Kurhekar et al.
column (1975)
Birds' brain Petroleum ether: Florisil GLC/ECD 70 0.05 mg/kg Ludke (1976)
samples ethyl ether column
Cows' milk Diethyl ether: Silica gel GLC/ECD 90 0.0001 mg/kg Baldwin et al.
hexane + ether (1976)
Hens' eggs Hexane:acetone Silica gel GLC/ECD 76 0.05 mg/kg Baldwin et al.
(yolks) (1976)
Crops, soil, Hydrocarbon Florisil:Celite + Reduction with 75-100 1 mg/kg Terriere (1964)
milk, animal solvent magnesia column, metallic sodium;
tissues (Skellysolve after alkaline phenyl azide
B) + isopropanol hydrolysis, if colorimetry
appropriate
-a GC-MS, gas chromatography-mass spectometry; GLC-ECD, gas-liquid chromatography-electron capture detection
Thier & Stijve (1986) reported a comparative study among 53
laboratories in Switzerland on the analysis of a vegetable fat spiked
with 13 organochlorine and five organophosphorus compounds. Endrin was
present at a concentration of 0.08 mg/kg and was identified by 77% of
the laboratories.
Some of the methods that are used for the analysis of endrin are
summarized in Table 3; the estimates given of the accuracy of the
procedures and the limits of detection refer to the specific
investigations and are not absolute values. The percentage recoveries
are an indication of the accuracy of the methods; the precision of
individual method is of interest particularly in regard to
inter-laboratory comparisons.
The many publications on specific procedures are reviewed in the
Codex Alimentarius Commission publication Recommendations for Methods
of Analysis of Pesticide Residues, CAC/PR8-1986 (FAO/WHO, 1986a).
That review lists 14 individual publications; it also lists the
following compendia of methods, which may be consulted.
-- Official Methods of Analysis of the Association of Official
Analytical Chemists, 14th Edition, 1984
-- Pesticide Analytical Manual, Washington DC, Food and Drug
Administration
-- Manual on Analytical Methods for Pesticide Residues in Foods,
Ottawa, Health Protection Branch, Health and Welfare Canada, 1985
-- Methodensammlung zur Rückstandsanalytik von
Pflanzenschutzmitteln (Methods for Analysing Residues of Plant
Protection Agents), Weinheim, Verlag Chemie GmbH, 1984
-- Chemistry Laboratory Guidebook, Washington DC, US Department
of Agriculture
Whatever procedure is adopted should be carried out following the
requirements of the Codex Alimentarius Commission publication Codex
Guidelines on Good Laboratory Practice in Pesticide Residue Analysis,
CAC/PR7-1984 (FAO/WHO, 1984).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Endrin does not occur naturally.
3.2 Man-made sources
3.2.1 Production levels and processes, uses
Endrin is a foliar insecticide which acts against a wide range of
agricultural pests at doses of the active material of 0.2-0.5 kg/ha.
It has a broad spectrum of control and is particularly effective
against Lepidoptera. It is used mainly on cotton but also against
pests of rice, sugar cane, maize, and other crops. It is also used as
a rodenticide (IARC, 1974). An endrin emulsion of 2% killed 40% of
Achatina fulica snails, an agricultural pest, in India (Singh,
1988).
A general indication of the possible uses of endrin can be
derived from the maximal residue limits recommended by FAO/WHO (1986b;
see section 10).
3.2.1.1 World production figures
Endrin was developed by J. Hyman & Co. and licensed to be
manufactured by Shell International Chemical Co. and Velsicol Chemical
Co. in 1950 (Thompson, 1976). It was made in the USA by Shell and
Velsicol and in the Netherlands by Shell. Its use has been banned in
many countries and severely restricted in others (Donoso et al., 1979;
Gips, 1987; Pearce, 1987). Shell discontinued manufacture of endrin in
1982; it is still manufactured in Mexico.
Whetstone (1964) estimated that 2.3-4.5 million kg of endrin were
sold in the USA in 1962. Imports of endrin into Japan in 1970 were 72
000 kg. The annual quantities of endrin that were used in paddy rice
production in Bali over the period 1963-72 varied from 171 to 10 700
kg (Machbub et al., 1988). After 1972, endrin was no longer used.
3.2.1.2 Manufacturing process
Endrin is produced by condensing vinyl chloride with
hexachloro-cyclopentadiene, dehydrochlorinating the adduct, and
subsequent reaction with cyclopentadiene to form isodrin, which is
epoxidized by peracetic or perbenzoic acid (Whetstone, 1964). The
intermediate isodrin can be manufactured via 1,2,3,4,7,7-
hexachloronorbornadiene (US EPA, 1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Endrin can enter the air by volatilization, evaporation, and
aerial drift during application, and as a vapour from manufacturing
and formulating plants. Most studies showed rapid volatilization
following application to soils and crops, the extent of vaporization
depending upon a large number of factors, including soil organic
matter, moisture content, air humidity, air flow, and surface area of
plants (Donoso et al., 1979).
4.1.2 Water
Endrin can reach surface water by several routes, including
effluents and waste disposal from endrin manufacturing and formulating
plants and careless aerial application, but by far the most important
route of contamination is surface run-off from soil and crops. Run-off
is affected by numerous, complex factors, such as intensity of
precipitation, irrigation practices, soil permeability, topographic
relief, organic content of the soil, and the degree of vegetative
cover. Soils of low permeability and low organic content allow copious
run-off after heavy precipitation (Donoso et al., 1979). Contamination
of surface water by industrial effluents and careless practices and
disposal (such as washing of drums and spray equipment in streams)
results in regional effects.
In 1961, studies were conducted in the Bayou Yokely basin in
Louisiana, USA, where 3300 acres (1335 ha) of sugar-cane were treated
with nearly 2000 lb (907 kg) of endrin between June and August. Of 18
water samples taken between April and November, six contained endrin
at levels of 0.001-0.36 µg/litre, with an average of 0.1 µg/litre. In
1964, the area was treated with 1200 lb (544 kg) of endrin, and the
pattern of residues was the same. The mean residue levels in samples
taken in September were 0.44 µg/litre in grab samples and 0.53
µg/litre in carbon adsorption samples; after three months, the average
levels were 0.03 and 0.04 µg/litre, respectively. Sediment samples
contained 165 µg/kg; after three months, this level had decreased to
70 µg/kg (Lauer et al., 1966).
Another, less important source of water contamination is run-off
from endrin-coated seeds. Marston et al. (1969) found that although
approximately 11% of the initial amount was washed off by water under
laboratory conditions, in field conditions the loss was smaller. The
total amount detected in the watershed 6 days after aerial application
of endrin-coated seed was 0.12% of the applied dose. The highest
concentration found in the water was 0.07 µg/litre.
A third possible source of contamination is fall-out by
precipitation in the form of rain and snow, but the measured levels
are negligible (see section 5.1.3.2).
4.1.3 Soil
The major source of endrin in soil is from direct application to
soil and crops. The amount of endrin that reaches the soil depends on
the type of crop and the method of application. The fate of endrin in
soil determines the degree to which the rest of the environment (water
and atmosphere) is contaminated. In soil, endrin can be retained,
transported, or degraded, depending on a large number of interrelated
factors (Donoso et al., 1979). When endrin was applied to tall, dense
crops such as tobacco, no residue appeared in the soil; when it was
applied to soil, the amount that remained depended on the retentive
ability of the soil. Although endrin has strong absorptive properties
in soils such as clay and sandy loam, limited residues were found. Far
greater retention was found in soils with a high organic content, in
which it was adsorbed quickly and was difficult to remove. The degree
to which endrin was retained in the soil depended not only on the soil
type but on numerous other factors such as volatilization, leaching,
wind erosion, surface run-off, and crop uptake (Harris et al., 1966).
In general, the persistence of endrin is highly dependent upon local
conditions, and residue levels can range from traces to milligrams per
kilogram. Its half-life in soil can be as long as 12 years (Donoso et
al., 1979).
The factors that affect the degree to which endrin is retained in
soil (Donoso et al., 1979) can be generalized as follows:
(a) Endrin appears to be less persistent if it is applied to the
soil surface or to crops rather than being mixed into the
soil.
(b) Volatilization and photodecomposition are the primary routes
for the disappearance of endrin from soil surfaces.
(c) Microbial degradation of endrin occurs anaerobically and is
accelerated by conditions such as flooding and soil depth.
(d) Soil cultivation and crop rotation accelerate the
dissipation of endrin.
(e) When the percentage of organic matter is high, as in muck -
soils, the persistence of eldrin is greater. In sandy soils,
volatilization is high and persistence is low.
4.1.4 Soil-plants
River and basin sediment was brought on land near Rotterdam, the
Netherlands, after dredging. Once the sediment had settled for several
years, the land was used for agriculture. Some of the sediment came
from a basin near a pesticide manufacturing plant and was contaminated
with many organochlorine hydrocarbons, including the pesticides
hexachlorobenzene, aldrin, dieldrin, and endrin. The mean
concentration of endrin in the sediment of the basin near the plant
(expressed in mg/kg on a dry weight basis) was 0.48 (range, 0.01-2.6)
in 1976 and 0.59 (< 0.01-3.6) in 1977. In crops, the concentration of
endrin ranged from none detected to 0.06 mg/kg of product; in carrots,
however, levels up to 0.73 mg/kg were found (Wegman et al., 1981).
4.2 Abiotic degradation
When endrin was heated to above 200 °C, as can occur during
gas-liquid chromatography at 230 °C, the molecule was isomerized to a
ketone, delta-ketoendrin (1, Fig. 1) and an aldehyde (3). A minor
product of the thermal rearrangement was an isomeric alcohol (4).
Endrin is also transformed to delta-ketoendrin (1) under
acid-catalysed conditions (Phillips et al., 1962).
Irradiation with ultraviolet light for 48 h also results in
rearrangement to this ketone (37%) and, to a much lesser extent, to
the aldehyde (9%) (Rosen et al., 1966; Plimmer, 1972; Mukerjee, 1985).
Endrin underwent a photolytic reaction in hexane and in cyclohexane
after irradiation at 253.7 and 300 nm, resulting in a half-cage
ketone, pentachloro photoproduct (2), in 80% yield. This photolytic
product has also been identified in the field and was found to be
highly resistant to oxidation and reduction (Plimmer, 1972; Zabik et
al., 1971; Mukerjee, 1985). When an acetone solution of endrin was
irradiated with light from a mercury lamp in a quartz cell for 24 h,
three metabolites were formed by the loss of one chlorine atom from
the initially produced delta-ketoendrin; one of these was compound 2
(Dureja et al., 1987).
Endrin has been reported to isomerize to delta-ketoendrin during
5 years' storage in the dark at room temperature (Plimmer, 1972.
In sunlight, mainly the ketone is formed (Soto & Deichmann, 1967;
Rosen, 1972); approximately 50% isomerization to the ketone took place
within 7 ± 2 days with exposure to intense summer sun (Burton &
Pollard, 1974).
The photochemical products are important as terminal residues:
delta-ketoendrin was found on cotton plants and on cabbage and apple
leaves after application of endrin (Plimmer, 1971; Mukerjee, 1985).
4.3 Biotransformation
The mechanisms by which endrin is removed from the environment
include photodecomposition and bacterial degradation. These factors
and their effects on the persistence of endrin have been reviewed by
the US Environmental Protection Agency (Donoso et al., 1979).
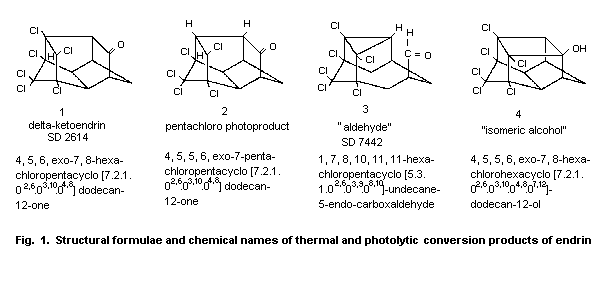 4.3.1 Biodegradation
Microbial degradation of endrin depends on the presence of an
appropriate microbial species and suitable soil conditions; it occurs
under anaerobic conditions (Donoso et al., 1979). Biodegradation is
aided by fungi and bacteria such as Trichoderma, Pseudomonas, and
Bacillus. The major transformation product is delta-ketoendrin
(Patil et al., 1970).
About 150 isolates from various soil samples were screened to
investigate the role of these microorganisms in degrading endrin; 25
of the 150 isolates were active. At least seven metabolites were
found, but conversion of endrin into the ketoendrin was common
throughout (Matsumura et al., 1971).
4.3.2 Bioaccumulation and biomagnification
The bioconcentration factors cited below are simple ratios of the
exposure concentration and the concentration in organic tissues. They
should be used with caution as indicators of bioaccumulation
potential: a high bioconcentration factor can represent little uptake
of a low concentration, and a low bioconcentration factor can be found
with considerable uptake of a high concentration. The bioconcentration
factor should therefore always be cited with the pertinent exposure
concentration of endrin.
Soil invertebrates such as slugs and earthworms had
bioconcentration factors of 14 to 103. Bioconcentration factors in a
number of aquatic organisms are given in Table 4. These ratios differ
extensively between different types of aquatic organisms.
Bioconcentration factors of 140 to 222 were found for four blue-green
algae (Microcystis aeruginosa, Anabaena cylindrica, Scenedesmus
quadricauda, and an Oedogonium species) after 7 days' exposure to
endrin at a concentration of 1 mg/litre of water( Vance & Drummond,
1969). In a study of the accumulation of endrin in stoneflies
(Pteronarcys dorsata) exposed to 0.03, 0.07, and 0.15 µg/litre of
water, the bioconcentration factor ranged from 1130 to 348, decreasing
with increasing water concentrations over the 28-day exposure period
(Anderson & DeFoe, 1980). In bullheads (Ictalurus melas), the
bioconcentration factor was 3700 after exposure for 4 days to 0.60
µg/litre and 6200 after exposure for 7 days to 0.26 µg/litre (Anderson
& DeFoe, 1980). The bioconcentration factors for endrin in sub-adults
of leopard frogs (Rana sphenocephala) exposed to 0.01, 0.012, 0.016,
0.022, and 0.030 mg/litre were 71.4, 34.4, 51.8, 59.4, and 94.3,
respectively. Sub-adults exposed to 0.01, 0.012, and 0.016 mg/litre
for 96 h and sacrificed 60 h later had bioconcentration factors of
6.1, 4.8, and 1.2, respectively (Hall & Swineford, 1980), indicating
a relatively rapid elimination of residues. In daphnids and molluscs,
a direct linear relationship was found between the logarithm of the
equilibrium bioconcentration factor (and the reciprocal clearance rate
constant) and the log P octanol/water partition coefficient for
non-degradable, lipophilic compounds with partition coefficients
ranging from 2 to 6. This relationship permits calculation of the
times required for equilibrium and for significant bioconcentration of
lipophilic chemicals, which were found to be shorter for molluscs than
for daphnids. The equilibrium biotic concentration for both molluscs
and daphnids decreased with increasing chemical hydrophobicity. The
relationship between the bioconcentration factor and log P
octanol/water partition coefficient was linear for compounds that did
not attain equilibrium within a finite exposure time (Hawker &
Connell, 1986).
In a study of the bioaccumulation of endrin from food by lobsters
(Homarus americanus), endrin dissolved in methanol was added to sea
water, and mussel tissue was soaked in the solution for 2 h to provide
a concentration of endrin of 4.7 mg/kg wet weight. Lobsters were fed
the prepared food every other day for 2 weeks, and excretion was
followed for an additional 4 weeks during which time the lobsters were
fed uncontaminated tissue. Liver and muscle were analysed from two or
three lobsters sampled after feedings 1, 2, 3, 5, and 7, and from one
or two lobsters sampled during the excretion phase at 1, 2, and 4
weeks. The concentration of endrin reached a maximum of 1.95 mg/kg wet
weight in the liver after 2 weeks of feeding; this level declined by
about 65% after 4 weeks of excretion. The time to 90% equilibrium
(uptake) was 15 weeks, and the time to 50% clearance (excretion) was
4 weeks (McLeese et al., 1980).
Bluegill sunfish (Lepomis macrochirus) exposed to water
containing 14C-labelled endrin at 1 µg/litre at temperatures of
20-22 °C rapidly absorbed the radioactivity, and, within 48 h, 91% of
the radioactive endrin had been taken up (6% was lost by
volatilization from a control tank without fish). Within 8 days after
the fish had been replaced in clean water, less than 15% of the
absorbed label had been eliminated; for three fish left for a longer
period, the half-life of loss was about 4 weeks, the loss curve being
linear (Sundershan & Khan, 1980). Endrin accumulated rapidly in the
tissues of channel catfish (Ictalurus punctatus) exposed to nominal
concentrations of 0.04, 0.4, or 4.0 mg/kg of diet for 198 days. After
that time, all groups were fed an endrin-free diet. Endrin was not
detected 28 days later in fish that had received 0.04 or 0.4 mg/kg,
and the level in the group that had received 4.0 mg/kg decreased to
0.011 mg/kg of tissue in 28 days and was below the limit of detection
within 41 days (Argyle et al., 1973). Similar results were obtained
for Leiostomus xantharus exposed to 0.05 µg/litre of water: at the
end of the study at 5 months, a residue level of 78 µg/kg tissue was
found, and no endrin was detected in fish after 18 days in
uncontaminated water (Lowe, 1966). Endrin thus seems to disappear
rapidly from tissues. In 20 Tilapia zilli (Alexandria strain) fry
(3.36 cm, 825 mg) exposed to 0.025 µg/litre (one-tenth of the 96-h
LC50) for 28 days, the total content of endrin was 327.4, 167.4,
297.6, 446.5, and 595.4 µg/kg after 4, 7, 14, 21, and 28 days,
respectively (El-Sebae, 1987).
Table 4. Bioconcentration factors for endrin in aquatic species
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Clam 1 5 days 480 Duke & Dumas
(Mercenaria (1974)
mercenaria)
Mussel 10 24 days 38 Ryan et al.
(Hyridella (1972)
australis)
Eastern oyster 0.05 7 day 2780 Mason & Rowe
(Crassostrea (1976)
virginica)
Water flea 1.0 1 day 2600 Metcalf et al.
(Daphnia (1973)
magna)
Fathead 0.015 - 10 000 Mount & Putnicki
minnow (1966)
(Pimephales
promelas)
Spot 0.05 8 months 1340 Lowe (1966)
(Leiostomus
xanthurus)
Flag fish 0.3 - 10 000 Hermanutz
(Jordanella 0.21 15 days 7 900 (1974)
floridae) 0.29 18 400 Hermanutz et
0.39 7 100 al. (1985)
Table 4 (contd)
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Mosquito 1.0 1 day 2 100 Metcalf et al.
larvae (Culex (1973)
ipiensquinque
fasciatus)
Mosquito fish 1.0 1 day 800 Metcalf et al.
(Gambusia (1973)
affinis)
Channel catfish 0.5 5-19 400-760 Argyle et al.
(Ictalurus (1973)
punctatus)
Sheepshead minnow (Cyprinodon variegatus) were exposed for 23
weeks to endrin at levels of 0.027-0.72 µg/litre of water, from the
embryonic stage through hatching until adulthood and spawning (see
section 7.2.2.2). Four-week-old juvenile fish accumulated 2500 times
the concentration in the water, adults, 6400 times, and their eggs,
5700 times (Hansen et al., 1977).
The transfer of endrin through the food chain
lichen-reindeer-humans was studied in the northern part of Sweden by
analysing lichen (Cladonia alpestris), a major food source for
reindeer during the winter, together with samples of tissues from
reindeer, which are eaten in considerable quantities by Lapps. One
4-year-old reindeer was slaughtered in 1979 and a 3-year-old in 1981,
and muscle and liver were taken for analysis. The annual uptake by
reindeer was 2.0 mg. The average level of endrin in lichen was 1.91
(range, 1.27-2.78) µg/kg; 1.45 and 2.4 µg/kg were found in the muscle
samples from the two reindeer and 0.55 and 0.72 µg/kg in liver. The
calculated transfer of endrin from lichen to reindeer was 0.7%. The
estimated annual consumption of reindeer muscle by Lapps was 70 kg for
males and 32 kg for women; consumption of liver was 3 and 1.1 kg,
respectively. The annual intake of endrin was thus 30.3 µg for males
and 13.8 µg for females (Villeneuve et al.,1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Many of the data reported in this chapter are measurements taken
at a time when endrin was used much more widely than at present or
with little control or restriction. They are therefore a reflection of
a historical situation in many countries. These data are included in
the document as an indication of the result of indiscriminate use and
disposal of endrin. Data from countries where endrin may still be used
are scarce or unavailable.
5.1 Environmental levels
The levels of residues associated with the use of endrin in
agriculture or with the discharge of industrial effluents containing
endrin are summarized in Tables 5-9; the levels of residues less
easily associated with specific uses or discharges are given in Table
10.
5.1.1 Air
A critical summary of studies on the atmospheric levels of
pesticides in the USA, e.g., in community air, was made by Donoso et
al. (1979). Some of their conclusions are worth repeating: "Endrin
concentrations are highest in the atmosphere over agricultural areas
and probably reach their peak levels during the pesticide use season.
Of all urban communities those surrounded by farmlands run the highest
chance of atmospheric contamination. Urban communities far removed
from agricultural areas are unlikely to experience significant
contamination." The maximum level of endrin in air, 58.5 ng/m3, was
found in a rural town in an agricultural area in the south of the USA,
but the normal weekly variation was between 0.8 and 6.5 ng/m3
(Stanley et al., 1971). In a later study of the same town, the average
annual atmospheric levels were 3.2 ng/m3 in 1972, 2.3 ng/m3 in
1973,and 5.3 ng/m3 in 1974, with the highest levels in August; in
1974, this was 27.2 ng/m3 (Arthur et al., 1976). The results of a
national monitoring programme for pesticides in the air of various
states of the USA showed the occasional presence of endrin over
agricultural areas at levels of the same order of magnitude: mean of
positive samples (8%), 2.6 ng/m3, with a maximum value of 19.2
ng/m3 (Kutz et al., 1976).
Endrin was not found in rain-water collected at different
location in the United Kingdom, using a method with a detection level
of 1 ng/litre of water (Tarrant & Tatton, 1968), nor in atmospheric
air (Abbott et al., 1966); however, endrin has never been used
extensively in the United Kingdom.
The mean daily intake of endrin by inhalation in the western part
of the Netherlands was calculated on the basis of an air concentration
of 41 pg/m3 (maximum, 300 pg/m3) to be 0.8 µg/day or 0.3 mg/year,
on the basis of air samples taken in the period 1975-81 (Guicherit &
Schulting, 1985).
Table 5. Concentrations of endrin in organisms collected in the Netherlands, 1965-71
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Coast Mussel (Mystilus edulis); 22 0.029 0.009-0.056 Composites of 25 Koeman (1971)
1965 composites of flesh mussels from 22
sampling stations
Netherlands Fish, 3 species; 103 0.13 0.07-0.45 Food of the sandwich Koeman et al. (1967)
1965 whole body tern
1966 Fish, 3 species; 37 0.10 0.07 -0.29
whole body
Sandwich tern
(Sterna sandvincensis)
1965 Liver 8 0.23 0.07-0.80 Shot or killed
1965-66 Liver 25 0.49 0.10-1.3 Found dead
1965-66 Egg 33 0.19 0.08-0.36
Wadden Sea Mussel (2 species); 20/4 LD LD Limit of detection, Koeman (1971)
1969 composites of flesh 0.005 mg/kg
Coast Mussel (Mytilus edulis); 199/8 < 0.016 LD-0.024 Koeman (1971)
1970 composites of flesh
Wadden Sea Zooplankton (marine) 1 LD LD Koeman (1971)
1969-70
Shrimp (Crangon 50/1 LD LD
vulgaris)
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Marine fish (4 species); 37/5 0.014 0.008-0.034
composites of whole
body
1967 Freshwater fish 28 LD LD-0.02 Measurable concentration
(3 species) (0.02 mg/kg) in one fish
only; limit of detection,
0.005 mg/kg
1970 Pike (whole body) 10 LD LD Limit of detection,
0.005 mg/kg
1971 Roach (whole body) 6 LD LD
1968-69 Hawks and falcons 16 < 0.1 LD-0.16 Birds found
dead or dying; Koeman et al. (1969)
(4 species); liver measurable concentration
(0.16 mg/kg) in one hawk
(buzzard)
Owls (2 species); liver 3 < 0.1 LD-0.13 Measurable concentration
(0.13 mg/kg) in one
long-eared owl; limit of
detection not specified
1970 Sandwich tern eggs 10 LD LD Limit of detection, Koeman (1971)
0.02-0.008 mg/kg
1971 Grey heron 27/4 LD LD
(Ardea cinerea);
composite of eggs
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
1969-71 Sparrowhawk 28/3 LD LD
(Accipiter nisus);
composite of eggs
a Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
b LD, limit of detection
Table 6. Concentrations of endrin in samples collected in North America
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Mississippi River
USA
December 1963 Channel catfish; blood 3 0.44 0.41-0.56 Found dead or dying in areas Anon. (1964)
of extensive fish kills
December 1963 Fish, various species; 24 0.18 0.14-0.26
blood
January- Fish, various species; 82 0.06 LD-0.21 Caught alive; limit of
February 1964 blood detection not specified
July 1964- Water 12 < 0.01 LD-0.01 4 samples contained Novak & Rao (1965)
June 1965 measurable concentrations
(0.01 mg/kg or litre)
Mud 12 < 0.01 LD-0.01
Oysters 12 LD LD Limit of detection,
0.005 mg/kg or litre
Shrimp 12 LD LD
Fish (2 species) 24 < 0.01 LD-0.02 9 samples of fish contained
measurable concentrations:
8 of 0.01 mg/kg and 1 of
0.02 mg/kg
Mississippi, USA Eastern oysters 470 LD LD 15 or more oysters per Butler (1973)
1965-72 (Crassostrea virginica); composite from 8 sampling
composites of flesh stations; limit of detection,
0.01 mg/kg
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Bayous in the Water 148 LD LD-0.0002 4 samples contained Rowe et al. (1971)
Mississippi delta, measurable amounts
USA, October (0.00009-0.0002 mg/litre),
1968-May 1969 4 samples contained traces;
remainder less than limit of
detection
Sediment 44 LD LD-0.005 7 samples contained
measurable amounts
(0.004-0.005 mg/kg); one
sample contained a trace
Eastern oyster 111 LD LD-0.006 79 samples contained
(Crassostrea virginica) < 0.001 mg/kg
Mississippi Water 26 LD LD Samples collected from Leard et al. (1980)
stream systems 13 sampling stations in
1972-73 5 major river basins; limit
of detection, 0.0005 mg/litre
Freshwater bivalves 58 LD LD-0.1 Residues below limit of
(7 species); flesh detection (0.02 mg/kg), except
for traces (< 0.1) in 1973 in
one river which drains from an
agricultural area
Ontario, 3 Fish (9 species) LD LD Residues below limit of Miles & Harris
streams, 1971 detection, 0.01 mg/kg (1973)
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Gulf coast Whole fish 139/48 < 0.02 LD-0.27 Reseidues below limit of Henderson et al.
streams, USA (various species); detection (0.001 mg/kg) (1969)
composites in 33 composites
Mississippi 657/202 < 0.01 LD-0.11 Residues in 184 composites
River system, below limit of detection
USA (0.001 mg/kg)
Louisiana, USA Brown pelican Eggs collected from nests of Blus et al. (1979)
(Pelecanus occidentalis); birds transplanted as nestlings
eggs from Florida, 1968-76; limit
1971 3 0.10 0.08-0.12 of detection not specified
1972 12 0.18 0.11-0.29
1973 21 0.16 0.03-0.46
1974 25 0.30 LD-0.73
1975 30 0.50 0.29-1.06
1976 25 0.29 LD-1.47
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 7. Concentrations of endrin in organisms collected in a
cotton-growing area in the Republic of Chad in 1969
Sample No. of Concentration (mg/kg) Comments
samples Mean Range
Fish, two 31 0.02 LD-0.083 Cotton-growing area,
species endrin and DDT used
for pest control; limit
Kingfishers and 46 0.02 LD-0.075 of detection, 0.008
cormorants; liver mg/kg
Birds, non-aquatic, Birds found dead
various species soon after insecticide
Brain 12 0.51 0.10-0.77 application; deaths
Liver 12 0.88 0.13-1.42 of some birds attributed
to endrin
From Everaarts et al. (1971); LD, limit of detection
5.1.2 Soil, sediments, and sewage sludge
5.1.2.1 Soil
In the US National Soil Monitoring Program, 1486 soil samples
from 37 states were analysed in 1971. Fourteen samples were found to
contain endrin, at a geometric mean level of < 0.001 (maximum,
0.02-1.00) mg/kg dry weight (Carey et al., 1978). The mean endrin
concentration in 29 soil samples in Kyushu District, Japan, was 0.183
mg/kg (range, 0.016-0.629 mg/kg) dry matter (Suzuki et al., 1973).
5.1.2.2 Sediments
In 1964, levels in the sediment of Cypress Creek, Memphis, TN,
USA, upstream and downstream of a pesticide manufacturing plant,
reached 12 800 mg/kg dry weight. In 1967, water from the Creek
contained levels of 0.27-2.03 µg/litre and sediment contained levels
of 47.4-10 676 mg/kg dry weight (Barthel et al., 1969).
Endrin was found in 17% of samples of bottom sediment from 59
sites on the Detroit River, USA, at levels up to 43 µg/kg (limit of
detection, 1.0 µg/kg) (Hamdy & Post, 1985). No endrin was detected in
sediment samples collected in 1980-82 from riverine and pothole
wetlands at 17 locations in the north-central USA (Martin & Hartman,
1985) or in samples of sediment from 34 stations on the upper Great
Lakes in 1974 (< 1 µg/kg) (Glooschenko et al., 1976).
Table 8. Concentrations of endrin in organisms collected in a rice-growing area in Wageningen, Surinam
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
October 1971 Snail kite (Rostrhamus 5/1 LD LD -- Pesticides, including endrin, applied
sociabilis); brain /liver | to rice fields
|
Black vulture (Coragyps 5/1 LD LD |
astratus); brain/liver |
|
Egrets (3 species); brain/ 30/1 LD LD | Samples collected at end of growing
liver |-- season before insecticide application
| for next growing season; limit of
Purple gallinule 10/1 LD LD | detection, 0.01 mg/kg
(Porphyrula martinica); |
brain/breast muscle |
|
Spectacled caiman (Caiman 10/1 LD LD |
crocodilus); brain/liver --
November 1971 Snail (Pomocea sp.) 10/1 LD LD --
|
Frog (Pseudis paradoxa); 6/1 LD LD | Found dead after application of
whole-body composites | pentachlorophenol; lower limit of
| detection, 0.01 mg/kg
Kwi kwi (Hoplosternum 8/1 LD LD |
littorale); whole-body |
composites --
Table 8. (contd)
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
Srieba (Astyanax bimaculatus); 8/1 LD 0.1 -- Found dead after application
whole-body composites | of pentachlorophenol; lower
|-- limit of detection 0.01 mg/kg
|
Krobia (Cichlasoma 8/1 LD LD |
bimaculatum); whole-body |
composites --
Fish (3 species listed above); 21/3 3.36 1.96-5.35 Found dead after application
whole-body composites of endrin
28 November- Snail kite; brain 17 LD LD Found dead; deaths
4 December 1971 attributed to pentachlorophenol
poisoning
2-9 December 1971 Aquatic birds (4 species); 5 0.11 0.06-0.16 Found dead after application
brain of endrin
2-9 December 1971 Wattled jacana 1 2.71 Death attributed to endrin
(Jacana jacana) poisoning
5-11 December 1971 Common egret (Egretta alba); 2 0.23 0.14-0.32 Found dead or sick in roost
brain
2-11 December 1971 Common egret (Egretta alba); Found dead or sick in rice
Brain 9 0.25 fields; about half the total
Liver/kidney 7-9 0.08 endrin was applied during
the first half of December
From Vermeer et al. (1974)
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 9. Concentrations of endrin collected in drainage water from irrigated land, California, USA
Geographical Type of sample No. of Concentration (mg/kg or mg/litre) Comments
area and year samples
Mean Range
Tule Lake and Water 44 0.000011 LD-0.0001 Limit of detection, < 0.000003 mg/litre
Klamath Lake,
National
Wild-life
Refuge,
USA, 1964
Refuge, Northern Suspended matter 8 0.011 LD-0.058 --
California, USA, Vascular plants 7 0.006 LD-0.013 |
April 1965- (two species) |-- Limit of detection, 0.005 mg/kg
February 1967 Algae (Cladophora sp.) 5 0.007 LD-0.022 |
Clam homogenates 3 0.013 LD-0.034 --
Gonidea sp.)
Fish (Siphateles sp.) 5 0.05 0.004-0.198 Samples collected at a pumping station
discharging water from irrigated land.
Peak concentrations of endrin occurred
during the growing season when endrin
was applied.
From Godsil & Johnson (1968); LD, limit of detection
Table 10. Concentrations of endrin in environmental samples; residues not associated with particular local use or industrial
effluent
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
North America
North Carolina, Soil (tobacco fields) 19 LD LD Reeves et al.
USA,1971 Sediment (ponds) 40 LD LD Limit of detection, (1977)
Frog (Rana sp.) 13 LD LD-0.01 0.01 mg/kg
Turtle (4 species) 41 LD LD-0.01
Bluegill (Lepomis 20 LD LD
macrochirus)
Tiger beetle 23 0.02 LD-0.05
(Megacephala
carolina)
Rice-growing Invertebrates, aquatic 1313/24 LD LD-trace A total of 192 dead or Flickinger &
area, Gulf Coast, and terrestrial (various dying birds were found in King (1972)
Texas, USA, species); whole-body three rice-growing areas in
1967-71 composites of live which rice seed dressed
specimens, except for with aldrin/ceresan had
4 composites of been used. Endrin residues
crayfish attributed to use in cotton-
growing areas. Limit of
1968 Fish (4 species); 542/4 LD LD detection not defined;
whole-body composites trace found in one
composite of dead
1968 Cricket frog (Acris 18/3 LD LD crayfish
crepeitans blanchardi);
whole-body composites
1968-70 Turtles (2 species); 5/2 LD LD
whole-body composites
of live specimens
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Snakes (3 species); sick 3 LD LD
specimens
Bobcat (sick) and dead 2 LD LD
rice rat; brain
Great horned owl; dead 2 LD LD
specimen; brain
Rice- growing Aquatic birds (10 26 0.22 LD-0.4
area, Gulf Coast species) found dead
USA, 1967-71 or dying; brain
1967 Fulvous tree duck 14 0.1 LD-0.3
(Dendrocygna bicolor);
eggs
Galveston Bay Oyster composites 10 0.01 LD-0.02 Limit of detection, Casper (1967)
Texas, USA, 1964 0.01 mg/kg
National Monitoring Fish (various species); 400 93% of samples below Henderson et
Program: Great Lakes whole-body composites limit of detection, al. (1969)
and major river 0.001 mg/kg
basins, USA (excluding
Gulf Coast, Mississippi
River system; see
Table 6); 1967-68
Atlantic coast streams 741/141 0.002 LD-1.50
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Great Lakes drainage Fish (various species); 378/66 0.001 LD-0.02
whole-body composites
Hudson Bay, Canada, 51/13 LD LD
drainage
Colorado River, USA 112/24 0.008 LD-0.71
Interior basins 120/25 0.001 LD-0.01
California, USA, 90/24 0.002 LD-0.02
streams
Columbia River, USA, 246/64 0.001 LD-0.01
systems
Pacific coast, USA, 83/20 LD LD
streams
Alaska, USA, streams 105/24 LD LD
National Monitoring Fish (various species); 666/147 LD LD Limit of detection, Henderson et
Program; 50 sampling whole-body composites 0.005 mg/kg al. (1971)
stations, USA, 1969
Estuaries, Giant Pacific oyster 1656/138 0.005 LD-0.01 Measurable concentration Modin (1969)
California, USA (Crassostrea gigas); in only one oyster; limit
1966-67 Mussel (Mytilus edulis); 432/36 LD LD of detection, 0.01 mg/kg
composites of shellfish
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 108-162/54 0.06 LD-0.4 Limit of detection, Crockett et
Mississippi, USA fish farms; composites 0.01 mg/kg; 13 al. (1975)
1970 of edible portions composites contained
< 0.01 mg/kg
Intensive cotton- 0.063 (0.030- 2 composites contained
growing areas, 0.122)a > 0.3 mg/kg. Significantly
Mississippi, USA higher residues in intensive
cotton-growing areas
Less intensive cotton- 0.010 (0.005- Crockett et
growing areas, 00.019)a al. (1975)
Mississippi, USA
Major watersheds, Fish (various species); 582/58 LD LD Limit of detection not Veith et al.
USA, 1976 whole-body composites specified. Intermediate (1979)
in the manufacture of
cyclodiene insecticides
detected (mass
spectrometry) in Wabash
River, Indiana
Major watersheds Fish (various species); 138/6 LD LD Limit of detection not Veith et al.
near Great Lakes, whole-body composites specified. Endrin (1981)
USA, 1978 identified by mass
spectometry in fish from
Wabash River, Indiana,
together with manufacturing
intermediates (concentration
not quantified)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 50 0.05 LD-0.41 Limit of detection, 0.01 Hawthorne
Mississippi, USA, fish farms, edible portion mg/kg; 14 fish contained et al. (1974)
1970 < 0.01 mg/kg
Continental rise Bathyl-demersal fish 4 LD LD Limit of detection, Meith-Avcin
south-east of (Antimora rostrata); 0.01 mg/kg. Samples et al. (1973)
Cape Hatteras, USA, liver collected by trawling at a
1972 depth of 2500 m
Lake Michigan, USA, Amphipods (Pontoporeia 24/8 0.08 0.04-0.33 Limit of detection, Peterson &
1969-72 affinis) collected from 0.005 mg/kg Ellarson
oesophagi of old squaws (1978)
December 1969 Old squaws (Clangula 37 0.18 0.1-0.2 Birds caught in fishing
hyemalis); carcasses nets or shot
March-April 1970 44 0.28 0.2-0.4
December 1970- 108 0.31 0.1-0.9
May 1971
January-February 8 0.6 0.2-1.0
1972
Northwest Territories 99 0.1 LD-0.3
and wintering areas
other than Lake
Michigan, 1971-73
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Canada and USA, Bald eagles (Haliaeetus 29 0.02 LD-0.1 Limit of detection, Reichel et al.
1965 leucocephalus) found 0.05 mg/kg. (1969)
dead; brain Concentration in 24
specimens below limit
of detection
Connecticut & Bald eagles found dead; 2 LD LD-0.1 Limit of detection not Reichel et al.
Florida, USA, brain, liver, carcass defined; apparent (1969)
1967-68 concentration of
0.1 mg/kg in Florida
eagle not conformed
by thin-layer
chromatography
Continental USA Bald eagle; brain Limit of detection, Mulhern et al.
1966 21 LD LD 0.05 mg/kg (1970)
1967 21 LD LD
1968 26 LD LD
1969 Bald eagle; brain 28 LD LD Limit of detection, Belisle et
0.05 mg/kg al. (1972)
1970 11 LD LD
1971-72 Bald eagle; brain 37 LD LD Limit of detection, Cromartie et
0.05 mg/kg al. (1975)
1973-74 Bald eagle; brain 81 LD LD Limit of detection, Prouty et al.
0.05 mg/kg (1977)
1975 Bald eagle; brain 49 0.07 LD-0.50 Limit of detection, Kaiser et al.
0.05 mg/kg. (1980)
Concentrations in 46
specimens < 0.05 mg/kg
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
1976 50 0.08 LD-0.71 Concentrations in 44
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisong
1977 69 0.08 LD-1.2 Concentrations in 64
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisoning
Wisconsin, Maine, Bald eagle; eggs 26 LD LD Limit of detection, Krantz et al.
Florida, USA, 0.05 mg/kg (1970)
1968
USA, Golden eagle (Aquila 102 LD LD-0.3 Limit of detection, Reidinger &
1964-71 chrysaetos) found dead 0.1 mg/kg. Concentrations Crabtree
or dying; body fat in 97 specimens below (1974)
limit of detection
Coast of California, Gray whale 23 LD LD Wolman &
USA, 1968-69 (Eschrichtius robustus); Wilson (1970)
blubber
Sperm whale (Physeter 6 LD LD
catodon); blubber
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
South Atlantic and Small cetaceans (10 69 LD LD-0.24 Limit of detection, O'Shea et al.
Pacific Oceans, species); blubber, 0.1 mg/kg. Measurable (1980)
1968-69 brain, muscle concentrations (0.22 and
0.24 mg/kg) found in two
specimens
Maryland, USA, Little brown bat (Myotis 87 LD LD Limit of detection, Clark &
1976 lucifugus); carcass 0.1 mg/kg Krynitsky
(1978)
Missouri, USA Gray bat (Myotis 20 LD LD Limit of detection, Clark et al.
1976-77 grisescens) found dead; 0.1 mg/kg (1980)
carcass (lipid basis)
Washington Sate 18 bird species (total Blus et al.
(orchards), number of birds, 91) (1983)
October 1981- Brain 78 LD-> 0.8
July 1982 Eggs 53 LD-1.7
Detroit River, Herring gulls (Larus LD LD Struger et al.
Niagara River, argentatus); eggs (1985)
Saginaw Bay, USA,
1978-82
North-west Fish species; 700 0.008 LD-0.026 Stout (1980)
Atlantic Ocean, muscle
Gulf of Mexico,
USA, 1973-75
South-east Montana, Merlins (Falco LD LD Becker & Sieg
USA, 1978-81 columbarius); eggs (1987)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
New Jersey, Snapping turtles 11 LD LD Limit of detection, Albers et al.
Maryland, USA (Chelydra serpentina) 0.1 mg/kg (1986)
Florida, USA Snail kite (Rostrhamus LD LD Limit of detection, Sykes (1985)
sociabilis); eggs, nestlings 0.05 mg/kg
Missouri, USA, Gray bats (Myotis 7 LD LD Clawson &
1982 grisescens) Clark (1989)
Red bats (Lasiurus 7 LD LD
borealis)
Pipstrelles (Pipistrellus 2 LD LD
subflavus)
Denver, Colorado, Tree swallows 32 LD LD Deweese et al.
USA, 1980-81 (Tachycineta bicolor) (1985)
North-east Alberta, Otter (Lutra canadensis); 158 LD LD Limit of detection, Somers et al.
Canada, 1980-82 carcass 0.001 mg/kg (1987)
Africa
Lake Nakuru, Fish (Tilapia grahami); 10-20/2 LD LD Limit of detection: Koeman &
Kenya, 1970 whole-body composites fish, 0.002 mg/kg; Santiago
African cormorant birds, 0.009 mg/kg (1972)
(Phalacrocorax africanus) 3 LD LD
liver
White pelican 1 LD LD
(Pelicanus onocratalus);
Lesser flamingo 5 LD LD
(Phoeniconaias minor)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Europe
Province of Leon, Kestrels (Falco 4 - LD-2.0 Liver, 0.01; brain, 0.016; Sierra &
Spain, 1986 tnnunculus); 5 organs kidneys, 0.027; muscle, Santiago
or tissues 0.054 mg/kg (1987)
Sparrowhawk 3 LD-2.0 Liver, 0.139; kidneys, 0.4;
(Accipiter nisus) fat, 1.068; brain, 0.031;
muscle, 0.047 mg/kg
Red kite 2 LD-2.0 Kidneys, 0.005; brain,
(Milvus milvus) 0.103; fat, 2.035 mg/kg
1984-87 Barn owl (Tyto alba) 0.001-0.22 Liver, 0.036; brain, 0.052; Sierra et al.
kidneys, 0.034; fat, 0.014; (1987)
muscle, 0.020 mg/kg
North Sea Gadus morhua and 12 0.0001-0.0023 Von Westernhagen
Merlangius merlangus; 4 < 0.001-0.0011 et al. (1987)
ovary
North Sea Merlangius merlangus Von Westernhagen
Ovary 56 LD-0.001 et al. (1989)
Testis 16 LD
Liver 30 LD
Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed; LD, limit of detection
a95% confidence interval
None of 60 samples of bottom deposit collected in 1974 from 19
rivers and their estuaries in Japan contained endrin (< 0.01 mg/kg)
(Japanese Environmental Agency, 1975).
No endrin was detected in sediment and particulates from the
River Elbe in Germany in 1983-85 (Sturm et al., 1986). Sediment from
Rotterdam Harbour contained a total of 3-59 µg/kg aldrin, dieldrin,
and endrin. No endrin was found at seven sites in the Elbe Estuary
(Japenga et al., 1987). A housing estate in the Netherlands,
comprising about 800 houses and public buildings, was built in 1983
directly on a 4-m-thick layer of harbour sludge transferred in 1962-64
from about 20 harbour basins in Rotterdam and the industrial area
around the Nieuwe Waterweg. Organic solvents, polycyclic aromatic
hydrocarbons, heavy metals, and endrin and related pesticides, were
detected in the sludge. One-third of the soil samples collected in the
gardens (71 locations), 0-40 cm below the surface, contained endrin
and related pesticides at a mean concentration of 1.2 mg/kg and a
maximal concentration of 19.5 mg/kg dry weight (Van Wynen & Stijkel,
1988).
In surface sediments from five sites in Manukan Harbour, New
Zealand, only traces (none detected to < 0.1 µg/kg dry weight) of
endrin were found (Fox et al., 1988).
Particulates from two sites in the Shatt al Arab River in Iraq
contained endrin at 84 and 154 µg/kg, and a site in the Tigris River
contained 217 µg/kg. No endrin was found in the Euphrates River. The
mean concentration of endrin in surface and subsurface sediment from
the Shatt al Arab River ranged from 3 to 18 (range, none detected to
32) µg/kg; no endrin was found in surface and subsurface sediment from
the Tigris River. None was found in surface sediment from the
Euphrates River, but in subsurface sediment a mean concentration of 11
(5-25) µg/kg was detected (DouAbul et al., 1988).
5.1.2.3 Sewage sludge
Endrin was found in only a few of 444 sludge samples analysed
from sewage treatment works in the United Kingdom. The mean
concentration was 0.11 mg/kg of sludge, with a range of 0.01-0.71
mg/kg (McIntyre & Lester, 1984). All samples of non-disinfected
influent at a pilot plant in Jefferson Parish, LA, USA, contained
endrin, at an average concentration of 0.67 (0.25-1.58) ng/litre
(Lykins et al., 1986).
Sludge from three main waste water treatment plants in Kuwait was
analysed over a 6-month period in 1984-85. Two grab samples were taken
from each plant every month to give a total of 36 samples. The mean
endrin levels for the three plants were 0.02, 0.02, and 0.06 mg/kg
(Samhan & Ghobrial, 1987). Sewage plant effluents before and after
treatment were analysed in Baghdad (Iraq) in 1982-83. Endrin was found
in 8/15 samples taken before treatment, at a mean concentration of
0.291 (0.081-2.637) µg/litre, and in 6/15 samples taken after
treatment, at a mean concentration of 0.194 (0.072-1.197) µg/litre
(Al-Omar et al., 1985a).
5.1.3 Water
5.1.3.1 Surface water
Data on the concentrations of endrin in surface water concern
mainly those regions in the USA where use of endrin was widespread,
such as in Mississippi and Missouri, over the period 1957-65. The
highest concentrations were found in 1963 in the Lower Mississippi,
with a maximum level of 0.214 µg/litre. The concentrations and the
rate of occurrence decreased considerably later (Breidenbach et al.,
1967). In a survey in 1964-68, a maximal level of 0.133 µg/litre was
reported to have been found in the Missouri basin in 1967. No endrin
was detected in 1968 (Lichtenberg et al., 1970). In 1974, the
concentrations in the Lower Mississippi was 0.0045 µg/litre in
August-November (Brodtmann, 1976).
In one sample from the Potomac River, at Quantico, endrin and
endrin aldehyde were identified at concentrations of 0.005 and 0.006
µg/litre, respectively (Hall et al., 1987). Endrin was not found in
the waters near the Los Angeles County ocean outfalls (< 0.00005
µg/litre) (Green et al., 1986) or in surface water in Louisiana in
1980 (< 1.0 ng/litre) (McFall et al., 1985). In a programme to
monitor surface water in the USA in 1976-80, endrin was found in only
0.1% of samples, at a maximum value of 0.04 µg/litre (Carey & Kutz,
1985).
No endrin was found in water from 33 sites in the Upper Great
Lakes in Canada (< 0.01 µg/litre) (Glooschenko et al., 1976). In
Ontario, where endrin was used only sparingly, no residues were found
in 1971 or 1975-77 (Miles & Harris, 1973; Frank et al., 1981). In
water samples taken 1 m below the surface at 14 stations on Lake
Ontario in 1983, endrin was found in concentrations of
0.000044-0.000145 µg/litre (Biberhofer & Stevens, 1987).
In a survey of the aquatic environment in The Netherlands,
including drinking-water, 1826 samples were taken at 99 sampling sites
between September 1969 and 1977; traces of endrin were reported
occasionally (Wegman & Greve, 1978, 1980). Studies of surface water in
other areas in Europe failed to show the presence of endrin (Wilson
Committee, 1969; Engst & Knoll, 1973; Uhnak et al., 1974; Galassi &
Provini, 1981;Hrubec, 1988). In 1984-85, water from a number of rivers
in Germany contained endrin at levels of none detected to 0.30
µg/litre (Braun ,1985); surface water in Greece occasionally contained
levels of 0.0003-0.0004 µg/litre (Albanis et al., 1986).
No endrin was found in surface or drinking-water in the state of
Sa Paulo (Brazil) (Lara & Barreto, 1972), but it was found in water
reservoirs of basins in Sa Paulo at concentrations of none detected
to 1.02 µg/litre (Celeste & Caceres, 1987; Caceres et al., 1987).
Endrin was also found accidentally in two lagoons in north-west Mexico
(Rosales et al., 1985).
None of 60 water samples collected in 1974 from 19 rivers and
their estuaries in Japan contained endrin (< 0.1 µg/litre) (Japanese
Environmental Agency, 1975).
Endrin was found at five places in the River Nile at
concentrations of 0.0038-0.0189 µg/litre in March-September 1982
(El-Dib & Badawy, 1985). In analyses of the water of the Shatt al
Arab, Euphrates, and Tigris Rivers, endrin was found only in the
Euphrates River, but in all samples at a mean concentration of 0.024
(0.014-0.036) µg/litre (DouAbul et al., 1988). It occurred in 75% of
samples of urban, industrial, and continental water from the Moroccan
Mediterranean coast, at concentrations of none detected to 13 µg/litre
(Kassabi et al., 1988). Three of 15 grab samples of surface water
sources in Southern Africa (Orange Free State) contained endrin, at a
concentration of 2-4 µg/litre (Hassett et al., 1987).
Endrin was present in the Kalinadi River in India as a result of
runoff, especially from agricultural areas, at a concentration of 2
µg/litre (Kudesia & Bali, 1985). In the Chao Phraya River and klongs
in Bangkok, Thailand, no endrin (< 0.001 µg/litre) was found in 1984
(Onodera & Tabucanon, 1986). Analyses in Bali of 16 samples of river
water in the dry season and 15 samples in the rainy season showed the
presence of endrin at 40 µg/litre once, in the rainy season (Machbub
et al., 1988).
5.1.3.2 Rain and snow
No endrin was found (limit of detection, 1-2 ng/litre) in
atmospheric precipitation in the form of snow (17 samples in 1976) and
rain (81 samples in 1976 and 1977) on the Canadian side of the Great
Lakes and inland in areas remote from any nearby industrial or urban
contamination (Strachan et al., 1980). Four of 16 samples of
rain-water collected at four sites in Canada had levels of
0.00013-0.00044 µg/litre and another sample had 0.0048 µg/litre; no
endrin was detected in the other 11 samples. The mean endrin contents
in samples taken at another site in 1977, 1981, 1983, and 1984 were
none detected, 0.000065, 0.000085, and 0.000049 µg/litre, respectively
(Strachan, 1988). Endrin was not detected in snow samples collected at
12 sites in the Northwest Territories of Canada in 1985-86 (Gregor &
Gummer, 1989).
5.1.3.3 Drinking-water
Data obtained in 1964-67 from selected municipal drinking-water
treatment plants in Mississippi and Missouri, USA, showed that the
concentration in approximately 10% of the samples exceeded 0.1
µg/litre in the first year but that the concentrations were lower in
1965-67 (Schafer et al., 1969). The most recent study on US
drinking-water was done on finished water in New Orleans, LA, in 1974,
where the highest concentration measured was 4 ng/litre (US EPA,
1974).
During 1976, endrin was found at a mean concentration of 4
ng/litre (range, 1-7 ng/litre) in drinking-water in Ottawa, Canada
(Williams et al., 1978).
The mean concentration of endrin in drinking-water at the
El-Abbasia station, Egypt, in 1986 was 3.507 ± 1.45 ng/litre in 10
samples taken before purification and 1.845 ± 1.29 ng/litre after
purification (Abdel-Razik et al., 1988).
Drinking-water from the North Coast region of New South Wales,
Australia, was analysed in 1986-87: 147 of 659 samples contained
traces of endrin (none detected [< 0.005] to 0.05 µg/litre) (Ang et
al., 1989).
5.1.3.4 Groundwater
Water in wells used as a source of water for mixing pesticides
in fruit orchards in West Virginia (USA) was found to contain endrin
at about 1 ng/litre in 1985 and in 1986. The water in these wells was
not used for drinking-water. Endrin had not been used in the area
since 1970, and the authors cite their results as evidence for the
persistence of endrin and its capacity to contaminate groundwater many
years after cessation of use (Hogmire et al., 1990).
5.1.4 Organisms in the environment
5.1.4.1 Birds
Endrin was found in the carcasses of four of 16 turkey vultures
(Cathartes aura) in southern California, USA, in 1981, at levels of
0.11-0.23 mg/kg wet weight, but in none of six common ravens (Corvus
corax). It was also found in two of four vulture eggs, at 0.10
(range, none detected to 0.52) mg/kg wet weight, but in none of 30
raven eggs (Wiemeyer et al., 1986).
Endrin was found in eggs of shag (Phalacrocorax aristotelis)
and cormorants (Phalacrocorax carbo) at one of five collection sites
in the east, south-east, and south of Ireland, at a geometric mean
concentration of 0.30 (range, 0.06-1.60) µg/kg (Wilson & Earley,
1986). Eggs from two species of passerine birds, three species of
gull, four species of tern, and the night heron were collected in
Italy in 1982-83. Endrin was found in 30 eggs of the night heron
(Nicticorax nycticorax), at an average concentration of 0.11
(0.03-0.27) mg/kg, in 50 eggs of the gull-billed tern (Gelochelidon
nilotica), at a concentration of 0.28 (0.05-1.31) mg/kg, and in 38
eggs of the tree sparrow (Passer montanus) and 33 eggs of the hooded
crow (Corvus corone), at concentrations of 0.17 (0.09-0.33) and 0.21
(0.07-0.31) mg/kg. It was not detected in eggs of the other species
(Fasola et al., 1987).
No endrin was found in 98 eggs or in the livers of 112 nestlings
of rooks (Corvus frugilegus) collected from five rookeries in
northern Germany in 1982-83 (Beyerbach et al., 1987) or in 45 eggs and
the livers of eight young lapwings (Vanellus vanellus) collected in
1984 and 1986 (Beyerbach et al., 1988).
Detectable residues of the commonest organochlorine pesticides
were found in 0.9% of 112 pools (mostly of 10 birds) of starlings
(Sturnus vulgaris) collected in 129 sites in the USA in 1979 and in
1.6% of 129 pools in 1982. In most states, no endrin was detected, but
levels of 0.01 and 0.17 mg/kg wet weight were found in two (Bunck et
al., 1987).
Endrin was present at microgram levels per kilogram of wet weight
in 272 samples of liver, muscle, fat, and eggs from northern fulmars
(Fulmarus glacialis), black-legged kittiwakes (Rissa tridactyla),
and thick-billed murres (Uria lomvia) collected in 1975-77 on Prince
Leopold Island, Northwest Territories, Canada (Nettleship & Peakall,
1987). It was found in 8 of 108 carcasses of herons analysed in the
USA since 1966, at levels of 0.10-0.86 mg/kg wet weight (Ohlendorf et
al., 1981) but was not found in 255 pools of wings from black ducks
(Anas rubripes) and mallards (A. platyrhynchos) collected in the
USA in 1981-82 (Prouty & Bunck, 1986).
Endrin was not detected in six eggs of Forster's tern (Sterna
forsteri) collected on Green Bay and Lake Poygan, Michigan, USA in
1983 (Kubiak et al., 1989). None was found in a total of 107 eggs
collected in 1975-80 from 10 species of colonial waterbirds nesting in
areas around Green Bay and Lake Michigan. The species were little
gulls (Lares minutes), green-backed herons (Butorides striatus),
black terns (Chlidonias niger), herring gulls (L. argentatus),
ring-billed gulls (L. delawarensis), common terns (S. hirundo),
Forster's tern (S. forsteri), double-crested cormorants
(Phalcrocorax auritis), black-crowned night herons (Nycticorax
nycticorax), and cattle egrets (Bubulcus ibis). The limit of
detection was 0.1 mg/kg in 1977 and 0.05 mg/kg in 1978 (Heinz et al.,
1985).
Of five eggs from peregrine falcons (Falco peregrinus)
collected in Arizona, USA, in 1978-82, one collected in 1978 contained
endrin at 0.20 mg/kg dry weight, one collected in 1981 contained no
detectable amount (< 0.01 mg/kg) and three collected in 1982
contained 0.02-0.04 mg/kg (Ellis et al., 1989).
No endrin was detected in 27 eggs from tree sparrows (Passer
montanus), 4 eggs from house martins (Delichon urbica), 28 eggs
from white storks (Ciconia ciconia), or eggs from nine other species
of bird in Germany in 1984. The livers of 25 nestling, 13 young, and
17 adult white storks also contained no detectable level of this
pesticide (limit of detection, 0.001 mg/kg) (Heidmann et al., 1989).
5.1.4.2 Fish and shellfish
The endrin concentrations in red mullet (Mullet barbatus)
collected at six locations in the Pagassitikos Gulf (Greece) in
1986-87 were < 0.005-0.5 µg/kg fresh weight of fillets (Satsmadjis et
al., 1988). The mean concentrations in liver, brain, kidneys, and
muscle of 22 trout (Salmo trutta fario L.) taken from four rivers in
Leon, Spain, in 1985 were 0.104, 0.123, 0.157, and 0.157 mg/kg wet
weight. The incidence in the four organs was 4.54-22.73% (Teran &
Sierra, 1987). Endrin was found in 29 samples of fish collected in
Italy, at a median concentration of 0.019 mg/kg (Cantoni et al.,
1988). Organochlorine compounds were measured in three samples of
liver from cod (Gadus morhua) collected in three areas of the North
Sea in 1977-87; endrin was present at a concentration of < 5 µg/kg of
product (De Boer, 1989).
Endrin was not detected (< 0.01 mg/kg) in two or three replicate
samples, each comprising three to five bluegill (Lepomis macrochirus)
and common carp (Cyprinus carpio), collected from downstream sites
exposed to irrigated agriculture and from non-irrigated upstream sites
on the San Joaquin River and tributaries in California, USA (Saiki &
Schmitt, 1986). Endrin was also not found in water near Los Angeles
County ocean outfalls (< 0.00005 µg/litre) or in mussels (Mytilus
californianus) (< 0.1 µg/kg wet weight) that had been suspended at
the monitoring site for 2 months to provide a measure of the
bioaccumulation of chlorinated hydrocarbon contaminants (Green et al.,
1986). No endrin was detected in fish samples taken at nine locations
in north-central USA (Martin & Hartman, 1985), and endrin was not
detectable (< 0.001 mg/kg) in 527 samples of edible fin fish
harvested from Chesapeake Bay and its tributaries (Maryland) over the
period 1976-80 or in 20 samples of roe and gonadal tissue (Eisenberg
& Topping, 1985).
No detectable quantity (< 1 µg/kg) of endrin was found in two
species of crayfish (Procambarus clarkii and P. acutus)
commercially harvested from dual-cropped ponds and from waters of the
Atchafalaya River Basin and the Mississippi River in southern
Louisiana, or in sediment and water collected from several ponds and
at the Basin three times during 1986 and 1987 (Madden et al., 1989).
Endrin was measured at levels of 0.4 and 66 µg/kg in American
eels (Anguilla rostrata) sampled at various sites between Lake
Ontario and the mouth of the St Lawrence river in 1982 (Castonguay et
al., 1989). Endrin was not detectable (< 0.002 mg/kg) in 'most'
composite samples (1-15 fish of 10 different species) collected from
10 sites on the Great Lakes and tributaries between 1980 and 1981,
although in a few cases concentrations up to 0.01 mg/kg were found
(Devault, 1985). Endrin was not present (< 0.005 mg/kg) in fillets of
Fall Run Coho salmon (Oncorhynchus kisutch) taken from 14 sites on
the Great Lakes in 1984. In most cases, three samples per site were
analysed, and the fish were 2-3 years old (Devault et al., 1988).
Johnson et al. (1988) measured the input of organochlorine pesticides
from precipitation and runoff to five small lakes peripheral to the
Canadian Great Lakes and the levels of residues in fish caught in the
lakes. While endrin was detectable in precipitation (at 0.46 and 0.54
ng/litre at the two sampling sites), none was measured in runoff water
and no detectable residue was found in fish.
The mean concentrations of endrin in 13 commercially important
fish species collected in the north-west Arabian Gulf varied between
1 and 28 µg/kg, and those in five species collected from Hor al-Hammar
Lake in Iraq in 1985 were 3-67 µg/kg wet weight of edible tissue.
Endrin residues were detected in approximately 90% of the fish
(DouAbul et al., 1987a). Samples of Barbus xanthopetrus collected in
the Shatt al Arab River and in Hor al Hammar Lake contained average
concentrations of 4 (none detected to 9) and 20 (11-27) µg/kg, while
Indian shed (Tenualosa ilistra) from the Shatt al Arab River
contained 80 (57-108) µg/kg (wet weight). Shrimp (Metapanaeus
affinis) did not contain endrin (DouAbul et al., 1987b). In 1984,
B. xanthopetrus from the River contained a mean concentration of 16
µg/kg wet weight, and those from the Lake, 154 µg/kg (range, 13-355);
Indian shed had mean concentrations of 41-147 µg/kg (range, none
detected to 236) (DouAbul et al., 1987c). Freshwater mussel
(Corbicula fluminea) collected in the Shatt al Arab River contained
166-540 µg/kg (range, 140-583) (DouAbul et al., 1988). Endrin was
present at concentrations of 1.9-12.2 µg/kg of muscle tissue (wet
weight) in three fish species and at 0.88-7.7 µg/kg in three Tilapia
species collected near Alexandria, Egypt, in 1985 (El Nabawi et al.,
1987).
Endrin was present at 0.003-0.004 mg/kg in black pomfret
(Parastromateus niger), mackerel (Rastrelliger kanagurta), and
marine vala (Chirocentrus sp.) and at 0.08 mg/kg in tuna (Euthynnus
affinis) collected off the Indian coast (Radhakrishnan & Antony,
1989). It was found in one sample of fish at 0.019 mg/kg wet weight
and in one shellfish sample at 0.034 mg/kg but in none of 312 other
specimens of 11 types of fish, crustaceans, and molluscs obtained from
five sites in Java, Indonesia (limit of detection, 0.01 mg/kg) (Koeman
et al., 1974).
No endrin was found (< 0.005 mg/kg) in 60 samples of fish and
shellfish collected in 19 rivers and their estuaries in Japan in 1974
(Japanese Environmental Agency, 1975).
The median concentration of endrin in the eggs of 15 adult
chinook salmon (Onchorhynchus tshawytscha) collected in Lake
Michigan in 1982 was 23.5 µg/kg wet weight (range, 3.9-126.3) (Giesy
et al., 1986).
Composite samples of whole fish of selected species were
collected in 1983 near the shores of 13 tributaries of Lake Michigan
and Grand Traverse Bay. Two of each of the following species were
collected from each site: common carp (Cyprinus carpio), bowfin
(Amia calva), channel catfish (Ictalurus punctatus), pumpkinseed
(Lepomis gibbosus), rock bass (Ambloplites rupestris), small-mouth
bass (Micropterus dolomieui), large-mouth bass (M. salmoides),
lake trout (Salvelinus namaycush), and pike (Esox lucius); the
composites comprised 3-11 fish. Endrin was not detected (limit, 0.005
mg/kg) (Camanzo et al., 1987).
Yellow perch (Perca flavencens) were sampled in eight
reservoirs and lakes in Ohio and Wisconsin, USA, in 1978-79. Endrin
was found in four fish at levels of 0.008-0.02 mg/kg, which were much
lower than the levels found of polychlorinated biphenyls, DDT and
dieldrin (Carline & Lawal, 1985).
5.1.4.3 Mixed species
Herons (Nyctanassa violacea), water snakes (Natrix spp.),
raccoons (Procyon lotor), channel catfish (Ictalurus punctatus),
crappies (Pomoxis spp.), frogs (Rana spp.), and crawfish
(Procambarus clarkii) were collected from three watersheds in
Louisiana, USA in 1978-79. Endrin was found in a heron at 0.014 mg/kg
and in a catfish at 0.022 mg/kg, but in no other case (limit of
detection, < 0.05 mg/kg) (Dowd et al., 1985).
5.1.5 Other food and feed
5.1.5.1 Cereals
Endrin has been used extensively for the control of insect pests
in rice. Typically, one to four applications are made, depending on
local conditions, the last application usually not later than one
month before harvest. Data on residue levels are available from India
(1969-70), Thailand (1968-70), the Philippines, Indonesia (1966), and
Venezuela (1969). The levels in polished rice were 0.01-0.04 mg/kg of
product (mean, 0.014 mg/kg), except in India where higher levels in
the order of 0.12 mg/kg were found. Bran, which is used mainly as a
component of poultry feed, contained a mean level of 0.35 mg/kg
(range, < 0.01-2.3 mg/kg), and low levels of delta-ketoendrin were
found (FAO/WHO, 1971).
Endrin has been used to only a limited extent on grain crops. The
residues in different types of treated grains in the USA were
generally below 0.05 mg/kg of product, except in oats in which levels
up to 0.5 mg/kg were found. In India, up to five applications on
sorghum gave residue levels below 0.02 mg/kg; in the USA, the levels
in sorghum were below 0.05 mg/kg. Straw of cereals contains higher
levels: rice straw had up to 3 mg/kg,, and sorghum straw up to 0.4
mg/kg (FAO/WHO, 1971).
Wheat imported into the United Kingdom in 1987-88 did not contain
endrin (< 0.01 mg/kg) (Osborne et al., 1989).
5.1.5.2 Fruit and vegetables
Endrin is occasionally used for control of field mice (voles). No
residue was found in apples at harvest (detection limit, 0.01-0.002
mg/kg of whole fruit) when it was sprayed on the ground under trees in
orchards in autumn or spring. The levels were sometimes higher in
fallen fruit, ranging from < 0.002 to 0.02 mg/kg of product (Horsfall
et al., 1970; FAO/WHO, 1971).
Only 14 of 15 000 samples of fruit and vegetables imported into
Sweden during the period 1981-84 contained endrin, at a maximum
concentration of 0.02 mg/kg (Anderson, 1986). The pesticide analysis
programme of the Swedish National Food Administration on fruit and
vegetables, including potatoes, showed no residue of endrin above the
limit of detection of 0.02 mg/kg in 13 724 samples analysed in 1985-87
(B.G. Ericsson, personal communication, 1990). The mean endrin
concentration in 137 samples of grape products (including seeds,
skins, marc and lees) in Italy was 6.2-16.2 µg/kg (Marinelli et al.,
1986). Seven of 306 samples of apples (five types) collected in
1980-83 from five regions of Italy contained endrin (limit of
detection, 0.001 mg/kg) (Foschi et al., 1985).
In Pakistan, in 16 samples of cucumber sprayed at the time of
maturity with a 0.05% endrin solution at a rate of 100 gallons/acre
(1123 litres/ha), the endrin concentrations ranged from 3.04 to 6.69
mg/kg. The residues persisted in the edible portion of cucumber up to
14 days and diminished thereafter (Illahi et al., 1986). Endrin was
found in 17% of samples of peas collected from fields and markets in
Faisalabad, Pakistan, at a level of 1.3-4.32 µg/kg. The residues
persisted for up to 12 days and then decreased (Illahi et al., 1987).
No endrin was found (< 0.02 mg/kg) in 141 samples of fruit and
vegetables from Pakistan in 1982-83 (Masud & Farhat, 1985).
In an analysis of soya bean and soya bean straw in a US
monitoring programme, seven of 177 samples of soya beans contained a
geometrical mean of < 0.001 (maximum, 0.03) mg/kg, and one of eight
straw samples contained < 0.01 mg/kg (Carey et al., 1978). Endrin was
used in up to four applications on sugar-cane in the USA, with an
interval of 45 days or longer between the last application and
harvest. The residues found in cane were usually < 0.05 mg/kg of
product (FAO/WHO, 1971).
5.1.5.3 Meat, poultry, and chicken eggs
Bovine fat (40 samples), pig fat (45 samples), calf fat (45
samples), sheep fat (22 samples), poultry fat (42 samples), and eggs
(44 samples) analysed in the Netherlands in 1983 had a median endrin
concentration of < 0.04 mg/kg (Dutch Agricultural Advisory Commission
on Environmental Pollutants, 1983). No endrin was found (detection
limit, 0.005 mg/kg) in samples of beef, pork, goat, mutton, poultry,
or eggs analaysed in Italy in 1985-87 (Cantoni et al., 1988) or in
'most' samples of pork, rabbit, or poultry analysed in
Rheinland/Pfalz, Germany, in 1981-84 (Kampe, 1985). Dietary surveys in
the United Kingdom demonstrated no endrin in meat (detection limit,
0.02 mg/kg) (United Kingdom Ministry of Agriculture, Fisheries and
Food, 1989).
Endrin was present in 10.8% of 2032 samples of bovine fat from
carcasses collected from slaughterhouses in Brazil, at a mean level of
0.01 mg/kg; the highest level was 0.09 mg/kg of tissue (De Paula
Carvalho et al., 1984). Endrin was present in hens' eggs from four of
five areas in Mexico, at concentrations of 0.004-0.11 mg/kg of whole
egg, and in 11 of 16 samples of chicken meat, at an average
concentration of 0.12 (none detected to 0.6) mg/kg on a fat basis
(Albert, 1990).
Endrin was detected in 86 of 221 samples of hens' eggs (78 native
and 143 commercial) collected in 1975-77 in Iran, at a mean
concentration of 0.017 (range, 0.003-0.13) mg/kg (Hashemy-Tonkabony &
Mosstofian, 1979). No endrin was found (limit of detection, 0.02
mg/kg) in samples of about 25 eggs of sawah ducks collected on 11
local markets in Java, Indonesia, in 1972 (Koeman et al., 1974).
It was found in 14 of 367 hens' eggs collected from 61 farms in
11 districts of Kenya in 1984; in three of the eggs, the level was >
0.2 mg/kg (Mugambi et al., 1989).
Heating, baking, frying, and steaming of tissues obtained from
broilers fed endrin at 10 mg/kg of diet for 8 weeks did not
significantly reduce the level of residues: raw, 28.2; baked, 20.8;
fried, 22.7; and steamed, 19.4 mg/kg of dry tissue (Ritchey et al.,
1972).
5.1.5.4 Milk and milk products
The mean endrin concentration in 20 samples of fresh buffalo milk
in Kalubia, Egypt, was 0.02 mg/kg of milk fat (range, < 0.01-0.03
mg/kg (Abdou et al., 1983). Cows' milk (39 samples) collected in four
areas of Bagdad, Iraq, in 1981-82 contained a mean of 60 (none
detected to 400) µg/litre (Al-Omar et al., 1985b).
The average concentration of endrin in 10 samples of evaporated
cows' milk from three main cities in the agricultural region of Mexico
was < 0.007 mg/litre of milk fat (Albert et al.,1982). Endrin was
found in powdered milk at an average concentration of 0.06 mg/kg and
in cheese at a concentration of 10-27.2 mg/kg on a fat basis (Albert,
1990).
The level of endrin in milk in the USA was < 0.001 mg/litre (on
a fat basis) (FAO/WHO, 1971). No endrin (< 0.5 µg/litre) was found in
308 samples from bulk transports of milk collected in Ontario, Canada,
in 1977 (Frank et al., 1979) or in 359 samples collected in 1983
(Frank et al., 1985).
No residue was found (detection limit, < 0.005 mg/kg) in samples
of milk, cream, butter, and cheese in Italy (Cantoni et al., 1988) or
in 12 samples of cows' milk collected in 1984-86 from different areas
of Spain (< 0.01 mg/kg fat) (Barcelo & Puignou, 1987).
5.1.5.5 Fat and oils
The most important use of endrin is for the control of insects in
cotton, the number of applications being 1-12; cottonseed oil is used
for cooking and for the manufacture of margarine, while the extracted
cake is used as cattle feed. Endrin is thus present both in the
cottonseed and in the edible oil and cakes. In a study of the
extraction processes, it was found that alkali washing and bleaching
had no marked effect but that deodorization reduced the endrin levels
to below the limit of detection (0.03 mg/kg) (Smith et al., 1968).
In field studies carried out in the USA, cottonseed contained
endrin at a maximum of 0.1 mg/kg, although the levels were usually
much lower. delta-Ketoendrin was not detected. The levels in crude,
decolourized, and deodorized oil in Venezuela and Brazil were all <
0.02 mg/kg of product. Spot samples of refined cottonseed oil from
California, USA, contained < 0.03 mg of endrin and < 0.02 mg of
delta-ketoendrin ( limits of detection) (FAO/WHO, 1971).
One-hundred-and-ten samples of raw oil and of oil at various
stages of processing, i.e., neutralized, hydrogenated, decolourized,
deodorized, and shortenings, were collected from seven oil processing
factories in Iran in 1974. Endrin was found only in raw and
neutralized vegetable oils, at concentrations of 0.004-0.005 mg/litre.
Raw imported and native oils contained < 0.01 mg/litre, except for
native sunflower oil which contained 0.026 mg/litre (Hashemy-Tonkabony
& Soleimani-Amiri, 1976).
Endrin was found in 60 samples of six varieties of the major
edible oils and oil seeds used in India, including groundnut, sesame,
mustard, coconut, and hydrogenated vegetable oils, collected from a
market in Lucknow. Vegetable oil contained 6 µg/litre, mustard oil, 72
µg/litre, and sesame oil, 1690 µg/kg. Of the different types of oil
seeds, only mustard seed contained endrin, at 22 µg/kg (Dikshith et
al., 1989a).
Endrin was found at a mean concentration of 0.184 (0.097-0.288)
mg/kg in samples of cod-liver oil analysed in Germany in 1985 (Ali,
1986). No residue was found in vegetable oils and fats imported into
the United Kingdom (detection limits, 0.02 and 0.001 mg/kg,
respectively) (Abbot et al., 1969).
5.1.5.6 Animal feed
Residues of endrin in pressed cottonseed cakes arise primarily
from the 1-5% of oil left in the cake after extraction. The residues
in cakes from Brazil, India, the USA, and Venezuela were mainly <
0.01-0.02 mg/kg product, levels up to 0.08 mg/kg were found
occasionally (FAO/WHO, 1971). The mean concentration of endrin in 32
samples of cattle feed from a local market in India was 0.020 mg/kg
(range, 0.013-0.027 mg/kg) (Dikshith et al., 1989b). No endrin was
found in 79 samples of cattle feed in Pakistan (Parveen & Masud,
1987). Endrin was not present in samples of domestic and imported
animal feed analysed in the USA in 1981-86 (Luke et al., 1988).
None of 42 samples of chicken feed collected from 61 farms in 11
districts of Kenya in 1984 contained endrin (Mugambi et al., 1989).
5.1.6 Miscellaneous products
Endrin was found in 5 of 25 tobacco samples imported into Germany
at concentrations of 25-50 µg/kg (Cetinkaya, 1988). No endrin was
found in cigarettes of 14 brands collected in Finland in 1960-84
(Mussalo-Rauhamaa et al., 1986). An average content of 0.006
µg/cigarette (range, none detected to 0.02 µg/cigarette) was found in
Switzerland (Zimmerli & Marek, 1973).
When raw cotton imported into Germany from 15 countries was
analysed, endrin was found in samples from the USSR and Mexico at a
concentration of 3 µg/kg (Cetinkaya & Schenek, 1987).
5.2 Exposure of the general population
5.2.1 Total-diet studies
Studies on complete prepared meals in the USA, started in May
1961 and continued to the present, have shown the occasional presence
of small amounts of endrin (Williams, 1964; Cummings, 1965, 1966;
Duggan et al., 1966, 1967; Martin & Duggan, 1968; Corneliussen, 1969,
1970, 1972; Manske & Corneliussen, 1974; Manske & Johnson, 1975;
Johnson & Manske, 1976, 1977; Manske & Johnson, 1977; Johnson et al.,
1981a, 1984). These measurements indicate that the total average daily
intake of endrin from food decreased from 0.009 µg/kg body weight in
1965 to 0.0005 µg/kg body weight in 1970 (Duggan & Lipscomb, 1969;
Duggan & Corneliussen, 1972), with a further decrease subsequently. In
total-diet studies of adults in the USA, representative foods were
purchased in 27 US cities in 1980-82; the daily intake of endrin was
found to be < 0.001 µg/kg body weight in 1978, but none was detected
in 1979, 1980, or 1981-82 (Gartrell et al., 1986a).
Further studies involved retail purchase of 234 food items
representative of the total diet of eight US population groups in
1982-84 and preparing them for consumption. The daily intake of endrin
in the groups, which included people aged 6-11 months, 2 years, 14-16
years (females), 14-16 years (males), 25-30 years (females), 25-30
years (males), 60-65 years (females), and 60-65 years (males), was
0.1-0.2 ng/kg body weight (FDA, 1988; Gunderson, 1988).
Endrin was not present in the total diets of infants and toddlers
in the USA during 1974-75 (Johnson et al., 1979). It was found in one
infant food sample at 0.011 mg/kg and in one sample of toddler food at
0.009 mg/kg of food in a study in 1975-76 (Johnson et al., 1981b).
Very low residue levels were found occasionally in market-basket
samples representing the average 2-week diets of infants (98 samples)
and toddlers (110 samples) collected in 10 cities in four geographic
areas of the USA in 1977-78 (Podrebarac, 1984). In total-diet studies
of infants and toddlers in the USA, representative foods were
purchased in 13 US cities in 1980-82; the daily intake of endrin by
infants was found to be < 0.001 µg/kg body weight in 1978, and that
by toddlers, < 0.001 µg/kg body weight in 1979, but none was detected
in the other years (Gartrell et al., 1986b).
Fresh food was bought from four retail grocery stores in Toronto,
Canada, in 1985 and combined in five food composites: fresh meat and
eggs, root vegetables (including potatoes), fresh fruit, leafy and
other surface vegetables, and cows' milk. The concentrations of endrin
in the five composites were used to estimate the annual dietary intake
of endrin from products in Ontario. Endrin was detected in all
composites except eggs and meat; the concentrations were 0.32 µg/kg in
leafy vegetables, 0.27 µg/kg in fruit, 0.37 µg/kg in root vegetables,
and 0.27 µg/kg in milk. The total annual intake was estimated to be
31.8 µg/person (Davies, 1988).
In a total-diet study carried out in the United Kingdom in
1985-88, 25 samples were obtained in 1984-85 which comprised the 16
food groups considered most likely to contain residues of
organochlorine compounds. No endrin was detected (limit of detection,
0.001-0.02 mg/kg, depending on the food group). Endrin was also not
detected (< 0.01 mg/kg) in 176 samples of pulses purchased from
retail outlets in 1986-87, except in 3 of 20 samples of mung beans in
which a mean value of < 0.01 mg/kg (range, none detected to 0.06
mg/kg) was found (United Kingdom Ministry of Agriculture, Fisheries
and Food, 1989). No endrin was detected in complete prepared meals
during surveys in the United Kingdom in 1965 (Robinson & McGill, 1966;
McGill & Robinson, 1968). Similar results were obtained in Switzerland
in 1973 (Zimmerli & Marek, 1973), and very low levels were found in
two of 73 samples analysed in 1985 (Wüthrich et al., 1985). No endrin
residues were found in total-diet studies carried out in the
Netherlands in 1976-78 (De Vos et al., 1984).
5.2.2 Levels in human tissues
Although the concentrations of many chlorinated hydrocarbon
insecticides, such as DDT, dieldrin, hexachlorocyclohexanes, and
hexachlorobenzene, and of their metabolites in blood or adipose tissue
of the general population or of occupationally exposed workers have
been found to be an excellent index of the level of exposure of the
general population, this is not the case for endrin, because it is
eliminated rapidly.
5.2.2.1 Adipose tissue
Except in a few cases, endrin was not demonstrated in adipose
tissue samples from the general population in the USA in 1962-66
(Hoffman et al., 1964, 1967), 1964 (Hayes et al., 1965; Zavon et al.,
1965), 1970-74 (Kutz et al., 1979a,b), and 1975-79 (US EPA, 1983);
Canada in 1967-68 (Kadis et al., 1970); Mexico in 1975 (Albert et al.,
1980); Argentina in 1968-69; (Wassermann et al., 1969); Belgium in
1968-69 (Wit, 1971); the United Kingdom in 1961 (Hunter et al., 1963),
1964 (Robinson et al., 1965), and 1965-67 (Egan et al., 1965; Abbott
et al., 1968, 1972); the Netherlands in 1969 (Wit, 1971); Switzerland
in 1972 (Zimmerli & Marek, 1973); Germany in 1970 (Acker & Schulte,
1974); France in 1971 (Fournier et al., 1972); Spain in 1978 (Herrera
Marteache et al., 1978); India in 1964 (Dale et al., 1965); or Western
Australia in 1965-66 (Wassermann et al., 1968).
No endrin was found in 91 samples of adipose tissue obtained at
autopsy in Kingston, Ontario, Canada, in 1979 and 1981 or in 84
samples from Ottawa in 1980 and 1981 (Williams et al., 1984), or in
adipose tissue obtained at autopsy from 92 males and 49 females in
Ontario municipalities in 1984 (limit of detection, 2.4 µg/kg)
(Williams et al., 1988).
These results indicate that endrin is either absent or present at
very low levels in the adipose tissue of the general population. It is
therefore surprising that Kanitz & Castello (1966) reported the
presence of endrin in nine adipose tissue samples from Liguria, Italy,
at a mean concentration of 0.93 mg/kg of tissue. The highest
concentration was 2.49 mg/kg. Pavan et al. (1987) found endrin at 0.1
and 0.3 mg/kg in 2 of 92 samples of adipose tissue obtained at surgery
from people living in the Province of Turin, Italy. In areas where
endrin has been used extensively, however, such as India and the lower
Mississippi, it has never been found in human adipose tissue (Brooks,
1974).
One of 62 adipose tissue samples obtained at surgery from people
in Ciudad Juarez, Mexico, in 1977-78 contained endrin at 0.02 mg/kg
(Redetzke et al., 1983).
5.2.2.2 Organs
In samples of liver, kidney, gonad, and brain obtained from the
general population of Alberta (Canada), no residue of endrin was
detected (Kadis et al., 1970).
5.2.2.3 Blood
No endrin was detected (limit of detection, 0.01 mg/kg) in 4000
blood samples from the general US population in 1976-80 (US E P A,
1983), or in areas where endrin has been used extensively, such as
India and the lower Mississippi (Brooks, 1974), or in 26 blood samples
from the general population in Nigeria (Atuma, 1985).
5.2.2.4 Breast milk
Endrin was not detected in breast milk in studies in the USA in
1966-78 (Strassman & Kutz, 1977; Currie et al., 1979; Kutz et al.,
1979a; Barnett et al., 1979), in El Salvador and Guatemala (De Campos
& Olszyna-Marzys, 1979), in Belgium, Italy, and The Netherlands
(Kanitz & Castello, 1966; Hendrickx & Maes, 1969; Wegman & Greve,
1974), and in Japan (Yakushiji et al., 1979). No endrin was detected
(< 0.01 mg/litre) in 50 breast milk samples from mothers (aged 18-32
years) in Leiden, The Netherlands, in 1969 (Tuinstra, 1971). It was
found in one of 12 samples from mothers aged 21-37 in Pavia, Italy, in
1988, at a concentration of 0.01 µg/kg of whole milk, but not in four
samples collected in Crotone, southern Italy (Bianchi et al., 1988).
5.2.2.5 Appraisal of exposure of the general population
The occasional presence of low concentrations of endrin in the
air of areas where endrin is used in agriculture cannot be considered
a significant source of contamination for the general public. The very
low concentrations that have been found in surface and drinking-water
are also of little significance for public health.
The source of exposure that may be relevant is dietary intake.
Apart from accidental contamination, however, intake of endrin by the
general population in the countries examined has been and is still far
below the maximum acceptable daily intake of 0.2 µg/kg body weight
established by the Joint FAO/WHO Meeting in 1970 (FAO/WHO, 1971). This
applies equally to the total intake, when the intake from dietary
sources is added to that from air and water.
Endrin has not been demonstrated in the large number of samples
of organs, adipose tissue, blood, and breast milk analysed in
different countries, even in areas where endrin is or was used
extensively.
5.3 Occupational exposure during manufacture, formulation and use
5.3.1 Manufacture and formulation
Endrin has not been detected in the blood, plasma, or urine of
workers exposed occupationally to endrin (Hayes & Curley, 1968; Jager,
1970). Endrin was detected in blood only after accidental
over-exposure. Jager (1970) estimated that the threshold level of
endrin in the blood, below which signs or symptoms of intoxication do
not occur, lies between 0.05 and 0.10 mg/litre. He estimated the
half-life of endrin in the blood to be in the order of 24 h.
The total exposure of workers in a manufacturing and formulation
plant was estimated on the basis of determinations of the quantity of
the endrin metabolite anti-12-hydroxyendrin in urine. Urine of
workers exposed to endrin for seven days had concentrations of up to
360 µg/g of creatinine; no unchanged endrin was found. Assuming an
average daily excretion of 1.5 g creatinine per day, the total daily
excretion of anti-12-hydroxyendrin in the urine may be up to 540 µg.
This gives a minimal absorption of 0.5 mg endrin, indicating that
inhalation of dusts and absorption through the skin may be significant
during occupational exposures in manufacture. It is not unreasonable
to assume that, as in other species, approximately half of all the
endrin absorbed is excreted in the urine as anti-12-hydroxyendrin,
since both endrin and this metabolite are present in the faeces of
workers (Ottevanger &Van Sittert, 1979; Baldwin & Hutson, 1980 ).
Thus, 1 mg/day may be the more accurate figure for exposure in this
manufacturing plant. The concentration of anti-12-hydroxyendrin in
urine decreased more slowly than the concentrations of endrin in
blood, with a half-life of 55-75 h (Van Sittert, 1985).
5.3.2 Application
Endrin is applied in agriculture by high-pressure spraying with
a hand gun, spraying orchards with a power air blast or boom to
control mice, dusting potatoes, spraying row crops, or application
from aircraft. These methods of application result in dermal and
respiratory exposures.
The potential dermal and respiratory exposure of workers applying
endrin formulations in the field has been quantified in a few studies.
Respiratory exposure to endrin during spraying of orchards,
high-pressure spraying of crops, and piloting of aircraft varied from
0.01 to 0.14 mg/h; dermal exposure during such activities was
0.01-1.64 mg/h. The activity that caused the most exposure was dusting
potatoes, which was associated with a respiratory exposure of 0.41
mg/h and a dermal exposure of 18.7 mg/h. Total exposure, calculated as
a percentage of a toxic dose/h = {dermal exposure (mg/h) +
[respiratory exposure (mg/h) x 10]} [dermal LD50 (mg/kg) x 70] x
100, was 0.21-1.5% (Durham & Wolfe, 1962) (see Table 11). These
figures show that although endrin is acutely highly toxic it can be
used safely when reasonable precautions are taken (Wolfe et al., 1963;
Jegier, 1964; Wolfe et al., 1967; Hayes, 1975).
Endrin was not found in the blood of 20 pesticide sprayers or in
19 controls in Choluteca, southern Honduras (Steinberg et al., 1989).
5.3.3 Appraisal of occupational exposure
No residues were found in healthy workers. The range of threshold
levels of endrin in blood below which no sign or symptom of
intoxication occurs has been estimated to be 50-100 µg/litre. The
half-life of endrin in blood may be in the order of 24 h following
occupational exposure. The concentration of anti-12-hydroxyendrin in
the urine decreased more slowly than the concentration of endrin in
blood, with a half-life of 55-75 h.
Table 11. Studies on potential exposure of agricultural workers to endrin, using direct methods
Activity Exposure
Respiratory Dermal Totala (%) toxic Reference
(mg/h) dose/h
mg/m3 mg/h
Spraying orchard cover crops for mouse 0.01b 2.6 0.21 Wolfe et al. (1963)
control
High-pressure hand-gun spraying orchard 0.01b 3 0.25 Wolfe et al. (1967)
cover crops for mouse control
Operating air-blast or boom sprayers treating 0.01b 2.5 0.21 Wolfe et al. (1967)
orchard cover crops for mouse control
Dusting potatoes 0.41b 18.7 1.5 Wolfe et al. (1963)
Spraying row crops NDb,c 0.15 (0.01)d Jegier (1964)
Piloting during air application of spray 0.05 0.08b 1.18 0.29 (0.16)e Jegier (1964)
aFor a 70-kg man on the basis of dermal LD50 for male rats (18 mg/kg body weight) using the formula given
by Durham & Wolfe (1962)
bMeasured by respirator pad
cNot detected
dOriginal values calculated on the basis of maximal exposure; recalculated values shown in parentheses are
based on mean exposure.
eCalculation based on published data on dermal and respiratory exposure (note in Wolfe et al., 1967)
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Laboratory animals
6.1.1.1 Oral administration
Rat: One male rat was fed 14C-endrin at a level of 30 mg/kg
of diet for 8 days. About 60-70% was excreted on the first day; after
three days, the faeces contained more then 80% of the administered
radiolabel; by day 9, 84% had been excreted; and there appeared to be
a level of saturation after 6-7 days of feeding. Only 0.5% was found
in the urine. About 75-80% of the label in the faeces was in at least
two different metabolites. The adipose tissue stored 3-4 mg/kg, giving
a storage rate of about 10 (FAO/WHO, 1971).
After female rats were given a single oral dose of 14C-endrin
at 16, 64, or 128 µg/kg body weight, excretion was rapid. The
biological half-life of the doses of 16 and 64 µg/kg was 1-2 days;
however, that of 128 µg/kg was approximately 6 days, showing that
excretion of higher doses is slower (Korte et al., 1970).
Six CFE rats of each sex were treated with a single oral dose of
0.5 mg 14C-endrin in arachis oil (approximately equivalent to
2.5-3.0 mg/kg body weight), and the radiolabel excreted in urine and
faeces was measured over 3 days. The animals were then killed and the
radiolabel measured in tissues. A sex difference was noted in the rate
of elimination in faeces: 66% of the dose was excreted in 3 days by
males and 37% by females; excretion was slower in females but tended
to increase daily between days 1 and 3. Small quantities of radiolabel
were excreted in the urine, females excreting three times more than
males. No radiolabel was found in exhaled air (Hutson et al., 1975).
The results are summarized in Tables 12 and 13.
Three rats of each sex were each given a single oral dose of 8 µg
14C-endrin in peanut oil (by gavage) daily for 12 days. A steady
state (at which the excreted amount equalled the daily intake) was
reached after about 6 days. Females stored about twice as much (27%)
as males (14%). The radiolabel was excreted mainly in the faeces:
after the first 24 h, 70-75% of the radiolabel was found in faeces as
hydrophilic metabolites; subsequently, only metabolites were present.
Four days after the last dose, males retained only 5% and females 15%
of the administered radiolabel (Klein et al., 1968; Korte et al.,
1970).
Table 12. Rates of excretion of radiolabel by rats treated with a
single oral dose of 14C-endrin (percentage of
radioactivity administered)
Sex Urine Faeces
Day 1 Day 2 Day 3 Day 1 Day 2 Day 3
Male 1.3 0.6 0.6 30.6 14.4 21.2
Female 1.8 2.5 2.9 2.3 10.7 24.2
Table 13. Recovery of radiolabel in rat tissues 3 days after a single
oral dose of 14C-endrin (percentage of radioactivity
administered)
Sex Urine Faeces Liver Kidneys Fat Skin Remaining Total
carcass
Males 2.7 66.2 1.2 0.6 1.7 2.3 12.2 86.9
Females 7.5 37.2 2.0 0.4 8.0 4.0 28.1 87.2
Rabbit: A Dutch strain male rabbit was given two oral doses of
4.7 mg 14C-endrin in olive oil at an interval of 14 days. Between
days 1 and 13, 37% of the first dose was excreted in the urine and 49%
in the faeces; the second dose was eliminated similarly. By day 49,
50% had been excreted in urine and 47% in faeces. Faecal excretion was
rapid, being almost complete within 24 h, and consisted virtually
entirely of unchanged endrin. The urine contained only metabolites
(Bedford et al., 1975a). The excretion of metabolites in rabbits thus
appears to differ considerably from that in rats: approximately half
of a dose of 14C-endrin is excreted in the urine of rabbits and
approximately 2% in rats; endrin metabolites are excreted in rat
faeces over several days (after a single oral dose), whereas in
rabbits faecal excretion is rapid, being almost complete within 24 h,
and consists virtually entirely of unchanged endrin. The probable
explanation is that the molecular weight threshold for biliary
secretion of anions is 325 ± 50 in rats and 475 ± 50 in rabbits (Hirom
et al., 1972). The glucuronide and sulfate conjugates of
monohydroxyendrin have molecular weights of 572 and 470, respectively.
Therefore, conjugates of the endrin metabolites are eliminated in the
bile and faeces of rats and in the urine in rabbits.
Dog: Three beagles were fed a diet containing endrin at a
concentration equivalent to 0.1 mg/kg body weight for 128 days; two
other animals were used as controls. The concentration of endrin in
blood was determined at weekly intervals. The time to reach a plateau
in blood was less than 1 week, and no significant increase in the
concentration of endrin in blood was found during this period. The
average concentration between day 9 and day 128 was 4 µg/litre. The
concentration of endrin in eight organs and tissues of dogs killed 7
days after termination of exposure was < 0.2 mg/kg of tissue; except
that the spleen of one dog contained 2.6 mg/kg, and adipose tissue
contained 0.2-0.8 mg/kg (Richardson et al., 1967).
6.1.1.2 Intravenous administration
Mouse: The concentrations of endrin were determined in tissues
from groups of five adult male CFI mice given endrin intravenously at
5 mg/kg body weight (LD90) in dimethyl sulfoxide. The concentrations
prior to convulsions (about 10 min after injection) were approximately
60 mg/kg in liver, 20 mg/kg in brain and omental fat, and
approximately 5 mg/litre in blood. The concentration in whole brain 15
min after an intravenous dose of 1.5 mg/kg body weight (the dose that
caused ataxia in 90% of animals; TD90) was 9.4 mg/kg. No endrin was
detected in the bile of animals with a bile fistula dosed
intravenously with the TD90 in samples collected after 0.5, 1, or 2
h (Walsh & Fink, 1972).
Rat: Male Holtzman rats with or without a bile fistula were
given a single intravenous dose of 14C-endrin at 0.25 m/kg body
weight. More than 90% of the excreted radiolabel was found in the
faeces of intact animals over the 7-day period after dosing or in the
bile of animals with fistulas over 4 days. The mean total percentage
recovery of administered radiolabel in faeces, urine, and carcasses
was 97% from intact animals 7 days after dosing, and 94% from animals
with a bile fistula 4 days after dosing (Cole et al., 1970). No
unchanged endrin was found in bile; the major metabolite was
anti-12-hydroxyendrin (see section 6.2.1).
Rapid excretion was observed in rats given two intravenous
injections of 14C-endrin at 0.1 mg/kg body weight at an interval of
4 days. Excretion of the radiolabel was exponential and occurred
mainly with faeces; only hydrophilic metabolites were present. With a
dose of 200 µg/kg body weight, male rats retained 5.2% and females
12.1% of the administered dose 20 days after the second injection. The
biological half-life of endrin after a single intravenous dose of 200
µg/kg body weight was 2.5-3 days in male rats and 4 days in females
(Klein et al., 1968; Korte et al., 1970; Brooks, 1974).
Rabbit: When rabbits were given 14C-endrin intravenously, the
radiolabel was excreted mainly in the urine and only as metabolites.
A probable explanation for the difference in excretion pattern after
oral and intravenous administration is that much lower doses
(micrograms compared with milligrams) were given intravenously (Korte
et al., 1970).
6.1.2 Domestic animals
Twelve cows were fed hay from endrin-sprayed alfalfa containing
an average of 1.9, 2.8, or 3.7 mg/kg endrin; the average daily intake
of individual animals ranged from 0.04 to 0.11 mg/kg body weight. The
average concentrations of endrin in the milk were < 0.05, 0.14, and
0.15 mg/litre, respectively. When endrin dissolved in soya bean oil
was fed to 11 dairy cows, levels > 1 mg/kg body weight were required
in order for significant quantities of endrin to be detected in milk
(Ely et al., 1957).
Dairy cows (eight Jerseys and six Guernseys) were fed diets
containing endrin at 0, 0.1, 0.25, 0.75, or 2.0 mg/kg of diet for 12
weeks. No residues were found (limit of detection, 0.01 mg/litre) in
the milk of animals that received 0.1 mg/kg, but up to 0.02 mg/litre
was found in milk of animals fed 0.25 mg/kg and up to 0.04 mg/litre
with 0.75 mg/kg of diet; the highest dose resulted in residues in milk
of 0.1 mg/litre. The endrin content of brain, heart, liver, kidneys,
body fat, and muscles was < 0.1 mg/kg, but renal fat contained up to
0.8 mg/kg (Kiigemagi et al., 1958).
The concentrations of endrin in milk of cows given feed
containing endrin at approximately 0.05, 0.14, and 0.30 mg/kg of whole
feed for 5 weeks were 0.004, 0.01, and 0.018 mg/litre, respectively
(Williams et al., 1964). A steady (plateau) level in milk was reached
after about 15 days.
The concentration of endrin in the milk of dairy cows given feed
contaminated with relatively low levels of endrin rose rapidly within
a few hours to days and levelled off at a plateau characteristic for
each concentration in the feed. The average milk:diet ratio for endrin
was 0.07 for feed levels of 0.05-0.3 mg/kg of diet (Biehl & Buck,
1987).
Two lactating cows were fed 14C-endrin for 21 days at an
overall dietary concentration of 0.1 mg/kg of diet, which was
considered to be comparable to the highest dose that cows are likely
to receive in cottonseed cake. Excretion of radiolabel in milk, urine,
and faeces reached a plateau 4-9 days after start of treatment.
Approximately 3% of the radiolabel was excreted in milk, 65% in urine,
and 20% in faeces. Unchanged endrin was not found in urine, but about
30% of the radiolabel in faeces and all of the 0.003-0.006 mg/litre
found in milk was endrin. The concentration of endrin equivalent
residues was 0.001-0.002 mg/kg in meat and 0.02-0.10 mg/kg in fat;
most of the radiolabel in fat consisted of endrin (Baldwin et al.,
1976).
Steers, hogs, and lambs fed diets containing endrin at 0, 0.1,
0.25, or 0.75 mg/kg of diet for 12 weeks had residues of < 0.1 mg/kg
in red meat, liver, and kidneys and of 0.02-0.2 mg/kg in body fat.
Feeding endrin at 2 mg/kg of diet to steers for 12 weeks resulted in
residues of 0.9 mg/kg in fat and 0.2-0.3 mg/kg in red meat, liver and
kidneys (Terriere et al., 1958). The biotransfer factors for endrin in
beef and milk were directly proportional to the octanol-water
partition coefficients, while the bioconcentration factor for endrin
in vegetation was inversely proportional to the square root of the
octanol-water partition coefficient (Travis & Arms, 1988).
Six weeks after the start of feeding seven Delaware X New
Hampshire male chicks and eight weeks after the start of feeding seven
White Leghorn pullets a diet containing endrin at 0.1 mg/kg, the
residues in eggs and meat were < 0.1 mg/kg and that in fat, 0.6
mg/kg. At a dietary level of 0.25 mg/kg, the residue levels were
0.2-0.3 mg/kg in eggs, 0.1 mg/kg in breast meat, and about 1 mg/kg in
fat. With 0.75 mg/kg of diet, the levels were 0.4 mg/kg in eggs, 0.24
mg/kg in breast meat, and 3.1 mg/kg in fat (Terriere et al., 1959).
14C-Endrin was administered daily in corn oil in gelatin
capsules to six laying hens at a concentration equivalent to 0.13
mg/kg of total diet for 21 weeks. Ingestion and elimination in eggs
and excreta were almost balanced after about 16 weeks. The residue
levels in eggs were 0.11-0.18 mg/kg and were found in the yolk; none
of the known metabolites was detected. The levels of endrin equivalent
were about 0.01 mg/kg in breast meat and 0.1 mg/kg in leg meat; higher
levels were found in the liver (0.47 mg/kg), kidneys (0.17 mg/kg), and
fat (1 mg/kg). The residues were accounted for by unchanged endrin,
except in the liver and kidneys, where part probably consisted of
polar metabolites. About 50% of the administered radiolabel was
excreted in the faeces, 10% of which was in unchanged endrin (Baldwin
et al., 1976).
6.1.3 Human beings
The concentrations of endrin in the blood of workmen exposed to
endrin were generally below the level of detection: endrin was not
found in plasma (< 3 µg/litre) or fat (< 0.03 mg/kg) of workers
exposed to endrin for an average of 88 days (Hayes & Curley, 1968). No
endrin was found in the blood of healthy people working in an endrin
manufacturing plant between 1964 and 1970, at an initial limit of
detection of 10 µg/litre, improved after 1965 to 5 µg/litre (Jager,
1970).
Residues of endrin have been found in blood only in individuals
with signs of recent intoxication or who have recently had excessive
exposure (see section 9.2.2). Endrin appears to be eliminated rapidly
from the human body.
6.1.4 Systems in vitro
Isolated liver preparations from Holtzman rats were perfused with
a solution containing 14C-endrin at 0.003 mg/ml. Within 1 h, 50% of
the radiolabel appeared in the bile; and in 6 h, more than 90% of the
total label was found (Cole et al., 1970). With the same dose,
radiolabel appeared 2-12 times faster in the bile of livers isolated
from male rats as in that of livers from females, which may account
for the lower toxicity and lower storage of endrin in adipose tissue
in male rats (Klevay, 1971). After perfusion of albino rat liver with
a physiological solution containing 40 µg of 14C-endrin, both endrin
and hydrophilic metabolites were found (Altmeier & Korte, 1969).
6.2 Biotransformation
Information on the metabolism of endrin up to 1967 was reviewed
(Soto & Deichmann, 1967; Brooks, 1969).
6.2.1 Experimental animals
A number of investigations have been carried out since 1970 to
elucidate the identity of several metabolites of endrin in rats
(Baldwin et al., 1970; Richardson et al., 1970; Hutson et al., 1975;
Bedford & Hutson, 1976), rabbits (Bedford et al., 1975a; Hutson,
1981), cows and chickens (Baldwin et al., 1976). The 12-hydroxy
derivative was reported to be present in the faeces of rats (Baldwin
et al., 1970), and the hydroxyl group was assigned tentatively as syn
to the epoxide ring. The stereochemical configuration was subsequently
shown to be anti to the epoxide group (Baldwin et al., 1973), and
this configuration was confirmed by the synthesis of anti-12-
hydroxyendrin (also called 9- anti-hydroxyendrin) (Bedford & Harrod,
1973; Bedford et al., 1986a). The chemical structures of these
compounds are shown in Figure 2; the chemical names are given in Annex
I.
Formation of anti-12-hydroxyendrin ( III), together with its
sulfate and glucuronide conjugates, is considered to be the major
route of metabolism of endrin. Four other metabolites have been
reported, but their concentrations are generally smaller than that of
anti-12-hydroxyendrin and its conjugates. These four metabolites are
syn-12-hydroxyendrin ( II; tentative identification),
3-hydroxyendrin ( IV; synthesized and structure confirmed by Bedford
et al., 1986b), 12-ketoendrin ( V), and the product of formal
hydroxylation of endrin, the 4,5- trans-dihydroisodrindiol ( VI;
tentative structure). The trans-diol ( VI) is a minor metabolite in
both rats and rabbits; it may be formed via an oxidation-reduction
pathway involving intermediates of the corresponding ketol ( VII).
Each of the hydroxy compounds is also excreted partly as its sulfate
or glucuronide in the urine of animals (Bedford et al., 1975a,b;
Hutson et al., 1975).
The three monohydroxylated derivatives of endrin, syn- and
anti-12-hydroxyendrin ( II and III) and 3-hydroxyendrin ( IV),
are the products of the action of liver microsomal monooxygenases on
endrin (Bedford & Hutson, 1976). These alcohols are also conjugated to
glucuronides and sulfates to some extent in the liver. Comparative
metabolic studies with rat liver microsome preparations have shown
that free syn-12-hydroxyendrin, but not its free anti-isomer, is
the precursor of 12-ketoendrin ( V) (Hutson & Hoadley, 1974).
Rats exhibit a sex difference in the rate of metabolism. The
major metabolite in animals of each sex is anti-12-hydroxyendrin,
which is excreted via the bile as the glucuronide; this undergoes
enterohepatic circulation and is eliminated as the aglycone in the
faeces, together with two minor metabolites, 3-hydroxyendrin and
4,5- trans-dihydroisodrindiol. Male rats produce the metabolite at a
higher rate than do females. The major urinary metabolite in male CFE
rats was 12-ketoendrin, while females excreted mainly
anti-12-hydroxyendrin O-sulfate. Endrin and the lipophilic
metabolite 12-ketoendrin were the major compounds found in the organs
and tissues of male and female CFE rats 3 days after a single oral
dose of endrin, but the ratio of endrin:12-ketoendrin was 2/1 in
females and 1/8 in males. Thus, 12-ketoendrin constituted most of the
radiolabel in the liver and kidneys of males and endrin that in the
kidneys of females (Hutson et al., 1975; Hutson, 1981).
The metabolism of endrin in rabbits is superficially different
from that in rats. The major metabolite is still
anti-12-hydroxyendrin, but it is conjugated with sulfate and
eliminated in the urine. Some syn-12-hydroxyendrin was also detected
as its sulfate in urine, and perhaps conjugation and elimination
prevented further oxidation to 12-ketoendrin. The respective
glucuronide conjugates were also eliminated in the urine, as were the
glucuronides of 3-hydroxyendrin and the 4,5- trans-diol ( VI)
(Bedford et al., 1975b; Hutson, 1981).
Studies with 14C-endrin in lactating cows showed that the
residues in milk and body fat consisted of unchanged endrin, although
traces of 12-ketoendrin were consistently found in fat. As in rats,
anti-12-hydroxyendrin was the major metabolite in urine and faeces,
the urine being the major excretory route, as in rabbits.
12-Ketoendrin and syn-12-hydroxyendrin were minor metabolites in cow
urine (Baldwin et al., 1976). Thus, although the metabolic pathways of
endrin in cows are qualitatively similar to those in rats and rabbits,
quantitative differences are seen in faecal and urinary excretion.
In hens, only endrin was found as a residue in meat, fat, and
eggs. Unchanged endrin accounted for about 10% of the radiolabel in
excreta, and the major metabolite was anti-12-hydroxyendrin and its
sulfate conjugate. No 12-ketoendrin was detected in tissues, eggs, or
excreta. The metabolism of endrin in hens is fundamentally similar to
that in rats, rabbits, and cows, except that they produce neither
syn-12-hydroxyendrin nor the related 12-ketoendrin. The rate of
metabolism, however, was much lower than in cows (Baldwin et al.,
1976). The absence of 12-ketoendrin in birds was confirmed in a study
of four species killed by endrin (Stickel et al., 1979). Hutson et al.
4.3.1 Biodegradation
Microbial degradation of endrin depends on the presence of an
appropriate microbial species and suitable soil conditions; it occurs
under anaerobic conditions (Donoso et al., 1979). Biodegradation is
aided by fungi and bacteria such as Trichoderma, Pseudomonas, and
Bacillus. The major transformation product is delta-ketoendrin
(Patil et al., 1970).
About 150 isolates from various soil samples were screened to
investigate the role of these microorganisms in degrading endrin; 25
of the 150 isolates were active. At least seven metabolites were
found, but conversion of endrin into the ketoendrin was common
throughout (Matsumura et al., 1971).
4.3.2 Bioaccumulation and biomagnification
The bioconcentration factors cited below are simple ratios of the
exposure concentration and the concentration in organic tissues. They
should be used with caution as indicators of bioaccumulation
potential: a high bioconcentration factor can represent little uptake
of a low concentration, and a low bioconcentration factor can be found
with considerable uptake of a high concentration. The bioconcentration
factor should therefore always be cited with the pertinent exposure
concentration of endrin.
Soil invertebrates such as slugs and earthworms had
bioconcentration factors of 14 to 103. Bioconcentration factors in a
number of aquatic organisms are given in Table 4. These ratios differ
extensively between different types of aquatic organisms.
Bioconcentration factors of 140 to 222 were found for four blue-green
algae (Microcystis aeruginosa, Anabaena cylindrica, Scenedesmus
quadricauda, and an Oedogonium species) after 7 days' exposure to
endrin at a concentration of 1 mg/litre of water( Vance & Drummond,
1969). In a study of the accumulation of endrin in stoneflies
(Pteronarcys dorsata) exposed to 0.03, 0.07, and 0.15 µg/litre of
water, the bioconcentration factor ranged from 1130 to 348, decreasing
with increasing water concentrations over the 28-day exposure period
(Anderson & DeFoe, 1980). In bullheads (Ictalurus melas), the
bioconcentration factor was 3700 after exposure for 4 days to 0.60
µg/litre and 6200 after exposure for 7 days to 0.26 µg/litre (Anderson
& DeFoe, 1980). The bioconcentration factors for endrin in sub-adults
of leopard frogs (Rana sphenocephala) exposed to 0.01, 0.012, 0.016,
0.022, and 0.030 mg/litre were 71.4, 34.4, 51.8, 59.4, and 94.3,
respectively. Sub-adults exposed to 0.01, 0.012, and 0.016 mg/litre
for 96 h and sacrificed 60 h later had bioconcentration factors of
6.1, 4.8, and 1.2, respectively (Hall & Swineford, 1980), indicating
a relatively rapid elimination of residues. In daphnids and molluscs,
a direct linear relationship was found between the logarithm of the
equilibrium bioconcentration factor (and the reciprocal clearance rate
constant) and the log P octanol/water partition coefficient for
non-degradable, lipophilic compounds with partition coefficients
ranging from 2 to 6. This relationship permits calculation of the
times required for equilibrium and for significant bioconcentration of
lipophilic chemicals, which were found to be shorter for molluscs than
for daphnids. The equilibrium biotic concentration for both molluscs
and daphnids decreased with increasing chemical hydrophobicity. The
relationship between the bioconcentration factor and log P
octanol/water partition coefficient was linear for compounds that did
not attain equilibrium within a finite exposure time (Hawker &
Connell, 1986).
In a study of the bioaccumulation of endrin from food by lobsters
(Homarus americanus), endrin dissolved in methanol was added to sea
water, and mussel tissue was soaked in the solution for 2 h to provide
a concentration of endrin of 4.7 mg/kg wet weight. Lobsters were fed
the prepared food every other day for 2 weeks, and excretion was
followed for an additional 4 weeks during which time the lobsters were
fed uncontaminated tissue. Liver and muscle were analysed from two or
three lobsters sampled after feedings 1, 2, 3, 5, and 7, and from one
or two lobsters sampled during the excretion phase at 1, 2, and 4
weeks. The concentration of endrin reached a maximum of 1.95 mg/kg wet
weight in the liver after 2 weeks of feeding; this level declined by
about 65% after 4 weeks of excretion. The time to 90% equilibrium
(uptake) was 15 weeks, and the time to 50% clearance (excretion) was
4 weeks (McLeese et al., 1980).
Bluegill sunfish (Lepomis macrochirus) exposed to water
containing 14C-labelled endrin at 1 µg/litre at temperatures of
20-22 °C rapidly absorbed the radioactivity, and, within 48 h, 91% of
the radioactive endrin had been taken up (6% was lost by
volatilization from a control tank without fish). Within 8 days after
the fish had been replaced in clean water, less than 15% of the
absorbed label had been eliminated; for three fish left for a longer
period, the half-life of loss was about 4 weeks, the loss curve being
linear (Sundershan & Khan, 1980). Endrin accumulated rapidly in the
tissues of channel catfish (Ictalurus punctatus) exposed to nominal
concentrations of 0.04, 0.4, or 4.0 mg/kg of diet for 198 days. After
that time, all groups were fed an endrin-free diet. Endrin was not
detected 28 days later in fish that had received 0.04 or 0.4 mg/kg,
and the level in the group that had received 4.0 mg/kg decreased to
0.011 mg/kg of tissue in 28 days and was below the limit of detection
within 41 days (Argyle et al., 1973). Similar results were obtained
for Leiostomus xantharus exposed to 0.05 µg/litre of water: at the
end of the study at 5 months, a residue level of 78 µg/kg tissue was
found, and no endrin was detected in fish after 18 days in
uncontaminated water (Lowe, 1966). Endrin thus seems to disappear
rapidly from tissues. In 20 Tilapia zilli (Alexandria strain) fry
(3.36 cm, 825 mg) exposed to 0.025 µg/litre (one-tenth of the 96-h
LC50) for 28 days, the total content of endrin was 327.4, 167.4,
297.6, 446.5, and 595.4 µg/kg after 4, 7, 14, 21, and 28 days,
respectively (El-Sebae, 1987).
Table 4. Bioconcentration factors for endrin in aquatic species
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Clam 1 5 days 480 Duke & Dumas
(Mercenaria (1974)
mercenaria)
Mussel 10 24 days 38 Ryan et al.
(Hyridella (1972)
australis)
Eastern oyster 0.05 7 day 2780 Mason & Rowe
(Crassostrea (1976)
virginica)
Water flea 1.0 1 day 2600 Metcalf et al.
(Daphnia (1973)
magna)
Fathead 0.015 - 10 000 Mount & Putnicki
minnow (1966)
(Pimephales
promelas)
Spot 0.05 8 months 1340 Lowe (1966)
(Leiostomus
xanthurus)
Flag fish 0.3 - 10 000 Hermanutz
(Jordanella 0.21 15 days 7 900 (1974)
floridae) 0.29 18 400 Hermanutz et
0.39 7 100 al. (1985)
Table 4 (contd)
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Mosquito 1.0 1 day 2 100 Metcalf et al.
larvae (Culex (1973)
ipiensquinque
fasciatus)
Mosquito fish 1.0 1 day 800 Metcalf et al.
(Gambusia (1973)
affinis)
Channel catfish 0.5 5-19 400-760 Argyle et al.
(Ictalurus (1973)
punctatus)
Sheepshead minnow (Cyprinodon variegatus) were exposed for 23
weeks to endrin at levels of 0.027-0.72 µg/litre of water, from the
embryonic stage through hatching until adulthood and spawning (see
section 7.2.2.2). Four-week-old juvenile fish accumulated 2500 times
the concentration in the water, adults, 6400 times, and their eggs,
5700 times (Hansen et al., 1977).
The transfer of endrin through the food chain
lichen-reindeer-humans was studied in the northern part of Sweden by
analysing lichen (Cladonia alpestris), a major food source for
reindeer during the winter, together with samples of tissues from
reindeer, which are eaten in considerable quantities by Lapps. One
4-year-old reindeer was slaughtered in 1979 and a 3-year-old in 1981,
and muscle and liver were taken for analysis. The annual uptake by
reindeer was 2.0 mg. The average level of endrin in lichen was 1.91
(range, 1.27-2.78) µg/kg; 1.45 and 2.4 µg/kg were found in the muscle
samples from the two reindeer and 0.55 and 0.72 µg/kg in liver. The
calculated transfer of endrin from lichen to reindeer was 0.7%. The
estimated annual consumption of reindeer muscle by Lapps was 70 kg for
males and 32 kg for women; consumption of liver was 3 and 1.1 kg,
respectively. The annual intake of endrin was thus 30.3 µg for males
and 13.8 µg for females (Villeneuve et al.,1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Many of the data reported in this chapter are measurements taken
at a time when endrin was used much more widely than at present or
with little control or restriction. They are therefore a reflection of
a historical situation in many countries. These data are included in
the document as an indication of the result of indiscriminate use and
disposal of endrin. Data from countries where endrin may still be used
are scarce or unavailable.
5.1 Environmental levels
The levels of residues associated with the use of endrin in
agriculture or with the discharge of industrial effluents containing
endrin are summarized in Tables 5-9; the levels of residues less
easily associated with specific uses or discharges are given in Table
10.
5.1.1 Air
A critical summary of studies on the atmospheric levels of
pesticides in the USA, e.g., in community air, was made by Donoso et
al. (1979). Some of their conclusions are worth repeating: "Endrin
concentrations are highest in the atmosphere over agricultural areas
and probably reach their peak levels during the pesticide use season.
Of all urban communities those surrounded by farmlands run the highest
chance of atmospheric contamination. Urban communities far removed
from agricultural areas are unlikely to experience significant
contamination." The maximum level of endrin in air, 58.5 ng/m3, was
found in a rural town in an agricultural area in the south of the USA,
but the normal weekly variation was between 0.8 and 6.5 ng/m3
(Stanley et al., 1971). In a later study of the same town, the average
annual atmospheric levels were 3.2 ng/m3 in 1972, 2.3 ng/m3 in
1973,and 5.3 ng/m3 in 1974, with the highest levels in August; in
1974, this was 27.2 ng/m3 (Arthur et al., 1976). The results of a
national monitoring programme for pesticides in the air of various
states of the USA showed the occasional presence of endrin over
agricultural areas at levels of the same order of magnitude: mean of
positive samples (8%), 2.6 ng/m3, with a maximum value of 19.2
ng/m3 (Kutz et al., 1976).
Endrin was not found in rain-water collected at different
location in the United Kingdom, using a method with a detection level
of 1 ng/litre of water (Tarrant & Tatton, 1968), nor in atmospheric
air (Abbott et al., 1966); however, endrin has never been used
extensively in the United Kingdom.
The mean daily intake of endrin by inhalation in the western part
of the Netherlands was calculated on the basis of an air concentration
of 41 pg/m3 (maximum, 300 pg/m3) to be 0.8 µg/day or 0.3 mg/year,
on the basis of air samples taken in the period 1975-81 (Guicherit &
Schulting, 1985).
Table 5. Concentrations of endrin in organisms collected in the Netherlands, 1965-71
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Coast Mussel (Mystilus edulis); 22 0.029 0.009-0.056 Composites of 25 Koeman (1971)
1965 composites of flesh mussels from 22
sampling stations
Netherlands Fish, 3 species; 103 0.13 0.07-0.45 Food of the sandwich Koeman et al. (1967)
1965 whole body tern
1966 Fish, 3 species; 37 0.10 0.07 -0.29
whole body
Sandwich tern
(Sterna sandvincensis)
1965 Liver 8 0.23 0.07-0.80 Shot or killed
1965-66 Liver 25 0.49 0.10-1.3 Found dead
1965-66 Egg 33 0.19 0.08-0.36
Wadden Sea Mussel (2 species); 20/4 LD LD Limit of detection, Koeman (1971)
1969 composites of flesh 0.005 mg/kg
Coast Mussel (Mytilus edulis); 199/8 < 0.016 LD-0.024 Koeman (1971)
1970 composites of flesh
Wadden Sea Zooplankton (marine) 1 LD LD Koeman (1971)
1969-70
Shrimp (Crangon 50/1 LD LD
vulgaris)
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Marine fish (4 species); 37/5 0.014 0.008-0.034
composites of whole
body
1967 Freshwater fish 28 LD LD-0.02 Measurable concentration
(3 species) (0.02 mg/kg) in one fish
only; limit of detection,
0.005 mg/kg
1970 Pike (whole body) 10 LD LD Limit of detection,
0.005 mg/kg
1971 Roach (whole body) 6 LD LD
1968-69 Hawks and falcons 16 < 0.1 LD-0.16 Birds found
dead or dying; Koeman et al. (1969)
(4 species); liver measurable concentration
(0.16 mg/kg) in one hawk
(buzzard)
Owls (2 species); liver 3 < 0.1 LD-0.13 Measurable concentration
(0.13 mg/kg) in one
long-eared owl; limit of
detection not specified
1970 Sandwich tern eggs 10 LD LD Limit of detection, Koeman (1971)
0.02-0.008 mg/kg
1971 Grey heron 27/4 LD LD
(Ardea cinerea);
composite of eggs
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
1969-71 Sparrowhawk 28/3 LD LD
(Accipiter nisus);
composite of eggs
a Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
b LD, limit of detection
Table 6. Concentrations of endrin in samples collected in North America
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Mississippi River
USA
December 1963 Channel catfish; blood 3 0.44 0.41-0.56 Found dead or dying in areas Anon. (1964)
of extensive fish kills
December 1963 Fish, various species; 24 0.18 0.14-0.26
blood
January- Fish, various species; 82 0.06 LD-0.21 Caught alive; limit of
February 1964 blood detection not specified
July 1964- Water 12 < 0.01 LD-0.01 4 samples contained Novak & Rao (1965)
June 1965 measurable concentrations
(0.01 mg/kg or litre)
Mud 12 < 0.01 LD-0.01
Oysters 12 LD LD Limit of detection,
0.005 mg/kg or litre
Shrimp 12 LD LD
Fish (2 species) 24 < 0.01 LD-0.02 9 samples of fish contained
measurable concentrations:
8 of 0.01 mg/kg and 1 of
0.02 mg/kg
Mississippi, USA Eastern oysters 470 LD LD 15 or more oysters per Butler (1973)
1965-72 (Crassostrea virginica); composite from 8 sampling
composites of flesh stations; limit of detection,
0.01 mg/kg
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Bayous in the Water 148 LD LD-0.0002 4 samples contained Rowe et al. (1971)
Mississippi delta, measurable amounts
USA, October (0.00009-0.0002 mg/litre),
1968-May 1969 4 samples contained traces;
remainder less than limit of
detection
Sediment 44 LD LD-0.005 7 samples contained
measurable amounts
(0.004-0.005 mg/kg); one
sample contained a trace
Eastern oyster 111 LD LD-0.006 79 samples contained
(Crassostrea virginica) < 0.001 mg/kg
Mississippi Water 26 LD LD Samples collected from Leard et al. (1980)
stream systems 13 sampling stations in
1972-73 5 major river basins; limit
of detection, 0.0005 mg/litre
Freshwater bivalves 58 LD LD-0.1 Residues below limit of
(7 species); flesh detection (0.02 mg/kg), except
for traces (< 0.1) in 1973 in
one river which drains from an
agricultural area
Ontario, 3 Fish (9 species) LD LD Residues below limit of Miles & Harris
streams, 1971 detection, 0.01 mg/kg (1973)
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Gulf coast Whole fish 139/48 < 0.02 LD-0.27 Reseidues below limit of Henderson et al.
streams, USA (various species); detection (0.001 mg/kg) (1969)
composites in 33 composites
Mississippi 657/202 < 0.01 LD-0.11 Residues in 184 composites
River system, below limit of detection
USA (0.001 mg/kg)
Louisiana, USA Brown pelican Eggs collected from nests of Blus et al. (1979)
(Pelecanus occidentalis); birds transplanted as nestlings
eggs from Florida, 1968-76; limit
1971 3 0.10 0.08-0.12 of detection not specified
1972 12 0.18 0.11-0.29
1973 21 0.16 0.03-0.46
1974 25 0.30 LD-0.73
1975 30 0.50 0.29-1.06
1976 25 0.29 LD-1.47
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 7. Concentrations of endrin in organisms collected in a
cotton-growing area in the Republic of Chad in 1969
Sample No. of Concentration (mg/kg) Comments
samples Mean Range
Fish, two 31 0.02 LD-0.083 Cotton-growing area,
species endrin and DDT used
for pest control; limit
Kingfishers and 46 0.02 LD-0.075 of detection, 0.008
cormorants; liver mg/kg
Birds, non-aquatic, Birds found dead
various species soon after insecticide
Brain 12 0.51 0.10-0.77 application; deaths
Liver 12 0.88 0.13-1.42 of some birds attributed
to endrin
From Everaarts et al. (1971); LD, limit of detection
5.1.2 Soil, sediments, and sewage sludge
5.1.2.1 Soil
In the US National Soil Monitoring Program, 1486 soil samples
from 37 states were analysed in 1971. Fourteen samples were found to
contain endrin, at a geometric mean level of < 0.001 (maximum,
0.02-1.00) mg/kg dry weight (Carey et al., 1978). The mean endrin
concentration in 29 soil samples in Kyushu District, Japan, was 0.183
mg/kg (range, 0.016-0.629 mg/kg) dry matter (Suzuki et al., 1973).
5.1.2.2 Sediments
In 1964, levels in the sediment of Cypress Creek, Memphis, TN,
USA, upstream and downstream of a pesticide manufacturing plant,
reached 12 800 mg/kg dry weight. In 1967, water from the Creek
contained levels of 0.27-2.03 µg/litre and sediment contained levels
of 47.4-10 676 mg/kg dry weight (Barthel et al., 1969).
Endrin was found in 17% of samples of bottom sediment from 59
sites on the Detroit River, USA, at levels up to 43 µg/kg (limit of
detection, 1.0 µg/kg) (Hamdy & Post, 1985). No endrin was detected in
sediment samples collected in 1980-82 from riverine and pothole
wetlands at 17 locations in the north-central USA (Martin & Hartman,
1985) or in samples of sediment from 34 stations on the upper Great
Lakes in 1974 (< 1 µg/kg) (Glooschenko et al., 1976).
Table 8. Concentrations of endrin in organisms collected in a rice-growing area in Wageningen, Surinam
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
October 1971 Snail kite (Rostrhamus 5/1 LD LD -- Pesticides, including endrin, applied
sociabilis); brain /liver | to rice fields
|
Black vulture (Coragyps 5/1 LD LD |
astratus); brain/liver |
|
Egrets (3 species); brain/ 30/1 LD LD | Samples collected at end of growing
liver |-- season before insecticide application
| for next growing season; limit of
Purple gallinule 10/1 LD LD | detection, 0.01 mg/kg
(Porphyrula martinica); |
brain/breast muscle |
|
Spectacled caiman (Caiman 10/1 LD LD |
crocodilus); brain/liver --
November 1971 Snail (Pomocea sp.) 10/1 LD LD --
|
Frog (Pseudis paradoxa); 6/1 LD LD | Found dead after application of
whole-body composites | pentachlorophenol; lower limit of
| detection, 0.01 mg/kg
Kwi kwi (Hoplosternum 8/1 LD LD |
littorale); whole-body |
composites --
Table 8. (contd)
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
Srieba (Astyanax bimaculatus); 8/1 LD 0.1 -- Found dead after application
whole-body composites | of pentachlorophenol; lower
|-- limit of detection 0.01 mg/kg
|
Krobia (Cichlasoma 8/1 LD LD |
bimaculatum); whole-body |
composites --
Fish (3 species listed above); 21/3 3.36 1.96-5.35 Found dead after application
whole-body composites of endrin
28 November- Snail kite; brain 17 LD LD Found dead; deaths
4 December 1971 attributed to pentachlorophenol
poisoning
2-9 December 1971 Aquatic birds (4 species); 5 0.11 0.06-0.16 Found dead after application
brain of endrin
2-9 December 1971 Wattled jacana 1 2.71 Death attributed to endrin
(Jacana jacana) poisoning
5-11 December 1971 Common egret (Egretta alba); 2 0.23 0.14-0.32 Found dead or sick in roost
brain
2-11 December 1971 Common egret (Egretta alba); Found dead or sick in rice
Brain 9 0.25 fields; about half the total
Liver/kidney 7-9 0.08 endrin was applied during
the first half of December
From Vermeer et al. (1974)
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 9. Concentrations of endrin collected in drainage water from irrigated land, California, USA
Geographical Type of sample No. of Concentration (mg/kg or mg/litre) Comments
area and year samples
Mean Range
Tule Lake and Water 44 0.000011 LD-0.0001 Limit of detection, < 0.000003 mg/litre
Klamath Lake,
National
Wild-life
Refuge,
USA, 1964
Refuge, Northern Suspended matter 8 0.011 LD-0.058 --
California, USA, Vascular plants 7 0.006 LD-0.013 |
April 1965- (two species) |-- Limit of detection, 0.005 mg/kg
February 1967 Algae (Cladophora sp.) 5 0.007 LD-0.022 |
Clam homogenates 3 0.013 LD-0.034 --
Gonidea sp.)
Fish (Siphateles sp.) 5 0.05 0.004-0.198 Samples collected at a pumping station
discharging water from irrigated land.
Peak concentrations of endrin occurred
during the growing season when endrin
was applied.
From Godsil & Johnson (1968); LD, limit of detection
Table 10. Concentrations of endrin in environmental samples; residues not associated with particular local use or industrial
effluent
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
North America
North Carolina, Soil (tobacco fields) 19 LD LD Reeves et al.
USA,1971 Sediment (ponds) 40 LD LD Limit of detection, (1977)
Frog (Rana sp.) 13 LD LD-0.01 0.01 mg/kg
Turtle (4 species) 41 LD LD-0.01
Bluegill (Lepomis 20 LD LD
macrochirus)
Tiger beetle 23 0.02 LD-0.05
(Megacephala
carolina)
Rice-growing Invertebrates, aquatic 1313/24 LD LD-trace A total of 192 dead or Flickinger &
area, Gulf Coast, and terrestrial (various dying birds were found in King (1972)
Texas, USA, species); whole-body three rice-growing areas in
1967-71 composites of live which rice seed dressed
specimens, except for with aldrin/ceresan had
4 composites of been used. Endrin residues
crayfish attributed to use in cotton-
growing areas. Limit of
1968 Fish (4 species); 542/4 LD LD detection not defined;
whole-body composites trace found in one
composite of dead
1968 Cricket frog (Acris 18/3 LD LD crayfish
crepeitans blanchardi);
whole-body composites
1968-70 Turtles (2 species); 5/2 LD LD
whole-body composites
of live specimens
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Snakes (3 species); sick 3 LD LD
specimens
Bobcat (sick) and dead 2 LD LD
rice rat; brain
Great horned owl; dead 2 LD LD
specimen; brain
Rice- growing Aquatic birds (10 26 0.22 LD-0.4
area, Gulf Coast species) found dead
USA, 1967-71 or dying; brain
1967 Fulvous tree duck 14 0.1 LD-0.3
(Dendrocygna bicolor);
eggs
Galveston Bay Oyster composites 10 0.01 LD-0.02 Limit of detection, Casper (1967)
Texas, USA, 1964 0.01 mg/kg
National Monitoring Fish (various species); 400 93% of samples below Henderson et
Program: Great Lakes whole-body composites limit of detection, al. (1969)
and major river 0.001 mg/kg
basins, USA (excluding
Gulf Coast, Mississippi
River system; see
Table 6); 1967-68
Atlantic coast streams 741/141 0.002 LD-1.50
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Great Lakes drainage Fish (various species); 378/66 0.001 LD-0.02
whole-body composites
Hudson Bay, Canada, 51/13 LD LD
drainage
Colorado River, USA 112/24 0.008 LD-0.71
Interior basins 120/25 0.001 LD-0.01
California, USA, 90/24 0.002 LD-0.02
streams
Columbia River, USA, 246/64 0.001 LD-0.01
systems
Pacific coast, USA, 83/20 LD LD
streams
Alaska, USA, streams 105/24 LD LD
National Monitoring Fish (various species); 666/147 LD LD Limit of detection, Henderson et
Program; 50 sampling whole-body composites 0.005 mg/kg al. (1971)
stations, USA, 1969
Estuaries, Giant Pacific oyster 1656/138 0.005 LD-0.01 Measurable concentration Modin (1969)
California, USA (Crassostrea gigas); in only one oyster; limit
1966-67 Mussel (Mytilus edulis); 432/36 LD LD of detection, 0.01 mg/kg
composites of shellfish
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 108-162/54 0.06 LD-0.4 Limit of detection, Crockett et
Mississippi, USA fish farms; composites 0.01 mg/kg; 13 al. (1975)
1970 of edible portions composites contained
< 0.01 mg/kg
Intensive cotton- 0.063 (0.030- 2 composites contained
growing areas, 0.122)a > 0.3 mg/kg. Significantly
Mississippi, USA higher residues in intensive
cotton-growing areas
Less intensive cotton- 0.010 (0.005- Crockett et
growing areas, 00.019)a al. (1975)
Mississippi, USA
Major watersheds, Fish (various species); 582/58 LD LD Limit of detection not Veith et al.
USA, 1976 whole-body composites specified. Intermediate (1979)
in the manufacture of
cyclodiene insecticides
detected (mass
spectrometry) in Wabash
River, Indiana
Major watersheds Fish (various species); 138/6 LD LD Limit of detection not Veith et al.
near Great Lakes, whole-body composites specified. Endrin (1981)
USA, 1978 identified by mass
spectometry in fish from
Wabash River, Indiana,
together with manufacturing
intermediates (concentration
not quantified)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 50 0.05 LD-0.41 Limit of detection, 0.01 Hawthorne
Mississippi, USA, fish farms, edible portion mg/kg; 14 fish contained et al. (1974)
1970 < 0.01 mg/kg
Continental rise Bathyl-demersal fish 4 LD LD Limit of detection, Meith-Avcin
south-east of (Antimora rostrata); 0.01 mg/kg. Samples et al. (1973)
Cape Hatteras, USA, liver collected by trawling at a
1972 depth of 2500 m
Lake Michigan, USA, Amphipods (Pontoporeia 24/8 0.08 0.04-0.33 Limit of detection, Peterson &
1969-72 affinis) collected from 0.005 mg/kg Ellarson
oesophagi of old squaws (1978)
December 1969 Old squaws (Clangula 37 0.18 0.1-0.2 Birds caught in fishing
hyemalis); carcasses nets or shot
March-April 1970 44 0.28 0.2-0.4
December 1970- 108 0.31 0.1-0.9
May 1971
January-February 8 0.6 0.2-1.0
1972
Northwest Territories 99 0.1 LD-0.3
and wintering areas
other than Lake
Michigan, 1971-73
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Canada and USA, Bald eagles (Haliaeetus 29 0.02 LD-0.1 Limit of detection, Reichel et al.
1965 leucocephalus) found 0.05 mg/kg. (1969)
dead; brain Concentration in 24
specimens below limit
of detection
Connecticut & Bald eagles found dead; 2 LD LD-0.1 Limit of detection not Reichel et al.
Florida, USA, brain, liver, carcass defined; apparent (1969)
1967-68 concentration of
0.1 mg/kg in Florida
eagle not conformed
by thin-layer
chromatography
Continental USA Bald eagle; brain Limit of detection, Mulhern et al.
1966 21 LD LD 0.05 mg/kg (1970)
1967 21 LD LD
1968 26 LD LD
1969 Bald eagle; brain 28 LD LD Limit of detection, Belisle et
0.05 mg/kg al. (1972)
1970 11 LD LD
1971-72 Bald eagle; brain 37 LD LD Limit of detection, Cromartie et
0.05 mg/kg al. (1975)
1973-74 Bald eagle; brain 81 LD LD Limit of detection, Prouty et al.
0.05 mg/kg (1977)
1975 Bald eagle; brain 49 0.07 LD-0.50 Limit of detection, Kaiser et al.
0.05 mg/kg. (1980)
Concentrations in 46
specimens < 0.05 mg/kg
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
1976 50 0.08 LD-0.71 Concentrations in 44
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisong
1977 69 0.08 LD-1.2 Concentrations in 64
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisoning
Wisconsin, Maine, Bald eagle; eggs 26 LD LD Limit of detection, Krantz et al.
Florida, USA, 0.05 mg/kg (1970)
1968
USA, Golden eagle (Aquila 102 LD LD-0.3 Limit of detection, Reidinger &
1964-71 chrysaetos) found dead 0.1 mg/kg. Concentrations Crabtree
or dying; body fat in 97 specimens below (1974)
limit of detection
Coast of California, Gray whale 23 LD LD Wolman &
USA, 1968-69 (Eschrichtius robustus); Wilson (1970)
blubber
Sperm whale (Physeter 6 LD LD
catodon); blubber
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
South Atlantic and Small cetaceans (10 69 LD LD-0.24 Limit of detection, O'Shea et al.
Pacific Oceans, species); blubber, 0.1 mg/kg. Measurable (1980)
1968-69 brain, muscle concentrations (0.22 and
0.24 mg/kg) found in two
specimens
Maryland, USA, Little brown bat (Myotis 87 LD LD Limit of detection, Clark &
1976 lucifugus); carcass 0.1 mg/kg Krynitsky
(1978)
Missouri, USA Gray bat (Myotis 20 LD LD Limit of detection, Clark et al.
1976-77 grisescens) found dead; 0.1 mg/kg (1980)
carcass (lipid basis)
Washington Sate 18 bird species (total Blus et al.
(orchards), number of birds, 91) (1983)
October 1981- Brain 78 LD-> 0.8
July 1982 Eggs 53 LD-1.7
Detroit River, Herring gulls (Larus LD LD Struger et al.
Niagara River, argentatus); eggs (1985)
Saginaw Bay, USA,
1978-82
North-west Fish species; 700 0.008 LD-0.026 Stout (1980)
Atlantic Ocean, muscle
Gulf of Mexico,
USA, 1973-75
South-east Montana, Merlins (Falco LD LD Becker & Sieg
USA, 1978-81 columbarius); eggs (1987)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
New Jersey, Snapping turtles 11 LD LD Limit of detection, Albers et al.
Maryland, USA (Chelydra serpentina) 0.1 mg/kg (1986)
Florida, USA Snail kite (Rostrhamus LD LD Limit of detection, Sykes (1985)
sociabilis); eggs, nestlings 0.05 mg/kg
Missouri, USA, Gray bats (Myotis 7 LD LD Clawson &
1982 grisescens) Clark (1989)
Red bats (Lasiurus 7 LD LD
borealis)
Pipstrelles (Pipistrellus 2 LD LD
subflavus)
Denver, Colorado, Tree swallows 32 LD LD Deweese et al.
USA, 1980-81 (Tachycineta bicolor) (1985)
North-east Alberta, Otter (Lutra canadensis); 158 LD LD Limit of detection, Somers et al.
Canada, 1980-82 carcass 0.001 mg/kg (1987)
Africa
Lake Nakuru, Fish (Tilapia grahami); 10-20/2 LD LD Limit of detection: Koeman &
Kenya, 1970 whole-body composites fish, 0.002 mg/kg; Santiago
African cormorant birds, 0.009 mg/kg (1972)
(Phalacrocorax africanus) 3 LD LD
liver
White pelican 1 LD LD
(Pelicanus onocratalus);
Lesser flamingo 5 LD LD
(Phoeniconaias minor)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Europe
Province of Leon, Kestrels (Falco 4 - LD-2.0 Liver, 0.01; brain, 0.016; Sierra &
Spain, 1986 tnnunculus); 5 organs kidneys, 0.027; muscle, Santiago
or tissues 0.054 mg/kg (1987)
Sparrowhawk 3 LD-2.0 Liver, 0.139; kidneys, 0.4;
(Accipiter nisus) fat, 1.068; brain, 0.031;
muscle, 0.047 mg/kg
Red kite 2 LD-2.0 Kidneys, 0.005; brain,
(Milvus milvus) 0.103; fat, 2.035 mg/kg
1984-87 Barn owl (Tyto alba) 0.001-0.22 Liver, 0.036; brain, 0.052; Sierra et al.
kidneys, 0.034; fat, 0.014; (1987)
muscle, 0.020 mg/kg
North Sea Gadus morhua and 12 0.0001-0.0023 Von Westernhagen
Merlangius merlangus; 4 < 0.001-0.0011 et al. (1987)
ovary
North Sea Merlangius merlangus Von Westernhagen
Ovary 56 LD-0.001 et al. (1989)
Testis 16 LD
Liver 30 LD
Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed; LD, limit of detection
a95% confidence interval
None of 60 samples of bottom deposit collected in 1974 from 19
rivers and their estuaries in Japan contained endrin (< 0.01 mg/kg)
(Japanese Environmental Agency, 1975).
No endrin was detected in sediment and particulates from the
River Elbe in Germany in 1983-85 (Sturm et al., 1986). Sediment from
Rotterdam Harbour contained a total of 3-59 µg/kg aldrin, dieldrin,
and endrin. No endrin was found at seven sites in the Elbe Estuary
(Japenga et al., 1987). A housing estate in the Netherlands,
comprising about 800 houses and public buildings, was built in 1983
directly on a 4-m-thick layer of harbour sludge transferred in 1962-64
from about 20 harbour basins in Rotterdam and the industrial area
around the Nieuwe Waterweg. Organic solvents, polycyclic aromatic
hydrocarbons, heavy metals, and endrin and related pesticides, were
detected in the sludge. One-third of the soil samples collected in the
gardens (71 locations), 0-40 cm below the surface, contained endrin
and related pesticides at a mean concentration of 1.2 mg/kg and a
maximal concentration of 19.5 mg/kg dry weight (Van Wynen & Stijkel,
1988).
In surface sediments from five sites in Manukan Harbour, New
Zealand, only traces (none detected to < 0.1 µg/kg dry weight) of
endrin were found (Fox et al., 1988).
Particulates from two sites in the Shatt al Arab River in Iraq
contained endrin at 84 and 154 µg/kg, and a site in the Tigris River
contained 217 µg/kg. No endrin was found in the Euphrates River. The
mean concentration of endrin in surface and subsurface sediment from
the Shatt al Arab River ranged from 3 to 18 (range, none detected to
32) µg/kg; no endrin was found in surface and subsurface sediment from
the Tigris River. None was found in surface sediment from the
Euphrates River, but in subsurface sediment a mean concentration of 11
(5-25) µg/kg was detected (DouAbul et al., 1988).
5.1.2.3 Sewage sludge
Endrin was found in only a few of 444 sludge samples analysed
from sewage treatment works in the United Kingdom. The mean
concentration was 0.11 mg/kg of sludge, with a range of 0.01-0.71
mg/kg (McIntyre & Lester, 1984). All samples of non-disinfected
influent at a pilot plant in Jefferson Parish, LA, USA, contained
endrin, at an average concentration of 0.67 (0.25-1.58) ng/litre
(Lykins et al., 1986).
Sludge from three main waste water treatment plants in Kuwait was
analysed over a 6-month period in 1984-85. Two grab samples were taken
from each plant every month to give a total of 36 samples. The mean
endrin levels for the three plants were 0.02, 0.02, and 0.06 mg/kg
(Samhan & Ghobrial, 1987). Sewage plant effluents before and after
treatment were analysed in Baghdad (Iraq) in 1982-83. Endrin was found
in 8/15 samples taken before treatment, at a mean concentration of
0.291 (0.081-2.637) µg/litre, and in 6/15 samples taken after
treatment, at a mean concentration of 0.194 (0.072-1.197) µg/litre
(Al-Omar et al., 1985a).
5.1.3 Water
5.1.3.1 Surface water
Data on the concentrations of endrin in surface water concern
mainly those regions in the USA where use of endrin was widespread,
such as in Mississippi and Missouri, over the period 1957-65. The
highest concentrations were found in 1963 in the Lower Mississippi,
with a maximum level of 0.214 µg/litre. The concentrations and the
rate of occurrence decreased considerably later (Breidenbach et al.,
1967). In a survey in 1964-68, a maximal level of 0.133 µg/litre was
reported to have been found in the Missouri basin in 1967. No endrin
was detected in 1968 (Lichtenberg et al., 1970). In 1974, the
concentrations in the Lower Mississippi was 0.0045 µg/litre in
August-November (Brodtmann, 1976).
In one sample from the Potomac River, at Quantico, endrin and
endrin aldehyde were identified at concentrations of 0.005 and 0.006
µg/litre, respectively (Hall et al., 1987). Endrin was not found in
the waters near the Los Angeles County ocean outfalls (< 0.00005
µg/litre) (Green et al., 1986) or in surface water in Louisiana in
1980 (< 1.0 ng/litre) (McFall et al., 1985). In a programme to
monitor surface water in the USA in 1976-80, endrin was found in only
0.1% of samples, at a maximum value of 0.04 µg/litre (Carey & Kutz,
1985).
No endrin was found in water from 33 sites in the Upper Great
Lakes in Canada (< 0.01 µg/litre) (Glooschenko et al., 1976). In
Ontario, where endrin was used only sparingly, no residues were found
in 1971 or 1975-77 (Miles & Harris, 1973; Frank et al., 1981). In
water samples taken 1 m below the surface at 14 stations on Lake
Ontario in 1983, endrin was found in concentrations of
0.000044-0.000145 µg/litre (Biberhofer & Stevens, 1987).
In a survey of the aquatic environment in The Netherlands,
including drinking-water, 1826 samples were taken at 99 sampling sites
between September 1969 and 1977; traces of endrin were reported
occasionally (Wegman & Greve, 1978, 1980). Studies of surface water in
other areas in Europe failed to show the presence of endrin (Wilson
Committee, 1969; Engst & Knoll, 1973; Uhnak et al., 1974; Galassi &
Provini, 1981;Hrubec, 1988). In 1984-85, water from a number of rivers
in Germany contained endrin at levels of none detected to 0.30
µg/litre (Braun ,1985); surface water in Greece occasionally contained
levels of 0.0003-0.0004 µg/litre (Albanis et al., 1986).
No endrin was found in surface or drinking-water in the state of
Sa Paulo (Brazil) (Lara & Barreto, 1972), but it was found in water
reservoirs of basins in Sa Paulo at concentrations of none detected
to 1.02 µg/litre (Celeste & Caceres, 1987; Caceres et al., 1987).
Endrin was also found accidentally in two lagoons in north-west Mexico
(Rosales et al., 1985).
None of 60 water samples collected in 1974 from 19 rivers and
their estuaries in Japan contained endrin (< 0.1 µg/litre) (Japanese
Environmental Agency, 1975).
Endrin was found at five places in the River Nile at
concentrations of 0.0038-0.0189 µg/litre in March-September 1982
(El-Dib & Badawy, 1985). In analyses of the water of the Shatt al
Arab, Euphrates, and Tigris Rivers, endrin was found only in the
Euphrates River, but in all samples at a mean concentration of 0.024
(0.014-0.036) µg/litre (DouAbul et al., 1988). It occurred in 75% of
samples of urban, industrial, and continental water from the Moroccan
Mediterranean coast, at concentrations of none detected to 13 µg/litre
(Kassabi et al., 1988). Three of 15 grab samples of surface water
sources in Southern Africa (Orange Free State) contained endrin, at a
concentration of 2-4 µg/litre (Hassett et al., 1987).
Endrin was present in the Kalinadi River in India as a result of
runoff, especially from agricultural areas, at a concentration of 2
µg/litre (Kudesia & Bali, 1985). In the Chao Phraya River and klongs
in Bangkok, Thailand, no endrin (< 0.001 µg/litre) was found in 1984
(Onodera & Tabucanon, 1986). Analyses in Bali of 16 samples of river
water in the dry season and 15 samples in the rainy season showed the
presence of endrin at 40 µg/litre once, in the rainy season (Machbub
et al., 1988).
5.1.3.2 Rain and snow
No endrin was found (limit of detection, 1-2 ng/litre) in
atmospheric precipitation in the form of snow (17 samples in 1976) and
rain (81 samples in 1976 and 1977) on the Canadian side of the Great
Lakes and inland in areas remote from any nearby industrial or urban
contamination (Strachan et al., 1980). Four of 16 samples of
rain-water collected at four sites in Canada had levels of
0.00013-0.00044 µg/litre and another sample had 0.0048 µg/litre; no
endrin was detected in the other 11 samples. The mean endrin contents
in samples taken at another site in 1977, 1981, 1983, and 1984 were
none detected, 0.000065, 0.000085, and 0.000049 µg/litre, respectively
(Strachan, 1988). Endrin was not detected in snow samples collected at
12 sites in the Northwest Territories of Canada in 1985-86 (Gregor &
Gummer, 1989).
5.1.3.3 Drinking-water
Data obtained in 1964-67 from selected municipal drinking-water
treatment plants in Mississippi and Missouri, USA, showed that the
concentration in approximately 10% of the samples exceeded 0.1
µg/litre in the first year but that the concentrations were lower in
1965-67 (Schafer et al., 1969). The most recent study on US
drinking-water was done on finished water in New Orleans, LA, in 1974,
where the highest concentration measured was 4 ng/litre (US EPA,
1974).
During 1976, endrin was found at a mean concentration of 4
ng/litre (range, 1-7 ng/litre) in drinking-water in Ottawa, Canada
(Williams et al., 1978).
The mean concentration of endrin in drinking-water at the
El-Abbasia station, Egypt, in 1986 was 3.507 ± 1.45 ng/litre in 10
samples taken before purification and 1.845 ± 1.29 ng/litre after
purification (Abdel-Razik et al., 1988).
Drinking-water from the North Coast region of New South Wales,
Australia, was analysed in 1986-87: 147 of 659 samples contained
traces of endrin (none detected [< 0.005] to 0.05 µg/litre) (Ang et
al., 1989).
5.1.3.4 Groundwater
Water in wells used as a source of water for mixing pesticides
in fruit orchards in West Virginia (USA) was found to contain endrin
at about 1 ng/litre in 1985 and in 1986. The water in these wells was
not used for drinking-water. Endrin had not been used in the area
since 1970, and the authors cite their results as evidence for the
persistence of endrin and its capacity to contaminate groundwater many
years after cessation of use (Hogmire et al., 1990).
5.1.4 Organisms in the environment
5.1.4.1 Birds
Endrin was found in the carcasses of four of 16 turkey vultures
(Cathartes aura) in southern California, USA, in 1981, at levels of
0.11-0.23 mg/kg wet weight, but in none of six common ravens (Corvus
corax). It was also found in two of four vulture eggs, at 0.10
(range, none detected to 0.52) mg/kg wet weight, but in none of 30
raven eggs (Wiemeyer et al., 1986).
Endrin was found in eggs of shag (Phalacrocorax aristotelis)
and cormorants (Phalacrocorax carbo) at one of five collection sites
in the east, south-east, and south of Ireland, at a geometric mean
concentration of 0.30 (range, 0.06-1.60) µg/kg (Wilson & Earley,
1986). Eggs from two species of passerine birds, three species of
gull, four species of tern, and the night heron were collected in
Italy in 1982-83. Endrin was found in 30 eggs of the night heron
(Nicticorax nycticorax), at an average concentration of 0.11
(0.03-0.27) mg/kg, in 50 eggs of the gull-billed tern (Gelochelidon
nilotica), at a concentration of 0.28 (0.05-1.31) mg/kg, and in 38
eggs of the tree sparrow (Passer montanus) and 33 eggs of the hooded
crow (Corvus corone), at concentrations of 0.17 (0.09-0.33) and 0.21
(0.07-0.31) mg/kg. It was not detected in eggs of the other species
(Fasola et al., 1987).
No endrin was found in 98 eggs or in the livers of 112 nestlings
of rooks (Corvus frugilegus) collected from five rookeries in
northern Germany in 1982-83 (Beyerbach et al., 1987) or in 45 eggs and
the livers of eight young lapwings (Vanellus vanellus) collected in
1984 and 1986 (Beyerbach et al., 1988).
Detectable residues of the commonest organochlorine pesticides
were found in 0.9% of 112 pools (mostly of 10 birds) of starlings
(Sturnus vulgaris) collected in 129 sites in the USA in 1979 and in
1.6% of 129 pools in 1982. In most states, no endrin was detected, but
levels of 0.01 and 0.17 mg/kg wet weight were found in two (Bunck et
al., 1987).
Endrin was present at microgram levels per kilogram of wet weight
in 272 samples of liver, muscle, fat, and eggs from northern fulmars
(Fulmarus glacialis), black-legged kittiwakes (Rissa tridactyla),
and thick-billed murres (Uria lomvia) collected in 1975-77 on Prince
Leopold Island, Northwest Territories, Canada (Nettleship & Peakall,
1987). It was found in 8 of 108 carcasses of herons analysed in the
USA since 1966, at levels of 0.10-0.86 mg/kg wet weight (Ohlendorf et
al., 1981) but was not found in 255 pools of wings from black ducks
(Anas rubripes) and mallards (A. platyrhynchos) collected in the
USA in 1981-82 (Prouty & Bunck, 1986).
Endrin was not detected in six eggs of Forster's tern (Sterna
forsteri) collected on Green Bay and Lake Poygan, Michigan, USA in
1983 (Kubiak et al., 1989). None was found in a total of 107 eggs
collected in 1975-80 from 10 species of colonial waterbirds nesting in
areas around Green Bay and Lake Michigan. The species were little
gulls (Lares minutes), green-backed herons (Butorides striatus),
black terns (Chlidonias niger), herring gulls (L. argentatus),
ring-billed gulls (L. delawarensis), common terns (S. hirundo),
Forster's tern (S. forsteri), double-crested cormorants
(Phalcrocorax auritis), black-crowned night herons (Nycticorax
nycticorax), and cattle egrets (Bubulcus ibis). The limit of
detection was 0.1 mg/kg in 1977 and 0.05 mg/kg in 1978 (Heinz et al.,
1985).
Of five eggs from peregrine falcons (Falco peregrinus)
collected in Arizona, USA, in 1978-82, one collected in 1978 contained
endrin at 0.20 mg/kg dry weight, one collected in 1981 contained no
detectable amount (< 0.01 mg/kg) and three collected in 1982
contained 0.02-0.04 mg/kg (Ellis et al., 1989).
No endrin was detected in 27 eggs from tree sparrows (Passer
montanus), 4 eggs from house martins (Delichon urbica), 28 eggs
from white storks (Ciconia ciconia), or eggs from nine other species
of bird in Germany in 1984. The livers of 25 nestling, 13 young, and
17 adult white storks also contained no detectable level of this
pesticide (limit of detection, 0.001 mg/kg) (Heidmann et al., 1989).
5.1.4.2 Fish and shellfish
The endrin concentrations in red mullet (Mullet barbatus)
collected at six locations in the Pagassitikos Gulf (Greece) in
1986-87 were < 0.005-0.5 µg/kg fresh weight of fillets (Satsmadjis et
al., 1988). The mean concentrations in liver, brain, kidneys, and
muscle of 22 trout (Salmo trutta fario L.) taken from four rivers in
Leon, Spain, in 1985 were 0.104, 0.123, 0.157, and 0.157 mg/kg wet
weight. The incidence in the four organs was 4.54-22.73% (Teran &
Sierra, 1987). Endrin was found in 29 samples of fish collected in
Italy, at a median concentration of 0.019 mg/kg (Cantoni et al.,
1988). Organochlorine compounds were measured in three samples of
liver from cod (Gadus morhua) collected in three areas of the North
Sea in 1977-87; endrin was present at a concentration of < 5 µg/kg of
product (De Boer, 1989).
Endrin was not detected (< 0.01 mg/kg) in two or three replicate
samples, each comprising three to five bluegill (Lepomis macrochirus)
and common carp (Cyprinus carpio), collected from downstream sites
exposed to irrigated agriculture and from non-irrigated upstream sites
on the San Joaquin River and tributaries in California, USA (Saiki &
Schmitt, 1986). Endrin was also not found in water near Los Angeles
County ocean outfalls (< 0.00005 µg/litre) or in mussels (Mytilus
californianus) (< 0.1 µg/kg wet weight) that had been suspended at
the monitoring site for 2 months to provide a measure of the
bioaccumulation of chlorinated hydrocarbon contaminants (Green et al.,
1986). No endrin was detected in fish samples taken at nine locations
in north-central USA (Martin & Hartman, 1985), and endrin was not
detectable (< 0.001 mg/kg) in 527 samples of edible fin fish
harvested from Chesapeake Bay and its tributaries (Maryland) over the
period 1976-80 or in 20 samples of roe and gonadal tissue (Eisenberg
& Topping, 1985).
No detectable quantity (< 1 µg/kg) of endrin was found in two
species of crayfish (Procambarus clarkii and P. acutus)
commercially harvested from dual-cropped ponds and from waters of the
Atchafalaya River Basin and the Mississippi River in southern
Louisiana, or in sediment and water collected from several ponds and
at the Basin three times during 1986 and 1987 (Madden et al., 1989).
Endrin was measured at levels of 0.4 and 66 µg/kg in American
eels (Anguilla rostrata) sampled at various sites between Lake
Ontario and the mouth of the St Lawrence river in 1982 (Castonguay et
al., 1989). Endrin was not detectable (< 0.002 mg/kg) in 'most'
composite samples (1-15 fish of 10 different species) collected from
10 sites on the Great Lakes and tributaries between 1980 and 1981,
although in a few cases concentrations up to 0.01 mg/kg were found
(Devault, 1985). Endrin was not present (< 0.005 mg/kg) in fillets of
Fall Run Coho salmon (Oncorhynchus kisutch) taken from 14 sites on
the Great Lakes in 1984. In most cases, three samples per site were
analysed, and the fish were 2-3 years old (Devault et al., 1988).
Johnson et al. (1988) measured the input of organochlorine pesticides
from precipitation and runoff to five small lakes peripheral to the
Canadian Great Lakes and the levels of residues in fish caught in the
lakes. While endrin was detectable in precipitation (at 0.46 and 0.54
ng/litre at the two sampling sites), none was measured in runoff water
and no detectable residue was found in fish.
The mean concentrations of endrin in 13 commercially important
fish species collected in the north-west Arabian Gulf varied between
1 and 28 µg/kg, and those in five species collected from Hor al-Hammar
Lake in Iraq in 1985 were 3-67 µg/kg wet weight of edible tissue.
Endrin residues were detected in approximately 90% of the fish
(DouAbul et al., 1987a). Samples of Barbus xanthopetrus collected in
the Shatt al Arab River and in Hor al Hammar Lake contained average
concentrations of 4 (none detected to 9) and 20 (11-27) µg/kg, while
Indian shed (Tenualosa ilistra) from the Shatt al Arab River
contained 80 (57-108) µg/kg (wet weight). Shrimp (Metapanaeus
affinis) did not contain endrin (DouAbul et al., 1987b). In 1984,
B. xanthopetrus from the River contained a mean concentration of 16
µg/kg wet weight, and those from the Lake, 154 µg/kg (range, 13-355);
Indian shed had mean concentrations of 41-147 µg/kg (range, none
detected to 236) (DouAbul et al., 1987c). Freshwater mussel
(Corbicula fluminea) collected in the Shatt al Arab River contained
166-540 µg/kg (range, 140-583) (DouAbul et al., 1988). Endrin was
present at concentrations of 1.9-12.2 µg/kg of muscle tissue (wet
weight) in three fish species and at 0.88-7.7 µg/kg in three Tilapia
species collected near Alexandria, Egypt, in 1985 (El Nabawi et al.,
1987).
Endrin was present at 0.003-0.004 mg/kg in black pomfret
(Parastromateus niger), mackerel (Rastrelliger kanagurta), and
marine vala (Chirocentrus sp.) and at 0.08 mg/kg in tuna (Euthynnus
affinis) collected off the Indian coast (Radhakrishnan & Antony,
1989). It was found in one sample of fish at 0.019 mg/kg wet weight
and in one shellfish sample at 0.034 mg/kg but in none of 312 other
specimens of 11 types of fish, crustaceans, and molluscs obtained from
five sites in Java, Indonesia (limit of detection, 0.01 mg/kg) (Koeman
et al., 1974).
No endrin was found (< 0.005 mg/kg) in 60 samples of fish and
shellfish collected in 19 rivers and their estuaries in Japan in 1974
(Japanese Environmental Agency, 1975).
The median concentration of endrin in the eggs of 15 adult
chinook salmon (Onchorhynchus tshawytscha) collected in Lake
Michigan in 1982 was 23.5 µg/kg wet weight (range, 3.9-126.3) (Giesy
et al., 1986).
Composite samples of whole fish of selected species were
collected in 1983 near the shores of 13 tributaries of Lake Michigan
and Grand Traverse Bay. Two of each of the following species were
collected from each site: common carp (Cyprinus carpio), bowfin
(Amia calva), channel catfish (Ictalurus punctatus), pumpkinseed
(Lepomis gibbosus), rock bass (Ambloplites rupestris), small-mouth
bass (Micropterus dolomieui), large-mouth bass (M. salmoides),
lake trout (Salvelinus namaycush), and pike (Esox lucius); the
composites comprised 3-11 fish. Endrin was not detected (limit, 0.005
mg/kg) (Camanzo et al., 1987).
Yellow perch (Perca flavencens) were sampled in eight
reservoirs and lakes in Ohio and Wisconsin, USA, in 1978-79. Endrin
was found in four fish at levels of 0.008-0.02 mg/kg, which were much
lower than the levels found of polychlorinated biphenyls, DDT and
dieldrin (Carline & Lawal, 1985).
5.1.4.3 Mixed species
Herons (Nyctanassa violacea), water snakes (Natrix spp.),
raccoons (Procyon lotor), channel catfish (Ictalurus punctatus),
crappies (Pomoxis spp.), frogs (Rana spp.), and crawfish
(Procambarus clarkii) were collected from three watersheds in
Louisiana, USA in 1978-79. Endrin was found in a heron at 0.014 mg/kg
and in a catfish at 0.022 mg/kg, but in no other case (limit of
detection, < 0.05 mg/kg) (Dowd et al., 1985).
5.1.5 Other food and feed
5.1.5.1 Cereals
Endrin has been used extensively for the control of insect pests
in rice. Typically, one to four applications are made, depending on
local conditions, the last application usually not later than one
month before harvest. Data on residue levels are available from India
(1969-70), Thailand (1968-70), the Philippines, Indonesia (1966), and
Venezuela (1969). The levels in polished rice were 0.01-0.04 mg/kg of
product (mean, 0.014 mg/kg), except in India where higher levels in
the order of 0.12 mg/kg were found. Bran, which is used mainly as a
component of poultry feed, contained a mean level of 0.35 mg/kg
(range, < 0.01-2.3 mg/kg), and low levels of delta-ketoendrin were
found (FAO/WHO, 1971).
Endrin has been used to only a limited extent on grain crops. The
residues in different types of treated grains in the USA were
generally below 0.05 mg/kg of product, except in oats in which levels
up to 0.5 mg/kg were found. In India, up to five applications on
sorghum gave residue levels below 0.02 mg/kg; in the USA, the levels
in sorghum were below 0.05 mg/kg. Straw of cereals contains higher
levels: rice straw had up to 3 mg/kg,, and sorghum straw up to 0.4
mg/kg (FAO/WHO, 1971).
Wheat imported into the United Kingdom in 1987-88 did not contain
endrin (< 0.01 mg/kg) (Osborne et al., 1989).
5.1.5.2 Fruit and vegetables
Endrin is occasionally used for control of field mice (voles). No
residue was found in apples at harvest (detection limit, 0.01-0.002
mg/kg of whole fruit) when it was sprayed on the ground under trees in
orchards in autumn or spring. The levels were sometimes higher in
fallen fruit, ranging from < 0.002 to 0.02 mg/kg of product (Horsfall
et al., 1970; FAO/WHO, 1971).
Only 14 of 15 000 samples of fruit and vegetables imported into
Sweden during the period 1981-84 contained endrin, at a maximum
concentration of 0.02 mg/kg (Anderson, 1986). The pesticide analysis
programme of the Swedish National Food Administration on fruit and
vegetables, including potatoes, showed no residue of endrin above the
limit of detection of 0.02 mg/kg in 13 724 samples analysed in 1985-87
(B.G. Ericsson, personal communication, 1990). The mean endrin
concentration in 137 samples of grape products (including seeds,
skins, marc and lees) in Italy was 6.2-16.2 µg/kg (Marinelli et al.,
1986). Seven of 306 samples of apples (five types) collected in
1980-83 from five regions of Italy contained endrin (limit of
detection, 0.001 mg/kg) (Foschi et al., 1985).
In Pakistan, in 16 samples of cucumber sprayed at the time of
maturity with a 0.05% endrin solution at a rate of 100 gallons/acre
(1123 litres/ha), the endrin concentrations ranged from 3.04 to 6.69
mg/kg. The residues persisted in the edible portion of cucumber up to
14 days and diminished thereafter (Illahi et al., 1986). Endrin was
found in 17% of samples of peas collected from fields and markets in
Faisalabad, Pakistan, at a level of 1.3-4.32 µg/kg. The residues
persisted for up to 12 days and then decreased (Illahi et al., 1987).
No endrin was found (< 0.02 mg/kg) in 141 samples of fruit and
vegetables from Pakistan in 1982-83 (Masud & Farhat, 1985).
In an analysis of soya bean and soya bean straw in a US
monitoring programme, seven of 177 samples of soya beans contained a
geometrical mean of < 0.001 (maximum, 0.03) mg/kg, and one of eight
straw samples contained < 0.01 mg/kg (Carey et al., 1978). Endrin was
used in up to four applications on sugar-cane in the USA, with an
interval of 45 days or longer between the last application and
harvest. The residues found in cane were usually < 0.05 mg/kg of
product (FAO/WHO, 1971).
5.1.5.3 Meat, poultry, and chicken eggs
Bovine fat (40 samples), pig fat (45 samples), calf fat (45
samples), sheep fat (22 samples), poultry fat (42 samples), and eggs
(44 samples) analysed in the Netherlands in 1983 had a median endrin
concentration of < 0.04 mg/kg (Dutch Agricultural Advisory Commission
on Environmental Pollutants, 1983). No endrin was found (detection
limit, 0.005 mg/kg) in samples of beef, pork, goat, mutton, poultry,
or eggs analaysed in Italy in 1985-87 (Cantoni et al., 1988) or in
'most' samples of pork, rabbit, or poultry analysed in
Rheinland/Pfalz, Germany, in 1981-84 (Kampe, 1985). Dietary surveys in
the United Kingdom demonstrated no endrin in meat (detection limit,
0.02 mg/kg) (United Kingdom Ministry of Agriculture, Fisheries and
Food, 1989).
Endrin was present in 10.8% of 2032 samples of bovine fat from
carcasses collected from slaughterhouses in Brazil, at a mean level of
0.01 mg/kg; the highest level was 0.09 mg/kg of tissue (De Paula
Carvalho et al., 1984). Endrin was present in hens' eggs from four of
five areas in Mexico, at concentrations of 0.004-0.11 mg/kg of whole
egg, and in 11 of 16 samples of chicken meat, at an average
concentration of 0.12 (none detected to 0.6) mg/kg on a fat basis
(Albert, 1990).
Endrin was detected in 86 of 221 samples of hens' eggs (78 native
and 143 commercial) collected in 1975-77 in Iran, at a mean
concentration of 0.017 (range, 0.003-0.13) mg/kg (Hashemy-Tonkabony &
Mosstofian, 1979). No endrin was found (limit of detection, 0.02
mg/kg) in samples of about 25 eggs of sawah ducks collected on 11
local markets in Java, Indonesia, in 1972 (Koeman et al., 1974).
It was found in 14 of 367 hens' eggs collected from 61 farms in
11 districts of Kenya in 1984; in three of the eggs, the level was >
0.2 mg/kg (Mugambi et al., 1989).
Heating, baking, frying, and steaming of tissues obtained from
broilers fed endrin at 10 mg/kg of diet for 8 weeks did not
significantly reduce the level of residues: raw, 28.2; baked, 20.8;
fried, 22.7; and steamed, 19.4 mg/kg of dry tissue (Ritchey et al.,
1972).
5.1.5.4 Milk and milk products
The mean endrin concentration in 20 samples of fresh buffalo milk
in Kalubia, Egypt, was 0.02 mg/kg of milk fat (range, < 0.01-0.03
mg/kg (Abdou et al., 1983). Cows' milk (39 samples) collected in four
areas of Bagdad, Iraq, in 1981-82 contained a mean of 60 (none
detected to 400) µg/litre (Al-Omar et al., 1985b).
The average concentration of endrin in 10 samples of evaporated
cows' milk from three main cities in the agricultural region of Mexico
was < 0.007 mg/litre of milk fat (Albert et al.,1982). Endrin was
found in powdered milk at an average concentration of 0.06 mg/kg and
in cheese at a concentration of 10-27.2 mg/kg on a fat basis (Albert,
1990).
The level of endrin in milk in the USA was < 0.001 mg/litre (on
a fat basis) (FAO/WHO, 1971). No endrin (< 0.5 µg/litre) was found in
308 samples from bulk transports of milk collected in Ontario, Canada,
in 1977 (Frank et al., 1979) or in 359 samples collected in 1983
(Frank et al., 1985).
No residue was found (detection limit, < 0.005 mg/kg) in samples
of milk, cream, butter, and cheese in Italy (Cantoni et al., 1988) or
in 12 samples of cows' milk collected in 1984-86 from different areas
of Spain (< 0.01 mg/kg fat) (Barcelo & Puignou, 1987).
5.1.5.5 Fat and oils
The most important use of endrin is for the control of insects in
cotton, the number of applications being 1-12; cottonseed oil is used
for cooking and for the manufacture of margarine, while the extracted
cake is used as cattle feed. Endrin is thus present both in the
cottonseed and in the edible oil and cakes. In a study of the
extraction processes, it was found that alkali washing and bleaching
had no marked effect but that deodorization reduced the endrin levels
to below the limit of detection (0.03 mg/kg) (Smith et al., 1968).
In field studies carried out in the USA, cottonseed contained
endrin at a maximum of 0.1 mg/kg, although the levels were usually
much lower. delta-Ketoendrin was not detected. The levels in crude,
decolourized, and deodorized oil in Venezuela and Brazil were all <
0.02 mg/kg of product. Spot samples of refined cottonseed oil from
California, USA, contained < 0.03 mg of endrin and < 0.02 mg of
delta-ketoendrin ( limits of detection) (FAO/WHO, 1971).
One-hundred-and-ten samples of raw oil and of oil at various
stages of processing, i.e., neutralized, hydrogenated, decolourized,
deodorized, and shortenings, were collected from seven oil processing
factories in Iran in 1974. Endrin was found only in raw and
neutralized vegetable oils, at concentrations of 0.004-0.005 mg/litre.
Raw imported and native oils contained < 0.01 mg/litre, except for
native sunflower oil which contained 0.026 mg/litre (Hashemy-Tonkabony
& Soleimani-Amiri, 1976).
Endrin was found in 60 samples of six varieties of the major
edible oils and oil seeds used in India, including groundnut, sesame,
mustard, coconut, and hydrogenated vegetable oils, collected from a
market in Lucknow. Vegetable oil contained 6 µg/litre, mustard oil, 72
µg/litre, and sesame oil, 1690 µg/kg. Of the different types of oil
seeds, only mustard seed contained endrin, at 22 µg/kg (Dikshith et
al., 1989a).
Endrin was found at a mean concentration of 0.184 (0.097-0.288)
mg/kg in samples of cod-liver oil analysed in Germany in 1985 (Ali,
1986). No residue was found in vegetable oils and fats imported into
the United Kingdom (detection limits, 0.02 and 0.001 mg/kg,
respectively) (Abbot et al., 1969).
5.1.5.6 Animal feed
Residues of endrin in pressed cottonseed cakes arise primarily
from the 1-5% of oil left in the cake after extraction. The residues
in cakes from Brazil, India, the USA, and Venezuela were mainly <
0.01-0.02 mg/kg product, levels up to 0.08 mg/kg were found
occasionally (FAO/WHO, 1971). The mean concentration of endrin in 32
samples of cattle feed from a local market in India was 0.020 mg/kg
(range, 0.013-0.027 mg/kg) (Dikshith et al., 1989b). No endrin was
found in 79 samples of cattle feed in Pakistan (Parveen & Masud,
1987). Endrin was not present in samples of domestic and imported
animal feed analysed in the USA in 1981-86 (Luke et al., 1988).
None of 42 samples of chicken feed collected from 61 farms in 11
districts of Kenya in 1984 contained endrin (Mugambi et al., 1989).
5.1.6 Miscellaneous products
Endrin was found in 5 of 25 tobacco samples imported into Germany
at concentrations of 25-50 µg/kg (Cetinkaya, 1988). No endrin was
found in cigarettes of 14 brands collected in Finland in 1960-84
(Mussalo-Rauhamaa et al., 1986). An average content of 0.006
µg/cigarette (range, none detected to 0.02 µg/cigarette) was found in
Switzerland (Zimmerli & Marek, 1973).
When raw cotton imported into Germany from 15 countries was
analysed, endrin was found in samples from the USSR and Mexico at a
concentration of 3 µg/kg (Cetinkaya & Schenek, 1987).
5.2 Exposure of the general population
5.2.1 Total-diet studies
Studies on complete prepared meals in the USA, started in May
1961 and continued to the present, have shown the occasional presence
of small amounts of endrin (Williams, 1964; Cummings, 1965, 1966;
Duggan et al., 1966, 1967; Martin & Duggan, 1968; Corneliussen, 1969,
1970, 1972; Manske & Corneliussen, 1974; Manske & Johnson, 1975;
Johnson & Manske, 1976, 1977; Manske & Johnson, 1977; Johnson et al.,
1981a, 1984). These measurements indicate that the total average daily
intake of endrin from food decreased from 0.009 µg/kg body weight in
1965 to 0.0005 µg/kg body weight in 1970 (Duggan & Lipscomb, 1969;
Duggan & Corneliussen, 1972), with a further decrease subsequently. In
total-diet studies of adults in the USA, representative foods were
purchased in 27 US cities in 1980-82; the daily intake of endrin was
found to be < 0.001 µg/kg body weight in 1978, but none was detected
in 1979, 1980, or 1981-82 (Gartrell et al., 1986a).
Further studies involved retail purchase of 234 food items
representative of the total diet of eight US population groups in
1982-84 and preparing them for consumption. The daily intake of endrin
in the groups, which included people aged 6-11 months, 2 years, 14-16
years (females), 14-16 years (males), 25-30 years (females), 25-30
years (males), 60-65 years (females), and 60-65 years (males), was
0.1-0.2 ng/kg body weight (FDA, 1988; Gunderson, 1988).
Endrin was not present in the total diets of infants and toddlers
in the USA during 1974-75 (Johnson et al., 1979). It was found in one
infant food sample at 0.011 mg/kg and in one sample of toddler food at
0.009 mg/kg of food in a study in 1975-76 (Johnson et al., 1981b).
Very low residue levels were found occasionally in market-basket
samples representing the average 2-week diets of infants (98 samples)
and toddlers (110 samples) collected in 10 cities in four geographic
areas of the USA in 1977-78 (Podrebarac, 1984). In total-diet studies
of infants and toddlers in the USA, representative foods were
purchased in 13 US cities in 1980-82; the daily intake of endrin by
infants was found to be < 0.001 µg/kg body weight in 1978, and that
by toddlers, < 0.001 µg/kg body weight in 1979, but none was detected
in the other years (Gartrell et al., 1986b).
Fresh food was bought from four retail grocery stores in Toronto,
Canada, in 1985 and combined in five food composites: fresh meat and
eggs, root vegetables (including potatoes), fresh fruit, leafy and
other surface vegetables, and cows' milk. The concentrations of endrin
in the five composites were used to estimate the annual dietary intake
of endrin from products in Ontario. Endrin was detected in all
composites except eggs and meat; the concentrations were 0.32 µg/kg in
leafy vegetables, 0.27 µg/kg in fruit, 0.37 µg/kg in root vegetables,
and 0.27 µg/kg in milk. The total annual intake was estimated to be
31.8 µg/person (Davies, 1988).
In a total-diet study carried out in the United Kingdom in
1985-88, 25 samples were obtained in 1984-85 which comprised the 16
food groups considered most likely to contain residues of
organochlorine compounds. No endrin was detected (limit of detection,
0.001-0.02 mg/kg, depending on the food group). Endrin was also not
detected (< 0.01 mg/kg) in 176 samples of pulses purchased from
retail outlets in 1986-87, except in 3 of 20 samples of mung beans in
which a mean value of < 0.01 mg/kg (range, none detected to 0.06
mg/kg) was found (United Kingdom Ministry of Agriculture, Fisheries
and Food, 1989). No endrin was detected in complete prepared meals
during surveys in the United Kingdom in 1965 (Robinson & McGill, 1966;
McGill & Robinson, 1968). Similar results were obtained in Switzerland
in 1973 (Zimmerli & Marek, 1973), and very low levels were found in
two of 73 samples analysed in 1985 (Wüthrich et al., 1985). No endrin
residues were found in total-diet studies carried out in the
Netherlands in 1976-78 (De Vos et al., 1984).
5.2.2 Levels in human tissues
Although the concentrations of many chlorinated hydrocarbon
insecticides, such as DDT, dieldrin, hexachlorocyclohexanes, and
hexachlorobenzene, and of their metabolites in blood or adipose tissue
of the general population or of occupationally exposed workers have
been found to be an excellent index of the level of exposure of the
general population, this is not the case for endrin, because it is
eliminated rapidly.
5.2.2.1 Adipose tissue
Except in a few cases, endrin was not demonstrated in adipose
tissue samples from the general population in the USA in 1962-66
(Hoffman et al., 1964, 1967), 1964 (Hayes et al., 1965; Zavon et al.,
1965), 1970-74 (Kutz et al., 1979a,b), and 1975-79 (US EPA, 1983);
Canada in 1967-68 (Kadis et al., 1970); Mexico in 1975 (Albert et al.,
1980); Argentina in 1968-69; (Wassermann et al., 1969); Belgium in
1968-69 (Wit, 1971); the United Kingdom in 1961 (Hunter et al., 1963),
1964 (Robinson et al., 1965), and 1965-67 (Egan et al., 1965; Abbott
et al., 1968, 1972); the Netherlands in 1969 (Wit, 1971); Switzerland
in 1972 (Zimmerli & Marek, 1973); Germany in 1970 (Acker & Schulte,
1974); France in 1971 (Fournier et al., 1972); Spain in 1978 (Herrera
Marteache et al., 1978); India in 1964 (Dale et al., 1965); or Western
Australia in 1965-66 (Wassermann et al., 1968).
No endrin was found in 91 samples of adipose tissue obtained at
autopsy in Kingston, Ontario, Canada, in 1979 and 1981 or in 84
samples from Ottawa in 1980 and 1981 (Williams et al., 1984), or in
adipose tissue obtained at autopsy from 92 males and 49 females in
Ontario municipalities in 1984 (limit of detection, 2.4 µg/kg)
(Williams et al., 1988).
These results indicate that endrin is either absent or present at
very low levels in the adipose tissue of the general population. It is
therefore surprising that Kanitz & Castello (1966) reported the
presence of endrin in nine adipose tissue samples from Liguria, Italy,
at a mean concentration of 0.93 mg/kg of tissue. The highest
concentration was 2.49 mg/kg. Pavan et al. (1987) found endrin at 0.1
and 0.3 mg/kg in 2 of 92 samples of adipose tissue obtained at surgery
from people living in the Province of Turin, Italy. In areas where
endrin has been used extensively, however, such as India and the lower
Mississippi, it has never been found in human adipose tissue (Brooks,
1974).
One of 62 adipose tissue samples obtained at surgery from people
in Ciudad Juarez, Mexico, in 1977-78 contained endrin at 0.02 mg/kg
(Redetzke et al., 1983).
5.2.2.2 Organs
In samples of liver, kidney, gonad, and brain obtained from the
general population of Alberta (Canada), no residue of endrin was
detected (Kadis et al., 1970).
5.2.2.3 Blood
No endrin was detected (limit of detection, 0.01 mg/kg) in 4000
blood samples from the general US population in 1976-80 (US E P A,
1983), or in areas where endrin has been used extensively, such as
India and the lower Mississippi (Brooks, 1974), or in 26 blood samples
from the general population in Nigeria (Atuma, 1985).
5.2.2.4 Breast milk
Endrin was not detected in breast milk in studies in the USA in
1966-78 (Strassman & Kutz, 1977; Currie et al., 1979; Kutz et al.,
1979a; Barnett et al., 1979), in El Salvador and Guatemala (De Campos
& Olszyna-Marzys, 1979), in Belgium, Italy, and The Netherlands
(Kanitz & Castello, 1966; Hendrickx & Maes, 1969; Wegman & Greve,
1974), and in Japan (Yakushiji et al., 1979). No endrin was detected
(< 0.01 mg/litre) in 50 breast milk samples from mothers (aged 18-32
years) in Leiden, The Netherlands, in 1969 (Tuinstra, 1971). It was
found in one of 12 samples from mothers aged 21-37 in Pavia, Italy, in
1988, at a concentration of 0.01 µg/kg of whole milk, but not in four
samples collected in Crotone, southern Italy (Bianchi et al., 1988).
5.2.2.5 Appraisal of exposure of the general population
The occasional presence of low concentrations of endrin in the
air of areas where endrin is used in agriculture cannot be considered
a significant source of contamination for the general public. The very
low concentrations that have been found in surface and drinking-water
are also of little significance for public health.
The source of exposure that may be relevant is dietary intake.
Apart from accidental contamination, however, intake of endrin by the
general population in the countries examined has been and is still far
below the maximum acceptable daily intake of 0.2 µg/kg body weight
established by the Joint FAO/WHO Meeting in 1970 (FAO/WHO, 1971). This
applies equally to the total intake, when the intake from dietary
sources is added to that from air and water.
Endrin has not been demonstrated in the large number of samples
of organs, adipose tissue, blood, and breast milk analysed in
different countries, even in areas where endrin is or was used
extensively.
5.3 Occupational exposure during manufacture, formulation and use
5.3.1 Manufacture and formulation
Endrin has not been detected in the blood, plasma, or urine of
workers exposed occupationally to endrin (Hayes & Curley, 1968; Jager,
1970). Endrin was detected in blood only after accidental
over-exposure. Jager (1970) estimated that the threshold level of
endrin in the blood, below which signs or symptoms of intoxication do
not occur, lies between 0.05 and 0.10 mg/litre. He estimated the
half-life of endrin in the blood to be in the order of 24 h.
The total exposure of workers in a manufacturing and formulation
plant was estimated on the basis of determinations of the quantity of
the endrin metabolite anti-12-hydroxyendrin in urine. Urine of
workers exposed to endrin for seven days had concentrations of up to
360 µg/g of creatinine; no unchanged endrin was found. Assuming an
average daily excretion of 1.5 g creatinine per day, the total daily
excretion of anti-12-hydroxyendrin in the urine may be up to 540 µg.
This gives a minimal absorption of 0.5 mg endrin, indicating that
inhalation of dusts and absorption through the skin may be significant
during occupational exposures in manufacture. It is not unreasonable
to assume that, as in other species, approximately half of all the
endrin absorbed is excreted in the urine as anti-12-hydroxyendrin,
since both endrin and this metabolite are present in the faeces of
workers (Ottevanger &Van Sittert, 1979; Baldwin & Hutson, 1980 ).
Thus, 1 mg/day may be the more accurate figure for exposure in this
manufacturing plant. The concentration of anti-12-hydroxyendrin in
urine decreased more slowly than the concentrations of endrin in
blood, with a half-life of 55-75 h (Van Sittert, 1985).
5.3.2 Application
Endrin is applied in agriculture by high-pressure spraying with
a hand gun, spraying orchards with a power air blast or boom to
control mice, dusting potatoes, spraying row crops, or application
from aircraft. These methods of application result in dermal and
respiratory exposures.
The potential dermal and respiratory exposure of workers applying
endrin formulations in the field has been quantified in a few studies.
Respiratory exposure to endrin during spraying of orchards,
high-pressure spraying of crops, and piloting of aircraft varied from
0.01 to 0.14 mg/h; dermal exposure during such activities was
0.01-1.64 mg/h. The activity that caused the most exposure was dusting
potatoes, which was associated with a respiratory exposure of 0.41
mg/h and a dermal exposure of 18.7 mg/h. Total exposure, calculated as
a percentage of a toxic dose/h = {dermal exposure (mg/h) +
[respiratory exposure (mg/h) x 10]} [dermal LD50 (mg/kg) x 70] x
100, was 0.21-1.5% (Durham & Wolfe, 1962) (see Table 11). These
figures show that although endrin is acutely highly toxic it can be
used safely when reasonable precautions are taken (Wolfe et al., 1963;
Jegier, 1964; Wolfe et al., 1967; Hayes, 1975).
Endrin was not found in the blood of 20 pesticide sprayers or in
19 controls in Choluteca, southern Honduras (Steinberg et al., 1989).
5.3.3 Appraisal of occupational exposure
No residues were found in healthy workers. The range of threshold
levels of endrin in blood below which no sign or symptom of
intoxication occurs has been estimated to be 50-100 µg/litre. The
half-life of endrin in blood may be in the order of 24 h following
occupational exposure. The concentration of anti-12-hydroxyendrin in
the urine decreased more slowly than the concentration of endrin in
blood, with a half-life of 55-75 h.
Table 11. Studies on potential exposure of agricultural workers to endrin, using direct methods
Activity Exposure
Respiratory Dermal Totala (%) toxic Reference
(mg/h) dose/h
mg/m3 mg/h
Spraying orchard cover crops for mouse 0.01b 2.6 0.21 Wolfe et al. (1963)
control
High-pressure hand-gun spraying orchard 0.01b 3 0.25 Wolfe et al. (1967)
cover crops for mouse control
Operating air-blast or boom sprayers treating 0.01b 2.5 0.21 Wolfe et al. (1967)
orchard cover crops for mouse control
Dusting potatoes 0.41b 18.7 1.5 Wolfe et al. (1963)
Spraying row crops NDb,c 0.15 (0.01)d Jegier (1964)
Piloting during air application of spray 0.05 0.08b 1.18 0.29 (0.16)e Jegier (1964)
aFor a 70-kg man on the basis of dermal LD50 for male rats (18 mg/kg body weight) using the formula given
by Durham & Wolfe (1962)
bMeasured by respirator pad
cNot detected
dOriginal values calculated on the basis of maximal exposure; recalculated values shown in parentheses are
based on mean exposure.
eCalculation based on published data on dermal and respiratory exposure (note in Wolfe et al., 1967)
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Laboratory animals
6.1.1.1 Oral administration
Rat: One male rat was fed 14C-endrin at a level of 30 mg/kg
of diet for 8 days. About 60-70% was excreted on the first day; after
three days, the faeces contained more then 80% of the administered
radiolabel; by day 9, 84% had been excreted; and there appeared to be
a level of saturation after 6-7 days of feeding. Only 0.5% was found
in the urine. About 75-80% of the label in the faeces was in at least
two different metabolites. The adipose tissue stored 3-4 mg/kg, giving
a storage rate of about 10 (FAO/WHO, 1971).
After female rats were given a single oral dose of 14C-endrin
at 16, 64, or 128 µg/kg body weight, excretion was rapid. The
biological half-life of the doses of 16 and 64 µg/kg was 1-2 days;
however, that of 128 µg/kg was approximately 6 days, showing that
excretion of higher doses is slower (Korte et al., 1970).
Six CFE rats of each sex were treated with a single oral dose of
0.5 mg 14C-endrin in arachis oil (approximately equivalent to
2.5-3.0 mg/kg body weight), and the radiolabel excreted in urine and
faeces was measured over 3 days. The animals were then killed and the
radiolabel measured in tissues. A sex difference was noted in the rate
of elimination in faeces: 66% of the dose was excreted in 3 days by
males and 37% by females; excretion was slower in females but tended
to increase daily between days 1 and 3. Small quantities of radiolabel
were excreted in the urine, females excreting three times more than
males. No radiolabel was found in exhaled air (Hutson et al., 1975).
The results are summarized in Tables 12 and 13.
Three rats of each sex were each given a single oral dose of 8 µg
14C-endrin in peanut oil (by gavage) daily for 12 days. A steady
state (at which the excreted amount equalled the daily intake) was
reached after about 6 days. Females stored about twice as much (27%)
as males (14%). The radiolabel was excreted mainly in the faeces:
after the first 24 h, 70-75% of the radiolabel was found in faeces as
hydrophilic metabolites; subsequently, only metabolites were present.
Four days after the last dose, males retained only 5% and females 15%
of the administered radiolabel (Klein et al., 1968; Korte et al.,
1970).
Table 12. Rates of excretion of radiolabel by rats treated with a
single oral dose of 14C-endrin (percentage of
radioactivity administered)
Sex Urine Faeces
Day 1 Day 2 Day 3 Day 1 Day 2 Day 3
Male 1.3 0.6 0.6 30.6 14.4 21.2
Female 1.8 2.5 2.9 2.3 10.7 24.2
Table 13. Recovery of radiolabel in rat tissues 3 days after a single
oral dose of 14C-endrin (percentage of radioactivity
administered)
Sex Urine Faeces Liver Kidneys Fat Skin Remaining Total
carcass
Males 2.7 66.2 1.2 0.6 1.7 2.3 12.2 86.9
Females 7.5 37.2 2.0 0.4 8.0 4.0 28.1 87.2
Rabbit: A Dutch strain male rabbit was given two oral doses of
4.7 mg 14C-endrin in olive oil at an interval of 14 days. Between
days 1 and 13, 37% of the first dose was excreted in the urine and 49%
in the faeces; the second dose was eliminated similarly. By day 49,
50% had been excreted in urine and 47% in faeces. Faecal excretion was
rapid, being almost complete within 24 h, and consisted virtually
entirely of unchanged endrin. The urine contained only metabolites
(Bedford et al., 1975a). The excretion of metabolites in rabbits thus
appears to differ considerably from that in rats: approximately half
of a dose of 14C-endrin is excreted in the urine of rabbits and
approximately 2% in rats; endrin metabolites are excreted in rat
faeces over several days (after a single oral dose), whereas in
rabbits faecal excretion is rapid, being almost complete within 24 h,
and consists virtually entirely of unchanged endrin. The probable
explanation is that the molecular weight threshold for biliary
secretion of anions is 325 ± 50 in rats and 475 ± 50 in rabbits (Hirom
et al., 1972). The glucuronide and sulfate conjugates of
monohydroxyendrin have molecular weights of 572 and 470, respectively.
Therefore, conjugates of the endrin metabolites are eliminated in the
bile and faeces of rats and in the urine in rabbits.
Dog: Three beagles were fed a diet containing endrin at a
concentration equivalent to 0.1 mg/kg body weight for 128 days; two
other animals were used as controls. The concentration of endrin in
blood was determined at weekly intervals. The time to reach a plateau
in blood was less than 1 week, and no significant increase in the
concentration of endrin in blood was found during this period. The
average concentration between day 9 and day 128 was 4 µg/litre. The
concentration of endrin in eight organs and tissues of dogs killed 7
days after termination of exposure was < 0.2 mg/kg of tissue; except
that the spleen of one dog contained 2.6 mg/kg, and adipose tissue
contained 0.2-0.8 mg/kg (Richardson et al., 1967).
6.1.1.2 Intravenous administration
Mouse: The concentrations of endrin were determined in tissues
from groups of five adult male CFI mice given endrin intravenously at
5 mg/kg body weight (LD90) in dimethyl sulfoxide. The concentrations
prior to convulsions (about 10 min after injection) were approximately
60 mg/kg in liver, 20 mg/kg in brain and omental fat, and
approximately 5 mg/litre in blood. The concentration in whole brain 15
min after an intravenous dose of 1.5 mg/kg body weight (the dose that
caused ataxia in 90% of animals; TD90) was 9.4 mg/kg. No endrin was
detected in the bile of animals with a bile fistula dosed
intravenously with the TD90 in samples collected after 0.5, 1, or 2
h (Walsh & Fink, 1972).
Rat: Male Holtzman rats with or without a bile fistula were
given a single intravenous dose of 14C-endrin at 0.25 m/kg body
weight. More than 90% of the excreted radiolabel was found in the
faeces of intact animals over the 7-day period after dosing or in the
bile of animals with fistulas over 4 days. The mean total percentage
recovery of administered radiolabel in faeces, urine, and carcasses
was 97% from intact animals 7 days after dosing, and 94% from animals
with a bile fistula 4 days after dosing (Cole et al., 1970). No
unchanged endrin was found in bile; the major metabolite was
anti-12-hydroxyendrin (see section 6.2.1).
Rapid excretion was observed in rats given two intravenous
injections of 14C-endrin at 0.1 mg/kg body weight at an interval of
4 days. Excretion of the radiolabel was exponential and occurred
mainly with faeces; only hydrophilic metabolites were present. With a
dose of 200 µg/kg body weight, male rats retained 5.2% and females
12.1% of the administered dose 20 days after the second injection. The
biological half-life of endrin after a single intravenous dose of 200
µg/kg body weight was 2.5-3 days in male rats and 4 days in females
(Klein et al., 1968; Korte et al., 1970; Brooks, 1974).
Rabbit: When rabbits were given 14C-endrin intravenously, the
radiolabel was excreted mainly in the urine and only as metabolites.
A probable explanation for the difference in excretion pattern after
oral and intravenous administration is that much lower doses
(micrograms compared with milligrams) were given intravenously (Korte
et al., 1970).
6.1.2 Domestic animals
Twelve cows were fed hay from endrin-sprayed alfalfa containing
an average of 1.9, 2.8, or 3.7 mg/kg endrin; the average daily intake
of individual animals ranged from 0.04 to 0.11 mg/kg body weight. The
average concentrations of endrin in the milk were < 0.05, 0.14, and
0.15 mg/litre, respectively. When endrin dissolved in soya bean oil
was fed to 11 dairy cows, levels > 1 mg/kg body weight were required
in order for significant quantities of endrin to be detected in milk
(Ely et al., 1957).
Dairy cows (eight Jerseys and six Guernseys) were fed diets
containing endrin at 0, 0.1, 0.25, 0.75, or 2.0 mg/kg of diet for 12
weeks. No residues were found (limit of detection, 0.01 mg/litre) in
the milk of animals that received 0.1 mg/kg, but up to 0.02 mg/litre
was found in milk of animals fed 0.25 mg/kg and up to 0.04 mg/litre
with 0.75 mg/kg of diet; the highest dose resulted in residues in milk
of 0.1 mg/litre. The endrin content of brain, heart, liver, kidneys,
body fat, and muscles was < 0.1 mg/kg, but renal fat contained up to
0.8 mg/kg (Kiigemagi et al., 1958).
The concentrations of endrin in milk of cows given feed
containing endrin at approximately 0.05, 0.14, and 0.30 mg/kg of whole
feed for 5 weeks were 0.004, 0.01, and 0.018 mg/litre, respectively
(Williams et al., 1964). A steady (plateau) level in milk was reached
after about 15 days.
The concentration of endrin in the milk of dairy cows given feed
contaminated with relatively low levels of endrin rose rapidly within
a few hours to days and levelled off at a plateau characteristic for
each concentration in the feed. The average milk:diet ratio for endrin
was 0.07 for feed levels of 0.05-0.3 mg/kg of diet (Biehl & Buck,
1987).
Two lactating cows were fed 14C-endrin for 21 days at an
overall dietary concentration of 0.1 mg/kg of diet, which was
considered to be comparable to the highest dose that cows are likely
to receive in cottonseed cake. Excretion of radiolabel in milk, urine,
and faeces reached a plateau 4-9 days after start of treatment.
Approximately 3% of the radiolabel was excreted in milk, 65% in urine,
and 20% in faeces. Unchanged endrin was not found in urine, but about
30% of the radiolabel in faeces and all of the 0.003-0.006 mg/litre
found in milk was endrin. The concentration of endrin equivalent
residues was 0.001-0.002 mg/kg in meat and 0.02-0.10 mg/kg in fat;
most of the radiolabel in fat consisted of endrin (Baldwin et al.,
1976).
Steers, hogs, and lambs fed diets containing endrin at 0, 0.1,
0.25, or 0.75 mg/kg of diet for 12 weeks had residues of < 0.1 mg/kg
in red meat, liver, and kidneys and of 0.02-0.2 mg/kg in body fat.
Feeding endrin at 2 mg/kg of diet to steers for 12 weeks resulted in
residues of 0.9 mg/kg in fat and 0.2-0.3 mg/kg in red meat, liver and
kidneys (Terriere et al., 1958). The biotransfer factors for endrin in
beef and milk were directly proportional to the octanol-water
partition coefficients, while the bioconcentration factor for endrin
in vegetation was inversely proportional to the square root of the
octanol-water partition coefficient (Travis & Arms, 1988).
Six weeks after the start of feeding seven Delaware X New
Hampshire male chicks and eight weeks after the start of feeding seven
White Leghorn pullets a diet containing endrin at 0.1 mg/kg, the
residues in eggs and meat were < 0.1 mg/kg and that in fat, 0.6
mg/kg. At a dietary level of 0.25 mg/kg, the residue levels were
0.2-0.3 mg/kg in eggs, 0.1 mg/kg in breast meat, and about 1 mg/kg in
fat. With 0.75 mg/kg of diet, the levels were 0.4 mg/kg in eggs, 0.24
mg/kg in breast meat, and 3.1 mg/kg in fat (Terriere et al., 1959).
14C-Endrin was administered daily in corn oil in gelatin
capsules to six laying hens at a concentration equivalent to 0.13
mg/kg of total diet for 21 weeks. Ingestion and elimination in eggs
and excreta were almost balanced after about 16 weeks. The residue
levels in eggs were 0.11-0.18 mg/kg and were found in the yolk; none
of the known metabolites was detected. The levels of endrin equivalent
were about 0.01 mg/kg in breast meat and 0.1 mg/kg in leg meat; higher
levels were found in the liver (0.47 mg/kg), kidneys (0.17 mg/kg), and
fat (1 mg/kg). The residues were accounted for by unchanged endrin,
except in the liver and kidneys, where part probably consisted of
polar metabolites. About 50% of the administered radiolabel was
excreted in the faeces, 10% of which was in unchanged endrin (Baldwin
et al., 1976).
6.1.3 Human beings
The concentrations of endrin in the blood of workmen exposed to
endrin were generally below the level of detection: endrin was not
found in plasma (< 3 µg/litre) or fat (< 0.03 mg/kg) of workers
exposed to endrin for an average of 88 days (Hayes & Curley, 1968). No
endrin was found in the blood of healthy people working in an endrin
manufacturing plant between 1964 and 1970, at an initial limit of
detection of 10 µg/litre, improved after 1965 to 5 µg/litre (Jager,
1970).
Residues of endrin have been found in blood only in individuals
with signs of recent intoxication or who have recently had excessive
exposure (see section 9.2.2). Endrin appears to be eliminated rapidly
from the human body.
6.1.4 Systems in vitro
Isolated liver preparations from Holtzman rats were perfused with
a solution containing 14C-endrin at 0.003 mg/ml. Within 1 h, 50% of
the radiolabel appeared in the bile; and in 6 h, more than 90% of the
total label was found (Cole et al., 1970). With the same dose,
radiolabel appeared 2-12 times faster in the bile of livers isolated
from male rats as in that of livers from females, which may account
for the lower toxicity and lower storage of endrin in adipose tissue
in male rats (Klevay, 1971). After perfusion of albino rat liver with
a physiological solution containing 40 µg of 14C-endrin, both endrin
and hydrophilic metabolites were found (Altmeier & Korte, 1969).
6.2 Biotransformation
Information on the metabolism of endrin up to 1967 was reviewed
(Soto & Deichmann, 1967; Brooks, 1969).
6.2.1 Experimental animals
A number of investigations have been carried out since 1970 to
elucidate the identity of several metabolites of endrin in rats
(Baldwin et al., 1970; Richardson et al., 1970; Hutson et al., 1975;
Bedford & Hutson, 1976), rabbits (Bedford et al., 1975a; Hutson,
1981), cows and chickens (Baldwin et al., 1976). The 12-hydroxy
derivative was reported to be present in the faeces of rats (Baldwin
et al., 1970), and the hydroxyl group was assigned tentatively as syn
to the epoxide ring. The stereochemical configuration was subsequently
shown to be anti to the epoxide group (Baldwin et al., 1973), and
this configuration was confirmed by the synthesis of anti-12-
hydroxyendrin (also called 9- anti-hydroxyendrin) (Bedford & Harrod,
1973; Bedford et al., 1986a). The chemical structures of these
compounds are shown in Figure 2; the chemical names are given in Annex
I.
Formation of anti-12-hydroxyendrin ( III), together with its
sulfate and glucuronide conjugates, is considered to be the major
route of metabolism of endrin. Four other metabolites have been
reported, but their concentrations are generally smaller than that of
anti-12-hydroxyendrin and its conjugates. These four metabolites are
syn-12-hydroxyendrin ( II; tentative identification),
3-hydroxyendrin ( IV; synthesized and structure confirmed by Bedford
et al., 1986b), 12-ketoendrin ( V), and the product of formal
hydroxylation of endrin, the 4,5- trans-dihydroisodrindiol ( VI;
tentative structure). The trans-diol ( VI) is a minor metabolite in
both rats and rabbits; it may be formed via an oxidation-reduction
pathway involving intermediates of the corresponding ketol ( VII).
Each of the hydroxy compounds is also excreted partly as its sulfate
or glucuronide in the urine of animals (Bedford et al., 1975a,b;
Hutson et al., 1975).
The three monohydroxylated derivatives of endrin, syn- and
anti-12-hydroxyendrin ( II and III) and 3-hydroxyendrin ( IV),
are the products of the action of liver microsomal monooxygenases on
endrin (Bedford & Hutson, 1976). These alcohols are also conjugated to
glucuronides and sulfates to some extent in the liver. Comparative
metabolic studies with rat liver microsome preparations have shown
that free syn-12-hydroxyendrin, but not its free anti-isomer, is
the precursor of 12-ketoendrin ( V) (Hutson & Hoadley, 1974).
Rats exhibit a sex difference in the rate of metabolism. The
major metabolite in animals of each sex is anti-12-hydroxyendrin,
which is excreted via the bile as the glucuronide; this undergoes
enterohepatic circulation and is eliminated as the aglycone in the
faeces, together with two minor metabolites, 3-hydroxyendrin and
4,5- trans-dihydroisodrindiol. Male rats produce the metabolite at a
higher rate than do females. The major urinary metabolite in male CFE
rats was 12-ketoendrin, while females excreted mainly
anti-12-hydroxyendrin O-sulfate. Endrin and the lipophilic
metabolite 12-ketoendrin were the major compounds found in the organs
and tissues of male and female CFE rats 3 days after a single oral
dose of endrin, but the ratio of endrin:12-ketoendrin was 2/1 in
females and 1/8 in males. Thus, 12-ketoendrin constituted most of the
radiolabel in the liver and kidneys of males and endrin that in the
kidneys of females (Hutson et al., 1975; Hutson, 1981).
The metabolism of endrin in rabbits is superficially different
from that in rats. The major metabolite is still
anti-12-hydroxyendrin, but it is conjugated with sulfate and
eliminated in the urine. Some syn-12-hydroxyendrin was also detected
as its sulfate in urine, and perhaps conjugation and elimination
prevented further oxidation to 12-ketoendrin. The respective
glucuronide conjugates were also eliminated in the urine, as were the
glucuronides of 3-hydroxyendrin and the 4,5- trans-diol ( VI)
(Bedford et al., 1975b; Hutson, 1981).
Studies with 14C-endrin in lactating cows showed that the
residues in milk and body fat consisted of unchanged endrin, although
traces of 12-ketoendrin were consistently found in fat. As in rats,
anti-12-hydroxyendrin was the major metabolite in urine and faeces,
the urine being the major excretory route, as in rabbits.
12-Ketoendrin and syn-12-hydroxyendrin were minor metabolites in cow
urine (Baldwin et al., 1976). Thus, although the metabolic pathways of
endrin in cows are qualitatively similar to those in rats and rabbits,
quantitative differences are seen in faecal and urinary excretion.
In hens, only endrin was found as a residue in meat, fat, and
eggs. Unchanged endrin accounted for about 10% of the radiolabel in
excreta, and the major metabolite was anti-12-hydroxyendrin and its
sulfate conjugate. No 12-ketoendrin was detected in tissues, eggs, or
excreta. The metabolism of endrin in hens is fundamentally similar to
that in rats, rabbits, and cows, except that they produce neither
syn-12-hydroxyendrin nor the related 12-ketoendrin. The rate of
metabolism, however, was much lower than in cows (Baldwin et al.,
1976). The absence of 12-ketoendrin in birds was confirmed in a study
of four species killed by endrin (Stickel et al., 1979). Hutson et al.
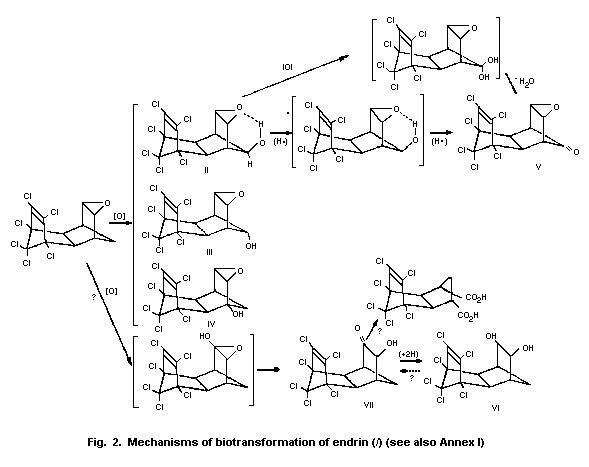 (1975) suggested that the acute toxicity of endrin in birds is not
associated with the formation of 12-ketoendrin.
6.2.2 Human beings
No endrin was found (limit of detection, 0.0016 mg/litre) in 14
samples of urine from workers exposed to aldrin, dieldrin, and endrin,
even though workers with a complete work history had been exposed to
endrin for an average of 2160 h (Hayes & Curley, 1968). Endrin was not
detected in urine from five men and five women (Cueto & Hayes, 1962;
Cueto & Biros, 1967). No unchanged endrin was found in the urine of
Dutch workers exposed to endrin, but it occurred in the faeces (Jager,
1970; Baldwin & Hutson, 1980).
Neither 3-hydroxyendrin nor the diol was detected in urine or
faeces (Hutson, 1981). anti-12-Hydroxyendrin was present in the
urine of workers exposed to endrin, and the glucuronide was found in
the faeces. Concentrations of up to 0.36 mg/g of creatinine were found
in urine after 7 days, accompanied by a sharp rise in the level of
D-glutaric acid (excreted in the urine of mammals as a metabolite of
D-glucuronolactone [Marsh, 1963]), indicating that liver enzyme
induction may have occurred. The levels tended to decrease over the
weekend (Ottevanger & Van Sittert, 1979). Endrin,
anti-12-hydroxyendrin, 12-ketoendrin, and the beta-glucuronide of
anti-12-hydroxyendrin were not found in the blood of workers at a
Dutch plant for the manufacture of endrin (limit of detection, 2
µg/litre). Both endrin and anti-12-hydroxyendrin were found in the
faeces, and all urine samples contained the beta-glucuronide of
anti-12-hydroxyendrin up to a concentration of 0.14 mg/litre as
anti-12-hydroxyendrin (Baldwin & Hutson, 1980).
Hydroxylation at anti-C-12 is relatively rapid and accounts for
the rapid metabolism of endrin. Even syn-12-hydroxyendrin is
hydroxylated rapidly at its anti-C-12 position, affording
12-ketoendrin (Hutson, 1981).
As neither endrin nor its metabolites were found in the blood of
exposed, healthy workers, exposure can be measured by determining
anti-12-hydroxyendrin in urine. A quantitative relationship between
exposure to endrin and the concentration of this metabolite cannot be
established, however, owing to lack of data.
6.2.3 Microorganisms
In mixed anaerobic microbial populations developed using inocula
from soil, freshwater mud, sheep rumen, and chicken litter, endrin
(like other cyclodiene compounds) was monodechlorinated at the
methylene bridge carbon atom. Neither endrin nor any other compound
was further metabolized. The 10 obligate anaerobic bacteria that made
up the mixed population were subsequently isolated in pure culture. Of
these, only Clostridium bifermentans, C. glycolium, and other
Clostridium species were capable of dehalogenation, but at a rate
that was much slower than that of the mixed population (Maule et al.,
1987).
6.2.4 Plants
Three experiments were carried out on tobacco plants. In the
first experiment, 2.08 mg of 14C-endrin were applied to the leaves
with free aeration during the experimental period. In the second test,
the same dose was applied but with little aeration; and in the third,
plants were exposed to 1.04 mg of 14C-endrin with little aeration.
An initial residue level of 50-100 mg/kg was found on leaves in all
three experiments, but, subsequently, less residue was found on plants
with free aeration. Six weeks after treatment, 30-47% of radiolabel
was recovered in residues, which consisted of endrin and hydrophilic
substances (Weisgerber et al., 1969; Donoso et al., 1979).
The leaves of cotton plants were treated with 4.2 mg of
14C-endrin, and the application was repeated after 2 and again after
6 weeks, at which time parathion was also applied. At harvest,
two-thirds of the radiolabel had evaporated, and the total residue in
cotton seed was 0.333 mg/kg. Endrin and two groups of degradation
products were found in the plants; one of these products (possibly
delta-ketoendrin) was only slightly more hydrophilic than endrin, and
the other was very hydrophilic. Most of the metabolites were found on
the surface of the leaves. When delta-ketoendrin was applied to white
cabbage, it disappeared more slowly than endrin, with the formation of
hydrophilic metabolites (Korte, 1969).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
The interactions of halogenated pesticides and microorganisms
have been reviewed extensively (Pfister, 1972).
In three Willamette valley soils (USA) treated with endrin at 0,
1 or 10 mg/kg, no effect was found 30 days after application on the
function and activity of the microbial population, the decomposition
of native organic matter, the transformation of native soil nitrogen,
ammonification of peptone, or nitrification of ammonium sulphate
(Bollen & Tu, 1971). Even at an annual application rate of 5 lb/acre
(5.6 kg/ha) for 5 years, no effect was seen on the numbers or kinds of
soil fungi, the numbers of bacteria, the decomposition rate of organic
matter (measured by CO2 production), or the oxidation of ammonium to
nitrate (Martin et al., 1959).
Endrin at a concentration of 100 mg/kg of soil had no effect on
denitrification in soil under anaerobic incubation for 5 days at 30 °C
or in an isolated denitrifying bacterium (Bollag & Henninger, 1976).
A concentration of 1000 mg/kg had no effect on methanogenesis, sulfate
reduction, or carbon dioxide evolution in anaerobic salt-marsh
sediments (Kiene & Capone, 1984).
The growth rates of two strains of blue-green algae were
decreased in the presence of endrin at a concentration of 0.29
µg/litre (Batterton et al., 1971), and the productivity of many forms
of natural phytoplankton in estuarine waters was decreased by 46% when
they were exposed to 1 mg/litre (Butler, 1963).
7.2 Aquatic organisms
7.2.1 Invertebrates
Acute toxicity of endrin to invertebrates is given in Tables 14
and 15.
A static system was used to study the toxicity of endrin to a
polychaete worm (Nereis virens) in water and sediment, in which sea
water or sediment (containing 17% sand, 83% clay, and 2% organic
carbon) was present at a temperature of 9-10 °C for 12 days. None of
the worms in sea water died after exposure to endrin at 0.11 mg/litre
for 12 days, but two of five worms exposed to 28 mg/kg in sediment
died in this period (McLeese et al., 1982).
Table 14. Acute toxicity of endrin to freshwater invertebrates
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Red snail Static 48-h LC50 7200 Hashimoto & Nishiuchi
Indoplanorbis exustus (1981)
Marsh snail Static 48-h LC50 9500 Hashimoto & Nishiuchi
Semisulcospira libertina (1981)
Snail Static 48-h LC50 12 000 Hashimoto & Nishiuchi
Physa acuta (1981)
Water flea Static 18-23 48-h LC50 160 Thurston et al.(1985)
Daphnia magna Static 21 44 7.1 48-h LC50 4.2 Mayer & Ellersieck (1986)
Static 18 48 7.4 48-h LC50 41-74 Mayer & Ellersieck (1986)
Static 22-24 240 8.0 96-h LC50 59 Elnabarawy et al. (1986)
Water flea Static 15 44 7.1 48-h LC50 20 Mayer & Ellersieck (1986)
Daphnia pulex Static 22-24 240 8.0 96-h LC50 30 Elnabarawy et al. (1986)
Water flea Static 22-24 240 8.0 96-h LC50 24 Elnabarawy et al. (1986)
Daphnia reticulata
Water flea Static 21 44 7.1 48-h LC50 45 Mayer & Ellersieck (1986)
Simocephalus serrulatus Static 15 44 7.1 48-h LC50 26 Mayer & Ellersieck (1986)
Water flea Adult Static 21 44 7.1 48-h LC50 1.8 Mayer & Ellersieck (1986)
Cypridopsis vidua
Sow bug (isopod) Adult Static 15 44 7.1 96-h LC50 1.5 Mayer & Ellersieck (1986)
Asellus brevicaudus
Scud Adult Static 21 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
Gammarus fasciatus Adult Static 15 272 7.4 96-h LC50 1.3 Mayer & Ellersieck (1986)
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Scud Adult Static 21 44 7.1 96-h LC50 3.0 Mayer & Ellersieck (1986)
Gammarus lacustris
Crayfish 3-5 Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Orconectes nais weeks
Adult Static 21 272 7.4 96-h LC50 320 Mayer & Ellersieck (1986)
Crayfish 0.4-2.0 g Flow 18-23 96-h LC50 > 89 Thurston et al. (1985)
Orconectes immunis
Red crayfish Static 48-h LC50 300 Muncy & Oliver (1963)
Procambarus clarki
Tantytarsus Static 18-23 48-h LC50 0.84 Thurston et al. (1985)
Tantytarsus dissimilis
Glass shrimp Adult Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Palaemonetes Adult Flow 21 272 7.4 96-h LC50 0.5 Mayer & Ellersieck (1986)
kadiakensis
Stonefly Larvae Static 15 44 7.1 96-h LC50 > 0.18 Mayer & Ellersieck (1986)
Acroneuria sp.
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.076 Mayer & Ellersieck (1986)
Claasenia sabulosa
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.54 Mayer & Ellersieck (1986)
Pteronarcella badia
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.25 Mayer & Ellersieck (1986)
Pteronarcys californica
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Mayfly Larvae Static 15 44 7.1 96-h LC50 0.9 Mayer & Ellersieck (1986)
Baetis sp.
Mayfly Larvae Static 15 44 7.1 96-h LC50 62 Mayer & Ellersieck (1986)
Hexagenia bilineata
Damselfly Larvae Static 21 44 7.1 96-h LC50 2.4 Mayer & Ellersieck (1986)
Ischnura verticalis Larvae Static 21 272 7.4 96-h LC50 2.1
Snipe fly Larvae Static 15 44 7.1 96-h LC50 4.6 Mayer & Ellersieck (1986)
Atherix variegata
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
Table 15. Acute toxicity of endrin to estuarine and marine invertebrates
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Sand shrimp Static 96-h LC50 1.7 Eisler (1970a)
Crangon septemspinosa Static 96-h LC50 0.2-2.0 McLeese & Metcalfe (1980)
Static 96-h LC50 4-120 McLeese & Metcalfe (1980)
Brown shrimp Juvenile Flow 15 26 48-h LC50 0.2 Mayer (1987)
Penaeus aztecus
Pink shrimp Juvenile Flow 17 30 48-h LC50 0.2 Mayer (1987)
Penaeus duorarum Adult Flow 17 28 96-h LC50 0.037
Grass shrimp Larvae Flow 25 13 96-h LC50 1.2 Mayer (1987)
Palaemonetes pugio Juvenile Flow 25 23 96-h LC50 0.35
Adult Flow 25 21 96-h LC50 0.69
Blue crab Juvenile Flow 11 16 48-h LC50 15 Mayer (1987)
Callinectes sapidus
Hermit crab 96-h LC50 1.2 Eisler (1970a)
Pagurus longicarpus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
The mean 96-h LC50 for the oligochaetes Stylodrilus
heringianus and Limnodrilus hoffmeisteri exposed to sediment from
Lake Michigan contaminated with 14C-endrin was 2588 ± 1974 mg/kg dry
weight of sediment in four assays and 2725 ± 955 mg/kg in two assays.
The toxicity to L. hoffmeisteri appeared to be reduced in the
presence of S. heringianus. The 96-h EC50 burrowing avoidance
values were 15.3-19 mg/kg for S. heringianus and 59 mg/kg of
sediment for L. hoffmeisteri (Keilty et al., 1988a).
Sediment reworking by L. hoffmeisteri alone and with
S. heringianus was measured by monitoring the burial of a 137Cs
marker layer in sediments dosed with 12C- and 14C-endrin at
concentrations of 5.5-81 400 µg/kg of dry sediment. With low endrin
concentrations, the marker layer burial rate did not suggest
stimulation of reworking by either L. hoffmeisteri or
S. heringianus. At higher concentrations, the reworking rates were
equal to or slower than control rates at the beginning of the
experiment but decreased thereafter. The presence of S. heringianus
appeared to enhance the reworking response of L. hoffmeisteri. A
reduction in the post-experimental mortality and an increase in the
dry weight of L. hoffmeisteri in tests with the two species implies
that L. hoffmeisteri benefits from the presence of S. heringianus,
although the reverse was not observed. High concentrations of endrin
in the upper 3 cm of the final sediment showed that the worms had
transported the contaminant upward. The bioaccumulation factor for
S. heringianus ranged from 9.7 to 43.8 and was consistently three to
four times greater than that for L. hoffmeisteri (1.7-13.6) (Keilty
et al., 1988b).
The reworking rates of S. heringianus in microcosms containing
sediments dosed with 14C-endrin at 3.1-42 000 µg/kg of dry matter
were measured at 10 °C by monitoring a 137Cs marker layer buried in
contaminated and uncontaminated microcosms. Alterations in reworking
rates were observed at endrin concentrations 5.5 orders of magnitude
below the LC50 of 1650 mg/kg. At the lower concentrations, a
possible stimulatory effect on the marker layer burial rate in the
first 300-600 h was followed by a significant decrease relative to the
controls. At the higher concentrations, the rates were equal or slower
during the first 600 h and decreased dramatically in the last 600 h.
Mortality was 9.3-28% at 11 500 and 42 000 µg/kg and 0-6.7% at all the
other concentrations tested, including controls. The dry weights of
the worms at the end of the experiment were inversely related to the
high concentrations. The bioaccumulation factors ranged from 34 to 67
on the basis of grams of dry organism to grams of dry sediment (Keilty
et al., 1988c).
The effect of addition of endrin at 50 mg/kg dry weight of
sediment on protein utilization by S. heringianus was examined on
days 4, 8, 20, 28, 39, and 69. A slight increase in the relative
percentage of protein to total body weight was observed, but the
authors concluded that estimation of total protein is not a useful
measure of sublethal responses (Keilty & Stehly, 1989).
The total organic carbon content of sediment had little apparent
effect on the toxicity of endrin in the freshwater amphipod Hyalella
azteca. The 10-day LC50 for endrin in sediment (dry-weight basis)
was 4.4 µg/litre at 3.0% total organic carbon and 6.0 µg/litre at
11.2% carbon (Nebeker et al., 1989).
The EC50s in the green sea urchin (Strongylocentrotus
droebachiensis), the purple sea urchin (S. purpuratus), the red
sea urchin (S. franciscanus), and the sand dollar (Dendraster
excentricus) were 103-441 µg/litre for sperm in a static system and
221-> 362 µg/litre for embryos in a continuous flow of sea water
(temperature, 8.2-8.4 °C; salinity, 30.0 parts per thousand; pH
7.8-8.1 for the sea urchins and 12.5-13.0 °C, 30.0 parts per thousand,
and pH 8.0-8.1 for sand dollar embryos), both with an exposure time of
120 h. In a larval test of static exposure of Dungeness crab (Cancer
magister), the EC50 was 2.0 µg/litre (Dinnel et al., 1989).
Endrin was tested at 0, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.5,
5.0, and 10 mg/litre for its effects on embryos of the American oyster
(Crassostrea virginica) and their larvae. Fertilized eggs were
studied after 48 h, and survival and growth of veliger larvae were
studied in 2-day old larvae and in larvae kept for a further 12 days
at 24 °C. The results varied considerably. The estimated concentration
that would cause an approximately 50% reduction in the number of eggs
that develop into normal straight-hinge larvae, calculated by
interpolation from the data, was 0.79 µg/litre and that at which 50%
of the larvae survived was > 10.0 mg/litre (Davis & Hidu, 1969).
In the mysid shrimp Mysidopsis bahia, exposed for the complete
life cycle, acute lethality (over 96 h) was observed with endrin at
120 ng/litre; increased oxygen consumption was measurable within 24 h
of exposure. The lowest-observed-effect level for chronic lethality
was 60 ng/litre; sub-lethal effects on growth (reduced by day 4 of
exposure) and oxygen consumption (increased by day 10 of exposure)
were observed before death (over 20 days). Reduced reproductive
capacity (assessed as production of young) was observed at 30 ng/litre
over 20 days--the time to full maturity (McKenney, 1986).
Behavioural changes were observed in stoneflies (Pteronarcys
dorsata) within 4 days of exposure to 96.1% endrin at 0.07 µg/litre
and in caddis flies (Brachycentrus americanus) at 0.15 µg/litre. The
28-day LC50 was < 0.03 µg/litre for caddis flies and 0.07 µg/litre
for stoneflies (Anderson & DeFoe, 1980).
7.2.2 Fish
7.2.2.1 Acute toxicity
Endrin is highly toxic for both freshwater and marine fish. The
available data are summarized in Tables 16 and 17.
7.2.2.2 Short-term toxicity
Channel catfish (Ictalurus punctatus) were exposed continuously
to renewed solutions of endrin in water at 15 and 22 °C. Measured
endrin concentrations of 0.25-0.30 µg/litre were found to be acutely
toxic to the fish within 10 days or less. None of the fish survived
blood concentrations exceeding 0.28 mg/litre, a well-defined threshold
concentration of endrin in blood, and none died at less than 0.23
mg/litre. The concentration of endrin in the blood of fish exposed to
lethal concentrations in water for periods insufficient to cause death
were markedly lower than that in fish that died from exposure to the
same water (Mount et al., 1966).
The 28-day LC50 for 96.1% eldrin in bullheads (Ictalurus
melas) was 0.10 µg/litre (Anderson & DeFoe, 1980).
In larval fathead minnows (< 24 h old) exposed continuously to
endrin (98%) for 28-30 days in a flow-through system, growth was the
most sensitive parameter. A 48-h exposure to 0.62 µg/litre caused
significant reduction in growth, and survival was reduced at 1.21
µg/litre; with a 72-h exposure, growth was reduced at 0.63 µg/litre,
and all fish died at 1.15 µg/litre. Continuous exposure to 0.38
µg/litre for 30 days significantly reduced growth, and all fish died
at 0.73 µg/litre (Jarvinen et al., 1988).
Sheephead minnows (Cyprinodon variegatus) were exposed
continuously for 23 weeks to endrin from the embryonic stage through
hatching, until adulthood and spawning. The average exposure
concentrations were 0 (control), 0.027, 0.077, 0.12, 0.31, and 0.72
µg/litre. The resultant progeny were monitored to determine effects on
their survival, growth, and reproduction. Embryos exposed to 0.31 and
0.72 µg/litre hatched early; all fry exposed to 0.72 µg/litre died by
day 9 of exposure. At 0.31 µg/litre, fry were initially stunted and
some died. Survivors seemed unaffected until maturity, when some
females died during spawning; fewer eggs were fertile, and survival of
exposed progeny was decreased. No significant effect was observed
throughout the life cycle at an exposure concentration of 0.12
µg/litre (Hansen et al., 1977).
Endrin was tested in flagfish (Jordanella floridae) at 0.21,
0.29, and 0.39 µg/litre for 30 days. Only the highest concentration
decreased survival, and the two highest dose levels affected the mean
number of eggs produced (Hermanutz et al., 1985).
Table 16. Acute toxicity of endrin to freshwater fish
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Tilupa sp. Static 15 44 7.1 96-h LC50 12 Mayer & Ellersieck (1986)
Coho salmon Static 16 44 7.1 96-h LC50 0.089 Mayer & Ellersieck (1986)
Oncorhynchus kisutch 1.9 g Static 20 96-h LC50 0.27 Katz & Chadwick (1961)
Chinook salmon 6-8 g Static 20 96-h LC50 0.92 Katz & Chadwick (1961)
Oncorhynchus
tshawytscha
Cutthroat trout 1.0 g Static 13 44 7.1 96-h LC50 > 1.0 Mayer & Ellersieck (1986)
Salmo clarki
Rainbow trout 1.0 g Static 13 44 7.1 96-h LC50 0.75 Mayer & Ellersieck (1986)
Oncorhynchus mykiss 1.0 g Static 13 272 7.4 96-h LC50 0.74
1.4 g Static 2 44 7.1 96-h LC50 2.4
1.4 g Static 7 44 7.1 96-h LC50 1.4
1.4 g Static 13 44 7.1 96-h LC50 1.11
1.4 g Static 18 44 7.1 96-h LC50 0.75
0.6-8.0 g Flow 18-23 96-h LC50 0.3 Thurston et al. (1985)
Goldfish 1-4 g Flow 12 314 7.6 96-h LC50 0.44 Mayer & Ellersieck (1986)
Carrassius auratus Flow 18-23 96-h LC50 0.95 Thurston et al. (1985)
Static 48-h LC50 1.0 Hashimoto & Nishiuchi
(1981)
Carp Flow 12 314 7.6 96-h LC50 0.32 Mayer & Ellersieck (1986)
Cyprinus carpio Static 48-h LC50 0.84 Hashimoto & Nishiuchi
(1981)
Medaka Static 48-h LC50 1.4 Hashimoto & Nishiuchi
Oryzias latipes (1981)
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Pond loach Static 48-h LC50 4.9 Hashimoto & Nishiuchi
Misgurnus (1981)
anguilicaudatus
Fathead minnow 1.2 g Static 18 44 7.1 96-h LC50 1.8 Mayer & Ellersieck (1986)
Pimephales promelas 0.9 g Flow 12 314 7.6 96-h LC50 0.24
Static 24-h LC50 12 Kagan et al. (1986)
Larvae Static 25-26 46 7.1-8.3 96-h LC50 0.7 Jarvinen et al. (1988)
0.2-1.0 g Flow 18-23 96-h LC50 0.65 Thurston et al. (1985)
Bluntnose minnow Static 96-h LC50 0.29 Johnson (1968)
Pimephales notatus
Black bullhead 1.5 g Static 24 44 7.1 96-h LC50 1.13 Mayer & Ellersieck (1986)
Ictalurus melas
Channel catfish 5.2 g Static 18 44 7.1 96-h LC50 1.9 Mayer & Ellersieck (1986)
Ictalurus punctatus 1.4 g Static 24 44 7.1 96-h LC50 0.32
Mosquito fish 0.6 g Static 17 44 7.1 96-h LC50 1.1 Mayer & Ellersieck (1986)
Gambusia affinis 0.222 g Static 25 96-h LC50 5.27 El-Sebae (1987)
Bluegill 1.5 g Static 18 44 7.1 96-h LC50 0.61 Mayer & Ellersieck (1986)
Lepomis macrochirus 0.5 g Static 18 272 7.4 96-h LC50 0.53
1.3 g Static 7 44 7.1 96-h LC50 0.73
1.3 g Static 13 44 7.1 96-h LC50 0.68
1.3 g Static 18 44 7.1 96-h LC50 0.41
1.3 g Static 24 44 7.1 96-h LC50 0.37
1.3 g Static 29 44 7.1 96-h LC50 0.19
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Largemouth bass 2.5 g Static 18 272 7.4 96-h LC50 0.31 Mayer & Ellersieck (1986)
Micropterus salmoides
Yellow perch Flow 12 314 7.6 96-h LC50 0.15 Mayer & Ellersieck (1986)
Perca flavescens
Tilapia 1.1 g Static 24 44 7.1 96-h LC50 < 5.6 Mayer & Ellersieck (1986)
Tilapia mossambica
Tilapia (Behera strain) 0.825 g Static 25 96-h LC50 10.09 El-Sebae (1987)
Tilapia zilli
Tilapia (Alexandria
strain) 0.825 g Static 25 96-h LC50 0.26 El-Sebae (1987)
Tilapia zilli
Guppy Static 20 96-h LC50 0.9 Katz & Chadwick (1961)
Poecilia reticulata
Flagfish 2-3 days Static 24-26 43-48 6.9-7.8 96-h LC50 0.85 Hermanutz et al. (1985)
Jordanella floridae
aStatic, static condition (water unchanged for the duration of the test); flow, flow-through conditions; endrin
concentration in water maintained continuously.
Table 17. Acute toxicity of endrin to estuarine and marine fish
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
American eel 57 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Anguilla rostrata
Atlantic riverside 54 mm Static 20 24 96-h LC50 0.05 Eisler (1970b)
Menidia menidia
Blue head 90 mm Static 20 24 96-h LC50 0.1 Eisler (1970b)
Thalassoma bifasciatum
Gulf menhaden Juvenile Flow 27 29 24-h LC50 0.8 Mayer (1987)
Brevoortia patronus
Sheepshead minnow Juvenile Flow 14 30 48-h LC50 1.0 Mayer (1987)
Cyprinodon variegatus Juvenile Flow 30 24 96-h LC50 0.34
Adult Flow 18 18 96-h LC50 0.38
Adult Flow 30 16 96-h LC50 0.36
Longnose killifish Juvenile Flow 25 19 24-h LC50 0.23 Mayer (1987)
Fundulus similis
Striped killifish 40 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
Fundulus majalis
Mummichog 51 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Fundulus heteroclitus
Sailfin molly Adult Flow 20 27 96-h LC50 0.63 Mayer (1987)
Poecilia latipinna
Spot Juvenile Flow 12 24 48-h LC50 0.3 Mayer (1987)
Leiostomus xanthurus
Table 17. (contd)
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Striped mullet Juvenile Flow 14 30 48-h LC50 0.4 Mayer (1987)
Mugil cephalus 83 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
White mullet Juvenile Flow 29 48-h LC50 2.6 Butler (1963)
Mugil curema
Northern puffer 131 mm Static 20 24 96-h LC50 3.1 Eisler (1970b)
Sphaeroidus maculatus
Striped bass 2.7 g Static 16-18 28 96-h LC50 0.09 Korn & Earnest (1974)
Morone saxatilis
Shiner perch 1.2-11 g Static 13 26 96-h LC50 0.8 Earnest & Benville (1972)
Cymatogaster aggregata 1.2-11 g Int. flow 13 26 96-h LC50 0.12
Dwarf perch 1.2-11 g Static 13 18 96-h LC50 0.6 Earnest & Benville (1972)
Micrometrus minimus 1.2-11 g Int. flow 13 28 96-h LC50 0.13
Threespine stickleback 0.3 g Static 20 25 96-h LC50 1.5 Katz & Chadwick (1961)
Gasterosteus aculeatus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; int. flow,
intermittent flow-through conditions; endrin concentration in water maintained continuously
7.2.2.3 Studies of resistance
Populations of mosquito fish (Gambusia affinis) developed high
levels of resistance to endrin and other cyclodiene insecticides as a
result of inadvertent exposure to agricultural sprays. Susceptible
fish (male) showed a LC50 of 8.3 mg/litre and resistant fish, 161
mg/litre. Genetic crossing studies show that endrin resistance is
inherited as a single, autosomal, intermediate gene (Yarbrough et al.,
1986).
Pesticide-susceptible and -resistant mosquito fish were exposed
to 14C-endrin at 20 or 1000 µg/litre, and liver and brain were
assayed to determine any difference in distribution, uptake, and nerve
binding patterns (Fabacher & Chambers, 1976). The results are
summarized in Table 18. Endrin was taken up faster by brain and liver
from susceptible fish than resistant fish. In resistant fish, at least
at a high lethal concentration (1000 µg/litre), endrin entered the
brain slowly and accumulated in the liver, suggesting a more efficient
blood-brain barrier in resistant than in susceptible fish. Extraction
studies provided some evidence that endrin binds more readily to
nonessential protein complexes in the nervous tissue of resistant
fish, consequently decreasing the amount of endrin available to
produce a toxic effect.
Table 18. Mean quantities of endrin (in mg/kg tissue) in brain and
liver of susceptible and resistant mosquito fish
Genotype At 20 µg/litre At 1000 µg/litre
Brain Liver Brain:liver Brain Liver Brain:liver
Susceptible 16.98 33.28 0.51 149.31 160.27 0.93
Resistant 8.83 16.84 0.52 57.52 353.42 0.16
Susceptible: 1.90 1.90 1.00 2.60 0.45 5.80
resistant
Cell membrane fractions from resistant mosquito fish bound more
endrin than those from susceptible fish, and mitochondria from the
liver of the resistant genotype bound less endrin than those from
susceptible fish. Differences in endrin uptake, retention of endrin by
brain cell membranes, a blood-brain barrier, and a structural
difference in myelin mayaccount for the resistance of some mosquito
fish to endrin (Wells & Yarbrough, 1972).
In resistant and non-resistant populations of golden shiner
(Notemigonus crysoleucas), blue gill sunfish (Lepomis macrochirus),
and green sunfish (Lepomis cyanellus), the median tolerated limit at
36 h was 3.0, 1.5, and 3.4 µg/litre for non-resistant strains and 310,
300, and 160 µg/litre for resistant fish, respectively (Ferguson et
al., 1964).
7.2.2.4 Interaction with other chemicals
The joint action of endrin with malathion on mortality in
flagfish (Jordanella floridae) consisted of enhanced effects at
concentrations that had no effect when the substances were tested
individually. The effects of the mixture on growth followed a simple
additive model. Malathion did not modify the effect of endrin on egg
production. In a separate test, malathion did not affect the uptake or
elimination of endrin (Hermanutz et al., 1985).
In a study of the interaction between the accumulation and
elimination of 14C-endrin and 14C-DDT in mosquito fish (Gambusia
affinis), fish about 4 cm long were exposed to a nominal
concentration of 3.94 nM endrin or DDT, or to a mixture of the
compounds. Prior exposure to DDT for 4 h generally reduced the
accumulation of endrin in serum, gall-bladder, and whole bodies,
whereas prior exposure to endrin for 4 h had little effect on DDT
accumulation. Simultaneous exposure to DDT and endrin reduced the
accumulation of DDT in the gall-bladder over the 4 h of exposure and
in the whole bodies during the first hours, and it reduced the
accumulation of endrin in gall-bladder and in the whole body. Endrin
levels in fish exposed subsequently only to DDT or DDE were
significantly higher in gall-bladder and were reduced in the whole
body over 4 h. The interactions observed may be the result of
competition for and/or displacement of insecticides from mutual
binding sites (Denison et al., 1985).
In a study of the relative binding and competition between
organochlorine pesticides for serum binding sites, incubation with
serum from mosquito fish led to their association primarily with the
vitellogenin/lipoprotein and albumin fractions. Preincubation of serum
with endrin significantly reduced the quantity of 3H-DDT that was
bound subsequently, while the reverse was not observed. Although the
reason for the apparent quantitative decrease in binding is unknown,
this phenomenon may be of toxicological importance (Denison &
Yarbrough, 1985).
7.2.2.5 Special studies
Fingerlings of carp (Cyprinus carpio) exposed to endrin at the
LC50 (0.0065 mg/kg) for 24 h showed clear inhibition of ý-amylase
activity in the liver (Datta & Ghose, 1985).
A group of 240 rainbow trout (Salmo gairdneri) were exposed to
endrin at 0.12-0.15 µg/litre for 30 days; one untreated and one
solvent control group were used. On day 30, 10 fish from each group
were sacrificed and examined for the ability of peritoneal macrophages
to phagocytize latex beads. The remaining fish were immunized with 10
µg of Yersinia ruckeri O-antigen and exposure to endrin continued.
Assays for migration inhibition factor, plaque forming cells, and
serum agglutination titre were performed 2, 14, and 30 days after
inoculation, and serum was collected from all fish to determine the
cortisol concentration. Exposure to endrin had no effect on the
phagocytic ability of peritoneal macrophages, but the responses in the
three assays were significantly reduced in comparison with the control
values. Serum cortisol concentrations were significantly elevated in
the endrin-treated fish. The study did not, however, elucidate the
mechanism of immune suppression, other than showing that a stress
response had occurred (Bennett & Wolke, 1987a). In another study,
therefore, control fish were fed cortisol at 20 mg/kg and metyrapone
at 35 mg/kg body weight, and endrin-exposed fish received metyrapone
at 35 mg/kg body weight per day in the diet. The fish that received
cortisol had significantly reduced responses in all three assays; but
in the endrin-exposed fish that received metyrapone, the migration
inhibition factor response was completely restored, the plaque forming
cell response was restored to 61%, and serum agglutination titres to
69%. These results indicate that elevated serum cortisol concentration
plays a central role in repressing the immune response (Bennett &
Wolke, 1987b).
The concentrations of serum glucose, liver and muscle glycogen,
cortisol, protein, and cholesterol were determined in carp (Cyprinus
carpio) exposed to endrin at 2 µg/litre for 6, 24, and 72 h. Only
the concentration of cortisol in serum was clearly decreased (Gluth &
Hanke, 1985).
7.2.3 Amphibia
The acute toxicity of endrin to amphibians is summarized in
Table 19.
7.3 Terrestrial organisms
The acute oral toxicity of endrin for terrestrial animals is
high. The available LD50 values are summarized in Table 20.
7.3.1 Honey bees
The 48-h LD50 of endrin in worker honey bees (Apis mellifera)
using a dusting technique was 2.02 µg/bee (Atkins et al., 1973). The
LD50 for bees after contact was 0.65 µg/bee, and the acute oral
LD50 was 0.46 µg/bee (Oomen, 1986).
Table 19. Acute toxicity of endrin to amphibians
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCo3/l) (mg/1) (mg/l)
Bullfrog 2-5 g Flow 18-23 96-h LC50 2.5 Thurston et al. (1985)
Rana catesbiana
Leopard frog Eggs Flow 20 100 7.2-7.5 24-h LC50 2.5 Hall & Swineford (1980)
Rana spenocephala Larvae Flow 20 100 7.2-7.5 96-h LC50 6
Subadult Flow 20 100 7.2-7.5 96-h LC50 5
Frog 0.5 g Static 14 20 6.2 96-h LC50 0.21 Khangarot et al. (1985)
Rana hexadactyla
Western chorus frog Tadpole Static 15 44 7.1 96-h LC50 120 Mayer & Ellersieck (1986)
Pseudacris triseriata
Fowlers toad Tadpole Static 15 44 7.1 96-h LC50 180 Mayer & Ellersieck (1986)
Bufo woodhousei
fowleri
aStatic,static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin concentration in water
maintained continuously
Table 20. Acute oral LD50s of endrin for terrestrial species
Species LD50 Reference
(mg/kg body weight)
Birds
Mallard 5.6 (2.7-11.7) Hudson et al. (1984)
(Anas platyrhynchos)
Pigeon 2.0-5.0
(Columbia livia)
Pheasant 1.8 (1.1-2.8)
(Phasianus colchicus)
Sharp-tailed grouse 1.06 (0.552-2.04)
(Pedioecetes phasia
nellus)
California quail 1.19 (0.857-1.65)
Redwinged blackbird 2.37 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling 2.37-3.16
(Sturnus vulgaris)
Quail 4.22
(Coturnix coturnix)
Mammals
Big brown bat 5-8 Luckens & Davis (1965)
(Eptesicus fuscus)
Pine mouse (Microtus 2.6/19.0 Petrella et al. (1975)
pitymys pinetorum) 1.3/36.4 Webb et al. (1973)
(susceptible/
resistant)
7.3.2 Birds
7.3.2.1 Acute toxicity
The LD50s of endrin for some bird species are given in Table
20.
7.3.2.2 Short-term toxicity
Groups of 40 one-day-old quail were fed endrin at dietary levels
of 0, 0.5, 1, 5, 10, 20, or 50 mg/kg of diet. Survival was adversely
affected in all test groups, and there were no survivors beyond two
weeks among birds fed 10 mg/kg or more. Food consumption was
abnormally low, and symptoms involved lack of muscular coordination,
tremors, and occasional convulsive movements. Similar results were
obtained in 40 one-day-old pheasants fed endrin at dietary levels of
5 or 20 mg/kg, none of which survived beyond 8 days (Dewitt, 1965).
Groups of 20 seven-day-old chicks were unaffected by diets
containing endrin at 0, 1.5, or 3 mg/kg. When the concentration was
increased to 6 or 12 mg/kg, the birds became highly excitable and
failed to gain weight in comparison with controls. The survival rates
over a 12-week period were 85 and 5%, respectively, compared with 100%
in the controls (Sherman & Rosenberg, 1954).
The LC50 values for 2-3-week-old bobwhite quail (Colinus
virginianus), Japanese quail (Coturnix coturnix japonica),
ring-necked pheasants (Phasianus colchicus), and mallards (Anas
platyrhynchos) (8-13 birds per group) fed endrin in their diet for
5 days followed by 3 days of untreated diet, were 14-22 mg/kg diet
(Hill et al., 1975; Hill & Camardese, 1986).
7.3.2.3 Studies of reproduction
In a study of reproduction in pheasants, a diet containing endrin
at 10 mg/kg reduced egg production and chick survival; diets
containing up to 2 mg/kg did not affect egg production, fertility,
hatchability, or chick survival (Dewitt, 1965).
Groups of five female and two male mallard ducks (Anas
platyrhynchos) were administered diets containing endrin at 0, 0.5,
or 3.0 mg/kg for a 12-week oviposition period. Egg production was not
affected. The eggs were incubated, and infertile eggs, embryo
survival, and hatchability were measured. Fertility and hatchability
were not affected, although a 9.6% drop in embryo survival was
observed in the group that had received the highest dose. Endrin
residues in body fat were 3.4 mg/kg of tissue in the group that
received 0.5 mg/kg and 19.3 mg/kg in the group that received 3.0
mg/kg. The concentrations were higher in females than in males. The
endrin residue levels in eggs were none detected in the controls, 0.43
mg/kg in the group fed 0.5 mg/kg, and 2.75 mg/kg in the group fed 3.0
mg/kg (Roylance et al., 1985).
Three groups of 27 pairs of mallards were fed endrin at 0, 1, or
3 mg/kg of dry duck mash from December to the summer to investigate
the influence on reproduction and health. Birds fed 1 mg/kg reproduced
as well as the controls; they had significantly greater success in
hatching fertile eggs than did those fed 0 or 3 mg/kg and their
clutches hatched earlier (not significantly) than those of birds fed
3 mg/kg. Endrin accumulated in eggs to a mean level of 1.1 mg/kg (wet
weight) in the group fed 1 mg/kg and 2.9 mg/kg in the group fed 3
mg/kg. The concentration of endrin in adipose tissue was four to seven
times higher than that in eggs (Spann et al., 1986).
7.3.2.4 Interaction with other chemicals
The toxicity of combinations of chlordane and endrin was studied
in 14-week-old male and female bobwhite quail. Eight birds received
10 mg/kg chlordane in the diet for 10 weeks; 20 quail were treated
with 10 mg/kg chlordane for 10 weeks followed immediately by 10 mg/kg
endrin (98%) in the diet; a fourth group of 20 birds received only 10
mg/kg endrin in the diet. The pesticides were dissolved in propylene
glycol. After 9-10 days on a control diet, survivors were sacrificed
and their brains dissected. No deaths occurred among the birds fed the
control diet or 10 mg/kg chlordane. With endrin alone, 15 birds died,
and with the combination 14 birds died. In birds that received endrin
alone, the residue levels in the brain were 0.34-1.84 mg/kg in those
that died and 0.28-0.62 mg/kg in the survivors. In the birds fed
chlordane and endrin, the residue levels were 0.17-1.25 mg/kg in birds
that died and 0.14-0.56 mg/kg in survivors. Birds treated with the
combination had considerably more chlordane residues in their brains
than did those fed chlordane alone. The main conclusion of this study
was that the additive toxicity of closely related chemicals should be
taken into account in diagnosing cause of death (Ludke, 1976).
7.3.2.5 Special studies
The influence of endrin at 5 and 10 mg/kg of feed on the
activity of various enzymes in the serum of juvenile cockerels was
studied. The greatest increases in activity were measured for
glutamate oxalacetate transaminase, cholinesterase, and alkaline
phosphatase. Smaller increases were observed for creatine kinase,
glutamate dehydrogenase, ý-hydroxybutyrate dehydrogenase, and
phosphohexose isomerase (Horn et al., 1987).
7.3.2.6 Behavioural studies
The effect of a sub-lethal dose of endrin (2 mg/kg diet) on
avoidance responseswas studied in eight pens of 25 seven-day-old
Coturnix quail chicks for 14 days. The stimulus used to elicit
avoidance was a moving silhouette, and the response was measured
daily. Group avoidance response was significantly suppressed by
exposure to endrin, but the behaviour returned to normal after 2 days
on untreated diet (Kreitzer & Heinz, 1974).
Adult male bobwhite quail (Colinus virginianus) were fed a diet
containing endrin at 0.1 or 1.0 mg/kg for 138 days (beginning at 3
days of age), and then their performance in five non-spatial
discrimination reversal tasks was studied. Treated birds made 36-139%
more errors than controls, and birds fed the lower dose made
significantly more errors than those given the higher dose after
reversal 3 or 4 in the first three tests. The effects of endrin were
reversed after 50 days on untreated feed. The principal effect of
endrin was to impair the birds' ability to solve a novel problem. The
mean levels of endrin residues in the brain were 0.075 mg/kg wet
weight in those given the lower dose and 0.35 mg/kg for those on the
higher dose (Kreitzer, 1980).
7.3.3 Mammals
7.3.3.1 Toxicity
The LC50 values for short-tailed male and female shrews
(Blarina brevicauda) aged 180, 105-150, and 30-75 days were 87-174
mg/kg diet for 14 days (Blus, 1978).
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus maniculatus) of various ages were fed endrin at 0, 1, 2,
4, or 7 mg/kg of diet for intermittent periods, between which the
animals were either fed a normal diet or were subjected to 48-h
starvation. The animals were sacrificed by exposing them to cold
stress at -16 °C and the time of death recorded. No influence was
found on litter production, frequency, or mean litter size. At the
higher levels of feeding, postnatal mortality before weaning was
increased. Significant parental mortality occurred at 4 mg/kg and
higher and appeared to be dose-dependent (Morris, 1968). (Remark:
Since the animals in this study were captured in the field and the
periods of feeding alternated with short periods of starvation in an
effort to simulate possible conditions in the field, this study is of
only limited value).
The effects of endrin at 8.0 oz/acre (0.56 kg/ha) on unenclosed
field populations of meadow voles (Microtus pennsylvanicus) and deer
mice (Peromyscus maniculatus) were investigated in 1966-68. Animals
were trapped live on adjacent 7-acre (2.8 ha) plots each summer at
regular intervals, before and after a single application of endrin.
Immediate, significant declines in the number of voles were seen on
the experimental plot, but no long-term toxicological effects were
observed. The population rapidly recovered, exceeding the initial and
control numbers in all three years. The experimental vole population
thus appears to have responded to endrin as it would to a local
depopulation by trapping. The mouse population decreased significantly
after the application of endrin in 1966 and did not recover, and the
highly unstable, transitory population on the experimental plot
indicated a long-term toxicological effect (Morris, 1970, 1972).
7.3.3.2 Studies of resistance
14C-Endrin in corn oil was administered to a resistant and a
susceptible strain of pine mice (Microtus pitymus pinetorum) orally
at 0.5 mg/kg body weight, as follows: days 1-5, unlabelled endrin;
days 6-14, 14C-endrin; and day 15 unlabelled endrin. Total recovery
of 14C in both faeces and urine was 76% for the resistant strain and
53% for the susceptible strain. The two strains produced the same
major faecal and urine metabolite, but the resistant strain produced
about twice the quantity as the susceptible strain. The quantitative
differences in the excretion of more polar endrin metabolites may
indicate metabolic differences between the two strains and,
consequently, the greater tolerance of the resistant strain to toxic
effects (Petrella et al., 1975). The major metabolite was identified
as anti-12-hydroxyendrin; one of the other more polar metabolites,
found in minor quantities, was suggested to be a tertiary alcohol of
endrin (Petrella et al., 1977).
The degree of toxicity of endrin in first-generation progeny of
susceptible and resistant strains and a cross of the two strains of
pine mice was studied by Webb et al. (1973). The LD50 for offspring
of susceptible x susceptible parentage was 5.0 mg/kg body weight; that
for resistant x resistant, 21.1 mg/kg; and that for susceptible x
resistant, 8.6 mg/kg. These results offer preliminary support for a
genetic mechanism with intermediate dominance. An increase in
resistance against the toxic effects of endrin was demonstrated in
wild pine mice trapped in orchards where endrin had been used for
years. The oral LD50 in susceptible mice was about 3 mg/kg body
weight and that in resistant mice, an average of 36 mg/kg body weight.
The increased resistance appeared to be heritable in the first
generation (Webb & Horsfall, 1967; Webb et al., 1973). Although
differences in the rate of metabolism of endrin could be demonstrated,
especially in the activity of mixed-function oxidase, these did not
appear to be sufficiently large to explain the resistance (Hartgrove
et al., 1977).
7.4 Effects in the field
Episodes have been reported in which endrin was concluded to be
the cause of death in fish and birds. Numerous fish kills were
reported from the sugar-cane growing areas of Louisiana in 1960-63. No
association with variables such as dissolved oxygen, pH, or
temperature was found, but following the development of sensitive
analytical techniques it was concluded that the fish had been killed
by endrin (Mount & Putnicki, 1966). Surface runoff from fields was
reported to be the main source of the endrin that contaminated the
rivers (Lauer et al., 1966), although effluent from an insecticide
plant may have contributed since the fish contained two chemicals
involved in endrin manufacture (Mount & Putnicki, 1966). Levels of
endrin found in studies of fish in the wild are given in section
5.1.4.2.
Declines in the population of brown pelicans in Louisiana were
attributed to endrin, although at least six other organochlorine
pesticides and polychlorinated biphenyls were found in the animals
(Blus et al., 1975; King et al., 1977). The eggs of brown pelicans
(Pelecanus occidentalis) in Texas, USA, were examined for endrin
residues in 1975-81. The compound was recovered only in 1975, in 15 of
18 eggs, at levels of 0.1-0.3 mg/kg. In the same year, the highest
levels of endrin were found in pelican eggs in Louisiana, and this
maximum coincided with the deaths of large numbers of brown and white
pelicans (P. erhyrorhyncos) (King et al., 1985).
Endrin was found in one of ten eggs of the American white pelican
(Pelecanus erythrorhynchos) collected in 1969, at 0.20 mg/kg, and in
two of 35 samples collected in 1981, at up to 0.18 mg/kg wet weight.
Brains of pelicans found dead in the period 1975-81 had levels up to
0.80 mg/kg. No endrin was found in eggs of the western grebe
(Aechmophorus accidentalis) collected in 1981. It was concluded that
endrin had caused some of the deaths among pelicans in California
(Boellstorff et al., 1985).
The death of sandwich terns in The Netherlands was attributed to
the discharge of a combination of endrin and related pesticides into
an estuary from a manufacturing plant (Koeman et al., 1967, 1969;
Koeman, 1971).
On several occasions in Victoria, Australia, large numbers of
wild birds, in particular pigeons (Columba livia), sparrows (Passer
domesticus) and Indian mynahs (Gracula religiosa), were observed
to be paralysed or in convulsions (Reece et al., 1985). The crops and
livers contained endrin at levels of up to 1.2 mg/kg.
Fulvous whistling ducks (Dendrocygna bicolor), which nest in
rice fields along the south-eastern coast of Texas, USA, suffered a
major decline in population in the late 1960s, which was attributed to
exposure to dieldrin or aldrin. Organochlorine pesticides were
determined in 1983 in the carcasses of 15 adult ducks immediately
after their arrival in Texas from Mexico in the spring and before
departure from Mexico in the autumn. Four of the ducks with high
levels of dieldrin residues also had residues of endrin; and four
other ducks, collected in 1967 and 1969, had endrin residues. The
geometric mean levels in the different years were 0.03-0.08 mg/kg wet
weight; in juveniles in 1960-69, the geometric mean level was 0.16
mg/kg (Flickinger et al., 1986).
The effects of endrin on wildlife were studied in 1981-83 in
fruit orchards in Washington, USA. A single application of endrin
after harvest resulted in acute and chronic toxicity to a variety of
avian species; most deaths occurred soon after the application, but
several raptors died during the spring and summer. The brains of 73 of
125 birds contained endrin at < 0.10-0.80 mg/kg; detectable levels
occurred most frequently in the brains of galliforms and falconiforms.
The species in which the greatest numbers of deaths attributed to
endrin occurred include California quail (Callipepta california),
chukars (Alectoris chukar), and common barn owls (Tyto alba). Of
the 97 eggs analysed, 68 contained detectable endrin residues: 51 had
levels of < 0.10 mg/kg, and the eggs of 10 species contained
0.01-0.17 mg/kg wet weight (range, none detected to 1.67). The authors
concluded that endrin was toxic to wildlife, although there was no
evidence that it affected reproductive success or population level
(Blus et al., 1989).
Levels found in birds in the wild are also given in section
5.1.4.1.
7.5 Appraisal of effects on organisms in the environment
Use of endrin in agriculture is the major source of its presence
in the environment, but discharge of waste material from manufacturing
and formulating plants has contributed to local contamination.
World-wide monitoring surveys have shown that the concentrations of
endrin in the biosphere are generally very low (Table 10), both
absolutely and relatively: The levels of residues of other
organochlorine compounds, particularly DDE and polychlorinated
biphenyls, are generally 100 times or higher than those of endrin.
Toxicologically significant levels of endrin residues have been
found locally in fish and other organisms, particularly in cases in
which endrin was applied near rivers and lakes and when runoff
occurred into waterways. Residues may also occur when endrin is used
as a seed dressing or in bait to control rodents.
The most serious adverse ecological effects that have been
reported were the fish kills (and associated adverse effects on brown
pelican populations) in the Mississippi River system in the USA.
Although the initial evidence for ascribing these effects to endrin
was circumstantial, the results of analyses of dead fish were
considered to confirm a causal relationship; there is little doubt
that endrin was a contributory factor in at least some of these fish
kills. The evidence that endrin was the primary cause of the decline
in the brown pelican population is less convincing, since the harmful
effects on reproductive success have been attributed to DDE and other
factors (Blus et al., 1974, 1979).
In summary, agricultural application of endrin should be such as
to avoid or minimize contamination of waterways, either by
overspraying or runoff or by leaching from dressed seed in
rice-growing areas. The effects of the use of baits containing endrin
for rodent control on non-target organisms should be assessed in the
light of local circumstances. Finally, effluents from manufacturing
and formulating plants must be treated adequately before being
discharged into waterways.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO
The toxicology and risk assessment of endrin have been reviewed
(US EPA, 1987a,b; Anon., 1988a,b).
8.1 Acute toxicity of technical-grade endrin
8.1.1 Oral administration
Endrin is highly toxic when given by the oral route and is more
acutely toxic to mammals than its stereoisomer dieldrin (WHO, 1989),
with an acute oral LD50 of 7.5-17.8 mg/kg body weight (Table 21),
compared with 50-60 mg/kg for dieldrin. There appears to be a
sex-dependent sensitivity to the acute effects of endrin, female
animals being more sensitive than males. A species-dependent
sensitivity has also been reported, monkeys and cats being more
susceptible than mice and rats.
Signs of intoxication may include increased irritability and
tremor, followed by tonic-clonic convulsions, ataxia, dyspnoea,
gasping, and cyanosis. Convulsions usually occur 30-60 min after an
oral dose, and death may occur within 24 h after the administration of
a lethal dose (Speck & Maaske, 1958). Animals that survive poisoning
recover completely with no delayed or persistent effect.
8.1.2 Dermal administration
The acute dermal LD50s for technical endrin in various animal
species are given in Table 22. Endrin is highly toxic when applied as
a solution in hydrocarbon solvents but moderately toxic when applied
as a dry powder. The signs of poisoning are similar to those seen
after oral administration.
8.1.3 Parenteral administration
The acute LD50s for technical-grade endrin given by parenteral
routes of administration are shown in Table 23.
8.1.4 Toxicity of metabolites and isomers
8.1.4.1 Mammalian metabolites
In a comparative study, the acute oral LD50s of endrin and
three of its metabolites were determined in rats (Table 24). When the
brains of some of the rats were analysed for the presence of endrin
and its metabolites, the concentration of 12-ketoendrin in male rats
given endrin at 60 mg/kg body weight was found to be higher (mean, 0.3
mg/kg) than that of endrin (0.07 mg/kg) 22 h after dosing. In male
rats intoxicated with syn-12-hydroxyendrin or 12-ketoendrin (at 16
mg/kg body weight each), the concentrations of 12-ketoendrin in the
brain 30 min after dosing were much higher (mean values, 1.9 and 1.4
Table 21. Oral LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Mouse Corn oil 8.6 - Spynu (1964)
Mouse Unknown 13 13 Gray et al. (1981)
Rat (4-5 weeks) Peanut oil 28.8 16.8 Treon et al.(1955)
Rat (6 months) Peanut oil 43.4 7.3 Treon et al. (1955)
Rat (6 months) Peanut oil 40.0 - Speck & Maaske (1958)
Rat (adult) Peanut oil 17.8 7.5 Gaines (1960)
Rat (7 weeks) Cottonseed oil 27 - Boyd & Stefec (1969)
Rat (12-14 weeks) Dimethyl sulfoxide 5.6a 5.3a Bedford et al. (1975b)
Rat (12-13 weeks) Corn oil 8.9 4.0 Carter & Simpson (1978)
Rat Corn oil 9.0 - Spynu (1964)
Rat Unknown 4 4 Gray et al. (1981)
Rabbit Peanut oil - 7-10 Treon et al. (1955)
Guinea- pig Peanut oil 36.0 16.0 Treon et al. (1955)
Hamster (golden Syrian) Corn oil - 18.6 Chernoff et al. (1979)
Hamster (golden Syrian)
(6 weeks) Unknown 12 17.0 Cabral et al. (1979)
Hamster Unknown 18 18 Gray et al. (1981)
Cat Cod liver oil Lethal dose: Ressang et al. (1958);
3-6 Cook & Casteel (1985);
Casteel & Cook (1985)
Monkey (Macacus mulatta) Peanut oil 3 Treon et al. (1955)
Monkey (Macacus speciosa) Unknown 12 Barth (1967)
Domestic goat Unknown 25-50 Tucker & Crabtree (1970)
Mule deer Unknown 6.25-12.5 Hudson et al. (1984)
aEndrin of 99% purity
Table 22. Dermal LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Rat Xylene 18 15 Gaines (1960,1969)
Rat Shellsol A 10-20 5-10 Carter & Simpson (1978)
Rat Toluene approx. 10 approx. 10 Carter & Simpson (1978)
Rat Corn oil 12.5 - Spynu (1964)
Rabbit None - Minimum lethal Treon et al. (1955)
dose: 60-94
Cat Cod-liver oil Lethal dose: Ressang et al. (1958)
approx. 150
Table 23. Parental LD50s for technical- grade endrin
Species Route Vehicle LD50 (mg/kg Reference
body weight)
Mouse Intraperitoneal Corn oil 5.6 Graves & Bradley
(1965)
Mouse Intravenous Dimethyl 2.3 Walsh & Fink
sulfoxide (1970, 1972)
Dog Intravenous Ethanol 3 Hinshaw et al.
(1966)
Table 24. Acute oral toxicity of mammalian metabolites of
endrin in rats
Compound Oral LD50 (mg/kg body weight)
Male Female
Mean 95% CI Mean 95% CI
Endrin 5.6 3.0-7.9 5.3 3.6-7.4
anti-12-Hydroxyendrin 2.4 2.0-3.0 5.5 4.2-7.2
syn-12-Hydroxyendrin 1.2 0.6-1.7 2.8 0.8-4.0
12-Ketoendrin 1.1 0.7-1.5 0.8 0.5-1.2
a95% CI, 95% confidence interval
mg/kg, respectively) than those in the brains of rats given a similar
but non-toxic dose of anti-12-hydroxyendrin (mean, 0.09 mg/kg) and
killed at the same time. The signs of intoxication were similar to
those of endrin (Bedford et al., 1975b).
These results suggest that 12-ketoendrin may be the acute
toxicant in rats. The production of 12-ketoendrin varies greatly from
one mammalian species to another, however, and none has been detected
in birds of various species that were killed by endrin (Stickel et
al., 1979).
8.1.4.2 Isomers
As described in section 4.2, endrin is changed under the
influence of sunlight into delta-ketoendrin. The acute toxicity of
this isomer is given in Table 25. It is less toxic than endrin, and,
like endrin, it is more toxic to female than to male rats. The signs
of intoxication are similar to those seen with endrin.
The acute tocixity of the endrin aldehyde has been reported to be
> 500 mg/kg body weight in male mice (Phillips et al., 1962).
Table 25. Acute toxicity of delta-ketoendrin
Sex and Route LD50 (mg/kg Reference
species body weight)
Male rats Oral 120-180 Soto & Deichmann
(1967)
Female rats Oral 10-36
Male rats Oral 62.1 (53.3-72.2) Stanford Research
Institute (1954)
Rats Intravenous 5 Soto & Deichmann
(1967)
Male rats Intraperitoneal 82 Stanford Research
Institute (1953)
Male mice Oral 23.6 (19.9-28.0) Stanford Research
Institute (1954)
Male mice Intraperitoneal 16.7 Stanford Research
Institute (1953)
8.1.5 Acute toxicity of formulated material
8.1.5.1 Oral and dermal administration
Oral and dermal LD50 values for formulated endrin in rats
(Muir, 1970) are presented in Table 26. Dry formulations were
administered orally as 1-2% aqueous suspensions and dermally in both
dry form and as 2-5% aqueous suspensions. In general, the type of
formulation did not significantly alter the acute oral toxicity of
endrin. The dermal toxicity of the 50% wettable powder was similar to
that of the 20% emulsifiable concentrate; the 2% field strength dust
was the least toxic.
Ten rabbits (body weight, 2.4-4.1 kg) were treated with an
emulsifiable concentrate containing 19.4% endrin on clipped skin at a
dose of 200 mg/kg body weight, and the material was allowed to remain
in contact with the skin for 24 h. Four of the 10 animals died within
48 h (Anderson et al., 1953). Two of 10 rabbits (body weight,
2.0-2.5 kg) treated similarly with a 25% dust concentrate died within
48 h (Hine et al., 1954).
Table 26. Oral and dermal LD50s for endrin formulations in rats
Formulation LD50 (mg/kg body weight)
Oral Dermal
Formulation Active Formulation Active
material material
20% Emulsifiable 20 4.20 52.20 (undiluted) 10.90
concentrate
50% Wettable 7.6 3.80 21.80 (dry) 10.90
powder 14.40 (aqueous) 7.20
2% Field strength 275 5.50 5720 (dry) 114.40
dust 1140 (aqueous) 22.80
From Muir (1970)
8.1.5.2 Inhalation
Ten adult rats were exposed for 1 h to a mist of of an
emulsifiable concentrate containing 19.4% by weight of endrin in
xylene, at a concentration of endrin slightly exceeding 2000 mg/m3
of air, and were observed for 48 h. The particle size of the mist and
other details of exposure were not reported. Three of the animals died
1-14 h after exposure (Anderson et al., 1953).
Groups of 10 Long Evans rats were exposed for 1 h to 25% and 30%
endrin dust concentrates at a concentration of 2000 mg/m3 of air.
Particle size and other details of exposure were not provided. Five
rats exposed to the 30% and three exposed to the 25% dust died within
48 h after exposure (Hine et al., 1954).
8.2 Short-term exposure
8.2.1 Oral administration
8.2.1.1 Mouse
Feeding studies were conducted to estimate the maximum tolerated
doses of endrin in B6C3F1 mice. Groups of five males and five females
were given a normal diet or one containing endrin at 2.5-20 mg/kg for
6 weeks, followed by observation for another 2 weeks. Three males and
four females given 10 mg/kg died, but no mortality occurred at 5
mg/kg. No data were provided on animals fed 20 mg/kg.
Hyperexcitability was observed in male mice given doses > 5 mg/kg of
diet. Mean body weight gains were comparable with those of controls.
The maximum tolerated dose was calculated by extrapolation to be 5
mg/kg of diet (NCI, 1978, 1979).
8.2.1.2 Rat
Groups of three male and two to three female Carworth rats,
either 29 days or 6 months old, received daily doses of endrin at 1,
2, or 5 mg/kg body weight in peanut oil by gavage, on five days per
week for 67-72 days. All rats given 1 mg/kg survived; the increased
mortality in the other groups was dose-related: 2/5 females at 2 mg/kg
and 3/3 males at 5 mg/kg day died. Pathological findings at autopsy
included diffuse degenerative changes in the liver, kidneys, and
brain, while survivors showed no such changes. All treated animals
lost weight and developed hypersensitivity to stimuli (Treon et al.,
1955).
Groups of five male and five female adult Sprague-Dawley rats
were given diets containing technical-grade endrin at 0, 1, 5, 25, 50,
or 100 mg/kg diet over a maximal period of 16 weeks and were observed
for behaviour, weight gain, feed consumption, mortality rate, and
symptoms of toxicity. Alkaline phosphatase levels, determined once a
week, were higher in rats fed endrin than in the control group, and
the total average feed consumption of treated rats was less than that
of the control group. All rats fed 100 mg/kg of diet died during the
first two weeks of the study, and only two female rats fed 50 mg/kg of
diet survived the experiment. All male rats given 1 mg/kg and all
female rats given 1 and 5 mg/kg of diet survived. Males appeared to be
more susceptible than females to endrin in this study. The symptoms of
intoxication were hypersensitivity to stimuli and convulsions:
hypersensitivity was noted in all rats, and convulsions occurred among
rats receiving 25, 50, and 100 mg/kg diet. Weight loss was
dose-dependent and significant in all rats treated with endrin (Nelson
et al., 1956).
To estimate the maximum tolerated doses of endrin in
Osborne-Mendel rats, groups of five males and five females were given
diets with or without endrin for 6 weeks, followed by observation for
another 2 weeks. Endrin was added to the diet in two-fold increasing
concentrations of 2.5-80 mg/kg. Mortality was not increased at 10
mg/kg, and mean body weight gain was no different from that in
controls. At 20 mg/kg of diet, one animal of each sex died. The
maximum tolerated dose was calculated by extrapolation to be 15 mg/kg
diet (NCI, 1978, 1979).
8.2.1.3 Rabbit
Four of five female rabbits given an oral dose of endrin at 1
mg/kg body weight on five days per week died following the
administration of 2, 30, 35, and 50 doses, respectively. The fifth
rabbit survived 50 doses over a period of 10 weeks. Diffuse
degenerative changes were observed in the liver and kidneys but not in
the brain (Treon et al., 1955).
8.2.1.4 Dog
Dogs (mainly two per group) fed endrin at 5-50 mg/kg of diet died
within 50 days. They regurgitated their food, became lethargic,
salivated, and later refused to eat; they became emaciated and
developed respiratory distress and signs of stimulation of the central
nervous system. Diffuse degenerative lesions in the brain, heart,
liver, and kidneys, together with pulmonary hyperaemia and oedema were
observed. Three dogs fed diets containing 4 mg/kg of diet for 6 months
survived, but they showed reduced body weight gain and a slight
increase in liver:body weight ratio; no histopathological change was
observed. At 3 mg/kg of diet or less, growth was normal (Treon et al.,
1955).
Beagles (one male and one female/group; control group, only one
dog) were fed diets containing endrin at 0, 1, or 3 mg/kg for 80
weeks. No sign of intoxication was observed, and the weight gain of
treated animals was comparable to that of controls. The ratios of
kidney and heart to body weight were increased at 3 mg but not at 1 mg
(about 0.045 mg/kg body weight). No histopathological change was found
in the viscera (Treon et al., 1955).
Groups of three male and three female pure-bred beagle dogs
(4-6 months old) were fed endrin in the diet at 0, 0.1, 0.5, 1, 2, or
4 mg/kg for two years. Additional groups of four male and four female
dogs were fed endrin at 0, 1, or 4 mg/kg of diet. Two males and two
females of each group were killed after 6 and 12 months of feeding; no
other death occurred during the study. Convulsions were observed in
three dogs at 4 mg and in one female at 2 mg; no other sign of
intoxication or illness was apparent during the study. No adverse
effect was noted on growth, food consumption, haematology, or
urinalysis, and no compound-related change was found in serum alkaline
phosphatase, prothrombin time, or any of the other clinical chemical
parameters measured at regular intervals. All organ weights, relative
as well as absolute, were normal, except for occasional, slight
increases in liver weight in some of the females at 2 and 4 mg in the
diet. The only histopathological change found was a slight to moderate
vacuolation of liver cells in dogs fed 2 and 4 mg in the diet. No
renal change was observed in any of the dogs (Jolley et al., 1969).
8.2.1.5 Domestic animals
Sheep and cattle fed diets containing endrin at 2.5 or 5 mg/kg
for 112 days showed no indication of harmful effects (details not
given) (Radeleff, 1956). The convulsions and muscular tremors that
were induced in six 10-18-month-old male buffalo calves administered
a 20% emulsion of endrin led to a significant rise in lactic acid
concentration in the blood of the animals, possibly due to excessive
production of the acid inside the fasciculating muscles (Verma et al.,
1970).
8.2.2 Inhalation
Three mice, three rats, two guinea-pigs, two hamsters, four
rabbits, and one cat were exposed to sublimed endrin vapour at an
actual concentration of 5.44 mg/m3 for 7 h/day on 5 days/week for up
to 26 weeks. Two rabbits died after 26 and 90 exposures, respectively,
and one mouse died after 22 exposures. No convulsions were observed,
and all other animals survived. Surviving rabbits showed a
granulomatous type of pneumonitis; no histological change was found in
the other surviving animals (Treon et al., 1955).
8.2.3 Dermal administration
Three female rabbits with intact skin died after 19, 19, and 25
applications, respectively, of endrin as a dry powder at 150 m/kg body
weight for 2 h/day on 5 days/week. Applications of 75 mg/kg resulted
in the death of one of three rabbits after 8 weeks; the other two
survived for 13-14 weeks--the end of exposure. Convulsions, tremors,
and twitching of the facial muscles were the main signs of
intoxication. Two of five rabbits (dose not specified) showed severe
fatty degeneration of the liver (Treon et al., 1955).
8.3 Skin irritation
Dry powdered endrin was applied repeatedly at a dose of 75 or 150
mg/kg body weight for 2 h/day, 5 days/week for up to 14 weeks on
intact or abraded skin of female rabbits (see section 8.2.3). No skin
irritation was observed. Single applications of endrin as dry powder
at doses up to 250 mg/kg body weight for 24 h on rabbit skin caused no
gross or microscopic damage to the skin of the animals (Treon et al.,
1955).
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Mouse
CFW mice (20 males and 20 females) were fed diets containing
endrin (96%) at 0 and 5 mg/kg for 120 days, beginning 30 days before
mating. Significant parental mortality (32%) and reduced litter size
were observed, but fertility, fecundity, and the number of litters
produced per pair were not affected (Good & Ware, 1969).
8.4.1.2 Rat
Forty male and 80 female Long-Evans rats were fed endrin in the
diet at 0, 0.1, 1, or 3 mg/kg over three generations, each generation
breeding once. No difference in appearance, behaviour, body weight, or
number or size of litters was seen. The weights of the liver, kidneys,
and brain were normal, and no histopathological abnormality was seen
in third-generation weanlings. The only significant effect was
increased mortality of pups in the second and third generations of
rats fed 3 mg/kg (Hine, 1965).
Ten male and 20 female Long-Evans rats were treated similarly,
but each generation bred twice. Weanling rats were mated after 79 days
on the diets (when they were 100 days old). All pups from the first
litters were discarded at weaning, and the parent rats were mated
again. Randomly selected pups from the second litters were maintained
on the diets and mated when 100 days old; this was done for three
generations. The number of pups in each litter was counted on the day
of birth and on the fifth day; on the twenty-first day, the weanlings
were counted and weighed and either sacrificed or saved for
continuation. Parent rats were weighed, sacrificed, and examined
grossly when no longer needed. Ten male and 10 female F3b weanlings
each from the controls and the highest dose-level group and five males
and five females from the 0.1 and 1.0 mg groups were autopsied. Body,
liver, kidney, and brain weights were recorded, and sections of these
organs and from heart, lung, spleen, and testis were studied
histologically. Appearance, behaviour, body weight, number and size of
litters, organ weights, and histopathological appearance of F3b
weanlings were comparable with those in control animals. No effect on
reproduction was observed in rats fed diets containing endrin at 2
mg/kg over three generations (Hine, 1968).
8.4.2 Embryotoxicity and teratogenicity
8.4.2.1 Mouse
Groups of 10 CD-1 mice were given a single oral dose of endrin
(99%) at 2.5 mg/kg body weight (stated to be half the LD50) in corn
oil by gavage on day 9 of gestation; an untreated and a vehicle
control group were also used. Fetuses were examined on day 18. No
significant effect was observed on intrauterine death or fetal weight,
but the incidence of total anomalies was increased over that in
controls: 2/117 fetuses had cleft palates, three had open eye, and two
had other anomalies. No data on maternal toxicity were reported
(Ottolenghi et al., 1974).
These results could not be repeated by Kavlock et al. Female CD-1
mice were given endrin (99%) at 0 (vehicle), 0.5, or 1.0 (groups of 40
mice), or 1.5 or 2.0 mg/kg body weight (groups of 20 mice) in corn oil
by gavage on days 7-17 of gestation. The animals were killed on day
18. Maternal deaths occurred in the 1.5 and 2 mg/kg groups, reduced
maternal weight gain was observed at and above 1 mg/kg, and maternal
liver weight was increased at 0.5 mg/kg and higher. Fetal weight and
skeletal, and visceral maturity were adversely affected at doses of 1
mg/kg and above. No teratogenic effect or embryonic lethality was
observed, even at doses that caused maternal death (Kavlock et al.,
1981, 1987).
In a study of the effects of acute alterations in maternal health
status upon fetal development in the mouse, groups of 21 or 40
pregnant CD-1 mice were given a single oral dose of technical-grade
endrin at 0 (vehicle), 7 or 9 mg/kg body weight in corn oil on day 8
of gestation. The animals were killed on day 18 of gestation. Three of
21 animals given 7 mg/kg (14%) and 19/40 mice gievn 9 mg/kg (47%)
died. Maternal weight gain was decreased in both test groups; the
total number of implantation sites and number of viable litters were
not affected, but fetal weight was reduced. Delays in ossification of
the skeleton and an increased incidence of supernumary lumbar ribs
were observed. Although three fetuses from one litter in the 9 mg/kg
group had fused ribs, no significant increase in the incidence of
malformations was found. A statistically significant, linear, inverse
relationship between maternal weight gain and the presence of
supernumary ribs in their offspring was found (Kavlock et al., 1985).
8.4.2.2 Rat
Five groups of 25 female CD rats were administered endrin (97%)
in methocel in oral doses of 0, 0.1, 0.5, or 2 mg/kg body weight per
day on days 6-15 of gestation, or vitamin A, used as a positive
control. The animals were killed on day 20. The largest dose of endrin
caused maternal toxicity, as evidenced by weight loss and mortality
(two animals). The fetuses showed some slight growth retardation (not
significant) but no increase in intrauterine death rate. No effect
attributable to endrin was seen on the mean number of viable fetuses,
post-implantation losses, implantations, corpora lutea, fetal sex
ratio, or fetal external, soft-tissue, or skeletal abnormalities. Bent
ribs were observed in 6/522 fetuses treated with endrin, but not in
relation to dose. An increase in delayed ossification in sternebrae
and skull of fetuses was seen in the treated groups in comparison with
the untreated control group. Animals given vitamin A had a
significantly increased number of post-implantation losses and
malformed fetuses (Goldentahl, 1978a).
Groups of 32, 15, 28, 30, and 15 female CD rats were given oral
doses of endrin (99%) in corn oil at 0, 0.075, 0.15, 0.30, or 0.45
mg/kg body weight on days 7-20 of gestation. Rats were killed on day
21. Maternal weight gain was reduced at dose levels above 0.15 mg/kg,
but no increase in maternal liver weight was found. Fetal mortality,
weight, degree of skeletal and visceral maturation, and incidences of
skeletal and visceral anomalies showed no dose-related effect
(Kavlock et al., 1981).
8.4.2.3 Hamster
Three groups of golden Syrian hamsters (7, 24, and 8
animals/group) received a single oral dose of endrin (99%) in corn
oil at 5 mg/kg body weight (stated to be half the LD50) on day 7,
8, and 9 of gestation, respectively. Two control groups were used,
consisting of 57 untreated and 41 vehicle controls. The animals were
killed on day 14. The number of resorptions and of dead fetuses was
increased after treatment on days 7 or 8 and to a lesser extent in the
vehicle controls. The live fetuses in all three treated groups showed
significant growth retardation when compared with controls. The
incidence of anomalies was high only after treatment on day 8:
congenital abnormalities were seen in 28% of fetuses, with open eye in
22%, webbed foot in 16%, cleft palate in 5%, and fused ribs in 8%. The
anomalies that appeared to be increased significantly but to almost
the same extent at all three stages were fused ribs and cleft palate
(Ottolenghi et al., 1974).
These results could not be repeated by other workers. Groups of
27-29 golden Syrian hamsters were administered oral doses of endrin
(97%) in methocel at 0 (two control groups), 0.1, 0.75, or 2.5 mg/kg
body weight on days 4-13 of gestation. The animals were killed on
day 14. Body weight gains of the animals given 2.5 mg/kg were
slightly reduced. Maternal appearance, behaviour, and survival, mean
number of viable fetuses, post-implantation losses, implantations,
corpora lutea, fetal body weight, and crown-rump length showed no
change attributable to treatment. The number of malformations in
fetuses was not increased, but ossification of the sternebrae and
certain ribs was delayed (Goldentahl, 1978b).
Groups of 18-87 golden Syrian hamsters (LVG strain) were given
endrin (98%) as a solution in corn oil by gavage either as a single
dose of 0.5, 1.5, 5, 7.5, or 10 mg/kg body weight on day 8 of
pregnancy or as multiple daily doses of 0.75, 1.5, 2.5, or 3.5 mg/kg
body weight on days 5-14 of pregnancy. All animals were killed on day
15. With single doses, no effect was found on maternal survival,
pregnancy rate, weight change or liver:body weight ratio. The only
sign of maternal toxicity was the occurrence of transient convulsions
2 h after dosing in one hamster given 10 mg. No compound-related
difference was noted in the number of implantation sites, fetal death
rate, or fetal weight; indicators of skeletal maturity were not
affected. A dose-related increase in the incidence of fused ribs was
found in the groups given 7.5 and 10 mg/kg; increased incidences of
meningo-encephaloceles were observed at 5 mg/kg and above, with no
dose-response relationship. No other compound-related skeletal or
visceral anomaly was noted. In the study of multiple doses, maternal
toxicity (reduced weight gain and increased mortality) and fetal
toxicity (increased mortality, reduced weight, reduced skeletal
ossification, and an increased percentage of irregular
supra-occipitalis) were observed at doses of 1.5 mg/kg and higher. No
treatment-related maternal or fetal effect occurred at 0.75 mg/kg per
day (Chernoff et al., 1979).
8.4.2.4 Perinatal behavioural development
Rats exposed perinatally to endrin at 0, 0.075, 0.15, or 0.3
mg/kg body weight from gestation day 7 through day 15 of lactation
showed no mortality and no influence on survival or growth. Pups of
mothers exposed to 0.15 or 0.3 mg were more active than those of
mothers exposed to 0.075 mg or those in the control group. No clear
difference in ambulation was noted, and at 90 days of age there was no
difference (Gray et al., 1981; Kavlock et al., 1987).
Golden Syrian hamsters (LVG strain) given endrin (98%) at 0 or
1.5 mg/kg body weight per day by gastric intubation on days 5-14 of
gestation had a persistent increase in locomotor activity. Offspring
of treated hamsters ambulated more than the controls in the open field
at 15 days, and long-term observation of activity in the figure-8 maze
indicated that a significant increase in this behaviour was still
present at 125 days of age. Other behaviour patterns, including
sexual, rearing and running, and wheel behaviour, were unaffected.
Dams repeatedly exposed to endrin at 0.75 and 1.5 mg/kg body weight
were markedly hypoactive under the same testing conditions in which
the pups were hyperactive. The dose of 1.5 mg/kg body weight killed
more than half of the dams (Gray et al., 1981; Kavlock et al., 1987).
8.4.3 Appraisal of reproductive effects
Endrin had no reproductive effects in three generations of rats
at a level of 2 mg/kg of diet, equivalent to 0.1 mg/kg body weight. It
had no teratogenic effect in mice, rats, or hamsters after oral
exposure during the period of organogenesis. The significance of the
anomalies observed in mice and hamsters by Ottolenghi et al. (1974) is
uncertain. Studies in the same strain of the same species using more
rigorous protocols and larger numbers of animals could not confirm
their findings.
The lowest-observed-adverse-effect level for maternal toxicity
was 1.0 mg/kg body weight in mice, 0.3 mg/kg body weight in rats, and
1.5 mg/kg body weight in hamsters. Embryotoxicity was observed at
doses of 1 mg/kg body weight in mice and 1.5 mg/kg body weight in
hamsters. The overall no-observed-adverse-effect levels in mice, rats,
and hamsters were 0.5, 0.15, and 0.75 mg/kg body weight, respectively
(Table 27).
8.5 Mutagenicity and related end-points
8.5.1 Effects on microorganisms
Endrin was not mutagenic in numerous studies using Salmonella
typhimurium (TA98, TA100, TA1535, TA1537, TA1538, TA1950, TA1978,
SL4525, SL4700), Escherichia coli (WP2 uvrA, WP2 uvr-, Gal
Rs, WP2, hcr, p3478, W3100), K-12 (Pol A1+/Pol1-), WP67,
CM611, and CM571 Bacillus subtilis (M45), Saccharomyces cerevisiae
(D3, D7), and Serratia marcescens (a21, a742), with or without
metabolic activation by rat or mouse liver S9 (Fahrig, 1974; Van Dijck
& van de Voorde, 1976; Ercegovich & Rashid, 1977; Simmon et al., 1977;
Nishimura et al., 1982; Waters et al., 1982; Glatt et al., 1983;
Moriya et al., 1983; Rashid & Mumma, 1986).
No mutagenic effect was observed in S. typhimurium strains
TA98, TA100, TA1535, or TA1537, with and without metabolic activation
with S9 from livers of Aroclor 1254-induced rats and hamsters in the
presence of five concentrations of endrin (0-10 000 µg/plate) (Zeiger,
1987; Zeiger et al., 1987). No mutagenic effect was observed in
S. typhimurium strains TA97, TA98, TA100, or TA102 with and without
metabolic activation with Aroclor 1254-induced rat liver microsome
fraction in the presence of seven concentrations of endrin (99.0%),
from 1 ng/plate up to 1 mg/plate (Mersch-Sundermann et al., 1988).
8.5.2 Point mutations in mammalian cells
Endrin was weakly mutagenic in 6-thioguanine-resistant mouse
FM3A cells (Morita & Umeda, 1984; abstract only).
8.5.3 Dominant lethal mutations
Endrin did not show detectable dominant lethality when given as
a single intraperitoneal dose (0.76 or 3.8 mg/kg body weight) or daily
oral doses (0.1 or 0.25 mg/kg body weight) for 5 days to seven or nine
male ICR/Ha Swiss mice, respectively. This study involved a sequential
mating procedure, in which one male was housed with three females for
one week, repeated for 8 weeks (Epstein et al., 1972).
8.5.4 Chromosomal and cytogenetic effects
Endrin at 10-5 and 10-4 M, but not at 10-6, produced a
dose-related increase in the percentage of M1 metaphases and a
dose-related decrease in that of M3 metaphases at 48 h in treated
LAZ-007 human lympoid cells. This effect is closely related to the
reduced rate of cell proliferation induced by endrin (Sobti et al.,
1983).
Intratesticular injection of 0.25 mg endrin in saline to three
albino rats doubled the percentage of chromosomal changes in
comparison with that in the single control when the testes were
studied histologically 10 days after the injection. Changes were
scored in 70-75 cells/animal (Dikshith & Datta, 1973). The use of a
single dose does not aid interpretation, and the increase in
chromosomal abnormalities may be related to cytotoxicity rather than
to a genetic effect. The relevance of this type of study in
mutagenicity testing is unknown.
Table 27. Teratogenicity and effects on reproduction of oral administration of endrin
Animal Exposure NOAEL LOAEL Reference
(strain) period
Rat (Long Evans) 3 generations, 1 litter 1 mg/kg diet 3 mg/kg diet (0.15 mg/kg bw): Hine (1965)
(0.05 mg/kg bw) increased mortality of pups in
F2 and F3 generations
Rat (Long Evans) 3 generations, 2 litters 2 mg/kg diet Hine (1968)
(0.1 mg/kg bw)
Mice (CD-1) Days 7-17 of gestation 0.5 mg/kg bw 1 mg/kg bw: maternal and fetal Kavlock et al. (1981,
weight, skeletal and visceral 1987)
maturity
Rat (CD) Days 7-20 of gestation 0.15 mg/kg bw 0.30 mg/kg bw: reduced Kavlock et al. (1981)
maternal weight gain
Rat (CD) Days 6-15 of gestation 0.5 mg/kg bw 2 mg/kg bw: maternal toxicity Goldentahll (1978a)
Hamster Days 5-14 of gestation 0.75 mg/kg bw 1.5 mg/kg bw: maternal and Chernoff et al. (1979)
fetal toxicity
Chromosomal studies were carried out on lymphocytes from eight
male workers exposed to endrin and from six unexposed workers from the
same work area. No increase in the frequency of chromosomal
abnormalities was found, whether taken individually or collectively
(Dean, 1977).
Chromosomal aberrations were found in meiotic cells of barley and
somatic cells of barley and Vicia faba grown from endrin-treated
seeds (Wuu & Grant, 1966, 1967a,b). After treatment of root tips with
0.1% endrin (EC20 solution) for 1.5-2 h at 10 °C, the function of the
spindle was destroyed and did not interfere with the spreading of the
chromosomes during squash preparation. The centromeric region became
distinct and visible in prophase-metaphase chromosomes. At higher
concentrations contraction, stickiness, and fragmentation of
chromosomes were seen (Bhowmik, 1978).
8.5.5 Host-mediated effects
In two studies, male CF1 mice were given single oral doses of
endrin in dimethyl sulfoxide at 3.75 or 7.5 mg/kg body weight. Control
mice were dosed with the solvent, and positive control groups were
given a single oral dose of ethylmethanesulfonate at 400 mg/kg body
weight. Saccharomyces cerevisiae JD1 suspensions were then injected
intraperitoneally into each mouse, and the suspensions of
S. cerevisiae were harvested and analysed after 5 h. No increase in
mitotic gene conversion was detected (Brooks, 1976).
8.5.6 Sister chromatid exchange
Endrin at concentrations of 10-6-10-4 mol/litre in dimethyl
sulfoxide (the latter dose was a cytotoxic concentration) failed to
increase the frequency of sister chromatid exchange significantly over
the control value in rat liver microsomal S9-activated and unactivated
incubation experiments using human lymphoid cells of the LAZ-007 cell
line (Sobti et al., 1983).
8.5.7 Effects in Drosophila melanogaster
Endrin was not mutagenic to Drosophila melanogaster after
injection at 0.2 µlitre of a 0.001% aqueous solution, in the Muller-5
test for recessive lethal mutations on the X-chromosome (Benes & Sram,
1969).
8.5.8 Effects on DNA
Endrin at 10-3 or 3 x 10-3 mol/litre did not induce mutation
in the adult rat liver epithelial culture/hypoxanthineguanine
phosphoribosyl transferase assay (Williams, 1979).
DNA repair was not elicited in primary cultures of hepatocytes
from CD-1 mice, Fischer 344 rats, or Syrian hamsters exposed to endrin
for 18 h together with tritium-labelled thymine deoxyribonucleotide
for incorporation during repair synthesis. DNA repair was measured
autoradiographically. In rat and hamster liver cell cultures, a
concentration of 10-3 mol/litre and in mouse liver cell cultures,
10-4 mol/litre endrin was tested (Maslansky & Williams, 1981).
Endrin did not induce unscheduled DNA synthesis in human lung
fibroblast cells with or without metabolic activation by rat liver
microsomes (five concentrations were tested, but they were not given
in the paper) (Waters et al., 1982).
Endrin at eight concentrations ranging from 0.5 up to 1000
nmol/ml did not induce unscheduled DNA synthesis in primary rat
hepatocytes or in a modified Ames test utilizing concentration
gradient plates and 10 bacterial tester strains (eight S. typhimurium
and two E. coli) (Probst et al., 1981)
8.5.9 Appraisal of mutagenicity and related end-points
Garrett et al. (1986) evaluated the activity of endrin in a
series of tests: for reverse mutation (point/gene mutations in
prokaryotes), forward mutation (point/gene mutations in eukaryotes),
differential toxicity (primary DNA damage in prokaryotes), enhanced
mitotic recombination, gene conversion and crossing-over, unscheduled
DNA synthesis (primary DNA damage in eukaryotes), sister chromatid
exchange, chromosomal breakage, and dominant lethality (chromosomal
effects). Endrin gave negative results in all these tests.
The vast majority of the data indicate that endrin is not
genotoxic; however, many of the studies would not reach current
standards, or they give insufficient data to allow an independent
assessment.
8.6 Long-term exposure
Groups of 20 male and 20 female Carworth rats (28 days old) were
given diets containing endrin at 0, 1, 5, 25, 50, or 100 mg/kg. With
100 mg, two males and one female survived for 2 years; with 50 mg,
four males but no female survived; and with 25 mg, 11 males and 5
females survived. Survival at the lower concentrations was comparable
to that of the control group. Males appeared to be less susceptible
than females to the toxic action of endrin. Signs of intoxication,
hypersensitivity to external stimuli, and occasional convulsions were
observed only at the two highest levels. The weight gain of females
fed 1, 5, or 25 mg/kg of diet was equal to or greater than that of the
controls after 40 weeks of feeding. In males fed 5 mg, growth
retardation occurred during the first 20 weeks only, while males that
received 25 mg showed significant reduction in body weight gain. The
body weight gain of males fed 1 mg was comparable to that of controls.
The liver:body weight ratios were increased in males fed 5 mg or more
and in females fed diets with 25 mg or more. Histopathological
examination of animals that died during exposure to the three higher
dietary levels revealed diffuse degeneration of the liver, kidneys,
brain, and adrenal glands. The few survivors at 50 and 100 mg showed
degenerative changes in the liver only. No histopathological change
was found in surviving rats fed 1, 5, or 25 mg/kg diet. There was no
increase in the incidence of neoplasia in the treated groups compared
to the control group (Treon et al., 1955).
This study indicates a no-effect level for endrin of 1 mg/kg of
diet (about 0.05 mg/kg body weight) but is inadequate in several
respects, e.g., survival rate, details of pathology, and
haematological and clinical chemical data are not reported.
8.7 Carcinogenicity
8.7.1 Oral administration
8.7.1.1 Mouse
Groups of 100 male and female C57B1/6J mice, an inbred strain
with a low incidence of tumours, and C3D2F1/J mice, a hybrid strain
with a high incidence of hepatomas in males and a high incidence of
mammary tumours in females, were fed endrin (99%) at dietary
concentrations of 0.3 or 3 mg/kg from the age of five weeks throughout
life. A control group consisted of 200 mice of each sex of each
strain. Except for all groups of female animals of the C3D2F1/J
strain, this part of the experiment was terminated at the 78th week
because of the early occurrence of high numbers of mammary
fibroadenomas in 70-90% of control and treated mice. Survival, growth,
food intake, and haematology were not impaired. Mice of both strains
fed 3 mg/kg diet occasionally developed convulsions in the early
stages of feeding but recovered and survived without signs of illness.
They generally showed the typical histological changes in the liver
characteristic of high doses of chlorinated hydrocarbon insecticides.
No effect was observed on mice fed 0.3 mg/kg diet. The tumour
incidence and type of tumours were not influenced by the feeding of
endrin, and it had no influence on the incidence of fibroadenomas in
female C3D2F1/J mice (Witherup et al., 1970).
Groups of 50 B6C3F1 mice of each sex were given endrin in the
diet for 80 weeks and were then observed for a further 10 or 11 weeks.
The initial doses of endrin (97%) (2.5 and 5 mg/kg of diet) were
poorly tolerated by males and were therefore reduced after 25 weeks to
1.2 and 2.5 mg/kg diet for males; but females received 2.5 and 5 mg/kg
diet during the whole experiment. The time-weighted average doses were
1.6 and 3.2 mg/kg diet for males and 2.5 and 5.0 mg/kg diet for
females. Matched controls consisted of groups of 10 mice of each sex;
pooled controls, used for statistical evaluation, consisted of the
matched control groups combined with 50 untreated male and 50
untreated female mice from similar bioassays of other chemicals. All
surviving mice were killed at 90 or 91 weeks. Mean body weight was not
affected, but the survival of males at the high dose was lower than
that of the controls. The survival of the low-dose males could not be
evaluated due to accidental administration of excessive quantities of
endrin to this group during week 66. The tumour (neoplastic lesions in
the liver) incidences in the high-dose males were higher than those of
the pooled or matched controls, but not significantly so, and the
increase was not considered to be related to the administration of
endrin (Fredrickson, 1978;NCI, 1978).
8.7.1.2 Rat
A study of groups of 20 male and 20 female Carworth rats
administered endrin at 0, 1, 5, 25, 50, and 100 mg/kg of diet was
reviewed in Section 8.6.1.1. Bearing in mind the limitations of this
study, such as small group sizes and low survival at the high doses,
no evidence of an increase in the incidence of neoplasia was found in
any of the groups (Treon et al., 1955).
Groups of 50 weanling Osborne-Mendel rats of each sex were fed
diets containing endrin (98%) at 2, 6, or 12 mg/kg for 29 months. The
control groups consisted of 100 males and 100 females. During the
first 10 weeks of the study, only half the nominal dietary
concentrations of endrin were fed. Signs of toxicity occurred in a few
animals in all treatment groups, mainly in females, and included
episodes of tremor and clonic convulsions with 'outcries', the
incidence of these signs being dose-related. Weight gain was
unaffected, and the survival rates in control and treated rats were
similar. The liver:body weight ratios were unaffected. A moderate (not
dose-related) increase in the incidence of centrilobular cloudy
swelling in the liver and of cloudy swelling of the renal tubular
epithelium was observed. The lungs of the animals fed endrin exhibited
a moderate increase in the incidence of congestion and focal
haemorrhages. The tumour incidence in the treatment groups was
comparable with that in control rats, and no difference in the type of
tumours was found (Deichmann et al., 1970a,b; Deichmann & MacDonald,
1971).
In a life-time study, groups of 24 male and 24 female
Osborne-Mendel rats (22 days old) were fed diets containing endrin at
0, 0.1, 1, 5, 10, or 25 mg/kg. Because 50% of the rats at 25 mg/kg
died within the first week, this group was restarted with 32-day-old
rats. Survival did not appear to be affected by treatment. The highest
incidence of malignant tumours in male and female rats occurred at 0.1
mg/kg, but the malignancies were not dose-related. Treated male rats
had a higher incidence of renal disease than controls, but this also
was not dose-related (details not available) (Reuber, 1978).
Groups of 50 Osborne-Mendel rats of each sex were fed endrin
(97%) in their diet for 80 weeks and then observed for 31 or 34 weeks.
Males received doses of 2.5 or 5 mg/kg diet; in females, the initial
doses of 5 and 10 mg/kg of diet were poorly tolerated and were reduced
after 9 weeks to 2.5 and 5 mg/kg. The time-weighted average doses were
2.5 and 5.0 mg/kg for males and 3 and 6 mg/kg for females. Matched
controls consisted of groups of 10 rats of each sex; pooled controls
used for statistical evaluation consisted of the matched control
groups combined with 40 untreated male and 40 untreated female rats
from similar bioassays of other chemicals. All surviving rats were
killed at 100-114 weeks. Body weights and survival were not affected
by administration of endrin. A slight increase in the incidences of
pituitary and thyroid tumours was observed, but no consistent
statistical significance or dose-response relationship was found
(Fredrickson, 1978; NCI, 1978).
8.7.1.3 Tumour promotion
No significant increase in the development of preneoplastic
changes (hyperplastic nodules) was observed in rat liver after partial
hepatectomy and initiation with N-nitrosodimethylamine or
N-2-fluorenylacetamide in combination with the administration of
endrin (Ito et al., 1980).
In vitro, endrin at levels of above 2.5 µg/ml appeared to
inhibit metabolic cooperation in the hypoxanthineguanine
phosphoribosyl transferase system using wild-type
6-thioguanine-sensitive V79 cells and variant 6-thioguanine-resistant
cells. Such inhibition is reported to be an index of potential tumour
promoting activity, although the test has not been validated (Kurata
et al., 1982).
Endrin stimulated protein kinase C activity in vitro only
slightly, whereas a representative endogenous ligand of protein kinase
C, syn-1,2-didecanoylglycerol, stimulated protein kinase C to a
maximal velocity (Moser & Smart, 1989).
8.7.2 Appraisal of carcinogenicity
One of several studies in mice suggests an increased incidence of
nonmalignant tumours in animals of one sex, but this study was
considered inadequate for assessing carcinogenicity because an
increased number of tumours was seen in controls. A second study using
a different mouse strain did not corroborate the increase in tumour
incidence. Several long-term feeding studies in rats provide no
evidence of a carcinogenic effect of endrin. Its tumour promoting
activity was tested in vitro using protein kinase C stimulation and
ATPase inhibition; the results do not suggest any overwhelming effect
in these systems. After a careful review of this evidence, and taking
into consideration the fact that most of the data indicate that endrin
is not genotoxic, the Task Group concluded that the data are
insufficient to indicate that endrin is a carcinogenic hazard to
humans.
8.8 Special studies
8.8.1 Nervous system
8.8.1.1 Electrophysiological studies
The effects of endrin on bioelectrogenesis was studied in
anaesthetized pigeons and squirrel monkeys with chronically implanted
electrodes. Endrin was administered intravenously to pigeons at doses
of 0.5-4 mg/kg body weight. Doses of 2-4 mg/kg and higher caused
seizure activity throughout the telencephalon; the lower dose levels
caused activity only in the ectostriatum, a telecephalic visual
projection area. At doses of 0.5-2 mg/kg body weight, endrin caused a
specific increase in the evocation of potentials in the ectostriatum
by stimulation of the nucleus rotundus, a diencephalic visual
projection area. Reticular formation functions were not or little
affected. Administration of endrin to squirrel monkeys at doses of
0.2-3 mg/kg body weight on 5 days/week intramuscularly in corn oil and
saline emulsion induced characteristic changes in the
electro-encephalogram (EEG), culminating in electrographic seizures;
these were transient and disappeared when endrin administration was
stopped. Seizures reappeared under stress conditions, however, several
months after endrin treatment (Revzin, 1966, 1980).
Groups of 20-60 Sprague-Dawley rats with previously implanted
electrodes were given endrin in peanut oil orally at 0.8, 1.7, or 3.5
mg/kg body weight per day on 5 days/week for 28 weeks. Dose-dependent
mortality occurred during the first week and again at the end of the
study. Most changes in the EEG were seen after one week of exposure:
these included severe bursts of multiple spikes accompanied by clonic
convulsions; other animals had runs of spikes without full-fledged
convulsions. The convulsions were usually preceded by a period of
hyperventilation. After a further week of exposure, the rats showed
normal EEG traces. Some irregular slow-wave activity was seen in
animals that were moribund in the last month of feeding (Speck &
Maaske, 1958).
The convulsive properties of endrin at 1-2 mg/kg body weight
were studied by intravenous injection in locally anaesthetized,
paralyzed male cats, in which electrodes were placed in the
subcortical structures of the brain. Endrin was dissolved in ethanol
(which itself stimulates or inhibits the central nervous system,
depending on dose). Changes in the EEG and evoked responses were
studied. Hypersynchrony, rhythmic bursts of spikes and waves, and
isolated spikes characterized the preictal state. Seizures were always
bilateral and symmetrical and of a general tonic-clonic type.
Responses in sensory and motor cortexes to sensory nerve stimulation
were enhanced three to five fold. The authors concluded that endrin is
directly toxic to the mammalian nervous system, is a potent rapidly
acting convulsant, and does not require metabolic activation to an
active metabolite (Joy, 1976).
8.8.1.2 Histopathological studies
Male CD1 Swiss mice were administered endrin or sesame oil daily
by intraperitoneal injection in gradually increasing doses of 1.5-4.0
mg/kg for 4-20 days. Electron microscopic examination of sciatic nerve
tissue revealed no morphological change in myelinated nerve fibres,
myelin, or associated Schwann cells, but morphological alterations
were observed in unmyelinated nerve fibres and associated Schwann
cells: axons were swollen, microtubules and neurofilaments showed
dissolution, axoplasm was replaced by large clear vesicles,
vacuolization was present, and Schwann cells and adaxonal spaces also
contained vesicles (Walker & Phillips, 1987; abstract only).
8.8.1.3 Neurotransmitter systems
gamma-Aminobutyric acid systems: The role of the inhibitory
neuro-transmitter of the central nervous system, gamma-aminobutyric
acid (GABA),in the production of convulsions is well established.
Polychloro-cycloalkane insecticides such as endrin have a potent
excitatory action on the nervous system, and the interaction between
GABA function and endrin has been studied.
Endrin strongly inhibited GABA-dependent 36Cl uptake by mouse
brain vesicles, with an IC50 (the concentration required to cause
50% inhibition) of 2.8 µmol/litre. Inhibition was confined to that
portion of 36Cl uptake that is GABA-dependent. The result
demonstrates disruption of GABA ionophore function in mammalian brain,
possibly providing the principal mechanism of toxicity (Bloomquist &
Soderlund, 1985). In a comparison of the inhibitory potential of
several polychlorocycloalkane insecticides on GABA-dependent 36Cl
uptake, the most potent inhibitor was 12-ketoendrin, followed by
isobenzan, endrin, and then dieldrin, heptachlor epoxide, aldrin,
heptachlor and lindane. This order closely parallels their acute
toxicities (Bloomquist et al., 1986).
The effect of these chemicals was also studied in the tert
butylcyclo-phosphorothioate (TBPS) system, which has been shown to
bind convulsants with varying affinities. The IC50 for endrin on
35S-TBPS binding was 0.22 µmol/litre and that for 12-ketoendrin,
0.036 µmol/litre. These were the most potent inhibitors of TBPS
binding, and there was a significant linear correlation between 36Cl
flux and TBPS binding (Bloomquist et al., 1986).
In vitro, endrin inhibited 35S-TBPS binding in tissue from
male Swiss-Webster mice with an IC50 of 18 nmol/litre (range, 4-90).
In vivo, doses representing 25, 50, and 100% of the LD50 (8 mg/kg
intraperitoneally) inhibited 35S-TBPS binding with IC50s of 77 ±
7 nmol/litre (LD50) and 39 ± 6 nmol/litre (LD50/2); no inhibition
was observed at LD50/4, indicating a possible no-observed-adverse-
effect level. Brain P2 membranes of treated mice contained endrin and
12-ketoendrin. The finding that the brains of treated mice contained
sufficient endrin or its biotransformed products to achieve TBPS
binding and that this was correlated with the severity of the
poisoning indicates that the acute toxicity of endrin to mammals is
regulated by GABA (Cole & Casida, 1986).
GABA-induced 36Cl flux into membrane microsacs was inhibited
by endrin at 3.9 ± 0.2 nmol/mg protein, which also suggests that
endrin inhibits the function of this receptor (Abalis et al., 1985,
1986). The IC50 for 36Cl influx was 0.19 ± 0.06 µM and that for
35S-TBPS binding was 0.003 µM (Gant et al., 1987).
Endrin inhibited both insect and rat GABA receptors in a
dose-related, non-competitive manner. It acts in a similar manner on
the GABA receptors in the central nervous system of the two species.
The blocking action may involve non-competitive binding to an
allosteric site associated with the receptor's chloride channel
(Wafford et al., 1989a).
Endrin potentially inhibits 35S-TBPS binding to rat brain
membranes and also potentiates the protective effect of NaCl (200 mM)
against heat inactivation of 3H-flunitrazepam binding sites on the
same membranes. The time courses of heat inactivation of these binding
sites in the presence of NaCl and saturating concentrations of endrin
revealed monophasic components constituting about 88% of the binding
sites (Squires & Saederup, 1989).
Endrin has also been shown to inhibit GABA-ergic function in
Torpedo fish (Matsumoto et al., 1988), chicken embryos (Seifert,
1988, 1989), the mosquito fish (Gambusia affinis) (Bonner &
Yarbrough, 1989), and the cockroach (Periplaneta americana) (Wafford
et al., 1989b).
Other amine systems: Studies on the effects of orally
administered endrin on the content of biogenic amines in the brain of
rats did not contribute to an understanding of the convulsive action
of endrin (Miller & Fink, 1973; Hrdina et al., 1974).
Cyclic AMP metabolism: Endrin did not affect adenylate cyclase
activity or inhibit the activity levels of synaptosomal
phosphodiesterase, enzymes involved in cyclic AMP metabolism, in rat
brain. The authors interpreted their results to support their
postulation that organochlorine insecticides exert their neurotoxic
action by selective inhibition of ATPases in synaptosomes (Kodavanti
et al., 1988).
ATPase systems: Inhibition of rat brain Na+-K+ATPase by
chlorinated insecticides varied considerably: endrin and dieldrin were
the least active in inhibiting both this enzyme and K+-stimulated
para-nitrophenyl phosphatase at a concentration of 2 x 10-5
mol/litre. Results of experiments on ATP-32Pi exchange suggest
that DDT is a powerful inhibitor of oxidative phosphorylation, which
may lead to depletion of ATP. This effect was much less evident with
endrin (Folmar, 1978).
Endrin caused about 15% inhibition of the activity of
Na+-K+ATPase in rat brain synaptosomes at the highest
concentration tested, 120 µM, and oligomycin-sensitive Mg2+-ATPase
in rat brain synaptosomes was significantly inhibited in a
concentration-dependent manner, to a maximal inhibition of 33% at the
highest dose. Endrin did not inhibit oligomycin-insensitive
Mg2+-ATPase, and it did not affect K+-stimulated
para-nitrophenyl phosphatase from rat brain synaptosomes; this
enzyme represents the dephosphorylation step of the overall reaction
to the Na+-K+ATPase. Oligomycin-sensitive Mg2+-ATPase in beef
heart mitochondria was significantly inhibited. The results of this
study suggest that the ATPase system in rat heart and central nervous
system is not selectively inhibited by endrin (Mehrotra et al., 1989).
Sodium channel: It has been demonstrated using voltage clamp
techniques in single cells that application of DDT prolongs the sodium
current, which in turn decreases the depolarizing after-potential to
initiate repetitive after-discharges in the cell. The repetitive
after-discharges facilitate synaptic transmission and result in
nervous system hyperexcitability, which at the functional level is
registered as tremors and eventually convulsions and death (Narahasi,
1987). Even if less than 1% of the sodium channels respond in this
manner to insecticides, it is sufficient to cause toxicity in the
animal. Narahasi (1987) reported these effects with pyrethroids and a
series of DDT analogues; such studies have not been carried out with
endrin. Lund & Narahasi (1983) suggested that because of the
similarity in the symptomatology of intoxication by the family of
organochlorine insecticides, the target site of endrin may also be the
sodium channels.
8.8.1.4 Appraisal of effects on the nervous system
The effect of endrin on the nervous system has received attention
because it has the well established ability to cause convulsions
following acute exposures. Endrin causes considerable changes in EEG
activity, which are associated with convulsions, at intramuscular
doses in experimental animals as low as 0.2 mg/kg body weight.
The probable underlying mechanisms are associated with a
dose-related, non-competitive inhibition of the GABA-ergic
neurotransmitter system. This is an inhibitory system, and removal of
its action leads to increased excitation in the nervous system. While
inhibition of GABA-ergic function is common to a number of
polychlorocycloalkane insecticides, endrin, and particularly its
metabolite 12-ketoendrin, have been shown to be extremely potent
inhibitors of this function. It appears therefore that the acute
toxicity of endrin is due to disruption of GABA-related mechanisms.
8.8.2 Cardiovascular system
Studies have been conducted on the physiological effects of
endrin on the peripheral vascular system, renal function, renal
haemodynamics, and the cardiovascular system of the dog (Emerson et
al., 1963, 1964; Reins et al., 1964; Emerson, 1965; Emerson & Hinshaw,
1965; Reins et al., 1966; Hinshaw et al., 1966; Reddy et al., 1967).
After a lethal dose of endrin was administered intravenously, most of
the effects appeared to be the direct or indirect result of the
stimulating action of endrin on the central nervous system.
Bradycardia, hypertension, salivation, hyperexcitability, tonic-clonic
convulsions, increased body temperature, leukocytosis,
haemoconcentration, and decreased blood pH were seen. Elevation of
cerebral venous pressure and cerebrospinal fluid pressure were also
prominent features. Increased levels of adrenaline and noradrenaline
in blood plasma cause increased venous return and cardiac output and
increased arterial blood pressure in the absence of a rise in total
peripheral resistance. There was a large increase in total limb
vascular resistance and also a decrease in renal blood flow due to
arteriolar vasoconstriction. In studies on intact dogs and isolated
heart-lung preparations, high doses of endrin appeared to have a toxic
action on the left ventricle of the heart, causing sudden left heart
failure.
Aldrin, dieldrin, and endrin inhibited rat brain synaptosomal
and heart sarcoplasmic reticulum in vitro in a
concentration-dependent manner. Calmodulin-depleted Ca2+ pump
activity was insensitive to the action of these compounds. Oral
administration of endrin at 0.5-10 mg/kg to rats similarly decreased
Ca2+ pump activity, in addition to decreasing the levels of
calmodulin in both brain and heart, indicating disruption of membrane
Ca2+ transport mechanisms. Exogenous addition of calmodulin
(1-20 µg) effectively reversed the endrin-induced inhibition. Ca2+
pump activity in brain is more sensitive to endrin than that in heart.
The results indicate that endrin may produce neurotoxic effects by
altering calmodulin-regulated calcium-dependent events in neurons
(Mehrotra et al., 1989).
8.8.3 Effects on liver enzymes
It is well known that chlorinated hydrocarbon insecticides such
as DDT and dieldrin stimulate hepatic microsomal drug metabolism,
stimulating the activity of enzymes for the metabolism of drugs and
endogenous compounds such as hormones (Kinoshita & Kempf, 1970).
8.8.3.1 Mouse
A single oral, convulsive dose of endrin (20 mg/kg body weight)
dissolved in corn oil was administered to 9-week-old male
Swiss-Webster mice. Control groups consisted of a group of untreated
mice and a group receiving corn oil. When convulsions began, blood
serum was examined for serum glutamic oxaloacetic transaminase, serum
glutamic pyruvic transaminase, and serum lactic dehydrogenase. The
activities of the three enzymes were significantly increased above
those seen in the two control groups (Luckens & Phelps, 1969).
After intraperitoneal injection of a single dose of endrin at
6.25 mg/kg body weight to mice, hexobarbital sleeping time was
decreased, starting 3 h after the injection and lasting for 3 days
(Hart & Fouts, 1963). Stimulating effects on the hepatic
mixed-function oxidase system were reported in ICR mice after single
oral doses of 4 and 10 mg/kg body weight (Hartgrove et al., 1977).
8.8.3.2 Rat
Feeding Sprague-Dawley rats on diets containing endrin at 1, 5,
25, 50, or 100 mg/kg for 16 weeks caused high mortality in all groups,
especially among male rats. The serum alkaline phosphatase
concentration was reported to be dose-relatedly increased in all
groups as compared to control animals. The effect was clearest in the
groups fed 25 mg/kg of diet or more (Nelson et al., 1956).
In strain FW 49 rats, a single oral dose of endrin at 5 mg/kg
body weight had no effect on pentobarbital sleeping time; 10 mg/kg
caused a significant reduction, which, however, disappeared after 10
days (Schwabe & Wendling, 1967).
Endrin caused a significant shortening of the duration of the
paralysis induced by zoxazolamine in male Sprague-Dawley rats aged
5-6 weeks. Endrin was injected intraperitoneally at 2 mg/kg body
weight daily for 3 days, and zoxazolamine was injected
intraperitoneally on the fourth day (Truhaut et al., 1974).
Male rats given single oral doses of 2.5, 3.75, or 5.0 mg/kg body
weight showed no effect on the various parameters (details not given)
of mixed-function oxidase activity after 12 h, but the level of
microsomal protein and electron transport components per gram of liver
were significantly increased after 108 h, in a dose-dependent fashion.
Thiopentone and pentobarbital sleeping times were reduced by a 24-h
prior intraperitoneal injection of endrin at 5 mg/kg body weight
(Kachole & Pawar, 1977).
A single oral dose of endrin at 10 mg/kg body weight to male
albino rats increased serum glutamic oxaloacetic transaminase and
glutamic pyruvic transaminase activities, and decreased ATPase, acid-
and alkaline phosphatase, succinic dehydrogenase, and
glucose-6-phosphatase activities significantly 2-48 h after treatment
(Meena et al., 1978). After three successive daily oral doses of
endrin at 15 mg/kg body weight to Sprague-Dawley rats, significant
increases in total lipids and triglycerides in liver and in serum
glutamic pyruvic transaminase activity were seen (Borady et al.,
1983).
When two groups of six adult female rats were fed 0 or 28.7 µg/kg
body weight, endrin accumulated in the liver (5.47 mg/kg),and its
concentration in blood increased progressively up to 28 days. Growth
was depressed. The activities of the enzymes aspartate amino
transferase and alanine amino transferase were slightly increased
(Illahi et al., 1986). Similar results were obtained in a study in
which rats were fed 20 µg/kg body weight for 28 days (Illahi et al.,
1987).
8.8.3.3 Guinea-pig
Groups of six female guinea-pigs were administered three
successive intraperitoneal injections of endrin in sunflower oil at 3
mg/kg body weight, and liver and kidneys were studied 24 h after the
last injection. Treatment caused a significant increase in liver
weight and a decrease in hepatic microsomal protein content; renal
weight and renal microsomal protein content were not affected. Hepatic
cytochrome b5 and cytochrome-c reductase activities were increased,
while cytochrome P450 and total haem levels were significantly
decreased. Related to the decrease in cytochrome P450 was a decrease
in TPNH-linked aminopyrine-N-demethylation, but an increase in
DPNH-linked demethylation was related to the increase in cytochrome b5
and cytochrome-c reductase. Lipid peroxidation was increased in both
liver and kidneys (Pawar & Kachole, 1978).
8.8.3.4 In-vitro studies
To test the possibility that phenobarbital induces cytochrome
P450p indirectly by increasing the availability of endogenous
glucocorticoids in the liver, phenobarbital and phenobarbital-like
inducers, including endrin, were added to primary monolayer cultures
of adult Sprague-Dawley rat hepatocytes incubated in serum-free medium
without glucocorticoids. De-novo synthesis of cytochrome P450p,
measured as increased incorporation of 3H-leucine into
immunoprecipitable P450p protein, was increased. Endrin at a
concentration of 1x10-5 M was half as potent as phenobarbital at 2
x 10-3 M (Schuetz et al., 1986).
8.8.4 Miscellaneous studies
Endrin inhibited rabbit muscle lactate dehydrogenase in vitro
(Hendrickson & Bowden, 1976). Exposure of isolated rat enterocytes to
endrin reduced the efficiency of the neuropeptide vasoactive
intestinal peptide after stimulation of cyclic AMP accumulation, as
was observed with lindane (Carrero et al., 1989).
Endrin at single oral doses of 25 mg/kg body weight or daily
doses of 1 mg/kg body weight daily for 8 days induced various shifts
in the mobilization of the ions of biologically important metals such
as magnesium, iron, zinc, and copper from liver, kidneys, brain,
heart, spleen, and blood (Coleman et al., 1968; Lawrence et al.,
1968). Rats receiving intraperitoneal injections of 1 mg/kg body
weight in peanut oil over periods up to 19 days showed no alteration
in the concentrations of serum proteins or serum lipoproteins,
separated by paper electrophoresis, or of albumin, alpha 1, alpha 2,
beta, or gamma globulins. Protein-bound sialic acid and methylpentose
were increased only temporarily; the level of bound hexose increased
with time and that of bound hexosamine decreased (Coleman, 1968).
Rats receiving a single oral dose of 50 mg/kg body weight, daily
intraperitoneal doses of 2 mg/kg body weight, or daily intramuscular
injections of 0.5 or 2.0 mg/kg body weight for 45 days showed
increased activity of a number of the enzymes that are involved in
gluconeogenesis in liver cells and cells of the renal cortex. A
significant decrease was noted in hepatic glycogen, an increase in
blood glucose and urea, as well as a significant rise in hepatic and
renal pyruvate carboxylase, phosphoenol pyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase. Furthermore,
endogenous levels of cyclic AMP were increased (Kacew et al., 1973;
Singhal & Kacew, 1976).
8.8.5 Factors that influence toxicity
8.8.5.1 Nutrition
The nutritional state of Wistar rats was found to alter their
susceptibility to the acute toxic action of endrin. Three groups of
approximately 100 rats were fed a normal diet, a diet containing
casein as the only source of protein, or a low protein diet for 28
days, and the acute toxicity of endrin was determined after a single
intragastric administration. The following LD50 values were
calculated: 27 mg, 17 mg, and 7 mg/kg body weight, respectively (Boyd
& Stefec, 1969).
8.8.5.2 Potentiation
The acute oral LD50s of equitoxic doses of combinations of 10
pesticides, including endrin, were studied in Swiss mice. No evidence
of potentiation was seen with combinations with dieldrin, diazinon,
malathion, toxaphene, parathion, DDT, or dioxathion, but more than
additive effects, i.e., possible potentiation, were found with
chlordane and possibly with aldrin (Keplinger & Deichmann, 1967).
Five groups of 20 male and 20 female Sprague-Dawley rats were fed
for 91 days on a diet containing a combination of 15 'persistent'
chemicals added at concentrations of 0, 1, 10, 100, and 1000 times the
water quality objective applied in Canada. For endrin, these
corresponded to 0.002, 0.02, 0.2, and 2.0 µg/kg of diet. No effect on
food intake, growth, clinical chemistry, bone marrow, or
histopathology were observed. It was concluded that the presence of
these chemicals at 1000 times the water quality objective had no
toxicological effect (Cote et al., 1985).
Six male and six female Sprague-Dawley rats were fed a control
diet or diets containing endrin at 5 or 10 mg/kg, endrin aldehyde at
10 mg/kg, or endrin ketone at 5 mg/kg for 15 days, at which time three
to six rats from each treatment group were given a single
intraperitoneal dose of carbon tetrachloride at 100 µlitre/kg body
weight in corn oil (1 mg/kg). Levels of serum enzymes, bile flow, and
biliary excretion of an anionic model compound, phenolphthalein
glucuronide, were measured on day 16. Dietary treatment with endrin at
either dose level did not elevate serum enzyme levels. Treatment with
5 mg/kg significantly reduced bile flow and a corresponding reduction
in phenolphthalein glucuronide excretion, whereas the 10 mg/kg dose
reduced only phenolphthalein glucuronide excretion in male rats.
Female rats treated with either dose showed a dose-dependent
choleretic effect with a commensurate increase in phenolphthalein
glucuronide excretion. Treatment of rats with endrin and carbon
tetrachloride did not result in potentiation of hepatobiliary
functions. The levels of some serum enzymes were elevated (two-fold)
in rats given endrin plus carbon tetrachloride over those in rats
given endrin or carbon tetrachloride alone, indicating an additive
interaction. Dietary treatment with endrin aldehyde slightly increased
the levels of serum glutamic oxaloacetic transaminase and glutamic
pyruvic transaminase; and endrin ketone induced a small elevation in
glutamic pyruvic transaminase levels. Neither compound altered bile
flow or biliary phenolphthalein glucuronide excretion. Combination
with carbon tetrachloride increased the levels of some serum enzymes
(two-fold) over those seen with the aldehyde or the ketone or carbon
tetrachloride alone (Young & Mehendale, 1986).
9. EFFECTS ON HUMAN BEINGS
9.1 Exposure of the general population
9.1.1 Acute toxicity
In mild cases of poisoning, dizziness, weakness of the legs,
abdominal discomfort, nausea, and vomiting have been reported. Some
patients have complained of temporary deafness or were slightly
disorientated or aggressive. The onset of poisoning is variable and
may occur 0.5-10 h after consumption of contaminated food or
contamination of the skin; the interval is usually 1-4 h, depending on
the quantity ingested. Severe poisoning is manifested by sudden
epileptiform fits, with frothing at the mouth, facial congestion, and
violent convulsive movements of the limbs, sometimes leading to
dislocation of a shoulder or other injury. The fits may last for
several minutes and may be followed by a period of semiconsciousness
for 15-30 min or until the next fit. In general, these convulsions
occur suddenly, with no prodromal sign or symptom. An uncommon but
very serious symptom observed in two children was hyperthermia (41 °C
or higher); the high fever was followed by decerebrate rigidity. In
fatal cases, death occurs within 2-12 h. In survivors, recovery is
rapid, within 24 h, and uneventful, although some patients have
complained of headache, dizziness, weakness, and anorexia for several
weeks (Davis & Lewis, 1956; Jacobziner & Raybin, 1959; Hoogendam et
al., 1962; Hayes, 1963; Weeks, 1967; Hayes, 1982). After clinical
recovery, EEG changes consisting of bilateral synchronous theta-wave
activity and occasional bilateral synchronous spike and wave
complexes, believed to be associated with brain stem irritation, may
still be found and may persist for up to several weeks (Hoogendam et
al., 1962, 1965; Weeks, 1967).
9.1.2 Poisoning incidents
Hayes (1982) reviewed poisoning cases caused by endrin. Outbreaks
of acute intoxication due to endrin have occurred by contamination of
flour during transport in railway cars. A first episode, which was
well studied, occurred in 1956 in Wales, United Kingdom (Davis &
Lewis, 1956): At least 59 people were ill enough to require medical
treatment, and at least 100 more had some symptoms, which were not
severe enough to require medical advice. No one died. On the basis of
the concentration of endrin in bread prepared from the flour (150
mg/kg), Hayes (1963) estimated that 0.20-0.25 mg/kg body weight may
cause a single convulsion and that the dose necessary to produce
repeated convulsions is about 1 mg/kg body weight. Karplus (1971)
estimated the lethal dose in man to be approximately 10 mg/kg body
weight.
A few conflicting data are available on the concentration of
endrin in the tissues of victims of fatal intoxication. Hayes (1982)
quoted levels of 7-10 mg/kg in the liver and 0.7-4.4 mg/kg in the
brain; however, 10-fold lower levels were reported in the tissues of
autopsied victims of an outbreak of poisoning caused by ingestion of
bread prepared from contaminated flour in the Middle East (Curley et
al., 1970). In another incident, two sacks of contaminated flour
contained endrin at 184.5 and 234.5 mg/kg, and the bread and rolls
prepared from the contaminated flour contained 125.67-176.11 mg/kg.
The levels of endrin in serum, collected 30 min, 20 h, and 30 h after
convulsions in one person were 0.053, 0.038, and 0.021 mg/litre,
respectively; three other cases had 0.003-0.004 mg/litre of blood
serum 9-19 h after convulsions. One of these three people had no
symptoms (Coble et al., 1967). The reported serum or blood levels of
endrin associated with convulsions must be interpreted in the context
of the rapid removal of endrin from blood and the often significant
time lag in taking blood samples after convulsions. When the time
between convulsion and blood sampling is long, the endrin levels
reported are likely to be much lower than those at the time of the
convulsion.
Four outbreaks of endrin intoxication occurred in Doha (Qatar)
and Hofuf (Saudi Arabia) in 1967, during which 874 people were
hospitalized of whom 26 died; another 500-750 people showed symptoms
of intoxication but required no hospitalization. These outbreaks were
due to contamination of flour by endrin leaking from drums during
shipment. The endrin concentrations found in bread were 48-1807 mg/kg,
and those in the blood of patients were 0.007-0.032 mg/litre (Weeks,
1967; Curley et al., 1970).
Between July and September 1984, an epidemic of endrin poisoning
occurred in Pakistan, resulting in acute convulsions. In 18 of 21
affected villages surveyed, 70% (106/152) of the cases for which age
was known were in children aged 1-9 years; 9.8% (19/194) of the
affected people died. A composite sugar sample taken from the houses
of three cases contained endrin at 0.04 mg/kg. Endrin was detected in
the blood of 12/18 patients, at levels of 0.3-254.0 µg/litre of serum.
It was also determined in brain, kidneys, adipose tissue, and liver of
one person and found at levels of 1680, 1760, 4010, 1430 µg/kg
respectively (Anon., 1984; Hill et al., 1986; Rowley et al., 1987).
In mid-March 1988, three members of a family in Orange County,
California, USA, became ill within 1 h of eating taquitos (baked corn
shell filled with spicy meat and salad). Two of the three had multiple
grand mal seizures. Subsequently, two other people were reported to
have had seizures less than 12 h after eating taquitos. All five
patients had obtained the taquitos from the same shop within 5 days.
The food was analysed, and the presence of endrin was confirmed but
not quantified. The origin of the endrin could not be identified
(Anon., 1989).
An episode of acute endrin poisoning was reported in 33 Mexican
children, who had sudden seizures without sensory alterations (Singh
& West, 1985).
Several other cases have been published of single accidental or
intentional intoxications, in children and in adults (Jacobziner &
Raybin, 1959; Karplus, 1971). Reddy et al. (1966) described 60 cases
of fatal endrin poisoning out of 95 encountered in India after the
introduction of endrin in agricultural work as an insecticide in 1959.
The majority of the cases were suicidal. Froth, petechial
haemorrhages, a kerosene-like smell and massive pulmonary oedema were
the characteristic autopsy findings. Respiratory failure was the most
common cause of death. The authors concluded that the toxic dose of
endrin is 5-50 mg/kg body weight or about 1 g; the lethal dose is
about 6 g. In a poisoning case in a 19-year-old male who ingested an
unknown amount of endrin, convulsions and gross pulmonary oedema were
found (Jedeikin et al., 1979). No histological changes were found in
the liver. At least some of the pulmonary changes seen in such cases
may be due to aspiration of the petroleum hydrocarbon solvent in
formulations of endrin.
A case of polyneuropathy of the Guillain-Barré type was
attributed to exposure to a mixture of DDT and endrin. Since
convulsions were not recorded, the causal relationship remains
doubtful (Jenkins & Toole, 1964).
In a fatal case of endrin poisoning, ingestion of 12 g of endrin
by a 49-year-old man caused convulsions (persisting for 4 days),
hypersalivation, hyperthermia, renal insufficiency, thrombocytopenia,
and recurrent hypotension. Death followed after 11 days due to
pulmonary complications (infection and haemorrhage) and hypoxaemia
causing bradycardia and cardiac arrest. The endrin concentrations in
blood 4 h and 6 and 11 days after ingestion were 450, 86 and 71
µg/litre. Endrin levels in adipose tissue, heart, brain, kidneys, and
liver, 11 days after ingestion were 89.5, 0.87, 0.89, 0.55, and 1.32
mg/kg, respectively (Runhaar et al., 1985).
The medical treatment of endrin poisoning is described in Annex
II.
9.2 Occupational exposure
9.2.1 Factory workers
No fatal case has been reported due to occupational exposure in
manufacturing and formulating plants (Van Raalte, 1965; Jager, 1970),
which may be due in part to underreporting but is also certainly due
to the fact that occupational exposure involving the absorption of
lethal doses occurs rarely under practical circumstances. Furthermore,
the rapid metabolism of endrin minimizes build-up of toxic levels in
tissues during normal working days.
Several cases of acute, non-fatal poisoning occurred in a
manufacturing plant in The Netherlands due to accidental over-exposure
to endrin (Jager, 1970). Endrin had been manufactured in this plant
since 1957. During the first 9 years of production of aldrin,
dieldrin, and endrin in the plant, 17 cases of poisoning with
convulsions occurred, five of which involved more than one convulsion.
Three of the cases were due to acute over-exposure to endrin among
workers who were handling these materials at high concentrations every
day. There was no fatality during 1300 man-years of exposure. No
evidence was found of skin sensitization, and there was no case of
permanent, partial, or complete incapacity. No difference was seen in
absenteeism due to illness among these workers in comparison with
those in other plants, and the results of liver function tests and
complete blood cell counts were within normal limits. In the cases of
poisoning, recovery from clinical and neurological signs, including
EEG tracings, was rapid and complete (Hoogendam et al., 1962, 1965;
Jager, 1970; Versteeg & Jager, 1973).
A series of studies has been published on the results of
continuing medical supervision of workers in this plant. A
complementary follow-up of 189 workers and of 52 workers who had left
employment at the plant for various reasons was published in 1973
(Versteeg & Jager, 1973). These workers had been exposed to endrin for
up to 14.5 years in 1973. In agreement with data published from a
study of 71 workers in an endrin manufacturing plant in the USA (Hayes
& Curley, 1968), endrin was not found in the blood of these workers,
except in cases of accidental, acute over-exposure. Medical
supervision of workers employed in the manufacture and formulation of
endrin and other pesticides for 1-19 years (average, 12 years), data
on absenteeism, the results of tests for liver function and blood
chemistry, blood morphology, urine analysis, the occurrence of
sensitization, the pattern and course of EEG changes in cases of
poisoning, other medical studies (including electrocardiography, chest
x rays, blood pressure, body weight), and the incidence and pattern of
diseases, including the occurrence of malignant growths, showed no
difference between workers exposed to endrin and other chemical plant
operators. Residues of endrin were not found in plasma (< 3 µg/litre)
or in adipose tissue (< 0.03 mg/kg).
A significant difference was found between workers exposed to
aldrin and dieldrin only, workers not exposed to insecticides, and
workers exposed to endrin only: endrin workers had lower blood levels
of the DDT metabolite para,para'-DDE than the other workers, and the
levels were lower than those in the general population of the
surrounding area, although DDT and related compounds had never been
manufactured in the plant. A second parameter that was compared was
excretion of 6-beta-hydrocortisol in the urine. (Increased activity of
the drug-metabolizing enzyme system increases the activity of the
oxidative pathway by which 6-beta-hydroxylase converts endogenous
cortisol to 6-beta-hydrocortisol and thus, relatively, diminishes the
contribution of the reductive pathway, leading to excretion of
17-hydroxycorticosteroids.) The ratio of the urinary excretion of
6-beta-hydroxycortisol to that of 17-hydroxysteroids was significantly
higher in the endrin workers than in workers not exposed to endrin
(Jager, 1970).
A third parameter of this enzyme system that was studied was
urinary excretion of D-glucaric acid (an end-product of the glucuronic
acid pathway in the liver), which has been shown to increase after
exposure to microsomal enzyme-stimulating compounds, like endrin
(Hunter et al., 1971; Notten & Henderson, 1975). In the endrin
workers, urinary excretion of D-glucaric acid after a week of exposure
increased significantly over pre-exposure levels and those in a
control group of workers. Excretion diminished again after 3 days
without exposure (Hunter et al., 1972; Ottevanger &Van Sittert, 1979;
Vrij-Standhardt et al., 1979; Van Sittert, 1985).
Since anti-12-hydroxyendrin is the only metabolite found in the
urine of endrin-exposed workers, a study was initiated to find whether
there is a correlation between the quantity of this metabolite and
that of D-glucaric acid excreted in the urine. A positive relationship
was found between excretion of the endrin metabolite and of D-glucaric
acid after 7 days. After exposure was discontinued, excretion of
anti-12-hydroxyendrin decreased faster than that of D-glucaric acid.
The fact that endrin-exposed workers had D-glucaric acid levels within
the normal range after 6 weeks indicates that enzyme induction in
endrin workers is reversible. The authors concluded that a urinary
level of anti-12-hydroxyendrin of 0.130 µg/g of creatinine is the
threshold exposure level, below which enzyme induction is not produced
(Ottevanger & Van Sittert, 1979; Van Sittert, 1985). Endrin did not
increase total urinary porphyrin excretion over that in a control
group of employees (Strik, 1979; Nagelsmit et al., 1979;
Vrij-Standhardt et al., 1979).
In a follow-up mortality study of the same group of workers,
vital status and cause of death were assessed for 232 of a group of
more than 1000 workers. This group was selected because they had
experienced high exposures in the initial years of manufacture and
formulation and because of the long periods of exposure (mean, 11
years; range, 4-27) and observation (mean, 24 years; range, 4-29).
Total observed mortality was 25, whereas 38 deaths were expected on
the basis of mortality statistics for the male Dutch population. Of
the nine cancer deaths, three were due to lung cancer; the remaining
six were due to cancers of stomach, pancreas, bladder, and kidney,
multiple myeloma, and cerebral glioma. It was concluded that the
pesticides manufactured had no specific carcinogenic activity
(Ribbens, 1985).
9.2.2 Dose-response relationships
It has not been possible to establish a dose-response
relationship between single or repeated oral exposures and endrin
concentrations in blood, adipose tissue, or organs and severity of
intoxication, because the actual oral intake in the accidental cases
was not known, and the onset of symptoms of intoxication and the time
of measuring concentrations of endrin in blood, organs, or tissues
were not comparable (Davis & Lewis, 1956; Hayes, 1963; Coble et al.,
1967; Weeks, 1967; Curley et al., 1970; Karplus, 1971; Hayes, 1982;
Anon., 1984).
Blood samples have been analysed in three cases of acute
over-exposure (Table 28): A formulator and an operator were
accidentally splashed with a 20% endrin emulsifiable concentrate,
which was washed off within 10 min. Neither developed signs or
symptoms of intoxication. The third case was in a formulator who
handled technical-grade endrin powder without wearing a dust-mask. He
had convulsions 4 h after starting work, but after treatment recovered
fully the next day. Blood samples from four colleagues working next to
him, but wearing dust-masks, were also examined. The author estimated
that the threshold level of endrin in the blood below which no sign or
symptom of intoxication occurs is 50-100 µg/litre and that the
half-life of endrin in blood is in the order of 24 h (Jager, 1970).
9.2.3 Exposures to mixtures
A retrospective mortality study was carried out on workers
employed in the manufacture of heptachlor and endrin in a plant in
Tennessee, USA, between 1952 and 1976. The study comprised 835 men who
had worked for more than 3 months up to 20 years at the plant. No
overall excess of deaths from cancer was found; however, there was an
excess of deaths from cerebrovascular disease (7 observed, 2.3
expected) (Wang & MacMahon, 1979).
A further retrospective cohort study was conducted to examine the
mortality of workers employed in the manufacture of chlordane,
heptachlor, DDT, aldrin/dieldrin, and endrin in a plant in Colorado,
USA, where endrin was manufactured from 1953 until 1965. Approximately
2100 workers who had been employed for at least 6 months in the plants
were involved. No excess of cerebrovascular disease was observed
(Ditraglia et al., 1981).
Neither study proves conclusively that exposure to these
organochlorine insecticides is associated with increased prevalence of
malignancy or other cause of death, but they are limited in design and
in the desciption of exposure.
A field study was carried out in 1983 in the Ivory Coast to
assess the health hazards associated with the handling and application
by hand-held sprayers of an ultra-low volume formulation consisting of
endrin at 85 g/litre, DDT at 333 g/litre, and methylparathion at 85
g/litre in petroleum solvent. Groups of five or six farmers sprayed 3
litre/ha of the formulation 4-6 weeks after sowing cotton and again 15
or 30 days after the first application. The spray apparatus was filled
and cleaned by the same men. The recommended protective clothing was
worn only rarely, and the handling and application techniques were
careless, resulting in many cases in appreciable skin contamination.
No adverse health effect was observed. Absorption of endrin was
monitored by measuring the concentration of anti-12-hydroxyendrin in
spot samples of urine collected about 20 h after spraying. The mean
concentrations after the first, second, and third applications were
0.34 (range, 0.04-0.59), 0.52 (range, 0.09-1.4), and 0.45 mg/g of
creatinine (range, 0.0-0.92). One person who had handled and sprayed
the formulation carefully still had anti-12-hydroxyendrin in the
urine after the third application, but at a very low level (0.03 mg/g
of creatinine). Measurements of para-nitrophenol, a metabolite of
methyl-parathion, in urine indicated that the rate of metabolism of
methylparathion was increased as a result of enzyme induction by
endrin in the liver (Kummer & Van Sittert, 1984, 1986). It was
concluded that endrin accumulated in most of the farmers after three
applications within a short period. An increase to toxic levels might
result if spraying were more frequent and at shorter intervals and if
the recommended clothing was not worn.
9.2.4 Appraisal of effects of occupational exposures
Endrin is a very toxic compound. Several episodes of fatal and
non-fatal poisoning have occurred, mostly from accidental
contamination of food and also from intentional (suicidal) ingestion.
The lethal oral dose is estimated to be 10 mg/kg body weight. In
non-fatal cases, recovery is rapid and complete within a few days. The
oral dose that causes a single convulsion is estimated to be 0.25
mg/kg body weight, and that which induces repeated convulsions, 1.0
mg/kg body weight.
Exposure of workers to endrin for long periods did not induce
adverse effects that were attributed to this compound, although
occasional cases of acute, non-fatal intoxication due to accidental
over-exposure have occurred. Endrin was not detected in the blood of
workers exposed to endrin at < 3.0 µg/litre. The threshold level of
endrin in blood that results in intoxication is estimated to be 50-100
µg/litre. Absorption of a toxic dose is therefore unlikely during
occupational exposure if recommended controls and precautions are
used. In fatal cases, endrin concentrations in blood as high as 450
µg/litre have been reported; however, it is not possible to establish
a dose-response relationship. Since endrin is not normally found in
air, water, or food, except under conditions of contamination,
exposure of the general population is not significant.
Table 28. Concentrations of endrin in blood from acutely over-exposed workers
Case Time of first Endrin concentration (µg/litre)
sampling
First 12 h 24 h 36 h 5 days
sample later later later later
Formulator 1 h after 90 ND
accident
Operator 40 min after 27 25 ND
accident
Formulator Directly after 80 20 ND
without dust-mask convulsion
Four colleagues Same time ND-10
with dust-masks time as above
ND, not detectable (< 5 µg/litre)
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Endrin was evaluated by the Joint FAO/WHO Expert Committee on
Pesticide Residues in 1963, 1965, and 1970 (FAO/WHO, 1964, 1965,
1971). In 1970, the Committee established an acceptable daily intake
(ADI) for humans of 0-0.0002 mg/kg body weight, which was based on the
level that causes no toxicological effect in rats and dogs, 1 mg/kg of
diet (equivalent to 0.05 mg/kg body weight per day in rats and 0.025
mg/kg body weight per day in dogs).
The Joint FAO/WHO Codex Alimentarius Commission has published
maximum residue limits for endrin (Table 29; (FAO/WHO, 1986b).
Table 29. Codex maximum limits for the sum of residues of
endrin and delta-ketoendrin
Commodity Maximum residue limit
(mg/kg product)
Apples 0.02a
Barley 0.02a
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02a
Eggs (without shells) 0.2
Meat (carcass fat) 0.1b
Milk 0.0008b
Poultry (carcass fat) 1
Rice (husked or polished) 0.02a
Sorghum 0.02a
Sweet maize 0.02a
Wheat 0.02a
aAt or near the limit of detection
bExtraneous residue limit
The International Agency for Research on Cancer (IARC) concluded
in 1974 and 1987 that there was inadequate evidence for the
carcinogenicity of endrin in experimental animals and that the
evidence from studies in humans was inadequate. Endrin was therefore
classified in Group 3: not classifiable as to its carcinogenicity to
humans (IARC, 1974, 1987).
In 1988, the Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified technical-grade endrin as
highly hazardous in normal use (WHO, 1992). A data sheet on endrin was
issued in 1978 (WHO/FAO, 1975).
REFERENCES
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1985) High-affinity
stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic Biochem Physiol, 24: 95-102.
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1986) Effects of insecticides
on GABA-induced chloride influx into rat brain microsacs. J Toxicol
Environ Health, 18: 13-23.
Abbott DC, Harrison RB, Tatton JO'G, & Thomson J (1966) Organochlorine
pesticides in the atmosphere. Nature, 211(5046): 259-261.
Abbott DC, Goulding R, & Tatton JO'G (1968) Organochlorine pesticide
residues in human fat in Great Britain. Br Med J, iii: 146-149.
Abbott DC, Holmes DC, & Tatton JO'G (1969) Pesticide residues in the
total diet in England and Wales, 1966-67. Organochlorine pesticide
residues in the total diet. J Sci Food Agric, 20(4): 245-259.
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in United Kingdom. Br Med J, ii: 553-556
Abdel-Razik M, Marzouk MAH, Mowafy LE, & Abdel-Kader MA (1988)
Pesticide residues in the River Nile water, Egypt. Pak J Sci Ind Res,
31(11): 795-797.
Abdou SM, Abdel-Gawaad AA, Abdel-Amaim E, Abdel-Hady SM, & El-Alfy MB
(1983) Organochlorine pesticide residues in buffaloes milk in Kalubia
province and the effect of the presence of insecticides on coagulation
time. Egypt J Dairy Sci, 11: 197-203.
Acker L & Schulte E (1974) [Chlorinated hydrocarbons in human fat.]
Naturwissenschaften, 61: 32 (in German).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Seasonal fluctuations of
organochlorine and triazines pesticides in the aquatic system of
Ioannina Basin (Greece). Sci Total Environ, 58: 243-253.
Albers PH, Sileo L, & Mulhern BM (1986) Effects of environmental
contaminants on snapping turtles of a tidal wetland. Arch Environ
Contam Toxicol, 15: 39-49.
Albert LA (1990) Environmental contamination in Mexican food. In:
Hriagu JO & Simmons MS ed., Food contamination from environmental
sources. New York, John Wiley and Sons, pp 542-577.
Albert L, Mendez F, Cebrian ME, & Portales A (1980) Organochlorine
pesticide residues in human adipose tissue in Mexico. Results of a
preliminary study in three Mexican cities. Arch Environ Health, 35(5):
262-269.
Albert LA, Vega P, & Nava E (1982) [Organochlorine pesticides. VI.
Organochlorine pesticide residues in Mexican evaporated milks.]
Biotica, 7(3): 473-482 (in Spanish).
Alford-Stevens AL, Eichelberger JW, & Budde WL (1988) Multi-laboratory
study of automated determination of polychlorinated biphenyls and
chlorinated pesticides in water, soil and sediment by gas
chromatography/mass spectrometry. Environ Sci Technol, 22: 304-312.
Ali SL (1986) [Pesticide residues and traces of heavy metals in cod
liver oil.] Pharm Ztg, 131(38): 2288-2290 (in German).
Al-Omar MA, Al-Ogaily NH, Tawfiq SJ, & Al-Bassoumy M (1985a) Residue
levels of organochlorine insecticides in sewage plant effluent. J Biol
Sci Res, 16(1): 145-151.
Al-Omar MA, Tameesh AH, & Al-Ogaily NH (1985b) Dairy product
contamination with organochlorine insecticide residues in Bagdad
district. J Biol Sci Res, 16(1): 133-144.
Altmeier G & Korte F (1969) [Contributions to ecological chemistry
(XXIV). Metabolism of endrin-14C in perfused rats' livers.]
Tetrahedron Lett, 49: 4269-4271 (in German).
Anderson A (1986) Monitoring and biased sampling of pesticide residues
in fruits and vegetables. Methods and results, 1981-1984. Var Foda,
38(Suppl. 1): 8-55.
Anderson RL & Defoe DL (1980) Toxicity and bioaccumulation of endrin
and methoxychlor in aquatic invertebrates and fish. Environ Pollut
(Ser A), 22: 111-121.
Anderson HH, Hine CH, Kodama JJ, & Critchlow JK (1953) Class B
Determination on HI-1185 Endrin Emulsifiable Concentrate, San
Francisco, University of California, School of Medicine (UC Report No.
213).
Ang C, Meleady K, & Wallace L (1989) Pesticide residues in drinking
water in the north coast region of New South Wales, Australia,
1986-87. Bull Environ Contam Toxicol, 42: 595-602.
Anon. (1964) Report on investigation of fish kills in Lower
Mississippi River, Atchafalaya River and Gulf of Mexico. Washington,
DC, US Department of Health, Education, and Welfare, Public Health
Service, Division of Water Supply and Pollution Control.
Anon (1979) Determination of residues of organochlorine pesticides in
animal fats and eggs. Report of the committee for analytical methods
for residues of pesticides and veterinary products in foodstuffs of
the Ministry of Agriculture, Fisheries and Food. Analyst, 104:
425-433.
Anon. (1984) Acute convulsions associated with endrin
poisoning--Pakistan. Morb Mortal Wkly Rep, 33(49): 687-695.
Anon. (1988a) Introduction--US Environmental Protection Agency Office
of Drinking Water health advisories. Rev Environ Contam Toxicol, 104:
1-8.
Anon. (1988b) Endrin. Rev Environ Contam Toxico,l 104: 103-114.
Anon. (1989) Endrin poisoning associated with taquito ingestion,
California. Morb Mortal Wkly Rep, 38(19): 345-347.
Argyle RJ, Williams GC, & Dupree HK (1973) Endrin uptake and release
by fingerling channel catfish (Ictalurus punctatus).
J Fish Res Board Canada, 30(11): 1743-1744.
Arthur RD, Cain JD, & Barrentine BF (1976) Atmospheric levels of
pesticides in the Mississippi Delta. Bull Environ Contam Toxicol,
15(2): 129-134.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees: Laboratory studies.
Riverside, University of California, Department of Entomology (Rev.
9/73 (M-16)).
Atuma SS (1985) Accumulation of organochlorine insecticides in the
blood of the general population of Nigeria. Toxicol Environ Chem,10:
77-82.
Baldwin MK & Hutson DH (1980) Analysis of human urine for a metabolite
of endrin by chemical oxidation and gas-liquid chromatography as an
indicator of exposure to endrin. Analyst, 105: 60-65.
Baldwin MK, Robinson J, & Parke DV (1970) Metabolism of endrin in the
rat. J Agric Food Chem, 18(6): 1117-1123.
Baldwin MK, Davis RA, & Burns DT (1973) Structural studies and
photochemical rearrangement of an animal metabolite of HEOD, the
active component of dieldrin. Pestic Sci, 4: 227-237.
Baldwin MK, Crayford JV, Hutson DH, & Street DL (1976) The metabolism
and residues of 14C-endrin in lactating cows and laying hens. Pestic
Sci, 7(6): 575-594.
Barcelo D & Puignou LG (1987) [Pesticide residue in Spanish U.H.T.
milks determined by high resolution gas chromatography.] Rev Agroquim
Tecnol Aliment, 27(4): 583-589 (in Spanish).
Barnett RW, D'Ercole AJ, Cain JD, & Arthur RD (1979) Organochlorine
pesticide residues in human milk samples from women living in
northwest and northeast Mississippi, 1973-75. Pestic Monit J, 13(2):
47-51.
Barth RAJ (1967) Pesticide toxicity in primates. Tulane, University of
Louisiana, Division of Hygiene and Tropical Medicine (Thesis).
Barthel WF, Hawthorne JC, Ford JH, Bolton GC, McDowell LL, Grissinger
EH, & Parsons DA (1969) Pesticide residues in sediments of the Lower
Mississippi River and its tributaries. Pestic Monit J, 3(1): 8-68.
Batterton JC, Boush JC, & Matsumura F (1971) Growth response of
blue-green algae to aldrin, dieldrin, endrin and their metabolites.
Bull Environ Contam Toxicol, 6(6): 589-594.
Becker DM & Sieg CH (1987) Egg shell quality and organochlorine
residues in eggs of merlins Falco columbarius in southeastern
Montana. Can Field-Nat, 101: 369-372.
Bedford CT (1974) Von Baeyer/IUPAC names and abbreviated chemical
names of metabolites and artifacts of aldrin (HHDN), dieldrin (HEOD)
and endrin. Pestic Sci, 5: 473-489.
Bedford CT & Harrod RK (1973) Synthesis of anti-12-hydroxyendrin and
12-ketoendrin, the two major mammalian metabolites of endrin.
Chemosphere, 4: 163-168.
Bedford CT & Hutson DH (1976) The comparative metabolism in rodents of
the isomeric insecticides dieldrin and endrin. Chem Ind, 1976:
440-447.
Bedford CT, Harrod RK, Hoadley EC, & Hutson DH (1975a) The metabolic
fate of endrin in the rabbit. Xenobiotica, 5(8): 485-500.
Bedford CT, Hutson DH, & Natoff IL (1975b) The acute toxicity of
endrin and its metabolites to rats. Toxicol Appl Pharmacol, 33:
115-121.
Bedford CT, Crane AE, & Harrod RK (1986a) Synthesis and confirmation
of structure of four mammalian metabolites of dieldrin and endrin.
Pestic Sci, 17: 659-667.
Bedford CT, Crane AE, Smith EH, & Wellard NK (1986b) Synthesis of
endrin metabolites. Part. 2: Total synthesis and confirmation of the
structure of 3-hydroxyendrin. Pestic Sci, 17: 33-47.
Belisle AA, Reichel WL, Locke LN, Lamont TG, Mulhern BM, Prouty RM,
DeWolf RB, & Cromartie E (1972) Residues in fish, wildlife and
estuaries. Pestic Monit J, 6: 133-138.
Benes V & Sram R (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind Med, 38(12): 442-444.
Bennett RO & Wolke RE (1987a) The effect of sublethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. I. Evaluation of serum
cortisol concentrations and immune responsiveness. J Fish Biol, 31(3):
375-385.
Bennett RO & Wolke RE (1987b) The effect of sub-lethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. II. The effect of
altering serum cortisol concentrations on the immune response. J Fish
Biol, 31(3): 387-394.
Benson WR (1969) Note on nomenclature of dieldrin and related
compounds. J Assoc Off Anal Chem, 52(5): 1109-1111.
Beyerbach M, Buthe A, Heidmann WA, Dettmer R, & Knuwer H (1987)
[Chlorinated hydrocarbons in eggs and livers of rooks (Corvus
frugilegus) from rookeries in Lower Saxony (northern Germany).]
J Ornitol, 128(3): 277-290 (in German with English summary).
Beyerbach M, Buthe A, Heidmann WA, Knuwer H, & Russel-Sinn HA (1988)
[The burden of dieldrin and other chlorinated hydrocarbons on the
lapwing (Vanellus vanellus).] J Ornitol, 129(3): 353-361 (in
German).
Bhowmik G (1978) Pretreating properties of endrin on plant
chromosomes. Letter to the Editor. Curr Sci, 47(15): 546-547.
Bianchi A, Tateo F, Nava C, Tateo S, Santamaria L, Berte F, &
Santagati G (1988) Presence of organophosphate and organochlorine
pesticides in the milk of women. Med Biol Environ, 16: 931-942.
Biberhofer J & Stevens RJJ (1987) Organochlorine contaminants in
ambient waters of Lake Ontario. Ottawa, Inland Water Directorate,
Waters Quality Branch,pp. 1-11 (Can Sci Ser (87) V51.159).
Biehl ML & Buck WB (1987) Chemical contaminants: their metabolism and
their residues. J Food Prot, 50(12): 1058-1073.
Bloomquist JR & Soderlund DM (1985) Neurotoxic insecticides inhibit
GABA-dependent chloride uptake by mouse brain vesicles. Biochem
Biophys Res Commun, 133(1): 37-43.
Bloomquist JR, Adams PM, & Soderlund DM (1986) Inhibition of
gamma-aminobutyric acid-stimulated chlorine flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
Blus LJ (1978) Short-tailed shrews: toxicity and residue relationship
of DDT, dieldrin and endrin. Arch Environ Contam Toxicol, 7: 83-98.
Blus LJ, Joanen T, Belisle AA, & Prouty RM (1975) The brown pelican
and certain environmental pollutants in Louisiana. Bull Environ Contam
Toxicol, 13: 646-655.
Blus L, Cromartie E, McNease L, & Joanen T (1979) Brown pelican:
population status, reproductive success, and organochlorine residues
in Louisiana, 1971-1976. Bull. Environ Contam Toxicol, 22: 128-135.
Blus LJ, Henny CJ, Kaiser TE, & Grove RA (1983) Effects on wildlife
from use of endrin in Washington State orchards. In: Fox GA & Hall RJ
ed. Transactions of the 48th North American Wildlife Conference on
Environmental Contaminants and Wildlife. Washington, DC, Wildlife
Management Institute, pp. 159-174.
Blus LJ, Henny CJ, & Grove RA (1989) Rise and fall of endrin usage in
Washington State fruit orchards: effects on wildlife. Environ Pollut,
60: 331-349.
Boellstorff DE, Ohlendorf HM, Anderson DW, O'Neill EJ, Keith JO, &
Prouty RM (1985) Organochlorine chemical residues in white pelicans
and western grebes from the Klamath Basin, California. Arch. Environ
Contam Toxicol, 14: 485-493.
Bollag JM & Henninger NM (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J Environ
Qual, 5(1): 15-18.
Bollen WB & Tu CM (1971) Influence of endrin on soil microbial
populations and their activity. Washington, DC, US Department of
Agriculture, Forest Service, pp 1-4 (Research Paper PNW 114).
Bonner JC & Yarbrough JD (1989) Role of the brain
t-butyl-bicyclophosphorothionate receptor in vertebrate resistance to
endrin, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane and
cypermethrin. J Pharmacol Exp Ther, 249(1): 149-154.
Borady AMA, Mikhail TH, Awadailah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23(1-2): 33-44.
Boyd EM & Stefec J (1969) Dietary protein and pesticide toxicity with
particular reference to endrin. Can Med Assoc J, 101: 335-339.
Braun F (1985) [PCN and chlorine-based pesticides in some Bavarian
rivers.]. Munch. Beitr. Abwasser Fisch Flussbiol, 39: 115-124 (in
German).
Breidenbach AW, Gunnerson CG, Kawahara FK, Lichtenberg JJ, & Green RS
(1967) Chlorinated hydrocarbon pesticides in major river basins,
1957-65. Public Health Rep, 82(2): 139-156.
Brodtmann NV. Jr (1976) Continuous analysis of chlorinated hydrocarbon
pesticides in the Lower Mississippi River. Bull Environ Contam
Toxicol, 15(1): 33-39.
Brooks GT (1969) The metabolism of diene-organochlorine (cyclodiene)
insecticides. Residue Rev, 27: 81-138.
Brooks GT (1974) Chlorinated insecticides, technology and application.
Cleveland, Ohio, CRC Press, vol. 1, pp 85-99 & vol 2, pp 94-97.
Brooks TM (1976) Mutagenicity studies with endrin in the host-mediated
assay. Unpublished report No. TLGR.0112.76, Sittingbourne, Kent, Shell
Research, submitted to WHO by Shell.
Bunck CM, Prouty RM, & Krynitsky AJ (1987) Residues of organochlorine
pesticides and polychlorobiphenyls in starlings (Sturnus vulgaris),
from the continental United States, 1982. Environ Monit Assess, 8:
59-75.
Burton WB & Pollard GE (1974) Rate of photochemical isomerization of
endrin in sunlight. Bull Environ Contam Toxicol, 12(1): 113-116.
Butler PA (1963) Commercial fisheries investigations. Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp 11-25
(Circular No. 167).
Butler PA (1973) Organochlorine residues in estuarine mollusks,
1965-1972. Pestic Monit J, 6(4): 238-362.
Cabral JRP, Raitano F, Mollner T, Bronczyk S, & Shubik P (1979) Acute
toxicity of pesticides in hamsters. Abstract: Eighteenth Annual
Meeting No. 384. Toxicol Appl Pharmacol, 48: A192.
Caceres O, Tundisi JG, & Castellan OAM (1987) Residues of
organochloric pesticides in reservoirs in Sao Paulo State. Cienc Cult,
39(3): 259-264.
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in near-shore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13(3): 296-309.
Cantoni C, Fabbris F, Rogledi R, & Campagnari A (1988) [Organochlorine
pesticides in foods of animal origin found during the 1985-1987
biennium.] Ind Aliment, 27(1): 6-8 (in Italian).
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ Monit Assess, 5(2): 155-163.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1978) Pesticide
residue levels in soils and crops, 1971--national soils monitoring
program (III). Pestic Monit J, 12(3): 117-136.
Carline RF & Lawal MV (1985) Contaminants and bilateral asymmetry in
yellow perch. Environ Toxicol Chem, 4: 543-547.
Carrero I, Fernandez-Moreno MD, Perez-Albarsanz MA, & Prieto JC (1989)
Lindane effect upon the vasoactive intestinal peptide
receptor/effector system in rat enterocytes. Biochem Biophys Res
Commun, 159(3): 1391-1396.
Carter BI & Simpson BJE (1978) Toxicity of insecticides: The acute
oral and percutaneous toxicities of two endrin bleed samples and of
technical endrin in Shellsol A and in toluene. Unpublished report No.
TLTR.0001.78, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Casper VL (1967) Galveston Bay pesticide study--water and oyster
samples analyzed for pesticide residues following mosquito control
program. Pestic Monit J, 1(3): 13-15.
Casteel SW & Cook WO (1985) Endrin toxicosis in a cat. J Am Vet Med
Assoc, 186(9): 988-989.
Castonguay M, Dutil J-D, & Desjardins C (1989) Distinction between
American eels (Anguilla rostrata) of different geographical origins
on the basis of their organochlorine contaminant levels. Can J Fish
Aquat Sci, 46: 836-843.
Celeste M de F & Caceres O (1987) [Chlorinated pesticide residues in
waters of Ribeirïo do Lobo (Broa) reservoir and its tributaries.]
Cienc Cult, 39(1): 66-70 (in Portuguese with English summary).
Cetinkaya M (1988) [Organochloro-pesticide residues in tobacco from
European cigarette brands.] Chem Mikrobiol Technol Lebensm, 11:
100-103 (in German with English summary).
Cetinkaya M & Schenek A (1987) [Investigation of
organochloro-pesticide residues in various raw cotton samples.] Chem
Mikrobiol Technol, Lebensm, 10: 150-153 (in German with English
summary).
Chau ASY (1974) Confirmation of pesticide residues identity. VII.
Solid matrix derivation procedure for the simultaneous confirmation of
heptachlor and endrin residues in the presence of large quantities of
polychlorinated biphenyls. J Assoc Off Anal Chem, 57(3): 585-591.
Chau ASY & Cochrane WP (1969) Cyclodiene chemistry. III. Derivative
formation for the identification of heptachlor, heptachlorepoxide,
cis-chlordane, trans-chlordane, dieldrin and endrin pesticide residues
by gaschromatography. J Assoc Off Anal Chem, 52: 1220-1226.
Chau ASY & Cochrane WP (1971) Chromous chloride reductions. VI.
Derivative formation for the simultaneous identification of heptachlor
and endrin pesticide residues by gas chromatography. J Assoc Off Anal
Chem, 54(5): 1124-1131.
Chernoff N, Kavlock RJ, Hanisch RC, Whitehouse DA, Gray JA, Gray LE
Jr, & Sovocool GW (1979) Perinatal toxicity of endrin in rodents. I.
Fetotoxic effects of prenatal exposure in hamsters. Toxicology, 13:
155-165.
Clark DR Jr & Krynitsky A (1978) Organochlorine residues and
reproduction in the little brown bat, Laurel, Maryland--June 1976.
Pestic. Monit J, 12(3): 113-116.
Clark DR Jr, Laval RK, & Krynitsky AJ (1980) Dieldrin and heptachlor
residues in dead gray bats, Franklin County, Missouri--1976 versus
1977. Pestic Monit J, 13(4): 137-140.
Clawson RL & Clark DR (1989) Pesticide contamination of endangered
gray bats and their food base in Boone County, Missouri, 1982. Bull
Environ Contam Toxicol, 42: 431-437.
Coble Y, Hildebrandt P, Davis J, Raasch F, & Curley A (1967) Acute
endrin poisoning. J Am Med Assoc, 202: 489-493.
Cole LM & Casida JE (1986) Polychlorocycloalkane insecticide-induced
convulsions in mice in relation to disruption of the GABA-regulated
chloride ionophore. Life Sci, 39: 1855-1862.
Cole JF, Klevay LM, & Zavon MR (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol Appl Pharmacol,
16: 547-555.
Coleman RL (1968) Endrin induced alterations in bound carbohydrates in
rat serum. Bull Environ Contam Toxicol, 3(6): 348-353.
Coleman RL, Lawrence CH, & Sowell WL (1968) Trace metal alterations
following subacute exposure to endrin. Bull. Environ Contam Toxicol,
3(5): 284-295.
Cook WO & Casteel SW (1985) A suspected case of endrin toxicosis in a
cat. Vet Hum Toxicol, 27(2): 111.
Corneliussen PE (1969) Pesticide residues in total diet samples (IV).
Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Pesticide residues in total diet samples (V).
Pestic Monit J, 4(3): 89-105.
Corneliussen PE (1972) Pesticide residues in total diet samples (VI).
Pestic Monit J, 5(4): 313-330.
Cote MG, Plaa GL, Valli VE, & Villeneuve DC (1985) Subchronic effects
of a mixture of 'persistent' chemicals found in the Great Lakes. Bull
Environ Contam Toxicol, 34: 285-290.
Crockett AB, Wiersma GB, Tai H, & Mitchell W (1975) Pesticide and
mercury residues in commercially grown catfish. Pestic Monit J, 8(4):
235-240.
Cromartie E, Reichel WL, Locke LN, Belisle AA, Kaiser TE, Lamont TG,
Mulhern BM, Prouty RM, & Swineford DM (1975) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1971-72. Pestic Monit J, 9(1): 11-14.
Cueto C Jr & Biros FJ (1967) Chlorinated insecticides and related
materials in human urine. Toxicol Appl Pharmacol, 10: 261-269.
Cueto C Jr & Hayes WJ Jr (1962) The detection of dieldrin metabolites
in human urine. Agric Food Chem, 10(5): 366-369.
Cummings JG (1965) Pesticide residues in the total diet samples. J
Assoc Off Anal Chem, 48(6): 1177-1180.
Cummings JG (1966) Pesticides in the total diet. Residue Rev, 16:
30-45.
Curley A, Jennings RW, Mann HT, & Sedlak V (1970) Measurement of
endrin following epidemics of poisoning. Bull Environ Contam Toxicol,
5(1): 24-29.
Currie RA, Kadis VW, Breitkreitz WE, Cunnignham GB, & Bruns GW (1979)
Pesticide residues in human milk, Alberta, Canada--1966-70, 1977-78.
Pestic Monit J, 13(2): 52-55.
Dale WE, Copeland F, & Hayes WJ Jr (1965) Chlorinated insecticides in
the body fat of people in India. Bull World Health Organ, 33: 471-477.
Datta SK & Ghose KC (1985) Toxic effect of endrin on the
hepatopancreas of a teleost, Cyprinus carpio. Indian Biol, 17(1):
37-41.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
Davis GM & Lewis I (1956) Outbreak of food poisoning from bread made
of chemically contaminated flour. Br Med J, ii: 393-398.
Davis HC & Hidu H (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish Bull , 67(2): 393-404.
Dean BJ (1977) Chromosome studies on workers employed in an endrin
manufacturing plant. Unpublished report No. TLGR.0008.77,
Sittingbourne, Kent, Shell Research, submitted to WHO by Shell.
De Boer J (1989) Organochlorine compounds and bromodiphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere,, 18(11/12): 2131-2140.
De Campos M & Olszyna-Marzys AE (1979) Contamination of human milk
with chlorinated pesticides in Guatemala and El Salvador. Arch Environ
Contam Toxicol, 8: 43-58.
Deichmann WB & MacDonald WE (1971) Organochlorine pesticides and human
health. Food Cosmet Toxicol, 9(1): 91-103.
Deichmann WB, MacDonald WE, Blum E, Bevilacqua M, Radomski J,
Keplinger M, & Balkus M (1970a) Tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Ind Med, 39(10): 426-434.
Deichmann WB, MacDonald WE, Radomski J, Blum E, Bevilacqua M, &
Keplinger M (1970b) The tumorigenicity of aldrin, dieldrin, and endrin
in the albino rat. Ind Med, 39(7): 314.
Denison MS & Yarbrough JD (1985) Binding of insecticides to serum
proteins in mosquitofish (Gambusia affinis). Comp Biochem Physiol,
81C(1): 105-107.
Denison MS, Chambers JE, & Yarbrough JD (1985) Short-term interactions
between DDT and endrin accumulation and elimination in mosquitofish
(Gambusia affinis). Arch Environ Contam Toxicol, 14: 315-320.
De Paula Carvalho JP, Niskikawa AM, Aranha S, & Fay EF (1984)
[Organochlorine pesticide residues in bovine fat.] Biol Sao Paulo,
50(2): 39-48 (in Portuguese).
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Devault DS, Clark JM, & Lahvis G (1988) Contaminants and trends in
fall run coho salmon. J Great Lakes Res, 14(1): 23-33.
De Vos RH, van Dokkum W, Olthof PDA, Quiryns JK, Muys T, & van der
Poll JM (1984) Pesticides and other chemical residues in Dutch total
diet samples (June 1976-July 1978). Food Chem Toxicol 22(1): 11-21.
Deweese LR, Cohen RR, & Stafford CJ (1985) Organochlorine residues and
egg shell measurements for tree swallows Tachycineta bicolor in
Colorado. Bull Environ Contam Toxicol, 35: 767-775.
Dewitt JB (1965) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J Agric Food Chem, 4(10): 863-866.
Dikshith TSS & Datta KK (1973) Endrin-induced cytological changes in
albino rats. Bull Environ Contam Toxicol, 9(2): 65-69.
Dikshith TSS, Kumar SN, Raizada RB, & Srivastava MK (1989a)
Organochlorine insecticide residues in cattle feed. Bull Environ
Contam Toxicol, 43: 691-696.
Dikshith TSS, Kumar SN, Tandon GS, Raizada RB, & Ray PK (1989b)
Pesticide residues in edible oils and oil seeds. Bull Environ Contam
Toxicol, 42: 50-56.
Dinnel PA, Link JM, Stober QJ, Letourneau MW, & Roberts WE (1989)
Comparative sensitivity of sea urchin sperm bioassays to metals and
pesticides. Arch Environ Contam Toxicol, 18: 748-755.
Ditraglia D, Brown DP, Namekata T, & Iverson N (1981) Mortality study
of workers employed at organochlorine pesticide manufacturing plants.
Scand J Work Environ Health, 7(suppl 4): 140-146.
Donahue JF, Burse VW, Head SL, & Andrews JS (1988) Comparison of two
techniques for quantifying environmental contaminants in human serum.
Life Sci, 43: 2257-2264.
Donoso J, Dorigan J, Fuller B, Gordon J, Kornreich M, Saari S, Thomas
L, & Walker P (1979) Reviews of the environmental effects of
pollutants. XIII. Endrin. Oak Ridge, Tennessee, Oak Ridge National
Laboratory (EPA-600/1-79-005).
DouAbul AAZ, Al-Saad HT, Al-Obaidy SZ, & Al-Rekabi HN (1987a) Residues
of organochlorine pesticides in fish from the Arabian Gulf. Water Air
Soil Pollut, 35: 187-194.
DouAbul AAZ, Al-Saad HT, & Al-Rekabi HN (1987b) Residues of
organochlorine pesticides in environmental samples from the Shatt
al-Arab River, Iraq. Environ Pollut, 43: 175-187.
DouAbul AAZ, Al-Omar M, Al-Obaidy S, & Al-Ogaily N (1987c)
Organochlorine pesticide residues in fish from the Shatt al-Arab
River, Iraq. Bull Environ Contam Toxicol, 38: 674-680.
DouAbul AAZ, Al-Saad HT, Al-Timari AA, & Al-Rekabi HN (1988)
Tigris-Euphrates delta: a major source of pesticides to the Shatt
al-Arab River (Iraq). Arch Environ Contam Toxicol, 17: 405-418.
Dowd PF, Mayfield GU, Coulon DP, Graves JB, & Newsom JD (1985)
Organochlorine residues in animals from three Louisiana watersheds in
1978 and 1979. Bull Environ Contam Toxicol, 34: 832-841.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-1970. Pestic Monit J,
5(4): 331-341.
Duggan RE & Lipscomb GQ (1969) Dietary intake of pesticide chemicals
in the United States (II), June 1966-April 1968. Pestic Monit J, 2(4):
153-162.
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in total
diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Pesticide residues in total
diet samples (II). Pestic Monit J, 1(2): 2-12.
Duke TW & Dumas DP (1974) Implications of pesticide residues in the
coastal environment. In: Vernberg FJ & Vernberg WB, ed. Pollution and
physiology of marine organisms. New York, Academic Press, pp 137-164.
Dureja P, Walia S, & Mukerjee SK (1987) New photometabolites of
endrin. Indian J Chem, 26G: 898-899.
Durham WF & Wolfe HR (1962) Measurement of the exposure of workers to
pesticides. Bull World Health Organ, 26: 75-91.
Dutch Agricultural Advisory Commission on Environmental Pollutants
(1983) Annual report. The Hague, Ministry of Agriculture, Management
of Nature and Fisheries.
Earnest RD & Benville PE Jr (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game, 58(2): 127-132.
Egan H, Goulding R, Roburn J, & Tatton JO'G (1965) Organochlorine
pesticide residues in human fat and human milk. Br Med J, ii: 66.
Eisenberg M & Topping JJ (1985) Organochlorine residues in finfish
from Maryland waters 1976-1980. J Environ Sci Health B20(6): 729-742.
Eisler R (1970a) Latent effects of insecticide intoxication to marine
molluscs. Hydrobiologia, 36(3-4): 345-352.
Eisler R (1970b) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish. Tech Paper Bur Sport
Fish Wildl, 46: 1-12.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCB's in
river Nile water, Egypt. Bull Environ Contam Toxicol, 34: 126-133.
Ellis DH, Deweese LR, Grubb TG, Kiff LF, Smith DG, Jarman WM, &
Peakall DB (1989) Pesticide residues in Arizona peregrine falcon eggs
and prey. Bull Environ Contam Toxicol, 42: 57-64.
Elnabarawy MT, Welter AN, & Robideau RR (1986) Relative sensitivity of
three daphnid species to selected organic and inorganic chemicals.
Environ Toxicol Chem, 5: 393-398.
El Nabawi A, Heinzow B, & Kruse H (1987) Residue levels of
organochlorine chemicals and polychlorinated biphenyls in fish from
the Alexandria Region, Egypt. Arch Environ Contam Toxicol, 16:
689-696.
El-Sebae AH (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region. In:
Research on the toxicity, persistence, bioaccumulation,
carcinogenicity and mutagenicity of selected substances (Activity G).
Final reports on projects dealing with toxicity (1983-1985). Athens,
United Nations Environment Programme (Mediterranean Action Plan (MAP),
Technical Reports Series No. 10).
Ely RE, Moore LA, Carter RH, & App BA (1957) Excretion of endrin in
the milk of cows fed endrin-sprayed alfalfa and technical endrin. J
Econ Entomol, 50(3): 348-349.
Emerson TE Jr (1965) Mechanisms of hemoconcentration in the dog during
acute endrin insecticide poisoning. Can J Physiol Pharmacol, 43:
793-800.
Emerson TE Jr & Hinshaw LB (1965) Peripheral vascular effects of the
insecticide endrin. Can J Physiol Pharmacol,43: 531-539.
Emerson TE Jr, Brake CM, & Hinshaw LB (1963) Mechanism of Action of
the Insecticide Endrin. Oklahoma City, Oklahoma, Civil Aeromedical
Research Institute (Report No. 63-16).
Emerson TE Jr, Brake CM, & Hinshaw LB (1964) Cardiovascular effects of
the insecticide endrin. Can J Physiol Pharmacol, 42: 41-51.
Engst R & Knoll R (1973) [On the contamination of surface, rain and
drinking waters with chlorinated hydrocarbons.] Nahrung, 17(8):
837-851 (in German with English summary).
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection of
chemical mutagens by the dominant lethal assay in the mouse. Toxicol
Appl Pharmacol, 23: 288-325.
Ercegovich CD & Rashid KA (1977) Mutagenesis induced in mutant strains
of Salmonella typhimurium by pesticides. In: Abstracts of the 174th
ACS National Meeting, Chicago, Illinois. Washington, DC, American
Chemical Society, Division of Pesticide Chemistry (Abstract No. 43).
Everaarts JM, Koeman JH, & Brader, L (1971) Contribution à l'étude des
effets sur quelques éléments de la faune sauvage des insecticides
organo-chlorés utilisés au Tchad en culture cotonnière. Cotton Fibre
Trop, 26(4): 385-394.
Fabacher DL & Chambers H (1976) Uptake and storage of 14C-labelled
endrin by the livers and brains of pesticide-susceptible and resistant
mosquitofish. Bull Environ Contam Toxicol, 16(2): 203-207.
Fahrig R (1974) Comparative mutagenicity studies with pesticides. In:
Montesano R & Tomatis L ed. Chemical carcinogenesis essays. Lyon,
International Agency for Research on Cancer, pp 161-181 (IARC
Scientific Publications No. 10).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No. PL:1963/13;
WHO/Food Add./23).
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. Geneva, World Health Organization (FAO Meeting Report No.
PL:1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1971) Joint meeting of FAO Working Party of Experts and the
WHO Expert Group on Pesticide Residues. 1970 Evaluation of some
pesticide residues in food. Geneva, World Health Organization
(AGP:1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1984) Codex guidelines on good practice in pesticide residue
analysis. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986a) Recommendations for methods of analysis of pesticide
residues. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR8-1986).
FAO/WHO (1986b) Codex maximum limits for pesticide residues. Rome,
Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme,
Food and Agriculture Organization of the United Nations, p 33-IV (FAO
CAC Vol. XIII, ed. 2).
Fasola M, Vecchio I, Caccialanza G, Gandini C, & Kitsos M (1987)
Trends of organochlorine residues in eggs of birds from Italy, 1977 to
1985. Environ Pollut, 48: 25-36.
FDA (1988) Food and Drug Administration pesticide program. Residues in
foods--1987. J Assoc Off Anal Chem, 71(6): 156A-174A.
Ferguson DE, Culley DD, & Cotton WD (1964) Resistance to chlorinated
hydrocarbon insecticides in three species of freshwater fish.
Bioscience, 14: 43-44.
Flickinger EL & King KA (1972) Some effects of aldrin-treated rice on
Gulf Coast wildlife. J Wildl Manage, 36: 706-727.
Flickinger EL, Mitchell CA, & Krynitsky AJ (1986) Dieldrin and endrin
residues in fulvous whistling ducks in Texas in 1983. J. Field
Ornithol, 57(2): 85-192.
Folmar LC (1978) In vitro inhibition of rat brain ATPase, pNPPase,
and ATP-32Pi exchange by chlorinated-diphenyl ethanes and cyclodiene
insecticides. Bull Environ Contam Toxicol, 19: 481-488.
Foschi F, Natali G, Guberti MG, Camisani MG, Taccheto Barbina M,
Spessotto C, & Bargarolo L (1985) [Study on residues of argicultural
chemicals in apples.] Inf Fitopatol, 12: 14-20 (in Italian).
Fournier E, Treich I, Campagne L, & Capelle N (1972) Pesticides
organo-chlorés dans le tissu adipeux d'êtres humains en France. Eur J
Toxicol, 1(1): 11-26.
Fox ME, Roper DS, & Thrush SF (1988) Organochlorine contaminants in
surficial sediments of Manukau Harbour, New Zealand. Mar Pollut
Bull,19(7): 333-336.
Frank R, Braun HE, Holdrinet M, Sirons GJ, Smith EH, & Dixon DW (1979)
Organochlorine insecticides and industrial pollutants in the milk
supply of Southern Ontario, Cananda--1977. J Food Prot, 42(1): 31-37.
Frank R, Braun HE, & Holdrinet MVH (1981) Residues from past uses of
organochlorine pesticides and PCB in waters draining eleven
agricultural watersheds in Southern Ontario, Canada, 1975-1977. Sci
Total Environ, 20: 255-276.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario--1983. J Food Prot, 48(6):
499-504.
Fredrickson DS (1978) Report on bioassay of endrin for possible
carcinogenicity. Fed Reg, 43(225): 54298.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Galassi S & Provini A (1981) Chlorinated pesticides and PCBs contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gant DB, Eldefrawi ME, & Eldefrawi AT (1987) Cyclodiene insecticides
inhibit GABAa receptor-regulated chloride transport. Toxicol Appl
Pharmacol, 88(3): 313-321.
Garrett NE, Stack HF, & Waters MD (1986) Evaluation of the genetic
activity profiles of 65 pesticides. Mutat Res, 168(3): 301-325.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements, and other chemicals in adult total diet
samples October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off Anal
Chem, 69(1): 123-145.
Giesy JP, Mewsted J, & Garling DL (1986) Relationships between
chlorinated hydrocarbon concentrations and rearing mortality of
chinook salmon (Onchorhynchus tshawytscha) eggs from Lake Michigan.
J Great Lakes Res, 12(1): 82-98.
Gips T (1987) Breaking the pesticide habit--Alternatives to 12
hazardous pesticides (International Alliance for Sustainable
Agriculture (IASA) Publication No. 1987-2).
Glatt H, Jung R, & Oesch F (1983) Bacterial mutagenicity investigation
of epoxides: drugs, drug metabolites, steroids and pesticides. Mutat
Res, 11: 99-118.
Glooschenko WA, Strachan WMJ, & Sampson RCJ (1976) Distribution of
pesticides and polychlorinated biphenyls in water, sediments and
seston of the Upper Great Lakes--1974. Pestic Monit J, 10(2): 61-67.
Gluth G & Hanke W (1985) A comparison of physiological changes in
carp, Cyprinus carpio, induced by several pollutants at sub-lethal
concentrations. I. The dependency on exposure time. Ecotoxicol Environ
Saf, 9: 179-188.
Godsil PJ & Johnson WC (1968) Pesticide monitoring of the aquatic
biota of the Tule Lake National Wildlife Refuge. Pestic Monit J, 1(4):
21-26.
Goerlitz DF & Law LM (1974) Determination of chlorinated insecticides
in suspended sediment and bottom material. J Assoc Off Anal Chem,
57(1): 176-181.
Goldentahl EI (1978a) Teratology study in rats. Unpublished report No.
163-488, International Research and Development Corporation, submitted
to WHO by Shell.
Goldentahl EI (1978b) Teratology study in hamsters. Unpublished report
No. 163-478, International Research and Development Corporation,
submitted to WHO by Shell.
Good EE & Ware GW (1969) Effects of insecticides on reproduction in
the laboratory mouse. Toxicol Appl Pharmacol, 14: 201-203.
Graves JB & Bradley JR (1965) Response of Swiss albino mice to
intraperitoneal injection of endrin. J Econ Entomol, 58(1): 178-179.
Gray LE Jr, Kavlock RJ, Chernoff N, Gray JA, & McLamb J (1981)
Perinatal toxicity of endrin in rodents. III. Alterations of
behavioural ontogeny. Toxicology, 21: 187-202.
Green DR, Stull JK, & Heesen TC (1986) Determination of chlorinated
hydrocarbons in coastal waters using a moored in situ sampler and
transported live mussels. Mar Pollut Bull, 17(7): 324-329.
Gregor DJ & Gummer WD (1989) Evidence of atmospheric transport and
deposition of organochlorine pesticides and polychlorinated biphenyls
in Canadian Arctic snow. Environ Sci Technol, 23: 561-565.
Gübeli T & Clerc JT (1988) [Detection of pesticide residues in ethanol
extracts of plants.] Pharm Acta Helv, 63(3): 85-89 (in German).
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984,
dietary intakes of pesticides, selected elements and other chemicals.
J Assoc Off Anal Chem, 71(6): 1200-1209.
Hall RJ & Swineford D (1980) Toxic effects of endrin and toxaphene on
the southern leopard frog Rana sphenocephala. In: Mellanby, K. ed.
Environmental pollution. Barking, Essex, Applied Science Publishing,
pp 53-65.
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hamdy Y & Post L (1985) Distribution of mercury, trace organics and
other heavy metals in Detroit River sediments. J Great Lakes Res,
11(3): 353-365.
Hansen DJ, Schimmel SC, & Forester J (1977) Endrin: effects on the
entire life cycle of a saltwater fish, Cyprinodon variegatus. J
Toxicol Environ Health, 3: 721-733.
Harris CR, Sans WW, & Miles JRW (1966) Exploratory studies on the
occurrence of organochlorine insecticides residues in agricultural
soils in southwestern Ontario. J Agric Food Chem,14(4): 398-403.
Hart LG & Fouts JR (1963) Effects of acute and chronic DDT
administration in hepatic microsomal drug metabolism in the rat
(28686). Proc Soc Exp Biol Med,114: 388-392.
Hartgrove RW Jr, Hundley SG, & Webb RE (1977) Characterization of the
hepatic mixed function oxidase system in endrin resistant and
-suspectible pinevoles. Pestic Biochem Physiol, 7(2): 146-153.
Hashemy-Tonkabony SE & Mosstofian B (1979) Chlorinated pesticide
residues in chicken egg. Poult Sci 58(6): 1432-1434.
Hashemy-Tonkabony SE & Soleimani-Amiri MJ (1976) Detection and
determination of chlorinated pesticide residues in raw and various
stages of processed vegetable oil. J Am Oil Chem Soc, 53(12): 752-753.
Hashimoto I & Nishiuchi S (1981) Establishment of bioassay methods for
evaluation of acute toxicity of pesticides to aquatic organisms. J
Pestic Sci, 6: 257-264.
Hassett AJ, Viljoen PT, & Liebenberg JJE (1987) An assessment of
chlorinated pesticides in the major surface water resources of the
Orange Free State during the period September 1984 to September
1985.Water SA, 13(3): 133-136.
Hawker DW & Connell DW (1986) Bioconcentration of lipophilic compounds
by some aquatic organisms. Ecotoxicol Environ Saf, 11: 184-197.
Hawthorne JC, Ford JH, & Markin GP (1974) Residues of mirex and other
chlorinated pesticides in commercially raised catfish. Bull Environ
Contam Toxicol, 11(3): 258-264.
Hayes WJ Jr (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department of
Health, Education, and Welfare, Communicable Disease Center,
Toxicology Section, pp 68-70.
Hayes WJ Jr (1975) Toxicology of pesticides, Baltimore,
Maryland,Williams & Wilkins, pp 288-294.
Hayes WJ Jr (1982) Pesticides studied in man, Baltimore, Maryland,
Williams and Wilkins, pp 247-251.
Hayes WJ Jr & Curley A (1968) Storage and excretion of dieldrin and
related compounds. Effect of occupational exposure. Arch Environ
Health, 16(2): 155-162.
Hayes WJ Jr, Dale WE, & Burse VW (1965) Chlorinated hydrocarbon
pesticides in the fat of people in New Orleans. Life Sci, 4:
1611-1615.
Heidmann WA, Büthe A, Beyerbach M, Löhmer R, & Rüssel-Sinn HA (1989)
[Chlorinated hydrocarbons of some bird species breeding in the inland
of Lower Saxony.] J Ornitol, 130(3): 311-320 (in German with English
summary).
Heinz GH, Erdman TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henderson C, Johnson WL, & Inglis A (1969) Organochlorine insecticide
residues in fish. National pesticides monitoring program. Pestic Monit
J, 3: 145-171.
Henderson C, Inglis A, & Johnson WL (1971) Organochlorine insecticide
residues in fish. Fall 1969, National Pesticide Monitoring Program.
Pestic Monit J, 5: 1-11.
Hendrickson CM & Bowden JA (1976) In vitro inhibition of lactic acid
dehydrogenase by insecticidal polychlorinated hydrocarbons. 2.
Inhibition by dieldrin and related compounds. J Agric Food Chem,
24(4): 756-759.
Hendrickx A & Maes R (1969) The excretion of chlorinated hydrocarbon
insecticides in human mother milk. J Pharm Belg, 24(9-10): 459-463.
Hermanutz R (1974) Quarterly report. Duluth, Minnesota, US
Environmental Protection Agency, National Water Quality Laboratory.
Hermanutz RO, Eaton JG, & Mueller LH (1985) Toxicity of endrin and
malathion mixtures to flagfish (Jordanella floridae). Arch Environ
Contam Toxicol, 14: 307-314.
Hernandez FH, Lopez Benet FJ, Escriche JM, & Ubeda JCB (1987) Sulfuric
acid cleanup and KOH-ethanol treatment for confirmation of
organochlorine pesticides and polychlorinated biphenyls: application
to wastewater samples. J Assoc Off Anal Chem, 70(4): 727-733.
Herrera Marteache A, Polo Villar LM, Jodral Villarejo M, Polo Villar
G, Mallol J, & Pozo Lora R (1978) [Organochlorine pesticide residues
in human fat in Spain.] Rev San Hig Publico, 52: 1125-1144 (in Spanish
with English summary).
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, p 147 (Fish
and Wildlife Technical Report No. 2).
Hill EF, Health RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report: Wildlife No. 191).
Hill RH Jr, Needham LL, & Liddle JA (1986) The laboratory's role in
environmental health emergency investigations. Clin Toxicol, 24(5):
363-374.
Hine CH (1965) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 2, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH (1968) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 7, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH, Anderson HH, Kodama JK, & Gutenberg EF (1954) Class B
evaluation of endrin compositions. Unpublished report No. 223, San
Francisco, CA, University of California School of Medicine, submitted
to WHO by Shell.
Hinshaw LB, Solomon LA, Reins DA, Fiorica V, & Emerson TE (1966)
Effects of the insecticide endrin on the cardiovascular system of the
dog. J Pharmacol Exp Ther, 153(2): 225-236.
Hirom PC, Millburn P, Smith RL, & Williams RT (1972) Species
variations in the threshold molecular-weight factor for the biliary
excretion of organic anions. Biochem J, 129: 1071-1077.
Hoffman WS, Fishbein WI, & Andelman MB (1964) The pesticide content of
human fat tissue. Arch Environ Health, 9: 387-394.
Hoffman WS, Adler H, Fishbein WI, & Bauer FC (1967) Relation of
pesticide concentration in fat to pathological changes in tissues.
Arch Environ Health, 15: 758-765.
Hogmire HW, Weaver JE, & Brooks JL (1990) Survey for pesticides in
wells associated with apple and peach orchards in West Virginia. Bull
Environ Contam Toxicol, 44: 81-86.
Holden AV (1970) International cooperative study of organo-chlorine
pesticide residues in terrestrial and aquatic wildlife, 1967/1968.
Pestic Monit J, 4(3): 117-135.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1962) Electroencephalograms
in insecticide toxicity. Arch Environ Health, 4: 86-94.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1965) Nine years toxicity
control in insecticide plants. Arch Environ Health, 10: 441-448.
Horn H, Hartner L, & von Faber H (1987) [On the suitability of liver
function tests in birds for the ecotoxicological evaluation of
environmental chemicals.] Dtsch Tierarztl Wochenschr, 94: 1-48 (in
German with English summary).
Horsfall F Jr, Webb RE, Price NO, & Young RW (1970) Residues in apples
subsequent to ground sprays of endrin. J Agric Food Chem, 18: 221-223.
Hrdina PD, Singhal RL, & Peters DAV (1974) Changes in brain biogenic
amines and body temperature after cyclodiene insecticides. Toxicol
Appl Pharmacol, 29(1): 119.
Hrubec J (1988) [Pesticides and drinking-water.] H2O, 21(11):
278-282 (in Dutch).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 153).
Hunter CG, Robinson J, & Richardson A (1963) Chlorinated insecticide
content of human body fat in southern England. Br Med J, i: 221-224.
Hunter J, Maxwell JD, Carrella M, Stewart DW, & Williams R (1971)
Urinary-D-glucaric acid excretion as a test for hepatic enzyme
induction in man. Lancet, 20 March: 572-575.
Hunter J, Maxwell JD, Stewart DW, Williams R, Robinson J, & Richardson
A (1972) Increased hepatic microsomal enzymes activity from
occupational exposure to certain organochlorine pesticides. Nature,
237: 399-401.
Hutson DH (1981) The metabolism of insecticides in man. In: Hutson DH
& Roberts TR ed. Progress in pesticide biochemistry. New York, John
Wiley and Sons, vol 1, pp 287-333.
Hutson DH & Hoadley EC (1974) The oxidation of a cyclic alcohol
(12-hydroxyendrin) to a ketone (12-ketoendrin) by microsomal
mono-oxygenation. Chemosphere, 5:205-210.
Hutson DH, Baldwin MK, & Hoadley EC (1975) Detoxication and
bioactivation of endrin in the rat. Xenobiotica, 5(11): 697-714.
IARC (1974) Endrin. In:Some organochlorine pesticides. Lyon,
International Agency for Research on Cancer, pp. 157-171 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Volume 5).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p. 63 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Illahi A, Amin N, Hashmi AS, Nawaz M, & Naeem-ur Rahman (1986)
Incidence of endrin residues in cucumber and its effects on the
biological system of rats. J Pak Med Assoc, 36(8): 209-211.
Illahi A, Roohi J, & Hashmi AS (1987) Present status of endrin
residues in peas and its effect on biological systems. J Pure Appl
Sci, 6(1): 1-4.
Ito N, Tatematsu M, Nakanishi K, Hasegawa R, Takano T, Imaida K, &
Ogiso T (1980) The effects of various chemicals on the development of
hyperplastic liver nodules in hepatectomized rats treated with
N-nitrosodimethylamine or N-2-fluorenylacetamide. Gann, 71: 832-842.
Jacobziner H & Raybin HW (1959) Briefs on accidental chemical
poisonings in New York City. Poisoning by insecticide (endrin). NY
State J Med, 59: 2017-2022.
Jager KW (1970) Aldrin, dieldrin, endrin and telodrin. An
epidemiological study of long-term occupational exposure. Amsterdam,
Elsevier Science Publishers.
Japanese Environmental Agency (1975) Environmental survey report on
chemical substances in FY 1974. Unpublished report, December 1975,
Tokyo, Environmental Health Department, Planning and Coordination
Bureau.
Japenga J, Wagenaar WJ, Smedes F, & Salomons W (1987) A new, rapid
clean-up procedure for the simultaneous determination of different
groups of organic micropollutants in sediments; application in two
European estuarine sediment studies. Environ Technol Lett, 8(1): 9-20.
Jarvinen AW, Tanner DK, & Kline ER (1988) Toxicity of chlorpyrifos,
endrin, or fenvalerate to fathead minnows following episodic or
continuous exposure. Ecotoxicol Environ Saf, 15: 78-95.
Jedeikin R, Kaplan R, Shapira A, Radwan H, & Hoffman S (1979) The
successful use of 'high level' PEEP in near fatal endrin poisoning.
Crit Care Med, 7(4): 168-170.
Jegier Z (1964) Health hazards in insecticide spraying of crops. Arch
Environ Health, 8: 670-674.
Jenkins RB & Toole JF (1964) Polyneuropathy following exposure to
insecticides. Arch Intern Med, 113: 691-695.
Johnson DW (1968) Pesticides and fishes--a review of selected
literature. Trans Am Fish Soc, 97(4) 398-424.
Johnson RD & Manske DD (1976) Pesticide residues in total diet samples
(IX). Pestic Monit J, 9(4): 157-169.
Johnson RD & Manske DD (1977) Pesticide and other chemical residues in
total diet samples (XI). Pestic Monit J, 11(3): 116-131.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1979) Pesticide and
other chemical residues in infant and toddler total diet samples, (I),
August 1974-July 1975. Pestic Monit J, 13(3): 87-98.
Johnson RD, Manske DD, & Podrebarac DS (1981a) Pesticide, metal, and
other chemical residues in adult total diet samples, (XII), August
1975-July 1976. Pestic Monit J, 15(1): 54-71.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1981b) Pesticide,
heavy metal, and other chemical residues in infant and toddler total
diet samples, (II), August 1975-July 1976. Pestic Monit J, 15(1):
39-50.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1984) Pesticide,
metal, and other chemical residues in adult total diet samples,
(XIII), August 1976-July 1977. J Assoc Off Anal Chem, 67(1): 154-166.
Johnson MG, Kelso JRM, & George SE (1988) Loadings of organochlorine
contaminants and trace elements to two Ontario lake systems and their
concentrations in fish. Can J Fish Aquat Sci, 45: 170-178.
Jolley WP, Stemmer KL, Grande F, Richmon J, & Pfitzer EA (1969) The
effects exerted upon beagle dogs during a period of two years by the
introduction of 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8-dimethanonaphthalene
into their daily diets. Unpublished report, Cincinnati, Ohio,
Kettering Laboratory, submitted to WHO by Shell.
Joy RM (1976) Convulsive properties of chlorinated hydrocarbon
insecticides in the cat central nervous system. Toxicol Appl
Pharmacol, 35: 95-106.
Kacew S, Sutherland DJB, & Singhal RL (1973) Biochemical changes
following chronic administration of heptachlor, heptachlor epoxide and
endrin to male rats. Environ Physiol Biochem, 3: 221-229.
Kachole MS & Pawar SS (1977) Effect of endrin on microsomal electron
transport reactions. Part I: Sleeping time, electron transport
components and protection due to pre-treatments. Abstracts of the 1976
Annual General Meeting of Biochemists. J Biochem, 14(1): 45.
Kadis VW, Breitkreitz WE, & Jonasson OJ (1970) Insecticide levels in
human tissues of Alberta residents. Can J Public Health, 61(5):
413-416.
Kagan J, Kagan ED, & Seigneurie E (1986) Alpha-terthienyl, a powerful
fish poison with light-dependent activity. Chemosphere, 15(1): 49-57.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Lamont TG, Mulhern BM,
Prouty RM, Stafford CJ, & Swineford DM (1980) Organochlorine
pesticide, PCB, PBB residues and necropsy data for bald eagles from 29
states--1975-77. Pestic Monit J, 13: 145-149.
Kampe W (1985) [Pesticide residues in animal feeding-stuffs.] Dtsch
Tierarztl Wochenschr, 92(6): 228-231 (in German).
Kanitz S & Castello G (1966) [The presence of residues of some
pesticides in human fatty tissue and in some foods. Initial results of
a survey carried out in Liguria.] G Ig Med Prev, 7: 1-19 (in Italian).
Karplus M (1971) [Endrin poisoning in children.] Harefuah, 18(3):
113-116 (in Hebrew with English summary).
Kassabi M, ElHraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kathpal T & Dewan RS (1975) Improved clean-up technique for the
estimation of endosulfan and endrin residues. J Assoc Off Anal Chem,
58(5): 1076-1078.
Katz M & Chadwick GG (1961) Toxicity of endrin to some Pacific
Northwest fishes.Trans Am Fish Soc, 90: 394-397.
Kavlock RJ, Chernoff N, Hanisch RC, Gray J, Rogers E, & Gray LE Jr
(1981) Perinatal toxicity of endrin in rodents. II. Fetotoxic effects
of prenatal exposure in rats and mice. Toxicology, 21: 141-150.
Kavlock RJ, Chernoff N, & Rogers EH (1985) The effect of acute
maternal toxicity on fetal development in the mouse. Teratog Carcinog
Mutagen, 5: 3-13.
Kavlock RJ, Rogers JM, Gray LE, & Chernoff N (1987) Postnatal
alterations in development resulting from prenatal exposure to
pesticides. In: Pesticide science and biotechnology, Proceedings of
the 6th International Congress on Pesticide Chemicals, pp 561-564.
Keilty TJ & Stehly GR (1989) Preliminary investigation of protein
utilization by an aquatic earthworm in response to sublethal stress.
Bull Environ Contam Toxicol, 43: 350-354.
Keilty TJ, White DS, & Landrum PF (1988a) Short-term lethality and
sediment avoidance assays with endrin-contaminated sediment and two
Oligochaetes from Lake Michigan. Arch Environ Contam Toxicol, 17:
95-101.
Keilty TJ, White DS, & Landrum PF (1988b) Sublethal responses to
endrin in sediment by Limnodrilus hoffmeisteri (Tubificidae) and in
mixed-culture with Stylodrilus heringianus (Lumbriculidae). Aquat
Toxicol, 13: 227-250.
Keilty TJ, White DS, & Landrum PF (1988c) Sublethal responses to
endrin in sediment by Stylodrilus heringianus (Lumbriculidae) as
measured by a 137cesium marker layer technique. Aquat Toxicol, 13:
251-270.
Keplinger MK & Deichmann WB (1967) Acute toxicity of combinations of
pesticides. Toxicol Appl Pharmacol, 10: 586-595.
Khangarot BS, Sehgal A, & Bhasin MK (1985) Man and biosphere--studies
on the Sikkim Himalayas. Part 6: Toxicity of selected pesticides to
frog tadpole, Rana hexadactyla (Lesson). Acta Hydrochim Hydrobiol,
13(3): 391-394.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci Health,
A21(1): 15-53.
Kiene RP & Capone DG (1984) Effects of organic pollutants on
methanogenesis, sulfate reduction and carbon dioxide evolution in salt
marsh sediments. Mar Environ Res 13: 141-160.
,
Kiigemagi U, Sprowls RG, & Terriere LC (1958) Endrin content of milk
and body tissues of dairy cows receiving endrin daily in their diet.
J Agric Food Chem, 6(7): 518-521.
King KA, Flickinger EL, & Hildebrand HH (1977) The decline of brown
pelicans on the Lousiana and Texas Gulf Coast. Southwest Nat, 21(4):
417-431.
King KA, Blankinship DR, Payne E, Krynitsky AJ, & Hensler GL (1985)
Brown pelican populations and pollutants in Texas, 1975-1981. Wilson
Bull, 97(2): 201-214.
Kinoshita FK & Kempf CK (1970) Quantitative measurement of hepatic
microsomal enzyme induction after dietary intake of chlorinated
hydrocarbon insecticides. Toxicol Appl Pharmacol, 17: 288.
Klein W, Mueller W, & Korte F (1968) [Insecticides in the metabolism.
XVI. Excretion, distribution and metabolism of endrin 14C in rats.]
Liebigs Ann Chem,713: 180-185.
Klevay LM (1971) Endrin excretion by the isolated perfused rat liver:
a sexual difference. Proc Soc Exp Biol Med, 136: 878-879.
Kodavanti PRS, Mehrotra BD, Chetty SC, & Desaiah D (1988) Effect of
selected insecticides on rat brain synaptosomal adenylate cyclase and
phosphodiesterase. J Toxicol Environ Health, 25: 207-215.
Koeman JH (1971) [The occurrence and the toxicological implications of
some chlorinated hydrocarbons in the Dutch coastal area in the period
1965-70], Utrecht, Rijks University, pp 88, 96 (Thesis) (in Dutch).
Koeman JH, Oskamp AAG, Veen J, Brouwer E, Rooth J, Zwart P, van de
Brock E, & van Genderen H (1967) Insecticides as a factor in the
mortality of the sandwich tern (Sterna sandvicensis). A preliminary
communication. Meded Fac Landbouwwet Rijksuniv Gent, 32: 841-854.
Koeman JH, Vink JAJ, & de Goeij JJM (1969) Causes of mortality in
birds of prey and owls in the Netherlands in the winter of 1968-69.
Andrea, 57: 67-76.
Koeman JH, Pennings JH, de Goeij JJM, Tjioe PS, Olindo PM, & Hopcraft
J (1972) A preliminary survey of the possible contamination of Lake
Nakuru in Kenya with some metals and chlorinated hydrocarbon
pesticides. J Appl Ecol, 9(2): 411-416.
Koeman JH, Pennings JH, Rosanto R, Soemarwoto O, Tjioe PS, Blancke S,
Kusumadinata S, & Djajadiredja RR (1974) Metals and chlorinated
hydrocarbon pesticides in samples of fish, sawah-duck eggs,
crustaceans and molluscs collected in Indonesia in April and May 1972.
Unpublished report, Wageningen-Bandung, University of Wageningen, The
Netherlands.
Korn S & Earnest R (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game, 60(3):
128-131.
Korte F (1969) Summary of results in 1969. Unpublished report,
submitted to WHO by Shell.
Korte F, Klein W, Weisgerber I, Kaul R, Mueller W, & Djirsurai A
(1970) Recent results in studies on the fate of chlorinated
insecticides. In: Deichmann WB, Radomski JL, & Penalver RA, ed.
Proceedings of the Sixth Conference on Toxicology and Occupational
Medicine, Pesticide Symposia. Miami, Florida, Halos & Associates,
Inc., pp 51-56.
Krantz WC, Mulhern BM, Bagley GE, Sprunt A, Ligas FJ, & Robertson WC
Jr (1970) Organochlorine and heavy metal residues in bald eagle eggs.
Pestic Monit J, 4(3): 136-140.
Kreitzer JF (1980) Effects of toxaphene and endrin at very low dietary
concentrations on discrimination acquisition and reversal in bobwhite
quail Colinus virginianus. Environ Pollut (Ser A), 23: 217-230.
Kreitzer JF & Heinz GH (1974) The effect of sub-lethal dosages of five
pesticides and a polychlorinated biphenyl on the avoidance response of
Coturnix quail chicks. Environ Pollut, 6: 21-29.
Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA,
Sileo L, Docjerty DE, & Erdman TC (1989) Microcontaminants and
reproductive impairment of the Forster's tern on Green Bay, Lake
Michigan--1983. Arch Environ Contam Toxicol, 18: 706-727.
Kudesia VP & Bali NP (1985) A study of pesticides in Kalinadi River
and evaluation of toxicity of some pesticides on fish Clarias
batrachus. Acta Cienc Indica, 10C(4): 245-254.
Kummer R & Van Sittert NJ (1984) Field study on health effects in
farmers applying an endrin/DDT/MEP formulation by hand-held ULV to
cotton in Ivory Coast. Unpublished report, The Hague, Shell
Internationale Petroleum Maatschappij, submitted to WHO by Shell.
Kummer R & Van Sittert NJ (1986) Field studies on health effects from
the application of two organophosphorus insecticide formulations by
hand-held ULV to cotton. Toxicol Lett, 33: 7-24.
Kurata M, Hirose K, & Umeda M (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
Kurhekar MP, D'Souza FC, & Meghal SK (1975) Rapid method for
extracting aldrin, dieldrin, and endrin from visceral material. J
Assoc Off Anal Chem, 58(3): 548-550.
Kutz FW, Yobs AR, & Yang HSC (1976) National pesticide monitoring
programs. In: Lee RE Jr, ed. Air pollution from pesticides and
agricultural processes. Cleveland, Ohio, CRC Press, pp 95-136.
Kutz F, Strassman S, & Yobs AR (1979a) Survey of pesticide residues
and their metabolites in the general population of the United States.
In: Berlin A, Wolff AH, & Hasegawa Y ed. Use of biological specimens
to assess human exposure to environmental pollutants, The Hague,
Martinus Nijhoff, pp 267-274.
Kutz FW, Strassman SC, & Sperling JF (1979b) Survey of selected
organochlorine pesticides in the general population of the United
States. Fiscal years 1970-1975. Ann NY Acad Sci, 320: 60-68.
Lara WH & Barreto HHC (1972) [Chlorinated pesticide residues in
water.] Rev Inst Adolfo Lutz, 32: 69-74 (in Portuguese with English
summary).
Lauer GJ, Nicholson HP, Cox WS, & Teasley JI (1966) Pesticide
contamination of surface waters by sugar cane farming in Louisiana.
Trans Am Fish Soc, 95(3): 310-316.
Lawrence CH, Coleman RL, & Sowell WL (1968) Endrin induced trace metal
alterations following acute exposure. Bull Environ Contam Toxicol,
3(4): 229-239.
Leard RL, Grantham BJ, & Pessoney GF (1980) Use of selected freshwater
bivalves for monitoring organochlorine pesticide residues in major
Mississippi stream systems, 1972-73. Pestic Monit J, 14(2): 47-52.
Lebel GL & Williams DT (1986) Determination of halogenated
contaminants in human adipose tissue. J Assoc Off Anal Chem, 69(3):
451-458.
Lichtenberg JJ, Eichelberger JW, Dressman RC, & Longbottom JE (1970)
Pesticides in surface waters of the United States; a 5-year summary,
1964-1968. Pestic Monit J, 4(2): 71-86.
Lopez-Avila V, Schoen S, Milanes J, & Beckert WF (1988)
Single-laboratory evaluation of EPA method 8080 for determination of
chlorinated pesticides and polychlorinated biphenyls in hazardous
wastes. J Assoc Off Anal Chem, 71(2): 375-387.
Lowe JI (1966) Some effects of endrin on estuarine fishes. In:
Proceedings of the 19th Annual Conference of the Southeast Association
of Game and Fish Commissioners, pp 271-276.
Luckens MM & Davis WH (1965) Toxicity of dieldrin and endrin to bats.
Nature, 207(4999): 879-880.
Luckens MM & Phelps KI (1969) Serum enzyme patterns in acute poisoning
with organochlorine insecticides. J Pharm Sci, 58(5): 569-572.
Ludke JL (1976) Organochlorine pesticide residues associated with
mortality: additivity of chlordane and endrin. Bull Environ Contam
Toxicol, 16(3): 253-260.
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multi-residue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-433.
Lund AE & Narahasi T (1983) Kinetics of sodium channel modification as
the basis for the variation in the nerve membrane effects of
pyrethroids and DDT analogs. Pestic Biochem Physiol, 20: 203-206.
Lykins BW Jr, Koffskey WE, & Miller RG (1986) Chemical products and
toxicologic effects of disinfection. J Am Water Works Assoc, 78(11):
66-75.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14(9): 1253-1265.
McGill AEJ & Robinson J (1968) Organochlorine insecticide residues in
complete prepared meals: a 12-month survey in SE England. Food Cosmet
Toxicol, 6: 45-57.
Machbub B, Ludwig HF, & Gunaratnam D (1988) Environmental impact from
agrochemicals in Bali (Indonesia). Environ Monit Assess, 11: 1-23.
McIntyre AE & Lester JN (1984) Occurrence and distribution of
persistent organochlorine compounds in UK sewage sludges. Water Air
Soil Pollut, 23: 397-415.
McKenney CL Jr (1986) Critical responses of populations of crustacea
to toxicants. Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Environmental Research
Brief EPA/600/M-86/004.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol 25: 921-928.
McLeese DW, Metcalfe CD, & Pezzack DS (1980) Bioaccumulation of
chlorobiphenyls and endrin from food by lobsters (Homarus
americanus). Bull Environ Contam Toxicol 25: 161-168.
McLeese DW, Burridge LE, & van Dinter J (1982) Toxicities of five
organochlorine compounds in water and sediment to Nereis virens. Bull
Environ Contam Toxicol 28: 216-220.
Madden JD, Finerty MW, & Grodner RM (1989) Survey of persistent
pesticide residues in the edible tissues of wild and pond-raised
Louisiana crayfish and their habitat. Bull Environ Contam Toxicol, 43:
779-784.
Manske DD & Corneliussen PE (1974) Pesticide residues in total diet
samples (VII). Pestic Monit J, 8(2): 110-124.
Manske DD & Johnson RD (1975) Pesticide residues in total diet samples
(VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Pesticide and other chemical residues in
total diet samples (X). Pestic Monit J, 10(4): 134-148.
Marinelli P, Stracciari GL, & Anfossi P (1986) [Presence of
organochlorinated pesticides in some wine-making by-products.] Zoot
Nutr Anim, 12: 479-486 (in Italian with English summary).
Marsh C (1963) Metabolism of D-glucuronolactone in mammalian systems.
Conversion of D-glucuronolactone into D-glucaric acid by tissue
preparations. Biochem J, 87: 82-90.
Marston RB, Tyo RM, & Middendorff SC (1969) Endrin in water from
treated Douglas fir seed. Pestic Monit J, 2(4): 167-171.
Martin RJ & Duggan RE (1968) Pesticide residues in total diet samples
(III). Pestic Monit J, 1(4): 11-20.
Martin DB & Hartman WA (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North Central United States. J Assoc Off Anal Chem, 68(4): 712-717.
Martin JP, Harding RB, Cannell GH, & Anderson L (1959) Influence of
five annual field applications of organic insecticides on soil
biological and physical properties. Soil Sci, 87: 334-338.
Maslansky CJ & Williams GM (1981) Evidence for an epigenetic mode of
action in organochlorine pesticide hepatocarcinogenicity. A lack of
genotoxicity in rat, mouse and hamster hepatocytes. J Toxicol Environ
Health, 8: 121-130.
Mason JW & Rowe OR (1976) The accumulation and loss of dieldrin and
endrin in the eastern oyster. Arch Environ Contam Toxicol, 4(3):
349-360.
Masud SZ & Farhat S (1985) Pesticide residues in foodstuffs in
Pakistan--organochlorine pesticides in fruits and vegetables. Pak J
Sci Ind Res, 28(6): 417-422.
Matsumoto K, Eldefrawi ME, & Eldefrawi AT (1988) Action of
polychlorocycloalkane insecticides on binding of
[35S]t-butylbicyclophosphorothionate to Torpedo electric organ
membranes and stereospecificity of binding site. Toxicol Appl
Pharmacol, 95: 220-229.
Matsumura F, Khanvilkar VG, Patil KC, & Boush GM (1971) Metabolism of
endrin by certain soil microorganisms. J Agric Food Chem, 19(1):
27-31.
Maule A, Plyte S, & Quirk AV (1987) Dehalogenation of organochlorine
insecticides by mixed anaerobic microbial populations. Pestic Biochem
Physiol, 27: 229-236.
Mayer, FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Environmental Research Laboratory (Environmental Research Brief
EPA/600/8-87/017).
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior Fish
and Wildlife Service (Resource publication 160).
Meena K, Gupta PK, & Bawa SR (1978) Endrin-induced toxicity in normal
and irradiated rats. Environ Res, 16: 373-382.
Mehrotra BD, Moorthy, KS, Reddy SR, & Desaiah D (1989) Effects of
cyclodiene compounds on calcium pump activity in rat brain and heart.
Toxicology, 54: 17-29.
Meith-Avcin N, Warlen SM, & Barber RT (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 meters.
Environ Lett, 5(4): 215-221.
Mersch-Sundermann V, Dickgiesser N, Hablizel U, & Gruber B (1988)
[Examination of mutagenicity of organic microcontaminations on the
environment. I. Communication: the mutagenicity of selected herbicides
and insecticides with the Salmonella-microsome-test (Ames-test) in
consideration of the pathogenetic potence of contaminated ground- and
drinking-water.] Zbl Bakteriol Hyg B, 186: 247-260 (in German).
Metcalf RL, Kapoor IP, Lu PY, Schuth CK, & Sherman P (1973) Model
ecosystem studies of the environmental fate of six organochlorine
pesticides. Environ Health Perspect, 4: 35-44.
Miles JRW & Harris CR (1973) Organochlorine insecticides residues in
streams draining agricultural, urban agricultural and resort areas of
Ontario, Canada, 1971. Pestic Monit J, 6(4): 363-368.
Miller PE & Fink GB (1973) Brain serotonin level and pentylenetetrazol
seizure threshold in dieldrin and endrin treated mice. Proc West
Pharmacol Soc, 16: 195-197.
Modin JC (1969) Chlorinated hydrocarbon pesticides in California bays
and estuaries. Pestic Monit J, 3(1): 1-7.
Morita H & Umeda M (1984) Detection of mutagenicity of various
compounds by FM3A cell system. Mutat Res, 130(5): 371.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay system. Mutat Res, 116: 185-216.
Morris RD (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse, Peromyscus maniculatus. Can J Zool,
46(5): 951-958.
Morris RD (1970) The effects of endrin on Microtus and Peromyscus.
I. Unenclosed field populations. Can J Zool, 48: 695-708.
Morris RD (1972) The effects of endrin on Microtus and Peromyscus.
II. Enclosed field populations. Can J Zool, 50(6): 885-896.
Moser GJ & Smart RC (1989) Hepatic tumour-promoting chlorinated
hydrocarbons stimulate protein kinase C activity. Carcinogenesis,
10(5): 851-856.
Mount DI & Putnicki GJ (1966) Summary report of the 1963 Mississippi
fish kill. North Am Wildl Nat Res Conf Trans, 31: 177-184.
Mount DI, Vigor LW, & Schafer ML (1966) Endrin: use of concentration
in blood to diagnose acute toxicity in fish. Science, 152: 1388-1390.
Mugambi JM, Kanja L, Maitho TE, Skaare JU, & Lokken P (1989)
Organochlorine pesticide residues in domestic fowl (Gallus
domesticus) eggs from Central Kenya. J Sci Food Agric, 48: 165-176.
Muir CMC (1970) The acute oral and percutaneous toxicities to rats of
formulations of aldrin, dieldrin or endrin. Unpublished report No.
TLGR.0020.70, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Mukerjee SK (1985) The environmental photodegradation of pesticides.
Indian J Agric Chem, 18(1): 1-9.
Mulhern BM, Reichel WL, Locke LN, Lamont TG, Belisle A, Cromartie E,
Bagerly GE, & Prouty R (1970) Organochlorine residues and autopsy data
from bald eagles. 1966-1968. Pestic Monit J, 4: 141-144.
Muncy RJ & Oliver AD Jr (1963) Toxicity of ten insecticides to the red
crawfish, Procambarus clarki (Girard). Trans Am Fish Soc, 92:
428-431.
Mussalo-Rauhamaa H, Salmela SS, Leppanen A, & Pyysalo H (1986)
Cigarettes as a source of some trace and heavy metals and pesticides
in man. Arch Environ Health, 41(1): 49-55.
Nagelsmit A, Vliet PW, van Wiel-Wetzels WAM, van der Wielard MJ, Strik
JJTWA, Ottevanger CF, & van Sittert NJ (1979) Porphyrins as possible
parameters for exposure to hexachlorocyclopentadiene, allylchloride,
epichlorohydrin and endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biochemical Press,
pp 55-61.
Narahasi T (1987) Effects of toxic agents on neural membranes. In:
Lowndes HE,ed. Electrophysiology in neurotoxicology. Boca Raton,
Florida, CRC Press, vol 1, pp 23-44.
NCI (1978) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute (DHEW Publication No. NIH 179-812)
NCI (1979) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute, 110 pp (Carcinogenesis Technical Report Series No. 12; NTIS
PB-288461)
Nebeker AV, Schuytema GS, Griffis WL, Barbitta JA, & Carey LA (1989)
Effect of sediment organic carbon on survival of Hyalella azteca
exposed to DDT and endrin. Environ Toxicol Chem, 8: 705-718.
Nelson SC, Bahler TL, Hartwell WV, Greenwood DA, & Harris LE (1956)
Serum alkaline phosphatase levels, weight changes and mortality rates
of rats fed endrin. J Agric Food Chem, 4(8): 696-700.
Nettleship DN & Peakall DB (1987) Organochlorine residue levels in
three high Arctic species of colonially-breeding seabirds from Prince
Leopold Island. Mar Pollut Bull, 18(8): 434-438.
NIOSH (1989) Manual of analytical methods. Endrin: Method No. 5519.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 1-4.
Nishimura N, Nishimura H, & Oshima H (1982) Survey on mutagenicity of
pesticides by the Salmonella-microsome test. J Aichi Med Univ Assoc,
10(4): 305-312.
Notten WRF & Henderson PT (1975) Alteration in urinary D-glucaric acid
excretion as an indication of exposition to xenobiotics. In:
Proceedings of the International Symposium--Environment and Health,
CEC/EPA/WHO, Paris, 1974.
Novak AF & Rao MRR (1965) Food safety program: endrin monitoring in
the Mississippi River. Science, 150: 1751.
Ohlendorf HM, Swineford DM, & Locke LN (1981) Organochlorine residues
and mortality of herons. Pestic Monit J, 14(4): 125-135.
Onodera S & Tabucanon MS (1986) Organochlorine pesticide residues in
the lower Chao Phraya River and klongs along the river at Bangkok
metropolitan area, 1982-1984. J Sci Soc Thailand, 12: 225-238.
Oomen PA (1986) A sequential scheme for evaluating the hazard of
pesticides to bees, Apis mellifera. Meded Fac Landbouwwet Rijksuniv
Gent, 51(3b): 1205-1213.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis K (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
O'Shea TJ, Brownell RL Jr, Clarke DR Jr, Walker WA, Gay ML, & Lamont
TG (1980) Organochlorine pollutants in small cetaceans from the
Pacific and South Atlantic Oceans, November 1968-June 1976. Pestic
Monit J, 14:(2): 35-46.
Ottevanger CF & Van Sittert NJ (1979) Relation between anti-12-hydroxy
endrin excretion and enzyme induction in workers involved in the
manufacture of endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 123-129.
Ottolenghi AD, Haseman JK, & Suggs F (1974) Teratogenic effects of
aldrin, dieldrin and endrin in hamsters and mice. Teratology, 9:
11-16.
Parveen Z & Masud SZ (1987) Organochlorine pesticide residues in
cattle feed samples in Karachi, Pakistan. J Sci Ind Res; 30(7):
513-516.
Patil KC, Matsumura F, & Boush GM (1970) Degradation of endrin,
aldrin, and DDT by soil microorganisms. Appl Microbiol, 19(5):
879-881.
Pavan I, Buglione E, Pettinati L, Perrelli G, Rubino GF, Bicchi C,
D'Amato A, Carlino F, Bugiani M, & Polizzi S (1987) [Accumulation of
organochlorine pesticides in human adipose tissue. data from the
province of Turin (Italy).] Med Lav, 78(3): 219-228 (in Italian).
Pawar SS & Kachole MS (1978) Hepatic and renal microsomal electron
transport reactions in endrin treated female guinea pigs. Bull Environ
Contam Toxicol, 20: 199-205.
Pearce F (1987) Pesticide deaths: the price of the green revolution.
New Sci, 114: 30.
Peterson SR & Ellarson RS (1978) pp'-DDE, polychlorinated biphenyls,
and endrin in old squaws in North America, 1969-73. Pestic Monit J,
11(4): 170-181.
Petrella VJ, Fox JP, & Webb RE (1975) Endrin metabolism in
endrin-susceptible and resistant strains of pine mice. Toxicol Appl
Pharmacol, 34: 283-291.
Petrella VJ, McKinney JD, Fox JP, & Webb RE (1977) Identification of
metabolites of endrin. Metabolism in endrin susceptible and resistant
strains of pine mice. J Agric Food Chem, 25(2): 393-398.
Pfister RM (1972) Interactions of halogenated pesticides and
microorganisms: a review. CRC Crit Rev Microbiol, 21(1): 1-33.
Phillips DD, Pollard GE, & Soloway SB (1962) Thermal isomerization of
endrin and its behaviour in gas chromatography. J Agric Food Chem,
10(3): 217-221.
Plimmer JR (1972) Photochemistry of organochlorine insecticides. In:
Tahori AS, ed. Proceedings of the Ssecond international IUPAC congress
of pesticides chemistry. New York, Gordon & Breach,vol 1, pp 413-432.
Podrebarac DS (1984) Pesticide, heavy metal, and other chemical
residues in infant and toddler total diet samples (IV). October
1977-September 1978. J Assoc Off Anal Chem, 67(1): 166-175.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environ Mutagen, 3: 11-32.
Prouty RM & Bunck C (1986) Organochlorine residues in adult mallard
and black duck wings, 1981-1982. Environ Monit Assess, 6: 49-57.
Prouty RM, Reichel WL, Locke LN, Belisle AA, Cromartie E, Kaiser TE,
Lamont TG, Mulhern BM, & Swineford DM (1977) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1973-74. Pestic Monit J, 11: 134-137.
Radeleff RD (1956) Hazards to livestock of insecticides used in
mosquito control. Mosq News, 16(2): 79-80.
Radhakrishnan AG & Antony PD (1989) Pesticide residues in marine
fishes. Fish Technol, 26: 60-61.
Rashid KA & Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. J Environ Sci Health, B21(4): 319-334.
Reddy DB, Edward VD, Abraham GJS, & Rao KV (1966) Fatal endrin
poisoning. A detailed autopsy, histopathological and experimental
study. J Indian Med Assoc, 46(3): 121-124.
Reddy DB, Abraham GJS, Edward VD, Naganna B, & Mathalli M (1967)
Further observations on endrin poisoning. J Indian Med Prof, 13(10):
5946, 5967-5968.
Redetzke K., Gonzalez AA, & Applegate HG (1983) Organochlorine
pesticides in adipose tissue of persons from Ciudad Juarez, Mexico. J
Environ Health, 46(1): 25-27.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 117(20): 525-527.
Reeves RG, Woodham DW, Ganyard MC, & Bond CA (1977) Preliminary
monitoring of agricultural pesticides in a cooperative tobacco pest
management project in North Carolina, 1971--first-year study. Pestic
Monit J, 11(2): 99-106.
Reichel WL, Lamont TG, Cromartie E, & Locke LN (1969) Residues in two
bald eagles suspected of pesticide poisoning. Bull Environ Contam
Toxicol, 4(1): 24-30.
Reidinger RF & Crabtree DG (1974) Organochlorine residues in golden
eagles, United States--March 1964-July 1971. Pestic Monit J, 8(1):
37-43.
Reins DA, Holmes DD, & Hinshaw LB (1964) Acute and chronic effects of
the insecticide endrin on renal function and renal hemodynamics. Can
J Physiol Pharmacol, 42(5): 599-608.
Reins DA, Rieger JA Jr, Stavinoha WB, & Hinshaw LB (1966) Effect of
endrin on venous return and catecholamine release in the dog. Can J
Physiol Pharmacol, 44: 59-67.
Ressang AA, Titus I, Andar RS, & Soedarmo D (1958) Aldrin, dieldrin
and endrin intoxication in cats. Commun Vet, 2(2): 71-88.
Reuber MD (1978) Carcinomas, sarcomas and other lesions in
Osborne-Mendel rats ingesting endrin. Exp Cell Biol, 46(3): 129-145.
Revzin AM (1966) Effects of endrin on telencephalic function in the
pigeon. Toxicol Appl Pharmacol, 9(1): 75-83.
Revzin AM (1980) Some acute and chronic effects of endrin on the
brains of pigeons and monkeys. In: Proceedings of the symposium on the
biological impact of pesticides in the environment, pp 134-141.
Ribbens PH (1985) Mortality study of industrial workers exposed to
aldrin, dieldrin and endrin. Arch Occup Environ Health, 56(2): 75-79.
Richardson LA, Lane JR, Gardner WS, Peeler JT, & Campbell JE (1967)
Relationship of dietary intake to concentration of dieldrin and endrin
in dogs. Bull Environ Contam Toxicol, 2(4): 207-219.
Richardson A, Robinson J, & Baldwin MK (1970) Metabolism of endrin in
the rat. Chem Ind, 1970,: 502-503.
Ritchey SJ, Young RW, & Essary EO (1972) Effects of heating and
cooking method on chlorinated hydrocarbon residues in chicken tissues.
J Agric Food Chem, 20: 291-293.
Robinson J & McGill AEJ (1966) Organochlorine insecticide residues in
complete prepared meals in Great Britain during 1965. Nature,
212(5066): 1037-1038.
Robinson J, Richardson A, Hunter CG, Crabtree AN, & Rees HJ (1965)
Organochlorine insecticide content of human adipose tissue in
south-eastern England. Br J Ind Med, 22: 220-229.
Roos AH, van Munsteren AJ, Nab FM, & Tuinstra LGMT (1987) Universal
extraction/clean-up procedure for screening of pesticides by
extraction with ethylacetate and size exclusion chromatography. Anal
Chim Acta, 196: 95-102.
Rosales MTL, Escalona RL, Alarcon RM, & Zamora V (1985) Organochlorine
hydrocarbon residues in sediments of two different lagoons of
Northwest Mexico. Bull Environ Contam Toxicol, 35: 322-330.
Rosen JD (1972) Conversion of pesticides under environmental
conditions. In: Coulston F & Korte F ed. Environmental quality.
Stuttgart, George Thieme, vol 1, pp 85-96.
Rosen JD, Sutherland DJ, & Lipton GR (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull
Environ Contam Toxicol, 1: 133-140.
Rowe DR, Canter LW, Snyder PJ, & Mason JW (1971) Dieldrin and endrin
concentrations in a Louisiana estuary. Pestic Monit J, 4(4): 177-183.
Rowley DL, Rab MA, Hardjotanojo W, Liddle J, Burse VW, Saleem M, Sokal
D, Falk H, & Head SL (1987) Convulsions caused by endrin poisoning in
Pakistan. Pediatrics, 79(6): 928-934.
Roylance KJ, Jorgensen CD, Booth GM, & Carter MW (1985) Effects of
dietary endrin on reproduction of Mallard ducks (Anas
platyrhynchos). Arch Environ Contam Toxicol, 14: 705-711.
Runhaar EA, Sangster B, Greve PA, & Voortman M (1985) A case of fatal
endrin poisoning. Hum Toxicol, 4: 241-247.
Ryan S, Bacher GJ, & Martin AA (1972) The mussel Hyridella australis
as a biological monitor of the pesticide endrin in fresh water.
Search, 3(11-12): 446-447.
Safe S & Hutzinger O (1979) Mass spectrometry of pesticides and
pollutants. Boca Raton, Florida, CRC Press, Inc.
Saiki MK & Schmitt CJ (1986) Organochlorine chemical residues in
bluegills and common carp from the irrigated San Joaquin Valley floor,
California. Arch Environ Contam Toxicol, 15: 357-366.
Samhan O & Ghobrial F (1987) Trace metals and chlorinated hydrocarbons
in sewage sludges of Kuwait. Water Air Soil Pollut, 36: 239-246.
Satsmadjis J, Georgakopoulos-Gregoriades E, & Voutsinou-Taliadouri F
(1988) Red mullet contamination by PCBs and chlorinated pesticides in
the Pagassitikos Gulf, Greece. Mar Pollut Bull, 19(3): 136-138.
Schafer ML, Peeler JT, Gardner WS, & Campbell JE (1969) Pesticides in
drinking water--waters from the Mississippi and Missouri rivers.
Environ Sci Technol,3(12): 1261-1269.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12: 355-382.
Scheutz EG, Wrighton SA, Safe SH, & Guzelian PS (1986) Regulation of
cytochrome P-450p by phenobarbital and phenobarbital-like inducers in
adult rat hepatocytes in primary monolayer culture and in vivo.
Biochemistry, 25: 1124-1133.
Schwabe U & Wendling I (1967) [Stimulation of drug metabolism by low
doses of DDT and other chlorinated hydrocarbon insecticides.]
Arzneimittel-forschung, 17(5): 614-618 (in German).
Seifert J (1988) Ontogenesis and properties of the convulsant
recognition site(s) of the gamma-aminobutyric acid (GABA) receptor
complex in chicken embryo. Eur J Pharmacol,151: 443-448.
Seifert J (1989) Teratogenesis of polychlorocycloalkane insecticides
in chicken embryos resulting from their interactions at the convulsant
recognition sites of the GABA (pro)receptor complex. Bull Environ
Contam Toxicol, 42: 707-715.
Sherman M & Rosenberg M (1954) Subchronic toxicity of four chlorinated
dimethanonaphthalene insecticides to chicks. J Econ Entomol, 47 (6):
1082-1083.
Sierra M & Santiago D (1987) Organochlorine pesticide levels in barn
owls collected in Leon, Spain. Bull Environ Contam Toxicol, 38:
261-265.
Sierra M, Teran MT, Gallego A, Diez MJ, & Santiago D (1987)
Organochlorine contamination in three species of diurnal raptors in
Leon, Spain. Bull Environ Contam Toxicol, 38: 254-260.
Simmon VF, Kauhanen K, & Tardiff RC (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North Holland Biomedical Press, pp 249-258.
Singh MN (1988) Mortality response of Achatina fulica to various
pesticides. J Environ Biol, 9(2): 157-162.
Singh PDA & West ME (1985) Acute pesticide poisoning in the Caribbean.
West Indian Med J, 34: 75-83.
Singhal RL & Kacew S (1976) The role of cyclic AMP in chlorinated
hydrocarbon-induced toxicity. Fed Proc, 35(14): 2618-2623.
Smith KJ, Polen PB, de Vries DM, & Coon FB (1968) Removal of
chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J Am Oil Chem Soc, 45: 866-869.
Sobti RC, Krishan A, & Davies J (1983) Cytokinetic and cytogenetic
effect of agricultural chemicals on human lymphoid cells in vitro.
II. Organochlorine pesticides. Arch Toxicol, 52: 221-231.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
northeastern Alberta otters. Bull Environ Contam Toxicol, 39: 783-790.
Soto AR & Deichmann WB (1967) Major metabolism and acute toxicity of
aldrin, dieldrin and endrin. Environ Res, 1(4): 307-322.
Spann JW, Heinz GH, & Hulse CS (1986) Reproduction and health of
mallards fed endrin. Environ Toxicol Chem, 5: 755-759.
Speck LB & Maaske CA (1958) The effects of chronic and acute exposure
of rats to endrin. Arch Ind Health, 18: 268-272.
Spynu EI (1964) On the toxicology of new organic chloride insecticides
obtained by diene synthesis on the basis of hexachlorocyclopentadiene.
Gig Tr Prof Zabol, 4: 30-35.
Squires RF & Saederup E (1989) Polychlorinated convulsant insecticides
potentiate the protective effect of NaCl against heat inactivation of
[3H] fluonitrazepam binding sites. J Neurochem, 52: 537-543.
Stanford Research Institute (1953) Unpublished letter Report No. 1
Ref. Project No. B-868 November 10, Stanford, CA, submitted to WHO by
Shell.
Stanford Research Institute (1954) Unpublished letter Report No. 3
Ref. Project No. B-868 February 1, Stanford, CA, submitted to WHO by
Shell.
Stanley CW, Barney JE II, Helton MR, & Yobs AR (1971) Measurement of
atmospheric levels of pesticides. Environ Sci Technol, 5: 430-435.
Steinberg KK, Garza A, Bueso JA, Burse VW, & Phillips DL (1989) Serum
pesticide concentrations in farming cooperatives in Honduras. Bull
Environ Contam Toxicol, 42: 643-650.
Stickel WH, Kaiser TE, & Reichel WL (1979) Endrin versus 12-ketoendrin
in birds and rodents. In: Kenaga EE ed. Avian and mammalian wildlife
toxicology, Philadeplphia,, American Society for Testing and
Materials, pp 61-68 (ASTM STP 693).
Stout VF (1980) Organochlorine residues in fishes from the Northwest
Atlantic Ocean and Gulf of Mexico. Fish Bull, 78(1): 51-58.
Strachan WMJ (1988) Toxic contaminants in rainfall in Canada: 1984.
Environ Toxicol Chem, 7: 871-877.
Strachan WMJ, Huneault H, Schertzer WM, & Elder FC (1980)
Organochlorines in precipitation in the Great Lakes region. In: Afghan
BK & MacKay D ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment. New York, Plenum Publishing Corp., pp 387-396.
Strassman SC & Kutz FW (1977) Insecticide residues in human milk from
Arkansas and Mississippi, 1973-1974. Pestic Monit J, 10(4): 130-133.
Strik JJTWA (1979) The occurrence of chronic hepatic porphyria in man,
caused by halogenated hydrocarbons. In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Elsevier/North Holland
Biomedical Press, p 3.
Struger J, Weseloh D, Hallett DJ, & Mineau P (1985) Organochlorine
contaminants in herring gull eggs from the Detroit and Niagara Rivers
and Saginaw Bay (1978-1982): contaminant discriminants. J Great Lakes
Res, 11(3): 223-230.
Sturm R, Knauth HD, Reinhardt KH, & Gandrasz J (1986) Distribution of
chlorinated hydrocarbons in sediment and seston of the River Elbe.
Wasser, 67: 23-38.
Sundershan P & Khan MAQ (1980) Metabolic fate of [14C] endrin in
bluegill fish. Pestic Biochem Physiol, 14: 5-12.
Suzuki M & Morimoto M (986) High resolution chemically bonded
fused-silica capillary gas chromatography of organochlorine
insecticides and related compounds in arable soil samples. J High
Resol Chromatogr Chromatogr Commun, 9: 296-298.
Suzuki M, Yamato Y, & Watanabe T (1973) Multiple organochlorine
pesticide residues in Japan. Bull Environ Contam Toxicol, 10(3):
145-150.
Suzuki K, Nagayoshi H, & Kashiwa T (1974) The systematic separation
and identification of pesticide in the first division. Agric Biol
Chem, 38(2): 279-285.
Sykes PW Jr (1985) Pesticide concentrations in snail kite eggs and
nestlings in Florida. Condor, 87: 438.
Tarrant KR & Tatton JO'G (1968) Organochlorine pesticides in rainwater
in the British Isles. Nature, 219(5155): 725-727.
Telling GM, Sissons DJ, & Brinkman HW (1977) Determination of
organochlorine insecticide residues in fatty food stuffs using a
clean-up technique based on a single column of activated alumina. J
Chromatogr, 137: 405-423.
Teran MT & Sierra M (1987) Organochlorine insecticides in trout,
Salmo trutta fario L. taken from four rivers in Leon, Spain. Bull
Environ Contam Toxicol, 38: 247-253.
Terriere LC (1964) Endrin. In: Zweig, G ed. Analytical methods for
pesticides, plant growth regulators and food additives. New York,
Academic Press, vol 2, pp 209-222.
Terriere LC, Kiigemagi U, & England DC (1958) Endrin content of body
tissues of steers, lambs and hogs receiving endrin in their daily
diet. J Agric Food Chem, 6(7): 516-518.
Terriere LC, Arscott GH, & Kiigemagi U (1959) The endrin content of
eggs and body tissue of poultry receiving endrin in their daily diet.
J Agric Food Chem, 7(7): 502-504.
Thier HP & Stijve T (1986) [Results of an inter-laboratory comparison
of analyses of analyses of organochlorine and organophosphorus
pesticide residues in fat.] Lebensmittelchem Gerichtl Chem, 40: 73-75
(in German).
Thompson JF (1976) Manual of analytical quality control for pesticides
and related compounds in human and environmental samples. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Office of Research and Development, Health Effects Research Laboratory
(EPA-600/1-76-017).
Thurston R, Gilfoil TA, Meyn EL, Zajdel RK, Aoki TI, & Veith GD (1985)
Comparative toxicity of ten organic chemicals to ten common aquatic
species. Water Res, 19(9): 1145-1155.
Travis CC & Arms AD (1988) Bioconcentration of organics in beef, milk
and vegetation. Environ Sci Technol, 22: 271-274.
Treon JF, Cleveland FP, & Cappel J (1955) Toxicity of endrin for
laboratory animals. J Agric Food Chem, 3(10): 842-848.
Truhaut R, Do Phuoc H, & Phu Lich N (1974) Influence de
l'administration de pesticides organo-halogénés et de
polychloro-biphényles sur le métabolisme de la zoxazolamine chez le
rat. CR Acad Sci Paris Sér D, 278: 3003-3006.
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Bureau of Sport Fisheries and Wildlife,
Denver Wildlife Center (Resource Publication No. 84).
Tuinstra LGMT (1971) Organochlorine insecticide residues in human milk
in the Leiden region. Neth Milk Dairy J, 25: 24-32.
Uhnak J, Sackmauerova M, Szokolay A, & Pal'usova O (1974) The use of
an electron capture detector for the determination of pesticides in
water. J Chromatogr 91: 545-547.
United Kingdom Ministry of Agriculture, Fisheries and Food (1989)
Report of the Working Party on Pesticide Residues 1985-1988. London,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
US EPA (1974) New Orleans area water supply study (draft analytical
report). Dallas, Texas, US Environmental Protection Agency, Lower
Mississippi River Facility, Surveillance and Analysis Divisions,
Region VI.
US EPA (1983) Findings of selected chemical residues in human blood
serum and adipose tissue: Endrin. Environ News, 9 May.
US EPA (1985) Hexachloronorbornadiene; Proposed submission of notice
of manufacturer, import or processing and determination of significant
new use. Fed Reg, 50(36): 7351-7356.
US EPA (1987a) Health effects assessment for endrin. Cincinnati, Ohio,
US Environmental Protection Agency, Ofice of Research and Development,
Environmental Criteria and Assessment Office, 31 pp (Report
PB88-180237).
US EPA (1987b) Health advisories for 16 pesticides. Washington, DC, US
Environmental Protection Agency, Office of Drinking Water, 18 pp
(Report PB87-200176).
Vance BD & Drummond W (1969) Biological concentration of pesticides by
algae. J Am Water Works Assoc, 61: 360-362.
Van Dijck P & van de Voorde H (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded Fac Landbouww
Rijksuniv Gent, 41(2): 1491-1498.
Van Raalte HGS (1965) [Some aspects of pesticide toxicity.]
Unpublished paper presented at the Conference on Occupational Health,
Caracas, Venezuela; The Hague. Shell, International Petroleum Company,
Health, Safety and Environment Division (in Spanish).
Van Sittert NJ (1985) Biological monitoring of bestrijdingsmiddelen;
Coronel-PAOG Nascholingssymposium. Unpublished paper, Amsterdam, Vrije
Universiteit, February 1985 (in Dutch).
Van Wynen JH & Stykel A (1988) Health risk assessment of residents
living on harbour sludge. Arch Occup Environ Health, 61: 77-87.
Veith GD, Kuehl DW, Leonard EN, Puglisi FA, & Lemke AE (1979)
Polychlorinated biphenyls and other organic chemical residues in fish
from major watersheds of the United States, 1976. Pestic Monit J,
13(1): 1-11.
Veith GD, Kuehl DW, Leonard EN, Welch K, & Pratt G (1981)
Polychlorinated biphenyls and other organic chemical residues in fish
from major United States watersheds near the Great Lakes, 1978. Pestic
Monit J, 15(1): 1-8.
Verma MP, Bahga HS, Soni BK, & Singh SP (1970) Effect of insecticides
on lactic acid concentration in the blood of buffalo-calves. Indian
Vet J, 47(12): 1056-1058.
Vermeer K, Risebrough RW, Spaans AL, & Reynolds LM (1974) Pesticide
effects on fishes and birds in rice fields in Surinam, South America.
Environ Pollut, 7: 217-236.
Versteeg JPJ & Jager KW (1973) Long-term occupational exposure to the
insecticides aldrin, dieldrin, endrin and telodrin. Br J Ind Med, 30:
201-202.
Villeneuve JP, Holm E, & Cattini C (1985) Transfer of chlorinated
hydrocarbons in the food chain lichen --> reindeer --> man.
Chemosphere, 14(11/12): 1651-1658.
Von Westernhagen H, Dethlefsen V, Cameron P, & Janssen D (1987)
Chlorinated hydrocarbon residues in gonads of marine fish and effects
on reproduction. Sarsia, 72: 419-422.
Von Westernhagen H, Cameron P, Dethlefsen V, & Janssen D (1989)
Chlorinated hydrocarbons in North Sea whiting (Merlangius merlangus)
and effects on reproduction. I. Tissue burden and hatching success.
Helgolander Meeresunters 43: 45-60.
Vrij-Standhardt WG, Strik JJTWA, Ottevanger CF, & van Sittert NJ
(1979) Urinary D-glucaric acid and urinary total porphyrin excretion
in workers exposed to endrin. In: Strik JJTWA & Koeman JH ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 113-121.
Wafford KA, Sattelle DB, Gant DB, Eldefrawi AT, & Eldefrawi ME (1989a)
Non-competitive inhibition of GABA receptors in insect and vertebrate
CNS by endrin and lindane. Pestic Biochem Physiol, 33: 213-219.
Wafford KA, Lummis SCR, & Sattelle DB (1989b) Block of an insect
central nervous system GABA receptor by cyclodiene and cyclohexane
insecticides. Proc R Soc Lond, B237: 53-61.
Walker JJ & Phillips DE (1987) An electron microscopic study of endrin
induced alterations in unmyelinated fibers of mouse sciatic nerve.
Neurotoxicology, 8(1): 55-64.
Walsh GM & Fink GB (1970) Temporal aspects of acute endrin toxicity in
mice. Proc West Pharmacol Soc, 13: 81-83.
Walsh GM & Fink GB (1972) Comparative toxicity and ditribution of
endrin and dieldrin after intravenous administration in mice. Toxicol
Appl Pharmacol, 23: 408-416.
Wang HH & MacMahon B (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J Occup Med, 21(11):745-748.
Wassermann M, Curnow DH, Forte PN, & Groner Y (1968) Storage of
organochlorine pesticides in the body fat of people in Western
Australia. Int J Ind Med Surg, 37(4): 295-300.
Wassermann M, Francone MP, Wassermann D, Mariani F, & Groner J (1969)
[Organochlorine pesticide content of the fatty tissue of the general
public in Argentina.] Sem Med, 134(16): 459-462 (in Spanish).
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. Basic Life Sci, 21: 275-326.
Webb RE & Horsfall F Jr (1967) Endrin resistance in pine mouse.
Science, 156: 1762.
Webb RE, Hartgrove RW, Randolph WC, Petrella VJ, & Horsfall F Jr
(1973) Toxicity studies in endrin-susceptible and resistant strains of
pine mice. Toxicol Appl Pharmacol, 25(1): 42-47.
Weeks DE (1967) Endrin food-poisoning. A report on four outbreaks
caused by two separate shipments of endrin-contaminated flour. Bull
World Health Organ, 37: 499-512.
Wegman RCC & Greve PA (1974) Levels of organochlorine pesticides and
inorganic bromide in human milk. Meded Fac Landbouwwet Rijksuniv Gent,
39: 1301-1310.
Wegman RCC & Greve PA (1978) Organochlorines, cholinesterase
inhibitors and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic Monit J, 12(3): 149-162.
Wegman RCC & Greve PA (1980) Halogenated hydrocarbons in Dutch water
samples over the years 1969-1977. Environ Sci Res, 16: 405-415.
Wegman RCC & Hofstee AWM (1982) Determination of organochlorines in
river sediment by capillary gas chromatography. Water Res, 16:
1265-1272.
Wegman RCC, Hofstee AWM, & Greve PA (1981) Uptake of organochlorines
by plants growing on river and basin sediment. Meded Fac Landbouwwet
Rijksuniv Gent, 46(1): 359-365.
Weisgerber I, Klein W, & Korte F (1969) [Disappearance of residues and
metabolism of endrin-(14C) in tobacco.] Liebigs Ann Chem, 729:
193-197 (in German with English abstract).
Wells MR & Yarbrough JD (1972) Vertebrate insecticide resistance:
in vivo and in vitro endrin binding to cellular fractions from
brain and liver tissues of Gambusia. J Agric Food Chem, 20(1): 14-16.
Whetstone RR (1964) Chlorocarbons and chlorohydrocarbons: chlorinated
derivatives of cyclopentadiene. In: Kirk-Othmer encyclopedia of
chemical technology, 2nd ed., New York, John Wiley and Sons, vol 5, pp
240-252.
WHO (1989) Environmental Health Criteria No. 91: Aldrin and dieldrin.
Geneva, World Health Organization, 335 pp.
WHO (1992) The WHO recommended classification of pesticides by hazard.
Guidelines to classification 1992-1993. Geneva, World Health
Organization (WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 1: Endrin. Geneva, World
Health Organization (VBC/DS/75.1).
Wiemeyer SN, Jurek RM, & Moore JF (1986) Environmental contaminants in
surrogates, foods and feathers of California condors (Gymnogyps
californianus). Environ Monit Assess, 6: 91-111.
Williams S (1964) Pesticide residues in total diet samples. J Assoc
Off Anal Chem, 47(5): 815-821.
Williams GM (1979) Liver cell culture systems for the study of
hepatocarcinogenesis. In: Margison GP ed Advances in medical oncology
research and education: Carcinogenesis, New York, Pergamon Press, vol
1, pp 273-280.
Williams S, Mills PA, & McDowell RE (1964) Residues in milk of cows
fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J Asoc Off Agric Chem, 47: 1124-1128.
Williams DT, Benoit FM, McNeil EE, & Otson R (1978) Organochlorine
pesticide levels in Ottawa drinking water, 1976. Pestic Monit J,
12(3): 163-166.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71(2): 410-414.
Wilson Committee (1969) Report by the Advisory Committee on Pesticides
and Other Toxic Chemicals. Further review of certain persistent
organochlorine pesticides used in Great Britain. London, Her Majesty's
Stationery Office, pp 90-100.
Wilson JG & Earley JJ (1986) Pesticide and PCB levels in the eggs of
shag Phalacrocorax aristotelis and cormorant Phalacrocorax carbo
from Ireland. Environ Pollut (Ser B), 12: 15-26.
Wit SL (1971) [Persistent insecticides in Dutch body fat.] Chem
Weekbl, 67(5): 11-14 (in Dutch).
Witherup S, Stemmer KL, Taylor P, & Bietsch P (1970) The Incidence of
Neoplasms in Two Strains of Mice Sustained on Diets Containing Endrin.
Unpublished report, Cincinnati, Ohio, Kettering Laboratory, submitted
to WHO by Shell.
Wolfe HR, Durham WF, & Armstrong JF (1963) Health hazards of the
pesticides endrin and dieldrin: hazards in some agricultural uses in
the Pacific Northwest. Arch Environ Health, 6: 458-464.
Wolfe HR, Durham WF, & Armstrong JF (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolman AA & Wilson AJ Jr (1970) Occurrence of pesticides in whales.
Pestic Monit J, 4(1): 8-10.
Wüthrich C, Müller F, Blaser O, & Marek B (1985) [Pesticides and other
chemical residues in Swiss diet samples.] Mitt Geb Lebensmittel Hyg,
76: 260-276 (in German with English summary).
Wuu KD & Grant WF (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare).
Can J Genet Cytol, 8: 481-501.
Wuu KD & Grant WF (1967a) Chromosomal aberrations induced by
pesticides in meiotic cells of barley. Cytologia, 32: 31-41.
Wuu KD & Grant WF (1967b) Chromosomal aberrations induced in somatic
cells of Vicia faba by pesticides. Nucleus, 10(1): 37-46.
Yakushiji T, Watanabe I, Kuwabara K, Yoshida S, Hori S, Fukushima S,
Kashimoto T, Koyama K, & Kunita N (1979) Levels of organochlorine
pesticides and polychlorinated biphenyls (PCBs) in mothers milk
collected in Osaka prefecture from 1969-1976. Arch Environ Contam
Toxicol, 8: 59-66.
Yarbrough JD, Roush RT, Bonner JC, & Wise DA (1986) Monogenic
inheritance of cyclodiene insecticide resistance in mosquitofish,
Gambusia affinis. Experientia (Basel), 42: 851-853.
Young RA & Mehendale HM (1986) Effect of endrin and endrin derivatives
on hepatobiliary function and carbontetrachloride-
induced hepatotoxicity in male and female rats. Food Chem Toxicol,
24(8): 863-868.
Zabik MJ, Schuetz RD, Burton WL, & Pape BE (1971) Photochemistry of
bioactive compounds. Studies of a major photolytic product of endrin.
J Agric Food Chem, 19(2): 308-313.
Zavon MR, Hine CH, & Parker KD (1965) Chlorinated hydrocarbon
insecticides in human body fat in the United States. J Am Med Assoc,
193(10): 181-183.
Zeiger E (1987) Carcinogenicity of mutagens: predictive capability of
the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer
Res, 47: 1287-1296.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environ Mutagen, 9 (suppl9): 1-110.
Zimmerli B & Marek B (1973) [The pesticide load of the Swiss
population.] Mitt Geb Lebensmittel Hyg,, 64(4): 459-479 (in German
with English summary).
ANNEX I CHEMICAL NAMES OF ENDRIN AND ITS METABOLITES
Two main systems are currently used for the nomenclature of
cyclodiene insecticides: 'polyhydroaromatic' names, used by Chemical
Abstracts (American Chemical Society) and the International Union for
Pure and Applied Chemistry (IUPAC), and the von Baeyer/IUPAC system
for polycyclic aliphatic compounds. Benson (1969) and Bedford (1974)
proposed that the latter system be used for the cyclodiene
insecticides.
The 'polyaromatic' system has, unfortunately, been subject to
historical variation, and there are differences between the IUPAC,
British and American conventions for defining the three-dimensional
stereochemistry in this system. As a consequence of differences in the
numbering of carbon atoms in the two systems and the modification of
the Chemical Abstracts 'polyaromatic' name for dieldrin since 1971,
considerable confusion can arise in the nomenclature of metabolites.
The possible misunderstandings that may occur, particularly among
people who are not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that are given to
the major metabolite of endrin; this one compound may be designated
as:
anti-9-hydroxyendrin (former Chemical Abstracts system)
anti-8-hydroxyendrin (current Chemical Abstracts system)
anti-12-hydroxyendrin (von Baeyer/IUPAC system).
A useful discussion of nomenclature was given by Brooks (1974).
The chemical names for endrin and its metabolites are summarized
in Table 30.
Table 30. Chemical nomenclature of endrin and its metobolites
Trivial namesa Polycyclic aliphatic name (von Baeyer/ IUPAC) Alternative or former names
Endrin (I) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-2,3-7, 6- 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,
endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]- 4a,5,6,7,8,8a-octahydro-1,4- endo,endo-
dodec-9-ene 5,8-demethanonaphthalene (former CAS
name)
1aa,2b,2ab,3a,6a,6b,7b,7a)-3,4,5,6,9,9-
Hexachloro-1a,2,2a,3,6,6a,7,7a,-octahydro-
2,7:3,6-dimethanonaphth[2,3- b]oxirene
(current CAS name)
(IR,4S,4aS,5S,7R,8R,8aR)-1,2,3,4,10,10-
Hexachloro-1,4,4a,5,6,7,8,8a-octahydro-6,7-
epoxy-1,5:5,8-dimethanonaphthalene
(current IUPAC name)
syn-12-Hydroxyendrin (II) 1,8,9,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- syn-hydroxyendrin) ( syn-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8- endo-
tetracyclo[6.2.1.13,6.02,7] dodec-9-ene
anti-12-Hydroxyendrin (III) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- anti-hydroxyendrin) ( anti-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8-
endo-tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
3-Hydroxyendrin (IV) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-3-
(5-hydroxyendrin) hydroxy-2,3-7,6- endo-2,1-7,8- endo-tetracyclo-
[6.2.1.13,6.02,7] dodec-9-ene
12-Ketoendrin (V) 1,8,9,10,11,11-Hexachloro-4,5 - exo-epoxy-2,3-7,6-
(9-ketoendrin) endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]-
dodec-12-one
Endrin trans-diol (IV) 1,8,9,10,11,11-Hexachloro-4,5-( exo)trans-dihydroxy-
2,3-7,6- endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]
dodec-9-ene
aRoman numerals in parentheses refer to the structures in Figure 2.
ANNEX II
MEDICAL TREATMENT OF ENDRIN POISONING
1. Symptoms of poisoning
Endrin is readily absorbed and is toxic when taken by mouth, by
skin contact (especially liquid formulations), and by inhalation. It
stimulates the central nervous system, and an oral dose of 0.25 mg/kg
body weight can cause convulsions in humans. Following accidental
ingestion or gross over-exposure, symptoms appear between 20 min and
12 h and may include headache, dizziness, nausea, vomiting, weakness
in the legs, and convulsions, sometimes leading to death.
Organochlorine compounds can cause respiratory depression, and
they may sensitize the heart to endogenous catecholamines, leading to
ventricular fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis, and if
necessary blood gases should be checked. Use of an
electrocardiographic monitor is recommended if symptoms are severe.
Endrin is eliminated very quickly from the blood and can be
detected for only 1 or 2 days even after massive over-exposures. Signs
and symptoms of poisoning occur only at levels in whole blood of above
0.05 µg/ml.
2. Medical treatment
Medical treatment is largely symptomatic and supportive and is
directed against convulsions and hypoxia. If endrin is swallowed, the
stomach should be emptied as soon as possible by careful gastric
lavage (with a cuffed endotracheal tube already in place), avoiding
aspiration into the lungs. In a rural situation where this is not
feasible, vomiting should be induced immediately. This should be
followed by (intragastric) administration of 50 g of activated
charcoal and 30 g of magnesium or sodium sulfate in a 30% aqueous
solution. Oily purgatives are contraindicated, and no fats, oils, or
milk should be given.
If convulsions occur, anticonvulsants should be given immediately
but slowly, and repeated as necessary. Diazepam can be given at 10 mg
(children, 1-5 mg) intravenously; thiopental sodium or hexobarbital
sodium can be given intravenously at a dose of 10 mg/kg, with a
maximum total dose of up to 750 mg for an adult; or paraldehyde can be
given at 5 ml by intramuscular injection. These short-acting
anticonvulsants should always be followed by phenobarbital given
orally at 3 mg/kg (up to 200 mg for an adult), or phenobarbital sodium
given intramuscularly at 3 mg/kg (up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline,
should never be given.
The airway must be kept unobstructed. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
A guideline for the management of major status epilepticus is
added as Annex III.
ANNEX III
MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
1. Initial management
1. Assess the patient, verify the diagnosis, remove false
teeth, place the patient in a lateral semi-prone position,
and establish an airway.
2. Give diazepam intravenously (see Note 1, below), usually at
10 mg in 2 ml (0.15-0.25 mg/kg), followed immediately by a
further 10 mg (2 ml) over 1-2 min. This may be repeated
according to response.
3. Take blood to measure levels of anticonvulsant drug,
ethanol, and blood sugar (5 ml of blood in a sugar tube); a
sample to measure calcium (5 ml in a plain tube); and a drop
of blood to determine blood glucose.
4. If the latter measurement shows low blood glucose level, 25
ml of 50% glucose should be given intravenously, preferably
by catheter and not into a small distal vein. If ethanol is
likely to be present, give thiamine intravenously at 100 mg.
5. Give phenytoin intravenously at 250 mg in 5 ml (10-15 mg/kg)
no faster than 50 mg (1 ml)/min by infusion pump or slow
intravenous injection (see Note 2, below).
2. If fits continue, transfer patient to the intensive care unit and
consult an anaesthetist
6. Give chlormethiazole intravenously at 8 mg/ml: a loading
dose of up to 800 mg (100 ml) over 10 min at 10 ml/min,
maintained with 0.5-1 ml/min (4-8 mg).
7. Give thiopentone intravenously at 5 mg/kg as a loading dose,
then 1-3 mg/kg per h to a maximum blood level of 100
mg/litre.
8. If this fails, consult a neurologist.
3. Notes
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there
is concurrent use of other central nervous system depressant
drugs, especially phenobarbital.
aAdapted from guidelines issued at Guy's Hospital, London
Diazepam must not be given intramuscularly:
--added to an intravenous infusion
--with phenobarbital, unless artificial ventilation is
available.
Rectal administration of diazepam (using a rectal
administration set), at 5 or 10 mg in 2.5 ml, may be used
for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
--intramuscularly
--by central line
--into a dextrose infusion
--with any other drug.
Intravenously administered phenytoin should be monitored by
continuous electrocardiographic recording. If this is not
available, it may be safer to use a diluted solution of 250
mg (5 ml) in 250 ml of normal saline, no faster than 50
mg/min. The diluted solution should be used immediately
provided there is no evidence of precipitation (this use of
phenytoin is not licensed).
4. Options
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate deep intramuscular
injection or 10 ml diluted in 100 ml of normal saline given
intravenously over 10-15 min. Note: Paraldehyde should be
administered only with glass syringes.
2. Phenobarbital: 200 mg/ml, should not be given intravenously
except when artificial ventilation is available, and not at
all if the patient normally takes phenobarbital. The maximal
rate of infusion is 100 mg/min to a maximum dose of 15
mg/kg.
3. Lignocaine: 100 mg by slow intravenous injection, followed
by 50-100 mg in 250 ml of 5% dextrose at 1-2 mg/min. Note:
This treatment must be monitored by electrocardigram.
4. Diazepam: 10 mg in 2 ml intravenously, or 40 mg in 500 ml of
5% dextrose at a maximal infusion rate of 100 mg/h.
5. Sodium valproate: 400 mg in 4 ml, or 400-800 mg
intravenously over 3-5 min (up to 10 mg/kg), followed by
intravenous. infusion to a maximum of 2.5 g/day
(unlicensed).
5. Paediatric doses
For children, dosing should be adapted as follows:
Diazepam: 0.2-0.3 mg/kg intravenously
Phenytoin: 10-20 mg/kg intravenously
Chlormethiazole: 5-10 mg/kg per h, equivalent to
0.6-1.25 ml/kg per h
RESUME ET EVALUATION; CONCLUSIONS; RECOMMANDATIONS
Résumé et évaluation
Exposition
L'endrine est un insecticide organochloré utilisé depuis les
années 1950 contre toute sorte de ravageurs, qui s'attaquent notamment
au coton mais également au riz, à la canne à sucre, au maïs et à
d'autres cultures. On l'utilise également comme rodenticide et
avicide. Il est disponible dans le commerce sous forme de poudres, de
granulés, de pâtes et de concentrés émulsionnables.
L'endrine pénètre principalement dans l'atmosphère par
volatilisation et dispersion. En général, la volatilisation se produit
après épandage sur le sol et sur les récoltes et elle est tributaire
de nombreux facteurs comme la teneur en matières organiques et en eau
du sol, l'humidité, les courants aériens et l'aire foliaire des
végétaux.
C'est principalement par lessivage à partir du sol que se produit
la contamination des eaux superficielles. Les précipitations, qu'il
s'agisse de neige ou de pluie, n'ont qu'une part négligeable dans
cette contamination.
Localement, une contamination peut également se produire par
suite du déversement d'effluents industriels ou de négligences dans
les techniques d'épandage.
C'est principalement par suite d'un épandage direct sur les
terrains et les récoltes que l'endrine pénètre dans le sol. Elle peut
y être retenue, transportée ou dégradée, en fonction d'un certain
nombre de facteurs. C'est dans les sols riches en matières organiques
que la rétention est la plus importante. La persistance de l'endrine
dépend dans une large mesure des conditions locales; sa demi-vie dans
le sol peut aller jusqu'à 12 ans. La disparition de l'endrine présente
en surface s'effectue principalement par volatilisation et
photodécomposition. Sous l'influence de la lumière solaire
(rayonnement ultra-violet), l'endrine est isomérisée en delta-
cétoendrine. En présence de lumière solaire intense, on a observé une
isomérisation de 50% de l'endrine en l'espace de sept jours.
L'isomérisation peut également s'effectuer par action microbienne
(champignons et bactéries), notamment en anaérobiose.
Les invertébrés aquatiques et les poissons absorbent rapidement
l'endrine présente dans l'eau mais, transvasés dans une eau non
contaminée, les poissons exposés éliminent sans délai le pesticide. En
cas d'exposition continue, le facteur de bioconcentration peut
atteindre 14-18 000.
Il est possible que les invertébrés terricoles absorbent
facilement l'endrine. La présence occasionnelle de faibles quantités
d'endrine dans l'air ainsi que dans les eaux de surface, notamment
destinées à la consommation, en zone agricole, n'a guère d'importance
au point de vue de la santé publique. La seule vole d'exposition
importante est la voie alimentaire. En général, toutefois, les
quantités ingérées se situent très largement en-dessous de la dose
journalière admissible qui a été fixée à 0,0002 mg/kg de poids
corporel en 1970 (FAO/OMS, 1971).
1.2 Absorption, métabolisme et excrétion
Contrairement à la dieldrine, son stéréoisomère, l'endrine est
rapidement métabolisée par l'organisme animal et, comparativement à
d'autres composés de structure chimique semblable, elle s'accumule
très peu dans les tissus adipeux.
L'absorption et l'excrétion sont rapides après administration
orale à des rats et la demi-vie biologique se situe entre 1 et 6 jours
selon les quantités ingérées. Un régime stationnaire, c'est à dire un
état d'équilibre entre la quantité excrétée et la dose ingérée,
s'établit au bout de 6 jours. On constate une différence entre les
deux sexes en ce sens que les mâles excrètent l'endrine et ses
métabolites par la voie biliaire plus rapidement que les femelles,
d'où une moindre accumulation de pesticides dans les tissus adipeux
des mâles. Les rats excrètent ce composé principalement dans leurs
matières fécales sous forme d'endrine, d' anti-12-hydroxyendrine
ainsi que sous la forme d'un dérivé hydroxyle de l'endrine, en
l'espace de 24 heures (70-75 %); un troisième métabolite, la 12-
cétoendrine, s'accumule dans les tissus. Les lapins excrètent 50% des
métabolites de l'endrine par la voie urinaire, l'excrétion urinaire
n'étant que de 2% chez le rat; dans les matières fécales des lapins,
on ne retrouve que de l'endrine non modifiée.
Des vaches à qui l'on avait administré de l'endrine à raison de
0, 1 mg/kg de nourriture pendant 21 jours en ont excrété jusqu'à 65%
sous forme de métabolites urinaires, 20% sous forme de métabolites
fécaux ou d'endrine non modifiée et 3% dans leur lait, cette fois,
principalement sous forme d'endrine non modifiée. Chez ces vaches, les
résidus atteignaient 0,003-0,006 mg/litre dans le lait, 0,001-0,002
mg/kg dans la viande, et 0,02-0,1 mg/kg dans la graisse.
Chez des poules pondeuses ayant reçu une alimentation additionnée
d'endrine, on a observé des résidus (selon la dose ingérée) qui
atteignaient 0.1 mg/kg dans la chair, 1 mg/kg dans la graisse, 0,2-0,3
mg/kg dans les oeufs (jaune), 0,2 mg/kg dans les reins et 0,5 mg/kg
dans le foie. Sauf dans le cas du foie et des reins, les résidus
présents étaient essentiellement formés d'endrine non modifiée.
Environ 50% de la quantité d'endrine administrée a été excrétée dans
les matières fécales, principalement sous la forme de métabolites.
Chez l'homme, le rat, le lapin, la vache et la poule, le
principal métabolite de l'endrine est l' anti-12-hydroxyendrine,
accompagnée de ses sulfo- et glucuro-conjugués. On trouve également 4
autres métabolites, mais en quantités minimes. Dans les tissus et le
lait on retrouve essentiellement de l'endrine non modifiée. Après
épandage sur des végétaux, on a retrouvé de l'endrine sous forme non
modifiée ainsi que deux produits de transformation hydrophiles.
1.3 Effets sur les êtres vivants dans leur milieu naturel
L'endrine n'exerce que des effets minimes sur les bactéries et
les champignons terricoles. Aux doses de 10-1000 mg/kg de terre, le
composé n'a aucun effet sur la décomposition des matières organiques,
sur la dénitrification ou sur la production de méthane. L'endrine est
très toxique pour les poissons, les invertébrés aquatiques et le
phytoplancton; la CL 50 à 96 h, est dans la plupart des cas
inférieure à 1,0 µg/litre. La dose nocive la plus faible observée au
cours d'un test portant sur le cycle évolutif d'un crevette,
Mysidopsis bahia, était de 30 ng/litre.
Les épreuves de toxicité aiguë effectuées sur des organismes
aquatiques ont été pratiquées dans des aquariums ne comportant pas de
sédiments, on peut penser que la présence de sédiments atténue l'effet
de l'endrine. D'ailleurs la présence de sédiments fortement contaminés
n'a guère eu d'effet sur les espèces de pleine eau, ce qui incite à
penser que l'endrine fixée aux sédiments présente une faible
biodisponibilité. On n'a pas pratiqué d'épreuves sur des animaux
aquatiques vivant dans les sédiments.
Pour les mammifères terrestres et les oiseaux, la DL50 est de
l'ordre de 1,0-10,0 mg/kg de poids corporel. Des canards de l'espèce
Anas platyrhynchos qui avaient reçu pendant 12 semaines de l'endrine
dans leur nourriture à des doses allant jusqu'à 3,0 mg/kg de poids
corporel, n'ont présenté aucun effet délétère que ce soit sur la
ponte, la fécondité ou l'éclosion des oeufs.
Il semblerait que certaines espèces d'invertébrés aquatiques, de
poissons et de petits mammifères résistent à l'action toxique de
l'endrine; d'ailleurs l'exposition à divers pesticides organochlorés
a pu entraîner la sélection de souches résistantes à l'endrine.
Dans des zones où existent des décharges industrielles et où
l'endrine peut être entraînée par ruissellement à partir des champs
traités, on a observé une mortalité parmi les poissons; par ailleurs,
le déclin des populations de pélicans bruns (en Louisiane) et de
caugeks (aux Pays-Bas) a été attribuée à une exposition à l'endrine et
à d'autres dérivés halogénés.
1.4 Effets sur les animaux d'expérience et sur les systèmes in vitro
L'endrine est un pesticide fortement toxique dont les signes
d'intoxication sont de type neurologiques. Chez les animaux de
laboratoire, la DL50 par voie orale de l'endrine de qualité
technique se situe dans les imites de 3-43 mg/kg de poids corporel; la
DL50 dermique va de 5-20 mg/kg de poids corporel pour le rat. Il n'y
a pas de différence notable concernant la toxicité aiguë par voie
orale et percutanée entre le produit technique et les diverses
formulations (concentrés émulsionnables ou poudres mouillables).
Des épreuves de courte durée portant sur la toxicité par voie
orale de l'endrine ont été effectuées sur (les souris, des rats, des
lapins, des chiens et autres animaux domestiques. Chez les souris et
les rats, les doses maximales tolérées ont été respectivement de 5 et
15 mg/kg de nourriture pendant 6 semaines (soit l'équivalent de 0,7
mg/kg de poids corporel). Les rats ont survécus à une dose de 1 mg/kg
de nourriture pendant 16 semaines (soit l'équivalent de 0,05 mg/kg de
poids corporel); les lapins sont morts après avoir reçu à plusieurs
reprises une dose de 1 mg/kg de poids corporel. chez le chien, une
dose de 1 mg/kg de nourriture (soit approximativement 0,025 mg/kg de
poids corporel) administrée sur une période de 2 ans, n'a produit
aucun effet.
Du point de vue neurologique, les signes d'intoxication observés
sont dus à l'inhibition de la fonction de l'acide gamma-aminobutyrique
(GABA) à faible dose. Comme les autres hydrocarbures chlorés
insecticides, l'endrine agit également au niveau du foie et la
stimulation des systèmes enzymatiques intervenant dans le métabolisme
des autres substances chimiques se manifeste, notamment chez la
souris, par une diminution de la durée du sommeil induit par
l'hexobarbital.
Des doses de 75-150 mg/kg appliquées sur l'épiderme des lapins
sous forme de poudre sèche, tous les jours pendant deux heures ont
entraîné des convulsions et la mort chez ces animaux sans toutefois
provoquer d'irritation cutanée. Cette intoxication par voie générale
sans irritation locale mérite d'être signalée.
Des études de toxicité et de cancérogénicité à long terme ont été
effectuées sur des souris et des rats. Aucun effet cancérogène n'a été
observé mais ces études présentaient un certain nombre d'insuffisances
notamment la faible survie des animaux. Lors d'une étude de deux ans
sur des rats traités par de l'endrine administrée dans leur
nourriture, on a estimé à 1 mg/kg de nourriture, soit l'équivalent
d'environ 0,05 mg/kg de poids corporel, la dose sans effets toxiques
observables. Après administration d'endrine avec des quantité
infinitésimales de substances chimiques cancérogènes pour l'animal, il
n'a pas été possible de mettre en évidence d'effet tumoro-promoteur.
Le Groupe de travail en a conclu que les données sont insuffisantes
pour permettre de considérer l'endrine comme cancérogène pour l'homme.
Plusieurs études ont également révélé que l'endrine n'était pas
génotoxique.
Dans la plupart des études, l'endrine s'est révélée non
tératogène pour la souris, le rat ou le hamster, même à des doses
toxiques pour la mère ou le foetus. La dose sans effet nocif
observable a été évaluée à 0,5 mg/kg de poids corporel chez la souris
et le rat et à 0,75 mg/kg de poids corporel chez les hamsters.
L'endrine n'a pas eu d'effets sur la reproduction des rats suivis
pendant trois générations qui en recevaient dans leur nourriture à
raison de 2 mg/kg (soit environ 0, 1 mg/kg de poids corporel).
Un certain nombre de métabolites de l'endrine sont plus ou moins
toxiques que le composé initial. Ainsi la delta-cétoendrine est moins
toxique de l'endrine, en revanche la 12-cétoendrine est considérée
comme le métabolite le plus toxique de l'endrine pour les mammifères,
avec une DL50 par voie orale de 0,8-1,1 mg/kg de poids corporel chez
le rat.
1.5 Effets sur l'homme
Plusieurs cas d'intoxication mortels ou non mortels consécutifs
à un accident ou à une tentative de suicide ont été observés. Les cas
d'intoxication aiguë non mortels résultant d'une, surexposition
accidentelle ont été observés chez les ouvriers d'une usine de
production d'endrine. On estime que par voie orale, la dose mortelle
est (l'environ 10 mg/kg de poids corporel, une dose unique prise par
voie orale de 0,25-1,0 mg/kg de poids corporel peut provoquer des
convulsions.
C'est au niveau du système nerveux central que l'endrine exerce
principalement son action. Après exposition à dose toxique, des signes
d'intoxication peuvent faire leur apparition et se manifestent sous la
forme d'un hyperexcitabilité et de convulsions, la mort pouvant
survenir dans les 2-12 heures suivant l'exposition si un traitement
approprié n'est pas institué immédiatement. En revanche, après une
intoxication non mortelle, la récupération est rapide et complète.
L'endrine ne s'accumule pas dans le corps humain de manière
importante. Chez 232 travailleurs exposés de par leur profession, on
n'a pas constaté d'effets indésirables à long terme (durée
d'exposition 4-27 ans) lors des examens médicaux pratiqués (durée de
l'observation 2-29 ans). Le seul effet observé, indirectement
d'ailleurs, consistait en une stimulation réversible des enzymes
pharmacométabolisantes.
Des analyses ont été pratiquées dans de nombreux pays sur un
grand nombre d'échantillons de tissus adipeux, de sang et de lait
maternel sans qu'il soit possible de mettre en évidence la présence
d'endrine. Le Groupe de travail attribue l'absence d'endrine dans ces
échantillons à la faible exposition de la population général à ce
pesticide et à sa métabolisation rapide.
En revanche la présence d'endrine a été décelée dans le sang (à
des concentrations atteignant 450 µg/litre) et dans les tissus adipeux
(à la dose de 89,5 mg/kg) chez les personnes décédées d'une
intoxication accidentelle. Dans les conditions normales, on n'a pas
retrouvé d'endrine chez les travailleurs exposés. Le seuil
d'apparition des symptômes d'intoxication est estimé à 50-100 µg/litre
de sang. On pense que la demi-vie de l'endrine dans le sang est de
l'ordre de 24 heures.
2. Conclusions
L'endrine est un 'Insecticide qui présente une très forte
toxicité aiguë. Il peut entraîner des intoxications graves en cas
d'exposition excessive due à une manipulation négligente lors de sa
production, de son utilisation ou par suite de la consommation
d'aliments contaminés. L'exposition de la population générale est
principalement due à la présence de résidus dans les denrées
alimentaires; toutefois on estime que la quantité d'endrine ingérée
est en général très inférieure à la dose journalière admissible fixée
par le Comité FAO/OMS d'experts des résidus de pesticides. Il n'y a
pas de danger pour la population générale qui résulterait d'une
exposition de ce genre à l'endrine. Moyennant de bonnes méthodes de
travail et le respect des mesure d'hygiène et de sécurité, l'endrine
ne devrait pas constituer un danger pour les ouvriers exposés.
Il est évident que des rejets incontrôlés d'endrine lors de la
production, de la formulation et de l'utilisation de ce pesticide
peuvent créer des problèmes écologiques dus à sa forte toxicité. Il
n'est pas possible d'être aussi catégorique en ce qui concerne les
effets que peut avoir son utilisation en agriculture sur la faune et
la flore, encore que l'entraînement par ruissellement du pesticide
puisse constituer une menace pour les poissons et les oiseaux
piscivores. Le déclin des populations de certaines espèces d'oiseaux
a été attribué à la présence de résidus élevés de divers organochlorés
dans les tissus des adultes et dans les oeufs. On a procédé au dosage
de l'endrine chez certaines de ces espèces; toutefois il est difficile
de faire la part des différents organochlorés qui sont en cause.
3. Recommandations
1. L'endrine ne doit être utilisée qu'en cas de nécessité et
seulement lorsqu'il n'existe pas d'autre produit moins
toxique.
2. Afin de préserver la santé et le bien-être des travailleurs
et de la population générale, on ne doit confier la
manipulation et l'épandage qu'à des personnes bien encadrées
et bien formées qui appliqueront des mesures de sécurité
convenables et épandront le produit conformément aux règles
de bonne pratique en la matière.
3. Il convient de s'entourer de toute les précautions
nécessaires lors de la production, de la formulation, de
l'utilisation en agriculture et du rejet de l'endrine afin
de contaminer le moins possible l'environnement et en
particulier les eaux de surface.
4. Les personnes qui sont habituellement exposées à l'endrine
doivent subir des examens médicaux périodiques.
5. Il faut poursuivre l'étude épidémiologique des travailleurs
exposés.
6. Dans les pays où l'on utilise encore de l'endrine, on devra
contrôler la présence de résidus d'endrine dans les denrées
alimentaires.
7. Au cas où l'on continuerait à utiliser de l'endrine, il
faudrait obtenir davantage de données sur la présence, la
destinée ultime et la toxicité de la 12-cétoendrine et de la
delta-cétoendrine.
RESUMEN Y EVALUACION; CONCLUSIONES; RECOMENDACIONES
1. Resumen y evaluación
1.1 Exposición
La endrina es un insecticida organoclorado que se utiliza desde
los años cincuenta para combatir muy diversas plagas agricolas, sobre
todo en el algodón aunque también en el arroz, la caña de azúcar, el
maíz y otros cultivos. Se utiliza asimismo como rodenticida. En el
comercio se encuentra en forma de polvos, gránulos, pastas y
concentrado emulsionable.
La endrina se incorpora al aire principalmente por volatilización
y arrastre aéreo. En general, la volatilización tiene lugar después de
aplicarla a suelos y cultivos y depende de muchos factores, como el
contenido de materia orgánica y agua del suelo, la humedad, el flujo
de aire y la superficie cultivada.
La vía más importante de contaminación de las aguas de superficie
es la escorrentía desde el suelo. La contaminación por precipitación
en forma de nieve o lluvia es insignificante. Puede producirse
contaminación local del medio debida a efluentes industriales y
prácticas de aplicación poco meticulosas.
La principal fuente de endrina en el suelo es la aplicación
directa a éste y a los cultivos. Puede quedar retenida, ser
transportada o degradarse en el suelo, atendiendo a diversos factores.
La retención más intensa se produce en suelos con contenido elevado de
materia orgánica. La persistencia de la endrina depende en gran medida
de las condiciones locales; su semivida en el suelo puede llegar a los
12 años. La volatilización y la fotodescomposición son los principales
factores de la desaparición de la endrina de las superficies del
suelo. La luz del sol (luz ultravioleta) induce la formación del
isómero delta-cetoendrina. En verano, bajo insolación intensa, se
observó que alrededor del 50% de la endrina se isomerizaba a esta
cetoendrina en un plazo de siete días. Se produce transformación
microbiana (en hongos y bacterias), especialmente en condiciones
anaerobias, originándose la misma sustancia.
Los invertebrados acuáticos y los peces absorben rápidamente la
endrina a partir del agua, si bien los peces expuestos transferidos a
agua no contaminada pierden el plaguicida rápidamente. Se han
registrado factores de bioconcentración de 14-18 000 tras una
exposición continua. Los invertebrados del suelo también absorben
fácilmente el compuesto.
La presencia ocasional de niveles reducidos de endrina en el aire
y en las aguas de superficie y de bebida en zonas agrícolas reviste
escasa importancia desde el punto de vista de la salud pública. La
única exposición que merece consideración es la ingesta en la dieta.
En general, no obstante, los niveles comunicados de ingesta se
encuentran muy por debajo de la ingesta diaria admisible de 0,0002
mg/kg de peso corporal, establecida en 1970 (FAO/OMS, 1971).
1.2 Absorción, metabolismo y excreción
A diferencia de la dieldrina, su estereoisómero, la endrina se
metaboliza rápidamente en los animales, y se acumula en muy pequeña
cantidad en las grasas en comparación con compuestos de estructura
química análoga.
En la rata, tanto la absorción como la excreción tras la
administración oral se producen rápidamente; su semivida biológica es
de 1-6 días, según la dosis administrada. Al cabo de 6 días se alcanza
un estado de equilibrio en el que la cantidad excretada es igual a la
ingesta diaria. Se observan diferencias de un sexo a otro: los machos
excretan endrina y metabolitos con la bilis mucho más deprisa que las
hembras, lo que produce una acumulación menor en el tejido adiposo de
aquéllos. Las ratas excretan este compuesto principalmente en las
heces en forma de endrina, anti-12-hidroxiendrina, y un derivado
hidroxilado durante las primeras horas (70-75%); un tercer metabolito,
la 12-cetoendrina, se acumula enlos tejidos. El conejo excreta el 50%
de los metabolitos del compuesto enla orina, mientras que en la rata
sólo el 2% se excreta por esta vía; en lasheces del conejo sólo se
detecta endrina sin alterar.
Las vacas a las que se administró endrina a razón de 0,1 mg/kg de
la dieta durante 21 días excretaron hasta el 65% en forma de
metabolitos en la orina, el 20% en las heces, parcialmente en forma de
endrina no alterada, y el 3% en la leche, también principalmente en
forma de endrina. Estas vacas presentaron niveles residuales de
0,003-0,006 mg/litro en la leche, 0,001-0,002 mg/kg en la carne, y
0,02-0,1 mg/kg en la grasa.
En gallinas ponedoras a las que se administró endrina por vía
oral seobservaron niveles residuales (dependientes de la dosis
administrada) de hasta 0,1 mg/kg en la carne, 1 mg/kg en la grasa,
0,2-0,3 mg/kg en los huevos (yema), 0,2 mg/kg en el riñón y 0,5 mg/kg
en el hígado. Salvo en el hígado y el riñón, los residuos encontrados
estaban formados principalmente por endrina no alterada. Alrededor del
50% de la endrina administrada se excretó en las heces, principalmente
en forma de metabolitos.
En el ser humano, la rata, el conejo, la vaca y la gallina, el
principal metabolito biotransformado de la endrina es la
anti-12-hidroxiendrina, junto con su sulfato y su glucur nido
conjugados. Se encontraron cuatro metabolitos más, si bien en
cantidades muy reducidas. En los tejidos corporales y en la leche se
encuentra sobre todo endrina inalterada. Tras la aplicación de este
plaguicida a plantas, seidentificaron endrina inalterada y dos
productos de transformación hidrófilos.
1.3 Efectos en los organismos del medio ambiente
El efecto de la endrina en las bacterias y los hongos del suelo
es mínimo. Con dosis de 10-1000 mg/kg de suelo no se observó efecto
alguno en la descomposición de materia orgánica, la desnitrificación
ni la generación de metano. La endrina es sumamente tóxica para los
peces, los invertebrados acuáticos y el fitoplancton: los valores de
la CL50 a las 96 horas se encuentran en su mayoría por debajo de 1,0
µg/litro. El nivel sin observación de efectos más bajo en un ensayo de
ciclo biológico del crustáceo Mysidopsis bahia se fijó en 30 ng/litro.
Los ensayos comunicados sobre la toxicidad aguda de la endrina
para los organismos acuáticos se llevaron a cabo en acuarios sin
sedimentos; cabría esperar que la presencia de sedimentos atenuara el
efecto del insecticida. Los sedimentos muy contaminados ejercieron
escaso efecto en las especies de aguas libres, lo que indica que la
endrina ligada a los sedimentos tiene una biodisponibilidad reducida.
Aún no se han llevado a cabo ensayos en animales acuáticos que viven
en los sedimentos.
La DL50 para mamíferos terrestres y aves oscila entre 1,0 y
10,0 mg/kg de peso corporal. Los patos silvestres a los que se
administraron 3,0 mg/kg de peso corporal durante 12 semanas no
mostraron efecto alguno en la producción de huevos, la fertilidad o la
eclosión.
Ciertas especies de invertebrados acuáticos, peces y mamíferos de
pequeño tama o son resistentes a la toxicidad de la endrina; la
exposición a diversos plaguicidas organoclorados llevó a la selección
de estirpes resistentes a la endrina.
Se observaron muertes masivas de peces en zonas de escorrentía
agrícola y descargas industriales; el declive de las poblaciones de
pelícanos pardos (en Luisiana, EE.UU.) y de golondrinas de mar
(Thalasseus sandvicensis) en los Países Bajos se ha atribuido a la
exposición a la endrina en combinación con otras sustancias químicas
halogenadas.
1.4 Efectos en animales de experimentación in vitro
La endrina es un plaguicida sumamente tóxico; los signos de
intoxicación son de carácter neurotóxico. La DL50 por vía oral de la
endrina de calidad técnica en animales de laboratorio oscila entre 3
y 43 mg/kg de peso corporal; la DL50 por vía cutánea en la rata es
de 5-20 mg/kg peso corporal. No se ha encontrado ninguna diferencia en
la toxicidad aguda por vía oral o cutánea entre los productos de
calidad técnica y los formulados (concentrado emulsionable y polvos
humectables).
Se han llevado a cabo experimentos de breve duración para
estudiar la toxicidad por vía oral en el ratón, la rata, el conejo, el
perro y animales domésticos. En el ratón y la rata, las dosis máximas
toleradas durante 6 semanas fueron 5 y 15 mg/kg de la dieta
(equivalentes a 0,7 mg/kg de peso corporal), respectivamente. Las
ratas sobrevivieron tras una exposición a 1 mg/kg de la dieta
(equivalente a 0,05 mg/kg de peso corporal) durante 16 semanas; los
conejos murieron tras recibir dosis repetidas de 1 mg/kg de peso
corporal. En el perro, no se observó efecto alguno tras la
administración de 1 mg/kg de la dieta (equivalente aproximadamente a
0,025 mg/kg de peso corporal) durante más de 2 años.
La base neuroógica de los signos de intoxicación observados es la
inhibición de la función del ácido gamma-aminobutírico (GABA) con
dosis reducidas. Al igual que otros insecticidas a base de
hidrocarburos clorados, la endrina afecta también al hígado, y se
observa claramente la estimulación de sistemas enzimáticos que
participan en el metabolismo de otras sustancias químicas, como lo
demuestra, por ejemplo, la menor duración del sueño por hexobarbital
en el ratón.
Con dosis de 75-150 mg/kg aplicadas por vía cutánea en forma de
polvo seco durante 2 horas al día se produjeron convulsiones y la
muerte en el conejo pero sin irritación cutánea. Esta toxicidad
sistémica sin irritación en el lugar de contacto resulta muy notable.
Se han llevado a cabo en ratones y ratas estudios prolongados de
toxicidad y carcinogenicidad. No se observó efecto carcinogénico, pero
estos estudios tenían ciertos defectos, entre ellos la reducida
supervivencia de los animales. El nivel sin observación de efectos en
cuanto a la toxicidad en un estudio de dos años de duración en la rata
fue de 1 mg/kg de la dieta (equivalente a unos 0,05 mg/kg de peso
corporal). No se demostró ningún efecto de favorecimiento de tumores
cuando se ensayó la endrina en combinación con cantidades submínimas
de sustancias químicas de conocido efecto carcinogénico en los
animales. El Grupo de Trabajo concluyó que los datos de que se dispone
no bastan para indicar que la endrina supone un riesgo carcinogénico
para el ser humano.
En varios estudios se observó que la endrina no es genotóxica.
En la mayoría de los estudios no resultó teratogénica para el
ratón, la rata o el hámster, ni siquiera con dosis suficientes para
provocar toxicidad materna o fetal. El nivel sin observación de
efectos adversos fue de 0,5 mg/kg de peso corporal en ratones y ratas
y de 0,75 mg/kg de peso corporal en el hámster. La endrina no indujo
efecto alguno en la reproducción de ratas estudiadas durante tres
generaciones cuando se administró a razón de 2 mg/kg de la dieta (unos
0,1 mg/kg de peso corporal).
Algunos metabolitos de la endrina tienen toxicidades agudas
iguales o más altas que el compuesto originario. El producto de
transformación, la delta-cetoendrina, es menos tóxico que la endrina,
pero la 12-cetoendrina se considera el metabolito más tóxico en los
mamíferos, con una DL50 por vía oral en la rata de 0,8-1,1 mg/kg de
peso corporal.
1.5 Efectos en el ser humano
Se han producido varios episodios de intoxicación mortal y no
mortal, tanto accidentales como suicidas. Los casos de intoxicación
aguda no mortal debida a exposición excesiva accidental se observaron
en trabajadores de una planta de fabricación de endrina. Se ha
calculado que la dosis que por vía oral provoca la muerte es de
aproximadamente 10 mg/kg de peso corporal; la dosis única por vía oral
que provoca convulsiones se fijó en 0,25-1,0 mg/kg de peso corporal.
El lugar principal de acción de la endrina es el sistema nervioso
central. La exposición del ser humano a una dosis tóxica puede
producir al cabo de pocas horas signos y síntomas de intoxicación
tales como excitabilidad y convulsiones; la muerte puede producirse en
las 2-12 horas que siguen a la exposición si no se administra
inmediatamente el tratamiento apropiado. La recuperación después de
una intoxicación no mortal es rápida y completa.
La endrina no se acumula en el cuerpo humano en grado
significativo. No se comunicaron efectos adversos a largo plazo en 232
trabajadores expuestos (duración de la exposición: 4-27 años) bajo
supervisión médica (tiempo de observación: 4-29 años). El único efecto
observado fueron pruebas indirectas de una estimulación reversible de
las enzimas metabolizadoras de fármacos.
No se detectó endrina en prácticamente ninguna muestra de tejido
adiposo, sangre y leche humana analizadas en numerosos países. El
Grupo de Trabajo atribuyó la ausencia de endrina en las muestras
humanas a la baja exposición de la población general a este plaguicida
y a su rápido metabolismo.
La endrina se detectó en la sangre (con concentraciones de hasta
450 µg/litro) y en el tejido adiposo (en concentraciones de 89,5
mg/kg) en casos de envenenamiento accidental mortal. No se encontró
endrina en los trabajadores en circunstancias normales. El nivel
umbral de endrina en la sangre por debajo del cual no se produce
ningún signo o síntoma de intoxicación se ha fijado en 50-100
µg/litro. La semivida de la endrina en la sangre puede ser del orden
de 24 horas.
2. Conclusiones
La endrina es un insecticida con elevada toxicidad aguda. Puede
provocar envenenamiento grave en casos de exposición excesiva
provocada por un manejo poco meticuloso durante su fabricación y uso
o por el consumo de alimentos contaminados. El público está expuesto
a la endrina principalmente por sus residuos en los alimentos; no
obstante, los niveles de ingesta de endrina que se han comunicado
están por lo general muy por debajo de la ingesta diaria admisible
establecida por la FAO/OMS. Esas exposiciones en principio no
constituyen un riesgo para la salud de la población general. Cuando se
aplican buenas prácticas de trabajo, medidas higiénicas y precauciones
de seguridad, es poco probable que la endrina suponga un riesgo para
los trabajadores expuestos.
Está claro que las descargas no controladas de endrina durante su
manufactura, formulación y uso pueden originar graves problemas
ambientales asociados a su elevada toxicidad. Los efectos del uso
agrícola del insecticida en la fauna y la flora están menos claros, si
bien los peces y las aves ictívoras están expuestos por la escorrentía
a partir de las superficies. El declive de las poblaciones de algunas
especies de aves se ha atribuido a la presencia de niveles elevados de
residuos de diversos compuestos organoclorados en los tejidos de
adultos y en los huevos. Se ha medido la endrina presente en algunas
de estas especies, pero es muy difícil separar los efectos de los
distintos compuestos organoclorados presentes.
3. Recomendaciones
1. No debe utilizarse la endrina a menos que sea indispensable
y sólo cuando no se disponga de una alternativa menos
tóxica.
2. Para la salud y el bienestar de los trabajadores y de la
población general, el manejo y el uso de la endrina se
confiarán sólo a operarios bien supervisados y adiestrados
que apliquen las medidas de seguridad adecuadas y utilicen
la endrina de acuerdo con las prácticas agrícolas correctas.
3. La fabricación, la formulación, el uso agrícola y la
evacuación de endrina se tratarán cuidadosamente para
reducir al mínimo la contaminación del medio, en particular
de las aguas de superficie.
4. Las personas expuestas regularmente a la endrina deben
someterse a revisiones médicas periódicas.
5. Proseguirán los estudios epidemiológicos sobre las
poblaciones de trabajadores expuestos.
6. En los países en los que aún se usa la endrina, deben
vigilarse sus residuos en los alimentos.
7. Si sigue utilizándose la endrina, debe obtenerse más
información sobre la presencia, el destino último y la
toxicidad de la 12-cetoendrina y la delta-cetoendrina.
(1975) suggested that the acute toxicity of endrin in birds is not
associated with the formation of 12-ketoendrin.
6.2.2 Human beings
No endrin was found (limit of detection, 0.0016 mg/litre) in 14
samples of urine from workers exposed to aldrin, dieldrin, and endrin,
even though workers with a complete work history had been exposed to
endrin for an average of 2160 h (Hayes & Curley, 1968). Endrin was not
detected in urine from five men and five women (Cueto & Hayes, 1962;
Cueto & Biros, 1967). No unchanged endrin was found in the urine of
Dutch workers exposed to endrin, but it occurred in the faeces (Jager,
1970; Baldwin & Hutson, 1980).
Neither 3-hydroxyendrin nor the diol was detected in urine or
faeces (Hutson, 1981). anti-12-Hydroxyendrin was present in the
urine of workers exposed to endrin, and the glucuronide was found in
the faeces. Concentrations of up to 0.36 mg/g of creatinine were found
in urine after 7 days, accompanied by a sharp rise in the level of
D-glutaric acid (excreted in the urine of mammals as a metabolite of
D-glucuronolactone [Marsh, 1963]), indicating that liver enzyme
induction may have occurred. The levels tended to decrease over the
weekend (Ottevanger & Van Sittert, 1979). Endrin,
anti-12-hydroxyendrin, 12-ketoendrin, and the beta-glucuronide of
anti-12-hydroxyendrin were not found in the blood of workers at a
Dutch plant for the manufacture of endrin (limit of detection, 2
µg/litre). Both endrin and anti-12-hydroxyendrin were found in the
faeces, and all urine samples contained the beta-glucuronide of
anti-12-hydroxyendrin up to a concentration of 0.14 mg/litre as
anti-12-hydroxyendrin (Baldwin & Hutson, 1980).
Hydroxylation at anti-C-12 is relatively rapid and accounts for
the rapid metabolism of endrin. Even syn-12-hydroxyendrin is
hydroxylated rapidly at its anti-C-12 position, affording
12-ketoendrin (Hutson, 1981).
As neither endrin nor its metabolites were found in the blood of
exposed, healthy workers, exposure can be measured by determining
anti-12-hydroxyendrin in urine. A quantitative relationship between
exposure to endrin and the concentration of this metabolite cannot be
established, however, owing to lack of data.
6.2.3 Microorganisms
In mixed anaerobic microbial populations developed using inocula
from soil, freshwater mud, sheep rumen, and chicken litter, endrin
(like other cyclodiene compounds) was monodechlorinated at the
methylene bridge carbon atom. Neither endrin nor any other compound
was further metabolized. The 10 obligate anaerobic bacteria that made
up the mixed population were subsequently isolated in pure culture. Of
these, only Clostridium bifermentans, C. glycolium, and other
Clostridium species were capable of dehalogenation, but at a rate
that was much slower than that of the mixed population (Maule et al.,
1987).
6.2.4 Plants
Three experiments were carried out on tobacco plants. In the
first experiment, 2.08 mg of 14C-endrin were applied to the leaves
with free aeration during the experimental period. In the second test,
the same dose was applied but with little aeration; and in the third,
plants were exposed to 1.04 mg of 14C-endrin with little aeration.
An initial residue level of 50-100 mg/kg was found on leaves in all
three experiments, but, subsequently, less residue was found on plants
with free aeration. Six weeks after treatment, 30-47% of radiolabel
was recovered in residues, which consisted of endrin and hydrophilic
substances (Weisgerber et al., 1969; Donoso et al., 1979).
The leaves of cotton plants were treated with 4.2 mg of
14C-endrin, and the application was repeated after 2 and again after
6 weeks, at which time parathion was also applied. At harvest,
two-thirds of the radiolabel had evaporated, and the total residue in
cotton seed was 0.333 mg/kg. Endrin and two groups of degradation
products were found in the plants; one of these products (possibly
delta-ketoendrin) was only slightly more hydrophilic than endrin, and
the other was very hydrophilic. Most of the metabolites were found on
the surface of the leaves. When delta-ketoendrin was applied to white
cabbage, it disappeared more slowly than endrin, with the formation of
hydrophilic metabolites (Korte, 1969).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
The interactions of halogenated pesticides and microorganisms
have been reviewed extensively (Pfister, 1972).
In three Willamette valley soils (USA) treated with endrin at 0,
1 or 10 mg/kg, no effect was found 30 days after application on the
function and activity of the microbial population, the decomposition
of native organic matter, the transformation of native soil nitrogen,
ammonification of peptone, or nitrification of ammonium sulphate
(Bollen & Tu, 1971). Even at an annual application rate of 5 lb/acre
(5.6 kg/ha) for 5 years, no effect was seen on the numbers or kinds of
soil fungi, the numbers of bacteria, the decomposition rate of organic
matter (measured by CO2 production), or the oxidation of ammonium to
nitrate (Martin et al., 1959).
Endrin at a concentration of 100 mg/kg of soil had no effect on
denitrification in soil under anaerobic incubation for 5 days at 30 °C
or in an isolated denitrifying bacterium (Bollag & Henninger, 1976).
A concentration of 1000 mg/kg had no effect on methanogenesis, sulfate
reduction, or carbon dioxide evolution in anaerobic salt-marsh
sediments (Kiene & Capone, 1984).
The growth rates of two strains of blue-green algae were
decreased in the presence of endrin at a concentration of 0.29
µg/litre (Batterton et al., 1971), and the productivity of many forms
of natural phytoplankton in estuarine waters was decreased by 46% when
they were exposed to 1 mg/litre (Butler, 1963).
7.2 Aquatic organisms
7.2.1 Invertebrates
Acute toxicity of endrin to invertebrates is given in Tables 14
and 15.
A static system was used to study the toxicity of endrin to a
polychaete worm (Nereis virens) in water and sediment, in which sea
water or sediment (containing 17% sand, 83% clay, and 2% organic
carbon) was present at a temperature of 9-10 °C for 12 days. None of
the worms in sea water died after exposure to endrin at 0.11 mg/litre
for 12 days, but two of five worms exposed to 28 mg/kg in sediment
died in this period (McLeese et al., 1982).
Table 14. Acute toxicity of endrin to freshwater invertebrates
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Red snail Static 48-h LC50 7200 Hashimoto & Nishiuchi
Indoplanorbis exustus (1981)
Marsh snail Static 48-h LC50 9500 Hashimoto & Nishiuchi
Semisulcospira libertina (1981)
Snail Static 48-h LC50 12 000 Hashimoto & Nishiuchi
Physa acuta (1981)
Water flea Static 18-23 48-h LC50 160 Thurston et al.(1985)
Daphnia magna Static 21 44 7.1 48-h LC50 4.2 Mayer & Ellersieck (1986)
Static 18 48 7.4 48-h LC50 41-74 Mayer & Ellersieck (1986)
Static 22-24 240 8.0 96-h LC50 59 Elnabarawy et al. (1986)
Water flea Static 15 44 7.1 48-h LC50 20 Mayer & Ellersieck (1986)
Daphnia pulex Static 22-24 240 8.0 96-h LC50 30 Elnabarawy et al. (1986)
Water flea Static 22-24 240 8.0 96-h LC50 24 Elnabarawy et al. (1986)
Daphnia reticulata
Water flea Static 21 44 7.1 48-h LC50 45 Mayer & Ellersieck (1986)
Simocephalus serrulatus Static 15 44 7.1 48-h LC50 26 Mayer & Ellersieck (1986)
Water flea Adult Static 21 44 7.1 48-h LC50 1.8 Mayer & Ellersieck (1986)
Cypridopsis vidua
Sow bug (isopod) Adult Static 15 44 7.1 96-h LC50 1.5 Mayer & Ellersieck (1986)
Asellus brevicaudus
Scud Adult Static 21 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
Gammarus fasciatus Adult Static 15 272 7.4 96-h LC50 1.3 Mayer & Ellersieck (1986)
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Scud Adult Static 21 44 7.1 96-h LC50 3.0 Mayer & Ellersieck (1986)
Gammarus lacustris
Crayfish 3-5 Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Orconectes nais weeks
Adult Static 21 272 7.4 96-h LC50 320 Mayer & Ellersieck (1986)
Crayfish 0.4-2.0 g Flow 18-23 96-h LC50 > 89 Thurston et al. (1985)
Orconectes immunis
Red crayfish Static 48-h LC50 300 Muncy & Oliver (1963)
Procambarus clarki
Tantytarsus Static 18-23 48-h LC50 0.84 Thurston et al. (1985)
Tantytarsus dissimilis
Glass shrimp Adult Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Palaemonetes Adult Flow 21 272 7.4 96-h LC50 0.5 Mayer & Ellersieck (1986)
kadiakensis
Stonefly Larvae Static 15 44 7.1 96-h LC50 > 0.18 Mayer & Ellersieck (1986)
Acroneuria sp.
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.076 Mayer & Ellersieck (1986)
Claasenia sabulosa
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.54 Mayer & Ellersieck (1986)
Pteronarcella badia
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.25 Mayer & Ellersieck (1986)
Pteronarcys californica
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Mayfly Larvae Static 15 44 7.1 96-h LC50 0.9 Mayer & Ellersieck (1986)
Baetis sp.
Mayfly Larvae Static 15 44 7.1 96-h LC50 62 Mayer & Ellersieck (1986)
Hexagenia bilineata
Damselfly Larvae Static 21 44 7.1 96-h LC50 2.4 Mayer & Ellersieck (1986)
Ischnura verticalis Larvae Static 21 272 7.4 96-h LC50 2.1
Snipe fly Larvae Static 15 44 7.1 96-h LC50 4.6 Mayer & Ellersieck (1986)
Atherix variegata
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
Table 15. Acute toxicity of endrin to estuarine and marine invertebrates
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Sand shrimp Static 96-h LC50 1.7 Eisler (1970a)
Crangon septemspinosa Static 96-h LC50 0.2-2.0 McLeese & Metcalfe (1980)
Static 96-h LC50 4-120 McLeese & Metcalfe (1980)
Brown shrimp Juvenile Flow 15 26 48-h LC50 0.2 Mayer (1987)
Penaeus aztecus
Pink shrimp Juvenile Flow 17 30 48-h LC50 0.2 Mayer (1987)
Penaeus duorarum Adult Flow 17 28 96-h LC50 0.037
Grass shrimp Larvae Flow 25 13 96-h LC50 1.2 Mayer (1987)
Palaemonetes pugio Juvenile Flow 25 23 96-h LC50 0.35
Adult Flow 25 21 96-h LC50 0.69
Blue crab Juvenile Flow 11 16 48-h LC50 15 Mayer (1987)
Callinectes sapidus
Hermit crab 96-h LC50 1.2 Eisler (1970a)
Pagurus longicarpus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
The mean 96-h LC50 for the oligochaetes Stylodrilus
heringianus and Limnodrilus hoffmeisteri exposed to sediment from
Lake Michigan contaminated with 14C-endrin was 2588 ± 1974 mg/kg dry
weight of sediment in four assays and 2725 ± 955 mg/kg in two assays.
The toxicity to L. hoffmeisteri appeared to be reduced in the
presence of S. heringianus. The 96-h EC50 burrowing avoidance
values were 15.3-19 mg/kg for S. heringianus and 59 mg/kg of
sediment for L. hoffmeisteri (Keilty et al., 1988a).
Sediment reworking by L. hoffmeisteri alone and with
S. heringianus was measured by monitoring the burial of a 137Cs
marker layer in sediments dosed with 12C- and 14C-endrin at
concentrations of 5.5-81 400 µg/kg of dry sediment. With low endrin
concentrations, the marker layer burial rate did not suggest
stimulation of reworking by either L. hoffmeisteri or
S. heringianus. At higher concentrations, the reworking rates were
equal to or slower than control rates at the beginning of the
experiment but decreased thereafter. The presence of S. heringianus
appeared to enhance the reworking response of L. hoffmeisteri. A
reduction in the post-experimental mortality and an increase in the
dry weight of L. hoffmeisteri in tests with the two species implies
that L. hoffmeisteri benefits from the presence of S. heringianus,
although the reverse was not observed. High concentrations of endrin
in the upper 3 cm of the final sediment showed that the worms had
transported the contaminant upward. The bioaccumulation factor for
S. heringianus ranged from 9.7 to 43.8 and was consistently three to
four times greater than that for L. hoffmeisteri (1.7-13.6) (Keilty
et al., 1988b).
The reworking rates of S. heringianus in microcosms containing
sediments dosed with 14C-endrin at 3.1-42 000 µg/kg of dry matter
were measured at 10 °C by monitoring a 137Cs marker layer buried in
contaminated and uncontaminated microcosms. Alterations in reworking
rates were observed at endrin concentrations 5.5 orders of magnitude
below the LC50 of 1650 mg/kg. At the lower concentrations, a
possible stimulatory effect on the marker layer burial rate in the
first 300-600 h was followed by a significant decrease relative to the
controls. At the higher concentrations, the rates were equal or slower
during the first 600 h and decreased dramatically in the last 600 h.
Mortality was 9.3-28% at 11 500 and 42 000 µg/kg and 0-6.7% at all the
other concentrations tested, including controls. The dry weights of
the worms at the end of the experiment were inversely related to the
high concentrations. The bioaccumulation factors ranged from 34 to 67
on the basis of grams of dry organism to grams of dry sediment (Keilty
et al., 1988c).
The effect of addition of endrin at 50 mg/kg dry weight of
sediment on protein utilization by S. heringianus was examined on
days 4, 8, 20, 28, 39, and 69. A slight increase in the relative
percentage of protein to total body weight was observed, but the
authors concluded that estimation of total protein is not a useful
measure of sublethal responses (Keilty & Stehly, 1989).
The total organic carbon content of sediment had little apparent
effect on the toxicity of endrin in the freshwater amphipod Hyalella
azteca. The 10-day LC50 for endrin in sediment (dry-weight basis)
was 4.4 µg/litre at 3.0% total organic carbon and 6.0 µg/litre at
11.2% carbon (Nebeker et al., 1989).
The EC50s in the green sea urchin (Strongylocentrotus
droebachiensis), the purple sea urchin (S. purpuratus), the red
sea urchin (S. franciscanus), and the sand dollar (Dendraster
excentricus) were 103-441 µg/litre for sperm in a static system and
221-> 362 µg/litre for embryos in a continuous flow of sea water
(temperature, 8.2-8.4 °C; salinity, 30.0 parts per thousand; pH
7.8-8.1 for the sea urchins and 12.5-13.0 °C, 30.0 parts per thousand,
and pH 8.0-8.1 for sand dollar embryos), both with an exposure time of
120 h. In a larval test of static exposure of Dungeness crab (Cancer
magister), the EC50 was 2.0 µg/litre (Dinnel et al., 1989).
Endrin was tested at 0, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.5,
5.0, and 10 mg/litre for its effects on embryos of the American oyster
(Crassostrea virginica) and their larvae. Fertilized eggs were
studied after 48 h, and survival and growth of veliger larvae were
studied in 2-day old larvae and in larvae kept for a further 12 days
at 24 °C. The results varied considerably. The estimated concentration
that would cause an approximately 50% reduction in the number of eggs
that develop into normal straight-hinge larvae, calculated by
interpolation from the data, was 0.79 µg/litre and that at which 50%
of the larvae survived was > 10.0 mg/litre (Davis & Hidu, 1969).
In the mysid shrimp Mysidopsis bahia, exposed for the complete
life cycle, acute lethality (over 96 h) was observed with endrin at
120 ng/litre; increased oxygen consumption was measurable within 24 h
of exposure. The lowest-observed-effect level for chronic lethality
was 60 ng/litre; sub-lethal effects on growth (reduced by day 4 of
exposure) and oxygen consumption (increased by day 10 of exposure)
were observed before death (over 20 days). Reduced reproductive
capacity (assessed as production of young) was observed at 30 ng/litre
over 20 days--the time to full maturity (McKenney, 1986).
Behavioural changes were observed in stoneflies (Pteronarcys
dorsata) within 4 days of exposure to 96.1% endrin at 0.07 µg/litre
and in caddis flies (Brachycentrus americanus) at 0.15 µg/litre. The
28-day LC50 was < 0.03 µg/litre for caddis flies and 0.07 µg/litre
for stoneflies (Anderson & DeFoe, 1980).
7.2.2 Fish
7.2.2.1 Acute toxicity
Endrin is highly toxic for both freshwater and marine fish. The
available data are summarized in Tables 16 and 17.
7.2.2.2 Short-term toxicity
Channel catfish (Ictalurus punctatus) were exposed continuously
to renewed solutions of endrin in water at 15 and 22 °C. Measured
endrin concentrations of 0.25-0.30 µg/litre were found to be acutely
toxic to the fish within 10 days or less. None of the fish survived
blood concentrations exceeding 0.28 mg/litre, a well-defined threshold
concentration of endrin in blood, and none died at less than 0.23
mg/litre. The concentration of endrin in the blood of fish exposed to
lethal concentrations in water for periods insufficient to cause death
were markedly lower than that in fish that died from exposure to the
same water (Mount et al., 1966).
The 28-day LC50 for 96.1% eldrin in bullheads (Ictalurus
melas) was 0.10 µg/litre (Anderson & DeFoe, 1980).
In larval fathead minnows (< 24 h old) exposed continuously to
endrin (98%) for 28-30 days in a flow-through system, growth was the
most sensitive parameter. A 48-h exposure to 0.62 µg/litre caused
significant reduction in growth, and survival was reduced at 1.21
µg/litre; with a 72-h exposure, growth was reduced at 0.63 µg/litre,
and all fish died at 1.15 µg/litre. Continuous exposure to 0.38
µg/litre for 30 days significantly reduced growth, and all fish died
at 0.73 µg/litre (Jarvinen et al., 1988).
Sheephead minnows (Cyprinodon variegatus) were exposed
continuously for 23 weeks to endrin from the embryonic stage through
hatching, until adulthood and spawning. The average exposure
concentrations were 0 (control), 0.027, 0.077, 0.12, 0.31, and 0.72
µg/litre. The resultant progeny were monitored to determine effects on
their survival, growth, and reproduction. Embryos exposed to 0.31 and
0.72 µg/litre hatched early; all fry exposed to 0.72 µg/litre died by
day 9 of exposure. At 0.31 µg/litre, fry were initially stunted and
some died. Survivors seemed unaffected until maturity, when some
females died during spawning; fewer eggs were fertile, and survival of
exposed progeny was decreased. No significant effect was observed
throughout the life cycle at an exposure concentration of 0.12
µg/litre (Hansen et al., 1977).
Endrin was tested in flagfish (Jordanella floridae) at 0.21,
0.29, and 0.39 µg/litre for 30 days. Only the highest concentration
decreased survival, and the two highest dose levels affected the mean
number of eggs produced (Hermanutz et al., 1985).
Table 16. Acute toxicity of endrin to freshwater fish
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Tilupa sp. Static 15 44 7.1 96-h LC50 12 Mayer & Ellersieck (1986)
Coho salmon Static 16 44 7.1 96-h LC50 0.089 Mayer & Ellersieck (1986)
Oncorhynchus kisutch 1.9 g Static 20 96-h LC50 0.27 Katz & Chadwick (1961)
Chinook salmon 6-8 g Static 20 96-h LC50 0.92 Katz & Chadwick (1961)
Oncorhynchus
tshawytscha
Cutthroat trout 1.0 g Static 13 44 7.1 96-h LC50 > 1.0 Mayer & Ellersieck (1986)
Salmo clarki
Rainbow trout 1.0 g Static 13 44 7.1 96-h LC50 0.75 Mayer & Ellersieck (1986)
Oncorhynchus mykiss 1.0 g Static 13 272 7.4 96-h LC50 0.74
1.4 g Static 2 44 7.1 96-h LC50 2.4
1.4 g Static 7 44 7.1 96-h LC50 1.4
1.4 g Static 13 44 7.1 96-h LC50 1.11
1.4 g Static 18 44 7.1 96-h LC50 0.75
0.6-8.0 g Flow 18-23 96-h LC50 0.3 Thurston et al. (1985)
Goldfish 1-4 g Flow 12 314 7.6 96-h LC50 0.44 Mayer & Ellersieck (1986)
Carrassius auratus Flow 18-23 96-h LC50 0.95 Thurston et al. (1985)
Static 48-h LC50 1.0 Hashimoto & Nishiuchi
(1981)
Carp Flow 12 314 7.6 96-h LC50 0.32 Mayer & Ellersieck (1986)
Cyprinus carpio Static 48-h LC50 0.84 Hashimoto & Nishiuchi
(1981)
Medaka Static 48-h LC50 1.4 Hashimoto & Nishiuchi
Oryzias latipes (1981)
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Pond loach Static 48-h LC50 4.9 Hashimoto & Nishiuchi
Misgurnus (1981)
anguilicaudatus
Fathead minnow 1.2 g Static 18 44 7.1 96-h LC50 1.8 Mayer & Ellersieck (1986)
Pimephales promelas 0.9 g Flow 12 314 7.6 96-h LC50 0.24
Static 24-h LC50 12 Kagan et al. (1986)
Larvae Static 25-26 46 7.1-8.3 96-h LC50 0.7 Jarvinen et al. (1988)
0.2-1.0 g Flow 18-23 96-h LC50 0.65 Thurston et al. (1985)
Bluntnose minnow Static 96-h LC50 0.29 Johnson (1968)
Pimephales notatus
Black bullhead 1.5 g Static 24 44 7.1 96-h LC50 1.13 Mayer & Ellersieck (1986)
Ictalurus melas
Channel catfish 5.2 g Static 18 44 7.1 96-h LC50 1.9 Mayer & Ellersieck (1986)
Ictalurus punctatus 1.4 g Static 24 44 7.1 96-h LC50 0.32
Mosquito fish 0.6 g Static 17 44 7.1 96-h LC50 1.1 Mayer & Ellersieck (1986)
Gambusia affinis 0.222 g Static 25 96-h LC50 5.27 El-Sebae (1987)
Bluegill 1.5 g Static 18 44 7.1 96-h LC50 0.61 Mayer & Ellersieck (1986)
Lepomis macrochirus 0.5 g Static 18 272 7.4 96-h LC50 0.53
1.3 g Static 7 44 7.1 96-h LC50 0.73
1.3 g Static 13 44 7.1 96-h LC50 0.68
1.3 g Static 18 44 7.1 96-h LC50 0.41
1.3 g Static 24 44 7.1 96-h LC50 0.37
1.3 g Static 29 44 7.1 96-h LC50 0.19
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Largemouth bass 2.5 g Static 18 272 7.4 96-h LC50 0.31 Mayer & Ellersieck (1986)
Micropterus salmoides
Yellow perch Flow 12 314 7.6 96-h LC50 0.15 Mayer & Ellersieck (1986)
Perca flavescens
Tilapia 1.1 g Static 24 44 7.1 96-h LC50 < 5.6 Mayer & Ellersieck (1986)
Tilapia mossambica
Tilapia (Behera strain) 0.825 g Static 25 96-h LC50 10.09 El-Sebae (1987)
Tilapia zilli
Tilapia (Alexandria
strain) 0.825 g Static 25 96-h LC50 0.26 El-Sebae (1987)
Tilapia zilli
Guppy Static 20 96-h LC50 0.9 Katz & Chadwick (1961)
Poecilia reticulata
Flagfish 2-3 days Static 24-26 43-48 6.9-7.8 96-h LC50 0.85 Hermanutz et al. (1985)
Jordanella floridae
aStatic, static condition (water unchanged for the duration of the test); flow, flow-through conditions; endrin
concentration in water maintained continuously.
Table 17. Acute toxicity of endrin to estuarine and marine fish
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
American eel 57 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Anguilla rostrata
Atlantic riverside 54 mm Static 20 24 96-h LC50 0.05 Eisler (1970b)
Menidia menidia
Blue head 90 mm Static 20 24 96-h LC50 0.1 Eisler (1970b)
Thalassoma bifasciatum
Gulf menhaden Juvenile Flow 27 29 24-h LC50 0.8 Mayer (1987)
Brevoortia patronus
Sheepshead minnow Juvenile Flow 14 30 48-h LC50 1.0 Mayer (1987)
Cyprinodon variegatus Juvenile Flow 30 24 96-h LC50 0.34
Adult Flow 18 18 96-h LC50 0.38
Adult Flow 30 16 96-h LC50 0.36
Longnose killifish Juvenile Flow 25 19 24-h LC50 0.23 Mayer (1987)
Fundulus similis
Striped killifish 40 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
Fundulus majalis
Mummichog 51 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Fundulus heteroclitus
Sailfin molly Adult Flow 20 27 96-h LC50 0.63 Mayer (1987)
Poecilia latipinna
Spot Juvenile Flow 12 24 48-h LC50 0.3 Mayer (1987)
Leiostomus xanthurus
Table 17. (contd)
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Striped mullet Juvenile Flow 14 30 48-h LC50 0.4 Mayer (1987)
Mugil cephalus 83 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
White mullet Juvenile Flow 29 48-h LC50 2.6 Butler (1963)
Mugil curema
Northern puffer 131 mm Static 20 24 96-h LC50 3.1 Eisler (1970b)
Sphaeroidus maculatus
Striped bass 2.7 g Static 16-18 28 96-h LC50 0.09 Korn & Earnest (1974)
Morone saxatilis
Shiner perch 1.2-11 g Static 13 26 96-h LC50 0.8 Earnest & Benville (1972)
Cymatogaster aggregata 1.2-11 g Int. flow 13 26 96-h LC50 0.12
Dwarf perch 1.2-11 g Static 13 18 96-h LC50 0.6 Earnest & Benville (1972)
Micrometrus minimus 1.2-11 g Int. flow 13 28 96-h LC50 0.13
Threespine stickleback 0.3 g Static 20 25 96-h LC50 1.5 Katz & Chadwick (1961)
Gasterosteus aculeatus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; int. flow,
intermittent flow-through conditions; endrin concentration in water maintained continuously
7.2.2.3 Studies of resistance
Populations of mosquito fish (Gambusia affinis) developed high
levels of resistance to endrin and other cyclodiene insecticides as a
result of inadvertent exposure to agricultural sprays. Susceptible
fish (male) showed a LC50 of 8.3 mg/litre and resistant fish, 161
mg/litre. Genetic crossing studies show that endrin resistance is
inherited as a single, autosomal, intermediate gene (Yarbrough et al.,
1986).
Pesticide-susceptible and -resistant mosquito fish were exposed
to 14C-endrin at 20 or 1000 µg/litre, and liver and brain were
assayed to determine any difference in distribution, uptake, and nerve
binding patterns (Fabacher & Chambers, 1976). The results are
summarized in Table 18. Endrin was taken up faster by brain and liver
from susceptible fish than resistant fish. In resistant fish, at least
at a high lethal concentration (1000 µg/litre), endrin entered the
brain slowly and accumulated in the liver, suggesting a more efficient
blood-brain barrier in resistant than in susceptible fish. Extraction
studies provided some evidence that endrin binds more readily to
nonessential protein complexes in the nervous tissue of resistant
fish, consequently decreasing the amount of endrin available to
produce a toxic effect.
Table 18. Mean quantities of endrin (in mg/kg tissue) in brain and
liver of susceptible and resistant mosquito fish
Genotype At 20 µg/litre At 1000 µg/litre
Brain Liver Brain:liver Brain Liver Brain:liver
Susceptible 16.98 33.28 0.51 149.31 160.27 0.93
Resistant 8.83 16.84 0.52 57.52 353.42 0.16
Susceptible: 1.90 1.90 1.00 2.60 0.45 5.80
resistant
Cell membrane fractions from resistant mosquito fish bound more
endrin than those from susceptible fish, and mitochondria from the
liver of the resistant genotype bound less endrin than those from
susceptible fish. Differences in endrin uptake, retention of endrin by
brain cell membranes, a blood-brain barrier, and a structural
difference in myelin mayaccount for the resistance of some mosquito
fish to endrin (Wells & Yarbrough, 1972).
In resistant and non-resistant populations of golden shiner
(Notemigonus crysoleucas), blue gill sunfish (Lepomis macrochirus),
and green sunfish (Lepomis cyanellus), the median tolerated limit at
36 h was 3.0, 1.5, and 3.4 µg/litre for non-resistant strains and 310,
300, and 160 µg/litre for resistant fish, respectively (Ferguson et
al., 1964).
7.2.2.4 Interaction with other chemicals
The joint action of endrin with malathion on mortality in
flagfish (Jordanella floridae) consisted of enhanced effects at
concentrations that had no effect when the substances were tested
individually. The effects of the mixture on growth followed a simple
additive model. Malathion did not modify the effect of endrin on egg
production. In a separate test, malathion did not affect the uptake or
elimination of endrin (Hermanutz et al., 1985).
In a study of the interaction between the accumulation and
elimination of 14C-endrin and 14C-DDT in mosquito fish (Gambusia
affinis), fish about 4 cm long were exposed to a nominal
concentration of 3.94 nM endrin or DDT, or to a mixture of the
compounds. Prior exposure to DDT for 4 h generally reduced the
accumulation of endrin in serum, gall-bladder, and whole bodies,
whereas prior exposure to endrin for 4 h had little effect on DDT
accumulation. Simultaneous exposure to DDT and endrin reduced the
accumulation of DDT in the gall-bladder over the 4 h of exposure and
in the whole bodies during the first hours, and it reduced the
accumulation of endrin in gall-bladder and in the whole body. Endrin
levels in fish exposed subsequently only to DDT or DDE were
significantly higher in gall-bladder and were reduced in the whole
body over 4 h. The interactions observed may be the result of
competition for and/or displacement of insecticides from mutual
binding sites (Denison et al., 1985).
In a study of the relative binding and competition between
organochlorine pesticides for serum binding sites, incubation with
serum from mosquito fish led to their association primarily with the
vitellogenin/lipoprotein and albumin fractions. Preincubation of serum
with endrin significantly reduced the quantity of 3H-DDT that was
bound subsequently, while the reverse was not observed. Although the
reason for the apparent quantitative decrease in binding is unknown,
this phenomenon may be of toxicological importance (Denison &
Yarbrough, 1985).
7.2.2.5 Special studies
Fingerlings of carp (Cyprinus carpio) exposed to endrin at the
LC50 (0.0065 mg/kg) for 24 h showed clear inhibition of ý-amylase
activity in the liver (Datta & Ghose, 1985).
A group of 240 rainbow trout (Salmo gairdneri) were exposed to
endrin at 0.12-0.15 µg/litre for 30 days; one untreated and one
solvent control group were used. On day 30, 10 fish from each group
were sacrificed and examined for the ability of peritoneal macrophages
to phagocytize latex beads. The remaining fish were immunized with 10
µg of Yersinia ruckeri O-antigen and exposure to endrin continued.
Assays for migration inhibition factor, plaque forming cells, and
serum agglutination titre were performed 2, 14, and 30 days after
inoculation, and serum was collected from all fish to determine the
cortisol concentration. Exposure to endrin had no effect on the
phagocytic ability of peritoneal macrophages, but the responses in the
three assays were significantly reduced in comparison with the control
values. Serum cortisol concentrations were significantly elevated in
the endrin-treated fish. The study did not, however, elucidate the
mechanism of immune suppression, other than showing that a stress
response had occurred (Bennett & Wolke, 1987a). In another study,
therefore, control fish were fed cortisol at 20 mg/kg and metyrapone
at 35 mg/kg body weight, and endrin-exposed fish received metyrapone
at 35 mg/kg body weight per day in the diet. The fish that received
cortisol had significantly reduced responses in all three assays; but
in the endrin-exposed fish that received metyrapone, the migration
inhibition factor response was completely restored, the plaque forming
cell response was restored to 61%, and serum agglutination titres to
69%. These results indicate that elevated serum cortisol concentration
plays a central role in repressing the immune response (Bennett &
Wolke, 1987b).
The concentrations of serum glucose, liver and muscle glycogen,
cortisol, protein, and cholesterol were determined in carp (Cyprinus
carpio) exposed to endrin at 2 µg/litre for 6, 24, and 72 h. Only
the concentration of cortisol in serum was clearly decreased (Gluth &
Hanke, 1985).
7.2.3 Amphibia
The acute toxicity of endrin to amphibians is summarized in
Table 19.
7.3 Terrestrial organisms
The acute oral toxicity of endrin for terrestrial animals is
high. The available LD50 values are summarized in Table 20.
7.3.1 Honey bees
The 48-h LD50 of endrin in worker honey bees (Apis mellifera)
using a dusting technique was 2.02 µg/bee (Atkins et al., 1973). The
LD50 for bees after contact was 0.65 µg/bee, and the acute oral
LD50 was 0.46 µg/bee (Oomen, 1986).
Table 19. Acute toxicity of endrin to amphibians
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCo3/l) (mg/1) (mg/l)
Bullfrog 2-5 g Flow 18-23 96-h LC50 2.5 Thurston et al. (1985)
Rana catesbiana
Leopard frog Eggs Flow 20 100 7.2-7.5 24-h LC50 2.5 Hall & Swineford (1980)
Rana spenocephala Larvae Flow 20 100 7.2-7.5 96-h LC50 6
Subadult Flow 20 100 7.2-7.5 96-h LC50 5
Frog 0.5 g Static 14 20 6.2 96-h LC50 0.21 Khangarot et al. (1985)
Rana hexadactyla
Western chorus frog Tadpole Static 15 44 7.1 96-h LC50 120 Mayer & Ellersieck (1986)
Pseudacris triseriata
Fowlers toad Tadpole Static 15 44 7.1 96-h LC50 180 Mayer & Ellersieck (1986)
Bufo woodhousei
fowleri
aStatic,static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin concentration in water
maintained continuously
Table 20. Acute oral LD50s of endrin for terrestrial species
Species LD50 Reference
(mg/kg body weight)
Birds
Mallard 5.6 (2.7-11.7) Hudson et al. (1984)
(Anas platyrhynchos)
Pigeon 2.0-5.0
(Columbia livia)
Pheasant 1.8 (1.1-2.8)
(Phasianus colchicus)
Sharp-tailed grouse 1.06 (0.552-2.04)
(Pedioecetes phasia
nellus)
California quail 1.19 (0.857-1.65)
Redwinged blackbird 2.37 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling 2.37-3.16
(Sturnus vulgaris)
Quail 4.22
(Coturnix coturnix)
Mammals
Big brown bat 5-8 Luckens & Davis (1965)
(Eptesicus fuscus)
Pine mouse (Microtus 2.6/19.0 Petrella et al. (1975)
pitymys pinetorum) 1.3/36.4 Webb et al. (1973)
(susceptible/
resistant)
7.3.2 Birds
7.3.2.1 Acute toxicity
The LD50s of endrin for some bird species are given in Table
20.
7.3.2.2 Short-term toxicity
Groups of 40 one-day-old quail were fed endrin at dietary levels
of 0, 0.5, 1, 5, 10, 20, or 50 mg/kg of diet. Survival was adversely
affected in all test groups, and there were no survivors beyond two
weeks among birds fed 10 mg/kg or more. Food consumption was
abnormally low, and symptoms involved lack of muscular coordination,
tremors, and occasional convulsive movements. Similar results were
obtained in 40 one-day-old pheasants fed endrin at dietary levels of
5 or 20 mg/kg, none of which survived beyond 8 days (Dewitt, 1965).
Groups of 20 seven-day-old chicks were unaffected by diets
containing endrin at 0, 1.5, or 3 mg/kg. When the concentration was
increased to 6 or 12 mg/kg, the birds became highly excitable and
failed to gain weight in comparison with controls. The survival rates
over a 12-week period were 85 and 5%, respectively, compared with 100%
in the controls (Sherman & Rosenberg, 1954).
The LC50 values for 2-3-week-old bobwhite quail (Colinus
virginianus), Japanese quail (Coturnix coturnix japonica),
ring-necked pheasants (Phasianus colchicus), and mallards (Anas
platyrhynchos) (8-13 birds per group) fed endrin in their diet for
5 days followed by 3 days of untreated diet, were 14-22 mg/kg diet
(Hill et al., 1975; Hill & Camardese, 1986).
7.3.2.3 Studies of reproduction
In a study of reproduction in pheasants, a diet containing endrin
at 10 mg/kg reduced egg production and chick survival; diets
containing up to 2 mg/kg did not affect egg production, fertility,
hatchability, or chick survival (Dewitt, 1965).
Groups of five female and two male mallard ducks (Anas
platyrhynchos) were administered diets containing endrin at 0, 0.5,
or 3.0 mg/kg for a 12-week oviposition period. Egg production was not
affected. The eggs were incubated, and infertile eggs, embryo
survival, and hatchability were measured. Fertility and hatchability
were not affected, although a 9.6% drop in embryo survival was
observed in the group that had received the highest dose. Endrin
residues in body fat were 3.4 mg/kg of tissue in the group that
received 0.5 mg/kg and 19.3 mg/kg in the group that received 3.0
mg/kg. The concentrations were higher in females than in males. The
endrin residue levels in eggs were none detected in the controls, 0.43
mg/kg in the group fed 0.5 mg/kg, and 2.75 mg/kg in the group fed 3.0
mg/kg (Roylance et al., 1985).
Three groups of 27 pairs of mallards were fed endrin at 0, 1, or
3 mg/kg of dry duck mash from December to the summer to investigate
the influence on reproduction and health. Birds fed 1 mg/kg reproduced
as well as the controls; they had significantly greater success in
hatching fertile eggs than did those fed 0 or 3 mg/kg and their
clutches hatched earlier (not significantly) than those of birds fed
3 mg/kg. Endrin accumulated in eggs to a mean level of 1.1 mg/kg (wet
weight) in the group fed 1 mg/kg and 2.9 mg/kg in the group fed 3
mg/kg. The concentration of endrin in adipose tissue was four to seven
times higher than that in eggs (Spann et al., 1986).
7.3.2.4 Interaction with other chemicals
The toxicity of combinations of chlordane and endrin was studied
in 14-week-old male and female bobwhite quail. Eight birds received
10 mg/kg chlordane in the diet for 10 weeks; 20 quail were treated
with 10 mg/kg chlordane for 10 weeks followed immediately by 10 mg/kg
endrin (98%) in the diet; a fourth group of 20 birds received only 10
mg/kg endrin in the diet. The pesticides were dissolved in propylene
glycol. After 9-10 days on a control diet, survivors were sacrificed
and their brains dissected. No deaths occurred among the birds fed the
control diet or 10 mg/kg chlordane. With endrin alone, 15 birds died,
and with the combination 14 birds died. In birds that received endrin
alone, the residue levels in the brain were 0.34-1.84 mg/kg in those
that died and 0.28-0.62 mg/kg in the survivors. In the birds fed
chlordane and endrin, the residue levels were 0.17-1.25 mg/kg in birds
that died and 0.14-0.56 mg/kg in survivors. Birds treated with the
combination had considerably more chlordane residues in their brains
than did those fed chlordane alone. The main conclusion of this study
was that the additive toxicity of closely related chemicals should be
taken into account in diagnosing cause of death (Ludke, 1976).
7.3.2.5 Special studies
The influence of endrin at 5 and 10 mg/kg of feed on the
activity of various enzymes in the serum of juvenile cockerels was
studied. The greatest increases in activity were measured for
glutamate oxalacetate transaminase, cholinesterase, and alkaline
phosphatase. Smaller increases were observed for creatine kinase,
glutamate dehydrogenase, ý-hydroxybutyrate dehydrogenase, and
phosphohexose isomerase (Horn et al., 1987).
7.3.2.6 Behavioural studies
The effect of a sub-lethal dose of endrin (2 mg/kg diet) on
avoidance responseswas studied in eight pens of 25 seven-day-old
Coturnix quail chicks for 14 days. The stimulus used to elicit
avoidance was a moving silhouette, and the response was measured
daily. Group avoidance response was significantly suppressed by
exposure to endrin, but the behaviour returned to normal after 2 days
on untreated diet (Kreitzer & Heinz, 1974).
Adult male bobwhite quail (Colinus virginianus) were fed a diet
containing endrin at 0.1 or 1.0 mg/kg for 138 days (beginning at 3
days of age), and then their performance in five non-spatial
discrimination reversal tasks was studied. Treated birds made 36-139%
more errors than controls, and birds fed the lower dose made
significantly more errors than those given the higher dose after
reversal 3 or 4 in the first three tests. The effects of endrin were
reversed after 50 days on untreated feed. The principal effect of
endrin was to impair the birds' ability to solve a novel problem. The
mean levels of endrin residues in the brain were 0.075 mg/kg wet
weight in those given the lower dose and 0.35 mg/kg for those on the
higher dose (Kreitzer, 1980).
7.3.3 Mammals
7.3.3.1 Toxicity
The LC50 values for short-tailed male and female shrews
(Blarina brevicauda) aged 180, 105-150, and 30-75 days were 87-174
mg/kg diet for 14 days (Blus, 1978).
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus maniculatus) of various ages were fed endrin at 0, 1, 2,
4, or 7 mg/kg of diet for intermittent periods, between which the
animals were either fed a normal diet or were subjected to 48-h
starvation. The animals were sacrificed by exposing them to cold
stress at -16 °C and the time of death recorded. No influence was
found on litter production, frequency, or mean litter size. At the
higher levels of feeding, postnatal mortality before weaning was
increased. Significant parental mortality occurred at 4 mg/kg and
higher and appeared to be dose-dependent (Morris, 1968). (Remark:
Since the animals in this study were captured in the field and the
periods of feeding alternated with short periods of starvation in an
effort to simulate possible conditions in the field, this study is of
only limited value).
The effects of endrin at 8.0 oz/acre (0.56 kg/ha) on unenclosed
field populations of meadow voles (Microtus pennsylvanicus) and deer
mice (Peromyscus maniculatus) were investigated in 1966-68. Animals
were trapped live on adjacent 7-acre (2.8 ha) plots each summer at
regular intervals, before and after a single application of endrin.
Immediate, significant declines in the number of voles were seen on
the experimental plot, but no long-term toxicological effects were
observed. The population rapidly recovered, exceeding the initial and
control numbers in all three years. The experimental vole population
thus appears to have responded to endrin as it would to a local
depopulation by trapping. The mouse population decreased significantly
after the application of endrin in 1966 and did not recover, and the
highly unstable, transitory population on the experimental plot
indicated a long-term toxicological effect (Morris, 1970, 1972).
7.3.3.2 Studies of resistance
14C-Endrin in corn oil was administered to a resistant and a
susceptible strain of pine mice (Microtus pitymus pinetorum) orally
at 0.5 mg/kg body weight, as follows: days 1-5, unlabelled endrin;
days 6-14, 14C-endrin; and day 15 unlabelled endrin. Total recovery
of 14C in both faeces and urine was 76% for the resistant strain and
53% for the susceptible strain. The two strains produced the same
major faecal and urine metabolite, but the resistant strain produced
about twice the quantity as the susceptible strain. The quantitative
differences in the excretion of more polar endrin metabolites may
indicate metabolic differences between the two strains and,
consequently, the greater tolerance of the resistant strain to toxic
effects (Petrella et al., 1975). The major metabolite was identified
as anti-12-hydroxyendrin; one of the other more polar metabolites,
found in minor quantities, was suggested to be a tertiary alcohol of
endrin (Petrella et al., 1977).
The degree of toxicity of endrin in first-generation progeny of
susceptible and resistant strains and a cross of the two strains of
pine mice was studied by Webb et al. (1973). The LD50 for offspring
of susceptible x susceptible parentage was 5.0 mg/kg body weight; that
for resistant x resistant, 21.1 mg/kg; and that for susceptible x
resistant, 8.6 mg/kg. These results offer preliminary support for a
genetic mechanism with intermediate dominance. An increase in
resistance against the toxic effects of endrin was demonstrated in
wild pine mice trapped in orchards where endrin had been used for
years. The oral LD50 in susceptible mice was about 3 mg/kg body
weight and that in resistant mice, an average of 36 mg/kg body weight.
The increased resistance appeared to be heritable in the first
generation (Webb & Horsfall, 1967; Webb et al., 1973). Although
differences in the rate of metabolism of endrin could be demonstrated,
especially in the activity of mixed-function oxidase, these did not
appear to be sufficiently large to explain the resistance (Hartgrove
et al., 1977).
7.4 Effects in the field
Episodes have been reported in which endrin was concluded to be
the cause of death in fish and birds. Numerous fish kills were
reported from the sugar-cane growing areas of Louisiana in 1960-63. No
association with variables such as dissolved oxygen, pH, or
temperature was found, but following the development of sensitive
analytical techniques it was concluded that the fish had been killed
by endrin (Mount & Putnicki, 1966). Surface runoff from fields was
reported to be the main source of the endrin that contaminated the
rivers (Lauer et al., 1966), although effluent from an insecticide
plant may have contributed since the fish contained two chemicals
involved in endrin manufacture (Mount & Putnicki, 1966). Levels of
endrin found in studies of fish in the wild are given in section
5.1.4.2.
Declines in the population of brown pelicans in Louisiana were
attributed to endrin, although at least six other organochlorine
pesticides and polychlorinated biphenyls were found in the animals
(Blus et al., 1975; King et al., 1977). The eggs of brown pelicans
(Pelecanus occidentalis) in Texas, USA, were examined for endrin
residues in 1975-81. The compound was recovered only in 1975, in 15 of
18 eggs, at levels of 0.1-0.3 mg/kg. In the same year, the highest
levels of endrin were found in pelican eggs in Louisiana, and this
maximum coincided with the deaths of large numbers of brown and white
pelicans (P. erhyrorhyncos) (King et al., 1985).
Endrin was found in one of ten eggs of the American white pelican
(Pelecanus erythrorhynchos) collected in 1969, at 0.20 mg/kg, and in
two of 35 samples collected in 1981, at up to 0.18 mg/kg wet weight.
Brains of pelicans found dead in the period 1975-81 had levels up to
0.80 mg/kg. No endrin was found in eggs of the western grebe
(Aechmophorus accidentalis) collected in 1981. It was concluded that
endrin had caused some of the deaths among pelicans in California
(Boellstorff et al., 1985).
The death of sandwich terns in The Netherlands was attributed to
the discharge of a combination of endrin and related pesticides into
an estuary from a manufacturing plant (Koeman et al., 1967, 1969;
Koeman, 1971).
On several occasions in Victoria, Australia, large numbers of
wild birds, in particular pigeons (Columba livia), sparrows (Passer
domesticus) and Indian mynahs (Gracula religiosa), were observed
to be paralysed or in convulsions (Reece et al., 1985). The crops and
livers contained endrin at levels of up to 1.2 mg/kg.
Fulvous whistling ducks (Dendrocygna bicolor), which nest in
rice fields along the south-eastern coast of Texas, USA, suffered a
major decline in population in the late 1960s, which was attributed to
exposure to dieldrin or aldrin. Organochlorine pesticides were
determined in 1983 in the carcasses of 15 adult ducks immediately
after their arrival in Texas from Mexico in the spring and before
departure from Mexico in the autumn. Four of the ducks with high
levels of dieldrin residues also had residues of endrin; and four
other ducks, collected in 1967 and 1969, had endrin residues. The
geometric mean levels in the different years were 0.03-0.08 mg/kg wet
weight; in juveniles in 1960-69, the geometric mean level was 0.16
mg/kg (Flickinger et al., 1986).
The effects of endrin on wildlife were studied in 1981-83 in
fruit orchards in Washington, USA. A single application of endrin
after harvest resulted in acute and chronic toxicity to a variety of
avian species; most deaths occurred soon after the application, but
several raptors died during the spring and summer. The brains of 73 of
125 birds contained endrin at < 0.10-0.80 mg/kg; detectable levels
occurred most frequently in the brains of galliforms and falconiforms.
The species in which the greatest numbers of deaths attributed to
endrin occurred include California quail (Callipepta california),
chukars (Alectoris chukar), and common barn owls (Tyto alba). Of
the 97 eggs analysed, 68 contained detectable endrin residues: 51 had
levels of < 0.10 mg/kg, and the eggs of 10 species contained
0.01-0.17 mg/kg wet weight (range, none detected to 1.67). The authors
concluded that endrin was toxic to wildlife, although there was no
evidence that it affected reproductive success or population level
(Blus et al., 1989).
Levels found in birds in the wild are also given in section
5.1.4.1.
7.5 Appraisal of effects on organisms in the environment
Use of endrin in agriculture is the major source of its presence
in the environment, but discharge of waste material from manufacturing
and formulating plants has contributed to local contamination.
World-wide monitoring surveys have shown that the concentrations of
endrin in the biosphere are generally very low (Table 10), both
absolutely and relatively: The levels of residues of other
organochlorine compounds, particularly DDE and polychlorinated
biphenyls, are generally 100 times or higher than those of endrin.
Toxicologically significant levels of endrin residues have been
found locally in fish and other organisms, particularly in cases in
which endrin was applied near rivers and lakes and when runoff
occurred into waterways. Residues may also occur when endrin is used
as a seed dressing or in bait to control rodents.
The most serious adverse ecological effects that have been
reported were the fish kills (and associated adverse effects on brown
pelican populations) in the Mississippi River system in the USA.
Although the initial evidence for ascribing these effects to endrin
was circumstantial, the results of analyses of dead fish were
considered to confirm a causal relationship; there is little doubt
that endrin was a contributory factor in at least some of these fish
kills. The evidence that endrin was the primary cause of the decline
in the brown pelican population is less convincing, since the harmful
effects on reproductive success have been attributed to DDE and other
factors (Blus et al., 1974, 1979).
In summary, agricultural application of endrin should be such as
to avoid or minimize contamination of waterways, either by
overspraying or runoff or by leaching from dressed seed in
rice-growing areas. The effects of the use of baits containing endrin
for rodent control on non-target organisms should be assessed in the
light of local circumstances. Finally, effluents from manufacturing
and formulating plants must be treated adequately before being
discharged into waterways.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO
The toxicology and risk assessment of endrin have been reviewed
(US EPA, 1987a,b; Anon., 1988a,b).
8.1 Acute toxicity of technical-grade endrin
8.1.1 Oral administration
Endrin is highly toxic when given by the oral route and is more
acutely toxic to mammals than its stereoisomer dieldrin (WHO, 1989),
with an acute oral LD50 of 7.5-17.8 mg/kg body weight (Table 21),
compared with 50-60 mg/kg for dieldrin. There appears to be a
sex-dependent sensitivity to the acute effects of endrin, female
animals being more sensitive than males. A species-dependent
sensitivity has also been reported, monkeys and cats being more
susceptible than mice and rats.
Signs of intoxication may include increased irritability and
tremor, followed by tonic-clonic convulsions, ataxia, dyspnoea,
gasping, and cyanosis. Convulsions usually occur 30-60 min after an
oral dose, and death may occur within 24 h after the administration of
a lethal dose (Speck & Maaske, 1958). Animals that survive poisoning
recover completely with no delayed or persistent effect.
8.1.2 Dermal administration
The acute dermal LD50s for technical endrin in various animal
species are given in Table 22. Endrin is highly toxic when applied as
a solution in hydrocarbon solvents but moderately toxic when applied
as a dry powder. The signs of poisoning are similar to those seen
after oral administration.
8.1.3 Parenteral administration
The acute LD50s for technical-grade endrin given by parenteral
routes of administration are shown in Table 23.
8.1.4 Toxicity of metabolites and isomers
8.1.4.1 Mammalian metabolites
In a comparative study, the acute oral LD50s of endrin and
three of its metabolites were determined in rats (Table 24). When the
brains of some of the rats were analysed for the presence of endrin
and its metabolites, the concentration of 12-ketoendrin in male rats
given endrin at 60 mg/kg body weight was found to be higher (mean, 0.3
mg/kg) than that of endrin (0.07 mg/kg) 22 h after dosing. In male
rats intoxicated with syn-12-hydroxyendrin or 12-ketoendrin (at 16
mg/kg body weight each), the concentrations of 12-ketoendrin in the
brain 30 min after dosing were much higher (mean values, 1.9 and 1.4
Table 21. Oral LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Mouse Corn oil 8.6 - Spynu (1964)
Mouse Unknown 13 13 Gray et al. (1981)
Rat (4-5 weeks) Peanut oil 28.8 16.8 Treon et al.(1955)
Rat (6 months) Peanut oil 43.4 7.3 Treon et al. (1955)
Rat (6 months) Peanut oil 40.0 - Speck & Maaske (1958)
Rat (adult) Peanut oil 17.8 7.5 Gaines (1960)
Rat (7 weeks) Cottonseed oil 27 - Boyd & Stefec (1969)
Rat (12-14 weeks) Dimethyl sulfoxide 5.6a 5.3a Bedford et al. (1975b)
Rat (12-13 weeks) Corn oil 8.9 4.0 Carter & Simpson (1978)
Rat Corn oil 9.0 - Spynu (1964)
Rat Unknown 4 4 Gray et al. (1981)
Rabbit Peanut oil - 7-10 Treon et al. (1955)
Guinea- pig Peanut oil 36.0 16.0 Treon et al. (1955)
Hamster (golden Syrian) Corn oil - 18.6 Chernoff et al. (1979)
Hamster (golden Syrian)
(6 weeks) Unknown 12 17.0 Cabral et al. (1979)
Hamster Unknown 18 18 Gray et al. (1981)
Cat Cod liver oil Lethal dose: Ressang et al. (1958);
3-6 Cook & Casteel (1985);
Casteel & Cook (1985)
Monkey (Macacus mulatta) Peanut oil 3 Treon et al. (1955)
Monkey (Macacus speciosa) Unknown 12 Barth (1967)
Domestic goat Unknown 25-50 Tucker & Crabtree (1970)
Mule deer Unknown 6.25-12.5 Hudson et al. (1984)
aEndrin of 99% purity
Table 22. Dermal LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Rat Xylene 18 15 Gaines (1960,1969)
Rat Shellsol A 10-20 5-10 Carter & Simpson (1978)
Rat Toluene approx. 10 approx. 10 Carter & Simpson (1978)
Rat Corn oil 12.5 - Spynu (1964)
Rabbit None - Minimum lethal Treon et al. (1955)
dose: 60-94
Cat Cod-liver oil Lethal dose: Ressang et al. (1958)
approx. 150
Table 23. Parental LD50s for technical- grade endrin
Species Route Vehicle LD50 (mg/kg Reference
body weight)
Mouse Intraperitoneal Corn oil 5.6 Graves & Bradley
(1965)
Mouse Intravenous Dimethyl 2.3 Walsh & Fink
sulfoxide (1970, 1972)
Dog Intravenous Ethanol 3 Hinshaw et al.
(1966)
Table 24. Acute oral toxicity of mammalian metabolites of
endrin in rats
Compound Oral LD50 (mg/kg body weight)
Male Female
Mean 95% CI Mean 95% CI
Endrin 5.6 3.0-7.9 5.3 3.6-7.4
anti-12-Hydroxyendrin 2.4 2.0-3.0 5.5 4.2-7.2
syn-12-Hydroxyendrin 1.2 0.6-1.7 2.8 0.8-4.0
12-Ketoendrin 1.1 0.7-1.5 0.8 0.5-1.2
a95% CI, 95% confidence interval
mg/kg, respectively) than those in the brains of rats given a similar
but non-toxic dose of anti-12-hydroxyendrin (mean, 0.09 mg/kg) and
killed at the same time. The signs of intoxication were similar to
those of endrin (Bedford et al., 1975b).
These results suggest that 12-ketoendrin may be the acute
toxicant in rats. The production of 12-ketoendrin varies greatly from
one mammalian species to another, however, and none has been detected
in birds of various species that were killed by endrin (Stickel et
al., 1979).
8.1.4.2 Isomers
As described in section 4.2, endrin is changed under the
influence of sunlight into delta-ketoendrin. The acute toxicity of
this isomer is given in Table 25. It is less toxic than endrin, and,
like endrin, it is more toxic to female than to male rats. The signs
of intoxication are similar to those seen with endrin.
The acute tocixity of the endrin aldehyde has been reported to be
> 500 mg/kg body weight in male mice (Phillips et al., 1962).
Table 25. Acute toxicity of delta-ketoendrin
Sex and Route LD50 (mg/kg Reference
species body weight)
Male rats Oral 120-180 Soto & Deichmann
(1967)
Female rats Oral 10-36
Male rats Oral 62.1 (53.3-72.2) Stanford Research
Institute (1954)
Rats Intravenous 5 Soto & Deichmann
(1967)
Male rats Intraperitoneal 82 Stanford Research
Institute (1953)
Male mice Oral 23.6 (19.9-28.0) Stanford Research
Institute (1954)
Male mice Intraperitoneal 16.7 Stanford Research
Institute (1953)
8.1.5 Acute toxicity of formulated material
8.1.5.1 Oral and dermal administration
Oral and dermal LD50 values for formulated endrin in rats
(Muir, 1970) are presented in Table 26. Dry formulations were
administered orally as 1-2% aqueous suspensions and dermally in both
dry form and as 2-5% aqueous suspensions. In general, the type of
formulation did not significantly alter the acute oral toxicity of
endrin. The dermal toxicity of the 50% wettable powder was similar to
that of the 20% emulsifiable concentrate; the 2% field strength dust
was the least toxic.
Ten rabbits (body weight, 2.4-4.1 kg) were treated with an
emulsifiable concentrate containing 19.4% endrin on clipped skin at a
dose of 200 mg/kg body weight, and the material was allowed to remain
in contact with the skin for 24 h. Four of the 10 animals died within
48 h (Anderson et al., 1953). Two of 10 rabbits (body weight,
2.0-2.5 kg) treated similarly with a 25% dust concentrate died within
48 h (Hine et al., 1954).
Table 26. Oral and dermal LD50s for endrin formulations in rats
Formulation LD50 (mg/kg body weight)
Oral Dermal
Formulation Active Formulation Active
material material
20% Emulsifiable 20 4.20 52.20 (undiluted) 10.90
concentrate
50% Wettable 7.6 3.80 21.80 (dry) 10.90
powder 14.40 (aqueous) 7.20
2% Field strength 275 5.50 5720 (dry) 114.40
dust 1140 (aqueous) 22.80
From Muir (1970)
8.1.5.2 Inhalation
Ten adult rats were exposed for 1 h to a mist of of an
emulsifiable concentrate containing 19.4% by weight of endrin in
xylene, at a concentration of endrin slightly exceeding 2000 mg/m3
of air, and were observed for 48 h. The particle size of the mist and
other details of exposure were not reported. Three of the animals died
1-14 h after exposure (Anderson et al., 1953).
Groups of 10 Long Evans rats were exposed for 1 h to 25% and 30%
endrin dust concentrates at a concentration of 2000 mg/m3 of air.
Particle size and other details of exposure were not provided. Five
rats exposed to the 30% and three exposed to the 25% dust died within
48 h after exposure (Hine et al., 1954).
8.2 Short-term exposure
8.2.1 Oral administration
8.2.1.1 Mouse
Feeding studies were conducted to estimate the maximum tolerated
doses of endrin in B6C3F1 mice. Groups of five males and five females
were given a normal diet or one containing endrin at 2.5-20 mg/kg for
6 weeks, followed by observation for another 2 weeks. Three males and
four females given 10 mg/kg died, but no mortality occurred at 5
mg/kg. No data were provided on animals fed 20 mg/kg.
Hyperexcitability was observed in male mice given doses > 5 mg/kg of
diet. Mean body weight gains were comparable with those of controls.
The maximum tolerated dose was calculated by extrapolation to be 5
mg/kg of diet (NCI, 1978, 1979).
8.2.1.2 Rat
Groups of three male and two to three female Carworth rats,
either 29 days or 6 months old, received daily doses of endrin at 1,
2, or 5 mg/kg body weight in peanut oil by gavage, on five days per
week for 67-72 days. All rats given 1 mg/kg survived; the increased
mortality in the other groups was dose-related: 2/5 females at 2 mg/kg
and 3/3 males at 5 mg/kg day died. Pathological findings at autopsy
included diffuse degenerative changes in the liver, kidneys, and
brain, while survivors showed no such changes. All treated animals
lost weight and developed hypersensitivity to stimuli (Treon et al.,
1955).
Groups of five male and five female adult Sprague-Dawley rats
were given diets containing technical-grade endrin at 0, 1, 5, 25, 50,
or 100 mg/kg diet over a maximal period of 16 weeks and were observed
for behaviour, weight gain, feed consumption, mortality rate, and
symptoms of toxicity. Alkaline phosphatase levels, determined once a
week, were higher in rats fed endrin than in the control group, and
the total average feed consumption of treated rats was less than that
of the control group. All rats fed 100 mg/kg of diet died during the
first two weeks of the study, and only two female rats fed 50 mg/kg of
diet survived the experiment. All male rats given 1 mg/kg and all
female rats given 1 and 5 mg/kg of diet survived. Males appeared to be
more susceptible than females to endrin in this study. The symptoms of
intoxication were hypersensitivity to stimuli and convulsions:
hypersensitivity was noted in all rats, and convulsions occurred among
rats receiving 25, 50, and 100 mg/kg diet. Weight loss was
dose-dependent and significant in all rats treated with endrin (Nelson
et al., 1956).
To estimate the maximum tolerated doses of endrin in
Osborne-Mendel rats, groups of five males and five females were given
diets with or without endrin for 6 weeks, followed by observation for
another 2 weeks. Endrin was added to the diet in two-fold increasing
concentrations of 2.5-80 mg/kg. Mortality was not increased at 10
mg/kg, and mean body weight gain was no different from that in
controls. At 20 mg/kg of diet, one animal of each sex died. The
maximum tolerated dose was calculated by extrapolation to be 15 mg/kg
diet (NCI, 1978, 1979).
8.2.1.3 Rabbit
Four of five female rabbits given an oral dose of endrin at 1
mg/kg body weight on five days per week died following the
administration of 2, 30, 35, and 50 doses, respectively. The fifth
rabbit survived 50 doses over a period of 10 weeks. Diffuse
degenerative changes were observed in the liver and kidneys but not in
the brain (Treon et al., 1955).
8.2.1.4 Dog
Dogs (mainly two per group) fed endrin at 5-50 mg/kg of diet died
within 50 days. They regurgitated their food, became lethargic,
salivated, and later refused to eat; they became emaciated and
developed respiratory distress and signs of stimulation of the central
nervous system. Diffuse degenerative lesions in the brain, heart,
liver, and kidneys, together with pulmonary hyperaemia and oedema were
observed. Three dogs fed diets containing 4 mg/kg of diet for 6 months
survived, but they showed reduced body weight gain and a slight
increase in liver:body weight ratio; no histopathological change was
observed. At 3 mg/kg of diet or less, growth was normal (Treon et al.,
1955).
Beagles (one male and one female/group; control group, only one
dog) were fed diets containing endrin at 0, 1, or 3 mg/kg for 80
weeks. No sign of intoxication was observed, and the weight gain of
treated animals was comparable to that of controls. The ratios of
kidney and heart to body weight were increased at 3 mg but not at 1 mg
(about 0.045 mg/kg body weight). No histopathological change was found
in the viscera (Treon et al., 1955).
Groups of three male and three female pure-bred beagle dogs
(4-6 months old) were fed endrin in the diet at 0, 0.1, 0.5, 1, 2, or
4 mg/kg for two years. Additional groups of four male and four female
dogs were fed endrin at 0, 1, or 4 mg/kg of diet. Two males and two
females of each group were killed after 6 and 12 months of feeding; no
other death occurred during the study. Convulsions were observed in
three dogs at 4 mg and in one female at 2 mg; no other sign of
intoxication or illness was apparent during the study. No adverse
effect was noted on growth, food consumption, haematology, or
urinalysis, and no compound-related change was found in serum alkaline
phosphatase, prothrombin time, or any of the other clinical chemical
parameters measured at regular intervals. All organ weights, relative
as well as absolute, were normal, except for occasional, slight
increases in liver weight in some of the females at 2 and 4 mg in the
diet. The only histopathological change found was a slight to moderate
vacuolation of liver cells in dogs fed 2 and 4 mg in the diet. No
renal change was observed in any of the dogs (Jolley et al., 1969).
8.2.1.5 Domestic animals
Sheep and cattle fed diets containing endrin at 2.5 or 5 mg/kg
for 112 days showed no indication of harmful effects (details not
given) (Radeleff, 1956). The convulsions and muscular tremors that
were induced in six 10-18-month-old male buffalo calves administered
a 20% emulsion of endrin led to a significant rise in lactic acid
concentration in the blood of the animals, possibly due to excessive
production of the acid inside the fasciculating muscles (Verma et al.,
1970).
8.2.2 Inhalation
Three mice, three rats, two guinea-pigs, two hamsters, four
rabbits, and one cat were exposed to sublimed endrin vapour at an
actual concentration of 5.44 mg/m3 for 7 h/day on 5 days/week for up
to 26 weeks. Two rabbits died after 26 and 90 exposures, respectively,
and one mouse died after 22 exposures. No convulsions were observed,
and all other animals survived. Surviving rabbits showed a
granulomatous type of pneumonitis; no histological change was found in
the other surviving animals (Treon et al., 1955).
8.2.3 Dermal administration
Three female rabbits with intact skin died after 19, 19, and 25
applications, respectively, of endrin as a dry powder at 150 m/kg body
weight for 2 h/day on 5 days/week. Applications of 75 mg/kg resulted
in the death of one of three rabbits after 8 weeks; the other two
survived for 13-14 weeks--the end of exposure. Convulsions, tremors,
and twitching of the facial muscles were the main signs of
intoxication. Two of five rabbits (dose not specified) showed severe
fatty degeneration of the liver (Treon et al., 1955).
8.3 Skin irritation
Dry powdered endrin was applied repeatedly at a dose of 75 or 150
mg/kg body weight for 2 h/day, 5 days/week for up to 14 weeks on
intact or abraded skin of female rabbits (see section 8.2.3). No skin
irritation was observed. Single applications of endrin as dry powder
at doses up to 250 mg/kg body weight for 24 h on rabbit skin caused no
gross or microscopic damage to the skin of the animals (Treon et al.,
1955).
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Mouse
CFW mice (20 males and 20 females) were fed diets containing
endrin (96%) at 0 and 5 mg/kg for 120 days, beginning 30 days before
mating. Significant parental mortality (32%) and reduced litter size
were observed, but fertility, fecundity, and the number of litters
produced per pair were not affected (Good & Ware, 1969).
8.4.1.2 Rat
Forty male and 80 female Long-Evans rats were fed endrin in the
diet at 0, 0.1, 1, or 3 mg/kg over three generations, each generation
breeding once. No difference in appearance, behaviour, body weight, or
number or size of litters was seen. The weights of the liver, kidneys,
and brain were normal, and no histopathological abnormality was seen
in third-generation weanlings. The only significant effect was
increased mortality of pups in the second and third generations of
rats fed 3 mg/kg (Hine, 1965).
Ten male and 20 female Long-Evans rats were treated similarly,
but each generation bred twice. Weanling rats were mated after 79 days
on the diets (when they were 100 days old). All pups from the first
litters were discarded at weaning, and the parent rats were mated
again. Randomly selected pups from the second litters were maintained
on the diets and mated when 100 days old; this was done for three
generations. The number of pups in each litter was counted on the day
of birth and on the fifth day; on the twenty-first day, the weanlings
were counted and weighed and either sacrificed or saved for
continuation. Parent rats were weighed, sacrificed, and examined
grossly when no longer needed. Ten male and 10 female F3b weanlings
each from the controls and the highest dose-level group and five males
and five females from the 0.1 and 1.0 mg groups were autopsied. Body,
liver, kidney, and brain weights were recorded, and sections of these
organs and from heart, lung, spleen, and testis were studied
histologically. Appearance, behaviour, body weight, number and size of
litters, organ weights, and histopathological appearance of F3b
weanlings were comparable with those in control animals. No effect on
reproduction was observed in rats fed diets containing endrin at 2
mg/kg over three generations (Hine, 1968).
8.4.2 Embryotoxicity and teratogenicity
8.4.2.1 Mouse
Groups of 10 CD-1 mice were given a single oral dose of endrin
(99%) at 2.5 mg/kg body weight (stated to be half the LD50) in corn
oil by gavage on day 9 of gestation; an untreated and a vehicle
control group were also used. Fetuses were examined on day 18. No
significant effect was observed on intrauterine death or fetal weight,
but the incidence of total anomalies was increased over that in
controls: 2/117 fetuses had cleft palates, three had open eye, and two
had other anomalies. No data on maternal toxicity were reported
(Ottolenghi et al., 1974).
These results could not be repeated by Kavlock et al. Female CD-1
mice were given endrin (99%) at 0 (vehicle), 0.5, or 1.0 (groups of 40
mice), or 1.5 or 2.0 mg/kg body weight (groups of 20 mice) in corn oil
by gavage on days 7-17 of gestation. The animals were killed on day
18. Maternal deaths occurred in the 1.5 and 2 mg/kg groups, reduced
maternal weight gain was observed at and above 1 mg/kg, and maternal
liver weight was increased at 0.5 mg/kg and higher. Fetal weight and
skeletal, and visceral maturity were adversely affected at doses of 1
mg/kg and above. No teratogenic effect or embryonic lethality was
observed, even at doses that caused maternal death (Kavlock et al.,
1981, 1987).
In a study of the effects of acute alterations in maternal health
status upon fetal development in the mouse, groups of 21 or 40
pregnant CD-1 mice were given a single oral dose of technical-grade
endrin at 0 (vehicle), 7 or 9 mg/kg body weight in corn oil on day 8
of gestation. The animals were killed on day 18 of gestation. Three of
21 animals given 7 mg/kg (14%) and 19/40 mice gievn 9 mg/kg (47%)
died. Maternal weight gain was decreased in both test groups; the
total number of implantation sites and number of viable litters were
not affected, but fetal weight was reduced. Delays in ossification of
the skeleton and an increased incidence of supernumary lumbar ribs
were observed. Although three fetuses from one litter in the 9 mg/kg
group had fused ribs, no significant increase in the incidence of
malformations was found. A statistically significant, linear, inverse
relationship between maternal weight gain and the presence of
supernumary ribs in their offspring was found (Kavlock et al., 1985).
8.4.2.2 Rat
Five groups of 25 female CD rats were administered endrin (97%)
in methocel in oral doses of 0, 0.1, 0.5, or 2 mg/kg body weight per
day on days 6-15 of gestation, or vitamin A, used as a positive
control. The animals were killed on day 20. The largest dose of endrin
caused maternal toxicity, as evidenced by weight loss and mortality
(two animals). The fetuses showed some slight growth retardation (not
significant) but no increase in intrauterine death rate. No effect
attributable to endrin was seen on the mean number of viable fetuses,
post-implantation losses, implantations, corpora lutea, fetal sex
ratio, or fetal external, soft-tissue, or skeletal abnormalities. Bent
ribs were observed in 6/522 fetuses treated with endrin, but not in
relation to dose. An increase in delayed ossification in sternebrae
and skull of fetuses was seen in the treated groups in comparison with
the untreated control group. Animals given vitamin A had a
significantly increased number of post-implantation losses and
malformed fetuses (Goldentahl, 1978a).
Groups of 32, 15, 28, 30, and 15 female CD rats were given oral
doses of endrin (99%) in corn oil at 0, 0.075, 0.15, 0.30, or 0.45
mg/kg body weight on days 7-20 of gestation. Rats were killed on day
21. Maternal weight gain was reduced at dose levels above 0.15 mg/kg,
but no increase in maternal liver weight was found. Fetal mortality,
weight, degree of skeletal and visceral maturation, and incidences of
skeletal and visceral anomalies showed no dose-related effect
(Kavlock et al., 1981).
8.4.2.3 Hamster
Three groups of golden Syrian hamsters (7, 24, and 8
animals/group) received a single oral dose of endrin (99%) in corn
oil at 5 mg/kg body weight (stated to be half the LD50) on day 7,
8, and 9 of gestation, respectively. Two control groups were used,
consisting of 57 untreated and 41 vehicle controls. The animals were
killed on day 14. The number of resorptions and of dead fetuses was
increased after treatment on days 7 or 8 and to a lesser extent in the
vehicle controls. The live fetuses in all three treated groups showed
significant growth retardation when compared with controls. The
incidence of anomalies was high only after treatment on day 8:
congenital abnormalities were seen in 28% of fetuses, with open eye in
22%, webbed foot in 16%, cleft palate in 5%, and fused ribs in 8%. The
anomalies that appeared to be increased significantly but to almost
the same extent at all three stages were fused ribs and cleft palate
(Ottolenghi et al., 1974).
These results could not be repeated by other workers. Groups of
27-29 golden Syrian hamsters were administered oral doses of endrin
(97%) in methocel at 0 (two control groups), 0.1, 0.75, or 2.5 mg/kg
body weight on days 4-13 of gestation. The animals were killed on
day 14. Body weight gains of the animals given 2.5 mg/kg were
slightly reduced. Maternal appearance, behaviour, and survival, mean
number of viable fetuses, post-implantation losses, implantations,
corpora lutea, fetal body weight, and crown-rump length showed no
change attributable to treatment. The number of malformations in
fetuses was not increased, but ossification of the sternebrae and
certain ribs was delayed (Goldentahl, 1978b).
Groups of 18-87 golden Syrian hamsters (LVG strain) were given
endrin (98%) as a solution in corn oil by gavage either as a single
dose of 0.5, 1.5, 5, 7.5, or 10 mg/kg body weight on day 8 of
pregnancy or as multiple daily doses of 0.75, 1.5, 2.5, or 3.5 mg/kg
body weight on days 5-14 of pregnancy. All animals were killed on day
15. With single doses, no effect was found on maternal survival,
pregnancy rate, weight change or liver:body weight ratio. The only
sign of maternal toxicity was the occurrence of transient convulsions
2 h after dosing in one hamster given 10 mg. No compound-related
difference was noted in the number of implantation sites, fetal death
rate, or fetal weight; indicators of skeletal maturity were not
affected. A dose-related increase in the incidence of fused ribs was
found in the groups given 7.5 and 10 mg/kg; increased incidences of
meningo-encephaloceles were observed at 5 mg/kg and above, with no
dose-response relationship. No other compound-related skeletal or
visceral anomaly was noted. In the study of multiple doses, maternal
toxicity (reduced weight gain and increased mortality) and fetal
toxicity (increased mortality, reduced weight, reduced skeletal
ossification, and an increased percentage of irregular
supra-occipitalis) were observed at doses of 1.5 mg/kg and higher. No
treatment-related maternal or fetal effect occurred at 0.75 mg/kg per
day (Chernoff et al., 1979).
8.4.2.4 Perinatal behavioural development
Rats exposed perinatally to endrin at 0, 0.075, 0.15, or 0.3
mg/kg body weight from gestation day 7 through day 15 of lactation
showed no mortality and no influence on survival or growth. Pups of
mothers exposed to 0.15 or 0.3 mg were more active than those of
mothers exposed to 0.075 mg or those in the control group. No clear
difference in ambulation was noted, and at 90 days of age there was no
difference (Gray et al., 1981; Kavlock et al., 1987).
Golden Syrian hamsters (LVG strain) given endrin (98%) at 0 or
1.5 mg/kg body weight per day by gastric intubation on days 5-14 of
gestation had a persistent increase in locomotor activity. Offspring
of treated hamsters ambulated more than the controls in the open field
at 15 days, and long-term observation of activity in the figure-8 maze
indicated that a significant increase in this behaviour was still
present at 125 days of age. Other behaviour patterns, including
sexual, rearing and running, and wheel behaviour, were unaffected.
Dams repeatedly exposed to endrin at 0.75 and 1.5 mg/kg body weight
were markedly hypoactive under the same testing conditions in which
the pups were hyperactive. The dose of 1.5 mg/kg body weight killed
more than half of the dams (Gray et al., 1981; Kavlock et al., 1987).
8.4.3 Appraisal of reproductive effects
Endrin had no reproductive effects in three generations of rats
at a level of 2 mg/kg of diet, equivalent to 0.1 mg/kg body weight. It
had no teratogenic effect in mice, rats, or hamsters after oral
exposure during the period of organogenesis. The significance of the
anomalies observed in mice and hamsters by Ottolenghi et al. (1974) is
uncertain. Studies in the same strain of the same species using more
rigorous protocols and larger numbers of animals could not confirm
their findings.
The lowest-observed-adverse-effect level for maternal toxicity
was 1.0 mg/kg body weight in mice, 0.3 mg/kg body weight in rats, and
1.5 mg/kg body weight in hamsters. Embryotoxicity was observed at
doses of 1 mg/kg body weight in mice and 1.5 mg/kg body weight in
hamsters. The overall no-observed-adverse-effect levels in mice, rats,
and hamsters were 0.5, 0.15, and 0.75 mg/kg body weight, respectively
(Table 27).
8.5 Mutagenicity and related end-points
8.5.1 Effects on microorganisms
Endrin was not mutagenic in numerous studies using Salmonella
typhimurium (TA98, TA100, TA1535, TA1537, TA1538, TA1950, TA1978,
SL4525, SL4700), Escherichia coli (WP2 uvrA, WP2 uvr-, Gal
Rs, WP2, hcr, p3478, W3100), K-12 (Pol A1+/Pol1-), WP67,
CM611, and CM571 Bacillus subtilis (M45), Saccharomyces cerevisiae
(D3, D7), and Serratia marcescens (a21, a742), with or without
metabolic activation by rat or mouse liver S9 (Fahrig, 1974; Van Dijck
& van de Voorde, 1976; Ercegovich & Rashid, 1977; Simmon et al., 1977;
Nishimura et al., 1982; Waters et al., 1982; Glatt et al., 1983;
Moriya et al., 1983; Rashid & Mumma, 1986).
No mutagenic effect was observed in S. typhimurium strains
TA98, TA100, TA1535, or TA1537, with and without metabolic activation
with S9 from livers of Aroclor 1254-induced rats and hamsters in the
presence of five concentrations of endrin (0-10 000 µg/plate) (Zeiger,
1987; Zeiger et al., 1987). No mutagenic effect was observed in
S. typhimurium strains TA97, TA98, TA100, or TA102 with and without
metabolic activation with Aroclor 1254-induced rat liver microsome
fraction in the presence of seven concentrations of endrin (99.0%),
from 1 ng/plate up to 1 mg/plate (Mersch-Sundermann et al., 1988).
8.5.2 Point mutations in mammalian cells
Endrin was weakly mutagenic in 6-thioguanine-resistant mouse
FM3A cells (Morita & Umeda, 1984; abstract only).
8.5.3 Dominant lethal mutations
Endrin did not show detectable dominant lethality when given as
a single intraperitoneal dose (0.76 or 3.8 mg/kg body weight) or daily
oral doses (0.1 or 0.25 mg/kg body weight) for 5 days to seven or nine
male ICR/Ha Swiss mice, respectively. This study involved a sequential
mating procedure, in which one male was housed with three females for
one week, repeated for 8 weeks (Epstein et al., 1972).
8.5.4 Chromosomal and cytogenetic effects
Endrin at 10-5 and 10-4 M, but not at 10-6, produced a
dose-related increase in the percentage of M1 metaphases and a
dose-related decrease in that of M3 metaphases at 48 h in treated
LAZ-007 human lympoid cells. This effect is closely related to the
reduced rate of cell proliferation induced by endrin (Sobti et al.,
1983).
Intratesticular injection of 0.25 mg endrin in saline to three
albino rats doubled the percentage of chromosomal changes in
comparison with that in the single control when the testes were
studied histologically 10 days after the injection. Changes were
scored in 70-75 cells/animal (Dikshith & Datta, 1973). The use of a
single dose does not aid interpretation, and the increase in
chromosomal abnormalities may be related to cytotoxicity rather than
to a genetic effect. The relevance of this type of study in
mutagenicity testing is unknown.
Table 27. Teratogenicity and effects on reproduction of oral administration of endrin
Animal Exposure NOAEL LOAEL Reference
(strain) period
Rat (Long Evans) 3 generations, 1 litter 1 mg/kg diet 3 mg/kg diet (0.15 mg/kg bw): Hine (1965)
(0.05 mg/kg bw) increased mortality of pups in
F2 and F3 generations
Rat (Long Evans) 3 generations, 2 litters 2 mg/kg diet Hine (1968)
(0.1 mg/kg bw)
Mice (CD-1) Days 7-17 of gestation 0.5 mg/kg bw 1 mg/kg bw: maternal and fetal Kavlock et al. (1981,
weight, skeletal and visceral 1987)
maturity
Rat (CD) Days 7-20 of gestation 0.15 mg/kg bw 0.30 mg/kg bw: reduced Kavlock et al. (1981)
maternal weight gain
Rat (CD) Days 6-15 of gestation 0.5 mg/kg bw 2 mg/kg bw: maternal toxicity Goldentahll (1978a)
Hamster Days 5-14 of gestation 0.75 mg/kg bw 1.5 mg/kg bw: maternal and Chernoff et al. (1979)
fetal toxicity
Chromosomal studies were carried out on lymphocytes from eight
male workers exposed to endrin and from six unexposed workers from the
same work area. No increase in the frequency of chromosomal
abnormalities was found, whether taken individually or collectively
(Dean, 1977).
Chromosomal aberrations were found in meiotic cells of barley and
somatic cells of barley and Vicia faba grown from endrin-treated
seeds (Wuu & Grant, 1966, 1967a,b). After treatment of root tips with
0.1% endrin (EC20 solution) for 1.5-2 h at 10 °C, the function of the
spindle was destroyed and did not interfere with the spreading of the
chromosomes during squash preparation. The centromeric region became
distinct and visible in prophase-metaphase chromosomes. At higher
concentrations contraction, stickiness, and fragmentation of
chromosomes were seen (Bhowmik, 1978).
8.5.5 Host-mediated effects
In two studies, male CF1 mice were given single oral doses of
endrin in dimethyl sulfoxide at 3.75 or 7.5 mg/kg body weight. Control
mice were dosed with the solvent, and positive control groups were
given a single oral dose of ethylmethanesulfonate at 400 mg/kg body
weight. Saccharomyces cerevisiae JD1 suspensions were then injected
intraperitoneally into each mouse, and the suspensions of
S. cerevisiae were harvested and analysed after 5 h. No increase in
mitotic gene conversion was detected (Brooks, 1976).
8.5.6 Sister chromatid exchange
Endrin at concentrations of 10-6-10-4 mol/litre in dimethyl
sulfoxide (the latter dose was a cytotoxic concentration) failed to
increase the frequency of sister chromatid exchange significantly over
the control value in rat liver microsomal S9-activated and unactivated
incubation experiments using human lymphoid cells of the LAZ-007 cell
line (Sobti et al., 1983).
8.5.7 Effects in Drosophila melanogaster
Endrin was not mutagenic to Drosophila melanogaster after
injection at 0.2 µlitre of a 0.001% aqueous solution, in the Muller-5
test for recessive lethal mutations on the X-chromosome (Benes & Sram,
1969).
8.5.8 Effects on DNA
Endrin at 10-3 or 3 x 10-3 mol/litre did not induce mutation
in the adult rat liver epithelial culture/hypoxanthineguanine
phosphoribosyl transferase assay (Williams, 1979).
DNA repair was not elicited in primary cultures of hepatocytes
from CD-1 mice, Fischer 344 rats, or Syrian hamsters exposed to endrin
for 18 h together with tritium-labelled thymine deoxyribonucleotide
for incorporation during repair synthesis. DNA repair was measured
autoradiographically. In rat and hamster liver cell cultures, a
concentration of 10-3 mol/litre and in mouse liver cell cultures,
10-4 mol/litre endrin was tested (Maslansky & Williams, 1981).
Endrin did not induce unscheduled DNA synthesis in human lung
fibroblast cells with or without metabolic activation by rat liver
microsomes (five concentrations were tested, but they were not given
in the paper) (Waters et al., 1982).
Endrin at eight concentrations ranging from 0.5 up to 1000
nmol/ml did not induce unscheduled DNA synthesis in primary rat
hepatocytes or in a modified Ames test utilizing concentration
gradient plates and 10 bacterial tester strains (eight S. typhimurium
and two E. coli) (Probst et al., 1981)
8.5.9 Appraisal of mutagenicity and related end-points
Garrett et al. (1986) evaluated the activity of endrin in a
series of tests: for reverse mutation (point/gene mutations in
prokaryotes), forward mutation (point/gene mutations in eukaryotes),
differential toxicity (primary DNA damage in prokaryotes), enhanced
mitotic recombination, gene conversion and crossing-over, unscheduled
DNA synthesis (primary DNA damage in eukaryotes), sister chromatid
exchange, chromosomal breakage, and dominant lethality (chromosomal
effects). Endrin gave negative results in all these tests.
The vast majority of the data indicate that endrin is not
genotoxic; however, many of the studies would not reach current
standards, or they give insufficient data to allow an independent
assessment.
8.6 Long-term exposure
Groups of 20 male and 20 female Carworth rats (28 days old) were
given diets containing endrin at 0, 1, 5, 25, 50, or 100 mg/kg. With
100 mg, two males and one female survived for 2 years; with 50 mg,
four males but no female survived; and with 25 mg, 11 males and 5
females survived. Survival at the lower concentrations was comparable
to that of the control group. Males appeared to be less susceptible
than females to the toxic action of endrin. Signs of intoxication,
hypersensitivity to external stimuli, and occasional convulsions were
observed only at the two highest levels. The weight gain of females
fed 1, 5, or 25 mg/kg of diet was equal to or greater than that of the
controls after 40 weeks of feeding. In males fed 5 mg, growth
retardation occurred during the first 20 weeks only, while males that
received 25 mg showed significant reduction in body weight gain. The
body weight gain of males fed 1 mg was comparable to that of controls.
The liver:body weight ratios were increased in males fed 5 mg or more
and in females fed diets with 25 mg or more. Histopathological
examination of animals that died during exposure to the three higher
dietary levels revealed diffuse degeneration of the liver, kidneys,
brain, and adrenal glands. The few survivors at 50 and 100 mg showed
degenerative changes in the liver only. No histopathological change
was found in surviving rats fed 1, 5, or 25 mg/kg diet. There was no
increase in the incidence of neoplasia in the treated groups compared
to the control group (Treon et al., 1955).
This study indicates a no-effect level for endrin of 1 mg/kg of
diet (about 0.05 mg/kg body weight) but is inadequate in several
respects, e.g., survival rate, details of pathology, and
haematological and clinical chemical data are not reported.
8.7 Carcinogenicity
8.7.1 Oral administration
8.7.1.1 Mouse
Groups of 100 male and female C57B1/6J mice, an inbred strain
with a low incidence of tumours, and C3D2F1/J mice, a hybrid strain
with a high incidence of hepatomas in males and a high incidence of
mammary tumours in females, were fed endrin (99%) at dietary
concentrations of 0.3 or 3 mg/kg from the age of five weeks throughout
life. A control group consisted of 200 mice of each sex of each
strain. Except for all groups of female animals of the C3D2F1/J
strain, this part of the experiment was terminated at the 78th week
because of the early occurrence of high numbers of mammary
fibroadenomas in 70-90% of control and treated mice. Survival, growth,
food intake, and haematology were not impaired. Mice of both strains
fed 3 mg/kg diet occasionally developed convulsions in the early
stages of feeding but recovered and survived without signs of illness.
They generally showed the typical histological changes in the liver
characteristic of high doses of chlorinated hydrocarbon insecticides.
No effect was observed on mice fed 0.3 mg/kg diet. The tumour
incidence and type of tumours were not influenced by the feeding of
endrin, and it had no influence on the incidence of fibroadenomas in
female C3D2F1/J mice (Witherup et al., 1970).
Groups of 50 B6C3F1 mice of each sex were given endrin in the
diet for 80 weeks and were then observed for a further 10 or 11 weeks.
The initial doses of endrin (97%) (2.5 and 5 mg/kg of diet) were
poorly tolerated by males and were therefore reduced after 25 weeks to
1.2 and 2.5 mg/kg diet for males; but females received 2.5 and 5 mg/kg
diet during the whole experiment. The time-weighted average doses were
1.6 and 3.2 mg/kg diet for males and 2.5 and 5.0 mg/kg diet for
females. Matched controls consisted of groups of 10 mice of each sex;
pooled controls, used for statistical evaluation, consisted of the
matched control groups combined with 50 untreated male and 50
untreated female mice from similar bioassays of other chemicals. All
surviving mice were killed at 90 or 91 weeks. Mean body weight was not
affected, but the survival of males at the high dose was lower than
that of the controls. The survival of the low-dose males could not be
evaluated due to accidental administration of excessive quantities of
endrin to this group during week 66. The tumour (neoplastic lesions in
the liver) incidences in the high-dose males were higher than those of
the pooled or matched controls, but not significantly so, and the
increase was not considered to be related to the administration of
endrin (Fredrickson, 1978;NCI, 1978).
8.7.1.2 Rat
A study of groups of 20 male and 20 female Carworth rats
administered endrin at 0, 1, 5, 25, 50, and 100 mg/kg of diet was
reviewed in Section 8.6.1.1. Bearing in mind the limitations of this
study, such as small group sizes and low survival at the high doses,
no evidence of an increase in the incidence of neoplasia was found in
any of the groups (Treon et al., 1955).
Groups of 50 weanling Osborne-Mendel rats of each sex were fed
diets containing endrin (98%) at 2, 6, or 12 mg/kg for 29 months. The
control groups consisted of 100 males and 100 females. During the
first 10 weeks of the study, only half the nominal dietary
concentrations of endrin were fed. Signs of toxicity occurred in a few
animals in all treatment groups, mainly in females, and included
episodes of tremor and clonic convulsions with 'outcries', the
incidence of these signs being dose-related. Weight gain was
unaffected, and the survival rates in control and treated rats were
similar. The liver:body weight ratios were unaffected. A moderate (not
dose-related) increase in the incidence of centrilobular cloudy
swelling in the liver and of cloudy swelling of the renal tubular
epithelium was observed. The lungs of the animals fed endrin exhibited
a moderate increase in the incidence of congestion and focal
haemorrhages. The tumour incidence in the treatment groups was
comparable with that in control rats, and no difference in the type of
tumours was found (Deichmann et al., 1970a,b; Deichmann & MacDonald,
1971).
In a life-time study, groups of 24 male and 24 female
Osborne-Mendel rats (22 days old) were fed diets containing endrin at
0, 0.1, 1, 5, 10, or 25 mg/kg. Because 50% of the rats at 25 mg/kg
died within the first week, this group was restarted with 32-day-old
rats. Survival did not appear to be affected by treatment. The highest
incidence of malignant tumours in male and female rats occurred at 0.1
mg/kg, but the malignancies were not dose-related. Treated male rats
had a higher incidence of renal disease than controls, but this also
was not dose-related (details not available) (Reuber, 1978).
Groups of 50 Osborne-Mendel rats of each sex were fed endrin
(97%) in their diet for 80 weeks and then observed for 31 or 34 weeks.
Males received doses of 2.5 or 5 mg/kg diet; in females, the initial
doses of 5 and 10 mg/kg of diet were poorly tolerated and were reduced
after 9 weeks to 2.5 and 5 mg/kg. The time-weighted average doses were
2.5 and 5.0 mg/kg for males and 3 and 6 mg/kg for females. Matched
controls consisted of groups of 10 rats of each sex; pooled controls
used for statistical evaluation consisted of the matched control
groups combined with 40 untreated male and 40 untreated female rats
from similar bioassays of other chemicals. All surviving rats were
killed at 100-114 weeks. Body weights and survival were not affected
by administration of endrin. A slight increase in the incidences of
pituitary and thyroid tumours was observed, but no consistent
statistical significance or dose-response relationship was found
(Fredrickson, 1978; NCI, 1978).
8.7.1.3 Tumour promotion
No significant increase in the development of preneoplastic
changes (hyperplastic nodules) was observed in rat liver after partial
hepatectomy and initiation with N-nitrosodimethylamine or
N-2-fluorenylacetamide in combination with the administration of
endrin (Ito et al., 1980).
In vitro, endrin at levels of above 2.5 µg/ml appeared to
inhibit metabolic cooperation in the hypoxanthineguanine
phosphoribosyl transferase system using wild-type
6-thioguanine-sensitive V79 cells and variant 6-thioguanine-resistant
cells. Such inhibition is reported to be an index of potential tumour
promoting activity, although the test has not been validated (Kurata
et al., 1982).
Endrin stimulated protein kinase C activity in vitro only
slightly, whereas a representative endogenous ligand of protein kinase
C, syn-1,2-didecanoylglycerol, stimulated protein kinase C to a
maximal velocity (Moser & Smart, 1989).
8.7.2 Appraisal of carcinogenicity
One of several studies in mice suggests an increased incidence of
nonmalignant tumours in animals of one sex, but this study was
considered inadequate for assessing carcinogenicity because an
increased number of tumours was seen in controls. A second study using
a different mouse strain did not corroborate the increase in tumour
incidence. Several long-term feeding studies in rats provide no
evidence of a carcinogenic effect of endrin. Its tumour promoting
activity was tested in vitro using protein kinase C stimulation and
ATPase inhibition; the results do not suggest any overwhelming effect
in these systems. After a careful review of this evidence, and taking
into consideration the fact that most of the data indicate that endrin
is not genotoxic, the Task Group concluded that the data are
insufficient to indicate that endrin is a carcinogenic hazard to
humans.
8.8 Special studies
8.8.1 Nervous system
8.8.1.1 Electrophysiological studies
The effects of endrin on bioelectrogenesis was studied in
anaesthetized pigeons and squirrel monkeys with chronically implanted
electrodes. Endrin was administered intravenously to pigeons at doses
of 0.5-4 mg/kg body weight. Doses of 2-4 mg/kg and higher caused
seizure activity throughout the telencephalon; the lower dose levels
caused activity only in the ectostriatum, a telecephalic visual
projection area. At doses of 0.5-2 mg/kg body weight, endrin caused a
specific increase in the evocation of potentials in the ectostriatum
by stimulation of the nucleus rotundus, a diencephalic visual
projection area. Reticular formation functions were not or little
affected. Administration of endrin to squirrel monkeys at doses of
0.2-3 mg/kg body weight on 5 days/week intramuscularly in corn oil and
saline emulsion induced characteristic changes in the
electro-encephalogram (EEG), culminating in electrographic seizures;
these were transient and disappeared when endrin administration was
stopped. Seizures reappeared under stress conditions, however, several
months after endrin treatment (Revzin, 1966, 1980).
Groups of 20-60 Sprague-Dawley rats with previously implanted
electrodes were given endrin in peanut oil orally at 0.8, 1.7, or 3.5
mg/kg body weight per day on 5 days/week for 28 weeks. Dose-dependent
mortality occurred during the first week and again at the end of the
study. Most changes in the EEG were seen after one week of exposure:
these included severe bursts of multiple spikes accompanied by clonic
convulsions; other animals had runs of spikes without full-fledged
convulsions. The convulsions were usually preceded by a period of
hyperventilation. After a further week of exposure, the rats showed
normal EEG traces. Some irregular slow-wave activity was seen in
animals that were moribund in the last month of feeding (Speck &
Maaske, 1958).
The convulsive properties of endrin at 1-2 mg/kg body weight
were studied by intravenous injection in locally anaesthetized,
paralyzed male cats, in which electrodes were placed in the
subcortical structures of the brain. Endrin was dissolved in ethanol
(which itself stimulates or inhibits the central nervous system,
depending on dose). Changes in the EEG and evoked responses were
studied. Hypersynchrony, rhythmic bursts of spikes and waves, and
isolated spikes characterized the preictal state. Seizures were always
bilateral and symmetrical and of a general tonic-clonic type.
Responses in sensory and motor cortexes to sensory nerve stimulation
were enhanced three to five fold. The authors concluded that endrin is
directly toxic to the mammalian nervous system, is a potent rapidly
acting convulsant, and does not require metabolic activation to an
active metabolite (Joy, 1976).
8.8.1.2 Histopathological studies
Male CD1 Swiss mice were administered endrin or sesame oil daily
by intraperitoneal injection in gradually increasing doses of 1.5-4.0
mg/kg for 4-20 days. Electron microscopic examination of sciatic nerve
tissue revealed no morphological change in myelinated nerve fibres,
myelin, or associated Schwann cells, but morphological alterations
were observed in unmyelinated nerve fibres and associated Schwann
cells: axons were swollen, microtubules and neurofilaments showed
dissolution, axoplasm was replaced by large clear vesicles,
vacuolization was present, and Schwann cells and adaxonal spaces also
contained vesicles (Walker & Phillips, 1987; abstract only).
8.8.1.3 Neurotransmitter systems
gamma-Aminobutyric acid systems: The role of the inhibitory
neuro-transmitter of the central nervous system, gamma-aminobutyric
acid (GABA),in the production of convulsions is well established.
Polychloro-cycloalkane insecticides such as endrin have a potent
excitatory action on the nervous system, and the interaction between
GABA function and endrin has been studied.
Endrin strongly inhibited GABA-dependent 36Cl uptake by mouse
brain vesicles, with an IC50 (the concentration required to cause
50% inhibition) of 2.8 µmol/litre. Inhibition was confined to that
portion of 36Cl uptake that is GABA-dependent. The result
demonstrates disruption of GABA ionophore function in mammalian brain,
possibly providing the principal mechanism of toxicity (Bloomquist &
Soderlund, 1985). In a comparison of the inhibitory potential of
several polychlorocycloalkane insecticides on GABA-dependent 36Cl
uptake, the most potent inhibitor was 12-ketoendrin, followed by
isobenzan, endrin, and then dieldrin, heptachlor epoxide, aldrin,
heptachlor and lindane. This order closely parallels their acute
toxicities (Bloomquist et al., 1986).
The effect of these chemicals was also studied in the tert
butylcyclo-phosphorothioate (TBPS) system, which has been shown to
bind convulsants with varying affinities. The IC50 for endrin on
35S-TBPS binding was 0.22 µmol/litre and that for 12-ketoendrin,
0.036 µmol/litre. These were the most potent inhibitors of TBPS
binding, and there was a significant linear correlation between 36Cl
flux and TBPS binding (Bloomquist et al., 1986).
In vitro, endrin inhibited 35S-TBPS binding in tissue from
male Swiss-Webster mice with an IC50 of 18 nmol/litre (range, 4-90).
In vivo, doses representing 25, 50, and 100% of the LD50 (8 mg/kg
intraperitoneally) inhibited 35S-TBPS binding with IC50s of 77 ±
7 nmol/litre (LD50) and 39 ± 6 nmol/litre (LD50/2); no inhibition
was observed at LD50/4, indicating a possible no-observed-adverse-
effect level. Brain P2 membranes of treated mice contained endrin and
12-ketoendrin. The finding that the brains of treated mice contained
sufficient endrin or its biotransformed products to achieve TBPS
binding and that this was correlated with the severity of the
poisoning indicates that the acute toxicity of endrin to mammals is
regulated by GABA (Cole & Casida, 1986).
GABA-induced 36Cl flux into membrane microsacs was inhibited
by endrin at 3.9 ± 0.2 nmol/mg protein, which also suggests that
endrin inhibits the function of this receptor (Abalis et al., 1985,
1986). The IC50 for 36Cl influx was 0.19 ± 0.06 µM and that for
35S-TBPS binding was 0.003 µM (Gant et al., 1987).
Endrin inhibited both insect and rat GABA receptors in a
dose-related, non-competitive manner. It acts in a similar manner on
the GABA receptors in the central nervous system of the two species.
The blocking action may involve non-competitive binding to an
allosteric site associated with the receptor's chloride channel
(Wafford et al., 1989a).
Endrin potentially inhibits 35S-TBPS binding to rat brain
membranes and also potentiates the protective effect of NaCl (200 mM)
against heat inactivation of 3H-flunitrazepam binding sites on the
same membranes. The time courses of heat inactivation of these binding
sites in the presence of NaCl and saturating concentrations of endrin
revealed monophasic components constituting about 88% of the binding
sites (Squires & Saederup, 1989).
Endrin has also been shown to inhibit GABA-ergic function in
Torpedo fish (Matsumoto et al., 1988), chicken embryos (Seifert,
1988, 1989), the mosquito fish (Gambusia affinis) (Bonner &
Yarbrough, 1989), and the cockroach (Periplaneta americana) (Wafford
et al., 1989b).
Other amine systems: Studies on the effects of orally
administered endrin on the content of biogenic amines in the brain of
rats did not contribute to an understanding of the convulsive action
of endrin (Miller & Fink, 1973; Hrdina et al., 1974).
Cyclic AMP metabolism: Endrin did not affect adenylate cyclase
activity or inhibit the activity levels of synaptosomal
phosphodiesterase, enzymes involved in cyclic AMP metabolism, in rat
brain. The authors interpreted their results to support their
postulation that organochlorine insecticides exert their neurotoxic
action by selective inhibition of ATPases in synaptosomes (Kodavanti
et al., 1988).
ATPase systems: Inhibition of rat brain Na+-K+ATPase by
chlorinated insecticides varied considerably: endrin and dieldrin were
the least active in inhibiting both this enzyme and K+-stimulated
para-nitrophenyl phosphatase at a concentration of 2 x 10-5
mol/litre. Results of experiments on ATP-32Pi exchange suggest
that DDT is a powerful inhibitor of oxidative phosphorylation, which
may lead to depletion of ATP. This effect was much less evident with
endrin (Folmar, 1978).
Endrin caused about 15% inhibition of the activity of
Na+-K+ATPase in rat brain synaptosomes at the highest
concentration tested, 120 µM, and oligomycin-sensitive Mg2+-ATPase
in rat brain synaptosomes was significantly inhibited in a
concentration-dependent manner, to a maximal inhibition of 33% at the
highest dose. Endrin did not inhibit oligomycin-insensitive
Mg2+-ATPase, and it did not affect K+-stimulated
para-nitrophenyl phosphatase from rat brain synaptosomes; this
enzyme represents the dephosphorylation step of the overall reaction
to the Na+-K+ATPase. Oligomycin-sensitive Mg2+-ATPase in beef
heart mitochondria was significantly inhibited. The results of this
study suggest that the ATPase system in rat heart and central nervous
system is not selectively inhibited by endrin (Mehrotra et al., 1989).
Sodium channel: It has been demonstrated using voltage clamp
techniques in single cells that application of DDT prolongs the sodium
current, which in turn decreases the depolarizing after-potential to
initiate repetitive after-discharges in the cell. The repetitive
after-discharges facilitate synaptic transmission and result in
nervous system hyperexcitability, which at the functional level is
registered as tremors and eventually convulsions and death (Narahasi,
1987). Even if less than 1% of the sodium channels respond in this
manner to insecticides, it is sufficient to cause toxicity in the
animal. Narahasi (1987) reported these effects with pyrethroids and a
series of DDT analogues; such studies have not been carried out with
endrin. Lund & Narahasi (1983) suggested that because of the
similarity in the symptomatology of intoxication by the family of
organochlorine insecticides, the target site of endrin may also be the
sodium channels.
8.8.1.4 Appraisal of effects on the nervous system
The effect of endrin on the nervous system has received attention
because it has the well established ability to cause convulsions
following acute exposures. Endrin causes considerable changes in EEG
activity, which are associated with convulsions, at intramuscular
doses in experimental animals as low as 0.2 mg/kg body weight.
The probable underlying mechanisms are associated with a
dose-related, non-competitive inhibition of the GABA-ergic
neurotransmitter system. This is an inhibitory system, and removal of
its action leads to increased excitation in the nervous system. While
inhibition of GABA-ergic function is common to a number of
polychlorocycloalkane insecticides, endrin, and particularly its
metabolite 12-ketoendrin, have been shown to be extremely potent
inhibitors of this function. It appears therefore that the acute
toxicity of endrin is due to disruption of GABA-related mechanisms.
8.8.2 Cardiovascular system
Studies have been conducted on the physiological effects of
endrin on the peripheral vascular system, renal function, renal
haemodynamics, and the cardiovascular system of the dog (Emerson et
al., 1963, 1964; Reins et al., 1964; Emerson, 1965; Emerson & Hinshaw,
1965; Reins et al., 1966; Hinshaw et al., 1966; Reddy et al., 1967).
After a lethal dose of endrin was administered intravenously, most of
the effects appeared to be the direct or indirect result of the
stimulating action of endrin on the central nervous system.
Bradycardia, hypertension, salivation, hyperexcitability, tonic-clonic
convulsions, increased body temperature, leukocytosis,
haemoconcentration, and decreased blood pH were seen. Elevation of
cerebral venous pressure and cerebrospinal fluid pressure were also
prominent features. Increased levels of adrenaline and noradrenaline
in blood plasma cause increased venous return and cardiac output and
increased arterial blood pressure in the absence of a rise in total
peripheral resistance. There was a large increase in total limb
vascular resistance and also a decrease in renal blood flow due to
arteriolar vasoconstriction. In studies on intact dogs and isolated
heart-lung preparations, high doses of endrin appeared to have a toxic
action on the left ventricle of the heart, causing sudden left heart
failure.
Aldrin, dieldrin, and endrin inhibited rat brain synaptosomal
and heart sarcoplasmic reticulum in vitro in a
concentration-dependent manner. Calmodulin-depleted Ca2+ pump
activity was insensitive to the action of these compounds. Oral
administration of endrin at 0.5-10 mg/kg to rats similarly decreased
Ca2+ pump activity, in addition to decreasing the levels of
calmodulin in both brain and heart, indicating disruption of membrane
Ca2+ transport mechanisms. Exogenous addition of calmodulin
(1-20 µg) effectively reversed the endrin-induced inhibition. Ca2+
pump activity in brain is more sensitive to endrin than that in heart.
The results indicate that endrin may produce neurotoxic effects by
altering calmodulin-regulated calcium-dependent events in neurons
(Mehrotra et al., 1989).
8.8.3 Effects on liver enzymes
It is well known that chlorinated hydrocarbon insecticides such
as DDT and dieldrin stimulate hepatic microsomal drug metabolism,
stimulating the activity of enzymes for the metabolism of drugs and
endogenous compounds such as hormones (Kinoshita & Kempf, 1970).
8.8.3.1 Mouse
A single oral, convulsive dose of endrin (20 mg/kg body weight)
dissolved in corn oil was administered to 9-week-old male
Swiss-Webster mice. Control groups consisted of a group of untreated
mice and a group receiving corn oil. When convulsions began, blood
serum was examined for serum glutamic oxaloacetic transaminase, serum
glutamic pyruvic transaminase, and serum lactic dehydrogenase. The
activities of the three enzymes were significantly increased above
those seen in the two control groups (Luckens & Phelps, 1969).
After intraperitoneal injection of a single dose of endrin at
6.25 mg/kg body weight to mice, hexobarbital sleeping time was
decreased, starting 3 h after the injection and lasting for 3 days
(Hart & Fouts, 1963). Stimulating effects on the hepatic
mixed-function oxidase system were reported in ICR mice after single
oral doses of 4 and 10 mg/kg body weight (Hartgrove et al., 1977).
8.8.3.2 Rat
Feeding Sprague-Dawley rats on diets containing endrin at 1, 5,
25, 50, or 100 mg/kg for 16 weeks caused high mortality in all groups,
especially among male rats. The serum alkaline phosphatase
concentration was reported to be dose-relatedly increased in all
groups as compared to control animals. The effect was clearest in the
groups fed 25 mg/kg of diet or more (Nelson et al., 1956).
In strain FW 49 rats, a single oral dose of endrin at 5 mg/kg
body weight had no effect on pentobarbital sleeping time; 10 mg/kg
caused a significant reduction, which, however, disappeared after 10
days (Schwabe & Wendling, 1967).
Endrin caused a significant shortening of the duration of the
paralysis induced by zoxazolamine in male Sprague-Dawley rats aged
5-6 weeks. Endrin was injected intraperitoneally at 2 mg/kg body
weight daily for 3 days, and zoxazolamine was injected
intraperitoneally on the fourth day (Truhaut et al., 1974).
Male rats given single oral doses of 2.5, 3.75, or 5.0 mg/kg body
weight showed no effect on the various parameters (details not given)
of mixed-function oxidase activity after 12 h, but the level of
microsomal protein and electron transport components per gram of liver
were significantly increased after 108 h, in a dose-dependent fashion.
Thiopentone and pentobarbital sleeping times were reduced by a 24-h
prior intraperitoneal injection of endrin at 5 mg/kg body weight
(Kachole & Pawar, 1977).
A single oral dose of endrin at 10 mg/kg body weight to male
albino rats increased serum glutamic oxaloacetic transaminase and
glutamic pyruvic transaminase activities, and decreased ATPase, acid-
and alkaline phosphatase, succinic dehydrogenase, and
glucose-6-phosphatase activities significantly 2-48 h after treatment
(Meena et al., 1978). After three successive daily oral doses of
endrin at 15 mg/kg body weight to Sprague-Dawley rats, significant
increases in total lipids and triglycerides in liver and in serum
glutamic pyruvic transaminase activity were seen (Borady et al.,
1983).
When two groups of six adult female rats were fed 0 or 28.7 µg/kg
body weight, endrin accumulated in the liver (5.47 mg/kg),and its
concentration in blood increased progressively up to 28 days. Growth
was depressed. The activities of the enzymes aspartate amino
transferase and alanine amino transferase were slightly increased
(Illahi et al., 1986). Similar results were obtained in a study in
which rats were fed 20 µg/kg body weight for 28 days (Illahi et al.,
1987).
8.8.3.3 Guinea-pig
Groups of six female guinea-pigs were administered three
successive intraperitoneal injections of endrin in sunflower oil at 3
mg/kg body weight, and liver and kidneys were studied 24 h after the
last injection. Treatment caused a significant increase in liver
weight and a decrease in hepatic microsomal protein content; renal
weight and renal microsomal protein content were not affected. Hepatic
cytochrome b5 and cytochrome-c reductase activities were increased,
while cytochrome P450 and total haem levels were significantly
decreased. Related to the decrease in cytochrome P450 was a decrease
in TPNH-linked aminopyrine-N-demethylation, but an increase in
DPNH-linked demethylation was related to the increase in cytochrome b5
and cytochrome-c reductase. Lipid peroxidation was increased in both
liver and kidneys (Pawar & Kachole, 1978).
8.8.3.4 In-vitro studies
To test the possibility that phenobarbital induces cytochrome
P450p indirectly by increasing the availability of endogenous
glucocorticoids in the liver, phenobarbital and phenobarbital-like
inducers, including endrin, were added to primary monolayer cultures
of adult Sprague-Dawley rat hepatocytes incubated in serum-free medium
without glucocorticoids. De-novo synthesis of cytochrome P450p,
measured as increased incorporation of 3H-leucine into
immunoprecipitable P450p protein, was increased. Endrin at a
concentration of 1x10-5 M was half as potent as phenobarbital at 2
x 10-3 M (Schuetz et al., 1986).
8.8.4 Miscellaneous studies
Endrin inhibited rabbit muscle lactate dehydrogenase in vitro
(Hendrickson & Bowden, 1976). Exposure of isolated rat enterocytes to
endrin reduced the efficiency of the neuropeptide vasoactive
intestinal peptide after stimulation of cyclic AMP accumulation, as
was observed with lindane (Carrero et al., 1989).
Endrin at single oral doses of 25 mg/kg body weight or daily
doses of 1 mg/kg body weight daily for 8 days induced various shifts
in the mobilization of the ions of biologically important metals such
as magnesium, iron, zinc, and copper from liver, kidneys, brain,
heart, spleen, and blood (Coleman et al., 1968; Lawrence et al.,
1968). Rats receiving intraperitoneal injections of 1 mg/kg body
weight in peanut oil over periods up to 19 days showed no alteration
in the concentrations of serum proteins or serum lipoproteins,
separated by paper electrophoresis, or of albumin, alpha 1, alpha 2,
beta, or gamma globulins. Protein-bound sialic acid and methylpentose
were increased only temporarily; the level of bound hexose increased
with time and that of bound hexosamine decreased (Coleman, 1968).
Rats receiving a single oral dose of 50 mg/kg body weight, daily
intraperitoneal doses of 2 mg/kg body weight, or daily intramuscular
injections of 0.5 or 2.0 mg/kg body weight for 45 days showed
increased activity of a number of the enzymes that are involved in
gluconeogenesis in liver cells and cells of the renal cortex. A
significant decrease was noted in hepatic glycogen, an increase in
blood glucose and urea, as well as a significant rise in hepatic and
renal pyruvate carboxylase, phosphoenol pyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase. Furthermore,
endogenous levels of cyclic AMP were increased (Kacew et al., 1973;
Singhal & Kacew, 1976).
8.8.5 Factors that influence toxicity
8.8.5.1 Nutrition
The nutritional state of Wistar rats was found to alter their
susceptibility to the acute toxic action of endrin. Three groups of
approximately 100 rats were fed a normal diet, a diet containing
casein as the only source of protein, or a low protein diet for 28
days, and the acute toxicity of endrin was determined after a single
intragastric administration. The following LD50 values were
calculated: 27 mg, 17 mg, and 7 mg/kg body weight, respectively (Boyd
& Stefec, 1969).
8.8.5.2 Potentiation
The acute oral LD50s of equitoxic doses of combinations of 10
pesticides, including endrin, were studied in Swiss mice. No evidence
of potentiation was seen with combinations with dieldrin, diazinon,
malathion, toxaphene, parathion, DDT, or dioxathion, but more than
additive effects, i.e., possible potentiation, were found with
chlordane and possibly with aldrin (Keplinger & Deichmann, 1967).
Five groups of 20 male and 20 female Sprague-Dawley rats were fed
for 91 days on a diet containing a combination of 15 'persistent'
chemicals added at concentrations of 0, 1, 10, 100, and 1000 times the
water quality objective applied in Canada. For endrin, these
corresponded to 0.002, 0.02, 0.2, and 2.0 µg/kg of diet. No effect on
food intake, growth, clinical chemistry, bone marrow, or
histopathology were observed. It was concluded that the presence of
these chemicals at 1000 times the water quality objective had no
toxicological effect (Cote et al., 1985).
Six male and six female Sprague-Dawley rats were fed a control
diet or diets containing endrin at 5 or 10 mg/kg, endrin aldehyde at
10 mg/kg, or endrin ketone at 5 mg/kg for 15 days, at which time three
to six rats from each treatment group were given a single
intraperitoneal dose of carbon tetrachloride at 100 µlitre/kg body
weight in corn oil (1 mg/kg). Levels of serum enzymes, bile flow, and
biliary excretion of an anionic model compound, phenolphthalein
glucuronide, were measured on day 16. Dietary treatment with endrin at
either dose level did not elevate serum enzyme levels. Treatment with
5 mg/kg significantly reduced bile flow and a corresponding reduction
in phenolphthalein glucuronide excretion, whereas the 10 mg/kg dose
reduced only phenolphthalein glucuronide excretion in male rats.
Female rats treated with either dose showed a dose-dependent
choleretic effect with a commensurate increase in phenolphthalein
glucuronide excretion. Treatment of rats with endrin and carbon
tetrachloride did not result in potentiation of hepatobiliary
functions. The levels of some serum enzymes were elevated (two-fold)
in rats given endrin plus carbon tetrachloride over those in rats
given endrin or carbon tetrachloride alone, indicating an additive
interaction. Dietary treatment with endrin aldehyde slightly increased
the levels of serum glutamic oxaloacetic transaminase and glutamic
pyruvic transaminase; and endrin ketone induced a small elevation in
glutamic pyruvic transaminase levels. Neither compound altered bile
flow or biliary phenolphthalein glucuronide excretion. Combination
with carbon tetrachloride increased the levels of some serum enzymes
(two-fold) over those seen with the aldehyde or the ketone or carbon
tetrachloride alone (Young & Mehendale, 1986).
9. EFFECTS ON HUMAN BEINGS
9.1 Exposure of the general population
9.1.1 Acute toxicity
In mild cases of poisoning, dizziness, weakness of the legs,
abdominal discomfort, nausea, and vomiting have been reported. Some
patients have complained of temporary deafness or were slightly
disorientated or aggressive. The onset of poisoning is variable and
may occur 0.5-10 h after consumption of contaminated food or
contamination of the skin; the interval is usually 1-4 h, depending on
the quantity ingested. Severe poisoning is manifested by sudden
epileptiform fits, with frothing at the mouth, facial congestion, and
violent convulsive movements of the limbs, sometimes leading to
dislocation of a shoulder or other injury. The fits may last for
several minutes and may be followed by a period of semiconsciousness
for 15-30 min or until the next fit. In general, these convulsions
occur suddenly, with no prodromal sign or symptom. An uncommon but
very serious symptom observed in two children was hyperthermia (41 °C
or higher); the high fever was followed by decerebrate rigidity. In
fatal cases, death occurs within 2-12 h. In survivors, recovery is
rapid, within 24 h, and uneventful, although some patients have
complained of headache, dizziness, weakness, and anorexia for several
weeks (Davis & Lewis, 1956; Jacobziner & Raybin, 1959; Hoogendam et
al., 1962; Hayes, 1963; Weeks, 1967; Hayes, 1982). After clinical
recovery, EEG changes consisting of bilateral synchronous theta-wave
activity and occasional bilateral synchronous spike and wave
complexes, believed to be associated with brain stem irritation, may
still be found and may persist for up to several weeks (Hoogendam et
al., 1962, 1965; Weeks, 1967).
9.1.2 Poisoning incidents
Hayes (1982) reviewed poisoning cases caused by endrin. Outbreaks
of acute intoxication due to endrin have occurred by contamination of
flour during transport in railway cars. A first episode, which was
well studied, occurred in 1956 in Wales, United Kingdom (Davis &
Lewis, 1956): At least 59 people were ill enough to require medical
treatment, and at least 100 more had some symptoms, which were not
severe enough to require medical advice. No one died. On the basis of
the concentration of endrin in bread prepared from the flour (150
mg/kg), Hayes (1963) estimated that 0.20-0.25 mg/kg body weight may
cause a single convulsion and that the dose necessary to produce
repeated convulsions is about 1 mg/kg body weight. Karplus (1971)
estimated the lethal dose in man to be approximately 10 mg/kg body
weight.
A few conflicting data are available on the concentration of
endrin in the tissues of victims of fatal intoxication. Hayes (1982)
quoted levels of 7-10 mg/kg in the liver and 0.7-4.4 mg/kg in the
brain; however, 10-fold lower levels were reported in the tissues of
autopsied victims of an outbreak of poisoning caused by ingestion of
bread prepared from contaminated flour in the Middle East (Curley et
al., 1970). In another incident, two sacks of contaminated flour
contained endrin at 184.5 and 234.5 mg/kg, and the bread and rolls
prepared from the contaminated flour contained 125.67-176.11 mg/kg.
The levels of endrin in serum, collected 30 min, 20 h, and 30 h after
convulsions in one person were 0.053, 0.038, and 0.021 mg/litre,
respectively; three other cases had 0.003-0.004 mg/litre of blood
serum 9-19 h after convulsions. One of these three people had no
symptoms (Coble et al., 1967). The reported serum or blood levels of
endrin associated with convulsions must be interpreted in the context
of the rapid removal of endrin from blood and the often significant
time lag in taking blood samples after convulsions. When the time
between convulsion and blood sampling is long, the endrin levels
reported are likely to be much lower than those at the time of the
convulsion.
Four outbreaks of endrin intoxication occurred in Doha (Qatar)
and Hofuf (Saudi Arabia) in 1967, during which 874 people were
hospitalized of whom 26 died; another 500-750 people showed symptoms
of intoxication but required no hospitalization. These outbreaks were
due to contamination of flour by endrin leaking from drums during
shipment. The endrin concentrations found in bread were 48-1807 mg/kg,
and those in the blood of patients were 0.007-0.032 mg/litre (Weeks,
1967; Curley et al., 1970).
Between July and September 1984, an epidemic of endrin poisoning
occurred in Pakistan, resulting in acute convulsions. In 18 of 21
affected villages surveyed, 70% (106/152) of the cases for which age
was known were in children aged 1-9 years; 9.8% (19/194) of the
affected people died. A composite sugar sample taken from the houses
of three cases contained endrin at 0.04 mg/kg. Endrin was detected in
the blood of 12/18 patients, at levels of 0.3-254.0 µg/litre of serum.
It was also determined in brain, kidneys, adipose tissue, and liver of
one person and found at levels of 1680, 1760, 4010, 1430 µg/kg
respectively (Anon., 1984; Hill et al., 1986; Rowley et al., 1987).
In mid-March 1988, three members of a family in Orange County,
California, USA, became ill within 1 h of eating taquitos (baked corn
shell filled with spicy meat and salad). Two of the three had multiple
grand mal seizures. Subsequently, two other people were reported to
have had seizures less than 12 h after eating taquitos. All five
patients had obtained the taquitos from the same shop within 5 days.
The food was analysed, and the presence of endrin was confirmed but
not quantified. The origin of the endrin could not be identified
(Anon., 1989).
An episode of acute endrin poisoning was reported in 33 Mexican
children, who had sudden seizures without sensory alterations (Singh
& West, 1985).
Several other cases have been published of single accidental or
intentional intoxications, in children and in adults (Jacobziner &
Raybin, 1959; Karplus, 1971). Reddy et al. (1966) described 60 cases
of fatal endrin poisoning out of 95 encountered in India after the
introduction of endrin in agricultural work as an insecticide in 1959.
The majority of the cases were suicidal. Froth, petechial
haemorrhages, a kerosene-like smell and massive pulmonary oedema were
the characteristic autopsy findings. Respiratory failure was the most
common cause of death. The authors concluded that the toxic dose of
endrin is 5-50 mg/kg body weight or about 1 g; the lethal dose is
about 6 g. In a poisoning case in a 19-year-old male who ingested an
unknown amount of endrin, convulsions and gross pulmonary oedema were
found (Jedeikin et al., 1979). No histological changes were found in
the liver. At least some of the pulmonary changes seen in such cases
may be due to aspiration of the petroleum hydrocarbon solvent in
formulations of endrin.
A case of polyneuropathy of the Guillain-Barré type was
attributed to exposure to a mixture of DDT and endrin. Since
convulsions were not recorded, the causal relationship remains
doubtful (Jenkins & Toole, 1964).
In a fatal case of endrin poisoning, ingestion of 12 g of endrin
by a 49-year-old man caused convulsions (persisting for 4 days),
hypersalivation, hyperthermia, renal insufficiency, thrombocytopenia,
and recurrent hypotension. Death followed after 11 days due to
pulmonary complications (infection and haemorrhage) and hypoxaemia
causing bradycardia and cardiac arrest. The endrin concentrations in
blood 4 h and 6 and 11 days after ingestion were 450, 86 and 71
µg/litre. Endrin levels in adipose tissue, heart, brain, kidneys, and
liver, 11 days after ingestion were 89.5, 0.87, 0.89, 0.55, and 1.32
mg/kg, respectively (Runhaar et al., 1985).
The medical treatment of endrin poisoning is described in Annex
II.
9.2 Occupational exposure
9.2.1 Factory workers
No fatal case has been reported due to occupational exposure in
manufacturing and formulating plants (Van Raalte, 1965; Jager, 1970),
which may be due in part to underreporting but is also certainly due
to the fact that occupational exposure involving the absorption of
lethal doses occurs rarely under practical circumstances. Furthermore,
the rapid metabolism of endrin minimizes build-up of toxic levels in
tissues during normal working days.
Several cases of acute, non-fatal poisoning occurred in a
manufacturing plant in The Netherlands due to accidental over-exposure
to endrin (Jager, 1970). Endrin had been manufactured in this plant
since 1957. During the first 9 years of production of aldrin,
dieldrin, and endrin in the plant, 17 cases of poisoning with
convulsions occurred, five of which involved more than one convulsion.
Three of the cases were due to acute over-exposure to endrin among
workers who were handling these materials at high concentrations every
day. There was no fatality during 1300 man-years of exposure. No
evidence was found of skin sensitization, and there was no case of
permanent, partial, or complete incapacity. No difference was seen in
absenteeism due to illness among these workers in comparison with
those in other plants, and the results of liver function tests and
complete blood cell counts were within normal limits. In the cases of
poisoning, recovery from clinical and neurological signs, including
EEG tracings, was rapid and complete (Hoogendam et al., 1962, 1965;
Jager, 1970; Versteeg & Jager, 1973).
A series of studies has been published on the results of
continuing medical supervision of workers in this plant. A
complementary follow-up of 189 workers and of 52 workers who had left
employment at the plant for various reasons was published in 1973
(Versteeg & Jager, 1973). These workers had been exposed to endrin for
up to 14.5 years in 1973. In agreement with data published from a
study of 71 workers in an endrin manufacturing plant in the USA (Hayes
& Curley, 1968), endrin was not found in the blood of these workers,
except in cases of accidental, acute over-exposure. Medical
supervision of workers employed in the manufacture and formulation of
endrin and other pesticides for 1-19 years (average, 12 years), data
on absenteeism, the results of tests for liver function and blood
chemistry, blood morphology, urine analysis, the occurrence of
sensitization, the pattern and course of EEG changes in cases of
poisoning, other medical studies (including electrocardiography, chest
x rays, blood pressure, body weight), and the incidence and pattern of
diseases, including the occurrence of malignant growths, showed no
difference between workers exposed to endrin and other chemical plant
operators. Residues of endrin were not found in plasma (< 3 µg/litre)
or in adipose tissue (< 0.03 mg/kg).
A significant difference was found between workers exposed to
aldrin and dieldrin only, workers not exposed to insecticides, and
workers exposed to endrin only: endrin workers had lower blood levels
of the DDT metabolite para,para'-DDE than the other workers, and the
levels were lower than those in the general population of the
surrounding area, although DDT and related compounds had never been
manufactured in the plant. A second parameter that was compared was
excretion of 6-beta-hydrocortisol in the urine. (Increased activity of
the drug-metabolizing enzyme system increases the activity of the
oxidative pathway by which 6-beta-hydroxylase converts endogenous
cortisol to 6-beta-hydrocortisol and thus, relatively, diminishes the
contribution of the reductive pathway, leading to excretion of
17-hydroxycorticosteroids.) The ratio of the urinary excretion of
6-beta-hydroxycortisol to that of 17-hydroxysteroids was significantly
higher in the endrin workers than in workers not exposed to endrin
(Jager, 1970).
A third parameter of this enzyme system that was studied was
urinary excretion of D-glucaric acid (an end-product of the glucuronic
acid pathway in the liver), which has been shown to increase after
exposure to microsomal enzyme-stimulating compounds, like endrin
(Hunter et al., 1971; Notten & Henderson, 1975). In the endrin
workers, urinary excretion of D-glucaric acid after a week of exposure
increased significantly over pre-exposure levels and those in a
control group of workers. Excretion diminished again after 3 days
without exposure (Hunter et al., 1972; Ottevanger &Van Sittert, 1979;
Vrij-Standhardt et al., 1979; Van Sittert, 1985).
Since anti-12-hydroxyendrin is the only metabolite found in the
urine of endrin-exposed workers, a study was initiated to find whether
there is a correlation between the quantity of this metabolite and
that of D-glucaric acid excreted in the urine. A positive relationship
was found between excretion of the endrin metabolite and of D-glucaric
acid after 7 days. After exposure was discontinued, excretion of
anti-12-hydroxyendrin decreased faster than that of D-glucaric acid.
The fact that endrin-exposed workers had D-glucaric acid levels within
the normal range after 6 weeks indicates that enzyme induction in
endrin workers is reversible. The authors concluded that a urinary
level of anti-12-hydroxyendrin of 0.130 µg/g of creatinine is the
threshold exposure level, below which enzyme induction is not produced
(Ottevanger & Van Sittert, 1979; Van Sittert, 1985). Endrin did not
increase total urinary porphyrin excretion over that in a control
group of employees (Strik, 1979; Nagelsmit et al., 1979;
Vrij-Standhardt et al., 1979).
In a follow-up mortality study of the same group of workers,
vital status and cause of death were assessed for 232 of a group of
more than 1000 workers. This group was selected because they had
experienced high exposures in the initial years of manufacture and
formulation and because of the long periods of exposure (mean, 11
years; range, 4-27) and observation (mean, 24 years; range, 4-29).
Total observed mortality was 25, whereas 38 deaths were expected on
the basis of mortality statistics for the male Dutch population. Of
the nine cancer deaths, three were due to lung cancer; the remaining
six were due to cancers of stomach, pancreas, bladder, and kidney,
multiple myeloma, and cerebral glioma. It was concluded that the
pesticides manufactured had no specific carcinogenic activity
(Ribbens, 1985).
9.2.2 Dose-response relationships
It has not been possible to establish a dose-response
relationship between single or repeated oral exposures and endrin
concentrations in blood, adipose tissue, or organs and severity of
intoxication, because the actual oral intake in the accidental cases
was not known, and the onset of symptoms of intoxication and the time
of measuring concentrations of endrin in blood, organs, or tissues
were not comparable (Davis & Lewis, 1956; Hayes, 1963; Coble et al.,
1967; Weeks, 1967; Curley et al., 1970; Karplus, 1971; Hayes, 1982;
Anon., 1984).
Blood samples have been analysed in three cases of acute
over-exposure (Table 28): A formulator and an operator were
accidentally splashed with a 20% endrin emulsifiable concentrate,
which was washed off within 10 min. Neither developed signs or
symptoms of intoxication. The third case was in a formulator who
handled technical-grade endrin powder without wearing a dust-mask. He
had convulsions 4 h after starting work, but after treatment recovered
fully the next day. Blood samples from four colleagues working next to
him, but wearing dust-masks, were also examined. The author estimated
that the threshold level of endrin in the blood below which no sign or
symptom of intoxication occurs is 50-100 µg/litre and that the
half-life of endrin in blood is in the order of 24 h (Jager, 1970).
9.2.3 Exposures to mixtures
A retrospective mortality study was carried out on workers
employed in the manufacture of heptachlor and endrin in a plant in
Tennessee, USA, between 1952 and 1976. The study comprised 835 men who
had worked for more than 3 months up to 20 years at the plant. No
overall excess of deaths from cancer was found; however, there was an
excess of deaths from cerebrovascular disease (7 observed, 2.3
expected) (Wang & MacMahon, 1979).
A further retrospective cohort study was conducted to examine the
mortality of workers employed in the manufacture of chlordane,
heptachlor, DDT, aldrin/dieldrin, and endrin in a plant in Colorado,
USA, where endrin was manufactured from 1953 until 1965. Approximately
2100 workers who had been employed for at least 6 months in the plants
were involved. No excess of cerebrovascular disease was observed
(Ditraglia et al., 1981).
Neither study proves conclusively that exposure to these
organochlorine insecticides is associated with increased prevalence of
malignancy or other cause of death, but they are limited in design and
in the desciption of exposure.
A field study was carried out in 1983 in the Ivory Coast to
assess the health hazards associated with the handling and application
by hand-held sprayers of an ultra-low volume formulation consisting of
endrin at 85 g/litre, DDT at 333 g/litre, and methylparathion at 85
g/litre in petroleum solvent. Groups of five or six farmers sprayed 3
litre/ha of the formulation 4-6 weeks after sowing cotton and again 15
or 30 days after the first application. The spray apparatus was filled
and cleaned by the same men. The recommended protective clothing was
worn only rarely, and the handling and application techniques were
careless, resulting in many cases in appreciable skin contamination.
No adverse health effect was observed. Absorption of endrin was
monitored by measuring the concentration of anti-12-hydroxyendrin in
spot samples of urine collected about 20 h after spraying. The mean
concentrations after the first, second, and third applications were
0.34 (range, 0.04-0.59), 0.52 (range, 0.09-1.4), and 0.45 mg/g of
creatinine (range, 0.0-0.92). One person who had handled and sprayed
the formulation carefully still had anti-12-hydroxyendrin in the
urine after the third application, but at a very low level (0.03 mg/g
of creatinine). Measurements of para-nitrophenol, a metabolite of
methyl-parathion, in urine indicated that the rate of metabolism of
methylparathion was increased as a result of enzyme induction by
endrin in the liver (Kummer & Van Sittert, 1984, 1986). It was
concluded that endrin accumulated in most of the farmers after three
applications within a short period. An increase to toxic levels might
result if spraying were more frequent and at shorter intervals and if
the recommended clothing was not worn.
9.2.4 Appraisal of effects of occupational exposures
Endrin is a very toxic compound. Several episodes of fatal and
non-fatal poisoning have occurred, mostly from accidental
contamination of food and also from intentional (suicidal) ingestion.
The lethal oral dose is estimated to be 10 mg/kg body weight. In
non-fatal cases, recovery is rapid and complete within a few days. The
oral dose that causes a single convulsion is estimated to be 0.25
mg/kg body weight, and that which induces repeated convulsions, 1.0
mg/kg body weight.
Exposure of workers to endrin for long periods did not induce
adverse effects that were attributed to this compound, although
occasional cases of acute, non-fatal intoxication due to accidental
over-exposure have occurred. Endrin was not detected in the blood of
workers exposed to endrin at < 3.0 µg/litre. The threshold level of
endrin in blood that results in intoxication is estimated to be 50-100
µg/litre. Absorption of a toxic dose is therefore unlikely during
occupational exposure if recommended controls and precautions are
used. In fatal cases, endrin concentrations in blood as high as 450
µg/litre have been reported; however, it is not possible to establish
a dose-response relationship. Since endrin is not normally found in
air, water, or food, except under conditions of contamination,
exposure of the general population is not significant.
Table 28. Concentrations of endrin in blood from acutely over-exposed workers
Case Time of first Endrin concentration (µg/litre)
sampling
First 12 h 24 h 36 h 5 days
sample later later later later
Formulator 1 h after 90 ND
accident
Operator 40 min after 27 25 ND
accident
Formulator Directly after 80 20 ND
without dust-mask convulsion
Four colleagues Same time ND-10
with dust-masks time as above
ND, not detectable (< 5 µg/litre)
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Endrin was evaluated by the Joint FAO/WHO Expert Committee on
Pesticide Residues in 1963, 1965, and 1970 (FAO/WHO, 1964, 1965,
1971). In 1970, the Committee established an acceptable daily intake
(ADI) for humans of 0-0.0002 mg/kg body weight, which was based on the
level that causes no toxicological effect in rats and dogs, 1 mg/kg of
diet (equivalent to 0.05 mg/kg body weight per day in rats and 0.025
mg/kg body weight per day in dogs).
The Joint FAO/WHO Codex Alimentarius Commission has published
maximum residue limits for endrin (Table 29; (FAO/WHO, 1986b).
Table 29. Codex maximum limits for the sum of residues of
endrin and delta-ketoendrin
Commodity Maximum residue limit
(mg/kg product)
Apples 0.02a
Barley 0.02a
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02a
Eggs (without shells) 0.2
Meat (carcass fat) 0.1b
Milk 0.0008b
Poultry (carcass fat) 1
Rice (husked or polished) 0.02a
Sorghum 0.02a
Sweet maize 0.02a
Wheat 0.02a
aAt or near the limit of detection
bExtraneous residue limit
The International Agency for Research on Cancer (IARC) concluded
in 1974 and 1987 that there was inadequate evidence for the
carcinogenicity of endrin in experimental animals and that the
evidence from studies in humans was inadequate. Endrin was therefore
classified in Group 3: not classifiable as to its carcinogenicity to
humans (IARC, 1974, 1987).
In 1988, the Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified technical-grade endrin as
highly hazardous in normal use (WHO, 1992). A data sheet on endrin was
issued in 1978 (WHO/FAO, 1975).
REFERENCES
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1985) High-affinity
stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic Biochem Physiol, 24: 95-102.
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1986) Effects of insecticides
on GABA-induced chloride influx into rat brain microsacs. J Toxicol
Environ Health, 18: 13-23.
Abbott DC, Harrison RB, Tatton JO'G, & Thomson J (1966) Organochlorine
pesticides in the atmosphere. Nature, 211(5046): 259-261.
Abbott DC, Goulding R, & Tatton JO'G (1968) Organochlorine pesticide
residues in human fat in Great Britain. Br Med J, iii: 146-149.
Abbott DC, Holmes DC, & Tatton JO'G (1969) Pesticide residues in the
total diet in England and Wales, 1966-67. Organochlorine pesticide
residues in the total diet. J Sci Food Agric, 20(4): 245-259.
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in United Kingdom. Br Med J, ii: 553-556
Abdel-Razik M, Marzouk MAH, Mowafy LE, & Abdel-Kader MA (1988)
Pesticide residues in the River Nile water, Egypt. Pak J Sci Ind Res,
31(11): 795-797.
Abdou SM, Abdel-Gawaad AA, Abdel-Amaim E, Abdel-Hady SM, & El-Alfy MB
(1983) Organochlorine pesticide residues in buffaloes milk in Kalubia
province and the effect of the presence of insecticides on coagulation
time. Egypt J Dairy Sci, 11: 197-203.
Acker L & Schulte E (1974) [Chlorinated hydrocarbons in human fat.]
Naturwissenschaften, 61: 32 (in German).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Seasonal fluctuations of
organochlorine and triazines pesticides in the aquatic system of
Ioannina Basin (Greece). Sci Total Environ, 58: 243-253.
Albers PH, Sileo L, & Mulhern BM (1986) Effects of environmental
contaminants on snapping turtles of a tidal wetland. Arch Environ
Contam Toxicol, 15: 39-49.
Albert LA (1990) Environmental contamination in Mexican food. In:
Hriagu JO & Simmons MS ed., Food contamination from environmental
sources. New York, John Wiley and Sons, pp 542-577.
Albert L, Mendez F, Cebrian ME, & Portales A (1980) Organochlorine
pesticide residues in human adipose tissue in Mexico. Results of a
preliminary study in three Mexican cities. Arch Environ Health, 35(5):
262-269.
Albert LA, Vega P, & Nava E (1982) [Organochlorine pesticides. VI.
Organochlorine pesticide residues in Mexican evaporated milks.]
Biotica, 7(3): 473-482 (in Spanish).
Alford-Stevens AL, Eichelberger JW, & Budde WL (1988) Multi-laboratory
study of automated determination of polychlorinated biphenyls and
chlorinated pesticides in water, soil and sediment by gas
chromatography/mass spectrometry. Environ Sci Technol, 22: 304-312.
Ali SL (1986) [Pesticide residues and traces of heavy metals in cod
liver oil.] Pharm Ztg, 131(38): 2288-2290 (in German).
Al-Omar MA, Al-Ogaily NH, Tawfiq SJ, & Al-Bassoumy M (1985a) Residue
levels of organochlorine insecticides in sewage plant effluent. J Biol
Sci Res, 16(1): 145-151.
Al-Omar MA, Tameesh AH, & Al-Ogaily NH (1985b) Dairy product
contamination with organochlorine insecticide residues in Bagdad
district. J Biol Sci Res, 16(1): 133-144.
Altmeier G & Korte F (1969) [Contributions to ecological chemistry
(XXIV). Metabolism of endrin-14C in perfused rats' livers.]
Tetrahedron Lett, 49: 4269-4271 (in German).
Anderson A (1986) Monitoring and biased sampling of pesticide residues
in fruits and vegetables. Methods and results, 1981-1984. Var Foda,
38(Suppl. 1): 8-55.
Anderson RL & Defoe DL (1980) Toxicity and bioaccumulation of endrin
and methoxychlor in aquatic invertebrates and fish. Environ Pollut
(Ser A), 22: 111-121.
Anderson HH, Hine CH, Kodama JJ, & Critchlow JK (1953) Class B
Determination on HI-1185 Endrin Emulsifiable Concentrate, San
Francisco, University of California, School of Medicine (UC Report No.
213).
Ang C, Meleady K, & Wallace L (1989) Pesticide residues in drinking
water in the north coast region of New South Wales, Australia,
1986-87. Bull Environ Contam Toxicol, 42: 595-602.
Anon. (1964) Report on investigation of fish kills in Lower
Mississippi River, Atchafalaya River and Gulf of Mexico. Washington,
DC, US Department of Health, Education, and Welfare, Public Health
Service, Division of Water Supply and Pollution Control.
Anon (1979) Determination of residues of organochlorine pesticides in
animal fats and eggs. Report of the committee for analytical methods
for residues of pesticides and veterinary products in foodstuffs of
the Ministry of Agriculture, Fisheries and Food. Analyst, 104:
425-433.
Anon. (1984) Acute convulsions associated with endrin
poisoning--Pakistan. Morb Mortal Wkly Rep, 33(49): 687-695.
Anon. (1988a) Introduction--US Environmental Protection Agency Office
of Drinking Water health advisories. Rev Environ Contam Toxicol, 104:
1-8.
Anon. (1988b) Endrin. Rev Environ Contam Toxico,l 104: 103-114.
Anon. (1989) Endrin poisoning associated with taquito ingestion,
California. Morb Mortal Wkly Rep, 38(19): 345-347.
Argyle RJ, Williams GC, & Dupree HK (1973) Endrin uptake and release
by fingerling channel catfish (Ictalurus punctatus).
J Fish Res Board Canada, 30(11): 1743-1744.
Arthur RD, Cain JD, & Barrentine BF (1976) Atmospheric levels of
pesticides in the Mississippi Delta. Bull Environ Contam Toxicol,
15(2): 129-134.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees: Laboratory studies.
Riverside, University of California, Department of Entomology (Rev.
9/73 (M-16)).
Atuma SS (1985) Accumulation of organochlorine insecticides in the
blood of the general population of Nigeria. Toxicol Environ Chem,10:
77-82.
Baldwin MK & Hutson DH (1980) Analysis of human urine for a metabolite
of endrin by chemical oxidation and gas-liquid chromatography as an
indicator of exposure to endrin. Analyst, 105: 60-65.
Baldwin MK, Robinson J, & Parke DV (1970) Metabolism of endrin in the
rat. J Agric Food Chem, 18(6): 1117-1123.
Baldwin MK, Davis RA, & Burns DT (1973) Structural studies and
photochemical rearrangement of an animal metabolite of HEOD, the
active component of dieldrin. Pestic Sci, 4: 227-237.
Baldwin MK, Crayford JV, Hutson DH, & Street DL (1976) The metabolism
and residues of 14C-endrin in lactating cows and laying hens. Pestic
Sci, 7(6): 575-594.
Barcelo D & Puignou LG (1987) [Pesticide residue in Spanish U.H.T.
milks determined by high resolution gas chromatography.] Rev Agroquim
Tecnol Aliment, 27(4): 583-589 (in Spanish).
Barnett RW, D'Ercole AJ, Cain JD, & Arthur RD (1979) Organochlorine
pesticide residues in human milk samples from women living in
northwest and northeast Mississippi, 1973-75. Pestic Monit J, 13(2):
47-51.
Barth RAJ (1967) Pesticide toxicity in primates. Tulane, University of
Louisiana, Division of Hygiene and Tropical Medicine (Thesis).
Barthel WF, Hawthorne JC, Ford JH, Bolton GC, McDowell LL, Grissinger
EH, & Parsons DA (1969) Pesticide residues in sediments of the Lower
Mississippi River and its tributaries. Pestic Monit J, 3(1): 8-68.
Batterton JC, Boush JC, & Matsumura F (1971) Growth response of
blue-green algae to aldrin, dieldrin, endrin and their metabolites.
Bull Environ Contam Toxicol, 6(6): 589-594.
Becker DM & Sieg CH (1987) Egg shell quality and organochlorine
residues in eggs of merlins Falco columbarius in southeastern
Montana. Can Field-Nat, 101: 369-372.
Bedford CT (1974) Von Baeyer/IUPAC names and abbreviated chemical
names of metabolites and artifacts of aldrin (HHDN), dieldrin (HEOD)
and endrin. Pestic Sci, 5: 473-489.
Bedford CT & Harrod RK (1973) Synthesis of anti-12-hydroxyendrin and
12-ketoendrin, the two major mammalian metabolites of endrin.
Chemosphere, 4: 163-168.
Bedford CT & Hutson DH (1976) The comparative metabolism in rodents of
the isomeric insecticides dieldrin and endrin. Chem Ind, 1976:
440-447.
Bedford CT, Harrod RK, Hoadley EC, & Hutson DH (1975a) The metabolic
fate of endrin in the rabbit. Xenobiotica, 5(8): 485-500.
Bedford CT, Hutson DH, & Natoff IL (1975b) The acute toxicity of
endrin and its metabolites to rats. Toxicol Appl Pharmacol, 33:
115-121.
Bedford CT, Crane AE, & Harrod RK (1986a) Synthesis and confirmation
of structure of four mammalian metabolites of dieldrin and endrin.
Pestic Sci, 17: 659-667.
Bedford CT, Crane AE, Smith EH, & Wellard NK (1986b) Synthesis of
endrin metabolites. Part. 2: Total synthesis and confirmation of the
structure of 3-hydroxyendrin. Pestic Sci, 17: 33-47.
Belisle AA, Reichel WL, Locke LN, Lamont TG, Mulhern BM, Prouty RM,
DeWolf RB, & Cromartie E (1972) Residues in fish, wildlife and
estuaries. Pestic Monit J, 6: 133-138.
Benes V & Sram R (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind Med, 38(12): 442-444.
Bennett RO & Wolke RE (1987a) The effect of sublethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. I. Evaluation of serum
cortisol concentrations and immune responsiveness. J Fish Biol, 31(3):
375-385.
Bennett RO & Wolke RE (1987b) The effect of sub-lethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. II. The effect of
altering serum cortisol concentrations on the immune response. J Fish
Biol, 31(3): 387-394.
Benson WR (1969) Note on nomenclature of dieldrin and related
compounds. J Assoc Off Anal Chem, 52(5): 1109-1111.
Beyerbach M, Buthe A, Heidmann WA, Dettmer R, & Knuwer H (1987)
[Chlorinated hydrocarbons in eggs and livers of rooks (Corvus
frugilegus) from rookeries in Lower Saxony (northern Germany).]
J Ornitol, 128(3): 277-290 (in German with English summary).
Beyerbach M, Buthe A, Heidmann WA, Knuwer H, & Russel-Sinn HA (1988)
[The burden of dieldrin and other chlorinated hydrocarbons on the
lapwing (Vanellus vanellus).] J Ornitol, 129(3): 353-361 (in
German).
Bhowmik G (1978) Pretreating properties of endrin on plant
chromosomes. Letter to the Editor. Curr Sci, 47(15): 546-547.
Bianchi A, Tateo F, Nava C, Tateo S, Santamaria L, Berte F, &
Santagati G (1988) Presence of organophosphate and organochlorine
pesticides in the milk of women. Med Biol Environ, 16: 931-942.
Biberhofer J & Stevens RJJ (1987) Organochlorine contaminants in
ambient waters of Lake Ontario. Ottawa, Inland Water Directorate,
Waters Quality Branch,pp. 1-11 (Can Sci Ser (87) V51.159).
Biehl ML & Buck WB (1987) Chemical contaminants: their metabolism and
their residues. J Food Prot, 50(12): 1058-1073.
Bloomquist JR & Soderlund DM (1985) Neurotoxic insecticides inhibit
GABA-dependent chloride uptake by mouse brain vesicles. Biochem
Biophys Res Commun, 133(1): 37-43.
Bloomquist JR, Adams PM, & Soderlund DM (1986) Inhibition of
gamma-aminobutyric acid-stimulated chlorine flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
Blus LJ (1978) Short-tailed shrews: toxicity and residue relationship
of DDT, dieldrin and endrin. Arch Environ Contam Toxicol, 7: 83-98.
Blus LJ, Joanen T, Belisle AA, & Prouty RM (1975) The brown pelican
and certain environmental pollutants in Louisiana. Bull Environ Contam
Toxicol, 13: 646-655.
Blus L, Cromartie E, McNease L, & Joanen T (1979) Brown pelican:
population status, reproductive success, and organochlorine residues
in Louisiana, 1971-1976. Bull. Environ Contam Toxicol, 22: 128-135.
Blus LJ, Henny CJ, Kaiser TE, & Grove RA (1983) Effects on wildlife
from use of endrin in Washington State orchards. In: Fox GA & Hall RJ
ed. Transactions of the 48th North American Wildlife Conference on
Environmental Contaminants and Wildlife. Washington, DC, Wildlife
Management Institute, pp. 159-174.
Blus LJ, Henny CJ, & Grove RA (1989) Rise and fall of endrin usage in
Washington State fruit orchards: effects on wildlife. Environ Pollut,
60: 331-349.
Boellstorff DE, Ohlendorf HM, Anderson DW, O'Neill EJ, Keith JO, &
Prouty RM (1985) Organochlorine chemical residues in white pelicans
and western grebes from the Klamath Basin, California. Arch. Environ
Contam Toxicol, 14: 485-493.
Bollag JM & Henninger NM (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J Environ
Qual, 5(1): 15-18.
Bollen WB & Tu CM (1971) Influence of endrin on soil microbial
populations and their activity. Washington, DC, US Department of
Agriculture, Forest Service, pp 1-4 (Research Paper PNW 114).
Bonner JC & Yarbrough JD (1989) Role of the brain
t-butyl-bicyclophosphorothionate receptor in vertebrate resistance to
endrin, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane and
cypermethrin. J Pharmacol Exp Ther, 249(1): 149-154.
Borady AMA, Mikhail TH, Awadailah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23(1-2): 33-44.
Boyd EM & Stefec J (1969) Dietary protein and pesticide toxicity with
particular reference to endrin. Can Med Assoc J, 101: 335-339.
Braun F (1985) [PCN and chlorine-based pesticides in some Bavarian
rivers.]. Munch. Beitr. Abwasser Fisch Flussbiol, 39: 115-124 (in
German).
Breidenbach AW, Gunnerson CG, Kawahara FK, Lichtenberg JJ, & Green RS
(1967) Chlorinated hydrocarbon pesticides in major river basins,
1957-65. Public Health Rep, 82(2): 139-156.
Brodtmann NV. Jr (1976) Continuous analysis of chlorinated hydrocarbon
pesticides in the Lower Mississippi River. Bull Environ Contam
Toxicol, 15(1): 33-39.
Brooks GT (1969) The metabolism of diene-organochlorine (cyclodiene)
insecticides. Residue Rev, 27: 81-138.
Brooks GT (1974) Chlorinated insecticides, technology and application.
Cleveland, Ohio, CRC Press, vol. 1, pp 85-99 & vol 2, pp 94-97.
Brooks TM (1976) Mutagenicity studies with endrin in the host-mediated
assay. Unpublished report No. TLGR.0112.76, Sittingbourne, Kent, Shell
Research, submitted to WHO by Shell.
Bunck CM, Prouty RM, & Krynitsky AJ (1987) Residues of organochlorine
pesticides and polychlorobiphenyls in starlings (Sturnus vulgaris),
from the continental United States, 1982. Environ Monit Assess, 8:
59-75.
Burton WB & Pollard GE (1974) Rate of photochemical isomerization of
endrin in sunlight. Bull Environ Contam Toxicol, 12(1): 113-116.
Butler PA (1963) Commercial fisheries investigations. Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp 11-25
(Circular No. 167).
Butler PA (1973) Organochlorine residues in estuarine mollusks,
1965-1972. Pestic Monit J, 6(4): 238-362.
Cabral JRP, Raitano F, Mollner T, Bronczyk S, & Shubik P (1979) Acute
toxicity of pesticides in hamsters. Abstract: Eighteenth Annual
Meeting No. 384. Toxicol Appl Pharmacol, 48: A192.
Caceres O, Tundisi JG, & Castellan OAM (1987) Residues of
organochloric pesticides in reservoirs in Sao Paulo State. Cienc Cult,
39(3): 259-264.
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in near-shore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13(3): 296-309.
Cantoni C, Fabbris F, Rogledi R, & Campagnari A (1988) [Organochlorine
pesticides in foods of animal origin found during the 1985-1987
biennium.] Ind Aliment, 27(1): 6-8 (in Italian).
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ Monit Assess, 5(2): 155-163.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1978) Pesticide
residue levels in soils and crops, 1971--national soils monitoring
program (III). Pestic Monit J, 12(3): 117-136.
Carline RF & Lawal MV (1985) Contaminants and bilateral asymmetry in
yellow perch. Environ Toxicol Chem, 4: 543-547.
Carrero I, Fernandez-Moreno MD, Perez-Albarsanz MA, & Prieto JC (1989)
Lindane effect upon the vasoactive intestinal peptide
receptor/effector system in rat enterocytes. Biochem Biophys Res
Commun, 159(3): 1391-1396.
Carter BI & Simpson BJE (1978) Toxicity of insecticides: The acute
oral and percutaneous toxicities of two endrin bleed samples and of
technical endrin in Shellsol A and in toluene. Unpublished report No.
TLTR.0001.78, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Casper VL (1967) Galveston Bay pesticide study--water and oyster
samples analyzed for pesticide residues following mosquito control
program. Pestic Monit J, 1(3): 13-15.
Casteel SW & Cook WO (1985) Endrin toxicosis in a cat. J Am Vet Med
Assoc, 186(9): 988-989.
Castonguay M, Dutil J-D, & Desjardins C (1989) Distinction between
American eels (Anguilla rostrata) of different geographical origins
on the basis of their organochlorine contaminant levels. Can J Fish
Aquat Sci, 46: 836-843.
Celeste M de F & Caceres O (1987) [Chlorinated pesticide residues in
waters of Ribeirïo do Lobo (Broa) reservoir and its tributaries.]
Cienc Cult, 39(1): 66-70 (in Portuguese with English summary).
Cetinkaya M (1988) [Organochloro-pesticide residues in tobacco from
European cigarette brands.] Chem Mikrobiol Technol Lebensm, 11:
100-103 (in German with English summary).
Cetinkaya M & Schenek A (1987) [Investigation of
organochloro-pesticide residues in various raw cotton samples.] Chem
Mikrobiol Technol, Lebensm, 10: 150-153 (in German with English
summary).
Chau ASY (1974) Confirmation of pesticide residues identity. VII.
Solid matrix derivation procedure for the simultaneous confirmation of
heptachlor and endrin residues in the presence of large quantities of
polychlorinated biphenyls. J Assoc Off Anal Chem, 57(3): 585-591.
Chau ASY & Cochrane WP (1969) Cyclodiene chemistry. III. Derivative
formation for the identification of heptachlor, heptachlorepoxide,
cis-chlordane, trans-chlordane, dieldrin and endrin pesticide residues
by gaschromatography. J Assoc Off Anal Chem, 52: 1220-1226.
Chau ASY & Cochrane WP (1971) Chromous chloride reductions. VI.
Derivative formation for the simultaneous identification of heptachlor
and endrin pesticide residues by gas chromatography. J Assoc Off Anal
Chem, 54(5): 1124-1131.
Chernoff N, Kavlock RJ, Hanisch RC, Whitehouse DA, Gray JA, Gray LE
Jr, & Sovocool GW (1979) Perinatal toxicity of endrin in rodents. I.
Fetotoxic effects of prenatal exposure in hamsters. Toxicology, 13:
155-165.
Clark DR Jr & Krynitsky A (1978) Organochlorine residues and
reproduction in the little brown bat, Laurel, Maryland--June 1976.
Pestic. Monit J, 12(3): 113-116.
Clark DR Jr, Laval RK, & Krynitsky AJ (1980) Dieldrin and heptachlor
residues in dead gray bats, Franklin County, Missouri--1976 versus
1977. Pestic Monit J, 13(4): 137-140.
Clawson RL & Clark DR (1989) Pesticide contamination of endangered
gray bats and their food base in Boone County, Missouri, 1982. Bull
Environ Contam Toxicol, 42: 431-437.
Coble Y, Hildebrandt P, Davis J, Raasch F, & Curley A (1967) Acute
endrin poisoning. J Am Med Assoc, 202: 489-493.
Cole LM & Casida JE (1986) Polychlorocycloalkane insecticide-induced
convulsions in mice in relation to disruption of the GABA-regulated
chloride ionophore. Life Sci, 39: 1855-1862.
Cole JF, Klevay LM, & Zavon MR (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol Appl Pharmacol,
16: 547-555.
Coleman RL (1968) Endrin induced alterations in bound carbohydrates in
rat serum. Bull Environ Contam Toxicol, 3(6): 348-353.
Coleman RL, Lawrence CH, & Sowell WL (1968) Trace metal alterations
following subacute exposure to endrin. Bull. Environ Contam Toxicol,
3(5): 284-295.
Cook WO & Casteel SW (1985) A suspected case of endrin toxicosis in a
cat. Vet Hum Toxicol, 27(2): 111.
Corneliussen PE (1969) Pesticide residues in total diet samples (IV).
Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Pesticide residues in total diet samples (V).
Pestic Monit J, 4(3): 89-105.
Corneliussen PE (1972) Pesticide residues in total diet samples (VI).
Pestic Monit J, 5(4): 313-330.
Cote MG, Plaa GL, Valli VE, & Villeneuve DC (1985) Subchronic effects
of a mixture of 'persistent' chemicals found in the Great Lakes. Bull
Environ Contam Toxicol, 34: 285-290.
Crockett AB, Wiersma GB, Tai H, & Mitchell W (1975) Pesticide and
mercury residues in commercially grown catfish. Pestic Monit J, 8(4):
235-240.
Cromartie E, Reichel WL, Locke LN, Belisle AA, Kaiser TE, Lamont TG,
Mulhern BM, Prouty RM, & Swineford DM (1975) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1971-72. Pestic Monit J, 9(1): 11-14.
Cueto C Jr & Biros FJ (1967) Chlorinated insecticides and related
materials in human urine. Toxicol Appl Pharmacol, 10: 261-269.
Cueto C Jr & Hayes WJ Jr (1962) The detection of dieldrin metabolites
in human urine. Agric Food Chem, 10(5): 366-369.
Cummings JG (1965) Pesticide residues in the total diet samples. J
Assoc Off Anal Chem, 48(6): 1177-1180.
Cummings JG (1966) Pesticides in the total diet. Residue Rev, 16:
30-45.
Curley A, Jennings RW, Mann HT, & Sedlak V (1970) Measurement of
endrin following epidemics of poisoning. Bull Environ Contam Toxicol,
5(1): 24-29.
Currie RA, Kadis VW, Breitkreitz WE, Cunnignham GB, & Bruns GW (1979)
Pesticide residues in human milk, Alberta, Canada--1966-70, 1977-78.
Pestic Monit J, 13(2): 52-55.
Dale WE, Copeland F, & Hayes WJ Jr (1965) Chlorinated insecticides in
the body fat of people in India. Bull World Health Organ, 33: 471-477.
Datta SK & Ghose KC (1985) Toxic effect of endrin on the
hepatopancreas of a teleost, Cyprinus carpio. Indian Biol, 17(1):
37-41.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
Davis GM & Lewis I (1956) Outbreak of food poisoning from bread made
of chemically contaminated flour. Br Med J, ii: 393-398.
Davis HC & Hidu H (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish Bull , 67(2): 393-404.
Dean BJ (1977) Chromosome studies on workers employed in an endrin
manufacturing plant. Unpublished report No. TLGR.0008.77,
Sittingbourne, Kent, Shell Research, submitted to WHO by Shell.
De Boer J (1989) Organochlorine compounds and bromodiphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere,, 18(11/12): 2131-2140.
De Campos M & Olszyna-Marzys AE (1979) Contamination of human milk
with chlorinated pesticides in Guatemala and El Salvador. Arch Environ
Contam Toxicol, 8: 43-58.
Deichmann WB & MacDonald WE (1971) Organochlorine pesticides and human
health. Food Cosmet Toxicol, 9(1): 91-103.
Deichmann WB, MacDonald WE, Blum E, Bevilacqua M, Radomski J,
Keplinger M, & Balkus M (1970a) Tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Ind Med, 39(10): 426-434.
Deichmann WB, MacDonald WE, Radomski J, Blum E, Bevilacqua M, &
Keplinger M (1970b) The tumorigenicity of aldrin, dieldrin, and endrin
in the albino rat. Ind Med, 39(7): 314.
Denison MS & Yarbrough JD (1985) Binding of insecticides to serum
proteins in mosquitofish (Gambusia affinis). Comp Biochem Physiol,
81C(1): 105-107.
Denison MS, Chambers JE, & Yarbrough JD (1985) Short-term interactions
between DDT and endrin accumulation and elimination in mosquitofish
(Gambusia affinis). Arch Environ Contam Toxicol, 14: 315-320.
De Paula Carvalho JP, Niskikawa AM, Aranha S, & Fay EF (1984)
[Organochlorine pesticide residues in bovine fat.] Biol Sao Paulo,
50(2): 39-48 (in Portuguese).
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Devault DS, Clark JM, & Lahvis G (1988) Contaminants and trends in
fall run coho salmon. J Great Lakes Res, 14(1): 23-33.
De Vos RH, van Dokkum W, Olthof PDA, Quiryns JK, Muys T, & van der
Poll JM (1984) Pesticides and other chemical residues in Dutch total
diet samples (June 1976-July 1978). Food Chem Toxicol 22(1): 11-21.
Deweese LR, Cohen RR, & Stafford CJ (1985) Organochlorine residues and
egg shell measurements for tree swallows Tachycineta bicolor in
Colorado. Bull Environ Contam Toxicol, 35: 767-775.
Dewitt JB (1965) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J Agric Food Chem, 4(10): 863-866.
Dikshith TSS & Datta KK (1973) Endrin-induced cytological changes in
albino rats. Bull Environ Contam Toxicol, 9(2): 65-69.
Dikshith TSS, Kumar SN, Raizada RB, & Srivastava MK (1989a)
Organochlorine insecticide residues in cattle feed. Bull Environ
Contam Toxicol, 43: 691-696.
Dikshith TSS, Kumar SN, Tandon GS, Raizada RB, & Ray PK (1989b)
Pesticide residues in edible oils and oil seeds. Bull Environ Contam
Toxicol, 42: 50-56.
Dinnel PA, Link JM, Stober QJ, Letourneau MW, & Roberts WE (1989)
Comparative sensitivity of sea urchin sperm bioassays to metals and
pesticides. Arch Environ Contam Toxicol, 18: 748-755.
Ditraglia D, Brown DP, Namekata T, & Iverson N (1981) Mortality study
of workers employed at organochlorine pesticide manufacturing plants.
Scand J Work Environ Health, 7(suppl 4): 140-146.
Donahue JF, Burse VW, Head SL, & Andrews JS (1988) Comparison of two
techniques for quantifying environmental contaminants in human serum.
Life Sci, 43: 2257-2264.
Donoso J, Dorigan J, Fuller B, Gordon J, Kornreich M, Saari S, Thomas
L, & Walker P (1979) Reviews of the environmental effects of
pollutants. XIII. Endrin. Oak Ridge, Tennessee, Oak Ridge National
Laboratory (EPA-600/1-79-005).
DouAbul AAZ, Al-Saad HT, Al-Obaidy SZ, & Al-Rekabi HN (1987a) Residues
of organochlorine pesticides in fish from the Arabian Gulf. Water Air
Soil Pollut, 35: 187-194.
DouAbul AAZ, Al-Saad HT, & Al-Rekabi HN (1987b) Residues of
organochlorine pesticides in environmental samples from the Shatt
al-Arab River, Iraq. Environ Pollut, 43: 175-187.
DouAbul AAZ, Al-Omar M, Al-Obaidy S, & Al-Ogaily N (1987c)
Organochlorine pesticide residues in fish from the Shatt al-Arab
River, Iraq. Bull Environ Contam Toxicol, 38: 674-680.
DouAbul AAZ, Al-Saad HT, Al-Timari AA, & Al-Rekabi HN (1988)
Tigris-Euphrates delta: a major source of pesticides to the Shatt
al-Arab River (Iraq). Arch Environ Contam Toxicol, 17: 405-418.
Dowd PF, Mayfield GU, Coulon DP, Graves JB, & Newsom JD (1985)
Organochlorine residues in animals from three Louisiana watersheds in
1978 and 1979. Bull Environ Contam Toxicol, 34: 832-841.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-1970. Pestic Monit J,
5(4): 331-341.
Duggan RE & Lipscomb GQ (1969) Dietary intake of pesticide chemicals
in the United States (II), June 1966-April 1968. Pestic Monit J, 2(4):
153-162.
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in total
diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Pesticide residues in total
diet samples (II). Pestic Monit J, 1(2): 2-12.
Duke TW & Dumas DP (1974) Implications of pesticide residues in the
coastal environment. In: Vernberg FJ & Vernberg WB, ed. Pollution and
physiology of marine organisms. New York, Academic Press, pp 137-164.
Dureja P, Walia S, & Mukerjee SK (1987) New photometabolites of
endrin. Indian J Chem, 26G: 898-899.
Durham WF & Wolfe HR (1962) Measurement of the exposure of workers to
pesticides. Bull World Health Organ, 26: 75-91.
Dutch Agricultural Advisory Commission on Environmental Pollutants
(1983) Annual report. The Hague, Ministry of Agriculture, Management
of Nature and Fisheries.
Earnest RD & Benville PE Jr (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game, 58(2): 127-132.
Egan H, Goulding R, Roburn J, & Tatton JO'G (1965) Organochlorine
pesticide residues in human fat and human milk. Br Med J, ii: 66.
Eisenberg M & Topping JJ (1985) Organochlorine residues in finfish
from Maryland waters 1976-1980. J Environ Sci Health B20(6): 729-742.
Eisler R (1970a) Latent effects of insecticide intoxication to marine
molluscs. Hydrobiologia, 36(3-4): 345-352.
Eisler R (1970b) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish. Tech Paper Bur Sport
Fish Wildl, 46: 1-12.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCB's in
river Nile water, Egypt. Bull Environ Contam Toxicol, 34: 126-133.
Ellis DH, Deweese LR, Grubb TG, Kiff LF, Smith DG, Jarman WM, &
Peakall DB (1989) Pesticide residues in Arizona peregrine falcon eggs
and prey. Bull Environ Contam Toxicol, 42: 57-64.
Elnabarawy MT, Welter AN, & Robideau RR (1986) Relative sensitivity of
three daphnid species to selected organic and inorganic chemicals.
Environ Toxicol Chem, 5: 393-398.
El Nabawi A, Heinzow B, & Kruse H (1987) Residue levels of
organochlorine chemicals and polychlorinated biphenyls in fish from
the Alexandria Region, Egypt. Arch Environ Contam Toxicol, 16:
689-696.
El-Sebae AH (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region. In:
Research on the toxicity, persistence, bioaccumulation,
carcinogenicity and mutagenicity of selected substances (Activity G).
Final reports on projects dealing with toxicity (1983-1985). Athens,
United Nations Environment Programme (Mediterranean Action Plan (MAP),
Technical Reports Series No. 10).
Ely RE, Moore LA, Carter RH, & App BA (1957) Excretion of endrin in
the milk of cows fed endrin-sprayed alfalfa and technical endrin. J
Econ Entomol, 50(3): 348-349.
Emerson TE Jr (1965) Mechanisms of hemoconcentration in the dog during
acute endrin insecticide poisoning. Can J Physiol Pharmacol, 43:
793-800.
Emerson TE Jr & Hinshaw LB (1965) Peripheral vascular effects of the
insecticide endrin. Can J Physiol Pharmacol,43: 531-539.
Emerson TE Jr, Brake CM, & Hinshaw LB (1963) Mechanism of Action of
the Insecticide Endrin. Oklahoma City, Oklahoma, Civil Aeromedical
Research Institute (Report No. 63-16).
Emerson TE Jr, Brake CM, & Hinshaw LB (1964) Cardiovascular effects of
the insecticide endrin. Can J Physiol Pharmacol, 42: 41-51.
Engst R & Knoll R (1973) [On the contamination of surface, rain and
drinking waters with chlorinated hydrocarbons.] Nahrung, 17(8):
837-851 (in German with English summary).
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection of
chemical mutagens by the dominant lethal assay in the mouse. Toxicol
Appl Pharmacol, 23: 288-325.
Ercegovich CD & Rashid KA (1977) Mutagenesis induced in mutant strains
of Salmonella typhimurium by pesticides. In: Abstracts of the 174th
ACS National Meeting, Chicago, Illinois. Washington, DC, American
Chemical Society, Division of Pesticide Chemistry (Abstract No. 43).
Everaarts JM, Koeman JH, & Brader, L (1971) Contribution à l'étude des
effets sur quelques éléments de la faune sauvage des insecticides
organo-chlorés utilisés au Tchad en culture cotonnière. Cotton Fibre
Trop, 26(4): 385-394.
Fabacher DL & Chambers H (1976) Uptake and storage of 14C-labelled
endrin by the livers and brains of pesticide-susceptible and resistant
mosquitofish. Bull Environ Contam Toxicol, 16(2): 203-207.
Fahrig R (1974) Comparative mutagenicity studies with pesticides. In:
Montesano R & Tomatis L ed. Chemical carcinogenesis essays. Lyon,
International Agency for Research on Cancer, pp 161-181 (IARC
Scientific Publications No. 10).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No. PL:1963/13;
WHO/Food Add./23).
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. Geneva, World Health Organization (FAO Meeting Report No.
PL:1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1971) Joint meeting of FAO Working Party of Experts and the
WHO Expert Group on Pesticide Residues. 1970 Evaluation of some
pesticide residues in food. Geneva, World Health Organization
(AGP:1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1984) Codex guidelines on good practice in pesticide residue
analysis. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986a) Recommendations for methods of analysis of pesticide
residues. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR8-1986).
FAO/WHO (1986b) Codex maximum limits for pesticide residues. Rome,
Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme,
Food and Agriculture Organization of the United Nations, p 33-IV (FAO
CAC Vol. XIII, ed. 2).
Fasola M, Vecchio I, Caccialanza G, Gandini C, & Kitsos M (1987)
Trends of organochlorine residues in eggs of birds from Italy, 1977 to
1985. Environ Pollut, 48: 25-36.
FDA (1988) Food and Drug Administration pesticide program. Residues in
foods--1987. J Assoc Off Anal Chem, 71(6): 156A-174A.
Ferguson DE, Culley DD, & Cotton WD (1964) Resistance to chlorinated
hydrocarbon insecticides in three species of freshwater fish.
Bioscience, 14: 43-44.
Flickinger EL & King KA (1972) Some effects of aldrin-treated rice on
Gulf Coast wildlife. J Wildl Manage, 36: 706-727.
Flickinger EL, Mitchell CA, & Krynitsky AJ (1986) Dieldrin and endrin
residues in fulvous whistling ducks in Texas in 1983. J. Field
Ornithol, 57(2): 85-192.
Folmar LC (1978) In vitro inhibition of rat brain ATPase, pNPPase,
and ATP-32Pi exchange by chlorinated-diphenyl ethanes and cyclodiene
insecticides. Bull Environ Contam Toxicol, 19: 481-488.
Foschi F, Natali G, Guberti MG, Camisani MG, Taccheto Barbina M,
Spessotto C, & Bargarolo L (1985) [Study on residues of argicultural
chemicals in apples.] Inf Fitopatol, 12: 14-20 (in Italian).
Fournier E, Treich I, Campagne L, & Capelle N (1972) Pesticides
organo-chlorés dans le tissu adipeux d'êtres humains en France. Eur J
Toxicol, 1(1): 11-26.
Fox ME, Roper DS, & Thrush SF (1988) Organochlorine contaminants in
surficial sediments of Manukau Harbour, New Zealand. Mar Pollut
Bull,19(7): 333-336.
Frank R, Braun HE, Holdrinet M, Sirons GJ, Smith EH, & Dixon DW (1979)
Organochlorine insecticides and industrial pollutants in the milk
supply of Southern Ontario, Cananda--1977. J Food Prot, 42(1): 31-37.
Frank R, Braun HE, & Holdrinet MVH (1981) Residues from past uses of
organochlorine pesticides and PCB in waters draining eleven
agricultural watersheds in Southern Ontario, Canada, 1975-1977. Sci
Total Environ, 20: 255-276.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario--1983. J Food Prot, 48(6):
499-504.
Fredrickson DS (1978) Report on bioassay of endrin for possible
carcinogenicity. Fed Reg, 43(225): 54298.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Galassi S & Provini A (1981) Chlorinated pesticides and PCBs contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gant DB, Eldefrawi ME, & Eldefrawi AT (1987) Cyclodiene insecticides
inhibit GABAa receptor-regulated chloride transport. Toxicol Appl
Pharmacol, 88(3): 313-321.
Garrett NE, Stack HF, & Waters MD (1986) Evaluation of the genetic
activity profiles of 65 pesticides. Mutat Res, 168(3): 301-325.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements, and other chemicals in adult total diet
samples October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off Anal
Chem, 69(1): 123-145.
Giesy JP, Mewsted J, & Garling DL (1986) Relationships between
chlorinated hydrocarbon concentrations and rearing mortality of
chinook salmon (Onchorhynchus tshawytscha) eggs from Lake Michigan.
J Great Lakes Res, 12(1): 82-98.
Gips T (1987) Breaking the pesticide habit--Alternatives to 12
hazardous pesticides (International Alliance for Sustainable
Agriculture (IASA) Publication No. 1987-2).
Glatt H, Jung R, & Oesch F (1983) Bacterial mutagenicity investigation
of epoxides: drugs, drug metabolites, steroids and pesticides. Mutat
Res, 11: 99-118.
Glooschenko WA, Strachan WMJ, & Sampson RCJ (1976) Distribution of
pesticides and polychlorinated biphenyls in water, sediments and
seston of the Upper Great Lakes--1974. Pestic Monit J, 10(2): 61-67.
Gluth G & Hanke W (1985) A comparison of physiological changes in
carp, Cyprinus carpio, induced by several pollutants at sub-lethal
concentrations. I. The dependency on exposure time. Ecotoxicol Environ
Saf, 9: 179-188.
Godsil PJ & Johnson WC (1968) Pesticide monitoring of the aquatic
biota of the Tule Lake National Wildlife Refuge. Pestic Monit J, 1(4):
21-26.
Goerlitz DF & Law LM (1974) Determination of chlorinated insecticides
in suspended sediment and bottom material. J Assoc Off Anal Chem,
57(1): 176-181.
Goldentahl EI (1978a) Teratology study in rats. Unpublished report No.
163-488, International Research and Development Corporation, submitted
to WHO by Shell.
Goldentahl EI (1978b) Teratology study in hamsters. Unpublished report
No. 163-478, International Research and Development Corporation,
submitted to WHO by Shell.
Good EE & Ware GW (1969) Effects of insecticides on reproduction in
the laboratory mouse. Toxicol Appl Pharmacol, 14: 201-203.
Graves JB & Bradley JR (1965) Response of Swiss albino mice to
intraperitoneal injection of endrin. J Econ Entomol, 58(1): 178-179.
Gray LE Jr, Kavlock RJ, Chernoff N, Gray JA, & McLamb J (1981)
Perinatal toxicity of endrin in rodents. III. Alterations of
behavioural ontogeny. Toxicology, 21: 187-202.
Green DR, Stull JK, & Heesen TC (1986) Determination of chlorinated
hydrocarbons in coastal waters using a moored in situ sampler and
transported live mussels. Mar Pollut Bull, 17(7): 324-329.
Gregor DJ & Gummer WD (1989) Evidence of atmospheric transport and
deposition of organochlorine pesticides and polychlorinated biphenyls
in Canadian Arctic snow. Environ Sci Technol, 23: 561-565.
Gübeli T & Clerc JT (1988) [Detection of pesticide residues in ethanol
extracts of plants.] Pharm Acta Helv, 63(3): 85-89 (in German).
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984,
dietary intakes of pesticides, selected elements and other chemicals.
J Assoc Off Anal Chem, 71(6): 1200-1209.
Hall RJ & Swineford D (1980) Toxic effects of endrin and toxaphene on
the southern leopard frog Rana sphenocephala. In: Mellanby, K. ed.
Environmental pollution. Barking, Essex, Applied Science Publishing,
pp 53-65.
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hamdy Y & Post L (1985) Distribution of mercury, trace organics and
other heavy metals in Detroit River sediments. J Great Lakes Res,
11(3): 353-365.
Hansen DJ, Schimmel SC, & Forester J (1977) Endrin: effects on the
entire life cycle of a saltwater fish, Cyprinodon variegatus. J
Toxicol Environ Health, 3: 721-733.
Harris CR, Sans WW, & Miles JRW (1966) Exploratory studies on the
occurrence of organochlorine insecticides residues in agricultural
soils in southwestern Ontario. J Agric Food Chem,14(4): 398-403.
Hart LG & Fouts JR (1963) Effects of acute and chronic DDT
administration in hepatic microsomal drug metabolism in the rat
(28686). Proc Soc Exp Biol Med,114: 388-392.
Hartgrove RW Jr, Hundley SG, & Webb RE (1977) Characterization of the
hepatic mixed function oxidase system in endrin resistant and
-suspectible pinevoles. Pestic Biochem Physiol, 7(2): 146-153.
Hashemy-Tonkabony SE & Mosstofian B (1979) Chlorinated pesticide
residues in chicken egg. Poult Sci 58(6): 1432-1434.
Hashemy-Tonkabony SE & Soleimani-Amiri MJ (1976) Detection and
determination of chlorinated pesticide residues in raw and various
stages of processed vegetable oil. J Am Oil Chem Soc, 53(12): 752-753.
Hashimoto I & Nishiuchi S (1981) Establishment of bioassay methods for
evaluation of acute toxicity of pesticides to aquatic organisms. J
Pestic Sci, 6: 257-264.
Hassett AJ, Viljoen PT, & Liebenberg JJE (1987) An assessment of
chlorinated pesticides in the major surface water resources of the
Orange Free State during the period September 1984 to September
1985.Water SA, 13(3): 133-136.
Hawker DW & Connell DW (1986) Bioconcentration of lipophilic compounds
by some aquatic organisms. Ecotoxicol Environ Saf, 11: 184-197.
Hawthorne JC, Ford JH, & Markin GP (1974) Residues of mirex and other
chlorinated pesticides in commercially raised catfish. Bull Environ
Contam Toxicol, 11(3): 258-264.
Hayes WJ Jr (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department of
Health, Education, and Welfare, Communicable Disease Center,
Toxicology Section, pp 68-70.
Hayes WJ Jr (1975) Toxicology of pesticides, Baltimore,
Maryland,Williams & Wilkins, pp 288-294.
Hayes WJ Jr (1982) Pesticides studied in man, Baltimore, Maryland,
Williams and Wilkins, pp 247-251.
Hayes WJ Jr & Curley A (1968) Storage and excretion of dieldrin and
related compounds. Effect of occupational exposure. Arch Environ
Health, 16(2): 155-162.
Hayes WJ Jr, Dale WE, & Burse VW (1965) Chlorinated hydrocarbon
pesticides in the fat of people in New Orleans. Life Sci, 4:
1611-1615.
Heidmann WA, Büthe A, Beyerbach M, Löhmer R, & Rüssel-Sinn HA (1989)
[Chlorinated hydrocarbons of some bird species breeding in the inland
of Lower Saxony.] J Ornitol, 130(3): 311-320 (in German with English
summary).
Heinz GH, Erdman TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henderson C, Johnson WL, & Inglis A (1969) Organochlorine insecticide
residues in fish. National pesticides monitoring program. Pestic Monit
J, 3: 145-171.
Henderson C, Inglis A, & Johnson WL (1971) Organochlorine insecticide
residues in fish. Fall 1969, National Pesticide Monitoring Program.
Pestic Monit J, 5: 1-11.
Hendrickson CM & Bowden JA (1976) In vitro inhibition of lactic acid
dehydrogenase by insecticidal polychlorinated hydrocarbons. 2.
Inhibition by dieldrin and related compounds. J Agric Food Chem,
24(4): 756-759.
Hendrickx A & Maes R (1969) The excretion of chlorinated hydrocarbon
insecticides in human mother milk. J Pharm Belg, 24(9-10): 459-463.
Hermanutz R (1974) Quarterly report. Duluth, Minnesota, US
Environmental Protection Agency, National Water Quality Laboratory.
Hermanutz RO, Eaton JG, & Mueller LH (1985) Toxicity of endrin and
malathion mixtures to flagfish (Jordanella floridae). Arch Environ
Contam Toxicol, 14: 307-314.
Hernandez FH, Lopez Benet FJ, Escriche JM, & Ubeda JCB (1987) Sulfuric
acid cleanup and KOH-ethanol treatment for confirmation of
organochlorine pesticides and polychlorinated biphenyls: application
to wastewater samples. J Assoc Off Anal Chem, 70(4): 727-733.
Herrera Marteache A, Polo Villar LM, Jodral Villarejo M, Polo Villar
G, Mallol J, & Pozo Lora R (1978) [Organochlorine pesticide residues
in human fat in Spain.] Rev San Hig Publico, 52: 1125-1144 (in Spanish
with English summary).
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, p 147 (Fish
and Wildlife Technical Report No. 2).
Hill EF, Health RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report: Wildlife No. 191).
Hill RH Jr, Needham LL, & Liddle JA (1986) The laboratory's role in
environmental health emergency investigations. Clin Toxicol, 24(5):
363-374.
Hine CH (1965) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 2, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH (1968) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 7, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH, Anderson HH, Kodama JK, & Gutenberg EF (1954) Class B
evaluation of endrin compositions. Unpublished report No. 223, San
Francisco, CA, University of California School of Medicine, submitted
to WHO by Shell.
Hinshaw LB, Solomon LA, Reins DA, Fiorica V, & Emerson TE (1966)
Effects of the insecticide endrin on the cardiovascular system of the
dog. J Pharmacol Exp Ther, 153(2): 225-236.
Hirom PC, Millburn P, Smith RL, & Williams RT (1972) Species
variations in the threshold molecular-weight factor for the biliary
excretion of organic anions. Biochem J, 129: 1071-1077.
Hoffman WS, Fishbein WI, & Andelman MB (1964) The pesticide content of
human fat tissue. Arch Environ Health, 9: 387-394.
Hoffman WS, Adler H, Fishbein WI, & Bauer FC (1967) Relation of
pesticide concentration in fat to pathological changes in tissues.
Arch Environ Health, 15: 758-765.
Hogmire HW, Weaver JE, & Brooks JL (1990) Survey for pesticides in
wells associated with apple and peach orchards in West Virginia. Bull
Environ Contam Toxicol, 44: 81-86.
Holden AV (1970) International cooperative study of organo-chlorine
pesticide residues in terrestrial and aquatic wildlife, 1967/1968.
Pestic Monit J, 4(3): 117-135.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1962) Electroencephalograms
in insecticide toxicity. Arch Environ Health, 4: 86-94.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1965) Nine years toxicity
control in insecticide plants. Arch Environ Health, 10: 441-448.
Horn H, Hartner L, & von Faber H (1987) [On the suitability of liver
function tests in birds for the ecotoxicological evaluation of
environmental chemicals.] Dtsch Tierarztl Wochenschr, 94: 1-48 (in
German with English summary).
Horsfall F Jr, Webb RE, Price NO, & Young RW (1970) Residues in apples
subsequent to ground sprays of endrin. J Agric Food Chem, 18: 221-223.
Hrdina PD, Singhal RL, & Peters DAV (1974) Changes in brain biogenic
amines and body temperature after cyclodiene insecticides. Toxicol
Appl Pharmacol, 29(1): 119.
Hrubec J (1988) [Pesticides and drinking-water.] H2O, 21(11):
278-282 (in Dutch).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 153).
Hunter CG, Robinson J, & Richardson A (1963) Chlorinated insecticide
content of human body fat in southern England. Br Med J, i: 221-224.
Hunter J, Maxwell JD, Carrella M, Stewart DW, & Williams R (1971)
Urinary-D-glucaric acid excretion as a test for hepatic enzyme
induction in man. Lancet, 20 March: 572-575.
Hunter J, Maxwell JD, Stewart DW, Williams R, Robinson J, & Richardson
A (1972) Increased hepatic microsomal enzymes activity from
occupational exposure to certain organochlorine pesticides. Nature,
237: 399-401.
Hutson DH (1981) The metabolism of insecticides in man. In: Hutson DH
& Roberts TR ed. Progress in pesticide biochemistry. New York, John
Wiley and Sons, vol 1, pp 287-333.
Hutson DH & Hoadley EC (1974) The oxidation of a cyclic alcohol
(12-hydroxyendrin) to a ketone (12-ketoendrin) by microsomal
mono-oxygenation. Chemosphere, 5:205-210.
Hutson DH, Baldwin MK, & Hoadley EC (1975) Detoxication and
bioactivation of endrin in the rat. Xenobiotica, 5(11): 697-714.
IARC (1974) Endrin. In:Some organochlorine pesticides. Lyon,
International Agency for Research on Cancer, pp. 157-171 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Volume 5).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p. 63 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Illahi A, Amin N, Hashmi AS, Nawaz M, & Naeem-ur Rahman (1986)
Incidence of endrin residues in cucumber and its effects on the
biological system of rats. J Pak Med Assoc, 36(8): 209-211.
Illahi A, Roohi J, & Hashmi AS (1987) Present status of endrin
residues in peas and its effect on biological systems. J Pure Appl
Sci, 6(1): 1-4.
Ito N, Tatematsu M, Nakanishi K, Hasegawa R, Takano T, Imaida K, &
Ogiso T (1980) The effects of various chemicals on the development of
hyperplastic liver nodules in hepatectomized rats treated with
N-nitrosodimethylamine or N-2-fluorenylacetamide. Gann, 71: 832-842.
Jacobziner H & Raybin HW (1959) Briefs on accidental chemical
poisonings in New York City. Poisoning by insecticide (endrin). NY
State J Med, 59: 2017-2022.
Jager KW (1970) Aldrin, dieldrin, endrin and telodrin. An
epidemiological study of long-term occupational exposure. Amsterdam,
Elsevier Science Publishers.
Japanese Environmental Agency (1975) Environmental survey report on
chemical substances in FY 1974. Unpublished report, December 1975,
Tokyo, Environmental Health Department, Planning and Coordination
Bureau.
Japenga J, Wagenaar WJ, Smedes F, & Salomons W (1987) A new, rapid
clean-up procedure for the simultaneous determination of different
groups of organic micropollutants in sediments; application in two
European estuarine sediment studies. Environ Technol Lett, 8(1): 9-20.
Jarvinen AW, Tanner DK, & Kline ER (1988) Toxicity of chlorpyrifos,
endrin, or fenvalerate to fathead minnows following episodic or
continuous exposure. Ecotoxicol Environ Saf, 15: 78-95.
Jedeikin R, Kaplan R, Shapira A, Radwan H, & Hoffman S (1979) The
successful use of 'high level' PEEP in near fatal endrin poisoning.
Crit Care Med, 7(4): 168-170.
Jegier Z (1964) Health hazards in insecticide spraying of crops. Arch
Environ Health, 8: 670-674.
Jenkins RB & Toole JF (1964) Polyneuropathy following exposure to
insecticides. Arch Intern Med, 113: 691-695.
Johnson DW (1968) Pesticides and fishes--a review of selected
literature. Trans Am Fish Soc, 97(4) 398-424.
Johnson RD & Manske DD (1976) Pesticide residues in total diet samples
(IX). Pestic Monit J, 9(4): 157-169.
Johnson RD & Manske DD (1977) Pesticide and other chemical residues in
total diet samples (XI). Pestic Monit J, 11(3): 116-131.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1979) Pesticide and
other chemical residues in infant and toddler total diet samples, (I),
August 1974-July 1975. Pestic Monit J, 13(3): 87-98.
Johnson RD, Manske DD, & Podrebarac DS (1981a) Pesticide, metal, and
other chemical residues in adult total diet samples, (XII), August
1975-July 1976. Pestic Monit J, 15(1): 54-71.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1981b) Pesticide,
heavy metal, and other chemical residues in infant and toddler total
diet samples, (II), August 1975-July 1976. Pestic Monit J, 15(1):
39-50.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1984) Pesticide,
metal, and other chemical residues in adult total diet samples,
(XIII), August 1976-July 1977. J Assoc Off Anal Chem, 67(1): 154-166.
Johnson MG, Kelso JRM, & George SE (1988) Loadings of organochlorine
contaminants and trace elements to two Ontario lake systems and their
concentrations in fish. Can J Fish Aquat Sci, 45: 170-178.
Jolley WP, Stemmer KL, Grande F, Richmon J, & Pfitzer EA (1969) The
effects exerted upon beagle dogs during a period of two years by the
introduction of 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8-dimethanonaphthalene
into their daily diets. Unpublished report, Cincinnati, Ohio,
Kettering Laboratory, submitted to WHO by Shell.
Joy RM (1976) Convulsive properties of chlorinated hydrocarbon
insecticides in the cat central nervous system. Toxicol Appl
Pharmacol, 35: 95-106.
Kacew S, Sutherland DJB, & Singhal RL (1973) Biochemical changes
following chronic administration of heptachlor, heptachlor epoxide and
endrin to male rats. Environ Physiol Biochem, 3: 221-229.
Kachole MS & Pawar SS (1977) Effect of endrin on microsomal electron
transport reactions. Part I: Sleeping time, electron transport
components and protection due to pre-treatments. Abstracts of the 1976
Annual General Meeting of Biochemists. J Biochem, 14(1): 45.
Kadis VW, Breitkreitz WE, & Jonasson OJ (1970) Insecticide levels in
human tissues of Alberta residents. Can J Public Health, 61(5):
413-416.
Kagan J, Kagan ED, & Seigneurie E (1986) Alpha-terthienyl, a powerful
fish poison with light-dependent activity. Chemosphere, 15(1): 49-57.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Lamont TG, Mulhern BM,
Prouty RM, Stafford CJ, & Swineford DM (1980) Organochlorine
pesticide, PCB, PBB residues and necropsy data for bald eagles from 29
states--1975-77. Pestic Monit J, 13: 145-149.
Kampe W (1985) [Pesticide residues in animal feeding-stuffs.] Dtsch
Tierarztl Wochenschr, 92(6): 228-231 (in German).
Kanitz S & Castello G (1966) [The presence of residues of some
pesticides in human fatty tissue and in some foods. Initial results of
a survey carried out in Liguria.] G Ig Med Prev, 7: 1-19 (in Italian).
Karplus M (1971) [Endrin poisoning in children.] Harefuah, 18(3):
113-116 (in Hebrew with English summary).
Kassabi M, ElHraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kathpal T & Dewan RS (1975) Improved clean-up technique for the
estimation of endosulfan and endrin residues. J Assoc Off Anal Chem,
58(5): 1076-1078.
Katz M & Chadwick GG (1961) Toxicity of endrin to some Pacific
Northwest fishes.Trans Am Fish Soc, 90: 394-397.
Kavlock RJ, Chernoff N, Hanisch RC, Gray J, Rogers E, & Gray LE Jr
(1981) Perinatal toxicity of endrin in rodents. II. Fetotoxic effects
of prenatal exposure in rats and mice. Toxicology, 21: 141-150.
Kavlock RJ, Chernoff N, & Rogers EH (1985) The effect of acute
maternal toxicity on fetal development in the mouse. Teratog Carcinog
Mutagen, 5: 3-13.
Kavlock RJ, Rogers JM, Gray LE, & Chernoff N (1987) Postnatal
alterations in development resulting from prenatal exposure to
pesticides. In: Pesticide science and biotechnology, Proceedings of
the 6th International Congress on Pesticide Chemicals, pp 561-564.
Keilty TJ & Stehly GR (1989) Preliminary investigation of protein
utilization by an aquatic earthworm in response to sublethal stress.
Bull Environ Contam Toxicol, 43: 350-354.
Keilty TJ, White DS, & Landrum PF (1988a) Short-term lethality and
sediment avoidance assays with endrin-contaminated sediment and two
Oligochaetes from Lake Michigan. Arch Environ Contam Toxicol, 17:
95-101.
Keilty TJ, White DS, & Landrum PF (1988b) Sublethal responses to
endrin in sediment by Limnodrilus hoffmeisteri (Tubificidae) and in
mixed-culture with Stylodrilus heringianus (Lumbriculidae). Aquat
Toxicol, 13: 227-250.
Keilty TJ, White DS, & Landrum PF (1988c) Sublethal responses to
endrin in sediment by Stylodrilus heringianus (Lumbriculidae) as
measured by a 137cesium marker layer technique. Aquat Toxicol, 13:
251-270.
Keplinger MK & Deichmann WB (1967) Acute toxicity of combinations of
pesticides. Toxicol Appl Pharmacol, 10: 586-595.
Khangarot BS, Sehgal A, & Bhasin MK (1985) Man and biosphere--studies
on the Sikkim Himalayas. Part 6: Toxicity of selected pesticides to
frog tadpole, Rana hexadactyla (Lesson). Acta Hydrochim Hydrobiol,
13(3): 391-394.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci Health,
A21(1): 15-53.
Kiene RP & Capone DG (1984) Effects of organic pollutants on
methanogenesis, sulfate reduction and carbon dioxide evolution in salt
marsh sediments. Mar Environ Res 13: 141-160.
,
Kiigemagi U, Sprowls RG, & Terriere LC (1958) Endrin content of milk
and body tissues of dairy cows receiving endrin daily in their diet.
J Agric Food Chem, 6(7): 518-521.
King KA, Flickinger EL, & Hildebrand HH (1977) The decline of brown
pelicans on the Lousiana and Texas Gulf Coast. Southwest Nat, 21(4):
417-431.
King KA, Blankinship DR, Payne E, Krynitsky AJ, & Hensler GL (1985)
Brown pelican populations and pollutants in Texas, 1975-1981. Wilson
Bull, 97(2): 201-214.
Kinoshita FK & Kempf CK (1970) Quantitative measurement of hepatic
microsomal enzyme induction after dietary intake of chlorinated
hydrocarbon insecticides. Toxicol Appl Pharmacol, 17: 288.
Klein W, Mueller W, & Korte F (1968) [Insecticides in the metabolism.
XVI. Excretion, distribution and metabolism of endrin 14C in rats.]
Liebigs Ann Chem,713: 180-185.
Klevay LM (1971) Endrin excretion by the isolated perfused rat liver:
a sexual difference. Proc Soc Exp Biol Med, 136: 878-879.
Kodavanti PRS, Mehrotra BD, Chetty SC, & Desaiah D (1988) Effect of
selected insecticides on rat brain synaptosomal adenylate cyclase and
phosphodiesterase. J Toxicol Environ Health, 25: 207-215.
Koeman JH (1971) [The occurrence and the toxicological implications of
some chlorinated hydrocarbons in the Dutch coastal area in the period
1965-70], Utrecht, Rijks University, pp 88, 96 (Thesis) (in Dutch).
Koeman JH, Oskamp AAG, Veen J, Brouwer E, Rooth J, Zwart P, van de
Brock E, & van Genderen H (1967) Insecticides as a factor in the
mortality of the sandwich tern (Sterna sandvicensis). A preliminary
communication. Meded Fac Landbouwwet Rijksuniv Gent, 32: 841-854.
Koeman JH, Vink JAJ, & de Goeij JJM (1969) Causes of mortality in
birds of prey and owls in the Netherlands in the winter of 1968-69.
Andrea, 57: 67-76.
Koeman JH, Pennings JH, de Goeij JJM, Tjioe PS, Olindo PM, & Hopcraft
J (1972) A preliminary survey of the possible contamination of Lake
Nakuru in Kenya with some metals and chlorinated hydrocarbon
pesticides. J Appl Ecol, 9(2): 411-416.
Koeman JH, Pennings JH, Rosanto R, Soemarwoto O, Tjioe PS, Blancke S,
Kusumadinata S, & Djajadiredja RR (1974) Metals and chlorinated
hydrocarbon pesticides in samples of fish, sawah-duck eggs,
crustaceans and molluscs collected in Indonesia in April and May 1972.
Unpublished report, Wageningen-Bandung, University of Wageningen, The
Netherlands.
Korn S & Earnest R (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game, 60(3):
128-131.
Korte F (1969) Summary of results in 1969. Unpublished report,
submitted to WHO by Shell.
Korte F, Klein W, Weisgerber I, Kaul R, Mueller W, & Djirsurai A
(1970) Recent results in studies on the fate of chlorinated
insecticides. In: Deichmann WB, Radomski JL, & Penalver RA, ed.
Proceedings of the Sixth Conference on Toxicology and Occupational
Medicine, Pesticide Symposia. Miami, Florida, Halos & Associates,
Inc., pp 51-56.
Krantz WC, Mulhern BM, Bagley GE, Sprunt A, Ligas FJ, & Robertson WC
Jr (1970) Organochlorine and heavy metal residues in bald eagle eggs.
Pestic Monit J, 4(3): 136-140.
Kreitzer JF (1980) Effects of toxaphene and endrin at very low dietary
concentrations on discrimination acquisition and reversal in bobwhite
quail Colinus virginianus. Environ Pollut (Ser A), 23: 217-230.
Kreitzer JF & Heinz GH (1974) The effect of sub-lethal dosages of five
pesticides and a polychlorinated biphenyl on the avoidance response of
Coturnix quail chicks. Environ Pollut, 6: 21-29.
Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA,
Sileo L, Docjerty DE, & Erdman TC (1989) Microcontaminants and
reproductive impairment of the Forster's tern on Green Bay, Lake
Michigan--1983. Arch Environ Contam Toxicol, 18: 706-727.
Kudesia VP & Bali NP (1985) A study of pesticides in Kalinadi River
and evaluation of toxicity of some pesticides on fish Clarias
batrachus. Acta Cienc Indica, 10C(4): 245-254.
Kummer R & Van Sittert NJ (1984) Field study on health effects in
farmers applying an endrin/DDT/MEP formulation by hand-held ULV to
cotton in Ivory Coast. Unpublished report, The Hague, Shell
Internationale Petroleum Maatschappij, submitted to WHO by Shell.
Kummer R & Van Sittert NJ (1986) Field studies on health effects from
the application of two organophosphorus insecticide formulations by
hand-held ULV to cotton. Toxicol Lett, 33: 7-24.
Kurata M, Hirose K, & Umeda M (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
Kurhekar MP, D'Souza FC, & Meghal SK (1975) Rapid method for
extracting aldrin, dieldrin, and endrin from visceral material. J
Assoc Off Anal Chem, 58(3): 548-550.
Kutz FW, Yobs AR, & Yang HSC (1976) National pesticide monitoring
programs. In: Lee RE Jr, ed. Air pollution from pesticides and
agricultural processes. Cleveland, Ohio, CRC Press, pp 95-136.
Kutz F, Strassman S, & Yobs AR (1979a) Survey of pesticide residues
and their metabolites in the general population of the United States.
In: Berlin A, Wolff AH, & Hasegawa Y ed. Use of biological specimens
to assess human exposure to environmental pollutants, The Hague,
Martinus Nijhoff, pp 267-274.
Kutz FW, Strassman SC, & Sperling JF (1979b) Survey of selected
organochlorine pesticides in the general population of the United
States. Fiscal years 1970-1975. Ann NY Acad Sci, 320: 60-68.
Lara WH & Barreto HHC (1972) [Chlorinated pesticide residues in
water.] Rev Inst Adolfo Lutz, 32: 69-74 (in Portuguese with English
summary).
Lauer GJ, Nicholson HP, Cox WS, & Teasley JI (1966) Pesticide
contamination of surface waters by sugar cane farming in Louisiana.
Trans Am Fish Soc, 95(3): 310-316.
Lawrence CH, Coleman RL, & Sowell WL (1968) Endrin induced trace metal
alterations following acute exposure. Bull Environ Contam Toxicol,
3(4): 229-239.
Leard RL, Grantham BJ, & Pessoney GF (1980) Use of selected freshwater
bivalves for monitoring organochlorine pesticide residues in major
Mississippi stream systems, 1972-73. Pestic Monit J, 14(2): 47-52.
Lebel GL & Williams DT (1986) Determination of halogenated
contaminants in human adipose tissue. J Assoc Off Anal Chem, 69(3):
451-458.
Lichtenberg JJ, Eichelberger JW, Dressman RC, & Longbottom JE (1970)
Pesticides in surface waters of the United States; a 5-year summary,
1964-1968. Pestic Monit J, 4(2): 71-86.
Lopez-Avila V, Schoen S, Milanes J, & Beckert WF (1988)
Single-laboratory evaluation of EPA method 8080 for determination of
chlorinated pesticides and polychlorinated biphenyls in hazardous
wastes. J Assoc Off Anal Chem, 71(2): 375-387.
Lowe JI (1966) Some effects of endrin on estuarine fishes. In:
Proceedings of the 19th Annual Conference of the Southeast Association
of Game and Fish Commissioners, pp 271-276.
Luckens MM & Davis WH (1965) Toxicity of dieldrin and endrin to bats.
Nature, 207(4999): 879-880.
Luckens MM & Phelps KI (1969) Serum enzyme patterns in acute poisoning
with organochlorine insecticides. J Pharm Sci, 58(5): 569-572.
Ludke JL (1976) Organochlorine pesticide residues associated with
mortality: additivity of chlordane and endrin. Bull Environ Contam
Toxicol, 16(3): 253-260.
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multi-residue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-433.
Lund AE & Narahasi T (1983) Kinetics of sodium channel modification as
the basis for the variation in the nerve membrane effects of
pyrethroids and DDT analogs. Pestic Biochem Physiol, 20: 203-206.
Lykins BW Jr, Koffskey WE, & Miller RG (1986) Chemical products and
toxicologic effects of disinfection. J Am Water Works Assoc, 78(11):
66-75.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14(9): 1253-1265.
McGill AEJ & Robinson J (1968) Organochlorine insecticide residues in
complete prepared meals: a 12-month survey in SE England. Food Cosmet
Toxicol, 6: 45-57.
Machbub B, Ludwig HF, & Gunaratnam D (1988) Environmental impact from
agrochemicals in Bali (Indonesia). Environ Monit Assess, 11: 1-23.
McIntyre AE & Lester JN (1984) Occurrence and distribution of
persistent organochlorine compounds in UK sewage sludges. Water Air
Soil Pollut, 23: 397-415.
McKenney CL Jr (1986) Critical responses of populations of crustacea
to toxicants. Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Environmental Research
Brief EPA/600/M-86/004.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol 25: 921-928.
McLeese DW, Metcalfe CD, & Pezzack DS (1980) Bioaccumulation of
chlorobiphenyls and endrin from food by lobsters (Homarus
americanus). Bull Environ Contam Toxicol 25: 161-168.
McLeese DW, Burridge LE, & van Dinter J (1982) Toxicities of five
organochlorine compounds in water and sediment to Nereis virens. Bull
Environ Contam Toxicol 28: 216-220.
Madden JD, Finerty MW, & Grodner RM (1989) Survey of persistent
pesticide residues in the edible tissues of wild and pond-raised
Louisiana crayfish and their habitat. Bull Environ Contam Toxicol, 43:
779-784.
Manske DD & Corneliussen PE (1974) Pesticide residues in total diet
samples (VII). Pestic Monit J, 8(2): 110-124.
Manske DD & Johnson RD (1975) Pesticide residues in total diet samples
(VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Pesticide and other chemical residues in
total diet samples (X). Pestic Monit J, 10(4): 134-148.
Marinelli P, Stracciari GL, & Anfossi P (1986) [Presence of
organochlorinated pesticides in some wine-making by-products.] Zoot
Nutr Anim, 12: 479-486 (in Italian with English summary).
Marsh C (1963) Metabolism of D-glucuronolactone in mammalian systems.
Conversion of D-glucuronolactone into D-glucaric acid by tissue
preparations. Biochem J, 87: 82-90.
Marston RB, Tyo RM, & Middendorff SC (1969) Endrin in water from
treated Douglas fir seed. Pestic Monit J, 2(4): 167-171.
Martin RJ & Duggan RE (1968) Pesticide residues in total diet samples
(III). Pestic Monit J, 1(4): 11-20.
Martin DB & Hartman WA (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North Central United States. J Assoc Off Anal Chem, 68(4): 712-717.
Martin JP, Harding RB, Cannell GH, & Anderson L (1959) Influence of
five annual field applications of organic insecticides on soil
biological and physical properties. Soil Sci, 87: 334-338.
Maslansky CJ & Williams GM (1981) Evidence for an epigenetic mode of
action in organochlorine pesticide hepatocarcinogenicity. A lack of
genotoxicity in rat, mouse and hamster hepatocytes. J Toxicol Environ
Health, 8: 121-130.
Mason JW & Rowe OR (1976) The accumulation and loss of dieldrin and
endrin in the eastern oyster. Arch Environ Contam Toxicol, 4(3):
349-360.
Masud SZ & Farhat S (1985) Pesticide residues in foodstuffs in
Pakistan--organochlorine pesticides in fruits and vegetables. Pak J
Sci Ind Res, 28(6): 417-422.
Matsumoto K, Eldefrawi ME, & Eldefrawi AT (1988) Action of
polychlorocycloalkane insecticides on binding of
[35S]t-butylbicyclophosphorothionate to Torpedo electric organ
membranes and stereospecificity of binding site. Toxicol Appl
Pharmacol, 95: 220-229.
Matsumura F, Khanvilkar VG, Patil KC, & Boush GM (1971) Metabolism of
endrin by certain soil microorganisms. J Agric Food Chem, 19(1):
27-31.
Maule A, Plyte S, & Quirk AV (1987) Dehalogenation of organochlorine
insecticides by mixed anaerobic microbial populations. Pestic Biochem
Physiol, 27: 229-236.
Mayer, FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Environmental Research Laboratory (Environmental Research Brief
EPA/600/8-87/017).
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior Fish
and Wildlife Service (Resource publication 160).
Meena K, Gupta PK, & Bawa SR (1978) Endrin-induced toxicity in normal
and irradiated rats. Environ Res, 16: 373-382.
Mehrotra BD, Moorthy, KS, Reddy SR, & Desaiah D (1989) Effects of
cyclodiene compounds on calcium pump activity in rat brain and heart.
Toxicology, 54: 17-29.
Meith-Avcin N, Warlen SM, & Barber RT (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 meters.
Environ Lett, 5(4): 215-221.
Mersch-Sundermann V, Dickgiesser N, Hablizel U, & Gruber B (1988)
[Examination of mutagenicity of organic microcontaminations on the
environment. I. Communication: the mutagenicity of selected herbicides
and insecticides with the Salmonella-microsome-test (Ames-test) in
consideration of the pathogenetic potence of contaminated ground- and
drinking-water.] Zbl Bakteriol Hyg B, 186: 247-260 (in German).
Metcalf RL, Kapoor IP, Lu PY, Schuth CK, & Sherman P (1973) Model
ecosystem studies of the environmental fate of six organochlorine
pesticides. Environ Health Perspect, 4: 35-44.
Miles JRW & Harris CR (1973) Organochlorine insecticides residues in
streams draining agricultural, urban agricultural and resort areas of
Ontario, Canada, 1971. Pestic Monit J, 6(4): 363-368.
Miller PE & Fink GB (1973) Brain serotonin level and pentylenetetrazol
seizure threshold in dieldrin and endrin treated mice. Proc West
Pharmacol Soc, 16: 195-197.
Modin JC (1969) Chlorinated hydrocarbon pesticides in California bays
and estuaries. Pestic Monit J, 3(1): 1-7.
Morita H & Umeda M (1984) Detection of mutagenicity of various
compounds by FM3A cell system. Mutat Res, 130(5): 371.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay system. Mutat Res, 116: 185-216.
Morris RD (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse, Peromyscus maniculatus. Can J Zool,
46(5): 951-958.
Morris RD (1970) The effects of endrin on Microtus and Peromyscus.
I. Unenclosed field populations. Can J Zool, 48: 695-708.
Morris RD (1972) The effects of endrin on Microtus and Peromyscus.
II. Enclosed field populations. Can J Zool, 50(6): 885-896.
Moser GJ & Smart RC (1989) Hepatic tumour-promoting chlorinated
hydrocarbons stimulate protein kinase C activity. Carcinogenesis,
10(5): 851-856.
Mount DI & Putnicki GJ (1966) Summary report of the 1963 Mississippi
fish kill. North Am Wildl Nat Res Conf Trans, 31: 177-184.
Mount DI, Vigor LW, & Schafer ML (1966) Endrin: use of concentration
in blood to diagnose acute toxicity in fish. Science, 152: 1388-1390.
Mugambi JM, Kanja L, Maitho TE, Skaare JU, & Lokken P (1989)
Organochlorine pesticide residues in domestic fowl (Gallus
domesticus) eggs from Central Kenya. J Sci Food Agric, 48: 165-176.
Muir CMC (1970) The acute oral and percutaneous toxicities to rats of
formulations of aldrin, dieldrin or endrin. Unpublished report No.
TLGR.0020.70, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Mukerjee SK (1985) The environmental photodegradation of pesticides.
Indian J Agric Chem, 18(1): 1-9.
Mulhern BM, Reichel WL, Locke LN, Lamont TG, Belisle A, Cromartie E,
Bagerly GE, & Prouty R (1970) Organochlorine residues and autopsy data
from bald eagles. 1966-1968. Pestic Monit J, 4: 141-144.
Muncy RJ & Oliver AD Jr (1963) Toxicity of ten insecticides to the red
crawfish, Procambarus clarki (Girard). Trans Am Fish Soc, 92:
428-431.
Mussalo-Rauhamaa H, Salmela SS, Leppanen A, & Pyysalo H (1986)
Cigarettes as a source of some trace and heavy metals and pesticides
in man. Arch Environ Health, 41(1): 49-55.
Nagelsmit A, Vliet PW, van Wiel-Wetzels WAM, van der Wielard MJ, Strik
JJTWA, Ottevanger CF, & van Sittert NJ (1979) Porphyrins as possible
parameters for exposure to hexachlorocyclopentadiene, allylchloride,
epichlorohydrin and endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biochemical Press,
pp 55-61.
Narahasi T (1987) Effects of toxic agents on neural membranes. In:
Lowndes HE,ed. Electrophysiology in neurotoxicology. Boca Raton,
Florida, CRC Press, vol 1, pp 23-44.
NCI (1978) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute (DHEW Publication No. NIH 179-812)
NCI (1979) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute, 110 pp (Carcinogenesis Technical Report Series No. 12; NTIS
PB-288461)
Nebeker AV, Schuytema GS, Griffis WL, Barbitta JA, & Carey LA (1989)
Effect of sediment organic carbon on survival of Hyalella azteca
exposed to DDT and endrin. Environ Toxicol Chem, 8: 705-718.
Nelson SC, Bahler TL, Hartwell WV, Greenwood DA, & Harris LE (1956)
Serum alkaline phosphatase levels, weight changes and mortality rates
of rats fed endrin. J Agric Food Chem, 4(8): 696-700.
Nettleship DN & Peakall DB (1987) Organochlorine residue levels in
three high Arctic species of colonially-breeding seabirds from Prince
Leopold Island. Mar Pollut Bull, 18(8): 434-438.
NIOSH (1989) Manual of analytical methods. Endrin: Method No. 5519.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 1-4.
Nishimura N, Nishimura H, & Oshima H (1982) Survey on mutagenicity of
pesticides by the Salmonella-microsome test. J Aichi Med Univ Assoc,
10(4): 305-312.
Notten WRF & Henderson PT (1975) Alteration in urinary D-glucaric acid
excretion as an indication of exposition to xenobiotics. In:
Proceedings of the International Symposium--Environment and Health,
CEC/EPA/WHO, Paris, 1974.
Novak AF & Rao MRR (1965) Food safety program: endrin monitoring in
the Mississippi River. Science, 150: 1751.
Ohlendorf HM, Swineford DM, & Locke LN (1981) Organochlorine residues
and mortality of herons. Pestic Monit J, 14(4): 125-135.
Onodera S & Tabucanon MS (1986) Organochlorine pesticide residues in
the lower Chao Phraya River and klongs along the river at Bangkok
metropolitan area, 1982-1984. J Sci Soc Thailand, 12: 225-238.
Oomen PA (1986) A sequential scheme for evaluating the hazard of
pesticides to bees, Apis mellifera. Meded Fac Landbouwwet Rijksuniv
Gent, 51(3b): 1205-1213.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis K (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
O'Shea TJ, Brownell RL Jr, Clarke DR Jr, Walker WA, Gay ML, & Lamont
TG (1980) Organochlorine pollutants in small cetaceans from the
Pacific and South Atlantic Oceans, November 1968-June 1976. Pestic
Monit J, 14:(2): 35-46.
Ottevanger CF & Van Sittert NJ (1979) Relation between anti-12-hydroxy
endrin excretion and enzyme induction in workers involved in the
manufacture of endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 123-129.
Ottolenghi AD, Haseman JK, & Suggs F (1974) Teratogenic effects of
aldrin, dieldrin and endrin in hamsters and mice. Teratology, 9:
11-16.
Parveen Z & Masud SZ (1987) Organochlorine pesticide residues in
cattle feed samples in Karachi, Pakistan. J Sci Ind Res; 30(7):
513-516.
Patil KC, Matsumura F, & Boush GM (1970) Degradation of endrin,
aldrin, and DDT by soil microorganisms. Appl Microbiol, 19(5):
879-881.
Pavan I, Buglione E, Pettinati L, Perrelli G, Rubino GF, Bicchi C,
D'Amato A, Carlino F, Bugiani M, & Polizzi S (1987) [Accumulation of
organochlorine pesticides in human adipose tissue. data from the
province of Turin (Italy).] Med Lav, 78(3): 219-228 (in Italian).
Pawar SS & Kachole MS (1978) Hepatic and renal microsomal electron
transport reactions in endrin treated female guinea pigs. Bull Environ
Contam Toxicol, 20: 199-205.
Pearce F (1987) Pesticide deaths: the price of the green revolution.
New Sci, 114: 30.
Peterson SR & Ellarson RS (1978) pp'-DDE, polychlorinated biphenyls,
and endrin in old squaws in North America, 1969-73. Pestic Monit J,
11(4): 170-181.
Petrella VJ, Fox JP, & Webb RE (1975) Endrin metabolism in
endrin-susceptible and resistant strains of pine mice. Toxicol Appl
Pharmacol, 34: 283-291.
Petrella VJ, McKinney JD, Fox JP, & Webb RE (1977) Identification of
metabolites of endrin. Metabolism in endrin susceptible and resistant
strains of pine mice. J Agric Food Chem, 25(2): 393-398.
Pfister RM (1972) Interactions of halogenated pesticides and
microorganisms: a review. CRC Crit Rev Microbiol, 21(1): 1-33.
Phillips DD, Pollard GE, & Soloway SB (1962) Thermal isomerization of
endrin and its behaviour in gas chromatography. J Agric Food Chem,
10(3): 217-221.
Plimmer JR (1972) Photochemistry of organochlorine insecticides. In:
Tahori AS, ed. Proceedings of the Ssecond international IUPAC congress
of pesticides chemistry. New York, Gordon & Breach,vol 1, pp 413-432.
Podrebarac DS (1984) Pesticide, heavy metal, and other chemical
residues in infant and toddler total diet samples (IV). October
1977-September 1978. J Assoc Off Anal Chem, 67(1): 166-175.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environ Mutagen, 3: 11-32.
Prouty RM & Bunck C (1986) Organochlorine residues in adult mallard
and black duck wings, 1981-1982. Environ Monit Assess, 6: 49-57.
Prouty RM, Reichel WL, Locke LN, Belisle AA, Cromartie E, Kaiser TE,
Lamont TG, Mulhern BM, & Swineford DM (1977) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1973-74. Pestic Monit J, 11: 134-137.
Radeleff RD (1956) Hazards to livestock of insecticides used in
mosquito control. Mosq News, 16(2): 79-80.
Radhakrishnan AG & Antony PD (1989) Pesticide residues in marine
fishes. Fish Technol, 26: 60-61.
Rashid KA & Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. J Environ Sci Health, B21(4): 319-334.
Reddy DB, Edward VD, Abraham GJS, & Rao KV (1966) Fatal endrin
poisoning. A detailed autopsy, histopathological and experimental
study. J Indian Med Assoc, 46(3): 121-124.
Reddy DB, Abraham GJS, Edward VD, Naganna B, & Mathalli M (1967)
Further observations on endrin poisoning. J Indian Med Prof, 13(10):
5946, 5967-5968.
Redetzke K., Gonzalez AA, & Applegate HG (1983) Organochlorine
pesticides in adipose tissue of persons from Ciudad Juarez, Mexico. J
Environ Health, 46(1): 25-27.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 117(20): 525-527.
Reeves RG, Woodham DW, Ganyard MC, & Bond CA (1977) Preliminary
monitoring of agricultural pesticides in a cooperative tobacco pest
management project in North Carolina, 1971--first-year study. Pestic
Monit J, 11(2): 99-106.
Reichel WL, Lamont TG, Cromartie E, & Locke LN (1969) Residues in two
bald eagles suspected of pesticide poisoning. Bull Environ Contam
Toxicol, 4(1): 24-30.
Reidinger RF & Crabtree DG (1974) Organochlorine residues in golden
eagles, United States--March 1964-July 1971. Pestic Monit J, 8(1):
37-43.
Reins DA, Holmes DD, & Hinshaw LB (1964) Acute and chronic effects of
the insecticide endrin on renal function and renal hemodynamics. Can
J Physiol Pharmacol, 42(5): 599-608.
Reins DA, Rieger JA Jr, Stavinoha WB, & Hinshaw LB (1966) Effect of
endrin on venous return and catecholamine release in the dog. Can J
Physiol Pharmacol, 44: 59-67.
Ressang AA, Titus I, Andar RS, & Soedarmo D (1958) Aldrin, dieldrin
and endrin intoxication in cats. Commun Vet, 2(2): 71-88.
Reuber MD (1978) Carcinomas, sarcomas and other lesions in
Osborne-Mendel rats ingesting endrin. Exp Cell Biol, 46(3): 129-145.
Revzin AM (1966) Effects of endrin on telencephalic function in the
pigeon. Toxicol Appl Pharmacol, 9(1): 75-83.
Revzin AM (1980) Some acute and chronic effects of endrin on the
brains of pigeons and monkeys. In: Proceedings of the symposium on the
biological impact of pesticides in the environment, pp 134-141.
Ribbens PH (1985) Mortality study of industrial workers exposed to
aldrin, dieldrin and endrin. Arch Occup Environ Health, 56(2): 75-79.
Richardson LA, Lane JR, Gardner WS, Peeler JT, & Campbell JE (1967)
Relationship of dietary intake to concentration of dieldrin and endrin
in dogs. Bull Environ Contam Toxicol, 2(4): 207-219.
Richardson A, Robinson J, & Baldwin MK (1970) Metabolism of endrin in
the rat. Chem Ind, 1970,: 502-503.
Ritchey SJ, Young RW, & Essary EO (1972) Effects of heating and
cooking method on chlorinated hydrocarbon residues in chicken tissues.
J Agric Food Chem, 20: 291-293.
Robinson J & McGill AEJ (1966) Organochlorine insecticide residues in
complete prepared meals in Great Britain during 1965. Nature,
212(5066): 1037-1038.
Robinson J, Richardson A, Hunter CG, Crabtree AN, & Rees HJ (1965)
Organochlorine insecticide content of human adipose tissue in
south-eastern England. Br J Ind Med, 22: 220-229.
Roos AH, van Munsteren AJ, Nab FM, & Tuinstra LGMT (1987) Universal
extraction/clean-up procedure for screening of pesticides by
extraction with ethylacetate and size exclusion chromatography. Anal
Chim Acta, 196: 95-102.
Rosales MTL, Escalona RL, Alarcon RM, & Zamora V (1985) Organochlorine
hydrocarbon residues in sediments of two different lagoons of
Northwest Mexico. Bull Environ Contam Toxicol, 35: 322-330.
Rosen JD (1972) Conversion of pesticides under environmental
conditions. In: Coulston F & Korte F ed. Environmental quality.
Stuttgart, George Thieme, vol 1, pp 85-96.
Rosen JD, Sutherland DJ, & Lipton GR (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull
Environ Contam Toxicol, 1: 133-140.
Rowe DR, Canter LW, Snyder PJ, & Mason JW (1971) Dieldrin and endrin
concentrations in a Louisiana estuary. Pestic Monit J, 4(4): 177-183.
Rowley DL, Rab MA, Hardjotanojo W, Liddle J, Burse VW, Saleem M, Sokal
D, Falk H, & Head SL (1987) Convulsions caused by endrin poisoning in
Pakistan. Pediatrics, 79(6): 928-934.
Roylance KJ, Jorgensen CD, Booth GM, & Carter MW (1985) Effects of
dietary endrin on reproduction of Mallard ducks (Anas
platyrhynchos). Arch Environ Contam Toxicol, 14: 705-711.
Runhaar EA, Sangster B, Greve PA, & Voortman M (1985) A case of fatal
endrin poisoning. Hum Toxicol, 4: 241-247.
Ryan S, Bacher GJ, & Martin AA (1972) The mussel Hyridella australis
as a biological monitor of the pesticide endrin in fresh water.
Search, 3(11-12): 446-447.
Safe S & Hutzinger O (1979) Mass spectrometry of pesticides and
pollutants. Boca Raton, Florida, CRC Press, Inc.
Saiki MK & Schmitt CJ (1986) Organochlorine chemical residues in
bluegills and common carp from the irrigated San Joaquin Valley floor,
California. Arch Environ Contam Toxicol, 15: 357-366.
Samhan O & Ghobrial F (1987) Trace metals and chlorinated hydrocarbons
in sewage sludges of Kuwait. Water Air Soil Pollut, 36: 239-246.
Satsmadjis J, Georgakopoulos-Gregoriades E, & Voutsinou-Taliadouri F
(1988) Red mullet contamination by PCBs and chlorinated pesticides in
the Pagassitikos Gulf, Greece. Mar Pollut Bull, 19(3): 136-138.
Schafer ML, Peeler JT, Gardner WS, & Campbell JE (1969) Pesticides in
drinking water--waters from the Mississippi and Missouri rivers.
Environ Sci Technol,3(12): 1261-1269.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12: 355-382.
Scheutz EG, Wrighton SA, Safe SH, & Guzelian PS (1986) Regulation of
cytochrome P-450p by phenobarbital and phenobarbital-like inducers in
adult rat hepatocytes in primary monolayer culture and in vivo.
Biochemistry, 25: 1124-1133.
Schwabe U & Wendling I (1967) [Stimulation of drug metabolism by low
doses of DDT and other chlorinated hydrocarbon insecticides.]
Arzneimittel-forschung, 17(5): 614-618 (in German).
Seifert J (1988) Ontogenesis and properties of the convulsant
recognition site(s) of the gamma-aminobutyric acid (GABA) receptor
complex in chicken embryo. Eur J Pharmacol,151: 443-448.
Seifert J (1989) Teratogenesis of polychlorocycloalkane insecticides
in chicken embryos resulting from their interactions at the convulsant
recognition sites of the GABA (pro)receptor complex. Bull Environ
Contam Toxicol, 42: 707-715.
Sherman M & Rosenberg M (1954) Subchronic toxicity of four chlorinated
dimethanonaphthalene insecticides to chicks. J Econ Entomol, 47 (6):
1082-1083.
Sierra M & Santiago D (1987) Organochlorine pesticide levels in barn
owls collected in Leon, Spain. Bull Environ Contam Toxicol, 38:
261-265.
Sierra M, Teran MT, Gallego A, Diez MJ, & Santiago D (1987)
Organochlorine contamination in three species of diurnal raptors in
Leon, Spain. Bull Environ Contam Toxicol, 38: 254-260.
Simmon VF, Kauhanen K, & Tardiff RC (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North Holland Biomedical Press, pp 249-258.
Singh MN (1988) Mortality response of Achatina fulica to various
pesticides. J Environ Biol, 9(2): 157-162.
Singh PDA & West ME (1985) Acute pesticide poisoning in the Caribbean.
West Indian Med J, 34: 75-83.
Singhal RL & Kacew S (1976) The role of cyclic AMP in chlorinated
hydrocarbon-induced toxicity. Fed Proc, 35(14): 2618-2623.
Smith KJ, Polen PB, de Vries DM, & Coon FB (1968) Removal of
chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J Am Oil Chem Soc, 45: 866-869.
Sobti RC, Krishan A, & Davies J (1983) Cytokinetic and cytogenetic
effect of agricultural chemicals on human lymphoid cells in vitro.
II. Organochlorine pesticides. Arch Toxicol, 52: 221-231.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
northeastern Alberta otters. Bull Environ Contam Toxicol, 39: 783-790.
Soto AR & Deichmann WB (1967) Major metabolism and acute toxicity of
aldrin, dieldrin and endrin. Environ Res, 1(4): 307-322.
Spann JW, Heinz GH, & Hulse CS (1986) Reproduction and health of
mallards fed endrin. Environ Toxicol Chem, 5: 755-759.
Speck LB & Maaske CA (1958) The effects of chronic and acute exposure
of rats to endrin. Arch Ind Health, 18: 268-272.
Spynu EI (1964) On the toxicology of new organic chloride insecticides
obtained by diene synthesis on the basis of hexachlorocyclopentadiene.
Gig Tr Prof Zabol, 4: 30-35.
Squires RF & Saederup E (1989) Polychlorinated convulsant insecticides
potentiate the protective effect of NaCl against heat inactivation of
[3H] fluonitrazepam binding sites. J Neurochem, 52: 537-543.
Stanford Research Institute (1953) Unpublished letter Report No. 1
Ref. Project No. B-868 November 10, Stanford, CA, submitted to WHO by
Shell.
Stanford Research Institute (1954) Unpublished letter Report No. 3
Ref. Project No. B-868 February 1, Stanford, CA, submitted to WHO by
Shell.
Stanley CW, Barney JE II, Helton MR, & Yobs AR (1971) Measurement of
atmospheric levels of pesticides. Environ Sci Technol, 5: 430-435.
Steinberg KK, Garza A, Bueso JA, Burse VW, & Phillips DL (1989) Serum
pesticide concentrations in farming cooperatives in Honduras. Bull
Environ Contam Toxicol, 42: 643-650.
Stickel WH, Kaiser TE, & Reichel WL (1979) Endrin versus 12-ketoendrin
in birds and rodents. In: Kenaga EE ed. Avian and mammalian wildlife
toxicology, Philadeplphia,, American Society for Testing and
Materials, pp 61-68 (ASTM STP 693).
Stout VF (1980) Organochlorine residues in fishes from the Northwest
Atlantic Ocean and Gulf of Mexico. Fish Bull, 78(1): 51-58.
Strachan WMJ (1988) Toxic contaminants in rainfall in Canada: 1984.
Environ Toxicol Chem, 7: 871-877.
Strachan WMJ, Huneault H, Schertzer WM, & Elder FC (1980)
Organochlorines in precipitation in the Great Lakes region. In: Afghan
BK & MacKay D ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment. New York, Plenum Publishing Corp., pp 387-396.
Strassman SC & Kutz FW (1977) Insecticide residues in human milk from
Arkansas and Mississippi, 1973-1974. Pestic Monit J, 10(4): 130-133.
Strik JJTWA (1979) The occurrence of chronic hepatic porphyria in man,
caused by halogenated hydrocarbons. In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Elsevier/North Holland
Biomedical Press, p 3.
Struger J, Weseloh D, Hallett DJ, & Mineau P (1985) Organochlorine
contaminants in herring gull eggs from the Detroit and Niagara Rivers
and Saginaw Bay (1978-1982): contaminant discriminants. J Great Lakes
Res, 11(3): 223-230.
Sturm R, Knauth HD, Reinhardt KH, & Gandrasz J (1986) Distribution of
chlorinated hydrocarbons in sediment and seston of the River Elbe.
Wasser, 67: 23-38.
Sundershan P & Khan MAQ (1980) Metabolic fate of [14C] endrin in
bluegill fish. Pestic Biochem Physiol, 14: 5-12.
Suzuki M & Morimoto M (986) High resolution chemically bonded
fused-silica capillary gas chromatography of organochlorine
insecticides and related compounds in arable soil samples. J High
Resol Chromatogr Chromatogr Commun, 9: 296-298.
Suzuki M, Yamato Y, & Watanabe T (1973) Multiple organochlorine
pesticide residues in Japan. Bull Environ Contam Toxicol, 10(3):
145-150.
Suzuki K, Nagayoshi H, & Kashiwa T (1974) The systematic separation
and identification of pesticide in the first division. Agric Biol
Chem, 38(2): 279-285.
Sykes PW Jr (1985) Pesticide concentrations in snail kite eggs and
nestlings in Florida. Condor, 87: 438.
Tarrant KR & Tatton JO'G (1968) Organochlorine pesticides in rainwater
in the British Isles. Nature, 219(5155): 725-727.
Telling GM, Sissons DJ, & Brinkman HW (1977) Determination of
organochlorine insecticide residues in fatty food stuffs using a
clean-up technique based on a single column of activated alumina. J
Chromatogr, 137: 405-423.
Teran MT & Sierra M (1987) Organochlorine insecticides in trout,
Salmo trutta fario L. taken from four rivers in Leon, Spain. Bull
Environ Contam Toxicol, 38: 247-253.
Terriere LC (1964) Endrin. In: Zweig, G ed. Analytical methods for
pesticides, plant growth regulators and food additives. New York,
Academic Press, vol 2, pp 209-222.
Terriere LC, Kiigemagi U, & England DC (1958) Endrin content of body
tissues of steers, lambs and hogs receiving endrin in their daily
diet. J Agric Food Chem, 6(7): 516-518.
Terriere LC, Arscott GH, & Kiigemagi U (1959) The endrin content of
eggs and body tissue of poultry receiving endrin in their daily diet.
J Agric Food Chem, 7(7): 502-504.
Thier HP & Stijve T (1986) [Results of an inter-laboratory comparison
of analyses of analyses of organochlorine and organophosphorus
pesticide residues in fat.] Lebensmittelchem Gerichtl Chem, 40: 73-75
(in German).
Thompson JF (1976) Manual of analytical quality control for pesticides
and related compounds in human and environmental samples. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Office of Research and Development, Health Effects Research Laboratory
(EPA-600/1-76-017).
Thurston R, Gilfoil TA, Meyn EL, Zajdel RK, Aoki TI, & Veith GD (1985)
Comparative toxicity of ten organic chemicals to ten common aquatic
species. Water Res, 19(9): 1145-1155.
Travis CC & Arms AD (1988) Bioconcentration of organics in beef, milk
and vegetation. Environ Sci Technol, 22: 271-274.
Treon JF, Cleveland FP, & Cappel J (1955) Toxicity of endrin for
laboratory animals. J Agric Food Chem, 3(10): 842-848.
Truhaut R, Do Phuoc H, & Phu Lich N (1974) Influence de
l'administration de pesticides organo-halogénés et de
polychloro-biphényles sur le métabolisme de la zoxazolamine chez le
rat. CR Acad Sci Paris Sér D, 278: 3003-3006.
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Bureau of Sport Fisheries and Wildlife,
Denver Wildlife Center (Resource Publication No. 84).
Tuinstra LGMT (1971) Organochlorine insecticide residues in human milk
in the Leiden region. Neth Milk Dairy J, 25: 24-32.
Uhnak J, Sackmauerova M, Szokolay A, & Pal'usova O (1974) The use of
an electron capture detector for the determination of pesticides in
water. J Chromatogr 91: 545-547.
United Kingdom Ministry of Agriculture, Fisheries and Food (1989)
Report of the Working Party on Pesticide Residues 1985-1988. London,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
US EPA (1974) New Orleans area water supply study (draft analytical
report). Dallas, Texas, US Environmental Protection Agency, Lower
Mississippi River Facility, Surveillance and Analysis Divisions,
Region VI.
US EPA (1983) Findings of selected chemical residues in human blood
serum and adipose tissue: Endrin. Environ News, 9 May.
US EPA (1985) Hexachloronorbornadiene; Proposed submission of notice
of manufacturer, import or processing and determination of significant
new use. Fed Reg, 50(36): 7351-7356.
US EPA (1987a) Health effects assessment for endrin. Cincinnati, Ohio,
US Environmental Protection Agency, Ofice of Research and Development,
Environmental Criteria and Assessment Office, 31 pp (Report
PB88-180237).
US EPA (1987b) Health advisories for 16 pesticides. Washington, DC, US
Environmental Protection Agency, Office of Drinking Water, 18 pp
(Report PB87-200176).
Vance BD & Drummond W (1969) Biological concentration of pesticides by
algae. J Am Water Works Assoc, 61: 360-362.
Van Dijck P & van de Voorde H (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded Fac Landbouww
Rijksuniv Gent, 41(2): 1491-1498.
Van Raalte HGS (1965) [Some aspects of pesticide toxicity.]
Unpublished paper presented at the Conference on Occupational Health,
Caracas, Venezuela; The Hague. Shell, International Petroleum Company,
Health, Safety and Environment Division (in Spanish).
Van Sittert NJ (1985) Biological monitoring of bestrijdingsmiddelen;
Coronel-PAOG Nascholingssymposium. Unpublished paper, Amsterdam, Vrije
Universiteit, February 1985 (in Dutch).
Van Wynen JH & Stykel A (1988) Health risk assessment of residents
living on harbour sludge. Arch Occup Environ Health, 61: 77-87.
Veith GD, Kuehl DW, Leonard EN, Puglisi FA, & Lemke AE (1979)
Polychlorinated biphenyls and other organic chemical residues in fish
from major watersheds of the United States, 1976. Pestic Monit J,
13(1): 1-11.
Veith GD, Kuehl DW, Leonard EN, Welch K, & Pratt G (1981)
Polychlorinated biphenyls and other organic chemical residues in fish
from major United States watersheds near the Great Lakes, 1978. Pestic
Monit J, 15(1): 1-8.
Verma MP, Bahga HS, Soni BK, & Singh SP (1970) Effect of insecticides
on lactic acid concentration in the blood of buffalo-calves. Indian
Vet J, 47(12): 1056-1058.
Vermeer K, Risebrough RW, Spaans AL, & Reynolds LM (1974) Pesticide
effects on fishes and birds in rice fields in Surinam, South America.
Environ Pollut, 7: 217-236.
Versteeg JPJ & Jager KW (1973) Long-term occupational exposure to the
insecticides aldrin, dieldrin, endrin and telodrin. Br J Ind Med, 30:
201-202.
Villeneuve JP, Holm E, & Cattini C (1985) Transfer of chlorinated
hydrocarbons in the food chain lichen --> reindeer --> man.
Chemosphere, 14(11/12): 1651-1658.
Von Westernhagen H, Dethlefsen V, Cameron P, & Janssen D (1987)
Chlorinated hydrocarbon residues in gonads of marine fish and effects
on reproduction. Sarsia, 72: 419-422.
Von Westernhagen H, Cameron P, Dethlefsen V, & Janssen D (1989)
Chlorinated hydrocarbons in North Sea whiting (Merlangius merlangus)
and effects on reproduction. I. Tissue burden and hatching success.
Helgolander Meeresunters 43: 45-60.
Vrij-Standhardt WG, Strik JJTWA, Ottevanger CF, & van Sittert NJ
(1979) Urinary D-glucaric acid and urinary total porphyrin excretion
in workers exposed to endrin. In: Strik JJTWA & Koeman JH ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 113-121.
Wafford KA, Sattelle DB, Gant DB, Eldefrawi AT, & Eldefrawi ME (1989a)
Non-competitive inhibition of GABA receptors in insect and vertebrate
CNS by endrin and lindane. Pestic Biochem Physiol, 33: 213-219.
Wafford KA, Lummis SCR, & Sattelle DB (1989b) Block of an insect
central nervous system GABA receptor by cyclodiene and cyclohexane
insecticides. Proc R Soc Lond, B237: 53-61.
Walker JJ & Phillips DE (1987) An electron microscopic study of endrin
induced alterations in unmyelinated fibers of mouse sciatic nerve.
Neurotoxicology, 8(1): 55-64.
Walsh GM & Fink GB (1970) Temporal aspects of acute endrin toxicity in
mice. Proc West Pharmacol Soc, 13: 81-83.
Walsh GM & Fink GB (1972) Comparative toxicity and ditribution of
endrin and dieldrin after intravenous administration in mice. Toxicol
Appl Pharmacol, 23: 408-416.
Wang HH & MacMahon B (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J Occup Med, 21(11):745-748.
Wassermann M, Curnow DH, Forte PN, & Groner Y (1968) Storage of
organochlorine pesticides in the body fat of people in Western
Australia. Int J Ind Med Surg, 37(4): 295-300.
Wassermann M, Francone MP, Wassermann D, Mariani F, & Groner J (1969)
[Organochlorine pesticide content of the fatty tissue of the general
public in Argentina.] Sem Med, 134(16): 459-462 (in Spanish).
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. Basic Life Sci, 21: 275-326.
Webb RE & Horsfall F Jr (1967) Endrin resistance in pine mouse.
Science, 156: 1762.
Webb RE, Hartgrove RW, Randolph WC, Petrella VJ, & Horsfall F Jr
(1973) Toxicity studies in endrin-susceptible and resistant strains of
pine mice. Toxicol Appl Pharmacol, 25(1): 42-47.
Weeks DE (1967) Endrin food-poisoning. A report on four outbreaks
caused by two separate shipments of endrin-contaminated flour. Bull
World Health Organ, 37: 499-512.
Wegman RCC & Greve PA (1974) Levels of organochlorine pesticides and
inorganic bromide in human milk. Meded Fac Landbouwwet Rijksuniv Gent,
39: 1301-1310.
Wegman RCC & Greve PA (1978) Organochlorines, cholinesterase
inhibitors and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic Monit J, 12(3): 149-162.
Wegman RCC & Greve PA (1980) Halogenated hydrocarbons in Dutch water
samples over the years 1969-1977. Environ Sci Res, 16: 405-415.
Wegman RCC & Hofstee AWM (1982) Determination of organochlorines in
river sediment by capillary gas chromatography. Water Res, 16:
1265-1272.
Wegman RCC, Hofstee AWM, & Greve PA (1981) Uptake of organochlorines
by plants growing on river and basin sediment. Meded Fac Landbouwwet
Rijksuniv Gent, 46(1): 359-365.
Weisgerber I, Klein W, & Korte F (1969) [Disappearance of residues and
metabolism of endrin-(14C) in tobacco.] Liebigs Ann Chem, 729:
193-197 (in German with English abstract).
Wells MR & Yarbrough JD (1972) Vertebrate insecticide resistance:
in vivo and in vitro endrin binding to cellular fractions from
brain and liver tissues of Gambusia. J Agric Food Chem, 20(1): 14-16.
Whetstone RR (1964) Chlorocarbons and chlorohydrocarbons: chlorinated
derivatives of cyclopentadiene. In: Kirk-Othmer encyclopedia of
chemical technology, 2nd ed., New York, John Wiley and Sons, vol 5, pp
240-252.
WHO (1989) Environmental Health Criteria No. 91: Aldrin and dieldrin.
Geneva, World Health Organization, 335 pp.
WHO (1992) The WHO recommended classification of pesticides by hazard.
Guidelines to classification 1992-1993. Geneva, World Health
Organization (WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 1: Endrin. Geneva, World
Health Organization (VBC/DS/75.1).
Wiemeyer SN, Jurek RM, & Moore JF (1986) Environmental contaminants in
surrogates, foods and feathers of California condors (Gymnogyps
californianus). Environ Monit Assess, 6: 91-111.
Williams S (1964) Pesticide residues in total diet samples. J Assoc
Off Anal Chem, 47(5): 815-821.
Williams GM (1979) Liver cell culture systems for the study of
hepatocarcinogenesis. In: Margison GP ed Advances in medical oncology
research and education: Carcinogenesis, New York, Pergamon Press, vol
1, pp 273-280.
Williams S, Mills PA, & McDowell RE (1964) Residues in milk of cows
fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J Asoc Off Agric Chem, 47: 1124-1128.
Williams DT, Benoit FM, McNeil EE, & Otson R (1978) Organochlorine
pesticide levels in Ottawa drinking water, 1976. Pestic Monit J,
12(3): 163-166.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71(2): 410-414.
Wilson Committee (1969) Report by the Advisory Committee on Pesticides
and Other Toxic Chemicals. Further review of certain persistent
organochlorine pesticides used in Great Britain. London, Her Majesty's
Stationery Office, pp 90-100.
Wilson JG & Earley JJ (1986) Pesticide and PCB levels in the eggs of
shag Phalacrocorax aristotelis and cormorant Phalacrocorax carbo
from Ireland. Environ Pollut (Ser B), 12: 15-26.
Wit SL (1971) [Persistent insecticides in Dutch body fat.] Chem
Weekbl, 67(5): 11-14 (in Dutch).
Witherup S, Stemmer KL, Taylor P, & Bietsch P (1970) The Incidence of
Neoplasms in Two Strains of Mice Sustained on Diets Containing Endrin.
Unpublished report, Cincinnati, Ohio, Kettering Laboratory, submitted
to WHO by Shell.
Wolfe HR, Durham WF, & Armstrong JF (1963) Health hazards of the
pesticides endrin and dieldrin: hazards in some agricultural uses in
the Pacific Northwest. Arch Environ Health, 6: 458-464.
Wolfe HR, Durham WF, & Armstrong JF (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolman AA & Wilson AJ Jr (1970) Occurrence of pesticides in whales.
Pestic Monit J, 4(1): 8-10.
Wüthrich C, Müller F, Blaser O, & Marek B (1985) [Pesticides and other
chemical residues in Swiss diet samples.] Mitt Geb Lebensmittel Hyg,
76: 260-276 (in German with English summary).
Wuu KD & Grant WF (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare).
Can J Genet Cytol, 8: 481-501.
Wuu KD & Grant WF (1967a) Chromosomal aberrations induced by
pesticides in meiotic cells of barley. Cytologia, 32: 31-41.
Wuu KD & Grant WF (1967b) Chromosomal aberrations induced in somatic
cells of Vicia faba by pesticides. Nucleus, 10(1): 37-46.
Yakushiji T, Watanabe I, Kuwabara K, Yoshida S, Hori S, Fukushima S,
Kashimoto T, Koyama K, & Kunita N (1979) Levels of organochlorine
pesticides and polychlorinated biphenyls (PCBs) in mothers milk
collected in Osaka prefecture from 1969-1976. Arch Environ Contam
Toxicol, 8: 59-66.
Yarbrough JD, Roush RT, Bonner JC, & Wise DA (1986) Monogenic
inheritance of cyclodiene insecticide resistance in mosquitofish,
Gambusia affinis. Experientia (Basel), 42: 851-853.
Young RA & Mehendale HM (1986) Effect of endrin and endrin derivatives
on hepatobiliary function and carbontetrachloride-
induced hepatotoxicity in male and female rats. Food Chem Toxicol,
24(8): 863-868.
Zabik MJ, Schuetz RD, Burton WL, & Pape BE (1971) Photochemistry of
bioactive compounds. Studies of a major photolytic product of endrin.
J Agric Food Chem, 19(2): 308-313.
Zavon MR, Hine CH, & Parker KD (1965) Chlorinated hydrocarbon
insecticides in human body fat in the United States. J Am Med Assoc,
193(10): 181-183.
Zeiger E (1987) Carcinogenicity of mutagens: predictive capability of
the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer
Res, 47: 1287-1296.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environ Mutagen, 9 (suppl9): 1-110.
Zimmerli B & Marek B (1973) [The pesticide load of the Swiss
population.] Mitt Geb Lebensmittel Hyg,, 64(4): 459-479 (in German
with English summary).
ANNEX I CHEMICAL NAMES OF ENDRIN AND ITS METABOLITES
Two main systems are currently used for the nomenclature of
cyclodiene insecticides: 'polyhydroaromatic' names, used by Chemical
Abstracts (American Chemical Society) and the International Union for
Pure and Applied Chemistry (IUPAC), and the von Baeyer/IUPAC system
for polycyclic aliphatic compounds. Benson (1969) and Bedford (1974)
proposed that the latter system be used for the cyclodiene
insecticides.
The 'polyaromatic' system has, unfortunately, been subject to
historical variation, and there are differences between the IUPAC,
British and American conventions for defining the three-dimensional
stereochemistry in this system. As a consequence of differences in the
numbering of carbon atoms in the two systems and the modification of
the Chemical Abstracts 'polyaromatic' name for dieldrin since 1971,
considerable confusion can arise in the nomenclature of metabolites.
The possible misunderstandings that may occur, particularly among
people who are not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that are given to
the major metabolite of endrin; this one compound may be designated
as:
anti-9-hydroxyendrin (former Chemical Abstracts system)
anti-8-hydroxyendrin (current Chemical Abstracts system)
anti-12-hydroxyendrin (von Baeyer/IUPAC system).
A useful discussion of nomenclature was given by Brooks (1974).
The chemical names for endrin and its metabolites are summarized
in Table 30.
Table 30. Chemical nomenclature of endrin and its metobolites
Trivial namesa Polycyclic aliphatic name (von Baeyer/ IUPAC) Alternative or former names
Endrin (I) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-2,3-7, 6- 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,
endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]- 4a,5,6,7,8,8a-octahydro-1,4- endo,endo-
dodec-9-ene 5,8-demethanonaphthalene (former CAS
name)
1aa,2b,2ab,3a,6a,6b,7b,7a)-3,4,5,6,9,9-
Hexachloro-1a,2,2a,3,6,6a,7,7a,-octahydro-
2,7:3,6-dimethanonaphth[2,3- b]oxirene
(current CAS name)
(IR,4S,4aS,5S,7R,8R,8aR)-1,2,3,4,10,10-
Hexachloro-1,4,4a,5,6,7,8,8a-octahydro-6,7-
epoxy-1,5:5,8-dimethanonaphthalene
(current IUPAC name)
syn-12-Hydroxyendrin (II) 1,8,9,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- syn-hydroxyendrin) ( syn-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8- endo-
tetracyclo[6.2.1.13,6.02,7] dodec-9-ene
anti-12-Hydroxyendrin (III) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- anti-hydroxyendrin) ( anti-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8-
endo-tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
3-Hydroxyendrin (IV) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-3-
(5-hydroxyendrin) hydroxy-2,3-7,6- endo-2,1-7,8- endo-tetracyclo-
[6.2.1.13,6.02,7] dodec-9-ene
12-Ketoendrin (V) 1,8,9,10,11,11-Hexachloro-4,5 - exo-epoxy-2,3-7,6-
(9-ketoendrin) endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]-
dodec-12-one
Endrin trans-diol (IV) 1,8,9,10,11,11-Hexachloro-4,5-( exo)trans-dihydroxy-
2,3-7,6- endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]
dodec-9-ene
aRoman numerals in parentheses refer to the structures in Figure 2.
ANNEX II
MEDICAL TREATMENT OF ENDRIN POISONING
1. Symptoms of poisoning
Endrin is readily absorbed and is toxic when taken by mouth, by
skin contact (especially liquid formulations), and by inhalation. It
stimulates the central nervous system, and an oral dose of 0.25 mg/kg
body weight can cause convulsions in humans. Following accidental
ingestion or gross over-exposure, symptoms appear between 20 min and
12 h and may include headache, dizziness, nausea, vomiting, weakness
in the legs, and convulsions, sometimes leading to death.
Organochlorine compounds can cause respiratory depression, and
they may sensitize the heart to endogenous catecholamines, leading to
ventricular fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis, and if
necessary blood gases should be checked. Use of an
electrocardiographic monitor is recommended if symptoms are severe.
Endrin is eliminated very quickly from the blood and can be
detected for only 1 or 2 days even after massive over-exposures. Signs
and symptoms of poisoning occur only at levels in whole blood of above
0.05 µg/ml.
2. Medical treatment
Medical treatment is largely symptomatic and supportive and is
directed against convulsions and hypoxia. If endrin is swallowed, the
stomach should be emptied as soon as possible by careful gastric
lavage (with a cuffed endotracheal tube already in place), avoiding
aspiration into the lungs. In a rural situation where this is not
feasible, vomiting should be induced immediately. This should be
followed by (intragastric) administration of 50 g of activated
charcoal and 30 g of magnesium or sodium sulfate in a 30% aqueous
solution. Oily purgatives are contraindicated, and no fats, oils, or
milk should be given.
If convulsions occur, anticonvulsants should be given immediately
but slowly, and repeated as necessary. Diazepam can be given at 10 mg
(children, 1-5 mg) intravenously; thiopental sodium or hexobarbital
sodium can be given intravenously at a dose of 10 mg/kg, with a
maximum total dose of up to 750 mg for an adult; or paraldehyde can be
given at 5 ml by intramuscular injection. These short-acting
anticonvulsants should always be followed by phenobarbital given
orally at 3 mg/kg (up to 200 mg for an adult), or phenobarbital sodium
given intramuscularly at 3 mg/kg (up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline,
should never be given.
The airway must be kept unobstructed. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
A guideline for the management of major status epilepticus is
added as Annex III.
ANNEX III
MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
1. Initial management
1. Assess the patient, verify the diagnosis, remove false
teeth, place the patient in a lateral semi-prone position,
and establish an airway.
2. Give diazepam intravenously (see Note 1, below), usually at
10 mg in 2 ml (0.15-0.25 mg/kg), followed immediately by a
further 10 mg (2 ml) over 1-2 min. This may be repeated
according to response.
3. Take blood to measure levels of anticonvulsant drug,
ethanol, and blood sugar (5 ml of blood in a sugar tube); a
sample to measure calcium (5 ml in a plain tube); and a drop
of blood to determine blood glucose.
4. If the latter measurement shows low blood glucose level, 25
ml of 50% glucose should be given intravenously, preferably
by catheter and not into a small distal vein. If ethanol is
likely to be present, give thiamine intravenously at 100 mg.
5. Give phenytoin intravenously at 250 mg in 5 ml (10-15 mg/kg)
no faster than 50 mg (1 ml)/min by infusion pump or slow
intravenous injection (see Note 2, below).
2. If fits continue, transfer patient to the intensive care unit and
consult an anaesthetist
6. Give chlormethiazole intravenously at 8 mg/ml: a loading
dose of up to 800 mg (100 ml) over 10 min at 10 ml/min,
maintained with 0.5-1 ml/min (4-8 mg).
7. Give thiopentone intravenously at 5 mg/kg as a loading dose,
then 1-3 mg/kg per h to a maximum blood level of 100
mg/litre.
8. If this fails, consult a neurologist.
3. Notes
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there
is concurrent use of other central nervous system depressant
drugs, especially phenobarbital.
aAdapted from guidelines issued at Guy's Hospital, London
Diazepam must not be given intramuscularly:
--added to an intravenous infusion
--with phenobarbital, unless artificial ventilation is
available.
Rectal administration of diazepam (using a rectal
administration set), at 5 or 10 mg in 2.5 ml, may be used
for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
--intramuscularly
--by central line
--into a dextrose infusion
--with any other drug.
Intravenously administered phenytoin should be monitored by
continuous electrocardiographic recording. If this is not
available, it may be safer to use a diluted solution of 250
mg (5 ml) in 250 ml of normal saline, no faster than 50
mg/min. The diluted solution should be used immediately
provided there is no evidence of precipitation (this use of
phenytoin is not licensed).
4. Options
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate deep intramuscular
injection or 10 ml diluted in 100 ml of normal saline given
intravenously over 10-15 min. Note: Paraldehyde should be
administered only with glass syringes.
2. Phenobarbital: 200 mg/ml, should not be given intravenously
except when artificial ventilation is available, and not at
all if the patient normally takes phenobarbital. The maximal
rate of infusion is 100 mg/min to a maximum dose of 15
mg/kg.
3. Lignocaine: 100 mg by slow intravenous injection, followed
by 50-100 mg in 250 ml of 5% dextrose at 1-2 mg/min. Note:
This treatment must be monitored by electrocardigram.
4. Diazepam: 10 mg in 2 ml intravenously, or 40 mg in 500 ml of
5% dextrose at a maximal infusion rate of 100 mg/h.
5. Sodium valproate: 400 mg in 4 ml, or 400-800 mg
intravenously over 3-5 min (up to 10 mg/kg), followed by
intravenous. infusion to a maximum of 2.5 g/day
(unlicensed).
5. Paediatric doses
For children, dosing should be adapted as follows:
Diazepam: 0.2-0.3 mg/kg intravenously
Phenytoin: 10-20 mg/kg intravenously
Chlormethiazole: 5-10 mg/kg per h, equivalent to
0.6-1.25 ml/kg per h
RESUME ET EVALUATION; CONCLUSIONS; RECOMMANDATIONS
Résumé et évaluation
Exposition
L'endrine est un insecticide organochloré utilisé depuis les
années 1950 contre toute sorte de ravageurs, qui s'attaquent notamment
au coton mais également au riz, à la canne à sucre, au maïs et à
d'autres cultures. On l'utilise également comme rodenticide et
avicide. Il est disponible dans le commerce sous forme de poudres, de
granulés, de pâtes et de concentrés émulsionnables.
L'endrine pénètre principalement dans l'atmosphère par
volatilisation et dispersion. En général, la volatilisation se produit
après épandage sur le sol et sur les récoltes et elle est tributaire
de nombreux facteurs comme la teneur en matières organiques et en eau
du sol, l'humidité, les courants aériens et l'aire foliaire des
végétaux.
C'est principalement par lessivage à partir du sol que se produit
la contamination des eaux superficielles. Les précipitations, qu'il
s'agisse de neige ou de pluie, n'ont qu'une part négligeable dans
cette contamination.
Localement, une contamination peut également se produire par
suite du déversement d'effluents industriels ou de négligences dans
les techniques d'épandage.
C'est principalement par suite d'un épandage direct sur les
terrains et les récoltes que l'endrine pénètre dans le sol. Elle peut
y être retenue, transportée ou dégradée, en fonction d'un certain
nombre de facteurs. C'est dans les sols riches en matières organiques
que la rétention est la plus importante. La persistance de l'endrine
dépend dans une large mesure des conditions locales; sa demi-vie dans
le sol peut aller jusqu'à 12 ans. La disparition de l'endrine présente
en surface s'effectue principalement par volatilisation et
photodécomposition. Sous l'influence de la lumière solaire
(rayonnement ultra-violet), l'endrine est isomérisée en delta-
cétoendrine. En présence de lumière solaire intense, on a observé une
isomérisation de 50% de l'endrine en l'espace de sept jours.
L'isomérisation peut également s'effectuer par action microbienne
(champignons et bactéries), notamment en anaérobiose.
Les invertébrés aquatiques et les poissons absorbent rapidement
l'endrine présente dans l'eau mais, transvasés dans une eau non
contaminée, les poissons exposés éliminent sans délai le pesticide. En
cas d'exposition continue, le facteur de bioconcentration peut
atteindre 14-18 000.
Il est possible que les invertébrés terricoles absorbent
facilement l'endrine. La présence occasionnelle de faibles quantités
d'endrine dans l'air ainsi que dans les eaux de surface, notamment
destinées à la consommation, en zone agricole, n'a guère d'importance
au point de vue de la santé publique. La seule vole d'exposition
importante est la voie alimentaire. En général, toutefois, les
quantités ingérées se situent très largement en-dessous de la dose
journalière admissible qui a été fixée à 0,0002 mg/kg de poids
corporel en 1970 (FAO/OMS, 1971).
1.2 Absorption, métabolisme et excrétion
Contrairement à la dieldrine, son stéréoisomère, l'endrine est
rapidement métabolisée par l'organisme animal et, comparativement à
d'autres composés de structure chimique semblable, elle s'accumule
très peu dans les tissus adipeux.
L'absorption et l'excrétion sont rapides après administration
orale à des rats et la demi-vie biologique se situe entre 1 et 6 jours
selon les quantités ingérées. Un régime stationnaire, c'est à dire un
état d'équilibre entre la quantité excrétée et la dose ingérée,
s'établit au bout de 6 jours. On constate une différence entre les
deux sexes en ce sens que les mâles excrètent l'endrine et ses
métabolites par la voie biliaire plus rapidement que les femelles,
d'où une moindre accumulation de pesticides dans les tissus adipeux
des mâles. Les rats excrètent ce composé principalement dans leurs
matières fécales sous forme d'endrine, d' anti-12-hydroxyendrine
ainsi que sous la forme d'un dérivé hydroxyle de l'endrine, en
l'espace de 24 heures (70-75 %); un troisième métabolite, la 12-
cétoendrine, s'accumule dans les tissus. Les lapins excrètent 50% des
métabolites de l'endrine par la voie urinaire, l'excrétion urinaire
n'étant que de 2% chez le rat; dans les matières fécales des lapins,
on ne retrouve que de l'endrine non modifiée.
Des vaches à qui l'on avait administré de l'endrine à raison de
0, 1 mg/kg de nourriture pendant 21 jours en ont excrété jusqu'à 65%
sous forme de métabolites urinaires, 20% sous forme de métabolites
fécaux ou d'endrine non modifiée et 3% dans leur lait, cette fois,
principalement sous forme d'endrine non modifiée. Chez ces vaches, les
résidus atteignaient 0,003-0,006 mg/litre dans le lait, 0,001-0,002
mg/kg dans la viande, et 0,02-0,1 mg/kg dans la graisse.
Chez des poules pondeuses ayant reçu une alimentation additionnée
d'endrine, on a observé des résidus (selon la dose ingérée) qui
atteignaient 0.1 mg/kg dans la chair, 1 mg/kg dans la graisse, 0,2-0,3
mg/kg dans les oeufs (jaune), 0,2 mg/kg dans les reins et 0,5 mg/kg
dans le foie. Sauf dans le cas du foie et des reins, les résidus
présents étaient essentiellement formés d'endrine non modifiée.
Environ 50% de la quantité d'endrine administrée a été excrétée dans
les matières fécales, principalement sous la forme de métabolites.
Chez l'homme, le rat, le lapin, la vache et la poule, le
principal métabolite de l'endrine est l' anti-12-hydroxyendrine,
accompagnée de ses sulfo- et glucuro-conjugués. On trouve également 4
autres métabolites, mais en quantités minimes. Dans les tissus et le
lait on retrouve essentiellement de l'endrine non modifiée. Après
épandage sur des végétaux, on a retrouvé de l'endrine sous forme non
modifiée ainsi que deux produits de transformation hydrophiles.
1.3 Effets sur les êtres vivants dans leur milieu naturel
L'endrine n'exerce que des effets minimes sur les bactéries et
les champignons terricoles. Aux doses de 10-1000 mg/kg de terre, le
composé n'a aucun effet sur la décomposition des matières organiques,
sur la dénitrification ou sur la production de méthane. L'endrine est
très toxique pour les poissons, les invertébrés aquatiques et le
phytoplancton; la CL 50 à 96 h, est dans la plupart des cas
inférieure à 1,0 µg/litre. La dose nocive la plus faible observée au
cours d'un test portant sur le cycle évolutif d'un crevette,
Mysidopsis bahia, était de 30 ng/litre.
Les épreuves de toxicité aiguë effectuées sur des organismes
aquatiques ont été pratiquées dans des aquariums ne comportant pas de
sédiments, on peut penser que la présence de sédiments atténue l'effet
de l'endrine. D'ailleurs la présence de sédiments fortement contaminés
n'a guère eu d'effet sur les espèces de pleine eau, ce qui incite à
penser que l'endrine fixée aux sédiments présente une faible
biodisponibilité. On n'a pas pratiqué d'épreuves sur des animaux
aquatiques vivant dans les sédiments.
Pour les mammifères terrestres et les oiseaux, la DL50 est de
l'ordre de 1,0-10,0 mg/kg de poids corporel. Des canards de l'espèce
Anas platyrhynchos qui avaient reçu pendant 12 semaines de l'endrine
dans leur nourriture à des doses allant jusqu'à 3,0 mg/kg de poids
corporel, n'ont présenté aucun effet délétère que ce soit sur la
ponte, la fécondité ou l'éclosion des oeufs.
Il semblerait que certaines espèces d'invertébrés aquatiques, de
poissons et de petits mammifères résistent à l'action toxique de
l'endrine; d'ailleurs l'exposition à divers pesticides organochlorés
a pu entraîner la sélection de souches résistantes à l'endrine.
Dans des zones où existent des décharges industrielles et où
l'endrine peut être entraînée par ruissellement à partir des champs
traités, on a observé une mortalité parmi les poissons; par ailleurs,
le déclin des populations de pélicans bruns (en Louisiane) et de
caugeks (aux Pays-Bas) a été attribuée à une exposition à l'endrine et
à d'autres dérivés halogénés.
1.4 Effets sur les animaux d'expérience et sur les systèmes in vitro
L'endrine est un pesticide fortement toxique dont les signes
d'intoxication sont de type neurologiques. Chez les animaux de
laboratoire, la DL50 par voie orale de l'endrine de qualité
technique se situe dans les imites de 3-43 mg/kg de poids corporel; la
DL50 dermique va de 5-20 mg/kg de poids corporel pour le rat. Il n'y
a pas de différence notable concernant la toxicité aiguë par voie
orale et percutanée entre le produit technique et les diverses
formulations (concentrés émulsionnables ou poudres mouillables).
Des épreuves de courte durée portant sur la toxicité par voie
orale de l'endrine ont été effectuées sur (les souris, des rats, des
lapins, des chiens et autres animaux domestiques. Chez les souris et
les rats, les doses maximales tolérées ont été respectivement de 5 et
15 mg/kg de nourriture pendant 6 semaines (soit l'équivalent de 0,7
mg/kg de poids corporel). Les rats ont survécus à une dose de 1 mg/kg
de nourriture pendant 16 semaines (soit l'équivalent de 0,05 mg/kg de
poids corporel); les lapins sont morts après avoir reçu à plusieurs
reprises une dose de 1 mg/kg de poids corporel. chez le chien, une
dose de 1 mg/kg de nourriture (soit approximativement 0,025 mg/kg de
poids corporel) administrée sur une période de 2 ans, n'a produit
aucun effet.
Du point de vue neurologique, les signes d'intoxication observés
sont dus à l'inhibition de la fonction de l'acide gamma-aminobutyrique
(GABA) à faible dose. Comme les autres hydrocarbures chlorés
insecticides, l'endrine agit également au niveau du foie et la
stimulation des systèmes enzymatiques intervenant dans le métabolisme
des autres substances chimiques se manifeste, notamment chez la
souris, par une diminution de la durée du sommeil induit par
l'hexobarbital.
Des doses de 75-150 mg/kg appliquées sur l'épiderme des lapins
sous forme de poudre sèche, tous les jours pendant deux heures ont
entraîné des convulsions et la mort chez ces animaux sans toutefois
provoquer d'irritation cutanée. Cette intoxication par voie générale
sans irritation locale mérite d'être signalée.
Des études de toxicité et de cancérogénicité à long terme ont été
effectuées sur des souris et des rats. Aucun effet cancérogène n'a été
observé mais ces études présentaient un certain nombre d'insuffisances
notamment la faible survie des animaux. Lors d'une étude de deux ans
sur des rats traités par de l'endrine administrée dans leur
nourriture, on a estimé à 1 mg/kg de nourriture, soit l'équivalent
d'environ 0,05 mg/kg de poids corporel, la dose sans effets toxiques
observables. Après administration d'endrine avec des quantité
infinitésimales de substances chimiques cancérogènes pour l'animal, il
n'a pas été possible de mettre en évidence d'effet tumoro-promoteur.
Le Groupe de travail en a conclu que les données sont insuffisantes
pour permettre de considérer l'endrine comme cancérogène pour l'homme.
Plusieurs études ont également révélé que l'endrine n'était pas
génotoxique.
Dans la plupart des études, l'endrine s'est révélée non
tératogène pour la souris, le rat ou le hamster, même à des doses
toxiques pour la mère ou le foetus. La dose sans effet nocif
observable a été évaluée à 0,5 mg/kg de poids corporel chez la souris
et le rat et à 0,75 mg/kg de poids corporel chez les hamsters.
L'endrine n'a pas eu d'effets sur la reproduction des rats suivis
pendant trois générations qui en recevaient dans leur nourriture à
raison de 2 mg/kg (soit environ 0, 1 mg/kg de poids corporel).
Un certain nombre de métabolites de l'endrine sont plus ou moins
toxiques que le composé initial. Ainsi la delta-cétoendrine est moins
toxique de l'endrine, en revanche la 12-cétoendrine est considérée
comme le métabolite le plus toxique de l'endrine pour les mammifères,
avec une DL50 par voie orale de 0,8-1,1 mg/kg de poids corporel chez
le rat.
1.5 Effets sur l'homme
Plusieurs cas d'intoxication mortels ou non mortels consécutifs
à un accident ou à une tentative de suicide ont été observés. Les cas
d'intoxication aiguë non mortels résultant d'une, surexposition
accidentelle ont été observés chez les ouvriers d'une usine de
production d'endrine. On estime que par voie orale, la dose mortelle
est (l'environ 10 mg/kg de poids corporel, une dose unique prise par
voie orale de 0,25-1,0 mg/kg de poids corporel peut provoquer des
convulsions.
C'est au niveau du système nerveux central que l'endrine exerce
principalement son action. Après exposition à dose toxique, des signes
d'intoxication peuvent faire leur apparition et se manifestent sous la
forme d'un hyperexcitabilité et de convulsions, la mort pouvant
survenir dans les 2-12 heures suivant l'exposition si un traitement
approprié n'est pas institué immédiatement. En revanche, après une
intoxication non mortelle, la récupération est rapide et complète.
L'endrine ne s'accumule pas dans le corps humain de manière
importante. Chez 232 travailleurs exposés de par leur profession, on
n'a pas constaté d'effets indésirables à long terme (durée
d'exposition 4-27 ans) lors des examens médicaux pratiqués (durée de
l'observation 2-29 ans). Le seul effet observé, indirectement
d'ailleurs, consistait en une stimulation réversible des enzymes
pharmacométabolisantes.
Des analyses ont été pratiquées dans de nombreux pays sur un
grand nombre d'échantillons de tissus adipeux, de sang et de lait
maternel sans qu'il soit possible de mettre en évidence la présence
d'endrine. Le Groupe de travail attribue l'absence d'endrine dans ces
échantillons à la faible exposition de la population général à ce
pesticide et à sa métabolisation rapide.
En revanche la présence d'endrine a été décelée dans le sang (à
des concentrations atteignant 450 µg/litre) et dans les tissus adipeux
(à la dose de 89,5 mg/kg) chez les personnes décédées d'une
intoxication accidentelle. Dans les conditions normales, on n'a pas
retrouvé d'endrine chez les travailleurs exposés. Le seuil
d'apparition des symptômes d'intoxication est estimé à 50-100 µg/litre
de sang. On pense que la demi-vie de l'endrine dans le sang est de
l'ordre de 24 heures.
2. Conclusions
L'endrine est un 'Insecticide qui présente une très forte
toxicité aiguë. Il peut entraîner des intoxications graves en cas
d'exposition excessive due à une manipulation négligente lors de sa
production, de son utilisation ou par suite de la consommation
d'aliments contaminés. L'exposition de la population générale est
principalement due à la présence de résidus dans les denrées
alimentaires; toutefois on estime que la quantité d'endrine ingérée
est en général très inférieure à la dose journalière admissible fixée
par le Comité FAO/OMS d'experts des résidus de pesticides. Il n'y a
pas de danger pour la population générale qui résulterait d'une
exposition de ce genre à l'endrine. Moyennant de bonnes méthodes de
travail et le respect des mesure d'hygiène et de sécurité, l'endrine
ne devrait pas constituer un danger pour les ouvriers exposés.
Il est évident que des rejets incontrôlés d'endrine lors de la
production, de la formulation et de l'utilisation de ce pesticide
peuvent créer des problèmes écologiques dus à sa forte toxicité. Il
n'est pas possible d'être aussi catégorique en ce qui concerne les
effets que peut avoir son utilisation en agriculture sur la faune et
la flore, encore que l'entraînement par ruissellement du pesticide
puisse constituer une menace pour les poissons et les oiseaux
piscivores. Le déclin des populations de certaines espèces d'oiseaux
a été attribué à la présence de résidus élevés de divers organochlorés
dans les tissus des adultes et dans les oeufs. On a procédé au dosage
de l'endrine chez certaines de ces espèces; toutefois il est difficile
de faire la part des différents organochlorés qui sont en cause.
3. Recommandations
1. L'endrine ne doit être utilisée qu'en cas de nécessité et
seulement lorsqu'il n'existe pas d'autre produit moins
toxique.
2. Afin de préserver la santé et le bien-être des travailleurs
et de la population générale, on ne doit confier la
manipulation et l'épandage qu'à des personnes bien encadrées
et bien formées qui appliqueront des mesures de sécurité
convenables et épandront le produit conformément aux règles
de bonne pratique en la matière.
3. Il convient de s'entourer de toute les précautions
nécessaires lors de la production, de la formulation, de
l'utilisation en agriculture et du rejet de l'endrine afin
de contaminer le moins possible l'environnement et en
particulier les eaux de surface.
4. Les personnes qui sont habituellement exposées à l'endrine
doivent subir des examens médicaux périodiques.
5. Il faut poursuivre l'étude épidémiologique des travailleurs
exposés.
6. Dans les pays où l'on utilise encore de l'endrine, on devra
contrôler la présence de résidus d'endrine dans les denrées
alimentaires.
7. Au cas où l'on continuerait à utiliser de l'endrine, il
faudrait obtenir davantage de données sur la présence, la
destinée ultime et la toxicité de la 12-cétoendrine et de la
delta-cétoendrine.
RESUMEN Y EVALUACION; CONCLUSIONES; RECOMENDACIONES
1. Resumen y evaluación
1.1 Exposición
La endrina es un insecticida organoclorado que se utiliza desde
los años cincuenta para combatir muy diversas plagas agricolas, sobre
todo en el algodón aunque también en el arroz, la caña de azúcar, el
maíz y otros cultivos. Se utiliza asimismo como rodenticida. En el
comercio se encuentra en forma de polvos, gránulos, pastas y
concentrado emulsionable.
La endrina se incorpora al aire principalmente por volatilización
y arrastre aéreo. En general, la volatilización tiene lugar después de
aplicarla a suelos y cultivos y depende de muchos factores, como el
contenido de materia orgánica y agua del suelo, la humedad, el flujo
de aire y la superficie cultivada.
La vía más importante de contaminación de las aguas de superficie
es la escorrentía desde el suelo. La contaminación por precipitación
en forma de nieve o lluvia es insignificante. Puede producirse
contaminación local del medio debida a efluentes industriales y
prácticas de aplicación poco meticulosas.
La principal fuente de endrina en el suelo es la aplicación
directa a éste y a los cultivos. Puede quedar retenida, ser
transportada o degradarse en el suelo, atendiendo a diversos factores.
La retención más intensa se produce en suelos con contenido elevado de
materia orgánica. La persistencia de la endrina depende en gran medida
de las condiciones locales; su semivida en el suelo puede llegar a los
12 años. La volatilización y la fotodescomposición son los principales
factores de la desaparición de la endrina de las superficies del
suelo. La luz del sol (luz ultravioleta) induce la formación del
isómero delta-cetoendrina. En verano, bajo insolación intensa, se
observó que alrededor del 50% de la endrina se isomerizaba a esta
cetoendrina en un plazo de siete días. Se produce transformación
microbiana (en hongos y bacterias), especialmente en condiciones
anaerobias, originándose la misma sustancia.
Los invertebrados acuáticos y los peces absorben rápidamente la
endrina a partir del agua, si bien los peces expuestos transferidos a
agua no contaminada pierden el plaguicida rápidamente. Se han
registrado factores de bioconcentración de 14-18 000 tras una
exposición continua. Los invertebrados del suelo también absorben
fácilmente el compuesto.
La presencia ocasional de niveles reducidos de endrina en el aire
y en las aguas de superficie y de bebida en zonas agrícolas reviste
escasa importancia desde el punto de vista de la salud pública. La
única exposición que merece consideración es la ingesta en la dieta.
En general, no obstante, los niveles comunicados de ingesta se
encuentran muy por debajo de la ingesta diaria admisible de 0,0002
mg/kg de peso corporal, establecida en 1970 (FAO/OMS, 1971).
1.2 Absorción, metabolismo y excreción
A diferencia de la dieldrina, su estereoisómero, la endrina se
metaboliza rápidamente en los animales, y se acumula en muy pequeña
cantidad en las grasas en comparación con compuestos de estructura
química análoga.
En la rata, tanto la absorción como la excreción tras la
administración oral se producen rápidamente; su semivida biológica es
de 1-6 días, según la dosis administrada. Al cabo de 6 días se alcanza
un estado de equilibrio en el que la cantidad excretada es igual a la
ingesta diaria. Se observan diferencias de un sexo a otro: los machos
excretan endrina y metabolitos con la bilis mucho más deprisa que las
hembras, lo que produce una acumulación menor en el tejido adiposo de
aquéllos. Las ratas excretan este compuesto principalmente en las
heces en forma de endrina, anti-12-hidroxiendrina, y un derivado
hidroxilado durante las primeras horas (70-75%); un tercer metabolito,
la 12-cetoendrina, se acumula enlos tejidos. El conejo excreta el 50%
de los metabolitos del compuesto enla orina, mientras que en la rata
sólo el 2% se excreta por esta vía; en lasheces del conejo sólo se
detecta endrina sin alterar.
Las vacas a las que se administró endrina a razón de 0,1 mg/kg de
la dieta durante 21 días excretaron hasta el 65% en forma de
metabolitos en la orina, el 20% en las heces, parcialmente en forma de
endrina no alterada, y el 3% en la leche, también principalmente en
forma de endrina. Estas vacas presentaron niveles residuales de
0,003-0,006 mg/litro en la leche, 0,001-0,002 mg/kg en la carne, y
0,02-0,1 mg/kg en la grasa.
En gallinas ponedoras a las que se administró endrina por vía
oral seobservaron niveles residuales (dependientes de la dosis
administrada) de hasta 0,1 mg/kg en la carne, 1 mg/kg en la grasa,
0,2-0,3 mg/kg en los huevos (yema), 0,2 mg/kg en el riñón y 0,5 mg/kg
en el hígado. Salvo en el hígado y el riñón, los residuos encontrados
estaban formados principalmente por endrina no alterada. Alrededor del
50% de la endrina administrada se excretó en las heces, principalmente
en forma de metabolitos.
En el ser humano, la rata, el conejo, la vaca y la gallina, el
principal metabolito biotransformado de la endrina es la
anti-12-hidroxiendrina, junto con su sulfato y su glucur nido
conjugados. Se encontraron cuatro metabolitos más, si bien en
cantidades muy reducidas. En los tejidos corporales y en la leche se
encuentra sobre todo endrina inalterada. Tras la aplicación de este
plaguicida a plantas, seidentificaron endrina inalterada y dos
productos de transformación hidrófilos.
1.3 Efectos en los organismos del medio ambiente
El efecto de la endrina en las bacterias y los hongos del suelo
es mínimo. Con dosis de 10-1000 mg/kg de suelo no se observó efecto
alguno en la descomposición de materia orgánica, la desnitrificación
ni la generación de metano. La endrina es sumamente tóxica para los
peces, los invertebrados acuáticos y el fitoplancton: los valores de
la CL50 a las 96 horas se encuentran en su mayoría por debajo de 1,0
µg/litro. El nivel sin observación de efectos más bajo en un ensayo de
ciclo biológico del crustáceo Mysidopsis bahia se fijó en 30 ng/litro.
Los ensayos comunicados sobre la toxicidad aguda de la endrina
para los organismos acuáticos se llevaron a cabo en acuarios sin
sedimentos; cabría esperar que la presencia de sedimentos atenuara el
efecto del insecticida. Los sedimentos muy contaminados ejercieron
escaso efecto en las especies de aguas libres, lo que indica que la
endrina ligada a los sedimentos tiene una biodisponibilidad reducida.
Aún no se han llevado a cabo ensayos en animales acuáticos que viven
en los sedimentos.
La DL50 para mamíferos terrestres y aves oscila entre 1,0 y
10,0 mg/kg de peso corporal. Los patos silvestres a los que se
administraron 3,0 mg/kg de peso corporal durante 12 semanas no
mostraron efecto alguno en la producción de huevos, la fertilidad o la
eclosión.
Ciertas especies de invertebrados acuáticos, peces y mamíferos de
pequeño tama o son resistentes a la toxicidad de la endrina; la
exposición a diversos plaguicidas organoclorados llevó a la selección
de estirpes resistentes a la endrina.
Se observaron muertes masivas de peces en zonas de escorrentía
agrícola y descargas industriales; el declive de las poblaciones de
pelícanos pardos (en Luisiana, EE.UU.) y de golondrinas de mar
(Thalasseus sandvicensis) en los Países Bajos se ha atribuido a la
exposición a la endrina en combinación con otras sustancias químicas
halogenadas.
1.4 Efectos en animales de experimentación in vitro
La endrina es un plaguicida sumamente tóxico; los signos de
intoxicación son de carácter neurotóxico. La DL50 por vía oral de la
endrina de calidad técnica en animales de laboratorio oscila entre 3
y 43 mg/kg de peso corporal; la DL50 por vía cutánea en la rata es
de 5-20 mg/kg peso corporal. No se ha encontrado ninguna diferencia en
la toxicidad aguda por vía oral o cutánea entre los productos de
calidad técnica y los formulados (concentrado emulsionable y polvos
humectables).
Se han llevado a cabo experimentos de breve duración para
estudiar la toxicidad por vía oral en el ratón, la rata, el conejo, el
perro y animales domésticos. En el ratón y la rata, las dosis máximas
toleradas durante 6 semanas fueron 5 y 15 mg/kg de la dieta
(equivalentes a 0,7 mg/kg de peso corporal), respectivamente. Las
ratas sobrevivieron tras una exposición a 1 mg/kg de la dieta
(equivalente a 0,05 mg/kg de peso corporal) durante 16 semanas; los
conejos murieron tras recibir dosis repetidas de 1 mg/kg de peso
corporal. En el perro, no se observó efecto alguno tras la
administración de 1 mg/kg de la dieta (equivalente aproximadamente a
0,025 mg/kg de peso corporal) durante más de 2 años.
La base neuroógica de los signos de intoxicación observados es la
inhibición de la función del ácido gamma-aminobutírico (GABA) con
dosis reducidas. Al igual que otros insecticidas a base de
hidrocarburos clorados, la endrina afecta también al hígado, y se
observa claramente la estimulación de sistemas enzimáticos que
participan en el metabolismo de otras sustancias químicas, como lo
demuestra, por ejemplo, la menor duración del sueño por hexobarbital
en el ratón.
Con dosis de 75-150 mg/kg aplicadas por vía cutánea en forma de
polvo seco durante 2 horas al día se produjeron convulsiones y la
muerte en el conejo pero sin irritación cutánea. Esta toxicidad
sistémica sin irritación en el lugar de contacto resulta muy notable.
Se han llevado a cabo en ratones y ratas estudios prolongados de
toxicidad y carcinogenicidad. No se observó efecto carcinogénico, pero
estos estudios tenían ciertos defectos, entre ellos la reducida
supervivencia de los animales. El nivel sin observación de efectos en
cuanto a la toxicidad en un estudio de dos años de duración en la rata
fue de 1 mg/kg de la dieta (equivalente a unos 0,05 mg/kg de peso
corporal). No se demostró ningún efecto de favorecimiento de tumores
cuando se ensayó la endrina en combinación con cantidades submínimas
de sustancias químicas de conocido efecto carcinogénico en los
animales. El Grupo de Trabajo concluyó que los datos de que se dispone
no bastan para indicar que la endrina supone un riesgo carcinogénico
para el ser humano.
En varios estudios se observó que la endrina no es genotóxica.
En la mayoría de los estudios no resultó teratogénica para el
ratón, la rata o el hámster, ni siquiera con dosis suficientes para
provocar toxicidad materna o fetal. El nivel sin observación de
efectos adversos fue de 0,5 mg/kg de peso corporal en ratones y ratas
y de 0,75 mg/kg de peso corporal en el hámster. La endrina no indujo
efecto alguno en la reproducción de ratas estudiadas durante tres
generaciones cuando se administró a razón de 2 mg/kg de la dieta (unos
0,1 mg/kg de peso corporal).
Algunos metabolitos de la endrina tienen toxicidades agudas
iguales o más altas que el compuesto originario. El producto de
transformación, la delta-cetoendrina, es menos tóxico que la endrina,
pero la 12-cetoendrina se considera el metabolito más tóxico en los
mamíferos, con una DL50 por vía oral en la rata de 0,8-1,1 mg/kg de
peso corporal.
1.5 Efectos en el ser humano
Se han producido varios episodios de intoxicación mortal y no
mortal, tanto accidentales como suicidas. Los casos de intoxicación
aguda no mortal debida a exposición excesiva accidental se observaron
en trabajadores de una planta de fabricación de endrina. Se ha
calculado que la dosis que por vía oral provoca la muerte es de
aproximadamente 10 mg/kg de peso corporal; la dosis única por vía oral
que provoca convulsiones se fijó en 0,25-1,0 mg/kg de peso corporal.
El lugar principal de acción de la endrina es el sistema nervioso
central. La exposición del ser humano a una dosis tóxica puede
producir al cabo de pocas horas signos y síntomas de intoxicación
tales como excitabilidad y convulsiones; la muerte puede producirse en
las 2-12 horas que siguen a la exposición si no se administra
inmediatamente el tratamiento apropiado. La recuperación después de
una intoxicación no mortal es rápida y completa.
La endrina no se acumula en el cuerpo humano en grado
significativo. No se comunicaron efectos adversos a largo plazo en 232
trabajadores expuestos (duración de la exposición: 4-27 años) bajo
supervisión médica (tiempo de observación: 4-29 años). El único efecto
observado fueron pruebas indirectas de una estimulación reversible de
las enzimas metabolizadoras de fármacos.
No se detectó endrina en prácticamente ninguna muestra de tejido
adiposo, sangre y leche humana analizadas en numerosos países. El
Grupo de Trabajo atribuyó la ausencia de endrina en las muestras
humanas a la baja exposición de la población general a este plaguicida
y a su rápido metabolismo.
La endrina se detectó en la sangre (con concentraciones de hasta
450 µg/litro) y en el tejido adiposo (en concentraciones de 89,5
mg/kg) en casos de envenenamiento accidental mortal. No se encontró
endrina en los trabajadores en circunstancias normales. El nivel
umbral de endrina en la sangre por debajo del cual no se produce
ningún signo o síntoma de intoxicación se ha fijado en 50-100
µg/litro. La semivida de la endrina en la sangre puede ser del orden
de 24 horas.
2. Conclusiones
La endrina es un insecticida con elevada toxicidad aguda. Puede
provocar envenenamiento grave en casos de exposición excesiva
provocada por un manejo poco meticuloso durante su fabricación y uso
o por el consumo de alimentos contaminados. El público está expuesto
a la endrina principalmente por sus residuos en los alimentos; no
obstante, los niveles de ingesta de endrina que se han comunicado
están por lo general muy por debajo de la ingesta diaria admisible
establecida por la FAO/OMS. Esas exposiciones en principio no
constituyen un riesgo para la salud de la población general. Cuando se
aplican buenas prácticas de trabajo, medidas higiénicas y precauciones
de seguridad, es poco probable que la endrina suponga un riesgo para
los trabajadores expuestos.
Está claro que las descargas no controladas de endrina durante su
manufactura, formulación y uso pueden originar graves problemas
ambientales asociados a su elevada toxicidad. Los efectos del uso
agrícola del insecticida en la fauna y la flora están menos claros, si
bien los peces y las aves ictívoras están expuestos por la escorrentía
a partir de las superficies. El declive de las poblaciones de algunas
especies de aves se ha atribuido a la presencia de niveles elevados de
residuos de diversos compuestos organoclorados en los tejidos de
adultos y en los huevos. Se ha medido la endrina presente en algunas
de estas especies, pero es muy difícil separar los efectos de los
distintos compuestos organoclorados presentes.
3. Recomendaciones
1. No debe utilizarse la endrina a menos que sea indispensable
y sólo cuando no se disponga de una alternativa menos
tóxica.
2. Para la salud y el bienestar de los trabajadores y de la
población general, el manejo y el uso de la endrina se
confiarán sólo a operarios bien supervisados y adiestrados
que apliquen las medidas de seguridad adecuadas y utilicen
la endrina de acuerdo con las prácticas agrícolas correctas.
3. La fabricación, la formulación, el uso agrícola y la
evacuación de endrina se tratarán cuidadosamente para
reducir al mínimo la contaminación del medio, en particular
de las aguas de superficie.
4. Las personas expuestas regularmente a la endrina deben
someterse a revisiones médicas periódicas.
5. Proseguirán los estudios epidemiológicos sobre las
poblaciones de trabajadores expuestos.
6. En los países en los que aún se usa la endrina, deben
vigilarse sus residuos en los alimentos.
7. Si sigue utilizándose la endrina, debe obtenerse más
información sobre la presencia, el destino último y la
toxicidad de la 12-cetoendrina y la delta-cetoendrina.
 Endrin is the endo,endo stereoisomer of dieldrin
Empirical formula: C12H8Cl6O
Relative molecular mass: 380.93
Common name: Endrin
CAS registry number:
Endrin is the endo,endo stereoisomer of dieldrin
Empirical formula: C12H8Cl6O
Relative molecular mass: 380.93
Common name: Endrin
CAS registry number:  4.3.1 Biodegradation
Microbial degradation of endrin depends on the presence of an
appropriate microbial species and suitable soil conditions; it occurs
under anaerobic conditions (Donoso et al., 1979). Biodegradation is
aided by fungi and bacteria such as Trichoderma, Pseudomonas, and
Bacillus. The major transformation product is delta-ketoendrin
(Patil et al., 1970).
About 150 isolates from various soil samples were screened to
investigate the role of these microorganisms in degrading endrin; 25
of the 150 isolates were active. At least seven metabolites were
found, but conversion of endrin into the ketoendrin was common
throughout (Matsumura et al., 1971).
4.3.2 Bioaccumulation and biomagnification
The bioconcentration factors cited below are simple ratios of the
exposure concentration and the concentration in organic tissues. They
should be used with caution as indicators of bioaccumulation
potential: a high bioconcentration factor can represent little uptake
of a low concentration, and a low bioconcentration factor can be found
with considerable uptake of a high concentration. The bioconcentration
factor should therefore always be cited with the pertinent exposure
concentration of endrin.
Soil invertebrates such as slugs and earthworms had
bioconcentration factors of 14 to 103. Bioconcentration factors in a
number of aquatic organisms are given in Table 4. These ratios differ
extensively between different types of aquatic organisms.
Bioconcentration factors of 140 to 222 were found for four blue-green
algae (Microcystis aeruginosa, Anabaena cylindrica, Scenedesmus
quadricauda, and an Oedogonium species) after 7 days' exposure to
endrin at a concentration of 1 mg/litre of water( Vance & Drummond,
1969). In a study of the accumulation of endrin in stoneflies
(Pteronarcys dorsata) exposed to 0.03, 0.07, and 0.15 µg/litre of
water, the bioconcentration factor ranged from 1130 to 348, decreasing
with increasing water concentrations over the 28-day exposure period
(Anderson & DeFoe, 1980). In bullheads (Ictalurus melas), the
bioconcentration factor was 3700 after exposure for 4 days to 0.60
µg/litre and 6200 after exposure for 7 days to 0.26 µg/litre (Anderson
& DeFoe, 1980). The bioconcentration factors for endrin in sub-adults
of leopard frogs (Rana sphenocephala) exposed to 0.01, 0.012, 0.016,
0.022, and 0.030 mg/litre were 71.4, 34.4, 51.8, 59.4, and 94.3,
respectively. Sub-adults exposed to 0.01, 0.012, and 0.016 mg/litre
for 96 h and sacrificed 60 h later had bioconcentration factors of
6.1, 4.8, and 1.2, respectively (Hall & Swineford, 1980), indicating
a relatively rapid elimination of residues. In daphnids and molluscs,
a direct linear relationship was found between the logarithm of the
equilibrium bioconcentration factor (and the reciprocal clearance rate
constant) and the log P octanol/water partition coefficient for
non-degradable, lipophilic compounds with partition coefficients
ranging from 2 to 6. This relationship permits calculation of the
times required for equilibrium and for significant bioconcentration of
lipophilic chemicals, which were found to be shorter for molluscs than
for daphnids. The equilibrium biotic concentration for both molluscs
and daphnids decreased with increasing chemical hydrophobicity. The
relationship between the bioconcentration factor and log P
octanol/water partition coefficient was linear for compounds that did
not attain equilibrium within a finite exposure time (Hawker &
Connell, 1986).
In a study of the bioaccumulation of endrin from food by lobsters
(Homarus americanus), endrin dissolved in methanol was added to sea
water, and mussel tissue was soaked in the solution for 2 h to provide
a concentration of endrin of 4.7 mg/kg wet weight. Lobsters were fed
the prepared food every other day for 2 weeks, and excretion was
followed for an additional 4 weeks during which time the lobsters were
fed uncontaminated tissue. Liver and muscle were analysed from two or
three lobsters sampled after feedings 1, 2, 3, 5, and 7, and from one
or two lobsters sampled during the excretion phase at 1, 2, and 4
weeks. The concentration of endrin reached a maximum of 1.95 mg/kg wet
weight in the liver after 2 weeks of feeding; this level declined by
about 65% after 4 weeks of excretion. The time to 90% equilibrium
(uptake) was 15 weeks, and the time to 50% clearance (excretion) was
4 weeks (McLeese et al., 1980).
Bluegill sunfish (Lepomis macrochirus) exposed to water
containing 14C-labelled endrin at 1 µg/litre at temperatures of
20-22 °C rapidly absorbed the radioactivity, and, within 48 h, 91% of
the radioactive endrin had been taken up (6% was lost by
volatilization from a control tank without fish). Within 8 days after
the fish had been replaced in clean water, less than 15% of the
absorbed label had been eliminated; for three fish left for a longer
period, the half-life of loss was about 4 weeks, the loss curve being
linear (Sundershan & Khan, 1980). Endrin accumulated rapidly in the
tissues of channel catfish (Ictalurus punctatus) exposed to nominal
concentrations of 0.04, 0.4, or 4.0 mg/kg of diet for 198 days. After
that time, all groups were fed an endrin-free diet. Endrin was not
detected 28 days later in fish that had received 0.04 or 0.4 mg/kg,
and the level in the group that had received 4.0 mg/kg decreased to
0.011 mg/kg of tissue in 28 days and was below the limit of detection
within 41 days (Argyle et al., 1973). Similar results were obtained
for Leiostomus xantharus exposed to 0.05 µg/litre of water: at the
end of the study at 5 months, a residue level of 78 µg/kg tissue was
found, and no endrin was detected in fish after 18 days in
uncontaminated water (Lowe, 1966). Endrin thus seems to disappear
rapidly from tissues. In 20 Tilapia zilli (Alexandria strain) fry
(3.36 cm, 825 mg) exposed to 0.025 µg/litre (one-tenth of the 96-h
LC50) for 28 days, the total content of endrin was 327.4, 167.4,
297.6, 446.5, and 595.4 µg/kg after 4, 7, 14, 21, and 28 days,
respectively (El-Sebae, 1987).
Table 4. Bioconcentration factors for endrin in aquatic species
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Clam 1 5 days 480 Duke & Dumas
(Mercenaria (1974)
mercenaria)
Mussel 10 24 days 38 Ryan et al.
(Hyridella (1972)
australis)
Eastern oyster 0.05 7 day 2780 Mason & Rowe
(Crassostrea (1976)
virginica)
Water flea 1.0 1 day 2600 Metcalf et al.
(Daphnia (1973)
magna)
Fathead 0.015 - 10 000 Mount & Putnicki
minnow (1966)
(Pimephales
promelas)
Spot 0.05 8 months 1340 Lowe (1966)
(Leiostomus
xanthurus)
Flag fish 0.3 - 10 000 Hermanutz
(Jordanella 0.21 15 days 7 900 (1974)
floridae) 0.29 18 400 Hermanutz et
0.39 7 100 al. (1985)
Table 4 (contd)
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Mosquito 1.0 1 day 2 100 Metcalf et al.
larvae (Culex (1973)
ipiensquinque
fasciatus)
Mosquito fish 1.0 1 day 800 Metcalf et al.
(Gambusia (1973)
affinis)
Channel catfish 0.5 5-19 400-760 Argyle et al.
(Ictalurus (1973)
punctatus)
Sheepshead minnow (Cyprinodon variegatus) were exposed for 23
weeks to endrin at levels of 0.027-0.72 µg/litre of water, from the
embryonic stage through hatching until adulthood and spawning (see
section 7.2.2.2). Four-week-old juvenile fish accumulated 2500 times
the concentration in the water, adults, 6400 times, and their eggs,
5700 times (Hansen et al., 1977).
The transfer of endrin through the food chain
lichen-reindeer-humans was studied in the northern part of Sweden by
analysing lichen (Cladonia alpestris), a major food source for
reindeer during the winter, together with samples of tissues from
reindeer, which are eaten in considerable quantities by Lapps. One
4-year-old reindeer was slaughtered in 1979 and a 3-year-old in 1981,
and muscle and liver were taken for analysis. The annual uptake by
reindeer was 2.0 mg. The average level of endrin in lichen was 1.91
(range, 1.27-2.78) µg/kg; 1.45 and 2.4 µg/kg were found in the muscle
samples from the two reindeer and 0.55 and 0.72 µg/kg in liver. The
calculated transfer of endrin from lichen to reindeer was 0.7%. The
estimated annual consumption of reindeer muscle by Lapps was 70 kg for
males and 32 kg for women; consumption of liver was 3 and 1.1 kg,
respectively. The annual intake of endrin was thus 30.3 µg for males
and 13.8 µg for females (Villeneuve et al.,1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Many of the data reported in this chapter are measurements taken
at a time when endrin was used much more widely than at present or
with little control or restriction. They are therefore a reflection of
a historical situation in many countries. These data are included in
the document as an indication of the result of indiscriminate use and
disposal of endrin. Data from countries where endrin may still be used
are scarce or unavailable.
5.1 Environmental levels
The levels of residues associated with the use of endrin in
agriculture or with the discharge of industrial effluents containing
endrin are summarized in Tables 5-9; the levels of residues less
easily associated with specific uses or discharges are given in Table
10.
5.1.1 Air
A critical summary of studies on the atmospheric levels of
pesticides in the USA, e.g., in community air, was made by Donoso et
al. (1979). Some of their conclusions are worth repeating: "Endrin
concentrations are highest in the atmosphere over agricultural areas
and probably reach their peak levels during the pesticide use season.
Of all urban communities those surrounded by farmlands run the highest
chance of atmospheric contamination. Urban communities far removed
from agricultural areas are unlikely to experience significant
contamination." The maximum level of endrin in air, 58.5 ng/m3, was
found in a rural town in an agricultural area in the south of the USA,
but the normal weekly variation was between 0.8 and 6.5 ng/m3
(Stanley et al., 1971). In a later study of the same town, the average
annual atmospheric levels were 3.2 ng/m3 in 1972, 2.3 ng/m3 in
1973,and 5.3 ng/m3 in 1974, with the highest levels in August; in
1974, this was 27.2 ng/m3 (Arthur et al., 1976). The results of a
national monitoring programme for pesticides in the air of various
states of the USA showed the occasional presence of endrin over
agricultural areas at levels of the same order of magnitude: mean of
positive samples (8%), 2.6 ng/m3, with a maximum value of 19.2
ng/m3 (Kutz et al., 1976).
Endrin was not found in rain-water collected at different
location in the United Kingdom, using a method with a detection level
of 1 ng/litre of water (Tarrant & Tatton, 1968), nor in atmospheric
air (Abbott et al., 1966); however, endrin has never been used
extensively in the United Kingdom.
The mean daily intake of endrin by inhalation in the western part
of the Netherlands was calculated on the basis of an air concentration
of 41 pg/m3 (maximum, 300 pg/m3) to be 0.8 µg/day or 0.3 mg/year,
on the basis of air samples taken in the period 1975-81 (Guicherit &
Schulting, 1985).
Table 5. Concentrations of endrin in organisms collected in the Netherlands, 1965-71
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Coast Mussel (Mystilus edulis); 22 0.029 0.009-0.056 Composites of 25 Koeman (1971)
1965 composites of flesh mussels from 22
sampling stations
Netherlands Fish, 3 species; 103 0.13 0.07-0.45 Food of the sandwich Koeman et al. (1967)
1965 whole body tern
1966 Fish, 3 species; 37 0.10 0.07 -0.29
whole body
Sandwich tern
(Sterna sandvincensis)
1965 Liver 8 0.23 0.07-0.80 Shot or killed
1965-66 Liver 25 0.49 0.10-1.3 Found dead
1965-66 Egg 33 0.19 0.08-0.36
Wadden Sea Mussel (2 species); 20/4 LD LD Limit of detection, Koeman (1971)
1969 composites of flesh 0.005 mg/kg
Coast Mussel (Mytilus edulis); 199/8 < 0.016 LD-0.024 Koeman (1971)
1970 composites of flesh
Wadden Sea Zooplankton (marine) 1 LD LD Koeman (1971)
1969-70
Shrimp (Crangon 50/1 LD LD
vulgaris)
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Marine fish (4 species); 37/5 0.014 0.008-0.034
composites of whole
body
1967 Freshwater fish 28 LD LD-0.02 Measurable concentration
(3 species) (0.02 mg/kg) in one fish
only; limit of detection,
0.005 mg/kg
1970 Pike (whole body) 10 LD LD Limit of detection,
0.005 mg/kg
1971 Roach (whole body) 6 LD LD
1968-69 Hawks and falcons 16 < 0.1 LD-0.16 Birds found
dead or dying; Koeman et al. (1969)
(4 species); liver measurable concentration
(0.16 mg/kg) in one hawk
(buzzard)
Owls (2 species); liver 3 < 0.1 LD-0.13 Measurable concentration
(0.13 mg/kg) in one
long-eared owl; limit of
detection not specified
1970 Sandwich tern eggs 10 LD LD Limit of detection, Koeman (1971)
0.02-0.008 mg/kg
1971 Grey heron 27/4 LD LD
(Ardea cinerea);
composite of eggs
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
1969-71 Sparrowhawk 28/3 LD LD
(Accipiter nisus);
composite of eggs
a Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
b LD, limit of detection
Table 6. Concentrations of endrin in samples collected in North America
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Mississippi River
USA
December 1963 Channel catfish; blood 3 0.44 0.41-0.56 Found dead or dying in areas Anon. (1964)
of extensive fish kills
December 1963 Fish, various species; 24 0.18 0.14-0.26
blood
January- Fish, various species; 82 0.06 LD-0.21 Caught alive; limit of
February 1964 blood detection not specified
July 1964- Water 12 < 0.01 LD-0.01 4 samples contained Novak & Rao (1965)
June 1965 measurable concentrations
(0.01 mg/kg or litre)
Mud 12 < 0.01 LD-0.01
Oysters 12 LD LD Limit of detection,
0.005 mg/kg or litre
Shrimp 12 LD LD
Fish (2 species) 24 < 0.01 LD-0.02 9 samples of fish contained
measurable concentrations:
8 of 0.01 mg/kg and 1 of
0.02 mg/kg
Mississippi, USA Eastern oysters 470 LD LD 15 or more oysters per Butler (1973)
1965-72 (Crassostrea virginica); composite from 8 sampling
composites of flesh stations; limit of detection,
0.01 mg/kg
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Bayous in the Water 148 LD LD-0.0002 4 samples contained Rowe et al. (1971)
Mississippi delta, measurable amounts
USA, October (0.00009-0.0002 mg/litre),
1968-May 1969 4 samples contained traces;
remainder less than limit of
detection
Sediment 44 LD LD-0.005 7 samples contained
measurable amounts
(0.004-0.005 mg/kg); one
sample contained a trace
Eastern oyster 111 LD LD-0.006 79 samples contained
(Crassostrea virginica) < 0.001 mg/kg
Mississippi Water 26 LD LD Samples collected from Leard et al. (1980)
stream systems 13 sampling stations in
1972-73 5 major river basins; limit
of detection, 0.0005 mg/litre
Freshwater bivalves 58 LD LD-0.1 Residues below limit of
(7 species); flesh detection (0.02 mg/kg), except
for traces (< 0.1) in 1973 in
one river which drains from an
agricultural area
Ontario, 3 Fish (9 species) LD LD Residues below limit of Miles & Harris
streams, 1971 detection, 0.01 mg/kg (1973)
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Gulf coast Whole fish 139/48 < 0.02 LD-0.27 Reseidues below limit of Henderson et al.
streams, USA (various species); detection (0.001 mg/kg) (1969)
composites in 33 composites
Mississippi 657/202 < 0.01 LD-0.11 Residues in 184 composites
River system, below limit of detection
USA (0.001 mg/kg)
Louisiana, USA Brown pelican Eggs collected from nests of Blus et al. (1979)
(Pelecanus occidentalis); birds transplanted as nestlings
eggs from Florida, 1968-76; limit
1971 3 0.10 0.08-0.12 of detection not specified
1972 12 0.18 0.11-0.29
1973 21 0.16 0.03-0.46
1974 25 0.30 LD-0.73
1975 30 0.50 0.29-1.06
1976 25 0.29 LD-1.47
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 7. Concentrations of endrin in organisms collected in a
cotton-growing area in the Republic of Chad in 1969
Sample No. of Concentration (mg/kg) Comments
samples Mean Range
Fish, two 31 0.02 LD-0.083 Cotton-growing area,
species endrin and DDT used
for pest control; limit
Kingfishers and 46 0.02 LD-0.075 of detection, 0.008
cormorants; liver mg/kg
Birds, non-aquatic, Birds found dead
various species soon after insecticide
Brain 12 0.51 0.10-0.77 application; deaths
Liver 12 0.88 0.13-1.42 of some birds attributed
to endrin
From Everaarts et al. (1971); LD, limit of detection
5.1.2 Soil, sediments, and sewage sludge
5.1.2.1 Soil
In the US National Soil Monitoring Program, 1486 soil samples
from 37 states were analysed in 1971. Fourteen samples were found to
contain endrin, at a geometric mean level of < 0.001 (maximum,
0.02-1.00) mg/kg dry weight (Carey et al., 1978). The mean endrin
concentration in 29 soil samples in Kyushu District, Japan, was 0.183
mg/kg (range, 0.016-0.629 mg/kg) dry matter (Suzuki et al., 1973).
5.1.2.2 Sediments
In 1964, levels in the sediment of Cypress Creek, Memphis, TN,
USA, upstream and downstream of a pesticide manufacturing plant,
reached 12 800 mg/kg dry weight. In 1967, water from the Creek
contained levels of 0.27-2.03 µg/litre and sediment contained levels
of 47.4-10 676 mg/kg dry weight (Barthel et al., 1969).
Endrin was found in 17% of samples of bottom sediment from 59
sites on the Detroit River, USA, at levels up to 43 µg/kg (limit of
detection, 1.0 µg/kg) (Hamdy & Post, 1985). No endrin was detected in
sediment samples collected in 1980-82 from riverine and pothole
wetlands at 17 locations in the north-central USA (Martin & Hartman,
1985) or in samples of sediment from 34 stations on the upper Great
Lakes in 1974 (< 1 µg/kg) (Glooschenko et al., 1976).
Table 8. Concentrations of endrin in organisms collected in a rice-growing area in Wageningen, Surinam
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
October 1971 Snail kite (Rostrhamus 5/1 LD LD -- Pesticides, including endrin, applied
sociabilis); brain /liver | to rice fields
|
Black vulture (Coragyps 5/1 LD LD |
astratus); brain/liver |
|
Egrets (3 species); brain/ 30/1 LD LD | Samples collected at end of growing
liver |-- season before insecticide application
| for next growing season; limit of
Purple gallinule 10/1 LD LD | detection, 0.01 mg/kg
(Porphyrula martinica); |
brain/breast muscle |
|
Spectacled caiman (Caiman 10/1 LD LD |
crocodilus); brain/liver --
November 1971 Snail (Pomocea sp.) 10/1 LD LD --
|
Frog (Pseudis paradoxa); 6/1 LD LD | Found dead after application of
whole-body composites | pentachlorophenol; lower limit of
| detection, 0.01 mg/kg
Kwi kwi (Hoplosternum 8/1 LD LD |
littorale); whole-body |
composites --
Table 8. (contd)
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
Srieba (Astyanax bimaculatus); 8/1 LD 0.1 -- Found dead after application
whole-body composites | of pentachlorophenol; lower
|-- limit of detection 0.01 mg/kg
|
Krobia (Cichlasoma 8/1 LD LD |
bimaculatum); whole-body |
composites --
Fish (3 species listed above); 21/3 3.36 1.96-5.35 Found dead after application
whole-body composites of endrin
28 November- Snail kite; brain 17 LD LD Found dead; deaths
4 December 1971 attributed to pentachlorophenol
poisoning
2-9 December 1971 Aquatic birds (4 species); 5 0.11 0.06-0.16 Found dead after application
brain of endrin
2-9 December 1971 Wattled jacana 1 2.71 Death attributed to endrin
(Jacana jacana) poisoning
5-11 December 1971 Common egret (Egretta alba); 2 0.23 0.14-0.32 Found dead or sick in roost
brain
2-11 December 1971 Common egret (Egretta alba); Found dead or sick in rice
Brain 9 0.25 fields; about half the total
Liver/kidney 7-9 0.08 endrin was applied during
the first half of December
From Vermeer et al. (1974)
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 9. Concentrations of endrin collected in drainage water from irrigated land, California, USA
Geographical Type of sample No. of Concentration (mg/kg or mg/litre) Comments
area and year samples
Mean Range
Tule Lake and Water 44 0.000011 LD-0.0001 Limit of detection, < 0.000003 mg/litre
Klamath Lake,
National
Wild-life
Refuge,
USA, 1964
Refuge, Northern Suspended matter 8 0.011 LD-0.058 --
California, USA, Vascular plants 7 0.006 LD-0.013 |
April 1965- (two species) |-- Limit of detection, 0.005 mg/kg
February 1967 Algae (Cladophora sp.) 5 0.007 LD-0.022 |
Clam homogenates 3 0.013 LD-0.034 --
Gonidea sp.)
Fish (Siphateles sp.) 5 0.05 0.004-0.198 Samples collected at a pumping station
discharging water from irrigated land.
Peak concentrations of endrin occurred
during the growing season when endrin
was applied.
From Godsil & Johnson (1968); LD, limit of detection
Table 10. Concentrations of endrin in environmental samples; residues not associated with particular local use or industrial
effluent
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
North America
North Carolina, Soil (tobacco fields) 19 LD LD Reeves et al.
USA,1971 Sediment (ponds) 40 LD LD Limit of detection, (1977)
Frog (Rana sp.) 13 LD LD-0.01 0.01 mg/kg
Turtle (4 species) 41 LD LD-0.01
Bluegill (Lepomis 20 LD LD
macrochirus)
Tiger beetle 23 0.02 LD-0.05
(Megacephala
carolina)
Rice-growing Invertebrates, aquatic 1313/24 LD LD-trace A total of 192 dead or Flickinger &
area, Gulf Coast, and terrestrial (various dying birds were found in King (1972)
Texas, USA, species); whole-body three rice-growing areas in
1967-71 composites of live which rice seed dressed
specimens, except for with aldrin/ceresan had
4 composites of been used. Endrin residues
crayfish attributed to use in cotton-
growing areas. Limit of
1968 Fish (4 species); 542/4 LD LD detection not defined;
whole-body composites trace found in one
composite of dead
1968 Cricket frog (Acris 18/3 LD LD crayfish
crepeitans blanchardi);
whole-body composites
1968-70 Turtles (2 species); 5/2 LD LD
whole-body composites
of live specimens
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Snakes (3 species); sick 3 LD LD
specimens
Bobcat (sick) and dead 2 LD LD
rice rat; brain
Great horned owl; dead 2 LD LD
specimen; brain
Rice- growing Aquatic birds (10 26 0.22 LD-0.4
area, Gulf Coast species) found dead
USA, 1967-71 or dying; brain
1967 Fulvous tree duck 14 0.1 LD-0.3
(Dendrocygna bicolor);
eggs
Galveston Bay Oyster composites 10 0.01 LD-0.02 Limit of detection, Casper (1967)
Texas, USA, 1964 0.01 mg/kg
National Monitoring Fish (various species); 400 93% of samples below Henderson et
Program: Great Lakes whole-body composites limit of detection, al. (1969)
and major river 0.001 mg/kg
basins, USA (excluding
Gulf Coast, Mississippi
River system; see
Table 6); 1967-68
Atlantic coast streams 741/141 0.002 LD-1.50
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Great Lakes drainage Fish (various species); 378/66 0.001 LD-0.02
whole-body composites
Hudson Bay, Canada, 51/13 LD LD
drainage
Colorado River, USA 112/24 0.008 LD-0.71
Interior basins 120/25 0.001 LD-0.01
California, USA, 90/24 0.002 LD-0.02
streams
Columbia River, USA, 246/64 0.001 LD-0.01
systems
Pacific coast, USA, 83/20 LD LD
streams
Alaska, USA, streams 105/24 LD LD
National Monitoring Fish (various species); 666/147 LD LD Limit of detection, Henderson et
Program; 50 sampling whole-body composites 0.005 mg/kg al. (1971)
stations, USA, 1969
Estuaries, Giant Pacific oyster 1656/138 0.005 LD-0.01 Measurable concentration Modin (1969)
California, USA (Crassostrea gigas); in only one oyster; limit
1966-67 Mussel (Mytilus edulis); 432/36 LD LD of detection, 0.01 mg/kg
composites of shellfish
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 108-162/54 0.06 LD-0.4 Limit of detection, Crockett et
Mississippi, USA fish farms; composites 0.01 mg/kg; 13 al. (1975)
1970 of edible portions composites contained
< 0.01 mg/kg
Intensive cotton- 0.063 (0.030- 2 composites contained
growing areas, 0.122)a > 0.3 mg/kg. Significantly
Mississippi, USA higher residues in intensive
cotton-growing areas
Less intensive cotton- 0.010 (0.005- Crockett et
growing areas, 00.019)a al. (1975)
Mississippi, USA
Major watersheds, Fish (various species); 582/58 LD LD Limit of detection not Veith et al.
USA, 1976 whole-body composites specified. Intermediate (1979)
in the manufacture of
cyclodiene insecticides
detected (mass
spectrometry) in Wabash
River, Indiana
Major watersheds Fish (various species); 138/6 LD LD Limit of detection not Veith et al.
near Great Lakes, whole-body composites specified. Endrin (1981)
USA, 1978 identified by mass
spectometry in fish from
Wabash River, Indiana,
together with manufacturing
intermediates (concentration
not quantified)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 50 0.05 LD-0.41 Limit of detection, 0.01 Hawthorne
Mississippi, USA, fish farms, edible portion mg/kg; 14 fish contained et al. (1974)
1970 < 0.01 mg/kg
Continental rise Bathyl-demersal fish 4 LD LD Limit of detection, Meith-Avcin
south-east of (Antimora rostrata); 0.01 mg/kg. Samples et al. (1973)
Cape Hatteras, USA, liver collected by trawling at a
1972 depth of 2500 m
Lake Michigan, USA, Amphipods (Pontoporeia 24/8 0.08 0.04-0.33 Limit of detection, Peterson &
1969-72 affinis) collected from 0.005 mg/kg Ellarson
oesophagi of old squaws (1978)
December 1969 Old squaws (Clangula 37 0.18 0.1-0.2 Birds caught in fishing
hyemalis); carcasses nets or shot
March-April 1970 44 0.28 0.2-0.4
December 1970- 108 0.31 0.1-0.9
May 1971
January-February 8 0.6 0.2-1.0
1972
Northwest Territories 99 0.1 LD-0.3
and wintering areas
other than Lake
Michigan, 1971-73
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Canada and USA, Bald eagles (Haliaeetus 29 0.02 LD-0.1 Limit of detection, Reichel et al.
1965 leucocephalus) found 0.05 mg/kg. (1969)
dead; brain Concentration in 24
specimens below limit
of detection
Connecticut & Bald eagles found dead; 2 LD LD-0.1 Limit of detection not Reichel et al.
Florida, USA, brain, liver, carcass defined; apparent (1969)
1967-68 concentration of
0.1 mg/kg in Florida
eagle not conformed
by thin-layer
chromatography
Continental USA Bald eagle; brain Limit of detection, Mulhern et al.
1966 21 LD LD 0.05 mg/kg (1970)
1967 21 LD LD
1968 26 LD LD
1969 Bald eagle; brain 28 LD LD Limit of detection, Belisle et
0.05 mg/kg al. (1972)
1970 11 LD LD
1971-72 Bald eagle; brain 37 LD LD Limit of detection, Cromartie et
0.05 mg/kg al. (1975)
1973-74 Bald eagle; brain 81 LD LD Limit of detection, Prouty et al.
0.05 mg/kg (1977)
1975 Bald eagle; brain 49 0.07 LD-0.50 Limit of detection, Kaiser et al.
0.05 mg/kg. (1980)
Concentrations in 46
specimens < 0.05 mg/kg
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
1976 50 0.08 LD-0.71 Concentrations in 44
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisong
1977 69 0.08 LD-1.2 Concentrations in 64
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisoning
Wisconsin, Maine, Bald eagle; eggs 26 LD LD Limit of detection, Krantz et al.
Florida, USA, 0.05 mg/kg (1970)
1968
USA, Golden eagle (Aquila 102 LD LD-0.3 Limit of detection, Reidinger &
1964-71 chrysaetos) found dead 0.1 mg/kg. Concentrations Crabtree
or dying; body fat in 97 specimens below (1974)
limit of detection
Coast of California, Gray whale 23 LD LD Wolman &
USA, 1968-69 (Eschrichtius robustus); Wilson (1970)
blubber
Sperm whale (Physeter 6 LD LD
catodon); blubber
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
South Atlantic and Small cetaceans (10 69 LD LD-0.24 Limit of detection, O'Shea et al.
Pacific Oceans, species); blubber, 0.1 mg/kg. Measurable (1980)
1968-69 brain, muscle concentrations (0.22 and
0.24 mg/kg) found in two
specimens
Maryland, USA, Little brown bat (Myotis 87 LD LD Limit of detection, Clark &
1976 lucifugus); carcass 0.1 mg/kg Krynitsky
(1978)
Missouri, USA Gray bat (Myotis 20 LD LD Limit of detection, Clark et al.
1976-77 grisescens) found dead; 0.1 mg/kg (1980)
carcass (lipid basis)
Washington Sate 18 bird species (total Blus et al.
(orchards), number of birds, 91) (1983)
October 1981- Brain 78 LD-> 0.8
July 1982 Eggs 53 LD-1.7
Detroit River, Herring gulls (Larus LD LD Struger et al.
Niagara River, argentatus); eggs (1985)
Saginaw Bay, USA,
1978-82
North-west Fish species; 700 0.008 LD-0.026 Stout (1980)
Atlantic Ocean, muscle
Gulf of Mexico,
USA, 1973-75
South-east Montana, Merlins (Falco LD LD Becker & Sieg
USA, 1978-81 columbarius); eggs (1987)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
New Jersey, Snapping turtles 11 LD LD Limit of detection, Albers et al.
Maryland, USA (Chelydra serpentina) 0.1 mg/kg (1986)
Florida, USA Snail kite (Rostrhamus LD LD Limit of detection, Sykes (1985)
sociabilis); eggs, nestlings 0.05 mg/kg
Missouri, USA, Gray bats (Myotis 7 LD LD Clawson &
1982 grisescens) Clark (1989)
Red bats (Lasiurus 7 LD LD
borealis)
Pipstrelles (Pipistrellus 2 LD LD
subflavus)
Denver, Colorado, Tree swallows 32 LD LD Deweese et al.
USA, 1980-81 (Tachycineta bicolor) (1985)
North-east Alberta, Otter (Lutra canadensis); 158 LD LD Limit of detection, Somers et al.
Canada, 1980-82 carcass 0.001 mg/kg (1987)
Africa
Lake Nakuru, Fish (Tilapia grahami); 10-20/2 LD LD Limit of detection: Koeman &
Kenya, 1970 whole-body composites fish, 0.002 mg/kg; Santiago
African cormorant birds, 0.009 mg/kg (1972)
(Phalacrocorax africanus) 3 LD LD
liver
White pelican 1 LD LD
(Pelicanus onocratalus);
Lesser flamingo 5 LD LD
(Phoeniconaias minor)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Europe
Province of Leon, Kestrels (Falco 4 - LD-2.0 Liver, 0.01; brain, 0.016; Sierra &
Spain, 1986 tnnunculus); 5 organs kidneys, 0.027; muscle, Santiago
or tissues 0.054 mg/kg (1987)
Sparrowhawk 3 LD-2.0 Liver, 0.139; kidneys, 0.4;
(Accipiter nisus) fat, 1.068; brain, 0.031;
muscle, 0.047 mg/kg
Red kite 2 LD-2.0 Kidneys, 0.005; brain,
(Milvus milvus) 0.103; fat, 2.035 mg/kg
1984-87 Barn owl (Tyto alba) 0.001-0.22 Liver, 0.036; brain, 0.052; Sierra et al.
kidneys, 0.034; fat, 0.014; (1987)
muscle, 0.020 mg/kg
North Sea Gadus morhua and 12 0.0001-0.0023 Von Westernhagen
Merlangius merlangus; 4 < 0.001-0.0011 et al. (1987)
ovary
North Sea Merlangius merlangus Von Westernhagen
Ovary 56 LD-0.001 et al. (1989)
Testis 16 LD
Liver 30 LD
Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed; LD, limit of detection
a95% confidence interval
None of 60 samples of bottom deposit collected in 1974 from 19
rivers and their estuaries in Japan contained endrin (< 0.01 mg/kg)
(Japanese Environmental Agency, 1975).
No endrin was detected in sediment and particulates from the
River Elbe in Germany in 1983-85 (Sturm et al., 1986). Sediment from
Rotterdam Harbour contained a total of 3-59 µg/kg aldrin, dieldrin,
and endrin. No endrin was found at seven sites in the Elbe Estuary
(Japenga et al., 1987). A housing estate in the Netherlands,
comprising about 800 houses and public buildings, was built in 1983
directly on a 4-m-thick layer of harbour sludge transferred in 1962-64
from about 20 harbour basins in Rotterdam and the industrial area
around the Nieuwe Waterweg. Organic solvents, polycyclic aromatic
hydrocarbons, heavy metals, and endrin and related pesticides, were
detected in the sludge. One-third of the soil samples collected in the
gardens (71 locations), 0-40 cm below the surface, contained endrin
and related pesticides at a mean concentration of 1.2 mg/kg and a
maximal concentration of 19.5 mg/kg dry weight (Van Wynen & Stijkel,
1988).
In surface sediments from five sites in Manukan Harbour, New
Zealand, only traces (none detected to < 0.1 µg/kg dry weight) of
endrin were found (Fox et al., 1988).
Particulates from two sites in the Shatt al Arab River in Iraq
contained endrin at 84 and 154 µg/kg, and a site in the Tigris River
contained 217 µg/kg. No endrin was found in the Euphrates River. The
mean concentration of endrin in surface and subsurface sediment from
the Shatt al Arab River ranged from 3 to 18 (range, none detected to
32) µg/kg; no endrin was found in surface and subsurface sediment from
the Tigris River. None was found in surface sediment from the
Euphrates River, but in subsurface sediment a mean concentration of 11
(5-25) µg/kg was detected (DouAbul et al., 1988).
5.1.2.3 Sewage sludge
Endrin was found in only a few of 444 sludge samples analysed
from sewage treatment works in the United Kingdom. The mean
concentration was 0.11 mg/kg of sludge, with a range of 0.01-0.71
mg/kg (McIntyre & Lester, 1984). All samples of non-disinfected
influent at a pilot plant in Jefferson Parish, LA, USA, contained
endrin, at an average concentration of 0.67 (0.25-1.58) ng/litre
(Lykins et al., 1986).
Sludge from three main waste water treatment plants in Kuwait was
analysed over a 6-month period in 1984-85. Two grab samples were taken
from each plant every month to give a total of 36 samples. The mean
endrin levels for the three plants were 0.02, 0.02, and 0.06 mg/kg
(Samhan & Ghobrial, 1987). Sewage plant effluents before and after
treatment were analysed in Baghdad (Iraq) in 1982-83. Endrin was found
in 8/15 samples taken before treatment, at a mean concentration of
0.291 (0.081-2.637) µg/litre, and in 6/15 samples taken after
treatment, at a mean concentration of 0.194 (0.072-1.197) µg/litre
(Al-Omar et al., 1985a).
5.1.3 Water
5.1.3.1 Surface water
Data on the concentrations of endrin in surface water concern
mainly those regions in the USA where use of endrin was widespread,
such as in Mississippi and Missouri, over the period 1957-65. The
highest concentrations were found in 1963 in the Lower Mississippi,
with a maximum level of 0.214 µg/litre. The concentrations and the
rate of occurrence decreased considerably later (Breidenbach et al.,
1967). In a survey in 1964-68, a maximal level of 0.133 µg/litre was
reported to have been found in the Missouri basin in 1967. No endrin
was detected in 1968 (Lichtenberg et al., 1970). In 1974, the
concentrations in the Lower Mississippi was 0.0045 µg/litre in
August-November (Brodtmann, 1976).
In one sample from the Potomac River, at Quantico, endrin and
endrin aldehyde were identified at concentrations of 0.005 and 0.006
µg/litre, respectively (Hall et al., 1987). Endrin was not found in
the waters near the Los Angeles County ocean outfalls (< 0.00005
µg/litre) (Green et al., 1986) or in surface water in Louisiana in
1980 (< 1.0 ng/litre) (McFall et al., 1985). In a programme to
monitor surface water in the USA in 1976-80, endrin was found in only
0.1% of samples, at a maximum value of 0.04 µg/litre (Carey & Kutz,
1985).
No endrin was found in water from 33 sites in the Upper Great
Lakes in Canada (< 0.01 µg/litre) (Glooschenko et al., 1976). In
Ontario, where endrin was used only sparingly, no residues were found
in 1971 or 1975-77 (Miles & Harris, 1973; Frank et al., 1981). In
water samples taken 1 m below the surface at 14 stations on Lake
Ontario in 1983, endrin was found in concentrations of
0.000044-0.000145 µg/litre (Biberhofer & Stevens, 1987).
In a survey of the aquatic environment in The Netherlands,
including drinking-water, 1826 samples were taken at 99 sampling sites
between September 1969 and 1977; traces of endrin were reported
occasionally (Wegman & Greve, 1978, 1980). Studies of surface water in
other areas in Europe failed to show the presence of endrin (Wilson
Committee, 1969; Engst & Knoll, 1973; Uhnak et al., 1974; Galassi &
Provini, 1981;Hrubec, 1988). In 1984-85, water from a number of rivers
in Germany contained endrin at levels of none detected to 0.30
µg/litre (Braun ,1985); surface water in Greece occasionally contained
levels of 0.0003-0.0004 µg/litre (Albanis et al., 1986).
No endrin was found in surface or drinking-water in the state of
Sa Paulo (Brazil) (Lara & Barreto, 1972), but it was found in water
reservoirs of basins in Sa Paulo at concentrations of none detected
to 1.02 µg/litre (Celeste & Caceres, 1987; Caceres et al., 1987).
Endrin was also found accidentally in two lagoons in north-west Mexico
(Rosales et al., 1985).
None of 60 water samples collected in 1974 from 19 rivers and
their estuaries in Japan contained endrin (< 0.1 µg/litre) (Japanese
Environmental Agency, 1975).
Endrin was found at five places in the River Nile at
concentrations of 0.0038-0.0189 µg/litre in March-September 1982
(El-Dib & Badawy, 1985). In analyses of the water of the Shatt al
Arab, Euphrates, and Tigris Rivers, endrin was found only in the
Euphrates River, but in all samples at a mean concentration of 0.024
(0.014-0.036) µg/litre (DouAbul et al., 1988). It occurred in 75% of
samples of urban, industrial, and continental water from the Moroccan
Mediterranean coast, at concentrations of none detected to 13 µg/litre
(Kassabi et al., 1988). Three of 15 grab samples of surface water
sources in Southern Africa (Orange Free State) contained endrin, at a
concentration of 2-4 µg/litre (Hassett et al., 1987).
Endrin was present in the Kalinadi River in India as a result of
runoff, especially from agricultural areas, at a concentration of 2
µg/litre (Kudesia & Bali, 1985). In the Chao Phraya River and klongs
in Bangkok, Thailand, no endrin (< 0.001 µg/litre) was found in 1984
(Onodera & Tabucanon, 1986). Analyses in Bali of 16 samples of river
water in the dry season and 15 samples in the rainy season showed the
presence of endrin at 40 µg/litre once, in the rainy season (Machbub
et al., 1988).
5.1.3.2 Rain and snow
No endrin was found (limit of detection, 1-2 ng/litre) in
atmospheric precipitation in the form of snow (17 samples in 1976) and
rain (81 samples in 1976 and 1977) on the Canadian side of the Great
Lakes and inland in areas remote from any nearby industrial or urban
contamination (Strachan et al., 1980). Four of 16 samples of
rain-water collected at four sites in Canada had levels of
0.00013-0.00044 µg/litre and another sample had 0.0048 µg/litre; no
endrin was detected in the other 11 samples. The mean endrin contents
in samples taken at another site in 1977, 1981, 1983, and 1984 were
none detected, 0.000065, 0.000085, and 0.000049 µg/litre, respectively
(Strachan, 1988). Endrin was not detected in snow samples collected at
12 sites in the Northwest Territories of Canada in 1985-86 (Gregor &
Gummer, 1989).
5.1.3.3 Drinking-water
Data obtained in 1964-67 from selected municipal drinking-water
treatment plants in Mississippi and Missouri, USA, showed that the
concentration in approximately 10% of the samples exceeded 0.1
µg/litre in the first year but that the concentrations were lower in
1965-67 (Schafer et al., 1969). The most recent study on US
drinking-water was done on finished water in New Orleans, LA, in 1974,
where the highest concentration measured was 4 ng/litre (US EPA,
1974).
During 1976, endrin was found at a mean concentration of 4
ng/litre (range, 1-7 ng/litre) in drinking-water in Ottawa, Canada
(Williams et al., 1978).
The mean concentration of endrin in drinking-water at the
El-Abbasia station, Egypt, in 1986 was 3.507 ± 1.45 ng/litre in 10
samples taken before purification and 1.845 ± 1.29 ng/litre after
purification (Abdel-Razik et al., 1988).
Drinking-water from the North Coast region of New South Wales,
Australia, was analysed in 1986-87: 147 of 659 samples contained
traces of endrin (none detected [< 0.005] to 0.05 µg/litre) (Ang et
al., 1989).
5.1.3.4 Groundwater
Water in wells used as a source of water for mixing pesticides
in fruit orchards in West Virginia (USA) was found to contain endrin
at about 1 ng/litre in 1985 and in 1986. The water in these wells was
not used for drinking-water. Endrin had not been used in the area
since 1970, and the authors cite their results as evidence for the
persistence of endrin and its capacity to contaminate groundwater many
years after cessation of use (Hogmire et al., 1990).
5.1.4 Organisms in the environment
5.1.4.1 Birds
Endrin was found in the carcasses of four of 16 turkey vultures
(Cathartes aura) in southern California, USA, in 1981, at levels of
0.11-0.23 mg/kg wet weight, but in none of six common ravens (Corvus
corax). It was also found in two of four vulture eggs, at 0.10
(range, none detected to 0.52) mg/kg wet weight, but in none of 30
raven eggs (Wiemeyer et al., 1986).
Endrin was found in eggs of shag (Phalacrocorax aristotelis)
and cormorants (Phalacrocorax carbo) at one of five collection sites
in the east, south-east, and south of Ireland, at a geometric mean
concentration of 0.30 (range, 0.06-1.60) µg/kg (Wilson & Earley,
1986). Eggs from two species of passerine birds, three species of
gull, four species of tern, and the night heron were collected in
Italy in 1982-83. Endrin was found in 30 eggs of the night heron
(Nicticorax nycticorax), at an average concentration of 0.11
(0.03-0.27) mg/kg, in 50 eggs of the gull-billed tern (Gelochelidon
nilotica), at a concentration of 0.28 (0.05-1.31) mg/kg, and in 38
eggs of the tree sparrow (Passer montanus) and 33 eggs of the hooded
crow (Corvus corone), at concentrations of 0.17 (0.09-0.33) and 0.21
(0.07-0.31) mg/kg. It was not detected in eggs of the other species
(Fasola et al., 1987).
No endrin was found in 98 eggs or in the livers of 112 nestlings
of rooks (Corvus frugilegus) collected from five rookeries in
northern Germany in 1982-83 (Beyerbach et al., 1987) or in 45 eggs and
the livers of eight young lapwings (Vanellus vanellus) collected in
1984 and 1986 (Beyerbach et al., 1988).
Detectable residues of the commonest organochlorine pesticides
were found in 0.9% of 112 pools (mostly of 10 birds) of starlings
(Sturnus vulgaris) collected in 129 sites in the USA in 1979 and in
1.6% of 129 pools in 1982. In most states, no endrin was detected, but
levels of 0.01 and 0.17 mg/kg wet weight were found in two (Bunck et
al., 1987).
Endrin was present at microgram levels per kilogram of wet weight
in 272 samples of liver, muscle, fat, and eggs from northern fulmars
(Fulmarus glacialis), black-legged kittiwakes (Rissa tridactyla),
and thick-billed murres (Uria lomvia) collected in 1975-77 on Prince
Leopold Island, Northwest Territories, Canada (Nettleship & Peakall,
1987). It was found in 8 of 108 carcasses of herons analysed in the
USA since 1966, at levels of 0.10-0.86 mg/kg wet weight (Ohlendorf et
al., 1981) but was not found in 255 pools of wings from black ducks
(Anas rubripes) and mallards (A. platyrhynchos) collected in the
USA in 1981-82 (Prouty & Bunck, 1986).
Endrin was not detected in six eggs of Forster's tern (Sterna
forsteri) collected on Green Bay and Lake Poygan, Michigan, USA in
1983 (Kubiak et al., 1989). None was found in a total of 107 eggs
collected in 1975-80 from 10 species of colonial waterbirds nesting in
areas around Green Bay and Lake Michigan. The species were little
gulls (Lares minutes), green-backed herons (Butorides striatus),
black terns (Chlidonias niger), herring gulls (L. argentatus),
ring-billed gulls (L. delawarensis), common terns (S. hirundo),
Forster's tern (S. forsteri), double-crested cormorants
(Phalcrocorax auritis), black-crowned night herons (Nycticorax
nycticorax), and cattle egrets (Bubulcus ibis). The limit of
detection was 0.1 mg/kg in 1977 and 0.05 mg/kg in 1978 (Heinz et al.,
1985).
Of five eggs from peregrine falcons (Falco peregrinus)
collected in Arizona, USA, in 1978-82, one collected in 1978 contained
endrin at 0.20 mg/kg dry weight, one collected in 1981 contained no
detectable amount (< 0.01 mg/kg) and three collected in 1982
contained 0.02-0.04 mg/kg (Ellis et al., 1989).
No endrin was detected in 27 eggs from tree sparrows (Passer
montanus), 4 eggs from house martins (Delichon urbica), 28 eggs
from white storks (Ciconia ciconia), or eggs from nine other species
of bird in Germany in 1984. The livers of 25 nestling, 13 young, and
17 adult white storks also contained no detectable level of this
pesticide (limit of detection, 0.001 mg/kg) (Heidmann et al., 1989).
5.1.4.2 Fish and shellfish
The endrin concentrations in red mullet (Mullet barbatus)
collected at six locations in the Pagassitikos Gulf (Greece) in
1986-87 were < 0.005-0.5 µg/kg fresh weight of fillets (Satsmadjis et
al., 1988). The mean concentrations in liver, brain, kidneys, and
muscle of 22 trout (Salmo trutta fario L.) taken from four rivers in
Leon, Spain, in 1985 were 0.104, 0.123, 0.157, and 0.157 mg/kg wet
weight. The incidence in the four organs was 4.54-22.73% (Teran &
Sierra, 1987). Endrin was found in 29 samples of fish collected in
Italy, at a median concentration of 0.019 mg/kg (Cantoni et al.,
1988). Organochlorine compounds were measured in three samples of
liver from cod (Gadus morhua) collected in three areas of the North
Sea in 1977-87; endrin was present at a concentration of < 5 µg/kg of
product (De Boer, 1989).
Endrin was not detected (< 0.01 mg/kg) in two or three replicate
samples, each comprising three to five bluegill (Lepomis macrochirus)
and common carp (Cyprinus carpio), collected from downstream sites
exposed to irrigated agriculture and from non-irrigated upstream sites
on the San Joaquin River and tributaries in California, USA (Saiki &
Schmitt, 1986). Endrin was also not found in water near Los Angeles
County ocean outfalls (< 0.00005 µg/litre) or in mussels (Mytilus
californianus) (< 0.1 µg/kg wet weight) that had been suspended at
the monitoring site for 2 months to provide a measure of the
bioaccumulation of chlorinated hydrocarbon contaminants (Green et al.,
1986). No endrin was detected in fish samples taken at nine locations
in north-central USA (Martin & Hartman, 1985), and endrin was not
detectable (< 0.001 mg/kg) in 527 samples of edible fin fish
harvested from Chesapeake Bay and its tributaries (Maryland) over the
period 1976-80 or in 20 samples of roe and gonadal tissue (Eisenberg
& Topping, 1985).
No detectable quantity (< 1 µg/kg) of endrin was found in two
species of crayfish (Procambarus clarkii and P. acutus)
commercially harvested from dual-cropped ponds and from waters of the
Atchafalaya River Basin and the Mississippi River in southern
Louisiana, or in sediment and water collected from several ponds and
at the Basin three times during 1986 and 1987 (Madden et al., 1989).
Endrin was measured at levels of 0.4 and 66 µg/kg in American
eels (Anguilla rostrata) sampled at various sites between Lake
Ontario and the mouth of the St Lawrence river in 1982 (Castonguay et
al., 1989). Endrin was not detectable (< 0.002 mg/kg) in 'most'
composite samples (1-15 fish of 10 different species) collected from
10 sites on the Great Lakes and tributaries between 1980 and 1981,
although in a few cases concentrations up to 0.01 mg/kg were found
(Devault, 1985). Endrin was not present (< 0.005 mg/kg) in fillets of
Fall Run Coho salmon (Oncorhynchus kisutch) taken from 14 sites on
the Great Lakes in 1984. In most cases, three samples per site were
analysed, and the fish were 2-3 years old (Devault et al., 1988).
Johnson et al. (1988) measured the input of organochlorine pesticides
from precipitation and runoff to five small lakes peripheral to the
Canadian Great Lakes and the levels of residues in fish caught in the
lakes. While endrin was detectable in precipitation (at 0.46 and 0.54
ng/litre at the two sampling sites), none was measured in runoff water
and no detectable residue was found in fish.
The mean concentrations of endrin in 13 commercially important
fish species collected in the north-west Arabian Gulf varied between
1 and 28 µg/kg, and those in five species collected from Hor al-Hammar
Lake in Iraq in 1985 were 3-67 µg/kg wet weight of edible tissue.
Endrin residues were detected in approximately 90% of the fish
(DouAbul et al., 1987a). Samples of Barbus xanthopetrus collected in
the Shatt al Arab River and in Hor al Hammar Lake contained average
concentrations of 4 (none detected to 9) and 20 (11-27) µg/kg, while
Indian shed (Tenualosa ilistra) from the Shatt al Arab River
contained 80 (57-108) µg/kg (wet weight). Shrimp (Metapanaeus
affinis) did not contain endrin (DouAbul et al., 1987b). In 1984,
B. xanthopetrus from the River contained a mean concentration of 16
µg/kg wet weight, and those from the Lake, 154 µg/kg (range, 13-355);
Indian shed had mean concentrations of 41-147 µg/kg (range, none
detected to 236) (DouAbul et al., 1987c). Freshwater mussel
(Corbicula fluminea) collected in the Shatt al Arab River contained
166-540 µg/kg (range, 140-583) (DouAbul et al., 1988). Endrin was
present at concentrations of 1.9-12.2 µg/kg of muscle tissue (wet
weight) in three fish species and at 0.88-7.7 µg/kg in three Tilapia
species collected near Alexandria, Egypt, in 1985 (El Nabawi et al.,
1987).
Endrin was present at 0.003-0.004 mg/kg in black pomfret
(Parastromateus niger), mackerel (Rastrelliger kanagurta), and
marine vala (Chirocentrus sp.) and at 0.08 mg/kg in tuna (Euthynnus
affinis) collected off the Indian coast (Radhakrishnan & Antony,
1989). It was found in one sample of fish at 0.019 mg/kg wet weight
and in one shellfish sample at 0.034 mg/kg but in none of 312 other
specimens of 11 types of fish, crustaceans, and molluscs obtained from
five sites in Java, Indonesia (limit of detection, 0.01 mg/kg) (Koeman
et al., 1974).
No endrin was found (< 0.005 mg/kg) in 60 samples of fish and
shellfish collected in 19 rivers and their estuaries in Japan in 1974
(Japanese Environmental Agency, 1975).
The median concentration of endrin in the eggs of 15 adult
chinook salmon (Onchorhynchus tshawytscha) collected in Lake
Michigan in 1982 was 23.5 µg/kg wet weight (range, 3.9-126.3) (Giesy
et al., 1986).
Composite samples of whole fish of selected species were
collected in 1983 near the shores of 13 tributaries of Lake Michigan
and Grand Traverse Bay. Two of each of the following species were
collected from each site: common carp (Cyprinus carpio), bowfin
(Amia calva), channel catfish (Ictalurus punctatus), pumpkinseed
(Lepomis gibbosus), rock bass (Ambloplites rupestris), small-mouth
bass (Micropterus dolomieui), large-mouth bass (M. salmoides),
lake trout (Salvelinus namaycush), and pike (Esox lucius); the
composites comprised 3-11 fish. Endrin was not detected (limit, 0.005
mg/kg) (Camanzo et al., 1987).
Yellow perch (Perca flavencens) were sampled in eight
reservoirs and lakes in Ohio and Wisconsin, USA, in 1978-79. Endrin
was found in four fish at levels of 0.008-0.02 mg/kg, which were much
lower than the levels found of polychlorinated biphenyls, DDT and
dieldrin (Carline & Lawal, 1985).
5.1.4.3 Mixed species
Herons (Nyctanassa violacea), water snakes (Natrix spp.),
raccoons (Procyon lotor), channel catfish (Ictalurus punctatus),
crappies (Pomoxis spp.), frogs (Rana spp.), and crawfish
(Procambarus clarkii) were collected from three watersheds in
Louisiana, USA in 1978-79. Endrin was found in a heron at 0.014 mg/kg
and in a catfish at 0.022 mg/kg, but in no other case (limit of
detection, < 0.05 mg/kg) (Dowd et al., 1985).
5.1.5 Other food and feed
5.1.5.1 Cereals
Endrin has been used extensively for the control of insect pests
in rice. Typically, one to four applications are made, depending on
local conditions, the last application usually not later than one
month before harvest. Data on residue levels are available from India
(1969-70), Thailand (1968-70), the Philippines, Indonesia (1966), and
Venezuela (1969). The levels in polished rice were 0.01-0.04 mg/kg of
product (mean, 0.014 mg/kg), except in India where higher levels in
the order of 0.12 mg/kg were found. Bran, which is used mainly as a
component of poultry feed, contained a mean level of 0.35 mg/kg
(range, < 0.01-2.3 mg/kg), and low levels of delta-ketoendrin were
found (FAO/WHO, 1971).
Endrin has been used to only a limited extent on grain crops. The
residues in different types of treated grains in the USA were
generally below 0.05 mg/kg of product, except in oats in which levels
up to 0.5 mg/kg were found. In India, up to five applications on
sorghum gave residue levels below 0.02 mg/kg; in the USA, the levels
in sorghum were below 0.05 mg/kg. Straw of cereals contains higher
levels: rice straw had up to 3 mg/kg,, and sorghum straw up to 0.4
mg/kg (FAO/WHO, 1971).
Wheat imported into the United Kingdom in 1987-88 did not contain
endrin (< 0.01 mg/kg) (Osborne et al., 1989).
5.1.5.2 Fruit and vegetables
Endrin is occasionally used for control of field mice (voles). No
residue was found in apples at harvest (detection limit, 0.01-0.002
mg/kg of whole fruit) when it was sprayed on the ground under trees in
orchards in autumn or spring. The levels were sometimes higher in
fallen fruit, ranging from < 0.002 to 0.02 mg/kg of product (Horsfall
et al., 1970; FAO/WHO, 1971).
Only 14 of 15 000 samples of fruit and vegetables imported into
Sweden during the period 1981-84 contained endrin, at a maximum
concentration of 0.02 mg/kg (Anderson, 1986). The pesticide analysis
programme of the Swedish National Food Administration on fruit and
vegetables, including potatoes, showed no residue of endrin above the
limit of detection of 0.02 mg/kg in 13 724 samples analysed in 1985-87
(B.G. Ericsson, personal communication, 1990). The mean endrin
concentration in 137 samples of grape products (including seeds,
skins, marc and lees) in Italy was 6.2-16.2 µg/kg (Marinelli et al.,
1986). Seven of 306 samples of apples (five types) collected in
1980-83 from five regions of Italy contained endrin (limit of
detection, 0.001 mg/kg) (Foschi et al., 1985).
In Pakistan, in 16 samples of cucumber sprayed at the time of
maturity with a 0.05% endrin solution at a rate of 100 gallons/acre
(1123 litres/ha), the endrin concentrations ranged from 3.04 to 6.69
mg/kg. The residues persisted in the edible portion of cucumber up to
14 days and diminished thereafter (Illahi et al., 1986). Endrin was
found in 17% of samples of peas collected from fields and markets in
Faisalabad, Pakistan, at a level of 1.3-4.32 µg/kg. The residues
persisted for up to 12 days and then decreased (Illahi et al., 1987).
No endrin was found (< 0.02 mg/kg) in 141 samples of fruit and
vegetables from Pakistan in 1982-83 (Masud & Farhat, 1985).
In an analysis of soya bean and soya bean straw in a US
monitoring programme, seven of 177 samples of soya beans contained a
geometrical mean of < 0.001 (maximum, 0.03) mg/kg, and one of eight
straw samples contained < 0.01 mg/kg (Carey et al., 1978). Endrin was
used in up to four applications on sugar-cane in the USA, with an
interval of 45 days or longer between the last application and
harvest. The residues found in cane were usually < 0.05 mg/kg of
product (FAO/WHO, 1971).
5.1.5.3 Meat, poultry, and chicken eggs
Bovine fat (40 samples), pig fat (45 samples), calf fat (45
samples), sheep fat (22 samples), poultry fat (42 samples), and eggs
(44 samples) analysed in the Netherlands in 1983 had a median endrin
concentration of < 0.04 mg/kg (Dutch Agricultural Advisory Commission
on Environmental Pollutants, 1983). No endrin was found (detection
limit, 0.005 mg/kg) in samples of beef, pork, goat, mutton, poultry,
or eggs analaysed in Italy in 1985-87 (Cantoni et al., 1988) or in
'most' samples of pork, rabbit, or poultry analysed in
Rheinland/Pfalz, Germany, in 1981-84 (Kampe, 1985). Dietary surveys in
the United Kingdom demonstrated no endrin in meat (detection limit,
0.02 mg/kg) (United Kingdom Ministry of Agriculture, Fisheries and
Food, 1989).
Endrin was present in 10.8% of 2032 samples of bovine fat from
carcasses collected from slaughterhouses in Brazil, at a mean level of
0.01 mg/kg; the highest level was 0.09 mg/kg of tissue (De Paula
Carvalho et al., 1984). Endrin was present in hens' eggs from four of
five areas in Mexico, at concentrations of 0.004-0.11 mg/kg of whole
egg, and in 11 of 16 samples of chicken meat, at an average
concentration of 0.12 (none detected to 0.6) mg/kg on a fat basis
(Albert, 1990).
Endrin was detected in 86 of 221 samples of hens' eggs (78 native
and 143 commercial) collected in 1975-77 in Iran, at a mean
concentration of 0.017 (range, 0.003-0.13) mg/kg (Hashemy-Tonkabony &
Mosstofian, 1979). No endrin was found (limit of detection, 0.02
mg/kg) in samples of about 25 eggs of sawah ducks collected on 11
local markets in Java, Indonesia, in 1972 (Koeman et al., 1974).
It was found in 14 of 367 hens' eggs collected from 61 farms in
11 districts of Kenya in 1984; in three of the eggs, the level was >
0.2 mg/kg (Mugambi et al., 1989).
Heating, baking, frying, and steaming of tissues obtained from
broilers fed endrin at 10 mg/kg of diet for 8 weeks did not
significantly reduce the level of residues: raw, 28.2; baked, 20.8;
fried, 22.7; and steamed, 19.4 mg/kg of dry tissue (Ritchey et al.,
1972).
5.1.5.4 Milk and milk products
The mean endrin concentration in 20 samples of fresh buffalo milk
in Kalubia, Egypt, was 0.02 mg/kg of milk fat (range, < 0.01-0.03
mg/kg (Abdou et al., 1983). Cows' milk (39 samples) collected in four
areas of Bagdad, Iraq, in 1981-82 contained a mean of 60 (none
detected to 400) µg/litre (Al-Omar et al., 1985b).
The average concentration of endrin in 10 samples of evaporated
cows' milk from three main cities in the agricultural region of Mexico
was < 0.007 mg/litre of milk fat (Albert et al.,1982). Endrin was
found in powdered milk at an average concentration of 0.06 mg/kg and
in cheese at a concentration of 10-27.2 mg/kg on a fat basis (Albert,
1990).
The level of endrin in milk in the USA was < 0.001 mg/litre (on
a fat basis) (FAO/WHO, 1971). No endrin (< 0.5 µg/litre) was found in
308 samples from bulk transports of milk collected in Ontario, Canada,
in 1977 (Frank et al., 1979) or in 359 samples collected in 1983
(Frank et al., 1985).
No residue was found (detection limit, < 0.005 mg/kg) in samples
of milk, cream, butter, and cheese in Italy (Cantoni et al., 1988) or
in 12 samples of cows' milk collected in 1984-86 from different areas
of Spain (< 0.01 mg/kg fat) (Barcelo & Puignou, 1987).
5.1.5.5 Fat and oils
The most important use of endrin is for the control of insects in
cotton, the number of applications being 1-12; cottonseed oil is used
for cooking and for the manufacture of margarine, while the extracted
cake is used as cattle feed. Endrin is thus present both in the
cottonseed and in the edible oil and cakes. In a study of the
extraction processes, it was found that alkali washing and bleaching
had no marked effect but that deodorization reduced the endrin levels
to below the limit of detection (0.03 mg/kg) (Smith et al., 1968).
In field studies carried out in the USA, cottonseed contained
endrin at a maximum of 0.1 mg/kg, although the levels were usually
much lower. delta-Ketoendrin was not detected. The levels in crude,
decolourized, and deodorized oil in Venezuela and Brazil were all <
0.02 mg/kg of product. Spot samples of refined cottonseed oil from
California, USA, contained < 0.03 mg of endrin and < 0.02 mg of
delta-ketoendrin ( limits of detection) (FAO/WHO, 1971).
One-hundred-and-ten samples of raw oil and of oil at various
stages of processing, i.e., neutralized, hydrogenated, decolourized,
deodorized, and shortenings, were collected from seven oil processing
factories in Iran in 1974. Endrin was found only in raw and
neutralized vegetable oils, at concentrations of 0.004-0.005 mg/litre.
Raw imported and native oils contained < 0.01 mg/litre, except for
native sunflower oil which contained 0.026 mg/litre (Hashemy-Tonkabony
& Soleimani-Amiri, 1976).
Endrin was found in 60 samples of six varieties of the major
edible oils and oil seeds used in India, including groundnut, sesame,
mustard, coconut, and hydrogenated vegetable oils, collected from a
market in Lucknow. Vegetable oil contained 6 µg/litre, mustard oil, 72
µg/litre, and sesame oil, 1690 µg/kg. Of the different types of oil
seeds, only mustard seed contained endrin, at 22 µg/kg (Dikshith et
al., 1989a).
Endrin was found at a mean concentration of 0.184 (0.097-0.288)
mg/kg in samples of cod-liver oil analysed in Germany in 1985 (Ali,
1986). No residue was found in vegetable oils and fats imported into
the United Kingdom (detection limits, 0.02 and 0.001 mg/kg,
respectively) (Abbot et al., 1969).
5.1.5.6 Animal feed
Residues of endrin in pressed cottonseed cakes arise primarily
from the 1-5% of oil left in the cake after extraction. The residues
in cakes from Brazil, India, the USA, and Venezuela were mainly <
0.01-0.02 mg/kg product, levels up to 0.08 mg/kg were found
occasionally (FAO/WHO, 1971). The mean concentration of endrin in 32
samples of cattle feed from a local market in India was 0.020 mg/kg
(range, 0.013-0.027 mg/kg) (Dikshith et al., 1989b). No endrin was
found in 79 samples of cattle feed in Pakistan (Parveen & Masud,
1987). Endrin was not present in samples of domestic and imported
animal feed analysed in the USA in 1981-86 (Luke et al., 1988).
None of 42 samples of chicken feed collected from 61 farms in 11
districts of Kenya in 1984 contained endrin (Mugambi et al., 1989).
5.1.6 Miscellaneous products
Endrin was found in 5 of 25 tobacco samples imported into Germany
at concentrations of 25-50 µg/kg (Cetinkaya, 1988). No endrin was
found in cigarettes of 14 brands collected in Finland in 1960-84
(Mussalo-Rauhamaa et al., 1986). An average content of 0.006
µg/cigarette (range, none detected to 0.02 µg/cigarette) was found in
Switzerland (Zimmerli & Marek, 1973).
When raw cotton imported into Germany from 15 countries was
analysed, endrin was found in samples from the USSR and Mexico at a
concentration of 3 µg/kg (Cetinkaya & Schenek, 1987).
5.2 Exposure of the general population
5.2.1 Total-diet studies
Studies on complete prepared meals in the USA, started in May
1961 and continued to the present, have shown the occasional presence
of small amounts of endrin (Williams, 1964; Cummings, 1965, 1966;
Duggan et al., 1966, 1967; Martin & Duggan, 1968; Corneliussen, 1969,
1970, 1972; Manske & Corneliussen, 1974; Manske & Johnson, 1975;
Johnson & Manske, 1976, 1977; Manske & Johnson, 1977; Johnson et al.,
1981a, 1984). These measurements indicate that the total average daily
intake of endrin from food decreased from 0.009 µg/kg body weight in
1965 to 0.0005 µg/kg body weight in 1970 (Duggan & Lipscomb, 1969;
Duggan & Corneliussen, 1972), with a further decrease subsequently. In
total-diet studies of adults in the USA, representative foods were
purchased in 27 US cities in 1980-82; the daily intake of endrin was
found to be < 0.001 µg/kg body weight in 1978, but none was detected
in 1979, 1980, or 1981-82 (Gartrell et al., 1986a).
Further studies involved retail purchase of 234 food items
representative of the total diet of eight US population groups in
1982-84 and preparing them for consumption. The daily intake of endrin
in the groups, which included people aged 6-11 months, 2 years, 14-16
years (females), 14-16 years (males), 25-30 years (females), 25-30
years (males), 60-65 years (females), and 60-65 years (males), was
0.1-0.2 ng/kg body weight (FDA, 1988; Gunderson, 1988).
Endrin was not present in the total diets of infants and toddlers
in the USA during 1974-75 (Johnson et al., 1979). It was found in one
infant food sample at 0.011 mg/kg and in one sample of toddler food at
0.009 mg/kg of food in a study in 1975-76 (Johnson et al., 1981b).
Very low residue levels were found occasionally in market-basket
samples representing the average 2-week diets of infants (98 samples)
and toddlers (110 samples) collected in 10 cities in four geographic
areas of the USA in 1977-78 (Podrebarac, 1984). In total-diet studies
of infants and toddlers in the USA, representative foods were
purchased in 13 US cities in 1980-82; the daily intake of endrin by
infants was found to be < 0.001 µg/kg body weight in 1978, and that
by toddlers, < 0.001 µg/kg body weight in 1979, but none was detected
in the other years (Gartrell et al., 1986b).
Fresh food was bought from four retail grocery stores in Toronto,
Canada, in 1985 and combined in five food composites: fresh meat and
eggs, root vegetables (including potatoes), fresh fruit, leafy and
other surface vegetables, and cows' milk. The concentrations of endrin
in the five composites were used to estimate the annual dietary intake
of endrin from products in Ontario. Endrin was detected in all
composites except eggs and meat; the concentrations were 0.32 µg/kg in
leafy vegetables, 0.27 µg/kg in fruit, 0.37 µg/kg in root vegetables,
and 0.27 µg/kg in milk. The total annual intake was estimated to be
31.8 µg/person (Davies, 1988).
In a total-diet study carried out in the United Kingdom in
1985-88, 25 samples were obtained in 1984-85 which comprised the 16
food groups considered most likely to contain residues of
organochlorine compounds. No endrin was detected (limit of detection,
0.001-0.02 mg/kg, depending on the food group). Endrin was also not
detected (< 0.01 mg/kg) in 176 samples of pulses purchased from
retail outlets in 1986-87, except in 3 of 20 samples of mung beans in
which a mean value of < 0.01 mg/kg (range, none detected to 0.06
mg/kg) was found (United Kingdom Ministry of Agriculture, Fisheries
and Food, 1989). No endrin was detected in complete prepared meals
during surveys in the United Kingdom in 1965 (Robinson & McGill, 1966;
McGill & Robinson, 1968). Similar results were obtained in Switzerland
in 1973 (Zimmerli & Marek, 1973), and very low levels were found in
two of 73 samples analysed in 1985 (Wüthrich et al., 1985). No endrin
residues were found in total-diet studies carried out in the
Netherlands in 1976-78 (De Vos et al., 1984).
5.2.2 Levels in human tissues
Although the concentrations of many chlorinated hydrocarbon
insecticides, such as DDT, dieldrin, hexachlorocyclohexanes, and
hexachlorobenzene, and of their metabolites in blood or adipose tissue
of the general population or of occupationally exposed workers have
been found to be an excellent index of the level of exposure of the
general population, this is not the case for endrin, because it is
eliminated rapidly.
5.2.2.1 Adipose tissue
Except in a few cases, endrin was not demonstrated in adipose
tissue samples from the general population in the USA in 1962-66
(Hoffman et al., 1964, 1967), 1964 (Hayes et al., 1965; Zavon et al.,
1965), 1970-74 (Kutz et al., 1979a,b), and 1975-79 (US EPA, 1983);
Canada in 1967-68 (Kadis et al., 1970); Mexico in 1975 (Albert et al.,
1980); Argentina in 1968-69; (Wassermann et al., 1969); Belgium in
1968-69 (Wit, 1971); the United Kingdom in 1961 (Hunter et al., 1963),
1964 (Robinson et al., 1965), and 1965-67 (Egan et al., 1965; Abbott
et al., 1968, 1972); the Netherlands in 1969 (Wit, 1971); Switzerland
in 1972 (Zimmerli & Marek, 1973); Germany in 1970 (Acker & Schulte,
1974); France in 1971 (Fournier et al., 1972); Spain in 1978 (Herrera
Marteache et al., 1978); India in 1964 (Dale et al., 1965); or Western
Australia in 1965-66 (Wassermann et al., 1968).
No endrin was found in 91 samples of adipose tissue obtained at
autopsy in Kingston, Ontario, Canada, in 1979 and 1981 or in 84
samples from Ottawa in 1980 and 1981 (Williams et al., 1984), or in
adipose tissue obtained at autopsy from 92 males and 49 females in
Ontario municipalities in 1984 (limit of detection, 2.4 µg/kg)
(Williams et al., 1988).
These results indicate that endrin is either absent or present at
very low levels in the adipose tissue of the general population. It is
therefore surprising that Kanitz & Castello (1966) reported the
presence of endrin in nine adipose tissue samples from Liguria, Italy,
at a mean concentration of 0.93 mg/kg of tissue. The highest
concentration was 2.49 mg/kg. Pavan et al. (1987) found endrin at 0.1
and 0.3 mg/kg in 2 of 92 samples of adipose tissue obtained at surgery
from people living in the Province of Turin, Italy. In areas where
endrin has been used extensively, however, such as India and the lower
Mississippi, it has never been found in human adipose tissue (Brooks,
1974).
One of 62 adipose tissue samples obtained at surgery from people
in Ciudad Juarez, Mexico, in 1977-78 contained endrin at 0.02 mg/kg
(Redetzke et al., 1983).
5.2.2.2 Organs
In samples of liver, kidney, gonad, and brain obtained from the
general population of Alberta (Canada), no residue of endrin was
detected (Kadis et al., 1970).
5.2.2.3 Blood
No endrin was detected (limit of detection, 0.01 mg/kg) in 4000
blood samples from the general US population in 1976-80 (US E P A,
1983), or in areas where endrin has been used extensively, such as
India and the lower Mississippi (Brooks, 1974), or in 26 blood samples
from the general population in Nigeria (Atuma, 1985).
5.2.2.4 Breast milk
Endrin was not detected in breast milk in studies in the USA in
1966-78 (Strassman & Kutz, 1977; Currie et al., 1979; Kutz et al.,
1979a; Barnett et al., 1979), in El Salvador and Guatemala (De Campos
& Olszyna-Marzys, 1979), in Belgium, Italy, and The Netherlands
(Kanitz & Castello, 1966; Hendrickx & Maes, 1969; Wegman & Greve,
1974), and in Japan (Yakushiji et al., 1979). No endrin was detected
(< 0.01 mg/litre) in 50 breast milk samples from mothers (aged 18-32
years) in Leiden, The Netherlands, in 1969 (Tuinstra, 1971). It was
found in one of 12 samples from mothers aged 21-37 in Pavia, Italy, in
1988, at a concentration of 0.01 µg/kg of whole milk, but not in four
samples collected in Crotone, southern Italy (Bianchi et al., 1988).
5.2.2.5 Appraisal of exposure of the general population
The occasional presence of low concentrations of endrin in the
air of areas where endrin is used in agriculture cannot be considered
a significant source of contamination for the general public. The very
low concentrations that have been found in surface and drinking-water
are also of little significance for public health.
The source of exposure that may be relevant is dietary intake.
Apart from accidental contamination, however, intake of endrin by the
general population in the countries examined has been and is still far
below the maximum acceptable daily intake of 0.2 µg/kg body weight
established by the Joint FAO/WHO Meeting in 1970 (FAO/WHO, 1971). This
applies equally to the total intake, when the intake from dietary
sources is added to that from air and water.
Endrin has not been demonstrated in the large number of samples
of organs, adipose tissue, blood, and breast milk analysed in
different countries, even in areas where endrin is or was used
extensively.
5.3 Occupational exposure during manufacture, formulation and use
5.3.1 Manufacture and formulation
Endrin has not been detected in the blood, plasma, or urine of
workers exposed occupationally to endrin (Hayes & Curley, 1968; Jager,
1970). Endrin was detected in blood only after accidental
over-exposure. Jager (1970) estimated that the threshold level of
endrin in the blood, below which signs or symptoms of intoxication do
not occur, lies between 0.05 and 0.10 mg/litre. He estimated the
half-life of endrin in the blood to be in the order of 24 h.
The total exposure of workers in a manufacturing and formulation
plant was estimated on the basis of determinations of the quantity of
the endrin metabolite anti-12-hydroxyendrin in urine. Urine of
workers exposed to endrin for seven days had concentrations of up to
360 µg/g of creatinine; no unchanged endrin was found. Assuming an
average daily excretion of 1.5 g creatinine per day, the total daily
excretion of anti-12-hydroxyendrin in the urine may be up to 540 µg.
This gives a minimal absorption of 0.5 mg endrin, indicating that
inhalation of dusts and absorption through the skin may be significant
during occupational exposures in manufacture. It is not unreasonable
to assume that, as in other species, approximately half of all the
endrin absorbed is excreted in the urine as anti-12-hydroxyendrin,
since both endrin and this metabolite are present in the faeces of
workers (Ottevanger &Van Sittert, 1979; Baldwin & Hutson, 1980 ).
Thus, 1 mg/day may be the more accurate figure for exposure in this
manufacturing plant. The concentration of anti-12-hydroxyendrin in
urine decreased more slowly than the concentrations of endrin in
blood, with a half-life of 55-75 h (Van Sittert, 1985).
5.3.2 Application
Endrin is applied in agriculture by high-pressure spraying with
a hand gun, spraying orchards with a power air blast or boom to
control mice, dusting potatoes, spraying row crops, or application
from aircraft. These methods of application result in dermal and
respiratory exposures.
The potential dermal and respiratory exposure of workers applying
endrin formulations in the field has been quantified in a few studies.
Respiratory exposure to endrin during spraying of orchards,
high-pressure spraying of crops, and piloting of aircraft varied from
0.01 to 0.14 mg/h; dermal exposure during such activities was
0.01-1.64 mg/h. The activity that caused the most exposure was dusting
potatoes, which was associated with a respiratory exposure of 0.41
mg/h and a dermal exposure of 18.7 mg/h. Total exposure, calculated as
a percentage of a toxic dose/h = {dermal exposure (mg/h) +
[respiratory exposure (mg/h) x 10]} [dermal LD50 (mg/kg) x 70] x
100, was 0.21-1.5% (Durham & Wolfe, 1962) (see Table 11). These
figures show that although endrin is acutely highly toxic it can be
used safely when reasonable precautions are taken (Wolfe et al., 1963;
Jegier, 1964; Wolfe et al., 1967; Hayes, 1975).
Endrin was not found in the blood of 20 pesticide sprayers or in
19 controls in Choluteca, southern Honduras (Steinberg et al., 1989).
5.3.3 Appraisal of occupational exposure
No residues were found in healthy workers. The range of threshold
levels of endrin in blood below which no sign or symptom of
intoxication occurs has been estimated to be 50-100 µg/litre. The
half-life of endrin in blood may be in the order of 24 h following
occupational exposure. The concentration of anti-12-hydroxyendrin in
the urine decreased more slowly than the concentration of endrin in
blood, with a half-life of 55-75 h.
Table 11. Studies on potential exposure of agricultural workers to endrin, using direct methods
Activity Exposure
Respiratory Dermal Totala (%) toxic Reference
(mg/h) dose/h
mg/m3 mg/h
Spraying orchard cover crops for mouse 0.01b 2.6 0.21 Wolfe et al. (1963)
control
High-pressure hand-gun spraying orchard 0.01b 3 0.25 Wolfe et al. (1967)
cover crops for mouse control
Operating air-blast or boom sprayers treating 0.01b 2.5 0.21 Wolfe et al. (1967)
orchard cover crops for mouse control
Dusting potatoes 0.41b 18.7 1.5 Wolfe et al. (1963)
Spraying row crops NDb,c 0.15 (0.01)d Jegier (1964)
Piloting during air application of spray 0.05 0.08b 1.18 0.29 (0.16)e Jegier (1964)
aFor a 70-kg man on the basis of dermal LD50 for male rats (18 mg/kg body weight) using the formula given
by Durham & Wolfe (1962)
bMeasured by respirator pad
cNot detected
dOriginal values calculated on the basis of maximal exposure; recalculated values shown in parentheses are
based on mean exposure.
eCalculation based on published data on dermal and respiratory exposure (note in Wolfe et al., 1967)
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Laboratory animals
6.1.1.1 Oral administration
Rat: One male rat was fed 14C-endrin at a level of 30 mg/kg
of diet for 8 days. About 60-70% was excreted on the first day; after
three days, the faeces contained more then 80% of the administered
radiolabel; by day 9, 84% had been excreted; and there appeared to be
a level of saturation after 6-7 days of feeding. Only 0.5% was found
in the urine. About 75-80% of the label in the faeces was in at least
two different metabolites. The adipose tissue stored 3-4 mg/kg, giving
a storage rate of about 10 (FAO/WHO, 1971).
After female rats were given a single oral dose of 14C-endrin
at 16, 64, or 128 µg/kg body weight, excretion was rapid. The
biological half-life of the doses of 16 and 64 µg/kg was 1-2 days;
however, that of 128 µg/kg was approximately 6 days, showing that
excretion of higher doses is slower (Korte et al., 1970).
Six CFE rats of each sex were treated with a single oral dose of
0.5 mg 14C-endrin in arachis oil (approximately equivalent to
2.5-3.0 mg/kg body weight), and the radiolabel excreted in urine and
faeces was measured over 3 days. The animals were then killed and the
radiolabel measured in tissues. A sex difference was noted in the rate
of elimination in faeces: 66% of the dose was excreted in 3 days by
males and 37% by females; excretion was slower in females but tended
to increase daily between days 1 and 3. Small quantities of radiolabel
were excreted in the urine, females excreting three times more than
males. No radiolabel was found in exhaled air (Hutson et al., 1975).
The results are summarized in Tables 12 and 13.
Three rats of each sex were each given a single oral dose of 8 µg
14C-endrin in peanut oil (by gavage) daily for 12 days. A steady
state (at which the excreted amount equalled the daily intake) was
reached after about 6 days. Females stored about twice as much (27%)
as males (14%). The radiolabel was excreted mainly in the faeces:
after the first 24 h, 70-75% of the radiolabel was found in faeces as
hydrophilic metabolites; subsequently, only metabolites were present.
Four days after the last dose, males retained only 5% and females 15%
of the administered radiolabel (Klein et al., 1968; Korte et al.,
1970).
Table 12. Rates of excretion of radiolabel by rats treated with a
single oral dose of 14C-endrin (percentage of
radioactivity administered)
Sex Urine Faeces
Day 1 Day 2 Day 3 Day 1 Day 2 Day 3
Male 1.3 0.6 0.6 30.6 14.4 21.2
Female 1.8 2.5 2.9 2.3 10.7 24.2
Table 13. Recovery of radiolabel in rat tissues 3 days after a single
oral dose of 14C-endrin (percentage of radioactivity
administered)
Sex Urine Faeces Liver Kidneys Fat Skin Remaining Total
carcass
Males 2.7 66.2 1.2 0.6 1.7 2.3 12.2 86.9
Females 7.5 37.2 2.0 0.4 8.0 4.0 28.1 87.2
Rabbit: A Dutch strain male rabbit was given two oral doses of
4.7 mg 14C-endrin in olive oil at an interval of 14 days. Between
days 1 and 13, 37% of the first dose was excreted in the urine and 49%
in the faeces; the second dose was eliminated similarly. By day 49,
50% had been excreted in urine and 47% in faeces. Faecal excretion was
rapid, being almost complete within 24 h, and consisted virtually
entirely of unchanged endrin. The urine contained only metabolites
(Bedford et al., 1975a). The excretion of metabolites in rabbits thus
appears to differ considerably from that in rats: approximately half
of a dose of 14C-endrin is excreted in the urine of rabbits and
approximately 2% in rats; endrin metabolites are excreted in rat
faeces over several days (after a single oral dose), whereas in
rabbits faecal excretion is rapid, being almost complete within 24 h,
and consists virtually entirely of unchanged endrin. The probable
explanation is that the molecular weight threshold for biliary
secretion of anions is 325 ± 50 in rats and 475 ± 50 in rabbits (Hirom
et al., 1972). The glucuronide and sulfate conjugates of
monohydroxyendrin have molecular weights of 572 and 470, respectively.
Therefore, conjugates of the endrin metabolites are eliminated in the
bile and faeces of rats and in the urine in rabbits.
Dog: Three beagles were fed a diet containing endrin at a
concentration equivalent to 0.1 mg/kg body weight for 128 days; two
other animals were used as controls. The concentration of endrin in
blood was determined at weekly intervals. The time to reach a plateau
in blood was less than 1 week, and no significant increase in the
concentration of endrin in blood was found during this period. The
average concentration between day 9 and day 128 was 4 µg/litre. The
concentration of endrin in eight organs and tissues of dogs killed 7
days after termination of exposure was < 0.2 mg/kg of tissue; except
that the spleen of one dog contained 2.6 mg/kg, and adipose tissue
contained 0.2-0.8 mg/kg (Richardson et al., 1967).
6.1.1.2 Intravenous administration
Mouse: The concentrations of endrin were determined in tissues
from groups of five adult male CFI mice given endrin intravenously at
5 mg/kg body weight (LD90) in dimethyl sulfoxide. The concentrations
prior to convulsions (about 10 min after injection) were approximately
60 mg/kg in liver, 20 mg/kg in brain and omental fat, and
approximately 5 mg/litre in blood. The concentration in whole brain 15
min after an intravenous dose of 1.5 mg/kg body weight (the dose that
caused ataxia in 90% of animals; TD90) was 9.4 mg/kg. No endrin was
detected in the bile of animals with a bile fistula dosed
intravenously with the TD90 in samples collected after 0.5, 1, or 2
h (Walsh & Fink, 1972).
Rat: Male Holtzman rats with or without a bile fistula were
given a single intravenous dose of 14C-endrin at 0.25 m/kg body
weight. More than 90% of the excreted radiolabel was found in the
faeces of intact animals over the 7-day period after dosing or in the
bile of animals with fistulas over 4 days. The mean total percentage
recovery of administered radiolabel in faeces, urine, and carcasses
was 97% from intact animals 7 days after dosing, and 94% from animals
with a bile fistula 4 days after dosing (Cole et al., 1970). No
unchanged endrin was found in bile; the major metabolite was
anti-12-hydroxyendrin (see section 6.2.1).
Rapid excretion was observed in rats given two intravenous
injections of 14C-endrin at 0.1 mg/kg body weight at an interval of
4 days. Excretion of the radiolabel was exponential and occurred
mainly with faeces; only hydrophilic metabolites were present. With a
dose of 200 µg/kg body weight, male rats retained 5.2% and females
12.1% of the administered dose 20 days after the second injection. The
biological half-life of endrin after a single intravenous dose of 200
µg/kg body weight was 2.5-3 days in male rats and 4 days in females
(Klein et al., 1968; Korte et al., 1970; Brooks, 1974).
Rabbit: When rabbits were given 14C-endrin intravenously, the
radiolabel was excreted mainly in the urine and only as metabolites.
A probable explanation for the difference in excretion pattern after
oral and intravenous administration is that much lower doses
(micrograms compared with milligrams) were given intravenously (Korte
et al., 1970).
6.1.2 Domestic animals
Twelve cows were fed hay from endrin-sprayed alfalfa containing
an average of 1.9, 2.8, or 3.7 mg/kg endrin; the average daily intake
of individual animals ranged from 0.04 to 0.11 mg/kg body weight. The
average concentrations of endrin in the milk were < 0.05, 0.14, and
0.15 mg/litre, respectively. When endrin dissolved in soya bean oil
was fed to 11 dairy cows, levels > 1 mg/kg body weight were required
in order for significant quantities of endrin to be detected in milk
(Ely et al., 1957).
Dairy cows (eight Jerseys and six Guernseys) were fed diets
containing endrin at 0, 0.1, 0.25, 0.75, or 2.0 mg/kg of diet for 12
weeks. No residues were found (limit of detection, 0.01 mg/litre) in
the milk of animals that received 0.1 mg/kg, but up to 0.02 mg/litre
was found in milk of animals fed 0.25 mg/kg and up to 0.04 mg/litre
with 0.75 mg/kg of diet; the highest dose resulted in residues in milk
of 0.1 mg/litre. The endrin content of brain, heart, liver, kidneys,
body fat, and muscles was < 0.1 mg/kg, but renal fat contained up to
0.8 mg/kg (Kiigemagi et al., 1958).
The concentrations of endrin in milk of cows given feed
containing endrin at approximately 0.05, 0.14, and 0.30 mg/kg of whole
feed for 5 weeks were 0.004, 0.01, and 0.018 mg/litre, respectively
(Williams et al., 1964). A steady (plateau) level in milk was reached
after about 15 days.
The concentration of endrin in the milk of dairy cows given feed
contaminated with relatively low levels of endrin rose rapidly within
a few hours to days and levelled off at a plateau characteristic for
each concentration in the feed. The average milk:diet ratio for endrin
was 0.07 for feed levels of 0.05-0.3 mg/kg of diet (Biehl & Buck,
1987).
Two lactating cows were fed 14C-endrin for 21 days at an
overall dietary concentration of 0.1 mg/kg of diet, which was
considered to be comparable to the highest dose that cows are likely
to receive in cottonseed cake. Excretion of radiolabel in milk, urine,
and faeces reached a plateau 4-9 days after start of treatment.
Approximately 3% of the radiolabel was excreted in milk, 65% in urine,
and 20% in faeces. Unchanged endrin was not found in urine, but about
30% of the radiolabel in faeces and all of the 0.003-0.006 mg/litre
found in milk was endrin. The concentration of endrin equivalent
residues was 0.001-0.002 mg/kg in meat and 0.02-0.10 mg/kg in fat;
most of the radiolabel in fat consisted of endrin (Baldwin et al.,
1976).
Steers, hogs, and lambs fed diets containing endrin at 0, 0.1,
0.25, or 0.75 mg/kg of diet for 12 weeks had residues of < 0.1 mg/kg
in red meat, liver, and kidneys and of 0.02-0.2 mg/kg in body fat.
Feeding endrin at 2 mg/kg of diet to steers for 12 weeks resulted in
residues of 0.9 mg/kg in fat and 0.2-0.3 mg/kg in red meat, liver and
kidneys (Terriere et al., 1958). The biotransfer factors for endrin in
beef and milk were directly proportional to the octanol-water
partition coefficients, while the bioconcentration factor for endrin
in vegetation was inversely proportional to the square root of the
octanol-water partition coefficient (Travis & Arms, 1988).
Six weeks after the start of feeding seven Delaware X New
Hampshire male chicks and eight weeks after the start of feeding seven
White Leghorn pullets a diet containing endrin at 0.1 mg/kg, the
residues in eggs and meat were < 0.1 mg/kg and that in fat, 0.6
mg/kg. At a dietary level of 0.25 mg/kg, the residue levels were
0.2-0.3 mg/kg in eggs, 0.1 mg/kg in breast meat, and about 1 mg/kg in
fat. With 0.75 mg/kg of diet, the levels were 0.4 mg/kg in eggs, 0.24
mg/kg in breast meat, and 3.1 mg/kg in fat (Terriere et al., 1959).
14C-Endrin was administered daily in corn oil in gelatin
capsules to six laying hens at a concentration equivalent to 0.13
mg/kg of total diet for 21 weeks. Ingestion and elimination in eggs
and excreta were almost balanced after about 16 weeks. The residue
levels in eggs were 0.11-0.18 mg/kg and were found in the yolk; none
of the known metabolites was detected. The levels of endrin equivalent
were about 0.01 mg/kg in breast meat and 0.1 mg/kg in leg meat; higher
levels were found in the liver (0.47 mg/kg), kidneys (0.17 mg/kg), and
fat (1 mg/kg). The residues were accounted for by unchanged endrin,
except in the liver and kidneys, where part probably consisted of
polar metabolites. About 50% of the administered radiolabel was
excreted in the faeces, 10% of which was in unchanged endrin (Baldwin
et al., 1976).
6.1.3 Human beings
The concentrations of endrin in the blood of workmen exposed to
endrin were generally below the level of detection: endrin was not
found in plasma (< 3 µg/litre) or fat (< 0.03 mg/kg) of workers
exposed to endrin for an average of 88 days (Hayes & Curley, 1968). No
endrin was found in the blood of healthy people working in an endrin
manufacturing plant between 1964 and 1970, at an initial limit of
detection of 10 µg/litre, improved after 1965 to 5 µg/litre (Jager,
1970).
Residues of endrin have been found in blood only in individuals
with signs of recent intoxication or who have recently had excessive
exposure (see section 9.2.2). Endrin appears to be eliminated rapidly
from the human body.
6.1.4 Systems in vitro
Isolated liver preparations from Holtzman rats were perfused with
a solution containing 14C-endrin at 0.003 mg/ml. Within 1 h, 50% of
the radiolabel appeared in the bile; and in 6 h, more than 90% of the
total label was found (Cole et al., 1970). With the same dose,
radiolabel appeared 2-12 times faster in the bile of livers isolated
from male rats as in that of livers from females, which may account
for the lower toxicity and lower storage of endrin in adipose tissue
in male rats (Klevay, 1971). After perfusion of albino rat liver with
a physiological solution containing 40 µg of 14C-endrin, both endrin
and hydrophilic metabolites were found (Altmeier & Korte, 1969).
6.2 Biotransformation
Information on the metabolism of endrin up to 1967 was reviewed
(Soto & Deichmann, 1967; Brooks, 1969).
6.2.1 Experimental animals
A number of investigations have been carried out since 1970 to
elucidate the identity of several metabolites of endrin in rats
(Baldwin et al., 1970; Richardson et al., 1970; Hutson et al., 1975;
Bedford & Hutson, 1976), rabbits (Bedford et al., 1975a; Hutson,
1981), cows and chickens (Baldwin et al., 1976). The 12-hydroxy
derivative was reported to be present in the faeces of rats (Baldwin
et al., 1970), and the hydroxyl group was assigned tentatively as syn
to the epoxide ring. The stereochemical configuration was subsequently
shown to be anti to the epoxide group (Baldwin et al., 1973), and
this configuration was confirmed by the synthesis of anti-12-
hydroxyendrin (also called 9- anti-hydroxyendrin) (Bedford & Harrod,
1973; Bedford et al., 1986a). The chemical structures of these
compounds are shown in Figure 2; the chemical names are given in Annex
I.
Formation of anti-12-hydroxyendrin ( III), together with its
sulfate and glucuronide conjugates, is considered to be the major
route of metabolism of endrin. Four other metabolites have been
reported, but their concentrations are generally smaller than that of
anti-12-hydroxyendrin and its conjugates. These four metabolites are
syn-12-hydroxyendrin ( II; tentative identification),
3-hydroxyendrin ( IV; synthesized and structure confirmed by Bedford
et al., 1986b), 12-ketoendrin ( V), and the product of formal
hydroxylation of endrin, the 4,5- trans-dihydroisodrindiol ( VI;
tentative structure). The trans-diol ( VI) is a minor metabolite in
both rats and rabbits; it may be formed via an oxidation-reduction
pathway involving intermediates of the corresponding ketol ( VII).
Each of the hydroxy compounds is also excreted partly as its sulfate
or glucuronide in the urine of animals (Bedford et al., 1975a,b;
Hutson et al., 1975).
The three monohydroxylated derivatives of endrin, syn- and
anti-12-hydroxyendrin ( II and III) and 3-hydroxyendrin ( IV),
are the products of the action of liver microsomal monooxygenases on
endrin (Bedford & Hutson, 1976). These alcohols are also conjugated to
glucuronides and sulfates to some extent in the liver. Comparative
metabolic studies with rat liver microsome preparations have shown
that free syn-12-hydroxyendrin, but not its free anti-isomer, is
the precursor of 12-ketoendrin ( V) (Hutson & Hoadley, 1974).
Rats exhibit a sex difference in the rate of metabolism. The
major metabolite in animals of each sex is anti-12-hydroxyendrin,
which is excreted via the bile as the glucuronide; this undergoes
enterohepatic circulation and is eliminated as the aglycone in the
faeces, together with two minor metabolites, 3-hydroxyendrin and
4,5- trans-dihydroisodrindiol. Male rats produce the metabolite at a
higher rate than do females. The major urinary metabolite in male CFE
rats was 12-ketoendrin, while females excreted mainly
anti-12-hydroxyendrin O-sulfate. Endrin and the lipophilic
metabolite 12-ketoendrin were the major compounds found in the organs
and tissues of male and female CFE rats 3 days after a single oral
dose of endrin, but the ratio of endrin:12-ketoendrin was 2/1 in
females and 1/8 in males. Thus, 12-ketoendrin constituted most of the
radiolabel in the liver and kidneys of males and endrin that in the
kidneys of females (Hutson et al., 1975; Hutson, 1981).
The metabolism of endrin in rabbits is superficially different
from that in rats. The major metabolite is still
anti-12-hydroxyendrin, but it is conjugated with sulfate and
eliminated in the urine. Some syn-12-hydroxyendrin was also detected
as its sulfate in urine, and perhaps conjugation and elimination
prevented further oxidation to 12-ketoendrin. The respective
glucuronide conjugates were also eliminated in the urine, as were the
glucuronides of 3-hydroxyendrin and the 4,5- trans-diol ( VI)
(Bedford et al., 1975b; Hutson, 1981).
Studies with 14C-endrin in lactating cows showed that the
residues in milk and body fat consisted of unchanged endrin, although
traces of 12-ketoendrin were consistently found in fat. As in rats,
anti-12-hydroxyendrin was the major metabolite in urine and faeces,
the urine being the major excretory route, as in rabbits.
12-Ketoendrin and syn-12-hydroxyendrin were minor metabolites in cow
urine (Baldwin et al., 1976). Thus, although the metabolic pathways of
endrin in cows are qualitatively similar to those in rats and rabbits,
quantitative differences are seen in faecal and urinary excretion.
In hens, only endrin was found as a residue in meat, fat, and
eggs. Unchanged endrin accounted for about 10% of the radiolabel in
excreta, and the major metabolite was anti-12-hydroxyendrin and its
sulfate conjugate. No 12-ketoendrin was detected in tissues, eggs, or
excreta. The metabolism of endrin in hens is fundamentally similar to
that in rats, rabbits, and cows, except that they produce neither
syn-12-hydroxyendrin nor the related 12-ketoendrin. The rate of
metabolism, however, was much lower than in cows (Baldwin et al.,
1976). The absence of 12-ketoendrin in birds was confirmed in a study
of four species killed by endrin (Stickel et al., 1979). Hutson et al.
4.3.1 Biodegradation
Microbial degradation of endrin depends on the presence of an
appropriate microbial species and suitable soil conditions; it occurs
under anaerobic conditions (Donoso et al., 1979). Biodegradation is
aided by fungi and bacteria such as Trichoderma, Pseudomonas, and
Bacillus. The major transformation product is delta-ketoendrin
(Patil et al., 1970).
About 150 isolates from various soil samples were screened to
investigate the role of these microorganisms in degrading endrin; 25
of the 150 isolates were active. At least seven metabolites were
found, but conversion of endrin into the ketoendrin was common
throughout (Matsumura et al., 1971).
4.3.2 Bioaccumulation and biomagnification
The bioconcentration factors cited below are simple ratios of the
exposure concentration and the concentration in organic tissues. They
should be used with caution as indicators of bioaccumulation
potential: a high bioconcentration factor can represent little uptake
of a low concentration, and a low bioconcentration factor can be found
with considerable uptake of a high concentration. The bioconcentration
factor should therefore always be cited with the pertinent exposure
concentration of endrin.
Soil invertebrates such as slugs and earthworms had
bioconcentration factors of 14 to 103. Bioconcentration factors in a
number of aquatic organisms are given in Table 4. These ratios differ
extensively between different types of aquatic organisms.
Bioconcentration factors of 140 to 222 were found for four blue-green
algae (Microcystis aeruginosa, Anabaena cylindrica, Scenedesmus
quadricauda, and an Oedogonium species) after 7 days' exposure to
endrin at a concentration of 1 mg/litre of water( Vance & Drummond,
1969). In a study of the accumulation of endrin in stoneflies
(Pteronarcys dorsata) exposed to 0.03, 0.07, and 0.15 µg/litre of
water, the bioconcentration factor ranged from 1130 to 348, decreasing
with increasing water concentrations over the 28-day exposure period
(Anderson & DeFoe, 1980). In bullheads (Ictalurus melas), the
bioconcentration factor was 3700 after exposure for 4 days to 0.60
µg/litre and 6200 after exposure for 7 days to 0.26 µg/litre (Anderson
& DeFoe, 1980). The bioconcentration factors for endrin in sub-adults
of leopard frogs (Rana sphenocephala) exposed to 0.01, 0.012, 0.016,
0.022, and 0.030 mg/litre were 71.4, 34.4, 51.8, 59.4, and 94.3,
respectively. Sub-adults exposed to 0.01, 0.012, and 0.016 mg/litre
for 96 h and sacrificed 60 h later had bioconcentration factors of
6.1, 4.8, and 1.2, respectively (Hall & Swineford, 1980), indicating
a relatively rapid elimination of residues. In daphnids and molluscs,
a direct linear relationship was found between the logarithm of the
equilibrium bioconcentration factor (and the reciprocal clearance rate
constant) and the log P octanol/water partition coefficient for
non-degradable, lipophilic compounds with partition coefficients
ranging from 2 to 6. This relationship permits calculation of the
times required for equilibrium and for significant bioconcentration of
lipophilic chemicals, which were found to be shorter for molluscs than
for daphnids. The equilibrium biotic concentration for both molluscs
and daphnids decreased with increasing chemical hydrophobicity. The
relationship between the bioconcentration factor and log P
octanol/water partition coefficient was linear for compounds that did
not attain equilibrium within a finite exposure time (Hawker &
Connell, 1986).
In a study of the bioaccumulation of endrin from food by lobsters
(Homarus americanus), endrin dissolved in methanol was added to sea
water, and mussel tissue was soaked in the solution for 2 h to provide
a concentration of endrin of 4.7 mg/kg wet weight. Lobsters were fed
the prepared food every other day for 2 weeks, and excretion was
followed for an additional 4 weeks during which time the lobsters were
fed uncontaminated tissue. Liver and muscle were analysed from two or
three lobsters sampled after feedings 1, 2, 3, 5, and 7, and from one
or two lobsters sampled during the excretion phase at 1, 2, and 4
weeks. The concentration of endrin reached a maximum of 1.95 mg/kg wet
weight in the liver after 2 weeks of feeding; this level declined by
about 65% after 4 weeks of excretion. The time to 90% equilibrium
(uptake) was 15 weeks, and the time to 50% clearance (excretion) was
4 weeks (McLeese et al., 1980).
Bluegill sunfish (Lepomis macrochirus) exposed to water
containing 14C-labelled endrin at 1 µg/litre at temperatures of
20-22 °C rapidly absorbed the radioactivity, and, within 48 h, 91% of
the radioactive endrin had been taken up (6% was lost by
volatilization from a control tank without fish). Within 8 days after
the fish had been replaced in clean water, less than 15% of the
absorbed label had been eliminated; for three fish left for a longer
period, the half-life of loss was about 4 weeks, the loss curve being
linear (Sundershan & Khan, 1980). Endrin accumulated rapidly in the
tissues of channel catfish (Ictalurus punctatus) exposed to nominal
concentrations of 0.04, 0.4, or 4.0 mg/kg of diet for 198 days. After
that time, all groups were fed an endrin-free diet. Endrin was not
detected 28 days later in fish that had received 0.04 or 0.4 mg/kg,
and the level in the group that had received 4.0 mg/kg decreased to
0.011 mg/kg of tissue in 28 days and was below the limit of detection
within 41 days (Argyle et al., 1973). Similar results were obtained
for Leiostomus xantharus exposed to 0.05 µg/litre of water: at the
end of the study at 5 months, a residue level of 78 µg/kg tissue was
found, and no endrin was detected in fish after 18 days in
uncontaminated water (Lowe, 1966). Endrin thus seems to disappear
rapidly from tissues. In 20 Tilapia zilli (Alexandria strain) fry
(3.36 cm, 825 mg) exposed to 0.025 µg/litre (one-tenth of the 96-h
LC50) for 28 days, the total content of endrin was 327.4, 167.4,
297.6, 446.5, and 595.4 µg/kg after 4, 7, 14, 21, and 28 days,
respectively (El-Sebae, 1987).
Table 4. Bioconcentration factors for endrin in aquatic species
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Clam 1 5 days 480 Duke & Dumas
(Mercenaria (1974)
mercenaria)
Mussel 10 24 days 38 Ryan et al.
(Hyridella (1972)
australis)
Eastern oyster 0.05 7 day 2780 Mason & Rowe
(Crassostrea (1976)
virginica)
Water flea 1.0 1 day 2600 Metcalf et al.
(Daphnia (1973)
magna)
Fathead 0.015 - 10 000 Mount & Putnicki
minnow (1966)
(Pimephales
promelas)
Spot 0.05 8 months 1340 Lowe (1966)
(Leiostomus
xanthurus)
Flag fish 0.3 - 10 000 Hermanutz
(Jordanella 0.21 15 days 7 900 (1974)
floridae) 0.29 18 400 Hermanutz et
0.39 7 100 al. (1985)
Table 4 (contd)
Species Concentration Length of Bioconcentration Reference
of endrin in exposure factor
water (µg/litre)
Mosquito 1.0 1 day 2 100 Metcalf et al.
larvae (Culex (1973)
ipiensquinque
fasciatus)
Mosquito fish 1.0 1 day 800 Metcalf et al.
(Gambusia (1973)
affinis)
Channel catfish 0.5 5-19 400-760 Argyle et al.
(Ictalurus (1973)
punctatus)
Sheepshead minnow (Cyprinodon variegatus) were exposed for 23
weeks to endrin at levels of 0.027-0.72 µg/litre of water, from the
embryonic stage through hatching until adulthood and spawning (see
section 7.2.2.2). Four-week-old juvenile fish accumulated 2500 times
the concentration in the water, adults, 6400 times, and their eggs,
5700 times (Hansen et al., 1977).
The transfer of endrin through the food chain
lichen-reindeer-humans was studied in the northern part of Sweden by
analysing lichen (Cladonia alpestris), a major food source for
reindeer during the winter, together with samples of tissues from
reindeer, which are eaten in considerable quantities by Lapps. One
4-year-old reindeer was slaughtered in 1979 and a 3-year-old in 1981,
and muscle and liver were taken for analysis. The annual uptake by
reindeer was 2.0 mg. The average level of endrin in lichen was 1.91
(range, 1.27-2.78) µg/kg; 1.45 and 2.4 µg/kg were found in the muscle
samples from the two reindeer and 0.55 and 0.72 µg/kg in liver. The
calculated transfer of endrin from lichen to reindeer was 0.7%. The
estimated annual consumption of reindeer muscle by Lapps was 70 kg for
males and 32 kg for women; consumption of liver was 3 and 1.1 kg,
respectively. The annual intake of endrin was thus 30.3 µg for males
and 13.8 µg for females (Villeneuve et al.,1985).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Many of the data reported in this chapter are measurements taken
at a time when endrin was used much more widely than at present or
with little control or restriction. They are therefore a reflection of
a historical situation in many countries. These data are included in
the document as an indication of the result of indiscriminate use and
disposal of endrin. Data from countries where endrin may still be used
are scarce or unavailable.
5.1 Environmental levels
The levels of residues associated with the use of endrin in
agriculture or with the discharge of industrial effluents containing
endrin are summarized in Tables 5-9; the levels of residues less
easily associated with specific uses or discharges are given in Table
10.
5.1.1 Air
A critical summary of studies on the atmospheric levels of
pesticides in the USA, e.g., in community air, was made by Donoso et
al. (1979). Some of their conclusions are worth repeating: "Endrin
concentrations are highest in the atmosphere over agricultural areas
and probably reach their peak levels during the pesticide use season.
Of all urban communities those surrounded by farmlands run the highest
chance of atmospheric contamination. Urban communities far removed
from agricultural areas are unlikely to experience significant
contamination." The maximum level of endrin in air, 58.5 ng/m3, was
found in a rural town in an agricultural area in the south of the USA,
but the normal weekly variation was between 0.8 and 6.5 ng/m3
(Stanley et al., 1971). In a later study of the same town, the average
annual atmospheric levels were 3.2 ng/m3 in 1972, 2.3 ng/m3 in
1973,and 5.3 ng/m3 in 1974, with the highest levels in August; in
1974, this was 27.2 ng/m3 (Arthur et al., 1976). The results of a
national monitoring programme for pesticides in the air of various
states of the USA showed the occasional presence of endrin over
agricultural areas at levels of the same order of magnitude: mean of
positive samples (8%), 2.6 ng/m3, with a maximum value of 19.2
ng/m3 (Kutz et al., 1976).
Endrin was not found in rain-water collected at different
location in the United Kingdom, using a method with a detection level
of 1 ng/litre of water (Tarrant & Tatton, 1968), nor in atmospheric
air (Abbott et al., 1966); however, endrin has never been used
extensively in the United Kingdom.
The mean daily intake of endrin by inhalation in the western part
of the Netherlands was calculated on the basis of an air concentration
of 41 pg/m3 (maximum, 300 pg/m3) to be 0.8 µg/day or 0.3 mg/year,
on the basis of air samples taken in the period 1975-81 (Guicherit &
Schulting, 1985).
Table 5. Concentrations of endrin in organisms collected in the Netherlands, 1965-71
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Coast Mussel (Mystilus edulis); 22 0.029 0.009-0.056 Composites of 25 Koeman (1971)
1965 composites of flesh mussels from 22
sampling stations
Netherlands Fish, 3 species; 103 0.13 0.07-0.45 Food of the sandwich Koeman et al. (1967)
1965 whole body tern
1966 Fish, 3 species; 37 0.10 0.07 -0.29
whole body
Sandwich tern
(Sterna sandvincensis)
1965 Liver 8 0.23 0.07-0.80 Shot or killed
1965-66 Liver 25 0.49 0.10-1.3 Found dead
1965-66 Egg 33 0.19 0.08-0.36
Wadden Sea Mussel (2 species); 20/4 LD LD Limit of detection, Koeman (1971)
1969 composites of flesh 0.005 mg/kg
Coast Mussel (Mytilus edulis); 199/8 < 0.016 LD-0.024 Koeman (1971)
1970 composites of flesh
Wadden Sea Zooplankton (marine) 1 LD LD Koeman (1971)
1969-70
Shrimp (Crangon 50/1 LD LD
vulgaris)
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Marine fish (4 species); 37/5 0.014 0.008-0.034
composites of whole
body
1967 Freshwater fish 28 LD LD-0.02 Measurable concentration
(3 species) (0.02 mg/kg) in one fish
only; limit of detection,
0.005 mg/kg
1970 Pike (whole body) 10 LD LD Limit of detection,
0.005 mg/kg
1971 Roach (whole body) 6 LD LD
1968-69 Hawks and falcons 16 < 0.1 LD-0.16 Birds found
dead or dying; Koeman et al. (1969)
(4 species); liver measurable concentration
(0.16 mg/kg) in one hawk
(buzzard)
Owls (2 species); liver 3 < 0.1 LD-0.13 Measurable concentration
(0.13 mg/kg) in one
long-eared owl; limit of
detection not specified
1970 Sandwich tern eggs 10 LD LD Limit of detection, Koeman (1971)
0.02-0.008 mg/kg
1971 Grey heron 27/4 LD LD
(Ardea cinerea);
composite of eggs
Table 5. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
1969-71 Sparrowhawk 28/3 LD LD
(Accipiter nisus);
composite of eggs
a Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
b LD, limit of detection
Table 6. Concentrations of endrin in samples collected in North America
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Mississippi River
USA
December 1963 Channel catfish; blood 3 0.44 0.41-0.56 Found dead or dying in areas Anon. (1964)
of extensive fish kills
December 1963 Fish, various species; 24 0.18 0.14-0.26
blood
January- Fish, various species; 82 0.06 LD-0.21 Caught alive; limit of
February 1964 blood detection not specified
July 1964- Water 12 < 0.01 LD-0.01 4 samples contained Novak & Rao (1965)
June 1965 measurable concentrations
(0.01 mg/kg or litre)
Mud 12 < 0.01 LD-0.01
Oysters 12 LD LD Limit of detection,
0.005 mg/kg or litre
Shrimp 12 LD LD
Fish (2 species) 24 < 0.01 LD-0.02 9 samples of fish contained
measurable concentrations:
8 of 0.01 mg/kg and 1 of
0.02 mg/kg
Mississippi, USA Eastern oysters 470 LD LD 15 or more oysters per Butler (1973)
1965-72 (Crassostrea virginica); composite from 8 sampling
composites of flesh stations; limit of detection,
0.01 mg/kg
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Bayous in the Water 148 LD LD-0.0002 4 samples contained Rowe et al. (1971)
Mississippi delta, measurable amounts
USA, October (0.00009-0.0002 mg/litre),
1968-May 1969 4 samples contained traces;
remainder less than limit of
detection
Sediment 44 LD LD-0.005 7 samples contained
measurable amounts
(0.004-0.005 mg/kg); one
sample contained a trace
Eastern oyster 111 LD LD-0.006 79 samples contained
(Crassostrea virginica) < 0.001 mg/kg
Mississippi Water 26 LD LD Samples collected from Leard et al. (1980)
stream systems 13 sampling stations in
1972-73 5 major river basins; limit
of detection, 0.0005 mg/litre
Freshwater bivalves 58 LD LD-0.1 Residues below limit of
(7 species); flesh detection (0.02 mg/kg), except
for traces (< 0.1) in 1973 in
one river which drains from an
agricultural area
Ontario, 3 Fish (9 species) LD LD Residues below limit of Miles & Harris
streams, 1971 detection, 0.01 mg/kg (1973)
Table 6. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samplesa
Mean Rangeb
Gulf coast Whole fish 139/48 < 0.02 LD-0.27 Reseidues below limit of Henderson et al.
streams, USA (various species); detection (0.001 mg/kg) (1969)
composites in 33 composites
Mississippi 657/202 < 0.01 LD-0.11 Residues in 184 composites
River system, below limit of detection
USA (0.001 mg/kg)
Louisiana, USA Brown pelican Eggs collected from nests of Blus et al. (1979)
(Pelecanus occidentalis); birds transplanted as nestlings
eggs from Florida, 1968-76; limit
1971 3 0.10 0.08-0.12 of detection not specified
1972 12 0.18 0.11-0.29
1973 21 0.16 0.03-0.46
1974 25 0.30 LD-0.73
1975 30 0.50 0.29-1.06
1976 25 0.29 LD-1.47
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 7. Concentrations of endrin in organisms collected in a
cotton-growing area in the Republic of Chad in 1969
Sample No. of Concentration (mg/kg) Comments
samples Mean Range
Fish, two 31 0.02 LD-0.083 Cotton-growing area,
species endrin and DDT used
for pest control; limit
Kingfishers and 46 0.02 LD-0.075 of detection, 0.008
cormorants; liver mg/kg
Birds, non-aquatic, Birds found dead
various species soon after insecticide
Brain 12 0.51 0.10-0.77 application; deaths
Liver 12 0.88 0.13-1.42 of some birds attributed
to endrin
From Everaarts et al. (1971); LD, limit of detection
5.1.2 Soil, sediments, and sewage sludge
5.1.2.1 Soil
In the US National Soil Monitoring Program, 1486 soil samples
from 37 states were analysed in 1971. Fourteen samples were found to
contain endrin, at a geometric mean level of < 0.001 (maximum,
0.02-1.00) mg/kg dry weight (Carey et al., 1978). The mean endrin
concentration in 29 soil samples in Kyushu District, Japan, was 0.183
mg/kg (range, 0.016-0.629 mg/kg) dry matter (Suzuki et al., 1973).
5.1.2.2 Sediments
In 1964, levels in the sediment of Cypress Creek, Memphis, TN,
USA, upstream and downstream of a pesticide manufacturing plant,
reached 12 800 mg/kg dry weight. In 1967, water from the Creek
contained levels of 0.27-2.03 µg/litre and sediment contained levels
of 47.4-10 676 mg/kg dry weight (Barthel et al., 1969).
Endrin was found in 17% of samples of bottom sediment from 59
sites on the Detroit River, USA, at levels up to 43 µg/kg (limit of
detection, 1.0 µg/kg) (Hamdy & Post, 1985). No endrin was detected in
sediment samples collected in 1980-82 from riverine and pothole
wetlands at 17 locations in the north-central USA (Martin & Hartman,
1985) or in samples of sediment from 34 stations on the upper Great
Lakes in 1974 (< 1 µg/kg) (Glooschenko et al., 1976).
Table 8. Concentrations of endrin in organisms collected in a rice-growing area in Wageningen, Surinam
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
October 1971 Snail kite (Rostrhamus 5/1 LD LD -- Pesticides, including endrin, applied
sociabilis); brain /liver | to rice fields
|
Black vulture (Coragyps 5/1 LD LD |
astratus); brain/liver |
|
Egrets (3 species); brain/ 30/1 LD LD | Samples collected at end of growing
liver |-- season before insecticide application
| for next growing season; limit of
Purple gallinule 10/1 LD LD | detection, 0.01 mg/kg
(Porphyrula martinica); |
brain/breast muscle |
|
Spectacled caiman (Caiman 10/1 LD LD |
crocodilus); brain/liver --
November 1971 Snail (Pomocea sp.) 10/1 LD LD --
|
Frog (Pseudis paradoxa); 6/1 LD LD | Found dead after application of
whole-body composites | pentachlorophenol; lower limit of
| detection, 0.01 mg/kg
Kwi kwi (Hoplosternum 8/1 LD LD |
littorale); whole-body |
composites --
Table 8. (contd)
Date Type of sample No. of Concentration (mg/kg)b Comments
samplesa
Mean Rangeb
Srieba (Astyanax bimaculatus); 8/1 LD 0.1 -- Found dead after application
whole-body composites | of pentachlorophenol; lower
|-- limit of detection 0.01 mg/kg
|
Krobia (Cichlasoma 8/1 LD LD |
bimaculatum); whole-body |
composites --
Fish (3 species listed above); 21/3 3.36 1.96-5.35 Found dead after application
whole-body composites of endrin
28 November- Snail kite; brain 17 LD LD Found dead; deaths
4 December 1971 attributed to pentachlorophenol
poisoning
2-9 December 1971 Aquatic birds (4 species); 5 0.11 0.06-0.16 Found dead after application
brain of endrin
2-9 December 1971 Wattled jacana 1 2.71 Death attributed to endrin
(Jacana jacana) poisoning
5-11 December 1971 Common egret (Egretta alba); 2 0.23 0.14-0.32 Found dead or sick in roost
brain
2-11 December 1971 Common egret (Egretta alba); Found dead or sick in rice
Brain 9 0.25 fields; about half the total
Liver/kidney 7-9 0.08 endrin was applied during
the first half of December
From Vermeer et al. (1974)
aSample numbers expressed as n/m correspond to n individuals sampled in m composites analysed
bLD, limit of detection
Table 9. Concentrations of endrin collected in drainage water from irrigated land, California, USA
Geographical Type of sample No. of Concentration (mg/kg or mg/litre) Comments
area and year samples
Mean Range
Tule Lake and Water 44 0.000011 LD-0.0001 Limit of detection, < 0.000003 mg/litre
Klamath Lake,
National
Wild-life
Refuge,
USA, 1964
Refuge, Northern Suspended matter 8 0.011 LD-0.058 --
California, USA, Vascular plants 7 0.006 LD-0.013 |
April 1965- (two species) |-- Limit of detection, 0.005 mg/kg
February 1967 Algae (Cladophora sp.) 5 0.007 LD-0.022 |
Clam homogenates 3 0.013 LD-0.034 --
Gonidea sp.)
Fish (Siphateles sp.) 5 0.05 0.004-0.198 Samples collected at a pumping station
discharging water from irrigated land.
Peak concentrations of endrin occurred
during the growing season when endrin
was applied.
From Godsil & Johnson (1968); LD, limit of detection
Table 10. Concentrations of endrin in environmental samples; residues not associated with particular local use or industrial
effluent
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
North America
North Carolina, Soil (tobacco fields) 19 LD LD Reeves et al.
USA,1971 Sediment (ponds) 40 LD LD Limit of detection, (1977)
Frog (Rana sp.) 13 LD LD-0.01 0.01 mg/kg
Turtle (4 species) 41 LD LD-0.01
Bluegill (Lepomis 20 LD LD
macrochirus)
Tiger beetle 23 0.02 LD-0.05
(Megacephala
carolina)
Rice-growing Invertebrates, aquatic 1313/24 LD LD-trace A total of 192 dead or Flickinger &
area, Gulf Coast, and terrestrial (various dying birds were found in King (1972)
Texas, USA, species); whole-body three rice-growing areas in
1967-71 composites of live which rice seed dressed
specimens, except for with aldrin/ceresan had
4 composites of been used. Endrin residues
crayfish attributed to use in cotton-
growing areas. Limit of
1968 Fish (4 species); 542/4 LD LD detection not defined;
whole-body composites trace found in one
composite of dead
1968 Cricket frog (Acris 18/3 LD LD crayfish
crepeitans blanchardi);
whole-body composites
1968-70 Turtles (2 species); 5/2 LD LD
whole-body composites
of live specimens
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Snakes (3 species); sick 3 LD LD
specimens
Bobcat (sick) and dead 2 LD LD
rice rat; brain
Great horned owl; dead 2 LD LD
specimen; brain
Rice- growing Aquatic birds (10 26 0.22 LD-0.4
area, Gulf Coast species) found dead
USA, 1967-71 or dying; brain
1967 Fulvous tree duck 14 0.1 LD-0.3
(Dendrocygna bicolor);
eggs
Galveston Bay Oyster composites 10 0.01 LD-0.02 Limit of detection, Casper (1967)
Texas, USA, 1964 0.01 mg/kg
National Monitoring Fish (various species); 400 93% of samples below Henderson et
Program: Great Lakes whole-body composites limit of detection, al. (1969)
and major river 0.001 mg/kg
basins, USA (excluding
Gulf Coast, Mississippi
River system; see
Table 6); 1967-68
Atlantic coast streams 741/141 0.002 LD-1.50
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Great Lakes drainage Fish (various species); 378/66 0.001 LD-0.02
whole-body composites
Hudson Bay, Canada, 51/13 LD LD
drainage
Colorado River, USA 112/24 0.008 LD-0.71
Interior basins 120/25 0.001 LD-0.01
California, USA, 90/24 0.002 LD-0.02
streams
Columbia River, USA, 246/64 0.001 LD-0.01
systems
Pacific coast, USA, 83/20 LD LD
streams
Alaska, USA, streams 105/24 LD LD
National Monitoring Fish (various species); 666/147 LD LD Limit of detection, Henderson et
Program; 50 sampling whole-body composites 0.005 mg/kg al. (1971)
stations, USA, 1969
Estuaries, Giant Pacific oyster 1656/138 0.005 LD-0.01 Measurable concentration Modin (1969)
California, USA (Crassostrea gigas); in only one oyster; limit
1966-67 Mussel (Mytilus edulis); 432/36 LD LD of detection, 0.01 mg/kg
composites of shellfish
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 108-162/54 0.06 LD-0.4 Limit of detection, Crockett et
Mississippi, USA fish farms; composites 0.01 mg/kg; 13 al. (1975)
1970 of edible portions composites contained
< 0.01 mg/kg
Intensive cotton- 0.063 (0.030- 2 composites contained
growing areas, 0.122)a > 0.3 mg/kg. Significantly
Mississippi, USA higher residues in intensive
cotton-growing areas
Less intensive cotton- 0.010 (0.005- Crockett et
growing areas, 00.019)a al. (1975)
Mississippi, USA
Major watersheds, Fish (various species); 582/58 LD LD Limit of detection not Veith et al.
USA, 1976 whole-body composites specified. Intermediate (1979)
in the manufacture of
cyclodiene insecticides
detected (mass
spectrometry) in Wabash
River, Indiana
Major watersheds Fish (various species); 138/6 LD LD Limit of detection not Veith et al.
near Great Lakes, whole-body composites specified. Endrin (1981)
USA, 1978 identified by mass
spectometry in fish from
Wabash River, Indiana,
together with manufacturing
intermediates (concentration
not quantified)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Arkansas and Catfish from commercial 50 0.05 LD-0.41 Limit of detection, 0.01 Hawthorne
Mississippi, USA, fish farms, edible portion mg/kg; 14 fish contained et al. (1974)
1970 < 0.01 mg/kg
Continental rise Bathyl-demersal fish 4 LD LD Limit of detection, Meith-Avcin
south-east of (Antimora rostrata); 0.01 mg/kg. Samples et al. (1973)
Cape Hatteras, USA, liver collected by trawling at a
1972 depth of 2500 m
Lake Michigan, USA, Amphipods (Pontoporeia 24/8 0.08 0.04-0.33 Limit of detection, Peterson &
1969-72 affinis) collected from 0.005 mg/kg Ellarson
oesophagi of old squaws (1978)
December 1969 Old squaws (Clangula 37 0.18 0.1-0.2 Birds caught in fishing
hyemalis); carcasses nets or shot
March-April 1970 44 0.28 0.2-0.4
December 1970- 108 0.31 0.1-0.9
May 1971
January-February 8 0.6 0.2-1.0
1972
Northwest Territories 99 0.1 LD-0.3
and wintering areas
other than Lake
Michigan, 1971-73
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Canada and USA, Bald eagles (Haliaeetus 29 0.02 LD-0.1 Limit of detection, Reichel et al.
1965 leucocephalus) found 0.05 mg/kg. (1969)
dead; brain Concentration in 24
specimens below limit
of detection
Connecticut & Bald eagles found dead; 2 LD LD-0.1 Limit of detection not Reichel et al.
Florida, USA, brain, liver, carcass defined; apparent (1969)
1967-68 concentration of
0.1 mg/kg in Florida
eagle not conformed
by thin-layer
chromatography
Continental USA Bald eagle; brain Limit of detection, Mulhern et al.
1966 21 LD LD 0.05 mg/kg (1970)
1967 21 LD LD
1968 26 LD LD
1969 Bald eagle; brain 28 LD LD Limit of detection, Belisle et
0.05 mg/kg al. (1972)
1970 11 LD LD
1971-72 Bald eagle; brain 37 LD LD Limit of detection, Cromartie et
0.05 mg/kg al. (1975)
1973-74 Bald eagle; brain 81 LD LD Limit of detection, Prouty et al.
0.05 mg/kg (1977)
1975 Bald eagle; brain 49 0.07 LD-0.50 Limit of detection, Kaiser et al.
0.05 mg/kg. (1980)
Concentrations in 46
specimens < 0.05 mg/kg
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
1976 50 0.08 LD-0.71 Concentrations in 44
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisong
1977 69 0.08 LD-1.2 Concentrations in 64
specimens below limit of
detection. Death of one
eagle attributed to endrin
poisoning
Wisconsin, Maine, Bald eagle; eggs 26 LD LD Limit of detection, Krantz et al.
Florida, USA, 0.05 mg/kg (1970)
1968
USA, Golden eagle (Aquila 102 LD LD-0.3 Limit of detection, Reidinger &
1964-71 chrysaetos) found dead 0.1 mg/kg. Concentrations Crabtree
or dying; body fat in 97 specimens below (1974)
limit of detection
Coast of California, Gray whale 23 LD LD Wolman &
USA, 1968-69 (Eschrichtius robustus); Wilson (1970)
blubber
Sperm whale (Physeter 6 LD LD
catodon); blubber
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
South Atlantic and Small cetaceans (10 69 LD LD-0.24 Limit of detection, O'Shea et al.
Pacific Oceans, species); blubber, 0.1 mg/kg. Measurable (1980)
1968-69 brain, muscle concentrations (0.22 and
0.24 mg/kg) found in two
specimens
Maryland, USA, Little brown bat (Myotis 87 LD LD Limit of detection, Clark &
1976 lucifugus); carcass 0.1 mg/kg Krynitsky
(1978)
Missouri, USA Gray bat (Myotis 20 LD LD Limit of detection, Clark et al.
1976-77 grisescens) found dead; 0.1 mg/kg (1980)
carcass (lipid basis)
Washington Sate 18 bird species (total Blus et al.
(orchards), number of birds, 91) (1983)
October 1981- Brain 78 LD-> 0.8
July 1982 Eggs 53 LD-1.7
Detroit River, Herring gulls (Larus LD LD Struger et al.
Niagara River, argentatus); eggs (1985)
Saginaw Bay, USA,
1978-82
North-west Fish species; 700 0.008 LD-0.026 Stout (1980)
Atlantic Ocean, muscle
Gulf of Mexico,
USA, 1973-75
South-east Montana, Merlins (Falco LD LD Becker & Sieg
USA, 1978-81 columbarius); eggs (1987)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
New Jersey, Snapping turtles 11 LD LD Limit of detection, Albers et al.
Maryland, USA (Chelydra serpentina) 0.1 mg/kg (1986)
Florida, USA Snail kite (Rostrhamus LD LD Limit of detection, Sykes (1985)
sociabilis); eggs, nestlings 0.05 mg/kg
Missouri, USA, Gray bats (Myotis 7 LD LD Clawson &
1982 grisescens) Clark (1989)
Red bats (Lasiurus 7 LD LD
borealis)
Pipstrelles (Pipistrellus 2 LD LD
subflavus)
Denver, Colorado, Tree swallows 32 LD LD Deweese et al.
USA, 1980-81 (Tachycineta bicolor) (1985)
North-east Alberta, Otter (Lutra canadensis); 158 LD LD Limit of detection, Somers et al.
Canada, 1980-82 carcass 0.001 mg/kg (1987)
Africa
Lake Nakuru, Fish (Tilapia grahami); 10-20/2 LD LD Limit of detection: Koeman &
Kenya, 1970 whole-body composites fish, 0.002 mg/kg; Santiago
African cormorant birds, 0.009 mg/kg (1972)
(Phalacrocorax africanus) 3 LD LD
liver
White pelican 1 LD LD
(Pelicanus onocratalus);
Lesser flamingo 5 LD LD
(Phoeniconaias minor)
Table 10. (contd)
Place and Type of sample No. of Concentration (mg/kg) Comments Reference
period samples
Mean Range
Europe
Province of Leon, Kestrels (Falco 4 - LD-2.0 Liver, 0.01; brain, 0.016; Sierra &
Spain, 1986 tnnunculus); 5 organs kidneys, 0.027; muscle, Santiago
or tissues 0.054 mg/kg (1987)
Sparrowhawk 3 LD-2.0 Liver, 0.139; kidneys, 0.4;
(Accipiter nisus) fat, 1.068; brain, 0.031;
muscle, 0.047 mg/kg
Red kite 2 LD-2.0 Kidneys, 0.005; brain,
(Milvus milvus) 0.103; fat, 2.035 mg/kg
1984-87 Barn owl (Tyto alba) 0.001-0.22 Liver, 0.036; brain, 0.052; Sierra et al.
kidneys, 0.034; fat, 0.014; (1987)
muscle, 0.020 mg/kg
North Sea Gadus morhua and 12 0.0001-0.0023 Von Westernhagen
Merlangius merlangus; 4 < 0.001-0.0011 et al. (1987)
ovary
North Sea Merlangius merlangus Von Westernhagen
Ovary 56 LD-0.001 et al. (1989)
Testis 16 LD
Liver 30 LD
Sample numbers expressed as n/m correspond to n individuals sampled in m composites analysed; LD, limit of detection
a95% confidence interval
None of 60 samples of bottom deposit collected in 1974 from 19
rivers and their estuaries in Japan contained endrin (< 0.01 mg/kg)
(Japanese Environmental Agency, 1975).
No endrin was detected in sediment and particulates from the
River Elbe in Germany in 1983-85 (Sturm et al., 1986). Sediment from
Rotterdam Harbour contained a total of 3-59 µg/kg aldrin, dieldrin,
and endrin. No endrin was found at seven sites in the Elbe Estuary
(Japenga et al., 1987). A housing estate in the Netherlands,
comprising about 800 houses and public buildings, was built in 1983
directly on a 4-m-thick layer of harbour sludge transferred in 1962-64
from about 20 harbour basins in Rotterdam and the industrial area
around the Nieuwe Waterweg. Organic solvents, polycyclic aromatic
hydrocarbons, heavy metals, and endrin and related pesticides, were
detected in the sludge. One-third of the soil samples collected in the
gardens (71 locations), 0-40 cm below the surface, contained endrin
and related pesticides at a mean concentration of 1.2 mg/kg and a
maximal concentration of 19.5 mg/kg dry weight (Van Wynen & Stijkel,
1988).
In surface sediments from five sites in Manukan Harbour, New
Zealand, only traces (none detected to < 0.1 µg/kg dry weight) of
endrin were found (Fox et al., 1988).
Particulates from two sites in the Shatt al Arab River in Iraq
contained endrin at 84 and 154 µg/kg, and a site in the Tigris River
contained 217 µg/kg. No endrin was found in the Euphrates River. The
mean concentration of endrin in surface and subsurface sediment from
the Shatt al Arab River ranged from 3 to 18 (range, none detected to
32) µg/kg; no endrin was found in surface and subsurface sediment from
the Tigris River. None was found in surface sediment from the
Euphrates River, but in subsurface sediment a mean concentration of 11
(5-25) µg/kg was detected (DouAbul et al., 1988).
5.1.2.3 Sewage sludge
Endrin was found in only a few of 444 sludge samples analysed
from sewage treatment works in the United Kingdom. The mean
concentration was 0.11 mg/kg of sludge, with a range of 0.01-0.71
mg/kg (McIntyre & Lester, 1984). All samples of non-disinfected
influent at a pilot plant in Jefferson Parish, LA, USA, contained
endrin, at an average concentration of 0.67 (0.25-1.58) ng/litre
(Lykins et al., 1986).
Sludge from three main waste water treatment plants in Kuwait was
analysed over a 6-month period in 1984-85. Two grab samples were taken
from each plant every month to give a total of 36 samples. The mean
endrin levels for the three plants were 0.02, 0.02, and 0.06 mg/kg
(Samhan & Ghobrial, 1987). Sewage plant effluents before and after
treatment were analysed in Baghdad (Iraq) in 1982-83. Endrin was found
in 8/15 samples taken before treatment, at a mean concentration of
0.291 (0.081-2.637) µg/litre, and in 6/15 samples taken after
treatment, at a mean concentration of 0.194 (0.072-1.197) µg/litre
(Al-Omar et al., 1985a).
5.1.3 Water
5.1.3.1 Surface water
Data on the concentrations of endrin in surface water concern
mainly those regions in the USA where use of endrin was widespread,
such as in Mississippi and Missouri, over the period 1957-65. The
highest concentrations were found in 1963 in the Lower Mississippi,
with a maximum level of 0.214 µg/litre. The concentrations and the
rate of occurrence decreased considerably later (Breidenbach et al.,
1967). In a survey in 1964-68, a maximal level of 0.133 µg/litre was
reported to have been found in the Missouri basin in 1967. No endrin
was detected in 1968 (Lichtenberg et al., 1970). In 1974, the
concentrations in the Lower Mississippi was 0.0045 µg/litre in
August-November (Brodtmann, 1976).
In one sample from the Potomac River, at Quantico, endrin and
endrin aldehyde were identified at concentrations of 0.005 and 0.006
µg/litre, respectively (Hall et al., 1987). Endrin was not found in
the waters near the Los Angeles County ocean outfalls (< 0.00005
µg/litre) (Green et al., 1986) or in surface water in Louisiana in
1980 (< 1.0 ng/litre) (McFall et al., 1985). In a programme to
monitor surface water in the USA in 1976-80, endrin was found in only
0.1% of samples, at a maximum value of 0.04 µg/litre (Carey & Kutz,
1985).
No endrin was found in water from 33 sites in the Upper Great
Lakes in Canada (< 0.01 µg/litre) (Glooschenko et al., 1976). In
Ontario, where endrin was used only sparingly, no residues were found
in 1971 or 1975-77 (Miles & Harris, 1973; Frank et al., 1981). In
water samples taken 1 m below the surface at 14 stations on Lake
Ontario in 1983, endrin was found in concentrations of
0.000044-0.000145 µg/litre (Biberhofer & Stevens, 1987).
In a survey of the aquatic environment in The Netherlands,
including drinking-water, 1826 samples were taken at 99 sampling sites
between September 1969 and 1977; traces of endrin were reported
occasionally (Wegman & Greve, 1978, 1980). Studies of surface water in
other areas in Europe failed to show the presence of endrin (Wilson
Committee, 1969; Engst & Knoll, 1973; Uhnak et al., 1974; Galassi &
Provini, 1981;Hrubec, 1988). In 1984-85, water from a number of rivers
in Germany contained endrin at levels of none detected to 0.30
µg/litre (Braun ,1985); surface water in Greece occasionally contained
levels of 0.0003-0.0004 µg/litre (Albanis et al., 1986).
No endrin was found in surface or drinking-water in the state of
Sa Paulo (Brazil) (Lara & Barreto, 1972), but it was found in water
reservoirs of basins in Sa Paulo at concentrations of none detected
to 1.02 µg/litre (Celeste & Caceres, 1987; Caceres et al., 1987).
Endrin was also found accidentally in two lagoons in north-west Mexico
(Rosales et al., 1985).
None of 60 water samples collected in 1974 from 19 rivers and
their estuaries in Japan contained endrin (< 0.1 µg/litre) (Japanese
Environmental Agency, 1975).
Endrin was found at five places in the River Nile at
concentrations of 0.0038-0.0189 µg/litre in March-September 1982
(El-Dib & Badawy, 1985). In analyses of the water of the Shatt al
Arab, Euphrates, and Tigris Rivers, endrin was found only in the
Euphrates River, but in all samples at a mean concentration of 0.024
(0.014-0.036) µg/litre (DouAbul et al., 1988). It occurred in 75% of
samples of urban, industrial, and continental water from the Moroccan
Mediterranean coast, at concentrations of none detected to 13 µg/litre
(Kassabi et al., 1988). Three of 15 grab samples of surface water
sources in Southern Africa (Orange Free State) contained endrin, at a
concentration of 2-4 µg/litre (Hassett et al., 1987).
Endrin was present in the Kalinadi River in India as a result of
runoff, especially from agricultural areas, at a concentration of 2
µg/litre (Kudesia & Bali, 1985). In the Chao Phraya River and klongs
in Bangkok, Thailand, no endrin (< 0.001 µg/litre) was found in 1984
(Onodera & Tabucanon, 1986). Analyses in Bali of 16 samples of river
water in the dry season and 15 samples in the rainy season showed the
presence of endrin at 40 µg/litre once, in the rainy season (Machbub
et al., 1988).
5.1.3.2 Rain and snow
No endrin was found (limit of detection, 1-2 ng/litre) in
atmospheric precipitation in the form of snow (17 samples in 1976) and
rain (81 samples in 1976 and 1977) on the Canadian side of the Great
Lakes and inland in areas remote from any nearby industrial or urban
contamination (Strachan et al., 1980). Four of 16 samples of
rain-water collected at four sites in Canada had levels of
0.00013-0.00044 µg/litre and another sample had 0.0048 µg/litre; no
endrin was detected in the other 11 samples. The mean endrin contents
in samples taken at another site in 1977, 1981, 1983, and 1984 were
none detected, 0.000065, 0.000085, and 0.000049 µg/litre, respectively
(Strachan, 1988). Endrin was not detected in snow samples collected at
12 sites in the Northwest Territories of Canada in 1985-86 (Gregor &
Gummer, 1989).
5.1.3.3 Drinking-water
Data obtained in 1964-67 from selected municipal drinking-water
treatment plants in Mississippi and Missouri, USA, showed that the
concentration in approximately 10% of the samples exceeded 0.1
µg/litre in the first year but that the concentrations were lower in
1965-67 (Schafer et al., 1969). The most recent study on US
drinking-water was done on finished water in New Orleans, LA, in 1974,
where the highest concentration measured was 4 ng/litre (US EPA,
1974).
During 1976, endrin was found at a mean concentration of 4
ng/litre (range, 1-7 ng/litre) in drinking-water in Ottawa, Canada
(Williams et al., 1978).
The mean concentration of endrin in drinking-water at the
El-Abbasia station, Egypt, in 1986 was 3.507 ± 1.45 ng/litre in 10
samples taken before purification and 1.845 ± 1.29 ng/litre after
purification (Abdel-Razik et al., 1988).
Drinking-water from the North Coast region of New South Wales,
Australia, was analysed in 1986-87: 147 of 659 samples contained
traces of endrin (none detected [< 0.005] to 0.05 µg/litre) (Ang et
al., 1989).
5.1.3.4 Groundwater
Water in wells used as a source of water for mixing pesticides
in fruit orchards in West Virginia (USA) was found to contain endrin
at about 1 ng/litre in 1985 and in 1986. The water in these wells was
not used for drinking-water. Endrin had not been used in the area
since 1970, and the authors cite their results as evidence for the
persistence of endrin and its capacity to contaminate groundwater many
years after cessation of use (Hogmire et al., 1990).
5.1.4 Organisms in the environment
5.1.4.1 Birds
Endrin was found in the carcasses of four of 16 turkey vultures
(Cathartes aura) in southern California, USA, in 1981, at levels of
0.11-0.23 mg/kg wet weight, but in none of six common ravens (Corvus
corax). It was also found in two of four vulture eggs, at 0.10
(range, none detected to 0.52) mg/kg wet weight, but in none of 30
raven eggs (Wiemeyer et al., 1986).
Endrin was found in eggs of shag (Phalacrocorax aristotelis)
and cormorants (Phalacrocorax carbo) at one of five collection sites
in the east, south-east, and south of Ireland, at a geometric mean
concentration of 0.30 (range, 0.06-1.60) µg/kg (Wilson & Earley,
1986). Eggs from two species of passerine birds, three species of
gull, four species of tern, and the night heron were collected in
Italy in 1982-83. Endrin was found in 30 eggs of the night heron
(Nicticorax nycticorax), at an average concentration of 0.11
(0.03-0.27) mg/kg, in 50 eggs of the gull-billed tern (Gelochelidon
nilotica), at a concentration of 0.28 (0.05-1.31) mg/kg, and in 38
eggs of the tree sparrow (Passer montanus) and 33 eggs of the hooded
crow (Corvus corone), at concentrations of 0.17 (0.09-0.33) and 0.21
(0.07-0.31) mg/kg. It was not detected in eggs of the other species
(Fasola et al., 1987).
No endrin was found in 98 eggs or in the livers of 112 nestlings
of rooks (Corvus frugilegus) collected from five rookeries in
northern Germany in 1982-83 (Beyerbach et al., 1987) or in 45 eggs and
the livers of eight young lapwings (Vanellus vanellus) collected in
1984 and 1986 (Beyerbach et al., 1988).
Detectable residues of the commonest organochlorine pesticides
were found in 0.9% of 112 pools (mostly of 10 birds) of starlings
(Sturnus vulgaris) collected in 129 sites in the USA in 1979 and in
1.6% of 129 pools in 1982. In most states, no endrin was detected, but
levels of 0.01 and 0.17 mg/kg wet weight were found in two (Bunck et
al., 1987).
Endrin was present at microgram levels per kilogram of wet weight
in 272 samples of liver, muscle, fat, and eggs from northern fulmars
(Fulmarus glacialis), black-legged kittiwakes (Rissa tridactyla),
and thick-billed murres (Uria lomvia) collected in 1975-77 on Prince
Leopold Island, Northwest Territories, Canada (Nettleship & Peakall,
1987). It was found in 8 of 108 carcasses of herons analysed in the
USA since 1966, at levels of 0.10-0.86 mg/kg wet weight (Ohlendorf et
al., 1981) but was not found in 255 pools of wings from black ducks
(Anas rubripes) and mallards (A. platyrhynchos) collected in the
USA in 1981-82 (Prouty & Bunck, 1986).
Endrin was not detected in six eggs of Forster's tern (Sterna
forsteri) collected on Green Bay and Lake Poygan, Michigan, USA in
1983 (Kubiak et al., 1989). None was found in a total of 107 eggs
collected in 1975-80 from 10 species of colonial waterbirds nesting in
areas around Green Bay and Lake Michigan. The species were little
gulls (Lares minutes), green-backed herons (Butorides striatus),
black terns (Chlidonias niger), herring gulls (L. argentatus),
ring-billed gulls (L. delawarensis), common terns (S. hirundo),
Forster's tern (S. forsteri), double-crested cormorants
(Phalcrocorax auritis), black-crowned night herons (Nycticorax
nycticorax), and cattle egrets (Bubulcus ibis). The limit of
detection was 0.1 mg/kg in 1977 and 0.05 mg/kg in 1978 (Heinz et al.,
1985).
Of five eggs from peregrine falcons (Falco peregrinus)
collected in Arizona, USA, in 1978-82, one collected in 1978 contained
endrin at 0.20 mg/kg dry weight, one collected in 1981 contained no
detectable amount (< 0.01 mg/kg) and three collected in 1982
contained 0.02-0.04 mg/kg (Ellis et al., 1989).
No endrin was detected in 27 eggs from tree sparrows (Passer
montanus), 4 eggs from house martins (Delichon urbica), 28 eggs
from white storks (Ciconia ciconia), or eggs from nine other species
of bird in Germany in 1984. The livers of 25 nestling, 13 young, and
17 adult white storks also contained no detectable level of this
pesticide (limit of detection, 0.001 mg/kg) (Heidmann et al., 1989).
5.1.4.2 Fish and shellfish
The endrin concentrations in red mullet (Mullet barbatus)
collected at six locations in the Pagassitikos Gulf (Greece) in
1986-87 were < 0.005-0.5 µg/kg fresh weight of fillets (Satsmadjis et
al., 1988). The mean concentrations in liver, brain, kidneys, and
muscle of 22 trout (Salmo trutta fario L.) taken from four rivers in
Leon, Spain, in 1985 were 0.104, 0.123, 0.157, and 0.157 mg/kg wet
weight. The incidence in the four organs was 4.54-22.73% (Teran &
Sierra, 1987). Endrin was found in 29 samples of fish collected in
Italy, at a median concentration of 0.019 mg/kg (Cantoni et al.,
1988). Organochlorine compounds were measured in three samples of
liver from cod (Gadus morhua) collected in three areas of the North
Sea in 1977-87; endrin was present at a concentration of < 5 µg/kg of
product (De Boer, 1989).
Endrin was not detected (< 0.01 mg/kg) in two or three replicate
samples, each comprising three to five bluegill (Lepomis macrochirus)
and common carp (Cyprinus carpio), collected from downstream sites
exposed to irrigated agriculture and from non-irrigated upstream sites
on the San Joaquin River and tributaries in California, USA (Saiki &
Schmitt, 1986). Endrin was also not found in water near Los Angeles
County ocean outfalls (< 0.00005 µg/litre) or in mussels (Mytilus
californianus) (< 0.1 µg/kg wet weight) that had been suspended at
the monitoring site for 2 months to provide a measure of the
bioaccumulation of chlorinated hydrocarbon contaminants (Green et al.,
1986). No endrin was detected in fish samples taken at nine locations
in north-central USA (Martin & Hartman, 1985), and endrin was not
detectable (< 0.001 mg/kg) in 527 samples of edible fin fish
harvested from Chesapeake Bay and its tributaries (Maryland) over the
period 1976-80 or in 20 samples of roe and gonadal tissue (Eisenberg
& Topping, 1985).
No detectable quantity (< 1 µg/kg) of endrin was found in two
species of crayfish (Procambarus clarkii and P. acutus)
commercially harvested from dual-cropped ponds and from waters of the
Atchafalaya River Basin and the Mississippi River in southern
Louisiana, or in sediment and water collected from several ponds and
at the Basin three times during 1986 and 1987 (Madden et al., 1989).
Endrin was measured at levels of 0.4 and 66 µg/kg in American
eels (Anguilla rostrata) sampled at various sites between Lake
Ontario and the mouth of the St Lawrence river in 1982 (Castonguay et
al., 1989). Endrin was not detectable (< 0.002 mg/kg) in 'most'
composite samples (1-15 fish of 10 different species) collected from
10 sites on the Great Lakes and tributaries between 1980 and 1981,
although in a few cases concentrations up to 0.01 mg/kg were found
(Devault, 1985). Endrin was not present (< 0.005 mg/kg) in fillets of
Fall Run Coho salmon (Oncorhynchus kisutch) taken from 14 sites on
the Great Lakes in 1984. In most cases, three samples per site were
analysed, and the fish were 2-3 years old (Devault et al., 1988).
Johnson et al. (1988) measured the input of organochlorine pesticides
from precipitation and runoff to five small lakes peripheral to the
Canadian Great Lakes and the levels of residues in fish caught in the
lakes. While endrin was detectable in precipitation (at 0.46 and 0.54
ng/litre at the two sampling sites), none was measured in runoff water
and no detectable residue was found in fish.
The mean concentrations of endrin in 13 commercially important
fish species collected in the north-west Arabian Gulf varied between
1 and 28 µg/kg, and those in five species collected from Hor al-Hammar
Lake in Iraq in 1985 were 3-67 µg/kg wet weight of edible tissue.
Endrin residues were detected in approximately 90% of the fish
(DouAbul et al., 1987a). Samples of Barbus xanthopetrus collected in
the Shatt al Arab River and in Hor al Hammar Lake contained average
concentrations of 4 (none detected to 9) and 20 (11-27) µg/kg, while
Indian shed (Tenualosa ilistra) from the Shatt al Arab River
contained 80 (57-108) µg/kg (wet weight). Shrimp (Metapanaeus
affinis) did not contain endrin (DouAbul et al., 1987b). In 1984,
B. xanthopetrus from the River contained a mean concentration of 16
µg/kg wet weight, and those from the Lake, 154 µg/kg (range, 13-355);
Indian shed had mean concentrations of 41-147 µg/kg (range, none
detected to 236) (DouAbul et al., 1987c). Freshwater mussel
(Corbicula fluminea) collected in the Shatt al Arab River contained
166-540 µg/kg (range, 140-583) (DouAbul et al., 1988). Endrin was
present at concentrations of 1.9-12.2 µg/kg of muscle tissue (wet
weight) in three fish species and at 0.88-7.7 µg/kg in three Tilapia
species collected near Alexandria, Egypt, in 1985 (El Nabawi et al.,
1987).
Endrin was present at 0.003-0.004 mg/kg in black pomfret
(Parastromateus niger), mackerel (Rastrelliger kanagurta), and
marine vala (Chirocentrus sp.) and at 0.08 mg/kg in tuna (Euthynnus
affinis) collected off the Indian coast (Radhakrishnan & Antony,
1989). It was found in one sample of fish at 0.019 mg/kg wet weight
and in one shellfish sample at 0.034 mg/kg but in none of 312 other
specimens of 11 types of fish, crustaceans, and molluscs obtained from
five sites in Java, Indonesia (limit of detection, 0.01 mg/kg) (Koeman
et al., 1974).
No endrin was found (< 0.005 mg/kg) in 60 samples of fish and
shellfish collected in 19 rivers and their estuaries in Japan in 1974
(Japanese Environmental Agency, 1975).
The median concentration of endrin in the eggs of 15 adult
chinook salmon (Onchorhynchus tshawytscha) collected in Lake
Michigan in 1982 was 23.5 µg/kg wet weight (range, 3.9-126.3) (Giesy
et al., 1986).
Composite samples of whole fish of selected species were
collected in 1983 near the shores of 13 tributaries of Lake Michigan
and Grand Traverse Bay. Two of each of the following species were
collected from each site: common carp (Cyprinus carpio), bowfin
(Amia calva), channel catfish (Ictalurus punctatus), pumpkinseed
(Lepomis gibbosus), rock bass (Ambloplites rupestris), small-mouth
bass (Micropterus dolomieui), large-mouth bass (M. salmoides),
lake trout (Salvelinus namaycush), and pike (Esox lucius); the
composites comprised 3-11 fish. Endrin was not detected (limit, 0.005
mg/kg) (Camanzo et al., 1987).
Yellow perch (Perca flavencens) were sampled in eight
reservoirs and lakes in Ohio and Wisconsin, USA, in 1978-79. Endrin
was found in four fish at levels of 0.008-0.02 mg/kg, which were much
lower than the levels found of polychlorinated biphenyls, DDT and
dieldrin (Carline & Lawal, 1985).
5.1.4.3 Mixed species
Herons (Nyctanassa violacea), water snakes (Natrix spp.),
raccoons (Procyon lotor), channel catfish (Ictalurus punctatus),
crappies (Pomoxis spp.), frogs (Rana spp.), and crawfish
(Procambarus clarkii) were collected from three watersheds in
Louisiana, USA in 1978-79. Endrin was found in a heron at 0.014 mg/kg
and in a catfish at 0.022 mg/kg, but in no other case (limit of
detection, < 0.05 mg/kg) (Dowd et al., 1985).
5.1.5 Other food and feed
5.1.5.1 Cereals
Endrin has been used extensively for the control of insect pests
in rice. Typically, one to four applications are made, depending on
local conditions, the last application usually not later than one
month before harvest. Data on residue levels are available from India
(1969-70), Thailand (1968-70), the Philippines, Indonesia (1966), and
Venezuela (1969). The levels in polished rice were 0.01-0.04 mg/kg of
product (mean, 0.014 mg/kg), except in India where higher levels in
the order of 0.12 mg/kg were found. Bran, which is used mainly as a
component of poultry feed, contained a mean level of 0.35 mg/kg
(range, < 0.01-2.3 mg/kg), and low levels of delta-ketoendrin were
found (FAO/WHO, 1971).
Endrin has been used to only a limited extent on grain crops. The
residues in different types of treated grains in the USA were
generally below 0.05 mg/kg of product, except in oats in which levels
up to 0.5 mg/kg were found. In India, up to five applications on
sorghum gave residue levels below 0.02 mg/kg; in the USA, the levels
in sorghum were below 0.05 mg/kg. Straw of cereals contains higher
levels: rice straw had up to 3 mg/kg,, and sorghum straw up to 0.4
mg/kg (FAO/WHO, 1971).
Wheat imported into the United Kingdom in 1987-88 did not contain
endrin (< 0.01 mg/kg) (Osborne et al., 1989).
5.1.5.2 Fruit and vegetables
Endrin is occasionally used for control of field mice (voles). No
residue was found in apples at harvest (detection limit, 0.01-0.002
mg/kg of whole fruit) when it was sprayed on the ground under trees in
orchards in autumn or spring. The levels were sometimes higher in
fallen fruit, ranging from < 0.002 to 0.02 mg/kg of product (Horsfall
et al., 1970; FAO/WHO, 1971).
Only 14 of 15 000 samples of fruit and vegetables imported into
Sweden during the period 1981-84 contained endrin, at a maximum
concentration of 0.02 mg/kg (Anderson, 1986). The pesticide analysis
programme of the Swedish National Food Administration on fruit and
vegetables, including potatoes, showed no residue of endrin above the
limit of detection of 0.02 mg/kg in 13 724 samples analysed in 1985-87
(B.G. Ericsson, personal communication, 1990). The mean endrin
concentration in 137 samples of grape products (including seeds,
skins, marc and lees) in Italy was 6.2-16.2 µg/kg (Marinelli et al.,
1986). Seven of 306 samples of apples (five types) collected in
1980-83 from five regions of Italy contained endrin (limit of
detection, 0.001 mg/kg) (Foschi et al., 1985).
In Pakistan, in 16 samples of cucumber sprayed at the time of
maturity with a 0.05% endrin solution at a rate of 100 gallons/acre
(1123 litres/ha), the endrin concentrations ranged from 3.04 to 6.69
mg/kg. The residues persisted in the edible portion of cucumber up to
14 days and diminished thereafter (Illahi et al., 1986). Endrin was
found in 17% of samples of peas collected from fields and markets in
Faisalabad, Pakistan, at a level of 1.3-4.32 µg/kg. The residues
persisted for up to 12 days and then decreased (Illahi et al., 1987).
No endrin was found (< 0.02 mg/kg) in 141 samples of fruit and
vegetables from Pakistan in 1982-83 (Masud & Farhat, 1985).
In an analysis of soya bean and soya bean straw in a US
monitoring programme, seven of 177 samples of soya beans contained a
geometrical mean of < 0.001 (maximum, 0.03) mg/kg, and one of eight
straw samples contained < 0.01 mg/kg (Carey et al., 1978). Endrin was
used in up to four applications on sugar-cane in the USA, with an
interval of 45 days or longer between the last application and
harvest. The residues found in cane were usually < 0.05 mg/kg of
product (FAO/WHO, 1971).
5.1.5.3 Meat, poultry, and chicken eggs
Bovine fat (40 samples), pig fat (45 samples), calf fat (45
samples), sheep fat (22 samples), poultry fat (42 samples), and eggs
(44 samples) analysed in the Netherlands in 1983 had a median endrin
concentration of < 0.04 mg/kg (Dutch Agricultural Advisory Commission
on Environmental Pollutants, 1983). No endrin was found (detection
limit, 0.005 mg/kg) in samples of beef, pork, goat, mutton, poultry,
or eggs analaysed in Italy in 1985-87 (Cantoni et al., 1988) or in
'most' samples of pork, rabbit, or poultry analysed in
Rheinland/Pfalz, Germany, in 1981-84 (Kampe, 1985). Dietary surveys in
the United Kingdom demonstrated no endrin in meat (detection limit,
0.02 mg/kg) (United Kingdom Ministry of Agriculture, Fisheries and
Food, 1989).
Endrin was present in 10.8% of 2032 samples of bovine fat from
carcasses collected from slaughterhouses in Brazil, at a mean level of
0.01 mg/kg; the highest level was 0.09 mg/kg of tissue (De Paula
Carvalho et al., 1984). Endrin was present in hens' eggs from four of
five areas in Mexico, at concentrations of 0.004-0.11 mg/kg of whole
egg, and in 11 of 16 samples of chicken meat, at an average
concentration of 0.12 (none detected to 0.6) mg/kg on a fat basis
(Albert, 1990).
Endrin was detected in 86 of 221 samples of hens' eggs (78 native
and 143 commercial) collected in 1975-77 in Iran, at a mean
concentration of 0.017 (range, 0.003-0.13) mg/kg (Hashemy-Tonkabony &
Mosstofian, 1979). No endrin was found (limit of detection, 0.02
mg/kg) in samples of about 25 eggs of sawah ducks collected on 11
local markets in Java, Indonesia, in 1972 (Koeman et al., 1974).
It was found in 14 of 367 hens' eggs collected from 61 farms in
11 districts of Kenya in 1984; in three of the eggs, the level was >
0.2 mg/kg (Mugambi et al., 1989).
Heating, baking, frying, and steaming of tissues obtained from
broilers fed endrin at 10 mg/kg of diet for 8 weeks did not
significantly reduce the level of residues: raw, 28.2; baked, 20.8;
fried, 22.7; and steamed, 19.4 mg/kg of dry tissue (Ritchey et al.,
1972).
5.1.5.4 Milk and milk products
The mean endrin concentration in 20 samples of fresh buffalo milk
in Kalubia, Egypt, was 0.02 mg/kg of milk fat (range, < 0.01-0.03
mg/kg (Abdou et al., 1983). Cows' milk (39 samples) collected in four
areas of Bagdad, Iraq, in 1981-82 contained a mean of 60 (none
detected to 400) µg/litre (Al-Omar et al., 1985b).
The average concentration of endrin in 10 samples of evaporated
cows' milk from three main cities in the agricultural region of Mexico
was < 0.007 mg/litre of milk fat (Albert et al.,1982). Endrin was
found in powdered milk at an average concentration of 0.06 mg/kg and
in cheese at a concentration of 10-27.2 mg/kg on a fat basis (Albert,
1990).
The level of endrin in milk in the USA was < 0.001 mg/litre (on
a fat basis) (FAO/WHO, 1971). No endrin (< 0.5 µg/litre) was found in
308 samples from bulk transports of milk collected in Ontario, Canada,
in 1977 (Frank et al., 1979) or in 359 samples collected in 1983
(Frank et al., 1985).
No residue was found (detection limit, < 0.005 mg/kg) in samples
of milk, cream, butter, and cheese in Italy (Cantoni et al., 1988) or
in 12 samples of cows' milk collected in 1984-86 from different areas
of Spain (< 0.01 mg/kg fat) (Barcelo & Puignou, 1987).
5.1.5.5 Fat and oils
The most important use of endrin is for the control of insects in
cotton, the number of applications being 1-12; cottonseed oil is used
for cooking and for the manufacture of margarine, while the extracted
cake is used as cattle feed. Endrin is thus present both in the
cottonseed and in the edible oil and cakes. In a study of the
extraction processes, it was found that alkali washing and bleaching
had no marked effect but that deodorization reduced the endrin levels
to below the limit of detection (0.03 mg/kg) (Smith et al., 1968).
In field studies carried out in the USA, cottonseed contained
endrin at a maximum of 0.1 mg/kg, although the levels were usually
much lower. delta-Ketoendrin was not detected. The levels in crude,
decolourized, and deodorized oil in Venezuela and Brazil were all <
0.02 mg/kg of product. Spot samples of refined cottonseed oil from
California, USA, contained < 0.03 mg of endrin and < 0.02 mg of
delta-ketoendrin ( limits of detection) (FAO/WHO, 1971).
One-hundred-and-ten samples of raw oil and of oil at various
stages of processing, i.e., neutralized, hydrogenated, decolourized,
deodorized, and shortenings, were collected from seven oil processing
factories in Iran in 1974. Endrin was found only in raw and
neutralized vegetable oils, at concentrations of 0.004-0.005 mg/litre.
Raw imported and native oils contained < 0.01 mg/litre, except for
native sunflower oil which contained 0.026 mg/litre (Hashemy-Tonkabony
& Soleimani-Amiri, 1976).
Endrin was found in 60 samples of six varieties of the major
edible oils and oil seeds used in India, including groundnut, sesame,
mustard, coconut, and hydrogenated vegetable oils, collected from a
market in Lucknow. Vegetable oil contained 6 µg/litre, mustard oil, 72
µg/litre, and sesame oil, 1690 µg/kg. Of the different types of oil
seeds, only mustard seed contained endrin, at 22 µg/kg (Dikshith et
al., 1989a).
Endrin was found at a mean concentration of 0.184 (0.097-0.288)
mg/kg in samples of cod-liver oil analysed in Germany in 1985 (Ali,
1986). No residue was found in vegetable oils and fats imported into
the United Kingdom (detection limits, 0.02 and 0.001 mg/kg,
respectively) (Abbot et al., 1969).
5.1.5.6 Animal feed
Residues of endrin in pressed cottonseed cakes arise primarily
from the 1-5% of oil left in the cake after extraction. The residues
in cakes from Brazil, India, the USA, and Venezuela were mainly <
0.01-0.02 mg/kg product, levels up to 0.08 mg/kg were found
occasionally (FAO/WHO, 1971). The mean concentration of endrin in 32
samples of cattle feed from a local market in India was 0.020 mg/kg
(range, 0.013-0.027 mg/kg) (Dikshith et al., 1989b). No endrin was
found in 79 samples of cattle feed in Pakistan (Parveen & Masud,
1987). Endrin was not present in samples of domestic and imported
animal feed analysed in the USA in 1981-86 (Luke et al., 1988).
None of 42 samples of chicken feed collected from 61 farms in 11
districts of Kenya in 1984 contained endrin (Mugambi et al., 1989).
5.1.6 Miscellaneous products
Endrin was found in 5 of 25 tobacco samples imported into Germany
at concentrations of 25-50 µg/kg (Cetinkaya, 1988). No endrin was
found in cigarettes of 14 brands collected in Finland in 1960-84
(Mussalo-Rauhamaa et al., 1986). An average content of 0.006
µg/cigarette (range, none detected to 0.02 µg/cigarette) was found in
Switzerland (Zimmerli & Marek, 1973).
When raw cotton imported into Germany from 15 countries was
analysed, endrin was found in samples from the USSR and Mexico at a
concentration of 3 µg/kg (Cetinkaya & Schenek, 1987).
5.2 Exposure of the general population
5.2.1 Total-diet studies
Studies on complete prepared meals in the USA, started in May
1961 and continued to the present, have shown the occasional presence
of small amounts of endrin (Williams, 1964; Cummings, 1965, 1966;
Duggan et al., 1966, 1967; Martin & Duggan, 1968; Corneliussen, 1969,
1970, 1972; Manske & Corneliussen, 1974; Manske & Johnson, 1975;
Johnson & Manske, 1976, 1977; Manske & Johnson, 1977; Johnson et al.,
1981a, 1984). These measurements indicate that the total average daily
intake of endrin from food decreased from 0.009 µg/kg body weight in
1965 to 0.0005 µg/kg body weight in 1970 (Duggan & Lipscomb, 1969;
Duggan & Corneliussen, 1972), with a further decrease subsequently. In
total-diet studies of adults in the USA, representative foods were
purchased in 27 US cities in 1980-82; the daily intake of endrin was
found to be < 0.001 µg/kg body weight in 1978, but none was detected
in 1979, 1980, or 1981-82 (Gartrell et al., 1986a).
Further studies involved retail purchase of 234 food items
representative of the total diet of eight US population groups in
1982-84 and preparing them for consumption. The daily intake of endrin
in the groups, which included people aged 6-11 months, 2 years, 14-16
years (females), 14-16 years (males), 25-30 years (females), 25-30
years (males), 60-65 years (females), and 60-65 years (males), was
0.1-0.2 ng/kg body weight (FDA, 1988; Gunderson, 1988).
Endrin was not present in the total diets of infants and toddlers
in the USA during 1974-75 (Johnson et al., 1979). It was found in one
infant food sample at 0.011 mg/kg and in one sample of toddler food at
0.009 mg/kg of food in a study in 1975-76 (Johnson et al., 1981b).
Very low residue levels were found occasionally in market-basket
samples representing the average 2-week diets of infants (98 samples)
and toddlers (110 samples) collected in 10 cities in four geographic
areas of the USA in 1977-78 (Podrebarac, 1984). In total-diet studies
of infants and toddlers in the USA, representative foods were
purchased in 13 US cities in 1980-82; the daily intake of endrin by
infants was found to be < 0.001 µg/kg body weight in 1978, and that
by toddlers, < 0.001 µg/kg body weight in 1979, but none was detected
in the other years (Gartrell et al., 1986b).
Fresh food was bought from four retail grocery stores in Toronto,
Canada, in 1985 and combined in five food composites: fresh meat and
eggs, root vegetables (including potatoes), fresh fruit, leafy and
other surface vegetables, and cows' milk. The concentrations of endrin
in the five composites were used to estimate the annual dietary intake
of endrin from products in Ontario. Endrin was detected in all
composites except eggs and meat; the concentrations were 0.32 µg/kg in
leafy vegetables, 0.27 µg/kg in fruit, 0.37 µg/kg in root vegetables,
and 0.27 µg/kg in milk. The total annual intake was estimated to be
31.8 µg/person (Davies, 1988).
In a total-diet study carried out in the United Kingdom in
1985-88, 25 samples were obtained in 1984-85 which comprised the 16
food groups considered most likely to contain residues of
organochlorine compounds. No endrin was detected (limit of detection,
0.001-0.02 mg/kg, depending on the food group). Endrin was also not
detected (< 0.01 mg/kg) in 176 samples of pulses purchased from
retail outlets in 1986-87, except in 3 of 20 samples of mung beans in
which a mean value of < 0.01 mg/kg (range, none detected to 0.06
mg/kg) was found (United Kingdom Ministry of Agriculture, Fisheries
and Food, 1989). No endrin was detected in complete prepared meals
during surveys in the United Kingdom in 1965 (Robinson & McGill, 1966;
McGill & Robinson, 1968). Similar results were obtained in Switzerland
in 1973 (Zimmerli & Marek, 1973), and very low levels were found in
two of 73 samples analysed in 1985 (Wüthrich et al., 1985). No endrin
residues were found in total-diet studies carried out in the
Netherlands in 1976-78 (De Vos et al., 1984).
5.2.2 Levels in human tissues
Although the concentrations of many chlorinated hydrocarbon
insecticides, such as DDT, dieldrin, hexachlorocyclohexanes, and
hexachlorobenzene, and of their metabolites in blood or adipose tissue
of the general population or of occupationally exposed workers have
been found to be an excellent index of the level of exposure of the
general population, this is not the case for endrin, because it is
eliminated rapidly.
5.2.2.1 Adipose tissue
Except in a few cases, endrin was not demonstrated in adipose
tissue samples from the general population in the USA in 1962-66
(Hoffman et al., 1964, 1967), 1964 (Hayes et al., 1965; Zavon et al.,
1965), 1970-74 (Kutz et al., 1979a,b), and 1975-79 (US EPA, 1983);
Canada in 1967-68 (Kadis et al., 1970); Mexico in 1975 (Albert et al.,
1980); Argentina in 1968-69; (Wassermann et al., 1969); Belgium in
1968-69 (Wit, 1971); the United Kingdom in 1961 (Hunter et al., 1963),
1964 (Robinson et al., 1965), and 1965-67 (Egan et al., 1965; Abbott
et al., 1968, 1972); the Netherlands in 1969 (Wit, 1971); Switzerland
in 1972 (Zimmerli & Marek, 1973); Germany in 1970 (Acker & Schulte,
1974); France in 1971 (Fournier et al., 1972); Spain in 1978 (Herrera
Marteache et al., 1978); India in 1964 (Dale et al., 1965); or Western
Australia in 1965-66 (Wassermann et al., 1968).
No endrin was found in 91 samples of adipose tissue obtained at
autopsy in Kingston, Ontario, Canada, in 1979 and 1981 or in 84
samples from Ottawa in 1980 and 1981 (Williams et al., 1984), or in
adipose tissue obtained at autopsy from 92 males and 49 females in
Ontario municipalities in 1984 (limit of detection, 2.4 µg/kg)
(Williams et al., 1988).
These results indicate that endrin is either absent or present at
very low levels in the adipose tissue of the general population. It is
therefore surprising that Kanitz & Castello (1966) reported the
presence of endrin in nine adipose tissue samples from Liguria, Italy,
at a mean concentration of 0.93 mg/kg of tissue. The highest
concentration was 2.49 mg/kg. Pavan et al. (1987) found endrin at 0.1
and 0.3 mg/kg in 2 of 92 samples of adipose tissue obtained at surgery
from people living in the Province of Turin, Italy. In areas where
endrin has been used extensively, however, such as India and the lower
Mississippi, it has never been found in human adipose tissue (Brooks,
1974).
One of 62 adipose tissue samples obtained at surgery from people
in Ciudad Juarez, Mexico, in 1977-78 contained endrin at 0.02 mg/kg
(Redetzke et al., 1983).
5.2.2.2 Organs
In samples of liver, kidney, gonad, and brain obtained from the
general population of Alberta (Canada), no residue of endrin was
detected (Kadis et al., 1970).
5.2.2.3 Blood
No endrin was detected (limit of detection, 0.01 mg/kg) in 4000
blood samples from the general US population in 1976-80 (US E P A,
1983), or in areas where endrin has been used extensively, such as
India and the lower Mississippi (Brooks, 1974), or in 26 blood samples
from the general population in Nigeria (Atuma, 1985).
5.2.2.4 Breast milk
Endrin was not detected in breast milk in studies in the USA in
1966-78 (Strassman & Kutz, 1977; Currie et al., 1979; Kutz et al.,
1979a; Barnett et al., 1979), in El Salvador and Guatemala (De Campos
& Olszyna-Marzys, 1979), in Belgium, Italy, and The Netherlands
(Kanitz & Castello, 1966; Hendrickx & Maes, 1969; Wegman & Greve,
1974), and in Japan (Yakushiji et al., 1979). No endrin was detected
(< 0.01 mg/litre) in 50 breast milk samples from mothers (aged 18-32
years) in Leiden, The Netherlands, in 1969 (Tuinstra, 1971). It was
found in one of 12 samples from mothers aged 21-37 in Pavia, Italy, in
1988, at a concentration of 0.01 µg/kg of whole milk, but not in four
samples collected in Crotone, southern Italy (Bianchi et al., 1988).
5.2.2.5 Appraisal of exposure of the general population
The occasional presence of low concentrations of endrin in the
air of areas where endrin is used in agriculture cannot be considered
a significant source of contamination for the general public. The very
low concentrations that have been found in surface and drinking-water
are also of little significance for public health.
The source of exposure that may be relevant is dietary intake.
Apart from accidental contamination, however, intake of endrin by the
general population in the countries examined has been and is still far
below the maximum acceptable daily intake of 0.2 µg/kg body weight
established by the Joint FAO/WHO Meeting in 1970 (FAO/WHO, 1971). This
applies equally to the total intake, when the intake from dietary
sources is added to that from air and water.
Endrin has not been demonstrated in the large number of samples
of organs, adipose tissue, blood, and breast milk analysed in
different countries, even in areas where endrin is or was used
extensively.
5.3 Occupational exposure during manufacture, formulation and use
5.3.1 Manufacture and formulation
Endrin has not been detected in the blood, plasma, or urine of
workers exposed occupationally to endrin (Hayes & Curley, 1968; Jager,
1970). Endrin was detected in blood only after accidental
over-exposure. Jager (1970) estimated that the threshold level of
endrin in the blood, below which signs or symptoms of intoxication do
not occur, lies between 0.05 and 0.10 mg/litre. He estimated the
half-life of endrin in the blood to be in the order of 24 h.
The total exposure of workers in a manufacturing and formulation
plant was estimated on the basis of determinations of the quantity of
the endrin metabolite anti-12-hydroxyendrin in urine. Urine of
workers exposed to endrin for seven days had concentrations of up to
360 µg/g of creatinine; no unchanged endrin was found. Assuming an
average daily excretion of 1.5 g creatinine per day, the total daily
excretion of anti-12-hydroxyendrin in the urine may be up to 540 µg.
This gives a minimal absorption of 0.5 mg endrin, indicating that
inhalation of dusts and absorption through the skin may be significant
during occupational exposures in manufacture. It is not unreasonable
to assume that, as in other species, approximately half of all the
endrin absorbed is excreted in the urine as anti-12-hydroxyendrin,
since both endrin and this metabolite are present in the faeces of
workers (Ottevanger &Van Sittert, 1979; Baldwin & Hutson, 1980 ).
Thus, 1 mg/day may be the more accurate figure for exposure in this
manufacturing plant. The concentration of anti-12-hydroxyendrin in
urine decreased more slowly than the concentrations of endrin in
blood, with a half-life of 55-75 h (Van Sittert, 1985).
5.3.2 Application
Endrin is applied in agriculture by high-pressure spraying with
a hand gun, spraying orchards with a power air blast or boom to
control mice, dusting potatoes, spraying row crops, or application
from aircraft. These methods of application result in dermal and
respiratory exposures.
The potential dermal and respiratory exposure of workers applying
endrin formulations in the field has been quantified in a few studies.
Respiratory exposure to endrin during spraying of orchards,
high-pressure spraying of crops, and piloting of aircraft varied from
0.01 to 0.14 mg/h; dermal exposure during such activities was
0.01-1.64 mg/h. The activity that caused the most exposure was dusting
potatoes, which was associated with a respiratory exposure of 0.41
mg/h and a dermal exposure of 18.7 mg/h. Total exposure, calculated as
a percentage of a toxic dose/h = {dermal exposure (mg/h) +
[respiratory exposure (mg/h) x 10]} [dermal LD50 (mg/kg) x 70] x
100, was 0.21-1.5% (Durham & Wolfe, 1962) (see Table 11). These
figures show that although endrin is acutely highly toxic it can be
used safely when reasonable precautions are taken (Wolfe et al., 1963;
Jegier, 1964; Wolfe et al., 1967; Hayes, 1975).
Endrin was not found in the blood of 20 pesticide sprayers or in
19 controls in Choluteca, southern Honduras (Steinberg et al., 1989).
5.3.3 Appraisal of occupational exposure
No residues were found in healthy workers. The range of threshold
levels of endrin in blood below which no sign or symptom of
intoxication occurs has been estimated to be 50-100 µg/litre. The
half-life of endrin in blood may be in the order of 24 h following
occupational exposure. The concentration of anti-12-hydroxyendrin in
the urine decreased more slowly than the concentration of endrin in
blood, with a half-life of 55-75 h.
Table 11. Studies on potential exposure of agricultural workers to endrin, using direct methods
Activity Exposure
Respiratory Dermal Totala (%) toxic Reference
(mg/h) dose/h
mg/m3 mg/h
Spraying orchard cover crops for mouse 0.01b 2.6 0.21 Wolfe et al. (1963)
control
High-pressure hand-gun spraying orchard 0.01b 3 0.25 Wolfe et al. (1967)
cover crops for mouse control
Operating air-blast or boom sprayers treating 0.01b 2.5 0.21 Wolfe et al. (1967)
orchard cover crops for mouse control
Dusting potatoes 0.41b 18.7 1.5 Wolfe et al. (1963)
Spraying row crops NDb,c 0.15 (0.01)d Jegier (1964)
Piloting during air application of spray 0.05 0.08b 1.18 0.29 (0.16)e Jegier (1964)
aFor a 70-kg man on the basis of dermal LD50 for male rats (18 mg/kg body weight) using the formula given
by Durham & Wolfe (1962)
bMeasured by respirator pad
cNot detected
dOriginal values calculated on the basis of maximal exposure; recalculated values shown in parentheses are
based on mean exposure.
eCalculation based on published data on dermal and respiratory exposure (note in Wolfe et al., 1967)
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and elimination
6.1.1 Laboratory animals
6.1.1.1 Oral administration
Rat: One male rat was fed 14C-endrin at a level of 30 mg/kg
of diet for 8 days. About 60-70% was excreted on the first day; after
three days, the faeces contained more then 80% of the administered
radiolabel; by day 9, 84% had been excreted; and there appeared to be
a level of saturation after 6-7 days of feeding. Only 0.5% was found
in the urine. About 75-80% of the label in the faeces was in at least
two different metabolites. The adipose tissue stored 3-4 mg/kg, giving
a storage rate of about 10 (FAO/WHO, 1971).
After female rats were given a single oral dose of 14C-endrin
at 16, 64, or 128 µg/kg body weight, excretion was rapid. The
biological half-life of the doses of 16 and 64 µg/kg was 1-2 days;
however, that of 128 µg/kg was approximately 6 days, showing that
excretion of higher doses is slower (Korte et al., 1970).
Six CFE rats of each sex were treated with a single oral dose of
0.5 mg 14C-endrin in arachis oil (approximately equivalent to
2.5-3.0 mg/kg body weight), and the radiolabel excreted in urine and
faeces was measured over 3 days. The animals were then killed and the
radiolabel measured in tissues. A sex difference was noted in the rate
of elimination in faeces: 66% of the dose was excreted in 3 days by
males and 37% by females; excretion was slower in females but tended
to increase daily between days 1 and 3. Small quantities of radiolabel
were excreted in the urine, females excreting three times more than
males. No radiolabel was found in exhaled air (Hutson et al., 1975).
The results are summarized in Tables 12 and 13.
Three rats of each sex were each given a single oral dose of 8 µg
14C-endrin in peanut oil (by gavage) daily for 12 days. A steady
state (at which the excreted amount equalled the daily intake) was
reached after about 6 days. Females stored about twice as much (27%)
as males (14%). The radiolabel was excreted mainly in the faeces:
after the first 24 h, 70-75% of the radiolabel was found in faeces as
hydrophilic metabolites; subsequently, only metabolites were present.
Four days after the last dose, males retained only 5% and females 15%
of the administered radiolabel (Klein et al., 1968; Korte et al.,
1970).
Table 12. Rates of excretion of radiolabel by rats treated with a
single oral dose of 14C-endrin (percentage of
radioactivity administered)
Sex Urine Faeces
Day 1 Day 2 Day 3 Day 1 Day 2 Day 3
Male 1.3 0.6 0.6 30.6 14.4 21.2
Female 1.8 2.5 2.9 2.3 10.7 24.2
Table 13. Recovery of radiolabel in rat tissues 3 days after a single
oral dose of 14C-endrin (percentage of radioactivity
administered)
Sex Urine Faeces Liver Kidneys Fat Skin Remaining Total
carcass
Males 2.7 66.2 1.2 0.6 1.7 2.3 12.2 86.9
Females 7.5 37.2 2.0 0.4 8.0 4.0 28.1 87.2
Rabbit: A Dutch strain male rabbit was given two oral doses of
4.7 mg 14C-endrin in olive oil at an interval of 14 days. Between
days 1 and 13, 37% of the first dose was excreted in the urine and 49%
in the faeces; the second dose was eliminated similarly. By day 49,
50% had been excreted in urine and 47% in faeces. Faecal excretion was
rapid, being almost complete within 24 h, and consisted virtually
entirely of unchanged endrin. The urine contained only metabolites
(Bedford et al., 1975a). The excretion of metabolites in rabbits thus
appears to differ considerably from that in rats: approximately half
of a dose of 14C-endrin is excreted in the urine of rabbits and
approximately 2% in rats; endrin metabolites are excreted in rat
faeces over several days (after a single oral dose), whereas in
rabbits faecal excretion is rapid, being almost complete within 24 h,
and consists virtually entirely of unchanged endrin. The probable
explanation is that the molecular weight threshold for biliary
secretion of anions is 325 ± 50 in rats and 475 ± 50 in rabbits (Hirom
et al., 1972). The glucuronide and sulfate conjugates of
monohydroxyendrin have molecular weights of 572 and 470, respectively.
Therefore, conjugates of the endrin metabolites are eliminated in the
bile and faeces of rats and in the urine in rabbits.
Dog: Three beagles were fed a diet containing endrin at a
concentration equivalent to 0.1 mg/kg body weight for 128 days; two
other animals were used as controls. The concentration of endrin in
blood was determined at weekly intervals. The time to reach a plateau
in blood was less than 1 week, and no significant increase in the
concentration of endrin in blood was found during this period. The
average concentration between day 9 and day 128 was 4 µg/litre. The
concentration of endrin in eight organs and tissues of dogs killed 7
days after termination of exposure was < 0.2 mg/kg of tissue; except
that the spleen of one dog contained 2.6 mg/kg, and adipose tissue
contained 0.2-0.8 mg/kg (Richardson et al., 1967).
6.1.1.2 Intravenous administration
Mouse: The concentrations of endrin were determined in tissues
from groups of five adult male CFI mice given endrin intravenously at
5 mg/kg body weight (LD90) in dimethyl sulfoxide. The concentrations
prior to convulsions (about 10 min after injection) were approximately
60 mg/kg in liver, 20 mg/kg in brain and omental fat, and
approximately 5 mg/litre in blood. The concentration in whole brain 15
min after an intravenous dose of 1.5 mg/kg body weight (the dose that
caused ataxia in 90% of animals; TD90) was 9.4 mg/kg. No endrin was
detected in the bile of animals with a bile fistula dosed
intravenously with the TD90 in samples collected after 0.5, 1, or 2
h (Walsh & Fink, 1972).
Rat: Male Holtzman rats with or without a bile fistula were
given a single intravenous dose of 14C-endrin at 0.25 m/kg body
weight. More than 90% of the excreted radiolabel was found in the
faeces of intact animals over the 7-day period after dosing or in the
bile of animals with fistulas over 4 days. The mean total percentage
recovery of administered radiolabel in faeces, urine, and carcasses
was 97% from intact animals 7 days after dosing, and 94% from animals
with a bile fistula 4 days after dosing (Cole et al., 1970). No
unchanged endrin was found in bile; the major metabolite was
anti-12-hydroxyendrin (see section 6.2.1).
Rapid excretion was observed in rats given two intravenous
injections of 14C-endrin at 0.1 mg/kg body weight at an interval of
4 days. Excretion of the radiolabel was exponential and occurred
mainly with faeces; only hydrophilic metabolites were present. With a
dose of 200 µg/kg body weight, male rats retained 5.2% and females
12.1% of the administered dose 20 days after the second injection. The
biological half-life of endrin after a single intravenous dose of 200
µg/kg body weight was 2.5-3 days in male rats and 4 days in females
(Klein et al., 1968; Korte et al., 1970; Brooks, 1974).
Rabbit: When rabbits were given 14C-endrin intravenously, the
radiolabel was excreted mainly in the urine and only as metabolites.
A probable explanation for the difference in excretion pattern after
oral and intravenous administration is that much lower doses
(micrograms compared with milligrams) were given intravenously (Korte
et al., 1970).
6.1.2 Domestic animals
Twelve cows were fed hay from endrin-sprayed alfalfa containing
an average of 1.9, 2.8, or 3.7 mg/kg endrin; the average daily intake
of individual animals ranged from 0.04 to 0.11 mg/kg body weight. The
average concentrations of endrin in the milk were < 0.05, 0.14, and
0.15 mg/litre, respectively. When endrin dissolved in soya bean oil
was fed to 11 dairy cows, levels > 1 mg/kg body weight were required
in order for significant quantities of endrin to be detected in milk
(Ely et al., 1957).
Dairy cows (eight Jerseys and six Guernseys) were fed diets
containing endrin at 0, 0.1, 0.25, 0.75, or 2.0 mg/kg of diet for 12
weeks. No residues were found (limit of detection, 0.01 mg/litre) in
the milk of animals that received 0.1 mg/kg, but up to 0.02 mg/litre
was found in milk of animals fed 0.25 mg/kg and up to 0.04 mg/litre
with 0.75 mg/kg of diet; the highest dose resulted in residues in milk
of 0.1 mg/litre. The endrin content of brain, heart, liver, kidneys,
body fat, and muscles was < 0.1 mg/kg, but renal fat contained up to
0.8 mg/kg (Kiigemagi et al., 1958).
The concentrations of endrin in milk of cows given feed
containing endrin at approximately 0.05, 0.14, and 0.30 mg/kg of whole
feed for 5 weeks were 0.004, 0.01, and 0.018 mg/litre, respectively
(Williams et al., 1964). A steady (plateau) level in milk was reached
after about 15 days.
The concentration of endrin in the milk of dairy cows given feed
contaminated with relatively low levels of endrin rose rapidly within
a few hours to days and levelled off at a plateau characteristic for
each concentration in the feed. The average milk:diet ratio for endrin
was 0.07 for feed levels of 0.05-0.3 mg/kg of diet (Biehl & Buck,
1987).
Two lactating cows were fed 14C-endrin for 21 days at an
overall dietary concentration of 0.1 mg/kg of diet, which was
considered to be comparable to the highest dose that cows are likely
to receive in cottonseed cake. Excretion of radiolabel in milk, urine,
and faeces reached a plateau 4-9 days after start of treatment.
Approximately 3% of the radiolabel was excreted in milk, 65% in urine,
and 20% in faeces. Unchanged endrin was not found in urine, but about
30% of the radiolabel in faeces and all of the 0.003-0.006 mg/litre
found in milk was endrin. The concentration of endrin equivalent
residues was 0.001-0.002 mg/kg in meat and 0.02-0.10 mg/kg in fat;
most of the radiolabel in fat consisted of endrin (Baldwin et al.,
1976).
Steers, hogs, and lambs fed diets containing endrin at 0, 0.1,
0.25, or 0.75 mg/kg of diet for 12 weeks had residues of < 0.1 mg/kg
in red meat, liver, and kidneys and of 0.02-0.2 mg/kg in body fat.
Feeding endrin at 2 mg/kg of diet to steers for 12 weeks resulted in
residues of 0.9 mg/kg in fat and 0.2-0.3 mg/kg in red meat, liver and
kidneys (Terriere et al., 1958). The biotransfer factors for endrin in
beef and milk were directly proportional to the octanol-water
partition coefficients, while the bioconcentration factor for endrin
in vegetation was inversely proportional to the square root of the
octanol-water partition coefficient (Travis & Arms, 1988).
Six weeks after the start of feeding seven Delaware X New
Hampshire male chicks and eight weeks after the start of feeding seven
White Leghorn pullets a diet containing endrin at 0.1 mg/kg, the
residues in eggs and meat were < 0.1 mg/kg and that in fat, 0.6
mg/kg. At a dietary level of 0.25 mg/kg, the residue levels were
0.2-0.3 mg/kg in eggs, 0.1 mg/kg in breast meat, and about 1 mg/kg in
fat. With 0.75 mg/kg of diet, the levels were 0.4 mg/kg in eggs, 0.24
mg/kg in breast meat, and 3.1 mg/kg in fat (Terriere et al., 1959).
14C-Endrin was administered daily in corn oil in gelatin
capsules to six laying hens at a concentration equivalent to 0.13
mg/kg of total diet for 21 weeks. Ingestion and elimination in eggs
and excreta were almost balanced after about 16 weeks. The residue
levels in eggs were 0.11-0.18 mg/kg and were found in the yolk; none
of the known metabolites was detected. The levels of endrin equivalent
were about 0.01 mg/kg in breast meat and 0.1 mg/kg in leg meat; higher
levels were found in the liver (0.47 mg/kg), kidneys (0.17 mg/kg), and
fat (1 mg/kg). The residues were accounted for by unchanged endrin,
except in the liver and kidneys, where part probably consisted of
polar metabolites. About 50% of the administered radiolabel was
excreted in the faeces, 10% of which was in unchanged endrin (Baldwin
et al., 1976).
6.1.3 Human beings
The concentrations of endrin in the blood of workmen exposed to
endrin were generally below the level of detection: endrin was not
found in plasma (< 3 µg/litre) or fat (< 0.03 mg/kg) of workers
exposed to endrin for an average of 88 days (Hayes & Curley, 1968). No
endrin was found in the blood of healthy people working in an endrin
manufacturing plant between 1964 and 1970, at an initial limit of
detection of 10 µg/litre, improved after 1965 to 5 µg/litre (Jager,
1970).
Residues of endrin have been found in blood only in individuals
with signs of recent intoxication or who have recently had excessive
exposure (see section 9.2.2). Endrin appears to be eliminated rapidly
from the human body.
6.1.4 Systems in vitro
Isolated liver preparations from Holtzman rats were perfused with
a solution containing 14C-endrin at 0.003 mg/ml. Within 1 h, 50% of
the radiolabel appeared in the bile; and in 6 h, more than 90% of the
total label was found (Cole et al., 1970). With the same dose,
radiolabel appeared 2-12 times faster in the bile of livers isolated
from male rats as in that of livers from females, which may account
for the lower toxicity and lower storage of endrin in adipose tissue
in male rats (Klevay, 1971). After perfusion of albino rat liver with
a physiological solution containing 40 µg of 14C-endrin, both endrin
and hydrophilic metabolites were found (Altmeier & Korte, 1969).
6.2 Biotransformation
Information on the metabolism of endrin up to 1967 was reviewed
(Soto & Deichmann, 1967; Brooks, 1969).
6.2.1 Experimental animals
A number of investigations have been carried out since 1970 to
elucidate the identity of several metabolites of endrin in rats
(Baldwin et al., 1970; Richardson et al., 1970; Hutson et al., 1975;
Bedford & Hutson, 1976), rabbits (Bedford et al., 1975a; Hutson,
1981), cows and chickens (Baldwin et al., 1976). The 12-hydroxy
derivative was reported to be present in the faeces of rats (Baldwin
et al., 1970), and the hydroxyl group was assigned tentatively as syn
to the epoxide ring. The stereochemical configuration was subsequently
shown to be anti to the epoxide group (Baldwin et al., 1973), and
this configuration was confirmed by the synthesis of anti-12-
hydroxyendrin (also called 9- anti-hydroxyendrin) (Bedford & Harrod,
1973; Bedford et al., 1986a). The chemical structures of these
compounds are shown in Figure 2; the chemical names are given in Annex
I.
Formation of anti-12-hydroxyendrin ( III), together with its
sulfate and glucuronide conjugates, is considered to be the major
route of metabolism of endrin. Four other metabolites have been
reported, but their concentrations are generally smaller than that of
anti-12-hydroxyendrin and its conjugates. These four metabolites are
syn-12-hydroxyendrin ( II; tentative identification),
3-hydroxyendrin ( IV; synthesized and structure confirmed by Bedford
et al., 1986b), 12-ketoendrin ( V), and the product of formal
hydroxylation of endrin, the 4,5- trans-dihydroisodrindiol ( VI;
tentative structure). The trans-diol ( VI) is a minor metabolite in
both rats and rabbits; it may be formed via an oxidation-reduction
pathway involving intermediates of the corresponding ketol ( VII).
Each of the hydroxy compounds is also excreted partly as its sulfate
or glucuronide in the urine of animals (Bedford et al., 1975a,b;
Hutson et al., 1975).
The three monohydroxylated derivatives of endrin, syn- and
anti-12-hydroxyendrin ( II and III) and 3-hydroxyendrin ( IV),
are the products of the action of liver microsomal monooxygenases on
endrin (Bedford & Hutson, 1976). These alcohols are also conjugated to
glucuronides and sulfates to some extent in the liver. Comparative
metabolic studies with rat liver microsome preparations have shown
that free syn-12-hydroxyendrin, but not its free anti-isomer, is
the precursor of 12-ketoendrin ( V) (Hutson & Hoadley, 1974).
Rats exhibit a sex difference in the rate of metabolism. The
major metabolite in animals of each sex is anti-12-hydroxyendrin,
which is excreted via the bile as the glucuronide; this undergoes
enterohepatic circulation and is eliminated as the aglycone in the
faeces, together with two minor metabolites, 3-hydroxyendrin and
4,5- trans-dihydroisodrindiol. Male rats produce the metabolite at a
higher rate than do females. The major urinary metabolite in male CFE
rats was 12-ketoendrin, while females excreted mainly
anti-12-hydroxyendrin O-sulfate. Endrin and the lipophilic
metabolite 12-ketoendrin were the major compounds found in the organs
and tissues of male and female CFE rats 3 days after a single oral
dose of endrin, but the ratio of endrin:12-ketoendrin was 2/1 in
females and 1/8 in males. Thus, 12-ketoendrin constituted most of the
radiolabel in the liver and kidneys of males and endrin that in the
kidneys of females (Hutson et al., 1975; Hutson, 1981).
The metabolism of endrin in rabbits is superficially different
from that in rats. The major metabolite is still
anti-12-hydroxyendrin, but it is conjugated with sulfate and
eliminated in the urine. Some syn-12-hydroxyendrin was also detected
as its sulfate in urine, and perhaps conjugation and elimination
prevented further oxidation to 12-ketoendrin. The respective
glucuronide conjugates were also eliminated in the urine, as were the
glucuronides of 3-hydroxyendrin and the 4,5- trans-diol ( VI)
(Bedford et al., 1975b; Hutson, 1981).
Studies with 14C-endrin in lactating cows showed that the
residues in milk and body fat consisted of unchanged endrin, although
traces of 12-ketoendrin were consistently found in fat. As in rats,
anti-12-hydroxyendrin was the major metabolite in urine and faeces,
the urine being the major excretory route, as in rabbits.
12-Ketoendrin and syn-12-hydroxyendrin were minor metabolites in cow
urine (Baldwin et al., 1976). Thus, although the metabolic pathways of
endrin in cows are qualitatively similar to those in rats and rabbits,
quantitative differences are seen in faecal and urinary excretion.
In hens, only endrin was found as a residue in meat, fat, and
eggs. Unchanged endrin accounted for about 10% of the radiolabel in
excreta, and the major metabolite was anti-12-hydroxyendrin and its
sulfate conjugate. No 12-ketoendrin was detected in tissues, eggs, or
excreta. The metabolism of endrin in hens is fundamentally similar to
that in rats, rabbits, and cows, except that they produce neither
syn-12-hydroxyendrin nor the related 12-ketoendrin. The rate of
metabolism, however, was much lower than in cows (Baldwin et al.,
1976). The absence of 12-ketoendrin in birds was confirmed in a study
of four species killed by endrin (Stickel et al., 1979). Hutson et al.
 (1975) suggested that the acute toxicity of endrin in birds is not
associated with the formation of 12-ketoendrin.
6.2.2 Human beings
No endrin was found (limit of detection, 0.0016 mg/litre) in 14
samples of urine from workers exposed to aldrin, dieldrin, and endrin,
even though workers with a complete work history had been exposed to
endrin for an average of 2160 h (Hayes & Curley, 1968). Endrin was not
detected in urine from five men and five women (Cueto & Hayes, 1962;
Cueto & Biros, 1967). No unchanged endrin was found in the urine of
Dutch workers exposed to endrin, but it occurred in the faeces (Jager,
1970; Baldwin & Hutson, 1980).
Neither 3-hydroxyendrin nor the diol was detected in urine or
faeces (Hutson, 1981). anti-12-Hydroxyendrin was present in the
urine of workers exposed to endrin, and the glucuronide was found in
the faeces. Concentrations of up to 0.36 mg/g of creatinine were found
in urine after 7 days, accompanied by a sharp rise in the level of
D-glutaric acid (excreted in the urine of mammals as a metabolite of
D-glucuronolactone [Marsh, 1963]), indicating that liver enzyme
induction may have occurred. The levels tended to decrease over the
weekend (Ottevanger & Van Sittert, 1979). Endrin,
anti-12-hydroxyendrin, 12-ketoendrin, and the beta-glucuronide of
anti-12-hydroxyendrin were not found in the blood of workers at a
Dutch plant for the manufacture of endrin (limit of detection, 2
µg/litre). Both endrin and anti-12-hydroxyendrin were found in the
faeces, and all urine samples contained the beta-glucuronide of
anti-12-hydroxyendrin up to a concentration of 0.14 mg/litre as
anti-12-hydroxyendrin (Baldwin & Hutson, 1980).
Hydroxylation at anti-C-12 is relatively rapid and accounts for
the rapid metabolism of endrin. Even syn-12-hydroxyendrin is
hydroxylated rapidly at its anti-C-12 position, affording
12-ketoendrin (Hutson, 1981).
As neither endrin nor its metabolites were found in the blood of
exposed, healthy workers, exposure can be measured by determining
anti-12-hydroxyendrin in urine. A quantitative relationship between
exposure to endrin and the concentration of this metabolite cannot be
established, however, owing to lack of data.
6.2.3 Microorganisms
In mixed anaerobic microbial populations developed using inocula
from soil, freshwater mud, sheep rumen, and chicken litter, endrin
(like other cyclodiene compounds) was monodechlorinated at the
methylene bridge carbon atom. Neither endrin nor any other compound
was further metabolized. The 10 obligate anaerobic bacteria that made
up the mixed population were subsequently isolated in pure culture. Of
these, only Clostridium bifermentans, C. glycolium, and other
Clostridium species were capable of dehalogenation, but at a rate
that was much slower than that of the mixed population (Maule et al.,
1987).
6.2.4 Plants
Three experiments were carried out on tobacco plants. In the
first experiment, 2.08 mg of 14C-endrin were applied to the leaves
with free aeration during the experimental period. In the second test,
the same dose was applied but with little aeration; and in the third,
plants were exposed to 1.04 mg of 14C-endrin with little aeration.
An initial residue level of 50-100 mg/kg was found on leaves in all
three experiments, but, subsequently, less residue was found on plants
with free aeration. Six weeks after treatment, 30-47% of radiolabel
was recovered in residues, which consisted of endrin and hydrophilic
substances (Weisgerber et al., 1969; Donoso et al., 1979).
The leaves of cotton plants were treated with 4.2 mg of
14C-endrin, and the application was repeated after 2 and again after
6 weeks, at which time parathion was also applied. At harvest,
two-thirds of the radiolabel had evaporated, and the total residue in
cotton seed was 0.333 mg/kg. Endrin and two groups of degradation
products were found in the plants; one of these products (possibly
delta-ketoendrin) was only slightly more hydrophilic than endrin, and
the other was very hydrophilic. Most of the metabolites were found on
the surface of the leaves. When delta-ketoendrin was applied to white
cabbage, it disappeared more slowly than endrin, with the formation of
hydrophilic metabolites (Korte, 1969).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
The interactions of halogenated pesticides and microorganisms
have been reviewed extensively (Pfister, 1972).
In three Willamette valley soils (USA) treated with endrin at 0,
1 or 10 mg/kg, no effect was found 30 days after application on the
function and activity of the microbial population, the decomposition
of native organic matter, the transformation of native soil nitrogen,
ammonification of peptone, or nitrification of ammonium sulphate
(Bollen & Tu, 1971). Even at an annual application rate of 5 lb/acre
(5.6 kg/ha) for 5 years, no effect was seen on the numbers or kinds of
soil fungi, the numbers of bacteria, the decomposition rate of organic
matter (measured by CO2 production), or the oxidation of ammonium to
nitrate (Martin et al., 1959).
Endrin at a concentration of 100 mg/kg of soil had no effect on
denitrification in soil under anaerobic incubation for 5 days at 30 °C
or in an isolated denitrifying bacterium (Bollag & Henninger, 1976).
A concentration of 1000 mg/kg had no effect on methanogenesis, sulfate
reduction, or carbon dioxide evolution in anaerobic salt-marsh
sediments (Kiene & Capone, 1984).
The growth rates of two strains of blue-green algae were
decreased in the presence of endrin at a concentration of 0.29
µg/litre (Batterton et al., 1971), and the productivity of many forms
of natural phytoplankton in estuarine waters was decreased by 46% when
they were exposed to 1 mg/litre (Butler, 1963).
7.2 Aquatic organisms
7.2.1 Invertebrates
Acute toxicity of endrin to invertebrates is given in Tables 14
and 15.
A static system was used to study the toxicity of endrin to a
polychaete worm (Nereis virens) in water and sediment, in which sea
water or sediment (containing 17% sand, 83% clay, and 2% organic
carbon) was present at a temperature of 9-10 °C for 12 days. None of
the worms in sea water died after exposure to endrin at 0.11 mg/litre
for 12 days, but two of five worms exposed to 28 mg/kg in sediment
died in this period (McLeese et al., 1982).
Table 14. Acute toxicity of endrin to freshwater invertebrates
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Red snail Static 48-h LC50 7200 Hashimoto & Nishiuchi
Indoplanorbis exustus (1981)
Marsh snail Static 48-h LC50 9500 Hashimoto & Nishiuchi
Semisulcospira libertina (1981)
Snail Static 48-h LC50 12 000 Hashimoto & Nishiuchi
Physa acuta (1981)
Water flea Static 18-23 48-h LC50 160 Thurston et al.(1985)
Daphnia magna Static 21 44 7.1 48-h LC50 4.2 Mayer & Ellersieck (1986)
Static 18 48 7.4 48-h LC50 41-74 Mayer & Ellersieck (1986)
Static 22-24 240 8.0 96-h LC50 59 Elnabarawy et al. (1986)
Water flea Static 15 44 7.1 48-h LC50 20 Mayer & Ellersieck (1986)
Daphnia pulex Static 22-24 240 8.0 96-h LC50 30 Elnabarawy et al. (1986)
Water flea Static 22-24 240 8.0 96-h LC50 24 Elnabarawy et al. (1986)
Daphnia reticulata
Water flea Static 21 44 7.1 48-h LC50 45 Mayer & Ellersieck (1986)
Simocephalus serrulatus Static 15 44 7.1 48-h LC50 26 Mayer & Ellersieck (1986)
Water flea Adult Static 21 44 7.1 48-h LC50 1.8 Mayer & Ellersieck (1986)
Cypridopsis vidua
Sow bug (isopod) Adult Static 15 44 7.1 96-h LC50 1.5 Mayer & Ellersieck (1986)
Asellus brevicaudus
Scud Adult Static 21 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
Gammarus fasciatus Adult Static 15 272 7.4 96-h LC50 1.3 Mayer & Ellersieck (1986)
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Scud Adult Static 21 44 7.1 96-h LC50 3.0 Mayer & Ellersieck (1986)
Gammarus lacustris
Crayfish 3-5 Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Orconectes nais weeks
Adult Static 21 272 7.4 96-h LC50 320 Mayer & Ellersieck (1986)
Crayfish 0.4-2.0 g Flow 18-23 96-h LC50 > 89 Thurston et al. (1985)
Orconectes immunis
Red crayfish Static 48-h LC50 300 Muncy & Oliver (1963)
Procambarus clarki
Tantytarsus Static 18-23 48-h LC50 0.84 Thurston et al. (1985)
Tantytarsus dissimilis
Glass shrimp Adult Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Palaemonetes Adult Flow 21 272 7.4 96-h LC50 0.5 Mayer & Ellersieck (1986)
kadiakensis
Stonefly Larvae Static 15 44 7.1 96-h LC50 > 0.18 Mayer & Ellersieck (1986)
Acroneuria sp.
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.076 Mayer & Ellersieck (1986)
Claasenia sabulosa
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.54 Mayer & Ellersieck (1986)
Pteronarcella badia
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.25 Mayer & Ellersieck (1986)
Pteronarcys californica
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Mayfly Larvae Static 15 44 7.1 96-h LC50 0.9 Mayer & Ellersieck (1986)
Baetis sp.
Mayfly Larvae Static 15 44 7.1 96-h LC50 62 Mayer & Ellersieck (1986)
Hexagenia bilineata
Damselfly Larvae Static 21 44 7.1 96-h LC50 2.4 Mayer & Ellersieck (1986)
Ischnura verticalis Larvae Static 21 272 7.4 96-h LC50 2.1
Snipe fly Larvae Static 15 44 7.1 96-h LC50 4.6 Mayer & Ellersieck (1986)
Atherix variegata
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
Table 15. Acute toxicity of endrin to estuarine and marine invertebrates
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Sand shrimp Static 96-h LC50 1.7 Eisler (1970a)
Crangon septemspinosa Static 96-h LC50 0.2-2.0 McLeese & Metcalfe (1980)
Static 96-h LC50 4-120 McLeese & Metcalfe (1980)
Brown shrimp Juvenile Flow 15 26 48-h LC50 0.2 Mayer (1987)
Penaeus aztecus
Pink shrimp Juvenile Flow 17 30 48-h LC50 0.2 Mayer (1987)
Penaeus duorarum Adult Flow 17 28 96-h LC50 0.037
Grass shrimp Larvae Flow 25 13 96-h LC50 1.2 Mayer (1987)
Palaemonetes pugio Juvenile Flow 25 23 96-h LC50 0.35
Adult Flow 25 21 96-h LC50 0.69
Blue crab Juvenile Flow 11 16 48-h LC50 15 Mayer (1987)
Callinectes sapidus
Hermit crab 96-h LC50 1.2 Eisler (1970a)
Pagurus longicarpus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
The mean 96-h LC50 for the oligochaetes Stylodrilus
heringianus and Limnodrilus hoffmeisteri exposed to sediment from
Lake Michigan contaminated with 14C-endrin was 2588 ± 1974 mg/kg dry
weight of sediment in four assays and 2725 ± 955 mg/kg in two assays.
The toxicity to L. hoffmeisteri appeared to be reduced in the
presence of S. heringianus. The 96-h EC50 burrowing avoidance
values were 15.3-19 mg/kg for S. heringianus and 59 mg/kg of
sediment for L. hoffmeisteri (Keilty et al., 1988a).
Sediment reworking by L. hoffmeisteri alone and with
S. heringianus was measured by monitoring the burial of a 137Cs
marker layer in sediments dosed with 12C- and 14C-endrin at
concentrations of 5.5-81 400 µg/kg of dry sediment. With low endrin
concentrations, the marker layer burial rate did not suggest
stimulation of reworking by either L. hoffmeisteri or
S. heringianus. At higher concentrations, the reworking rates were
equal to or slower than control rates at the beginning of the
experiment but decreased thereafter. The presence of S. heringianus
appeared to enhance the reworking response of L. hoffmeisteri. A
reduction in the post-experimental mortality and an increase in the
dry weight of L. hoffmeisteri in tests with the two species implies
that L. hoffmeisteri benefits from the presence of S. heringianus,
although the reverse was not observed. High concentrations of endrin
in the upper 3 cm of the final sediment showed that the worms had
transported the contaminant upward. The bioaccumulation factor for
S. heringianus ranged from 9.7 to 43.8 and was consistently three to
four times greater than that for L. hoffmeisteri (1.7-13.6) (Keilty
et al., 1988b).
The reworking rates of S. heringianus in microcosms containing
sediments dosed with 14C-endrin at 3.1-42 000 µg/kg of dry matter
were measured at 10 °C by monitoring a 137Cs marker layer buried in
contaminated and uncontaminated microcosms. Alterations in reworking
rates were observed at endrin concentrations 5.5 orders of magnitude
below the LC50 of 1650 mg/kg. At the lower concentrations, a
possible stimulatory effect on the marker layer burial rate in the
first 300-600 h was followed by a significant decrease relative to the
controls. At the higher concentrations, the rates were equal or slower
during the first 600 h and decreased dramatically in the last 600 h.
Mortality was 9.3-28% at 11 500 and 42 000 µg/kg and 0-6.7% at all the
other concentrations tested, including controls. The dry weights of
the worms at the end of the experiment were inversely related to the
high concentrations. The bioaccumulation factors ranged from 34 to 67
on the basis of grams of dry organism to grams of dry sediment (Keilty
et al., 1988c).
The effect of addition of endrin at 50 mg/kg dry weight of
sediment on protein utilization by S. heringianus was examined on
days 4, 8, 20, 28, 39, and 69. A slight increase in the relative
percentage of protein to total body weight was observed, but the
authors concluded that estimation of total protein is not a useful
measure of sublethal responses (Keilty & Stehly, 1989).
The total organic carbon content of sediment had little apparent
effect on the toxicity of endrin in the freshwater amphipod Hyalella
azteca. The 10-day LC50 for endrin in sediment (dry-weight basis)
was 4.4 µg/litre at 3.0% total organic carbon and 6.0 µg/litre at
11.2% carbon (Nebeker et al., 1989).
The EC50s in the green sea urchin (Strongylocentrotus
droebachiensis), the purple sea urchin (S. purpuratus), the red
sea urchin (S. franciscanus), and the sand dollar (Dendraster
excentricus) were 103-441 µg/litre for sperm in a static system and
221-> 362 µg/litre for embryos in a continuous flow of sea water
(temperature, 8.2-8.4 °C; salinity, 30.0 parts per thousand; pH
7.8-8.1 for the sea urchins and 12.5-13.0 °C, 30.0 parts per thousand,
and pH 8.0-8.1 for sand dollar embryos), both with an exposure time of
120 h. In a larval test of static exposure of Dungeness crab (Cancer
magister), the EC50 was 2.0 µg/litre (Dinnel et al., 1989).
Endrin was tested at 0, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.5,
5.0, and 10 mg/litre for its effects on embryos of the American oyster
(Crassostrea virginica) and their larvae. Fertilized eggs were
studied after 48 h, and survival and growth of veliger larvae were
studied in 2-day old larvae and in larvae kept for a further 12 days
at 24 °C. The results varied considerably. The estimated concentration
that would cause an approximately 50% reduction in the number of eggs
that develop into normal straight-hinge larvae, calculated by
interpolation from the data, was 0.79 µg/litre and that at which 50%
of the larvae survived was > 10.0 mg/litre (Davis & Hidu, 1969).
In the mysid shrimp Mysidopsis bahia, exposed for the complete
life cycle, acute lethality (over 96 h) was observed with endrin at
120 ng/litre; increased oxygen consumption was measurable within 24 h
of exposure. The lowest-observed-effect level for chronic lethality
was 60 ng/litre; sub-lethal effects on growth (reduced by day 4 of
exposure) and oxygen consumption (increased by day 10 of exposure)
were observed before death (over 20 days). Reduced reproductive
capacity (assessed as production of young) was observed at 30 ng/litre
over 20 days--the time to full maturity (McKenney, 1986).
Behavioural changes were observed in stoneflies (Pteronarcys
dorsata) within 4 days of exposure to 96.1% endrin at 0.07 µg/litre
and in caddis flies (Brachycentrus americanus) at 0.15 µg/litre. The
28-day LC50 was < 0.03 µg/litre for caddis flies and 0.07 µg/litre
for stoneflies (Anderson & DeFoe, 1980).
7.2.2 Fish
7.2.2.1 Acute toxicity
Endrin is highly toxic for both freshwater and marine fish. The
available data are summarized in Tables 16 and 17.
7.2.2.2 Short-term toxicity
Channel catfish (Ictalurus punctatus) were exposed continuously
to renewed solutions of endrin in water at 15 and 22 °C. Measured
endrin concentrations of 0.25-0.30 µg/litre were found to be acutely
toxic to the fish within 10 days or less. None of the fish survived
blood concentrations exceeding 0.28 mg/litre, a well-defined threshold
concentration of endrin in blood, and none died at less than 0.23
mg/litre. The concentration of endrin in the blood of fish exposed to
lethal concentrations in water for periods insufficient to cause death
were markedly lower than that in fish that died from exposure to the
same water (Mount et al., 1966).
The 28-day LC50 for 96.1% eldrin in bullheads (Ictalurus
melas) was 0.10 µg/litre (Anderson & DeFoe, 1980).
In larval fathead minnows (< 24 h old) exposed continuously to
endrin (98%) for 28-30 days in a flow-through system, growth was the
most sensitive parameter. A 48-h exposure to 0.62 µg/litre caused
significant reduction in growth, and survival was reduced at 1.21
µg/litre; with a 72-h exposure, growth was reduced at 0.63 µg/litre,
and all fish died at 1.15 µg/litre. Continuous exposure to 0.38
µg/litre for 30 days significantly reduced growth, and all fish died
at 0.73 µg/litre (Jarvinen et al., 1988).
Sheephead minnows (Cyprinodon variegatus) were exposed
continuously for 23 weeks to endrin from the embryonic stage through
hatching, until adulthood and spawning. The average exposure
concentrations were 0 (control), 0.027, 0.077, 0.12, 0.31, and 0.72
µg/litre. The resultant progeny were monitored to determine effects on
their survival, growth, and reproduction. Embryos exposed to 0.31 and
0.72 µg/litre hatched early; all fry exposed to 0.72 µg/litre died by
day 9 of exposure. At 0.31 µg/litre, fry were initially stunted and
some died. Survivors seemed unaffected until maturity, when some
females died during spawning; fewer eggs were fertile, and survival of
exposed progeny was decreased. No significant effect was observed
throughout the life cycle at an exposure concentration of 0.12
µg/litre (Hansen et al., 1977).
Endrin was tested in flagfish (Jordanella floridae) at 0.21,
0.29, and 0.39 µg/litre for 30 days. Only the highest concentration
decreased survival, and the two highest dose levels affected the mean
number of eggs produced (Hermanutz et al., 1985).
Table 16. Acute toxicity of endrin to freshwater fish
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Tilupa sp. Static 15 44 7.1 96-h LC50 12 Mayer & Ellersieck (1986)
Coho salmon Static 16 44 7.1 96-h LC50 0.089 Mayer & Ellersieck (1986)
Oncorhynchus kisutch 1.9 g Static 20 96-h LC50 0.27 Katz & Chadwick (1961)
Chinook salmon 6-8 g Static 20 96-h LC50 0.92 Katz & Chadwick (1961)
Oncorhynchus
tshawytscha
Cutthroat trout 1.0 g Static 13 44 7.1 96-h LC50 > 1.0 Mayer & Ellersieck (1986)
Salmo clarki
Rainbow trout 1.0 g Static 13 44 7.1 96-h LC50 0.75 Mayer & Ellersieck (1986)
Oncorhynchus mykiss 1.0 g Static 13 272 7.4 96-h LC50 0.74
1.4 g Static 2 44 7.1 96-h LC50 2.4
1.4 g Static 7 44 7.1 96-h LC50 1.4
1.4 g Static 13 44 7.1 96-h LC50 1.11
1.4 g Static 18 44 7.1 96-h LC50 0.75
0.6-8.0 g Flow 18-23 96-h LC50 0.3 Thurston et al. (1985)
Goldfish 1-4 g Flow 12 314 7.6 96-h LC50 0.44 Mayer & Ellersieck (1986)
Carrassius auratus Flow 18-23 96-h LC50 0.95 Thurston et al. (1985)
Static 48-h LC50 1.0 Hashimoto & Nishiuchi
(1981)
Carp Flow 12 314 7.6 96-h LC50 0.32 Mayer & Ellersieck (1986)
Cyprinus carpio Static 48-h LC50 0.84 Hashimoto & Nishiuchi
(1981)
Medaka Static 48-h LC50 1.4 Hashimoto & Nishiuchi
Oryzias latipes (1981)
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Pond loach Static 48-h LC50 4.9 Hashimoto & Nishiuchi
Misgurnus (1981)
anguilicaudatus
Fathead minnow 1.2 g Static 18 44 7.1 96-h LC50 1.8 Mayer & Ellersieck (1986)
Pimephales promelas 0.9 g Flow 12 314 7.6 96-h LC50 0.24
Static 24-h LC50 12 Kagan et al. (1986)
Larvae Static 25-26 46 7.1-8.3 96-h LC50 0.7 Jarvinen et al. (1988)
0.2-1.0 g Flow 18-23 96-h LC50 0.65 Thurston et al. (1985)
Bluntnose minnow Static 96-h LC50 0.29 Johnson (1968)
Pimephales notatus
Black bullhead 1.5 g Static 24 44 7.1 96-h LC50 1.13 Mayer & Ellersieck (1986)
Ictalurus melas
Channel catfish 5.2 g Static 18 44 7.1 96-h LC50 1.9 Mayer & Ellersieck (1986)
Ictalurus punctatus 1.4 g Static 24 44 7.1 96-h LC50 0.32
Mosquito fish 0.6 g Static 17 44 7.1 96-h LC50 1.1 Mayer & Ellersieck (1986)
Gambusia affinis 0.222 g Static 25 96-h LC50 5.27 El-Sebae (1987)
Bluegill 1.5 g Static 18 44 7.1 96-h LC50 0.61 Mayer & Ellersieck (1986)
Lepomis macrochirus 0.5 g Static 18 272 7.4 96-h LC50 0.53
1.3 g Static 7 44 7.1 96-h LC50 0.73
1.3 g Static 13 44 7.1 96-h LC50 0.68
1.3 g Static 18 44 7.1 96-h LC50 0.41
1.3 g Static 24 44 7.1 96-h LC50 0.37
1.3 g Static 29 44 7.1 96-h LC50 0.19
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Largemouth bass 2.5 g Static 18 272 7.4 96-h LC50 0.31 Mayer & Ellersieck (1986)
Micropterus salmoides
Yellow perch Flow 12 314 7.6 96-h LC50 0.15 Mayer & Ellersieck (1986)
Perca flavescens
Tilapia 1.1 g Static 24 44 7.1 96-h LC50 < 5.6 Mayer & Ellersieck (1986)
Tilapia mossambica
Tilapia (Behera strain) 0.825 g Static 25 96-h LC50 10.09 El-Sebae (1987)
Tilapia zilli
Tilapia (Alexandria
strain) 0.825 g Static 25 96-h LC50 0.26 El-Sebae (1987)
Tilapia zilli
Guppy Static 20 96-h LC50 0.9 Katz & Chadwick (1961)
Poecilia reticulata
Flagfish 2-3 days Static 24-26 43-48 6.9-7.8 96-h LC50 0.85 Hermanutz et al. (1985)
Jordanella floridae
aStatic, static condition (water unchanged for the duration of the test); flow, flow-through conditions; endrin
concentration in water maintained continuously.
Table 17. Acute toxicity of endrin to estuarine and marine fish
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
American eel 57 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Anguilla rostrata
Atlantic riverside 54 mm Static 20 24 96-h LC50 0.05 Eisler (1970b)
Menidia menidia
Blue head 90 mm Static 20 24 96-h LC50 0.1 Eisler (1970b)
Thalassoma bifasciatum
Gulf menhaden Juvenile Flow 27 29 24-h LC50 0.8 Mayer (1987)
Brevoortia patronus
Sheepshead minnow Juvenile Flow 14 30 48-h LC50 1.0 Mayer (1987)
Cyprinodon variegatus Juvenile Flow 30 24 96-h LC50 0.34
Adult Flow 18 18 96-h LC50 0.38
Adult Flow 30 16 96-h LC50 0.36
Longnose killifish Juvenile Flow 25 19 24-h LC50 0.23 Mayer (1987)
Fundulus similis
Striped killifish 40 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
Fundulus majalis
Mummichog 51 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Fundulus heteroclitus
Sailfin molly Adult Flow 20 27 96-h LC50 0.63 Mayer (1987)
Poecilia latipinna
Spot Juvenile Flow 12 24 48-h LC50 0.3 Mayer (1987)
Leiostomus xanthurus
Table 17. (contd)
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Striped mullet Juvenile Flow 14 30 48-h LC50 0.4 Mayer (1987)
Mugil cephalus 83 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
White mullet Juvenile Flow 29 48-h LC50 2.6 Butler (1963)
Mugil curema
Northern puffer 131 mm Static 20 24 96-h LC50 3.1 Eisler (1970b)
Sphaeroidus maculatus
Striped bass 2.7 g Static 16-18 28 96-h LC50 0.09 Korn & Earnest (1974)
Morone saxatilis
Shiner perch 1.2-11 g Static 13 26 96-h LC50 0.8 Earnest & Benville (1972)
Cymatogaster aggregata 1.2-11 g Int. flow 13 26 96-h LC50 0.12
Dwarf perch 1.2-11 g Static 13 18 96-h LC50 0.6 Earnest & Benville (1972)
Micrometrus minimus 1.2-11 g Int. flow 13 28 96-h LC50 0.13
Threespine stickleback 0.3 g Static 20 25 96-h LC50 1.5 Katz & Chadwick (1961)
Gasterosteus aculeatus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; int. flow,
intermittent flow-through conditions; endrin concentration in water maintained continuously
7.2.2.3 Studies of resistance
Populations of mosquito fish (Gambusia affinis) developed high
levels of resistance to endrin and other cyclodiene insecticides as a
result of inadvertent exposure to agricultural sprays. Susceptible
fish (male) showed a LC50 of 8.3 mg/litre and resistant fish, 161
mg/litre. Genetic crossing studies show that endrin resistance is
inherited as a single, autosomal, intermediate gene (Yarbrough et al.,
1986).
Pesticide-susceptible and -resistant mosquito fish were exposed
to 14C-endrin at 20 or 1000 µg/litre, and liver and brain were
assayed to determine any difference in distribution, uptake, and nerve
binding patterns (Fabacher & Chambers, 1976). The results are
summarized in Table 18. Endrin was taken up faster by brain and liver
from susceptible fish than resistant fish. In resistant fish, at least
at a high lethal concentration (1000 µg/litre), endrin entered the
brain slowly and accumulated in the liver, suggesting a more efficient
blood-brain barrier in resistant than in susceptible fish. Extraction
studies provided some evidence that endrin binds more readily to
nonessential protein complexes in the nervous tissue of resistant
fish, consequently decreasing the amount of endrin available to
produce a toxic effect.
Table 18. Mean quantities of endrin (in mg/kg tissue) in brain and
liver of susceptible and resistant mosquito fish
Genotype At 20 µg/litre At 1000 µg/litre
Brain Liver Brain:liver Brain Liver Brain:liver
Susceptible 16.98 33.28 0.51 149.31 160.27 0.93
Resistant 8.83 16.84 0.52 57.52 353.42 0.16
Susceptible: 1.90 1.90 1.00 2.60 0.45 5.80
resistant
Cell membrane fractions from resistant mosquito fish bound more
endrin than those from susceptible fish, and mitochondria from the
liver of the resistant genotype bound less endrin than those from
susceptible fish. Differences in endrin uptake, retention of endrin by
brain cell membranes, a blood-brain barrier, and a structural
difference in myelin mayaccount for the resistance of some mosquito
fish to endrin (Wells & Yarbrough, 1972).
In resistant and non-resistant populations of golden shiner
(Notemigonus crysoleucas), blue gill sunfish (Lepomis macrochirus),
and green sunfish (Lepomis cyanellus), the median tolerated limit at
36 h was 3.0, 1.5, and 3.4 µg/litre for non-resistant strains and 310,
300, and 160 µg/litre for resistant fish, respectively (Ferguson et
al., 1964).
7.2.2.4 Interaction with other chemicals
The joint action of endrin with malathion on mortality in
flagfish (Jordanella floridae) consisted of enhanced effects at
concentrations that had no effect when the substances were tested
individually. The effects of the mixture on growth followed a simple
additive model. Malathion did not modify the effect of endrin on egg
production. In a separate test, malathion did not affect the uptake or
elimination of endrin (Hermanutz et al., 1985).
In a study of the interaction between the accumulation and
elimination of 14C-endrin and 14C-DDT in mosquito fish (Gambusia
affinis), fish about 4 cm long were exposed to a nominal
concentration of 3.94 nM endrin or DDT, or to a mixture of the
compounds. Prior exposure to DDT for 4 h generally reduced the
accumulation of endrin in serum, gall-bladder, and whole bodies,
whereas prior exposure to endrin for 4 h had little effect on DDT
accumulation. Simultaneous exposure to DDT and endrin reduced the
accumulation of DDT in the gall-bladder over the 4 h of exposure and
in the whole bodies during the first hours, and it reduced the
accumulation of endrin in gall-bladder and in the whole body. Endrin
levels in fish exposed subsequently only to DDT or DDE were
significantly higher in gall-bladder and were reduced in the whole
body over 4 h. The interactions observed may be the result of
competition for and/or displacement of insecticides from mutual
binding sites (Denison et al., 1985).
In a study of the relative binding and competition between
organochlorine pesticides for serum binding sites, incubation with
serum from mosquito fish led to their association primarily with the
vitellogenin/lipoprotein and albumin fractions. Preincubation of serum
with endrin significantly reduced the quantity of 3H-DDT that was
bound subsequently, while the reverse was not observed. Although the
reason for the apparent quantitative decrease in binding is unknown,
this phenomenon may be of toxicological importance (Denison &
Yarbrough, 1985).
7.2.2.5 Special studies
Fingerlings of carp (Cyprinus carpio) exposed to endrin at the
LC50 (0.0065 mg/kg) for 24 h showed clear inhibition of ý-amylase
activity in the liver (Datta & Ghose, 1985).
A group of 240 rainbow trout (Salmo gairdneri) were exposed to
endrin at 0.12-0.15 µg/litre for 30 days; one untreated and one
solvent control group were used. On day 30, 10 fish from each group
were sacrificed and examined for the ability of peritoneal macrophages
to phagocytize latex beads. The remaining fish were immunized with 10
µg of Yersinia ruckeri O-antigen and exposure to endrin continued.
Assays for migration inhibition factor, plaque forming cells, and
serum agglutination titre were performed 2, 14, and 30 days after
inoculation, and serum was collected from all fish to determine the
cortisol concentration. Exposure to endrin had no effect on the
phagocytic ability of peritoneal macrophages, but the responses in the
three assays were significantly reduced in comparison with the control
values. Serum cortisol concentrations were significantly elevated in
the endrin-treated fish. The study did not, however, elucidate the
mechanism of immune suppression, other than showing that a stress
response had occurred (Bennett & Wolke, 1987a). In another study,
therefore, control fish were fed cortisol at 20 mg/kg and metyrapone
at 35 mg/kg body weight, and endrin-exposed fish received metyrapone
at 35 mg/kg body weight per day in the diet. The fish that received
cortisol had significantly reduced responses in all three assays; but
in the endrin-exposed fish that received metyrapone, the migration
inhibition factor response was completely restored, the plaque forming
cell response was restored to 61%, and serum agglutination titres to
69%. These results indicate that elevated serum cortisol concentration
plays a central role in repressing the immune response (Bennett &
Wolke, 1987b).
The concentrations of serum glucose, liver and muscle glycogen,
cortisol, protein, and cholesterol were determined in carp (Cyprinus
carpio) exposed to endrin at 2 µg/litre for 6, 24, and 72 h. Only
the concentration of cortisol in serum was clearly decreased (Gluth &
Hanke, 1985).
7.2.3 Amphibia
The acute toxicity of endrin to amphibians is summarized in
Table 19.
7.3 Terrestrial organisms
The acute oral toxicity of endrin for terrestrial animals is
high. The available LD50 values are summarized in Table 20.
7.3.1 Honey bees
The 48-h LD50 of endrin in worker honey bees (Apis mellifera)
using a dusting technique was 2.02 µg/bee (Atkins et al., 1973). The
LD50 for bees after contact was 0.65 µg/bee, and the acute oral
LD50 was 0.46 µg/bee (Oomen, 1986).
Table 19. Acute toxicity of endrin to amphibians
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCo3/l) (mg/1) (mg/l)
Bullfrog 2-5 g Flow 18-23 96-h LC50 2.5 Thurston et al. (1985)
Rana catesbiana
Leopard frog Eggs Flow 20 100 7.2-7.5 24-h LC50 2.5 Hall & Swineford (1980)
Rana spenocephala Larvae Flow 20 100 7.2-7.5 96-h LC50 6
Subadult Flow 20 100 7.2-7.5 96-h LC50 5
Frog 0.5 g Static 14 20 6.2 96-h LC50 0.21 Khangarot et al. (1985)
Rana hexadactyla
Western chorus frog Tadpole Static 15 44 7.1 96-h LC50 120 Mayer & Ellersieck (1986)
Pseudacris triseriata
Fowlers toad Tadpole Static 15 44 7.1 96-h LC50 180 Mayer & Ellersieck (1986)
Bufo woodhousei
fowleri
aStatic,static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin concentration in water
maintained continuously
Table 20. Acute oral LD50s of endrin for terrestrial species
Species LD50 Reference
(mg/kg body weight)
Birds
Mallard 5.6 (2.7-11.7) Hudson et al. (1984)
(Anas platyrhynchos)
Pigeon 2.0-5.0
(Columbia livia)
Pheasant 1.8 (1.1-2.8)
(Phasianus colchicus)
Sharp-tailed grouse 1.06 (0.552-2.04)
(Pedioecetes phasia
nellus)
California quail 1.19 (0.857-1.65)
Redwinged blackbird 2.37 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling 2.37-3.16
(Sturnus vulgaris)
Quail 4.22
(Coturnix coturnix)
Mammals
Big brown bat 5-8 Luckens & Davis (1965)
(Eptesicus fuscus)
Pine mouse (Microtus 2.6/19.0 Petrella et al. (1975)
pitymys pinetorum) 1.3/36.4 Webb et al. (1973)
(susceptible/
resistant)
7.3.2 Birds
7.3.2.1 Acute toxicity
The LD50s of endrin for some bird species are given in Table
20.
7.3.2.2 Short-term toxicity
Groups of 40 one-day-old quail were fed endrin at dietary levels
of 0, 0.5, 1, 5, 10, 20, or 50 mg/kg of diet. Survival was adversely
affected in all test groups, and there were no survivors beyond two
weeks among birds fed 10 mg/kg or more. Food consumption was
abnormally low, and symptoms involved lack of muscular coordination,
tremors, and occasional convulsive movements. Similar results were
obtained in 40 one-day-old pheasants fed endrin at dietary levels of
5 or 20 mg/kg, none of which survived beyond 8 days (Dewitt, 1965).
Groups of 20 seven-day-old chicks were unaffected by diets
containing endrin at 0, 1.5, or 3 mg/kg. When the concentration was
increased to 6 or 12 mg/kg, the birds became highly excitable and
failed to gain weight in comparison with controls. The survival rates
over a 12-week period were 85 and 5%, respectively, compared with 100%
in the controls (Sherman & Rosenberg, 1954).
The LC50 values for 2-3-week-old bobwhite quail (Colinus
virginianus), Japanese quail (Coturnix coturnix japonica),
ring-necked pheasants (Phasianus colchicus), and mallards (Anas
platyrhynchos) (8-13 birds per group) fed endrin in their diet for
5 days followed by 3 days of untreated diet, were 14-22 mg/kg diet
(Hill et al., 1975; Hill & Camardese, 1986).
7.3.2.3 Studies of reproduction
In a study of reproduction in pheasants, a diet containing endrin
at 10 mg/kg reduced egg production and chick survival; diets
containing up to 2 mg/kg did not affect egg production, fertility,
hatchability, or chick survival (Dewitt, 1965).
Groups of five female and two male mallard ducks (Anas
platyrhynchos) were administered diets containing endrin at 0, 0.5,
or 3.0 mg/kg for a 12-week oviposition period. Egg production was not
affected. The eggs were incubated, and infertile eggs, embryo
survival, and hatchability were measured. Fertility and hatchability
were not affected, although a 9.6% drop in embryo survival was
observed in the group that had received the highest dose. Endrin
residues in body fat were 3.4 mg/kg of tissue in the group that
received 0.5 mg/kg and 19.3 mg/kg in the group that received 3.0
mg/kg. The concentrations were higher in females than in males. The
endrin residue levels in eggs were none detected in the controls, 0.43
mg/kg in the group fed 0.5 mg/kg, and 2.75 mg/kg in the group fed 3.0
mg/kg (Roylance et al., 1985).
Three groups of 27 pairs of mallards were fed endrin at 0, 1, or
3 mg/kg of dry duck mash from December to the summer to investigate
the influence on reproduction and health. Birds fed 1 mg/kg reproduced
as well as the controls; they had significantly greater success in
hatching fertile eggs than did those fed 0 or 3 mg/kg and their
clutches hatched earlier (not significantly) than those of birds fed
3 mg/kg. Endrin accumulated in eggs to a mean level of 1.1 mg/kg (wet
weight) in the group fed 1 mg/kg and 2.9 mg/kg in the group fed 3
mg/kg. The concentration of endrin in adipose tissue was four to seven
times higher than that in eggs (Spann et al., 1986).
7.3.2.4 Interaction with other chemicals
The toxicity of combinations of chlordane and endrin was studied
in 14-week-old male and female bobwhite quail. Eight birds received
10 mg/kg chlordane in the diet for 10 weeks; 20 quail were treated
with 10 mg/kg chlordane for 10 weeks followed immediately by 10 mg/kg
endrin (98%) in the diet; a fourth group of 20 birds received only 10
mg/kg endrin in the diet. The pesticides were dissolved in propylene
glycol. After 9-10 days on a control diet, survivors were sacrificed
and their brains dissected. No deaths occurred among the birds fed the
control diet or 10 mg/kg chlordane. With endrin alone, 15 birds died,
and with the combination 14 birds died. In birds that received endrin
alone, the residue levels in the brain were 0.34-1.84 mg/kg in those
that died and 0.28-0.62 mg/kg in the survivors. In the birds fed
chlordane and endrin, the residue levels were 0.17-1.25 mg/kg in birds
that died and 0.14-0.56 mg/kg in survivors. Birds treated with the
combination had considerably more chlordane residues in their brains
than did those fed chlordane alone. The main conclusion of this study
was that the additive toxicity of closely related chemicals should be
taken into account in diagnosing cause of death (Ludke, 1976).
7.3.2.5 Special studies
The influence of endrin at 5 and 10 mg/kg of feed on the
activity of various enzymes in the serum of juvenile cockerels was
studied. The greatest increases in activity were measured for
glutamate oxalacetate transaminase, cholinesterase, and alkaline
phosphatase. Smaller increases were observed for creatine kinase,
glutamate dehydrogenase, ý-hydroxybutyrate dehydrogenase, and
phosphohexose isomerase (Horn et al., 1987).
7.3.2.6 Behavioural studies
The effect of a sub-lethal dose of endrin (2 mg/kg diet) on
avoidance responseswas studied in eight pens of 25 seven-day-old
Coturnix quail chicks for 14 days. The stimulus used to elicit
avoidance was a moving silhouette, and the response was measured
daily. Group avoidance response was significantly suppressed by
exposure to endrin, but the behaviour returned to normal after 2 days
on untreated diet (Kreitzer & Heinz, 1974).
Adult male bobwhite quail (Colinus virginianus) were fed a diet
containing endrin at 0.1 or 1.0 mg/kg for 138 days (beginning at 3
days of age), and then their performance in five non-spatial
discrimination reversal tasks was studied. Treated birds made 36-139%
more errors than controls, and birds fed the lower dose made
significantly more errors than those given the higher dose after
reversal 3 or 4 in the first three tests. The effects of endrin were
reversed after 50 days on untreated feed. The principal effect of
endrin was to impair the birds' ability to solve a novel problem. The
mean levels of endrin residues in the brain were 0.075 mg/kg wet
weight in those given the lower dose and 0.35 mg/kg for those on the
higher dose (Kreitzer, 1980).
7.3.3 Mammals
7.3.3.1 Toxicity
The LC50 values for short-tailed male and female shrews
(Blarina brevicauda) aged 180, 105-150, and 30-75 days were 87-174
mg/kg diet for 14 days (Blus, 1978).
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus maniculatus) of various ages were fed endrin at 0, 1, 2,
4, or 7 mg/kg of diet for intermittent periods, between which the
animals were either fed a normal diet or were subjected to 48-h
starvation. The animals were sacrificed by exposing them to cold
stress at -16 °C and the time of death recorded. No influence was
found on litter production, frequency, or mean litter size. At the
higher levels of feeding, postnatal mortality before weaning was
increased. Significant parental mortality occurred at 4 mg/kg and
higher and appeared to be dose-dependent (Morris, 1968). (Remark:
Since the animals in this study were captured in the field and the
periods of feeding alternated with short periods of starvation in an
effort to simulate possible conditions in the field, this study is of
only limited value).
The effects of endrin at 8.0 oz/acre (0.56 kg/ha) on unenclosed
field populations of meadow voles (Microtus pennsylvanicus) and deer
mice (Peromyscus maniculatus) were investigated in 1966-68. Animals
were trapped live on adjacent 7-acre (2.8 ha) plots each summer at
regular intervals, before and after a single application of endrin.
Immediate, significant declines in the number of voles were seen on
the experimental plot, but no long-term toxicological effects were
observed. The population rapidly recovered, exceeding the initial and
control numbers in all three years. The experimental vole population
thus appears to have responded to endrin as it would to a local
depopulation by trapping. The mouse population decreased significantly
after the application of endrin in 1966 and did not recover, and the
highly unstable, transitory population on the experimental plot
indicated a long-term toxicological effect (Morris, 1970, 1972).
7.3.3.2 Studies of resistance
14C-Endrin in corn oil was administered to a resistant and a
susceptible strain of pine mice (Microtus pitymus pinetorum) orally
at 0.5 mg/kg body weight, as follows: days 1-5, unlabelled endrin;
days 6-14, 14C-endrin; and day 15 unlabelled endrin. Total recovery
of 14C in both faeces and urine was 76% for the resistant strain and
53% for the susceptible strain. The two strains produced the same
major faecal and urine metabolite, but the resistant strain produced
about twice the quantity as the susceptible strain. The quantitative
differences in the excretion of more polar endrin metabolites may
indicate metabolic differences between the two strains and,
consequently, the greater tolerance of the resistant strain to toxic
effects (Petrella et al., 1975). The major metabolite was identified
as anti-12-hydroxyendrin; one of the other more polar metabolites,
found in minor quantities, was suggested to be a tertiary alcohol of
endrin (Petrella et al., 1977).
The degree of toxicity of endrin in first-generation progeny of
susceptible and resistant strains and a cross of the two strains of
pine mice was studied by Webb et al. (1973). The LD50 for offspring
of susceptible x susceptible parentage was 5.0 mg/kg body weight; that
for resistant x resistant, 21.1 mg/kg; and that for susceptible x
resistant, 8.6 mg/kg. These results offer preliminary support for a
genetic mechanism with intermediate dominance. An increase in
resistance against the toxic effects of endrin was demonstrated in
wild pine mice trapped in orchards where endrin had been used for
years. The oral LD50 in susceptible mice was about 3 mg/kg body
weight and that in resistant mice, an average of 36 mg/kg body weight.
The increased resistance appeared to be heritable in the first
generation (Webb & Horsfall, 1967; Webb et al., 1973). Although
differences in the rate of metabolism of endrin could be demonstrated,
especially in the activity of mixed-function oxidase, these did not
appear to be sufficiently large to explain the resistance (Hartgrove
et al., 1977).
7.4 Effects in the field
Episodes have been reported in which endrin was concluded to be
the cause of death in fish and birds. Numerous fish kills were
reported from the sugar-cane growing areas of Louisiana in 1960-63. No
association with variables such as dissolved oxygen, pH, or
temperature was found, but following the development of sensitive
analytical techniques it was concluded that the fish had been killed
by endrin (Mount & Putnicki, 1966). Surface runoff from fields was
reported to be the main source of the endrin that contaminated the
rivers (Lauer et al., 1966), although effluent from an insecticide
plant may have contributed since the fish contained two chemicals
involved in endrin manufacture (Mount & Putnicki, 1966). Levels of
endrin found in studies of fish in the wild are given in section
5.1.4.2.
Declines in the population of brown pelicans in Louisiana were
attributed to endrin, although at least six other organochlorine
pesticides and polychlorinated biphenyls were found in the animals
(Blus et al., 1975; King et al., 1977). The eggs of brown pelicans
(Pelecanus occidentalis) in Texas, USA, were examined for endrin
residues in 1975-81. The compound was recovered only in 1975, in 15 of
18 eggs, at levels of 0.1-0.3 mg/kg. In the same year, the highest
levels of endrin were found in pelican eggs in Louisiana, and this
maximum coincided with the deaths of large numbers of brown and white
pelicans (P. erhyrorhyncos) (King et al., 1985).
Endrin was found in one of ten eggs of the American white pelican
(Pelecanus erythrorhynchos) collected in 1969, at 0.20 mg/kg, and in
two of 35 samples collected in 1981, at up to 0.18 mg/kg wet weight.
Brains of pelicans found dead in the period 1975-81 had levels up to
0.80 mg/kg. No endrin was found in eggs of the western grebe
(Aechmophorus accidentalis) collected in 1981. It was concluded that
endrin had caused some of the deaths among pelicans in California
(Boellstorff et al., 1985).
The death of sandwich terns in The Netherlands was attributed to
the discharge of a combination of endrin and related pesticides into
an estuary from a manufacturing plant (Koeman et al., 1967, 1969;
Koeman, 1971).
On several occasions in Victoria, Australia, large numbers of
wild birds, in particular pigeons (Columba livia), sparrows (Passer
domesticus) and Indian mynahs (Gracula religiosa), were observed
to be paralysed or in convulsions (Reece et al., 1985). The crops and
livers contained endrin at levels of up to 1.2 mg/kg.
Fulvous whistling ducks (Dendrocygna bicolor), which nest in
rice fields along the south-eastern coast of Texas, USA, suffered a
major decline in population in the late 1960s, which was attributed to
exposure to dieldrin or aldrin. Organochlorine pesticides were
determined in 1983 in the carcasses of 15 adult ducks immediately
after their arrival in Texas from Mexico in the spring and before
departure from Mexico in the autumn. Four of the ducks with high
levels of dieldrin residues also had residues of endrin; and four
other ducks, collected in 1967 and 1969, had endrin residues. The
geometric mean levels in the different years were 0.03-0.08 mg/kg wet
weight; in juveniles in 1960-69, the geometric mean level was 0.16
mg/kg (Flickinger et al., 1986).
The effects of endrin on wildlife were studied in 1981-83 in
fruit orchards in Washington, USA. A single application of endrin
after harvest resulted in acute and chronic toxicity to a variety of
avian species; most deaths occurred soon after the application, but
several raptors died during the spring and summer. The brains of 73 of
125 birds contained endrin at < 0.10-0.80 mg/kg; detectable levels
occurred most frequently in the brains of galliforms and falconiforms.
The species in which the greatest numbers of deaths attributed to
endrin occurred include California quail (Callipepta california),
chukars (Alectoris chukar), and common barn owls (Tyto alba). Of
the 97 eggs analysed, 68 contained detectable endrin residues: 51 had
levels of < 0.10 mg/kg, and the eggs of 10 species contained
0.01-0.17 mg/kg wet weight (range, none detected to 1.67). The authors
concluded that endrin was toxic to wildlife, although there was no
evidence that it affected reproductive success or population level
(Blus et al., 1989).
Levels found in birds in the wild are also given in section
5.1.4.1.
7.5 Appraisal of effects on organisms in the environment
Use of endrin in agriculture is the major source of its presence
in the environment, but discharge of waste material from manufacturing
and formulating plants has contributed to local contamination.
World-wide monitoring surveys have shown that the concentrations of
endrin in the biosphere are generally very low (Table 10), both
absolutely and relatively: The levels of residues of other
organochlorine compounds, particularly DDE and polychlorinated
biphenyls, are generally 100 times or higher than those of endrin.
Toxicologically significant levels of endrin residues have been
found locally in fish and other organisms, particularly in cases in
which endrin was applied near rivers and lakes and when runoff
occurred into waterways. Residues may also occur when endrin is used
as a seed dressing or in bait to control rodents.
The most serious adverse ecological effects that have been
reported were the fish kills (and associated adverse effects on brown
pelican populations) in the Mississippi River system in the USA.
Although the initial evidence for ascribing these effects to endrin
was circumstantial, the results of analyses of dead fish were
considered to confirm a causal relationship; there is little doubt
that endrin was a contributory factor in at least some of these fish
kills. The evidence that endrin was the primary cause of the decline
in the brown pelican population is less convincing, since the harmful
effects on reproductive success have been attributed to DDE and other
factors (Blus et al., 1974, 1979).
In summary, agricultural application of endrin should be such as
to avoid or minimize contamination of waterways, either by
overspraying or runoff or by leaching from dressed seed in
rice-growing areas. The effects of the use of baits containing endrin
for rodent control on non-target organisms should be assessed in the
light of local circumstances. Finally, effluents from manufacturing
and formulating plants must be treated adequately before being
discharged into waterways.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO
The toxicology and risk assessment of endrin have been reviewed
(US EPA, 1987a,b; Anon., 1988a,b).
8.1 Acute toxicity of technical-grade endrin
8.1.1 Oral administration
Endrin is highly toxic when given by the oral route and is more
acutely toxic to mammals than its stereoisomer dieldrin (WHO, 1989),
with an acute oral LD50 of 7.5-17.8 mg/kg body weight (Table 21),
compared with 50-60 mg/kg for dieldrin. There appears to be a
sex-dependent sensitivity to the acute effects of endrin, female
animals being more sensitive than males. A species-dependent
sensitivity has also been reported, monkeys and cats being more
susceptible than mice and rats.
Signs of intoxication may include increased irritability and
tremor, followed by tonic-clonic convulsions, ataxia, dyspnoea,
gasping, and cyanosis. Convulsions usually occur 30-60 min after an
oral dose, and death may occur within 24 h after the administration of
a lethal dose (Speck & Maaske, 1958). Animals that survive poisoning
recover completely with no delayed or persistent effect.
8.1.2 Dermal administration
The acute dermal LD50s for technical endrin in various animal
species are given in Table 22. Endrin is highly toxic when applied as
a solution in hydrocarbon solvents but moderately toxic when applied
as a dry powder. The signs of poisoning are similar to those seen
after oral administration.
8.1.3 Parenteral administration
The acute LD50s for technical-grade endrin given by parenteral
routes of administration are shown in Table 23.
8.1.4 Toxicity of metabolites and isomers
8.1.4.1 Mammalian metabolites
In a comparative study, the acute oral LD50s of endrin and
three of its metabolites were determined in rats (Table 24). When the
brains of some of the rats were analysed for the presence of endrin
and its metabolites, the concentration of 12-ketoendrin in male rats
given endrin at 60 mg/kg body weight was found to be higher (mean, 0.3
mg/kg) than that of endrin (0.07 mg/kg) 22 h after dosing. In male
rats intoxicated with syn-12-hydroxyendrin or 12-ketoendrin (at 16
mg/kg body weight each), the concentrations of 12-ketoendrin in the
brain 30 min after dosing were much higher (mean values, 1.9 and 1.4
Table 21. Oral LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Mouse Corn oil 8.6 - Spynu (1964)
Mouse Unknown 13 13 Gray et al. (1981)
Rat (4-5 weeks) Peanut oil 28.8 16.8 Treon et al.(1955)
Rat (6 months) Peanut oil 43.4 7.3 Treon et al. (1955)
Rat (6 months) Peanut oil 40.0 - Speck & Maaske (1958)
Rat (adult) Peanut oil 17.8 7.5 Gaines (1960)
Rat (7 weeks) Cottonseed oil 27 - Boyd & Stefec (1969)
Rat (12-14 weeks) Dimethyl sulfoxide 5.6a 5.3a Bedford et al. (1975b)
Rat (12-13 weeks) Corn oil 8.9 4.0 Carter & Simpson (1978)
Rat Corn oil 9.0 - Spynu (1964)
Rat Unknown 4 4 Gray et al. (1981)
Rabbit Peanut oil - 7-10 Treon et al. (1955)
Guinea- pig Peanut oil 36.0 16.0 Treon et al. (1955)
Hamster (golden Syrian) Corn oil - 18.6 Chernoff et al. (1979)
Hamster (golden Syrian)
(6 weeks) Unknown 12 17.0 Cabral et al. (1979)
Hamster Unknown 18 18 Gray et al. (1981)
Cat Cod liver oil Lethal dose: Ressang et al. (1958);
3-6 Cook & Casteel (1985);
Casteel & Cook (1985)
Monkey (Macacus mulatta) Peanut oil 3 Treon et al. (1955)
Monkey (Macacus speciosa) Unknown 12 Barth (1967)
Domestic goat Unknown 25-50 Tucker & Crabtree (1970)
Mule deer Unknown 6.25-12.5 Hudson et al. (1984)
aEndrin of 99% purity
Table 22. Dermal LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Rat Xylene 18 15 Gaines (1960,1969)
Rat Shellsol A 10-20 5-10 Carter & Simpson (1978)
Rat Toluene approx. 10 approx. 10 Carter & Simpson (1978)
Rat Corn oil 12.5 - Spynu (1964)
Rabbit None - Minimum lethal Treon et al. (1955)
dose: 60-94
Cat Cod-liver oil Lethal dose: Ressang et al. (1958)
approx. 150
Table 23. Parental LD50s for technical- grade endrin
Species Route Vehicle LD50 (mg/kg Reference
body weight)
Mouse Intraperitoneal Corn oil 5.6 Graves & Bradley
(1965)
Mouse Intravenous Dimethyl 2.3 Walsh & Fink
sulfoxide (1970, 1972)
Dog Intravenous Ethanol 3 Hinshaw et al.
(1966)
Table 24. Acute oral toxicity of mammalian metabolites of
endrin in rats
Compound Oral LD50 (mg/kg body weight)
Male Female
Mean 95% CI Mean 95% CI
Endrin 5.6 3.0-7.9 5.3 3.6-7.4
anti-12-Hydroxyendrin 2.4 2.0-3.0 5.5 4.2-7.2
syn-12-Hydroxyendrin 1.2 0.6-1.7 2.8 0.8-4.0
12-Ketoendrin 1.1 0.7-1.5 0.8 0.5-1.2
a95% CI, 95% confidence interval
mg/kg, respectively) than those in the brains of rats given a similar
but non-toxic dose of anti-12-hydroxyendrin (mean, 0.09 mg/kg) and
killed at the same time. The signs of intoxication were similar to
those of endrin (Bedford et al., 1975b).
These results suggest that 12-ketoendrin may be the acute
toxicant in rats. The production of 12-ketoendrin varies greatly from
one mammalian species to another, however, and none has been detected
in birds of various species that were killed by endrin (Stickel et
al., 1979).
8.1.4.2 Isomers
As described in section 4.2, endrin is changed under the
influence of sunlight into delta-ketoendrin. The acute toxicity of
this isomer is given in Table 25. It is less toxic than endrin, and,
like endrin, it is more toxic to female than to male rats. The signs
of intoxication are similar to those seen with endrin.
The acute tocixity of the endrin aldehyde has been reported to be
> 500 mg/kg body weight in male mice (Phillips et al., 1962).
Table 25. Acute toxicity of delta-ketoendrin
Sex and Route LD50 (mg/kg Reference
species body weight)
Male rats Oral 120-180 Soto & Deichmann
(1967)
Female rats Oral 10-36
Male rats Oral 62.1 (53.3-72.2) Stanford Research
Institute (1954)
Rats Intravenous 5 Soto & Deichmann
(1967)
Male rats Intraperitoneal 82 Stanford Research
Institute (1953)
Male mice Oral 23.6 (19.9-28.0) Stanford Research
Institute (1954)
Male mice Intraperitoneal 16.7 Stanford Research
Institute (1953)
8.1.5 Acute toxicity of formulated material
8.1.5.1 Oral and dermal administration
Oral and dermal LD50 values for formulated endrin in rats
(Muir, 1970) are presented in Table 26. Dry formulations were
administered orally as 1-2% aqueous suspensions and dermally in both
dry form and as 2-5% aqueous suspensions. In general, the type of
formulation did not significantly alter the acute oral toxicity of
endrin. The dermal toxicity of the 50% wettable powder was similar to
that of the 20% emulsifiable concentrate; the 2% field strength dust
was the least toxic.
Ten rabbits (body weight, 2.4-4.1 kg) were treated with an
emulsifiable concentrate containing 19.4% endrin on clipped skin at a
dose of 200 mg/kg body weight, and the material was allowed to remain
in contact with the skin for 24 h. Four of the 10 animals died within
48 h (Anderson et al., 1953). Two of 10 rabbits (body weight,
2.0-2.5 kg) treated similarly with a 25% dust concentrate died within
48 h (Hine et al., 1954).
Table 26. Oral and dermal LD50s for endrin formulations in rats
Formulation LD50 (mg/kg body weight)
Oral Dermal
Formulation Active Formulation Active
material material
20% Emulsifiable 20 4.20 52.20 (undiluted) 10.90
concentrate
50% Wettable 7.6 3.80 21.80 (dry) 10.90
powder 14.40 (aqueous) 7.20
2% Field strength 275 5.50 5720 (dry) 114.40
dust 1140 (aqueous) 22.80
From Muir (1970)
8.1.5.2 Inhalation
Ten adult rats were exposed for 1 h to a mist of of an
emulsifiable concentrate containing 19.4% by weight of endrin in
xylene, at a concentration of endrin slightly exceeding 2000 mg/m3
of air, and were observed for 48 h. The particle size of the mist and
other details of exposure were not reported. Three of the animals died
1-14 h after exposure (Anderson et al., 1953).
Groups of 10 Long Evans rats were exposed for 1 h to 25% and 30%
endrin dust concentrates at a concentration of 2000 mg/m3 of air.
Particle size and other details of exposure were not provided. Five
rats exposed to the 30% and three exposed to the 25% dust died within
48 h after exposure (Hine et al., 1954).
8.2 Short-term exposure
8.2.1 Oral administration
8.2.1.1 Mouse
Feeding studies were conducted to estimate the maximum tolerated
doses of endrin in B6C3F1 mice. Groups of five males and five females
were given a normal diet or one containing endrin at 2.5-20 mg/kg for
6 weeks, followed by observation for another 2 weeks. Three males and
four females given 10 mg/kg died, but no mortality occurred at 5
mg/kg. No data were provided on animals fed 20 mg/kg.
Hyperexcitability was observed in male mice given doses > 5 mg/kg of
diet. Mean body weight gains were comparable with those of controls.
The maximum tolerated dose was calculated by extrapolation to be 5
mg/kg of diet (NCI, 1978, 1979).
8.2.1.2 Rat
Groups of three male and two to three female Carworth rats,
either 29 days or 6 months old, received daily doses of endrin at 1,
2, or 5 mg/kg body weight in peanut oil by gavage, on five days per
week for 67-72 days. All rats given 1 mg/kg survived; the increased
mortality in the other groups was dose-related: 2/5 females at 2 mg/kg
and 3/3 males at 5 mg/kg day died. Pathological findings at autopsy
included diffuse degenerative changes in the liver, kidneys, and
brain, while survivors showed no such changes. All treated animals
lost weight and developed hypersensitivity to stimuli (Treon et al.,
1955).
Groups of five male and five female adult Sprague-Dawley rats
were given diets containing technical-grade endrin at 0, 1, 5, 25, 50,
or 100 mg/kg diet over a maximal period of 16 weeks and were observed
for behaviour, weight gain, feed consumption, mortality rate, and
symptoms of toxicity. Alkaline phosphatase levels, determined once a
week, were higher in rats fed endrin than in the control group, and
the total average feed consumption of treated rats was less than that
of the control group. All rats fed 100 mg/kg of diet died during the
first two weeks of the study, and only two female rats fed 50 mg/kg of
diet survived the experiment. All male rats given 1 mg/kg and all
female rats given 1 and 5 mg/kg of diet survived. Males appeared to be
more susceptible than females to endrin in this study. The symptoms of
intoxication were hypersensitivity to stimuli and convulsions:
hypersensitivity was noted in all rats, and convulsions occurred among
rats receiving 25, 50, and 100 mg/kg diet. Weight loss was
dose-dependent and significant in all rats treated with endrin (Nelson
et al., 1956).
To estimate the maximum tolerated doses of endrin in
Osborne-Mendel rats, groups of five males and five females were given
diets with or without endrin for 6 weeks, followed by observation for
another 2 weeks. Endrin was added to the diet in two-fold increasing
concentrations of 2.5-80 mg/kg. Mortality was not increased at 10
mg/kg, and mean body weight gain was no different from that in
controls. At 20 mg/kg of diet, one animal of each sex died. The
maximum tolerated dose was calculated by extrapolation to be 15 mg/kg
diet (NCI, 1978, 1979).
8.2.1.3 Rabbit
Four of five female rabbits given an oral dose of endrin at 1
mg/kg body weight on five days per week died following the
administration of 2, 30, 35, and 50 doses, respectively. The fifth
rabbit survived 50 doses over a period of 10 weeks. Diffuse
degenerative changes were observed in the liver and kidneys but not in
the brain (Treon et al., 1955).
8.2.1.4 Dog
Dogs (mainly two per group) fed endrin at 5-50 mg/kg of diet died
within 50 days. They regurgitated their food, became lethargic,
salivated, and later refused to eat; they became emaciated and
developed respiratory distress and signs of stimulation of the central
nervous system. Diffuse degenerative lesions in the brain, heart,
liver, and kidneys, together with pulmonary hyperaemia and oedema were
observed. Three dogs fed diets containing 4 mg/kg of diet for 6 months
survived, but they showed reduced body weight gain and a slight
increase in liver:body weight ratio; no histopathological change was
observed. At 3 mg/kg of diet or less, growth was normal (Treon et al.,
1955).
Beagles (one male and one female/group; control group, only one
dog) were fed diets containing endrin at 0, 1, or 3 mg/kg for 80
weeks. No sign of intoxication was observed, and the weight gain of
treated animals was comparable to that of controls. The ratios of
kidney and heart to body weight were increased at 3 mg but not at 1 mg
(about 0.045 mg/kg body weight). No histopathological change was found
in the viscera (Treon et al., 1955).
Groups of three male and three female pure-bred beagle dogs
(4-6 months old) were fed endrin in the diet at 0, 0.1, 0.5, 1, 2, or
4 mg/kg for two years. Additional groups of four male and four female
dogs were fed endrin at 0, 1, or 4 mg/kg of diet. Two males and two
females of each group were killed after 6 and 12 months of feeding; no
other death occurred during the study. Convulsions were observed in
three dogs at 4 mg and in one female at 2 mg; no other sign of
intoxication or illness was apparent during the study. No adverse
effect was noted on growth, food consumption, haematology, or
urinalysis, and no compound-related change was found in serum alkaline
phosphatase, prothrombin time, or any of the other clinical chemical
parameters measured at regular intervals. All organ weights, relative
as well as absolute, were normal, except for occasional, slight
increases in liver weight in some of the females at 2 and 4 mg in the
diet. The only histopathological change found was a slight to moderate
vacuolation of liver cells in dogs fed 2 and 4 mg in the diet. No
renal change was observed in any of the dogs (Jolley et al., 1969).
8.2.1.5 Domestic animals
Sheep and cattle fed diets containing endrin at 2.5 or 5 mg/kg
for 112 days showed no indication of harmful effects (details not
given) (Radeleff, 1956). The convulsions and muscular tremors that
were induced in six 10-18-month-old male buffalo calves administered
a 20% emulsion of endrin led to a significant rise in lactic acid
concentration in the blood of the animals, possibly due to excessive
production of the acid inside the fasciculating muscles (Verma et al.,
1970).
8.2.2 Inhalation
Three mice, three rats, two guinea-pigs, two hamsters, four
rabbits, and one cat were exposed to sublimed endrin vapour at an
actual concentration of 5.44 mg/m3 for 7 h/day on 5 days/week for up
to 26 weeks. Two rabbits died after 26 and 90 exposures, respectively,
and one mouse died after 22 exposures. No convulsions were observed,
and all other animals survived. Surviving rabbits showed a
granulomatous type of pneumonitis; no histological change was found in
the other surviving animals (Treon et al., 1955).
8.2.3 Dermal administration
Three female rabbits with intact skin died after 19, 19, and 25
applications, respectively, of endrin as a dry powder at 150 m/kg body
weight for 2 h/day on 5 days/week. Applications of 75 mg/kg resulted
in the death of one of three rabbits after 8 weeks; the other two
survived for 13-14 weeks--the end of exposure. Convulsions, tremors,
and twitching of the facial muscles were the main signs of
intoxication. Two of five rabbits (dose not specified) showed severe
fatty degeneration of the liver (Treon et al., 1955).
8.3 Skin irritation
Dry powdered endrin was applied repeatedly at a dose of 75 or 150
mg/kg body weight for 2 h/day, 5 days/week for up to 14 weeks on
intact or abraded skin of female rabbits (see section 8.2.3). No skin
irritation was observed. Single applications of endrin as dry powder
at doses up to 250 mg/kg body weight for 24 h on rabbit skin caused no
gross or microscopic damage to the skin of the animals (Treon et al.,
1955).
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Mouse
CFW mice (20 males and 20 females) were fed diets containing
endrin (96%) at 0 and 5 mg/kg for 120 days, beginning 30 days before
mating. Significant parental mortality (32%) and reduced litter size
were observed, but fertility, fecundity, and the number of litters
produced per pair were not affected (Good & Ware, 1969).
8.4.1.2 Rat
Forty male and 80 female Long-Evans rats were fed endrin in the
diet at 0, 0.1, 1, or 3 mg/kg over three generations, each generation
breeding once. No difference in appearance, behaviour, body weight, or
number or size of litters was seen. The weights of the liver, kidneys,
and brain were normal, and no histopathological abnormality was seen
in third-generation weanlings. The only significant effect was
increased mortality of pups in the second and third generations of
rats fed 3 mg/kg (Hine, 1965).
Ten male and 20 female Long-Evans rats were treated similarly,
but each generation bred twice. Weanling rats were mated after 79 days
on the diets (when they were 100 days old). All pups from the first
litters were discarded at weaning, and the parent rats were mated
again. Randomly selected pups from the second litters were maintained
on the diets and mated when 100 days old; this was done for three
generations. The number of pups in each litter was counted on the day
of birth and on the fifth day; on the twenty-first day, the weanlings
were counted and weighed and either sacrificed or saved for
continuation. Parent rats were weighed, sacrificed, and examined
grossly when no longer needed. Ten male and 10 female F3b weanlings
each from the controls and the highest dose-level group and five males
and five females from the 0.1 and 1.0 mg groups were autopsied. Body,
liver, kidney, and brain weights were recorded, and sections of these
organs and from heart, lung, spleen, and testis were studied
histologically. Appearance, behaviour, body weight, number and size of
litters, organ weights, and histopathological appearance of F3b
weanlings were comparable with those in control animals. No effect on
reproduction was observed in rats fed diets containing endrin at 2
mg/kg over three generations (Hine, 1968).
8.4.2 Embryotoxicity and teratogenicity
8.4.2.1 Mouse
Groups of 10 CD-1 mice were given a single oral dose of endrin
(99%) at 2.5 mg/kg body weight (stated to be half the LD50) in corn
oil by gavage on day 9 of gestation; an untreated and a vehicle
control group were also used. Fetuses were examined on day 18. No
significant effect was observed on intrauterine death or fetal weight,
but the incidence of total anomalies was increased over that in
controls: 2/117 fetuses had cleft palates, three had open eye, and two
had other anomalies. No data on maternal toxicity were reported
(Ottolenghi et al., 1974).
These results could not be repeated by Kavlock et al. Female CD-1
mice were given endrin (99%) at 0 (vehicle), 0.5, or 1.0 (groups of 40
mice), or 1.5 or 2.0 mg/kg body weight (groups of 20 mice) in corn oil
by gavage on days 7-17 of gestation. The animals were killed on day
18. Maternal deaths occurred in the 1.5 and 2 mg/kg groups, reduced
maternal weight gain was observed at and above 1 mg/kg, and maternal
liver weight was increased at 0.5 mg/kg and higher. Fetal weight and
skeletal, and visceral maturity were adversely affected at doses of 1
mg/kg and above. No teratogenic effect or embryonic lethality was
observed, even at doses that caused maternal death (Kavlock et al.,
1981, 1987).
In a study of the effects of acute alterations in maternal health
status upon fetal development in the mouse, groups of 21 or 40
pregnant CD-1 mice were given a single oral dose of technical-grade
endrin at 0 (vehicle), 7 or 9 mg/kg body weight in corn oil on day 8
of gestation. The animals were killed on day 18 of gestation. Three of
21 animals given 7 mg/kg (14%) and 19/40 mice gievn 9 mg/kg (47%)
died. Maternal weight gain was decreased in both test groups; the
total number of implantation sites and number of viable litters were
not affected, but fetal weight was reduced. Delays in ossification of
the skeleton and an increased incidence of supernumary lumbar ribs
were observed. Although three fetuses from one litter in the 9 mg/kg
group had fused ribs, no significant increase in the incidence of
malformations was found. A statistically significant, linear, inverse
relationship between maternal weight gain and the presence of
supernumary ribs in their offspring was found (Kavlock et al., 1985).
8.4.2.2 Rat
Five groups of 25 female CD rats were administered endrin (97%)
in methocel in oral doses of 0, 0.1, 0.5, or 2 mg/kg body weight per
day on days 6-15 of gestation, or vitamin A, used as a positive
control. The animals were killed on day 20. The largest dose of endrin
caused maternal toxicity, as evidenced by weight loss and mortality
(two animals). The fetuses showed some slight growth retardation (not
significant) but no increase in intrauterine death rate. No effect
attributable to endrin was seen on the mean number of viable fetuses,
post-implantation losses, implantations, corpora lutea, fetal sex
ratio, or fetal external, soft-tissue, or skeletal abnormalities. Bent
ribs were observed in 6/522 fetuses treated with endrin, but not in
relation to dose. An increase in delayed ossification in sternebrae
and skull of fetuses was seen in the treated groups in comparison with
the untreated control group. Animals given vitamin A had a
significantly increased number of post-implantation losses and
malformed fetuses (Goldentahl, 1978a).
Groups of 32, 15, 28, 30, and 15 female CD rats were given oral
doses of endrin (99%) in corn oil at 0, 0.075, 0.15, 0.30, or 0.45
mg/kg body weight on days 7-20 of gestation. Rats were killed on day
21. Maternal weight gain was reduced at dose levels above 0.15 mg/kg,
but no increase in maternal liver weight was found. Fetal mortality,
weight, degree of skeletal and visceral maturation, and incidences of
skeletal and visceral anomalies showed no dose-related effect
(Kavlock et al., 1981).
8.4.2.3 Hamster
Three groups of golden Syrian hamsters (7, 24, and 8
animals/group) received a single oral dose of endrin (99%) in corn
oil at 5 mg/kg body weight (stated to be half the LD50) on day 7,
8, and 9 of gestation, respectively. Two control groups were used,
consisting of 57 untreated and 41 vehicle controls. The animals were
killed on day 14. The number of resorptions and of dead fetuses was
increased after treatment on days 7 or 8 and to a lesser extent in the
vehicle controls. The live fetuses in all three treated groups showed
significant growth retardation when compared with controls. The
incidence of anomalies was high only after treatment on day 8:
congenital abnormalities were seen in 28% of fetuses, with open eye in
22%, webbed foot in 16%, cleft palate in 5%, and fused ribs in 8%. The
anomalies that appeared to be increased significantly but to almost
the same extent at all three stages were fused ribs and cleft palate
(Ottolenghi et al., 1974).
These results could not be repeated by other workers. Groups of
27-29 golden Syrian hamsters were administered oral doses of endrin
(97%) in methocel at 0 (two control groups), 0.1, 0.75, or 2.5 mg/kg
body weight on days 4-13 of gestation. The animals were killed on
day 14. Body weight gains of the animals given 2.5 mg/kg were
slightly reduced. Maternal appearance, behaviour, and survival, mean
number of viable fetuses, post-implantation losses, implantations,
corpora lutea, fetal body weight, and crown-rump length showed no
change attributable to treatment. The number of malformations in
fetuses was not increased, but ossification of the sternebrae and
certain ribs was delayed (Goldentahl, 1978b).
Groups of 18-87 golden Syrian hamsters (LVG strain) were given
endrin (98%) as a solution in corn oil by gavage either as a single
dose of 0.5, 1.5, 5, 7.5, or 10 mg/kg body weight on day 8 of
pregnancy or as multiple daily doses of 0.75, 1.5, 2.5, or 3.5 mg/kg
body weight on days 5-14 of pregnancy. All animals were killed on day
15. With single doses, no effect was found on maternal survival,
pregnancy rate, weight change or liver:body weight ratio. The only
sign of maternal toxicity was the occurrence of transient convulsions
2 h after dosing in one hamster given 10 mg. No compound-related
difference was noted in the number of implantation sites, fetal death
rate, or fetal weight; indicators of skeletal maturity were not
affected. A dose-related increase in the incidence of fused ribs was
found in the groups given 7.5 and 10 mg/kg; increased incidences of
meningo-encephaloceles were observed at 5 mg/kg and above, with no
dose-response relationship. No other compound-related skeletal or
visceral anomaly was noted. In the study of multiple doses, maternal
toxicity (reduced weight gain and increased mortality) and fetal
toxicity (increased mortality, reduced weight, reduced skeletal
ossification, and an increased percentage of irregular
supra-occipitalis) were observed at doses of 1.5 mg/kg and higher. No
treatment-related maternal or fetal effect occurred at 0.75 mg/kg per
day (Chernoff et al., 1979).
8.4.2.4 Perinatal behavioural development
Rats exposed perinatally to endrin at 0, 0.075, 0.15, or 0.3
mg/kg body weight from gestation day 7 through day 15 of lactation
showed no mortality and no influence on survival or growth. Pups of
mothers exposed to 0.15 or 0.3 mg were more active than those of
mothers exposed to 0.075 mg or those in the control group. No clear
difference in ambulation was noted, and at 90 days of age there was no
difference (Gray et al., 1981; Kavlock et al., 1987).
Golden Syrian hamsters (LVG strain) given endrin (98%) at 0 or
1.5 mg/kg body weight per day by gastric intubation on days 5-14 of
gestation had a persistent increase in locomotor activity. Offspring
of treated hamsters ambulated more than the controls in the open field
at 15 days, and long-term observation of activity in the figure-8 maze
indicated that a significant increase in this behaviour was still
present at 125 days of age. Other behaviour patterns, including
sexual, rearing and running, and wheel behaviour, were unaffected.
Dams repeatedly exposed to endrin at 0.75 and 1.5 mg/kg body weight
were markedly hypoactive under the same testing conditions in which
the pups were hyperactive. The dose of 1.5 mg/kg body weight killed
more than half of the dams (Gray et al., 1981; Kavlock et al., 1987).
8.4.3 Appraisal of reproductive effects
Endrin had no reproductive effects in three generations of rats
at a level of 2 mg/kg of diet, equivalent to 0.1 mg/kg body weight. It
had no teratogenic effect in mice, rats, or hamsters after oral
exposure during the period of organogenesis. The significance of the
anomalies observed in mice and hamsters by Ottolenghi et al. (1974) is
uncertain. Studies in the same strain of the same species using more
rigorous protocols and larger numbers of animals could not confirm
their findings.
The lowest-observed-adverse-effect level for maternal toxicity
was 1.0 mg/kg body weight in mice, 0.3 mg/kg body weight in rats, and
1.5 mg/kg body weight in hamsters. Embryotoxicity was observed at
doses of 1 mg/kg body weight in mice and 1.5 mg/kg body weight in
hamsters. The overall no-observed-adverse-effect levels in mice, rats,
and hamsters were 0.5, 0.15, and 0.75 mg/kg body weight, respectively
(Table 27).
8.5 Mutagenicity and related end-points
8.5.1 Effects on microorganisms
Endrin was not mutagenic in numerous studies using Salmonella
typhimurium (TA98, TA100, TA1535, TA1537, TA1538, TA1950, TA1978,
SL4525, SL4700), Escherichia coli (WP2 uvrA, WP2 uvr-, Gal
Rs, WP2, hcr, p3478, W3100), K-12 (Pol A1+/Pol1-), WP67,
CM611, and CM571 Bacillus subtilis (M45), Saccharomyces cerevisiae
(D3, D7), and Serratia marcescens (a21, a742), with or without
metabolic activation by rat or mouse liver S9 (Fahrig, 1974; Van Dijck
& van de Voorde, 1976; Ercegovich & Rashid, 1977; Simmon et al., 1977;
Nishimura et al., 1982; Waters et al., 1982; Glatt et al., 1983;
Moriya et al., 1983; Rashid & Mumma, 1986).
No mutagenic effect was observed in S. typhimurium strains
TA98, TA100, TA1535, or TA1537, with and without metabolic activation
with S9 from livers of Aroclor 1254-induced rats and hamsters in the
presence of five concentrations of endrin (0-10 000 µg/plate) (Zeiger,
1987; Zeiger et al., 1987). No mutagenic effect was observed in
S. typhimurium strains TA97, TA98, TA100, or TA102 with and without
metabolic activation with Aroclor 1254-induced rat liver microsome
fraction in the presence of seven concentrations of endrin (99.0%),
from 1 ng/plate up to 1 mg/plate (Mersch-Sundermann et al., 1988).
8.5.2 Point mutations in mammalian cells
Endrin was weakly mutagenic in 6-thioguanine-resistant mouse
FM3A cells (Morita & Umeda, 1984; abstract only).
8.5.3 Dominant lethal mutations
Endrin did not show detectable dominant lethality when given as
a single intraperitoneal dose (0.76 or 3.8 mg/kg body weight) or daily
oral doses (0.1 or 0.25 mg/kg body weight) for 5 days to seven or nine
male ICR/Ha Swiss mice, respectively. This study involved a sequential
mating procedure, in which one male was housed with three females for
one week, repeated for 8 weeks (Epstein et al., 1972).
8.5.4 Chromosomal and cytogenetic effects
Endrin at 10-5 and 10-4 M, but not at 10-6, produced a
dose-related increase in the percentage of M1 metaphases and a
dose-related decrease in that of M3 metaphases at 48 h in treated
LAZ-007 human lympoid cells. This effect is closely related to the
reduced rate of cell proliferation induced by endrin (Sobti et al.,
1983).
Intratesticular injection of 0.25 mg endrin in saline to three
albino rats doubled the percentage of chromosomal changes in
comparison with that in the single control when the testes were
studied histologically 10 days after the injection. Changes were
scored in 70-75 cells/animal (Dikshith & Datta, 1973). The use of a
single dose does not aid interpretation, and the increase in
chromosomal abnormalities may be related to cytotoxicity rather than
to a genetic effect. The relevance of this type of study in
mutagenicity testing is unknown.
Table 27. Teratogenicity and effects on reproduction of oral administration of endrin
Animal Exposure NOAEL LOAEL Reference
(strain) period
Rat (Long Evans) 3 generations, 1 litter 1 mg/kg diet 3 mg/kg diet (0.15 mg/kg bw): Hine (1965)
(0.05 mg/kg bw) increased mortality of pups in
F2 and F3 generations
Rat (Long Evans) 3 generations, 2 litters 2 mg/kg diet Hine (1968)
(0.1 mg/kg bw)
Mice (CD-1) Days 7-17 of gestation 0.5 mg/kg bw 1 mg/kg bw: maternal and fetal Kavlock et al. (1981,
weight, skeletal and visceral 1987)
maturity
Rat (CD) Days 7-20 of gestation 0.15 mg/kg bw 0.30 mg/kg bw: reduced Kavlock et al. (1981)
maternal weight gain
Rat (CD) Days 6-15 of gestation 0.5 mg/kg bw 2 mg/kg bw: maternal toxicity Goldentahll (1978a)
Hamster Days 5-14 of gestation 0.75 mg/kg bw 1.5 mg/kg bw: maternal and Chernoff et al. (1979)
fetal toxicity
Chromosomal studies were carried out on lymphocytes from eight
male workers exposed to endrin and from six unexposed workers from the
same work area. No increase in the frequency of chromosomal
abnormalities was found, whether taken individually or collectively
(Dean, 1977).
Chromosomal aberrations were found in meiotic cells of barley and
somatic cells of barley and Vicia faba grown from endrin-treated
seeds (Wuu & Grant, 1966, 1967a,b). After treatment of root tips with
0.1% endrin (EC20 solution) for 1.5-2 h at 10 °C, the function of the
spindle was destroyed and did not interfere with the spreading of the
chromosomes during squash preparation. The centromeric region became
distinct and visible in prophase-metaphase chromosomes. At higher
concentrations contraction, stickiness, and fragmentation of
chromosomes were seen (Bhowmik, 1978).
8.5.5 Host-mediated effects
In two studies, male CF1 mice were given single oral doses of
endrin in dimethyl sulfoxide at 3.75 or 7.5 mg/kg body weight. Control
mice were dosed with the solvent, and positive control groups were
given a single oral dose of ethylmethanesulfonate at 400 mg/kg body
weight. Saccharomyces cerevisiae JD1 suspensions were then injected
intraperitoneally into each mouse, and the suspensions of
S. cerevisiae were harvested and analysed after 5 h. No increase in
mitotic gene conversion was detected (Brooks, 1976).
8.5.6 Sister chromatid exchange
Endrin at concentrations of 10-6-10-4 mol/litre in dimethyl
sulfoxide (the latter dose was a cytotoxic concentration) failed to
increase the frequency of sister chromatid exchange significantly over
the control value in rat liver microsomal S9-activated and unactivated
incubation experiments using human lymphoid cells of the LAZ-007 cell
line (Sobti et al., 1983).
8.5.7 Effects in Drosophila melanogaster
Endrin was not mutagenic to Drosophila melanogaster after
injection at 0.2 µlitre of a 0.001% aqueous solution, in the Muller-5
test for recessive lethal mutations on the X-chromosome (Benes & Sram,
1969).
8.5.8 Effects on DNA
Endrin at 10-3 or 3 x 10-3 mol/litre did not induce mutation
in the adult rat liver epithelial culture/hypoxanthineguanine
phosphoribosyl transferase assay (Williams, 1979).
DNA repair was not elicited in primary cultures of hepatocytes
from CD-1 mice, Fischer 344 rats, or Syrian hamsters exposed to endrin
for 18 h together with tritium-labelled thymine deoxyribonucleotide
for incorporation during repair synthesis. DNA repair was measured
autoradiographically. In rat and hamster liver cell cultures, a
concentration of 10-3 mol/litre and in mouse liver cell cultures,
10-4 mol/litre endrin was tested (Maslansky & Williams, 1981).
Endrin did not induce unscheduled DNA synthesis in human lung
fibroblast cells with or without metabolic activation by rat liver
microsomes (five concentrations were tested, but they were not given
in the paper) (Waters et al., 1982).
Endrin at eight concentrations ranging from 0.5 up to 1000
nmol/ml did not induce unscheduled DNA synthesis in primary rat
hepatocytes or in a modified Ames test utilizing concentration
gradient plates and 10 bacterial tester strains (eight S. typhimurium
and two E. coli) (Probst et al., 1981)
8.5.9 Appraisal of mutagenicity and related end-points
Garrett et al. (1986) evaluated the activity of endrin in a
series of tests: for reverse mutation (point/gene mutations in
prokaryotes), forward mutation (point/gene mutations in eukaryotes),
differential toxicity (primary DNA damage in prokaryotes), enhanced
mitotic recombination, gene conversion and crossing-over, unscheduled
DNA synthesis (primary DNA damage in eukaryotes), sister chromatid
exchange, chromosomal breakage, and dominant lethality (chromosomal
effects). Endrin gave negative results in all these tests.
The vast majority of the data indicate that endrin is not
genotoxic; however, many of the studies would not reach current
standards, or they give insufficient data to allow an independent
assessment.
8.6 Long-term exposure
Groups of 20 male and 20 female Carworth rats (28 days old) were
given diets containing endrin at 0, 1, 5, 25, 50, or 100 mg/kg. With
100 mg, two males and one female survived for 2 years; with 50 mg,
four males but no female survived; and with 25 mg, 11 males and 5
females survived. Survival at the lower concentrations was comparable
to that of the control group. Males appeared to be less susceptible
than females to the toxic action of endrin. Signs of intoxication,
hypersensitivity to external stimuli, and occasional convulsions were
observed only at the two highest levels. The weight gain of females
fed 1, 5, or 25 mg/kg of diet was equal to or greater than that of the
controls after 40 weeks of feeding. In males fed 5 mg, growth
retardation occurred during the first 20 weeks only, while males that
received 25 mg showed significant reduction in body weight gain. The
body weight gain of males fed 1 mg was comparable to that of controls.
The liver:body weight ratios were increased in males fed 5 mg or more
and in females fed diets with 25 mg or more. Histopathological
examination of animals that died during exposure to the three higher
dietary levels revealed diffuse degeneration of the liver, kidneys,
brain, and adrenal glands. The few survivors at 50 and 100 mg showed
degenerative changes in the liver only. No histopathological change
was found in surviving rats fed 1, 5, or 25 mg/kg diet. There was no
increase in the incidence of neoplasia in the treated groups compared
to the control group (Treon et al., 1955).
This study indicates a no-effect level for endrin of 1 mg/kg of
diet (about 0.05 mg/kg body weight) but is inadequate in several
respects, e.g., survival rate, details of pathology, and
haematological and clinical chemical data are not reported.
8.7 Carcinogenicity
8.7.1 Oral administration
8.7.1.1 Mouse
Groups of 100 male and female C57B1/6J mice, an inbred strain
with a low incidence of tumours, and C3D2F1/J mice, a hybrid strain
with a high incidence of hepatomas in males and a high incidence of
mammary tumours in females, were fed endrin (99%) at dietary
concentrations of 0.3 or 3 mg/kg from the age of five weeks throughout
life. A control group consisted of 200 mice of each sex of each
strain. Except for all groups of female animals of the C3D2F1/J
strain, this part of the experiment was terminated at the 78th week
because of the early occurrence of high numbers of mammary
fibroadenomas in 70-90% of control and treated mice. Survival, growth,
food intake, and haematology were not impaired. Mice of both strains
fed 3 mg/kg diet occasionally developed convulsions in the early
stages of feeding but recovered and survived without signs of illness.
They generally showed the typical histological changes in the liver
characteristic of high doses of chlorinated hydrocarbon insecticides.
No effect was observed on mice fed 0.3 mg/kg diet. The tumour
incidence and type of tumours were not influenced by the feeding of
endrin, and it had no influence on the incidence of fibroadenomas in
female C3D2F1/J mice (Witherup et al., 1970).
Groups of 50 B6C3F1 mice of each sex were given endrin in the
diet for 80 weeks and were then observed for a further 10 or 11 weeks.
The initial doses of endrin (97%) (2.5 and 5 mg/kg of diet) were
poorly tolerated by males and were therefore reduced after 25 weeks to
1.2 and 2.5 mg/kg diet for males; but females received 2.5 and 5 mg/kg
diet during the whole experiment. The time-weighted average doses were
1.6 and 3.2 mg/kg diet for males and 2.5 and 5.0 mg/kg diet for
females. Matched controls consisted of groups of 10 mice of each sex;
pooled controls, used for statistical evaluation, consisted of the
matched control groups combined with 50 untreated male and 50
untreated female mice from similar bioassays of other chemicals. All
surviving mice were killed at 90 or 91 weeks. Mean body weight was not
affected, but the survival of males at the high dose was lower than
that of the controls. The survival of the low-dose males could not be
evaluated due to accidental administration of excessive quantities of
endrin to this group during week 66. The tumour (neoplastic lesions in
the liver) incidences in the high-dose males were higher than those of
the pooled or matched controls, but not significantly so, and the
increase was not considered to be related to the administration of
endrin (Fredrickson, 1978;NCI, 1978).
8.7.1.2 Rat
A study of groups of 20 male and 20 female Carworth rats
administered endrin at 0, 1, 5, 25, 50, and 100 mg/kg of diet was
reviewed in Section 8.6.1.1. Bearing in mind the limitations of this
study, such as small group sizes and low survival at the high doses,
no evidence of an increase in the incidence of neoplasia was found in
any of the groups (Treon et al., 1955).
Groups of 50 weanling Osborne-Mendel rats of each sex were fed
diets containing endrin (98%) at 2, 6, or 12 mg/kg for 29 months. The
control groups consisted of 100 males and 100 females. During the
first 10 weeks of the study, only half the nominal dietary
concentrations of endrin were fed. Signs of toxicity occurred in a few
animals in all treatment groups, mainly in females, and included
episodes of tremor and clonic convulsions with 'outcries', the
incidence of these signs being dose-related. Weight gain was
unaffected, and the survival rates in control and treated rats were
similar. The liver:body weight ratios were unaffected. A moderate (not
dose-related) increase in the incidence of centrilobular cloudy
swelling in the liver and of cloudy swelling of the renal tubular
epithelium was observed. The lungs of the animals fed endrin exhibited
a moderate increase in the incidence of congestion and focal
haemorrhages. The tumour incidence in the treatment groups was
comparable with that in control rats, and no difference in the type of
tumours was found (Deichmann et al., 1970a,b; Deichmann & MacDonald,
1971).
In a life-time study, groups of 24 male and 24 female
Osborne-Mendel rats (22 days old) were fed diets containing endrin at
0, 0.1, 1, 5, 10, or 25 mg/kg. Because 50% of the rats at 25 mg/kg
died within the first week, this group was restarted with 32-day-old
rats. Survival did not appear to be affected by treatment. The highest
incidence of malignant tumours in male and female rats occurred at 0.1
mg/kg, but the malignancies were not dose-related. Treated male rats
had a higher incidence of renal disease than controls, but this also
was not dose-related (details not available) (Reuber, 1978).
Groups of 50 Osborne-Mendel rats of each sex were fed endrin
(97%) in their diet for 80 weeks and then observed for 31 or 34 weeks.
Males received doses of 2.5 or 5 mg/kg diet; in females, the initial
doses of 5 and 10 mg/kg of diet were poorly tolerated and were reduced
after 9 weeks to 2.5 and 5 mg/kg. The time-weighted average doses were
2.5 and 5.0 mg/kg for males and 3 and 6 mg/kg for females. Matched
controls consisted of groups of 10 rats of each sex; pooled controls
used for statistical evaluation consisted of the matched control
groups combined with 40 untreated male and 40 untreated female rats
from similar bioassays of other chemicals. All surviving rats were
killed at 100-114 weeks. Body weights and survival were not affected
by administration of endrin. A slight increase in the incidences of
pituitary and thyroid tumours was observed, but no consistent
statistical significance or dose-response relationship was found
(Fredrickson, 1978; NCI, 1978).
8.7.1.3 Tumour promotion
No significant increase in the development of preneoplastic
changes (hyperplastic nodules) was observed in rat liver after partial
hepatectomy and initiation with N-nitrosodimethylamine or
N-2-fluorenylacetamide in combination with the administration of
endrin (Ito et al., 1980).
In vitro, endrin at levels of above 2.5 µg/ml appeared to
inhibit metabolic cooperation in the hypoxanthineguanine
phosphoribosyl transferase system using wild-type
6-thioguanine-sensitive V79 cells and variant 6-thioguanine-resistant
cells. Such inhibition is reported to be an index of potential tumour
promoting activity, although the test has not been validated (Kurata
et al., 1982).
Endrin stimulated protein kinase C activity in vitro only
slightly, whereas a representative endogenous ligand of protein kinase
C, syn-1,2-didecanoylglycerol, stimulated protein kinase C to a
maximal velocity (Moser & Smart, 1989).
8.7.2 Appraisal of carcinogenicity
One of several studies in mice suggests an increased incidence of
nonmalignant tumours in animals of one sex, but this study was
considered inadequate for assessing carcinogenicity because an
increased number of tumours was seen in controls. A second study using
a different mouse strain did not corroborate the increase in tumour
incidence. Several long-term feeding studies in rats provide no
evidence of a carcinogenic effect of endrin. Its tumour promoting
activity was tested in vitro using protein kinase C stimulation and
ATPase inhibition; the results do not suggest any overwhelming effect
in these systems. After a careful review of this evidence, and taking
into consideration the fact that most of the data indicate that endrin
is not genotoxic, the Task Group concluded that the data are
insufficient to indicate that endrin is a carcinogenic hazard to
humans.
8.8 Special studies
8.8.1 Nervous system
8.8.1.1 Electrophysiological studies
The effects of endrin on bioelectrogenesis was studied in
anaesthetized pigeons and squirrel monkeys with chronically implanted
electrodes. Endrin was administered intravenously to pigeons at doses
of 0.5-4 mg/kg body weight. Doses of 2-4 mg/kg and higher caused
seizure activity throughout the telencephalon; the lower dose levels
caused activity only in the ectostriatum, a telecephalic visual
projection area. At doses of 0.5-2 mg/kg body weight, endrin caused a
specific increase in the evocation of potentials in the ectostriatum
by stimulation of the nucleus rotundus, a diencephalic visual
projection area. Reticular formation functions were not or little
affected. Administration of endrin to squirrel monkeys at doses of
0.2-3 mg/kg body weight on 5 days/week intramuscularly in corn oil and
saline emulsion induced characteristic changes in the
electro-encephalogram (EEG), culminating in electrographic seizures;
these were transient and disappeared when endrin administration was
stopped. Seizures reappeared under stress conditions, however, several
months after endrin treatment (Revzin, 1966, 1980).
Groups of 20-60 Sprague-Dawley rats with previously implanted
electrodes were given endrin in peanut oil orally at 0.8, 1.7, or 3.5
mg/kg body weight per day on 5 days/week for 28 weeks. Dose-dependent
mortality occurred during the first week and again at the end of the
study. Most changes in the EEG were seen after one week of exposure:
these included severe bursts of multiple spikes accompanied by clonic
convulsions; other animals had runs of spikes without full-fledged
convulsions. The convulsions were usually preceded by a period of
hyperventilation. After a further week of exposure, the rats showed
normal EEG traces. Some irregular slow-wave activity was seen in
animals that were moribund in the last month of feeding (Speck &
Maaske, 1958).
The convulsive properties of endrin at 1-2 mg/kg body weight
were studied by intravenous injection in locally anaesthetized,
paralyzed male cats, in which electrodes were placed in the
subcortical structures of the brain. Endrin was dissolved in ethanol
(which itself stimulates or inhibits the central nervous system,
depending on dose). Changes in the EEG and evoked responses were
studied. Hypersynchrony, rhythmic bursts of spikes and waves, and
isolated spikes characterized the preictal state. Seizures were always
bilateral and symmetrical and of a general tonic-clonic type.
Responses in sensory and motor cortexes to sensory nerve stimulation
were enhanced three to five fold. The authors concluded that endrin is
directly toxic to the mammalian nervous system, is a potent rapidly
acting convulsant, and does not require metabolic activation to an
active metabolite (Joy, 1976).
8.8.1.2 Histopathological studies
Male CD1 Swiss mice were administered endrin or sesame oil daily
by intraperitoneal injection in gradually increasing doses of 1.5-4.0
mg/kg for 4-20 days. Electron microscopic examination of sciatic nerve
tissue revealed no morphological change in myelinated nerve fibres,
myelin, or associated Schwann cells, but morphological alterations
were observed in unmyelinated nerve fibres and associated Schwann
cells: axons were swollen, microtubules and neurofilaments showed
dissolution, axoplasm was replaced by large clear vesicles,
vacuolization was present, and Schwann cells and adaxonal spaces also
contained vesicles (Walker & Phillips, 1987; abstract only).
8.8.1.3 Neurotransmitter systems
gamma-Aminobutyric acid systems: The role of the inhibitory
neuro-transmitter of the central nervous system, gamma-aminobutyric
acid (GABA),in the production of convulsions is well established.
Polychloro-cycloalkane insecticides such as endrin have a potent
excitatory action on the nervous system, and the interaction between
GABA function and endrin has been studied.
Endrin strongly inhibited GABA-dependent 36Cl uptake by mouse
brain vesicles, with an IC50 (the concentration required to cause
50% inhibition) of 2.8 µmol/litre. Inhibition was confined to that
portion of 36Cl uptake that is GABA-dependent. The result
demonstrates disruption of GABA ionophore function in mammalian brain,
possibly providing the principal mechanism of toxicity (Bloomquist &
Soderlund, 1985). In a comparison of the inhibitory potential of
several polychlorocycloalkane insecticides on GABA-dependent 36Cl
uptake, the most potent inhibitor was 12-ketoendrin, followed by
isobenzan, endrin, and then dieldrin, heptachlor epoxide, aldrin,
heptachlor and lindane. This order closely parallels their acute
toxicities (Bloomquist et al., 1986).
The effect of these chemicals was also studied in the tert
butylcyclo-phosphorothioate (TBPS) system, which has been shown to
bind convulsants with varying affinities. The IC50 for endrin on
35S-TBPS binding was 0.22 µmol/litre and that for 12-ketoendrin,
0.036 µmol/litre. These were the most potent inhibitors of TBPS
binding, and there was a significant linear correlation between 36Cl
flux and TBPS binding (Bloomquist et al., 1986).
In vitro, endrin inhibited 35S-TBPS binding in tissue from
male Swiss-Webster mice with an IC50 of 18 nmol/litre (range, 4-90).
In vivo, doses representing 25, 50, and 100% of the LD50 (8 mg/kg
intraperitoneally) inhibited 35S-TBPS binding with IC50s of 77 ±
7 nmol/litre (LD50) and 39 ± 6 nmol/litre (LD50/2); no inhibition
was observed at LD50/4, indicating a possible no-observed-adverse-
effect level. Brain P2 membranes of treated mice contained endrin and
12-ketoendrin. The finding that the brains of treated mice contained
sufficient endrin or its biotransformed products to achieve TBPS
binding and that this was correlated with the severity of the
poisoning indicates that the acute toxicity of endrin to mammals is
regulated by GABA (Cole & Casida, 1986).
GABA-induced 36Cl flux into membrane microsacs was inhibited
by endrin at 3.9 ± 0.2 nmol/mg protein, which also suggests that
endrin inhibits the function of this receptor (Abalis et al., 1985,
1986). The IC50 for 36Cl influx was 0.19 ± 0.06 µM and that for
35S-TBPS binding was 0.003 µM (Gant et al., 1987).
Endrin inhibited both insect and rat GABA receptors in a
dose-related, non-competitive manner. It acts in a similar manner on
the GABA receptors in the central nervous system of the two species.
The blocking action may involve non-competitive binding to an
allosteric site associated with the receptor's chloride channel
(Wafford et al., 1989a).
Endrin potentially inhibits 35S-TBPS binding to rat brain
membranes and also potentiates the protective effect of NaCl (200 mM)
against heat inactivation of 3H-flunitrazepam binding sites on the
same membranes. The time courses of heat inactivation of these binding
sites in the presence of NaCl and saturating concentrations of endrin
revealed monophasic components constituting about 88% of the binding
sites (Squires & Saederup, 1989).
Endrin has also been shown to inhibit GABA-ergic function in
Torpedo fish (Matsumoto et al., 1988), chicken embryos (Seifert,
1988, 1989), the mosquito fish (Gambusia affinis) (Bonner &
Yarbrough, 1989), and the cockroach (Periplaneta americana) (Wafford
et al., 1989b).
Other amine systems: Studies on the effects of orally
administered endrin on the content of biogenic amines in the brain of
rats did not contribute to an understanding of the convulsive action
of endrin (Miller & Fink, 1973; Hrdina et al., 1974).
Cyclic AMP metabolism: Endrin did not affect adenylate cyclase
activity or inhibit the activity levels of synaptosomal
phosphodiesterase, enzymes involved in cyclic AMP metabolism, in rat
brain. The authors interpreted their results to support their
postulation that organochlorine insecticides exert their neurotoxic
action by selective inhibition of ATPases in synaptosomes (Kodavanti
et al., 1988).
ATPase systems: Inhibition of rat brain Na+-K+ATPase by
chlorinated insecticides varied considerably: endrin and dieldrin were
the least active in inhibiting both this enzyme and K+-stimulated
para-nitrophenyl phosphatase at a concentration of 2 x 10-5
mol/litre. Results of experiments on ATP-32Pi exchange suggest
that DDT is a powerful inhibitor of oxidative phosphorylation, which
may lead to depletion of ATP. This effect was much less evident with
endrin (Folmar, 1978).
Endrin caused about 15% inhibition of the activity of
Na+-K+ATPase in rat brain synaptosomes at the highest
concentration tested, 120 µM, and oligomycin-sensitive Mg2+-ATPase
in rat brain synaptosomes was significantly inhibited in a
concentration-dependent manner, to a maximal inhibition of 33% at the
highest dose. Endrin did not inhibit oligomycin-insensitive
Mg2+-ATPase, and it did not affect K+-stimulated
para-nitrophenyl phosphatase from rat brain synaptosomes; this
enzyme represents the dephosphorylation step of the overall reaction
to the Na+-K+ATPase. Oligomycin-sensitive Mg2+-ATPase in beef
heart mitochondria was significantly inhibited. The results of this
study suggest that the ATPase system in rat heart and central nervous
system is not selectively inhibited by endrin (Mehrotra et al., 1989).
Sodium channel: It has been demonstrated using voltage clamp
techniques in single cells that application of DDT prolongs the sodium
current, which in turn decreases the depolarizing after-potential to
initiate repetitive after-discharges in the cell. The repetitive
after-discharges facilitate synaptic transmission and result in
nervous system hyperexcitability, which at the functional level is
registered as tremors and eventually convulsions and death (Narahasi,
1987). Even if less than 1% of the sodium channels respond in this
manner to insecticides, it is sufficient to cause toxicity in the
animal. Narahasi (1987) reported these effects with pyrethroids and a
series of DDT analogues; such studies have not been carried out with
endrin. Lund & Narahasi (1983) suggested that because of the
similarity in the symptomatology of intoxication by the family of
organochlorine insecticides, the target site of endrin may also be the
sodium channels.
8.8.1.4 Appraisal of effects on the nervous system
The effect of endrin on the nervous system has received attention
because it has the well established ability to cause convulsions
following acute exposures. Endrin causes considerable changes in EEG
activity, which are associated with convulsions, at intramuscular
doses in experimental animals as low as 0.2 mg/kg body weight.
The probable underlying mechanisms are associated with a
dose-related, non-competitive inhibition of the GABA-ergic
neurotransmitter system. This is an inhibitory system, and removal of
its action leads to increased excitation in the nervous system. While
inhibition of GABA-ergic function is common to a number of
polychlorocycloalkane insecticides, endrin, and particularly its
metabolite 12-ketoendrin, have been shown to be extremely potent
inhibitors of this function. It appears therefore that the acute
toxicity of endrin is due to disruption of GABA-related mechanisms.
8.8.2 Cardiovascular system
Studies have been conducted on the physiological effects of
endrin on the peripheral vascular system, renal function, renal
haemodynamics, and the cardiovascular system of the dog (Emerson et
al., 1963, 1964; Reins et al., 1964; Emerson, 1965; Emerson & Hinshaw,
1965; Reins et al., 1966; Hinshaw et al., 1966; Reddy et al., 1967).
After a lethal dose of endrin was administered intravenously, most of
the effects appeared to be the direct or indirect result of the
stimulating action of endrin on the central nervous system.
Bradycardia, hypertension, salivation, hyperexcitability, tonic-clonic
convulsions, increased body temperature, leukocytosis,
haemoconcentration, and decreased blood pH were seen. Elevation of
cerebral venous pressure and cerebrospinal fluid pressure were also
prominent features. Increased levels of adrenaline and noradrenaline
in blood plasma cause increased venous return and cardiac output and
increased arterial blood pressure in the absence of a rise in total
peripheral resistance. There was a large increase in total limb
vascular resistance and also a decrease in renal blood flow due to
arteriolar vasoconstriction. In studies on intact dogs and isolated
heart-lung preparations, high doses of endrin appeared to have a toxic
action on the left ventricle of the heart, causing sudden left heart
failure.
Aldrin, dieldrin, and endrin inhibited rat brain synaptosomal
and heart sarcoplasmic reticulum in vitro in a
concentration-dependent manner. Calmodulin-depleted Ca2+ pump
activity was insensitive to the action of these compounds. Oral
administration of endrin at 0.5-10 mg/kg to rats similarly decreased
Ca2+ pump activity, in addition to decreasing the levels of
calmodulin in both brain and heart, indicating disruption of membrane
Ca2+ transport mechanisms. Exogenous addition of calmodulin
(1-20 µg) effectively reversed the endrin-induced inhibition. Ca2+
pump activity in brain is more sensitive to endrin than that in heart.
The results indicate that endrin may produce neurotoxic effects by
altering calmodulin-regulated calcium-dependent events in neurons
(Mehrotra et al., 1989).
8.8.3 Effects on liver enzymes
It is well known that chlorinated hydrocarbon insecticides such
as DDT and dieldrin stimulate hepatic microsomal drug metabolism,
stimulating the activity of enzymes for the metabolism of drugs and
endogenous compounds such as hormones (Kinoshita & Kempf, 1970).
8.8.3.1 Mouse
A single oral, convulsive dose of endrin (20 mg/kg body weight)
dissolved in corn oil was administered to 9-week-old male
Swiss-Webster mice. Control groups consisted of a group of untreated
mice and a group receiving corn oil. When convulsions began, blood
serum was examined for serum glutamic oxaloacetic transaminase, serum
glutamic pyruvic transaminase, and serum lactic dehydrogenase. The
activities of the three enzymes were significantly increased above
those seen in the two control groups (Luckens & Phelps, 1969).
After intraperitoneal injection of a single dose of endrin at
6.25 mg/kg body weight to mice, hexobarbital sleeping time was
decreased, starting 3 h after the injection and lasting for 3 days
(Hart & Fouts, 1963). Stimulating effects on the hepatic
mixed-function oxidase system were reported in ICR mice after single
oral doses of 4 and 10 mg/kg body weight (Hartgrove et al., 1977).
8.8.3.2 Rat
Feeding Sprague-Dawley rats on diets containing endrin at 1, 5,
25, 50, or 100 mg/kg for 16 weeks caused high mortality in all groups,
especially among male rats. The serum alkaline phosphatase
concentration was reported to be dose-relatedly increased in all
groups as compared to control animals. The effect was clearest in the
groups fed 25 mg/kg of diet or more (Nelson et al., 1956).
In strain FW 49 rats, a single oral dose of endrin at 5 mg/kg
body weight had no effect on pentobarbital sleeping time; 10 mg/kg
caused a significant reduction, which, however, disappeared after 10
days (Schwabe & Wendling, 1967).
Endrin caused a significant shortening of the duration of the
paralysis induced by zoxazolamine in male Sprague-Dawley rats aged
5-6 weeks. Endrin was injected intraperitoneally at 2 mg/kg body
weight daily for 3 days, and zoxazolamine was injected
intraperitoneally on the fourth day (Truhaut et al., 1974).
Male rats given single oral doses of 2.5, 3.75, or 5.0 mg/kg body
weight showed no effect on the various parameters (details not given)
of mixed-function oxidase activity after 12 h, but the level of
microsomal protein and electron transport components per gram of liver
were significantly increased after 108 h, in a dose-dependent fashion.
Thiopentone and pentobarbital sleeping times were reduced by a 24-h
prior intraperitoneal injection of endrin at 5 mg/kg body weight
(Kachole & Pawar, 1977).
A single oral dose of endrin at 10 mg/kg body weight to male
albino rats increased serum glutamic oxaloacetic transaminase and
glutamic pyruvic transaminase activities, and decreased ATPase, acid-
and alkaline phosphatase, succinic dehydrogenase, and
glucose-6-phosphatase activities significantly 2-48 h after treatment
(Meena et al., 1978). After three successive daily oral doses of
endrin at 15 mg/kg body weight to Sprague-Dawley rats, significant
increases in total lipids and triglycerides in liver and in serum
glutamic pyruvic transaminase activity were seen (Borady et al.,
1983).
When two groups of six adult female rats were fed 0 or 28.7 µg/kg
body weight, endrin accumulated in the liver (5.47 mg/kg),and its
concentration in blood increased progressively up to 28 days. Growth
was depressed. The activities of the enzymes aspartate amino
transferase and alanine amino transferase were slightly increased
(Illahi et al., 1986). Similar results were obtained in a study in
which rats were fed 20 µg/kg body weight for 28 days (Illahi et al.,
1987).
8.8.3.3 Guinea-pig
Groups of six female guinea-pigs were administered three
successive intraperitoneal injections of endrin in sunflower oil at 3
mg/kg body weight, and liver and kidneys were studied 24 h after the
last injection. Treatment caused a significant increase in liver
weight and a decrease in hepatic microsomal protein content; renal
weight and renal microsomal protein content were not affected. Hepatic
cytochrome b5 and cytochrome-c reductase activities were increased,
while cytochrome P450 and total haem levels were significantly
decreased. Related to the decrease in cytochrome P450 was a decrease
in TPNH-linked aminopyrine-N-demethylation, but an increase in
DPNH-linked demethylation was related to the increase in cytochrome b5
and cytochrome-c reductase. Lipid peroxidation was increased in both
liver and kidneys (Pawar & Kachole, 1978).
8.8.3.4 In-vitro studies
To test the possibility that phenobarbital induces cytochrome
P450p indirectly by increasing the availability of endogenous
glucocorticoids in the liver, phenobarbital and phenobarbital-like
inducers, including endrin, were added to primary monolayer cultures
of adult Sprague-Dawley rat hepatocytes incubated in serum-free medium
without glucocorticoids. De-novo synthesis of cytochrome P450p,
measured as increased incorporation of 3H-leucine into
immunoprecipitable P450p protein, was increased. Endrin at a
concentration of 1x10-5 M was half as potent as phenobarbital at 2
x 10-3 M (Schuetz et al., 1986).
8.8.4 Miscellaneous studies
Endrin inhibited rabbit muscle lactate dehydrogenase in vitro
(Hendrickson & Bowden, 1976). Exposure of isolated rat enterocytes to
endrin reduced the efficiency of the neuropeptide vasoactive
intestinal peptide after stimulation of cyclic AMP accumulation, as
was observed with lindane (Carrero et al., 1989).
Endrin at single oral doses of 25 mg/kg body weight or daily
doses of 1 mg/kg body weight daily for 8 days induced various shifts
in the mobilization of the ions of biologically important metals such
as magnesium, iron, zinc, and copper from liver, kidneys, brain,
heart, spleen, and blood (Coleman et al., 1968; Lawrence et al.,
1968). Rats receiving intraperitoneal injections of 1 mg/kg body
weight in peanut oil over periods up to 19 days showed no alteration
in the concentrations of serum proteins or serum lipoproteins,
separated by paper electrophoresis, or of albumin, alpha 1, alpha 2,
beta, or gamma globulins. Protein-bound sialic acid and methylpentose
were increased only temporarily; the level of bound hexose increased
with time and that of bound hexosamine decreased (Coleman, 1968).
Rats receiving a single oral dose of 50 mg/kg body weight, daily
intraperitoneal doses of 2 mg/kg body weight, or daily intramuscular
injections of 0.5 or 2.0 mg/kg body weight for 45 days showed
increased activity of a number of the enzymes that are involved in
gluconeogenesis in liver cells and cells of the renal cortex. A
significant decrease was noted in hepatic glycogen, an increase in
blood glucose and urea, as well as a significant rise in hepatic and
renal pyruvate carboxylase, phosphoenol pyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase. Furthermore,
endogenous levels of cyclic AMP were increased (Kacew et al., 1973;
Singhal & Kacew, 1976).
8.8.5 Factors that influence toxicity
8.8.5.1 Nutrition
The nutritional state of Wistar rats was found to alter their
susceptibility to the acute toxic action of endrin. Three groups of
approximately 100 rats were fed a normal diet, a diet containing
casein as the only source of protein, or a low protein diet for 28
days, and the acute toxicity of endrin was determined after a single
intragastric administration. The following LD50 values were
calculated: 27 mg, 17 mg, and 7 mg/kg body weight, respectively (Boyd
& Stefec, 1969).
8.8.5.2 Potentiation
The acute oral LD50s of equitoxic doses of combinations of 10
pesticides, including endrin, were studied in Swiss mice. No evidence
of potentiation was seen with combinations with dieldrin, diazinon,
malathion, toxaphene, parathion, DDT, or dioxathion, but more than
additive effects, i.e., possible potentiation, were found with
chlordane and possibly with aldrin (Keplinger & Deichmann, 1967).
Five groups of 20 male and 20 female Sprague-Dawley rats were fed
for 91 days on a diet containing a combination of 15 'persistent'
chemicals added at concentrations of 0, 1, 10, 100, and 1000 times the
water quality objective applied in Canada. For endrin, these
corresponded to 0.002, 0.02, 0.2, and 2.0 µg/kg of diet. No effect on
food intake, growth, clinical chemistry, bone marrow, or
histopathology were observed. It was concluded that the presence of
these chemicals at 1000 times the water quality objective had no
toxicological effect (Cote et al., 1985).
Six male and six female Sprague-Dawley rats were fed a control
diet or diets containing endrin at 5 or 10 mg/kg, endrin aldehyde at
10 mg/kg, or endrin ketone at 5 mg/kg for 15 days, at which time three
to six rats from each treatment group were given a single
intraperitoneal dose of carbon tetrachloride at 100 µlitre/kg body
weight in corn oil (1 mg/kg). Levels of serum enzymes, bile flow, and
biliary excretion of an anionic model compound, phenolphthalein
glucuronide, were measured on day 16. Dietary treatment with endrin at
either dose level did not elevate serum enzyme levels. Treatment with
5 mg/kg significantly reduced bile flow and a corresponding reduction
in phenolphthalein glucuronide excretion, whereas the 10 mg/kg dose
reduced only phenolphthalein glucuronide excretion in male rats.
Female rats treated with either dose showed a dose-dependent
choleretic effect with a commensurate increase in phenolphthalein
glucuronide excretion. Treatment of rats with endrin and carbon
tetrachloride did not result in potentiation of hepatobiliary
functions. The levels of some serum enzymes were elevated (two-fold)
in rats given endrin plus carbon tetrachloride over those in rats
given endrin or carbon tetrachloride alone, indicating an additive
interaction. Dietary treatment with endrin aldehyde slightly increased
the levels of serum glutamic oxaloacetic transaminase and glutamic
pyruvic transaminase; and endrin ketone induced a small elevation in
glutamic pyruvic transaminase levels. Neither compound altered bile
flow or biliary phenolphthalein glucuronide excretion. Combination
with carbon tetrachloride increased the levels of some serum enzymes
(two-fold) over those seen with the aldehyde or the ketone or carbon
tetrachloride alone (Young & Mehendale, 1986).
9. EFFECTS ON HUMAN BEINGS
9.1 Exposure of the general population
9.1.1 Acute toxicity
In mild cases of poisoning, dizziness, weakness of the legs,
abdominal discomfort, nausea, and vomiting have been reported. Some
patients have complained of temporary deafness or were slightly
disorientated or aggressive. The onset of poisoning is variable and
may occur 0.5-10 h after consumption of contaminated food or
contamination of the skin; the interval is usually 1-4 h, depending on
the quantity ingested. Severe poisoning is manifested by sudden
epileptiform fits, with frothing at the mouth, facial congestion, and
violent convulsive movements of the limbs, sometimes leading to
dislocation of a shoulder or other injury. The fits may last for
several minutes and may be followed by a period of semiconsciousness
for 15-30 min or until the next fit. In general, these convulsions
occur suddenly, with no prodromal sign or symptom. An uncommon but
very serious symptom observed in two children was hyperthermia (41 °C
or higher); the high fever was followed by decerebrate rigidity. In
fatal cases, death occurs within 2-12 h. In survivors, recovery is
rapid, within 24 h, and uneventful, although some patients have
complained of headache, dizziness, weakness, and anorexia for several
weeks (Davis & Lewis, 1956; Jacobziner & Raybin, 1959; Hoogendam et
al., 1962; Hayes, 1963; Weeks, 1967; Hayes, 1982). After clinical
recovery, EEG changes consisting of bilateral synchronous theta-wave
activity and occasional bilateral synchronous spike and wave
complexes, believed to be associated with brain stem irritation, may
still be found and may persist for up to several weeks (Hoogendam et
al., 1962, 1965; Weeks, 1967).
9.1.2 Poisoning incidents
Hayes (1982) reviewed poisoning cases caused by endrin. Outbreaks
of acute intoxication due to endrin have occurred by contamination of
flour during transport in railway cars. A first episode, which was
well studied, occurred in 1956 in Wales, United Kingdom (Davis &
Lewis, 1956): At least 59 people were ill enough to require medical
treatment, and at least 100 more had some symptoms, which were not
severe enough to require medical advice. No one died. On the basis of
the concentration of endrin in bread prepared from the flour (150
mg/kg), Hayes (1963) estimated that 0.20-0.25 mg/kg body weight may
cause a single convulsion and that the dose necessary to produce
repeated convulsions is about 1 mg/kg body weight. Karplus (1971)
estimated the lethal dose in man to be approximately 10 mg/kg body
weight.
A few conflicting data are available on the concentration of
endrin in the tissues of victims of fatal intoxication. Hayes (1982)
quoted levels of 7-10 mg/kg in the liver and 0.7-4.4 mg/kg in the
brain; however, 10-fold lower levels were reported in the tissues of
autopsied victims of an outbreak of poisoning caused by ingestion of
bread prepared from contaminated flour in the Middle East (Curley et
al., 1970). In another incident, two sacks of contaminated flour
contained endrin at 184.5 and 234.5 mg/kg, and the bread and rolls
prepared from the contaminated flour contained 125.67-176.11 mg/kg.
The levels of endrin in serum, collected 30 min, 20 h, and 30 h after
convulsions in one person were 0.053, 0.038, and 0.021 mg/litre,
respectively; three other cases had 0.003-0.004 mg/litre of blood
serum 9-19 h after convulsions. One of these three people had no
symptoms (Coble et al., 1967). The reported serum or blood levels of
endrin associated with convulsions must be interpreted in the context
of the rapid removal of endrin from blood and the often significant
time lag in taking blood samples after convulsions. When the time
between convulsion and blood sampling is long, the endrin levels
reported are likely to be much lower than those at the time of the
convulsion.
Four outbreaks of endrin intoxication occurred in Doha (Qatar)
and Hofuf (Saudi Arabia) in 1967, during which 874 people were
hospitalized of whom 26 died; another 500-750 people showed symptoms
of intoxication but required no hospitalization. These outbreaks were
due to contamination of flour by endrin leaking from drums during
shipment. The endrin concentrations found in bread were 48-1807 mg/kg,
and those in the blood of patients were 0.007-0.032 mg/litre (Weeks,
1967; Curley et al., 1970).
Between July and September 1984, an epidemic of endrin poisoning
occurred in Pakistan, resulting in acute convulsions. In 18 of 21
affected villages surveyed, 70% (106/152) of the cases for which age
was known were in children aged 1-9 years; 9.8% (19/194) of the
affected people died. A composite sugar sample taken from the houses
of three cases contained endrin at 0.04 mg/kg. Endrin was detected in
the blood of 12/18 patients, at levels of 0.3-254.0 µg/litre of serum.
It was also determined in brain, kidneys, adipose tissue, and liver of
one person and found at levels of 1680, 1760, 4010, 1430 µg/kg
respectively (Anon., 1984; Hill et al., 1986; Rowley et al., 1987).
In mid-March 1988, three members of a family in Orange County,
California, USA, became ill within 1 h of eating taquitos (baked corn
shell filled with spicy meat and salad). Two of the three had multiple
grand mal seizures. Subsequently, two other people were reported to
have had seizures less than 12 h after eating taquitos. All five
patients had obtained the taquitos from the same shop within 5 days.
The food was analysed, and the presence of endrin was confirmed but
not quantified. The origin of the endrin could not be identified
(Anon., 1989).
An episode of acute endrin poisoning was reported in 33 Mexican
children, who had sudden seizures without sensory alterations (Singh
& West, 1985).
Several other cases have been published of single accidental or
intentional intoxications, in children and in adults (Jacobziner &
Raybin, 1959; Karplus, 1971). Reddy et al. (1966) described 60 cases
of fatal endrin poisoning out of 95 encountered in India after the
introduction of endrin in agricultural work as an insecticide in 1959.
The majority of the cases were suicidal. Froth, petechial
haemorrhages, a kerosene-like smell and massive pulmonary oedema were
the characteristic autopsy findings. Respiratory failure was the most
common cause of death. The authors concluded that the toxic dose of
endrin is 5-50 mg/kg body weight or about 1 g; the lethal dose is
about 6 g. In a poisoning case in a 19-year-old male who ingested an
unknown amount of endrin, convulsions and gross pulmonary oedema were
found (Jedeikin et al., 1979). No histological changes were found in
the liver. At least some of the pulmonary changes seen in such cases
may be due to aspiration of the petroleum hydrocarbon solvent in
formulations of endrin.
A case of polyneuropathy of the Guillain-Barré type was
attributed to exposure to a mixture of DDT and endrin. Since
convulsions were not recorded, the causal relationship remains
doubtful (Jenkins & Toole, 1964).
In a fatal case of endrin poisoning, ingestion of 12 g of endrin
by a 49-year-old man caused convulsions (persisting for 4 days),
hypersalivation, hyperthermia, renal insufficiency, thrombocytopenia,
and recurrent hypotension. Death followed after 11 days due to
pulmonary complications (infection and haemorrhage) and hypoxaemia
causing bradycardia and cardiac arrest. The endrin concentrations in
blood 4 h and 6 and 11 days after ingestion were 450, 86 and 71
µg/litre. Endrin levels in adipose tissue, heart, brain, kidneys, and
liver, 11 days after ingestion were 89.5, 0.87, 0.89, 0.55, and 1.32
mg/kg, respectively (Runhaar et al., 1985).
The medical treatment of endrin poisoning is described in Annex
II.
9.2 Occupational exposure
9.2.1 Factory workers
No fatal case has been reported due to occupational exposure in
manufacturing and formulating plants (Van Raalte, 1965; Jager, 1970),
which may be due in part to underreporting but is also certainly due
to the fact that occupational exposure involving the absorption of
lethal doses occurs rarely under practical circumstances. Furthermore,
the rapid metabolism of endrin minimizes build-up of toxic levels in
tissues during normal working days.
Several cases of acute, non-fatal poisoning occurred in a
manufacturing plant in The Netherlands due to accidental over-exposure
to endrin (Jager, 1970). Endrin had been manufactured in this plant
since 1957. During the first 9 years of production of aldrin,
dieldrin, and endrin in the plant, 17 cases of poisoning with
convulsions occurred, five of which involved more than one convulsion.
Three of the cases were due to acute over-exposure to endrin among
workers who were handling these materials at high concentrations every
day. There was no fatality during 1300 man-years of exposure. No
evidence was found of skin sensitization, and there was no case of
permanent, partial, or complete incapacity. No difference was seen in
absenteeism due to illness among these workers in comparison with
those in other plants, and the results of liver function tests and
complete blood cell counts were within normal limits. In the cases of
poisoning, recovery from clinical and neurological signs, including
EEG tracings, was rapid and complete (Hoogendam et al., 1962, 1965;
Jager, 1970; Versteeg & Jager, 1973).
A series of studies has been published on the results of
continuing medical supervision of workers in this plant. A
complementary follow-up of 189 workers and of 52 workers who had left
employment at the plant for various reasons was published in 1973
(Versteeg & Jager, 1973). These workers had been exposed to endrin for
up to 14.5 years in 1973. In agreement with data published from a
study of 71 workers in an endrin manufacturing plant in the USA (Hayes
& Curley, 1968), endrin was not found in the blood of these workers,
except in cases of accidental, acute over-exposure. Medical
supervision of workers employed in the manufacture and formulation of
endrin and other pesticides for 1-19 years (average, 12 years), data
on absenteeism, the results of tests for liver function and blood
chemistry, blood morphology, urine analysis, the occurrence of
sensitization, the pattern and course of EEG changes in cases of
poisoning, other medical studies (including electrocardiography, chest
x rays, blood pressure, body weight), and the incidence and pattern of
diseases, including the occurrence of malignant growths, showed no
difference between workers exposed to endrin and other chemical plant
operators. Residues of endrin were not found in plasma (< 3 µg/litre)
or in adipose tissue (< 0.03 mg/kg).
A significant difference was found between workers exposed to
aldrin and dieldrin only, workers not exposed to insecticides, and
workers exposed to endrin only: endrin workers had lower blood levels
of the DDT metabolite para,para'-DDE than the other workers, and the
levels were lower than those in the general population of the
surrounding area, although DDT and related compounds had never been
manufactured in the plant. A second parameter that was compared was
excretion of 6-beta-hydrocortisol in the urine. (Increased activity of
the drug-metabolizing enzyme system increases the activity of the
oxidative pathway by which 6-beta-hydroxylase converts endogenous
cortisol to 6-beta-hydrocortisol and thus, relatively, diminishes the
contribution of the reductive pathway, leading to excretion of
17-hydroxycorticosteroids.) The ratio of the urinary excretion of
6-beta-hydroxycortisol to that of 17-hydroxysteroids was significantly
higher in the endrin workers than in workers not exposed to endrin
(Jager, 1970).
A third parameter of this enzyme system that was studied was
urinary excretion of D-glucaric acid (an end-product of the glucuronic
acid pathway in the liver), which has been shown to increase after
exposure to microsomal enzyme-stimulating compounds, like endrin
(Hunter et al., 1971; Notten & Henderson, 1975). In the endrin
workers, urinary excretion of D-glucaric acid after a week of exposure
increased significantly over pre-exposure levels and those in a
control group of workers. Excretion diminished again after 3 days
without exposure (Hunter et al., 1972; Ottevanger &Van Sittert, 1979;
Vrij-Standhardt et al., 1979; Van Sittert, 1985).
Since anti-12-hydroxyendrin is the only metabolite found in the
urine of endrin-exposed workers, a study was initiated to find whether
there is a correlation between the quantity of this metabolite and
that of D-glucaric acid excreted in the urine. A positive relationship
was found between excretion of the endrin metabolite and of D-glucaric
acid after 7 days. After exposure was discontinued, excretion of
anti-12-hydroxyendrin decreased faster than that of D-glucaric acid.
The fact that endrin-exposed workers had D-glucaric acid levels within
the normal range after 6 weeks indicates that enzyme induction in
endrin workers is reversible. The authors concluded that a urinary
level of anti-12-hydroxyendrin of 0.130 µg/g of creatinine is the
threshold exposure level, below which enzyme induction is not produced
(Ottevanger & Van Sittert, 1979; Van Sittert, 1985). Endrin did not
increase total urinary porphyrin excretion over that in a control
group of employees (Strik, 1979; Nagelsmit et al., 1979;
Vrij-Standhardt et al., 1979).
In a follow-up mortality study of the same group of workers,
vital status and cause of death were assessed for 232 of a group of
more than 1000 workers. This group was selected because they had
experienced high exposures in the initial years of manufacture and
formulation and because of the long periods of exposure (mean, 11
years; range, 4-27) and observation (mean, 24 years; range, 4-29).
Total observed mortality was 25, whereas 38 deaths were expected on
the basis of mortality statistics for the male Dutch population. Of
the nine cancer deaths, three were due to lung cancer; the remaining
six were due to cancers of stomach, pancreas, bladder, and kidney,
multiple myeloma, and cerebral glioma. It was concluded that the
pesticides manufactured had no specific carcinogenic activity
(Ribbens, 1985).
9.2.2 Dose-response relationships
It has not been possible to establish a dose-response
relationship between single or repeated oral exposures and endrin
concentrations in blood, adipose tissue, or organs and severity of
intoxication, because the actual oral intake in the accidental cases
was not known, and the onset of symptoms of intoxication and the time
of measuring concentrations of endrin in blood, organs, or tissues
were not comparable (Davis & Lewis, 1956; Hayes, 1963; Coble et al.,
1967; Weeks, 1967; Curley et al., 1970; Karplus, 1971; Hayes, 1982;
Anon., 1984).
Blood samples have been analysed in three cases of acute
over-exposure (Table 28): A formulator and an operator were
accidentally splashed with a 20% endrin emulsifiable concentrate,
which was washed off within 10 min. Neither developed signs or
symptoms of intoxication. The third case was in a formulator who
handled technical-grade endrin powder without wearing a dust-mask. He
had convulsions 4 h after starting work, but after treatment recovered
fully the next day. Blood samples from four colleagues working next to
him, but wearing dust-masks, were also examined. The author estimated
that the threshold level of endrin in the blood below which no sign or
symptom of intoxication occurs is 50-100 µg/litre and that the
half-life of endrin in blood is in the order of 24 h (Jager, 1970).
9.2.3 Exposures to mixtures
A retrospective mortality study was carried out on workers
employed in the manufacture of heptachlor and endrin in a plant in
Tennessee, USA, between 1952 and 1976. The study comprised 835 men who
had worked for more than 3 months up to 20 years at the plant. No
overall excess of deaths from cancer was found; however, there was an
excess of deaths from cerebrovascular disease (7 observed, 2.3
expected) (Wang & MacMahon, 1979).
A further retrospective cohort study was conducted to examine the
mortality of workers employed in the manufacture of chlordane,
heptachlor, DDT, aldrin/dieldrin, and endrin in a plant in Colorado,
USA, where endrin was manufactured from 1953 until 1965. Approximately
2100 workers who had been employed for at least 6 months in the plants
were involved. No excess of cerebrovascular disease was observed
(Ditraglia et al., 1981).
Neither study proves conclusively that exposure to these
organochlorine insecticides is associated with increased prevalence of
malignancy or other cause of death, but they are limited in design and
in the desciption of exposure.
A field study was carried out in 1983 in the Ivory Coast to
assess the health hazards associated with the handling and application
by hand-held sprayers of an ultra-low volume formulation consisting of
endrin at 85 g/litre, DDT at 333 g/litre, and methylparathion at 85
g/litre in petroleum solvent. Groups of five or six farmers sprayed 3
litre/ha of the formulation 4-6 weeks after sowing cotton and again 15
or 30 days after the first application. The spray apparatus was filled
and cleaned by the same men. The recommended protective clothing was
worn only rarely, and the handling and application techniques were
careless, resulting in many cases in appreciable skin contamination.
No adverse health effect was observed. Absorption of endrin was
monitored by measuring the concentration of anti-12-hydroxyendrin in
spot samples of urine collected about 20 h after spraying. The mean
concentrations after the first, second, and third applications were
0.34 (range, 0.04-0.59), 0.52 (range, 0.09-1.4), and 0.45 mg/g of
creatinine (range, 0.0-0.92). One person who had handled and sprayed
the formulation carefully still had anti-12-hydroxyendrin in the
urine after the third application, but at a very low level (0.03 mg/g
of creatinine). Measurements of para-nitrophenol, a metabolite of
methyl-parathion, in urine indicated that the rate of metabolism of
methylparathion was increased as a result of enzyme induction by
endrin in the liver (Kummer & Van Sittert, 1984, 1986). It was
concluded that endrin accumulated in most of the farmers after three
applications within a short period. An increase to toxic levels might
result if spraying were more frequent and at shorter intervals and if
the recommended clothing was not worn.
9.2.4 Appraisal of effects of occupational exposures
Endrin is a very toxic compound. Several episodes of fatal and
non-fatal poisoning have occurred, mostly from accidental
contamination of food and also from intentional (suicidal) ingestion.
The lethal oral dose is estimated to be 10 mg/kg body weight. In
non-fatal cases, recovery is rapid and complete within a few days. The
oral dose that causes a single convulsion is estimated to be 0.25
mg/kg body weight, and that which induces repeated convulsions, 1.0
mg/kg body weight.
Exposure of workers to endrin for long periods did not induce
adverse effects that were attributed to this compound, although
occasional cases of acute, non-fatal intoxication due to accidental
over-exposure have occurred. Endrin was not detected in the blood of
workers exposed to endrin at < 3.0 µg/litre. The threshold level of
endrin in blood that results in intoxication is estimated to be 50-100
µg/litre. Absorption of a toxic dose is therefore unlikely during
occupational exposure if recommended controls and precautions are
used. In fatal cases, endrin concentrations in blood as high as 450
µg/litre have been reported; however, it is not possible to establish
a dose-response relationship. Since endrin is not normally found in
air, water, or food, except under conditions of contamination,
exposure of the general population is not significant.
Table 28. Concentrations of endrin in blood from acutely over-exposed workers
Case Time of first Endrin concentration (µg/litre)
sampling
First 12 h 24 h 36 h 5 days
sample later later later later
Formulator 1 h after 90 ND
accident
Operator 40 min after 27 25 ND
accident
Formulator Directly after 80 20 ND
without dust-mask convulsion
Four colleagues Same time ND-10
with dust-masks time as above
ND, not detectable (< 5 µg/litre)
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Endrin was evaluated by the Joint FAO/WHO Expert Committee on
Pesticide Residues in 1963, 1965, and 1970 (FAO/WHO, 1964, 1965,
1971). In 1970, the Committee established an acceptable daily intake
(ADI) for humans of 0-0.0002 mg/kg body weight, which was based on the
level that causes no toxicological effect in rats and dogs, 1 mg/kg of
diet (equivalent to 0.05 mg/kg body weight per day in rats and 0.025
mg/kg body weight per day in dogs).
The Joint FAO/WHO Codex Alimentarius Commission has published
maximum residue limits for endrin (Table 29; (FAO/WHO, 1986b).
Table 29. Codex maximum limits for the sum of residues of
endrin and delta-ketoendrin
Commodity Maximum residue limit
(mg/kg product)
Apples 0.02a
Barley 0.02a
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02a
Eggs (without shells) 0.2
Meat (carcass fat) 0.1b
Milk 0.0008b
Poultry (carcass fat) 1
Rice (husked or polished) 0.02a
Sorghum 0.02a
Sweet maize 0.02a
Wheat 0.02a
aAt or near the limit of detection
bExtraneous residue limit
The International Agency for Research on Cancer (IARC) concluded
in 1974 and 1987 that there was inadequate evidence for the
carcinogenicity of endrin in experimental animals and that the
evidence from studies in humans was inadequate. Endrin was therefore
classified in Group 3: not classifiable as to its carcinogenicity to
humans (IARC, 1974, 1987).
In 1988, the Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified technical-grade endrin as
highly hazardous in normal use (WHO, 1992). A data sheet on endrin was
issued in 1978 (WHO/FAO, 1975).
REFERENCES
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1985) High-affinity
stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic Biochem Physiol, 24: 95-102.
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1986) Effects of insecticides
on GABA-induced chloride influx into rat brain microsacs. J Toxicol
Environ Health, 18: 13-23.
Abbott DC, Harrison RB, Tatton JO'G, & Thomson J (1966) Organochlorine
pesticides in the atmosphere. Nature, 211(5046): 259-261.
Abbott DC, Goulding R, & Tatton JO'G (1968) Organochlorine pesticide
residues in human fat in Great Britain. Br Med J, iii: 146-149.
Abbott DC, Holmes DC, & Tatton JO'G (1969) Pesticide residues in the
total diet in England and Wales, 1966-67. Organochlorine pesticide
residues in the total diet. J Sci Food Agric, 20(4): 245-259.
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in United Kingdom. Br Med J, ii: 553-556
Abdel-Razik M, Marzouk MAH, Mowafy LE, & Abdel-Kader MA (1988)
Pesticide residues in the River Nile water, Egypt. Pak J Sci Ind Res,
31(11): 795-797.
Abdou SM, Abdel-Gawaad AA, Abdel-Amaim E, Abdel-Hady SM, & El-Alfy MB
(1983) Organochlorine pesticide residues in buffaloes milk in Kalubia
province and the effect of the presence of insecticides on coagulation
time. Egypt J Dairy Sci, 11: 197-203.
Acker L & Schulte E (1974) [Chlorinated hydrocarbons in human fat.]
Naturwissenschaften, 61: 32 (in German).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Seasonal fluctuations of
organochlorine and triazines pesticides in the aquatic system of
Ioannina Basin (Greece). Sci Total Environ, 58: 243-253.
Albers PH, Sileo L, & Mulhern BM (1986) Effects of environmental
contaminants on snapping turtles of a tidal wetland. Arch Environ
Contam Toxicol, 15: 39-49.
Albert LA (1990) Environmental contamination in Mexican food. In:
Hriagu JO & Simmons MS ed., Food contamination from environmental
sources. New York, John Wiley and Sons, pp 542-577.
Albert L, Mendez F, Cebrian ME, & Portales A (1980) Organochlorine
pesticide residues in human adipose tissue in Mexico. Results of a
preliminary study in three Mexican cities. Arch Environ Health, 35(5):
262-269.
Albert LA, Vega P, & Nava E (1982) [Organochlorine pesticides. VI.
Organochlorine pesticide residues in Mexican evaporated milks.]
Biotica, 7(3): 473-482 (in Spanish).
Alford-Stevens AL, Eichelberger JW, & Budde WL (1988) Multi-laboratory
study of automated determination of polychlorinated biphenyls and
chlorinated pesticides in water, soil and sediment by gas
chromatography/mass spectrometry. Environ Sci Technol, 22: 304-312.
Ali SL (1986) [Pesticide residues and traces of heavy metals in cod
liver oil.] Pharm Ztg, 131(38): 2288-2290 (in German).
Al-Omar MA, Al-Ogaily NH, Tawfiq SJ, & Al-Bassoumy M (1985a) Residue
levels of organochlorine insecticides in sewage plant effluent. J Biol
Sci Res, 16(1): 145-151.
Al-Omar MA, Tameesh AH, & Al-Ogaily NH (1985b) Dairy product
contamination with organochlorine insecticide residues in Bagdad
district. J Biol Sci Res, 16(1): 133-144.
Altmeier G & Korte F (1969) [Contributions to ecological chemistry
(XXIV). Metabolism of endrin-14C in perfused rats' livers.]
Tetrahedron Lett, 49: 4269-4271 (in German).
Anderson A (1986) Monitoring and biased sampling of pesticide residues
in fruits and vegetables. Methods and results, 1981-1984. Var Foda,
38(Suppl. 1): 8-55.
Anderson RL & Defoe DL (1980) Toxicity and bioaccumulation of endrin
and methoxychlor in aquatic invertebrates and fish. Environ Pollut
(Ser A), 22: 111-121.
Anderson HH, Hine CH, Kodama JJ, & Critchlow JK (1953) Class B
Determination on HI-1185 Endrin Emulsifiable Concentrate, San
Francisco, University of California, School of Medicine (UC Report No.
213).
Ang C, Meleady K, & Wallace L (1989) Pesticide residues in drinking
water in the north coast region of New South Wales, Australia,
1986-87. Bull Environ Contam Toxicol, 42: 595-602.
Anon. (1964) Report on investigation of fish kills in Lower
Mississippi River, Atchafalaya River and Gulf of Mexico. Washington,
DC, US Department of Health, Education, and Welfare, Public Health
Service, Division of Water Supply and Pollution Control.
Anon (1979) Determination of residues of organochlorine pesticides in
animal fats and eggs. Report of the committee for analytical methods
for residues of pesticides and veterinary products in foodstuffs of
the Ministry of Agriculture, Fisheries and Food. Analyst, 104:
425-433.
Anon. (1984) Acute convulsions associated with endrin
poisoning--Pakistan. Morb Mortal Wkly Rep, 33(49): 687-695.
Anon. (1988a) Introduction--US Environmental Protection Agency Office
of Drinking Water health advisories. Rev Environ Contam Toxicol, 104:
1-8.
Anon. (1988b) Endrin. Rev Environ Contam Toxico,l 104: 103-114.
Anon. (1989) Endrin poisoning associated with taquito ingestion,
California. Morb Mortal Wkly Rep, 38(19): 345-347.
Argyle RJ, Williams GC, & Dupree HK (1973) Endrin uptake and release
by fingerling channel catfish (Ictalurus punctatus).
J Fish Res Board Canada, 30(11): 1743-1744.
Arthur RD, Cain JD, & Barrentine BF (1976) Atmospheric levels of
pesticides in the Mississippi Delta. Bull Environ Contam Toxicol,
15(2): 129-134.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees: Laboratory studies.
Riverside, University of California, Department of Entomology (Rev.
9/73 (M-16)).
Atuma SS (1985) Accumulation of organochlorine insecticides in the
blood of the general population of Nigeria. Toxicol Environ Chem,10:
77-82.
Baldwin MK & Hutson DH (1980) Analysis of human urine for a metabolite
of endrin by chemical oxidation and gas-liquid chromatography as an
indicator of exposure to endrin. Analyst, 105: 60-65.
Baldwin MK, Robinson J, & Parke DV (1970) Metabolism of endrin in the
rat. J Agric Food Chem, 18(6): 1117-1123.
Baldwin MK, Davis RA, & Burns DT (1973) Structural studies and
photochemical rearrangement of an animal metabolite of HEOD, the
active component of dieldrin. Pestic Sci, 4: 227-237.
Baldwin MK, Crayford JV, Hutson DH, & Street DL (1976) The metabolism
and residues of 14C-endrin in lactating cows and laying hens. Pestic
Sci, 7(6): 575-594.
Barcelo D & Puignou LG (1987) [Pesticide residue in Spanish U.H.T.
milks determined by high resolution gas chromatography.] Rev Agroquim
Tecnol Aliment, 27(4): 583-589 (in Spanish).
Barnett RW, D'Ercole AJ, Cain JD, & Arthur RD (1979) Organochlorine
pesticide residues in human milk samples from women living in
northwest and northeast Mississippi, 1973-75. Pestic Monit J, 13(2):
47-51.
Barth RAJ (1967) Pesticide toxicity in primates. Tulane, University of
Louisiana, Division of Hygiene and Tropical Medicine (Thesis).
Barthel WF, Hawthorne JC, Ford JH, Bolton GC, McDowell LL, Grissinger
EH, & Parsons DA (1969) Pesticide residues in sediments of the Lower
Mississippi River and its tributaries. Pestic Monit J, 3(1): 8-68.
Batterton JC, Boush JC, & Matsumura F (1971) Growth response of
blue-green algae to aldrin, dieldrin, endrin and their metabolites.
Bull Environ Contam Toxicol, 6(6): 589-594.
Becker DM & Sieg CH (1987) Egg shell quality and organochlorine
residues in eggs of merlins Falco columbarius in southeastern
Montana. Can Field-Nat, 101: 369-372.
Bedford CT (1974) Von Baeyer/IUPAC names and abbreviated chemical
names of metabolites and artifacts of aldrin (HHDN), dieldrin (HEOD)
and endrin. Pestic Sci, 5: 473-489.
Bedford CT & Harrod RK (1973) Synthesis of anti-12-hydroxyendrin and
12-ketoendrin, the two major mammalian metabolites of endrin.
Chemosphere, 4: 163-168.
Bedford CT & Hutson DH (1976) The comparative metabolism in rodents of
the isomeric insecticides dieldrin and endrin. Chem Ind, 1976:
440-447.
Bedford CT, Harrod RK, Hoadley EC, & Hutson DH (1975a) The metabolic
fate of endrin in the rabbit. Xenobiotica, 5(8): 485-500.
Bedford CT, Hutson DH, & Natoff IL (1975b) The acute toxicity of
endrin and its metabolites to rats. Toxicol Appl Pharmacol, 33:
115-121.
Bedford CT, Crane AE, & Harrod RK (1986a) Synthesis and confirmation
of structure of four mammalian metabolites of dieldrin and endrin.
Pestic Sci, 17: 659-667.
Bedford CT, Crane AE, Smith EH, & Wellard NK (1986b) Synthesis of
endrin metabolites. Part. 2: Total synthesis and confirmation of the
structure of 3-hydroxyendrin. Pestic Sci, 17: 33-47.
Belisle AA, Reichel WL, Locke LN, Lamont TG, Mulhern BM, Prouty RM,
DeWolf RB, & Cromartie E (1972) Residues in fish, wildlife and
estuaries. Pestic Monit J, 6: 133-138.
Benes V & Sram R (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind Med, 38(12): 442-444.
Bennett RO & Wolke RE (1987a) The effect of sublethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. I. Evaluation of serum
cortisol concentrations and immune responsiveness. J Fish Biol, 31(3):
375-385.
Bennett RO & Wolke RE (1987b) The effect of sub-lethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. II. The effect of
altering serum cortisol concentrations on the immune response. J Fish
Biol, 31(3): 387-394.
Benson WR (1969) Note on nomenclature of dieldrin and related
compounds. J Assoc Off Anal Chem, 52(5): 1109-1111.
Beyerbach M, Buthe A, Heidmann WA, Dettmer R, & Knuwer H (1987)
[Chlorinated hydrocarbons in eggs and livers of rooks (Corvus
frugilegus) from rookeries in Lower Saxony (northern Germany).]
J Ornitol, 128(3): 277-290 (in German with English summary).
Beyerbach M, Buthe A, Heidmann WA, Knuwer H, & Russel-Sinn HA (1988)
[The burden of dieldrin and other chlorinated hydrocarbons on the
lapwing (Vanellus vanellus).] J Ornitol, 129(3): 353-361 (in
German).
Bhowmik G (1978) Pretreating properties of endrin on plant
chromosomes. Letter to the Editor. Curr Sci, 47(15): 546-547.
Bianchi A, Tateo F, Nava C, Tateo S, Santamaria L, Berte F, &
Santagati G (1988) Presence of organophosphate and organochlorine
pesticides in the milk of women. Med Biol Environ, 16: 931-942.
Biberhofer J & Stevens RJJ (1987) Organochlorine contaminants in
ambient waters of Lake Ontario. Ottawa, Inland Water Directorate,
Waters Quality Branch,pp. 1-11 (Can Sci Ser (87) V51.159).
Biehl ML & Buck WB (1987) Chemical contaminants: their metabolism and
their residues. J Food Prot, 50(12): 1058-1073.
Bloomquist JR & Soderlund DM (1985) Neurotoxic insecticides inhibit
GABA-dependent chloride uptake by mouse brain vesicles. Biochem
Biophys Res Commun, 133(1): 37-43.
Bloomquist JR, Adams PM, & Soderlund DM (1986) Inhibition of
gamma-aminobutyric acid-stimulated chlorine flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
Blus LJ (1978) Short-tailed shrews: toxicity and residue relationship
of DDT, dieldrin and endrin. Arch Environ Contam Toxicol, 7: 83-98.
Blus LJ, Joanen T, Belisle AA, & Prouty RM (1975) The brown pelican
and certain environmental pollutants in Louisiana. Bull Environ Contam
Toxicol, 13: 646-655.
Blus L, Cromartie E, McNease L, & Joanen T (1979) Brown pelican:
population status, reproductive success, and organochlorine residues
in Louisiana, 1971-1976. Bull. Environ Contam Toxicol, 22: 128-135.
Blus LJ, Henny CJ, Kaiser TE, & Grove RA (1983) Effects on wildlife
from use of endrin in Washington State orchards. In: Fox GA & Hall RJ
ed. Transactions of the 48th North American Wildlife Conference on
Environmental Contaminants and Wildlife. Washington, DC, Wildlife
Management Institute, pp. 159-174.
Blus LJ, Henny CJ, & Grove RA (1989) Rise and fall of endrin usage in
Washington State fruit orchards: effects on wildlife. Environ Pollut,
60: 331-349.
Boellstorff DE, Ohlendorf HM, Anderson DW, O'Neill EJ, Keith JO, &
Prouty RM (1985) Organochlorine chemical residues in white pelicans
and western grebes from the Klamath Basin, California. Arch. Environ
Contam Toxicol, 14: 485-493.
Bollag JM & Henninger NM (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J Environ
Qual, 5(1): 15-18.
Bollen WB & Tu CM (1971) Influence of endrin on soil microbial
populations and their activity. Washington, DC, US Department of
Agriculture, Forest Service, pp 1-4 (Research Paper PNW 114).
Bonner JC & Yarbrough JD (1989) Role of the brain
t-butyl-bicyclophosphorothionate receptor in vertebrate resistance to
endrin, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane and
cypermethrin. J Pharmacol Exp Ther, 249(1): 149-154.
Borady AMA, Mikhail TH, Awadailah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23(1-2): 33-44.
Boyd EM & Stefec J (1969) Dietary protein and pesticide toxicity with
particular reference to endrin. Can Med Assoc J, 101: 335-339.
Braun F (1985) [PCN and chlorine-based pesticides in some Bavarian
rivers.]. Munch. Beitr. Abwasser Fisch Flussbiol, 39: 115-124 (in
German).
Breidenbach AW, Gunnerson CG, Kawahara FK, Lichtenberg JJ, & Green RS
(1967) Chlorinated hydrocarbon pesticides in major river basins,
1957-65. Public Health Rep, 82(2): 139-156.
Brodtmann NV. Jr (1976) Continuous analysis of chlorinated hydrocarbon
pesticides in the Lower Mississippi River. Bull Environ Contam
Toxicol, 15(1): 33-39.
Brooks GT (1969) The metabolism of diene-organochlorine (cyclodiene)
insecticides. Residue Rev, 27: 81-138.
Brooks GT (1974) Chlorinated insecticides, technology and application.
Cleveland, Ohio, CRC Press, vol. 1, pp 85-99 & vol 2, pp 94-97.
Brooks TM (1976) Mutagenicity studies with endrin in the host-mediated
assay. Unpublished report No. TLGR.0112.76, Sittingbourne, Kent, Shell
Research, submitted to WHO by Shell.
Bunck CM, Prouty RM, & Krynitsky AJ (1987) Residues of organochlorine
pesticides and polychlorobiphenyls in starlings (Sturnus vulgaris),
from the continental United States, 1982. Environ Monit Assess, 8:
59-75.
Burton WB & Pollard GE (1974) Rate of photochemical isomerization of
endrin in sunlight. Bull Environ Contam Toxicol, 12(1): 113-116.
Butler PA (1963) Commercial fisheries investigations. Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp 11-25
(Circular No. 167).
Butler PA (1973) Organochlorine residues in estuarine mollusks,
1965-1972. Pestic Monit J, 6(4): 238-362.
Cabral JRP, Raitano F, Mollner T, Bronczyk S, & Shubik P (1979) Acute
toxicity of pesticides in hamsters. Abstract: Eighteenth Annual
Meeting No. 384. Toxicol Appl Pharmacol, 48: A192.
Caceres O, Tundisi JG, & Castellan OAM (1987) Residues of
organochloric pesticides in reservoirs in Sao Paulo State. Cienc Cult,
39(3): 259-264.
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in near-shore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13(3): 296-309.
Cantoni C, Fabbris F, Rogledi R, & Campagnari A (1988) [Organochlorine
pesticides in foods of animal origin found during the 1985-1987
biennium.] Ind Aliment, 27(1): 6-8 (in Italian).
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ Monit Assess, 5(2): 155-163.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1978) Pesticide
residue levels in soils and crops, 1971--national soils monitoring
program (III). Pestic Monit J, 12(3): 117-136.
Carline RF & Lawal MV (1985) Contaminants and bilateral asymmetry in
yellow perch. Environ Toxicol Chem, 4: 543-547.
Carrero I, Fernandez-Moreno MD, Perez-Albarsanz MA, & Prieto JC (1989)
Lindane effect upon the vasoactive intestinal peptide
receptor/effector system in rat enterocytes. Biochem Biophys Res
Commun, 159(3): 1391-1396.
Carter BI & Simpson BJE (1978) Toxicity of insecticides: The acute
oral and percutaneous toxicities of two endrin bleed samples and of
technical endrin in Shellsol A and in toluene. Unpublished report No.
TLTR.0001.78, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Casper VL (1967) Galveston Bay pesticide study--water and oyster
samples analyzed for pesticide residues following mosquito control
program. Pestic Monit J, 1(3): 13-15.
Casteel SW & Cook WO (1985) Endrin toxicosis in a cat. J Am Vet Med
Assoc, 186(9): 988-989.
Castonguay M, Dutil J-D, & Desjardins C (1989) Distinction between
American eels (Anguilla rostrata) of different geographical origins
on the basis of their organochlorine contaminant levels. Can J Fish
Aquat Sci, 46: 836-843.
Celeste M de F & Caceres O (1987) [Chlorinated pesticide residues in
waters of Ribeirïo do Lobo (Broa) reservoir and its tributaries.]
Cienc Cult, 39(1): 66-70 (in Portuguese with English summary).
Cetinkaya M (1988) [Organochloro-pesticide residues in tobacco from
European cigarette brands.] Chem Mikrobiol Technol Lebensm, 11:
100-103 (in German with English summary).
Cetinkaya M & Schenek A (1987) [Investigation of
organochloro-pesticide residues in various raw cotton samples.] Chem
Mikrobiol Technol, Lebensm, 10: 150-153 (in German with English
summary).
Chau ASY (1974) Confirmation of pesticide residues identity. VII.
Solid matrix derivation procedure for the simultaneous confirmation of
heptachlor and endrin residues in the presence of large quantities of
polychlorinated biphenyls. J Assoc Off Anal Chem, 57(3): 585-591.
Chau ASY & Cochrane WP (1969) Cyclodiene chemistry. III. Derivative
formation for the identification of heptachlor, heptachlorepoxide,
cis-chlordane, trans-chlordane, dieldrin and endrin pesticide residues
by gaschromatography. J Assoc Off Anal Chem, 52: 1220-1226.
Chau ASY & Cochrane WP (1971) Chromous chloride reductions. VI.
Derivative formation for the simultaneous identification of heptachlor
and endrin pesticide residues by gas chromatography. J Assoc Off Anal
Chem, 54(5): 1124-1131.
Chernoff N, Kavlock RJ, Hanisch RC, Whitehouse DA, Gray JA, Gray LE
Jr, & Sovocool GW (1979) Perinatal toxicity of endrin in rodents. I.
Fetotoxic effects of prenatal exposure in hamsters. Toxicology, 13:
155-165.
Clark DR Jr & Krynitsky A (1978) Organochlorine residues and
reproduction in the little brown bat, Laurel, Maryland--June 1976.
Pestic. Monit J, 12(3): 113-116.
Clark DR Jr, Laval RK, & Krynitsky AJ (1980) Dieldrin and heptachlor
residues in dead gray bats, Franklin County, Missouri--1976 versus
1977. Pestic Monit J, 13(4): 137-140.
Clawson RL & Clark DR (1989) Pesticide contamination of endangered
gray bats and their food base in Boone County, Missouri, 1982. Bull
Environ Contam Toxicol, 42: 431-437.
Coble Y, Hildebrandt P, Davis J, Raasch F, & Curley A (1967) Acute
endrin poisoning. J Am Med Assoc, 202: 489-493.
Cole LM & Casida JE (1986) Polychlorocycloalkane insecticide-induced
convulsions in mice in relation to disruption of the GABA-regulated
chloride ionophore. Life Sci, 39: 1855-1862.
Cole JF, Klevay LM, & Zavon MR (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol Appl Pharmacol,
16: 547-555.
Coleman RL (1968) Endrin induced alterations in bound carbohydrates in
rat serum. Bull Environ Contam Toxicol, 3(6): 348-353.
Coleman RL, Lawrence CH, & Sowell WL (1968) Trace metal alterations
following subacute exposure to endrin. Bull. Environ Contam Toxicol,
3(5): 284-295.
Cook WO & Casteel SW (1985) A suspected case of endrin toxicosis in a
cat. Vet Hum Toxicol, 27(2): 111.
Corneliussen PE (1969) Pesticide residues in total diet samples (IV).
Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Pesticide residues in total diet samples (V).
Pestic Monit J, 4(3): 89-105.
Corneliussen PE (1972) Pesticide residues in total diet samples (VI).
Pestic Monit J, 5(4): 313-330.
Cote MG, Plaa GL, Valli VE, & Villeneuve DC (1985) Subchronic effects
of a mixture of 'persistent' chemicals found in the Great Lakes. Bull
Environ Contam Toxicol, 34: 285-290.
Crockett AB, Wiersma GB, Tai H, & Mitchell W (1975) Pesticide and
mercury residues in commercially grown catfish. Pestic Monit J, 8(4):
235-240.
Cromartie E, Reichel WL, Locke LN, Belisle AA, Kaiser TE, Lamont TG,
Mulhern BM, Prouty RM, & Swineford DM (1975) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1971-72. Pestic Monit J, 9(1): 11-14.
Cueto C Jr & Biros FJ (1967) Chlorinated insecticides and related
materials in human urine. Toxicol Appl Pharmacol, 10: 261-269.
Cueto C Jr & Hayes WJ Jr (1962) The detection of dieldrin metabolites
in human urine. Agric Food Chem, 10(5): 366-369.
Cummings JG (1965) Pesticide residues in the total diet samples. J
Assoc Off Anal Chem, 48(6): 1177-1180.
Cummings JG (1966) Pesticides in the total diet. Residue Rev, 16:
30-45.
Curley A, Jennings RW, Mann HT, & Sedlak V (1970) Measurement of
endrin following epidemics of poisoning. Bull Environ Contam Toxicol,
5(1): 24-29.
Currie RA, Kadis VW, Breitkreitz WE, Cunnignham GB, & Bruns GW (1979)
Pesticide residues in human milk, Alberta, Canada--1966-70, 1977-78.
Pestic Monit J, 13(2): 52-55.
Dale WE, Copeland F, & Hayes WJ Jr (1965) Chlorinated insecticides in
the body fat of people in India. Bull World Health Organ, 33: 471-477.
Datta SK & Ghose KC (1985) Toxic effect of endrin on the
hepatopancreas of a teleost, Cyprinus carpio. Indian Biol, 17(1):
37-41.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
Davis GM & Lewis I (1956) Outbreak of food poisoning from bread made
of chemically contaminated flour. Br Med J, ii: 393-398.
Davis HC & Hidu H (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish Bull , 67(2): 393-404.
Dean BJ (1977) Chromosome studies on workers employed in an endrin
manufacturing plant. Unpublished report No. TLGR.0008.77,
Sittingbourne, Kent, Shell Research, submitted to WHO by Shell.
De Boer J (1989) Organochlorine compounds and bromodiphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere,, 18(11/12): 2131-2140.
De Campos M & Olszyna-Marzys AE (1979) Contamination of human milk
with chlorinated pesticides in Guatemala and El Salvador. Arch Environ
Contam Toxicol, 8: 43-58.
Deichmann WB & MacDonald WE (1971) Organochlorine pesticides and human
health. Food Cosmet Toxicol, 9(1): 91-103.
Deichmann WB, MacDonald WE, Blum E, Bevilacqua M, Radomski J,
Keplinger M, & Balkus M (1970a) Tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Ind Med, 39(10): 426-434.
Deichmann WB, MacDonald WE, Radomski J, Blum E, Bevilacqua M, &
Keplinger M (1970b) The tumorigenicity of aldrin, dieldrin, and endrin
in the albino rat. Ind Med, 39(7): 314.
Denison MS & Yarbrough JD (1985) Binding of insecticides to serum
proteins in mosquitofish (Gambusia affinis). Comp Biochem Physiol,
81C(1): 105-107.
Denison MS, Chambers JE, & Yarbrough JD (1985) Short-term interactions
between DDT and endrin accumulation and elimination in mosquitofish
(Gambusia affinis). Arch Environ Contam Toxicol, 14: 315-320.
De Paula Carvalho JP, Niskikawa AM, Aranha S, & Fay EF (1984)
[Organochlorine pesticide residues in bovine fat.] Biol Sao Paulo,
50(2): 39-48 (in Portuguese).
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Devault DS, Clark JM, & Lahvis G (1988) Contaminants and trends in
fall run coho salmon. J Great Lakes Res, 14(1): 23-33.
De Vos RH, van Dokkum W, Olthof PDA, Quiryns JK, Muys T, & van der
Poll JM (1984) Pesticides and other chemical residues in Dutch total
diet samples (June 1976-July 1978). Food Chem Toxicol 22(1): 11-21.
Deweese LR, Cohen RR, & Stafford CJ (1985) Organochlorine residues and
egg shell measurements for tree swallows Tachycineta bicolor in
Colorado. Bull Environ Contam Toxicol, 35: 767-775.
Dewitt JB (1965) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J Agric Food Chem, 4(10): 863-866.
Dikshith TSS & Datta KK (1973) Endrin-induced cytological changes in
albino rats. Bull Environ Contam Toxicol, 9(2): 65-69.
Dikshith TSS, Kumar SN, Raizada RB, & Srivastava MK (1989a)
Organochlorine insecticide residues in cattle feed. Bull Environ
Contam Toxicol, 43: 691-696.
Dikshith TSS, Kumar SN, Tandon GS, Raizada RB, & Ray PK (1989b)
Pesticide residues in edible oils and oil seeds. Bull Environ Contam
Toxicol, 42: 50-56.
Dinnel PA, Link JM, Stober QJ, Letourneau MW, & Roberts WE (1989)
Comparative sensitivity of sea urchin sperm bioassays to metals and
pesticides. Arch Environ Contam Toxicol, 18: 748-755.
Ditraglia D, Brown DP, Namekata T, & Iverson N (1981) Mortality study
of workers employed at organochlorine pesticide manufacturing plants.
Scand J Work Environ Health, 7(suppl 4): 140-146.
Donahue JF, Burse VW, Head SL, & Andrews JS (1988) Comparison of two
techniques for quantifying environmental contaminants in human serum.
Life Sci, 43: 2257-2264.
Donoso J, Dorigan J, Fuller B, Gordon J, Kornreich M, Saari S, Thomas
L, & Walker P (1979) Reviews of the environmental effects of
pollutants. XIII. Endrin. Oak Ridge, Tennessee, Oak Ridge National
Laboratory (EPA-600/1-79-005).
DouAbul AAZ, Al-Saad HT, Al-Obaidy SZ, & Al-Rekabi HN (1987a) Residues
of organochlorine pesticides in fish from the Arabian Gulf. Water Air
Soil Pollut, 35: 187-194.
DouAbul AAZ, Al-Saad HT, & Al-Rekabi HN (1987b) Residues of
organochlorine pesticides in environmental samples from the Shatt
al-Arab River, Iraq. Environ Pollut, 43: 175-187.
DouAbul AAZ, Al-Omar M, Al-Obaidy S, & Al-Ogaily N (1987c)
Organochlorine pesticide residues in fish from the Shatt al-Arab
River, Iraq. Bull Environ Contam Toxicol, 38: 674-680.
DouAbul AAZ, Al-Saad HT, Al-Timari AA, & Al-Rekabi HN (1988)
Tigris-Euphrates delta: a major source of pesticides to the Shatt
al-Arab River (Iraq). Arch Environ Contam Toxicol, 17: 405-418.
Dowd PF, Mayfield GU, Coulon DP, Graves JB, & Newsom JD (1985)
Organochlorine residues in animals from three Louisiana watersheds in
1978 and 1979. Bull Environ Contam Toxicol, 34: 832-841.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-1970. Pestic Monit J,
5(4): 331-341.
Duggan RE & Lipscomb GQ (1969) Dietary intake of pesticide chemicals
in the United States (II), June 1966-April 1968. Pestic Monit J, 2(4):
153-162.
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in total
diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Pesticide residues in total
diet samples (II). Pestic Monit J, 1(2): 2-12.
Duke TW & Dumas DP (1974) Implications of pesticide residues in the
coastal environment. In: Vernberg FJ & Vernberg WB, ed. Pollution and
physiology of marine organisms. New York, Academic Press, pp 137-164.
Dureja P, Walia S, & Mukerjee SK (1987) New photometabolites of
endrin. Indian J Chem, 26G: 898-899.
Durham WF & Wolfe HR (1962) Measurement of the exposure of workers to
pesticides. Bull World Health Organ, 26: 75-91.
Dutch Agricultural Advisory Commission on Environmental Pollutants
(1983) Annual report. The Hague, Ministry of Agriculture, Management
of Nature and Fisheries.
Earnest RD & Benville PE Jr (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game, 58(2): 127-132.
Egan H, Goulding R, Roburn J, & Tatton JO'G (1965) Organochlorine
pesticide residues in human fat and human milk. Br Med J, ii: 66.
Eisenberg M & Topping JJ (1985) Organochlorine residues in finfish
from Maryland waters 1976-1980. J Environ Sci Health B20(6): 729-742.
Eisler R (1970a) Latent effects of insecticide intoxication to marine
molluscs. Hydrobiologia, 36(3-4): 345-352.
Eisler R (1970b) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish. Tech Paper Bur Sport
Fish Wildl, 46: 1-12.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCB's in
river Nile water, Egypt. Bull Environ Contam Toxicol, 34: 126-133.
Ellis DH, Deweese LR, Grubb TG, Kiff LF, Smith DG, Jarman WM, &
Peakall DB (1989) Pesticide residues in Arizona peregrine falcon eggs
and prey. Bull Environ Contam Toxicol, 42: 57-64.
Elnabarawy MT, Welter AN, & Robideau RR (1986) Relative sensitivity of
three daphnid species to selected organic and inorganic chemicals.
Environ Toxicol Chem, 5: 393-398.
El Nabawi A, Heinzow B, & Kruse H (1987) Residue levels of
organochlorine chemicals and polychlorinated biphenyls in fish from
the Alexandria Region, Egypt. Arch Environ Contam Toxicol, 16:
689-696.
El-Sebae AH (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region. In:
Research on the toxicity, persistence, bioaccumulation,
carcinogenicity and mutagenicity of selected substances (Activity G).
Final reports on projects dealing with toxicity (1983-1985). Athens,
United Nations Environment Programme (Mediterranean Action Plan (MAP),
Technical Reports Series No. 10).
Ely RE, Moore LA, Carter RH, & App BA (1957) Excretion of endrin in
the milk of cows fed endrin-sprayed alfalfa and technical endrin. J
Econ Entomol, 50(3): 348-349.
Emerson TE Jr (1965) Mechanisms of hemoconcentration in the dog during
acute endrin insecticide poisoning. Can J Physiol Pharmacol, 43:
793-800.
Emerson TE Jr & Hinshaw LB (1965) Peripheral vascular effects of the
insecticide endrin. Can J Physiol Pharmacol,43: 531-539.
Emerson TE Jr, Brake CM, & Hinshaw LB (1963) Mechanism of Action of
the Insecticide Endrin. Oklahoma City, Oklahoma, Civil Aeromedical
Research Institute (Report No. 63-16).
Emerson TE Jr, Brake CM, & Hinshaw LB (1964) Cardiovascular effects of
the insecticide endrin. Can J Physiol Pharmacol, 42: 41-51.
Engst R & Knoll R (1973) [On the contamination of surface, rain and
drinking waters with chlorinated hydrocarbons.] Nahrung, 17(8):
837-851 (in German with English summary).
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection of
chemical mutagens by the dominant lethal assay in the mouse. Toxicol
Appl Pharmacol, 23: 288-325.
Ercegovich CD & Rashid KA (1977) Mutagenesis induced in mutant strains
of Salmonella typhimurium by pesticides. In: Abstracts of the 174th
ACS National Meeting, Chicago, Illinois. Washington, DC, American
Chemical Society, Division of Pesticide Chemistry (Abstract No. 43).
Everaarts JM, Koeman JH, & Brader, L (1971) Contribution à l'étude des
effets sur quelques éléments de la faune sauvage des insecticides
organo-chlorés utilisés au Tchad en culture cotonnière. Cotton Fibre
Trop, 26(4): 385-394.
Fabacher DL & Chambers H (1976) Uptake and storage of 14C-labelled
endrin by the livers and brains of pesticide-susceptible and resistant
mosquitofish. Bull Environ Contam Toxicol, 16(2): 203-207.
Fahrig R (1974) Comparative mutagenicity studies with pesticides. In:
Montesano R & Tomatis L ed. Chemical carcinogenesis essays. Lyon,
International Agency for Research on Cancer, pp 161-181 (IARC
Scientific Publications No. 10).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No. PL:1963/13;
WHO/Food Add./23).
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. Geneva, World Health Organization (FAO Meeting Report No.
PL:1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1971) Joint meeting of FAO Working Party of Experts and the
WHO Expert Group on Pesticide Residues. 1970 Evaluation of some
pesticide residues in food. Geneva, World Health Organization
(AGP:1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1984) Codex guidelines on good practice in pesticide residue
analysis. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986a) Recommendations for methods of analysis of pesticide
residues. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR8-1986).
FAO/WHO (1986b) Codex maximum limits for pesticide residues. Rome,
Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme,
Food and Agriculture Organization of the United Nations, p 33-IV (FAO
CAC Vol. XIII, ed. 2).
Fasola M, Vecchio I, Caccialanza G, Gandini C, & Kitsos M (1987)
Trends of organochlorine residues in eggs of birds from Italy, 1977 to
1985. Environ Pollut, 48: 25-36.
FDA (1988) Food and Drug Administration pesticide program. Residues in
foods--1987. J Assoc Off Anal Chem, 71(6): 156A-174A.
Ferguson DE, Culley DD, & Cotton WD (1964) Resistance to chlorinated
hydrocarbon insecticides in three species of freshwater fish.
Bioscience, 14: 43-44.
Flickinger EL & King KA (1972) Some effects of aldrin-treated rice on
Gulf Coast wildlife. J Wildl Manage, 36: 706-727.
Flickinger EL, Mitchell CA, & Krynitsky AJ (1986) Dieldrin and endrin
residues in fulvous whistling ducks in Texas in 1983. J. Field
Ornithol, 57(2): 85-192.
Folmar LC (1978) In vitro inhibition of rat brain ATPase, pNPPase,
and ATP-32Pi exchange by chlorinated-diphenyl ethanes and cyclodiene
insecticides. Bull Environ Contam Toxicol, 19: 481-488.
Foschi F, Natali G, Guberti MG, Camisani MG, Taccheto Barbina M,
Spessotto C, & Bargarolo L (1985) [Study on residues of argicultural
chemicals in apples.] Inf Fitopatol, 12: 14-20 (in Italian).
Fournier E, Treich I, Campagne L, & Capelle N (1972) Pesticides
organo-chlorés dans le tissu adipeux d'êtres humains en France. Eur J
Toxicol, 1(1): 11-26.
Fox ME, Roper DS, & Thrush SF (1988) Organochlorine contaminants in
surficial sediments of Manukau Harbour, New Zealand. Mar Pollut
Bull,19(7): 333-336.
Frank R, Braun HE, Holdrinet M, Sirons GJ, Smith EH, & Dixon DW (1979)
Organochlorine insecticides and industrial pollutants in the milk
supply of Southern Ontario, Cananda--1977. J Food Prot, 42(1): 31-37.
Frank R, Braun HE, & Holdrinet MVH (1981) Residues from past uses of
organochlorine pesticides and PCB in waters draining eleven
agricultural watersheds in Southern Ontario, Canada, 1975-1977. Sci
Total Environ, 20: 255-276.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario--1983. J Food Prot, 48(6):
499-504.
Fredrickson DS (1978) Report on bioassay of endrin for possible
carcinogenicity. Fed Reg, 43(225): 54298.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Galassi S & Provini A (1981) Chlorinated pesticides and PCBs contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gant DB, Eldefrawi ME, & Eldefrawi AT (1987) Cyclodiene insecticides
inhibit GABAa receptor-regulated chloride transport. Toxicol Appl
Pharmacol, 88(3): 313-321.
Garrett NE, Stack HF, & Waters MD (1986) Evaluation of the genetic
activity profiles of 65 pesticides. Mutat Res, 168(3): 301-325.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements, and other chemicals in adult total diet
samples October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off Anal
Chem, 69(1): 123-145.
Giesy JP, Mewsted J, & Garling DL (1986) Relationships between
chlorinated hydrocarbon concentrations and rearing mortality of
chinook salmon (Onchorhynchus tshawytscha) eggs from Lake Michigan.
J Great Lakes Res, 12(1): 82-98.
Gips T (1987) Breaking the pesticide habit--Alternatives to 12
hazardous pesticides (International Alliance for Sustainable
Agriculture (IASA) Publication No. 1987-2).
Glatt H, Jung R, & Oesch F (1983) Bacterial mutagenicity investigation
of epoxides: drugs, drug metabolites, steroids and pesticides. Mutat
Res, 11: 99-118.
Glooschenko WA, Strachan WMJ, & Sampson RCJ (1976) Distribution of
pesticides and polychlorinated biphenyls in water, sediments and
seston of the Upper Great Lakes--1974. Pestic Monit J, 10(2): 61-67.
Gluth G & Hanke W (1985) A comparison of physiological changes in
carp, Cyprinus carpio, induced by several pollutants at sub-lethal
concentrations. I. The dependency on exposure time. Ecotoxicol Environ
Saf, 9: 179-188.
Godsil PJ & Johnson WC (1968) Pesticide monitoring of the aquatic
biota of the Tule Lake National Wildlife Refuge. Pestic Monit J, 1(4):
21-26.
Goerlitz DF & Law LM (1974) Determination of chlorinated insecticides
in suspended sediment and bottom material. J Assoc Off Anal Chem,
57(1): 176-181.
Goldentahl EI (1978a) Teratology study in rats. Unpublished report No.
163-488, International Research and Development Corporation, submitted
to WHO by Shell.
Goldentahl EI (1978b) Teratology study in hamsters. Unpublished report
No. 163-478, International Research and Development Corporation,
submitted to WHO by Shell.
Good EE & Ware GW (1969) Effects of insecticides on reproduction in
the laboratory mouse. Toxicol Appl Pharmacol, 14: 201-203.
Graves JB & Bradley JR (1965) Response of Swiss albino mice to
intraperitoneal injection of endrin. J Econ Entomol, 58(1): 178-179.
Gray LE Jr, Kavlock RJ, Chernoff N, Gray JA, & McLamb J (1981)
Perinatal toxicity of endrin in rodents. III. Alterations of
behavioural ontogeny. Toxicology, 21: 187-202.
Green DR, Stull JK, & Heesen TC (1986) Determination of chlorinated
hydrocarbons in coastal waters using a moored in situ sampler and
transported live mussels. Mar Pollut Bull, 17(7): 324-329.
Gregor DJ & Gummer WD (1989) Evidence of atmospheric transport and
deposition of organochlorine pesticides and polychlorinated biphenyls
in Canadian Arctic snow. Environ Sci Technol, 23: 561-565.
Gübeli T & Clerc JT (1988) [Detection of pesticide residues in ethanol
extracts of plants.] Pharm Acta Helv, 63(3): 85-89 (in German).
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984,
dietary intakes of pesticides, selected elements and other chemicals.
J Assoc Off Anal Chem, 71(6): 1200-1209.
Hall RJ & Swineford D (1980) Toxic effects of endrin and toxaphene on
the southern leopard frog Rana sphenocephala. In: Mellanby, K. ed.
Environmental pollution. Barking, Essex, Applied Science Publishing,
pp 53-65.
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hamdy Y & Post L (1985) Distribution of mercury, trace organics and
other heavy metals in Detroit River sediments. J Great Lakes Res,
11(3): 353-365.
Hansen DJ, Schimmel SC, & Forester J (1977) Endrin: effects on the
entire life cycle of a saltwater fish, Cyprinodon variegatus. J
Toxicol Environ Health, 3: 721-733.
Harris CR, Sans WW, & Miles JRW (1966) Exploratory studies on the
occurrence of organochlorine insecticides residues in agricultural
soils in southwestern Ontario. J Agric Food Chem,14(4): 398-403.
Hart LG & Fouts JR (1963) Effects of acute and chronic DDT
administration in hepatic microsomal drug metabolism in the rat
(28686). Proc Soc Exp Biol Med,114: 388-392.
Hartgrove RW Jr, Hundley SG, & Webb RE (1977) Characterization of the
hepatic mixed function oxidase system in endrin resistant and
-suspectible pinevoles. Pestic Biochem Physiol, 7(2): 146-153.
Hashemy-Tonkabony SE & Mosstofian B (1979) Chlorinated pesticide
residues in chicken egg. Poult Sci 58(6): 1432-1434.
Hashemy-Tonkabony SE & Soleimani-Amiri MJ (1976) Detection and
determination of chlorinated pesticide residues in raw and various
stages of processed vegetable oil. J Am Oil Chem Soc, 53(12): 752-753.
Hashimoto I & Nishiuchi S (1981) Establishment of bioassay methods for
evaluation of acute toxicity of pesticides to aquatic organisms. J
Pestic Sci, 6: 257-264.
Hassett AJ, Viljoen PT, & Liebenberg JJE (1987) An assessment of
chlorinated pesticides in the major surface water resources of the
Orange Free State during the period September 1984 to September
1985.Water SA, 13(3): 133-136.
Hawker DW & Connell DW (1986) Bioconcentration of lipophilic compounds
by some aquatic organisms. Ecotoxicol Environ Saf, 11: 184-197.
Hawthorne JC, Ford JH, & Markin GP (1974) Residues of mirex and other
chlorinated pesticides in commercially raised catfish. Bull Environ
Contam Toxicol, 11(3): 258-264.
Hayes WJ Jr (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department of
Health, Education, and Welfare, Communicable Disease Center,
Toxicology Section, pp 68-70.
Hayes WJ Jr (1975) Toxicology of pesticides, Baltimore,
Maryland,Williams & Wilkins, pp 288-294.
Hayes WJ Jr (1982) Pesticides studied in man, Baltimore, Maryland,
Williams and Wilkins, pp 247-251.
Hayes WJ Jr & Curley A (1968) Storage and excretion of dieldrin and
related compounds. Effect of occupational exposure. Arch Environ
Health, 16(2): 155-162.
Hayes WJ Jr, Dale WE, & Burse VW (1965) Chlorinated hydrocarbon
pesticides in the fat of people in New Orleans. Life Sci, 4:
1611-1615.
Heidmann WA, Büthe A, Beyerbach M, Löhmer R, & Rüssel-Sinn HA (1989)
[Chlorinated hydrocarbons of some bird species breeding in the inland
of Lower Saxony.] J Ornitol, 130(3): 311-320 (in German with English
summary).
Heinz GH, Erdman TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henderson C, Johnson WL, & Inglis A (1969) Organochlorine insecticide
residues in fish. National pesticides monitoring program. Pestic Monit
J, 3: 145-171.
Henderson C, Inglis A, & Johnson WL (1971) Organochlorine insecticide
residues in fish. Fall 1969, National Pesticide Monitoring Program.
Pestic Monit J, 5: 1-11.
Hendrickson CM & Bowden JA (1976) In vitro inhibition of lactic acid
dehydrogenase by insecticidal polychlorinated hydrocarbons. 2.
Inhibition by dieldrin and related compounds. J Agric Food Chem,
24(4): 756-759.
Hendrickx A & Maes R (1969) The excretion of chlorinated hydrocarbon
insecticides in human mother milk. J Pharm Belg, 24(9-10): 459-463.
Hermanutz R (1974) Quarterly report. Duluth, Minnesota, US
Environmental Protection Agency, National Water Quality Laboratory.
Hermanutz RO, Eaton JG, & Mueller LH (1985) Toxicity of endrin and
malathion mixtures to flagfish (Jordanella floridae). Arch Environ
Contam Toxicol, 14: 307-314.
Hernandez FH, Lopez Benet FJ, Escriche JM, & Ubeda JCB (1987) Sulfuric
acid cleanup and KOH-ethanol treatment for confirmation of
organochlorine pesticides and polychlorinated biphenyls: application
to wastewater samples. J Assoc Off Anal Chem, 70(4): 727-733.
Herrera Marteache A, Polo Villar LM, Jodral Villarejo M, Polo Villar
G, Mallol J, & Pozo Lora R (1978) [Organochlorine pesticide residues
in human fat in Spain.] Rev San Hig Publico, 52: 1125-1144 (in Spanish
with English summary).
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, p 147 (Fish
and Wildlife Technical Report No. 2).
Hill EF, Health RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report: Wildlife No. 191).
Hill RH Jr, Needham LL, & Liddle JA (1986) The laboratory's role in
environmental health emergency investigations. Clin Toxicol, 24(5):
363-374.
Hine CH (1965) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 2, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH (1968) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 7, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH, Anderson HH, Kodama JK, & Gutenberg EF (1954) Class B
evaluation of endrin compositions. Unpublished report No. 223, San
Francisco, CA, University of California School of Medicine, submitted
to WHO by Shell.
Hinshaw LB, Solomon LA, Reins DA, Fiorica V, & Emerson TE (1966)
Effects of the insecticide endrin on the cardiovascular system of the
dog. J Pharmacol Exp Ther, 153(2): 225-236.
Hirom PC, Millburn P, Smith RL, & Williams RT (1972) Species
variations in the threshold molecular-weight factor for the biliary
excretion of organic anions. Biochem J, 129: 1071-1077.
Hoffman WS, Fishbein WI, & Andelman MB (1964) The pesticide content of
human fat tissue. Arch Environ Health, 9: 387-394.
Hoffman WS, Adler H, Fishbein WI, & Bauer FC (1967) Relation of
pesticide concentration in fat to pathological changes in tissues.
Arch Environ Health, 15: 758-765.
Hogmire HW, Weaver JE, & Brooks JL (1990) Survey for pesticides in
wells associated with apple and peach orchards in West Virginia. Bull
Environ Contam Toxicol, 44: 81-86.
Holden AV (1970) International cooperative study of organo-chlorine
pesticide residues in terrestrial and aquatic wildlife, 1967/1968.
Pestic Monit J, 4(3): 117-135.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1962) Electroencephalograms
in insecticide toxicity. Arch Environ Health, 4: 86-94.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1965) Nine years toxicity
control in insecticide plants. Arch Environ Health, 10: 441-448.
Horn H, Hartner L, & von Faber H (1987) [On the suitability of liver
function tests in birds for the ecotoxicological evaluation of
environmental chemicals.] Dtsch Tierarztl Wochenschr, 94: 1-48 (in
German with English summary).
Horsfall F Jr, Webb RE, Price NO, & Young RW (1970) Residues in apples
subsequent to ground sprays of endrin. J Agric Food Chem, 18: 221-223.
Hrdina PD, Singhal RL, & Peters DAV (1974) Changes in brain biogenic
amines and body temperature after cyclodiene insecticides. Toxicol
Appl Pharmacol, 29(1): 119.
Hrubec J (1988) [Pesticides and drinking-water.] H2O, 21(11):
278-282 (in Dutch).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 153).
Hunter CG, Robinson J, & Richardson A (1963) Chlorinated insecticide
content of human body fat in southern England. Br Med J, i: 221-224.
Hunter J, Maxwell JD, Carrella M, Stewart DW, & Williams R (1971)
Urinary-D-glucaric acid excretion as a test for hepatic enzyme
induction in man. Lancet, 20 March: 572-575.
Hunter J, Maxwell JD, Stewart DW, Williams R, Robinson J, & Richardson
A (1972) Increased hepatic microsomal enzymes activity from
occupational exposure to certain organochlorine pesticides. Nature,
237: 399-401.
Hutson DH (1981) The metabolism of insecticides in man. In: Hutson DH
& Roberts TR ed. Progress in pesticide biochemistry. New York, John
Wiley and Sons, vol 1, pp 287-333.
Hutson DH & Hoadley EC (1974) The oxidation of a cyclic alcohol
(12-hydroxyendrin) to a ketone (12-ketoendrin) by microsomal
mono-oxygenation. Chemosphere, 5:205-210.
Hutson DH, Baldwin MK, & Hoadley EC (1975) Detoxication and
bioactivation of endrin in the rat. Xenobiotica, 5(11): 697-714.
IARC (1974) Endrin. In:Some organochlorine pesticides. Lyon,
International Agency for Research on Cancer, pp. 157-171 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Volume 5).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p. 63 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Illahi A, Amin N, Hashmi AS, Nawaz M, & Naeem-ur Rahman (1986)
Incidence of endrin residues in cucumber and its effects on the
biological system of rats. J Pak Med Assoc, 36(8): 209-211.
Illahi A, Roohi J, & Hashmi AS (1987) Present status of endrin
residues in peas and its effect on biological systems. J Pure Appl
Sci, 6(1): 1-4.
Ito N, Tatematsu M, Nakanishi K, Hasegawa R, Takano T, Imaida K, &
Ogiso T (1980) The effects of various chemicals on the development of
hyperplastic liver nodules in hepatectomized rats treated with
N-nitrosodimethylamine or N-2-fluorenylacetamide. Gann, 71: 832-842.
Jacobziner H & Raybin HW (1959) Briefs on accidental chemical
poisonings in New York City. Poisoning by insecticide (endrin). NY
State J Med, 59: 2017-2022.
Jager KW (1970) Aldrin, dieldrin, endrin and telodrin. An
epidemiological study of long-term occupational exposure. Amsterdam,
Elsevier Science Publishers.
Japanese Environmental Agency (1975) Environmental survey report on
chemical substances in FY 1974. Unpublished report, December 1975,
Tokyo, Environmental Health Department, Planning and Coordination
Bureau.
Japenga J, Wagenaar WJ, Smedes F, & Salomons W (1987) A new, rapid
clean-up procedure for the simultaneous determination of different
groups of organic micropollutants in sediments; application in two
European estuarine sediment studies. Environ Technol Lett, 8(1): 9-20.
Jarvinen AW, Tanner DK, & Kline ER (1988) Toxicity of chlorpyrifos,
endrin, or fenvalerate to fathead minnows following episodic or
continuous exposure. Ecotoxicol Environ Saf, 15: 78-95.
Jedeikin R, Kaplan R, Shapira A, Radwan H, & Hoffman S (1979) The
successful use of 'high level' PEEP in near fatal endrin poisoning.
Crit Care Med, 7(4): 168-170.
Jegier Z (1964) Health hazards in insecticide spraying of crops. Arch
Environ Health, 8: 670-674.
Jenkins RB & Toole JF (1964) Polyneuropathy following exposure to
insecticides. Arch Intern Med, 113: 691-695.
Johnson DW (1968) Pesticides and fishes--a review of selected
literature. Trans Am Fish Soc, 97(4) 398-424.
Johnson RD & Manske DD (1976) Pesticide residues in total diet samples
(IX). Pestic Monit J, 9(4): 157-169.
Johnson RD & Manske DD (1977) Pesticide and other chemical residues in
total diet samples (XI). Pestic Monit J, 11(3): 116-131.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1979) Pesticide and
other chemical residues in infant and toddler total diet samples, (I),
August 1974-July 1975. Pestic Monit J, 13(3): 87-98.
Johnson RD, Manske DD, & Podrebarac DS (1981a) Pesticide, metal, and
other chemical residues in adult total diet samples, (XII), August
1975-July 1976. Pestic Monit J, 15(1): 54-71.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1981b) Pesticide,
heavy metal, and other chemical residues in infant and toddler total
diet samples, (II), August 1975-July 1976. Pestic Monit J, 15(1):
39-50.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1984) Pesticide,
metal, and other chemical residues in adult total diet samples,
(XIII), August 1976-July 1977. J Assoc Off Anal Chem, 67(1): 154-166.
Johnson MG, Kelso JRM, & George SE (1988) Loadings of organochlorine
contaminants and trace elements to two Ontario lake systems and their
concentrations in fish. Can J Fish Aquat Sci, 45: 170-178.
Jolley WP, Stemmer KL, Grande F, Richmon J, & Pfitzer EA (1969) The
effects exerted upon beagle dogs during a period of two years by the
introduction of 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8-dimethanonaphthalene
into their daily diets. Unpublished report, Cincinnati, Ohio,
Kettering Laboratory, submitted to WHO by Shell.
Joy RM (1976) Convulsive properties of chlorinated hydrocarbon
insecticides in the cat central nervous system. Toxicol Appl
Pharmacol, 35: 95-106.
Kacew S, Sutherland DJB, & Singhal RL (1973) Biochemical changes
following chronic administration of heptachlor, heptachlor epoxide and
endrin to male rats. Environ Physiol Biochem, 3: 221-229.
Kachole MS & Pawar SS (1977) Effect of endrin on microsomal electron
transport reactions. Part I: Sleeping time, electron transport
components and protection due to pre-treatments. Abstracts of the 1976
Annual General Meeting of Biochemists. J Biochem, 14(1): 45.
Kadis VW, Breitkreitz WE, & Jonasson OJ (1970) Insecticide levels in
human tissues of Alberta residents. Can J Public Health, 61(5):
413-416.
Kagan J, Kagan ED, & Seigneurie E (1986) Alpha-terthienyl, a powerful
fish poison with light-dependent activity. Chemosphere, 15(1): 49-57.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Lamont TG, Mulhern BM,
Prouty RM, Stafford CJ, & Swineford DM (1980) Organochlorine
pesticide, PCB, PBB residues and necropsy data for bald eagles from 29
states--1975-77. Pestic Monit J, 13: 145-149.
Kampe W (1985) [Pesticide residues in animal feeding-stuffs.] Dtsch
Tierarztl Wochenschr, 92(6): 228-231 (in German).
Kanitz S & Castello G (1966) [The presence of residues of some
pesticides in human fatty tissue and in some foods. Initial results of
a survey carried out in Liguria.] G Ig Med Prev, 7: 1-19 (in Italian).
Karplus M (1971) [Endrin poisoning in children.] Harefuah, 18(3):
113-116 (in Hebrew with English summary).
Kassabi M, ElHraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kathpal T & Dewan RS (1975) Improved clean-up technique for the
estimation of endosulfan and endrin residues. J Assoc Off Anal Chem,
58(5): 1076-1078.
Katz M & Chadwick GG (1961) Toxicity of endrin to some Pacific
Northwest fishes.Trans Am Fish Soc, 90: 394-397.
Kavlock RJ, Chernoff N, Hanisch RC, Gray J, Rogers E, & Gray LE Jr
(1981) Perinatal toxicity of endrin in rodents. II. Fetotoxic effects
of prenatal exposure in rats and mice. Toxicology, 21: 141-150.
Kavlock RJ, Chernoff N, & Rogers EH (1985) The effect of acute
maternal toxicity on fetal development in the mouse. Teratog Carcinog
Mutagen, 5: 3-13.
Kavlock RJ, Rogers JM, Gray LE, & Chernoff N (1987) Postnatal
alterations in development resulting from prenatal exposure to
pesticides. In: Pesticide science and biotechnology, Proceedings of
the 6th International Congress on Pesticide Chemicals, pp 561-564.
Keilty TJ & Stehly GR (1989) Preliminary investigation of protein
utilization by an aquatic earthworm in response to sublethal stress.
Bull Environ Contam Toxicol, 43: 350-354.
Keilty TJ, White DS, & Landrum PF (1988a) Short-term lethality and
sediment avoidance assays with endrin-contaminated sediment and two
Oligochaetes from Lake Michigan. Arch Environ Contam Toxicol, 17:
95-101.
Keilty TJ, White DS, & Landrum PF (1988b) Sublethal responses to
endrin in sediment by Limnodrilus hoffmeisteri (Tubificidae) and in
mixed-culture with Stylodrilus heringianus (Lumbriculidae). Aquat
Toxicol, 13: 227-250.
Keilty TJ, White DS, & Landrum PF (1988c) Sublethal responses to
endrin in sediment by Stylodrilus heringianus (Lumbriculidae) as
measured by a 137cesium marker layer technique. Aquat Toxicol, 13:
251-270.
Keplinger MK & Deichmann WB (1967) Acute toxicity of combinations of
pesticides. Toxicol Appl Pharmacol, 10: 586-595.
Khangarot BS, Sehgal A, & Bhasin MK (1985) Man and biosphere--studies
on the Sikkim Himalayas. Part 6: Toxicity of selected pesticides to
frog tadpole, Rana hexadactyla (Lesson). Acta Hydrochim Hydrobiol,
13(3): 391-394.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci Health,
A21(1): 15-53.
Kiene RP & Capone DG (1984) Effects of organic pollutants on
methanogenesis, sulfate reduction and carbon dioxide evolution in salt
marsh sediments. Mar Environ Res 13: 141-160.
,
Kiigemagi U, Sprowls RG, & Terriere LC (1958) Endrin content of milk
and body tissues of dairy cows receiving endrin daily in their diet.
J Agric Food Chem, 6(7): 518-521.
King KA, Flickinger EL, & Hildebrand HH (1977) The decline of brown
pelicans on the Lousiana and Texas Gulf Coast. Southwest Nat, 21(4):
417-431.
King KA, Blankinship DR, Payne E, Krynitsky AJ, & Hensler GL (1985)
Brown pelican populations and pollutants in Texas, 1975-1981. Wilson
Bull, 97(2): 201-214.
Kinoshita FK & Kempf CK (1970) Quantitative measurement of hepatic
microsomal enzyme induction after dietary intake of chlorinated
hydrocarbon insecticides. Toxicol Appl Pharmacol, 17: 288.
Klein W, Mueller W, & Korte F (1968) [Insecticides in the metabolism.
XVI. Excretion, distribution and metabolism of endrin 14C in rats.]
Liebigs Ann Chem,713: 180-185.
Klevay LM (1971) Endrin excretion by the isolated perfused rat liver:
a sexual difference. Proc Soc Exp Biol Med, 136: 878-879.
Kodavanti PRS, Mehrotra BD, Chetty SC, & Desaiah D (1988) Effect of
selected insecticides on rat brain synaptosomal adenylate cyclase and
phosphodiesterase. J Toxicol Environ Health, 25: 207-215.
Koeman JH (1971) [The occurrence and the toxicological implications of
some chlorinated hydrocarbons in the Dutch coastal area in the period
1965-70], Utrecht, Rijks University, pp 88, 96 (Thesis) (in Dutch).
Koeman JH, Oskamp AAG, Veen J, Brouwer E, Rooth J, Zwart P, van de
Brock E, & van Genderen H (1967) Insecticides as a factor in the
mortality of the sandwich tern (Sterna sandvicensis). A preliminary
communication. Meded Fac Landbouwwet Rijksuniv Gent, 32: 841-854.
Koeman JH, Vink JAJ, & de Goeij JJM (1969) Causes of mortality in
birds of prey and owls in the Netherlands in the winter of 1968-69.
Andrea, 57: 67-76.
Koeman JH, Pennings JH, de Goeij JJM, Tjioe PS, Olindo PM, & Hopcraft
J (1972) A preliminary survey of the possible contamination of Lake
Nakuru in Kenya with some metals and chlorinated hydrocarbon
pesticides. J Appl Ecol, 9(2): 411-416.
Koeman JH, Pennings JH, Rosanto R, Soemarwoto O, Tjioe PS, Blancke S,
Kusumadinata S, & Djajadiredja RR (1974) Metals and chlorinated
hydrocarbon pesticides in samples of fish, sawah-duck eggs,
crustaceans and molluscs collected in Indonesia in April and May 1972.
Unpublished report, Wageningen-Bandung, University of Wageningen, The
Netherlands.
Korn S & Earnest R (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game, 60(3):
128-131.
Korte F (1969) Summary of results in 1969. Unpublished report,
submitted to WHO by Shell.
Korte F, Klein W, Weisgerber I, Kaul R, Mueller W, & Djirsurai A
(1970) Recent results in studies on the fate of chlorinated
insecticides. In: Deichmann WB, Radomski JL, & Penalver RA, ed.
Proceedings of the Sixth Conference on Toxicology and Occupational
Medicine, Pesticide Symposia. Miami, Florida, Halos & Associates,
Inc., pp 51-56.
Krantz WC, Mulhern BM, Bagley GE, Sprunt A, Ligas FJ, & Robertson WC
Jr (1970) Organochlorine and heavy metal residues in bald eagle eggs.
Pestic Monit J, 4(3): 136-140.
Kreitzer JF (1980) Effects of toxaphene and endrin at very low dietary
concentrations on discrimination acquisition and reversal in bobwhite
quail Colinus virginianus. Environ Pollut (Ser A), 23: 217-230.
Kreitzer JF & Heinz GH (1974) The effect of sub-lethal dosages of five
pesticides and a polychlorinated biphenyl on the avoidance response of
Coturnix quail chicks. Environ Pollut, 6: 21-29.
Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA,
Sileo L, Docjerty DE, & Erdman TC (1989) Microcontaminants and
reproductive impairment of the Forster's tern on Green Bay, Lake
Michigan--1983. Arch Environ Contam Toxicol, 18: 706-727.
Kudesia VP & Bali NP (1985) A study of pesticides in Kalinadi River
and evaluation of toxicity of some pesticides on fish Clarias
batrachus. Acta Cienc Indica, 10C(4): 245-254.
Kummer R & Van Sittert NJ (1984) Field study on health effects in
farmers applying an endrin/DDT/MEP formulation by hand-held ULV to
cotton in Ivory Coast. Unpublished report, The Hague, Shell
Internationale Petroleum Maatschappij, submitted to WHO by Shell.
Kummer R & Van Sittert NJ (1986) Field studies on health effects from
the application of two organophosphorus insecticide formulations by
hand-held ULV to cotton. Toxicol Lett, 33: 7-24.
Kurata M, Hirose K, & Umeda M (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
Kurhekar MP, D'Souza FC, & Meghal SK (1975) Rapid method for
extracting aldrin, dieldrin, and endrin from visceral material. J
Assoc Off Anal Chem, 58(3): 548-550.
Kutz FW, Yobs AR, & Yang HSC (1976) National pesticide monitoring
programs. In: Lee RE Jr, ed. Air pollution from pesticides and
agricultural processes. Cleveland, Ohio, CRC Press, pp 95-136.
Kutz F, Strassman S, & Yobs AR (1979a) Survey of pesticide residues
and their metabolites in the general population of the United States.
In: Berlin A, Wolff AH, & Hasegawa Y ed. Use of biological specimens
to assess human exposure to environmental pollutants, The Hague,
Martinus Nijhoff, pp 267-274.
Kutz FW, Strassman SC, & Sperling JF (1979b) Survey of selected
organochlorine pesticides in the general population of the United
States. Fiscal years 1970-1975. Ann NY Acad Sci, 320: 60-68.
Lara WH & Barreto HHC (1972) [Chlorinated pesticide residues in
water.] Rev Inst Adolfo Lutz, 32: 69-74 (in Portuguese with English
summary).
Lauer GJ, Nicholson HP, Cox WS, & Teasley JI (1966) Pesticide
contamination of surface waters by sugar cane farming in Louisiana.
Trans Am Fish Soc, 95(3): 310-316.
Lawrence CH, Coleman RL, & Sowell WL (1968) Endrin induced trace metal
alterations following acute exposure. Bull Environ Contam Toxicol,
3(4): 229-239.
Leard RL, Grantham BJ, & Pessoney GF (1980) Use of selected freshwater
bivalves for monitoring organochlorine pesticide residues in major
Mississippi stream systems, 1972-73. Pestic Monit J, 14(2): 47-52.
Lebel GL & Williams DT (1986) Determination of halogenated
contaminants in human adipose tissue. J Assoc Off Anal Chem, 69(3):
451-458.
Lichtenberg JJ, Eichelberger JW, Dressman RC, & Longbottom JE (1970)
Pesticides in surface waters of the United States; a 5-year summary,
1964-1968. Pestic Monit J, 4(2): 71-86.
Lopez-Avila V, Schoen S, Milanes J, & Beckert WF (1988)
Single-laboratory evaluation of EPA method 8080 for determination of
chlorinated pesticides and polychlorinated biphenyls in hazardous
wastes. J Assoc Off Anal Chem, 71(2): 375-387.
Lowe JI (1966) Some effects of endrin on estuarine fishes. In:
Proceedings of the 19th Annual Conference of the Southeast Association
of Game and Fish Commissioners, pp 271-276.
Luckens MM & Davis WH (1965) Toxicity of dieldrin and endrin to bats.
Nature, 207(4999): 879-880.
Luckens MM & Phelps KI (1969) Serum enzyme patterns in acute poisoning
with organochlorine insecticides. J Pharm Sci, 58(5): 569-572.
Ludke JL (1976) Organochlorine pesticide residues associated with
mortality: additivity of chlordane and endrin. Bull Environ Contam
Toxicol, 16(3): 253-260.
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multi-residue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-433.
Lund AE & Narahasi T (1983) Kinetics of sodium channel modification as
the basis for the variation in the nerve membrane effects of
pyrethroids and DDT analogs. Pestic Biochem Physiol, 20: 203-206.
Lykins BW Jr, Koffskey WE, & Miller RG (1986) Chemical products and
toxicologic effects of disinfection. J Am Water Works Assoc, 78(11):
66-75.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14(9): 1253-1265.
McGill AEJ & Robinson J (1968) Organochlorine insecticide residues in
complete prepared meals: a 12-month survey in SE England. Food Cosmet
Toxicol, 6: 45-57.
Machbub B, Ludwig HF, & Gunaratnam D (1988) Environmental impact from
agrochemicals in Bali (Indonesia). Environ Monit Assess, 11: 1-23.
McIntyre AE & Lester JN (1984) Occurrence and distribution of
persistent organochlorine compounds in UK sewage sludges. Water Air
Soil Pollut, 23: 397-415.
McKenney CL Jr (1986) Critical responses of populations of crustacea
to toxicants. Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Environmental Research
Brief EPA/600/M-86/004.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol 25: 921-928.
McLeese DW, Metcalfe CD, & Pezzack DS (1980) Bioaccumulation of
chlorobiphenyls and endrin from food by lobsters (Homarus
americanus). Bull Environ Contam Toxicol 25: 161-168.
McLeese DW, Burridge LE, & van Dinter J (1982) Toxicities of five
organochlorine compounds in water and sediment to Nereis virens. Bull
Environ Contam Toxicol 28: 216-220.
Madden JD, Finerty MW, & Grodner RM (1989) Survey of persistent
pesticide residues in the edible tissues of wild and pond-raised
Louisiana crayfish and their habitat. Bull Environ Contam Toxicol, 43:
779-784.
Manske DD & Corneliussen PE (1974) Pesticide residues in total diet
samples (VII). Pestic Monit J, 8(2): 110-124.
Manske DD & Johnson RD (1975) Pesticide residues in total diet samples
(VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Pesticide and other chemical residues in
total diet samples (X). Pestic Monit J, 10(4): 134-148.
Marinelli P, Stracciari GL, & Anfossi P (1986) [Presence of
organochlorinated pesticides in some wine-making by-products.] Zoot
Nutr Anim, 12: 479-486 (in Italian with English summary).
Marsh C (1963) Metabolism of D-glucuronolactone in mammalian systems.
Conversion of D-glucuronolactone into D-glucaric acid by tissue
preparations. Biochem J, 87: 82-90.
Marston RB, Tyo RM, & Middendorff SC (1969) Endrin in water from
treated Douglas fir seed. Pestic Monit J, 2(4): 167-171.
Martin RJ & Duggan RE (1968) Pesticide residues in total diet samples
(III). Pestic Monit J, 1(4): 11-20.
Martin DB & Hartman WA (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North Central United States. J Assoc Off Anal Chem, 68(4): 712-717.
Martin JP, Harding RB, Cannell GH, & Anderson L (1959) Influence of
five annual field applications of organic insecticides on soil
biological and physical properties. Soil Sci, 87: 334-338.
Maslansky CJ & Williams GM (1981) Evidence for an epigenetic mode of
action in organochlorine pesticide hepatocarcinogenicity. A lack of
genotoxicity in rat, mouse and hamster hepatocytes. J Toxicol Environ
Health, 8: 121-130.
Mason JW & Rowe OR (1976) The accumulation and loss of dieldrin and
endrin in the eastern oyster. Arch Environ Contam Toxicol, 4(3):
349-360.
Masud SZ & Farhat S (1985) Pesticide residues in foodstuffs in
Pakistan--organochlorine pesticides in fruits and vegetables. Pak J
Sci Ind Res, 28(6): 417-422.
Matsumoto K, Eldefrawi ME, & Eldefrawi AT (1988) Action of
polychlorocycloalkane insecticides on binding of
[35S]t-butylbicyclophosphorothionate to Torpedo electric organ
membranes and stereospecificity of binding site. Toxicol Appl
Pharmacol, 95: 220-229.
Matsumura F, Khanvilkar VG, Patil KC, & Boush GM (1971) Metabolism of
endrin by certain soil microorganisms. J Agric Food Chem, 19(1):
27-31.
Maule A, Plyte S, & Quirk AV (1987) Dehalogenation of organochlorine
insecticides by mixed anaerobic microbial populations. Pestic Biochem
Physiol, 27: 229-236.
Mayer, FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Environmental Research Laboratory (Environmental Research Brief
EPA/600/8-87/017).
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior Fish
and Wildlife Service (Resource publication 160).
Meena K, Gupta PK, & Bawa SR (1978) Endrin-induced toxicity in normal
and irradiated rats. Environ Res, 16: 373-382.
Mehrotra BD, Moorthy, KS, Reddy SR, & Desaiah D (1989) Effects of
cyclodiene compounds on calcium pump activity in rat brain and heart.
Toxicology, 54: 17-29.
Meith-Avcin N, Warlen SM, & Barber RT (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 meters.
Environ Lett, 5(4): 215-221.
Mersch-Sundermann V, Dickgiesser N, Hablizel U, & Gruber B (1988)
[Examination of mutagenicity of organic microcontaminations on the
environment. I. Communication: the mutagenicity of selected herbicides
and insecticides with the Salmonella-microsome-test (Ames-test) in
consideration of the pathogenetic potence of contaminated ground- and
drinking-water.] Zbl Bakteriol Hyg B, 186: 247-260 (in German).
Metcalf RL, Kapoor IP, Lu PY, Schuth CK, & Sherman P (1973) Model
ecosystem studies of the environmental fate of six organochlorine
pesticides. Environ Health Perspect, 4: 35-44.
Miles JRW & Harris CR (1973) Organochlorine insecticides residues in
streams draining agricultural, urban agricultural and resort areas of
Ontario, Canada, 1971. Pestic Monit J, 6(4): 363-368.
Miller PE & Fink GB (1973) Brain serotonin level and pentylenetetrazol
seizure threshold in dieldrin and endrin treated mice. Proc West
Pharmacol Soc, 16: 195-197.
Modin JC (1969) Chlorinated hydrocarbon pesticides in California bays
and estuaries. Pestic Monit J, 3(1): 1-7.
Morita H & Umeda M (1984) Detection of mutagenicity of various
compounds by FM3A cell system. Mutat Res, 130(5): 371.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay system. Mutat Res, 116: 185-216.
Morris RD (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse, Peromyscus maniculatus. Can J Zool,
46(5): 951-958.
Morris RD (1970) The effects of endrin on Microtus and Peromyscus.
I. Unenclosed field populations. Can J Zool, 48: 695-708.
Morris RD (1972) The effects of endrin on Microtus and Peromyscus.
II. Enclosed field populations. Can J Zool, 50(6): 885-896.
Moser GJ & Smart RC (1989) Hepatic tumour-promoting chlorinated
hydrocarbons stimulate protein kinase C activity. Carcinogenesis,
10(5): 851-856.
Mount DI & Putnicki GJ (1966) Summary report of the 1963 Mississippi
fish kill. North Am Wildl Nat Res Conf Trans, 31: 177-184.
Mount DI, Vigor LW, & Schafer ML (1966) Endrin: use of concentration
in blood to diagnose acute toxicity in fish. Science, 152: 1388-1390.
Mugambi JM, Kanja L, Maitho TE, Skaare JU, & Lokken P (1989)
Organochlorine pesticide residues in domestic fowl (Gallus
domesticus) eggs from Central Kenya. J Sci Food Agric, 48: 165-176.
Muir CMC (1970) The acute oral and percutaneous toxicities to rats of
formulations of aldrin, dieldrin or endrin. Unpublished report No.
TLGR.0020.70, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Mukerjee SK (1985) The environmental photodegradation of pesticides.
Indian J Agric Chem, 18(1): 1-9.
Mulhern BM, Reichel WL, Locke LN, Lamont TG, Belisle A, Cromartie E,
Bagerly GE, & Prouty R (1970) Organochlorine residues and autopsy data
from bald eagles. 1966-1968. Pestic Monit J, 4: 141-144.
Muncy RJ & Oliver AD Jr (1963) Toxicity of ten insecticides to the red
crawfish, Procambarus clarki (Girard). Trans Am Fish Soc, 92:
428-431.
Mussalo-Rauhamaa H, Salmela SS, Leppanen A, & Pyysalo H (1986)
Cigarettes as a source of some trace and heavy metals and pesticides
in man. Arch Environ Health, 41(1): 49-55.
Nagelsmit A, Vliet PW, van Wiel-Wetzels WAM, van der Wielard MJ, Strik
JJTWA, Ottevanger CF, & van Sittert NJ (1979) Porphyrins as possible
parameters for exposure to hexachlorocyclopentadiene, allylchloride,
epichlorohydrin and endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biochemical Press,
pp 55-61.
Narahasi T (1987) Effects of toxic agents on neural membranes. In:
Lowndes HE,ed. Electrophysiology in neurotoxicology. Boca Raton,
Florida, CRC Press, vol 1, pp 23-44.
NCI (1978) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute (DHEW Publication No. NIH 179-812)
NCI (1979) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute, 110 pp (Carcinogenesis Technical Report Series No. 12; NTIS
PB-288461)
Nebeker AV, Schuytema GS, Griffis WL, Barbitta JA, & Carey LA (1989)
Effect of sediment organic carbon on survival of Hyalella azteca
exposed to DDT and endrin. Environ Toxicol Chem, 8: 705-718.
Nelson SC, Bahler TL, Hartwell WV, Greenwood DA, & Harris LE (1956)
Serum alkaline phosphatase levels, weight changes and mortality rates
of rats fed endrin. J Agric Food Chem, 4(8): 696-700.
Nettleship DN & Peakall DB (1987) Organochlorine residue levels in
three high Arctic species of colonially-breeding seabirds from Prince
Leopold Island. Mar Pollut Bull, 18(8): 434-438.
NIOSH (1989) Manual of analytical methods. Endrin: Method No. 5519.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 1-4.
Nishimura N, Nishimura H, & Oshima H (1982) Survey on mutagenicity of
pesticides by the Salmonella-microsome test. J Aichi Med Univ Assoc,
10(4): 305-312.
Notten WRF & Henderson PT (1975) Alteration in urinary D-glucaric acid
excretion as an indication of exposition to xenobiotics. In:
Proceedings of the International Symposium--Environment and Health,
CEC/EPA/WHO, Paris, 1974.
Novak AF & Rao MRR (1965) Food safety program: endrin monitoring in
the Mississippi River. Science, 150: 1751.
Ohlendorf HM, Swineford DM, & Locke LN (1981) Organochlorine residues
and mortality of herons. Pestic Monit J, 14(4): 125-135.
Onodera S & Tabucanon MS (1986) Organochlorine pesticide residues in
the lower Chao Phraya River and klongs along the river at Bangkok
metropolitan area, 1982-1984. J Sci Soc Thailand, 12: 225-238.
Oomen PA (1986) A sequential scheme for evaluating the hazard of
pesticides to bees, Apis mellifera. Meded Fac Landbouwwet Rijksuniv
Gent, 51(3b): 1205-1213.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis K (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
O'Shea TJ, Brownell RL Jr, Clarke DR Jr, Walker WA, Gay ML, & Lamont
TG (1980) Organochlorine pollutants in small cetaceans from the
Pacific and South Atlantic Oceans, November 1968-June 1976. Pestic
Monit J, 14:(2): 35-46.
Ottevanger CF & Van Sittert NJ (1979) Relation between anti-12-hydroxy
endrin excretion and enzyme induction in workers involved in the
manufacture of endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 123-129.
Ottolenghi AD, Haseman JK, & Suggs F (1974) Teratogenic effects of
aldrin, dieldrin and endrin in hamsters and mice. Teratology, 9:
11-16.
Parveen Z & Masud SZ (1987) Organochlorine pesticide residues in
cattle feed samples in Karachi, Pakistan. J Sci Ind Res; 30(7):
513-516.
Patil KC, Matsumura F, & Boush GM (1970) Degradation of endrin,
aldrin, and DDT by soil microorganisms. Appl Microbiol, 19(5):
879-881.
Pavan I, Buglione E, Pettinati L, Perrelli G, Rubino GF, Bicchi C,
D'Amato A, Carlino F, Bugiani M, & Polizzi S (1987) [Accumulation of
organochlorine pesticides in human adipose tissue. data from the
province of Turin (Italy).] Med Lav, 78(3): 219-228 (in Italian).
Pawar SS & Kachole MS (1978) Hepatic and renal microsomal electron
transport reactions in endrin treated female guinea pigs. Bull Environ
Contam Toxicol, 20: 199-205.
Pearce F (1987) Pesticide deaths: the price of the green revolution.
New Sci, 114: 30.
Peterson SR & Ellarson RS (1978) pp'-DDE, polychlorinated biphenyls,
and endrin in old squaws in North America, 1969-73. Pestic Monit J,
11(4): 170-181.
Petrella VJ, Fox JP, & Webb RE (1975) Endrin metabolism in
endrin-susceptible and resistant strains of pine mice. Toxicol Appl
Pharmacol, 34: 283-291.
Petrella VJ, McKinney JD, Fox JP, & Webb RE (1977) Identification of
metabolites of endrin. Metabolism in endrin susceptible and resistant
strains of pine mice. J Agric Food Chem, 25(2): 393-398.
Pfister RM (1972) Interactions of halogenated pesticides and
microorganisms: a review. CRC Crit Rev Microbiol, 21(1): 1-33.
Phillips DD, Pollard GE, & Soloway SB (1962) Thermal isomerization of
endrin and its behaviour in gas chromatography. J Agric Food Chem,
10(3): 217-221.
Plimmer JR (1972) Photochemistry of organochlorine insecticides. In:
Tahori AS, ed. Proceedings of the Ssecond international IUPAC congress
of pesticides chemistry. New York, Gordon & Breach,vol 1, pp 413-432.
Podrebarac DS (1984) Pesticide, heavy metal, and other chemical
residues in infant and toddler total diet samples (IV). October
1977-September 1978. J Assoc Off Anal Chem, 67(1): 166-175.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environ Mutagen, 3: 11-32.
Prouty RM & Bunck C (1986) Organochlorine residues in adult mallard
and black duck wings, 1981-1982. Environ Monit Assess, 6: 49-57.
Prouty RM, Reichel WL, Locke LN, Belisle AA, Cromartie E, Kaiser TE,
Lamont TG, Mulhern BM, & Swineford DM (1977) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1973-74. Pestic Monit J, 11: 134-137.
Radeleff RD (1956) Hazards to livestock of insecticides used in
mosquito control. Mosq News, 16(2): 79-80.
Radhakrishnan AG & Antony PD (1989) Pesticide residues in marine
fishes. Fish Technol, 26: 60-61.
Rashid KA & Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. J Environ Sci Health, B21(4): 319-334.
Reddy DB, Edward VD, Abraham GJS, & Rao KV (1966) Fatal endrin
poisoning. A detailed autopsy, histopathological and experimental
study. J Indian Med Assoc, 46(3): 121-124.
Reddy DB, Abraham GJS, Edward VD, Naganna B, & Mathalli M (1967)
Further observations on endrin poisoning. J Indian Med Prof, 13(10):
5946, 5967-5968.
Redetzke K., Gonzalez AA, & Applegate HG (1983) Organochlorine
pesticides in adipose tissue of persons from Ciudad Juarez, Mexico. J
Environ Health, 46(1): 25-27.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 117(20): 525-527.
Reeves RG, Woodham DW, Ganyard MC, & Bond CA (1977) Preliminary
monitoring of agricultural pesticides in a cooperative tobacco pest
management project in North Carolina, 1971--first-year study. Pestic
Monit J, 11(2): 99-106.
Reichel WL, Lamont TG, Cromartie E, & Locke LN (1969) Residues in two
bald eagles suspected of pesticide poisoning. Bull Environ Contam
Toxicol, 4(1): 24-30.
Reidinger RF & Crabtree DG (1974) Organochlorine residues in golden
eagles, United States--March 1964-July 1971. Pestic Monit J, 8(1):
37-43.
Reins DA, Holmes DD, & Hinshaw LB (1964) Acute and chronic effects of
the insecticide endrin on renal function and renal hemodynamics. Can
J Physiol Pharmacol, 42(5): 599-608.
Reins DA, Rieger JA Jr, Stavinoha WB, & Hinshaw LB (1966) Effect of
endrin on venous return and catecholamine release in the dog. Can J
Physiol Pharmacol, 44: 59-67.
Ressang AA, Titus I, Andar RS, & Soedarmo D (1958) Aldrin, dieldrin
and endrin intoxication in cats. Commun Vet, 2(2): 71-88.
Reuber MD (1978) Carcinomas, sarcomas and other lesions in
Osborne-Mendel rats ingesting endrin. Exp Cell Biol, 46(3): 129-145.
Revzin AM (1966) Effects of endrin on telencephalic function in the
pigeon. Toxicol Appl Pharmacol, 9(1): 75-83.
Revzin AM (1980) Some acute and chronic effects of endrin on the
brains of pigeons and monkeys. In: Proceedings of the symposium on the
biological impact of pesticides in the environment, pp 134-141.
Ribbens PH (1985) Mortality study of industrial workers exposed to
aldrin, dieldrin and endrin. Arch Occup Environ Health, 56(2): 75-79.
Richardson LA, Lane JR, Gardner WS, Peeler JT, & Campbell JE (1967)
Relationship of dietary intake to concentration of dieldrin and endrin
in dogs. Bull Environ Contam Toxicol, 2(4): 207-219.
Richardson A, Robinson J, & Baldwin MK (1970) Metabolism of endrin in
the rat. Chem Ind, 1970,: 502-503.
Ritchey SJ, Young RW, & Essary EO (1972) Effects of heating and
cooking method on chlorinated hydrocarbon residues in chicken tissues.
J Agric Food Chem, 20: 291-293.
Robinson J & McGill AEJ (1966) Organochlorine insecticide residues in
complete prepared meals in Great Britain during 1965. Nature,
212(5066): 1037-1038.
Robinson J, Richardson A, Hunter CG, Crabtree AN, & Rees HJ (1965)
Organochlorine insecticide content of human adipose tissue in
south-eastern England. Br J Ind Med, 22: 220-229.
Roos AH, van Munsteren AJ, Nab FM, & Tuinstra LGMT (1987) Universal
extraction/clean-up procedure for screening of pesticides by
extraction with ethylacetate and size exclusion chromatography. Anal
Chim Acta, 196: 95-102.
Rosales MTL, Escalona RL, Alarcon RM, & Zamora V (1985) Organochlorine
hydrocarbon residues in sediments of two different lagoons of
Northwest Mexico. Bull Environ Contam Toxicol, 35: 322-330.
Rosen JD (1972) Conversion of pesticides under environmental
conditions. In: Coulston F & Korte F ed. Environmental quality.
Stuttgart, George Thieme, vol 1, pp 85-96.
Rosen JD, Sutherland DJ, & Lipton GR (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull
Environ Contam Toxicol, 1: 133-140.
Rowe DR, Canter LW, Snyder PJ, & Mason JW (1971) Dieldrin and endrin
concentrations in a Louisiana estuary. Pestic Monit J, 4(4): 177-183.
Rowley DL, Rab MA, Hardjotanojo W, Liddle J, Burse VW, Saleem M, Sokal
D, Falk H, & Head SL (1987) Convulsions caused by endrin poisoning in
Pakistan. Pediatrics, 79(6): 928-934.
Roylance KJ, Jorgensen CD, Booth GM, & Carter MW (1985) Effects of
dietary endrin on reproduction of Mallard ducks (Anas
platyrhynchos). Arch Environ Contam Toxicol, 14: 705-711.
Runhaar EA, Sangster B, Greve PA, & Voortman M (1985) A case of fatal
endrin poisoning. Hum Toxicol, 4: 241-247.
Ryan S, Bacher GJ, & Martin AA (1972) The mussel Hyridella australis
as a biological monitor of the pesticide endrin in fresh water.
Search, 3(11-12): 446-447.
Safe S & Hutzinger O (1979) Mass spectrometry of pesticides and
pollutants. Boca Raton, Florida, CRC Press, Inc.
Saiki MK & Schmitt CJ (1986) Organochlorine chemical residues in
bluegills and common carp from the irrigated San Joaquin Valley floor,
California. Arch Environ Contam Toxicol, 15: 357-366.
Samhan O & Ghobrial F (1987) Trace metals and chlorinated hydrocarbons
in sewage sludges of Kuwait. Water Air Soil Pollut, 36: 239-246.
Satsmadjis J, Georgakopoulos-Gregoriades E, & Voutsinou-Taliadouri F
(1988) Red mullet contamination by PCBs and chlorinated pesticides in
the Pagassitikos Gulf, Greece. Mar Pollut Bull, 19(3): 136-138.
Schafer ML, Peeler JT, Gardner WS, & Campbell JE (1969) Pesticides in
drinking water--waters from the Mississippi and Missouri rivers.
Environ Sci Technol,3(12): 1261-1269.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12: 355-382.
Scheutz EG, Wrighton SA, Safe SH, & Guzelian PS (1986) Regulation of
cytochrome P-450p by phenobarbital and phenobarbital-like inducers in
adult rat hepatocytes in primary monolayer culture and in vivo.
Biochemistry, 25: 1124-1133.
Schwabe U & Wendling I (1967) [Stimulation of drug metabolism by low
doses of DDT and other chlorinated hydrocarbon insecticides.]
Arzneimittel-forschung, 17(5): 614-618 (in German).
Seifert J (1988) Ontogenesis and properties of the convulsant
recognition site(s) of the gamma-aminobutyric acid (GABA) receptor
complex in chicken embryo. Eur J Pharmacol,151: 443-448.
Seifert J (1989) Teratogenesis of polychlorocycloalkane insecticides
in chicken embryos resulting from their interactions at the convulsant
recognition sites of the GABA (pro)receptor complex. Bull Environ
Contam Toxicol, 42: 707-715.
Sherman M & Rosenberg M (1954) Subchronic toxicity of four chlorinated
dimethanonaphthalene insecticides to chicks. J Econ Entomol, 47 (6):
1082-1083.
Sierra M & Santiago D (1987) Organochlorine pesticide levels in barn
owls collected in Leon, Spain. Bull Environ Contam Toxicol, 38:
261-265.
Sierra M, Teran MT, Gallego A, Diez MJ, & Santiago D (1987)
Organochlorine contamination in three species of diurnal raptors in
Leon, Spain. Bull Environ Contam Toxicol, 38: 254-260.
Simmon VF, Kauhanen K, & Tardiff RC (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North Holland Biomedical Press, pp 249-258.
Singh MN (1988) Mortality response of Achatina fulica to various
pesticides. J Environ Biol, 9(2): 157-162.
Singh PDA & West ME (1985) Acute pesticide poisoning in the Caribbean.
West Indian Med J, 34: 75-83.
Singhal RL & Kacew S (1976) The role of cyclic AMP in chlorinated
hydrocarbon-induced toxicity. Fed Proc, 35(14): 2618-2623.
Smith KJ, Polen PB, de Vries DM, & Coon FB (1968) Removal of
chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J Am Oil Chem Soc, 45: 866-869.
Sobti RC, Krishan A, & Davies J (1983) Cytokinetic and cytogenetic
effect of agricultural chemicals on human lymphoid cells in vitro.
II. Organochlorine pesticides. Arch Toxicol, 52: 221-231.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
northeastern Alberta otters. Bull Environ Contam Toxicol, 39: 783-790.
Soto AR & Deichmann WB (1967) Major metabolism and acute toxicity of
aldrin, dieldrin and endrin. Environ Res, 1(4): 307-322.
Spann JW, Heinz GH, & Hulse CS (1986) Reproduction and health of
mallards fed endrin. Environ Toxicol Chem, 5: 755-759.
Speck LB & Maaske CA (1958) The effects of chronic and acute exposure
of rats to endrin. Arch Ind Health, 18: 268-272.
Spynu EI (1964) On the toxicology of new organic chloride insecticides
obtained by diene synthesis on the basis of hexachlorocyclopentadiene.
Gig Tr Prof Zabol, 4: 30-35.
Squires RF & Saederup E (1989) Polychlorinated convulsant insecticides
potentiate the protective effect of NaCl against heat inactivation of
[3H] fluonitrazepam binding sites. J Neurochem, 52: 537-543.
Stanford Research Institute (1953) Unpublished letter Report No. 1
Ref. Project No. B-868 November 10, Stanford, CA, submitted to WHO by
Shell.
Stanford Research Institute (1954) Unpublished letter Report No. 3
Ref. Project No. B-868 February 1, Stanford, CA, submitted to WHO by
Shell.
Stanley CW, Barney JE II, Helton MR, & Yobs AR (1971) Measurement of
atmospheric levels of pesticides. Environ Sci Technol, 5: 430-435.
Steinberg KK, Garza A, Bueso JA, Burse VW, & Phillips DL (1989) Serum
pesticide concentrations in farming cooperatives in Honduras. Bull
Environ Contam Toxicol, 42: 643-650.
Stickel WH, Kaiser TE, & Reichel WL (1979) Endrin versus 12-ketoendrin
in birds and rodents. In: Kenaga EE ed. Avian and mammalian wildlife
toxicology, Philadeplphia,, American Society for Testing and
Materials, pp 61-68 (ASTM STP 693).
Stout VF (1980) Organochlorine residues in fishes from the Northwest
Atlantic Ocean and Gulf of Mexico. Fish Bull, 78(1): 51-58.
Strachan WMJ (1988) Toxic contaminants in rainfall in Canada: 1984.
Environ Toxicol Chem, 7: 871-877.
Strachan WMJ, Huneault H, Schertzer WM, & Elder FC (1980)
Organochlorines in precipitation in the Great Lakes region. In: Afghan
BK & MacKay D ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment. New York, Plenum Publishing Corp., pp 387-396.
Strassman SC & Kutz FW (1977) Insecticide residues in human milk from
Arkansas and Mississippi, 1973-1974. Pestic Monit J, 10(4): 130-133.
Strik JJTWA (1979) The occurrence of chronic hepatic porphyria in man,
caused by halogenated hydrocarbons. In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Elsevier/North Holland
Biomedical Press, p 3.
Struger J, Weseloh D, Hallett DJ, & Mineau P (1985) Organochlorine
contaminants in herring gull eggs from the Detroit and Niagara Rivers
and Saginaw Bay (1978-1982): contaminant discriminants. J Great Lakes
Res, 11(3): 223-230.
Sturm R, Knauth HD, Reinhardt KH, & Gandrasz J (1986) Distribution of
chlorinated hydrocarbons in sediment and seston of the River Elbe.
Wasser, 67: 23-38.
Sundershan P & Khan MAQ (1980) Metabolic fate of [14C] endrin in
bluegill fish. Pestic Biochem Physiol, 14: 5-12.
Suzuki M & Morimoto M (986) High resolution chemically bonded
fused-silica capillary gas chromatography of organochlorine
insecticides and related compounds in arable soil samples. J High
Resol Chromatogr Chromatogr Commun, 9: 296-298.
Suzuki M, Yamato Y, & Watanabe T (1973) Multiple organochlorine
pesticide residues in Japan. Bull Environ Contam Toxicol, 10(3):
145-150.
Suzuki K, Nagayoshi H, & Kashiwa T (1974) The systematic separation
and identification of pesticide in the first division. Agric Biol
Chem, 38(2): 279-285.
Sykes PW Jr (1985) Pesticide concentrations in snail kite eggs and
nestlings in Florida. Condor, 87: 438.
Tarrant KR & Tatton JO'G (1968) Organochlorine pesticides in rainwater
in the British Isles. Nature, 219(5155): 725-727.
Telling GM, Sissons DJ, & Brinkman HW (1977) Determination of
organochlorine insecticide residues in fatty food stuffs using a
clean-up technique based on a single column of activated alumina. J
Chromatogr, 137: 405-423.
Teran MT & Sierra M (1987) Organochlorine insecticides in trout,
Salmo trutta fario L. taken from four rivers in Leon, Spain. Bull
Environ Contam Toxicol, 38: 247-253.
Terriere LC (1964) Endrin. In: Zweig, G ed. Analytical methods for
pesticides, plant growth regulators and food additives. New York,
Academic Press, vol 2, pp 209-222.
Terriere LC, Kiigemagi U, & England DC (1958) Endrin content of body
tissues of steers, lambs and hogs receiving endrin in their daily
diet. J Agric Food Chem, 6(7): 516-518.
Terriere LC, Arscott GH, & Kiigemagi U (1959) The endrin content of
eggs and body tissue of poultry receiving endrin in their daily diet.
J Agric Food Chem, 7(7): 502-504.
Thier HP & Stijve T (1986) [Results of an inter-laboratory comparison
of analyses of analyses of organochlorine and organophosphorus
pesticide residues in fat.] Lebensmittelchem Gerichtl Chem, 40: 73-75
(in German).
Thompson JF (1976) Manual of analytical quality control for pesticides
and related compounds in human and environmental samples. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Office of Research and Development, Health Effects Research Laboratory
(EPA-600/1-76-017).
Thurston R, Gilfoil TA, Meyn EL, Zajdel RK, Aoki TI, & Veith GD (1985)
Comparative toxicity of ten organic chemicals to ten common aquatic
species. Water Res, 19(9): 1145-1155.
Travis CC & Arms AD (1988) Bioconcentration of organics in beef, milk
and vegetation. Environ Sci Technol, 22: 271-274.
Treon JF, Cleveland FP, & Cappel J (1955) Toxicity of endrin for
laboratory animals. J Agric Food Chem, 3(10): 842-848.
Truhaut R, Do Phuoc H, & Phu Lich N (1974) Influence de
l'administration de pesticides organo-halogénés et de
polychloro-biphényles sur le métabolisme de la zoxazolamine chez le
rat. CR Acad Sci Paris Sér D, 278: 3003-3006.
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Bureau of Sport Fisheries and Wildlife,
Denver Wildlife Center (Resource Publication No. 84).
Tuinstra LGMT (1971) Organochlorine insecticide residues in human milk
in the Leiden region. Neth Milk Dairy J, 25: 24-32.
Uhnak J, Sackmauerova M, Szokolay A, & Pal'usova O (1974) The use of
an electron capture detector for the determination of pesticides in
water. J Chromatogr 91: 545-547.
United Kingdom Ministry of Agriculture, Fisheries and Food (1989)
Report of the Working Party on Pesticide Residues 1985-1988. London,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
US EPA (1974) New Orleans area water supply study (draft analytical
report). Dallas, Texas, US Environmental Protection Agency, Lower
Mississippi River Facility, Surveillance and Analysis Divisions,
Region VI.
US EPA (1983) Findings of selected chemical residues in human blood
serum and adipose tissue: Endrin. Environ News, 9 May.
US EPA (1985) Hexachloronorbornadiene; Proposed submission of notice
of manufacturer, import or processing and determination of significant
new use. Fed Reg, 50(36): 7351-7356.
US EPA (1987a) Health effects assessment for endrin. Cincinnati, Ohio,
US Environmental Protection Agency, Ofice of Research and Development,
Environmental Criteria and Assessment Office, 31 pp (Report
PB88-180237).
US EPA (1987b) Health advisories for 16 pesticides. Washington, DC, US
Environmental Protection Agency, Office of Drinking Water, 18 pp
(Report PB87-200176).
Vance BD & Drummond W (1969) Biological concentration of pesticides by
algae. J Am Water Works Assoc, 61: 360-362.
Van Dijck P & van de Voorde H (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded Fac Landbouww
Rijksuniv Gent, 41(2): 1491-1498.
Van Raalte HGS (1965) [Some aspects of pesticide toxicity.]
Unpublished paper presented at the Conference on Occupational Health,
Caracas, Venezuela; The Hague. Shell, International Petroleum Company,
Health, Safety and Environment Division (in Spanish).
Van Sittert NJ (1985) Biological monitoring of bestrijdingsmiddelen;
Coronel-PAOG Nascholingssymposium. Unpublished paper, Amsterdam, Vrije
Universiteit, February 1985 (in Dutch).
Van Wynen JH & Stykel A (1988) Health risk assessment of residents
living on harbour sludge. Arch Occup Environ Health, 61: 77-87.
Veith GD, Kuehl DW, Leonard EN, Puglisi FA, & Lemke AE (1979)
Polychlorinated biphenyls and other organic chemical residues in fish
from major watersheds of the United States, 1976. Pestic Monit J,
13(1): 1-11.
Veith GD, Kuehl DW, Leonard EN, Welch K, & Pratt G (1981)
Polychlorinated biphenyls and other organic chemical residues in fish
from major United States watersheds near the Great Lakes, 1978. Pestic
Monit J, 15(1): 1-8.
Verma MP, Bahga HS, Soni BK, & Singh SP (1970) Effect of insecticides
on lactic acid concentration in the blood of buffalo-calves. Indian
Vet J, 47(12): 1056-1058.
Vermeer K, Risebrough RW, Spaans AL, & Reynolds LM (1974) Pesticide
effects on fishes and birds in rice fields in Surinam, South America.
Environ Pollut, 7: 217-236.
Versteeg JPJ & Jager KW (1973) Long-term occupational exposure to the
insecticides aldrin, dieldrin, endrin and telodrin. Br J Ind Med, 30:
201-202.
Villeneuve JP, Holm E, & Cattini C (1985) Transfer of chlorinated
hydrocarbons in the food chain lichen --> reindeer --> man.
Chemosphere, 14(11/12): 1651-1658.
Von Westernhagen H, Dethlefsen V, Cameron P, & Janssen D (1987)
Chlorinated hydrocarbon residues in gonads of marine fish and effects
on reproduction. Sarsia, 72: 419-422.
Von Westernhagen H, Cameron P, Dethlefsen V, & Janssen D (1989)
Chlorinated hydrocarbons in North Sea whiting (Merlangius merlangus)
and effects on reproduction. I. Tissue burden and hatching success.
Helgolander Meeresunters 43: 45-60.
Vrij-Standhardt WG, Strik JJTWA, Ottevanger CF, & van Sittert NJ
(1979) Urinary D-glucaric acid and urinary total porphyrin excretion
in workers exposed to endrin. In: Strik JJTWA & Koeman JH ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 113-121.
Wafford KA, Sattelle DB, Gant DB, Eldefrawi AT, & Eldefrawi ME (1989a)
Non-competitive inhibition of GABA receptors in insect and vertebrate
CNS by endrin and lindane. Pestic Biochem Physiol, 33: 213-219.
Wafford KA, Lummis SCR, & Sattelle DB (1989b) Block of an insect
central nervous system GABA receptor by cyclodiene and cyclohexane
insecticides. Proc R Soc Lond, B237: 53-61.
Walker JJ & Phillips DE (1987) An electron microscopic study of endrin
induced alterations in unmyelinated fibers of mouse sciatic nerve.
Neurotoxicology, 8(1): 55-64.
Walsh GM & Fink GB (1970) Temporal aspects of acute endrin toxicity in
mice. Proc West Pharmacol Soc, 13: 81-83.
Walsh GM & Fink GB (1972) Comparative toxicity and ditribution of
endrin and dieldrin after intravenous administration in mice. Toxicol
Appl Pharmacol, 23: 408-416.
Wang HH & MacMahon B (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J Occup Med, 21(11):745-748.
Wassermann M, Curnow DH, Forte PN, & Groner Y (1968) Storage of
organochlorine pesticides in the body fat of people in Western
Australia. Int J Ind Med Surg, 37(4): 295-300.
Wassermann M, Francone MP, Wassermann D, Mariani F, & Groner J (1969)
[Organochlorine pesticide content of the fatty tissue of the general
public in Argentina.] Sem Med, 134(16): 459-462 (in Spanish).
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. Basic Life Sci, 21: 275-326.
Webb RE & Horsfall F Jr (1967) Endrin resistance in pine mouse.
Science, 156: 1762.
Webb RE, Hartgrove RW, Randolph WC, Petrella VJ, & Horsfall F Jr
(1973) Toxicity studies in endrin-susceptible and resistant strains of
pine mice. Toxicol Appl Pharmacol, 25(1): 42-47.
Weeks DE (1967) Endrin food-poisoning. A report on four outbreaks
caused by two separate shipments of endrin-contaminated flour. Bull
World Health Organ, 37: 499-512.
Wegman RCC & Greve PA (1974) Levels of organochlorine pesticides and
inorganic bromide in human milk. Meded Fac Landbouwwet Rijksuniv Gent,
39: 1301-1310.
Wegman RCC & Greve PA (1978) Organochlorines, cholinesterase
inhibitors and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic Monit J, 12(3): 149-162.
Wegman RCC & Greve PA (1980) Halogenated hydrocarbons in Dutch water
samples over the years 1969-1977. Environ Sci Res, 16: 405-415.
Wegman RCC & Hofstee AWM (1982) Determination of organochlorines in
river sediment by capillary gas chromatography. Water Res, 16:
1265-1272.
Wegman RCC, Hofstee AWM, & Greve PA (1981) Uptake of organochlorines
by plants growing on river and basin sediment. Meded Fac Landbouwwet
Rijksuniv Gent, 46(1): 359-365.
Weisgerber I, Klein W, & Korte F (1969) [Disappearance of residues and
metabolism of endrin-(14C) in tobacco.] Liebigs Ann Chem, 729:
193-197 (in German with English abstract).
Wells MR & Yarbrough JD (1972) Vertebrate insecticide resistance:
in vivo and in vitro endrin binding to cellular fractions from
brain and liver tissues of Gambusia. J Agric Food Chem, 20(1): 14-16.
Whetstone RR (1964) Chlorocarbons and chlorohydrocarbons: chlorinated
derivatives of cyclopentadiene. In: Kirk-Othmer encyclopedia of
chemical technology, 2nd ed., New York, John Wiley and Sons, vol 5, pp
240-252.
WHO (1989) Environmental Health Criteria No. 91: Aldrin and dieldrin.
Geneva, World Health Organization, 335 pp.
WHO (1992) The WHO recommended classification of pesticides by hazard.
Guidelines to classification 1992-1993. Geneva, World Health
Organization (WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 1: Endrin. Geneva, World
Health Organization (VBC/DS/75.1).
Wiemeyer SN, Jurek RM, & Moore JF (1986) Environmental contaminants in
surrogates, foods and feathers of California condors (Gymnogyps
californianus). Environ Monit Assess, 6: 91-111.
Williams S (1964) Pesticide residues in total diet samples. J Assoc
Off Anal Chem, 47(5): 815-821.
Williams GM (1979) Liver cell culture systems for the study of
hepatocarcinogenesis. In: Margison GP ed Advances in medical oncology
research and education: Carcinogenesis, New York, Pergamon Press, vol
1, pp 273-280.
Williams S, Mills PA, & McDowell RE (1964) Residues in milk of cows
fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J Asoc Off Agric Chem, 47: 1124-1128.
Williams DT, Benoit FM, McNeil EE, & Otson R (1978) Organochlorine
pesticide levels in Ottawa drinking water, 1976. Pestic Monit J,
12(3): 163-166.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71(2): 410-414.
Wilson Committee (1969) Report by the Advisory Committee on Pesticides
and Other Toxic Chemicals. Further review of certain persistent
organochlorine pesticides used in Great Britain. London, Her Majesty's
Stationery Office, pp 90-100.
Wilson JG & Earley JJ (1986) Pesticide and PCB levels in the eggs of
shag Phalacrocorax aristotelis and cormorant Phalacrocorax carbo
from Ireland. Environ Pollut (Ser B), 12: 15-26.
Wit SL (1971) [Persistent insecticides in Dutch body fat.] Chem
Weekbl, 67(5): 11-14 (in Dutch).
Witherup S, Stemmer KL, Taylor P, & Bietsch P (1970) The Incidence of
Neoplasms in Two Strains of Mice Sustained on Diets Containing Endrin.
Unpublished report, Cincinnati, Ohio, Kettering Laboratory, submitted
to WHO by Shell.
Wolfe HR, Durham WF, & Armstrong JF (1963) Health hazards of the
pesticides endrin and dieldrin: hazards in some agricultural uses in
the Pacific Northwest. Arch Environ Health, 6: 458-464.
Wolfe HR, Durham WF, & Armstrong JF (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolman AA & Wilson AJ Jr (1970) Occurrence of pesticides in whales.
Pestic Monit J, 4(1): 8-10.
Wüthrich C, Müller F, Blaser O, & Marek B (1985) [Pesticides and other
chemical residues in Swiss diet samples.] Mitt Geb Lebensmittel Hyg,
76: 260-276 (in German with English summary).
Wuu KD & Grant WF (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare).
Can J Genet Cytol, 8: 481-501.
Wuu KD & Grant WF (1967a) Chromosomal aberrations induced by
pesticides in meiotic cells of barley. Cytologia, 32: 31-41.
Wuu KD & Grant WF (1967b) Chromosomal aberrations induced in somatic
cells of Vicia faba by pesticides. Nucleus, 10(1): 37-46.
Yakushiji T, Watanabe I, Kuwabara K, Yoshida S, Hori S, Fukushima S,
Kashimoto T, Koyama K, & Kunita N (1979) Levels of organochlorine
pesticides and polychlorinated biphenyls (PCBs) in mothers milk
collected in Osaka prefecture from 1969-1976. Arch Environ Contam
Toxicol, 8: 59-66.
Yarbrough JD, Roush RT, Bonner JC, & Wise DA (1986) Monogenic
inheritance of cyclodiene insecticide resistance in mosquitofish,
Gambusia affinis. Experientia (Basel), 42: 851-853.
Young RA & Mehendale HM (1986) Effect of endrin and endrin derivatives
on hepatobiliary function and carbontetrachloride-
induced hepatotoxicity in male and female rats. Food Chem Toxicol,
24(8): 863-868.
Zabik MJ, Schuetz RD, Burton WL, & Pape BE (1971) Photochemistry of
bioactive compounds. Studies of a major photolytic product of endrin.
J Agric Food Chem, 19(2): 308-313.
Zavon MR, Hine CH, & Parker KD (1965) Chlorinated hydrocarbon
insecticides in human body fat in the United States. J Am Med Assoc,
193(10): 181-183.
Zeiger E (1987) Carcinogenicity of mutagens: predictive capability of
the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer
Res, 47: 1287-1296.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environ Mutagen, 9 (suppl9): 1-110.
Zimmerli B & Marek B (1973) [The pesticide load of the Swiss
population.] Mitt Geb Lebensmittel Hyg,, 64(4): 459-479 (in German
with English summary).
ANNEX I CHEMICAL NAMES OF ENDRIN AND ITS METABOLITES
Two main systems are currently used for the nomenclature of
cyclodiene insecticides: 'polyhydroaromatic' names, used by Chemical
Abstracts (American Chemical Society) and the International Union for
Pure and Applied Chemistry (IUPAC), and the von Baeyer/IUPAC system
for polycyclic aliphatic compounds. Benson (1969) and Bedford (1974)
proposed that the latter system be used for the cyclodiene
insecticides.
The 'polyaromatic' system has, unfortunately, been subject to
historical variation, and there are differences between the IUPAC,
British and American conventions for defining the three-dimensional
stereochemistry in this system. As a consequence of differences in the
numbering of carbon atoms in the two systems and the modification of
the Chemical Abstracts 'polyaromatic' name for dieldrin since 1971,
considerable confusion can arise in the nomenclature of metabolites.
The possible misunderstandings that may occur, particularly among
people who are not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that are given to
the major metabolite of endrin; this one compound may be designated
as:
anti-9-hydroxyendrin (former Chemical Abstracts system)
anti-8-hydroxyendrin (current Chemical Abstracts system)
anti-12-hydroxyendrin (von Baeyer/IUPAC system).
A useful discussion of nomenclature was given by Brooks (1974).
The chemical names for endrin and its metabolites are summarized
in Table 30.
Table 30. Chemical nomenclature of endrin and its metobolites
Trivial namesa Polycyclic aliphatic name (von Baeyer/ IUPAC) Alternative or former names
Endrin (I) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-2,3-7, 6- 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,
endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]- 4a,5,6,7,8,8a-octahydro-1,4- endo,endo-
dodec-9-ene 5,8-demethanonaphthalene (former CAS
name)
1aa,2b,2ab,3a,6a,6b,7b,7a)-3,4,5,6,9,9-
Hexachloro-1a,2,2a,3,6,6a,7,7a,-octahydro-
2,7:3,6-dimethanonaphth[2,3- b]oxirene
(current CAS name)
(IR,4S,4aS,5S,7R,8R,8aR)-1,2,3,4,10,10-
Hexachloro-1,4,4a,5,6,7,8,8a-octahydro-6,7-
epoxy-1,5:5,8-dimethanonaphthalene
(current IUPAC name)
syn-12-Hydroxyendrin (II) 1,8,9,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- syn-hydroxyendrin) ( syn-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8- endo-
tetracyclo[6.2.1.13,6.02,7] dodec-9-ene
anti-12-Hydroxyendrin (III) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- anti-hydroxyendrin) ( anti-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8-
endo-tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
3-Hydroxyendrin (IV) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-3-
(5-hydroxyendrin) hydroxy-2,3-7,6- endo-2,1-7,8- endo-tetracyclo-
[6.2.1.13,6.02,7] dodec-9-ene
12-Ketoendrin (V) 1,8,9,10,11,11-Hexachloro-4,5 - exo-epoxy-2,3-7,6-
(9-ketoendrin) endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]-
dodec-12-one
Endrin trans-diol (IV) 1,8,9,10,11,11-Hexachloro-4,5-( exo)trans-dihydroxy-
2,3-7,6- endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]
dodec-9-ene
aRoman numerals in parentheses refer to the structures in Figure 2.
ANNEX II
MEDICAL TREATMENT OF ENDRIN POISONING
1. Symptoms of poisoning
Endrin is readily absorbed and is toxic when taken by mouth, by
skin contact (especially liquid formulations), and by inhalation. It
stimulates the central nervous system, and an oral dose of 0.25 mg/kg
body weight can cause convulsions in humans. Following accidental
ingestion or gross over-exposure, symptoms appear between 20 min and
12 h and may include headache, dizziness, nausea, vomiting, weakness
in the legs, and convulsions, sometimes leading to death.
Organochlorine compounds can cause respiratory depression, and
they may sensitize the heart to endogenous catecholamines, leading to
ventricular fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis, and if
necessary blood gases should be checked. Use of an
electrocardiographic monitor is recommended if symptoms are severe.
Endrin is eliminated very quickly from the blood and can be
detected for only 1 or 2 days even after massive over-exposures. Signs
and symptoms of poisoning occur only at levels in whole blood of above
0.05 µg/ml.
2. Medical treatment
Medical treatment is largely symptomatic and supportive and is
directed against convulsions and hypoxia. If endrin is swallowed, the
stomach should be emptied as soon as possible by careful gastric
lavage (with a cuffed endotracheal tube already in place), avoiding
aspiration into the lungs. In a rural situation where this is not
feasible, vomiting should be induced immediately. This should be
followed by (intragastric) administration of 50 g of activated
charcoal and 30 g of magnesium or sodium sulfate in a 30% aqueous
solution. Oily purgatives are contraindicated, and no fats, oils, or
milk should be given.
If convulsions occur, anticonvulsants should be given immediately
but slowly, and repeated as necessary. Diazepam can be given at 10 mg
(children, 1-5 mg) intravenously; thiopental sodium or hexobarbital
sodium can be given intravenously at a dose of 10 mg/kg, with a
maximum total dose of up to 750 mg for an adult; or paraldehyde can be
given at 5 ml by intramuscular injection. These short-acting
anticonvulsants should always be followed by phenobarbital given
orally at 3 mg/kg (up to 200 mg for an adult), or phenobarbital sodium
given intramuscularly at 3 mg/kg (up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline,
should never be given.
The airway must be kept unobstructed. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
A guideline for the management of major status epilepticus is
added as Annex III.
ANNEX III
MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
1. Initial management
1. Assess the patient, verify the diagnosis, remove false
teeth, place the patient in a lateral semi-prone position,
and establish an airway.
2. Give diazepam intravenously (see Note 1, below), usually at
10 mg in 2 ml (0.15-0.25 mg/kg), followed immediately by a
further 10 mg (2 ml) over 1-2 min. This may be repeated
according to response.
3. Take blood to measure levels of anticonvulsant drug,
ethanol, and blood sugar (5 ml of blood in a sugar tube); a
sample to measure calcium (5 ml in a plain tube); and a drop
of blood to determine blood glucose.
4. If the latter measurement shows low blood glucose level, 25
ml of 50% glucose should be given intravenously, preferably
by catheter and not into a small distal vein. If ethanol is
likely to be present, give thiamine intravenously at 100 mg.
5. Give phenytoin intravenously at 250 mg in 5 ml (10-15 mg/kg)
no faster than 50 mg (1 ml)/min by infusion pump or slow
intravenous injection (see Note 2, below).
2. If fits continue, transfer patient to the intensive care unit and
consult an anaesthetist
6. Give chlormethiazole intravenously at 8 mg/ml: a loading
dose of up to 800 mg (100 ml) over 10 min at 10 ml/min,
maintained with 0.5-1 ml/min (4-8 mg).
7. Give thiopentone intravenously at 5 mg/kg as a loading dose,
then 1-3 mg/kg per h to a maximum blood level of 100
mg/litre.
8. If this fails, consult a neurologist.
3. Notes
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there
is concurrent use of other central nervous system depressant
drugs, especially phenobarbital.
aAdapted from guidelines issued at Guy's Hospital, London
Diazepam must not be given intramuscularly:
--added to an intravenous infusion
--with phenobarbital, unless artificial ventilation is
available.
Rectal administration of diazepam (using a rectal
administration set), at 5 or 10 mg in 2.5 ml, may be used
for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
--intramuscularly
--by central line
--into a dextrose infusion
--with any other drug.
Intravenously administered phenytoin should be monitored by
continuous electrocardiographic recording. If this is not
available, it may be safer to use a diluted solution of 250
mg (5 ml) in 250 ml of normal saline, no faster than 50
mg/min. The diluted solution should be used immediately
provided there is no evidence of precipitation (this use of
phenytoin is not licensed).
4. Options
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate deep intramuscular
injection or 10 ml diluted in 100 ml of normal saline given
intravenously over 10-15 min. Note: Paraldehyde should be
administered only with glass syringes.
2. Phenobarbital: 200 mg/ml, should not be given intravenously
except when artificial ventilation is available, and not at
all if the patient normally takes phenobarbital. The maximal
rate of infusion is 100 mg/min to a maximum dose of 15
mg/kg.
3. Lignocaine: 100 mg by slow intravenous injection, followed
by 50-100 mg in 250 ml of 5% dextrose at 1-2 mg/min. Note:
This treatment must be monitored by electrocardigram.
4. Diazepam: 10 mg in 2 ml intravenously, or 40 mg in 500 ml of
5% dextrose at a maximal infusion rate of 100 mg/h.
5. Sodium valproate: 400 mg in 4 ml, or 400-800 mg
intravenously over 3-5 min (up to 10 mg/kg), followed by
intravenous. infusion to a maximum of 2.5 g/day
(unlicensed).
5. Paediatric doses
For children, dosing should be adapted as follows:
Diazepam: 0.2-0.3 mg/kg intravenously
Phenytoin: 10-20 mg/kg intravenously
Chlormethiazole: 5-10 mg/kg per h, equivalent to
0.6-1.25 ml/kg per h
RESUME ET EVALUATION; CONCLUSIONS; RECOMMANDATIONS
Résumé et évaluation
Exposition
L'endrine est un insecticide organochloré utilisé depuis les
années 1950 contre toute sorte de ravageurs, qui s'attaquent notamment
au coton mais également au riz, à la canne à sucre, au maïs et à
d'autres cultures. On l'utilise également comme rodenticide et
avicide. Il est disponible dans le commerce sous forme de poudres, de
granulés, de pâtes et de concentrés émulsionnables.
L'endrine pénètre principalement dans l'atmosphère par
volatilisation et dispersion. En général, la volatilisation se produit
après épandage sur le sol et sur les récoltes et elle est tributaire
de nombreux facteurs comme la teneur en matières organiques et en eau
du sol, l'humidité, les courants aériens et l'aire foliaire des
végétaux.
C'est principalement par lessivage à partir du sol que se produit
la contamination des eaux superficielles. Les précipitations, qu'il
s'agisse de neige ou de pluie, n'ont qu'une part négligeable dans
cette contamination.
Localement, une contamination peut également se produire par
suite du déversement d'effluents industriels ou de négligences dans
les techniques d'épandage.
C'est principalement par suite d'un épandage direct sur les
terrains et les récoltes que l'endrine pénètre dans le sol. Elle peut
y être retenue, transportée ou dégradée, en fonction d'un certain
nombre de facteurs. C'est dans les sols riches en matières organiques
que la rétention est la plus importante. La persistance de l'endrine
dépend dans une large mesure des conditions locales; sa demi-vie dans
le sol peut aller jusqu'à 12 ans. La disparition de l'endrine présente
en surface s'effectue principalement par volatilisation et
photodécomposition. Sous l'influence de la lumière solaire
(rayonnement ultra-violet), l'endrine est isomérisée en delta-
cétoendrine. En présence de lumière solaire intense, on a observé une
isomérisation de 50% de l'endrine en l'espace de sept jours.
L'isomérisation peut également s'effectuer par action microbienne
(champignons et bactéries), notamment en anaérobiose.
Les invertébrés aquatiques et les poissons absorbent rapidement
l'endrine présente dans l'eau mais, transvasés dans une eau non
contaminée, les poissons exposés éliminent sans délai le pesticide. En
cas d'exposition continue, le facteur de bioconcentration peut
atteindre 14-18 000.
Il est possible que les invertébrés terricoles absorbent
facilement l'endrine. La présence occasionnelle de faibles quantités
d'endrine dans l'air ainsi que dans les eaux de surface, notamment
destinées à la consommation, en zone agricole, n'a guère d'importance
au point de vue de la santé publique. La seule vole d'exposition
importante est la voie alimentaire. En général, toutefois, les
quantités ingérées se situent très largement en-dessous de la dose
journalière admissible qui a été fixée à 0,0002 mg/kg de poids
corporel en 1970 (FAO/OMS, 1971).
1.2 Absorption, métabolisme et excrétion
Contrairement à la dieldrine, son stéréoisomère, l'endrine est
rapidement métabolisée par l'organisme animal et, comparativement à
d'autres composés de structure chimique semblable, elle s'accumule
très peu dans les tissus adipeux.
L'absorption et l'excrétion sont rapides après administration
orale à des rats et la demi-vie biologique se situe entre 1 et 6 jours
selon les quantités ingérées. Un régime stationnaire, c'est à dire un
état d'équilibre entre la quantité excrétée et la dose ingérée,
s'établit au bout de 6 jours. On constate une différence entre les
deux sexes en ce sens que les mâles excrètent l'endrine et ses
métabolites par la voie biliaire plus rapidement que les femelles,
d'où une moindre accumulation de pesticides dans les tissus adipeux
des mâles. Les rats excrètent ce composé principalement dans leurs
matières fécales sous forme d'endrine, d' anti-12-hydroxyendrine
ainsi que sous la forme d'un dérivé hydroxyle de l'endrine, en
l'espace de 24 heures (70-75 %); un troisième métabolite, la 12-
cétoendrine, s'accumule dans les tissus. Les lapins excrètent 50% des
métabolites de l'endrine par la voie urinaire, l'excrétion urinaire
n'étant que de 2% chez le rat; dans les matières fécales des lapins,
on ne retrouve que de l'endrine non modifiée.
Des vaches à qui l'on avait administré de l'endrine à raison de
0, 1 mg/kg de nourriture pendant 21 jours en ont excrété jusqu'à 65%
sous forme de métabolites urinaires, 20% sous forme de métabolites
fécaux ou d'endrine non modifiée et 3% dans leur lait, cette fois,
principalement sous forme d'endrine non modifiée. Chez ces vaches, les
résidus atteignaient 0,003-0,006 mg/litre dans le lait, 0,001-0,002
mg/kg dans la viande, et 0,02-0,1 mg/kg dans la graisse.
Chez des poules pondeuses ayant reçu une alimentation additionnée
d'endrine, on a observé des résidus (selon la dose ingérée) qui
atteignaient 0.1 mg/kg dans la chair, 1 mg/kg dans la graisse, 0,2-0,3
mg/kg dans les oeufs (jaune), 0,2 mg/kg dans les reins et 0,5 mg/kg
dans le foie. Sauf dans le cas du foie et des reins, les résidus
présents étaient essentiellement formés d'endrine non modifiée.
Environ 50% de la quantité d'endrine administrée a été excrétée dans
les matières fécales, principalement sous la forme de métabolites.
Chez l'homme, le rat, le lapin, la vache et la poule, le
principal métabolite de l'endrine est l' anti-12-hydroxyendrine,
accompagnée de ses sulfo- et glucuro-conjugués. On trouve également 4
autres métabolites, mais en quantités minimes. Dans les tissus et le
lait on retrouve essentiellement de l'endrine non modifiée. Après
épandage sur des végétaux, on a retrouvé de l'endrine sous forme non
modifiée ainsi que deux produits de transformation hydrophiles.
1.3 Effets sur les êtres vivants dans leur milieu naturel
L'endrine n'exerce que des effets minimes sur les bactéries et
les champignons terricoles. Aux doses de 10-1000 mg/kg de terre, le
composé n'a aucun effet sur la décomposition des matières organiques,
sur la dénitrification ou sur la production de méthane. L'endrine est
très toxique pour les poissons, les invertébrés aquatiques et le
phytoplancton; la CL 50 à 96 h, est dans la plupart des cas
inférieure à 1,0 µg/litre. La dose nocive la plus faible observée au
cours d'un test portant sur le cycle évolutif d'un crevette,
Mysidopsis bahia, était de 30 ng/litre.
Les épreuves de toxicité aiguë effectuées sur des organismes
aquatiques ont été pratiquées dans des aquariums ne comportant pas de
sédiments, on peut penser que la présence de sédiments atténue l'effet
de l'endrine. D'ailleurs la présence de sédiments fortement contaminés
n'a guère eu d'effet sur les espèces de pleine eau, ce qui incite à
penser que l'endrine fixée aux sédiments présente une faible
biodisponibilité. On n'a pas pratiqué d'épreuves sur des animaux
aquatiques vivant dans les sédiments.
Pour les mammifères terrestres et les oiseaux, la DL50 est de
l'ordre de 1,0-10,0 mg/kg de poids corporel. Des canards de l'espèce
Anas platyrhynchos qui avaient reçu pendant 12 semaines de l'endrine
dans leur nourriture à des doses allant jusqu'à 3,0 mg/kg de poids
corporel, n'ont présenté aucun effet délétère que ce soit sur la
ponte, la fécondité ou l'éclosion des oeufs.
Il semblerait que certaines espèces d'invertébrés aquatiques, de
poissons et de petits mammifères résistent à l'action toxique de
l'endrine; d'ailleurs l'exposition à divers pesticides organochlorés
a pu entraîner la sélection de souches résistantes à l'endrine.
Dans des zones où existent des décharges industrielles et où
l'endrine peut être entraînée par ruissellement à partir des champs
traités, on a observé une mortalité parmi les poissons; par ailleurs,
le déclin des populations de pélicans bruns (en Louisiane) et de
caugeks (aux Pays-Bas) a été attribuée à une exposition à l'endrine et
à d'autres dérivés halogénés.
1.4 Effets sur les animaux d'expérience et sur les systèmes in vitro
L'endrine est un pesticide fortement toxique dont les signes
d'intoxication sont de type neurologiques. Chez les animaux de
laboratoire, la DL50 par voie orale de l'endrine de qualité
technique se situe dans les imites de 3-43 mg/kg de poids corporel; la
DL50 dermique va de 5-20 mg/kg de poids corporel pour le rat. Il n'y
a pas de différence notable concernant la toxicité aiguë par voie
orale et percutanée entre le produit technique et les diverses
formulations (concentrés émulsionnables ou poudres mouillables).
Des épreuves de courte durée portant sur la toxicité par voie
orale de l'endrine ont été effectuées sur (les souris, des rats, des
lapins, des chiens et autres animaux domestiques. Chez les souris et
les rats, les doses maximales tolérées ont été respectivement de 5 et
15 mg/kg de nourriture pendant 6 semaines (soit l'équivalent de 0,7
mg/kg de poids corporel). Les rats ont survécus à une dose de 1 mg/kg
de nourriture pendant 16 semaines (soit l'équivalent de 0,05 mg/kg de
poids corporel); les lapins sont morts après avoir reçu à plusieurs
reprises une dose de 1 mg/kg de poids corporel. chez le chien, une
dose de 1 mg/kg de nourriture (soit approximativement 0,025 mg/kg de
poids corporel) administrée sur une période de 2 ans, n'a produit
aucun effet.
Du point de vue neurologique, les signes d'intoxication observés
sont dus à l'inhibition de la fonction de l'acide gamma-aminobutyrique
(GABA) à faible dose. Comme les autres hydrocarbures chlorés
insecticides, l'endrine agit également au niveau du foie et la
stimulation des systèmes enzymatiques intervenant dans le métabolisme
des autres substances chimiques se manifeste, notamment chez la
souris, par une diminution de la durée du sommeil induit par
l'hexobarbital.
Des doses de 75-150 mg/kg appliquées sur l'épiderme des lapins
sous forme de poudre sèche, tous les jours pendant deux heures ont
entraîné des convulsions et la mort chez ces animaux sans toutefois
provoquer d'irritation cutanée. Cette intoxication par voie générale
sans irritation locale mérite d'être signalée.
Des études de toxicité et de cancérogénicité à long terme ont été
effectuées sur des souris et des rats. Aucun effet cancérogène n'a été
observé mais ces études présentaient un certain nombre d'insuffisances
notamment la faible survie des animaux. Lors d'une étude de deux ans
sur des rats traités par de l'endrine administrée dans leur
nourriture, on a estimé à 1 mg/kg de nourriture, soit l'équivalent
d'environ 0,05 mg/kg de poids corporel, la dose sans effets toxiques
observables. Après administration d'endrine avec des quantité
infinitésimales de substances chimiques cancérogènes pour l'animal, il
n'a pas été possible de mettre en évidence d'effet tumoro-promoteur.
Le Groupe de travail en a conclu que les données sont insuffisantes
pour permettre de considérer l'endrine comme cancérogène pour l'homme.
Plusieurs études ont également révélé que l'endrine n'était pas
génotoxique.
Dans la plupart des études, l'endrine s'est révélée non
tératogène pour la souris, le rat ou le hamster, même à des doses
toxiques pour la mère ou le foetus. La dose sans effet nocif
observable a été évaluée à 0,5 mg/kg de poids corporel chez la souris
et le rat et à 0,75 mg/kg de poids corporel chez les hamsters.
L'endrine n'a pas eu d'effets sur la reproduction des rats suivis
pendant trois générations qui en recevaient dans leur nourriture à
raison de 2 mg/kg (soit environ 0, 1 mg/kg de poids corporel).
Un certain nombre de métabolites de l'endrine sont plus ou moins
toxiques que le composé initial. Ainsi la delta-cétoendrine est moins
toxique de l'endrine, en revanche la 12-cétoendrine est considérée
comme le métabolite le plus toxique de l'endrine pour les mammifères,
avec une DL50 par voie orale de 0,8-1,1 mg/kg de poids corporel chez
le rat.
1.5 Effets sur l'homme
Plusieurs cas d'intoxication mortels ou non mortels consécutifs
à un accident ou à une tentative de suicide ont été observés. Les cas
d'intoxication aiguë non mortels résultant d'une, surexposition
accidentelle ont été observés chez les ouvriers d'une usine de
production d'endrine. On estime que par voie orale, la dose mortelle
est (l'environ 10 mg/kg de poids corporel, une dose unique prise par
voie orale de 0,25-1,0 mg/kg de poids corporel peut provoquer des
convulsions.
C'est au niveau du système nerveux central que l'endrine exerce
principalement son action. Après exposition à dose toxique, des signes
d'intoxication peuvent faire leur apparition et se manifestent sous la
forme d'un hyperexcitabilité et de convulsions, la mort pouvant
survenir dans les 2-12 heures suivant l'exposition si un traitement
approprié n'est pas institué immédiatement. En revanche, après une
intoxication non mortelle, la récupération est rapide et complète.
L'endrine ne s'accumule pas dans le corps humain de manière
importante. Chez 232 travailleurs exposés de par leur profession, on
n'a pas constaté d'effets indésirables à long terme (durée
d'exposition 4-27 ans) lors des examens médicaux pratiqués (durée de
l'observation 2-29 ans). Le seul effet observé, indirectement
d'ailleurs, consistait en une stimulation réversible des enzymes
pharmacométabolisantes.
Des analyses ont été pratiquées dans de nombreux pays sur un
grand nombre d'échantillons de tissus adipeux, de sang et de lait
maternel sans qu'il soit possible de mettre en évidence la présence
d'endrine. Le Groupe de travail attribue l'absence d'endrine dans ces
échantillons à la faible exposition de la population général à ce
pesticide et à sa métabolisation rapide.
En revanche la présence d'endrine a été décelée dans le sang (à
des concentrations atteignant 450 µg/litre) et dans les tissus adipeux
(à la dose de 89,5 mg/kg) chez les personnes décédées d'une
intoxication accidentelle. Dans les conditions normales, on n'a pas
retrouvé d'endrine chez les travailleurs exposés. Le seuil
d'apparition des symptômes d'intoxication est estimé à 50-100 µg/litre
de sang. On pense que la demi-vie de l'endrine dans le sang est de
l'ordre de 24 heures.
2. Conclusions
L'endrine est un 'Insecticide qui présente une très forte
toxicité aiguë. Il peut entraîner des intoxications graves en cas
d'exposition excessive due à une manipulation négligente lors de sa
production, de son utilisation ou par suite de la consommation
d'aliments contaminés. L'exposition de la population générale est
principalement due à la présence de résidus dans les denrées
alimentaires; toutefois on estime que la quantité d'endrine ingérée
est en général très inférieure à la dose journalière admissible fixée
par le Comité FAO/OMS d'experts des résidus de pesticides. Il n'y a
pas de danger pour la population générale qui résulterait d'une
exposition de ce genre à l'endrine. Moyennant de bonnes méthodes de
travail et le respect des mesure d'hygiène et de sécurité, l'endrine
ne devrait pas constituer un danger pour les ouvriers exposés.
Il est évident que des rejets incontrôlés d'endrine lors de la
production, de la formulation et de l'utilisation de ce pesticide
peuvent créer des problèmes écologiques dus à sa forte toxicité. Il
n'est pas possible d'être aussi catégorique en ce qui concerne les
effets que peut avoir son utilisation en agriculture sur la faune et
la flore, encore que l'entraînement par ruissellement du pesticide
puisse constituer une menace pour les poissons et les oiseaux
piscivores. Le déclin des populations de certaines espèces d'oiseaux
a été attribué à la présence de résidus élevés de divers organochlorés
dans les tissus des adultes et dans les oeufs. On a procédé au dosage
de l'endrine chez certaines de ces espèces; toutefois il est difficile
de faire la part des différents organochlorés qui sont en cause.
3. Recommandations
1. L'endrine ne doit être utilisée qu'en cas de nécessité et
seulement lorsqu'il n'existe pas d'autre produit moins
toxique.
2. Afin de préserver la santé et le bien-être des travailleurs
et de la population générale, on ne doit confier la
manipulation et l'épandage qu'à des personnes bien encadrées
et bien formées qui appliqueront des mesures de sécurité
convenables et épandront le produit conformément aux règles
de bonne pratique en la matière.
3. Il convient de s'entourer de toute les précautions
nécessaires lors de la production, de la formulation, de
l'utilisation en agriculture et du rejet de l'endrine afin
de contaminer le moins possible l'environnement et en
particulier les eaux de surface.
4. Les personnes qui sont habituellement exposées à l'endrine
doivent subir des examens médicaux périodiques.
5. Il faut poursuivre l'étude épidémiologique des travailleurs
exposés.
6. Dans les pays où l'on utilise encore de l'endrine, on devra
contrôler la présence de résidus d'endrine dans les denrées
alimentaires.
7. Au cas où l'on continuerait à utiliser de l'endrine, il
faudrait obtenir davantage de données sur la présence, la
destinée ultime et la toxicité de la 12-cétoendrine et de la
delta-cétoendrine.
RESUMEN Y EVALUACION; CONCLUSIONES; RECOMENDACIONES
1. Resumen y evaluación
1.1 Exposición
La endrina es un insecticida organoclorado que se utiliza desde
los años cincuenta para combatir muy diversas plagas agricolas, sobre
todo en el algodón aunque también en el arroz, la caña de azúcar, el
maíz y otros cultivos. Se utiliza asimismo como rodenticida. En el
comercio se encuentra en forma de polvos, gránulos, pastas y
concentrado emulsionable.
La endrina se incorpora al aire principalmente por volatilización
y arrastre aéreo. En general, la volatilización tiene lugar después de
aplicarla a suelos y cultivos y depende de muchos factores, como el
contenido de materia orgánica y agua del suelo, la humedad, el flujo
de aire y la superficie cultivada.
La vía más importante de contaminación de las aguas de superficie
es la escorrentía desde el suelo. La contaminación por precipitación
en forma de nieve o lluvia es insignificante. Puede producirse
contaminación local del medio debida a efluentes industriales y
prácticas de aplicación poco meticulosas.
La principal fuente de endrina en el suelo es la aplicación
directa a éste y a los cultivos. Puede quedar retenida, ser
transportada o degradarse en el suelo, atendiendo a diversos factores.
La retención más intensa se produce en suelos con contenido elevado de
materia orgánica. La persistencia de la endrina depende en gran medida
de las condiciones locales; su semivida en el suelo puede llegar a los
12 años. La volatilización y la fotodescomposición son los principales
factores de la desaparición de la endrina de las superficies del
suelo. La luz del sol (luz ultravioleta) induce la formación del
isómero delta-cetoendrina. En verano, bajo insolación intensa, se
observó que alrededor del 50% de la endrina se isomerizaba a esta
cetoendrina en un plazo de siete días. Se produce transformación
microbiana (en hongos y bacterias), especialmente en condiciones
anaerobias, originándose la misma sustancia.
Los invertebrados acuáticos y los peces absorben rápidamente la
endrina a partir del agua, si bien los peces expuestos transferidos a
agua no contaminada pierden el plaguicida rápidamente. Se han
registrado factores de bioconcentración de 14-18 000 tras una
exposición continua. Los invertebrados del suelo también absorben
fácilmente el compuesto.
La presencia ocasional de niveles reducidos de endrina en el aire
y en las aguas de superficie y de bebida en zonas agrícolas reviste
escasa importancia desde el punto de vista de la salud pública. La
única exposición que merece consideración es la ingesta en la dieta.
En general, no obstante, los niveles comunicados de ingesta se
encuentran muy por debajo de la ingesta diaria admisible de 0,0002
mg/kg de peso corporal, establecida en 1970 (FAO/OMS, 1971).
1.2 Absorción, metabolismo y excreción
A diferencia de la dieldrina, su estereoisómero, la endrina se
metaboliza rápidamente en los animales, y se acumula en muy pequeña
cantidad en las grasas en comparación con compuestos de estructura
química análoga.
En la rata, tanto la absorción como la excreción tras la
administración oral se producen rápidamente; su semivida biológica es
de 1-6 días, según la dosis administrada. Al cabo de 6 días se alcanza
un estado de equilibrio en el que la cantidad excretada es igual a la
ingesta diaria. Se observan diferencias de un sexo a otro: los machos
excretan endrina y metabolitos con la bilis mucho más deprisa que las
hembras, lo que produce una acumulación menor en el tejido adiposo de
aquéllos. Las ratas excretan este compuesto principalmente en las
heces en forma de endrina, anti-12-hidroxiendrina, y un derivado
hidroxilado durante las primeras horas (70-75%); un tercer metabolito,
la 12-cetoendrina, se acumula enlos tejidos. El conejo excreta el 50%
de los metabolitos del compuesto enla orina, mientras que en la rata
sólo el 2% se excreta por esta vía; en lasheces del conejo sólo se
detecta endrina sin alterar.
Las vacas a las que se administró endrina a razón de 0,1 mg/kg de
la dieta durante 21 días excretaron hasta el 65% en forma de
metabolitos en la orina, el 20% en las heces, parcialmente en forma de
endrina no alterada, y el 3% en la leche, también principalmente en
forma de endrina. Estas vacas presentaron niveles residuales de
0,003-0,006 mg/litro en la leche, 0,001-0,002 mg/kg en la carne, y
0,02-0,1 mg/kg en la grasa.
En gallinas ponedoras a las que se administró endrina por vía
oral seobservaron niveles residuales (dependientes de la dosis
administrada) de hasta 0,1 mg/kg en la carne, 1 mg/kg en la grasa,
0,2-0,3 mg/kg en los huevos (yema), 0,2 mg/kg en el riñón y 0,5 mg/kg
en el hígado. Salvo en el hígado y el riñón, los residuos encontrados
estaban formados principalmente por endrina no alterada. Alrededor del
50% de la endrina administrada se excretó en las heces, principalmente
en forma de metabolitos.
En el ser humano, la rata, el conejo, la vaca y la gallina, el
principal metabolito biotransformado de la endrina es la
anti-12-hidroxiendrina, junto con su sulfato y su glucur nido
conjugados. Se encontraron cuatro metabolitos más, si bien en
cantidades muy reducidas. En los tejidos corporales y en la leche se
encuentra sobre todo endrina inalterada. Tras la aplicación de este
plaguicida a plantas, seidentificaron endrina inalterada y dos
productos de transformación hidrófilos.
1.3 Efectos en los organismos del medio ambiente
El efecto de la endrina en las bacterias y los hongos del suelo
es mínimo. Con dosis de 10-1000 mg/kg de suelo no se observó efecto
alguno en la descomposición de materia orgánica, la desnitrificación
ni la generación de metano. La endrina es sumamente tóxica para los
peces, los invertebrados acuáticos y el fitoplancton: los valores de
la CL50 a las 96 horas se encuentran en su mayoría por debajo de 1,0
µg/litro. El nivel sin observación de efectos más bajo en un ensayo de
ciclo biológico del crustáceo Mysidopsis bahia se fijó en 30 ng/litro.
Los ensayos comunicados sobre la toxicidad aguda de la endrina
para los organismos acuáticos se llevaron a cabo en acuarios sin
sedimentos; cabría esperar que la presencia de sedimentos atenuara el
efecto del insecticida. Los sedimentos muy contaminados ejercieron
escaso efecto en las especies de aguas libres, lo que indica que la
endrina ligada a los sedimentos tiene una biodisponibilidad reducida.
Aún no se han llevado a cabo ensayos en animales acuáticos que viven
en los sedimentos.
La DL50 para mamíferos terrestres y aves oscila entre 1,0 y
10,0 mg/kg de peso corporal. Los patos silvestres a los que se
administraron 3,0 mg/kg de peso corporal durante 12 semanas no
mostraron efecto alguno en la producción de huevos, la fertilidad o la
eclosión.
Ciertas especies de invertebrados acuáticos, peces y mamíferos de
pequeño tama o son resistentes a la toxicidad de la endrina; la
exposición a diversos plaguicidas organoclorados llevó a la selección
de estirpes resistentes a la endrina.
Se observaron muertes masivas de peces en zonas de escorrentía
agrícola y descargas industriales; el declive de las poblaciones de
pelícanos pardos (en Luisiana, EE.UU.) y de golondrinas de mar
(Thalasseus sandvicensis) en los Países Bajos se ha atribuido a la
exposición a la endrina en combinación con otras sustancias químicas
halogenadas.
1.4 Efectos en animales de experimentación in vitro
La endrina es un plaguicida sumamente tóxico; los signos de
intoxicación son de carácter neurotóxico. La DL50 por vía oral de la
endrina de calidad técnica en animales de laboratorio oscila entre 3
y 43 mg/kg de peso corporal; la DL50 por vía cutánea en la rata es
de 5-20 mg/kg peso corporal. No se ha encontrado ninguna diferencia en
la toxicidad aguda por vía oral o cutánea entre los productos de
calidad técnica y los formulados (concentrado emulsionable y polvos
humectables).
Se han llevado a cabo experimentos de breve duración para
estudiar la toxicidad por vía oral en el ratón, la rata, el conejo, el
perro y animales domésticos. En el ratón y la rata, las dosis máximas
toleradas durante 6 semanas fueron 5 y 15 mg/kg de la dieta
(equivalentes a 0,7 mg/kg de peso corporal), respectivamente. Las
ratas sobrevivieron tras una exposición a 1 mg/kg de la dieta
(equivalente a 0,05 mg/kg de peso corporal) durante 16 semanas; los
conejos murieron tras recibir dosis repetidas de 1 mg/kg de peso
corporal. En el perro, no se observó efecto alguno tras la
administración de 1 mg/kg de la dieta (equivalente aproximadamente a
0,025 mg/kg de peso corporal) durante más de 2 años.
La base neuroógica de los signos de intoxicación observados es la
inhibición de la función del ácido gamma-aminobutírico (GABA) con
dosis reducidas. Al igual que otros insecticidas a base de
hidrocarburos clorados, la endrina afecta también al hígado, y se
observa claramente la estimulación de sistemas enzimáticos que
participan en el metabolismo de otras sustancias químicas, como lo
demuestra, por ejemplo, la menor duración del sueño por hexobarbital
en el ratón.
Con dosis de 75-150 mg/kg aplicadas por vía cutánea en forma de
polvo seco durante 2 horas al día se produjeron convulsiones y la
muerte en el conejo pero sin irritación cutánea. Esta toxicidad
sistémica sin irritación en el lugar de contacto resulta muy notable.
Se han llevado a cabo en ratones y ratas estudios prolongados de
toxicidad y carcinogenicidad. No se observó efecto carcinogénico, pero
estos estudios tenían ciertos defectos, entre ellos la reducida
supervivencia de los animales. El nivel sin observación de efectos en
cuanto a la toxicidad en un estudio de dos años de duración en la rata
fue de 1 mg/kg de la dieta (equivalente a unos 0,05 mg/kg de peso
corporal). No se demostró ningún efecto de favorecimiento de tumores
cuando se ensayó la endrina en combinación con cantidades submínimas
de sustancias químicas de conocido efecto carcinogénico en los
animales. El Grupo de Trabajo concluyó que los datos de que se dispone
no bastan para indicar que la endrina supone un riesgo carcinogénico
para el ser humano.
En varios estudios se observó que la endrina no es genotóxica.
En la mayoría de los estudios no resultó teratogénica para el
ratón, la rata o el hámster, ni siquiera con dosis suficientes para
provocar toxicidad materna o fetal. El nivel sin observación de
efectos adversos fue de 0,5 mg/kg de peso corporal en ratones y ratas
y de 0,75 mg/kg de peso corporal en el hámster. La endrina no indujo
efecto alguno en la reproducción de ratas estudiadas durante tres
generaciones cuando se administró a razón de 2 mg/kg de la dieta (unos
0,1 mg/kg de peso corporal).
Algunos metabolitos de la endrina tienen toxicidades agudas
iguales o más altas que el compuesto originario. El producto de
transformación, la delta-cetoendrina, es menos tóxico que la endrina,
pero la 12-cetoendrina se considera el metabolito más tóxico en los
mamíferos, con una DL50 por vía oral en la rata de 0,8-1,1 mg/kg de
peso corporal.
1.5 Efectos en el ser humano
Se han producido varios episodios de intoxicación mortal y no
mortal, tanto accidentales como suicidas. Los casos de intoxicación
aguda no mortal debida a exposición excesiva accidental se observaron
en trabajadores de una planta de fabricación de endrina. Se ha
calculado que la dosis que por vía oral provoca la muerte es de
aproximadamente 10 mg/kg de peso corporal; la dosis única por vía oral
que provoca convulsiones se fijó en 0,25-1,0 mg/kg de peso corporal.
El lugar principal de acción de la endrina es el sistema nervioso
central. La exposición del ser humano a una dosis tóxica puede
producir al cabo de pocas horas signos y síntomas de intoxicación
tales como excitabilidad y convulsiones; la muerte puede producirse en
las 2-12 horas que siguen a la exposición si no se administra
inmediatamente el tratamiento apropiado. La recuperación después de
una intoxicación no mortal es rápida y completa.
La endrina no se acumula en el cuerpo humano en grado
significativo. No se comunicaron efectos adversos a largo plazo en 232
trabajadores expuestos (duración de la exposición: 4-27 años) bajo
supervisión médica (tiempo de observación: 4-29 años). El único efecto
observado fueron pruebas indirectas de una estimulación reversible de
las enzimas metabolizadoras de fármacos.
No se detectó endrina en prácticamente ninguna muestra de tejido
adiposo, sangre y leche humana analizadas en numerosos países. El
Grupo de Trabajo atribuyó la ausencia de endrina en las muestras
humanas a la baja exposición de la población general a este plaguicida
y a su rápido metabolismo.
La endrina se detectó en la sangre (con concentraciones de hasta
450 µg/litro) y en el tejido adiposo (en concentraciones de 89,5
mg/kg) en casos de envenenamiento accidental mortal. No se encontró
endrina en los trabajadores en circunstancias normales. El nivel
umbral de endrina en la sangre por debajo del cual no se produce
ningún signo o síntoma de intoxicación se ha fijado en 50-100
µg/litro. La semivida de la endrina en la sangre puede ser del orden
de 24 horas.
2. Conclusiones
La endrina es un insecticida con elevada toxicidad aguda. Puede
provocar envenenamiento grave en casos de exposición excesiva
provocada por un manejo poco meticuloso durante su fabricación y uso
o por el consumo de alimentos contaminados. El público está expuesto
a la endrina principalmente por sus residuos en los alimentos; no
obstante, los niveles de ingesta de endrina que se han comunicado
están por lo general muy por debajo de la ingesta diaria admisible
establecida por la FAO/OMS. Esas exposiciones en principio no
constituyen un riesgo para la salud de la población general. Cuando se
aplican buenas prácticas de trabajo, medidas higiénicas y precauciones
de seguridad, es poco probable que la endrina suponga un riesgo para
los trabajadores expuestos.
Está claro que las descargas no controladas de endrina durante su
manufactura, formulación y uso pueden originar graves problemas
ambientales asociados a su elevada toxicidad. Los efectos del uso
agrícola del insecticida en la fauna y la flora están menos claros, si
bien los peces y las aves ictívoras están expuestos por la escorrentía
a partir de las superficies. El declive de las poblaciones de algunas
especies de aves se ha atribuido a la presencia de niveles elevados de
residuos de diversos compuestos organoclorados en los tejidos de
adultos y en los huevos. Se ha medido la endrina presente en algunas
de estas especies, pero es muy difícil separar los efectos de los
distintos compuestos organoclorados presentes.
3. Recomendaciones
1. No debe utilizarse la endrina a menos que sea indispensable
y sólo cuando no se disponga de una alternativa menos
tóxica.
2. Para la salud y el bienestar de los trabajadores y de la
población general, el manejo y el uso de la endrina se
confiarán sólo a operarios bien supervisados y adiestrados
que apliquen las medidas de seguridad adecuadas y utilicen
la endrina de acuerdo con las prácticas agrícolas correctas.
3. La fabricación, la formulación, el uso agrícola y la
evacuación de endrina se tratarán cuidadosamente para
reducir al mínimo la contaminación del medio, en particular
de las aguas de superficie.
4. Las personas expuestas regularmente a la endrina deben
someterse a revisiones médicas periódicas.
5. Proseguirán los estudios epidemiológicos sobre las
poblaciones de trabajadores expuestos.
6. En los países en los que aún se usa la endrina, deben
vigilarse sus residuos en los alimentos.
7. Si sigue utilizándose la endrina, debe obtenerse más
información sobre la presencia, el destino último y la
toxicidad de la 12-cetoendrina y la delta-cetoendrina.
(1975) suggested that the acute toxicity of endrin in birds is not
associated with the formation of 12-ketoendrin.
6.2.2 Human beings
No endrin was found (limit of detection, 0.0016 mg/litre) in 14
samples of urine from workers exposed to aldrin, dieldrin, and endrin,
even though workers with a complete work history had been exposed to
endrin for an average of 2160 h (Hayes & Curley, 1968). Endrin was not
detected in urine from five men and five women (Cueto & Hayes, 1962;
Cueto & Biros, 1967). No unchanged endrin was found in the urine of
Dutch workers exposed to endrin, but it occurred in the faeces (Jager,
1970; Baldwin & Hutson, 1980).
Neither 3-hydroxyendrin nor the diol was detected in urine or
faeces (Hutson, 1981). anti-12-Hydroxyendrin was present in the
urine of workers exposed to endrin, and the glucuronide was found in
the faeces. Concentrations of up to 0.36 mg/g of creatinine were found
in urine after 7 days, accompanied by a sharp rise in the level of
D-glutaric acid (excreted in the urine of mammals as a metabolite of
D-glucuronolactone [Marsh, 1963]), indicating that liver enzyme
induction may have occurred. The levels tended to decrease over the
weekend (Ottevanger & Van Sittert, 1979). Endrin,
anti-12-hydroxyendrin, 12-ketoendrin, and the beta-glucuronide of
anti-12-hydroxyendrin were not found in the blood of workers at a
Dutch plant for the manufacture of endrin (limit of detection, 2
µg/litre). Both endrin and anti-12-hydroxyendrin were found in the
faeces, and all urine samples contained the beta-glucuronide of
anti-12-hydroxyendrin up to a concentration of 0.14 mg/litre as
anti-12-hydroxyendrin (Baldwin & Hutson, 1980).
Hydroxylation at anti-C-12 is relatively rapid and accounts for
the rapid metabolism of endrin. Even syn-12-hydroxyendrin is
hydroxylated rapidly at its anti-C-12 position, affording
12-ketoendrin (Hutson, 1981).
As neither endrin nor its metabolites were found in the blood of
exposed, healthy workers, exposure can be measured by determining
anti-12-hydroxyendrin in urine. A quantitative relationship between
exposure to endrin and the concentration of this metabolite cannot be
established, however, owing to lack of data.
6.2.3 Microorganisms
In mixed anaerobic microbial populations developed using inocula
from soil, freshwater mud, sheep rumen, and chicken litter, endrin
(like other cyclodiene compounds) was monodechlorinated at the
methylene bridge carbon atom. Neither endrin nor any other compound
was further metabolized. The 10 obligate anaerobic bacteria that made
up the mixed population were subsequently isolated in pure culture. Of
these, only Clostridium bifermentans, C. glycolium, and other
Clostridium species were capable of dehalogenation, but at a rate
that was much slower than that of the mixed population (Maule et al.,
1987).
6.2.4 Plants
Three experiments were carried out on tobacco plants. In the
first experiment, 2.08 mg of 14C-endrin were applied to the leaves
with free aeration during the experimental period. In the second test,
the same dose was applied but with little aeration; and in the third,
plants were exposed to 1.04 mg of 14C-endrin with little aeration.
An initial residue level of 50-100 mg/kg was found on leaves in all
three experiments, but, subsequently, less residue was found on plants
with free aeration. Six weeks after treatment, 30-47% of radiolabel
was recovered in residues, which consisted of endrin and hydrophilic
substances (Weisgerber et al., 1969; Donoso et al., 1979).
The leaves of cotton plants were treated with 4.2 mg of
14C-endrin, and the application was repeated after 2 and again after
6 weeks, at which time parathion was also applied. At harvest,
two-thirds of the radiolabel had evaporated, and the total residue in
cotton seed was 0.333 mg/kg. Endrin and two groups of degradation
products were found in the plants; one of these products (possibly
delta-ketoendrin) was only slightly more hydrophilic than endrin, and
the other was very hydrophilic. Most of the metabolites were found on
the surface of the leaves. When delta-ketoendrin was applied to white
cabbage, it disappeared more slowly than endrin, with the formation of
hydrophilic metabolites (Korte, 1969).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
The interactions of halogenated pesticides and microorganisms
have been reviewed extensively (Pfister, 1972).
In three Willamette valley soils (USA) treated with endrin at 0,
1 or 10 mg/kg, no effect was found 30 days after application on the
function and activity of the microbial population, the decomposition
of native organic matter, the transformation of native soil nitrogen,
ammonification of peptone, or nitrification of ammonium sulphate
(Bollen & Tu, 1971). Even at an annual application rate of 5 lb/acre
(5.6 kg/ha) for 5 years, no effect was seen on the numbers or kinds of
soil fungi, the numbers of bacteria, the decomposition rate of organic
matter (measured by CO2 production), or the oxidation of ammonium to
nitrate (Martin et al., 1959).
Endrin at a concentration of 100 mg/kg of soil had no effect on
denitrification in soil under anaerobic incubation for 5 days at 30 °C
or in an isolated denitrifying bacterium (Bollag & Henninger, 1976).
A concentration of 1000 mg/kg had no effect on methanogenesis, sulfate
reduction, or carbon dioxide evolution in anaerobic salt-marsh
sediments (Kiene & Capone, 1984).
The growth rates of two strains of blue-green algae were
decreased in the presence of endrin at a concentration of 0.29
µg/litre (Batterton et al., 1971), and the productivity of many forms
of natural phytoplankton in estuarine waters was decreased by 46% when
they were exposed to 1 mg/litre (Butler, 1963).
7.2 Aquatic organisms
7.2.1 Invertebrates
Acute toxicity of endrin to invertebrates is given in Tables 14
and 15.
A static system was used to study the toxicity of endrin to a
polychaete worm (Nereis virens) in water and sediment, in which sea
water or sediment (containing 17% sand, 83% clay, and 2% organic
carbon) was present at a temperature of 9-10 °C for 12 days. None of
the worms in sea water died after exposure to endrin at 0.11 mg/litre
for 12 days, but two of five worms exposed to 28 mg/kg in sediment
died in this period (McLeese et al., 1982).
Table 14. Acute toxicity of endrin to freshwater invertebrates
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Red snail Static 48-h LC50 7200 Hashimoto & Nishiuchi
Indoplanorbis exustus (1981)
Marsh snail Static 48-h LC50 9500 Hashimoto & Nishiuchi
Semisulcospira libertina (1981)
Snail Static 48-h LC50 12 000 Hashimoto & Nishiuchi
Physa acuta (1981)
Water flea Static 18-23 48-h LC50 160 Thurston et al.(1985)
Daphnia magna Static 21 44 7.1 48-h LC50 4.2 Mayer & Ellersieck (1986)
Static 18 48 7.4 48-h LC50 41-74 Mayer & Ellersieck (1986)
Static 22-24 240 8.0 96-h LC50 59 Elnabarawy et al. (1986)
Water flea Static 15 44 7.1 48-h LC50 20 Mayer & Ellersieck (1986)
Daphnia pulex Static 22-24 240 8.0 96-h LC50 30 Elnabarawy et al. (1986)
Water flea Static 22-24 240 8.0 96-h LC50 24 Elnabarawy et al. (1986)
Daphnia reticulata
Water flea Static 21 44 7.1 48-h LC50 45 Mayer & Ellersieck (1986)
Simocephalus serrulatus Static 15 44 7.1 48-h LC50 26 Mayer & Ellersieck (1986)
Water flea Adult Static 21 44 7.1 48-h LC50 1.8 Mayer & Ellersieck (1986)
Cypridopsis vidua
Sow bug (isopod) Adult Static 15 44 7.1 96-h LC50 1.5 Mayer & Ellersieck (1986)
Asellus brevicaudus
Scud Adult Static 21 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
Gammarus fasciatus Adult Static 15 272 7.4 96-h LC50 1.3 Mayer & Ellersieck (1986)
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Scud Adult Static 21 44 7.1 96-h LC50 3.0 Mayer & Ellersieck (1986)
Gammarus lacustris
Crayfish 3-5 Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Orconectes nais weeks
Adult Static 21 272 7.4 96-h LC50 320 Mayer & Ellersieck (1986)
Crayfish 0.4-2.0 g Flow 18-23 96-h LC50 > 89 Thurston et al. (1985)
Orconectes immunis
Red crayfish Static 48-h LC50 300 Muncy & Oliver (1963)
Procambarus clarki
Tantytarsus Static 18-23 48-h LC50 0.84 Thurston et al. (1985)
Tantytarsus dissimilis
Glass shrimp Adult Static 21 272 7.4 96-h LC50 3.2 Mayer & Ellersieck (1986)
Palaemonetes Adult Flow 21 272 7.4 96-h LC50 0.5 Mayer & Ellersieck (1986)
kadiakensis
Stonefly Larvae Static 15 44 7.1 96-h LC50 > 0.18 Mayer & Ellersieck (1986)
Acroneuria sp.
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.076 Mayer & Ellersieck (1986)
Claasenia sabulosa
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.54 Mayer & Ellersieck (1986)
Pteronarcella badia
Stonefly Larvae Static 15 44 7.1 96-h LC50 0.25 Mayer & Ellersieck (1986)
Pteronarcys californica
Table 14. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (mg/l)
Mayfly Larvae Static 15 44 7.1 96-h LC50 0.9 Mayer & Ellersieck (1986)
Baetis sp.
Mayfly Larvae Static 15 44 7.1 96-h LC50 62 Mayer & Ellersieck (1986)
Hexagenia bilineata
Damselfly Larvae Static 21 44 7.1 96-h LC50 2.4 Mayer & Ellersieck (1986)
Ischnura verticalis Larvae Static 21 272 7.4 96-h LC50 2.1
Snipe fly Larvae Static 15 44 7.1 96-h LC50 4.6 Mayer & Ellersieck (1986)
Atherix variegata
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
Table 15. Acute toxicity of endrin to estuarine and marine invertebrates
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Sand shrimp Static 96-h LC50 1.7 Eisler (1970a)
Crangon septemspinosa Static 96-h LC50 0.2-2.0 McLeese & Metcalfe (1980)
Static 96-h LC50 4-120 McLeese & Metcalfe (1980)
Brown shrimp Juvenile Flow 15 26 48-h LC50 0.2 Mayer (1987)
Penaeus aztecus
Pink shrimp Juvenile Flow 17 30 48-h LC50 0.2 Mayer (1987)
Penaeus duorarum Adult Flow 17 28 96-h LC50 0.037
Grass shrimp Larvae Flow 25 13 96-h LC50 1.2 Mayer (1987)
Palaemonetes pugio Juvenile Flow 25 23 96-h LC50 0.35
Adult Flow 25 21 96-h LC50 0.69
Blue crab Juvenile Flow 11 16 48-h LC50 15 Mayer (1987)
Callinectes sapidus
Hermit crab 96-h LC50 1.2 Eisler (1970a)
Pagurus longicarpus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin
concentration in water maintained continuously
The mean 96-h LC50 for the oligochaetes Stylodrilus
heringianus and Limnodrilus hoffmeisteri exposed to sediment from
Lake Michigan contaminated with 14C-endrin was 2588 ± 1974 mg/kg dry
weight of sediment in four assays and 2725 ± 955 mg/kg in two assays.
The toxicity to L. hoffmeisteri appeared to be reduced in the
presence of S. heringianus. The 96-h EC50 burrowing avoidance
values were 15.3-19 mg/kg for S. heringianus and 59 mg/kg of
sediment for L. hoffmeisteri (Keilty et al., 1988a).
Sediment reworking by L. hoffmeisteri alone and with
S. heringianus was measured by monitoring the burial of a 137Cs
marker layer in sediments dosed with 12C- and 14C-endrin at
concentrations of 5.5-81 400 µg/kg of dry sediment. With low endrin
concentrations, the marker layer burial rate did not suggest
stimulation of reworking by either L. hoffmeisteri or
S. heringianus. At higher concentrations, the reworking rates were
equal to or slower than control rates at the beginning of the
experiment but decreased thereafter. The presence of S. heringianus
appeared to enhance the reworking response of L. hoffmeisteri. A
reduction in the post-experimental mortality and an increase in the
dry weight of L. hoffmeisteri in tests with the two species implies
that L. hoffmeisteri benefits from the presence of S. heringianus,
although the reverse was not observed. High concentrations of endrin
in the upper 3 cm of the final sediment showed that the worms had
transported the contaminant upward. The bioaccumulation factor for
S. heringianus ranged from 9.7 to 43.8 and was consistently three to
four times greater than that for L. hoffmeisteri (1.7-13.6) (Keilty
et al., 1988b).
The reworking rates of S. heringianus in microcosms containing
sediments dosed with 14C-endrin at 3.1-42 000 µg/kg of dry matter
were measured at 10 °C by monitoring a 137Cs marker layer buried in
contaminated and uncontaminated microcosms. Alterations in reworking
rates were observed at endrin concentrations 5.5 orders of magnitude
below the LC50 of 1650 mg/kg. At the lower concentrations, a
possible stimulatory effect on the marker layer burial rate in the
first 300-600 h was followed by a significant decrease relative to the
controls. At the higher concentrations, the rates were equal or slower
during the first 600 h and decreased dramatically in the last 600 h.
Mortality was 9.3-28% at 11 500 and 42 000 µg/kg and 0-6.7% at all the
other concentrations tested, including controls. The dry weights of
the worms at the end of the experiment were inversely related to the
high concentrations. The bioaccumulation factors ranged from 34 to 67
on the basis of grams of dry organism to grams of dry sediment (Keilty
et al., 1988c).
The effect of addition of endrin at 50 mg/kg dry weight of
sediment on protein utilization by S. heringianus was examined on
days 4, 8, 20, 28, 39, and 69. A slight increase in the relative
percentage of protein to total body weight was observed, but the
authors concluded that estimation of total protein is not a useful
measure of sublethal responses (Keilty & Stehly, 1989).
The total organic carbon content of sediment had little apparent
effect on the toxicity of endrin in the freshwater amphipod Hyalella
azteca. The 10-day LC50 for endrin in sediment (dry-weight basis)
was 4.4 µg/litre at 3.0% total organic carbon and 6.0 µg/litre at
11.2% carbon (Nebeker et al., 1989).
The EC50s in the green sea urchin (Strongylocentrotus
droebachiensis), the purple sea urchin (S. purpuratus), the red
sea urchin (S. franciscanus), and the sand dollar (Dendraster
excentricus) were 103-441 µg/litre for sperm in a static system and
221-> 362 µg/litre for embryos in a continuous flow of sea water
(temperature, 8.2-8.4 °C; salinity, 30.0 parts per thousand; pH
7.8-8.1 for the sea urchins and 12.5-13.0 °C, 30.0 parts per thousand,
and pH 8.0-8.1 for sand dollar embryos), both with an exposure time of
120 h. In a larval test of static exposure of Dungeness crab (Cancer
magister), the EC50 was 2.0 µg/litre (Dinnel et al., 1989).
Endrin was tested at 0, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.5,
5.0, and 10 mg/litre for its effects on embryos of the American oyster
(Crassostrea virginica) and their larvae. Fertilized eggs were
studied after 48 h, and survival and growth of veliger larvae were
studied in 2-day old larvae and in larvae kept for a further 12 days
at 24 °C. The results varied considerably. The estimated concentration
that would cause an approximately 50% reduction in the number of eggs
that develop into normal straight-hinge larvae, calculated by
interpolation from the data, was 0.79 µg/litre and that at which 50%
of the larvae survived was > 10.0 mg/litre (Davis & Hidu, 1969).
In the mysid shrimp Mysidopsis bahia, exposed for the complete
life cycle, acute lethality (over 96 h) was observed with endrin at
120 ng/litre; increased oxygen consumption was measurable within 24 h
of exposure. The lowest-observed-effect level for chronic lethality
was 60 ng/litre; sub-lethal effects on growth (reduced by day 4 of
exposure) and oxygen consumption (increased by day 10 of exposure)
were observed before death (over 20 days). Reduced reproductive
capacity (assessed as production of young) was observed at 30 ng/litre
over 20 days--the time to full maturity (McKenney, 1986).
Behavioural changes were observed in stoneflies (Pteronarcys
dorsata) within 4 days of exposure to 96.1% endrin at 0.07 µg/litre
and in caddis flies (Brachycentrus americanus) at 0.15 µg/litre. The
28-day LC50 was < 0.03 µg/litre for caddis flies and 0.07 µg/litre
for stoneflies (Anderson & DeFoe, 1980).
7.2.2 Fish
7.2.2.1 Acute toxicity
Endrin is highly toxic for both freshwater and marine fish. The
available data are summarized in Tables 16 and 17.
7.2.2.2 Short-term toxicity
Channel catfish (Ictalurus punctatus) were exposed continuously
to renewed solutions of endrin in water at 15 and 22 °C. Measured
endrin concentrations of 0.25-0.30 µg/litre were found to be acutely
toxic to the fish within 10 days or less. None of the fish survived
blood concentrations exceeding 0.28 mg/litre, a well-defined threshold
concentration of endrin in blood, and none died at less than 0.23
mg/litre. The concentration of endrin in the blood of fish exposed to
lethal concentrations in water for periods insufficient to cause death
were markedly lower than that in fish that died from exposure to the
same water (Mount et al., 1966).
The 28-day LC50 for 96.1% eldrin in bullheads (Ictalurus
melas) was 0.10 µg/litre (Anderson & DeFoe, 1980).
In larval fathead minnows (< 24 h old) exposed continuously to
endrin (98%) for 28-30 days in a flow-through system, growth was the
most sensitive parameter. A 48-h exposure to 0.62 µg/litre caused
significant reduction in growth, and survival was reduced at 1.21
µg/litre; with a 72-h exposure, growth was reduced at 0.63 µg/litre,
and all fish died at 1.15 µg/litre. Continuous exposure to 0.38
µg/litre for 30 days significantly reduced growth, and all fish died
at 0.73 µg/litre (Jarvinen et al., 1988).
Sheephead minnows (Cyprinodon variegatus) were exposed
continuously for 23 weeks to endrin from the embryonic stage through
hatching, until adulthood and spawning. The average exposure
concentrations were 0 (control), 0.027, 0.077, 0.12, 0.31, and 0.72
µg/litre. The resultant progeny were monitored to determine effects on
their survival, growth, and reproduction. Embryos exposed to 0.31 and
0.72 µg/litre hatched early; all fry exposed to 0.72 µg/litre died by
day 9 of exposure. At 0.31 µg/litre, fry were initially stunted and
some died. Survivors seemed unaffected until maturity, when some
females died during spawning; fewer eggs were fertile, and survival of
exposed progeny was decreased. No significant effect was observed
throughout the life cycle at an exposure concentration of 0.12
µg/litre (Hansen et al., 1977).
Endrin was tested in flagfish (Jordanella floridae) at 0.21,
0.29, and 0.39 µg/litre for 30 days. Only the highest concentration
decreased survival, and the two highest dose levels affected the mean
number of eggs produced (Hermanutz et al., 1985).
Table 16. Acute toxicity of endrin to freshwater fish
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Tilupa sp. Static 15 44 7.1 96-h LC50 12 Mayer & Ellersieck (1986)
Coho salmon Static 16 44 7.1 96-h LC50 0.089 Mayer & Ellersieck (1986)
Oncorhynchus kisutch 1.9 g Static 20 96-h LC50 0.27 Katz & Chadwick (1961)
Chinook salmon 6-8 g Static 20 96-h LC50 0.92 Katz & Chadwick (1961)
Oncorhynchus
tshawytscha
Cutthroat trout 1.0 g Static 13 44 7.1 96-h LC50 > 1.0 Mayer & Ellersieck (1986)
Salmo clarki
Rainbow trout 1.0 g Static 13 44 7.1 96-h LC50 0.75 Mayer & Ellersieck (1986)
Oncorhynchus mykiss 1.0 g Static 13 272 7.4 96-h LC50 0.74
1.4 g Static 2 44 7.1 96-h LC50 2.4
1.4 g Static 7 44 7.1 96-h LC50 1.4
1.4 g Static 13 44 7.1 96-h LC50 1.11
1.4 g Static 18 44 7.1 96-h LC50 0.75
0.6-8.0 g Flow 18-23 96-h LC50 0.3 Thurston et al. (1985)
Goldfish 1-4 g Flow 12 314 7.6 96-h LC50 0.44 Mayer & Ellersieck (1986)
Carrassius auratus Flow 18-23 96-h LC50 0.95 Thurston et al. (1985)
Static 48-h LC50 1.0 Hashimoto & Nishiuchi
(1981)
Carp Flow 12 314 7.6 96-h LC50 0.32 Mayer & Ellersieck (1986)
Cyprinus carpio Static 48-h LC50 0.84 Hashimoto & Nishiuchi
(1981)
Medaka Static 48-h LC50 1.4 Hashimoto & Nishiuchi
Oryzias latipes (1981)
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Pond loach Static 48-h LC50 4.9 Hashimoto & Nishiuchi
Misgurnus (1981)
anguilicaudatus
Fathead minnow 1.2 g Static 18 44 7.1 96-h LC50 1.8 Mayer & Ellersieck (1986)
Pimephales promelas 0.9 g Flow 12 314 7.6 96-h LC50 0.24
Static 24-h LC50 12 Kagan et al. (1986)
Larvae Static 25-26 46 7.1-8.3 96-h LC50 0.7 Jarvinen et al. (1988)
0.2-1.0 g Flow 18-23 96-h LC50 0.65 Thurston et al. (1985)
Bluntnose minnow Static 96-h LC50 0.29 Johnson (1968)
Pimephales notatus
Black bullhead 1.5 g Static 24 44 7.1 96-h LC50 1.13 Mayer & Ellersieck (1986)
Ictalurus melas
Channel catfish 5.2 g Static 18 44 7.1 96-h LC50 1.9 Mayer & Ellersieck (1986)
Ictalurus punctatus 1.4 g Static 24 44 7.1 96-h LC50 0.32
Mosquito fish 0.6 g Static 17 44 7.1 96-h LC50 1.1 Mayer & Ellersieck (1986)
Gambusia affinis 0.222 g Static 25 96-h LC50 5.27 El-Sebae (1987)
Bluegill 1.5 g Static 18 44 7.1 96-h LC50 0.61 Mayer & Ellersieck (1986)
Lepomis macrochirus 0.5 g Static 18 272 7.4 96-h LC50 0.53
1.3 g Static 7 44 7.1 96-h LC50 0.73
1.3 g Static 13 44 7.1 96-h LC50 0.68
1.3 g Static 18 44 7.1 96-h LC50 0.41
1.3 g Static 24 44 7.1 96-h LC50 0.37
1.3 g Static 29 44 7.1 96-h LC50 0.19
Table 16. (contd)
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCO3/l) (µ g/l)
Largemouth bass 2.5 g Static 18 272 7.4 96-h LC50 0.31 Mayer & Ellersieck (1986)
Micropterus salmoides
Yellow perch Flow 12 314 7.6 96-h LC50 0.15 Mayer & Ellersieck (1986)
Perca flavescens
Tilapia 1.1 g Static 24 44 7.1 96-h LC50 < 5.6 Mayer & Ellersieck (1986)
Tilapia mossambica
Tilapia (Behera strain) 0.825 g Static 25 96-h LC50 10.09 El-Sebae (1987)
Tilapia zilli
Tilapia (Alexandria
strain) 0.825 g Static 25 96-h LC50 0.26 El-Sebae (1987)
Tilapia zilli
Guppy Static 20 96-h LC50 0.9 Katz & Chadwick (1961)
Poecilia reticulata
Flagfish 2-3 days Static 24-26 43-48 6.9-7.8 96-h LC50 0.85 Hermanutz et al. (1985)
Jordanella floridae
aStatic, static condition (water unchanged for the duration of the test); flow, flow-through conditions; endrin
concentration in water maintained continuously.
Table 17. Acute toxicity of endrin to estuarine and marine fish
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
American eel 57 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Anguilla rostrata
Atlantic riverside 54 mm Static 20 24 96-h LC50 0.05 Eisler (1970b)
Menidia menidia
Blue head 90 mm Static 20 24 96-h LC50 0.1 Eisler (1970b)
Thalassoma bifasciatum
Gulf menhaden Juvenile Flow 27 29 24-h LC50 0.8 Mayer (1987)
Brevoortia patronus
Sheepshead minnow Juvenile Flow 14 30 48-h LC50 1.0 Mayer (1987)
Cyprinodon variegatus Juvenile Flow 30 24 96-h LC50 0.34
Adult Flow 18 18 96-h LC50 0.38
Adult Flow 30 16 96-h LC50 0.36
Longnose killifish Juvenile Flow 25 19 24-h LC50 0.23 Mayer (1987)
Fundulus similis
Striped killifish 40 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
Fundulus majalis
Mummichog 51 mm Static 20 24 96-h LC50 0.6 Eisler (1970b)
Fundulus heteroclitus
Sailfin molly Adult Flow 20 27 96-h LC50 0.63 Mayer (1987)
Poecilia latipinna
Spot Juvenile Flow 12 24 48-h LC50 0.3 Mayer (1987)
Leiostomus xanthurus
Table 17. (contd)
Organism Size/ Static/ Temp. Salinity Parameter Concentration Reference
age flowa (°C) (%) (µg/l)
Striped mullet Juvenile Flow 14 30 48-h LC50 0.4 Mayer (1987)
Mugil cephalus 83 mm Static 20 24 96-h LC50 0.3 Eisler (1970b)
White mullet Juvenile Flow 29 48-h LC50 2.6 Butler (1963)
Mugil curema
Northern puffer 131 mm Static 20 24 96-h LC50 3.1 Eisler (1970b)
Sphaeroidus maculatus
Striped bass 2.7 g Static 16-18 28 96-h LC50 0.09 Korn & Earnest (1974)
Morone saxatilis
Shiner perch 1.2-11 g Static 13 26 96-h LC50 0.8 Earnest & Benville (1972)
Cymatogaster aggregata 1.2-11 g Int. flow 13 26 96-h LC50 0.12
Dwarf perch 1.2-11 g Static 13 18 96-h LC50 0.6 Earnest & Benville (1972)
Micrometrus minimus 1.2-11 g Int. flow 13 28 96-h LC50 0.13
Threespine stickleback 0.3 g Static 20 25 96-h LC50 1.5 Katz & Chadwick (1961)
Gasterosteus aculeatus
aStatic, static conditions (water unchanged for duration of test); flow, flow-through conditions; int. flow,
intermittent flow-through conditions; endrin concentration in water maintained continuously
7.2.2.3 Studies of resistance
Populations of mosquito fish (Gambusia affinis) developed high
levels of resistance to endrin and other cyclodiene insecticides as a
result of inadvertent exposure to agricultural sprays. Susceptible
fish (male) showed a LC50 of 8.3 mg/litre and resistant fish, 161
mg/litre. Genetic crossing studies show that endrin resistance is
inherited as a single, autosomal, intermediate gene (Yarbrough et al.,
1986).
Pesticide-susceptible and -resistant mosquito fish were exposed
to 14C-endrin at 20 or 1000 µg/litre, and liver and brain were
assayed to determine any difference in distribution, uptake, and nerve
binding patterns (Fabacher & Chambers, 1976). The results are
summarized in Table 18. Endrin was taken up faster by brain and liver
from susceptible fish than resistant fish. In resistant fish, at least
at a high lethal concentration (1000 µg/litre), endrin entered the
brain slowly and accumulated in the liver, suggesting a more efficient
blood-brain barrier in resistant than in susceptible fish. Extraction
studies provided some evidence that endrin binds more readily to
nonessential protein complexes in the nervous tissue of resistant
fish, consequently decreasing the amount of endrin available to
produce a toxic effect.
Table 18. Mean quantities of endrin (in mg/kg tissue) in brain and
liver of susceptible and resistant mosquito fish
Genotype At 20 µg/litre At 1000 µg/litre
Brain Liver Brain:liver Brain Liver Brain:liver
Susceptible 16.98 33.28 0.51 149.31 160.27 0.93
Resistant 8.83 16.84 0.52 57.52 353.42 0.16
Susceptible: 1.90 1.90 1.00 2.60 0.45 5.80
resistant
Cell membrane fractions from resistant mosquito fish bound more
endrin than those from susceptible fish, and mitochondria from the
liver of the resistant genotype bound less endrin than those from
susceptible fish. Differences in endrin uptake, retention of endrin by
brain cell membranes, a blood-brain barrier, and a structural
difference in myelin mayaccount for the resistance of some mosquito
fish to endrin (Wells & Yarbrough, 1972).
In resistant and non-resistant populations of golden shiner
(Notemigonus crysoleucas), blue gill sunfish (Lepomis macrochirus),
and green sunfish (Lepomis cyanellus), the median tolerated limit at
36 h was 3.0, 1.5, and 3.4 µg/litre for non-resistant strains and 310,
300, and 160 µg/litre for resistant fish, respectively (Ferguson et
al., 1964).
7.2.2.4 Interaction with other chemicals
The joint action of endrin with malathion on mortality in
flagfish (Jordanella floridae) consisted of enhanced effects at
concentrations that had no effect when the substances were tested
individually. The effects of the mixture on growth followed a simple
additive model. Malathion did not modify the effect of endrin on egg
production. In a separate test, malathion did not affect the uptake or
elimination of endrin (Hermanutz et al., 1985).
In a study of the interaction between the accumulation and
elimination of 14C-endrin and 14C-DDT in mosquito fish (Gambusia
affinis), fish about 4 cm long were exposed to a nominal
concentration of 3.94 nM endrin or DDT, or to a mixture of the
compounds. Prior exposure to DDT for 4 h generally reduced the
accumulation of endrin in serum, gall-bladder, and whole bodies,
whereas prior exposure to endrin for 4 h had little effect on DDT
accumulation. Simultaneous exposure to DDT and endrin reduced the
accumulation of DDT in the gall-bladder over the 4 h of exposure and
in the whole bodies during the first hours, and it reduced the
accumulation of endrin in gall-bladder and in the whole body. Endrin
levels in fish exposed subsequently only to DDT or DDE were
significantly higher in gall-bladder and were reduced in the whole
body over 4 h. The interactions observed may be the result of
competition for and/or displacement of insecticides from mutual
binding sites (Denison et al., 1985).
In a study of the relative binding and competition between
organochlorine pesticides for serum binding sites, incubation with
serum from mosquito fish led to their association primarily with the
vitellogenin/lipoprotein and albumin fractions. Preincubation of serum
with endrin significantly reduced the quantity of 3H-DDT that was
bound subsequently, while the reverse was not observed. Although the
reason for the apparent quantitative decrease in binding is unknown,
this phenomenon may be of toxicological importance (Denison &
Yarbrough, 1985).
7.2.2.5 Special studies
Fingerlings of carp (Cyprinus carpio) exposed to endrin at the
LC50 (0.0065 mg/kg) for 24 h showed clear inhibition of ý-amylase
activity in the liver (Datta & Ghose, 1985).
A group of 240 rainbow trout (Salmo gairdneri) were exposed to
endrin at 0.12-0.15 µg/litre for 30 days; one untreated and one
solvent control group were used. On day 30, 10 fish from each group
were sacrificed and examined for the ability of peritoneal macrophages
to phagocytize latex beads. The remaining fish were immunized with 10
µg of Yersinia ruckeri O-antigen and exposure to endrin continued.
Assays for migration inhibition factor, plaque forming cells, and
serum agglutination titre were performed 2, 14, and 30 days after
inoculation, and serum was collected from all fish to determine the
cortisol concentration. Exposure to endrin had no effect on the
phagocytic ability of peritoneal macrophages, but the responses in the
three assays were significantly reduced in comparison with the control
values. Serum cortisol concentrations were significantly elevated in
the endrin-treated fish. The study did not, however, elucidate the
mechanism of immune suppression, other than showing that a stress
response had occurred (Bennett & Wolke, 1987a). In another study,
therefore, control fish were fed cortisol at 20 mg/kg and metyrapone
at 35 mg/kg body weight, and endrin-exposed fish received metyrapone
at 35 mg/kg body weight per day in the diet. The fish that received
cortisol had significantly reduced responses in all three assays; but
in the endrin-exposed fish that received metyrapone, the migration
inhibition factor response was completely restored, the plaque forming
cell response was restored to 61%, and serum agglutination titres to
69%. These results indicate that elevated serum cortisol concentration
plays a central role in repressing the immune response (Bennett &
Wolke, 1987b).
The concentrations of serum glucose, liver and muscle glycogen,
cortisol, protein, and cholesterol were determined in carp (Cyprinus
carpio) exposed to endrin at 2 µg/litre for 6, 24, and 72 h. Only
the concentration of cortisol in serum was clearly decreased (Gluth &
Hanke, 1985).
7.2.3 Amphibia
The acute toxicity of endrin to amphibians is summarized in
Table 19.
7.3 Terrestrial organisms
The acute oral toxicity of endrin for terrestrial animals is
high. The available LD50 values are summarized in Table 20.
7.3.1 Honey bees
The 48-h LD50 of endrin in worker honey bees (Apis mellifera)
using a dusting technique was 2.02 µg/bee (Atkins et al., 1973). The
LD50 for bees after contact was 0.65 µg/bee, and the acute oral
LD50 was 0.46 µg/bee (Oomen, 1986).
Table 19. Acute toxicity of endrin to amphibians
Organism Size/ Static/ Temp. Hardness pH Parameter Concentration Reference
age flowa (°C) (mg CaCo3/l) (mg/1) (mg/l)
Bullfrog 2-5 g Flow 18-23 96-h LC50 2.5 Thurston et al. (1985)
Rana catesbiana
Leopard frog Eggs Flow 20 100 7.2-7.5 24-h LC50 2.5 Hall & Swineford (1980)
Rana spenocephala Larvae Flow 20 100 7.2-7.5 96-h LC50 6
Subadult Flow 20 100 7.2-7.5 96-h LC50 5
Frog 0.5 g Static 14 20 6.2 96-h LC50 0.21 Khangarot et al. (1985)
Rana hexadactyla
Western chorus frog Tadpole Static 15 44 7.1 96-h LC50 120 Mayer & Ellersieck (1986)
Pseudacris triseriata
Fowlers toad Tadpole Static 15 44 7.1 96-h LC50 180 Mayer & Ellersieck (1986)
Bufo woodhousei
fowleri
aStatic,static conditions (water unchanged for duration of test); flow, flow-through conditions; endrin concentration in water
maintained continuously
Table 20. Acute oral LD50s of endrin for terrestrial species
Species LD50 Reference
(mg/kg body weight)
Birds
Mallard 5.6 (2.7-11.7) Hudson et al. (1984)
(Anas platyrhynchos)
Pigeon 2.0-5.0
(Columbia livia)
Pheasant 1.8 (1.1-2.8)
(Phasianus colchicus)
Sharp-tailed grouse 1.06 (0.552-2.04)
(Pedioecetes phasia
nellus)
California quail 1.19 (0.857-1.65)
Redwinged blackbird 2.37 Schafer et al. (1983)
(Agelaius phoeniceus)
Starling 2.37-3.16
(Sturnus vulgaris)
Quail 4.22
(Coturnix coturnix)
Mammals
Big brown bat 5-8 Luckens & Davis (1965)
(Eptesicus fuscus)
Pine mouse (Microtus 2.6/19.0 Petrella et al. (1975)
pitymys pinetorum) 1.3/36.4 Webb et al. (1973)
(susceptible/
resistant)
7.3.2 Birds
7.3.2.1 Acute toxicity
The LD50s of endrin for some bird species are given in Table
20.
7.3.2.2 Short-term toxicity
Groups of 40 one-day-old quail were fed endrin at dietary levels
of 0, 0.5, 1, 5, 10, 20, or 50 mg/kg of diet. Survival was adversely
affected in all test groups, and there were no survivors beyond two
weeks among birds fed 10 mg/kg or more. Food consumption was
abnormally low, and symptoms involved lack of muscular coordination,
tremors, and occasional convulsive movements. Similar results were
obtained in 40 one-day-old pheasants fed endrin at dietary levels of
5 or 20 mg/kg, none of which survived beyond 8 days (Dewitt, 1965).
Groups of 20 seven-day-old chicks were unaffected by diets
containing endrin at 0, 1.5, or 3 mg/kg. When the concentration was
increased to 6 or 12 mg/kg, the birds became highly excitable and
failed to gain weight in comparison with controls. The survival rates
over a 12-week period were 85 and 5%, respectively, compared with 100%
in the controls (Sherman & Rosenberg, 1954).
The LC50 values for 2-3-week-old bobwhite quail (Colinus
virginianus), Japanese quail (Coturnix coturnix japonica),
ring-necked pheasants (Phasianus colchicus), and mallards (Anas
platyrhynchos) (8-13 birds per group) fed endrin in their diet for
5 days followed by 3 days of untreated diet, were 14-22 mg/kg diet
(Hill et al., 1975; Hill & Camardese, 1986).
7.3.2.3 Studies of reproduction
In a study of reproduction in pheasants, a diet containing endrin
at 10 mg/kg reduced egg production and chick survival; diets
containing up to 2 mg/kg did not affect egg production, fertility,
hatchability, or chick survival (Dewitt, 1965).
Groups of five female and two male mallard ducks (Anas
platyrhynchos) were administered diets containing endrin at 0, 0.5,
or 3.0 mg/kg for a 12-week oviposition period. Egg production was not
affected. The eggs were incubated, and infertile eggs, embryo
survival, and hatchability were measured. Fertility and hatchability
were not affected, although a 9.6% drop in embryo survival was
observed in the group that had received the highest dose. Endrin
residues in body fat were 3.4 mg/kg of tissue in the group that
received 0.5 mg/kg and 19.3 mg/kg in the group that received 3.0
mg/kg. The concentrations were higher in females than in males. The
endrin residue levels in eggs were none detected in the controls, 0.43
mg/kg in the group fed 0.5 mg/kg, and 2.75 mg/kg in the group fed 3.0
mg/kg (Roylance et al., 1985).
Three groups of 27 pairs of mallards were fed endrin at 0, 1, or
3 mg/kg of dry duck mash from December to the summer to investigate
the influence on reproduction and health. Birds fed 1 mg/kg reproduced
as well as the controls; they had significantly greater success in
hatching fertile eggs than did those fed 0 or 3 mg/kg and their
clutches hatched earlier (not significantly) than those of birds fed
3 mg/kg. Endrin accumulated in eggs to a mean level of 1.1 mg/kg (wet
weight) in the group fed 1 mg/kg and 2.9 mg/kg in the group fed 3
mg/kg. The concentration of endrin in adipose tissue was four to seven
times higher than that in eggs (Spann et al., 1986).
7.3.2.4 Interaction with other chemicals
The toxicity of combinations of chlordane and endrin was studied
in 14-week-old male and female bobwhite quail. Eight birds received
10 mg/kg chlordane in the diet for 10 weeks; 20 quail were treated
with 10 mg/kg chlordane for 10 weeks followed immediately by 10 mg/kg
endrin (98%) in the diet; a fourth group of 20 birds received only 10
mg/kg endrin in the diet. The pesticides were dissolved in propylene
glycol. After 9-10 days on a control diet, survivors were sacrificed
and their brains dissected. No deaths occurred among the birds fed the
control diet or 10 mg/kg chlordane. With endrin alone, 15 birds died,
and with the combination 14 birds died. In birds that received endrin
alone, the residue levels in the brain were 0.34-1.84 mg/kg in those
that died and 0.28-0.62 mg/kg in the survivors. In the birds fed
chlordane and endrin, the residue levels were 0.17-1.25 mg/kg in birds
that died and 0.14-0.56 mg/kg in survivors. Birds treated with the
combination had considerably more chlordane residues in their brains
than did those fed chlordane alone. The main conclusion of this study
was that the additive toxicity of closely related chemicals should be
taken into account in diagnosing cause of death (Ludke, 1976).
7.3.2.5 Special studies
The influence of endrin at 5 and 10 mg/kg of feed on the
activity of various enzymes in the serum of juvenile cockerels was
studied. The greatest increases in activity were measured for
glutamate oxalacetate transaminase, cholinesterase, and alkaline
phosphatase. Smaller increases were observed for creatine kinase,
glutamate dehydrogenase, ý-hydroxybutyrate dehydrogenase, and
phosphohexose isomerase (Horn et al., 1987).
7.3.2.6 Behavioural studies
The effect of a sub-lethal dose of endrin (2 mg/kg diet) on
avoidance responseswas studied in eight pens of 25 seven-day-old
Coturnix quail chicks for 14 days. The stimulus used to elicit
avoidance was a moving silhouette, and the response was measured
daily. Group avoidance response was significantly suppressed by
exposure to endrin, but the behaviour returned to normal after 2 days
on untreated diet (Kreitzer & Heinz, 1974).
Adult male bobwhite quail (Colinus virginianus) were fed a diet
containing endrin at 0.1 or 1.0 mg/kg for 138 days (beginning at 3
days of age), and then their performance in five non-spatial
discrimination reversal tasks was studied. Treated birds made 36-139%
more errors than controls, and birds fed the lower dose made
significantly more errors than those given the higher dose after
reversal 3 or 4 in the first three tests. The effects of endrin were
reversed after 50 days on untreated feed. The principal effect of
endrin was to impair the birds' ability to solve a novel problem. The
mean levels of endrin residues in the brain were 0.075 mg/kg wet
weight in those given the lower dose and 0.35 mg/kg for those on the
higher dose (Kreitzer, 1980).
7.3.3 Mammals
7.3.3.1 Toxicity
The LC50 values for short-tailed male and female shrews
(Blarina brevicauda) aged 180, 105-150, and 30-75 days were 87-174
mg/kg diet for 14 days (Blus, 1978).
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus maniculatus) of various ages were fed endrin at 0, 1, 2,
4, or 7 mg/kg of diet for intermittent periods, between which the
animals were either fed a normal diet or were subjected to 48-h
starvation. The animals were sacrificed by exposing them to cold
stress at -16 °C and the time of death recorded. No influence was
found on litter production, frequency, or mean litter size. At the
higher levels of feeding, postnatal mortality before weaning was
increased. Significant parental mortality occurred at 4 mg/kg and
higher and appeared to be dose-dependent (Morris, 1968). (Remark:
Since the animals in this study were captured in the field and the
periods of feeding alternated with short periods of starvation in an
effort to simulate possible conditions in the field, this study is of
only limited value).
The effects of endrin at 8.0 oz/acre (0.56 kg/ha) on unenclosed
field populations of meadow voles (Microtus pennsylvanicus) and deer
mice (Peromyscus maniculatus) were investigated in 1966-68. Animals
were trapped live on adjacent 7-acre (2.8 ha) plots each summer at
regular intervals, before and after a single application of endrin.
Immediate, significant declines in the number of voles were seen on
the experimental plot, but no long-term toxicological effects were
observed. The population rapidly recovered, exceeding the initial and
control numbers in all three years. The experimental vole population
thus appears to have responded to endrin as it would to a local
depopulation by trapping. The mouse population decreased significantly
after the application of endrin in 1966 and did not recover, and the
highly unstable, transitory population on the experimental plot
indicated a long-term toxicological effect (Morris, 1970, 1972).
7.3.3.2 Studies of resistance
14C-Endrin in corn oil was administered to a resistant and a
susceptible strain of pine mice (Microtus pitymus pinetorum) orally
at 0.5 mg/kg body weight, as follows: days 1-5, unlabelled endrin;
days 6-14, 14C-endrin; and day 15 unlabelled endrin. Total recovery
of 14C in both faeces and urine was 76% for the resistant strain and
53% for the susceptible strain. The two strains produced the same
major faecal and urine metabolite, but the resistant strain produced
about twice the quantity as the susceptible strain. The quantitative
differences in the excretion of more polar endrin metabolites may
indicate metabolic differences between the two strains and,
consequently, the greater tolerance of the resistant strain to toxic
effects (Petrella et al., 1975). The major metabolite was identified
as anti-12-hydroxyendrin; one of the other more polar metabolites,
found in minor quantities, was suggested to be a tertiary alcohol of
endrin (Petrella et al., 1977).
The degree of toxicity of endrin in first-generation progeny of
susceptible and resistant strains and a cross of the two strains of
pine mice was studied by Webb et al. (1973). The LD50 for offspring
of susceptible x susceptible parentage was 5.0 mg/kg body weight; that
for resistant x resistant, 21.1 mg/kg; and that for susceptible x
resistant, 8.6 mg/kg. These results offer preliminary support for a
genetic mechanism with intermediate dominance. An increase in
resistance against the toxic effects of endrin was demonstrated in
wild pine mice trapped in orchards where endrin had been used for
years. The oral LD50 in susceptible mice was about 3 mg/kg body
weight and that in resistant mice, an average of 36 mg/kg body weight.
The increased resistance appeared to be heritable in the first
generation (Webb & Horsfall, 1967; Webb et al., 1973). Although
differences in the rate of metabolism of endrin could be demonstrated,
especially in the activity of mixed-function oxidase, these did not
appear to be sufficiently large to explain the resistance (Hartgrove
et al., 1977).
7.4 Effects in the field
Episodes have been reported in which endrin was concluded to be
the cause of death in fish and birds. Numerous fish kills were
reported from the sugar-cane growing areas of Louisiana in 1960-63. No
association with variables such as dissolved oxygen, pH, or
temperature was found, but following the development of sensitive
analytical techniques it was concluded that the fish had been killed
by endrin (Mount & Putnicki, 1966). Surface runoff from fields was
reported to be the main source of the endrin that contaminated the
rivers (Lauer et al., 1966), although effluent from an insecticide
plant may have contributed since the fish contained two chemicals
involved in endrin manufacture (Mount & Putnicki, 1966). Levels of
endrin found in studies of fish in the wild are given in section
5.1.4.2.
Declines in the population of brown pelicans in Louisiana were
attributed to endrin, although at least six other organochlorine
pesticides and polychlorinated biphenyls were found in the animals
(Blus et al., 1975; King et al., 1977). The eggs of brown pelicans
(Pelecanus occidentalis) in Texas, USA, were examined for endrin
residues in 1975-81. The compound was recovered only in 1975, in 15 of
18 eggs, at levels of 0.1-0.3 mg/kg. In the same year, the highest
levels of endrin were found in pelican eggs in Louisiana, and this
maximum coincided with the deaths of large numbers of brown and white
pelicans (P. erhyrorhyncos) (King et al., 1985).
Endrin was found in one of ten eggs of the American white pelican
(Pelecanus erythrorhynchos) collected in 1969, at 0.20 mg/kg, and in
two of 35 samples collected in 1981, at up to 0.18 mg/kg wet weight.
Brains of pelicans found dead in the period 1975-81 had levels up to
0.80 mg/kg. No endrin was found in eggs of the western grebe
(Aechmophorus accidentalis) collected in 1981. It was concluded that
endrin had caused some of the deaths among pelicans in California
(Boellstorff et al., 1985).
The death of sandwich terns in The Netherlands was attributed to
the discharge of a combination of endrin and related pesticides into
an estuary from a manufacturing plant (Koeman et al., 1967, 1969;
Koeman, 1971).
On several occasions in Victoria, Australia, large numbers of
wild birds, in particular pigeons (Columba livia), sparrows (Passer
domesticus) and Indian mynahs (Gracula religiosa), were observed
to be paralysed or in convulsions (Reece et al., 1985). The crops and
livers contained endrin at levels of up to 1.2 mg/kg.
Fulvous whistling ducks (Dendrocygna bicolor), which nest in
rice fields along the south-eastern coast of Texas, USA, suffered a
major decline in population in the late 1960s, which was attributed to
exposure to dieldrin or aldrin. Organochlorine pesticides were
determined in 1983 in the carcasses of 15 adult ducks immediately
after their arrival in Texas from Mexico in the spring and before
departure from Mexico in the autumn. Four of the ducks with high
levels of dieldrin residues also had residues of endrin; and four
other ducks, collected in 1967 and 1969, had endrin residues. The
geometric mean levels in the different years were 0.03-0.08 mg/kg wet
weight; in juveniles in 1960-69, the geometric mean level was 0.16
mg/kg (Flickinger et al., 1986).
The effects of endrin on wildlife were studied in 1981-83 in
fruit orchards in Washington, USA. A single application of endrin
after harvest resulted in acute and chronic toxicity to a variety of
avian species; most deaths occurred soon after the application, but
several raptors died during the spring and summer. The brains of 73 of
125 birds contained endrin at < 0.10-0.80 mg/kg; detectable levels
occurred most frequently in the brains of galliforms and falconiforms.
The species in which the greatest numbers of deaths attributed to
endrin occurred include California quail (Callipepta california),
chukars (Alectoris chukar), and common barn owls (Tyto alba). Of
the 97 eggs analysed, 68 contained detectable endrin residues: 51 had
levels of < 0.10 mg/kg, and the eggs of 10 species contained
0.01-0.17 mg/kg wet weight (range, none detected to 1.67). The authors
concluded that endrin was toxic to wildlife, although there was no
evidence that it affected reproductive success or population level
(Blus et al., 1989).
Levels found in birds in the wild are also given in section
5.1.4.1.
7.5 Appraisal of effects on organisms in the environment
Use of endrin in agriculture is the major source of its presence
in the environment, but discharge of waste material from manufacturing
and formulating plants has contributed to local contamination.
World-wide monitoring surveys have shown that the concentrations of
endrin in the biosphere are generally very low (Table 10), both
absolutely and relatively: The levels of residues of other
organochlorine compounds, particularly DDE and polychlorinated
biphenyls, are generally 100 times or higher than those of endrin.
Toxicologically significant levels of endrin residues have been
found locally in fish and other organisms, particularly in cases in
which endrin was applied near rivers and lakes and when runoff
occurred into waterways. Residues may also occur when endrin is used
as a seed dressing or in bait to control rodents.
The most serious adverse ecological effects that have been
reported were the fish kills (and associated adverse effects on brown
pelican populations) in the Mississippi River system in the USA.
Although the initial evidence for ascribing these effects to endrin
was circumstantial, the results of analyses of dead fish were
considered to confirm a causal relationship; there is little doubt
that endrin was a contributory factor in at least some of these fish
kills. The evidence that endrin was the primary cause of the decline
in the brown pelican population is less convincing, since the harmful
effects on reproductive success have been attributed to DDE and other
factors (Blus et al., 1974, 1979).
In summary, agricultural application of endrin should be such as
to avoid or minimize contamination of waterways, either by
overspraying or runoff or by leaching from dressed seed in
rice-growing areas. The effects of the use of baits containing endrin
for rodent control on non-target organisms should be assessed in the
light of local circumstances. Finally, effluents from manufacturing
and formulating plants must be treated adequately before being
discharged into waterways.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO
The toxicology and risk assessment of endrin have been reviewed
(US EPA, 1987a,b; Anon., 1988a,b).
8.1 Acute toxicity of technical-grade endrin
8.1.1 Oral administration
Endrin is highly toxic when given by the oral route and is more
acutely toxic to mammals than its stereoisomer dieldrin (WHO, 1989),
with an acute oral LD50 of 7.5-17.8 mg/kg body weight (Table 21),
compared with 50-60 mg/kg for dieldrin. There appears to be a
sex-dependent sensitivity to the acute effects of endrin, female
animals being more sensitive than males. A species-dependent
sensitivity has also been reported, monkeys and cats being more
susceptible than mice and rats.
Signs of intoxication may include increased irritability and
tremor, followed by tonic-clonic convulsions, ataxia, dyspnoea,
gasping, and cyanosis. Convulsions usually occur 30-60 min after an
oral dose, and death may occur within 24 h after the administration of
a lethal dose (Speck & Maaske, 1958). Animals that survive poisoning
recover completely with no delayed or persistent effect.
8.1.2 Dermal administration
The acute dermal LD50s for technical endrin in various animal
species are given in Table 22. Endrin is highly toxic when applied as
a solution in hydrocarbon solvents but moderately toxic when applied
as a dry powder. The signs of poisoning are similar to those seen
after oral administration.
8.1.3 Parenteral administration
The acute LD50s for technical-grade endrin given by parenteral
routes of administration are shown in Table 23.
8.1.4 Toxicity of metabolites and isomers
8.1.4.1 Mammalian metabolites
In a comparative study, the acute oral LD50s of endrin and
three of its metabolites were determined in rats (Table 24). When the
brains of some of the rats were analysed for the presence of endrin
and its metabolites, the concentration of 12-ketoendrin in male rats
given endrin at 60 mg/kg body weight was found to be higher (mean, 0.3
mg/kg) than that of endrin (0.07 mg/kg) 22 h after dosing. In male
rats intoxicated with syn-12-hydroxyendrin or 12-ketoendrin (at 16
mg/kg body weight each), the concentrations of 12-ketoendrin in the
brain 30 min after dosing were much higher (mean values, 1.9 and 1.4
Table 21. Oral LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Mouse Corn oil 8.6 - Spynu (1964)
Mouse Unknown 13 13 Gray et al. (1981)
Rat (4-5 weeks) Peanut oil 28.8 16.8 Treon et al.(1955)
Rat (6 months) Peanut oil 43.4 7.3 Treon et al. (1955)
Rat (6 months) Peanut oil 40.0 - Speck & Maaske (1958)
Rat (adult) Peanut oil 17.8 7.5 Gaines (1960)
Rat (7 weeks) Cottonseed oil 27 - Boyd & Stefec (1969)
Rat (12-14 weeks) Dimethyl sulfoxide 5.6a 5.3a Bedford et al. (1975b)
Rat (12-13 weeks) Corn oil 8.9 4.0 Carter & Simpson (1978)
Rat Corn oil 9.0 - Spynu (1964)
Rat Unknown 4 4 Gray et al. (1981)
Rabbit Peanut oil - 7-10 Treon et al. (1955)
Guinea- pig Peanut oil 36.0 16.0 Treon et al. (1955)
Hamster (golden Syrian) Corn oil - 18.6 Chernoff et al. (1979)
Hamster (golden Syrian)
(6 weeks) Unknown 12 17.0 Cabral et al. (1979)
Hamster Unknown 18 18 Gray et al. (1981)
Cat Cod liver oil Lethal dose: Ressang et al. (1958);
3-6 Cook & Casteel (1985);
Casteel & Cook (1985)
Monkey (Macacus mulatta) Peanut oil 3 Treon et al. (1955)
Monkey (Macacus speciosa) Unknown 12 Barth (1967)
Domestic goat Unknown 25-50 Tucker & Crabtree (1970)
Mule deer Unknown 6.25-12.5 Hudson et al. (1984)
aEndrin of 99% purity
Table 22. Dermal LD50s for technical-grade endrin
Species (age) Vehicle LD50(mg/kg body weight) Reference
Males Females
Rat Xylene 18 15 Gaines (1960,1969)
Rat Shellsol A 10-20 5-10 Carter & Simpson (1978)
Rat Toluene approx. 10 approx. 10 Carter & Simpson (1978)
Rat Corn oil 12.5 - Spynu (1964)
Rabbit None - Minimum lethal Treon et al. (1955)
dose: 60-94
Cat Cod-liver oil Lethal dose: Ressang et al. (1958)
approx. 150
Table 23. Parental LD50s for technical- grade endrin
Species Route Vehicle LD50 (mg/kg Reference
body weight)
Mouse Intraperitoneal Corn oil 5.6 Graves & Bradley
(1965)
Mouse Intravenous Dimethyl 2.3 Walsh & Fink
sulfoxide (1970, 1972)
Dog Intravenous Ethanol 3 Hinshaw et al.
(1966)
Table 24. Acute oral toxicity of mammalian metabolites of
endrin in rats
Compound Oral LD50 (mg/kg body weight)
Male Female
Mean 95% CI Mean 95% CI
Endrin 5.6 3.0-7.9 5.3 3.6-7.4
anti-12-Hydroxyendrin 2.4 2.0-3.0 5.5 4.2-7.2
syn-12-Hydroxyendrin 1.2 0.6-1.7 2.8 0.8-4.0
12-Ketoendrin 1.1 0.7-1.5 0.8 0.5-1.2
a95% CI, 95% confidence interval
mg/kg, respectively) than those in the brains of rats given a similar
but non-toxic dose of anti-12-hydroxyendrin (mean, 0.09 mg/kg) and
killed at the same time. The signs of intoxication were similar to
those of endrin (Bedford et al., 1975b).
These results suggest that 12-ketoendrin may be the acute
toxicant in rats. The production of 12-ketoendrin varies greatly from
one mammalian species to another, however, and none has been detected
in birds of various species that were killed by endrin (Stickel et
al., 1979).
8.1.4.2 Isomers
As described in section 4.2, endrin is changed under the
influence of sunlight into delta-ketoendrin. The acute toxicity of
this isomer is given in Table 25. It is less toxic than endrin, and,
like endrin, it is more toxic to female than to male rats. The signs
of intoxication are similar to those seen with endrin.
The acute tocixity of the endrin aldehyde has been reported to be
> 500 mg/kg body weight in male mice (Phillips et al., 1962).
Table 25. Acute toxicity of delta-ketoendrin
Sex and Route LD50 (mg/kg Reference
species body weight)
Male rats Oral 120-180 Soto & Deichmann
(1967)
Female rats Oral 10-36
Male rats Oral 62.1 (53.3-72.2) Stanford Research
Institute (1954)
Rats Intravenous 5 Soto & Deichmann
(1967)
Male rats Intraperitoneal 82 Stanford Research
Institute (1953)
Male mice Oral 23.6 (19.9-28.0) Stanford Research
Institute (1954)
Male mice Intraperitoneal 16.7 Stanford Research
Institute (1953)
8.1.5 Acute toxicity of formulated material
8.1.5.1 Oral and dermal administration
Oral and dermal LD50 values for formulated endrin in rats
(Muir, 1970) are presented in Table 26. Dry formulations were
administered orally as 1-2% aqueous suspensions and dermally in both
dry form and as 2-5% aqueous suspensions. In general, the type of
formulation did not significantly alter the acute oral toxicity of
endrin. The dermal toxicity of the 50% wettable powder was similar to
that of the 20% emulsifiable concentrate; the 2% field strength dust
was the least toxic.
Ten rabbits (body weight, 2.4-4.1 kg) were treated with an
emulsifiable concentrate containing 19.4% endrin on clipped skin at a
dose of 200 mg/kg body weight, and the material was allowed to remain
in contact with the skin for 24 h. Four of the 10 animals died within
48 h (Anderson et al., 1953). Two of 10 rabbits (body weight,
2.0-2.5 kg) treated similarly with a 25% dust concentrate died within
48 h (Hine et al., 1954).
Table 26. Oral and dermal LD50s for endrin formulations in rats
Formulation LD50 (mg/kg body weight)
Oral Dermal
Formulation Active Formulation Active
material material
20% Emulsifiable 20 4.20 52.20 (undiluted) 10.90
concentrate
50% Wettable 7.6 3.80 21.80 (dry) 10.90
powder 14.40 (aqueous) 7.20
2% Field strength 275 5.50 5720 (dry) 114.40
dust 1140 (aqueous) 22.80
From Muir (1970)
8.1.5.2 Inhalation
Ten adult rats were exposed for 1 h to a mist of of an
emulsifiable concentrate containing 19.4% by weight of endrin in
xylene, at a concentration of endrin slightly exceeding 2000 mg/m3
of air, and were observed for 48 h. The particle size of the mist and
other details of exposure were not reported. Three of the animals died
1-14 h after exposure (Anderson et al., 1953).
Groups of 10 Long Evans rats were exposed for 1 h to 25% and 30%
endrin dust concentrates at a concentration of 2000 mg/m3 of air.
Particle size and other details of exposure were not provided. Five
rats exposed to the 30% and three exposed to the 25% dust died within
48 h after exposure (Hine et al., 1954).
8.2 Short-term exposure
8.2.1 Oral administration
8.2.1.1 Mouse
Feeding studies were conducted to estimate the maximum tolerated
doses of endrin in B6C3F1 mice. Groups of five males and five females
were given a normal diet or one containing endrin at 2.5-20 mg/kg for
6 weeks, followed by observation for another 2 weeks. Three males and
four females given 10 mg/kg died, but no mortality occurred at 5
mg/kg. No data were provided on animals fed 20 mg/kg.
Hyperexcitability was observed in male mice given doses > 5 mg/kg of
diet. Mean body weight gains were comparable with those of controls.
The maximum tolerated dose was calculated by extrapolation to be 5
mg/kg of diet (NCI, 1978, 1979).
8.2.1.2 Rat
Groups of three male and two to three female Carworth rats,
either 29 days or 6 months old, received daily doses of endrin at 1,
2, or 5 mg/kg body weight in peanut oil by gavage, on five days per
week for 67-72 days. All rats given 1 mg/kg survived; the increased
mortality in the other groups was dose-related: 2/5 females at 2 mg/kg
and 3/3 males at 5 mg/kg day died. Pathological findings at autopsy
included diffuse degenerative changes in the liver, kidneys, and
brain, while survivors showed no such changes. All treated animals
lost weight and developed hypersensitivity to stimuli (Treon et al.,
1955).
Groups of five male and five female adult Sprague-Dawley rats
were given diets containing technical-grade endrin at 0, 1, 5, 25, 50,
or 100 mg/kg diet over a maximal period of 16 weeks and were observed
for behaviour, weight gain, feed consumption, mortality rate, and
symptoms of toxicity. Alkaline phosphatase levels, determined once a
week, were higher in rats fed endrin than in the control group, and
the total average feed consumption of treated rats was less than that
of the control group. All rats fed 100 mg/kg of diet died during the
first two weeks of the study, and only two female rats fed 50 mg/kg of
diet survived the experiment. All male rats given 1 mg/kg and all
female rats given 1 and 5 mg/kg of diet survived. Males appeared to be
more susceptible than females to endrin in this study. The symptoms of
intoxication were hypersensitivity to stimuli and convulsions:
hypersensitivity was noted in all rats, and convulsions occurred among
rats receiving 25, 50, and 100 mg/kg diet. Weight loss was
dose-dependent and significant in all rats treated with endrin (Nelson
et al., 1956).
To estimate the maximum tolerated doses of endrin in
Osborne-Mendel rats, groups of five males and five females were given
diets with or without endrin for 6 weeks, followed by observation for
another 2 weeks. Endrin was added to the diet in two-fold increasing
concentrations of 2.5-80 mg/kg. Mortality was not increased at 10
mg/kg, and mean body weight gain was no different from that in
controls. At 20 mg/kg of diet, one animal of each sex died. The
maximum tolerated dose was calculated by extrapolation to be 15 mg/kg
diet (NCI, 1978, 1979).
8.2.1.3 Rabbit
Four of five female rabbits given an oral dose of endrin at 1
mg/kg body weight on five days per week died following the
administration of 2, 30, 35, and 50 doses, respectively. The fifth
rabbit survived 50 doses over a period of 10 weeks. Diffuse
degenerative changes were observed in the liver and kidneys but not in
the brain (Treon et al., 1955).
8.2.1.4 Dog
Dogs (mainly two per group) fed endrin at 5-50 mg/kg of diet died
within 50 days. They regurgitated their food, became lethargic,
salivated, and later refused to eat; they became emaciated and
developed respiratory distress and signs of stimulation of the central
nervous system. Diffuse degenerative lesions in the brain, heart,
liver, and kidneys, together with pulmonary hyperaemia and oedema were
observed. Three dogs fed diets containing 4 mg/kg of diet for 6 months
survived, but they showed reduced body weight gain and a slight
increase in liver:body weight ratio; no histopathological change was
observed. At 3 mg/kg of diet or less, growth was normal (Treon et al.,
1955).
Beagles (one male and one female/group; control group, only one
dog) were fed diets containing endrin at 0, 1, or 3 mg/kg for 80
weeks. No sign of intoxication was observed, and the weight gain of
treated animals was comparable to that of controls. The ratios of
kidney and heart to body weight were increased at 3 mg but not at 1 mg
(about 0.045 mg/kg body weight). No histopathological change was found
in the viscera (Treon et al., 1955).
Groups of three male and three female pure-bred beagle dogs
(4-6 months old) were fed endrin in the diet at 0, 0.1, 0.5, 1, 2, or
4 mg/kg for two years. Additional groups of four male and four female
dogs were fed endrin at 0, 1, or 4 mg/kg of diet. Two males and two
females of each group were killed after 6 and 12 months of feeding; no
other death occurred during the study. Convulsions were observed in
three dogs at 4 mg and in one female at 2 mg; no other sign of
intoxication or illness was apparent during the study. No adverse
effect was noted on growth, food consumption, haematology, or
urinalysis, and no compound-related change was found in serum alkaline
phosphatase, prothrombin time, or any of the other clinical chemical
parameters measured at regular intervals. All organ weights, relative
as well as absolute, were normal, except for occasional, slight
increases in liver weight in some of the females at 2 and 4 mg in the
diet. The only histopathological change found was a slight to moderate
vacuolation of liver cells in dogs fed 2 and 4 mg in the diet. No
renal change was observed in any of the dogs (Jolley et al., 1969).
8.2.1.5 Domestic animals
Sheep and cattle fed diets containing endrin at 2.5 or 5 mg/kg
for 112 days showed no indication of harmful effects (details not
given) (Radeleff, 1956). The convulsions and muscular tremors that
were induced in six 10-18-month-old male buffalo calves administered
a 20% emulsion of endrin led to a significant rise in lactic acid
concentration in the blood of the animals, possibly due to excessive
production of the acid inside the fasciculating muscles (Verma et al.,
1970).
8.2.2 Inhalation
Three mice, three rats, two guinea-pigs, two hamsters, four
rabbits, and one cat were exposed to sublimed endrin vapour at an
actual concentration of 5.44 mg/m3 for 7 h/day on 5 days/week for up
to 26 weeks. Two rabbits died after 26 and 90 exposures, respectively,
and one mouse died after 22 exposures. No convulsions were observed,
and all other animals survived. Surviving rabbits showed a
granulomatous type of pneumonitis; no histological change was found in
the other surviving animals (Treon et al., 1955).
8.2.3 Dermal administration
Three female rabbits with intact skin died after 19, 19, and 25
applications, respectively, of endrin as a dry powder at 150 m/kg body
weight for 2 h/day on 5 days/week. Applications of 75 mg/kg resulted
in the death of one of three rabbits after 8 weeks; the other two
survived for 13-14 weeks--the end of exposure. Convulsions, tremors,
and twitching of the facial muscles were the main signs of
intoxication. Two of five rabbits (dose not specified) showed severe
fatty degeneration of the liver (Treon et al., 1955).
8.3 Skin irritation
Dry powdered endrin was applied repeatedly at a dose of 75 or 150
mg/kg body weight for 2 h/day, 5 days/week for up to 14 weeks on
intact or abraded skin of female rabbits (see section 8.2.3). No skin
irritation was observed. Single applications of endrin as dry powder
at doses up to 250 mg/kg body weight for 24 h on rabbit skin caused no
gross or microscopic damage to the skin of the animals (Treon et al.,
1955).
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Mouse
CFW mice (20 males and 20 females) were fed diets containing
endrin (96%) at 0 and 5 mg/kg for 120 days, beginning 30 days before
mating. Significant parental mortality (32%) and reduced litter size
were observed, but fertility, fecundity, and the number of litters
produced per pair were not affected (Good & Ware, 1969).
8.4.1.2 Rat
Forty male and 80 female Long-Evans rats were fed endrin in the
diet at 0, 0.1, 1, or 3 mg/kg over three generations, each generation
breeding once. No difference in appearance, behaviour, body weight, or
number or size of litters was seen. The weights of the liver, kidneys,
and brain were normal, and no histopathological abnormality was seen
in third-generation weanlings. The only significant effect was
increased mortality of pups in the second and third generations of
rats fed 3 mg/kg (Hine, 1965).
Ten male and 20 female Long-Evans rats were treated similarly,
but each generation bred twice. Weanling rats were mated after 79 days
on the diets (when they were 100 days old). All pups from the first
litters were discarded at weaning, and the parent rats were mated
again. Randomly selected pups from the second litters were maintained
on the diets and mated when 100 days old; this was done for three
generations. The number of pups in each litter was counted on the day
of birth and on the fifth day; on the twenty-first day, the weanlings
were counted and weighed and either sacrificed or saved for
continuation. Parent rats were weighed, sacrificed, and examined
grossly when no longer needed. Ten male and 10 female F3b weanlings
each from the controls and the highest dose-level group and five males
and five females from the 0.1 and 1.0 mg groups were autopsied. Body,
liver, kidney, and brain weights were recorded, and sections of these
organs and from heart, lung, spleen, and testis were studied
histologically. Appearance, behaviour, body weight, number and size of
litters, organ weights, and histopathological appearance of F3b
weanlings were comparable with those in control animals. No effect on
reproduction was observed in rats fed diets containing endrin at 2
mg/kg over three generations (Hine, 1968).
8.4.2 Embryotoxicity and teratogenicity
8.4.2.1 Mouse
Groups of 10 CD-1 mice were given a single oral dose of endrin
(99%) at 2.5 mg/kg body weight (stated to be half the LD50) in corn
oil by gavage on day 9 of gestation; an untreated and a vehicle
control group were also used. Fetuses were examined on day 18. No
significant effect was observed on intrauterine death or fetal weight,
but the incidence of total anomalies was increased over that in
controls: 2/117 fetuses had cleft palates, three had open eye, and two
had other anomalies. No data on maternal toxicity were reported
(Ottolenghi et al., 1974).
These results could not be repeated by Kavlock et al. Female CD-1
mice were given endrin (99%) at 0 (vehicle), 0.5, or 1.0 (groups of 40
mice), or 1.5 or 2.0 mg/kg body weight (groups of 20 mice) in corn oil
by gavage on days 7-17 of gestation. The animals were killed on day
18. Maternal deaths occurred in the 1.5 and 2 mg/kg groups, reduced
maternal weight gain was observed at and above 1 mg/kg, and maternal
liver weight was increased at 0.5 mg/kg and higher. Fetal weight and
skeletal, and visceral maturity were adversely affected at doses of 1
mg/kg and above. No teratogenic effect or embryonic lethality was
observed, even at doses that caused maternal death (Kavlock et al.,
1981, 1987).
In a study of the effects of acute alterations in maternal health
status upon fetal development in the mouse, groups of 21 or 40
pregnant CD-1 mice were given a single oral dose of technical-grade
endrin at 0 (vehicle), 7 or 9 mg/kg body weight in corn oil on day 8
of gestation. The animals were killed on day 18 of gestation. Three of
21 animals given 7 mg/kg (14%) and 19/40 mice gievn 9 mg/kg (47%)
died. Maternal weight gain was decreased in both test groups; the
total number of implantation sites and number of viable litters were
not affected, but fetal weight was reduced. Delays in ossification of
the skeleton and an increased incidence of supernumary lumbar ribs
were observed. Although three fetuses from one litter in the 9 mg/kg
group had fused ribs, no significant increase in the incidence of
malformations was found. A statistically significant, linear, inverse
relationship between maternal weight gain and the presence of
supernumary ribs in their offspring was found (Kavlock et al., 1985).
8.4.2.2 Rat
Five groups of 25 female CD rats were administered endrin (97%)
in methocel in oral doses of 0, 0.1, 0.5, or 2 mg/kg body weight per
day on days 6-15 of gestation, or vitamin A, used as a positive
control. The animals were killed on day 20. The largest dose of endrin
caused maternal toxicity, as evidenced by weight loss and mortality
(two animals). The fetuses showed some slight growth retardation (not
significant) but no increase in intrauterine death rate. No effect
attributable to endrin was seen on the mean number of viable fetuses,
post-implantation losses, implantations, corpora lutea, fetal sex
ratio, or fetal external, soft-tissue, or skeletal abnormalities. Bent
ribs were observed in 6/522 fetuses treated with endrin, but not in
relation to dose. An increase in delayed ossification in sternebrae
and skull of fetuses was seen in the treated groups in comparison with
the untreated control group. Animals given vitamin A had a
significantly increased number of post-implantation losses and
malformed fetuses (Goldentahl, 1978a).
Groups of 32, 15, 28, 30, and 15 female CD rats were given oral
doses of endrin (99%) in corn oil at 0, 0.075, 0.15, 0.30, or 0.45
mg/kg body weight on days 7-20 of gestation. Rats were killed on day
21. Maternal weight gain was reduced at dose levels above 0.15 mg/kg,
but no increase in maternal liver weight was found. Fetal mortality,
weight, degree of skeletal and visceral maturation, and incidences of
skeletal and visceral anomalies showed no dose-related effect
(Kavlock et al., 1981).
8.4.2.3 Hamster
Three groups of golden Syrian hamsters (7, 24, and 8
animals/group) received a single oral dose of endrin (99%) in corn
oil at 5 mg/kg body weight (stated to be half the LD50) on day 7,
8, and 9 of gestation, respectively. Two control groups were used,
consisting of 57 untreated and 41 vehicle controls. The animals were
killed on day 14. The number of resorptions and of dead fetuses was
increased after treatment on days 7 or 8 and to a lesser extent in the
vehicle controls. The live fetuses in all three treated groups showed
significant growth retardation when compared with controls. The
incidence of anomalies was high only after treatment on day 8:
congenital abnormalities were seen in 28% of fetuses, with open eye in
22%, webbed foot in 16%, cleft palate in 5%, and fused ribs in 8%. The
anomalies that appeared to be increased significantly but to almost
the same extent at all three stages were fused ribs and cleft palate
(Ottolenghi et al., 1974).
These results could not be repeated by other workers. Groups of
27-29 golden Syrian hamsters were administered oral doses of endrin
(97%) in methocel at 0 (two control groups), 0.1, 0.75, or 2.5 mg/kg
body weight on days 4-13 of gestation. The animals were killed on
day 14. Body weight gains of the animals given 2.5 mg/kg were
slightly reduced. Maternal appearance, behaviour, and survival, mean
number of viable fetuses, post-implantation losses, implantations,
corpora lutea, fetal body weight, and crown-rump length showed no
change attributable to treatment. The number of malformations in
fetuses was not increased, but ossification of the sternebrae and
certain ribs was delayed (Goldentahl, 1978b).
Groups of 18-87 golden Syrian hamsters (LVG strain) were given
endrin (98%) as a solution in corn oil by gavage either as a single
dose of 0.5, 1.5, 5, 7.5, or 10 mg/kg body weight on day 8 of
pregnancy or as multiple daily doses of 0.75, 1.5, 2.5, or 3.5 mg/kg
body weight on days 5-14 of pregnancy. All animals were killed on day
15. With single doses, no effect was found on maternal survival,
pregnancy rate, weight change or liver:body weight ratio. The only
sign of maternal toxicity was the occurrence of transient convulsions
2 h after dosing in one hamster given 10 mg. No compound-related
difference was noted in the number of implantation sites, fetal death
rate, or fetal weight; indicators of skeletal maturity were not
affected. A dose-related increase in the incidence of fused ribs was
found in the groups given 7.5 and 10 mg/kg; increased incidences of
meningo-encephaloceles were observed at 5 mg/kg and above, with no
dose-response relationship. No other compound-related skeletal or
visceral anomaly was noted. In the study of multiple doses, maternal
toxicity (reduced weight gain and increased mortality) and fetal
toxicity (increased mortality, reduced weight, reduced skeletal
ossification, and an increased percentage of irregular
supra-occipitalis) were observed at doses of 1.5 mg/kg and higher. No
treatment-related maternal or fetal effect occurred at 0.75 mg/kg per
day (Chernoff et al., 1979).
8.4.2.4 Perinatal behavioural development
Rats exposed perinatally to endrin at 0, 0.075, 0.15, or 0.3
mg/kg body weight from gestation day 7 through day 15 of lactation
showed no mortality and no influence on survival or growth. Pups of
mothers exposed to 0.15 or 0.3 mg were more active than those of
mothers exposed to 0.075 mg or those in the control group. No clear
difference in ambulation was noted, and at 90 days of age there was no
difference (Gray et al., 1981; Kavlock et al., 1987).
Golden Syrian hamsters (LVG strain) given endrin (98%) at 0 or
1.5 mg/kg body weight per day by gastric intubation on days 5-14 of
gestation had a persistent increase in locomotor activity. Offspring
of treated hamsters ambulated more than the controls in the open field
at 15 days, and long-term observation of activity in the figure-8 maze
indicated that a significant increase in this behaviour was still
present at 125 days of age. Other behaviour patterns, including
sexual, rearing and running, and wheel behaviour, were unaffected.
Dams repeatedly exposed to endrin at 0.75 and 1.5 mg/kg body weight
were markedly hypoactive under the same testing conditions in which
the pups were hyperactive. The dose of 1.5 mg/kg body weight killed
more than half of the dams (Gray et al., 1981; Kavlock et al., 1987).
8.4.3 Appraisal of reproductive effects
Endrin had no reproductive effects in three generations of rats
at a level of 2 mg/kg of diet, equivalent to 0.1 mg/kg body weight. It
had no teratogenic effect in mice, rats, or hamsters after oral
exposure during the period of organogenesis. The significance of the
anomalies observed in mice and hamsters by Ottolenghi et al. (1974) is
uncertain. Studies in the same strain of the same species using more
rigorous protocols and larger numbers of animals could not confirm
their findings.
The lowest-observed-adverse-effect level for maternal toxicity
was 1.0 mg/kg body weight in mice, 0.3 mg/kg body weight in rats, and
1.5 mg/kg body weight in hamsters. Embryotoxicity was observed at
doses of 1 mg/kg body weight in mice and 1.5 mg/kg body weight in
hamsters. The overall no-observed-adverse-effect levels in mice, rats,
and hamsters were 0.5, 0.15, and 0.75 mg/kg body weight, respectively
(Table 27).
8.5 Mutagenicity and related end-points
8.5.1 Effects on microorganisms
Endrin was not mutagenic in numerous studies using Salmonella
typhimurium (TA98, TA100, TA1535, TA1537, TA1538, TA1950, TA1978,
SL4525, SL4700), Escherichia coli (WP2 uvrA, WP2 uvr-, Gal
Rs, WP2, hcr, p3478, W3100), K-12 (Pol A1+/Pol1-), WP67,
CM611, and CM571 Bacillus subtilis (M45), Saccharomyces cerevisiae
(D3, D7), and Serratia marcescens (a21, a742), with or without
metabolic activation by rat or mouse liver S9 (Fahrig, 1974; Van Dijck
& van de Voorde, 1976; Ercegovich & Rashid, 1977; Simmon et al., 1977;
Nishimura et al., 1982; Waters et al., 1982; Glatt et al., 1983;
Moriya et al., 1983; Rashid & Mumma, 1986).
No mutagenic effect was observed in S. typhimurium strains
TA98, TA100, TA1535, or TA1537, with and without metabolic activation
with S9 from livers of Aroclor 1254-induced rats and hamsters in the
presence of five concentrations of endrin (0-10 000 µg/plate) (Zeiger,
1987; Zeiger et al., 1987). No mutagenic effect was observed in
S. typhimurium strains TA97, TA98, TA100, or TA102 with and without
metabolic activation with Aroclor 1254-induced rat liver microsome
fraction in the presence of seven concentrations of endrin (99.0%),
from 1 ng/plate up to 1 mg/plate (Mersch-Sundermann et al., 1988).
8.5.2 Point mutations in mammalian cells
Endrin was weakly mutagenic in 6-thioguanine-resistant mouse
FM3A cells (Morita & Umeda, 1984; abstract only).
8.5.3 Dominant lethal mutations
Endrin did not show detectable dominant lethality when given as
a single intraperitoneal dose (0.76 or 3.8 mg/kg body weight) or daily
oral doses (0.1 or 0.25 mg/kg body weight) for 5 days to seven or nine
male ICR/Ha Swiss mice, respectively. This study involved a sequential
mating procedure, in which one male was housed with three females for
one week, repeated for 8 weeks (Epstein et al., 1972).
8.5.4 Chromosomal and cytogenetic effects
Endrin at 10-5 and 10-4 M, but not at 10-6, produced a
dose-related increase in the percentage of M1 metaphases and a
dose-related decrease in that of M3 metaphases at 48 h in treated
LAZ-007 human lympoid cells. This effect is closely related to the
reduced rate of cell proliferation induced by endrin (Sobti et al.,
1983).
Intratesticular injection of 0.25 mg endrin in saline to three
albino rats doubled the percentage of chromosomal changes in
comparison with that in the single control when the testes were
studied histologically 10 days after the injection. Changes were
scored in 70-75 cells/animal (Dikshith & Datta, 1973). The use of a
single dose does not aid interpretation, and the increase in
chromosomal abnormalities may be related to cytotoxicity rather than
to a genetic effect. The relevance of this type of study in
mutagenicity testing is unknown.
Table 27. Teratogenicity and effects on reproduction of oral administration of endrin
Animal Exposure NOAEL LOAEL Reference
(strain) period
Rat (Long Evans) 3 generations, 1 litter 1 mg/kg diet 3 mg/kg diet (0.15 mg/kg bw): Hine (1965)
(0.05 mg/kg bw) increased mortality of pups in
F2 and F3 generations
Rat (Long Evans) 3 generations, 2 litters 2 mg/kg diet Hine (1968)
(0.1 mg/kg bw)
Mice (CD-1) Days 7-17 of gestation 0.5 mg/kg bw 1 mg/kg bw: maternal and fetal Kavlock et al. (1981,
weight, skeletal and visceral 1987)
maturity
Rat (CD) Days 7-20 of gestation 0.15 mg/kg bw 0.30 mg/kg bw: reduced Kavlock et al. (1981)
maternal weight gain
Rat (CD) Days 6-15 of gestation 0.5 mg/kg bw 2 mg/kg bw: maternal toxicity Goldentahll (1978a)
Hamster Days 5-14 of gestation 0.75 mg/kg bw 1.5 mg/kg bw: maternal and Chernoff et al. (1979)
fetal toxicity
Chromosomal studies were carried out on lymphocytes from eight
male workers exposed to endrin and from six unexposed workers from the
same work area. No increase in the frequency of chromosomal
abnormalities was found, whether taken individually or collectively
(Dean, 1977).
Chromosomal aberrations were found in meiotic cells of barley and
somatic cells of barley and Vicia faba grown from endrin-treated
seeds (Wuu & Grant, 1966, 1967a,b). After treatment of root tips with
0.1% endrin (EC20 solution) for 1.5-2 h at 10 °C, the function of the
spindle was destroyed and did not interfere with the spreading of the
chromosomes during squash preparation. The centromeric region became
distinct and visible in prophase-metaphase chromosomes. At higher
concentrations contraction, stickiness, and fragmentation of
chromosomes were seen (Bhowmik, 1978).
8.5.5 Host-mediated effects
In two studies, male CF1 mice were given single oral doses of
endrin in dimethyl sulfoxide at 3.75 or 7.5 mg/kg body weight. Control
mice were dosed with the solvent, and positive control groups were
given a single oral dose of ethylmethanesulfonate at 400 mg/kg body
weight. Saccharomyces cerevisiae JD1 suspensions were then injected
intraperitoneally into each mouse, and the suspensions of
S. cerevisiae were harvested and analysed after 5 h. No increase in
mitotic gene conversion was detected (Brooks, 1976).
8.5.6 Sister chromatid exchange
Endrin at concentrations of 10-6-10-4 mol/litre in dimethyl
sulfoxide (the latter dose was a cytotoxic concentration) failed to
increase the frequency of sister chromatid exchange significantly over
the control value in rat liver microsomal S9-activated and unactivated
incubation experiments using human lymphoid cells of the LAZ-007 cell
line (Sobti et al., 1983).
8.5.7 Effects in Drosophila melanogaster
Endrin was not mutagenic to Drosophila melanogaster after
injection at 0.2 µlitre of a 0.001% aqueous solution, in the Muller-5
test for recessive lethal mutations on the X-chromosome (Benes & Sram,
1969).
8.5.8 Effects on DNA
Endrin at 10-3 or 3 x 10-3 mol/litre did not induce mutation
in the adult rat liver epithelial culture/hypoxanthineguanine
phosphoribosyl transferase assay (Williams, 1979).
DNA repair was not elicited in primary cultures of hepatocytes
from CD-1 mice, Fischer 344 rats, or Syrian hamsters exposed to endrin
for 18 h together with tritium-labelled thymine deoxyribonucleotide
for incorporation during repair synthesis. DNA repair was measured
autoradiographically. In rat and hamster liver cell cultures, a
concentration of 10-3 mol/litre and in mouse liver cell cultures,
10-4 mol/litre endrin was tested (Maslansky & Williams, 1981).
Endrin did not induce unscheduled DNA synthesis in human lung
fibroblast cells with or without metabolic activation by rat liver
microsomes (five concentrations were tested, but they were not given
in the paper) (Waters et al., 1982).
Endrin at eight concentrations ranging from 0.5 up to 1000
nmol/ml did not induce unscheduled DNA synthesis in primary rat
hepatocytes or in a modified Ames test utilizing concentration
gradient plates and 10 bacterial tester strains (eight S. typhimurium
and two E. coli) (Probst et al., 1981)
8.5.9 Appraisal of mutagenicity and related end-points
Garrett et al. (1986) evaluated the activity of endrin in a
series of tests: for reverse mutation (point/gene mutations in
prokaryotes), forward mutation (point/gene mutations in eukaryotes),
differential toxicity (primary DNA damage in prokaryotes), enhanced
mitotic recombination, gene conversion and crossing-over, unscheduled
DNA synthesis (primary DNA damage in eukaryotes), sister chromatid
exchange, chromosomal breakage, and dominant lethality (chromosomal
effects). Endrin gave negative results in all these tests.
The vast majority of the data indicate that endrin is not
genotoxic; however, many of the studies would not reach current
standards, or they give insufficient data to allow an independent
assessment.
8.6 Long-term exposure
Groups of 20 male and 20 female Carworth rats (28 days old) were
given diets containing endrin at 0, 1, 5, 25, 50, or 100 mg/kg. With
100 mg, two males and one female survived for 2 years; with 50 mg,
four males but no female survived; and with 25 mg, 11 males and 5
females survived. Survival at the lower concentrations was comparable
to that of the control group. Males appeared to be less susceptible
than females to the toxic action of endrin. Signs of intoxication,
hypersensitivity to external stimuli, and occasional convulsions were
observed only at the two highest levels. The weight gain of females
fed 1, 5, or 25 mg/kg of diet was equal to or greater than that of the
controls after 40 weeks of feeding. In males fed 5 mg, growth
retardation occurred during the first 20 weeks only, while males that
received 25 mg showed significant reduction in body weight gain. The
body weight gain of males fed 1 mg was comparable to that of controls.
The liver:body weight ratios were increased in males fed 5 mg or more
and in females fed diets with 25 mg or more. Histopathological
examination of animals that died during exposure to the three higher
dietary levels revealed diffuse degeneration of the liver, kidneys,
brain, and adrenal glands. The few survivors at 50 and 100 mg showed
degenerative changes in the liver only. No histopathological change
was found in surviving rats fed 1, 5, or 25 mg/kg diet. There was no
increase in the incidence of neoplasia in the treated groups compared
to the control group (Treon et al., 1955).
This study indicates a no-effect level for endrin of 1 mg/kg of
diet (about 0.05 mg/kg body weight) but is inadequate in several
respects, e.g., survival rate, details of pathology, and
haematological and clinical chemical data are not reported.
8.7 Carcinogenicity
8.7.1 Oral administration
8.7.1.1 Mouse
Groups of 100 male and female C57B1/6J mice, an inbred strain
with a low incidence of tumours, and C3D2F1/J mice, a hybrid strain
with a high incidence of hepatomas in males and a high incidence of
mammary tumours in females, were fed endrin (99%) at dietary
concentrations of 0.3 or 3 mg/kg from the age of five weeks throughout
life. A control group consisted of 200 mice of each sex of each
strain. Except for all groups of female animals of the C3D2F1/J
strain, this part of the experiment was terminated at the 78th week
because of the early occurrence of high numbers of mammary
fibroadenomas in 70-90% of control and treated mice. Survival, growth,
food intake, and haematology were not impaired. Mice of both strains
fed 3 mg/kg diet occasionally developed convulsions in the early
stages of feeding but recovered and survived without signs of illness.
They generally showed the typical histological changes in the liver
characteristic of high doses of chlorinated hydrocarbon insecticides.
No effect was observed on mice fed 0.3 mg/kg diet. The tumour
incidence and type of tumours were not influenced by the feeding of
endrin, and it had no influence on the incidence of fibroadenomas in
female C3D2F1/J mice (Witherup et al., 1970).
Groups of 50 B6C3F1 mice of each sex were given endrin in the
diet for 80 weeks and were then observed for a further 10 or 11 weeks.
The initial doses of endrin (97%) (2.5 and 5 mg/kg of diet) were
poorly tolerated by males and were therefore reduced after 25 weeks to
1.2 and 2.5 mg/kg diet for males; but females received 2.5 and 5 mg/kg
diet during the whole experiment. The time-weighted average doses were
1.6 and 3.2 mg/kg diet for males and 2.5 and 5.0 mg/kg diet for
females. Matched controls consisted of groups of 10 mice of each sex;
pooled controls, used for statistical evaluation, consisted of the
matched control groups combined with 50 untreated male and 50
untreated female mice from similar bioassays of other chemicals. All
surviving mice were killed at 90 or 91 weeks. Mean body weight was not
affected, but the survival of males at the high dose was lower than
that of the controls. The survival of the low-dose males could not be
evaluated due to accidental administration of excessive quantities of
endrin to this group during week 66. The tumour (neoplastic lesions in
the liver) incidences in the high-dose males were higher than those of
the pooled or matched controls, but not significantly so, and the
increase was not considered to be related to the administration of
endrin (Fredrickson, 1978;NCI, 1978).
8.7.1.2 Rat
A study of groups of 20 male and 20 female Carworth rats
administered endrin at 0, 1, 5, 25, 50, and 100 mg/kg of diet was
reviewed in Section 8.6.1.1. Bearing in mind the limitations of this
study, such as small group sizes and low survival at the high doses,
no evidence of an increase in the incidence of neoplasia was found in
any of the groups (Treon et al., 1955).
Groups of 50 weanling Osborne-Mendel rats of each sex were fed
diets containing endrin (98%) at 2, 6, or 12 mg/kg for 29 months. The
control groups consisted of 100 males and 100 females. During the
first 10 weeks of the study, only half the nominal dietary
concentrations of endrin were fed. Signs of toxicity occurred in a few
animals in all treatment groups, mainly in females, and included
episodes of tremor and clonic convulsions with 'outcries', the
incidence of these signs being dose-related. Weight gain was
unaffected, and the survival rates in control and treated rats were
similar. The liver:body weight ratios were unaffected. A moderate (not
dose-related) increase in the incidence of centrilobular cloudy
swelling in the liver and of cloudy swelling of the renal tubular
epithelium was observed. The lungs of the animals fed endrin exhibited
a moderate increase in the incidence of congestion and focal
haemorrhages. The tumour incidence in the treatment groups was
comparable with that in control rats, and no difference in the type of
tumours was found (Deichmann et al., 1970a,b; Deichmann & MacDonald,
1971).
In a life-time study, groups of 24 male and 24 female
Osborne-Mendel rats (22 days old) were fed diets containing endrin at
0, 0.1, 1, 5, 10, or 25 mg/kg. Because 50% of the rats at 25 mg/kg
died within the first week, this group was restarted with 32-day-old
rats. Survival did not appear to be affected by treatment. The highest
incidence of malignant tumours in male and female rats occurred at 0.1
mg/kg, but the malignancies were not dose-related. Treated male rats
had a higher incidence of renal disease than controls, but this also
was not dose-related (details not available) (Reuber, 1978).
Groups of 50 Osborne-Mendel rats of each sex were fed endrin
(97%) in their diet for 80 weeks and then observed for 31 or 34 weeks.
Males received doses of 2.5 or 5 mg/kg diet; in females, the initial
doses of 5 and 10 mg/kg of diet were poorly tolerated and were reduced
after 9 weeks to 2.5 and 5 mg/kg. The time-weighted average doses were
2.5 and 5.0 mg/kg for males and 3 and 6 mg/kg for females. Matched
controls consisted of groups of 10 rats of each sex; pooled controls
used for statistical evaluation consisted of the matched control
groups combined with 40 untreated male and 40 untreated female rats
from similar bioassays of other chemicals. All surviving rats were
killed at 100-114 weeks. Body weights and survival were not affected
by administration of endrin. A slight increase in the incidences of
pituitary and thyroid tumours was observed, but no consistent
statistical significance or dose-response relationship was found
(Fredrickson, 1978; NCI, 1978).
8.7.1.3 Tumour promotion
No significant increase in the development of preneoplastic
changes (hyperplastic nodules) was observed in rat liver after partial
hepatectomy and initiation with N-nitrosodimethylamine or
N-2-fluorenylacetamide in combination with the administration of
endrin (Ito et al., 1980).
In vitro, endrin at levels of above 2.5 µg/ml appeared to
inhibit metabolic cooperation in the hypoxanthineguanine
phosphoribosyl transferase system using wild-type
6-thioguanine-sensitive V79 cells and variant 6-thioguanine-resistant
cells. Such inhibition is reported to be an index of potential tumour
promoting activity, although the test has not been validated (Kurata
et al., 1982).
Endrin stimulated protein kinase C activity in vitro only
slightly, whereas a representative endogenous ligand of protein kinase
C, syn-1,2-didecanoylglycerol, stimulated protein kinase C to a
maximal velocity (Moser & Smart, 1989).
8.7.2 Appraisal of carcinogenicity
One of several studies in mice suggests an increased incidence of
nonmalignant tumours in animals of one sex, but this study was
considered inadequate for assessing carcinogenicity because an
increased number of tumours was seen in controls. A second study using
a different mouse strain did not corroborate the increase in tumour
incidence. Several long-term feeding studies in rats provide no
evidence of a carcinogenic effect of endrin. Its tumour promoting
activity was tested in vitro using protein kinase C stimulation and
ATPase inhibition; the results do not suggest any overwhelming effect
in these systems. After a careful review of this evidence, and taking
into consideration the fact that most of the data indicate that endrin
is not genotoxic, the Task Group concluded that the data are
insufficient to indicate that endrin is a carcinogenic hazard to
humans.
8.8 Special studies
8.8.1 Nervous system
8.8.1.1 Electrophysiological studies
The effects of endrin on bioelectrogenesis was studied in
anaesthetized pigeons and squirrel monkeys with chronically implanted
electrodes. Endrin was administered intravenously to pigeons at doses
of 0.5-4 mg/kg body weight. Doses of 2-4 mg/kg and higher caused
seizure activity throughout the telencephalon; the lower dose levels
caused activity only in the ectostriatum, a telecephalic visual
projection area. At doses of 0.5-2 mg/kg body weight, endrin caused a
specific increase in the evocation of potentials in the ectostriatum
by stimulation of the nucleus rotundus, a diencephalic visual
projection area. Reticular formation functions were not or little
affected. Administration of endrin to squirrel monkeys at doses of
0.2-3 mg/kg body weight on 5 days/week intramuscularly in corn oil and
saline emulsion induced characteristic changes in the
electro-encephalogram (EEG), culminating in electrographic seizures;
these were transient and disappeared when endrin administration was
stopped. Seizures reappeared under stress conditions, however, several
months after endrin treatment (Revzin, 1966, 1980).
Groups of 20-60 Sprague-Dawley rats with previously implanted
electrodes were given endrin in peanut oil orally at 0.8, 1.7, or 3.5
mg/kg body weight per day on 5 days/week for 28 weeks. Dose-dependent
mortality occurred during the first week and again at the end of the
study. Most changes in the EEG were seen after one week of exposure:
these included severe bursts of multiple spikes accompanied by clonic
convulsions; other animals had runs of spikes without full-fledged
convulsions. The convulsions were usually preceded by a period of
hyperventilation. After a further week of exposure, the rats showed
normal EEG traces. Some irregular slow-wave activity was seen in
animals that were moribund in the last month of feeding (Speck &
Maaske, 1958).
The convulsive properties of endrin at 1-2 mg/kg body weight
were studied by intravenous injection in locally anaesthetized,
paralyzed male cats, in which electrodes were placed in the
subcortical structures of the brain. Endrin was dissolved in ethanol
(which itself stimulates or inhibits the central nervous system,
depending on dose). Changes in the EEG and evoked responses were
studied. Hypersynchrony, rhythmic bursts of spikes and waves, and
isolated spikes characterized the preictal state. Seizures were always
bilateral and symmetrical and of a general tonic-clonic type.
Responses in sensory and motor cortexes to sensory nerve stimulation
were enhanced three to five fold. The authors concluded that endrin is
directly toxic to the mammalian nervous system, is a potent rapidly
acting convulsant, and does not require metabolic activation to an
active metabolite (Joy, 1976).
8.8.1.2 Histopathological studies
Male CD1 Swiss mice were administered endrin or sesame oil daily
by intraperitoneal injection in gradually increasing doses of 1.5-4.0
mg/kg for 4-20 days. Electron microscopic examination of sciatic nerve
tissue revealed no morphological change in myelinated nerve fibres,
myelin, or associated Schwann cells, but morphological alterations
were observed in unmyelinated nerve fibres and associated Schwann
cells: axons were swollen, microtubules and neurofilaments showed
dissolution, axoplasm was replaced by large clear vesicles,
vacuolization was present, and Schwann cells and adaxonal spaces also
contained vesicles (Walker & Phillips, 1987; abstract only).
8.8.1.3 Neurotransmitter systems
gamma-Aminobutyric acid systems: The role of the inhibitory
neuro-transmitter of the central nervous system, gamma-aminobutyric
acid (GABA),in the production of convulsions is well established.
Polychloro-cycloalkane insecticides such as endrin have a potent
excitatory action on the nervous system, and the interaction between
GABA function and endrin has been studied.
Endrin strongly inhibited GABA-dependent 36Cl uptake by mouse
brain vesicles, with an IC50 (the concentration required to cause
50% inhibition) of 2.8 µmol/litre. Inhibition was confined to that
portion of 36Cl uptake that is GABA-dependent. The result
demonstrates disruption of GABA ionophore function in mammalian brain,
possibly providing the principal mechanism of toxicity (Bloomquist &
Soderlund, 1985). In a comparison of the inhibitory potential of
several polychlorocycloalkane insecticides on GABA-dependent 36Cl
uptake, the most potent inhibitor was 12-ketoendrin, followed by
isobenzan, endrin, and then dieldrin, heptachlor epoxide, aldrin,
heptachlor and lindane. This order closely parallels their acute
toxicities (Bloomquist et al., 1986).
The effect of these chemicals was also studied in the tert
butylcyclo-phosphorothioate (TBPS) system, which has been shown to
bind convulsants with varying affinities. The IC50 for endrin on
35S-TBPS binding was 0.22 µmol/litre and that for 12-ketoendrin,
0.036 µmol/litre. These were the most potent inhibitors of TBPS
binding, and there was a significant linear correlation between 36Cl
flux and TBPS binding (Bloomquist et al., 1986).
In vitro, endrin inhibited 35S-TBPS binding in tissue from
male Swiss-Webster mice with an IC50 of 18 nmol/litre (range, 4-90).
In vivo, doses representing 25, 50, and 100% of the LD50 (8 mg/kg
intraperitoneally) inhibited 35S-TBPS binding with IC50s of 77 ±
7 nmol/litre (LD50) and 39 ± 6 nmol/litre (LD50/2); no inhibition
was observed at LD50/4, indicating a possible no-observed-adverse-
effect level. Brain P2 membranes of treated mice contained endrin and
12-ketoendrin. The finding that the brains of treated mice contained
sufficient endrin or its biotransformed products to achieve TBPS
binding and that this was correlated with the severity of the
poisoning indicates that the acute toxicity of endrin to mammals is
regulated by GABA (Cole & Casida, 1986).
GABA-induced 36Cl flux into membrane microsacs was inhibited
by endrin at 3.9 ± 0.2 nmol/mg protein, which also suggests that
endrin inhibits the function of this receptor (Abalis et al., 1985,
1986). The IC50 for 36Cl influx was 0.19 ± 0.06 µM and that for
35S-TBPS binding was 0.003 µM (Gant et al., 1987).
Endrin inhibited both insect and rat GABA receptors in a
dose-related, non-competitive manner. It acts in a similar manner on
the GABA receptors in the central nervous system of the two species.
The blocking action may involve non-competitive binding to an
allosteric site associated with the receptor's chloride channel
(Wafford et al., 1989a).
Endrin potentially inhibits 35S-TBPS binding to rat brain
membranes and also potentiates the protective effect of NaCl (200 mM)
against heat inactivation of 3H-flunitrazepam binding sites on the
same membranes. The time courses of heat inactivation of these binding
sites in the presence of NaCl and saturating concentrations of endrin
revealed monophasic components constituting about 88% of the binding
sites (Squires & Saederup, 1989).
Endrin has also been shown to inhibit GABA-ergic function in
Torpedo fish (Matsumoto et al., 1988), chicken embryos (Seifert,
1988, 1989), the mosquito fish (Gambusia affinis) (Bonner &
Yarbrough, 1989), and the cockroach (Periplaneta americana) (Wafford
et al., 1989b).
Other amine systems: Studies on the effects of orally
administered endrin on the content of biogenic amines in the brain of
rats did not contribute to an understanding of the convulsive action
of endrin (Miller & Fink, 1973; Hrdina et al., 1974).
Cyclic AMP metabolism: Endrin did not affect adenylate cyclase
activity or inhibit the activity levels of synaptosomal
phosphodiesterase, enzymes involved in cyclic AMP metabolism, in rat
brain. The authors interpreted their results to support their
postulation that organochlorine insecticides exert their neurotoxic
action by selective inhibition of ATPases in synaptosomes (Kodavanti
et al., 1988).
ATPase systems: Inhibition of rat brain Na+-K+ATPase by
chlorinated insecticides varied considerably: endrin and dieldrin were
the least active in inhibiting both this enzyme and K+-stimulated
para-nitrophenyl phosphatase at a concentration of 2 x 10-5
mol/litre. Results of experiments on ATP-32Pi exchange suggest
that DDT is a powerful inhibitor of oxidative phosphorylation, which
may lead to depletion of ATP. This effect was much less evident with
endrin (Folmar, 1978).
Endrin caused about 15% inhibition of the activity of
Na+-K+ATPase in rat brain synaptosomes at the highest
concentration tested, 120 µM, and oligomycin-sensitive Mg2+-ATPase
in rat brain synaptosomes was significantly inhibited in a
concentration-dependent manner, to a maximal inhibition of 33% at the
highest dose. Endrin did not inhibit oligomycin-insensitive
Mg2+-ATPase, and it did not affect K+-stimulated
para-nitrophenyl phosphatase from rat brain synaptosomes; this
enzyme represents the dephosphorylation step of the overall reaction
to the Na+-K+ATPase. Oligomycin-sensitive Mg2+-ATPase in beef
heart mitochondria was significantly inhibited. The results of this
study suggest that the ATPase system in rat heart and central nervous
system is not selectively inhibited by endrin (Mehrotra et al., 1989).
Sodium channel: It has been demonstrated using voltage clamp
techniques in single cells that application of DDT prolongs the sodium
current, which in turn decreases the depolarizing after-potential to
initiate repetitive after-discharges in the cell. The repetitive
after-discharges facilitate synaptic transmission and result in
nervous system hyperexcitability, which at the functional level is
registered as tremors and eventually convulsions and death (Narahasi,
1987). Even if less than 1% of the sodium channels respond in this
manner to insecticides, it is sufficient to cause toxicity in the
animal. Narahasi (1987) reported these effects with pyrethroids and a
series of DDT analogues; such studies have not been carried out with
endrin. Lund & Narahasi (1983) suggested that because of the
similarity in the symptomatology of intoxication by the family of
organochlorine insecticides, the target site of endrin may also be the
sodium channels.
8.8.1.4 Appraisal of effects on the nervous system
The effect of endrin on the nervous system has received attention
because it has the well established ability to cause convulsions
following acute exposures. Endrin causes considerable changes in EEG
activity, which are associated with convulsions, at intramuscular
doses in experimental animals as low as 0.2 mg/kg body weight.
The probable underlying mechanisms are associated with a
dose-related, non-competitive inhibition of the GABA-ergic
neurotransmitter system. This is an inhibitory system, and removal of
its action leads to increased excitation in the nervous system. While
inhibition of GABA-ergic function is common to a number of
polychlorocycloalkane insecticides, endrin, and particularly its
metabolite 12-ketoendrin, have been shown to be extremely potent
inhibitors of this function. It appears therefore that the acute
toxicity of endrin is due to disruption of GABA-related mechanisms.
8.8.2 Cardiovascular system
Studies have been conducted on the physiological effects of
endrin on the peripheral vascular system, renal function, renal
haemodynamics, and the cardiovascular system of the dog (Emerson et
al., 1963, 1964; Reins et al., 1964; Emerson, 1965; Emerson & Hinshaw,
1965; Reins et al., 1966; Hinshaw et al., 1966; Reddy et al., 1967).
After a lethal dose of endrin was administered intravenously, most of
the effects appeared to be the direct or indirect result of the
stimulating action of endrin on the central nervous system.
Bradycardia, hypertension, salivation, hyperexcitability, tonic-clonic
convulsions, increased body temperature, leukocytosis,
haemoconcentration, and decreased blood pH were seen. Elevation of
cerebral venous pressure and cerebrospinal fluid pressure were also
prominent features. Increased levels of adrenaline and noradrenaline
in blood plasma cause increased venous return and cardiac output and
increased arterial blood pressure in the absence of a rise in total
peripheral resistance. There was a large increase in total limb
vascular resistance and also a decrease in renal blood flow due to
arteriolar vasoconstriction. In studies on intact dogs and isolated
heart-lung preparations, high doses of endrin appeared to have a toxic
action on the left ventricle of the heart, causing sudden left heart
failure.
Aldrin, dieldrin, and endrin inhibited rat brain synaptosomal
and heart sarcoplasmic reticulum in vitro in a
concentration-dependent manner. Calmodulin-depleted Ca2+ pump
activity was insensitive to the action of these compounds. Oral
administration of endrin at 0.5-10 mg/kg to rats similarly decreased
Ca2+ pump activity, in addition to decreasing the levels of
calmodulin in both brain and heart, indicating disruption of membrane
Ca2+ transport mechanisms. Exogenous addition of calmodulin
(1-20 µg) effectively reversed the endrin-induced inhibition. Ca2+
pump activity in brain is more sensitive to endrin than that in heart.
The results indicate that endrin may produce neurotoxic effects by
altering calmodulin-regulated calcium-dependent events in neurons
(Mehrotra et al., 1989).
8.8.3 Effects on liver enzymes
It is well known that chlorinated hydrocarbon insecticides such
as DDT and dieldrin stimulate hepatic microsomal drug metabolism,
stimulating the activity of enzymes for the metabolism of drugs and
endogenous compounds such as hormones (Kinoshita & Kempf, 1970).
8.8.3.1 Mouse
A single oral, convulsive dose of endrin (20 mg/kg body weight)
dissolved in corn oil was administered to 9-week-old male
Swiss-Webster mice. Control groups consisted of a group of untreated
mice and a group receiving corn oil. When convulsions began, blood
serum was examined for serum glutamic oxaloacetic transaminase, serum
glutamic pyruvic transaminase, and serum lactic dehydrogenase. The
activities of the three enzymes were significantly increased above
those seen in the two control groups (Luckens & Phelps, 1969).
After intraperitoneal injection of a single dose of endrin at
6.25 mg/kg body weight to mice, hexobarbital sleeping time was
decreased, starting 3 h after the injection and lasting for 3 days
(Hart & Fouts, 1963). Stimulating effects on the hepatic
mixed-function oxidase system were reported in ICR mice after single
oral doses of 4 and 10 mg/kg body weight (Hartgrove et al., 1977).
8.8.3.2 Rat
Feeding Sprague-Dawley rats on diets containing endrin at 1, 5,
25, 50, or 100 mg/kg for 16 weeks caused high mortality in all groups,
especially among male rats. The serum alkaline phosphatase
concentration was reported to be dose-relatedly increased in all
groups as compared to control animals. The effect was clearest in the
groups fed 25 mg/kg of diet or more (Nelson et al., 1956).
In strain FW 49 rats, a single oral dose of endrin at 5 mg/kg
body weight had no effect on pentobarbital sleeping time; 10 mg/kg
caused a significant reduction, which, however, disappeared after 10
days (Schwabe & Wendling, 1967).
Endrin caused a significant shortening of the duration of the
paralysis induced by zoxazolamine in male Sprague-Dawley rats aged
5-6 weeks. Endrin was injected intraperitoneally at 2 mg/kg body
weight daily for 3 days, and zoxazolamine was injected
intraperitoneally on the fourth day (Truhaut et al., 1974).
Male rats given single oral doses of 2.5, 3.75, or 5.0 mg/kg body
weight showed no effect on the various parameters (details not given)
of mixed-function oxidase activity after 12 h, but the level of
microsomal protein and electron transport components per gram of liver
were significantly increased after 108 h, in a dose-dependent fashion.
Thiopentone and pentobarbital sleeping times were reduced by a 24-h
prior intraperitoneal injection of endrin at 5 mg/kg body weight
(Kachole & Pawar, 1977).
A single oral dose of endrin at 10 mg/kg body weight to male
albino rats increased serum glutamic oxaloacetic transaminase and
glutamic pyruvic transaminase activities, and decreased ATPase, acid-
and alkaline phosphatase, succinic dehydrogenase, and
glucose-6-phosphatase activities significantly 2-48 h after treatment
(Meena et al., 1978). After three successive daily oral doses of
endrin at 15 mg/kg body weight to Sprague-Dawley rats, significant
increases in total lipids and triglycerides in liver and in serum
glutamic pyruvic transaminase activity were seen (Borady et al.,
1983).
When two groups of six adult female rats were fed 0 or 28.7 µg/kg
body weight, endrin accumulated in the liver (5.47 mg/kg),and its
concentration in blood increased progressively up to 28 days. Growth
was depressed. The activities of the enzymes aspartate amino
transferase and alanine amino transferase were slightly increased
(Illahi et al., 1986). Similar results were obtained in a study in
which rats were fed 20 µg/kg body weight for 28 days (Illahi et al.,
1987).
8.8.3.3 Guinea-pig
Groups of six female guinea-pigs were administered three
successive intraperitoneal injections of endrin in sunflower oil at 3
mg/kg body weight, and liver and kidneys were studied 24 h after the
last injection. Treatment caused a significant increase in liver
weight and a decrease in hepatic microsomal protein content; renal
weight and renal microsomal protein content were not affected. Hepatic
cytochrome b5 and cytochrome-c reductase activities were increased,
while cytochrome P450 and total haem levels were significantly
decreased. Related to the decrease in cytochrome P450 was a decrease
in TPNH-linked aminopyrine-N-demethylation, but an increase in
DPNH-linked demethylation was related to the increase in cytochrome b5
and cytochrome-c reductase. Lipid peroxidation was increased in both
liver and kidneys (Pawar & Kachole, 1978).
8.8.3.4 In-vitro studies
To test the possibility that phenobarbital induces cytochrome
P450p indirectly by increasing the availability of endogenous
glucocorticoids in the liver, phenobarbital and phenobarbital-like
inducers, including endrin, were added to primary monolayer cultures
of adult Sprague-Dawley rat hepatocytes incubated in serum-free medium
without glucocorticoids. De-novo synthesis of cytochrome P450p,
measured as increased incorporation of 3H-leucine into
immunoprecipitable P450p protein, was increased. Endrin at a
concentration of 1x10-5 M was half as potent as phenobarbital at 2
x 10-3 M (Schuetz et al., 1986).
8.8.4 Miscellaneous studies
Endrin inhibited rabbit muscle lactate dehydrogenase in vitro
(Hendrickson & Bowden, 1976). Exposure of isolated rat enterocytes to
endrin reduced the efficiency of the neuropeptide vasoactive
intestinal peptide after stimulation of cyclic AMP accumulation, as
was observed with lindane (Carrero et al., 1989).
Endrin at single oral doses of 25 mg/kg body weight or daily
doses of 1 mg/kg body weight daily for 8 days induced various shifts
in the mobilization of the ions of biologically important metals such
as magnesium, iron, zinc, and copper from liver, kidneys, brain,
heart, spleen, and blood (Coleman et al., 1968; Lawrence et al.,
1968). Rats receiving intraperitoneal injections of 1 mg/kg body
weight in peanut oil over periods up to 19 days showed no alteration
in the concentrations of serum proteins or serum lipoproteins,
separated by paper electrophoresis, or of albumin, alpha 1, alpha 2,
beta, or gamma globulins. Protein-bound sialic acid and methylpentose
were increased only temporarily; the level of bound hexose increased
with time and that of bound hexosamine decreased (Coleman, 1968).
Rats receiving a single oral dose of 50 mg/kg body weight, daily
intraperitoneal doses of 2 mg/kg body weight, or daily intramuscular
injections of 0.5 or 2.0 mg/kg body weight for 45 days showed
increased activity of a number of the enzymes that are involved in
gluconeogenesis in liver cells and cells of the renal cortex. A
significant decrease was noted in hepatic glycogen, an increase in
blood glucose and urea, as well as a significant rise in hepatic and
renal pyruvate carboxylase, phosphoenol pyruvate carboxykinase,
fructose-1,6-diphosphatase, and glucose-6-phosphatase. Furthermore,
endogenous levels of cyclic AMP were increased (Kacew et al., 1973;
Singhal & Kacew, 1976).
8.8.5 Factors that influence toxicity
8.8.5.1 Nutrition
The nutritional state of Wistar rats was found to alter their
susceptibility to the acute toxic action of endrin. Three groups of
approximately 100 rats were fed a normal diet, a diet containing
casein as the only source of protein, or a low protein diet for 28
days, and the acute toxicity of endrin was determined after a single
intragastric administration. The following LD50 values were
calculated: 27 mg, 17 mg, and 7 mg/kg body weight, respectively (Boyd
& Stefec, 1969).
8.8.5.2 Potentiation
The acute oral LD50s of equitoxic doses of combinations of 10
pesticides, including endrin, were studied in Swiss mice. No evidence
of potentiation was seen with combinations with dieldrin, diazinon,
malathion, toxaphene, parathion, DDT, or dioxathion, but more than
additive effects, i.e., possible potentiation, were found with
chlordane and possibly with aldrin (Keplinger & Deichmann, 1967).
Five groups of 20 male and 20 female Sprague-Dawley rats were fed
for 91 days on a diet containing a combination of 15 'persistent'
chemicals added at concentrations of 0, 1, 10, 100, and 1000 times the
water quality objective applied in Canada. For endrin, these
corresponded to 0.002, 0.02, 0.2, and 2.0 µg/kg of diet. No effect on
food intake, growth, clinical chemistry, bone marrow, or
histopathology were observed. It was concluded that the presence of
these chemicals at 1000 times the water quality objective had no
toxicological effect (Cote et al., 1985).
Six male and six female Sprague-Dawley rats were fed a control
diet or diets containing endrin at 5 or 10 mg/kg, endrin aldehyde at
10 mg/kg, or endrin ketone at 5 mg/kg for 15 days, at which time three
to six rats from each treatment group were given a single
intraperitoneal dose of carbon tetrachloride at 100 µlitre/kg body
weight in corn oil (1 mg/kg). Levels of serum enzymes, bile flow, and
biliary excretion of an anionic model compound, phenolphthalein
glucuronide, were measured on day 16. Dietary treatment with endrin at
either dose level did not elevate serum enzyme levels. Treatment with
5 mg/kg significantly reduced bile flow and a corresponding reduction
in phenolphthalein glucuronide excretion, whereas the 10 mg/kg dose
reduced only phenolphthalein glucuronide excretion in male rats.
Female rats treated with either dose showed a dose-dependent
choleretic effect with a commensurate increase in phenolphthalein
glucuronide excretion. Treatment of rats with endrin and carbon
tetrachloride did not result in potentiation of hepatobiliary
functions. The levels of some serum enzymes were elevated (two-fold)
in rats given endrin plus carbon tetrachloride over those in rats
given endrin or carbon tetrachloride alone, indicating an additive
interaction. Dietary treatment with endrin aldehyde slightly increased
the levels of serum glutamic oxaloacetic transaminase and glutamic
pyruvic transaminase; and endrin ketone induced a small elevation in
glutamic pyruvic transaminase levels. Neither compound altered bile
flow or biliary phenolphthalein glucuronide excretion. Combination
with carbon tetrachloride increased the levels of some serum enzymes
(two-fold) over those seen with the aldehyde or the ketone or carbon
tetrachloride alone (Young & Mehendale, 1986).
9. EFFECTS ON HUMAN BEINGS
9.1 Exposure of the general population
9.1.1 Acute toxicity
In mild cases of poisoning, dizziness, weakness of the legs,
abdominal discomfort, nausea, and vomiting have been reported. Some
patients have complained of temporary deafness or were slightly
disorientated or aggressive. The onset of poisoning is variable and
may occur 0.5-10 h after consumption of contaminated food or
contamination of the skin; the interval is usually 1-4 h, depending on
the quantity ingested. Severe poisoning is manifested by sudden
epileptiform fits, with frothing at the mouth, facial congestion, and
violent convulsive movements of the limbs, sometimes leading to
dislocation of a shoulder or other injury. The fits may last for
several minutes and may be followed by a period of semiconsciousness
for 15-30 min or until the next fit. In general, these convulsions
occur suddenly, with no prodromal sign or symptom. An uncommon but
very serious symptom observed in two children was hyperthermia (41 °C
or higher); the high fever was followed by decerebrate rigidity. In
fatal cases, death occurs within 2-12 h. In survivors, recovery is
rapid, within 24 h, and uneventful, although some patients have
complained of headache, dizziness, weakness, and anorexia for several
weeks (Davis & Lewis, 1956; Jacobziner & Raybin, 1959; Hoogendam et
al., 1962; Hayes, 1963; Weeks, 1967; Hayes, 1982). After clinical
recovery, EEG changes consisting of bilateral synchronous theta-wave
activity and occasional bilateral synchronous spike and wave
complexes, believed to be associated with brain stem irritation, may
still be found and may persist for up to several weeks (Hoogendam et
al., 1962, 1965; Weeks, 1967).
9.1.2 Poisoning incidents
Hayes (1982) reviewed poisoning cases caused by endrin. Outbreaks
of acute intoxication due to endrin have occurred by contamination of
flour during transport in railway cars. A first episode, which was
well studied, occurred in 1956 in Wales, United Kingdom (Davis &
Lewis, 1956): At least 59 people were ill enough to require medical
treatment, and at least 100 more had some symptoms, which were not
severe enough to require medical advice. No one died. On the basis of
the concentration of endrin in bread prepared from the flour (150
mg/kg), Hayes (1963) estimated that 0.20-0.25 mg/kg body weight may
cause a single convulsion and that the dose necessary to produce
repeated convulsions is about 1 mg/kg body weight. Karplus (1971)
estimated the lethal dose in man to be approximately 10 mg/kg body
weight.
A few conflicting data are available on the concentration of
endrin in the tissues of victims of fatal intoxication. Hayes (1982)
quoted levels of 7-10 mg/kg in the liver and 0.7-4.4 mg/kg in the
brain; however, 10-fold lower levels were reported in the tissues of
autopsied victims of an outbreak of poisoning caused by ingestion of
bread prepared from contaminated flour in the Middle East (Curley et
al., 1970). In another incident, two sacks of contaminated flour
contained endrin at 184.5 and 234.5 mg/kg, and the bread and rolls
prepared from the contaminated flour contained 125.67-176.11 mg/kg.
The levels of endrin in serum, collected 30 min, 20 h, and 30 h after
convulsions in one person were 0.053, 0.038, and 0.021 mg/litre,
respectively; three other cases had 0.003-0.004 mg/litre of blood
serum 9-19 h after convulsions. One of these three people had no
symptoms (Coble et al., 1967). The reported serum or blood levels of
endrin associated with convulsions must be interpreted in the context
of the rapid removal of endrin from blood and the often significant
time lag in taking blood samples after convulsions. When the time
between convulsion and blood sampling is long, the endrin levels
reported are likely to be much lower than those at the time of the
convulsion.
Four outbreaks of endrin intoxication occurred in Doha (Qatar)
and Hofuf (Saudi Arabia) in 1967, during which 874 people were
hospitalized of whom 26 died; another 500-750 people showed symptoms
of intoxication but required no hospitalization. These outbreaks were
due to contamination of flour by endrin leaking from drums during
shipment. The endrin concentrations found in bread were 48-1807 mg/kg,
and those in the blood of patients were 0.007-0.032 mg/litre (Weeks,
1967; Curley et al., 1970).
Between July and September 1984, an epidemic of endrin poisoning
occurred in Pakistan, resulting in acute convulsions. In 18 of 21
affected villages surveyed, 70% (106/152) of the cases for which age
was known were in children aged 1-9 years; 9.8% (19/194) of the
affected people died. A composite sugar sample taken from the houses
of three cases contained endrin at 0.04 mg/kg. Endrin was detected in
the blood of 12/18 patients, at levels of 0.3-254.0 µg/litre of serum.
It was also determined in brain, kidneys, adipose tissue, and liver of
one person and found at levels of 1680, 1760, 4010, 1430 µg/kg
respectively (Anon., 1984; Hill et al., 1986; Rowley et al., 1987).
In mid-March 1988, three members of a family in Orange County,
California, USA, became ill within 1 h of eating taquitos (baked corn
shell filled with spicy meat and salad). Two of the three had multiple
grand mal seizures. Subsequently, two other people were reported to
have had seizures less than 12 h after eating taquitos. All five
patients had obtained the taquitos from the same shop within 5 days.
The food was analysed, and the presence of endrin was confirmed but
not quantified. The origin of the endrin could not be identified
(Anon., 1989).
An episode of acute endrin poisoning was reported in 33 Mexican
children, who had sudden seizures without sensory alterations (Singh
& West, 1985).
Several other cases have been published of single accidental or
intentional intoxications, in children and in adults (Jacobziner &
Raybin, 1959; Karplus, 1971). Reddy et al. (1966) described 60 cases
of fatal endrin poisoning out of 95 encountered in India after the
introduction of endrin in agricultural work as an insecticide in 1959.
The majority of the cases were suicidal. Froth, petechial
haemorrhages, a kerosene-like smell and massive pulmonary oedema were
the characteristic autopsy findings. Respiratory failure was the most
common cause of death. The authors concluded that the toxic dose of
endrin is 5-50 mg/kg body weight or about 1 g; the lethal dose is
about 6 g. In a poisoning case in a 19-year-old male who ingested an
unknown amount of endrin, convulsions and gross pulmonary oedema were
found (Jedeikin et al., 1979). No histological changes were found in
the liver. At least some of the pulmonary changes seen in such cases
may be due to aspiration of the petroleum hydrocarbon solvent in
formulations of endrin.
A case of polyneuropathy of the Guillain-Barré type was
attributed to exposure to a mixture of DDT and endrin. Since
convulsions were not recorded, the causal relationship remains
doubtful (Jenkins & Toole, 1964).
In a fatal case of endrin poisoning, ingestion of 12 g of endrin
by a 49-year-old man caused convulsions (persisting for 4 days),
hypersalivation, hyperthermia, renal insufficiency, thrombocytopenia,
and recurrent hypotension. Death followed after 11 days due to
pulmonary complications (infection and haemorrhage) and hypoxaemia
causing bradycardia and cardiac arrest. The endrin concentrations in
blood 4 h and 6 and 11 days after ingestion were 450, 86 and 71
µg/litre. Endrin levels in adipose tissue, heart, brain, kidneys, and
liver, 11 days after ingestion were 89.5, 0.87, 0.89, 0.55, and 1.32
mg/kg, respectively (Runhaar et al., 1985).
The medical treatment of endrin poisoning is described in Annex
II.
9.2 Occupational exposure
9.2.1 Factory workers
No fatal case has been reported due to occupational exposure in
manufacturing and formulating plants (Van Raalte, 1965; Jager, 1970),
which may be due in part to underreporting but is also certainly due
to the fact that occupational exposure involving the absorption of
lethal doses occurs rarely under practical circumstances. Furthermore,
the rapid metabolism of endrin minimizes build-up of toxic levels in
tissues during normal working days.
Several cases of acute, non-fatal poisoning occurred in a
manufacturing plant in The Netherlands due to accidental over-exposure
to endrin (Jager, 1970). Endrin had been manufactured in this plant
since 1957. During the first 9 years of production of aldrin,
dieldrin, and endrin in the plant, 17 cases of poisoning with
convulsions occurred, five of which involved more than one convulsion.
Three of the cases were due to acute over-exposure to endrin among
workers who were handling these materials at high concentrations every
day. There was no fatality during 1300 man-years of exposure. No
evidence was found of skin sensitization, and there was no case of
permanent, partial, or complete incapacity. No difference was seen in
absenteeism due to illness among these workers in comparison with
those in other plants, and the results of liver function tests and
complete blood cell counts were within normal limits. In the cases of
poisoning, recovery from clinical and neurological signs, including
EEG tracings, was rapid and complete (Hoogendam et al., 1962, 1965;
Jager, 1970; Versteeg & Jager, 1973).
A series of studies has been published on the results of
continuing medical supervision of workers in this plant. A
complementary follow-up of 189 workers and of 52 workers who had left
employment at the plant for various reasons was published in 1973
(Versteeg & Jager, 1973). These workers had been exposed to endrin for
up to 14.5 years in 1973. In agreement with data published from a
study of 71 workers in an endrin manufacturing plant in the USA (Hayes
& Curley, 1968), endrin was not found in the blood of these workers,
except in cases of accidental, acute over-exposure. Medical
supervision of workers employed in the manufacture and formulation of
endrin and other pesticides for 1-19 years (average, 12 years), data
on absenteeism, the results of tests for liver function and blood
chemistry, blood morphology, urine analysis, the occurrence of
sensitization, the pattern and course of EEG changes in cases of
poisoning, other medical studies (including electrocardiography, chest
x rays, blood pressure, body weight), and the incidence and pattern of
diseases, including the occurrence of malignant growths, showed no
difference between workers exposed to endrin and other chemical plant
operators. Residues of endrin were not found in plasma (< 3 µg/litre)
or in adipose tissue (< 0.03 mg/kg).
A significant difference was found between workers exposed to
aldrin and dieldrin only, workers not exposed to insecticides, and
workers exposed to endrin only: endrin workers had lower blood levels
of the DDT metabolite para,para'-DDE than the other workers, and the
levels were lower than those in the general population of the
surrounding area, although DDT and related compounds had never been
manufactured in the plant. A second parameter that was compared was
excretion of 6-beta-hydrocortisol in the urine. (Increased activity of
the drug-metabolizing enzyme system increases the activity of the
oxidative pathway by which 6-beta-hydroxylase converts endogenous
cortisol to 6-beta-hydrocortisol and thus, relatively, diminishes the
contribution of the reductive pathway, leading to excretion of
17-hydroxycorticosteroids.) The ratio of the urinary excretion of
6-beta-hydroxycortisol to that of 17-hydroxysteroids was significantly
higher in the endrin workers than in workers not exposed to endrin
(Jager, 1970).
A third parameter of this enzyme system that was studied was
urinary excretion of D-glucaric acid (an end-product of the glucuronic
acid pathway in the liver), which has been shown to increase after
exposure to microsomal enzyme-stimulating compounds, like endrin
(Hunter et al., 1971; Notten & Henderson, 1975). In the endrin
workers, urinary excretion of D-glucaric acid after a week of exposure
increased significantly over pre-exposure levels and those in a
control group of workers. Excretion diminished again after 3 days
without exposure (Hunter et al., 1972; Ottevanger &Van Sittert, 1979;
Vrij-Standhardt et al., 1979; Van Sittert, 1985).
Since anti-12-hydroxyendrin is the only metabolite found in the
urine of endrin-exposed workers, a study was initiated to find whether
there is a correlation between the quantity of this metabolite and
that of D-glucaric acid excreted in the urine. A positive relationship
was found between excretion of the endrin metabolite and of D-glucaric
acid after 7 days. After exposure was discontinued, excretion of
anti-12-hydroxyendrin decreased faster than that of D-glucaric acid.
The fact that endrin-exposed workers had D-glucaric acid levels within
the normal range after 6 weeks indicates that enzyme induction in
endrin workers is reversible. The authors concluded that a urinary
level of anti-12-hydroxyendrin of 0.130 µg/g of creatinine is the
threshold exposure level, below which enzyme induction is not produced
(Ottevanger & Van Sittert, 1979; Van Sittert, 1985). Endrin did not
increase total urinary porphyrin excretion over that in a control
group of employees (Strik, 1979; Nagelsmit et al., 1979;
Vrij-Standhardt et al., 1979).
In a follow-up mortality study of the same group of workers,
vital status and cause of death were assessed for 232 of a group of
more than 1000 workers. This group was selected because they had
experienced high exposures in the initial years of manufacture and
formulation and because of the long periods of exposure (mean, 11
years; range, 4-27) and observation (mean, 24 years; range, 4-29).
Total observed mortality was 25, whereas 38 deaths were expected on
the basis of mortality statistics for the male Dutch population. Of
the nine cancer deaths, three were due to lung cancer; the remaining
six were due to cancers of stomach, pancreas, bladder, and kidney,
multiple myeloma, and cerebral glioma. It was concluded that the
pesticides manufactured had no specific carcinogenic activity
(Ribbens, 1985).
9.2.2 Dose-response relationships
It has not been possible to establish a dose-response
relationship between single or repeated oral exposures and endrin
concentrations in blood, adipose tissue, or organs and severity of
intoxication, because the actual oral intake in the accidental cases
was not known, and the onset of symptoms of intoxication and the time
of measuring concentrations of endrin in blood, organs, or tissues
were not comparable (Davis & Lewis, 1956; Hayes, 1963; Coble et al.,
1967; Weeks, 1967; Curley et al., 1970; Karplus, 1971; Hayes, 1982;
Anon., 1984).
Blood samples have been analysed in three cases of acute
over-exposure (Table 28): A formulator and an operator were
accidentally splashed with a 20% endrin emulsifiable concentrate,
which was washed off within 10 min. Neither developed signs or
symptoms of intoxication. The third case was in a formulator who
handled technical-grade endrin powder without wearing a dust-mask. He
had convulsions 4 h after starting work, but after treatment recovered
fully the next day. Blood samples from four colleagues working next to
him, but wearing dust-masks, were also examined. The author estimated
that the threshold level of endrin in the blood below which no sign or
symptom of intoxication occurs is 50-100 µg/litre and that the
half-life of endrin in blood is in the order of 24 h (Jager, 1970).
9.2.3 Exposures to mixtures
A retrospective mortality study was carried out on workers
employed in the manufacture of heptachlor and endrin in a plant in
Tennessee, USA, between 1952 and 1976. The study comprised 835 men who
had worked for more than 3 months up to 20 years at the plant. No
overall excess of deaths from cancer was found; however, there was an
excess of deaths from cerebrovascular disease (7 observed, 2.3
expected) (Wang & MacMahon, 1979).
A further retrospective cohort study was conducted to examine the
mortality of workers employed in the manufacture of chlordane,
heptachlor, DDT, aldrin/dieldrin, and endrin in a plant in Colorado,
USA, where endrin was manufactured from 1953 until 1965. Approximately
2100 workers who had been employed for at least 6 months in the plants
were involved. No excess of cerebrovascular disease was observed
(Ditraglia et al., 1981).
Neither study proves conclusively that exposure to these
organochlorine insecticides is associated with increased prevalence of
malignancy or other cause of death, but they are limited in design and
in the desciption of exposure.
A field study was carried out in 1983 in the Ivory Coast to
assess the health hazards associated with the handling and application
by hand-held sprayers of an ultra-low volume formulation consisting of
endrin at 85 g/litre, DDT at 333 g/litre, and methylparathion at 85
g/litre in petroleum solvent. Groups of five or six farmers sprayed 3
litre/ha of the formulation 4-6 weeks after sowing cotton and again 15
or 30 days after the first application. The spray apparatus was filled
and cleaned by the same men. The recommended protective clothing was
worn only rarely, and the handling and application techniques were
careless, resulting in many cases in appreciable skin contamination.
No adverse health effect was observed. Absorption of endrin was
monitored by measuring the concentration of anti-12-hydroxyendrin in
spot samples of urine collected about 20 h after spraying. The mean
concentrations after the first, second, and third applications were
0.34 (range, 0.04-0.59), 0.52 (range, 0.09-1.4), and 0.45 mg/g of
creatinine (range, 0.0-0.92). One person who had handled and sprayed
the formulation carefully still had anti-12-hydroxyendrin in the
urine after the third application, but at a very low level (0.03 mg/g
of creatinine). Measurements of para-nitrophenol, a metabolite of
methyl-parathion, in urine indicated that the rate of metabolism of
methylparathion was increased as a result of enzyme induction by
endrin in the liver (Kummer & Van Sittert, 1984, 1986). It was
concluded that endrin accumulated in most of the farmers after three
applications within a short period. An increase to toxic levels might
result if spraying were more frequent and at shorter intervals and if
the recommended clothing was not worn.
9.2.4 Appraisal of effects of occupational exposures
Endrin is a very toxic compound. Several episodes of fatal and
non-fatal poisoning have occurred, mostly from accidental
contamination of food and also from intentional (suicidal) ingestion.
The lethal oral dose is estimated to be 10 mg/kg body weight. In
non-fatal cases, recovery is rapid and complete within a few days. The
oral dose that causes a single convulsion is estimated to be 0.25
mg/kg body weight, and that which induces repeated convulsions, 1.0
mg/kg body weight.
Exposure of workers to endrin for long periods did not induce
adverse effects that were attributed to this compound, although
occasional cases of acute, non-fatal intoxication due to accidental
over-exposure have occurred. Endrin was not detected in the blood of
workers exposed to endrin at < 3.0 µg/litre. The threshold level of
endrin in blood that results in intoxication is estimated to be 50-100
µg/litre. Absorption of a toxic dose is therefore unlikely during
occupational exposure if recommended controls and precautions are
used. In fatal cases, endrin concentrations in blood as high as 450
µg/litre have been reported; however, it is not possible to establish
a dose-response relationship. Since endrin is not normally found in
air, water, or food, except under conditions of contamination,
exposure of the general population is not significant.
Table 28. Concentrations of endrin in blood from acutely over-exposed workers
Case Time of first Endrin concentration (µg/litre)
sampling
First 12 h 24 h 36 h 5 days
sample later later later later
Formulator 1 h after 90 ND
accident
Operator 40 min after 27 25 ND
accident
Formulator Directly after 80 20 ND
without dust-mask convulsion
Four colleagues Same time ND-10
with dust-masks time as above
ND, not detectable (< 5 µg/litre)
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Endrin was evaluated by the Joint FAO/WHO Expert Committee on
Pesticide Residues in 1963, 1965, and 1970 (FAO/WHO, 1964, 1965,
1971). In 1970, the Committee established an acceptable daily intake
(ADI) for humans of 0-0.0002 mg/kg body weight, which was based on the
level that causes no toxicological effect in rats and dogs, 1 mg/kg of
diet (equivalent to 0.05 mg/kg body weight per day in rats and 0.025
mg/kg body weight per day in dogs).
The Joint FAO/WHO Codex Alimentarius Commission has published
maximum residue limits for endrin (Table 29; (FAO/WHO, 1986b).
Table 29. Codex maximum limits for the sum of residues of
endrin and delta-ketoendrin
Commodity Maximum residue limit
(mg/kg product)
Apples 0.02a
Barley 0.02a
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02a
Eggs (without shells) 0.2
Meat (carcass fat) 0.1b
Milk 0.0008b
Poultry (carcass fat) 1
Rice (husked or polished) 0.02a
Sorghum 0.02a
Sweet maize 0.02a
Wheat 0.02a
aAt or near the limit of detection
bExtraneous residue limit
The International Agency for Research on Cancer (IARC) concluded
in 1974 and 1987 that there was inadequate evidence for the
carcinogenicity of endrin in experimental animals and that the
evidence from studies in humans was inadequate. Endrin was therefore
classified in Group 3: not classifiable as to its carcinogenicity to
humans (IARC, 1974, 1987).
In 1988, the Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified technical-grade endrin as
highly hazardous in normal use (WHO, 1992). A data sheet on endrin was
issued in 1978 (WHO/FAO, 1975).
REFERENCES
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1985) High-affinity
stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic Biochem Physiol, 24: 95-102.
Abalis IM, Eldefrawi ME, & Eldefrawi AT (1986) Effects of insecticides
on GABA-induced chloride influx into rat brain microsacs. J Toxicol
Environ Health, 18: 13-23.
Abbott DC, Harrison RB, Tatton JO'G, & Thomson J (1966) Organochlorine
pesticides in the atmosphere. Nature, 211(5046): 259-261.
Abbott DC, Goulding R, & Tatton JO'G (1968) Organochlorine pesticide
residues in human fat in Great Britain. Br Med J, iii: 146-149.
Abbott DC, Holmes DC, & Tatton JO'G (1969) Pesticide residues in the
total diet in England and Wales, 1966-67. Organochlorine pesticide
residues in the total diet. J Sci Food Agric, 20(4): 245-259.
Abbott DC, Collins GB, & Goulding R (1972) Organochlorine pesticide
residues in human fat in United Kingdom. Br Med J, ii: 553-556
Abdel-Razik M, Marzouk MAH, Mowafy LE, & Abdel-Kader MA (1988)
Pesticide residues in the River Nile water, Egypt. Pak J Sci Ind Res,
31(11): 795-797.
Abdou SM, Abdel-Gawaad AA, Abdel-Amaim E, Abdel-Hady SM, & El-Alfy MB
(1983) Organochlorine pesticide residues in buffaloes milk in Kalubia
province and the effect of the presence of insecticides on coagulation
time. Egypt J Dairy Sci, 11: 197-203.
Acker L & Schulte E (1974) [Chlorinated hydrocarbons in human fat.]
Naturwissenschaften, 61: 32 (in German).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Seasonal fluctuations of
organochlorine and triazines pesticides in the aquatic system of
Ioannina Basin (Greece). Sci Total Environ, 58: 243-253.
Albers PH, Sileo L, & Mulhern BM (1986) Effects of environmental
contaminants on snapping turtles of a tidal wetland. Arch Environ
Contam Toxicol, 15: 39-49.
Albert LA (1990) Environmental contamination in Mexican food. In:
Hriagu JO & Simmons MS ed., Food contamination from environmental
sources. New York, John Wiley and Sons, pp 542-577.
Albert L, Mendez F, Cebrian ME, & Portales A (1980) Organochlorine
pesticide residues in human adipose tissue in Mexico. Results of a
preliminary study in three Mexican cities. Arch Environ Health, 35(5):
262-269.
Albert LA, Vega P, & Nava E (1982) [Organochlorine pesticides. VI.
Organochlorine pesticide residues in Mexican evaporated milks.]
Biotica, 7(3): 473-482 (in Spanish).
Alford-Stevens AL, Eichelberger JW, & Budde WL (1988) Multi-laboratory
study of automated determination of polychlorinated biphenyls and
chlorinated pesticides in water, soil and sediment by gas
chromatography/mass spectrometry. Environ Sci Technol, 22: 304-312.
Ali SL (1986) [Pesticide residues and traces of heavy metals in cod
liver oil.] Pharm Ztg, 131(38): 2288-2290 (in German).
Al-Omar MA, Al-Ogaily NH, Tawfiq SJ, & Al-Bassoumy M (1985a) Residue
levels of organochlorine insecticides in sewage plant effluent. J Biol
Sci Res, 16(1): 145-151.
Al-Omar MA, Tameesh AH, & Al-Ogaily NH (1985b) Dairy product
contamination with organochlorine insecticide residues in Bagdad
district. J Biol Sci Res, 16(1): 133-144.
Altmeier G & Korte F (1969) [Contributions to ecological chemistry
(XXIV). Metabolism of endrin-14C in perfused rats' livers.]
Tetrahedron Lett, 49: 4269-4271 (in German).
Anderson A (1986) Monitoring and biased sampling of pesticide residues
in fruits and vegetables. Methods and results, 1981-1984. Var Foda,
38(Suppl. 1): 8-55.
Anderson RL & Defoe DL (1980) Toxicity and bioaccumulation of endrin
and methoxychlor in aquatic invertebrates and fish. Environ Pollut
(Ser A), 22: 111-121.
Anderson HH, Hine CH, Kodama JJ, & Critchlow JK (1953) Class B
Determination on HI-1185 Endrin Emulsifiable Concentrate, San
Francisco, University of California, School of Medicine (UC Report No.
213).
Ang C, Meleady K, & Wallace L (1989) Pesticide residues in drinking
water in the north coast region of New South Wales, Australia,
1986-87. Bull Environ Contam Toxicol, 42: 595-602.
Anon. (1964) Report on investigation of fish kills in Lower
Mississippi River, Atchafalaya River and Gulf of Mexico. Washington,
DC, US Department of Health, Education, and Welfare, Public Health
Service, Division of Water Supply and Pollution Control.
Anon (1979) Determination of residues of organochlorine pesticides in
animal fats and eggs. Report of the committee for analytical methods
for residues of pesticides and veterinary products in foodstuffs of
the Ministry of Agriculture, Fisheries and Food. Analyst, 104:
425-433.
Anon. (1984) Acute convulsions associated with endrin
poisoning--Pakistan. Morb Mortal Wkly Rep, 33(49): 687-695.
Anon. (1988a) Introduction--US Environmental Protection Agency Office
of Drinking Water health advisories. Rev Environ Contam Toxicol, 104:
1-8.
Anon. (1988b) Endrin. Rev Environ Contam Toxico,l 104: 103-114.
Anon. (1989) Endrin poisoning associated with taquito ingestion,
California. Morb Mortal Wkly Rep, 38(19): 345-347.
Argyle RJ, Williams GC, & Dupree HK (1973) Endrin uptake and release
by fingerling channel catfish (Ictalurus punctatus).
J Fish Res Board Canada, 30(11): 1743-1744.
Arthur RD, Cain JD, & Barrentine BF (1976) Atmospheric levels of
pesticides in the Mississippi Delta. Bull Environ Contam Toxicol,
15(2): 129-134.
Atkins EL, Greywood EA, & MacDonald RL (1973) Toxicity of pesticides
and other agricultural chemicals to honey bees: Laboratory studies.
Riverside, University of California, Department of Entomology (Rev.
9/73 (M-16)).
Atuma SS (1985) Accumulation of organochlorine insecticides in the
blood of the general population of Nigeria. Toxicol Environ Chem,10:
77-82.
Baldwin MK & Hutson DH (1980) Analysis of human urine for a metabolite
of endrin by chemical oxidation and gas-liquid chromatography as an
indicator of exposure to endrin. Analyst, 105: 60-65.
Baldwin MK, Robinson J, & Parke DV (1970) Metabolism of endrin in the
rat. J Agric Food Chem, 18(6): 1117-1123.
Baldwin MK, Davis RA, & Burns DT (1973) Structural studies and
photochemical rearrangement of an animal metabolite of HEOD, the
active component of dieldrin. Pestic Sci, 4: 227-237.
Baldwin MK, Crayford JV, Hutson DH, & Street DL (1976) The metabolism
and residues of 14C-endrin in lactating cows and laying hens. Pestic
Sci, 7(6): 575-594.
Barcelo D & Puignou LG (1987) [Pesticide residue in Spanish U.H.T.
milks determined by high resolution gas chromatography.] Rev Agroquim
Tecnol Aliment, 27(4): 583-589 (in Spanish).
Barnett RW, D'Ercole AJ, Cain JD, & Arthur RD (1979) Organochlorine
pesticide residues in human milk samples from women living in
northwest and northeast Mississippi, 1973-75. Pestic Monit J, 13(2):
47-51.
Barth RAJ (1967) Pesticide toxicity in primates. Tulane, University of
Louisiana, Division of Hygiene and Tropical Medicine (Thesis).
Barthel WF, Hawthorne JC, Ford JH, Bolton GC, McDowell LL, Grissinger
EH, & Parsons DA (1969) Pesticide residues in sediments of the Lower
Mississippi River and its tributaries. Pestic Monit J, 3(1): 8-68.
Batterton JC, Boush JC, & Matsumura F (1971) Growth response of
blue-green algae to aldrin, dieldrin, endrin and their metabolites.
Bull Environ Contam Toxicol, 6(6): 589-594.
Becker DM & Sieg CH (1987) Egg shell quality and organochlorine
residues in eggs of merlins Falco columbarius in southeastern
Montana. Can Field-Nat, 101: 369-372.
Bedford CT (1974) Von Baeyer/IUPAC names and abbreviated chemical
names of metabolites and artifacts of aldrin (HHDN), dieldrin (HEOD)
and endrin. Pestic Sci, 5: 473-489.
Bedford CT & Harrod RK (1973) Synthesis of anti-12-hydroxyendrin and
12-ketoendrin, the two major mammalian metabolites of endrin.
Chemosphere, 4: 163-168.
Bedford CT & Hutson DH (1976) The comparative metabolism in rodents of
the isomeric insecticides dieldrin and endrin. Chem Ind, 1976:
440-447.
Bedford CT, Harrod RK, Hoadley EC, & Hutson DH (1975a) The metabolic
fate of endrin in the rabbit. Xenobiotica, 5(8): 485-500.
Bedford CT, Hutson DH, & Natoff IL (1975b) The acute toxicity of
endrin and its metabolites to rats. Toxicol Appl Pharmacol, 33:
115-121.
Bedford CT, Crane AE, & Harrod RK (1986a) Synthesis and confirmation
of structure of four mammalian metabolites of dieldrin and endrin.
Pestic Sci, 17: 659-667.
Bedford CT, Crane AE, Smith EH, & Wellard NK (1986b) Synthesis of
endrin metabolites. Part. 2: Total synthesis and confirmation of the
structure of 3-hydroxyendrin. Pestic Sci, 17: 33-47.
Belisle AA, Reichel WL, Locke LN, Lamont TG, Mulhern BM, Prouty RM,
DeWolf RB, & Cromartie E (1972) Residues in fish, wildlife and
estuaries. Pestic Monit J, 6: 133-138.
Benes V & Sram R (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Ind Med, 38(12): 442-444.
Bennett RO & Wolke RE (1987a) The effect of sublethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. I. Evaluation of serum
cortisol concentrations and immune responsiveness. J Fish Biol, 31(3):
375-385.
Bennett RO & Wolke RE (1987b) The effect of sub-lethal endrin exposure
on rainbow trout, Salmo gairdneri Richardson. II. The effect of
altering serum cortisol concentrations on the immune response. J Fish
Biol, 31(3): 387-394.
Benson WR (1969) Note on nomenclature of dieldrin and related
compounds. J Assoc Off Anal Chem, 52(5): 1109-1111.
Beyerbach M, Buthe A, Heidmann WA, Dettmer R, & Knuwer H (1987)
[Chlorinated hydrocarbons in eggs and livers of rooks (Corvus
frugilegus) from rookeries in Lower Saxony (northern Germany).]
J Ornitol, 128(3): 277-290 (in German with English summary).
Beyerbach M, Buthe A, Heidmann WA, Knuwer H, & Russel-Sinn HA (1988)
[The burden of dieldrin and other chlorinated hydrocarbons on the
lapwing (Vanellus vanellus).] J Ornitol, 129(3): 353-361 (in
German).
Bhowmik G (1978) Pretreating properties of endrin on plant
chromosomes. Letter to the Editor. Curr Sci, 47(15): 546-547.
Bianchi A, Tateo F, Nava C, Tateo S, Santamaria L, Berte F, &
Santagati G (1988) Presence of organophosphate and organochlorine
pesticides in the milk of women. Med Biol Environ, 16: 931-942.
Biberhofer J & Stevens RJJ (1987) Organochlorine contaminants in
ambient waters of Lake Ontario. Ottawa, Inland Water Directorate,
Waters Quality Branch,pp. 1-11 (Can Sci Ser (87) V51.159).
Biehl ML & Buck WB (1987) Chemical contaminants: their metabolism and
their residues. J Food Prot, 50(12): 1058-1073.
Bloomquist JR & Soderlund DM (1985) Neurotoxic insecticides inhibit
GABA-dependent chloride uptake by mouse brain vesicles. Biochem
Biophys Res Commun, 133(1): 37-43.
Bloomquist JR, Adams PM, & Soderlund DM (1986) Inhibition of
gamma-aminobutyric acid-stimulated chlorine flux in mouse brain
vesicles by polychlorocycloalkane and pyrethroid insecticides.
Neurotoxicology, 7(3): 11-20.
Blus LJ (1978) Short-tailed shrews: toxicity and residue relationship
of DDT, dieldrin and endrin. Arch Environ Contam Toxicol, 7: 83-98.
Blus LJ, Joanen T, Belisle AA, & Prouty RM (1975) The brown pelican
and certain environmental pollutants in Louisiana. Bull Environ Contam
Toxicol, 13: 646-655.
Blus L, Cromartie E, McNease L, & Joanen T (1979) Brown pelican:
population status, reproductive success, and organochlorine residues
in Louisiana, 1971-1976. Bull. Environ Contam Toxicol, 22: 128-135.
Blus LJ, Henny CJ, Kaiser TE, & Grove RA (1983) Effects on wildlife
from use of endrin in Washington State orchards. In: Fox GA & Hall RJ
ed. Transactions of the 48th North American Wildlife Conference on
Environmental Contaminants and Wildlife. Washington, DC, Wildlife
Management Institute, pp. 159-174.
Blus LJ, Henny CJ, & Grove RA (1989) Rise and fall of endrin usage in
Washington State fruit orchards: effects on wildlife. Environ Pollut,
60: 331-349.
Boellstorff DE, Ohlendorf HM, Anderson DW, O'Neill EJ, Keith JO, &
Prouty RM (1985) Organochlorine chemical residues in white pelicans
and western grebes from the Klamath Basin, California. Arch. Environ
Contam Toxicol, 14: 485-493.
Bollag JM & Henninger NM (1976) Influence of pesticides on
denitrification in soil and with an isolated bacterium. J Environ
Qual, 5(1): 15-18.
Bollen WB & Tu CM (1971) Influence of endrin on soil microbial
populations and their activity. Washington, DC, US Department of
Agriculture, Forest Service, pp 1-4 (Research Paper PNW 114).
Bonner JC & Yarbrough JD (1989) Role of the brain
t-butyl-bicyclophosphorothionate receptor in vertebrate resistance to
endrin, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane and
cypermethrin. J Pharmacol Exp Ther, 249(1): 149-154.
Borady AMA, Mikhail TH, Awadailah R, Ibrahim KA, & Kamar GAR (1983)
Effect of some insecticides on fat metabolism and blood enzymes in
rats. Egypt J Anim Prod, 23(1-2): 33-44.
Boyd EM & Stefec J (1969) Dietary protein and pesticide toxicity with
particular reference to endrin. Can Med Assoc J, 101: 335-339.
Braun F (1985) [PCN and chlorine-based pesticides in some Bavarian
rivers.]. Munch. Beitr. Abwasser Fisch Flussbiol, 39: 115-124 (in
German).
Breidenbach AW, Gunnerson CG, Kawahara FK, Lichtenberg JJ, & Green RS
(1967) Chlorinated hydrocarbon pesticides in major river basins,
1957-65. Public Health Rep, 82(2): 139-156.
Brodtmann NV. Jr (1976) Continuous analysis of chlorinated hydrocarbon
pesticides in the Lower Mississippi River. Bull Environ Contam
Toxicol, 15(1): 33-39.
Brooks GT (1969) The metabolism of diene-organochlorine (cyclodiene)
insecticides. Residue Rev, 27: 81-138.
Brooks GT (1974) Chlorinated insecticides, technology and application.
Cleveland, Ohio, CRC Press, vol. 1, pp 85-99 & vol 2, pp 94-97.
Brooks TM (1976) Mutagenicity studies with endrin in the host-mediated
assay. Unpublished report No. TLGR.0112.76, Sittingbourne, Kent, Shell
Research, submitted to WHO by Shell.
Bunck CM, Prouty RM, & Krynitsky AJ (1987) Residues of organochlorine
pesticides and polychlorobiphenyls in starlings (Sturnus vulgaris),
from the continental United States, 1982. Environ Monit Assess, 8:
59-75.
Burton WB & Pollard GE (1974) Rate of photochemical isomerization of
endrin in sunlight. Bull Environ Contam Toxicol, 12(1): 113-116.
Butler PA (1963) Commercial fisheries investigations. Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp 11-25
(Circular No. 167).
Butler PA (1973) Organochlorine residues in estuarine mollusks,
1965-1972. Pestic Monit J, 6(4): 238-362.
Cabral JRP, Raitano F, Mollner T, Bronczyk S, & Shubik P (1979) Acute
toxicity of pesticides in hamsters. Abstract: Eighteenth Annual
Meeting No. 384. Toxicol Appl Pharmacol, 48: A192.
Caceres O, Tundisi JG, & Castellan OAM (1987) Residues of
organochloric pesticides in reservoirs in Sao Paulo State. Cienc Cult,
39(3): 259-264.
Camanzo J, Rice CP, Jude DJ, & Rossmann R (1987) Organic priority
pollutants in near-shore fish from 14 Lake Michigan tributaries and
embayments, 1983. J Great Lakes Res, 13(3): 296-309.
Cantoni C, Fabbris F, Rogledi R, & Campagnari A (1988) [Organochlorine
pesticides in foods of animal origin found during the 1985-1987
biennium.] Ind Aliment, 27(1): 6-8 (in Italian).
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ Monit Assess, 5(2): 155-163.
Carey AE, Gowen JA, Tai H, Mitchell WG, & Wiersma GB (1978) Pesticide
residue levels in soils and crops, 1971--national soils monitoring
program (III). Pestic Monit J, 12(3): 117-136.
Carline RF & Lawal MV (1985) Contaminants and bilateral asymmetry in
yellow perch. Environ Toxicol Chem, 4: 543-547.
Carrero I, Fernandez-Moreno MD, Perez-Albarsanz MA, & Prieto JC (1989)
Lindane effect upon the vasoactive intestinal peptide
receptor/effector system in rat enterocytes. Biochem Biophys Res
Commun, 159(3): 1391-1396.
Carter BI & Simpson BJE (1978) Toxicity of insecticides: The acute
oral and percutaneous toxicities of two endrin bleed samples and of
technical endrin in Shellsol A and in toluene. Unpublished report No.
TLTR.0001.78, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Casper VL (1967) Galveston Bay pesticide study--water and oyster
samples analyzed for pesticide residues following mosquito control
program. Pestic Monit J, 1(3): 13-15.
Casteel SW & Cook WO (1985) Endrin toxicosis in a cat. J Am Vet Med
Assoc, 186(9): 988-989.
Castonguay M, Dutil J-D, & Desjardins C (1989) Distinction between
American eels (Anguilla rostrata) of different geographical origins
on the basis of their organochlorine contaminant levels. Can J Fish
Aquat Sci, 46: 836-843.
Celeste M de F & Caceres O (1987) [Chlorinated pesticide residues in
waters of Ribeirïo do Lobo (Broa) reservoir and its tributaries.]
Cienc Cult, 39(1): 66-70 (in Portuguese with English summary).
Cetinkaya M (1988) [Organochloro-pesticide residues in tobacco from
European cigarette brands.] Chem Mikrobiol Technol Lebensm, 11:
100-103 (in German with English summary).
Cetinkaya M & Schenek A (1987) [Investigation of
organochloro-pesticide residues in various raw cotton samples.] Chem
Mikrobiol Technol, Lebensm, 10: 150-153 (in German with English
summary).
Chau ASY (1974) Confirmation of pesticide residues identity. VII.
Solid matrix derivation procedure for the simultaneous confirmation of
heptachlor and endrin residues in the presence of large quantities of
polychlorinated biphenyls. J Assoc Off Anal Chem, 57(3): 585-591.
Chau ASY & Cochrane WP (1969) Cyclodiene chemistry. III. Derivative
formation for the identification of heptachlor, heptachlorepoxide,
cis-chlordane, trans-chlordane, dieldrin and endrin pesticide residues
by gaschromatography. J Assoc Off Anal Chem, 52: 1220-1226.
Chau ASY & Cochrane WP (1971) Chromous chloride reductions. VI.
Derivative formation for the simultaneous identification of heptachlor
and endrin pesticide residues by gas chromatography. J Assoc Off Anal
Chem, 54(5): 1124-1131.
Chernoff N, Kavlock RJ, Hanisch RC, Whitehouse DA, Gray JA, Gray LE
Jr, & Sovocool GW (1979) Perinatal toxicity of endrin in rodents. I.
Fetotoxic effects of prenatal exposure in hamsters. Toxicology, 13:
155-165.
Clark DR Jr & Krynitsky A (1978) Organochlorine residues and
reproduction in the little brown bat, Laurel, Maryland--June 1976.
Pestic. Monit J, 12(3): 113-116.
Clark DR Jr, Laval RK, & Krynitsky AJ (1980) Dieldrin and heptachlor
residues in dead gray bats, Franklin County, Missouri--1976 versus
1977. Pestic Monit J, 13(4): 137-140.
Clawson RL & Clark DR (1989) Pesticide contamination of endangered
gray bats and their food base in Boone County, Missouri, 1982. Bull
Environ Contam Toxicol, 42: 431-437.
Coble Y, Hildebrandt P, Davis J, Raasch F, & Curley A (1967) Acute
endrin poisoning. J Am Med Assoc, 202: 489-493.
Cole LM & Casida JE (1986) Polychlorocycloalkane insecticide-induced
convulsions in mice in relation to disruption of the GABA-regulated
chloride ionophore. Life Sci, 39: 1855-1862.
Cole JF, Klevay LM, & Zavon MR (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol Appl Pharmacol,
16: 547-555.
Coleman RL (1968) Endrin induced alterations in bound carbohydrates in
rat serum. Bull Environ Contam Toxicol, 3(6): 348-353.
Coleman RL, Lawrence CH, & Sowell WL (1968) Trace metal alterations
following subacute exposure to endrin. Bull. Environ Contam Toxicol,
3(5): 284-295.
Cook WO & Casteel SW (1985) A suspected case of endrin toxicosis in a
cat. Vet Hum Toxicol, 27(2): 111.
Corneliussen PE (1969) Pesticide residues in total diet samples (IV).
Pestic Monit J, 2(4): 140-152.
Corneliussen PE (1970) Pesticide residues in total diet samples (V).
Pestic Monit J, 4(3): 89-105.
Corneliussen PE (1972) Pesticide residues in total diet samples (VI).
Pestic Monit J, 5(4): 313-330.
Cote MG, Plaa GL, Valli VE, & Villeneuve DC (1985) Subchronic effects
of a mixture of 'persistent' chemicals found in the Great Lakes. Bull
Environ Contam Toxicol, 34: 285-290.
Crockett AB, Wiersma GB, Tai H, & Mitchell W (1975) Pesticide and
mercury residues in commercially grown catfish. Pestic Monit J, 8(4):
235-240.
Cromartie E, Reichel WL, Locke LN, Belisle AA, Kaiser TE, Lamont TG,
Mulhern BM, Prouty RM, & Swineford DM (1975) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1971-72. Pestic Monit J, 9(1): 11-14.
Cueto C Jr & Biros FJ (1967) Chlorinated insecticides and related
materials in human urine. Toxicol Appl Pharmacol, 10: 261-269.
Cueto C Jr & Hayes WJ Jr (1962) The detection of dieldrin metabolites
in human urine. Agric Food Chem, 10(5): 366-369.
Cummings JG (1965) Pesticide residues in the total diet samples. J
Assoc Off Anal Chem, 48(6): 1177-1180.
Cummings JG (1966) Pesticides in the total diet. Residue Rev, 16:
30-45.
Curley A, Jennings RW, Mann HT, & Sedlak V (1970) Measurement of
endrin following epidemics of poisoning. Bull Environ Contam Toxicol,
5(1): 24-29.
Currie RA, Kadis VW, Breitkreitz WE, Cunnignham GB, & Bruns GW (1979)
Pesticide residues in human milk, Alberta, Canada--1966-70, 1977-78.
Pestic Monit J, 13(2): 52-55.
Dale WE, Copeland F, & Hayes WJ Jr (1965) Chlorinated insecticides in
the body fat of people in India. Bull World Health Organ, 33: 471-477.
Datta SK & Ghose KC (1985) Toxic effect of endrin on the
hepatopancreas of a teleost, Cyprinus carpio. Indian Biol, 17(1):
37-41.
Davies K (1988) Concentrations and dietary intake of selected
organochlorines, including PCBs, PCDDs and PCDFs in fresh food
composites grown in Ontario, Canada. Chemosphere, 17(2): 263-276.
Davis GM & Lewis I (1956) Outbreak of food poisoning from bread made
of chemically contaminated flour. Br Med J, ii: 393-398.
Davis HC & Hidu H (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish Bull , 67(2): 393-404.
Dean BJ (1977) Chromosome studies on workers employed in an endrin
manufacturing plant. Unpublished report No. TLGR.0008.77,
Sittingbourne, Kent, Shell Research, submitted to WHO by Shell.
De Boer J (1989) Organochlorine compounds and bromodiphenylethers in
livers of Atlantic cod (Gadus morhua) from the North Sea, 1977-1987.
Chemosphere,, 18(11/12): 2131-2140.
De Campos M & Olszyna-Marzys AE (1979) Contamination of human milk
with chlorinated pesticides in Guatemala and El Salvador. Arch Environ
Contam Toxicol, 8: 43-58.
Deichmann WB & MacDonald WE (1971) Organochlorine pesticides and human
health. Food Cosmet Toxicol, 9(1): 91-103.
Deichmann WB, MacDonald WE, Blum E, Bevilacqua M, Radomski J,
Keplinger M, & Balkus M (1970a) Tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Ind Med, 39(10): 426-434.
Deichmann WB, MacDonald WE, Radomski J, Blum E, Bevilacqua M, &
Keplinger M (1970b) The tumorigenicity of aldrin, dieldrin, and endrin
in the albino rat. Ind Med, 39(7): 314.
Denison MS & Yarbrough JD (1985) Binding of insecticides to serum
proteins in mosquitofish (Gambusia affinis). Comp Biochem Physiol,
81C(1): 105-107.
Denison MS, Chambers JE, & Yarbrough JD (1985) Short-term interactions
between DDT and endrin accumulation and elimination in mosquitofish
(Gambusia affinis). Arch Environ Contam Toxicol, 14: 315-320.
De Paula Carvalho JP, Niskikawa AM, Aranha S, & Fay EF (1984)
[Organochlorine pesticide residues in bovine fat.] Biol Sao Paulo,
50(2): 39-48 (in Portuguese).
Devault DS (1985) Contaminants in fish from Great Lakes harbors and
tributary mouths. Arch Environ Contam Toxicol, 14: 587-594.
Devault DS, Clark JM, & Lahvis G (1988) Contaminants and trends in
fall run coho salmon. J Great Lakes Res, 14(1): 23-33.
De Vos RH, van Dokkum W, Olthof PDA, Quiryns JK, Muys T, & van der
Poll JM (1984) Pesticides and other chemical residues in Dutch total
diet samples (June 1976-July 1978). Food Chem Toxicol 22(1): 11-21.
Deweese LR, Cohen RR, & Stafford CJ (1985) Organochlorine residues and
egg shell measurements for tree swallows Tachycineta bicolor in
Colorado. Bull Environ Contam Toxicol, 35: 767-775.
Dewitt JB (1965) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J Agric Food Chem, 4(10): 863-866.
Dikshith TSS & Datta KK (1973) Endrin-induced cytological changes in
albino rats. Bull Environ Contam Toxicol, 9(2): 65-69.
Dikshith TSS, Kumar SN, Raizada RB, & Srivastava MK (1989a)
Organochlorine insecticide residues in cattle feed. Bull Environ
Contam Toxicol, 43: 691-696.
Dikshith TSS, Kumar SN, Tandon GS, Raizada RB, & Ray PK (1989b)
Pesticide residues in edible oils and oil seeds. Bull Environ Contam
Toxicol, 42: 50-56.
Dinnel PA, Link JM, Stober QJ, Letourneau MW, & Roberts WE (1989)
Comparative sensitivity of sea urchin sperm bioassays to metals and
pesticides. Arch Environ Contam Toxicol, 18: 748-755.
Ditraglia D, Brown DP, Namekata T, & Iverson N (1981) Mortality study
of workers employed at organochlorine pesticide manufacturing plants.
Scand J Work Environ Health, 7(suppl 4): 140-146.
Donahue JF, Burse VW, Head SL, & Andrews JS (1988) Comparison of two
techniques for quantifying environmental contaminants in human serum.
Life Sci, 43: 2257-2264.
Donoso J, Dorigan J, Fuller B, Gordon J, Kornreich M, Saari S, Thomas
L, & Walker P (1979) Reviews of the environmental effects of
pollutants. XIII. Endrin. Oak Ridge, Tennessee, Oak Ridge National
Laboratory (EPA-600/1-79-005).
DouAbul AAZ, Al-Saad HT, Al-Obaidy SZ, & Al-Rekabi HN (1987a) Residues
of organochlorine pesticides in fish from the Arabian Gulf. Water Air
Soil Pollut, 35: 187-194.
DouAbul AAZ, Al-Saad HT, & Al-Rekabi HN (1987b) Residues of
organochlorine pesticides in environmental samples from the Shatt
al-Arab River, Iraq. Environ Pollut, 43: 175-187.
DouAbul AAZ, Al-Omar M, Al-Obaidy S, & Al-Ogaily N (1987c)
Organochlorine pesticide residues in fish from the Shatt al-Arab
River, Iraq. Bull Environ Contam Toxicol, 38: 674-680.
DouAbul AAZ, Al-Saad HT, Al-Timari AA, & Al-Rekabi HN (1988)
Tigris-Euphrates delta: a major source of pesticides to the Shatt
al-Arab River (Iraq). Arch Environ Contam Toxicol, 17: 405-418.
Dowd PF, Mayfield GU, Coulon DP, Graves JB, & Newsom JD (1985)
Organochlorine residues in animals from three Louisiana watersheds in
1978 and 1979. Bull Environ Contam Toxicol, 34: 832-841.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III), June 1968-1970. Pestic Monit J,
5(4): 331-341.
Duggan RE & Lipscomb GQ (1969) Dietary intake of pesticide chemicals
in the United States (II), June 1966-April 1968. Pestic Monit J, 2(4):
153-162.
Duggan RE, Barry HC, & Johnson LY (1966) Pesticide residues in total
diet samples. Science, 151: 101-104.
Duggan RE, Barry HC, & Johnson LY (1967) Pesticide residues in total
diet samples (II). Pestic Monit J, 1(2): 2-12.
Duke TW & Dumas DP (1974) Implications of pesticide residues in the
coastal environment. In: Vernberg FJ & Vernberg WB, ed. Pollution and
physiology of marine organisms. New York, Academic Press, pp 137-164.
Dureja P, Walia S, & Mukerjee SK (1987) New photometabolites of
endrin. Indian J Chem, 26G: 898-899.
Durham WF & Wolfe HR (1962) Measurement of the exposure of workers to
pesticides. Bull World Health Organ, 26: 75-91.
Dutch Agricultural Advisory Commission on Environmental Pollutants
(1983) Annual report. The Hague, Ministry of Agriculture, Management
of Nature and Fisheries.
Earnest RD & Benville PE Jr (1972) Acute toxicity of four
organochlorine insecticides to two species of surf perch. California
Fish Game, 58(2): 127-132.
Egan H, Goulding R, Roburn J, & Tatton JO'G (1965) Organochlorine
pesticide residues in human fat and human milk. Br Med J, ii: 66.
Eisenberg M & Topping JJ (1985) Organochlorine residues in finfish
from Maryland waters 1976-1980. J Environ Sci Health B20(6): 729-742.
Eisler R (1970a) Latent effects of insecticide intoxication to marine
molluscs. Hydrobiologia, 36(3-4): 345-352.
Eisler R (1970b) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish. Tech Paper Bur Sport
Fish Wildl, 46: 1-12.
El-Dib MA & Badawy MI (1985) Organochlorine insecticides and PCB's in
river Nile water, Egypt. Bull Environ Contam Toxicol, 34: 126-133.
Ellis DH, Deweese LR, Grubb TG, Kiff LF, Smith DG, Jarman WM, &
Peakall DB (1989) Pesticide residues in Arizona peregrine falcon eggs
and prey. Bull Environ Contam Toxicol, 42: 57-64.
Elnabarawy MT, Welter AN, & Robideau RR (1986) Relative sensitivity of
three daphnid species to selected organic and inorganic chemicals.
Environ Toxicol Chem, 5: 393-398.
El Nabawi A, Heinzow B, & Kruse H (1987) Residue levels of
organochlorine chemicals and polychlorinated biphenyls in fish from
the Alexandria Region, Egypt. Arch Environ Contam Toxicol, 16:
689-696.
El-Sebae AH (1987) Acute and chronic toxicity to marine biota of
widely used dispersants, PCBs, chlorinated pesticides and their
combinations and their biomagnification in Alexandria region. In:
Research on the toxicity, persistence, bioaccumulation,
carcinogenicity and mutagenicity of selected substances (Activity G).
Final reports on projects dealing with toxicity (1983-1985). Athens,
United Nations Environment Programme (Mediterranean Action Plan (MAP),
Technical Reports Series No. 10).
Ely RE, Moore LA, Carter RH, & App BA (1957) Excretion of endrin in
the milk of cows fed endrin-sprayed alfalfa and technical endrin. J
Econ Entomol, 50(3): 348-349.
Emerson TE Jr (1965) Mechanisms of hemoconcentration in the dog during
acute endrin insecticide poisoning. Can J Physiol Pharmacol, 43:
793-800.
Emerson TE Jr & Hinshaw LB (1965) Peripheral vascular effects of the
insecticide endrin. Can J Physiol Pharmacol,43: 531-539.
Emerson TE Jr, Brake CM, & Hinshaw LB (1963) Mechanism of Action of
the Insecticide Endrin. Oklahoma City, Oklahoma, Civil Aeromedical
Research Institute (Report No. 63-16).
Emerson TE Jr, Brake CM, & Hinshaw LB (1964) Cardiovascular effects of
the insecticide endrin. Can J Physiol Pharmacol, 42: 41-51.
Engst R & Knoll R (1973) [On the contamination of surface, rain and
drinking waters with chlorinated hydrocarbons.] Nahrung, 17(8):
837-851 (in German with English summary).
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection of
chemical mutagens by the dominant lethal assay in the mouse. Toxicol
Appl Pharmacol, 23: 288-325.
Ercegovich CD & Rashid KA (1977) Mutagenesis induced in mutant strains
of Salmonella typhimurium by pesticides. In: Abstracts of the 174th
ACS National Meeting, Chicago, Illinois. Washington, DC, American
Chemical Society, Division of Pesticide Chemistry (Abstract No. 43).
Everaarts JM, Koeman JH, & Brader, L (1971) Contribution à l'étude des
effets sur quelques éléments de la faune sauvage des insecticides
organo-chlorés utilisés au Tchad en culture cotonnière. Cotton Fibre
Trop, 26(4): 385-394.
Fabacher DL & Chambers H (1976) Uptake and storage of 14C-labelled
endrin by the livers and brains of pesticide-susceptible and resistant
mosquitofish. Bull Environ Contam Toxicol, 16(2): 203-207.
Fahrig R (1974) Comparative mutagenicity studies with pesticides. In:
Montesano R & Tomatis L ed. Chemical carcinogenesis essays. Lyon,
International Agency for Research on Cancer, pp 161-181 (IARC
Scientific Publications No. 10).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No. PL:1963/13;
WHO/Food Add./23).
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. Geneva, World Health Organization (FAO Meeting Report No.
PL:1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1971) Joint meeting of FAO Working Party of Experts and the
WHO Expert Group on Pesticide Residues. 1970 Evaluation of some
pesticide residues in food. Geneva, World Health Organization
(AGP:1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1984) Codex guidelines on good practice in pesticide residue
analysis. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986a) Recommendations for methods of analysis of pesticide
residues. Rome, Codex Alimentarius Commission, Food and Agriculture
Organization of the United Nations (CAC/PR8-1986).
FAO/WHO (1986b) Codex maximum limits for pesticide residues. Rome,
Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme,
Food and Agriculture Organization of the United Nations, p 33-IV (FAO
CAC Vol. XIII, ed. 2).
Fasola M, Vecchio I, Caccialanza G, Gandini C, & Kitsos M (1987)
Trends of organochlorine residues in eggs of birds from Italy, 1977 to
1985. Environ Pollut, 48: 25-36.
FDA (1988) Food and Drug Administration pesticide program. Residues in
foods--1987. J Assoc Off Anal Chem, 71(6): 156A-174A.
Ferguson DE, Culley DD, & Cotton WD (1964) Resistance to chlorinated
hydrocarbon insecticides in three species of freshwater fish.
Bioscience, 14: 43-44.
Flickinger EL & King KA (1972) Some effects of aldrin-treated rice on
Gulf Coast wildlife. J Wildl Manage, 36: 706-727.
Flickinger EL, Mitchell CA, & Krynitsky AJ (1986) Dieldrin and endrin
residues in fulvous whistling ducks in Texas in 1983. J. Field
Ornithol, 57(2): 85-192.
Folmar LC (1978) In vitro inhibition of rat brain ATPase, pNPPase,
and ATP-32Pi exchange by chlorinated-diphenyl ethanes and cyclodiene
insecticides. Bull Environ Contam Toxicol, 19: 481-488.
Foschi F, Natali G, Guberti MG, Camisani MG, Taccheto Barbina M,
Spessotto C, & Bargarolo L (1985) [Study on residues of argicultural
chemicals in apples.] Inf Fitopatol, 12: 14-20 (in Italian).
Fournier E, Treich I, Campagne L, & Capelle N (1972) Pesticides
organo-chlorés dans le tissu adipeux d'êtres humains en France. Eur J
Toxicol, 1(1): 11-26.
Fox ME, Roper DS, & Thrush SF (1988) Organochlorine contaminants in
surficial sediments of Manukau Harbour, New Zealand. Mar Pollut
Bull,19(7): 333-336.
Frank R, Braun HE, Holdrinet M, Sirons GJ, Smith EH, & Dixon DW (1979)
Organochlorine insecticides and industrial pollutants in the milk
supply of Southern Ontario, Cananda--1977. J Food Prot, 42(1): 31-37.
Frank R, Braun HE, & Holdrinet MVH (1981) Residues from past uses of
organochlorine pesticides and PCB in waters draining eleven
agricultural watersheds in Southern Ontario, Canada, 1975-1977. Sci
Total Environ, 20: 255-276.
Frank R, Braun HE, Sirons GH, Rasper J, & Ward GG (1985)
Organochlorine and organophosphorus insecticides and industrial
pollutants in the milk supplies of Ontario--1983. J Food Prot, 48(6):
499-504.
Fredrickson DS (1978) Report on bioassay of endrin for possible
carcinogenicity. Fed Reg, 43(225): 54298.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Galassi S & Provini A (1981) Chlorinated pesticides and PCBs contents
of the two main tributaries into the Adriatic Sea. Sci Total Environ,
17: 51-57.
Gant DB, Eldefrawi ME, & Eldefrawi AT (1987) Cyclodiene insecticides
inhibit GABAa receptor-regulated chloride transport. Toxicol Appl
Pharmacol, 88(3): 313-321.
Garrett NE, Stack HF, & Waters MD (1986) Evaluation of the genetic
activity profiles of 65 pesticides. Mutat Res, 168(3): 301-325.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements, and other chemicals in adult total diet
samples October 1980-March 1982. J Assoc Off Anal Chem, 69(1):
146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off Anal
Chem, 69(1): 123-145.
Giesy JP, Mewsted J, & Garling DL (1986) Relationships between
chlorinated hydrocarbon concentrations and rearing mortality of
chinook salmon (Onchorhynchus tshawytscha) eggs from Lake Michigan.
J Great Lakes Res, 12(1): 82-98.
Gips T (1987) Breaking the pesticide habit--Alternatives to 12
hazardous pesticides (International Alliance for Sustainable
Agriculture (IASA) Publication No. 1987-2).
Glatt H, Jung R, & Oesch F (1983) Bacterial mutagenicity investigation
of epoxides: drugs, drug metabolites, steroids and pesticides. Mutat
Res, 11: 99-118.
Glooschenko WA, Strachan WMJ, & Sampson RCJ (1976) Distribution of
pesticides and polychlorinated biphenyls in water, sediments and
seston of the Upper Great Lakes--1974. Pestic Monit J, 10(2): 61-67.
Gluth G & Hanke W (1985) A comparison of physiological changes in
carp, Cyprinus carpio, induced by several pollutants at sub-lethal
concentrations. I. The dependency on exposure time. Ecotoxicol Environ
Saf, 9: 179-188.
Godsil PJ & Johnson WC (1968) Pesticide monitoring of the aquatic
biota of the Tule Lake National Wildlife Refuge. Pestic Monit J, 1(4):
21-26.
Goerlitz DF & Law LM (1974) Determination of chlorinated insecticides
in suspended sediment and bottom material. J Assoc Off Anal Chem,
57(1): 176-181.
Goldentahl EI (1978a) Teratology study in rats. Unpublished report No.
163-488, International Research and Development Corporation, submitted
to WHO by Shell.
Goldentahl EI (1978b) Teratology study in hamsters. Unpublished report
No. 163-478, International Research and Development Corporation,
submitted to WHO by Shell.
Good EE & Ware GW (1969) Effects of insecticides on reproduction in
the laboratory mouse. Toxicol Appl Pharmacol, 14: 201-203.
Graves JB & Bradley JR (1965) Response of Swiss albino mice to
intraperitoneal injection of endrin. J Econ Entomol, 58(1): 178-179.
Gray LE Jr, Kavlock RJ, Chernoff N, Gray JA, & McLamb J (1981)
Perinatal toxicity of endrin in rodents. III. Alterations of
behavioural ontogeny. Toxicology, 21: 187-202.
Green DR, Stull JK, & Heesen TC (1986) Determination of chlorinated
hydrocarbons in coastal waters using a moored in situ sampler and
transported live mussels. Mar Pollut Bull, 17(7): 324-329.
Gregor DJ & Gummer WD (1989) Evidence of atmospheric transport and
deposition of organochlorine pesticides and polychlorinated biphenyls
in Canadian Arctic snow. Environ Sci Technol, 23: 561-565.
Gübeli T & Clerc JT (1988) [Detection of pesticide residues in ethanol
extracts of plants.] Pharm Acta Helv, 63(3): 85-89 (in German).
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984,
dietary intakes of pesticides, selected elements and other chemicals.
J Assoc Off Anal Chem, 71(6): 1200-1209.
Hall RJ & Swineford D (1980) Toxic effects of endrin and toxaphene on
the southern leopard frog Rana sphenocephala. In: Mellanby, K. ed.
Environmental pollution. Barking, Essex, Applied Science Publishing,
pp 53-65.
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hamdy Y & Post L (1985) Distribution of mercury, trace organics and
other heavy metals in Detroit River sediments. J Great Lakes Res,
11(3): 353-365.
Hansen DJ, Schimmel SC, & Forester J (1977) Endrin: effects on the
entire life cycle of a saltwater fish, Cyprinodon variegatus. J
Toxicol Environ Health, 3: 721-733.
Harris CR, Sans WW, & Miles JRW (1966) Exploratory studies on the
occurrence of organochlorine insecticides residues in agricultural
soils in southwestern Ontario. J Agric Food Chem,14(4): 398-403.
Hart LG & Fouts JR (1963) Effects of acute and chronic DDT
administration in hepatic microsomal drug metabolism in the rat
(28686). Proc Soc Exp Biol Med,114: 388-392.
Hartgrove RW Jr, Hundley SG, & Webb RE (1977) Characterization of the
hepatic mixed function oxidase system in endrin resistant and
-suspectible pinevoles. Pestic Biochem Physiol, 7(2): 146-153.
Hashemy-Tonkabony SE & Mosstofian B (1979) Chlorinated pesticide
residues in chicken egg. Poult Sci 58(6): 1432-1434.
Hashemy-Tonkabony SE & Soleimani-Amiri MJ (1976) Detection and
determination of chlorinated pesticide residues in raw and various
stages of processed vegetable oil. J Am Oil Chem Soc, 53(12): 752-753.
Hashimoto I & Nishiuchi S (1981) Establishment of bioassay methods for
evaluation of acute toxicity of pesticides to aquatic organisms. J
Pestic Sci, 6: 257-264.
Hassett AJ, Viljoen PT, & Liebenberg JJE (1987) An assessment of
chlorinated pesticides in the major surface water resources of the
Orange Free State during the period September 1984 to September
1985.Water SA, 13(3): 133-136.
Hawker DW & Connell DW (1986) Bioconcentration of lipophilic compounds
by some aquatic organisms. Ecotoxicol Environ Saf, 11: 184-197.
Hawthorne JC, Ford JH, & Markin GP (1974) Residues of mirex and other
chlorinated pesticides in commercially raised catfish. Bull Environ
Contam Toxicol, 11(3): 258-264.
Hayes WJ Jr (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department of
Health, Education, and Welfare, Communicable Disease Center,
Toxicology Section, pp 68-70.
Hayes WJ Jr (1975) Toxicology of pesticides, Baltimore,
Maryland,Williams & Wilkins, pp 288-294.
Hayes WJ Jr (1982) Pesticides studied in man, Baltimore, Maryland,
Williams and Wilkins, pp 247-251.
Hayes WJ Jr & Curley A (1968) Storage and excretion of dieldrin and
related compounds. Effect of occupational exposure. Arch Environ
Health, 16(2): 155-162.
Hayes WJ Jr, Dale WE, & Burse VW (1965) Chlorinated hydrocarbon
pesticides in the fat of people in New Orleans. Life Sci, 4:
1611-1615.
Heidmann WA, Büthe A, Beyerbach M, Löhmer R, & Rüssel-Sinn HA (1989)
[Chlorinated hydrocarbons of some bird species breeding in the inland
of Lower Saxony.] J Ornitol, 130(3): 311-320 (in German with English
summary).
Heinz GH, Erdman TC, Haseltine SD, & Stafford C (1985) Contaminant
levels in colonial waterbirds from Green Bay and Lake Michigan,
1975-80. Environ Monit Assess, 5: 223-236.
Henderson C, Johnson WL, & Inglis A (1969) Organochlorine insecticide
residues in fish. National pesticides monitoring program. Pestic Monit
J, 3: 145-171.
Henderson C, Inglis A, & Johnson WL (1971) Organochlorine insecticide
residues in fish. Fall 1969, National Pesticide Monitoring Program.
Pestic Monit J, 5: 1-11.
Hendrickson CM & Bowden JA (1976) In vitro inhibition of lactic acid
dehydrogenase by insecticidal polychlorinated hydrocarbons. 2.
Inhibition by dieldrin and related compounds. J Agric Food Chem,
24(4): 756-759.
Hendrickx A & Maes R (1969) The excretion of chlorinated hydrocarbon
insecticides in human mother milk. J Pharm Belg, 24(9-10): 459-463.
Hermanutz R (1974) Quarterly report. Duluth, Minnesota, US
Environmental Protection Agency, National Water Quality Laboratory.
Hermanutz RO, Eaton JG, & Mueller LH (1985) Toxicity of endrin and
malathion mixtures to flagfish (Jordanella floridae). Arch Environ
Contam Toxicol, 14: 307-314.
Hernandez FH, Lopez Benet FJ, Escriche JM, & Ubeda JCB (1987) Sulfuric
acid cleanup and KOH-ethanol treatment for confirmation of
organochlorine pesticides and polychlorinated biphenyls: application
to wastewater samples. J Assoc Off Anal Chem, 70(4): 727-733.
Herrera Marteache A, Polo Villar LM, Jodral Villarejo M, Polo Villar
G, Mallol J, & Pozo Lora R (1978) [Organochlorine pesticide residues
in human fat in Spain.] Rev San Hig Publico, 52: 1125-1144 (in Spanish
with English summary).
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington, DC,
US Department of the Interior, Fish and Wildlife Service, p 147 (Fish
and Wildlife Technical Report No. 2).
Hill EF, Health RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report: Wildlife No. 191).
Hill RH Jr, Needham LL, & Liddle JA (1986) The laboratory's role in
environmental health emergency investigations. Clin Toxicol, 24(5):
363-374.
Hine CH (1965) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 2, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH (1968) Results of reproduction study of rats fed diets
containing endrin insecticide over three generations. Unpublished
report No. 7, San Francisco, CA, Hine Laboratories, submitted to WHO
by Shell.
Hine CH, Anderson HH, Kodama JK, & Gutenberg EF (1954) Class B
evaluation of endrin compositions. Unpublished report No. 223, San
Francisco, CA, University of California School of Medicine, submitted
to WHO by Shell.
Hinshaw LB, Solomon LA, Reins DA, Fiorica V, & Emerson TE (1966)
Effects of the insecticide endrin on the cardiovascular system of the
dog. J Pharmacol Exp Ther, 153(2): 225-236.
Hirom PC, Millburn P, Smith RL, & Williams RT (1972) Species
variations in the threshold molecular-weight factor for the biliary
excretion of organic anions. Biochem J, 129: 1071-1077.
Hoffman WS, Fishbein WI, & Andelman MB (1964) The pesticide content of
human fat tissue. Arch Environ Health, 9: 387-394.
Hoffman WS, Adler H, Fishbein WI, & Bauer FC (1967) Relation of
pesticide concentration in fat to pathological changes in tissues.
Arch Environ Health, 15: 758-765.
Hogmire HW, Weaver JE, & Brooks JL (1990) Survey for pesticides in
wells associated with apple and peach orchards in West Virginia. Bull
Environ Contam Toxicol, 44: 81-86.
Holden AV (1970) International cooperative study of organo-chlorine
pesticide residues in terrestrial and aquatic wildlife, 1967/1968.
Pestic Monit J, 4(3): 117-135.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1962) Electroencephalograms
in insecticide toxicity. Arch Environ Health, 4: 86-94.
Hoogendam I, Versteeg JPJ, & de Vlieger M (1965) Nine years toxicity
control in insecticide plants. Arch Environ Health, 10: 441-448.
Horn H, Hartner L, & von Faber H (1987) [On the suitability of liver
function tests in birds for the ecotoxicological evaluation of
environmental chemicals.] Dtsch Tierarztl Wochenschr, 94: 1-48 (in
German with English summary).
Horsfall F Jr, Webb RE, Price NO, & Young RW (1970) Residues in apples
subsequent to ground sprays of endrin. J Agric Food Chem, 18: 221-223.
Hrdina PD, Singhal RL, & Peters DAV (1974) Changes in brain biogenic
amines and body temperature after cyclodiene insecticides. Toxicol
Appl Pharmacol, 29(1): 119.
Hrubec J (1988) [Pesticides and drinking-water.] H2O, 21(11):
278-282 (in Dutch).
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 153).
Hunter CG, Robinson J, & Richardson A (1963) Chlorinated insecticide
content of human body fat in southern England. Br Med J, i: 221-224.
Hunter J, Maxwell JD, Carrella M, Stewart DW, & Williams R (1971)
Urinary-D-glucaric acid excretion as a test for hepatic enzyme
induction in man. Lancet, 20 March: 572-575.
Hunter J, Maxwell JD, Stewart DW, Williams R, Robinson J, & Richardson
A (1972) Increased hepatic microsomal enzymes activity from
occupational exposure to certain organochlorine pesticides. Nature,
237: 399-401.
Hutson DH (1981) The metabolism of insecticides in man. In: Hutson DH
& Roberts TR ed. Progress in pesticide biochemistry. New York, John
Wiley and Sons, vol 1, pp 287-333.
Hutson DH & Hoadley EC (1974) The oxidation of a cyclic alcohol
(12-hydroxyendrin) to a ketone (12-ketoendrin) by microsomal
mono-oxygenation. Chemosphere, 5:205-210.
Hutson DH, Baldwin MK, & Hoadley EC (1975) Detoxication and
bioactivation of endrin in the rat. Xenobiotica, 5(11): 697-714.
IARC (1974) Endrin. In:Some organochlorine pesticides. Lyon,
International Agency for Research on Cancer, pp. 157-171 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Volume 5).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC Monographs volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p. 63 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Illahi A, Amin N, Hashmi AS, Nawaz M, & Naeem-ur Rahman (1986)
Incidence of endrin residues in cucumber and its effects on the
biological system of rats. J Pak Med Assoc, 36(8): 209-211.
Illahi A, Roohi J, & Hashmi AS (1987) Present status of endrin
residues in peas and its effect on biological systems. J Pure Appl
Sci, 6(1): 1-4.
Ito N, Tatematsu M, Nakanishi K, Hasegawa R, Takano T, Imaida K, &
Ogiso T (1980) The effects of various chemicals on the development of
hyperplastic liver nodules in hepatectomized rats treated with
N-nitrosodimethylamine or N-2-fluorenylacetamide. Gann, 71: 832-842.
Jacobziner H & Raybin HW (1959) Briefs on accidental chemical
poisonings in New York City. Poisoning by insecticide (endrin). NY
State J Med, 59: 2017-2022.
Jager KW (1970) Aldrin, dieldrin, endrin and telodrin. An
epidemiological study of long-term occupational exposure. Amsterdam,
Elsevier Science Publishers.
Japanese Environmental Agency (1975) Environmental survey report on
chemical substances in FY 1974. Unpublished report, December 1975,
Tokyo, Environmental Health Department, Planning and Coordination
Bureau.
Japenga J, Wagenaar WJ, Smedes F, & Salomons W (1987) A new, rapid
clean-up procedure for the simultaneous determination of different
groups of organic micropollutants in sediments; application in two
European estuarine sediment studies. Environ Technol Lett, 8(1): 9-20.
Jarvinen AW, Tanner DK, & Kline ER (1988) Toxicity of chlorpyrifos,
endrin, or fenvalerate to fathead minnows following episodic or
continuous exposure. Ecotoxicol Environ Saf, 15: 78-95.
Jedeikin R, Kaplan R, Shapira A, Radwan H, & Hoffman S (1979) The
successful use of 'high level' PEEP in near fatal endrin poisoning.
Crit Care Med, 7(4): 168-170.
Jegier Z (1964) Health hazards in insecticide spraying of crops. Arch
Environ Health, 8: 670-674.
Jenkins RB & Toole JF (1964) Polyneuropathy following exposure to
insecticides. Arch Intern Med, 113: 691-695.
Johnson DW (1968) Pesticides and fishes--a review of selected
literature. Trans Am Fish Soc, 97(4) 398-424.
Johnson RD & Manske DD (1976) Pesticide residues in total diet samples
(IX). Pestic Monit J, 9(4): 157-169.
Johnson RD & Manske DD (1977) Pesticide and other chemical residues in
total diet samples (XI). Pestic Monit J, 11(3): 116-131.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1979) Pesticide and
other chemical residues in infant and toddler total diet samples, (I),
August 1974-July 1975. Pestic Monit J, 13(3): 87-98.
Johnson RD, Manske DD, & Podrebarac DS (1981a) Pesticide, metal, and
other chemical residues in adult total diet samples, (XII), August
1975-July 1976. Pestic Monit J, 15(1): 54-71.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1981b) Pesticide,
heavy metal, and other chemical residues in infant and toddler total
diet samples, (II), August 1975-July 1976. Pestic Monit J, 15(1):
39-50.
Johnson RD, Manske DD, New DH, & Podrebarac DS (1984) Pesticide,
metal, and other chemical residues in adult total diet samples,
(XIII), August 1976-July 1977. J Assoc Off Anal Chem, 67(1): 154-166.
Johnson MG, Kelso JRM, & George SE (1988) Loadings of organochlorine
contaminants and trace elements to two Ontario lake systems and their
concentrations in fish. Can J Fish Aquat Sci, 45: 170-178.
Jolley WP, Stemmer KL, Grande F, Richmon J, & Pfitzer EA (1969) The
effects exerted upon beagle dogs during a period of two years by the
introduction of 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8-dimethanonaphthalene
into their daily diets. Unpublished report, Cincinnati, Ohio,
Kettering Laboratory, submitted to WHO by Shell.
Joy RM (1976) Convulsive properties of chlorinated hydrocarbon
insecticides in the cat central nervous system. Toxicol Appl
Pharmacol, 35: 95-106.
Kacew S, Sutherland DJB, & Singhal RL (1973) Biochemical changes
following chronic administration of heptachlor, heptachlor epoxide and
endrin to male rats. Environ Physiol Biochem, 3: 221-229.
Kachole MS & Pawar SS (1977) Effect of endrin on microsomal electron
transport reactions. Part I: Sleeping time, electron transport
components and protection due to pre-treatments. Abstracts of the 1976
Annual General Meeting of Biochemists. J Biochem, 14(1): 45.
Kadis VW, Breitkreitz WE, & Jonasson OJ (1970) Insecticide levels in
human tissues of Alberta residents. Can J Public Health, 61(5):
413-416.
Kagan J, Kagan ED, & Seigneurie E (1986) Alpha-terthienyl, a powerful
fish poison with light-dependent activity. Chemosphere, 15(1): 49-57.
Kaiser TE, Reichel WL, Locke LN, Cromartie E, Lamont TG, Mulhern BM,
Prouty RM, Stafford CJ, & Swineford DM (1980) Organochlorine
pesticide, PCB, PBB residues and necropsy data for bald eagles from 29
states--1975-77. Pestic Monit J, 13: 145-149.
Kampe W (1985) [Pesticide residues in animal feeding-stuffs.] Dtsch
Tierarztl Wochenschr, 92(6): 228-231 (in German).
Kanitz S & Castello G (1966) [The presence of residues of some
pesticides in human fatty tissue and in some foods. Initial results of
a survey carried out in Liguria.] G Ig Med Prev, 7: 1-19 (in Italian).
Karplus M (1971) [Endrin poisoning in children.] Harefuah, 18(3):
113-116 (in Hebrew with English summary).
Kassabi M, ElHraiki A, & Nader B (1988) Contamination of urban,
industrial and continental waters by chlorinated hydrocarbon
pesticides along the mediterranean coast of Morocco. Sci Total
Environ, 71: 209-214.
Kathpal T & Dewan RS (1975) Improved clean-up technique for the
estimation of endosulfan and endrin residues. J Assoc Off Anal Chem,
58(5): 1076-1078.
Katz M & Chadwick GG (1961) Toxicity of endrin to some Pacific
Northwest fishes.Trans Am Fish Soc, 90: 394-397.
Kavlock RJ, Chernoff N, Hanisch RC, Gray J, Rogers E, & Gray LE Jr
(1981) Perinatal toxicity of endrin in rodents. II. Fetotoxic effects
of prenatal exposure in rats and mice. Toxicology, 21: 141-150.
Kavlock RJ, Chernoff N, & Rogers EH (1985) The effect of acute
maternal toxicity on fetal development in the mouse. Teratog Carcinog
Mutagen, 5: 3-13.
Kavlock RJ, Rogers JM, Gray LE, & Chernoff N (1987) Postnatal
alterations in development resulting from prenatal exposure to
pesticides. In: Pesticide science and biotechnology, Proceedings of
the 6th International Congress on Pesticide Chemicals, pp 561-564.
Keilty TJ & Stehly GR (1989) Preliminary investigation of protein
utilization by an aquatic earthworm in response to sublethal stress.
Bull Environ Contam Toxicol, 43: 350-354.
Keilty TJ, White DS, & Landrum PF (1988a) Short-term lethality and
sediment avoidance assays with endrin-contaminated sediment and two
Oligochaetes from Lake Michigan. Arch Environ Contam Toxicol, 17:
95-101.
Keilty TJ, White DS, & Landrum PF (1988b) Sublethal responses to
endrin in sediment by Limnodrilus hoffmeisteri (Tubificidae) and in
mixed-culture with Stylodrilus heringianus (Lumbriculidae). Aquat
Toxicol, 13: 227-250.
Keilty TJ, White DS, & Landrum PF (1988c) Sublethal responses to
endrin in sediment by Stylodrilus heringianus (Lumbriculidae) as
measured by a 137cesium marker layer technique. Aquat Toxicol, 13:
251-270.
Keplinger MK & Deichmann WB (1967) Acute toxicity of combinations of
pesticides. Toxicol Appl Pharmacol, 10: 586-595.
Khangarot BS, Sehgal A, & Bhasin MK (1985) Man and biosphere--studies
on the Sikkim Himalayas. Part 6: Toxicity of selected pesticides to
frog tadpole, Rana hexadactyla (Lesson). Acta Hydrochim Hydrobiol,
13(3): 391-394.
Kiang PH & Grob RL (1986) Development of a screening method for the
determination of 49 priority pollutants in soil. J Environ Sci Health,
A21(1): 15-53.
Kiene RP & Capone DG (1984) Effects of organic pollutants on
methanogenesis, sulfate reduction and carbon dioxide evolution in salt
marsh sediments. Mar Environ Res 13: 141-160.
,
Kiigemagi U, Sprowls RG, & Terriere LC (1958) Endrin content of milk
and body tissues of dairy cows receiving endrin daily in their diet.
J Agric Food Chem, 6(7): 518-521.
King KA, Flickinger EL, & Hildebrand HH (1977) The decline of brown
pelicans on the Lousiana and Texas Gulf Coast. Southwest Nat, 21(4):
417-431.
King KA, Blankinship DR, Payne E, Krynitsky AJ, & Hensler GL (1985)
Brown pelican populations and pollutants in Texas, 1975-1981. Wilson
Bull, 97(2): 201-214.
Kinoshita FK & Kempf CK (1970) Quantitative measurement of hepatic
microsomal enzyme induction after dietary intake of chlorinated
hydrocarbon insecticides. Toxicol Appl Pharmacol, 17: 288.
Klein W, Mueller W, & Korte F (1968) [Insecticides in the metabolism.
XVI. Excretion, distribution and metabolism of endrin 14C in rats.]
Liebigs Ann Chem,713: 180-185.
Klevay LM (1971) Endrin excretion by the isolated perfused rat liver:
a sexual difference. Proc Soc Exp Biol Med, 136: 878-879.
Kodavanti PRS, Mehrotra BD, Chetty SC, & Desaiah D (1988) Effect of
selected insecticides on rat brain synaptosomal adenylate cyclase and
phosphodiesterase. J Toxicol Environ Health, 25: 207-215.
Koeman JH (1971) [The occurrence and the toxicological implications of
some chlorinated hydrocarbons in the Dutch coastal area in the period
1965-70], Utrecht, Rijks University, pp 88, 96 (Thesis) (in Dutch).
Koeman JH, Oskamp AAG, Veen J, Brouwer E, Rooth J, Zwart P, van de
Brock E, & van Genderen H (1967) Insecticides as a factor in the
mortality of the sandwich tern (Sterna sandvicensis). A preliminary
communication. Meded Fac Landbouwwet Rijksuniv Gent, 32: 841-854.
Koeman JH, Vink JAJ, & de Goeij JJM (1969) Causes of mortality in
birds of prey and owls in the Netherlands in the winter of 1968-69.
Andrea, 57: 67-76.
Koeman JH, Pennings JH, de Goeij JJM, Tjioe PS, Olindo PM, & Hopcraft
J (1972) A preliminary survey of the possible contamination of Lake
Nakuru in Kenya with some metals and chlorinated hydrocarbon
pesticides. J Appl Ecol, 9(2): 411-416.
Koeman JH, Pennings JH, Rosanto R, Soemarwoto O, Tjioe PS, Blancke S,
Kusumadinata S, & Djajadiredja RR (1974) Metals and chlorinated
hydrocarbon pesticides in samples of fish, sawah-duck eggs,
crustaceans and molluscs collected in Indonesia in April and May 1972.
Unpublished report, Wageningen-Bandung, University of Wageningen, The
Netherlands.
Korn S & Earnest R (1974) Acute toxicity of twenty insecticides to
striped bass, Morone saxatilis. California Fish Game, 60(3):
128-131.
Korte F (1969) Summary of results in 1969. Unpublished report,
submitted to WHO by Shell.
Korte F, Klein W, Weisgerber I, Kaul R, Mueller W, & Djirsurai A
(1970) Recent results in studies on the fate of chlorinated
insecticides. In: Deichmann WB, Radomski JL, & Penalver RA, ed.
Proceedings of the Sixth Conference on Toxicology and Occupational
Medicine, Pesticide Symposia. Miami, Florida, Halos & Associates,
Inc., pp 51-56.
Krantz WC, Mulhern BM, Bagley GE, Sprunt A, Ligas FJ, & Robertson WC
Jr (1970) Organochlorine and heavy metal residues in bald eagle eggs.
Pestic Monit J, 4(3): 136-140.
Kreitzer JF (1980) Effects of toxaphene and endrin at very low dietary
concentrations on discrimination acquisition and reversal in bobwhite
quail Colinus virginianus. Environ Pollut (Ser A), 23: 217-230.
Kreitzer JF & Heinz GH (1974) The effect of sub-lethal dosages of five
pesticides and a polychlorinated biphenyl on the avoidance response of
Coturnix quail chicks. Environ Pollut, 6: 21-29.
Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA,
Sileo L, Docjerty DE, & Erdman TC (1989) Microcontaminants and
reproductive impairment of the Forster's tern on Green Bay, Lake
Michigan--1983. Arch Environ Contam Toxicol, 18: 706-727.
Kudesia VP & Bali NP (1985) A study of pesticides in Kalinadi River
and evaluation of toxicity of some pesticides on fish Clarias
batrachus. Acta Cienc Indica, 10C(4): 245-254.
Kummer R & Van Sittert NJ (1984) Field study on health effects in
farmers applying an endrin/DDT/MEP formulation by hand-held ULV to
cotton in Ivory Coast. Unpublished report, The Hague, Shell
Internationale Petroleum Maatschappij, submitted to WHO by Shell.
Kummer R & Van Sittert NJ (1986) Field studies on health effects from
the application of two organophosphorus insecticide formulations by
hand-held ULV to cotton. Toxicol Lett, 33: 7-24.
Kurata M, Hirose K, & Umeda M (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
Kurhekar MP, D'Souza FC, & Meghal SK (1975) Rapid method for
extracting aldrin, dieldrin, and endrin from visceral material. J
Assoc Off Anal Chem, 58(3): 548-550.
Kutz FW, Yobs AR, & Yang HSC (1976) National pesticide monitoring
programs. In: Lee RE Jr, ed. Air pollution from pesticides and
agricultural processes. Cleveland, Ohio, CRC Press, pp 95-136.
Kutz F, Strassman S, & Yobs AR (1979a) Survey of pesticide residues
and their metabolites in the general population of the United States.
In: Berlin A, Wolff AH, & Hasegawa Y ed. Use of biological specimens
to assess human exposure to environmental pollutants, The Hague,
Martinus Nijhoff, pp 267-274.
Kutz FW, Strassman SC, & Sperling JF (1979b) Survey of selected
organochlorine pesticides in the general population of the United
States. Fiscal years 1970-1975. Ann NY Acad Sci, 320: 60-68.
Lara WH & Barreto HHC (1972) [Chlorinated pesticide residues in
water.] Rev Inst Adolfo Lutz, 32: 69-74 (in Portuguese with English
summary).
Lauer GJ, Nicholson HP, Cox WS, & Teasley JI (1966) Pesticide
contamination of surface waters by sugar cane farming in Louisiana.
Trans Am Fish Soc, 95(3): 310-316.
Lawrence CH, Coleman RL, & Sowell WL (1968) Endrin induced trace metal
alterations following acute exposure. Bull Environ Contam Toxicol,
3(4): 229-239.
Leard RL, Grantham BJ, & Pessoney GF (1980) Use of selected freshwater
bivalves for monitoring organochlorine pesticide residues in major
Mississippi stream systems, 1972-73. Pestic Monit J, 14(2): 47-52.
Lebel GL & Williams DT (1986) Determination of halogenated
contaminants in human adipose tissue. J Assoc Off Anal Chem, 69(3):
451-458.
Lichtenberg JJ, Eichelberger JW, Dressman RC, & Longbottom JE (1970)
Pesticides in surface waters of the United States; a 5-year summary,
1964-1968. Pestic Monit J, 4(2): 71-86.
Lopez-Avila V, Schoen S, Milanes J, & Beckert WF (1988)
Single-laboratory evaluation of EPA method 8080 for determination of
chlorinated pesticides and polychlorinated biphenyls in hazardous
wastes. J Assoc Off Anal Chem, 71(2): 375-387.
Lowe JI (1966) Some effects of endrin on estuarine fishes. In:
Proceedings of the 19th Annual Conference of the Southeast Association
of Game and Fish Commissioners, pp 271-276.
Luckens MM & Davis WH (1965) Toxicity of dieldrin and endrin to bats.
Nature, 207(4999): 879-880.
Luckens MM & Phelps KI (1969) Serum enzyme patterns in acute poisoning
with organochlorine insecticides. J Pharm Sci, 58(5): 569-572.
Ludke JL (1976) Organochlorine pesticide residues associated with
mortality: additivity of chlordane and endrin. Bull Environ Contam
Toxicol, 16(3): 253-260.
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multi-residue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-433.
Lund AE & Narahasi T (1983) Kinetics of sodium channel modification as
the basis for the variation in the nerve membrane effects of
pyrethroids and DDT analogs. Pestic Biochem Physiol, 20: 203-206.
Lykins BW Jr, Koffskey WE, & Miller RG (1986) Chemical products and
toxicologic effects of disinfection. J Am Water Works Assoc, 78(11):
66-75.
McFall JA, Antoine SR, & Deleon IR (1985) Organics in the water column
of Lake Pontchartrain. Chemosphere, 14(9): 1253-1265.
McGill AEJ & Robinson J (1968) Organochlorine insecticide residues in
complete prepared meals: a 12-month survey in SE England. Food Cosmet
Toxicol, 6: 45-57.
Machbub B, Ludwig HF, & Gunaratnam D (1988) Environmental impact from
agrochemicals in Bali (Indonesia). Environ Monit Assess, 11: 1-23.
McIntyre AE & Lester JN (1984) Occurrence and distribution of
persistent organochlorine compounds in UK sewage sludges. Water Air
Soil Pollut, 23: 397-415.
McKenney CL Jr (1986) Critical responses of populations of crustacea
to toxicants. Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Environmental Research
Brief EPA/600/M-86/004.
McLeese DW & Metcalfe CD (1980) Toxicities of eight organochlorine
compounds in sediment and seawater to Crangon septemspinosa. Bull
Environ Contam Toxicol 25: 921-928.
McLeese DW, Metcalfe CD, & Pezzack DS (1980) Bioaccumulation of
chlorobiphenyls and endrin from food by lobsters (Homarus
americanus). Bull Environ Contam Toxicol 25: 161-168.
McLeese DW, Burridge LE, & van Dinter J (1982) Toxicities of five
organochlorine compounds in water and sediment to Nereis virens. Bull
Environ Contam Toxicol 28: 216-220.
Madden JD, Finerty MW, & Grodner RM (1989) Survey of persistent
pesticide residues in the edible tissues of wild and pond-raised
Louisiana crayfish and their habitat. Bull Environ Contam Toxicol, 43:
779-784.
Manske DD & Corneliussen PE (1974) Pesticide residues in total diet
samples (VII). Pestic Monit J, 8(2): 110-124.
Manske DD & Johnson RD (1975) Pesticide residues in total diet samples
(VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Pesticide and other chemical residues in
total diet samples (X). Pestic Monit J, 10(4): 134-148.
Marinelli P, Stracciari GL, & Anfossi P (1986) [Presence of
organochlorinated pesticides in some wine-making by-products.] Zoot
Nutr Anim, 12: 479-486 (in Italian with English summary).
Marsh C (1963) Metabolism of D-glucuronolactone in mammalian systems.
Conversion of D-glucuronolactone into D-glucaric acid by tissue
preparations. Biochem J, 87: 82-90.
Marston RB, Tyo RM, & Middendorff SC (1969) Endrin in water from
treated Douglas fir seed. Pestic Monit J, 2(4): 167-171.
Martin RJ & Duggan RE (1968) Pesticide residues in total diet samples
(III). Pestic Monit J, 1(4): 11-20.
Martin DB & Hartman WA (1985) Organochlorine pesticides and
polychlorinated biphenyls in sediment and fish from wetlands in the
North Central United States. J Assoc Off Anal Chem, 68(4): 712-717.
Martin JP, Harding RB, Cannell GH, & Anderson L (1959) Influence of
five annual field applications of organic insecticides on soil
biological and physical properties. Soil Sci, 87: 334-338.
Maslansky CJ & Williams GM (1981) Evidence for an epigenetic mode of
action in organochlorine pesticide hepatocarcinogenicity. A lack of
genotoxicity in rat, mouse and hamster hepatocytes. J Toxicol Environ
Health, 8: 121-130.
Mason JW & Rowe OR (1976) The accumulation and loss of dieldrin and
endrin in the eastern oyster. Arch Environ Contam Toxicol, 4(3):
349-360.
Masud SZ & Farhat S (1985) Pesticide residues in foodstuffs in
Pakistan--organochlorine pesticides in fruits and vegetables. Pak J
Sci Ind Res, 28(6): 417-422.
Matsumoto K, Eldefrawi ME, & Eldefrawi AT (1988) Action of
polychlorocycloalkane insecticides on binding of
[35S]t-butylbicyclophosphorothionate to Torpedo electric organ
membranes and stereospecificity of binding site. Toxicol Appl
Pharmacol, 95: 220-229.
Matsumura F, Khanvilkar VG, Patil KC, & Boush GM (1971) Metabolism of
endrin by certain soil microorganisms. J Agric Food Chem, 19(1):
27-31.
Maule A, Plyte S, & Quirk AV (1987) Dehalogenation of organochlorine
insecticides by mixed anaerobic microbial populations. Pestic Biochem
Physiol, 27: 229-236.
Mayer, FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Environmental Research Laboratory (Environmental Research Brief
EPA/600/8-87/017).
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior Fish
and Wildlife Service (Resource publication 160).
Meena K, Gupta PK, & Bawa SR (1978) Endrin-induced toxicity in normal
and irradiated rats. Environ Res, 16: 373-382.
Mehrotra BD, Moorthy, KS, Reddy SR, & Desaiah D (1989) Effects of
cyclodiene compounds on calcium pump activity in rat brain and heart.
Toxicology, 54: 17-29.
Meith-Avcin N, Warlen SM, & Barber RT (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 meters.
Environ Lett, 5(4): 215-221.
Mersch-Sundermann V, Dickgiesser N, Hablizel U, & Gruber B (1988)
[Examination of mutagenicity of organic microcontaminations on the
environment. I. Communication: the mutagenicity of selected herbicides
and insecticides with the Salmonella-microsome-test (Ames-test) in
consideration of the pathogenetic potence of contaminated ground- and
drinking-water.] Zbl Bakteriol Hyg B, 186: 247-260 (in German).
Metcalf RL, Kapoor IP, Lu PY, Schuth CK, & Sherman P (1973) Model
ecosystem studies of the environmental fate of six organochlorine
pesticides. Environ Health Perspect, 4: 35-44.
Miles JRW & Harris CR (1973) Organochlorine insecticides residues in
streams draining agricultural, urban agricultural and resort areas of
Ontario, Canada, 1971. Pestic Monit J, 6(4): 363-368.
Miller PE & Fink GB (1973) Brain serotonin level and pentylenetetrazol
seizure threshold in dieldrin and endrin treated mice. Proc West
Pharmacol Soc, 16: 195-197.
Modin JC (1969) Chlorinated hydrocarbon pesticides in California bays
and estuaries. Pestic Monit J, 3(1): 1-7.
Morita H & Umeda M (1984) Detection of mutagenicity of various
compounds by FM3A cell system. Mutat Res, 130(5): 371.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay system. Mutat Res, 116: 185-216.
Morris RD (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse, Peromyscus maniculatus. Can J Zool,
46(5): 951-958.
Morris RD (1970) The effects of endrin on Microtus and Peromyscus.
I. Unenclosed field populations. Can J Zool, 48: 695-708.
Morris RD (1972) The effects of endrin on Microtus and Peromyscus.
II. Enclosed field populations. Can J Zool, 50(6): 885-896.
Moser GJ & Smart RC (1989) Hepatic tumour-promoting chlorinated
hydrocarbons stimulate protein kinase C activity. Carcinogenesis,
10(5): 851-856.
Mount DI & Putnicki GJ (1966) Summary report of the 1963 Mississippi
fish kill. North Am Wildl Nat Res Conf Trans, 31: 177-184.
Mount DI, Vigor LW, & Schafer ML (1966) Endrin: use of concentration
in blood to diagnose acute toxicity in fish. Science, 152: 1388-1390.
Mugambi JM, Kanja L, Maitho TE, Skaare JU, & Lokken P (1989)
Organochlorine pesticide residues in domestic fowl (Gallus
domesticus) eggs from Central Kenya. J Sci Food Agric, 48: 165-176.
Muir CMC (1970) The acute oral and percutaneous toxicities to rats of
formulations of aldrin, dieldrin or endrin. Unpublished report No.
TLGR.0020.70, Sittingbourne, Kent, Shell Research, submitted to WHO by
Shell.
Mukerjee SK (1985) The environmental photodegradation of pesticides.
Indian J Agric Chem, 18(1): 1-9.
Mulhern BM, Reichel WL, Locke LN, Lamont TG, Belisle A, Cromartie E,
Bagerly GE, & Prouty R (1970) Organochlorine residues and autopsy data
from bald eagles. 1966-1968. Pestic Monit J, 4: 141-144.
Muncy RJ & Oliver AD Jr (1963) Toxicity of ten insecticides to the red
crawfish, Procambarus clarki (Girard). Trans Am Fish Soc, 92:
428-431.
Mussalo-Rauhamaa H, Salmela SS, Leppanen A, & Pyysalo H (1986)
Cigarettes as a source of some trace and heavy metals and pesticides
in man. Arch Environ Health, 41(1): 49-55.
Nagelsmit A, Vliet PW, van Wiel-Wetzels WAM, van der Wielard MJ, Strik
JJTWA, Ottevanger CF, & van Sittert NJ (1979) Porphyrins as possible
parameters for exposure to hexachlorocyclopentadiene, allylchloride,
epichlorohydrin and endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biochemical Press,
pp 55-61.
Narahasi T (1987) Effects of toxic agents on neural membranes. In:
Lowndes HE,ed. Electrophysiology in neurotoxicology. Boca Raton,
Florida, CRC Press, vol 1, pp 23-44.
NCI (1978) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute (DHEW Publication No. NIH 179-812)
NCI (1979) Bioassay of endrin for possible carcinogenicity. Bethesda,
Maryland, Department of Health Education, and Welfare, National Cancer
Institute, 110 pp (Carcinogenesis Technical Report Series No. 12; NTIS
PB-288461)
Nebeker AV, Schuytema GS, Griffis WL, Barbitta JA, & Carey LA (1989)
Effect of sediment organic carbon on survival of Hyalella azteca
exposed to DDT and endrin. Environ Toxicol Chem, 8: 705-718.
Nelson SC, Bahler TL, Hartwell WV, Greenwood DA, & Harris LE (1956)
Serum alkaline phosphatase levels, weight changes and mortality rates
of rats fed endrin. J Agric Food Chem, 4(8): 696-700.
Nettleship DN & Peakall DB (1987) Organochlorine residue levels in
three high Arctic species of colonially-breeding seabirds from Prince
Leopold Island. Mar Pollut Bull, 18(8): 434-438.
NIOSH (1989) Manual of analytical methods. Endrin: Method No. 5519.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 1-4.
Nishimura N, Nishimura H, & Oshima H (1982) Survey on mutagenicity of
pesticides by the Salmonella-microsome test. J Aichi Med Univ Assoc,
10(4): 305-312.
Notten WRF & Henderson PT (1975) Alteration in urinary D-glucaric acid
excretion as an indication of exposition to xenobiotics. In:
Proceedings of the International Symposium--Environment and Health,
CEC/EPA/WHO, Paris, 1974.
Novak AF & Rao MRR (1965) Food safety program: endrin monitoring in
the Mississippi River. Science, 150: 1751.
Ohlendorf HM, Swineford DM, & Locke LN (1981) Organochlorine residues
and mortality of herons. Pestic Monit J, 14(4): 125-135.
Onodera S & Tabucanon MS (1986) Organochlorine pesticide residues in
the lower Chao Phraya River and klongs along the river at Bangkok
metropolitan area, 1982-1984. J Sci Soc Thailand, 12: 225-238.
Oomen PA (1986) A sequential scheme for evaluating the hazard of
pesticides to bees, Apis mellifera. Meded Fac Landbouwwet Rijksuniv
Gent, 51(3b): 1205-1213.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis K (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
O'Shea TJ, Brownell RL Jr, Clarke DR Jr, Walker WA, Gay ML, & Lamont
TG (1980) Organochlorine pollutants in small cetaceans from the
Pacific and South Atlantic Oceans, November 1968-June 1976. Pestic
Monit J, 14:(2): 35-46.
Ottevanger CF & Van Sittert NJ (1979) Relation between anti-12-hydroxy
endrin excretion and enzyme induction in workers involved in the
manufacture of endrin. In: Strik JJTWA & Koeman JH, ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 123-129.
Ottolenghi AD, Haseman JK, & Suggs F (1974) Teratogenic effects of
aldrin, dieldrin and endrin in hamsters and mice. Teratology, 9:
11-16.
Parveen Z & Masud SZ (1987) Organochlorine pesticide residues in
cattle feed samples in Karachi, Pakistan. J Sci Ind Res; 30(7):
513-516.
Patil KC, Matsumura F, & Boush GM (1970) Degradation of endrin,
aldrin, and DDT by soil microorganisms. Appl Microbiol, 19(5):
879-881.
Pavan I, Buglione E, Pettinati L, Perrelli G, Rubino GF, Bicchi C,
D'Amato A, Carlino F, Bugiani M, & Polizzi S (1987) [Accumulation of
organochlorine pesticides in human adipose tissue. data from the
province of Turin (Italy).] Med Lav, 78(3): 219-228 (in Italian).
Pawar SS & Kachole MS (1978) Hepatic and renal microsomal electron
transport reactions in endrin treated female guinea pigs. Bull Environ
Contam Toxicol, 20: 199-205.
Pearce F (1987) Pesticide deaths: the price of the green revolution.
New Sci, 114: 30.
Peterson SR & Ellarson RS (1978) pp'-DDE, polychlorinated biphenyls,
and endrin in old squaws in North America, 1969-73. Pestic Monit J,
11(4): 170-181.
Petrella VJ, Fox JP, & Webb RE (1975) Endrin metabolism in
endrin-susceptible and resistant strains of pine mice. Toxicol Appl
Pharmacol, 34: 283-291.
Petrella VJ, McKinney JD, Fox JP, & Webb RE (1977) Identification of
metabolites of endrin. Metabolism in endrin susceptible and resistant
strains of pine mice. J Agric Food Chem, 25(2): 393-398.
Pfister RM (1972) Interactions of halogenated pesticides and
microorganisms: a review. CRC Crit Rev Microbiol, 21(1): 1-33.
Phillips DD, Pollard GE, & Soloway SB (1962) Thermal isomerization of
endrin and its behaviour in gas chromatography. J Agric Food Chem,
10(3): 217-221.
Plimmer JR (1972) Photochemistry of organochlorine insecticides. In:
Tahori AS, ed. Proceedings of the Ssecond international IUPAC congress
of pesticides chemistry. New York, Gordon & Breach,vol 1, pp 413-432.
Podrebarac DS (1984) Pesticide, heavy metal, and other chemical
residues in infant and toddler total diet samples (IV). October
1977-September 1978. J Assoc Off Anal Chem, 67(1): 166-175.
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB (1981)
Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte
cultures: a comparison with bacterial mutagenicity using 218
compounds. Environ Mutagen, 3: 11-32.
Prouty RM & Bunck C (1986) Organochlorine residues in adult mallard
and black duck wings, 1981-1982. Environ Monit Assess, 6: 49-57.
Prouty RM, Reichel WL, Locke LN, Belisle AA, Cromartie E, Kaiser TE,
Lamont TG, Mulhern BM, & Swineford DM (1977) Residues of
organochlorine pesticides and polychlorinated biphenyls and autopsy
data for bald eagles, 1973-74. Pestic Monit J, 11: 134-137.
Radeleff RD (1956) Hazards to livestock of insecticides used in
mosquito control. Mosq News, 16(2): 79-80.
Radhakrishnan AG & Antony PD (1989) Pesticide residues in marine
fishes. Fish Technol, 26: 60-61.
Rashid KA & Mumma RO (1986) Screening pesticides for their ability to
damage bacterial DNA. J Environ Sci Health, B21(4): 319-334.
Reddy DB, Edward VD, Abraham GJS, & Rao KV (1966) Fatal endrin
poisoning. A detailed autopsy, histopathological and experimental
study. J Indian Med Assoc, 46(3): 121-124.
Reddy DB, Abraham GJS, Edward VD, Naganna B, & Mathalli M (1967)
Further observations on endrin poisoning. J Indian Med Prof, 13(10):
5946, 5967-5968.
Redetzke K., Gonzalez AA, & Applegate HG (1983) Organochlorine
pesticides in adipose tissue of persons from Ciudad Juarez, Mexico. J
Environ Health, 46(1): 25-27.
Reece RL, Scott PC, Forsyth WM, Gould JA, & Barr DA (1985) Toxicity
episodes involving agricultural chemicals and other substances in
birds in Victoria, Australia. Vet Rec, 117(20): 525-527.
Reeves RG, Woodham DW, Ganyard MC, & Bond CA (1977) Preliminary
monitoring of agricultural pesticides in a cooperative tobacco pest
management project in North Carolina, 1971--first-year study. Pestic
Monit J, 11(2): 99-106.
Reichel WL, Lamont TG, Cromartie E, & Locke LN (1969) Residues in two
bald eagles suspected of pesticide poisoning. Bull Environ Contam
Toxicol, 4(1): 24-30.
Reidinger RF & Crabtree DG (1974) Organochlorine residues in golden
eagles, United States--March 1964-July 1971. Pestic Monit J, 8(1):
37-43.
Reins DA, Holmes DD, & Hinshaw LB (1964) Acute and chronic effects of
the insecticide endrin on renal function and renal hemodynamics. Can
J Physiol Pharmacol, 42(5): 599-608.
Reins DA, Rieger JA Jr, Stavinoha WB, & Hinshaw LB (1966) Effect of
endrin on venous return and catecholamine release in the dog. Can J
Physiol Pharmacol, 44: 59-67.
Ressang AA, Titus I, Andar RS, & Soedarmo D (1958) Aldrin, dieldrin
and endrin intoxication in cats. Commun Vet, 2(2): 71-88.
Reuber MD (1978) Carcinomas, sarcomas and other lesions in
Osborne-Mendel rats ingesting endrin. Exp Cell Biol, 46(3): 129-145.
Revzin AM (1966) Effects of endrin on telencephalic function in the
pigeon. Toxicol Appl Pharmacol, 9(1): 75-83.
Revzin AM (1980) Some acute and chronic effects of endrin on the
brains of pigeons and monkeys. In: Proceedings of the symposium on the
biological impact of pesticides in the environment, pp 134-141.
Ribbens PH (1985) Mortality study of industrial workers exposed to
aldrin, dieldrin and endrin. Arch Occup Environ Health, 56(2): 75-79.
Richardson LA, Lane JR, Gardner WS, Peeler JT, & Campbell JE (1967)
Relationship of dietary intake to concentration of dieldrin and endrin
in dogs. Bull Environ Contam Toxicol, 2(4): 207-219.
Richardson A, Robinson J, & Baldwin MK (1970) Metabolism of endrin in
the rat. Chem Ind, 1970,: 502-503.
Ritchey SJ, Young RW, & Essary EO (1972) Effects of heating and
cooking method on chlorinated hydrocarbon residues in chicken tissues.
J Agric Food Chem, 20: 291-293.
Robinson J & McGill AEJ (1966) Organochlorine insecticide residues in
complete prepared meals in Great Britain during 1965. Nature,
212(5066): 1037-1038.
Robinson J, Richardson A, Hunter CG, Crabtree AN, & Rees HJ (1965)
Organochlorine insecticide content of human adipose tissue in
south-eastern England. Br J Ind Med, 22: 220-229.
Roos AH, van Munsteren AJ, Nab FM, & Tuinstra LGMT (1987) Universal
extraction/clean-up procedure for screening of pesticides by
extraction with ethylacetate and size exclusion chromatography. Anal
Chim Acta, 196: 95-102.
Rosales MTL, Escalona RL, Alarcon RM, & Zamora V (1985) Organochlorine
hydrocarbon residues in sediments of two different lagoons of
Northwest Mexico. Bull Environ Contam Toxicol, 35: 322-330.
Rosen JD (1972) Conversion of pesticides under environmental
conditions. In: Coulston F & Korte F ed. Environmental quality.
Stuttgart, George Thieme, vol 1, pp 85-96.
Rosen JD, Sutherland DJ, & Lipton GR (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull
Environ Contam Toxicol, 1: 133-140.
Rowe DR, Canter LW, Snyder PJ, & Mason JW (1971) Dieldrin and endrin
concentrations in a Louisiana estuary. Pestic Monit J, 4(4): 177-183.
Rowley DL, Rab MA, Hardjotanojo W, Liddle J, Burse VW, Saleem M, Sokal
D, Falk H, & Head SL (1987) Convulsions caused by endrin poisoning in
Pakistan. Pediatrics, 79(6): 928-934.
Roylance KJ, Jorgensen CD, Booth GM, & Carter MW (1985) Effects of
dietary endrin on reproduction of Mallard ducks (Anas
platyrhynchos). Arch Environ Contam Toxicol, 14: 705-711.
Runhaar EA, Sangster B, Greve PA, & Voortman M (1985) A case of fatal
endrin poisoning. Hum Toxicol, 4: 241-247.
Ryan S, Bacher GJ, & Martin AA (1972) The mussel Hyridella australis
as a biological monitor of the pesticide endrin in fresh water.
Search, 3(11-12): 446-447.
Safe S & Hutzinger O (1979) Mass spectrometry of pesticides and
pollutants. Boca Raton, Florida, CRC Press, Inc.
Saiki MK & Schmitt CJ (1986) Organochlorine chemical residues in
bluegills and common carp from the irrigated San Joaquin Valley floor,
California. Arch Environ Contam Toxicol, 15: 357-366.
Samhan O & Ghobrial F (1987) Trace metals and chlorinated hydrocarbons
in sewage sludges of Kuwait. Water Air Soil Pollut, 36: 239-246.
Satsmadjis J, Georgakopoulos-Gregoriades E, & Voutsinou-Taliadouri F
(1988) Red mullet contamination by PCBs and chlorinated pesticides in
the Pagassitikos Gulf, Greece. Mar Pollut Bull, 19(3): 136-138.
Schafer ML, Peeler JT, Gardner WS, & Campbell JE (1969) Pesticides in
drinking water--waters from the Mississippi and Missouri rivers.
Environ Sci Technol,3(12): 1261-1269.
Schafer EW Jr, Bowles WA Jr, & Hurlbut J (1983) The acute oral
toxicity, repellency, and hazard potential of 998 chemicals to one or
more species of wild and domestic birds. Arch Environ Contam Toxicol,
12: 355-382.
Scheutz EG, Wrighton SA, Safe SH, & Guzelian PS (1986) Regulation of
cytochrome P-450p by phenobarbital and phenobarbital-like inducers in
adult rat hepatocytes in primary monolayer culture and in vivo.
Biochemistry, 25: 1124-1133.
Schwabe U & Wendling I (1967) [Stimulation of drug metabolism by low
doses of DDT and other chlorinated hydrocarbon insecticides.]
Arzneimittel-forschung, 17(5): 614-618 (in German).
Seifert J (1988) Ontogenesis and properties of the convulsant
recognition site(s) of the gamma-aminobutyric acid (GABA) receptor
complex in chicken embryo. Eur J Pharmacol,151: 443-448.
Seifert J (1989) Teratogenesis of polychlorocycloalkane insecticides
in chicken embryos resulting from their interactions at the convulsant
recognition sites of the GABA (pro)receptor complex. Bull Environ
Contam Toxicol, 42: 707-715.
Sherman M & Rosenberg M (1954) Subchronic toxicity of four chlorinated
dimethanonaphthalene insecticides to chicks. J Econ Entomol, 47 (6):
1082-1083.
Sierra M & Santiago D (1987) Organochlorine pesticide levels in barn
owls collected in Leon, Spain. Bull Environ Contam Toxicol, 38:
261-265.
Sierra M, Teran MT, Gallego A, Diez MJ, & Santiago D (1987)
Organochlorine contamination in three species of diurnal raptors in
Leon, Spain. Bull Environ Contam Toxicol, 38: 254-260.
Simmon VF, Kauhanen K, & Tardiff RC (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North Holland Biomedical Press, pp 249-258.
Singh MN (1988) Mortality response of Achatina fulica to various
pesticides. J Environ Biol, 9(2): 157-162.
Singh PDA & West ME (1985) Acute pesticide poisoning in the Caribbean.
West Indian Med J, 34: 75-83.
Singhal RL & Kacew S (1976) The role of cyclic AMP in chlorinated
hydrocarbon-induced toxicity. Fed Proc, 35(14): 2618-2623.
Smith KJ, Polen PB, de Vries DM, & Coon FB (1968) Removal of
chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J Am Oil Chem Soc, 45: 866-869.
Sobti RC, Krishan A, & Davies J (1983) Cytokinetic and cytogenetic
effect of agricultural chemicals on human lymphoid cells in vitro.
II. Organochlorine pesticides. Arch Toxicol, 52: 221-231.
Somers JD, Goski BC, & Barrett MW (1987) Organochlorine residues in
northeastern Alberta otters. Bull Environ Contam Toxicol, 39: 783-790.
Soto AR & Deichmann WB (1967) Major metabolism and acute toxicity of
aldrin, dieldrin and endrin. Environ Res, 1(4): 307-322.
Spann JW, Heinz GH, & Hulse CS (1986) Reproduction and health of
mallards fed endrin. Environ Toxicol Chem, 5: 755-759.
Speck LB & Maaske CA (1958) The effects of chronic and acute exposure
of rats to endrin. Arch Ind Health, 18: 268-272.
Spynu EI (1964) On the toxicology of new organic chloride insecticides
obtained by diene synthesis on the basis of hexachlorocyclopentadiene.
Gig Tr Prof Zabol, 4: 30-35.
Squires RF & Saederup E (1989) Polychlorinated convulsant insecticides
potentiate the protective effect of NaCl against heat inactivation of
[3H] fluonitrazepam binding sites. J Neurochem, 52: 537-543.
Stanford Research Institute (1953) Unpublished letter Report No. 1
Ref. Project No. B-868 November 10, Stanford, CA, submitted to WHO by
Shell.
Stanford Research Institute (1954) Unpublished letter Report No. 3
Ref. Project No. B-868 February 1, Stanford, CA, submitted to WHO by
Shell.
Stanley CW, Barney JE II, Helton MR, & Yobs AR (1971) Measurement of
atmospheric levels of pesticides. Environ Sci Technol, 5: 430-435.
Steinberg KK, Garza A, Bueso JA, Burse VW, & Phillips DL (1989) Serum
pesticide concentrations in farming cooperatives in Honduras. Bull
Environ Contam Toxicol, 42: 643-650.
Stickel WH, Kaiser TE, & Reichel WL (1979) Endrin versus 12-ketoendrin
in birds and rodents. In: Kenaga EE ed. Avian and mammalian wildlife
toxicology, Philadeplphia,, American Society for Testing and
Materials, pp 61-68 (ASTM STP 693).
Stout VF (1980) Organochlorine residues in fishes from the Northwest
Atlantic Ocean and Gulf of Mexico. Fish Bull, 78(1): 51-58.
Strachan WMJ (1988) Toxic contaminants in rainfall in Canada: 1984.
Environ Toxicol Chem, 7: 871-877.
Strachan WMJ, Huneault H, Schertzer WM, & Elder FC (1980)
Organochlorines in precipitation in the Great Lakes region. In: Afghan
BK & MacKay D ed. Hydrocarbons and halogenated hydrocarbons in the
aquatic environment. New York, Plenum Publishing Corp., pp 387-396.
Strassman SC & Kutz FW (1977) Insecticide residues in human milk from
Arkansas and Mississippi, 1973-1974. Pestic Monit J, 10(4): 130-133.
Strik JJTWA (1979) The occurrence of chronic hepatic porphyria in man,
caused by halogenated hydrocarbons. In: Strik JJTWA & Koeman JH ed.
Chemical porphyria in man. Amsterdam, Elsevier/North Holland
Biomedical Press, p 3.
Struger J, Weseloh D, Hallett DJ, & Mineau P (1985) Organochlorine
contaminants in herring gull eggs from the Detroit and Niagara Rivers
and Saginaw Bay (1978-1982): contaminant discriminants. J Great Lakes
Res, 11(3): 223-230.
Sturm R, Knauth HD, Reinhardt KH, & Gandrasz J (1986) Distribution of
chlorinated hydrocarbons in sediment and seston of the River Elbe.
Wasser, 67: 23-38.
Sundershan P & Khan MAQ (1980) Metabolic fate of [14C] endrin in
bluegill fish. Pestic Biochem Physiol, 14: 5-12.
Suzuki M & Morimoto M (986) High resolution chemically bonded
fused-silica capillary gas chromatography of organochlorine
insecticides and related compounds in arable soil samples. J High
Resol Chromatogr Chromatogr Commun, 9: 296-298.
Suzuki M, Yamato Y, & Watanabe T (1973) Multiple organochlorine
pesticide residues in Japan. Bull Environ Contam Toxicol, 10(3):
145-150.
Suzuki K, Nagayoshi H, & Kashiwa T (1974) The systematic separation
and identification of pesticide in the first division. Agric Biol
Chem, 38(2): 279-285.
Sykes PW Jr (1985) Pesticide concentrations in snail kite eggs and
nestlings in Florida. Condor, 87: 438.
Tarrant KR & Tatton JO'G (1968) Organochlorine pesticides in rainwater
in the British Isles. Nature, 219(5155): 725-727.
Telling GM, Sissons DJ, & Brinkman HW (1977) Determination of
organochlorine insecticide residues in fatty food stuffs using a
clean-up technique based on a single column of activated alumina. J
Chromatogr, 137: 405-423.
Teran MT & Sierra M (1987) Organochlorine insecticides in trout,
Salmo trutta fario L. taken from four rivers in Leon, Spain. Bull
Environ Contam Toxicol, 38: 247-253.
Terriere LC (1964) Endrin. In: Zweig, G ed. Analytical methods for
pesticides, plant growth regulators and food additives. New York,
Academic Press, vol 2, pp 209-222.
Terriere LC, Kiigemagi U, & England DC (1958) Endrin content of body
tissues of steers, lambs and hogs receiving endrin in their daily
diet. J Agric Food Chem, 6(7): 516-518.
Terriere LC, Arscott GH, & Kiigemagi U (1959) The endrin content of
eggs and body tissue of poultry receiving endrin in their daily diet.
J Agric Food Chem, 7(7): 502-504.
Thier HP & Stijve T (1986) [Results of an inter-laboratory comparison
of analyses of analyses of organochlorine and organophosphorus
pesticide residues in fat.] Lebensmittelchem Gerichtl Chem, 40: 73-75
(in German).
Thompson JF (1976) Manual of analytical quality control for pesticides
and related compounds in human and environmental samples. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Office of Research and Development, Health Effects Research Laboratory
(EPA-600/1-76-017).
Thurston R, Gilfoil TA, Meyn EL, Zajdel RK, Aoki TI, & Veith GD (1985)
Comparative toxicity of ten organic chemicals to ten common aquatic
species. Water Res, 19(9): 1145-1155.
Travis CC & Arms AD (1988) Bioconcentration of organics in beef, milk
and vegetation. Environ Sci Technol, 22: 271-274.
Treon JF, Cleveland FP, & Cappel J (1955) Toxicity of endrin for
laboratory animals. J Agric Food Chem, 3(10): 842-848.
Truhaut R, Do Phuoc H, & Phu Lich N (1974) Influence de
l'administration de pesticides organo-halogénés et de
polychloro-biphényles sur le métabolisme de la zoxazolamine chez le
rat. CR Acad Sci Paris Sér D, 278: 3003-3006.
Tucker RK & Crabtree DG (1970) Handbook of toxicity of pesticides to
wildlife. Washington, DC, US Bureau of Sport Fisheries and Wildlife,
Denver Wildlife Center (Resource Publication No. 84).
Tuinstra LGMT (1971) Organochlorine insecticide residues in human milk
in the Leiden region. Neth Milk Dairy J, 25: 24-32.
Uhnak J, Sackmauerova M, Szokolay A, & Pal'usova O (1974) The use of
an electron capture detector for the determination of pesticides in
water. J Chromatogr 91: 545-547.
United Kingdom Ministry of Agriculture, Fisheries and Food (1989)
Report of the Working Party on Pesticide Residues 1985-1988. London,
Her Majesty's Stationery Office (Food Surveillance Paper No. 25).
US EPA (1974) New Orleans area water supply study (draft analytical
report). Dallas, Texas, US Environmental Protection Agency, Lower
Mississippi River Facility, Surveillance and Analysis Divisions,
Region VI.
US EPA (1983) Findings of selected chemical residues in human blood
serum and adipose tissue: Endrin. Environ News, 9 May.
US EPA (1985) Hexachloronorbornadiene; Proposed submission of notice
of manufacturer, import or processing and determination of significant
new use. Fed Reg, 50(36): 7351-7356.
US EPA (1987a) Health effects assessment for endrin. Cincinnati, Ohio,
US Environmental Protection Agency, Ofice of Research and Development,
Environmental Criteria and Assessment Office, 31 pp (Report
PB88-180237).
US EPA (1987b) Health advisories for 16 pesticides. Washington, DC, US
Environmental Protection Agency, Office of Drinking Water, 18 pp
(Report PB87-200176).
Vance BD & Drummond W (1969) Biological concentration of pesticides by
algae. J Am Water Works Assoc, 61: 360-362.
Van Dijck P & van de Voorde H (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded Fac Landbouww
Rijksuniv Gent, 41(2): 1491-1498.
Van Raalte HGS (1965) [Some aspects of pesticide toxicity.]
Unpublished paper presented at the Conference on Occupational Health,
Caracas, Venezuela; The Hague. Shell, International Petroleum Company,
Health, Safety and Environment Division (in Spanish).
Van Sittert NJ (1985) Biological monitoring of bestrijdingsmiddelen;
Coronel-PAOG Nascholingssymposium. Unpublished paper, Amsterdam, Vrije
Universiteit, February 1985 (in Dutch).
Van Wynen JH & Stykel A (1988) Health risk assessment of residents
living on harbour sludge. Arch Occup Environ Health, 61: 77-87.
Veith GD, Kuehl DW, Leonard EN, Puglisi FA, & Lemke AE (1979)
Polychlorinated biphenyls and other organic chemical residues in fish
from major watersheds of the United States, 1976. Pestic Monit J,
13(1): 1-11.
Veith GD, Kuehl DW, Leonard EN, Welch K, & Pratt G (1981)
Polychlorinated biphenyls and other organic chemical residues in fish
from major United States watersheds near the Great Lakes, 1978. Pestic
Monit J, 15(1): 1-8.
Verma MP, Bahga HS, Soni BK, & Singh SP (1970) Effect of insecticides
on lactic acid concentration in the blood of buffalo-calves. Indian
Vet J, 47(12): 1056-1058.
Vermeer K, Risebrough RW, Spaans AL, & Reynolds LM (1974) Pesticide
effects on fishes and birds in rice fields in Surinam, South America.
Environ Pollut, 7: 217-236.
Versteeg JPJ & Jager KW (1973) Long-term occupational exposure to the
insecticides aldrin, dieldrin, endrin and telodrin. Br J Ind Med, 30:
201-202.
Villeneuve JP, Holm E, & Cattini C (1985) Transfer of chlorinated
hydrocarbons in the food chain lichen --> reindeer --> man.
Chemosphere, 14(11/12): 1651-1658.
Von Westernhagen H, Dethlefsen V, Cameron P, & Janssen D (1987)
Chlorinated hydrocarbon residues in gonads of marine fish and effects
on reproduction. Sarsia, 72: 419-422.
Von Westernhagen H, Cameron P, Dethlefsen V, & Janssen D (1989)
Chlorinated hydrocarbons in North Sea whiting (Merlangius merlangus)
and effects on reproduction. I. Tissue burden and hatching success.
Helgolander Meeresunters 43: 45-60.
Vrij-Standhardt WG, Strik JJTWA, Ottevanger CF, & van Sittert NJ
(1979) Urinary D-glucaric acid and urinary total porphyrin excretion
in workers exposed to endrin. In: Strik JJTWA & Koeman JH ed. Chemical
porphyria in man. Amsterdam, Elsevier/North Holland Biomedical Press,
pp 113-121.
Wafford KA, Sattelle DB, Gant DB, Eldefrawi AT, & Eldefrawi ME (1989a)
Non-competitive inhibition of GABA receptors in insect and vertebrate
CNS by endrin and lindane. Pestic Biochem Physiol, 33: 213-219.
Wafford KA, Lummis SCR, & Sattelle DB (1989b) Block of an insect
central nervous system GABA receptor by cyclodiene and cyclohexane
insecticides. Proc R Soc Lond, B237: 53-61.
Walker JJ & Phillips DE (1987) An electron microscopic study of endrin
induced alterations in unmyelinated fibers of mouse sciatic nerve.
Neurotoxicology, 8(1): 55-64.
Walsh GM & Fink GB (1970) Temporal aspects of acute endrin toxicity in
mice. Proc West Pharmacol Soc, 13: 81-83.
Walsh GM & Fink GB (1972) Comparative toxicity and ditribution of
endrin and dieldrin after intravenous administration in mice. Toxicol
Appl Pharmacol, 23: 408-416.
Wang HH & MacMahon B (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J Occup Med, 21(11):745-748.
Wassermann M, Curnow DH, Forte PN, & Groner Y (1968) Storage of
organochlorine pesticides in the body fat of people in Western
Australia. Int J Ind Med Surg, 37(4): 295-300.
Wassermann M, Francone MP, Wassermann D, Mariani F, & Groner J (1969)
[Organochlorine pesticide content of the fatty tissue of the general
public in Argentina.] Sem Med, 134(16): 459-462 (in Spanish).
Waters MD, Sandhu SS, Simmon VF, Mortelmans KE, Mitchell AD, Jorgenson
TA, Jones DCL, Valencia R, & Garrett NE (1982) Study of pesticide
genotoxicity. Basic Life Sci, 21: 275-326.
Webb RE & Horsfall F Jr (1967) Endrin resistance in pine mouse.
Science, 156: 1762.
Webb RE, Hartgrove RW, Randolph WC, Petrella VJ, & Horsfall F Jr
(1973) Toxicity studies in endrin-susceptible and resistant strains of
pine mice. Toxicol Appl Pharmacol, 25(1): 42-47.
Weeks DE (1967) Endrin food-poisoning. A report on four outbreaks
caused by two separate shipments of endrin-contaminated flour. Bull
World Health Organ, 37: 499-512.
Wegman RCC & Greve PA (1974) Levels of organochlorine pesticides and
inorganic bromide in human milk. Meded Fac Landbouwwet Rijksuniv Gent,
39: 1301-1310.
Wegman RCC & Greve PA (1978) Organochlorines, cholinesterase
inhibitors and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic Monit J, 12(3): 149-162.
Wegman RCC & Greve PA (1980) Halogenated hydrocarbons in Dutch water
samples over the years 1969-1977. Environ Sci Res, 16: 405-415.
Wegman RCC & Hofstee AWM (1982) Determination of organochlorines in
river sediment by capillary gas chromatography. Water Res, 16:
1265-1272.
Wegman RCC, Hofstee AWM, & Greve PA (1981) Uptake of organochlorines
by plants growing on river and basin sediment. Meded Fac Landbouwwet
Rijksuniv Gent, 46(1): 359-365.
Weisgerber I, Klein W, & Korte F (1969) [Disappearance of residues and
metabolism of endrin-(14C) in tobacco.] Liebigs Ann Chem, 729:
193-197 (in German with English abstract).
Wells MR & Yarbrough JD (1972) Vertebrate insecticide resistance:
in vivo and in vitro endrin binding to cellular fractions from
brain and liver tissues of Gambusia. J Agric Food Chem, 20(1): 14-16.
Whetstone RR (1964) Chlorocarbons and chlorohydrocarbons: chlorinated
derivatives of cyclopentadiene. In: Kirk-Othmer encyclopedia of
chemical technology, 2nd ed., New York, John Wiley and Sons, vol 5, pp
240-252.
WHO (1989) Environmental Health Criteria No. 91: Aldrin and dieldrin.
Geneva, World Health Organization, 335 pp.
WHO (1992) The WHO recommended classification of pesticides by hazard.
Guidelines to classification 1992-1993. Geneva, World Health
Organization (WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 1: Endrin. Geneva, World
Health Organization (VBC/DS/75.1).
Wiemeyer SN, Jurek RM, & Moore JF (1986) Environmental contaminants in
surrogates, foods and feathers of California condors (Gymnogyps
californianus). Environ Monit Assess, 6: 91-111.
Williams S (1964) Pesticide residues in total diet samples. J Assoc
Off Anal Chem, 47(5): 815-821.
Williams GM (1979) Liver cell culture systems for the study of
hepatocarcinogenesis. In: Margison GP ed Advances in medical oncology
research and education: Carcinogenesis, New York, Pergamon Press, vol
1, pp 273-280.
Williams S, Mills PA, & McDowell RE (1964) Residues in milk of cows
fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J Asoc Off Agric Chem, 47: 1124-1128.
Williams DT, Benoit FM, McNeil EE, & Otson R (1978) Organochlorine
pesticide levels in Ottawa drinking water, 1976. Pestic Monit J,
12(3): 163-166.
Williams DT, Lebel GL, & Junkins E (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples from
two Ontario municipalities. J Toxicol Environ Health, 13: 19-29.
Williams DT, Lebel GL, & Junkins E (1988) Organohalogen residues in
human adipose autopsy samples from six Ontario municipalities. J Assoc
Off Anal Chem, 71(2): 410-414.
Wilson Committee (1969) Report by the Advisory Committee on Pesticides
and Other Toxic Chemicals. Further review of certain persistent
organochlorine pesticides used in Great Britain. London, Her Majesty's
Stationery Office, pp 90-100.
Wilson JG & Earley JJ (1986) Pesticide and PCB levels in the eggs of
shag Phalacrocorax aristotelis and cormorant Phalacrocorax carbo
from Ireland. Environ Pollut (Ser B), 12: 15-26.
Wit SL (1971) [Persistent insecticides in Dutch body fat.] Chem
Weekbl, 67(5): 11-14 (in Dutch).
Witherup S, Stemmer KL, Taylor P, & Bietsch P (1970) The Incidence of
Neoplasms in Two Strains of Mice Sustained on Diets Containing Endrin.
Unpublished report, Cincinnati, Ohio, Kettering Laboratory, submitted
to WHO by Shell.
Wolfe HR, Durham WF, & Armstrong JF (1963) Health hazards of the
pesticides endrin and dieldrin: hazards in some agricultural uses in
the Pacific Northwest. Arch Environ Health, 6: 458-464.
Wolfe HR, Durham WF, & Armstrong JF (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolman AA & Wilson AJ Jr (1970) Occurrence of pesticides in whales.
Pestic Monit J, 4(1): 8-10.
Wüthrich C, Müller F, Blaser O, & Marek B (1985) [Pesticides and other
chemical residues in Swiss diet samples.] Mitt Geb Lebensmittel Hyg,
76: 260-276 (in German with English summary).
Wuu KD & Grant WF (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare).
Can J Genet Cytol, 8: 481-501.
Wuu KD & Grant WF (1967a) Chromosomal aberrations induced by
pesticides in meiotic cells of barley. Cytologia, 32: 31-41.
Wuu KD & Grant WF (1967b) Chromosomal aberrations induced in somatic
cells of Vicia faba by pesticides. Nucleus, 10(1): 37-46.
Yakushiji T, Watanabe I, Kuwabara K, Yoshida S, Hori S, Fukushima S,
Kashimoto T, Koyama K, & Kunita N (1979) Levels of organochlorine
pesticides and polychlorinated biphenyls (PCBs) in mothers milk
collected in Osaka prefecture from 1969-1976. Arch Environ Contam
Toxicol, 8: 59-66.
Yarbrough JD, Roush RT, Bonner JC, & Wise DA (1986) Monogenic
inheritance of cyclodiene insecticide resistance in mosquitofish,
Gambusia affinis. Experientia (Basel), 42: 851-853.
Young RA & Mehendale HM (1986) Effect of endrin and endrin derivatives
on hepatobiliary function and carbontetrachloride-
induced hepatotoxicity in male and female rats. Food Chem Toxicol,
24(8): 863-868.
Zabik MJ, Schuetz RD, Burton WL, & Pape BE (1971) Photochemistry of
bioactive compounds. Studies of a major photolytic product of endrin.
J Agric Food Chem, 19(2): 308-313.
Zavon MR, Hine CH, & Parker KD (1965) Chlorinated hydrocarbon
insecticides in human body fat in the United States. J Am Med Assoc,
193(10): 181-183.
Zeiger E (1987) Carcinogenicity of mutagens: predictive capability of
the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer
Res, 47: 1287-1296.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity tests: III. Results from the testing
of 255 chemicals. Environ Mutagen, 9 (suppl9): 1-110.
Zimmerli B & Marek B (1973) [The pesticide load of the Swiss
population.] Mitt Geb Lebensmittel Hyg,, 64(4): 459-479 (in German
with English summary).
ANNEX I CHEMICAL NAMES OF ENDRIN AND ITS METABOLITES
Two main systems are currently used for the nomenclature of
cyclodiene insecticides: 'polyhydroaromatic' names, used by Chemical
Abstracts (American Chemical Society) and the International Union for
Pure and Applied Chemistry (IUPAC), and the von Baeyer/IUPAC system
for polycyclic aliphatic compounds. Benson (1969) and Bedford (1974)
proposed that the latter system be used for the cyclodiene
insecticides.
The 'polyaromatic' system has, unfortunately, been subject to
historical variation, and there are differences between the IUPAC,
British and American conventions for defining the three-dimensional
stereochemistry in this system. As a consequence of differences in the
numbering of carbon atoms in the two systems and the modification of
the Chemical Abstracts 'polyaromatic' name for dieldrin since 1971,
considerable confusion can arise in the nomenclature of metabolites.
The possible misunderstandings that may occur, particularly among
people who are not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that are given to
the major metabolite of endrin; this one compound may be designated
as:
anti-9-hydroxyendrin (former Chemical Abstracts system)
anti-8-hydroxyendrin (current Chemical Abstracts system)
anti-12-hydroxyendrin (von Baeyer/IUPAC system).
A useful discussion of nomenclature was given by Brooks (1974).
The chemical names for endrin and its metabolites are summarized
in Table 30.
Table 30. Chemical nomenclature of endrin and its metobolites
Trivial namesa Polycyclic aliphatic name (von Baeyer/ IUPAC) Alternative or former names
Endrin (I) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-2,3-7, 6- 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,
endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]- 4a,5,6,7,8,8a-octahydro-1,4- endo,endo-
dodec-9-ene 5,8-demethanonaphthalene (former CAS
name)
1aa,2b,2ab,3a,6a,6b,7b,7a)-3,4,5,6,9,9-
Hexachloro-1a,2,2a,3,6,6a,7,7a,-octahydro-
2,7:3,6-dimethanonaphth[2,3- b]oxirene
(current CAS name)
(IR,4S,4aS,5S,7R,8R,8aR)-1,2,3,4,10,10-
Hexachloro-1,4,4a,5,6,7,8,8a-octahydro-6,7-
epoxy-1,5:5,8-dimethanonaphthalene
(current IUPAC name)
syn-12-Hydroxyendrin (II) 1,8,9,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- syn-hydroxyendrin) ( syn-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8- endo-
tetracyclo[6.2.1.13,6.02,7] dodec-9-ene
anti-12-Hydroxyendrin (III) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-12-
(9- anti-hydroxyendrin) ( anti-epoxy)hydroxy-2,3-7,6- endo-2,1-7,8-
endo-tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
3-Hydroxyendrin (IV) 1,8,9,10,11,11-Hexachloro-4,5- exo-epoxy-3-
(5-hydroxyendrin) hydroxy-2,3-7,6- endo-2,1-7,8- endo-tetracyclo-
[6.2.1.13,6.02,7] dodec-9-ene
12-Ketoendrin (V) 1,8,9,10,11,11-Hexachloro-4,5 - exo-epoxy-2,3-7,6-
(9-ketoendrin) endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]-
dodec-12-one
Endrin trans-diol (IV) 1,8,9,10,11,11-Hexachloro-4,5-( exo)trans-dihydroxy-
2,3-7,6- endo-2,1-7,8- endo-tetracyclo[6.2.1.13,6.02,7]
dodec-9-ene
aRoman numerals in parentheses refer to the structures in Figure 2.
ANNEX II
MEDICAL TREATMENT OF ENDRIN POISONING
1. Symptoms of poisoning
Endrin is readily absorbed and is toxic when taken by mouth, by
skin contact (especially liquid formulations), and by inhalation. It
stimulates the central nervous system, and an oral dose of 0.25 mg/kg
body weight can cause convulsions in humans. Following accidental
ingestion or gross over-exposure, symptoms appear between 20 min and
12 h and may include headache, dizziness, nausea, vomiting, weakness
in the legs, and convulsions, sometimes leading to death.
Organochlorine compounds can cause respiratory depression, and
they may sensitize the heart to endogenous catecholamines, leading to
ventricular fibrillation and cardiac arrest in severe cases.
Respiratory depression may lead to metabolic acidosis, and if
necessary blood gases should be checked. Use of an
electrocardiographic monitor is recommended if symptoms are severe.
Endrin is eliminated very quickly from the blood and can be
detected for only 1 or 2 days even after massive over-exposures. Signs
and symptoms of poisoning occur only at levels in whole blood of above
0.05 µg/ml.
2. Medical treatment
Medical treatment is largely symptomatic and supportive and is
directed against convulsions and hypoxia. If endrin is swallowed, the
stomach should be emptied as soon as possible by careful gastric
lavage (with a cuffed endotracheal tube already in place), avoiding
aspiration into the lungs. In a rural situation where this is not
feasible, vomiting should be induced immediately. This should be
followed by (intragastric) administration of 50 g of activated
charcoal and 30 g of magnesium or sodium sulfate in a 30% aqueous
solution. Oily purgatives are contraindicated, and no fats, oils, or
milk should be given.
If convulsions occur, anticonvulsants should be given immediately
but slowly, and repeated as necessary. Diazepam can be given at 10 mg
(children, 1-5 mg) intravenously; thiopental sodium or hexobarbital
sodium can be given intravenously at a dose of 10 mg/kg, with a
maximum total dose of up to 750 mg for an adult; or paraldehyde can be
given at 5 ml by intramuscular injection. These short-acting
anticonvulsants should always be followed by phenobarbital given
orally at 3 mg/kg (up to 200 mg for an adult), or phenobarbital sodium
given intramuscularly at 3 mg/kg (up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline,
should never be given.
The airway must be kept unobstructed. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
A guideline for the management of major status epilepticus is
added as Annex III.
ANNEX III
MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
1. Initial management
1. Assess the patient, verify the diagnosis, remove false
teeth, place the patient in a lateral semi-prone position,
and establish an airway.
2. Give diazepam intravenously (see Note 1, below), usually at
10 mg in 2 ml (0.15-0.25 mg/kg), followed immediately by a
further 10 mg (2 ml) over 1-2 min. This may be repeated
according to response.
3. Take blood to measure levels of anticonvulsant drug,
ethanol, and blood sugar (5 ml of blood in a sugar tube); a
sample to measure calcium (5 ml in a plain tube); and a drop
of blood to determine blood glucose.
4. If the latter measurement shows low blood glucose level, 25
ml of 50% glucose should be given intravenously, preferably
by catheter and not into a small distal vein. If ethanol is
likely to be present, give thiamine intravenously at 100 mg.
5. Give phenytoin intravenously at 250 mg in 5 ml (10-15 mg/kg)
no faster than 50 mg (1 ml)/min by infusion pump or slow
intravenous injection (see Note 2, below).
2. If fits continue, transfer patient to the intensive care unit and
consult an anaesthetist
6. Give chlormethiazole intravenously at 8 mg/ml: a loading
dose of up to 800 mg (100 ml) over 10 min at 10 ml/min,
maintained with 0.5-1 ml/min (4-8 mg).
7. Give thiopentone intravenously at 5 mg/kg as a loading dose,
then 1-3 mg/kg per h to a maximum blood level of 100
mg/litre.
8. If this fails, consult a neurologist.
3. Notes
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there
is concurrent use of other central nervous system depressant
drugs, especially phenobarbital.
aAdapted from guidelines issued at Guy's Hospital, London
Diazepam must not be given intramuscularly:
--added to an intravenous infusion
--with phenobarbital, unless artificial ventilation is
available.
Rectal administration of diazepam (using a rectal
administration set), at 5 or 10 mg in 2.5 ml, may be used
for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
--intramuscularly
--by central line
--into a dextrose infusion
--with any other drug.
Intravenously administered phenytoin should be monitored by
continuous electrocardiographic recording. If this is not
available, it may be safer to use a diluted solution of 250
mg (5 ml) in 250 ml of normal saline, no faster than 50
mg/min. The diluted solution should be used immediately
provided there is no evidence of precipitation (this use of
phenytoin is not licensed).
4. Options
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate deep intramuscular
injection or 10 ml diluted in 100 ml of normal saline given
intravenously over 10-15 min. Note: Paraldehyde should be
administered only with glass syringes.
2. Phenobarbital: 200 mg/ml, should not be given intravenously
except when artificial ventilation is available, and not at
all if the patient normally takes phenobarbital. The maximal
rate of infusion is 100 mg/min to a maximum dose of 15
mg/kg.
3. Lignocaine: 100 mg by slow intravenous injection, followed
by 50-100 mg in 250 ml of 5% dextrose at 1-2 mg/min. Note:
This treatment must be monitored by electrocardigram.
4. Diazepam: 10 mg in 2 ml intravenously, or 40 mg in 500 ml of
5% dextrose at a maximal infusion rate of 100 mg/h.
5. Sodium valproate: 400 mg in 4 ml, or 400-800 mg
intravenously over 3-5 min (up to 10 mg/kg), followed by
intravenous. infusion to a maximum of 2.5 g/day
(unlicensed).
5. Paediatric doses
For children, dosing should be adapted as follows:
Diazepam: 0.2-0.3 mg/kg intravenously
Phenytoin: 10-20 mg/kg intravenously
Chlormethiazole: 5-10 mg/kg per h, equivalent to
0.6-1.25 ml/kg per h
RESUME ET EVALUATION; CONCLUSIONS; RECOMMANDATIONS
Résumé et évaluation
Exposition
L'endrine est un insecticide organochloré utilisé depuis les
années 1950 contre toute sorte de ravageurs, qui s'attaquent notamment
au coton mais également au riz, à la canne à sucre, au maïs et à
d'autres cultures. On l'utilise également comme rodenticide et
avicide. Il est disponible dans le commerce sous forme de poudres, de
granulés, de pâtes et de concentrés émulsionnables.
L'endrine pénètre principalement dans l'atmosphère par
volatilisation et dispersion. En général, la volatilisation se produit
après épandage sur le sol et sur les récoltes et elle est tributaire
de nombreux facteurs comme la teneur en matières organiques et en eau
du sol, l'humidité, les courants aériens et l'aire foliaire des
végétaux.
C'est principalement par lessivage à partir du sol que se produit
la contamination des eaux superficielles. Les précipitations, qu'il
s'agisse de neige ou de pluie, n'ont qu'une part négligeable dans
cette contamination.
Localement, une contamination peut également se produire par
suite du déversement d'effluents industriels ou de négligences dans
les techniques d'épandage.
C'est principalement par suite d'un épandage direct sur les
terrains et les récoltes que l'endrine pénètre dans le sol. Elle peut
y être retenue, transportée ou dégradée, en fonction d'un certain
nombre de facteurs. C'est dans les sols riches en matières organiques
que la rétention est la plus importante. La persistance de l'endrine
dépend dans une large mesure des conditions locales; sa demi-vie dans
le sol peut aller jusqu'à 12 ans. La disparition de l'endrine présente
en surface s'effectue principalement par volatilisation et
photodécomposition. Sous l'influence de la lumière solaire
(rayonnement ultra-violet), l'endrine est isomérisée en delta-
cétoendrine. En présence de lumière solaire intense, on a observé une
isomérisation de 50% de l'endrine en l'espace de sept jours.
L'isomérisation peut également s'effectuer par action microbienne
(champignons et bactéries), notamment en anaérobiose.
Les invertébrés aquatiques et les poissons absorbent rapidement
l'endrine présente dans l'eau mais, transvasés dans une eau non
contaminée, les poissons exposés éliminent sans délai le pesticide. En
cas d'exposition continue, le facteur de bioconcentration peut
atteindre 14-18 000.
Il est possible que les invertébrés terricoles absorbent
facilement l'endrine. La présence occasionnelle de faibles quantités
d'endrine dans l'air ainsi que dans les eaux de surface, notamment
destinées à la consommation, en zone agricole, n'a guère d'importance
au point de vue de la santé publique. La seule vole d'exposition
importante est la voie alimentaire. En général, toutefois, les
quantités ingérées se situent très largement en-dessous de la dose
journalière admissible qui a été fixée à 0,0002 mg/kg de poids
corporel en 1970 (FAO/OMS, 1971).
1.2 Absorption, métabolisme et excrétion
Contrairement à la dieldrine, son stéréoisomère, l'endrine est
rapidement métabolisée par l'organisme animal et, comparativement à
d'autres composés de structure chimique semblable, elle s'accumule
très peu dans les tissus adipeux.
L'absorption et l'excrétion sont rapides après administration
orale à des rats et la demi-vie biologique se situe entre 1 et 6 jours
selon les quantités ingérées. Un régime stationnaire, c'est à dire un
état d'équilibre entre la quantité excrétée et la dose ingérée,
s'établit au bout de 6 jours. On constate une différence entre les
deux sexes en ce sens que les mâles excrètent l'endrine et ses
métabolites par la voie biliaire plus rapidement que les femelles,
d'où une moindre accumulation de pesticides dans les tissus adipeux
des mâles. Les rats excrètent ce composé principalement dans leurs
matières fécales sous forme d'endrine, d' anti-12-hydroxyendrine
ainsi que sous la forme d'un dérivé hydroxyle de l'endrine, en
l'espace de 24 heures (70-75 %); un troisième métabolite, la 12-
cétoendrine, s'accumule dans les tissus. Les lapins excrètent 50% des
métabolites de l'endrine par la voie urinaire, l'excrétion urinaire
n'étant que de 2% chez le rat; dans les matières fécales des lapins,
on ne retrouve que de l'endrine non modifiée.
Des vaches à qui l'on avait administré de l'endrine à raison de
0, 1 mg/kg de nourriture pendant 21 jours en ont excrété jusqu'à 65%
sous forme de métabolites urinaires, 20% sous forme de métabolites
fécaux ou d'endrine non modifiée et 3% dans leur lait, cette fois,
principalement sous forme d'endrine non modifiée. Chez ces vaches, les
résidus atteignaient 0,003-0,006 mg/litre dans le lait, 0,001-0,002
mg/kg dans la viande, et 0,02-0,1 mg/kg dans la graisse.
Chez des poules pondeuses ayant reçu une alimentation additionnée
d'endrine, on a observé des résidus (selon la dose ingérée) qui
atteignaient 0.1 mg/kg dans la chair, 1 mg/kg dans la graisse, 0,2-0,3
mg/kg dans les oeufs (jaune), 0,2 mg/kg dans les reins et 0,5 mg/kg
dans le foie. Sauf dans le cas du foie et des reins, les résidus
présents étaient essentiellement formés d'endrine non modifiée.
Environ 50% de la quantité d'endrine administrée a été excrétée dans
les matières fécales, principalement sous la forme de métabolites.
Chez l'homme, le rat, le lapin, la vache et la poule, le
principal métabolite de l'endrine est l' anti-12-hydroxyendrine,
accompagnée de ses sulfo- et glucuro-conjugués. On trouve également 4
autres métabolites, mais en quantités minimes. Dans les tissus et le
lait on retrouve essentiellement de l'endrine non modifiée. Après
épandage sur des végétaux, on a retrouvé de l'endrine sous forme non
modifiée ainsi que deux produits de transformation hydrophiles.
1.3 Effets sur les êtres vivants dans leur milieu naturel
L'endrine n'exerce que des effets minimes sur les bactéries et
les champignons terricoles. Aux doses de 10-1000 mg/kg de terre, le
composé n'a aucun effet sur la décomposition des matières organiques,
sur la dénitrification ou sur la production de méthane. L'endrine est
très toxique pour les poissons, les invertébrés aquatiques et le
phytoplancton; la CL 50 à 96 h, est dans la plupart des cas
inférieure à 1,0 µg/litre. La dose nocive la plus faible observée au
cours d'un test portant sur le cycle évolutif d'un crevette,
Mysidopsis bahia, était de 30 ng/litre.
Les épreuves de toxicité aiguë effectuées sur des organismes
aquatiques ont été pratiquées dans des aquariums ne comportant pas de
sédiments, on peut penser que la présence de sédiments atténue l'effet
de l'endrine. D'ailleurs la présence de sédiments fortement contaminés
n'a guère eu d'effet sur les espèces de pleine eau, ce qui incite à
penser que l'endrine fixée aux sédiments présente une faible
biodisponibilité. On n'a pas pratiqué d'épreuves sur des animaux
aquatiques vivant dans les sédiments.
Pour les mammifères terrestres et les oiseaux, la DL50 est de
l'ordre de 1,0-10,0 mg/kg de poids corporel. Des canards de l'espèce
Anas platyrhynchos qui avaient reçu pendant 12 semaines de l'endrine
dans leur nourriture à des doses allant jusqu'à 3,0 mg/kg de poids
corporel, n'ont présenté aucun effet délétère que ce soit sur la
ponte, la fécondité ou l'éclosion des oeufs.
Il semblerait que certaines espèces d'invertébrés aquatiques, de
poissons et de petits mammifères résistent à l'action toxique de
l'endrine; d'ailleurs l'exposition à divers pesticides organochlorés
a pu entraîner la sélection de souches résistantes à l'endrine.
Dans des zones où existent des décharges industrielles et où
l'endrine peut être entraînée par ruissellement à partir des champs
traités, on a observé une mortalité parmi les poissons; par ailleurs,
le déclin des populations de pélicans bruns (en Louisiane) et de
caugeks (aux Pays-Bas) a été attribuée à une exposition à l'endrine et
à d'autres dérivés halogénés.
1.4 Effets sur les animaux d'expérience et sur les systèmes in vitro
L'endrine est un pesticide fortement toxique dont les signes
d'intoxication sont de type neurologiques. Chez les animaux de
laboratoire, la DL50 par voie orale de l'endrine de qualité
technique se situe dans les imites de 3-43 mg/kg de poids corporel; la
DL50 dermique va de 5-20 mg/kg de poids corporel pour le rat. Il n'y
a pas de différence notable concernant la toxicité aiguë par voie
orale et percutanée entre le produit technique et les diverses
formulations (concentrés émulsionnables ou poudres mouillables).
Des épreuves de courte durée portant sur la toxicité par voie
orale de l'endrine ont été effectuées sur (les souris, des rats, des
lapins, des chiens et autres animaux domestiques. Chez les souris et
les rats, les doses maximales tolérées ont été respectivement de 5 et
15 mg/kg de nourriture pendant 6 semaines (soit l'équivalent de 0,7
mg/kg de poids corporel). Les rats ont survécus à une dose de 1 mg/kg
de nourriture pendant 16 semaines (soit l'équivalent de 0,05 mg/kg de
poids corporel); les lapins sont morts après avoir reçu à plusieurs
reprises une dose de 1 mg/kg de poids corporel. chez le chien, une
dose de 1 mg/kg de nourriture (soit approximativement 0,025 mg/kg de
poids corporel) administrée sur une période de 2 ans, n'a produit
aucun effet.
Du point de vue neurologique, les signes d'intoxication observés
sont dus à l'inhibition de la fonction de l'acide gamma-aminobutyrique
(GABA) à faible dose. Comme les autres hydrocarbures chlorés
insecticides, l'endrine agit également au niveau du foie et la
stimulation des systèmes enzymatiques intervenant dans le métabolisme
des autres substances chimiques se manifeste, notamment chez la
souris, par une diminution de la durée du sommeil induit par
l'hexobarbital.
Des doses de 75-150 mg/kg appliquées sur l'épiderme des lapins
sous forme de poudre sèche, tous les jours pendant deux heures ont
entraîné des convulsions et la mort chez ces animaux sans toutefois
provoquer d'irritation cutanée. Cette intoxication par voie générale
sans irritation locale mérite d'être signalée.
Des études de toxicité et de cancérogénicité à long terme ont été
effectuées sur des souris et des rats. Aucun effet cancérogène n'a été
observé mais ces études présentaient un certain nombre d'insuffisances
notamment la faible survie des animaux. Lors d'une étude de deux ans
sur des rats traités par de l'endrine administrée dans leur
nourriture, on a estimé à 1 mg/kg de nourriture, soit l'équivalent
d'environ 0,05 mg/kg de poids corporel, la dose sans effets toxiques
observables. Après administration d'endrine avec des quantité
infinitésimales de substances chimiques cancérogènes pour l'animal, il
n'a pas été possible de mettre en évidence d'effet tumoro-promoteur.
Le Groupe de travail en a conclu que les données sont insuffisantes
pour permettre de considérer l'endrine comme cancérogène pour l'homme.
Plusieurs études ont également révélé que l'endrine n'était pas
génotoxique.
Dans la plupart des études, l'endrine s'est révélée non
tératogène pour la souris, le rat ou le hamster, même à des doses
toxiques pour la mère ou le foetus. La dose sans effet nocif
observable a été évaluée à 0,5 mg/kg de poids corporel chez la souris
et le rat et à 0,75 mg/kg de poids corporel chez les hamsters.
L'endrine n'a pas eu d'effets sur la reproduction des rats suivis
pendant trois générations qui en recevaient dans leur nourriture à
raison de 2 mg/kg (soit environ 0, 1 mg/kg de poids corporel).
Un certain nombre de métabolites de l'endrine sont plus ou moins
toxiques que le composé initial. Ainsi la delta-cétoendrine est moins
toxique de l'endrine, en revanche la 12-cétoendrine est considérée
comme le métabolite le plus toxique de l'endrine pour les mammifères,
avec une DL50 par voie orale de 0,8-1,1 mg/kg de poids corporel chez
le rat.
1.5 Effets sur l'homme
Plusieurs cas d'intoxication mortels ou non mortels consécutifs
à un accident ou à une tentative de suicide ont été observés. Les cas
d'intoxication aiguë non mortels résultant d'une, surexposition
accidentelle ont été observés chez les ouvriers d'une usine de
production d'endrine. On estime que par voie orale, la dose mortelle
est (l'environ 10 mg/kg de poids corporel, une dose unique prise par
voie orale de 0,25-1,0 mg/kg de poids corporel peut provoquer des
convulsions.
C'est au niveau du système nerveux central que l'endrine exerce
principalement son action. Après exposition à dose toxique, des signes
d'intoxication peuvent faire leur apparition et se manifestent sous la
forme d'un hyperexcitabilité et de convulsions, la mort pouvant
survenir dans les 2-12 heures suivant l'exposition si un traitement
approprié n'est pas institué immédiatement. En revanche, après une
intoxication non mortelle, la récupération est rapide et complète.
L'endrine ne s'accumule pas dans le corps humain de manière
importante. Chez 232 travailleurs exposés de par leur profession, on
n'a pas constaté d'effets indésirables à long terme (durée
d'exposition 4-27 ans) lors des examens médicaux pratiqués (durée de
l'observation 2-29 ans). Le seul effet observé, indirectement
d'ailleurs, consistait en une stimulation réversible des enzymes
pharmacométabolisantes.
Des analyses ont été pratiquées dans de nombreux pays sur un
grand nombre d'échantillons de tissus adipeux, de sang et de lait
maternel sans qu'il soit possible de mettre en évidence la présence
d'endrine. Le Groupe de travail attribue l'absence d'endrine dans ces
échantillons à la faible exposition de la population général à ce
pesticide et à sa métabolisation rapide.
En revanche la présence d'endrine a été décelée dans le sang (à
des concentrations atteignant 450 µg/litre) et dans les tissus adipeux
(à la dose de 89,5 mg/kg) chez les personnes décédées d'une
intoxication accidentelle. Dans les conditions normales, on n'a pas
retrouvé d'endrine chez les travailleurs exposés. Le seuil
d'apparition des symptômes d'intoxication est estimé à 50-100 µg/litre
de sang. On pense que la demi-vie de l'endrine dans le sang est de
l'ordre de 24 heures.
2. Conclusions
L'endrine est un 'Insecticide qui présente une très forte
toxicité aiguë. Il peut entraîner des intoxications graves en cas
d'exposition excessive due à une manipulation négligente lors de sa
production, de son utilisation ou par suite de la consommation
d'aliments contaminés. L'exposition de la population générale est
principalement due à la présence de résidus dans les denrées
alimentaires; toutefois on estime que la quantité d'endrine ingérée
est en général très inférieure à la dose journalière admissible fixée
par le Comité FAO/OMS d'experts des résidus de pesticides. Il n'y a
pas de danger pour la population générale qui résulterait d'une
exposition de ce genre à l'endrine. Moyennant de bonnes méthodes de
travail et le respect des mesure d'hygiène et de sécurité, l'endrine
ne devrait pas constituer un danger pour les ouvriers exposés.
Il est évident que des rejets incontrôlés d'endrine lors de la
production, de la formulation et de l'utilisation de ce pesticide
peuvent créer des problèmes écologiques dus à sa forte toxicité. Il
n'est pas possible d'être aussi catégorique en ce qui concerne les
effets que peut avoir son utilisation en agriculture sur la faune et
la flore, encore que l'entraînement par ruissellement du pesticide
puisse constituer une menace pour les poissons et les oiseaux
piscivores. Le déclin des populations de certaines espèces d'oiseaux
a été attribué à la présence de résidus élevés de divers organochlorés
dans les tissus des adultes et dans les oeufs. On a procédé au dosage
de l'endrine chez certaines de ces espèces; toutefois il est difficile
de faire la part des différents organochlorés qui sont en cause.
3. Recommandations
1. L'endrine ne doit être utilisée qu'en cas de nécessité et
seulement lorsqu'il n'existe pas d'autre produit moins
toxique.
2. Afin de préserver la santé et le bien-être des travailleurs
et de la population générale, on ne doit confier la
manipulation et l'épandage qu'à des personnes bien encadrées
et bien formées qui appliqueront des mesures de sécurité
convenables et épandront le produit conformément aux règles
de bonne pratique en la matière.
3. Il convient de s'entourer de toute les précautions
nécessaires lors de la production, de la formulation, de
l'utilisation en agriculture et du rejet de l'endrine afin
de contaminer le moins possible l'environnement et en
particulier les eaux de surface.
4. Les personnes qui sont habituellement exposées à l'endrine
doivent subir des examens médicaux périodiques.
5. Il faut poursuivre l'étude épidémiologique des travailleurs
exposés.
6. Dans les pays où l'on utilise encore de l'endrine, on devra
contrôler la présence de résidus d'endrine dans les denrées
alimentaires.
7. Au cas où l'on continuerait à utiliser de l'endrine, il
faudrait obtenir davantage de données sur la présence, la
destinée ultime et la toxicité de la 12-cétoendrine et de la
delta-cétoendrine.
RESUMEN Y EVALUACION; CONCLUSIONES; RECOMENDACIONES
1. Resumen y evaluación
1.1 Exposición
La endrina es un insecticida organoclorado que se utiliza desde
los años cincuenta para combatir muy diversas plagas agricolas, sobre
todo en el algodón aunque también en el arroz, la caña de azúcar, el
maíz y otros cultivos. Se utiliza asimismo como rodenticida. En el
comercio se encuentra en forma de polvos, gránulos, pastas y
concentrado emulsionable.
La endrina se incorpora al aire principalmente por volatilización
y arrastre aéreo. En general, la volatilización tiene lugar después de
aplicarla a suelos y cultivos y depende de muchos factores, como el
contenido de materia orgánica y agua del suelo, la humedad, el flujo
de aire y la superficie cultivada.
La vía más importante de contaminación de las aguas de superficie
es la escorrentía desde el suelo. La contaminación por precipitación
en forma de nieve o lluvia es insignificante. Puede producirse
contaminación local del medio debida a efluentes industriales y
prácticas de aplicación poco meticulosas.
La principal fuente de endrina en el suelo es la aplicación
directa a éste y a los cultivos. Puede quedar retenida, ser
transportada o degradarse en el suelo, atendiendo a diversos factores.
La retención más intensa se produce en suelos con contenido elevado de
materia orgánica. La persistencia de la endrina depende en gran medida
de las condiciones locales; su semivida en el suelo puede llegar a los
12 años. La volatilización y la fotodescomposición son los principales
factores de la desaparición de la endrina de las superficies del
suelo. La luz del sol (luz ultravioleta) induce la formación del
isómero delta-cetoendrina. En verano, bajo insolación intensa, se
observó que alrededor del 50% de la endrina se isomerizaba a esta
cetoendrina en un plazo de siete días. Se produce transformación
microbiana (en hongos y bacterias), especialmente en condiciones
anaerobias, originándose la misma sustancia.
Los invertebrados acuáticos y los peces absorben rápidamente la
endrina a partir del agua, si bien los peces expuestos transferidos a
agua no contaminada pierden el plaguicida rápidamente. Se han
registrado factores de bioconcentración de 14-18 000 tras una
exposición continua. Los invertebrados del suelo también absorben
fácilmente el compuesto.
La presencia ocasional de niveles reducidos de endrina en el aire
y en las aguas de superficie y de bebida en zonas agrícolas reviste
escasa importancia desde el punto de vista de la salud pública. La
única exposición que merece consideración es la ingesta en la dieta.
En general, no obstante, los niveles comunicados de ingesta se
encuentran muy por debajo de la ingesta diaria admisible de 0,0002
mg/kg de peso corporal, establecida en 1970 (FAO/OMS, 1971).
1.2 Absorción, metabolismo y excreción
A diferencia de la dieldrina, su estereoisómero, la endrina se
metaboliza rápidamente en los animales, y se acumula en muy pequeña
cantidad en las grasas en comparación con compuestos de estructura
química análoga.
En la rata, tanto la absorción como la excreción tras la
administración oral se producen rápidamente; su semivida biológica es
de 1-6 días, según la dosis administrada. Al cabo de 6 días se alcanza
un estado de equilibrio en el que la cantidad excretada es igual a la
ingesta diaria. Se observan diferencias de un sexo a otro: los machos
excretan endrina y metabolitos con la bilis mucho más deprisa que las
hembras, lo que produce una acumulación menor en el tejido adiposo de
aquéllos. Las ratas excretan este compuesto principalmente en las
heces en forma de endrina, anti-12-hidroxiendrina, y un derivado
hidroxilado durante las primeras horas (70-75%); un tercer metabolito,
la 12-cetoendrina, se acumula enlos tejidos. El conejo excreta el 50%
de los metabolitos del compuesto enla orina, mientras que en la rata
sólo el 2% se excreta por esta vía; en lasheces del conejo sólo se
detecta endrina sin alterar.
Las vacas a las que se administró endrina a razón de 0,1 mg/kg de
la dieta durante 21 días excretaron hasta el 65% en forma de
metabolitos en la orina, el 20% en las heces, parcialmente en forma de
endrina no alterada, y el 3% en la leche, también principalmente en
forma de endrina. Estas vacas presentaron niveles residuales de
0,003-0,006 mg/litro en la leche, 0,001-0,002 mg/kg en la carne, y
0,02-0,1 mg/kg en la grasa.
En gallinas ponedoras a las que se administró endrina por vía
oral seobservaron niveles residuales (dependientes de la dosis
administrada) de hasta 0,1 mg/kg en la carne, 1 mg/kg en la grasa,
0,2-0,3 mg/kg en los huevos (yema), 0,2 mg/kg en el riñón y 0,5 mg/kg
en el hígado. Salvo en el hígado y el riñón, los residuos encontrados
estaban formados principalmente por endrina no alterada. Alrededor del
50% de la endrina administrada se excretó en las heces, principalmente
en forma de metabolitos.
En el ser humano, la rata, el conejo, la vaca y la gallina, el
principal metabolito biotransformado de la endrina es la
anti-12-hidroxiendrina, junto con su sulfato y su glucur nido
conjugados. Se encontraron cuatro metabolitos más, si bien en
cantidades muy reducidas. En los tejidos corporales y en la leche se
encuentra sobre todo endrina inalterada. Tras la aplicación de este
plaguicida a plantas, seidentificaron endrina inalterada y dos
productos de transformación hidrófilos.
1.3 Efectos en los organismos del medio ambiente
El efecto de la endrina en las bacterias y los hongos del suelo
es mínimo. Con dosis de 10-1000 mg/kg de suelo no se observó efecto
alguno en la descomposición de materia orgánica, la desnitrificación
ni la generación de metano. La endrina es sumamente tóxica para los
peces, los invertebrados acuáticos y el fitoplancton: los valores de
la CL50 a las 96 horas se encuentran en su mayoría por debajo de 1,0
µg/litro. El nivel sin observación de efectos más bajo en un ensayo de
ciclo biológico del crustáceo Mysidopsis bahia se fijó en 30 ng/litro.
Los ensayos comunicados sobre la toxicidad aguda de la endrina
para los organismos acuáticos se llevaron a cabo en acuarios sin
sedimentos; cabría esperar que la presencia de sedimentos atenuara el
efecto del insecticida. Los sedimentos muy contaminados ejercieron
escaso efecto en las especies de aguas libres, lo que indica que la
endrina ligada a los sedimentos tiene una biodisponibilidad reducida.
Aún no se han llevado a cabo ensayos en animales acuáticos que viven
en los sedimentos.
La DL50 para mamíferos terrestres y aves oscila entre 1,0 y
10,0 mg/kg de peso corporal. Los patos silvestres a los que se
administraron 3,0 mg/kg de peso corporal durante 12 semanas no
mostraron efecto alguno en la producción de huevos, la fertilidad o la
eclosión.
Ciertas especies de invertebrados acuáticos, peces y mamíferos de
pequeño tama o son resistentes a la toxicidad de la endrina; la
exposición a diversos plaguicidas organoclorados llevó a la selección
de estirpes resistentes a la endrina.
Se observaron muertes masivas de peces en zonas de escorrentía
agrícola y descargas industriales; el declive de las poblaciones de
pelícanos pardos (en Luisiana, EE.UU.) y de golondrinas de mar
(Thalasseus sandvicensis) en los Países Bajos se ha atribuido a la
exposición a la endrina en combinación con otras sustancias químicas
halogenadas.
1.4 Efectos en animales de experimentación in vitro
La endrina es un plaguicida sumamente tóxico; los signos de
intoxicación son de carácter neurotóxico. La DL50 por vía oral de la
endrina de calidad técnica en animales de laboratorio oscila entre 3
y 43 mg/kg de peso corporal; la DL50 por vía cutánea en la rata es
de 5-20 mg/kg peso corporal. No se ha encontrado ninguna diferencia en
la toxicidad aguda por vía oral o cutánea entre los productos de
calidad técnica y los formulados (concentrado emulsionable y polvos
humectables).
Se han llevado a cabo experimentos de breve duración para
estudiar la toxicidad por vía oral en el ratón, la rata, el conejo, el
perro y animales domésticos. En el ratón y la rata, las dosis máximas
toleradas durante 6 semanas fueron 5 y 15 mg/kg de la dieta
(equivalentes a 0,7 mg/kg de peso corporal), respectivamente. Las
ratas sobrevivieron tras una exposición a 1 mg/kg de la dieta
(equivalente a 0,05 mg/kg de peso corporal) durante 16 semanas; los
conejos murieron tras recibir dosis repetidas de 1 mg/kg de peso
corporal. En el perro, no se observó efecto alguno tras la
administración de 1 mg/kg de la dieta (equivalente aproximadamente a
0,025 mg/kg de peso corporal) durante más de 2 años.
La base neuroógica de los signos de intoxicación observados es la
inhibición de la función del ácido gamma-aminobutírico (GABA) con
dosis reducidas. Al igual que otros insecticidas a base de
hidrocarburos clorados, la endrina afecta también al hígado, y se
observa claramente la estimulación de sistemas enzimáticos que
participan en el metabolismo de otras sustancias químicas, como lo
demuestra, por ejemplo, la menor duración del sueño por hexobarbital
en el ratón.
Con dosis de 75-150 mg/kg aplicadas por vía cutánea en forma de
polvo seco durante 2 horas al día se produjeron convulsiones y la
muerte en el conejo pero sin irritación cutánea. Esta toxicidad
sistémica sin irritación en el lugar de contacto resulta muy notable.
Se han llevado a cabo en ratones y ratas estudios prolongados de
toxicidad y carcinogenicidad. No se observó efecto carcinogénico, pero
estos estudios tenían ciertos defectos, entre ellos la reducida
supervivencia de los animales. El nivel sin observación de efectos en
cuanto a la toxicidad en un estudio de dos años de duración en la rata
fue de 1 mg/kg de la dieta (equivalente a unos 0,05 mg/kg de peso
corporal). No se demostró ningún efecto de favorecimiento de tumores
cuando se ensayó la endrina en combinación con cantidades submínimas
de sustancias químicas de conocido efecto carcinogénico en los
animales. El Grupo de Trabajo concluyó que los datos de que se dispone
no bastan para indicar que la endrina supone un riesgo carcinogénico
para el ser humano.
En varios estudios se observó que la endrina no es genotóxica.
En la mayoría de los estudios no resultó teratogénica para el
ratón, la rata o el hámster, ni siquiera con dosis suficientes para
provocar toxicidad materna o fetal. El nivel sin observación de
efectos adversos fue de 0,5 mg/kg de peso corporal en ratones y ratas
y de 0,75 mg/kg de peso corporal en el hámster. La endrina no indujo
efecto alguno en la reproducción de ratas estudiadas durante tres
generaciones cuando se administró a razón de 2 mg/kg de la dieta (unos
0,1 mg/kg de peso corporal).
Algunos metabolitos de la endrina tienen toxicidades agudas
iguales o más altas que el compuesto originario. El producto de
transformación, la delta-cetoendrina, es menos tóxico que la endrina,
pero la 12-cetoendrina se considera el metabolito más tóxico en los
mamíferos, con una DL50 por vía oral en la rata de 0,8-1,1 mg/kg de
peso corporal.
1.5 Efectos en el ser humano
Se han producido varios episodios de intoxicación mortal y no
mortal, tanto accidentales como suicidas. Los casos de intoxicación
aguda no mortal debida a exposición excesiva accidental se observaron
en trabajadores de una planta de fabricación de endrina. Se ha
calculado que la dosis que por vía oral provoca la muerte es de
aproximadamente 10 mg/kg de peso corporal; la dosis única por vía oral
que provoca convulsiones se fijó en 0,25-1,0 mg/kg de peso corporal.
El lugar principal de acción de la endrina es el sistema nervioso
central. La exposición del ser humano a una dosis tóxica puede
producir al cabo de pocas horas signos y síntomas de intoxicación
tales como excitabilidad y convulsiones; la muerte puede producirse en
las 2-12 horas que siguen a la exposición si no se administra
inmediatamente el tratamiento apropiado. La recuperación después de
una intoxicación no mortal es rápida y completa.
La endrina no se acumula en el cuerpo humano en grado
significativo. No se comunicaron efectos adversos a largo plazo en 232
trabajadores expuestos (duración de la exposición: 4-27 años) bajo
supervisión médica (tiempo de observación: 4-29 años). El único efecto
observado fueron pruebas indirectas de una estimulación reversible de
las enzimas metabolizadoras de fármacos.
No se detectó endrina en prácticamente ninguna muestra de tejido
adiposo, sangre y leche humana analizadas en numerosos países. El
Grupo de Trabajo atribuyó la ausencia de endrina en las muestras
humanas a la baja exposición de la población general a este plaguicida
y a su rápido metabolismo.
La endrina se detectó en la sangre (con concentraciones de hasta
450 µg/litro) y en el tejido adiposo (en concentraciones de 89,5
mg/kg) en casos de envenenamiento accidental mortal. No se encontró
endrina en los trabajadores en circunstancias normales. El nivel
umbral de endrina en la sangre por debajo del cual no se produce
ningún signo o síntoma de intoxicación se ha fijado en 50-100
µg/litro. La semivida de la endrina en la sangre puede ser del orden
de 24 horas.
2. Conclusiones
La endrina es un insecticida con elevada toxicidad aguda. Puede
provocar envenenamiento grave en casos de exposición excesiva
provocada por un manejo poco meticuloso durante su fabricación y uso
o por el consumo de alimentos contaminados. El público está expuesto
a la endrina principalmente por sus residuos en los alimentos; no
obstante, los niveles de ingesta de endrina que se han comunicado
están por lo general muy por debajo de la ingesta diaria admisible
establecida por la FAO/OMS. Esas exposiciones en principio no
constituyen un riesgo para la salud de la población general. Cuando se
aplican buenas prácticas de trabajo, medidas higiénicas y precauciones
de seguridad, es poco probable que la endrina suponga un riesgo para
los trabajadores expuestos.
Está claro que las descargas no controladas de endrina durante su
manufactura, formulación y uso pueden originar graves problemas
ambientales asociados a su elevada toxicidad. Los efectos del uso
agrícola del insecticida en la fauna y la flora están menos claros, si
bien los peces y las aves ictívoras están expuestos por la escorrentía
a partir de las superficies. El declive de las poblaciones de algunas
especies de aves se ha atribuido a la presencia de niveles elevados de
residuos de diversos compuestos organoclorados en los tejidos de
adultos y en los huevos. Se ha medido la endrina presente en algunas
de estas especies, pero es muy difícil separar los efectos de los
distintos compuestos organoclorados presentes.
3. Recomendaciones
1. No debe utilizarse la endrina a menos que sea indispensable
y sólo cuando no se disponga de una alternativa menos
tóxica.
2. Para la salud y el bienestar de los trabajadores y de la
población general, el manejo y el uso de la endrina se
confiarán sólo a operarios bien supervisados y adiestrados
que apliquen las medidas de seguridad adecuadas y utilicen
la endrina de acuerdo con las prácticas agrícolas correctas.
3. La fabricación, la formulación, el uso agrícola y la
evacuación de endrina se tratarán cuidadosamente para
reducir al mínimo la contaminación del medio, en particular
de las aguas de superficie.
4. Las personas expuestas regularmente a la endrina deben
someterse a revisiones médicas periódicas.
5. Proseguirán los estudios epidemiológicos sobre las
poblaciones de trabajadores expuestos.
6. En los países en los que aún se usa la endrina, deben
vigilarse sus residuos en los alimentos.
7. Si sigue utilizándose la endrina, debe obtenerse más
información sobre la presencia, el destino último y la
toxicidad de la 12-cetoendrina y la delta-cetoendrina.
