
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 128
CHLOROBENZENES OTHER THAN HEXACHLOROBENZENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms M.E. Meek and Ms M.J. Giddings,
Health and Welfare Canada
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1991
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Chlorobenzenes other than hexachlorobenzene
(Environmental health criteria: 128)
1. Chlorobenzenes - adverse effects
2. Chlorobenzenes - toxicity
3. Environmental exposure
4. Environmental pollutants I. Series
ISBN 92 4 157128 4 (NLM Classification QV 633)
ISSN 0250-863X
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the impression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of every country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Sources of human and environmental exposure
1.2.1. Production figures
1.2.2. Uses
1.2.3. Release of chlorobenzenes into the environment
1.3. Environmental transport, distribution, and transformation
1.3.1. Degradation
1.3.2. Fate
1.4. Environmental levels and human exposure
1.4.1. Chlorobenzenes in the environment
1.4.2. Human exposure
1.4.2.1 General population
1.4.2.2 Occupational
1.5. Kinetics and metabolism
1.6. Effects on aquatic organisms in the environment
1.7. Effects on experimental animals and in vitro systems
1.8. Effects on humans
1.8.1. General population
1.8.2. Occupational exposure
1.9. Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.3. Organoleptic properties
2.4. Conversion factors
2.5. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production
3.2.2. Uses
3.2.3. Sources in the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution
4.2. Persistence and fate
4.2.1. Persistence
4.2.2. Abiotic degradation
4.2.2.1 Photolysis
4.2.2.2 Hydrolytic and oxidative reactions
4.2.3. Biodegradation and biotransformation
4.2.4. Bioaccumulation
4.2.5. Biomagnification
4.2.6. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food
5.1.5. Human milk
5.1.6. Consumer products
5.2. Human exposure from all sources
5.2.1. General population
5.2.2. Occupational exposure
5.3. Human monitoring data
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Binding to protein
6.6. Effects on metabolizing enzymes
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.1.1. Bacteria and protozoa
7.1.2. Unicellular algae
7.2. Aquatic organisms
7.2.1. Plants
7.2.2. Invertebrates
7.2.3. Fish
7.3. Terrestrial biota
7.4. Model ecosystems
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Skin and eye irritation, skin sensitization
8.3. Short-term exposures
8.4. Long-term exposures
8.5. Chronic toxicity and carcinogenicity
8.6. Mutagenicity and related endpoints
8.6.1. In vitro systems
8.6.2. In vivo tests on experimental animals
8.6.3. Human in vivo studies
8.7. Developmental and reproductive effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.1.1. General population exposure
9.1.2. Occupational exposure
9.2. Epidemiological Studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure of the general population
10.1.2. Occupational exposure
10.1.3. Toxic effects
10.1.4. Risk evaluation
10.1.4.1 General population
10.1.4.2 Occupationally exposed population
10.2. Evaluation of effects on the environment
10.2.1. Levels of exposure
10.2.2. Fate
10.2.3. Bioavailability and bioaccumulation
10.2.4. Degradation
10.2.5. Persistence
10.2.6. Toxic effects on organisms
10.2.7. Risk evaluation
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations
11.2.1. Public health measures
11.2.2. Human health risk evaluation
11.2.3. Environmental risk evaluation
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROBENZENES
OTHER THAN HEXACHLOROBENZENE
Members
Dr U. G. Ahlborg, Karolinska Institute, Institute of Environmental
Medicine, General Toxicology, Stockholm, Sweden
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, Cambridgeshire, England
(Vice-Chairman)
Dr P. E. T. Douben, Research Institute for Nature Management, Arnhem,
Netherlands
Dr R. J. Fielder, Department of Health, MED TEH Division, Hannibal
House, London, England
Dr R. A. Jedrychowski, Institute of Occupational Medicine, Lodz,
Poland
Dr S. K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India (Chairman)
Dr T. Lakhanisky, Institut d'Hygiène et d'Epidémiologie, Brussels,
Belgium
Dr D. C. Villeneuve, Health Protection Branch, Environmental Health
Centre, Tunneys Pasture, Ottawa, Ontario, Canada
Dr R. S. H. Yang, National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA (present address: College
of Veterinary Medicine and Biomedical Sciences, Colorado State
University, Fort Collins, Colorado, USA)
Observers
Dr L. Caillard, Rhone-Poulenc, Service Toxicologie, Les Miroirs,
Paris, France
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA (Secretary)
Ms M.J. Giddings, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Tunneys Pasture, Ottawa, Ontario,
Canada (Temporary Adviser, Co-Rapporteur)
Ms M.E. Meek, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Tunneys Pasture, Ottawa, Ontario,
Canada (Temporary Adviser, Co-Rapporteur)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROBENZENES
OTHER THAN HEXACHLOROBENZENE
A WHO Task Group on Environmental Health Criteria for Chlorobenzenes
other than Hexachlorobenzene met at the Institut d'Hygiène et
d'Epidémiologie, Brussels, Belgium, from 25 to 29 June 1990. Dr T.
Lakhanisky opened the meeting and welcomed the Members on behalf of
the host institute, and on behalf of the Ministère de la Santé
Publique et de l'Environnement, who sponsored the meeting. Dr G.C.
Becking addressed the meeting on behalf of the three cooperating
organizations of the IPCS (UNEP, ILO, WHO). The Task Group reviewed
and revised the draft criteria document, and made an evaluation of the
risks for human health and the environment from exposure to
chlorobenzenes other than hexachlorobenzene.
The drafts of this document were prepared by Ms M.E. Meek and Ms M.J.
Giddings, Health and Welfare Canada, Health Protection Branch, Ottawa,
Canada. Dr G.C. Becking, IPCS Interregional Research Unit, WHO,
Research Triangle Park, North Carolina, was responsible for the
overall scientific content of the document, and Mrs M.O. Head, Oxford,
England, for the editing.
The Secretariat wishes to acknowledge the extensive comments from: Dr
U. Schlottmann, Federal Ministry of the Environment, Germany
(chemistry and environmental effects), and Dr R. Fielder, Department
of Health, United Kingdom (effects on experimental animals), during
the initial review of the document.
Dr S. Dobson, Co-Chairman of the Task Group, and Dr P.E.T. Douben
deserve special thanks for their significant contributions and
revisions of the draft document during the meeting, particularly the
sections dealing with environmental effects.
The efforts of all who helped in the preparation and finalization of
this publication are gratefully acknowledged.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400/7985850).
1. SUMMARY
This publication focuses on the risks for human health and the
environment from exposures to: monochlorobenzene (MCB);
dichlorobenzenes (DCB); trichlorobenzenes (TCB); tetrachloro-benzenes
(TeCB); and pentachlorobenzene (PeCB). Chlorine substitution is
indicated as follows: 1,2-dichlorobenzene (1,2-DCB);
1,2,3-trichlorobenzene (1,2,3-TCB), etc.
1.1 Identity, Physical and Chemical Properties, Analytical Methods
Chlorobenzenes are cyclic aromatic compounds formed by the addition of
1-6 atoms of chlorine to the benzene ring. This yields 12 compounds:
monochlorobenzene, three isomeric forms each of di-, tri-, and
tetrachlorobenzenes, as well as penta- and hexachlorobenzenes.
Chlorobenzenes are white crystalline solids at room temperature,
except for MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB, which are colourless
liquids. In general, the water solubility of chlorobenzene compounds
is low, decreasing with increased chlorination. Flammability is low,
the octanol/water partition coefficients are moderate to high,
increasing with increasing chlorination, and the vapour pressures are
low to moderate, decreasing with increasing chlorination. The taste
and odour thresholds are low, particularly for the lower chlorinated
compounds.
Commercial chlorobenzenes, even when purified, contain various amounts
of closely related isomers. For example, pure MCB may contain as much
as 0.05 % benzene and 0.1 % DCBs, while technical 1,2-DCB may contain
up to 19 % of the other DCBs, 1 % TCBs, and up to 0.05 % MCB. No
evidence of contamination by polychlorinated dibenzo- p-dioxins
(PCDDs) and dibenzofurans (PCDFs) has been reported.
A large number of sampling techniques have been developed for
chlorobenzenes, depending on the medium. These range from solvent
extraction procedures for aqueous media, to the use of absorbents for
airborne compounds. The analytical technique of choice for the
determination of chlorobenzenes in environmental samples is gas-liquid
chromatography (GLC).
1.2 Sources of Human and Environmental Exposure
1.2.1 Production figures
Available data on chlorobenzene production levels are from the period
1980-83, when global production was estimated to be 568 x 106 kg,
though the use of chlorobenzenes has declined in some countries since
then. About 50 % of this amount was manufactured within the USA and
the remainder primarily in Western Europe and Japan. MCB accounted for
70 % of the global production, 1,2-DCB, 1,4-DCB, and 1,2,4-TCB being
produced at 22 x 106, 24 x 106, and 1.2-3.7 x 106 kg,
respectively.
MCB and DCBs are produced by the direct chlorination of benzene in the
liquid phase, using a catalyst, while TCBs and TeCBs are produced by
the direct chlorination of appropriate chlorobenzene isomers, in the
presence of a metal catalyst.
1.2.2 Uses
Chlorobenzenes are used mainly as intermediates in the synthesis of
pesticides and other chemicals; 1,4-DCB is used in space deodorants
and as a moth repellent. The higher chlorinated benzenes (TCBs and
1,2,3,4-TeCB) have been used as components of dielectric fluids.
1.2.3 Release of chlorobenzenes into the environment
The release of chlorobenzenes into the environment occurs primarily
during manufacture, and through the dispersive nature of their uses.
For example, in the USA, between 0.1 and 0.2 % of the 1983 production
of 130 x 106 kg of MCB was estimated to have been lost to the
environment. Releases of chlorobenzenes from waste disposal, including
incineration of municipal waste, are much lower. However, the
incineration of chlorobenzenes may lead to the emission of PCDDs and
PCDFs.
1.3 Environmental Transport, Distribution, and Transformation
1.3.1 Degradation
Chlorobenzenes are removed from the environment principally by
biological, and, to a lesser extent, by non-biological mechanisms;
however, they are considered moderately persistent in water, air, and
sediments. Residence times in water of 1 day in rivers and over 100
days in ground water have been reported. In air, chemical and
photolytic reactions are presumed to be the predominant pathways for
chlorobenzene degradation, with residence times in the range of 13-116
days reported for MCB, DCBs, and an unspecified TCB isomer.
Many microorganisms from sediments and sewage sludge have been shown
to degrade chlorobenzenes. It would appear that the higher chlorinated
compounds are less readily degraded, and such degradation occurs only
under aerobic conditions. Under anaerobic conditions in soil and
ground water, DCB, TCBs, and PeCBs are usually resistant to microbial
degradation.
1.3.2 Fate
Chlorobenzenes released into the aquatic environment will be
redistributed preferentially to the air and to sediment (particularly
organically rich sediments). Limited information has shown that levels
1000 times those found in water have been detected in sediments,
particularly in highly industrialized regions. Retention of
chlorobenzenes in soil increases with the organic content of the soil;
there is a positive correlation between the degree of chlorination of
the compound and its adsorption on organic matter. Limited evidence is
available showing that sediment-bound residues are bioavailable to
organisms; i.e., aquatic invertebrates can take up residues from
sediment, and plants, from soil.
1.4 Environmental Levels and Human Exposure
1.4.1 Chlorobenzenes in the environment
Mean levels of chlorobenzenes (mono- to tri-) in ambient air are of
the order of 0.1 µg/m3, with maximum levels of up to 100 µg/m3. No
data are available on levels of TeCB and PeCB in ambient air, though
these chemicals have been detected in fly ash from municipal
incinerators. Levels of chlorobenzenes in indoor air are similar to
those in ambient air; however, levels much higher than those in the
ambient air have been reported in heavily polluted areas, and in
enclosed spaces where chlorobenzene-containing products have been
used.
Chlorobenzenes (mono- to penta-) have been detected in surface waters
in the ng/litre-µg/litre range, with occasional levels of up to tenths
of one mg/litre reported near industrial sources. Levels of
chlorobenzenes in industrial waste waters may be higher and vary
according to the nature of the processes used.
All chlorobenzene congeners have been detected in the drinking-water
samples analysed. The lower chlorinated compounds were found most
frequently and in the highest concentrations, with the 1,4-DCB isomer
predominating; however, the mean concentrations of any chlorobenzene
detected have generally been less than 1 µg/litre and have rarely
exceeded 50 µg/litre.
Data from well-designed monitoring programmes on chlorobenzene levels
in food have not been found; available information has mainly been
confined to concentrations in fish in the vicinity of industrial
sources and to isolated incidents of contamination of meat products.
All chlorobenzene isomers (mono- to penta-) were detected in
freshwater trout, with levels ranging from 0.1 to 16 µg/kg. In another
study, levels of total chlorobenzenes in freshwater fish varied from
a mean of 0.2 mg/kg fat in lightly polluted areas to 1.8 mg/kg fat in
an industrialized area. There is some indication that concentrations
of chlorobenzenes in freshwater fish increase with increasing degree
of chlorination of the compound. The few studies available indicate
levels of 1,4-DCB in some marine fish of 0.05 mg/kg (wet weight).
In the available studies on chlorobenzene levels in meat and milk,
limited primarily to samples from contaminated areas, concentrations
of 0.02-5 µg/kg have been reported.
In 2 surveys of human milk, the levels of all chlorobenzene congeners,
except MCB, were quantified. In one study, the levels of DCBs averaged
25 µg/kg milk, whereas the TCB and TeCB isomers and PeCB were found at
mean levels of less than 5 µg/kg milk. Levels in the second survey
were much lower, mean concentrations ranging from 1 µg/kg (1,2,3-TCB
and PeCB) to a maximum of 6 µg/kg (1,3- and 1,4-dichlorobenzene).
1.4.2 Human exposure
1.4.2.1 General population
On the basis of limited data, the daily intake of chlorobenzenes
within the general population appears to be greatest from air,
particularly for the lower, more volatile compounds (0.2-0.9 µ/kg body
weight). Intake from food compared with that from other sources
increases with increasing degree of chlorination; food contributes a
greater percentage of the total daily intake of TeCBs and PeCB than
air. However, exposure levels for such congeners are likely to be less
than 0.05 µg/kg body weight. A limited number of studies have shown
that, on a body weight basis, breast-fed infants may receive a higher
dose of chlorobenzenes than members of the adult population.
1.4.2.2 Occupational
It is not possible to make an accurate quantification of occupational
exposure to chlorobenzenes on the basis of available data. However,
levels of 1,4-DCB ranged between 42 and 288 mg/m3 in one plant, and
levels of MCB of up to 18.7 mg/m3 were found in other chemical
plants.
1.5 Kinetics and Metabolism
All chlorobenzenes appear to be absorbed readily from the
gastrointestinal and respiratory tracts in humans and experimental
animals, with absorption influenced by the position of the chlorine in
different isomers of the same congener. The chlorobenzenes are less
readily absorbed through the skin.
After rapid distribution to highly perfused organs in experimental
animals, absorbed chlorobenzenes accumulate primarily in the fatty
tissue, with smaller amounts in the liver and other organs.
Chlorobenzenes have been shown to cross the placenta, and have been
found in the fetal brain. In general, accumulation is greater for the
more highly chlorinated congeners. There is considerable variation,
however, in the accumulation of different isomers of the same
congener.
In both humans and experimental animals, the metabolism of
chlorobenzenes proceeds via microsomal oxidation to the corresponding
chlorophenol. These chlorophenols can be excreted in the urine as
mercapturic acids, or as glucuronic acid or sulfate conjugates. TeCB
and PeCB are metabolized at a slower rate and remain in the tissues
for longer periods than the monochloro- to trichloro- congeners. Some
of the chlorobenzenes induce a wide range of enzyme systems including
those involved in oxidative, reductive, conjugation, and hydrolytic
pathways.
In general, elimination of the higher chlorinated benzenes is slower
than that of the MCB and DCB congeners, and a greater proportion of
the tri- to penta- congeners are eliminated unchanged in the faeces.
For example, 17% of a dose of 1,2,4-TCB was eliminated in the faeces
after 7 days, whereas 91-97% of 1,4-DCB was eliminated as metabolites
in the urine after 5 days. The position of the chlorine atoms on the
benzene ring is also an important determinant of the rate of
metabolism and elimination, the isomers with two adjacent
unsubstituted carbon atoms being more rapidly metabolized and
eliminated.
1.6 Effects on Aquatic Organisms in the Environment
Available information on the effects of chlorobenzenes on the
environment is mainly focused on acute effects on aquatic organisms.
In general, toxicity increases with the degree of chlorination of the
benzene ring. While MCB, 1,2-DCB, 1,3-DCB, 1,2,4-TCB, 1,3,5-TCB, and
1,2,4,5-TeCB all exhibit a low toxicity for microorganisms, the
toxicity of the TCBs and TeCBs is, with the exception of 1,2,4,5-TeCB,
slightly higher than that of the other compounds; in unicellular
aquatic algae, EC50 values for 96-h cell growth or chlorophyll a
production ranged from over 300 mg/litre for MCB to approximately 1
mg/litre for 1,2,3,5-TeCB. Some aquatic invertebrates appear more
sensitive to chlorobenzenes, but levels required for 48- or 96-h
lethality are still near, or well above, 1 mg/litre (e.g., Daphnia
magna at 2.4 mg/litre for 1,2-DCB, and up to 530 mg/litre for
1,2,4,5-TeCB).
The 96-h LC50 for bluegill sunfish ranged between 0.3 mg/litre for
PeCB and 24 mg/litre for MCB. In embryo-larval assays, the chronic
toxicity limits for DCBs varied between 0.76 and 2.0 mg/litre for the
fathead minnow; in the estuarine sheepshead minnow, the chronic
toxicity limits for 1,2,4-TCB and 1,2,4,5-TeCB were 0.22 and
0.13 mg/litre, respectively. Newly-hatched goldfish and large-mouth
bass were the most susceptible life-stage with LC50s (96-h) of 1 and
0.05 mg/litre, respectively, for MCB.
No data are available on the effects of chlorobenzenes on terrestrial
systems.
1.7 Effects on Experimental Animals and In Vitro Systems
With few exceptions, the chlorobenzenes are only moderately toxic for
experimental animals, on an acute basis, and, generally, have oral
LD50s greater than 1000 mg/kg body weight; from the limited data
available, dermal LD50s are higher. The ingestion of a lethal dose
leads to respiratory paralysis, while the inhalation of high doses
causes local irritation and depression of the central nervous system.
Acute exposures to non-lethal doses of chlorobenzenes induce toxic
effects on the liver, kidneys, adrenal glands, mucous membranes, and
brain, and effects on metabolizing enzymes.
Studies on skin and eye irritation caused by chlorobenzenes have been
restricted to 1,2,4-TCB and 1,2-DCB. Both produce severe discomfort,
but no permanent damage was noted after direct application to the
rabbit eye. 1,2,4-TCB is mildly irritating to the skin and may lead to
dermatitis after repeated or prolonged contact. No evidence of
sensitization was found.
Short-term exposures (5-21 days) of rats and mice to MCB and DCBs at
hundreds of mg/kg body weight resulted in liver damage and
haematological changes indicative of bone marrow damage. Liver damage
was also the major adverse effect noted after the short-term exposure
of rats or rabbits to other chlorobenzenes (TCB-PeCB), at doses
slightly lower than those for MCB and DCBs. Several of the
chlorobenzene isomers studied induced porphyria, the isomers with
para chlorine atoms being the most active (i.e., 1,4-DCB, 1,2,4-TCB,
1,2,3,,4-TeCB, and PeCB). The general order of toxicity noted for
TeCBs and PeCB after short-term exposure was: 1,2,4,5-TeCB
>PeCB>1,2,3,4- and 1,2,3,5-TeCB, which correlated well with the
levels found in fat and liver.
Long-term exposure studies (up to 6 months) on several species of
experimental animals indicated a trend for the toxicity of
chlorobenzenes to increase with increased ring chlorination. However,
there was considerable variation in the long-term toxicities of
different isomers of the same congener. For example, 1,4-DCB appeared
to be much less toxic than 1,2-DCB. There was a good correlation
between toxicity and the degree of accumulation of the compound in the
body tissues, female animals being less sensitive than males. Major
target organs were the liver and kidney; at higher doses, effects on
the haematopoietic system were reported and thyroid toxicity was noted
in studies on 1,2,4,5-TeCB and PeCB.
In a bioassay for the carcinogenicity of MCB, there was an increased
incidence of hepatic neoplastic nodules in the high-dose group
(120 mg/kg body weight) of male F344 rats, but no treatment-related
increases in tumour incidence in female F344 rats or male or female
B6C3F1 mice. There was no evidence for the carcino-genicity of
1,2-DCB in male or female F344 rats or B6C3F1 mice (60 or 120 mg/kg
body weight).
In a bioassay for the carcinogenicity of 1,4-DCB, there was a
dose-related increase in renal tubular cell adenocarcinomas in male
F344 rats and an increase in hepatocellular carcinomas and adenomas in
both sexes of B6C3F1 mice. No evidence of carcinogenicity was
reported in male and female Wistar rats, or female Swiss mice,
following inhalation of slightly higher doses of 1,4-DCB (estimated to
be 400 mg/kg per day for rats and 790 mg/kg per day for mice) for
shorter periods. However, available data indicate that the induction
of renal tumours by 1,4-DCB in male F344 rats and the associated
severe nephropathy and hyaline droplet formation are species- and
sex-specific responses associated with the reabsorption of
alpha-2-microglobulin.
Available data are inadequate for the assessment of the
carcinogenicity of the higher chlorinated benzenes (tri- to penta-).
Although available data from in vitro and in vivo assays for
isomers other than 1,4-DCB are limited, chlorobenzenes do not appear
to be mutagenic. On the basis of a more extensive database for
1,4-DCB, it can be concluded that this compound has no mutagenic
potential, either in vivo or in vitro.
There has been no evidence that chlorobenzenes are teratogenic in rats
and rabbits. The administration of MCB and DCBs to rats or rabbits via
inhalation at concentrations >2000 mg/m3 (approximately 550 mg/kg
body weight per day) and, orally, at concentrations >500 mg/kg body
weight, resulted in minor embryotoxic and fetotoxic effects. However,
such doses were clearly toxic to the mother. Although there is some
evidence that TCBs, TeCBs, and PeCB are embryotoxic and fetotoxic at
doses that are not toxic for the mother, available data are
inconsistent.
1.8 Effects on Humans
1.8.1 General population
Reports on the effects of CBs on the general population are restricted
to case reports from accidents and/or the misuse of products
containing the lower chlorinated benzenes (MCB, 1,2-DCB, 1,4-DCB, and
an unspecified isomer of TCB). Little or no information is available
on dose, chemical purity, or dose:time relationships and observed
effects, such as myeloblastic leukaemia, rhinitis, glomerulonephritis,
pulmonary granulomatosis, dizziness, tremor, ataxia, polyneuritis, and
jaundice, cannot be quantified.
No epidemiological studies on the health effects of chlorobenzenes in
the general population have been reported.
1.8.2 Occupational exposure
During the manufacture and use of chlorobenzenes, clinical symptoms
and signs of excessive exposure include: central nervous system
effects and irritation of the eyes and upper respiratory tract (MCB);
haematological disorders (1,2-DCB); and central nervous system
effects, hardening of the skin, and haematological disorders including
anaemia (1,4-DCB). However, such symptoms come only from case reports,
and are difficult to quantify, since little information on actual
levels, chemical purity, or dose:time relationships is available.
The few epidemiological studies on workers exposed to chlorobenzenes
that have been reported concern only MCB, 1,2-DCB, 1,4-DCB, and
1,2,4,5-TeCB. Although effects on the nervous system, on neonatal
development, and on the skin have been reported after MCB exposures,
the 3 studies were not adequate for assessing risk, because of
methodological problems, such as exposure assessment, mixed exposures,
and lack of control groups. Similar criticism can be made of the study
on 1,4-DCB, in which eye and nose irritation was reported, as well as
the study in which chromosomal aberrations resulting from exposure to
unspecified levels of 1,2-DCB and 1,2,4,5-TeCB were reported.
1.9 Conclusions
If good industrial practices are followed, the risks associated with
occupational exposure to chlorobenzenes are considered to be minimal.
The present risk assessment also indicates that current concentrations
of chlorobenzenes in the environment pose a minimal risk for the
general population, except in the case of the misuse of
chlorobenzene-based products or their uncontrolled discharge into the
environment. However, this assessment is based on limited monitoring
data and additional information is needed to substantiate this
conclusion. Reduction of the widespread use and disposal of
chlorobenzenes should, however, be considered because:
(a) Chlorobenzenes may act as precursors for the formation of
polychlorinated dibenzodioxins/polychlorinated dibenzofurans
(PCDDs/PCDFs), e.g., in incineration processes.
(b) These chemicals can lead to taste and odour problems in
drinking-water and fish.
(c) Residues persist in organically-rich anaerobic sediments and
soils, and ground water.
For most chlorobenzenes, the assessment of risk has been based on
non-neoplastic effects. However, neoplastic effects were taken into
consideration in the risk assessment for MCB and 1,4-DCB. Available
data indicate that the observed increase in renal tumours in rats
caused by 1,4-DCB is a species- and sex-specific response that is
unlikely to be relevant for humans. On the basis of evidence of
increased DNA replication in the mouse liver and the increased
incidence of hepatocellular adenomas and carcinomas in mice, 1,4-DCB
may act as a non-genotoxic carcinogen in the rodent liver. The
increased incidence of hepatic neoplastic nodules observed in the
high-dose group of male rats in a bioassay for carcinogenicity
indicates that MCB may also be a non-genotoxic carcinogen.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
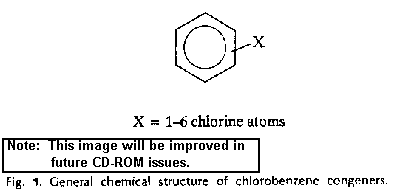
The chlorinated benzenes are cyclic aromatic compounds in which the
hydrogen atoms of the benzene ring have been replaced by 1-6 chlorine
substituents (Fig. 1). This substitution yields 12 compounds,
including: monochlorobenzene, 3 isomeric forms of dichlorobenzene, 3
isomers of trichlorobenzene, 3 isomers of tetrachlorobenzene,
pentachlorobenzene, and hexachlorobenzene. The identification features
for the congeners ranging from mono to pentachlorobenzene are
summarized in Table 1.
 Hexachlorobenzene is the subject of a separate Environmental Health
Criteria publication and will not be evaluated here.
2.1.2 Technical product
There are no widely established trade specifications for commercial
chlorobenzenes. Pure commercial monochlorobenzene may contain 0.05 %
or less of benzene and up to 0.1 % of dichlorobenzenes. Technical
grade 1,2-dichlorobenzene contains up to 19 % of the other 2
dichlorobenzene isomers, 1 % of trichlorobenzenes, and up to 0.05 % of
monochlorobenzene, while purified 1,2-dichlorobenzene contains up to
0.05 % of monochlorobenzene and 0.2 % of 1,2,4-trichlorobenzene.
Technical grade 1,4-dichlorobenzene contains up to a total of 0.1 % of
mono- and trichlorobenzenes and 0.5 % of each of the other
dichlorobenzene isomers. Commercial 1,2,4-trichlorobenzene may contain
up to 0.1 % of mono-chlorobenzene, 0.5 % of dichlorobenzenes, and
0.5 % of tetrachlorobenzenes (Kao & Poffenberger, 1979).
Polychlorinated dibenzodioxins or dibenzofurans were not detected in
trichlorobenzenes, tetrachlorobenzenes, or penta-chlorobenzene (Buser,
1979).
2.2 Physical and Chemical Properties
The physical and chemical properties of the chlorobenzenes (mono- to
penta-) are presented in Table 2.
MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB are colourless liquids, while all
other congeners are white crystalline solids at room temperature. In
general, the solubility of chlorobenzenes in water is poor (decreasing
with increasing chlorination), flammability is low, the octanol/water
partition coefficients are moderate to high (increasing with
increasing chlorination), and vapour pressures are low to moderate
(decreasing with increasing chlorination).
2.3 Organoleptic Properties
The odour and taste thresholds for different isomers of the same
chlorobenzene appear to be similar: 0.01-0.02 mg/litre for MCB and
0.001-0.002 mg/litre for both 1,2-DCB and 1,4-DCB (Varshavskaya,
1968). Piet et al. (1980) reported that the odour thresholds for 1,2-
and 1,4-dichlorobenzenes in Rhine tap water were 10 and 0.3 µg/litre
respectively, while 1,2,4-TCB was detected at a level of 5 µg/litre.
Using available experimental data, Amoore & Hautala (1983) determined
water-dilution odour thresholds for MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB to be 0.050, 0.024, 0.011, and 0.064 mg/litre (ppm),
respectively. Odour thresholds in air for these compounds are 0.68,
0.30, 0.18, and 1.4 µlitre/litre (ppm), respectively. Fomenko (1965)
reported that the thresholds for smell and taste for 1,2,4,5-TeCB were
0.006 mg/litre and 0.0064 mg/litre, respectively.
2.4 Conversion Factors
At 25 °C and 101.3 kPa, the conversion factors for chlorobenzenes in
air are as follows:
monochlorobenzene: 1 ppm=4.55 mg/m3: 1 mg/m3=0.22 ppm
dichlorobenzenes: 1 ppm=6.00 mg/m3: 1 mg/m3=0.17 ppm
trichlorobenzenes: 1 ppm=7.42 mg/m3: 1 mg/m3=0.13 ppm
tetrachlorobenzenes: 1 ppm=8.83 mg/m3: 1 mg/m3=0.11 ppm
pentachlorobenzene: 1 ppm=10.24 mg/m3: 1 mg/m3=0.10 ppm
Table 1. Information on the identity of chlorobenzenes
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
Monochlorobenzene MCB C6H5Cl 112.6 chlorobenzene
(108-90-7) phenyl chloride
1,2-dichlorobenzene 1,2-DCB C6H4Cl2 147.0 ortho-dichlorobenzene
(95-50-1) o-dichlorobenzene
1,3-dichlorobenzene 1,3-DCB C6H4Cl2 147.0 meta-dichlorobenzene
(541-73-1) m-dichlorobenzene
1,4-dichlorobenzene 1,4-DCB C6H4Cl2 147.0 para-dichlorobenzene
(106-46-7) p-dichlorobenzene
1,2,3-trichlorobenzene 1,2,3-TCB C6H3Cl3 181.5 vic-trichlorobenzene
(87-61-6) v-trichlorobenzene
1,2,6-trichlorobenzene
1,2,4-trichlorobenzene 1,2,4-TCB C6H3Cl3 181.5 1,2,4-trichlorobenzol
(120-82-1)
1,3,5-trichlorobenzene 1,3,5-TCB C6H3Cl3 181.5 s-trichlorobenzene
(108-70-3) TCBA
sym-trichlorobenzene
1,2,3,4-tetrachlorobenzene 1,2,3,4-TeCB C6H2Cl4 215.9 benzene, 1,2,3,4-
(634-66-2) tetrachloro-
1,2,3,5-tetrachlorobenzene 1,2,3,5-TeCB C6H2Cl4 215.9 benzene, 1,2,3,5-
(634-90-2) tetrachloro-
Table 1 (continued)
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
1,2,4,5-tetrachlorobenzene 1,2,4,5-TeCB C6H2Cl4 215.9 benzene, tetrachloride
(95-94-3) benzene, 1,2,4,5-
tetrachloro-
s-tetrachlorobenzene
Pentachlorobenzene PeCB C6HCl5 250.3 1,2,3,4,5-
(608-93-5) pentachloro-benzene
QCB
a Chemical Abstract Services registry number.
b R.M.M. - Relative molecular mass.
Table 2. Physical and chemical properties
Solubility Log Henry's Soil Blood/air
Compound Melting Boiling Vapour Densityf in water at octanol/water Law sorption partition
point point pressure 25 °C (mol/ partition constant coefficient coefficientj
(°C)a (°C)a at 25 °C litre) coefficientg (kPa m3/ (KOC)i
(Pa) (mg/litre)g mol)h
MCB -45.6 132.0 1665b 1.105820/4 2.6x10-3 2.98 0.377 466 30.8
(293)
1,2-DCB -17.0 180.5 197b 1.304820/4 6.2x10-4 3.38 0.198 987 423
(91.1)
1,3-DCB -24.7 173.0 269b 1.288420/4 8.4x10-4 3.48 0.366 1070 201.4
(123)
1,4-DCB 53.1 174.0 90c 1,247520/4 2.1x10-4 3.38 0.160 1470 NA
(30.9)
1,2,3-TCB 53.5 218.5 17.3d NA 6.7x10-5 4.04 0.306 3680 NA
(12.2)
1,2,4-TCB 17.0 213.5 45.3d 1.454220/4 2.5x10-4 3.98 0.439 2670 NA
(45.3)
1,3,5-TCB 63.5 208761 24.0d NA 2.2x10-5 4.02 0.233 NA NA
(3.99)
1,2,3,4-TeCB 47.5 254.0 5.2c NA 5.6x10-5 4.55 0.261 NA NA
(12.1)
1,2,3,5-TeCB 54.5 246.0 9.8c NA 1.3x10-5 4.65 0.593 8560 NA
(2.81)
Table 2 (continued)
Solubility Log Henry's Soil Blood/air
Compound Melting Boiling Vapour Densityf in water at octanol/water Law sorption partition
point point pressure 25 °C (mol/ partition constant coefficient coefficientj
(°C)a (°C)a at 25 °C litre) coefficientg (kPa m3/ (KOC)i
(Pa) (mg/litre)g mol)h
1,2,4,5-TeCB 139.5 243.6 0.72c NA 1.0x10-5 4.51 0.261 6990 NA
(2.16)
PeCB 86.0 277.0 133 at 1.834216.5 3.3x10-6 5.03 0.977 58 700 NA
98.6 °Ce (0.83)
a Melting points are rounded to the nearest 0.1 °C; Boiling points are at atmospheric pressure (760 mm), unless otherwise indicated
by a superscript (Weast, 1986).
b Vapour pressures obtained from the Antoine equation: log10p(kPa) = A-B/(T+C) - 0.8751 presented by Kao & Poffenberger (1979),
together with the values for the Antoiine constants (A,B,C).T = temperature in °C.
c From: MacKay et al. (1982). The value was derived from experimental data obtained above 25 °C and extrapolated to 25 °C, taking into
account the phase change from liquid to solid.
d Vapour pressures obtained from the equation: log10p(10-3torr) = -(A/T) + B and values for the constants (A and B) are presented by
Sears & Hopke (1949).T = absolute temperature.
e From: Stull (1947).
f Density is relative to water, otherwise it has the dimensions g/ml. A superscript indicates the temperature of the liquid and a
subscript indicates the temperature of water to which the density is referred (Weast, 1986).
g From: Miller et al. (1984).
h From: MacKay & Shiu (1981).
i Derived from: Karlokoff et al. (1979).
j From: Sato & Nakajima (1979).
NA - values either not given in the reference indicated or not found in the literature.
2.5 Analytical Methods
Some methods for the sampling and determination of chlorobenzenes in
various environmental media and human tissues and fluids are
presented in Table 3.
The analytical technique of choice for the determination of
chlorobenzenes in environmental samples is gas-liquid chromatography
(GLC). However, the methods of collection and preparation of samples
for GLC analysis vary considerably, depending on the medium and the
laboratory. Columns with silicone-based stationary phases or Tenax
resins, and electron capture detectors, appear to be the most widely
used.
Tenax-GC resins appear to be the most commonly used absorbent for
the air sampling of chlorobenzenes (Sievers et al., 1980; Krost et
al., 1982; Pellizzari, 1982), though XAD resins have also been used
(Langhorst & Nestrick, 1979). Air pollutants collected on Tenax-GC
resins can be desorbed directly on to the GLC column by heating the
absorber. XAD resins can be extracted with carbon tetrachloride, an
aliquot of which can then be injected into a gas chromatograph
(Langhorst & Nestrick, 1979).
Solvent extraction is a simple and effective technique for
recovering chlorobenzenes from water samples. Hexane, pentane, and a
1:1 mixture of cyclohexane and diethyl ether have been identified as
suitable extraction solvents for these compounds (Oliver & Bothen,
1980; Piet et al., 1980; Otson & Williams, 1981). Alternatively,
preconcentration of the chlorobenzenes on organic resins, such as
Chromosorb 102 and Tenax-GC, is also effective (Oliver & Bothen,
1980; Pankow & Isabelle, 1982). The purge-trap method is also often
used to concentrate the volatile halogenated benzenes before
analysis using GC (Jungclaus et al., 1978; Pereira & Hughes, 1980;
Otson & Williams, 1982).
The extraction of chlorobenzenes from aquatic sediments or soil can
be achieved by solvent or Soxhlet extraction (Oliver & Bothen, 1982;
Lopez-Avila et al., 1983; Onuska & Terry, 1985). Solvents commonly
used are acetone and/or hexane. The extract is generally dried using
sodium sulfate, followed by clean-up on a Florisil column before GLC
analysis.
For the detection of chlorobenzenes in fish samples, solvent or
Soxhlet extraction with subsequent clean-up on Florisil and GC
analysis with electron capture detection have commonly been used
(Lunde & Ofstad, 1976; Kuehl et al., 1980; Oliver & Bothen, 1982).
Vacuum extraction and the direct purge and trap method have also
been used to quantify levels of MCB in fish tissue (Hiatt, 1981).
Table 3. Analytical methods for chlorobenzenesa
Matrix Sampling, extraction Analytical method Detection limitsb Reference
air continuous flow, aircraft trap purged in oven at NA Sievers et al. (1980)
sampling port; sorbent traps 220 °C with He; capillary
with 4 changes column (30 m x 0.3 mm),
gas chromatography-mass
spectrometry (GC-MS) data
system
air 4-h samples collected on silanized glass column; GC MCB 3.2 Langhorst & Nestrick
Amberlite XAD-Z resin at with photoionization detector DCBs 4.2 (1979)
100-200 ml/min; desorbed TCBs 5.9
TeCB 7.1
PeCB 9.2
water 500 ml with chromosorb 102, or GC analysis, glass capillary MCB 0.5 Oliver & Bothen
3.1 litres with 75 ml pentane columns; electron capture DCBs 0.001 (1980)
TCBs 0.0001
TeCB 0.00005
PeCB 0.00001
water 40 ml with automated purge and GC analysis with FID Otson & Williams
trap; inert gas bubbled through simultaneous use of flame MCB < 0.1 (1982)
purged compounds directly on ionization detector (FID) 1,2-DCB 0.2
to column and Hall electrolytic 1,3-DCB 0.1
conductivity detector (HECD) 1,4-DCB 0.1
HECD
MCB 0.1
1,2-DCB 0.1
1,3-DCB 0.1
1,4-DCB 0.1
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
water liquid-liquid extraction of 120 ml GC analysis using 63Ni FID Otson & Williams
water with 38:1 water:hexane electron capture detector MCB 5 (1981)
(ECD), FID or HECD 1,2-DCB 2
1,4-DCB 2
1,2,4-TCB 2
ECD
MCB ND
1,2-DCB 5
1,4-DCB 5
1,2,4-TCB < 1
HECD
MCB 1
1,2-DCB < 1
1,4-DCB < 1
1,2,4-TCB < 1
water extraction of 4 litres water with compounds desorbed directly NA Pankow & Isabelle
Tenax-GC 35/60 mesh; from glass column of (1982)
centrifugation or vacuum Tenax-GC into GC by flash
dessication of wet cartridge heating; flame ionization
to remove water detector
water extraction of 1-litre sample with glass capillary column NA Piet et al. (1980)
20 ml cyclohexane-diethylether coupled to electron detector
(1:1) on line with FID detector
water adsorption on 1 g of activated GC analysis, FID detector MCB concentration range: Blanchard & Hardy
charcoal in exposure chamber; 0.058-19.4 mg/litre (1985)
charcoal desorbed with 5 ml of
carbon disulfide for >30 min
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
sediment Soxhlet extraction of 10-15 g GC analysis on glass MCB 1500 Oliver & Bothen
with 41% hexane/59% acetone; capillary columns; electron DCBs 5 (1982)
back-extracted with water to capture detector TCBs 0.4
remove acetone, through Na2SO4 TeCBs 0.2
and evaporated to 10 ml; PeCB 0.05
clean-up on Na2SO4 + deactivated
Florisil column
sediment 10 g sediment treated by steam identification by relative 1,3-DCB 1.5 Onuska & Terry
distillation, soxhlet or retention-time matching after 1,3,5-TCB 1.0 (1985)
ultrasonic extraction; clean-up ECD 1,2,4-TCB 0.8
with mercury only needed when 1,2,3-TCB 0.8
sulfur present 1,2,3,5-TeCB 0.5
1,2,4,5-TeCB 0.5
1,2,3,4-TeCB 0.5
PeCB 0.4
fish 15 g fish soxhlet extracted; GC analysis on glass MCB 1500 Oliver & Bothen
clean-up with combination of capillary column, ECD DCBs 5 (1982)
alumina, silica gel, florisil and detector TCBs 0.4
acidified florisil (fish), after TeCBS 0.2
removal of lipids PeCB 0.05
blood hexane extraction on Synder borosilicate glass column, DCBs approx. 2 Bristol et al.
column using 3 g for GC and GC analysis; electron capture TCBs approx. 1.5 (1982)
710 g for GC/MS detector or GC/MS system TeCBs approx. 1
PeCB approx. 1
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
blood CCl4 extraction of 5 g of blood silanized glass column; GC Blood Langhorst & Nestrick
urine or 20 g urine, silica gel column analysis with photoionization MCB approx.23 (1979)
chromatography (CCl4 eluent) detector DCBs approx. 4
TCBs approx. 5
TeCBs approx. 6
PeCB approx. 9
Urine
MCB approx. 6
DCBs approx. 6
TCBs approx. 1
TeCBs approx. 2
PeCB approx. 2
blood 0.1-1 ml GC sample diluted to Tenax adsorbent heated and NA Balkon & Leary (1979)
urine 5 ml with water and placed in a volatiles analysed by GC/MS
bubbler for purging on to Tenax for detection and
in liquid sample concentrator identification in a screening
procedure
blood hexane/isopropanol extraction of GC analysis, electron capture NA Lunde & Bjorseth
approximately 25 g; H2SO4 detector (1977)
digestion of hexane phase
adipose tissue extraction of tissue with GC analysis, capillary DCBs ND LeBel & Williams
acetone-hexane, then fractionated column, ECD detection; 1,3,5-TCB 11.0 µg/kg (1986)
by gel permeation chromatography compounds 5.9 µg/kg
(GPC); clean-up on Florisil column confirmed by gas 1,2,3,5-TeCB 13.1 µg/kg
chromatography-mass spectrometry 1,2,3,4-TeCB 4.8 µg/kg
with selected ion monitoring PeCB 1.9 µg/kg
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
urine solutions stirred and heated to Analysis by GC/FID MCB Michael et al.
blood 50 °C, headspace above the blood 98c (1980)
adipose tissue solution purged on to Tenax GC urine 86c
cartridges; cartridges dessicated adipose 13c
using anhydrous calcium sulfate
and thermally desorbed DCB
blood 86c
urine 79c
adipose 57c
urine 5 ml samples: GC equipped with an electron urine 94c McKinney et al.
blood Urine: acidified with 0.5 ml capture (tritium) detector blood 78c (1970)
concentrated HCl, then extracted
with benzene Extracts dried over
anhydrous sodium sulfate
Blood: plasma extracted with
benzene, then dried with
anhydrous sodium sulfate
adipose tissue 2-g samples extracted with analysis by GC with electron NA Mes et al. (1982)
benzene:acetone (1:19 v/v); capture detector;
repeated evaporation with hexane confirmation by GLC; monitored
to remove traces of benzene; by mass spectrometry
fat-free extract chromatographed
on Florisil-silicic acid column
Table 3 (continued)
a Often, the primary aim of the analyses was quantification of organochlorine compounds, other than chlorobenzenes. In these cases,
the clean-up procedures were quite complicated, because of the need to separate different organochlorine pesticide residues, prior
to chromatographic analysis.
b Detection limits reported in µg/m3 for air and µg/litre or µg/kg for other media, unless noted otherwise.
NA - information not available in the paper.
ND - not detected during analysis.
c Indicates recovery percentages from spiked samples.
Solvent extraction is also used in the determination of
chlorobenzenes in biological matrices, such as blood and urine. For
less volatile compounds (tri-, tetra-, and pentachlorobenzenes),
solvent extraction is followed by column chromatographic clean up
and quantification (Lamparski et al., 1980; Mes et al., 1982). For
the more volatile compounds (mono-, dichlorobenzenes), a modified
purge-trap method with a capillary GC can be used (Michael et al.,
1980). The chlorobenzenes are then quantified using a GC with
detection by electron capture (McKinney et al., 1970; Morita et al.,
1975; Lunde & Bjorseth, 1977), photoionization (Langhorst &
Nestrick, 1979), or mass spectrometry (Balkon & Leary, 1979; Bristol
et al., 1982; LeBel & Williams, 1986).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
Natural sources of chlorobenzenes in the general environment have
not been identified; however, 1,2,3,4-TeCB has been identified in
the oil of a marsh grass (Miles et al., 1973).
3.2 Man-made Sources
3.2.1 Production
Monochlorobenzene and the dichlorobenzenes are produced commercially
by the direct chlorination of benzene in the liquid phase, in the
presence of a Lewis acid catalyst, such as ferric chloride. In the
liquid-phase chlorination of monochlorobenzene, 1,2-, and
1,4-dichlorobenzenes are the predominant products. Trichlorobenzenes
result from the chlorination of dichlorobenzenes with ferric
chloride, while tetrachlorobenzenes are produced by the addition of
chlorine to trichlorobenzenes in the presence of an aluminium
catalyst. Tetrachlorobenzenes can be used as the precursor in
pentachlorobenzene production (US EPA, 1985). Pentachloro-benzene is
also produced by the denitrification of penta-chloronitrobenzene and
the reductive dechlorination of hexa-chlorobenzene (Renner & Mücke,
1986).
About 50% of the world production of all chlorobenzenes
(estimated from data in US EPA (1985) to be 568 x 106 kg in 1983)
is manufactured in the USA. The remainder is produced mainly in
Western Europe and Japan. Monochlorobenzene makes up approximately
70 % of total world production of all chlorobenzenes.
Data on current, global chlorobenzene production volumes are not
available in readily retrievable references. Summaries of production
levels in 1980 and 1983 have been published and are presented in
Table 4 (IARC, 1982; US EPA, 1985). Although these may provide some
indication of present production levels, it appears that PeCB
production has ceased within the USA, and that the use of
chlorobenzenes as chemical intermediates has decreased. Therefore,
the actual level of production is probably less than that shown in
Table 4.
No information was found on the production of TCB, TeCB, and PeCB
congeners outside the USA. However, in 1979, the estimated
production of 1,4-DCB in Japan was 27.5 x 106 kg and that of
1,2-DCB was 13 x 106 kg (IARC, 1982).
Table 4. Production levels in the USA and possible uses of chlorinated benzenes
Chemical Major usesa Estimated annual
production in the USA
MCB Intermediate in the manufacture of chloronitrobenzenes, diphenyl 130 x 106 kg in 1980
oxide, DDT, and silicones; as a process solvent for methylene
diisocyanate, adhesives, polishes, waxes, pharmaceutical products,
and natural rubber; as a degrading solvent
1,2-DCB In the manufacture of 3,4-dichloroaniline; as a solvent for a wide 22 x 106 kg in 1980
range of organic materials and for oxides of non-ferrous metals; as a
solvent carrier in the production of toluene diisocyanate; in the
manufacture of dyes; as a fumigant and insecticide; in degreasing
hides and wool; in metal polishes; in industrial odour control; in
cleaners for drains
1,3-DCB As a fumigant and insecticide NA
1,4-DCB As a moth repellent, general insecticide, germicide, space deodorant; 24 x 106 kg in 1980
in the manufacture of 2,5-dichloroaniline and dyes; as a chemical
intermediate; in pharmaceutical products; in agricultural fumigants
1,2,3-TCB Apart from use as a chemical intermediate, the uses are the same as 23-74 x 103 kg
those 1,2,4-trichlorobenzene
1,2,4-TCB As an intermediate in the manufacture of herbicides; dye carrier, 1.2-3.7 x 106 kg
dielectric fluid; solvent; heat-transfer medium
1,3,5-TCB Solvent for products melting at high-temperatures; coolant in 1.1-2.1 x 105 kg
electrical insulators; heat-transfer medium, lubricant, and synthetic
transformer oil; termite preparation and insecticide; in dyes
Table 4 (continued)
Chemical Major usesa Estimated annual
production in the USA
1,2,3,4-TeCB Component in dielectric fluids; in the synthesis of fungicides NA
1,2,3,5-TeCB NA NA
1,2,4,5-TeCB Intermediate for herbicides and defoliants; insecticide; NA
moisture-resistant impregnant; in electric insulation; in packing
protection
PeCB Formerly in a pesticide used to combat oyster drills; chemical Not manufactured in
intermediate the USAa
a From: US EPA (1985).
NA - not available.
The total production capacity for all chlorobenzenes in Western
Europe during 1980 was estimated to be greater than 208 x 106 kg
(IARC, 1982).
Although data on production levels are scarce, it is apparent from
available information that chlorobenzenes (in particular MCB and
DCBs) are produced in high volumes. Use patterns shown in Table 4,
and estimated losses to the environment shown in Table 5, indicate a
high potential for human exposure and environmental contamination.
Table 5. Estimated quantities (kg) of chlorobenzenes lost to the environment
during manufacture in relation to total 1983 productiona
Chlorobenzene Losses during Losses to Total production
manufacture environment
MCB 1.9-3.0 x 105 1.5-2.6 x 105 130 x 106
1,2-DCB 1.1-2.1 x 105 30 x 103 22 x 106
1,3-DCB 2-6 x 102 NA NA
1,4-DCB 1.8-2.8 x 105 1.7-2.7 x 105 24 x 106
1,2,3-TCB 0.6-2 x 103 <1 x 102 23-74 x 103
1,2,4-TCB 3-10 x 103 3-9 x 102 1.2-3.7 x 106
1,3,5-TCB import import 1.1-2.1 x 105
TeCB NA NA NA
PeCB not manufactured NA NA
a Values calculated from US EPA (1985).
NA - data not available.
3.2.2 Uses
Use patterns may vary considerably among countries. A summary of the
uses of chlorinated benzenes in the USA is presented in Table 4.
Chlorobenzenes are used mainly as intermediates in the synthesis of
other chemicals, and as pesticides. The 1,4-DCB isomer is commonly
used in space deodorants and moth repellents, and several of the
higher chlorinated benzenes (TCBs, 1,2,3,4-TeCB) have been used in
dielectric fluids.
MCB also has potential as a functional fluid in external combustion
Rankine engines (Curran, 1981) and as a component in heat transfer
fluids in solar energy collectors (Boy-Marcotte, 1980).
The 1,4-DCB isomer is also being used in the USA as an intermediate
in the production of polyphenylene sulfide resin, an engineering
plastic with electrical and automotive applications.
3.2.3 Sources in the environment
Incineration of organochlorine and hydrocarbon polymers in the
presence of chlorine may result in the atmospheric release of
chlorobenzenes, though quantities are small in relation to the total
mass of carbon compounds incinerated (Ahling et al., 1978;
Lahaniatis et al., 1981a). Incineration of chlorobenzenes most
probably leads to the formation of polychlorinated dibenzodioxins
and dibenzofurans, as indicated by experimental studies on the
pyrolysis of various TCBs, TeCBs, and PeCB (Buser, 1979). Although,
in experimental studies, chlorobenzenes have been formed in
reactions between benzene and sodium hypochlorite (Hofler et al.,
1983), evidence that they are generated during public water
treatment is slight (Otson et al., 1982a).
On the basis of measurements of concentrations in flue gases from
all municipal waste incinerators in Sweden (N=24), the maximum
contribution of chlorobenzenes (di- to hexa-) to ambient air was
calculated, in 1985, to be 590 kg (Ahlborg & Victorin, 1987).
Average emissions of total chlorobenzenes from small-scale wood
burners for dry wood, in closed fireplace ovens, during 2-h sampling
periods, ranged from 24 to 80 µg/kg dry fuel (Rudling et al., 1980).
Several of the chlorinated benzenes have been identified as
microbial metabolites of lindane degradation (Macholz & Kujawa,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The transport and fate of chlorobenzenes in the environment has not
been well characterized. However, it is possible to draw some
conclusions based on the physical and chemical properties of the
compounds and the results of a limited number of laboratory and
field studies.
4.1 Transport and Distribution
The water solubility, saturated vapour pressure, and partition
coefficients (Henry's Law constant, KH; soil sorption, Koc;
octanol/ water, Kow; blood/air), useful for the prediction of the
transport and distribution of the chlorobenzenes in the environment,
are presented in Table 2.
As shown by Henry's Law constant (KH - the equilibrium
distribution coefficient of a compound between air and water), all
chlorobenzenes released into the aquatic environment will evaporate
preferentially from water to the atmosphere, despite their high
relative molecular masses and comparatively low vapour pressures
(MacKay & Wolkoff, 1973). From these data, it can also be predicted
that the preferential distribution from water to air will decrease
with increasing chlorination. In a study on the volatility of MCB in
a model aquatic ecosystem, 96% of the compound was released to the
atmosphere (Lu & Metcalf, 1975). In experimental studies by Garrison
& Hill (1972), 99% of the test compounds MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB had evaporated from aerated distilled solutions within 4
h. In non-aerated solutions, evaporation was complete within 72 h.
The results of a 1-year field study on Lake Zurich, Switzerland,
confirmed that most of the 1,4-DCB present in the water was
transferred to the atmosphere. The half-life of the compound was
estimated to be approximately 100 days, 67% being lost to the
atmosphere; 2% entering lake sediments and 31% being present in the
lake outflow (Schwarzenbach et al., 1979). Wilson et al. (1981)
studied the transport of a mixture in water of more than 10 organic
chemicals, including MCB, 1,4-DCB, and 1,2,4-TCB, through a column
of sandy soil having a low organic matter content, over a 21-day
period. They reported that up to 50% of the MCB evaporated and
approximately 50% of all 3 chlorobenzenes was degraded or was
unaccounted for, indicating that the compounds are likely to leach
into ground water.
4.2 Persistence and Fate
The chlorobenzenes are environmentally persistent compounds, the
most likely degradation mechanisms being photochemical reactions and
microbial action. While bioconcentration has been demonstrated, the
potential for biomagnification in food chains has not been
investigated. Soils that are rich in organic matter and aquatic
sediments are probably the major environmental sinks for these
compounds.
4.2.1 Persistence
In water, 1,2-DCB and 1,2,4-TCB are considered moderately persistent
compounds with half-lives ranging from 1 day in rivers to 10 days in
lakes and 100 days in ground waters (Zoeteman et al., 1980).
Concentrations may be rapidly reduced with aerobic biological
degradation or volatilization, but chlorobenzenes are extremely
persistent under anaerobic conditions, or where volatilization
cannot occur, i.e., in ground water.
Turbulence is a major factor in the elimination of these compounds
from surface waters. Turbulence increases volatilization and
bio-degradation. It may also lead to more rapid photochemical
degradation through the propagation of sensitized photolysis and the
increased frequency of exposure of water particles to surface
sunlight (Zoeteman et al., 1980).
Wakeham et al. (1983) studied the fate and persistence of MCB,
1,4-DCB, and 1,2,4-TCB in tanks containing seawater and associated
planktonic and microbial communities, with simulated tidal
turbulence and seasonal temperature regimes (spring, summer,
winter). It was suggested that removal processes other than
volatilization, such as biodegradation and sorption on to particles,
are probably not very important for 1,4-DCB and 1,2,4-TCB, but that
MCB is subject to rapid biodegradation under the relatively warm
spring and summer water temperatures, when microbial activity is
greater than in winter.
Chlorobenzenes in the air are degraded by chemical or sunlight-
catalysed reactions, or they may be adsorbed onto particles that
settle or are removed with rain. In a 2-week study on air samples
from California and Arizona, Singh et al. (1981) estimated the
residence times of MCB, DCBs, and an unspecified TCB isomer to be
13, 18.6, and 116.0 days, respectively.
In soils, the DCBs, TCBs, and PeCB are usually resistant to
micro-bial degradation; primary degradation products are the
chloro-phenols (Ballschmiter & Scholz, 1980). In experiments using
radiolabelled 1,2,3- and 1,2,4-TCBs on fresh field soil, the
observed degradation rates were very slow, 0.35 and 1.00 nmol/day
per 20 g soil, respectively (Marinucci & Bartha, 1979). These
investigators also observed that evaporation of the chlorobenzenes
was reduced by increasing the amounts of organic material in the
soil. In another experiment using 14C-labelled MCB, 1,2- and
1,4-DCBs, and 1,2,3- and 1,2,4-TCBs in soil, Haider et al. (1974)
found that 18.3%, 1.1% and 20.3% of MCB, DCBs, and TCBs,
respectively, were released as carbon dioxide.
4.2.2 Abiotic degradation
The higher chlorinated chlorobenzenes are not particularly reactive
compounds and would, therefore, be expected to disappear only slowly
in the environment through chemical degradation. Photolysis and
oxidative and hydrolytic reactions are pathways by which the
compounds may be abiotically degraded.
4.2.2.1 Photolysis
Although chlorobenzenes absorb light only weakly above 290 nm, some
photodegradation can occur when they are irradiated with sunlight,
or light containing an equivalent broad spectrum of wavelengths.
Uyeta et al. (1976) demonstrated that chlorobenzenes (other than
1,2,3,5-TeCB and PeCB, which were not examined) form polychlorinated
biphenyls when irradiated with sunlight. However, the yields of
polychlorinated biphenyls were less than 1% of the initial amount of
chlorobenzene. Of the compounds tested, 1,2,3-TCB and 1,2,4,5-TeCB
were the most resistant to photodegradation, while 1,2,4-TCB and
1,2,3,4-TeCB were the most easily degraded. The number of chlorine
atoms in the polychlorinated biphenyl photoproducts was 1 less than
the number contained in 2 molecules of the parent chlorobenzene,
i.e., monochlorobenzene yielded a monochlorobiphenyl,
dichlorobenzenes yielded tri-chlorobiphenyls and so on. Hydrochloric
acid was also a reaction product. On the basis of these results, it
was suggested that the photoformation of polychlorinated biphenyls
from chlorobenzenes involves free radical reactions based on the
dehydrochlorination of 1 molecule from 2 molecules of the parent
chlorobenzene.
Studies on direct photodegradation, either with direct sunlight or
artificial light simulating natural conditions, suggest that the
chlorobenzenes can be photodegraded, though the reactions may be
slow (Crosby & Hamadmad, 1971; Akermark et al., 1976; Uyeta et al.,
1976; Choudhry et al., 1979; Choudhry & Webster, 1985). For example,
the half-life of 1,4-DCB, under artificial sunlight irradiation, was
estimated to be 115.5 h (Hanai et al., 1985). This value was
considerably greater than the half-lives of other air pollutants
(i.e., tetrachloroethylene, trichloroethylene, benzene, toluene,
ethylbenzene, 1,2,4-trimethylbenzene, n-octane, and n-nonane)
under similar conditions.
Reductive dechlorination is the main photochemical reaction that
occurs in proton-donating solvents and there is evidence that the
solvent is involved with the electronically excited reactant
molecule in the transition state complex. Photodegradation of the
tri- and tetrachlorobenzenes, using acetonitrile as the solvent in a
1:1 ratio with water, has been reported; however, it should be noted
that acetonitrile would not be present in this ratio under normal
environmental conditions (Choudhry et al., 1979; Choudhry & Webster,
1985). Some form of hydrogen-donating entity, such as a solvent
molecule or another chlorobenzene molecule, appears necessary for
the photochemical dechlorination of chlorobenzenes at wave-lengths
above 290 nm. It has been speculated (Akermark et al., 1976) that
such hydrogen-donating "photosensitizers" may be found in
naturally occurring organic substances and that
photodecomposition may be important as a degradative pathway, given
the general physical and chemical stability of the chlorobenzenes.
In addition to direct photolysis, chlorobenzenes may also be removed
from the environment by reaction with molecular species that are
photochemically produced from other atmospheric pollutants. Such a
possibility has been suggested, on the basis of studies involving
simulated atmospheric environments, for interactions between
monochlorobenzene or 1,4-dichlorobenzene and oxides of nitrogen
(Dilling et al., 1976; Kanno & Nojima, 1979; Nojima & Kanno, 1980).
Reaction mechanisms and rates of disappearance of the compounds were
poorly defined in these studies.
4.2.2.2 Hydrolytic and oxidative reactions
It is unlikely that simple hydrolysis is an important degradation
pathway for the chlorobenzenes in the environment.
Cupitt (1980) suggested that MCB and the DCBs may be removed from
the troposphere by reaction with hydroxyl radicals (considered to
be the most potentially reactive species in the troposphere),
and possibly also by reaction with ozone. This investigator used
estimated rate constants for the reaction with hydroxyl radicals
(assumed to have a tropospheric concentration of 1 x 106
molecules/cm3) and ozone (tropospheric concentration, 1 x 1012
molecules/cm3) to predict atmospheric residence times of 28 days
and 39 days for MCB and the DCBs, respectively.
Calculations by Cupitt (1980) suggest that ozonolysis contributes
very little to the removal of the compounds, because the rate
constants for the reaction of hydroxyl radicals with the
chlorobenzenes are some 9 or 10 orders of magnitude greater than
those for the corresponding reactions with ozone.
4.2.3 Biodegradation and biotransformation
The degradation of chlorobenzenes by microorganisms has been
reported in several studies using various substrates, such as soil,
sediment, and sewage sludge (Table 6). It can be speculated, from a
perusal of these data, that the more highly chlorinated benzenes are
not degraded microbiologically as readily as the less chlorinated
congeners; however, the data are insufficient to draw definitive
conclusions. Garrison & Hill, (1972) found that MCB, 1,2-DCB, and
1,4-DCB were completely volatized in less than one day from
solutions containing mixed cultures of aerobic organisms, but that
2% of 1,2,4-TCB remained after 80 h.
The major degradation mechanism is oxidative dechlorination leading
to the formation of hydroxylated aromatic compounds (mainly
phenols), followed by ring fission and, eventually, mineralization
to carbon dioxide and water. It has been suggested that, like
polychlorinated biphenyls, chlorobenzenes appear to be attacked by
microorganisms only under aerobic conditions (Kobayashi & Rittman,
1982; Bouwer & McCarty, 1984).
Schwarzenbach et al. (1983) studied the movement of 1,4-DCB from a
polluted river in Switzerland through a ground water aquifer to a
series of wells. Correlation between the indicators of
microbiological metabolic activity and the observed decrease in
concentrations of 1,4-DCB with increasing distance of the wells from
the river was taken as evidence of the biotransformation of 1,4-DCB
in the aquifer system. On certain occasions, the persistence of
1,4-DCB was well correlated with anoxic conditions that prevailed in
parts of the aquifer, suggesting that the biotransformation of the
compound is minimal under anaerobic conditions. These findings were
confirmed in laboratory experiments using sediments from this
aquifer. Results showed that the DCBs were transformed only under
aerobic conditions and that the rates of transformation were
different with each isomer, 1,4-DCB degrading at the faster rate
(Kuhn et al., 1985).
4.2.4 Bioaccumulation
The bioaccumulation of chlorobenzenes by aquatic organisms is
determined by their relative water and lipid solubility (thus
reflecting the octanol/water partition coefficients) and the number
of chlorine substitutions. Uptake from water increases with
increasing chlorination. The coefficient of adsorption on sediment
influences the uptake into terrestrial plants and sediment-living
aquatic invertebrates; the degree of chlorination is also correlated
with uptake.
Table 6. Degradation of chlorobenzenes by miroorganisms
Chlorobenzene; Organism Substrate Rate Remarks
Reference
MCB, DCBs, Pseudomonas sp. synthetic NAa DCBs metabolized to dichlorophenols and
TCBs and TeCBs medium dichloropyrocatechols; MCB, TCBs, and
Ballschmiter & TeCBs metabolized to their respective
Scholz (1980) chlorophenols
MCB, DCBs, and NA synthetic no significant degradation medium seeded with sewage effluent and
1,2,4-TCB medium observed after 11 weeks strictly maintained under denitrifying
Bouwer & conditions
McCarty(1983)
MCB NA estuarine half-life = 75 days radiolabelled compound used,
Lee & Ryan sediments; half-life = 150 days degradation rate measured by
(1979) estuarine waters evolution of radiolabelled CO2;
considerable reduction in rate observed
when temperature reduced to 9-13 °C
MCB Pseudomonas synthetic not measured P. putida grown with toluene as the
Gibson et al. putida medium sole carbon source, oxidized MCB to
(1968) 3-chlorocatechol
MCB planktonic and sea water spring half-life = 21 days tanks contained 13 m3 sea water
Wakeham et al. microbial summer half-life = 4.6 days with simulated turbulence and
(1983) winter half-life = 13 days seasonal patterns
Table 6 (continued)
Chlorobenzene; Organism Substrate Rate Remarks
Reference
MCB microbial strain synthetic NAa culture isolated from soil and sewage
Reineke & WR 1306 medium and was sensitive to sudden increases
Knackmuss in MCB concentrations, resulting
(1984) in prolonged lag phase or disturbed
exponential phase; 3-chlorocatechol
isolated from culture fluid;
organisms did not oxidize isomeric DCBs
1,2-DCB Acinetobacter activated >90% disappearance in mixture of 4 bacterial genera and 1 yeast,
Davis et al. + sewage sludge 7 days glucose as sole carbon source, incubation
(1981) Alcaligenes temperature 28 °C; some DCB may have been
+ lost by evaporation
Flavobacterium
+
Pseudomonas
+
Rhodotorula
1,4-DCB planktonic and sea water spring half-life = 18 days tanks contained 13 m3 sea water with
Wakeham et al. microbial summer half-life = 10 days simulated turbulence and seasonal patterns
(1983) winter half-life = 13 days
1,4-DCB microbial flora ground water NAa under aerobic conditions, concentrations
Schwarzenbach present aquifer of 1,4-DCB decreased with increasing
et al. (1983) distance of wells from the polluted
1,2,4-TCB NA activated after 5 days, 56% converted to radiolabelled compound used, degradation
Simmons et al. sludge CO2; 23% converted to polar measured by evolution of radiolabelled CO2
(1977) metabolites; 7% evaporated
Table 6 (continued)
Chlorobenzene; Organism Substrate Rate Remarks
Reference
1,2,3-TCB and NA soil mineralization rates nmol/day radiolabelled compounds applied at
1,2,4-TCB per 20 g soil: 1,2,3-: 0.33, 50 mg/kg soil, mineralization measured by
Marinucci & 0.38; 1,2,4-: 1.09, 0.93, evolution of radiolabelled CO2; both TCBs
Bartha 1.37 poisoned metabolic action of soil
(1979) bacteria; 1,2,3-TCB yielded 2,3- and
2,6-dichlorophenol; 1,2,4-TCB yielded
2,4-, 2,5- and 3,4-dichlorophenol
1,2,4-TCB planktonic and sea water spring half-life = 22 days tanks contained 13 m3 sea water with
Wakeham et al. microbial summer half-life = 11 days simulated turbulence and seasonal patterns
(1983) winter half-life = 12 days
PeCB NA soil half-life = 194, 345 days compounds applied to soil samples at
Beck & Hansen concentrations equivalent to 10 kg/ha,
(1974) concentrations measured using gas
chromatography; duplicate experiments, no
explanation given for differences in
half-lives measured
a NA - not available.
Topp et al. (1986) compared the uptake in plants of chlorobenzenes
from the soil and via the air in closed, aerated laboratory systems.
A negative correlation was demonstrated between the bioconcentration
factor (BCF) and the soil adsorption coefficient (based on soil
organic matter content) for the uptake into the roots of barley. The
adsorption of chlorobenzenes on soil organic matter increased with
increasing chlorination. However, expression of uptake in barley
roots in relation to the soil interstitial water concentration of
the chlorobenzenes produced a positive correlation between the BCF
and the octanol/water partition coefficients. Higher chlorinated
chlorobenzenes, therefore, are most readily taken up by the plant
roots, when they are available in soil interstitial water. This will
occur particularly in sandy soils with a low organic matter content.
Uptake of volatilized chlorobenzenes in leaves was extremely low
compared with root uptake. The correlation between uptake and
physical properties demonstrated in barley did not hold for corn;
the authors stated that the uptake of lipophilic compounds by
lipid-rich plants, or plants with oil channels, was unpredictable .
In a later study, Topp et al. (1989) studied the uptake and
distribution of 14C-labelled 1,2,4-TCB and PeCB in barley. The BCF
concentration decreased with time of exposure; this was a dilution
effect as the plant grew. The total load of chlorobenzene increased
over the whole growing period of the plant, but the rate of uptake
was greater in the early growth period. Uptake increased with
increasing chlorination but decreased in relation to the soil
concentration (BCF fell with increasing chlorination). There was
evidence of metabolism of the chlorobenzenes in the plant, with the
level of the parent compound falling over the course of the
experiment in relation to the rate of metabolism, and the levels of
uncharacterized"bound" residues. After growth in soil containing 2
µg each of 1,2,4-TCB and PeCB/kg (dry weight), harvested barley
grain contained 73 and 82 µg/plant, respectively. The concentrations
in the dry grain were 0.05 and
0.06 mg/kg for 1,2,4-TCB and PeCB, respectively.
Khezovich & Harrison (1988) used closed, flow-through bioassay
systems to investigate the bioavailability to chiromonid midge
larvae of sediment-bound MCB, 1,2-DCB, and 1,2,4-TCB. A sediment
with a high organic matter content (14.5%) was compared with a
sediment with a low organic content (3.6%). The bioconcentration of
the chlorobenzenes increased with increasing chlorination. The
experiment was run without equilibrium between the sediment and the
overlying water (flow-through of uncontaminated water) and after
equilibration of recirculated water. Most of the uptake of
chlorobenzenes occurred from the interstitial water between sediment
particles and the results of bioconcentration were best correlated
with the concentrations of the chlorobenzenes in the interstitial
water. Under non-equilibrium conditions, bioconcentration factors
were 5, 29, and 225 for MCB, 1,2-DCB, and 1,2,4-TCB, respectively.
Köneman & Van Leeuwan (1980) exposed guppies to 116 µg
1,4-DCB/litre, 48 µg 1,2,3-TCB/litre, 43 µg 1,3,5-TCB/litre, 12 µg
1,2,3,5-TeCB/litre, or 1.2 µg PeCB/litre for 19 days. The fish were
fed daily on commercial fish food. Concentrations in fish were
expressed in mg/kg. The results showed an increase in the rate of
uptake with increasing level of chlorination of the benzene ring.
After exposure, the fish were kept for 9 weeks in clear water to
study the rate of elimination. The rate constant of loss of 1,4-DCB
was described by a one-compartment model and was relatively high
(1.00/day). For the other CBs, the losses showed a clear biphasic
pattern with a decrease in the first, rapid rate of loss with
increasing level of chlorination. Consequently, the level of
bioaccumulation went up with increasing chlorination.
In a study performed by Opperhuizen & Stokkel (1988), 1-year-old
guppies were exposed for 42 days to 1,2,3,4-TeCB or PeCB at µg/litre
levels. There were 3 groups of fish: one with contaminated
Chromosorb (artificial sediment) added, one with uncontaminated
Chromosorb, and one without Chromosorb. The concentration of PeCB in
the water was reduced by the presence of contaminated sediment,
while neither type of particle affected the TeCB concentration in
the water. Addition of uncontaminated particles did not affect the
increase in chlorobenzene residues in the fish. However, the
presence of contaminated particles resulted in higher concentrations
of PeCB in exposed fish than in control fish. No effects were seen
with TeCB. The authors attributed this to the low levels of TeCB on
the particles compared with levels in the water. They concluded that
the influence of contaminated particles on the bioconcentration of
hydrophobic chemicals by fish depends on the hydrophobicity of the
chemicals. The particles may act as a source of the compounds.
Van Hoogen & Opperhuizen (1988) exposed guppies in acute toxicity
tests to 1,2,3-TCB, 1,2,3,4-TeCB, or PeCB in a continuous-flow
system. Fish died having reached the lethal dose of chlorobenzene
for fish, which was between 2.0 and 2.5 mmol/kg and was independent
of the exposure concentration. The authors suggested that this value
was not affected by the route of administration. In addition, the
level of chlorination did not influence the lethal dose expressed in
mmol/kg. When uptake and elimination rate constants were calculated,
any combination of exposure time and concentration required to reach
the lethal dose could be calculated.
4.2.5 Biomagnification
No studies were found concerning the possibility that concentrations
of chlorobenzenes may increase as they move up the food chain.
4.2.6 Ultimate fate following use
As discussed in section 4.1, there is a preferential exchange of
chlorobenzenes from water to the atmosphere. However, in natural
waters containing appreciable amounts of suspended organic matter,
chlorobenzenes may be retained and transported within the aquatic
environment. The accumulation of chlorobenzenes in aquatic sediments
is striking, concentrations being at least 1000 times higher than
those found in the water. Available data suggest, therefore, that
soils rich in organic matter may be a major environmental sink for
these compounds (Elder et al., 1981; Oliver & Nicol, 1982).
Historical evidence for the persistence of chlorobenzenes and for
sediments acting as an environmental sink for these compounds has
been reported by Durham & Oliver (1983). Radionuclide measurements
were used to construct age profiles for Lake Ontario sediments. The
age of lake bottom sediments near the mouth of the Niagara River was
correlated with the concentrations of chlorobenzenes found in the
sediment samples. Over an 80-year period, the concentrations of all
chlorobenzene isomers increased from 0.4 to 15 µg/litre in 1898-1904
to a peak of 18 to 1100 µg/litre in 1959-67, and then declined to 6
to 110 µg/litre in 1980-81. The rise and fall of chlorobenzene
levels closely followed the rise and fall of the total USA
production figures for all chlorobenzenes for a similar period. The
Niagara River is considered to be a major source of chlorobenzene
pollution in Lake Ontario (Oliver & Nicol, 1982).
In Canada, recent data indicate that levels of chlorobenzenes in
sediments are highest in the industrialized Central Region (Ontario
and Quebec). Mean concentrations of the dichlorobenzenes (the
congeners that are present at the highest levels) were 130 µg/kg for
the 1,2-isomer and 46 µg/kg for both the 1,3- and 1,4-isomers. Mean
levels of other congeners, which were also present at elevated
concentrations (above individual detection limits), were 43 µg/kg,
39 µg/kg, and 29 µg/kg for 1,2,3,4-TeCB, PeCB, and 1,2,4-TCB,
respectively (NAQUADAT, 1987).
When the proportion of each congener (di- to penta-) to the total
chlorobenzene content of the water of the Niagara River (a major
source) was compared with that found in the sediment of Lake
Ontario, Oliver et al. (1989) concluded that "increasing the
chlorine content on the benzene ring leads to higher relative
accumulation of the chemicals in sediments". DCBs, TCBs, TeCBs, and
PeCB constituted 68%, 21%, 8%, and 2% of the total chlorobenzenes
(di- to penta-) measured in the water samples from the Niagara
River; comparable values for sediment in Lake Ontario were 12%, 31%,
21%, and 9%, respectively.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental Levels
5.1.1 Air
Levels of chlorobenzenes in ambient outdoor air are presented in
Table 7. Although the available data are insufficient to make a
reliable estimate of human exposure from the atmosphere, it can be
concluded that mean levels of chlorobenzenes (mono- to tri-) in
ambient air are in the tenths of µg/m3 range; however, maximum
values can range up to 100 µg/m3. Seasonal variations in the
concentrations of 1,4-DCB in ambient air have also been reported,
with concentrations increasing with increasing temperature (Hanai et
al., 1985). No data are available concerning levels of
tetrachloro-benzene and pentachlorobenzene in the ambient air,
though these congeners have been detected (but not quantified) in
the fly ash from municipal incinerators (Eiceman et al., 1979,
1981).
Chlorobenzenes have also been detected in rainwater, presumably
through transfer from the ambient air; Pankow et al. (1983) found
all 3 DCB isomers and 1,2,4-TCB, at levels of less than 10 ng/litre
at selected sites in Oregon and California. In the United Kingdom,
1,4-DCB was detected in rainwater at a level of 0.01 ± 0.005
µg/litre (Fielding et al., 1981).
In general, the levels of the chlorobenzenes in indoor air (Table 8)
are similar to those in ambient air. However, in several cases,
levels have been much higher. For example, concentrations found in
basements in the Love Canal area (up to 190 µg/m3 for total
dichlorobenzenes) and in the wardrobe of a Tokyo residence (up to
1700 µg 1,4-DCB/m3 detected in 1 sample) may be explained by the
proximity of a chemical dump and the use of 1,4-DCB as a moth
repellent, respectively.
5.1.2 Water
Chlorinated benzenes have been detected in sewage sludge, municipal
waste water, surface and ground waters, and in drinking-water.
However, in 12 sewage sludges in the United Kingdom, the
concentrations of chlorobenzenes ranged from <0.01 mg/kg dry weight
for PeCB to 40.2 mg/kg dry weight for 1,3-DCB, with a general
reduction in concentration with increased chlorine substitution
(Rogers et al., 1989).
Table 7. Chlorobenzenes in outdoor air
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
MCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.9 (2.3)
Phoenix, Arizona 0.9 (2.3)
Oakland, California 0.45 (1.4)
Harkov et al. (1983) 38 (35) USA; cities:
Newark, New Jersey 0.5
Elizabeth, New Jersey 0.4
Camden, New Jersey 0.3
Levine et al. (1985) 9 USA
Hamilton, Ohio ND - 43
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites 0.2 - 3.6
and landfills in New (20.4)
Jersey
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.4 (0.4)
Pellizzari et al. (1986) USA; cities:
20 Greenboro, North Carolina median 0.029 (0.57)
11 Houston, Texas median 0.045 (1.3)
71 Elizabeth/Bayonnne, New Jersey median 0.43 (6.3)
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Environment Canada (1986) Canada
46 (43) Montreal 0.2 (1.0)
100 (83) Toronto 0.38 (2.2)
1,2-DCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.08 (0.3)
Phoenix, Arizona 0.14 (1.4)
Oakland, California 0.22 (0.2)
Bauer (1981) 89c Germany; city and environs:
Bochum 8.3
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites 0.03 - 3.2 (25.1)
and landfills in New Jersey
Pellizzari et al. (1986) USA
25 Los Angeles - winter median 0.20 (0.55)
23 Los Angeles - summer median 0.03 (0.82)
10 Antioch/West, Pittsburgh median 0.02 (0.58)
Wallace (1986); USA
Wallace et al. (1985) 24 Los Angeles (February) 0.2
24 Los Angeles (May) 0.8
10 Contra Costa (June) 0.07
175 Elizabeth/Bayonne, New Jersey 0.17
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Environment Canada (1986) Canada
40 (3) Montreal 0.0 (0.1)
70 (1) Toronto 0.02 (0.1)
Harkov et al. (1983) 38 (29) USA; cities:
Newark, New Jersey 0.18
Elizabeth, New Jersey 0.12
Camden, New Jersey 0.06
1,3-DCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.05 (0.02)
Phoenix, Arizona 0.05 (0.17)
Oakland, California 0.04 (0.1)
Bauer (1981) West Germany; city and environs:
89c Bochum 3.7
Levine et al. (1985) USA
9 Hamilton, Ohio ND - 100
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.6 (<0.6)
Environment Canada (1986) Canada
67 (8) Toronto 0.02 (0.30)
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
1,3-, 1,4-DCBs
Wallace (1986); Wallace et al. (1985, USA
1986) 86 Elizabeth/Bayonne, New Jersey (1981) 1.7
60 Elizabeth/Bayonne, New Jersey (1982) 1.3
9 Elizabeth/Bayonne, New Jersey (1983) 1.2
24 Los Angeles (Feb.) 2.2
24 Los Angeles (May) 0.8
10 Contra Costa (June) 0.3
Pellizzari et al. (1986) USA
85 Elizabeth/Bayonne, New Jersey (1981) median 0.8 (13)
71 Elizabeth/Bayonne, New Jersey (1982) median 1.2 (7.6)
9 Elizabeth/Bayonne, New Jersey (1983) median 0.32 (4.6)
25 Los Angeles (winter) median 1.8 (21)
2 Los Angeles (summer) median 0.72 (2.4)
10 Antioch/West, Pittsburgh median 0.25 (1.0)
1-DCB
Morita & Ohi (1975) Tokyo, Japan
3 (3) city centre 2.7 - 4.2
3 (3) suburbs 1.5 - 2.4
Harkov et al. (1983) 38 (32) USA
Newark, New Jersey 0.3
Elizabeth, New Jersey 0.4
Camden, New Jersey 0.2
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites and 0.2 - 5.2 (50.4)
landfills in New Jersey
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.6 (<0.6)
Environment Canada (1986) Canada
46 (46) Montreal 0.3 (0.8)
72 (69) Toronto 0.37 (2.14)
De Bortoli et al. (1985) 15 (3) Northern Italy
various towns <5(4)
1,2,3-TCB
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.8 (<0.8)
Bauer (1981) 89c Germany; city and environs:
Bochum 0.40
1,2,4-TCB
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.8 (<0.8)
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.05 (0.25)
Phoenix, Arizona 0.02 (0.08)
Oakland, California 0.02 (0.11)
1,3,5-TCB
Bauer (1981) 89c Germany; city and environs:
Bochum 0.5
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Lebret (1985) Netherlands
Ede and Rotterdam median <0.8(<0.8)
a Number of positive observations given in brackets where possible.
NA - not available.
* - Samples obtained during 2-weeks mobile sampling in the cities indicated; samples taken hourly, 24 h/day.
b Maximum values are in brackets.
ND - not detected.
c Samples obtained sporadically, over a 6-month period, from 3 locations within the city and its environs; averages from 89 samples.
Table 8. Chlorobenzenes in indoor air
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
MCB
Potenta & Saunders (1983) USA - urban Office 2 (2) 1.8 - 3.2
Pellizzari (1982) USA - urban house basements 6 (5) ND - 4.2
(Love Canal area)
Lebret (1985) Netherlands - urban Ede - post-war homes 134 median <0.4 (0.4)
Ede - <6-year-old homes 96 median <0.4 (27)
Rotterdam 89 median <0.4 (3)
Wallace et al. (1984) USA Personal air samples:
New Jersey subjects 160 (115) 0.05 - 26.5 median 0.61
North Carolina subjects 60 (13) 0.05 - 3.66
Pellizzari et al. (1986) USA Greensboro, North Carolina 20 median 0.026 (0.7)
Houston, Texas 11 median 0.035 (0.75)
Elizabeth/Bayonne, New Jersey 71 median 0.42 (6.6)
DCBc
Pellizzari et al. (1982) USA house basements 6 (6) 0.65 - 190
(Love Canal area)
Pellizzari et al. (1986) USA Greensboro, North Carolina 20 median 0.09 (60)
Baton Rouge/Geismar, 27 median 2.1 (120)
Louisiana
Houston, Texas 11 median 5.5 (21)
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
1,2-DCB
Wallace (1986) USA Personal air samples:
Los Angeles (Feb) 115 0.4
Los Angeles (May) 50 0.3
Contra Costa (June) 68 0.6
Pellizzari et al. (1986) USA Los Angeles - winter 25 median 0.12 (2.5)
Los Angeles - summer 23 median 0.03 (11)
Antioch/West, Pittsburg - 10 median 0.03 (0.49)
summer
Otson & Benoit (1986) Canada - urban houses 9 (6.5)
1,3-DCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.6 (9)
Ede - <6-year-old homes 96 median <0.6 (6)
Rotterdam 89 median <0.6 (6)
1,4-DCB
Lebret (1985) Netherlands Ede - post-war homes 134 median 2 (138)
Ede - <6-year-old homes 96 median <0.6 (240)
Rotterdam 89 median <0.6 (299)
Morita & Ohi (1975) Japan - urban home bedroom 1 (1) 105
closet 1 (1) 315
wardrobe 1 (1) 1700
Potenta & Saunders (1983) USA - urban office 2 (1) trace
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
De Bortoli et al. (1985) Northern Italy houses 15 (9) 55(230)
1,3-, 1,4-DCBs
Wallace (1986); Wallace et al. USA Personal air samples:
(1985, 1986) Elizabeth/Bayonne, 340 45
New Jersey - fall
Elizabeth/Bayonne, 150 50
New Jersey - summer
Elizabeth/Bayonne, 49 71
New Jersey - winter
Los Angeles - Feb 115 18
Los Angeles - May 50 12
Contra Costa - June 68 5.5
Pellizzari et al. (1982) USA Elizabeth/Bayonne, 85 median 2.8 (915)
New Jersey - fall
Elizabeth/Bayonne, 71 median 2.6 (1550)
New Jersey - summer
Elizabeth/Bayonne, 9 median 1.2 (120)
New Jersey - winter
Los Angeles - winter 25 median 2.8 (214)
Los Angeles - summer 23 median 1.0 (170)
Antioch/West, Pittsburgh 10 median 0.44 (7.5)
TCBsc
Pellizzari et al. (1986) USA house basements 6 (6) 0.07 - 33
(Love Canal area)
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
1,2,3-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (3)
Ede - <6-year-old homes 96 median <0.8 (28)
Rotterdam 89 median <0.8 (3)
1,2,4-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (15)
Ede - <6-year-old homes 96 median <0.8 (33)
Rotterdam 89 median <0.8 (<0.8)
1,3,5-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (8)
Ede - <6-year-old homes 96 median <0.8 (5)
Rotterdam 89 median <0.8 (<0.8)
TeCBsc
Pellizzari (1982) USA house basements 6 (6) 0.03 - 20
(Love Canal area)
PeCB
Pellizzari (1982) USA house basements 6 (4) trace - 0.49
(Love Canal area)
a Number of positive observations given in brackets where available.
b Mean concentrations in µg/m3; maximum values obtained are given in brackets.
ND - not detected.
c Sum of all isomers.
Data on levels of the lower chlorinated benzenes (MCB, DCBs, and
TCBs) in waste water indicate that MCB is detected the most often
and at the highest concentrations, occasionally exceeding 1
mg/litre. Reported levels of DCB and TCB isomers have been lower. In
a survey of industrial waste waters in the USA (44 264 samples),
levels of MCB averaged 667 µg/litre and ranged from 11 to 6400
µg/litre. Other chlorobenzenes detected were 1,2-DCB (12-860
µg/litre; mean 141 µg/litre); 1,3-DCB (10-39 µg/litre; mean 21
µg/litre); 1,4-DCB (10-410 µg/litre; xmean 79 µg/litre); and
1,2,4-TCB (12-607 µg/litre; mean 161 µg/litre) (Neptune, 1980). In a
survey of waste water throughout the USA, concentrations of 1,2-DCB
ranged from 15 to 690 µg/litre and concentrations of 1,2,4-TCB
ranged from 0.25 to 500 µg/litre (Ware & West, 1977). A survey of 4
municipal treatment plants in Georgia revealed levels of DCB and TCB
in the influent of 3-146 µg/litre and 1-60 µg/litre, respectively.
Concentrations in the effluent ranged from 0 to 268 µg/litre and 0
to 13 µg/litre, respectively (Gaffney, 1976). The increase in DCB
levels in the effluent was believed to be the result of chlorination
during the secondary phase of the waste water treatment. In waste
water from major municipal treatment plants in Southern California,
concentrations of DCB ranged from 0.2 to 435 µg/litre, while
concentrations of 1,2,4-TCB and 1,3,5-TCB were 130 µg/litre and
<0.2 µg/litre, respectively (Young & Heesen, 1978).
Oliver & Nicol (1982) reported that the concentrations of
dichloro-benzenes in raw water in the Great Lakes were much greater
than those of the more highly chlorinated congeners, the 1,4-isomer
being present at the highest level. This isomer was particularly
prevalent in effluent from sewage-treatment plants.
Data on the levels of chlorobenzenes (mono- to penta-) in surface
waters are presented in Table 9. It is difficult to draw general
conclusions concerning levels of chlorobenzenes that are commonly
present in surface water, because of the paucity of available data
and the considerable variations in reported concentrations, mainly
owing to differences in the proximity to industrial sources. In
general, it appears that levels are in the ng-µg/litre range;
however, levels in the vicinity of industrial sources may
occasionally range up to 0.1 mg/litre.
The levels of chlorobenzenes found in some drinking-water supplies
are shown in Table 10. Although chlorobenzenes are unlikely to be
changed chemically during the treatment, it is possible that some
may be lost at the aeration and chlorination stages, because the
compounds tend to be redistributed preferentially to air from water.
It has been suggested, however, that water chlorination itself may
produce chlorobenzenes by the reaction of chlorine (or one of its
aqueous species) with organic material (both natural and man-made)
in the raw water supply (Carlson et al., 1975; Glaze et al., 1978;
Höfler et al., 1983). For example, Höfler et al. (1983) demonstrated
that all of the chlorobenzenes were detected following the reaction
of benzene (tenths of a µmol/litre) with aqueous sodium
hypochlorite. There is slight evidence from surveys of
chlorobenzenes in drinking-water that they may be formed during
treatment; Otson et al. (1982a,b) observed that the frequency of
detection of MCB at 30 Canadian water treatment facilities was less
for raw water than for drinking-water samples (5 and 18%,
respectively); however, levels were too low to permit
quantification.
In general, the lower chlorinated benzenes are detected more
frequently in drinking-water and are present in higher
concentrations; however, even for these compounds, mean levels are
generally less than 1 µg/litre and rarely exceed 50 µg/litre. Most
of the dichloro-benzene in drinking-water is present as the
1,4-isomer, probably because of its release into surface water from
urinal deodorant blocks (Oliver & Nichol, 1982).
5.1.3 Soil
Very few published data are available on the levels of
chlorobenzenes in soil. With the exception of soil near poorly
maintained waste sites, the presence of chlorobenzenes in soil would
appear to be associated with pesticide use. For example, PeCB has
been detected at a level of 0.09 mg/kg in fields treated with
quintozene in Finland (Rautapaa et al., 1977).
5.1.4 Food
Little information is available concerning the presence of
chloro-benzenes (mono- to penta-) in food, but levels in various
seafoods have been measured. Concentrations of 1,4-DCB in mackerel,
caught off the coast of Japan, averaged 0.05 mg/kg on a whole-fish,
wet-weight basis (Morita et al., 1975). All of the chlorobenzenes
(mono- to penta-) were detected in trout from the Great Lakes at
levels ranging from 0.1 to 16 µg/kg whole-fish, wet weight (Oliver &
Nicol, 1982). With the exception of MCB, chlorobenzenes (di- to
penta-) were measured in samples of sprats from south-east Norway in
the vicinity of an unspecified "source of contamination". Levels of
PeCB were highest, ranging from 0.01 to 3.7 mg/kg, while levels of
trichloro- and tetrachlorobenzenes ranged between <0.01 and 0.5
mg/kg (Lunde & Ofstad, 1976). Levels of total chlorobenzenes in the
edible tissue of freshwater fish from highly polluted and industrial
areas of Yugoslavia were 1.8 mg/kg, on a fat basis, whereas levels
in fish from lightly polluted agricultural and woodland areas, and
in marine fish, were 0.2 mg/kg and 0.4 mg/kg, respectively (Jan &
Malnersic, 1980). The 1,4-DCB isomer was identified among other
volatile organic compounds collected from watercress (Spence &
Tucknott, 1983). The source of the compound in the plant matter was
not identified, but it is likely that it was present in the water in
which the plants were grown.
Table 9. Chlorinated benzenes in surface watersa
Chemical Location Levelsb Reference
MCB Glatt River, Germany + US EPA (1985)
Delaware River ND - 7000 Sheldon & Hites (1978)
Ohio River ND - >10 000 US EPA (1985)
DCBs Delaware Riverc ND - 400 Sheldon & Hites (1978)
Great Lakesd 32 - 71 (27) Oliver & Nicol (1982)
Grand River, Canada ND - 77 (11) Oliver & Nicol (1982)
Niagara Falls, New York + Elder et al. (1981)
1,2-DCB Atlantic Region, Canada <20 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 3.9 - 240 (23) Oliver & Nicol (1984)
1,3-DCB Atlantic Region, Canada <2 - 310 (22) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 2.1 - 110 (11) Oliver & Nicol (1984)
1,4-DCB Glatt River, Germany 30 - 900 US EPA (1985)
Ohio River ND - >10 000 US EPA (1985)
Atlantic Region, Canada <20 - 130 (22) NAQUADAT (1987)
Great Lakes trace - >100 Otson (1987)
Niagara-on-the-Lake, Canada 9.0 - 310 (36) Oliver & Nicol (1984)
TCBs Merrimack River, Massachusettsc 100 - 500 Hites (1973)
Delaware Riverc ND - 1000 Sheldon & Hites (1978)
Niagara Falls, New York 100 - 8000 Elder et al. (1981)
Great Lakesd 0.1 - 1.6 (0.5) Oliver & Nicol (1982)
Grand River, Canadad ND - 8.7 (2.1) Oliver & Nicol (1982)
Atlantic Region, Canada <4.0 NAQUADAT (1987)
Table 9 (continued)
Chemical Location Levelsb Reference
1,2,4-TCB Niagara-on-the-Lake, Canada 5.8 - 120 (16) Oliver & Nicol (1984)
1,2,3-TCB Niagara-on-the-Lake, Canada 1.4 - 30 (3.5) Oliver & Nicol (1984)
1,3,5-TCB Niagara-on-the-Lake, Canada 0.19 - 6.8 (0.84) Oliver & Nicol (1984)
TeCBs Niagara Falls, New Yorkc 100 - 200 000 Elder et al (1981)
Great Lakesc ND - 0.8 (0.12) Oliver & Nicol (1982)
Grand River, Canadad ND - 0.2 (0.05) Oliver & Nicol (1982)
1,2,3,5-TeCB Atlantic Region, Canada <2 - 20 (2.0) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.10 - 1.4 (0.41) Oliver & Nicol (1984)
1,2,4,5-TeCB Altantic Region, Canada <2 - 4 (2.0) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.39 - 9.3 (2.0) Oliver & Nicol (1984)
1,2,3,4-TeCB Atlantic Region, Canada <2 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 1.4 - 36 (4.5) Oliver & Nicol (1984)
PeCB Niagara Falls, New York ND - 100 000 Elder et al. (1981)
Great Lakes ND - 0.6 (0.12) Oliver & Nicol (1982)
Grand River, Canada ND - 0.1 (0.05) Oliver & Nicol (1982)
Atlantic Region, Canada <2.0 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.34 - 6.4 (1.3) Oliver & Nicol (1984)
a Modified from US EPA (1985).
b Range in ng/litre unless indicated.
ND - not detected.
+ - detected.
c Unidentified isomers.
d All isomers.
Table 10. Chlorobenzene levels in drinking-water supplies
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
MCB
Otson et al. (1982a, 1982b) < 1 µg/litre NA 90(16) samples from 30 water treatment
plants in Canada; maximum of
5 µg/litre in one instance
US EPA (1980a) approx 7 µg/litre 0.1-27 µg/litre NA 8 locations in the USA
Barkley et al. (1980) 25 ng/litre 10-60 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
Wallace et al. (1984) NA 0.02-0.02 µg/litre 75(0) water supply of 9 New Jersey homes
Wallace et al. (1984) 0.03 µg/litreb 0.03-0.03 µg/litre 45(0) work water samples of 9 New Jersey
subjects
Wallace et al. (1984) NA 0.03-3.10 µg/litre 30(2) Water supply of 3 North Carolina
homes
Wallace et al. (1984) NA 0.25-0.25 µg/litre 18(0) work water samples of 3 North
Carolina homes
DCBs
US EPA (1980b) NA 1-3 µg/litre NA National survey of the USA
Barkley et al. (1980) 159 ng/litre 10-800 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
1,2-DCB
Oliver & Nicol (1982) 3 ng/litre ND-7 ng/litre NA 3 cities by Lake Ontario
Table 10 (continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
1,3-DCB
Oliver & Nicol (1982) 1 ng/litre ND-2 ng/litre NA 3 cities by Lake Ontario
Otson et al. (1982a,b) < 1 µg/litre NA 90(1) samples from 30 water treatment
plants in Canada; maximum of
1 µg/litre
1,4-DCB
Oliver & Nicol (1982) 13 ng/litre 8-20 ng/litre NA 3 cities by lake Ontario
Fielding et al. (1981) 0.05 µg/litre 0.01-0.08 µg/litre 26(6) from 4 selected sites in England;
reported levels do not include a
sample affected by the use of "air
freshener" blocks containing
1,4-DCB (4 µg/litre)
Otson et al. (1982a,b) < 1 µg/litre NA 90(6) samples from 30 water treatment
plants in Canada; maximum of
<1 µg/litre
TCBs
Barkley et al. (1980) 500 ng/litre 300-760 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
1,2,3-TCB
US EPA (1980a) NA 21-46 µg/litre NA Catawba, North Carolina
Oliver & Nicol (1982) 0.1 ng/litre 0.1-0.1 ng/litre NA 3 cities by Lake Ontario
Table 10 (continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
1,2,4-TCB
US EPA (1980a) 33.6 µg/litre 0.007-275 µg/litre NA 8 locations in the USA (does not
include one sample where levels of
500 µg/litre were detected)
Oliver & Nicol (1982) 2 ng/litre 1-4 ng/litre NA 3 cities by Lake Ontario
1,3,5-TCB
US EPA (1980a) 2.2 µg/litre 0.006-26 µg/litre NA 8 locations in the USA
Oliver & Nicol (1982) ND ND NA 3 cities by Lake Ontario
TeCBs
Barkley et al. (1980) 927 ng/litre ND-2000 ng/litre 9(6) Old Love Canal, Niagara Falls, USA
1,2,3,5-TeCB
Oliver & Nicol (1982) ND ND NA 3 cities by Lake Ontario
1,2,4,5-TeCB
Oliver & Nicol (1982) 0.2 ng/litre ND-0.3 ng/litre NA 3 cities by Lake Ontario
1,2,3,4-TeCB
Oliver & Nicol (1982) 0.3 ng/litre 0.1-0.4 ng/litre NA 3 cities by Lake Ontario
PeCB
Oliver & Nicol (1982) 0.04 ng/litre 0.03-0.05 ng/litre NA 3 cities by Lake Ontario
Table 10 continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
Barkley et al. (1980) NA ND-240 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
a Number of positive observations given in brackets where possible.
NA - not available.
b Median concentration.
ND - not detected.
In an isolated incident in England, tainted pork (lean and fat meat)
contained 5-20 mg 1,4-dichlorobenzene/kg (Watson & Patterson, 1982).
The 1,4-isomer of DCB was also present in other batches of pork from
the same source, and other meat products from various commercial
sources; however, concentrations were less than 25 µg/kg. It has
been suggested that the source of the chlorobenzenes in pork was the
use of organochlorine pesticides in animal husbandry and their
subsequent metabolism by the animals to chlorobenzenes (Mottram
et al., 1983). Chlorobenzenes have been detected in cows' milk as
well as beef meat in Yugoslavia. In cows' milk, residues ("as in"
basis) for the DCBs ranged from "not detected" (1,3-DCB) to 5.3
µg/kg (1,4-DCB). TCB and TeCB levels ranged from 0.7 µg/kg
(1,2,4-TCB) to 1.5 µg/kg (1,2,3-TCB) and 0.02 µg/kg (1,2,3,4-TeCB)
to 1.10 µg/kg (1,2,3,5-TeCB), respectively. In beef, the DCB
concentrations ranged from "not detected" (1,3-DCB) to 5.0 µg/kg
(1,4-DCB), while TCB and TeCB levels were 1.0 µg/kg (1,2,4-TCB) to
1.8 µg/kg (1,2,3-TCB) and 0.02 µg/kg (1,2,3,4-TeCB) to 1.00 µg/kg
(1,2,3,5- and/or 1,2,4,5-TeCB), respectively. PeCB was detected in
both milk and meat at a concentration of 0.05 µg/kg (Jan, 1983b).
Jan (1980) identified chlorobenzenes (except MCB) in the oils of 9
different seeds, the highest level being 0.09 mg/kg (1,4-DCB) in
corn. DCBs and TCBs have also been detected in dried legumes. Levels
of DCBs ranged from 1.8 to 52.9 µg/kg, while TCB levels ranged from
3.0 to 8.9 µg/kg. These vegetables were grown under controlled
conditions with no pesticide application, indicating that the origin
of the chlorobenzenes was probably environmental contamination
(Lovegren et al., 1979). TeCB residues have also been reported in
potatoes. In composite samples of boiled, fried, and baked potatoes,
levels of 1,2,4,5-TeCB were 0.095, 0.051, and 0.110 mg/kg,
respectively. The authors stated that the higher levels in the baked
potatoes were probably attributable to the fact that the potatoes
were not peeled prior to analysis (Heikes et al., 1979).
An isolated instance of PeCB contamination of cooked ham (present in
only 1 out of 37 samples tested) has also been reported; the level
measured was 0.05 mg/kg (Greve, 1973). PeCB has also been detected
in peanut butter in the USA; concentrations in 11 samples ranged
from 1.8 to 62 µg/kg, the average being 16 µg/kg (Heikes, 1980).
PeCB at 0.03 mg/kg was reported in Finnish rye grown on soils
previously treated with Quintozene (Rautapää et al., 1977).
5.1.5 Human milk
Available data indicate that human milk may be a source of exposure
to chlorobenzenes (mono- to penta-) for suckling infants. MCB was
found in 5 out of 8 samples of human milk in the USA. DCBs (isomers
not specified) were found in all of the 8 samples, whileTCBs
(isomers not specified) were found in only 1 out of the 8 samples;
however, levels were not quantified (Pellizzari et al., 1982). Jan
(1983a) detected all chlorobenzene isomers (except MCB, for which
analysis was not conducted) in the breast milk (3-5 days after
parturition) of women in a Yugoslavian hospital (Table 11). The
concentrations of DCBs were highest, averaging 25 µg/kg, on a whole
milk basis, and ranging up to 35 µg/kg for the 1,4-isomer. The mean
concentrations of the isomers of tri-, tetra, and
pentachlorobenzenes were <5 µg/kg (whole milk). Davies & Mes
(1987) also detected chlorobenzene residues (di- to penta-) in the
breast milk (3-4 weeks after parturition) of Canadian women at mean
levels ranging from <1 µg/kg (1,2,3-TCB and PeCB) to a maximum of 6
µg/kg (1,3- + 1,4-DCB) in whole milk. Concentrations in the breast
milk of women of the indigenous population were slightly higher
(Table 11). Tetrachlorobenzenes were detected, but not quantified.
Recoveries ranged from 69% (1,2-DCB) to 100% (1,2,4-TCB).
5.1.6 Consumer products
As discussed in section 3, chlorobenzenes are used in a wide variety
of consumer and commercial products. However, there is little
information concerning the levels of chlorobenzenes present as
contaminants in consumer products. It is reasonable to expect,
however, that chlorobenzenes could be incidental contaminants in
chemical products, such as solvent formulations and pesticides.
Airborne levels of 1,4-DCB, possibly associated with its use as a
moth repellent, are presented in Table 8 (section 5).
5.2 Human Exposure from All Sources
5.2.1 General population
Although available data are limited and vary widely, it is possible
to estimate an approximate intake of chlorobenzenes from various
sources (Table 12). The limited data suggest that the daily intake
of chlorobenzenes from the air might be considerably greater than
that from food and drinking-water. It can also be concluded that the
intake from air is greatest for the lower, more volatile
chlorobenzenes and that intake from food, compared with that from
other sources, increases with the degree of chlorination. These
conclusions are also supported by data on concentrations of
chlorobenzenes in human tissues, fluids, and exhaled air, presented
in Table 13.
On a body weight basis, breast-fed infants may receive higher doses
of the chlorobenzenes than members of the adult population. Assuming
a daily intake of 0.714 litres of milk for a 5-kg infant, estimated
intakes for infants based on levels measured in breast milk in
Yugoslavia (Jan, 1983a) are 5.57 µg/kg, 1.0 µg/kg, 0.43 µg/kg, and
0.1 µg/kg body weight for total DCBs, TCBs, TeCBs, and PeCB,
respectively. On the basis of recent Canadian data (Mes et al.,
Table 11. Levels of chlorobenzenes (mono- to penta-) in human milk
Compound Country Average level
µg/kg whole milk
(range)c
1,2-DCB Yugoslaviaa 9(5-12)
Canada-national surveyb 2.9
Canada-indigenous populationb 8.1
1,3-DCB Yugoslavia <5
1,4-DCB Yugoslavia 25(5-35)
1,3- & 1,4-DCB Canada-national survey 6.1
Canada-indigenous population ND
1,2,3-TCB Yugoslavia 5(2-10)
Canada-national survey 0.3
Canada-indigenous population 0.1
1,2,4-TCB Yugoslavia 1(ND-4)
Canada-national survey 0.6
Canada-indigenous population 1.2
1,3,5-TCB Yugoslavia 1(ND-3)
Canada-national survey ND
Canada-indigenous population 0.4
1,2,3,4-TeCB Yugoslavia 1(ND-3)
1,2,3,5- & Yugoslavia 2(ND-5)
1,2,4,5-TeCB
PeCB Yugoslavia 0.7(ND-3)
Canada-national survey 0.1
Canada-indigenous population trace
a Jan (1983a).
b Davies & Mes (1987).
c ND - not detectable (detection limits not specified).
Table 12. Possible daily exposure (µg/kg) body weight to total chlorobenzenes from
various sources
Ambient air Ambient air Drinking-water Food
(US)a (Canada)a (Canada)b (Canada)c
MCB 0.882 0.166 < 0.029 NAd
DCB 0.930 0.203 0.00049 0.0013
TCB 0.039 NAd 0.00006 0.0010
TeCB NAd NAd 0.000014 0.0015
PeCB NAd NAd 0.0000011 0.0011
a Air intakes, calculated from mean outdoor levels in Canada and the USA
presented in Table 7, assuming a daily inspired volume of 20 m3 for a
70-kg adult.
b Drinking-water intakes for chlorobenzenes were estimated from the levels in
Canadian drinking-water presented in Table 10, and assuming a daily tapwater
consumption of 2 litres for a 70 kg adult.
c Food intakes were estimated using the mean concentrations of chlorobenzenes
in the tissues of fish from the Great Lakes (Oliver & Nicol, 1982), assuming
a daily intake of 18.5 g fish for a 70-kg adult (Statistics Canada, 1981).
d NA - not available.
1986), estimated intakes are 1.29 µg/kg, 0.29 µg/kg, and <0.14µg/kg
body weight for total DCBs, TCBs, and PeCB, respectively. In view of
the small number of samples analysed and the lack of relevant data
for most regions, these figures should be considered to provide only
rough approximations of the relative intakes from various sources.
5.2.2 Occupational exposure
Few data are available on the levels of the chlorobenzenes in the
workplace. Levels of 1,4-DCB in the atmosphere of facilities
manufacturing, for example, air fresheners, urinal cakes, or moth
repellents, could be quite high, taking into account the volatile
nature of the compound. In one manufacturing plant, concentrations
of 1,4-DCB ranged from 42 to 288 mg/m3, the average being
204 mg/m3 (Ware & West, 1977). Levels of MCB in chemical plants
ranging up to 18.7 mg/m3 have been reported (Cohen et al., 1981).
Very low levels of MCB (1.8-3.2 µg/m3) and a trace of 1,4-DCB have
been found in office air (Table 8). These values are less than the
occupational standards in several countries, which vary between 350
and 450 mg/m3 (IRPTC, 1986).
5.3 Human Monitoring Data
Some data are available on the concentrations of chlorobenzenes in
human blood and adipose tissue (Table 13). The 1,4-isomer of DCB was
detected in all samples of adipose tissue (49/49), from hospitals in
Tokyo, at levels ranging from 0.2 to 11.7 mg/kg (Morita & Ohi, 1975;
Morita et al., 1975). The 1,2,4,5-isomer of TeCB was detected in all
samples (15/15), but levels were several orders of magnitude less
than those for 1,4-DCB (0.008-0.039 mg/kg) (Morita et al., 1975).
Levels of PeCB, detected in 92 out of 99 samples of adipose tissue
from autopsies of accident victims throughout Canada were somewhat
similar to those reported for 1,2,4,5-TeCB in Tokyo; concentrations
ranged from 0.001 to 0.020 mg/kg (Mes et al., 1982).
MCB was detected in the blood of 8 out of 9 residents of the
polluted Love Canal area at concentrations up to 17µg/litre;
however, levels in a control population were not determined (Barkley
et al., 1980). Although the different isomers of DCB were detected
more frequently in the blood of residents of the Love Canal area
(7-18 out of 36 compared with 2 out of 12 in the control
population), the levels of 1,2- and 1,3-DCB were not significantly
different from those in"unexposed" volunteers (i.e., <10 ng/g).
Levels of 1,4-DCB in the blood of Love Canal residents ranged from 4
to 36 ng/g, whereas concentrations in "unexposed" volunteers ranged
from 2 to 9 ng/g (Bristol et al., 1982). The 1,4-isomer of DCB was
detected in all samples of the blood of 6 Tokyo residents at levels
ranging from 4 to 16 µg/litre (Morita & Ohi, 1975). The 1,2,4,5-TeCB
isomer was detected in the blood of 1 out of 36 residents of the
Love Canal area at a concentration of 2 ng/g, but was not detected
in any of 12 "unexposed" volunteers (Bristol et al., 1982). Even in
occupationally exposed populations, levels of chlorobenzenes in the
blood are low; Lunde & Bjorseth (1977) reported detecting PeCB in
the blood of 9 out of 17 workers at concentrations ranging from 0.06
to 0.26 ng/g.
Table 13. Chlorobenzenes in human tissues, fluids, and exhaled air
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
MCB
Barkley et al. (1980) residents of the Old Love Canal area urine ND - 120 ng/litre (6/9)
blood ND - 17 µg/litre (8/9)
breath ND - trace (1/9)
Wallace et al. (1984) samples from 9 individuals from New Jersey and 3 from North breath 0.07 - 8.15 µg/m3 (31/66)
Carolina
DCB
Barkley et al. (1980) residents of the Old Love Canal area urine ND - 39 µg/litre (7/9)
blood 0.15 - 68 µg/litre (9/9)
breath ND - 5000 ng/m3 (7/9)
Antoine et al. (1986) patients in the USA with possible sensitivities to blood ND - 31 µg/litre (NA/250)
synthetic chemicals
1,3- + 1,4-DCB
Wallace et al. (1984) samples from individuals from North Carolina breath 0.09 - 0.76 µg/m3 (4/5)
Wallace (1986) samples from residents of cities in New Jersey and California breath 2.9 - 8.1 µg/m3 (NA/660)
1,2-DCB
Wallace (1986) samples from residents of Los Angeles breath 0.08 - 0.1 µg/m3 (NA/201)
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 1-4 µg/litre (9/36)
workers) volunteers: 3-4 µg/litre (2/12)
Table 13 (continued)
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
1,3-DCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 3-8 µg/kg (7/36)
workers) volunteers: 3-8 µg/kg (2/12)
1,4-DCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 4-26 µg/kg (18/36)
employees) volunteers: 2-9 µg/kg (2/12)
Morita & Ohi (1975) adipose tissue samples from medical examiners and 3 Tokyo adipose 0.2 - 11.7 mg/kg (34/34)
hospitals; tissue
blood from 6 healthy volunteers blood 4 - 16 µg/litre (6/6)
Morita et al. (1975) samples from hospitals or medical examiners in Tokyo adipose 0.2 - 9.9 mg/kg (15/15)
tissue
Pagnotto & Walkley (1965) analysis for urinary dichlorophenol in workers in urine 10 - 233 mg/litred
various stages of p-dichlorobenzene production
TCB
Barkley et al. (1980) residents of the Old Love Canal area breath ND - 90 ng/m3 (2/9)
1,3,5-TCB
Bristol et al. (1982)
residents of the Love Canal area and 9 volunteers (laboratory blood residents: 0.7 µg/kg (1/36)
employees) volunteers: ND (0/12)
TeCB
Barkley et al. (1980)
residents of the Old Love Canal area blood ND - 2.6 µg/litre (1/99)
breath ND - 180 ng/m3 (2/9)
Table 13 (continued)
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
1,2,4,5-TeCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 2 µg/kg (1/36)
employees) volunteers: ND (0/12)
Morita et al. (1975) samples from hospitals or medical examiners in Tokyo adipose 0.008 - 0.039 mg/kg (15/15)
tissue
PeCB
Lunde & Bjorseth (1977) samples obtained from 17 employees working at various stages in blood 0.06 - 0.26 µg/kg (9/17)
magnesium production
Mes et al. (1982) samples obtained from autopsies of accident victims across adipose 0.001 - 0.020 mg/kg (92/99)
Canada tissue
Barkley et al. (1980) residents of the Old Love Canal area breath ND - 70 ng/m3 (1/90)
Williams et al. (1988) samples obtained from autopsies of individuals from 6 Canadian adipose ND - 43 mg/kg (9/141)
Great Lakes municipalities tissue
a Isomers specified where available.
b Number of subjects specified where available.
c ND - not detected.
d Levels of dichlorophenol found in urine.
6. KINETICS AND METABOLISM
With the exception of limited information concerning MCB and 1,4-DCB
in man, available data on the kinetics and metabolism of
chlorobenzenes (mono- to penta-) have been obtained from studies on
experimental animals.
6.1 Absorption
Although few quantitative data are available, the results of
experimental studies on animals (section 8) and human case reports
of poisonings (section 9) indicate that the chlorobenzenes are
readily absorbed from the gastrointestinal and respiratory tracts.
Available data also indicate that the position of the chlorines in
different isomers of the same congener influences absorption. It is
probable that the rate of transport of the chlorobenzenes (mono- to
penta-) across the gut wall also varies as a function of exogenous
factors, particularly the presence of bile and of dietary lipids in
the gastrointestinal tract, as is the case for hexachlorobenzene
(Koss & Koransky, 1977).
Quantitative data on absorption can be derived from studies on
experimental animals, the most important of these (summarized in
Table 14) are limited to the gastrointestinal tract. For example,
following intragastric administration of 1.5 g of 1,4-DCB to
Chinchilla rabbits, Azouz et al. (1955) did not detect any of the
unchanged compound in the faeces, implying that, under the
conditions of the study, total absorption had occurred. Hawkins et
al. (1980) reported that only 9% (the unabsorbed portion) of a
single radio-labelled dose of 250 mg 1,4-DCB/kg body weight was
present in the faeces of rats with cannulated bile ducts, at 24 h.
Six days following administration by stomach tube of 500 mg/kg body
weight of the 1,2,3,4-, 1,2,3,5- and 1,2,4,5-isomers of TeCB to
Chinchilla rabbits, 5%, 14%, and 16%, respectively, of the
administered doses were recovered unchanged in the faeces (Jondorf
et al., 1958). The percentages of the administered doses recovered
unchanged in the gut contents were 0.5%, 1.4%, and 6.2%,
respectively, suggesting that gastrointestinal absorption of the
compounds is relatively efficient and that the chlorine positions on
the molecule may influence the process. In rhesus monkeys, Rozman
et al. (1979) reported that 95% of an oral dose of 0.5 mg PeCB/kg
body weight was absorbed, as indicated by faecal elimination in the
first 4 days.
6.2 Distribution
The results of available studies indicate that, following
absorption, the chlorobenzenes are initially rapidly distributed to
highly perfused tissues, but then accumulate in fatty tissues,
because of their lipophilic nature (Jondorf et al., 1958; Jacobs
et al., 1974; Villeneuve & Khera, 1975; Jacobs et al., 1977; Kimura
et al., 1979; Hawkins et al., 1980; Linder et al., 1980; Smith &
Carlson, 1980; Chu et al., 1983; Sullivan et al., 1983; Tanaka
et al., 1986; Chu et al., 1987). Transplacental transfer (PeCB) into
the fetal brain and liver tissues has also been observed (Villeneuve
& Khera, 1975). Available data also indicate that, in general,
accumulation is greatest for the more highly chlorinated congeners,
but that it can vary enormously for different isomers of the same
chlorobenzene. For example, when 0.59 g of 14C-labelled MCB was
administered twice daily for 7 days to Dutch rabbits, only 0.05% of
the radiolabel was found in the tissues over the following 7-day
period (Smith et al., 1972). Following oral administration of 500 mg
1,3,5-TCB/kg to Chinchilla rabbits, only 5% of the administered dose
was present in the fat at 8 days, whereas 22% of a similar dose of
PeCB, administered subcutaneously, was present in the fat of the
same species at 10 days (Parke & Williams, 1960). Jondorf et al.
(1958) reported that 25%, 11%, and 5% of an oral dose of 500 mg/kg
was recovered in the fat of Chinchilla rabbits for the 1,2,4,5-,
1,2,3,5- and 1,2,3,4-isomers of TeCB, respectively, at 6 days.
Similarly, Chu et al. (1983) reported that the 1,2,4,5-isomer of
TeCB accumulated in the fat and liver at much higher levels (by 2
orders of magnitude) than the 1,2,3,5 and 1,2,3,4-isomers, following
oral administration for 28 days to rats of diets containing 0.5,
5.0, 50, or 500 mg chlorobenzene/kg, reflecting wide differences in
metabolic rates (section 6.3).
Peak levels in fat, which occurred 6-12 h after oral administration
of 200 or 800 mg 1,4-DCB/kg body weight to rats, were 50 times those
in the blood (Kimura et al., 1979). Braun et al. (1978) reported
that the ratio of 1,2,4,5-TeCB concentrations in fat to those in
plasma in beagle dogs was 650, following 1 month of oral
administration of 5 mg/kg body weight per day. This ratio decreased
steadily to about 280 by the end of the administration period (2
years) and increased rapidly in the 20 months following treatment.
Levels of the same isomer of the chlorobenzenes in various tissues
appear to be similar, regardless of the route of administration.
Hawkins et al. (1980) reported that the concentrations in rat
tissues at 24 h were similar following inhalation of 6000 mg
14C-labelled 1,4-DCB/m3 (1000 ppm) for 3 h/day or administration
of 250 mg/kg body weight per day orally, or subcutaneously, for up
to 10 days. At low levels of exposure, accumulation in adipose
tissue appears to be dose-related, whereas at high levels there
appears to be a disproportionate increase in the adipose tissue
burden. For example, Jacobs et al. (1974) reported a dose-related
accumulation of 1,2-DCB in the abdominal and renal adipose tissue of
rats following administration of a mixture of organic chemicals
including 1,2-DCB at doses of 0.4, 0.8, or 2 mg/kg diet per day for
4-12 weeks. On the other hand, when rats inhaled 14C-labelled MCB
at 455, 1820, or 3185 mg/m3 (100, 400, or 700 ppm) for 1 or 5
days, tissue concentrations increased in proportion to the level of
exposure, except for those in adipose tissue, which increased more
than 30-fold between 455 and 3185 mg/m3 (Sullivan et al., 1983).
Smith & Carlson (1980) demonstrated that starvation for 4 days did
not have any effects on the distribution of 1,2,4-TCB in the fat or
liver of rats following oral administration of 181.5 mg/kg
(1 mmol/kg body weight) per day for 7 days.
6.3 Metabolic Transformation
The chlorobenzenes are metabolized by microsomal oxidation and
proceed principally, either directly or through the formation of a
metastable arene oxide intermediate, to form the corresponding
chlorophenols. These chlorophenols can be excreted in the urine as
mercapturic acids, formed by conjugation with glutathione, or as
glucuronic acid or sulfate conjugates; they may also be eliminated
unchanged, mainly in expired air or faeces.
The results of available studies concerning the metabolism and
elimination of the chlorobenzenes are presented in Table 14. More
detailed comparative metabolic profiles for each of the
chlorobenzenes in rabbits, following oral administration of 0.5 g/kg
body weight, are presented in Table 15. On the basis of the results
of such studies, it can be concluded that: (a) metabolic
transformation of the chlorobenzenes decreases with increasing
degree of chlorination; and (b) metabolism and elimination of the
higher chlorinated congeners is slower and a greater proportion of
the compound is eliminated unchanged in the faeces or expired air.
The position of the chlorine atoms on the benzene ring is also an
important determinant of the rates of metabolism and elimination,
with chlorobenzenes with 2 adjacent unsubstituted carbon atoms
(e.g., 1,2,3-TCB, 1,2,3,4-TeCB) being more rapidly metabolized and
eliminated than congeners having a similar degree of chlorination,
but lacking unsubstituted carbon atoms. The presence of the two
adjacent unsubstituted carbon atoms facilitates the formation of
arene oxides. For isomers without adjacent unsubstituted carbon
atoms, intermediate reactions take place in which chlorine atoms are
shifted to adjacent carbons ("the NIH-shift") to form compounds that
can then form epoxide intermediates (Daly et al., 1972; Jerina &
Daly, 1974).
Table 14. Metabolism and elimination in experimental animals
Compound; Speciesa Study protocol Results
Reference
MCB Dutch rabbit oral administration (gavage) of 19.6% of administered label in urine, 1.05% in
Smith- 0.59 g of 14C-MCB, twice a day faeces and 0.05% in tissues; urinary metabolites
Lindsay et al. for 4 days; collection of urine were glucuronides (33.6%), ethereal sulfates
(1972) and faeces for 7 days (33.9%), mercapturic acids (23.8%), diphenols
(4.17%), monophenols (2.84%) and
3,4-dihydro-3,4-dihydroxy-chlorobenzene (0.57%)
MCB male Wistar intraperitoneal injection of urinary metabolites in 24 h: 4-chlorocatechol
Yoshida & rat 2 mmol/kg body weight in olive (1.6%), 2-chlorophenol (1.6%), 4-chlorophenol
Hara (1985) oil; collection of urine for 3 (4.8%), 3-chlorophenol (3.6%), and
days prior to, and for 4 days 4-chlorophenylmercapturic acid (19.9%)
following, the injection
1,4-DCB female CFY inhalation of 6000 mg after 5 days dosing, compound metabolized and
Hawkins rat 14C-1,4-DCB/m3 3 h/day, or daily eliminated primarily in the urine (91-97%); for
et al. (1980) oral or subcutaneous doses all routes of exposure, primarily 2,5-DCP sulfate
(250 mg/kg) for up to 10 days; (46-54% of total eliminated) and 2,5-DCP
administration of a dose glucuronide in the urine (31-34%) and bile
(250 mg/kg) to rats with (30-42%)
cannulated bile ducts
1,4-DCB Chinchilla oral administration (intragastric metabolism primarily through oxidation to 3,4-DCP
1,2-DCB rabbit in olive oil) of 1.5 g (500 mg/kg) (from 1,2-DCB) and 2,5-DCP (from 1,4-DCB) and
Azouz et al. 1,4-DCB or 500 mg/kg 1,2-DCB eliminated in the urine in the form of glucuronic
(1955) (intragastric in water) and sulfuric acid conjugates; metabolism and
elimination complete in 5-6 days for 1,2-DCB and
>6 days for 1,4-DCB; not detected in the faeces
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB male Charles oral (gastric intubation) or about 84% of the oral dose and 78% of iv dose
Lingg et al. River rat and intravenous administration of eliminated by rats and 40% (oral) and 22% (iv)
(1982) female rhesus 10 mg/kg by rhesus monkeys in the urine in 24 h; faecal
monkey elimination accounted for 11% (oral) and 7% (iv)
in rats, and <1% from both routes in monkeys;
48-61% comprised an isomeric pair of
3,4,6-trichloro-3,5-cyclohexadiene-1,2-diol
glucuronides, 14-37% comprised glucuronides of
2,4,5-TCP and 2,3,5-TCP, and 1-37% was
unconjugated TCPs; in the rats, 60-62% of the
urinary metabolites comprised 2 isomers
(2,4,5- and 2,3,5-) of
N-acetyl-S-(trichlorophenyl)-L-cysteine, 28-33%
comprised the 2,4,5- and 2,3,5-isomers of
trichlorothiophenol, with 1% 2,4,5-, and 10% 2,3,5-TCPs
1,2,4-TCB male Wistar oral administration of approximately 66% and 17% eliminated in the urine
Tanaka et al. rat 50 mg 14C-1,2,4-TCB/kg and faeces, respectively, in 7 days; 2.1%
(1986) exhaled; tissue residues evenly distributed, with
the exception of the adipose tissue in which
concentrations were consistently slightly higher;
principal metabolites in the urine were free
2,4,5- and 2,3,5-TCP and their conjugates; minor
metabolites in the urine were 5- or 6-sulfhydryl,
methylthio, methyl-sulfoxide, and methylsulfone
derivatives of TCB; DCBs and TCB were present in
expired air
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB Chinchilla oral dose (gavage in arachis oil) at 5 days, the urinary metabolites of the
1,2,3-TCB rabbit of 500 mg/kg body weight 1,2,4-isomer were glucuronide conjugates (27%),
1,3,5-TCB sulfuric acid conjugates (11%), and 2,3,5- and
Jondorf 2,4,5-trichlorophenylmercapturic acid (0.3%),
et al. (1955) major phenols being 2,4,5- and 2,3,5-TCP; the
1,2,3-isomer was metabolized to 2,3,4-TCP, and
3,4,5-TCP to a lesser extent and to small amounts
of 3,4,5-trichlorocatechol, with 50% of the dose
being eliminated in the urine as glucuronic acid
conjugates, 12% as sulfuric acid conjugates, and
0.3% as 2,3,4-trichlorophenyl-mercapturic acid;
for the 1,3,5-isomer, 20% was eliminated as
sulfuric acid conjugates, no mercapturic acid was
found, 2,4,6-TCP was the only phenol detected in
the urine and some unchanged 1,3,5-TCB was
present in faeces; the 1,2,3-isomer most rapidly
metabolized; the rate of metabolism in descending
order was: 1,2,3- > 1,2,4- > 1,3,5-TCB
1,2,3-TCB male single oral dose (gavage in corn elimination data obtained only for 1,3,5- and
1,2,4-TCB Sprague-Dawley oil) of 10 mg/kg body weight of 1,2,3-isomers; both rapidly eliminated in the
1,3,5-TCB rat each 14C-labelled isomer urine and faeces:
Chu et al. urine faeces total
(1987) 1,3,5- 24 h 47.1% 35.8% 82.9%
48 h 50.3% 38.3% 88.6%
1,2,3- 24 h 56.3% 35.6% 91.9%
48 h 58.5% 36.8% 95.3%
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB male rabbit intraperitoneal injection in major urinary metabolites of 1,2,4-TCB were
1,2,3-TCB vegetable oil; 60 - 75 mg/kg body 2,4,5- and 2,3,5-TCP; the major metabolite of
1,3,5-TCB weight 1,2,3-TCB was 2,3,4-TCP with 2,3,6- and 3,4,5-TCP
Kohli et al. as minor urinary metabolites; 1,3,5-TCB was
(1976) metabolized to 2,3,5- and 2,4,6-TCP and a third
more polar metabolite
1,3,5-TCB Chinchilla oral administration (gavage in at day 8, 13% of administered dose was in the
Parke & female rabbit arachis oil) of 500 mg/kg faeces, 12% exhaled, 23% (4% as MCB) in the gut,
Williams 5% in the pelt, 5% in depot fat, and 22% in the
(1960) carcass; <10% eliminated as the major urinary
metabolite of 2,4,6-TCP; minor metabolites
included 4-chlorocatechol and 4-MCP
1,2,4,5-TeCB Sprague-Dawley oral gavage in corn oil; 10 mg/kg for the 1,2,3,4- and 1,2,3,5- isomers,
1,2,3,5-TeCB rat body weight TeCB approximately 46-51% eliminated in the urine and
1,2,3,4-TeCB faeces within 48 h following administration,
Chu et al. whereas only 8% of the 1,2,4,5-isomer was
(1984b) eliminated in the same period; most of the
1,2,4,5-isomer was eliminated in the urine, the
1,2,3,4-isomer mainly in the faeces and the
1,2,3,5-isomer equally between the urine and
faeces; metabolites in decreasing order were as
follows: 1,2,3,4-TeCB: 2,3,4,5- and
2,3,4,6-TeCP, and traces of tetrachlorthiophenol
and 2,3,4-TCP; 1,2,3,5-TeCB: 2,3,4,6-TeCP,
isomeric mercaptotrichlorophenols, and a TCP;
1,2,4,5-TeCB: 2,3,5,6-TeCP, tetrachloroquinol,
and a TCP
and a TCP
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4,5-TeCB male rabbit intraperitoneal injection in 1,2,3,5-TeCB the most extensively metabolized
1,2,3,5-TeCB vegetable oil; 60-75 mg/kg body isomer, yielding
1,2,3,4-TeCB weight 2,3,4,5-, 2,3,5,6-, and 2,3,4,6-TeCP;
Kohli et al. 1,2,3,4-TeCB was metabolized to 2,3,4,5- and
(1976) 2,3,4,6-TeCP and 1,2,4,5-TeCB yielded only 2,3,5,6-TeCP
1,2,3,4-TeCB Squirrel oral administration (gavage in major urinary metabolite was
Schwartz monkey corn oil) of 100 mg/kg 14C-TeCB N-acetyl-S-(2,3,4,5-tetrachlorophenyl) accounting
et al. (1985) for 85% in urine; minor urinary metabolite was 2,3,4,5-TeCP
1,2,3,4-TeCB Squirrel single oral dose of one of the main pathway of elimination for all isomers was
1,2,3,5-TeCB monkey 14C-labelled isomers dissolved faecal elimination - 38%, 36%, and 18% for
1,2,4,5-TeCB in corn oil, twice a week for 3 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-TeCB isomers,
Schwartz weeks, at the following doses; respectively; 1.2% of 1,2,3,4- and less than 0.1%
et al. (1987) 1,2,3,4- 100 mg/kg body weight; of the 1,2,3,5- and 1,2,4,5-TeCB isomers were
1,2,3,5- 100 mg/kg body weight eliminated in the urine; 1,2,3,4-isomer-Faeces:
1,2,4,5- 50 mg/kg body weight; 50% as the parent compound, metabolites included
urine and faeces collected 2,3,4,5-tetrachlorophenol (22%),
N-acetyl-S-(2,3,4,5-tetrachlorophenyl)-cysteine
(18%), 2,3,4,5-tetrachlorophenyl sulfinic acid
(3%), 2,4,5-trichlorophenyl methyl sulfoxide
(0.6%), and 2,3,4,5-tetrachlorophenyl methyl
sulfide (0.2%); urine:
N-acetyl-S-(2,3,4,5-tetrachlorophenyl)-cysteine
(85%) and 2,3,4,5-tetrachlorophenol (15%).
1,2,3,5-isomer-Faeces: 50% as unchanged TeCB,
2,3,4,5-tetrachlorophenol (2%),
2,3,4,6-tetrachlorophenol (14%),
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
2,3,5,6-tetrachlorophenol (9%), and
2,3,4,6-tetrachlorophenyl sulfinic acid (15%);
urine: nondetectable; 1,2,4,5-TeCB-Faeces: >99%
as unchanged parent compound; urine:
nondetectable
PeCB Rhesus oral gavage of 0.5 mg/kg body approximately 12% of the administered dose
Rozman monkey weight 14C-PeCB eliminated in the urine after
et al. (1979) 40 days and 24% in the faeces (99%
unmetabolized); the 2,3,4,5-, 2,3,5,6-, and
1,2,3,4- isomers of TeCP identified as
metabolites; no sex-related differences in
metabolism
PeCB male rabbit intraperitoneal injection in urinary metabolites were 2,3,4,5-TeCP and PeCP,
Kohli et al. vegetable oil; 300 mg both detected at 1% of the administered dose, 10
(1976) days after dosing
PeCB male Wistar oral administration (gavage in major urinary metabolites: 2,3,4,5-TeCP and PeCP;
Engst et al. rat sunflower oil) of 8 mg/kg PeCB, 2,3,4,6-TeCP, and/or 2,3,5,6-TeCP present
(1976) in free form; TCP (isomer not specified),
2,4,6-TCP and 1,2,3,4-TeCB present in small
concentrations
PeCB female rat intraperitoneal administration (in over a 4-day period, 3% eliminated in unchanged
Koss & olive oil) of 403 µmol/kg body form; urinary metabolites: PeCP (9%),
Koransky weight 2,3,4,5-TeCP, tetrachlorohydroquinone, a
(1977) hydroxylated chlorothiocompound, and traces of
another isomer of TeCP; PeCP,
tetrachlorohydroquinone, tetrachlorophenol, and
hydroxylated chlorothio- compound present in faeces
Table 14 (continued)
a Strain specified where available.
MCP - monochlorophenol.
DCP - dichlorophenol.
TCP - trichlorophenol.
TeCP - tetrachlorophenol.
PeCP - pentachlorophenol.
MCB is metabolized to an arene oxide as the first stage
intermediate, the major urinary metabolites being 4-chlorocatechol
(and conjugates) and 4-chlorophenyl-mercapturic acid. Three isomeric
chlorophenols (2-, 3-, or 4-chlorophenol) and 3-chloro-catechol are
minor metabolites.
The availability of tissue glutathione and the extent of the
conjugation of the catechol and chlorophenol metabolites appear to
play an important role in species differences in the metabolism of
MCB. Examination of the metabolic profile, 24 h following
administration of MCB (dosage unspecified), in 13 species including
man, indicated that the pattern was quantitatively similar in all
species; the profile in man was most similar to that in the
guinea-pig with metabolites in human urine identified as
4-chlorocatechol, 4-chlorophenol, and 4-chlorophenylmercapturic acid
(Williams et al., 1975). Data from an additional comparative study
indicate that 4-chlorocatechol is the main urinary metabolite of
MCB in man (Ogata & Shimada, 1983). In rats, rabbits, and mice
administered 0.5, 1.0, or 2.0 mmol MCB/kg body weight,
intra-peritoneally, the ratios of urinary 4-chlorophenylmercapturic
acid: 4-chlorocatechol were 9.1, 7.2, 6.1; 1.6, 1.8, 1.8; and 7.3,
7.8, 6.4, in the various species, respectively. In man, however,
following ingestion of 3 repeated doses of 0.3 mmol MCB/kg body
weight, or inhalation (concentration not specified) of MCB, the
comparable ratios were considerably lower, 0.002 and 0.007,
respectively, because of the extremely small amounts of
4-chlorophenylmercapturic acid present in the urine.
Saturation of glutathione conjugation capacity has been observed and
may play a role in the manifestation of toxic effects from MCB
exposures. For example, Sullivan et al. (1983) reported a
disproportionate increase in the respiratory elimination of
unchanged compound and a dose-dependent decrease in the mercapturic
acid percentage of urinary metabolites from 68% at 455 mg/m3 (100
ppm) to 51% at 3185 mg/m3 (700 ppm), following inhalation by rats
for 1 or 5 days. Yoshida & Hara (1985) observed a transient decrease
in hepatic glutathione related to urinary levels of mercapturic acid
following intraperitoneal injection of rats with 2 mmol MCB/kg. A
transient decrease in urinary taurine levels was also observed,
which the authors attributed to depression of the oxidative
degradation of cysteine by the increase in glutathione synthesis.
The authors further suggested that the levels of sulfur-containing
dietary amino acids may have an effect on the metabolism or toxicity
of chlorobenzenes for which the major metabolite is mercapturic
acid.
Table 15. Metabolites of chlorobenzenes in rabbits following ingestion
of 0.5 g/kg body weighta
Percentage of dose eliminated as:
Compound Time of Mercapturic Monophenolsb Catecholsb Total O- Percentage of dose eliminated
elimination Acid conjugatesc unchanged
(days)d
MCB 1-2 25 2-3 27 47 27 in expired air
1,2-DCB 5 5 40 4 69 NSf
1,3-DCB 5 11 25 3 37 NSf
1,4-DCB 5 0 35 6e 63 NSf
in faeces in tissue
1,2,3-TCB 5 0.3 78 trg 62 0 NSf
1,2,4-TCB 5 0.4 42 trg 38 0 NSf
1,3,5-TCB 8 0 9 0 23 10 51
1,2,3,4-TeCB 6 0 34 trg 43 5 10
1,2,3,5-TeCB 6 0 5 0 8 14 23
1,2,4,5-TeCB 6 0 2 0 5 16 48
PeCB 6 0 <1 0 9 5 50
Table 15 (cont'd)
a From: Williams (1959).
b In some cases these numbers give the amounts isolated.
c Conjugated glucuronic acid plus ethereal sulfate.
d Metabolites produced only at a very low rate after this time.
e Quinols.
f NS - not stated.
g tr - trace.
The metabolism of the dichlorobenzenes in most animal species
proceeds through the formation of arene oxide intermediates but,
unlike that of MCB, it results primarily in the excretion of
dichlorophenols, generally as conjugates of glucuronic and sulfuric
acids. Mercapturic acids and catechols are only minor metabolites.
For 1,4-DCB, 2,5-dichloroquinol is also a minor metabolite. In
general, mercapturic acids and catechols do not appear to be formed
during the metabolism of 1,4-DCB in animals. However, Hawkins et al.
(1980) reported the presence of small amounts of "a mercapturic acid
of 1,4-DCB" in the urine during the 5-day period following exposure
of rats to 1,4-DCB via inhalation (6000 mg/m3, 3 h/day) or orally
or subcutaneously at (250 mg/kg body weight per day) for up to 10
days. Small amounts (<0.03% of the total dose) of sulfur-containing
metabolites, namely 2,5-dichlorophenyl methyl sulfoxide, and
2,5-dichlorophenyl methyl sulfone, have also been identified
following oral administration of 1,4-DCB to rats (Kimura et al.,
1979).
Limited available data indicate that the rate of metabolism of
1,2-DCB is slightly greater than that for the 1,4-isomer. Azouz et
al. (1955) reported that the metabolism and elimination of 1,2-DCB
and 1,4-DCB in rabbits was complete in 5-6 days, and >6 days,
respectively, following oral administration of 500 mg/kg.
Dichlorophenol has been detected in the urine of workers exposed to
1,4-DCB. Levels ranged from 10 to 233 mg/litre in an unspecified
number of workers employed at various stages in the production of
1,4-DCB (Pagnotto & Walkley, 1965).
The principal metabolic products of the trichlorobenzenes, which are
formed following generation of intermediate arene oxides, are
trichlorophenols (TCP) (2,3,4-TCP for the 1,2,3-isomer; 2,4,6-TCP
for the 1,3,5-isomer, and both 2,3,5-TCP and 2,4,5-TCP for the
1,2,4-isomer). Minor metabolites include trichlorocatechols (for the
1,2,3- and 1,2,4-isomers of TCB), other trichlorophenols (for all
isomers of TCB), trichlorophenyl-mercapturic acids (for the 1,2,3-
and 1,2,4-isomers of TCB), and 4-chlorophenol and 4-chloro-catechol
(for the 1,3,5-isomer of TCB). Kohli et al. (1976) also reported the
presence of a polar urinary metabolite ("possibly DCB with 2
hydroxyl and 1 methoxyl substituent") following intraperitoneal
injection of rabbits with 60-75 mg/kg of 1,3,5-TCB. Several
additional metabolites have been reported following administration
of 1,2,4-TCB to various species, including 5- or 6-sulhydryl,
methylthio, methylsulfoxide, and methylsulfone derivatives of TCB
(minor metabolites in rats) (Tanaka et al., 1986), 2,4,5- and
2,3,5-trichlorothiophenol and the 2,4,5- and 2,3,5-isomers of
N-acetyl- S-(trichlorophenyl)-L-cysteine (major metabolites in
rats) (Lingg et al., 1982), and an isomeric pair of
3,4,6-trichloro-3,5-cyclo-hexadiene-1,2-diol glucuronides (major
metabolites in monkeys) (Lingg et al., 1982).
The most rapidly metabolized of the 3 isomers is 1,2,3-TCB, with
over 60% of an orally administered dose of 500 mg/kg body weight
being eliminated in the urine of rabbits in a 5-day period, compared
with 38% and 28% for the 1,2,4- and 1,3,5-isomers, respectively
(Jondorf et al., 1955).
Available data also indicate that there are considerable species
differences in the metabolism of the trichlorobenzenes. In a study
by Lingg et al. (1982), in which 10 mg 1,2,4-TCB/kg body weight was
administered orally or intravenously to both rats and rhesus
mon-keys, the metabolic profile of the two species varied
considerably. In the monkey, a stereo isomeric pair of
3,4,6-trichloro-3,5-cyclo-hexadiene-
1,2-diol glucuronides accounted for over half of the urinary
metabolites while, in the rat, the 2,4,5- and
2,3,5- N-acetyl- S-(trichlorophenyl)-L-cysteine isomers accounted
for the majority of the urinary metabolites. In addition to the
metabolic pathway being species specific, 2-3 times as much
14C-TCB was excreted in 24 h in the rat as in the monkey.
The tetrachlorobenzenes are slowly metabolized, principally to 3
tetrachlorophenols (2,3,4,5-, 2,3,4,6-, and 2,3,5,6-isomers). Arene
oxides are postulated to be metabolic intermediates and "NIH shifts"
are required in most of the postulated pathways. Schwartz et al.
(1985) recently reported that the major urinary metabolite
(accounting for 85% of the radioactivity in the urine) following
oral administration of 100 mg 1,2,3,4-TeCB/kg body weight to
squirrel monkeys was N-acetyl- S-(2,3,4,5-tetrachlorophenyl)
cysteine. A minor metabolite of the 1,2,3,4-TeCB isomer was
2,3,4-trichlorophenol. Tetrachlorophenols are major metabolites of
TeCBs in rats (Chu et al., 1984b). Isomeric mercaptotrichlorophenols
and a trichlorophenol have been identified as metabolites of
1,2,3,5-TeCB in rats; tetrachloroquinol and a trichlorophenol have
also been detected in this species following administration of
1,2,4,5-TeCB (Chu et al, 1984b).
The 1,2,4,5-isomer is not well metabolized and tends to remain in
the tissues for a considerable time in the unchanged state. For
example, following the oral administration of 500 mg/kg body weight
to rabbits, 48% of the 1,2,4,5-isomer was detected unchanged in the
tissues at 6 days, compared with 10% for the 1,2,3,4 isomer and 23%
for the 1,2,3,5-isomer (Jondorf et al., 1958). Following oral
administration of 10 mg/kg body weight to rats, 46-51% of the
1,2,3,4- and 1,2,3,5-isomers was eliminated in the urine and faeces
within 48 h, whereas only 8% of the 1,2,4,5-isomer was eliminated in
the same period (Chu et al., 1984b).
Available data also indicate that there are species differences in
the metabolism of tetrachlorobenzenes. In a recent study described
in Table 14, the tetrachlorobenzenes were not as extensively
metabolized in squirrel monkeys as in other species, and metabolites
were eliminated exclusively in the faeces (Schwartz et al., 1987).
PeCB may be metabolized primarily to pentachlorophenol by direct
oxidation or to 2,3,4,5-tetrachlorophenol via an arene oxide
intermediate. Other compounds that have been identified as
metabolites of PeCB include the 1,2,3,4- and 2,3,5,6-isomers of
tetrachlorophenol in monkeys (Rozman et al., 1979), and the
2,3,4,6-isomer of tetrachlorophenol, an unspecified isomer of
trichorophenol, 2,4,6-trichlorophenol, 1,2,3,4-TeCB, and
tetra-chlorohydroquinone in rats (Engst et al., 1976; Koss &
Koransky, 1977).
In in vitro studies on rat liver microsomes, the principal primary
metabolites of pentachlorobenzene were pentachlorophenol and
2,3,4,6-tetrachlorophenol in a ratio of 4:1 (den Besten et al.,
1989). Minor metabolites included the 2,3,4,5- and 2,3,5,6-isomers
of tetrachlorophenol. In addition, para- and ortho-
tetrachlorohydroquinone were formed as secondary metabolites. The
authors suggested that these compounds may be involved in the
covalent binding of microsomal protein.
6.4 Elimination and Excretion
While there is variation among different isomers of the same
congener and among species, generally, the metabolism and excretion
of the higher chlorinated benzenes is slower than that of mono- and
dichlorobenzenes, and a greater proportion of the compound is
eliminated unchanged in the faeces or expired air. This is
illustrated by the data on the metabolism and elimination of the
various chlorobenzenes in the rabbit following the oral
administration of 500 mg/kg body weight, which are presented in
Table 15 (Williams, 1959). Hawkins et al. (1980) reported that
1,4-DCB was eliminated primarily in the rat urine (91-97%) in the
5-day period following inhalation, subcutaneous, or oral exposure.
It has also been reported that 78-84% of an oral or intravenous dose
of 10 mg 1,2,4-TCB/kg body weight was eliminated in the urine and
that 7-11% was eliminated in the faeces of rats at 24 h; in monkeys,
the comparable values were 40% and <1% (Lingg et al., 1982). In
rats receiving an oral dose of 50 mg 1,2,4-TCB/kg, approximately 66%
and 17% were eliminated in the urine and faeces, respectively, in 7
days; 2.1% was exhaled (Tanaka et al., 1986). Rozman et al. (1979)
reported that approximately 12% of an oral dose of 0.5 mg PeCB/kg
was eliminated in the urine of rhesus monkeys and 24% in the faeces
(99% unmetabolized) within 40 days. In a study by Koss & Koransky
(1977), it was observed that 3% was excreted unchanged during a
4-day period following administration of 403 µmol PeCB/kg body
weight. The higher chlorobenzenes tend to be stored in the adipose
depots of the body at higher levels than monochloro- and
dichloro-congeners (section 6.2, Table 14).
Available data on the half-lives of the chlorobenzenes in the
tissues of experimental animals are restricted to the
trichlorobenzenes, the 1,2,4,5-isomer of TeCB, and PeCB. Chu et al.
(1987) reported that the terminal half-lives for 1,2,3-TCB,
1,2,4-TCB, and 1,3,5-TCB in rats were 145, 93, and 68 h,
respectively. It was reported by Braun et al. (1978) that the
half-lives for the elimination of 1,2,4,5-TeCB from the fat and
plasma of beagle dogs were 111 and 104 days, respectively. In rhesus
monkeys, the estimated half-life of PeCB (tissue unspecified) was
2-3 months (Rozman et al., 1979).
6.5 Binding to Protein
The results of several studies indicate that the metabolites of MCB
and DCB, most likely the arene oxide intermediate or unconjugated
chlorophenols, covalently bind to rat kidney and liver tissues and,
in some cases, induce damage (Oesch et al., 1973; Reid, 1973; Reid &
Krishna, 1973). The binding and toxic effects of MCB were prevented
by piperonyl butoxide, cyclohexene oxide, and glutathione (Oesch et
al., 1973; Reid, 1973; Reid & Krishna, 1973) and were reduced by
3-methylcholanthrene (Reid, 1973; Reid & Krishna, 1973). The binding
of 1,2-DCB to rat liver protein was enhanced by pretreatment with
phenobarbital; the 1,4-isomer of DCB did not bind to the same extent
and was less hepatotoxic than the 1,2-isomer (Reid & Krishna, 1973).
6.6 Effects on Metabolizing Enzymes
The chlorobenzenes are broad inducers of metabolic reactions
including both oxidative and reductive, as well as conjugation
hydrolytic pathways. The effects of the different chlorobenzenes on
the metabolizing enzymes vary considerably.
The 1,4-isomer of dichlorobenzene and 1,2,4-trichlorobenzene induced
cytochrome-c-reductase, cytochrome P-450, EPN-detoxification
( O-ethyl O-p-nitrophenyl phenylphosphonothioate), glucuronyl
transferase, benzopyrene hydroxylase, and azoreductase following
oral administration of doses of up to 40 mg/kg per day for 14 days
in the rat. In contrast, administration of doses of MCB as high as
800 mg/kg per day had no effects on these enzymes (Carlson &
Tardiff, 1976).
The cytochrome P-450 content in rat liver and the enzymes,
aminopyrine demethylase and aniline hydroxylase, were induced by
1,4-DCB, 1,3,5-TCB, 1,2,4,5-TeCB, and PeCB, but not by MCB, after
administration of oral doses of between 125 and 500 mg/kg body
weight, daily, for 1 or 3 days. Aniline hydroxylase activity did
increase, however, with high doses of monochlorobenzene (1000 mg/kg
body weight) (Ariyoshi et al., 1975). MCB has also been reported to
induce epoxide hydrolase in rat liver (Oesch et al., 1973). There
was, therefore, no consistent pattern of enzyme induction in
relation to the degree of chlorination of the chlorobenzenes. The
most potent inducer was found to be 1,2,4-TCB (Ariyoshi et al.,
1981).
The induction of hepatic metabolizing enzymes in rats treated with
trichlorobenzenes differs from isomer to isomer. The 1,2,4-isomer
induced NADPH-cytochrome-c-reductase, acetanilide esterase,
arylesterase, and procaine esterase, but not cytochrome P-450 or
acetanilide hydroxylase, following oral administration to rats of
0.1 mmol/kg per day for 14 days. On the other hand, the 1,3,5-isomer
induced acetanilide hydroxylase, acetanilide esterase, and procaine
esterase, but not arylesterase (Carlson et al., 1979; Carlson,
1980).
In pregnant rats, 1,2,4-TCB, 1,2,4,5-TeCB, and 1,2,3,4-TeCB all
induced hepatic cytochrome P-450, aninopyrine N-demethylase, and
ethoxy resorufin- O-deethylase. In addition, 1,2,4-TCB and
1,2,3,4-TeCB increased NADPH-cytochrome-c-reductase, glucuronyl
transferase for p-nitrophenol, and glutathione- S-transferase
activities (Kitchin & Ebron, 1983a,b,c).
Although the induction of metabolizing enzymes by PeCB has been less
well studied, it has been shown to induce the O-dealkylation of
7-ethoxycoumarin in rats following the ingestion of 0.05% in the
diet, indicating that it stimulates the cytochrome P-450 system
(Goerz et al., 1978).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The database on the effects of chlorobenzenes on organisms in the
environment is restricted to studies on acute toxicity in aquatic
systems. Only a few studies have been reported on the long-term
effects of chlorobenzenes on the aquatic environment, and none on
the effects of chlorobenzenes on non-mammalian terrestrial animals
and aquatic vascular plants.
7.1 Microorganisms
7.1.1 Bacteria and protozoa
The toxicity thresholds of MCB and 1,2-DCB for protozoa and bacteria
have been reported by Bringmann & Kuhn (1980). MCB levels in excess
of 3500 µmol/litre were noted in studies on Entosiphon sulcatum,
Uronema parduczi, and Chilomonas paramecium. The authors studied
the toxicity threshold over a 72-h period in an open system, where
an inhibition of cell growth greater than 3% was considered a
significant effect. Toxicity thresholds for 1,2-DCB in the same
three species were reported to be: >435µmol/litre for E. sulcatum,
544 µmol/litre for U. parduczi , and >410 µmol/litre for C.
paramecium. With regard to bacteria, Boyles (1980) reported that a
level of 270 µmol 1,2-DCB/litre resulted in a 50% reduction in the
growth rate of Vibrio natriegens (EC50, 40-min). In Pseudomonas
putida, Bringmann & Kuhn (1980) reported 16-h toxicity thresholds
for MCB and 1,2-DCB of 150 and 100 µmol/litre, respectively.
Using Tetrahymena pyriformis, Yoshioka et al. (1985) reported
EC50 concentrations (cell proliferation) of 51, 130, 20, 0.91, and
30 mg/litre for 1,2-DCB, 1,3-DCB, 1,2,4,5-TeCB, 1,2,4-TCB, and
1,3,5-TCB, respectively.
7.1.2 Unicellular algae
The acute toxicities of various chlorobenzenes for unicellular algae
are summarized in Table 16.
In general, the acute toxicities of chlorobenzenes for aquatic
unicellular algae, whether measured according to the inhibition of
chlorophyll a production or cellular proliferation, increase with
increasing chlorination. MCB was reported to have a 96-h EC50 of
232 mg/litre in Selenastrum capricornutum, whereas 1,2,3,5-TeCB
showed a 96-h EC50 of 17.2 mg/litre in this species; both were
measured according to the inhibition of chlorophyll a production.
The estuarine alga, Skeletonema costatum, appears to be more
sensitive to the acute effects of 1,2,3,5-TeCB; a 96-h EC50 of 0.7
mg/litre has been reported by the US EPA (1980a). Calamari et al.
(1983) demonstrated the comparable toxicity of 1,2-DCB, 1,4-DCB,
1,2,3-TCB, and 1,2,4-TCB in the green alga Selenastrum
capricornutum. MCB was less toxic (Table 16).
In the freshwater alga Ankistrodesmus falcatus, a 4-h EC50 for
primary productivity of 0.005 mmol/litre was shown for PeCB, whereas
MCB had an EC50 of 0.44 mmol/litre, under the same conditions
(Wong et al., 1984). Canton et al. (1985) reported EC50s for
growth of 17, 31, and 31 mg/litre, for 1,2-DCB, 1,3-DCB, and
1,4-DCB, respectively.
7.2 Aquatic Organisms
7.2.1 Plants
No data have been reported on the effects of chlorinated benzenes on
freshwater or saltwater vascular plants.
7.2.2 Invertebrates
Available data for the acute toxicity of chlorobenzenes in aquatic
invertebrates are given in Table 17. Most of the studies were
carried out at high chlorobenzene concentrations, yielding LC50
values that sometimes exceeded the solubilities of the compounds,
particularly those with higher levels of chlorination (see for
example LeBlanc, 1980).
In a study by Calamari et al. (1983), an enclosed static system was
used in which the authors measured the concentration of 1,4-DCB
throughout the test period. A 24-h IC50 (immobilization
concentration) of 1.6 mg/litre was reported for 1,4-DCB; the 24-h
IC50 for 1,2-DCB was 0.78 mg/litre. Such experimental data
probably reflect more accurately the true toxicity values than those
from an open, static apparatus, relying on nominal concentrations of
chlorobenzenes.
Hermens et al. (1985) determined the no-observed-effect
concentration (NOEC) on growth and the concentration inhibiting
growth of Daphnia magna by 50 % (EC50) in acute exposures to
chlorobenzenes. Values for the NOEC in µmol/litre were 0.59 for
1,2,3,4-TeCB and 1.14 for PeCB.
When LC50s (48-h) and LC0s were determined for a range of
chlorobenzenes (LeBlanc, 1980) for Daphnia magna, there was no
trend in toxicity in relation to the degree of chlorination. Wide
differences in response to different isomers of the same congener
were seen (Table 17).
Table 16. Acute toxicity of chlorobenzenes for aquatic unicellular algae
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Freshwater species
Selenastrum capricornutum MCB 232.0 EC50 (96-h) chlorophyll a
US EPA (1980a) 224.0 EC50 (96-h) cell growth
1,2,4-TCB 35.3 EC50 (96-h) chlorophyll a
36.7 EC50 (96-h) cell growth
1,2,3,5-TeCB 17.2 EC50 (96-h) chlorophyll a
17.7 EC50 (96-h) cell growth
1,2,4,5-TeCB 52.9 EC50 (96-h) chlorophyll a
46.8 EC50 (96-h) cell growth
PeCB 6.78 EC50 (96-h) chlorophyll a
6.63 EC50 (96-h) cell growth
EC50 (96-h) cell growth
Selenastrum capricornutum 1,2-DCB 91.6 EC50 (96-h) chlorophyll a
US EPA (1980b) 98.0 EC50 (96-h) cell growth
1,2-DCB 179.0 EC50 (96-h) chlorophyll a
149.0 EC50 (96-h) cell growth
1,4-DCB 98.1 EC50 (96-h) chlorophyll a
96.7 EC50 (96-h) cell growth
EC50 (96-h) cell growth
Table 16 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Selenastrum capricornutum MCB 12.5 EC50 (96-h) cell growth
Calamari et al. (1983)a 33.0 EC50 (3-h) decreased photosynthesis
1,2-DCB 2.2 EC50 (96-h) cell growth
10.0 EC50 (3-h) decreased photosynthesis
1,4-DCB 1.6 EC50 (96-h) cell growth
5.2 EC50 (3-h) decreased photosynthesis
1,2,3-TCB 0.9 EC50 (96-h) cell growth
2.2 EC50 (3-h) decreased photosynthesis
1,2,4-TCB 1.4 EC50 (96-h) cell growth
3.9 EC50 (3-h) decreased photosynthesis
Chlorella vulgaris MCB 99.1 EC50 (3-h) cell growth
Hutchinson et al. (1980) 1,2,3-TCB 6.2 EC50 (3-h) cell growth
1,2,3,5-TeCB 2.51 EC50 (3-h) cell growth
Ankistrodesmus falcatus MCB 50 EC50 (4-h) primary productivity 14C uptake
(acicularis) 1,2-DCB 20 EC50 (4-h) primary productivity 14C uptake
Wong et al. (1984)a 1,3-DCB 23 EC50 (4-h) primary productivity 14C uptake
1,4-DCB 20 EC50 (4-h) primary productivity 14C uptake
1,2,3-TCB 6 EC50 (4-h) primary productivity 14C uptake
1,2,4-TCB 6 EC50 (4-h) primary productivity 14C uptake
1,3,5-TCB 9 EC50 (4-h) primary productivity 14C uptake
1,2,3,4-TeCB 4 EC50 (4-h) primary productivity 14C uptake
1,2,3,5-TeCB 3 EC50 (4-h) primary productivity 14C uptake
1,2,4,5-TeCB 5 EC50 (4-h) primary productivity 14C uptake
PeCB 1.3 EC50 (4-h) primary productivity 14C uptake
Table 16 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Chlamydomas angulosa MCB 56.9 EC50 (3-h) cell growth
Hutchinson et al. (1980) 1,2,3-TCB 3.45 EC50 (3-h) cell growth
1,2,3,5-TeCB 1.58 EC50 (3-h) cell growth
Skeletonema costatum MCB 343.0 EC50 (96-h) chlorophyll a
US EPA (1980a) 341.0 EC50 (96-h) cell growth
1,2,4-TCB 8.75 EC50 (96-h) chlorophyll a
8.93 EC50 (96-h) cell growth
1,2,3,5-TeCB 0.83 EC50 (96-h) chlorophyll a
0.70 EC50 (96-h) cell growth
1,2,4,5-TeCB 7.10 EC50 (96-h) chlorophyll a
7.30 EC50 (96-h) cell growth
PeCB 2.23 EC50 (96-h) chlorophyll a
1.98 EC50 (96-h) cell growth
Skeletonema costatum 1,2-DCB 44.2 EC50 (96-h) chlorophyll a
US EPA (1980b) 44.1 EC50 (96-h) cell growth
1,3-DCB 52.8 EC50 (96-h) chlorophyll a
49.6 EC50 (96-h) cell growth
1,4-DCB 54.8 EC50 (96-h) chlorophyll a
59.1 EC50 (96-h) cell growth
a Carried out using a closed system, with measured concentrations of chlorobenzenes.
Table 17. Acute toxicity of chlorobenzenes for aquatic invertebrates
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Freshwater species
Daphnia magna MCB 86 (64-120) LC50 (48-h) immobilization
Le Blanc (1980)a 10 LC0 (48-h) NOEL
1,2-DCB 2.4 (1.9-3.0) LC50 (48-h) immobilization
0.36 LC0 (48-h) NOEL
1,3-DCB 28 (21-34) LC50 (48-h) immobilization
6 LC0 (48-h) NOEL
1,4-DCB 11 (6.6-19.0) LC50 (48-h) immobilization
0.68 LC0 (48-h) NOEL
1,2,4-TCB 50 (7.2-130) LC50 (48-h) immobilization
<2.4 LC0 (48-h) NOEL
1,2,3,5-TeCB 9.7 (6.6-14.0) LC50 (48-h) immobilization
<1.1 LC0 (48-h) NOEL
1,2,4,5-TeCB >530 LC50 (48-h) immobilization
320 LC0 (48-h) NOEL
PeCB 5.3 (4.1-7.2) LC50 (48-h) immobilization
1.3 LC0 (48-h) NOEL
Daphnia magna MCB 4.3 LC50 (24-h) immobilization
Calarmari et al. (1983)b 1,2-DCB 0.78 LC50 (24-h) immobilization
1,4-DCB 1.6 LC50 (24-h) immobilization
1,2,3-TCB 0.35 LC50 (24-h) immobilization
Daphnia magna MCB 51.6 mmol/m3 LC50 (48-h) lethality
Abernethy et al. (1986) 1,2-DCB 16.0 mmol/m3 LC50 (48-h) lethality
1,4-DCB -- LC50 (48-h) lethality
1,2,3-TCB 8.0 mmol/m3 LC50 (48-h) lethality
1,2,3,5-TeCB 4.0 mmol/m3 LC50 (48-h) lethality
PeCB 1.2 mmol/m3 LC50 (48-h) lethality
Table 17 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Seawater species
Tanytarsus dissimilis 1,2-DCB 11.76 LC50 (48-h) lethality
US EPA (1980b)b 1,4-DCB 13.0 LC50 (48-h) lethality
Mysidopsis bahia MCB 16.4 LC50/EC50 (96-h) lethality
US EPA (1980a) 1,2,4-TCB 0.45 LC50/EC50 (96-h) lethality
1,2,3,5-TeCB 0.34 LC50/EC50 (96-h) lethality
1,2,4,5-TeCB 1.48 LC50/EC50 (96-h) lethality
PeCB 0.16 LC50/EC50 (96-h) lethality
Mysidopsis bahia 1,2-DCB 1.97 LC50/EC50 (96-h) lethality
US EPA (1980b)b 1,3-DCB 2.85 LC50/EC50 (96-h) lethality
1,4-DCB 1.99 LC50/EC50 (96-h) lethality
Paleaemonetes pugio 1,2,4-TCB 0.54 LC50 (96-h) lethality
Clark et al. (1987)c
a Closed static system; nominal concentrations.
b Aquatic concentrations of CB measured; static conditions employed.
c A flow-through system was used and concentrations of water-borne TCB measured.
Calamari et al. (1983) exposed 30 specimens of Daphnia magna to a
range of chlorobenzenes in closed bottles for 14 days, to obtain 3
broods. The higher chlorinated materials had lower effective
concentrations on fertility (14-day EC50s) ranging from
2.5 mg/litre for MCB to 0.20 mg/litre for 1,2,3 TCB.
When saltwater crustacea were exposed to high chlorobenzene
concentrations (55 µmol/litre), Grosch (1973) found that a single
24-h exposure of adult Artemia salina (brine shrimp) to 1,3,5-TCB
resulted in a decreased life span, a delay in the onset of the first
brood, and a decreased number of offspring. The US EPA (1980b)
reported that the LC50 values for the exposure of Mysidopsis
bahia (mysid shrimp) were similar for the 3 DCB isomers (1.97
mg/litre for 1,2- and 1,4-DCB, and 2.85 mg/litre for 1,3-DCB).
However, these tests were carried out in open static systems and the
reported values may not reflect accurately the actual toxicity of
these chemicals.
The toxic effects of 1,4-DCB and 1,3,5-TCB on the embryonic and
larval stages of clams (Mercenaria mercenaria) and oysters
(Crassostrea virginica) were studied by Davis & Hidu (1969).
Embryos, initially at the 2-cell stage, were exposed to 1,4-DCB or
1,3,5-TCB for 48 h. The median toxicity levels (TL50) for embryo
development to normal larvae were >680 µmol/litre and >55
µmol/litre for clam eggs exposed to 1,4-DCB and 1,3,5-TCB,
respectively.
7.2.3 Fish
The results of studies on the acute toxicity of chlorobenzenes for
fish are presented in Table 18. A comparison of Tables 17 and 18
shows a similar degree of sensitivity to chlorobenzenes between
bluegills (Lepomis macrochirus) and Daphnia magna. With a few
exceptions, open static systems were used in the acute toxicity
tests on fish, without measurements of actual concentrations in the
water. With the exception of 1,2,3,5-TeCB, the acute toxicity of
chlorobenzenes in several fish species increased with increased
chlorination, and was well correlated with the log P (octanol:water
partition coefficient) value (Calamari et al., 1983). Buccafusco
et al. (1981) stated that undissolved, preciptated chlorobenzenes
were present in the test vessels. The LC50s, therefore, are
probably underestimates of the toxicity of chlorobenzenes to fish.
In addition to the acute toxicity studies summarized in Table 18,
several studies have been reported on the effects of chlorobenzenes
on various developmental stages (i.e., embryo, larval, juvenile,
etc.) of freshwater and saltwater fish (Birge et al., 1979; US EPA,
1980a,b). Such embryo-larval studies more accurately reflect the
long-term toxicities arising from continuous exposures to low levels
of chlorobenzenes, at sensitive life-stages, than the acute LC50
studies.
Using the freshwater fathead minnow (Pimephales promelas) in an
embryo larval assay, long-term toxicity limits of 2.0, 1.51, and
0.76 mg/litre were reported for 1,2-, 1,3-, and 1,4-DCB,
respectively, by the US EPA (1980b). Long-term toxicity limits in
the estuarine sheepshead minnow for 1,2,4-TCB and 1,2,4,5-TeCB were
0.22 and 0.13 mg/litre, respectively (US EPA, 1980a); in the
freshwater fathead minnow (Pimephales promelas), long-term
toxicity limit values of 0.71, and 0.32 mg/litre were reported for
1,2,4-TCB and 1,2,3,4-TeCB, respectively (US EPA, 1980a).
Birge et al. (1979) exposed rainbow trout eggs to MCB at a range of
concentrations from 0.09 mg/litre, for 16 days; all concentrations
were lethal and, thus, a no-observed-effect level could not be
determined. Goldfish (Carassius auratus) and largemouth bass
(Micropterus salmonides) were less sensitive to MCB. Larvae were
more susceptible to MCB than eggs. For the goldfish, the LC50 with
exposure up to hatching was 3.5 mg/litre in soft water and 2.4
mg/litre in hard water. Exposure until 4 days after hatching
resulted in an LC50 of 1 mg/litre. Largemouth bass showed an
LC50 of 0.3 mg/litre, at hatching, and an LC50 of 0.05 mg/litre,
4 days after hatching.
7.3 Terrestrial Biota
No data are available.
7.4 Model Ecosystems
In a study by Tagatz et al. (1985), sand-filled aquaria were used in
which macrobenthic animal communities were allowed to develop. In
one test, colonization of the system by planktonic larvae for 50
days, was followed by 6 days of exposure to waterborne
concentrations of 1,2,4-TCB. In a second study, colonization was
allowed to proceed for 8 weeks in the presence of sediment
containing 1,2,4-TCB. The same types of organisms were affected,
whether the TCB was waterborne or sediment bound. However, community
structure was affected at waterborne concentrations (measured) that
were 2 orders of magnitude lower than those of the sediment-bound
TCB. The lowest waterborne TCB concentrations that affected the
number of individuals were: 0.04 mg/litre for molluscs; 0.4 mg/litre
for arthropods; and 4 mg/litre for annelids. At 4 mg/litre, the
average number of species was significantly lower than that in the
control aquaria.
Table 18. Acute toxicity of chlorobenzenes for fish
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
Freshwater species
Salmo gairdneri
(rainbow trout)
Calamari et al. (1983) FB; M MCB 4.1 LC50 (48-h) lethality
1,2-DCB 2.3
1,4-DCB 1.18
1,2,3-TCB 0.71
1,2,4-TCB 1.95
US EPA (1980b) FB; M 1,2-DCB 1.58 LC50 (96-h) lethality
1,4-DCB 1.12
Pimephales promelas
(fathead minnow)
US EPA (1980b) FB; M 1,3-DCB 7.79 LC50 (96-h) lethality
1,4-DCB 4.0
US EPA (1980a) FB; M 1,2,3,4-TeCB 1.07 LC50 (96-h) lethality
Lepomis macrochirus
(bluegill)
Buccafusco et al. (1981) Sb; U MCB 16 (13-20) LC50 (96-h) lethality
1,2-DCB 5.6 (4.8-6.6)
1,3-DCB 5.0 (3.9-6.2)
1,4-DCB 4.3 (3.9-4.8)
Table 18 (continued)
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
1,2,4-TCB 3.4 (2.7-4.1)
1,2,3,5-TeCB 6.4 (5.2-8.1)
1,2,4,5-TeCB 1.6 (1.3-1.8)
PeCB 0.25 (0.18-0.32)
Salt-water species
Cyprinodon variegatus
(sheepshead minnow)
Heitmuller et al. (1981) S; U MCB 10 (8.8-12) LC50 (96-h) lethality
6.2 LC0 (96-h) NOEL lethality
1,2-DCB 9.7 (9.0-10.0) LC50 (96-h) lethality
1,3-DCB 7.8 (6.8-8.7) LC50 (96-h) lethality
4.2 LC0 (96-h) NOEL lethality
1,4-DCB 7.4 (6.8-7.9) LC50 (96-h) lethality
5.6 LC0 (96-h) NOEL lethality
1,2,4-TCB 21 (17-26) LC50 (96-h) lethality
15 LC0 (96-h) NOEL lethality
Table 18 (continued)
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
Heitmuller et al. (1981) 1,2,3,5-TeCB 3.7 (3.3-4.1) LC50 (96-h) lethality
(continued)
1.0 LC0 (96-h) NOEL lethality
1,2,4,5-TeCB 0.8 (0.7-1.1) LC50 (96-h) lethality
0.3 LC0 (96-h) NOEL lethality
PeCB 0.8 (0.4-1.8) LC50 (96-h) lethality
0.3 LC0 (96-h) NOEL lethality
Menidia beryllina
(tidewater silverside)
Dawson et al. (1977) S; U 1,2-DCB 7.3 LC50 (96-h) lethality
a S - static bioassay;
U - actual concentrations in system not monitored;
FB - flow-through conditions for bioassay;
M - concentrations of chlorobenzenes in experimental media determined by monitoring.
b Closed system.
The effects of 1,2,4-TCB on a natural freshwater phytoplankton
community were reported by Schauerte et al. (1985). A water meadow,
rich in both Daphnia and phytoplankton, was divided into
approximately 200 litre test cells to study the effects of 0.25 mg
1,2,4-TCB/litre over a period of 22 days, with a half-time of 13.7
days. No chemical-related effects on the abundance and diversity of
the phytoplankton community were noted. However, the high toxicity
(greater than 90% death) for Daphnia may have resulted in algal
blooms from fewer grazing species. The authors noted that the
toxicity of 1,2,4-TCB for Daphnia was much higher in the natural
system than was observed by Bringman & Kuhn (1982) in the laboratory
(0.25 mg/litre compared with 1.2-21 mg/litre).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposure
The results of the more recent and well documented acute toxicity
studies on the chlorobenzenes in experimental animals are presented
in Table 19. For isomers for which there were few available studies,
quantitative data (i.e., LC50s or LD50s) from early
investigations have also been included. A more complete review of
these early studies is included in US EPA (1985).
Inhalation of sufficiently high single doses of volatile
chlorobenzenes causes local respiratory irritation and depression of
the central nervous system; ingestion of lethal doses leads to
respiratory paralysis. Effects on the liver, kidneys, adrenal
glands, mucous membranes, brain ganglion cells, and metabolizing
enzymes have also been observed following acute exposure to
non-lethal doses of the chlorobenzenes.
Data concerning the acute toxicity of the higher chlorinated
compounds are sparse; some conclusions concerning the relative acute
toxicity of the different congeners can be drawn, however, from
examination of the oral LD50s in rats in the more recent and well
documented studies. On the basis of both mass and molar quantities,
the higher chlorinated benzenes appear, in general, to be more
acutely toxic than the mono- and dichlorobenzenes; however, for any
one isomer, there is a wide range of LD50s. In general, the dermal
LD50s are considerably higher than those for ingestion, probably,
in some cases, as a result of evaporation losses from the skin
(though this is less likely with occlusive dressings) together with
relatively poor dermal absorption. Inhalation LC50s have varied
widely, even for the same congeners, principally because of large
differences in the durations of exposure in the various studies.
Acute exposure to monochlorobenzene via inhalation causes sensory
irritation of the respiratory system, after several minutes;
exposure for periods ranging from several minutes to hours causes
narcosis and central nervous system (CNS) depression, which can
result in death. The LC50s (6-h exposure period) for male
Sprague-Dawley rats and SPF-OF1 female mice were 13 490 mg/m3
(2965 ppm) and 8581 mg/m3 (1886 ppm), respectively (Bonnet et al.,
1979, 1982). De Ceaurriz et al. (1981) reported that inhalation of
4796 mg MCB/m3 (1054 ppm) for 5 min reduced the respiratory rate
in Swiss-OF1 mice by 50% (RD50), indicating respiratory
irritation.
LD50 values for the ingestion of MCB (gavage in corn oil) were
approximately 4000 mg/kg body weight in male and female F344 rats;
B6C3F1 mice were more sensitive with 100% lethality at doses
exceeding 1000 mg/kg and 2000 mg/kg for males and females,
respectively (Kluwe et al., 1985). Clinical symptoms included
hyperpnoea, ataxia, laboured breathing, prostration, and death from
respiratory paralysis.
Systemic effects of acute toxic doses of MCB included damage to the
liver and kidney, and effects on bile and pancreatic flow.
Circulating alanine aminotransferase (ALT) was increased in 50% of
CF-1 strain albino mice following a single intraperitoneal
adminis-tration of approximately 428 mg/kg (Shelton & Weber, 1981).
In Sprague-Dawley rats, single intraperitoneal administration of the
two highest doses of 1104 mg/kg and 1655 mg/kg body weight resulted
in increases in plasma ALT and centrilobular necrosis. Increased
liver to body weight ratios were observed at all doses
(225-1655 mg/kg body weight) (Dalich & Larson, 1985). In C57
Black/6J male mice, coagulation necrosis of the proximal renal
tubules was observed following intraperitoneal administration of 760
mg MCB/kg body weight; Sprague-Dawley rats were somewhat less
sensitive having only swollen, vacuolated convoluted tubules at
doses of 1047 mg/kg body weight (Reid, 1973). Yang et al. (1979)
reported increased bile duct-pancreatic flow in Holtzman male rats
administered 563 mg MCB/kg body weight (5 mmol/kg)
intraperitoneally. The investigators suggested that these effects
were distinct from those on the liver, since they occurred in the
absence of liver damage.
Effects of acute exposure to the dichlorobenzenes are similar to
those for MCB, resulting in CNS depression following inhalation, and
respiratory paralysis following ingestion. Limited available data
indicate that the 1,2-isomer is more toxic than the 1,4-isomer. In
the inhalation studies of Bonnet et al. (1979, 1982), the 1,2-isomer
of dichlorobenzene was somewhat more toxic than MCB, the LC50s
(6-h exposure) in both Sprague-Dawley rats and SPF-OF1 female mice
(9192 mg/m3 and 7416 mg/m3, respectively) being less than those
for MCB (13 490 mg/m3 and 8581 mg/m3, respectively). Increases
in circulating hepatic enzymes were reported 24 h after exposure of
Sprague-Dawley rats to levels of 1,2-DCB greater than 1830 mg/m3
(Brondeau et al., 1983).
Few recent data are available concerning the acute toxicity of the
dichlorobenzenes after oral administration. Hollingsworth et al.
(1958) reported that no deaths occurred in guinea-pigs following
ingestion (gavage in olive oil) of 800 mg/kg body weight of the
1,2-isomer, whereas there was 100% lethality at 2000 mg/kg body
weight. For the 1,4-isomer, these investigators reported that 2800
mg/kg and 4000 mg/kg body weight were lethal for 100% of guinea-pigs
and rats, respectively (Hollingsworth et al., 1956). There were no
deaths in guinea-pigs and rats exposed to 1600 mg/kg and 1000 mg/kg
body weight, respectively.
Table 19. Acute toxicity of chlorobenzenes for experimental animals
Compound; Speciesa Routeb Dosec Resultsd
Reference
MCB Sprague-Dawley male rat; inhalation NA (6-h exposure) 14-day LC50 in rats: 13 490 mg/m3
Bonnet et al. (1979, 12 animals/group; SPF-OF1 observation period (2965 ppm)
1982) female mouse; 20-25/group LC50 in mice: 8581 mg/m3
(1886 ppm)
MCB Swiss OF1 male mouse; inhalation NA (5-min exposure) RD50: 4796 mg/m3
De Ceaurriz et al. 6 animals/group (1054 ppm)
(1981)
MCB Fischer 344 rat; B6C3F1 oral (gavage in 0, 250, 500, 1000, LD50 in rats: about
Kluwe et al. (1985) mouse; 5 of each sex/group corn oil) 4000 mg/kg 4000 mg/kg (males and
females) (35.5 µmol/kg);
100% lethality in mice:
>1000 mg/kg (males),
>2000 mg/kg (females)
MCB Sprague-Dawley male rat; ip (in corn oil) 0, 225, 552, 1104, LD50 - 1655 mg/kg
Dalich & Larson 3-5 animals/group 1655 mg/kg (2.0, 4.9, 9.8, (14.7 mmol/kg); depression
(1985) 14.7 mmol/kg); 72-h in hepatic glutathione and
observation period increased liver to body
weight ratios at all doses;
centrilobular necrosis and
increase in plasma ALT only
at 2 highest doses (1104 and
1655 mg/kg)
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB Sprague-Dawley male rat; inhalation NA (6-h exposure); 17-day LC50 in rats: 9192 mg/m3
Bonnet et al. (1979, 12 animals/group observation period (1532 ppm)
1982) SPF-OF1 female mouse; LC50 in mice: 7416 mg/m3
20-25 animals/group (1236 ppm)
1,2-DCB rat; 5-20 animals/group inhalation 0, 3234, 4926, 5646, LD50 approx. 5862 mg/m3
Hollingsworth et al. 5862 mg/m3 (0, 539, 821, (lethal to 4/5); marked
(1958) 941, 977 ppm); 7-h hepatic centrilobular
exposure necrosis and cloudy swelling
in the renal tubular
epithelium; increase in
liver and kidney weights
1,2-DCB Sprague-Dawley male rat; inhalation 0, 1224, 1830, 2556, 3654, significant increase in
Brondeau et al. 8 animals/group 4644 mg/m3 (0, 204, 305, serum hepatic enzymes
(1983) 426, 609, 774 ppm); (GLDH, ALT and SDH) at
4-h exposure levels >1830 mg/m3
1,2-DCB Albino guinea-pig; oral (intubation 800 or 2000 mg/kg (305 ppm) no deaths at 100%
Hollingsworth et al. 10 animals (mixed in olive oil) 800 mg/kg; lethality at
(1958) sex)/group 2000 mg/kg
1,4-DCB guinea-pig; rat; 5-15 oral (intubation 1000 or 4000 mg/kg in rats; 100% lethality in
Hollingsworth et al. animals (mixed sex)/group in olive oil) 1600 or 2800 mg/kg in guinea-pigs at 2800 mg/kg
(1956) guinea-pigs and in rats at 4000 mg/kg;
no deaths at 1600 and
1000 mg/kg, respectively
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB male Sprague-Dawley rat or ip (in sesame oil) 1,2-DCB: 195 mg/kg 1,2-DCB: minimal hepatic
1,3-DCB C57 Black/6J mouse (1.33 mmol/kg) necrosis
1,4-DCB 1,3-DCB: 193 mg/kg 1,3-DCB: normal to minimal
Reid (1973) (1.31 mmol/kg) hepatic necrosis
1,4-DCB: 500 mg/kg 1,4-DCB: little or no
(3.40 mmol/kg) effect
1,2,4-TCB CFE rat; CF1 mouse; 4 of oral (gavage) NA; 10-day observation LD50 in rats: 756 mg/kg
Brown et al. (1969) each sex/group period LD50 in mice: 766 mg/kg
CFE rat; 4 of each percutaneous LD50 in rats: 6139 mg/kg
sex/group (topical
administration
on dorso-lumbar
region for 24 h)
1,2,4-TCB ddY mouse; 10 of each oral 123, 160, 207, 269, 350, LD50 in males: 300 mg/kg
Yamamoto et al. sex/group 455, 591, 769 mg/kg LD50 in females: 305 mg/kg
(1978)
1,2,4-TCB Holtzman male rat ip (in sesame oil) 0, 907 mg/kg (0.5 mmol/kg) increased bile
1,3,5-TCB duct-pancreatic flow with
Yang et al. (1979) both isomers, but
1,2,4-isomer 4 more potent;
increase in serum GPT with
the 1,3,5-isomer
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,3,4-TeCB Sprague-Dawley rat; 10 of oral (gavage in 0, 200 - 4000 mg/kg LD50s in males:
1,2,3,5-TeCB each sex/group corn oil) (5 doses) 1,2,3,4-TeCB: 1470 mg/kg
1,2,4,5-TeCB 1,2,4,5-TeCB: 3105 mg/kg
Chu et al. (1983, 1,2,3,5-TeCB: 2297 mg/kg
1984a) LD50s in females:
1,2,3,4-TeCB: 1167 mg/kg
1,2,3,5-TeCB: 1727 mg/kg
1,2,4,5-TeCB mouse, rat, rabbit oral (gavage in NA LD50 in mice:
Fomenko (1965) sunflower oil or 1035 mg/kg (sunflower oil)
starch) 2650 mg/kg (starch)
LD50 in rats and rabbits:
1500 mg/kg (vehicle
unspecified)
PeCB Sherman rat - adult (male oral (gavage in rats: LD50 in male adult rats:
Linder et al. (1980) and female) and weanling peanut oil) 600 - 1500 mg/kg 1125 mg/kg
(female); Swiss Webster mice: LD50 in female adult rats:
mouse; 750 - 1500 mg/kg 1080 mg/kg
10 of each sex/group LD50 in weanling female
rats: 940 mg/kg
LD50 in male mice:
1175 mg/kg
LD50 in female mice:
1370 mg/kg
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
PeCB Sherman rat; 10 of each percutaneous 2500 mg/kg no clinical signs of
Linder et al. (1980) sex/group (dissolved in toxicity
xylene and
applied to back
and shoulder
area)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
NA - not available.
ip - intraperitoneal.
d LC50 - median lethal concentration.
LD50 - median lethal dose.
RD50 - dose producing a 50% decrease in respiratory rate.
ALT - alanine aminotransferase.
GLDH - glutamate dehydrogenase.
SDH - sorbitol dehydrogenase.
Reid (1973) reported minimal hepatic necrosis in Sprague-Dawley rats
or C57Black/6J mice receiving 1,2- or 1,3-DCB at 195 mg/kg body
weight (about 1.32 mmol/kg) intraperitoneally; no such effects were
observed following intraperitoneal administration of 500 mg/kg body
weight (3.4 mmol/kg) of the 1,4-isomer. Yang et al. (1979) reported
increased bile duct-pancreatic flow following administration of
735 mg 1,2-DCB/kg body weight (5 mmol/kg) to male Holtzman rats.
Similar effects were not observed following the administration of
the same dose of the 1,4-isomer.
There are no reliable quantitative data on acute toxicity following
the inhalation of the trichlorobenzenes.
Similar LD50s were reported for CFE rats and CF1 mice ingesting
1,2,4-TCB (756 mg/kg and 766 mg/kg body weight, respectively) (Brown
et al., 1969). Symptoms included depressed activity at lower doses
and extensor convulsions at lethal doses.
The single percutaneous LD50 in CFE rats, determined by the same
investigators, was about an order of magnitude higher than the oral
LD50 (6139 mg/kg body weight).
As was observed for MCB and the 1,2-isomer of DCB, intraperitoneal
administration of the 1,2,4- and 1,3,5-isomers of trichlorobenzene
at 907 mg/kg body weight (5.0 mmol/kg) increased bile
duct-pancreatic flow in Holtzman male rats (Yang et al., 1979). The
1,2,4-isomer was 4 times more potent in inducing this effect;
however, an increase in serum alanine aminotransferase (ALT)
resulted from administration of the 1,3,5-isomer.
Data on the acute toxicity of the tetrachlorobenzenes are restricted
to the oral route of administration. In male Sprague-Dawley rats,
the LD50s for 1,2,3,4-, 1,2,4,5-, and 1,2,3,5-TeCB were
1470 mg/kg, 3105 mg/kg, and 2297 mg/kg body weight, respectively
(Chu et al., 1983, 1984a). In females, the LD50 values were
1167 mg/kg and 1727 mg/kg body weight for 1,2,3,4- and 1,2,3,5-TeCB,
respectively. Clinical signs included depression, flaccid muscle
tone, prostration, piloerection, loose stool, hypothermia,
dacryorrhoea, and coma. At post-mortem, the gastrointestinal tract
was distended and slightly haemorrhagic.
Linder et al. (1980) reported LD50s for PeCB (gavage in peanut
oil) in adult male and female Sherman rats of 1125 mg/kg and
1080 mg/kg body weight, respectively. The value in weanling female
rats was 940 mg/kg body weight. In male and female Swiss Webster
mice, the LD50s were 1175 mg/kg and 1370 mg/kg body weight,
respectively. Decreased activity and tremors were observed in both
species at sublethal dosage levels; the kidneys, liver, and adrenal
glands of rats were also enlarged. In some rats, the gastric mucosa
was hyperaemic, and a slight reddish fluorescence of the
gastro-intestinal tract, suggesting porphyria, was observed in both
rats and mice, under ultraviolet light.
There were no clinical signs of toxicity in rats following
percutaneous administration of 2500 mg PeCB/kg body weight.
8.2 Skin and Eye Irritation, Skin Sensitization
The results of studies on the potential of chlorobenzenes to induce
skin and eye irritation and skin sensitization are presented in
Table 20. These investigations were mainly restricted to the
1,2,4-isomer of trichlorobenzene, which, on the basis of available
data, is mildly irritating, and causes dermatitis after repeated or
prolonged contact, probably because of its degreasing action on the
skin. Direct contact of the eyes with either 1,2-DCB or 1,2,4-TCB is
painful, but does not produce permanent damage; moreover, there was
no evidence of sensitization in the limited studies available.
8.3 Short-term Exposures
The results of short-term studies on the effects of chlorobenzenes
on experimental animals are presented in Table 21. Effects following
the exposure of rats and mice to repeated daily doses of
chlorobenzenes for periods of up to 28 days were confined mainly to
the liver; other target organs included the thyroid, kidney, and
lung in rats and the bone marrow in mice.
In evaluating repeated dose studies in which chlorobenzenes were
administered in the diet, it is important to note that the dose
received by the exposed animals might have been substantially lower
than that indicated by the nominal concentrations, particularly for
the more volatile lower chlorinated congeners. In a short-term
bioassay for 1,2,4,5-TeCB, conducted recently by NTP (1989a), there
was an 8% loss from the diet in 7 days, though corn oil had been
added to minimize volatilization. On the basis of the physical and
chemical properties of chlorobenzene congeners of lower chlorination
than 1,2,4,5-TeCB, volatilization from the diet may have been even
greater.
The results of 14-day studies, in which relatively high doses of MCB
and the two isomers of DCB (1,2- and 1,4-) (up to 8000 mg/kg body
weight) were administered by gavage to F344 rats and B6C3F1 mice,
were consistent with those of the acute toxicity studies; the
1,2-isomer of DCB was slightly more toxic than MCB, as indicated by
the doses that caused decreases in body weight gain and deaths. The
1,4-isomer of DCB was considerably less toxic (NTP, 1983a,b, 1987).
Table 20. Skin and eye irritation; sensitization in experimental annimals
Compound; Speciesa Routeb Dose Results
Reference
Irritation
1,2-DCB rabbit (2) application to the 2 drops undiluted slight to moderate pain and slight
Hollingsworth et al. eyes compound conjunctival irritation, clearing
(1958) in 7 days; prompt washing
immediately after contact reduced
pain and irritation
1,4-DCB rabbit inhalation 4.6 to 4.8 mg/litre tremors, weakness, lateral
Pike (1944) (765 to 798 ppm) nystagmous, corneal and optical
8h/day for nerve oedema, death
62 exposures over
83 days
1,2,4-TCB rabbit - 4 of each dermal (topical 1 ml, 6 h/day very weak irritant, based on
Brown et al. (1969) sex/group application on (3 days) visual inspection and
shorn backs - histopathology 7 days after first
covered) application - fissuring typical
of degreasing action;
rabbit - 4 of each dermal (topical rabbit: 1 ml
sex/group application on guinea-pigs: 0.5 ml, spongiosis, acanthosis and
guinea-pig - 5 of each shorn backs - 5 days/week (3 weeks) parakeratosis in rabbits
sex/group uncovered and guinea-pigs - some
inflammation in the superficial
dermis of rabbits exposed for 3
weeks rabbits exposed for 3 weeks
Table 20 (continued)
Compound; Speciesa Routeb Dose Results
Reference
1,2,4-TCB rabbit application to the irritation; pain, severe
Brown et al. (1969) eyes conjunctivitis, chemosis and
discharge, but without corneal
involvement; swollen lids
1,2,4-TCB New Zealand albino dermal (topical 0, 30, 150, 450 mg/kg, dermal irritation - fur matted by
(70% + 30% rabbit - 5 of each application on 5 days/week (4 weeks) fine, white, bran-like scales
1,2,3-TCB) sex/group shorn backs) with variable degrees of
Rao et al. (1982) erythema, fissures, erosions,
and ulcers; inflammation and
thickening of the epidermis; size
of affected area varied with
dose and increased
severity at 450 mg/kg
1,2,4-TCB ddY mouse - dermal 100, 70, 40, 1 or 0% erythema with 100% TCB (4/8) and
Yamamoto et al. 8 animals/group TCB 70% TCB (2/8); no remarkable
(1978) change in histology
1,2-4-TCB New Zealand white dermal (topical 0, 5, 25, 100% dose-related dermal irritation;
Powers et al. rabbit - 12 of each application on (0.2 ml), 3 times acanthosis and hyperkeratosis
(1975) sex/group ventral surface weekly (13 weeks)
of ear)
Table 20 (continued)
Compound; Speciesa Routeb Dose Results
Reference
Sensitization
1,2,4-TCB guinea-pig intradermal 1 day/week for no overt signs of skin
Brown et al. (1969) injection or 3 successive weeks; sensitization
topical application challenge after
on shorn backs (0.1% 10 days
w/v in light liquid
paraffin)
a Strain and number of animals/group specified where available.
b Vehicle specified, where available.
With the exception of 1,2,4,5-TeCB, administration by gavage (for
periods of from 5 to 15 days) of all of the chlorinated (mono to
penta-) benzene isomers examined caused hepatic porphyria,
characterized by increased levels of porphyrins and porphyrin
precursors in the liver and excreta. 1,3-DCB, 1,3,5-TCB, and
1,2,3,5-TeCB were not examined (Rimington & Ziegler, 1963). The most
active isomers in inducing porphyria were those with 2 chlorine
atoms in a para position to one another (1,4-DCB, 1,2,4-TCB, and
1,2,3,4-TeCB). Liver damage was most severe (intense necrosis and
fatty changes over large areas) in animals treated with high doses
of MCB, 1,2-DCB, or 1,2,4-TCB (maximum doses ranged from 455 to 1140
mg/kg body weight per day, by gavage, for the different isomers).
The other chlorinated benzenes (1,4-DCB, 1,2,3-TCB, 1,2,3,4-TeCB,
and 1,2,4,5-TeCB) produced degeneration of individual liver cells or
focal necrosis in the central, midzonal, and periportal regions.
Hepatomegaly was common in most porphyric rats.
Hepatic porphyria was also observed in rats administered 800 mg
1,3-DCB/kg body weight per day, by gavage, for 5 days (Poland et
al., 1971). Rao et al. (1982) reported an increase in urinary
coproporphyrin in male rabbits treated dermally with 450 mg
1,2,4-TCB/kg body weight per day, for 5 days/week, over 4 weeks.
Fatty degeneration of the liver and changes in blood cell counts
indicative of bone marrow damage, were observed following inhalation
by Swiss white mice of about 1250 mg MCB/m3 or 500 mg TCB/m3
(isomer not specified) for 7 h/day, over 3 weeks (Zub, 1978).
Necrotic foci in the liver were also observed following the dermal
application to guinea-pigs of 0.5 ml undiluted 1,2,4-TCB, for 5
days/ week, over 3 weeks (Brown et al., 1969).
Effects on the adrenal glands and uterus have also been noted in a
study in which 1,2,4-TCB at 250 or 500 mg/kg body weight per day was
administered intraperitoneally for 3 days to immature female rats
(Robinson et al., 1981). There was a decrease in uterine weight and
hepatomegaly at both doses and a decrease in body weight and
increase in adrenal weight at the high dose (500 mg/kg).
The most extensive short-term study (28 days) completed to date on
the toxicity of the higher chlorobenzenes was conducted by Chu
et al. (1983) on 3 isomers of TeCB and PeCB. Levels administered in
the diet of 0.5, 5.0, 50, or 500 mg/kg (ppm) for TeCB, and 5, 50, or
500 mg/kg (ppm) for PeCB, did not cause effects on body weight gain
or food consumption, or induce clinical signs of toxicity. No
haematological aberrations were observed; however, dose-dependent
effects were seen, principally, in the liver, but also in the
thyroid, kidney, and lungs in Sprague-Dawley rats. While the results
of acute toxicity studies indicated that the 1,2,4,5-isomer was the
least toxic of the TeCBs, it was the most toxic congener in the
28-day studies. This was well supported by the observation that it
was present at highest concentrations in fat and liver. The order of
toxicity for the TeCBs and PeCB, which was well correlated with
tissue concentrations, was as follows: 1,2,4,5-TeCB>PeCB> 1,2,3,4-
and 1,2,3,5-TeCB. Moderate to severe histopathological hepatic
changes (e.g., cytoplasmic ballooning and anisokaryosis of
hepatocytes) and increases in liver weight were observed in rats
exposed to 1,2,4,5-TeCB or PeCB at levels of about 50 mg/kg body
weight per day. Histopathological changes in the thyroid (i.e.,
increase in epithelial height and angular collapse of thyroid
follicles with a reduction in colloid density), kidney (i.e.,
eosinophilic inclusions in proximal convoluted tubules), and lung
(i.e., focal alveolar emphysema and inflammation) were mild, even at
the highest dose levels. Although all isomers of TeCB and PeCB
induced hepatic enzymes, liver porphyrin concentrations were not
affected. The data suggest that the position of the chlorine
substituents in TeCBs affects the tissue accumulation and toxicity
of these chemicals.
The toxicity of 1,2,4,5-TeCB following short-term administration (14
days) of 0, 30, 100, 1000, or 3000 mg/kg (ppm) in the diet to
B6C3F1 mice or F344 rats was also investigated recently in an NTP
bioassay (NTP, 1989a). In rats, there were no deaths in any dose
group, though compound-related clinical signs were observed in the
high-dose group. There were also decreases in food consumption and
final mean body weights of both sexes at 3000 mg/kg, increases in
liver weights in males at 1000 mg/kg or more and in females at
3000 mg/kg, and abnormal hyaline droplets in the renal cortical
epithelium of exposed males. In mice, all animals in the 3000 mg/kg
dose group died before the end of exposure. There was also a
significant increase in liver weights in males at 1000 mg/kg and in
females at 300 or 1000 mg/kg. Depletion and necrosis of lymphoid
tissue of the spleen, thymus, and lymph nodes in both sexes were
also observed, particularly in moribund or early death animals.
Concentrations of 0, 100, 330, 1000, 3300, or 10 000 PeCB mg/kg
(ppm) in the diet have also been administered in short-term studies
(15 days) to F344 rats and B6C3F1 mice (NTP, 1989b). All rats in
the 10 000 mg/kg dose group died by day 7, and final mean body
weights in both sexes were lower at 3300 mg/kg. There were also
significant increases in liver weights at all doses except 100 mg/kg
(females), and increases in kidney weights and abnormal hyaline
droplet formation in the renal cortical epithelium in males at all
doses. Centrilobular hepatocellular hypertrophy was observed in
males at 330 and 1000 mg/kg and in females at 1000 and 3300 mg/kg.
At 10 000 mg/kg, there was depletion of thymic lymphocytes and
hyperkeratosis of the forestomach in both sexes, and forestomach
acanthosis in females. In mice, all animals in the 2 highest dose
groups (3300 and 10 000 mg/kg) died by day 10. There was a
significant increase in liver weights in both sexes at 330 and 1000
mg/kg, and mild to moderate depletion of thymic lymphocytes, due to
lymphocyte necrosis, in moribund animals or those that died early.
Table 21. Short-term toxicity of chlorobenzenes in experimental animals
Compound; Speciesa Routeb Dosec Resultsd
Reference
MCB Fischer-344 rat; oral (gavage in rats: 0, 125, 250, rats: prostration, reduced response to stimuli
NTP (1983a); B6C3F1 mouse - corn oil) 500, 1000, or and death of all animals at 1000 and 2000 mg/kg;
Kluwe et al. 5 of each 2000 mg/kg per day mice: no clinical signs of toxicity or deaths
(1985) sex/group mice: 0, 30, 60,
125, 250, or
500 mg/kg per day
(14 days)
MCB male albino rat oral (gavage in maximum dose - hepatic porphyria and hepatomegaly in porphyric
Rimington & - paraffin) 1140 mg/kg per day rats; severe liver damage (intense necrosis and
Ziegler 3 animals/group (5 days) fatty change) at high doses; weight and appetite
(1963) loss; decrease in haemoglobin levels
MCB Swiss white inhalation 0, 2500 mg/m3 fatty degeneration of the liver with "acute
Zub (1978) mouse - 5 of (2.5 mg/litre) yellow atrophy"; changes in blood cell counts
each sex/group reduced to 1250 mg/m3 indicative of bone marrow damage; loss of
(7 h/day - 3 weeks) appetite, general emaciation and marked
somnolence with death of the animals at the
higher dose
1,2-DCB Fischer-344 rat; oral (gavage in rats: 0, 60, 125, 500, rats: dose-related decrease in weight gain (more
NTP (1983b) B6C3F1 mice - corn oil) or 1000 mg/kg per day than 10% in males at highest dose); death of all
5 of each mice: 0, 60, 125, or animals at 1000 mg/kg; mice: death of 1 male and
sex/group 500 mg/kg per day 1 female in high dose (500 mg/kg) group
(14 days)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB male albino rat - oral (gavage in maximum dose - results similar to those for MCB with exception
Rimington & 3 animals/group liquid paraffin) 455 mg/kg per that there was no decrease in haemoglobin levels
Ziegler day (15 days)
(1963)
1,3-DCB Sherman male rat - oral (gavage in 0, 800 mg/kg per day hepatic porphyria; peak effect at 3 days
Poland et al. 4 animals/group peanut oil) (9 days)
(1971)
1,4-DCB male albino rat - oral (gavage in maximum dose - hepatic porphyria; non-necrotic liver cell
Rimington & 3 animals/group liquid paraffin) 770 mg/kg per day degeneration; weight and appetite loss
Ziegler (5 days)
(1963)
1,4-DCB Fischer-344 rat; oral (gavage in rats: 0, 500, 1000, rats: death of all animals at 3 highest doses;
NTP (1987) B6C3F1 mouse - corn oil) 2000, 4000, or decrease in body weight gain at lower doses;
5 of each 8000 mg/kg per day mice: no compound-related deaths or decreases in
sex/group mice: 0, 60, 125, 250, body weight gain
500, or 1000 (14 days)
1,2,3-TCB male albino rat - oral (gavage in maximum dose - hepatic porphyria; non-necrotic liver cell
Rimington & 3 animals/group 1% cellofas) 785 mg/kg per day degeneration; weight and appetite loss
Ziegler (7 days)
(1963)
1,2,4-TCB male albino rat - oral (gavage in maximum dose - results similar to those for MCB with the
Rimington & 3 animals/group 1% cellofas) 730 mg/kg per day exception that there was no decrease in
Ziegler (15 days) haemoglobin levels
(1963)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,4-TCB rabbit (1 of each percutaneous rabbits - 1 ml/day death of some guinea-pigs - necrotic foci in
Brown et al. sex) and (applied on guinea-pigs - livers
(1969) guinea-pig (5 of shaved backs) 0.5 ml/day
each sex) (5 days/week for
3 weeks)
1,2,4-TCB New Zealand albino percutaneous 0, 30, 150, or systemic effects only at 450 mg/kg - increase in
(70% + 30% rabbit - 5 of each (applied on 450 mg/kg per day urinary coproporphyrin in males and slight pallor
1,2,3-TCB) sex/group shaved backs) (5 days/week for of liver in both sexes at necropsy; NOEL -
Rao et al. 4 weeks) 150 mg/kg per day
(1982)
1,2,4-TCB Charles River intraperitoneal 0, 250 or 500 mg/kg decrease in uterus weights and increase in liver
Robinson et al. immature female rat (in corn oil) per day (3 days) weight at both doses; decrease in body weight and
(1981) - 9-10 increase in adrenal weight at high dose
animals/group (500 mg/kg)
1,2,4-TCB Alderley Park SPF inhalation 0, 148, 519, or lethargy and retarded weight gain at 2 highest
(up to 20% rat - 2-4 of each 1484 mg/m3 (0, 20, 70, doses; lacrimation at 519 mg/m3; no observed
1,2,3-TCB) sex/group or 200 ppm) effects at 148 mg/m3
Gage (1970) (6 h/day, 5 days/week
for 3 or 4 weeks)
1,3,5-TCB CD (outbred inhalation 0, 10, 100, or increase in liver to body weight ratio at highest
Sasmore et al. albino) rat - 20 1000 mg/m3 (6 h/day, 5 dose level (1000 mg/m3)
(1983) of each sex/group days/week for 4 weeks)
TCB Swiss white mouse - inhalation 0, 500 mg/m3 fatty degeneration of the liver; changes in blood
(isomer not 5 of each sex/group (0.5 mg/litre) cell counts indicative of bone marrow damage;
specified) (7 h/day for 3 weeks) loss of appetite, hypoactive
Zub (1978)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,4,5-TeCB albino rat (male) - oral (gavage in maximum dose - no effect on urinary porphyrin levels, possibly
Rimington & 3 animals/group 1% cellofas) 905 mg/kg per day because of poor absorption
Ziegler (1963) (5 days)
1,2,4,5-TeCB F344/N rat - oral (mixed in 1% 0, 30, 100, 300, 1000, no deaths at any dose; compound-related clinical
NTP (1989a) 5 of each corn oil added to or 3000 mg/kg (14 days) signs included tremors, lethargy, thin appearance,
sex/group feed) rough coats, ataxia, and chromodacryorrhoea in
both sexes at 3000 mg/kg and rapid breathing in
females at 3000 mg/kg; decrease in final mean
body weights at 3000 mg/kg (18% in males; 15% in
females); 20% decrease in food consumption at
3000 mg/kg; at >300 mg/kg, increases in absolute
and relative kidney weights (males only); liver
congestion in males at >1000 mg/kg and in females
at 3000 mg/kg; abnormal hyaline droplets in the
renal cortical epithelium of exposed male rats
(doses unspecified)
1,2,4,5-TeCB B6C3F1 mouse - oral (mixed in 1% 0, 30, 100, 300, 1000, all animals in the 3000 mg/kg dose group died;
NTP (1989a) 5 of each sex/group corn oil added to or 3000 mg/kg (14 days) compound-related clinical signs included tremors,
feed) rapid breathing, lethargy, hunched posture, rough
coats, dyspnoea, and prostration in both sexes at
3000 mg/kg and thin appearance in females at
3000 mg/kg; significant increase in absolute and
relative liver weights in males at 1000 mg/kg and
in females at 300 or 1000 mg/kg; at 3000 mg/kg,
depletion and necrosis of lymphoid tissue of the
spleen, thymus, and lymph nodes in both sexes
(these changes frequently observed in moribund or
early-death animals)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,3,4-TeCB albino rat (male) - oral (gavage in maximum dose - results similar to those for 1,2,3-TCB and hair
Rimington & 3 animals/group liquid paraffin) 660 mg/kg per day loss
Ziegler (1963) (10 days)
1,2,3,4-TeCB Sprague-Dawley rat oral (diet) TeCBs: 0, 0.5, 5.0, 50, no effect on body weight gain, food consumption;
1,2,4,5-TeCB - 10 of each or 500 mg/kg diet (28 no clinical signs of toxicity; no haematological
1,2,3,5-TeCB sex/group days) PeCB: 0. 5.0, 50, aberrations; increase in serum cholesterol for
PeCB or 500 mg/kg diet 1,2,4,5-TeCB at 500 mg/kg; induction of hepatic
Chu et al. (28 days) enzymes by all isomers; increase in liver weight
(1983) for 1,2,4,5-TeCB and PeCB at 500 mg/kg;
dose-dependent histological changes (e.g.,
cytoplasmic vacuolation) in the liver (moderate to
severe) for 1,2,4,5-TeCB at 500 mg/kg, mild in all
other cases - PeCB > 1,2,3,4-TeCB and
1,2,3,5-TeCB; dose-dependent accumulation of
TeCBs in fat and liver - greatest for 1,2,4,5-TeCB
and PeCB; mild thyroid changes for 1,2,4,5-TeCB
and PeCB at 500 mg/kg, very mild in all other
cases; mild changes in kidney and lung
PeCB F344/N rat - 5 of oral (in feed) 0, 100, 330, 1000, all animals in the 10 000 mg/kg dose group died
NTP (1989b) each sex/group 3300, or 10 000 mg/kg by day 7; at 3300 mg/kg, final mean body weights
(15 days) were 23% and 15% lower in males and females,
respectively; feed consumption lower in week 1
and higher in week 2; significant increase in
absolute and relative liver weights in all dose
groups except at 100 mg/kg (females); in males,
significant increases in absolute kidney weights
at 100, 330, and 1000 mg/kg and significant
increase in relative kidney weights at all doses;
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
significant decrease in the absolute weights
of the kidney, thymus, heart, and lung of both
sexes at 3300 mg/kg possibly attributable to the
lower weight gain in these animals; excessive
abnormal hyaline droplets in the renal cortical
epithelium in males (0/5, 1/5, 5/5, 5/5, 2/5, 0/5
in control and increasing dose groups,
respectively); centrilobular hepatocellular
hypertrophy in males at 330 and 1000 mg/kg, and in
females at 1000 and 3300 mg/kg; at 10 000 mg/kg,
depletion of thymic lymphocytes and hyperkeratosis
(mild to moderate) of the forestomach in both
sexes and forestomach acanthosis in females
PeCB B6C3F1 mouse - oral (in feed) 0, 100, 330, 1000, all mice in 3300 or 10 000 mg/kg dose groups died
NTP (1989b) 5 of each 3300, or 10 000 mg/kg by day 10; clinical signs of toxicity in animals
sex/group (15 days) exposed to 3300 mg/kg prior to death included
tremors, lethargy, hunched posture, and paralysis
(both sexes) and dyspnoea in females; significant
increase in absolute and relative liver weights
at 330 and 1000 mg/kg (both sexes); no
compound-related lesions in surviving animals, but
mild-to-moderate depletion of thymic lymphocytes,
due to lymphocyte necrosis, in moribund or
early-death animals
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
8.4 Long-term Exposures
The results of selected studies of the toxicity of the
chlorobenzenes after long-term exposures are presented in Table 22.
The data included in the table have been restricted to
investigations for which analyses of, at least, body weight gain,
survival, clinical signs of toxicity, clinical chemistry,
haematology, and histopathology of major organs and tissues have
been conducted. No-observed-effect levels (NOEL),
no-observed-adverse-effect levels (NOAEL), and lowest-observed-
adverse-effect levels (LOAEL), determined on the basis of the
results of these studies, are also presented in Table 22. Additional
studies, in which analyses were more limited, are referenced in the
text.
As in the case of the single and short-term exposure studies, there
are comparatively more data on the toxicity of the lower chlorinated
congeners (i.e., mono- and dichlorobenzenes) after long-term
exposure than there are for the tri-, tetra-, and
pentachlorobenzenes.
Because of the paucity of available data on some of the congeners
and the wide variations in study design and duration, it is
difficult to draw very specific conclusions about structure-activity
relationships with respect to the toxicity of the chlorobenzenes
after long-term exposures. However, on the basis of available data,
it appears that, with the exception of 1,4-DCB, which has relatively
low long-term toxicity, there is a trend for toxicity to increase
with increased chlorination of the benzene ring for many of the
endpoints examined. However, it should be noted that the variations
in the long-term toxicity of different isomers of the same congener
are, in some cases, considerable. The toxicity of the compounds also
appears to be well correlated with the extent of accumulation in the
body tissues, and, in general, female animals appear to be less
sensitive to the chlorinated benzenes than males.
Administration of MCB via inhalation or ingestion to rats, mice,
rabbits, or dogs has caused reductions in both body weight gain and
survival at high doses, and hepatic and renal toxicity, as indicated
by increases in serum enzymes, liver and kidney weights,
histo-pathological changes, and necrosis (Irish, 1963; Knapp et al.,
1971; Dilley, 1977; NTP, 1983a). At high doses, depression of bone
marrow activity in mice (Zub, 1978) and myeloid depletion of the
thymus, spleen, or bone marrow in rats and mice (NTP, 1983a) have
also been observed. Inhibited chronaxia of antagonistic muscles, an
increase in blood cholinesterase, and a decrease in serum
alpha-globulin in rats have also been reported at considerably lower
doses (0.1 mg/m3) (Tarkhova, 1965); however, it is difficult to
assess the validity of these results on the basis of the published
data.
The no-observed-effect level for the long-term inhalation of MCB (32
exposures of 7 h/day, over 44 days) was approximately 910 mg/m3 in
rats (Irish, 1963). For ingestion, the no-observed-effect level was
50-125 mg/kg body weight in rats, and 125 mg/kg body weight in mice
(Knapp et al., 1971; NTP, 1983a) (Table 22).
The effects of long-term exposure of rats, guinea-pigs, rabbits, and
mice to the DCBs, via inhalation or ingestion, have been similar to
those of MCB. Reductions in body weight gain and reduced survival
have resulted from exposures to high doses of DCBs. Hepatic and
renal toxicity, as indicated by increases in liver and kidney
weights, as well as histopathological changes and necrosis, have
also been reported (Hollingsworth et al., 1956, 1958; NTP, 1983b,
1987). Increases in porphyrin excretion, and lymphoid depletion of
the thymus and spleen in rats, and multifocal mineralization of the
myocardial fibres and skeletal muscles in mice have also been
observed following administration of high doses of 1,2-DCB (NTP,
1983b).
As was observed in the acute and short-term studies, the 1,4-isomer
appears to be considerably less toxic than 1,2-DCB. While the NOEL
for the long-term inhalation of 1,2-DCB, derived from available
studies, was 560 mg/m3 for rats, guinea-pigs, rabbits, and monkeys
(Hollingsworth et al., 1958), the NOEL for the 1,4-isomer, was
950 mg/m3 in mice, rabbits, and monkeys, and 580 mg/m3 in rats
and guinea-pigs (Hollingsworth et al., 1956). The NOEL for ingestion
of the 1,2-isomer in male mice was 125 mg/kg body weight (NTP,
1983b) compared with values for the 1,4-isomer of 337.5 mg/kg body
weight in mice and 300-600 mg/kg body weight in rats (NTP, 1987).
Most of the data on the trichlorobenzenes are restricted to
1,2,4-TCB, the most widely used isomer. Inhalation of 1,2,4-TCB in
long-term studies on rabbits, rats, dogs, and monkeys resulted in
liver and kidney damage, as indicated by increases in organ weight
and transient histopathological changes (Coate et al., 1977; Kociba
et al., 1981). The NOELs were 742 mg/m3 in rabbits and monkeys
(Coate et al., 1977) and 223 mg/m3 in dogs (Kociba et al., 1981).
Increases in porphyrin excretion have also been observed in various
species following the inhalation of 1,2,4-TCB (Watanabe et al.,
1978; Kociba et al., 1981); the no-observed-adverse-effect level
(NOAEL) in rats for this effect was reported to be 22.3 mg/m3.
On the basis of the results of one long-term inhalation study, the
1,3,5-isomer appears to be somewhat less toxic than 1,2,4-TCB,
possibly owing to low absorption of this relatively non-volatile
chemical. The only notable effects resulting from the long-term
administration of doses up to 1000 mg/m3 to rats were squamous
metaplasia and hyperplasia of the respiratory epithelium of the
nasal passages at the highest dose (NOEL - 100 mg/m3) (Sasmore
et al., 1983).
Table 22. Toxicity of chlorobenzenes after long-term exposures
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Inhalation
MCB Sprague-Dawley 0, 341, increase in food intake (rats); reduction in SGOT; 341 mg/m3 "marginally
Dilley (1977) rat (male) and 1138 mg/m3, increase in liver weight (both species) and toxic concentration"
rabbit (male); 7 h/day, congestion of liver (rabbits, 1138 mg/m3); increase
32 animals/ 5 days/week in kidney weight and tubular and interstitial
group for 24 weeks lesions (both species); lesions of the adrenal
cortex (rats); small changes in red cell parameters
(both species)
MCB rat, rabbit, 910, 2161, at 4550 mg/m3, increase in mortality (guinea-pigs), 910 mg/m3 (NOEL)
Irish (1963) and guinea-pig or 4550 mg/m3, slight depression in growth, histopathological 2161 mg/m3 (LOAEL)
7 h/day, changes in the lungs, liver, and kidney; at 2161
5 days/week mg/m3, slight increase in liver weight and slight
for 32 histopathological changes in the liver; no effects
exposures at 910 mg/m3
over 44 days
1,2-DCB rat: 20 of 0, 290, or decrease in spleen weight (M guinea-pigs) not 560 mg/m3 (NOEL)
Hollingsworth each 560 mg/m3, believed to be treatment related; no other effects
et al. (1958) sex/group; 7 h/day, on growth, mortality, organ weights, haematological
guinea-pig: 5 days/week or urinalysis parameters; no histopathological
8 of each for 6-7 changes
sex/group; months
rabbit: 7 of
each
sex/group;
monkey:
2 females
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB rat, 0, 580, or growth depression (guinea-pig), increase in liver 580 mg/m3 (rats, (NOEL)
Hollingsworth guinea-pig, 950 mg/m3, and kidney weight, cloudy swelling and granular guinea-pigs);
et al. (1956) rabbit, mouse, 7 h/day, degeneration of the liver in rats at 950 mg/m3 950 mg/m3 (monkeys,
and monkey; 5 days/week (NOEL) rabbits, mice);
1-10 for 5-7 950 mg/m3 (rats,
animals/group months (LOAEL) guinea-pigs)
1,3,5-TCB CD rat - 20 of 0, 10, 100, squamous metaplasia and hyperplasia in the 100 mg/m3 (NOEL)
Sasmore et al. each sex/group or 1000 respiratory epithelium of the nasal passages at
(1983) mg/m3, 6 h/day, 1000 mg/m3
5 days/week
for 13 weeks
1,2,4-TCB Sprague-Dawley 0, 223, or increased liver weight (rats and dogs) and kidney 742 mg/m3 (rabbits);
(99.41%) rat (male) - 742 mg/m3, weight at 742 mg/m3; increased urinary excretion of 223 mg/m3 (dogs) (NOEL)
Kociba et al. 20/group; New (0, 30, or porphyrins at >223 mg/m3 (rats), considered to
(1981) Zealand albino 100 ppm) be physiological rather than toxic effect
rabbit (male) 7 h/day,
- 4/group; 5 days/week
beagle dogs for 30
(male) - exposures
2/group (44 days)
1,2,4-TCB Sprague-Dawley 0, 22.3, or slight reversible increase in urinary porphyrins 22.3 mg/m3 (M) (NOAEL)
Watanabe rat (male and 74.2 mg/m3 at 74.2 mg/m3
et al. (1978) female) (0, 3, or
10 ppm),
6 h/day,
5 days/week
for 3 months
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4-TCB Sprague-Dawley 0, 186, 371, no effect on pulmonary function or operant behaviour 742 mg/m3 (NOEL)
(99.07%) albino rat or 742 mg/m3 in monkeys; transient changes in the liver
Coate et al. (male) - (0, 25, 50, (hepatocytomegaly) and kidney (hyaline degeneration
(1977) 30/group; New or 100 ppm) of the cortex) in rats, at all doses; no
Zealand white 7 h/day, abnormal opthalmic changes in rabbits and monkeys
albino rabbit 5 days/week
(male) - for 26 weeks
16/group;
cynomolgus
monkey (male)
- 9/group
Ingestion
MCB Fischer-344 0, 60, 125, reduced survival and reduction in body weight gain 125 mg/kg per day
Kluwe et al. rat; B6C3F1 250, 500, or at >250 mg/kg; increase in SAP and SGGPT at >500 (NOEL);
(1985) mouse - 10 of 750 mg/kg mg/kg (F rats); polyuria and porphyrinuria at >500 250 mg/kg per day
each sex/group per day, mg/kg (rats and F mice); increase in liver porphyrin (LOAEL)
5 days/week at >500 mg/kg (F rats); dose-dependent increase in
for 13 weeks; liver weight, centrilobular hepato-cellular
gavage in degeneration and necrosis; slight increase in kidney
corn oil weight, degeneration and focal necrosis of the
proximal renal tubules; slight decrease in spleen
weight with lymphoid or myeloid depletion of the
thymus, spleen, or bone marrow at >250 mg/kg
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
MCB dog - 8 27.25, 54.5, in high-dose group, 50% mortality, low blood sugar, 54.5 mg/kg per day
Knapp et al. animals/group or 272.5 mg/kg increase in SGPT and SAP, increase in total(NOAEL);
(1971) per day, bilirubin and cholesterol in some animals, 272.5 mg/kg per day
5 days/week histopathological changes in liver, kidneys, (LOAEL)
for 93 days; gastrointestinal mucosa, and haematopoietic tissue
capsule and increase in immature leukocytes; in
intermediate- and low-dose groups, no consistent
toxic effects
MCB rat 12.5, 50, or in high-dose group, retarded growth in males; 50 mg/kg per day
Knapp et al. 250 mg/kg increase in liver and kidney weight at >50 mg/kg; no (NOEL);
(1971) per day for significant histopathological changes 250 mg/kg per day
93-99 days; (LOAEL)
diet
1,2-DCB Fischer-344 0, 30, 60, reduced survival at 500 mg/kg (mice and F rats) and 125 mg/kg per day
NTP (1983b) rat; B6C3F1 125, 250, or reduction in body weight gain at 500 mg/kg (F rats); (NOEL);
mouse - 10 of 500 mg/kg no increase in serum hepatic enzymes; increase in 250 mg/kg per day
each sex/group per day, urinary porphyrin at 500 mg/kg (rats and F mice), (LOAEL)
5 days/week but no increase in hepatic porphyrin; increase in
for 13 liver weight/body weight ratio (>125 mg/kg - rats,
weeks; 500 mg/kg - F mice); centrilobular hepatic
gavage in degeneration and necrosis (>125 mg/kg - rats;
corn oil >250 mg/kg - M mice); renal tubular degeneration
(500 mg/kg - M rats); decrease in spleen/body weight
ratio (>30 mg/kg - F mice) and decrease in thymus/-
body weight ratio (500 mg/kg - M rats) and lymphoid
depletion of the thymus and spleen at 500 mg/kg in
mice; in rats, slight decrease in haemoglobin levels
and haematocrit and in red blood cell counts (M), at
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2-DCB 500 mg/kg; in mice, multifocal mineralization of
NTP (1983b) myocardial fibres of heart and skeletal muscle at
cont'd 500 mg/kg
1,4-DCB Fischer 344/N 0, 37.5, 75, no compound-related deaths or changes in body weight 600 mg/kg per day (F);
NTP (1987) rat - 10 of 150, 300, or gain; increase in the incidence and severity of 300 mg/kg per day (M)
each sex/group 600 mg/kg kidney cortical tubular degeneration at 600 mg/kg (NOEL);
per day, (M) 600 mg/kg per day (M)
5 days/week (LOAEL)
for 13
weeks;
gavage in
corn oil
1,4-DCB B6C3F1 mouse - 0, 84.4, no compound-related deaths or change in body weight 337.5 mg/kg per day
NTP (1987) 10 of each 168.8, gain; mild to moderate centrilobular (NOEL);
sex/group 337.5, 675, hepatocytomegaly at 900 mg/kg; minimal to 675 mg/kg per day
or 900 mg/kg mild hepatocytomegaly at 675 mg/kg (LOAEL)
per day,
5 days/week
for 13
weeks;
gavage in
corn oil
1,2,4-TCB ICR-JCL mouse 600 mg/kg no abnormal weight changes in organs; no macroscopic
Goto et al. (male) diet (78 or histological lesions in liver
(1972) - 20 animals/- g/kg per
group day for
6 months;
diet
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3-TCB Sprague-Dawley 0, 1, 10, no clinical signs of toxicity; deaths of one 1,2,3,-TCB:
1,2,4-TCB rat 100, 1000 high-dose female (not treatment related) and one 7.7 mg/kg per day;
1,3,5-TCB (weanling); mg/kg diet male control (no apparent cause); statistically 1,2,4-TCB:
(>99%) 5 animals each mixed with significant growth suppression in males exposed to 7.8 mg/kg per day;
Côté et al. sex/group corn oil in 10 or 1000 mg 1,2,3-TCB/kg; nephrosis in one male 1,3,5-TCB:
(1988) the diet exposed to 1000 mg 1,2,4-TCB/kg; significant 7.6 mg/kg per day
(males: 0, increase in liver/body weight ratios in males (NOAEL)
0.07-0.08, exposed to high dose of all 3 TCBs; significant
0.78-0.81, increase in serum AH (males) and APDM (both sexes)
7.6-7.8, in animals exposed to 1000 mg 1,2,4-TCB/kg;
78-82 mg/kg "qualitatively similar" changes, mild to moderate
per day; (all TCB isomers) observed in the liver, thyroid,
females: 0, and kidney, significant at high-dose levels only and
0.11-0.13, more severe in males; histopathological changes
1.3-1.5, include: liver: mild to moderate increase in
12-17, cytoplasmic volume and anisokaryosis of hepatocytes;
101-146 all isomers produced fatty infiltration-most severe
mg/kg per at 1000 mg 1,2,4-TCB/kg; thyroid: mild to moderate
day) changes (high-dose groups) including reduction in
follicular size, increased epithelial height and
reduced colloid density; kidney: moderate changes in
the convoluted tubules of males exposed to 1000 mg
1,3,5-TCB/kg
1,2,4,5 TeCB albino rat 0, 0.001, increase in content of SH groups in serum, increases
Fomenko 0.005, or in hemoglobin and reticulocytes of the peripheral
(1965) 0.05 mg/kg blood at >0.005 mg/kg; disorders in the
per day for glycogen-forming function of the liver
8 months
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB B6C3F1 mouse - 0, 30, 100, compound-related clinical signs included tremors 300 mg/kg diet (NOEL);
NTP (1989a) 10 of each 300, 1000, (females), prostration, lethargy, hunched posture, 1000 mg/kg diet (LOAEL)
sex/group or 2000 and rough hair coats (both sexes) at 2000 mg/kg;
mg/kg diet 2/10 female mice in the 2000 mg/kg dose group were
for 13 killed in moribound condition, before termination of
weeks; mixed the study; decrease in final mean body weights of
in 1% corn exposed mice (all doses in males; 30 mg/kg and >1000
oil added to mg/kg for females); decrease in food consumption in
the diet males (at 2000 mg/kg) and females (>1000 mg/kg); at
2000 mg/kg, absolute liver weights (both sexes)
approximately 3 times those of controls; absolute
liver weights increased in females at 30 mg/kg and
>1000 mg/kg and in males at 100 mg/kg and
>1000 mg/kg; relative liver weights increased in
females at >1000 mg/kg and in males at >100 mg/kg;
minimal to mild centrilobular hepatocellular
hypertrophy (both sexes) at 1000 mg/kg (7/10 M; 7/10
F) and 2000 mg/kg (10/10 M; 9/10 F); minimal to mild
individual hepatocyte degeneration at 1000 mg/kg
(9/10 M; 5/10 F); hepatocellular necrosis in males
at 2000 mg/kg (4/10) and in females at 30 mg/kg
(1/10) (not considered to be treatment-related),
1000 mg/kg (1/10) and 2000 mg/kg (1/10);
mineralization of the heart in males at 300 (2/10)
and 2000 (3/10) mg/kg with relationship to treatment
unclear; at doses >1000 mg/kg (both sexes),
increases in serum sorbitol dehydrogenase and
alanine aminotransferase activity; thyroid
follicular hypertrophy in males at >300 mg/kg and in
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB females at >100 mg/kg; at 2000 mg/kg in males and
NTP (1989a) >1000 mg/kg in females, decreased hemoglobin
concentrations, mean corpuscular hemoglobin,
haematocrit, and mean cell volume
1,2,4,5-TeCB F344/N rat - 0, 30, 100, significant decrease in body weights of both sexes 30 mg/kg diet (F)
NTP (1989a) 20 of each 300, 1000, at >1000 mg/kg; compound-related clinical symptoms (NOEL);
sex/group or 2000 included hypoactivity and lethargy; significant 100 mg/kg diet (F)
mg/kg diet increases in absolute (>300 and >100 mg/kg) and (LOAEL)
for 13 relative (>300 and >30 mg/kg) liver weights for
weeks; mixed males and females, respectively; significant
in 1% corn increases in absolute (>300 mg/kg - both sexes) and
oil and relative kidney weights (M >100 mg/kg, F >300
added to the mg/kg); thyroid follicular cell hypertrophy
feed (M >300 mg/kg, F >100 mg/kg); "primary hypothyroid
state" (M >300 mg/kg, F >30 mg/kg); centrilobular
hypertrophy in both sexes (>1000 mg/kg); "hyaline
droplet nephropathy" particularly in males (>100
mg/kg); in females, renal cortical tubular
regeneration (>100 mg/kg) and renal tubular
epithelial accumulation of an unidentified
yellow-brown pigment (2000 mg/kg); in males,
decreases in haematocrit value, haemoglobin
concentrations, and erythrocyte counts at >1000
mg/kg and increases in platelet count and serum
albumin at >100 mg/kg; in females, increase in serum
albumin at 2000 mg/kg and decrease in mean cell
volume at >1000 mg/k3g
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3,4-TeCB Sprague-Dawley 0, 0.5, 5.0, for 1,2,4,5-TeCB - increase in hepatic AH and APDM 1,2,3,4-TeCB:
1,2,3,5-TeCB rat - 15 of 50, or at >50 mg/kg (M) 500 mg/kg (F); at 500 mg/kg 3.4 mg/kg per day (M);
1,2,4,5-TeCB each sex/group 500 mg/kg increase in serum cholesterol, haemoglobin levels, 42 mg/kg per day (F)
(>99.5%) diet for 90 haematocrit, liver and kidney weights with (NOEL);
Chu et al. days; in dose-dependent changes in severity and prevalence of 34 mg/kg per day (M)
(1984a) corn oil in kidney and liver lesions; dose increase in serum (LOAEL)
diet cholesterol, haemoglobin levels and haematocrit at
500 mg/kg; increase in liver and kidney weights at 1,2,3,5-TeCB:
500 mg/kg; dose-dependent changes in severity and 0.42 mg/kg per day (F);
prevalence of renal lesions - (extensive epithelial 3.4 mg/kg per day (M)
necrosis with large intratubular casts at 500 mg/kg) (NOEL);
and of hepatic lesions (accentuation of zonation, 4.2 mg/kg per day (F);
increase in cytoplasmic homogeneity, aggravated 34 mg/kg per day (M)
basophilia, anisokaryosis and pyknotic (LOAEL)
hepatocellular nuclei); dose-dependent accumulation 1,2,4,5-TeCB:
in fat and liver; for the other isomers, changes in 0.034 mg/kg per
kidney and liver much milder, little accumulation in day (M);
fat and liver; males more susceptible than females 4.2 mg/kg per day (F)
(NOEL);
0.34 mg/kg per
day (M);
42 mg/kg per day (F)
(LOAEL)
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB Sherman rat - males: 6 - dose-dependent accumulation in adipose tissue; no 16-31 mg/kg per day (F)
(99.1%) 10 of each 140 mg/kg evidence of porphyria; increase in liver weight at (NOEL);
Linder et al. sex/group per day (0, >500 mg/kg; increase in adrenal (M) and kidney 27-63 mg/kg per day
(1980) 125, or 1000 weight at 1000 mg/kg (and increase in kidney weight (LOAEL)
mg/kg diet) in M at 125 mg/kg); hypertrophy of hepatic cells at
for 100 >500 mg/kg; dose-related increase in hyaline
days; droplets in kidney (M) at >125 mg/kg (at 1000 mg/kg,
females: 7 - focal areas of tubular atrophy and interstitial
150 mg/kg lymphocytic infiltration); decrease in haemoglobin
per day (0, levels, increase in WBC at 1000 mg/kg; decrease in
125, 250, RBC and haematocrit (M) at 1000 mg/kg
500, or 1000
mg/kg diet)
for 180 days
PeCB F344/N rat - 0, 33, 100, decrease in mean body weights of males (>1000 mg/kg) 33 mg/kg (M)
NTP (1989b) 20 of each 330, 1000, and females in all dose groups; increase in absolute 330 mg/kg (F) (NOEL);
sex/group or 2000 liver weights in males at >100 mg/kg and in females 100 mg/kg (M)
mg/kg for 13 at 100 and >1000 mg/kg; increase in relative liver 1000 mg/kg (F) (LOAEL)
weeks in weights in males at >33 mg/kg and in females at
feed >100 mg/kg; increase in
absolute kidney weights in males at >330 mg/kg and
in females at >1000 mg/kg; increase in relative
kidney weights in males >100 mg/kg and in females at
100 and >1000 mg/kg; at >1000 mg/kg, decreases in
hematocrit values, hemoglobin concentrations, mean
corpuscular hemoglobin, and mean cell volume in both
sexes; increases in serum albumin (males, >1000
mg/kg; females, >330 mg/kg); increase in
reticulocyte (males at 2000 mg/kg) and platelet
(males, >1000 mg/kg) counts and creatinine
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB concentrations (males, >1000 mg/kg); lower platelet
NTP (1989b) counts in females at all doses but within normal
(continued) variation; significant increase in serum sorbitol
dehydrogenase activity in males (>1000 mg/kg) and
females (at concentrations as low as 100 mg/kg);
compound-related urinary effects included
significant increase in glucose concentrations
(males, >330 mg/kg; females >1000 mg/kg), increased
protein concentrations (both sexes >1000 mg/kg; more
pronounced in males) and significantly increased
urine volume (males, >1000 mg/kg; females at 2000
mg/kg on day 90); compound-related effects on the
thyroid included decreased free thyroxin and total
thyroxin at all doses in males and >100 mg/kg in
females; incidence of abnormal sperm increased in
males at 330 mg/kg (70%) and 2000 mg/kg (100%)
(sperm of males receiving 1000 mg/kg not examined);
dose-related renal lesions of males included
hyaline droplets of the cortical tubular epithelium
(>100 mg/kg), medullary tubular dilatation
(>100 mg/kg),
medullary collecting tubule mineralization (>330
mg/kg) and tubular cortex regeneration and cortex
chronic inflammation (>100 mg/kg) (also seen in some
control animals); tubular cortex regeneration in
females only at 2000 mg/kg; tubular cortex
pigmentation (males, 2000 mg/kg; females
>1000 mg/kg) and cortex protein casts (both sexes at
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB 2000 ppm); hepatic centrilobular hypertrophy in
NTP (1989b) males at >330 ppm and in females at >1000 ppm;
(continued) hepatic pigmentation and periportal cytoplasmic
vacuolization in females at >1000 ppm; follicular
cell hypertrophy in both sexes at >1000 ppm
PeCB B6C3F1 mouse - 0, 33, 100, compound-related clinical signs included ventral 100 mg/kg (F) (NOEL);
NTP (1989b) 20 of each 330, 1000, body swelling and ruffled fur in both sexes at 330 mg/kg (F)
sex/group or 2000 mg/kg, 2000 mg/kg; significant increase in males in (LOAEL)
for 13 weeks absolute kidney weight at >330 mg/kg and relative
in feed kidney weights at >1000 mg/kg; increase in absolute
liver weights (males, >100 mg/kg; females,
>330 mg/kg); increase in relative liver weights
(both sexes, >330 mg/kg); increases in hemoglobin of
both sexes at >2000 mg/kg (although within normal
variation); increase in serum sorbitol dehydrogenase
activity (both sexes, >1000 mg/kg); decrease in
total thyroxin concentration in both sexes at
concentrations >33 mg/kg; at end of study, liver
porphyrin increased in both sexes at >1000 mg/kg;
minimal-to-moderate centrilobular hepatocellular
hypertrophy in males at all dose levels and in
females at >330 mg/kg; occasional necrosis of
hypertrophied hepatocytes (considered secondary to
the hypertrophy) observed in both sexes (males, 33
and 2000 mg/kg; females in control, 330 and
2000 mg/kg dose groups)
Table 22 (continued)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses reported as mg/kg body weight, unless specified.
d M - male.
F - female.
WBC - white blood cells.
RBC - red blood cells.
ALH - microsomal aniline hydroxylase.
SGOT - serum glutamic-oxaloacetic transaminase.
SGPT - serum glutamic-pyruvic transaminase.
SAP - serum alkaline phosphatase.
ALT - alanine aminotransferase.
APDM - aminopyrine demethylase.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOAEL - lowest-observed-adverse-effect level.
Reports of investigations of the long-term toxicity of the
tetra-chlorobenzenes following inhalation have not been found.
However, in a 90-day study to compare the toxicities of TeCB isomers
administered in the diet to rats, renal and hepatic toxicity were
observed, as indicated by increases in organ weights and
histopathological changes (Chu et al., 1984a). As was seen in a
short-term study conducted by the same investigators, the
1,2,4,5-isomer was the most toxic of the TeCBs; this may be
attributable to its comparatively greater accumulation in fat and
liver. The authors concluded that the observed changes were similar
to those resulting from 28-day exposures to the same doses, but that
renal lesions induced by the highest concentration of 1,2,4,5-TeCB
(30-40 mg/kg) were more severe. The NOEL in male rats adminis-tered
the 1,2,4,5-isomer was 0.034 mg/kg body weight; the value for
females was considerably higher (4.2 mg/kg body weight). The NOEL
for the other isomers in male rats was 3.4 mg/kg body weight; in
females, these values were 42 and 0.42 mg/kg body weight for the
1,2,3,4- and 1,2,3,5-isomers, respectively.
In a 13-week study on F344 rats, which was completed recently by the
US National Toxicology Program, 1,2,4,5-TeCB was mixed in corn oil
(1%) and added to the diet at concentrations of 0, 30, 100, 300,
1000, or 2000 mg/kg (NTP, 1989a). There were significant decreases
in body weights, compound-related clinical symptoms,
histopathological changes in the liver, and haematological effects
at the highest doses; increases in the weights of both liver and
kidney, renal histopathological effects, and effects on the thyroid
were seen at lower doses. The NOEL for histopathological effects in
females was considered by the investigators to be 30 mg/kg diet
(approximately 1.95 mg/kg body weight); a NOEL in males could not be
established.
In a similar study, in which B6C3F1 mice were administered a diet
containing 0, 30, 100, 300, 1000, or 2000 mg 1,2,4,5-TeCB/kg for 13
weeks, there were significant decreases in body weights and food
consumption, compound-related clinical signs, increases in liver
weights and circulating hepatic enzymes, histopathological changes
in the liver, and haematological effects, at the highest doses. The
NOEL in both males and females was considered to be 300 mg/kg diet
(approximately 48 mg/kg body weight) (NTP, 1989a).
Only limited data are available on the long-term toxicity of
penta-chlorobenzene. Ingestion of high doses by rats resulted in
hepatic and renal toxicity, as indicated by increases in organ
weights and histopathological changes (Linder et al., 1980),
increases in adrenal weight (Linder et al., 1980), and increased
excretion of porphyrins (Goerz et al., 1978). The NOEL in female
rats, derived from the results of Linder et al. (1980), was
16-31 mg/kg.
In a recently completed 13-week study conducted by NTP (1989b), F344
rats and B6C3F1 mice were fed diets containing 0, 33, 100, 330,
1000, or 2000 mg PeCB/kg. Decreases were seen in the mean body
weights of male rats at 1000 mg/kg or more and in females at all
dose levels. Increased liver weights were seen at the lower doses
and histopathological effects on the liver, at the higher doses. In
males, there were increases in kidney weights and renal
histo-pathological effects at lower doses; these effects were
observed only at higher doses in females. Functional effects were
observed in the kidney in both sexes at the highest doses and in the
thyroid at all doses, with histopathological effects occurring only
at the highest doses (1000 or 2000 mg/kg diet). There were also
haematological effects at doses exceeding 1000 mg/kg in males and
330 mg/kg in females. The incidence of abnormal sperm in males was
also increased at the dose levels at which it was examined (330 and
2000 mg/kg diet). On the basis of histopathological lesions, the
authors considered the NOEL to be 33 mg/kg diet in male rats, and
330 mg/kg diet in females (approximately 2.0 and 21.5 mg/kg body
weight, respectively).
In mice, there were compound-related clinical signs at the highest
dose. Increased kidney weights were observed at the highest doses
and functional effects on the thyroid at all doses. There were
increases in liver weights at lower doses and histopathological
effects on the liver at all doses in males and at doses of 330 mg/kg
diet or more in females. On the basis of the histopathological
lesions, the authors considered the NOEL in female mice to be 100
mg/kg diet (approximately 18.3 mg/kg body weight); no NOEL in males
could be established.
8.5 Chronic Toxicity and Carcinogenicity
The protocols and results of the few studies on chronic toxicity and
carcinogenicity of the chlorobenzenes are summarized in Table 23.
The United States National Toxicology Program (NTP) has studied the
chronic toxicity and carcinogenicity for rats and mice of MCB, and
the 1,2- and 1,4-isomers of DCB (NTP, 1983a,b, 1987), following oral
administration. The results of an additional investigations by
Loeser & Litchfield (1983) on the chronic toxicity and
carcino-genicity of 1,4-DCB for rats and mice have also been
reported.
Only one rather limited investigation on the chronic toxicity and
carcinogenicity of the TCBs (1,2,4-isomer) following skin painting
in mice has been identified (Yamamoto et al., 1982) and studies on
the chronic toxicity of the TeCBs are confined to a very limited
investigation involving the oral administration of the
1,2,4,5-isomer to dogs (Braun et al., 1978). No data were found on
the chronic toxicity and carcinogenicity of PeCB.
Table 23. Chronic toxicity and carcinogenicity of chlorobenzenes
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Inhalation
1,4-DCB SPF Alderlely 0, 450, or 3000 mg/m3 increase in liver and kidney weights, 450 mg/m3
Loeser & Park Wistar (0, 75 or 500 ppm); 5h urinary protein, and coproporphyrin at (NOEL)
Litchfield -derived rat /day, 5 days/week for 3000 mg/m3; no treatment-related tumours 3000 mg/m3
(1983) - 76-79 of each 76 weeks; killed at 102 (LOAEL)
sex/group weeks
1,4-DCB SPF Swiss 0, 450, or 3000 mg/m3 no treatment-related toxic effects or 3000 mg/m3 (NOEL)
Loeser & (Alderley Park (0, 75, or 500 ppm); 5h tumours; one osteosarcoma in nasal sinus
Litchfield strain) mouse /day, 5 days/week for 57 at 450 mg/m3
(1983) (female) - weeks; killed at 75-76
75/group weeks
Ingestion
MCB Fischer 344 rat - 0, 60, or 120 mg/kg per significant increase in hepatic 60 mg/kg per day (M),
(99%) 50 of each day (5 days/week for 103 neoplastic nodules (M) at 120 mg/kg 120 mg/kg per day (F)
NTP (1983a); sex/group weeks); gavage in corn in comparison with vehicle and pooled (NOEL);
Kluwe et al. oil controls, but no carcinomas at this 120 mg/kg per day (M)
(1985) dose; marginally significant (LOAEL)
dose-response trend; rare tumours in 3
exposed animals - 1 renal tubular cell
adenocarcinoma (F) and 2 transitional
cell papillomas of the bladder (M);
decrease in pituitary tumours
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
MCB B6C3F1 mouse - 50 males: 0, 30, or 60 mg no treatment-related toxic effects or 60 mg/kg per day (M),
(99%) of each sex/group /kg per day; females: 0, tumours 120 mg/kg per day (F)
NTP (1983a) 60, or 120 mg/kg per day (NOEL)
Kluwe et al. (5 days/week for
(1985) 103 weeks); gavage in
1,2-DCB Fischer-344 rat - 0, 60, or 120 mg/kg per decrease in survival and body weight 120 mg/kg per day (F)
(>99%) 50 of each day (5 days/week for gain (M) at 120 mg/kg; increase in body (NOEL)
NTP (1983b) sex/group 103 weeks); gavage in weight gain (F) after 32 weeks; increase
corn oil in adrenal phacochromocytomas at 60
mg/kg - probably not compound-related
1,2-DCB B6C3F1 mouse - 0, 60, or 120 mg/kg per dose-related trend for increase in 60 mg/kg per day (M),
(>99%) 50 of each day (5 days/week for tubular regeneration in the kidney (M at 120 mg/kg per day (F)
NTP (1983b) sex/group 103 weeks); gavage in 120 mg/kg); positive trend for incidence (NOEL);
corn oil of malignant histiocytic lymphomas in 120 mg/kg per day (M)
both sexes, but negative trend for (LOAEL)
malignant lymphocytic lymphomas;
significant positive trend in
alveolar/bronchiolar carcinomas in males
not significant when combined with
adenomas; dose-related decrease in
hepatocellular adenomas (M)
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB Fischer 344 rat - males: 0, 150, or reduced survival (M) and body weight
(>99%) 50 of each 300 mg/kg per day gain at 300 mg/kg; increased severity of
NTP (1987) sex/group females: 0, 300, or nephropathy and incidence of hyperplasia
600 mg/kg per day of the parathyroid at >150 mg/kg (M) and
(5 days/week for dose-related increase in nephropathy (F)
103 weeks); gavage in at >300 mg/kg; dose-related increase in
corn oil renal tubular cell adenocarcinomas (M);
tubular cell adenoma in high-dose groups
(M); marginal increase in mononuclear
leukaemias (M)
1,4-DCB B6C3F1 mouse - 0, 300,or 600 mg/kg per increased incidence of hepatic lesions;
(>99%) 50 of each day (5 days/week for nephropathy (M), renal tubular
NTP (1987) sex/group 103 weeks); gavage in regeneration (F); thyroid gland
corn oil follicular cell hyperplasia (M)
(positive trend in F); adrenal medullary
hyperplasia (M) and focal hyperplasia of
the adrenal gland capsule (M); positive
trend and increase in hepatocellular
carcinomas at 600 mg/kg and
hepatocellular adenomas
(M >300 mg/kg; F >600 mg/kg);
hepatoblastomas in 4 high-dose males;
positive trend for phaeochromocytomas
f adrenal gland with increased incidence
at 600 mg/kg (M); marginal trend in
follicular cell adenomas of the thyroid
(F)
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB Beagle dog - 5 mg/kg per day (2 increases in SAP and total bilirubin at
Braun et al. 2 of each years; killed at 3 years 24 months returned to normal, 3 months
(1978) sex/group (no 8 months); diet after exposure (no measurement of
concurrent urinary, haematological, or clinical
control group) chemistry, during exposure); no
morphological changes after the 20-month
recovery phase (no gross or histological
examination during, or at the end of,
exposure)
Dermal
1,2,4-TCB Slc:ddY mouse - 0.03 ml of 0, 30%, or clinical symptoms of toxicity including
Yamamoto 75 of each 60% solution (twice/week hysterical excitement, acceleration of
et al. (1982) sex/group (50 of for 2 years); dissolved spontaneous activity, and panting;
each sex/control in acetone decreased survival (F-both doses, M-high
group) dose); thickening and keratinization of
epidermis followed by inflammation;
increase in spleen weight at both doses
(M-low dose, F-high dose); minor
haematological changes at both doses;
authors report no increase in tumour
incidence attributable to TCB - however
data inadequate for evaluation
Table 23 (continued)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
d SAP - serum alkaline phosphatase.
M - male.
F - female.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOAEL - lowest-observed-adverse-effect level.
In the NTP bioassays on MCB, there was no convincing evidence of
compound-related toxicity in either rats or mice administered doses
of up to 120 mg/kg body weight per day for 103 weeks. Evidence for
mild hepatocellular necrosis in rats was equivocal and, though there
was a significant decrease in the survival of male rats in the
high-dose group (120 mg/kg), the absence of marked toxic lesions did
not support a causal relationship with MCB. The doses administered
in the long-term bioassay were not significantly less than those at
which toxic effects were observed in the NTP 13-week studies (LOAEL
= 250 mg/kg per day), indicating little potential for progressive
toxicity with continued MCB administration for more than 13 weeks
(NTP, 1983a; Kluwe et al., 1985).
A significant increase in neoplastic nodules in the liver was noted
in the high-dose group of male rats administered MCB (incidence,
2/50, 4/49, and 8/49 at 0, 60, and 120 mg/kg body weight,
respectively). However, no hepatocellular carcinomas were found and
analysis of the combined data on neoplastic nodules and
hepato-cellular carcinomas reduced the significance of the increase
in tumour incidence. It was concluded that the NTP study provided
some, but not clear, evidence of carcinogenicity in male Fischer 344
rats, based on the increased incidence of hepatic nodules, but that
there was no evidence of carcinogenicity in female Fischer 344 rats
or in male or female B6C3F1 mice (NTP, 1983a).
In the NTP bioassay for the carcinogenesis of 1,2-DCB, the only
evidence of toxicity was a decrease in survival and body weight gain
in male rats and an increase in tubular regeneration of the kidney
in male mice, both of which occurred at the highest dose level (120
mg/kg body weight per day for 103 weeks) (NTP, 1983b). The rates of
survival until the end of the study in the 3 groups of male rats
were 42/50 (84%), 36/50 (72%), and 19/50 (38%) for 0, 60 mg/kg, and
120 mg/kg body weight, respectively. Although the survival rate in
the high-dose group was significantly different from those in the
low-dose and vehicle-control groups in the male rat, this may have
been the result of accidents occurring during the dosing by gavage
(NTP, 1983b). In mice administered 1,2-DCB, there was a dose-related
trend in the tubular regeneration of the kidney in males only, with
an increased incidence at the highest dose (120 mg/kg body weight)
(control, 17%; 60 mg/kg, 24%; 120 mg/kg, 35%); otherwise, there was
no other evidence of non-neoplastic toxicity (NTP, 1983b).
In rats administered 1,2-DCB in the NTP study, the only tumours that
occurred in excess were adrenal phaeochromocytomas in the low-dose
group of male rats (incidence in controls, 9/50; low-dose, 16/50 and
high-dose, 6/49), which were not considered to be treatment-related.
In mice, there was a positive trend in the incidence of malignant
histiocytic lymphomas in both sexes; however, the combined incidence
of all types of lymphomas was not significantly greater than that in
the controls for mice of either sex. Similarly, alveolar/bronchiolar
carcinomas occurred in male mice with a statistically significant
positive trend; there were, however, no significant trends when the
incidences of alveolar/bronchiolar adenomas and carcinomas were
combined. Thus, the NTP concluded that, under the conditions of
these studies, there was no evidence for the carcinogenicity of
1,2-DCB in male or female F344 rats or B6C3F1 mice.
There are 2 bioassays that are relevant to the assessment of the
chronic toxicity and carcinogenicity of 1,4-DCB: an inhalation study
on rats and mice (Loeser & Litchfield, 1983) and an NTP bioassay
involving ingestion by the same species (NTP, 1987).
Loeser & Litchfield (1983) reported increases in liver and kidney
weights, as well as in urinary protein and coproporphyrin, in the
high-dose group when rats were administered 0, 450, or 3000 mg
1,4-DCB/m3, for 5 h/day, 5 days/week, over 76 weeks, followed by
36 weeks without exposure. There were also some statistically
significant changes in blood biochemical and haematological
parameters; however, there was no indication of a dose-response
relationship in this regard. There was no evidence of toxicity in
mice following administration of the same doses for a shorter period
of 57 weeks, followed by 19 weeks without exposure. No
treatment-related effects on the incidence, multiplicity, or
malignancy of tumours were observed in either species. The
relatively short exposure period (76 weeks in rats, 57 weeks in
mice) and the high early mortality in mice may have decreased the
sensitivity of this carcinogenicity bioassay.
In the NTP (1987) study, 1,4-DCB was administered, by gavage, in
corn oil, for 5 days/week, over 103 weeks. Groups of 50 male F344
rats received 150 or 300 mg/kg body weight per day, while groups of
50 female F344 rats and 50 male and 50 female B6C3F1 mice received
300 or 600 mg/kg body weight per day. Under this regimen, there was
an increased incidence of non-neoplastic liver lesions in male and
female mice, including cytomegaly and karyomegaly, hepatocullular
degeneration, and individual cell necrosis. Also, 1,4-DCB
administration resulted in an increased incidence of nephropathy in
male mice and renal tubular regeneration in female mice.
Non-neoplastic lesions in male rats after 1,4-DCB administration
included an increased incidence and severity of nephropathy, an
increased incidence of epithelial hyperplasia of the renal pelvis,
mineralization of the collecting tubules in the renal medulla, and
focal hyperplasia of the renal tubular epithelium. An increased
incidence of nephropathy was noted in both low- and high-dose female
rats compared with the vehicle controls.
As reported by NTP (1987), administration of 1,4-DCB resulted in a
dose-related increase in the incidence of tubular cell
adenocarcinomas of the kidney in male rats (1/50, 3/50, and 7/50 in
the control, mid-, and high-dose groups, respectively); an uncommon
malignant tumour was found in F344 rats, but only in 4/1098 of the
NTP historical controls fed corn oil by gavage. An additional
tubular cell adenoma was observed in a high-dose male rat. No
tubular cell tumours were found in either treated or vehicle-control
female rats. A marginal increase in the incidence of mono-nuclear
cell leukaemia in treated male rats was noted compared with controls
(5/50, 7/50, and 11/50 in control, mid- and high-dose groups,
respectively).
In mice, there were positive trends in the incidence of
hepatocellular adenomas, hepatocellular carcinomas, and
hepatocellular adenomas and carcinomas (combined) in males and
females, the incidences in the high-dose groups being significantly
greater than those in the vehicle controls. In low-dose male mice,
the incidences of hepatocellular adenomas and adenomas or carcinomas
(combined) were also significantly increased over those in the
vehicle controls (combined incidence 17/50, 22/49, and 40/50 in
control, low-, and high-dose groups of males; combined incidence
15/50, 10/48, and 36/50 in control, low-, and high-dose groups of
females) (NTP, 1987). Hepatoblastomas occurred in 4 high-dose male
mice, each of which also bore hepatocellular carcinomas. This rare
type of hepatocellular carcinoma had not been observed by NTP in
1091 control (corn oil by gavage) male mice and had only been seen
in 1/2080 untreated female mice. Marginal increases were observed in
the incidences of pheochromocytomas of the adrenal gland in male
mice (controls, 0/47; low-dose group, 2/48; and high-dose group,
2/49) reaching statistical significance in the high-dose group
(P=0.035), and in the incidence of follicular cell adenomas of the
thyroid gland in female mice (controls, 0/48; low-dose group, 0/45;
and high-dose group, 3/46).
On the basis of these results, the NTP (1987) concluded that there
was clear evidence of the carcinogenicity of 1,4-DCB in male F344/N
rats, and clear evidence of carcinogenicity in both male and female
B6C3F1 mice. There was no evidence of carcinogenicity in female
F344/N rats.
It is possible that the discrepancies between the results of Loeser
& Litchfield (1983) and NTP (1987) are the result of differences in
experimental design. Loeser & Litchfield (1983) administered
approximately 390 mg/kg body weight per day via inhalation to rats
and approximately 790 mg/kg to mice. Although these doses were
higher than those used by NTP (1987), mice were exposed for only 57
weeks and rats for only 76 weeks. The strains of animals used and
the nature of administration also varied between the 2 studies
(i.e., continuous inhalation for 5 h/day versus bolus dose by
gavage).
The induction of kidney tumours in the male rat is believed to be
associated with hyaline droplet formation resulting in a
characteristic alpha-2-microglobulin nephropathy (hyaline droplet
nephropathy). Such effects, specific for the male rat, have been
shown to occur with a number of chemicals, including 1,4-DCB,
unleaded gasoline, isophorone, decalin, and limonene, and the
mechanism has been established (Short et al., 1987; Goldsworthy et
al., 1988; Murty et al., 1988; Charbonneau et al., 1989). It
involves increased formation of protein droplets and crystalloid
accumulation in the cytoplasm of the P2 segment of the proximal
tubule with a marked increase in cell proliferation. The hyaline
droplets formed are composed of lysosomes containing excess
alpha-2-microglobulin and protein-bound chemical. Increased cell
turnover in the P2 segment of the kidneys of male rats may be
related to the slow catabolism of this accumulated protein within
the lysosomes leading, in some cases, to renal tubular
adenocarcinomas in the male rat. Alpha-2-microglobulin occurs in
large amounts in the male Fisher 344 rat, but is not seen in
significant amounts in the female rat, mouse, or man (Olson et al.,
1990).
For 1,4-DCB, there is much evidence to support the involvement of
hyaline droplet accumulation in the induction of the tubular cell
adenocarcinomas in the male rat. Bomhard et al. (1988) demonstrated
hyaline droplet accumulation in male, but not in female, rats
following exposure to 1,4-DCB. Charbonneau et al. (1989) reported
that 1,4-DCB (single exposure of F344 rats to 300 or 500 mg/kg body
weight in corn oil) increased protein droplet formation and cell
proliferation in male but not female rat kidneys, whereas equimolar
doses of 1,2-DCB did not have any effect. The maximum amount of
radiolabel reversibly bound to alpha-2-microglobulin by
C14-labelled 1,4-DCB was also twice that for an equimolar dose of
C14-labelled 1,2-DCB.
Available data are inadequate for the assessment of the chronic
toxicity and carcinogenicity of the higher chlorinated benzenes
(tri- to penta-). Clinical signs of toxicity, decreased survival,
increased organ weights, keratinization of the epidermis, and minor
haemato-logical effects were reported in Slc:ddy mice treated
dermally with 0.03 ml of a 30 or 60% solution of 1,2,4-TCB, twice a
week, over 2 years (Yamamoto et al., 1982). No increase in tumour
incidence, attributable to TCB, was reported.
An increase in serum alkaline phosphatase and total bilirubin
following 24 months of exposure of beagle dogs to 5 mg
1,2,4,5-TeCB/kg body weight per day in the diet has been reported
(Braun et al., 1978). The values for these parameters returned to
normal 3 months after cessation of exposure, and there were no
morpho-logical changes after a 20-month recovery phase. However, the
results of this study are inadequate for the assessment of chronic
toxicity or carcinogenicity. No measurements were made of urinary,
haematological, or clinical chemistry parameters during exposure,
and no histopathological examination was carried out at the end of
exposure. Moreover, there were only 2 animals of each sex in the
exposed group, only 1 dose level was examined, there was no
concurrent control group, and the period of administration (2 years)
was short in relation to the life span of the dog.
No data are available on the chronic toxicity or carcinogenicity of
pentachlorobenzene.
8.6 Mutagenicity and Related Endpoints
8.6.1 In vitro systems
When tested in S. typhimurium strains TA98, TA100, TA1535, or
TA1537, with, or without, the addition of an S9 fraction from the
liver of Aroclor(R) 1254-treated rats, MCB did not show any
mutagenic potential (Haworth et al., 1983). In another study in
S. typhimurium using a slightly lower dose (1.28 µl/plate) and
adding strain TA1538, Shimizu et al. (1983) reported that there was
no evidence for the mutagenicity of MCB in this system.
The dichlorobenzenes (1,2-, 1,3-, and 1,4-DCB) have been studied for
mutagenic effects in S. typhimurium , with, and without, metabolic
activation. Haworth et al., (1983) studied a mixture of DCB isomers
in 4 strains with, and without, activation with S9 supernatant from
the livers of rats treated with Aroclor(R) 1254. No mutagenicity
was noted. Using a similar protocol with 5 strains of
S. typhimurium, at doses of up to approximately 1.3 µl/plate,
Shimizu et al. (1983) reported that neither 1,2- nor 1,3-DCB was
mutagenic. 1,4-DCB, at doses up to 6.6 mg/plate, was also found to
be negative in S. typhimurium, under the conditions outlined by
Shimizu et al. (1983).
No evidence was reported by Haworth et al. (1983) that mixed TeCBs
and PeCB were mutagenic, when assayed in S. typhimurium strains
TA98, TA100, TA1535, and TA1537 with, and without, metabolic
activation. Using a similar protocol, Schoeny et al. (1979) reported
that 1,2,4-TCB was not mutagenic in S. typhimurium.
When tested for forward mutations in L5178Y/TK+/- mouse lymphoma
cells, 1,4-DCB was negative when assayed without metabolic
activation and the results were equivocal with activation with S9
from Aroclor(R) 1254-induced rat liver (NTP, 1987). No evidence
was reported by Perocco et al. (1983) for the induction by 1,4-DCB
of unscheduled DNA synthesis in human lymphocytes in vitro.
The induction in vitro of chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster ovary (CHO) cells by
1,4-DCB was studied by Galloway et al. (1985). Experiments were
carried out with, and without, S9 from the livers of male rats
induced with Aroclor(R) 1254. No chromosomal effects were
reported. Using cultured Chinese hamster lung fibroblast cells,
with, and without, activation by S9 from livers of rats induced with
the PCB KC-400, Sofuni et al. (1985) reported that treatment with
1,4-DCB, 1,2,3-TCB, 1,2,4-TCB, or 1,3,5-TCB did not cause
chromosomal aberrations.
In unpublished studies reviewed by the Task Group, 1,4-DCB did not
induce mutation at the HGPRT locus in CHO cells (Bayer, 1986a),
unscheduled DNA synthesis in HeLa cells (Milone, 1986a), or
chromosome aberrations in human lymphocytes (Milone, 1986b), in
either the presence or absence of an exogenous metabolic activation
system. 1,4-DCB did not induce cell transformation in the Balb/3T3
assay (Bayer, 1986b).
8.6.2 In vivo tests on experimental animals
Very few studies have been reported on the in vivo genotoxicity of
chlorobenzenes.
No increase in micronucleated red blood cells was reported after
oral administration to male mice of 600, 900, 1000, 1500, or 1800 mg
1,4-DCB/kg body weight, by gavage, for 5 days/week, over 13 weeks.
Female mice were similarly treated with 1200, 1500, or 1800 mg/kg
body weight (NTP, 1987). These results support those from a
previously unpublished study, reviewed by Loeser & Litchfield
(1983), in which treatment of rats, via inhalation with doses of
1,4-DCB as high as 4092 mg/m3 (682 ppm) for 2 h/day, or 3000
mg/m3 (500 ppm) for 5 h/day, over 3 months, did not result in any
increase in chromosomal abnormalities in bone marrow cells.
A study of the clastogenic activity of halogenated benzenes in mice
has been published by Mohtashamipur et al. (1987). MCB, 1,2-, 1,3-,
or 1,4-DCB, or 1,2,3-, 1,2,4-, or 1,3,5-TCB, administered by
intraperitoneal injection in corn oil to groups of 5 mice, resulted
in a dose-related increase in the formation of micronucleated
poly-chromatic erythrocytes. The doses used were between
approximately 15 and 70% of the respective LD50s, given in 2
doses, separated by a 24-h period. Bone marrow samples were taken
30 h after the first injection. Doses were similar to those given by
gavage for 13 weeks in other studies (NTP, 1987). However, this
result was not confirmed in studies by other workers, also using the
interperitoneal route (Heibold, 1988). Groups of mice were
administered doses of 2 x 177.5 and 2 x 355 mg/kg in solution in
corn oil, intraperitoneally (doses similar to those inducing an
effect in the study by Mohtashamipur et al., 1987). The two doses
were given 24 h apart, and the cells were harvested 6 h later.
Evidence of a reduction in erythropoiesis was seen at both dose
levels; however, there was no indication of micronuclei induction at
either dose level. The ability of orally administered 1,4-DCB to
produce micronuclei in bone marrow has also been investigated
(Heibold, 1986). There was no evidence of any increase in
micronuclei following the administration of a single oral dose of
2500 mg/kg body weight and the harvesting of the bone marrow at 24,
48, and 72 h.
The ability of 1,4-DCB to produce unscheduled DNA synthesis (UDS) in
the liver, or effects on DNA replication (S phase), was investigated
following oral exposure of B6C3F1 mice to the compound (Steinmetz
& Spanggord, 1987). Mice were given doses of 300, 600, or 1000 mg
1,4-DCB/kg body weight in corn oil and hepatocytes were harvested 16
and 48 h later. There was no evidence of any increase in UDS at any
of the dose levels studied. The small increase in S-phase DNA
replication seen was statistically significant in males only. These
data indicate that 1,4-DCB does not produce DNA damage in the mouse
liver following oral exposure.
The production of chromosome damage in the bone marrow of mice by
1,4-DCB has been investigated by a number of workers. Negative
results were obtained in all except one case. In addition, negative
results were obtained in an assay of DNA damage (UDS) in the liver
of mice following oral exposure to 1,4-DCB. These results indicate
that 1,4-DCB does not have any mutagenic potential in in vivo
studies.
MCB and 1,4-DCB bind covalently to DNA in the mouse liver, kidney,
and lung, while only MCB binds to the DNA in rat tissue (Grilli
et al., 1985; Lattanzi et al., 1989). The levels of binding for both
compounds are low.
8.6.3 Human in vivo studies
Workers in a pathology laboratory (8 male/18 female, average age 35
years) were accidently exposed to vapours of 1,2-DCB, for 8 h/day,
over 4 days. Chromosome analyses on peripheral leukocytes revealed a
marked increase in chromosomal aberrations compared with controls.
The controls (8 male/8 female, average age 34 years) were from other
pathology laboratories where the occupational exposures to chemicals
were similar to that of the DCB-exposed population. In the 1345
cells studied in the exposed workers, 120 exhibited chromosomal
alterations (84 single breaks and 86 double breaks) compared with 19
cells with aberrations in the 942 cells examined in the control
group. Analyses carried out 6 months after exposure indicated that
the alterations were reversible (Zapata-Gayon et al., 1982).
Although no air monitoring was carried out, the symptoms reported by
22 out of the 26 subjects were consistent with those produced at
exposure levels in excess of 600 mg/m3 (100 ppm), described by
Hollingsworth et al. (1958).
In workers (24 male/1 female) producing 1,2,4,5-TeCB in a chemical
plant, in which several organochlorine and organophosphorus
pesticides were also produced, there were increases (significance
not reported) in the total number of chromatid gaps and breaks,
labile chromosome-type aberrations (acentric fragment, ring
chromosomes, and dicentric chromosomes), and stable chromosome-type
aberrations (deletions, inversions, and translocations), compared
with those in 14 workers from another type of factory and 49
unexposed controls. No workplace monitoring for exposure levels was
carried out and workers were exposed to several other compounds.
Thus, it is difficult to assess the potential risks in other groups
exposed to TeCB on the basis of these results (Kiraly et al., 1979).
8.7 Developmental and Reproductive Effects
The results of studies concerning the embryotoxicity, fetotoxicity,
teratogenicity, and reproductive effects of the chlorobenzenes are
presented in Table 24. Effect levels derived in these studies for
both mothers and offspring are also presented in this table. In
contrast to the lack of information on acute, short-term, long-term,
and carcinogenic effects, comparatively more data are available on
the potential embryotoxic, fetotoxic, and teratogenic effects of the
higher chlorinated benzenes. Furthermore, the TCBs, TeCBs, and PeCB
have been better studied in this regard than MCB and the isomers of
DCB.
There has been no evidence in any of the studies conducted to date
that the chlorobenzenes (mono- to penta-) are teratogenic in animal
species. Some relatively minor embryotoxic and fetotoxic effects
have been observed for the lower chlorinated benzenes (MCB and
DCBs), but only at dose levels that were toxic for the mother. For
example, there was a slight delay in skeletal development
(ossification) in the fetuses of pregnant rats exposed to 2864 mg
MCB/m3, a dose that induced decreases in body weight gain in the
mothers (John et al., 1984). Similarly, the ossification of cervical
vertebrae in fetuses of pregnant rats exposed to 2400 mg
1,2-DCB/m3 was delayed; this dose also induced decreases in body
weight gain and food consumption in the mothers (Hayes et al.,
1985). However, in both of these studies, there was no convincing
evidence of embryotoxic, fetotoxic, or teratogenic effects in
rabbits exposed to MCB or 1,2-DCB via inhalation, even at dose
levels that were maternally toxic (John et al., 1984; Hayes et al.,
1985). In fetuses of pregnant rabbits exposed to 4720 mg
1,4-DCB/m3, there was an increase in the incidence of
retro-oesophageal right subclavian artery, a minor variation in the
circulatory system that is often observed in control litters; at
this dose, the maternal body weight gain was also decreased (Hayes
et al., 1985). In an additional study, Giavini et al. (1986)
administered 1,4-DCB, in corn oil, by gavage, at doses of 250, 500,
750, or 1000 mg/kg body weight per day, between days 6 and 15 of
gestation. Effects were noted only at doses greater than 500 mg/kg
per day. These included an increased frequency of extra ribs, a
reduction in fetal weight, and an increase in skeletal
abnormalities. These dose levels also induced decreases in body
weight gain and food consumption in the mothers.
There is some evidence that the higher chlorinated benzenes (TCBs,
TeCBs, PeCB) are embryotoxic or fetotoxic at dose levels that are
not maternally toxic. However, available data are not consistent and
the toxicities of the various isomers of the TCBs and TeCBs for the
mother and fetus vary considerably. For example, the 1,2,4-isomer
was the most maternally toxic of the 3 TCB isomers administered by
ingestion to pregnant rats in a study conducted by Black et al.
(1988); changes in haematological parameters occurred at doses as
low as 150 mg/kg per day. At a lower dose (75 mg/kg), there were
mild histological changes in the lenses of pups of exposed mothers;
however, these changes were not observed at higher doses (150 or
300 mg/kg) and were unlikely, therefore, to be treatment-related.
Kitchin & Ebron (1983a) observed growth retardation in the embryos
of pregnant rats administered 360 mg 1,2,4-TCB/kg body weight on
days 9-13, a dose that caused some lethality, reduced body weight
gain, and produced moderate hepatocellular hypertrophy in mothers.
Although less toxic for the mothers than 1,2,4-TCB, the 1,3,5-isomer
of trichlorobenzene induced mild changes in the lenses of pups of
pregnant rats administered doses as low as 150 mg/kg body weight by
gavage; there was no significant maternal toxicity at this dose
(Black et al., 1988). For the 1,2,3-isomer, there were no effects in
offspring at any dose level (up to 600 mg/kg body weight), even
though a level of 300 mg/kg was toxic to the mothers. The authors of
this study did not discuss the significance of the observed
histological changes in the lenses (areas of cellular disorientation
and disaggregation with ballooning and granular degeneration) of the
pups of mothers administered the 1,3,5-isomer, but concluded that
there was no evidence that any of the TCB congeners were teratogenic
or fetotoxic.
Kacew et al. (1984) reported that the 1,2,4,5-isomer was the most
toxic of the TeCBs for both mothers and fetuses, in a study in which
all 3 isomers were administered to pregnant rats by gavage (death of
9/10 animals at 200 mg/kg and increase in serum cholesterol at
50 mg/kg body weight). The toxicity was well correlated with the
greater accumulation of 1,2,4,5-TeCB in maternal and fetal tissues.
There was a decrease in the number of live fetuses in pregnant rats
administered 50 mg 1,2,4,5-TeCB/kg body weight, a dose that induced
minor changes (increases in serum cholesterol) in exposed mothers.
Table 24. Developmental and reproductive studies on chlorobenzenes
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Developmental Studies:
Inhalation
MCB Fischer 344 rat rats: 0, 341, 955, or rats: maternal toxicity (decreased body weight gain 955 mg/m3 (F);
(99.98%) (pregnant), 2684 mg/m3 (0, 75, 210, or and increased liver weight) at 2684 mg/m3 - slight (NOEL);
John et al. 32-33/group; 590 ppm), 6 h/day on days delay in skeletal development (ossification) in 2684 mg/m3 (F)
(1984) New Zealand white 6-15 of gestation; rabbits: fetuses at this dose; rabbits: maternal toxicity *2684 mg/m3
rabbit, (pregnant), 0, 341, 955 or 2684 mg/m3 (increased liver weight) at >2684 mg/m3; significant (O) (LOAEL)
30-32/group (0, 75, 210, or 590 ppm) increase in resorptions at 2684 mg/m3 (second
6 h/day on days 6-18 of study), but within historical range; no
gestation; or rabbits: 0, treatment-related effects on fetus
45, 136, 341, or 2684 mg/m3
(0, 10, 30, 75 or 590 ppm)
6 h/day on days 6-18 of
gestation
1,2-DCB Fischer 344 rat rats: 0, 600, 1200, or rats: maternal toxicity at all doses; increased rats:
(98.81%) (pregnant), 2400 mg/m3, 6 h/day on days liver weight at 2400 mg/m3; significant increase in 600 mg/m3 (F);
Hayes 30-32/group; 6-15 of gestation; rabbits: spurs on first lumbar vertebra and delayed *2400 mg/m3
et al. New Zealand white doses as above - on days 6-18 ossification of sternebrae in fetuses at 1200 mg/m3 (O) (LOAEL)
(1985) rabbit (pregnant), of gestation not considered to be treatment related; delayed
28-30/group ossification of cervical vertebra centra in fetuses rabbits:
at 2400 mg/m3; rabbits: maternal toxicity (decreased *2400 mg/m3
body weight gain) at all doses; ratio of male:female (O) (NOEL);
offspring significantly different from 50:50 at 1200 600 mg/m3 (F)
mg/m3 not considered to be treatment related; no (LOAEL)
other embryotoxic, fetotoxic or teratogenic effects
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB New Zealand white 0, 590, 1770, or slight maternal toxicity (decreased body weight 1770 mg/m3 (F)
(99.9%) rabbit (pregnant), 4720 mg/m3, 6 h/day on days gain) at 4720 mg/m3; significant increase in (NOEL)
Hayes 29-30/group 6-18 of gestation retroesophageal right subclavian artery at 4720 4720 mg/m3 (F)
et al. mg/m3, not considered to be a teratogenic response *4720 mg/m3
(1985) (O) (LOAEL)
1,4-DCB SPF rat (pregnant), 0, 450, 1200, or no evidence of maternal toxicity, embryo-, or
Loeser & 20/group 3000 mg/m3 (0, 75, 200 or 500 fetotoxicity or teratogenicity
Litchfield ppm), 6 h/day on days 6-15
(1983) of gestation
Developmental Studies:
Ingestion
1,4-DCB CD rat (pregnant), 0, 250, 500, 750, or maternal toxicity (decreased body weight gain and 250 mg/kg per
Giavini 11-16/group 1000 mg/kg per day on days food consumption) at >500 mg/kg; reduction in fetal day (F) (NOEL)
et al. 6-15 of gestation; gavage in weight at 1000 mg/kg per day; fetotoxic effects 500 mg/kg per
(1986) corn oil included increase in fetal skeletal variations at day (F)
>750 mg/kg, dose-related increase in the frequency *500 mg/kg per
of extra ribs at >500 mg/kg day (O)
(LOAEL)
1,2,3-TCB Sprague-Dawley rat 1,2,4-TCB: 0, 75, 150, or maternal toxicity - reduced body weight gain at 600 1,2,3-TCB:
1,2,4-TCB (pregnant); 14/group 300 mg/kg per day; 1,2,3-TCB mg/kg (1,3,5-TCB), increased liver weight at 600 300 mg/kg per
1,3,5-TCB and 1,3,5-TCB: 0, 150, 300 or mg/kg (1,2,3- and 1,3,5-TCB) and 300 mg/kg day (F);
(99.5%) 600 mg/kg per day on days (1,2,4-TCB), decreased haemoglobin levels and *600 mg/kg per
Ruddick 6-15 of gestation; gavage in haematocrit, generally at highest dose (all 3 day (O) (NOEL)
et al. corn oil isomers), decreased RBC at 300 mg/kg (1,2,3-TCB),
(1983); 150 and 300 mg/kg (1,2,4-TCB), and 150 and 1,2,4-TCB:
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Black 600 mg/kg (1,3,5-TCB), mild treatment-related 75 mg/kg per
et al. histopathological changes in spleen, liver, and day (F);
(1988) thyroid, generally at highest dose (all three *300 mg/kg per
isomers); mild histological changes in the lenses of day (O)
pups at all doses (1,3,5-TCB) and 75 mg/kg (NOEL);
(1,2,4-TCB); no significant accumulation in maternal 150 mg/kg per
or fetal tissue day (F)
(LOAEL)
1,3,5-TCB:
300 mg/kg per
day (F)
(NOEL);
150 mg/kg per
day (O)
(LOEL);
600 mg/kg per
day (F)
(LOAEL)
1,2,4-TCB Sprague-Dawley CD 0, 36, 120, 360, or maternal toxicity - all animals died at 120 mg/kg per
(>99%) rat (pregnant); 1200 mg/kg per day on days 1200 mg/kg, some lethality and reduced body weight day (F)
Kitchin >6/group 9-13 of gestation; gavage in gain at 360 mg/kg, moderate hepato- cellular (NOEL);
& Ebron corn oil hypertrophy at 360 mg/kg, and moderate to severe 360 mg/kg per
(1983a) multifocal hepatic necrosis at 1200 mg/kg; day (F);
embryonic growth retardation at 360 mg/kg *360 mg/kg per
day (O)
(LOAEL)
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3,4-TeCB Sprague-Dawley 0, 50, 100, or 200 mg/kg per maternal toxicity - death of 9/10 animals at 1,2,3,4-TeCB:
1,2,3,5-TeCB rat (pregnant); day on days 6-15 of 200 mg/kg and increased serum cholesterol at >50 200 mg/kg per
1,2,4,5-TeCB 10/group gestation; gavage in corn oil mg/kg (1,2,4,5-TeCB); greater toxicity of day (F)
(99.5%) 1,2,4,5-TeCB correlated with its accumulation in (NOEL);
Kacew tissue; decrease in number of live fetuses at 200 mg/kg per
et al. 200 mg/kg (1,2,3,4-TeCB, 1,2,3,5-TeCB); decrease in day (O)
(1984) number of live fetuses at 50 mg/kg (1,2,4,5-TeCB), (LOAEL)
probably not treatment-related
1,2,3,5-TeCB:
200 mg/kg per
day (F)
(NOEL);
200 mg/kg per
day (O)
(LOAEL)
1,2,4,5-TeCB:
*50 mg/kg per
day (O)
(LOEL);
50 mg/kg per
day (F)
(LOAEL)
1,2,4,5-TeCB Sprague-Dawley CD 0, 30, 100, 300, or maternal toxicity - decrease in body weight gain 300 mg/kg per
(>98%) rat (pregnant) 1000 mg/kg per day on days and slight centrilobular hypertrophy (3/9) at day (F);
Kitchin & 9-13 of gestation; gavage 1000 mg/kg; no embryo- or fetotoxic effects *1000 mg/kg
Ebron in 1.5% gum tragacanth or evidence of teratogenicity per day (O)
(1983c) (NOEL);
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1000 mg/kg per
day (F)
(LOAEL)
1,2,3,4-TeCB Sprague-Dawley CD 0, 100, 300, or 1000 mg/kg maternal toxicity - death of 7/19 animals, decrease 100 mg/kg per
(>98%) rat (pregnant) per day on days 9-13 of in body weight gain and liver weight, and minimal day (F)
Kitchin & gestation; gavage in 1.5% gum to moderate hepatocellular hypertrophy (2/9) at (NOEL);
Ebron tragacanth 1000 mg/kg, death of 1/10 and minimal 300 mg/kg per
(1983b) hepatocellular hypertrophy (2/9) at 300 mg/kg; day (F);
decrease in yolk sac diameter, embryonic crown-rump *300 mg/kg per
length and head length, at 300 mg/kg (not examined day (O)
at 1000 mg/kg because of maternal lethality) (LOAEL)
PeCB Wistar rat 0, 50, 100, or 200 mg/kg per maternal toxicity - non-significant reduction in 200 mg/kg per
Villeneuve (pregnant) 20/group day on days 6-15 of body weight gain at 200 mg/kg; increased incidence day (F)
& Khera gestation; gavage in corn oil of extra ribs at >50 mg/kg, and sternal defects and (NOAEL);
(1975) decreased fetal weight at 200 mg/kg 50 mg/kg per
day (O) (LOEL)
PeCB CD-1 mouse 0, 50, or 100 mg/kg per day on maternal toxicity - increase in liver weight (both *100 mg/kg per
(>97%) (pregnant); 9-10 days 6-15 of gestation; oral - doses); no embryotoxic, fetotoxic, or teratogenic day (O)
Courtney litters/group (6 gastric intubation in corn oil effects (NOEL);
et al. litters - control 50 mg/kg per
(1977) group) day (F)
(LOAEL)
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Reproductive Studies:
Inhalation
MCB Sprague-Dawley CD 0, 50, 150 or 450 ppm for 10 hepatocellular hypertrophy and renal changes in F0
Nair rat; 30 of each weeks prior to mating through and F1 males (>150 ppm); incidence of bilateral
et al. sex/group weaning of the F2 generation degeneration of the testicular germinal epithelium
(1987) increased in F0 adults at 450 ppm but relation to
MCB unclear since not observed in F1.
Reproductive Studies:
Ingestion
1,2,4-TCB Charles River rat 0, 25, 100, or 400 mg/kg no effects on fertility, growth, viability, approx.
Robinson (pregnant); 17-23 (during pregnancy through to locomotor activity or blood chemical analyses; 50 mg/kg per
et al. litters/group weaning of F2 generation). For decrease in water intake of some F0 groups at day (F)
(1981) F0, doses ranged from 8.3-134 400 mg/kg; enlarged adrenals in F0 and F1 at 95 (NOEL);
mg/kg at day 29 and 2.5-54 days at 400 mg/kg approx.
mg/kg at day 83 (mating at day 50 mg/kg per
90); mixed with Tween 20 in day (O)
drinking-water (LOAEL)
PeCB Sherman rat; 10 females: 7-150 mg/kg per day maternal toxicity - increased liver weight and approx.
(99.1%) of each sex/group (0, 125, 250, 500, or 1000 hypertrophy of hepatic cells at >500 mg/kg, 17-31 mg/kg
Linder ppm), 180 days (from 4-5 decreased haemoglobin levels at 1000 mg/kg and per day (F)
et al. weeks of age to mating, dose-dependent accumulation in adipose tissue; (NOEL);
(1980) through gestation and pre-weaning growth rates decreased at higher approx.
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
lactation) males: 6-140 concentrations, tremors in suckling pups at 17-31 mg/kg
mg/kg per day (0, 125, or >250 mg/kg and significant mortality in pups at per day (O);
1000 ppm), 100 days (through 1000 mg/kg approx.
mating); diet 27-64 mg/kg
per day (F)
(LOAEL)
a and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified otherwise.
d RBC - red blood cells.
F - females.
O - offspring.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOEL - lowest-observed-effect level.
* - maternally toxic dose.
However, the decrease in the number of live fetuses was not observed
at a higher concentration of 1,2,4,5-TeCB (100 mg/kg body weight)
and, therefore, may not have been treatment-related. The only toxic
effect observed in the mothers or fetuses, when pregnant rats
ingested either the 1,2,3,4- or the 1,2,3,5-isomer, was a decrease
in the number of live fetuses at the highest dose (200 mg/kg body
weight).
In contrast, Kitchin & Ebron (1983b) reported that the
1,2,3,4-isomer of TeCB was more toxic for both mothers and fetuses
than the 1,2,4,5-isomer (Kitchin & Ebron, 1983c). The LOAEL in
mothers administered the 1,2,3,4-isomer by gavage, was 300 mg/kg
body weight; the LOAEL for the 1,2,4,5-isomer was 1000 mg/kg
(compared with 50 mg/kg in the study of Kacew et al., 1984).
Embryonic growth retardation was observed in offspring administered
the maternally toxic dose (300 mg/kg body weight) of the
1,2,3,4-isomer, only.
For pentachlorobenzene, Villeneuve & Khera (1975) reported an
increased incidence of extra ribs in the fetuses of pregnant rats
administered oral doses as low as 50 mg/kg body weight, and an
increased incidence of sternal defects and decreased fetal weight
following ingestion of 200 mg/kg; these doses did not induce
significant toxic effects in the mothers. The results contrast with
those of Courtney et al. (1977) in which increases in liver weights
were observed in pregnant mice administered 50 or 100 mg PeCB/kg,
but no embryotoxic, fetotoxic, or teratogenic effects.
The possible reproductive effects of the chlorobenzenes have not
been well studied; only 3 relevant studies have been conducted.
There were no treatment-related adverse reproductive effects in a 2-
generation study in which rats were exposed via inhalation to MCB at
227.5, 682.5, or 2047.5 mg/m3 (50, 150, or 450 ppm), though there
was evidence of hepatotoxicity and nephrotoxicity in male rats in
the F0 and F1 generations (Nair et al., 1987). Robinson et al.
(1981) did not find any significant effects on fertility, growth,
viability, locomotor activity, or the chemical composition of the
blood in rats administered 1,2,4-TCB, at levels of up to
approximately 54 mg/kg, during pregnancy and through to weaning of
the F2 generation. However, enlarged adrenal glands were observed
in the F0 and F1 generations at 95 days in the highest dose
group.
When rats were administered PeCB in the diet from 4 to 5 weeks of
age, and during gestation and lactation, Linder et al. (1980) noted
maternal toxicity (LOAEL approx. 27-64 mg/kg per day), tremors in
suckling pups (LOAEL approx. 17-31 mg/kg per day), and, at higher
doses, decreases in pre-weaning growth rates, and mortality of pups.
9. EFFECTS ON HUMANS
9.1 Case Reports
9.1.1 General population exposure
Data on the health effects of exposure of the general population to
chlorobenzenes are restricted to case reports mainly concerning
accidental exposure to, or misuse of, products containing the lower
chlorinated benzenes (MCB, 1,2- or 1,4-DCB, and an unspecified
isomer of TCB). Many of these reports are from the early literature
with no indication of the purity of the chlorobenzene or the actual
dose/time relationship.
Reversible acute effects on the central nervous system (loss of
reflexes, cyanosis leading to unconsciousness, with head and neck
twitching) were observed in a 2-year-old boy following the ingestion
of 5-10 ml of MCB (Reich, 1934). An adult female showed nausea,
shortness of breath, sleepiness, and haematuria, 1 week after an
acute exposure to DCB used as a termiticide (Nill, 1936). Two cases
of acute myeloblastic leukaemia were reported in females who had
been repeatedly exposed (over 1 year) to DCBs (primarily 1,2-DCB)
during its use as a cleaning solution (Girard et al., 1969). There
have been numerous case reports of adverse effects of 1,4-DCB,
because of its availability to the public as a moth repellent and
air freshener. Reported effects of acute or short-term exposure (all
of which were reversible) include acute haemolytic anaemia
(Hallowell, 1959; Campbell & Davidson, 1970), respiratory irritation
(rhinitis) (Cotter, 1953), and allergic purpura of the skin and
glomerulonephritis (Nalbandian & Pearce, 1965). Effects on the lungs
(pulmonary granulomatosis), (Weller & Crellin, 1953), blood
(anaemia), central nervous system effects including dizziness,
numbness, and incoordination, "mental sluggishness", tremor, and
polyneuritis (Frank & Cohen, 1961), and liver damage, including
yellow atrophy and jaundice (Berliner, 1939; Cotter, 1953) have been
recorded in case reports of persons exposed to 1,4-DCB for prolonged
periods (several months to 15 years) . Limb and truncal ataxia,
dysarthria, hyporeflexia, hypotonus, and mild proximal limb weakness
were reported in a 25-year-old woman exposed to 1,4-DCB during a
period of 6 years, under very unusual circumstances, i.e., through
extensive use of mothballs in her bedclothes and wardrobe. She
developed difficulty in speech and gait. Exposure was not
quantified, but was probably high, according to the observed effects
and reported use (Miyai et al., 1988).
There has also been a case report of aplastic anaemia in a
68-year-old woman with long-term exposure through the soaking of her
husband's work clothes in TCB (isomer not identified) (Girard
et al., 1969).
9.1.2 Occupational exposure
Most of the available data on the adverse effects of occupational
exposure to chlorobenzenes come from case reports. Many of these
cases are poorly described with regard to chemical purity, dose/time
relationships, and possible confounding factors, and thus provide
very little information relevant to the assessment of human health
risks.
Case reports concerning MCB in occupationally exposed populations
have been restricted to symptoms of effects on the central nervous
system (headaches, numbness, and lethargy) and irritation of the
eyes and upper respiratory tract (Girard et al., 1969) after
exposures for periods of up to 6 years, in the presence of several
other chemicals.
In workers exposed to 1,2-DCB, or mixtures of chlorobenzenes
containing mainly 1,2-DCB, there have been case reports of:
haematological disorders, including anaemia in 1 male worker filling
barrels for 8 years (Girard et al., 1969; US EPA, 1985); chronic
lymphoid leukaemia (2 cases) after inhalation exposures of 10 and 16
years, respectively (Girard et al., 1969); and myelocytic/
myeloblastic leukaemia (1 case) after 22 years in a dyestuff plant
(Tolot et al., 1969).
In workers exposed to 1,4-DCB, probably often in combination with
several other chemicals, there have been case reports of
haemato-logical disorders, including anaemia (Petit & Champeix,
1948; Cotter, 1953; Harden & Baetjer, 1978); splenomegaly (2 cases)
(Sumers et al., 1952; Cotter, 1953); effects on the gastrointestinal
tract (Sumers et al., 1952; Cotter, 1953; Wallgren, 1953); drying or
hardening of the skin (Cotter, 1953; Harden & Baetjer, 1978); and
symptoms of effects on the CNS, including finger tremors, stronger
muscle reflexes, and ankle contractions (Wallgren, 1953).
Only two case reports have been identified concerning
trichloro-benzene. Ehrlicher (1968) reported massive haemoptysis in
an adult male who had inhaled TCB vapours for several hours, during
the repair of a pump, and Popovki et al. (1980) reported 7 cases of
chloracne in 15 TCB production workers, exposed for 2-6 months.
However, the isomer was not specified in either report, and no
details of confounding factors and doses received were available for
evaluation.
9.2 Epidemiological Studies
Only a few epidemiological studies have been carried out on workers
exposed to chlorobenzenes other than hexachlorobenzene. Many of
these studies have been carried out on groups of workers
simultaneously exposed to other chemicals with known adverse effects
on man (e.g., benzene, aromatic and/or chlorinated solvents).
Furthermore, most of the studies have not been fully described with
regard to methods and confounding factors, making them inadequate
for use in risk assessments.
In an early cross-sectional study, 3 groups of workers were examined
for the general adverse effects of solvent vapours during the
production of perchlorovinyl lacquer. Two health examinations were
carried out in the course of 1 year. Group 1 included 28 workers (25
of whom had been employed on the job for 1 year or more), reported
to have been exposed only to chlorobenzene at levels ranging between
0.034 and 1.44 mg/litre (Rozenbaum et al., 1947). Group 2 consisted
of 12 workers in the central chemical laboratory working on
long-term experiments with chlorobenzene. Group 3 included 14 female
workers in the lacquer plant, who were exposed to benzene (0.02-0.4
mg/litre) as well as to chlorobenzene (0.06-0.6 mg/litre). There was
no unexposed control group. No significant adverse effects were
reported for workers in Groups 2 and 3; however, workers in Group 1
reported headaches, dizziness, drowsiness, and dyspeptic disorders.
Numerical data concerning the prevalence of these disorders were not
given. No effects on "internal organs" that could be attributed to
chlorobenzene exposure were noted; however, the first
neuropathological examination indicated numbness and stiffness in
the extremities of 8 workers, convulsive muscle contractions in the
fingers of 9, and hypoaesthesia of the hand in 4. The deviations
noted were reported to have returned to normal by the second
examination.
Workers producing enamel-insulated wire are exposed to both MCB and
tricresol. An examination of 311 female workers for the possible
effects of these chemicals on gynaecological and obstetrical
functions was reported by Syrovadko & Malysheva (1977). Levels of
exposure to MCB varied from 11 to 429 mg/m3 (mean, 72.3 mg/m3)
and those to tricresol from 0.8 to 18.7 mg/m3 (mean, 4.3 mg/m3).
Similar health parameters were examined in some 15 000 women in
other occupations and the general population in the region. The
frequency of various gynaecological dysfunctions was 2.6-9.4 times
higher in the exposed workers than in women outside the plant, but
was similar to that in the controls from within the enamel-wire
plant. Lost-time was 2.5 times greater in the enamellers than in
in-plant controls. The authors reported marked hormonal
disturbances, but did not quantify these results. An analysis of 190
births in the enameller group, and 152 from the in-plant controls,
revealed an increase in birth anomalies (10 versus 3.9%) in the
exposed group. Congenital heart defects (26.3%) were the most
prevalent. Neonatal development appeared to be compromised in
infants of exposed mothers, as evidenced by patterns of
breast-feeding and body weight gain. These results are difficult to
interpret, however, with respect to MCB, owing to concomitant
exposure to another chemical and a lack of quantitative data.
MCB was considered to be the causative agent in 26 out of 1951 cases
of work-related dermatosis observed at the "Shanghai Institute
for Occupational Disease Prevention in the Chemical Industry"
between 1970 and 1982; reported manifestations were eczematous
dermatitis, pigmentation, and neurodermatitis (Zong & Ma, 1985).
In periodic medical examinations of 58 workers exposed to various
concentrations of 1,4-DCB, there was no evidence of "organic injury
or untoward haematological effects". It was concluded, however,
that, at levels ranging between 300 and 480 mg/m3 (50 and 80 ppm),
1,4-DCB was irritating to the eyes and nose, the irritation becoming
severe at a level of 960 mg/m3 (160 ppm). Details of the study
design in this early investigation were incomplete (Hollingsworth et
al., 1956).
In the only identified cross-sectional epidemiological study of
workers exposed to 1,2-DCB, there was no evidence of "organic injury
or untoward haematological effects" in an unspecified number of
workers exposed to mean levels of 90 mg/m3 (15 ppm) 1,2-DCB
(Hollingsworth et al., 1958). However, details of the study design
were not presented in the account of this early investigation.
As described in section 8, there was an increase in the total number
of chromosomal aberrations (primarily single and double breaks) in
the peripheral leukocytes of 26 laboratory workers exposed for 4
days, 8 h/day, to 1,2-DCB vapour compared with a control group of 11
unexposed laboratory personnel (Zapata-Gayon et al., 1982). Of the
white cells analysed (1345, exposed; 942, control), 8.92% of the
exposed cells contained aberrations compared with 2.02% in the
control group. In 15 exposed subjects examined at 6 months, only
double breaks were significantly increased. Although mention was
made of a strong odour of DCB in the room and all subjects suffered
mucosal irritation, no determination of exposure levels was carried
out.
Only one study on workers exposed to TeCB was found. The peripheral
lymphocytes of workers producing 1,2,4,5-TeCB in a
pesticide-manufacturing complex were examined for chromosomal
aberrations. Exposure levels were not reported; however, workers (24
male and 1 female) were considered to be exposed to only TeCB and
they were compared with 14 other workers, minimally exposed to
chemicals, as well as a group from the local community. An increase
(significance unspecified) in the total number of chromatid-type,
labile (acentric fragment and dicentric chromosome), and stable
(deletion, inversion, and translocation) chromosomal aberrations was
reported. No follow-up studies of these findings have been reported.
No case reports or epidemiological studies on workers exposed to
PeCB were found.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure of the general population
Exposure to chlorobenzenes other than hexachlorobenzene may occur
via ingestion, inhalation, or dermal contact (section 5.2.1). The
general population is thought to be exposed to the lower chlorinated
congeners mainly through inhalation, whereas a greater proportion of
the total daily intake of the higher chlorinated compounds is
ingested in food. Dermal exposure may occur if consumers use
chlorobenzene-based products without appropriate care and
protection.
Exposure of the general population through the ingestion of
drinking-water and food and via inhalation has been estimated (Table
12). However, these estimates are based on few data from a limited
number of countries and, therefore, must be considered tentative
until further monitoring data become available.
On the basis of the limited data available, the total daily intakes
of MCB and the various DCB isomers are estimated to be less than 1
µg/kg body weight. The intakes of the higher chlorinated benzenes
are lower, probably, less than 0.05 µg/kg body weight per day.
Dichlorobenzenes, TCBs, TeCBs, and PeCB have been detected in human
milk, the DCBs (particularly the 1,4-isomer) being present in the
highest concentrations. On the basis of the limited data available
from a small number of countries, it is estimated that, on a body
weight basis, the intake of chlorobenzenes in breast-fed infants is
greater than that in the adult population,.
10.1.2 Occupational exposure
Very few data are available to serve as a basis for the estimation
of occupational exposure. Air levels of MCB of up to 18.7 mg/m3
have been detected in chemical plants, whereas levels of 1,4-DCB in
one manufacturing plant averaged 204 mg/m3 (42-288 mg/m3). On
the basis of established exposure limits, workers may be exposed
daily to maximum concentrations of 350 mg/m3 for MCB, 450 mg/m3
for 1,4-DCB, and 300 mg/m3 for 1,2-DCB.
10.1.3 Toxic effects
Acute lethal doses of the lower chlorinated benzenes (MCB and DCBs)
in experimental animals cause narcosis and CNS depression, damage to
the liver and kidneys, as well as respiratory paralysis. Inhalation
LC50s for MCB have been reported to range from approximately
9000 mg/m3 in mice to 13 000 mg/m3 in rats. Inhalation LC50
values for 1,2-DCB are similar. With few exceptions, oral LD50
values in the species examined to date have been >1000 mg/kg body
weight for all congeners, and, according to the limited data
available, dermal LD50s are higher.
In general, effects following short-term and long-term exposures to
chlorobenzenes are similar to those following acute exposure and
include damage to the liver and kidneys, and, at higher doses,
effects on the thyroid gland in rats and on the bone marrow in mice.
Of the few chlorobenzenes tested, only 1,2,4-TCB has been shown to
cause dermatitis after prolonged contact with skin.
In a bioassay for the carcinogenicity of MCB, there was an increased
incidence of hepatic neoplastic nodules in the high-dose group
(120 mg/kg body weight) of male F344 rats, but no treatment-related
increases in tumour incidence in female F344 rats or male and female
B6C3F1 mice. There was no evidence for the carcino-genicity of
1,2-DCB in male or female F344 rats or B6C3F1 mice (60 or
120 mg/kg body weight).
In a bioassay for the carcinogenicity of 1,4-DCB, there was a
dose-related increase in renal tubular cell adenocarcinomas in male
F344 rats and an increase in hepatocellular adenomas and carcinomas
in both sexes of B6C3F1 mice. No evidence of carcinogenicity was
reported in male and female Wistar rats or female Swiss mice
following inhalation of slightly higher doses of 1,4-DCB (estimated
to be 400 mg/kg per day for rats and 790 mg/kg per day for mice) for
shorter periods of time. Available data indicate, however, that
renal tumours in male F344 rats and the associated severe
nephropathy and hyaline droplet formation, induced by 1,4-DCB, are
species- and sex-specific responses associated with the
re-absorption of alpha-2-microglobulin.
Available data are inadequate to assess the carcinogenicity of the
higher chlorinated benzenes (tri- to penta-).
Although available data from in vitro and in vivo assays of
isomers other than 1,4-DCB are limited, chlorobenzenes do not appear
to be mutagenic. On the basis of a more extensive database for
1,4-DCB, it can be concluded that this compound has no mutagenic
potential, either in vivo or in vitro.
In studies conducted to date, there has been no evidence that
chlorobenzenes are teratogenic in animal species. Exposure of rats
or rabbits to MCB or DCBs, via inhalation at levels exceeding
2400 mg/m3, resulted in minor embryotoxic and fetotoxic effects.
However, such doses were clearly toxic for the mother. Although
there is some evidence that TCBs, TeCBs, and PeCB are embryo-toxic
and fetotoxic at doses that are not toxic for the mother, available
data are inconsistent.
The lowest reported NOELs or LOAELs for each of the chlorobenzene
isomers in long-term exposure studies and studies on chronic
toxicity, teratogenicity, and developmental and reproductive
toxicity, of acceptable design, are summarized in Table 25.
Toxic effects in humans following acute exposures to MCB, 1,2-DCB,
and 1,4-DCB include CNS depression, haematuria, haemolytic anaemia,
rhinitis, tremor, ataxia, polyneuritis, and jaundice. No reports
have been published on the possible health effects that might result
from long-term exposures to low levels of chlorobenzenes in the
general environment. Effects attributed to occupational exposures to
chlorobenzenes have been confounded by exposures to several
chemicals and the limitations of the epidemiological studies
conducted to date; however, they include CNS depression, dermatitis,
and eye and nose irritation. No dose-response data are available
from these epidemiological studies.
10.1.4 Risk evaluation
The following is an example of a risk assessment procedure for
setting Tolerable Daily Intakes (TDIs) for chlorobenzenes other than
hexachlorobenzene. This is only an illustration and may need to be
adjusted to take into account local considerations and any
additional scientific data that are reported.
10.1.4.1 General population
The general population appears to be exposed to low levels of
chlorinated benzenes (see Table 12, section 5). Exposure to the
major chlorobenzenes other than hexachlorobenzene, for non-
occupationally-exposed populations, is estimated to be less than 2
µg/kg body weight per day. Over 90% of the total exposure is to MCB
and DCBs, principally from air.
For comparison with these estimated exposures, TDIs can be
calculated from the no-observed-effect levels (NOELs) from
long-term, chronic teratogenicity, and developmental reproductive
toxicity studies on experimental animals. TDIs, based on the lowest
NOELs reported in studies of acceptable design, using uncertainty
factors established on the basis of the principles outlined by WHO
(1987), are listed in Table 26.
Table 25. Summary of lowest reported NOELs (or LOAELs) for the
inhalation and ingestion of chlorobenzenes other than
hexachlorobenzene
Inhalation
Compound Reported NOEL Species Exposure
(mg/m3) period
MCB 341 (LOAEL) rat, rabbit 24 weeks
1,4-DCB 450 rat; mousea 76 weeks
1,3,5-TCB 100 rat 13 weeks
1,2,4-TCB 22.3 rat 13 weeks
Ingestion (dietary incorporation or gavage)
Compound Reported NOEL Species Exposure
(mg/kg body weight period
per day)
MCB 60 rat 103 weeks
1,2-DCB 60 mouse 103 weeks
1,4-DCB 150 (LOAEL) rat 103 weeks
1,2,4-TCB 7.8 rat 13 weeks
1,2,3-TCB 7.7 rat 13 weeks
1,3,5-TCB 7.6 rat 13 weeks
1,2,4,5-TeCB 0.034 rat 13 weeks
1,2,3,4-TeCB 3.4 rat 13 weeks
1,2,3,5-TeCB 0.42 rat 13 weeks
PeCB 3.3 rat 13 weeks
a = In this bioassay, only female mice were included.
For most chlorobenzenes, the TDIs are based on non-neoplastic
effects. Neoplastic effects were taken into consideration in the
derivation of the uncertainty factors for MCB and 1,4-DCB. However,
available data indicate that the observed increase in renal tumours
in rats caused by 1,4-DCB is a species- and sex-specific response
that is unlikely to be relevant to humans. It should be noted,
however, that on the basis of evidence of increased DNA replication
in the mouse liver, and the increased incidence of hepatocellular
adenomas and carcinomas in mice in a bioassay for the
carcinogenicity of 1,4-DCB, this compound may act as a non-genotoxic
carcinogen in the rodent liver. On the basis of the increased
incidence of hepatic neoplastic nodules observed in the high-dose
group of male rats in a bioassay for the carcinogenicity of MCB,
this may also be true for this compound.
As stated above, with the exception of individuals who use
chlorobenzene-based products without appropriate care and
protection, non-occupationally exposed humans are exposed to levels
of chlorobenzenes well below the derived TDIs, indicating that the
anticipated health hazards for the general population from exposure
to chlorobenzenes other than hexachlorobenzene are minimal. However,
the odour thresholds of chlorobenzenes range between 0.1 and 3
µg/litre in drinking-water, thus, necessitating continuous pollution
control measures to ensure aesthetically acceptable water supplies.
Table 26. Calculated Tolerable Daily Intakes for chlorobenzenes
other than hexachlorobenzene
Inhalation
Compound Reported NOEL Uncertainty Estimated
(mg/m3) factor TDIb
MCB 341 (LOAEL) 1000 0.5
1,4-DCB 450 500 1
1,3,5-TCB 100 500 0.2
1,2,4-TCB 22.3 500 0.05
Ingestion
Compound Reported NOEL Uncertainty Estimated
(mg/kg body weight factor TDIa
per day
MCB 60 500 0.1
1,2-DCB 60 100 0.60
1,4-DCB 150 (LOAEL) 1000 0.1
1,2,4-TCB 7.8 500 0.02
1,2,3-TCB 7.7 500 0.02
1,3,5-TCB 7.6 500 0.02
1,2,4,5-TeCB 0.034 500 0.0001
1,2,3,4-TeCB 3.4 500 0.01
1,2,3,5-TeCB 0.42 500 0.001
PeCB 3.3 500 0.01
a = Values were rounded up according to the judgement of the
panel.
10.1.4.2 Occupationally exposed population
Workers can be exposed to levels as high as 2.7 mg/kg body weight
for MCB and 29.1 mg/kg body weight for 1,4-DCB. These estimates may
not be representative, however, since they are based on only two
reports.
If good industrial hygiene practices are followed, the risks
associated with occupational exposure to chlorobenzenes are
considered to be minimal.
10.2 Evaluation of Effects on the Environment
10.2.1 Levels of exposure
Chlorobenzene isomers have been found in air, surface water, ground
water, drinking-water, municipal waste water, rain water, soils, and
sediments. Most monitoring has been limited to levels in air and the
aquatic environment.
In surface waters, levels of total chlorobenzenes are in the
ng-µg/litre range; however, values as high as 0.1 mg/litre have been
reported near industrial sources. Examples of levels in waste water
average about 700 µg/litre for MCB and less than 170 µg/litre for
the other congeners.
10.2.2 Fate
Chlorobenzenes released into the aquatic environment will become
distributed preferentially to the air and to sediments (particularly
organically rich sediments). Levels 1000 times those found in water
have been detected in sediments, particularly those in highly
industrialized regions, though available data are limited. The
retention of chlorobenzenes in soil increases with the organic
content of the soil; the degree of chlorination is positively
correlated with adsorption.
10.2.3 Bioavailability and bioaccumulation
The bioavailability of chlorobenzenes is inversely correlated with
the organic matter content of soil and sediment. Accumulation
studies are limited, but organisms have been shown to accumulate
chlorobenzenes from water, soil, and aquatic sediment. Adsorption on
organic sediment increases with increasing level of chlorination,
which may reduce bioavailability; however, uptake into, and
retention by, organisms also increases with increasing level of
chlorination.
10.2.4 Degradation
Chlorobenzenes are eliminated from the environment by both abiotic
and biotic degradation, the latter appearing the more important.
Photolysis and hydrolytic reactions are possible processes, but
aerobic degradation constitutes the major route of breakdown for the
removal of chlorobenzene residues. Many microorganisms are capable
of degrading chlorobenzenes (section 4.2.3, Table 6); however, the
more highly chlorinated congeners are degraded microbiologically at
a slower rate than the less chlorinated benzenes. This degradation
does not appear to occur anaerobically.
10.2.5 Persistence
In water, chlorobenzenes are considered moderately persistent with
half-times of approximately 1 day in rivers, 10 days in lakes, and
more than 100 days in ground water. They are much more persistent
under the anaerobic conditions usually found in sediment and ground
water.
10.2.6 Toxic effects on organisms
The toxicities of chlorobenzenes for microorganisms, invertebrates,
and fish are comparable; with a few exceptions, the EC50 and
LC50 values fall within the several mg/litre range (Tables 16, 17,
and 18). In general, toxicity increases with increased chlorination
of the benzene ring. For example, in the bluegill sunfish
(L. macrochirus), the following 96-h LC50 values were reported:
MCB (>16 mg/litre); 1,2,4-TCB (3.4 mg/litre); 1,2,4,5-TeCB (1.6
mg/litre) and PeCB (0.3 mg/litre).
No data on the effects of chlorobenzenes on terrestrial biota were
found; however, data from studies on laboratory mammals suggest a
low risk for terrestrial mammals (see section 10.1.3).
10.2.7 Risk evaluation
There are insufficient data on (a) the quantities of
chlorobenzenes entering the environment and their subsequent
dynamics, and (b) toxicity studies on many chlorobenzene isomers,
carried out under realistic environmental conditions. It is,
therefore, impossible to predict quantitatively the impact on the
environment of widespread low-level contamination by chlorobenzenes
other than hexachlorobenzene.
In virtually all instances within the aquatic ecosystem, the levels
at which acute effects occur in experimental studies are many times
higher than the environmental levels monitored at present (mg/litre
range versus µg/litre range). Organisms will be exposed only
transiently to chlorobenzenes in surface water (because of
volatility and adsorption on sediment) and will be mainly exposed to
chlorobenzenes in the interstitial water of sediments. Few studies
on organisms living in sediments have been conducted, despite
evidence for bioavailability. Similarly, there are no data on the
transfer of chlorobenzenes in the food chain.
Only in the case of accidental spills or uncontrolled industrial
discharges would ambient concentrations approach toxic levels.
However, the continued discharge of chlorobenzenes into the aquatic
environment should be avoided, to prevent the build up of persistent
residues in sediment and/or ground water.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
If good industrial practices are followed, the risks associated with
occupational exposure to chlorobenzenes are considered to be
minimal. The present risk assessment also indicates that current
concentrations of chlorobenzenes in the environment pose a minimal
risk for the general population, except in the case of the misuse of
chlorobenzene-based products or uncontrolled discharge to the
environment. However, this assessment is based on limited monitoring
data; additional information is needed to substantiate this
conclusion. The reduction of the widespread use and disposal of
chlorobenzenes should, however, be considered because:
* Chlorobenzenes may act as precursors for the formation of
PCDDs/PCDFs, e.g., in incineration processes;
* These chemicals can lead to taste and odour problems in
drinking-water and fish;
* Residues persist in organically-rich anaerobic sediments and
soils, and ground water.
For most chlorobenzenes, the assessment of risk was based on
non-neoplastic effects. However, neoplastic effects were taken into
consideration in the risk assessments for MCB and 1,4-DCB. Available
data indicate that the observed increase in renal tumours in rats
caused by 1,4-DCB is a species- and sex-specific response that is
unlikely to be relevant to humans. On the basis of evidence of
increased DNA replication in the mouse liver and the increased
incidence of hepatocellular adenomas and carcinomas in mice, 1,4-DCB
may act as a non-genotoxic carcinogen in the rodent liver. MCB may
also be a non-genotoxic carcinogen, in view of the increased
incidence of hepatic neoplastic nodules observed in the high-dose
group of male rats in a bioassay for carcinogenicity.
11.2. Recommendations
11.2.1 Public health measures
The present risk assessment indicates that current concentrations of
chlorobenzenes in the environment pose a minimal risk for humans.
However, this assessment is based on limited monitoring data and
additional information is needed to substantiate this conclusion.
The reduction of the widespread use and disposal of chlorobenzenes
should, however, be considered because:
* Chlorobenzenes may act as precursors for the formation of
PCDDs/PCDFs, e.g., in incineration processes;
* These chemicals can lead to taste and odour problems in
drinking-water and fish;
* Residues persist in organically-rich anaerobic sediments and
soils, and ground water.
More information is needed on the fate of chlorinated benzenes at
high temperatures, in order to better assess the hazards associated
with their disposal by incineration.
11.2.2 Human health risk evaluation
In order to improve the database available for the assessment of the
risks of chlorobenzenes for human health, it is recommended that:
1. The IPCS should coordinate the collection of industrial
production data for chlorobenzenes.
2. Additional monitoring data should be collected so that a more
accurate assessment of human exposure can be made. In
particular, additional information is desirable on the
concentrations in different geographical areas, especially with
regard to the levels in food of the highly chlorinated
congeners.
3. There is a lack of chronic toxicity data on the higher
chlorinated benzenes. On the basis of subchronic toxicity data,
it appears that 1,2,4,5-TeCB is the most toxic congener and
should be examined in long-term bioassays or studied with
regard to possible species-specific mechanisms.
4. Since ambient air is believed to be the major source of
exposure to the chlorinated benzenes for humans, more
information is required on the photoreactivity and
photodegradation of these compounds.
There is a lack of experimental data on the interaction between
chlorobenzenes and other chemicals. As a general recommendation,
research on the toxicity of chemical mixtures, and the consideration
of toxicological interaction in the risk assessment process is
encouraged.
11.2.3 Environmental risk evaluation
Persistent residues of chlorobenzenes have been reported in organic
sediments and limited information indicates that these residues are
bioavailable. The following recommendations are made to improve the
understanding of the environmental distribution, fate, and impact of
chlorobenzenes:
1. Residues of chlorobenzenes in sediment should be included in
monitoring programmes.
2. The extent to which the presence of organic matter governs the
distribution of chlorobenzenes between water, sediment, and air
should be further studied.
3. Further research into the role of, and uptake from sediment
(aquatic sediment and soil) by, biota is needed, together with
studies on possible transfer in the food chains.
4. The capacity of a wider range of organisms to metabolize
chlorobenzenes should be assessed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL
BODIES
The carcinogenicity of 1,2-DCB and 1,4-DCB has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987). Data
on the carcinogenicity of both compounds for humans were considered
inadequate. There was inadequate evidence for carcinogenicity to
animals for 1,2-DCB and sufficient evidence for 1,4-DCB.
In the Guidelines for drinking-water quality (WHO, 1984), the
following guideline values were developed for chlorobenzenes: MCB, 3
µg/litre; 1,2-DCB, 0.3 µg/litre; and 1,4-DCB, 0.1 µg/litre. All
values represent 10% of the taste and odour threshold value.
Regulatory standards established by national bodies in different
countries and the EEC are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1986).
REFERENCES
ABERNATHY, S., BOBRA, R.M., SHIU, W.Y., WELLS, P.G., & MACKAY, D.
(1986) Acute lethal toxicity of hydrocarbons and chlorinated
hydrocarbons to two planktonic crustaceans: The key role of
organism-water partitioning. Aquat. Toxicol., 8: 163-174.
AHBORG, U.G. & VICTORIN, K. (1987) Impact on health of chlorinated
dioxins and other trace organic emissions. Waste Manag. Res., 5:
203-224.
AHLING, B. (1987) Formation of chlorinated hydrocarbons during
combustion of poly(vinyl chloride). Chemosphere, 10: 799-806.
AMOORE, J.E. & HAVTALA, E. (1983) Odor as an aid to chemical safety:
Odor thresholds compared with threshold limit values and
volatilities for 214 industrial chemicals in air and water dilution.
J. appl. Toxicol., 3(6):272-290.
AKERMARK, B., BAECKSTROM, P., WESTLIN, U.E., GOTHE, R., &
WACHTMEISTER, C.A. (1976) Photochemical dechlorination of
1,2,4-trichlorobenzene. Acta chem. Scand., B30: 49-52.
ANTOINE, S.R., DELEON, I., & O'DELL-SMITH, R.M. (1986)
Environmentally significant volatile organic pollutants in human
blood. Bull. environ. Contam. Toxicol., 36: 364-371.
ARIYOSHI, T.M., IDEGUCHI, K., ISHIZUKA, Y., IWASAKI, K., & ARAKAKI,
M. (1975) Relationship between chemical structure and activity. I.
Effects of the number of chlorine atoms in chlorinated benzenes on
the components of drug-metabolizing system and the hepatic
constituents. Chem. Pharm. Bull., 23 (4): 817-823.
ARIYOSHI, T., YASUMATSU, F., SUETSUGU, M., KOKUBA, Y., HAMASAKI, K.,
& ARIZONO, K. (1981) Effects of halogenated compounds on the
cytochrome P-450 content and the activities of enzymes in
biosynthesis and degradation of heme. J. Pharmacobiodyn., 4(2):
4-5.
AZOUZ, W.M., PARKE, D.V., & WILLIAMS, R.T. (1955) Studies in
detoxication: The metabolism of halogenobenzenes. ortho- and
para-dichlorobenzenes. Biochem. J., 59(3): 410-415.
BALKON, J. & LEARY, J.A. (1979) An initial report on a comprehensive
quantitative, screening procedure for volatile compounds of forensic
and environmental interest in human biofluids by GC/MS. J. anal.
Toxicol., 3: 213-215.
BALLSCHMITER, K. & SCHOLZ, C. (1980) Microbial decomposition of
chlorinated aromatic substances. VI. Formation of dichlorophenols
and dichloropyrocatechol from dichlorobenzenes in a micromolar
solution by Pseudomonas species. Chemosphere, 9 (7-8): 457-467.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS, B.S.H., KIRKPATRICK, M.,
MICHEAL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment-a multimedia
environmental study. Biomed. mass Spectrom., 7(4): 139-147.
BAUER, U. (1981) [Human exposure to environmental
chemicals-investigations on volatile organic halogenated compounds
in water air, food, and human tissues. III. Communication: results
of investigations.] Zbl. Bakteriol. Hyg., I. Abt. Orig. B., 174:
200-237 (in German).
BAYER, A.G. (1986a) Para-dichlorobenzene in the CHO HGPRT forward
mutation assay. Report prepared by Litton Bionetics, USA, submitted
to WHO by Bayer, A.G., Institute of Toxicology (Unpublished).
BAYER, A.G. (1986b) Para-dichlorobenzene in the in vitro
transformation of Balb/3T3 cells assay. Report prepared by Litton
Bionetics, USA, submitted to WHO by Bayer, A.G., Institute of
Toxicology (Unpublished).
BECK, J. & HANSEN, K.E. (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in
soil. Pestic. Sci., 5: 41-48.
BERLINER, M.L. (1939) Cataract following the inhalation of
paradichlorobenzene vapor. Arch. Ophthalmol., 22: 1023-1034.
BIRGE, W.J., BLACK, J.A. & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Lexington, Kentucky, 69
pp (EPA Report No. 560/11-79-007; NTIS Report No. PB80-101637).
BLACK, W.D., VALLI, V.E.O., RUDDICK, J.A., & VILLENEUVE, D.C. (1988)
Assessment of teratogenic potential of 1,2,3-, 1,2,4- and
1,3,5-trichlorobenzenes in rats. Bull. environ. Contam. Toxicol.,
41: 719-726.
BLANCHARD, R.D. & HARDY, J.K. (1985) Use of a permeation sampler in
the collection of 23 volatile organic priority pollutants. Anal.
Chem., 57(12): 2349-2351.
BOMHARD, E., LUCKHAUS, G., VOIGT, W.-H., & LOESER, E. (1988)
Induction of light hydrocarbon nephropathy by p-dichlorobenzene.
Arch. Toxicol., 61: 433-439.
BONNET, P., RAOULT, G., & GRADISKI, D. (1979) Concentrations
lethales 50 des principaux hydrocarbons aromatiques. Arch. Mal.
prof., 40(8-9): 805-810.
BONNET, P., MORELE, Y., RAOULT, G., ZISSU, D., & GRADISKI, D. (1982)
Determination de la concentration lethale 50 des hydrocarbones
aromatiques chez le rat. Arch. Mal. prof., 63(4): 461-465.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformation of halogenated
organic compounds under denitrification conditions. Appl. environ.
Microbiol., 45(4): 13295-1299.
BOUWER, E.J. & MCCARTY, P.L. (1984) Modeling of trace organics
biotransformation in the subsurface. Ground Water, 22(4): 433-440.
BOYLES, D.T. (1980) Toxicity of hydrocarbons and their halogenated
derivatives in an aqueous environment. In: Afgan, B.K. & Mackay, D.,
ed. Hydrocarbons and halogenated hydrocarbons in the aquatic
environment, New York and London, Plenum Press, pp. 545-557.
BOY-MARCOTTE, J.L. (1980) 100-1000 kW(el) medium-power distributed-
collector solar system. Electr. Power Syst. Res., 3: 41-51.
BRAUN, W.H., SUNG, L.Y., KEYES, D.G., & KOCIBA, R.J. (1978)
Pharmacokinetic and toxicological evaluation of dogs fed
1,2,4,5-tetra-chlorobenzene in the diet for two years. J. environ.
Pathol. Toxicol., 2: 225-233.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14(3):
231-241.
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Wasser abwasser Fors., 15(1): 1-6 (in
German).
BRISTOL, D.W., CRIST, H.L., LEWIS, R.G., MACLEOD, K.E., & SOVOCOOL,
G.W. (1982) Chemical analysis of human blood for assessment of
environmental exposure to semivolatile organochlorine chemical
contaminants. J. anal. Toxicol., 6: 269-275.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DECEAURRIZ, J. (1983)
Short-inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BROWN, V.K.H., MUIR, C., & THORPE, E. (1969) The acute toxicity and
skin irritant properties of 1,2,4-trichlorobenzene. Ann. occup.
Hyg., 12: 209-212.
BUCCAFUSCO, R.J., ELLS, S.J. & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to bluegill (Lepomis macrochirus). Bull.
Environ. Contam. Toxicol., 26: 446-452.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) from the pyrolysis of chlorobenzenes. Chemosphere, 6:
415-424.
CALARMARI, D., GALASSI, S., SETTI, F. & VIGHI, M. (1983) Toxicity of
selected chlorobenzenes to aquatic organisms. Chemosphere, 12(2):
253-262.
CAMPBELL, D.M. & DAVIDSON, R.J.L. (1970) Toxic haemolytic anaemia in
pregnancy due to a pica for paradichlorobenzene. J. Obstet.
Gynaecol. Br. Commonw., 77: 657-659.
CANTON, J.H., SLOOFF, W., KOOL, H.J. & STRUYS, J. (1985) Toxicity,
biodegradability, and accumulation of a number of C/N-containing
compounds for classification and establishing water quality
criteria. Regulat. Toxicol. Pharmacol., 5: 123-131.
CARLSON, G.P. (1980) Effects of halogenated benzenes on arylesterase
activity in vivo and in vitro. Res. Comm. chem. Pathol.
Pharmacol., 30 (2): 361-364.
CARLSON, G.P. & TARDIFF, R.G. (1976) Effect of chlorinated benzenes
on the metabolism of foreign organic compounds. Toxicol. appl.
Pharmacol., 36: 383-394.
CARLSON, G.P., DZIEZAK, J.D., & JOHNSON, K.M. (l979) Effect of
halogenated benzenes on acetanilide esterase, acetanilide
hydroxilase and procaine esterase in rats. Res. Comm. chem. Pathol.
Pharmacol., 25(1): 181-184.
CARLSON, R.M., CARLSON, R.E., KOPPERMAN, H.L., & CAPLE, R. (1975)
Facile incorporation of chlorine into aromatic systems during
aqueous chlorination process. Environ. Sci. Technol., 9: 674-675.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J., & SWENBERG,
J.A. (1989) Involvement of reversible binding to a-2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol. appl.
Pharmacol., 99: 122-132.
CHOUDHRY, G.G. & WEBSTER, G.R.B. (1985) Photochemistry of
halogenated benzene derivatives. V. Photolytic reductive
dechlorination and and isomerization of tetrachlorobenzenes in
acetonitrile-water mixtures. Toxicol. environ. Chem., 9: 291-308.
CHOUDHRY, G.G., ROOF, A.A.M., & HUTZINGER, O. (1979) Photochemistry
of halogenated benzene derivatives. I. Trichlorobenzenes: reductive
dechlorination, isomerization and formation of polychlorobiphenyls.
Tetrahed. Lett., 22: 2059-2062.
CHU, I., VILLENEUVE, D., SECOURS, V., & VALLI, V.E. (1983)
Comparative toxicity of 1,2,3,4-, 1,2,4,5-, and
1,2,3,5-tetrachlorobenzene in the rat: results of acute and subacute
studies. J. Toxicol. environ. Health, 11(5): 663-667.
CHU, I., VILLENEUVE, D.C., VALLI, V.E., & SECOURS, V.E. (1984a)
Toxicity of 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-tetrachlorobenzene in
the rat: results of a 90-day feeding study. Drug Chem. Toxicol.,
7(2): 113-127.
CHU, I., VILLENEUVE, D.C., VIAU, A., BARNES, C.R., BENOIT, F.M., &
QIN, Y.H. (1984b) Metabolism of 1,2,3,4-, 1,2,3,5-, and
1,2,4,5-tetrachlorobenzene in the rat. J. Toxicol. environ. Health,
13(4-6): 777-786.
CHU, I., MURDOCH, D.J., VILLENEUVE, D.C. & VIAU, A. (1987) Tissue
distribution and elimination of trichlorobenzenes in the rat. J.
environ. Sci. Health, B22(4): 439-453.
CLARK, J.R., PATRICK, J.M. MOORE, J.C., & LORES, E.M. (1987)
Waterborne and sediment-source toxicities of six organic chemicals
to grass shrimp (Palaemonetes pugio) and amphioxus (Branchiostoma
caribaeum). Arch. environ. Contam. Toxicol., 16: 401-407.
COATE, W.B., SCHOENFISCH, W.H., LEWIS, T.R., & BUSEY, W.M. (1977)
Chronic inhalation exposure of rats, rabbits, and monkeys to
1,2,4-trichlorobenzene. Arch. environ. Health, 32(6): 249-255.
COHEN, J.M., DAWSON, R., & KOKETSU, M. (l981) Extent-of-exposure
survey of monochlorobenzene. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, Div. Surveillance, Hazard
Evaluations and Field Studies, NIOSH (PB 82-183963).
COTE, M., CHU, I., VILLENEUVE, D.C., SECOURS, V.E., & VALLI, V.E.
(1988) Trichlorobenzenes: Results of a thirteen week feeding study
in the rat. Drug chem. Toxicol., 11(1): 11-28.
COTTER, L.H. (1953) Paradichlorobenzene poisoning from insecticides.
N.Y. State J. Med., 53: 1690-1692.
COURTNEY, K.D., ANDREWS, J.E., & EBRON, M.T. (1977) Teratology study
of pentachlorobenzene in mice: no teratogenic effect at 50 or 100
mg/kg/day from day 6 to day 15 of gestation. IRCS med. Sci.: Libr.
Compend., 5: 587.
CROSBY, D.G. & HAMADMAD, N. (1971) The photoreduction of
pentachlorobenzenes. J. agric. food Chem., 19(6): 1171-1174.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment. Washington, US Environmental Protection Agency (Report
EPA-600/3-80-084) (NTIS PB80-221948).
CURRAN, H.M. (1981) Use of organic working fluids in Rancine
engines. J. Energy, 5(4): 218-223.
DALICH, G.M. & LARSON, R.E. (1985) Temporal and dose-response
features of monochlorobenzene hepatotoxicity in rats. Fundam. appl.
Toxicol., 5: 105-116.
DALY, J.W., JERINA, D.M., & WITKOP, B. (1972) Arene oxides and the
NIH shift: the metabolism, toxicity and carcinogenicity of aromatic
compounds. Experimentia(Basel), 28: 1129-1149.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters on survival and growth of the
larvae. US Fish. Bull., 67: 393-404.
DAVIS, E.M., MURRAY, H.E., LIEHR, J.G., & POWERS, E.L. (1981) Basic
microbial degradation rates and chemical byproducts of selected
organic compounds. Water Res., 15: 1125-1127.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of some
organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The
acute toxicity of 47 industrial chemicals to fresh and saltwater
fishes. J. hazard. Mat., 1: 303-318.
DEBORTOLI, M., KNOPPEL, H., PECCHIO, E., PEIL, A., ROGORA, L.,
SCHAUENBURG, H., SCHLITT, H., & VISSERS, H. (1985) Measurement of
indoor air quality and comparison with ambient air: a study on 15
homes in northern Italy. Luxembourg, Commission of the European
Communities (EUR 9656 EN).
DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DEN BESTEN, C., PETERS, M.M.C.G. & VAN BLADEREN, P.J. (1989) The
metabolism of pentachlorobenzene by rat liver microsomes: The nature
of the reactive intermediates formed. Biochem. biophys.Res. Comm.,
163(3): 1275-1281.
DILLEY, J.V. (1977) Toxic evaluation of inhaled chlorobenzene
(monochlorobenzene). Springfield, Virginia, National Technical
Information Service, US Department of Commerce (PB-276 623).
DILLING, W.L., BREDEWEG, C.J., & TEFERTILLER, N.B. (1976) Organic
photochemistry: simulated atmospheric photodecomposition rates of
methylene chloride, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene and other compounds. Environ. Sci. Technol.,
10(4): 351-356.
DURHAM, R.W. & OLIVER, B.G. (1983) History of Lake Ontario
contamination from the Niagara River by sediment radiodating and
chlorinated hydrocarbon analysis. J. Great Lakes Res., 9(2):
160-168.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral, neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
EHRLICHER, H. (1968) [Observations and experiences in industry
concerning the toxicity (physiopathologic effect) of chlorated
benzene vapours (mono- to hexachlorobenzene).] Zentrabl.
Arbeitsmed., 18: 204-205 (in German).
EICEMAN, G.A., CLEMENT, R.E., & KASAREK, F.W. (1979) Analysis of fly
ash from municipal incinerators for trace organic compounds. Anal.
Chem., 51(14): 2343-2350.
EICEMAN, G.A., CLEMENT, R.E., & KASAREK, F.W. (1981) Variations in
concentrations of organic compounds including polychlorinated
dibenzo- p-dioxins and polynuclear aromatic hydrocarbons in fly
ash from a municipal incinerator. Anal. Chem., 53(7): 955-959.
ELDER, V.A., PROCTOR, B.L., & HITES, R.A. (1981) Organic compounds
found near dump sites in Niagara Falls, New York. Environ. Sci.
Technol., 15(10): 1237-1243.
ENGST, R., MACHOLZ, R.M., KUJAWA, M., LEWERENZ, H-J., & PLASS, R.
(1976) The metabolism of lindane and its metabolites
gamma-2,3,4,5,6-pentachlorocyclohexene, pentachlorobenzene and
pentachlorophenol in rats and the pathways of lindane metabolism. J.
environ. Sci. Health. Bull., B11(2): 905-117.
ENVIRONMENT CANADA (1986) Ambient air concentrations of volatile
organic compounds in Toronto and Montreal. Ottawa, Environment
Canada, Pollution Measurement Division.
EUROPEAN PATENT APPLICATION 36,270, September 23, 1981.
FIELDING, M., GIBSON, T.M., & JAMES, H.A. (1981) Levels of
trichloroethylene and p-dichlorobenzene in groundwaters. Environ.
Technol. Lett., 2(12): 545-550.
FOMENKO, V.N. (1965) Determination of the maximum permissible
concentration of tetrachlorobenzene in water basins. Hyg. Sanit.,
30: 8-15.
FRANK, S.B. & COHEN, H.J. (1961) Fixed drug eruption due to paradi-
chlorobenzene. NY State J. Med., 61: 4079.
GAFFNEY, P.E. (1976) Carpet and rug industry case study: I. Water
and wastewater treatment plant operation. J. Water Pollut. Contr.
Fed., 48: 2590-2598.
GAGE, J.C. (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br.ö òJ. industr. Med., 27: 1-18.
GALLOWAY, S., BLOOM, A., RESNICK, M., MARGOLIN, B., NAKAMURA, F.,
ARCHER, P., & ZEIGER, E. (1985) Development of a standard protocol
for in vitro cytogenetic testing with Chinese Hamster ovary cells:
comparison of results for 22 compounds in two laboratories. Environ.
Mutagen., 7: 1-51.
GIAVINI, E., BOCCIA, M.L., PRATI, M., & VISMARA, C. (1986)
Teratologic evaluation of p-dichlorobenzene in the rat. Bull.
environ. Contam. Toxicol., 37: 164-168.
GIBSON, D.T., KOCH, J.R., SCHULD, C.L., & KALLIO, R.E. (1968)
Oxidative degradation of aromatic hydrocarbons by microorganisms.
II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry,
7(11): 3795-3802.
GIRARD, R., MARTIN, P., & BOURRET, J. (1969) [Haemopathies graves et
exposition à des dérivés chlorés du benzène (a propos de 7 cas). J.
Med. Lyon, 50(1164): 771-773.
GOERZ, G., VIZETHUM, W., BOLSEN, K., KRIEG, Th., & LISSNER, R.
(1978) [Hexachlorobenzene (HBC) induced porphyria in rats. Influence
of HBC-metabolites on the biosynthesis of heme.] Arch. dermatol.
Res., 263: 189-196 (in German).
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L., & POPP, J.A. (1988)
Potential role of 2µ - globulin, protein droplet accumulation, and
cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicol.appl. Pharmacol., 96: 367-379.
GOTO, M., HATTORI, M., MIYAGAWA, T., & ENOMOTO, M. (1972)[Hepatoma
generation in mice following application of high doses of HCH
(hexachlorocyclohexane)-isomers.] Chemosphere, 6: 279-282. (in
German).
GREVE, P.A. (1973) Pentachlorobenzene as a contaminant of animal
feed. Med. Fac. Landbouwwet. Rigksonio. Gent., 38: 775-784.
GRILLI, S., ARFELLINI, G., COLACCI, A., MAZZULLO, M., & PRODI, G.
(1985) In vivo and in vitro covalent binding of chlorobenzene to
nucleic acids. Jpn. J. Cancer Res. (Gann)., 76: 745-751.
GROSCH, D.S. (1973) Reproduction tests: the toxicity for Artemia
of derivatives from non-persistent pesticides. Biol. Bull., 145:
340-351.
HAIDER, K., JAGNOW, G., KOHNEN, R., & LIM, S.U. (1974) [Degradation
of chlorinated benzenes, phenols, and cyclohexane derivatives by
benzene and phenol utilizing soil bacteria under aerobic
conditions.] Acta Microbiol., 96(3): 183-200 (in German).
HALLOWELL, M. (1959) Acute haemolytic anaemia following the
ingestion of paradichlorobenzene. Arch. Dis. Childhood (London),
34: 74-75.
HANAI, Y., KATOU, T., JIMMA, T., & NOMURA, K. (1985) [The movement
of p-dichlorobenzene in the atmosphere.] Yokohama Kokuritsu Daigaku
Kankyo Kagaku Kenkyu Senta Kiyo, 12: 31-39 (in Japanese).
HARDEN., R.A. & BAETJER, A.M. (1978) Aplastic anemia following
exposure to paradichlorobenzene and naphthalene. J. occup. Med.,
20(12): 820-822.
HARKOV, R., GIANTI, S.J.Jr., BOZZELLI, J.W., & LAREGINA, J.E. (1985)
Monitoring volatile organic compounds at hazardous and sanitary
landfills in New Jersey. J. environ. Sci. Health, A20(5): 491-501.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., & LIOY, P.J. (1983)
Measurement of selected volatile organic compounds at three
locations in New Jersey during the summer season. J. Air Pollut.
Contr. Assoc., 33(12): 1177-1183.
HAWKINS, D.R., CHASSEAUD, L.F., WOODHOUSE, R.N., & CRESSWELL, D.G.
(1980) The distribution, excretion and biotransformation of
p-di-chloro[14]benzene in rats after repeated inhalation, oral
and subcutaneous doses. Xenobiotica, 10(2): 81-95.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.,
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutag., Suppl. 1, 5: 3-142.
HAYES, W.C., HANLEY, T.R., GUSHOW, T.S., JOHNSON, K.A., & JOHN, J.
A. (1985) Teratogenic potential of inhaled dichlorobenzenes in rats
and rabbits. Fundam. appl. Toxicol., 5(1): 190-202.
HEIKES, D.L. (1980) Residues of pentachlorobenzene and related
compounds in peanut butter. Bull. environ. Contam. Toxicol., 24:
338-343.
HEIKES, D.L., GRIFFITT, K.R., & CRAUN, J.C. (1979) Residues of
tetrachloro-nitrobenzene and related compounds in potatoes. Bull.
environ. Contam. Toxicol., 21: 563-568.
HEITMULLER, P.T., HOLLISTAR, T.A., & PARRISH, P.R. (1981) Acute
toxicity of 54 industrial chemicals to sheepshead minnows
(Cyprinodon variegatus). Bull. environ. Contam. Toxicol., 27:
596-604.
HERBOLD, B.A. (1986) Investigation of p-dichlorobenzene
1,4-dichlorobenzene for clastogenic effects in mice using the
micronucleus test. Bayer, A.G., Institute of Toxicology (Unpublished
report No. 14694 submitted to WHO) .
HERBOLD, B.A. (1988) p-Dichlorobenzene micronucleus test on the
mouse to evaluate clastogenic effects. Bayer, A.G., Institute og
Toxicology (Unpublished report no. 16902 submitted to WHO).
HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R. (1985)
Quantitative structure activity relationships and mixture toxicity
studies of alcohols and chlorohydrocarbons: effects on growth of
Daphnia magna. Aquat. Toxicol., 6: 209-217.
HIATT, M.H. (1981) Analysis of fish and sediment for volatile
priority polutants. Anal. Chem., 53(9): 1541-1543.
HITES, R.A. (1973) Analysis of trace organic compounds in New
England rivers. J. Chromatogr. Sci., 11: 570-574.
HOFLER, M., LAHANIATIS, E.S., BIENIEK, D., & KORTE, F. (1983)
[Reactionbehaviour of benzene at ppm concentrations during
chlorination withsodium hypochlorite in an aqueous solution.]
Chemosphere, 12(2): 217-224 (in German).
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., HOYLE, H.R., & SPENCER,
H. (1956) Toxicity of paradichlorobenzene; determinants on
experimental animals and human subjects. Arch. ind. Health, 14:
138-147.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., TORKELSON, T.R., & ADAM,
E.M. (1958) Toxicity of o-dichlorobenzene; studies on animals and
industrial experience. Arch. Ind. Health, 17: 180-187.
IARC (1982) Ortho and para dichlorobenzenes. In: Some industrial
chemicals and dyestuffs. Lyon, International Agency for Research on
Cancer, pp. 213-238 (Monographs on the Evaluation of Carcinogenic
Risk of Chemicals to Man, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: an update of
IARC Monographs, Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, pp. 192-193 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic. In: Patty,
F.A., ed. Industrial hygiene and toxicology, 2nd ed. New York,
Inter-Science Publishers, pp. 1333-1340.
IRPTC (1987) IRPTC Legal File 1986, Vol. 1. Geneva, International
Register of Potentially Toxic Chemicals, United National Environment
Programme.
JACOBS, A., BLANGETTI, M., & HELLMUND, E. (1974) Accumulation of
noxious chlorinated substances from Rhine River water in the fatty
tissue of rats. Vom Wass., 43: 259-274 (in German).
JACOBS, A., HELLMUND, E., & EBERLE, S.H. (1977) [Accumulation of
trace water pollutants in living organisms: time and dose dependence
of the accumulation of hexachlorobenzene and tetrachlorobenzene
derivatives in rats.] Vom Wass., 48: 255-272 (in German).
JAN, J. (1980) Chlorinated benzene residues in some seeds.
Chemosphere, 9: 165-167.
JAN, J. (1983a) Chlorobenzene residues in human fat and milk. Bull.
environ. Contam. Toxicol., 30: 595-599.
JAN, J. (1983b) Chlorobenzene residues in market milk and meat.
Mitt. Gebiete Lebensm. Hyg., 74(4): 420-425.
JAN, J. & MALNERSIC, S. (1980) Chlorinated benzene residues in fish
in Slovenia (Yugoslavia). Bull. environ. Contam. Toxicol., 24:824-827.
JERINA, D.M. & DALY, J.W. (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185(4151): 573-582.
JOHN, J.A., HAYES, W.C., HANLEY, T.R., Jr, JOHNSON, K.A., GUSHOW, T.
S., & RAO, K.S. (1984) Inhalation teratology study on
monochlorobenzene in rats and rabbits. Toxicol. appl. Pharmacol.,
76: 365-373.
JONDORF, W.R., PARKE, D.V., & WILLIAMS, R.T. (1955) Studies in
detoxication: the metabolism of halogenobenzenes. 1,2,3-, 1,2,4-,
and 1,3,5-tri-chlorobenzenes. Biochem. J., 61: 512-521.
JONDORF, W.R., PARKE, D.V., & WILLIAMS, R.T. (1958) Studies in
detoxication: the metabolism of halogenobenzenes 1,2,3,4-, 1,2,3,5-,
and 1,2,4,5-tetrachlorobenzenes. Biochem. J., 69: 181-189.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial wastewater: a case study of their
environmental impact. Environ. Sci. Technol., 12(1): 88-96.
KACEW, S., RUDDICK, J.A., PARULEKAR, M., VALLI, V.E., CHU, I., &
VILLENEUVE, D.C. (1984) A teratological evaluation and analysis of
fetal tissue levels following administration of tetrachlorobenzene
isomers to the rat. Teratology, 29: 21-27.
KANNO, S. & NOJIMA, K. (1979) Studies on photochemistry of aromatic
hydrocarbons. V. Photochemical reaction of chlorobenzene with
nitrogen oxides in air. Chemosphere, 4: 225-232.
KAO, C.I. & POFFENBERGER, N. (1979) Chlorinated benzenes. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed. Toronto,
Ontario, John Wiley and Sons, Vol. 5, pp. 797-808.
KARLOKOFF, S.W., BROWN, D.S., & SCOTT, T.S. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13:
241-249.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies on
halogenated benzenes (pentachloro-, pentachloronitro- and
hexabromo-) in rats. Toxicology, 5: 117-122.
KIMURA, R., HAYASHI, T., SATO, M., AIMOTO, T., & MURATA, T. (1979)
Identification of sulfur-containing metabolites of
p-dichlorobenzene and their disposition in rats. J. Pharmacobio-
Dyn., 2: 237-244.
KIRALY, J., SZENTESI, I., RUZICSKA, M., & CZEIZE, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. environ. Contam. Toxicol., 8: 309-319.
KITCHIN, K.T. & EBRON, M.T. (1983a) Maternal hepatic and embryonic
effects of 1,2,4-trichlorobenzene in the rat. Environ. Res., 31:
362-373.
KITCHIN, K.T. & EBRON, M.T. (1983b) Maternal hepatic and embryonic
effects of 1,2,3,4-tetrachlorobenzene in the rat. Toxicologist,
26: 243-256.
KITCHIN, K.T. & EBRON, M.T. (1983c) Maternal hepatic effects of
1,2,4,5-tetrachlorobenzene in the rat. Environ. Res., 32:
134-144.
KLUWE, W.M., DILL, G., PERSING, A., & PETERS, A. (1985) Toxic
response to acute, subchronic, and chronic oral administrations of
monochlorobenzene to rodents. J. Toxicol. environ. Health, 15(6):
745-767.
KNAPP, W.K., Jr, BUSEY, W.M., & KUNDZINS, W. (1971) Subacute oral
toxicity of monochlorobenzene in dogs and rats. Toxicol. appl.
Pharmacol., 19(2): 393 (Abstract)
KNEZOVICH, J.P. & HARRISON, F.L. (1988) The bioavailability of
sediment-sorbed chlorobenzenes to larvae of the midge, Chironomus
decorus. Ecotoxicol. environ. Saf., 15: 226-241.
KOBAYASHI, H. & RITTMAN, B.E. (1982) Microbial removal of hazardous
organic compounds. Environ. Sci. Technol., 16(3): 170A-183A.
KOCIBA, R.J., LEONG, B.J.K., & HEFNER, R.E. Jr (1981) Subchronic
toxicity study of 1,2,4-trichlorobenzene in the rat, rabbit and
beagle dog. Drug Chem. Toxicol., 4(3): 229-249.
KOHLI, J., JONES, D., & SAFE, S. (1976) The metabolism of higher
chlorinated benzene isomers. Can. J. Biochem., 54(3): 203-208.
KONEMANN, H. & VAN LEEUWEN, K. (1980) Toxicokinetics in fish:
accumulation and elimination of six chlorobenzenes by guppies.
Chemosphere, 9: 3-19.
KOSS, G. & KORANSKY, W. (1977) Pentachlorophenol in different
species of vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. Environ. Sci. Res., 12: 131-137.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal. Chem.,
54: 810-817.
KUEHL, D.W., LEONARD, E.N., WELCH, K.J., & VEITH, G.D. (1980)
Identification of hazardous organic chemicals in fish from the
Ashtabula River, Ohio, and Wabash River, Indiana. J. Assoc. Off.
Anal. Chem., 63(6): 1238-1244.
KUHN, E.P., COLBERG, P.J., SCHNOOR, J.L., WANNER, O., ZEHNER, A.J.
B., & SCHWARZENBACH, R.P. (1985) Microbial transformations of
substituted benzenes during infiltration of river water to
ground-water: laboratory column studies. Environ. Sci. Technol.,
19: 961-968.
LAHANIATIS, E.S., BIENIEK, D., VOLLNER, L., & KORTE, F. (1981a)
[Formation of organochlorine compounds in the combustion of
chlorine-containing polymers.] Chemosphere, 10(8): 935-943 (in
German)
LAHANIATIS, E.S., ROAS, R., BIENIEK, D., KLEIN, W., & KORTE, F.
(1981b) [Formation of chlorinated organic compounds in combustion of
polyethylenes in the presence of sodium chloride.] Chemosphere,
10(11-12): 1321-1326 (in German).
LAMPARSKI, L.L., LANGHORST, M.L., NESTRICK, T.J., & CUTIE, S. (1980)
Gas-liquid chromatographic determination of chlorinated benzenes and
phenols in selected biological matrices. J. Assoc. Off. Anal. Chem.,
63(1): 27-32.
LANGHORST, M.L. & NESTRICK, T.J. (1979) Determination of
chlorobenzenes in air and biological samples by gas chromatography
with photoionization detection. Anal. Chem., 51(12): 2018-2025.
LATTANZI, G., BARTOLI, S., BONORA, B., COLACCI, A., GRILLI, S.,
NIERO, A., & MAZZULLO, M. (1989) The different genotoxicity of
p-dichlorobenzene in mouse and rat: Measurement of the in vivo
and in vitro covalent interaction with nucleic acids. Tumori,
75: 305-310.
LAY, J.P., SCHAUERTE, W., MULLER, A., KLEIN, W., & KORTE, F. (1985)
Long-term effects of 1,2,4-trichlorobenzene on freshwater plankton
in an outdoor-model ecosystem. Bull. environ. Contam. Toxicol.,
34: 761-769.
LEBEL, G.L. & WILLIAMS, D.T. (1986) Determination of halogenated
contaminants in human adipose tissue. J. Assoc. Off. Anal. Chem.,
69(3): 451-458.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol, 24:
684-691.
LEBRET, E. (1985) Air pollution in Dutch homes: an exploratory study
in environmental epidemiology. Wageningen, The Netherlands,
Department of Air Pollution, Department of Environmental and
Tropical Health, Wageningen Agricultural University (Report R-138).
LEE, R.P. & RYAN, C. (1979) Microbial degradation of organochlorine
compounds in esturine waters and sediments. In: Proceedings of the
Workshop: Microbial Degradation of Pollutants in Marine
Environments, Pensacola Beach, Florida, April 9-14, 1978.
Springfield, Virginia, US Environmental Protection Agency,
pp.443-450 (Contract No., EPA-600/9-79-012. NTIS: PB298254).
LEVINE, S.P., COSTELLO, R.J., GERACI, C.L., & CONLIN K.A. (1985) Air
monitoring at the drum bulking process of a hazardous waste remedial
action site. Am. Ind. Hyg. Assoc. J., 46(4): 192-196.
LINDER, R., SCOTTI, T., GOLDSTEIN, J., MCELROY, K., & WALSH, D.
(1980) Acute and subchronic toxicity of pentachlorobenzene. J.
environ. Pathol. Toxicol., 4(5-6): 183-196.
LINGG, R.D., KAYLOR, W.H., PYLE, S.M., KOPFLER, F.C., SMITH, C.C.,
WOLFE, G.F., & CRAGG, S. (1982) Comparative metabolism of
1,2,4-trichlorobenzene in the rat and rhesus monkey. Drug Metab.
Disp., 10(2): 134-141.
LOESER, E. & LITCHFIELD, M.H. (1983) Review of recent toxicology
studies on p- dichlorobenzene. Food.cChem. Toxicol., 21(6):
825-832.
LOPEZ-AVILA, V., NORTHCUTT, R., ONSTOT, J., WICKHAM, M., & BILLETS,
S. (1983) Determination of 51 priority organic compounds after
extraction from standard reference materials. Anal. Chem., 55(6):
881-889.
LOVEGREN, N.V., FISHER, G.S., LEGENDRE, M.G., & SCHULLER, W.H.
(1979) Volatile constituents of dried legumes. J. agric. food Chem.,
27(4): 851-853.
LU, P-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Persp., 10: 269-284.
LUNDE, G. & BJORSETH, A. (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci.
total Environ., 8: 241-246.
LUNDE, G. & OFSTAD, E.B. (1976) Determination of fat-soluble
chlorinated compounds in fish. Z. anal. Chem., 282: 395-399.
MACHOLZ, R.M. & KUJAWA, M. (1985) Recent state of lindane
metabolism. Part III. Res. Rev., 94: 119-149.
MACKAY, D., BOBRA, A., CHAN, D.W., & SHIU, W.Y. (1982) Vapour
pressure correlations for low-volatility environmental chemicals.
Environ. Sci. Technol., 16: 645-649.
MACKAY, D. & SHIU, W.Y. (1981) A critical review of Henry's Law
Constants for chemicals of environmental interest. J. phys. chem.
Ref. Data, 10(4): 1175-1199.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of
low-solubility contaminants from water bodies to atmosphere.
Environ. Sci. Technol., 7(7): 611-614.
MARINUCCI, A.C. & BARTHA, R. (1979) Biodegradation of 1,2,3- and
1,2,4-trichlorobenzene in soil and in liquid enrichment culture.
Appl. environ. Microbiol., 38(5): 8ll-817.
MCKINNEY, J.D., FISHBEIN, C.E., FLETCHER, C.E., & BARTEL, W.F.,
(1970) Electron-capture gas chromatography of paradichlorobenzene
metabolites as a measure of exposure. Bull. environ. Contam.
Toxicol., 5(4): 354-361.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28(1): 97-104.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk of
Canadian women. Food Addit. Contam., 3(4): 313-322.
MICHAEL, L.C., ERICKSON, M.D., PARKS, S.P., & PELLIZZARI, E.D.
(1980) Volatile environmental pollutants in biological matrices with
a headspace purge technique. Anal. Chem., 52(12): 1836-1841.
MILES, D.H., MODY, N.V., & MINYARD, J.P. (1973) Constituents of
marsh grass: survey of the essential oils in Juncus roemerianus.
Phyto-chemistry, 12: 1399-1404.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. eng. Data, 29: 184-190.
MILONE, M.F. (1986a) Capacity of para-dichlorobenzene to induce
unscheduled DNA synthesis in cultured Hela cells, Bayer Chemical Co.
(unpublished report submitted to WHO).
MILONE, M.F. (1986b) Chromosome aberrations in human lymphocytes
cultured in vitro and treated with p-dichlorobenzene, Bayer
Chemical Co., (Unpublished report submitted to WHO ).
MIYAI, I., HIRONO, N., FUJITA, M., & KAMEYAMA, M. (1988) Reversible
ataxia following chronic exposure to paradichlorobenzene. J. Neurol.
Neurosurg. Psychiat., 51: 453-454.
MOHTASHAMIPUR, E., TRIEBEL, R., STRAETER, H., & NORPOTH, K. (1987)
The bone marrow clastogenicity of eight halogenated benzenes in male
NMRI mice. Mutagenesis, 2(2): 111-113.
MORITA, M. & OHI, G. (1975) Para-dichlorobenzene in human tissue and
atmosphere in Tokyo metropolitan area. Environ. Pollut., 8:
269-274.
MORITA, M., MIMURA, S., OHI, G., YAGYU, H., & NISHIZAWA, T. (1975) A
systematic determination of chlorinated benzenes in human adipose
tissue. Environ. Pollut., 9: 175-179.
MOTTRAM, D.S., PSOMAS, I.E., & PATTERSON, R.L.S. (1983) Chlorinated
residues in the adipose tissue of pigs treated with
hexachlorocyclohexane. J. Sci. Food Agric., 34: 378-387.
MURTY, C.V.R., OLSON, M.J. GARG, B.D., & RAY, A.K. (1988)
Hydrocarbon induced hyaline droplet nephropathy in male rats during
senescence. Toxicol. appl. Pharmacol., y6: 380-392.
NAIR, R.S., BARTER, J.A., SCHROEDER, R.E. KNEZEVICH, A., & STACK,
C.R. (1987) A two-generation reproduction study with
monochlorobenzene vapor in rats. Fund. appl. Toxicol., 9: 678-686.
NALBANDIAN, R.M. & PEARCE, J.F. (1965) Allergic purpura induced by
exposure to p- dichlorobenzene. J. Am. Med. Assoc., 194(7):
828-829.
NAQUADAT (1987) National Water Quality Data Bank, Inland Waters
Directorate, Water Quality Branch, Environment Canada, Ottawa.
NEPTUNE, D. (1980) Descriptive statistic for detected priority
pollutants and tabulation listings. Washington, DC, US EPA (Office
of Water Planning and Standards, TRDB-0280-001).
NILL, J.P. (1936) Effects of dichlorobenzene. J. Am. Med. Assoc.,
107: 607-608.
NOJIMA, K. & KANNO, S. (1980) Studies on photochemistry of aromatic
hydrocarbons. VII. Photochemical reaction of p-dichlorobenzene
with nitrogen oxides in air. Chemosphere, 9: 437-440.
NTP (1985a) NTP technical report on the carcinogenesis studies of
chlorobenzene (CAS No. 108-90-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, North Carolina, National
Toxicology Program, US Department of Health and Human Services,
pp.228 (NTP TR 261).
NTP (1985b) NTP technical report on the carcinogenesis studies of
1,2-dichlorobenzene (CAS No. 95-50-1) in F344/N rats and B6C3F1
mice (gavage studies). Research Triangle Park, North Carolina,
National Toxicology Program, US Department of Health and Human
Services, pp.192 (NTP TR 255) .
NTP (1987) NTP technical report on the toxicology and
carcinogenicity studies of 1,4-dichlorobenzene (CAS No. 106-46-7) in
F344/N rats and B6C3F1 mice (gavage studies . Research Triangle
Park, North Carolina, National Toxicology Program, US Department of
Health and Human Services, pp.198 (NTP TR 319) .
NTP (1989a) Draft NTP report on the toxicity studies of
1,2,4,5-tetra-chlorobenzene in F344/N rats and B6C3F1 mice (feed
studies). Research Triangle Park, North Carolina, National
Toxicology Program, US Department of Health and Human Services (NTP
Tox 7).
NTP (1989b) Draft NTP report on the toxicity studies of
Pentachlorobenzene in F344/N rats and B6C3F1 mice (feed studies).
Research Triangle Park, North Carolina, National Toxicology Program,
US Department of Health and Human Services (NTP Tox 6) .
OESCH, F., JERINA, D.M., DALY, J.W., & RICE, J.M. (1973) Induction,
activation and inhibition of epoxide hydrase: an anomalous
prevention of chlorobenzene-induced hepatotoxicity by an inhibitor
of epoxide hydrase. Chem-Biol. Interact., 6(3): 189-202.
OGATA, M. & SHIMADA, Y. (1983) Differences in urinary
monochlorobenzene metabolites between rats and humans. Int. Arch.
occup. Environ. Health, 53: 51-57.
OLIVER, B.G. & BOTHEN, K.D. (1980) Determination of chlorobenzenes
in water by capillary gas chromatography. Anal. Chem., 52:
2066-2069.
OLIVER, B.G. & BOTHEN, K.D. (1982) Extraction and clean-up
procedures for measuring chlorobenzenes in sediments and fish by
capillary gas chromatography. Int. J. environ. anal. Chem., 12:
131-139.
OLIVER, B.G. & NICOL, K.D. (1982) Chlorobenzenes in sediments,
water, and selected fish from lakes Superior, Huron, Erie, and
Ontario. Environ. Sci. Technol., 16: 532-536.
OLIVER, B.G., & NICOL, K.D. (1984) Chlorinated contaminants in the
Niagara River, 1981-1983. Sci. total Environ., 39: 57-70.
OLIVER, B.G., CHARLTON, M.N., & DURHAM, R.W. (1989) Distribution,
redistribution and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
Sediments. Environ. Sci. Technol., 23: 200-208.
OLSON, M.J., JOHNSON, J.T., & REIDY, C.A. (1990) A comparison of
male rat & human urinary proteins: implications for human resistance
to hyaline droplet nephropathy. Toxicol. appl. Pharmacol., 102:
524-536.
ONUSKA, F.I. & TERRY, K.A. (1985) Determination of chlorinated
benzenes in bottom sediment samples by WCOT column gas
chromatography. Anal. Chem., 57: 801-805.
OPPERHUIZEN, A. & STOKKEL, R.C.A.M. (1988) Influence of contaminated
particles on the bioaccumulation of hydrophobic organic
micropollutants in fish. Environ. Pollut., 51: 165-177.
OTSON, R. (1987) Purgeable organics in Great Lakes raw and treated
water. Int. J. environ. anal. Chem., 31: 43-53.
OTSON, R. & BENOIT, F.M. (1986) Surveys of selected organics in
residential air. In; Walkenshaw, D.S. ed. Transactions - Indoor air
quality in cold climates - Hazards and abatement measures, April
1985, Pittsburgh, PA, Air Pollution Control Association, pp 224-236.
OTSON, R. & WILLIAMS, D.T. (1981) Evaluation of a liquid-liquid
extraction technique for water pollutants. J. Chromatogr., 212:
187-197.
OTSON, R. & WILLIAMS, D.T. (1982) Headspace chromatographic
determination of water pollutants. Anal. Chem., 54(6): 942-946.
OTSON, R., WILLIAMS, D.T., & BIGGS, D.C. (1982a) Relationship
between raw water quality, treatment, and occurence of organics in
Canadian potable water. Bull. environ. Contam. Toxicol., 28:
396-403.
OTSON, R., WILLIAMS, D.T., & BOTHWELL, P.D. (1982b) Volatile organic
compounds in water at thirty Canadian potable water treatment
facilities. J. Assoc. Off. Anal. Chem., 65(6): 1370-1374.
PAGNOTTO, L.D. & WALKLEY, J.E. (1965) Urinary dichlorophenol as an
index of para-dichlorobenzene exposure. Am. Ind. Hyg. Assoc. J.,
26: 137-142.
PANKOW, J.F. & ISABELLE, L.M. (1982) Adsorption-thermal desorption
as a method for the determination of low levels of aqueous organics.
J. Chromatog., 237: 25-39.
PANKOW, J.F., ISABELLE, L.M., ASHER, W.E., KRISTENSEN, T.J., &
PETERSON, M.E. (1983) Organic compounds in Los Angeles and Portland
rain: identities, concentrations, and operative scavenging
mechanisms. In: Pruppacher, H.R., Semonin, R.G., & Slinen, W.G.N.,
ed. Precipitation Scavenging, Dry Deposition, and Resususpensions:
Proceedings of the Fourth International Conference, Santa Monica,
California, 29 November-3 December, 1982,. Elsevier, New York. Vol.
1, 403-415.
PARKE, D.V. & WILLIAMS, R.T. (1960) Studies in detoxication: the
halogeno-benzenes (a) penta- and hexa-chlorobenzenes, (b)
further observations on 1,3,5-trichlorobenzene. Biochem. J., 74:
5-9.
PELLIZZARI, E.D. (1982) Analysis for organic vapour emissions near
industrial and chemical waste disposal sites. Environ. Sci.
Technol., 16: 781-785.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERIKSON, M.D. (1982) Purgeable organic compounds
in mother's milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PELLIZZARI, E.D., HARTWELL, T.D., PERRITT, R.L., SPARACINO, C.M.,
SHELDON, L.S., ZELON, H.S., WHITMORE, R.W., BREEN, J.J., & WALLACE,
L. (1986) Comparison of indoor and outdoor residential levels of
volatile organic chemicals in five U.S. geographical areas. Environ.
Int., 12(6): 619-623.
PEREIRA, W.E. & HUGHES, B.A. (1980) Determination of selected
volatile organic priority pollutants in water by computerized
spectrometry. J. Am. Water Works Assoc., 72(4): 220-230.
PEROCCO, P., BOLOGNESI, S., & ALBERGHINI, W. (1983) Toxic activity
of seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16: 69-75.
PIET, G.J., SLINGERLAND, P., BIJLSMA, G.H., & MORRA, C. (1980) Fast
quantitative analysis of a wide variety of halogenated compounds in
surface-, drinking-, and groundwater. Environ. Sci. Res., 16:
69-80
PIKE, M.H. (1944) Ocular pathology due to organic compounds.
Michigan State Med. Assoc. J., 43: 581-584.
POLAND, A., GOLDSTEIN, J., HICKMAN, P., & BURSE, V.W. (1971) A
reciprocal relationship between the induction of -aminolevulinic
acid synthetase and drug metabolism produced by m-dichlorobenzene.
Biochem. Pharmacol., 2: 1281-1290.
POPOVKI, P., ORUSEV, T., URUMOVA, E., BLAGOEVA, L., & TRPOVSKI, V.
(1980) [Skin changes of workers employed in trichlorobenzene
production.] Arh. Hig. Rada Toksikol., 31: 177-184 (in
Serbo-Croat, with French abstract).
POTENTA, E.J. & SAUNDERS, M.F. (1983) Indoor air quality
investigation of chronic health problems in a Manhattan office
space. In:Proceedings of the 76th Annual Meeting of the Air
Pollution Control Association, Atlanta, Georgia, 19-24 June, 1983,
Pittsburgh, Air Pollution Control Association, pp. 2-12.
POWERS, M.B., COATE, W.B., & LEWIS, T.R. (1975) Repeated topical
applications of 1,2,4-trichlorobenzene: effects on rabbit ears.
Arch. environ. Health, 30: 165-167.
RAO, K.S., JOHNSON, K.A., & HENCK, J.W. (1982) Subchronic dermal
toxicity study of trichlorobenzene in the rabbit. Drug chem.
Toxicol., 5(3): 249-263.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene in some
soils and plants in Finland. Ann. Agric. Fenn., 16: 277-282.
REICH, H. (1934) [Puran(R) poisoning in a 2-year-old child].
Schweizerische Medzinische Wochenschrift, 64: 223-224. (in German)
REID, W.D. (1973) Mechanism of renal necrosis induced by
bromobenzene or chlorobenzene. Exp. mol. Pathol., 19: 197-214.
REID, W.D. & KRISHNA, G. (1973) Centrolobular hepatic necrosis
related to covalent binding of metabolites of halogenated aromatic
hydrocarbons. Exp. Mol. Path., 18: 80-99.
REINEKE, W., KNACKMUSS, H-J. (1984) Microbial metabolism of
haloaromatics: isolation and properties of chlorobenzene-degrading
bacterium. Appl. environ. Microb., 47(2): 395-402.
RENNER, G. & MUCKE, W. (1986) Transformation of pentachlorophenol.
Part 1: metabolism in animals and man. Toxicol. environ. Chem.,
11: 9-29.
RIMINGTON, C. & ZIEGLER, G. (1963) Experimental porphyria in rats
induced by chlorinated benzenes. Biochem. Pharmacol., 12:
1387-1397.
ROBINSON, K.S., KAVLOCK, R.J., CHERNOFF, N., & GRAY, L.E. (1981)
Multigeneration study of 1,2,4-trichlorobenzene in rats. J. Toxicol.
environ. Health, 8: 489-500.
ROGERS, H.R, CAMPBELL, J.A., CRATHORNE, B. & DOBBS, A.J. (1989) The
occurrence of chlorobenzenes and permethrins in twelve U.K. sewage
sludges. Water Res., 23(7): 913-921.
ROZENBAUM, N.D., BLOCK, R.S., KREMNAVA, S.N., GINZBURG, S.L., &
POZHARISKII, I.V. (1947) [Use of chlorobenzene as a solvent from the
standpoint of industrial hygiene.] Gig. i Sanit., 12(1): 21-24(in
Russian).
ROZMAN, K., WILLIAMS, J., MUELLER, W.F., COULSTON, F., & KORTE, F.
(1979) Metabolism and pharmacokinetics of pentachlorobenzene in the
rhesus monkey. Bull. environ. Contam. Toxicol., 22: 190-195.
RUDDICK, J.A., BLACK, W.D., VILLENEUVE, D.C., & VALLI, V.E. (1983) A
teratological evaluation following oral administration of trichloro-
and dichlorobenzene isomers to the rat. Teratology, 27(2): 73A.
RUDLING, L., AHLING, B. & LOFROTH, G. (1980) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in a small central heating furnace and from combustion of wood in
closed fireplace stoves, Solna, Sweden, Swedish Environment
Protection Board (SNV PM 1331) (in Swedish, with English summary).
SASMORE, D.P., MITOMA, C., TYSON, C.A., & JOHNSON, J.S. (1983)
Subchronic inhalation toxicity of 1,3,5-trichlorobenzene. Drug chem.
Toxicol., 6(3): 241-258.
SATO, A. & NAKAJIMA, T. (1979) A structure-activity relationship of
some chlorinated hydrocarbons. Arch. environ. Health, 34(2):
69-75.
SCHOENY, R.S., SMITH, C.C., & LOPER, J.C. (1979) Non-mutagenicity
for Salmonella of the chlorinatd hydrocarbons Aroclor 1254,
1,2,4-tri-chlorobenzene, Mirex and Kepone. Mutat. Res., 68(2):
125-132.
SCHWARTZ, H., CHU, I., VILLENEUVE, D.C., VIAU, A., & BENOIT, F.M.
(1985) Metabolites of 1,2,3,4-tetrachlorobenzene in monkey urine. J.
Toxicol. environ. Health, 15(5): 603-607.
SCHWARTZ, H., CHU, I., VILLENEUVE, D.C., & BENOIT, F.M. (1987)
Metabolism of 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-tetrachlorobenzene in
the Squirrel monkey. J. Toxicol. environ. Health, 22: 341-350.
SCHWARZENBACH, R.P., MOLNAR-KUBICA, E., GIGER, W., & WAKEHAM, S.G.
(1979) Distribution, residence time and fluxes of tetrachlorethylene
and 1,4-dichlorobenzene in Lake Zurich, Switzerland. Environ. Sci.
Technol., 13(11): 1367-1373.
SCHWARZENBACH, R.P., GIGER, W., HOEHN, E., & SCHNEIDER, J.K. (1983)
Behavior of organic compounds during infiltration of river water to
groundwater. Field Stud.environ. Sci. Technol., 17: 472-479.
SEARS, G.W. & HOPKE, E.R. (1949) Vapor pressures of the isomeric
trichlorobenzenes in the low pressure region. J. Am. Chem. Soc.,
71: 2575-2576.
SELANDER, H. G., JERINA, D.M., & DALY, J.W. (1975) Metabolism of
chlorobenzene with hepatic microsomes and solubilized cytochrome
P-450 systems. Arch. Biochem. Biophys., 168: 309-321.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the Delaware
River. Environ, Sci. Technol., 12(10): 1188-1194.
SHELTON, D.W. & WEBER, L.J. (1981) Quantification of the joint of
effects of mixtures of hepatotoxic agents: evaluation of a
theoretical model in mice. Environ. Res., 26: 33-41.
SHIMIZU, M., YASUI, Y., & MATSUMOTO, N. (1983) Structural
specificity of aromatic compounds with special reference to
mutagenic activity in Salmonella typhimurium: a series of
chloro-or fluoro-nitrobenzene derivatives. Mutat. Res., 116:
217-238.
SHORT, B.G., BURNETT, V.L., COX, M.G., BUS, J.S., & SWENBERG, J.A.
(1987) Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Lab. Invest., 57(5):
564-577.
SIMMONS, P., BRANSON, D., & BAILEY, R. (1977)
1,2,4-Trichlorobenzene: biodegradable or not. Text. Chem. Color.,
9(9): 211-213.
SIEVERS, R.E., BARKLEY, R.M., DENNEY, D.W., HARVEY, S.D., ROBERTS,
J. M., & DELANY, A.C. (1980) Gas chromatographic and mass
spectrometric analysis of volatile organics in the atmosphere. In:
Sampling and analysis of toxic organics in the atmosphere.
Philadelphia, American Society for Testing and Materials, pp. 3-21
(ASTM STP 721).
SINGH, H.B., SALAS, L.J., SMITH, A.J., & SHIGEISHI, M. (1981)
Measurements of some potentially hazardous organic chemicals in
urban environments. Atmos. Environ., 15: 601-612.
SMITH, J.R.L., SHAW, B.A., & FOULKES, D.M. (1972) Mechanisms of
mammalian hydroxylation: some novel metabolites of chlorobenzene.
Xenobiotica, 2(3): 215-226.
SMITH, E.N. & CARLSON, G.P. (1980) Various pharmacokinetic
parameters in relation to enzyme-inducing abilities of
1,3,4-trichlorobenzene and 1,2,4-tribromobenzene. J. Toxicol.
environ. Health, 6(4): 737-749.
SOFUNI, T., HAYASHI, M., MATSUOKA, A., SAWADA, M., HATANAKA, M., &
ISHIDATE, M.JR. (1985) [Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells.] Eisei Shikenjo
Hokoku, 103: 64-75. (in Japanese).
SPENCE, R-M.M. & TUCKNOTT, O.G. (1983) The examination of the
headspace volatiles of watercress. J. Sci. Food Agric., 34:
768-772.
STATISTICS CANADA (1981) Apparent per capita food consumption in
Canada. Ottawa, Queen's Printer (Cat. 32-229, Annual, Part II).
STEINMETZ, K.L., SPANGGORD, R.J. (1987) Examination of the potential
of p- dichlorobenzene to induce unscheduled DNA synthesis or DNA
replication in the in vivo-in vitro mouse hepatocyte DNA repair
assay (Task 1). (Unpublished report prepared by SRI International,
USA, for US Chemical Manufacturers Association and submitted to WHO
by ICI, Central Toxicology Laboratory ).
STULL, D.R. (1947) Vapor pressure of pure substances: organic
compounds. Ind. Eng. Chem., 39: 517-539.
SULLIVAN, T.M., BORN, G.S., CARLSON, G.P., & KESSLER, W.V. (1983)
The pharmacokinetics of inhaled chlorobenzene in the rat. Toxicol.
appl. Pharmacol., 71: 194-203.
SUMERS, J. FUHRMAN, M. & KELMAN, A. (1952) Hepatitis with
concomitant esophageal varices following exposure to mothball
vapors. N.Y. State med. J., 52: 1048-1049.
SYROVADKO, O.N. & MALYSHEVA, Z.V. (1977) [Working conditions and
their effect on some specific functions of women engaged in the
manufacture of enamel-insulated wires.] Gig. Tr. Prof. Zabol., 4:
25-28 (in Russian)
TAGATZ, M.E., PLAIA, G.R., & DEANS, C.H. (1985) Effects of 1,2,4-
trichlorobenzene on estuarine macrobenthic communities exposed via
water and sediment. Ecotoxicol. environ. Safety, 10: 351-360.
TANAKA, A., SATO, M., TSUCHIYA, T., ADACHI, T., NIIMURA, T., &
YAMAHA, T. (1986) Excretion, distribution and metabolism of
1,2,4-trichlorobenzene in rats. Arch. Toxicol., 59: 82-88.
TARKHOVA, L.P. (1965) [Materials for determining the maximum
permissible concentration of chlorobensol in atmospheric air.] Gig.
i Sanit., 30: 327-333 (in Russian).
TOLOT, F., SOUBRIER, B., BRESSON, J.-R., & MARTIN, P. (1969) Myélose
proliférative d'évolution rapide. Role étiologigue possible des
dérivés chlorés du benzène. J. Med. Lyon, 50(164): 761-780.
TOPP, E., SCHEUNERT, I., ATTAR, A,. & KORTE, F. (1986) Factors
affecting the utake of 14C-labelled organic chemicals by plants
from soil. Ecotoxicol. Environ. Safety, 11: 219-228.
TOPP, E., SCHEUNERT, I., & KORTE, F. (1989) Kinetics of the uptake
of 14C-labelled chlorinated benzenes from soil by plants.
Ecotoxicol. environ. Safety, 17: 157-166.
US EPA (1980a) Ambient water quality criteria for chlorinated
benzenes. Washington, DC, Office of Water Regulations and
Standards, US Environmental Protection Agency (Report EPA
440/5-80-028, PB81-117392).
US EPA (1980b) Ambient water quality criteria for dichlorobenzenes.
Washington, DC, Office of Water Regulations and Standards, US
Environmental Protection Agency (Report EPA 440/5-80-039,
PB81-117509).
US EPA (1985) Health assessment document for chlorinated benzenes.
Final Report. Washington, DC, Office of Health and Environment
Assessment, US Environmental Protection Agency ( Report
EPA/600/8-84/015F).
UYETA, M., TAUE, S., CHIKASAWA, K., & MAZAKI, M. (1976)
Photoformation of polychlorinated biphenyls from chlorinated
benzenes. Nature (Lond.), 264: 583-584.
VAN HOOGEN, G. & OPPERHUIZEN, A. (1988) Toxicokinetics of
chlorobenzenes in fish. Environ. Toxicol. Chem., 7: 213-219.
VARSHAVSKAYA, S.P. (1968) Comparative toxicological characteristics
of chlorobenzene and dichlorobenzene (ortho- and para-isomers) in
relation to the sanitary protection of water bodies. Hyg. Sanit.,
33(10-12): 17-23.
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (pentachloro- pentachloronitro-, and
hexabromo-) in rats. Environ. Physiol. Biochem., 5(5): 328-331.
WAKEHAM, S.G., DAVIS, A.C., & KARAS, J.L. (1983) Mesocosm
experiments to determine the fate and persistence of volatile
organic compounds in coastal seawater. Environ. Sci. Technol., 17:
611-617.
WALLACE, L.A. (1986) Personal exposures, indoor and outdoor air
concentrations and exhaled breath concentrations of selected
volatile organic compounds measured for 600 residents of New Jersey,
North Dakota, North Carolina and California. Toxicol. environ.
Chem., 12(3-4): 215-236.
WALLACE, L.A., PELLIZZARI, E., HARTWELL, T., ROSENZWEIG, M.,
ERIKSON, M., SPARACINO, C., & ZELON, H. (1984) Personal exposure to
volatile organic compounds. I. Direct measurements in breathing-zone
air, drinking water, food, and exhaled breath. Environ. Res., 35:
293-319.
WALLACE, L.A., PELLIZZARI, E.D., HARTWELL, T.D., SPARACINO, C.M.,
SHELDON, L.S.,& ZELON, H. (1985) Personal exposures, indoor-outdoor
relationships, and breath levels of toxic air pollutants measured
for 355 persons in New Jersey. Atmos. Environ., 19(10): 1651-1661.
WALLACE, L.A., PELLIZZARI, E., HARTWELL, T., ZELON, H., SPARACINO,
C., PERRITT, R., & WHITMORE, R. (1986) Concentrations of 20 volatile
organic compounds in the air and drinking water of 350 residents of
New Jersey compared with concentrations in their exhaled breath. J.
occup. Med., 28(8): 603-608.
WALLGREN, K. (1953) [Chronic poisoning in the production of moth
deterrents mainly composed of p-dichlorobenzene]. Zentralbl.
Arbeitsmed. Arbeitsschutz, 3: 14-15 (in German).
WARE, S.A. & WEST, W.L. (1977) Investigation of selected potential
environmental contaminants: halogenated benzenes. Washington, DC,
297 pp. (US EPA Report, EPA 560/2-77-004, NTIS PB 273 206).
WATANABE, P.G., KOCIBA, R.J., HEFNER, R.E. JR., YAKEL, H.O., &
LEONG, B.K.J. (1978) Subchronic toxicity studies of
1,2,4-trichlorobenzene in experimental animals. Toxicol. appl.
Pharmacol., 45(1): 332-333 (Abstract).
WATSON, A. & PATTERSON, R.L.S. (1982) Tainting of pork meat by 1,4-
dichlorobenzene. J. Sci. Food Agric., 33: 103-105.
WEAST, R.C., ed. (1986) CRC handbook of chemistry and physics, 67th
ed., Boca Raton, Florida, The Chemical Rubber Company.
WELLER, R.W. & CRELLIN, A.J. (1953) Pulmonary granulomatosis
following extensive use of paradichlorobenzene. Arch. intern. Med.,
91: 408-413.
WHO (1984) Guidelines for drinking-water quality. Vol. 1:
Recommendations, Geneva, World Health Organization, 130 pp.
WHO (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food.
Geneva, World HealthOrganization, 174pp.
WILLIAMS, R.T. (1959) The metabolism of halogenated aromatic
hydrocarbons, In: Detoxification mechanisms, 2nd ed., London,
Chapman and Hall Ltd., pp. 237-258.
WILLIAMS, R.T., HIROM, P.C., & RENWICK, A.G. (1975) Species
variation in the metabolism of some organic halogen compounds, In:
Mcintyre, A.D. & Mills, C.F., ed., Ecolical and toxicological
Research, New York and London, Plenum Press, pp. 91-105.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1988) Organohalogen
residues in human adipose autopsy samples from six Ontario
municipalities. J. Assoc. Off. Anal. Chem., 71(2): 410-414.
WILSON, J.T., ENFIELD, C.G., DUNLAP, W.J., COSBY, R.L., FOSTER,
D.A., & BASKIN, L.B. (1981) Transport and fate of selected organic
pollutants in a sandy soil. J. environ. Qual., 10(4): 501-506.
WONG, P.T.S., CHAU, Y.K., RHAMEY, J.S., & DOCKER, M. (1984)
Relationship between water solubility of chlorobenzenes and their
effects on a freshwater green alga. Chemosphere, 13(9): 991-996.
YAMAMOTO, H., TANIGUCHI, Y., IMAI, S., OHNO, Y., & TSUBURA, Y.
(1978) [Acute toxicity and local irritation tests of
trichlorobenzene (TCB) on ddY mice.] J. Tara Med. Assoc., 29:
569-573 (in Japanese).
YAMAMOTO, H., OHNO, Y., NAKAMORI, K., OKUYAMA, T., IMAI, S., &
TSUBURA, Y. (1982) [Chronic toxicity and carcinogenicity test of
1,2,4-trichlorobenzene on mice by dermal painting.] J. Nara Med.
Assoc., 33(2): 132-145 (in Japanese).
YANG, K.H., PETERSON, R.E., & FUJIMOTO, J.M. (1979) Increased bile
duct-pancreatic fluid flow in benzene-treated rats. Toxicol. appl.
Pharmacol., 47(3): 505-514.
YOSHIDA, M. & HARA, I. (1985) Composition of urinary metabolites and
variation of urinary taurine levels in rats injected with
chlorobenzene. Ind. Health, 23(3): 239-243.
YOSHIOKA, Y., OSE, Y., & SATO, T. (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci. tot. Environ., 43:
149-157.
YOUNG, D.R. & HEESEN, T.C. (1978) DDT, PCB, and chlorinated benzenes
in the marine ecosystem off Southern California. In: Jolley, R.L.,
Gorshev, H., & Hamilton, D.H., Jr, ed., Water chlorination.
Environmental impact and health effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 2, pp. 267-290.
ZAPATA-GAYON, C., ZAPATA-GAYON, N., & GONZALEZ-ANGULO, A. (1982)
Clastogenic chromosomal aberrations in 26 individuals accidentally
exposed to ortho dichlorobenzene vapors in the National Medical
Center in Mexico City. Arch. environ. Health, 37(4): 231-235.
ZOETEMAN, B.C.J., HARMSEN, K., LINDERS, J.B.H.J., MORRA, C.F.H., &
SLOOFF, W. (1980) Persistent organic pollutants in river water and
ground water of the Netherlands. Chemosphere, 9: 231-249.
ZONG, Z. & MA, A. (1985) [Statistical analysis of 1951 cases of
occupational dermatosis in Shanghai chemical plants.,] Zhonghua
Yufanguixue Zazhi, 19(2): 90-92 (in Chinese).
ZUB, M. (1978) Reactivity of the white blood cell system to toxic
action benzene and its derivatives. Acta biol. Cracoviensia, 21:
163-174.
RESUME
La présente publication traite des risques pour la santé humaine et
l'environnement qui découlent de l'exposition au monochloro-benzène
(MCB), aux dichlorobenzènes (DCB), aux trichlorobenzènes (TCB), aux
tétrachlorobenzènes (TeCB) et au pentachlorobenzène (PeCB). La
substitution par le chlore est indiquée comme suit:
1,2-dichlorobenzène (1,2-DCB); 1,2,3-trichlorobenzène (1,2,3-TCB),
etc.
1. Identitée, proprietes physiques et chimiques, et methodes
d'analyse
Les chlorobenzènes sont des dérivés aromatiques cycliques formés par
addition de 1 à 6 atomes de chlore au noyau benzénique. On obtient
ainsi 12 composés: le monochlorobenzène, trois isomères pour chacun
des di-, tri et tétrachlorobenzènes, ainsi que le penta- et
l'hexachlorobenzène.
Les chlorobenzènes sont des solides blancs cristallins à la
température ambiante, sauf dans le cas du MCB, du 1,2-DCB, du
1,3-DCB et du 1,2,4-TCB qui sont des liquides incolores. En général,
ils sont peu solubles dans l'eau, leur solubilité diminuant à mesure
que la substitution par le chlore augmente. Ils sont peu
inflammables, leurs coéfficients de partage octanol/eau sont moyens
à élevés et augmentent avec le nombre d'atomes de chlore; la tension
de vapeur est basse à moyenne et diminue lorsque la substitution
augmente. Les seuils gustatif et olfactif sont bas, en particulier
dans le cas des dérivés les moins chlorés.
Même lorsqu'ils sont purifiés, les chlorobenzènes du commerce
contiennent en proportions diverses, des isomères très voisins. Par
exemple, le MCB pur peut contenir jusqu'à 0,05% de benzène et 0,1%
de DCB; le 1,2-DCB technique pouvant contenir jusqu'à 19 % des
autres DCB, 1% de TCB et jusqu'à 0,05% de MCB. Rien n'indique qu'il
puisse y avoir contamination par des dibenzo- p-dioxines
polychlorées (PCDD) ni par des polychlorodibenzofuranes (PCDF).
Un grand nombre de techniques de prélèvement ont été mises au point
pour l'échantillonnage des chlorobenzènes, techniques qui sont
fonction du milieu. Elles vont de l'extraction par solvant dans le
cas des milieux aqueux à l'utilisation de substances absorbantes
pour les composés en suspension dans l'air. La chromatographie
gaz-liquide (GLC) est la technique de choix pour le dosage du
chlorobenzène dans des échantillons provenant de l'environnement.
2. Sources d'exposition humaine et environnementale
2.1 Chiffres de production
D'après les données dont on dispose sur le volume de la production
des chlorobenzènes et qui correspondent à la période 1980-83, la
production mondiale serait de 568 x 106 kg; cependant,
l'utilisation des chlorobenzènes a reculé dans certains pays depuis
lors. Environ 50 % de cette quantité a été produite aux Etats-Unis
d'Amérique et le reste, essentiellement en Europe occidentale et au
Japon. Le MCB correspondait à 70% de la production mondiale, la
production du 1,2-DCB, du 1,4-DCB et du 1,2,4-TCB étant
respectivement de 22 x 106, 4 x 106, et 1,2-3,7 x 106 kg.
Le MCB et les DCB sont obtenus par chloration directe du benzène en
phase liquide en présence d'un catalyseur, alors que les TCB et les
TeCB sont produits par chloration directe des chlorobenzènes
isomères convenables, en présence d'un catalyseur métallique.
2.2 Usages
On utilise principalement les chlorobenzènes comme intermédiaires
dans la synthèse des pesticides et d'autres produits chimiques; le
1,4-DCB est utilisé dans les désodorisants d'ambiance et comme
répulsif contre les mites. Les chlorobenzènes les plus substitués
(TCB et 1,2,3,4-TeCB) entrent dans la composition des fluides
diélectriques.
2.3 Liberation de chlorobenzenes dans l'environnement
Il peut y avoir libération de chlorobenzènes dans l'environnement,
principalement lors de la production de ces substances, qu i sont
en outre amenées à être dispersées lors d'un certain nombre de leurs
utilisations. Par exemple, aux Etats-Unis d'Amérique, entre 0,1 et
0,2% de la production de 1983 (qui correspondait à 130 x 106
tonnes de MCB a sans doute été dispersée dans l'environnement. Les
quantités libérées lors du rejet de déchets, et notamment
l'incinération de déchets municipaux, sont beaucoup plus faibles.
Toutefois, l'incinération des chlorobenzènes peut conduire à
l'émission de PCDD et de PCDF.
3. Transport, repartition et transformation dans l'environnement
3.1 Degradation
C'est principalement par voie biologique et, dans une moindre
mesure, par des processus abiotiques que les chlorobenzènes
disparaissent de l'environnement. Toutefois, on estime qu'ils sont
moyennement persistants dans l'eau, l'air et les sédiments. On a
fait état de temps de séjour dans l'eau allant d'une journée dans
les rivières à plus de 100 jours dans les eaux souterraines. D ans
l'air, la dégradation des chlorobenzènes s'effectue principalement,
semble-t-il, par voie chimique et par photolyse, la durée de séjour
se situant entre 13 et 116 jours pour le MCB, les DCB et un isomère
du TCB non précisé.
On a montré que nombre de microorganismes présents dans les
sédiments et les boues d'effluents dégradaient les chlorobenzènes.
Il semblerait que les composés les plus substitués soient les plus
difficiles à dégrader, la dégradation s'effectuant uniquement en
aérobiose. Dans les conditions d'anaérobiose du sol et les eaux
souterraines, le DCB, les TCB et les PeCB résistent en général à la
dégradation microbienne.
3.2 Destinee
Les chlorobenzènes qui sont libérés dans le milieu aquatique se
redistribuent préférentiellement dans l'air et les sédiments (en
particulier dans les sédiments riches en matières organiques).
D'après les renseignements limités dont on dispose, il semble que
l'on trouve dans les sédiments, notamment les sédiments des régions
très industrialisées, des quantités 1000 fois plus élevées que
celles qui sont présentes dans l'eau. La rétention dans le sol
augmente avec la teneur de celui-ci en matières organiques; il
existe une corrélation positive entre le degré de chloration du
composé et son adsorption sur les matières organiques. Quelques
données montrent que les résidus liés aux sédiments sont
biodisponibles; c'est ainsi que les invertébrés aquatiques sont
susceptibles de fixer les résidus présents sur les sédiments, les
plantes et dans le sol.
4. Concentrations dans l'environnement et exposition humaine
4.1 Les chlorobenzènes dans l'environnement
Les concentrations moyennes en chlorobenzènes (mono- à tri-) dans
l'air ambiant sont de l'ordre de 0,1 µg/m3, avec des valeurs
maximales allant jusqu'à 100 µg/m3. On ne dispose d'aucune donnée
sur les teneurs en TeCB et PeCB de l'air ambiant, encore que ces
substances aient été décelées dans des cendres volantes provenant
d'incinérateurs municipaux. Les concentrations de chlorobenzènes
dans l'air intérieur sont analogues à celles que l'on trouve dans
l'air extérieur; toutefois, on a fait état de valeurs beaucoup plus
élevées que dans l'air ambiant dans des régions très polluées, ainsi
que dans des espaces confinés où l'on avait utilisé des produits
contenant du chlorobenzène.
Des chlorobenzènes (mono- à penta-) ont été décelés dans des eaux
superficielles à des concentrations de l'ordre du ng/litre-µg/litre,
avec quelquefois des valeurs atteignant quelques dixièmes de
mg/litre à proximité d'installations industrielles. La teneur en
chlorobenzènes des eaux résiduaires industrielles peut être encore
plus élevée et varier en fonction de la nature des procédés mis en
oeuvre.
Dans les échantillons d'eau de boisson analysés on a décelé la
présence de tous les chlorobenzènes. Ce sont les composés les moins
chlorés qui étaient le plus fréquemment présents et aux
concentrations les plus fortes, avec prédominance de l'isomère
1,4-DCB; toutefois, les concentrations moyennes étaient généralement
inférieures à 1 µg/litre et ne dépassaient que rarement 50 µg/litre,
quel que soit le composé.
On n'a pas trouvé de données sur la teneur des aliments en
chlorobenzènes qui proviennent de programmes de surveillance bien
conçus; les renseignements disponibles se limitent principalement à
la concentration dans le poisson à proximité d'installations
industrielles ou à des incidents isolés de contamination de produits
carnés. Tous les isomères du chlorobenzène (mono- à penta-) ont été
décelés dans des truites d'eau douce, à des teneurs allant de 0,1 à
16 µg/kg. Dans une autre étude, les teneurs des poissons d'eau douce
en chlorobenzènes totaux variaient en moyenne de 0,2 mg/kg de
graisse dans les régions peu polluées à 1,8 mg/kg dans une zone
industrialisée. Il existe certains indices de l'augmentation de la
concentration en chlorobenzènes dans le poisson d'eau douce avec le
degré de chloration du composé. En ce qui concerne le poisson de
mer, les quelques études disponibles donnent une teneur en 1,4-DCB
de 0,05 mg/kg de poids humide.
Les résultats dont on dispose au sujet des taux de chlorobenzène
dans la viande et le lait et qui concernent principalement les
régions contaminées, sont de l'ordre de 0,02 à 5 µg/kg.
Lors de deux enquêtes sur le lait humain, on a procédé au dosage de
la totalité des chlorobenzènes, sauf le MCB. Dans une des études,
les concentrations de DCB étaient égales en moyenne à 25 µg/kg de
lait alors qu'elles étaient inférieures à 5 µg/kg dans le cas du
TCB, des isomères du TeCB et du PeCB. Dans la seconde enquête, les
concentrations étaient beaucoup plus basses puisqu'en moyenne elles
allaient de 1 µg/kg (1,2,3-TCB et PeCB) à un maximum de 6 µg/kg (1,3
et 1,4-dichlorobenzène).
4.2 Exposition humaine
4.2.1 Population generale
Sur la base des données limitées dont on dispose, il semble que
c'est à partir de l'air que l'apport journalier de chlorobenzènes
dans la population générale est le plus élevé, en particulier en ce
qui concerne les dérivés les moins chlorés qui sont plus volatils
(0,2-0,9 µg/kg de poids corporel). Par comparaison avec l'apport
d'autres origines, l'apport d'origine alimentaire augmente à mesure
qu'augmente le degré de chloration; ainsi, l'alimentation contribue
davantage que l'air à la dose journalière de TeCB et de PeCB.
Toutefois, les niveaux d'exposition dans ce cas restent
vraisem-blablement au-dessous de 0,05 µg/kg de poids corporel.
Quelques études, en nombre limité, ont montré que les enfants
nourris au sein risquaient de recevoir une dose de chlorobenzène
plus forte, rapportée au poids corporel, que les adultes.
4.2.2 Contexte professionnel
Il n'est pas possible de chiffrer avec précision l'exposition
professionnelle au chlorobenzène sur la base des données
disponibles. Toutefois, dans une unité de production, on a trouvé
des teneurs en 1,4-DCB allant de 42 à 288 mg/m3 et dans d'autres
usines chimiques, la concentration de MCB pouvait atteindre 18,7
mg/m3.
5. Cinetique et metabolisme
Tous les chlorobenzènes sont facilement résorbés dans les voies
digestives et respiratoires chez l'homme et les animaux
d'expérience, la résorption dépendant de la position de l'atome de
chlore pour les différents isomères d'un même dérivé.
Chez l'animal d'expérience, après une rapide répartition dans les
organes très irrigués, les chlorobenzèn s'accumulent principalement
dans les tissus adipeux, une quantité plus faible passant dans le
foie et les autres organes. On a montré que les chlorobenzènes
étaient capables de traverser la barrière placentaire et l'on en a
trouvé dans le cerveau de foetus. En général, ces dérivés
s'accumulent d'autant plus qu'ils sont plus chlorés. Toutefois, les
variations sont considérables en ce qui concerne les différents
isomères d'un même dérivé.
Chez l'homme et l'animal d'expérience, le métabolisme des
chlorobenzènes conduit au chlorophénol correspondant par oxydation
microsomique. Ces chlorophénols peuvent être excrétés dans l'urine
sous forme d'acides mercapturiques, d'acide glucuronique ou de
sulfo-conjugués. Le TeCB et le PeCB sont métabolisés plus lentement
et séjournent dans les tissus plus longtemps que les dérivés
monochlorés, dichlorés et trichlorés. Certains des chlorobenzènes
induisent toute une variété de systèmes enzymatiques et notamment
ceux qui interviennent dans les processus d'oxydation, de réduction,
de conjugaison et d'hydrolyse.
En général, les dérivés les plus chlorés s'éliminent plus lentement
que le MCB et le DCB, la proportion des dérivés tétra et
pentachlorés éliminés tels quels dans les matières fécales étant
plus importante. C'est ainsi que 17 % d'une dose 1,2,4-TCB a été
éliminé dans les matières fécales au bout de sept jours alors que 91
à 97 % d'une dose de 1,4 TCB était éliminé sous forme de métabolites
dans les urines au bout de cinq jours. La position des atomes de
chlore sur le noyau benzénique conditionne également de manière
importante la vitesse de métabolisation et d'élimination, les
isomères possédant deux atomes de carbone adjacents non substitués
étant métabolisés et éliminés plus rapidement.
6. Effets sur les organismes aquatiques dans leur milieu naturel
Les renseignements dont on dispose sur les effets des
chloro-benzènes au niveau de l'environnement, portent principalement
sur leur toxicité aiguë pour les organismes aquatiques. En général,
la toxicité augmente avec le degré de chloration du noyau
benzénique. Alors que le MCB, le 1,2-DCB, le 1,3-DCB, le 1,2,4-TCB,
le 1,3,5-TCB et le 1,2,4,5-TeCB sont tous peu toxiques pour les
micro-organismes, la toxicité du TCB et des TeCBs est, à l'exception
du 1,2,4,5-TeCB, plus élevée que celle des autres composés; chez les
algues unicellulaires, la CL50 à 96 heures (pour la croissance
cellulaire ou la production de chlorophylle) allait de plus de 300
mg/litre dans le cas du MCB à environ 1 mg/litre dans le cas du
1,2,3,5-TeCB. Certains invertébrés aquatiques paraissent plus
sensibles aux chlorobenzènes, toutefois les concentrations
nécessaires pour entraîner une mortalité à 48 ou 96 heures restent
encore proches de 1 mg/litre ou nettement supérieures (par exemple
2,4 mg/litre dans le cas du 1,2-DCB pour
Daphnia magna et jusqu'à 530 mg/litre dans le cas du
1,2,4,5-TeCB).
Les valeurs de la CL50 à 96 heures pour Lepomis machrochirus
allaient de 0,3 mg/litre dans le cas du TeCB à 24 mg/litre dans le
cas du MCB. L'étude de la toxicité chronique sur des stades
embryo-larvaires a donné des limites allant de 0,76 à 2,0 mg/litre
dans le cas des DCBs pour Pimephales promelas; de 0,22 et de 0,13
mg/litre respectivement dans le cas du 1,2,4-TCB et du 1,2,4,5-TeCB
pour un autre vairon des eaux estuarielles. Les stades les plus
sensibles dans le cas du MCB sont les alevins nouvellements éclos de
poissons rouges et de Micropterus salmoides, avec une CL50 à 96
heures respectivement égale à 1 et 0,05 mg/litre.
On ne dispose d'aucune donnée concernant les effets des
chlorobenzènes sur les organismes terrestres.
7. Effets sur les animaux d'experience et sur les systemes
in vitro
A quelques exceptions près, les chlorobenzènes ne présentent qu'une
toxicité aiguë modérée pour les animaux d'expérience et en général
la DL50 par voie orale est supérieure à 1000 mg/kg de poids
corporel; la DL50 dermique est plus élevée mais les données
disponibles à ce sujet sont limitées. L'ingestion de doses mortelles
entraîne une paralysie respiratoire, l'inhalation de fortes doses
entraînant une irritation locale et une dépression du système
nerveux central. Une intoxication aiguë par des doses non mortelles
de chlorobenzène entraîne des effets au niveau du foie, des reins,
des surrénales, des muqueuses, du cerveau ainsi que sur les enzymes
métabolisantes.
On n'a étudié l'irritation cutanée et oculaire due au chlorobenzène
que dans le cas du 1,2,4-TCB et du 1,2-DCB. Ces deux composés
causent une gêne très importante mais on n'a pas constaté de lésion
permanente après application directe dans l'oeil de lapins. Le
1,2,4-TCB est légèrement irritant pour la peau et peut provoquer une
dermatite par suite d'un contact répété ou prolongé. On n'a constaté
aucun signe de sensibilisation.
Des rats et des souris exposés pendant de courtes périodes (5 et 21
jours) à du MCB et des DCBs, à doses de plusieurs centaines de mg/kg
de poids corporel ont présenté des lésions hépatiques et des
anomalies hématologiques indiquant une atteinte de la moelle
osseuse. Après exposition de brève durée de rats et de lapins à
d'autres chlorobenzènes (TCD et PeCB) à des doses légèrement plus
faibles que celles de MCB et de DCB, ce sont les lésions hépatiques
qui constituaient le principal effet nocif constaté. Plusieurs des
isomères étudiés ont provoqué une porphyrie, les isomères porteurs
d'atomes de chlore en para, étant les plus actifs de ce point de
vue (c'est-à-dire 1,4-DCB, 1,2,4-TCB, 1,2,3,4-TeCB et PeCB). L'ordre
décroissant de toxicité générale dans le cas des TeCBs et du PeCB
après exposition de brève durée était le suivant : 1,2,4,5-TeCB
PeCB 1,2,3,4- et 1,2,3,5-TeCB, en bonne corrélation avec les
concentrations retrouvées dans les graisses et le foie.
Des études d'exposition au long cours (jusqu'à six mois) portant sur
plusieurs espèces d'animaux d'expérience ont fait ressortir une
tendance à l'augmentation de la toxicité avec le degré de chloration
du noyau benzénique. Toutefois pour un même dérivé on constatait des
variations importantes de cette toxicité selon l'isomère en cause.
Par exemple 1,4-DCB s'est révélé beaucoup moins toxique que le
1,2-DCB.
Il y avait une bonne corrélation entre la toxicité d'un composé
donné et son degré d'accumulation dans les tissus, les femelles
étant moins sensibles que les mâles. Les principaux organes cibles
étaient le foie et le rein; à dose plus élevée, on a signalé des
effets sur le système hématopoïetique et une toxicité thyroïdienne a
été observée dans les études portant sur le 1,2,4,5-TeCB et le PeCB.
Une étude de cancérogénicité portant sur le MCB a fait ressortir un
accroissement de l'incidence des nodules néoplasiques du foie dans
le groupe de rats mâles F344 soumis à la dose la plus élevée (120
mg/kg de poids corporel); toutefois on n'a pas noté d'accroissement
de l'incidence tumorale, lié au traitement, chez ces rats, mâles ou
femelles ou chez des souris femelles B6C3F1. Du 1,2-DCB a été
administré à des rats F344 mâles et femelles et à des souris B6C3F1
(60 ou 120 mg/kg de poids corporel), sans qu'on puisse observer de
signe de cancérogénicité.
Lors d'une étude de cancérogénicité portant le 1,4-DCB, on a noté un
accroissement, lié à la dose, de la fréquence des adénocar-cinomes
des tubules rénaux chez des rats mâles F344 ainsi qu'une
augmentation de celle des carcinomes et des adénomes
hépatocellulaires chez des souris B6C3F1 des deux sexes. Chez des
rats Wistar mâles et femelles ainsi que des souris Swiss femelles on
n'a noté aucun signe de cancérogénicité après exposition de ces
animaux par inhalation à des doses légèrement plus élevées de
1,4-DCB (dose estimée à 400 mg/kg par jour pour les rats et à 790
mg/kg par jour pour les souris) pendant des périodes plus courtes.
Toutefois les données disponibles indiquent que l'induction de
tumeurs rénales par le 1,4-DCB chez les rats F344 mâles et la
néphropathie grave avec formation d'inclusions hyalines, qui lui
sont associées, constituent des réactions spécifiques de l'espèce et
du sexe, liées à la réabsorption de l'alpha-2-microglobuline.
Les données disponibles sont insuffisantes pour qu'on puisse évaluer
le pouvoir cancérogène des chlorobenzènes fortement chlorés (tri,
tétra et pentachlorobenzènes).
On ne dispose que de données limitées résultant d'expériences
in vitro et in vivo, à propos des isomères autres que le
1,4-DCB, toutefois les chlorobenzènes ne semblent pas être
mutagènes. Sur la base de données plus nombreuses concernant le
1,4-DCB, on peut conclure que ce composé n'a aucun pouvoir mutagène
in vivo ou in vitro.
Rien n'indique non plus que les chlorobenzènes soient tératogènes
pour le rat ou le lapin. L'administration de MCB et de DCBs à des
rats ou des lapins par la voie respiratoire à une concentration
supérieure à 2000 mg/m3 (environ 550 mg/kg de poids corporel et
par jour) et, par voie orale, à des concentrations supérieures à
500 mg/kg de poids corporel, a entraîné des effets embryotoxiques et
fétotoxiques minimes. Toutefois ces doses étaient nettement toxiques
pour les femelles gravides. Selon certains indices, les TCBs, les
TeCBs et le PeCB seraient embryotoxiques et fétotoxiques à des doses
non toxiques pour les femelles gravides, cependant les données
disponibles ne sont pas cohérentes.
8. Effets sur l'homme
8.1 Population generale
Les rapports dont on dispose sur les effets des chlorobenzènes dans
la population générale se limitent à des rapports ponctuels sur
des cas d'accidents ou d'erreurs de manipulations de composés
contenant des chlorobenzènes peu substitués (MCB, 1,2-DCB, 1,4-DCB
ainsi qu'un isomère non précisé du TCB). On n'a pratiquement aucun
renseignement sur les doses, la pureté chimique, les relations
doses/temps et les effets observés, tels qu'une leucémie
myéloblastique, une rhinite, une glomérulonéphrite, une
granulomatose pulmonaire, des sensations vébrieuses, des
tremblements, de l'ataxie, une polynévrite et un ictère - tous
effets qui ne peuvent être évalués quantitativement.
Aucune étude épidémiologique concernant les effets des
chlorobenzènes sur la santé au sein de la population générale n'a
été rapportée.
8.2 Exposition professionnelle
Au cours de la fabrication et de l'utilisation du chlorobenzène, on
peut observer la symptomatologie suivante qui résulte d'une
exposition excessive : effets sur le système nerveux central et
irritation des yeux et des voies respiratoires supérieures (MCB);
troubles hématologiques (1,2-DCB); effets sur le système nerveux
central, durcissement de l'épiderme et troubles hématologiques -
notamment anémie (1,4-DCB). Toutefois ces symptômes n'ont été
décrits que lors de cas d'intoxications et sont difficiles à évaluer
quantitativement car on ne possède guère de renseignements sur les
concentrations réelles, la pureté chimique ou les relations
dose/temps.
Peu d'études é pidémiologiques ont été consacrées à l'exposition des
travailleurs aux chlorobenzènes; elles ne portent que sur le MCB,
1,2-DCB, 1,4-DCB et le 1,2,4,5-TeCB. On a signalé des effets sur le
système nerveux, sur le développement néonatal et sur la peau après
exposition au MCB mais les trois études en cause ne permettaient pas
d'évaluer le risque pour des raisons d'ordre méthodologique qui
tenaient notamment à l'évaluation de l'exposition, au fait que
l'exposition n'était pas uniforme et qu'il n'y avait pas de groupe
témoin. On peut émettre des critiques analogues à l'encontre de
l'étude consacrée au 1,4-DCB, étude au cours de laquelle on a
observé une irritation des muqueuses oculaires et nasales ainsi que
de l'étude faisant état d'aberrations chromosomiques à la suite
d'une exposition à des doses non précisées de 1,2-DCB et
1,2,4,5-TeCB.
9. Conclusions
Dans la mesure où les opérations industrielles sont effectuées
conformément aux normes de bonne pratique, les risques qu'impliq ue
une exposition professionnelle au chlorobenzène peuvent être
considérés comme minimes. A l'heure actuelle, les concentrations de
chlorobenzène dans l'environnement ne font courir qu'un risque
minimum à la population générale sauf dans le cas d'erreurs de
manipulation de produits à base de chlorobenzène ou de leur rejet
inconsidéré dans le milieu ambiant. Cependant cette évaluation se
base sur des données de surveillance limitées et il faudrait
disposer de renseignements complémentaires pour confirmer cette
conclusion. Il faudrait cependant envisager de réduire l'usage et
les rejets de chlorobenzène pour les raisons suivantes:
a) Les chlorobenzènes peuvent servir de précurseurs dans la
formation de dibenzodioxines polychlorés et de dibenzofuranes
polychlorés, par exemple lors de l'incinération de déchets.
b) Ces produits peuvent communiquer un goût ou une odeur
déplaisants à l'eau de boisson et au poisson.
c) Des résidus persistent en condition d'anaérobiose dans les
sédiments, des sols et les eaux souterraines riches en matières
organiques.
Pour la plupart des chlorobenzènes, l'évaluation du risque repose
sur leurs effets non tumorigènes. Toutefois, on a tenu compte des
effets tumorigènes lors de l'évaluation du risque dans le cas du MCB
et du 1,4-DCB. Les données disponibles montrent que la fréquence
accrue de tumeurs rénales chez des rats exposés à du 1,4-DCB
constituent une réaction typique de cette espèce, liée au sexe, qui
ne saurait être extrapolée à l'homme. En se basant sur le fait
qu'après exposition à du 1,4-DCB, il y a accroissement de la
réplication de l'ADN dans le foie de souris et accroissement de la
fréquence des adénomes et des carcinomes hépatocellulaires chez ces
mêmes animaux, on peut penser que ce produit est susceptible de se
comporter comme un cancérogène non génotoxique dans le foie des
rongeurs. La fréquence accrue de nodules hépatiques néoplasiques
observée dans un groupe de rats mâles soumis à une dose élevée de
MCB lors d'une étude de cancérogénicité indique également que ce
dernier produit peut se comporter comme un cancérogène non
génotoxique.
RESUMEN
La presente publicación se centra en los riesgos que tiene para la
salud humana y el medio ambiente la exposición a los siguientes
compuestos: monoclorobenceno (MCB), diclorobencenos (DCB),
triclorobencenos (TCB); tetraclorobencenos (TeCB) y
pentacloro-benceno (PeCB). La sustitución del cloro se indica de la
manera siguiente: 1,2-diclorobenceno (1,2-DCB);
1,2,3-triclorobenceno (1,2,3-TCB), etc.
1 Identidad, propiedades fisicas y quimicas y metodos analiticos
Los clorobencenos son compuestos aromáticos cíclicos formados por la
adición de 1 a 6 átomos de cloro al anillo de benceno. De esta
manera se obtienen 12 compuestos: monoclorobenceno, tres formas
isómeras de di-, tri- y tetraclorobencenos, así como penta- y
hexaclorobencenos.
Los clorobencenos son sólidos cristalinos de color blanco a
temperatura ambiente, excepto el MCB, el 1,2-DCB, el 1,3-DCB y el
1,2,4-TCB, que son líquidos incoloros. La solubilidad de los
clorobencenos en agua es en general baja, disminuyendo al aumentar
el número de átomes de cloro. La inflamabilidad es escasa, los
coeficientes de reparto octanol/agua son de moderados a altos y
aumentan con el número de átomes de cloro, y las presiones de vapor
son de bajas a moderadas, disminuyendo al aumentar el grado de
cloración. El umbral de sabor y olor son bajos, en particular para
los compuestos menos clorados.
Los clorobencenos comerciales, incluso los purificados, contienen
distintas cantidades de isómeros estrechamente relacionados. Por
ejemplo, el MCB puro puede contener hasta un 0,05% de benceno y un
0,1% de DCB, mientras que el 1,2-DCB de calidad técnica puede
contener hasta un 19% de los otros DCB, un 1% de TCB y hasta un
0,05% de MCB. No se ha informado de la existencia de pruebas de
contaminación por dibenzo- p-dioxinas policloradas (DDPC) y
dibenzofuranos policlorados (DFPC).
Se han puesto a punto, en función del medio, un gran número de
técnicas de muestreo para clorobencenos. Abarcan desde los métodos
de extracción con disolventes para los medios acuosos hasta el uso
de absorbentes para los compuestos presentes en el aire. La técnica
analítica de elección para la determinación de clorobencenos en
muestras obtenidas del medio ambiente es la cromatografía
gas-líquido (CGL).
2 Fuentes de exposicion humana y ambiental
2.1 Cifras de produccion
Los datos disponibles sobre las cifras de producción de
clorobencenos corresponden al período 1980-83, cuando la producción
mundial se estimaba en 568 x 106 kg, aunque la utilización de
clorobencenos ha disminuido posteriormente en algunos países.
Alrededor del 50% de esta cantidad se fabricaba en los Estados
Unidos, y la mayor parte del resto en Europa occidental y el Japón.
El 70% de la producción mundial era de MCB, y de 1,2-DCB, 1,4-DCB y
1,2,4-TCB se producían respectivamente 22 x 106, 4 x 106 y 1,2-3,7 x
106 kg.
El MCB y el DCB se obtienen por cloración directa del benceno en la
fase líquida utilizando un catalizador, mientras que los TCB y los
TeCB se producen mediante la cloración directa de los isómeros de
clorobenceno apropiados en presencia de un catalizador metálico.
2.2 Aplicaciones
Los clorobencenos se utilizan sobre todo como intermediarios en la
síntesis de plaguicidas y de otros productos químicos; el 1,4-DCB se
usa en los desodorante para ambientes cerrados y como repelente de
las polillas. Los bencenos con mayor número de átomos de cloro (TCB
y 1,2,3,4-TeCB) se han empleado como componentes de fluidos
dieléctricos.
2.3 Liberacion de clorobencenos en el medio ambiente
La liberación de clorobencenos en el medio ambiente tiene lugar
fundamentalmente durante la fabricación y por la naturaleza
dispersiva de sus aplicaciones. En los Estados Unidos, por ejemplo,
se estima que de las 130 x 106 toneladas de MCB producidas en 1983
se perdió en el medio ambiente el 0,1-0,2%. La liberación de
clorobencenos a partir de la eliminación de residuos, inclusive la
incineración de vertidos municipales, es mucho más baja. Sin
embargo, la incineración de clorobencenos puede producir emisiones
de DDPC y DFPC.
3 Transporte, distribucion y transformacion en el medio
ambiente
3.1 Degradacion
La eliminación de los clorobencenos del medio ambiente se efectúa
principalmente mediante mecanismos biológicos y, en menor medida,
por otros sistemas; sin embargo, se considera que su presencia es
moderadamente persistente en el agua, el aire y los sedimentos. Se
ha informado que su tiempo de permanencia en el agua es de un día en
la de río y de más de 100 días en la subterránea. En el aire, parece
que las vías predominantes de degradación del clorobenceno son las
reacciones químicas y fotolíticas, con tiempos de permanencia que
para el MCB, los DCB y un isómero sin especificar del TCB se ha
informado que oscilan entre 13 y 116 días. Se ha demostrado que
muchos microorganismos presentes en los sedimentos y los fangos
cloacales degradan los clorobencenos. Parece ser que los compuestos
con mayor número de átomos de cloro se descomponen menos fácilmente,
y tal proceso sólo tiene lugar en condiciones aerobias. El DCB, los
TCB y los PeCB presentes en el suelo y en el agua subterránea en
condiciones anaerobias suelen resistir la degradación microbiana.
3.2 Destino final
Los clorobencenos que se liberan en el medio acuático se
redistribuyen de manera preferente en el aire y en los sedimentos
(sobre todo en los ricos en materia orgánica). Son limitadas las
informaciones según las cuales en los sedimentos se han detectado
niveles 1000 veces superiores a los del agua, en particular en
regiones muy industrializadas. La retención de clorobencenos en el
suelo aumenta con el contenido de éste en materia orgánica; existe
una correlación positiva entre el grado de cloración del compuesto y
su adsorción a la materia orgánica. Hay pruebas limitadas que
indican que los residuos unidos a los sedimentos están
biodisponibles para los organismos, es decir, que los invertebrados
acuáticos pueden captar residuos de los sedimentos, y las plantas,
del suelo.
4 Niveles medio ambientales y exposicion humana
4.1 Clorobencenos en el medio ambiente
Los niveles medios de clorobencenos (de mono- a tri-) en el aire del
medio ambiente son del orden de 0,1 µg/m3, con niveles máximos de
hasta 100 µg/m3. No se dispone de datos sobre los niveles de TeCB
y PeCB en el aire, aunque estos compuestos químicos se han detectado
en cenizas volantes procedentes de incineradores municipales. Los
niveles de clorobencenos en el aire de los espacios cerrados son
similares a los presentes en el aire exterior; sin embargo, se han
comunicado concentraciones muy superiores en zonas intensamente
contaminadas y en espacios cerrados donde se habían utilizado
productos con clorobenceno.
Se han detectado clorobencenos (de mono- a penta-) en aguas
superficiales con concentraciones del orden de ng/litro-µg/litro,
con niveles ocasionales de hasta décimas de mg/litro en las
cercanías de fuentes industriales. Los niveles de clorobencenos en
las aguas residuales industriales pueden ser más elevados y varían
en función de la naturaleza de los procesos utilizados.
En los análisis de muestras de agua de bebida se han detectado todos
los tipos de clorobencenos. Los compuestos menos clorados eran los
que se encontraban con mayor frecuencia y en concentraciones más
elevadas, con predominio del isómero 1,4-DCB; sin embargo, las
concentraciones medias de todos los clorobencenos detectados han
sido en general inferiores a 1 µg/litro y raramente han superado los
50 µg/litro.
No se han encontrado datos procedentes de programas de vigilancia
bien formulados sobre los niveles de clorobencenos en los alimentos;
la información disponible se ha limitado principalmente a las
concentraciones en los peces de zonas cercanas a fuentes
industriales y a casos aislados de contaminación de productos
cárnicos. En truchas de agua dulce se detectaron todos los isómeros
del clorobenceno (de mono- a penta-), en concentraciones que
oscilaban entre 0,1 y 16 µg/kg. En otro estudio, los niveles totales
de clorobencenos en peces de agua dulce variaron de una media de 0,2
mg/kg de grasa en las zonas ligeramente contaminadas a 1,8 mg/kg de
grasa en una industrializada. Existen algunos indicios de que las
concentraciones de clorobencenos en los peces de agua dulce aumentan
a medida que es mayor el grado de cloración del producto. En los
escasos estudios disponibles de algunos peces marinos se señalan
niveles de 1,4-DCB de 0,05 mg/kg (peso fresco).
En los estudios conocidos sobre los niveles de clorobencenos en la
carne y en la leche, limitados principalmente a muestras procedentes
de zonas contaminadas, se han comunicado concentraciones de 0,02-5
µg/kg.
En dos estudios de la leche materna se determinaron
cuantitativamente las concentraciones de todos los clorobencenos,
excepto el MCB. En el primero, el promedio de los niveles de DBC era
de 25 µg/kg de leche, mientras que los valores medios relativos a
los isómeros del TCB y el TeCB y al PeCB fueron inferiores a 5 µg/kg
de leche. Los niveles en el segundo estudio fueron mucho más bajos,
con concentraciones medias que oscilaban entre 1 µg/kilo (1,2,3-TCB
y PeCB) y un máximo de 6 µg/kg (1,3- y 1,4-diclorobenceno).
4.2 Exposicion humana
4.2.1 Poblacion general
De acuerdo con los limitados datos disponibles, la ingestión diaria
de clorobencenos por la población general procede en su mayor parte
del aire, en particular de los compuestos inferiores, más volátiles
(0,2-0,9 µg/kg de peso corporal). La ingestión a partir de los
alimentos en comparación con otras fuentes aumenta al elevarse el
grado de cloración; Los alimentos contribuyen a un porcentaje más
alto de la ingestión diaria total de TeCB y PeCB que el aire. Sin
embargo, los niveles de exposición para este tipo de compuestos son
probablemente inferiores a 0,05 µg/kg de peso corporal. En un número
limitado de estudios se ha puesto de manifiesto que, teniendo en
cuenta el peso corporal, los lactantes alimentados con leche materna
pueden recibir una dosis más alta de clorobencenos que las personas
adultas.
4.2.2 Profesional
Con los datos de que se dispone no es posible cuantificar con
exactitud la exposición profesional a los clorobencenos. Sin
embargo, en una fábrica se encontraron niveles de 1,4-DCB que
oscilaban entre 42 y 288 mg/m3, y en otras instalaciones químicas
las concentraciones de MCB eran de hasta 18,7 mg/m3.
5 Cinetica y metabolismo
Parece que todos los clorobencenos se absorben fácilmente de los
tractos gastrointestinal y respiratorio en el hombre y en los
animales de experimentación; influye en la absorción la posición de
los átomos de cloro en los distintos isómeros del mismo compuesto.
Los clorobencenos se absorben menos fácilmente a través de la piel.
En los animales de experimentación, tras una rápida distribución en
órganos con elevada perfusión, los clorobencenos absorbidos se
acumulan sobre todo en el tejido adiposo, con cantidades menores en
el hígado y en otros órganos. Se ha demostrado que atraviesan la
placenta, y se han detectado en el cerebro del feto. En general, la
acumulación es mayor en el caso de los compuestos más clorados. Sin
embargo, hay una considerable diferencia de acumulación entre los
distintos isómeros del mismo compuesto.
El metabolismo de los clorobencenos en la especie humana y en los
animales de experimentación sigue la vía de la oxidación microsómica
para formar el clorofenol correspondiente. Estos clorofenoles se
pueden excretar en la orina en forma de ácidos mercaptúricos, o bien
como ácido glucurónico o conjugados de sulfato. El TeCB y el PeCB se
metabolizan a menor velocidad y permanecen en los tejidos durante
más tiempo que el grupo de los monocloro- a los triclorobencenos.
Algunos de estos clorobencenos inducen una amplia gama de sistemas
enzimáticos, entre ellos los que participan en las vías de
oxidación, reducción, conjugación e hidrólisis.
En general, la eliminación de los bencenos con mayor grado de
cloración es más lenta que la de los MCB y DCB, y es mayor la
proporción de los compuestos tri-, tetra y pentaclorobencenos que se
eliminan inalterados en las heces. Por ejemplo, el 17% de una dosis
de 1,2,4-TCB se eliminó en las heces al cabo de 7 días, mientras que
el 91-97% del 1,4-DCB se excretó en la orina en forma de metabolitos
a los 5 días. La posición de los átomos de cloro en el anillo
bencénico es también un factor importante del que depende la
velocidad de metabolización y eliminación; los isómeros con dos
átomos de carbono adyacentes sin sustituir se metabolizan y eliminan
más rápidamente.
6. Efectos en los organismos acuaticos del medio ambiente
La información disponible acerca de los efectos de los clorobencenos
en el medio ambiente se centra principalmente en sus efectos agudos
sobre los animales acuáticos. En general, la toxicidad aumenta con
el grado de cloración del anillo bencénico. Mientras que los MCB,
1,2-DCB, 1,3-DCB, 1,2,4-TCB, 1,3,5-TCB y 1,2,4,5-TeCB presentan una
baja toxicidad para los microorganismos, la de los TCB y TeCB es, a
excepción del 1,2,4,5-TeCB, ligeramente superior a la de los otros
compuestos; en algas unicelulares acuáticas, los valores de la CE50
para el crecimiento celular o la producción de clorofila a en 96
horas variaron de más de 300 mg/litro para el MCB hasta
aproximadamente 1 mg/litro para el 1,2,3,5-TeCB. Algunos
invertebrados acuáticos muestran mayor sensibilidad a los
clorobencenos, pero los niveles necesarios para la letalidad en 48 ó
96 horas son todavía próximos, o muy superiores, a 1 mg/litro (por
ejemplo, para Daphnia magna son de 2,4 mg/litro en el caso del
1,2-DCB y de hasta 530 mg/litro en el del 1,2,4,5-TeCB).
La CL50 a las 96 horas para Lepomis macrochirus osciló entre 0,3
mg/litro para el PeCB y 24 mg/litro para el MCB. En ensayos con
embriones-larvas, los límites de toxicidad crónica para los DCB
oscilaron entre 0,76 y 2,0 mg/litro para Pimephales promelas; en
la variedad de Aplodinotus grunniens de los estuarios, los límites
de la toxicidad crónica para el 1,2,4-TCB y 1,2,4,5-TeCB fueron de
0,22 y 0,13 mg/litro respectivamente. En el caso de Carassius
auratus y de la perca atruchada, los alevines recién nacidos
constituyeron la etapa vital m s sensible, con CL50 (a las 96 h)
de 1 y 0,05 mg/litro, respectivamente, para el MCB.
No se conocen datos sobre los clorobencenos en los sistemas
terrestres.
7. Efectos en los animales de experimentacion y en los
sistemas in vitro
Salvo algunas excepciones, los clorobencenos son sólo moderadamente
tóxicos para los animales de experimentación en cuanto a la
toxicidad aguda, y en general la DL50 por vía oral es superior a
los 1000 mg/kg de peso corporal; según los limitados datos
disponibles, la DL50 por vía cutánea es más alta. La ingestión de
una dosis letal produce parálisis respiratoria, mientras que la
inhalación de dosis elevadas causa irritación local y depresión del
sistema nervioso central. La exposición aguda a dosis no letales de
clorobencenos induce efectos tóxicos en el hígado, los riñones, las
glándulas suprarrenales, las adrenales membranas mucosas y el
cerebro, y tiene efectos sobre las enzimas metabolizantes.
Los estudios sobre la irritación de la piel y de los ojos ocasionada
por los clorobencenos se han limitado al 1,2,4-TCB y al 1,2-DCB.
Ambos producen graves molestias, pero no se observaron lesiones
permanentes tras su aplicación directa a los ojos de conejos. El
1,2,4-TCB es ligeramente irritante para la piel, pudiendo ocasionar
dermatitis por contacto repetido o prolongado. No se encontraron
pruebas de sensibilización.
La exposición breve (5-21 días) de ratas y ratones al MCB y a los
DCB en concentraciones del orden de cientos de mg/kg de peso
corporal se tradujo en lesiones hepáticas y en cambios hemáticos
indicativos de lesiones en la médula ósea. Las lesiones hepáticas
fueron también el principal efecto nocivo observado tras la
exposición breve de ratas y ratones a otros clorobencenos
(TCB-PeCB), en dosis ligeramente menores que las utilizadas con el
MCB y los DCB. Varios de los isómeros estudiados indujeron porfiria,
siendo los más activos los que tienen los átomos de cloro en
posición para (es decir, 1,4-DCB, 1,2,4-TCB, 1,2,3,4-TeCB y PeCB).
El orden general de toxicidad observado para los TeCB y el PeCB tras
una exposición breve fue el siguiente: 1,2,4,5-TeCB PeCB 1,2,3,4- y
1,2,3,5-TeCB, lo que guarda una correlación adecuada con los niveles
encontrados en la grasa y en el hígado.
Los estudios de exposición a largo plazo (hasta seis meses) en
varias especies de animales de experimentación pusieron de
manifiesto que la toxicidad de los clorobencenos tendía a aumentar
con el grado de cloración del anillo. Sin embargo, fue considerable
la variación de la toxicidad a largo plazo de los distintos isómeros
de un mismo compuesto. Por ejemplo, el 1,4-DCB demostró ser mucho
menos tóxico que el 1,2-DCB. Se observó una correlación positiva
entre la toxicidad y el grado de acumulación del compuesto en los
tejidos, siendo las hembras menos sensibles que los machos. Los
órganos más afectados fueron el hígado y los riñones; con dosis más
elevadas se describieron efectos en el sistema hematopoyético, y en
estudios con el 1,2,4,5-TeCB y el PeCB se observó toxicidad en el
tiroides.
En un bioensayo para determinar la carcinogenicidad del MCB, aumentó
la frecuencia de nódulos neoplásicos hepáticos en el grupo de dosis
alta (120 mg/kg de peso corporal) de ratas macho F344, pero no hubo
aumento de la incidencia de tumores asociada con el tratamiento en
ratas hembra F344 o en ratones macho o hembra B6C3F1. No se
obtuvieron pruebas de la carcinogenicidad del 1,2-DCB en ratas
machos o hembras F344 ni en ratones B6C3F1 (60 ó 120 mg/kg de peso
corporal).
En un bioensayo para determinar la carcinogenicidad del 1,4-DCB, se
registró un incremento de la formación de adenocarcinomas de las
células de los túbulos renales relacionado con la dosis en ratas
macho F344 y un aumento de los carcinomas y adenomas hepatocelulares
en ambos sexos de ratones B6C3F1. No se comunicaron signos de
carcinogenicidad en ratas Wistar macho y hembra, ni en hembras de
raton suizo tras la inhalación de dosis ligeramente más altas de
1,4-DCB (estimadas en 400 mg/kg al día para las ratas y 790 mg/kg al
día para los ratones) durante períodos más cortos. Sin embargo, los
datos disponibles indican que la inducción de tumores renales por el
1,4-DCB en ratas macho F344 y la inducción asociada de nefropatia
grave y de la formación de gotitas hialinas son respuestas
específicas de la especie y del sexo, asociadas a la reabsorción de
alfa-2-microglobulina.
Los datos disponibles son insuficientes para valorar la
carcinogenicidad de los bencenos con mayor grado de cloración (de
tri- a penta-).
Aunque los datos obtenidos en ensayos in vitro e in vivo para
isómeros distintos del 1,4-DCB son limitados, los clorobencenos no
parecen tener efectos mutagénicos. Teniendo en cuenta la base de
datos más amplia del 1,4-DCB, se puede concluir que este compuesto
carece de potencial mutagénico, tanto in vivo como in vitro.
No hay pruebas de teratogenicidad de los clorobencenos en ratas y en
conejos. La administración de MCB y de distintos DCB a ratas o
conejos por vía respiratoria en concentraciones superiores a
2000 mg/m3 (unos 550 mg/kg de peso corporal al día) y por vía oral
a concentraciones de >500 mg/kg de peso corporal dio como resultado
efectos embriotóxicos y fetotóxicos de poca consideración. Sin
embargo, esas dosis fueron claramente tóxicas para la madre. Aunque
hay algunas pruebas de embriotoxicidad y fetotoxicidad de los TCB,
los TeCB y los PeCB en dosis que no son tóxicas para la madre, los
datos disponibles son contradictorios.
8. Efectos en el ser humano
8.1 Poblacion general
Los informes acerca de los efectos de los clorobencenos en la
población general se limitan a los casos notificados de accidentes
y/o de uso indebido de productos con los bencenos menos clorados
(MCB, 1,2-DCB, 1,4-DCB y un isómero de TCB sin especificar). Se
dispone de información escasa o nula sobre las dosis, la pureza
química o las relaciones dosis:tiempo y no es posible determinar
cuantitativamente los efectos observados, como leucemia
mieloblástica, rinitis, glomerulonefritis, granulomatosis pulmonar,
mareo, temblor, ataxia, polineuritis e ictericia.
No se han comunicado de estudios epidemiológicos sobre los efectos
de los clorobencenos en la salud de la población general.
8.2 Exposicion profesional
Entre los síntomas y los signos clínicos derivados de la exposición
excesiva durante la fabricación y el uso de los clorobencenos cabe
mencionar los siguientes: efectos en el sistema nervioso central
(SNC) e irritación de los ojos y del tracto respiratorio superior
(MCB); trastornos hematológicos (1,2-DCB); y efectos en el SNC,
endurecimiento de la piel y trastornos hematológicos, incluso anemia
(1,4-DCB). Sin embargo, tales síntomas proceden sólo de informes de
casos aislados y son difíciles de determinar cuantitativamente,
puesto que se dispone de escasa información relativa a los niveles
reales, la pureza química o las relaciones dosis:tiempo.
Los pocos estudios epidemiológicos sobre trabajadores expuestos a
clorobencenos que se han publicado se refieren solamente al MCB, el
1,2-DCB, el 1,4-DCB y el 1,2,4,5-TeCB. Aunque se ha informado de
efectos en el sistema nervioso, en el desarrollo neonatal y en la
piel tras la exposición al MCB, ninguno de los tres estudios fue
suficiente para evaluar el riesgo, a causa de problemas
metodo-lógicos, como la valoración de la exposición, las
exposiciones mixtas y la ausencia de grupos testigo. Una crítica
parecida merece el estudio sobre el 1,4-DCB, en el que se informó de
irritación ocular y nasal, así como el estudio en el que se
describieron aberraciones cromosómicas como consecuencia de la
exposición a niveles no especificados de 1,2-DCB y 1,2,4,5-TeCB.
9 Conclusiones
Si se aplica una práctica industrial correcta, los riesgos asociados
con la exposición profesional a los clorobencenos se pueden
considerar mínimos. La evaluación del riesgo en la actualidad pone
de manifiesto también que las concentraciones actuales de
clorobencenos en el medio ambiente representan un riesgo
insignificante para la población general, excepto en el caso de uso
indebido de productos que contienen clorobencenos o de su liberación
incontrolada en el medio ambiente. Sin embargo, esta valoración se
basa en datos limitados de vigilancia, y se necesita más información
para justificar esta conclusión. Se debería considerar, sin embargo,
la posibilidad de reducir el uso y la eliminación generalizados de
clorobencenos por los siguientes motivos:
a) Los clorobencenos pueden actuar como precursores de la
formación de dibenzodioxinas policloradas/dibenzofuranos
policlorados (DDPC/DFPC), por ejemplo en los procesos de
incineración.
b) Estos productos químicos pueden ocasionar alteraciones del
sabor y el olor del agua de bebida y del pescado.
c) Los residuos persisten en los sedimentos y suelos anaerobios
ricos en materia orgánica y en el agua subterránea.
En la mayor parte de los clorobencenos, la evaluación del riesgo se
ha basado en efectos no neoplásicos. Sin embargo, los efectos
neoplásicos se tuvieron en cuenta en la valoración del riesgo del
MCB y el 1,4-DCB. Los datos disponibles indican que el aumento
observado de los tumores renales en ratas debido al 1,4-DCB es una
respuesta específica de la especie y del sexo, probablemente sin
importancia para la especie humana. De acuerdo con las pruebas de
aumento de la replicación del ADN en el hígado de ratón y la mayor
incidencia de adenomas y carcinomas hepato-celulares en ratones, el
1,4-DCB puede actuar como agente carcinógeno no genotóxico en el
hígado de los roedores. La mayor incidencia de nódulos neoplásicos
hepáticos observada en el grupo de ratas macho que recibió dosis
altas en un bioensayo de carcinogenicidad indica que también el MCB
puede ser un agente carcinógeno no genotóxico.
Hexachlorobenzene is the subject of a separate Environmental Health
Criteria publication and will not be evaluated here.
2.1.2 Technical product
There are no widely established trade specifications for commercial
chlorobenzenes. Pure commercial monochlorobenzene may contain 0.05 %
or less of benzene and up to 0.1 % of dichlorobenzenes. Technical
grade 1,2-dichlorobenzene contains up to 19 % of the other 2
dichlorobenzene isomers, 1 % of trichlorobenzenes, and up to 0.05 % of
monochlorobenzene, while purified 1,2-dichlorobenzene contains up to
0.05 % of monochlorobenzene and 0.2 % of 1,2,4-trichlorobenzene.
Technical grade 1,4-dichlorobenzene contains up to a total of 0.1 % of
mono- and trichlorobenzenes and 0.5 % of each of the other
dichlorobenzene isomers. Commercial 1,2,4-trichlorobenzene may contain
up to 0.1 % of mono-chlorobenzene, 0.5 % of dichlorobenzenes, and
0.5 % of tetrachlorobenzenes (Kao & Poffenberger, 1979).
Polychlorinated dibenzodioxins or dibenzofurans were not detected in
trichlorobenzenes, tetrachlorobenzenes, or penta-chlorobenzene (Buser,
1979).
2.2 Physical and Chemical Properties
The physical and chemical properties of the chlorobenzenes (mono- to
penta-) are presented in Table 2.
MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB are colourless liquids, while all
other congeners are white crystalline solids at room temperature. In
general, the solubility of chlorobenzenes in water is poor (decreasing
with increasing chlorination), flammability is low, the octanol/water
partition coefficients are moderate to high (increasing with
increasing chlorination), and vapour pressures are low to moderate
(decreasing with increasing chlorination).
2.3 Organoleptic Properties
The odour and taste thresholds for different isomers of the same
chlorobenzene appear to be similar: 0.01-0.02 mg/litre for MCB and
0.001-0.002 mg/litre for both 1,2-DCB and 1,4-DCB (Varshavskaya,
1968). Piet et al. (1980) reported that the odour thresholds for 1,2-
and 1,4-dichlorobenzenes in Rhine tap water were 10 and 0.3 µg/litre
respectively, while 1,2,4-TCB was detected at a level of 5 µg/litre.
Using available experimental data, Amoore & Hautala (1983) determined
water-dilution odour thresholds for MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB to be 0.050, 0.024, 0.011, and 0.064 mg/litre (ppm),
respectively. Odour thresholds in air for these compounds are 0.68,
0.30, 0.18, and 1.4 µlitre/litre (ppm), respectively. Fomenko (1965)
reported that the thresholds for smell and taste for 1,2,4,5-TeCB were
0.006 mg/litre and 0.0064 mg/litre, respectively.
2.4 Conversion Factors
At 25 °C and 101.3 kPa, the conversion factors for chlorobenzenes in
air are as follows:
monochlorobenzene: 1 ppm=4.55 mg/m3: 1 mg/m3=0.22 ppm
dichlorobenzenes: 1 ppm=6.00 mg/m3: 1 mg/m3=0.17 ppm
trichlorobenzenes: 1 ppm=7.42 mg/m3: 1 mg/m3=0.13 ppm
tetrachlorobenzenes: 1 ppm=8.83 mg/m3: 1 mg/m3=0.11 ppm
pentachlorobenzene: 1 ppm=10.24 mg/m3: 1 mg/m3=0.10 ppm
Table 1. Information on the identity of chlorobenzenes
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
Monochlorobenzene MCB C6H5Cl 112.6 chlorobenzene
(108-90-7) phenyl chloride
1,2-dichlorobenzene 1,2-DCB C6H4Cl2 147.0 ortho-dichlorobenzene
(95-50-1) o-dichlorobenzene
1,3-dichlorobenzene 1,3-DCB C6H4Cl2 147.0 meta-dichlorobenzene
(541-73-1) m-dichlorobenzene
1,4-dichlorobenzene 1,4-DCB C6H4Cl2 147.0 para-dichlorobenzene
(106-46-7) p-dichlorobenzene
1,2,3-trichlorobenzene 1,2,3-TCB C6H3Cl3 181.5 vic-trichlorobenzene
(87-61-6) v-trichlorobenzene
1,2,6-trichlorobenzene
1,2,4-trichlorobenzene 1,2,4-TCB C6H3Cl3 181.5 1,2,4-trichlorobenzol
(120-82-1)
1,3,5-trichlorobenzene 1,3,5-TCB C6H3Cl3 181.5 s-trichlorobenzene
(108-70-3) TCBA
sym-trichlorobenzene
1,2,3,4-tetrachlorobenzene 1,2,3,4-TeCB C6H2Cl4 215.9 benzene, 1,2,3,4-
(634-66-2) tetrachloro-
1,2,3,5-tetrachlorobenzene 1,2,3,5-TeCB C6H2Cl4 215.9 benzene, 1,2,3,5-
(634-90-2) tetrachloro-
Table 1 (continued)
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
1,2,4,5-tetrachlorobenzene 1,2,4,5-TeCB C6H2Cl4 215.9 benzene, tetrachloride
(95-94-3) benzene, 1,2,4,5-
tetrachloro-
s-tetrachlorobenzene
Pentachlorobenzene PeCB C6HCl5 250.3 1,2,3,4,5-
(608-93-5) pentachloro-benzene
QCB
a Chemical Abstract Services registry number.
b R.M.M. - Relative molecular mass.
Table 2. Physical and chemical properties
Solubility Log Henry's Soil Blood/air
Compound Melting Boiling Vapour Densityf in water at octanol/water Law sorption partition
point point pressure 25 °C (mol/ partition constant coefficient coefficientj
(°C)a (°C)a at 25 °C litre) coefficientg (kPa m3/ (KOC)i
(Pa) (mg/litre)g mol)h
MCB -45.6 132.0 1665b 1.105820/4 2.6x10-3 2.98 0.377 466 30.8
(293)
1,2-DCB -17.0 180.5 197b 1.304820/4 6.2x10-4 3.38 0.198 987 423
(91.1)
1,3-DCB -24.7 173.0 269b 1.288420/4 8.4x10-4 3.48 0.366 1070 201.4
(123)
1,4-DCB 53.1 174.0 90c 1,247520/4 2.1x10-4 3.38 0.160 1470 NA
(30.9)
1,2,3-TCB 53.5 218.5 17.3d NA 6.7x10-5 4.04 0.306 3680 NA
(12.2)
1,2,4-TCB 17.0 213.5 45.3d 1.454220/4 2.5x10-4 3.98 0.439 2670 NA
(45.3)
1,3,5-TCB 63.5 208761 24.0d NA 2.2x10-5 4.02 0.233 NA NA
(3.99)
1,2,3,4-TeCB 47.5 254.0 5.2c NA 5.6x10-5 4.55 0.261 NA NA
(12.1)
1,2,3,5-TeCB 54.5 246.0 9.8c NA 1.3x10-5 4.65 0.593 8560 NA
(2.81)
Table 2 (continued)
Solubility Log Henry's Soil Blood/air
Compound Melting Boiling Vapour Densityf in water at octanol/water Law sorption partition
point point pressure 25 °C (mol/ partition constant coefficient coefficientj
(°C)a (°C)a at 25 °C litre) coefficientg (kPa m3/ (KOC)i
(Pa) (mg/litre)g mol)h
1,2,4,5-TeCB 139.5 243.6 0.72c NA 1.0x10-5 4.51 0.261 6990 NA
(2.16)
PeCB 86.0 277.0 133 at 1.834216.5 3.3x10-6 5.03 0.977 58 700 NA
98.6 °Ce (0.83)
a Melting points are rounded to the nearest 0.1 °C; Boiling points are at atmospheric pressure (760 mm), unless otherwise indicated
by a superscript (Weast, 1986).
b Vapour pressures obtained from the Antoine equation: log10p(kPa) = A-B/(T+C) - 0.8751 presented by Kao & Poffenberger (1979),
together with the values for the Antoiine constants (A,B,C).T = temperature in °C.
c From: MacKay et al. (1982). The value was derived from experimental data obtained above 25 °C and extrapolated to 25 °C, taking into
account the phase change from liquid to solid.
d Vapour pressures obtained from the equation: log10p(10-3torr) = -(A/T) + B and values for the constants (A and B) are presented by
Sears & Hopke (1949).T = absolute temperature.
e From: Stull (1947).
f Density is relative to water, otherwise it has the dimensions g/ml. A superscript indicates the temperature of the liquid and a
subscript indicates the temperature of water to which the density is referred (Weast, 1986).
g From: Miller et al. (1984).
h From: MacKay & Shiu (1981).
i Derived from: Karlokoff et al. (1979).
j From: Sato & Nakajima (1979).
NA - values either not given in the reference indicated or not found in the literature.
2.5 Analytical Methods
Some methods for the sampling and determination of chlorobenzenes in
various environmental media and human tissues and fluids are
presented in Table 3.
The analytical technique of choice for the determination of
chlorobenzenes in environmental samples is gas-liquid chromatography
(GLC). However, the methods of collection and preparation of samples
for GLC analysis vary considerably, depending on the medium and the
laboratory. Columns with silicone-based stationary phases or Tenax
resins, and electron capture detectors, appear to be the most widely
used.
Tenax-GC resins appear to be the most commonly used absorbent for
the air sampling of chlorobenzenes (Sievers et al., 1980; Krost et
al., 1982; Pellizzari, 1982), though XAD resins have also been used
(Langhorst & Nestrick, 1979). Air pollutants collected on Tenax-GC
resins can be desorbed directly on to the GLC column by heating the
absorber. XAD resins can be extracted with carbon tetrachloride, an
aliquot of which can then be injected into a gas chromatograph
(Langhorst & Nestrick, 1979).
Solvent extraction is a simple and effective technique for
recovering chlorobenzenes from water samples. Hexane, pentane, and a
1:1 mixture of cyclohexane and diethyl ether have been identified as
suitable extraction solvents for these compounds (Oliver & Bothen,
1980; Piet et al., 1980; Otson & Williams, 1981). Alternatively,
preconcentration of the chlorobenzenes on organic resins, such as
Chromosorb 102 and Tenax-GC, is also effective (Oliver & Bothen,
1980; Pankow & Isabelle, 1982). The purge-trap method is also often
used to concentrate the volatile halogenated benzenes before
analysis using GC (Jungclaus et al., 1978; Pereira & Hughes, 1980;
Otson & Williams, 1982).
The extraction of chlorobenzenes from aquatic sediments or soil can
be achieved by solvent or Soxhlet extraction (Oliver & Bothen, 1982;
Lopez-Avila et al., 1983; Onuska & Terry, 1985). Solvents commonly
used are acetone and/or hexane. The extract is generally dried using
sodium sulfate, followed by clean-up on a Florisil column before GLC
analysis.
For the detection of chlorobenzenes in fish samples, solvent or
Soxhlet extraction with subsequent clean-up on Florisil and GC
analysis with electron capture detection have commonly been used
(Lunde & Ofstad, 1976; Kuehl et al., 1980; Oliver & Bothen, 1982).
Vacuum extraction and the direct purge and trap method have also
been used to quantify levels of MCB in fish tissue (Hiatt, 1981).
Table 3. Analytical methods for chlorobenzenesa
Matrix Sampling, extraction Analytical method Detection limitsb Reference
air continuous flow, aircraft trap purged in oven at NA Sievers et al. (1980)
sampling port; sorbent traps 220 °C with He; capillary
with 4 changes column (30 m x 0.3 mm),
gas chromatography-mass
spectrometry (GC-MS) data
system
air 4-h samples collected on silanized glass column; GC MCB 3.2 Langhorst & Nestrick
Amberlite XAD-Z resin at with photoionization detector DCBs 4.2 (1979)
100-200 ml/min; desorbed TCBs 5.9
TeCB 7.1
PeCB 9.2
water 500 ml with chromosorb 102, or GC analysis, glass capillary MCB 0.5 Oliver & Bothen
3.1 litres with 75 ml pentane columns; electron capture DCBs 0.001 (1980)
TCBs 0.0001
TeCB 0.00005
PeCB 0.00001
water 40 ml with automated purge and GC analysis with FID Otson & Williams
trap; inert gas bubbled through simultaneous use of flame MCB < 0.1 (1982)
purged compounds directly on ionization detector (FID) 1,2-DCB 0.2
to column and Hall electrolytic 1,3-DCB 0.1
conductivity detector (HECD) 1,4-DCB 0.1
HECD
MCB 0.1
1,2-DCB 0.1
1,3-DCB 0.1
1,4-DCB 0.1
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
water liquid-liquid extraction of 120 ml GC analysis using 63Ni FID Otson & Williams
water with 38:1 water:hexane electron capture detector MCB 5 (1981)
(ECD), FID or HECD 1,2-DCB 2
1,4-DCB 2
1,2,4-TCB 2
ECD
MCB ND
1,2-DCB 5
1,4-DCB 5
1,2,4-TCB < 1
HECD
MCB 1
1,2-DCB < 1
1,4-DCB < 1
1,2,4-TCB < 1
water extraction of 4 litres water with compounds desorbed directly NA Pankow & Isabelle
Tenax-GC 35/60 mesh; from glass column of (1982)
centrifugation or vacuum Tenax-GC into GC by flash
dessication of wet cartridge heating; flame ionization
to remove water detector
water extraction of 1-litre sample with glass capillary column NA Piet et al. (1980)
20 ml cyclohexane-diethylether coupled to electron detector
(1:1) on line with FID detector
water adsorption on 1 g of activated GC analysis, FID detector MCB concentration range: Blanchard & Hardy
charcoal in exposure chamber; 0.058-19.4 mg/litre (1985)
charcoal desorbed with 5 ml of
carbon disulfide for >30 min
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
sediment Soxhlet extraction of 10-15 g GC analysis on glass MCB 1500 Oliver & Bothen
with 41% hexane/59% acetone; capillary columns; electron DCBs 5 (1982)
back-extracted with water to capture detector TCBs 0.4
remove acetone, through Na2SO4 TeCBs 0.2
and evaporated to 10 ml; PeCB 0.05
clean-up on Na2SO4 + deactivated
Florisil column
sediment 10 g sediment treated by steam identification by relative 1,3-DCB 1.5 Onuska & Terry
distillation, soxhlet or retention-time matching after 1,3,5-TCB 1.0 (1985)
ultrasonic extraction; clean-up ECD 1,2,4-TCB 0.8
with mercury only needed when 1,2,3-TCB 0.8
sulfur present 1,2,3,5-TeCB 0.5
1,2,4,5-TeCB 0.5
1,2,3,4-TeCB 0.5
PeCB 0.4
fish 15 g fish soxhlet extracted; GC analysis on glass MCB 1500 Oliver & Bothen
clean-up with combination of capillary column, ECD DCBs 5 (1982)
alumina, silica gel, florisil and detector TCBs 0.4
acidified florisil (fish), after TeCBS 0.2
removal of lipids PeCB 0.05
blood hexane extraction on Synder borosilicate glass column, DCBs approx. 2 Bristol et al.
column using 3 g for GC and GC analysis; electron capture TCBs approx. 1.5 (1982)
710 g for GC/MS detector or GC/MS system TeCBs approx. 1
PeCB approx. 1
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
blood CCl4 extraction of 5 g of blood silanized glass column; GC Blood Langhorst & Nestrick
urine or 20 g urine, silica gel column analysis with photoionization MCB approx.23 (1979)
chromatography (CCl4 eluent) detector DCBs approx. 4
TCBs approx. 5
TeCBs approx. 6
PeCB approx. 9
Urine
MCB approx. 6
DCBs approx. 6
TCBs approx. 1
TeCBs approx. 2
PeCB approx. 2
blood 0.1-1 ml GC sample diluted to Tenax adsorbent heated and NA Balkon & Leary (1979)
urine 5 ml with water and placed in a volatiles analysed by GC/MS
bubbler for purging on to Tenax for detection and
in liquid sample concentrator identification in a screening
procedure
blood hexane/isopropanol extraction of GC analysis, electron capture NA Lunde & Bjorseth
approximately 25 g; H2SO4 detector (1977)
digestion of hexane phase
adipose tissue extraction of tissue with GC analysis, capillary DCBs ND LeBel & Williams
acetone-hexane, then fractionated column, ECD detection; 1,3,5-TCB 11.0 µg/kg (1986)
by gel permeation chromatography compounds 5.9 µg/kg
(GPC); clean-up on Florisil column confirmed by gas 1,2,3,5-TeCB 13.1 µg/kg
chromatography-mass spectrometry 1,2,3,4-TeCB 4.8 µg/kg
with selected ion monitoring PeCB 1.9 µg/kg
Table 3 (continued)
Matrix Sampling, extraction Analytical method Detection limitsb Reference
urine solutions stirred and heated to Analysis by GC/FID MCB Michael et al.
blood 50 °C, headspace above the blood 98c (1980)
adipose tissue solution purged on to Tenax GC urine 86c
cartridges; cartridges dessicated adipose 13c
using anhydrous calcium sulfate
and thermally desorbed DCB
blood 86c
urine 79c
adipose 57c
urine 5 ml samples: GC equipped with an electron urine 94c McKinney et al.
blood Urine: acidified with 0.5 ml capture (tritium) detector blood 78c (1970)
concentrated HCl, then extracted
with benzene Extracts dried over
anhydrous sodium sulfate
Blood: plasma extracted with
benzene, then dried with
anhydrous sodium sulfate
adipose tissue 2-g samples extracted with analysis by GC with electron NA Mes et al. (1982)
benzene:acetone (1:19 v/v); capture detector;
repeated evaporation with hexane confirmation by GLC; monitored
to remove traces of benzene; by mass spectrometry
fat-free extract chromatographed
on Florisil-silicic acid column
Table 3 (continued)
a Often, the primary aim of the analyses was quantification of organochlorine compounds, other than chlorobenzenes. In these cases,
the clean-up procedures were quite complicated, because of the need to separate different organochlorine pesticide residues, prior
to chromatographic analysis.
b Detection limits reported in µg/m3 for air and µg/litre or µg/kg for other media, unless noted otherwise.
NA - information not available in the paper.
ND - not detected during analysis.
c Indicates recovery percentages from spiked samples.
Solvent extraction is also used in the determination of
chlorobenzenes in biological matrices, such as blood and urine. For
less volatile compounds (tri-, tetra-, and pentachlorobenzenes),
solvent extraction is followed by column chromatographic clean up
and quantification (Lamparski et al., 1980; Mes et al., 1982). For
the more volatile compounds (mono-, dichlorobenzenes), a modified
purge-trap method with a capillary GC can be used (Michael et al.,
1980). The chlorobenzenes are then quantified using a GC with
detection by electron capture (McKinney et al., 1970; Morita et al.,
1975; Lunde & Bjorseth, 1977), photoionization (Langhorst &
Nestrick, 1979), or mass spectrometry (Balkon & Leary, 1979; Bristol
et al., 1982; LeBel & Williams, 1986).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
Natural sources of chlorobenzenes in the general environment have
not been identified; however, 1,2,3,4-TeCB has been identified in
the oil of a marsh grass (Miles et al., 1973).
3.2 Man-made Sources
3.2.1 Production
Monochlorobenzene and the dichlorobenzenes are produced commercially
by the direct chlorination of benzene in the liquid phase, in the
presence of a Lewis acid catalyst, such as ferric chloride. In the
liquid-phase chlorination of monochlorobenzene, 1,2-, and
1,4-dichlorobenzenes are the predominant products. Trichlorobenzenes
result from the chlorination of dichlorobenzenes with ferric
chloride, while tetrachlorobenzenes are produced by the addition of
chlorine to trichlorobenzenes in the presence of an aluminium
catalyst. Tetrachlorobenzenes can be used as the precursor in
pentachlorobenzene production (US EPA, 1985). Pentachloro-benzene is
also produced by the denitrification of penta-chloronitrobenzene and
the reductive dechlorination of hexa-chlorobenzene (Renner & Mücke,
1986).
About 50% of the world production of all chlorobenzenes
(estimated from data in US EPA (1985) to be 568 x 106 kg in 1983)
is manufactured in the USA. The remainder is produced mainly in
Western Europe and Japan. Monochlorobenzene makes up approximately
70 % of total world production of all chlorobenzenes.
Data on current, global chlorobenzene production volumes are not
available in readily retrievable references. Summaries of production
levels in 1980 and 1983 have been published and are presented in
Table 4 (IARC, 1982; US EPA, 1985). Although these may provide some
indication of present production levels, it appears that PeCB
production has ceased within the USA, and that the use of
chlorobenzenes as chemical intermediates has decreased. Therefore,
the actual level of production is probably less than that shown in
Table 4.
No information was found on the production of TCB, TeCB, and PeCB
congeners outside the USA. However, in 1979, the estimated
production of 1,4-DCB in Japan was 27.5 x 106 kg and that of
1,2-DCB was 13 x 106 kg (IARC, 1982).
Table 4. Production levels in the USA and possible uses of chlorinated benzenes
Chemical Major usesa Estimated annual
production in the USA
MCB Intermediate in the manufacture of chloronitrobenzenes, diphenyl 130 x 106 kg in 1980
oxide, DDT, and silicones; as a process solvent for methylene
diisocyanate, adhesives, polishes, waxes, pharmaceutical products,
and natural rubber; as a degrading solvent
1,2-DCB In the manufacture of 3,4-dichloroaniline; as a solvent for a wide 22 x 106 kg in 1980
range of organic materials and for oxides of non-ferrous metals; as a
solvent carrier in the production of toluene diisocyanate; in the
manufacture of dyes; as a fumigant and insecticide; in degreasing
hides and wool; in metal polishes; in industrial odour control; in
cleaners for drains
1,3-DCB As a fumigant and insecticide NA
1,4-DCB As a moth repellent, general insecticide, germicide, space deodorant; 24 x 106 kg in 1980
in the manufacture of 2,5-dichloroaniline and dyes; as a chemical
intermediate; in pharmaceutical products; in agricultural fumigants
1,2,3-TCB Apart from use as a chemical intermediate, the uses are the same as 23-74 x 103 kg
those 1,2,4-trichlorobenzene
1,2,4-TCB As an intermediate in the manufacture of herbicides; dye carrier, 1.2-3.7 x 106 kg
dielectric fluid; solvent; heat-transfer medium
1,3,5-TCB Solvent for products melting at high-temperatures; coolant in 1.1-2.1 x 105 kg
electrical insulators; heat-transfer medium, lubricant, and synthetic
transformer oil; termite preparation and insecticide; in dyes
Table 4 (continued)
Chemical Major usesa Estimated annual
production in the USA
1,2,3,4-TeCB Component in dielectric fluids; in the synthesis of fungicides NA
1,2,3,5-TeCB NA NA
1,2,4,5-TeCB Intermediate for herbicides and defoliants; insecticide; NA
moisture-resistant impregnant; in electric insulation; in packing
protection
PeCB Formerly in a pesticide used to combat oyster drills; chemical Not manufactured in
intermediate the USAa
a From: US EPA (1985).
NA - not available.
The total production capacity for all chlorobenzenes in Western
Europe during 1980 was estimated to be greater than 208 x 106 kg
(IARC, 1982).
Although data on production levels are scarce, it is apparent from
available information that chlorobenzenes (in particular MCB and
DCBs) are produced in high volumes. Use patterns shown in Table 4,
and estimated losses to the environment shown in Table 5, indicate a
high potential for human exposure and environmental contamination.
Table 5. Estimated quantities (kg) of chlorobenzenes lost to the environment
during manufacture in relation to total 1983 productiona
Chlorobenzene Losses during Losses to Total production
manufacture environment
MCB 1.9-3.0 x 105 1.5-2.6 x 105 130 x 106
1,2-DCB 1.1-2.1 x 105 30 x 103 22 x 106
1,3-DCB 2-6 x 102 NA NA
1,4-DCB 1.8-2.8 x 105 1.7-2.7 x 105 24 x 106
1,2,3-TCB 0.6-2 x 103 <1 x 102 23-74 x 103
1,2,4-TCB 3-10 x 103 3-9 x 102 1.2-3.7 x 106
1,3,5-TCB import import 1.1-2.1 x 105
TeCB NA NA NA
PeCB not manufactured NA NA
a Values calculated from US EPA (1985).
NA - data not available.
3.2.2 Uses
Use patterns may vary considerably among countries. A summary of the
uses of chlorinated benzenes in the USA is presented in Table 4.
Chlorobenzenes are used mainly as intermediates in the synthesis of
other chemicals, and as pesticides. The 1,4-DCB isomer is commonly
used in space deodorants and moth repellents, and several of the
higher chlorinated benzenes (TCBs, 1,2,3,4-TeCB) have been used in
dielectric fluids.
MCB also has potential as a functional fluid in external combustion
Rankine engines (Curran, 1981) and as a component in heat transfer
fluids in solar energy collectors (Boy-Marcotte, 1980).
The 1,4-DCB isomer is also being used in the USA as an intermediate
in the production of polyphenylene sulfide resin, an engineering
plastic with electrical and automotive applications.
3.2.3 Sources in the environment
Incineration of organochlorine and hydrocarbon polymers in the
presence of chlorine may result in the atmospheric release of
chlorobenzenes, though quantities are small in relation to the total
mass of carbon compounds incinerated (Ahling et al., 1978;
Lahaniatis et al., 1981a). Incineration of chlorobenzenes most
probably leads to the formation of polychlorinated dibenzodioxins
and dibenzofurans, as indicated by experimental studies on the
pyrolysis of various TCBs, TeCBs, and PeCB (Buser, 1979). Although,
in experimental studies, chlorobenzenes have been formed in
reactions between benzene and sodium hypochlorite (Hofler et al.,
1983), evidence that they are generated during public water
treatment is slight (Otson et al., 1982a).
On the basis of measurements of concentrations in flue gases from
all municipal waste incinerators in Sweden (N=24), the maximum
contribution of chlorobenzenes (di- to hexa-) to ambient air was
calculated, in 1985, to be 590 kg (Ahlborg & Victorin, 1987).
Average emissions of total chlorobenzenes from small-scale wood
burners for dry wood, in closed fireplace ovens, during 2-h sampling
periods, ranged from 24 to 80 µg/kg dry fuel (Rudling et al., 1980).
Several of the chlorinated benzenes have been identified as
microbial metabolites of lindane degradation (Macholz & Kujawa,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The transport and fate of chlorobenzenes in the environment has not
been well characterized. However, it is possible to draw some
conclusions based on the physical and chemical properties of the
compounds and the results of a limited number of laboratory and
field studies.
4.1 Transport and Distribution
The water solubility, saturated vapour pressure, and partition
coefficients (Henry's Law constant, KH; soil sorption, Koc;
octanol/ water, Kow; blood/air), useful for the prediction of the
transport and distribution of the chlorobenzenes in the environment,
are presented in Table 2.
As shown by Henry's Law constant (KH - the equilibrium
distribution coefficient of a compound between air and water), all
chlorobenzenes released into the aquatic environment will evaporate
preferentially from water to the atmosphere, despite their high
relative molecular masses and comparatively low vapour pressures
(MacKay & Wolkoff, 1973). From these data, it can also be predicted
that the preferential distribution from water to air will decrease
with increasing chlorination. In a study on the volatility of MCB in
a model aquatic ecosystem, 96% of the compound was released to the
atmosphere (Lu & Metcalf, 1975). In experimental studies by Garrison
& Hill (1972), 99% of the test compounds MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB had evaporated from aerated distilled solutions within 4
h. In non-aerated solutions, evaporation was complete within 72 h.
The results of a 1-year field study on Lake Zurich, Switzerland,
confirmed that most of the 1,4-DCB present in the water was
transferred to the atmosphere. The half-life of the compound was
estimated to be approximately 100 days, 67% being lost to the
atmosphere; 2% entering lake sediments and 31% being present in the
lake outflow (Schwarzenbach et al., 1979). Wilson et al. (1981)
studied the transport of a mixture in water of more than 10 organic
chemicals, including MCB, 1,4-DCB, and 1,2,4-TCB, through a column
of sandy soil having a low organic matter content, over a 21-day
period. They reported that up to 50% of the MCB evaporated and
approximately 50% of all 3 chlorobenzenes was degraded or was
unaccounted for, indicating that the compounds are likely to leach
into ground water.
4.2 Persistence and Fate
The chlorobenzenes are environmentally persistent compounds, the
most likely degradation mechanisms being photochemical reactions and
microbial action. While bioconcentration has been demonstrated, the
potential for biomagnification in food chains has not been
investigated. Soils that are rich in organic matter and aquatic
sediments are probably the major environmental sinks for these
compounds.
4.2.1 Persistence
In water, 1,2-DCB and 1,2,4-TCB are considered moderately persistent
compounds with half-lives ranging from 1 day in rivers to 10 days in
lakes and 100 days in ground waters (Zoeteman et al., 1980).
Concentrations may be rapidly reduced with aerobic biological
degradation or volatilization, but chlorobenzenes are extremely
persistent under anaerobic conditions, or where volatilization
cannot occur, i.e., in ground water.
Turbulence is a major factor in the elimination of these compounds
from surface waters. Turbulence increases volatilization and
bio-degradation. It may also lead to more rapid photochemical
degradation through the propagation of sensitized photolysis and the
increased frequency of exposure of water particles to surface
sunlight (Zoeteman et al., 1980).
Wakeham et al. (1983) studied the fate and persistence of MCB,
1,4-DCB, and 1,2,4-TCB in tanks containing seawater and associated
planktonic and microbial communities, with simulated tidal
turbulence and seasonal temperature regimes (spring, summer,
winter). It was suggested that removal processes other than
volatilization, such as biodegradation and sorption on to particles,
are probably not very important for 1,4-DCB and 1,2,4-TCB, but that
MCB is subject to rapid biodegradation under the relatively warm
spring and summer water temperatures, when microbial activity is
greater than in winter.
Chlorobenzenes in the air are degraded by chemical or sunlight-
catalysed reactions, or they may be adsorbed onto particles that
settle or are removed with rain. In a 2-week study on air samples
from California and Arizona, Singh et al. (1981) estimated the
residence times of MCB, DCBs, and an unspecified TCB isomer to be
13, 18.6, and 116.0 days, respectively.
In soils, the DCBs, TCBs, and PeCB are usually resistant to
micro-bial degradation; primary degradation products are the
chloro-phenols (Ballschmiter & Scholz, 1980). In experiments using
radiolabelled 1,2,3- and 1,2,4-TCBs on fresh field soil, the
observed degradation rates were very slow, 0.35 and 1.00 nmol/day
per 20 g soil, respectively (Marinucci & Bartha, 1979). These
investigators also observed that evaporation of the chlorobenzenes
was reduced by increasing the amounts of organic material in the
soil. In another experiment using 14C-labelled MCB, 1,2- and
1,4-DCBs, and 1,2,3- and 1,2,4-TCBs in soil, Haider et al. (1974)
found that 18.3%, 1.1% and 20.3% of MCB, DCBs, and TCBs,
respectively, were released as carbon dioxide.
4.2.2 Abiotic degradation
The higher chlorinated chlorobenzenes are not particularly reactive
compounds and would, therefore, be expected to disappear only slowly
in the environment through chemical degradation. Photolysis and
oxidative and hydrolytic reactions are pathways by which the
compounds may be abiotically degraded.
4.2.2.1 Photolysis
Although chlorobenzenes absorb light only weakly above 290 nm, some
photodegradation can occur when they are irradiated with sunlight,
or light containing an equivalent broad spectrum of wavelengths.
Uyeta et al. (1976) demonstrated that chlorobenzenes (other than
1,2,3,5-TeCB and PeCB, which were not examined) form polychlorinated
biphenyls when irradiated with sunlight. However, the yields of
polychlorinated biphenyls were less than 1% of the initial amount of
chlorobenzene. Of the compounds tested, 1,2,3-TCB and 1,2,4,5-TeCB
were the most resistant to photodegradation, while 1,2,4-TCB and
1,2,3,4-TeCB were the most easily degraded. The number of chlorine
atoms in the polychlorinated biphenyl photoproducts was 1 less than
the number contained in 2 molecules of the parent chlorobenzene,
i.e., monochlorobenzene yielded a monochlorobiphenyl,
dichlorobenzenes yielded tri-chlorobiphenyls and so on. Hydrochloric
acid was also a reaction product. On the basis of these results, it
was suggested that the photoformation of polychlorinated biphenyls
from chlorobenzenes involves free radical reactions based on the
dehydrochlorination of 1 molecule from 2 molecules of the parent
chlorobenzene.
Studies on direct photodegradation, either with direct sunlight or
artificial light simulating natural conditions, suggest that the
chlorobenzenes can be photodegraded, though the reactions may be
slow (Crosby & Hamadmad, 1971; Akermark et al., 1976; Uyeta et al.,
1976; Choudhry et al., 1979; Choudhry & Webster, 1985). For example,
the half-life of 1,4-DCB, under artificial sunlight irradiation, was
estimated to be 115.5 h (Hanai et al., 1985). This value was
considerably greater than the half-lives of other air pollutants
(i.e., tetrachloroethylene, trichloroethylene, benzene, toluene,
ethylbenzene, 1,2,4-trimethylbenzene, n-octane, and n-nonane)
under similar conditions.
Reductive dechlorination is the main photochemical reaction that
occurs in proton-donating solvents and there is evidence that the
solvent is involved with the electronically excited reactant
molecule in the transition state complex. Photodegradation of the
tri- and tetrachlorobenzenes, using acetonitrile as the solvent in a
1:1 ratio with water, has been reported; however, it should be noted
that acetonitrile would not be present in this ratio under normal
environmental conditions (Choudhry et al., 1979; Choudhry & Webster,
1985). Some form of hydrogen-donating entity, such as a solvent
molecule or another chlorobenzene molecule, appears necessary for
the photochemical dechlorination of chlorobenzenes at wave-lengths
above 290 nm. It has been speculated (Akermark et al., 1976) that
such hydrogen-donating "photosensitizers" may be found in
naturally occurring organic substances and that
photodecomposition may be important as a degradative pathway, given
the general physical and chemical stability of the chlorobenzenes.
In addition to direct photolysis, chlorobenzenes may also be removed
from the environment by reaction with molecular species that are
photochemically produced from other atmospheric pollutants. Such a
possibility has been suggested, on the basis of studies involving
simulated atmospheric environments, for interactions between
monochlorobenzene or 1,4-dichlorobenzene and oxides of nitrogen
(Dilling et al., 1976; Kanno & Nojima, 1979; Nojima & Kanno, 1980).
Reaction mechanisms and rates of disappearance of the compounds were
poorly defined in these studies.
4.2.2.2 Hydrolytic and oxidative reactions
It is unlikely that simple hydrolysis is an important degradation
pathway for the chlorobenzenes in the environment.
Cupitt (1980) suggested that MCB and the DCBs may be removed from
the troposphere by reaction with hydroxyl radicals (considered to
be the most potentially reactive species in the troposphere),
and possibly also by reaction with ozone. This investigator used
estimated rate constants for the reaction with hydroxyl radicals
(assumed to have a tropospheric concentration of 1 x 106
molecules/cm3) and ozone (tropospheric concentration, 1 x 1012
molecules/cm3) to predict atmospheric residence times of 28 days
and 39 days for MCB and the DCBs, respectively.
Calculations by Cupitt (1980) suggest that ozonolysis contributes
very little to the removal of the compounds, because the rate
constants for the reaction of hydroxyl radicals with the
chlorobenzenes are some 9 or 10 orders of magnitude greater than
those for the corresponding reactions with ozone.
4.2.3 Biodegradation and biotransformation
The degradation of chlorobenzenes by microorganisms has been
reported in several studies using various substrates, such as soil,
sediment, and sewage sludge (Table 6). It can be speculated, from a
perusal of these data, that the more highly chlorinated benzenes are
not degraded microbiologically as readily as the less chlorinated
congeners; however, the data are insufficient to draw definitive
conclusions. Garrison & Hill, (1972) found that MCB, 1,2-DCB, and
1,4-DCB were completely volatized in less than one day from
solutions containing mixed cultures of aerobic organisms, but that
2% of 1,2,4-TCB remained after 80 h.
The major degradation mechanism is oxidative dechlorination leading
to the formation of hydroxylated aromatic compounds (mainly
phenols), followed by ring fission and, eventually, mineralization
to carbon dioxide and water. It has been suggested that, like
polychlorinated biphenyls, chlorobenzenes appear to be attacked by
microorganisms only under aerobic conditions (Kobayashi & Rittman,
1982; Bouwer & McCarty, 1984).
Schwarzenbach et al. (1983) studied the movement of 1,4-DCB from a
polluted river in Switzerland through a ground water aquifer to a
series of wells. Correlation between the indicators of
microbiological metabolic activity and the observed decrease in
concentrations of 1,4-DCB with increasing distance of the wells from
the river was taken as evidence of the biotransformation of 1,4-DCB
in the aquifer system. On certain occasions, the persistence of
1,4-DCB was well correlated with anoxic conditions that prevailed in
parts of the aquifer, suggesting that the biotransformation of the
compound is minimal under anaerobic conditions. These findings were
confirmed in laboratory experiments using sediments from this
aquifer. Results showed that the DCBs were transformed only under
aerobic conditions and that the rates of transformation were
different with each isomer, 1,4-DCB degrading at the faster rate
(Kuhn et al., 1985).
4.2.4 Bioaccumulation
The bioaccumulation of chlorobenzenes by aquatic organisms is
determined by their relative water and lipid solubility (thus
reflecting the octanol/water partition coefficients) and the number
of chlorine substitutions. Uptake from water increases with
increasing chlorination. The coefficient of adsorption on sediment
influences the uptake into terrestrial plants and sediment-living
aquatic invertebrates; the degree of chlorination is also correlated
with uptake.
Table 6. Degradation of chlorobenzenes by miroorganisms
Chlorobenzene; Organism Substrate Rate Remarks
Reference
MCB, DCBs, Pseudomonas sp. synthetic NAa DCBs metabolized to dichlorophenols and
TCBs and TeCBs medium dichloropyrocatechols; MCB, TCBs, and
Ballschmiter & TeCBs metabolized to their respective
Scholz (1980) chlorophenols
MCB, DCBs, and NA synthetic no significant degradation medium seeded with sewage effluent and
1,2,4-TCB medium observed after 11 weeks strictly maintained under denitrifying
Bouwer & conditions
McCarty(1983)
MCB NA estuarine half-life = 75 days radiolabelled compound used,
Lee & Ryan sediments; half-life = 150 days degradation rate measured by
(1979) estuarine waters evolution of radiolabelled CO2;
considerable reduction in rate observed
when temperature reduced to 9-13 °C
MCB Pseudomonas synthetic not measured P. putida grown with toluene as the
Gibson et al. putida medium sole carbon source, oxidized MCB to
(1968) 3-chlorocatechol
MCB planktonic and sea water spring half-life = 21 days tanks contained 13 m3 sea water
Wakeham et al. microbial summer half-life = 4.6 days with simulated turbulence and
(1983) winter half-life = 13 days seasonal patterns
Table 6 (continued)
Chlorobenzene; Organism Substrate Rate Remarks
Reference
MCB microbial strain synthetic NAa culture isolated from soil and sewage
Reineke & WR 1306 medium and was sensitive to sudden increases
Knackmuss in MCB concentrations, resulting
(1984) in prolonged lag phase or disturbed
exponential phase; 3-chlorocatechol
isolated from culture fluid;
organisms did not oxidize isomeric DCBs
1,2-DCB Acinetobacter activated >90% disappearance in mixture of 4 bacterial genera and 1 yeast,
Davis et al. + sewage sludge 7 days glucose as sole carbon source, incubation
(1981) Alcaligenes temperature 28 °C; some DCB may have been
+ lost by evaporation
Flavobacterium
+
Pseudomonas
+
Rhodotorula
1,4-DCB planktonic and sea water spring half-life = 18 days tanks contained 13 m3 sea water with
Wakeham et al. microbial summer half-life = 10 days simulated turbulence and seasonal patterns
(1983) winter half-life = 13 days
1,4-DCB microbial flora ground water NAa under aerobic conditions, concentrations
Schwarzenbach present aquifer of 1,4-DCB decreased with increasing
et al. (1983) distance of wells from the polluted
1,2,4-TCB NA activated after 5 days, 56% converted to radiolabelled compound used, degradation
Simmons et al. sludge CO2; 23% converted to polar measured by evolution of radiolabelled CO2
(1977) metabolites; 7% evaporated
Table 6 (continued)
Chlorobenzene; Organism Substrate Rate Remarks
Reference
1,2,3-TCB and NA soil mineralization rates nmol/day radiolabelled compounds applied at
1,2,4-TCB per 20 g soil: 1,2,3-: 0.33, 50 mg/kg soil, mineralization measured by
Marinucci & 0.38; 1,2,4-: 1.09, 0.93, evolution of radiolabelled CO2; both TCBs
Bartha 1.37 poisoned metabolic action of soil
(1979) bacteria; 1,2,3-TCB yielded 2,3- and
2,6-dichlorophenol; 1,2,4-TCB yielded
2,4-, 2,5- and 3,4-dichlorophenol
1,2,4-TCB planktonic and sea water spring half-life = 22 days tanks contained 13 m3 sea water with
Wakeham et al. microbial summer half-life = 11 days simulated turbulence and seasonal patterns
(1983) winter half-life = 12 days
PeCB NA soil half-life = 194, 345 days compounds applied to soil samples at
Beck & Hansen concentrations equivalent to 10 kg/ha,
(1974) concentrations measured using gas
chromatography; duplicate experiments, no
explanation given for differences in
half-lives measured
a NA - not available.
Topp et al. (1986) compared the uptake in plants of chlorobenzenes
from the soil and via the air in closed, aerated laboratory systems.
A negative correlation was demonstrated between the bioconcentration
factor (BCF) and the soil adsorption coefficient (based on soil
organic matter content) for the uptake into the roots of barley. The
adsorption of chlorobenzenes on soil organic matter increased with
increasing chlorination. However, expression of uptake in barley
roots in relation to the soil interstitial water concentration of
the chlorobenzenes produced a positive correlation between the BCF
and the octanol/water partition coefficients. Higher chlorinated
chlorobenzenes, therefore, are most readily taken up by the plant
roots, when they are available in soil interstitial water. This will
occur particularly in sandy soils with a low organic matter content.
Uptake of volatilized chlorobenzenes in leaves was extremely low
compared with root uptake. The correlation between uptake and
physical properties demonstrated in barley did not hold for corn;
the authors stated that the uptake of lipophilic compounds by
lipid-rich plants, or plants with oil channels, was unpredictable .
In a later study, Topp et al. (1989) studied the uptake and
distribution of 14C-labelled 1,2,4-TCB and PeCB in barley. The BCF
concentration decreased with time of exposure; this was a dilution
effect as the plant grew. The total load of chlorobenzene increased
over the whole growing period of the plant, but the rate of uptake
was greater in the early growth period. Uptake increased with
increasing chlorination but decreased in relation to the soil
concentration (BCF fell with increasing chlorination). There was
evidence of metabolism of the chlorobenzenes in the plant, with the
level of the parent compound falling over the course of the
experiment in relation to the rate of metabolism, and the levels of
uncharacterized"bound" residues. After growth in soil containing 2
µg each of 1,2,4-TCB and PeCB/kg (dry weight), harvested barley
grain contained 73 and 82 µg/plant, respectively. The concentrations
in the dry grain were 0.05 and
0.06 mg/kg for 1,2,4-TCB and PeCB, respectively.
Khezovich & Harrison (1988) used closed, flow-through bioassay
systems to investigate the bioavailability to chiromonid midge
larvae of sediment-bound MCB, 1,2-DCB, and 1,2,4-TCB. A sediment
with a high organic matter content (14.5%) was compared with a
sediment with a low organic content (3.6%). The bioconcentration of
the chlorobenzenes increased with increasing chlorination. The
experiment was run without equilibrium between the sediment and the
overlying water (flow-through of uncontaminated water) and after
equilibration of recirculated water. Most of the uptake of
chlorobenzenes occurred from the interstitial water between sediment
particles and the results of bioconcentration were best correlated
with the concentrations of the chlorobenzenes in the interstitial
water. Under non-equilibrium conditions, bioconcentration factors
were 5, 29, and 225 for MCB, 1,2-DCB, and 1,2,4-TCB, respectively.
Köneman & Van Leeuwan (1980) exposed guppies to 116 µg
1,4-DCB/litre, 48 µg 1,2,3-TCB/litre, 43 µg 1,3,5-TCB/litre, 12 µg
1,2,3,5-TeCB/litre, or 1.2 µg PeCB/litre for 19 days. The fish were
fed daily on commercial fish food. Concentrations in fish were
expressed in mg/kg. The results showed an increase in the rate of
uptake with increasing level of chlorination of the benzene ring.
After exposure, the fish were kept for 9 weeks in clear water to
study the rate of elimination. The rate constant of loss of 1,4-DCB
was described by a one-compartment model and was relatively high
(1.00/day). For the other CBs, the losses showed a clear biphasic
pattern with a decrease in the first, rapid rate of loss with
increasing level of chlorination. Consequently, the level of
bioaccumulation went up with increasing chlorination.
In a study performed by Opperhuizen & Stokkel (1988), 1-year-old
guppies were exposed for 42 days to 1,2,3,4-TeCB or PeCB at µg/litre
levels. There were 3 groups of fish: one with contaminated
Chromosorb (artificial sediment) added, one with uncontaminated
Chromosorb, and one without Chromosorb. The concentration of PeCB in
the water was reduced by the presence of contaminated sediment,
while neither type of particle affected the TeCB concentration in
the water. Addition of uncontaminated particles did not affect the
increase in chlorobenzene residues in the fish. However, the
presence of contaminated particles resulted in higher concentrations
of PeCB in exposed fish than in control fish. No effects were seen
with TeCB. The authors attributed this to the low levels of TeCB on
the particles compared with levels in the water. They concluded that
the influence of contaminated particles on the bioconcentration of
hydrophobic chemicals by fish depends on the hydrophobicity of the
chemicals. The particles may act as a source of the compounds.
Van Hoogen & Opperhuizen (1988) exposed guppies in acute toxicity
tests to 1,2,3-TCB, 1,2,3,4-TeCB, or PeCB in a continuous-flow
system. Fish died having reached the lethal dose of chlorobenzene
for fish, which was between 2.0 and 2.5 mmol/kg and was independent
of the exposure concentration. The authors suggested that this value
was not affected by the route of administration. In addition, the
level of chlorination did not influence the lethal dose expressed in
mmol/kg. When uptake and elimination rate constants were calculated,
any combination of exposure time and concentration required to reach
the lethal dose could be calculated.
4.2.5 Biomagnification
No studies were found concerning the possibility that concentrations
of chlorobenzenes may increase as they move up the food chain.
4.2.6 Ultimate fate following use
As discussed in section 4.1, there is a preferential exchange of
chlorobenzenes from water to the atmosphere. However, in natural
waters containing appreciable amounts of suspended organic matter,
chlorobenzenes may be retained and transported within the aquatic
environment. The accumulation of chlorobenzenes in aquatic sediments
is striking, concentrations being at least 1000 times higher than
those found in the water. Available data suggest, therefore, that
soils rich in organic matter may be a major environmental sink for
these compounds (Elder et al., 1981; Oliver & Nicol, 1982).
Historical evidence for the persistence of chlorobenzenes and for
sediments acting as an environmental sink for these compounds has
been reported by Durham & Oliver (1983). Radionuclide measurements
were used to construct age profiles for Lake Ontario sediments. The
age of lake bottom sediments near the mouth of the Niagara River was
correlated with the concentrations of chlorobenzenes found in the
sediment samples. Over an 80-year period, the concentrations of all
chlorobenzene isomers increased from 0.4 to 15 µg/litre in 1898-1904
to a peak of 18 to 1100 µg/litre in 1959-67, and then declined to 6
to 110 µg/litre in 1980-81. The rise and fall of chlorobenzene
levels closely followed the rise and fall of the total USA
production figures for all chlorobenzenes for a similar period. The
Niagara River is considered to be a major source of chlorobenzene
pollution in Lake Ontario (Oliver & Nicol, 1982).
In Canada, recent data indicate that levels of chlorobenzenes in
sediments are highest in the industrialized Central Region (Ontario
and Quebec). Mean concentrations of the dichlorobenzenes (the
congeners that are present at the highest levels) were 130 µg/kg for
the 1,2-isomer and 46 µg/kg for both the 1,3- and 1,4-isomers. Mean
levels of other congeners, which were also present at elevated
concentrations (above individual detection limits), were 43 µg/kg,
39 µg/kg, and 29 µg/kg for 1,2,3,4-TeCB, PeCB, and 1,2,4-TCB,
respectively (NAQUADAT, 1987).
When the proportion of each congener (di- to penta-) to the total
chlorobenzene content of the water of the Niagara River (a major
source) was compared with that found in the sediment of Lake
Ontario, Oliver et al. (1989) concluded that "increasing the
chlorine content on the benzene ring leads to higher relative
accumulation of the chemicals in sediments". DCBs, TCBs, TeCBs, and
PeCB constituted 68%, 21%, 8%, and 2% of the total chlorobenzenes
(di- to penta-) measured in the water samples from the Niagara
River; comparable values for sediment in Lake Ontario were 12%, 31%,
21%, and 9%, respectively.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental Levels
5.1.1 Air
Levels of chlorobenzenes in ambient outdoor air are presented in
Table 7. Although the available data are insufficient to make a
reliable estimate of human exposure from the atmosphere, it can be
concluded that mean levels of chlorobenzenes (mono- to tri-) in
ambient air are in the tenths of µg/m3 range; however, maximum
values can range up to 100 µg/m3. Seasonal variations in the
concentrations of 1,4-DCB in ambient air have also been reported,
with concentrations increasing with increasing temperature (Hanai et
al., 1985). No data are available concerning levels of
tetrachloro-benzene and pentachlorobenzene in the ambient air,
though these congeners have been detected (but not quantified) in
the fly ash from municipal incinerators (Eiceman et al., 1979,
1981).
Chlorobenzenes have also been detected in rainwater, presumably
through transfer from the ambient air; Pankow et al. (1983) found
all 3 DCB isomers and 1,2,4-TCB, at levels of less than 10 ng/litre
at selected sites in Oregon and California. In the United Kingdom,
1,4-DCB was detected in rainwater at a level of 0.01 ± 0.005
µg/litre (Fielding et al., 1981).
In general, the levels of the chlorobenzenes in indoor air (Table 8)
are similar to those in ambient air. However, in several cases,
levels have been much higher. For example, concentrations found in
basements in the Love Canal area (up to 190 µg/m3 for total
dichlorobenzenes) and in the wardrobe of a Tokyo residence (up to
1700 µg 1,4-DCB/m3 detected in 1 sample) may be explained by the
proximity of a chemical dump and the use of 1,4-DCB as a moth
repellent, respectively.
5.1.2 Water
Chlorinated benzenes have been detected in sewage sludge, municipal
waste water, surface and ground waters, and in drinking-water.
However, in 12 sewage sludges in the United Kingdom, the
concentrations of chlorobenzenes ranged from <0.01 mg/kg dry weight
for PeCB to 40.2 mg/kg dry weight for 1,3-DCB, with a general
reduction in concentration with increased chlorine substitution
(Rogers et al., 1989).
Table 7. Chlorobenzenes in outdoor air
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
MCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.9 (2.3)
Phoenix, Arizona 0.9 (2.3)
Oakland, California 0.45 (1.4)
Harkov et al. (1983) 38 (35) USA; cities:
Newark, New Jersey 0.5
Elizabeth, New Jersey 0.4
Camden, New Jersey 0.3
Levine et al. (1985) 9 USA
Hamilton, Ohio ND - 43
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites 0.2 - 3.6
and landfills in New (20.4)
Jersey
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.4 (0.4)
Pellizzari et al. (1986) USA; cities:
20 Greenboro, North Carolina median 0.029 (0.57)
11 Houston, Texas median 0.045 (1.3)
71 Elizabeth/Bayonnne, New Jersey median 0.43 (6.3)
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Environment Canada (1986) Canada
46 (43) Montreal 0.2 (1.0)
100 (83) Toronto 0.38 (2.2)
1,2-DCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.08 (0.3)
Phoenix, Arizona 0.14 (1.4)
Oakland, California 0.22 (0.2)
Bauer (1981) 89c Germany; city and environs:
Bochum 8.3
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites 0.03 - 3.2 (25.1)
and landfills in New Jersey
Pellizzari et al. (1986) USA
25 Los Angeles - winter median 0.20 (0.55)
23 Los Angeles - summer median 0.03 (0.82)
10 Antioch/West, Pittsburgh median 0.02 (0.58)
Wallace (1986); USA
Wallace et al. (1985) 24 Los Angeles (February) 0.2
24 Los Angeles (May) 0.8
10 Contra Costa (June) 0.07
175 Elizabeth/Bayonne, New Jersey 0.17
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Environment Canada (1986) Canada
40 (3) Montreal 0.0 (0.1)
70 (1) Toronto 0.02 (0.1)
Harkov et al. (1983) 38 (29) USA; cities:
Newark, New Jersey 0.18
Elizabeth, New Jersey 0.12
Camden, New Jersey 0.06
1,3-DCB
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.05 (0.02)
Phoenix, Arizona 0.05 (0.17)
Oakland, California 0.04 (0.1)
Bauer (1981) West Germany; city and environs:
89c Bochum 3.7
Levine et al. (1985) USA
9 Hamilton, Ohio ND - 100
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.6 (<0.6)
Environment Canada (1986) Canada
67 (8) Toronto 0.02 (0.30)
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
1,3-, 1,4-DCBs
Wallace (1986); Wallace et al. (1985, USA
1986) 86 Elizabeth/Bayonne, New Jersey (1981) 1.7
60 Elizabeth/Bayonne, New Jersey (1982) 1.3
9 Elizabeth/Bayonne, New Jersey (1983) 1.2
24 Los Angeles (Feb.) 2.2
24 Los Angeles (May) 0.8
10 Contra Costa (June) 0.3
Pellizzari et al. (1986) USA
85 Elizabeth/Bayonne, New Jersey (1981) median 0.8 (13)
71 Elizabeth/Bayonne, New Jersey (1982) median 1.2 (7.6)
9 Elizabeth/Bayonne, New Jersey (1983) median 0.32 (4.6)
25 Los Angeles (winter) median 1.8 (21)
2 Los Angeles (summer) median 0.72 (2.4)
10 Antioch/West, Pittsburgh median 0.25 (1.0)
1-DCB
Morita & Ohi (1975) Tokyo, Japan
3 (3) city centre 2.7 - 4.2
3 (3) suburbs 1.5 - 2.4
Harkov et al. (1983) 38 (32) USA
Newark, New Jersey 0.3
Elizabeth, New Jersey 0.4
Camden, New Jersey 0.2
Harkov et al. (1985) 7 (7) USA
Hazardous waste sites and 0.2 - 5.2 (50.4)
landfills in New Jersey
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.6 (<0.6)
Environment Canada (1986) Canada
46 (46) Montreal 0.3 (0.8)
72 (69) Toronto 0.37 (2.14)
De Bortoli et al. (1985) 15 (3) Northern Italy
various towns <5(4)
1,2,3-TCB
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.8 (<0.8)
Bauer (1981) 89c Germany; city and environs:
Bochum 0.40
1,2,4-TCB
Lebret (1985) NA Netherlands
Ede and Rotterdam median <0.8 (<0.8)
Singh et al. (1981) * USA; cities:
Los Angeles, California 0.05 (0.25)
Phoenix, Arizona 0.02 (0.08)
Oakland, California 0.02 (0.11)
1,3,5-TCB
Bauer (1981) 89c Germany; city and environs:
Bochum 0.5
Table 7 (continued)
Compound; Number of Location Concentrationb
Reference Samplesa (µg/m3)
Lebret (1985) Netherlands
Ede and Rotterdam median <0.8(<0.8)
a Number of positive observations given in brackets where possible.
NA - not available.
* - Samples obtained during 2-weeks mobile sampling in the cities indicated; samples taken hourly, 24 h/day.
b Maximum values are in brackets.
ND - not detected.
c Samples obtained sporadically, over a 6-month period, from 3 locations within the city and its environs; averages from 89 samples.
Table 8. Chlorobenzenes in indoor air
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
MCB
Potenta & Saunders (1983) USA - urban Office 2 (2) 1.8 - 3.2
Pellizzari (1982) USA - urban house basements 6 (5) ND - 4.2
(Love Canal area)
Lebret (1985) Netherlands - urban Ede - post-war homes 134 median <0.4 (0.4)
Ede - <6-year-old homes 96 median <0.4 (27)
Rotterdam 89 median <0.4 (3)
Wallace et al. (1984) USA Personal air samples:
New Jersey subjects 160 (115) 0.05 - 26.5 median 0.61
North Carolina subjects 60 (13) 0.05 - 3.66
Pellizzari et al. (1986) USA Greensboro, North Carolina 20 median 0.026 (0.7)
Houston, Texas 11 median 0.035 (0.75)
Elizabeth/Bayonne, New Jersey 71 median 0.42 (6.6)
DCBc
Pellizzari et al. (1982) USA house basements 6 (6) 0.65 - 190
(Love Canal area)
Pellizzari et al. (1986) USA Greensboro, North Carolina 20 median 0.09 (60)
Baton Rouge/Geismar, 27 median 2.1 (120)
Louisiana
Houston, Texas 11 median 5.5 (21)
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
1,2-DCB
Wallace (1986) USA Personal air samples:
Los Angeles (Feb) 115 0.4
Los Angeles (May) 50 0.3
Contra Costa (June) 68 0.6
Pellizzari et al. (1986) USA Los Angeles - winter 25 median 0.12 (2.5)
Los Angeles - summer 23 median 0.03 (11)
Antioch/West, Pittsburg - 10 median 0.03 (0.49)
summer
Otson & Benoit (1986) Canada - urban houses 9 (6.5)
1,3-DCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.6 (9)
Ede - <6-year-old homes 96 median <0.6 (6)
Rotterdam 89 median <0.6 (6)
1,4-DCB
Lebret (1985) Netherlands Ede - post-war homes 134 median 2 (138)
Ede - <6-year-old homes 96 median <0.6 (240)
Rotterdam 89 median <0.6 (299)
Morita & Ohi (1975) Japan - urban home bedroom 1 (1) 105
closet 1 (1) 315
wardrobe 1 (1) 1700
Potenta & Saunders (1983) USA - urban office 2 (1) trace
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
De Bortoli et al. (1985) Northern Italy houses 15 (9) 55(230)
1,3-, 1,4-DCBs
Wallace (1986); Wallace et al. USA Personal air samples:
(1985, 1986) Elizabeth/Bayonne, 340 45
New Jersey - fall
Elizabeth/Bayonne, 150 50
New Jersey - summer
Elizabeth/Bayonne, 49 71
New Jersey - winter
Los Angeles - Feb 115 18
Los Angeles - May 50 12
Contra Costa - June 68 5.5
Pellizzari et al. (1982) USA Elizabeth/Bayonne, 85 median 2.8 (915)
New Jersey - fall
Elizabeth/Bayonne, 71 median 2.6 (1550)
New Jersey - summer
Elizabeth/Bayonne, 9 median 1.2 (120)
New Jersey - winter
Los Angeles - winter 25 median 2.8 (214)
Los Angeles - summer 23 median 1.0 (170)
Antioch/West, Pittsburgh 10 median 0.44 (7.5)
TCBsc
Pellizzari et al. (1986) USA house basements 6 (6) 0.07 - 33
(Love Canal area)
Table 8 (continued)
Compound; Sample Location Numbera Concentrationb
Reference (µg/m3)
1,2,3-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (3)
Ede - <6-year-old homes 96 median <0.8 (28)
Rotterdam 89 median <0.8 (3)
1,2,4-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (15)
Ede - <6-year-old homes 96 median <0.8 (33)
Rotterdam 89 median <0.8 (<0.8)
1,3,5-TCB
Lebret (1985) Netherlands Ede - post-war homes 134 median <0.8 (8)
Ede - <6-year-old homes 96 median <0.8 (5)
Rotterdam 89 median <0.8 (<0.8)
TeCBsc
Pellizzari (1982) USA house basements 6 (6) 0.03 - 20
(Love Canal area)
PeCB
Pellizzari (1982) USA house basements 6 (4) trace - 0.49
(Love Canal area)
a Number of positive observations given in brackets where available.
b Mean concentrations in µg/m3; maximum values obtained are given in brackets.
ND - not detected.
c Sum of all isomers.
Data on levels of the lower chlorinated benzenes (MCB, DCBs, and
TCBs) in waste water indicate that MCB is detected the most often
and at the highest concentrations, occasionally exceeding 1
mg/litre. Reported levels of DCB and TCB isomers have been lower. In
a survey of industrial waste waters in the USA (44 264 samples),
levels of MCB averaged 667 µg/litre and ranged from 11 to 6400
µg/litre. Other chlorobenzenes detected were 1,2-DCB (12-860
µg/litre; mean 141 µg/litre); 1,3-DCB (10-39 µg/litre; mean 21
µg/litre); 1,4-DCB (10-410 µg/litre; xmean 79 µg/litre); and
1,2,4-TCB (12-607 µg/litre; mean 161 µg/litre) (Neptune, 1980). In a
survey of waste water throughout the USA, concentrations of 1,2-DCB
ranged from 15 to 690 µg/litre and concentrations of 1,2,4-TCB
ranged from 0.25 to 500 µg/litre (Ware & West, 1977). A survey of 4
municipal treatment plants in Georgia revealed levels of DCB and TCB
in the influent of 3-146 µg/litre and 1-60 µg/litre, respectively.
Concentrations in the effluent ranged from 0 to 268 µg/litre and 0
to 13 µg/litre, respectively (Gaffney, 1976). The increase in DCB
levels in the effluent was believed to be the result of chlorination
during the secondary phase of the waste water treatment. In waste
water from major municipal treatment plants in Southern California,
concentrations of DCB ranged from 0.2 to 435 µg/litre, while
concentrations of 1,2,4-TCB and 1,3,5-TCB were 130 µg/litre and
<0.2 µg/litre, respectively (Young & Heesen, 1978).
Oliver & Nicol (1982) reported that the concentrations of
dichloro-benzenes in raw water in the Great Lakes were much greater
than those of the more highly chlorinated congeners, the 1,4-isomer
being present at the highest level. This isomer was particularly
prevalent in effluent from sewage-treatment plants.
Data on the levels of chlorobenzenes (mono- to penta-) in surface
waters are presented in Table 9. It is difficult to draw general
conclusions concerning levels of chlorobenzenes that are commonly
present in surface water, because of the paucity of available data
and the considerable variations in reported concentrations, mainly
owing to differences in the proximity to industrial sources. In
general, it appears that levels are in the ng-µg/litre range;
however, levels in the vicinity of industrial sources may
occasionally range up to 0.1 mg/litre.
The levels of chlorobenzenes found in some drinking-water supplies
are shown in Table 10. Although chlorobenzenes are unlikely to be
changed chemically during the treatment, it is possible that some
may be lost at the aeration and chlorination stages, because the
compounds tend to be redistributed preferentially to air from water.
It has been suggested, however, that water chlorination itself may
produce chlorobenzenes by the reaction of chlorine (or one of its
aqueous species) with organic material (both natural and man-made)
in the raw water supply (Carlson et al., 1975; Glaze et al., 1978;
Höfler et al., 1983). For example, Höfler et al. (1983) demonstrated
that all of the chlorobenzenes were detected following the reaction
of benzene (tenths of a µmol/litre) with aqueous sodium
hypochlorite. There is slight evidence from surveys of
chlorobenzenes in drinking-water that they may be formed during
treatment; Otson et al. (1982a,b) observed that the frequency of
detection of MCB at 30 Canadian water treatment facilities was less
for raw water than for drinking-water samples (5 and 18%,
respectively); however, levels were too low to permit
quantification.
In general, the lower chlorinated benzenes are detected more
frequently in drinking-water and are present in higher
concentrations; however, even for these compounds, mean levels are
generally less than 1 µg/litre and rarely exceed 50 µg/litre. Most
of the dichloro-benzene in drinking-water is present as the
1,4-isomer, probably because of its release into surface water from
urinal deodorant blocks (Oliver & Nichol, 1982).
5.1.3 Soil
Very few published data are available on the levels of
chlorobenzenes in soil. With the exception of soil near poorly
maintained waste sites, the presence of chlorobenzenes in soil would
appear to be associated with pesticide use. For example, PeCB has
been detected at a level of 0.09 mg/kg in fields treated with
quintozene in Finland (Rautapaa et al., 1977).
5.1.4 Food
Little information is available concerning the presence of
chloro-benzenes (mono- to penta-) in food, but levels in various
seafoods have been measured. Concentrations of 1,4-DCB in mackerel,
caught off the coast of Japan, averaged 0.05 mg/kg on a whole-fish,
wet-weight basis (Morita et al., 1975). All of the chlorobenzenes
(mono- to penta-) were detected in trout from the Great Lakes at
levels ranging from 0.1 to 16 µg/kg whole-fish, wet weight (Oliver &
Nicol, 1982). With the exception of MCB, chlorobenzenes (di- to
penta-) were measured in samples of sprats from south-east Norway in
the vicinity of an unspecified "source of contamination". Levels of
PeCB were highest, ranging from 0.01 to 3.7 mg/kg, while levels of
trichloro- and tetrachlorobenzenes ranged between <0.01 and 0.5
mg/kg (Lunde & Ofstad, 1976). Levels of total chlorobenzenes in the
edible tissue of freshwater fish from highly polluted and industrial
areas of Yugoslavia were 1.8 mg/kg, on a fat basis, whereas levels
in fish from lightly polluted agricultural and woodland areas, and
in marine fish, were 0.2 mg/kg and 0.4 mg/kg, respectively (Jan &
Malnersic, 1980). The 1,4-DCB isomer was identified among other
volatile organic compounds collected from watercress (Spence &
Tucknott, 1983). The source of the compound in the plant matter was
not identified, but it is likely that it was present in the water in
which the plants were grown.
Table 9. Chlorinated benzenes in surface watersa
Chemical Location Levelsb Reference
MCB Glatt River, Germany + US EPA (1985)
Delaware River ND - 7000 Sheldon & Hites (1978)
Ohio River ND - >10 000 US EPA (1985)
DCBs Delaware Riverc ND - 400 Sheldon & Hites (1978)
Great Lakesd 32 - 71 (27) Oliver & Nicol (1982)
Grand River, Canada ND - 77 (11) Oliver & Nicol (1982)
Niagara Falls, New York + Elder et al. (1981)
1,2-DCB Atlantic Region, Canada <20 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 3.9 - 240 (23) Oliver & Nicol (1984)
1,3-DCB Atlantic Region, Canada <2 - 310 (22) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 2.1 - 110 (11) Oliver & Nicol (1984)
1,4-DCB Glatt River, Germany 30 - 900 US EPA (1985)
Ohio River ND - >10 000 US EPA (1985)
Atlantic Region, Canada <20 - 130 (22) NAQUADAT (1987)
Great Lakes trace - >100 Otson (1987)
Niagara-on-the-Lake, Canada 9.0 - 310 (36) Oliver & Nicol (1984)
TCBs Merrimack River, Massachusettsc 100 - 500 Hites (1973)
Delaware Riverc ND - 1000 Sheldon & Hites (1978)
Niagara Falls, New York 100 - 8000 Elder et al. (1981)
Great Lakesd 0.1 - 1.6 (0.5) Oliver & Nicol (1982)
Grand River, Canadad ND - 8.7 (2.1) Oliver & Nicol (1982)
Atlantic Region, Canada <4.0 NAQUADAT (1987)
Table 9 (continued)
Chemical Location Levelsb Reference
1,2,4-TCB Niagara-on-the-Lake, Canada 5.8 - 120 (16) Oliver & Nicol (1984)
1,2,3-TCB Niagara-on-the-Lake, Canada 1.4 - 30 (3.5) Oliver & Nicol (1984)
1,3,5-TCB Niagara-on-the-Lake, Canada 0.19 - 6.8 (0.84) Oliver & Nicol (1984)
TeCBs Niagara Falls, New Yorkc 100 - 200 000 Elder et al (1981)
Great Lakesc ND - 0.8 (0.12) Oliver & Nicol (1982)
Grand River, Canadad ND - 0.2 (0.05) Oliver & Nicol (1982)
1,2,3,5-TeCB Atlantic Region, Canada <2 - 20 (2.0) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.10 - 1.4 (0.41) Oliver & Nicol (1984)
1,2,4,5-TeCB Altantic Region, Canada <2 - 4 (2.0) NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.39 - 9.3 (2.0) Oliver & Nicol (1984)
1,2,3,4-TeCB Atlantic Region, Canada <2 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 1.4 - 36 (4.5) Oliver & Nicol (1984)
PeCB Niagara Falls, New York ND - 100 000 Elder et al. (1981)
Great Lakes ND - 0.6 (0.12) Oliver & Nicol (1982)
Grand River, Canada ND - 0.1 (0.05) Oliver & Nicol (1982)
Atlantic Region, Canada <2.0 NAQUADAT (1987)
Niagara-on-the-Lake, Canada 0.34 - 6.4 (1.3) Oliver & Nicol (1984)
a Modified from US EPA (1985).
b Range in ng/litre unless indicated.
ND - not detected.
+ - detected.
c Unidentified isomers.
d All isomers.
Table 10. Chlorobenzene levels in drinking-water supplies
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
MCB
Otson et al. (1982a, 1982b) < 1 µg/litre NA 90(16) samples from 30 water treatment
plants in Canada; maximum of
5 µg/litre in one instance
US EPA (1980a) approx 7 µg/litre 0.1-27 µg/litre NA 8 locations in the USA
Barkley et al. (1980) 25 ng/litre 10-60 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
Wallace et al. (1984) NA 0.02-0.02 µg/litre 75(0) water supply of 9 New Jersey homes
Wallace et al. (1984) 0.03 µg/litreb 0.03-0.03 µg/litre 45(0) work water samples of 9 New Jersey
subjects
Wallace et al. (1984) NA 0.03-3.10 µg/litre 30(2) Water supply of 3 North Carolina
homes
Wallace et al. (1984) NA 0.25-0.25 µg/litre 18(0) work water samples of 3 North
Carolina homes
DCBs
US EPA (1980b) NA 1-3 µg/litre NA National survey of the USA
Barkley et al. (1980) 159 ng/litre 10-800 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
1,2-DCB
Oliver & Nicol (1982) 3 ng/litre ND-7 ng/litre NA 3 cities by Lake Ontario
Table 10 (continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
1,3-DCB
Oliver & Nicol (1982) 1 ng/litre ND-2 ng/litre NA 3 cities by Lake Ontario
Otson et al. (1982a,b) < 1 µg/litre NA 90(1) samples from 30 water treatment
plants in Canada; maximum of
1 µg/litre
1,4-DCB
Oliver & Nicol (1982) 13 ng/litre 8-20 ng/litre NA 3 cities by lake Ontario
Fielding et al. (1981) 0.05 µg/litre 0.01-0.08 µg/litre 26(6) from 4 selected sites in England;
reported levels do not include a
sample affected by the use of "air
freshener" blocks containing
1,4-DCB (4 µg/litre)
Otson et al. (1982a,b) < 1 µg/litre NA 90(6) samples from 30 water treatment
plants in Canada; maximum of
<1 µg/litre
TCBs
Barkley et al. (1980) 500 ng/litre 300-760 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
1,2,3-TCB
US EPA (1980a) NA 21-46 µg/litre NA Catawba, North Carolina
Oliver & Nicol (1982) 0.1 ng/litre 0.1-0.1 ng/litre NA 3 cities by Lake Ontario
Table 10 (continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
1,2,4-TCB
US EPA (1980a) 33.6 µg/litre 0.007-275 µg/litre NA 8 locations in the USA (does not
include one sample where levels of
500 µg/litre were detected)
Oliver & Nicol (1982) 2 ng/litre 1-4 ng/litre NA 3 cities by Lake Ontario
1,3,5-TCB
US EPA (1980a) 2.2 µg/litre 0.006-26 µg/litre NA 8 locations in the USA
Oliver & Nicol (1982) ND ND NA 3 cities by Lake Ontario
TeCBs
Barkley et al. (1980) 927 ng/litre ND-2000 ng/litre 9(6) Old Love Canal, Niagara Falls, USA
1,2,3,5-TeCB
Oliver & Nicol (1982) ND ND NA 3 cities by Lake Ontario
1,2,4,5-TeCB
Oliver & Nicol (1982) 0.2 ng/litre ND-0.3 ng/litre NA 3 cities by Lake Ontario
1,2,3,4-TeCB
Oliver & Nicol (1982) 0.3 ng/litre 0.1-0.4 ng/litre NA 3 cities by Lake Ontario
PeCB
Oliver & Nicol (1982) 0.04 ng/litre 0.03-0.05 ng/litre NA 3 cities by Lake Ontario
Table 10 continued)
Compound: Reference Concentration Number of Remarks
samplesa
Mean Range
Barkley et al. (1980) NA ND-240 ng/litre 9(9) Old Love Canal, Niagara Falls, USA
a Number of positive observations given in brackets where possible.
NA - not available.
b Median concentration.
ND - not detected.
In an isolated incident in England, tainted pork (lean and fat meat)
contained 5-20 mg 1,4-dichlorobenzene/kg (Watson & Patterson, 1982).
The 1,4-isomer of DCB was also present in other batches of pork from
the same source, and other meat products from various commercial
sources; however, concentrations were less than 25 µg/kg. It has
been suggested that the source of the chlorobenzenes in pork was the
use of organochlorine pesticides in animal husbandry and their
subsequent metabolism by the animals to chlorobenzenes (Mottram
et al., 1983). Chlorobenzenes have been detected in cows' milk as
well as beef meat in Yugoslavia. In cows' milk, residues ("as in"
basis) for the DCBs ranged from "not detected" (1,3-DCB) to 5.3
µg/kg (1,4-DCB). TCB and TeCB levels ranged from 0.7 µg/kg
(1,2,4-TCB) to 1.5 µg/kg (1,2,3-TCB) and 0.02 µg/kg (1,2,3,4-TeCB)
to 1.10 µg/kg (1,2,3,5-TeCB), respectively. In beef, the DCB
concentrations ranged from "not detected" (1,3-DCB) to 5.0 µg/kg
(1,4-DCB), while TCB and TeCB levels were 1.0 µg/kg (1,2,4-TCB) to
1.8 µg/kg (1,2,3-TCB) and 0.02 µg/kg (1,2,3,4-TeCB) to 1.00 µg/kg
(1,2,3,5- and/or 1,2,4,5-TeCB), respectively. PeCB was detected in
both milk and meat at a concentration of 0.05 µg/kg (Jan, 1983b).
Jan (1980) identified chlorobenzenes (except MCB) in the oils of 9
different seeds, the highest level being 0.09 mg/kg (1,4-DCB) in
corn. DCBs and TCBs have also been detected in dried legumes. Levels
of DCBs ranged from 1.8 to 52.9 µg/kg, while TCB levels ranged from
3.0 to 8.9 µg/kg. These vegetables were grown under controlled
conditions with no pesticide application, indicating that the origin
of the chlorobenzenes was probably environmental contamination
(Lovegren et al., 1979). TeCB residues have also been reported in
potatoes. In composite samples of boiled, fried, and baked potatoes,
levels of 1,2,4,5-TeCB were 0.095, 0.051, and 0.110 mg/kg,
respectively. The authors stated that the higher levels in the baked
potatoes were probably attributable to the fact that the potatoes
were not peeled prior to analysis (Heikes et al., 1979).
An isolated instance of PeCB contamination of cooked ham (present in
only 1 out of 37 samples tested) has also been reported; the level
measured was 0.05 mg/kg (Greve, 1973). PeCB has also been detected
in peanut butter in the USA; concentrations in 11 samples ranged
from 1.8 to 62 µg/kg, the average being 16 µg/kg (Heikes, 1980).
PeCB at 0.03 mg/kg was reported in Finnish rye grown on soils
previously treated with Quintozene (Rautapää et al., 1977).
5.1.5 Human milk
Available data indicate that human milk may be a source of exposure
to chlorobenzenes (mono- to penta-) for suckling infants. MCB was
found in 5 out of 8 samples of human milk in the USA. DCBs (isomers
not specified) were found in all of the 8 samples, whileTCBs
(isomers not specified) were found in only 1 out of the 8 samples;
however, levels were not quantified (Pellizzari et al., 1982). Jan
(1983a) detected all chlorobenzene isomers (except MCB, for which
analysis was not conducted) in the breast milk (3-5 days after
parturition) of women in a Yugoslavian hospital (Table 11). The
concentrations of DCBs were highest, averaging 25 µg/kg, on a whole
milk basis, and ranging up to 35 µg/kg for the 1,4-isomer. The mean
concentrations of the isomers of tri-, tetra, and
pentachlorobenzenes were <5 µg/kg (whole milk). Davies & Mes
(1987) also detected chlorobenzene residues (di- to penta-) in the
breast milk (3-4 weeks after parturition) of Canadian women at mean
levels ranging from <1 µg/kg (1,2,3-TCB and PeCB) to a maximum of 6
µg/kg (1,3- + 1,4-DCB) in whole milk. Concentrations in the breast
milk of women of the indigenous population were slightly higher
(Table 11). Tetrachlorobenzenes were detected, but not quantified.
Recoveries ranged from 69% (1,2-DCB) to 100% (1,2,4-TCB).
5.1.6 Consumer products
As discussed in section 3, chlorobenzenes are used in a wide variety
of consumer and commercial products. However, there is little
information concerning the levels of chlorobenzenes present as
contaminants in consumer products. It is reasonable to expect,
however, that chlorobenzenes could be incidental contaminants in
chemical products, such as solvent formulations and pesticides.
Airborne levels of 1,4-DCB, possibly associated with its use as a
moth repellent, are presented in Table 8 (section 5).
5.2 Human Exposure from All Sources
5.2.1 General population
Although available data are limited and vary widely, it is possible
to estimate an approximate intake of chlorobenzenes from various
sources (Table 12). The limited data suggest that the daily intake
of chlorobenzenes from the air might be considerably greater than
that from food and drinking-water. It can also be concluded that the
intake from air is greatest for the lower, more volatile
chlorobenzenes and that intake from food, compared with that from
other sources, increases with the degree of chlorination. These
conclusions are also supported by data on concentrations of
chlorobenzenes in human tissues, fluids, and exhaled air, presented
in Table 13.
On a body weight basis, breast-fed infants may receive higher doses
of the chlorobenzenes than members of the adult population. Assuming
a daily intake of 0.714 litres of milk for a 5-kg infant, estimated
intakes for infants based on levels measured in breast milk in
Yugoslavia (Jan, 1983a) are 5.57 µg/kg, 1.0 µg/kg, 0.43 µg/kg, and
0.1 µg/kg body weight for total DCBs, TCBs, TeCBs, and PeCB,
respectively. On the basis of recent Canadian data (Mes et al.,
Table 11. Levels of chlorobenzenes (mono- to penta-) in human milk
Compound Country Average level
µg/kg whole milk
(range)c
1,2-DCB Yugoslaviaa 9(5-12)
Canada-national surveyb 2.9
Canada-indigenous populationb 8.1
1,3-DCB Yugoslavia <5
1,4-DCB Yugoslavia 25(5-35)
1,3- & 1,4-DCB Canada-national survey 6.1
Canada-indigenous population ND
1,2,3-TCB Yugoslavia 5(2-10)
Canada-national survey 0.3
Canada-indigenous population 0.1
1,2,4-TCB Yugoslavia 1(ND-4)
Canada-national survey 0.6
Canada-indigenous population 1.2
1,3,5-TCB Yugoslavia 1(ND-3)
Canada-national survey ND
Canada-indigenous population 0.4
1,2,3,4-TeCB Yugoslavia 1(ND-3)
1,2,3,5- & Yugoslavia 2(ND-5)
1,2,4,5-TeCB
PeCB Yugoslavia 0.7(ND-3)
Canada-national survey 0.1
Canada-indigenous population trace
a Jan (1983a).
b Davies & Mes (1987).
c ND - not detectable (detection limits not specified).
Table 12. Possible daily exposure (µg/kg) body weight to total chlorobenzenes from
various sources
Ambient air Ambient air Drinking-water Food
(US)a (Canada)a (Canada)b (Canada)c
MCB 0.882 0.166 < 0.029 NAd
DCB 0.930 0.203 0.00049 0.0013
TCB 0.039 NAd 0.00006 0.0010
TeCB NAd NAd 0.000014 0.0015
PeCB NAd NAd 0.0000011 0.0011
a Air intakes, calculated from mean outdoor levels in Canada and the USA
presented in Table 7, assuming a daily inspired volume of 20 m3 for a
70-kg adult.
b Drinking-water intakes for chlorobenzenes were estimated from the levels in
Canadian drinking-water presented in Table 10, and assuming a daily tapwater
consumption of 2 litres for a 70 kg adult.
c Food intakes were estimated using the mean concentrations of chlorobenzenes
in the tissues of fish from the Great Lakes (Oliver & Nicol, 1982), assuming
a daily intake of 18.5 g fish for a 70-kg adult (Statistics Canada, 1981).
d NA - not available.
1986), estimated intakes are 1.29 µg/kg, 0.29 µg/kg, and <0.14µg/kg
body weight for total DCBs, TCBs, and PeCB, respectively. In view of
the small number of samples analysed and the lack of relevant data
for most regions, these figures should be considered to provide only
rough approximations of the relative intakes from various sources.
5.2.2 Occupational exposure
Few data are available on the levels of the chlorobenzenes in the
workplace. Levels of 1,4-DCB in the atmosphere of facilities
manufacturing, for example, air fresheners, urinal cakes, or moth
repellents, could be quite high, taking into account the volatile
nature of the compound. In one manufacturing plant, concentrations
of 1,4-DCB ranged from 42 to 288 mg/m3, the average being
204 mg/m3 (Ware & West, 1977). Levels of MCB in chemical plants
ranging up to 18.7 mg/m3 have been reported (Cohen et al., 1981).
Very low levels of MCB (1.8-3.2 µg/m3) and a trace of 1,4-DCB have
been found in office air (Table 8). These values are less than the
occupational standards in several countries, which vary between 350
and 450 mg/m3 (IRPTC, 1986).
5.3 Human Monitoring Data
Some data are available on the concentrations of chlorobenzenes in
human blood and adipose tissue (Table 13). The 1,4-isomer of DCB was
detected in all samples of adipose tissue (49/49), from hospitals in
Tokyo, at levels ranging from 0.2 to 11.7 mg/kg (Morita & Ohi, 1975;
Morita et al., 1975). The 1,2,4,5-isomer of TeCB was detected in all
samples (15/15), but levels were several orders of magnitude less
than those for 1,4-DCB (0.008-0.039 mg/kg) (Morita et al., 1975).
Levels of PeCB, detected in 92 out of 99 samples of adipose tissue
from autopsies of accident victims throughout Canada were somewhat
similar to those reported for 1,2,4,5-TeCB in Tokyo; concentrations
ranged from 0.001 to 0.020 mg/kg (Mes et al., 1982).
MCB was detected in the blood of 8 out of 9 residents of the
polluted Love Canal area at concentrations up to 17µg/litre;
however, levels in a control population were not determined (Barkley
et al., 1980). Although the different isomers of DCB were detected
more frequently in the blood of residents of the Love Canal area
(7-18 out of 36 compared with 2 out of 12 in the control
population), the levels of 1,2- and 1,3-DCB were not significantly
different from those in"unexposed" volunteers (i.e., <10 ng/g).
Levels of 1,4-DCB in the blood of Love Canal residents ranged from 4
to 36 ng/g, whereas concentrations in "unexposed" volunteers ranged
from 2 to 9 ng/g (Bristol et al., 1982). The 1,4-isomer of DCB was
detected in all samples of the blood of 6 Tokyo residents at levels
ranging from 4 to 16 µg/litre (Morita & Ohi, 1975). The 1,2,4,5-TeCB
isomer was detected in the blood of 1 out of 36 residents of the
Love Canal area at a concentration of 2 ng/g, but was not detected
in any of 12 "unexposed" volunteers (Bristol et al., 1982). Even in
occupationally exposed populations, levels of chlorobenzenes in the
blood are low; Lunde & Bjorseth (1977) reported detecting PeCB in
the blood of 9 out of 17 workers at concentrations ranging from 0.06
to 0.26 ng/g.
Table 13. Chlorobenzenes in human tissues, fluids, and exhaled air
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
MCB
Barkley et al. (1980) residents of the Old Love Canal area urine ND - 120 ng/litre (6/9)
blood ND - 17 µg/litre (8/9)
breath ND - trace (1/9)
Wallace et al. (1984) samples from 9 individuals from New Jersey and 3 from North breath 0.07 - 8.15 µg/m3 (31/66)
Carolina
DCB
Barkley et al. (1980) residents of the Old Love Canal area urine ND - 39 µg/litre (7/9)
blood 0.15 - 68 µg/litre (9/9)
breath ND - 5000 ng/m3 (7/9)
Antoine et al. (1986) patients in the USA with possible sensitivities to blood ND - 31 µg/litre (NA/250)
synthetic chemicals
1,3- + 1,4-DCB
Wallace et al. (1984) samples from individuals from North Carolina breath 0.09 - 0.76 µg/m3 (4/5)
Wallace (1986) samples from residents of cities in New Jersey and California breath 2.9 - 8.1 µg/m3 (NA/660)
1,2-DCB
Wallace (1986) samples from residents of Los Angeles breath 0.08 - 0.1 µg/m3 (NA/201)
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 1-4 µg/litre (9/36)
workers) volunteers: 3-4 µg/litre (2/12)
Table 13 (continued)
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
1,3-DCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 3-8 µg/kg (7/36)
workers) volunteers: 3-8 µg/kg (2/12)
1,4-DCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 4-26 µg/kg (18/36)
employees) volunteers: 2-9 µg/kg (2/12)
Morita & Ohi (1975) adipose tissue samples from medical examiners and 3 Tokyo adipose 0.2 - 11.7 mg/kg (34/34)
hospitals; tissue
blood from 6 healthy volunteers blood 4 - 16 µg/litre (6/6)
Morita et al. (1975) samples from hospitals or medical examiners in Tokyo adipose 0.2 - 9.9 mg/kg (15/15)
tissue
Pagnotto & Walkley (1965) analysis for urinary dichlorophenol in workers in urine 10 - 233 mg/litred
various stages of p-dichlorobenzene production
TCB
Barkley et al. (1980) residents of the Old Love Canal area breath ND - 90 ng/m3 (2/9)
1,3,5-TCB
Bristol et al. (1982)
residents of the Love Canal area and 9 volunteers (laboratory blood residents: 0.7 µg/kg (1/36)
employees) volunteers: ND (0/12)
TeCB
Barkley et al. (1980)
residents of the Old Love Canal area blood ND - 2.6 µg/litre (1/99)
breath ND - 180 ng/m3 (2/9)
Table 13 (continued)
Compounda; Reference Populationb Sample Concentration
matrix (no. of positives/no. of
samples)c
1,2,4,5-TeCB
Bristol et al. (1982) residents of the Love Canal area and 9 volunteers (laboratory blood residents: 2 µg/kg (1/36)
employees) volunteers: ND (0/12)
Morita et al. (1975) samples from hospitals or medical examiners in Tokyo adipose 0.008 - 0.039 mg/kg (15/15)
tissue
PeCB
Lunde & Bjorseth (1977) samples obtained from 17 employees working at various stages in blood 0.06 - 0.26 µg/kg (9/17)
magnesium production
Mes et al. (1982) samples obtained from autopsies of accident victims across adipose 0.001 - 0.020 mg/kg (92/99)
Canada tissue
Barkley et al. (1980) residents of the Old Love Canal area breath ND - 70 ng/m3 (1/90)
Williams et al. (1988) samples obtained from autopsies of individuals from 6 Canadian adipose ND - 43 mg/kg (9/141)
Great Lakes municipalities tissue
a Isomers specified where available.
b Number of subjects specified where available.
c ND - not detected.
d Levels of dichlorophenol found in urine.
6. KINETICS AND METABOLISM
With the exception of limited information concerning MCB and 1,4-DCB
in man, available data on the kinetics and metabolism of
chlorobenzenes (mono- to penta-) have been obtained from studies on
experimental animals.
6.1 Absorption
Although few quantitative data are available, the results of
experimental studies on animals (section 8) and human case reports
of poisonings (section 9) indicate that the chlorobenzenes are
readily absorbed from the gastrointestinal and respiratory tracts.
Available data also indicate that the position of the chlorines in
different isomers of the same congener influences absorption. It is
probable that the rate of transport of the chlorobenzenes (mono- to
penta-) across the gut wall also varies as a function of exogenous
factors, particularly the presence of bile and of dietary lipids in
the gastrointestinal tract, as is the case for hexachlorobenzene
(Koss & Koransky, 1977).
Quantitative data on absorption can be derived from studies on
experimental animals, the most important of these (summarized in
Table 14) are limited to the gastrointestinal tract. For example,
following intragastric administration of 1.5 g of 1,4-DCB to
Chinchilla rabbits, Azouz et al. (1955) did not detect any of the
unchanged compound in the faeces, implying that, under the
conditions of the study, total absorption had occurred. Hawkins et
al. (1980) reported that only 9% (the unabsorbed portion) of a
single radio-labelled dose of 250 mg 1,4-DCB/kg body weight was
present in the faeces of rats with cannulated bile ducts, at 24 h.
Six days following administration by stomach tube of 500 mg/kg body
weight of the 1,2,3,4-, 1,2,3,5- and 1,2,4,5-isomers of TeCB to
Chinchilla rabbits, 5%, 14%, and 16%, respectively, of the
administered doses were recovered unchanged in the faeces (Jondorf
et al., 1958). The percentages of the administered doses recovered
unchanged in the gut contents were 0.5%, 1.4%, and 6.2%,
respectively, suggesting that gastrointestinal absorption of the
compounds is relatively efficient and that the chlorine positions on
the molecule may influence the process. In rhesus monkeys, Rozman
et al. (1979) reported that 95% of an oral dose of 0.5 mg PeCB/kg
body weight was absorbed, as indicated by faecal elimination in the
first 4 days.
6.2 Distribution
The results of available studies indicate that, following
absorption, the chlorobenzenes are initially rapidly distributed to
highly perfused tissues, but then accumulate in fatty tissues,
because of their lipophilic nature (Jondorf et al., 1958; Jacobs
et al., 1974; Villeneuve & Khera, 1975; Jacobs et al., 1977; Kimura
et al., 1979; Hawkins et al., 1980; Linder et al., 1980; Smith &
Carlson, 1980; Chu et al., 1983; Sullivan et al., 1983; Tanaka
et al., 1986; Chu et al., 1987). Transplacental transfer (PeCB) into
the fetal brain and liver tissues has also been observed (Villeneuve
& Khera, 1975). Available data also indicate that, in general,
accumulation is greatest for the more highly chlorinated congeners,
but that it can vary enormously for different isomers of the same
chlorobenzene. For example, when 0.59 g of 14C-labelled MCB was
administered twice daily for 7 days to Dutch rabbits, only 0.05% of
the radiolabel was found in the tissues over the following 7-day
period (Smith et al., 1972). Following oral administration of 500 mg
1,3,5-TCB/kg to Chinchilla rabbits, only 5% of the administered dose
was present in the fat at 8 days, whereas 22% of a similar dose of
PeCB, administered subcutaneously, was present in the fat of the
same species at 10 days (Parke & Williams, 1960). Jondorf et al.
(1958) reported that 25%, 11%, and 5% of an oral dose of 500 mg/kg
was recovered in the fat of Chinchilla rabbits for the 1,2,4,5-,
1,2,3,5- and 1,2,3,4-isomers of TeCB, respectively, at 6 days.
Similarly, Chu et al. (1983) reported that the 1,2,4,5-isomer of
TeCB accumulated in the fat and liver at much higher levels (by 2
orders of magnitude) than the 1,2,3,5 and 1,2,3,4-isomers, following
oral administration for 28 days to rats of diets containing 0.5,
5.0, 50, or 500 mg chlorobenzene/kg, reflecting wide differences in
metabolic rates (section 6.3).
Peak levels in fat, which occurred 6-12 h after oral administration
of 200 or 800 mg 1,4-DCB/kg body weight to rats, were 50 times those
in the blood (Kimura et al., 1979). Braun et al. (1978) reported
that the ratio of 1,2,4,5-TeCB concentrations in fat to those in
plasma in beagle dogs was 650, following 1 month of oral
administration of 5 mg/kg body weight per day. This ratio decreased
steadily to about 280 by the end of the administration period (2
years) and increased rapidly in the 20 months following treatment.
Levels of the same isomer of the chlorobenzenes in various tissues
appear to be similar, regardless of the route of administration.
Hawkins et al. (1980) reported that the concentrations in rat
tissues at 24 h were similar following inhalation of 6000 mg
14C-labelled 1,4-DCB/m3 (1000 ppm) for 3 h/day or administration
of 250 mg/kg body weight per day orally, or subcutaneously, for up
to 10 days. At low levels of exposure, accumulation in adipose
tissue appears to be dose-related, whereas at high levels there
appears to be a disproportionate increase in the adipose tissue
burden. For example, Jacobs et al. (1974) reported a dose-related
accumulation of 1,2-DCB in the abdominal and renal adipose tissue of
rats following administration of a mixture of organic chemicals
including 1,2-DCB at doses of 0.4, 0.8, or 2 mg/kg diet per day for
4-12 weeks. On the other hand, when rats inhaled 14C-labelled MCB
at 455, 1820, or 3185 mg/m3 (100, 400, or 700 ppm) for 1 or 5
days, tissue concentrations increased in proportion to the level of
exposure, except for those in adipose tissue, which increased more
than 30-fold between 455 and 3185 mg/m3 (Sullivan et al., 1983).
Smith & Carlson (1980) demonstrated that starvation for 4 days did
not have any effects on the distribution of 1,2,4-TCB in the fat or
liver of rats following oral administration of 181.5 mg/kg
(1 mmol/kg body weight) per day for 7 days.
6.3 Metabolic Transformation
The chlorobenzenes are metabolized by microsomal oxidation and
proceed principally, either directly or through the formation of a
metastable arene oxide intermediate, to form the corresponding
chlorophenols. These chlorophenols can be excreted in the urine as
mercapturic acids, formed by conjugation with glutathione, or as
glucuronic acid or sulfate conjugates; they may also be eliminated
unchanged, mainly in expired air or faeces.
The results of available studies concerning the metabolism and
elimination of the chlorobenzenes are presented in Table 14. More
detailed comparative metabolic profiles for each of the
chlorobenzenes in rabbits, following oral administration of 0.5 g/kg
body weight, are presented in Table 15. On the basis of the results
of such studies, it can be concluded that: (a) metabolic
transformation of the chlorobenzenes decreases with increasing
degree of chlorination; and (b) metabolism and elimination of the
higher chlorinated congeners is slower and a greater proportion of
the compound is eliminated unchanged in the faeces or expired air.
The position of the chlorine atoms on the benzene ring is also an
important determinant of the rates of metabolism and elimination,
with chlorobenzenes with 2 adjacent unsubstituted carbon atoms
(e.g., 1,2,3-TCB, 1,2,3,4-TeCB) being more rapidly metabolized and
eliminated than congeners having a similar degree of chlorination,
but lacking unsubstituted carbon atoms. The presence of the two
adjacent unsubstituted carbon atoms facilitates the formation of
arene oxides. For isomers without adjacent unsubstituted carbon
atoms, intermediate reactions take place in which chlorine atoms are
shifted to adjacent carbons ("the NIH-shift") to form compounds that
can then form epoxide intermediates (Daly et al., 1972; Jerina &
Daly, 1974).
Table 14. Metabolism and elimination in experimental animals
Compound; Speciesa Study protocol Results
Reference
MCB Dutch rabbit oral administration (gavage) of 19.6% of administered label in urine, 1.05% in
Smith- 0.59 g of 14C-MCB, twice a day faeces and 0.05% in tissues; urinary metabolites
Lindsay et al. for 4 days; collection of urine were glucuronides (33.6%), ethereal sulfates
(1972) and faeces for 7 days (33.9%), mercapturic acids (23.8%), diphenols
(4.17%), monophenols (2.84%) and
3,4-dihydro-3,4-dihydroxy-chlorobenzene (0.57%)
MCB male Wistar intraperitoneal injection of urinary metabolites in 24 h: 4-chlorocatechol
Yoshida & rat 2 mmol/kg body weight in olive (1.6%), 2-chlorophenol (1.6%), 4-chlorophenol
Hara (1985) oil; collection of urine for 3 (4.8%), 3-chlorophenol (3.6%), and
days prior to, and for 4 days 4-chlorophenylmercapturic acid (19.9%)
following, the injection
1,4-DCB female CFY inhalation of 6000 mg after 5 days dosing, compound metabolized and
Hawkins rat 14C-1,4-DCB/m3 3 h/day, or daily eliminated primarily in the urine (91-97%); for
et al. (1980) oral or subcutaneous doses all routes of exposure, primarily 2,5-DCP sulfate
(250 mg/kg) for up to 10 days; (46-54% of total eliminated) and 2,5-DCP
administration of a dose glucuronide in the urine (31-34%) and bile
(250 mg/kg) to rats with (30-42%)
cannulated bile ducts
1,4-DCB Chinchilla oral administration (intragastric metabolism primarily through oxidation to 3,4-DCP
1,2-DCB rabbit in olive oil) of 1.5 g (500 mg/kg) (from 1,2-DCB) and 2,5-DCP (from 1,4-DCB) and
Azouz et al. 1,4-DCB or 500 mg/kg 1,2-DCB eliminated in the urine in the form of glucuronic
(1955) (intragastric in water) and sulfuric acid conjugates; metabolism and
elimination complete in 5-6 days for 1,2-DCB and
>6 days for 1,4-DCB; not detected in the faeces
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB male Charles oral (gastric intubation) or about 84% of the oral dose and 78% of iv dose
Lingg et al. River rat and intravenous administration of eliminated by rats and 40% (oral) and 22% (iv)
(1982) female rhesus 10 mg/kg by rhesus monkeys in the urine in 24 h; faecal
monkey elimination accounted for 11% (oral) and 7% (iv)
in rats, and <1% from both routes in monkeys;
48-61% comprised an isomeric pair of
3,4,6-trichloro-3,5-cyclohexadiene-1,2-diol
glucuronides, 14-37% comprised glucuronides of
2,4,5-TCP and 2,3,5-TCP, and 1-37% was
unconjugated TCPs; in the rats, 60-62% of the
urinary metabolites comprised 2 isomers
(2,4,5- and 2,3,5-) of
N-acetyl-S-(trichlorophenyl)-L-cysteine, 28-33%
comprised the 2,4,5- and 2,3,5-isomers of
trichlorothiophenol, with 1% 2,4,5-, and 10% 2,3,5-TCPs
1,2,4-TCB male Wistar oral administration of approximately 66% and 17% eliminated in the urine
Tanaka et al. rat 50 mg 14C-1,2,4-TCB/kg and faeces, respectively, in 7 days; 2.1%
(1986) exhaled; tissue residues evenly distributed, with
the exception of the adipose tissue in which
concentrations were consistently slightly higher;
principal metabolites in the urine were free
2,4,5- and 2,3,5-TCP and their conjugates; minor
metabolites in the urine were 5- or 6-sulfhydryl,
methylthio, methyl-sulfoxide, and methylsulfone
derivatives of TCB; DCBs and TCB were present in
expired air
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB Chinchilla oral dose (gavage in arachis oil) at 5 days, the urinary metabolites of the
1,2,3-TCB rabbit of 500 mg/kg body weight 1,2,4-isomer were glucuronide conjugates (27%),
1,3,5-TCB sulfuric acid conjugates (11%), and 2,3,5- and
Jondorf 2,4,5-trichlorophenylmercapturic acid (0.3%),
et al. (1955) major phenols being 2,4,5- and 2,3,5-TCP; the
1,2,3-isomer was metabolized to 2,3,4-TCP, and
3,4,5-TCP to a lesser extent and to small amounts
of 3,4,5-trichlorocatechol, with 50% of the dose
being eliminated in the urine as glucuronic acid
conjugates, 12% as sulfuric acid conjugates, and
0.3% as 2,3,4-trichlorophenyl-mercapturic acid;
for the 1,3,5-isomer, 20% was eliminated as
sulfuric acid conjugates, no mercapturic acid was
found, 2,4,6-TCP was the only phenol detected in
the urine and some unchanged 1,3,5-TCB was
present in faeces; the 1,2,3-isomer most rapidly
metabolized; the rate of metabolism in descending
order was: 1,2,3- > 1,2,4- > 1,3,5-TCB
1,2,3-TCB male single oral dose (gavage in corn elimination data obtained only for 1,3,5- and
1,2,4-TCB Sprague-Dawley oil) of 10 mg/kg body weight of 1,2,3-isomers; both rapidly eliminated in the
1,3,5-TCB rat each 14C-labelled isomer urine and faeces:
Chu et al. urine faeces total
(1987) 1,3,5- 24 h 47.1% 35.8% 82.9%
48 h 50.3% 38.3% 88.6%
1,2,3- 24 h 56.3% 35.6% 91.9%
48 h 58.5% 36.8% 95.3%
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4-TCB male rabbit intraperitoneal injection in major urinary metabolites of 1,2,4-TCB were
1,2,3-TCB vegetable oil; 60 - 75 mg/kg body 2,4,5- and 2,3,5-TCP; the major metabolite of
1,3,5-TCB weight 1,2,3-TCB was 2,3,4-TCP with 2,3,6- and 3,4,5-TCP
Kohli et al. as minor urinary metabolites; 1,3,5-TCB was
(1976) metabolized to 2,3,5- and 2,4,6-TCP and a third
more polar metabolite
1,3,5-TCB Chinchilla oral administration (gavage in at day 8, 13% of administered dose was in the
Parke & female rabbit arachis oil) of 500 mg/kg faeces, 12% exhaled, 23% (4% as MCB) in the gut,
Williams 5% in the pelt, 5% in depot fat, and 22% in the
(1960) carcass; <10% eliminated as the major urinary
metabolite of 2,4,6-TCP; minor metabolites
included 4-chlorocatechol and 4-MCP
1,2,4,5-TeCB Sprague-Dawley oral gavage in corn oil; 10 mg/kg for the 1,2,3,4- and 1,2,3,5- isomers,
1,2,3,5-TeCB rat body weight TeCB approximately 46-51% eliminated in the urine and
1,2,3,4-TeCB faeces within 48 h following administration,
Chu et al. whereas only 8% of the 1,2,4,5-isomer was
(1984b) eliminated in the same period; most of the
1,2,4,5-isomer was eliminated in the urine, the
1,2,3,4-isomer mainly in the faeces and the
1,2,3,5-isomer equally between the urine and
faeces; metabolites in decreasing order were as
follows: 1,2,3,4-TeCB: 2,3,4,5- and
2,3,4,6-TeCP, and traces of tetrachlorthiophenol
and 2,3,4-TCP; 1,2,3,5-TeCB: 2,3,4,6-TeCP,
isomeric mercaptotrichlorophenols, and a TCP;
1,2,4,5-TeCB: 2,3,5,6-TeCP, tetrachloroquinol,
and a TCP
and a TCP
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
1,2,4,5-TeCB male rabbit intraperitoneal injection in 1,2,3,5-TeCB the most extensively metabolized
1,2,3,5-TeCB vegetable oil; 60-75 mg/kg body isomer, yielding
1,2,3,4-TeCB weight 2,3,4,5-, 2,3,5,6-, and 2,3,4,6-TeCP;
Kohli et al. 1,2,3,4-TeCB was metabolized to 2,3,4,5- and
(1976) 2,3,4,6-TeCP and 1,2,4,5-TeCB yielded only 2,3,5,6-TeCP
1,2,3,4-TeCB Squirrel oral administration (gavage in major urinary metabolite was
Schwartz monkey corn oil) of 100 mg/kg 14C-TeCB N-acetyl-S-(2,3,4,5-tetrachlorophenyl) accounting
et al. (1985) for 85% in urine; minor urinary metabolite was 2,3,4,5-TeCP
1,2,3,4-TeCB Squirrel single oral dose of one of the main pathway of elimination for all isomers was
1,2,3,5-TeCB monkey 14C-labelled isomers dissolved faecal elimination - 38%, 36%, and 18% for
1,2,4,5-TeCB in corn oil, twice a week for 3 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-TeCB isomers,
Schwartz weeks, at the following doses; respectively; 1.2% of 1,2,3,4- and less than 0.1%
et al. (1987) 1,2,3,4- 100 mg/kg body weight; of the 1,2,3,5- and 1,2,4,5-TeCB isomers were
1,2,3,5- 100 mg/kg body weight eliminated in the urine; 1,2,3,4-isomer-Faeces:
1,2,4,5- 50 mg/kg body weight; 50% as the parent compound, metabolites included
urine and faeces collected 2,3,4,5-tetrachlorophenol (22%),
N-acetyl-S-(2,3,4,5-tetrachlorophenyl)-cysteine
(18%), 2,3,4,5-tetrachlorophenyl sulfinic acid
(3%), 2,4,5-trichlorophenyl methyl sulfoxide
(0.6%), and 2,3,4,5-tetrachlorophenyl methyl
sulfide (0.2%); urine:
N-acetyl-S-(2,3,4,5-tetrachlorophenyl)-cysteine
(85%) and 2,3,4,5-tetrachlorophenol (15%).
1,2,3,5-isomer-Faeces: 50% as unchanged TeCB,
2,3,4,5-tetrachlorophenol (2%),
2,3,4,6-tetrachlorophenol (14%),
Table 14 (continued)
Compound; Speciesa Study protocol Results
Reference
2,3,5,6-tetrachlorophenol (9%), and
2,3,4,6-tetrachlorophenyl sulfinic acid (15%);
urine: nondetectable; 1,2,4,5-TeCB-Faeces: >99%
as unchanged parent compound; urine:
nondetectable
PeCB Rhesus oral gavage of 0.5 mg/kg body approximately 12% of the administered dose
Rozman monkey weight 14C-PeCB eliminated in the urine after
et al. (1979) 40 days and 24% in the faeces (99%
unmetabolized); the 2,3,4,5-, 2,3,5,6-, and
1,2,3,4- isomers of TeCP identified as
metabolites; no sex-related differences in
metabolism
PeCB male rabbit intraperitoneal injection in urinary metabolites were 2,3,4,5-TeCP and PeCP,
Kohli et al. vegetable oil; 300 mg both detected at 1% of the administered dose, 10
(1976) days after dosing
PeCB male Wistar oral administration (gavage in major urinary metabolites: 2,3,4,5-TeCP and PeCP;
Engst et al. rat sunflower oil) of 8 mg/kg PeCB, 2,3,4,6-TeCP, and/or 2,3,5,6-TeCP present
(1976) in free form; TCP (isomer not specified),
2,4,6-TCP and 1,2,3,4-TeCB present in small
concentrations
PeCB female rat intraperitoneal administration (in over a 4-day period, 3% eliminated in unchanged
Koss & olive oil) of 403 µmol/kg body form; urinary metabolites: PeCP (9%),
Koransky weight 2,3,4,5-TeCP, tetrachlorohydroquinone, a
(1977) hydroxylated chlorothiocompound, and traces of
another isomer of TeCP; PeCP,
tetrachlorohydroquinone, tetrachlorophenol, and
hydroxylated chlorothio- compound present in faeces
Table 14 (continued)
a Strain specified where available.
MCP - monochlorophenol.
DCP - dichlorophenol.
TCP - trichlorophenol.
TeCP - tetrachlorophenol.
PeCP - pentachlorophenol.
MCB is metabolized to an arene oxide as the first stage
intermediate, the major urinary metabolites being 4-chlorocatechol
(and conjugates) and 4-chlorophenyl-mercapturic acid. Three isomeric
chlorophenols (2-, 3-, or 4-chlorophenol) and 3-chloro-catechol are
minor metabolites.
The availability of tissue glutathione and the extent of the
conjugation of the catechol and chlorophenol metabolites appear to
play an important role in species differences in the metabolism of
MCB. Examination of the metabolic profile, 24 h following
administration of MCB (dosage unspecified), in 13 species including
man, indicated that the pattern was quantitatively similar in all
species; the profile in man was most similar to that in the
guinea-pig with metabolites in human urine identified as
4-chlorocatechol, 4-chlorophenol, and 4-chlorophenylmercapturic acid
(Williams et al., 1975). Data from an additional comparative study
indicate that 4-chlorocatechol is the main urinary metabolite of
MCB in man (Ogata & Shimada, 1983). In rats, rabbits, and mice
administered 0.5, 1.0, or 2.0 mmol MCB/kg body weight,
intra-peritoneally, the ratios of urinary 4-chlorophenylmercapturic
acid: 4-chlorocatechol were 9.1, 7.2, 6.1; 1.6, 1.8, 1.8; and 7.3,
7.8, 6.4, in the various species, respectively. In man, however,
following ingestion of 3 repeated doses of 0.3 mmol MCB/kg body
weight, or inhalation (concentration not specified) of MCB, the
comparable ratios were considerably lower, 0.002 and 0.007,
respectively, because of the extremely small amounts of
4-chlorophenylmercapturic acid present in the urine.
Saturation of glutathione conjugation capacity has been observed and
may play a role in the manifestation of toxic effects from MCB
exposures. For example, Sullivan et al. (1983) reported a
disproportionate increase in the respiratory elimination of
unchanged compound and a dose-dependent decrease in the mercapturic
acid percentage of urinary metabolites from 68% at 455 mg/m3 (100
ppm) to 51% at 3185 mg/m3 (700 ppm), following inhalation by rats
for 1 or 5 days. Yoshida & Hara (1985) observed a transient decrease
in hepatic glutathione related to urinary levels of mercapturic acid
following intraperitoneal injection of rats with 2 mmol MCB/kg. A
transient decrease in urinary taurine levels was also observed,
which the authors attributed to depression of the oxidative
degradation of cysteine by the increase in glutathione synthesis.
The authors further suggested that the levels of sulfur-containing
dietary amino acids may have an effect on the metabolism or toxicity
of chlorobenzenes for which the major metabolite is mercapturic
acid.
Table 15. Metabolites of chlorobenzenes in rabbits following ingestion
of 0.5 g/kg body weighta
Percentage of dose eliminated as:
Compound Time of Mercapturic Monophenolsb Catecholsb Total O- Percentage of dose eliminated
elimination Acid conjugatesc unchanged
(days)d
MCB 1-2 25 2-3 27 47 27 in expired air
1,2-DCB 5 5 40 4 69 NSf
1,3-DCB 5 11 25 3 37 NSf
1,4-DCB 5 0 35 6e 63 NSf
in faeces in tissue
1,2,3-TCB 5 0.3 78 trg 62 0 NSf
1,2,4-TCB 5 0.4 42 trg 38 0 NSf
1,3,5-TCB 8 0 9 0 23 10 51
1,2,3,4-TeCB 6 0 34 trg 43 5 10
1,2,3,5-TeCB 6 0 5 0 8 14 23
1,2,4,5-TeCB 6 0 2 0 5 16 48
PeCB 6 0 <1 0 9 5 50
Table 15 (cont'd)
a From: Williams (1959).
b In some cases these numbers give the amounts isolated.
c Conjugated glucuronic acid plus ethereal sulfate.
d Metabolites produced only at a very low rate after this time.
e Quinols.
f NS - not stated.
g tr - trace.
The metabolism of the dichlorobenzenes in most animal species
proceeds through the formation of arene oxide intermediates but,
unlike that of MCB, it results primarily in the excretion of
dichlorophenols, generally as conjugates of glucuronic and sulfuric
acids. Mercapturic acids and catechols are only minor metabolites.
For 1,4-DCB, 2,5-dichloroquinol is also a minor metabolite. In
general, mercapturic acids and catechols do not appear to be formed
during the metabolism of 1,4-DCB in animals. However, Hawkins et al.
(1980) reported the presence of small amounts of "a mercapturic acid
of 1,4-DCB" in the urine during the 5-day period following exposure
of rats to 1,4-DCB via inhalation (6000 mg/m3, 3 h/day) or orally
or subcutaneously at (250 mg/kg body weight per day) for up to 10
days. Small amounts (<0.03% of the total dose) of sulfur-containing
metabolites, namely 2,5-dichlorophenyl methyl sulfoxide, and
2,5-dichlorophenyl methyl sulfone, have also been identified
following oral administration of 1,4-DCB to rats (Kimura et al.,
1979).
Limited available data indicate that the rate of metabolism of
1,2-DCB is slightly greater than that for the 1,4-isomer. Azouz et
al. (1955) reported that the metabolism and elimination of 1,2-DCB
and 1,4-DCB in rabbits was complete in 5-6 days, and >6 days,
respectively, following oral administration of 500 mg/kg.
Dichlorophenol has been detected in the urine of workers exposed to
1,4-DCB. Levels ranged from 10 to 233 mg/litre in an unspecified
number of workers employed at various stages in the production of
1,4-DCB (Pagnotto & Walkley, 1965).
The principal metabolic products of the trichlorobenzenes, which are
formed following generation of intermediate arene oxides, are
trichlorophenols (TCP) (2,3,4-TCP for the 1,2,3-isomer; 2,4,6-TCP
for the 1,3,5-isomer, and both 2,3,5-TCP and 2,4,5-TCP for the
1,2,4-isomer). Minor metabolites include trichlorocatechols (for the
1,2,3- and 1,2,4-isomers of TCB), other trichlorophenols (for all
isomers of TCB), trichlorophenyl-mercapturic acids (for the 1,2,3-
and 1,2,4-isomers of TCB), and 4-chlorophenol and 4-chloro-catechol
(for the 1,3,5-isomer of TCB). Kohli et al. (1976) also reported the
presence of a polar urinary metabolite ("possibly DCB with 2
hydroxyl and 1 methoxyl substituent") following intraperitoneal
injection of rabbits with 60-75 mg/kg of 1,3,5-TCB. Several
additional metabolites have been reported following administration
of 1,2,4-TCB to various species, including 5- or 6-sulhydryl,
methylthio, methylsulfoxide, and methylsulfone derivatives of TCB
(minor metabolites in rats) (Tanaka et al., 1986), 2,4,5- and
2,3,5-trichlorothiophenol and the 2,4,5- and 2,3,5-isomers of
N-acetyl- S-(trichlorophenyl)-L-cysteine (major metabolites in
rats) (Lingg et al., 1982), and an isomeric pair of
3,4,6-trichloro-3,5-cyclo-hexadiene-1,2-diol glucuronides (major
metabolites in monkeys) (Lingg et al., 1982).
The most rapidly metabolized of the 3 isomers is 1,2,3-TCB, with
over 60% of an orally administered dose of 500 mg/kg body weight
being eliminated in the urine of rabbits in a 5-day period, compared
with 38% and 28% for the 1,2,4- and 1,3,5-isomers, respectively
(Jondorf et al., 1955).
Available data also indicate that there are considerable species
differences in the metabolism of the trichlorobenzenes. In a study
by Lingg et al. (1982), in which 10 mg 1,2,4-TCB/kg body weight was
administered orally or intravenously to both rats and rhesus
mon-keys, the metabolic profile of the two species varied
considerably. In the monkey, a stereo isomeric pair of
3,4,6-trichloro-3,5-cyclo-hexadiene-
1,2-diol glucuronides accounted for over half of the urinary
metabolites while, in the rat, the 2,4,5- and
2,3,5- N-acetyl- S-(trichlorophenyl)-L-cysteine isomers accounted
for the majority of the urinary metabolites. In addition to the
metabolic pathway being species specific, 2-3 times as much
14C-TCB was excreted in 24 h in the rat as in the monkey.
The tetrachlorobenzenes are slowly metabolized, principally to 3
tetrachlorophenols (2,3,4,5-, 2,3,4,6-, and 2,3,5,6-isomers). Arene
oxides are postulated to be metabolic intermediates and "NIH shifts"
are required in most of the postulated pathways. Schwartz et al.
(1985) recently reported that the major urinary metabolite
(accounting for 85% of the radioactivity in the urine) following
oral administration of 100 mg 1,2,3,4-TeCB/kg body weight to
squirrel monkeys was N-acetyl- S-(2,3,4,5-tetrachlorophenyl)
cysteine. A minor metabolite of the 1,2,3,4-TeCB isomer was
2,3,4-trichlorophenol. Tetrachlorophenols are major metabolites of
TeCBs in rats (Chu et al., 1984b). Isomeric mercaptotrichlorophenols
and a trichlorophenol have been identified as metabolites of
1,2,3,5-TeCB in rats; tetrachloroquinol and a trichlorophenol have
also been detected in this species following administration of
1,2,4,5-TeCB (Chu et al, 1984b).
The 1,2,4,5-isomer is not well metabolized and tends to remain in
the tissues for a considerable time in the unchanged state. For
example, following the oral administration of 500 mg/kg body weight
to rabbits, 48% of the 1,2,4,5-isomer was detected unchanged in the
tissues at 6 days, compared with 10% for the 1,2,3,4 isomer and 23%
for the 1,2,3,5-isomer (Jondorf et al., 1958). Following oral
administration of 10 mg/kg body weight to rats, 46-51% of the
1,2,3,4- and 1,2,3,5-isomers was eliminated in the urine and faeces
within 48 h, whereas only 8% of the 1,2,4,5-isomer was eliminated in
the same period (Chu et al., 1984b).
Available data also indicate that there are species differences in
the metabolism of tetrachlorobenzenes. In a recent study described
in Table 14, the tetrachlorobenzenes were not as extensively
metabolized in squirrel monkeys as in other species, and metabolites
were eliminated exclusively in the faeces (Schwartz et al., 1987).
PeCB may be metabolized primarily to pentachlorophenol by direct
oxidation or to 2,3,4,5-tetrachlorophenol via an arene oxide
intermediate. Other compounds that have been identified as
metabolites of PeCB include the 1,2,3,4- and 2,3,5,6-isomers of
tetrachlorophenol in monkeys (Rozman et al., 1979), and the
2,3,4,6-isomer of tetrachlorophenol, an unspecified isomer of
trichorophenol, 2,4,6-trichlorophenol, 1,2,3,4-TeCB, and
tetra-chlorohydroquinone in rats (Engst et al., 1976; Koss &
Koransky, 1977).
In in vitro studies on rat liver microsomes, the principal primary
metabolites of pentachlorobenzene were pentachlorophenol and
2,3,4,6-tetrachlorophenol in a ratio of 4:1 (den Besten et al.,
1989). Minor metabolites included the 2,3,4,5- and 2,3,5,6-isomers
of tetrachlorophenol. In addition, para- and ortho-
tetrachlorohydroquinone were formed as secondary metabolites. The
authors suggested that these compounds may be involved in the
covalent binding of microsomal protein.
6.4 Elimination and Excretion
While there is variation among different isomers of the same
congener and among species, generally, the metabolism and excretion
of the higher chlorinated benzenes is slower than that of mono- and
dichlorobenzenes, and a greater proportion of the compound is
eliminated unchanged in the faeces or expired air. This is
illustrated by the data on the metabolism and elimination of the
various chlorobenzenes in the rabbit following the oral
administration of 500 mg/kg body weight, which are presented in
Table 15 (Williams, 1959). Hawkins et al. (1980) reported that
1,4-DCB was eliminated primarily in the rat urine (91-97%) in the
5-day period following inhalation, subcutaneous, or oral exposure.
It has also been reported that 78-84% of an oral or intravenous dose
of 10 mg 1,2,4-TCB/kg body weight was eliminated in the urine and
that 7-11% was eliminated in the faeces of rats at 24 h; in monkeys,
the comparable values were 40% and <1% (Lingg et al., 1982). In
rats receiving an oral dose of 50 mg 1,2,4-TCB/kg, approximately 66%
and 17% were eliminated in the urine and faeces, respectively, in 7
days; 2.1% was exhaled (Tanaka et al., 1986). Rozman et al. (1979)
reported that approximately 12% of an oral dose of 0.5 mg PeCB/kg
was eliminated in the urine of rhesus monkeys and 24% in the faeces
(99% unmetabolized) within 40 days. In a study by Koss & Koransky
(1977), it was observed that 3% was excreted unchanged during a
4-day period following administration of 403 µmol PeCB/kg body
weight. The higher chlorobenzenes tend to be stored in the adipose
depots of the body at higher levels than monochloro- and
dichloro-congeners (section 6.2, Table 14).
Available data on the half-lives of the chlorobenzenes in the
tissues of experimental animals are restricted to the
trichlorobenzenes, the 1,2,4,5-isomer of TeCB, and PeCB. Chu et al.
(1987) reported that the terminal half-lives for 1,2,3-TCB,
1,2,4-TCB, and 1,3,5-TCB in rats were 145, 93, and 68 h,
respectively. It was reported by Braun et al. (1978) that the
half-lives for the elimination of 1,2,4,5-TeCB from the fat and
plasma of beagle dogs were 111 and 104 days, respectively. In rhesus
monkeys, the estimated half-life of PeCB (tissue unspecified) was
2-3 months (Rozman et al., 1979).
6.5 Binding to Protein
The results of several studies indicate that the metabolites of MCB
and DCB, most likely the arene oxide intermediate or unconjugated
chlorophenols, covalently bind to rat kidney and liver tissues and,
in some cases, induce damage (Oesch et al., 1973; Reid, 1973; Reid &
Krishna, 1973). The binding and toxic effects of MCB were prevented
by piperonyl butoxide, cyclohexene oxide, and glutathione (Oesch et
al., 1973; Reid, 1973; Reid & Krishna, 1973) and were reduced by
3-methylcholanthrene (Reid, 1973; Reid & Krishna, 1973). The binding
of 1,2-DCB to rat liver protein was enhanced by pretreatment with
phenobarbital; the 1,4-isomer of DCB did not bind to the same extent
and was less hepatotoxic than the 1,2-isomer (Reid & Krishna, 1973).
6.6 Effects on Metabolizing Enzymes
The chlorobenzenes are broad inducers of metabolic reactions
including both oxidative and reductive, as well as conjugation
hydrolytic pathways. The effects of the different chlorobenzenes on
the metabolizing enzymes vary considerably.
The 1,4-isomer of dichlorobenzene and 1,2,4-trichlorobenzene induced
cytochrome-c-reductase, cytochrome P-450, EPN-detoxification
( O-ethyl O-p-nitrophenyl phenylphosphonothioate), glucuronyl
transferase, benzopyrene hydroxylase, and azoreductase following
oral administration of doses of up to 40 mg/kg per day for 14 days
in the rat. In contrast, administration of doses of MCB as high as
800 mg/kg per day had no effects on these enzymes (Carlson &
Tardiff, 1976).
The cytochrome P-450 content in rat liver and the enzymes,
aminopyrine demethylase and aniline hydroxylase, were induced by
1,4-DCB, 1,3,5-TCB, 1,2,4,5-TeCB, and PeCB, but not by MCB, after
administration of oral doses of between 125 and 500 mg/kg body
weight, daily, for 1 or 3 days. Aniline hydroxylase activity did
increase, however, with high doses of monochlorobenzene (1000 mg/kg
body weight) (Ariyoshi et al., 1975). MCB has also been reported to
induce epoxide hydrolase in rat liver (Oesch et al., 1973). There
was, therefore, no consistent pattern of enzyme induction in
relation to the degree of chlorination of the chlorobenzenes. The
most potent inducer was found to be 1,2,4-TCB (Ariyoshi et al.,
1981).
The induction of hepatic metabolizing enzymes in rats treated with
trichlorobenzenes differs from isomer to isomer. The 1,2,4-isomer
induced NADPH-cytochrome-c-reductase, acetanilide esterase,
arylesterase, and procaine esterase, but not cytochrome P-450 or
acetanilide hydroxylase, following oral administration to rats of
0.1 mmol/kg per day for 14 days. On the other hand, the 1,3,5-isomer
induced acetanilide hydroxylase, acetanilide esterase, and procaine
esterase, but not arylesterase (Carlson et al., 1979; Carlson,
1980).
In pregnant rats, 1,2,4-TCB, 1,2,4,5-TeCB, and 1,2,3,4-TeCB all
induced hepatic cytochrome P-450, aninopyrine N-demethylase, and
ethoxy resorufin- O-deethylase. In addition, 1,2,4-TCB and
1,2,3,4-TeCB increased NADPH-cytochrome-c-reductase, glucuronyl
transferase for p-nitrophenol, and glutathione- S-transferase
activities (Kitchin & Ebron, 1983a,b,c).
Although the induction of metabolizing enzymes by PeCB has been less
well studied, it has been shown to induce the O-dealkylation of
7-ethoxycoumarin in rats following the ingestion of 0.05% in the
diet, indicating that it stimulates the cytochrome P-450 system
(Goerz et al., 1978).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The database on the effects of chlorobenzenes on organisms in the
environment is restricted to studies on acute toxicity in aquatic
systems. Only a few studies have been reported on the long-term
effects of chlorobenzenes on the aquatic environment, and none on
the effects of chlorobenzenes on non-mammalian terrestrial animals
and aquatic vascular plants.
7.1 Microorganisms
7.1.1 Bacteria and protozoa
The toxicity thresholds of MCB and 1,2-DCB for protozoa and bacteria
have been reported by Bringmann & Kuhn (1980). MCB levels in excess
of 3500 µmol/litre were noted in studies on Entosiphon sulcatum,
Uronema parduczi, and Chilomonas paramecium. The authors studied
the toxicity threshold over a 72-h period in an open system, where
an inhibition of cell growth greater than 3% was considered a
significant effect. Toxicity thresholds for 1,2-DCB in the same
three species were reported to be: >435µmol/litre for E. sulcatum,
544 µmol/litre for U. parduczi , and >410 µmol/litre for C.
paramecium. With regard to bacteria, Boyles (1980) reported that a
level of 270 µmol 1,2-DCB/litre resulted in a 50% reduction in the
growth rate of Vibrio natriegens (EC50, 40-min). In Pseudomonas
putida, Bringmann & Kuhn (1980) reported 16-h toxicity thresholds
for MCB and 1,2-DCB of 150 and 100 µmol/litre, respectively.
Using Tetrahymena pyriformis, Yoshioka et al. (1985) reported
EC50 concentrations (cell proliferation) of 51, 130, 20, 0.91, and
30 mg/litre for 1,2-DCB, 1,3-DCB, 1,2,4,5-TeCB, 1,2,4-TCB, and
1,3,5-TCB, respectively.
7.1.2 Unicellular algae
The acute toxicities of various chlorobenzenes for unicellular algae
are summarized in Table 16.
In general, the acute toxicities of chlorobenzenes for aquatic
unicellular algae, whether measured according to the inhibition of
chlorophyll a production or cellular proliferation, increase with
increasing chlorination. MCB was reported to have a 96-h EC50 of
232 mg/litre in Selenastrum capricornutum, whereas 1,2,3,5-TeCB
showed a 96-h EC50 of 17.2 mg/litre in this species; both were
measured according to the inhibition of chlorophyll a production.
The estuarine alga, Skeletonema costatum, appears to be more
sensitive to the acute effects of 1,2,3,5-TeCB; a 96-h EC50 of 0.7
mg/litre has been reported by the US EPA (1980a). Calamari et al.
(1983) demonstrated the comparable toxicity of 1,2-DCB, 1,4-DCB,
1,2,3-TCB, and 1,2,4-TCB in the green alga Selenastrum
capricornutum. MCB was less toxic (Table 16).
In the freshwater alga Ankistrodesmus falcatus, a 4-h EC50 for
primary productivity of 0.005 mmol/litre was shown for PeCB, whereas
MCB had an EC50 of 0.44 mmol/litre, under the same conditions
(Wong et al., 1984). Canton et al. (1985) reported EC50s for
growth of 17, 31, and 31 mg/litre, for 1,2-DCB, 1,3-DCB, and
1,4-DCB, respectively.
7.2 Aquatic Organisms
7.2.1 Plants
No data have been reported on the effects of chlorinated benzenes on
freshwater or saltwater vascular plants.
7.2.2 Invertebrates
Available data for the acute toxicity of chlorobenzenes in aquatic
invertebrates are given in Table 17. Most of the studies were
carried out at high chlorobenzene concentrations, yielding LC50
values that sometimes exceeded the solubilities of the compounds,
particularly those with higher levels of chlorination (see for
example LeBlanc, 1980).
In a study by Calamari et al. (1983), an enclosed static system was
used in which the authors measured the concentration of 1,4-DCB
throughout the test period. A 24-h IC50 (immobilization
concentration) of 1.6 mg/litre was reported for 1,4-DCB; the 24-h
IC50 for 1,2-DCB was 0.78 mg/litre. Such experimental data
probably reflect more accurately the true toxicity values than those
from an open, static apparatus, relying on nominal concentrations of
chlorobenzenes.
Hermens et al. (1985) determined the no-observed-effect
concentration (NOEC) on growth and the concentration inhibiting
growth of Daphnia magna by 50 % (EC50) in acute exposures to
chlorobenzenes. Values for the NOEC in µmol/litre were 0.59 for
1,2,3,4-TeCB and 1.14 for PeCB.
When LC50s (48-h) and LC0s were determined for a range of
chlorobenzenes (LeBlanc, 1980) for Daphnia magna, there was no
trend in toxicity in relation to the degree of chlorination. Wide
differences in response to different isomers of the same congener
were seen (Table 17).
Table 16. Acute toxicity of chlorobenzenes for aquatic unicellular algae
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Freshwater species
Selenastrum capricornutum MCB 232.0 EC50 (96-h) chlorophyll a
US EPA (1980a) 224.0 EC50 (96-h) cell growth
1,2,4-TCB 35.3 EC50 (96-h) chlorophyll a
36.7 EC50 (96-h) cell growth
1,2,3,5-TeCB 17.2 EC50 (96-h) chlorophyll a
17.7 EC50 (96-h) cell growth
1,2,4,5-TeCB 52.9 EC50 (96-h) chlorophyll a
46.8 EC50 (96-h) cell growth
PeCB 6.78 EC50 (96-h) chlorophyll a
6.63 EC50 (96-h) cell growth
EC50 (96-h) cell growth
Selenastrum capricornutum 1,2-DCB 91.6 EC50 (96-h) chlorophyll a
US EPA (1980b) 98.0 EC50 (96-h) cell growth
1,2-DCB 179.0 EC50 (96-h) chlorophyll a
149.0 EC50 (96-h) cell growth
1,4-DCB 98.1 EC50 (96-h) chlorophyll a
96.7 EC50 (96-h) cell growth
EC50 (96-h) cell growth
Table 16 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Selenastrum capricornutum MCB 12.5 EC50 (96-h) cell growth
Calamari et al. (1983)a 33.0 EC50 (3-h) decreased photosynthesis
1,2-DCB 2.2 EC50 (96-h) cell growth
10.0 EC50 (3-h) decreased photosynthesis
1,4-DCB 1.6 EC50 (96-h) cell growth
5.2 EC50 (3-h) decreased photosynthesis
1,2,3-TCB 0.9 EC50 (96-h) cell growth
2.2 EC50 (3-h) decreased photosynthesis
1,2,4-TCB 1.4 EC50 (96-h) cell growth
3.9 EC50 (3-h) decreased photosynthesis
Chlorella vulgaris MCB 99.1 EC50 (3-h) cell growth
Hutchinson et al. (1980) 1,2,3-TCB 6.2 EC50 (3-h) cell growth
1,2,3,5-TeCB 2.51 EC50 (3-h) cell growth
Ankistrodesmus falcatus MCB 50 EC50 (4-h) primary productivity 14C uptake
(acicularis) 1,2-DCB 20 EC50 (4-h) primary productivity 14C uptake
Wong et al. (1984)a 1,3-DCB 23 EC50 (4-h) primary productivity 14C uptake
1,4-DCB 20 EC50 (4-h) primary productivity 14C uptake
1,2,3-TCB 6 EC50 (4-h) primary productivity 14C uptake
1,2,4-TCB 6 EC50 (4-h) primary productivity 14C uptake
1,3,5-TCB 9 EC50 (4-h) primary productivity 14C uptake
1,2,3,4-TeCB 4 EC50 (4-h) primary productivity 14C uptake
1,2,3,5-TeCB 3 EC50 (4-h) primary productivity 14C uptake
1,2,4,5-TeCB 5 EC50 (4-h) primary productivity 14C uptake
PeCB 1.3 EC50 (4-h) primary productivity 14C uptake
Table 16 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Chlamydomas angulosa MCB 56.9 EC50 (3-h) cell growth
Hutchinson et al. (1980) 1,2,3-TCB 3.45 EC50 (3-h) cell growth
1,2,3,5-TeCB 1.58 EC50 (3-h) cell growth
Skeletonema costatum MCB 343.0 EC50 (96-h) chlorophyll a
US EPA (1980a) 341.0 EC50 (96-h) cell growth
1,2,4-TCB 8.75 EC50 (96-h) chlorophyll a
8.93 EC50 (96-h) cell growth
1,2,3,5-TeCB 0.83 EC50 (96-h) chlorophyll a
0.70 EC50 (96-h) cell growth
1,2,4,5-TeCB 7.10 EC50 (96-h) chlorophyll a
7.30 EC50 (96-h) cell growth
PeCB 2.23 EC50 (96-h) chlorophyll a
1.98 EC50 (96-h) cell growth
Skeletonema costatum 1,2-DCB 44.2 EC50 (96-h) chlorophyll a
US EPA (1980b) 44.1 EC50 (96-h) cell growth
1,3-DCB 52.8 EC50 (96-h) chlorophyll a
49.6 EC50 (96-h) cell growth
1,4-DCB 54.8 EC50 (96-h) chlorophyll a
59.1 EC50 (96-h) cell growth
a Carried out using a closed system, with measured concentrations of chlorobenzenes.
Table 17. Acute toxicity of chlorobenzenes for aquatic invertebrates
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Freshwater species
Daphnia magna MCB 86 (64-120) LC50 (48-h) immobilization
Le Blanc (1980)a 10 LC0 (48-h) NOEL
1,2-DCB 2.4 (1.9-3.0) LC50 (48-h) immobilization
0.36 LC0 (48-h) NOEL
1,3-DCB 28 (21-34) LC50 (48-h) immobilization
6 LC0 (48-h) NOEL
1,4-DCB 11 (6.6-19.0) LC50 (48-h) immobilization
0.68 LC0 (48-h) NOEL
1,2,4-TCB 50 (7.2-130) LC50 (48-h) immobilization
<2.4 LC0 (48-h) NOEL
1,2,3,5-TeCB 9.7 (6.6-14.0) LC50 (48-h) immobilization
<1.1 LC0 (48-h) NOEL
1,2,4,5-TeCB >530 LC50 (48-h) immobilization
320 LC0 (48-h) NOEL
PeCB 5.3 (4.1-7.2) LC50 (48-h) immobilization
1.3 LC0 (48-h) NOEL
Daphnia magna MCB 4.3 LC50 (24-h) immobilization
Calarmari et al. (1983)b 1,2-DCB 0.78 LC50 (24-h) immobilization
1,4-DCB 1.6 LC50 (24-h) immobilization
1,2,3-TCB 0.35 LC50 (24-h) immobilization
Daphnia magna MCB 51.6 mmol/m3 LC50 (48-h) lethality
Abernethy et al. (1986) 1,2-DCB 16.0 mmol/m3 LC50 (48-h) lethality
1,4-DCB -- LC50 (48-h) lethality
1,2,3-TCB 8.0 mmol/m3 LC50 (48-h) lethality
1,2,3,5-TeCB 4.0 mmol/m3 LC50 (48-h) lethality
PeCB 1.2 mmol/m3 LC50 (48-h) lethality
Table 17 (continued)
Test organism; Reference Chlorobenzene Concentration Criterion
(mg/litre)
Seawater species
Tanytarsus dissimilis 1,2-DCB 11.76 LC50 (48-h) lethality
US EPA (1980b)b 1,4-DCB 13.0 LC50 (48-h) lethality
Mysidopsis bahia MCB 16.4 LC50/EC50 (96-h) lethality
US EPA (1980a) 1,2,4-TCB 0.45 LC50/EC50 (96-h) lethality
1,2,3,5-TeCB 0.34 LC50/EC50 (96-h) lethality
1,2,4,5-TeCB 1.48 LC50/EC50 (96-h) lethality
PeCB 0.16 LC50/EC50 (96-h) lethality
Mysidopsis bahia 1,2-DCB 1.97 LC50/EC50 (96-h) lethality
US EPA (1980b)b 1,3-DCB 2.85 LC50/EC50 (96-h) lethality
1,4-DCB 1.99 LC50/EC50 (96-h) lethality
Paleaemonetes pugio 1,2,4-TCB 0.54 LC50 (96-h) lethality
Clark et al. (1987)c
a Closed static system; nominal concentrations.
b Aquatic concentrations of CB measured; static conditions employed.
c A flow-through system was used and concentrations of water-borne TCB measured.
Calamari et al. (1983) exposed 30 specimens of Daphnia magna to a
range of chlorobenzenes in closed bottles for 14 days, to obtain 3
broods. The higher chlorinated materials had lower effective
concentrations on fertility (14-day EC50s) ranging from
2.5 mg/litre for MCB to 0.20 mg/litre for 1,2,3 TCB.
When saltwater crustacea were exposed to high chlorobenzene
concentrations (55 µmol/litre), Grosch (1973) found that a single
24-h exposure of adult Artemia salina (brine shrimp) to 1,3,5-TCB
resulted in a decreased life span, a delay in the onset of the first
brood, and a decreased number of offspring. The US EPA (1980b)
reported that the LC50 values for the exposure of Mysidopsis
bahia (mysid shrimp) were similar for the 3 DCB isomers (1.97
mg/litre for 1,2- and 1,4-DCB, and 2.85 mg/litre for 1,3-DCB).
However, these tests were carried out in open static systems and the
reported values may not reflect accurately the actual toxicity of
these chemicals.
The toxic effects of 1,4-DCB and 1,3,5-TCB on the embryonic and
larval stages of clams (Mercenaria mercenaria) and oysters
(Crassostrea virginica) were studied by Davis & Hidu (1969).
Embryos, initially at the 2-cell stage, were exposed to 1,4-DCB or
1,3,5-TCB for 48 h. The median toxicity levels (TL50) for embryo
development to normal larvae were >680 µmol/litre and >55
µmol/litre for clam eggs exposed to 1,4-DCB and 1,3,5-TCB,
respectively.
7.2.3 Fish
The results of studies on the acute toxicity of chlorobenzenes for
fish are presented in Table 18. A comparison of Tables 17 and 18
shows a similar degree of sensitivity to chlorobenzenes between
bluegills (Lepomis macrochirus) and Daphnia magna. With a few
exceptions, open static systems were used in the acute toxicity
tests on fish, without measurements of actual concentrations in the
water. With the exception of 1,2,3,5-TeCB, the acute toxicity of
chlorobenzenes in several fish species increased with increased
chlorination, and was well correlated with the log P (octanol:water
partition coefficient) value (Calamari et al., 1983). Buccafusco
et al. (1981) stated that undissolved, preciptated chlorobenzenes
were present in the test vessels. The LC50s, therefore, are
probably underestimates of the toxicity of chlorobenzenes to fish.
In addition to the acute toxicity studies summarized in Table 18,
several studies have been reported on the effects of chlorobenzenes
on various developmental stages (i.e., embryo, larval, juvenile,
etc.) of freshwater and saltwater fish (Birge et al., 1979; US EPA,
1980a,b). Such embryo-larval studies more accurately reflect the
long-term toxicities arising from continuous exposures to low levels
of chlorobenzenes, at sensitive life-stages, than the acute LC50
studies.
Using the freshwater fathead minnow (Pimephales promelas) in an
embryo larval assay, long-term toxicity limits of 2.0, 1.51, and
0.76 mg/litre were reported for 1,2-, 1,3-, and 1,4-DCB,
respectively, by the US EPA (1980b). Long-term toxicity limits in
the estuarine sheepshead minnow for 1,2,4-TCB and 1,2,4,5-TeCB were
0.22 and 0.13 mg/litre, respectively (US EPA, 1980a); in the
freshwater fathead minnow (Pimephales promelas), long-term
toxicity limit values of 0.71, and 0.32 mg/litre were reported for
1,2,4-TCB and 1,2,3,4-TeCB, respectively (US EPA, 1980a).
Birge et al. (1979) exposed rainbow trout eggs to MCB at a range of
concentrations from 0.09 mg/litre, for 16 days; all concentrations
were lethal and, thus, a no-observed-effect level could not be
determined. Goldfish (Carassius auratus) and largemouth bass
(Micropterus salmonides) were less sensitive to MCB. Larvae were
more susceptible to MCB than eggs. For the goldfish, the LC50 with
exposure up to hatching was 3.5 mg/litre in soft water and 2.4
mg/litre in hard water. Exposure until 4 days after hatching
resulted in an LC50 of 1 mg/litre. Largemouth bass showed an
LC50 of 0.3 mg/litre, at hatching, and an LC50 of 0.05 mg/litre,
4 days after hatching.
7.3 Terrestrial Biota
No data are available.
7.4 Model Ecosystems
In a study by Tagatz et al. (1985), sand-filled aquaria were used in
which macrobenthic animal communities were allowed to develop. In
one test, colonization of the system by planktonic larvae for 50
days, was followed by 6 days of exposure to waterborne
concentrations of 1,2,4-TCB. In a second study, colonization was
allowed to proceed for 8 weeks in the presence of sediment
containing 1,2,4-TCB. The same types of organisms were affected,
whether the TCB was waterborne or sediment bound. However, community
structure was affected at waterborne concentrations (measured) that
were 2 orders of magnitude lower than those of the sediment-bound
TCB. The lowest waterborne TCB concentrations that affected the
number of individuals were: 0.04 mg/litre for molluscs; 0.4 mg/litre
for arthropods; and 4 mg/litre for annelids. At 4 mg/litre, the
average number of species was significantly lower than that in the
control aquaria.
Table 18. Acute toxicity of chlorobenzenes for fish
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
Freshwater species
Salmo gairdneri
(rainbow trout)
Calamari et al. (1983) FB; M MCB 4.1 LC50 (48-h) lethality
1,2-DCB 2.3
1,4-DCB 1.18
1,2,3-TCB 0.71
1,2,4-TCB 1.95
US EPA (1980b) FB; M 1,2-DCB 1.58 LC50 (96-h) lethality
1,4-DCB 1.12
Pimephales promelas
(fathead minnow)
US EPA (1980b) FB; M 1,3-DCB 7.79 LC50 (96-h) lethality
1,4-DCB 4.0
US EPA (1980a) FB; M 1,2,3,4-TeCB 1.07 LC50 (96-h) lethality
Lepomis macrochirus
(bluegill)
Buccafusco et al. (1981) Sb; U MCB 16 (13-20) LC50 (96-h) lethality
1,2-DCB 5.6 (4.8-6.6)
1,3-DCB 5.0 (3.9-6.2)
1,4-DCB 4.3 (3.9-4.8)
Table 18 (continued)
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
1,2,4-TCB 3.4 (2.7-4.1)
1,2,3,5-TeCB 6.4 (5.2-8.1)
1,2,4,5-TeCB 1.6 (1.3-1.8)
PeCB 0.25 (0.18-0.32)
Salt-water species
Cyprinodon variegatus
(sheepshead minnow)
Heitmuller et al. (1981) S; U MCB 10 (8.8-12) LC50 (96-h) lethality
6.2 LC0 (96-h) NOEL lethality
1,2-DCB 9.7 (9.0-10.0) LC50 (96-h) lethality
1,3-DCB 7.8 (6.8-8.7) LC50 (96-h) lethality
4.2 LC0 (96-h) NOEL lethality
1,4-DCB 7.4 (6.8-7.9) LC50 (96-h) lethality
5.6 LC0 (96-h) NOEL lethality
1,2,4-TCB 21 (17-26) LC50 (96-h) lethality
15 LC0 (96-h) NOEL lethality
Table 18 (continued)
Test organism; Test Conditionsa Chlorobenzene Concentration Criterion
Reference (mg/litre)
Heitmuller et al. (1981) 1,2,3,5-TeCB 3.7 (3.3-4.1) LC50 (96-h) lethality
(continued)
1.0 LC0 (96-h) NOEL lethality
1,2,4,5-TeCB 0.8 (0.7-1.1) LC50 (96-h) lethality
0.3 LC0 (96-h) NOEL lethality
PeCB 0.8 (0.4-1.8) LC50 (96-h) lethality
0.3 LC0 (96-h) NOEL lethality
Menidia beryllina
(tidewater silverside)
Dawson et al. (1977) S; U 1,2-DCB 7.3 LC50 (96-h) lethality
a S - static bioassay;
U - actual concentrations in system not monitored;
FB - flow-through conditions for bioassay;
M - concentrations of chlorobenzenes in experimental media determined by monitoring.
b Closed system.
The effects of 1,2,4-TCB on a natural freshwater phytoplankton
community were reported by Schauerte et al. (1985). A water meadow,
rich in both Daphnia and phytoplankton, was divided into
approximately 200 litre test cells to study the effects of 0.25 mg
1,2,4-TCB/litre over a period of 22 days, with a half-time of 13.7
days. No chemical-related effects on the abundance and diversity of
the phytoplankton community were noted. However, the high toxicity
(greater than 90% death) for Daphnia may have resulted in algal
blooms from fewer grazing species. The authors noted that the
toxicity of 1,2,4-TCB for Daphnia was much higher in the natural
system than was observed by Bringman & Kuhn (1982) in the laboratory
(0.25 mg/litre compared with 1.2-21 mg/litre).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposure
The results of the more recent and well documented acute toxicity
studies on the chlorobenzenes in experimental animals are presented
in Table 19. For isomers for which there were few available studies,
quantitative data (i.e., LC50s or LD50s) from early
investigations have also been included. A more complete review of
these early studies is included in US EPA (1985).
Inhalation of sufficiently high single doses of volatile
chlorobenzenes causes local respiratory irritation and depression of
the central nervous system; ingestion of lethal doses leads to
respiratory paralysis. Effects on the liver, kidneys, adrenal
glands, mucous membranes, brain ganglion cells, and metabolizing
enzymes have also been observed following acute exposure to
non-lethal doses of the chlorobenzenes.
Data concerning the acute toxicity of the higher chlorinated
compounds are sparse; some conclusions concerning the relative acute
toxicity of the different congeners can be drawn, however, from
examination of the oral LD50s in rats in the more recent and well
documented studies. On the basis of both mass and molar quantities,
the higher chlorinated benzenes appear, in general, to be more
acutely toxic than the mono- and dichlorobenzenes; however, for any
one isomer, there is a wide range of LD50s. In general, the dermal
LD50s are considerably higher than those for ingestion, probably,
in some cases, as a result of evaporation losses from the skin
(though this is less likely with occlusive dressings) together with
relatively poor dermal absorption. Inhalation LC50s have varied
widely, even for the same congeners, principally because of large
differences in the durations of exposure in the various studies.
Acute exposure to monochlorobenzene via inhalation causes sensory
irritation of the respiratory system, after several minutes;
exposure for periods ranging from several minutes to hours causes
narcosis and central nervous system (CNS) depression, which can
result in death. The LC50s (6-h exposure period) for male
Sprague-Dawley rats and SPF-OF1 female mice were 13 490 mg/m3
(2965 ppm) and 8581 mg/m3 (1886 ppm), respectively (Bonnet et al.,
1979, 1982). De Ceaurriz et al. (1981) reported that inhalation of
4796 mg MCB/m3 (1054 ppm) for 5 min reduced the respiratory rate
in Swiss-OF1 mice by 50% (RD50), indicating respiratory
irritation.
LD50 values for the ingestion of MCB (gavage in corn oil) were
approximately 4000 mg/kg body weight in male and female F344 rats;
B6C3F1 mice were more sensitive with 100% lethality at doses
exceeding 1000 mg/kg and 2000 mg/kg for males and females,
respectively (Kluwe et al., 1985). Clinical symptoms included
hyperpnoea, ataxia, laboured breathing, prostration, and death from
respiratory paralysis.
Systemic effects of acute toxic doses of MCB included damage to the
liver and kidney, and effects on bile and pancreatic flow.
Circulating alanine aminotransferase (ALT) was increased in 50% of
CF-1 strain albino mice following a single intraperitoneal
adminis-tration of approximately 428 mg/kg (Shelton & Weber, 1981).
In Sprague-Dawley rats, single intraperitoneal administration of the
two highest doses of 1104 mg/kg and 1655 mg/kg body weight resulted
in increases in plasma ALT and centrilobular necrosis. Increased
liver to body weight ratios were observed at all doses
(225-1655 mg/kg body weight) (Dalich & Larson, 1985). In C57
Black/6J male mice, coagulation necrosis of the proximal renal
tubules was observed following intraperitoneal administration of 760
mg MCB/kg body weight; Sprague-Dawley rats were somewhat less
sensitive having only swollen, vacuolated convoluted tubules at
doses of 1047 mg/kg body weight (Reid, 1973). Yang et al. (1979)
reported increased bile duct-pancreatic flow in Holtzman male rats
administered 563 mg MCB/kg body weight (5 mmol/kg)
intraperitoneally. The investigators suggested that these effects
were distinct from those on the liver, since they occurred in the
absence of liver damage.
Effects of acute exposure to the dichlorobenzenes are similar to
those for MCB, resulting in CNS depression following inhalation, and
respiratory paralysis following ingestion. Limited available data
indicate that the 1,2-isomer is more toxic than the 1,4-isomer. In
the inhalation studies of Bonnet et al. (1979, 1982), the 1,2-isomer
of dichlorobenzene was somewhat more toxic than MCB, the LC50s
(6-h exposure) in both Sprague-Dawley rats and SPF-OF1 female mice
(9192 mg/m3 and 7416 mg/m3, respectively) being less than those
for MCB (13 490 mg/m3 and 8581 mg/m3, respectively). Increases
in circulating hepatic enzymes were reported 24 h after exposure of
Sprague-Dawley rats to levels of 1,2-DCB greater than 1830 mg/m3
(Brondeau et al., 1983).
Few recent data are available concerning the acute toxicity of the
dichlorobenzenes after oral administration. Hollingsworth et al.
(1958) reported that no deaths occurred in guinea-pigs following
ingestion (gavage in olive oil) of 800 mg/kg body weight of the
1,2-isomer, whereas there was 100% lethality at 2000 mg/kg body
weight. For the 1,4-isomer, these investigators reported that 2800
mg/kg and 4000 mg/kg body weight were lethal for 100% of guinea-pigs
and rats, respectively (Hollingsworth et al., 1956). There were no
deaths in guinea-pigs and rats exposed to 1600 mg/kg and 1000 mg/kg
body weight, respectively.
Table 19. Acute toxicity of chlorobenzenes for experimental animals
Compound; Speciesa Routeb Dosec Resultsd
Reference
MCB Sprague-Dawley male rat; inhalation NA (6-h exposure) 14-day LC50 in rats: 13 490 mg/m3
Bonnet et al. (1979, 12 animals/group; SPF-OF1 observation period (2965 ppm)
1982) female mouse; 20-25/group LC50 in mice: 8581 mg/m3
(1886 ppm)
MCB Swiss OF1 male mouse; inhalation NA (5-min exposure) RD50: 4796 mg/m3
De Ceaurriz et al. 6 animals/group (1054 ppm)
(1981)
MCB Fischer 344 rat; B6C3F1 oral (gavage in 0, 250, 500, 1000, LD50 in rats: about
Kluwe et al. (1985) mouse; 5 of each sex/group corn oil) 4000 mg/kg 4000 mg/kg (males and
females) (35.5 µmol/kg);
100% lethality in mice:
>1000 mg/kg (males),
>2000 mg/kg (females)
MCB Sprague-Dawley male rat; ip (in corn oil) 0, 225, 552, 1104, LD50 - 1655 mg/kg
Dalich & Larson 3-5 animals/group 1655 mg/kg (2.0, 4.9, 9.8, (14.7 mmol/kg); depression
(1985) 14.7 mmol/kg); 72-h in hepatic glutathione and
observation period increased liver to body
weight ratios at all doses;
centrilobular necrosis and
increase in plasma ALT only
at 2 highest doses (1104 and
1655 mg/kg)
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB Sprague-Dawley male rat; inhalation NA (6-h exposure); 17-day LC50 in rats: 9192 mg/m3
Bonnet et al. (1979, 12 animals/group observation period (1532 ppm)
1982) SPF-OF1 female mouse; LC50 in mice: 7416 mg/m3
20-25 animals/group (1236 ppm)
1,2-DCB rat; 5-20 animals/group inhalation 0, 3234, 4926, 5646, LD50 approx. 5862 mg/m3
Hollingsworth et al. 5862 mg/m3 (0, 539, 821, (lethal to 4/5); marked
(1958) 941, 977 ppm); 7-h hepatic centrilobular
exposure necrosis and cloudy swelling
in the renal tubular
epithelium; increase in
liver and kidney weights
1,2-DCB Sprague-Dawley male rat; inhalation 0, 1224, 1830, 2556, 3654, significant increase in
Brondeau et al. 8 animals/group 4644 mg/m3 (0, 204, 305, serum hepatic enzymes
(1983) 426, 609, 774 ppm); (GLDH, ALT and SDH) at
4-h exposure levels >1830 mg/m3
1,2-DCB Albino guinea-pig; oral (intubation 800 or 2000 mg/kg (305 ppm) no deaths at 100%
Hollingsworth et al. 10 animals (mixed in olive oil) 800 mg/kg; lethality at
(1958) sex)/group 2000 mg/kg
1,4-DCB guinea-pig; rat; 5-15 oral (intubation 1000 or 4000 mg/kg in rats; 100% lethality in
Hollingsworth et al. animals (mixed sex)/group in olive oil) 1600 or 2800 mg/kg in guinea-pigs at 2800 mg/kg
(1956) guinea-pigs and in rats at 4000 mg/kg;
no deaths at 1600 and
1000 mg/kg, respectively
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB male Sprague-Dawley rat or ip (in sesame oil) 1,2-DCB: 195 mg/kg 1,2-DCB: minimal hepatic
1,3-DCB C57 Black/6J mouse (1.33 mmol/kg) necrosis
1,4-DCB 1,3-DCB: 193 mg/kg 1,3-DCB: normal to minimal
Reid (1973) (1.31 mmol/kg) hepatic necrosis
1,4-DCB: 500 mg/kg 1,4-DCB: little or no
(3.40 mmol/kg) effect
1,2,4-TCB CFE rat; CF1 mouse; 4 of oral (gavage) NA; 10-day observation LD50 in rats: 756 mg/kg
Brown et al. (1969) each sex/group period LD50 in mice: 766 mg/kg
CFE rat; 4 of each percutaneous LD50 in rats: 6139 mg/kg
sex/group (topical
administration
on dorso-lumbar
region for 24 h)
1,2,4-TCB ddY mouse; 10 of each oral 123, 160, 207, 269, 350, LD50 in males: 300 mg/kg
Yamamoto et al. sex/group 455, 591, 769 mg/kg LD50 in females: 305 mg/kg
(1978)
1,2,4-TCB Holtzman male rat ip (in sesame oil) 0, 907 mg/kg (0.5 mmol/kg) increased bile
1,3,5-TCB duct-pancreatic flow with
Yang et al. (1979) both isomers, but
1,2,4-isomer 4 more potent;
increase in serum GPT with
the 1,3,5-isomer
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,3,4-TeCB Sprague-Dawley rat; 10 of oral (gavage in 0, 200 - 4000 mg/kg LD50s in males:
1,2,3,5-TeCB each sex/group corn oil) (5 doses) 1,2,3,4-TeCB: 1470 mg/kg
1,2,4,5-TeCB 1,2,4,5-TeCB: 3105 mg/kg
Chu et al. (1983, 1,2,3,5-TeCB: 2297 mg/kg
1984a) LD50s in females:
1,2,3,4-TeCB: 1167 mg/kg
1,2,3,5-TeCB: 1727 mg/kg
1,2,4,5-TeCB mouse, rat, rabbit oral (gavage in NA LD50 in mice:
Fomenko (1965) sunflower oil or 1035 mg/kg (sunflower oil)
starch) 2650 mg/kg (starch)
LD50 in rats and rabbits:
1500 mg/kg (vehicle
unspecified)
PeCB Sherman rat - adult (male oral (gavage in rats: LD50 in male adult rats:
Linder et al. (1980) and female) and weanling peanut oil) 600 - 1500 mg/kg 1125 mg/kg
(female); Swiss Webster mice: LD50 in female adult rats:
mouse; 750 - 1500 mg/kg 1080 mg/kg
10 of each sex/group LD50 in weanling female
rats: 940 mg/kg
LD50 in male mice:
1175 mg/kg
LD50 in female mice:
1370 mg/kg
Table 19 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
PeCB Sherman rat; 10 of each percutaneous 2500 mg/kg no clinical signs of
Linder et al. (1980) sex/group (dissolved in toxicity
xylene and
applied to back
and shoulder
area)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
NA - not available.
ip - intraperitoneal.
d LC50 - median lethal concentration.
LD50 - median lethal dose.
RD50 - dose producing a 50% decrease in respiratory rate.
ALT - alanine aminotransferase.
GLDH - glutamate dehydrogenase.
SDH - sorbitol dehydrogenase.
Reid (1973) reported minimal hepatic necrosis in Sprague-Dawley rats
or C57Black/6J mice receiving 1,2- or 1,3-DCB at 195 mg/kg body
weight (about 1.32 mmol/kg) intraperitoneally; no such effects were
observed following intraperitoneal administration of 500 mg/kg body
weight (3.4 mmol/kg) of the 1,4-isomer. Yang et al. (1979) reported
increased bile duct-pancreatic flow following administration of
735 mg 1,2-DCB/kg body weight (5 mmol/kg) to male Holtzman rats.
Similar effects were not observed following the administration of
the same dose of the 1,4-isomer.
There are no reliable quantitative data on acute toxicity following
the inhalation of the trichlorobenzenes.
Similar LD50s were reported for CFE rats and CF1 mice ingesting
1,2,4-TCB (756 mg/kg and 766 mg/kg body weight, respectively) (Brown
et al., 1969). Symptoms included depressed activity at lower doses
and extensor convulsions at lethal doses.
The single percutaneous LD50 in CFE rats, determined by the same
investigators, was about an order of magnitude higher than the oral
LD50 (6139 mg/kg body weight).
As was observed for MCB and the 1,2-isomer of DCB, intraperitoneal
administration of the 1,2,4- and 1,3,5-isomers of trichlorobenzene
at 907 mg/kg body weight (5.0 mmol/kg) increased bile
duct-pancreatic flow in Holtzman male rats (Yang et al., 1979). The
1,2,4-isomer was 4 times more potent in inducing this effect;
however, an increase in serum alanine aminotransferase (ALT)
resulted from administration of the 1,3,5-isomer.
Data on the acute toxicity of the tetrachlorobenzenes are restricted
to the oral route of administration. In male Sprague-Dawley rats,
the LD50s for 1,2,3,4-, 1,2,4,5-, and 1,2,3,5-TeCB were
1470 mg/kg, 3105 mg/kg, and 2297 mg/kg body weight, respectively
(Chu et al., 1983, 1984a). In females, the LD50 values were
1167 mg/kg and 1727 mg/kg body weight for 1,2,3,4- and 1,2,3,5-TeCB,
respectively. Clinical signs included depression, flaccid muscle
tone, prostration, piloerection, loose stool, hypothermia,
dacryorrhoea, and coma. At post-mortem, the gastrointestinal tract
was distended and slightly haemorrhagic.
Linder et al. (1980) reported LD50s for PeCB (gavage in peanut
oil) in adult male and female Sherman rats of 1125 mg/kg and
1080 mg/kg body weight, respectively. The value in weanling female
rats was 940 mg/kg body weight. In male and female Swiss Webster
mice, the LD50s were 1175 mg/kg and 1370 mg/kg body weight,
respectively. Decreased activity and tremors were observed in both
species at sublethal dosage levels; the kidneys, liver, and adrenal
glands of rats were also enlarged. In some rats, the gastric mucosa
was hyperaemic, and a slight reddish fluorescence of the
gastro-intestinal tract, suggesting porphyria, was observed in both
rats and mice, under ultraviolet light.
There were no clinical signs of toxicity in rats following
percutaneous administration of 2500 mg PeCB/kg body weight.
8.2 Skin and Eye Irritation, Skin Sensitization
The results of studies on the potential of chlorobenzenes to induce
skin and eye irritation and skin sensitization are presented in
Table 20. These investigations were mainly restricted to the
1,2,4-isomer of trichlorobenzene, which, on the basis of available
data, is mildly irritating, and causes dermatitis after repeated or
prolonged contact, probably because of its degreasing action on the
skin. Direct contact of the eyes with either 1,2-DCB or 1,2,4-TCB is
painful, but does not produce permanent damage; moreover, there was
no evidence of sensitization in the limited studies available.
8.3 Short-term Exposures
The results of short-term studies on the effects of chlorobenzenes
on experimental animals are presented in Table 21. Effects following
the exposure of rats and mice to repeated daily doses of
chlorobenzenes for periods of up to 28 days were confined mainly to
the liver; other target organs included the thyroid, kidney, and
lung in rats and the bone marrow in mice.
In evaluating repeated dose studies in which chlorobenzenes were
administered in the diet, it is important to note that the dose
received by the exposed animals might have been substantially lower
than that indicated by the nominal concentrations, particularly for
the more volatile lower chlorinated congeners. In a short-term
bioassay for 1,2,4,5-TeCB, conducted recently by NTP (1989a), there
was an 8% loss from the diet in 7 days, though corn oil had been
added to minimize volatilization. On the basis of the physical and
chemical properties of chlorobenzene congeners of lower chlorination
than 1,2,4,5-TeCB, volatilization from the diet may have been even
greater.
The results of 14-day studies, in which relatively high doses of MCB
and the two isomers of DCB (1,2- and 1,4-) (up to 8000 mg/kg body
weight) were administered by gavage to F344 rats and B6C3F1 mice,
were consistent with those of the acute toxicity studies; the
1,2-isomer of DCB was slightly more toxic than MCB, as indicated by
the doses that caused decreases in body weight gain and deaths. The
1,4-isomer of DCB was considerably less toxic (NTP, 1983a,b, 1987).
Table 20. Skin and eye irritation; sensitization in experimental annimals
Compound; Speciesa Routeb Dose Results
Reference
Irritation
1,2-DCB rabbit (2) application to the 2 drops undiluted slight to moderate pain and slight
Hollingsworth et al. eyes compound conjunctival irritation, clearing
(1958) in 7 days; prompt washing
immediately after contact reduced
pain and irritation
1,4-DCB rabbit inhalation 4.6 to 4.8 mg/litre tremors, weakness, lateral
Pike (1944) (765 to 798 ppm) nystagmous, corneal and optical
8h/day for nerve oedema, death
62 exposures over
83 days
1,2,4-TCB rabbit - 4 of each dermal (topical 1 ml, 6 h/day very weak irritant, based on
Brown et al. (1969) sex/group application on (3 days) visual inspection and
shorn backs - histopathology 7 days after first
covered) application - fissuring typical
of degreasing action;
rabbit - 4 of each dermal (topical rabbit: 1 ml
sex/group application on guinea-pigs: 0.5 ml, spongiosis, acanthosis and
guinea-pig - 5 of each shorn backs - 5 days/week (3 weeks) parakeratosis in rabbits
sex/group uncovered and guinea-pigs - some
inflammation in the superficial
dermis of rabbits exposed for 3
weeks rabbits exposed for 3 weeks
Table 20 (continued)
Compound; Speciesa Routeb Dose Results
Reference
1,2,4-TCB rabbit application to the irritation; pain, severe
Brown et al. (1969) eyes conjunctivitis, chemosis and
discharge, but without corneal
involvement; swollen lids
1,2,4-TCB New Zealand albino dermal (topical 0, 30, 150, 450 mg/kg, dermal irritation - fur matted by
(70% + 30% rabbit - 5 of each application on 5 days/week (4 weeks) fine, white, bran-like scales
1,2,3-TCB) sex/group shorn backs) with variable degrees of
Rao et al. (1982) erythema, fissures, erosions,
and ulcers; inflammation and
thickening of the epidermis; size
of affected area varied with
dose and increased
severity at 450 mg/kg
1,2,4-TCB ddY mouse - dermal 100, 70, 40, 1 or 0% erythema with 100% TCB (4/8) and
Yamamoto et al. 8 animals/group TCB 70% TCB (2/8); no remarkable
(1978) change in histology
1,2-4-TCB New Zealand white dermal (topical 0, 5, 25, 100% dose-related dermal irritation;
Powers et al. rabbit - 12 of each application on (0.2 ml), 3 times acanthosis and hyperkeratosis
(1975) sex/group ventral surface weekly (13 weeks)
of ear)
Table 20 (continued)
Compound; Speciesa Routeb Dose Results
Reference
Sensitization
1,2,4-TCB guinea-pig intradermal 1 day/week for no overt signs of skin
Brown et al. (1969) injection or 3 successive weeks; sensitization
topical application challenge after
on shorn backs (0.1% 10 days
w/v in light liquid
paraffin)
a Strain and number of animals/group specified where available.
b Vehicle specified, where available.
With the exception of 1,2,4,5-TeCB, administration by gavage (for
periods of from 5 to 15 days) of all of the chlorinated (mono to
penta-) benzene isomers examined caused hepatic porphyria,
characterized by increased levels of porphyrins and porphyrin
precursors in the liver and excreta. 1,3-DCB, 1,3,5-TCB, and
1,2,3,5-TeCB were not examined (Rimington & Ziegler, 1963). The most
active isomers in inducing porphyria were those with 2 chlorine
atoms in a para position to one another (1,4-DCB, 1,2,4-TCB, and
1,2,3,4-TeCB). Liver damage was most severe (intense necrosis and
fatty changes over large areas) in animals treated with high doses
of MCB, 1,2-DCB, or 1,2,4-TCB (maximum doses ranged from 455 to 1140
mg/kg body weight per day, by gavage, for the different isomers).
The other chlorinated benzenes (1,4-DCB, 1,2,3-TCB, 1,2,3,4-TeCB,
and 1,2,4,5-TeCB) produced degeneration of individual liver cells or
focal necrosis in the central, midzonal, and periportal regions.
Hepatomegaly was common in most porphyric rats.
Hepatic porphyria was also observed in rats administered 800 mg
1,3-DCB/kg body weight per day, by gavage, for 5 days (Poland et
al., 1971). Rao et al. (1982) reported an increase in urinary
coproporphyrin in male rabbits treated dermally with 450 mg
1,2,4-TCB/kg body weight per day, for 5 days/week, over 4 weeks.
Fatty degeneration of the liver and changes in blood cell counts
indicative of bone marrow damage, were observed following inhalation
by Swiss white mice of about 1250 mg MCB/m3 or 500 mg TCB/m3
(isomer not specified) for 7 h/day, over 3 weeks (Zub, 1978).
Necrotic foci in the liver were also observed following the dermal
application to guinea-pigs of 0.5 ml undiluted 1,2,4-TCB, for 5
days/ week, over 3 weeks (Brown et al., 1969).
Effects on the adrenal glands and uterus have also been noted in a
study in which 1,2,4-TCB at 250 or 500 mg/kg body weight per day was
administered intraperitoneally for 3 days to immature female rats
(Robinson et al., 1981). There was a decrease in uterine weight and
hepatomegaly at both doses and a decrease in body weight and
increase in adrenal weight at the high dose (500 mg/kg).
The most extensive short-term study (28 days) completed to date on
the toxicity of the higher chlorobenzenes was conducted by Chu
et al. (1983) on 3 isomers of TeCB and PeCB. Levels administered in
the diet of 0.5, 5.0, 50, or 500 mg/kg (ppm) for TeCB, and 5, 50, or
500 mg/kg (ppm) for PeCB, did not cause effects on body weight gain
or food consumption, or induce clinical signs of toxicity. No
haematological aberrations were observed; however, dose-dependent
effects were seen, principally, in the liver, but also in the
thyroid, kidney, and lungs in Sprague-Dawley rats. While the results
of acute toxicity studies indicated that the 1,2,4,5-isomer was the
least toxic of the TeCBs, it was the most toxic congener in the
28-day studies. This was well supported by the observation that it
was present at highest concentrations in fat and liver. The order of
toxicity for the TeCBs and PeCB, which was well correlated with
tissue concentrations, was as follows: 1,2,4,5-TeCB>PeCB> 1,2,3,4-
and 1,2,3,5-TeCB. Moderate to severe histopathological hepatic
changes (e.g., cytoplasmic ballooning and anisokaryosis of
hepatocytes) and increases in liver weight were observed in rats
exposed to 1,2,4,5-TeCB or PeCB at levels of about 50 mg/kg body
weight per day. Histopathological changes in the thyroid (i.e.,
increase in epithelial height and angular collapse of thyroid
follicles with a reduction in colloid density), kidney (i.e.,
eosinophilic inclusions in proximal convoluted tubules), and lung
(i.e., focal alveolar emphysema and inflammation) were mild, even at
the highest dose levels. Although all isomers of TeCB and PeCB
induced hepatic enzymes, liver porphyrin concentrations were not
affected. The data suggest that the position of the chlorine
substituents in TeCBs affects the tissue accumulation and toxicity
of these chemicals.
The toxicity of 1,2,4,5-TeCB following short-term administration (14
days) of 0, 30, 100, 1000, or 3000 mg/kg (ppm) in the diet to
B6C3F1 mice or F344 rats was also investigated recently in an NTP
bioassay (NTP, 1989a). In rats, there were no deaths in any dose
group, though compound-related clinical signs were observed in the
high-dose group. There were also decreases in food consumption and
final mean body weights of both sexes at 3000 mg/kg, increases in
liver weights in males at 1000 mg/kg or more and in females at
3000 mg/kg, and abnormal hyaline droplets in the renal cortical
epithelium of exposed males. In mice, all animals in the 3000 mg/kg
dose group died before the end of exposure. There was also a
significant increase in liver weights in males at 1000 mg/kg and in
females at 300 or 1000 mg/kg. Depletion and necrosis of lymphoid
tissue of the spleen, thymus, and lymph nodes in both sexes were
also observed, particularly in moribund or early death animals.
Concentrations of 0, 100, 330, 1000, 3300, or 10 000 PeCB mg/kg
(ppm) in the diet have also been administered in short-term studies
(15 days) to F344 rats and B6C3F1 mice (NTP, 1989b). All rats in
the 10 000 mg/kg dose group died by day 7, and final mean body
weights in both sexes were lower at 3300 mg/kg. There were also
significant increases in liver weights at all doses except 100 mg/kg
(females), and increases in kidney weights and abnormal hyaline
droplet formation in the renal cortical epithelium in males at all
doses. Centrilobular hepatocellular hypertrophy was observed in
males at 330 and 1000 mg/kg and in females at 1000 and 3300 mg/kg.
At 10 000 mg/kg, there was depletion of thymic lymphocytes and
hyperkeratosis of the forestomach in both sexes, and forestomach
acanthosis in females. In mice, all animals in the 2 highest dose
groups (3300 and 10 000 mg/kg) died by day 10. There was a
significant increase in liver weights in both sexes at 330 and 1000
mg/kg, and mild to moderate depletion of thymic lymphocytes, due to
lymphocyte necrosis, in moribund animals or those that died early.
Table 21. Short-term toxicity of chlorobenzenes in experimental animals
Compound; Speciesa Routeb Dosec Resultsd
Reference
MCB Fischer-344 rat; oral (gavage in rats: 0, 125, 250, rats: prostration, reduced response to stimuli
NTP (1983a); B6C3F1 mouse - corn oil) 500, 1000, or and death of all animals at 1000 and 2000 mg/kg;
Kluwe et al. 5 of each 2000 mg/kg per day mice: no clinical signs of toxicity or deaths
(1985) sex/group mice: 0, 30, 60,
125, 250, or
500 mg/kg per day
(14 days)
MCB male albino rat oral (gavage in maximum dose - hepatic porphyria and hepatomegaly in porphyric
Rimington & - paraffin) 1140 mg/kg per day rats; severe liver damage (intense necrosis and
Ziegler 3 animals/group (5 days) fatty change) at high doses; weight and appetite
(1963) loss; decrease in haemoglobin levels
MCB Swiss white inhalation 0, 2500 mg/m3 fatty degeneration of the liver with "acute
Zub (1978) mouse - 5 of (2.5 mg/litre) yellow atrophy"; changes in blood cell counts
each sex/group reduced to 1250 mg/m3 indicative of bone marrow damage; loss of
(7 h/day - 3 weeks) appetite, general emaciation and marked
somnolence with death of the animals at the
higher dose
1,2-DCB Fischer-344 rat; oral (gavage in rats: 0, 60, 125, 500, rats: dose-related decrease in weight gain (more
NTP (1983b) B6C3F1 mice - corn oil) or 1000 mg/kg per day than 10% in males at highest dose); death of all
5 of each mice: 0, 60, 125, or animals at 1000 mg/kg; mice: death of 1 male and
sex/group 500 mg/kg per day 1 female in high dose (500 mg/kg) group
(14 days)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2-DCB male albino rat - oral (gavage in maximum dose - results similar to those for MCB with exception
Rimington & 3 animals/group liquid paraffin) 455 mg/kg per that there was no decrease in haemoglobin levels
Ziegler day (15 days)
(1963)
1,3-DCB Sherman male rat - oral (gavage in 0, 800 mg/kg per day hepatic porphyria; peak effect at 3 days
Poland et al. 4 animals/group peanut oil) (9 days)
(1971)
1,4-DCB male albino rat - oral (gavage in maximum dose - hepatic porphyria; non-necrotic liver cell
Rimington & 3 animals/group liquid paraffin) 770 mg/kg per day degeneration; weight and appetite loss
Ziegler (5 days)
(1963)
1,4-DCB Fischer-344 rat; oral (gavage in rats: 0, 500, 1000, rats: death of all animals at 3 highest doses;
NTP (1987) B6C3F1 mouse - corn oil) 2000, 4000, or decrease in body weight gain at lower doses;
5 of each 8000 mg/kg per day mice: no compound-related deaths or decreases in
sex/group mice: 0, 60, 125, 250, body weight gain
500, or 1000 (14 days)
1,2,3-TCB male albino rat - oral (gavage in maximum dose - hepatic porphyria; non-necrotic liver cell
Rimington & 3 animals/group 1% cellofas) 785 mg/kg per day degeneration; weight and appetite loss
Ziegler (7 days)
(1963)
1,2,4-TCB male albino rat - oral (gavage in maximum dose - results similar to those for MCB with the
Rimington & 3 animals/group 1% cellofas) 730 mg/kg per day exception that there was no decrease in
Ziegler (15 days) haemoglobin levels
(1963)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,4-TCB rabbit (1 of each percutaneous rabbits - 1 ml/day death of some guinea-pigs - necrotic foci in
Brown et al. sex) and (applied on guinea-pigs - livers
(1969) guinea-pig (5 of shaved backs) 0.5 ml/day
each sex) (5 days/week for
3 weeks)
1,2,4-TCB New Zealand albino percutaneous 0, 30, 150, or systemic effects only at 450 mg/kg - increase in
(70% + 30% rabbit - 5 of each (applied on 450 mg/kg per day urinary coproporphyrin in males and slight pallor
1,2,3-TCB) sex/group shaved backs) (5 days/week for of liver in both sexes at necropsy; NOEL -
Rao et al. 4 weeks) 150 mg/kg per day
(1982)
1,2,4-TCB Charles River intraperitoneal 0, 250 or 500 mg/kg decrease in uterus weights and increase in liver
Robinson et al. immature female rat (in corn oil) per day (3 days) weight at both doses; decrease in body weight and
(1981) - 9-10 increase in adrenal weight at high dose
animals/group (500 mg/kg)
1,2,4-TCB Alderley Park SPF inhalation 0, 148, 519, or lethargy and retarded weight gain at 2 highest
(up to 20% rat - 2-4 of each 1484 mg/m3 (0, 20, 70, doses; lacrimation at 519 mg/m3; no observed
1,2,3-TCB) sex/group or 200 ppm) effects at 148 mg/m3
Gage (1970) (6 h/day, 5 days/week
for 3 or 4 weeks)
1,3,5-TCB CD (outbred inhalation 0, 10, 100, or increase in liver to body weight ratio at highest
Sasmore et al. albino) rat - 20 1000 mg/m3 (6 h/day, 5 dose level (1000 mg/m3)
(1983) of each sex/group days/week for 4 weeks)
TCB Swiss white mouse - inhalation 0, 500 mg/m3 fatty degeneration of the liver; changes in blood
(isomer not 5 of each sex/group (0.5 mg/litre) cell counts indicative of bone marrow damage;
specified) (7 h/day for 3 weeks) loss of appetite, hypoactive
Zub (1978)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,4,5-TeCB albino rat (male) - oral (gavage in maximum dose - no effect on urinary porphyrin levels, possibly
Rimington & 3 animals/group 1% cellofas) 905 mg/kg per day because of poor absorption
Ziegler (1963) (5 days)
1,2,4,5-TeCB F344/N rat - oral (mixed in 1% 0, 30, 100, 300, 1000, no deaths at any dose; compound-related clinical
NTP (1989a) 5 of each corn oil added to or 3000 mg/kg (14 days) signs included tremors, lethargy, thin appearance,
sex/group feed) rough coats, ataxia, and chromodacryorrhoea in
both sexes at 3000 mg/kg and rapid breathing in
females at 3000 mg/kg; decrease in final mean
body weights at 3000 mg/kg (18% in males; 15% in
females); 20% decrease in food consumption at
3000 mg/kg; at >300 mg/kg, increases in absolute
and relative kidney weights (males only); liver
congestion in males at >1000 mg/kg and in females
at 3000 mg/kg; abnormal hyaline droplets in the
renal cortical epithelium of exposed male rats
(doses unspecified)
1,2,4,5-TeCB B6C3F1 mouse - oral (mixed in 1% 0, 30, 100, 300, 1000, all animals in the 3000 mg/kg dose group died;
NTP (1989a) 5 of each sex/group corn oil added to or 3000 mg/kg (14 days) compound-related clinical signs included tremors,
feed) rapid breathing, lethargy, hunched posture, rough
coats, dyspnoea, and prostration in both sexes at
3000 mg/kg and thin appearance in females at
3000 mg/kg; significant increase in absolute and
relative liver weights in males at 1000 mg/kg and
in females at 300 or 1000 mg/kg; at 3000 mg/kg,
depletion and necrosis of lymphoid tissue of the
spleen, thymus, and lymph nodes in both sexes
(these changes frequently observed in moribund or
early-death animals)
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
1,2,3,4-TeCB albino rat (male) - oral (gavage in maximum dose - results similar to those for 1,2,3-TCB and hair
Rimington & 3 animals/group liquid paraffin) 660 mg/kg per day loss
Ziegler (1963) (10 days)
1,2,3,4-TeCB Sprague-Dawley rat oral (diet) TeCBs: 0, 0.5, 5.0, 50, no effect on body weight gain, food consumption;
1,2,4,5-TeCB - 10 of each or 500 mg/kg diet (28 no clinical signs of toxicity; no haematological
1,2,3,5-TeCB sex/group days) PeCB: 0. 5.0, 50, aberrations; increase in serum cholesterol for
PeCB or 500 mg/kg diet 1,2,4,5-TeCB at 500 mg/kg; induction of hepatic
Chu et al. (28 days) enzymes by all isomers; increase in liver weight
(1983) for 1,2,4,5-TeCB and PeCB at 500 mg/kg;
dose-dependent histological changes (e.g.,
cytoplasmic vacuolation) in the liver (moderate to
severe) for 1,2,4,5-TeCB at 500 mg/kg, mild in all
other cases - PeCB > 1,2,3,4-TeCB and
1,2,3,5-TeCB; dose-dependent accumulation of
TeCBs in fat and liver - greatest for 1,2,4,5-TeCB
and PeCB; mild thyroid changes for 1,2,4,5-TeCB
and PeCB at 500 mg/kg, very mild in all other
cases; mild changes in kidney and lung
PeCB F344/N rat - 5 of oral (in feed) 0, 100, 330, 1000, all animals in the 10 000 mg/kg dose group died
NTP (1989b) each sex/group 3300, or 10 000 mg/kg by day 7; at 3300 mg/kg, final mean body weights
(15 days) were 23% and 15% lower in males and females,
respectively; feed consumption lower in week 1
and higher in week 2; significant increase in
absolute and relative liver weights in all dose
groups except at 100 mg/kg (females); in males,
significant increases in absolute kidney weights
at 100, 330, and 1000 mg/kg and significant
increase in relative kidney weights at all doses;
Table 21 (continued)
Compound; Speciesa Routeb Dosec Resultsd
Reference
significant decrease in the absolute weights
of the kidney, thymus, heart, and lung of both
sexes at 3300 mg/kg possibly attributable to the
lower weight gain in these animals; excessive
abnormal hyaline droplets in the renal cortical
epithelium in males (0/5, 1/5, 5/5, 5/5, 2/5, 0/5
in control and increasing dose groups,
respectively); centrilobular hepatocellular
hypertrophy in males at 330 and 1000 mg/kg, and in
females at 1000 and 3300 mg/kg; at 10 000 mg/kg,
depletion of thymic lymphocytes and hyperkeratosis
(mild to moderate) of the forestomach in both
sexes and forestomach acanthosis in females
PeCB B6C3F1 mouse - oral (in feed) 0, 100, 330, 1000, all mice in 3300 or 10 000 mg/kg dose groups died
NTP (1989b) 5 of each 3300, or 10 000 mg/kg by day 10; clinical signs of toxicity in animals
sex/group (15 days) exposed to 3300 mg/kg prior to death included
tremors, lethargy, hunched posture, and paralysis
(both sexes) and dyspnoea in females; significant
increase in absolute and relative liver weights
at 330 and 1000 mg/kg (both sexes); no
compound-related lesions in surviving animals, but
mild-to-moderate depletion of thymic lymphocytes,
due to lymphocyte necrosis, in moribund or
early-death animals
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
8.4 Long-term Exposures
The results of selected studies of the toxicity of the
chlorobenzenes after long-term exposures are presented in Table 22.
The data included in the table have been restricted to
investigations for which analyses of, at least, body weight gain,
survival, clinical signs of toxicity, clinical chemistry,
haematology, and histopathology of major organs and tissues have
been conducted. No-observed-effect levels (NOEL),
no-observed-adverse-effect levels (NOAEL), and lowest-observed-
adverse-effect levels (LOAEL), determined on the basis of the
results of these studies, are also presented in Table 22. Additional
studies, in which analyses were more limited, are referenced in the
text.
As in the case of the single and short-term exposure studies, there
are comparatively more data on the toxicity of the lower chlorinated
congeners (i.e., mono- and dichlorobenzenes) after long-term
exposure than there are for the tri-, tetra-, and
pentachlorobenzenes.
Because of the paucity of available data on some of the congeners
and the wide variations in study design and duration, it is
difficult to draw very specific conclusions about structure-activity
relationships with respect to the toxicity of the chlorobenzenes
after long-term exposures. However, on the basis of available data,
it appears that, with the exception of 1,4-DCB, which has relatively
low long-term toxicity, there is a trend for toxicity to increase
with increased chlorination of the benzene ring for many of the
endpoints examined. However, it should be noted that the variations
in the long-term toxicity of different isomers of the same congener
are, in some cases, considerable. The toxicity of the compounds also
appears to be well correlated with the extent of accumulation in the
body tissues, and, in general, female animals appear to be less
sensitive to the chlorinated benzenes than males.
Administration of MCB via inhalation or ingestion to rats, mice,
rabbits, or dogs has caused reductions in both body weight gain and
survival at high doses, and hepatic and renal toxicity, as indicated
by increases in serum enzymes, liver and kidney weights,
histo-pathological changes, and necrosis (Irish, 1963; Knapp et al.,
1971; Dilley, 1977; NTP, 1983a). At high doses, depression of bone
marrow activity in mice (Zub, 1978) and myeloid depletion of the
thymus, spleen, or bone marrow in rats and mice (NTP, 1983a) have
also been observed. Inhibited chronaxia of antagonistic muscles, an
increase in blood cholinesterase, and a decrease in serum
alpha-globulin in rats have also been reported at considerably lower
doses (0.1 mg/m3) (Tarkhova, 1965); however, it is difficult to
assess the validity of these results on the basis of the published
data.
The no-observed-effect level for the long-term inhalation of MCB (32
exposures of 7 h/day, over 44 days) was approximately 910 mg/m3 in
rats (Irish, 1963). For ingestion, the no-observed-effect level was
50-125 mg/kg body weight in rats, and 125 mg/kg body weight in mice
(Knapp et al., 1971; NTP, 1983a) (Table 22).
The effects of long-term exposure of rats, guinea-pigs, rabbits, and
mice to the DCBs, via inhalation or ingestion, have been similar to
those of MCB. Reductions in body weight gain and reduced survival
have resulted from exposures to high doses of DCBs. Hepatic and
renal toxicity, as indicated by increases in liver and kidney
weights, as well as histopathological changes and necrosis, have
also been reported (Hollingsworth et al., 1956, 1958; NTP, 1983b,
1987). Increases in porphyrin excretion, and lymphoid depletion of
the thymus and spleen in rats, and multifocal mineralization of the
myocardial fibres and skeletal muscles in mice have also been
observed following administration of high doses of 1,2-DCB (NTP,
1983b).
As was observed in the acute and short-term studies, the 1,4-isomer
appears to be considerably less toxic than 1,2-DCB. While the NOEL
for the long-term inhalation of 1,2-DCB, derived from available
studies, was 560 mg/m3 for rats, guinea-pigs, rabbits, and monkeys
(Hollingsworth et al., 1958), the NOEL for the 1,4-isomer, was
950 mg/m3 in mice, rabbits, and monkeys, and 580 mg/m3 in rats
and guinea-pigs (Hollingsworth et al., 1956). The NOEL for ingestion
of the 1,2-isomer in male mice was 125 mg/kg body weight (NTP,
1983b) compared with values for the 1,4-isomer of 337.5 mg/kg body
weight in mice and 300-600 mg/kg body weight in rats (NTP, 1987).
Most of the data on the trichlorobenzenes are restricted to
1,2,4-TCB, the most widely used isomer. Inhalation of 1,2,4-TCB in
long-term studies on rabbits, rats, dogs, and monkeys resulted in
liver and kidney damage, as indicated by increases in organ weight
and transient histopathological changes (Coate et al., 1977; Kociba
et al., 1981). The NOELs were 742 mg/m3 in rabbits and monkeys
(Coate et al., 1977) and 223 mg/m3 in dogs (Kociba et al., 1981).
Increases in porphyrin excretion have also been observed in various
species following the inhalation of 1,2,4-TCB (Watanabe et al.,
1978; Kociba et al., 1981); the no-observed-adverse-effect level
(NOAEL) in rats for this effect was reported to be 22.3 mg/m3.
On the basis of the results of one long-term inhalation study, the
1,3,5-isomer appears to be somewhat less toxic than 1,2,4-TCB,
possibly owing to low absorption of this relatively non-volatile
chemical. The only notable effects resulting from the long-term
administration of doses up to 1000 mg/m3 to rats were squamous
metaplasia and hyperplasia of the respiratory epithelium of the
nasal passages at the highest dose (NOEL - 100 mg/m3) (Sasmore
et al., 1983).
Table 22. Toxicity of chlorobenzenes after long-term exposures
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Inhalation
MCB Sprague-Dawley 0, 341, increase in food intake (rats); reduction in SGOT; 341 mg/m3 "marginally
Dilley (1977) rat (male) and 1138 mg/m3, increase in liver weight (both species) and toxic concentration"
rabbit (male); 7 h/day, congestion of liver (rabbits, 1138 mg/m3); increase
32 animals/ 5 days/week in kidney weight and tubular and interstitial
group for 24 weeks lesions (both species); lesions of the adrenal
cortex (rats); small changes in red cell parameters
(both species)
MCB rat, rabbit, 910, 2161, at 4550 mg/m3, increase in mortality (guinea-pigs), 910 mg/m3 (NOEL)
Irish (1963) and guinea-pig or 4550 mg/m3, slight depression in growth, histopathological 2161 mg/m3 (LOAEL)
7 h/day, changes in the lungs, liver, and kidney; at 2161
5 days/week mg/m3, slight increase in liver weight and slight
for 32 histopathological changes in the liver; no effects
exposures at 910 mg/m3
over 44 days
1,2-DCB rat: 20 of 0, 290, or decrease in spleen weight (M guinea-pigs) not 560 mg/m3 (NOEL)
Hollingsworth each 560 mg/m3, believed to be treatment related; no other effects
et al. (1958) sex/group; 7 h/day, on growth, mortality, organ weights, haematological
guinea-pig: 5 days/week or urinalysis parameters; no histopathological
8 of each for 6-7 changes
sex/group; months
rabbit: 7 of
each
sex/group;
monkey:
2 females
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB rat, 0, 580, or growth depression (guinea-pig), increase in liver 580 mg/m3 (rats, (NOEL)
Hollingsworth guinea-pig, 950 mg/m3, and kidney weight, cloudy swelling and granular guinea-pigs);
et al. (1956) rabbit, mouse, 7 h/day, degeneration of the liver in rats at 950 mg/m3 950 mg/m3 (monkeys,
and monkey; 5 days/week (NOEL) rabbits, mice);
1-10 for 5-7 950 mg/m3 (rats,
animals/group months (LOAEL) guinea-pigs)
1,3,5-TCB CD rat - 20 of 0, 10, 100, squamous metaplasia and hyperplasia in the 100 mg/m3 (NOEL)
Sasmore et al. each sex/group or 1000 respiratory epithelium of the nasal passages at
(1983) mg/m3, 6 h/day, 1000 mg/m3
5 days/week
for 13 weeks
1,2,4-TCB Sprague-Dawley 0, 223, or increased liver weight (rats and dogs) and kidney 742 mg/m3 (rabbits);
(99.41%) rat (male) - 742 mg/m3, weight at 742 mg/m3; increased urinary excretion of 223 mg/m3 (dogs) (NOEL)
Kociba et al. 20/group; New (0, 30, or porphyrins at >223 mg/m3 (rats), considered to
(1981) Zealand albino 100 ppm) be physiological rather than toxic effect
rabbit (male) 7 h/day,
- 4/group; 5 days/week
beagle dogs for 30
(male) - exposures
2/group (44 days)
1,2,4-TCB Sprague-Dawley 0, 22.3, or slight reversible increase in urinary porphyrins 22.3 mg/m3 (M) (NOAEL)
Watanabe rat (male and 74.2 mg/m3 at 74.2 mg/m3
et al. (1978) female) (0, 3, or
10 ppm),
6 h/day,
5 days/week
for 3 months
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4-TCB Sprague-Dawley 0, 186, 371, no effect on pulmonary function or operant behaviour 742 mg/m3 (NOEL)
(99.07%) albino rat or 742 mg/m3 in monkeys; transient changes in the liver
Coate et al. (male) - (0, 25, 50, (hepatocytomegaly) and kidney (hyaline degeneration
(1977) 30/group; New or 100 ppm) of the cortex) in rats, at all doses; no
Zealand white 7 h/day, abnormal opthalmic changes in rabbits and monkeys
albino rabbit 5 days/week
(male) - for 26 weeks
16/group;
cynomolgus
monkey (male)
- 9/group
Ingestion
MCB Fischer-344 0, 60, 125, reduced survival and reduction in body weight gain 125 mg/kg per day
Kluwe et al. rat; B6C3F1 250, 500, or at >250 mg/kg; increase in SAP and SGGPT at >500 (NOEL);
(1985) mouse - 10 of 750 mg/kg mg/kg (F rats); polyuria and porphyrinuria at >500 250 mg/kg per day
each sex/group per day, mg/kg (rats and F mice); increase in liver porphyrin (LOAEL)
5 days/week at >500 mg/kg (F rats); dose-dependent increase in
for 13 weeks; liver weight, centrilobular hepato-cellular
gavage in degeneration and necrosis; slight increase in kidney
corn oil weight, degeneration and focal necrosis of the
proximal renal tubules; slight decrease in spleen
weight with lymphoid or myeloid depletion of the
thymus, spleen, or bone marrow at >250 mg/kg
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
MCB dog - 8 27.25, 54.5, in high-dose group, 50% mortality, low blood sugar, 54.5 mg/kg per day
Knapp et al. animals/group or 272.5 mg/kg increase in SGPT and SAP, increase in total(NOAEL);
(1971) per day, bilirubin and cholesterol in some animals, 272.5 mg/kg per day
5 days/week histopathological changes in liver, kidneys, (LOAEL)
for 93 days; gastrointestinal mucosa, and haematopoietic tissue
capsule and increase in immature leukocytes; in
intermediate- and low-dose groups, no consistent
toxic effects
MCB rat 12.5, 50, or in high-dose group, retarded growth in males; 50 mg/kg per day
Knapp et al. 250 mg/kg increase in liver and kidney weight at >50 mg/kg; no (NOEL);
(1971) per day for significant histopathological changes 250 mg/kg per day
93-99 days; (LOAEL)
diet
1,2-DCB Fischer-344 0, 30, 60, reduced survival at 500 mg/kg (mice and F rats) and 125 mg/kg per day
NTP (1983b) rat; B6C3F1 125, 250, or reduction in body weight gain at 500 mg/kg (F rats); (NOEL);
mouse - 10 of 500 mg/kg no increase in serum hepatic enzymes; increase in 250 mg/kg per day
each sex/group per day, urinary porphyrin at 500 mg/kg (rats and F mice), (LOAEL)
5 days/week but no increase in hepatic porphyrin; increase in
for 13 liver weight/body weight ratio (>125 mg/kg - rats,
weeks; 500 mg/kg - F mice); centrilobular hepatic
gavage in degeneration and necrosis (>125 mg/kg - rats;
corn oil >250 mg/kg - M mice); renal tubular degeneration
(500 mg/kg - M rats); decrease in spleen/body weight
ratio (>30 mg/kg - F mice) and decrease in thymus/-
body weight ratio (500 mg/kg - M rats) and lymphoid
depletion of the thymus and spleen at 500 mg/kg in
mice; in rats, slight decrease in haemoglobin levels
and haematocrit and in red blood cell counts (M), at
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2-DCB 500 mg/kg; in mice, multifocal mineralization of
NTP (1983b) myocardial fibres of heart and skeletal muscle at
cont'd 500 mg/kg
1,4-DCB Fischer 344/N 0, 37.5, 75, no compound-related deaths or changes in body weight 600 mg/kg per day (F);
NTP (1987) rat - 10 of 150, 300, or gain; increase in the incidence and severity of 300 mg/kg per day (M)
each sex/group 600 mg/kg kidney cortical tubular degeneration at 600 mg/kg (NOEL);
per day, (M) 600 mg/kg per day (M)
5 days/week (LOAEL)
for 13
weeks;
gavage in
corn oil
1,4-DCB B6C3F1 mouse - 0, 84.4, no compound-related deaths or change in body weight 337.5 mg/kg per day
NTP (1987) 10 of each 168.8, gain; mild to moderate centrilobular (NOEL);
sex/group 337.5, 675, hepatocytomegaly at 900 mg/kg; minimal to 675 mg/kg per day
or 900 mg/kg mild hepatocytomegaly at 675 mg/kg (LOAEL)
per day,
5 days/week
for 13
weeks;
gavage in
corn oil
1,2,4-TCB ICR-JCL mouse 600 mg/kg no abnormal weight changes in organs; no macroscopic
Goto et al. (male) diet (78 or histological lesions in liver
(1972) - 20 animals/- g/kg per
group day for
6 months;
diet
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3-TCB Sprague-Dawley 0, 1, 10, no clinical signs of toxicity; deaths of one 1,2,3,-TCB:
1,2,4-TCB rat 100, 1000 high-dose female (not treatment related) and one 7.7 mg/kg per day;
1,3,5-TCB (weanling); mg/kg diet male control (no apparent cause); statistically 1,2,4-TCB:
(>99%) 5 animals each mixed with significant growth suppression in males exposed to 7.8 mg/kg per day;
Côté et al. sex/group corn oil in 10 or 1000 mg 1,2,3-TCB/kg; nephrosis in one male 1,3,5-TCB:
(1988) the diet exposed to 1000 mg 1,2,4-TCB/kg; significant 7.6 mg/kg per day
(males: 0, increase in liver/body weight ratios in males (NOAEL)
0.07-0.08, exposed to high dose of all 3 TCBs; significant
0.78-0.81, increase in serum AH (males) and APDM (both sexes)
7.6-7.8, in animals exposed to 1000 mg 1,2,4-TCB/kg;
78-82 mg/kg "qualitatively similar" changes, mild to moderate
per day; (all TCB isomers) observed in the liver, thyroid,
females: 0, and kidney, significant at high-dose levels only and
0.11-0.13, more severe in males; histopathological changes
1.3-1.5, include: liver: mild to moderate increase in
12-17, cytoplasmic volume and anisokaryosis of hepatocytes;
101-146 all isomers produced fatty infiltration-most severe
mg/kg per at 1000 mg 1,2,4-TCB/kg; thyroid: mild to moderate
day) changes (high-dose groups) including reduction in
follicular size, increased epithelial height and
reduced colloid density; kidney: moderate changes in
the convoluted tubules of males exposed to 1000 mg
1,3,5-TCB/kg
1,2,4,5 TeCB albino rat 0, 0.001, increase in content of SH groups in serum, increases
Fomenko 0.005, or in hemoglobin and reticulocytes of the peripheral
(1965) 0.05 mg/kg blood at >0.005 mg/kg; disorders in the
per day for glycogen-forming function of the liver
8 months
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB B6C3F1 mouse - 0, 30, 100, compound-related clinical signs included tremors 300 mg/kg diet (NOEL);
NTP (1989a) 10 of each 300, 1000, (females), prostration, lethargy, hunched posture, 1000 mg/kg diet (LOAEL)
sex/group or 2000 and rough hair coats (both sexes) at 2000 mg/kg;
mg/kg diet 2/10 female mice in the 2000 mg/kg dose group were
for 13 killed in moribound condition, before termination of
weeks; mixed the study; decrease in final mean body weights of
in 1% corn exposed mice (all doses in males; 30 mg/kg and >1000
oil added to mg/kg for females); decrease in food consumption in
the diet males (at 2000 mg/kg) and females (>1000 mg/kg); at
2000 mg/kg, absolute liver weights (both sexes)
approximately 3 times those of controls; absolute
liver weights increased in females at 30 mg/kg and
>1000 mg/kg and in males at 100 mg/kg and
>1000 mg/kg; relative liver weights increased in
females at >1000 mg/kg and in males at >100 mg/kg;
minimal to mild centrilobular hepatocellular
hypertrophy (both sexes) at 1000 mg/kg (7/10 M; 7/10
F) and 2000 mg/kg (10/10 M; 9/10 F); minimal to mild
individual hepatocyte degeneration at 1000 mg/kg
(9/10 M; 5/10 F); hepatocellular necrosis in males
at 2000 mg/kg (4/10) and in females at 30 mg/kg
(1/10) (not considered to be treatment-related),
1000 mg/kg (1/10) and 2000 mg/kg (1/10);
mineralization of the heart in males at 300 (2/10)
and 2000 (3/10) mg/kg with relationship to treatment
unclear; at doses >1000 mg/kg (both sexes),
increases in serum sorbitol dehydrogenase and
alanine aminotransferase activity; thyroid
follicular hypertrophy in males at >300 mg/kg and in
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB females at >100 mg/kg; at 2000 mg/kg in males and
NTP (1989a) >1000 mg/kg in females, decreased hemoglobin
concentrations, mean corpuscular hemoglobin,
haematocrit, and mean cell volume
1,2,4,5-TeCB F344/N rat - 0, 30, 100, significant decrease in body weights of both sexes 30 mg/kg diet (F)
NTP (1989a) 20 of each 300, 1000, at >1000 mg/kg; compound-related clinical symptoms (NOEL);
sex/group or 2000 included hypoactivity and lethargy; significant 100 mg/kg diet (F)
mg/kg diet increases in absolute (>300 and >100 mg/kg) and (LOAEL)
for 13 relative (>300 and >30 mg/kg) liver weights for
weeks; mixed males and females, respectively; significant
in 1% corn increases in absolute (>300 mg/kg - both sexes) and
oil and relative kidney weights (M >100 mg/kg, F >300
added to the mg/kg); thyroid follicular cell hypertrophy
feed (M >300 mg/kg, F >100 mg/kg); "primary hypothyroid
state" (M >300 mg/kg, F >30 mg/kg); centrilobular
hypertrophy in both sexes (>1000 mg/kg); "hyaline
droplet nephropathy" particularly in males (>100
mg/kg); in females, renal cortical tubular
regeneration (>100 mg/kg) and renal tubular
epithelial accumulation of an unidentified
yellow-brown pigment (2000 mg/kg); in males,
decreases in haematocrit value, haemoglobin
concentrations, and erythrocyte counts at >1000
mg/kg and increases in platelet count and serum
albumin at >100 mg/kg; in females, increase in serum
albumin at 2000 mg/kg and decrease in mean cell
volume at >1000 mg/k3g
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3,4-TeCB Sprague-Dawley 0, 0.5, 5.0, for 1,2,4,5-TeCB - increase in hepatic AH and APDM 1,2,3,4-TeCB:
1,2,3,5-TeCB rat - 15 of 50, or at >50 mg/kg (M) 500 mg/kg (F); at 500 mg/kg 3.4 mg/kg per day (M);
1,2,4,5-TeCB each sex/group 500 mg/kg increase in serum cholesterol, haemoglobin levels, 42 mg/kg per day (F)
(>99.5%) diet for 90 haematocrit, liver and kidney weights with (NOEL);
Chu et al. days; in dose-dependent changes in severity and prevalence of 34 mg/kg per day (M)
(1984a) corn oil in kidney and liver lesions; dose increase in serum (LOAEL)
diet cholesterol, haemoglobin levels and haematocrit at
500 mg/kg; increase in liver and kidney weights at 1,2,3,5-TeCB:
500 mg/kg; dose-dependent changes in severity and 0.42 mg/kg per day (F);
prevalence of renal lesions - (extensive epithelial 3.4 mg/kg per day (M)
necrosis with large intratubular casts at 500 mg/kg) (NOEL);
and of hepatic lesions (accentuation of zonation, 4.2 mg/kg per day (F);
increase in cytoplasmic homogeneity, aggravated 34 mg/kg per day (M)
basophilia, anisokaryosis and pyknotic (LOAEL)
hepatocellular nuclei); dose-dependent accumulation 1,2,4,5-TeCB:
in fat and liver; for the other isomers, changes in 0.034 mg/kg per
kidney and liver much milder, little accumulation in day (M);
fat and liver; males more susceptible than females 4.2 mg/kg per day (F)
(NOEL);
0.34 mg/kg per
day (M);
42 mg/kg per day (F)
(LOAEL)
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB Sherman rat - males: 6 - dose-dependent accumulation in adipose tissue; no 16-31 mg/kg per day (F)
(99.1%) 10 of each 140 mg/kg evidence of porphyria; increase in liver weight at (NOEL);
Linder et al. sex/group per day (0, >500 mg/kg; increase in adrenal (M) and kidney 27-63 mg/kg per day
(1980) 125, or 1000 weight at 1000 mg/kg (and increase in kidney weight (LOAEL)
mg/kg diet) in M at 125 mg/kg); hypertrophy of hepatic cells at
for 100 >500 mg/kg; dose-related increase in hyaline
days; droplets in kidney (M) at >125 mg/kg (at 1000 mg/kg,
females: 7 - focal areas of tubular atrophy and interstitial
150 mg/kg lymphocytic infiltration); decrease in haemoglobin
per day (0, levels, increase in WBC at 1000 mg/kg; decrease in
125, 250, RBC and haematocrit (M) at 1000 mg/kg
500, or 1000
mg/kg diet)
for 180 days
PeCB F344/N rat - 0, 33, 100, decrease in mean body weights of males (>1000 mg/kg) 33 mg/kg (M)
NTP (1989b) 20 of each 330, 1000, and females in all dose groups; increase in absolute 330 mg/kg (F) (NOEL);
sex/group or 2000 liver weights in males at >100 mg/kg and in females 100 mg/kg (M)
mg/kg for 13 at 100 and >1000 mg/kg; increase in relative liver 1000 mg/kg (F) (LOAEL)
weeks in weights in males at >33 mg/kg and in females at
feed >100 mg/kg; increase in
absolute kidney weights in males at >330 mg/kg and
in females at >1000 mg/kg; increase in relative
kidney weights in males >100 mg/kg and in females at
100 and >1000 mg/kg; at >1000 mg/kg, decreases in
hematocrit values, hemoglobin concentrations, mean
corpuscular hemoglobin, and mean cell volume in both
sexes; increases in serum albumin (males, >1000
mg/kg; females, >330 mg/kg); increase in
reticulocyte (males at 2000 mg/kg) and platelet
(males, >1000 mg/kg) counts and creatinine
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB concentrations (males, >1000 mg/kg); lower platelet
NTP (1989b) counts in females at all doses but within normal
(continued) variation; significant increase in serum sorbitol
dehydrogenase activity in males (>1000 mg/kg) and
females (at concentrations as low as 100 mg/kg);
compound-related urinary effects included
significant increase in glucose concentrations
(males, >330 mg/kg; females >1000 mg/kg), increased
protein concentrations (both sexes >1000 mg/kg; more
pronounced in males) and significantly increased
urine volume (males, >1000 mg/kg; females at 2000
mg/kg on day 90); compound-related effects on the
thyroid included decreased free thyroxin and total
thyroxin at all doses in males and >100 mg/kg in
females; incidence of abnormal sperm increased in
males at 330 mg/kg (70%) and 2000 mg/kg (100%)
(sperm of males receiving 1000 mg/kg not examined);
dose-related renal lesions of males included
hyaline droplets of the cortical tubular epithelium
(>100 mg/kg), medullary tubular dilatation
(>100 mg/kg),
medullary collecting tubule mineralization (>330
mg/kg) and tubular cortex regeneration and cortex
chronic inflammation (>100 mg/kg) (also seen in some
control animals); tubular cortex regeneration in
females only at 2000 mg/kg; tubular cortex
pigmentation (males, 2000 mg/kg; females
>1000 mg/kg) and cortex protein casts (both sexes at
Table 22 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
PeCB 2000 ppm); hepatic centrilobular hypertrophy in
NTP (1989b) males at >330 ppm and in females at >1000 ppm;
(continued) hepatic pigmentation and periportal cytoplasmic
vacuolization in females at >1000 ppm; follicular
cell hypertrophy in both sexes at >1000 ppm
PeCB B6C3F1 mouse - 0, 33, 100, compound-related clinical signs included ventral 100 mg/kg (F) (NOEL);
NTP (1989b) 20 of each 330, 1000, body swelling and ruffled fur in both sexes at 330 mg/kg (F)
sex/group or 2000 mg/kg, 2000 mg/kg; significant increase in males in (LOAEL)
for 13 weeks absolute kidney weight at >330 mg/kg and relative
in feed kidney weights at >1000 mg/kg; increase in absolute
liver weights (males, >100 mg/kg; females,
>330 mg/kg); increase in relative liver weights
(both sexes, >330 mg/kg); increases in hemoglobin of
both sexes at >2000 mg/kg (although within normal
variation); increase in serum sorbitol dehydrogenase
activity (both sexes, >1000 mg/kg); decrease in
total thyroxin concentration in both sexes at
concentrations >33 mg/kg; at end of study, liver
porphyrin increased in both sexes at >1000 mg/kg;
minimal-to-moderate centrilobular hepatocellular
hypertrophy in males at all dose levels and in
females at >330 mg/kg; occasional necrosis of
hypertrophied hepatocytes (considered secondary to
the hypertrophy) observed in both sexes (males, 33
and 2000 mg/kg; females in control, 330 and
2000 mg/kg dose groups)
Table 22 (continued)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses reported as mg/kg body weight, unless specified.
d M - male.
F - female.
WBC - white blood cells.
RBC - red blood cells.
ALH - microsomal aniline hydroxylase.
SGOT - serum glutamic-oxaloacetic transaminase.
SGPT - serum glutamic-pyruvic transaminase.
SAP - serum alkaline phosphatase.
ALT - alanine aminotransferase.
APDM - aminopyrine demethylase.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOAEL - lowest-observed-adverse-effect level.
Reports of investigations of the long-term toxicity of the
tetra-chlorobenzenes following inhalation have not been found.
However, in a 90-day study to compare the toxicities of TeCB isomers
administered in the diet to rats, renal and hepatic toxicity were
observed, as indicated by increases in organ weights and
histopathological changes (Chu et al., 1984a). As was seen in a
short-term study conducted by the same investigators, the
1,2,4,5-isomer was the most toxic of the TeCBs; this may be
attributable to its comparatively greater accumulation in fat and
liver. The authors concluded that the observed changes were similar
to those resulting from 28-day exposures to the same doses, but that
renal lesions induced by the highest concentration of 1,2,4,5-TeCB
(30-40 mg/kg) were more severe. The NOEL in male rats adminis-tered
the 1,2,4,5-isomer was 0.034 mg/kg body weight; the value for
females was considerably higher (4.2 mg/kg body weight). The NOEL
for the other isomers in male rats was 3.4 mg/kg body weight; in
females, these values were 42 and 0.42 mg/kg body weight for the
1,2,3,4- and 1,2,3,5-isomers, respectively.
In a 13-week study on F344 rats, which was completed recently by the
US National Toxicology Program, 1,2,4,5-TeCB was mixed in corn oil
(1%) and added to the diet at concentrations of 0, 30, 100, 300,
1000, or 2000 mg/kg (NTP, 1989a). There were significant decreases
in body weights, compound-related clinical symptoms,
histopathological changes in the liver, and haematological effects
at the highest doses; increases in the weights of both liver and
kidney, renal histopathological effects, and effects on the thyroid
were seen at lower doses. The NOEL for histopathological effects in
females was considered by the investigators to be 30 mg/kg diet
(approximately 1.95 mg/kg body weight); a NOEL in males could not be
established.
In a similar study, in which B6C3F1 mice were administered a diet
containing 0, 30, 100, 300, 1000, or 2000 mg 1,2,4,5-TeCB/kg for 13
weeks, there were significant decreases in body weights and food
consumption, compound-related clinical signs, increases in liver
weights and circulating hepatic enzymes, histopathological changes
in the liver, and haematological effects, at the highest doses. The
NOEL in both males and females was considered to be 300 mg/kg diet
(approximately 48 mg/kg body weight) (NTP, 1989a).
Only limited data are available on the long-term toxicity of
penta-chlorobenzene. Ingestion of high doses by rats resulted in
hepatic and renal toxicity, as indicated by increases in organ
weights and histopathological changes (Linder et al., 1980),
increases in adrenal weight (Linder et al., 1980), and increased
excretion of porphyrins (Goerz et al., 1978). The NOEL in female
rats, derived from the results of Linder et al. (1980), was
16-31 mg/kg.
In a recently completed 13-week study conducted by NTP (1989b), F344
rats and B6C3F1 mice were fed diets containing 0, 33, 100, 330,
1000, or 2000 mg PeCB/kg. Decreases were seen in the mean body
weights of male rats at 1000 mg/kg or more and in females at all
dose levels. Increased liver weights were seen at the lower doses
and histopathological effects on the liver, at the higher doses. In
males, there were increases in kidney weights and renal
histo-pathological effects at lower doses; these effects were
observed only at higher doses in females. Functional effects were
observed in the kidney in both sexes at the highest doses and in the
thyroid at all doses, with histopathological effects occurring only
at the highest doses (1000 or 2000 mg/kg diet). There were also
haematological effects at doses exceeding 1000 mg/kg in males and
330 mg/kg in females. The incidence of abnormal sperm in males was
also increased at the dose levels at which it was examined (330 and
2000 mg/kg diet). On the basis of histopathological lesions, the
authors considered the NOEL to be 33 mg/kg diet in male rats, and
330 mg/kg diet in females (approximately 2.0 and 21.5 mg/kg body
weight, respectively).
In mice, there were compound-related clinical signs at the highest
dose. Increased kidney weights were observed at the highest doses
and functional effects on the thyroid at all doses. There were
increases in liver weights at lower doses and histopathological
effects on the liver at all doses in males and at doses of 330 mg/kg
diet or more in females. On the basis of the histopathological
lesions, the authors considered the NOEL in female mice to be 100
mg/kg diet (approximately 18.3 mg/kg body weight); no NOEL in males
could be established.
8.5 Chronic Toxicity and Carcinogenicity
The protocols and results of the few studies on chronic toxicity and
carcinogenicity of the chlorobenzenes are summarized in Table 23.
The United States National Toxicology Program (NTP) has studied the
chronic toxicity and carcinogenicity for rats and mice of MCB, and
the 1,2- and 1,4-isomers of DCB (NTP, 1983a,b, 1987), following oral
administration. The results of an additional investigations by
Loeser & Litchfield (1983) on the chronic toxicity and
carcino-genicity of 1,4-DCB for rats and mice have also been
reported.
Only one rather limited investigation on the chronic toxicity and
carcinogenicity of the TCBs (1,2,4-isomer) following skin painting
in mice has been identified (Yamamoto et al., 1982) and studies on
the chronic toxicity of the TeCBs are confined to a very limited
investigation involving the oral administration of the
1,2,4,5-isomer to dogs (Braun et al., 1978). No data were found on
the chronic toxicity and carcinogenicity of PeCB.
Table 23. Chronic toxicity and carcinogenicity of chlorobenzenes
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Inhalation
1,4-DCB SPF Alderlely 0, 450, or 3000 mg/m3 increase in liver and kidney weights, 450 mg/m3
Loeser & Park Wistar (0, 75 or 500 ppm); 5h urinary protein, and coproporphyrin at (NOEL)
Litchfield -derived rat /day, 5 days/week for 3000 mg/m3; no treatment-related tumours 3000 mg/m3
(1983) - 76-79 of each 76 weeks; killed at 102 (LOAEL)
sex/group weeks
1,4-DCB SPF Swiss 0, 450, or 3000 mg/m3 no treatment-related toxic effects or 3000 mg/m3 (NOEL)
Loeser & (Alderley Park (0, 75, or 500 ppm); 5h tumours; one osteosarcoma in nasal sinus
Litchfield strain) mouse /day, 5 days/week for 57 at 450 mg/m3
(1983) (female) - weeks; killed at 75-76
75/group weeks
Ingestion
MCB Fischer 344 rat - 0, 60, or 120 mg/kg per significant increase in hepatic 60 mg/kg per day (M),
(99%) 50 of each day (5 days/week for 103 neoplastic nodules (M) at 120 mg/kg 120 mg/kg per day (F)
NTP (1983a); sex/group weeks); gavage in corn in comparison with vehicle and pooled (NOEL);
Kluwe et al. oil controls, but no carcinomas at this 120 mg/kg per day (M)
(1985) dose; marginally significant (LOAEL)
dose-response trend; rare tumours in 3
exposed animals - 1 renal tubular cell
adenocarcinoma (F) and 2 transitional
cell papillomas of the bladder (M);
decrease in pituitary tumours
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
MCB B6C3F1 mouse - 50 males: 0, 30, or 60 mg no treatment-related toxic effects or 60 mg/kg per day (M),
(99%) of each sex/group /kg per day; females: 0, tumours 120 mg/kg per day (F)
NTP (1983a) 60, or 120 mg/kg per day (NOEL)
Kluwe et al. (5 days/week for
(1985) 103 weeks); gavage in
1,2-DCB Fischer-344 rat - 0, 60, or 120 mg/kg per decrease in survival and body weight 120 mg/kg per day (F)
(>99%) 50 of each day (5 days/week for gain (M) at 120 mg/kg; increase in body (NOEL)
NTP (1983b) sex/group 103 weeks); gavage in weight gain (F) after 32 weeks; increase
corn oil in adrenal phacochromocytomas at 60
mg/kg - probably not compound-related
1,2-DCB B6C3F1 mouse - 0, 60, or 120 mg/kg per dose-related trend for increase in 60 mg/kg per day (M),
(>99%) 50 of each day (5 days/week for tubular regeneration in the kidney (M at 120 mg/kg per day (F)
NTP (1983b) sex/group 103 weeks); gavage in 120 mg/kg); positive trend for incidence (NOEL);
corn oil of malignant histiocytic lymphomas in 120 mg/kg per day (M)
both sexes, but negative trend for (LOAEL)
malignant lymphocytic lymphomas;
significant positive trend in
alveolar/bronchiolar carcinomas in males
not significant when combined with
adenomas; dose-related decrease in
hepatocellular adenomas (M)
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB Fischer 344 rat - males: 0, 150, or reduced survival (M) and body weight
(>99%) 50 of each 300 mg/kg per day gain at 300 mg/kg; increased severity of
NTP (1987) sex/group females: 0, 300, or nephropathy and incidence of hyperplasia
600 mg/kg per day of the parathyroid at >150 mg/kg (M) and
(5 days/week for dose-related increase in nephropathy (F)
103 weeks); gavage in at >300 mg/kg; dose-related increase in
corn oil renal tubular cell adenocarcinomas (M);
tubular cell adenoma in high-dose groups
(M); marginal increase in mononuclear
leukaemias (M)
1,4-DCB B6C3F1 mouse - 0, 300,or 600 mg/kg per increased incidence of hepatic lesions;
(>99%) 50 of each day (5 days/week for nephropathy (M), renal tubular
NTP (1987) sex/group 103 weeks); gavage in regeneration (F); thyroid gland
corn oil follicular cell hyperplasia (M)
(positive trend in F); adrenal medullary
hyperplasia (M) and focal hyperplasia of
the adrenal gland capsule (M); positive
trend and increase in hepatocellular
carcinomas at 600 mg/kg and
hepatocellular adenomas
(M >300 mg/kg; F >600 mg/kg);
hepatoblastomas in 4 high-dose males;
positive trend for phaeochromocytomas
f adrenal gland with increased incidence
at 600 mg/kg (M); marginal trend in
follicular cell adenomas of the thyroid
(F)
Table 23 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,4,5-TeCB Beagle dog - 5 mg/kg per day (2 increases in SAP and total bilirubin at
Braun et al. 2 of each years; killed at 3 years 24 months returned to normal, 3 months
(1978) sex/group (no 8 months); diet after exposure (no measurement of
concurrent urinary, haematological, or clinical
control group) chemistry, during exposure); no
morphological changes after the 20-month
recovery phase (no gross or histological
examination during, or at the end of,
exposure)
Dermal
1,2,4-TCB Slc:ddY mouse - 0.03 ml of 0, 30%, or clinical symptoms of toxicity including
Yamamoto 75 of each 60% solution (twice/week hysterical excitement, acceleration of
et al. (1982) sex/group (50 of for 2 years); dissolved spontaneous activity, and panting;
each sex/control in acetone decreased survival (F-both doses, M-high
group) dose); thickening and keratinization of
epidermis followed by inflammation;
increase in spleen weight at both doses
(M-low dose, F-high dose); minor
haematological changes at both doses;
authors report no increase in tumour
incidence attributable to TCB - however
data inadequate for evaluation
Table 23 (continued)
a Strain and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified.
d SAP - serum alkaline phosphatase.
M - male.
F - female.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOAEL - lowest-observed-adverse-effect level.
In the NTP bioassays on MCB, there was no convincing evidence of
compound-related toxicity in either rats or mice administered doses
of up to 120 mg/kg body weight per day for 103 weeks. Evidence for
mild hepatocellular necrosis in rats was equivocal and, though there
was a significant decrease in the survival of male rats in the
high-dose group (120 mg/kg), the absence of marked toxic lesions did
not support a causal relationship with MCB. The doses administered
in the long-term bioassay were not significantly less than those at
which toxic effects were observed in the NTP 13-week studies (LOAEL
= 250 mg/kg per day), indicating little potential for progressive
toxicity with continued MCB administration for more than 13 weeks
(NTP, 1983a; Kluwe et al., 1985).
A significant increase in neoplastic nodules in the liver was noted
in the high-dose group of male rats administered MCB (incidence,
2/50, 4/49, and 8/49 at 0, 60, and 120 mg/kg body weight,
respectively). However, no hepatocellular carcinomas were found and
analysis of the combined data on neoplastic nodules and
hepato-cellular carcinomas reduced the significance of the increase
in tumour incidence. It was concluded that the NTP study provided
some, but not clear, evidence of carcinogenicity in male Fischer 344
rats, based on the increased incidence of hepatic nodules, but that
there was no evidence of carcinogenicity in female Fischer 344 rats
or in male or female B6C3F1 mice (NTP, 1983a).
In the NTP bioassay for the carcinogenesis of 1,2-DCB, the only
evidence of toxicity was a decrease in survival and body weight gain
in male rats and an increase in tubular regeneration of the kidney
in male mice, both of which occurred at the highest dose level (120
mg/kg body weight per day for 103 weeks) (NTP, 1983b). The rates of
survival until the end of the study in the 3 groups of male rats
were 42/50 (84%), 36/50 (72%), and 19/50 (38%) for 0, 60 mg/kg, and
120 mg/kg body weight, respectively. Although the survival rate in
the high-dose group was significantly different from those in the
low-dose and vehicle-control groups in the male rat, this may have
been the result of accidents occurring during the dosing by gavage
(NTP, 1983b). In mice administered 1,2-DCB, there was a dose-related
trend in the tubular regeneration of the kidney in males only, with
an increased incidence at the highest dose (120 mg/kg body weight)
(control, 17%; 60 mg/kg, 24%; 120 mg/kg, 35%); otherwise, there was
no other evidence of non-neoplastic toxicity (NTP, 1983b).
In rats administered 1,2-DCB in the NTP study, the only tumours that
occurred in excess were adrenal phaeochromocytomas in the low-dose
group of male rats (incidence in controls, 9/50; low-dose, 16/50 and
high-dose, 6/49), which were not considered to be treatment-related.
In mice, there was a positive trend in the incidence of malignant
histiocytic lymphomas in both sexes; however, the combined incidence
of all types of lymphomas was not significantly greater than that in
the controls for mice of either sex. Similarly, alveolar/bronchiolar
carcinomas occurred in male mice with a statistically significant
positive trend; there were, however, no significant trends when the
incidences of alveolar/bronchiolar adenomas and carcinomas were
combined. Thus, the NTP concluded that, under the conditions of
these studies, there was no evidence for the carcinogenicity of
1,2-DCB in male or female F344 rats or B6C3F1 mice.
There are 2 bioassays that are relevant to the assessment of the
chronic toxicity and carcinogenicity of 1,4-DCB: an inhalation study
on rats and mice (Loeser & Litchfield, 1983) and an NTP bioassay
involving ingestion by the same species (NTP, 1987).
Loeser & Litchfield (1983) reported increases in liver and kidney
weights, as well as in urinary protein and coproporphyrin, in the
high-dose group when rats were administered 0, 450, or 3000 mg
1,4-DCB/m3, for 5 h/day, 5 days/week, over 76 weeks, followed by
36 weeks without exposure. There were also some statistically
significant changes in blood biochemical and haematological
parameters; however, there was no indication of a dose-response
relationship in this regard. There was no evidence of toxicity in
mice following administration of the same doses for a shorter period
of 57 weeks, followed by 19 weeks without exposure. No
treatment-related effects on the incidence, multiplicity, or
malignancy of tumours were observed in either species. The
relatively short exposure period (76 weeks in rats, 57 weeks in
mice) and the high early mortality in mice may have decreased the
sensitivity of this carcinogenicity bioassay.
In the NTP (1987) study, 1,4-DCB was administered, by gavage, in
corn oil, for 5 days/week, over 103 weeks. Groups of 50 male F344
rats received 150 or 300 mg/kg body weight per day, while groups of
50 female F344 rats and 50 male and 50 female B6C3F1 mice received
300 or 600 mg/kg body weight per day. Under this regimen, there was
an increased incidence of non-neoplastic liver lesions in male and
female mice, including cytomegaly and karyomegaly, hepatocullular
degeneration, and individual cell necrosis. Also, 1,4-DCB
administration resulted in an increased incidence of nephropathy in
male mice and renal tubular regeneration in female mice.
Non-neoplastic lesions in male rats after 1,4-DCB administration
included an increased incidence and severity of nephropathy, an
increased incidence of epithelial hyperplasia of the renal pelvis,
mineralization of the collecting tubules in the renal medulla, and
focal hyperplasia of the renal tubular epithelium. An increased
incidence of nephropathy was noted in both low- and high-dose female
rats compared with the vehicle controls.
As reported by NTP (1987), administration of 1,4-DCB resulted in a
dose-related increase in the incidence of tubular cell
adenocarcinomas of the kidney in male rats (1/50, 3/50, and 7/50 in
the control, mid-, and high-dose groups, respectively); an uncommon
malignant tumour was found in F344 rats, but only in 4/1098 of the
NTP historical controls fed corn oil by gavage. An additional
tubular cell adenoma was observed in a high-dose male rat. No
tubular cell tumours were found in either treated or vehicle-control
female rats. A marginal increase in the incidence of mono-nuclear
cell leukaemia in treated male rats was noted compared with controls
(5/50, 7/50, and 11/50 in control, mid- and high-dose groups,
respectively).
In mice, there were positive trends in the incidence of
hepatocellular adenomas, hepatocellular carcinomas, and
hepatocellular adenomas and carcinomas (combined) in males and
females, the incidences in the high-dose groups being significantly
greater than those in the vehicle controls. In low-dose male mice,
the incidences of hepatocellular adenomas and adenomas or carcinomas
(combined) were also significantly increased over those in the
vehicle controls (combined incidence 17/50, 22/49, and 40/50 in
control, low-, and high-dose groups of males; combined incidence
15/50, 10/48, and 36/50 in control, low-, and high-dose groups of
females) (NTP, 1987). Hepatoblastomas occurred in 4 high-dose male
mice, each of which also bore hepatocellular carcinomas. This rare
type of hepatocellular carcinoma had not been observed by NTP in
1091 control (corn oil by gavage) male mice and had only been seen
in 1/2080 untreated female mice. Marginal increases were observed in
the incidences of pheochromocytomas of the adrenal gland in male
mice (controls, 0/47; low-dose group, 2/48; and high-dose group,
2/49) reaching statistical significance in the high-dose group
(P=0.035), and in the incidence of follicular cell adenomas of the
thyroid gland in female mice (controls, 0/48; low-dose group, 0/45;
and high-dose group, 3/46).
On the basis of these results, the NTP (1987) concluded that there
was clear evidence of the carcinogenicity of 1,4-DCB in male F344/N
rats, and clear evidence of carcinogenicity in both male and female
B6C3F1 mice. There was no evidence of carcinogenicity in female
F344/N rats.
It is possible that the discrepancies between the results of Loeser
& Litchfield (1983) and NTP (1987) are the result of differences in
experimental design. Loeser & Litchfield (1983) administered
approximately 390 mg/kg body weight per day via inhalation to rats
and approximately 790 mg/kg to mice. Although these doses were
higher than those used by NTP (1987), mice were exposed for only 57
weeks and rats for only 76 weeks. The strains of animals used and
the nature of administration also varied between the 2 studies
(i.e., continuous inhalation for 5 h/day versus bolus dose by
gavage).
The induction of kidney tumours in the male rat is believed to be
associated with hyaline droplet formation resulting in a
characteristic alpha-2-microglobulin nephropathy (hyaline droplet
nephropathy). Such effects, specific for the male rat, have been
shown to occur with a number of chemicals, including 1,4-DCB,
unleaded gasoline, isophorone, decalin, and limonene, and the
mechanism has been established (Short et al., 1987; Goldsworthy et
al., 1988; Murty et al., 1988; Charbonneau et al., 1989). It
involves increased formation of protein droplets and crystalloid
accumulation in the cytoplasm of the P2 segment of the proximal
tubule with a marked increase in cell proliferation. The hyaline
droplets formed are composed of lysosomes containing excess
alpha-2-microglobulin and protein-bound chemical. Increased cell
turnover in the P2 segment of the kidneys of male rats may be
related to the slow catabolism of this accumulated protein within
the lysosomes leading, in some cases, to renal tubular
adenocarcinomas in the male rat. Alpha-2-microglobulin occurs in
large amounts in the male Fisher 344 rat, but is not seen in
significant amounts in the female rat, mouse, or man (Olson et al.,
1990).
For 1,4-DCB, there is much evidence to support the involvement of
hyaline droplet accumulation in the induction of the tubular cell
adenocarcinomas in the male rat. Bomhard et al. (1988) demonstrated
hyaline droplet accumulation in male, but not in female, rats
following exposure to 1,4-DCB. Charbonneau et al. (1989) reported
that 1,4-DCB (single exposure of F344 rats to 300 or 500 mg/kg body
weight in corn oil) increased protein droplet formation and cell
proliferation in male but not female rat kidneys, whereas equimolar
doses of 1,2-DCB did not have any effect. The maximum amount of
radiolabel reversibly bound to alpha-2-microglobulin by
C14-labelled 1,4-DCB was also twice that for an equimolar dose of
C14-labelled 1,2-DCB.
Available data are inadequate for the assessment of the chronic
toxicity and carcinogenicity of the higher chlorinated benzenes
(tri- to penta-). Clinical signs of toxicity, decreased survival,
increased organ weights, keratinization of the epidermis, and minor
haemato-logical effects were reported in Slc:ddy mice treated
dermally with 0.03 ml of a 30 or 60% solution of 1,2,4-TCB, twice a
week, over 2 years (Yamamoto et al., 1982). No increase in tumour
incidence, attributable to TCB, was reported.
An increase in serum alkaline phosphatase and total bilirubin
following 24 months of exposure of beagle dogs to 5 mg
1,2,4,5-TeCB/kg body weight per day in the diet has been reported
(Braun et al., 1978). The values for these parameters returned to
normal 3 months after cessation of exposure, and there were no
morpho-logical changes after a 20-month recovery phase. However, the
results of this study are inadequate for the assessment of chronic
toxicity or carcinogenicity. No measurements were made of urinary,
haematological, or clinical chemistry parameters during exposure,
and no histopathological examination was carried out at the end of
exposure. Moreover, there were only 2 animals of each sex in the
exposed group, only 1 dose level was examined, there was no
concurrent control group, and the period of administration (2 years)
was short in relation to the life span of the dog.
No data are available on the chronic toxicity or carcinogenicity of
pentachlorobenzene.
8.6 Mutagenicity and Related Endpoints
8.6.1 In vitro systems
When tested in S. typhimurium strains TA98, TA100, TA1535, or
TA1537, with, or without, the addition of an S9 fraction from the
liver of Aroclor(R) 1254-treated rats, MCB did not show any
mutagenic potential (Haworth et al., 1983). In another study in
S. typhimurium using a slightly lower dose (1.28 µl/plate) and
adding strain TA1538, Shimizu et al. (1983) reported that there was
no evidence for the mutagenicity of MCB in this system.
The dichlorobenzenes (1,2-, 1,3-, and 1,4-DCB) have been studied for
mutagenic effects in S. typhimurium , with, and without, metabolic
activation. Haworth et al., (1983) studied a mixture of DCB isomers
in 4 strains with, and without, activation with S9 supernatant from
the livers of rats treated with Aroclor(R) 1254. No mutagenicity
was noted. Using a similar protocol with 5 strains of
S. typhimurium, at doses of up to approximately 1.3 µl/plate,
Shimizu et al. (1983) reported that neither 1,2- nor 1,3-DCB was
mutagenic. 1,4-DCB, at doses up to 6.6 mg/plate, was also found to
be negative in S. typhimurium, under the conditions outlined by
Shimizu et al. (1983).
No evidence was reported by Haworth et al. (1983) that mixed TeCBs
and PeCB were mutagenic, when assayed in S. typhimurium strains
TA98, TA100, TA1535, and TA1537 with, and without, metabolic
activation. Using a similar protocol, Schoeny et al. (1979) reported
that 1,2,4-TCB was not mutagenic in S. typhimurium.
When tested for forward mutations in L5178Y/TK+/- mouse lymphoma
cells, 1,4-DCB was negative when assayed without metabolic
activation and the results were equivocal with activation with S9
from Aroclor(R) 1254-induced rat liver (NTP, 1987). No evidence
was reported by Perocco et al. (1983) for the induction by 1,4-DCB
of unscheduled DNA synthesis in human lymphocytes in vitro.
The induction in vitro of chromosomal aberrations and
sister-chromatid exchanges in Chinese hamster ovary (CHO) cells by
1,4-DCB was studied by Galloway et al. (1985). Experiments were
carried out with, and without, S9 from the livers of male rats
induced with Aroclor(R) 1254. No chromosomal effects were
reported. Using cultured Chinese hamster lung fibroblast cells,
with, and without, activation by S9 from livers of rats induced with
the PCB KC-400, Sofuni et al. (1985) reported that treatment with
1,4-DCB, 1,2,3-TCB, 1,2,4-TCB, or 1,3,5-TCB did not cause
chromosomal aberrations.
In unpublished studies reviewed by the Task Group, 1,4-DCB did not
induce mutation at the HGPRT locus in CHO cells (Bayer, 1986a),
unscheduled DNA synthesis in HeLa cells (Milone, 1986a), or
chromosome aberrations in human lymphocytes (Milone, 1986b), in
either the presence or absence of an exogenous metabolic activation
system. 1,4-DCB did not induce cell transformation in the Balb/3T3
assay (Bayer, 1986b).
8.6.2 In vivo tests on experimental animals
Very few studies have been reported on the in vivo genotoxicity of
chlorobenzenes.
No increase in micronucleated red blood cells was reported after
oral administration to male mice of 600, 900, 1000, 1500, or 1800 mg
1,4-DCB/kg body weight, by gavage, for 5 days/week, over 13 weeks.
Female mice were similarly treated with 1200, 1500, or 1800 mg/kg
body weight (NTP, 1987). These results support those from a
previously unpublished study, reviewed by Loeser & Litchfield
(1983), in which treatment of rats, via inhalation with doses of
1,4-DCB as high as 4092 mg/m3 (682 ppm) for 2 h/day, or 3000
mg/m3 (500 ppm) for 5 h/day, over 3 months, did not result in any
increase in chromosomal abnormalities in bone marrow cells.
A study of the clastogenic activity of halogenated benzenes in mice
has been published by Mohtashamipur et al. (1987). MCB, 1,2-, 1,3-,
or 1,4-DCB, or 1,2,3-, 1,2,4-, or 1,3,5-TCB, administered by
intraperitoneal injection in corn oil to groups of 5 mice, resulted
in a dose-related increase in the formation of micronucleated
poly-chromatic erythrocytes. The doses used were between
approximately 15 and 70% of the respective LD50s, given in 2
doses, separated by a 24-h period. Bone marrow samples were taken
30 h after the first injection. Doses were similar to those given by
gavage for 13 weeks in other studies (NTP, 1987). However, this
result was not confirmed in studies by other workers, also using the
interperitoneal route (Heibold, 1988). Groups of mice were
administered doses of 2 x 177.5 and 2 x 355 mg/kg in solution in
corn oil, intraperitoneally (doses similar to those inducing an
effect in the study by Mohtashamipur et al., 1987). The two doses
were given 24 h apart, and the cells were harvested 6 h later.
Evidence of a reduction in erythropoiesis was seen at both dose
levels; however, there was no indication of micronuclei induction at
either dose level. The ability of orally administered 1,4-DCB to
produce micronuclei in bone marrow has also been investigated
(Heibold, 1986). There was no evidence of any increase in
micronuclei following the administration of a single oral dose of
2500 mg/kg body weight and the harvesting of the bone marrow at 24,
48, and 72 h.
The ability of 1,4-DCB to produce unscheduled DNA synthesis (UDS) in
the liver, or effects on DNA replication (S phase), was investigated
following oral exposure of B6C3F1 mice to the compound (Steinmetz
& Spanggord, 1987). Mice were given doses of 300, 600, or 1000 mg
1,4-DCB/kg body weight in corn oil and hepatocytes were harvested 16
and 48 h later. There was no evidence of any increase in UDS at any
of the dose levels studied. The small increase in S-phase DNA
replication seen was statistically significant in males only. These
data indicate that 1,4-DCB does not produce DNA damage in the mouse
liver following oral exposure.
The production of chromosome damage in the bone marrow of mice by
1,4-DCB has been investigated by a number of workers. Negative
results were obtained in all except one case. In addition, negative
results were obtained in an assay of DNA damage (UDS) in the liver
of mice following oral exposure to 1,4-DCB. These results indicate
that 1,4-DCB does not have any mutagenic potential in in vivo
studies.
MCB and 1,4-DCB bind covalently to DNA in the mouse liver, kidney,
and lung, while only MCB binds to the DNA in rat tissue (Grilli
et al., 1985; Lattanzi et al., 1989). The levels of binding for both
compounds are low.
8.6.3 Human in vivo studies
Workers in a pathology laboratory (8 male/18 female, average age 35
years) were accidently exposed to vapours of 1,2-DCB, for 8 h/day,
over 4 days. Chromosome analyses on peripheral leukocytes revealed a
marked increase in chromosomal aberrations compared with controls.
The controls (8 male/8 female, average age 34 years) were from other
pathology laboratories where the occupational exposures to chemicals
were similar to that of the DCB-exposed population. In the 1345
cells studied in the exposed workers, 120 exhibited chromosomal
alterations (84 single breaks and 86 double breaks) compared with 19
cells with aberrations in the 942 cells examined in the control
group. Analyses carried out 6 months after exposure indicated that
the alterations were reversible (Zapata-Gayon et al., 1982).
Although no air monitoring was carried out, the symptoms reported by
22 out of the 26 subjects were consistent with those produced at
exposure levels in excess of 600 mg/m3 (100 ppm), described by
Hollingsworth et al. (1958).
In workers (24 male/1 female) producing 1,2,4,5-TeCB in a chemical
plant, in which several organochlorine and organophosphorus
pesticides were also produced, there were increases (significance
not reported) in the total number of chromatid gaps and breaks,
labile chromosome-type aberrations (acentric fragment, ring
chromosomes, and dicentric chromosomes), and stable chromosome-type
aberrations (deletions, inversions, and translocations), compared
with those in 14 workers from another type of factory and 49
unexposed controls. No workplace monitoring for exposure levels was
carried out and workers were exposed to several other compounds.
Thus, it is difficult to assess the potential risks in other groups
exposed to TeCB on the basis of these results (Kiraly et al., 1979).
8.7 Developmental and Reproductive Effects
The results of studies concerning the embryotoxicity, fetotoxicity,
teratogenicity, and reproductive effects of the chlorobenzenes are
presented in Table 24. Effect levels derived in these studies for
both mothers and offspring are also presented in this table. In
contrast to the lack of information on acute, short-term, long-term,
and carcinogenic effects, comparatively more data are available on
the potential embryotoxic, fetotoxic, and teratogenic effects of the
higher chlorinated benzenes. Furthermore, the TCBs, TeCBs, and PeCB
have been better studied in this regard than MCB and the isomers of
DCB.
There has been no evidence in any of the studies conducted to date
that the chlorobenzenes (mono- to penta-) are teratogenic in animal
species. Some relatively minor embryotoxic and fetotoxic effects
have been observed for the lower chlorinated benzenes (MCB and
DCBs), but only at dose levels that were toxic for the mother. For
example, there was a slight delay in skeletal development
(ossification) in the fetuses of pregnant rats exposed to 2864 mg
MCB/m3, a dose that induced decreases in body weight gain in the
mothers (John et al., 1984). Similarly, the ossification of cervical
vertebrae in fetuses of pregnant rats exposed to 2400 mg
1,2-DCB/m3 was delayed; this dose also induced decreases in body
weight gain and food consumption in the mothers (Hayes et al.,
1985). However, in both of these studies, there was no convincing
evidence of embryotoxic, fetotoxic, or teratogenic effects in
rabbits exposed to MCB or 1,2-DCB via inhalation, even at dose
levels that were maternally toxic (John et al., 1984; Hayes et al.,
1985). In fetuses of pregnant rabbits exposed to 4720 mg
1,4-DCB/m3, there was an increase in the incidence of
retro-oesophageal right subclavian artery, a minor variation in the
circulatory system that is often observed in control litters; at
this dose, the maternal body weight gain was also decreased (Hayes
et al., 1985). In an additional study, Giavini et al. (1986)
administered 1,4-DCB, in corn oil, by gavage, at doses of 250, 500,
750, or 1000 mg/kg body weight per day, between days 6 and 15 of
gestation. Effects were noted only at doses greater than 500 mg/kg
per day. These included an increased frequency of extra ribs, a
reduction in fetal weight, and an increase in skeletal
abnormalities. These dose levels also induced decreases in body
weight gain and food consumption in the mothers.
There is some evidence that the higher chlorinated benzenes (TCBs,
TeCBs, PeCB) are embryotoxic or fetotoxic at dose levels that are
not maternally toxic. However, available data are not consistent and
the toxicities of the various isomers of the TCBs and TeCBs for the
mother and fetus vary considerably. For example, the 1,2,4-isomer
was the most maternally toxic of the 3 TCB isomers administered by
ingestion to pregnant rats in a study conducted by Black et al.
(1988); changes in haematological parameters occurred at doses as
low as 150 mg/kg per day. At a lower dose (75 mg/kg), there were
mild histological changes in the lenses of pups of exposed mothers;
however, these changes were not observed at higher doses (150 or
300 mg/kg) and were unlikely, therefore, to be treatment-related.
Kitchin & Ebron (1983a) observed growth retardation in the embryos
of pregnant rats administered 360 mg 1,2,4-TCB/kg body weight on
days 9-13, a dose that caused some lethality, reduced body weight
gain, and produced moderate hepatocellular hypertrophy in mothers.
Although less toxic for the mothers than 1,2,4-TCB, the 1,3,5-isomer
of trichlorobenzene induced mild changes in the lenses of pups of
pregnant rats administered doses as low as 150 mg/kg body weight by
gavage; there was no significant maternal toxicity at this dose
(Black et al., 1988). For the 1,2,3-isomer, there were no effects in
offspring at any dose level (up to 600 mg/kg body weight), even
though a level of 300 mg/kg was toxic to the mothers. The authors of
this study did not discuss the significance of the observed
histological changes in the lenses (areas of cellular disorientation
and disaggregation with ballooning and granular degeneration) of the
pups of mothers administered the 1,3,5-isomer, but concluded that
there was no evidence that any of the TCB congeners were teratogenic
or fetotoxic.
Kacew et al. (1984) reported that the 1,2,4,5-isomer was the most
toxic of the TeCBs for both mothers and fetuses, in a study in which
all 3 isomers were administered to pregnant rats by gavage (death of
9/10 animals at 200 mg/kg and increase in serum cholesterol at
50 mg/kg body weight). The toxicity was well correlated with the
greater accumulation of 1,2,4,5-TeCB in maternal and fetal tissues.
There was a decrease in the number of live fetuses in pregnant rats
administered 50 mg 1,2,4,5-TeCB/kg body weight, a dose that induced
minor changes (increases in serum cholesterol) in exposed mothers.
Table 24. Developmental and reproductive studies on chlorobenzenes
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Developmental Studies:
Inhalation
MCB Fischer 344 rat rats: 0, 341, 955, or rats: maternal toxicity (decreased body weight gain 955 mg/m3 (F);
(99.98%) (pregnant), 2684 mg/m3 (0, 75, 210, or and increased liver weight) at 2684 mg/m3 - slight (NOEL);
John et al. 32-33/group; 590 ppm), 6 h/day on days delay in skeletal development (ossification) in 2684 mg/m3 (F)
(1984) New Zealand white 6-15 of gestation; rabbits: fetuses at this dose; rabbits: maternal toxicity *2684 mg/m3
rabbit, (pregnant), 0, 341, 955 or 2684 mg/m3 (increased liver weight) at >2684 mg/m3; significant (O) (LOAEL)
30-32/group (0, 75, 210, or 590 ppm) increase in resorptions at 2684 mg/m3 (second
6 h/day on days 6-18 of study), but within historical range; no
gestation; or rabbits: 0, treatment-related effects on fetus
45, 136, 341, or 2684 mg/m3
(0, 10, 30, 75 or 590 ppm)
6 h/day on days 6-18 of
gestation
1,2-DCB Fischer 344 rat rats: 0, 600, 1200, or rats: maternal toxicity at all doses; increased rats:
(98.81%) (pregnant), 2400 mg/m3, 6 h/day on days liver weight at 2400 mg/m3; significant increase in 600 mg/m3 (F);
Hayes 30-32/group; 6-15 of gestation; rabbits: spurs on first lumbar vertebra and delayed *2400 mg/m3
et al. New Zealand white doses as above - on days 6-18 ossification of sternebrae in fetuses at 1200 mg/m3 (O) (LOAEL)
(1985) rabbit (pregnant), of gestation not considered to be treatment related; delayed
28-30/group ossification of cervical vertebra centra in fetuses rabbits:
at 2400 mg/m3; rabbits: maternal toxicity (decreased *2400 mg/m3
body weight gain) at all doses; ratio of male:female (O) (NOEL);
offspring significantly different from 50:50 at 1200 600 mg/m3 (F)
mg/m3 not considered to be treatment related; no (LOAEL)
other embryotoxic, fetotoxic or teratogenic effects
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,4-DCB New Zealand white 0, 590, 1770, or slight maternal toxicity (decreased body weight 1770 mg/m3 (F)
(99.9%) rabbit (pregnant), 4720 mg/m3, 6 h/day on days gain) at 4720 mg/m3; significant increase in (NOEL)
Hayes 29-30/group 6-18 of gestation retroesophageal right subclavian artery at 4720 4720 mg/m3 (F)
et al. mg/m3, not considered to be a teratogenic response *4720 mg/m3
(1985) (O) (LOAEL)
1,4-DCB SPF rat (pregnant), 0, 450, 1200, or no evidence of maternal toxicity, embryo-, or
Loeser & 20/group 3000 mg/m3 (0, 75, 200 or 500 fetotoxicity or teratogenicity
Litchfield ppm), 6 h/day on days 6-15
(1983) of gestation
Developmental Studies:
Ingestion
1,4-DCB CD rat (pregnant), 0, 250, 500, 750, or maternal toxicity (decreased body weight gain and 250 mg/kg per
Giavini 11-16/group 1000 mg/kg per day on days food consumption) at >500 mg/kg; reduction in fetal day (F) (NOEL)
et al. 6-15 of gestation; gavage in weight at 1000 mg/kg per day; fetotoxic effects 500 mg/kg per
(1986) corn oil included increase in fetal skeletal variations at day (F)
>750 mg/kg, dose-related increase in the frequency *500 mg/kg per
of extra ribs at >500 mg/kg day (O)
(LOAEL)
1,2,3-TCB Sprague-Dawley rat 1,2,4-TCB: 0, 75, 150, or maternal toxicity - reduced body weight gain at 600 1,2,3-TCB:
1,2,4-TCB (pregnant); 14/group 300 mg/kg per day; 1,2,3-TCB mg/kg (1,3,5-TCB), increased liver weight at 600 300 mg/kg per
1,3,5-TCB and 1,3,5-TCB: 0, 150, 300 or mg/kg (1,2,3- and 1,3,5-TCB) and 300 mg/kg day (F);
(99.5%) 600 mg/kg per day on days (1,2,4-TCB), decreased haemoglobin levels and *600 mg/kg per
Ruddick 6-15 of gestation; gavage in haematocrit, generally at highest dose (all 3 day (O) (NOEL)
et al. corn oil isomers), decreased RBC at 300 mg/kg (1,2,3-TCB),
(1983); 150 and 300 mg/kg (1,2,4-TCB), and 150 and 1,2,4-TCB:
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Black 600 mg/kg (1,3,5-TCB), mild treatment-related 75 mg/kg per
et al. histopathological changes in spleen, liver, and day (F);
(1988) thyroid, generally at highest dose (all three *300 mg/kg per
isomers); mild histological changes in the lenses of day (O)
pups at all doses (1,3,5-TCB) and 75 mg/kg (NOEL);
(1,2,4-TCB); no significant accumulation in maternal 150 mg/kg per
or fetal tissue day (F)
(LOAEL)
1,3,5-TCB:
300 mg/kg per
day (F)
(NOEL);
150 mg/kg per
day (O)
(LOEL);
600 mg/kg per
day (F)
(LOAEL)
1,2,4-TCB Sprague-Dawley CD 0, 36, 120, 360, or maternal toxicity - all animals died at 120 mg/kg per
(>99%) rat (pregnant); 1200 mg/kg per day on days 1200 mg/kg, some lethality and reduced body weight day (F)
Kitchin >6/group 9-13 of gestation; gavage in gain at 360 mg/kg, moderate hepato- cellular (NOEL);
& Ebron corn oil hypertrophy at 360 mg/kg, and moderate to severe 360 mg/kg per
(1983a) multifocal hepatic necrosis at 1200 mg/kg; day (F);
embryonic growth retardation at 360 mg/kg *360 mg/kg per
day (O)
(LOAEL)
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1,2,3,4-TeCB Sprague-Dawley 0, 50, 100, or 200 mg/kg per maternal toxicity - death of 9/10 animals at 1,2,3,4-TeCB:
1,2,3,5-TeCB rat (pregnant); day on days 6-15 of 200 mg/kg and increased serum cholesterol at >50 200 mg/kg per
1,2,4,5-TeCB 10/group gestation; gavage in corn oil mg/kg (1,2,4,5-TeCB); greater toxicity of day (F)
(99.5%) 1,2,4,5-TeCB correlated with its accumulation in (NOEL);
Kacew tissue; decrease in number of live fetuses at 200 mg/kg per
et al. 200 mg/kg (1,2,3,4-TeCB, 1,2,3,5-TeCB); decrease in day (O)
(1984) number of live fetuses at 50 mg/kg (1,2,4,5-TeCB), (LOAEL)
probably not treatment-related
1,2,3,5-TeCB:
200 mg/kg per
day (F)
(NOEL);
200 mg/kg per
day (O)
(LOAEL)
1,2,4,5-TeCB:
*50 mg/kg per
day (O)
(LOEL);
50 mg/kg per
day (F)
(LOAEL)
1,2,4,5-TeCB Sprague-Dawley CD 0, 30, 100, 300, or maternal toxicity - decrease in body weight gain 300 mg/kg per
(>98%) rat (pregnant) 1000 mg/kg per day on days and slight centrilobular hypertrophy (3/9) at day (F);
Kitchin & 9-13 of gestation; gavage 1000 mg/kg; no embryo- or fetotoxic effects *1000 mg/kg
Ebron in 1.5% gum tragacanth or evidence of teratogenicity per day (O)
(1983c) (NOEL);
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
1000 mg/kg per
day (F)
(LOAEL)
1,2,3,4-TeCB Sprague-Dawley CD 0, 100, 300, or 1000 mg/kg maternal toxicity - death of 7/19 animals, decrease 100 mg/kg per
(>98%) rat (pregnant) per day on days 9-13 of in body weight gain and liver weight, and minimal day (F)
Kitchin & gestation; gavage in 1.5% gum to moderate hepatocellular hypertrophy (2/9) at (NOEL);
Ebron tragacanth 1000 mg/kg, death of 1/10 and minimal 300 mg/kg per
(1983b) hepatocellular hypertrophy (2/9) at 300 mg/kg; day (F);
decrease in yolk sac diameter, embryonic crown-rump *300 mg/kg per
length and head length, at 300 mg/kg (not examined day (O)
at 1000 mg/kg because of maternal lethality) (LOAEL)
PeCB Wistar rat 0, 50, 100, or 200 mg/kg per maternal toxicity - non-significant reduction in 200 mg/kg per
Villeneuve (pregnant) 20/group day on days 6-15 of body weight gain at 200 mg/kg; increased incidence day (F)
& Khera gestation; gavage in corn oil of extra ribs at >50 mg/kg, and sternal defects and (NOAEL);
(1975) decreased fetal weight at 200 mg/kg 50 mg/kg per
day (O) (LOEL)
PeCB CD-1 mouse 0, 50, or 100 mg/kg per day on maternal toxicity - increase in liver weight (both *100 mg/kg per
(>97%) (pregnant); 9-10 days 6-15 of gestation; oral - doses); no embryotoxic, fetotoxic, or teratogenic day (O)
Courtney litters/group (6 gastric intubation in corn oil effects (NOEL);
et al. litters - control 50 mg/kg per
(1977) group) day (F)
(LOAEL)
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
Reproductive Studies:
Inhalation
MCB Sprague-Dawley CD 0, 50, 150 or 450 ppm for 10 hepatocellular hypertrophy and renal changes in F0
Nair rat; 30 of each weeks prior to mating through and F1 males (>150 ppm); incidence of bilateral
et al. sex/group weaning of the F2 generation degeneration of the testicular germinal epithelium
(1987) increased in F0 adults at 450 ppm but relation to
MCB unclear since not observed in F1.
Reproductive Studies:
Ingestion
1,2,4-TCB Charles River rat 0, 25, 100, or 400 mg/kg no effects on fertility, growth, viability, approx.
Robinson (pregnant); 17-23 (during pregnancy through to locomotor activity or blood chemical analyses; 50 mg/kg per
et al. litters/group weaning of F2 generation). For decrease in water intake of some F0 groups at day (F)
(1981) F0, doses ranged from 8.3-134 400 mg/kg; enlarged adrenals in F0 and F1 at 95 (NOEL);
mg/kg at day 29 and 2.5-54 days at 400 mg/kg approx.
mg/kg at day 83 (mating at day 50 mg/kg per
90); mixed with Tween 20 in day (O)
drinking-water (LOAEL)
PeCB Sherman rat; 10 females: 7-150 mg/kg per day maternal toxicity - increased liver weight and approx.
(99.1%) of each sex/group (0, 125, 250, 500, or 1000 hypertrophy of hepatic cells at >500 mg/kg, 17-31 mg/kg
Linder ppm), 180 days (from 4-5 decreased haemoglobin levels at 1000 mg/kg and per day (F)
et al. weeks of age to mating, dose-dependent accumulation in adipose tissue; (NOEL);
(1980) through gestation and pre-weaning growth rates decreased at higher approx.
Table 24 (continued)
Compound; Speciesa Doseb,c Resultsd Effect Levelse
Reference
lactation) males: 6-140 concentrations, tremors in suckling pups at 17-31 mg/kg
mg/kg per day (0, 125, or >250 mg/kg and significant mortality in pups at per day (O);
1000 ppm), 100 days (through 1000 mg/kg approx.
mating); diet 27-64 mg/kg
per day (F)
(LOAEL)
a and number of animals/group specified, where available.
b Vehicle specified, where available.
c Doses given as mg/kg body weight, unless specified otherwise.
d RBC - red blood cells.
F - females.
O - offspring.
e NOEL - no-observed-effect level.
NOAEL - no-observed-adverse-effect level.
LOEL - lowest-observed-effect level.
* - maternally toxic dose.
However, the decrease in the number of live fetuses was not observed
at a higher concentration of 1,2,4,5-TeCB (100 mg/kg body weight)
and, therefore, may not have been treatment-related. The only toxic
effect observed in the mothers or fetuses, when pregnant rats
ingested either the 1,2,3,4- or the 1,2,3,5-isomer, was a decrease
in the number of live fetuses at the highest dose (200 mg/kg body
weight).
In contrast, Kitchin & Ebron (1983b) reported that the
1,2,3,4-isomer of TeCB was more toxic for both mothers and fetuses
than the 1,2,4,5-isomer (Kitchin & Ebron, 1983c). The LOAEL in
mothers administered the 1,2,3,4-isomer by gavage, was 300 mg/kg
body weight; the LOAEL for the 1,2,4,5-isomer was 1000 mg/kg
(compared with 50 mg/kg in the study of Kacew et al., 1984).
Embryonic growth retardation was observed in offspring administered
the maternally toxic dose (300 mg/kg body weight) of the
1,2,3,4-isomer, only.
For pentachlorobenzene, Villeneuve & Khera (1975) reported an
increased incidence of extra ribs in the fetuses of pregnant rats
administered oral doses as low as 50 mg/kg body weight, and an
increased incidence of sternal defects and decreased fetal weight
following ingestion of 200 mg/kg; these doses did not induce
significant toxic effects in the mothers. The results contrast with
those of Courtney et al. (1977) in which increases in liver weights
were observed in pregnant mice administered 50 or 100 mg PeCB/kg,
but no embryotoxic, fetotoxic, or teratogenic effects.
The possible reproductive effects of the chlorobenzenes have not
been well studied; only 3 relevant studies have been conducted.
There were no treatment-related adverse reproductive effects in a 2-
generation study in which rats were exposed via inhalation to MCB at
227.5, 682.5, or 2047.5 mg/m3 (50, 150, or 450 ppm), though there
was evidence of hepatotoxicity and nephrotoxicity in male rats in
the F0 and F1 generations (Nair et al., 1987). Robinson et al.
(1981) did not find any significant effects on fertility, growth,
viability, locomotor activity, or the chemical composition of the
blood in rats administered 1,2,4-TCB, at levels of up to
approximately 54 mg/kg, during pregnancy and through to weaning of
the F2 generation. However, enlarged adrenal glands were observed
in the F0 and F1 generations at 95 days in the highest dose
group.
When rats were administered PeCB in the diet from 4 to 5 weeks of
age, and during gestation and lactation, Linder et al. (1980) noted
maternal toxicity (LOAEL approx. 27-64 mg/kg per day), tremors in
suckling pups (LOAEL approx. 17-31 mg/kg per day), and, at higher
doses, decreases in pre-weaning growth rates, and mortality of pups.
9. EFFECTS ON HUMANS
9.1 Case Reports
9.1.1 General population exposure
Data on the health effects of exposure of the general population to
chlorobenzenes are restricted to case reports mainly concerning
accidental exposure to, or misuse of, products containing the lower
chlorinated benzenes (MCB, 1,2- or 1,4-DCB, and an unspecified
isomer of TCB). Many of these reports are from the early literature
with no indication of the purity of the chlorobenzene or the actual
dose/time relationship.
Reversible acute effects on the central nervous system (loss of
reflexes, cyanosis leading to unconsciousness, with head and neck
twitching) were observed in a 2-year-old boy following the ingestion
of 5-10 ml of MCB (Reich, 1934). An adult female showed nausea,
shortness of breath, sleepiness, and haematuria, 1 week after an
acute exposure to DCB used as a termiticide (Nill, 1936). Two cases
of acute myeloblastic leukaemia were reported in females who had
been repeatedly exposed (over 1 year) to DCBs (primarily 1,2-DCB)
during its use as a cleaning solution (Girard et al., 1969). There
have been numerous case reports of adverse effects of 1,4-DCB,
because of its availability to the public as a moth repellent and
air freshener. Reported effects of acute or short-term exposure (all
of which were reversible) include acute haemolytic anaemia
(Hallowell, 1959; Campbell & Davidson, 1970), respiratory irritation
(rhinitis) (Cotter, 1953), and allergic purpura of the skin and
glomerulonephritis (Nalbandian & Pearce, 1965). Effects on the lungs
(pulmonary granulomatosis), (Weller & Crellin, 1953), blood
(anaemia), central nervous system effects including dizziness,
numbness, and incoordination, "mental sluggishness", tremor, and
polyneuritis (Frank & Cohen, 1961), and liver damage, including
yellow atrophy and jaundice (Berliner, 1939; Cotter, 1953) have been
recorded in case reports of persons exposed to 1,4-DCB for prolonged
periods (several months to 15 years) . Limb and truncal ataxia,
dysarthria, hyporeflexia, hypotonus, and mild proximal limb weakness
were reported in a 25-year-old woman exposed to 1,4-DCB during a
period of 6 years, under very unusual circumstances, i.e., through
extensive use of mothballs in her bedclothes and wardrobe. She
developed difficulty in speech and gait. Exposure was not
quantified, but was probably high, according to the observed effects
and reported use (Miyai et al., 1988).
There has also been a case report of aplastic anaemia in a
68-year-old woman with long-term exposure through the soaking of her
husband's work clothes in TCB (isomer not identified) (Girard
et al., 1969).
9.1.2 Occupational exposure
Most of the available data on the adverse effects of occupational
exposure to chlorobenzenes come from case reports. Many of these
cases are poorly described with regard to chemical purity, dose/time
relationships, and possible confounding factors, and thus provide
very little information relevant to the assessment of human health
risks.
Case reports concerning MCB in occupationally exposed populations
have been restricted to symptoms of effects on the central nervous
system (headaches, numbness, and lethargy) and irritation of the
eyes and upper respiratory tract (Girard et al., 1969) after
exposures for periods of up to 6 years, in the presence of several
other chemicals.
In workers exposed to 1,2-DCB, or mixtures of chlorobenzenes
containing mainly 1,2-DCB, there have been case reports of:
haematological disorders, including anaemia in 1 male worker filling
barrels for 8 years (Girard et al., 1969; US EPA, 1985); chronic
lymphoid leukaemia (2 cases) after inhalation exposures of 10 and 16
years, respectively (Girard et al., 1969); and myelocytic/
myeloblastic leukaemia (1 case) after 22 years in a dyestuff plant
(Tolot et al., 1969).
In workers exposed to 1,4-DCB, probably often in combination with
several other chemicals, there have been case reports of
haemato-logical disorders, including anaemia (Petit & Champeix,
1948; Cotter, 1953; Harden & Baetjer, 1978); splenomegaly (2 cases)
(Sumers et al., 1952; Cotter, 1953); effects on the gastrointestinal
tract (Sumers et al., 1952; Cotter, 1953; Wallgren, 1953); drying or
hardening of the skin (Cotter, 1953; Harden & Baetjer, 1978); and
symptoms of effects on the CNS, including finger tremors, stronger
muscle reflexes, and ankle contractions (Wallgren, 1953).
Only two case reports have been identified concerning
trichloro-benzene. Ehrlicher (1968) reported massive haemoptysis in
an adult male who had inhaled TCB vapours for several hours, during
the repair of a pump, and Popovki et al. (1980) reported 7 cases of
chloracne in 15 TCB production workers, exposed for 2-6 months.
However, the isomer was not specified in either report, and no
details of confounding factors and doses received were available for
evaluation.
9.2 Epidemiological Studies
Only a few epidemiological studies have been carried out on workers
exposed to chlorobenzenes other than hexachlorobenzene. Many of
these studies have been carried out on groups of workers
simultaneously exposed to other chemicals with known adverse effects
on man (e.g., benzene, aromatic and/or chlorinated solvents).
Furthermore, most of the studies have not been fully described with
regard to methods and confounding factors, making them inadequate
for use in risk assessments.
In an early cross-sectional study, 3 groups of workers were examined
for the general adverse effects of solvent vapours during the
production of perchlorovinyl lacquer. Two health examinations were
carried out in the course of 1 year. Group 1 included 28 workers (25
of whom had been employed on the job for 1 year or more), reported
to have been exposed only to chlorobenzene at levels ranging between
0.034 and 1.44 mg/litre (Rozenbaum et al., 1947). Group 2 consisted
of 12 workers in the central chemical laboratory working on
long-term experiments with chlorobenzene. Group 3 included 14 female
workers in the lacquer plant, who were exposed to benzene (0.02-0.4
mg/litre) as well as to chlorobenzene (0.06-0.6 mg/litre). There was
no unexposed control group. No significant adverse effects were
reported for workers in Groups 2 and 3; however, workers in Group 1
reported headaches, dizziness, drowsiness, and dyspeptic disorders.
Numerical data concerning the prevalence of these disorders were not
given. No effects on "internal organs" that could be attributed to
chlorobenzene exposure were noted; however, the first
neuropathological examination indicated numbness and stiffness in
the extremities of 8 workers, convulsive muscle contractions in the
fingers of 9, and hypoaesthesia of the hand in 4. The deviations
noted were reported to have returned to normal by the second
examination.
Workers producing enamel-insulated wire are exposed to both MCB and
tricresol. An examination of 311 female workers for the possible
effects of these chemicals on gynaecological and obstetrical
functions was reported by Syrovadko & Malysheva (1977). Levels of
exposure to MCB varied from 11 to 429 mg/m3 (mean, 72.3 mg/m3)
and those to tricresol from 0.8 to 18.7 mg/m3 (mean, 4.3 mg/m3).
Similar health parameters were examined in some 15 000 women in
other occupations and the general population in the region. The
frequency of various gynaecological dysfunctions was 2.6-9.4 times
higher in the exposed workers than in women outside the plant, but
was similar to that in the controls from within the enamel-wire
plant. Lost-time was 2.5 times greater in the enamellers than in
in-plant controls. The authors reported marked hormonal
disturbances, but did not quantify these results. An analysis of 190
births in the enameller group, and 152 from the in-plant controls,
revealed an increase in birth anomalies (10 versus 3.9%) in the
exposed group. Congenital heart defects (26.3%) were the most
prevalent. Neonatal development appeared to be compromised in
infants of exposed mothers, as evidenced by patterns of
breast-feeding and body weight gain. These results are difficult to
interpret, however, with respect to MCB, owing to concomitant
exposure to another chemical and a lack of quantitative data.
MCB was considered to be the causative agent in 26 out of 1951 cases
of work-related dermatosis observed at the "Shanghai Institute
for Occupational Disease Prevention in the Chemical Industry"
between 1970 and 1982; reported manifestations were eczematous
dermatitis, pigmentation, and neurodermatitis (Zong & Ma, 1985).
In periodic medical examinations of 58 workers exposed to various
concentrations of 1,4-DCB, there was no evidence of "organic injury
or untoward haematological effects". It was concluded, however,
that, at levels ranging between 300 and 480 mg/m3 (50 and 80 ppm),
1,4-DCB was irritating to the eyes and nose, the irritation becoming
severe at a level of 960 mg/m3 (160 ppm). Details of the study
design in this early investigation were incomplete (Hollingsworth et
al., 1956).
In the only identified cross-sectional epidemiological study of
workers exposed to 1,2-DCB, there was no evidence of "organic injury
or untoward haematological effects" in an unspecified number of
workers exposed to mean levels of 90 mg/m3 (15 ppm) 1,2-DCB
(Hollingsworth et al., 1958). However, details of the study design
were not presented in the account of this early investigation.
As described in section 8, there was an increase in the total number
of chromosomal aberrations (primarily single and double breaks) in
the peripheral leukocytes of 26 laboratory workers exposed for 4
days, 8 h/day, to 1,2-DCB vapour compared with a control group of 11
unexposed laboratory personnel (Zapata-Gayon et al., 1982). Of the
white cells analysed (1345, exposed; 942, control), 8.92% of the
exposed cells contained aberrations compared with 2.02% in the
control group. In 15 exposed subjects examined at 6 months, only
double breaks were significantly increased. Although mention was
made of a strong odour of DCB in the room and all subjects suffered
mucosal irritation, no determination of exposure levels was carried
out.
Only one study on workers exposed to TeCB was found. The peripheral
lymphocytes of workers producing 1,2,4,5-TeCB in a
pesticide-manufacturing complex were examined for chromosomal
aberrations. Exposure levels were not reported; however, workers (24
male and 1 female) were considered to be exposed to only TeCB and
they were compared with 14 other workers, minimally exposed to
chemicals, as well as a group from the local community. An increase
(significance unspecified) in the total number of chromatid-type,
labile (acentric fragment and dicentric chromosome), and stable
(deletion, inversion, and translocation) chromosomal aberrations was
reported. No follow-up studies of these findings have been reported.
No case reports or epidemiological studies on workers exposed to
PeCB were found.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure of the general population
Exposure to chlorobenzenes other than hexachlorobenzene may occur
via ingestion, inhalation, or dermal contact (section 5.2.1). The
general population is thought to be exposed to the lower chlorinated
congeners mainly through inhalation, whereas a greater proportion of
the total daily intake of the higher chlorinated compounds is
ingested in food. Dermal exposure may occur if consumers use
chlorobenzene-based products without appropriate care and
protection.
Exposure of the general population through the ingestion of
drinking-water and food and via inhalation has been estimated (Table
12). However, these estimates are based on few data from a limited
number of countries and, therefore, must be considered tentative
until further monitoring data become available.
On the basis of the limited data available, the total daily intakes
of MCB and the various DCB isomers are estimated to be less than 1
µg/kg body weight. The intakes of the higher chlorinated benzenes
are lower, probably, less than 0.05 µg/kg body weight per day.
Dichlorobenzenes, TCBs, TeCBs, and PeCB have been detected in human
milk, the DCBs (particularly the 1,4-isomer) being present in the
highest concentrations. On the basis of the limited data available
from a small number of countries, it is estimated that, on a body
weight basis, the intake of chlorobenzenes in breast-fed infants is
greater than that in the adult population,.
10.1.2 Occupational exposure
Very few data are available to serve as a basis for the estimation
of occupational exposure. Air levels of MCB of up to 18.7 mg/m3
have been detected in chemical plants, whereas levels of 1,4-DCB in
one manufacturing plant averaged 204 mg/m3 (42-288 mg/m3). On
the basis of established exposure limits, workers may be exposed
daily to maximum concentrations of 350 mg/m3 for MCB, 450 mg/m3
for 1,4-DCB, and 300 mg/m3 for 1,2-DCB.
10.1.3 Toxic effects
Acute lethal doses of the lower chlorinated benzenes (MCB and DCBs)
in experimental animals cause narcosis and CNS depression, damage to
the liver and kidneys, as well as respiratory paralysis. Inhalation
LC50s for MCB have been reported to range from approximately
9000 mg/m3 in mice to 13 000 mg/m3 in rats. Inhalation LC50
values for 1,2-DCB are similar. With few exceptions, oral LD50
values in the species examined to date have been >1000 mg/kg body
weight for all congeners, and, according to the limited data
available, dermal LD50s are higher.
In general, effects following short-term and long-term exposures to
chlorobenzenes are similar to those following acute exposure and
include damage to the liver and kidneys, and, at higher doses,
effects on the thyroid gland in rats and on the bone marrow in mice.
Of the few chlorobenzenes tested, only 1,2,4-TCB has been shown to
cause dermatitis after prolonged contact with skin.
In a bioassay for the carcinogenicity of MCB, there was an increased
incidence of hepatic neoplastic nodules in the high-dose group
(120 mg/kg body weight) of male F344 rats, but no treatment-related
increases in tumour incidence in female F344 rats or male and female
B6C3F1 mice. There was no evidence for the carcino-genicity of
1,2-DCB in male or female F344 rats or B6C3F1 mice (60 or
120 mg/kg body weight).
In a bioassay for the carcinogenicity of 1,4-DCB, there was a
dose-related increase in renal tubular cell adenocarcinomas in male
F344 rats and an increase in hepatocellular adenomas and carcinomas
in both sexes of B6C3F1 mice. No evidence of carcinogenicity was
reported in male and female Wistar rats or female Swiss mice
following inhalation of slightly higher doses of 1,4-DCB (estimated
to be 400 mg/kg per day for rats and 790 mg/kg per day for mice) for
shorter periods of time. Available data indicate, however, that
renal tumours in male F344 rats and the associated severe
nephropathy and hyaline droplet formation, induced by 1,4-DCB, are
species- and sex-specific responses associated with the
re-absorption of alpha-2-microglobulin.
Available data are inadequate to assess the carcinogenicity of the
higher chlorinated benzenes (tri- to penta-).
Although available data from in vitro and in vivo assays of
isomers other than 1,4-DCB are limited, chlorobenzenes do not appear
to be mutagenic. On the basis of a more extensive database for
1,4-DCB, it can be concluded that this compound has no mutagenic
potential, either in vivo or in vitro.
In studies conducted to date, there has been no evidence that
chlorobenzenes are teratogenic in animal species. Exposure of rats
or rabbits to MCB or DCBs, via inhalation at levels exceeding
2400 mg/m3, resulted in minor embryotoxic and fetotoxic effects.
However, such doses were clearly toxic for the mother. Although
there is some evidence that TCBs, TeCBs, and PeCB are embryo-toxic
and fetotoxic at doses that are not toxic for the mother, available
data are inconsistent.
The lowest reported NOELs or LOAELs for each of the chlorobenzene
isomers in long-term exposure studies and studies on chronic
toxicity, teratogenicity, and developmental and reproductive
toxicity, of acceptable design, are summarized in Table 25.
Toxic effects in humans following acute exposures to MCB, 1,2-DCB,
and 1,4-DCB include CNS depression, haematuria, haemolytic anaemia,
rhinitis, tremor, ataxia, polyneuritis, and jaundice. No reports
have been published on the possible health effects that might result
from long-term exposures to low levels of chlorobenzenes in the
general environment. Effects attributed to occupational exposures to
chlorobenzenes have been confounded by exposures to several
chemicals and the limitations of the epidemiological studies
conducted to date; however, they include CNS depression, dermatitis,
and eye and nose irritation. No dose-response data are available
from these epidemiological studies.
10.1.4 Risk evaluation
The following is an example of a risk assessment procedure for
setting Tolerable Daily Intakes (TDIs) for chlorobenzenes other than
hexachlorobenzene. This is only an illustration and may need to be
adjusted to take into account local considerations and any
additional scientific data that are reported.
10.1.4.1 General population
The general population appears to be exposed to low levels of
chlorinated benzenes (see Table 12, section 5). Exposure to the
major chlorobenzenes other than hexachlorobenzene, for non-
occupationally-exposed populations, is estimated to be less than 2
µg/kg body weight per day. Over 90% of the total exposure is to MCB
and DCBs, principally from air.
For comparison with these estimated exposures, TDIs can be
calculated from the no-observed-effect levels (NOELs) from
long-term, chronic teratogenicity, and developmental reproductive
toxicity studies on experimental animals. TDIs, based on the lowest
NOELs reported in studies of acceptable design, using uncertainty
factors established on the basis of the principles outlined by WHO
(1987), are listed in Table 26.
Table 25. Summary of lowest reported NOELs (or LOAELs) for the
inhalation and ingestion of chlorobenzenes other than
hexachlorobenzene
Inhalation
Compound Reported NOEL Species Exposure
(mg/m3) period
MCB 341 (LOAEL) rat, rabbit 24 weeks
1,4-DCB 450 rat; mousea 76 weeks
1,3,5-TCB 100 rat 13 weeks
1,2,4-TCB 22.3 rat 13 weeks
Ingestion (dietary incorporation or gavage)
Compound Reported NOEL Species Exposure
(mg/kg body weight period
per day)
MCB 60 rat 103 weeks
1,2-DCB 60 mouse 103 weeks
1,4-DCB 150 (LOAEL) rat 103 weeks
1,2,4-TCB 7.8 rat 13 weeks
1,2,3-TCB 7.7 rat 13 weeks
1,3,5-TCB 7.6 rat 13 weeks
1,2,4,5-TeCB 0.034 rat 13 weeks
1,2,3,4-TeCB 3.4 rat 13 weeks
1,2,3,5-TeCB 0.42 rat 13 weeks
PeCB 3.3 rat 13 weeks
a = In this bioassay, only female mice were included.
For most chlorobenzenes, the TDIs are based on non-neoplastic
effects. Neoplastic effects were taken into consideration in the
derivation of the uncertainty factors for MCB and 1,4-DCB. However,
available data indicate that the observed increase in renal tumours
in rats caused by 1,4-DCB is a species- and sex-specific response
that is unlikely to be relevant to humans. It should be noted,
however, that on the basis of evidence of increased DNA replication
in the mouse liver, and the increased incidence of hepatocellular
adenomas and carcinomas in mice in a bioassay for the
carcinogenicity of 1,4-DCB, this compound may act as a non-genotoxic
carcinogen in the rodent liver. On the basis of the increased
incidence of hepatic neoplastic nodules observed in the high-dose
group of male rats in a bioassay for the carcinogenicity of MCB,
this may also be true for this compound.
As stated above, with the exception of individuals who use
chlorobenzene-based products without appropriate care and
protection, non-occupationally exposed humans are exposed to levels
of chlorobenzenes well below the derived TDIs, indicating that the
anticipated health hazards for the general population from exposure
to chlorobenzenes other than hexachlorobenzene are minimal. However,
the odour thresholds of chlorobenzenes range between 0.1 and 3
µg/litre in drinking-water, thus, necessitating continuous pollution
control measures to ensure aesthetically acceptable water supplies.
Table 26. Calculated Tolerable Daily Intakes for chlorobenzenes
other than hexachlorobenzene
Inhalation
Compound Reported NOEL Uncertainty Estimated
(mg/m3) factor TDIb
MCB 341 (LOAEL) 1000 0.5
1,4-DCB 450 500 1
1,3,5-TCB 100 500 0.2
1,2,4-TCB 22.3 500 0.05
Ingestion
Compound Reported NOEL Uncertainty Estimated
(mg/kg body weight factor TDIa
per day
MCB 60 500 0.1
1,2-DCB 60 100 0.60
1,4-DCB 150 (LOAEL) 1000 0.1
1,2,4-TCB 7.8 500 0.02
1,2,3-TCB 7.7 500 0.02
1,3,5-TCB 7.6 500 0.02
1,2,4,5-TeCB 0.034 500 0.0001
1,2,3,4-TeCB 3.4 500 0.01
1,2,3,5-TeCB 0.42 500 0.001
PeCB 3.3 500 0.01
a = Values were rounded up according to the judgement of the
panel.
10.1.4.2 Occupationally exposed population
Workers can be exposed to levels as high as 2.7 mg/kg body weight
for MCB and 29.1 mg/kg body weight for 1,4-DCB. These estimates may
not be representative, however, since they are based on only two
reports.
If good industrial hygiene practices are followed, the risks
associated with occupational exposure to chlorobenzenes are
considered to be minimal.
10.2 Evaluation of Effects on the Environment
10.2.1 Levels of exposure
Chlorobenzene isomers have been found in air, surface water, ground
water, drinking-water, municipal waste water, rain water, soils, and
sediments. Most monitoring has been limited to levels in air and the
aquatic environment.
In surface waters, levels of total chlorobenzenes are in the
ng-µg/litre range; however, values as high as 0.1 mg/litre have been
reported near industrial sources. Examples of levels in waste water
average about 700 µg/litre for MCB and less than 170 µg/litre for
the other congeners.
10.2.2 Fate
Chlorobenzenes released into the aquatic environment will become
distributed preferentially to the air and to sediments (particularly
organically rich sediments). Levels 1000 times those found in water
have been detected in sediments, particularly those in highly
industrialized regions, though available data are limited. The
retention of chlorobenzenes in soil increases with the organic
content of the soil; the degree of chlorination is positively
correlated with adsorption.
10.2.3 Bioavailability and bioaccumulation
The bioavailability of chlorobenzenes is inversely correlated with
the organic matter content of soil and sediment. Accumulation
studies are limited, but organisms have been shown to accumulate
chlorobenzenes from water, soil, and aquatic sediment. Adsorption on
organic sediment increases with increasing level of chlorination,
which may reduce bioavailability; however, uptake into, and
retention by, organisms also increases with increasing level of
chlorination.
10.2.4 Degradation
Chlorobenzenes are eliminated from the environment by both abiotic
and biotic degradation, the latter appearing the more important.
Photolysis and hydrolytic reactions are possible processes, but
aerobic degradation constitutes the major route of breakdown for the
removal of chlorobenzene residues. Many microorganisms are capable
of degrading chlorobenzenes (section 4.2.3, Table 6); however, the
more highly chlorinated congeners are degraded microbiologically at
a slower rate than the less chlorinated benzenes. This degradation
does not appear to occur anaerobically.
10.2.5 Persistence
In water, chlorobenzenes are considered moderately persistent with
half-times of approximately 1 day in rivers, 10 days in lakes, and
more than 100 days in ground water. They are much more persistent
under the anaerobic conditions usually found in sediment and ground
water.
10.2.6 Toxic effects on organisms
The toxicities of chlorobenzenes for microorganisms, invertebrates,
and fish are comparable; with a few exceptions, the EC50 and
LC50 values fall within the several mg/litre range (Tables 16, 17,
and 18). In general, toxicity increases with increased chlorination
of the benzene ring. For example, in the bluegill sunfish
(L. macrochirus), the following 96-h LC50 values were reported:
MCB (>16 mg/litre); 1,2,4-TCB (3.4 mg/litre); 1,2,4,5-TeCB (1.6
mg/litre) and PeCB (0.3 mg/litre).
No data on the effects of chlorobenzenes on terrestrial biota were
found; however, data from studies on laboratory mammals suggest a
low risk for terrestrial mammals (see section 10.1.3).
10.2.7 Risk evaluation
There are insufficient data on (a) the quantities of
chlorobenzenes entering the environment and their subsequent
dynamics, and (b) toxicity studies on many chlorobenzene isomers,
carried out under realistic environmental conditions. It is,
therefore, impossible to predict quantitatively the impact on the
environment of widespread low-level contamination by chlorobenzenes
other than hexachlorobenzene.
In virtually all instances within the aquatic ecosystem, the levels
at which acute effects occur in experimental studies are many times
higher than the environmental levels monitored at present (mg/litre
range versus µg/litre range). Organisms will be exposed only
transiently to chlorobenzenes in surface water (because of
volatility and adsorption on sediment) and will be mainly exposed to
chlorobenzenes in the interstitial water of sediments. Few studies
on organisms living in sediments have been conducted, despite
evidence for bioavailability. Similarly, there are no data on the
transfer of chlorobenzenes in the food chain.
Only in the case of accidental spills or uncontrolled industrial
discharges would ambient concentrations approach toxic levels.
However, the continued discharge of chlorobenzenes into the aquatic
environment should be avoided, to prevent the build up of persistent
residues in sediment and/or ground water.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
If good industrial practices are followed, the risks associated with
occupational exposure to chlorobenzenes are considered to be
minimal. The present risk assessment also indicates that current
concentrations of chlorobenzenes in the environment pose a minimal
risk for the general population, except in the case of the misuse of
chlorobenzene-based products or uncontrolled discharge to the
environment. However, this assessment is based on limited monitoring
data; additional information is needed to substantiate this
conclusion. The reduction of the widespread use and disposal of
chlorobenzenes should, however, be considered because:
* Chlorobenzenes may act as precursors for the formation of
PCDDs/PCDFs, e.g., in incineration processes;
* These chemicals can lead to taste and odour problems in
drinking-water and fish;
* Residues persist in organically-rich anaerobic sediments and
soils, and ground water.
For most chlorobenzenes, the assessment of risk was based on
non-neoplastic effects. However, neoplastic effects were taken into
consideration in the risk assessments for MCB and 1,4-DCB. Available
data indicate that the observed increase in renal tumours in rats
caused by 1,4-DCB is a species- and sex-specific response that is
unlikely to be relevant to humans. On the basis of evidence of
increased DNA replication in the mouse liver and the increased
incidence of hepatocellular adenomas and carcinomas in mice, 1,4-DCB
may act as a non-genotoxic carcinogen in the rodent liver. MCB may
also be a non-genotoxic carcinogen, in view of the increased
incidence of hepatic neoplastic nodules observed in the high-dose
group of male rats in a bioassay for carcinogenicity.
11.2. Recommendations
11.2.1 Public health measures
The present risk assessment indicates that current concentrations of
chlorobenzenes in the environment pose a minimal risk for humans.
However, this assessment is based on limited monitoring data and
additional information is needed to substantiate this conclusion.
The reduction of the widespread use and disposal of chlorobenzenes
should, however, be considered because:
* Chlorobenzenes may act as precursors for the formation of
PCDDs/PCDFs, e.g., in incineration processes;
* These chemicals can lead to taste and odour problems in
drinking-water and fish;
* Residues persist in organically-rich anaerobic sediments and
soils, and ground water.
More information is needed on the fate of chlorinated benzenes at
high temperatures, in order to better assess the hazards associated
with their disposal by incineration.
11.2.2 Human health risk evaluation
In order to improve the database available for the assessment of the
risks of chlorobenzenes for human health, it is recommended that:
1. The IPCS should coordinate the collection of industrial
production data for chlorobenzenes.
2. Additional monitoring data should be collected so that a more
accurate assessment of human exposure can be made. In
particular, additional information is desirable on the
concentrations in different geographical areas, especially with
regard to the levels in food of the highly chlorinated
congeners.
3. There is a lack of chronic toxicity data on the higher
chlorinated benzenes. On the basis of subchronic toxicity data,
it appears that 1,2,4,5-TeCB is the most toxic congener and
should be examined in long-term bioassays or studied with
regard to possible species-specific mechanisms.
4. Since ambient air is believed to be the major source of
exposure to the chlorinated benzenes for humans, more
information is required on the photoreactivity and
photodegradation of these compounds.
There is a lack of experimental data on the interaction between
chlorobenzenes and other chemicals. As a general recommendation,
research on the toxicity of chemical mixtures, and the consideration
of toxicological interaction in the risk assessment process is
encouraged.
11.2.3 Environmental risk evaluation
Persistent residues of chlorobenzenes have been reported in organic
sediments and limited information indicates that these residues are
bioavailable. The following recommendations are made to improve the
understanding of the environmental distribution, fate, and impact of
chlorobenzenes:
1. Residues of chlorobenzenes in sediment should be included in
monitoring programmes.
2. The extent to which the presence of organic matter governs the
distribution of chlorobenzenes between water, sediment, and air
should be further studied.
3. Further research into the role of, and uptake from sediment
(aquatic sediment and soil) by, biota is needed, together with
studies on possible transfer in the food chains.
4. The capacity of a wider range of organisms to metabolize
chlorobenzenes should be assessed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL
BODIES
The carcinogenicity of 1,2-DCB and 1,4-DCB has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987). Data
on the carcinogenicity of both compounds for humans were considered
inadequate. There was inadequate evidence for carcinogenicity to
animals for 1,2-DCB and sufficient evidence for 1,4-DCB.
In the Guidelines for drinking-water quality (WHO, 1984), the
following guideline values were developed for chlorobenzenes: MCB, 3
µg/litre; 1,2-DCB, 0.3 µg/litre; and 1,4-DCB, 0.1 µg/litre. All
values represent 10% of the taste and odour threshold value.
Regulatory standards established by national bodies in different
countries and the EEC are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1986).
REFERENCES
ABERNATHY, S., BOBRA, R.M., SHIU, W.Y., WELLS, P.G., & MACKAY, D.
(1986) Acute lethal toxicity of hydrocarbons and chlorinated
hydrocarbons to two planktonic crustaceans: The key role of
organism-water partitioning. Aquat. Toxicol., 8: 163-174.
AHBORG, U.G. & VICTORIN, K. (1987) Impact on health of chlorinated
dioxins and other trace organic emissions. Waste Manag. Res., 5:
203-224.
AHLING, B. (1987) Formation of chlorinated hydrocarbons during
combustion of poly(vinyl chloride). Chemosphere, 10: 799-806.
AMOORE, J.E. & HAVTALA, E. (1983) Odor as an aid to chemical safety:
Odor thresholds compared with threshold limit values and
volatilities for 214 industrial chemicals in air and water dilution.
J. appl. Toxicol., 3(6):272-290.
AKERMARK, B., BAECKSTROM, P., WESTLIN, U.E., GOTHE, R., &
WACHTMEISTER, C.A. (1976) Photochemical dechlorination of
1,2,4-trichlorobenzene. Acta chem. Scand., B30: 49-52.
ANTOINE, S.R., DELEON, I., & O'DELL-SMITH, R.M. (1986)
Environmentally significant volatile organic pollutants in human
blood. Bull. environ. Contam. Toxicol., 36: 364-371.
ARIYOSHI, T.M., IDEGUCHI, K., ISHIZUKA, Y., IWASAKI, K., & ARAKAKI,
M. (1975) Relationship between chemical structure and activity. I.
Effects of the number of chlorine atoms in chlorinated benzenes on
the components of drug-metabolizing system and the hepatic
constituents. Chem. Pharm. Bull., 23 (4): 817-823.
ARIYOSHI, T., YASUMATSU, F., SUETSUGU, M., KOKUBA, Y., HAMASAKI, K.,
& ARIZONO, K. (1981) Effects of halogenated compounds on the
cytochrome P-450 content and the activities of enzymes in
biosynthesis and degradation of heme. J. Pharmacobiodyn., 4(2):
4-5.
AZOUZ, W.M., PARKE, D.V., & WILLIAMS, R.T. (1955) Studies in
detoxication: The metabolism of halogenobenzenes. ortho- and
para-dichlorobenzenes. Biochem. J., 59(3): 410-415.
BALKON, J. & LEARY, J.A. (1979) An initial report on a comprehensive
quantitative, screening procedure for volatile compounds of forensic
and environmental interest in human biofluids by GC/MS. J. anal.
Toxicol., 3: 213-215.
BALLSCHMITER, K. & SCHOLZ, C. (1980) Microbial decomposition of
chlorinated aromatic substances. VI. Formation of dichlorophenols
and dichloropyrocatechol from dichlorobenzenes in a micromolar
solution by Pseudomonas species. Chemosphere, 9 (7-8): 457-467.
BARKLEY, J., BUNCH, J., BURSEY, J.T., CASTILLO, N., COOPER, S.D.,
DAVIS, J.M., ERICKSON, M.D., HARRIS, B.S.H., KIRKPATRICK, M.,
MICHEAL, L.C., PARKS, S.P., PELLIZZARI, E.D., RAY, M., SMITH, D.,
TOMER, K.B., WAGNER, R., & ZWEIDINGER, R.A. (1980) Gas
chromatography mass spectrometry computer analysis of volatile
halogenated hydrocarbons in man and his environment-a multimedia
environmental study. Biomed. mass Spectrom., 7(4): 139-147.
BAUER, U. (1981) [Human exposure to environmental
chemicals-investigations on volatile organic halogenated compounds
in water air, food, and human tissues. III. Communication: results
of investigations.] Zbl. Bakteriol. Hyg., I. Abt. Orig. B., 174:
200-237 (in German).
BAYER, A.G. (1986a) Para-dichlorobenzene in the CHO HGPRT forward
mutation assay. Report prepared by Litton Bionetics, USA, submitted
to WHO by Bayer, A.G., Institute of Toxicology (Unpublished).
BAYER, A.G. (1986b) Para-dichlorobenzene in the in vitro
transformation of Balb/3T3 cells assay. Report prepared by Litton
Bionetics, USA, submitted to WHO by Bayer, A.G., Institute of
Toxicology (Unpublished).
BECK, J. & HANSEN, K.E. (1974) The degradation of quintozene,
pentachlorobenzene, hexachlorobenzene and pentachloroaniline in
soil. Pestic. Sci., 5: 41-48.
BERLINER, M.L. (1939) Cataract following the inhalation of
paradichlorobenzene vapor. Arch. Ophthalmol., 22: 1023-1034.
BIRGE, W.J., BLACK, J.A. & BRUSER, D.M. (1979) Toxicity of organic
chemicals to embryo-larval stages of fish. Lexington, Kentucky, 69
pp (EPA Report No. 560/11-79-007; NTIS Report No. PB80-101637).
BLACK, W.D., VALLI, V.E.O., RUDDICK, J.A., & VILLENEUVE, D.C. (1988)
Assessment of teratogenic potential of 1,2,3-, 1,2,4- and
1,3,5-trichlorobenzenes in rats. Bull. environ. Contam. Toxicol.,
41: 719-726.
BLANCHARD, R.D. & HARDY, J.K. (1985) Use of a permeation sampler in
the collection of 23 volatile organic priority pollutants. Anal.
Chem., 57(12): 2349-2351.
BOMHARD, E., LUCKHAUS, G., VOIGT, W.-H., & LOESER, E. (1988)
Induction of light hydrocarbon nephropathy by p-dichlorobenzene.
Arch. Toxicol., 61: 433-439.
BONNET, P., RAOULT, G., & GRADISKI, D. (1979) Concentrations
lethales 50 des principaux hydrocarbons aromatiques. Arch. Mal.
prof., 40(8-9): 805-810.
BONNET, P., MORELE, Y., RAOULT, G., ZISSU, D., & GRADISKI, D. (1982)
Determination de la concentration lethale 50 des hydrocarbones
aromatiques chez le rat. Arch. Mal. prof., 63(4): 461-465.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformation of halogenated
organic compounds under denitrification conditions. Appl. environ.
Microbiol., 45(4): 13295-1299.
BOUWER, E.J. & MCCARTY, P.L. (1984) Modeling of trace organics
biotransformation in the subsurface. Ground Water, 22(4): 433-440.
BOYLES, D.T. (1980) Toxicity of hydrocarbons and their halogenated
derivatives in an aqueous environment. In: Afgan, B.K. & Mackay, D.,
ed. Hydrocarbons and halogenated hydrocarbons in the aquatic
environment, New York and London, Plenum Press, pp. 545-557.
BOY-MARCOTTE, J.L. (1980) 100-1000 kW(el) medium-power distributed-
collector solar system. Electr. Power Syst. Res., 3: 41-51.
BRAUN, W.H., SUNG, L.Y., KEYES, D.G., & KOCIBA, R.J. (1978)
Pharmacokinetic and toxicological evaluation of dogs fed
1,2,4,5-tetra-chlorobenzene in the diet for two years. J. environ.
Pathol. Toxicol., 2: 225-233.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa in
the cell multiplication inhibition test. Water Res., 14(3):
231-241.
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna Straus tested by an improved
standardized procedure.] Wasser abwasser Fors., 15(1): 1-6 (in
German).
BRISTOL, D.W., CRIST, H.L., LEWIS, R.G., MACLEOD, K.E., & SOVOCOOL,
G.W. (1982) Chemical analysis of human blood for assessment of
environmental exposure to semivolatile organochlorine chemical
contaminants. J. anal. Toxicol., 6: 269-275.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DECEAURRIZ, J. (1983)
Short-inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BROWN, V.K.H., MUIR, C., & THORPE, E. (1969) The acute toxicity and
skin irritant properties of 1,2,4-trichlorobenzene. Ann. occup.
Hyg., 12: 209-212.
BUCCAFUSCO, R.J., ELLS, S.J. & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to bluegill (Lepomis macrochirus). Bull.
Environ. Contam. Toxicol., 26: 446-452.
BUSER, H.R. (1979) Formation of polychlorinated dibenzofurans
(PCDFs) from the pyrolysis of chlorobenzenes. Chemosphere, 6:
415-424.
CALARMARI, D., GALASSI, S., SETTI, F. & VIGHI, M. (1983) Toxicity of
selected chlorobenzenes to aquatic organisms. Chemosphere, 12(2):
253-262.
CAMPBELL, D.M. & DAVIDSON, R.J.L. (1970) Toxic haemolytic anaemia in
pregnancy due to a pica for paradichlorobenzene. J. Obstet.
Gynaecol. Br. Commonw., 77: 657-659.
CANTON, J.H., SLOOFF, W., KOOL, H.J. & STRUYS, J. (1985) Toxicity,
biodegradability, and accumulation of a number of C/N-containing
compounds for classification and establishing water quality
criteria. Regulat. Toxicol. Pharmacol., 5: 123-131.
CARLSON, G.P. (1980) Effects of halogenated benzenes on arylesterase
activity in vivo and in vitro. Res. Comm. chem. Pathol.
Pharmacol., 30 (2): 361-364.
CARLSON, G.P. & TARDIFF, R.G. (1976) Effect of chlorinated benzenes
on the metabolism of foreign organic compounds. Toxicol. appl.
Pharmacol., 36: 383-394.
CARLSON, G.P., DZIEZAK, J.D., & JOHNSON, K.M. (l979) Effect of
halogenated benzenes on acetanilide esterase, acetanilide
hydroxilase and procaine esterase in rats. Res. Comm. chem. Pathol.
Pharmacol., 25(1): 181-184.
CARLSON, R.M., CARLSON, R.E., KOPPERMAN, H.L., & CAPLE, R. (1975)
Facile incorporation of chlorine into aromatic systems during
aqueous chlorination process. Environ. Sci. Technol., 9: 674-675.
CHARBONNEAU, M., STRASSER, J., LOCK, E.A., TURNER, M.J., & SWENBERG,
J.A. (1989) Involvement of reversible binding to a-2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol. appl.
Pharmacol., 99: 122-132.
CHOUDHRY, G.G. & WEBSTER, G.R.B. (1985) Photochemistry of
halogenated benzene derivatives. V. Photolytic reductive
dechlorination and and isomerization of tetrachlorobenzenes in
acetonitrile-water mixtures. Toxicol. environ. Chem., 9: 291-308.
CHOUDHRY, G.G., ROOF, A.A.M., & HUTZINGER, O. (1979) Photochemistry
of halogenated benzene derivatives. I. Trichlorobenzenes: reductive
dechlorination, isomerization and formation of polychlorobiphenyls.
Tetrahed. Lett., 22: 2059-2062.
CHU, I., VILLENEUVE, D., SECOURS, V., & VALLI, V.E. (1983)
Comparative toxicity of 1,2,3,4-, 1,2,4,5-, and
1,2,3,5-tetrachlorobenzene in the rat: results of acute and subacute
studies. J. Toxicol. environ. Health, 11(5): 663-667.
CHU, I., VILLENEUVE, D.C., VALLI, V.E., & SECOURS, V.E. (1984a)
Toxicity of 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-tetrachlorobenzene in
the rat: results of a 90-day feeding study. Drug Chem. Toxicol.,
7(2): 113-127.
CHU, I., VILLENEUVE, D.C., VIAU, A., BARNES, C.R., BENOIT, F.M., &
QIN, Y.H. (1984b) Metabolism of 1,2,3,4-, 1,2,3,5-, and
1,2,4,5-tetrachlorobenzene in the rat. J. Toxicol. environ. Health,
13(4-6): 777-786.
CHU, I., MURDOCH, D.J., VILLENEUVE, D.C. & VIAU, A. (1987) Tissue
distribution and elimination of trichlorobenzenes in the rat. J.
environ. Sci. Health, B22(4): 439-453.
CLARK, J.R., PATRICK, J.M. MOORE, J.C., & LORES, E.M. (1987)
Waterborne and sediment-source toxicities of six organic chemicals
to grass shrimp (Palaemonetes pugio) and amphioxus (Branchiostoma
caribaeum). Arch. environ. Contam. Toxicol., 16: 401-407.
COATE, W.B., SCHOENFISCH, W.H., LEWIS, T.R., & BUSEY, W.M. (1977)
Chronic inhalation exposure of rats, rabbits, and monkeys to
1,2,4-trichlorobenzene. Arch. environ. Health, 32(6): 249-255.
COHEN, J.M., DAWSON, R., & KOKETSU, M. (l981) Extent-of-exposure
survey of monochlorobenzene. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, Div. Surveillance, Hazard
Evaluations and Field Studies, NIOSH (PB 82-183963).
COTE, M., CHU, I., VILLENEUVE, D.C., SECOURS, V.E., & VALLI, V.E.
(1988) Trichlorobenzenes: Results of a thirteen week feeding study
in the rat. Drug chem. Toxicol., 11(1): 11-28.
COTTER, L.H. (1953) Paradichlorobenzene poisoning from insecticides.
N.Y. State J. Med., 53: 1690-1692.
COURTNEY, K.D., ANDREWS, J.E., & EBRON, M.T. (1977) Teratology study
of pentachlorobenzene in mice: no teratogenic effect at 50 or 100
mg/kg/day from day 6 to day 15 of gestation. IRCS med. Sci.: Libr.
Compend., 5: 587.
CROSBY, D.G. & HAMADMAD, N. (1971) The photoreduction of
pentachlorobenzenes. J. agric. food Chem., 19(6): 1171-1174.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment. Washington, US Environmental Protection Agency (Report
EPA-600/3-80-084) (NTIS PB80-221948).
CURRAN, H.M. (1981) Use of organic working fluids in Rancine
engines. J. Energy, 5(4): 218-223.
DALICH, G.M. & LARSON, R.E. (1985) Temporal and dose-response
features of monochlorobenzene hepatotoxicity in rats. Fundam. appl.
Toxicol., 5: 105-116.
DALY, J.W., JERINA, D.M., & WITKOP, B. (1972) Arene oxides and the
NIH shift: the metabolism, toxicity and carcinogenicity of aromatic
compounds. Experimentia(Basel), 28: 1129-1149.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters on survival and growth of the
larvae. US Fish. Bull., 67: 393-404.
DAVIS, E.M., MURRAY, H.E., LIEHR, J.G., & POWERS, E.L. (1981) Basic
microbial degradation rates and chemical byproducts of selected
organic compounds. Water Res., 15: 1125-1127.
DAVIES, D. & MES, J. (1987) Comparison of the residue levels of some
organochlorine compounds in breast milk of the general and
indigenous Canadian populations. Bull. environ. Contam. Toxicol.,
39: 743-749.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The
acute toxicity of 47 industrial chemicals to fresh and saltwater
fishes. J. hazard. Mat., 1: 303-318.
DEBORTOLI, M., KNOPPEL, H., PECCHIO, E., PEIL, A., ROGORA, L.,
SCHAUENBURG, H., SCHLITT, H., & VISSERS, H. (1985) Measurement of
indoor air quality and comparison with ambient air: a study on 15
homes in northern Italy. Luxembourg, Commission of the European
Communities (EUR 9656 EN).
DE CEAURRIZ, J.C., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DEN BESTEN, C., PETERS, M.M.C.G. & VAN BLADEREN, P.J. (1989) The
metabolism of pentachlorobenzene by rat liver microsomes: The nature
of the reactive intermediates formed. Biochem. biophys.Res. Comm.,
163(3): 1275-1281.
DILLEY, J.V. (1977) Toxic evaluation of inhaled chlorobenzene
(monochlorobenzene). Springfield, Virginia, National Technical
Information Service, US Department of Commerce (PB-276 623).
DILLING, W.L., BREDEWEG, C.J., & TEFERTILLER, N.B. (1976) Organic
photochemistry: simulated atmospheric photodecomposition rates of
methylene chloride, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene and other compounds. Environ. Sci. Technol.,
10(4): 351-356.
DURHAM, R.W. & OLIVER, B.G. (1983) History of Lake Ontario
contamination from the Niagara River by sediment radiodating and
chlorinated hydrocarbon analysis. J. Great Lakes Res., 9(2):
160-168.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral, neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
EHRLICHER, H. (1968) [Observations and experiences in industry
concerning the toxicity (physiopathologic effect) of chlorated
benzene vapours (mono- to hexachlorobenzene).] Zentrabl.
Arbeitsmed., 18: 204-205 (in German).
EICEMAN, G.A., CLEMENT, R.E., & KASAREK, F.W. (1979) Analysis of fly
ash from municipal incinerators for trace organic compounds. Anal.
Chem., 51(14): 2343-2350.
EICEMAN, G.A., CLEMENT, R.E., & KASAREK, F.W. (1981) Variations in
concentrations of organic compounds including polychlorinated
dibenzo- p-dioxins and polynuclear aromatic hydrocarbons in fly
ash from a municipal incinerator. Anal. Chem., 53(7): 955-959.
ELDER, V.A., PROCTOR, B.L., & HITES, R.A. (1981) Organic compounds
found near dump sites in Niagara Falls, New York. Environ. Sci.
Technol., 15(10): 1237-1243.
ENGST, R., MACHOLZ, R.M., KUJAWA, M., LEWERENZ, H-J., & PLASS, R.
(1976) The metabolism of lindane and its metabolites
gamma-2,3,4,5,6-pentachlorocyclohexene, pentachlorobenzene and
pentachlorophenol in rats and the pathways of lindane metabolism. J.
environ. Sci. Health. Bull., B11(2): 905-117.
ENVIRONMENT CANADA (1986) Ambient air concentrations of volatile
organic compounds in Toronto and Montreal. Ottawa, Environment
Canada, Pollution Measurement Division.
EUROPEAN PATENT APPLICATION 36,270, September 23, 1981.
FIELDING, M., GIBSON, T.M., & JAMES, H.A. (1981) Levels of
trichloroethylene and p-dichlorobenzene in groundwaters. Environ.
Technol. Lett., 2(12): 545-550.
FOMENKO, V.N. (1965) Determination of the maximum permissible
concentration of tetrachlorobenzene in water basins. Hyg. Sanit.,
30: 8-15.
FRANK, S.B. & COHEN, H.J. (1961) Fixed drug eruption due to paradi-
chlorobenzene. NY State J. Med., 61: 4079.
GAFFNEY, P.E. (1976) Carpet and rug industry case study: I. Water
and wastewater treatment plant operation. J. Water Pollut. Contr.
Fed., 48: 2590-2598.
GAGE, J.C. (1970) The subacute inhalation toxicity of 109 industrial
chemicals. Br.ö òJ. industr. Med., 27: 1-18.
GALLOWAY, S., BLOOM, A., RESNICK, M., MARGOLIN, B., NAKAMURA, F.,
ARCHER, P., & ZEIGER, E. (1985) Development of a standard protocol
for in vitro cytogenetic testing with Chinese Hamster ovary cells:
comparison of results for 22 compounds in two laboratories. Environ.
Mutagen., 7: 1-51.
GIAVINI, E., BOCCIA, M.L., PRATI, M., & VISMARA, C. (1986)
Teratologic evaluation of p-dichlorobenzene in the rat. Bull.
environ. Contam. Toxicol., 37: 164-168.
GIBSON, D.T., KOCH, J.R., SCHULD, C.L., & KALLIO, R.E. (1968)
Oxidative degradation of aromatic hydrocarbons by microorganisms.
II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry,
7(11): 3795-3802.
GIRARD, R., MARTIN, P., & BOURRET, J. (1969) [Haemopathies graves et
exposition à des dérivés chlorés du benzène (a propos de 7 cas). J.
Med. Lyon, 50(1164): 771-773.
GOERZ, G., VIZETHUM, W., BOLSEN, K., KRIEG, Th., & LISSNER, R.
(1978) [Hexachlorobenzene (HBC) induced porphyria in rats. Influence
of HBC-metabolites on the biosynthesis of heme.] Arch. dermatol.
Res., 263: 189-196 (in German).
GOLDSWORTHY, T.L., LYGHT, O., BURNETT, V.L., & POPP, J.A. (1988)
Potential role of 2µ - globulin, protein droplet accumulation, and
cell replication in the renal carcinogenicity of rats exposed to
trichloroethylene, perchloroethylene, and pentachloroethane.
Toxicol.appl. Pharmacol., 96: 367-379.
GOTO, M., HATTORI, M., MIYAGAWA, T., & ENOMOTO, M. (1972)[Hepatoma
generation in mice following application of high doses of HCH
(hexachlorocyclohexane)-isomers.] Chemosphere, 6: 279-282. (in
German).
GREVE, P.A. (1973) Pentachlorobenzene as a contaminant of animal
feed. Med. Fac. Landbouwwet. Rigksonio. Gent., 38: 775-784.
GRILLI, S., ARFELLINI, G., COLACCI, A., MAZZULLO, M., & PRODI, G.
(1985) In vivo and in vitro covalent binding of chlorobenzene to
nucleic acids. Jpn. J. Cancer Res. (Gann)., 76: 745-751.
GROSCH, D.S. (1973) Reproduction tests: the toxicity for Artemia
of derivatives from non-persistent pesticides. Biol. Bull., 145:
340-351.
HAIDER, K., JAGNOW, G., KOHNEN, R., & LIM, S.U. (1974) [Degradation
of chlorinated benzenes, phenols, and cyclohexane derivatives by
benzene and phenol utilizing soil bacteria under aerobic
conditions.] Acta Microbiol., 96(3): 183-200 (in German).
HALLOWELL, M. (1959) Acute haemolytic anaemia following the
ingestion of paradichlorobenzene. Arch. Dis. Childhood (London),
34: 74-75.
HANAI, Y., KATOU, T., JIMMA, T., & NOMURA, K. (1985) [The movement
of p-dichlorobenzene in the atmosphere.] Yokohama Kokuritsu Daigaku
Kankyo Kagaku Kenkyu Senta Kiyo, 12: 31-39 (in Japanese).
HARDEN., R.A. & BAETJER, A.M. (1978) Aplastic anemia following
exposure to paradichlorobenzene and naphthalene. J. occup. Med.,
20(12): 820-822.
HARKOV, R., GIANTI, S.J.Jr., BOZZELLI, J.W., & LAREGINA, J.E. (1985)
Monitoring volatile organic compounds at hazardous and sanitary
landfills in New Jersey. J. environ. Sci. Health, A20(5): 491-501.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., & LIOY, P.J. (1983)
Measurement of selected volatile organic compounds at three
locations in New Jersey during the summer season. J. Air Pollut.
Contr. Assoc., 33(12): 1177-1183.
HAWKINS, D.R., CHASSEAUD, L.F., WOODHOUSE, R.N., & CRESSWELL, D.G.
(1980) The distribution, excretion and biotransformation of
p-di-chloro[14]benzene in rats after repeated inhalation, oral
and subcutaneous doses. Xenobiotica, 10(2): 81-95.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.,
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutag., Suppl. 1, 5: 3-142.
HAYES, W.C., HANLEY, T.R., GUSHOW, T.S., JOHNSON, K.A., & JOHN, J.
A. (1985) Teratogenic potential of inhaled dichlorobenzenes in rats
and rabbits. Fundam. appl. Toxicol., 5(1): 190-202.
HEIKES, D.L. (1980) Residues of pentachlorobenzene and related
compounds in peanut butter. Bull. environ. Contam. Toxicol., 24:
338-343.
HEIKES, D.L., GRIFFITT, K.R., & CRAUN, J.C. (1979) Residues of
tetrachloro-nitrobenzene and related compounds in potatoes. Bull.
environ. Contam. Toxicol., 21: 563-568.
HEITMULLER, P.T., HOLLISTAR, T.A., & PARRISH, P.R. (1981) Acute
toxicity of 54 industrial chemicals to sheepshead minnows
(Cyprinodon variegatus). Bull. environ. Contam. Toxicol., 27:
596-604.
HERBOLD, B.A. (1986) Investigation of p-dichlorobenzene
1,4-dichlorobenzene for clastogenic effects in mice using the
micronucleus test. Bayer, A.G., Institute of Toxicology (Unpublished
report No. 14694 submitted to WHO) .
HERBOLD, B.A. (1988) p-Dichlorobenzene micronucleus test on the
mouse to evaluate clastogenic effects. Bayer, A.G., Institute og
Toxicology (Unpublished report no. 16902 submitted to WHO).
HERMENS, J., BROEKHUYZEN, E., CANTON, H., & WEGMAN, R. (1985)
Quantitative structure activity relationships and mixture toxicity
studies of alcohols and chlorohydrocarbons: effects on growth of
Daphnia magna. Aquat. Toxicol., 6: 209-217.
HIATT, M.H. (1981) Analysis of fish and sediment for volatile
priority polutants. Anal. Chem., 53(9): 1541-1543.
HITES, R.A. (1973) Analysis of trace organic compounds in New
England rivers. J. Chromatogr. Sci., 11: 570-574.
HOFLER, M., LAHANIATIS, E.S., BIENIEK, D., & KORTE, F. (1983)
[Reactionbehaviour of benzene at ppm concentrations during
chlorination withsodium hypochlorite in an aqueous solution.]
Chemosphere, 12(2): 217-224 (in German).
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., HOYLE, H.R., & SPENCER,
H. (1956) Toxicity of paradichlorobenzene; determinants on
experimental animals and human subjects. Arch. ind. Health, 14:
138-147.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., TORKELSON, T.R., & ADAM,
E.M. (1958) Toxicity of o-dichlorobenzene; studies on animals and
industrial experience. Arch. Ind. Health, 17: 180-187.
IARC (1982) Ortho and para dichlorobenzenes. In: Some industrial
chemicals and dyestuffs. Lyon, International Agency for Research on
Cancer, pp. 213-238 (Monographs on the Evaluation of Carcinogenic
Risk of Chemicals to Man, Vol. 29).
IARC (1987) Overall evaluations of carcinogenicity: an update of
IARC Monographs, Volumes 1 to 42, Lyon, International Agency for
Research on Cancer, pp. 192-193 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Supplement 7).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic. In: Patty,
F.A., ed. Industrial hygiene and toxicology, 2nd ed. New York,
Inter-Science Publishers, pp. 1333-1340.
IRPTC (1987) IRPTC Legal File 1986, Vol. 1. Geneva, International
Register of Potentially Toxic Chemicals, United National Environment
Programme.
JACOBS, A., BLANGETTI, M., & HELLMUND, E. (1974) Accumulation of
noxious chlorinated substances from Rhine River water in the fatty
tissue of rats. Vom Wass., 43: 259-274 (in German).
JACOBS, A., HELLMUND, E., & EBERLE, S.H. (1977) [Accumulation of
trace water pollutants in living organisms: time and dose dependence
of the accumulation of hexachlorobenzene and tetrachlorobenzene
derivatives in rats.] Vom Wass., 48: 255-272 (in German).
JAN, J. (1980) Chlorinated benzene residues in some seeds.
Chemosphere, 9: 165-167.
JAN, J. (1983a) Chlorobenzene residues in human fat and milk. Bull.
environ. Contam. Toxicol., 30: 595-599.
JAN, J. (1983b) Chlorobenzene residues in market milk and meat.
Mitt. Gebiete Lebensm. Hyg., 74(4): 420-425.
JAN, J. & MALNERSIC, S. (1980) Chlorinated benzene residues in fish
in Slovenia (Yugoslavia). Bull. environ. Contam. Toxicol., 24:824-827.
JERINA, D.M. & DALY, J.W. (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185(4151): 573-582.
JOHN, J.A., HAYES, W.C., HANLEY, T.R., Jr, JOHNSON, K.A., GUSHOW, T.
S., & RAO, K.S. (1984) Inhalation teratology study on
monochlorobenzene in rats and rabbits. Toxicol. appl. Pharmacol.,
76: 365-373.
JONDORF, W.R., PARKE, D.V., & WILLIAMS, R.T. (1955) Studies in
detoxication: the metabolism of halogenobenzenes. 1,2,3-, 1,2,4-,
and 1,3,5-tri-chlorobenzenes. Biochem. J., 61: 512-521.
JONDORF, W.R., PARKE, D.V., & WILLIAMS, R.T. (1958) Studies in
detoxication: the metabolism of halogenobenzenes 1,2,3,4-, 1,2,3,5-,
and 1,2,4,5-tetrachlorobenzenes. Biochem. J., 69: 181-189.
JUNGCLAUS, G.A., LOPEZ-AVILA, V., & HITES, R.A. (1978) Organic
compounds in an industrial wastewater: a case study of their
environmental impact. Environ. Sci. Technol., 12(1): 88-96.
KACEW, S., RUDDICK, J.A., PARULEKAR, M., VALLI, V.E., CHU, I., &
VILLENEUVE, D.C. (1984) A teratological evaluation and analysis of
fetal tissue levels following administration of tetrachlorobenzene
isomers to the rat. Teratology, 29: 21-27.
KANNO, S. & NOJIMA, K. (1979) Studies on photochemistry of aromatic
hydrocarbons. V. Photochemical reaction of chlorobenzene with
nitrogen oxides in air. Chemosphere, 4: 225-232.
KAO, C.I. & POFFENBERGER, N. (1979) Chlorinated benzenes. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed. Toronto,
Ontario, John Wiley and Sons, Vol. 5, pp. 797-808.
KARLOKOFF, S.W., BROWN, D.S., & SCOTT, T.S. (1979) Sorption of
hydrophobic pollutants on natural sediments. Water Res., 13:
241-249.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies on
halogenated benzenes (pentachloro-, pentachloronitro- and
hexabromo-) in rats. Toxicology, 5: 117-122.
KIMURA, R., HAYASHI, T., SATO, M., AIMOTO, T., & MURATA, T. (1979)
Identification of sulfur-containing metabolites of
p-dichlorobenzene and their disposition in rats. J. Pharmacobio-
Dyn., 2: 237-244.
KIRALY, J., SZENTESI, I., RUZICSKA, M., & CZEIZE, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. environ. Contam. Toxicol., 8: 309-319.
KITCHIN, K.T. & EBRON, M.T. (1983a) Maternal hepatic and embryonic
effects of 1,2,4-trichlorobenzene in the rat. Environ. Res., 31:
362-373.
KITCHIN, K.T. & EBRON, M.T. (1983b) Maternal hepatic and embryonic
effects of 1,2,3,4-tetrachlorobenzene in the rat. Toxicologist,
26: 243-256.
KITCHIN, K.T. & EBRON, M.T. (1983c) Maternal hepatic effects of
1,2,4,5-tetrachlorobenzene in the rat. Environ. Res., 32:
134-144.
KLUWE, W.M., DILL, G., PERSING, A., & PETERS, A. (1985) Toxic
response to acute, subchronic, and chronic oral administrations of
monochlorobenzene to rodents. J. Toxicol. environ. Health, 15(6):
745-767.
KNAPP, W.K., Jr, BUSEY, W.M., & KUNDZINS, W. (1971) Subacute oral
toxicity of monochlorobenzene in dogs and rats. Toxicol. appl.
Pharmacol., 19(2): 393 (Abstract)
KNEZOVICH, J.P. & HARRISON, F.L. (1988) The bioavailability of
sediment-sorbed chlorobenzenes to larvae of the midge, Chironomus
decorus. Ecotoxicol. environ. Saf., 15: 226-241.
KOBAYASHI, H. & RITTMAN, B.E. (1982) Microbial removal of hazardous
organic compounds. Environ. Sci. Technol., 16(3): 170A-183A.
KOCIBA, R.J., LEONG, B.J.K., & HEFNER, R.E. Jr (1981) Subchronic
toxicity study of 1,2,4-trichlorobenzene in the rat, rabbit and
beagle dog. Drug Chem. Toxicol., 4(3): 229-249.
KOHLI, J., JONES, D., & SAFE, S. (1976) The metabolism of higher
chlorinated benzene isomers. Can. J. Biochem., 54(3): 203-208.
KONEMANN, H. & VAN LEEUWEN, K. (1980) Toxicokinetics in fish:
accumulation and elimination of six chlorobenzenes by guppies.
Chemosphere, 9: 3-19.
KOSS, G. & KORANSKY, W. (1977) Pentachlorophenol in different
species of vertebrates after administration of hexachlorobenzene and
pentachlorobenzene. Environ. Sci. Res., 12: 131-137.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal. Chem.,
54: 810-817.
KUEHL, D.W., LEONARD, E.N., WELCH, K.J., & VEITH, G.D. (1980)
Identification of hazardous organic chemicals in fish from the
Ashtabula River, Ohio, and Wabash River, Indiana. J. Assoc. Off.
Anal. Chem., 63(6): 1238-1244.
KUHN, E.P., COLBERG, P.J., SCHNOOR, J.L., WANNER, O., ZEHNER, A.J.
B., & SCHWARZENBACH, R.P. (1985) Microbial transformations of
substituted benzenes during infiltration of river water to
ground-water: laboratory column studies. Environ. Sci. Technol.,
19: 961-968.
LAHANIATIS, E.S., BIENIEK, D., VOLLNER, L., & KORTE, F. (1981a)
[Formation of organochlorine compounds in the combustion of
chlorine-containing polymers.] Chemosphere, 10(8): 935-943 (in
German)
LAHANIATIS, E.S., ROAS, R., BIENIEK, D., KLEIN, W., & KORTE, F.
(1981b) [Formation of chlorinated organic compounds in combustion of
polyethylenes in the presence of sodium chloride.] Chemosphere,
10(11-12): 1321-1326 (in German).
LAMPARSKI, L.L., LANGHORST, M.L., NESTRICK, T.J., & CUTIE, S. (1980)
Gas-liquid chromatographic determination of chlorinated benzenes and
phenols in selected biological matrices. J. Assoc. Off. Anal. Chem.,
63(1): 27-32.
LANGHORST, M.L. & NESTRICK, T.J. (1979) Determination of
chlorobenzenes in air and biological samples by gas chromatography
with photoionization detection. Anal. Chem., 51(12): 2018-2025.
LATTANZI, G., BARTOLI, S., BONORA, B., COLACCI, A., GRILLI, S.,
NIERO, A., & MAZZULLO, M. (1989) The different genotoxicity of
p-dichlorobenzene in mouse and rat: Measurement of the in vivo
and in vitro covalent interaction with nucleic acids. Tumori,
75: 305-310.
LAY, J.P., SCHAUERTE, W., MULLER, A., KLEIN, W., & KORTE, F. (1985)
Long-term effects of 1,2,4-trichlorobenzene on freshwater plankton
in an outdoor-model ecosystem. Bull. environ. Contam. Toxicol.,
34: 761-769.
LEBEL, G.L. & WILLIAMS, D.T. (1986) Determination of halogenated
contaminants in human adipose tissue. J. Assoc. Off. Anal. Chem.,
69(3): 451-458.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol, 24:
684-691.
LEBRET, E. (1985) Air pollution in Dutch homes: an exploratory study
in environmental epidemiology. Wageningen, The Netherlands,
Department of Air Pollution, Department of Environmental and
Tropical Health, Wageningen Agricultural University (Report R-138).
LEE, R.P. & RYAN, C. (1979) Microbial degradation of organochlorine
compounds in esturine waters and sediments. In: Proceedings of the
Workshop: Microbial Degradation of Pollutants in Marine
Environments, Pensacola Beach, Florida, April 9-14, 1978.
Springfield, Virginia, US Environmental Protection Agency,
pp.443-450 (Contract No., EPA-600/9-79-012. NTIS: PB298254).
LEVINE, S.P., COSTELLO, R.J., GERACI, C.L., & CONLIN K.A. (1985) Air
monitoring at the drum bulking process of a hazardous waste remedial
action site. Am. Ind. Hyg. Assoc. J., 46(4): 192-196.
LINDER, R., SCOTTI, T., GOLDSTEIN, J., MCELROY, K., & WALSH, D.
(1980) Acute and subchronic toxicity of pentachlorobenzene. J.
environ. Pathol. Toxicol., 4(5-6): 183-196.
LINGG, R.D., KAYLOR, W.H., PYLE, S.M., KOPFLER, F.C., SMITH, C.C.,
WOLFE, G.F., & CRAGG, S. (1982) Comparative metabolism of
1,2,4-trichlorobenzene in the rat and rhesus monkey. Drug Metab.
Disp., 10(2): 134-141.
LOESER, E. & LITCHFIELD, M.H. (1983) Review of recent toxicology
studies on p- dichlorobenzene. Food.cChem. Toxicol., 21(6):
825-832.
LOPEZ-AVILA, V., NORTHCUTT, R., ONSTOT, J., WICKHAM, M., & BILLETS,
S. (1983) Determination of 51 priority organic compounds after
extraction from standard reference materials. Anal. Chem., 55(6):
881-889.
LOVEGREN, N.V., FISHER, G.S., LEGENDRE, M.G., & SCHULLER, W.H.
(1979) Volatile constituents of dried legumes. J. agric. food Chem.,
27(4): 851-853.
LU, P-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Persp., 10: 269-284.
LUNDE, G. & BJORSETH, A. (1977) Human blood samples as indicators of
occupational exposure to persistent chlorinated hydrocarbons. Sci.
total Environ., 8: 241-246.
LUNDE, G. & OFSTAD, E.B. (1976) Determination of fat-soluble
chlorinated compounds in fish. Z. anal. Chem., 282: 395-399.
MACHOLZ, R.M. & KUJAWA, M. (1985) Recent state of lindane
metabolism. Part III. Res. Rev., 94: 119-149.
MACKAY, D., BOBRA, A., CHAN, D.W., & SHIU, W.Y. (1982) Vapour
pressure correlations for low-volatility environmental chemicals.
Environ. Sci. Technol., 16: 645-649.
MACKAY, D. & SHIU, W.Y. (1981) A critical review of Henry's Law
Constants for chemicals of environmental interest. J. phys. chem.
Ref. Data, 10(4): 1175-1199.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of
low-solubility contaminants from water bodies to atmosphere.
Environ. Sci. Technol., 7(7): 611-614.
MARINUCCI, A.C. & BARTHA, R. (1979) Biodegradation of 1,2,3- and
1,2,4-trichlorobenzene in soil and in liquid enrichment culture.
Appl. environ. Microbiol., 38(5): 8ll-817.
MCKINNEY, J.D., FISHBEIN, C.E., FLETCHER, C.E., & BARTEL, W.F.,
(1970) Electron-capture gas chromatography of paradichlorobenzene
metabolites as a measure of exposure. Bull. environ. Contam.
Toxicol., 5(4): 354-361.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 28(1): 97-104.
MES, J., DAVIES, D.J., TURTON, D., & SUN, W-F. (1986) Levels and
trends of chlorinated hydrocarbon contaminants in the breast milk of
Canadian women. Food Addit. Contam., 3(4): 313-322.
MICHAEL, L.C., ERICKSON, M.D., PARKS, S.P., & PELLIZZARI, E.D.
(1980) Volatile environmental pollutants in biological matrices with
a headspace purge technique. Anal. Chem., 52(12): 1836-1841.
MILES, D.H., MODY, N.V., & MINYARD, J.P. (1973) Constituents of
marsh grass: survey of the essential oils in Juncus roemerianus.
Phyto-chemistry, 12: 1399-1404.
MILLER, M.M., GHODBANE, S., WASIK, S.P., TEWARI, Y.B., & MARTIRE,
D.E. (1984) Aqueous solubilities, octanol/water partition
coefficients, and entropies of melting of chlorinated benzenes and
biphenyls. J. chem. eng. Data, 29: 184-190.
MILONE, M.F. (1986a) Capacity of para-dichlorobenzene to induce
unscheduled DNA synthesis in cultured Hela cells, Bayer Chemical Co.
(unpublished report submitted to WHO).
MILONE, M.F. (1986b) Chromosome aberrations in human lymphocytes
cultured in vitro and treated with p-dichlorobenzene, Bayer
Chemical Co., (Unpublished report submitted to WHO ).
MIYAI, I., HIRONO, N., FUJITA, M., & KAMEYAMA, M. (1988) Reversible
ataxia following chronic exposure to paradichlorobenzene. J. Neurol.
Neurosurg. Psychiat., 51: 453-454.
MOHTASHAMIPUR, E., TRIEBEL, R., STRAETER, H., & NORPOTH, K. (1987)
The bone marrow clastogenicity of eight halogenated benzenes in male
NMRI mice. Mutagenesis, 2(2): 111-113.
MORITA, M. & OHI, G. (1975) Para-dichlorobenzene in human tissue and
atmosphere in Tokyo metropolitan area. Environ. Pollut., 8:
269-274.
MORITA, M., MIMURA, S., OHI, G., YAGYU, H., & NISHIZAWA, T. (1975) A
systematic determination of chlorinated benzenes in human adipose
tissue. Environ. Pollut., 9: 175-179.
MOTTRAM, D.S., PSOMAS, I.E., & PATTERSON, R.L.S. (1983) Chlorinated
residues in the adipose tissue of pigs treated with
hexachlorocyclohexane. J. Sci. Food Agric., 34: 378-387.
MURTY, C.V.R., OLSON, M.J. GARG, B.D., & RAY, A.K. (1988)
Hydrocarbon induced hyaline droplet nephropathy in male rats during
senescence. Toxicol. appl. Pharmacol., y6: 380-392.
NAIR, R.S., BARTER, J.A., SCHROEDER, R.E. KNEZEVICH, A., & STACK,
C.R. (1987) A two-generation reproduction study with
monochlorobenzene vapor in rats. Fund. appl. Toxicol., 9: 678-686.
NALBANDIAN, R.M. & PEARCE, J.F. (1965) Allergic purpura induced by
exposure to p- dichlorobenzene. J. Am. Med. Assoc., 194(7):
828-829.
NAQUADAT (1987) National Water Quality Data Bank, Inland Waters
Directorate, Water Quality Branch, Environment Canada, Ottawa.
NEPTUNE, D. (1980) Descriptive statistic for detected priority
pollutants and tabulation listings. Washington, DC, US EPA (Office
of Water Planning and Standards, TRDB-0280-001).
NILL, J.P. (1936) Effects of dichlorobenzene. J. Am. Med. Assoc.,
107: 607-608.
NOJIMA, K. & KANNO, S. (1980) Studies on photochemistry of aromatic
hydrocarbons. VII. Photochemical reaction of p-dichlorobenzene
with nitrogen oxides in air. Chemosphere, 9: 437-440.
NTP (1985a) NTP technical report on the carcinogenesis studies of
chlorobenzene (CAS No. 108-90-7) in F344/N rats and B6C3F1 mice
(gavage studies). Research Triangle Park, North Carolina, National
Toxicology Program, US Department of Health and Human Services,
pp.228 (NTP TR 261).
NTP (1985b) NTP technical report on the carcinogenesis studies of
1,2-dichlorobenzene (CAS No. 95-50-1) in F344/N rats and B6C3F1
mice (gavage studies). Research Triangle Park, North Carolina,
National Toxicology Program, US Department of Health and Human
Services, pp.192 (NTP TR 255) .
NTP (1987) NTP technical report on the toxicology and
carcinogenicity studies of 1,4-dichlorobenzene (CAS No. 106-46-7) in
F344/N rats and B6C3F1 mice (gavage studies . Research Triangle
Park, North Carolina, National Toxicology Program, US Department of
Health and Human Services, pp.198 (NTP TR 319) .
NTP (1989a) Draft NTP report on the toxicity studies of
1,2,4,5-tetra-chlorobenzene in F344/N rats and B6C3F1 mice (feed
studies). Research Triangle Park, North Carolina, National
Toxicology Program, US Department of Health and Human Services (NTP
Tox 7).
NTP (1989b) Draft NTP report on the toxicity studies of
Pentachlorobenzene in F344/N rats and B6C3F1 mice (feed studies).
Research Triangle Park, North Carolina, National Toxicology Program,
US Department of Health and Human Services (NTP Tox 6) .
OESCH, F., JERINA, D.M., DALY, J.W., & RICE, J.M. (1973) Induction,
activation and inhibition of epoxide hydrase: an anomalous
prevention of chlorobenzene-induced hepatotoxicity by an inhibitor
of epoxide hydrase. Chem-Biol. Interact., 6(3): 189-202.
OGATA, M. & SHIMADA, Y. (1983) Differences in urinary
monochlorobenzene metabolites between rats and humans. Int. Arch.
occup. Environ. Health, 53: 51-57.
OLIVER, B.G. & BOTHEN, K.D. (1980) Determination of chlorobenzenes
in water by capillary gas chromatography. Anal. Chem., 52:
2066-2069.
OLIVER, B.G. & BOTHEN, K.D. (1982) Extraction and clean-up
procedures for measuring chlorobenzenes in sediments and fish by
capillary gas chromatography. Int. J. environ. anal. Chem., 12:
131-139.
OLIVER, B.G. & NICOL, K.D. (1982) Chlorobenzenes in sediments,
water, and selected fish from lakes Superior, Huron, Erie, and
Ontario. Environ. Sci. Technol., 16: 532-536.
OLIVER, B.G., & NICOL, K.D. (1984) Chlorinated contaminants in the
Niagara River, 1981-1983. Sci. total Environ., 39: 57-70.
OLIVER, B.G., CHARLTON, M.N., & DURHAM, R.W. (1989) Distribution,
redistribution and geochronology of polychlorinated biphenyl
congeners and other chlorinated hydrocarbons in Lake Ontario
Sediments. Environ. Sci. Technol., 23: 200-208.
OLSON, M.J., JOHNSON, J.T., & REIDY, C.A. (1990) A comparison of
male rat & human urinary proteins: implications for human resistance
to hyaline droplet nephropathy. Toxicol. appl. Pharmacol., 102:
524-536.
ONUSKA, F.I. & TERRY, K.A. (1985) Determination of chlorinated
benzenes in bottom sediment samples by WCOT column gas
chromatography. Anal. Chem., 57: 801-805.
OPPERHUIZEN, A. & STOKKEL, R.C.A.M. (1988) Influence of contaminated
particles on the bioaccumulation of hydrophobic organic
micropollutants in fish. Environ. Pollut., 51: 165-177.
OTSON, R. (1987) Purgeable organics in Great Lakes raw and treated
water. Int. J. environ. anal. Chem., 31: 43-53.
OTSON, R. & BENOIT, F.M. (1986) Surveys of selected organics in
residential air. In; Walkenshaw, D.S. ed. Transactions - Indoor air
quality in cold climates - Hazards and abatement measures, April
1985, Pittsburgh, PA, Air Pollution Control Association, pp 224-236.
OTSON, R. & WILLIAMS, D.T. (1981) Evaluation of a liquid-liquid
extraction technique for water pollutants. J. Chromatogr., 212:
187-197.
OTSON, R. & WILLIAMS, D.T. (1982) Headspace chromatographic
determination of water pollutants. Anal. Chem., 54(6): 942-946.
OTSON, R., WILLIAMS, D.T., & BIGGS, D.C. (1982a) Relationship
between raw water quality, treatment, and occurence of organics in
Canadian potable water. Bull. environ. Contam. Toxicol., 28:
396-403.
OTSON, R., WILLIAMS, D.T., & BOTHWELL, P.D. (1982b) Volatile organic
compounds in water at thirty Canadian potable water treatment
facilities. J. Assoc. Off. Anal. Chem., 65(6): 1370-1374.
PAGNOTTO, L.D. & WALKLEY, J.E. (1965) Urinary dichlorophenol as an
index of para-dichlorobenzene exposure. Am. Ind. Hyg. Assoc. J.,
26: 137-142.
PANKOW, J.F. & ISABELLE, L.M. (1982) Adsorption-thermal desorption
as a method for the determination of low levels of aqueous organics.
J. Chromatog., 237: 25-39.
PANKOW, J.F., ISABELLE, L.M., ASHER, W.E., KRISTENSEN, T.J., &
PETERSON, M.E. (1983) Organic compounds in Los Angeles and Portland
rain: identities, concentrations, and operative scavenging
mechanisms. In: Pruppacher, H.R., Semonin, R.G., & Slinen, W.G.N.,
ed. Precipitation Scavenging, Dry Deposition, and Resususpensions:
Proceedings of the Fourth International Conference, Santa Monica,
California, 29 November-3 December, 1982,. Elsevier, New York. Vol.
1, 403-415.
PARKE, D.V. & WILLIAMS, R.T. (1960) Studies in detoxication: the
halogeno-benzenes (a) penta- and hexa-chlorobenzenes, (b)
further observations on 1,3,5-trichlorobenzene. Biochem. J., 74:
5-9.
PELLIZZARI, E.D. (1982) Analysis for organic vapour emissions near
industrial and chemical waste disposal sites. Environ. Sci.
Technol., 16: 781-785.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERIKSON, M.D. (1982) Purgeable organic compounds
in mother's milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PELLIZZARI, E.D., HARTWELL, T.D., PERRITT, R.L., SPARACINO, C.M.,
SHELDON, L.S., ZELON, H.S., WHITMORE, R.W., BREEN, J.J., & WALLACE,
L. (1986) Comparison of indoor and outdoor residential levels of
volatile organic chemicals in five U.S. geographical areas. Environ.
Int., 12(6): 619-623.
PEREIRA, W.E. & HUGHES, B.A. (1980) Determination of selected
volatile organic priority pollutants in water by computerized
spectrometry. J. Am. Water Works Assoc., 72(4): 220-230.
PEROCCO, P., BOLOGNESI, S., & ALBERGHINI, W. (1983) Toxic activity
of seventeen industrial solvents and halogenated compounds on human
lymphocytes cultured in vitro. Toxicol. Lett., 16: 69-75.
PIET, G.J., SLINGERLAND, P., BIJLSMA, G.H., & MORRA, C. (1980) Fast
quantitative analysis of a wide variety of halogenated compounds in
surface-, drinking-, and groundwater. Environ. Sci. Res., 16:
69-80
PIKE, M.H. (1944) Ocular pathology due to organic compounds.
Michigan State Med. Assoc. J., 43: 581-584.
POLAND, A., GOLDSTEIN, J., HICKMAN, P., & BURSE, V.W. (1971) A
reciprocal relationship between the induction of -aminolevulinic
acid synthetase and drug metabolism produced by m-dichlorobenzene.
Biochem. Pharmacol., 2: 1281-1290.
POPOVKI, P., ORUSEV, T., URUMOVA, E., BLAGOEVA, L., & TRPOVSKI, V.
(1980) [Skin changes of workers employed in trichlorobenzene
production.] Arh. Hig. Rada Toksikol., 31: 177-184 (in
Serbo-Croat, with French abstract).
POTENTA, E.J. & SAUNDERS, M.F. (1983) Indoor air quality
investigation of chronic health problems in a Manhattan office
space. In:Proceedings of the 76th Annual Meeting of the Air
Pollution Control Association, Atlanta, Georgia, 19-24 June, 1983,
Pittsburgh, Air Pollution Control Association, pp. 2-12.
POWERS, M.B., COATE, W.B., & LEWIS, T.R. (1975) Repeated topical
applications of 1,2,4-trichlorobenzene: effects on rabbit ears.
Arch. environ. Health, 30: 165-167.
RAO, K.S., JOHNSON, K.A., & HENCK, J.W. (1982) Subchronic dermal
toxicity study of trichlorobenzene in the rabbit. Drug chem.
Toxicol., 5(3): 249-263.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene in some
soils and plants in Finland. Ann. Agric. Fenn., 16: 277-282.
REICH, H. (1934) [Puran(R) poisoning in a 2-year-old child].
Schweizerische Medzinische Wochenschrift, 64: 223-224. (in German)
REID, W.D. (1973) Mechanism of renal necrosis induced by
bromobenzene or chlorobenzene. Exp. mol. Pathol., 19: 197-214.
REID, W.D. & KRISHNA, G. (1973) Centrolobular hepatic necrosis
related to covalent binding of metabolites of halogenated aromatic
hydrocarbons. Exp. Mol. Path., 18: 80-99.
REINEKE, W., KNACKMUSS, H-J. (1984) Microbial metabolism of
haloaromatics: isolation and properties of chlorobenzene-degrading
bacterium. Appl. environ. Microb., 47(2): 395-402.
RENNER, G. & MUCKE, W. (1986) Transformation of pentachlorophenol.
Part 1: metabolism in animals and man. Toxicol. environ. Chem.,
11: 9-29.
RIMINGTON, C. & ZIEGLER, G. (1963) Experimental porphyria in rats
induced by chlorinated benzenes. Biochem. Pharmacol., 12:
1387-1397.
ROBINSON, K.S., KAVLOCK, R.J., CHERNOFF, N., & GRAY, L.E. (1981)
Multigeneration study of 1,2,4-trichlorobenzene in rats. J. Toxicol.
environ. Health, 8: 489-500.
ROGERS, H.R, CAMPBELL, J.A., CRATHORNE, B. & DOBBS, A.J. (1989) The
occurrence of chlorobenzenes and permethrins in twelve U.K. sewage
sludges. Water Res., 23(7): 913-921.
ROZENBAUM, N.D., BLOCK, R.S., KREMNAVA, S.N., GINZBURG, S.L., &
POZHARISKII, I.V. (1947) [Use of chlorobenzene as a solvent from the
standpoint of industrial hygiene.] Gig. i Sanit., 12(1): 21-24(in
Russian).
ROZMAN, K., WILLIAMS, J., MUELLER, W.F., COULSTON, F., & KORTE, F.
(1979) Metabolism and pharmacokinetics of pentachlorobenzene in the
rhesus monkey. Bull. environ. Contam. Toxicol., 22: 190-195.
RUDDICK, J.A., BLACK, W.D., VILLENEUVE, D.C., & VALLI, V.E. (1983) A
teratological evaluation following oral administration of trichloro-
and dichlorobenzene isomers to the rat. Teratology, 27(2): 73A.
RUDLING, L., AHLING, B. & LOFROTH, G. (1980) Chemical and biological
characterization of emissions from combustion of wood and wood-chips
in a small central heating furnace and from combustion of wood in
closed fireplace stoves, Solna, Sweden, Swedish Environment
Protection Board (SNV PM 1331) (in Swedish, with English summary).
SASMORE, D.P., MITOMA, C., TYSON, C.A., & JOHNSON, J.S. (1983)
Subchronic inhalation toxicity of 1,3,5-trichlorobenzene. Drug chem.
Toxicol., 6(3): 241-258.
SATO, A. & NAKAJIMA, T. (1979) A structure-activity relationship of
some chlorinated hydrocarbons. Arch. environ. Health, 34(2):
69-75.
SCHOENY, R.S., SMITH, C.C., & LOPER, J.C. (1979) Non-mutagenicity
for Salmonella of the chlorinatd hydrocarbons Aroclor 1254,
1,2,4-tri-chlorobenzene, Mirex and Kepone. Mutat. Res., 68(2):
125-132.
SCHWARTZ, H., CHU, I., VILLENEUVE, D.C., VIAU, A., & BENOIT, F.M.
(1985) Metabolites of 1,2,3,4-tetrachlorobenzene in monkey urine. J.
Toxicol. environ. Health, 15(5): 603-607.
SCHWARTZ, H., CHU, I., VILLENEUVE, D.C., & BENOIT, F.M. (1987)
Metabolism of 1,2,3,4-, 1,2,3,5-, and 1,2,4,5-tetrachlorobenzene in
the Squirrel monkey. J. Toxicol. environ. Health, 22: 341-350.
SCHWARZENBACH, R.P., MOLNAR-KUBICA, E., GIGER, W., & WAKEHAM, S.G.
(1979) Distribution, residence time and fluxes of tetrachlorethylene
and 1,4-dichlorobenzene in Lake Zurich, Switzerland. Environ. Sci.
Technol., 13(11): 1367-1373.
SCHWARZENBACH, R.P., GIGER, W., HOEHN, E., & SCHNEIDER, J.K. (1983)
Behavior of organic compounds during infiltration of river water to
groundwater. Field Stud.environ. Sci. Technol., 17: 472-479.
SEARS, G.W. & HOPKE, E.R. (1949) Vapor pressures of the isomeric
trichlorobenzenes in the low pressure region. J. Am. Chem. Soc.,
71: 2575-2576.
SELANDER, H. G., JERINA, D.M., & DALY, J.W. (1975) Metabolism of
chlorobenzene with hepatic microsomes and solubilized cytochrome
P-450 systems. Arch. Biochem. Biophys., 168: 309-321.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the Delaware
River. Environ, Sci. Technol., 12(10): 1188-1194.
SHELTON, D.W. & WEBER, L.J. (1981) Quantification of the joint of
effects of mixtures of hepatotoxic agents: evaluation of a
theoretical model in mice. Environ. Res., 26: 33-41.
SHIMIZU, M., YASUI, Y., & MATSUMOTO, N. (1983) Structural
specificity of aromatic compounds with special reference to
mutagenic activity in Salmonella typhimurium: a series of
chloro-or fluoro-nitrobenzene derivatives. Mutat. Res., 116:
217-238.
SHORT, B.G., BURNETT, V.L., COX, M.G., BUS, J.S., & SWENBERG, J.A.
(1987) Site-specific renal cytotoxicity and cell proliferation in
male rats exposed to petroleum hydrocarbons. Lab. Invest., 57(5):
564-577.
SIMMONS, P., BRANSON, D., & BAILEY, R. (1977)
1,2,4-Trichlorobenzene: biodegradable or not. Text. Chem. Color.,
9(9): 211-213.
SIEVERS, R.E., BARKLEY, R.M., DENNEY, D.W., HARVEY, S.D., ROBERTS,
J. M., & DELANY, A.C. (1980) Gas chromatographic and mass
spectrometric analysis of volatile organics in the atmosphere. In:
Sampling and analysis of toxic organics in the atmosphere.
Philadelphia, American Society for Testing and Materials, pp. 3-21
(ASTM STP 721).
SINGH, H.B., SALAS, L.J., SMITH, A.J., & SHIGEISHI, M. (1981)
Measurements of some potentially hazardous organic chemicals in
urban environments. Atmos. Environ., 15: 601-612.
SMITH, J.R.L., SHAW, B.A., & FOULKES, D.M. (1972) Mechanisms of
mammalian hydroxylation: some novel metabolites of chlorobenzene.
Xenobiotica, 2(3): 215-226.
SMITH, E.N. & CARLSON, G.P. (1980) Various pharmacokinetic
parameters in relation to enzyme-inducing abilities of
1,3,4-trichlorobenzene and 1,2,4-tribromobenzene. J. Toxicol.
environ. Health, 6(4): 737-749.
SOFUNI, T., HAYASHI, M., MATSUOKA, A., SAWADA, M., HATANAKA, M., &
ISHIDATE, M.JR. (1985) [Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells.] Eisei Shikenjo
Hokoku, 103: 64-75. (in Japanese).
SPENCE, R-M.M. & TUCKNOTT, O.G. (1983) The examination of the
headspace volatiles of watercress. J. Sci. Food Agric., 34:
768-772.
STATISTICS CANADA (1981) Apparent per capita food consumption in
Canada. Ottawa, Queen's Printer (Cat. 32-229, Annual, Part II).
STEINMETZ, K.L., SPANGGORD, R.J. (1987) Examination of the potential
of p- dichlorobenzene to induce unscheduled DNA synthesis or DNA
replication in the in vivo-in vitro mouse hepatocyte DNA repair
assay (Task 1). (Unpublished report prepared by SRI International,
USA, for US Chemical Manufacturers Association and submitted to WHO
by ICI, Central Toxicology Laboratory ).
STULL, D.R. (1947) Vapor pressure of pure substances: organic
compounds. Ind. Eng. Chem., 39: 517-539.
SULLIVAN, T.M., BORN, G.S., CARLSON, G.P., & KESSLER, W.V. (1983)
The pharmacokinetics of inhaled chlorobenzene in the rat. Toxicol.
appl. Pharmacol., 71: 194-203.
SUMERS, J. FUHRMAN, M. & KELMAN, A. (1952) Hepatitis with
concomitant esophageal varices following exposure to mothball
vapors. N.Y. State med. J., 52: 1048-1049.
SYROVADKO, O.N. & MALYSHEVA, Z.V. (1977) [Working conditions and
their effect on some specific functions of women engaged in the
manufacture of enamel-insulated wires.] Gig. Tr. Prof. Zabol., 4:
25-28 (in Russian)
TAGATZ, M.E., PLAIA, G.R., & DEANS, C.H. (1985) Effects of 1,2,4-
trichlorobenzene on estuarine macrobenthic communities exposed via
water and sediment. Ecotoxicol. environ. Safety, 10: 351-360.
TANAKA, A., SATO, M., TSUCHIYA, T., ADACHI, T., NIIMURA, T., &
YAMAHA, T. (1986) Excretion, distribution and metabolism of
1,2,4-trichlorobenzene in rats. Arch. Toxicol., 59: 82-88.
TARKHOVA, L.P. (1965) [Materials for determining the maximum
permissible concentration of chlorobensol in atmospheric air.] Gig.
i Sanit., 30: 327-333 (in Russian).
TOLOT, F., SOUBRIER, B., BRESSON, J.-R., & MARTIN, P. (1969) Myélose
proliférative d'évolution rapide. Role étiologigue possible des
dérivés chlorés du benzène. J. Med. Lyon, 50(164): 761-780.
TOPP, E., SCHEUNERT, I., ATTAR, A,. & KORTE, F. (1986) Factors
affecting the utake of 14C-labelled organic chemicals by plants
from soil. Ecotoxicol. Environ. Safety, 11: 219-228.
TOPP, E., SCHEUNERT, I., & KORTE, F. (1989) Kinetics of the uptake
of 14C-labelled chlorinated benzenes from soil by plants.
Ecotoxicol. environ. Safety, 17: 157-166.
US EPA (1980a) Ambient water quality criteria for chlorinated
benzenes. Washington, DC, Office of Water Regulations and
Standards, US Environmental Protection Agency (Report EPA
440/5-80-028, PB81-117392).
US EPA (1980b) Ambient water quality criteria for dichlorobenzenes.
Washington, DC, Office of Water Regulations and Standards, US
Environmental Protection Agency (Report EPA 440/5-80-039,
PB81-117509).
US EPA (1985) Health assessment document for chlorinated benzenes.
Final Report. Washington, DC, Office of Health and Environment
Assessment, US Environmental Protection Agency ( Report
EPA/600/8-84/015F).
UYETA, M., TAUE, S., CHIKASAWA, K., & MAZAKI, M. (1976)
Photoformation of polychlorinated biphenyls from chlorinated
benzenes. Nature (Lond.), 264: 583-584.
VAN HOOGEN, G. & OPPERHUIZEN, A. (1988) Toxicokinetics of
chlorobenzenes in fish. Environ. Toxicol. Chem., 7: 213-219.
VARSHAVSKAYA, S.P. (1968) Comparative toxicological characteristics
of chlorobenzene and dichlorobenzene (ortho- and para-isomers) in
relation to the sanitary protection of water bodies. Hyg. Sanit.,
33(10-12): 17-23.
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (pentachloro- pentachloronitro-, and
hexabromo-) in rats. Environ. Physiol. Biochem., 5(5): 328-331.
WAKEHAM, S.G., DAVIS, A.C., & KARAS, J.L. (1983) Mesocosm
experiments to determine the fate and persistence of volatile
organic compounds in coastal seawater. Environ. Sci. Technol., 17:
611-617.
WALLACE, L.A. (1986) Personal exposures, indoor and outdoor air
concentrations and exhaled breath concentrations of selected
volatile organic compounds measured for 600 residents of New Jersey,
North Dakota, North Carolina and California. Toxicol. environ.
Chem., 12(3-4): 215-236.
WALLACE, L.A., PELLIZZARI, E., HARTWELL, T., ROSENZWEIG, M.,
ERIKSON, M., SPARACINO, C., & ZELON, H. (1984) Personal exposure to
volatile organic compounds. I. Direct measurements in breathing-zone
air, drinking water, food, and exhaled breath. Environ. Res., 35:
293-319.
WALLACE, L.A., PELLIZZARI, E.D., HARTWELL, T.D., SPARACINO, C.M.,
SHELDON, L.S.,& ZELON, H. (1985) Personal exposures, indoor-outdoor
relationships, and breath levels of toxic air pollutants measured
for 355 persons in New Jersey. Atmos. Environ., 19(10): 1651-1661.
WALLACE, L.A., PELLIZZARI, E., HARTWELL, T., ZELON, H., SPARACINO,
C., PERRITT, R., & WHITMORE, R. (1986) Concentrations of 20 volatile
organic compounds in the air and drinking water of 350 residents of
New Jersey compared with concentrations in their exhaled breath. J.
occup. Med., 28(8): 603-608.
WALLGREN, K. (1953) [Chronic poisoning in the production of moth
deterrents mainly composed of p-dichlorobenzene]. Zentralbl.
Arbeitsmed. Arbeitsschutz, 3: 14-15 (in German).
WARE, S.A. & WEST, W.L. (1977) Investigation of selected potential
environmental contaminants: halogenated benzenes. Washington, DC,
297 pp. (US EPA Report, EPA 560/2-77-004, NTIS PB 273 206).
WATANABE, P.G., KOCIBA, R.J., HEFNER, R.E. JR., YAKEL, H.O., &
LEONG, B.K.J. (1978) Subchronic toxicity studies of
1,2,4-trichlorobenzene in experimental animals. Toxicol. appl.
Pharmacol., 45(1): 332-333 (Abstract).
WATSON, A. & PATTERSON, R.L.S. (1982) Tainting of pork meat by 1,4-
dichlorobenzene. J. Sci. Food Agric., 33: 103-105.
WEAST, R.C., ed. (1986) CRC handbook of chemistry and physics, 67th
ed., Boca Raton, Florida, The Chemical Rubber Company.
WELLER, R.W. & CRELLIN, A.J. (1953) Pulmonary granulomatosis
following extensive use of paradichlorobenzene. Arch. intern. Med.,
91: 408-413.
WHO (1984) Guidelines for drinking-water quality. Vol. 1:
Recommendations, Geneva, World Health Organization, 130 pp.
WHO (1987) Environmental Health Criteria 70: Principles for the
safety assessment of food additives and contaminants in food.
Geneva, World HealthOrganization, 174pp.
WILLIAMS, R.T. (1959) The metabolism of halogenated aromatic
hydrocarbons, In: Detoxification mechanisms, 2nd ed., London,
Chapman and Hall Ltd., pp. 237-258.
WILLIAMS, R.T., HIROM, P.C., & RENWICK, A.G. (1975) Species
variation in the metabolism of some organic halogen compounds, In:
Mcintyre, A.D. & Mills, C.F., ed., Ecolical and toxicological
Research, New York and London, Plenum Press, pp. 91-105.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1988) Organohalogen
residues in human adipose autopsy samples from six Ontario
municipalities. J. Assoc. Off. Anal. Chem., 71(2): 410-414.
WILSON, J.T., ENFIELD, C.G., DUNLAP, W.J., COSBY, R.L., FOSTER,
D.A., & BASKIN, L.B. (1981) Transport and fate of selected organic
pollutants in a sandy soil. J. environ. Qual., 10(4): 501-506.
WONG, P.T.S., CHAU, Y.K., RHAMEY, J.S., & DOCKER, M. (1984)
Relationship between water solubility of chlorobenzenes and their
effects on a freshwater green alga. Chemosphere, 13(9): 991-996.
YAMAMOTO, H., TANIGUCHI, Y., IMAI, S., OHNO, Y., & TSUBURA, Y.
(1978) [Acute toxicity and local irritation tests of
trichlorobenzene (TCB) on ddY mice.] J. Tara Med. Assoc., 29:
569-573 (in Japanese).
YAMAMOTO, H., OHNO, Y., NAKAMORI, K., OKUYAMA, T., IMAI, S., &
TSUBURA, Y. (1982) [Chronic toxicity and carcinogenicity test of
1,2,4-trichlorobenzene on mice by dermal painting.] J. Nara Med.
Assoc., 33(2): 132-145 (in Japanese).
YANG, K.H., PETERSON, R.E., & FUJIMOTO, J.M. (1979) Increased bile
duct-pancreatic fluid flow in benzene-treated rats. Toxicol. appl.
Pharmacol., 47(3): 505-514.
YOSHIDA, M. & HARA, I. (1985) Composition of urinary metabolites and
variation of urinary taurine levels in rats injected with
chlorobenzene. Ind. Health, 23(3): 239-243.
YOSHIOKA, Y., OSE, Y., & SATO, T. (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci. tot. Environ., 43:
149-157.
YOUNG, D.R. & HEESEN, T.C. (1978) DDT, PCB, and chlorinated benzenes
in the marine ecosystem off Southern California. In: Jolley, R.L.,
Gorshev, H., & Hamilton, D.H., Jr, ed., Water chlorination.
Environmental impact and health effects, Ann Arbor, Michigan, Ann
Arbor Science Publishers, Vol. 2, pp. 267-290.
ZAPATA-GAYON, C., ZAPATA-GAYON, N., & GONZALEZ-ANGULO, A. (1982)
Clastogenic chromosomal aberrations in 26 individuals accidentally
exposed to ortho dichlorobenzene vapors in the National Medical
Center in Mexico City. Arch. environ. Health, 37(4): 231-235.
ZOETEMAN, B.C.J., HARMSEN, K., LINDERS, J.B.H.J., MORRA, C.F.H., &
SLOOFF, W. (1980) Persistent organic pollutants in river water and
ground water of the Netherlands. Chemosphere, 9: 231-249.
ZONG, Z. & MA, A. (1985) [Statistical analysis of 1951 cases of
occupational dermatosis in Shanghai chemical plants.,] Zhonghua
Yufanguixue Zazhi, 19(2): 90-92 (in Chinese).
ZUB, M. (1978) Reactivity of the white blood cell system to toxic
action benzene and its derivatives. Acta biol. Cracoviensia, 21:
163-174.
RESUME
La présente publication traite des risques pour la santé humaine et
l'environnement qui découlent de l'exposition au monochloro-benzène
(MCB), aux dichlorobenzènes (DCB), aux trichlorobenzènes (TCB), aux
tétrachlorobenzènes (TeCB) et au pentachlorobenzène (PeCB). La
substitution par le chlore est indiquée comme suit:
1,2-dichlorobenzène (1,2-DCB); 1,2,3-trichlorobenzène (1,2,3-TCB),
etc.
1. Identitée, proprietes physiques et chimiques, et methodes
d'analyse
Les chlorobenzènes sont des dérivés aromatiques cycliques formés par
addition de 1 à 6 atomes de chlore au noyau benzénique. On obtient
ainsi 12 composés: le monochlorobenzène, trois isomères pour chacun
des di-, tri et tétrachlorobenzènes, ainsi que le penta- et
l'hexachlorobenzène.
Les chlorobenzènes sont des solides blancs cristallins à la
température ambiante, sauf dans le cas du MCB, du 1,2-DCB, du
1,3-DCB et du 1,2,4-TCB qui sont des liquides incolores. En général,
ils sont peu solubles dans l'eau, leur solubilité diminuant à mesure
que la substitution par le chlore augmente. Ils sont peu
inflammables, leurs coéfficients de partage octanol/eau sont moyens
à élevés et augmentent avec le nombre d'atomes de chlore; la tension
de vapeur est basse à moyenne et diminue lorsque la substitution
augmente. Les seuils gustatif et olfactif sont bas, en particulier
dans le cas des dérivés les moins chlorés.
Même lorsqu'ils sont purifiés, les chlorobenzènes du commerce
contiennent en proportions diverses, des isomères très voisins. Par
exemple, le MCB pur peut contenir jusqu'à 0,05% de benzène et 0,1%
de DCB; le 1,2-DCB technique pouvant contenir jusqu'à 19 % des
autres DCB, 1% de TCB et jusqu'à 0,05% de MCB. Rien n'indique qu'il
puisse y avoir contamination par des dibenzo- p-dioxines
polychlorées (PCDD) ni par des polychlorodibenzofuranes (PCDF).
Un grand nombre de techniques de prélèvement ont été mises au point
pour l'échantillonnage des chlorobenzènes, techniques qui sont
fonction du milieu. Elles vont de l'extraction par solvant dans le
cas des milieux aqueux à l'utilisation de substances absorbantes
pour les composés en suspension dans l'air. La chromatographie
gaz-liquide (GLC) est la technique de choix pour le dosage du
chlorobenzène dans des échantillons provenant de l'environnement.
2. Sources d'exposition humaine et environnementale
2.1 Chiffres de production
D'après les données dont on dispose sur le volume de la production
des chlorobenzènes et qui correspondent à la période 1980-83, la
production mondiale serait de 568 x 106 kg; cependant,
l'utilisation des chlorobenzènes a reculé dans certains pays depuis
lors. Environ 50 % de cette quantité a été produite aux Etats-Unis
d'Amérique et le reste, essentiellement en Europe occidentale et au
Japon. Le MCB correspondait à 70% de la production mondiale, la
production du 1,2-DCB, du 1,4-DCB et du 1,2,4-TCB étant
respectivement de 22 x 106, 4 x 106, et 1,2-3,7 x 106 kg.
Le MCB et les DCB sont obtenus par chloration directe du benzène en
phase liquide en présence d'un catalyseur, alors que les TCB et les
TeCB sont produits par chloration directe des chlorobenzènes
isomères convenables, en présence d'un catalyseur métallique.
2.2 Usages
On utilise principalement les chlorobenzènes comme intermédiaires
dans la synthèse des pesticides et d'autres produits chimiques; le
1,4-DCB est utilisé dans les désodorisants d'ambiance et comme
répulsif contre les mites. Les chlorobenzènes les plus substitués
(TCB et 1,2,3,4-TeCB) entrent dans la composition des fluides
diélectriques.
2.3 Liberation de chlorobenzenes dans l'environnement
Il peut y avoir libération de chlorobenzènes dans l'environnement,
principalement lors de la production de ces substances, qu i sont
en outre amenées à être dispersées lors d'un certain nombre de leurs
utilisations. Par exemple, aux Etats-Unis d'Amérique, entre 0,1 et
0,2% de la production de 1983 (qui correspondait à 130 x 106
tonnes de MCB a sans doute été dispersée dans l'environnement. Les
quantités libérées lors du rejet de déchets, et notamment
l'incinération de déchets municipaux, sont beaucoup plus faibles.
Toutefois, l'incinération des chlorobenzènes peut conduire à
l'émission de PCDD et de PCDF.
3. Transport, repartition et transformation dans l'environnement
3.1 Degradation
C'est principalement par voie biologique et, dans une moindre
mesure, par des processus abiotiques que les chlorobenzènes
disparaissent de l'environnement. Toutefois, on estime qu'ils sont
moyennement persistants dans l'eau, l'air et les sédiments. On a
fait état de temps de séjour dans l'eau allant d'une journée dans
les rivières à plus de 100 jours dans les eaux souterraines. D ans
l'air, la dégradation des chlorobenzènes s'effectue principalement,
semble-t-il, par voie chimique et par photolyse, la durée de séjour
se situant entre 13 et 116 jours pour le MCB, les DCB et un isomère
du TCB non précisé.
On a montré que nombre de microorganismes présents dans les
sédiments et les boues d'effluents dégradaient les chlorobenzènes.
Il semblerait que les composés les plus substitués soient les plus
difficiles à dégrader, la dégradation s'effectuant uniquement en
aérobiose. Dans les conditions d'anaérobiose du sol et les eaux
souterraines, le DCB, les TCB et les PeCB résistent en général à la
dégradation microbienne.
3.2 Destinee
Les chlorobenzènes qui sont libérés dans le milieu aquatique se
redistribuent préférentiellement dans l'air et les sédiments (en
particulier dans les sédiments riches en matières organiques).
D'après les renseignements limités dont on dispose, il semble que
l'on trouve dans les sédiments, notamment les sédiments des régions
très industrialisées, des quantités 1000 fois plus élevées que
celles qui sont présentes dans l'eau. La rétention dans le sol
augmente avec la teneur de celui-ci en matières organiques; il
existe une corrélation positive entre le degré de chloration du
composé et son adsorption sur les matières organiques. Quelques
données montrent que les résidus liés aux sédiments sont
biodisponibles; c'est ainsi que les invertébrés aquatiques sont
susceptibles de fixer les résidus présents sur les sédiments, les
plantes et dans le sol.
4. Concentrations dans l'environnement et exposition humaine
4.1 Les chlorobenzènes dans l'environnement
Les concentrations moyennes en chlorobenzènes (mono- à tri-) dans
l'air ambiant sont de l'ordre de 0,1 µg/m3, avec des valeurs
maximales allant jusqu'à 100 µg/m3. On ne dispose d'aucune donnée
sur les teneurs en TeCB et PeCB de l'air ambiant, encore que ces
substances aient été décelées dans des cendres volantes provenant
d'incinérateurs municipaux. Les concentrations de chlorobenzènes
dans l'air intérieur sont analogues à celles que l'on trouve dans
l'air extérieur; toutefois, on a fait état de valeurs beaucoup plus
élevées que dans l'air ambiant dans des régions très polluées, ainsi
que dans des espaces confinés où l'on avait utilisé des produits
contenant du chlorobenzène.
Des chlorobenzènes (mono- à penta-) ont été décelés dans des eaux
superficielles à des concentrations de l'ordre du ng/litre-µg/litre,
avec quelquefois des valeurs atteignant quelques dixièmes de
mg/litre à proximité d'installations industrielles. La teneur en
chlorobenzènes des eaux résiduaires industrielles peut être encore
plus élevée et varier en fonction de la nature des procédés mis en
oeuvre.
Dans les échantillons d'eau de boisson analysés on a décelé la
présence de tous les chlorobenzènes. Ce sont les composés les moins
chlorés qui étaient le plus fréquemment présents et aux
concentrations les plus fortes, avec prédominance de l'isomère
1,4-DCB; toutefois, les concentrations moyennes étaient généralement
inférieures à 1 µg/litre et ne dépassaient que rarement 50 µg/litre,
quel que soit le composé.
On n'a pas trouvé de données sur la teneur des aliments en
chlorobenzènes qui proviennent de programmes de surveillance bien
conçus; les renseignements disponibles se limitent principalement à
la concentration dans le poisson à proximité d'installations
industrielles ou à des incidents isolés de contamination de produits
carnés. Tous les isomères du chlorobenzène (mono- à penta-) ont été
décelés dans des truites d'eau douce, à des teneurs allant de 0,1 à
16 µg/kg. Dans une autre étude, les teneurs des poissons d'eau douce
en chlorobenzènes totaux variaient en moyenne de 0,2 mg/kg de
graisse dans les régions peu polluées à 1,8 mg/kg dans une zone
industrialisée. Il existe certains indices de l'augmentation de la
concentration en chlorobenzènes dans le poisson d'eau douce avec le
degré de chloration du composé. En ce qui concerne le poisson de
mer, les quelques études disponibles donnent une teneur en 1,4-DCB
de 0,05 mg/kg de poids humide.
Les résultats dont on dispose au sujet des taux de chlorobenzène
dans la viande et le lait et qui concernent principalement les
régions contaminées, sont de l'ordre de 0,02 à 5 µg/kg.
Lors de deux enquêtes sur le lait humain, on a procédé au dosage de
la totalité des chlorobenzènes, sauf le MCB. Dans une des études,
les concentrations de DCB étaient égales en moyenne à 25 µg/kg de
lait alors qu'elles étaient inférieures à 5 µg/kg dans le cas du
TCB, des isomères du TeCB et du PeCB. Dans la seconde enquête, les
concentrations étaient beaucoup plus basses puisqu'en moyenne elles
allaient de 1 µg/kg (1,2,3-TCB et PeCB) à un maximum de 6 µg/kg (1,3
et 1,4-dichlorobenzène).
4.2 Exposition humaine
4.2.1 Population generale
Sur la base des données limitées dont on dispose, il semble que
c'est à partir de l'air que l'apport journalier de chlorobenzènes
dans la population générale est le plus élevé, en particulier en ce
qui concerne les dérivés les moins chlorés qui sont plus volatils
(0,2-0,9 µg/kg de poids corporel). Par comparaison avec l'apport
d'autres origines, l'apport d'origine alimentaire augmente à mesure
qu'augmente le degré de chloration; ainsi, l'alimentation contribue
davantage que l'air à la dose journalière de TeCB et de PeCB.
Toutefois, les niveaux d'exposition dans ce cas restent
vraisem-blablement au-dessous de 0,05 µg/kg de poids corporel.
Quelques études, en nombre limité, ont montré que les enfants
nourris au sein risquaient de recevoir une dose de chlorobenzène
plus forte, rapportée au poids corporel, que les adultes.
4.2.2 Contexte professionnel
Il n'est pas possible de chiffrer avec précision l'exposition
professionnelle au chlorobenzène sur la base des données
disponibles. Toutefois, dans une unité de production, on a trouvé
des teneurs en 1,4-DCB allant de 42 à 288 mg/m3 et dans d'autres
usines chimiques, la concentration de MCB pouvait atteindre 18,7
mg/m3.
5. Cinetique et metabolisme
Tous les chlorobenzènes sont facilement résorbés dans les voies
digestives et respiratoires chez l'homme et les animaux
d'expérience, la résorption dépendant de la position de l'atome de
chlore pour les différents isomères d'un même dérivé.
Chez l'animal d'expérience, après une rapide répartition dans les
organes très irrigués, les chlorobenzèn s'accumulent principalement
dans les tissus adipeux, une quantité plus faible passant dans le
foie et les autres organes. On a montré que les chlorobenzènes
étaient capables de traverser la barrière placentaire et l'on en a
trouvé dans le cerveau de foetus. En général, ces dérivés
s'accumulent d'autant plus qu'ils sont plus chlorés. Toutefois, les
variations sont considérables en ce qui concerne les différents
isomères d'un même dérivé.
Chez l'homme et l'animal d'expérience, le métabolisme des
chlorobenzènes conduit au chlorophénol correspondant par oxydation
microsomique. Ces chlorophénols peuvent être excrétés dans l'urine
sous forme d'acides mercapturiques, d'acide glucuronique ou de
sulfo-conjugués. Le TeCB et le PeCB sont métabolisés plus lentement
et séjournent dans les tissus plus longtemps que les dérivés
monochlorés, dichlorés et trichlorés. Certains des chlorobenzènes
induisent toute une variété de systèmes enzymatiques et notamment
ceux qui interviennent dans les processus d'oxydation, de réduction,
de conjugaison et d'hydrolyse.
En général, les dérivés les plus chlorés s'éliminent plus lentement
que le MCB et le DCB, la proportion des dérivés tétra et
pentachlorés éliminés tels quels dans les matières fécales étant
plus importante. C'est ainsi que 17 % d'une dose 1,2,4-TCB a été
éliminé dans les matières fécales au bout de sept jours alors que 91
à 97 % d'une dose de 1,4 TCB était éliminé sous forme de métabolites
dans les urines au bout de cinq jours. La position des atomes de
chlore sur le noyau benzénique conditionne également de manière
importante la vitesse de métabolisation et d'élimination, les
isomères possédant deux atomes de carbone adjacents non substitués
étant métabolisés et éliminés plus rapidement.
6. Effets sur les organismes aquatiques dans leur milieu naturel
Les renseignements dont on dispose sur les effets des
chloro-benzènes au niveau de l'environnement, portent principalement
sur leur toxicité aiguë pour les organismes aquatiques. En général,
la toxicité augmente avec le degré de chloration du noyau
benzénique. Alors que le MCB, le 1,2-DCB, le 1,3-DCB, le 1,2,4-TCB,
le 1,3,5-TCB et le 1,2,4,5-TeCB sont tous peu toxiques pour les
micro-organismes, la toxicité du TCB et des TeCBs est, à l'exception
du 1,2,4,5-TeCB, plus élevée que celle des autres composés; chez les
algues unicellulaires, la CL50 à 96 heures (pour la croissance
cellulaire ou la production de chlorophylle) allait de plus de 300
mg/litre dans le cas du MCB à environ 1 mg/litre dans le cas du
1,2,3,5-TeCB. Certains invertébrés aquatiques paraissent plus
sensibles aux chlorobenzènes, toutefois les concentrations
nécessaires pour entraîner une mortalité à 48 ou 96 heures restent
encore proches de 1 mg/litre ou nettement supérieures (par exemple
2,4 mg/litre dans le cas du 1,2-DCB pour
Daphnia magna et jusqu'à 530 mg/litre dans le cas du
1,2,4,5-TeCB).
Les valeurs de la CL50 à 96 heures pour Lepomis machrochirus
allaient de 0,3 mg/litre dans le cas du TeCB à 24 mg/litre dans le
cas du MCB. L'étude de la toxicité chronique sur des stades
embryo-larvaires a donné des limites allant de 0,76 à 2,0 mg/litre
dans le cas des DCBs pour Pimephales promelas; de 0,22 et de 0,13
mg/litre respectivement dans le cas du 1,2,4-TCB et du 1,2,4,5-TeCB
pour un autre vairon des eaux estuarielles. Les stades les plus
sensibles dans le cas du MCB sont les alevins nouvellements éclos de
poissons rouges et de Micropterus salmoides, avec une CL50 à 96
heures respectivement égale à 1 et 0,05 mg/litre.
On ne dispose d'aucune donnée concernant les effets des
chlorobenzènes sur les organismes terrestres.
7. Effets sur les animaux d'experience et sur les systemes
in vitro
A quelques exceptions près, les chlorobenzènes ne présentent qu'une
toxicité aiguë modérée pour les animaux d'expérience et en général
la DL50 par voie orale est supérieure à 1000 mg/kg de poids
corporel; la DL50 dermique est plus élevée mais les données
disponibles à ce sujet sont limitées. L'ingestion de doses mortelles
entraîne une paralysie respiratoire, l'inhalation de fortes doses
entraînant une irritation locale et une dépression du système
nerveux central. Une intoxication aiguë par des doses non mortelles
de chlorobenzène entraîne des effets au niveau du foie, des reins,
des surrénales, des muqueuses, du cerveau ainsi que sur les enzymes
métabolisantes.
On n'a étudié l'irritation cutanée et oculaire due au chlorobenzène
que dans le cas du 1,2,4-TCB et du 1,2-DCB. Ces deux composés
causent une gêne très importante mais on n'a pas constaté de lésion
permanente après application directe dans l'oeil de lapins. Le
1,2,4-TCB est légèrement irritant pour la peau et peut provoquer une
dermatite par suite d'un contact répété ou prolongé. On n'a constaté
aucun signe de sensibilisation.
Des rats et des souris exposés pendant de courtes périodes (5 et 21
jours) à du MCB et des DCBs, à doses de plusieurs centaines de mg/kg
de poids corporel ont présenté des lésions hépatiques et des
anomalies hématologiques indiquant une atteinte de la moelle
osseuse. Après exposition de brève durée de rats et de lapins à
d'autres chlorobenzènes (TCD et PeCB) à des doses légèrement plus
faibles que celles de MCB et de DCB, ce sont les lésions hépatiques
qui constituaient le principal effet nocif constaté. Plusieurs des
isomères étudiés ont provoqué une porphyrie, les isomères porteurs
d'atomes de chlore en para, étant les plus actifs de ce point de
vue (c'est-à-dire 1,4-DCB, 1,2,4-TCB, 1,2,3,4-TeCB et PeCB). L'ordre
décroissant de toxicité générale dans le cas des TeCBs et du PeCB
après exposition de brève durée était le suivant : 1,2,4,5-TeCB
PeCB 1,2,3,4- et 1,2,3,5-TeCB, en bonne corrélation avec les
concentrations retrouvées dans les graisses et le foie.
Des études d'exposition au long cours (jusqu'à six mois) portant sur
plusieurs espèces d'animaux d'expérience ont fait ressortir une
tendance à l'augmentation de la toxicité avec le degré de chloration
du noyau benzénique. Toutefois pour un même dérivé on constatait des
variations importantes de cette toxicité selon l'isomère en cause.
Par exemple 1,4-DCB s'est révélé beaucoup moins toxique que le
1,2-DCB.
Il y avait une bonne corrélation entre la toxicité d'un composé
donné et son degré d'accumulation dans les tissus, les femelles
étant moins sensibles que les mâles. Les principaux organes cibles
étaient le foie et le rein; à dose plus élevée, on a signalé des
effets sur le système hématopoïetique et une toxicité thyroïdienne a
été observée dans les études portant sur le 1,2,4,5-TeCB et le PeCB.
Une étude de cancérogénicité portant sur le MCB a fait ressortir un
accroissement de l'incidence des nodules néoplasiques du foie dans
le groupe de rats mâles F344 soumis à la dose la plus élevée (120
mg/kg de poids corporel); toutefois on n'a pas noté d'accroissement
de l'incidence tumorale, lié au traitement, chez ces rats, mâles ou
femelles ou chez des souris femelles B6C3F1. Du 1,2-DCB a été
administré à des rats F344 mâles et femelles et à des souris B6C3F1
(60 ou 120 mg/kg de poids corporel), sans qu'on puisse observer de
signe de cancérogénicité.
Lors d'une étude de cancérogénicité portant le 1,4-DCB, on a noté un
accroissement, lié à la dose, de la fréquence des adénocar-cinomes
des tubules rénaux chez des rats mâles F344 ainsi qu'une
augmentation de celle des carcinomes et des adénomes
hépatocellulaires chez des souris B6C3F1 des deux sexes. Chez des
rats Wistar mâles et femelles ainsi que des souris Swiss femelles on
n'a noté aucun signe de cancérogénicité après exposition de ces
animaux par inhalation à des doses légèrement plus élevées de
1,4-DCB (dose estimée à 400 mg/kg par jour pour les rats et à 790
mg/kg par jour pour les souris) pendant des périodes plus courtes.
Toutefois les données disponibles indiquent que l'induction de
tumeurs rénales par le 1,4-DCB chez les rats F344 mâles et la
néphropathie grave avec formation d'inclusions hyalines, qui lui
sont associées, constituent des réactions spécifiques de l'espèce et
du sexe, liées à la réabsorption de l'alpha-2-microglobuline.
Les données disponibles sont insuffisantes pour qu'on puisse évaluer
le pouvoir cancérogène des chlorobenzènes fortement chlorés (tri,
tétra et pentachlorobenzènes).
On ne dispose que de données limitées résultant d'expériences
in vitro et in vivo, à propos des isomères autres que le
1,4-DCB, toutefois les chlorobenzènes ne semblent pas être
mutagènes. Sur la base de données plus nombreuses concernant le
1,4-DCB, on peut conclure que ce composé n'a aucun pouvoir mutagène
in vivo ou in vitro.
Rien n'indique non plus que les chlorobenzènes soient tératogènes
pour le rat ou le lapin. L'administration de MCB et de DCBs à des
rats ou des lapins par la voie respiratoire à une concentration
supérieure à 2000 mg/m3 (environ 550 mg/kg de poids corporel et
par jour) et, par voie orale, à des concentrations supérieures à
500 mg/kg de poids corporel, a entraîné des effets embryotoxiques et
fétotoxiques minimes. Toutefois ces doses étaient nettement toxiques
pour les femelles gravides. Selon certains indices, les TCBs, les
TeCBs et le PeCB seraient embryotoxiques et fétotoxiques à des doses
non toxiques pour les femelles gravides, cependant les données
disponibles ne sont pas cohérentes.
8. Effets sur l'homme
8.1 Population generale
Les rapports dont on dispose sur les effets des chlorobenzènes dans
la population générale se limitent à des rapports ponctuels sur
des cas d'accidents ou d'erreurs de manipulations de composés
contenant des chlorobenzènes peu substitués (MCB, 1,2-DCB, 1,4-DCB
ainsi qu'un isomère non précisé du TCB). On n'a pratiquement aucun
renseignement sur les doses, la pureté chimique, les relations
doses/temps et les effets observés, tels qu'une leucémie
myéloblastique, une rhinite, une glomérulonéphrite, une
granulomatose pulmonaire, des sensations vébrieuses, des
tremblements, de l'ataxie, une polynévrite et un ictère - tous
effets qui ne peuvent être évalués quantitativement.
Aucune étude épidémiologique concernant les effets des
chlorobenzènes sur la santé au sein de la population générale n'a
été rapportée.
8.2 Exposition professionnelle
Au cours de la fabrication et de l'utilisation du chlorobenzène, on
peut observer la symptomatologie suivante qui résulte d'une
exposition excessive : effets sur le système nerveux central et
irritation des yeux et des voies respiratoires supérieures (MCB);
troubles hématologiques (1,2-DCB); effets sur le système nerveux
central, durcissement de l'épiderme et troubles hématologiques -
notamment anémie (1,4-DCB). Toutefois ces symptômes n'ont été
décrits que lors de cas d'intoxications et sont difficiles à évaluer
quantitativement car on ne possède guère de renseignements sur les
concentrations réelles, la pureté chimique ou les relations
dose/temps.
Peu d'études é pidémiologiques ont été consacrées à l'exposition des
travailleurs aux chlorobenzènes; elles ne portent que sur le MCB,
1,2-DCB, 1,4-DCB et le 1,2,4,5-TeCB. On a signalé des effets sur le
système nerveux, sur le développement néonatal et sur la peau après
exposition au MCB mais les trois études en cause ne permettaient pas
d'évaluer le risque pour des raisons d'ordre méthodologique qui
tenaient notamment à l'évaluation de l'exposition, au fait que
l'exposition n'était pas uniforme et qu'il n'y avait pas de groupe
témoin. On peut émettre des critiques analogues à l'encontre de
l'étude consacrée au 1,4-DCB, étude au cours de laquelle on a
observé une irritation des muqueuses oculaires et nasales ainsi que
de l'étude faisant état d'aberrations chromosomiques à la suite
d'une exposition à des doses non précisées de 1,2-DCB et
1,2,4,5-TeCB.
9. Conclusions
Dans la mesure où les opérations industrielles sont effectuées
conformément aux normes de bonne pratique, les risques qu'impliq ue
une exposition professionnelle au chlorobenzène peuvent être
considérés comme minimes. A l'heure actuelle, les concentrations de
chlorobenzène dans l'environnement ne font courir qu'un risque
minimum à la population générale sauf dans le cas d'erreurs de
manipulation de produits à base de chlorobenzène ou de leur rejet
inconsidéré dans le milieu ambiant. Cependant cette évaluation se
base sur des données de surveillance limitées et il faudrait
disposer de renseignements complémentaires pour confirmer cette
conclusion. Il faudrait cependant envisager de réduire l'usage et
les rejets de chlorobenzène pour les raisons suivantes:
a) Les chlorobenzènes peuvent servir de précurseurs dans la
formation de dibenzodioxines polychlorés et de dibenzofuranes
polychlorés, par exemple lors de l'incinération de déchets.
b) Ces produits peuvent communiquer un goût ou une odeur
déplaisants à l'eau de boisson et au poisson.
c) Des résidus persistent en condition d'anaérobiose dans les
sédiments, des sols et les eaux souterraines riches en matières
organiques.
Pour la plupart des chlorobenzènes, l'évaluation du risque repose
sur leurs effets non tumorigènes. Toutefois, on a tenu compte des
effets tumorigènes lors de l'évaluation du risque dans le cas du MCB
et du 1,4-DCB. Les données disponibles montrent que la fréquence
accrue de tumeurs rénales chez des rats exposés à du 1,4-DCB
constituent une réaction typique de cette espèce, liée au sexe, qui
ne saurait être extrapolée à l'homme. En se basant sur le fait
qu'après exposition à du 1,4-DCB, il y a accroissement de la
réplication de l'ADN dans le foie de souris et accroissement de la
fréquence des adénomes et des carcinomes hépatocellulaires chez ces
mêmes animaux, on peut penser que ce produit est susceptible de se
comporter comme un cancérogène non génotoxique dans le foie des
rongeurs. La fréquence accrue de nodules hépatiques néoplasiques
observée dans un groupe de rats mâles soumis à une dose élevée de
MCB lors d'une étude de cancérogénicité indique également que ce
dernier produit peut se comporter comme un cancérogène non
génotoxique.
RESUMEN
La presente publicación se centra en los riesgos que tiene para la
salud humana y el medio ambiente la exposición a los siguientes
compuestos: monoclorobenceno (MCB), diclorobencenos (DCB),
triclorobencenos (TCB); tetraclorobencenos (TeCB) y
pentacloro-benceno (PeCB). La sustitución del cloro se indica de la
manera siguiente: 1,2-diclorobenceno (1,2-DCB);
1,2,3-triclorobenceno (1,2,3-TCB), etc.
1 Identidad, propiedades fisicas y quimicas y metodos analiticos
Los clorobencenos son compuestos aromáticos cíclicos formados por la
adición de 1 a 6 átomos de cloro al anillo de benceno. De esta
manera se obtienen 12 compuestos: monoclorobenceno, tres formas
isómeras de di-, tri- y tetraclorobencenos, así como penta- y
hexaclorobencenos.
Los clorobencenos son sólidos cristalinos de color blanco a
temperatura ambiente, excepto el MCB, el 1,2-DCB, el 1,3-DCB y el
1,2,4-TCB, que son líquidos incoloros. La solubilidad de los
clorobencenos en agua es en general baja, disminuyendo al aumentar
el número de átomes de cloro. La inflamabilidad es escasa, los
coeficientes de reparto octanol/agua son de moderados a altos y
aumentan con el número de átomes de cloro, y las presiones de vapor
son de bajas a moderadas, disminuyendo al aumentar el grado de
cloración. El umbral de sabor y olor son bajos, en particular para
los compuestos menos clorados.
Los clorobencenos comerciales, incluso los purificados, contienen
distintas cantidades de isómeros estrechamente relacionados. Por
ejemplo, el MCB puro puede contener hasta un 0,05% de benceno y un
0,1% de DCB, mientras que el 1,2-DCB de calidad técnica puede
contener hasta un 19% de los otros DCB, un 1% de TCB y hasta un
0,05% de MCB. No se ha informado de la existencia de pruebas de
contaminación por dibenzo- p-dioxinas policloradas (DDPC) y
dibenzofuranos policlorados (DFPC).
Se han puesto a punto, en función del medio, un gran número de
técnicas de muestreo para clorobencenos. Abarcan desde los métodos
de extracción con disolventes para los medios acuosos hasta el uso
de absorbentes para los compuestos presentes en el aire. La técnica
analítica de elección para la determinación de clorobencenos en
muestras obtenidas del medio ambiente es la cromatografía
gas-líquido (CGL).
2 Fuentes de exposicion humana y ambiental
2.1 Cifras de produccion
Los datos disponibles sobre las cifras de producción de
clorobencenos corresponden al período 1980-83, cuando la producción
mundial se estimaba en 568 x 106 kg, aunque la utilización de
clorobencenos ha disminuido posteriormente en algunos países.
Alrededor del 50% de esta cantidad se fabricaba en los Estados
Unidos, y la mayor parte del resto en Europa occidental y el Japón.
El 70% de la producción mundial era de MCB, y de 1,2-DCB, 1,4-DCB y
1,2,4-TCB se producían respectivamente 22 x 106, 4 x 106 y 1,2-3,7 x
106 kg.
El MCB y el DCB se obtienen por cloración directa del benceno en la
fase líquida utilizando un catalizador, mientras que los TCB y los
TeCB se producen mediante la cloración directa de los isómeros de
clorobenceno apropiados en presencia de un catalizador metálico.
2.2 Aplicaciones
Los clorobencenos se utilizan sobre todo como intermediarios en la
síntesis de plaguicidas y de otros productos químicos; el 1,4-DCB se
usa en los desodorante para ambientes cerrados y como repelente de
las polillas. Los bencenos con mayor número de átomos de cloro (TCB
y 1,2,3,4-TeCB) se han empleado como componentes de fluidos
dieléctricos.
2.3 Liberacion de clorobencenos en el medio ambiente
La liberación de clorobencenos en el medio ambiente tiene lugar
fundamentalmente durante la fabricación y por la naturaleza
dispersiva de sus aplicaciones. En los Estados Unidos, por ejemplo,
se estima que de las 130 x 106 toneladas de MCB producidas en 1983
se perdió en el medio ambiente el 0,1-0,2%. La liberación de
clorobencenos a partir de la eliminación de residuos, inclusive la
incineración de vertidos municipales, es mucho más baja. Sin
embargo, la incineración de clorobencenos puede producir emisiones
de DDPC y DFPC.
3 Transporte, distribucion y transformacion en el medio
ambiente
3.1 Degradacion
La eliminación de los clorobencenos del medio ambiente se efectúa
principalmente mediante mecanismos biológicos y, en menor medida,
por otros sistemas; sin embargo, se considera que su presencia es
moderadamente persistente en el agua, el aire y los sedimentos. Se
ha informado que su tiempo de permanencia en el agua es de un día en
la de río y de más de 100 días en la subterránea. En el aire, parece
que las vías predominantes de degradación del clorobenceno son las
reacciones químicas y fotolíticas, con tiempos de permanencia que
para el MCB, los DCB y un isómero sin especificar del TCB se ha
informado que oscilan entre 13 y 116 días. Se ha demostrado que
muchos microorganismos presentes en los sedimentos y los fangos
cloacales degradan los clorobencenos. Parece ser que los compuestos
con mayor número de átomos de cloro se descomponen menos fácilmente,
y tal proceso sólo tiene lugar en condiciones aerobias. El DCB, los
TCB y los PeCB presentes en el suelo y en el agua subterránea en
condiciones anaerobias suelen resistir la degradación microbiana.
3.2 Destino final
Los clorobencenos que se liberan en el medio acuático se
redistribuyen de manera preferente en el aire y en los sedimentos
(sobre todo en los ricos en materia orgánica). Son limitadas las
informaciones según las cuales en los sedimentos se han detectado
niveles 1000 veces superiores a los del agua, en particular en
regiones muy industrializadas. La retención de clorobencenos en el
suelo aumenta con el contenido de éste en materia orgánica; existe
una correlación positiva entre el grado de cloración del compuesto y
su adsorción a la materia orgánica. Hay pruebas limitadas que
indican que los residuos unidos a los sedimentos están
biodisponibles para los organismos, es decir, que los invertebrados
acuáticos pueden captar residuos de los sedimentos, y las plantas,
del suelo.
4 Niveles medio ambientales y exposicion humana
4.1 Clorobencenos en el medio ambiente
Los niveles medios de clorobencenos (de mono- a tri-) en el aire del
medio ambiente son del orden de 0,1 µg/m3, con niveles máximos de
hasta 100 µg/m3. No se dispone de datos sobre los niveles de TeCB
y PeCB en el aire, aunque estos compuestos químicos se han detectado
en cenizas volantes procedentes de incineradores municipales. Los
niveles de clorobencenos en el aire de los espacios cerrados son
similares a los presentes en el aire exterior; sin embargo, se han
comunicado concentraciones muy superiores en zonas intensamente
contaminadas y en espacios cerrados donde se habían utilizado
productos con clorobenceno.
Se han detectado clorobencenos (de mono- a penta-) en aguas
superficiales con concentraciones del orden de ng/litro-µg/litro,
con niveles ocasionales de hasta décimas de mg/litro en las
cercanías de fuentes industriales. Los niveles de clorobencenos en
las aguas residuales industriales pueden ser más elevados y varían
en función de la naturaleza de los procesos utilizados.
En los análisis de muestras de agua de bebida se han detectado todos
los tipos de clorobencenos. Los compuestos menos clorados eran los
que se encontraban con mayor frecuencia y en concentraciones más
elevadas, con predominio del isómero 1,4-DCB; sin embargo, las
concentraciones medias de todos los clorobencenos detectados han
sido en general inferiores a 1 µg/litro y raramente han superado los
50 µg/litro.
No se han encontrado datos procedentes de programas de vigilancia
bien formulados sobre los niveles de clorobencenos en los alimentos;
la información disponible se ha limitado principalmente a las
concentraciones en los peces de zonas cercanas a fuentes
industriales y a casos aislados de contaminación de productos
cárnicos. En truchas de agua dulce se detectaron todos los isómeros
del clorobenceno (de mono- a penta-), en concentraciones que
oscilaban entre 0,1 y 16 µg/kg. En otro estudio, los niveles totales
de clorobencenos en peces de agua dulce variaron de una media de 0,2
mg/kg de grasa en las zonas ligeramente contaminadas a 1,8 mg/kg de
grasa en una industrializada. Existen algunos indicios de que las
concentraciones de clorobencenos en los peces de agua dulce aumentan
a medida que es mayor el grado de cloración del producto. En los
escasos estudios disponibles de algunos peces marinos se señalan
niveles de 1,4-DCB de 0,05 mg/kg (peso fresco).
En los estudios conocidos sobre los niveles de clorobencenos en la
carne y en la leche, limitados principalmente a muestras procedentes
de zonas contaminadas, se han comunicado concentraciones de 0,02-5
µg/kg.
En dos estudios de la leche materna se determinaron
cuantitativamente las concentraciones de todos los clorobencenos,
excepto el MCB. En el primero, el promedio de los niveles de DBC era
de 25 µg/kg de leche, mientras que los valores medios relativos a
los isómeros del TCB y el TeCB y al PeCB fueron inferiores a 5 µg/kg
de leche. Los niveles en el segundo estudio fueron mucho más bajos,
con concentraciones medias que oscilaban entre 1 µg/kilo (1,2,3-TCB
y PeCB) y un máximo de 6 µg/kg (1,3- y 1,4-diclorobenceno).
4.2 Exposicion humana
4.2.1 Poblacion general
De acuerdo con los limitados datos disponibles, la ingestión diaria
de clorobencenos por la población general procede en su mayor parte
del aire, en particular de los compuestos inferiores, más volátiles
(0,2-0,9 µg/kg de peso corporal). La ingestión a partir de los
alimentos en comparación con otras fuentes aumenta al elevarse el
grado de cloración; Los alimentos contribuyen a un porcentaje más
alto de la ingestión diaria total de TeCB y PeCB que el aire. Sin
embargo, los niveles de exposición para este tipo de compuestos son
probablemente inferiores a 0,05 µg/kg de peso corporal. En un número
limitado de estudios se ha puesto de manifiesto que, teniendo en
cuenta el peso corporal, los lactantes alimentados con leche materna
pueden recibir una dosis más alta de clorobencenos que las personas
adultas.
4.2.2 Profesional
Con los datos de que se dispone no es posible cuantificar con
exactitud la exposición profesional a los clorobencenos. Sin
embargo, en una fábrica se encontraron niveles de 1,4-DCB que
oscilaban entre 42 y 288 mg/m3, y en otras instalaciones químicas
las concentraciones de MCB eran de hasta 18,7 mg/m3.
5 Cinetica y metabolismo
Parece que todos los clorobencenos se absorben fácilmente de los
tractos gastrointestinal y respiratorio en el hombre y en los
animales de experimentación; influye en la absorción la posición de
los átomos de cloro en los distintos isómeros del mismo compuesto.
Los clorobencenos se absorben menos fácilmente a través de la piel.
En los animales de experimentación, tras una rápida distribución en
órganos con elevada perfusión, los clorobencenos absorbidos se
acumulan sobre todo en el tejido adiposo, con cantidades menores en
el hígado y en otros órganos. Se ha demostrado que atraviesan la
placenta, y se han detectado en el cerebro del feto. En general, la
acumulación es mayor en el caso de los compuestos más clorados. Sin
embargo, hay una considerable diferencia de acumulación entre los
distintos isómeros del mismo compuesto.
El metabolismo de los clorobencenos en la especie humana y en los
animales de experimentación sigue la vía de la oxidación microsómica
para formar el clorofenol correspondiente. Estos clorofenoles se
pueden excretar en la orina en forma de ácidos mercaptúricos, o bien
como ácido glucurónico o conjugados de sulfato. El TeCB y el PeCB se
metabolizan a menor velocidad y permanecen en los tejidos durante
más tiempo que el grupo de los monocloro- a los triclorobencenos.
Algunos de estos clorobencenos inducen una amplia gama de sistemas
enzimáticos, entre ellos los que participan en las vías de
oxidación, reducción, conjugación e hidrólisis.
En general, la eliminación de los bencenos con mayor grado de
cloración es más lenta que la de los MCB y DCB, y es mayor la
proporción de los compuestos tri-, tetra y pentaclorobencenos que se
eliminan inalterados en las heces. Por ejemplo, el 17% de una dosis
de 1,2,4-TCB se eliminó en las heces al cabo de 7 días, mientras que
el 91-97% del 1,4-DCB se excretó en la orina en forma de metabolitos
a los 5 días. La posición de los átomos de cloro en el anillo
bencénico es también un factor importante del que depende la
velocidad de metabolización y eliminación; los isómeros con dos
átomos de carbono adyacentes sin sustituir se metabolizan y eliminan
más rápidamente.
6. Efectos en los organismos acuaticos del medio ambiente
La información disponible acerca de los efectos de los clorobencenos
en el medio ambiente se centra principalmente en sus efectos agudos
sobre los animales acuáticos. En general, la toxicidad aumenta con
el grado de cloración del anillo bencénico. Mientras que los MCB,
1,2-DCB, 1,3-DCB, 1,2,4-TCB, 1,3,5-TCB y 1,2,4,5-TeCB presentan una
baja toxicidad para los microorganismos, la de los TCB y TeCB es, a
excepción del 1,2,4,5-TeCB, ligeramente superior a la de los otros
compuestos; en algas unicelulares acuáticas, los valores de la CE50
para el crecimiento celular o la producción de clorofila a en 96
horas variaron de más de 300 mg/litro para el MCB hasta
aproximadamente 1 mg/litro para el 1,2,3,5-TeCB. Algunos
invertebrados acuáticos muestran mayor sensibilidad a los
clorobencenos, pero los niveles necesarios para la letalidad en 48 ó
96 horas son todavía próximos, o muy superiores, a 1 mg/litro (por
ejemplo, para Daphnia magna son de 2,4 mg/litro en el caso del
1,2-DCB y de hasta 530 mg/litro en el del 1,2,4,5-TeCB).
La CL50 a las 96 horas para Lepomis macrochirus osciló entre 0,3
mg/litro para el PeCB y 24 mg/litro para el MCB. En ensayos con
embriones-larvas, los límites de toxicidad crónica para los DCB
oscilaron entre 0,76 y 2,0 mg/litro para Pimephales promelas; en
la variedad de Aplodinotus grunniens de los estuarios, los límites
de la toxicidad crónica para el 1,2,4-TCB y 1,2,4,5-TeCB fueron de
0,22 y 0,13 mg/litro respectivamente. En el caso de Carassius
auratus y de la perca atruchada, los alevines recién nacidos
constituyeron la etapa vital m s sensible, con CL50 (a las 96 h)
de 1 y 0,05 mg/litro, respectivamente, para el MCB.
No se conocen datos sobre los clorobencenos en los sistemas
terrestres.
7. Efectos en los animales de experimentacion y en los
sistemas in vitro
Salvo algunas excepciones, los clorobencenos son sólo moderadamente
tóxicos para los animales de experimentación en cuanto a la
toxicidad aguda, y en general la DL50 por vía oral es superior a
los 1000 mg/kg de peso corporal; según los limitados datos
disponibles, la DL50 por vía cutánea es más alta. La ingestión de
una dosis letal produce parálisis respiratoria, mientras que la
inhalación de dosis elevadas causa irritación local y depresión del
sistema nervioso central. La exposición aguda a dosis no letales de
clorobencenos induce efectos tóxicos en el hígado, los riñones, las
glándulas suprarrenales, las adrenales membranas mucosas y el
cerebro, y tiene efectos sobre las enzimas metabolizantes.
Los estudios sobre la irritación de la piel y de los ojos ocasionada
por los clorobencenos se han limitado al 1,2,4-TCB y al 1,2-DCB.
Ambos producen graves molestias, pero no se observaron lesiones
permanentes tras su aplicación directa a los ojos de conejos. El
1,2,4-TCB es ligeramente irritante para la piel, pudiendo ocasionar
dermatitis por contacto repetido o prolongado. No se encontraron
pruebas de sensibilización.
La exposición breve (5-21 días) de ratas y ratones al MCB y a los
DCB en concentraciones del orden de cientos de mg/kg de peso
corporal se tradujo en lesiones hepáticas y en cambios hemáticos
indicativos de lesiones en la médula ósea. Las lesiones hepáticas
fueron también el principal efecto nocivo observado tras la
exposición breve de ratas y ratones a otros clorobencenos
(TCB-PeCB), en dosis ligeramente menores que las utilizadas con el
MCB y los DCB. Varios de los isómeros estudiados indujeron porfiria,
siendo los más activos los que tienen los átomos de cloro en
posición para (es decir, 1,4-DCB, 1,2,4-TCB, 1,2,3,4-TeCB y PeCB).
El orden general de toxicidad observado para los TeCB y el PeCB tras
una exposición breve fue el siguiente: 1,2,4,5-TeCB PeCB 1,2,3,4- y
1,2,3,5-TeCB, lo que guarda una correlación adecuada con los niveles
encontrados en la grasa y en el hígado.
Los estudios de exposición a largo plazo (hasta seis meses) en
varias especies de animales de experimentación pusieron de
manifiesto que la toxicidad de los clorobencenos tendía a aumentar
con el grado de cloración del anillo. Sin embargo, fue considerable
la variación de la toxicidad a largo plazo de los distintos isómeros
de un mismo compuesto. Por ejemplo, el 1,4-DCB demostró ser mucho
menos tóxico que el 1,2-DCB. Se observó una correlación positiva
entre la toxicidad y el grado de acumulación del compuesto en los
tejidos, siendo las hembras menos sensibles que los machos. Los
órganos más afectados fueron el hígado y los riñones; con dosis más
elevadas se describieron efectos en el sistema hematopoyético, y en
estudios con el 1,2,4,5-TeCB y el PeCB se observó toxicidad en el
tiroides.
En un bioensayo para determinar la carcinogenicidad del MCB, aumentó
la frecuencia de nódulos neoplásicos hepáticos en el grupo de dosis
alta (120 mg/kg de peso corporal) de ratas macho F344, pero no hubo
aumento de la incidencia de tumores asociada con el tratamiento en
ratas hembra F344 o en ratones macho o hembra B6C3F1. No se
obtuvieron pruebas de la carcinogenicidad del 1,2-DCB en ratas
machos o hembras F344 ni en ratones B6C3F1 (60 ó 120 mg/kg de peso
corporal).
En un bioensayo para determinar la carcinogenicidad del 1,4-DCB, se
registró un incremento de la formación de adenocarcinomas de las
células de los túbulos renales relacionado con la dosis en ratas
macho F344 y un aumento de los carcinomas y adenomas hepatocelulares
en ambos sexos de ratones B6C3F1. No se comunicaron signos de
carcinogenicidad en ratas Wistar macho y hembra, ni en hembras de
raton suizo tras la inhalación de dosis ligeramente más altas de
1,4-DCB (estimadas en 400 mg/kg al día para las ratas y 790 mg/kg al
día para los ratones) durante períodos más cortos. Sin embargo, los
datos disponibles indican que la inducción de tumores renales por el
1,4-DCB en ratas macho F344 y la inducción asociada de nefropatia
grave y de la formación de gotitas hialinas son respuestas
específicas de la especie y del sexo, asociadas a la reabsorción de
alfa-2-microglobulina.
Los datos disponibles son insuficientes para valorar la
carcinogenicidad de los bencenos con mayor grado de cloración (de
tri- a penta-).
Aunque los datos obtenidos en ensayos in vitro e in vivo para
isómeros distintos del 1,4-DCB son limitados, los clorobencenos no
parecen tener efectos mutagénicos. Teniendo en cuenta la base de
datos más amplia del 1,4-DCB, se puede concluir que este compuesto
carece de potencial mutagénico, tanto in vivo como in vitro.
No hay pruebas de teratogenicidad de los clorobencenos en ratas y en
conejos. La administración de MCB y de distintos DCB a ratas o
conejos por vía respiratoria en concentraciones superiores a
2000 mg/m3 (unos 550 mg/kg de peso corporal al día) y por vía oral
a concentraciones de >500 mg/kg de peso corporal dio como resultado
efectos embriotóxicos y fetotóxicos de poca consideración. Sin
embargo, esas dosis fueron claramente tóxicas para la madre. Aunque
hay algunas pruebas de embriotoxicidad y fetotoxicidad de los TCB,
los TeCB y los PeCB en dosis que no son tóxicas para la madre, los
datos disponibles son contradictorios.
8. Efectos en el ser humano
8.1 Poblacion general
Los informes acerca de los efectos de los clorobencenos en la
población general se limitan a los casos notificados de accidentes
y/o de uso indebido de productos con los bencenos menos clorados
(MCB, 1,2-DCB, 1,4-DCB y un isómero de TCB sin especificar). Se
dispone de información escasa o nula sobre las dosis, la pureza
química o las relaciones dosis:tiempo y no es posible determinar
cuantitativamente los efectos observados, como leucemia
mieloblástica, rinitis, glomerulonefritis, granulomatosis pulmonar,
mareo, temblor, ataxia, polineuritis e ictericia.
No se han comunicado de estudios epidemiológicos sobre los efectos
de los clorobencenos en la salud de la población general.
8.2 Exposicion profesional
Entre los síntomas y los signos clínicos derivados de la exposición
excesiva durante la fabricación y el uso de los clorobencenos cabe
mencionar los siguientes: efectos en el sistema nervioso central
(SNC) e irritación de los ojos y del tracto respiratorio superior
(MCB); trastornos hematológicos (1,2-DCB); y efectos en el SNC,
endurecimiento de la piel y trastornos hematológicos, incluso anemia
(1,4-DCB). Sin embargo, tales síntomas proceden sólo de informes de
casos aislados y son difíciles de determinar cuantitativamente,
puesto que se dispone de escasa información relativa a los niveles
reales, la pureza química o las relaciones dosis:tiempo.
Los pocos estudios epidemiológicos sobre trabajadores expuestos a
clorobencenos que se han publicado se refieren solamente al MCB, el
1,2-DCB, el 1,4-DCB y el 1,2,4,5-TeCB. Aunque se ha informado de
efectos en el sistema nervioso, en el desarrollo neonatal y en la
piel tras la exposición al MCB, ninguno de los tres estudios fue
suficiente para evaluar el riesgo, a causa de problemas
metodo-lógicos, como la valoración de la exposición, las
exposiciones mixtas y la ausencia de grupos testigo. Una crítica
parecida merece el estudio sobre el 1,4-DCB, en el que se informó de
irritación ocular y nasal, así como el estudio en el que se
describieron aberraciones cromosómicas como consecuencia de la
exposición a niveles no especificados de 1,2-DCB y 1,2,4,5-TeCB.
9 Conclusiones
Si se aplica una práctica industrial correcta, los riesgos asociados
con la exposición profesional a los clorobencenos se pueden
considerar mínimos. La evaluación del riesgo en la actualidad pone
de manifiesto también que las concentraciones actuales de
clorobencenos en el medio ambiente representan un riesgo
insignificante para la población general, excepto en el caso de uso
indebido de productos que contienen clorobencenos o de su liberación
incontrolada en el medio ambiente. Sin embargo, esta valoración se
basa en datos limitados de vigilancia, y se necesita más información
para justificar esta conclusión. Se debería considerar, sin embargo,
la posibilidad de reducir el uso y la eliminación generalizados de
clorobencenos por los siguientes motivos:
a) Los clorobencenos pueden actuar como precursores de la
formación de dibenzodioxinas policloradas/dibenzofuranos
policlorados (DDPC/DFPC), por ejemplo en los procesos de
incineración.
b) Estos productos químicos pueden ocasionar alteraciones del
sabor y el olor del agua de bebida y del pescado.
c) Los residuos persisten en los sedimentos y suelos anaerobios
ricos en materia orgánica y en el agua subterránea.
En la mayor parte de los clorobencenos, la evaluación del riesgo se
ha basado en efectos no neoplásicos. Sin embargo, los efectos
neoplásicos se tuvieron en cuenta en la valoración del riesgo del
MCB y el 1,4-DCB. Los datos disponibles indican que el aumento
observado de los tumores renales en ratas debido al 1,4-DCB es una
respuesta específica de la especie y del sexo, probablemente sin
importancia para la especie humana. De acuerdo con las pruebas de
aumento de la replicación del ADN en el hígado de ratón y la mayor
incidencia de adenomas y carcinomas hepato-celulares en ratones, el
1,4-DCB puede actuar como agente carcinógeno no genotóxico en el
hígado de los roedores. La mayor incidencia de nódulos neoplásicos
hepáticos observada en el grupo de ratas macho que recibió dosis
altas en un bioensayo de carcinogenicidad indica que también el MCB
puede ser un agente carcinógeno no genotóxico.
 Hexachlorobenzene is the subject of a separate Environmental Health
Criteria publication and will not be evaluated here.
2.1.2 Technical product
There are no widely established trade specifications for commercial
chlorobenzenes. Pure commercial monochlorobenzene may contain 0.05 %
or less of benzene and up to 0.1 % of dichlorobenzenes. Technical
grade 1,2-dichlorobenzene contains up to 19 % of the other 2
dichlorobenzene isomers, 1 % of trichlorobenzenes, and up to 0.05 % of
monochlorobenzene, while purified 1,2-dichlorobenzene contains up to
0.05 % of monochlorobenzene and 0.2 % of 1,2,4-trichlorobenzene.
Technical grade 1,4-dichlorobenzene contains up to a total of 0.1 % of
mono- and trichlorobenzenes and 0.5 % of each of the other
dichlorobenzene isomers. Commercial 1,2,4-trichlorobenzene may contain
up to 0.1 % of mono-chlorobenzene, 0.5 % of dichlorobenzenes, and
0.5 % of tetrachlorobenzenes (Kao & Poffenberger, 1979).
Polychlorinated dibenzodioxins or dibenzofurans were not detected in
trichlorobenzenes, tetrachlorobenzenes, or penta-chlorobenzene (Buser,
1979).
2.2 Physical and Chemical Properties
The physical and chemical properties of the chlorobenzenes (mono- to
penta-) are presented in Table 2.
MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB are colourless liquids, while all
other congeners are white crystalline solids at room temperature. In
general, the solubility of chlorobenzenes in water is poor (decreasing
with increasing chlorination), flammability is low, the octanol/water
partition coefficients are moderate to high (increasing with
increasing chlorination), and vapour pressures are low to moderate
(decreasing with increasing chlorination).
2.3 Organoleptic Properties
The odour and taste thresholds for different isomers of the same
chlorobenzene appear to be similar: 0.01-0.02 mg/litre for MCB and
0.001-0.002 mg/litre for both 1,2-DCB and 1,4-DCB (Varshavskaya,
1968). Piet et al. (1980) reported that the odour thresholds for 1,2-
and 1,4-dichlorobenzenes in Rhine tap water were 10 and 0.3 µg/litre
respectively, while 1,2,4-TCB was detected at a level of 5 µg/litre.
Using available experimental data, Amoore & Hautala (1983) determined
water-dilution odour thresholds for MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB to be 0.050, 0.024, 0.011, and 0.064 mg/litre (ppm),
respectively. Odour thresholds in air for these compounds are 0.68,
0.30, 0.18, and 1.4 µlitre/litre (ppm), respectively. Fomenko (1965)
reported that the thresholds for smell and taste for 1,2,4,5-TeCB were
0.006 mg/litre and 0.0064 mg/litre, respectively.
2.4 Conversion Factors
At 25 °C and 101.3 kPa, the conversion factors for chlorobenzenes in
air are as follows:
monochlorobenzene: 1 ppm=4.55 mg/m3: 1 mg/m3=0.22 ppm
dichlorobenzenes: 1 ppm=6.00 mg/m3: 1 mg/m3=0.17 ppm
trichlorobenzenes: 1 ppm=7.42 mg/m3: 1 mg/m3=0.13 ppm
tetrachlorobenzenes: 1 ppm=8.83 mg/m3: 1 mg/m3=0.11 ppm
pentachlorobenzene: 1 ppm=10.24 mg/m3: 1 mg/m3=0.10 ppm
Table 1. Information on the identity of chlorobenzenes
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
Monochlorobenzene MCB C6H5Cl 112.6 chlorobenzene
(
Hexachlorobenzene is the subject of a separate Environmental Health
Criteria publication and will not be evaluated here.
2.1.2 Technical product
There are no widely established trade specifications for commercial
chlorobenzenes. Pure commercial monochlorobenzene may contain 0.05 %
or less of benzene and up to 0.1 % of dichlorobenzenes. Technical
grade 1,2-dichlorobenzene contains up to 19 % of the other 2
dichlorobenzene isomers, 1 % of trichlorobenzenes, and up to 0.05 % of
monochlorobenzene, while purified 1,2-dichlorobenzene contains up to
0.05 % of monochlorobenzene and 0.2 % of 1,2,4-trichlorobenzene.
Technical grade 1,4-dichlorobenzene contains up to a total of 0.1 % of
mono- and trichlorobenzenes and 0.5 % of each of the other
dichlorobenzene isomers. Commercial 1,2,4-trichlorobenzene may contain
up to 0.1 % of mono-chlorobenzene, 0.5 % of dichlorobenzenes, and
0.5 % of tetrachlorobenzenes (Kao & Poffenberger, 1979).
Polychlorinated dibenzodioxins or dibenzofurans were not detected in
trichlorobenzenes, tetrachlorobenzenes, or penta-chlorobenzene (Buser,
1979).
2.2 Physical and Chemical Properties
The physical and chemical properties of the chlorobenzenes (mono- to
penta-) are presented in Table 2.
MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB are colourless liquids, while all
other congeners are white crystalline solids at room temperature. In
general, the solubility of chlorobenzenes in water is poor (decreasing
with increasing chlorination), flammability is low, the octanol/water
partition coefficients are moderate to high (increasing with
increasing chlorination), and vapour pressures are low to moderate
(decreasing with increasing chlorination).
2.3 Organoleptic Properties
The odour and taste thresholds for different isomers of the same
chlorobenzene appear to be similar: 0.01-0.02 mg/litre for MCB and
0.001-0.002 mg/litre for both 1,2-DCB and 1,4-DCB (Varshavskaya,
1968). Piet et al. (1980) reported that the odour thresholds for 1,2-
and 1,4-dichlorobenzenes in Rhine tap water were 10 and 0.3 µg/litre
respectively, while 1,2,4-TCB was detected at a level of 5 µg/litre.
Using available experimental data, Amoore & Hautala (1983) determined
water-dilution odour thresholds for MCB, 1,2-DCB, 1,4-DCB, and
1,2,4-TCB to be 0.050, 0.024, 0.011, and 0.064 mg/litre (ppm),
respectively. Odour thresholds in air for these compounds are 0.68,
0.30, 0.18, and 1.4 µlitre/litre (ppm), respectively. Fomenko (1965)
reported that the thresholds for smell and taste for 1,2,4,5-TeCB were
0.006 mg/litre and 0.0064 mg/litre, respectively.
2.4 Conversion Factors
At 25 °C and 101.3 kPa, the conversion factors for chlorobenzenes in
air are as follows:
monochlorobenzene: 1 ppm=4.55 mg/m3: 1 mg/m3=0.22 ppm
dichlorobenzenes: 1 ppm=6.00 mg/m3: 1 mg/m3=0.17 ppm
trichlorobenzenes: 1 ppm=7.42 mg/m3: 1 mg/m3=0.13 ppm
tetrachlorobenzenes: 1 ppm=8.83 mg/m3: 1 mg/m3=0.11 ppm
pentachlorobenzene: 1 ppm=10.24 mg/m3: 1 mg/m3=0.10 ppm
Table 1. Information on the identity of chlorobenzenes
Compound Congener Molecular R.M.M.b Synonyms
(CAS number)a identification formula
Monochlorobenzene MCB C6H5Cl 112.6 chlorobenzene
(