
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 120
HEXACHLOROCYCLOPENTADIENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by D.T. Reisman,
US Environmental Protection Agency, Cincinnati, USA
World Health Orgnization
Geneva, 1991
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Hexachlorocyclopentadiene.
(Environmental health criteria ; 120)
1.Hydrocarbons, Chlorinated - adverse effects 2.Hydrocarbons,
Chlorinated - toxicity 3.Environmental exposure 4.Environmental
pollutants I.Series
ISBN 92 4 157120 9 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROCYCLOPENTADIENE
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.2.1. Physical properties
2.2.2. Chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Air
2.4.2. Water
2.4.3. Soil
2.4.4. Biological media
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Other sources of exposure
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Overview
4.2. Transport and distribution between media
4.2.1. Air
4.2.2. Water
4.2.3. Soil
4.3. Biotransformation
4.3.1. Biodegradation
4.3.2. Bioconcentration, bioaccumulation, and biomagnification
4.4. Interactions with other physical and chemical factors
4.4.1. Phototransformation
4.4.2. Oxidation
4.5. Disposal and fate
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption, retention, distribution, metabolism,
elimination, and excretion
6.1.1. Oral
6.1.2. Inhalation
6.1.3. Dermal
6.1.4. Comparative studies
6.1.5. In vitro studies
6.2. Metabolic transformation
6.3. Reaction with body components
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.2.1. Freshwater aquatic life
7.2.2. Marine and estuarine aquatic life
7.3. Terrestrial organisms and wildlife
7.4. Population and ecosystem effects
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Acute toxicity studies
8.1.1. Acute oral, inhalation, and dermal toxicity
8.1.2. Eye and skin irritation
8.2. Short-term exposure
8.2.1. Oral
8.2.2. Short-term inhalation toxicity
8.2.3. Short-term dermal toxicity
8.3. Long-term exposure
8.3.1. Long-term oral toxicity
8.3.2. Long-term inhalation toxicity
8.3.3. Long-term dermal toxicity
8.3.4. Principal effects and target organs
8.4. Developmental and reproductive toxicity
8.5. Mutagenicity
8.6. Cell transformation
8.7. Carcinogenicity
9. EFFECTS ON HUMANS
9.1. General population exposure
9.2. Occupational exposure
9.3. Epidemiological studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations for protection of human health and the
environment
12. FURTHER RESEARCH
REFERENCES
APPENDIX 1
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR
HEXACHLOROCYCLOPENTADIENE
Members
Dr K. Abdo, National Institute of Environmental Health
Sciences, Division of Toxicology Research and Testing,
Research Triangle Park, North Carolina, USA
Professor C. Scott Clark, Department of Environmental
Health, University of Cincinnati, Cincinnati, Ohio,
USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom
Dr S.K. Kashyap, National Institute of Occupational
Health, Indian Council of Medical Research, Meghani
Nagar, Ahmedabad, India
Dr F. Matsumura, Department of Environmental Toxicology,
University of California, Davis, California, USA
Mr G. Welter, German Federal Environmental Protection
Agency, Berlin, Germany
Dr J. Withey, Environmental and Occupational Toxicology
Division, Environmental Health Centre, Tunney's
Pasture, Ottawa, Ontario, Canada (Chairman)
Dr Shou-zheng Xue, Department of Occupational Health,
School of Public Health, Shanghai Medical University,
Shanghai, China
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
(Secretary)
Mr D.J. Reisman, Environmental Criteria and Assessment
Office, US Environmental Protection Agency, Cincinnati,
Ohio, USA (Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria monographs as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria monographs,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemical, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 or 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR HEXACHLOROCYCLOPENTADIENE
A WHO Task Group on Environmental Health Criteria for
Hexachlorocyclopentadiene met in Cincinnati, USA, from 30
July to 3 August 1990. Dr Chris DeRosa opened the meeting
on behalf of the US Environmental Protection Agency in
Cincinnati. Dr B.H. Chen of the International Programme
on Chemical Safety (IPCS) welcomed the participants on
behalf of the Manager, IPCS, and the three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evalu-
ation of the risks for human health and the environment
from exposure to hexachlorocyclopentadiene.
The first draft of this monograph was prepared by Mr
D.J. Reisman of the US Environmental Protection Agency.
The second draft was also prepared by Mr Reisman, incor-
porating comments received following the circulation of
the first draft to the IPCS contact points for Environmen-
tal Health Criteria Monographs. Dr B.H. Chen and Dr P.G.
Jenkins, both members of the IPCS Central Unit, were
responsible for the overall scientific content and techni-
cal editing, respectively.
Financial support for the meeting was provided by the
US Environmental Protection Agency in Cincinnati.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
ABBREVIATIONS
ACGIH American Conference of Government Industrial Hygienists
BAF bioaccumulation factor
BCF bioconcentration factor
ECD electron capture detection
GC gas chromatography
HEX hexachlorocyclopentadiene
LAQL lowest analytically quantifiable level
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MS mass spectrometry
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
SD standard deviation
TWA time-weighted average
1. SUMMARY
Hexachlorocyclopentadiene (HEX) is a dense pale-yellow
or greenish-yellow, non-flammable liquid with a unique
pungent odour. It has a relative molecular mass of 272.77
and low solubility in water. HEX is highly reactive and
undergoes addition, substitution, and Diels-Alder reactions.
In the USA, the Velsicol Chemical Corporation is the
only company that currently produces HEX. In Europe, it
is produced by the Shell Chemical Corporation in the
Netherlands. Production data are proprietary, but it is
estimated that between 3600 and 6800 tonnes of HEX are
produced annually in the USA. In 1988, worldwide pro-
duction was approximately 15 000 tonnes (BUA, 1988).
Although HEX is used as an intermediate in the production
of many pesticides, some countries have restricted its use
in the production of certain organochlorine pesticides.
It is also used in the manufacture of flame retardants,
resins, and dyes.
During its manufacture and processing, small amounts
of HEX are released into the environment. It may also be
released when present as an impurity in some of the prod-
ucts for which it is an intermediate. HEX may be released
both during and after disposal. Only limited monitoring
data on the environmental levels of HEX are available.
These data suggest that it is present primarily in the
aquatic compartment and is associated with bottom sedi-
ments and organic matter except in locations where dis-
posal or release has occurred. In laboratory studies, HEX
readily sorbs to most types of soil particles. However,
leaching and movement in ground water have been reported.
In the USA, the total annual estimated release of HEX
into the environment is 5.9 tonnes (US EPA, 1989). In the
Federal Republic of Germany and the Netherlands, about
400-500 kg was emitted to the atmosphere in 1987 (BUA,
1988). Owing to the physical and chemical characteristics
of HEX, only a small fraction of these emissions would be
expected to persist.
Using the available laboratory data, the fate and
transport of HEX in the atmosphere have been modelled and
a tropospheric residence time of approximately 5 h has
been calculated. There have been reports of atmospheric
transport of HEX from an area where waste is stored and
from wet wells during the treatment of industrial wastes.
In water, HEX may undergo photolysis, hydrolysis, and
biodegradation. In shallow water, it has a photolytic
half-life of < 1 h. In deeper water where photolysis is
precluded, the hydrolytic half-life has been found to
range from several days to approximately 3 months, while
biodegradation is predicted to occur more slowly. HEX is
known to volatilize from surface water, the rate of vola-
tilization being affected by turbulence and by sorption
onto sediments.
Owing to its low solubility in water, HEX should be
relatively immobile in soil. However, HEX has been found
in ground water. Volatilization, which is most likely to
occur at the soil surface, is inversely related to the
levels of organic matter in the soil. The results of lab-
oratory studies indicate that chemical hydrolysis and
microbial metabolism, both aerobic and anaerobic, would be
expected to reduce HEX levels in soils.
The biomagnification potential of HEX should theoreti-
cally be substantial because of its high lipophilicity
(log octanol/water partition coefficient). However, this
has not been supported by experimental evidence. Studies
in laboratory animals have shown that 14C-HEX is both
metabolized and excreted within the first few hours after
oral dosing, with little being retained in the body.
Steady-state bioconcentration factors in fish are < 30.
Bioaccumulation factors derived from short-term model eco-
systems indicate a moderate accumulation potential. There-
fore, it would appear that HEX and its metabolites do not
persist or accumulate to any great extent in biological
systems.
Low concentrations of HEX have been shown to be toxic
to aquatic life. Lethality in acute exposures (48 to 96 h)
has been observed in both freshwater and marine crus-
taceans and fish at nominal concentrations of 32-180 µg
per litre in static exposure systems in which the water
was not renewed during the test. Since the photolytic
half-life is < 1 h, the HEX concentration would have
decreased substantially during the exposure period used in
these studies. In the only studies using flowing water and
measured HEX concentrations, 96-h LC50 values of 7 µg
per litre were obtained for the fathead minnow and a
marine shrimp. Tests with these two species yielded values
for LC10 of 3.7 and LC40 of 0.7 µg/litre, respectively.
Seven-day static tests with marine algae showed a
median reduction of growth (EC50) at nominal concen-
trations ranging from 3.5-100 µg/litre, depending on the
species.
In aqueous media, HEX is toxic to many microorganisms
at nominal concentrations of 0.2-10 mg/litre, i.e. levels
substantially higher than those needed to kill most
aquatic animals or plants. HEX appears to be less toxic
to microorganisms in soil than in aquatic media, probably
because of adsorption of HEX on the soil matrix.
Although exposure would be expected to be low, there
is insufficient information currently available to deter-
mine the effects of HEX exposure on terrestrial vegetation
or wildlife.
The absorption of unchanged HEX is minimized by its
reactivity with body membranes and tissues and especially
with the contents of the gastrointestinal tract. Most
radiolabelled 14C-HEX is retained by the kidneys, liver,
trachea, and lungs of animals after oral, dermal, or inha-
lation dosing. Absorbed HEX is metabolized and rapidly
excreted, predominantly in the urine, less in the faeces,
and < 1% in expired air. The terminal elimination time is
about 30 h, irrespective of the route of administration.
After inhalation or intravenous administration, no
unchanged HEX is excreted; the faecal and urinary metab-
olites have been isolated but not identified. The failure
to identify metabolites represents a major difficulty in
assessing the pharmacokinetics and potential mechanisms of
HEX action.
The acute inhalation LC50 (over a period of approxi-
mately 4 h) is 17.9 mg/m3 in male rats and 39.1 mg/m3 in
females. Although there are some interspecies differences
between guinea-pigs, rabbits, rats, and mice, HEX vapour
is highly toxic to all tested species. It appears to be
most toxic when administered by inhalation, as compared
with oral and dermal administration, and is a severe pri-
mary irritant. The systemic effects of acute exposure,
irrespective of the route of administration, include
pathological changes in the lungs, liver, kidneys, and
adrenal glands.
Short-term oral dosing of rats (38 mg/kg per day) and
mice (75 mg/kg per day) for 91 days produced nephrosis and
inflammation and hyperplasia of the forestomach. No overt
signs were noted when mice or rats were exposed by inha-
lation to 2.26 mg/m3 (0.2 ppm), 6 h/day, 5 days/week, for
14 weeks. At 1.69 mg/m3 (0.15 ppm) only mild irritation
was seen. Inhalation exposure of rats to 5.65 mg/m3 (0.5
ppm) for 30 weeks caused histopathological changes in the
liver, respiratory tract, and kidneys. A short-term inha-
lation study of HEX in mice and rats for 90 days showed
respiratory system effects at 4.52 mg/m3 (0.4 ppm) or
more. HEX has not been shown to be a mutagen in in vitro
assays, either with or without metabolic activation. It
was also inactive in mouse dominant lethal assays. It has
not been shown to be a teratogen in rats and mice by oral
exposure; there are no data for the teratogenicity of HEX
after inhalation exposure.
Only limited data are available on the human health
effects of HEX exposure. There have been isolated inci-
dents in which HEX caused severe irritation in the eyes,
nose, throat, and lungs. The irritation was usually of
short duration, with recovery beginning after exposure
ceased. There were no statistically significant differ-
ences in certain liver enzymes between exposed and control
groups after short-term exposure. The long-term human
health effects of continuous low-level exposure and/or
intermittent acute exposure are not known. Handlers of the
product and its waste, as well as sewage workers and resi-
dents near disposal sites, have been shown to be at risk
because of the potential for exposure to the chemical or
wastes from its manufacture.
The data base is not extensive or adequate to assess
the carcinogenicity of HEX. The US National Toxicology
Program (NTP) has conducted a lifetime animal inhalation
bioassay using both rats and mice. After the pathology
report has been produced, there will be a better under-
standing of the long-term effects of HEX exposure. An
assessment of carcinogenicity will have to be deferred
until the results of the NTP bioassay are available. The
International Agency for Research on Cancer evaluated the
existing data for HEX and classified it in Group 3 (which
indicates that because of major qualitative or quantitat-
ive limitations, the studies cannot be interpreted as
showing either the presence or absence of a carcinogenic
effect). Several epidemiological studies were cited in the
literature; there were no reports of an increase, attribu-
table to HEX or its metabolites, in the incidence of neo-
plasms at any site.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Hexachlorocyclopentadiene (HEX) is the most commonly
used name for the compound that is designated 1,2,3,4,5,5'-
hexachloro-1,3-cyclopentadiene by the International Union
of Pure and Applied Chemistry (IUPAC).
Chemical formula: C5Cl6
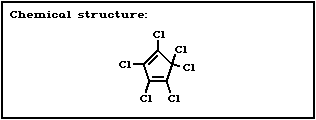 CAS and IUPAC 1,2,3,4,5,5'-hexachloro-1,3-cyclo-
name: pentadiene
Synonyms and Hexachlorocyclopentadiene, perchloro-
common trade cyclopentadiene, hexachloro-1,3-cyclo-
names: pentadiene, HEX, HCPD, HCCP,
HCCPD, C-56, HRS 1655, Graphlox
CAS registry number: 77-47-4
RTECS number: GY 1225000
CIS accession number: 7800117
EINECS number: 2010293
2.2. Physical and chemical properties
2.2.1. Physical properties
Hexachlorocyclopentadiene (98% pure) is a dense
liquid with low solubility in water (Table 1). It is
non-flammable and has a characteristic pungent musty
odour. The pure compound is a light lemon-yellow colour,
but impure HEX may have a greenish tinge (Stevens, 1979).
HEX (and quite possibly other substances) was reported to
have created a blue haze in an accident involving the
treatment of waste (Kominsky et al., 1980). A list of some
physical and chemical properties is presented in Table 1.
It appears that the compound is strongly adsorbed to soil
colloids. In spite of its low vapour pressure and high
boiling point, HEX volatilizes rapidly from water (Atallah
et al., 1980). According to the Handbook of Chemistry and
Physics (Weast & Astle, 1980), the ultraviolet-visible
lambdamax in heptane is 323 nm with a log molar absorptivity
of 3.2. This absorption band extends into the visible
spectrum, as shown by the yellow colour of HEX. Facile
homopolar carbon-chlorine bond scission might be expected
in sunlight or under fluorescent light. The infrared
spectrum has characteristic absorptions at 6.2, 8.1, 8.4,
8.8, 12.4, 14.1, and 14.7 µm. The mass spectrum of HEX
shows a weak molecular ion (M) at M/e 270, but a very
intense M-35 ion, making this latter ion suitable for
sensitive specific ion monitoring.
Table 1. Physical and chemical properties of hexachlorocyclopentadiene
------------------------------------------------------------------------
Property Value/description Reference
------------------------------------------------------------------------
Relative molecular mass 272.77 Stevens (1979)
Physical state (25 °C) pale yellow liquid Hawley (1977)
Odour pungent Hawley (1977)
Electronic absorption maximum 322 nm Wolfe et al. (1982)
(in 50% acetonitrile-water) (log e = 3.18)
Solubility (22 °C)
Water (mg/litre) 1.03-1.25 Chou & Griffin (1983)
Organic solvents miscible (hexane) Bell et al. (1978)
Vapour density (air = 1) 9.42 Verschueren (1977)
Vapour pressure
(25 °C) 10.7 Pa (0.08 mmHg) Irish (1963)
(25 °C) 10.7 Pa (0.08 mmHg) Wolfe et al. (1982)
(62 °C) 131 Pa (0.98 mmHg) Stevens (1979)
Relative density 1.717 (15 °C) Hawley (1977)
1.710 (20 °C) Stevens (1979)
1.702 (25 °C) Weast & Astle (1980)
Melting point (°C) -9.6 Hawley (1977)
-11.34 Stevens (1979)
Boiling point (°C) 239 at 103 kPa Hawley (1977);
(753 mmHg) Stevens (1979)
234 Irish (1963)
------------------------------------------------------------------------
Table 1 (contd.)
------------------------------------------------------------------------
Property Value/description Reference
------------------------------------------------------------------------
Octanol/water partition
coefficient (log Pow)
(measured): 5.04 ± 0.04 Wolfe et al. (1982)
(at 28 °C)a
(estimated): 5.51 Wolfe et al. (1982)
(measured): 5.51b Veith et al. (1979)
Octanol/water partition 1.1 (± 0.1) x 105 Wolfe et al. (1982)
coefficient (Pow) (28 °C)
Latent heat of vaporization 176.6 J/g Stevens (1979)
------------------------------------------------------------------------
a Measured by simple partition.
b Measured by HPLC.
2.2.2. Chemical properties
Hexachlorocyclopentadiene is a highly reactive diene
that readily undergoes addition and substitution reactions
and also participates in Diels-Alder reactions (Ungnade &
McBee, 1958). The products of the Diels-Alder reaction of
HEX with a compound containing a non-conjugated double
bond are generally 1:1 adducts containing a hexachlorobi-
cyclo(2,2,1)heptene structure; the monoene derived part of
the adduct is nearly always in the endoposition, rather
than the exoposition (Stevens, 1979).
Two excellent early reviews of the chemistry of HEX
were produced by Roberts (1958) and Ungnade & McBee
(1958). Look (1974) reviewed the formation of HEX adducts
of aromatic compounds and the by-products of the Diels-
Alder reaction.
2.3. Conversion factors
1 ppm = 11.3 mg/m3 1 mg/m3 = 0.088 ppm
2.4. Analytical methods
2.4.1. Air
The techniques used to collect samples of HEX vapour
in air involve the adsorption and concentration of the
vapour in liquid-filled impingers or solid sorbent-packed
cartridges.
Whitmore et al. (1977) pumped airborne vapours through
a miniature glass impinger tube containing hexane or
benzene and through a solid sorbent-packed tube
(Chromosorb(R) 102) tube. Sampling efficiency was found to
be 97% with hexane and 100% with benzene. The sampling
efficiency for the solid sorbent tube was 100%. The sensi-
tivity of the liquid impinger system was found to be
< 11.2 µg/m3 (< 1 ppb) in ambient air.
Kominsky & Wisseman (1978) collected HEX vapour on
Chromosorb(R) 102 (20/40 mesh) sorbent previously cleaned
by extraction with 1:1 acetone/methanol solvent to remove
interfering compounds. HEX was desorbed with carbon disul-
fide (68% efficiency) and analysed by gas chromatography-
flame ionization detection (Neumeister & Kurimo, 1978).
Dillon (1980) and Boyd et al. (1981) developed and
validated sampling and analytical methods for air samples
containing HEX. Methods were reliable at levels below the
8-h time-weighted average (TWA) and threshold limit value
(TLV) of 0.1 mg/m3 recommended by the American Confer-
ence of Governmental Industrial Hygienists (ACGIH).
The method developed by NIOSH, Physical and Chemical
Analytical Method No. 308 (NIOSH, 1979), used adsorption
on Porapak(R) T (80/100 mesh), desorption with hexane (100%
for 30 ng HEX on 50-100 mg adsorbent), and then analysis
by GC-63Ni electron capture detection (ECD). The solid
sorbent was cleaned by soxhlet extraction with 4:1 (v/v)
acetone/methanol (4 h) and hexane (4 h) and was dried
under vacuum overnight at 50-70 °C to ambient temperature.
The pyrex sampling tubes (7 cm long, 6 mm outside diam-
eter, 4 mm inside diameter) contained a 75-mg layer of
sorbent in the front and a 25-mg section in the back. Each
section was held in place by two silylated glass wool
plugs. A 5-mm long airspace was needed between the front
and back sections. A battery-operated sampling pump, which
drew air at 0.05 and 2.0 litre/min, was used for personal
sampling of workers. The lowest analytically quantifiable
level was 25 ng HEX/sorbent sample (using 1 ml of hexane-
desorbing solvent and a 1-h period of desorption by ultra-
sonification), and the upper limit was 2500 ng/sorbent
sample. The method was validated for air HEX concen-
trations that were between 13 and 865 µg/m3 at 25-28 °C
and with a relative humidity of 90% or more.
Gas chromatography has been considered the preferred
method for analysing HEX in air, using either flame ioniz-
ation collection or electron capture detection (Whitmore
et al., 1977; Neumeister & Kurimo, 1978; Chopra et al.,
1978; NIOSH, 1979). Gas chromatography/mass spectroscopy
(GC/MS) is necessary for confirmation (Eichler, 1978).
Gas chromatography with electron capture detection has
been reported to be the most sensitive analytical tech-
nique for HEX. The chromatographic response was stated to
be a linear and reproducible function of HEX concentration
over the range from approximately 5 to 142 ng/ml (25-710
pg injected), with a correlation coefficient of 0.9993 for
peak height measurement (NIOSH, 1979).
The lowest analytically quantifiable level (LAQL) of
HEX in air was found to be 25 ng/sorbent tube. This level
represented the smallest amount of HEX that could be
determined with a recovery of > 80% and a coefficient of
variation of < 10%. The desorption efficiency of 100% was
obtained by averaging the levels ranging from near the
LAQL of 25 ng to 1000 times the LAQL (NIOSH, 1979).
2.4.2. Water
Since HEX is sensitive to light in both organic and
aqueous solutions, the water samples, extracts, and stan-
dard HEX solutions to be used for laboratory examinations
must be protected from light. The rate of degradation
depends on the light intensity and wavelength, the half-
life of HEX being approximately 7 days when the solution
is exposed to ordinary lighting conditions in the labora-
tory (Benoit & Williams, 1981). Storing the HEX-containing
solutions in amber- or red-coloured (low actinic) glass-
ware is recommended for adequate protection (Benoit &
Williams, 1981).
XAD-2 resin extraction has been used to concentrate
HEX from large volumes of water. Solvent extraction of
water has also proved successful. The detection limit used
for the organic solvent extraction technique was 50 ng per
litre, as opposed to 0.5 ng/litre for the XAD-2 method.
When the solvent extraction method was used under subdued
lighting conditions in the laboratory, the efficiency of
recovery for an artificially loaded water sample was found
to be 79-88%. The authors concluded that the XAD-2 resin
could not be used to sample accurately quantitative
amounts of HEX in water, but it could be used to screen
samples qualitatively because of the low detection limit
(Benoit & Williams, 1981).
Lichtenberg et al. (1987) developed methods for the
sampling and analysis of organic pollutants, including
HEX, in water for the US Environmental Protection Agency
(US EPA). Their emphasis was on compound-specific methods,
such as GC/MS employing packed and capillary columns. For
organochlorine pesticides, methylene chlorine in hexane is
used for extraction.
Thielen et al. (1987) developed a technique combining
microextraction and capillary column gas chromatography
and applied it to plant discharge streams for repetitive
waste-water discharge permit analyses. Samples were col-
lected in amber bottles and sealed with Teflon-lined caps.
Hewlett-Packard 5880 gas chromatographs equipped with
flame ionization detectors, electron capture detectors,
and 7672A autosamplers were used for analyses. According
to the researchers, the overall effect of converting to
the microextraction/capillary-column procedure was both
cost-and time-saving, and instrumentation needs were cut
by half. A statistical comparison was made to determine
whether this technique was equivalent to purge-2nd-trap
and normal extraction methods. It was found that the
differences in precision were not significant above
2 µg/litre. However, the precision and accuracy of the
microextraction method was poor for HEX owing to its
instability and the fact that it is adsorbed onto sur-
faces. The final microextraction data yielded an average
HEX recovery (for 44 samples) of 99.27% (S.D. 18.94).
2.4.3. Soil
DeLeon et al. (1980a) developed a method for determin-
ing volatile and semi-volatile organochlorine compounds in
samples taken from the soil and from chemical waste-dis-
posal sites. This method used hexane extraction followed
by analysis of the extract with temperature-programmed GC
on high-resolution glass capillary columns using ECD.
GC/MS was used to confirm the presence of the chlorocar-
bons. The lowest detection limit was 10 µg/g.
2.4.4. Biological media
A method to determine levels of HEX in blood and urine
has been described by DeLeon et al. (1980b). This method
involves isolation of the compound from the blood or urine
sample by liquid-liquid extraction, GC analysis with ECD,
and confirmation by GC/MS. The best recoveries have been
obtained by using a toluene-acetonitrile extraction mix-
ture for blood assays and a petroleum ether extraction for
urine assays. In this method, the detection limits of HEX
were 50 ng/ml for blood and 10 ng/ml for urine. Studies by
the Velsicol Chemical Corporation have shown that up to
30% of the HEX can be lost if the extracts are concen-
trated to 0.1 ml. Quantitative recovery is possible only
for volumes of concentrate larger than 0.5 ml, which
limits the sensitivity of the DeLeon method. However, this
method may offer a sensitive process for monitoring occu-
pational exposure.
The Velsicol Chemical Corporation (1979) has developed
three analytical methods, which have been used for urine,
fish fillet, beef liver, beef skeletal muscle, beef adi-
pose tissue, beef kidney, chicken liver, chicken skeletal
muscle, and chicken adipose tissue. The respective recov-
eries were: 80 ± 10% (1-50 ppb), 81 ± 1%, 69 ± 4%,
88 ± 2%, 86 ± 5%, 71 ± 3%, 55 ± 9%, 76 ± 4%, and 85 ± 2%.
The limit of detection for HEX was 0.5 ppb.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
HEX is not found as a natural component in the
environment.
3.2. Man-made sources
Low levels of HEX are released into the environment
during its manufacture and during the manufacture of
products requiring HEX (US EPA, 1980c). It is also found
as an impurity and a degradation product in compounds
manufactured from HEX (Spehar et al., 1977; Chopra et al.,
1978).
3.2.1. Production levels and processes
Since there is only one producer of HEX in the USA and
one in Europe (in the Netherlands), production statistics
are considered to be confidential business information and
are not available to the public. Production estimates for
HEX based on the manufacture of chlorinated cyclodiene
pesticides in the early 1970s were approximately 22 700
tonnes/year (Lu et al., 1975). After restrictions were
established for the use of some pesticides produced from
HEX, USA production estimates were lowered to a range of
3600-6800 tonnes/year (US EPA, 1977). In 1988, worldwide
production volume was estimated to be approximately 15 000
tonnes (BUA, 1988).
Commercial HEX has various purities depending on the
method of synthesis. HEX made by the chlorination of
cyclopentadiene by alkaline hypochlorite at 40 °C, fol-
lowed by fractional distillation, is only 75% pure, and
contains many lower chlorinated cyclopentadienes and other
contaminants (e.g., hexachlorobenzene and octachlorocyclo-
pentene). Purities above 90% have been obtained by thermal
dechlorination of octachlorocyclopentene at 470-480 °C
(Stevens, 1979). The current specification for HEX
produced by the Velsicol Chemical Corporation at Memphis,
Tennessee, USA, which is used internally and sold to other
users, has a minimum purity of 97% (Velsicol Chemical
Corporation, 1984).
The nature and levels of HEX contaminants vary with
the method of production. The major contaminants found in
an industrial preparation of HEX (from Velsicol) were
octachlorocyclopentene (0.68%), hexachloro-1,3-butadiene
(1.11%), tetrachloroethane (0.09%), hexachlorobenzene
(0.04%), and pentachlorobenzene (0.02%). Another prep-
aration (from Shell International Petroleum in 1982) con-
tained up to 1.5% of octachlorocyclopentene and approxi-
mately 0.2% of hexachloro-1,3-butadiene (BUA, 1988).
3.2.2. Uses
HEX is the key intermediate in the manufacture of some
chlorinated cyclodiene pesticides (Fig. 1). These pesti-
cides include heptachlor, chlordane, aldrin, dieldrin,
endrin, mirex, pentac, and endosulfan. Another major use
of HEX is in the manufacture of flame retardants, such as
chlorendic anhydride, and Dechlorane Plus. It has been
estimated that the production volume is split equally
between fire retardant and pesticide use (BUA, 1988). HEX
is also used, to a lesser extent, in the manufacture of
resins and dyes (US EPA, 1980b), and was previously used
as a general biocide (Cole, 1954).
3.2.3. Other sources of exposure
Human and environmental exposure to HEX has occurred
as a result of releases at production and processing
facilities, during transport to disposal facilities, and
at land disposal sites.
In 1977, a waste transporter released an estimated
5.5 tonnes of HEX and octachlorocyclopentene, a co-
contaminant, into the sewers of Louisville, Kentucky,
which led to the contamination of several miles of sewer.
The waste-water treatment plant was temporarily closed
because of excessive exposure of workers to HEX. (Kominsky
& Wisseman, 1978; Morse et al., 1978, 1979). Releases from
the Memphis production facility have resulted in high
concentrations of HEX in waste water from the facility and
have led to HEX being present in the inflow to the
receiving waste-water treatment plant and in air at the
treatment plant. HEX has also been released from a waste
site in Montague, Michigan, USA (US EPA, 1980b).
The US EPA Toxic Chemical Release Inventory for 1987
revealed that over 4.5 tonnes of HEX was released at the
Velsicol facility in Marshall, Illinois, USA (most of it
from underground injection disposal), that over 540 kg was
released at the Velsicol facility in Memphis, and that a
similar quantity was released from the Occidental Chemical
Corporation at Niagara Falls, New York, USA. The latter
two releases were primarily to the air (US EPA, 1989).
CAS and IUPAC 1,2,3,4,5,5'-hexachloro-1,3-cyclo-
name: pentadiene
Synonyms and Hexachlorocyclopentadiene, perchloro-
common trade cyclopentadiene, hexachloro-1,3-cyclo-
names: pentadiene, HEX, HCPD, HCCP,
HCCPD, C-56, HRS 1655, Graphlox
CAS registry number: 77-47-4
RTECS number: GY 1225000
CIS accession number: 7800117
EINECS number: 2010293
2.2. Physical and chemical properties
2.2.1. Physical properties
Hexachlorocyclopentadiene (98% pure) is a dense
liquid with low solubility in water (Table 1). It is
non-flammable and has a characteristic pungent musty
odour. The pure compound is a light lemon-yellow colour,
but impure HEX may have a greenish tinge (Stevens, 1979).
HEX (and quite possibly other substances) was reported to
have created a blue haze in an accident involving the
treatment of waste (Kominsky et al., 1980). A list of some
physical and chemical properties is presented in Table 1.
It appears that the compound is strongly adsorbed to soil
colloids. In spite of its low vapour pressure and high
boiling point, HEX volatilizes rapidly from water (Atallah
et al., 1980). According to the Handbook of Chemistry and
Physics (Weast & Astle, 1980), the ultraviolet-visible
lambdamax in heptane is 323 nm with a log molar absorptivity
of 3.2. This absorption band extends into the visible
spectrum, as shown by the yellow colour of HEX. Facile
homopolar carbon-chlorine bond scission might be expected
in sunlight or under fluorescent light. The infrared
spectrum has characteristic absorptions at 6.2, 8.1, 8.4,
8.8, 12.4, 14.1, and 14.7 µm. The mass spectrum of HEX
shows a weak molecular ion (M) at M/e 270, but a very
intense M-35 ion, making this latter ion suitable for
sensitive specific ion monitoring.
Table 1. Physical and chemical properties of hexachlorocyclopentadiene
------------------------------------------------------------------------
Property Value/description Reference
------------------------------------------------------------------------
Relative molecular mass 272.77 Stevens (1979)
Physical state (25 °C) pale yellow liquid Hawley (1977)
Odour pungent Hawley (1977)
Electronic absorption maximum 322 nm Wolfe et al. (1982)
(in 50% acetonitrile-water) (log e = 3.18)
Solubility (22 °C)
Water (mg/litre) 1.03-1.25 Chou & Griffin (1983)
Organic solvents miscible (hexane) Bell et al. (1978)
Vapour density (air = 1) 9.42 Verschueren (1977)
Vapour pressure
(25 °C) 10.7 Pa (0.08 mmHg) Irish (1963)
(25 °C) 10.7 Pa (0.08 mmHg) Wolfe et al. (1982)
(62 °C) 131 Pa (0.98 mmHg) Stevens (1979)
Relative density 1.717 (15 °C) Hawley (1977)
1.710 (20 °C) Stevens (1979)
1.702 (25 °C) Weast & Astle (1980)
Melting point (°C) -9.6 Hawley (1977)
-11.34 Stevens (1979)
Boiling point (°C) 239 at 103 kPa Hawley (1977);
(753 mmHg) Stevens (1979)
234 Irish (1963)
------------------------------------------------------------------------
Table 1 (contd.)
------------------------------------------------------------------------
Property Value/description Reference
------------------------------------------------------------------------
Octanol/water partition
coefficient (log Pow)
(measured): 5.04 ± 0.04 Wolfe et al. (1982)
(at 28 °C)a
(estimated): 5.51 Wolfe et al. (1982)
(measured): 5.51b Veith et al. (1979)
Octanol/water partition 1.1 (± 0.1) x 105 Wolfe et al. (1982)
coefficient (Pow) (28 °C)
Latent heat of vaporization 176.6 J/g Stevens (1979)
------------------------------------------------------------------------
a Measured by simple partition.
b Measured by HPLC.
2.2.2. Chemical properties
Hexachlorocyclopentadiene is a highly reactive diene
that readily undergoes addition and substitution reactions
and also participates in Diels-Alder reactions (Ungnade &
McBee, 1958). The products of the Diels-Alder reaction of
HEX with a compound containing a non-conjugated double
bond are generally 1:1 adducts containing a hexachlorobi-
cyclo(2,2,1)heptene structure; the monoene derived part of
the adduct is nearly always in the endoposition, rather
than the exoposition (Stevens, 1979).
Two excellent early reviews of the chemistry of HEX
were produced by Roberts (1958) and Ungnade & McBee
(1958). Look (1974) reviewed the formation of HEX adducts
of aromatic compounds and the by-products of the Diels-
Alder reaction.
2.3. Conversion factors
1 ppm = 11.3 mg/m3 1 mg/m3 = 0.088 ppm
2.4. Analytical methods
2.4.1. Air
The techniques used to collect samples of HEX vapour
in air involve the adsorption and concentration of the
vapour in liquid-filled impingers or solid sorbent-packed
cartridges.
Whitmore et al. (1977) pumped airborne vapours through
a miniature glass impinger tube containing hexane or
benzene and through a solid sorbent-packed tube
(Chromosorb(R) 102) tube. Sampling efficiency was found to
be 97% with hexane and 100% with benzene. The sampling
efficiency for the solid sorbent tube was 100%. The sensi-
tivity of the liquid impinger system was found to be
< 11.2 µg/m3 (< 1 ppb) in ambient air.
Kominsky & Wisseman (1978) collected HEX vapour on
Chromosorb(R) 102 (20/40 mesh) sorbent previously cleaned
by extraction with 1:1 acetone/methanol solvent to remove
interfering compounds. HEX was desorbed with carbon disul-
fide (68% efficiency) and analysed by gas chromatography-
flame ionization detection (Neumeister & Kurimo, 1978).
Dillon (1980) and Boyd et al. (1981) developed and
validated sampling and analytical methods for air samples
containing HEX. Methods were reliable at levels below the
8-h time-weighted average (TWA) and threshold limit value
(TLV) of 0.1 mg/m3 recommended by the American Confer-
ence of Governmental Industrial Hygienists (ACGIH).
The method developed by NIOSH, Physical and Chemical
Analytical Method No. 308 (NIOSH, 1979), used adsorption
on Porapak(R) T (80/100 mesh), desorption with hexane (100%
for 30 ng HEX on 50-100 mg adsorbent), and then analysis
by GC-63Ni electron capture detection (ECD). The solid
sorbent was cleaned by soxhlet extraction with 4:1 (v/v)
acetone/methanol (4 h) and hexane (4 h) and was dried
under vacuum overnight at 50-70 °C to ambient temperature.
The pyrex sampling tubes (7 cm long, 6 mm outside diam-
eter, 4 mm inside diameter) contained a 75-mg layer of
sorbent in the front and a 25-mg section in the back. Each
section was held in place by two silylated glass wool
plugs. A 5-mm long airspace was needed between the front
and back sections. A battery-operated sampling pump, which
drew air at 0.05 and 2.0 litre/min, was used for personal
sampling of workers. The lowest analytically quantifiable
level was 25 ng HEX/sorbent sample (using 1 ml of hexane-
desorbing solvent and a 1-h period of desorption by ultra-
sonification), and the upper limit was 2500 ng/sorbent
sample. The method was validated for air HEX concen-
trations that were between 13 and 865 µg/m3 at 25-28 °C
and with a relative humidity of 90% or more.
Gas chromatography has been considered the preferred
method for analysing HEX in air, using either flame ioniz-
ation collection or electron capture detection (Whitmore
et al., 1977; Neumeister & Kurimo, 1978; Chopra et al.,
1978; NIOSH, 1979). Gas chromatography/mass spectroscopy
(GC/MS) is necessary for confirmation (Eichler, 1978).
Gas chromatography with electron capture detection has
been reported to be the most sensitive analytical tech-
nique for HEX. The chromatographic response was stated to
be a linear and reproducible function of HEX concentration
over the range from approximately 5 to 142 ng/ml (25-710
pg injected), with a correlation coefficient of 0.9993 for
peak height measurement (NIOSH, 1979).
The lowest analytically quantifiable level (LAQL) of
HEX in air was found to be 25 ng/sorbent tube. This level
represented the smallest amount of HEX that could be
determined with a recovery of > 80% and a coefficient of
variation of < 10%. The desorption efficiency of 100% was
obtained by averaging the levels ranging from near the
LAQL of 25 ng to 1000 times the LAQL (NIOSH, 1979).
2.4.2. Water
Since HEX is sensitive to light in both organic and
aqueous solutions, the water samples, extracts, and stan-
dard HEX solutions to be used for laboratory examinations
must be protected from light. The rate of degradation
depends on the light intensity and wavelength, the half-
life of HEX being approximately 7 days when the solution
is exposed to ordinary lighting conditions in the labora-
tory (Benoit & Williams, 1981). Storing the HEX-containing
solutions in amber- or red-coloured (low actinic) glass-
ware is recommended for adequate protection (Benoit &
Williams, 1981).
XAD-2 resin extraction has been used to concentrate
HEX from large volumes of water. Solvent extraction of
water has also proved successful. The detection limit used
for the organic solvent extraction technique was 50 ng per
litre, as opposed to 0.5 ng/litre for the XAD-2 method.
When the solvent extraction method was used under subdued
lighting conditions in the laboratory, the efficiency of
recovery for an artificially loaded water sample was found
to be 79-88%. The authors concluded that the XAD-2 resin
could not be used to sample accurately quantitative
amounts of HEX in water, but it could be used to screen
samples qualitatively because of the low detection limit
(Benoit & Williams, 1981).
Lichtenberg et al. (1987) developed methods for the
sampling and analysis of organic pollutants, including
HEX, in water for the US Environmental Protection Agency
(US EPA). Their emphasis was on compound-specific methods,
such as GC/MS employing packed and capillary columns. For
organochlorine pesticides, methylene chlorine in hexane is
used for extraction.
Thielen et al. (1987) developed a technique combining
microextraction and capillary column gas chromatography
and applied it to plant discharge streams for repetitive
waste-water discharge permit analyses. Samples were col-
lected in amber bottles and sealed with Teflon-lined caps.
Hewlett-Packard 5880 gas chromatographs equipped with
flame ionization detectors, electron capture detectors,
and 7672A autosamplers were used for analyses. According
to the researchers, the overall effect of converting to
the microextraction/capillary-column procedure was both
cost-and time-saving, and instrumentation needs were cut
by half. A statistical comparison was made to determine
whether this technique was equivalent to purge-2nd-trap
and normal extraction methods. It was found that the
differences in precision were not significant above
2 µg/litre. However, the precision and accuracy of the
microextraction method was poor for HEX owing to its
instability and the fact that it is adsorbed onto sur-
faces. The final microextraction data yielded an average
HEX recovery (for 44 samples) of 99.27% (S.D. 18.94).
2.4.3. Soil
DeLeon et al. (1980a) developed a method for determin-
ing volatile and semi-volatile organochlorine compounds in
samples taken from the soil and from chemical waste-dis-
posal sites. This method used hexane extraction followed
by analysis of the extract with temperature-programmed GC
on high-resolution glass capillary columns using ECD.
GC/MS was used to confirm the presence of the chlorocar-
bons. The lowest detection limit was 10 µg/g.
2.4.4. Biological media
A method to determine levels of HEX in blood and urine
has been described by DeLeon et al. (1980b). This method
involves isolation of the compound from the blood or urine
sample by liquid-liquid extraction, GC analysis with ECD,
and confirmation by GC/MS. The best recoveries have been
obtained by using a toluene-acetonitrile extraction mix-
ture for blood assays and a petroleum ether extraction for
urine assays. In this method, the detection limits of HEX
were 50 ng/ml for blood and 10 ng/ml for urine. Studies by
the Velsicol Chemical Corporation have shown that up to
30% of the HEX can be lost if the extracts are concen-
trated to 0.1 ml. Quantitative recovery is possible only
for volumes of concentrate larger than 0.5 ml, which
limits the sensitivity of the DeLeon method. However, this
method may offer a sensitive process for monitoring occu-
pational exposure.
The Velsicol Chemical Corporation (1979) has developed
three analytical methods, which have been used for urine,
fish fillet, beef liver, beef skeletal muscle, beef adi-
pose tissue, beef kidney, chicken liver, chicken skeletal
muscle, and chicken adipose tissue. The respective recov-
eries were: 80 ± 10% (1-50 ppb), 81 ± 1%, 69 ± 4%,
88 ± 2%, 86 ± 5%, 71 ± 3%, 55 ± 9%, 76 ± 4%, and 85 ± 2%.
The limit of detection for HEX was 0.5 ppb.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
HEX is not found as a natural component in the
environment.
3.2. Man-made sources
Low levels of HEX are released into the environment
during its manufacture and during the manufacture of
products requiring HEX (US EPA, 1980c). It is also found
as an impurity and a degradation product in compounds
manufactured from HEX (Spehar et al., 1977; Chopra et al.,
1978).
3.2.1. Production levels and processes
Since there is only one producer of HEX in the USA and
one in Europe (in the Netherlands), production statistics
are considered to be confidential business information and
are not available to the public. Production estimates for
HEX based on the manufacture of chlorinated cyclodiene
pesticides in the early 1970s were approximately 22 700
tonnes/year (Lu et al., 1975). After restrictions were
established for the use of some pesticides produced from
HEX, USA production estimates were lowered to a range of
3600-6800 tonnes/year (US EPA, 1977). In 1988, worldwide
production volume was estimated to be approximately 15 000
tonnes (BUA, 1988).
Commercial HEX has various purities depending on the
method of synthesis. HEX made by the chlorination of
cyclopentadiene by alkaline hypochlorite at 40 °C, fol-
lowed by fractional distillation, is only 75% pure, and
contains many lower chlorinated cyclopentadienes and other
contaminants (e.g., hexachlorobenzene and octachlorocyclo-
pentene). Purities above 90% have been obtained by thermal
dechlorination of octachlorocyclopentene at 470-480 °C
(Stevens, 1979). The current specification for HEX
produced by the Velsicol Chemical Corporation at Memphis,
Tennessee, USA, which is used internally and sold to other
users, has a minimum purity of 97% (Velsicol Chemical
Corporation, 1984).
The nature and levels of HEX contaminants vary with
the method of production. The major contaminants found in
an industrial preparation of HEX (from Velsicol) were
octachlorocyclopentene (0.68%), hexachloro-1,3-butadiene
(1.11%), tetrachloroethane (0.09%), hexachlorobenzene
(0.04%), and pentachlorobenzene (0.02%). Another prep-
aration (from Shell International Petroleum in 1982) con-
tained up to 1.5% of octachlorocyclopentene and approxi-
mately 0.2% of hexachloro-1,3-butadiene (BUA, 1988).
3.2.2. Uses
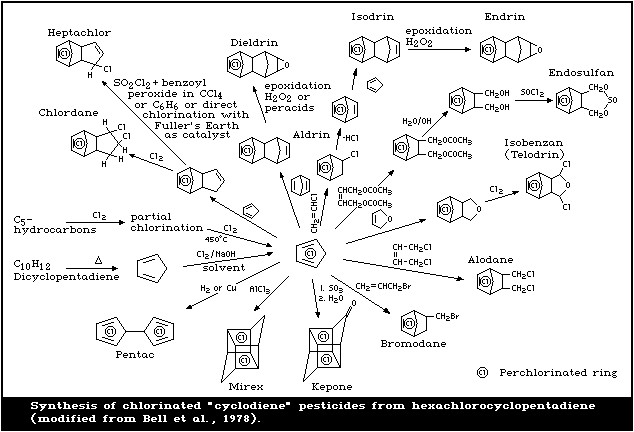
HEX is the key intermediate in the manufacture of some
chlorinated cyclodiene pesticides (Fig. 1). These pesti-
cides include heptachlor, chlordane, aldrin, dieldrin,
endrin, mirex, pentac, and endosulfan. Another major use
of HEX is in the manufacture of flame retardants, such as
chlorendic anhydride, and Dechlorane Plus. It has been
estimated that the production volume is split equally
between fire retardant and pesticide use (BUA, 1988). HEX
is also used, to a lesser extent, in the manufacture of
resins and dyes (US EPA, 1980b), and was previously used
as a general biocide (Cole, 1954).
3.2.3. Other sources of exposure
Human and environmental exposure to HEX has occurred
as a result of releases at production and processing
facilities, during transport to disposal facilities, and
at land disposal sites.
In 1977, a waste transporter released an estimated
5.5 tonnes of HEX and octachlorocyclopentene, a co-
contaminant, into the sewers of Louisville, Kentucky,
which led to the contamination of several miles of sewer.
The waste-water treatment plant was temporarily closed
because of excessive exposure of workers to HEX. (Kominsky
& Wisseman, 1978; Morse et al., 1978, 1979). Releases from
the Memphis production facility have resulted in high
concentrations of HEX in waste water from the facility and
have led to HEX being present in the inflow to the
receiving waste-water treatment plant and in air at the
treatment plant. HEX has also been released from a waste
site in Montague, Michigan, USA (US EPA, 1980b).
The US EPA Toxic Chemical Release Inventory for 1987
revealed that over 4.5 tonnes of HEX was released at the
Velsicol facility in Marshall, Illinois, USA (most of it
from underground injection disposal), that over 540 kg was
released at the Velsicol facility in Memphis, and that a
similar quantity was released from the Occidental Chemical
Corporation at Niagara Falls, New York, USA. The latter
two releases were primarily to the air (US EPA, 1989).
 4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Overviewa
The fate and transport of HEX in the atmosphere are
not well understood, but the available information
suggests that the compound does not persist. Atmospheric
transport of HEX from an area of stored waste has been
reported (Peters et al., 1981). Experimentally derived
constants for HEX in various environmental processes are
given in Table 2.
Table 2. Summary of constants used in the exposure
analysis modelling system (EXAMS)a
---------------------------------------------------
Constants Values used
---------------------------------------------------
Water solubility (Ks) 1.8 mg/litre
Henry's law constant (KH) 2.7 x 10-2 atm m3/mol
Octanol/water partition 1.1 x 105
coefficient (Pow)
Photolysis (kp) 3.9 h-1
Hydrolysis 4.0 x 10-3 h-1b
Oxidation (kox) 1 x 10-10 M-1 sec-1c
Biodegradation (kB) 1 x 10-5 ml org-1 h-1d
---------------------------------------------------
a Adapted from Wolfe et al. (1982).
b Extrapolated to 25 °C.
c Estimated value (Wolfe et al., 1982).
d This is a maximum value based on the observation
that there was no detectable difference in the
hydrolysis rate in either sterile or non-sterile
studies and measured organism numbers (plate
counts).
In water, HEX probably dissipates rapidly by means of
photolysis, hydrolysis, and biodegradation. In shallow
water (a few centimetres deep), it has a photolytic half-
life of approximately 0.2 h (Butz et al., 1982; Wolfe et
al., 1982). Chou et al. (1987) found this first-order
reaction to take even less time in full sunlight. In
deeper water where photolysis is precluded, hydrolysis and
biodegradation should become the key degradative processes
when there is little movement in the system. The hydro-
lytic half-life of HEX ranges from several days to
-----------------------------------------------------------
a Throughout this chapter, the terms sorb and sorption
are used in preference to absorb/adsorb and
absorption/adsorption.
approximately 3 months, and it is not strongly affected by
the pH in the environmental range (5-9), by salinity or by
the presence of suspended solids (Yu & Atallah, 1977a;
Wolfe et al., 1982). HEX is known to volatilize from water
(Kilzer et al., 1979; Weber, 1979). It is probable that
volatilization is limited by diffusion, i.e. loss from
deeper waters should occur very slowly unless vertical
mixing has taken place. Sorption on sediments may also
retard volatilization.
The fate and transport of HEX in soils are affected by
its strong tendency to sorb onto organic matter (Weber,
1979; Kenaga & Goring, 1980; Wolfe et al., 1982). Another
possibility is that HEX partitions to the interior of soil
particles and stays in loams and silt in a dissolved
state. HEX should be relatively immobile in soil because
of its high log P value (Briggs, 1973), but several inci-
dents in the USA have shown that this is not true in all
soil types (Sprinkle, 1978). Volatilization, which is
likely to occur primarily at the soil surface, is
inversely related to the organic matter level and water-
holding capacity of the soil (Kilzer et al., 1979). Leach-
ing of HEX by ground water can occur, while chemical
hydrolysis and microbial metabolism would be expected to
reduce levels in the environment. HEX is metabolized by a
number of unidentified soil microorganisms (Rieck,
1977b,c; Thuma et al., 1978).
The high lipophilicity and log Pow of HEX indicate a
high potential for bioaccumulation. However, in practice,
this potential is not realized because of metabolism and
elimination. Steady-state bioconcentration factors (BCFs)
in fish measured in 30-day flow-through systems were 29 or
less (Spehar et al., 1979; Veith et al., 1979). In a model
ecosystem study, BCF values for a range of aquatic organ-
isms were between 340 and 1600. These measurements did not
distinguish between the parent compound and the
metabolites and, therefore, should be regarded as over-
estimates of bioaccumulation.
4.2. Transport and distribution between media
4.2.1. Air
Little relevant information is available to predict
the fate of HEX in the air. Cupitt (1980) estimated its
tropospheric residence time to be approximately 5 h, based
on estimated rates of reaction with photochemically
produced hydroxyl radicals and ozone. The theoretical
reaction rates were calculated to be 59 x 10-12 and
8 x 10-18 cm3 molecule-1 sec-1, respectively. In
estimating the tropospheric residence time, or the time
for a quantity of HEX to be reduced to 1/e (or approxi-
mately 37%) of its original value, it was assumed that the
rate constants calculated at room temperature for both
reactions were valid in the ambient atmosphere, and that
the background concentrations of hydroxyl radical and
ozone were 106 and 1012 molecules cm3, respectively.
Direct atmospheric photolysis of HEX was also rated as
"probable", since HEX has a chromophore that absorbs
light in the solar spectral region, and is known to photo-
lyse in aqueous media. No attempt was made to estimate a
rate for atmospheric photolysis. Cupitt (1980) listed the
theoretical degradation products as phosgene, diacylchlor-
ides, ketones, and free chlorine radicals, all of which
would be likely to react with other elements and
compounds.
The vapour pressure and vapour density, water solu-
bility, sorption properties, rapid photolysis (Wolfe et
al., 1982), and high reactivity (Callahan et al., 1979) of
HEX are significant factors that affect its atmospheric
transport. The atmospheric transport of HEX vapour from a
closed waste site in Montague, Michigan, USA, was reported
by Peters et al. (1981). At an unspecified distance
downwind from the site, HEX was detected in the air at
concentrations of 0.36-0.59 µg/m3 (0.032-0.053 ppb).
Based on the concentration ratio of HEX and a tracer gas
released at a known rate, the average HEX emission rate
during the measurement period was calculated to be
0.26 g/h.
4.2.2. Water
In the event of release into shallow or flowing bodies
of water, degradative processes such as photolysis, hydro-
lysis, and biodegradation, as well as transport processes
involving volatilization and other physical loss mechan-
isms, would be expected to play a significant role in
dissipating HEX. In deeper, non-flowing bodies of water,
hydrolysis and biodegradation may become the predominant
processes in determining the fate of HEX.
HEX introduced into bodies of water may be transported
in either the undissolved, dissolved, or sorbed forms. In
its undissolved form, HEX will tend to sink because of its
high relative density, and it may then become concentrated
in deeper waters where photolysis and volatilization would
be precluded. Some HEX may be dissolved in water (up to
approximately 2 mg/litre) and then be dispersed with water
flow. The solubility of HEX in water, soil extracts, and
sanitary landfill leachates ranges from 1.03 to 1.25 mg
per litre (Chou & Griffin, 1983). It tends to sorb onto
organic matter and may then be transported with water flow
in a suspended form. Transport to the air may occur by
volatilization, which has been measured in laboratory
studies (Kilzer et al., 1979; Weber, 1979) and was pre-
dicted using the EXAMS model by Wolfe et al. (1982). How-
ever, suspended solids in surface water may be a major
factor in reducing volatilization.
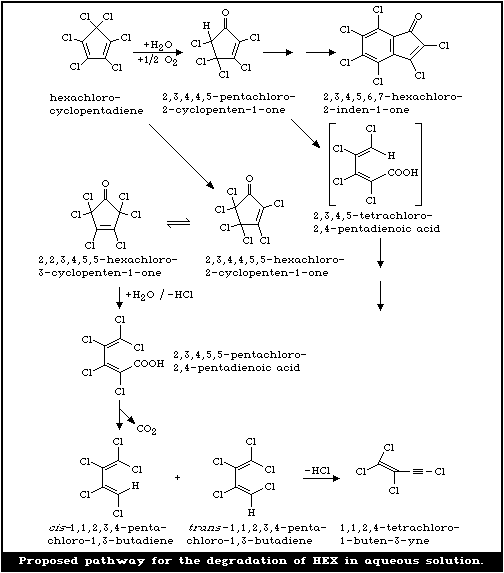
The photodegradation and degradation products of HEX
in aqueous solution have been studied in the laboratory
(Chou & Griffin, 1983; Chou et al., 1987). When aqueous
solutions containing 1.33 µg HEX/ml (a concentration
below its solubility in water) were exposed to sunlight,
the rate of photodegradation followed a first-order reac-
tion; the photolytical half-lives of HEX in tap water,
creek water, and distilled water in sunlight were all less
than 4 min. At least eight degradation products were posi-
tively or tentatively identified, 2,3,4,4,5-pentachloro-2-
cyclopentenone, hexachloro-2-cyclopentenone, and hexa-
chloro-3-cyclopentenone being the primary photodegradation
products. Secondary degradation products and other com-
pounds were formed through minor routes of degradation.
A proposed pathway for aqueous degradation is shown in
Fig. 2 (Chou & Griffin, 1983).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Overviewa
The fate and transport of HEX in the atmosphere are
not well understood, but the available information
suggests that the compound does not persist. Atmospheric
transport of HEX from an area of stored waste has been
reported (Peters et al., 1981). Experimentally derived
constants for HEX in various environmental processes are
given in Table 2.
Table 2. Summary of constants used in the exposure
analysis modelling system (EXAMS)a
---------------------------------------------------
Constants Values used
---------------------------------------------------
Water solubility (Ks) 1.8 mg/litre
Henry's law constant (KH) 2.7 x 10-2 atm m3/mol
Octanol/water partition 1.1 x 105
coefficient (Pow)
Photolysis (kp) 3.9 h-1
Hydrolysis 4.0 x 10-3 h-1b
Oxidation (kox) 1 x 10-10 M-1 sec-1c
Biodegradation (kB) 1 x 10-5 ml org-1 h-1d
---------------------------------------------------
a Adapted from Wolfe et al. (1982).
b Extrapolated to 25 °C.
c Estimated value (Wolfe et al., 1982).
d This is a maximum value based on the observation
that there was no detectable difference in the
hydrolysis rate in either sterile or non-sterile
studies and measured organism numbers (plate
counts).
In water, HEX probably dissipates rapidly by means of
photolysis, hydrolysis, and biodegradation. In shallow
water (a few centimetres deep), it has a photolytic half-
life of approximately 0.2 h (Butz et al., 1982; Wolfe et
al., 1982). Chou et al. (1987) found this first-order
reaction to take even less time in full sunlight. In
deeper water where photolysis is precluded, hydrolysis and
biodegradation should become the key degradative processes
when there is little movement in the system. The hydro-
lytic half-life of HEX ranges from several days to
-----------------------------------------------------------
a Throughout this chapter, the terms sorb and sorption
are used in preference to absorb/adsorb and
absorption/adsorption.
approximately 3 months, and it is not strongly affected by
the pH in the environmental range (5-9), by salinity or by
the presence of suspended solids (Yu & Atallah, 1977a;
Wolfe et al., 1982). HEX is known to volatilize from water
(Kilzer et al., 1979; Weber, 1979). It is probable that
volatilization is limited by diffusion, i.e. loss from
deeper waters should occur very slowly unless vertical
mixing has taken place. Sorption on sediments may also
retard volatilization.
The fate and transport of HEX in soils are affected by
its strong tendency to sorb onto organic matter (Weber,
1979; Kenaga & Goring, 1980; Wolfe et al., 1982). Another
possibility is that HEX partitions to the interior of soil
particles and stays in loams and silt in a dissolved
state. HEX should be relatively immobile in soil because
of its high log P value (Briggs, 1973), but several inci-
dents in the USA have shown that this is not true in all
soil types (Sprinkle, 1978). Volatilization, which is
likely to occur primarily at the soil surface, is
inversely related to the organic matter level and water-
holding capacity of the soil (Kilzer et al., 1979). Leach-
ing of HEX by ground water can occur, while chemical
hydrolysis and microbial metabolism would be expected to
reduce levels in the environment. HEX is metabolized by a
number of unidentified soil microorganisms (Rieck,
1977b,c; Thuma et al., 1978).
The high lipophilicity and log Pow of HEX indicate a
high potential for bioaccumulation. However, in practice,
this potential is not realized because of metabolism and
elimination. Steady-state bioconcentration factors (BCFs)
in fish measured in 30-day flow-through systems were 29 or
less (Spehar et al., 1979; Veith et al., 1979). In a model
ecosystem study, BCF values for a range of aquatic organ-
isms were between 340 and 1600. These measurements did not
distinguish between the parent compound and the
metabolites and, therefore, should be regarded as over-
estimates of bioaccumulation.
4.2. Transport and distribution between media
4.2.1. Air
Little relevant information is available to predict
the fate of HEX in the air. Cupitt (1980) estimated its
tropospheric residence time to be approximately 5 h, based
on estimated rates of reaction with photochemically
produced hydroxyl radicals and ozone. The theoretical
reaction rates were calculated to be 59 x 10-12 and
8 x 10-18 cm3 molecule-1 sec-1, respectively. In
estimating the tropospheric residence time, or the time
for a quantity of HEX to be reduced to 1/e (or approxi-
mately 37%) of its original value, it was assumed that the
rate constants calculated at room temperature for both
reactions were valid in the ambient atmosphere, and that
the background concentrations of hydroxyl radical and
ozone were 106 and 1012 molecules cm3, respectively.
Direct atmospheric photolysis of HEX was also rated as
"probable", since HEX has a chromophore that absorbs
light in the solar spectral region, and is known to photo-
lyse in aqueous media. No attempt was made to estimate a
rate for atmospheric photolysis. Cupitt (1980) listed the
theoretical degradation products as phosgene, diacylchlor-
ides, ketones, and free chlorine radicals, all of which
would be likely to react with other elements and
compounds.
The vapour pressure and vapour density, water solu-
bility, sorption properties, rapid photolysis (Wolfe et
al., 1982), and high reactivity (Callahan et al., 1979) of
HEX are significant factors that affect its atmospheric
transport. The atmospheric transport of HEX vapour from a
closed waste site in Montague, Michigan, USA, was reported
by Peters et al. (1981). At an unspecified distance
downwind from the site, HEX was detected in the air at
concentrations of 0.36-0.59 µg/m3 (0.032-0.053 ppb).
Based on the concentration ratio of HEX and a tracer gas
released at a known rate, the average HEX emission rate
during the measurement period was calculated to be
0.26 g/h.
4.2.2. Water
In the event of release into shallow or flowing bodies
of water, degradative processes such as photolysis, hydro-
lysis, and biodegradation, as well as transport processes
involving volatilization and other physical loss mechan-
isms, would be expected to play a significant role in
dissipating HEX. In deeper, non-flowing bodies of water,
hydrolysis and biodegradation may become the predominant
processes in determining the fate of HEX.
HEX introduced into bodies of water may be transported
in either the undissolved, dissolved, or sorbed forms. In
its undissolved form, HEX will tend to sink because of its
high relative density, and it may then become concentrated
in deeper waters where photolysis and volatilization would
be precluded. Some HEX may be dissolved in water (up to
approximately 2 mg/litre) and then be dispersed with water
flow. The solubility of HEX in water, soil extracts, and
sanitary landfill leachates ranges from 1.03 to 1.25 mg
per litre (Chou & Griffin, 1983). It tends to sorb onto
organic matter and may then be transported with water flow
in a suspended form. Transport to the air may occur by
volatilization, which has been measured in laboratory
studies (Kilzer et al., 1979; Weber, 1979) and was pre-
dicted using the EXAMS model by Wolfe et al. (1982). How-
ever, suspended solids in surface water may be a major
factor in reducing volatilization.
The photodegradation and degradation products of HEX
in aqueous solution have been studied in the laboratory
(Chou & Griffin, 1983; Chou et al., 1987). When aqueous
solutions containing 1.33 µg HEX/ml (a concentration
below its solubility in water) were exposed to sunlight,
the rate of photodegradation followed a first-order reac-
tion; the photolytical half-lives of HEX in tap water,
creek water, and distilled water in sunlight were all less
than 4 min. At least eight degradation products were posi-
tively or tentatively identified, 2,3,4,4,5-pentachloro-2-
cyclopentenone, hexachloro-2-cyclopentenone, and hexa-
chloro-3-cyclopentenone being the primary photodegradation
products. Secondary degradation products and other com-
pounds were formed through minor routes of degradation.
A proposed pathway for aqueous degradation is shown in
Fig. 2 (Chou & Griffin, 1983).
 Kilzer et al. (1979) determined the rate of 14C-HEX
volatilization from water as a function of the rate of
water evaporation. Bottles containing aqueous HEX sol-
utions (50 µg/litre) were kept at 25 °C without shaking.
The escaping vapour condensed on a "cold finger" and was
quantified by liquid scintillation spectroscopy. Based on
recovery of added label, the HEX volatilization rates for
the first and second hours of testing were calculated to
be 5.87 and 0.75%/ml of water, respectively. Since the
water evaporation rate was 0.8-1.5 ml/h, the evaporation
rates for HEX were within the ranges of 4.7-8.8 and 0.6-
1.1%/h, respectively. These results suggest that a fairly
rapid initial volatilization occurred at the water sur-
face, and that by the second hour diffusion of HEX to the
water surface may have been limiting because of the static
conditions of the test. If the rate observed during the
second hour had continued for the remaining 24 h, the
total loss would have been approximately 18-34%, i.e.
somewhat less than that observed in the test conducted by
Weber (1979) where unstoppered bottles were shaken.
At 25-30 °C and in the environmental pH range of 5-9,
the hydrolytic half-life of HEX was found to be approxi-
mately 3-11 days (Yu & Atallah, 1977a; Wolfe et al.,
1982). In a later study in which evaporation and photo-
chemical reactions were carefully prevented, the hydro-
lytic half-life was approximately 3 months (Chou &
Griffin, 1983). Hydrolysis is much slower than photolysis
(see Table 2) but may be a significant load-reducing
process in waters where photolysis and physical transport
processes are not important (i.e. in deep, non-flowing
waters). Wolfe et al. (1982) found HEX hydrolysis to be
independent of pH over the range of 3-10. The rate con-
stant was dependent on temperature at pH 7.0, and the
half-life was estimated to be 3.31, 1.71, and 0.64 days at
30, 40, and 50 °C, respectively. The addition of various
buffers or sodium chloride (0.5 mol/litre) did not affect
the hydrolysis rate constant, suggesting that the rate
constant obtained would be applicable to marine environ-
ments as well. The addition of natural sediments, suf-
ficient to sorb up to 92% of the compound, caused the rate
constant to vary by less than a factor of 2. It was
therefore concluded that sorption to sediments would not
significantly affect the rate of hydrolysis (Wolfe et al.,
1982).
4.2.3. Soil
When it is released on to soil, HEX is likely to sorb
strongly to any organic matter or humus present (Weber,
1979; Kenaga & Goring, 1980). The HEX concentrations
should decrease with time as populations of soil micro-
organisms that are better adapted to metabolize HEX
increase (Rieck, 1977b,c; Thuma et al., 1978). Volatiliz-
ation, photolysis, and various chemical processes may also
dissipate the compound in certain soil environments.
The main methods of transport for HEX applied to the
soil are (a) the movement of particles to which it is
sorbed and (b) volatilization. Other possibilities are
that HEX is sorbed on to soil colloids or that it par-
titions to the interior of soil particles and stays in
loams and silts in a dissolved state. No data are avail-
able pertaining to HEX transport on soil particles. How-
ever, in a few studies, the rate of volatilization from
soils has been reported and is discussed in the following
paragraphs.
Kilzer et al. (1979) found that 14C-HEX and its
degradation products volatilized from moist soils (sand,
loam, and humus) at a faster rate during the first hour of
the study than during the second hour. HEX (50 µg/kg) was
placed in bottles with each soil type, the bottles were
shaken vigorously, and they were then incubated for 2 h at
25 °C without shaking. The radiolabelled HEX condensed on
a "cold finger" and was quantified by liquid scintil-
lation counting. For sand, loam, and humus, the volatiliz-
ation rate was expressed as the percentages of applied
radioactivity per ml of evaporated water. For the first
hour the percentages were 0.83, 0.33, and 0.14%, respect-
ively, and for the second hour they were 0.23, 0.11, and
0.05%. For HEX and nine other tested chemicals, the
authors found that the volatilization rate from distilled
water could not be used to predict the rate from wetted
soils. Among the chemicals tested, there was no corre-
lation between water solubility or vapour pressure and
volatilization from soils. The volatilization rate for
HEX and its metabolites in soil was primarily dependent on
soil organic matter content, mainly because of the highly
sorptive characteristics of HEX.
In a model ecosystem study, Kloskowski et al. (1981)
applied 14C-HEX to 1 kg of humus sand soil (2 mg/kg) and
grew summer barley by keeping the system under an illumi-
nation of 10 000 lux (12 h light, 12 h dark) at 20-24 °C
in an enclosed 10-litre desiccator with an aeration of
10 ml/min. After 7 days, approximately 19.5% of the orig-
inal radioactivity was recovered in the form of 14CO2
evolved and 0.5% as volatilized organics. The level of
radiolabelled compounds in the plants was 13.4 mg/kg,
which represented a bioaccumulation factor of 7.1 (i.e.
plant residues divided by soil residues). It is not clear
whether the plants played a major role in the volatiliz-
ation or metabolic fate of HEX, but the total 14C recov-
ery was over 95%.
Rieck (1977c) measured the rate of volatilization of
HEX from Maury silt loam soils. After the application of
100 mg 14C-HEX to soil, the cumulative evaporation of HEX
and its non-polar metabolites (penta- and tetrachloro-
cyclopentadiene) on days 1, 2, 3, 5, 7, and 14 was 9.3,
10.2, 10.6, 10.8, 11.0, and 11.2%, respectively. The
results indicated that HEX evaporation to air occurred
mainly during the first day after application and was
probably associated with the surface soil only.
The soil sorption properties of compounds such as HEX
can be predicted from their soil organic carbon/water
partition coefficients (Koc). Kenaga (1980) examined
the sorption properties of 100 chemicals and concluded
that compounds with Koc values > 1000 are tightly bound
to soil components and are immobile in soils. Those with
values < 100 are sorbed less strongly and are considered
to be moderately to highly mobile. Thus, the theoretical
Koc value is useful as an indicator of potential soil
leachability or binding of the chemical. The Koc values
also indicate whether a chemical is likely to enter water
by leaching or by being sorbed to eroded soil particles.
Since Koc values for HEX are not available in the
literature, these values were calculated using the
following mathematical equation, developed by Kenaga &
Goring (1980) and Kenaga (1980):
log Koc = 3.64-0.55 (log WS)
where WS is water solubility (mg/litre), and the 95%
confidence limits are ± 1.23 orders of magnitude. The
calculated values of Koc for HEX using the reported water
solubility values of 2.1 mg/litre (Dal Monte & Yu, 1977),
1.8 mg/litre (Wolfe et al., 1982), and 0.805 mg/litre (Lu
et al., 1975) are 2903, 3159, and 4918, respectively.
Since these calculated Koc values are all > 1000, the
authors concluded that HEX is tightly bound to soil
components and immobile in the soil compartment. Simi-
larly, Briggs (1973) concluded that compounds with a log
octanol/water partition coefficient (log Pow) > 3.78 are
immobile in soil. Log Pow values for HEX of 5.04 (Wolfe
et al., 1982) and 5.51 (Veith et al., 1979) have been
measured.
In studies by Chou & Griffin (1983), the mobility of
HEX (C-56) in six soils was measured with several leaching
solvents using soil thin-layer chromatography (TLC) and
column leaching studies. It remained immobile in the soil
when leached with water, landfill leachate, or caustic
brine, but was highly mobile when leached with organic
solvents. A further conclusion was that several degra-
dation products of HEX migrated through soils faster than
HEX itself, and that the degradation products warranted
further study. The sorption capacity of HEX was highly
correlated with the total organic carbon (TOC) content of
soil materials (r2 = 0.97), which was the dominant soil
characterization parameter. Sorption appears to be pre-
dictable from the TOC content of soils (Chou & Griffin,
1983).
4.3. Biotransformation
4.3.1. Biodegradation
The metabolism of HEX by soil microorganisms is
apparently an important process in its environmental
degradation. Soil degradation is rapid under non-sterile
aerobic and anaerobic conditions, and indirect evidence
for microbial involvement has been reported by Rieck
(1977b,c). In one of his studies, Rieck (1977b) used
several types of treatments and soils of different pH to
determine whether the biodegradation of HEX in Maury silt
loam soil was biologically or chemically mediated, or
both. Soils were incubated in glass flasks covered with
perforated aluminum foil and kept on a laboratory shelf,
presumably exposed to ambient lighting through the flask
walls. When 14C-HEX was applied to non-sterile soil at
1 mg/kg, only 6.1% was recovered as non-polar material
(either HEX or non-polar degradation products) 7 days
after treatment, and approximately 71.7% was polar and
unextractable material. Adjustment of the pH to 4 or 8 had
little effect on these results. By comparison, in auto-
claved soil (the control), 36.1% of the applied dose was
recovered as non-polar material and only 33.4% was
recovered as polar and unextractable material. The degra-
dation of HEX under anaerobic (flooded) conditions
occurred at a slightly faster rate than under aerobic
conditions. However, no sterile, flooded control was used
to determine the effects of hydrolysis, which could have
accounted for the observed effect in this treatment. The
mean total recovery in all treatments decreased from 67%
at 7 days to 55% at 56 days. This decrease was attributed
to volatilization of HEX and/or its degradation products.
Volatilization from soil was examined in a further
experiment (Rieck, 1977c). In a 14-day study, radiocarbon
volatilized from non-sterile, 14C-HEX-treated soil was
trapped and assayed. A total of 20.2% of the applied 14C
was trapped: 11.2% in hexane and 9.0% in ethanolamine-
water. Most of the hexane fraction (9.3% of the applied
14C) was trapped during the first day, and probably
represented volatilized HEX. However, the ethanolamine-
water fraction, considered to represent evolved carbon
dioxide, was released gradually over the 14-day period.
In the soil analysis, non-polar (extractable) and polar
(extractable and unextractable) material accounted for 3.4
and 40.0% of the dose, respectively, during the 14 days;
total recovery was only 63.6% including volatilization.
No metabolic products were identified in the two studies
by Rieck (1977b,c).
Thuma et al. (1978) studied the feasibility of using
selected pure cultures (organisms not identified) to
biodegrade spills of hazardous chemicals, including HEX,
on soil. They tested 23 organisms and found that from
2-76% of the applied HEX had been removed from the aqueous
culture medium within 14 days. Seven of the 23 organisms
degraded more than 33% of the HEX within 14 days. Losses
of HEX by other means than biodegradation were accounted
for by using controls.
Atallah et al. (1980) conducted an aqueous aerobic
biodegradability study to determine whether HEX could be
degraded to CO2 and at what rate. The inoculum was a
mixed acclimated culture containing secondary municipal
waste effluent and several strains of Pseudomonas putida.
14C-labelled HEX was the sole source of carbon in the
study, with the exception of trace levels of vitamins.
Total removal of 14C, primarily as volatile organic com-
pounds, was > 80% during the first day in both uninocu-
lated (45 mg HEX/litre) and inoculated (4.5 and 45 mg
HEX/litre) media, although removal was slightly greater in
inoculated media. 14CO2 was released from both media,
indicating that CO2 was a product of hydrolysis as well
as of biodegradation. The rate of conversion to CO2 was
initially higher in the uninoculated media, but after 1
week, became higher in the inoculated media. This study
showed clearly that HEX can be biodegraded in aquatic
media under laboratory conditions. However, Wolfe et al.
(1982) failed to detect any difference between the HEX
degradation rates in hydrolysis experiments where non-
sterile natural sediments were added to water (10 g/litre)
and those where sterile sediment was used. They calculated
a relatively low value (1 x 10-5 ml org-1 h-1; see
Table 2) as a maximum biodegradation rate, and conse-
quently biodegradation was estimated to be a relatively
unimportant fate process in the EXAMS model (see Table 3).
These studies indicate that the persistence of HEX in
soil is brief, degradation of more than 90% of applied HEX
to non-polar products occurring within approximately 7
days. Factors contributing to this loss include abiotic
and biotic degradation processes and volatilization,
although the relative importance of each is difficult to
quantify.
4.3.2. Bioconcentration, bioaccumulation, and biomagnification
Bioaccumulation, sometimes also expressed as biologi-
cal persistence, is a consequence of the rate of elimin-
ation of a compound and the extent of adsorption.
The terminology used in this section conforms to that
used by Macek et al. (1979):
* bioconcentration implies that tissue residues result
only from simultaneous uptake and elimination from
exposure to the ambient environment (e.g., air for
terrestrial species or water for aquatic species);
* bioaccumulation considers all exposures (air, water,
and food) of an individual organism to be the source
of tissue residues;
* biomagnification defines the increase in tissue
residues observed at successively higher trophic
levels of a food web.
Table 3. Summary of results of computer simulation of the
fate and transport of hexachlorocyclopentadiene in four
typical aquatic environmentsa
---------------------------------------------------------------
River Pond Eutrophic Oligotrophic
lake lake
---------------------------------------------------------------
Distribution (%)
Water column 1.22 14 12.97 2.91b
Sediment 98.78 86 87.03 97.09
Recovery timec (days) 52 81 58 87
Load reduction (%) by processes:
Hydrolysis 8.04 17.85 8.29 16.50
Oxidation 0.00 0.00 0.00 0.00
Photolysis 18.68 80.39 89.18 82.41
Biodegradation 0.57 0.23 0.30 0.01
(biolysis)
Volatilization 0.69 1.33 1.56 1.08
Exportd 72.02 0.20 0.01 0.00
---------------------------------------------------------------
a Adapted from Wolfe et al. (1982), with correction applied.
b Value was incorrectly reported as 32.91 in original paper.
c The time needed to reduce steady-state concentrations by
97% (five half-lives).
d Physical loss from the system by any pathway other than
volatilization.
The log octanol/water partition coefficient of HEX has
been experimentally determined to be 5.04 (Wolfe et al.,
1982) and 5.51 (Veith et al., 1979), which would indicate
a substantial potential for bioconcentration, bioaccumu-
lation, and biomagnification. Actual determinations of
bioconcentration and bioaccumulation in several aquatic
organisms, however, indicate that HEX does not accumulate
to any great extent (Lu et al., 1975; Podowski & Khan,
1979, 1984; Spehar et al., 1979; Veith et al., 1979),
mainly because it is metabolized rapidly.
Podowski & Khan (1979, 1984) conducted several exper-
iments on the uptake, bioaccumulation, and elimination
of 14C-HEX in goldfish ( Carassius auratus) and concluded
that this species rapidly eliminates absorbed HEX. In one
experiment, fish were transferred daily into fresh sol-
utions of 14C-HEX for 16 days. This transfer of three
fish/jar resulted in an accumulative exposure of 240 µg
of HEX. Nominal HEX concentrations of 10 µg/litre re-
sulted in measured water concentrations (based on radio-
activity) in the range of 3.4-4.8 µg/litre, because of
rapid volatilization of the compound. Radioactivity
accumulated rapidly in fish tissue, reaching a maximum on
day 8 corresponding to approximately 6 mg HEX/kg. Since an
undetermined amount of the radioactivity was present as
metabolites, no bioconcentration factor could be calcu-
lated. From day 8 to day 16, tissue levels declined in
spite of the daily renewal of exposure solutions, indi-
cating that excretion of HEX and/or its metabolites was
occurring more rapidly than uptake. In a static exposure
to an initial measured HEX concentration of 5 µg per
litre, uptake of the radiolabel by the fish was to a level
corresponding to 1.6 mg HEX/kg on day 2, accompanied by a
slight decrease of HEX in the water. By day 4, approxi-
mately 50% of the radiolabel had been excreted, and the
radioactivity in the water increased. Over the following
12 days, the radioactivity in both water and fish declined
slowly.
Podowski & Khan (1979, 1984) also studied the elimin-
ation, metabolism, and tissue distribution of HEX injected
intraperitoneally into goldfish and concluded that gold-
fish eliminate injected HEX both rapidly and linearly
(the biological half-life was approximately 9 days). The
fish (27-45 g) were injected with 39.6 µg 14C-HEX and
analysed 3 days later. Of the 97% of the radiolabelled
dose accounted for, approximately 18.9% was eliminated by
the fish. Of the residue found in the fish, 47.2% was
extractable in organic solvent (little of the radio-
labelled material could be identified as HEX, which indi-
cated that extensive biotransformation had occurred),
10.6% consisted of water-soluble metabolites, and 20.3%
was unextractable. None of the metabolites were ident-
ified. The elimination was biphasic, consisting of a rapid
initial phase followed by a slower terminal phase.
In another part of these studies, the residual
activity in several fish tissues was assayed 2, 4, 6, and
8 days after an injection of 38.4 µg 14C-HEX per fish.
The activity corresponded to 0.2 and 0.3 µg HEX/kg in the
spinal cord and gills, respectively, concentrations that
remained constant throughout the 8-day period of the
study. Residues in the kidneys and bile increased within
the same period from 1-3 and 0-32 µg/kg, respectively,
indicating elimination by these routes. The authors stated
that the increase probably occurred from enhanced con-
version of the parent compound into polar products, which
could be excreted more easily. In the other tissues, all
residual levels decreased, leaving only the liver with a
level of more than 1 µg/kg. The metabolites were not
identified (Podowski & Khan, 1979, 1984).
Veith et al. (1979) determined a bioconcentration
factor (BCF) for HEX of 29 in the fat-head minnow
(Pimephales promelas). In a 32-day flow-through study,
30 fish were exposed to HEX at a mean concentration of
20.9 µg/litre. Five fish at a time were killed at 2, 4,
8, 16, 24, and 32 days for residue analysis. The study
was conducted using Lake Superior water at 25 °C (pH 7.5,
dissolved oxygen > 5.0 mg/litre, and hardness 41.5 mg
CaCO3/litre. On the basis of its estimated octanol/water
partition coefficient alone (log Pow = 5.51), a BCF of
approximately 9600 would have been predicted. However,
HEX did not bioconcentrate substantially, and therefore
deviated from the log P:log BCF relationship shown for
most of the other 29 chemicals tested by these researchers.
Lu et al. (1975) studied the fate of HEX in a model
terrestrial-aquatic ecosystem maintained at 26.7 °C with a
12-h photoperiod. The model ecosystem consisted of 50
sorghum (Sorghum vulgare) plants (7.62-10.16 cm tall) in
the terrestrial portion, while 10 snails (Physa sp.), 30
water fleas (Daphnia magna), filamentous green algae
(Oedogonium cardiacum), and a plankton culture were
added to the aquatic portion. The sorghum plants were
treated topically with 5.0 mg 14C-HEX in acetone to simu-
late a terrestrial application of 1.1 kg/hectare. Ten
early-fifth-instar caterpillar larvae (Estigmene acrea)
were placed on the plants. The insects consumed most of
the treated plant surface within 3-4 days. The faeces,
leaf grass, and the larvae themselves contaminated the
moist sand, permitting distribution of the radiolabelled
metabolites by water throughout the ecosystem. After 26
days, 300 mosquito larvae (Culex pipiens quinquefasciatus)
were added to the ecosystem, and on day 30 three mosquito
fish (Gambusia affinis) were added. The experiment was
terminated after 33 days, and the various parameters were
analysed. The radioactivity was then extracted with
diethyl ether from the water and with acetone from the
organisms. The results of thin-layer chromatographic
analysis of the extracts are presented in Table 4. Data
were not reported for Daphnia magna or the salt marsh
caterpillar. Uptake in this experiment occurred through
food as well as water, and therefore is termed bioaccumu-
lation rather than bioconcentration. Lu et al. (1975) used
the term ecological magnification to designate the bio-
accumulation factor (BAF). The BAF for HEX in fish was
448 (0.1076 mg/kg fish divided by 0.24 µg/litre water)
for the 3-day exposure period, indicating a moderate
potential for concentration (Kenaga, 1980). The BAFs in
algae (< 33-day exposure), snails (< 33-day exposure), and
mosquito larvae (7-day exposure) were reported to be 341,
1634, and 929, respectively (Lu et al., 1975).
Table 4. Relative distribution of hexachlorocyclopentadiene (HEX) and
its degradation products in a model ecosystema
------------------------------------------------------------------------
14C-HEX equivalents
Water Algae Snail Mosquito Fish
(mg/litre) (mg/kg) (mg/kg) larva (mg/kg)
(mg/kg)
------------------------------------------------------------------------
HEX 0.00024 0.0818 0.3922 0.2230 0.1076
Other extractable 0.00204 0.1632 0.3824 0.2542 0.1542
compounds
Total extractable14Cb 0.00228 0.2450 0.7746 0.4772 0.2618
Unextractable14C 0.00750 0.0094 0.0814 0.0104 0.0982
Total14Cc 0.00978 0.2544 0.8560 0.4876 0.3600
------------------------------------------------------------------------
a Source: Lu et al. (1975).
b Sum of HEX and other extractable compounds.
c Sum of total extractable and unextractable 14C.
Biomagnification, measured as the ratio of HEX
residues between trophic levels (e.g., snail/algae or
fish/mosquito), was far less substantial than bioconcen-
tration. Based on the HEX tissue residues, the snail/algae
ratio was 0.3922/0.0818 = 4.8 and the fish/mosquito ratio
was 0.1076/0.2230 = 0.48.
Lu et al. (1975) also studied the metabolism of HEX by
the organisms present in the model terrestrial-aquatic
ecosystem, but none of the products was identified except
for HEX. The authors reported that unmetabolized HEX re-
presented a large percentage of the total extractable 14C,
being 33% in algae, 50% in snail, 46% in mosquito, and 41%
in fish. The percentage of biodegradation was calculated
for each organism (unextractable 14C x 100/total 14C) and
found to be 4% for the algae (in < 33 days), 10% for the
snails (in < 33 days), 2% for the mosquitoes (in 7 days),
and 27% for the fish (in 3 days). However, these values
may underestimate the extent of metabolism, since acetone-
extractable polar compounds were not considered in the
calculations.
The Velsicol Chemical Corporation (1978) conducted
fish tissue residue studies in waters located below their
facility in Memphis, Tennessee, USA, and reported that HEX
was not detected in either catfish or carp, although
chlorinated compounds, including octachlorocyclopentadiene
(a common co-contaminant), were detected in the fish
tissue. This indicated that HEX was not accumulated. The
possible source of these other compounds was not
discussed. In a joint USA federal and state study of the
Mississippi River at locations above, around, and below
Memphis, Bennett (1982) reported that HEX was not detected
in any of the eight fish sample groups analysed by GC/MS.
4.4. Interactions with other physical and chemical factors
4.4.1. Phototransformation
Zepp et al. (1979) and Wolfe et al. (1982) reported
the results of US EPA studies on the rate of HEX photo-
transformation in water. Under a variety of sunlight
conditions, in both distilled and natural waters of 1-4 cm
depth, the phototransformation half-life was < 10 min.
Chou & Griffin (1983) determined a half-life of < 4 min at
740 j/m2. The addition of natural sediments to distilled
water containing HEX had little effect on the phototrans-
formation rate. These findings indicate that the dominant
mechanism of HEX phototransformation is direct absorption
of light by the chemical, rather than photosensitization
reactions involving other dissolved or suspended
materials.
The direct photoreaction of HEX in water was also
studied under controlled conditions in the laboratory
using a monochromatic light (313 nm) with a mercury lamp
source and appropriate filters. Phototransformation rate
constants, computed for the study location (Athens,
Georgia, USA, 34 °N latitude), agreed with those observed
in the sunlight experiments described above. Rate con-
stants were also computed for various times of day at a
latitude of 40 °N. The near-surface phototransformation
rate constant of HEX at this latitude on cloudless days
(averaged over both light and dark periods for 1 year) was
3.9 h-1, which corresponds to a very rapid half-life of
10.7 min (Zepp et al., 1979; Wolfe et al., 1982).
These laboratory researchers suggested that the pri-
mary phototransformation product was the hydrated form of
tetrachlorocyclopentadienone (C5Cl4O, TCPD), although
it was not isolated. Several chlorinated photoproducts
with a higher relative molecular mass than HEX were
detected by GC/MS analysis of the reaction mixture. Photo-
lysis of HEX in methanol gave a product identified as the
dimethyl ketal of TCPD (Wolfe et al., 1982). According to
Zepp et al. (1979), it is likely that TCPD exists predomi-
nantly in its hydrated form in the aquatic environment.
The compound was not isolated, supposedly because it
rapidly dimerizes or reacts to form products of higher
relative molecular mass. Chou et al. (1987) identified
2,3,4,4,5-pentachloro-2-cyclopentenone, hexachloro-2-cyclo-
pentenone, and hexachloro-3-cyclopentenone as the primary
photodegradation products, as well as several other pri-
mary and secondary ones (Chou & Griffin, 1983; Fig. 2).
Yu & Atallah (1977b) found that, at a concentration of
2.2 mg/litre in water, uniformly labelled 14C-HEX was
rapidly converted to water-soluble products upon
irradiation with light from a mercury vapour lamp (light
energy: 40-48% ultraviolet, 40-43% visible, remainder
infrared). In exposures lasting 0.5-5.0 h, 46-53% of the
radiolabel was recovered in the form of water-soluble
products (compared with 7% at initiation), whereas the
amount recovered by organic (petroleum ether) extraction
decreased with increasing exposure duration from 25% to 6%
(compared with 66% at initiation). HEX was not detected
among the photoproducts in the organic extraction. Chou
et al. (1987) also found that dimerization of degradation
products to form compounds of higher relative molecular
mass was only a minor route of degradation.
4.4.2. Oxidation
HEX would not be expected to be oxidized under ordi-
nary environmental conditions. In the laboratory, HEX
reacts with molecular oxygen at 95-105 °C to form a mix-
ture of hexachlorocyclopentenones (Molotsky & Ballweber,
1957). However, based on an estimated second-order oxi-
dation rate constant of 1 x 10-10 M-1 sec-1 at 25 °C
in water (Table 2), the EXAMS computer simulation of Wolfe
et al. (1982) predicted that HEX would not be oxidized in
the simulated river, pond, eutrophic lake or oligotrophic
lake (Table 3).
4.5. Disposal and fate
HEX and HEX-contaminated material and wastes are
disposed of in secure chemical landfills, by incineration,
and by deep well injection (US EPA, 1989). Additionally,
there are solid waste regulations in the USA because,
under the Resource Conservation and Recovery Act, HEX is
designated to be a toxic waste. German regulations are
similar to those of the USA, except that there is no deep
well injection (BUA, 1988).
Since the photodegradation products of HEX have been
identified only recently and because HEX has also been
found in areas where waste has not been disposed of for
years (US EPA, 1980c), it is difficult to determine its
fate in the environment.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
Releases of HEX into the atmosphere can result from
the production, processing, and use of HEX, the disposal
of wastes containing HEX, or from products contaminated
with HEX (Hunt & Brooks, 1984). Data sent to the US EPA
for 1987 regarding emission levels from companies in the
USA indicated that 1400 kg of HEX was emitted into the air
(US EPA, 1989). In the Federal Republic of Germany and
the Netherlands, about 400-500 kg was emitted to the air
in 1987 (BUA, 1988)
In September and October 1985, the Velsicol Chemical
Corporation determined concentrations of HEX at predeter-
mined locations around its production facilities in
Tennessee, USA. The study was designed to measure ambient
concentrations in the air during routine manufacturing
operations. Of the 25 samples collected, 15 were below the
analytical limit of detection, i.e. 0.03 µg (0.1 ppb).
The air HEX levels in the other samples ranged between 1
and 10 µg/m3 (0.1-0.9 ppb) when ambient temperatures
were between 4.4 °C and 27.7 °C (Velsicol Chemical Corpor-
ation, 1986).
The highest reported concentration of HEX measured in
homes in Tennessee was 0.10 µg/m3, while air levels at
the Memphis North treatment plant were as high as
39 µg/m3 (C.S. Clark et al., 1982; Elia et al., 1983).
In an air monitoring study on an abandoned waste site in
Michigan, the average HEX emission rate was 0.26 (± 0.05)
g/h. In May 1977, HEX was detected at a level of 633 µg
per m3 (56 ppb) in air samples collected from a waste
site in Montague, Michigan (US EPA, 1980c).
5.1.2. Water
Benoit & Williams (1981) sampled both untreated water
and drinking-water from a water treatment plant in Ottawa,
Canada. Using solvent extraction analysis with a detection
sensitivity of 50 ng/litre (or the XAD-2 resin extraction
method with a detection sensitivity of approximately 0.5
ng/litre), the authors did not detect any HEX in the
untreated water, but reported levels ranging from 57-110
ng/litre in the finished drinking-water. These results
suggest that HEX was introduced into the drinking-water
during the treatment process. However, the researchers did
not find the source of the HEX. Meier et al. (1985) found
that HEX can be produced through the chlorination of humic
acid.
Limited monitoring data from production sites revealed
that HEX was present in a spot sample at a level of 18
mg/litre (February 1977) and a range of 0.156-8.24 mg per
litre (over the month of January 1977) in the aqueous dis-
charge from the Memphis pesticide plant (US EPA, 1980c).
The calculated concentration of HEX in the Mississippi
River was 6 µg/litre (Carter, 1977). In the summer of
1977, shortly after these readings, a new waste-water
treatment plant began operation (Table 5). Prior to con-
struction of the plant, waste water flowed directly into
the Mississippi River or through one of its tributaries
(Elia et al., 1983). Voluntary improvements in controlling
the discharge from the Memphis plant resulted in reported
levels of 0.07 µg HEX/litre in the Mississippi River,
near the mouth of Wolf Creek (Velsicol Chemical Corpor-
ation, 1978). HEX has also been identified in the soil and
river sediments downstream from a USA manufacturing plant,
even after pesticide production was discontinued (US EPA,
1980c).
5.1.3. Soil
Ambient monitoring data for the terrestrial environ-
ment are not available, but it seems that these concen-
trations should be much lower than those in the aquatic
environment. Deposition of HEX from the atmospheric (and
aquatic) compartments into the terrestrial environment is
expected to be minimal. Similarly, direct release of HEX
into the terrestrial environment (i.e. as an impurity in
chlorinated pesticides) should be decreasing because of
regulatory controls on some products, with the possible
exceptions of disposal at waste sites, accidental spills,
and other illegal disposal methods.
Table 5. Concentrations of selected organic compounds in
influent waste water at the Memphis North treatment plant, 1978a
---------------------------------------------------------------
Concentration (µg/litre)b
Date No. of HEX HEX-BCHc HCBCHd Chlordane
samples
---------------------------------------------------------------
June 1 3 334 57 87
August 5 0.8 329 115 216
September 2 4 292 668 58
October-November 2 0.8 11 17 32
---------------------------------------------------------------
a From: Elia et al. (1983).
b Mean values for the number of samples indicated.
c Hexachlorobicycloheptadiene.
d Heptachlorobicycloheptene.
5.1.4. Food
HEX was qualitatively detected in fish samples taken
from water near a pesticide manufacturing plant in
Michigan (Spehar et al., 1977), but none was detected in
fish samples taken from the waters near the pesticide
manufacturing plant in Memphis (Velsicol Chemical Corpor-
ation, 1978; Bennett, 1982). No information regarding HEX
contamination of other foods is available.
5.2. General population exposure
There are insufficient data to determine the relative
contributions of the various sources of HEX to the
environment. There will be exposure to HEX present in
some commonly used pesticides, and possibly some flame
retardants, where HEX is a contaminant. The US EPA has
reported that exposure of humans to HEX from the air or
water should be extremely low, except in the case of
workers and residents near manufacturing, shipping, and
waste sites, and concluded that general population ex-
posure was not considered to be significant or substantial
(US EPA, 1982).
The only other estimates of relative source contri-
butions are from reports completed for the US EPA (Hunt &
Brooks, 1984) and the BUA (1988). The air releases from
manufacturing processes can come from the vents on reac-
tors, process and storage tanks, and as fugitive
emissions. Hunt & Brooks (1984) estimated that the total
quantity of HEX released from these sources was 8 tonnes
per year. In the Federal Republic of Germany and the
Netherlands, HEX emissions were estimated to be 400-500
kg/year. HEX can also be emitted into the air from the
incineration and landfilling of HEX-containing waste, the
most accurate estimate being 1 tonne per year. The total
annual estimated release of HEX to the environment in the
USA is 11.9 tonnes. These figures are only estimates
because of the limited available data. They are given
simply to indicate the relative magnitude of HEX emissions
into the environment.
Exposure limit values for various countries are given
in Appendix 1.
5.3. Occupational exposure
Occupational exposure can occur both at HEX production
and processing facilities and at other locations where
HEX-containing waste is present. For example, the highest
reported workplace air concentrations of HEX were measured
at the Louisville, Kentucky, USA, waste-water treatment
plant, which received a slug of HEX discharged by a waste
hauler. Four days after the plant was closed, air concen-
trations in the primary treatment area ranged from 3.05
to 11.0 mg/m3 (270 to 970 ppb) (Morse et al., 1979).
During the clean-up operations air concentrations as high
as 133 mg/m3 (11 800 ppb) were reported (Kominsky &
Wisseman, 1978).
In 1982, the Velsicol Chemical Corporation used the
Southern Research Institute (SRI) sampling method at the
Memphis (Tennessee) and Marshall (Illinois) facilities to
evaluate possible exposure of workers to HEX vapour and
the effectiveness of engineering controls. Tables 6 and 7
show the HEX concentrations measured at various points.
At the Memphis facility almost one-half of the worker
8-h time-weighted average (TWA) air HEX concentrations
were at or above the USA Threshold Limit Value of 0.11
mg/m3 (0.01 ppm) (OSHA, 1989). At the Marshall plant all
six TWA values were above 0.11 mg/m3. It should be noted
that in Tables 6 and 7 the results of employee monitoring
are reported without regard to respirator use. Respirators
are required to be worn in operations in these plants
where HEX exposure is possible.
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Appendix 1
(Table 21).
Table 6. Summary of hexachlorocyclopentadiene monitoring, Memphis, Tennessee, USAa
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
HEX Process operator 2 445 0.009-0.011 0.009
HEX No. 1 operator 5 432 0.006-0.033 0.015
HEX No. 2 process operator 5 418 0.006-0.029 0.014
HEX No. 2 cyclo operator 5 417 0.001-0.048 0.017
HEX No. 2 chlorine operator 6 415 0.004-0.0161 0.035
a) HEX Bottoms drumming 1 50 0.016
HEX Area sample control room 12 476 0.002-0.018 0.009
HEX Brinks filter cleaning 2 387 0.004-0.006 0.005
Formulations HEX drummers 4 407 0.002-2.0337 0.010
Material HEX railroad tank car 1 279 0.013 0.008
handling unloading
Endrin R2 filter operator 1 281 0.003
Endrin R1 operator 1 334 0.002
Chlorendic No. 1 operator 2 437 0.0077-0.0102 0.008
anhydride
Chlorendic No. 2 operator D34 2 440 0.0107-0.0198 0.014
anhydride
Chlorendic No. 2 operator R6 2 437 0.0065-0.0169 0.011
anhydride
Chlorendic Packaging operator 1 396 0.035 0.031
anhydride
----------------------------------------------------------------------------------------
Table 6 (contd.)
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
Chlorendic Area sample - control 3 475 0.0003-0.0014 0.001
anhydride room
Heptachlor No. 1 operator 2 407 0.007-0.009 0.007
Heptachlor No. 2 operator 2 415 0.006-0.009 0.007
Heptachlor 237 operator 2 392 0.006-0.019 0.011
Heptachlor Utility operator 1 363 0.006 0.005
Heptachlor Cleaning sparkler filter 3 44 0.002-0.005 0.0003
a) ceiling sample 1 15 0.006
----------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming
that the only chemical exposure occurred during the sampling period.
Table 7. Summary of hexachlorocyclopentadiene monitoring, Marshall, Illinois, USAa
--------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrations TWAb
(min) (ppm) (ppm)
--------------------------------------------------------------------------------------
Chlordane No. 1 operator 8 451 0.0091-0.0316 0.017
Chlordane No. 2 operator 8 455 0.008 -0.0195 0.013
Chlordane No. 3 operator 8 451 0.0002-0.0325 0.014
Chlordane Area sample - 13 433 0.0002-0.0254 0.016
North control room
Chlordane Area sample - 10 435 0.001-0.0276 0.015
South control room
Chlordane HEX filter changing 1 15 0.1322
Chlordane Waste handling HEX 6 307 0.0006-0.0606 0.020
a) HEX mud drumming - 2 15 0.0005-0.0061
ceiling sample
b) Loading HEX waste 2 15 0.1199-0.2325
truck - ceiling sample
c) Sump pit dumping - 2 15 0.0333-0.1129
ceiling sample
--------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming that the
only chemical exposure was during the sampling period.
6. KINETICS AND METABOLISM
6.1. Absorption, retention, distribution, metabolism,
elimination, and excretion
6.1.1. Oral
In a study by Mehendale (1977), male Sprague-Dawley
rats (225-250 g body weight) were administered 5 µmol
of 14C-HEX (approximately 5.5 mg/kg) by oral intubation
as 0.2 ml of a solution in corn oil. The total 14C ac-
tivity contained in the dose was approximately 1 µCi. The
animals were maintained in metabolism cages and the urine
and faeces were collected. About 35% of the administered
dose was collected in the urine and only 10% was collected
in the faeces. More than 87% of the 14C activity in the
urine and more than 60% of the activity in the faeces
appeared during the first day. Only a small amount
(approximately 0.5%) of the original dose was recovered
in the kidneys and liver. The author speculated that, in
view of the low total recovery of the administered dose, a
major part of the dose (> 50%) had been excreted through
the lung. This speculation was later proven to be unwar-
ranted because subsequent studies (Dorough, 1979), in
which exhaled air and lung and tracheal tissues were ana-
lysed, showed that this was not the case. There is strong
evidence to suggest that, after oral dosing with HEX, at
least part of the faecal contents contained a volatile
constituent that could be readily lost if the samples were
dried and powdered, as they were in this case. An extrac-
tion procedure, using the major tissues and excreta, fol-
lowed by thin-layer chromatography, showed that at least
four water-soluble (polar) metabolites were produced, but
not identified, after oral dosing.
In a study designed to re-examine some of the findings
and observations of Mehendale (1977), Dorough (1979)
investigated the accumulation, distribution, and excretion
of 14C-HEX after its administration to rats and mice
either as a single oral dose or as a component of their
diet. The principal results of this study were reported by
Dorough & Ranieri (1984). The animals used were male and
female Sprague-Dawley rats, weighing between 200 and 250 g
body weight, and male and female Sprague-Dawley albino
mice, weighing between 25 and 30 g. Two female rats were
dosed, by gavage, with HEX (20 mg/kg) in 0.9 ml of corn
oil and were immediately placed in separate metabolism
cages through which air was drawn at 600 ml/min. The
evacuated air was passed through two high efficiency
traps. Since less than 1% of the administered dose was
recovered from the traps, it was considered to be conclus-
ive evidence that the pulmonary route is not of major
importance in the excretion of HEX (Dorough, 1979).
Dorough (1979) conducted single dose studies by admin-
istering, with a dosing needle, either 2.5 or 25 mg 14C-
HEX/kg body weight (dissolved in 0.9 ml of corn oil for
rats and in 0.2-0.3 ml for mice). The animals were killed
at 1, 3, or 7 days after dosing, and samples of muscle,
brain, liver, kidneys, fat, and either ovaries or testes
were removed and analysed for 14C activity. Urine and
faeces were also collected during the period between
dosing and tissue sample collection. No appreciable dif-
ferences due to sex or species were found in the excretion
patterns. The liver, kidneys, and fat were the most
important deposition sites for 14C residues in both rats
and mice, the levels in the kidneys of rats and in the
liver of mice being the highest.
In the same study (Dorough, 1979), rats and mice were
also placed on diets containing 1, 5, or 25 mg 14C-HEX
per kg. Assuming a daily food intake of 15 g for rats and
5 g for mice, this would give daily dose rates of 0.066,
0.330, and 1.666 mg/kg for rats and 0.182, 0.910, and 4.55
mg/kg for mice. Feed was replaced in the feeders every
12 h to minimize the loss of 14C-HEX (from volatiliz-
ation), and the feeding study was carried out for 30 days.
During this period, rats and mice were killed at 1, 3, 7,
12, 15, or 30 days. The surviving animals were then
returned to a normal diet for up to 30 days and, during
this post-treatment period, animals were killed at 1, 3,
7, 15, or 30 days after the last exposure. The total
excretion (urine and faeces) of the radiolabel ranged from
63-79% of the consumed 14C-HEX, which was significantly
lower than that found in the single-dose study (73-96%).
In all cases, the liver, kidneys, and fat contained the
highest amounts of 14C, and it appeared that a steady
state for these levels was reached after 15 days of the
feeding phase. A good correlation was observed between
the level of HEX in the diet and the 14C-levels found in
all the examined tissues. In a separate experiment with
male rats, in which the bile duct was cannulated and a
single dose of 14C-HEX (25 mg/kg) was administered
orally, only 16% of the dose was excreted in the bile. The
extraction characteristics of the radiocarbon compounds in
the excreta showed that they were primarily polar metab-
olites, some of which were capable of being converted to
organic-soluble compounds after acid-catalysed hydrolysis.
In a comparative study of the pharmacokinetics
of 14C-HEX after intravenous and oral dosing, Yu &
Atallah (1981) dosed Sprague-Dawley rats (240-350 g body
weight) with either 3 or 6 mg 14C-HEX (specific activity:
0.267 mCi/mmol). The doses ranged from 8.5 to 25.6 mg/kg.
Shortly after oral dosing, 14C activity appeared in the
blood and reached a maximum after approximately 4 h.
The 14C activity appeared in most of the tissues
analysed at 8, 24, 48, 72, 96, and 120 h after dosing.
Following oral dosing, there were higher residue levels in
the kidneys and liver than in any other tissue, although
these levels were generally much lower than those observed
after intravenous dosing. For example, at 24 h after
dosing, the kidneys and liver were found to contain only
0.96 and 0.75%, respectively, of the administered oral
dose, while these organs retained 2.92 and 4.68%, respect-
ively, of the administered intravenous dose. A higher
proportion (15.07%) of the 14C activity was found in the
digestive system (duodenum and large and small intestines)
after oral dosing. Coupled with the increased rate and
extent of faecal excretion after oral administration
(approximately 72%), compared to that after intravenous
dosing (approximately 20%), this would suggest that only a
fraction of the orally administered dose was absorbed.
About 17% of the oral dose was excreted in the urine.
Both urinary and faecal metabolites were again
characterized as polar because of their insolubility in
organic solvents. Unchanged HEX was not detected in any
of the samples examined. Only 11% of the 14C content was
soluble in organic solvents and a further 32% was con-
verhydrolysis. This indicated, perhaps, the formation of
metabolic ester conjugates.
Lawrence & Dorough (1982) made a comparative study of
the uptake, disposition, and elimination of HEX after
administering radiolabelled 14C-HEX by the intravenous
(10 µg/kg), inhalation (24 µg/kg), and oral routes (6 mg
per kg) to Sprague-Dawley rats weighing between 175 and
250 g, respectively. They noted that while doses in the
microgram range were useful for monitoring the urinary and
faecal excretion of HEX, much higher doses (about 6 mg/kg
in 0.5 ml of corn oil, and with a 4-fold increase in
radiocarbon activity) were necessary to obtain levels in
the principal organs that could be measured with any pre-
cision. Indeed, the doses administered orally were some
250 and 600 times the inhaled and intravenous doses,
respectively. In agreement with other researchers, these
authors attributed the lack of measurable levels in the
organs, following the administration of low doses, to the
poor bioavailability of HEX when given by the oral route.
The total radiolabel recovery immediately after the admin-
istration of the dose was 98.0 ± 5.3% (mean ± S.D.). Rats
dosed orally eliminated 2-3 times more of the dose in the
faeces than those dosed by the intravenous or inhalation
route. A maximum blood level was reached at approximately
2 h after dosing. The peak was broad with similar blood
concentrations between 2 and 5 h, perhaps indicating that
absorption occurred along the gastrointestinal tract over
this period in a quasi-steady state with elimination.
Biliary excretion was again confirmed as being greater
after oral dosing than after intravenous or inhalation
dosing, but it still only accounted for 18% of the admin-
istered dose. This observation agreed with previous
studies and, more importantly, with the report of Yu &
Atallah (1981), who administered comparable dose levels by
the oral route. Lawrence & Dorough (1982) also reported
that the faecal material contained predominantly polar or
unextractable material, as did the bile. These authors
considered that this was a clear indication that 14C-HEX
was extensively metabolized to polar products by the gut
contents, since only approximately 50% of 14C-HEX was
recovered when it was added to rat stomach contents that
were then immediately extracted with hexane.
A more recent comparative study (El Dareer et al.,
1983) essentially confirmed the findings of Yu & Atallah
(1981) and Lawrence & Dorough (1982). Male Fischer-344
rats with an average body weight of 169 g were dosed at
a level of 4.1 and 61 mg/kg with approximately 1 ml of a
solution of 14C-HEX dissolved in a 1:1:4 mixture of
Emulphor EL620, ethanol, and water. Little radioactivity
appeared as exhaled 14CO2.
6.1.2. Inhalation
In studies by Dorough (1980) and Lawrence & Dorough
(1981, 1982), rats were exposed to 14C-HEX vapour in a
specially designed, single animal inhalation exposure sys-
tem. Each animal was exposed to the vapours in a rodent
respirator, with the exhaust vapours from the system pass-
ing through a filter pad made from expanded polyurethane
foam. The flow rate and concentration of HEX was measured
prior to and after passing through the respirator contain-
ing the exposed animal. The difference between the amounts
of HEX in the input and output was assumed to be equi-
valent to the retained dose. Rats were exposed for a
period of 1 h and received doses in air which ranged from
1.4 to 37.4 mg/kg body weight (Lawrence & Dorough, 1981).
Immediately after the 1-h exposure, the recovery of the
dose retained by the animal was 91.8 ± 8.5% (mean ± S.D.).
Exposed animals were immediately placed in metabolism
cages for 72 h, during which time faeces, urine, and
expired air were collected. The animals were then killed
and certain of their tissues analysed for 14C activity.
Less than 1% of the retained radiocarbon was expired
during the 24-h period immediately following exposure, and
no radiocarbon was detected as 14CO2. Only about 69%
of the inhaled dose was recovered, which was much lower
than that recovered after intravenous (85%) or oral dosing
(82%). Since recovery of the dose immediately after the
administration of the inhalation dose was approximately
92%, the reduced recovery during the 72-h post-dosing
period led to the speculation that a volatile metabolite
was formed during this period, but attempts to collect and
identify this metabolite were not successful.
No kinetic parameters were reported in either of the
publications by Lawrence & Dorough (1981, 1982), although
blood concentration-time data during the 1-h exposure and
the following 6 h were presented. Elimination during the
subsequent 6 h appeared to relate to a complex pharmaco-
kinetic model with a terminal rate comparable to that
reported for the intravenous route, the half-life being
approximately 30 h.
The elimination via the bile was relatively low (8%)
after inhalation exposure, compared with 13 and 18% after
intravenous or oral administration of the same dose
(5 µg/kg) (Lawrence & Dorough, 1982). The fraction of
the dose recovered in the faeces and urine (23 and 33%,
respectively) was about the same as that recovered after
the intravenous dose, except that more was recovered in
the urine than in the faeces after the inhalation
exposure, while the reverse was observed after the intra-
venous dose.
A comparative study of the uptake, distribution, and
elimination of 14C-HEX (El Dareer et al., 1983) confirmed
and extended the conclusions reached by Lawrence & Dorough
(1981, 1982) concerning pulmonary exposure. Individual
Fischer-344 rats weighing between 125 and 190 g (with an
average weight of 169 g) were placed in metabolism cages
and exposed by inhalation. The dose received by each rat
over a 2-h exposure period was calculated from the total
amount of radioactivity recovered from the tissues,
faeces, urine, and exhaled air. The animal fur was not
included. The dose received by the exposed animals was
between 1.3 and 1.8 mg/kg body weight. The animals were
killed at either 6 or 24 h after they were removed from
the inhalation exposure. Whole blood, plasma, liver,
kidneys, voluntary muscle (gastrocnemius), subcutaneous
fat, brain, skin (ears), and the residual carcass (except
for the skin and fur which were discarded) were analysed
for 14C activity, as were the urine, faeces, and exhaled
air. The principal sites of deposition were the lungs,
kidneys, and liver. Only approximately 1% of the radio-
label was identified as 14CO2. No intact HEX was found
in any of the tissues; the majority of the radiolabel
extracted was polar (water soluble). These findings were
similar to those of Lawrence & Dorough (1981, 1982).
6.1.3. Dermal
No studies on the pharmacokinetics or distribution of
dermally applied HEX were found in a survey of the pub-
lished literature. Although no qualitative studies or
estimates of the uptake of HEX through skin were found,
studies have been reported in which discoloration of the
skin was observed after the dermal application of HEX
(Treon at al., 1955; IRDC, 1972). In these reports, toxic
response, leading to death, was observed in several
instances, which would suggest that HEX was absorbed
transdermally into the systemic circulation.
6.1.4. Comparative studies
Each of the four major studies (Yu & Atallah, 1981;
Lawrence & Dorough, 1981, 1982; El Dareer et al., 1983) of
the uptake and distribution of HEX involved more than one
route of uptake. One objective of each of these studies
was to compare the exposure routes. The observations made
were as follows:
* The principal routes of excretion were via the urine
and faeces. Considerably more of the administered dose
was excreted in the faeces after oral administration
than after dosing by the intravenous or inhalation
route, probably as a consequence of the increased
biliary excretion after oral dosing and the interac-
tion or metabolism of the dose by gut and faecal con-
tents. More of the administered dose was excreted in
the urine than in the faeces after inhalation
exposure, while the reverse was the case after intra-
venous administration.
* Biliary excretion occurred after administration by
each of the three routes. For similar doses, the frac-
tion of the dose eliminated by this route was in the
order oral > intravenous > inhalation.
* Comparative distribution to the major organs and tis-
sues is presented in Tables 8, 9, and 10. The princi-
pal organs to which HEX was distributed by the sys-
temic circulation were the kidneys and liver. The
lungs and trachea contained the highest concentrations
of HEX after inhalation exposure.
* There was a significantly higher retention of 14C in
the carcass, at 72 h post-dosing, after dosing by the
inhalation and intravenous routes than after oral
dosing (Table 10).
6.1.5. In vitro studies
Yu & Atallah (1981) examined the ability of liver,
faecal, and gut homogenates to metabolize HEX in vitro.
In an apparent first-order kinetic process, HEX was
metabolized by gut content, faecal, and liver homogenates
with half-lives of 10.6, 1.6, and 14.2 h, respectively.
When mercuric chloride (HgCl2) was added to the gut and
faecal homogenates as a bacteriocide, the half-lives were
increased to 17.2 and 6.2 h, respectively, indicating that
the gut and faecal flora contributed significantly to the
metabolism of HEX. Denaturation of the liver homogenate
had virtually no effect on the in vitro metabolic rate
indicating, perhaps, that there was only limited involve-
ment of liver microsomes or other enzyme-dependent process.
Table 8. Distribution of radioactivity (expressed as percentage of administered
dose) from 14C-HEX in rats dosed by various routesa
---------------------------------------------------------------------------------
Oral dose Intravenous Inhalation dose
Low doseb High doseb doseb Group Ac Group Bb
(4.1 mg/kg) (61 mg/kg) 0.59 mg/kg (1.0 mg/kg) (1.4 mg/kg)
---------------------------------------------------------------------------------
Faeces 74.5 ± 2.8 65.3 ± 6.9 34.0 ± 1.0d 28.7 ± 4.3 47.5 ± 6.4
Urine 35.5 ± 2.5 28.7 ± 4.2 15.8 ± 1.4 41.0 ± 4.8 40.0 ± 6.6
Tissues 2.4 ± 0.6 2.4 ± 0.1 39.0 ± 1.0 28.9 ± 1.6 11.5 ± 0.8
CO2 0.8 ± 0.0 0.6 ± 0.0 0.1 ± 0.0 1.4 ± 0.3 1.0 ± 0.5
Other volatile 0.2 ± 0.0 0.3 ± 0.0 0.1 ± 0.0
compounds
Total recovery 118 ± 3.0e 97 ± 7.0 89 ± 2.0 100 100
---------------------------------------------------------------------------------
a Adapted from: El Dareer et al. (1983). Values represent the mean percentage
of dose ± S.D. for three rats.
b At 72 h after dosing or exposure.
c At 6 h after exposure.
d Plus intestinal contents.
e For an unexplained reason, the total recovery for this dose was higher than
theoretical. If the percent recoveries for this dose are "normalized" to
100%, differences in distribution for the two doses are minimal, indicating
that no saturable process is operative in this dose range.
Table 9. Fate of radiocarbon (expressed as percentage of
administered dose) after oral, inhalation, and intravenous
exposure of rats to 14C-HEXa
--------------------------------------------------------------
Cumulative percent of dose
Oralb Intravenousc Inhalationd
--------------------------------------------------------------
24-h
Urine 22.2 ± 1.8 18.3 ± 5.2 29.7 ± 4.5
Faeces 62.2 ± 8.0 21.1 ± 7.1 17.0 ± 7.5
48-h
Urine 24.0 ± 1.9 20.7 ± 5.6 32.5 ± 5.1
Faeces 67.7 ± 5.1 30.4 ± 1.7 21.0 ± 7.5
72-h
Urine 24.4 ± 1.9 22.1 ± 5.7 33.1 ± 4.5
Faeces 68.2 ± 5.1 47.4 ± 1.9 23.1 ± 5.7
Body 0.2 ± 0.2 15.7 ± 7.8 12.9 ± 4.7
Total Recovery 92.8 ± 4.7 85.2 ± 4.8 69.1 ± 9.6
--------------------------------------------------------------
a Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
b Dose (7 µg/kg body weight) administered in 0.5 ml corn oil.
c Dose (5 µg/kg body weight) administered in 0.2 ml
saline:propylene glycol:ethanol (10:4:1) by injection into
the femoral vein.
d Doses administered as vapours over a 1-h exposure period
to achieve doses of about 24 µg/kg body weight.
El Dareer et al. (1983) incubated 14C-HEX with homo-
genates of liver, faeces, and intestinal (large and small)
contents, as well as with whole blood and plasma. Samples
were taken at 0, 5, and 60 min. The results, presented in
Table 11, clearly demonstrated the chemical reactivity of
HEX and its ability to bind components of biological
material.
Table 10. Distribution of HEX equivalentsa in tissues and
excreta of rats 72 h after oral, inhalation, and intravenous
exposure to 14C-HEXb,c
-----------------------------------------------------------------
Sample Oral dose Inhaled dose Intravenous dose
(6 mg/kg)d (24 µg/kg) (10 µg/kg)
-----------------------------------------------------------------
ng/g of tissue
Trachea 292 ± 170 107.0 ± 65.0 3.3 ± 1.7
Lungs 420 ± 250 71.5 ± 55.2 14.9 ± 1.1
Liver 539 ± 72 3.6 ± 1.9 9.6 ± 1.1
Kidneys 3272 ± 84 29.5 ± 20.2 22.3 ± 0.6
Fat 311 ± 12 2.8 ± 0.4 2.3 ± 0.2
Remaining carcass 63 ± 40 1.3 ± 0.6 0.5 ± 0.1
percentage of dose
Whole body 2.8 ± 1.1 12.9 ± 4.7 31.0 ± 7.8
Urine 15.3 ± 3.3 33.1 ± 4.5 22.1 ± 5.7
Faeces 63.6 ± 8.5 23.1 ± 5.7 31.4 ± 1.9
Total recovery 81.7 ± 6.7 69.1 ± 9.6 84.6 ± 4.6
-----------------------------------------------------------------
a One HEX equivalent is defined as the amount of radiolabel
equivalent to 1 ng of HEX, based on the specific activity of
the dosing solution.
b Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
c All values are the mean ± S.D. of three replicates.
d It should be noted that the oral dose was 250 and 600 times
that of the inhaled and intravenous doses, respectively.
This was necessary because residues were not detected in
individual tissues of animals treated orally at doses of
5-25 µg/kg.
6.2. Metabolic transformation
No primary metabolites or conjugates of HEX have been
identified. The data available on the pharmacokinetics of
HEX after dosing by the oral, inhalation, and dermal
routes are presented in sections 6.1.1, 6.1.2, and 6.1.3.
In studies by Dorough (1980) and Lawrence & Dorough
(1982), the principal routes of excretion were shown to be
via the urine and faeces. No unchanged HEX was found in
either, indicating that HEX was involved in extensive
metabolism.
In studies with rats and mice fed a diet containing 1,
5, or 25 mg 14C-HEX/kg, 63-79% of the consumed HEX was
recovered in the urine and faeces (Dorough, 1979; Dorough
& Ranieri, 1984). The extraction characteristics of the
radiocarbon compounds in the excreta showed that they were
primarily polar metabolites, some of which were trans-
formed to organic-soluble compounds after acid-catalysed
hydrolysis.
Table 11. Extractability of 14C-HEX and radioactivity derived from
saline and various biological preparationsa
----------------------------------------------------------------------
Preparation Time First Extraction Second Extraction Pellet
(min) Organicb Aqueous Organic Aqueous
----------------------------------------------------------------------
Saline 0 99.6 (92.4) 0.4
5 99.1 (92.8) 0.9
60 98.8 (94.6) 1.2
Liver 0 55.0 (74.4) 8.0 24.5 1.0 11.6
5 42.8 (49.7) 15.2 15.0 4.7 22.2
60 11.1 18.8 5.9 2.4 51.8
Plasma 0 22.2 (61.7) 7.2 50.2 0.8 19.6
5 19.7 (66.3) 25.0 33.6 2.0 19.6
60 1.4 43.4 21. 3.9 30.2
Whole blood 0 16.2 (60.4) 3.8 27.9 1.2 50.8
5 2.8 21.6 13.4 1.6 60.6
60 0.6 27.4 12.0 1.4 58.6
Faeces 0 90.0 (93.7) 0.6 8.0 0.2 1.2
5 83.4 (87.8) 0.8 9.0 0.6 6.2
60 40.5 (61.0) 2.8 31.3 3.0 22.4
Intestinal 0 93.7 (94.7) 0.6 4.6 0.2 1.0
contents 5 82.8 (89.5) 1.6 8.6 1.0 5.9
60 66.3 (87.0) 4.6 15.4 2.4 11.3
----------------------------------------------------------------------
a From: El Dareer et al. (1983). Values represent the percentage of
the total radioactivity in the respective fraction.
b Values in parentheses represent the percentage of the
radioactivity in the fraction as HEX.
In a comparative study of the pharmacokinetics of HEX
after intravenous or oral dosing at 8.5-25.6 mg/kg (Yu &
Atallah, 1981), urinary and faecal metabolites were again
characterized as polar because of their poor solubility in
organic solvents. No unchanged HEX was found. Only 11%
of the 14C content of the excreta was soluble in organic
solvents, and a further 32% of the extract was converted
to organic-soluble compounds after acid-catalysed hydro-
lysis. This indicated, perhaps, that metabolite ester
conjugates had been formed.
Yu & Atallah (1981) also performed in vitro metabolic
studies in which they incubated HEX with liver, faecal,
and gut-content homogenates (see section 6.1.5).
After Lawrence & Dorough (1982) had dosed rats by the
oral, inhalation, and intravenous routes with 14C-
labelled HEX at 6 mg/kg, 24 µg/kg and 10 µg/kg, respect-
ively, they found that the faecal and bile contents con-
tained mostly polar metabolites. These authors suggested
that HEX was rapidly metabolized to polar products, since
only about 50% of the HEX was recovered when it was added
to rat gut contents and immediately extracted with n-hex-
ane. In addition, the authors noted that approximately
15.8 ± 4% of the radiolabel that appeared in the faeces
within 24 h after dosing was volatile, indicating that a
catabolite was probably produced.
El Dareer et al. (1983) dosed rats by the inhalation
route so that individual animals received between 1.3 and
1.8 mg/kg body weight over a 2-h exposure period. They
were killed 6 or 24 h after removal from exposure. No in-
tact HEX was found in any of the tissues, and the majority
of the extractable material was polar (water soluble), in
accordance with the findings of Lawrence & Dorough (1981,
1982). As a part of this same study, El Dareer et al.
(1983) incubated 14C-HEX with homogenates of liver,
faeces, and intestinal (large and small) contents, as well
as with whole blood and plasma. These in vitro studies
were designed to assess the reactivity and binding charac-
teristics of HEX. The results, presented in Table 8,
clearly show chemical lability of HEX and its ability to
bind the components of biological material (see section
6.1.2).
Despite these efforts to characterize HEX metabolism,
no metabolites were identified. This observation suggests
that an attempt to rectify this deficiency would be a high
priority.
6.3. Reaction with body components
The toxic mechanisms of hexachlorocyclopentadiene are
not well understood. HEX has been shown to react with
olefins and other organic molecules such as aromatic com-
pounds. Using the available data, especially those from
studies comparing the various routes of exposure, a very
general and hypothetical rationale can be developed to
suggest possible reactions with body tissue.
The reactivity of HEX shows a high potential for
transformation and reaction with other chemicals. Absorp-
tion from the gastrointestinal contents is relatively
inefficient, probably due to the interaction of HEX with
the gastrointestinal contents and metabolism by intestinal
flora. The fact that HEX did not appear to be interactive
with the gastrointestinal epithelia in the kinetic studies
discussed in this chapter was probably due to its dilution
in the carrier vehicle, as well as its interaction with,
and metabolism by, the gut contents. However, in short-
term repeated oral dosing studies (SRI, 1981a,b), at doses
of 19 mg/kg or more, inflammation and hyperplasia was
noted in the forestomach (see Table 16). In addition, the
interaction of HEX after dermal contact was marked by a
distinct discoloration of the skin. This suggests a "site
of uptake" interaction, probably similar to that observed
in the lungs after pulmonary uptake.
During inhalation and the passage of HEX through the
lung tissue to reach the systemic circulation, metabolism
to water-soluble compounds probably occurs and HEX is
eliminated through the kidneys. However, an intravenous
dose may be bound unchanged to blood components (e.g.,
haemoglobin) and remain attached until reaching the liver.
The relatively slow elimination of the radiolabel from the
systemic circulation after intravenous dosing with 14C-
HEX (approximate terminal half-life of 30 h) suggests a
bioaccumulation potential, at least for some of the
metabolites, since little HEX appears to remain in the
tissues.
Rand et al. (1982b) showed that the cellular level in
lung tissue underwent significant changes after HEX inha-
lation. HEX vapour, administered by the inhalation route,
in addition to binding to epithelial lung tissue, was
found to bind to the extracellular lining in the lung.
Binding to bronchiolar Clara cells, which contribute
important materials to the extracellular lining of the
peripheral airways, was observed after inhalation exposure
in rats and monkeys (Rand et al., 1982a). HEX was also
found, in in vitro studies, to bind to the components of
whole blood, plasma, liver, and faecal homogenates and to
gastrointestinal contents (El Dareer et al., 1983). Thus,
irrespective of the route of administration, the principal
sites of toxic action seem to be the lungs, liver, and
kidneys. This observation is supported by the results from
the toxicity testing reported in chapter 8.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The effects of HEX on microorganisms have been
studied in aqueous and soil systems. Many of the aqueous
concentrations used in these experiments exceeded the
upper limit of aqueous solubility (0.8-2.1 mg/litre).
These concentrations were usually obtained by using an
organic solvent vehicle to disperse the chemical in aque-
ous media. The environmental significance of the results
should be interpreted with this aspect in mind.
Cole (1953) inoculated 10 strains of common human and
animal pathogens into growth media that contained various
concentrations of HEX. The inhibiting concentration, or
lowest concentration in which no growth was observed after
96 h of contact, ranged from 1-10 mg HEX/litre. The
Addition of 5 or 10 mg HEX/litre to sewage effluent inocu-
lated with Salmonella typhosa was found to be more effec-
tive than similar concentrations of chlorine in reducing
counts of total bacteria, coliforms, and S. typhosa (Cole,
1954). Yowell (1951) also reported, in a patent appli-
cation, that HEX has antibacterial properties; standard
phenol coefficients for Ebertnella typhi (Salmonella
typhi) and Staphylococcus aureus were 25 and 33, at 21 and
23 mg HEX/litre, respectively. These findings indicate
that concentrations of HEX at or slightly above its aque-
ous solubility limit are toxic to several types of
pathogens.
In contrast, tests with other microorganisms have
shown some ability to withstand HEX exposure. Twenty-three
strains of organisms (type unspecified), when added to
aqueous media containing HEX at 1000 mg/litre, were able
to metabolize the compound to a varying degree. Analysis
of the medium after 14 days indicated a HEX removal of
2-76%, depending on the organism used (Thuma et al.,
1978).
Rieck (1977a) found no effects on natural populations
of bacteria, actinomycetes, or fungi after a 24-day incu-
bation of a sandy loam soil treated with 1 or 10 mg HEX/kg
dry weight. It was concluded that no significant detrimen-
tal effects on microbial populations would result from
contamination of soils with these levels of HEX.
The effects of HEX on three ecologically important
microbial processes have been reported (Butz & Atallah,
1980). Results on cellulose degradation by the fungus
Trichoderma longibrachiatum indicated that a suspension
of HEX inhibited cellulose degradation at a concentration
of 1 mg/litre or more in a liquid medium. The calculated
7-day EC50 was 1.1 mg/litre. Extrapolations for 1- and
3-day EC50 values were both reported to be 0.2 mg/litre.
The decrease in toxicity over the 7-day period was
attributed to adaptation by T. longibrachiatum.
HEX inhibited anaerobic sulfate reduction by the bac-
terium Desulfovibrio desulfuricans when HEX was present in
suspension in a liquid medium. After a 3-h contact period,
growth inhibition was observed at HEX concentrations of
10-100 mg/litre, and there was no growth at 500 or 1000
mg/litre. Similarly, growth inhibition was observed at 1
and 10 mg/litre after a 24-h contact period, and there was
no growth at 50-1000 mg/litre. HEX was considered to be
slightly toxic to D. desulfuricans (Butz & Atallah,
1980).
The third part of the study by these investigators
(Butz & Atallah, 1980) focused on the effects of HEX on
urea ammonification by a mixed microbial culture in moist
soil. The results indicated that HEX concentrations of
1-100 mg/kg (dry weight) were not toxic to soil organisms
responsible for urea ammonification. The EC50 increased
from 104 mg/kg at day 1 to 1374 mg/kg at day 14. The
authors suggested that the low toxicity and its decrease
over time in this experiment may have been due to adsorp-
tion of the toxicant onto soil particles, as well as to
potential adaptation by the organism. Adsorption onto soil
particles may also account for the lack of toxicity in the
study of Rieck (1977a).
Walsh (1981) reported unpublished data on the effects
of HEX on four species of marine algae, obtained according
to the method described by Walsh & Alexander (1980). The
7-day EC50 was calculated as the concentration that
caused a 50% decrease in growth compared with the control,
as estimated by absorbance at 525 nm. The 7-day EC50
values reported indicated a wide range of susceptibility
among the species tested. Isochrysis galbana and
Skeletonema costatum were the most susceptible species,
the average 7-day EC50 values being about 3.5 and 6.6 µg per
litre, respectively. The average value for Porphyridium
cruentum was 30 µg/litre, while that for Dunaliella
tertiolecta was 100 µg/litre. Other tests with S.
costatum indicated that the direct algicidal effect of HEX
was less pronounced than its effect on growth. After 48 h
of exposure to HEX at 25 µg/litre, mortality, as indi-
cated by staining and cell enumeration, was only 4%
(Walsh, 1983).
7.2. Aquatic organisms
7.2.1. Freshwater aquatic life
Several studies are available on the effects resulting
from exposure of freshwater aquatic life to various con-
centrations of HEX.
Results from acute toxicity tests with HEX have been
reported for a number of freshwater fish species (Table
12). The 96-h LC50 value for fathead minnow larvae in a
flow-through test with measured toxicant concentration was
7 µg/litre (Spehar et al., 1977, 1979). Values obtained
for adult fathead minnows in static tests with unmeasured
toxicant concentrations ranged from 59 to 180 µg/litre
(Henderson, 1956; Buccafusco & LeBlanc, 1977). Reported
96-h values for goldfish, channel catfish, and bluegills
were also within this range (Buccafusco & LeBlanc, 1977;
Podowski & Khan, 1979; Khan et al., 1981).
Sinhaseni et al. (1982) reported the biological
effects of HEX on rainbow trout (Salmo gairdneri) exposed
to 130 µg HEX/litre in a non-recirculating flow-through
chamber. Oxygen consumption, measured polarographically,
increased by 193% within 80 min and then gradually
decreased until the fish died (after approximately 5 h).
Vehicle controls showed no effects after 76 h of exposure.
When added to normal trout mitochondria, HEX increased
basal oxygen consumption. The authors concluded that HEX
uncoupled oxidative phosphorylation.
Sinhaseni et al. (1983) continued their research on
the respiratory effects of HEX on intact rainbow trout.
Acclimated rainbow trout were exposed to 130 µg HEX/litre
in a flow-through well-water circuit, which was designed
to allow measurements of oxygen consumption in fish.
Again, HEX increased oxygen consumption rates (186 ± 24%),
the maximum rates being nearly the same as in the previous
experiment (approximately 84 min). The oxygen consumption
decreased until death (after approximately 6.5 h). Control
trout, exposed to the same concentration of the vehicle
(acetone) used to disperse HEX, showed no changes. The
authors reported profound respiratory stimulation, and HEX
appeared to uncouple oxidative phosphorylation. Sinhaseni
et al. (1983) postulated that HEX intoxication in the
intact animal may be due to increased oxygen consumption
and impaired oxidative ATP synthesis resulting from the
mitochondrial uncoupling action of HEX.
Table 12. Acute toxicity data for freshwater species exposed to HEX
---------------------------------------------------------------------------------------------------------------------------
Acute
no-effect
Species Methoda LC50 (µg/litre)b concentration Comments Reference
24-h 48-h 96-h (µg/litre)
---------------------------------------------------------------------------------------------------------------------------
Cladoceran S,U 93.0 52.2 ND 32 17 °C, soft water Vilkas (1977)
Daphnia magna (78.9-109.6) (44.8-60.9)
Cladoceran S,U 130 39 ND 18 22 °C, soft water Buccafusco &
Daphnia magna (68-260) (30-52) Leblanc (1977)
Fathead minnow FT,M NR NR 7.0 3.7 25 °C, soft water Spehar et al.
(larvae, < 0.1 g) (1977, 1979)
Pimephales promelas
Fathead minnow (1-1.5 g) S,U 115 110 104 NR Hard water, Henderson
Pimephales promelas acetone soln. (1956)
93 78 78 NR Soft water,
acetone soln.
75 59 59 NR Hard water,
emulsion
(no acetone)
Fathead minnow (0.72 g) S,U 240 210 180 87 22 °C, soft water Buccafusco &
Pimephales promelas (170-320) (180-250) (160-220) Leblanc (1977)
Goldfish NR NR NR 78 NR No details given Podowski & Khan
Carassius auratus (1979)
Channel catfish (2.1 g) S,U 190 150 97 56 22 °C, soft water Buccafusco &
Ictalurus punctatus (140-250) (130-180) (81-120) Leblanc (1977)
Bluegill (0.45 g) S,U 170 150 130 65 22 °C, soft water Buccafusco &
Lepomis macrochirus (140-210) (120-180) (110-170) Leblanc (1977)
---------------------------------------------------------------------------------------------------------------------------
a S = static, FT = flow-through, U = unmeasured concentrations, M = measured concentrations.
b Numbers in parentheses show 95% confidence interval. ND = Not determined. NR = Not reported.
Spehar et al. (1977, 1979) conducted 30-day early-life
stage flow-through toxicity tests with fathead minnows
(Pimephales promelas) using measured concentrations and
1-day-old larvae. The 96-h LC50 value was 7 µg/litre.
The 96-h mortality data indicated a sharp toxicity
threshold, such that 94% survival was observed at 3.7 µg
per litre, 70% at 7.3 µg/litre, and 2% at 9.1 µg/litre.
At the end of the 30-day exposure period, mortality was
only slightly higher, with 90% survival at 3.7 µg/litre,
66% at 7.3 µg/litre, and 0% at 9.1 µg/litre. These
results indicated that the median lethal threshold, the
lowest concentration causing 50% mortality, was reached
within 4 days. In addition, the HEX residues found in
fathead minnows at the end of the 30-day tests were low
(< 0.1 µg/g), and a BCF value of < 11 was reported
(Spehar et al., 1979). The authors concluded that the
toxicity data and the BCF values indicated that HEX was
non-cumulative in fish, i.e. it did not bioconcentrate in
fish as a result of continuous low-level exposure to HEX.
The growth rate of surviving larvae, measured in terms of
both body length and weight, did not decrease signifi-
cantly at any of the concentrations tested, compared with
the controls. This was true even at 7.3 µg/litre, a level
greater than the calculated LC50 value. Based on these
toxicity and growth data, Spehar et al. (1977, 1979) con-
cluded that 3.7 µg/litre is the highest concentration of
HEX that produces no adverse effects on fathead minnow
larvae. No other chronic toxicity data for any freshwater
species are available.
7.2.2. Marine and estuarine aquatic life
Among marine invertebrates, the 96-h LC50 values for
HEX range from 7 to 371 µg/litre (Table 13) (US EPA,
1980a). Except where indicated, these results were
obtained from static tests with nominal concentrations of
HEX. The highest LC50 by far was for the polychaete
Neanthes arenaceodentata, which is an infaunal organism
living in the sediment. The two shrimp species tested
were more sensitive to HEX by a factor of 10 or more.
The static LC50 value reported by US EPA (1980a) for
the grass shrimp, Palaemonetes pugio, was slightly higher
than that for the mysid shrimp, Mysidopsis bahia (Table
13). However, the LC50 for the mysid shrimp was consider-
ably lower in a flow-through test than in the static test.
Similarly, the LC50 value was lower when calculated
from actual measurements of HEX concentrations in the test
solutions (measured concentration) than when calculated
according to the concentrations based on amounts orig-
inally added to test solutions (nominal concentrations).
Table 13. Acute toxicity data on estuarine marine
organisms exposed to HEXa
----------------------------------------------------------
Species Methodb Salinity 96-h LC50c
(o/oo) (µg/litre)
----------------------------------------------------------
Polychaete S,U 28 371
Neanthes arenaceodentata (297-484)
Grass shrimp S,U 22 42
Palaemonetes pugio (36-50)
Mysid shrimp S,U 24 32
Mysidopis bahia (27-37)
Mysid shrimp FT,U 24 12
Mysidopsis bahia (10-13)
Mysid shrimp FT,M 24 7
Mysidopsis bahia (6-8)
Pinfish S,U 22 48
Lagodon rhomboides (41-58)
Spot S,U 22 37
Leiostomus xanthurus (30-42)
Sheepshead minnow S,U 24 45
Cyprinodon variegatus (34-61)
----------------------------------------------------------
a Adapted from: US EPA (1980a) and Mayer (1987).
b S = static; FT = flow-through; U = unmeasured
concentrations; M = measured concentrations.
c Numbers in parentheses show 95% confidence interval.
The acute toxicity values for HEX were similar for
each of three estuarine and marine fish species tested (US
EPA, 1980a). The static 96-h LC50 values based on
unmeasured concentrations for spot, sheepshead minnow, and
pinfish varied only between 37 and 48 µg/litre (Table 13).
7.3. Terrestrial organisms and wildlife
In a USA patent application, HEX was reported to be
nontoxic to plants in concentrations at which it was an
effective fungicide (Yowell, 1951). Test solutions were
prepared by adding HEX at various proportions to attaclay
and a wetting agent, and they were then mixed with water.
The concentrations of HEX applied to plants as an aqueous
spray were 0.1, 0.2, 0.5, and 1.0%. Slight injury
(unspecified) to Coleus blumei was reported at 1.0% HEX,
but lower concentrations were not harmful. Similarly, HEX
was added to horticultural spray oil and an emulsifier at
various proportions and then mixed with water. The concen-
tations of HEX in the prepared spray were 0.25 and 0.5%.
No injury to C. blumei was observed at these concen-
trations. No data are available concerning the effects of
HEX on amphibians, reptiles, birds, or mammals other than
those routinely used in laboratory testing.
7.4. Population and ecosystem effects
The ecological effects of HEX have not been studied at
the ecosystem, population, or community levels.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Acute toxicity studies
8.1.1. Acute oral, inhalation, and dermal toxicity
The data from acute toxicity studies using HEX are
summarized in Table 14. Caution should be exercised in
comparing the various studies. The acute toxicity (LD50
and LC50) may be affected not only by the species and
age of the animals used in the experimental tests, but
also by the strain. In addition, the purity of the com-
pound and the nature of the contaminants may affect the
toxicity, as can the experimental method and the vehicle
used. These details (when specified by the researchers)
have been summarized in the table.
For the rat, oral LD50 values ranged from 425 to 926
mg/kg for males, and from 315 to 926 mg/kg for females.
Values for mice and rabbits were within the same range.
Acute inhalation experiments involved exposure
durations from 3.5 to 4 h, and LC50 values for male rats
ranged from 18.1 to 35.0 mg/m3 (1.6 to 3.1 ppm) and for
female rats from 35.0 to 40.0 mg/m3 (3.1 to 3.5 ppm).
The mouse, rabbit, and guinea-pig values ranged from 23.7
to 80.2 mg/m3 (2.1 to 7.1 ppm).
There are few data available on dermal toxicity.
LD50 values of < 200 mg/kg in male rabbits and 340-780
mg/kg in females have been reported.
In spite of variations in LD50 values in the differ-
ent studies, these data suggest that HEX is moderately
toxic when administered orally. The acute toxicity of HEX
by the dermal route is quite similar to its acute oral
toxicity. HEX is much more toxic by the inhalation route
of exposure than by the dermal or oral routes.
8.1.2. Eye and skin irritation
IRDC (1972) tested HEX for eye irritation by instil-
ling 0.1 ml HEX into the eyes of New Zealand white rabbits
for 5 min or 24 h before washing. All the rabbits died on
or before the ninth day of the observation period. Treon
et al. (1955) reported HEX to be a primary skin irritant
in rabbits (strain unspecified) at a dose level of 250
mg/kg, and IRDC (1972) reported HEX to be a dermal irri-
tant because of the oedema that appeared after application
of 0.5 ml HEX. In this study, intense discoloration of
the skin was noted. In the study of Treon et al. (1955),
monkeys (strain unspecified) were also tested, and dis-
coloration of the skin was noted even at low doses (0.05
ml of 10% HEX) (Table 15).
Table 14. Acute toxicity studies of HEX
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Oral LD50
Rat (unspecified) oral gavage M: 510 mg/kg Treon et al.
young adult (5% solution F: 690 mg/kg (1955)
of peanut oil)
Rat (Charles River CD) oral gavage M + F: 926 IDRC (1968)
young adult (corn oil) mg/kg
Rat (Sprague-Dawley) M + F: 651 Dorough (1979)
young adult mg/kg
Rat (Fischer-344) oral gavage M: 425 mg/kg SRI (1980a)
weanling (corn oil) F: 315 mg/kg
Mouse (unspecified) M + F: 600 Dorough (1979)
mg/kg
Mouse (B6C3F1) oral gavage M + F: 680 SRI (1980a)
weanling (corn oil) mg/kg
Rabbit (Albino, strain oral gavage F: 640 mg/kg Treon et al.
unspecified) adult (5% peanut oil) (1955)
Dermal LD50
Rabbit (unspecified) skin painted F: 780 mg/kg Treon et al.
adult (1955)
Rabbit (unspecified) skin painted M: < 200 mg/kg IDRC (1972)
adult F: 340 mg/kg
Inhalation LC50
Rat (Carworth) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 35.0 (1955)
mg/m3 (3.1 ppm)
Rat (Sprague-Dawley) inhalation 4.0-h LC50 Rand et al.
young adult M: 18.1 mg/m3 (1982a)
(1.6 ppm)
F: 39.6 mg/m3
(3.5 ppm)
Mouse (unspecified) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 23.7 (1955)
mg/m3 (2.1 ppm)
----------------------------------------------------------------------
Table 14 (contd.)
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Rabbit (unspecified) inhalation 3.5-h LC50 Treon et al.
adult F: 58.8 mg/m3 (1955)
(5.2 ppm)
Guinea-pig inhalation 3.5-h LC50 Treon et al.
(unspecified) adult M + F: 80.2 (1955)
mg/m3 (7.1 ppm)
----------------------------------------------------------------------
a M = male; F = female.
Table 15. Primary eye and dermal irritation
--------------------------------------------------------------------------------
Study Species (strain) Results Reference
age
--------------------------------------------------------------------------------
Primary eye Rabbit (New Zealand Severe eye irritant (0.1 IRDC (1972)
irritation white) adult ml for 5 min or 24 h); all
died by day 9 of study
Primary dermal Rabbit (unspecified) Moderate skin irritant Treon et al.
irritation adult (250 mg/kg); one (1955)
application
Primary dermal Rabbit (New Zealand Severe skin irritant IRDC (1972)
irritation white) adult (200 mg/kg); all males died
Primary dermal Monkey (unspecified) Mild skin discoloration Treon et al.
irritation adult (0.05 ml of 10% HEX solution) (1955)
--------------------------------------------------------------------------------
Shell Research Limited conducted a study of the skin-
sensitizing potential of 98.8% pure HEX (Shell Research
Limited, 1982). Guinea-pigs (310-370g) were placed at
random into single sex groups of 10 animals and housed in
groups of five animals. Range-finding tests were conducted
to determine the concentrations of test material to be
used for intradermal induction, topical induction, and
topical challenge. Two male and two female guinea-pigs
were injected intradermally on each side of the midline
with 0.1 ml of several dilutions (0.5, 1, 5, and 10 mg HEX
per ml HEX corn oil). Filter paper patches with 0.3 ml of
a 1% or 2% dilution of test material in corn oil were
applied to two other groups. On the basis of the results
of the range-finding studies, the following tests and HEX
concentrations were selected for use: intradermal induc-
tion, 0.5 mg/litre; topical induction, 20.5 mg/litre; and
topical challenge, 10 mg/ml (all in corn oil). In the
sensitizing test, all four animals given an intradermal
injection of 0.5 mg HEX/ml suffered necrosis, while top-
ical applications at 10 or 20.5 mg/litre produced slight
redness or no difference from surrounding skin. However,
all 20 test animals showed positive responses 24 and 48 h
after removal of the challenge patches. The researchers
concluded that HEX is a skin sensitizer.
8.2. Short-term exposure
8.2.1. Oral
In a range-finding study using groups of five male and
five female Fischer-344 rats, there were no deaths when 12
doses of 25, 50, or 100 mg/kg were given in 16 days (SRI,
1980b). With the same dosing schedule, one out of five
males and four out of five females died when the dose was
200 mg/kg, and five out of five males and four out of five
females died when the dose was 400 mg/kg. In the same
study, B6C3F1 mice died when given doses of 400 or 800
mg/kg, but not when given doses of 50, 100, or 200 mg/kg.
Both rats and mice exhibited pathological changes of the
stomach wall at all but the lowest dose level.
The short-term toxicity of HEX is summarized in Table
16. These short-term toxicity studies on B6C3F1 mice and
Fischer-344 rats were conducted by SRI (1981a,b) under
contract with the National Toxicology Program (NTP), and
the results were reported by Abdo et al. (1984). In the
mouse study (SRI, 1981a), HEX (94.3-97.4% pure) in corn
oil was administered by gavage at dose levels of 19, 38,
75, 150, and 300 mg/kg to 10 mice of each sex, 5 days/week
for 13 weeks (91 days). At the highest dose level (300
mg/kg), all 10 male mice died by day 8 and three females
died by day 14. In female mice, the liver was enlarged.
Toxic nephrosis in females at doses of 75 mg/kg or more
was characterized by distensions in the proximal convol-
uted tubules, with basophilia in the inner cortical zone
and cytoplasmic vacuolization. However, male mice admin-
istered 75 mg/kg or more did not show these effects. Dose
levels of 38 mg/kg or more caused lesions in the fore-
stomach, including ulceration in both males and females,
as well as increased kidney and liver weights. The no-
observed-adverse-effect level (NOAEL) in mice was 19 mg/kg
and the lowest-observed-adverse-effect level (LOAEL) was
38 mg/kg.
In the rat study (SRI, 1981b), HEX in corn oil was
administered by gavage at dose levels of 10, 19, 38, 75,
and 150 mg/kg to groups of 10 male and female F-344 rats.
At 38 mg/kg or more, mortality and toxic nephrosis were
observed in both males and females. The male rats treated
with 19 mg/kg showed no significant effects, but female
rats had lesions in the forestomach. Similar lesions were
observed in males given 38 mg/kg or more. There was a
dose-related depression of body weight gain (relative to
the controls) and female rats had increased kidney and
liver weights. The NOAEL in rats was 10 mg/kg, and the
lowest-observed-effect level (LOEL) was 19 mg/kg.
A summary of the results of these two experiments
appears in Table 17.
Table 16. Short-term toxicity of HEX
------------------------------------------------------------------------------------------------------
Study Species Dose Results Effects at LOEL Reference
or lowest dose
------------------------------------------------------------------------------------------------------
90-day rat 10, 19, 38, 75, and NOAEL: 10 mg/kg lesions of forestomach SRI (1981b)
feeding 150 mg/kg (by gavage) LOEL: 19 mg/kg in female rats at
study 19 mg/kg
14-week rat 0.11, 0.56, and 0.226 NOEL: 0.226 mg/m3 no statistically Rand et al.
inhalation mg/m3 (5 days/week) LOEL: NE significant effects (1982a)
study
14-week monkey 0.11, 0.56, and 0.226 NOEL: 0.2 ppm no effects noted Alexander
inhalation mg/m3 (5 days/week) LOEL: NE et al.
study (1980)
------------------------------------------------------------------------------------------------------
NE = not established.
NOAEL = no-observed-adverse-effect level.
NOEL = no-observed-effect level.
LOEL = lowest-observed-effect level.
Table 17. Toxicological parameters for mice and rats administered technical grade HEX
in corn oil by gavage for 91 daysa
---------------------------------------------------------------------------------------
Species Sex Dose Mortality Relative Forestomach Forestomach Toxic
(strain) (mg/kg) weight inflammation hyperplasia nephrosis
gainb
---------------------------------------------------------------------------------------
Mice male 0 1/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 36% 0/10 0/10 0/10
38 0/10 + 9% 2/10 2/10 0/10
75 0/10 - 9% 7/10 8/10 0/10
150 0/10 - 45% 7/10 9/10 0/10
300 10/10 7/10 8/10 0/10
Mice female 0 0/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 13% 0/10 0/10 0/10
38 0/10 - 13% 2/9 2/9 0/9
75 0/10 - 13% 6/10 9/10 10/10
150 0/10 - 25% 10/10 10/10 10/10
300 3/10 - 38% 7/9 9/9 7/10
Rats male 0 3/10 0/10 0/10 0/10
(F-344) 10 1/10 - 4% 0/10 0/10 0/10
19 1/10 - 8% 0/10 0/10 0/10
38 1/10 - 20% 4/10 5/10 10/10
75 3/10 - 49% 9/10 9/10 9/10
150 7/10 - 57% 8/9 8/9 8/10
Rats female 0 1/10 0% 0/10 0/10 0/10
(F-344) 10 2/10 + 4% 0/10 0/10 0/10
19 1/10 - 5% 2/10 2/10 0/10
38 1/10 - 2% 2/10 5/10 10/10
75 3/10 - 30% 9/10 9/10 10/10
150 5/10 - 33% 9/10 9/10 10/10
---------------------------------------------------------------------------------------
a From: SRI (1981a,b).
b Relative weight gain was calculated as follows:
Dose group value - control group value
-------------------------------------- x 100
Control group value
8.2.2. Short-term inhalation toxicity
Rand et al. (1982a) conducted a range-finding study in
which groups of 10 male and 10 female Sprague-Dawley rats
were exposed to atmospheres of 0.25, 1.24, or 5.65 mg
HEX/m3 (0.022, 0.11, or 0.5 ppm), 6 h/day, 5 days/week
for a total of 10 exposures. Nine male rats and one female
rat exposed to 5.65 mg/m3 were moribund after five to
seven exposures. These rats had dark red eyes, laboured
breathing, and pale extremities. There were no mortalities
in the other exposure groups. However, the males in the
medium- and high-dose groups lost weight during the study
and reduced mean liver weights and pathology were noted.
The NOAEL for HEX exposure in this study was 0.25 mg/m3
and the LOEL was 1.24 mg/m3.
Fourteen-week inhalation studies have been carried out
on rats and monkeys (Alexander et al., 1980; Rand et al.,
1982a,b) and the results are summarized in Table 16.
Groups of 40 male and 40 female Sprague-Dawley rats
weighing 160-224 g and groups of 12 Cynomolgus monkeys
weighing 1.5-2.5 kg were exposed to HEX, 6 h/day, 5 days
per week, for 14 weeks. Levels of exposure were 0, 0.11,
0.56, and 2.26 mg/m3 (0, 0.01, 0.05, and 0.20 ppm). In
monkeys, there were no mortalities, adverse clinical
signs, weight gain changes, pulmonary function changes,
eye lesions, haematological changes, clinical chemistry
abnormalities, or histopathological abnormalities at any
dose level tested. Thus, the NOEL for monkeys was at least
2.26 mg/m3 for this exposure period, but the LOEL was
not established. Male rats had a transient appearance of
dark-red eyes at 0.56 and 2.26 mg/m3. At 12 weeks, there
were marginal but not statistically significant increases
in haemoglobin concentration and erythrocyte count in
males exposed to 0.11 mg/m3, females exposed to 0.56
mg/m3, and males and females exposed to 2.26 mg/m3.
There were small but not statistically significant changes
in mean liver weight of all groups of treated rats and
similar changes in the kidneys of all treated males. No
treatment-related abnormalities in gross pathology or
histopathology were observed. On this basis, the NOEL in
rats was 2.26 mg/m3, but the LOEL was not established.
In a further study by Rand et al. (1982b), no ultra-
structural changes were observed in monkeys that could be
attributed to the inhalation of HEX vapour. Exposure was
identical to that of the previous study (Rand et al.,
1982a). There was a statistically significant (P < 0.01)
increase in the mean number of electronlucent inclusions
in the apex and base of the Clara cells in exposed ani-
mals, as compared with the controls. According to some
researchers (Evans et al., 1978), Clara cells respond to
injury by regression to a more primitive cell type. Rand
et al. (1982b) noted that some of the ultrastructural
changes in the exposed animals resembled those of the
Evans study. It is not known what effect these changes
might cause. The Clara cell contributes important
materials to the extracellular lining of the peripheral
airways, and if this effect of HEX vapour causes a change
in the content of the contributed material, then the
extracellular lining may be altered and breathing may be
subsequently impaired (Rand et al., 1982b). This obser-
vation of lung effects coincides with those of other
researchers (Dorough, 1979, 1980; Lawrence & Dorough,
1981, 1982). Furthermore, in inhalation studies with HEX
occasional statistically significant increases in the
haemoglobin level and red blood cell counts of rats have
been noted, which may be manifestations of the impairment
of respiratory functions.
In 1984, the US National Toxicology Program (NTP)
completed a short-term, 90-day HEX inhalation study on
B6C3F1 mice and F-344 rats (NTP, 1984a,b). In the basic
study, ten rats and ten mice of each sex were placed at
random into five exposure and control groups. The rats and
mice were exposed to nominal concentrations of 0.45, 1.70,
4.52, 11.3, or 22.6 mg/m3 (0.04, 0.15, 0.4, 1.0, or 2.0
ppm) for 6 h/day, 5 days/week for 13 weeks. In the female
mouse study, 6 out of 10 of the control animals died,
while none of the animals in the male control population
died. The six animal deaths in week 7 were attributed to
a defective feeder insert. Mortality in both the rats and
mice was high in the two highest-dose groups; all rats and
mice in these groups died in the first five weeks of the
study. Posterior paresis and listlessness were observed
in mice at 4.52 and 11.3 mg/m3. Compound-related histo-
pathological alterations were observed in the respiratory
tracts of male and female mice exposed to 1.7 mg/m3 or
more. These changes included: necrosis, acute and
chronic inflammation, hyperplasia or squamous metaplasia
of the nasal, laryngeal, tracheal, bronchial, and bronchi-
olar epithelia of the affected animals. No compound-
related effects were observed in mice at the lowest dose,
and so this level could be considered the NOEL (NTP,
1984a).
Clinical signs resulting from HEX exposure included
posterior paresis in all rats exposed to 1.7 mg/m3 or
more, listlessness in all rats exposed to 4.52 mg/m3 or
more, and eye irritation and respiratory distress in all
rats exposed to 11.3 mg/m3 or more. As in the case of
mice, HEX caused significant histological alterations in
the respiratory tracts of rats at the two highest doses.
Changes of a less-marked degree were observed in the
respiratory tracts of rats receiving 4.52 mg/m3. No
compound-related changes were seen in any organ of male
and female rats exposed to the lowest dose, and so this
level could be considered the NOEL. In addition, compound-
related effects of a secondary stress-related nature were
seen in a number of other organs of rats of both sexes at
the two highest doses. These includes lymphoid depletion
of the spleen and thymus, degeneration of the seminiferous
tubules and decreased lytoplasmic vacuolization of the
adrenal cortex.
Some basic clinical pathology and histopathology
examinations were undertaken simultaneously in both
studies (NTP, 1984a,b). All effects were similar to those
noted in the previous toxicity studies. In the rat study
(NTP, 1984b), HEX nephrotoxicity was examined in more
depth. HEX was not found to be nephrotoxic at these ex-
posure levels, nor did it appear that it was myelotoxic.
8.2.3. Short-term dermal toxicity
No short-term dermal toxicity studies are available.
8.3. Long-term exposure
8.3.1. Long-term oral toxicity
No long-term oral toxicity studies have been reported.
8.3.2. Long-term inhalation toxicity
In view of the absence of long-term studies on the
inhalation of HEX, the following studies were examined.
Treon et al. (1955) exposed guinea-pigs, rabbits, rats,
and mice to a HEX (89.5% pure) concentration of 3.73
mg/m3 (0.33 ppm), 7 h/day, 5 days/week, for 25-30
exposure days. The guinea-pigs survived 30 exposures,
whereas rats and mice did not survive five exposures, and
four out of six rabbits did not survive 25 exposures.
Using a lower concentration of 1.70 mg/m3 (0.15 ppm),
guinea-pigs, rabbits, and rats survived 150 7-h exposures
over a 7-month period. This level was too high to conduct
a long-term study in mice since four out of five animals
did not survive. The rats, guinea-pigs, and rabbits toler-
ated 1.7 mg/m3 and did not exhibit any treatment-related
effects. Thus, the NOEL for rats, guinea-pigs, and rabbits
was 1.7 mg/m3 over the 7-month period. Due to the high
mortality, a NOEL for mice could not be established.
A long-term (30 weeks) inhalation study in rats with
technical grade HEX (96% pure with hexachlorobuta-1,3-
diene and octachlorocyclopentene as impurities) was con-
ducted by the Shell Toxicology Laboratory (D.G. Clark et
al., 1982). Four groups of eight male and eight female
Wistar albino rats were exposed to HEX at nominal concen-
trations of 0, 0.56, 1.13, and 5.65 mg/m3 (0, 0.05, 0.1,
and 0.5 ppm), 6 h/day, 5 days/week, for 30 weeks and were
observed for a 14-week recovery period without HEX
exposure. At the highest dose level, four males and two
females died. In males, there was depressed body weight
gain at the highest dose, relative to controls, beginning
at 7 weeks of exposure and persisting throughout the
remainder of the study. Females in the two highest-dose
groups had lower body weights at the end of the recovery
period than did the controls. At the highest dose, there
were pulmonary degenerative changes, ranging from epi-
thelial hyperplasia and oedema to epithelial ulceration
and necrosis, in both sexes, the males being affected more
severely. There were also mild degenerative changes in
the liver (bile duct hyperplasia and inflammatory cell
infiltration) and kidneys (protein casts in tubules and
pigmented cortical tubules) in a few rats, and kidney
weights were significantly increased in the females at 30
weeks. After 30 weeks of study, no biologically signifi-
cant toxicity was noted in animals that were exposed to
0.56 or 1.13 mg/m3. Thus, the NOEL in rats exposed to
HEX vapour in this study (6 h/day, 5 days/week, for 30
weeks) was 0.56 mg/m3, and the LOEL was 1.13 mg/m3.
A long-term inhalation study by the National Toxi-
cology Program started in January 1986. The animal
exposure has been completed and results will be published
as soon as the pathology review is completed.
8.3.3. Long-term dermal toxicity
No long-term dermal toxicity studies have been
reported.
8.3.4. Principal effects and target organs
Repeated exposure of several animal species to levels
of HEX vapour in the range of 1.13 to 2.26 mg/m3 (0.1-0.2
ppm) has been found to cause pulmonary degenerative
changes (Treon et al., 1955; D.G. Clark et al., 1982; Rand
et al., 1982a,b). In addition, Treon et al. (1955)
reported diffuse degeneration of the brain, heart, and
adrenal glands and necrosis of the liver and kidney
tubules, together with severe pulmonary hyperaemia and
oedema. In many instances, acute bronchitis and intersti-
tial pneumonitis also occurred. Necrosis of the epithelium
of the primary, secondary, and tertiary bronchi was
observed. At later stages, reacting inflammatory cells
migrated into the wall and the mucosa of the bronchi and
alveoli. In rabbits, the walls of the alveoli were covered
by a hyaline or fibrinoid membrane. Possibly, some of the
changes found by Treon et al. (1955) were caused by
impurities in the HEX preparation. Acute exposure by the
oral and dermal routes also affects the respiratory system
(Kommineni, 1978; SRI, 1980a). Death from acute exposure
by any tested route seemed to be associated with respir-
atory failure (Lawrence & Dorough, 1982).
There are insufficient data to identify the site that
is the most sensitive to prolonged, repeated exposure to
HEX. In comparing routes of administration, researchers
found that damage to the lungs occurred regardless of
which route was used (Lawrence & Dorough, 1982). When HEX
is administered orally to animals, the kidneys may be the
most sensitive site, since short-term dosing of rats and
mice was found to cause nephrosis, especially in females
(SRI, 1981a,b). Although the oral route may not be a
significant route of exposure for human beings, the fact
that the kidneys are a possible target organ in short-term
exposure indicates that low-level, prolonged systemic
exposure from any ambient route may affect the kidneys.
The liver has also been shown, in several of the labora-
tory studies, to be affected by HEX.
The available literature does not cite any single
mechanism to explain HEX toxicity. HEX vapour irritates
the respiratory tract, leading to death by respiratory
failure after bronchopneumonia (D.G. Clark et al., 1982).
The degenerative changes that have been observed in the
liver and kidneys are mild and unlikely to contribute to
the chemical's lethality (NAS, 1978; SRI, 1980a,b).
The difficulty in studying HEX because of its high
reactivity and volatility has also created problems in
identifying its metabolites. Several questions remain as
to whether the same metabolites are formed after various
routes of exposure and whether it is the administered HEX
or its metabolites that cause the lung injuries seen with
various dosing regimens. Furthermore, the strong ability
of HEX to interact with other compounds, especially
organic molecules, can lead to many other effects, such as
haemoglobin binding. Little is known about the interac-
tions of HEX with other chemicals in animal or human
tissue.
8.4. Developmental and reproductive toxicity
The teratogenic potential of HEX has been evaluated in
pregnant Charles River CD-1 rats that were administered
HEX (98.25%) in corn oil, by gastric intubation, at dose
levels of 3, 10, and 30 mg/kg per day from days 6 to 15 of
gestation. A control group received the vehicle (corn oil)
at a dose volume of 10 ml/kg per day. All the rats sur-
vived, and there was no difference in mean maternal body
weight gain between the dosed groups and controls. There
were no differences in the mean number of implantations,
corpora lutea, live fetuses, mean fetal body weights, or
male/female sex ratios among any of the groups, and there
were no statistical differences in malformation or devel-
opmental variations, compared with the controls, when
external, soft tissue, and skeletal examinations were
performed (IRDC, 1978).
Murray et al. (1980) evaluated the teratogenic poten-
tial of HEX (98%) in CF-1 mice and New Zealand white
rabbits. Mice were dosed at 0, 5, 25, or 75 mg HEX/kg per
day by gavage during days 6-15 of gestation, while rabbits
received the same dose during days 6-18 of gestation. The
fertility of the treated mice and rabbits was not signifi-
cantly different from that of the control groups. In the
mice, there was no evidence of maternal toxicity, embryo-
toxicity, or teratogenic effects. A total of 249-374
fetuses (22-33 litters) was examined in each dose group.
In rabbits, maternal toxicity was noted at a dose level of
75 mg/kg (diarrhoea, weight loss, and mortality), but
there was no evidence of maternal toxicity at the lower
levels. There were no embryotoxic effects at any dose
level. Although there was a two-fold increase over con-
trols in the proportion of fetuses with 13 ribs at 75
mg/kg, this was considered to be a minor skeletal vari-
ation. The authors concluded that HEX was not teratogenic
at the levels tested (Murray et al., 1980).
Chernoff & Kavlock (1982) tested 28 compounds, includ-
ing HEX, by an in vivo screening procedure. According to
the researchers, the underlying hypothesis was that most
prenatal insults would manifest themselves postnatally as
reduced viability and/or impaired growth. Twenty-five
Oravid CD-1 mice were administered HEX orally at or near
the maternal minimal tolerated dose (MTD). The MTD was
considered to be that dose resulting in either significant
weight reduction during the treatment period, mortality,
or other signs of toxicity. There were no differences in
maternal weight gain, number of live offspring or average
weight between the HEX-treated animals and controls when
HEX was administered orally for 8-12 days at 45 mg/kg.
Gray & Kavlock (1984) extended the observation period
proposed by Chernoff & Kavlock (1982, 1983) to 250 days to
determine whether neonatal weight reductions persisted
throughout life and whether other serious abnormalities or
mortality resulted from exposure to HEX. CD-1 pregnant
mice were orally exposed to HEX (45 mg/kg) on days 8 to 12
of gestation, which is within the period of major embryo-
nic organogenesis. Females were weighed throughout dosing
and on day 19 of gestation. They were allowed to deliver
and the litters were counted and weighed at 1 and 3 days
of age. The animals were observed at approximately 250
days of age. During the postmortem examination of males,
body weight and the weights of the liver, testes, seminal
vesicles, and right kidney were recorded. HEX did not
produce any statistically significant developmental
effects in this study.
Studies on the teratogenic potential of inhaled HEX
were not found in the review of the scientific literature.
8.5. Mutagenicity
Goggelman et al. (1978) found that HEX was not muta-
genic, either before or after liver microsomal activation,
at 2.7 mmol/litre in an Escherichia coli K12 back-
mutation system. In this test there was a 70% survival of
bacteria at 72 h. HEX was not tested at higher concen-
trations because it was cytotoxic to E. coli. A previous
report from the same laboratory (Greim et al., 1977) indi-
cated that HEX was also non-mutagenic in Salmonella
typhimurium strains TA1535 (base-pair mutant) and TA1538
(frame-shift mutant) after liver microsomal activation.
However, no details of the concentrations tested were
given. Although tetrachlorocyclopentadiene is mutagenic
in these systems, probably through metabolic conversion to
the dienone, it appears that the chlorine atoms at the C-1
position of HEX hinder metabolic oxidation to the corre-
sponding acylating dienone (Greim et al., 1977).
A study conducted by the Industrial Bio-Test Labora-
tories (IBT, 1977) also suggested that HEX is not muta-
genic in S. typhimurium. Both liquid HEX and its vapour
were tested with and without metabolic activation. The
vapour test was carried out in desiccators with the TA100
strain of S. typhimurium only. It is not clear from
vapour test data that sufficient amounts of HEX or
adequate exposure times (30, 60, and 120 min) were used.
Longer exposures in the presence of HEX vapour may be
necessary for a potential mutagenic effect to be seen.
At concentrations of up to 1.25 mg/litre in the
presence of an S-9 liver activating system, HEX was not
mutagenic in the mouse lymphoma mutation assay. Mutageni-
city could not be evaluated at higher concentrations
because of the cytotoxicity of HEX (Litton Bionetics,
Inc., 1978a). This assay uses L5178Y cells that are het-
erozygous for thymidine kinase (TK+/-) and are sensitive.
The mutation is scored by cloning with bromodeoxyuridine
in the absence of thymidine. HEX is highly toxic to these
cells, particularly in the absence of an activating system
(at 0.04 ml/litre), and the positive control, dimethyl-
nitrosamine, was mutagenic at 0.5 ml/litre.
Williams (1978) found that HEX (10-6 mol/litre) was
inactive in the liver epithelial culture (hypoxanthine-
guanine-phosphoribosyl transferase locus) mutation assay.
At 10-5 mol/litre it also failed to stimulate DNA repair
in hepatocyte primary cultures. Negative results were also
obtained in an additional unscheduled DNA synthesis assay
(Brat, 1983).
One study provided by the US National Toxicology
Program (NTP) (Haworth et al., 1983) demonstrated a lack
of mutagenicity of HEX (98% pure). In S. typhimurium
strains TA98, TA100, TA1535, and TA1537, levels of up to
3.3 µg/plate were not mutagenic without activation, and
levels of up to 100 µg/plate were not mutagenic after
microsomal activation. Higher levels could not be tested
because of excessive cell dealth. Zimmering et al. (1985)
tested Drosophila by the sex-linked recessive lethal test
(SLRL), either by feeding doses of 40 ppm for 3 days or by
giving a single injection of 2000 ppm or 3000 ppm (volume
not specified). The vehicle used was 10% ethyl alcohol,
which did not totally dissolve the HEX. HEX (98%) was
first assayed in the SLRL test in adult feeding exper-
iments. When negative results were obtained, the chemical
was retested by injecting < 1 day-old Canton-S wild-type
Drosphila males. The results of the injection test were
reported to be inconclusive.
HEX has also been assayed in the mouse dominant lethal
test (Litton Bionetics, Inc., 1978b). In this assay, 0.1,
0.3, or 1.0 mg HEX/kg was administered by gavage to 10
male CD-1 mice for 5 days and the mice were then mated
throughout spermatogenesis (7 weeks). This test determines
whether the compound induces lethal genetic damage to the
germ cells of males. There was no evidence of dominant
lethal activity, at any dose level, based on any parameter
(e.g., fertility index, implantations per pregnancy,
average resorptions per pregnancy).
8.6. Cell transformation
The ability of HEX to induce morphological transfor-
mation of BALB/3T3 cells in vitro has been studied by
Litton Bionetics, Inc. (1977).
The selection of test doses was based on previous
cytotoxicity tests using a wide range of HEX concen-
trations at 0.0, 0.01, 0.02, 0.039, 0.078, and 0.156 mg
per litre. The cultures were exposed for 48 h, which was
followed by an incubation period of 3-4 weeks. The
cultures were observed daily. The doses selected allowed a
cell survival of 80-100% compared with controls (solvent
only). This high survival rate permitted an evaluation of
in vitro malignant transformation in cultures treated
with HEX as compared with the solvent controls. 3-Methyl-
cholanthrene at a dose level of 3 mg/litre was used as a
positive control. Results indicated that HEX was not
responsible for cell transformation.
8.7. Carcinogenicity
Bioassays of HEX for possible carcinogenicity have not
been conducted. As noted previously (section 8.3.2), the
NTP has completed a study on HEX for carcinogenicity by
the inhalation route in rats and mice, but the results
were not available when this monograph was being prepared.
9. EFFECTS ON HUMANS
9.1. General population exposure
There is very little detailed information available on
the human health effects of HEX exposure. Acute human
exposure has been reported in homes near waste sites where
disposal of HEX has occurred (C.S. Clark et al., 1982;
Elia et al., 1983). The odour threshold has been stated
to be 1.92 µg/m3 (0.17 ppb), but there appears to be
great individual variation. According to a study completed
by the A.D. Little Co. for the Occidental Chemical
Corporation, the 100% panel recognition concentration was
1.92 µg/m3 (0.17 ppb v/v)a, but the study design and
methodology were not reported. The US EPA (1982) estimated
that exposure of the general population to HEX in air
and/or water would be extremely low.
Treon et al. (1955) reported that members of a
research group conducting toxicity tests developed head-
aches when they were accidentally exposed to unknown
concentrations of HEX. The HEX escaped into the room when
an aerated exposure chamber was opened.
In a 48-block area surrounding a contaminated sewer
line in Kentucky, USA, questionnaires were sent to a
selected sample of residents. A total of 212 occupants
were surveyed. Only 3.8% of the residents reported an
unusual odour. The most common symptoms were stomach aches
(5.2%), burning or watery eyes (4.7%), and headaches
(4.7%). There was no association between the frequency of
symptoms and the distance of houses from the contaminated
sewer line. No significant ambient air concentrations of
HEX were found in these areas (Kominsky et al., 1978).
9.2. Occupational exposure
The US National Institute for Occupational Safety and
Health (NIOSH, 1980) stated that 1427 workers were occu-
pationally exposed to HEX. Officials from the Velsicol
Chemical Corporation estimated that 157 employees had been
potentially exposed to HEX in their production and pro-
cessing facilities.
A well-documented incident of acute human exposure to
HEX occurred in March 1977 at the Morris Forman Wastewater
Treatment Plant in Louisville, Kentucky, USA (Wilson et
al., 1978; Morse et al., 1979; Kominsky et al., 1980). The
details of the incident are included in the original NIOSH
Hazard Evaluation and Technical Assistance Report Number
TA-77-39 (Kominsky et al., 1978), which is available from
the US National Technical Information Service (NTIS). This
--------------------------------------------------------
a Memorandum from G. Leonardos to P. Levins on Hooker
special priority samples (odour properties).
treatment facility was contaminated with approximately 6
tonnes of HEX and octachlorocyclopentene, a waste product
of HEX manufacture (Morse et al., 1979). The contamination
was traced to an illegal dumping in one large sewer line
that passed through several populated areas. Concen-
trations of HEX detected in the sewage at the plant were
as high as 1000 mg/litre, and levels in the sewer line
were up to 100 mg/litre. Air samples taken from the sewer
line showed HEX concentrations to be as high as 4.5
mg/m3 (400 ppb). Although the airborne concentrations of
HEX at the time of the exposure in the treatment facility
were not known, airborne concentrations in the primary
treatment areas (screen and grit chambers) ranged between
3.05 and 10.96 mg/m3 (270 and 970 ppb) 4 days after the
plant had closed. It should be noted that the ACGIH
8 h-TWA for HEX was 0.1 mg/m3 (10 ppb) in 1977. Workers
tried to remove an odoriferous and sticky substance from
the bar screens and grit collection system by using steam
during clean-up of the contamination. This procedure pro-
duced a blue haze which permeated the primary treatment
area. The airborne HEX concentration of the blue haze was
reported to be 217 µg/m3 (19.2 ppm) (Kominsky et al., 1980).
The US Centers for Disease Control (CDC) and NIOSH
sent representatives to the plant with questionnaires
about the type and duration of symptoms (Morse et al.,
1979; Kominsky et al., 1980). In all, 193 employees were
identified as having been potentially exposed for 2 or
more days during the 2 weeks before the plant was closed
(Morse et al., 1979). The questionnaire was sent to each
of these 193 workers and 145 (75%) responded. Workers with
complaints of mucous membrane irritation were given a
physical examination, and blood and urine samples were
collected for clinical screening by an independent labora-
tory. Data were also collected on the exposure levels and
symptoms experienced by several people who had been
acutely exposed to the chemical vapours.
The results of the CDC and NIOSH questionnaires showed
that the odour of HEX had been detected by 94% of the
workers before the onset of symptoms. The most common
symptoms reported were eye irritation (59%), headaches
(45%), and throat irritation (27%) (Table 18). Of the 41
plant workers examined, six had physical signs of eye
irritation (i.e. lacrimation or redness) and five had
signs of skin irritation. Laboratory analyses of blood
and urine specimens from these workers showed marginal
increases in lactic dehydrogenase activity in 27% of cases
and proteinuria in 15%. Three weeks later, no abnormali-
ties were detected in the blood and urine tests. After six
weeks, some of the clinical symptoms persisted in 25-45%
of the employees (Morse et al., 1978).
Table 18. Symptoms of 145 waste-water treatment
plant employees exposed to HEX (Louisville,
Kentucky, USA, March 1977)a
----------------------------------------------------
Symptom No. of Percent of
employees employees
with symptoms with symptoms
----------------------------------------------------
Eye irritation 86 59
Headache 65 45
Throat irritation 39 27
Nausea 31 21
Skin irritation 29 20
Cough 28 19
Chest pain 28 19
Difficult breathing 23 16
Nervousness 21 14
Abdominal cramps 17 12
Decreased appetite 13 9
Decreased memory 6 4
Increased saliva 6 4
----------------------------------------------------
a From: Morse et al. (1978).
Although there was difficulty in measuring the level
of exposure received by the plant workers, more than 50%
of the clean-up crew were monitored. Laboratory tests
showed no significant abnormalities in renal function
tests, complete blood counts, or urinalysis, but several
minimal or mild abnormalities were found in liver function
tests (Kominsky et al., 1980). The abnormalities in 18 out
of 97 clean-up workers are listed in Table 19. These
people also had physical signs of mucous membrane irri-
tation. A more detailed correlation between acute exposure
level data and symptomatology was reported for nine adults
(Kominsky et al., 1980). The data appear in Table 20. The
exposure levels could not be estimated accurately because
of prior exposure or because the worker had used protec-
tive equipment.
Table 19. Abnormalities detected in clean-up
workers at the Morris Forman treatment plant,
Louisville, Kentucky, USAa
-------------------------------------------------
Laboratory test Normal range Abnormal results
Range No.b
-------------------------------------------------
Serum glutamate
oxaloacetate 7-40 mU/ml 40-49 5
transaminase 50-59 1
60-69 4
70-79 0
80-89 1
90-99 1
Serum alkaline 30-100 mU/ml 100-109 3
phosphatase 110-119 1
120-129 1
Serum total 0.15-10 mg/dl 1.0-1.9 1c
bilirubin
Serum lactate 100-225 mU/ml 230-239 1
dehydrogenase
-------------------------------------------------
a From: Kominsky et al. (1980).
b For individuals with more than one serial
blood test, only the most abnormal result is
tabulated.
c Associated with a serum glutamate oxaloacetate
transaminase activity of 66 mU/ml.
Table 20. Individual exposure symptomatology correlations at the Morris Forman treatment plant (Louisville, Kentucky, USA)a
-------------------------------------------------------------------------------------------------------------------------------------
No. of Estimated airborne Immediate symptoms Persistence of symptoms Laboratory
workers exposureb results
-------------------------------------------------------------------------------------------------------------------------------------
1 19.2 ppm HEX and 650 ppb lacrimation; skin irritation on face 1.5 h post-exposure: fatigue; normal results
OCCP for several seconds and neck; dyspnoea and chest erythema of exposed skin; eye 4 days
(no protective equipment) discomfort;nausea (several min later) irritation subsided in 1 day; post-exposurec
chest discomfort persisted
several days
3 7083 ppb HEX and 446 ppb lacrimation; irritation of exposed asymptomatic at 2 h, except normal results
OCCP for several seconds skin for soreness around eyes 7 days post-
(half-face respirator) exposure in
one workerc
2 40-52 ppb HEX and slight eye irritation no symptoms after cessation of normal results
9-21 ppb OCCP exposure 7 days post-
(half-face respirator) exposure in one
workerc
2 exact exposure unknown slight skin irritation faces felt "puffy" and "windburned" none available
(half-face respirator) for 1-2 days after exposure; this
was noted also by friends and
family; no residual skin lesions.
1 980 ppb HEX for 15 min; irritated eyes; nasal irritation eyes felt "dry and irritated" for none available
OCCP not measured and sinus congestion after 2 weeks 2-3 days after exposure; nasal
(no protective equipment) of intermittent exposures irritation ceased within 1-2 days
of cessation of exposure.
-------------------------------------------------------------------------------------------------------------------------------------
a From: Kominsky et al. (1980).
b OCCP = Octachlorocyclopentene.
c Laboratory work was same as carried out on clean-up crew.
Hazards to workers in treatment plants can result from
chemical compounds contained in the industrial waste
treated in municipal waste-water plants. The HEX-contain-
ing wastes from a pesticide manufacturer were treated in a
municipal waste-water treatment plant at Memphis,
Tennessee, USA. In 1978, the workers at this plant
reported symptoms similar to those reported by workers in
the Louisville plant referred to above. The air and waste-
water were monitored, and analyses of urine and blood,
liver function tests, and illness symptom questionnaires
were completed. Workers from another waste treatment
plant, where no pesticide wastes had been received, were
used as a control group. No statistical differences in
urine HEX concentrations or liver function tests between
the exposed and control groups were found, although
differences in levels of HEX-related compounds (co-
contaminants) were detected (Elia et al., 1983).
9.3. Epidemiological studies
Mortality studies have been carried out on workers
involved in the production of HEX or formulation of HEX
products. Shindell et al. (1980) studied a cohort of 783
current and former workers who had been employed at the
Velsicol Chemical Corporation plant at Marshall, Illinois,
between 1946 and 1979. The purpose of the study was to
evaluate the overall health status of all employees who
had been present during the manufacture of chlordane for 3
months or more. There were no significant differences in
mortality rates between these employees and the overall
USA population. The observed value for deaths from all
causes, including heart disease and cancer, was less than
the expected value in the overall USA population.
Shindell et al. (1981) completed another epidemiologi-
cal study for the Velsicol Chemical Corporation at its
Memphis, Tennessee, plant covering the period 1952-1979.
This coincided with the manufacture of heptachlor, a
pesticide made from HEX. The reseachers studied 1115
current and former employees who had worked for 3 months
or more. Again, there was no significant difference in
mortality between the control and exposure groups.
Concomitant with the study performed by Shindell et
al. (1980), Wang & MacMahon (1979) conducted a retrospec-
tive mortality study of workers employed at the Marshall
and Memphis plants, where chlordane and heptachlor were
manufactured between 1946 and 1976. They studied 1403
males who had worked at the plants for more than 3 months.
There were 113 observed deaths compared with 157 expected
deaths, giving a standardized mortality ratio (SMR) of 72.
Among the various causes of death, the two highest SMRs
were 134 for lung cancer and 183 for cerebrovascular
disease, but only the latter figure was statistically sig-
nificant (P < 0.05). The excess mortality due to cerebro-
vascular disease was not related to the duration of
exposure or to the latency period, and occurred only after
termination of employment. Shindell & Ulrich (1986)
updated their 1980 data set with additional worker data.
There was no information specific to HEX exposure.
Buncher et al. (1980) studied the mortality of workers
at a chemical plant that produced HEX. They examined 341
workers (287 male and 54 female), together with their
health records, who had worked at the plant for at least
90 days between 1 October 1953 and 31 December 1974. Their
vital status was determined through 1978. The expected
numbers of deaths, based on the USA population and
specific for sex, age, and calendar year, were calculated.
The SMR for all causes of death was 69. Deaths caused by
specific cancers, all cancers, and diseases of the circu-
latory and digestive systems were also fewer than
expected. The authors noted that the time that had elapsed
since the initial exposure (25 years at most) reduced the
power of the study to detect cancers that may have a
10-40 year latent period.
Similar studies have been performed on a cohort of
workers in the Shell International Petroleum plants in the
Netherlands. These reports have been reviewed extensively
in Environmental Health Criteria 91: Aldrin and Dieldrin
(WHO, 1989).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
The general population is not at risk from exposure to
HEX. However, people living near HEX processing and pro-
duction facilities, as well as handlers of the chemical
and its waste, are at risk. Exposure to HEX can occur
through several different routes. However, HEX is much
more toxic when inhaled than when ingested or following
dermal contact. Skin exposure studies have shown that HEX
can cause irritation, together with visceral changes that
are similar to those that result from oral administration.
Inhalation studies in animals have shown that HEX vapour
is very irritating, repeated exposure to 1.13-2.26 mg/m3
(0.1-0.2 ppm) causing pulmonary pathological changes.
Acute toxic symptoms, including headaches, nausea, dizzi-
ness, and respiratory distress, have been reported. Based
on a 90-day inhalation study in mice and rats, a NOEL of
0.45 mg/m3 (0.04 ppm) has been estimated. No information
is available on the long-term effects of a single exposure
or of continuous exposure to HEX.
A carcinogenic study of HEX has been completed (but
not evaluated) by the US National Toxicology Program. In
vitro mutagenicity and cell transformation tests yielded
negative results, as did an in vivo mouse dominant lethal
assay at the levels tested. There was no evidence of
teratogenicity in oral exposure studies in which three
species were examined.
The toxic effects of HEX exposure on the human respir-
atory system are of major concern. Although the long-term
toxicity data are limited, systemic toxic effects of HEX
inhalation have been observed after short-term exposure,
suggesting that long-term inhalation exposure to low con-
centrations of HEX could cause adverse health effects.
Limited epidemiological studies of workers exposed to HEX
at levels above those known to induce adverse health
effects have been conducted, although concomitant exposure
to other chemicals was known to have occurred. These
workers complained of headaches, eye and skin irritation,
nausea, dizziness, and respiratory distress, as did
individuals living near areas where there were HEX
releases.
10.2. Evaluation of effects on the environment
Release of HEX into the environment can result from
the production, processing and use of HEX, disposal of
waste containing HEX or from products contaminated with
HEX. Only a small proportion of the total amount of HEX
released into the environment from production, processing,
and use can be expected to persist beyond a few days.
However, waste disposal has resulted in HEX persisting in
soil, sediment, and ground water. There is little monitor-
ing information on the levels of HEX in air, water, and
sediment. The exposure of organisms in the aquatic
environment to HEX is therefore difficult to quantify.
HEX may undergo photolysis, hydrolysis, and biodegra-
dation. In water, photolysis is the dominant process in
direct sunlight, hydrolysis being the next most important
degradation route. Volatilization to the atmosphere occurs
from both water and soil. Biodegradation occurs in soil
under both aerobic and anaerobic conditions and in sewage
sludge. In water and aquatic sediment, biodegradation is
initially limited. Information on the adaptation of micro-
organisms to degrade HEX is limited. HEX has a calculated
tropospheric residence time of approximately 5 h.
Laboratory studies suggest that HEX is relatively
immobile in soil, particularly in soil with a high organic
content. However, leaching and movement in ground water
has been reported in field studies.
HEX has been shown to be toxic to aquatic life at a
level of 1-100 µg/litre. However, little information has
been obtained under field conditions (long-term exposure
at low concentration in the presence of sediment). The
actual hazard to aquatic life is, therefore, difficult to
assess. HEX is toxic to aquatic microorganisms but less
toxic to soil microorganisms. Exposure of terrestrial
organisms, except at or near disposal sites, would be
expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
* The general population is not at risk from exposure
to HEX, except in the case of people residing near
contaminated areas.
* The long-term human health effects of continuous low-
level exposure are not known. Handlers of the product
and its waste, as well as sewage workers, are at
risk.
* Results of laboratory studies indicate that HEX
should be degraded rapidly in the environment by
photolysis, hydrolysis, or biodegradation. The rela-
tive importance of these processes varies with the
medium. HEX is not a widespread environmental con-
taminant and the available data suggest that it is
only found associated with production, processing and
disposal sites. HEX does not bioaccumulate.
* Acute laboratory tests show that HEX is highly toxic
to aquatic microorganisms, invertebrates, and fish,
but less toxic to soil microorganisms. However,
information obtained under environmentally realistic
conditions is limited. The potential hazard to the
general environment is expected to be low.
11.2. Recommendations for protection of human health and
the environment
* Occupational exposure to HEX should be minimized by
the use of closed systems. Guidelines for the dis-
posal of HEX and HEX wastes should be followed.
* Environmental monitoring is needed to examine the
persistence and fate of HEX in all media near pro-
duction, processing and disposal sites, and also
hazardous waste incinerators. Monitoring data are
required for HEX in drinking-water, and in surface,
shower, and ground water.
12. FURTHER RESEARCH
* Biomarker technology should be developed to indicate
the possibility of past or current actions of HEX.
Such biomarkers could be stable metabolites derived
from HEX and its impurities that are present in the
original preparation.
* Research is needed on the metabolic, degradative, and
reactive products to understand the fate of HEX in
human beings and the environment.
* Further study of the apparent disparity between degra-
dation under laboratory conditions and that observed
in the environment is needed.
* The efficacy and safety of current disposal methods
should be evaluated and their present and future
health impacts assessed.
* Developmental and reproductive studies of HEX need to
be conducted, with emphasis on the inhalation route of
exposure.
* Methods for the early warning of the presence of low
levels of HEX should be developed.
REFERENCES
ABDO, K.M., MONTGOMERY, C.A., KLUWE, W.M., FARNELL, D.R., & PREJEAN, J.D.
(1984) Toxicity of hexachlorocyclopentadiene: subchronic (13-week)
administration by gavage to F344 rats and B6C3F1 mice. J. appl. Toxicol.,
4(2): 75-81.
ALEXANDER, D.J., CLARK, G.C., JACKSON, G.C., HARDY, C.J., STREET, A.E.,
HEYWOOD, R.H., BUIST, D., PRENTICE, D.E., & ISAACS, K.R. (1980)
Subchronic inhalation toxicity of hexachlorocyclopentadiene in monkeys
and rats, Huntingdon, Huntingdon Research Centre, 373 pp (Report
VCL14M/791081) (Prepared for Velsicol Chemical Corporation, Chicago).
ATALLAH, Y.H., WHITACRE, D.M., & BUTZ, R.G. (1980) Fate of
hexachlorocyclopentadiene in the environment. Paper presented at the 2nd
Chemical Congress of the North American Continent, Las Vegas, Nevada, 29
August, 1980, Chicago, Velsicol Chemical Corporation.
BELL, M.A., EWING, R.A., & LUTZ, G.A. (1978) Reviews of the environmental
effects of pollutants. XII. Hexachlorocyclopentadiene, Washington, DC, US
Environmental Protection Agency (Report EPA-600/1-78-047) (NTIS PB 80-
122963).
BENNETT, B. (1982) Hexachlorocyclopentadiene analyses of Mississippi
river fish, Athens, Georgia, US Environmental Protection Agency, Region
IV (Unpublished report).
BENOIT, F.M. & WILLIAMS, D.T. (1981) Determination of
hexachlorocyclopentadiene at the nanogram per liter level in drinking
water. Bull. environ. Contam. Toxicol., 27: 303-308.
BOYD, K.W., EMORY, M.B., & DILLON, H.K. (1981) Development of personal
sampling and analytical methods for organochlorine compounds. In:
Chemical hazards in the workplace, Washington, DC, American Chemical
Society, pp. 49-64 (ACS Symposium Series No. 149).
BRAT, S.V. (1983) The hepatocyte primary culture/DNA repair assay on
compound hexachlorocyclopentadiene using rat hepatocytes in culture,
Valhalla, New York, American Health Foundation, Naylor Dana Institute for
Disease Prevention.
BRIGGS, G.G. (1973) A simple relationship between soil adsorption of
organic chemicals and their octanol/water partition coefficients. In:
Proceedings of the 7th British Insecticide and Fungicide Conference,
Brighton, England, 19-22 November, 1973, Croydon, British Crop Protection
Council, Vol. 1, pp. 83-86 (Research Report).
BUA (BERATERGREMIUM FUR UMWELTRELEVANTE ALTSTOFFE) (1988)
[Hexachlorocyclopentadiene] Weinheim, Germany, VCH Verlagsgesellschaft
(BUA Report No. 25) (in German).
BUCCAFUSCO, R.J. & LEBLANC, G.A. (1977) Acute toxicity of
hexachlorocyclopentadiene to bluegill (Lepomis macrochirus), channel
catfish (Ictalurus punctatus), fathead minnow (Pimephales promelas), and
the water flea (Daphnia magna) (Unpublished report prepared for Velsicol
Chemical Corporation, Chicago).
BUNCHER, C.R., MOOMAW, C., & SIRKOSKI, E. (1980) Mortality study of
Montague Plant-Hooker Chemical, Cincinnati, Ohio, University of
Cincinnati Medical Center, Division of Epidemiology and Biostatistics
(Unpublished report prepared for Hooker Chemical Corporation).
BUTZ, R.G. & ATALLAH, Y.H. (1980) Effects of hexachlorocyclopentadiene on
three microbial functions (VCC Project No. 482428, Report No. 8)
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
BUTZ, R.G., YU, C.C., & ATALLAH, Y.H. (1982) Photolysis of
hexachlorocyclopentadiene in water. Ecotoxicol. environ. Saf., 6: 347-
357.
CALLAHAN, M.A., SLIMAK, M.W., GABEL, N.W., MAY, I.P., FOWLER, C.F.,
FREED, J.R., JENNINGS, P., DURFEE, R.L., WHITMORE, F.C., MAESTRI, B.,
MABEY, W.R., HOLT, B.R., & GOULD, C. (1979) Water-related environmental
fate of 129 priority pollutants: II. Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic
aromatic hydrocarbons, nitrosamines, miscellaneous compounds, Washington,
DC, US Environmental Protection Agency, Monitoring and Data Supervision
Division, Office of Water Planning and Standards (Report EPA-440/4-79-
029b).
CARTER, M.R. (1977) Legal affidavit field in the State of Georgia, Fulton
County, dated 14 June, 1977. Testimony concerning estimates of total
daily discharge of HEX from Velsicol Chemical Corporation, Atlanta,
Athens, Georgia, US Environmental Protection Agency.
CHERNOFF, N. & KAVLOCK, R.J. (1982) An in vivo teratology screen
utilizing pregnant mice. J. Toxicol. environ. Health, 10: 541-550.
CHERNOFF N. & KAVLOCK, R.J. (1983) A teratology test system which
utilizes postnatal growth and viability in the mouse. In: Waters, M.,
Sandhu, S., Lewtas, J., Claxton, L., Chernoff, N., & Nesnow, S., ed.
Short-term bioassays in the analysis of complex mixtures III, New York,
London, Plenum Publishing Corporation, pp. 417-427.
CHOPRA, N.M., CAMPBELL, B.S., & HURLEY, J.C. (1978) Systematic studies on
the breakdown of endosulfan in tobacco smokes: Isolation and
identification of the degradation products from the pyrolysis of
endosulfan I in a nitrogen atmosphere. J. agric. food Chem., 26: 255-258.
CHOU, S.F.J. & GRIFFIN, R.A. (1983) Soil, clay, and caustic soda effects
on solubility, sorption and mobility of hexachlorocyclopentadiene,
Springfield, Virginia, National Technical Information Service, 54 pp (PB
84-116060) (Environmental Geology Notes No. 104).
CHOU, S.F.J., GRIFFIN, R.A., CHOU, M.M, & LARSON, R.A. (1987) Products of
hexachlorocyclopentadiene (C-56) in aqueous solution. Environ. Toxicol.
Chem., 6: 371-376.
CLARK, C.S., MEYER, C.R., GARTSIDE, P.S., MAJETI, V.A., & SPECKER, B.
(1982) An environmental health survey of drinking water contamination by
leachate from a pesticide waste dump in Hardeman County, TN. Arch.
environ. Health, 37(1): 9-18.
CLARK, D.G., BLAIR, D., MARTIN, J., HENDY, R., PILCHER, A., & WIGGINS, D.
(1982) Thirty-week chronic inhalation study of hexachlorocyclopentadiene
(HEX) in rats, Tunstall, United Kingdom, Shell Toxicology Laboratory
(Experiment No. 1760, Report No. SBGR.81) (Permission for use granted by
M.J. Sloan, Shell Oil Co., Washington).
COLE, E.J. (1953) Chemotherapeutic and pharmacologic aspects of
hexachlorocyclopentadiene, Laramie, University of Wyoming, Department of
Veterinary Sciences and Bacteriology (Master's Thesis).
COLE, E.J. (1954) Treatment of sewage with hexachlorocyclopentadiene.
Appl. Microbiol., 2: 198-199.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment, Research Triangle Park, North Carolina, US Environmental
Protection Agency (Report EPA-600/3-80-084).
DAL MONTE, R.P. & YU, C.C. (1977) Water solubility of MC-984 and hex
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DELEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, P.C., REMELE, P.C.,
STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980a) Rapid gas
chromatographic method for the determination of volatile and semivolatile
organochlorine compounds in soil and chemical waste disposal site
samples. J. chromatogr. Sci., 18: 85-88.
DELEON, I.R., BROWN, N.J., COCCHIARA, J.P., MILES, S.K., & LASETER, J.L.
(1980b) Determination of trace levels of hexachlorocyclopentadiene and
octachlorocyclopentene in body fluids. J. anal. Toxicol., 4: 314-317.
DILLON, H.K. (1980) Development of air sampling and analytical methods
for toxic chlorinated organic compounds, Birmingham, Alabama, Southern
Research Institute, 76 pp (NTIS PB 80-193279).
DOROUGH, H.W. (1979) The accumulation, distribution and dissipation of
hexachlorocyclopentadiene (C56) in tissues of rats and mice, 27 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. (1980) Disposition of 14C-hexachlorocyclopentadiene (C56)
in rats following inhalation exposure, 53 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. & RANIERI, T.A. (1984) Distribution and elimination of
hexachlorocyclopentadiene in rats and mice. Drug chem. Toxicol., 7(1):
73-89.
EICHLER, D.L. (1978) Quantitative analyses of mixtures containing trace
amounts of pesticides. Internal memorandum to M.R. Zavon, Hooker
Chemicals and Plastics Corporation, Niagara Falls, New York, 3 pp.
EL DAREER, S.M., NOKER, P.E., TILLERY, K.F., & HILL, D.L. (1983)
Investigations on the basis for the differential toxicity of
hexachlorocyclopentadiene administered to rats by various routes. J.
Toxicol. environ. Health, 12: 203-211.
ELIA, V.J., CLARK, C.S., MAJETI, V.A., GARTSIDE, P.S., MACDONALD, T.,
RICHDALE, N., MEYER, C.R., VAN MEER, G.L., & HUNNINEN, K. (1983) Chemical
exposure at a municipal wastewater treatment plant. Environ. Res., 32:
360-371.
EVANS, M.J., CABRAL-ANDERSON, L.J., & FREEMAN, G. (1978) Role of the
Clara cell in renewal of the bronchiolar epithelium. Lab. Invest., 38:
648-655.
GOGGELMAN, W., BONSE, G., HENSCHLER, D., & CREIM, H. (1978) Mutagenicity
of chlorinated cyclopentadiene due to metabolic activation. Biochem.
Pharmacol., 27: 2927-2929.
GRAY, L.E., Jr & KAVLOCK, R.J. (1984) An extended evaluation of an in
vivo teratology screen utilizing postnatal growth and viability in the
mouse. Teratog. Carcinog. Mutagen., 4: 403-426.
GREIM, J., BIMBOES, D., GOGGELMANN, W., & KRAMER, M. (1977) Mutagenicity
and chromosomal aberrations as an analytical tool for in vitro detection
of mammalian enzyme-mediated formation reactive metabolites. Arch.
Toxicol., 39: 159-169.
HAWLEY, G.G., ed. (1977) Condensed chemical dictionary, 9th ed., New
York, Van Nostrand Reinhold Co.
HAWORTH, S., LOWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E. (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen., 5(Suppl. 1): 3-142.
HENDERSON, C. (1956) Bio-assay investigations for International Joint
Commission, Niagara Falls, New York, Hooker Electrochemical Company, 17
pp (Unpublished report).
HUNT, G.E. & BROOKS, G.W. (1984) Source assessment for
hexachlorocyclopentadiene, Research Triangle Park, North Carolina, Radian
Corporation (Unpublished report prepared for the US Environmental
Protection Agency).
IBT (1977) Mutagenicity of PCL-HEX incorporated in the test medium tested
against five strains of S. typhimurium and as a volatilate against tester
strain TA-100, Northbrood, Illinois, Industrial Bio-Test Laboratories.
IRDC (1968) Hexachlorocyclopentadiene and octachlorocyclopentene: Acute
oral toxicity LD50 in male albino rats, Mattawan, Michigan, International
Research and Development Corporation, 4 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
IRDC (1972) Acute toxicity studies in rats and rabbits, Mattawan,
Michigan, International Research and Development Corporation, 21 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
IRDC (1978) Hexachlorocyclopentadiene: Teratology study in rats,
Mattawan, Michigan, International Research and Development Corporation,
17 pp (Unpublished report prepared for Velsicol Chemical Corporation,
Chicago).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic.
Hexachlorocyclopentadiene. In: Patty, F.A., ed. Industrial hygiene and
toxicology, 2nd revised ed., New York, Chichester, Brisbane, Toronto,
John Wiley & Sons, pp. 1333-1363.
IRPTC (1989) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol. environ.
Safety, 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (1980) Relationship between water
solubility, soil sorption, octanol/water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
of Testing and Materials, pp. 78-115 (ASTM STP 707).
KHAN, M.A.Q., SUDERSHAN, P., FEROZ, M., & PODOWSKI, A.A. (1981)
Biotransformations of cyclodienes and their photoisomers and
hexachlorocyclopentadiene in mammals and fish. In: Khan, M.A.Q. &
Stanton, R.H., ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects, Oxford, New York, Pergamon Press, pp. 271-288.
KILZER, L., SCHEUNERT, I., GEYER, H., KLEIN, W., & KORTE, F. (1979)
Laboratory screening of the volatilization rates of organic chemicals
from water and soil. Chemosphere, 8: 751-761.
KLOSKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981) Laboratory
screening of distribution, conversion and mineralization of chemicals in
the soil-plant-system and comparison to outdoor experimental data.
Chemosphere, 10: 1089-1100.
KOMINSKY, J.R. & WISSEMAN, C.L. (1978) Morris Forman wastewater treatment
plant, Metropolitan Sewer District, Louisville, KY, Cincinnati, Ohio,
National Institute of Occupational Safety and Health (NIOSH Hazard
Evaluation and Technical Assistance Report No. TA 77-39) (NTIS PB 82-
178088).
KOMINSKY, J.R., WISSEMAN, C.L., & MORSE, D.L. (1980)
Hexachlorocyclopentadiene contamination of a municipal wastewater
treatment plant. Am. Ind. Hyg. Assoc. J., 41: 52.
KOMMINENI, C. (1978) Pathology reports. Animal portion of the Louisville
sewage study, Cincinnati, Ohio, National Institute of Occupational Safety
and Health (NIOSH Report No. TA 77-39).
LAWLESS, E.W., VON RUMKER, R., & FERGUSON, T.L. (1972) The pollution
potential in pesticide manufacturing, Springfield, Virginia, US
Department of Commerce, National Technical Information Service (NTIS PB
213782/3) (Prepared for the US Environmental Protection Agency by Midwest
Research Institute).
LAWRENCE, L.J. & DOROUGH, H.W. (1981) Retention and fate of inhaled
hexachlorocyclopentadiene in the rat. Bull. environ. Contam. Toxicol.,
26: 663-668.
LAWRENCE, L.J. & DOROUGH, H.W. (1982) Fate of inhaled
hexachlorocyclopentadiene in albino rats and comparison to the oral and
iv routes of administration. Fundam. appl. Toxicol., 2: 235-240.
LEVIN, A.A. (1982a) Hexachlorocyclopentadiene: Response to issues raised
at US EPA Test Rules Meeting of March 17, 1982, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Docket No.
40-8249078).
LEVIN, A.A. (1982b) Hexachloropentadiene: Follow-up and additional
information to the 17 March, 1982 Meeting, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
LICHTENBERG, J.J., LONGBOTTON, J.E., & BELLAR, T.A. (1987) Analytical
methods for the determination of volatile non-polar organic chemicals in
water and water-related environments. In: Suffet, I.H. & Malaiyandi, M.,
ed. Organic pollutants in water: Sampling, analysis and toxicity testing.
A Symposium of the 188th Meeting of the American Chemical Society,
Philadelphia, 29-31 August, 1984, Washington, DC, American Chemical
Society, pp. 63-81.
LITTON BIONETICS, INC. (1977) Evaluation of hexachlorocyclopentadiene; in
vitro malignant transformation in BALB/3T3 cells, Kensington, Maryland,
Litton Bionetics, Inc., 7 pp (LBI Project No. 29840) (Prepared for
Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978a) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse lymphoma forward mutation assay,
Kensington, Maryland, Litton Bionetics, inc., 10 pp (LBI Project No.
20839) (Prepared for Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978b) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse dominant lethal assay, Kensington,
Maryland, Litton Bionetics, Inc., 13 pp (LBI Project No. 20862).
LOOK, M. (1974) Hexachlorocyclopentadiene adducts of aromatic compounds
and their reaction products. Aldrichem. Acta, 7(2): 1974.
LU, P.Y., METCALF, R.L., HIRWE, A.S., & WILLIAMS, J.W. (1975) Evaluation
of environmental distribution and fate of hexachlorocyclopentadiene,
chlordane, heptachlor, and heptachlor epoxide in a laboratory model
ecosystem. J. agric. food Chem., 23: 967-973.
MACEK, R.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979) Considerations
in assessing the potential for, and significance of, biomagnification of
chemical residues in aquatic food chains. In: Marking, L. & Kimerle,
R.A., ed. Aquatic toxicology, Philadelphia, American Society for Testing
and Materials, pp. 251-268.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida, US Environmental Protection Agency
(Report EPA-600/8-017).
MEHENDALE, H.M. (1977) Chemical reactivity-absorption, retention,
metabolism and elimination of hexachlorocyclopentadiene. Environ. Health
Perspect., 21: 275-278.
MEIER, J.R., RINGHAND, H.P., COLEMAN, W.E., MUNCH, J.W., STREICHER, R.P.,
KAYLOR, W.H., & SCHENCK, K.M. (1985) Identification of mutagenic
compounds formed during chlorination of humic acid. Mutat. Res., 157:
111-122.
MOLOTSKY, H.M. & BALLWEBER, E.G. (1957) Hexachlorocyclopentenones: US
Patent No. 2,795,608, June 11, 1957, Chicago, Velsicol Chemical
Corporation.
MORSE, D.L., LANDRIGAN, P.J., & FLYNT, J.W. (1978) Internal CDC report
concerning hexachlorocyclopentadiene contamination of a municipal sewage
treatment plant, Louisville, Kentucky, Atlanta, Georgia, Centers for
Disease Control.
MORSE, D.L., KOMINSKY, J.R., & WISSEMAN, C.L., III (1979) Occupational
exposure to hexachlorocyclopentadiene (How safe is sewage?). J. Am. Med.
Soc., 241: 2177-2179.
MURRAY, F.J., SCHWETZ, B.A., BALMER, M.F., & STAPLES, R.E. (1980)
Teratogenic potential of hexachlorocyclopentadiene in mice and rabbits.
Toxicol. appl. Pharmacol., 53: 497-500.
NAS (1978) Kepone/mirex/hexachlorocyclopentadiene: An environmental
assessment, Washington, DC, National Academy of Sciences (NTIS PB 280-
289).
NEUMEISTER, C. & KURIMO, R. (1978) Determination of
hexachlorocyclopentadiene and octachlorocyclopentene in air. Presented at
the ACGIH Conference, Los Angeles, May 1978, Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
NIOSH (1979) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1-5 (DHEW
(NIOSH) Pub. No. 77-157-A).
NIOSH (1980) Quarterly hazard summary report: Hexachlorocyclopentadiene,
Cincinnati, Ohio, National Institute for Occupational Safety and Health.
NTP (1984a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 128 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
NTP (1984b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 196 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
OSHA (US DEPARTMENT OF LABOR, OCCUPATIONAL SAFETY AND HEALTH
ADMINISTRATION) (1989) Air contaminants: Final rule -
Hexachlorocyclopentadiene. Fed. Reg., 54(12): 2464.
PETERS, J.A., TACKETT, K.M., & EIMUTIS, E.C. (1981) Measurement of
fugitive hydrocarbon emissions from a chemical waste disposal site.
Presented at the 74th Annual Meeting of the Air Pollution Control
Association, Philadelphia, 21-26 June, 1981, Dayton, Ohio, Montesanto
Research Corporation.
PODOWSKI, A.A. & KHAN, M.A.Q. (1979) Fate of hexachlorocyclopentadiene in
goldfish (Carassius auratus). Paper presented at the American Chemical
Society Meeting, Honolulu, April 1979, Washington, DC, American Chemical
Society.
PODOWSKI, A.A. & KHAN, M.A.Q. (1984) Fate of hexachlorocyclopentadiene in
water and goldfish. Arch. environ. Contam. Toxicol., 13: 471-481.
RAND, G.M., NEES, P.O., CALO, C.J., ALEXANDER, D.J., & CLARK, G.C.
(1982a) Effects of inhalation exposure to hexachlorocyclopentadiene on
rats and monkeys. J. Toxicol. environ. Health, 9: 743-760.
RAND, G.M., NEES, P.O., CALO, C.J., CLARKE, G.C., & EDMONDSON, N.A.
(1982b) The Clara cell: An electron microscopy examination of the
terminal bronchioles of rats and monkeys following inhalation of
hexachlorocyclopentadiene. J. Toxicol. environ. Health, 10: 59-72.
RIECK, C.E. (1977a) Effect of hexachlorocyclopentadiene on soil microbe
populations, Lexington, Kentucky, University of Kentucky, Agronomy
Department (Unpublished report prepared for Velsicol Chemical
Corporation, Chicago).
RIECK, C.E. (1977b) Soil metabolism of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
RIECK, C.E. (1977c) Volatile products of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
ROBERTS, C.W. (1958) Chemistry of hexachlorocyclopentadiene. Chem. Ind.,
1 February: 110.
SHELL RESEARCH LIMITED (1982) Toxicology of insecticide intermediates:
The skin sensitizing potential of hexachlorocyclopentadiene, Tunstall,
United Kingdom, Shell Research Ltd., Sittingbourne Research Centre
(Report No. SBGR 82.225).
SHINDEL, S. & ULRICH, S. (1986) Mortality of workers employed in the
manufacture of chlordane. An update. J. occup. Med., 28(7): 497-501.
SHINDELL & ASSOCIATES (1980) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Marshall, Illinois,
January 1946-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SHINDELL & ASSOCIATES (1981) Report of the epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Memphis, Tennessee,
January 1952-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1982)
Hexachlorocyclopentadiene increases oxygen consumption by intact rainbow
trout and isolated heart mitochondria. Sixty-sixth Annual Meeting of the
Federation of American Societies for Experimental Biology, New Orleans,
15-23 April, 1982. Fed. Proc., 41(5): 1580 (abstract).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1983)
Respiratory effects of hexachlorocyclopentadiene on intact rainbow trout
(Salmo gairdneri) and on oxidative phosphorylation of isolated trout
heart mitochondria. Toxicol. appl. Pharmacol., 67: 215-223.
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1977) A rapid
assessment of the toxicity of three chlorinated cyclodiene insecticide
intermediates to fathead minnows, Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory (Report EPA-600/3-
77-099).
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1979) Toxicity
and bioaccumulation of hexachlorocyclopentadiene, hexachloronorbornadiene
and heptachloronorbonene in larval and early juvenile fathead minnows,
Pimephales promelas. Bull. environ. Contam. Toxicol., 21: 576-583.
SPRINKLE, C.L. (1978) Leachate migration from a pesticide. Waste disposal
site in Hardeman County, Tennessee, Washington, DC, US Department of
Interior, US Geological Survey (Waste Resources Investigations 78-128).
SRI (1980a) Acute toxicity report on hexachlorocyclopentadiene (C53607)
in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama, Southern
Research Institute, 44 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1980b) Repeated-dose toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama,
Southern Research Institute, 33 pp (Unpublished report prepared for the
National Toxicology Program).
SRI (1981a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 137 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1981b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 144 pp (Unpublished report prepared for the National
Toxicology Program).
STEVENS, J.E. (1979) Chlorinated derivatives of cyclopentadiene. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
Chichester, Brisbane, Toronto, John Wiley & Sons, Vol. 5, pp. 791-797.
THIELEN, D.R., OLSEN, G., DAVIS, A., BAJOR, E., STEFANOVSKI, J., &
CHODKOWSKI, J. (1987) An evaluation of microextraction/capillary column
gas chromatography for monitoring industrial outfalls. J. chromatogr.
Sci., 25(1): 12-16.
THUMA, N.K., O'NEILL, P.E., BROWNLEE, S.G., & VALENTINE, R.S. (1978)
Biodegradation of spilled hazardous materials. In: Control of hazardous
materials spills, Rockville, Maryland, Information Transfer, Inc., pp.
217-220.
TREON, J.F., CLEVELAND, F.P., & CAPPEL, J. (1955) The toxicity of
hexachlorocyclopentadiene. Arch. ind. Health, 11: 459-472.
UNGNADE, H.E. & MCBEE, E.T. (1958) The chemistry of
perchlorocyclopentenes and cyclopentadienes. Chem. Rev., 58: 249-254.
US EPA (1977) Chemical Hazard Information Profile:
Hexachlorocyclopentadiene (CHIP), Washington, DC, US Environmental
Protection Agency, TSCA Interagency Testing Committee.
US EPA (1980a) Summary of UWF Co-op Data on hexachlorocyclopentadiene and
hexachlorobutadiene, Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished laboratory data).
US EPA (1980b) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards (Report EPA-440/5-80-055)
(NTIS PB 292-436).
US EPA (1980c) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, office of Water Regulations and Standards (Report EPA-440/5-80-
055) (Unpublished data).
US EPA (1982) Hexachlorocyclopentadiene: Response to the Interagency
Testing Committee. Fed. Reg., 47(250): 58023-58025.
US EPA (1989) Toxic chemical release inventory: Online printout of April
1989, Washington, DC, US Environmental Protection Agency (Available from
the US National Library of Medicine).
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish.
Res. Board Can., 36: 1040-1048.
VELSICOL CHEMICAL CORPORATION (1978) TSCA Sec. 8(E): Submission 8EHQ-
06780208. Chlorinates in Mississippi River catfish and carp, 1978
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago)
(Submitted to the US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VELSICOL CHEMICAL CORPORATION (1979) Confirmation of HEX and HEX-BCH
residues in human urine. Analytical method No. 0682, Chicago, Velsicol
Chemical Corporation.
VELSICOL CHEMICAL CORPORATION (1984) Comments on draft health effects
document for hexachlorocyclopentadiene, Chicago, Velsicol Chemical
Corporation.
VELSICOL CHEMICAL CORPORATION (1986) Air sampling and monitoring at
Velsicol production facilities, Chicago, Velsicol Chemical Corporation.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Co.
VILKAS, A.G. (1977) The acute toxicity of hexachlorocyclopentadiene to
the water flea, Daphnia magna straus, Tarrytown, New York, Union Carbide
Environmental Services (UCES Project No. 11506-03-05) (Prepared for
Velsicol Chemical Corporation, Chicago).
WALSH, G.E. (1981) Effects of chlordane, heptachlor and
hexachlorocyclopentadiene on growth of marine unicellular algae, Gulf
Breeze, Florida, US Environmental Protection Agency, Environmental
Research Laboratory (Unpublished report).
WALSH, G.E. (1983) Cell death and inhibition of population growth of
marine unicellular algae by pesticides. Aquat. Toxicol., 3: 209-214.
WALSH, G.E. & ALEXANDER, S.V. (1980) A marine algal bioassay method:
Results with pesticides and industrial wastes. Water Air Soil Pollut.,
13: 45-55.
WANG, H.H. & MACMAHON, B. (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J. occup. Med., 21: 745-748.
WEAST, R.C. & ASTLE, M.J. (1980) CRC handbook of chemistry and physics,
60th ed., Boca Raton, Florida, CRC Press, Inc.
WEBER, J.B. (1979) Adsorption of HEX by Cape Fear loam soil, Research
Triangle Park, North Carolina State University (Unpublished report
prepared for Velsicol Chemical Corporation, Chicago).
WHITMORE, F.C., DURFEE, R.L., & KHATTAK, M.N. (1977) Evaluation of a
technique for sampling low concentrations of organic vapors in ambient
air, Atlanta, Georgia, US Environmental Protection Agency (NTIS PB 279-
672).
WHO (1989) Environmental Health Criteria 91: Aldrin and Dieldrin, Geneva,
World Health Organization, 335 pp.
WILLIAMS, C.M. (1978) Liver cell culture systems for the study of
hepatocarcinogens. Proceedings of the 12th International Cancer Congress,
XII. International Cancer Congress Symposium No. 2: Chemical Oncogenesis
and Mutagenesis, October 1978, New York, London, Plenum Publishing
Corporation.
WILSON, J.A., BALDWIN, C.P., & MCBRIDE, T.J. (1978) Case history:
Contamination of Louisville, Kentucky Morris Foreman treatment plant.
Hexachlorocyclopentadiene. In: Control of hazardous material spills,
Miami, Florida, Hazardous Material Control Research Institute, pp. 170-
177.
WOLFE, N.L., ZEPP, R.G., SCHLOTZHAVER, P., & SINK, M. (1982)
Transformation pathways of hexachlorocyclopentadiene in the aquatic
environment. Chemosphere, 11(2): 91-101.
YOWELL, H.L. (1951) Fungicidal compositions containing
hexachlorocyclopentadiene: US Patent 2538509, Washington, DC, US Patent
Office.
YU, C.C. & ATALLAH, Y.H. (1977a) HEX hydrolysis at various pHs and
temperatures, Chicago, Velsicol Chemical Corporation (Project No. 482428,
Report No. 8) (Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1977b) Photolysis of hexachlorocyclopentadiene,
Chicago, Velsicol Chemical Corporation (Project No. 482428, Report No. 4)
(Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1981) Pharmacokinetics and metabolism of
hexachlorocyclopentadiene in rats, Chicago, Velsicol Chemical Corporation
(Project No. 482428, Report No. 10).
ZEPP, R.G., BAUGHMAN, G.L., & SCHLOTZHAUER, P.F. (1979) Dynamics of
processes influencing the behavior of hexachlorocyclopentadiene in the
aquatic environment. Paper presented at the 178th National Meeting of the
American Chemical Society, Washington, 9-14 September, 1979, Washington,
DC, American Chemical Society, Division of Environmental Chemistry.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R.C. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ. Mutagen.,
7: 87-100.
APPENDIX 1
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Table 21.
Table 21. Guidelines, recommendations and standards used in various countries/areasa
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
Australia recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1983
weighted average
short-term exposure limit 0.3 (0.03)
Belgium recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1988
weighted average
Canada regulation air/occupational threshold limit value/time- 0.1 (0.01) 1980
weighted average
Canada regulation transport specific transportation 1987
regulations
Finland recommendation air/occupational time-weighted average 1.0 (0.1) 1989
skin short-term exposure limit 3 (0.3) 1989
Netherlands recommendation air/occupational time-weighted average/ 0.11 (0.01) 1986
occupational
Federal regulation waste "toxic waste" subject to 1981
Republic specific handling,
of Germany transport, treatment,
storage, and disposal
regulation/permits
Switzerland regulation air/occupational time-weighted average 0.1 (0.01) 1987
USA regulation water ambient 1 µg/litre 1980
water quality criteria
(organoleptic)
----------------------------------------------------------------------------------------------------
Table 21 (contd.)
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
USA (ACGIH) recommendation air/occupational time-weighted average 0.1 (0.01) 1980
USA regulation air/occupational time-weighted average 0.1 (0.01) 1989
USA regulation water/land notification of spill 1983
of 0.45 kg (1 lb)
in 24-h period
USA regulation waste transport "toxic waste" subject to 1980
specific handling,
transport, treatment,
storage and disposal
regulation/permits
USA draft drinking-water lifetime 7 µg/kg 1990
recommendation per day
USSR regulation air/occupational threshold limit value 0.01 (0.001) 1989
USSR regulation water maximum allowable 1 mg/litre 1985
concentration
USSR regulation air/ambient short-term exposure limit 0.001 1987
Yugoslavia regulation air/occupational time-weighted average 0.1 1985
----------------------------------------------------------------------------------------------------
a From: IRPTC (1989)
b Unless stated otherwise, units are mg/m3. The value in parts per million is given in parentheses.
RESUME
L'hexachlorocyclopentadiène (HEX) est un liquide dense
et ininflammable, de couleur jaune pâle à jaune verdâtre,
et qui possède une odeur piquante caractéristique. Le HEX
est très réactif; il donne lieu à des réactions d'addition
et de substitution et à des réactions de Diels-Alder.
Aux Etats-Unis d'Amérique, la Velsicol Chemical Cor-
poration est actuellement le seul producteur de HEX. En
Europe, il est produit aux Pays-Bas par la Société Shell.
Les chiffres de production sont confidentiels mais on
estime que 3600 à 6800 tonnes de HEX sont produites
actuellement aux Etats-Unis. En 1988, la production
mondiale était d'environ 15 000 tonnes (BUA, 1988). Le
HEX est utilisé comme intermédiaire dans la production de
nombreux pesticides, mais quelques pays en ont limité
l'emploi à la fabrication de certains pesticides organo-
chlorés. Il est également utilisé pour la fabrication de
retardateurs de flamme, de résines et de colorants.
Au cours de la production et de la transformation du
HEX, de petites quantités sont libérées dans l'environne-
ment. Ce peut être également le cas lorsqu'il constitue
une impureté de certains des produits pour lesquels il
sert d'intermédiaire. La libération de HEX peut interve-
nir pendant ou après le rejet. On ne dispose que de
données limitées sur la surveillance des concentrations de
cette substance dans l'environnement. D'après ces données,
il semble que le HEX soit présent essentiellement dans le
compartiment aquatique et y soit associé aux sédiments et
aux matières organiques, sauf là où il y a eu rejet ou
libération du produit. D'après les études en laboratoire,
il y a sorption du HEX par la plupart des particules du
sol. Toutefois, on a fait état d'un lessivage et d'un
mouvement dans les eaux souterraines.
Aux Etats-Unis d'Amérique, on estime que 5,9 tonnes de
HEX sont libérées annuellement dans le milieu (US EPA,
1989). En République fédérale d'Allemagne et aux Pays-
Bas, environ 400 à 500 kg de HEX ont été libérés dans
l'atmosphère en 1987 (BUA, 1988). En raison des propriétés
physiques et chimiques du HEX, il ne devrait subsister
qu'une faible fraction de ces émissions.
En s'appuyant sur les données de laboratoire disponi-
bles, on a modélisé la destinée et le transport du HEX
dans l'atmosphère et calculé que son temps de séjour dans
la troposphère était d'environ 5 h. On a fait état d'un
transport atmosphérique de HEX à partir d'une zone de
stockage de déchets et de puits au cours du traitement de
rejets industriels.
Dans l'eau, le HEX peut subir une photolyse, une
hydrolyse et une biodégradation. Dans les eaux peu
profondes son temps de demi-photolyse est inférieur à une
heure. Dans les eaux plus profondes où la photolyse est
exclue, le temps de demi-hydrolyse varie de plusieurs
jours à environ trois mois et la biodégradation est encore
beaucoup plus lente. Le HEX se volatilise à la surface de
l'eau à une vitesse qui dépend de la turbulence et du
degré de sorption par les sédiments.
En raison de sa faible solubilité dans l'eau, le HEX
devrait être relativement immobile dans le sol. Toutefois
on en a trouvé dans des eaux souterraines. La volatili-
sation de cette substance qui se produit très vraisembla-
blement à la surface du sol est d'autant plus importante
que la teneur du sol en matières organiques est plus
faible. Les résultats d'études en laboratoire indiquent
que l'hydrolyse chimique et la métabolisation microbienne,
qu'elles soient aérobie ou anaérobie, devraient réduire la
teneur des sols en HEX.
En principe, le HEX devrait avoir un pouvoir de bio-
amplification notable en raison de sa forte lipophilicité
(log du coefficient de partage octanol/eau). Toutefois
les données expérimentales ne corroborent pas cette hypo-
thèse. Des études sur animaux d'expérience ont montré que
le 14C-HEX est à la fois métabolisé et excrété dans les
quelques heures qui suivent l'administration par voie
orale, la rétention dans l'organisme étant très faible.
Le facteur de bioconcentration à l'état stationnaire est
inférieur à 30 chez les poissons. Les facteurs de bio-
accumulation calculés à partir de modèles d'écosystèmes à
court terme indiquent que le potentiel d'accumulation est
modéré. Il semblerait donc que le HEX et ses métabolites
ne persistent pas et ne s'accumulent pas en quantités
importantes dans les systèmes biologiques.
On a montré qu'à faibles concentrations, le HEX était
toxique pour la faune aquatique. Des cas de mortalité par
intoxication aiguë (exposition de 48 à 96 h) ont été
observés chez des crustacés et des poissons dulçaquicoles
et marins à des concentrations nominales de 32 à
180 µg/litre, dans des systèmes statiques dont l'eau
n'était pas renouvelée au cours de l'épreuve. Etant donné
que le temps de demi-photolyse est inférieur à une heure,
la concentration en HEX devrait avoir diminué de manière
importante au cours de la période d'exposition utilisée
dans ces études. Les seules études au cours desquelles on
a mesuré les concentrations de HEX dans de l'eau courante
ont donné une valeur de la CL50 à 96 h de 7 µg/litre
pour le vairon américain et une crevette de mer. Les
épreuves effectuées sur ces deux espèces ont donné,
respectivement pour la CL10 et la CL40, des valeurs de
3,7 et de 0,7 µg/litre.
Des épreuves statiques de sept jours effectuées sur
des algues marines à des concentrations nominales allant
de 3,5 à 100 µg/litre ont fait ressortir une réduction
moyenne de la croissance (CE50), qui était fonction de
l'espèce. En milieu aqueux, le HEX est toxique pour de
nombreux microorganismes à des concentrations nominales de
0,2 à 10 mg/litre, c'est-à-dire à des valeurs sensiblement
plus importantes que celles qui sont nécessaires pour tuer
la plupart des animaux et des plantes aquatiques. Le HEX
semble être moins toxique pour les microorganismes terri-
coles que pour les microorganismes aquatiques, probable-
ment en raison de l'adsorption du HEX sur la matrice
constituée par le sol.
On pense que l'exposition devrait être faible mais les
données actuellement disponibles sont insuffisantes pour
permettre de déterminer les effets de l'exposition au HEX
sur la flore et la faune terrestres.
La résorption du HEX non modifié est minimale du fait
de sa réactivité vis-à-vis des membranes et des tissus de
l'organisme et plus particulièrement, du contenu des voies
digestives. Après administration par voie orale, percuta-
née ou par inhalation, la majeure partie du 14C-HEX
radiomarqué est retenue au niveau des reins, du foie, de
la trachée et des poumons des animaux d'expérience. Une
fois résorbé, le HEX est métabolisé et rapidement excrété,
principalement dans les urines, un peu moins dans les
matières fécales et à raison de moins de 1%, dans l'air
expiré. La durée nécessaire pour l'élimination complète
est d'environ 30 h quelle que soit la voie d'adminis-
tration. Après inhalation ou administration par voie
intraveineuse, le composé parent ne se retrouve plus dans
les excréta; on a isolé des métabolites fécaux et uri-
naires mais ils n'ont pas été identifiés. C'est d'ailleurs
la raison pour laquelle il est très difficile de se faire
une idée de la pharmacocinétique du HEX et d'élucider son
mode d'action.
La CL50 par inhalation (sur une période d'environ
4 h) est de 17,9 mg/m3 chez le rat mâle et de 39,1 mg
par m3 chez la ratte. Bien qu'il existe quelques
différences d'une espèce à l'autre, notamment entre les
cobayes, les rats, les lapins et les souris, les vapeurs
de HEX sont extrêmement toxiques pour toutes les espèces
étudiées. C'est par inhalation qu'il se révèle le plus
toxique, par comparaison avec l'administration orale ou
percutanée, et il se montre également extrêmement irri-
tant. Quelle que soit la voie d'administration, toute
exposition aiguë entraîne des effets généraux pathologi-
ques au niveau des poumons, du foie, des reins et des
surrénales.
L'administration de HEX par voie orale à des rats
(30 mg/kg et par jour) et à des souris (75 mg/kg et par
jour) pendant 91 jours a déterminé une néphrose ainsi
qu'une inflammation et une hyperplasie de l'estomac
antérieur. On n'a pas relevé de signes manifestes après
exposition de souris ou de rats à qui l'on avait fait
inhaler du HEX à raison de 2,26 mg/m3 (0,2 ppm), six
heures par jour, cinq jours par semaine pendant 14
semaines. A la concentration de 1,69 mg/m3 (0,15 ppm),
on a observé seulement une petite irritation. Des rats
exposés de la même manière à 5,65 mg/m3 (0,5 ppm) de
HEX pendant 30 semaines ont présenté des modifications
histopathologiques au niveau du foie, des voies respi-
ratoires et des reins. Une autre étude du même genre
portant sur des rats et des souris et qui s'est prolongée
pendant 90 jours a révélé des effets respiratoires à
partir de 4,52 mg/m3 (0,4 ppm). Le HEX ne s'est pas
révélé mutagène lors d'épreuves in vitro, qu'il y ait ou
non activation métabolique. Il s'est également révélé
inactif dans les épreuves de dominance létale chez la
souris. Administré par voie orale à des rats et à des
souris, il ne s'est pas révélé tératogène, mais on ne
dispose d'aucune donnée relative à sa tératogénicité
éventuelle après exposition par voie respiratoire. On ne
dispose que de données limitées sur l'exposition humaine
au HEX et sur ses effets. On a observé des incidents
isolés au cours desquels il y a eu forte irritation des
yeux, du nez, de la gorge et des poumons. Cette irritation
était généralement brève, les victimes commençant à
récupérer dès cessation de l'exposition. Après une expo-
sition de courte durée on n'a noté aucune différence
statistiquement significative au niveau de certaines
enzymes hépatiques entre les groupes exposés et les
groupes témoins. On ignore quels peuvent être les effets
à longue échéance sur la santé humaine d'une exposition
continue à de faibles concentrations de HEX ou d'une
exposition intermittente à des fortes concentrations. Les
personnes qui sont amenées à manipuler cette substance ou
des déchets qui en contiennent, ainsi que les égoutiers,
travailleurs des usines de traitement d'eaux usées et
personnes qui résident à proximité de décharges, pour-
raient être exposés au risque du fait des possibilités de
contact avec cette substance ou avec les déchets qui
résultent de sa fabrication.
La base de données dont on dispose n'est pas suffi-
sante pour qu'on puisse évaluer la cancérogénicité du HEX.
Le Programme toxicologique national des Etats-Unis (NTP) a
procédé à des études d'inhalation sur des rats et des
souris pendant toute la durée de leur vie. Une fois qu'un
rapport sur la pathologie observée aura été publié, on
aura une meilleure idée des effets à long terme éventuels
de l'exposition au HEX. En ce qui concerne la cancéro-
génicité de cette substance, il faudra différer les tra-
vaux dans l'attente des résultats des épreuves effectuées
par le NTP. Le Centre international de recherche sur le
cancer a examiné les données existantes sur le HEX et l'a
classé dans le Groupe 3 (ce qui indique qu'en raison
d'insuffisances quantitatives ou qualitatives importantes,
il n'est pas possible de déterminer, au vu des résultats
disponibles, s'il y a présence ou absence d'effets
cancérogènes). Un certain nombre d'études épidémiologiques
sont citées dans la littérature; on n'a pas fait état
d'une incidence accrue de cancers, de localisation
quelconque, qui puisse être attribuée au HEX ou à ses
métabolites.
RESUMEN
El hexaclorociclopentadieno (HEX) es un líquido denso
de color amarillo pálido o verdoso, no inflamable, con un
olor acre característico. Su masa molecular relativa es de
272,77 y es poco soluble en agua. El HEX es muy reactivo
y experimenta reacciones de adición, sustitución y de
Diels-Alder.
En los EE.UU., la Velsicol Chemical Corporation es la
única empresa que actualmente produce HEX. En Europa lo
fabrica la Shell Chemical Corporation en los Países Bajos.
Los datos de producción son propiedad de las empresas,
pero se calcula que al año se producen en los EE.UU. entre
3600 y 6800 toneladas de HEX. En 1988, la producción
mundial fue de aproximadamente 15 000 toneladas (BUA,
1988). Aunque el HEX se utiliza como intermedio en la
producción de numerosos plaguicidas, algunos países han
restringido su empleo en la fabricación de ciertos plagui-
cidas organoclorados. También se utiliza en la producción
de pirorretardantes, resinas y tintes.
Durante la fabricación y elaboración del HEX, se
liberan pequeñas cantidades de la sustancia al medio
ambiente. También puede liberarse cuando aparece en forma
de impureza en algunos de los productos para los que sirve
de intermedio. El HEX puede liberarse tanto durante como
después de su evacuación. Sólo se dispone de datos limi-
tados de vigilancia de los niveles ambientales de HEX.
Esos datos indican que aparece principalmente en el
compartimento acuático y que se asocia a los sedimentos y
la materia orgánica del fondo salvo en los lugares en los
que se ha producido evacuación o liberación. En el labora-
torio, el HEX se adsorbe sin dificultad a la mayoría de
los tipos de partículas del suelo. Sin embargo, se han
comunicado casos de lixiviación y de movimiento en aguas
subterráneas.
En los EE.UU., se calcula que la liberación total de
HEX al medio ambiente en un año es de 5,9 toneladas (US
EPA, 1989). En la República Federal de Alemania y los
Países Bajos, se emitieron en 1987 alrededor de 400-500 kg
(BUA, 1988). Dadas las características físicas y químicas
del HEX, es de esperar que solo persista una pequeña
fracción de esas emisiones.
Basandose en los datos de laboratorio disponibles, se
ha formulado un modelo sobre el destino y el transporte
del HEX en la atmósfera y se ha calculado que tiene un
tiempo de residencia de aproximadamente 5 horas en la
troposfera. Se ha comunicado la existencia de transporte
atmosférico de HEX desde una zona en la que se almacenan
desechos y a partir de las cisternas durante el trata-
miento de desechos industriales.
En el agua, el HEX puede experimentar fotolisis,
hidrólisis y biodegradación. En aguas poco profundas,
tiene una semivida fotolítica de < 1 h. En aguas más
profundas, donde la fotolisis se ve impedida, se ha
observado que la semivida hidrolítica puede variar entre
varios días y aproximadamente tres meses, mientras que es
de prever que la biodegradación se produzca con más
lentitud. Se sabe que el HEX se volatiliza en las aguas
superficiales, y que la tasa de volatilización varia con
la turbulencia y con la adsorción a los sedimentos.
Debido a su baja solubilidad en el agua, el HEX debe
ser relativamente inmóvil en el suelo. Sin embargo, se ha
detectado la sustancia en aguas subterráneas. La volatili-
zación, que tiene más probabilidades de producirse en la
superficie del suelo, guarda relación inversa con los
niveles de materia orgánica en éste. Los resultados de
estudios en laboratorio indican que la hidrólisis química
y el metabolismo microbiano, tanto aeróbico como anaeró-
bico, deben reducir los niveles de HEX en los suelos.
En teoría, el potencial de biomagnificación del HEX
debe ser importante debido a su elevada lipofilia (log
coeficiente de partición octanol/agua). Este extremo, sin
embargo, no ha sido demostrado en pruebas experimentales.
Los estudios en animales de laboratorio han demostrado que
el 14C-HEX es metabolizado y excretado durante de las
primeras horas que siguen la administración de una dosis
por vía oral, y que la proporción que queda retenida en el
organismo es pequeña. En los peces, los factores de
bioconcentración en estado estable son < 30. Los factores
de bioacumulación derivados de modelos de ecosistemas a
corto plazo indican un moderado potencial de acumulación.
Así pues, parece que el HEX y sus metabolitos no persisten
ni se acumulan en gran medida en los sistemas biológicos.
Se ha demostrado que el HEX en bajas concentraciones
es tóxico para los organismos acuáticos. Se ha observado
letalidad en exposiciones agudas (48 a 96 h) en crustáceos
y peces de agua dulce y salada, en concentraciones nomi-
nales de 32-180 µg/litro en sistemas de exposición
estática en los que el agua no se renovó durante la
prueba. Puesto que la semivida fotolítica es < 1 h, la
concentración de HEX habría disminuido sustancialmente
durante el periodo de exposición utilizado en esos
estudios. En los únicos estudios en los que se usó agua
corriente y se midieron las concentraciones de HEX, se
obtuvieron valores de la CL50 en 96 horas de 7 µg por
litro en Pimephales promelas y un camarón de mar. Los
ensayos realizados con esas dos especies dieron un valor
de CL10 de 3,7 y un valor de CL40 de 0,7 µg por litro,
respectivamente.
En ensayos estáticos de siete días con algas marinas
se observó una reducción mediana del crecimiento (CE50)
a concentraciones nominales que variaron entre 3,5 y
100 µg/litro, según la especie.
En medios acuosos, el HEX resulta tóxico para
numerosos microorganismos en concentraciones nominales de
0,2-10 mg/litro, es decir, niveles sensiblemente mayores
que los necesarios para destruir a la mayoría de los
animales o vegetales acuáticos. El HEX parece menos tóxico
para los microorganismos en el suelo que en el medio
acuático, probablemente a causa de la adsorción del HEX a
la matriz del suelo.
Aunque cabe esperar que la exposición sea reducida,
actualmente no se dispone de bastante información para
determinar los efectos de la exposición al HEX en la
vegetación o la fauna terrestres.
La absorción de HEX sin modificar es mínima debido a
su reactividad con las membranas y los tejidos del organ-
ismo y especialmente con el contenido del tracto gastro-
intestinal. La mayor parte del 14C-HEX radiomarcado
queda retenido en el riñón, el hígado, la tráquea y los
pulmones de los animales tras la administración por vía
oral, cutánea o respiratoria. El HEX absorbido es metab-
olizado y excretado rápidamente, sobre todo en la orina,
menos en las heces y < 1% en el aire expirado. El tiempo
de eliminación terminal es de unas 30 horas, con inde-
pendencia de la vía de administración. Tras la inhalación
o la administración intravenosa, no se excreta HEX sin
modificar; los metabolitos fecales y urinarios se han
aislado pero no se han identificado. La falta de identifi-
cación de los metabolitos representa uno de los prin-
cipales obstaculos para evaluar la farmacocinética y los
mecanismos potenciales de acción del HEX.
En la rata, la CL50 aguda por inhalación (durante un
periodo de aproximadamente 4 h) es de 17,9 mg/m3 en el
macho y 39,1 mg/m3 en la hembra. Aunque hay algunas
diferencias interespecíficas entre cobayos, conejos, ratas
y ratones, los vapores de HEX son sumamente tóxicos para
todas las especies ensayadas. Su toxicidad parece máxima
cuando se administra por inhalación, en comparación con la
administración oral y cutánea, y es un irritante primario
fuerte. Los efectos sistémicos de la exposición aguda,
con independencia de la vía de administración, comprenden
cambios patológicos en el pulmón, el hígado, el riñón y
las glándulas suprarrenales.
La administración oral a corto plazo a ratas (38 mg/kg
al día) y ratones (75 mg/kg al día) durante 91 días
produjo nefrosis e inflamación e hiperplasia de la región
anterior del estómago. No se observaron signos manifiestos
cuando se expuso a ratas o ratones por inhalación a 2,26
mg/m3 (0,2 ppm), 6 h/día, 5 días/semana, durante 14
semanas. Con 1,69 mg/m3 (0,15 ppm) sólo se observó una
ligera irritación. La exposición de ratas a la inhalación
de 5,65 mg/m3 (0,5 ppm) durante 30 semanas provocó
cambios histopatológicos en el hígado, el tracto respira-
torio y el riñón. En un estudio de inhalación a corto
plazo de HEX en ratones y ratas durante 90 días se
observaron efectos en el sistema respiratorio a 4,52
mg/m3 (0,4 ppm) o más. No se ha demostrado que el HEX
sea mutagénico en ensayos in vitro , con o sin activación
metabólica. También resultó inactivo en ensayos de letali-
dad dominante en el ratón. Tampoco se ha demostrado que
sea teratogénico en ratas y ratones por exposición oral;
no se dispone de datos sobre la teratogenicidad del HEX
tras la exposición por inhalación.
Sólo se dispone de datos limitados sobre los efectos
de la exposición al HEX en la salud humana. Se han
producido incidentes aislados en los que el HEX provocó
fuerte irritación de los ojos, la nariz, la garganta y los
pulmones. Por lo general esa irritación fue breve, y la
recuperación se inició en cuanto cesó la exposición. No
se observaron diferencias estadísticamente significativas
en ciertas enzimas hepáticas entre grupos expuestos y
grupos testigo tras la exposición a corto plazo. Se
desconocen los efectos a largo plazo en la salud humana de
la exposición continua a bajos niveles y/o la exposición
aguda intermitente. Se ha demostrado que los manipuladores
del producto y de sus desechos, así como las personas que
trabajan en la depuración de aguas residuales o que viven
en las proximidades de los lugares de evacuación corren
riesgo debido al potencial de exposición a la sustancia
química o a los residuos de su fabricación.
La base de datos no es lo bastante amplia ni adecuada
para evaluar la carcinogenicidad del HEX. El Programa
Nacional de Toxicología de los EE. UU. ha llevado a cabo
un bioensayo de inhalación durante toda la vida en ratas y
ratones. Cuando se publique el informe patológico, se
comprenderá mejor los efectos a largo plazo de la exposi-
ción al HEX. La evaluación de la carcinogenicidad deberá
demorarse hasta que estén disponibles los resultados del
bioensayo del Programa. El Centro Internacional de Inves-
tigaciones sobre el Cáncer evaluó los datos existentes
para el HEX y clasificó la sustancia en el Grupo 3 (lo que
indica que, debido a limitaciones importantes de orden
cualitativo o cuantitativo, no puede interpretarse que los
estudios demuestren ni la existencia ni la ausencia de
efecto carcinogénico). En la bibliografía se citaron
varios estudios epidemiológicos; no se notificaron aumen-
tos de la incidencia de neoplasmas en ninguna localización
que pudieran atribuirse al HEX o sus metabolitos.
Kilzer et al. (1979) determined the rate of 14C-HEX
volatilization from water as a function of the rate of
water evaporation. Bottles containing aqueous HEX sol-
utions (50 µg/litre) were kept at 25 °C without shaking.
The escaping vapour condensed on a "cold finger" and was
quantified by liquid scintillation spectroscopy. Based on
recovery of added label, the HEX volatilization rates for
the first and second hours of testing were calculated to
be 5.87 and 0.75%/ml of water, respectively. Since the
water evaporation rate was 0.8-1.5 ml/h, the evaporation
rates for HEX were within the ranges of 4.7-8.8 and 0.6-
1.1%/h, respectively. These results suggest that a fairly
rapid initial volatilization occurred at the water sur-
face, and that by the second hour diffusion of HEX to the
water surface may have been limiting because of the static
conditions of the test. If the rate observed during the
second hour had continued for the remaining 24 h, the
total loss would have been approximately 18-34%, i.e.
somewhat less than that observed in the test conducted by
Weber (1979) where unstoppered bottles were shaken.
At 25-30 °C and in the environmental pH range of 5-9,
the hydrolytic half-life of HEX was found to be approxi-
mately 3-11 days (Yu & Atallah, 1977a; Wolfe et al.,
1982). In a later study in which evaporation and photo-
chemical reactions were carefully prevented, the hydro-
lytic half-life was approximately 3 months (Chou &
Griffin, 1983). Hydrolysis is much slower than photolysis
(see Table 2) but may be a significant load-reducing
process in waters where photolysis and physical transport
processes are not important (i.e. in deep, non-flowing
waters). Wolfe et al. (1982) found HEX hydrolysis to be
independent of pH over the range of 3-10. The rate con-
stant was dependent on temperature at pH 7.0, and the
half-life was estimated to be 3.31, 1.71, and 0.64 days at
30, 40, and 50 °C, respectively. The addition of various
buffers or sodium chloride (0.5 mol/litre) did not affect
the hydrolysis rate constant, suggesting that the rate
constant obtained would be applicable to marine environ-
ments as well. The addition of natural sediments, suf-
ficient to sorb up to 92% of the compound, caused the rate
constant to vary by less than a factor of 2. It was
therefore concluded that sorption to sediments would not
significantly affect the rate of hydrolysis (Wolfe et al.,
1982).
4.2.3. Soil
When it is released on to soil, HEX is likely to sorb
strongly to any organic matter or humus present (Weber,
1979; Kenaga & Goring, 1980). The HEX concentrations
should decrease with time as populations of soil micro-
organisms that are better adapted to metabolize HEX
increase (Rieck, 1977b,c; Thuma et al., 1978). Volatiliz-
ation, photolysis, and various chemical processes may also
dissipate the compound in certain soil environments.
The main methods of transport for HEX applied to the
soil are (a) the movement of particles to which it is
sorbed and (b) volatilization. Other possibilities are
that HEX is sorbed on to soil colloids or that it par-
titions to the interior of soil particles and stays in
loams and silts in a dissolved state. No data are avail-
able pertaining to HEX transport on soil particles. How-
ever, in a few studies, the rate of volatilization from
soils has been reported and is discussed in the following
paragraphs.
Kilzer et al. (1979) found that 14C-HEX and its
degradation products volatilized from moist soils (sand,
loam, and humus) at a faster rate during the first hour of
the study than during the second hour. HEX (50 µg/kg) was
placed in bottles with each soil type, the bottles were
shaken vigorously, and they were then incubated for 2 h at
25 °C without shaking. The radiolabelled HEX condensed on
a "cold finger" and was quantified by liquid scintil-
lation counting. For sand, loam, and humus, the volatiliz-
ation rate was expressed as the percentages of applied
radioactivity per ml of evaporated water. For the first
hour the percentages were 0.83, 0.33, and 0.14%, respect-
ively, and for the second hour they were 0.23, 0.11, and
0.05%. For HEX and nine other tested chemicals, the
authors found that the volatilization rate from distilled
water could not be used to predict the rate from wetted
soils. Among the chemicals tested, there was no corre-
lation between water solubility or vapour pressure and
volatilization from soils. The volatilization rate for
HEX and its metabolites in soil was primarily dependent on
soil organic matter content, mainly because of the highly
sorptive characteristics of HEX.
In a model ecosystem study, Kloskowski et al. (1981)
applied 14C-HEX to 1 kg of humus sand soil (2 mg/kg) and
grew summer barley by keeping the system under an illumi-
nation of 10 000 lux (12 h light, 12 h dark) at 20-24 °C
in an enclosed 10-litre desiccator with an aeration of
10 ml/min. After 7 days, approximately 19.5% of the orig-
inal radioactivity was recovered in the form of 14CO2
evolved and 0.5% as volatilized organics. The level of
radiolabelled compounds in the plants was 13.4 mg/kg,
which represented a bioaccumulation factor of 7.1 (i.e.
plant residues divided by soil residues). It is not clear
whether the plants played a major role in the volatiliz-
ation or metabolic fate of HEX, but the total 14C recov-
ery was over 95%.
Rieck (1977c) measured the rate of volatilization of
HEX from Maury silt loam soils. After the application of
100 mg 14C-HEX to soil, the cumulative evaporation of HEX
and its non-polar metabolites (penta- and tetrachloro-
cyclopentadiene) on days 1, 2, 3, 5, 7, and 14 was 9.3,
10.2, 10.6, 10.8, 11.0, and 11.2%, respectively. The
results indicated that HEX evaporation to air occurred
mainly during the first day after application and was
probably associated with the surface soil only.
The soil sorption properties of compounds such as HEX
can be predicted from their soil organic carbon/water
partition coefficients (Koc). Kenaga (1980) examined
the sorption properties of 100 chemicals and concluded
that compounds with Koc values > 1000 are tightly bound
to soil components and are immobile in soils. Those with
values < 100 are sorbed less strongly and are considered
to be moderately to highly mobile. Thus, the theoretical
Koc value is useful as an indicator of potential soil
leachability or binding of the chemical. The Koc values
also indicate whether a chemical is likely to enter water
by leaching or by being sorbed to eroded soil particles.
Since Koc values for HEX are not available in the
literature, these values were calculated using the
following mathematical equation, developed by Kenaga &
Goring (1980) and Kenaga (1980):
log Koc = 3.64-0.55 (log WS)
where WS is water solubility (mg/litre), and the 95%
confidence limits are ± 1.23 orders of magnitude. The
calculated values of Koc for HEX using the reported water
solubility values of 2.1 mg/litre (Dal Monte & Yu, 1977),
1.8 mg/litre (Wolfe et al., 1982), and 0.805 mg/litre (Lu
et al., 1975) are 2903, 3159, and 4918, respectively.
Since these calculated Koc values are all > 1000, the
authors concluded that HEX is tightly bound to soil
components and immobile in the soil compartment. Simi-
larly, Briggs (1973) concluded that compounds with a log
octanol/water partition coefficient (log Pow) > 3.78 are
immobile in soil. Log Pow values for HEX of 5.04 (Wolfe
et al., 1982) and 5.51 (Veith et al., 1979) have been
measured.
In studies by Chou & Griffin (1983), the mobility of
HEX (C-56) in six soils was measured with several leaching
solvents using soil thin-layer chromatography (TLC) and
column leaching studies. It remained immobile in the soil
when leached with water, landfill leachate, or caustic
brine, but was highly mobile when leached with organic
solvents. A further conclusion was that several degra-
dation products of HEX migrated through soils faster than
HEX itself, and that the degradation products warranted
further study. The sorption capacity of HEX was highly
correlated with the total organic carbon (TOC) content of
soil materials (r2 = 0.97), which was the dominant soil
characterization parameter. Sorption appears to be pre-
dictable from the TOC content of soils (Chou & Griffin,
1983).
4.3. Biotransformation
4.3.1. Biodegradation
The metabolism of HEX by soil microorganisms is
apparently an important process in its environmental
degradation. Soil degradation is rapid under non-sterile
aerobic and anaerobic conditions, and indirect evidence
for microbial involvement has been reported by Rieck
(1977b,c). In one of his studies, Rieck (1977b) used
several types of treatments and soils of different pH to
determine whether the biodegradation of HEX in Maury silt
loam soil was biologically or chemically mediated, or
both. Soils were incubated in glass flasks covered with
perforated aluminum foil and kept on a laboratory shelf,
presumably exposed to ambient lighting through the flask
walls. When 14C-HEX was applied to non-sterile soil at
1 mg/kg, only 6.1% was recovered as non-polar material
(either HEX or non-polar degradation products) 7 days
after treatment, and approximately 71.7% was polar and
unextractable material. Adjustment of the pH to 4 or 8 had
little effect on these results. By comparison, in auto-
claved soil (the control), 36.1% of the applied dose was
recovered as non-polar material and only 33.4% was
recovered as polar and unextractable material. The degra-
dation of HEX under anaerobic (flooded) conditions
occurred at a slightly faster rate than under aerobic
conditions. However, no sterile, flooded control was used
to determine the effects of hydrolysis, which could have
accounted for the observed effect in this treatment. The
mean total recovery in all treatments decreased from 67%
at 7 days to 55% at 56 days. This decrease was attributed
to volatilization of HEX and/or its degradation products.
Volatilization from soil was examined in a further
experiment (Rieck, 1977c). In a 14-day study, radiocarbon
volatilized from non-sterile, 14C-HEX-treated soil was
trapped and assayed. A total of 20.2% of the applied 14C
was trapped: 11.2% in hexane and 9.0% in ethanolamine-
water. Most of the hexane fraction (9.3% of the applied
14C) was trapped during the first day, and probably
represented volatilized HEX. However, the ethanolamine-
water fraction, considered to represent evolved carbon
dioxide, was released gradually over the 14-day period.
In the soil analysis, non-polar (extractable) and polar
(extractable and unextractable) material accounted for 3.4
and 40.0% of the dose, respectively, during the 14 days;
total recovery was only 63.6% including volatilization.
No metabolic products were identified in the two studies
by Rieck (1977b,c).
Thuma et al. (1978) studied the feasibility of using
selected pure cultures (organisms not identified) to
biodegrade spills of hazardous chemicals, including HEX,
on soil. They tested 23 organisms and found that from
2-76% of the applied HEX had been removed from the aqueous
culture medium within 14 days. Seven of the 23 organisms
degraded more than 33% of the HEX within 14 days. Losses
of HEX by other means than biodegradation were accounted
for by using controls.
Atallah et al. (1980) conducted an aqueous aerobic
biodegradability study to determine whether HEX could be
degraded to CO2 and at what rate. The inoculum was a
mixed acclimated culture containing secondary municipal
waste effluent and several strains of Pseudomonas putida.
14C-labelled HEX was the sole source of carbon in the
study, with the exception of trace levels of vitamins.
Total removal of 14C, primarily as volatile organic com-
pounds, was > 80% during the first day in both uninocu-
lated (45 mg HEX/litre) and inoculated (4.5 and 45 mg
HEX/litre) media, although removal was slightly greater in
inoculated media. 14CO2 was released from both media,
indicating that CO2 was a product of hydrolysis as well
as of biodegradation. The rate of conversion to CO2 was
initially higher in the uninoculated media, but after 1
week, became higher in the inoculated media. This study
showed clearly that HEX can be biodegraded in aquatic
media under laboratory conditions. However, Wolfe et al.
(1982) failed to detect any difference between the HEX
degradation rates in hydrolysis experiments where non-
sterile natural sediments were added to water (10 g/litre)
and those where sterile sediment was used. They calculated
a relatively low value (1 x 10-5 ml org-1 h-1; see
Table 2) as a maximum biodegradation rate, and conse-
quently biodegradation was estimated to be a relatively
unimportant fate process in the EXAMS model (see Table 3).
These studies indicate that the persistence of HEX in
soil is brief, degradation of more than 90% of applied HEX
to non-polar products occurring within approximately 7
days. Factors contributing to this loss include abiotic
and biotic degradation processes and volatilization,
although the relative importance of each is difficult to
quantify.
4.3.2. Bioconcentration, bioaccumulation, and biomagnification
Bioaccumulation, sometimes also expressed as biologi-
cal persistence, is a consequence of the rate of elimin-
ation of a compound and the extent of adsorption.
The terminology used in this section conforms to that
used by Macek et al. (1979):
* bioconcentration implies that tissue residues result
only from simultaneous uptake and elimination from
exposure to the ambient environment (e.g., air for
terrestrial species or water for aquatic species);
* bioaccumulation considers all exposures (air, water,
and food) of an individual organism to be the source
of tissue residues;
* biomagnification defines the increase in tissue
residues observed at successively higher trophic
levels of a food web.
Table 3. Summary of results of computer simulation of the
fate and transport of hexachlorocyclopentadiene in four
typical aquatic environmentsa
---------------------------------------------------------------
River Pond Eutrophic Oligotrophic
lake lake
---------------------------------------------------------------
Distribution (%)
Water column 1.22 14 12.97 2.91b
Sediment 98.78 86 87.03 97.09
Recovery timec (days) 52 81 58 87
Load reduction (%) by processes:
Hydrolysis 8.04 17.85 8.29 16.50
Oxidation 0.00 0.00 0.00 0.00
Photolysis 18.68 80.39 89.18 82.41
Biodegradation 0.57 0.23 0.30 0.01
(biolysis)
Volatilization 0.69 1.33 1.56 1.08
Exportd 72.02 0.20 0.01 0.00
---------------------------------------------------------------
a Adapted from Wolfe et al. (1982), with correction applied.
b Value was incorrectly reported as 32.91 in original paper.
c The time needed to reduce steady-state concentrations by
97% (five half-lives).
d Physical loss from the system by any pathway other than
volatilization.
The log octanol/water partition coefficient of HEX has
been experimentally determined to be 5.04 (Wolfe et al.,
1982) and 5.51 (Veith et al., 1979), which would indicate
a substantial potential for bioconcentration, bioaccumu-
lation, and biomagnification. Actual determinations of
bioconcentration and bioaccumulation in several aquatic
organisms, however, indicate that HEX does not accumulate
to any great extent (Lu et al., 1975; Podowski & Khan,
1979, 1984; Spehar et al., 1979; Veith et al., 1979),
mainly because it is metabolized rapidly.
Podowski & Khan (1979, 1984) conducted several exper-
iments on the uptake, bioaccumulation, and elimination
of 14C-HEX in goldfish ( Carassius auratus) and concluded
that this species rapidly eliminates absorbed HEX. In one
experiment, fish were transferred daily into fresh sol-
utions of 14C-HEX for 16 days. This transfer of three
fish/jar resulted in an accumulative exposure of 240 µg
of HEX. Nominal HEX concentrations of 10 µg/litre re-
sulted in measured water concentrations (based on radio-
activity) in the range of 3.4-4.8 µg/litre, because of
rapid volatilization of the compound. Radioactivity
accumulated rapidly in fish tissue, reaching a maximum on
day 8 corresponding to approximately 6 mg HEX/kg. Since an
undetermined amount of the radioactivity was present as
metabolites, no bioconcentration factor could be calcu-
lated. From day 8 to day 16, tissue levels declined in
spite of the daily renewal of exposure solutions, indi-
cating that excretion of HEX and/or its metabolites was
occurring more rapidly than uptake. In a static exposure
to an initial measured HEX concentration of 5 µg per
litre, uptake of the radiolabel by the fish was to a level
corresponding to 1.6 mg HEX/kg on day 2, accompanied by a
slight decrease of HEX in the water. By day 4, approxi-
mately 50% of the radiolabel had been excreted, and the
radioactivity in the water increased. Over the following
12 days, the radioactivity in both water and fish declined
slowly.
Podowski & Khan (1979, 1984) also studied the elimin-
ation, metabolism, and tissue distribution of HEX injected
intraperitoneally into goldfish and concluded that gold-
fish eliminate injected HEX both rapidly and linearly
(the biological half-life was approximately 9 days). The
fish (27-45 g) were injected with 39.6 µg 14C-HEX and
analysed 3 days later. Of the 97% of the radiolabelled
dose accounted for, approximately 18.9% was eliminated by
the fish. Of the residue found in the fish, 47.2% was
extractable in organic solvent (little of the radio-
labelled material could be identified as HEX, which indi-
cated that extensive biotransformation had occurred),
10.6% consisted of water-soluble metabolites, and 20.3%
was unextractable. None of the metabolites were ident-
ified. The elimination was biphasic, consisting of a rapid
initial phase followed by a slower terminal phase.
In another part of these studies, the residual
activity in several fish tissues was assayed 2, 4, 6, and
8 days after an injection of 38.4 µg 14C-HEX per fish.
The activity corresponded to 0.2 and 0.3 µg HEX/kg in the
spinal cord and gills, respectively, concentrations that
remained constant throughout the 8-day period of the
study. Residues in the kidneys and bile increased within
the same period from 1-3 and 0-32 µg/kg, respectively,
indicating elimination by these routes. The authors stated
that the increase probably occurred from enhanced con-
version of the parent compound into polar products, which
could be excreted more easily. In the other tissues, all
residual levels decreased, leaving only the liver with a
level of more than 1 µg/kg. The metabolites were not
identified (Podowski & Khan, 1979, 1984).
Veith et al. (1979) determined a bioconcentration
factor (BCF) for HEX of 29 in the fat-head minnow
(Pimephales promelas). In a 32-day flow-through study,
30 fish were exposed to HEX at a mean concentration of
20.9 µg/litre. Five fish at a time were killed at 2, 4,
8, 16, 24, and 32 days for residue analysis. The study
was conducted using Lake Superior water at 25 °C (pH 7.5,
dissolved oxygen > 5.0 mg/litre, and hardness 41.5 mg
CaCO3/litre. On the basis of its estimated octanol/water
partition coefficient alone (log Pow = 5.51), a BCF of
approximately 9600 would have been predicted. However,
HEX did not bioconcentrate substantially, and therefore
deviated from the log P:log BCF relationship shown for
most of the other 29 chemicals tested by these researchers.
Lu et al. (1975) studied the fate of HEX in a model
terrestrial-aquatic ecosystem maintained at 26.7 °C with a
12-h photoperiod. The model ecosystem consisted of 50
sorghum (Sorghum vulgare) plants (7.62-10.16 cm tall) in
the terrestrial portion, while 10 snails (Physa sp.), 30
water fleas (Daphnia magna), filamentous green algae
(Oedogonium cardiacum), and a plankton culture were
added to the aquatic portion. The sorghum plants were
treated topically with 5.0 mg 14C-HEX in acetone to simu-
late a terrestrial application of 1.1 kg/hectare. Ten
early-fifth-instar caterpillar larvae (Estigmene acrea)
were placed on the plants. The insects consumed most of
the treated plant surface within 3-4 days. The faeces,
leaf grass, and the larvae themselves contaminated the
moist sand, permitting distribution of the radiolabelled
metabolites by water throughout the ecosystem. After 26
days, 300 mosquito larvae (Culex pipiens quinquefasciatus)
were added to the ecosystem, and on day 30 three mosquito
fish (Gambusia affinis) were added. The experiment was
terminated after 33 days, and the various parameters were
analysed. The radioactivity was then extracted with
diethyl ether from the water and with acetone from the
organisms. The results of thin-layer chromatographic
analysis of the extracts are presented in Table 4. Data
were not reported for Daphnia magna or the salt marsh
caterpillar. Uptake in this experiment occurred through
food as well as water, and therefore is termed bioaccumu-
lation rather than bioconcentration. Lu et al. (1975) used
the term ecological magnification to designate the bio-
accumulation factor (BAF). The BAF for HEX in fish was
448 (0.1076 mg/kg fish divided by 0.24 µg/litre water)
for the 3-day exposure period, indicating a moderate
potential for concentration (Kenaga, 1980). The BAFs in
algae (< 33-day exposure), snails (< 33-day exposure), and
mosquito larvae (7-day exposure) were reported to be 341,
1634, and 929, respectively (Lu et al., 1975).
Table 4. Relative distribution of hexachlorocyclopentadiene (HEX) and
its degradation products in a model ecosystema
------------------------------------------------------------------------
14C-HEX equivalents
Water Algae Snail Mosquito Fish
(mg/litre) (mg/kg) (mg/kg) larva (mg/kg)
(mg/kg)
------------------------------------------------------------------------
HEX 0.00024 0.0818 0.3922 0.2230 0.1076
Other extractable 0.00204 0.1632 0.3824 0.2542 0.1542
compounds
Total extractable14Cb 0.00228 0.2450 0.7746 0.4772 0.2618
Unextractable14C 0.00750 0.0094 0.0814 0.0104 0.0982
Total14Cc 0.00978 0.2544 0.8560 0.4876 0.3600
------------------------------------------------------------------------
a Source: Lu et al. (1975).
b Sum of HEX and other extractable compounds.
c Sum of total extractable and unextractable 14C.
Biomagnification, measured as the ratio of HEX
residues between trophic levels (e.g., snail/algae or
fish/mosquito), was far less substantial than bioconcen-
tration. Based on the HEX tissue residues, the snail/algae
ratio was 0.3922/0.0818 = 4.8 and the fish/mosquito ratio
was 0.1076/0.2230 = 0.48.
Lu et al. (1975) also studied the metabolism of HEX by
the organisms present in the model terrestrial-aquatic
ecosystem, but none of the products was identified except
for HEX. The authors reported that unmetabolized HEX re-
presented a large percentage of the total extractable 14C,
being 33% in algae, 50% in snail, 46% in mosquito, and 41%
in fish. The percentage of biodegradation was calculated
for each organism (unextractable 14C x 100/total 14C) and
found to be 4% for the algae (in < 33 days), 10% for the
snails (in < 33 days), 2% for the mosquitoes (in 7 days),
and 27% for the fish (in 3 days). However, these values
may underestimate the extent of metabolism, since acetone-
extractable polar compounds were not considered in the
calculations.
The Velsicol Chemical Corporation (1978) conducted
fish tissue residue studies in waters located below their
facility in Memphis, Tennessee, USA, and reported that HEX
was not detected in either catfish or carp, although
chlorinated compounds, including octachlorocyclopentadiene
(a common co-contaminant), were detected in the fish
tissue. This indicated that HEX was not accumulated. The
possible source of these other compounds was not
discussed. In a joint USA federal and state study of the
Mississippi River at locations above, around, and below
Memphis, Bennett (1982) reported that HEX was not detected
in any of the eight fish sample groups analysed by GC/MS.
4.4. Interactions with other physical and chemical factors
4.4.1. Phototransformation
Zepp et al. (1979) and Wolfe et al. (1982) reported
the results of US EPA studies on the rate of HEX photo-
transformation in water. Under a variety of sunlight
conditions, in both distilled and natural waters of 1-4 cm
depth, the phototransformation half-life was < 10 min.
Chou & Griffin (1983) determined a half-life of < 4 min at
740 j/m2. The addition of natural sediments to distilled
water containing HEX had little effect on the phototrans-
formation rate. These findings indicate that the dominant
mechanism of HEX phototransformation is direct absorption
of light by the chemical, rather than photosensitization
reactions involving other dissolved or suspended
materials.
The direct photoreaction of HEX in water was also
studied under controlled conditions in the laboratory
using a monochromatic light (313 nm) with a mercury lamp
source and appropriate filters. Phototransformation rate
constants, computed for the study location (Athens,
Georgia, USA, 34 °N latitude), agreed with those observed
in the sunlight experiments described above. Rate con-
stants were also computed for various times of day at a
latitude of 40 °N. The near-surface phototransformation
rate constant of HEX at this latitude on cloudless days
(averaged over both light and dark periods for 1 year) was
3.9 h-1, which corresponds to a very rapid half-life of
10.7 min (Zepp et al., 1979; Wolfe et al., 1982).
These laboratory researchers suggested that the pri-
mary phototransformation product was the hydrated form of
tetrachlorocyclopentadienone (C5Cl4O, TCPD), although
it was not isolated. Several chlorinated photoproducts
with a higher relative molecular mass than HEX were
detected by GC/MS analysis of the reaction mixture. Photo-
lysis of HEX in methanol gave a product identified as the
dimethyl ketal of TCPD (Wolfe et al., 1982). According to
Zepp et al. (1979), it is likely that TCPD exists predomi-
nantly in its hydrated form in the aquatic environment.
The compound was not isolated, supposedly because it
rapidly dimerizes or reacts to form products of higher
relative molecular mass. Chou et al. (1987) identified
2,3,4,4,5-pentachloro-2-cyclopentenone, hexachloro-2-cyclo-
pentenone, and hexachloro-3-cyclopentenone as the primary
photodegradation products, as well as several other pri-
mary and secondary ones (Chou & Griffin, 1983; Fig. 2).
Yu & Atallah (1977b) found that, at a concentration of
2.2 mg/litre in water, uniformly labelled 14C-HEX was
rapidly converted to water-soluble products upon
irradiation with light from a mercury vapour lamp (light
energy: 40-48% ultraviolet, 40-43% visible, remainder
infrared). In exposures lasting 0.5-5.0 h, 46-53% of the
radiolabel was recovered in the form of water-soluble
products (compared with 7% at initiation), whereas the
amount recovered by organic (petroleum ether) extraction
decreased with increasing exposure duration from 25% to 6%
(compared with 66% at initiation). HEX was not detected
among the photoproducts in the organic extraction. Chou
et al. (1987) also found that dimerization of degradation
products to form compounds of higher relative molecular
mass was only a minor route of degradation.
4.4.2. Oxidation
HEX would not be expected to be oxidized under ordi-
nary environmental conditions. In the laboratory, HEX
reacts with molecular oxygen at 95-105 °C to form a mix-
ture of hexachlorocyclopentenones (Molotsky & Ballweber,
1957). However, based on an estimated second-order oxi-
dation rate constant of 1 x 10-10 M-1 sec-1 at 25 °C
in water (Table 2), the EXAMS computer simulation of Wolfe
et al. (1982) predicted that HEX would not be oxidized in
the simulated river, pond, eutrophic lake or oligotrophic
lake (Table 3).
4.5. Disposal and fate
HEX and HEX-contaminated material and wastes are
disposed of in secure chemical landfills, by incineration,
and by deep well injection (US EPA, 1989). Additionally,
there are solid waste regulations in the USA because,
under the Resource Conservation and Recovery Act, HEX is
designated to be a toxic waste. German regulations are
similar to those of the USA, except that there is no deep
well injection (BUA, 1988).
Since the photodegradation products of HEX have been
identified only recently and because HEX has also been
found in areas where waste has not been disposed of for
years (US EPA, 1980c), it is difficult to determine its
fate in the environment.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
Releases of HEX into the atmosphere can result from
the production, processing, and use of HEX, the disposal
of wastes containing HEX, or from products contaminated
with HEX (Hunt & Brooks, 1984). Data sent to the US EPA
for 1987 regarding emission levels from companies in the
USA indicated that 1400 kg of HEX was emitted into the air
(US EPA, 1989). In the Federal Republic of Germany and
the Netherlands, about 400-500 kg was emitted to the air
in 1987 (BUA, 1988)
In September and October 1985, the Velsicol Chemical
Corporation determined concentrations of HEX at predeter-
mined locations around its production facilities in
Tennessee, USA. The study was designed to measure ambient
concentrations in the air during routine manufacturing
operations. Of the 25 samples collected, 15 were below the
analytical limit of detection, i.e. 0.03 µg (0.1 ppb).
The air HEX levels in the other samples ranged between 1
and 10 µg/m3 (0.1-0.9 ppb) when ambient temperatures
were between 4.4 °C and 27.7 °C (Velsicol Chemical Corpor-
ation, 1986).
The highest reported concentration of HEX measured in
homes in Tennessee was 0.10 µg/m3, while air levels at
the Memphis North treatment plant were as high as
39 µg/m3 (C.S. Clark et al., 1982; Elia et al., 1983).
In an air monitoring study on an abandoned waste site in
Michigan, the average HEX emission rate was 0.26 (± 0.05)
g/h. In May 1977, HEX was detected at a level of 633 µg
per m3 (56 ppb) in air samples collected from a waste
site in Montague, Michigan (US EPA, 1980c).
5.1.2. Water
Benoit & Williams (1981) sampled both untreated water
and drinking-water from a water treatment plant in Ottawa,
Canada. Using solvent extraction analysis with a detection
sensitivity of 50 ng/litre (or the XAD-2 resin extraction
method with a detection sensitivity of approximately 0.5
ng/litre), the authors did not detect any HEX in the
untreated water, but reported levels ranging from 57-110
ng/litre in the finished drinking-water. These results
suggest that HEX was introduced into the drinking-water
during the treatment process. However, the researchers did
not find the source of the HEX. Meier et al. (1985) found
that HEX can be produced through the chlorination of humic
acid.
Limited monitoring data from production sites revealed
that HEX was present in a spot sample at a level of 18
mg/litre (February 1977) and a range of 0.156-8.24 mg per
litre (over the month of January 1977) in the aqueous dis-
charge from the Memphis pesticide plant (US EPA, 1980c).
The calculated concentration of HEX in the Mississippi
River was 6 µg/litre (Carter, 1977). In the summer of
1977, shortly after these readings, a new waste-water
treatment plant began operation (Table 5). Prior to con-
struction of the plant, waste water flowed directly into
the Mississippi River or through one of its tributaries
(Elia et al., 1983). Voluntary improvements in controlling
the discharge from the Memphis plant resulted in reported
levels of 0.07 µg HEX/litre in the Mississippi River,
near the mouth of Wolf Creek (Velsicol Chemical Corpor-
ation, 1978). HEX has also been identified in the soil and
river sediments downstream from a USA manufacturing plant,
even after pesticide production was discontinued (US EPA,
1980c).
5.1.3. Soil
Ambient monitoring data for the terrestrial environ-
ment are not available, but it seems that these concen-
trations should be much lower than those in the aquatic
environment. Deposition of HEX from the atmospheric (and
aquatic) compartments into the terrestrial environment is
expected to be minimal. Similarly, direct release of HEX
into the terrestrial environment (i.e. as an impurity in
chlorinated pesticides) should be decreasing because of
regulatory controls on some products, with the possible
exceptions of disposal at waste sites, accidental spills,
and other illegal disposal methods.
Table 5. Concentrations of selected organic compounds in
influent waste water at the Memphis North treatment plant, 1978a
---------------------------------------------------------------
Concentration (µg/litre)b
Date No. of HEX HEX-BCHc HCBCHd Chlordane
samples
---------------------------------------------------------------
June 1 3 334 57 87
August 5 0.8 329 115 216
September 2 4 292 668 58
October-November 2 0.8 11 17 32
---------------------------------------------------------------
a From: Elia et al. (1983).
b Mean values for the number of samples indicated.
c Hexachlorobicycloheptadiene.
d Heptachlorobicycloheptene.
5.1.4. Food
HEX was qualitatively detected in fish samples taken
from water near a pesticide manufacturing plant in
Michigan (Spehar et al., 1977), but none was detected in
fish samples taken from the waters near the pesticide
manufacturing plant in Memphis (Velsicol Chemical Corpor-
ation, 1978; Bennett, 1982). No information regarding HEX
contamination of other foods is available.
5.2. General population exposure
There are insufficient data to determine the relative
contributions of the various sources of HEX to the
environment. There will be exposure to HEX present in
some commonly used pesticides, and possibly some flame
retardants, where HEX is a contaminant. The US EPA has
reported that exposure of humans to HEX from the air or
water should be extremely low, except in the case of
workers and residents near manufacturing, shipping, and
waste sites, and concluded that general population ex-
posure was not considered to be significant or substantial
(US EPA, 1982).
The only other estimates of relative source contri-
butions are from reports completed for the US EPA (Hunt &
Brooks, 1984) and the BUA (1988). The air releases from
manufacturing processes can come from the vents on reac-
tors, process and storage tanks, and as fugitive
emissions. Hunt & Brooks (1984) estimated that the total
quantity of HEX released from these sources was 8 tonnes
per year. In the Federal Republic of Germany and the
Netherlands, HEX emissions were estimated to be 400-500
kg/year. HEX can also be emitted into the air from the
incineration and landfilling of HEX-containing waste, the
most accurate estimate being 1 tonne per year. The total
annual estimated release of HEX to the environment in the
USA is 11.9 tonnes. These figures are only estimates
because of the limited available data. They are given
simply to indicate the relative magnitude of HEX emissions
into the environment.
Exposure limit values for various countries are given
in Appendix 1.
5.3. Occupational exposure
Occupational exposure can occur both at HEX production
and processing facilities and at other locations where
HEX-containing waste is present. For example, the highest
reported workplace air concentrations of HEX were measured
at the Louisville, Kentucky, USA, waste-water treatment
plant, which received a slug of HEX discharged by a waste
hauler. Four days after the plant was closed, air concen-
trations in the primary treatment area ranged from 3.05
to 11.0 mg/m3 (270 to 970 ppb) (Morse et al., 1979).
During the clean-up operations air concentrations as high
as 133 mg/m3 (11 800 ppb) were reported (Kominsky &
Wisseman, 1978).
In 1982, the Velsicol Chemical Corporation used the
Southern Research Institute (SRI) sampling method at the
Memphis (Tennessee) and Marshall (Illinois) facilities to
evaluate possible exposure of workers to HEX vapour and
the effectiveness of engineering controls. Tables 6 and 7
show the HEX concentrations measured at various points.
At the Memphis facility almost one-half of the worker
8-h time-weighted average (TWA) air HEX concentrations
were at or above the USA Threshold Limit Value of 0.11
mg/m3 (0.01 ppm) (OSHA, 1989). At the Marshall plant all
six TWA values were above 0.11 mg/m3. It should be noted
that in Tables 6 and 7 the results of employee monitoring
are reported without regard to respirator use. Respirators
are required to be worn in operations in these plants
where HEX exposure is possible.
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Appendix 1
(Table 21).
Table 6. Summary of hexachlorocyclopentadiene monitoring, Memphis, Tennessee, USAa
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
HEX Process operator 2 445 0.009-0.011 0.009
HEX No. 1 operator 5 432 0.006-0.033 0.015
HEX No. 2 process operator 5 418 0.006-0.029 0.014
HEX No. 2 cyclo operator 5 417 0.001-0.048 0.017
HEX No. 2 chlorine operator 6 415 0.004-0.0161 0.035
a) HEX Bottoms drumming 1 50 0.016
HEX Area sample control room 12 476 0.002-0.018 0.009
HEX Brinks filter cleaning 2 387 0.004-0.006 0.005
Formulations HEX drummers 4 407 0.002-2.0337 0.010
Material HEX railroad tank car 1 279 0.013 0.008
handling unloading
Endrin R2 filter operator 1 281 0.003
Endrin R1 operator 1 334 0.002
Chlorendic No. 1 operator 2 437 0.0077-0.0102 0.008
anhydride
Chlorendic No. 2 operator D34 2 440 0.0107-0.0198 0.014
anhydride
Chlorendic No. 2 operator R6 2 437 0.0065-0.0169 0.011
anhydride
Chlorendic Packaging operator 1 396 0.035 0.031
anhydride
----------------------------------------------------------------------------------------
Table 6 (contd.)
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
Chlorendic Area sample - control 3 475 0.0003-0.0014 0.001
anhydride room
Heptachlor No. 1 operator 2 407 0.007-0.009 0.007
Heptachlor No. 2 operator 2 415 0.006-0.009 0.007
Heptachlor 237 operator 2 392 0.006-0.019 0.011
Heptachlor Utility operator 1 363 0.006 0.005
Heptachlor Cleaning sparkler filter 3 44 0.002-0.005 0.0003
a) ceiling sample 1 15 0.006
----------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming
that the only chemical exposure occurred during the sampling period.
Table 7. Summary of hexachlorocyclopentadiene monitoring, Marshall, Illinois, USAa
--------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrations TWAb
(min) (ppm) (ppm)
--------------------------------------------------------------------------------------
Chlordane No. 1 operator 8 451 0.0091-0.0316 0.017
Chlordane No. 2 operator 8 455 0.008 -0.0195 0.013
Chlordane No. 3 operator 8 451 0.0002-0.0325 0.014
Chlordane Area sample - 13 433 0.0002-0.0254 0.016
North control room
Chlordane Area sample - 10 435 0.001-0.0276 0.015
South control room
Chlordane HEX filter changing 1 15 0.1322
Chlordane Waste handling HEX 6 307 0.0006-0.0606 0.020
a) HEX mud drumming - 2 15 0.0005-0.0061
ceiling sample
b) Loading HEX waste 2 15 0.1199-0.2325
truck - ceiling sample
c) Sump pit dumping - 2 15 0.0333-0.1129
ceiling sample
--------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming that the
only chemical exposure was during the sampling period.
6. KINETICS AND METABOLISM
6.1. Absorption, retention, distribution, metabolism,
elimination, and excretion
6.1.1. Oral
In a study by Mehendale (1977), male Sprague-Dawley
rats (225-250 g body weight) were administered 5 µmol
of 14C-HEX (approximately 5.5 mg/kg) by oral intubation
as 0.2 ml of a solution in corn oil. The total 14C ac-
tivity contained in the dose was approximately 1 µCi. The
animals were maintained in metabolism cages and the urine
and faeces were collected. About 35% of the administered
dose was collected in the urine and only 10% was collected
in the faeces. More than 87% of the 14C activity in the
urine and more than 60% of the activity in the faeces
appeared during the first day. Only a small amount
(approximately 0.5%) of the original dose was recovered
in the kidneys and liver. The author speculated that, in
view of the low total recovery of the administered dose, a
major part of the dose (> 50%) had been excreted through
the lung. This speculation was later proven to be unwar-
ranted because subsequent studies (Dorough, 1979), in
which exhaled air and lung and tracheal tissues were ana-
lysed, showed that this was not the case. There is strong
evidence to suggest that, after oral dosing with HEX, at
least part of the faecal contents contained a volatile
constituent that could be readily lost if the samples were
dried and powdered, as they were in this case. An extrac-
tion procedure, using the major tissues and excreta, fol-
lowed by thin-layer chromatography, showed that at least
four water-soluble (polar) metabolites were produced, but
not identified, after oral dosing.
In a study designed to re-examine some of the findings
and observations of Mehendale (1977), Dorough (1979)
investigated the accumulation, distribution, and excretion
of 14C-HEX after its administration to rats and mice
either as a single oral dose or as a component of their
diet. The principal results of this study were reported by
Dorough & Ranieri (1984). The animals used were male and
female Sprague-Dawley rats, weighing between 200 and 250 g
body weight, and male and female Sprague-Dawley albino
mice, weighing between 25 and 30 g. Two female rats were
dosed, by gavage, with HEX (20 mg/kg) in 0.9 ml of corn
oil and were immediately placed in separate metabolism
cages through which air was drawn at 600 ml/min. The
evacuated air was passed through two high efficiency
traps. Since less than 1% of the administered dose was
recovered from the traps, it was considered to be conclus-
ive evidence that the pulmonary route is not of major
importance in the excretion of HEX (Dorough, 1979).
Dorough (1979) conducted single dose studies by admin-
istering, with a dosing needle, either 2.5 or 25 mg 14C-
HEX/kg body weight (dissolved in 0.9 ml of corn oil for
rats and in 0.2-0.3 ml for mice). The animals were killed
at 1, 3, or 7 days after dosing, and samples of muscle,
brain, liver, kidneys, fat, and either ovaries or testes
were removed and analysed for 14C activity. Urine and
faeces were also collected during the period between
dosing and tissue sample collection. No appreciable dif-
ferences due to sex or species were found in the excretion
patterns. The liver, kidneys, and fat were the most
important deposition sites for 14C residues in both rats
and mice, the levels in the kidneys of rats and in the
liver of mice being the highest.
In the same study (Dorough, 1979), rats and mice were
also placed on diets containing 1, 5, or 25 mg 14C-HEX
per kg. Assuming a daily food intake of 15 g for rats and
5 g for mice, this would give daily dose rates of 0.066,
0.330, and 1.666 mg/kg for rats and 0.182, 0.910, and 4.55
mg/kg for mice. Feed was replaced in the feeders every
12 h to minimize the loss of 14C-HEX (from volatiliz-
ation), and the feeding study was carried out for 30 days.
During this period, rats and mice were killed at 1, 3, 7,
12, 15, or 30 days. The surviving animals were then
returned to a normal diet for up to 30 days and, during
this post-treatment period, animals were killed at 1, 3,
7, 15, or 30 days after the last exposure. The total
excretion (urine and faeces) of the radiolabel ranged from
63-79% of the consumed 14C-HEX, which was significantly
lower than that found in the single-dose study (73-96%).
In all cases, the liver, kidneys, and fat contained the
highest amounts of 14C, and it appeared that a steady
state for these levels was reached after 15 days of the
feeding phase. A good correlation was observed between
the level of HEX in the diet and the 14C-levels found in
all the examined tissues. In a separate experiment with
male rats, in which the bile duct was cannulated and a
single dose of 14C-HEX (25 mg/kg) was administered
orally, only 16% of the dose was excreted in the bile. The
extraction characteristics of the radiocarbon compounds in
the excreta showed that they were primarily polar metab-
olites, some of which were capable of being converted to
organic-soluble compounds after acid-catalysed hydrolysis.
In a comparative study of the pharmacokinetics
of 14C-HEX after intravenous and oral dosing, Yu &
Atallah (1981) dosed Sprague-Dawley rats (240-350 g body
weight) with either 3 or 6 mg 14C-HEX (specific activity:
0.267 mCi/mmol). The doses ranged from 8.5 to 25.6 mg/kg.
Shortly after oral dosing, 14C activity appeared in the
blood and reached a maximum after approximately 4 h.
The 14C activity appeared in most of the tissues
analysed at 8, 24, 48, 72, 96, and 120 h after dosing.
Following oral dosing, there were higher residue levels in
the kidneys and liver than in any other tissue, although
these levels were generally much lower than those observed
after intravenous dosing. For example, at 24 h after
dosing, the kidneys and liver were found to contain only
0.96 and 0.75%, respectively, of the administered oral
dose, while these organs retained 2.92 and 4.68%, respect-
ively, of the administered intravenous dose. A higher
proportion (15.07%) of the 14C activity was found in the
digestive system (duodenum and large and small intestines)
after oral dosing. Coupled with the increased rate and
extent of faecal excretion after oral administration
(approximately 72%), compared to that after intravenous
dosing (approximately 20%), this would suggest that only a
fraction of the orally administered dose was absorbed.
About 17% of the oral dose was excreted in the urine.
Both urinary and faecal metabolites were again
characterized as polar because of their insolubility in
organic solvents. Unchanged HEX was not detected in any
of the samples examined. Only 11% of the 14C content was
soluble in organic solvents and a further 32% was con-
verhydrolysis. This indicated, perhaps, the formation of
metabolic ester conjugates.
Lawrence & Dorough (1982) made a comparative study of
the uptake, disposition, and elimination of HEX after
administering radiolabelled 14C-HEX by the intravenous
(10 µg/kg), inhalation (24 µg/kg), and oral routes (6 mg
per kg) to Sprague-Dawley rats weighing between 175 and
250 g, respectively. They noted that while doses in the
microgram range were useful for monitoring the urinary and
faecal excretion of HEX, much higher doses (about 6 mg/kg
in 0.5 ml of corn oil, and with a 4-fold increase in
radiocarbon activity) were necessary to obtain levels in
the principal organs that could be measured with any pre-
cision. Indeed, the doses administered orally were some
250 and 600 times the inhaled and intravenous doses,
respectively. In agreement with other researchers, these
authors attributed the lack of measurable levels in the
organs, following the administration of low doses, to the
poor bioavailability of HEX when given by the oral route.
The total radiolabel recovery immediately after the admin-
istration of the dose was 98.0 ± 5.3% (mean ± S.D.). Rats
dosed orally eliminated 2-3 times more of the dose in the
faeces than those dosed by the intravenous or inhalation
route. A maximum blood level was reached at approximately
2 h after dosing. The peak was broad with similar blood
concentrations between 2 and 5 h, perhaps indicating that
absorption occurred along the gastrointestinal tract over
this period in a quasi-steady state with elimination.
Biliary excretion was again confirmed as being greater
after oral dosing than after intravenous or inhalation
dosing, but it still only accounted for 18% of the admin-
istered dose. This observation agreed with previous
studies and, more importantly, with the report of Yu &
Atallah (1981), who administered comparable dose levels by
the oral route. Lawrence & Dorough (1982) also reported
that the faecal material contained predominantly polar or
unextractable material, as did the bile. These authors
considered that this was a clear indication that 14C-HEX
was extensively metabolized to polar products by the gut
contents, since only approximately 50% of 14C-HEX was
recovered when it was added to rat stomach contents that
were then immediately extracted with hexane.
A more recent comparative study (El Dareer et al.,
1983) essentially confirmed the findings of Yu & Atallah
(1981) and Lawrence & Dorough (1982). Male Fischer-344
rats with an average body weight of 169 g were dosed at
a level of 4.1 and 61 mg/kg with approximately 1 ml of a
solution of 14C-HEX dissolved in a 1:1:4 mixture of
Emulphor EL620, ethanol, and water. Little radioactivity
appeared as exhaled 14CO2.
6.1.2. Inhalation
In studies by Dorough (1980) and Lawrence & Dorough
(1981, 1982), rats were exposed to 14C-HEX vapour in a
specially designed, single animal inhalation exposure sys-
tem. Each animal was exposed to the vapours in a rodent
respirator, with the exhaust vapours from the system pass-
ing through a filter pad made from expanded polyurethane
foam. The flow rate and concentration of HEX was measured
prior to and after passing through the respirator contain-
ing the exposed animal. The difference between the amounts
of HEX in the input and output was assumed to be equi-
valent to the retained dose. Rats were exposed for a
period of 1 h and received doses in air which ranged from
1.4 to 37.4 mg/kg body weight (Lawrence & Dorough, 1981).
Immediately after the 1-h exposure, the recovery of the
dose retained by the animal was 91.8 ± 8.5% (mean ± S.D.).
Exposed animals were immediately placed in metabolism
cages for 72 h, during which time faeces, urine, and
expired air were collected. The animals were then killed
and certain of their tissues analysed for 14C activity.
Less than 1% of the retained radiocarbon was expired
during the 24-h period immediately following exposure, and
no radiocarbon was detected as 14CO2. Only about 69%
of the inhaled dose was recovered, which was much lower
than that recovered after intravenous (85%) or oral dosing
(82%). Since recovery of the dose immediately after the
administration of the inhalation dose was approximately
92%, the reduced recovery during the 72-h post-dosing
period led to the speculation that a volatile metabolite
was formed during this period, but attempts to collect and
identify this metabolite were not successful.
No kinetic parameters were reported in either of the
publications by Lawrence & Dorough (1981, 1982), although
blood concentration-time data during the 1-h exposure and
the following 6 h were presented. Elimination during the
subsequent 6 h appeared to relate to a complex pharmaco-
kinetic model with a terminal rate comparable to that
reported for the intravenous route, the half-life being
approximately 30 h.
The elimination via the bile was relatively low (8%)
after inhalation exposure, compared with 13 and 18% after
intravenous or oral administration of the same dose
(5 µg/kg) (Lawrence & Dorough, 1982). The fraction of
the dose recovered in the faeces and urine (23 and 33%,
respectively) was about the same as that recovered after
the intravenous dose, except that more was recovered in
the urine than in the faeces after the inhalation
exposure, while the reverse was observed after the intra-
venous dose.
A comparative study of the uptake, distribution, and
elimination of 14C-HEX (El Dareer et al., 1983) confirmed
and extended the conclusions reached by Lawrence & Dorough
(1981, 1982) concerning pulmonary exposure. Individual
Fischer-344 rats weighing between 125 and 190 g (with an
average weight of 169 g) were placed in metabolism cages
and exposed by inhalation. The dose received by each rat
over a 2-h exposure period was calculated from the total
amount of radioactivity recovered from the tissues,
faeces, urine, and exhaled air. The animal fur was not
included. The dose received by the exposed animals was
between 1.3 and 1.8 mg/kg body weight. The animals were
killed at either 6 or 24 h after they were removed from
the inhalation exposure. Whole blood, plasma, liver,
kidneys, voluntary muscle (gastrocnemius), subcutaneous
fat, brain, skin (ears), and the residual carcass (except
for the skin and fur which were discarded) were analysed
for 14C activity, as were the urine, faeces, and exhaled
air. The principal sites of deposition were the lungs,
kidneys, and liver. Only approximately 1% of the radio-
label was identified as 14CO2. No intact HEX was found
in any of the tissues; the majority of the radiolabel
extracted was polar (water soluble). These findings were
similar to those of Lawrence & Dorough (1981, 1982).
6.1.3. Dermal
No studies on the pharmacokinetics or distribution of
dermally applied HEX were found in a survey of the pub-
lished literature. Although no qualitative studies or
estimates of the uptake of HEX through skin were found,
studies have been reported in which discoloration of the
skin was observed after the dermal application of HEX
(Treon at al., 1955; IRDC, 1972). In these reports, toxic
response, leading to death, was observed in several
instances, which would suggest that HEX was absorbed
transdermally into the systemic circulation.
6.1.4. Comparative studies
Each of the four major studies (Yu & Atallah, 1981;
Lawrence & Dorough, 1981, 1982; El Dareer et al., 1983) of
the uptake and distribution of HEX involved more than one
route of uptake. One objective of each of these studies
was to compare the exposure routes. The observations made
were as follows:
* The principal routes of excretion were via the urine
and faeces. Considerably more of the administered dose
was excreted in the faeces after oral administration
than after dosing by the intravenous or inhalation
route, probably as a consequence of the increased
biliary excretion after oral dosing and the interac-
tion or metabolism of the dose by gut and faecal con-
tents. More of the administered dose was excreted in
the urine than in the faeces after inhalation
exposure, while the reverse was the case after intra-
venous administration.
* Biliary excretion occurred after administration by
each of the three routes. For similar doses, the frac-
tion of the dose eliminated by this route was in the
order oral > intravenous > inhalation.
* Comparative distribution to the major organs and tis-
sues is presented in Tables 8, 9, and 10. The princi-
pal organs to which HEX was distributed by the sys-
temic circulation were the kidneys and liver. The
lungs and trachea contained the highest concentrations
of HEX after inhalation exposure.
* There was a significantly higher retention of 14C in
the carcass, at 72 h post-dosing, after dosing by the
inhalation and intravenous routes than after oral
dosing (Table 10).
6.1.5. In vitro studies
Yu & Atallah (1981) examined the ability of liver,
faecal, and gut homogenates to metabolize HEX in vitro.
In an apparent first-order kinetic process, HEX was
metabolized by gut content, faecal, and liver homogenates
with half-lives of 10.6, 1.6, and 14.2 h, respectively.
When mercuric chloride (HgCl2) was added to the gut and
faecal homogenates as a bacteriocide, the half-lives were
increased to 17.2 and 6.2 h, respectively, indicating that
the gut and faecal flora contributed significantly to the
metabolism of HEX. Denaturation of the liver homogenate
had virtually no effect on the in vitro metabolic rate
indicating, perhaps, that there was only limited involve-
ment of liver microsomes or other enzyme-dependent process.
Table 8. Distribution of radioactivity (expressed as percentage of administered
dose) from 14C-HEX in rats dosed by various routesa
---------------------------------------------------------------------------------
Oral dose Intravenous Inhalation dose
Low doseb High doseb doseb Group Ac Group Bb
(4.1 mg/kg) (61 mg/kg) 0.59 mg/kg (1.0 mg/kg) (1.4 mg/kg)
---------------------------------------------------------------------------------
Faeces 74.5 ± 2.8 65.3 ± 6.9 34.0 ± 1.0d 28.7 ± 4.3 47.5 ± 6.4
Urine 35.5 ± 2.5 28.7 ± 4.2 15.8 ± 1.4 41.0 ± 4.8 40.0 ± 6.6
Tissues 2.4 ± 0.6 2.4 ± 0.1 39.0 ± 1.0 28.9 ± 1.6 11.5 ± 0.8
CO2 0.8 ± 0.0 0.6 ± 0.0 0.1 ± 0.0 1.4 ± 0.3 1.0 ± 0.5
Other volatile 0.2 ± 0.0 0.3 ± 0.0 0.1 ± 0.0
compounds
Total recovery 118 ± 3.0e 97 ± 7.0 89 ± 2.0 100 100
---------------------------------------------------------------------------------
a Adapted from: El Dareer et al. (1983). Values represent the mean percentage
of dose ± S.D. for three rats.
b At 72 h after dosing or exposure.
c At 6 h after exposure.
d Plus intestinal contents.
e For an unexplained reason, the total recovery for this dose was higher than
theoretical. If the percent recoveries for this dose are "normalized" to
100%, differences in distribution for the two doses are minimal, indicating
that no saturable process is operative in this dose range.
Table 9. Fate of radiocarbon (expressed as percentage of
administered dose) after oral, inhalation, and intravenous
exposure of rats to 14C-HEXa
--------------------------------------------------------------
Cumulative percent of dose
Oralb Intravenousc Inhalationd
--------------------------------------------------------------
24-h
Urine 22.2 ± 1.8 18.3 ± 5.2 29.7 ± 4.5
Faeces 62.2 ± 8.0 21.1 ± 7.1 17.0 ± 7.5
48-h
Urine 24.0 ± 1.9 20.7 ± 5.6 32.5 ± 5.1
Faeces 67.7 ± 5.1 30.4 ± 1.7 21.0 ± 7.5
72-h
Urine 24.4 ± 1.9 22.1 ± 5.7 33.1 ± 4.5
Faeces 68.2 ± 5.1 47.4 ± 1.9 23.1 ± 5.7
Body 0.2 ± 0.2 15.7 ± 7.8 12.9 ± 4.7
Total Recovery 92.8 ± 4.7 85.2 ± 4.8 69.1 ± 9.6
--------------------------------------------------------------
a Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
b Dose (7 µg/kg body weight) administered in 0.5 ml corn oil.
c Dose (5 µg/kg body weight) administered in 0.2 ml
saline:propylene glycol:ethanol (10:4:1) by injection into
the femoral vein.
d Doses administered as vapours over a 1-h exposure period
to achieve doses of about 24 µg/kg body weight.
El Dareer et al. (1983) incubated 14C-HEX with homo-
genates of liver, faeces, and intestinal (large and small)
contents, as well as with whole blood and plasma. Samples
were taken at 0, 5, and 60 min. The results, presented in
Table 11, clearly demonstrated the chemical reactivity of
HEX and its ability to bind components of biological
material.
Table 10. Distribution of HEX equivalentsa in tissues and
excreta of rats 72 h after oral, inhalation, and intravenous
exposure to 14C-HEXb,c
-----------------------------------------------------------------
Sample Oral dose Inhaled dose Intravenous dose
(6 mg/kg)d (24 µg/kg) (10 µg/kg)
-----------------------------------------------------------------
ng/g of tissue
Trachea 292 ± 170 107.0 ± 65.0 3.3 ± 1.7
Lungs 420 ± 250 71.5 ± 55.2 14.9 ± 1.1
Liver 539 ± 72 3.6 ± 1.9 9.6 ± 1.1
Kidneys 3272 ± 84 29.5 ± 20.2 22.3 ± 0.6
Fat 311 ± 12 2.8 ± 0.4 2.3 ± 0.2
Remaining carcass 63 ± 40 1.3 ± 0.6 0.5 ± 0.1
percentage of dose
Whole body 2.8 ± 1.1 12.9 ± 4.7 31.0 ± 7.8
Urine 15.3 ± 3.3 33.1 ± 4.5 22.1 ± 5.7
Faeces 63.6 ± 8.5 23.1 ± 5.7 31.4 ± 1.9
Total recovery 81.7 ± 6.7 69.1 ± 9.6 84.6 ± 4.6
-----------------------------------------------------------------
a One HEX equivalent is defined as the amount of radiolabel
equivalent to 1 ng of HEX, based on the specific activity of
the dosing solution.
b Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
c All values are the mean ± S.D. of three replicates.
d It should be noted that the oral dose was 250 and 600 times
that of the inhaled and intravenous doses, respectively.
This was necessary because residues were not detected in
individual tissues of animals treated orally at doses of
5-25 µg/kg.
6.2. Metabolic transformation
No primary metabolites or conjugates of HEX have been
identified. The data available on the pharmacokinetics of
HEX after dosing by the oral, inhalation, and dermal
routes are presented in sections 6.1.1, 6.1.2, and 6.1.3.
In studies by Dorough (1980) and Lawrence & Dorough
(1982), the principal routes of excretion were shown to be
via the urine and faeces. No unchanged HEX was found in
either, indicating that HEX was involved in extensive
metabolism.
In studies with rats and mice fed a diet containing 1,
5, or 25 mg 14C-HEX/kg, 63-79% of the consumed HEX was
recovered in the urine and faeces (Dorough, 1979; Dorough
& Ranieri, 1984). The extraction characteristics of the
radiocarbon compounds in the excreta showed that they were
primarily polar metabolites, some of which were trans-
formed to organic-soluble compounds after acid-catalysed
hydrolysis.
Table 11. Extractability of 14C-HEX and radioactivity derived from
saline and various biological preparationsa
----------------------------------------------------------------------
Preparation Time First Extraction Second Extraction Pellet
(min) Organicb Aqueous Organic Aqueous
----------------------------------------------------------------------
Saline 0 99.6 (92.4) 0.4
5 99.1 (92.8) 0.9
60 98.8 (94.6) 1.2
Liver 0 55.0 (74.4) 8.0 24.5 1.0 11.6
5 42.8 (49.7) 15.2 15.0 4.7 22.2
60 11.1 18.8 5.9 2.4 51.8
Plasma 0 22.2 (61.7) 7.2 50.2 0.8 19.6
5 19.7 (66.3) 25.0 33.6 2.0 19.6
60 1.4 43.4 21. 3.9 30.2
Whole blood 0 16.2 (60.4) 3.8 27.9 1.2 50.8
5 2.8 21.6 13.4 1.6 60.6
60 0.6 27.4 12.0 1.4 58.6
Faeces 0 90.0 (93.7) 0.6 8.0 0.2 1.2
5 83.4 (87.8) 0.8 9.0 0.6 6.2
60 40.5 (61.0) 2.8 31.3 3.0 22.4
Intestinal 0 93.7 (94.7) 0.6 4.6 0.2 1.0
contents 5 82.8 (89.5) 1.6 8.6 1.0 5.9
60 66.3 (87.0) 4.6 15.4 2.4 11.3
----------------------------------------------------------------------
a From: El Dareer et al. (1983). Values represent the percentage of
the total radioactivity in the respective fraction.
b Values in parentheses represent the percentage of the
radioactivity in the fraction as HEX.
In a comparative study of the pharmacokinetics of HEX
after intravenous or oral dosing at 8.5-25.6 mg/kg (Yu &
Atallah, 1981), urinary and faecal metabolites were again
characterized as polar because of their poor solubility in
organic solvents. No unchanged HEX was found. Only 11%
of the 14C content of the excreta was soluble in organic
solvents, and a further 32% of the extract was converted
to organic-soluble compounds after acid-catalysed hydro-
lysis. This indicated, perhaps, that metabolite ester
conjugates had been formed.
Yu & Atallah (1981) also performed in vitro metabolic
studies in which they incubated HEX with liver, faecal,
and gut-content homogenates (see section 6.1.5).
After Lawrence & Dorough (1982) had dosed rats by the
oral, inhalation, and intravenous routes with 14C-
labelled HEX at 6 mg/kg, 24 µg/kg and 10 µg/kg, respect-
ively, they found that the faecal and bile contents con-
tained mostly polar metabolites. These authors suggested
that HEX was rapidly metabolized to polar products, since
only about 50% of the HEX was recovered when it was added
to rat gut contents and immediately extracted with n-hex-
ane. In addition, the authors noted that approximately
15.8 ± 4% of the radiolabel that appeared in the faeces
within 24 h after dosing was volatile, indicating that a
catabolite was probably produced.
El Dareer et al. (1983) dosed rats by the inhalation
route so that individual animals received between 1.3 and
1.8 mg/kg body weight over a 2-h exposure period. They
were killed 6 or 24 h after removal from exposure. No in-
tact HEX was found in any of the tissues, and the majority
of the extractable material was polar (water soluble), in
accordance with the findings of Lawrence & Dorough (1981,
1982). As a part of this same study, El Dareer et al.
(1983) incubated 14C-HEX with homogenates of liver,
faeces, and intestinal (large and small) contents, as well
as with whole blood and plasma. These in vitro studies
were designed to assess the reactivity and binding charac-
teristics of HEX. The results, presented in Table 8,
clearly show chemical lability of HEX and its ability to
bind the components of biological material (see section
6.1.2).
Despite these efforts to characterize HEX metabolism,
no metabolites were identified. This observation suggests
that an attempt to rectify this deficiency would be a high
priority.
6.3. Reaction with body components
The toxic mechanisms of hexachlorocyclopentadiene are
not well understood. HEX has been shown to react with
olefins and other organic molecules such as aromatic com-
pounds. Using the available data, especially those from
studies comparing the various routes of exposure, a very
general and hypothetical rationale can be developed to
suggest possible reactions with body tissue.
The reactivity of HEX shows a high potential for
transformation and reaction with other chemicals. Absorp-
tion from the gastrointestinal contents is relatively
inefficient, probably due to the interaction of HEX with
the gastrointestinal contents and metabolism by intestinal
flora. The fact that HEX did not appear to be interactive
with the gastrointestinal epithelia in the kinetic studies
discussed in this chapter was probably due to its dilution
in the carrier vehicle, as well as its interaction with,
and metabolism by, the gut contents. However, in short-
term repeated oral dosing studies (SRI, 1981a,b), at doses
of 19 mg/kg or more, inflammation and hyperplasia was
noted in the forestomach (see Table 16). In addition, the
interaction of HEX after dermal contact was marked by a
distinct discoloration of the skin. This suggests a "site
of uptake" interaction, probably similar to that observed
in the lungs after pulmonary uptake.
During inhalation and the passage of HEX through the
lung tissue to reach the systemic circulation, metabolism
to water-soluble compounds probably occurs and HEX is
eliminated through the kidneys. However, an intravenous
dose may be bound unchanged to blood components (e.g.,
haemoglobin) and remain attached until reaching the liver.
The relatively slow elimination of the radiolabel from the
systemic circulation after intravenous dosing with 14C-
HEX (approximate terminal half-life of 30 h) suggests a
bioaccumulation potential, at least for some of the
metabolites, since little HEX appears to remain in the
tissues.
Rand et al. (1982b) showed that the cellular level in
lung tissue underwent significant changes after HEX inha-
lation. HEX vapour, administered by the inhalation route,
in addition to binding to epithelial lung tissue, was
found to bind to the extracellular lining in the lung.
Binding to bronchiolar Clara cells, which contribute
important materials to the extracellular lining of the
peripheral airways, was observed after inhalation exposure
in rats and monkeys (Rand et al., 1982a). HEX was also
found, in in vitro studies, to bind to the components of
whole blood, plasma, liver, and faecal homogenates and to
gastrointestinal contents (El Dareer et al., 1983). Thus,
irrespective of the route of administration, the principal
sites of toxic action seem to be the lungs, liver, and
kidneys. This observation is supported by the results from
the toxicity testing reported in chapter 8.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The effects of HEX on microorganisms have been
studied in aqueous and soil systems. Many of the aqueous
concentrations used in these experiments exceeded the
upper limit of aqueous solubility (0.8-2.1 mg/litre).
These concentrations were usually obtained by using an
organic solvent vehicle to disperse the chemical in aque-
ous media. The environmental significance of the results
should be interpreted with this aspect in mind.
Cole (1953) inoculated 10 strains of common human and
animal pathogens into growth media that contained various
concentrations of HEX. The inhibiting concentration, or
lowest concentration in which no growth was observed after
96 h of contact, ranged from 1-10 mg HEX/litre. The
Addition of 5 or 10 mg HEX/litre to sewage effluent inocu-
lated with Salmonella typhosa was found to be more effec-
tive than similar concentrations of chlorine in reducing
counts of total bacteria, coliforms, and S. typhosa (Cole,
1954). Yowell (1951) also reported, in a patent appli-
cation, that HEX has antibacterial properties; standard
phenol coefficients for Ebertnella typhi (Salmonella
typhi) and Staphylococcus aureus were 25 and 33, at 21 and
23 mg HEX/litre, respectively. These findings indicate
that concentrations of HEX at or slightly above its aque-
ous solubility limit are toxic to several types of
pathogens.
In contrast, tests with other microorganisms have
shown some ability to withstand HEX exposure. Twenty-three
strains of organisms (type unspecified), when added to
aqueous media containing HEX at 1000 mg/litre, were able
to metabolize the compound to a varying degree. Analysis
of the medium after 14 days indicated a HEX removal of
2-76%, depending on the organism used (Thuma et al.,
1978).
Rieck (1977a) found no effects on natural populations
of bacteria, actinomycetes, or fungi after a 24-day incu-
bation of a sandy loam soil treated with 1 or 10 mg HEX/kg
dry weight. It was concluded that no significant detrimen-
tal effects on microbial populations would result from
contamination of soils with these levels of HEX.
The effects of HEX on three ecologically important
microbial processes have been reported (Butz & Atallah,
1980). Results on cellulose degradation by the fungus
Trichoderma longibrachiatum indicated that a suspension
of HEX inhibited cellulose degradation at a concentration
of 1 mg/litre or more in a liquid medium. The calculated
7-day EC50 was 1.1 mg/litre. Extrapolations for 1- and
3-day EC50 values were both reported to be 0.2 mg/litre.
The decrease in toxicity over the 7-day period was
attributed to adaptation by T. longibrachiatum.
HEX inhibited anaerobic sulfate reduction by the bac-
terium Desulfovibrio desulfuricans when HEX was present in
suspension in a liquid medium. After a 3-h contact period,
growth inhibition was observed at HEX concentrations of
10-100 mg/litre, and there was no growth at 500 or 1000
mg/litre. Similarly, growth inhibition was observed at 1
and 10 mg/litre after a 24-h contact period, and there was
no growth at 50-1000 mg/litre. HEX was considered to be
slightly toxic to D. desulfuricans (Butz & Atallah,
1980).
The third part of the study by these investigators
(Butz & Atallah, 1980) focused on the effects of HEX on
urea ammonification by a mixed microbial culture in moist
soil. The results indicated that HEX concentrations of
1-100 mg/kg (dry weight) were not toxic to soil organisms
responsible for urea ammonification. The EC50 increased
from 104 mg/kg at day 1 to 1374 mg/kg at day 14. The
authors suggested that the low toxicity and its decrease
over time in this experiment may have been due to adsorp-
tion of the toxicant onto soil particles, as well as to
potential adaptation by the organism. Adsorption onto soil
particles may also account for the lack of toxicity in the
study of Rieck (1977a).
Walsh (1981) reported unpublished data on the effects
of HEX on four species of marine algae, obtained according
to the method described by Walsh & Alexander (1980). The
7-day EC50 was calculated as the concentration that
caused a 50% decrease in growth compared with the control,
as estimated by absorbance at 525 nm. The 7-day EC50
values reported indicated a wide range of susceptibility
among the species tested. Isochrysis galbana and
Skeletonema costatum were the most susceptible species,
the average 7-day EC50 values being about 3.5 and 6.6 µg per
litre, respectively. The average value for Porphyridium
cruentum was 30 µg/litre, while that for Dunaliella
tertiolecta was 100 µg/litre. Other tests with S.
costatum indicated that the direct algicidal effect of HEX
was less pronounced than its effect on growth. After 48 h
of exposure to HEX at 25 µg/litre, mortality, as indi-
cated by staining and cell enumeration, was only 4%
(Walsh, 1983).
7.2. Aquatic organisms
7.2.1. Freshwater aquatic life
Several studies are available on the effects resulting
from exposure of freshwater aquatic life to various con-
centrations of HEX.
Results from acute toxicity tests with HEX have been
reported for a number of freshwater fish species (Table
12). The 96-h LC50 value for fathead minnow larvae in a
flow-through test with measured toxicant concentration was
7 µg/litre (Spehar et al., 1977, 1979). Values obtained
for adult fathead minnows in static tests with unmeasured
toxicant concentrations ranged from 59 to 180 µg/litre
(Henderson, 1956; Buccafusco & LeBlanc, 1977). Reported
96-h values for goldfish, channel catfish, and bluegills
were also within this range (Buccafusco & LeBlanc, 1977;
Podowski & Khan, 1979; Khan et al., 1981).
Sinhaseni et al. (1982) reported the biological
effects of HEX on rainbow trout (Salmo gairdneri) exposed
to 130 µg HEX/litre in a non-recirculating flow-through
chamber. Oxygen consumption, measured polarographically,
increased by 193% within 80 min and then gradually
decreased until the fish died (after approximately 5 h).
Vehicle controls showed no effects after 76 h of exposure.
When added to normal trout mitochondria, HEX increased
basal oxygen consumption. The authors concluded that HEX
uncoupled oxidative phosphorylation.
Sinhaseni et al. (1983) continued their research on
the respiratory effects of HEX on intact rainbow trout.
Acclimated rainbow trout were exposed to 130 µg HEX/litre
in a flow-through well-water circuit, which was designed
to allow measurements of oxygen consumption in fish.
Again, HEX increased oxygen consumption rates (186 ± 24%),
the maximum rates being nearly the same as in the previous
experiment (approximately 84 min). The oxygen consumption
decreased until death (after approximately 6.5 h). Control
trout, exposed to the same concentration of the vehicle
(acetone) used to disperse HEX, showed no changes. The
authors reported profound respiratory stimulation, and HEX
appeared to uncouple oxidative phosphorylation. Sinhaseni
et al. (1983) postulated that HEX intoxication in the
intact animal may be due to increased oxygen consumption
and impaired oxidative ATP synthesis resulting from the
mitochondrial uncoupling action of HEX.
Table 12. Acute toxicity data for freshwater species exposed to HEX
---------------------------------------------------------------------------------------------------------------------------
Acute
no-effect
Species Methoda LC50 (µg/litre)b concentration Comments Reference
24-h 48-h 96-h (µg/litre)
---------------------------------------------------------------------------------------------------------------------------
Cladoceran S,U 93.0 52.2 ND 32 17 °C, soft water Vilkas (1977)
Daphnia magna (78.9-109.6) (44.8-60.9)
Cladoceran S,U 130 39 ND 18 22 °C, soft water Buccafusco &
Daphnia magna (68-260) (30-52) Leblanc (1977)
Fathead minnow FT,M NR NR 7.0 3.7 25 °C, soft water Spehar et al.
(larvae, < 0.1 g) (1977, 1979)
Pimephales promelas
Fathead minnow (1-1.5 g) S,U 115 110 104 NR Hard water, Henderson
Pimephales promelas acetone soln. (1956)
93 78 78 NR Soft water,
acetone soln.
75 59 59 NR Hard water,
emulsion
(no acetone)
Fathead minnow (0.72 g) S,U 240 210 180 87 22 °C, soft water Buccafusco &
Pimephales promelas (170-320) (180-250) (160-220) Leblanc (1977)
Goldfish NR NR NR 78 NR No details given Podowski & Khan
Carassius auratus (1979)
Channel catfish (2.1 g) S,U 190 150 97 56 22 °C, soft water Buccafusco &
Ictalurus punctatus (140-250) (130-180) (81-120) Leblanc (1977)
Bluegill (0.45 g) S,U 170 150 130 65 22 °C, soft water Buccafusco &
Lepomis macrochirus (140-210) (120-180) (110-170) Leblanc (1977)
---------------------------------------------------------------------------------------------------------------------------
a S = static, FT = flow-through, U = unmeasured concentrations, M = measured concentrations.
b Numbers in parentheses show 95% confidence interval. ND = Not determined. NR = Not reported.
Spehar et al. (1977, 1979) conducted 30-day early-life
stage flow-through toxicity tests with fathead minnows
(Pimephales promelas) using measured concentrations and
1-day-old larvae. The 96-h LC50 value was 7 µg/litre.
The 96-h mortality data indicated a sharp toxicity
threshold, such that 94% survival was observed at 3.7 µg
per litre, 70% at 7.3 µg/litre, and 2% at 9.1 µg/litre.
At the end of the 30-day exposure period, mortality was
only slightly higher, with 90% survival at 3.7 µg/litre,
66% at 7.3 µg/litre, and 0% at 9.1 µg/litre. These
results indicated that the median lethal threshold, the
lowest concentration causing 50% mortality, was reached
within 4 days. In addition, the HEX residues found in
fathead minnows at the end of the 30-day tests were low
(< 0.1 µg/g), and a BCF value of < 11 was reported
(Spehar et al., 1979). The authors concluded that the
toxicity data and the BCF values indicated that HEX was
non-cumulative in fish, i.e. it did not bioconcentrate in
fish as a result of continuous low-level exposure to HEX.
The growth rate of surviving larvae, measured in terms of
both body length and weight, did not decrease signifi-
cantly at any of the concentrations tested, compared with
the controls. This was true even at 7.3 µg/litre, a level
greater than the calculated LC50 value. Based on these
toxicity and growth data, Spehar et al. (1977, 1979) con-
cluded that 3.7 µg/litre is the highest concentration of
HEX that produces no adverse effects on fathead minnow
larvae. No other chronic toxicity data for any freshwater
species are available.
7.2.2. Marine and estuarine aquatic life
Among marine invertebrates, the 96-h LC50 values for
HEX range from 7 to 371 µg/litre (Table 13) (US EPA,
1980a). Except where indicated, these results were
obtained from static tests with nominal concentrations of
HEX. The highest LC50 by far was for the polychaete
Neanthes arenaceodentata, which is an infaunal organism
living in the sediment. The two shrimp species tested
were more sensitive to HEX by a factor of 10 or more.
The static LC50 value reported by US EPA (1980a) for
the grass shrimp, Palaemonetes pugio, was slightly higher
than that for the mysid shrimp, Mysidopsis bahia (Table
13). However, the LC50 for the mysid shrimp was consider-
ably lower in a flow-through test than in the static test.
Similarly, the LC50 value was lower when calculated
from actual measurements of HEX concentrations in the test
solutions (measured concentration) than when calculated
according to the concentrations based on amounts orig-
inally added to test solutions (nominal concentrations).
Table 13. Acute toxicity data on estuarine marine
organisms exposed to HEXa
----------------------------------------------------------
Species Methodb Salinity 96-h LC50c
(o/oo) (µg/litre)
----------------------------------------------------------
Polychaete S,U 28 371
Neanthes arenaceodentata (297-484)
Grass shrimp S,U 22 42
Palaemonetes pugio (36-50)
Mysid shrimp S,U 24 32
Mysidopis bahia (27-37)
Mysid shrimp FT,U 24 12
Mysidopsis bahia (10-13)
Mysid shrimp FT,M 24 7
Mysidopsis bahia (6-8)
Pinfish S,U 22 48
Lagodon rhomboides (41-58)
Spot S,U 22 37
Leiostomus xanthurus (30-42)
Sheepshead minnow S,U 24 45
Cyprinodon variegatus (34-61)
----------------------------------------------------------
a Adapted from: US EPA (1980a) and Mayer (1987).
b S = static; FT = flow-through; U = unmeasured
concentrations; M = measured concentrations.
c Numbers in parentheses show 95% confidence interval.
The acute toxicity values for HEX were similar for
each of three estuarine and marine fish species tested (US
EPA, 1980a). The static 96-h LC50 values based on
unmeasured concentrations for spot, sheepshead minnow, and
pinfish varied only between 37 and 48 µg/litre (Table 13).
7.3. Terrestrial organisms and wildlife
In a USA patent application, HEX was reported to be
nontoxic to plants in concentrations at which it was an
effective fungicide (Yowell, 1951). Test solutions were
prepared by adding HEX at various proportions to attaclay
and a wetting agent, and they were then mixed with water.
The concentrations of HEX applied to plants as an aqueous
spray were 0.1, 0.2, 0.5, and 1.0%. Slight injury
(unspecified) to Coleus blumei was reported at 1.0% HEX,
but lower concentrations were not harmful. Similarly, HEX
was added to horticultural spray oil and an emulsifier at
various proportions and then mixed with water. The concen-
tations of HEX in the prepared spray were 0.25 and 0.5%.
No injury to C. blumei was observed at these concen-
trations. No data are available concerning the effects of
HEX on amphibians, reptiles, birds, or mammals other than
those routinely used in laboratory testing.
7.4. Population and ecosystem effects
The ecological effects of HEX have not been studied at
the ecosystem, population, or community levels.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Acute toxicity studies
8.1.1. Acute oral, inhalation, and dermal toxicity
The data from acute toxicity studies using HEX are
summarized in Table 14. Caution should be exercised in
comparing the various studies. The acute toxicity (LD50
and LC50) may be affected not only by the species and
age of the animals used in the experimental tests, but
also by the strain. In addition, the purity of the com-
pound and the nature of the contaminants may affect the
toxicity, as can the experimental method and the vehicle
used. These details (when specified by the researchers)
have been summarized in the table.
For the rat, oral LD50 values ranged from 425 to 926
mg/kg for males, and from 315 to 926 mg/kg for females.
Values for mice and rabbits were within the same range.
Acute inhalation experiments involved exposure
durations from 3.5 to 4 h, and LC50 values for male rats
ranged from 18.1 to 35.0 mg/m3 (1.6 to 3.1 ppm) and for
female rats from 35.0 to 40.0 mg/m3 (3.1 to 3.5 ppm).
The mouse, rabbit, and guinea-pig values ranged from 23.7
to 80.2 mg/m3 (2.1 to 7.1 ppm).
There are few data available on dermal toxicity.
LD50 values of < 200 mg/kg in male rabbits and 340-780
mg/kg in females have been reported.
In spite of variations in LD50 values in the differ-
ent studies, these data suggest that HEX is moderately
toxic when administered orally. The acute toxicity of HEX
by the dermal route is quite similar to its acute oral
toxicity. HEX is much more toxic by the inhalation route
of exposure than by the dermal or oral routes.
8.1.2. Eye and skin irritation
IRDC (1972) tested HEX for eye irritation by instil-
ling 0.1 ml HEX into the eyes of New Zealand white rabbits
for 5 min or 24 h before washing. All the rabbits died on
or before the ninth day of the observation period. Treon
et al. (1955) reported HEX to be a primary skin irritant
in rabbits (strain unspecified) at a dose level of 250
mg/kg, and IRDC (1972) reported HEX to be a dermal irri-
tant because of the oedema that appeared after application
of 0.5 ml HEX. In this study, intense discoloration of
the skin was noted. In the study of Treon et al. (1955),
monkeys (strain unspecified) were also tested, and dis-
coloration of the skin was noted even at low doses (0.05
ml of 10% HEX) (Table 15).
Table 14. Acute toxicity studies of HEX
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Oral LD50
Rat (unspecified) oral gavage M: 510 mg/kg Treon et al.
young adult (5% solution F: 690 mg/kg (1955)
of peanut oil)
Rat (Charles River CD) oral gavage M + F: 926 IDRC (1968)
young adult (corn oil) mg/kg
Rat (Sprague-Dawley) M + F: 651 Dorough (1979)
young adult mg/kg
Rat (Fischer-344) oral gavage M: 425 mg/kg SRI (1980a)
weanling (corn oil) F: 315 mg/kg
Mouse (unspecified) M + F: 600 Dorough (1979)
mg/kg
Mouse (B6C3F1) oral gavage M + F: 680 SRI (1980a)
weanling (corn oil) mg/kg
Rabbit (Albino, strain oral gavage F: 640 mg/kg Treon et al.
unspecified) adult (5% peanut oil) (1955)
Dermal LD50
Rabbit (unspecified) skin painted F: 780 mg/kg Treon et al.
adult (1955)
Rabbit (unspecified) skin painted M: < 200 mg/kg IDRC (1972)
adult F: 340 mg/kg
Inhalation LC50
Rat (Carworth) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 35.0 (1955)
mg/m3 (3.1 ppm)
Rat (Sprague-Dawley) inhalation 4.0-h LC50 Rand et al.
young adult M: 18.1 mg/m3 (1982a)
(1.6 ppm)
F: 39.6 mg/m3
(3.5 ppm)
Mouse (unspecified) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 23.7 (1955)
mg/m3 (2.1 ppm)
----------------------------------------------------------------------
Table 14 (contd.)
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Rabbit (unspecified) inhalation 3.5-h LC50 Treon et al.
adult F: 58.8 mg/m3 (1955)
(5.2 ppm)
Guinea-pig inhalation 3.5-h LC50 Treon et al.
(unspecified) adult M + F: 80.2 (1955)
mg/m3 (7.1 ppm)
----------------------------------------------------------------------
a M = male; F = female.
Table 15. Primary eye and dermal irritation
--------------------------------------------------------------------------------
Study Species (strain) Results Reference
age
--------------------------------------------------------------------------------
Primary eye Rabbit (New Zealand Severe eye irritant (0.1 IRDC (1972)
irritation white) adult ml for 5 min or 24 h); all
died by day 9 of study
Primary dermal Rabbit (unspecified) Moderate skin irritant Treon et al.
irritation adult (250 mg/kg); one (1955)
application
Primary dermal Rabbit (New Zealand Severe skin irritant IRDC (1972)
irritation white) adult (200 mg/kg); all males died
Primary dermal Monkey (unspecified) Mild skin discoloration Treon et al.
irritation adult (0.05 ml of 10% HEX solution) (1955)
--------------------------------------------------------------------------------
Shell Research Limited conducted a study of the skin-
sensitizing potential of 98.8% pure HEX (Shell Research
Limited, 1982). Guinea-pigs (310-370g) were placed at
random into single sex groups of 10 animals and housed in
groups of five animals. Range-finding tests were conducted
to determine the concentrations of test material to be
used for intradermal induction, topical induction, and
topical challenge. Two male and two female guinea-pigs
were injected intradermally on each side of the midline
with 0.1 ml of several dilutions (0.5, 1, 5, and 10 mg HEX
per ml HEX corn oil). Filter paper patches with 0.3 ml of
a 1% or 2% dilution of test material in corn oil were
applied to two other groups. On the basis of the results
of the range-finding studies, the following tests and HEX
concentrations were selected for use: intradermal induc-
tion, 0.5 mg/litre; topical induction, 20.5 mg/litre; and
topical challenge, 10 mg/ml (all in corn oil). In the
sensitizing test, all four animals given an intradermal
injection of 0.5 mg HEX/ml suffered necrosis, while top-
ical applications at 10 or 20.5 mg/litre produced slight
redness or no difference from surrounding skin. However,
all 20 test animals showed positive responses 24 and 48 h
after removal of the challenge patches. The researchers
concluded that HEX is a skin sensitizer.
8.2. Short-term exposure
8.2.1. Oral
In a range-finding study using groups of five male and
five female Fischer-344 rats, there were no deaths when 12
doses of 25, 50, or 100 mg/kg were given in 16 days (SRI,
1980b). With the same dosing schedule, one out of five
males and four out of five females died when the dose was
200 mg/kg, and five out of five males and four out of five
females died when the dose was 400 mg/kg. In the same
study, B6C3F1 mice died when given doses of 400 or 800
mg/kg, but not when given doses of 50, 100, or 200 mg/kg.
Both rats and mice exhibited pathological changes of the
stomach wall at all but the lowest dose level.
The short-term toxicity of HEX is summarized in Table
16. These short-term toxicity studies on B6C3F1 mice and
Fischer-344 rats were conducted by SRI (1981a,b) under
contract with the National Toxicology Program (NTP), and
the results were reported by Abdo et al. (1984). In the
mouse study (SRI, 1981a), HEX (94.3-97.4% pure) in corn
oil was administered by gavage at dose levels of 19, 38,
75, 150, and 300 mg/kg to 10 mice of each sex, 5 days/week
for 13 weeks (91 days). At the highest dose level (300
mg/kg), all 10 male mice died by day 8 and three females
died by day 14. In female mice, the liver was enlarged.
Toxic nephrosis in females at doses of 75 mg/kg or more
was characterized by distensions in the proximal convol-
uted tubules, with basophilia in the inner cortical zone
and cytoplasmic vacuolization. However, male mice admin-
istered 75 mg/kg or more did not show these effects. Dose
levels of 38 mg/kg or more caused lesions in the fore-
stomach, including ulceration in both males and females,
as well as increased kidney and liver weights. The no-
observed-adverse-effect level (NOAEL) in mice was 19 mg/kg
and the lowest-observed-adverse-effect level (LOAEL) was
38 mg/kg.
In the rat study (SRI, 1981b), HEX in corn oil was
administered by gavage at dose levels of 10, 19, 38, 75,
and 150 mg/kg to groups of 10 male and female F-344 rats.
At 38 mg/kg or more, mortality and toxic nephrosis were
observed in both males and females. The male rats treated
with 19 mg/kg showed no significant effects, but female
rats had lesions in the forestomach. Similar lesions were
observed in males given 38 mg/kg or more. There was a
dose-related depression of body weight gain (relative to
the controls) and female rats had increased kidney and
liver weights. The NOAEL in rats was 10 mg/kg, and the
lowest-observed-effect level (LOEL) was 19 mg/kg.
A summary of the results of these two experiments
appears in Table 17.
Table 16. Short-term toxicity of HEX
------------------------------------------------------------------------------------------------------
Study Species Dose Results Effects at LOEL Reference
or lowest dose
------------------------------------------------------------------------------------------------------
90-day rat 10, 19, 38, 75, and NOAEL: 10 mg/kg lesions of forestomach SRI (1981b)
feeding 150 mg/kg (by gavage) LOEL: 19 mg/kg in female rats at
study 19 mg/kg
14-week rat 0.11, 0.56, and 0.226 NOEL: 0.226 mg/m3 no statistically Rand et al.
inhalation mg/m3 (5 days/week) LOEL: NE significant effects (1982a)
study
14-week monkey 0.11, 0.56, and 0.226 NOEL: 0.2 ppm no effects noted Alexander
inhalation mg/m3 (5 days/week) LOEL: NE et al.
study (1980)
------------------------------------------------------------------------------------------------------
NE = not established.
NOAEL = no-observed-adverse-effect level.
NOEL = no-observed-effect level.
LOEL = lowest-observed-effect level.
Table 17. Toxicological parameters for mice and rats administered technical grade HEX
in corn oil by gavage for 91 daysa
---------------------------------------------------------------------------------------
Species Sex Dose Mortality Relative Forestomach Forestomach Toxic
(strain) (mg/kg) weight inflammation hyperplasia nephrosis
gainb
---------------------------------------------------------------------------------------
Mice male 0 1/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 36% 0/10 0/10 0/10
38 0/10 + 9% 2/10 2/10 0/10
75 0/10 - 9% 7/10 8/10 0/10
150 0/10 - 45% 7/10 9/10 0/10
300 10/10 7/10 8/10 0/10
Mice female 0 0/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 13% 0/10 0/10 0/10
38 0/10 - 13% 2/9 2/9 0/9
75 0/10 - 13% 6/10 9/10 10/10
150 0/10 - 25% 10/10 10/10 10/10
300 3/10 - 38% 7/9 9/9 7/10
Rats male 0 3/10 0/10 0/10 0/10
(F-344) 10 1/10 - 4% 0/10 0/10 0/10
19 1/10 - 8% 0/10 0/10 0/10
38 1/10 - 20% 4/10 5/10 10/10
75 3/10 - 49% 9/10 9/10 9/10
150 7/10 - 57% 8/9 8/9 8/10
Rats female 0 1/10 0% 0/10 0/10 0/10
(F-344) 10 2/10 + 4% 0/10 0/10 0/10
19 1/10 - 5% 2/10 2/10 0/10
38 1/10 - 2% 2/10 5/10 10/10
75 3/10 - 30% 9/10 9/10 10/10
150 5/10 - 33% 9/10 9/10 10/10
---------------------------------------------------------------------------------------
a From: SRI (1981a,b).
b Relative weight gain was calculated as follows:
Dose group value - control group value
-------------------------------------- x 100
Control group value
8.2.2. Short-term inhalation toxicity
Rand et al. (1982a) conducted a range-finding study in
which groups of 10 male and 10 female Sprague-Dawley rats
were exposed to atmospheres of 0.25, 1.24, or 5.65 mg
HEX/m3 (0.022, 0.11, or 0.5 ppm), 6 h/day, 5 days/week
for a total of 10 exposures. Nine male rats and one female
rat exposed to 5.65 mg/m3 were moribund after five to
seven exposures. These rats had dark red eyes, laboured
breathing, and pale extremities. There were no mortalities
in the other exposure groups. However, the males in the
medium- and high-dose groups lost weight during the study
and reduced mean liver weights and pathology were noted.
The NOAEL for HEX exposure in this study was 0.25 mg/m3
and the LOEL was 1.24 mg/m3.
Fourteen-week inhalation studies have been carried out
on rats and monkeys (Alexander et al., 1980; Rand et al.,
1982a,b) and the results are summarized in Table 16.
Groups of 40 male and 40 female Sprague-Dawley rats
weighing 160-224 g and groups of 12 Cynomolgus monkeys
weighing 1.5-2.5 kg were exposed to HEX, 6 h/day, 5 days
per week, for 14 weeks. Levels of exposure were 0, 0.11,
0.56, and 2.26 mg/m3 (0, 0.01, 0.05, and 0.20 ppm). In
monkeys, there were no mortalities, adverse clinical
signs, weight gain changes, pulmonary function changes,
eye lesions, haematological changes, clinical chemistry
abnormalities, or histopathological abnormalities at any
dose level tested. Thus, the NOEL for monkeys was at least
2.26 mg/m3 for this exposure period, but the LOEL was
not established. Male rats had a transient appearance of
dark-red eyes at 0.56 and 2.26 mg/m3. At 12 weeks, there
were marginal but not statistically significant increases
in haemoglobin concentration and erythrocyte count in
males exposed to 0.11 mg/m3, females exposed to 0.56
mg/m3, and males and females exposed to 2.26 mg/m3.
There were small but not statistically significant changes
in mean liver weight of all groups of treated rats and
similar changes in the kidneys of all treated males. No
treatment-related abnormalities in gross pathology or
histopathology were observed. On this basis, the NOEL in
rats was 2.26 mg/m3, but the LOEL was not established.
In a further study by Rand et al. (1982b), no ultra-
structural changes were observed in monkeys that could be
attributed to the inhalation of HEX vapour. Exposure was
identical to that of the previous study (Rand et al.,
1982a). There was a statistically significant (P < 0.01)
increase in the mean number of electronlucent inclusions
in the apex and base of the Clara cells in exposed ani-
mals, as compared with the controls. According to some
researchers (Evans et al., 1978), Clara cells respond to
injury by regression to a more primitive cell type. Rand
et al. (1982b) noted that some of the ultrastructural
changes in the exposed animals resembled those of the
Evans study. It is not known what effect these changes
might cause. The Clara cell contributes important
materials to the extracellular lining of the peripheral
airways, and if this effect of HEX vapour causes a change
in the content of the contributed material, then the
extracellular lining may be altered and breathing may be
subsequently impaired (Rand et al., 1982b). This obser-
vation of lung effects coincides with those of other
researchers (Dorough, 1979, 1980; Lawrence & Dorough,
1981, 1982). Furthermore, in inhalation studies with HEX
occasional statistically significant increases in the
haemoglobin level and red blood cell counts of rats have
been noted, which may be manifestations of the impairment
of respiratory functions.
In 1984, the US National Toxicology Program (NTP)
completed a short-term, 90-day HEX inhalation study on
B6C3F1 mice and F-344 rats (NTP, 1984a,b). In the basic
study, ten rats and ten mice of each sex were placed at
random into five exposure and control groups. The rats and
mice were exposed to nominal concentrations of 0.45, 1.70,
4.52, 11.3, or 22.6 mg/m3 (0.04, 0.15, 0.4, 1.0, or 2.0
ppm) for 6 h/day, 5 days/week for 13 weeks. In the female
mouse study, 6 out of 10 of the control animals died,
while none of the animals in the male control population
died. The six animal deaths in week 7 were attributed to
a defective feeder insert. Mortality in both the rats and
mice was high in the two highest-dose groups; all rats and
mice in these groups died in the first five weeks of the
study. Posterior paresis and listlessness were observed
in mice at 4.52 and 11.3 mg/m3. Compound-related histo-
pathological alterations were observed in the respiratory
tracts of male and female mice exposed to 1.7 mg/m3 or
more. These changes included: necrosis, acute and
chronic inflammation, hyperplasia or squamous metaplasia
of the nasal, laryngeal, tracheal, bronchial, and bronchi-
olar epithelia of the affected animals. No compound-
related effects were observed in mice at the lowest dose,
and so this level could be considered the NOEL (NTP,
1984a).
Clinical signs resulting from HEX exposure included
posterior paresis in all rats exposed to 1.7 mg/m3 or
more, listlessness in all rats exposed to 4.52 mg/m3 or
more, and eye irritation and respiratory distress in all
rats exposed to 11.3 mg/m3 or more. As in the case of
mice, HEX caused significant histological alterations in
the respiratory tracts of rats at the two highest doses.
Changes of a less-marked degree were observed in the
respiratory tracts of rats receiving 4.52 mg/m3. No
compound-related changes were seen in any organ of male
and female rats exposed to the lowest dose, and so this
level could be considered the NOEL. In addition, compound-
related effects of a secondary stress-related nature were
seen in a number of other organs of rats of both sexes at
the two highest doses. These includes lymphoid depletion
of the spleen and thymus, degeneration of the seminiferous
tubules and decreased lytoplasmic vacuolization of the
adrenal cortex.
Some basic clinical pathology and histopathology
examinations were undertaken simultaneously in both
studies (NTP, 1984a,b). All effects were similar to those
noted in the previous toxicity studies. In the rat study
(NTP, 1984b), HEX nephrotoxicity was examined in more
depth. HEX was not found to be nephrotoxic at these ex-
posure levels, nor did it appear that it was myelotoxic.
8.2.3. Short-term dermal toxicity
No short-term dermal toxicity studies are available.
8.3. Long-term exposure
8.3.1. Long-term oral toxicity
No long-term oral toxicity studies have been reported.
8.3.2. Long-term inhalation toxicity
In view of the absence of long-term studies on the
inhalation of HEX, the following studies were examined.
Treon et al. (1955) exposed guinea-pigs, rabbits, rats,
and mice to a HEX (89.5% pure) concentration of 3.73
mg/m3 (0.33 ppm), 7 h/day, 5 days/week, for 25-30
exposure days. The guinea-pigs survived 30 exposures,
whereas rats and mice did not survive five exposures, and
four out of six rabbits did not survive 25 exposures.
Using a lower concentration of 1.70 mg/m3 (0.15 ppm),
guinea-pigs, rabbits, and rats survived 150 7-h exposures
over a 7-month period. This level was too high to conduct
a long-term study in mice since four out of five animals
did not survive. The rats, guinea-pigs, and rabbits toler-
ated 1.7 mg/m3 and did not exhibit any treatment-related
effects. Thus, the NOEL for rats, guinea-pigs, and rabbits
was 1.7 mg/m3 over the 7-month period. Due to the high
mortality, a NOEL for mice could not be established.
A long-term (30 weeks) inhalation study in rats with
technical grade HEX (96% pure with hexachlorobuta-1,3-
diene and octachlorocyclopentene as impurities) was con-
ducted by the Shell Toxicology Laboratory (D.G. Clark et
al., 1982). Four groups of eight male and eight female
Wistar albino rats were exposed to HEX at nominal concen-
trations of 0, 0.56, 1.13, and 5.65 mg/m3 (0, 0.05, 0.1,
and 0.5 ppm), 6 h/day, 5 days/week, for 30 weeks and were
observed for a 14-week recovery period without HEX
exposure. At the highest dose level, four males and two
females died. In males, there was depressed body weight
gain at the highest dose, relative to controls, beginning
at 7 weeks of exposure and persisting throughout the
remainder of the study. Females in the two highest-dose
groups had lower body weights at the end of the recovery
period than did the controls. At the highest dose, there
were pulmonary degenerative changes, ranging from epi-
thelial hyperplasia and oedema to epithelial ulceration
and necrosis, in both sexes, the males being affected more
severely. There were also mild degenerative changes in
the liver (bile duct hyperplasia and inflammatory cell
infiltration) and kidneys (protein casts in tubules and
pigmented cortical tubules) in a few rats, and kidney
weights were significantly increased in the females at 30
weeks. After 30 weeks of study, no biologically signifi-
cant toxicity was noted in animals that were exposed to
0.56 or 1.13 mg/m3. Thus, the NOEL in rats exposed to
HEX vapour in this study (6 h/day, 5 days/week, for 30
weeks) was 0.56 mg/m3, and the LOEL was 1.13 mg/m3.
A long-term inhalation study by the National Toxi-
cology Program started in January 1986. The animal
exposure has been completed and results will be published
as soon as the pathology review is completed.
8.3.3. Long-term dermal toxicity
No long-term dermal toxicity studies have been
reported.
8.3.4. Principal effects and target organs
Repeated exposure of several animal species to levels
of HEX vapour in the range of 1.13 to 2.26 mg/m3 (0.1-0.2
ppm) has been found to cause pulmonary degenerative
changes (Treon et al., 1955; D.G. Clark et al., 1982; Rand
et al., 1982a,b). In addition, Treon et al. (1955)
reported diffuse degeneration of the brain, heart, and
adrenal glands and necrosis of the liver and kidney
tubules, together with severe pulmonary hyperaemia and
oedema. In many instances, acute bronchitis and intersti-
tial pneumonitis also occurred. Necrosis of the epithelium
of the primary, secondary, and tertiary bronchi was
observed. At later stages, reacting inflammatory cells
migrated into the wall and the mucosa of the bronchi and
alveoli. In rabbits, the walls of the alveoli were covered
by a hyaline or fibrinoid membrane. Possibly, some of the
changes found by Treon et al. (1955) were caused by
impurities in the HEX preparation. Acute exposure by the
oral and dermal routes also affects the respiratory system
(Kommineni, 1978; SRI, 1980a). Death from acute exposure
by any tested route seemed to be associated with respir-
atory failure (Lawrence & Dorough, 1982).
There are insufficient data to identify the site that
is the most sensitive to prolonged, repeated exposure to
HEX. In comparing routes of administration, researchers
found that damage to the lungs occurred regardless of
which route was used (Lawrence & Dorough, 1982). When HEX
is administered orally to animals, the kidneys may be the
most sensitive site, since short-term dosing of rats and
mice was found to cause nephrosis, especially in females
(SRI, 1981a,b). Although the oral route may not be a
significant route of exposure for human beings, the fact
that the kidneys are a possible target organ in short-term
exposure indicates that low-level, prolonged systemic
exposure from any ambient route may affect the kidneys.
The liver has also been shown, in several of the labora-
tory studies, to be affected by HEX.
The available literature does not cite any single
mechanism to explain HEX toxicity. HEX vapour irritates
the respiratory tract, leading to death by respiratory
failure after bronchopneumonia (D.G. Clark et al., 1982).
The degenerative changes that have been observed in the
liver and kidneys are mild and unlikely to contribute to
the chemical's lethality (NAS, 1978; SRI, 1980a,b).
The difficulty in studying HEX because of its high
reactivity and volatility has also created problems in
identifying its metabolites. Several questions remain as
to whether the same metabolites are formed after various
routes of exposure and whether it is the administered HEX
or its metabolites that cause the lung injuries seen with
various dosing regimens. Furthermore, the strong ability
of HEX to interact with other compounds, especially
organic molecules, can lead to many other effects, such as
haemoglobin binding. Little is known about the interac-
tions of HEX with other chemicals in animal or human
tissue.
8.4. Developmental and reproductive toxicity
The teratogenic potential of HEX has been evaluated in
pregnant Charles River CD-1 rats that were administered
HEX (98.25%) in corn oil, by gastric intubation, at dose
levels of 3, 10, and 30 mg/kg per day from days 6 to 15 of
gestation. A control group received the vehicle (corn oil)
at a dose volume of 10 ml/kg per day. All the rats sur-
vived, and there was no difference in mean maternal body
weight gain between the dosed groups and controls. There
were no differences in the mean number of implantations,
corpora lutea, live fetuses, mean fetal body weights, or
male/female sex ratios among any of the groups, and there
were no statistical differences in malformation or devel-
opmental variations, compared with the controls, when
external, soft tissue, and skeletal examinations were
performed (IRDC, 1978).
Murray et al. (1980) evaluated the teratogenic poten-
tial of HEX (98%) in CF-1 mice and New Zealand white
rabbits. Mice were dosed at 0, 5, 25, or 75 mg HEX/kg per
day by gavage during days 6-15 of gestation, while rabbits
received the same dose during days 6-18 of gestation. The
fertility of the treated mice and rabbits was not signifi-
cantly different from that of the control groups. In the
mice, there was no evidence of maternal toxicity, embryo-
toxicity, or teratogenic effects. A total of 249-374
fetuses (22-33 litters) was examined in each dose group.
In rabbits, maternal toxicity was noted at a dose level of
75 mg/kg (diarrhoea, weight loss, and mortality), but
there was no evidence of maternal toxicity at the lower
levels. There were no embryotoxic effects at any dose
level. Although there was a two-fold increase over con-
trols in the proportion of fetuses with 13 ribs at 75
mg/kg, this was considered to be a minor skeletal vari-
ation. The authors concluded that HEX was not teratogenic
at the levels tested (Murray et al., 1980).
Chernoff & Kavlock (1982) tested 28 compounds, includ-
ing HEX, by an in vivo screening procedure. According to
the researchers, the underlying hypothesis was that most
prenatal insults would manifest themselves postnatally as
reduced viability and/or impaired growth. Twenty-five
Oravid CD-1 mice were administered HEX orally at or near
the maternal minimal tolerated dose (MTD). The MTD was
considered to be that dose resulting in either significant
weight reduction during the treatment period, mortality,
or other signs of toxicity. There were no differences in
maternal weight gain, number of live offspring or average
weight between the HEX-treated animals and controls when
HEX was administered orally for 8-12 days at 45 mg/kg.
Gray & Kavlock (1984) extended the observation period
proposed by Chernoff & Kavlock (1982, 1983) to 250 days to
determine whether neonatal weight reductions persisted
throughout life and whether other serious abnormalities or
mortality resulted from exposure to HEX. CD-1 pregnant
mice were orally exposed to HEX (45 mg/kg) on days 8 to 12
of gestation, which is within the period of major embryo-
nic organogenesis. Females were weighed throughout dosing
and on day 19 of gestation. They were allowed to deliver
and the litters were counted and weighed at 1 and 3 days
of age. The animals were observed at approximately 250
days of age. During the postmortem examination of males,
body weight and the weights of the liver, testes, seminal
vesicles, and right kidney were recorded. HEX did not
produce any statistically significant developmental
effects in this study.
Studies on the teratogenic potential of inhaled HEX
were not found in the review of the scientific literature.
8.5. Mutagenicity
Goggelman et al. (1978) found that HEX was not muta-
genic, either before or after liver microsomal activation,
at 2.7 mmol/litre in an Escherichia coli K12 back-
mutation system. In this test there was a 70% survival of
bacteria at 72 h. HEX was not tested at higher concen-
trations because it was cytotoxic to E. coli. A previous
report from the same laboratory (Greim et al., 1977) indi-
cated that HEX was also non-mutagenic in Salmonella
typhimurium strains TA1535 (base-pair mutant) and TA1538
(frame-shift mutant) after liver microsomal activation.
However, no details of the concentrations tested were
given. Although tetrachlorocyclopentadiene is mutagenic
in these systems, probably through metabolic conversion to
the dienone, it appears that the chlorine atoms at the C-1
position of HEX hinder metabolic oxidation to the corre-
sponding acylating dienone (Greim et al., 1977).
A study conducted by the Industrial Bio-Test Labora-
tories (IBT, 1977) also suggested that HEX is not muta-
genic in S. typhimurium. Both liquid HEX and its vapour
were tested with and without metabolic activation. The
vapour test was carried out in desiccators with the TA100
strain of S. typhimurium only. It is not clear from
vapour test data that sufficient amounts of HEX or
adequate exposure times (30, 60, and 120 min) were used.
Longer exposures in the presence of HEX vapour may be
necessary for a potential mutagenic effect to be seen.
At concentrations of up to 1.25 mg/litre in the
presence of an S-9 liver activating system, HEX was not
mutagenic in the mouse lymphoma mutation assay. Mutageni-
city could not be evaluated at higher concentrations
because of the cytotoxicity of HEX (Litton Bionetics,
Inc., 1978a). This assay uses L5178Y cells that are het-
erozygous for thymidine kinase (TK+/-) and are sensitive.
The mutation is scored by cloning with bromodeoxyuridine
in the absence of thymidine. HEX is highly toxic to these
cells, particularly in the absence of an activating system
(at 0.04 ml/litre), and the positive control, dimethyl-
nitrosamine, was mutagenic at 0.5 ml/litre.
Williams (1978) found that HEX (10-6 mol/litre) was
inactive in the liver epithelial culture (hypoxanthine-
guanine-phosphoribosyl transferase locus) mutation assay.
At 10-5 mol/litre it also failed to stimulate DNA repair
in hepatocyte primary cultures. Negative results were also
obtained in an additional unscheduled DNA synthesis assay
(Brat, 1983).
One study provided by the US National Toxicology
Program (NTP) (Haworth et al., 1983) demonstrated a lack
of mutagenicity of HEX (98% pure). In S. typhimurium
strains TA98, TA100, TA1535, and TA1537, levels of up to
3.3 µg/plate were not mutagenic without activation, and
levels of up to 100 µg/plate were not mutagenic after
microsomal activation. Higher levels could not be tested
because of excessive cell dealth. Zimmering et al. (1985)
tested Drosophila by the sex-linked recessive lethal test
(SLRL), either by feeding doses of 40 ppm for 3 days or by
giving a single injection of 2000 ppm or 3000 ppm (volume
not specified). The vehicle used was 10% ethyl alcohol,
which did not totally dissolve the HEX. HEX (98%) was
first assayed in the SLRL test in adult feeding exper-
iments. When negative results were obtained, the chemical
was retested by injecting < 1 day-old Canton-S wild-type
Drosphila males. The results of the injection test were
reported to be inconclusive.
HEX has also been assayed in the mouse dominant lethal
test (Litton Bionetics, Inc., 1978b). In this assay, 0.1,
0.3, or 1.0 mg HEX/kg was administered by gavage to 10
male CD-1 mice for 5 days and the mice were then mated
throughout spermatogenesis (7 weeks). This test determines
whether the compound induces lethal genetic damage to the
germ cells of males. There was no evidence of dominant
lethal activity, at any dose level, based on any parameter
(e.g., fertility index, implantations per pregnancy,
average resorptions per pregnancy).
8.6. Cell transformation
The ability of HEX to induce morphological transfor-
mation of BALB/3T3 cells in vitro has been studied by
Litton Bionetics, Inc. (1977).
The selection of test doses was based on previous
cytotoxicity tests using a wide range of HEX concen-
trations at 0.0, 0.01, 0.02, 0.039, 0.078, and 0.156 mg
per litre. The cultures were exposed for 48 h, which was
followed by an incubation period of 3-4 weeks. The
cultures were observed daily. The doses selected allowed a
cell survival of 80-100% compared with controls (solvent
only). This high survival rate permitted an evaluation of
in vitro malignant transformation in cultures treated
with HEX as compared with the solvent controls. 3-Methyl-
cholanthrene at a dose level of 3 mg/litre was used as a
positive control. Results indicated that HEX was not
responsible for cell transformation.
8.7. Carcinogenicity
Bioassays of HEX for possible carcinogenicity have not
been conducted. As noted previously (section 8.3.2), the
NTP has completed a study on HEX for carcinogenicity by
the inhalation route in rats and mice, but the results
were not available when this monograph was being prepared.
9. EFFECTS ON HUMANS
9.1. General population exposure
There is very little detailed information available on
the human health effects of HEX exposure. Acute human
exposure has been reported in homes near waste sites where
disposal of HEX has occurred (C.S. Clark et al., 1982;
Elia et al., 1983). The odour threshold has been stated
to be 1.92 µg/m3 (0.17 ppb), but there appears to be
great individual variation. According to a study completed
by the A.D. Little Co. for the Occidental Chemical
Corporation, the 100% panel recognition concentration was
1.92 µg/m3 (0.17 ppb v/v)a, but the study design and
methodology were not reported. The US EPA (1982) estimated
that exposure of the general population to HEX in air
and/or water would be extremely low.
Treon et al. (1955) reported that members of a
research group conducting toxicity tests developed head-
aches when they were accidentally exposed to unknown
concentrations of HEX. The HEX escaped into the room when
an aerated exposure chamber was opened.
In a 48-block area surrounding a contaminated sewer
line in Kentucky, USA, questionnaires were sent to a
selected sample of residents. A total of 212 occupants
were surveyed. Only 3.8% of the residents reported an
unusual odour. The most common symptoms were stomach aches
(5.2%), burning or watery eyes (4.7%), and headaches
(4.7%). There was no association between the frequency of
symptoms and the distance of houses from the contaminated
sewer line. No significant ambient air concentrations of
HEX were found in these areas (Kominsky et al., 1978).
9.2. Occupational exposure
The US National Institute for Occupational Safety and
Health (NIOSH, 1980) stated that 1427 workers were occu-
pationally exposed to HEX. Officials from the Velsicol
Chemical Corporation estimated that 157 employees had been
potentially exposed to HEX in their production and pro-
cessing facilities.
A well-documented incident of acute human exposure to
HEX occurred in March 1977 at the Morris Forman Wastewater
Treatment Plant in Louisville, Kentucky, USA (Wilson et
al., 1978; Morse et al., 1979; Kominsky et al., 1980). The
details of the incident are included in the original NIOSH
Hazard Evaluation and Technical Assistance Report Number
TA-77-39 (Kominsky et al., 1978), which is available from
the US National Technical Information Service (NTIS). This
--------------------------------------------------------
a Memorandum from G. Leonardos to P. Levins on Hooker
special priority samples (odour properties).
treatment facility was contaminated with approximately 6
tonnes of HEX and octachlorocyclopentene, a waste product
of HEX manufacture (Morse et al., 1979). The contamination
was traced to an illegal dumping in one large sewer line
that passed through several populated areas. Concen-
trations of HEX detected in the sewage at the plant were
as high as 1000 mg/litre, and levels in the sewer line
were up to 100 mg/litre. Air samples taken from the sewer
line showed HEX concentrations to be as high as 4.5
mg/m3 (400 ppb). Although the airborne concentrations of
HEX at the time of the exposure in the treatment facility
were not known, airborne concentrations in the primary
treatment areas (screen and grit chambers) ranged between
3.05 and 10.96 mg/m3 (270 and 970 ppb) 4 days after the
plant had closed. It should be noted that the ACGIH
8 h-TWA for HEX was 0.1 mg/m3 (10 ppb) in 1977. Workers
tried to remove an odoriferous and sticky substance from
the bar screens and grit collection system by using steam
during clean-up of the contamination. This procedure pro-
duced a blue haze which permeated the primary treatment
area. The airborne HEX concentration of the blue haze was
reported to be 217 µg/m3 (19.2 ppm) (Kominsky et al., 1980).
The US Centers for Disease Control (CDC) and NIOSH
sent representatives to the plant with questionnaires
about the type and duration of symptoms (Morse et al.,
1979; Kominsky et al., 1980). In all, 193 employees were
identified as having been potentially exposed for 2 or
more days during the 2 weeks before the plant was closed
(Morse et al., 1979). The questionnaire was sent to each
of these 193 workers and 145 (75%) responded. Workers with
complaints of mucous membrane irritation were given a
physical examination, and blood and urine samples were
collected for clinical screening by an independent labora-
tory. Data were also collected on the exposure levels and
symptoms experienced by several people who had been
acutely exposed to the chemical vapours.
The results of the CDC and NIOSH questionnaires showed
that the odour of HEX had been detected by 94% of the
workers before the onset of symptoms. The most common
symptoms reported were eye irritation (59%), headaches
(45%), and throat irritation (27%) (Table 18). Of the 41
plant workers examined, six had physical signs of eye
irritation (i.e. lacrimation or redness) and five had
signs of skin irritation. Laboratory analyses of blood
and urine specimens from these workers showed marginal
increases in lactic dehydrogenase activity in 27% of cases
and proteinuria in 15%. Three weeks later, no abnormali-
ties were detected in the blood and urine tests. After six
weeks, some of the clinical symptoms persisted in 25-45%
of the employees (Morse et al., 1978).
Table 18. Symptoms of 145 waste-water treatment
plant employees exposed to HEX (Louisville,
Kentucky, USA, March 1977)a
----------------------------------------------------
Symptom No. of Percent of
employees employees
with symptoms with symptoms
----------------------------------------------------
Eye irritation 86 59
Headache 65 45
Throat irritation 39 27
Nausea 31 21
Skin irritation 29 20
Cough 28 19
Chest pain 28 19
Difficult breathing 23 16
Nervousness 21 14
Abdominal cramps 17 12
Decreased appetite 13 9
Decreased memory 6 4
Increased saliva 6 4
----------------------------------------------------
a From: Morse et al. (1978).
Although there was difficulty in measuring the level
of exposure received by the plant workers, more than 50%
of the clean-up crew were monitored. Laboratory tests
showed no significant abnormalities in renal function
tests, complete blood counts, or urinalysis, but several
minimal or mild abnormalities were found in liver function
tests (Kominsky et al., 1980). The abnormalities in 18 out
of 97 clean-up workers are listed in Table 19. These
people also had physical signs of mucous membrane irri-
tation. A more detailed correlation between acute exposure
level data and symptomatology was reported for nine adults
(Kominsky et al., 1980). The data appear in Table 20. The
exposure levels could not be estimated accurately because
of prior exposure or because the worker had used protec-
tive equipment.
Table 19. Abnormalities detected in clean-up
workers at the Morris Forman treatment plant,
Louisville, Kentucky, USAa
-------------------------------------------------
Laboratory test Normal range Abnormal results
Range No.b
-------------------------------------------------
Serum glutamate
oxaloacetate 7-40 mU/ml 40-49 5
transaminase 50-59 1
60-69 4
70-79 0
80-89 1
90-99 1
Serum alkaline 30-100 mU/ml 100-109 3
phosphatase 110-119 1
120-129 1
Serum total 0.15-10 mg/dl 1.0-1.9 1c
bilirubin
Serum lactate 100-225 mU/ml 230-239 1
dehydrogenase
-------------------------------------------------
a From: Kominsky et al. (1980).
b For individuals with more than one serial
blood test, only the most abnormal result is
tabulated.
c Associated with a serum glutamate oxaloacetate
transaminase activity of 66 mU/ml.
Table 20. Individual exposure symptomatology correlations at the Morris Forman treatment plant (Louisville, Kentucky, USA)a
-------------------------------------------------------------------------------------------------------------------------------------
No. of Estimated airborne Immediate symptoms Persistence of symptoms Laboratory
workers exposureb results
-------------------------------------------------------------------------------------------------------------------------------------
1 19.2 ppm HEX and 650 ppb lacrimation; skin irritation on face 1.5 h post-exposure: fatigue; normal results
OCCP for several seconds and neck; dyspnoea and chest erythema of exposed skin; eye 4 days
(no protective equipment) discomfort;nausea (several min later) irritation subsided in 1 day; post-exposurec
chest discomfort persisted
several days
3 7083 ppb HEX and 446 ppb lacrimation; irritation of exposed asymptomatic at 2 h, except normal results
OCCP for several seconds skin for soreness around eyes 7 days post-
(half-face respirator) exposure in
one workerc
2 40-52 ppb HEX and slight eye irritation no symptoms after cessation of normal results
9-21 ppb OCCP exposure 7 days post-
(half-face respirator) exposure in one
workerc
2 exact exposure unknown slight skin irritation faces felt "puffy" and "windburned" none available
(half-face respirator) for 1-2 days after exposure; this
was noted also by friends and
family; no residual skin lesions.
1 980 ppb HEX for 15 min; irritated eyes; nasal irritation eyes felt "dry and irritated" for none available
OCCP not measured and sinus congestion after 2 weeks 2-3 days after exposure; nasal
(no protective equipment) of intermittent exposures irritation ceased within 1-2 days
of cessation of exposure.
-------------------------------------------------------------------------------------------------------------------------------------
a From: Kominsky et al. (1980).
b OCCP = Octachlorocyclopentene.
c Laboratory work was same as carried out on clean-up crew.
Hazards to workers in treatment plants can result from
chemical compounds contained in the industrial waste
treated in municipal waste-water plants. The HEX-contain-
ing wastes from a pesticide manufacturer were treated in a
municipal waste-water treatment plant at Memphis,
Tennessee, USA. In 1978, the workers at this plant
reported symptoms similar to those reported by workers in
the Louisville plant referred to above. The air and waste-
water were monitored, and analyses of urine and blood,
liver function tests, and illness symptom questionnaires
were completed. Workers from another waste treatment
plant, where no pesticide wastes had been received, were
used as a control group. No statistical differences in
urine HEX concentrations or liver function tests between
the exposed and control groups were found, although
differences in levels of HEX-related compounds (co-
contaminants) were detected (Elia et al., 1983).
9.3. Epidemiological studies
Mortality studies have been carried out on workers
involved in the production of HEX or formulation of HEX
products. Shindell et al. (1980) studied a cohort of 783
current and former workers who had been employed at the
Velsicol Chemical Corporation plant at Marshall, Illinois,
between 1946 and 1979. The purpose of the study was to
evaluate the overall health status of all employees who
had been present during the manufacture of chlordane for 3
months or more. There were no significant differences in
mortality rates between these employees and the overall
USA population. The observed value for deaths from all
causes, including heart disease and cancer, was less than
the expected value in the overall USA population.
Shindell et al. (1981) completed another epidemiologi-
cal study for the Velsicol Chemical Corporation at its
Memphis, Tennessee, plant covering the period 1952-1979.
This coincided with the manufacture of heptachlor, a
pesticide made from HEX. The reseachers studied 1115
current and former employees who had worked for 3 months
or more. Again, there was no significant difference in
mortality between the control and exposure groups.
Concomitant with the study performed by Shindell et
al. (1980), Wang & MacMahon (1979) conducted a retrospec-
tive mortality study of workers employed at the Marshall
and Memphis plants, where chlordane and heptachlor were
manufactured between 1946 and 1976. They studied 1403
males who had worked at the plants for more than 3 months.
There were 113 observed deaths compared with 157 expected
deaths, giving a standardized mortality ratio (SMR) of 72.
Among the various causes of death, the two highest SMRs
were 134 for lung cancer and 183 for cerebrovascular
disease, but only the latter figure was statistically sig-
nificant (P < 0.05). The excess mortality due to cerebro-
vascular disease was not related to the duration of
exposure or to the latency period, and occurred only after
termination of employment. Shindell & Ulrich (1986)
updated their 1980 data set with additional worker data.
There was no information specific to HEX exposure.
Buncher et al. (1980) studied the mortality of workers
at a chemical plant that produced HEX. They examined 341
workers (287 male and 54 female), together with their
health records, who had worked at the plant for at least
90 days between 1 October 1953 and 31 December 1974. Their
vital status was determined through 1978. The expected
numbers of deaths, based on the USA population and
specific for sex, age, and calendar year, were calculated.
The SMR for all causes of death was 69. Deaths caused by
specific cancers, all cancers, and diseases of the circu-
latory and digestive systems were also fewer than
expected. The authors noted that the time that had elapsed
since the initial exposure (25 years at most) reduced the
power of the study to detect cancers that may have a
10-40 year latent period.
Similar studies have been performed on a cohort of
workers in the Shell International Petroleum plants in the
Netherlands. These reports have been reviewed extensively
in Environmental Health Criteria 91: Aldrin and Dieldrin
(WHO, 1989).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
The general population is not at risk from exposure to
HEX. However, people living near HEX processing and pro-
duction facilities, as well as handlers of the chemical
and its waste, are at risk. Exposure to HEX can occur
through several different routes. However, HEX is much
more toxic when inhaled than when ingested or following
dermal contact. Skin exposure studies have shown that HEX
can cause irritation, together with visceral changes that
are similar to those that result from oral administration.
Inhalation studies in animals have shown that HEX vapour
is very irritating, repeated exposure to 1.13-2.26 mg/m3
(0.1-0.2 ppm) causing pulmonary pathological changes.
Acute toxic symptoms, including headaches, nausea, dizzi-
ness, and respiratory distress, have been reported. Based
on a 90-day inhalation study in mice and rats, a NOEL of
0.45 mg/m3 (0.04 ppm) has been estimated. No information
is available on the long-term effects of a single exposure
or of continuous exposure to HEX.
A carcinogenic study of HEX has been completed (but
not evaluated) by the US National Toxicology Program. In
vitro mutagenicity and cell transformation tests yielded
negative results, as did an in vivo mouse dominant lethal
assay at the levels tested. There was no evidence of
teratogenicity in oral exposure studies in which three
species were examined.
The toxic effects of HEX exposure on the human respir-
atory system are of major concern. Although the long-term
toxicity data are limited, systemic toxic effects of HEX
inhalation have been observed after short-term exposure,
suggesting that long-term inhalation exposure to low con-
centrations of HEX could cause adverse health effects.
Limited epidemiological studies of workers exposed to HEX
at levels above those known to induce adverse health
effects have been conducted, although concomitant exposure
to other chemicals was known to have occurred. These
workers complained of headaches, eye and skin irritation,
nausea, dizziness, and respiratory distress, as did
individuals living near areas where there were HEX
releases.
10.2. Evaluation of effects on the environment
Release of HEX into the environment can result from
the production, processing and use of HEX, disposal of
waste containing HEX or from products contaminated with
HEX. Only a small proportion of the total amount of HEX
released into the environment from production, processing,
and use can be expected to persist beyond a few days.
However, waste disposal has resulted in HEX persisting in
soil, sediment, and ground water. There is little monitor-
ing information on the levels of HEX in air, water, and
sediment. The exposure of organisms in the aquatic
environment to HEX is therefore difficult to quantify.
HEX may undergo photolysis, hydrolysis, and biodegra-
dation. In water, photolysis is the dominant process in
direct sunlight, hydrolysis being the next most important
degradation route. Volatilization to the atmosphere occurs
from both water and soil. Biodegradation occurs in soil
under both aerobic and anaerobic conditions and in sewage
sludge. In water and aquatic sediment, biodegradation is
initially limited. Information on the adaptation of micro-
organisms to degrade HEX is limited. HEX has a calculated
tropospheric residence time of approximately 5 h.
Laboratory studies suggest that HEX is relatively
immobile in soil, particularly in soil with a high organic
content. However, leaching and movement in ground water
has been reported in field studies.
HEX has been shown to be toxic to aquatic life at a
level of 1-100 µg/litre. However, little information has
been obtained under field conditions (long-term exposure
at low concentration in the presence of sediment). The
actual hazard to aquatic life is, therefore, difficult to
assess. HEX is toxic to aquatic microorganisms but less
toxic to soil microorganisms. Exposure of terrestrial
organisms, except at or near disposal sites, would be
expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
* The general population is not at risk from exposure
to HEX, except in the case of people residing near
contaminated areas.
* The long-term human health effects of continuous low-
level exposure are not known. Handlers of the product
and its waste, as well as sewage workers, are at
risk.
* Results of laboratory studies indicate that HEX
should be degraded rapidly in the environment by
photolysis, hydrolysis, or biodegradation. The rela-
tive importance of these processes varies with the
medium. HEX is not a widespread environmental con-
taminant and the available data suggest that it is
only found associated with production, processing and
disposal sites. HEX does not bioaccumulate.
* Acute laboratory tests show that HEX is highly toxic
to aquatic microorganisms, invertebrates, and fish,
but less toxic to soil microorganisms. However,
information obtained under environmentally realistic
conditions is limited. The potential hazard to the
general environment is expected to be low.
11.2. Recommendations for protection of human health and
the environment
* Occupational exposure to HEX should be minimized by
the use of closed systems. Guidelines for the dis-
posal of HEX and HEX wastes should be followed.
* Environmental monitoring is needed to examine the
persistence and fate of HEX in all media near pro-
duction, processing and disposal sites, and also
hazardous waste incinerators. Monitoring data are
required for HEX in drinking-water, and in surface,
shower, and ground water.
12. FURTHER RESEARCH
* Biomarker technology should be developed to indicate
the possibility of past or current actions of HEX.
Such biomarkers could be stable metabolites derived
from HEX and its impurities that are present in the
original preparation.
* Research is needed on the metabolic, degradative, and
reactive products to understand the fate of HEX in
human beings and the environment.
* Further study of the apparent disparity between degra-
dation under laboratory conditions and that observed
in the environment is needed.
* The efficacy and safety of current disposal methods
should be evaluated and their present and future
health impacts assessed.
* Developmental and reproductive studies of HEX need to
be conducted, with emphasis on the inhalation route of
exposure.
* Methods for the early warning of the presence of low
levels of HEX should be developed.
REFERENCES
ABDO, K.M., MONTGOMERY, C.A., KLUWE, W.M., FARNELL, D.R., & PREJEAN, J.D.
(1984) Toxicity of hexachlorocyclopentadiene: subchronic (13-week)
administration by gavage to F344 rats and B6C3F1 mice. J. appl. Toxicol.,
4(2): 75-81.
ALEXANDER, D.J., CLARK, G.C., JACKSON, G.C., HARDY, C.J., STREET, A.E.,
HEYWOOD, R.H., BUIST, D., PRENTICE, D.E., & ISAACS, K.R. (1980)
Subchronic inhalation toxicity of hexachlorocyclopentadiene in monkeys
and rats, Huntingdon, Huntingdon Research Centre, 373 pp (Report
VCL14M/791081) (Prepared for Velsicol Chemical Corporation, Chicago).
ATALLAH, Y.H., WHITACRE, D.M., & BUTZ, R.G. (1980) Fate of
hexachlorocyclopentadiene in the environment. Paper presented at the 2nd
Chemical Congress of the North American Continent, Las Vegas, Nevada, 29
August, 1980, Chicago, Velsicol Chemical Corporation.
BELL, M.A., EWING, R.A., & LUTZ, G.A. (1978) Reviews of the environmental
effects of pollutants. XII. Hexachlorocyclopentadiene, Washington, DC, US
Environmental Protection Agency (Report EPA-600/1-78-047) (NTIS PB 80-
122963).
BENNETT, B. (1982) Hexachlorocyclopentadiene analyses of Mississippi
river fish, Athens, Georgia, US Environmental Protection Agency, Region
IV (Unpublished report).
BENOIT, F.M. & WILLIAMS, D.T. (1981) Determination of
hexachlorocyclopentadiene at the nanogram per liter level in drinking
water. Bull. environ. Contam. Toxicol., 27: 303-308.
BOYD, K.W., EMORY, M.B., & DILLON, H.K. (1981) Development of personal
sampling and analytical methods for organochlorine compounds. In:
Chemical hazards in the workplace, Washington, DC, American Chemical
Society, pp. 49-64 (ACS Symposium Series No. 149).
BRAT, S.V. (1983) The hepatocyte primary culture/DNA repair assay on
compound hexachlorocyclopentadiene using rat hepatocytes in culture,
Valhalla, New York, American Health Foundation, Naylor Dana Institute for
Disease Prevention.
BRIGGS, G.G. (1973) A simple relationship between soil adsorption of
organic chemicals and their octanol/water partition coefficients. In:
Proceedings of the 7th British Insecticide and Fungicide Conference,
Brighton, England, 19-22 November, 1973, Croydon, British Crop Protection
Council, Vol. 1, pp. 83-86 (Research Report).
BUA (BERATERGREMIUM FUR UMWELTRELEVANTE ALTSTOFFE) (1988)
[Hexachlorocyclopentadiene] Weinheim, Germany, VCH Verlagsgesellschaft
(BUA Report No. 25) (in German).
BUCCAFUSCO, R.J. & LEBLANC, G.A. (1977) Acute toxicity of
hexachlorocyclopentadiene to bluegill (Lepomis macrochirus), channel
catfish (Ictalurus punctatus), fathead minnow (Pimephales promelas), and
the water flea (Daphnia magna) (Unpublished report prepared for Velsicol
Chemical Corporation, Chicago).
BUNCHER, C.R., MOOMAW, C., & SIRKOSKI, E. (1980) Mortality study of
Montague Plant-Hooker Chemical, Cincinnati, Ohio, University of
Cincinnati Medical Center, Division of Epidemiology and Biostatistics
(Unpublished report prepared for Hooker Chemical Corporation).
BUTZ, R.G. & ATALLAH, Y.H. (1980) Effects of hexachlorocyclopentadiene on
three microbial functions (VCC Project No. 482428, Report No. 8)
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
BUTZ, R.G., YU, C.C., & ATALLAH, Y.H. (1982) Photolysis of
hexachlorocyclopentadiene in water. Ecotoxicol. environ. Saf., 6: 347-
357.
CALLAHAN, M.A., SLIMAK, M.W., GABEL, N.W., MAY, I.P., FOWLER, C.F.,
FREED, J.R., JENNINGS, P., DURFEE, R.L., WHITMORE, F.C., MAESTRI, B.,
MABEY, W.R., HOLT, B.R., & GOULD, C. (1979) Water-related environmental
fate of 129 priority pollutants: II. Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic
aromatic hydrocarbons, nitrosamines, miscellaneous compounds, Washington,
DC, US Environmental Protection Agency, Monitoring and Data Supervision
Division, Office of Water Planning and Standards (Report EPA-440/4-79-
029b).
CARTER, M.R. (1977) Legal affidavit field in the State of Georgia, Fulton
County, dated 14 June, 1977. Testimony concerning estimates of total
daily discharge of HEX from Velsicol Chemical Corporation, Atlanta,
Athens, Georgia, US Environmental Protection Agency.
CHERNOFF, N. & KAVLOCK, R.J. (1982) An in vivo teratology screen
utilizing pregnant mice. J. Toxicol. environ. Health, 10: 541-550.
CHERNOFF N. & KAVLOCK, R.J. (1983) A teratology test system which
utilizes postnatal growth and viability in the mouse. In: Waters, M.,
Sandhu, S., Lewtas, J., Claxton, L., Chernoff, N., & Nesnow, S., ed.
Short-term bioassays in the analysis of complex mixtures III, New York,
London, Plenum Publishing Corporation, pp. 417-427.
CHOPRA, N.M., CAMPBELL, B.S., & HURLEY, J.C. (1978) Systematic studies on
the breakdown of endosulfan in tobacco smokes: Isolation and
identification of the degradation products from the pyrolysis of
endosulfan I in a nitrogen atmosphere. J. agric. food Chem., 26: 255-258.
CHOU, S.F.J. & GRIFFIN, R.A. (1983) Soil, clay, and caustic soda effects
on solubility, sorption and mobility of hexachlorocyclopentadiene,
Springfield, Virginia, National Technical Information Service, 54 pp (PB
84-116060) (Environmental Geology Notes No. 104).
CHOU, S.F.J., GRIFFIN, R.A., CHOU, M.M, & LARSON, R.A. (1987) Products of
hexachlorocyclopentadiene (C-56) in aqueous solution. Environ. Toxicol.
Chem., 6: 371-376.
CLARK, C.S., MEYER, C.R., GARTSIDE, P.S., MAJETI, V.A., & SPECKER, B.
(1982) An environmental health survey of drinking water contamination by
leachate from a pesticide waste dump in Hardeman County, TN. Arch.
environ. Health, 37(1): 9-18.
CLARK, D.G., BLAIR, D., MARTIN, J., HENDY, R., PILCHER, A., & WIGGINS, D.
(1982) Thirty-week chronic inhalation study of hexachlorocyclopentadiene
(HEX) in rats, Tunstall, United Kingdom, Shell Toxicology Laboratory
(Experiment No. 1760, Report No. SBGR.81) (Permission for use granted by
M.J. Sloan, Shell Oil Co., Washington).
COLE, E.J. (1953) Chemotherapeutic and pharmacologic aspects of
hexachlorocyclopentadiene, Laramie, University of Wyoming, Department of
Veterinary Sciences and Bacteriology (Master's Thesis).
COLE, E.J. (1954) Treatment of sewage with hexachlorocyclopentadiene.
Appl. Microbiol., 2: 198-199.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment, Research Triangle Park, North Carolina, US Environmental
Protection Agency (Report EPA-600/3-80-084).
DAL MONTE, R.P. & YU, C.C. (1977) Water solubility of MC-984 and hex
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DELEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, P.C., REMELE, P.C.,
STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980a) Rapid gas
chromatographic method for the determination of volatile and semivolatile
organochlorine compounds in soil and chemical waste disposal site
samples. J. chromatogr. Sci., 18: 85-88.
DELEON, I.R., BROWN, N.J., COCCHIARA, J.P., MILES, S.K., & LASETER, J.L.
(1980b) Determination of trace levels of hexachlorocyclopentadiene and
octachlorocyclopentene in body fluids. J. anal. Toxicol., 4: 314-317.
DILLON, H.K. (1980) Development of air sampling and analytical methods
for toxic chlorinated organic compounds, Birmingham, Alabama, Southern
Research Institute, 76 pp (NTIS PB 80-193279).
DOROUGH, H.W. (1979) The accumulation, distribution and dissipation of
hexachlorocyclopentadiene (C56) in tissues of rats and mice, 27 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. (1980) Disposition of 14C-hexachlorocyclopentadiene (C56)
in rats following inhalation exposure, 53 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. & RANIERI, T.A. (1984) Distribution and elimination of
hexachlorocyclopentadiene in rats and mice. Drug chem. Toxicol., 7(1):
73-89.
EICHLER, D.L. (1978) Quantitative analyses of mixtures containing trace
amounts of pesticides. Internal memorandum to M.R. Zavon, Hooker
Chemicals and Plastics Corporation, Niagara Falls, New York, 3 pp.
EL DAREER, S.M., NOKER, P.E., TILLERY, K.F., & HILL, D.L. (1983)
Investigations on the basis for the differential toxicity of
hexachlorocyclopentadiene administered to rats by various routes. J.
Toxicol. environ. Health, 12: 203-211.
ELIA, V.J., CLARK, C.S., MAJETI, V.A., GARTSIDE, P.S., MACDONALD, T.,
RICHDALE, N., MEYER, C.R., VAN MEER, G.L., & HUNNINEN, K. (1983) Chemical
exposure at a municipal wastewater treatment plant. Environ. Res., 32:
360-371.
EVANS, M.J., CABRAL-ANDERSON, L.J., & FREEMAN, G. (1978) Role of the
Clara cell in renewal of the bronchiolar epithelium. Lab. Invest., 38:
648-655.
GOGGELMAN, W., BONSE, G., HENSCHLER, D., & CREIM, H. (1978) Mutagenicity
of chlorinated cyclopentadiene due to metabolic activation. Biochem.
Pharmacol., 27: 2927-2929.
GRAY, L.E., Jr & KAVLOCK, R.J. (1984) An extended evaluation of an in
vivo teratology screen utilizing postnatal growth and viability in the
mouse. Teratog. Carcinog. Mutagen., 4: 403-426.
GREIM, J., BIMBOES, D., GOGGELMANN, W., & KRAMER, M. (1977) Mutagenicity
and chromosomal aberrations as an analytical tool for in vitro detection
of mammalian enzyme-mediated formation reactive metabolites. Arch.
Toxicol., 39: 159-169.
HAWLEY, G.G., ed. (1977) Condensed chemical dictionary, 9th ed., New
York, Van Nostrand Reinhold Co.
HAWORTH, S., LOWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E. (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen., 5(Suppl. 1): 3-142.
HENDERSON, C. (1956) Bio-assay investigations for International Joint
Commission, Niagara Falls, New York, Hooker Electrochemical Company, 17
pp (Unpublished report).
HUNT, G.E. & BROOKS, G.W. (1984) Source assessment for
hexachlorocyclopentadiene, Research Triangle Park, North Carolina, Radian
Corporation (Unpublished report prepared for the US Environmental
Protection Agency).
IBT (1977) Mutagenicity of PCL-HEX incorporated in the test medium tested
against five strains of S. typhimurium and as a volatilate against tester
strain TA-100, Northbrood, Illinois, Industrial Bio-Test Laboratories.
IRDC (1968) Hexachlorocyclopentadiene and octachlorocyclopentene: Acute
oral toxicity LD50 in male albino rats, Mattawan, Michigan, International
Research and Development Corporation, 4 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
IRDC (1972) Acute toxicity studies in rats and rabbits, Mattawan,
Michigan, International Research and Development Corporation, 21 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
IRDC (1978) Hexachlorocyclopentadiene: Teratology study in rats,
Mattawan, Michigan, International Research and Development Corporation,
17 pp (Unpublished report prepared for Velsicol Chemical Corporation,
Chicago).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic.
Hexachlorocyclopentadiene. In: Patty, F.A., ed. Industrial hygiene and
toxicology, 2nd revised ed., New York, Chichester, Brisbane, Toronto,
John Wiley & Sons, pp. 1333-1363.
IRPTC (1989) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol. environ.
Safety, 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (1980) Relationship between water
solubility, soil sorption, octanol/water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
of Testing and Materials, pp. 78-115 (ASTM STP 707).
KHAN, M.A.Q., SUDERSHAN, P., FEROZ, M., & PODOWSKI, A.A. (1981)
Biotransformations of cyclodienes and their photoisomers and
hexachlorocyclopentadiene in mammals and fish. In: Khan, M.A.Q. &
Stanton, R.H., ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects, Oxford, New York, Pergamon Press, pp. 271-288.
KILZER, L., SCHEUNERT, I., GEYER, H., KLEIN, W., & KORTE, F. (1979)
Laboratory screening of the volatilization rates of organic chemicals
from water and soil. Chemosphere, 8: 751-761.
KLOSKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981) Laboratory
screening of distribution, conversion and mineralization of chemicals in
the soil-plant-system and comparison to outdoor experimental data.
Chemosphere, 10: 1089-1100.
KOMINSKY, J.R. & WISSEMAN, C.L. (1978) Morris Forman wastewater treatment
plant, Metropolitan Sewer District, Louisville, KY, Cincinnati, Ohio,
National Institute of Occupational Safety and Health (NIOSH Hazard
Evaluation and Technical Assistance Report No. TA 77-39) (NTIS PB 82-
178088).
KOMINSKY, J.R., WISSEMAN, C.L., & MORSE, D.L. (1980)
Hexachlorocyclopentadiene contamination of a municipal wastewater
treatment plant. Am. Ind. Hyg. Assoc. J., 41: 52.
KOMMINENI, C. (1978) Pathology reports. Animal portion of the Louisville
sewage study, Cincinnati, Ohio, National Institute of Occupational Safety
and Health (NIOSH Report No. TA 77-39).
LAWLESS, E.W., VON RUMKER, R., & FERGUSON, T.L. (1972) The pollution
potential in pesticide manufacturing, Springfield, Virginia, US
Department of Commerce, National Technical Information Service (NTIS PB
213782/3) (Prepared for the US Environmental Protection Agency by Midwest
Research Institute).
LAWRENCE, L.J. & DOROUGH, H.W. (1981) Retention and fate of inhaled
hexachlorocyclopentadiene in the rat. Bull. environ. Contam. Toxicol.,
26: 663-668.
LAWRENCE, L.J. & DOROUGH, H.W. (1982) Fate of inhaled
hexachlorocyclopentadiene in albino rats and comparison to the oral and
iv routes of administration. Fundam. appl. Toxicol., 2: 235-240.
LEVIN, A.A. (1982a) Hexachlorocyclopentadiene: Response to issues raised
at US EPA Test Rules Meeting of March 17, 1982, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Docket No.
40-8249078).
LEVIN, A.A. (1982b) Hexachloropentadiene: Follow-up and additional
information to the 17 March, 1982 Meeting, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
LICHTENBERG, J.J., LONGBOTTON, J.E., & BELLAR, T.A. (1987) Analytical
methods for the determination of volatile non-polar organic chemicals in
water and water-related environments. In: Suffet, I.H. & Malaiyandi, M.,
ed. Organic pollutants in water: Sampling, analysis and toxicity testing.
A Symposium of the 188th Meeting of the American Chemical Society,
Philadelphia, 29-31 August, 1984, Washington, DC, American Chemical
Society, pp. 63-81.
LITTON BIONETICS, INC. (1977) Evaluation of hexachlorocyclopentadiene; in
vitro malignant transformation in BALB/3T3 cells, Kensington, Maryland,
Litton Bionetics, Inc., 7 pp (LBI Project No. 29840) (Prepared for
Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978a) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse lymphoma forward mutation assay,
Kensington, Maryland, Litton Bionetics, inc., 10 pp (LBI Project No.
20839) (Prepared for Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978b) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse dominant lethal assay, Kensington,
Maryland, Litton Bionetics, Inc., 13 pp (LBI Project No. 20862).
LOOK, M. (1974) Hexachlorocyclopentadiene adducts of aromatic compounds
and their reaction products. Aldrichem. Acta, 7(2): 1974.
LU, P.Y., METCALF, R.L., HIRWE, A.S., & WILLIAMS, J.W. (1975) Evaluation
of environmental distribution and fate of hexachlorocyclopentadiene,
chlordane, heptachlor, and heptachlor epoxide in a laboratory model
ecosystem. J. agric. food Chem., 23: 967-973.
MACEK, R.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979) Considerations
in assessing the potential for, and significance of, biomagnification of
chemical residues in aquatic food chains. In: Marking, L. & Kimerle,
R.A., ed. Aquatic toxicology, Philadelphia, American Society for Testing
and Materials, pp. 251-268.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida, US Environmental Protection Agency
(Report EPA-600/8-017).
MEHENDALE, H.M. (1977) Chemical reactivity-absorption, retention,
metabolism and elimination of hexachlorocyclopentadiene. Environ. Health
Perspect., 21: 275-278.
MEIER, J.R., RINGHAND, H.P., COLEMAN, W.E., MUNCH, J.W., STREICHER, R.P.,
KAYLOR, W.H., & SCHENCK, K.M. (1985) Identification of mutagenic
compounds formed during chlorination of humic acid. Mutat. Res., 157:
111-122.
MOLOTSKY, H.M. & BALLWEBER, E.G. (1957) Hexachlorocyclopentenones: US
Patent No. 2,795,608, June 11, 1957, Chicago, Velsicol Chemical
Corporation.
MORSE, D.L., LANDRIGAN, P.J., & FLYNT, J.W. (1978) Internal CDC report
concerning hexachlorocyclopentadiene contamination of a municipal sewage
treatment plant, Louisville, Kentucky, Atlanta, Georgia, Centers for
Disease Control.
MORSE, D.L., KOMINSKY, J.R., & WISSEMAN, C.L., III (1979) Occupational
exposure to hexachlorocyclopentadiene (How safe is sewage?). J. Am. Med.
Soc., 241: 2177-2179.
MURRAY, F.J., SCHWETZ, B.A., BALMER, M.F., & STAPLES, R.E. (1980)
Teratogenic potential of hexachlorocyclopentadiene in mice and rabbits.
Toxicol. appl. Pharmacol., 53: 497-500.
NAS (1978) Kepone/mirex/hexachlorocyclopentadiene: An environmental
assessment, Washington, DC, National Academy of Sciences (NTIS PB 280-
289).
NEUMEISTER, C. & KURIMO, R. (1978) Determination of
hexachlorocyclopentadiene and octachlorocyclopentene in air. Presented at
the ACGIH Conference, Los Angeles, May 1978, Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
NIOSH (1979) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1-5 (DHEW
(NIOSH) Pub. No. 77-157-A).
NIOSH (1980) Quarterly hazard summary report: Hexachlorocyclopentadiene,
Cincinnati, Ohio, National Institute for Occupational Safety and Health.
NTP (1984a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 128 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
NTP (1984b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 196 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
OSHA (US DEPARTMENT OF LABOR, OCCUPATIONAL SAFETY AND HEALTH
ADMINISTRATION) (1989) Air contaminants: Final rule -
Hexachlorocyclopentadiene. Fed. Reg., 54(12): 2464.
PETERS, J.A., TACKETT, K.M., & EIMUTIS, E.C. (1981) Measurement of
fugitive hydrocarbon emissions from a chemical waste disposal site.
Presented at the 74th Annual Meeting of the Air Pollution Control
Association, Philadelphia, 21-26 June, 1981, Dayton, Ohio, Montesanto
Research Corporation.
PODOWSKI, A.A. & KHAN, M.A.Q. (1979) Fate of hexachlorocyclopentadiene in
goldfish (Carassius auratus). Paper presented at the American Chemical
Society Meeting, Honolulu, April 1979, Washington, DC, American Chemical
Society.
PODOWSKI, A.A. & KHAN, M.A.Q. (1984) Fate of hexachlorocyclopentadiene in
water and goldfish. Arch. environ. Contam. Toxicol., 13: 471-481.
RAND, G.M., NEES, P.O., CALO, C.J., ALEXANDER, D.J., & CLARK, G.C.
(1982a) Effects of inhalation exposure to hexachlorocyclopentadiene on
rats and monkeys. J. Toxicol. environ. Health, 9: 743-760.
RAND, G.M., NEES, P.O., CALO, C.J., CLARKE, G.C., & EDMONDSON, N.A.
(1982b) The Clara cell: An electron microscopy examination of the
terminal bronchioles of rats and monkeys following inhalation of
hexachlorocyclopentadiene. J. Toxicol. environ. Health, 10: 59-72.
RIECK, C.E. (1977a) Effect of hexachlorocyclopentadiene on soil microbe
populations, Lexington, Kentucky, University of Kentucky, Agronomy
Department (Unpublished report prepared for Velsicol Chemical
Corporation, Chicago).
RIECK, C.E. (1977b) Soil metabolism of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
RIECK, C.E. (1977c) Volatile products of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
ROBERTS, C.W. (1958) Chemistry of hexachlorocyclopentadiene. Chem. Ind.,
1 February: 110.
SHELL RESEARCH LIMITED (1982) Toxicology of insecticide intermediates:
The skin sensitizing potential of hexachlorocyclopentadiene, Tunstall,
United Kingdom, Shell Research Ltd., Sittingbourne Research Centre
(Report No. SBGR 82.225).
SHINDEL, S. & ULRICH, S. (1986) Mortality of workers employed in the
manufacture of chlordane. An update. J. occup. Med., 28(7): 497-501.
SHINDELL & ASSOCIATES (1980) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Marshall, Illinois,
January 1946-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SHINDELL & ASSOCIATES (1981) Report of the epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Memphis, Tennessee,
January 1952-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1982)
Hexachlorocyclopentadiene increases oxygen consumption by intact rainbow
trout and isolated heart mitochondria. Sixty-sixth Annual Meeting of the
Federation of American Societies for Experimental Biology, New Orleans,
15-23 April, 1982. Fed. Proc., 41(5): 1580 (abstract).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1983)
Respiratory effects of hexachlorocyclopentadiene on intact rainbow trout
(Salmo gairdneri) and on oxidative phosphorylation of isolated trout
heart mitochondria. Toxicol. appl. Pharmacol., 67: 215-223.
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1977) A rapid
assessment of the toxicity of three chlorinated cyclodiene insecticide
intermediates to fathead minnows, Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory (Report EPA-600/3-
77-099).
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1979) Toxicity
and bioaccumulation of hexachlorocyclopentadiene, hexachloronorbornadiene
and heptachloronorbonene in larval and early juvenile fathead minnows,
Pimephales promelas. Bull. environ. Contam. Toxicol., 21: 576-583.
SPRINKLE, C.L. (1978) Leachate migration from a pesticide. Waste disposal
site in Hardeman County, Tennessee, Washington, DC, US Department of
Interior, US Geological Survey (Waste Resources Investigations 78-128).
SRI (1980a) Acute toxicity report on hexachlorocyclopentadiene (C53607)
in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama, Southern
Research Institute, 44 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1980b) Repeated-dose toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama,
Southern Research Institute, 33 pp (Unpublished report prepared for the
National Toxicology Program).
SRI (1981a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 137 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1981b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 144 pp (Unpublished report prepared for the National
Toxicology Program).
STEVENS, J.E. (1979) Chlorinated derivatives of cyclopentadiene. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
Chichester, Brisbane, Toronto, John Wiley & Sons, Vol. 5, pp. 791-797.
THIELEN, D.R., OLSEN, G., DAVIS, A., BAJOR, E., STEFANOVSKI, J., &
CHODKOWSKI, J. (1987) An evaluation of microextraction/capillary column
gas chromatography for monitoring industrial outfalls. J. chromatogr.
Sci., 25(1): 12-16.
THUMA, N.K., O'NEILL, P.E., BROWNLEE, S.G., & VALENTINE, R.S. (1978)
Biodegradation of spilled hazardous materials. In: Control of hazardous
materials spills, Rockville, Maryland, Information Transfer, Inc., pp.
217-220.
TREON, J.F., CLEVELAND, F.P., & CAPPEL, J. (1955) The toxicity of
hexachlorocyclopentadiene. Arch. ind. Health, 11: 459-472.
UNGNADE, H.E. & MCBEE, E.T. (1958) The chemistry of
perchlorocyclopentenes and cyclopentadienes. Chem. Rev., 58: 249-254.
US EPA (1977) Chemical Hazard Information Profile:
Hexachlorocyclopentadiene (CHIP), Washington, DC, US Environmental
Protection Agency, TSCA Interagency Testing Committee.
US EPA (1980a) Summary of UWF Co-op Data on hexachlorocyclopentadiene and
hexachlorobutadiene, Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished laboratory data).
US EPA (1980b) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards (Report EPA-440/5-80-055)
(NTIS PB 292-436).
US EPA (1980c) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, office of Water Regulations and Standards (Report EPA-440/5-80-
055) (Unpublished data).
US EPA (1982) Hexachlorocyclopentadiene: Response to the Interagency
Testing Committee. Fed. Reg., 47(250): 58023-58025.
US EPA (1989) Toxic chemical release inventory: Online printout of April
1989, Washington, DC, US Environmental Protection Agency (Available from
the US National Library of Medicine).
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish.
Res. Board Can., 36: 1040-1048.
VELSICOL CHEMICAL CORPORATION (1978) TSCA Sec. 8(E): Submission 8EHQ-
06780208. Chlorinates in Mississippi River catfish and carp, 1978
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago)
(Submitted to the US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VELSICOL CHEMICAL CORPORATION (1979) Confirmation of HEX and HEX-BCH
residues in human urine. Analytical method No. 0682, Chicago, Velsicol
Chemical Corporation.
VELSICOL CHEMICAL CORPORATION (1984) Comments on draft health effects
document for hexachlorocyclopentadiene, Chicago, Velsicol Chemical
Corporation.
VELSICOL CHEMICAL CORPORATION (1986) Air sampling and monitoring at
Velsicol production facilities, Chicago, Velsicol Chemical Corporation.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Co.
VILKAS, A.G. (1977) The acute toxicity of hexachlorocyclopentadiene to
the water flea, Daphnia magna straus, Tarrytown, New York, Union Carbide
Environmental Services (UCES Project No. 11506-03-05) (Prepared for
Velsicol Chemical Corporation, Chicago).
WALSH, G.E. (1981) Effects of chlordane, heptachlor and
hexachlorocyclopentadiene on growth of marine unicellular algae, Gulf
Breeze, Florida, US Environmental Protection Agency, Environmental
Research Laboratory (Unpublished report).
WALSH, G.E. (1983) Cell death and inhibition of population growth of
marine unicellular algae by pesticides. Aquat. Toxicol., 3: 209-214.
WALSH, G.E. & ALEXANDER, S.V. (1980) A marine algal bioassay method:
Results with pesticides and industrial wastes. Water Air Soil Pollut.,
13: 45-55.
WANG, H.H. & MACMAHON, B. (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J. occup. Med., 21: 745-748.
WEAST, R.C. & ASTLE, M.J. (1980) CRC handbook of chemistry and physics,
60th ed., Boca Raton, Florida, CRC Press, Inc.
WEBER, J.B. (1979) Adsorption of HEX by Cape Fear loam soil, Research
Triangle Park, North Carolina State University (Unpublished report
prepared for Velsicol Chemical Corporation, Chicago).
WHITMORE, F.C., DURFEE, R.L., & KHATTAK, M.N. (1977) Evaluation of a
technique for sampling low concentrations of organic vapors in ambient
air, Atlanta, Georgia, US Environmental Protection Agency (NTIS PB 279-
672).
WHO (1989) Environmental Health Criteria 91: Aldrin and Dieldrin, Geneva,
World Health Organization, 335 pp.
WILLIAMS, C.M. (1978) Liver cell culture systems for the study of
hepatocarcinogens. Proceedings of the 12th International Cancer Congress,
XII. International Cancer Congress Symposium No. 2: Chemical Oncogenesis
and Mutagenesis, October 1978, New York, London, Plenum Publishing
Corporation.
WILSON, J.A., BALDWIN, C.P., & MCBRIDE, T.J. (1978) Case history:
Contamination of Louisville, Kentucky Morris Foreman treatment plant.
Hexachlorocyclopentadiene. In: Control of hazardous material spills,
Miami, Florida, Hazardous Material Control Research Institute, pp. 170-
177.
WOLFE, N.L., ZEPP, R.G., SCHLOTZHAVER, P., & SINK, M. (1982)
Transformation pathways of hexachlorocyclopentadiene in the aquatic
environment. Chemosphere, 11(2): 91-101.
YOWELL, H.L. (1951) Fungicidal compositions containing
hexachlorocyclopentadiene: US Patent 2538509, Washington, DC, US Patent
Office.
YU, C.C. & ATALLAH, Y.H. (1977a) HEX hydrolysis at various pHs and
temperatures, Chicago, Velsicol Chemical Corporation (Project No. 482428,
Report No. 8) (Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1977b) Photolysis of hexachlorocyclopentadiene,
Chicago, Velsicol Chemical Corporation (Project No. 482428, Report No. 4)
(Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1981) Pharmacokinetics and metabolism of
hexachlorocyclopentadiene in rats, Chicago, Velsicol Chemical Corporation
(Project No. 482428, Report No. 10).
ZEPP, R.G., BAUGHMAN, G.L., & SCHLOTZHAUER, P.F. (1979) Dynamics of
processes influencing the behavior of hexachlorocyclopentadiene in the
aquatic environment. Paper presented at the 178th National Meeting of the
American Chemical Society, Washington, 9-14 September, 1979, Washington,
DC, American Chemical Society, Division of Environmental Chemistry.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R.C. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ. Mutagen.,
7: 87-100.
APPENDIX 1
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Table 21.
Table 21. Guidelines, recommendations and standards used in various countries/areasa
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
Australia recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1983
weighted average
short-term exposure limit 0.3 (0.03)
Belgium recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1988
weighted average
Canada regulation air/occupational threshold limit value/time- 0.1 (0.01) 1980
weighted average
Canada regulation transport specific transportation 1987
regulations
Finland recommendation air/occupational time-weighted average 1.0 (0.1) 1989
skin short-term exposure limit 3 (0.3) 1989
Netherlands recommendation air/occupational time-weighted average/ 0.11 (0.01) 1986
occupational
Federal regulation waste "toxic waste" subject to 1981
Republic specific handling,
of Germany transport, treatment,
storage, and disposal
regulation/permits
Switzerland regulation air/occupational time-weighted average 0.1 (0.01) 1987
USA regulation water ambient 1 µg/litre 1980
water quality criteria
(organoleptic)
----------------------------------------------------------------------------------------------------
Table 21 (contd.)
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
USA (ACGIH) recommendation air/occupational time-weighted average 0.1 (0.01) 1980
USA regulation air/occupational time-weighted average 0.1 (0.01) 1989
USA regulation water/land notification of spill 1983
of 0.45 kg (1 lb)
in 24-h period
USA regulation waste transport "toxic waste" subject to 1980
specific handling,
transport, treatment,
storage and disposal
regulation/permits
USA draft drinking-water lifetime 7 µg/kg 1990
recommendation per day
USSR regulation air/occupational threshold limit value 0.01 (0.001) 1989
USSR regulation water maximum allowable 1 mg/litre 1985
concentration
USSR regulation air/ambient short-term exposure limit 0.001 1987
Yugoslavia regulation air/occupational time-weighted average 0.1 1985
----------------------------------------------------------------------------------------------------
a From: IRPTC (1989)
b Unless stated otherwise, units are mg/m3. The value in parts per million is given in parentheses.
RESUME
L'hexachlorocyclopentadiène (HEX) est un liquide dense
et ininflammable, de couleur jaune pâle à jaune verdâtre,
et qui possède une odeur piquante caractéristique. Le HEX
est très réactif; il donne lieu à des réactions d'addition
et de substitution et à des réactions de Diels-Alder.
Aux Etats-Unis d'Amérique, la Velsicol Chemical Cor-
poration est actuellement le seul producteur de HEX. En
Europe, il est produit aux Pays-Bas par la Société Shell.
Les chiffres de production sont confidentiels mais on
estime que 3600 à 6800 tonnes de HEX sont produites
actuellement aux Etats-Unis. En 1988, la production
mondiale était d'environ 15 000 tonnes (BUA, 1988). Le
HEX est utilisé comme intermédiaire dans la production de
nombreux pesticides, mais quelques pays en ont limité
l'emploi à la fabrication de certains pesticides organo-
chlorés. Il est également utilisé pour la fabrication de
retardateurs de flamme, de résines et de colorants.
Au cours de la production et de la transformation du
HEX, de petites quantités sont libérées dans l'environne-
ment. Ce peut être également le cas lorsqu'il constitue
une impureté de certains des produits pour lesquels il
sert d'intermédiaire. La libération de HEX peut interve-
nir pendant ou après le rejet. On ne dispose que de
données limitées sur la surveillance des concentrations de
cette substance dans l'environnement. D'après ces données,
il semble que le HEX soit présent essentiellement dans le
compartiment aquatique et y soit associé aux sédiments et
aux matières organiques, sauf là où il y a eu rejet ou
libération du produit. D'après les études en laboratoire,
il y a sorption du HEX par la plupart des particules du
sol. Toutefois, on a fait état d'un lessivage et d'un
mouvement dans les eaux souterraines.
Aux Etats-Unis d'Amérique, on estime que 5,9 tonnes de
HEX sont libérées annuellement dans le milieu (US EPA,
1989). En République fédérale d'Allemagne et aux Pays-
Bas, environ 400 à 500 kg de HEX ont été libérés dans
l'atmosphère en 1987 (BUA, 1988). En raison des propriétés
physiques et chimiques du HEX, il ne devrait subsister
qu'une faible fraction de ces émissions.
En s'appuyant sur les données de laboratoire disponi-
bles, on a modélisé la destinée et le transport du HEX
dans l'atmosphère et calculé que son temps de séjour dans
la troposphère était d'environ 5 h. On a fait état d'un
transport atmosphérique de HEX à partir d'une zone de
stockage de déchets et de puits au cours du traitement de
rejets industriels.
Dans l'eau, le HEX peut subir une photolyse, une
hydrolyse et une biodégradation. Dans les eaux peu
profondes son temps de demi-photolyse est inférieur à une
heure. Dans les eaux plus profondes où la photolyse est
exclue, le temps de demi-hydrolyse varie de plusieurs
jours à environ trois mois et la biodégradation est encore
beaucoup plus lente. Le HEX se volatilise à la surface de
l'eau à une vitesse qui dépend de la turbulence et du
degré de sorption par les sédiments.
En raison de sa faible solubilité dans l'eau, le HEX
devrait être relativement immobile dans le sol. Toutefois
on en a trouvé dans des eaux souterraines. La volatili-
sation de cette substance qui se produit très vraisembla-
blement à la surface du sol est d'autant plus importante
que la teneur du sol en matières organiques est plus
faible. Les résultats d'études en laboratoire indiquent
que l'hydrolyse chimique et la métabolisation microbienne,
qu'elles soient aérobie ou anaérobie, devraient réduire la
teneur des sols en HEX.
En principe, le HEX devrait avoir un pouvoir de bio-
amplification notable en raison de sa forte lipophilicité
(log du coefficient de partage octanol/eau). Toutefois
les données expérimentales ne corroborent pas cette hypo-
thèse. Des études sur animaux d'expérience ont montré que
le 14C-HEX est à la fois métabolisé et excrété dans les
quelques heures qui suivent l'administration par voie
orale, la rétention dans l'organisme étant très faible.
Le facteur de bioconcentration à l'état stationnaire est
inférieur à 30 chez les poissons. Les facteurs de bio-
accumulation calculés à partir de modèles d'écosystèmes à
court terme indiquent que le potentiel d'accumulation est
modéré. Il semblerait donc que le HEX et ses métabolites
ne persistent pas et ne s'accumulent pas en quantités
importantes dans les systèmes biologiques.
On a montré qu'à faibles concentrations, le HEX était
toxique pour la faune aquatique. Des cas de mortalité par
intoxication aiguë (exposition de 48 à 96 h) ont été
observés chez des crustacés et des poissons dulçaquicoles
et marins à des concentrations nominales de 32 à
180 µg/litre, dans des systèmes statiques dont l'eau
n'était pas renouvelée au cours de l'épreuve. Etant donné
que le temps de demi-photolyse est inférieur à une heure,
la concentration en HEX devrait avoir diminué de manière
importante au cours de la période d'exposition utilisée
dans ces études. Les seules études au cours desquelles on
a mesuré les concentrations de HEX dans de l'eau courante
ont donné une valeur de la CL50 à 96 h de 7 µg/litre
pour le vairon américain et une crevette de mer. Les
épreuves effectuées sur ces deux espèces ont donné,
respectivement pour la CL10 et la CL40, des valeurs de
3,7 et de 0,7 µg/litre.
Des épreuves statiques de sept jours effectuées sur
des algues marines à des concentrations nominales allant
de 3,5 à 100 µg/litre ont fait ressortir une réduction
moyenne de la croissance (CE50), qui était fonction de
l'espèce. En milieu aqueux, le HEX est toxique pour de
nombreux microorganismes à des concentrations nominales de
0,2 à 10 mg/litre, c'est-à-dire à des valeurs sensiblement
plus importantes que celles qui sont nécessaires pour tuer
la plupart des animaux et des plantes aquatiques. Le HEX
semble être moins toxique pour les microorganismes terri-
coles que pour les microorganismes aquatiques, probable-
ment en raison de l'adsorption du HEX sur la matrice
constituée par le sol.
On pense que l'exposition devrait être faible mais les
données actuellement disponibles sont insuffisantes pour
permettre de déterminer les effets de l'exposition au HEX
sur la flore et la faune terrestres.
La résorption du HEX non modifié est minimale du fait
de sa réactivité vis-à-vis des membranes et des tissus de
l'organisme et plus particulièrement, du contenu des voies
digestives. Après administration par voie orale, percuta-
née ou par inhalation, la majeure partie du 14C-HEX
radiomarqué est retenue au niveau des reins, du foie, de
la trachée et des poumons des animaux d'expérience. Une
fois résorbé, le HEX est métabolisé et rapidement excrété,
principalement dans les urines, un peu moins dans les
matières fécales et à raison de moins de 1%, dans l'air
expiré. La durée nécessaire pour l'élimination complète
est d'environ 30 h quelle que soit la voie d'adminis-
tration. Après inhalation ou administration par voie
intraveineuse, le composé parent ne se retrouve plus dans
les excréta; on a isolé des métabolites fécaux et uri-
naires mais ils n'ont pas été identifiés. C'est d'ailleurs
la raison pour laquelle il est très difficile de se faire
une idée de la pharmacocinétique du HEX et d'élucider son
mode d'action.
La CL50 par inhalation (sur une période d'environ
4 h) est de 17,9 mg/m3 chez le rat mâle et de 39,1 mg
par m3 chez la ratte. Bien qu'il existe quelques
différences d'une espèce à l'autre, notamment entre les
cobayes, les rats, les lapins et les souris, les vapeurs
de HEX sont extrêmement toxiques pour toutes les espèces
étudiées. C'est par inhalation qu'il se révèle le plus
toxique, par comparaison avec l'administration orale ou
percutanée, et il se montre également extrêmement irri-
tant. Quelle que soit la voie d'administration, toute
exposition aiguë entraîne des effets généraux pathologi-
ques au niveau des poumons, du foie, des reins et des
surrénales.
L'administration de HEX par voie orale à des rats
(30 mg/kg et par jour) et à des souris (75 mg/kg et par
jour) pendant 91 jours a déterminé une néphrose ainsi
qu'une inflammation et une hyperplasie de l'estomac
antérieur. On n'a pas relevé de signes manifestes après
exposition de souris ou de rats à qui l'on avait fait
inhaler du HEX à raison de 2,26 mg/m3 (0,2 ppm), six
heures par jour, cinq jours par semaine pendant 14
semaines. A la concentration de 1,69 mg/m3 (0,15 ppm),
on a observé seulement une petite irritation. Des rats
exposés de la même manière à 5,65 mg/m3 (0,5 ppm) de
HEX pendant 30 semaines ont présenté des modifications
histopathologiques au niveau du foie, des voies respi-
ratoires et des reins. Une autre étude du même genre
portant sur des rats et des souris et qui s'est prolongée
pendant 90 jours a révélé des effets respiratoires à
partir de 4,52 mg/m3 (0,4 ppm). Le HEX ne s'est pas
révélé mutagène lors d'épreuves in vitro, qu'il y ait ou
non activation métabolique. Il s'est également révélé
inactif dans les épreuves de dominance létale chez la
souris. Administré par voie orale à des rats et à des
souris, il ne s'est pas révélé tératogène, mais on ne
dispose d'aucune donnée relative à sa tératogénicité
éventuelle après exposition par voie respiratoire. On ne
dispose que de données limitées sur l'exposition humaine
au HEX et sur ses effets. On a observé des incidents
isolés au cours desquels il y a eu forte irritation des
yeux, du nez, de la gorge et des poumons. Cette irritation
était généralement brève, les victimes commençant à
récupérer dès cessation de l'exposition. Après une expo-
sition de courte durée on n'a noté aucune différence
statistiquement significative au niveau de certaines
enzymes hépatiques entre les groupes exposés et les
groupes témoins. On ignore quels peuvent être les effets
à longue échéance sur la santé humaine d'une exposition
continue à de faibles concentrations de HEX ou d'une
exposition intermittente à des fortes concentrations. Les
personnes qui sont amenées à manipuler cette substance ou
des déchets qui en contiennent, ainsi que les égoutiers,
travailleurs des usines de traitement d'eaux usées et
personnes qui résident à proximité de décharges, pour-
raient être exposés au risque du fait des possibilités de
contact avec cette substance ou avec les déchets qui
résultent de sa fabrication.
La base de données dont on dispose n'est pas suffi-
sante pour qu'on puisse évaluer la cancérogénicité du HEX.
Le Programme toxicologique national des Etats-Unis (NTP) a
procédé à des études d'inhalation sur des rats et des
souris pendant toute la durée de leur vie. Une fois qu'un
rapport sur la pathologie observée aura été publié, on
aura une meilleure idée des effets à long terme éventuels
de l'exposition au HEX. En ce qui concerne la cancéro-
génicité de cette substance, il faudra différer les tra-
vaux dans l'attente des résultats des épreuves effectuées
par le NTP. Le Centre international de recherche sur le
cancer a examiné les données existantes sur le HEX et l'a
classé dans le Groupe 3 (ce qui indique qu'en raison
d'insuffisances quantitatives ou qualitatives importantes,
il n'est pas possible de déterminer, au vu des résultats
disponibles, s'il y a présence ou absence d'effets
cancérogènes). Un certain nombre d'études épidémiologiques
sont citées dans la littérature; on n'a pas fait état
d'une incidence accrue de cancers, de localisation
quelconque, qui puisse être attribuée au HEX ou à ses
métabolites.
RESUMEN
El hexaclorociclopentadieno (HEX) es un líquido denso
de color amarillo pálido o verdoso, no inflamable, con un
olor acre característico. Su masa molecular relativa es de
272,77 y es poco soluble en agua. El HEX es muy reactivo
y experimenta reacciones de adición, sustitución y de
Diels-Alder.
En los EE.UU., la Velsicol Chemical Corporation es la
única empresa que actualmente produce HEX. En Europa lo
fabrica la Shell Chemical Corporation en los Países Bajos.
Los datos de producción son propiedad de las empresas,
pero se calcula que al año se producen en los EE.UU. entre
3600 y 6800 toneladas de HEX. En 1988, la producción
mundial fue de aproximadamente 15 000 toneladas (BUA,
1988). Aunque el HEX se utiliza como intermedio en la
producción de numerosos plaguicidas, algunos países han
restringido su empleo en la fabricación de ciertos plagui-
cidas organoclorados. También se utiliza en la producción
de pirorretardantes, resinas y tintes.
Durante la fabricación y elaboración del HEX, se
liberan pequeñas cantidades de la sustancia al medio
ambiente. También puede liberarse cuando aparece en forma
de impureza en algunos de los productos para los que sirve
de intermedio. El HEX puede liberarse tanto durante como
después de su evacuación. Sólo se dispone de datos limi-
tados de vigilancia de los niveles ambientales de HEX.
Esos datos indican que aparece principalmente en el
compartimento acuático y que se asocia a los sedimentos y
la materia orgánica del fondo salvo en los lugares en los
que se ha producido evacuación o liberación. En el labora-
torio, el HEX se adsorbe sin dificultad a la mayoría de
los tipos de partículas del suelo. Sin embargo, se han
comunicado casos de lixiviación y de movimiento en aguas
subterráneas.
En los EE.UU., se calcula que la liberación total de
HEX al medio ambiente en un año es de 5,9 toneladas (US
EPA, 1989). En la República Federal de Alemania y los
Países Bajos, se emitieron en 1987 alrededor de 400-500 kg
(BUA, 1988). Dadas las características físicas y químicas
del HEX, es de esperar que solo persista una pequeña
fracción de esas emisiones.
Basandose en los datos de laboratorio disponibles, se
ha formulado un modelo sobre el destino y el transporte
del HEX en la atmósfera y se ha calculado que tiene un
tiempo de residencia de aproximadamente 5 horas en la
troposfera. Se ha comunicado la existencia de transporte
atmosférico de HEX desde una zona en la que se almacenan
desechos y a partir de las cisternas durante el trata-
miento de desechos industriales.
En el agua, el HEX puede experimentar fotolisis,
hidrólisis y biodegradación. En aguas poco profundas,
tiene una semivida fotolítica de < 1 h. En aguas más
profundas, donde la fotolisis se ve impedida, se ha
observado que la semivida hidrolítica puede variar entre
varios días y aproximadamente tres meses, mientras que es
de prever que la biodegradación se produzca con más
lentitud. Se sabe que el HEX se volatiliza en las aguas
superficiales, y que la tasa de volatilización varia con
la turbulencia y con la adsorción a los sedimentos.
Debido a su baja solubilidad en el agua, el HEX debe
ser relativamente inmóvil en el suelo. Sin embargo, se ha
detectado la sustancia en aguas subterráneas. La volatili-
zación, que tiene más probabilidades de producirse en la
superficie del suelo, guarda relación inversa con los
niveles de materia orgánica en éste. Los resultados de
estudios en laboratorio indican que la hidrólisis química
y el metabolismo microbiano, tanto aeróbico como anaeró-
bico, deben reducir los niveles de HEX en los suelos.
En teoría, el potencial de biomagnificación del HEX
debe ser importante debido a su elevada lipofilia (log
coeficiente de partición octanol/agua). Este extremo, sin
embargo, no ha sido demostrado en pruebas experimentales.
Los estudios en animales de laboratorio han demostrado que
el 14C-HEX es metabolizado y excretado durante de las
primeras horas que siguen la administración de una dosis
por vía oral, y que la proporción que queda retenida en el
organismo es pequeña. En los peces, los factores de
bioconcentración en estado estable son < 30. Los factores
de bioacumulación derivados de modelos de ecosistemas a
corto plazo indican un moderado potencial de acumulación.
Así pues, parece que el HEX y sus metabolitos no persisten
ni se acumulan en gran medida en los sistemas biológicos.
Se ha demostrado que el HEX en bajas concentraciones
es tóxico para los organismos acuáticos. Se ha observado
letalidad en exposiciones agudas (48 a 96 h) en crustáceos
y peces de agua dulce y salada, en concentraciones nomi-
nales de 32-180 µg/litro en sistemas de exposición
estática en los que el agua no se renovó durante la
prueba. Puesto que la semivida fotolítica es < 1 h, la
concentración de HEX habría disminuido sustancialmente
durante el periodo de exposición utilizado en esos
estudios. En los únicos estudios en los que se usó agua
corriente y se midieron las concentraciones de HEX, se
obtuvieron valores de la CL50 en 96 horas de 7 µg por
litro en Pimephales promelas y un camarón de mar. Los
ensayos realizados con esas dos especies dieron un valor
de CL10 de 3,7 y un valor de CL40 de 0,7 µg por litro,
respectivamente.
En ensayos estáticos de siete días con algas marinas
se observó una reducción mediana del crecimiento (CE50)
a concentraciones nominales que variaron entre 3,5 y
100 µg/litro, según la especie.
En medios acuosos, el HEX resulta tóxico para
numerosos microorganismos en concentraciones nominales de
0,2-10 mg/litro, es decir, niveles sensiblemente mayores
que los necesarios para destruir a la mayoría de los
animales o vegetales acuáticos. El HEX parece menos tóxico
para los microorganismos en el suelo que en el medio
acuático, probablemente a causa de la adsorción del HEX a
la matriz del suelo.
Aunque cabe esperar que la exposición sea reducida,
actualmente no se dispone de bastante información para
determinar los efectos de la exposición al HEX en la
vegetación o la fauna terrestres.
La absorción de HEX sin modificar es mínima debido a
su reactividad con las membranas y los tejidos del organ-
ismo y especialmente con el contenido del tracto gastro-
intestinal. La mayor parte del 14C-HEX radiomarcado
queda retenido en el riñón, el hígado, la tráquea y los
pulmones de los animales tras la administración por vía
oral, cutánea o respiratoria. El HEX absorbido es metab-
olizado y excretado rápidamente, sobre todo en la orina,
menos en las heces y < 1% en el aire expirado. El tiempo
de eliminación terminal es de unas 30 horas, con inde-
pendencia de la vía de administración. Tras la inhalación
o la administración intravenosa, no se excreta HEX sin
modificar; los metabolitos fecales y urinarios se han
aislado pero no se han identificado. La falta de identifi-
cación de los metabolitos representa uno de los prin-
cipales obstaculos para evaluar la farmacocinética y los
mecanismos potenciales de acción del HEX.
En la rata, la CL50 aguda por inhalación (durante un
periodo de aproximadamente 4 h) es de 17,9 mg/m3 en el
macho y 39,1 mg/m3 en la hembra. Aunque hay algunas
diferencias interespecíficas entre cobayos, conejos, ratas
y ratones, los vapores de HEX son sumamente tóxicos para
todas las especies ensayadas. Su toxicidad parece máxima
cuando se administra por inhalación, en comparación con la
administración oral y cutánea, y es un irritante primario
fuerte. Los efectos sistémicos de la exposición aguda,
con independencia de la vía de administración, comprenden
cambios patológicos en el pulmón, el hígado, el riñón y
las glándulas suprarrenales.
La administración oral a corto plazo a ratas (38 mg/kg
al día) y ratones (75 mg/kg al día) durante 91 días
produjo nefrosis e inflamación e hiperplasia de la región
anterior del estómago. No se observaron signos manifiestos
cuando se expuso a ratas o ratones por inhalación a 2,26
mg/m3 (0,2 ppm), 6 h/día, 5 días/semana, durante 14
semanas. Con 1,69 mg/m3 (0,15 ppm) sólo se observó una
ligera irritación. La exposición de ratas a la inhalación
de 5,65 mg/m3 (0,5 ppm) durante 30 semanas provocó
cambios histopatológicos en el hígado, el tracto respira-
torio y el riñón. En un estudio de inhalación a corto
plazo de HEX en ratones y ratas durante 90 días se
observaron efectos en el sistema respiratorio a 4,52
mg/m3 (0,4 ppm) o más. No se ha demostrado que el HEX
sea mutagénico en ensayos in vitro , con o sin activación
metabólica. También resultó inactivo en ensayos de letali-
dad dominante en el ratón. Tampoco se ha demostrado que
sea teratogénico en ratas y ratones por exposición oral;
no se dispone de datos sobre la teratogenicidad del HEX
tras la exposición por inhalación.
Sólo se dispone de datos limitados sobre los efectos
de la exposición al HEX en la salud humana. Se han
producido incidentes aislados en los que el HEX provocó
fuerte irritación de los ojos, la nariz, la garganta y los
pulmones. Por lo general esa irritación fue breve, y la
recuperación se inició en cuanto cesó la exposición. No
se observaron diferencias estadísticamente significativas
en ciertas enzimas hepáticas entre grupos expuestos y
grupos testigo tras la exposición a corto plazo. Se
desconocen los efectos a largo plazo en la salud humana de
la exposición continua a bajos niveles y/o la exposición
aguda intermitente. Se ha demostrado que los manipuladores
del producto y de sus desechos, así como las personas que
trabajan en la depuración de aguas residuales o que viven
en las proximidades de los lugares de evacuación corren
riesgo debido al potencial de exposición a la sustancia
química o a los residuos de su fabricación.
La base de datos no es lo bastante amplia ni adecuada
para evaluar la carcinogenicidad del HEX. El Programa
Nacional de Toxicología de los EE. UU. ha llevado a cabo
un bioensayo de inhalación durante toda la vida en ratas y
ratones. Cuando se publique el informe patológico, se
comprenderá mejor los efectos a largo plazo de la exposi-
ción al HEX. La evaluación de la carcinogenicidad deberá
demorarse hasta que estén disponibles los resultados del
bioensayo del Programa. El Centro Internacional de Inves-
tigaciones sobre el Cáncer evaluó los datos existentes
para el HEX y clasificó la sustancia en el Grupo 3 (lo que
indica que, debido a limitaciones importantes de orden
cualitativo o cuantitativo, no puede interpretarse que los
estudios demuestren ni la existencia ni la ausencia de
efecto carcinogénico). En la bibliografía se citaron
varios estudios epidemiológicos; no se notificaron aumen-
tos de la incidencia de neoplasmas en ninguna localización
que pudieran atribuirse al HEX o sus metabolitos.
See Also:
Toxicological Abbreviations
Hexachlorocyclopentadiene (HSG 63, 1991)
Hexachlorocyclopentadiene (ICSC)
 CAS and IUPAC 1,2,3,4,5,5'-hexachloro-1,3-cyclo-
name: pentadiene
Synonyms and Hexachlorocyclopentadiene, perchloro-
common trade cyclopentadiene, hexachloro-1,3-cyclo-
names: pentadiene, HEX, HCPD, HCCP,
HCCPD, C-56, HRS 1655, Graphlox
CAS registry number:
CAS and IUPAC 1,2,3,4,5,5'-hexachloro-1,3-cyclo-
name: pentadiene
Synonyms and Hexachlorocyclopentadiene, perchloro-
common trade cyclopentadiene, hexachloro-1,3-cyclo-
names: pentadiene, HEX, HCPD, HCCP,
HCCPD, C-56, HRS 1655, Graphlox
CAS registry number:  4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Overviewa
The fate and transport of HEX in the atmosphere are
not well understood, but the available information
suggests that the compound does not persist. Atmospheric
transport of HEX from an area of stored waste has been
reported (Peters et al., 1981). Experimentally derived
constants for HEX in various environmental processes are
given in Table 2.
Table 2. Summary of constants used in the exposure
analysis modelling system (EXAMS)a
---------------------------------------------------
Constants Values used
---------------------------------------------------
Water solubility (Ks) 1.8 mg/litre
Henry's law constant (KH) 2.7 x 10-2 atm m3/mol
Octanol/water partition 1.1 x 105
coefficient (Pow)
Photolysis (kp) 3.9 h-1
Hydrolysis 4.0 x 10-3 h-1b
Oxidation (kox) 1 x 10-10 M-1 sec-1c
Biodegradation (kB) 1 x 10-5 ml org-1 h-1d
---------------------------------------------------
a Adapted from Wolfe et al. (1982).
b Extrapolated to 25 °C.
c Estimated value (Wolfe et al., 1982).
d This is a maximum value based on the observation
that there was no detectable difference in the
hydrolysis rate in either sterile or non-sterile
studies and measured organism numbers (plate
counts).
In water, HEX probably dissipates rapidly by means of
photolysis, hydrolysis, and biodegradation. In shallow
water (a few centimetres deep), it has a photolytic half-
life of approximately 0.2 h (Butz et al., 1982; Wolfe et
al., 1982). Chou et al. (1987) found this first-order
reaction to take even less time in full sunlight. In
deeper water where photolysis is precluded, hydrolysis and
biodegradation should become the key degradative processes
when there is little movement in the system. The hydro-
lytic half-life of HEX ranges from several days to
-----------------------------------------------------------
a Throughout this chapter, the terms sorb and sorption
are used in preference to absorb/adsorb and
absorption/adsorption.
approximately 3 months, and it is not strongly affected by
the pH in the environmental range (5-9), by salinity or by
the presence of suspended solids (Yu & Atallah, 1977a;
Wolfe et al., 1982). HEX is known to volatilize from water
(Kilzer et al., 1979; Weber, 1979). It is probable that
volatilization is limited by diffusion, i.e. loss from
deeper waters should occur very slowly unless vertical
mixing has taken place. Sorption on sediments may also
retard volatilization.
The fate and transport of HEX in soils are affected by
its strong tendency to sorb onto organic matter (Weber,
1979; Kenaga & Goring, 1980; Wolfe et al., 1982). Another
possibility is that HEX partitions to the interior of soil
particles and stays in loams and silt in a dissolved
state. HEX should be relatively immobile in soil because
of its high log P value (Briggs, 1973), but several inci-
dents in the USA have shown that this is not true in all
soil types (Sprinkle, 1978). Volatilization, which is
likely to occur primarily at the soil surface, is
inversely related to the organic matter level and water-
holding capacity of the soil (Kilzer et al., 1979). Leach-
ing of HEX by ground water can occur, while chemical
hydrolysis and microbial metabolism would be expected to
reduce levels in the environment. HEX is metabolized by a
number of unidentified soil microorganisms (Rieck,
1977b,c; Thuma et al., 1978).
The high lipophilicity and log Pow of HEX indicate a
high potential for bioaccumulation. However, in practice,
this potential is not realized because of metabolism and
elimination. Steady-state bioconcentration factors (BCFs)
in fish measured in 30-day flow-through systems were 29 or
less (Spehar et al., 1979; Veith et al., 1979). In a model
ecosystem study, BCF values for a range of aquatic organ-
isms were between 340 and 1600. These measurements did not
distinguish between the parent compound and the
metabolites and, therefore, should be regarded as over-
estimates of bioaccumulation.
4.2. Transport and distribution between media
4.2.1. Air
Little relevant information is available to predict
the fate of HEX in the air. Cupitt (1980) estimated its
tropospheric residence time to be approximately 5 h, based
on estimated rates of reaction with photochemically
produced hydroxyl radicals and ozone. The theoretical
reaction rates were calculated to be 59 x 10-12 and
8 x 10-18 cm3 molecule-1 sec-1, respectively. In
estimating the tropospheric residence time, or the time
for a quantity of HEX to be reduced to 1/e (or approxi-
mately 37%) of its original value, it was assumed that the
rate constants calculated at room temperature for both
reactions were valid in the ambient atmosphere, and that
the background concentrations of hydroxyl radical and
ozone were 106 and 1012 molecules cm3, respectively.
Direct atmospheric photolysis of HEX was also rated as
"probable", since HEX has a chromophore that absorbs
light in the solar spectral region, and is known to photo-
lyse in aqueous media. No attempt was made to estimate a
rate for atmospheric photolysis. Cupitt (1980) listed the
theoretical degradation products as phosgene, diacylchlor-
ides, ketones, and free chlorine radicals, all of which
would be likely to react with other elements and
compounds.
The vapour pressure and vapour density, water solu-
bility, sorption properties, rapid photolysis (Wolfe et
al., 1982), and high reactivity (Callahan et al., 1979) of
HEX are significant factors that affect its atmospheric
transport. The atmospheric transport of HEX vapour from a
closed waste site in Montague, Michigan, USA, was reported
by Peters et al. (1981). At an unspecified distance
downwind from the site, HEX was detected in the air at
concentrations of 0.36-0.59 µg/m3 (0.032-0.053 ppb).
Based on the concentration ratio of HEX and a tracer gas
released at a known rate, the average HEX emission rate
during the measurement period was calculated to be
0.26 g/h.
4.2.2. Water
In the event of release into shallow or flowing bodies
of water, degradative processes such as photolysis, hydro-
lysis, and biodegradation, as well as transport processes
involving volatilization and other physical loss mechan-
isms, would be expected to play a significant role in
dissipating HEX. In deeper, non-flowing bodies of water,
hydrolysis and biodegradation may become the predominant
processes in determining the fate of HEX.
HEX introduced into bodies of water may be transported
in either the undissolved, dissolved, or sorbed forms. In
its undissolved form, HEX will tend to sink because of its
high relative density, and it may then become concentrated
in deeper waters where photolysis and volatilization would
be precluded. Some HEX may be dissolved in water (up to
approximately 2 mg/litre) and then be dispersed with water
flow. The solubility of HEX in water, soil extracts, and
sanitary landfill leachates ranges from 1.03 to 1.25 mg
per litre (Chou & Griffin, 1983). It tends to sorb onto
organic matter and may then be transported with water flow
in a suspended form. Transport to the air may occur by
volatilization, which has been measured in laboratory
studies (Kilzer et al., 1979; Weber, 1979) and was pre-
dicted using the EXAMS model by Wolfe et al. (1982). How-
ever, suspended solids in surface water may be a major
factor in reducing volatilization.
The photodegradation and degradation products of HEX
in aqueous solution have been studied in the laboratory
(Chou & Griffin, 1983; Chou et al., 1987). When aqueous
solutions containing 1.33 µg HEX/ml (a concentration
below its solubility in water) were exposed to sunlight,
the rate of photodegradation followed a first-order reac-
tion; the photolytical half-lives of HEX in tap water,
creek water, and distilled water in sunlight were all less
than 4 min. At least eight degradation products were posi-
tively or tentatively identified, 2,3,4,4,5-pentachloro-2-
cyclopentenone, hexachloro-2-cyclopentenone, and hexa-
chloro-3-cyclopentenone being the primary photodegradation
products. Secondary degradation products and other com-
pounds were formed through minor routes of degradation.
A proposed pathway for aqueous degradation is shown in
Fig. 2 (Chou & Griffin, 1983).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Overviewa
The fate and transport of HEX in the atmosphere are
not well understood, but the available information
suggests that the compound does not persist. Atmospheric
transport of HEX from an area of stored waste has been
reported (Peters et al., 1981). Experimentally derived
constants for HEX in various environmental processes are
given in Table 2.
Table 2. Summary of constants used in the exposure
analysis modelling system (EXAMS)a
---------------------------------------------------
Constants Values used
---------------------------------------------------
Water solubility (Ks) 1.8 mg/litre
Henry's law constant (KH) 2.7 x 10-2 atm m3/mol
Octanol/water partition 1.1 x 105
coefficient (Pow)
Photolysis (kp) 3.9 h-1
Hydrolysis 4.0 x 10-3 h-1b
Oxidation (kox) 1 x 10-10 M-1 sec-1c
Biodegradation (kB) 1 x 10-5 ml org-1 h-1d
---------------------------------------------------
a Adapted from Wolfe et al. (1982).
b Extrapolated to 25 °C.
c Estimated value (Wolfe et al., 1982).
d This is a maximum value based on the observation
that there was no detectable difference in the
hydrolysis rate in either sterile or non-sterile
studies and measured organism numbers (plate
counts).
In water, HEX probably dissipates rapidly by means of
photolysis, hydrolysis, and biodegradation. In shallow
water (a few centimetres deep), it has a photolytic half-
life of approximately 0.2 h (Butz et al., 1982; Wolfe et
al., 1982). Chou et al. (1987) found this first-order
reaction to take even less time in full sunlight. In
deeper water where photolysis is precluded, hydrolysis and
biodegradation should become the key degradative processes
when there is little movement in the system. The hydro-
lytic half-life of HEX ranges from several days to
-----------------------------------------------------------
a Throughout this chapter, the terms sorb and sorption
are used in preference to absorb/adsorb and
absorption/adsorption.
approximately 3 months, and it is not strongly affected by
the pH in the environmental range (5-9), by salinity or by
the presence of suspended solids (Yu & Atallah, 1977a;
Wolfe et al., 1982). HEX is known to volatilize from water
(Kilzer et al., 1979; Weber, 1979). It is probable that
volatilization is limited by diffusion, i.e. loss from
deeper waters should occur very slowly unless vertical
mixing has taken place. Sorption on sediments may also
retard volatilization.
The fate and transport of HEX in soils are affected by
its strong tendency to sorb onto organic matter (Weber,
1979; Kenaga & Goring, 1980; Wolfe et al., 1982). Another
possibility is that HEX partitions to the interior of soil
particles and stays in loams and silt in a dissolved
state. HEX should be relatively immobile in soil because
of its high log P value (Briggs, 1973), but several inci-
dents in the USA have shown that this is not true in all
soil types (Sprinkle, 1978). Volatilization, which is
likely to occur primarily at the soil surface, is
inversely related to the organic matter level and water-
holding capacity of the soil (Kilzer et al., 1979). Leach-
ing of HEX by ground water can occur, while chemical
hydrolysis and microbial metabolism would be expected to
reduce levels in the environment. HEX is metabolized by a
number of unidentified soil microorganisms (Rieck,
1977b,c; Thuma et al., 1978).
The high lipophilicity and log Pow of HEX indicate a
high potential for bioaccumulation. However, in practice,
this potential is not realized because of metabolism and
elimination. Steady-state bioconcentration factors (BCFs)
in fish measured in 30-day flow-through systems were 29 or
less (Spehar et al., 1979; Veith et al., 1979). In a model
ecosystem study, BCF values for a range of aquatic organ-
isms were between 340 and 1600. These measurements did not
distinguish between the parent compound and the
metabolites and, therefore, should be regarded as over-
estimates of bioaccumulation.
4.2. Transport and distribution between media
4.2.1. Air
Little relevant information is available to predict
the fate of HEX in the air. Cupitt (1980) estimated its
tropospheric residence time to be approximately 5 h, based
on estimated rates of reaction with photochemically
produced hydroxyl radicals and ozone. The theoretical
reaction rates were calculated to be 59 x 10-12 and
8 x 10-18 cm3 molecule-1 sec-1, respectively. In
estimating the tropospheric residence time, or the time
for a quantity of HEX to be reduced to 1/e (or approxi-
mately 37%) of its original value, it was assumed that the
rate constants calculated at room temperature for both
reactions were valid in the ambient atmosphere, and that
the background concentrations of hydroxyl radical and
ozone were 106 and 1012 molecules cm3, respectively.
Direct atmospheric photolysis of HEX was also rated as
"probable", since HEX has a chromophore that absorbs
light in the solar spectral region, and is known to photo-
lyse in aqueous media. No attempt was made to estimate a
rate for atmospheric photolysis. Cupitt (1980) listed the
theoretical degradation products as phosgene, diacylchlor-
ides, ketones, and free chlorine radicals, all of which
would be likely to react with other elements and
compounds.
The vapour pressure and vapour density, water solu-
bility, sorption properties, rapid photolysis (Wolfe et
al., 1982), and high reactivity (Callahan et al., 1979) of
HEX are significant factors that affect its atmospheric
transport. The atmospheric transport of HEX vapour from a
closed waste site in Montague, Michigan, USA, was reported
by Peters et al. (1981). At an unspecified distance
downwind from the site, HEX was detected in the air at
concentrations of 0.36-0.59 µg/m3 (0.032-0.053 ppb).
Based on the concentration ratio of HEX and a tracer gas
released at a known rate, the average HEX emission rate
during the measurement period was calculated to be
0.26 g/h.
4.2.2. Water
In the event of release into shallow or flowing bodies
of water, degradative processes such as photolysis, hydro-
lysis, and biodegradation, as well as transport processes
involving volatilization and other physical loss mechan-
isms, would be expected to play a significant role in
dissipating HEX. In deeper, non-flowing bodies of water,
hydrolysis and biodegradation may become the predominant
processes in determining the fate of HEX.
HEX introduced into bodies of water may be transported
in either the undissolved, dissolved, or sorbed forms. In
its undissolved form, HEX will tend to sink because of its
high relative density, and it may then become concentrated
in deeper waters where photolysis and volatilization would
be precluded. Some HEX may be dissolved in water (up to
approximately 2 mg/litre) and then be dispersed with water
flow. The solubility of HEX in water, soil extracts, and
sanitary landfill leachates ranges from 1.03 to 1.25 mg
per litre (Chou & Griffin, 1983). It tends to sorb onto
organic matter and may then be transported with water flow
in a suspended form. Transport to the air may occur by
volatilization, which has been measured in laboratory
studies (Kilzer et al., 1979; Weber, 1979) and was pre-
dicted using the EXAMS model by Wolfe et al. (1982). How-
ever, suspended solids in surface water may be a major
factor in reducing volatilization.
The photodegradation and degradation products of HEX
in aqueous solution have been studied in the laboratory
(Chou & Griffin, 1983; Chou et al., 1987). When aqueous
solutions containing 1.33 µg HEX/ml (a concentration
below its solubility in water) were exposed to sunlight,
the rate of photodegradation followed a first-order reac-
tion; the photolytical half-lives of HEX in tap water,
creek water, and distilled water in sunlight were all less
than 4 min. At least eight degradation products were posi-
tively or tentatively identified, 2,3,4,4,5-pentachloro-2-
cyclopentenone, hexachloro-2-cyclopentenone, and hexa-
chloro-3-cyclopentenone being the primary photodegradation
products. Secondary degradation products and other com-
pounds were formed through minor routes of degradation.
A proposed pathway for aqueous degradation is shown in
Fig. 2 (Chou & Griffin, 1983).
 Kilzer et al. (1979) determined the rate of 14C-HEX
volatilization from water as a function of the rate of
water evaporation. Bottles containing aqueous HEX sol-
utions (50 µg/litre) were kept at 25 °C without shaking.
The escaping vapour condensed on a "cold finger" and was
quantified by liquid scintillation spectroscopy. Based on
recovery of added label, the HEX volatilization rates for
the first and second hours of testing were calculated to
be 5.87 and 0.75%/ml of water, respectively. Since the
water evaporation rate was 0.8-1.5 ml/h, the evaporation
rates for HEX were within the ranges of 4.7-8.8 and 0.6-
1.1%/h, respectively. These results suggest that a fairly
rapid initial volatilization occurred at the water sur-
face, and that by the second hour diffusion of HEX to the
water surface may have been limiting because of the static
conditions of the test. If the rate observed during the
second hour had continued for the remaining 24 h, the
total loss would have been approximately 18-34%, i.e.
somewhat less than that observed in the test conducted by
Weber (1979) where unstoppered bottles were shaken.
At 25-30 °C and in the environmental pH range of 5-9,
the hydrolytic half-life of HEX was found to be approxi-
mately 3-11 days (Yu & Atallah, 1977a; Wolfe et al.,
1982). In a later study in which evaporation and photo-
chemical reactions were carefully prevented, the hydro-
lytic half-life was approximately 3 months (Chou &
Griffin, 1983). Hydrolysis is much slower than photolysis
(see Table 2) but may be a significant load-reducing
process in waters where photolysis and physical transport
processes are not important (i.e. in deep, non-flowing
waters). Wolfe et al. (1982) found HEX hydrolysis to be
independent of pH over the range of 3-10. The rate con-
stant was dependent on temperature at pH 7.0, and the
half-life was estimated to be 3.31, 1.71, and 0.64 days at
30, 40, and 50 °C, respectively. The addition of various
buffers or sodium chloride (0.5 mol/litre) did not affect
the hydrolysis rate constant, suggesting that the rate
constant obtained would be applicable to marine environ-
ments as well. The addition of natural sediments, suf-
ficient to sorb up to 92% of the compound, caused the rate
constant to vary by less than a factor of 2. It was
therefore concluded that sorption to sediments would not
significantly affect the rate of hydrolysis (Wolfe et al.,
1982).
4.2.3. Soil
When it is released on to soil, HEX is likely to sorb
strongly to any organic matter or humus present (Weber,
1979; Kenaga & Goring, 1980). The HEX concentrations
should decrease with time as populations of soil micro-
organisms that are better adapted to metabolize HEX
increase (Rieck, 1977b,c; Thuma et al., 1978). Volatiliz-
ation, photolysis, and various chemical processes may also
dissipate the compound in certain soil environments.
The main methods of transport for HEX applied to the
soil are (a) the movement of particles to which it is
sorbed and (b) volatilization. Other possibilities are
that HEX is sorbed on to soil colloids or that it par-
titions to the interior of soil particles and stays in
loams and silts in a dissolved state. No data are avail-
able pertaining to HEX transport on soil particles. How-
ever, in a few studies, the rate of volatilization from
soils has been reported and is discussed in the following
paragraphs.
Kilzer et al. (1979) found that 14C-HEX and its
degradation products volatilized from moist soils (sand,
loam, and humus) at a faster rate during the first hour of
the study than during the second hour. HEX (50 µg/kg) was
placed in bottles with each soil type, the bottles were
shaken vigorously, and they were then incubated for 2 h at
25 °C without shaking. The radiolabelled HEX condensed on
a "cold finger" and was quantified by liquid scintil-
lation counting. For sand, loam, and humus, the volatiliz-
ation rate was expressed as the percentages of applied
radioactivity per ml of evaporated water. For the first
hour the percentages were 0.83, 0.33, and 0.14%, respect-
ively, and for the second hour they were 0.23, 0.11, and
0.05%. For HEX and nine other tested chemicals, the
authors found that the volatilization rate from distilled
water could not be used to predict the rate from wetted
soils. Among the chemicals tested, there was no corre-
lation between water solubility or vapour pressure and
volatilization from soils. The volatilization rate for
HEX and its metabolites in soil was primarily dependent on
soil organic matter content, mainly because of the highly
sorptive characteristics of HEX.
In a model ecosystem study, Kloskowski et al. (1981)
applied 14C-HEX to 1 kg of humus sand soil (2 mg/kg) and
grew summer barley by keeping the system under an illumi-
nation of 10 000 lux (12 h light, 12 h dark) at 20-24 °C
in an enclosed 10-litre desiccator with an aeration of
10 ml/min. After 7 days, approximately 19.5% of the orig-
inal radioactivity was recovered in the form of 14CO2
evolved and 0.5% as volatilized organics. The level of
radiolabelled compounds in the plants was 13.4 mg/kg,
which represented a bioaccumulation factor of 7.1 (i.e.
plant residues divided by soil residues). It is not clear
whether the plants played a major role in the volatiliz-
ation or metabolic fate of HEX, but the total 14C recov-
ery was over 95%.
Rieck (1977c) measured the rate of volatilization of
HEX from Maury silt loam soils. After the application of
100 mg 14C-HEX to soil, the cumulative evaporation of HEX
and its non-polar metabolites (penta- and tetrachloro-
cyclopentadiene) on days 1, 2, 3, 5, 7, and 14 was 9.3,
10.2, 10.6, 10.8, 11.0, and 11.2%, respectively. The
results indicated that HEX evaporation to air occurred
mainly during the first day after application and was
probably associated with the surface soil only.
The soil sorption properties of compounds such as HEX
can be predicted from their soil organic carbon/water
partition coefficients (Koc). Kenaga (1980) examined
the sorption properties of 100 chemicals and concluded
that compounds with Koc values > 1000 are tightly bound
to soil components and are immobile in soils. Those with
values < 100 are sorbed less strongly and are considered
to be moderately to highly mobile. Thus, the theoretical
Koc value is useful as an indicator of potential soil
leachability or binding of the chemical. The Koc values
also indicate whether a chemical is likely to enter water
by leaching or by being sorbed to eroded soil particles.
Since Koc values for HEX are not available in the
literature, these values were calculated using the
following mathematical equation, developed by Kenaga &
Goring (1980) and Kenaga (1980):
log Koc = 3.64-0.55 (log WS)
where WS is water solubility (mg/litre), and the 95%
confidence limits are ± 1.23 orders of magnitude. The
calculated values of Koc for HEX using the reported water
solubility values of 2.1 mg/litre (Dal Monte & Yu, 1977),
1.8 mg/litre (Wolfe et al., 1982), and 0.805 mg/litre (Lu
et al., 1975) are 2903, 3159, and 4918, respectively.
Since these calculated Koc values are all > 1000, the
authors concluded that HEX is tightly bound to soil
components and immobile in the soil compartment. Simi-
larly, Briggs (1973) concluded that compounds with a log
octanol/water partition coefficient (log Pow) > 3.78 are
immobile in soil. Log Pow values for HEX of 5.04 (Wolfe
et al., 1982) and 5.51 (Veith et al., 1979) have been
measured.
In studies by Chou & Griffin (1983), the mobility of
HEX (C-56) in six soils was measured with several leaching
solvents using soil thin-layer chromatography (TLC) and
column leaching studies. It remained immobile in the soil
when leached with water, landfill leachate, or caustic
brine, but was highly mobile when leached with organic
solvents. A further conclusion was that several degra-
dation products of HEX migrated through soils faster than
HEX itself, and that the degradation products warranted
further study. The sorption capacity of HEX was highly
correlated with the total organic carbon (TOC) content of
soil materials (r2 = 0.97), which was the dominant soil
characterization parameter. Sorption appears to be pre-
dictable from the TOC content of soils (Chou & Griffin,
1983).
4.3. Biotransformation
4.3.1. Biodegradation
The metabolism of HEX by soil microorganisms is
apparently an important process in its environmental
degradation. Soil degradation is rapid under non-sterile
aerobic and anaerobic conditions, and indirect evidence
for microbial involvement has been reported by Rieck
(1977b,c). In one of his studies, Rieck (1977b) used
several types of treatments and soils of different pH to
determine whether the biodegradation of HEX in Maury silt
loam soil was biologically or chemically mediated, or
both. Soils were incubated in glass flasks covered with
perforated aluminum foil and kept on a laboratory shelf,
presumably exposed to ambient lighting through the flask
walls. When 14C-HEX was applied to non-sterile soil at
1 mg/kg, only 6.1% was recovered as non-polar material
(either HEX or non-polar degradation products) 7 days
after treatment, and approximately 71.7% was polar and
unextractable material. Adjustment of the pH to 4 or 8 had
little effect on these results. By comparison, in auto-
claved soil (the control), 36.1% of the applied dose was
recovered as non-polar material and only 33.4% was
recovered as polar and unextractable material. The degra-
dation of HEX under anaerobic (flooded) conditions
occurred at a slightly faster rate than under aerobic
conditions. However, no sterile, flooded control was used
to determine the effects of hydrolysis, which could have
accounted for the observed effect in this treatment. The
mean total recovery in all treatments decreased from 67%
at 7 days to 55% at 56 days. This decrease was attributed
to volatilization of HEX and/or its degradation products.
Volatilization from soil was examined in a further
experiment (Rieck, 1977c). In a 14-day study, radiocarbon
volatilized from non-sterile, 14C-HEX-treated soil was
trapped and assayed. A total of 20.2% of the applied 14C
was trapped: 11.2% in hexane and 9.0% in ethanolamine-
water. Most of the hexane fraction (9.3% of the applied
14C) was trapped during the first day, and probably
represented volatilized HEX. However, the ethanolamine-
water fraction, considered to represent evolved carbon
dioxide, was released gradually over the 14-day period.
In the soil analysis, non-polar (extractable) and polar
(extractable and unextractable) material accounted for 3.4
and 40.0% of the dose, respectively, during the 14 days;
total recovery was only 63.6% including volatilization.
No metabolic products were identified in the two studies
by Rieck (1977b,c).
Thuma et al. (1978) studied the feasibility of using
selected pure cultures (organisms not identified) to
biodegrade spills of hazardous chemicals, including HEX,
on soil. They tested 23 organisms and found that from
2-76% of the applied HEX had been removed from the aqueous
culture medium within 14 days. Seven of the 23 organisms
degraded more than 33% of the HEX within 14 days. Losses
of HEX by other means than biodegradation were accounted
for by using controls.
Atallah et al. (1980) conducted an aqueous aerobic
biodegradability study to determine whether HEX could be
degraded to CO2 and at what rate. The inoculum was a
mixed acclimated culture containing secondary municipal
waste effluent and several strains of Pseudomonas putida.
14C-labelled HEX was the sole source of carbon in the
study, with the exception of trace levels of vitamins.
Total removal of 14C, primarily as volatile organic com-
pounds, was > 80% during the first day in both uninocu-
lated (45 mg HEX/litre) and inoculated (4.5 and 45 mg
HEX/litre) media, although removal was slightly greater in
inoculated media. 14CO2 was released from both media,
indicating that CO2 was a product of hydrolysis as well
as of biodegradation. The rate of conversion to CO2 was
initially higher in the uninoculated media, but after 1
week, became higher in the inoculated media. This study
showed clearly that HEX can be biodegraded in aquatic
media under laboratory conditions. However, Wolfe et al.
(1982) failed to detect any difference between the HEX
degradation rates in hydrolysis experiments where non-
sterile natural sediments were added to water (10 g/litre)
and those where sterile sediment was used. They calculated
a relatively low value (1 x 10-5 ml org-1 h-1; see
Table 2) as a maximum biodegradation rate, and conse-
quently biodegradation was estimated to be a relatively
unimportant fate process in the EXAMS model (see Table 3).
These studies indicate that the persistence of HEX in
soil is brief, degradation of more than 90% of applied HEX
to non-polar products occurring within approximately 7
days. Factors contributing to this loss include abiotic
and biotic degradation processes and volatilization,
although the relative importance of each is difficult to
quantify.
4.3.2. Bioconcentration, bioaccumulation, and biomagnification
Bioaccumulation, sometimes also expressed as biologi-
cal persistence, is a consequence of the rate of elimin-
ation of a compound and the extent of adsorption.
The terminology used in this section conforms to that
used by Macek et al. (1979):
* bioconcentration implies that tissue residues result
only from simultaneous uptake and elimination from
exposure to the ambient environment (e.g., air for
terrestrial species or water for aquatic species);
* bioaccumulation considers all exposures (air, water,
and food) of an individual organism to be the source
of tissue residues;
* biomagnification defines the increase in tissue
residues observed at successively higher trophic
levels of a food web.
Table 3. Summary of results of computer simulation of the
fate and transport of hexachlorocyclopentadiene in four
typical aquatic environmentsa
---------------------------------------------------------------
River Pond Eutrophic Oligotrophic
lake lake
---------------------------------------------------------------
Distribution (%)
Water column 1.22 14 12.97 2.91b
Sediment 98.78 86 87.03 97.09
Recovery timec (days) 52 81 58 87
Load reduction (%) by processes:
Hydrolysis 8.04 17.85 8.29 16.50
Oxidation 0.00 0.00 0.00 0.00
Photolysis 18.68 80.39 89.18 82.41
Biodegradation 0.57 0.23 0.30 0.01
(biolysis)
Volatilization 0.69 1.33 1.56 1.08
Exportd 72.02 0.20 0.01 0.00
---------------------------------------------------------------
a Adapted from Wolfe et al. (1982), with correction applied.
b Value was incorrectly reported as 32.91 in original paper.
c The time needed to reduce steady-state concentrations by
97% (five half-lives).
d Physical loss from the system by any pathway other than
volatilization.
The log octanol/water partition coefficient of HEX has
been experimentally determined to be 5.04 (Wolfe et al.,
1982) and 5.51 (Veith et al., 1979), which would indicate
a substantial potential for bioconcentration, bioaccumu-
lation, and biomagnification. Actual determinations of
bioconcentration and bioaccumulation in several aquatic
organisms, however, indicate that HEX does not accumulate
to any great extent (Lu et al., 1975; Podowski & Khan,
1979, 1984; Spehar et al., 1979; Veith et al., 1979),
mainly because it is metabolized rapidly.
Podowski & Khan (1979, 1984) conducted several exper-
iments on the uptake, bioaccumulation, and elimination
of 14C-HEX in goldfish ( Carassius auratus) and concluded
that this species rapidly eliminates absorbed HEX. In one
experiment, fish were transferred daily into fresh sol-
utions of 14C-HEX for 16 days. This transfer of three
fish/jar resulted in an accumulative exposure of 240 µg
of HEX. Nominal HEX concentrations of 10 µg/litre re-
sulted in measured water concentrations (based on radio-
activity) in the range of 3.4-4.8 µg/litre, because of
rapid volatilization of the compound. Radioactivity
accumulated rapidly in fish tissue, reaching a maximum on
day 8 corresponding to approximately 6 mg HEX/kg. Since an
undetermined amount of the radioactivity was present as
metabolites, no bioconcentration factor could be calcu-
lated. From day 8 to day 16, tissue levels declined in
spite of the daily renewal of exposure solutions, indi-
cating that excretion of HEX and/or its metabolites was
occurring more rapidly than uptake. In a static exposure
to an initial measured HEX concentration of 5 µg per
litre, uptake of the radiolabel by the fish was to a level
corresponding to 1.6 mg HEX/kg on day 2, accompanied by a
slight decrease of HEX in the water. By day 4, approxi-
mately 50% of the radiolabel had been excreted, and the
radioactivity in the water increased. Over the following
12 days, the radioactivity in both water and fish declined
slowly.
Podowski & Khan (1979, 1984) also studied the elimin-
ation, metabolism, and tissue distribution of HEX injected
intraperitoneally into goldfish and concluded that gold-
fish eliminate injected HEX both rapidly and linearly
(the biological half-life was approximately 9 days). The
fish (27-45 g) were injected with 39.6 µg 14C-HEX and
analysed 3 days later. Of the 97% of the radiolabelled
dose accounted for, approximately 18.9% was eliminated by
the fish. Of the residue found in the fish, 47.2% was
extractable in organic solvent (little of the radio-
labelled material could be identified as HEX, which indi-
cated that extensive biotransformation had occurred),
10.6% consisted of water-soluble metabolites, and 20.3%
was unextractable. None of the metabolites were ident-
ified. The elimination was biphasic, consisting of a rapid
initial phase followed by a slower terminal phase.
In another part of these studies, the residual
activity in several fish tissues was assayed 2, 4, 6, and
8 days after an injection of 38.4 µg 14C-HEX per fish.
The activity corresponded to 0.2 and 0.3 µg HEX/kg in the
spinal cord and gills, respectively, concentrations that
remained constant throughout the 8-day period of the
study. Residues in the kidneys and bile increased within
the same period from 1-3 and 0-32 µg/kg, respectively,
indicating elimination by these routes. The authors stated
that the increase probably occurred from enhanced con-
version of the parent compound into polar products, which
could be excreted more easily. In the other tissues, all
residual levels decreased, leaving only the liver with a
level of more than 1 µg/kg. The metabolites were not
identified (Podowski & Khan, 1979, 1984).
Veith et al. (1979) determined a bioconcentration
factor (BCF) for HEX of 29 in the fat-head minnow
(Pimephales promelas). In a 32-day flow-through study,
30 fish were exposed to HEX at a mean concentration of
20.9 µg/litre. Five fish at a time were killed at 2, 4,
8, 16, 24, and 32 days for residue analysis. The study
was conducted using Lake Superior water at 25 °C (pH 7.5,
dissolved oxygen > 5.0 mg/litre, and hardness 41.5 mg
CaCO3/litre. On the basis of its estimated octanol/water
partition coefficient alone (log Pow = 5.51), a BCF of
approximately 9600 would have been predicted. However,
HEX did not bioconcentrate substantially, and therefore
deviated from the log P:log BCF relationship shown for
most of the other 29 chemicals tested by these researchers.
Lu et al. (1975) studied the fate of HEX in a model
terrestrial-aquatic ecosystem maintained at 26.7 °C with a
12-h photoperiod. The model ecosystem consisted of 50
sorghum (Sorghum vulgare) plants (7.62-10.16 cm tall) in
the terrestrial portion, while 10 snails (Physa sp.), 30
water fleas (Daphnia magna), filamentous green algae
(Oedogonium cardiacum), and a plankton culture were
added to the aquatic portion. The sorghum plants were
treated topically with 5.0 mg 14C-HEX in acetone to simu-
late a terrestrial application of 1.1 kg/hectare. Ten
early-fifth-instar caterpillar larvae (Estigmene acrea)
were placed on the plants. The insects consumed most of
the treated plant surface within 3-4 days. The faeces,
leaf grass, and the larvae themselves contaminated the
moist sand, permitting distribution of the radiolabelled
metabolites by water throughout the ecosystem. After 26
days, 300 mosquito larvae (Culex pipiens quinquefasciatus)
were added to the ecosystem, and on day 30 three mosquito
fish (Gambusia affinis) were added. The experiment was
terminated after 33 days, and the various parameters were
analysed. The radioactivity was then extracted with
diethyl ether from the water and with acetone from the
organisms. The results of thin-layer chromatographic
analysis of the extracts are presented in Table 4. Data
were not reported for Daphnia magna or the salt marsh
caterpillar. Uptake in this experiment occurred through
food as well as water, and therefore is termed bioaccumu-
lation rather than bioconcentration. Lu et al. (1975) used
the term ecological magnification to designate the bio-
accumulation factor (BAF). The BAF for HEX in fish was
448 (0.1076 mg/kg fish divided by 0.24 µg/litre water)
for the 3-day exposure period, indicating a moderate
potential for concentration (Kenaga, 1980). The BAFs in
algae (< 33-day exposure), snails (< 33-day exposure), and
mosquito larvae (7-day exposure) were reported to be 341,
1634, and 929, respectively (Lu et al., 1975).
Table 4. Relative distribution of hexachlorocyclopentadiene (HEX) and
its degradation products in a model ecosystema
------------------------------------------------------------------------
14C-HEX equivalents
Water Algae Snail Mosquito Fish
(mg/litre) (mg/kg) (mg/kg) larva (mg/kg)
(mg/kg)
------------------------------------------------------------------------
HEX 0.00024 0.0818 0.3922 0.2230 0.1076
Other extractable 0.00204 0.1632 0.3824 0.2542 0.1542
compounds
Total extractable14Cb 0.00228 0.2450 0.7746 0.4772 0.2618
Unextractable14C 0.00750 0.0094 0.0814 0.0104 0.0982
Total14Cc 0.00978 0.2544 0.8560 0.4876 0.3600
------------------------------------------------------------------------
a Source: Lu et al. (1975).
b Sum of HEX and other extractable compounds.
c Sum of total extractable and unextractable 14C.
Biomagnification, measured as the ratio of HEX
residues between trophic levels (e.g., snail/algae or
fish/mosquito), was far less substantial than bioconcen-
tration. Based on the HEX tissue residues, the snail/algae
ratio was 0.3922/0.0818 = 4.8 and the fish/mosquito ratio
was 0.1076/0.2230 = 0.48.
Lu et al. (1975) also studied the metabolism of HEX by
the organisms present in the model terrestrial-aquatic
ecosystem, but none of the products was identified except
for HEX. The authors reported that unmetabolized HEX re-
presented a large percentage of the total extractable 14C,
being 33% in algae, 50% in snail, 46% in mosquito, and 41%
in fish. The percentage of biodegradation was calculated
for each organism (unextractable 14C x 100/total 14C) and
found to be 4% for the algae (in < 33 days), 10% for the
snails (in < 33 days), 2% for the mosquitoes (in 7 days),
and 27% for the fish (in 3 days). However, these values
may underestimate the extent of metabolism, since acetone-
extractable polar compounds were not considered in the
calculations.
The Velsicol Chemical Corporation (1978) conducted
fish tissue residue studies in waters located below their
facility in Memphis, Tennessee, USA, and reported that HEX
was not detected in either catfish or carp, although
chlorinated compounds, including octachlorocyclopentadiene
(a common co-contaminant), were detected in the fish
tissue. This indicated that HEX was not accumulated. The
possible source of these other compounds was not
discussed. In a joint USA federal and state study of the
Mississippi River at locations above, around, and below
Memphis, Bennett (1982) reported that HEX was not detected
in any of the eight fish sample groups analysed by GC/MS.
4.4. Interactions with other physical and chemical factors
4.4.1. Phototransformation
Zepp et al. (1979) and Wolfe et al. (1982) reported
the results of US EPA studies on the rate of HEX photo-
transformation in water. Under a variety of sunlight
conditions, in both distilled and natural waters of 1-4 cm
depth, the phototransformation half-life was < 10 min.
Chou & Griffin (1983) determined a half-life of < 4 min at
740 j/m2. The addition of natural sediments to distilled
water containing HEX had little effect on the phototrans-
formation rate. These findings indicate that the dominant
mechanism of HEX phototransformation is direct absorption
of light by the chemical, rather than photosensitization
reactions involving other dissolved or suspended
materials.
The direct photoreaction of HEX in water was also
studied under controlled conditions in the laboratory
using a monochromatic light (313 nm) with a mercury lamp
source and appropriate filters. Phototransformation rate
constants, computed for the study location (Athens,
Georgia, USA, 34 °N latitude), agreed with those observed
in the sunlight experiments described above. Rate con-
stants were also computed for various times of day at a
latitude of 40 °N. The near-surface phototransformation
rate constant of HEX at this latitude on cloudless days
(averaged over both light and dark periods for 1 year) was
3.9 h-1, which corresponds to a very rapid half-life of
10.7 min (Zepp et al., 1979; Wolfe et al., 1982).
These laboratory researchers suggested that the pri-
mary phototransformation product was the hydrated form of
tetrachlorocyclopentadienone (C5Cl4O, TCPD), although
it was not isolated. Several chlorinated photoproducts
with a higher relative molecular mass than HEX were
detected by GC/MS analysis of the reaction mixture. Photo-
lysis of HEX in methanol gave a product identified as the
dimethyl ketal of TCPD (Wolfe et al., 1982). According to
Zepp et al. (1979), it is likely that TCPD exists predomi-
nantly in its hydrated form in the aquatic environment.
The compound was not isolated, supposedly because it
rapidly dimerizes or reacts to form products of higher
relative molecular mass. Chou et al. (1987) identified
2,3,4,4,5-pentachloro-2-cyclopentenone, hexachloro-2-cyclo-
pentenone, and hexachloro-3-cyclopentenone as the primary
photodegradation products, as well as several other pri-
mary and secondary ones (Chou & Griffin, 1983; Fig. 2).
Yu & Atallah (1977b) found that, at a concentration of
2.2 mg/litre in water, uniformly labelled 14C-HEX was
rapidly converted to water-soluble products upon
irradiation with light from a mercury vapour lamp (light
energy: 40-48% ultraviolet, 40-43% visible, remainder
infrared). In exposures lasting 0.5-5.0 h, 46-53% of the
radiolabel was recovered in the form of water-soluble
products (compared with 7% at initiation), whereas the
amount recovered by organic (petroleum ether) extraction
decreased with increasing exposure duration from 25% to 6%
(compared with 66% at initiation). HEX was not detected
among the photoproducts in the organic extraction. Chou
et al. (1987) also found that dimerization of degradation
products to form compounds of higher relative molecular
mass was only a minor route of degradation.
4.4.2. Oxidation
HEX would not be expected to be oxidized under ordi-
nary environmental conditions. In the laboratory, HEX
reacts with molecular oxygen at 95-105 °C to form a mix-
ture of hexachlorocyclopentenones (Molotsky & Ballweber,
1957). However, based on an estimated second-order oxi-
dation rate constant of 1 x 10-10 M-1 sec-1 at 25 °C
in water (Table 2), the EXAMS computer simulation of Wolfe
et al. (1982) predicted that HEX would not be oxidized in
the simulated river, pond, eutrophic lake or oligotrophic
lake (Table 3).
4.5. Disposal and fate
HEX and HEX-contaminated material and wastes are
disposed of in secure chemical landfills, by incineration,
and by deep well injection (US EPA, 1989). Additionally,
there are solid waste regulations in the USA because,
under the Resource Conservation and Recovery Act, HEX is
designated to be a toxic waste. German regulations are
similar to those of the USA, except that there is no deep
well injection (BUA, 1988).
Since the photodegradation products of HEX have been
identified only recently and because HEX has also been
found in areas where waste has not been disposed of for
years (US EPA, 1980c), it is difficult to determine its
fate in the environment.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
Releases of HEX into the atmosphere can result from
the production, processing, and use of HEX, the disposal
of wastes containing HEX, or from products contaminated
with HEX (Hunt & Brooks, 1984). Data sent to the US EPA
for 1987 regarding emission levels from companies in the
USA indicated that 1400 kg of HEX was emitted into the air
(US EPA, 1989). In the Federal Republic of Germany and
the Netherlands, about 400-500 kg was emitted to the air
in 1987 (BUA, 1988)
In September and October 1985, the Velsicol Chemical
Corporation determined concentrations of HEX at predeter-
mined locations around its production facilities in
Tennessee, USA. The study was designed to measure ambient
concentrations in the air during routine manufacturing
operations. Of the 25 samples collected, 15 were below the
analytical limit of detection, i.e. 0.03 µg (0.1 ppb).
The air HEX levels in the other samples ranged between 1
and 10 µg/m3 (0.1-0.9 ppb) when ambient temperatures
were between 4.4 °C and 27.7 °C (Velsicol Chemical Corpor-
ation, 1986).
The highest reported concentration of HEX measured in
homes in Tennessee was 0.10 µg/m3, while air levels at
the Memphis North treatment plant were as high as
39 µg/m3 (C.S. Clark et al., 1982; Elia et al., 1983).
In an air monitoring study on an abandoned waste site in
Michigan, the average HEX emission rate was 0.26 (± 0.05)
g/h. In May 1977, HEX was detected at a level of 633 µg
per m3 (56 ppb) in air samples collected from a waste
site in Montague, Michigan (US EPA, 1980c).
5.1.2. Water
Benoit & Williams (1981) sampled both untreated water
and drinking-water from a water treatment plant in Ottawa,
Canada. Using solvent extraction analysis with a detection
sensitivity of 50 ng/litre (or the XAD-2 resin extraction
method with a detection sensitivity of approximately 0.5
ng/litre), the authors did not detect any HEX in the
untreated water, but reported levels ranging from 57-110
ng/litre in the finished drinking-water. These results
suggest that HEX was introduced into the drinking-water
during the treatment process. However, the researchers did
not find the source of the HEX. Meier et al. (1985) found
that HEX can be produced through the chlorination of humic
acid.
Limited monitoring data from production sites revealed
that HEX was present in a spot sample at a level of 18
mg/litre (February 1977) and a range of 0.156-8.24 mg per
litre (over the month of January 1977) in the aqueous dis-
charge from the Memphis pesticide plant (US EPA, 1980c).
The calculated concentration of HEX in the Mississippi
River was 6 µg/litre (Carter, 1977). In the summer of
1977, shortly after these readings, a new waste-water
treatment plant began operation (Table 5). Prior to con-
struction of the plant, waste water flowed directly into
the Mississippi River or through one of its tributaries
(Elia et al., 1983). Voluntary improvements in controlling
the discharge from the Memphis plant resulted in reported
levels of 0.07 µg HEX/litre in the Mississippi River,
near the mouth of Wolf Creek (Velsicol Chemical Corpor-
ation, 1978). HEX has also been identified in the soil and
river sediments downstream from a USA manufacturing plant,
even after pesticide production was discontinued (US EPA,
1980c).
5.1.3. Soil
Ambient monitoring data for the terrestrial environ-
ment are not available, but it seems that these concen-
trations should be much lower than those in the aquatic
environment. Deposition of HEX from the atmospheric (and
aquatic) compartments into the terrestrial environment is
expected to be minimal. Similarly, direct release of HEX
into the terrestrial environment (i.e. as an impurity in
chlorinated pesticides) should be decreasing because of
regulatory controls on some products, with the possible
exceptions of disposal at waste sites, accidental spills,
and other illegal disposal methods.
Table 5. Concentrations of selected organic compounds in
influent waste water at the Memphis North treatment plant, 1978a
---------------------------------------------------------------
Concentration (µg/litre)b
Date No. of HEX HEX-BCHc HCBCHd Chlordane
samples
---------------------------------------------------------------
June 1 3 334 57 87
August 5 0.8 329 115 216
September 2 4 292 668 58
October-November 2 0.8 11 17 32
---------------------------------------------------------------
a From: Elia et al. (1983).
b Mean values for the number of samples indicated.
c Hexachlorobicycloheptadiene.
d Heptachlorobicycloheptene.
5.1.4. Food
HEX was qualitatively detected in fish samples taken
from water near a pesticide manufacturing plant in
Michigan (Spehar et al., 1977), but none was detected in
fish samples taken from the waters near the pesticide
manufacturing plant in Memphis (Velsicol Chemical Corpor-
ation, 1978; Bennett, 1982). No information regarding HEX
contamination of other foods is available.
5.2. General population exposure
There are insufficient data to determine the relative
contributions of the various sources of HEX to the
environment. There will be exposure to HEX present in
some commonly used pesticides, and possibly some flame
retardants, where HEX is a contaminant. The US EPA has
reported that exposure of humans to HEX from the air or
water should be extremely low, except in the case of
workers and residents near manufacturing, shipping, and
waste sites, and concluded that general population ex-
posure was not considered to be significant or substantial
(US EPA, 1982).
The only other estimates of relative source contri-
butions are from reports completed for the US EPA (Hunt &
Brooks, 1984) and the BUA (1988). The air releases from
manufacturing processes can come from the vents on reac-
tors, process and storage tanks, and as fugitive
emissions. Hunt & Brooks (1984) estimated that the total
quantity of HEX released from these sources was 8 tonnes
per year. In the Federal Republic of Germany and the
Netherlands, HEX emissions were estimated to be 400-500
kg/year. HEX can also be emitted into the air from the
incineration and landfilling of HEX-containing waste, the
most accurate estimate being 1 tonne per year. The total
annual estimated release of HEX to the environment in the
USA is 11.9 tonnes. These figures are only estimates
because of the limited available data. They are given
simply to indicate the relative magnitude of HEX emissions
into the environment.
Exposure limit values for various countries are given
in Appendix 1.
5.3. Occupational exposure
Occupational exposure can occur both at HEX production
and processing facilities and at other locations where
HEX-containing waste is present. For example, the highest
reported workplace air concentrations of HEX were measured
at the Louisville, Kentucky, USA, waste-water treatment
plant, which received a slug of HEX discharged by a waste
hauler. Four days after the plant was closed, air concen-
trations in the primary treatment area ranged from 3.05
to 11.0 mg/m3 (270 to 970 ppb) (Morse et al., 1979).
During the clean-up operations air concentrations as high
as 133 mg/m3 (11 800 ppb) were reported (Kominsky &
Wisseman, 1978).
In 1982, the Velsicol Chemical Corporation used the
Southern Research Institute (SRI) sampling method at the
Memphis (Tennessee) and Marshall (Illinois) facilities to
evaluate possible exposure of workers to HEX vapour and
the effectiveness of engineering controls. Tables 6 and 7
show the HEX concentrations measured at various points.
At the Memphis facility almost one-half of the worker
8-h time-weighted average (TWA) air HEX concentrations
were at or above the USA Threshold Limit Value of 0.11
mg/m3 (0.01 ppm) (OSHA, 1989). At the Marshall plant all
six TWA values were above 0.11 mg/m3. It should be noted
that in Tables 6 and 7 the results of employee monitoring
are reported without regard to respirator use. Respirators
are required to be worn in operations in these plants
where HEX exposure is possible.
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Appendix 1
(Table 21).
Table 6. Summary of hexachlorocyclopentadiene monitoring, Memphis, Tennessee, USAa
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
HEX Process operator 2 445 0.009-0.011 0.009
HEX No. 1 operator 5 432 0.006-0.033 0.015
HEX No. 2 process operator 5 418 0.006-0.029 0.014
HEX No. 2 cyclo operator 5 417 0.001-0.048 0.017
HEX No. 2 chlorine operator 6 415 0.004-0.0161 0.035
a) HEX Bottoms drumming 1 50 0.016
HEX Area sample control room 12 476 0.002-0.018 0.009
HEX Brinks filter cleaning 2 387 0.004-0.006 0.005
Formulations HEX drummers 4 407 0.002-2.0337 0.010
Material HEX railroad tank car 1 279 0.013 0.008
handling unloading
Endrin R2 filter operator 1 281 0.003
Endrin R1 operator 1 334 0.002
Chlorendic No. 1 operator 2 437 0.0077-0.0102 0.008
anhydride
Chlorendic No. 2 operator D34 2 440 0.0107-0.0198 0.014
anhydride
Chlorendic No. 2 operator R6 2 437 0.0065-0.0169 0.011
anhydride
Chlorendic Packaging operator 1 396 0.035 0.031
anhydride
----------------------------------------------------------------------------------------
Table 6 (contd.)
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
Chlorendic Area sample - control 3 475 0.0003-0.0014 0.001
anhydride room
Heptachlor No. 1 operator 2 407 0.007-0.009 0.007
Heptachlor No. 2 operator 2 415 0.006-0.009 0.007
Heptachlor 237 operator 2 392 0.006-0.019 0.011
Heptachlor Utility operator 1 363 0.006 0.005
Heptachlor Cleaning sparkler filter 3 44 0.002-0.005 0.0003
a) ceiling sample 1 15 0.006
----------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming
that the only chemical exposure occurred during the sampling period.
Table 7. Summary of hexachlorocyclopentadiene monitoring, Marshall, Illinois, USAa
--------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrations TWAb
(min) (ppm) (ppm)
--------------------------------------------------------------------------------------
Chlordane No. 1 operator 8 451 0.0091-0.0316 0.017
Chlordane No. 2 operator 8 455 0.008 -0.0195 0.013
Chlordane No. 3 operator 8 451 0.0002-0.0325 0.014
Chlordane Area sample - 13 433 0.0002-0.0254 0.016
North control room
Chlordane Area sample - 10 435 0.001-0.0276 0.015
South control room
Chlordane HEX filter changing 1 15 0.1322
Chlordane Waste handling HEX 6 307 0.0006-0.0606 0.020
a) HEX mud drumming - 2 15 0.0005-0.0061
ceiling sample
b) Loading HEX waste 2 15 0.1199-0.2325
truck - ceiling sample
c) Sump pit dumping - 2 15 0.0333-0.1129
ceiling sample
--------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming that the
only chemical exposure was during the sampling period.
6. KINETICS AND METABOLISM
6.1. Absorption, retention, distribution, metabolism,
elimination, and excretion
6.1.1. Oral
In a study by Mehendale (1977), male Sprague-Dawley
rats (225-250 g body weight) were administered 5 µmol
of 14C-HEX (approximately 5.5 mg/kg) by oral intubation
as 0.2 ml of a solution in corn oil. The total 14C ac-
tivity contained in the dose was approximately 1 µCi. The
animals were maintained in metabolism cages and the urine
and faeces were collected. About 35% of the administered
dose was collected in the urine and only 10% was collected
in the faeces. More than 87% of the 14C activity in the
urine and more than 60% of the activity in the faeces
appeared during the first day. Only a small amount
(approximately 0.5%) of the original dose was recovered
in the kidneys and liver. The author speculated that, in
view of the low total recovery of the administered dose, a
major part of the dose (> 50%) had been excreted through
the lung. This speculation was later proven to be unwar-
ranted because subsequent studies (Dorough, 1979), in
which exhaled air and lung and tracheal tissues were ana-
lysed, showed that this was not the case. There is strong
evidence to suggest that, after oral dosing with HEX, at
least part of the faecal contents contained a volatile
constituent that could be readily lost if the samples were
dried and powdered, as they were in this case. An extrac-
tion procedure, using the major tissues and excreta, fol-
lowed by thin-layer chromatography, showed that at least
four water-soluble (polar) metabolites were produced, but
not identified, after oral dosing.
In a study designed to re-examine some of the findings
and observations of Mehendale (1977), Dorough (1979)
investigated the accumulation, distribution, and excretion
of 14C-HEX after its administration to rats and mice
either as a single oral dose or as a component of their
diet. The principal results of this study were reported by
Dorough & Ranieri (1984). The animals used were male and
female Sprague-Dawley rats, weighing between 200 and 250 g
body weight, and male and female Sprague-Dawley albino
mice, weighing between 25 and 30 g. Two female rats were
dosed, by gavage, with HEX (20 mg/kg) in 0.9 ml of corn
oil and were immediately placed in separate metabolism
cages through which air was drawn at 600 ml/min. The
evacuated air was passed through two high efficiency
traps. Since less than 1% of the administered dose was
recovered from the traps, it was considered to be conclus-
ive evidence that the pulmonary route is not of major
importance in the excretion of HEX (Dorough, 1979).
Dorough (1979) conducted single dose studies by admin-
istering, with a dosing needle, either 2.5 or 25 mg 14C-
HEX/kg body weight (dissolved in 0.9 ml of corn oil for
rats and in 0.2-0.3 ml for mice). The animals were killed
at 1, 3, or 7 days after dosing, and samples of muscle,
brain, liver, kidneys, fat, and either ovaries or testes
were removed and analysed for 14C activity. Urine and
faeces were also collected during the period between
dosing and tissue sample collection. No appreciable dif-
ferences due to sex or species were found in the excretion
patterns. The liver, kidneys, and fat were the most
important deposition sites for 14C residues in both rats
and mice, the levels in the kidneys of rats and in the
liver of mice being the highest.
In the same study (Dorough, 1979), rats and mice were
also placed on diets containing 1, 5, or 25 mg 14C-HEX
per kg. Assuming a daily food intake of 15 g for rats and
5 g for mice, this would give daily dose rates of 0.066,
0.330, and 1.666 mg/kg for rats and 0.182, 0.910, and 4.55
mg/kg for mice. Feed was replaced in the feeders every
12 h to minimize the loss of 14C-HEX (from volatiliz-
ation), and the feeding study was carried out for 30 days.
During this period, rats and mice were killed at 1, 3, 7,
12, 15, or 30 days. The surviving animals were then
returned to a normal diet for up to 30 days and, during
this post-treatment period, animals were killed at 1, 3,
7, 15, or 30 days after the last exposure. The total
excretion (urine and faeces) of the radiolabel ranged from
63-79% of the consumed 14C-HEX, which was significantly
lower than that found in the single-dose study (73-96%).
In all cases, the liver, kidneys, and fat contained the
highest amounts of 14C, and it appeared that a steady
state for these levels was reached after 15 days of the
feeding phase. A good correlation was observed between
the level of HEX in the diet and the 14C-levels found in
all the examined tissues. In a separate experiment with
male rats, in which the bile duct was cannulated and a
single dose of 14C-HEX (25 mg/kg) was administered
orally, only 16% of the dose was excreted in the bile. The
extraction characteristics of the radiocarbon compounds in
the excreta showed that they were primarily polar metab-
olites, some of which were capable of being converted to
organic-soluble compounds after acid-catalysed hydrolysis.
In a comparative study of the pharmacokinetics
of 14C-HEX after intravenous and oral dosing, Yu &
Atallah (1981) dosed Sprague-Dawley rats (240-350 g body
weight) with either 3 or 6 mg 14C-HEX (specific activity:
0.267 mCi/mmol). The doses ranged from 8.5 to 25.6 mg/kg.
Shortly after oral dosing, 14C activity appeared in the
blood and reached a maximum after approximately 4 h.
The 14C activity appeared in most of the tissues
analysed at 8, 24, 48, 72, 96, and 120 h after dosing.
Following oral dosing, there were higher residue levels in
the kidneys and liver than in any other tissue, although
these levels were generally much lower than those observed
after intravenous dosing. For example, at 24 h after
dosing, the kidneys and liver were found to contain only
0.96 and 0.75%, respectively, of the administered oral
dose, while these organs retained 2.92 and 4.68%, respect-
ively, of the administered intravenous dose. A higher
proportion (15.07%) of the 14C activity was found in the
digestive system (duodenum and large and small intestines)
after oral dosing. Coupled with the increased rate and
extent of faecal excretion after oral administration
(approximately 72%), compared to that after intravenous
dosing (approximately 20%), this would suggest that only a
fraction of the orally administered dose was absorbed.
About 17% of the oral dose was excreted in the urine.
Both urinary and faecal metabolites were again
characterized as polar because of their insolubility in
organic solvents. Unchanged HEX was not detected in any
of the samples examined. Only 11% of the 14C content was
soluble in organic solvents and a further 32% was con-
verhydrolysis. This indicated, perhaps, the formation of
metabolic ester conjugates.
Lawrence & Dorough (1982) made a comparative study of
the uptake, disposition, and elimination of HEX after
administering radiolabelled 14C-HEX by the intravenous
(10 µg/kg), inhalation (24 µg/kg), and oral routes (6 mg
per kg) to Sprague-Dawley rats weighing between 175 and
250 g, respectively. They noted that while doses in the
microgram range were useful for monitoring the urinary and
faecal excretion of HEX, much higher doses (about 6 mg/kg
in 0.5 ml of corn oil, and with a 4-fold increase in
radiocarbon activity) were necessary to obtain levels in
the principal organs that could be measured with any pre-
cision. Indeed, the doses administered orally were some
250 and 600 times the inhaled and intravenous doses,
respectively. In agreement with other researchers, these
authors attributed the lack of measurable levels in the
organs, following the administration of low doses, to the
poor bioavailability of HEX when given by the oral route.
The total radiolabel recovery immediately after the admin-
istration of the dose was 98.0 ± 5.3% (mean ± S.D.). Rats
dosed orally eliminated 2-3 times more of the dose in the
faeces than those dosed by the intravenous or inhalation
route. A maximum blood level was reached at approximately
2 h after dosing. The peak was broad with similar blood
concentrations between 2 and 5 h, perhaps indicating that
absorption occurred along the gastrointestinal tract over
this period in a quasi-steady state with elimination.
Biliary excretion was again confirmed as being greater
after oral dosing than after intravenous or inhalation
dosing, but it still only accounted for 18% of the admin-
istered dose. This observation agreed with previous
studies and, more importantly, with the report of Yu &
Atallah (1981), who administered comparable dose levels by
the oral route. Lawrence & Dorough (1982) also reported
that the faecal material contained predominantly polar or
unextractable material, as did the bile. These authors
considered that this was a clear indication that 14C-HEX
was extensively metabolized to polar products by the gut
contents, since only approximately 50% of 14C-HEX was
recovered when it was added to rat stomach contents that
were then immediately extracted with hexane.
A more recent comparative study (El Dareer et al.,
1983) essentially confirmed the findings of Yu & Atallah
(1981) and Lawrence & Dorough (1982). Male Fischer-344
rats with an average body weight of 169 g were dosed at
a level of 4.1 and 61 mg/kg with approximately 1 ml of a
solution of 14C-HEX dissolved in a 1:1:4 mixture of
Emulphor EL620, ethanol, and water. Little radioactivity
appeared as exhaled 14CO2.
6.1.2. Inhalation
In studies by Dorough (1980) and Lawrence & Dorough
(1981, 1982), rats were exposed to 14C-HEX vapour in a
specially designed, single animal inhalation exposure sys-
tem. Each animal was exposed to the vapours in a rodent
respirator, with the exhaust vapours from the system pass-
ing through a filter pad made from expanded polyurethane
foam. The flow rate and concentration of HEX was measured
prior to and after passing through the respirator contain-
ing the exposed animal. The difference between the amounts
of HEX in the input and output was assumed to be equi-
valent to the retained dose. Rats were exposed for a
period of 1 h and received doses in air which ranged from
1.4 to 37.4 mg/kg body weight (Lawrence & Dorough, 1981).
Immediately after the 1-h exposure, the recovery of the
dose retained by the animal was 91.8 ± 8.5% (mean ± S.D.).
Exposed animals were immediately placed in metabolism
cages for 72 h, during which time faeces, urine, and
expired air were collected. The animals were then killed
and certain of their tissues analysed for 14C activity.
Less than 1% of the retained radiocarbon was expired
during the 24-h period immediately following exposure, and
no radiocarbon was detected as 14CO2. Only about 69%
of the inhaled dose was recovered, which was much lower
than that recovered after intravenous (85%) or oral dosing
(82%). Since recovery of the dose immediately after the
administration of the inhalation dose was approximately
92%, the reduced recovery during the 72-h post-dosing
period led to the speculation that a volatile metabolite
was formed during this period, but attempts to collect and
identify this metabolite were not successful.
No kinetic parameters were reported in either of the
publications by Lawrence & Dorough (1981, 1982), although
blood concentration-time data during the 1-h exposure and
the following 6 h were presented. Elimination during the
subsequent 6 h appeared to relate to a complex pharmaco-
kinetic model with a terminal rate comparable to that
reported for the intravenous route, the half-life being
approximately 30 h.
The elimination via the bile was relatively low (8%)
after inhalation exposure, compared with 13 and 18% after
intravenous or oral administration of the same dose
(5 µg/kg) (Lawrence & Dorough, 1982). The fraction of
the dose recovered in the faeces and urine (23 and 33%,
respectively) was about the same as that recovered after
the intravenous dose, except that more was recovered in
the urine than in the faeces after the inhalation
exposure, while the reverse was observed after the intra-
venous dose.
A comparative study of the uptake, distribution, and
elimination of 14C-HEX (El Dareer et al., 1983) confirmed
and extended the conclusions reached by Lawrence & Dorough
(1981, 1982) concerning pulmonary exposure. Individual
Fischer-344 rats weighing between 125 and 190 g (with an
average weight of 169 g) were placed in metabolism cages
and exposed by inhalation. The dose received by each rat
over a 2-h exposure period was calculated from the total
amount of radioactivity recovered from the tissues,
faeces, urine, and exhaled air. The animal fur was not
included. The dose received by the exposed animals was
between 1.3 and 1.8 mg/kg body weight. The animals were
killed at either 6 or 24 h after they were removed from
the inhalation exposure. Whole blood, plasma, liver,
kidneys, voluntary muscle (gastrocnemius), subcutaneous
fat, brain, skin (ears), and the residual carcass (except
for the skin and fur which were discarded) were analysed
for 14C activity, as were the urine, faeces, and exhaled
air. The principal sites of deposition were the lungs,
kidneys, and liver. Only approximately 1% of the radio-
label was identified as 14CO2. No intact HEX was found
in any of the tissues; the majority of the radiolabel
extracted was polar (water soluble). These findings were
similar to those of Lawrence & Dorough (1981, 1982).
6.1.3. Dermal
No studies on the pharmacokinetics or distribution of
dermally applied HEX were found in a survey of the pub-
lished literature. Although no qualitative studies or
estimates of the uptake of HEX through skin were found,
studies have been reported in which discoloration of the
skin was observed after the dermal application of HEX
(Treon at al., 1955; IRDC, 1972). In these reports, toxic
response, leading to death, was observed in several
instances, which would suggest that HEX was absorbed
transdermally into the systemic circulation.
6.1.4. Comparative studies
Each of the four major studies (Yu & Atallah, 1981;
Lawrence & Dorough, 1981, 1982; El Dareer et al., 1983) of
the uptake and distribution of HEX involved more than one
route of uptake. One objective of each of these studies
was to compare the exposure routes. The observations made
were as follows:
* The principal routes of excretion were via the urine
and faeces. Considerably more of the administered dose
was excreted in the faeces after oral administration
than after dosing by the intravenous or inhalation
route, probably as a consequence of the increased
biliary excretion after oral dosing and the interac-
tion or metabolism of the dose by gut and faecal con-
tents. More of the administered dose was excreted in
the urine than in the faeces after inhalation
exposure, while the reverse was the case after intra-
venous administration.
* Biliary excretion occurred after administration by
each of the three routes. For similar doses, the frac-
tion of the dose eliminated by this route was in the
order oral > intravenous > inhalation.
* Comparative distribution to the major organs and tis-
sues is presented in Tables 8, 9, and 10. The princi-
pal organs to which HEX was distributed by the sys-
temic circulation were the kidneys and liver. The
lungs and trachea contained the highest concentrations
of HEX after inhalation exposure.
* There was a significantly higher retention of 14C in
the carcass, at 72 h post-dosing, after dosing by the
inhalation and intravenous routes than after oral
dosing (Table 10).
6.1.5. In vitro studies
Yu & Atallah (1981) examined the ability of liver,
faecal, and gut homogenates to metabolize HEX in vitro.
In an apparent first-order kinetic process, HEX was
metabolized by gut content, faecal, and liver homogenates
with half-lives of 10.6, 1.6, and 14.2 h, respectively.
When mercuric chloride (HgCl2) was added to the gut and
faecal homogenates as a bacteriocide, the half-lives were
increased to 17.2 and 6.2 h, respectively, indicating that
the gut and faecal flora contributed significantly to the
metabolism of HEX. Denaturation of the liver homogenate
had virtually no effect on the in vitro metabolic rate
indicating, perhaps, that there was only limited involve-
ment of liver microsomes or other enzyme-dependent process.
Table 8. Distribution of radioactivity (expressed as percentage of administered
dose) from 14C-HEX in rats dosed by various routesa
---------------------------------------------------------------------------------
Oral dose Intravenous Inhalation dose
Low doseb High doseb doseb Group Ac Group Bb
(4.1 mg/kg) (61 mg/kg) 0.59 mg/kg (1.0 mg/kg) (1.4 mg/kg)
---------------------------------------------------------------------------------
Faeces 74.5 ± 2.8 65.3 ± 6.9 34.0 ± 1.0d 28.7 ± 4.3 47.5 ± 6.4
Urine 35.5 ± 2.5 28.7 ± 4.2 15.8 ± 1.4 41.0 ± 4.8 40.0 ± 6.6
Tissues 2.4 ± 0.6 2.4 ± 0.1 39.0 ± 1.0 28.9 ± 1.6 11.5 ± 0.8
CO2 0.8 ± 0.0 0.6 ± 0.0 0.1 ± 0.0 1.4 ± 0.3 1.0 ± 0.5
Other volatile 0.2 ± 0.0 0.3 ± 0.0 0.1 ± 0.0
compounds
Total recovery 118 ± 3.0e 97 ± 7.0 89 ± 2.0 100 100
---------------------------------------------------------------------------------
a Adapted from: El Dareer et al. (1983). Values represent the mean percentage
of dose ± S.D. for three rats.
b At 72 h after dosing or exposure.
c At 6 h after exposure.
d Plus intestinal contents.
e For an unexplained reason, the total recovery for this dose was higher than
theoretical. If the percent recoveries for this dose are "normalized" to
100%, differences in distribution for the two doses are minimal, indicating
that no saturable process is operative in this dose range.
Table 9. Fate of radiocarbon (expressed as percentage of
administered dose) after oral, inhalation, and intravenous
exposure of rats to 14C-HEXa
--------------------------------------------------------------
Cumulative percent of dose
Oralb Intravenousc Inhalationd
--------------------------------------------------------------
24-h
Urine 22.2 ± 1.8 18.3 ± 5.2 29.7 ± 4.5
Faeces 62.2 ± 8.0 21.1 ± 7.1 17.0 ± 7.5
48-h
Urine 24.0 ± 1.9 20.7 ± 5.6 32.5 ± 5.1
Faeces 67.7 ± 5.1 30.4 ± 1.7 21.0 ± 7.5
72-h
Urine 24.4 ± 1.9 22.1 ± 5.7 33.1 ± 4.5
Faeces 68.2 ± 5.1 47.4 ± 1.9 23.1 ± 5.7
Body 0.2 ± 0.2 15.7 ± 7.8 12.9 ± 4.7
Total Recovery 92.8 ± 4.7 85.2 ± 4.8 69.1 ± 9.6
--------------------------------------------------------------
a Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
b Dose (7 µg/kg body weight) administered in 0.5 ml corn oil.
c Dose (5 µg/kg body weight) administered in 0.2 ml
saline:propylene glycol:ethanol (10:4:1) by injection into
the femoral vein.
d Doses administered as vapours over a 1-h exposure period
to achieve doses of about 24 µg/kg body weight.
El Dareer et al. (1983) incubated 14C-HEX with homo-
genates of liver, faeces, and intestinal (large and small)
contents, as well as with whole blood and plasma. Samples
were taken at 0, 5, and 60 min. The results, presented in
Table 11, clearly demonstrated the chemical reactivity of
HEX and its ability to bind components of biological
material.
Table 10. Distribution of HEX equivalentsa in tissues and
excreta of rats 72 h after oral, inhalation, and intravenous
exposure to 14C-HEXb,c
-----------------------------------------------------------------
Sample Oral dose Inhaled dose Intravenous dose
(6 mg/kg)d (24 µg/kg) (10 µg/kg)
-----------------------------------------------------------------
ng/g of tissue
Trachea 292 ± 170 107.0 ± 65.0 3.3 ± 1.7
Lungs 420 ± 250 71.5 ± 55.2 14.9 ± 1.1
Liver 539 ± 72 3.6 ± 1.9 9.6 ± 1.1
Kidneys 3272 ± 84 29.5 ± 20.2 22.3 ± 0.6
Fat 311 ± 12 2.8 ± 0.4 2.3 ± 0.2
Remaining carcass 63 ± 40 1.3 ± 0.6 0.5 ± 0.1
percentage of dose
Whole body 2.8 ± 1.1 12.9 ± 4.7 31.0 ± 7.8
Urine 15.3 ± 3.3 33.1 ± 4.5 22.1 ± 5.7
Faeces 63.6 ± 8.5 23.1 ± 5.7 31.4 ± 1.9
Total recovery 81.7 ± 6.7 69.1 ± 9.6 84.6 ± 4.6
-----------------------------------------------------------------
a One HEX equivalent is defined as the amount of radiolabel
equivalent to 1 ng of HEX, based on the specific activity of
the dosing solution.
b Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
c All values are the mean ± S.D. of three replicates.
d It should be noted that the oral dose was 250 and 600 times
that of the inhaled and intravenous doses, respectively.
This was necessary because residues were not detected in
individual tissues of animals treated orally at doses of
5-25 µg/kg.
6.2. Metabolic transformation
No primary metabolites or conjugates of HEX have been
identified. The data available on the pharmacokinetics of
HEX after dosing by the oral, inhalation, and dermal
routes are presented in sections 6.1.1, 6.1.2, and 6.1.3.
In studies by Dorough (1980) and Lawrence & Dorough
(1982), the principal routes of excretion were shown to be
via the urine and faeces. No unchanged HEX was found in
either, indicating that HEX was involved in extensive
metabolism.
In studies with rats and mice fed a diet containing 1,
5, or 25 mg 14C-HEX/kg, 63-79% of the consumed HEX was
recovered in the urine and faeces (Dorough, 1979; Dorough
& Ranieri, 1984). The extraction characteristics of the
radiocarbon compounds in the excreta showed that they were
primarily polar metabolites, some of which were trans-
formed to organic-soluble compounds after acid-catalysed
hydrolysis.
Table 11. Extractability of 14C-HEX and radioactivity derived from
saline and various biological preparationsa
----------------------------------------------------------------------
Preparation Time First Extraction Second Extraction Pellet
(min) Organicb Aqueous Organic Aqueous
----------------------------------------------------------------------
Saline 0 99.6 (92.4) 0.4
5 99.1 (92.8) 0.9
60 98.8 (94.6) 1.2
Liver 0 55.0 (74.4) 8.0 24.5 1.0 11.6
5 42.8 (49.7) 15.2 15.0 4.7 22.2
60 11.1 18.8 5.9 2.4 51.8
Plasma 0 22.2 (61.7) 7.2 50.2 0.8 19.6
5 19.7 (66.3) 25.0 33.6 2.0 19.6
60 1.4 43.4 21. 3.9 30.2
Whole blood 0 16.2 (60.4) 3.8 27.9 1.2 50.8
5 2.8 21.6 13.4 1.6 60.6
60 0.6 27.4 12.0 1.4 58.6
Faeces 0 90.0 (93.7) 0.6 8.0 0.2 1.2
5 83.4 (87.8) 0.8 9.0 0.6 6.2
60 40.5 (61.0) 2.8 31.3 3.0 22.4
Intestinal 0 93.7 (94.7) 0.6 4.6 0.2 1.0
contents 5 82.8 (89.5) 1.6 8.6 1.0 5.9
60 66.3 (87.0) 4.6 15.4 2.4 11.3
----------------------------------------------------------------------
a From: El Dareer et al. (1983). Values represent the percentage of
the total radioactivity in the respective fraction.
b Values in parentheses represent the percentage of the
radioactivity in the fraction as HEX.
In a comparative study of the pharmacokinetics of HEX
after intravenous or oral dosing at 8.5-25.6 mg/kg (Yu &
Atallah, 1981), urinary and faecal metabolites were again
characterized as polar because of their poor solubility in
organic solvents. No unchanged HEX was found. Only 11%
of the 14C content of the excreta was soluble in organic
solvents, and a further 32% of the extract was converted
to organic-soluble compounds after acid-catalysed hydro-
lysis. This indicated, perhaps, that metabolite ester
conjugates had been formed.
Yu & Atallah (1981) also performed in vitro metabolic
studies in which they incubated HEX with liver, faecal,
and gut-content homogenates (see section 6.1.5).
After Lawrence & Dorough (1982) had dosed rats by the
oral, inhalation, and intravenous routes with 14C-
labelled HEX at 6 mg/kg, 24 µg/kg and 10 µg/kg, respect-
ively, they found that the faecal and bile contents con-
tained mostly polar metabolites. These authors suggested
that HEX was rapidly metabolized to polar products, since
only about 50% of the HEX was recovered when it was added
to rat gut contents and immediately extracted with n-hex-
ane. In addition, the authors noted that approximately
15.8 ± 4% of the radiolabel that appeared in the faeces
within 24 h after dosing was volatile, indicating that a
catabolite was probably produced.
El Dareer et al. (1983) dosed rats by the inhalation
route so that individual animals received between 1.3 and
1.8 mg/kg body weight over a 2-h exposure period. They
were killed 6 or 24 h after removal from exposure. No in-
tact HEX was found in any of the tissues, and the majority
of the extractable material was polar (water soluble), in
accordance with the findings of Lawrence & Dorough (1981,
1982). As a part of this same study, El Dareer et al.
(1983) incubated 14C-HEX with homogenates of liver,
faeces, and intestinal (large and small) contents, as well
as with whole blood and plasma. These in vitro studies
were designed to assess the reactivity and binding charac-
teristics of HEX. The results, presented in Table 8,
clearly show chemical lability of HEX and its ability to
bind the components of biological material (see section
6.1.2).
Despite these efforts to characterize HEX metabolism,
no metabolites were identified. This observation suggests
that an attempt to rectify this deficiency would be a high
priority.
6.3. Reaction with body components
The toxic mechanisms of hexachlorocyclopentadiene are
not well understood. HEX has been shown to react with
olefins and other organic molecules such as aromatic com-
pounds. Using the available data, especially those from
studies comparing the various routes of exposure, a very
general and hypothetical rationale can be developed to
suggest possible reactions with body tissue.
The reactivity of HEX shows a high potential for
transformation and reaction with other chemicals. Absorp-
tion from the gastrointestinal contents is relatively
inefficient, probably due to the interaction of HEX with
the gastrointestinal contents and metabolism by intestinal
flora. The fact that HEX did not appear to be interactive
with the gastrointestinal epithelia in the kinetic studies
discussed in this chapter was probably due to its dilution
in the carrier vehicle, as well as its interaction with,
and metabolism by, the gut contents. However, in short-
term repeated oral dosing studies (SRI, 1981a,b), at doses
of 19 mg/kg or more, inflammation and hyperplasia was
noted in the forestomach (see Table 16). In addition, the
interaction of HEX after dermal contact was marked by a
distinct discoloration of the skin. This suggests a "site
of uptake" interaction, probably similar to that observed
in the lungs after pulmonary uptake.
During inhalation and the passage of HEX through the
lung tissue to reach the systemic circulation, metabolism
to water-soluble compounds probably occurs and HEX is
eliminated through the kidneys. However, an intravenous
dose may be bound unchanged to blood components (e.g.,
haemoglobin) and remain attached until reaching the liver.
The relatively slow elimination of the radiolabel from the
systemic circulation after intravenous dosing with 14C-
HEX (approximate terminal half-life of 30 h) suggests a
bioaccumulation potential, at least for some of the
metabolites, since little HEX appears to remain in the
tissues.
Rand et al. (1982b) showed that the cellular level in
lung tissue underwent significant changes after HEX inha-
lation. HEX vapour, administered by the inhalation route,
in addition to binding to epithelial lung tissue, was
found to bind to the extracellular lining in the lung.
Binding to bronchiolar Clara cells, which contribute
important materials to the extracellular lining of the
peripheral airways, was observed after inhalation exposure
in rats and monkeys (Rand et al., 1982a). HEX was also
found, in in vitro studies, to bind to the components of
whole blood, plasma, liver, and faecal homogenates and to
gastrointestinal contents (El Dareer et al., 1983). Thus,
irrespective of the route of administration, the principal
sites of toxic action seem to be the lungs, liver, and
kidneys. This observation is supported by the results from
the toxicity testing reported in chapter 8.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The effects of HEX on microorganisms have been
studied in aqueous and soil systems. Many of the aqueous
concentrations used in these experiments exceeded the
upper limit of aqueous solubility (0.8-2.1 mg/litre).
These concentrations were usually obtained by using an
organic solvent vehicle to disperse the chemical in aque-
ous media. The environmental significance of the results
should be interpreted with this aspect in mind.
Cole (1953) inoculated 10 strains of common human and
animal pathogens into growth media that contained various
concentrations of HEX. The inhibiting concentration, or
lowest concentration in which no growth was observed after
96 h of contact, ranged from 1-10 mg HEX/litre. The
Addition of 5 or 10 mg HEX/litre to sewage effluent inocu-
lated with Salmonella typhosa was found to be more effec-
tive than similar concentrations of chlorine in reducing
counts of total bacteria, coliforms, and S. typhosa (Cole,
1954). Yowell (1951) also reported, in a patent appli-
cation, that HEX has antibacterial properties; standard
phenol coefficients for Ebertnella typhi (Salmonella
typhi) and Staphylococcus aureus were 25 and 33, at 21 and
23 mg HEX/litre, respectively. These findings indicate
that concentrations of HEX at or slightly above its aque-
ous solubility limit are toxic to several types of
pathogens.
In contrast, tests with other microorganisms have
shown some ability to withstand HEX exposure. Twenty-three
strains of organisms (type unspecified), when added to
aqueous media containing HEX at 1000 mg/litre, were able
to metabolize the compound to a varying degree. Analysis
of the medium after 14 days indicated a HEX removal of
2-76%, depending on the organism used (Thuma et al.,
1978).
Rieck (1977a) found no effects on natural populations
of bacteria, actinomycetes, or fungi after a 24-day incu-
bation of a sandy loam soil treated with 1 or 10 mg HEX/kg
dry weight. It was concluded that no significant detrimen-
tal effects on microbial populations would result from
contamination of soils with these levels of HEX.
The effects of HEX on three ecologically important
microbial processes have been reported (Butz & Atallah,
1980). Results on cellulose degradation by the fungus
Trichoderma longibrachiatum indicated that a suspension
of HEX inhibited cellulose degradation at a concentration
of 1 mg/litre or more in a liquid medium. The calculated
7-day EC50 was 1.1 mg/litre. Extrapolations for 1- and
3-day EC50 values were both reported to be 0.2 mg/litre.
The decrease in toxicity over the 7-day period was
attributed to adaptation by T. longibrachiatum.
HEX inhibited anaerobic sulfate reduction by the bac-
terium Desulfovibrio desulfuricans when HEX was present in
suspension in a liquid medium. After a 3-h contact period,
growth inhibition was observed at HEX concentrations of
10-100 mg/litre, and there was no growth at 500 or 1000
mg/litre. Similarly, growth inhibition was observed at 1
and 10 mg/litre after a 24-h contact period, and there was
no growth at 50-1000 mg/litre. HEX was considered to be
slightly toxic to D. desulfuricans (Butz & Atallah,
1980).
The third part of the study by these investigators
(Butz & Atallah, 1980) focused on the effects of HEX on
urea ammonification by a mixed microbial culture in moist
soil. The results indicated that HEX concentrations of
1-100 mg/kg (dry weight) were not toxic to soil organisms
responsible for urea ammonification. The EC50 increased
from 104 mg/kg at day 1 to 1374 mg/kg at day 14. The
authors suggested that the low toxicity and its decrease
over time in this experiment may have been due to adsorp-
tion of the toxicant onto soil particles, as well as to
potential adaptation by the organism. Adsorption onto soil
particles may also account for the lack of toxicity in the
study of Rieck (1977a).
Walsh (1981) reported unpublished data on the effects
of HEX on four species of marine algae, obtained according
to the method described by Walsh & Alexander (1980). The
7-day EC50 was calculated as the concentration that
caused a 50% decrease in growth compared with the control,
as estimated by absorbance at 525 nm. The 7-day EC50
values reported indicated a wide range of susceptibility
among the species tested. Isochrysis galbana and
Skeletonema costatum were the most susceptible species,
the average 7-day EC50 values being about 3.5 and 6.6 µg per
litre, respectively. The average value for Porphyridium
cruentum was 30 µg/litre, while that for Dunaliella
tertiolecta was 100 µg/litre. Other tests with S.
costatum indicated that the direct algicidal effect of HEX
was less pronounced than its effect on growth. After 48 h
of exposure to HEX at 25 µg/litre, mortality, as indi-
cated by staining and cell enumeration, was only 4%
(Walsh, 1983).
7.2. Aquatic organisms
7.2.1. Freshwater aquatic life
Several studies are available on the effects resulting
from exposure of freshwater aquatic life to various con-
centrations of HEX.
Results from acute toxicity tests with HEX have been
reported for a number of freshwater fish species (Table
12). The 96-h LC50 value for fathead minnow larvae in a
flow-through test with measured toxicant concentration was
7 µg/litre (Spehar et al., 1977, 1979). Values obtained
for adult fathead minnows in static tests with unmeasured
toxicant concentrations ranged from 59 to 180 µg/litre
(Henderson, 1956; Buccafusco & LeBlanc, 1977). Reported
96-h values for goldfish, channel catfish, and bluegills
were also within this range (Buccafusco & LeBlanc, 1977;
Podowski & Khan, 1979; Khan et al., 1981).
Sinhaseni et al. (1982) reported the biological
effects of HEX on rainbow trout (Salmo gairdneri) exposed
to 130 µg HEX/litre in a non-recirculating flow-through
chamber. Oxygen consumption, measured polarographically,
increased by 193% within 80 min and then gradually
decreased until the fish died (after approximately 5 h).
Vehicle controls showed no effects after 76 h of exposure.
When added to normal trout mitochondria, HEX increased
basal oxygen consumption. The authors concluded that HEX
uncoupled oxidative phosphorylation.
Sinhaseni et al. (1983) continued their research on
the respiratory effects of HEX on intact rainbow trout.
Acclimated rainbow trout were exposed to 130 µg HEX/litre
in a flow-through well-water circuit, which was designed
to allow measurements of oxygen consumption in fish.
Again, HEX increased oxygen consumption rates (186 ± 24%),
the maximum rates being nearly the same as in the previous
experiment (approximately 84 min). The oxygen consumption
decreased until death (after approximately 6.5 h). Control
trout, exposed to the same concentration of the vehicle
(acetone) used to disperse HEX, showed no changes. The
authors reported profound respiratory stimulation, and HEX
appeared to uncouple oxidative phosphorylation. Sinhaseni
et al. (1983) postulated that HEX intoxication in the
intact animal may be due to increased oxygen consumption
and impaired oxidative ATP synthesis resulting from the
mitochondrial uncoupling action of HEX.
Table 12. Acute toxicity data for freshwater species exposed to HEX
---------------------------------------------------------------------------------------------------------------------------
Acute
no-effect
Species Methoda LC50 (µg/litre)b concentration Comments Reference
24-h 48-h 96-h (µg/litre)
---------------------------------------------------------------------------------------------------------------------------
Cladoceran S,U 93.0 52.2 ND 32 17 °C, soft water Vilkas (1977)
Daphnia magna (78.9-109.6) (44.8-60.9)
Cladoceran S,U 130 39 ND 18 22 °C, soft water Buccafusco &
Daphnia magna (68-260) (30-52) Leblanc (1977)
Fathead minnow FT,M NR NR 7.0 3.7 25 °C, soft water Spehar et al.
(larvae, < 0.1 g) (1977, 1979)
Pimephales promelas
Fathead minnow (1-1.5 g) S,U 115 110 104 NR Hard water, Henderson
Pimephales promelas acetone soln. (1956)
93 78 78 NR Soft water,
acetone soln.
75 59 59 NR Hard water,
emulsion
(no acetone)
Fathead minnow (0.72 g) S,U 240 210 180 87 22 °C, soft water Buccafusco &
Pimephales promelas (170-320) (180-250) (160-220) Leblanc (1977)
Goldfish NR NR NR 78 NR No details given Podowski & Khan
Carassius auratus (1979)
Channel catfish (2.1 g) S,U 190 150 97 56 22 °C, soft water Buccafusco &
Ictalurus punctatus (140-250) (130-180) (81-120) Leblanc (1977)
Bluegill (0.45 g) S,U 170 150 130 65 22 °C, soft water Buccafusco &
Lepomis macrochirus (140-210) (120-180) (110-170) Leblanc (1977)
---------------------------------------------------------------------------------------------------------------------------
a S = static, FT = flow-through, U = unmeasured concentrations, M = measured concentrations.
b Numbers in parentheses show 95% confidence interval. ND = Not determined. NR = Not reported.
Spehar et al. (1977, 1979) conducted 30-day early-life
stage flow-through toxicity tests with fathead minnows
(Pimephales promelas) using measured concentrations and
1-day-old larvae. The 96-h LC50 value was 7 µg/litre.
The 96-h mortality data indicated a sharp toxicity
threshold, such that 94% survival was observed at 3.7 µg
per litre, 70% at 7.3 µg/litre, and 2% at 9.1 µg/litre.
At the end of the 30-day exposure period, mortality was
only slightly higher, with 90% survival at 3.7 µg/litre,
66% at 7.3 µg/litre, and 0% at 9.1 µg/litre. These
results indicated that the median lethal threshold, the
lowest concentration causing 50% mortality, was reached
within 4 days. In addition, the HEX residues found in
fathead minnows at the end of the 30-day tests were low
(< 0.1 µg/g), and a BCF value of < 11 was reported
(Spehar et al., 1979). The authors concluded that the
toxicity data and the BCF values indicated that HEX was
non-cumulative in fish, i.e. it did not bioconcentrate in
fish as a result of continuous low-level exposure to HEX.
The growth rate of surviving larvae, measured in terms of
both body length and weight, did not decrease signifi-
cantly at any of the concentrations tested, compared with
the controls. This was true even at 7.3 µg/litre, a level
greater than the calculated LC50 value. Based on these
toxicity and growth data, Spehar et al. (1977, 1979) con-
cluded that 3.7 µg/litre is the highest concentration of
HEX that produces no adverse effects on fathead minnow
larvae. No other chronic toxicity data for any freshwater
species are available.
7.2.2. Marine and estuarine aquatic life
Among marine invertebrates, the 96-h LC50 values for
HEX range from 7 to 371 µg/litre (Table 13) (US EPA,
1980a). Except where indicated, these results were
obtained from static tests with nominal concentrations of
HEX. The highest LC50 by far was for the polychaete
Neanthes arenaceodentata, which is an infaunal organism
living in the sediment. The two shrimp species tested
were more sensitive to HEX by a factor of 10 or more.
The static LC50 value reported by US EPA (1980a) for
the grass shrimp, Palaemonetes pugio, was slightly higher
than that for the mysid shrimp, Mysidopsis bahia (Table
13). However, the LC50 for the mysid shrimp was consider-
ably lower in a flow-through test than in the static test.
Similarly, the LC50 value was lower when calculated
from actual measurements of HEX concentrations in the test
solutions (measured concentration) than when calculated
according to the concentrations based on amounts orig-
inally added to test solutions (nominal concentrations).
Table 13. Acute toxicity data on estuarine marine
organisms exposed to HEXa
----------------------------------------------------------
Species Methodb Salinity 96-h LC50c
(o/oo) (µg/litre)
----------------------------------------------------------
Polychaete S,U 28 371
Neanthes arenaceodentata (297-484)
Grass shrimp S,U 22 42
Palaemonetes pugio (36-50)
Mysid shrimp S,U 24 32
Mysidopis bahia (27-37)
Mysid shrimp FT,U 24 12
Mysidopsis bahia (10-13)
Mysid shrimp FT,M 24 7
Mysidopsis bahia (6-8)
Pinfish S,U 22 48
Lagodon rhomboides (41-58)
Spot S,U 22 37
Leiostomus xanthurus (30-42)
Sheepshead minnow S,U 24 45
Cyprinodon variegatus (34-61)
----------------------------------------------------------
a Adapted from: US EPA (1980a) and Mayer (1987).
b S = static; FT = flow-through; U = unmeasured
concentrations; M = measured concentrations.
c Numbers in parentheses show 95% confidence interval.
The acute toxicity values for HEX were similar for
each of three estuarine and marine fish species tested (US
EPA, 1980a). The static 96-h LC50 values based on
unmeasured concentrations for spot, sheepshead minnow, and
pinfish varied only between 37 and 48 µg/litre (Table 13).
7.3. Terrestrial organisms and wildlife
In a USA patent application, HEX was reported to be
nontoxic to plants in concentrations at which it was an
effective fungicide (Yowell, 1951). Test solutions were
prepared by adding HEX at various proportions to attaclay
and a wetting agent, and they were then mixed with water.
The concentrations of HEX applied to plants as an aqueous
spray were 0.1, 0.2, 0.5, and 1.0%. Slight injury
(unspecified) to Coleus blumei was reported at 1.0% HEX,
but lower concentrations were not harmful. Similarly, HEX
was added to horticultural spray oil and an emulsifier at
various proportions and then mixed with water. The concen-
tations of HEX in the prepared spray were 0.25 and 0.5%.
No injury to C. blumei was observed at these concen-
trations. No data are available concerning the effects of
HEX on amphibians, reptiles, birds, or mammals other than
those routinely used in laboratory testing.
7.4. Population and ecosystem effects
The ecological effects of HEX have not been studied at
the ecosystem, population, or community levels.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Acute toxicity studies
8.1.1. Acute oral, inhalation, and dermal toxicity
The data from acute toxicity studies using HEX are
summarized in Table 14. Caution should be exercised in
comparing the various studies. The acute toxicity (LD50
and LC50) may be affected not only by the species and
age of the animals used in the experimental tests, but
also by the strain. In addition, the purity of the com-
pound and the nature of the contaminants may affect the
toxicity, as can the experimental method and the vehicle
used. These details (when specified by the researchers)
have been summarized in the table.
For the rat, oral LD50 values ranged from 425 to 926
mg/kg for males, and from 315 to 926 mg/kg for females.
Values for mice and rabbits were within the same range.
Acute inhalation experiments involved exposure
durations from 3.5 to 4 h, and LC50 values for male rats
ranged from 18.1 to 35.0 mg/m3 (1.6 to 3.1 ppm) and for
female rats from 35.0 to 40.0 mg/m3 (3.1 to 3.5 ppm).
The mouse, rabbit, and guinea-pig values ranged from 23.7
to 80.2 mg/m3 (2.1 to 7.1 ppm).
There are few data available on dermal toxicity.
LD50 values of < 200 mg/kg in male rabbits and 340-780
mg/kg in females have been reported.
In spite of variations in LD50 values in the differ-
ent studies, these data suggest that HEX is moderately
toxic when administered orally. The acute toxicity of HEX
by the dermal route is quite similar to its acute oral
toxicity. HEX is much more toxic by the inhalation route
of exposure than by the dermal or oral routes.
8.1.2. Eye and skin irritation
IRDC (1972) tested HEX for eye irritation by instil-
ling 0.1 ml HEX into the eyes of New Zealand white rabbits
for 5 min or 24 h before washing. All the rabbits died on
or before the ninth day of the observation period. Treon
et al. (1955) reported HEX to be a primary skin irritant
in rabbits (strain unspecified) at a dose level of 250
mg/kg, and IRDC (1972) reported HEX to be a dermal irri-
tant because of the oedema that appeared after application
of 0.5 ml HEX. In this study, intense discoloration of
the skin was noted. In the study of Treon et al. (1955),
monkeys (strain unspecified) were also tested, and dis-
coloration of the skin was noted even at low doses (0.05
ml of 10% HEX) (Table 15).
Table 14. Acute toxicity studies of HEX
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Oral LD50
Rat (unspecified) oral gavage M: 510 mg/kg Treon et al.
young adult (5% solution F: 690 mg/kg (1955)
of peanut oil)
Rat (Charles River CD) oral gavage M + F: 926 IDRC (1968)
young adult (corn oil) mg/kg
Rat (Sprague-Dawley) M + F: 651 Dorough (1979)
young adult mg/kg
Rat (Fischer-344) oral gavage M: 425 mg/kg SRI (1980a)
weanling (corn oil) F: 315 mg/kg
Mouse (unspecified) M + F: 600 Dorough (1979)
mg/kg
Mouse (B6C3F1) oral gavage M + F: 680 SRI (1980a)
weanling (corn oil) mg/kg
Rabbit (Albino, strain oral gavage F: 640 mg/kg Treon et al.
unspecified) adult (5% peanut oil) (1955)
Dermal LD50
Rabbit (unspecified) skin painted F: 780 mg/kg Treon et al.
adult (1955)
Rabbit (unspecified) skin painted M: < 200 mg/kg IDRC (1972)
adult F: 340 mg/kg
Inhalation LC50
Rat (Carworth) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 35.0 (1955)
mg/m3 (3.1 ppm)
Rat (Sprague-Dawley) inhalation 4.0-h LC50 Rand et al.
young adult M: 18.1 mg/m3 (1982a)
(1.6 ppm)
F: 39.6 mg/m3
(3.5 ppm)
Mouse (unspecified) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 23.7 (1955)
mg/m3 (2.1 ppm)
----------------------------------------------------------------------
Table 14 (contd.)
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Rabbit (unspecified) inhalation 3.5-h LC50 Treon et al.
adult F: 58.8 mg/m3 (1955)
(5.2 ppm)
Guinea-pig inhalation 3.5-h LC50 Treon et al.
(unspecified) adult M + F: 80.2 (1955)
mg/m3 (7.1 ppm)
----------------------------------------------------------------------
a M = male; F = female.
Table 15. Primary eye and dermal irritation
--------------------------------------------------------------------------------
Study Species (strain) Results Reference
age
--------------------------------------------------------------------------------
Primary eye Rabbit (New Zealand Severe eye irritant (0.1 IRDC (1972)
irritation white) adult ml for 5 min or 24 h); all
died by day 9 of study
Primary dermal Rabbit (unspecified) Moderate skin irritant Treon et al.
irritation adult (250 mg/kg); one (1955)
application
Primary dermal Rabbit (New Zealand Severe skin irritant IRDC (1972)
irritation white) adult (200 mg/kg); all males died
Primary dermal Monkey (unspecified) Mild skin discoloration Treon et al.
irritation adult (0.05 ml of 10% HEX solution) (1955)
--------------------------------------------------------------------------------
Shell Research Limited conducted a study of the skin-
sensitizing potential of 98.8% pure HEX (Shell Research
Limited, 1982). Guinea-pigs (310-370g) were placed at
random into single sex groups of 10 animals and housed in
groups of five animals. Range-finding tests were conducted
to determine the concentrations of test material to be
used for intradermal induction, topical induction, and
topical challenge. Two male and two female guinea-pigs
were injected intradermally on each side of the midline
with 0.1 ml of several dilutions (0.5, 1, 5, and 10 mg HEX
per ml HEX corn oil). Filter paper patches with 0.3 ml of
a 1% or 2% dilution of test material in corn oil were
applied to two other groups. On the basis of the results
of the range-finding studies, the following tests and HEX
concentrations were selected for use: intradermal induc-
tion, 0.5 mg/litre; topical induction, 20.5 mg/litre; and
topical challenge, 10 mg/ml (all in corn oil). In the
sensitizing test, all four animals given an intradermal
injection of 0.5 mg HEX/ml suffered necrosis, while top-
ical applications at 10 or 20.5 mg/litre produced slight
redness or no difference from surrounding skin. However,
all 20 test animals showed positive responses 24 and 48 h
after removal of the challenge patches. The researchers
concluded that HEX is a skin sensitizer.
8.2. Short-term exposure
8.2.1. Oral
In a range-finding study using groups of five male and
five female Fischer-344 rats, there were no deaths when 12
doses of 25, 50, or 100 mg/kg were given in 16 days (SRI,
1980b). With the same dosing schedule, one out of five
males and four out of five females died when the dose was
200 mg/kg, and five out of five males and four out of five
females died when the dose was 400 mg/kg. In the same
study, B6C3F1 mice died when given doses of 400 or 800
mg/kg, but not when given doses of 50, 100, or 200 mg/kg.
Both rats and mice exhibited pathological changes of the
stomach wall at all but the lowest dose level.
The short-term toxicity of HEX is summarized in Table
16. These short-term toxicity studies on B6C3F1 mice and
Fischer-344 rats were conducted by SRI (1981a,b) under
contract with the National Toxicology Program (NTP), and
the results were reported by Abdo et al. (1984). In the
mouse study (SRI, 1981a), HEX (94.3-97.4% pure) in corn
oil was administered by gavage at dose levels of 19, 38,
75, 150, and 300 mg/kg to 10 mice of each sex, 5 days/week
for 13 weeks (91 days). At the highest dose level (300
mg/kg), all 10 male mice died by day 8 and three females
died by day 14. In female mice, the liver was enlarged.
Toxic nephrosis in females at doses of 75 mg/kg or more
was characterized by distensions in the proximal convol-
uted tubules, with basophilia in the inner cortical zone
and cytoplasmic vacuolization. However, male mice admin-
istered 75 mg/kg or more did not show these effects. Dose
levels of 38 mg/kg or more caused lesions in the fore-
stomach, including ulceration in both males and females,
as well as increased kidney and liver weights. The no-
observed-adverse-effect level (NOAEL) in mice was 19 mg/kg
and the lowest-observed-adverse-effect level (LOAEL) was
38 mg/kg.
In the rat study (SRI, 1981b), HEX in corn oil was
administered by gavage at dose levels of 10, 19, 38, 75,
and 150 mg/kg to groups of 10 male and female F-344 rats.
At 38 mg/kg or more, mortality and toxic nephrosis were
observed in both males and females. The male rats treated
with 19 mg/kg showed no significant effects, but female
rats had lesions in the forestomach. Similar lesions were
observed in males given 38 mg/kg or more. There was a
dose-related depression of body weight gain (relative to
the controls) and female rats had increased kidney and
liver weights. The NOAEL in rats was 10 mg/kg, and the
lowest-observed-effect level (LOEL) was 19 mg/kg.
A summary of the results of these two experiments
appears in Table 17.
Table 16. Short-term toxicity of HEX
------------------------------------------------------------------------------------------------------
Study Species Dose Results Effects at LOEL Reference
or lowest dose
------------------------------------------------------------------------------------------------------
90-day rat 10, 19, 38, 75, and NOAEL: 10 mg/kg lesions of forestomach SRI (1981b)
feeding 150 mg/kg (by gavage) LOEL: 19 mg/kg in female rats at
study 19 mg/kg
14-week rat 0.11, 0.56, and 0.226 NOEL: 0.226 mg/m3 no statistically Rand et al.
inhalation mg/m3 (5 days/week) LOEL: NE significant effects (1982a)
study
14-week monkey 0.11, 0.56, and 0.226 NOEL: 0.2 ppm no effects noted Alexander
inhalation mg/m3 (5 days/week) LOEL: NE et al.
study (1980)
------------------------------------------------------------------------------------------------------
NE = not established.
NOAEL = no-observed-adverse-effect level.
NOEL = no-observed-effect level.
LOEL = lowest-observed-effect level.
Table 17. Toxicological parameters for mice and rats administered technical grade HEX
in corn oil by gavage for 91 daysa
---------------------------------------------------------------------------------------
Species Sex Dose Mortality Relative Forestomach Forestomach Toxic
(strain) (mg/kg) weight inflammation hyperplasia nephrosis
gainb
---------------------------------------------------------------------------------------
Mice male 0 1/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 36% 0/10 0/10 0/10
38 0/10 + 9% 2/10 2/10 0/10
75 0/10 - 9% 7/10 8/10 0/10
150 0/10 - 45% 7/10 9/10 0/10
300 10/10 7/10 8/10 0/10
Mice female 0 0/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 13% 0/10 0/10 0/10
38 0/10 - 13% 2/9 2/9 0/9
75 0/10 - 13% 6/10 9/10 10/10
150 0/10 - 25% 10/10 10/10 10/10
300 3/10 - 38% 7/9 9/9 7/10
Rats male 0 3/10 0/10 0/10 0/10
(F-344) 10 1/10 - 4% 0/10 0/10 0/10
19 1/10 - 8% 0/10 0/10 0/10
38 1/10 - 20% 4/10 5/10 10/10
75 3/10 - 49% 9/10 9/10 9/10
150 7/10 - 57% 8/9 8/9 8/10
Rats female 0 1/10 0% 0/10 0/10 0/10
(F-344) 10 2/10 + 4% 0/10 0/10 0/10
19 1/10 - 5% 2/10 2/10 0/10
38 1/10 - 2% 2/10 5/10 10/10
75 3/10 - 30% 9/10 9/10 10/10
150 5/10 - 33% 9/10 9/10 10/10
---------------------------------------------------------------------------------------
a From: SRI (1981a,b).
b Relative weight gain was calculated as follows:
Dose group value - control group value
-------------------------------------- x 100
Control group value
8.2.2. Short-term inhalation toxicity
Rand et al. (1982a) conducted a range-finding study in
which groups of 10 male and 10 female Sprague-Dawley rats
were exposed to atmospheres of 0.25, 1.24, or 5.65 mg
HEX/m3 (0.022, 0.11, or 0.5 ppm), 6 h/day, 5 days/week
for a total of 10 exposures. Nine male rats and one female
rat exposed to 5.65 mg/m3 were moribund after five to
seven exposures. These rats had dark red eyes, laboured
breathing, and pale extremities. There were no mortalities
in the other exposure groups. However, the males in the
medium- and high-dose groups lost weight during the study
and reduced mean liver weights and pathology were noted.
The NOAEL for HEX exposure in this study was 0.25 mg/m3
and the LOEL was 1.24 mg/m3.
Fourteen-week inhalation studies have been carried out
on rats and monkeys (Alexander et al., 1980; Rand et al.,
1982a,b) and the results are summarized in Table 16.
Groups of 40 male and 40 female Sprague-Dawley rats
weighing 160-224 g and groups of 12 Cynomolgus monkeys
weighing 1.5-2.5 kg were exposed to HEX, 6 h/day, 5 days
per week, for 14 weeks. Levels of exposure were 0, 0.11,
0.56, and 2.26 mg/m3 (0, 0.01, 0.05, and 0.20 ppm). In
monkeys, there were no mortalities, adverse clinical
signs, weight gain changes, pulmonary function changes,
eye lesions, haematological changes, clinical chemistry
abnormalities, or histopathological abnormalities at any
dose level tested. Thus, the NOEL for monkeys was at least
2.26 mg/m3 for this exposure period, but the LOEL was
not established. Male rats had a transient appearance of
dark-red eyes at 0.56 and 2.26 mg/m3. At 12 weeks, there
were marginal but not statistically significant increases
in haemoglobin concentration and erythrocyte count in
males exposed to 0.11 mg/m3, females exposed to 0.56
mg/m3, and males and females exposed to 2.26 mg/m3.
There were small but not statistically significant changes
in mean liver weight of all groups of treated rats and
similar changes in the kidneys of all treated males. No
treatment-related abnormalities in gross pathology or
histopathology were observed. On this basis, the NOEL in
rats was 2.26 mg/m3, but the LOEL was not established.
In a further study by Rand et al. (1982b), no ultra-
structural changes were observed in monkeys that could be
attributed to the inhalation of HEX vapour. Exposure was
identical to that of the previous study (Rand et al.,
1982a). There was a statistically significant (P < 0.01)
increase in the mean number of electronlucent inclusions
in the apex and base of the Clara cells in exposed ani-
mals, as compared with the controls. According to some
researchers (Evans et al., 1978), Clara cells respond to
injury by regression to a more primitive cell type. Rand
et al. (1982b) noted that some of the ultrastructural
changes in the exposed animals resembled those of the
Evans study. It is not known what effect these changes
might cause. The Clara cell contributes important
materials to the extracellular lining of the peripheral
airways, and if this effect of HEX vapour causes a change
in the content of the contributed material, then the
extracellular lining may be altered and breathing may be
subsequently impaired (Rand et al., 1982b). This obser-
vation of lung effects coincides with those of other
researchers (Dorough, 1979, 1980; Lawrence & Dorough,
1981, 1982). Furthermore, in inhalation studies with HEX
occasional statistically significant increases in the
haemoglobin level and red blood cell counts of rats have
been noted, which may be manifestations of the impairment
of respiratory functions.
In 1984, the US National Toxicology Program (NTP)
completed a short-term, 90-day HEX inhalation study on
B6C3F1 mice and F-344 rats (NTP, 1984a,b). In the basic
study, ten rats and ten mice of each sex were placed at
random into five exposure and control groups. The rats and
mice were exposed to nominal concentrations of 0.45, 1.70,
4.52, 11.3, or 22.6 mg/m3 (0.04, 0.15, 0.4, 1.0, or 2.0
ppm) for 6 h/day, 5 days/week for 13 weeks. In the female
mouse study, 6 out of 10 of the control animals died,
while none of the animals in the male control population
died. The six animal deaths in week 7 were attributed to
a defective feeder insert. Mortality in both the rats and
mice was high in the two highest-dose groups; all rats and
mice in these groups died in the first five weeks of the
study. Posterior paresis and listlessness were observed
in mice at 4.52 and 11.3 mg/m3. Compound-related histo-
pathological alterations were observed in the respiratory
tracts of male and female mice exposed to 1.7 mg/m3 or
more. These changes included: necrosis, acute and
chronic inflammation, hyperplasia or squamous metaplasia
of the nasal, laryngeal, tracheal, bronchial, and bronchi-
olar epithelia of the affected animals. No compound-
related effects were observed in mice at the lowest dose,
and so this level could be considered the NOEL (NTP,
1984a).
Clinical signs resulting from HEX exposure included
posterior paresis in all rats exposed to 1.7 mg/m3 or
more, listlessness in all rats exposed to 4.52 mg/m3 or
more, and eye irritation and respiratory distress in all
rats exposed to 11.3 mg/m3 or more. As in the case of
mice, HEX caused significant histological alterations in
the respiratory tracts of rats at the two highest doses.
Changes of a less-marked degree were observed in the
respiratory tracts of rats receiving 4.52 mg/m3. No
compound-related changes were seen in any organ of male
and female rats exposed to the lowest dose, and so this
level could be considered the NOEL. In addition, compound-
related effects of a secondary stress-related nature were
seen in a number of other organs of rats of both sexes at
the two highest doses. These includes lymphoid depletion
of the spleen and thymus, degeneration of the seminiferous
tubules and decreased lytoplasmic vacuolization of the
adrenal cortex.
Some basic clinical pathology and histopathology
examinations were undertaken simultaneously in both
studies (NTP, 1984a,b). All effects were similar to those
noted in the previous toxicity studies. In the rat study
(NTP, 1984b), HEX nephrotoxicity was examined in more
depth. HEX was not found to be nephrotoxic at these ex-
posure levels, nor did it appear that it was myelotoxic.
8.2.3. Short-term dermal toxicity
No short-term dermal toxicity studies are available.
8.3. Long-term exposure
8.3.1. Long-term oral toxicity
No long-term oral toxicity studies have been reported.
8.3.2. Long-term inhalation toxicity
In view of the absence of long-term studies on the
inhalation of HEX, the following studies were examined.
Treon et al. (1955) exposed guinea-pigs, rabbits, rats,
and mice to a HEX (89.5% pure) concentration of 3.73
mg/m3 (0.33 ppm), 7 h/day, 5 days/week, for 25-30
exposure days. The guinea-pigs survived 30 exposures,
whereas rats and mice did not survive five exposures, and
four out of six rabbits did not survive 25 exposures.
Using a lower concentration of 1.70 mg/m3 (0.15 ppm),
guinea-pigs, rabbits, and rats survived 150 7-h exposures
over a 7-month period. This level was too high to conduct
a long-term study in mice since four out of five animals
did not survive. The rats, guinea-pigs, and rabbits toler-
ated 1.7 mg/m3 and did not exhibit any treatment-related
effects. Thus, the NOEL for rats, guinea-pigs, and rabbits
was 1.7 mg/m3 over the 7-month period. Due to the high
mortality, a NOEL for mice could not be established.
A long-term (30 weeks) inhalation study in rats with
technical grade HEX (96% pure with hexachlorobuta-1,3-
diene and octachlorocyclopentene as impurities) was con-
ducted by the Shell Toxicology Laboratory (D.G. Clark et
al., 1982). Four groups of eight male and eight female
Wistar albino rats were exposed to HEX at nominal concen-
trations of 0, 0.56, 1.13, and 5.65 mg/m3 (0, 0.05, 0.1,
and 0.5 ppm), 6 h/day, 5 days/week, for 30 weeks and were
observed for a 14-week recovery period without HEX
exposure. At the highest dose level, four males and two
females died. In males, there was depressed body weight
gain at the highest dose, relative to controls, beginning
at 7 weeks of exposure and persisting throughout the
remainder of the study. Females in the two highest-dose
groups had lower body weights at the end of the recovery
period than did the controls. At the highest dose, there
were pulmonary degenerative changes, ranging from epi-
thelial hyperplasia and oedema to epithelial ulceration
and necrosis, in both sexes, the males being affected more
severely. There were also mild degenerative changes in
the liver (bile duct hyperplasia and inflammatory cell
infiltration) and kidneys (protein casts in tubules and
pigmented cortical tubules) in a few rats, and kidney
weights were significantly increased in the females at 30
weeks. After 30 weeks of study, no biologically signifi-
cant toxicity was noted in animals that were exposed to
0.56 or 1.13 mg/m3. Thus, the NOEL in rats exposed to
HEX vapour in this study (6 h/day, 5 days/week, for 30
weeks) was 0.56 mg/m3, and the LOEL was 1.13 mg/m3.
A long-term inhalation study by the National Toxi-
cology Program started in January 1986. The animal
exposure has been completed and results will be published
as soon as the pathology review is completed.
8.3.3. Long-term dermal toxicity
No long-term dermal toxicity studies have been
reported.
8.3.4. Principal effects and target organs
Repeated exposure of several animal species to levels
of HEX vapour in the range of 1.13 to 2.26 mg/m3 (0.1-0.2
ppm) has been found to cause pulmonary degenerative
changes (Treon et al., 1955; D.G. Clark et al., 1982; Rand
et al., 1982a,b). In addition, Treon et al. (1955)
reported diffuse degeneration of the brain, heart, and
adrenal glands and necrosis of the liver and kidney
tubules, together with severe pulmonary hyperaemia and
oedema. In many instances, acute bronchitis and intersti-
tial pneumonitis also occurred. Necrosis of the epithelium
of the primary, secondary, and tertiary bronchi was
observed. At later stages, reacting inflammatory cells
migrated into the wall and the mucosa of the bronchi and
alveoli. In rabbits, the walls of the alveoli were covered
by a hyaline or fibrinoid membrane. Possibly, some of the
changes found by Treon et al. (1955) were caused by
impurities in the HEX preparation. Acute exposure by the
oral and dermal routes also affects the respiratory system
(Kommineni, 1978; SRI, 1980a). Death from acute exposure
by any tested route seemed to be associated with respir-
atory failure (Lawrence & Dorough, 1982).
There are insufficient data to identify the site that
is the most sensitive to prolonged, repeated exposure to
HEX. In comparing routes of administration, researchers
found that damage to the lungs occurred regardless of
which route was used (Lawrence & Dorough, 1982). When HEX
is administered orally to animals, the kidneys may be the
most sensitive site, since short-term dosing of rats and
mice was found to cause nephrosis, especially in females
(SRI, 1981a,b). Although the oral route may not be a
significant route of exposure for human beings, the fact
that the kidneys are a possible target organ in short-term
exposure indicates that low-level, prolonged systemic
exposure from any ambient route may affect the kidneys.
The liver has also been shown, in several of the labora-
tory studies, to be affected by HEX.
The available literature does not cite any single
mechanism to explain HEX toxicity. HEX vapour irritates
the respiratory tract, leading to death by respiratory
failure after bronchopneumonia (D.G. Clark et al., 1982).
The degenerative changes that have been observed in the
liver and kidneys are mild and unlikely to contribute to
the chemical's lethality (NAS, 1978; SRI, 1980a,b).
The difficulty in studying HEX because of its high
reactivity and volatility has also created problems in
identifying its metabolites. Several questions remain as
to whether the same metabolites are formed after various
routes of exposure and whether it is the administered HEX
or its metabolites that cause the lung injuries seen with
various dosing regimens. Furthermore, the strong ability
of HEX to interact with other compounds, especially
organic molecules, can lead to many other effects, such as
haemoglobin binding. Little is known about the interac-
tions of HEX with other chemicals in animal or human
tissue.
8.4. Developmental and reproductive toxicity
The teratogenic potential of HEX has been evaluated in
pregnant Charles River CD-1 rats that were administered
HEX (98.25%) in corn oil, by gastric intubation, at dose
levels of 3, 10, and 30 mg/kg per day from days 6 to 15 of
gestation. A control group received the vehicle (corn oil)
at a dose volume of 10 ml/kg per day. All the rats sur-
vived, and there was no difference in mean maternal body
weight gain between the dosed groups and controls. There
were no differences in the mean number of implantations,
corpora lutea, live fetuses, mean fetal body weights, or
male/female sex ratios among any of the groups, and there
were no statistical differences in malformation or devel-
opmental variations, compared with the controls, when
external, soft tissue, and skeletal examinations were
performed (IRDC, 1978).
Murray et al. (1980) evaluated the teratogenic poten-
tial of HEX (98%) in CF-1 mice and New Zealand white
rabbits. Mice were dosed at 0, 5, 25, or 75 mg HEX/kg per
day by gavage during days 6-15 of gestation, while rabbits
received the same dose during days 6-18 of gestation. The
fertility of the treated mice and rabbits was not signifi-
cantly different from that of the control groups. In the
mice, there was no evidence of maternal toxicity, embryo-
toxicity, or teratogenic effects. A total of 249-374
fetuses (22-33 litters) was examined in each dose group.
In rabbits, maternal toxicity was noted at a dose level of
75 mg/kg (diarrhoea, weight loss, and mortality), but
there was no evidence of maternal toxicity at the lower
levels. There were no embryotoxic effects at any dose
level. Although there was a two-fold increase over con-
trols in the proportion of fetuses with 13 ribs at 75
mg/kg, this was considered to be a minor skeletal vari-
ation. The authors concluded that HEX was not teratogenic
at the levels tested (Murray et al., 1980).
Chernoff & Kavlock (1982) tested 28 compounds, includ-
ing HEX, by an in vivo screening procedure. According to
the researchers, the underlying hypothesis was that most
prenatal insults would manifest themselves postnatally as
reduced viability and/or impaired growth. Twenty-five
Oravid CD-1 mice were administered HEX orally at or near
the maternal minimal tolerated dose (MTD). The MTD was
considered to be that dose resulting in either significant
weight reduction during the treatment period, mortality,
or other signs of toxicity. There were no differences in
maternal weight gain, number of live offspring or average
weight between the HEX-treated animals and controls when
HEX was administered orally for 8-12 days at 45 mg/kg.
Gray & Kavlock (1984) extended the observation period
proposed by Chernoff & Kavlock (1982, 1983) to 250 days to
determine whether neonatal weight reductions persisted
throughout life and whether other serious abnormalities or
mortality resulted from exposure to HEX. CD-1 pregnant
mice were orally exposed to HEX (45 mg/kg) on days 8 to 12
of gestation, which is within the period of major embryo-
nic organogenesis. Females were weighed throughout dosing
and on day 19 of gestation. They were allowed to deliver
and the litters were counted and weighed at 1 and 3 days
of age. The animals were observed at approximately 250
days of age. During the postmortem examination of males,
body weight and the weights of the liver, testes, seminal
vesicles, and right kidney were recorded. HEX did not
produce any statistically significant developmental
effects in this study.
Studies on the teratogenic potential of inhaled HEX
were not found in the review of the scientific literature.
8.5. Mutagenicity
Goggelman et al. (1978) found that HEX was not muta-
genic, either before or after liver microsomal activation,
at 2.7 mmol/litre in an Escherichia coli K12 back-
mutation system. In this test there was a 70% survival of
bacteria at 72 h. HEX was not tested at higher concen-
trations because it was cytotoxic to E. coli. A previous
report from the same laboratory (Greim et al., 1977) indi-
cated that HEX was also non-mutagenic in Salmonella
typhimurium strains TA1535 (base-pair mutant) and TA1538
(frame-shift mutant) after liver microsomal activation.
However, no details of the concentrations tested were
given. Although tetrachlorocyclopentadiene is mutagenic
in these systems, probably through metabolic conversion to
the dienone, it appears that the chlorine atoms at the C-1
position of HEX hinder metabolic oxidation to the corre-
sponding acylating dienone (Greim et al., 1977).
A study conducted by the Industrial Bio-Test Labora-
tories (IBT, 1977) also suggested that HEX is not muta-
genic in S. typhimurium. Both liquid HEX and its vapour
were tested with and without metabolic activation. The
vapour test was carried out in desiccators with the TA100
strain of S. typhimurium only. It is not clear from
vapour test data that sufficient amounts of HEX or
adequate exposure times (30, 60, and 120 min) were used.
Longer exposures in the presence of HEX vapour may be
necessary for a potential mutagenic effect to be seen.
At concentrations of up to 1.25 mg/litre in the
presence of an S-9 liver activating system, HEX was not
mutagenic in the mouse lymphoma mutation assay. Mutageni-
city could not be evaluated at higher concentrations
because of the cytotoxicity of HEX (Litton Bionetics,
Inc., 1978a). This assay uses L5178Y cells that are het-
erozygous for thymidine kinase (TK+/-) and are sensitive.
The mutation is scored by cloning with bromodeoxyuridine
in the absence of thymidine. HEX is highly toxic to these
cells, particularly in the absence of an activating system
(at 0.04 ml/litre), and the positive control, dimethyl-
nitrosamine, was mutagenic at 0.5 ml/litre.
Williams (1978) found that HEX (10-6 mol/litre) was
inactive in the liver epithelial culture (hypoxanthine-
guanine-phosphoribosyl transferase locus) mutation assay.
At 10-5 mol/litre it also failed to stimulate DNA repair
in hepatocyte primary cultures. Negative results were also
obtained in an additional unscheduled DNA synthesis assay
(Brat, 1983).
One study provided by the US National Toxicology
Program (NTP) (Haworth et al., 1983) demonstrated a lack
of mutagenicity of HEX (98% pure). In S. typhimurium
strains TA98, TA100, TA1535, and TA1537, levels of up to
3.3 µg/plate were not mutagenic without activation, and
levels of up to 100 µg/plate were not mutagenic after
microsomal activation. Higher levels could not be tested
because of excessive cell dealth. Zimmering et al. (1985)
tested Drosophila by the sex-linked recessive lethal test
(SLRL), either by feeding doses of 40 ppm for 3 days or by
giving a single injection of 2000 ppm or 3000 ppm (volume
not specified). The vehicle used was 10% ethyl alcohol,
which did not totally dissolve the HEX. HEX (98%) was
first assayed in the SLRL test in adult feeding exper-
iments. When negative results were obtained, the chemical
was retested by injecting < 1 day-old Canton-S wild-type
Drosphila males. The results of the injection test were
reported to be inconclusive.
HEX has also been assayed in the mouse dominant lethal
test (Litton Bionetics, Inc., 1978b). In this assay, 0.1,
0.3, or 1.0 mg HEX/kg was administered by gavage to 10
male CD-1 mice for 5 days and the mice were then mated
throughout spermatogenesis (7 weeks). This test determines
whether the compound induces lethal genetic damage to the
germ cells of males. There was no evidence of dominant
lethal activity, at any dose level, based on any parameter
(e.g., fertility index, implantations per pregnancy,
average resorptions per pregnancy).
8.6. Cell transformation
The ability of HEX to induce morphological transfor-
mation of BALB/3T3 cells in vitro has been studied by
Litton Bionetics, Inc. (1977).
The selection of test doses was based on previous
cytotoxicity tests using a wide range of HEX concen-
trations at 0.0, 0.01, 0.02, 0.039, 0.078, and 0.156 mg
per litre. The cultures were exposed for 48 h, which was
followed by an incubation period of 3-4 weeks. The
cultures were observed daily. The doses selected allowed a
cell survival of 80-100% compared with controls (solvent
only). This high survival rate permitted an evaluation of
in vitro malignant transformation in cultures treated
with HEX as compared with the solvent controls. 3-Methyl-
cholanthrene at a dose level of 3 mg/litre was used as a
positive control. Results indicated that HEX was not
responsible for cell transformation.
8.7. Carcinogenicity
Bioassays of HEX for possible carcinogenicity have not
been conducted. As noted previously (section 8.3.2), the
NTP has completed a study on HEX for carcinogenicity by
the inhalation route in rats and mice, but the results
were not available when this monograph was being prepared.
9. EFFECTS ON HUMANS
9.1. General population exposure
There is very little detailed information available on
the human health effects of HEX exposure. Acute human
exposure has been reported in homes near waste sites where
disposal of HEX has occurred (C.S. Clark et al., 1982;
Elia et al., 1983). The odour threshold has been stated
to be 1.92 µg/m3 (0.17 ppb), but there appears to be
great individual variation. According to a study completed
by the A.D. Little Co. for the Occidental Chemical
Corporation, the 100% panel recognition concentration was
1.92 µg/m3 (0.17 ppb v/v)a, but the study design and
methodology were not reported. The US EPA (1982) estimated
that exposure of the general population to HEX in air
and/or water would be extremely low.
Treon et al. (1955) reported that members of a
research group conducting toxicity tests developed head-
aches when they were accidentally exposed to unknown
concentrations of HEX. The HEX escaped into the room when
an aerated exposure chamber was opened.
In a 48-block area surrounding a contaminated sewer
line in Kentucky, USA, questionnaires were sent to a
selected sample of residents. A total of 212 occupants
were surveyed. Only 3.8% of the residents reported an
unusual odour. The most common symptoms were stomach aches
(5.2%), burning or watery eyes (4.7%), and headaches
(4.7%). There was no association between the frequency of
symptoms and the distance of houses from the contaminated
sewer line. No significant ambient air concentrations of
HEX were found in these areas (Kominsky et al., 1978).
9.2. Occupational exposure
The US National Institute for Occupational Safety and
Health (NIOSH, 1980) stated that 1427 workers were occu-
pationally exposed to HEX. Officials from the Velsicol
Chemical Corporation estimated that 157 employees had been
potentially exposed to HEX in their production and pro-
cessing facilities.
A well-documented incident of acute human exposure to
HEX occurred in March 1977 at the Morris Forman Wastewater
Treatment Plant in Louisville, Kentucky, USA (Wilson et
al., 1978; Morse et al., 1979; Kominsky et al., 1980). The
details of the incident are included in the original NIOSH
Hazard Evaluation and Technical Assistance Report Number
TA-77-39 (Kominsky et al., 1978), which is available from
the US National Technical Information Service (NTIS). This
--------------------------------------------------------
a Memorandum from G. Leonardos to P. Levins on Hooker
special priority samples (odour properties).
treatment facility was contaminated with approximately 6
tonnes of HEX and octachlorocyclopentene, a waste product
of HEX manufacture (Morse et al., 1979). The contamination
was traced to an illegal dumping in one large sewer line
that passed through several populated areas. Concen-
trations of HEX detected in the sewage at the plant were
as high as 1000 mg/litre, and levels in the sewer line
were up to 100 mg/litre. Air samples taken from the sewer
line showed HEX concentrations to be as high as 4.5
mg/m3 (400 ppb). Although the airborne concentrations of
HEX at the time of the exposure in the treatment facility
were not known, airborne concentrations in the primary
treatment areas (screen and grit chambers) ranged between
3.05 and 10.96 mg/m3 (270 and 970 ppb) 4 days after the
plant had closed. It should be noted that the ACGIH
8 h-TWA for HEX was 0.1 mg/m3 (10 ppb) in 1977. Workers
tried to remove an odoriferous and sticky substance from
the bar screens and grit collection system by using steam
during clean-up of the contamination. This procedure pro-
duced a blue haze which permeated the primary treatment
area. The airborne HEX concentration of the blue haze was
reported to be 217 µg/m3 (19.2 ppm) (Kominsky et al., 1980).
The US Centers for Disease Control (CDC) and NIOSH
sent representatives to the plant with questionnaires
about the type and duration of symptoms (Morse et al.,
1979; Kominsky et al., 1980). In all, 193 employees were
identified as having been potentially exposed for 2 or
more days during the 2 weeks before the plant was closed
(Morse et al., 1979). The questionnaire was sent to each
of these 193 workers and 145 (75%) responded. Workers with
complaints of mucous membrane irritation were given a
physical examination, and blood and urine samples were
collected for clinical screening by an independent labora-
tory. Data were also collected on the exposure levels and
symptoms experienced by several people who had been
acutely exposed to the chemical vapours.
The results of the CDC and NIOSH questionnaires showed
that the odour of HEX had been detected by 94% of the
workers before the onset of symptoms. The most common
symptoms reported were eye irritation (59%), headaches
(45%), and throat irritation (27%) (Table 18). Of the 41
plant workers examined, six had physical signs of eye
irritation (i.e. lacrimation or redness) and five had
signs of skin irritation. Laboratory analyses of blood
and urine specimens from these workers showed marginal
increases in lactic dehydrogenase activity in 27% of cases
and proteinuria in 15%. Three weeks later, no abnormali-
ties were detected in the blood and urine tests. After six
weeks, some of the clinical symptoms persisted in 25-45%
of the employees (Morse et al., 1978).
Table 18. Symptoms of 145 waste-water treatment
plant employees exposed to HEX (Louisville,
Kentucky, USA, March 1977)a
----------------------------------------------------
Symptom No. of Percent of
employees employees
with symptoms with symptoms
----------------------------------------------------
Eye irritation 86 59
Headache 65 45
Throat irritation 39 27
Nausea 31 21
Skin irritation 29 20
Cough 28 19
Chest pain 28 19
Difficult breathing 23 16
Nervousness 21 14
Abdominal cramps 17 12
Decreased appetite 13 9
Decreased memory 6 4
Increased saliva 6 4
----------------------------------------------------
a From: Morse et al. (1978).
Although there was difficulty in measuring the level
of exposure received by the plant workers, more than 50%
of the clean-up crew were monitored. Laboratory tests
showed no significant abnormalities in renal function
tests, complete blood counts, or urinalysis, but several
minimal or mild abnormalities were found in liver function
tests (Kominsky et al., 1980). The abnormalities in 18 out
of 97 clean-up workers are listed in Table 19. These
people also had physical signs of mucous membrane irri-
tation. A more detailed correlation between acute exposure
level data and symptomatology was reported for nine adults
(Kominsky et al., 1980). The data appear in Table 20. The
exposure levels could not be estimated accurately because
of prior exposure or because the worker had used protec-
tive equipment.
Table 19. Abnormalities detected in clean-up
workers at the Morris Forman treatment plant,
Louisville, Kentucky, USAa
-------------------------------------------------
Laboratory test Normal range Abnormal results
Range No.b
-------------------------------------------------
Serum glutamate
oxaloacetate 7-40 mU/ml 40-49 5
transaminase 50-59 1
60-69 4
70-79 0
80-89 1
90-99 1
Serum alkaline 30-100 mU/ml 100-109 3
phosphatase 110-119 1
120-129 1
Serum total 0.15-10 mg/dl 1.0-1.9 1c
bilirubin
Serum lactate 100-225 mU/ml 230-239 1
dehydrogenase
-------------------------------------------------
a From: Kominsky et al. (1980).
b For individuals with more than one serial
blood test, only the most abnormal result is
tabulated.
c Associated with a serum glutamate oxaloacetate
transaminase activity of 66 mU/ml.
Table 20. Individual exposure symptomatology correlations at the Morris Forman treatment plant (Louisville, Kentucky, USA)a
-------------------------------------------------------------------------------------------------------------------------------------
No. of Estimated airborne Immediate symptoms Persistence of symptoms Laboratory
workers exposureb results
-------------------------------------------------------------------------------------------------------------------------------------
1 19.2 ppm HEX and 650 ppb lacrimation; skin irritation on face 1.5 h post-exposure: fatigue; normal results
OCCP for several seconds and neck; dyspnoea and chest erythema of exposed skin; eye 4 days
(no protective equipment) discomfort;nausea (several min later) irritation subsided in 1 day; post-exposurec
chest discomfort persisted
several days
3 7083 ppb HEX and 446 ppb lacrimation; irritation of exposed asymptomatic at 2 h, except normal results
OCCP for several seconds skin for soreness around eyes 7 days post-
(half-face respirator) exposure in
one workerc
2 40-52 ppb HEX and slight eye irritation no symptoms after cessation of normal results
9-21 ppb OCCP exposure 7 days post-
(half-face respirator) exposure in one
workerc
2 exact exposure unknown slight skin irritation faces felt "puffy" and "windburned" none available
(half-face respirator) for 1-2 days after exposure; this
was noted also by friends and
family; no residual skin lesions.
1 980 ppb HEX for 15 min; irritated eyes; nasal irritation eyes felt "dry and irritated" for none available
OCCP not measured and sinus congestion after 2 weeks 2-3 days after exposure; nasal
(no protective equipment) of intermittent exposures irritation ceased within 1-2 days
of cessation of exposure.
-------------------------------------------------------------------------------------------------------------------------------------
a From: Kominsky et al. (1980).
b OCCP = Octachlorocyclopentene.
c Laboratory work was same as carried out on clean-up crew.
Hazards to workers in treatment plants can result from
chemical compounds contained in the industrial waste
treated in municipal waste-water plants. The HEX-contain-
ing wastes from a pesticide manufacturer were treated in a
municipal waste-water treatment plant at Memphis,
Tennessee, USA. In 1978, the workers at this plant
reported symptoms similar to those reported by workers in
the Louisville plant referred to above. The air and waste-
water were monitored, and analyses of urine and blood,
liver function tests, and illness symptom questionnaires
were completed. Workers from another waste treatment
plant, where no pesticide wastes had been received, were
used as a control group. No statistical differences in
urine HEX concentrations or liver function tests between
the exposed and control groups were found, although
differences in levels of HEX-related compounds (co-
contaminants) were detected (Elia et al., 1983).
9.3. Epidemiological studies
Mortality studies have been carried out on workers
involved in the production of HEX or formulation of HEX
products. Shindell et al. (1980) studied a cohort of 783
current and former workers who had been employed at the
Velsicol Chemical Corporation plant at Marshall, Illinois,
between 1946 and 1979. The purpose of the study was to
evaluate the overall health status of all employees who
had been present during the manufacture of chlordane for 3
months or more. There were no significant differences in
mortality rates between these employees and the overall
USA population. The observed value for deaths from all
causes, including heart disease and cancer, was less than
the expected value in the overall USA population.
Shindell et al. (1981) completed another epidemiologi-
cal study for the Velsicol Chemical Corporation at its
Memphis, Tennessee, plant covering the period 1952-1979.
This coincided with the manufacture of heptachlor, a
pesticide made from HEX. The reseachers studied 1115
current and former employees who had worked for 3 months
or more. Again, there was no significant difference in
mortality between the control and exposure groups.
Concomitant with the study performed by Shindell et
al. (1980), Wang & MacMahon (1979) conducted a retrospec-
tive mortality study of workers employed at the Marshall
and Memphis plants, where chlordane and heptachlor were
manufactured between 1946 and 1976. They studied 1403
males who had worked at the plants for more than 3 months.
There were 113 observed deaths compared with 157 expected
deaths, giving a standardized mortality ratio (SMR) of 72.
Among the various causes of death, the two highest SMRs
were 134 for lung cancer and 183 for cerebrovascular
disease, but only the latter figure was statistically sig-
nificant (P < 0.05). The excess mortality due to cerebro-
vascular disease was not related to the duration of
exposure or to the latency period, and occurred only after
termination of employment. Shindell & Ulrich (1986)
updated their 1980 data set with additional worker data.
There was no information specific to HEX exposure.
Buncher et al. (1980) studied the mortality of workers
at a chemical plant that produced HEX. They examined 341
workers (287 male and 54 female), together with their
health records, who had worked at the plant for at least
90 days between 1 October 1953 and 31 December 1974. Their
vital status was determined through 1978. The expected
numbers of deaths, based on the USA population and
specific for sex, age, and calendar year, were calculated.
The SMR for all causes of death was 69. Deaths caused by
specific cancers, all cancers, and diseases of the circu-
latory and digestive systems were also fewer than
expected. The authors noted that the time that had elapsed
since the initial exposure (25 years at most) reduced the
power of the study to detect cancers that may have a
10-40 year latent period.
Similar studies have been performed on a cohort of
workers in the Shell International Petroleum plants in the
Netherlands. These reports have been reviewed extensively
in Environmental Health Criteria 91: Aldrin and Dieldrin
(WHO, 1989).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
The general population is not at risk from exposure to
HEX. However, people living near HEX processing and pro-
duction facilities, as well as handlers of the chemical
and its waste, are at risk. Exposure to HEX can occur
through several different routes. However, HEX is much
more toxic when inhaled than when ingested or following
dermal contact. Skin exposure studies have shown that HEX
can cause irritation, together with visceral changes that
are similar to those that result from oral administration.
Inhalation studies in animals have shown that HEX vapour
is very irritating, repeated exposure to 1.13-2.26 mg/m3
(0.1-0.2 ppm) causing pulmonary pathological changes.
Acute toxic symptoms, including headaches, nausea, dizzi-
ness, and respiratory distress, have been reported. Based
on a 90-day inhalation study in mice and rats, a NOEL of
0.45 mg/m3 (0.04 ppm) has been estimated. No information
is available on the long-term effects of a single exposure
or of continuous exposure to HEX.
A carcinogenic study of HEX has been completed (but
not evaluated) by the US National Toxicology Program. In
vitro mutagenicity and cell transformation tests yielded
negative results, as did an in vivo mouse dominant lethal
assay at the levels tested. There was no evidence of
teratogenicity in oral exposure studies in which three
species were examined.
The toxic effects of HEX exposure on the human respir-
atory system are of major concern. Although the long-term
toxicity data are limited, systemic toxic effects of HEX
inhalation have been observed after short-term exposure,
suggesting that long-term inhalation exposure to low con-
centrations of HEX could cause adverse health effects.
Limited epidemiological studies of workers exposed to HEX
at levels above those known to induce adverse health
effects have been conducted, although concomitant exposure
to other chemicals was known to have occurred. These
workers complained of headaches, eye and skin irritation,
nausea, dizziness, and respiratory distress, as did
individuals living near areas where there were HEX
releases.
10.2. Evaluation of effects on the environment
Release of HEX into the environment can result from
the production, processing and use of HEX, disposal of
waste containing HEX or from products contaminated with
HEX. Only a small proportion of the total amount of HEX
released into the environment from production, processing,
and use can be expected to persist beyond a few days.
However, waste disposal has resulted in HEX persisting in
soil, sediment, and ground water. There is little monitor-
ing information on the levels of HEX in air, water, and
sediment. The exposure of organisms in the aquatic
environment to HEX is therefore difficult to quantify.
HEX may undergo photolysis, hydrolysis, and biodegra-
dation. In water, photolysis is the dominant process in
direct sunlight, hydrolysis being the next most important
degradation route. Volatilization to the atmosphere occurs
from both water and soil. Biodegradation occurs in soil
under both aerobic and anaerobic conditions and in sewage
sludge. In water and aquatic sediment, biodegradation is
initially limited. Information on the adaptation of micro-
organisms to degrade HEX is limited. HEX has a calculated
tropospheric residence time of approximately 5 h.
Laboratory studies suggest that HEX is relatively
immobile in soil, particularly in soil with a high organic
content. However, leaching and movement in ground water
has been reported in field studies.
HEX has been shown to be toxic to aquatic life at a
level of 1-100 µg/litre. However, little information has
been obtained under field conditions (long-term exposure
at low concentration in the presence of sediment). The
actual hazard to aquatic life is, therefore, difficult to
assess. HEX is toxic to aquatic microorganisms but less
toxic to soil microorganisms. Exposure of terrestrial
organisms, except at or near disposal sites, would be
expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
* The general population is not at risk from exposure
to HEX, except in the case of people residing near
contaminated areas.
* The long-term human health effects of continuous low-
level exposure are not known. Handlers of the product
and its waste, as well as sewage workers, are at
risk.
* Results of laboratory studies indicate that HEX
should be degraded rapidly in the environment by
photolysis, hydrolysis, or biodegradation. The rela-
tive importance of these processes varies with the
medium. HEX is not a widespread environmental con-
taminant and the available data suggest that it is
only found associated with production, processing and
disposal sites. HEX does not bioaccumulate.
* Acute laboratory tests show that HEX is highly toxic
to aquatic microorganisms, invertebrates, and fish,
but less toxic to soil microorganisms. However,
information obtained under environmentally realistic
conditions is limited. The potential hazard to the
general environment is expected to be low.
11.2. Recommendations for protection of human health and
the environment
* Occupational exposure to HEX should be minimized by
the use of closed systems. Guidelines for the dis-
posal of HEX and HEX wastes should be followed.
* Environmental monitoring is needed to examine the
persistence and fate of HEX in all media near pro-
duction, processing and disposal sites, and also
hazardous waste incinerators. Monitoring data are
required for HEX in drinking-water, and in surface,
shower, and ground water.
12. FURTHER RESEARCH
* Biomarker technology should be developed to indicate
the possibility of past or current actions of HEX.
Such biomarkers could be stable metabolites derived
from HEX and its impurities that are present in the
original preparation.
* Research is needed on the metabolic, degradative, and
reactive products to understand the fate of HEX in
human beings and the environment.
* Further study of the apparent disparity between degra-
dation under laboratory conditions and that observed
in the environment is needed.
* The efficacy and safety of current disposal methods
should be evaluated and their present and future
health impacts assessed.
* Developmental and reproductive studies of HEX need to
be conducted, with emphasis on the inhalation route of
exposure.
* Methods for the early warning of the presence of low
levels of HEX should be developed.
REFERENCES
ABDO, K.M., MONTGOMERY, C.A., KLUWE, W.M., FARNELL, D.R., & PREJEAN, J.D.
(1984) Toxicity of hexachlorocyclopentadiene: subchronic (13-week)
administration by gavage to F344 rats and B6C3F1 mice. J. appl. Toxicol.,
4(2): 75-81.
ALEXANDER, D.J., CLARK, G.C., JACKSON, G.C., HARDY, C.J., STREET, A.E.,
HEYWOOD, R.H., BUIST, D., PRENTICE, D.E., & ISAACS, K.R. (1980)
Subchronic inhalation toxicity of hexachlorocyclopentadiene in monkeys
and rats, Huntingdon, Huntingdon Research Centre, 373 pp (Report
VCL14M/791081) (Prepared for Velsicol Chemical Corporation, Chicago).
ATALLAH, Y.H., WHITACRE, D.M., & BUTZ, R.G. (1980) Fate of
hexachlorocyclopentadiene in the environment. Paper presented at the 2nd
Chemical Congress of the North American Continent, Las Vegas, Nevada, 29
August, 1980, Chicago, Velsicol Chemical Corporation.
BELL, M.A., EWING, R.A., & LUTZ, G.A. (1978) Reviews of the environmental
effects of pollutants. XII. Hexachlorocyclopentadiene, Washington, DC, US
Environmental Protection Agency (Report EPA-600/1-78-047) (NTIS PB 80-
122963).
BENNETT, B. (1982) Hexachlorocyclopentadiene analyses of Mississippi
river fish, Athens, Georgia, US Environmental Protection Agency, Region
IV (Unpublished report).
BENOIT, F.M. & WILLIAMS, D.T. (1981) Determination of
hexachlorocyclopentadiene at the nanogram per liter level in drinking
water. Bull. environ. Contam. Toxicol., 27: 303-308.
BOYD, K.W., EMORY, M.B., & DILLON, H.K. (1981) Development of personal
sampling and analytical methods for organochlorine compounds. In:
Chemical hazards in the workplace, Washington, DC, American Chemical
Society, pp. 49-64 (ACS Symposium Series No. 149).
BRAT, S.V. (1983) The hepatocyte primary culture/DNA repair assay on
compound hexachlorocyclopentadiene using rat hepatocytes in culture,
Valhalla, New York, American Health Foundation, Naylor Dana Institute for
Disease Prevention.
BRIGGS, G.G. (1973) A simple relationship between soil adsorption of
organic chemicals and their octanol/water partition coefficients. In:
Proceedings of the 7th British Insecticide and Fungicide Conference,
Brighton, England, 19-22 November, 1973, Croydon, British Crop Protection
Council, Vol. 1, pp. 83-86 (Research Report).
BUA (BERATERGREMIUM FUR UMWELTRELEVANTE ALTSTOFFE) (1988)
[Hexachlorocyclopentadiene] Weinheim, Germany, VCH Verlagsgesellschaft
(BUA Report No. 25) (in German).
BUCCAFUSCO, R.J. & LEBLANC, G.A. (1977) Acute toxicity of
hexachlorocyclopentadiene to bluegill (Lepomis macrochirus), channel
catfish (Ictalurus punctatus), fathead minnow (Pimephales promelas), and
the water flea (Daphnia magna) (Unpublished report prepared for Velsicol
Chemical Corporation, Chicago).
BUNCHER, C.R., MOOMAW, C., & SIRKOSKI, E. (1980) Mortality study of
Montague Plant-Hooker Chemical, Cincinnati, Ohio, University of
Cincinnati Medical Center, Division of Epidemiology and Biostatistics
(Unpublished report prepared for Hooker Chemical Corporation).
BUTZ, R.G. & ATALLAH, Y.H. (1980) Effects of hexachlorocyclopentadiene on
three microbial functions (VCC Project No. 482428, Report No. 8)
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
BUTZ, R.G., YU, C.C., & ATALLAH, Y.H. (1982) Photolysis of
hexachlorocyclopentadiene in water. Ecotoxicol. environ. Saf., 6: 347-
357.
CALLAHAN, M.A., SLIMAK, M.W., GABEL, N.W., MAY, I.P., FOWLER, C.F.,
FREED, J.R., JENNINGS, P., DURFEE, R.L., WHITMORE, F.C., MAESTRI, B.,
MABEY, W.R., HOLT, B.R., & GOULD, C. (1979) Water-related environmental
fate of 129 priority pollutants: II. Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic
aromatic hydrocarbons, nitrosamines, miscellaneous compounds, Washington,
DC, US Environmental Protection Agency, Monitoring and Data Supervision
Division, Office of Water Planning and Standards (Report EPA-440/4-79-
029b).
CARTER, M.R. (1977) Legal affidavit field in the State of Georgia, Fulton
County, dated 14 June, 1977. Testimony concerning estimates of total
daily discharge of HEX from Velsicol Chemical Corporation, Atlanta,
Athens, Georgia, US Environmental Protection Agency.
CHERNOFF, N. & KAVLOCK, R.J. (1982) An in vivo teratology screen
utilizing pregnant mice. J. Toxicol. environ. Health, 10: 541-550.
CHERNOFF N. & KAVLOCK, R.J. (1983) A teratology test system which
utilizes postnatal growth and viability in the mouse. In: Waters, M.,
Sandhu, S., Lewtas, J., Claxton, L., Chernoff, N., & Nesnow, S., ed.
Short-term bioassays in the analysis of complex mixtures III, New York,
London, Plenum Publishing Corporation, pp. 417-427.
CHOPRA, N.M., CAMPBELL, B.S., & HURLEY, J.C. (1978) Systematic studies on
the breakdown of endosulfan in tobacco smokes: Isolation and
identification of the degradation products from the pyrolysis of
endosulfan I in a nitrogen atmosphere. J. agric. food Chem., 26: 255-258.
CHOU, S.F.J. & GRIFFIN, R.A. (1983) Soil, clay, and caustic soda effects
on solubility, sorption and mobility of hexachlorocyclopentadiene,
Springfield, Virginia, National Technical Information Service, 54 pp (PB
84-116060) (Environmental Geology Notes No. 104).
CHOU, S.F.J., GRIFFIN, R.A., CHOU, M.M, & LARSON, R.A. (1987) Products of
hexachlorocyclopentadiene (C-56) in aqueous solution. Environ. Toxicol.
Chem., 6: 371-376.
CLARK, C.S., MEYER, C.R., GARTSIDE, P.S., MAJETI, V.A., & SPECKER, B.
(1982) An environmental health survey of drinking water contamination by
leachate from a pesticide waste dump in Hardeman County, TN. Arch.
environ. Health, 37(1): 9-18.
CLARK, D.G., BLAIR, D., MARTIN, J., HENDY, R., PILCHER, A., & WIGGINS, D.
(1982) Thirty-week chronic inhalation study of hexachlorocyclopentadiene
(HEX) in rats, Tunstall, United Kingdom, Shell Toxicology Laboratory
(Experiment No. 1760, Report No. SBGR.81) (Permission for use granted by
M.J. Sloan, Shell Oil Co., Washington).
COLE, E.J. (1953) Chemotherapeutic and pharmacologic aspects of
hexachlorocyclopentadiene, Laramie, University of Wyoming, Department of
Veterinary Sciences and Bacteriology (Master's Thesis).
COLE, E.J. (1954) Treatment of sewage with hexachlorocyclopentadiene.
Appl. Microbiol., 2: 198-199.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment, Research Triangle Park, North Carolina, US Environmental
Protection Agency (Report EPA-600/3-80-084).
DAL MONTE, R.P. & YU, C.C. (1977) Water solubility of MC-984 and hex
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DELEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, P.C., REMELE, P.C.,
STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980a) Rapid gas
chromatographic method for the determination of volatile and semivolatile
organochlorine compounds in soil and chemical waste disposal site
samples. J. chromatogr. Sci., 18: 85-88.
DELEON, I.R., BROWN, N.J., COCCHIARA, J.P., MILES, S.K., & LASETER, J.L.
(1980b) Determination of trace levels of hexachlorocyclopentadiene and
octachlorocyclopentene in body fluids. J. anal. Toxicol., 4: 314-317.
DILLON, H.K. (1980) Development of air sampling and analytical methods
for toxic chlorinated organic compounds, Birmingham, Alabama, Southern
Research Institute, 76 pp (NTIS PB 80-193279).
DOROUGH, H.W. (1979) The accumulation, distribution and dissipation of
hexachlorocyclopentadiene (C56) in tissues of rats and mice, 27 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. (1980) Disposition of 14C-hexachlorocyclopentadiene (C56)
in rats following inhalation exposure, 53 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. & RANIERI, T.A. (1984) Distribution and elimination of
hexachlorocyclopentadiene in rats and mice. Drug chem. Toxicol., 7(1):
73-89.
EICHLER, D.L. (1978) Quantitative analyses of mixtures containing trace
amounts of pesticides. Internal memorandum to M.R. Zavon, Hooker
Chemicals and Plastics Corporation, Niagara Falls, New York, 3 pp.
EL DAREER, S.M., NOKER, P.E., TILLERY, K.F., & HILL, D.L. (1983)
Investigations on the basis for the differential toxicity of
hexachlorocyclopentadiene administered to rats by various routes. J.
Toxicol. environ. Health, 12: 203-211.
ELIA, V.J., CLARK, C.S., MAJETI, V.A., GARTSIDE, P.S., MACDONALD, T.,
RICHDALE, N., MEYER, C.R., VAN MEER, G.L., & HUNNINEN, K. (1983) Chemical
exposure at a municipal wastewater treatment plant. Environ. Res., 32:
360-371.
EVANS, M.J., CABRAL-ANDERSON, L.J., & FREEMAN, G. (1978) Role of the
Clara cell in renewal of the bronchiolar epithelium. Lab. Invest., 38:
648-655.
GOGGELMAN, W., BONSE, G., HENSCHLER, D., & CREIM, H. (1978) Mutagenicity
of chlorinated cyclopentadiene due to metabolic activation. Biochem.
Pharmacol., 27: 2927-2929.
GRAY, L.E., Jr & KAVLOCK, R.J. (1984) An extended evaluation of an in
vivo teratology screen utilizing postnatal growth and viability in the
mouse. Teratog. Carcinog. Mutagen., 4: 403-426.
GREIM, J., BIMBOES, D., GOGGELMANN, W., & KRAMER, M. (1977) Mutagenicity
and chromosomal aberrations as an analytical tool for in vitro detection
of mammalian enzyme-mediated formation reactive metabolites. Arch.
Toxicol., 39: 159-169.
HAWLEY, G.G., ed. (1977) Condensed chemical dictionary, 9th ed., New
York, Van Nostrand Reinhold Co.
HAWORTH, S., LOWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E. (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen., 5(Suppl. 1): 3-142.
HENDERSON, C. (1956) Bio-assay investigations for International Joint
Commission, Niagara Falls, New York, Hooker Electrochemical Company, 17
pp (Unpublished report).
HUNT, G.E. & BROOKS, G.W. (1984) Source assessment for
hexachlorocyclopentadiene, Research Triangle Park, North Carolina, Radian
Corporation (Unpublished report prepared for the US Environmental
Protection Agency).
IBT (1977) Mutagenicity of PCL-HEX incorporated in the test medium tested
against five strains of S. typhimurium and as a volatilate against tester
strain TA-100, Northbrood, Illinois, Industrial Bio-Test Laboratories.
IRDC (1968) Hexachlorocyclopentadiene and octachlorocyclopentene: Acute
oral toxicity LD50 in male albino rats, Mattawan, Michigan, International
Research and Development Corporation, 4 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
IRDC (1972) Acute toxicity studies in rats and rabbits, Mattawan,
Michigan, International Research and Development Corporation, 21 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
IRDC (1978) Hexachlorocyclopentadiene: Teratology study in rats,
Mattawan, Michigan, International Research and Development Corporation,
17 pp (Unpublished report prepared for Velsicol Chemical Corporation,
Chicago).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic.
Hexachlorocyclopentadiene. In: Patty, F.A., ed. Industrial hygiene and
toxicology, 2nd revised ed., New York, Chichester, Brisbane, Toronto,
John Wiley & Sons, pp. 1333-1363.
IRPTC (1989) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol. environ.
Safety, 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (1980) Relationship between water
solubility, soil sorption, octanol/water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
of Testing and Materials, pp. 78-115 (ASTM STP 707).
KHAN, M.A.Q., SUDERSHAN, P., FEROZ, M., & PODOWSKI, A.A. (1981)
Biotransformations of cyclodienes and their photoisomers and
hexachlorocyclopentadiene in mammals and fish. In: Khan, M.A.Q. &
Stanton, R.H., ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects, Oxford, New York, Pergamon Press, pp. 271-288.
KILZER, L., SCHEUNERT, I., GEYER, H., KLEIN, W., & KORTE, F. (1979)
Laboratory screening of the volatilization rates of organic chemicals
from water and soil. Chemosphere, 8: 751-761.
KLOSKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981) Laboratory
screening of distribution, conversion and mineralization of chemicals in
the soil-plant-system and comparison to outdoor experimental data.
Chemosphere, 10: 1089-1100.
KOMINSKY, J.R. & WISSEMAN, C.L. (1978) Morris Forman wastewater treatment
plant, Metropolitan Sewer District, Louisville, KY, Cincinnati, Ohio,
National Institute of Occupational Safety and Health (NIOSH Hazard
Evaluation and Technical Assistance Report No. TA 77-39) (NTIS PB 82-
178088).
KOMINSKY, J.R., WISSEMAN, C.L., & MORSE, D.L. (1980)
Hexachlorocyclopentadiene contamination of a municipal wastewater
treatment plant. Am. Ind. Hyg. Assoc. J., 41: 52.
KOMMINENI, C. (1978) Pathology reports. Animal portion of the Louisville
sewage study, Cincinnati, Ohio, National Institute of Occupational Safety
and Health (NIOSH Report No. TA 77-39).
LAWLESS, E.W., VON RUMKER, R., & FERGUSON, T.L. (1972) The pollution
potential in pesticide manufacturing, Springfield, Virginia, US
Department of Commerce, National Technical Information Service (NTIS PB
213782/3) (Prepared for the US Environmental Protection Agency by Midwest
Research Institute).
LAWRENCE, L.J. & DOROUGH, H.W. (1981) Retention and fate of inhaled
hexachlorocyclopentadiene in the rat. Bull. environ. Contam. Toxicol.,
26: 663-668.
LAWRENCE, L.J. & DOROUGH, H.W. (1982) Fate of inhaled
hexachlorocyclopentadiene in albino rats and comparison to the oral and
iv routes of administration. Fundam. appl. Toxicol., 2: 235-240.
LEVIN, A.A. (1982a) Hexachlorocyclopentadiene: Response to issues raised
at US EPA Test Rules Meeting of March 17, 1982, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Docket No.
40-8249078).
LEVIN, A.A. (1982b) Hexachloropentadiene: Follow-up and additional
information to the 17 March, 1982 Meeting, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
LICHTENBERG, J.J., LONGBOTTON, J.E., & BELLAR, T.A. (1987) Analytical
methods for the determination of volatile non-polar organic chemicals in
water and water-related environments. In: Suffet, I.H. & Malaiyandi, M.,
ed. Organic pollutants in water: Sampling, analysis and toxicity testing.
A Symposium of the 188th Meeting of the American Chemical Society,
Philadelphia, 29-31 August, 1984, Washington, DC, American Chemical
Society, pp. 63-81.
LITTON BIONETICS, INC. (1977) Evaluation of hexachlorocyclopentadiene; in
vitro malignant transformation in BALB/3T3 cells, Kensington, Maryland,
Litton Bionetics, Inc., 7 pp (LBI Project No. 29840) (Prepared for
Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978a) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse lymphoma forward mutation assay,
Kensington, Maryland, Litton Bionetics, inc., 10 pp (LBI Project No.
20839) (Prepared for Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978b) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse dominant lethal assay, Kensington,
Maryland, Litton Bionetics, Inc., 13 pp (LBI Project No. 20862).
LOOK, M. (1974) Hexachlorocyclopentadiene adducts of aromatic compounds
and their reaction products. Aldrichem. Acta, 7(2): 1974.
LU, P.Y., METCALF, R.L., HIRWE, A.S., & WILLIAMS, J.W. (1975) Evaluation
of environmental distribution and fate of hexachlorocyclopentadiene,
chlordane, heptachlor, and heptachlor epoxide in a laboratory model
ecosystem. J. agric. food Chem., 23: 967-973.
MACEK, R.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979) Considerations
in assessing the potential for, and significance of, biomagnification of
chemical residues in aquatic food chains. In: Marking, L. & Kimerle,
R.A., ed. Aquatic toxicology, Philadelphia, American Society for Testing
and Materials, pp. 251-268.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida, US Environmental Protection Agency
(Report EPA-600/8-017).
MEHENDALE, H.M. (1977) Chemical reactivity-absorption, retention,
metabolism and elimination of hexachlorocyclopentadiene. Environ. Health
Perspect., 21: 275-278.
MEIER, J.R., RINGHAND, H.P., COLEMAN, W.E., MUNCH, J.W., STREICHER, R.P.,
KAYLOR, W.H., & SCHENCK, K.M. (1985) Identification of mutagenic
compounds formed during chlorination of humic acid. Mutat. Res., 157:
111-122.
MOLOTSKY, H.M. & BALLWEBER, E.G. (1957) Hexachlorocyclopentenones: US
Patent No. 2,795,608, June 11, 1957, Chicago, Velsicol Chemical
Corporation.
MORSE, D.L., LANDRIGAN, P.J., & FLYNT, J.W. (1978) Internal CDC report
concerning hexachlorocyclopentadiene contamination of a municipal sewage
treatment plant, Louisville, Kentucky, Atlanta, Georgia, Centers for
Disease Control.
MORSE, D.L., KOMINSKY, J.R., & WISSEMAN, C.L., III (1979) Occupational
exposure to hexachlorocyclopentadiene (How safe is sewage?). J. Am. Med.
Soc., 241: 2177-2179.
MURRAY, F.J., SCHWETZ, B.A., BALMER, M.F., & STAPLES, R.E. (1980)
Teratogenic potential of hexachlorocyclopentadiene in mice and rabbits.
Toxicol. appl. Pharmacol., 53: 497-500.
NAS (1978) Kepone/mirex/hexachlorocyclopentadiene: An environmental
assessment, Washington, DC, National Academy of Sciences (NTIS PB 280-
289).
NEUMEISTER, C. & KURIMO, R. (1978) Determination of
hexachlorocyclopentadiene and octachlorocyclopentene in air. Presented at
the ACGIH Conference, Los Angeles, May 1978, Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
NIOSH (1979) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1-5 (DHEW
(NIOSH) Pub. No. 77-157-A).
NIOSH (1980) Quarterly hazard summary report: Hexachlorocyclopentadiene,
Cincinnati, Ohio, National Institute for Occupational Safety and Health.
NTP (1984a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 128 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
NTP (1984b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 196 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
OSHA (US DEPARTMENT OF LABOR, OCCUPATIONAL SAFETY AND HEALTH
ADMINISTRATION) (1989) Air contaminants: Final rule -
Hexachlorocyclopentadiene. Fed. Reg., 54(12): 2464.
PETERS, J.A., TACKETT, K.M., & EIMUTIS, E.C. (1981) Measurement of
fugitive hydrocarbon emissions from a chemical waste disposal site.
Presented at the 74th Annual Meeting of the Air Pollution Control
Association, Philadelphia, 21-26 June, 1981, Dayton, Ohio, Montesanto
Research Corporation.
PODOWSKI, A.A. & KHAN, M.A.Q. (1979) Fate of hexachlorocyclopentadiene in
goldfish (Carassius auratus). Paper presented at the American Chemical
Society Meeting, Honolulu, April 1979, Washington, DC, American Chemical
Society.
PODOWSKI, A.A. & KHAN, M.A.Q. (1984) Fate of hexachlorocyclopentadiene in
water and goldfish. Arch. environ. Contam. Toxicol., 13: 471-481.
RAND, G.M., NEES, P.O., CALO, C.J., ALEXANDER, D.J., & CLARK, G.C.
(1982a) Effects of inhalation exposure to hexachlorocyclopentadiene on
rats and monkeys. J. Toxicol. environ. Health, 9: 743-760.
RAND, G.M., NEES, P.O., CALO, C.J., CLARKE, G.C., & EDMONDSON, N.A.
(1982b) The Clara cell: An electron microscopy examination of the
terminal bronchioles of rats and monkeys following inhalation of
hexachlorocyclopentadiene. J. Toxicol. environ. Health, 10: 59-72.
RIECK, C.E. (1977a) Effect of hexachlorocyclopentadiene on soil microbe
populations, Lexington, Kentucky, University of Kentucky, Agronomy
Department (Unpublished report prepared for Velsicol Chemical
Corporation, Chicago).
RIECK, C.E. (1977b) Soil metabolism of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
RIECK, C.E. (1977c) Volatile products of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
ROBERTS, C.W. (1958) Chemistry of hexachlorocyclopentadiene. Chem. Ind.,
1 February: 110.
SHELL RESEARCH LIMITED (1982) Toxicology of insecticide intermediates:
The skin sensitizing potential of hexachlorocyclopentadiene, Tunstall,
United Kingdom, Shell Research Ltd., Sittingbourne Research Centre
(Report No. SBGR 82.225).
SHINDEL, S. & ULRICH, S. (1986) Mortality of workers employed in the
manufacture of chlordane. An update. J. occup. Med., 28(7): 497-501.
SHINDELL & ASSOCIATES (1980) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Marshall, Illinois,
January 1946-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SHINDELL & ASSOCIATES (1981) Report of the epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Memphis, Tennessee,
January 1952-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1982)
Hexachlorocyclopentadiene increases oxygen consumption by intact rainbow
trout and isolated heart mitochondria. Sixty-sixth Annual Meeting of the
Federation of American Societies for Experimental Biology, New Orleans,
15-23 April, 1982. Fed. Proc., 41(5): 1580 (abstract).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1983)
Respiratory effects of hexachlorocyclopentadiene on intact rainbow trout
(Salmo gairdneri) and on oxidative phosphorylation of isolated trout
heart mitochondria. Toxicol. appl. Pharmacol., 67: 215-223.
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1977) A rapid
assessment of the toxicity of three chlorinated cyclodiene insecticide
intermediates to fathead minnows, Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory (Report EPA-600/3-
77-099).
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1979) Toxicity
and bioaccumulation of hexachlorocyclopentadiene, hexachloronorbornadiene
and heptachloronorbonene in larval and early juvenile fathead minnows,
Pimephales promelas. Bull. environ. Contam. Toxicol., 21: 576-583.
SPRINKLE, C.L. (1978) Leachate migration from a pesticide. Waste disposal
site in Hardeman County, Tennessee, Washington, DC, US Department of
Interior, US Geological Survey (Waste Resources Investigations 78-128).
SRI (1980a) Acute toxicity report on hexachlorocyclopentadiene (C53607)
in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama, Southern
Research Institute, 44 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1980b) Repeated-dose toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama,
Southern Research Institute, 33 pp (Unpublished report prepared for the
National Toxicology Program).
SRI (1981a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 137 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1981b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 144 pp (Unpublished report prepared for the National
Toxicology Program).
STEVENS, J.E. (1979) Chlorinated derivatives of cyclopentadiene. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
Chichester, Brisbane, Toronto, John Wiley & Sons, Vol. 5, pp. 791-797.
THIELEN, D.R., OLSEN, G., DAVIS, A., BAJOR, E., STEFANOVSKI, J., &
CHODKOWSKI, J. (1987) An evaluation of microextraction/capillary column
gas chromatography for monitoring industrial outfalls. J. chromatogr.
Sci., 25(1): 12-16.
THUMA, N.K., O'NEILL, P.E., BROWNLEE, S.G., & VALENTINE, R.S. (1978)
Biodegradation of spilled hazardous materials. In: Control of hazardous
materials spills, Rockville, Maryland, Information Transfer, Inc., pp.
217-220.
TREON, J.F., CLEVELAND, F.P., & CAPPEL, J. (1955) The toxicity of
hexachlorocyclopentadiene. Arch. ind. Health, 11: 459-472.
UNGNADE, H.E. & MCBEE, E.T. (1958) The chemistry of
perchlorocyclopentenes and cyclopentadienes. Chem. Rev., 58: 249-254.
US EPA (1977) Chemical Hazard Information Profile:
Hexachlorocyclopentadiene (CHIP), Washington, DC, US Environmental
Protection Agency, TSCA Interagency Testing Committee.
US EPA (1980a) Summary of UWF Co-op Data on hexachlorocyclopentadiene and
hexachlorobutadiene, Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished laboratory data).
US EPA (1980b) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards (Report EPA-440/5-80-055)
(NTIS PB 292-436).
US EPA (1980c) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, office of Water Regulations and Standards (Report EPA-440/5-80-
055) (Unpublished data).
US EPA (1982) Hexachlorocyclopentadiene: Response to the Interagency
Testing Committee. Fed. Reg., 47(250): 58023-58025.
US EPA (1989) Toxic chemical release inventory: Online printout of April
1989, Washington, DC, US Environmental Protection Agency (Available from
the US National Library of Medicine).
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish.
Res. Board Can., 36: 1040-1048.
VELSICOL CHEMICAL CORPORATION (1978) TSCA Sec. 8(E): Submission 8EHQ-
06780208. Chlorinates in Mississippi River catfish and carp, 1978
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago)
(Submitted to the US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VELSICOL CHEMICAL CORPORATION (1979) Confirmation of HEX and HEX-BCH
residues in human urine. Analytical method No. 0682, Chicago, Velsicol
Chemical Corporation.
VELSICOL CHEMICAL CORPORATION (1984) Comments on draft health effects
document for hexachlorocyclopentadiene, Chicago, Velsicol Chemical
Corporation.
VELSICOL CHEMICAL CORPORATION (1986) Air sampling and monitoring at
Velsicol production facilities, Chicago, Velsicol Chemical Corporation.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Co.
VILKAS, A.G. (1977) The acute toxicity of hexachlorocyclopentadiene to
the water flea, Daphnia magna straus, Tarrytown, New York, Union Carbide
Environmental Services (UCES Project No. 11506-03-05) (Prepared for
Velsicol Chemical Corporation, Chicago).
WALSH, G.E. (1981) Effects of chlordane, heptachlor and
hexachlorocyclopentadiene on growth of marine unicellular algae, Gulf
Breeze, Florida, US Environmental Protection Agency, Environmental
Research Laboratory (Unpublished report).
WALSH, G.E. (1983) Cell death and inhibition of population growth of
marine unicellular algae by pesticides. Aquat. Toxicol., 3: 209-214.
WALSH, G.E. & ALEXANDER, S.V. (1980) A marine algal bioassay method:
Results with pesticides and industrial wastes. Water Air Soil Pollut.,
13: 45-55.
WANG, H.H. & MACMAHON, B. (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J. occup. Med., 21: 745-748.
WEAST, R.C. & ASTLE, M.J. (1980) CRC handbook of chemistry and physics,
60th ed., Boca Raton, Florida, CRC Press, Inc.
WEBER, J.B. (1979) Adsorption of HEX by Cape Fear loam soil, Research
Triangle Park, North Carolina State University (Unpublished report
prepared for Velsicol Chemical Corporation, Chicago).
WHITMORE, F.C., DURFEE, R.L., & KHATTAK, M.N. (1977) Evaluation of a
technique for sampling low concentrations of organic vapors in ambient
air, Atlanta, Georgia, US Environmental Protection Agency (NTIS PB 279-
672).
WHO (1989) Environmental Health Criteria 91: Aldrin and Dieldrin, Geneva,
World Health Organization, 335 pp.
WILLIAMS, C.M. (1978) Liver cell culture systems for the study of
hepatocarcinogens. Proceedings of the 12th International Cancer Congress,
XII. International Cancer Congress Symposium No. 2: Chemical Oncogenesis
and Mutagenesis, October 1978, New York, London, Plenum Publishing
Corporation.
WILSON, J.A., BALDWIN, C.P., & MCBRIDE, T.J. (1978) Case history:
Contamination of Louisville, Kentucky Morris Foreman treatment plant.
Hexachlorocyclopentadiene. In: Control of hazardous material spills,
Miami, Florida, Hazardous Material Control Research Institute, pp. 170-
177.
WOLFE, N.L., ZEPP, R.G., SCHLOTZHAVER, P., & SINK, M. (1982)
Transformation pathways of hexachlorocyclopentadiene in the aquatic
environment. Chemosphere, 11(2): 91-101.
YOWELL, H.L. (1951) Fungicidal compositions containing
hexachlorocyclopentadiene: US Patent 2538509, Washington, DC, US Patent
Office.
YU, C.C. & ATALLAH, Y.H. (1977a) HEX hydrolysis at various pHs and
temperatures, Chicago, Velsicol Chemical Corporation (Project No. 482428,
Report No. 8) (Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1977b) Photolysis of hexachlorocyclopentadiene,
Chicago, Velsicol Chemical Corporation (Project No. 482428, Report No. 4)
(Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1981) Pharmacokinetics and metabolism of
hexachlorocyclopentadiene in rats, Chicago, Velsicol Chemical Corporation
(Project No. 482428, Report No. 10).
ZEPP, R.G., BAUGHMAN, G.L., & SCHLOTZHAUER, P.F. (1979) Dynamics of
processes influencing the behavior of hexachlorocyclopentadiene in the
aquatic environment. Paper presented at the 178th National Meeting of the
American Chemical Society, Washington, 9-14 September, 1979, Washington,
DC, American Chemical Society, Division of Environmental Chemistry.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R.C. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ. Mutagen.,
7: 87-100.
APPENDIX 1
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Table 21.
Table 21. Guidelines, recommendations and standards used in various countries/areasa
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
Australia recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1983
weighted average
short-term exposure limit 0.3 (0.03)
Belgium recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1988
weighted average
Canada regulation air/occupational threshold limit value/time- 0.1 (0.01) 1980
weighted average
Canada regulation transport specific transportation 1987
regulations
Finland recommendation air/occupational time-weighted average 1.0 (0.1) 1989
skin short-term exposure limit 3 (0.3) 1989
Netherlands recommendation air/occupational time-weighted average/ 0.11 (0.01) 1986
occupational
Federal regulation waste "toxic waste" subject to 1981
Republic specific handling,
of Germany transport, treatment,
storage, and disposal
regulation/permits
Switzerland regulation air/occupational time-weighted average 0.1 (0.01) 1987
USA regulation water ambient 1 µg/litre 1980
water quality criteria
(organoleptic)
----------------------------------------------------------------------------------------------------
Table 21 (contd.)
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
USA (ACGIH) recommendation air/occupational time-weighted average 0.1 (0.01) 1980
USA regulation air/occupational time-weighted average 0.1 (0.01) 1989
USA regulation water/land notification of spill 1983
of 0.45 kg (1 lb)
in 24-h period
USA regulation waste transport "toxic waste" subject to 1980
specific handling,
transport, treatment,
storage and disposal
regulation/permits
USA draft drinking-water lifetime 7 µg/kg 1990
recommendation per day
USSR regulation air/occupational threshold limit value 0.01 (0.001) 1989
USSR regulation water maximum allowable 1 mg/litre 1985
concentration
USSR regulation air/ambient short-term exposure limit 0.001 1987
Yugoslavia regulation air/occupational time-weighted average 0.1 1985
----------------------------------------------------------------------------------------------------
a From: IRPTC (1989)
b Unless stated otherwise, units are mg/m3. The value in parts per million is given in parentheses.
RESUME
L'hexachlorocyclopentadiène (HEX) est un liquide dense
et ininflammable, de couleur jaune pâle à jaune verdâtre,
et qui possède une odeur piquante caractéristique. Le HEX
est très réactif; il donne lieu à des réactions d'addition
et de substitution et à des réactions de Diels-Alder.
Aux Etats-Unis d'Amérique, la Velsicol Chemical Cor-
poration est actuellement le seul producteur de HEX. En
Europe, il est produit aux Pays-Bas par la Société Shell.
Les chiffres de production sont confidentiels mais on
estime que 3600 à 6800 tonnes de HEX sont produites
actuellement aux Etats-Unis. En 1988, la production
mondiale était d'environ 15 000 tonnes (BUA, 1988). Le
HEX est utilisé comme intermédiaire dans la production de
nombreux pesticides, mais quelques pays en ont limité
l'emploi à la fabrication de certains pesticides organo-
chlorés. Il est également utilisé pour la fabrication de
retardateurs de flamme, de résines et de colorants.
Au cours de la production et de la transformation du
HEX, de petites quantités sont libérées dans l'environne-
ment. Ce peut être également le cas lorsqu'il constitue
une impureté de certains des produits pour lesquels il
sert d'intermédiaire. La libération de HEX peut interve-
nir pendant ou après le rejet. On ne dispose que de
données limitées sur la surveillance des concentrations de
cette substance dans l'environnement. D'après ces données,
il semble que le HEX soit présent essentiellement dans le
compartiment aquatique et y soit associé aux sédiments et
aux matières organiques, sauf là où il y a eu rejet ou
libération du produit. D'après les études en laboratoire,
il y a sorption du HEX par la plupart des particules du
sol. Toutefois, on a fait état d'un lessivage et d'un
mouvement dans les eaux souterraines.
Aux Etats-Unis d'Amérique, on estime que 5,9 tonnes de
HEX sont libérées annuellement dans le milieu (US EPA,
1989). En République fédérale d'Allemagne et aux Pays-
Bas, environ 400 à 500 kg de HEX ont été libérés dans
l'atmosphère en 1987 (BUA, 1988). En raison des propriétés
physiques et chimiques du HEX, il ne devrait subsister
qu'une faible fraction de ces émissions.
En s'appuyant sur les données de laboratoire disponi-
bles, on a modélisé la destinée et le transport du HEX
dans l'atmosphère et calculé que son temps de séjour dans
la troposphère était d'environ 5 h. On a fait état d'un
transport atmosphérique de HEX à partir d'une zone de
stockage de déchets et de puits au cours du traitement de
rejets industriels.
Dans l'eau, le HEX peut subir une photolyse, une
hydrolyse et une biodégradation. Dans les eaux peu
profondes son temps de demi-photolyse est inférieur à une
heure. Dans les eaux plus profondes où la photolyse est
exclue, le temps de demi-hydrolyse varie de plusieurs
jours à environ trois mois et la biodégradation est encore
beaucoup plus lente. Le HEX se volatilise à la surface de
l'eau à une vitesse qui dépend de la turbulence et du
degré de sorption par les sédiments.
En raison de sa faible solubilité dans l'eau, le HEX
devrait être relativement immobile dans le sol. Toutefois
on en a trouvé dans des eaux souterraines. La volatili-
sation de cette substance qui se produit très vraisembla-
blement à la surface du sol est d'autant plus importante
que la teneur du sol en matières organiques est plus
faible. Les résultats d'études en laboratoire indiquent
que l'hydrolyse chimique et la métabolisation microbienne,
qu'elles soient aérobie ou anaérobie, devraient réduire la
teneur des sols en HEX.
En principe, le HEX devrait avoir un pouvoir de bio-
amplification notable en raison de sa forte lipophilicité
(log du coefficient de partage octanol/eau). Toutefois
les données expérimentales ne corroborent pas cette hypo-
thèse. Des études sur animaux d'expérience ont montré que
le 14C-HEX est à la fois métabolisé et excrété dans les
quelques heures qui suivent l'administration par voie
orale, la rétention dans l'organisme étant très faible.
Le facteur de bioconcentration à l'état stationnaire est
inférieur à 30 chez les poissons. Les facteurs de bio-
accumulation calculés à partir de modèles d'écosystèmes à
court terme indiquent que le potentiel d'accumulation est
modéré. Il semblerait donc que le HEX et ses métabolites
ne persistent pas et ne s'accumulent pas en quantités
importantes dans les systèmes biologiques.
On a montré qu'à faibles concentrations, le HEX était
toxique pour la faune aquatique. Des cas de mortalité par
intoxication aiguë (exposition de 48 à 96 h) ont été
observés chez des crustacés et des poissons dulçaquicoles
et marins à des concentrations nominales de 32 à
180 µg/litre, dans des systèmes statiques dont l'eau
n'était pas renouvelée au cours de l'épreuve. Etant donné
que le temps de demi-photolyse est inférieur à une heure,
la concentration en HEX devrait avoir diminué de manière
importante au cours de la période d'exposition utilisée
dans ces études. Les seules études au cours desquelles on
a mesuré les concentrations de HEX dans de l'eau courante
ont donné une valeur de la CL50 à 96 h de 7 µg/litre
pour le vairon américain et une crevette de mer. Les
épreuves effectuées sur ces deux espèces ont donné,
respectivement pour la CL10 et la CL40, des valeurs de
3,7 et de 0,7 µg/litre.
Des épreuves statiques de sept jours effectuées sur
des algues marines à des concentrations nominales allant
de 3,5 à 100 µg/litre ont fait ressortir une réduction
moyenne de la croissance (CE50), qui était fonction de
l'espèce. En milieu aqueux, le HEX est toxique pour de
nombreux microorganismes à des concentrations nominales de
0,2 à 10 mg/litre, c'est-à-dire à des valeurs sensiblement
plus importantes que celles qui sont nécessaires pour tuer
la plupart des animaux et des plantes aquatiques. Le HEX
semble être moins toxique pour les microorganismes terri-
coles que pour les microorganismes aquatiques, probable-
ment en raison de l'adsorption du HEX sur la matrice
constituée par le sol.
On pense que l'exposition devrait être faible mais les
données actuellement disponibles sont insuffisantes pour
permettre de déterminer les effets de l'exposition au HEX
sur la flore et la faune terrestres.
La résorption du HEX non modifié est minimale du fait
de sa réactivité vis-à-vis des membranes et des tissus de
l'organisme et plus particulièrement, du contenu des voies
digestives. Après administration par voie orale, percuta-
née ou par inhalation, la majeure partie du 14C-HEX
radiomarqué est retenue au niveau des reins, du foie, de
la trachée et des poumons des animaux d'expérience. Une
fois résorbé, le HEX est métabolisé et rapidement excrété,
principalement dans les urines, un peu moins dans les
matières fécales et à raison de moins de 1%, dans l'air
expiré. La durée nécessaire pour l'élimination complète
est d'environ 30 h quelle que soit la voie d'adminis-
tration. Après inhalation ou administration par voie
intraveineuse, le composé parent ne se retrouve plus dans
les excréta; on a isolé des métabolites fécaux et uri-
naires mais ils n'ont pas été identifiés. C'est d'ailleurs
la raison pour laquelle il est très difficile de se faire
une idée de la pharmacocinétique du HEX et d'élucider son
mode d'action.
La CL50 par inhalation (sur une période d'environ
4 h) est de 17,9 mg/m3 chez le rat mâle et de 39,1 mg
par m3 chez la ratte. Bien qu'il existe quelques
différences d'une espèce à l'autre, notamment entre les
cobayes, les rats, les lapins et les souris, les vapeurs
de HEX sont extrêmement toxiques pour toutes les espèces
étudiées. C'est par inhalation qu'il se révèle le plus
toxique, par comparaison avec l'administration orale ou
percutanée, et il se montre également extrêmement irri-
tant. Quelle que soit la voie d'administration, toute
exposition aiguë entraîne des effets généraux pathologi-
ques au niveau des poumons, du foie, des reins et des
surrénales.
L'administration de HEX par voie orale à des rats
(30 mg/kg et par jour) et à des souris (75 mg/kg et par
jour) pendant 91 jours a déterminé une néphrose ainsi
qu'une inflammation et une hyperplasie de l'estomac
antérieur. On n'a pas relevé de signes manifestes après
exposition de souris ou de rats à qui l'on avait fait
inhaler du HEX à raison de 2,26 mg/m3 (0,2 ppm), six
heures par jour, cinq jours par semaine pendant 14
semaines. A la concentration de 1,69 mg/m3 (0,15 ppm),
on a observé seulement une petite irritation. Des rats
exposés de la même manière à 5,65 mg/m3 (0,5 ppm) de
HEX pendant 30 semaines ont présenté des modifications
histopathologiques au niveau du foie, des voies respi-
ratoires et des reins. Une autre étude du même genre
portant sur des rats et des souris et qui s'est prolongée
pendant 90 jours a révélé des effets respiratoires à
partir de 4,52 mg/m3 (0,4 ppm). Le HEX ne s'est pas
révélé mutagène lors d'épreuves in vitro, qu'il y ait ou
non activation métabolique. Il s'est également révélé
inactif dans les épreuves de dominance létale chez la
souris. Administré par voie orale à des rats et à des
souris, il ne s'est pas révélé tératogène, mais on ne
dispose d'aucune donnée relative à sa tératogénicité
éventuelle après exposition par voie respiratoire. On ne
dispose que de données limitées sur l'exposition humaine
au HEX et sur ses effets. On a observé des incidents
isolés au cours desquels il y a eu forte irritation des
yeux, du nez, de la gorge et des poumons. Cette irritation
était généralement brève, les victimes commençant à
récupérer dès cessation de l'exposition. Après une expo-
sition de courte durée on n'a noté aucune différence
statistiquement significative au niveau de certaines
enzymes hépatiques entre les groupes exposés et les
groupes témoins. On ignore quels peuvent être les effets
à longue échéance sur la santé humaine d'une exposition
continue à de faibles concentrations de HEX ou d'une
exposition intermittente à des fortes concentrations. Les
personnes qui sont amenées à manipuler cette substance ou
des déchets qui en contiennent, ainsi que les égoutiers,
travailleurs des usines de traitement d'eaux usées et
personnes qui résident à proximité de décharges, pour-
raient être exposés au risque du fait des possibilités de
contact avec cette substance ou avec les déchets qui
résultent de sa fabrication.
La base de données dont on dispose n'est pas suffi-
sante pour qu'on puisse évaluer la cancérogénicité du HEX.
Le Programme toxicologique national des Etats-Unis (NTP) a
procédé à des études d'inhalation sur des rats et des
souris pendant toute la durée de leur vie. Une fois qu'un
rapport sur la pathologie observée aura été publié, on
aura une meilleure idée des effets à long terme éventuels
de l'exposition au HEX. En ce qui concerne la cancéro-
génicité de cette substance, il faudra différer les tra-
vaux dans l'attente des résultats des épreuves effectuées
par le NTP. Le Centre international de recherche sur le
cancer a examiné les données existantes sur le HEX et l'a
classé dans le Groupe 3 (ce qui indique qu'en raison
d'insuffisances quantitatives ou qualitatives importantes,
il n'est pas possible de déterminer, au vu des résultats
disponibles, s'il y a présence ou absence d'effets
cancérogènes). Un certain nombre d'études épidémiologiques
sont citées dans la littérature; on n'a pas fait état
d'une incidence accrue de cancers, de localisation
quelconque, qui puisse être attribuée au HEX ou à ses
métabolites.
RESUMEN
El hexaclorociclopentadieno (HEX) es un líquido denso
de color amarillo pálido o verdoso, no inflamable, con un
olor acre característico. Su masa molecular relativa es de
272,77 y es poco soluble en agua. El HEX es muy reactivo
y experimenta reacciones de adición, sustitución y de
Diels-Alder.
En los EE.UU., la Velsicol Chemical Corporation es la
única empresa que actualmente produce HEX. En Europa lo
fabrica la Shell Chemical Corporation en los Países Bajos.
Los datos de producción son propiedad de las empresas,
pero se calcula que al año se producen en los EE.UU. entre
3600 y 6800 toneladas de HEX. En 1988, la producción
mundial fue de aproximadamente 15 000 toneladas (BUA,
1988). Aunque el HEX se utiliza como intermedio en la
producción de numerosos plaguicidas, algunos países han
restringido su empleo en la fabricación de ciertos plagui-
cidas organoclorados. También se utiliza en la producción
de pirorretardantes, resinas y tintes.
Durante la fabricación y elaboración del HEX, se
liberan pequeñas cantidades de la sustancia al medio
ambiente. También puede liberarse cuando aparece en forma
de impureza en algunos de los productos para los que sirve
de intermedio. El HEX puede liberarse tanto durante como
después de su evacuación. Sólo se dispone de datos limi-
tados de vigilancia de los niveles ambientales de HEX.
Esos datos indican que aparece principalmente en el
compartimento acuático y que se asocia a los sedimentos y
la materia orgánica del fondo salvo en los lugares en los
que se ha producido evacuación o liberación. En el labora-
torio, el HEX se adsorbe sin dificultad a la mayoría de
los tipos de partículas del suelo. Sin embargo, se han
comunicado casos de lixiviación y de movimiento en aguas
subterráneas.
En los EE.UU., se calcula que la liberación total de
HEX al medio ambiente en un año es de 5,9 toneladas (US
EPA, 1989). En la República Federal de Alemania y los
Países Bajos, se emitieron en 1987 alrededor de 400-500 kg
(BUA, 1988). Dadas las características físicas y químicas
del HEX, es de esperar que solo persista una pequeña
fracción de esas emisiones.
Basandose en los datos de laboratorio disponibles, se
ha formulado un modelo sobre el destino y el transporte
del HEX en la atmósfera y se ha calculado que tiene un
tiempo de residencia de aproximadamente 5 horas en la
troposfera. Se ha comunicado la existencia de transporte
atmosférico de HEX desde una zona en la que se almacenan
desechos y a partir de las cisternas durante el trata-
miento de desechos industriales.
En el agua, el HEX puede experimentar fotolisis,
hidrólisis y biodegradación. En aguas poco profundas,
tiene una semivida fotolítica de < 1 h. En aguas más
profundas, donde la fotolisis se ve impedida, se ha
observado que la semivida hidrolítica puede variar entre
varios días y aproximadamente tres meses, mientras que es
de prever que la biodegradación se produzca con más
lentitud. Se sabe que el HEX se volatiliza en las aguas
superficiales, y que la tasa de volatilización varia con
la turbulencia y con la adsorción a los sedimentos.
Debido a su baja solubilidad en el agua, el HEX debe
ser relativamente inmóvil en el suelo. Sin embargo, se ha
detectado la sustancia en aguas subterráneas. La volatili-
zación, que tiene más probabilidades de producirse en la
superficie del suelo, guarda relación inversa con los
niveles de materia orgánica en éste. Los resultados de
estudios en laboratorio indican que la hidrólisis química
y el metabolismo microbiano, tanto aeróbico como anaeró-
bico, deben reducir los niveles de HEX en los suelos.
En teoría, el potencial de biomagnificación del HEX
debe ser importante debido a su elevada lipofilia (log
coeficiente de partición octanol/agua). Este extremo, sin
embargo, no ha sido demostrado en pruebas experimentales.
Los estudios en animales de laboratorio han demostrado que
el 14C-HEX es metabolizado y excretado durante de las
primeras horas que siguen la administración de una dosis
por vía oral, y que la proporción que queda retenida en el
organismo es pequeña. En los peces, los factores de
bioconcentración en estado estable son < 30. Los factores
de bioacumulación derivados de modelos de ecosistemas a
corto plazo indican un moderado potencial de acumulación.
Así pues, parece que el HEX y sus metabolitos no persisten
ni se acumulan en gran medida en los sistemas biológicos.
Se ha demostrado que el HEX en bajas concentraciones
es tóxico para los organismos acuáticos. Se ha observado
letalidad en exposiciones agudas (48 a 96 h) en crustáceos
y peces de agua dulce y salada, en concentraciones nomi-
nales de 32-180 µg/litro en sistemas de exposición
estática en los que el agua no se renovó durante la
prueba. Puesto que la semivida fotolítica es < 1 h, la
concentración de HEX habría disminuido sustancialmente
durante el periodo de exposición utilizado en esos
estudios. En los únicos estudios en los que se usó agua
corriente y se midieron las concentraciones de HEX, se
obtuvieron valores de la CL50 en 96 horas de 7 µg por
litro en Pimephales promelas y un camarón de mar. Los
ensayos realizados con esas dos especies dieron un valor
de CL10 de 3,7 y un valor de CL40 de 0,7 µg por litro,
respectivamente.
En ensayos estáticos de siete días con algas marinas
se observó una reducción mediana del crecimiento (CE50)
a concentraciones nominales que variaron entre 3,5 y
100 µg/litro, según la especie.
En medios acuosos, el HEX resulta tóxico para
numerosos microorganismos en concentraciones nominales de
0,2-10 mg/litro, es decir, niveles sensiblemente mayores
que los necesarios para destruir a la mayoría de los
animales o vegetales acuáticos. El HEX parece menos tóxico
para los microorganismos en el suelo que en el medio
acuático, probablemente a causa de la adsorción del HEX a
la matriz del suelo.
Aunque cabe esperar que la exposición sea reducida,
actualmente no se dispone de bastante información para
determinar los efectos de la exposición al HEX en la
vegetación o la fauna terrestres.
La absorción de HEX sin modificar es mínima debido a
su reactividad con las membranas y los tejidos del organ-
ismo y especialmente con el contenido del tracto gastro-
intestinal. La mayor parte del 14C-HEX radiomarcado
queda retenido en el riñón, el hígado, la tráquea y los
pulmones de los animales tras la administración por vía
oral, cutánea o respiratoria. El HEX absorbido es metab-
olizado y excretado rápidamente, sobre todo en la orina,
menos en las heces y < 1% en el aire expirado. El tiempo
de eliminación terminal es de unas 30 horas, con inde-
pendencia de la vía de administración. Tras la inhalación
o la administración intravenosa, no se excreta HEX sin
modificar; los metabolitos fecales y urinarios se han
aislado pero no se han identificado. La falta de identifi-
cación de los metabolitos representa uno de los prin-
cipales obstaculos para evaluar la farmacocinética y los
mecanismos potenciales de acción del HEX.
En la rata, la CL50 aguda por inhalación (durante un
periodo de aproximadamente 4 h) es de 17,9 mg/m3 en el
macho y 39,1 mg/m3 en la hembra. Aunque hay algunas
diferencias interespecíficas entre cobayos, conejos, ratas
y ratones, los vapores de HEX son sumamente tóxicos para
todas las especies ensayadas. Su toxicidad parece máxima
cuando se administra por inhalación, en comparación con la
administración oral y cutánea, y es un irritante primario
fuerte. Los efectos sistémicos de la exposición aguda,
con independencia de la vía de administración, comprenden
cambios patológicos en el pulmón, el hígado, el riñón y
las glándulas suprarrenales.
La administración oral a corto plazo a ratas (38 mg/kg
al día) y ratones (75 mg/kg al día) durante 91 días
produjo nefrosis e inflamación e hiperplasia de la región
anterior del estómago. No se observaron signos manifiestos
cuando se expuso a ratas o ratones por inhalación a 2,26
mg/m3 (0,2 ppm), 6 h/día, 5 días/semana, durante 14
semanas. Con 1,69 mg/m3 (0,15 ppm) sólo se observó una
ligera irritación. La exposición de ratas a la inhalación
de 5,65 mg/m3 (0,5 ppm) durante 30 semanas provocó
cambios histopatológicos en el hígado, el tracto respira-
torio y el riñón. En un estudio de inhalación a corto
plazo de HEX en ratones y ratas durante 90 días se
observaron efectos en el sistema respiratorio a 4,52
mg/m3 (0,4 ppm) o más. No se ha demostrado que el HEX
sea mutagénico en ensayos in vitro , con o sin activación
metabólica. También resultó inactivo en ensayos de letali-
dad dominante en el ratón. Tampoco se ha demostrado que
sea teratogénico en ratas y ratones por exposición oral;
no se dispone de datos sobre la teratogenicidad del HEX
tras la exposición por inhalación.
Sólo se dispone de datos limitados sobre los efectos
de la exposición al HEX en la salud humana. Se han
producido incidentes aislados en los que el HEX provocó
fuerte irritación de los ojos, la nariz, la garganta y los
pulmones. Por lo general esa irritación fue breve, y la
recuperación se inició en cuanto cesó la exposición. No
se observaron diferencias estadísticamente significativas
en ciertas enzimas hepáticas entre grupos expuestos y
grupos testigo tras la exposición a corto plazo. Se
desconocen los efectos a largo plazo en la salud humana de
la exposición continua a bajos niveles y/o la exposición
aguda intermitente. Se ha demostrado que los manipuladores
del producto y de sus desechos, así como las personas que
trabajan en la depuración de aguas residuales o que viven
en las proximidades de los lugares de evacuación corren
riesgo debido al potencial de exposición a la sustancia
química o a los residuos de su fabricación.
La base de datos no es lo bastante amplia ni adecuada
para evaluar la carcinogenicidad del HEX. El Programa
Nacional de Toxicología de los EE. UU. ha llevado a cabo
un bioensayo de inhalación durante toda la vida en ratas y
ratones. Cuando se publique el informe patológico, se
comprenderá mejor los efectos a largo plazo de la exposi-
ción al HEX. La evaluación de la carcinogenicidad deberá
demorarse hasta que estén disponibles los resultados del
bioensayo del Programa. El Centro Internacional de Inves-
tigaciones sobre el Cáncer evaluó los datos existentes
para el HEX y clasificó la sustancia en el Grupo 3 (lo que
indica que, debido a limitaciones importantes de orden
cualitativo o cuantitativo, no puede interpretarse que los
estudios demuestren ni la existencia ni la ausencia de
efecto carcinogénico). En la bibliografía se citaron
varios estudios epidemiológicos; no se notificaron aumen-
tos de la incidencia de neoplasmas en ninguna localización
que pudieran atribuirse al HEX o sus metabolitos.
Kilzer et al. (1979) determined the rate of 14C-HEX
volatilization from water as a function of the rate of
water evaporation. Bottles containing aqueous HEX sol-
utions (50 µg/litre) were kept at 25 °C without shaking.
The escaping vapour condensed on a "cold finger" and was
quantified by liquid scintillation spectroscopy. Based on
recovery of added label, the HEX volatilization rates for
the first and second hours of testing were calculated to
be 5.87 and 0.75%/ml of water, respectively. Since the
water evaporation rate was 0.8-1.5 ml/h, the evaporation
rates for HEX were within the ranges of 4.7-8.8 and 0.6-
1.1%/h, respectively. These results suggest that a fairly
rapid initial volatilization occurred at the water sur-
face, and that by the second hour diffusion of HEX to the
water surface may have been limiting because of the static
conditions of the test. If the rate observed during the
second hour had continued for the remaining 24 h, the
total loss would have been approximately 18-34%, i.e.
somewhat less than that observed in the test conducted by
Weber (1979) where unstoppered bottles were shaken.
At 25-30 °C and in the environmental pH range of 5-9,
the hydrolytic half-life of HEX was found to be approxi-
mately 3-11 days (Yu & Atallah, 1977a; Wolfe et al.,
1982). In a later study in which evaporation and photo-
chemical reactions were carefully prevented, the hydro-
lytic half-life was approximately 3 months (Chou &
Griffin, 1983). Hydrolysis is much slower than photolysis
(see Table 2) but may be a significant load-reducing
process in waters where photolysis and physical transport
processes are not important (i.e. in deep, non-flowing
waters). Wolfe et al. (1982) found HEX hydrolysis to be
independent of pH over the range of 3-10. The rate con-
stant was dependent on temperature at pH 7.0, and the
half-life was estimated to be 3.31, 1.71, and 0.64 days at
30, 40, and 50 °C, respectively. The addition of various
buffers or sodium chloride (0.5 mol/litre) did not affect
the hydrolysis rate constant, suggesting that the rate
constant obtained would be applicable to marine environ-
ments as well. The addition of natural sediments, suf-
ficient to sorb up to 92% of the compound, caused the rate
constant to vary by less than a factor of 2. It was
therefore concluded that sorption to sediments would not
significantly affect the rate of hydrolysis (Wolfe et al.,
1982).
4.2.3. Soil
When it is released on to soil, HEX is likely to sorb
strongly to any organic matter or humus present (Weber,
1979; Kenaga & Goring, 1980). The HEX concentrations
should decrease with time as populations of soil micro-
organisms that are better adapted to metabolize HEX
increase (Rieck, 1977b,c; Thuma et al., 1978). Volatiliz-
ation, photolysis, and various chemical processes may also
dissipate the compound in certain soil environments.
The main methods of transport for HEX applied to the
soil are (a) the movement of particles to which it is
sorbed and (b) volatilization. Other possibilities are
that HEX is sorbed on to soil colloids or that it par-
titions to the interior of soil particles and stays in
loams and silts in a dissolved state. No data are avail-
able pertaining to HEX transport on soil particles. How-
ever, in a few studies, the rate of volatilization from
soils has been reported and is discussed in the following
paragraphs.
Kilzer et al. (1979) found that 14C-HEX and its
degradation products volatilized from moist soils (sand,
loam, and humus) at a faster rate during the first hour of
the study than during the second hour. HEX (50 µg/kg) was
placed in bottles with each soil type, the bottles were
shaken vigorously, and they were then incubated for 2 h at
25 °C without shaking. The radiolabelled HEX condensed on
a "cold finger" and was quantified by liquid scintil-
lation counting. For sand, loam, and humus, the volatiliz-
ation rate was expressed as the percentages of applied
radioactivity per ml of evaporated water. For the first
hour the percentages were 0.83, 0.33, and 0.14%, respect-
ively, and for the second hour they were 0.23, 0.11, and
0.05%. For HEX and nine other tested chemicals, the
authors found that the volatilization rate from distilled
water could not be used to predict the rate from wetted
soils. Among the chemicals tested, there was no corre-
lation between water solubility or vapour pressure and
volatilization from soils. The volatilization rate for
HEX and its metabolites in soil was primarily dependent on
soil organic matter content, mainly because of the highly
sorptive characteristics of HEX.
In a model ecosystem study, Kloskowski et al. (1981)
applied 14C-HEX to 1 kg of humus sand soil (2 mg/kg) and
grew summer barley by keeping the system under an illumi-
nation of 10 000 lux (12 h light, 12 h dark) at 20-24 °C
in an enclosed 10-litre desiccator with an aeration of
10 ml/min. After 7 days, approximately 19.5% of the orig-
inal radioactivity was recovered in the form of 14CO2
evolved and 0.5% as volatilized organics. The level of
radiolabelled compounds in the plants was 13.4 mg/kg,
which represented a bioaccumulation factor of 7.1 (i.e.
plant residues divided by soil residues). It is not clear
whether the plants played a major role in the volatiliz-
ation or metabolic fate of HEX, but the total 14C recov-
ery was over 95%.
Rieck (1977c) measured the rate of volatilization of
HEX from Maury silt loam soils. After the application of
100 mg 14C-HEX to soil, the cumulative evaporation of HEX
and its non-polar metabolites (penta- and tetrachloro-
cyclopentadiene) on days 1, 2, 3, 5, 7, and 14 was 9.3,
10.2, 10.6, 10.8, 11.0, and 11.2%, respectively. The
results indicated that HEX evaporation to air occurred
mainly during the first day after application and was
probably associated with the surface soil only.
The soil sorption properties of compounds such as HEX
can be predicted from their soil organic carbon/water
partition coefficients (Koc). Kenaga (1980) examined
the sorption properties of 100 chemicals and concluded
that compounds with Koc values > 1000 are tightly bound
to soil components and are immobile in soils. Those with
values < 100 are sorbed less strongly and are considered
to be moderately to highly mobile. Thus, the theoretical
Koc value is useful as an indicator of potential soil
leachability or binding of the chemical. The Koc values
also indicate whether a chemical is likely to enter water
by leaching or by being sorbed to eroded soil particles.
Since Koc values for HEX are not available in the
literature, these values were calculated using the
following mathematical equation, developed by Kenaga &
Goring (1980) and Kenaga (1980):
log Koc = 3.64-0.55 (log WS)
where WS is water solubility (mg/litre), and the 95%
confidence limits are ± 1.23 orders of magnitude. The
calculated values of Koc for HEX using the reported water
solubility values of 2.1 mg/litre (Dal Monte & Yu, 1977),
1.8 mg/litre (Wolfe et al., 1982), and 0.805 mg/litre (Lu
et al., 1975) are 2903, 3159, and 4918, respectively.
Since these calculated Koc values are all > 1000, the
authors concluded that HEX is tightly bound to soil
components and immobile in the soil compartment. Simi-
larly, Briggs (1973) concluded that compounds with a log
octanol/water partition coefficient (log Pow) > 3.78 are
immobile in soil. Log Pow values for HEX of 5.04 (Wolfe
et al., 1982) and 5.51 (Veith et al., 1979) have been
measured.
In studies by Chou & Griffin (1983), the mobility of
HEX (C-56) in six soils was measured with several leaching
solvents using soil thin-layer chromatography (TLC) and
column leaching studies. It remained immobile in the soil
when leached with water, landfill leachate, or caustic
brine, but was highly mobile when leached with organic
solvents. A further conclusion was that several degra-
dation products of HEX migrated through soils faster than
HEX itself, and that the degradation products warranted
further study. The sorption capacity of HEX was highly
correlated with the total organic carbon (TOC) content of
soil materials (r2 = 0.97), which was the dominant soil
characterization parameter. Sorption appears to be pre-
dictable from the TOC content of soils (Chou & Griffin,
1983).
4.3. Biotransformation
4.3.1. Biodegradation
The metabolism of HEX by soil microorganisms is
apparently an important process in its environmental
degradation. Soil degradation is rapid under non-sterile
aerobic and anaerobic conditions, and indirect evidence
for microbial involvement has been reported by Rieck
(1977b,c). In one of his studies, Rieck (1977b) used
several types of treatments and soils of different pH to
determine whether the biodegradation of HEX in Maury silt
loam soil was biologically or chemically mediated, or
both. Soils were incubated in glass flasks covered with
perforated aluminum foil and kept on a laboratory shelf,
presumably exposed to ambient lighting through the flask
walls. When 14C-HEX was applied to non-sterile soil at
1 mg/kg, only 6.1% was recovered as non-polar material
(either HEX or non-polar degradation products) 7 days
after treatment, and approximately 71.7% was polar and
unextractable material. Adjustment of the pH to 4 or 8 had
little effect on these results. By comparison, in auto-
claved soil (the control), 36.1% of the applied dose was
recovered as non-polar material and only 33.4% was
recovered as polar and unextractable material. The degra-
dation of HEX under anaerobic (flooded) conditions
occurred at a slightly faster rate than under aerobic
conditions. However, no sterile, flooded control was used
to determine the effects of hydrolysis, which could have
accounted for the observed effect in this treatment. The
mean total recovery in all treatments decreased from 67%
at 7 days to 55% at 56 days. This decrease was attributed
to volatilization of HEX and/or its degradation products.
Volatilization from soil was examined in a further
experiment (Rieck, 1977c). In a 14-day study, radiocarbon
volatilized from non-sterile, 14C-HEX-treated soil was
trapped and assayed. A total of 20.2% of the applied 14C
was trapped: 11.2% in hexane and 9.0% in ethanolamine-
water. Most of the hexane fraction (9.3% of the applied
14C) was trapped during the first day, and probably
represented volatilized HEX. However, the ethanolamine-
water fraction, considered to represent evolved carbon
dioxide, was released gradually over the 14-day period.
In the soil analysis, non-polar (extractable) and polar
(extractable and unextractable) material accounted for 3.4
and 40.0% of the dose, respectively, during the 14 days;
total recovery was only 63.6% including volatilization.
No metabolic products were identified in the two studies
by Rieck (1977b,c).
Thuma et al. (1978) studied the feasibility of using
selected pure cultures (organisms not identified) to
biodegrade spills of hazardous chemicals, including HEX,
on soil. They tested 23 organisms and found that from
2-76% of the applied HEX had been removed from the aqueous
culture medium within 14 days. Seven of the 23 organisms
degraded more than 33% of the HEX within 14 days. Losses
of HEX by other means than biodegradation were accounted
for by using controls.
Atallah et al. (1980) conducted an aqueous aerobic
biodegradability study to determine whether HEX could be
degraded to CO2 and at what rate. The inoculum was a
mixed acclimated culture containing secondary municipal
waste effluent and several strains of Pseudomonas putida.
14C-labelled HEX was the sole source of carbon in the
study, with the exception of trace levels of vitamins.
Total removal of 14C, primarily as volatile organic com-
pounds, was > 80% during the first day in both uninocu-
lated (45 mg HEX/litre) and inoculated (4.5 and 45 mg
HEX/litre) media, although removal was slightly greater in
inoculated media. 14CO2 was released from both media,
indicating that CO2 was a product of hydrolysis as well
as of biodegradation. The rate of conversion to CO2 was
initially higher in the uninoculated media, but after 1
week, became higher in the inoculated media. This study
showed clearly that HEX can be biodegraded in aquatic
media under laboratory conditions. However, Wolfe et al.
(1982) failed to detect any difference between the HEX
degradation rates in hydrolysis experiments where non-
sterile natural sediments were added to water (10 g/litre)
and those where sterile sediment was used. They calculated
a relatively low value (1 x 10-5 ml org-1 h-1; see
Table 2) as a maximum biodegradation rate, and conse-
quently biodegradation was estimated to be a relatively
unimportant fate process in the EXAMS model (see Table 3).
These studies indicate that the persistence of HEX in
soil is brief, degradation of more than 90% of applied HEX
to non-polar products occurring within approximately 7
days. Factors contributing to this loss include abiotic
and biotic degradation processes and volatilization,
although the relative importance of each is difficult to
quantify.
4.3.2. Bioconcentration, bioaccumulation, and biomagnification
Bioaccumulation, sometimes also expressed as biologi-
cal persistence, is a consequence of the rate of elimin-
ation of a compound and the extent of adsorption.
The terminology used in this section conforms to that
used by Macek et al. (1979):
* bioconcentration implies that tissue residues result
only from simultaneous uptake and elimination from
exposure to the ambient environment (e.g., air for
terrestrial species or water for aquatic species);
* bioaccumulation considers all exposures (air, water,
and food) of an individual organism to be the source
of tissue residues;
* biomagnification defines the increase in tissue
residues observed at successively higher trophic
levels of a food web.
Table 3. Summary of results of computer simulation of the
fate and transport of hexachlorocyclopentadiene in four
typical aquatic environmentsa
---------------------------------------------------------------
River Pond Eutrophic Oligotrophic
lake lake
---------------------------------------------------------------
Distribution (%)
Water column 1.22 14 12.97 2.91b
Sediment 98.78 86 87.03 97.09
Recovery timec (days) 52 81 58 87
Load reduction (%) by processes:
Hydrolysis 8.04 17.85 8.29 16.50
Oxidation 0.00 0.00 0.00 0.00
Photolysis 18.68 80.39 89.18 82.41
Biodegradation 0.57 0.23 0.30 0.01
(biolysis)
Volatilization 0.69 1.33 1.56 1.08
Exportd 72.02 0.20 0.01 0.00
---------------------------------------------------------------
a Adapted from Wolfe et al. (1982), with correction applied.
b Value was incorrectly reported as 32.91 in original paper.
c The time needed to reduce steady-state concentrations by
97% (five half-lives).
d Physical loss from the system by any pathway other than
volatilization.
The log octanol/water partition coefficient of HEX has
been experimentally determined to be 5.04 (Wolfe et al.,
1982) and 5.51 (Veith et al., 1979), which would indicate
a substantial potential for bioconcentration, bioaccumu-
lation, and biomagnification. Actual determinations of
bioconcentration and bioaccumulation in several aquatic
organisms, however, indicate that HEX does not accumulate
to any great extent (Lu et al., 1975; Podowski & Khan,
1979, 1984; Spehar et al., 1979; Veith et al., 1979),
mainly because it is metabolized rapidly.
Podowski & Khan (1979, 1984) conducted several exper-
iments on the uptake, bioaccumulation, and elimination
of 14C-HEX in goldfish ( Carassius auratus) and concluded
that this species rapidly eliminates absorbed HEX. In one
experiment, fish were transferred daily into fresh sol-
utions of 14C-HEX for 16 days. This transfer of three
fish/jar resulted in an accumulative exposure of 240 µg
of HEX. Nominal HEX concentrations of 10 µg/litre re-
sulted in measured water concentrations (based on radio-
activity) in the range of 3.4-4.8 µg/litre, because of
rapid volatilization of the compound. Radioactivity
accumulated rapidly in fish tissue, reaching a maximum on
day 8 corresponding to approximately 6 mg HEX/kg. Since an
undetermined amount of the radioactivity was present as
metabolites, no bioconcentration factor could be calcu-
lated. From day 8 to day 16, tissue levels declined in
spite of the daily renewal of exposure solutions, indi-
cating that excretion of HEX and/or its metabolites was
occurring more rapidly than uptake. In a static exposure
to an initial measured HEX concentration of 5 µg per
litre, uptake of the radiolabel by the fish was to a level
corresponding to 1.6 mg HEX/kg on day 2, accompanied by a
slight decrease of HEX in the water. By day 4, approxi-
mately 50% of the radiolabel had been excreted, and the
radioactivity in the water increased. Over the following
12 days, the radioactivity in both water and fish declined
slowly.
Podowski & Khan (1979, 1984) also studied the elimin-
ation, metabolism, and tissue distribution of HEX injected
intraperitoneally into goldfish and concluded that gold-
fish eliminate injected HEX both rapidly and linearly
(the biological half-life was approximately 9 days). The
fish (27-45 g) were injected with 39.6 µg 14C-HEX and
analysed 3 days later. Of the 97% of the radiolabelled
dose accounted for, approximately 18.9% was eliminated by
the fish. Of the residue found in the fish, 47.2% was
extractable in organic solvent (little of the radio-
labelled material could be identified as HEX, which indi-
cated that extensive biotransformation had occurred),
10.6% consisted of water-soluble metabolites, and 20.3%
was unextractable. None of the metabolites were ident-
ified. The elimination was biphasic, consisting of a rapid
initial phase followed by a slower terminal phase.
In another part of these studies, the residual
activity in several fish tissues was assayed 2, 4, 6, and
8 days after an injection of 38.4 µg 14C-HEX per fish.
The activity corresponded to 0.2 and 0.3 µg HEX/kg in the
spinal cord and gills, respectively, concentrations that
remained constant throughout the 8-day period of the
study. Residues in the kidneys and bile increased within
the same period from 1-3 and 0-32 µg/kg, respectively,
indicating elimination by these routes. The authors stated
that the increase probably occurred from enhanced con-
version of the parent compound into polar products, which
could be excreted more easily. In the other tissues, all
residual levels decreased, leaving only the liver with a
level of more than 1 µg/kg. The metabolites were not
identified (Podowski & Khan, 1979, 1984).
Veith et al. (1979) determined a bioconcentration
factor (BCF) for HEX of 29 in the fat-head minnow
(Pimephales promelas). In a 32-day flow-through study,
30 fish were exposed to HEX at a mean concentration of
20.9 µg/litre. Five fish at a time were killed at 2, 4,
8, 16, 24, and 32 days for residue analysis. The study
was conducted using Lake Superior water at 25 °C (pH 7.5,
dissolved oxygen > 5.0 mg/litre, and hardness 41.5 mg
CaCO3/litre. On the basis of its estimated octanol/water
partition coefficient alone (log Pow = 5.51), a BCF of
approximately 9600 would have been predicted. However,
HEX did not bioconcentrate substantially, and therefore
deviated from the log P:log BCF relationship shown for
most of the other 29 chemicals tested by these researchers.
Lu et al. (1975) studied the fate of HEX in a model
terrestrial-aquatic ecosystem maintained at 26.7 °C with a
12-h photoperiod. The model ecosystem consisted of 50
sorghum (Sorghum vulgare) plants (7.62-10.16 cm tall) in
the terrestrial portion, while 10 snails (Physa sp.), 30
water fleas (Daphnia magna), filamentous green algae
(Oedogonium cardiacum), and a plankton culture were
added to the aquatic portion. The sorghum plants were
treated topically with 5.0 mg 14C-HEX in acetone to simu-
late a terrestrial application of 1.1 kg/hectare. Ten
early-fifth-instar caterpillar larvae (Estigmene acrea)
were placed on the plants. The insects consumed most of
the treated plant surface within 3-4 days. The faeces,
leaf grass, and the larvae themselves contaminated the
moist sand, permitting distribution of the radiolabelled
metabolites by water throughout the ecosystem. After 26
days, 300 mosquito larvae (Culex pipiens quinquefasciatus)
were added to the ecosystem, and on day 30 three mosquito
fish (Gambusia affinis) were added. The experiment was
terminated after 33 days, and the various parameters were
analysed. The radioactivity was then extracted with
diethyl ether from the water and with acetone from the
organisms. The results of thin-layer chromatographic
analysis of the extracts are presented in Table 4. Data
were not reported for Daphnia magna or the salt marsh
caterpillar. Uptake in this experiment occurred through
food as well as water, and therefore is termed bioaccumu-
lation rather than bioconcentration. Lu et al. (1975) used
the term ecological magnification to designate the bio-
accumulation factor (BAF). The BAF for HEX in fish was
448 (0.1076 mg/kg fish divided by 0.24 µg/litre water)
for the 3-day exposure period, indicating a moderate
potential for concentration (Kenaga, 1980). The BAFs in
algae (< 33-day exposure), snails (< 33-day exposure), and
mosquito larvae (7-day exposure) were reported to be 341,
1634, and 929, respectively (Lu et al., 1975).
Table 4. Relative distribution of hexachlorocyclopentadiene (HEX) and
its degradation products in a model ecosystema
------------------------------------------------------------------------
14C-HEX equivalents
Water Algae Snail Mosquito Fish
(mg/litre) (mg/kg) (mg/kg) larva (mg/kg)
(mg/kg)
------------------------------------------------------------------------
HEX 0.00024 0.0818 0.3922 0.2230 0.1076
Other extractable 0.00204 0.1632 0.3824 0.2542 0.1542
compounds
Total extractable14Cb 0.00228 0.2450 0.7746 0.4772 0.2618
Unextractable14C 0.00750 0.0094 0.0814 0.0104 0.0982
Total14Cc 0.00978 0.2544 0.8560 0.4876 0.3600
------------------------------------------------------------------------
a Source: Lu et al. (1975).
b Sum of HEX and other extractable compounds.
c Sum of total extractable and unextractable 14C.
Biomagnification, measured as the ratio of HEX
residues between trophic levels (e.g., snail/algae or
fish/mosquito), was far less substantial than bioconcen-
tration. Based on the HEX tissue residues, the snail/algae
ratio was 0.3922/0.0818 = 4.8 and the fish/mosquito ratio
was 0.1076/0.2230 = 0.48.
Lu et al. (1975) also studied the metabolism of HEX by
the organisms present in the model terrestrial-aquatic
ecosystem, but none of the products was identified except
for HEX. The authors reported that unmetabolized HEX re-
presented a large percentage of the total extractable 14C,
being 33% in algae, 50% in snail, 46% in mosquito, and 41%
in fish. The percentage of biodegradation was calculated
for each organism (unextractable 14C x 100/total 14C) and
found to be 4% for the algae (in < 33 days), 10% for the
snails (in < 33 days), 2% for the mosquitoes (in 7 days),
and 27% for the fish (in 3 days). However, these values
may underestimate the extent of metabolism, since acetone-
extractable polar compounds were not considered in the
calculations.
The Velsicol Chemical Corporation (1978) conducted
fish tissue residue studies in waters located below their
facility in Memphis, Tennessee, USA, and reported that HEX
was not detected in either catfish or carp, although
chlorinated compounds, including octachlorocyclopentadiene
(a common co-contaminant), were detected in the fish
tissue. This indicated that HEX was not accumulated. The
possible source of these other compounds was not
discussed. In a joint USA federal and state study of the
Mississippi River at locations above, around, and below
Memphis, Bennett (1982) reported that HEX was not detected
in any of the eight fish sample groups analysed by GC/MS.
4.4. Interactions with other physical and chemical factors
4.4.1. Phototransformation
Zepp et al. (1979) and Wolfe et al. (1982) reported
the results of US EPA studies on the rate of HEX photo-
transformation in water. Under a variety of sunlight
conditions, in both distilled and natural waters of 1-4 cm
depth, the phototransformation half-life was < 10 min.
Chou & Griffin (1983) determined a half-life of < 4 min at
740 j/m2. The addition of natural sediments to distilled
water containing HEX had little effect on the phototrans-
formation rate. These findings indicate that the dominant
mechanism of HEX phototransformation is direct absorption
of light by the chemical, rather than photosensitization
reactions involving other dissolved or suspended
materials.
The direct photoreaction of HEX in water was also
studied under controlled conditions in the laboratory
using a monochromatic light (313 nm) with a mercury lamp
source and appropriate filters. Phototransformation rate
constants, computed for the study location (Athens,
Georgia, USA, 34 °N latitude), agreed with those observed
in the sunlight experiments described above. Rate con-
stants were also computed for various times of day at a
latitude of 40 °N. The near-surface phototransformation
rate constant of HEX at this latitude on cloudless days
(averaged over both light and dark periods for 1 year) was
3.9 h-1, which corresponds to a very rapid half-life of
10.7 min (Zepp et al., 1979; Wolfe et al., 1982).
These laboratory researchers suggested that the pri-
mary phototransformation product was the hydrated form of
tetrachlorocyclopentadienone (C5Cl4O, TCPD), although
it was not isolated. Several chlorinated photoproducts
with a higher relative molecular mass than HEX were
detected by GC/MS analysis of the reaction mixture. Photo-
lysis of HEX in methanol gave a product identified as the
dimethyl ketal of TCPD (Wolfe et al., 1982). According to
Zepp et al. (1979), it is likely that TCPD exists predomi-
nantly in its hydrated form in the aquatic environment.
The compound was not isolated, supposedly because it
rapidly dimerizes or reacts to form products of higher
relative molecular mass. Chou et al. (1987) identified
2,3,4,4,5-pentachloro-2-cyclopentenone, hexachloro-2-cyclo-
pentenone, and hexachloro-3-cyclopentenone as the primary
photodegradation products, as well as several other pri-
mary and secondary ones (Chou & Griffin, 1983; Fig. 2).
Yu & Atallah (1977b) found that, at a concentration of
2.2 mg/litre in water, uniformly labelled 14C-HEX was
rapidly converted to water-soluble products upon
irradiation with light from a mercury vapour lamp (light
energy: 40-48% ultraviolet, 40-43% visible, remainder
infrared). In exposures lasting 0.5-5.0 h, 46-53% of the
radiolabel was recovered in the form of water-soluble
products (compared with 7% at initiation), whereas the
amount recovered by organic (petroleum ether) extraction
decreased with increasing exposure duration from 25% to 6%
(compared with 66% at initiation). HEX was not detected
among the photoproducts in the organic extraction. Chou
et al. (1987) also found that dimerization of degradation
products to form compounds of higher relative molecular
mass was only a minor route of degradation.
4.4.2. Oxidation
HEX would not be expected to be oxidized under ordi-
nary environmental conditions. In the laboratory, HEX
reacts with molecular oxygen at 95-105 °C to form a mix-
ture of hexachlorocyclopentenones (Molotsky & Ballweber,
1957). However, based on an estimated second-order oxi-
dation rate constant of 1 x 10-10 M-1 sec-1 at 25 °C
in water (Table 2), the EXAMS computer simulation of Wolfe
et al. (1982) predicted that HEX would not be oxidized in
the simulated river, pond, eutrophic lake or oligotrophic
lake (Table 3).
4.5. Disposal and fate
HEX and HEX-contaminated material and wastes are
disposed of in secure chemical landfills, by incineration,
and by deep well injection (US EPA, 1989). Additionally,
there are solid waste regulations in the USA because,
under the Resource Conservation and Recovery Act, HEX is
designated to be a toxic waste. German regulations are
similar to those of the USA, except that there is no deep
well injection (BUA, 1988).
Since the photodegradation products of HEX have been
identified only recently and because HEX has also been
found in areas where waste has not been disposed of for
years (US EPA, 1980c), it is difficult to determine its
fate in the environment.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
Releases of HEX into the atmosphere can result from
the production, processing, and use of HEX, the disposal
of wastes containing HEX, or from products contaminated
with HEX (Hunt & Brooks, 1984). Data sent to the US EPA
for 1987 regarding emission levels from companies in the
USA indicated that 1400 kg of HEX was emitted into the air
(US EPA, 1989). In the Federal Republic of Germany and
the Netherlands, about 400-500 kg was emitted to the air
in 1987 (BUA, 1988)
In September and October 1985, the Velsicol Chemical
Corporation determined concentrations of HEX at predeter-
mined locations around its production facilities in
Tennessee, USA. The study was designed to measure ambient
concentrations in the air during routine manufacturing
operations. Of the 25 samples collected, 15 were below the
analytical limit of detection, i.e. 0.03 µg (0.1 ppb).
The air HEX levels in the other samples ranged between 1
and 10 µg/m3 (0.1-0.9 ppb) when ambient temperatures
were between 4.4 °C and 27.7 °C (Velsicol Chemical Corpor-
ation, 1986).
The highest reported concentration of HEX measured in
homes in Tennessee was 0.10 µg/m3, while air levels at
the Memphis North treatment plant were as high as
39 µg/m3 (C.S. Clark et al., 1982; Elia et al., 1983).
In an air monitoring study on an abandoned waste site in
Michigan, the average HEX emission rate was 0.26 (± 0.05)
g/h. In May 1977, HEX was detected at a level of 633 µg
per m3 (56 ppb) in air samples collected from a waste
site in Montague, Michigan (US EPA, 1980c).
5.1.2. Water
Benoit & Williams (1981) sampled both untreated water
and drinking-water from a water treatment plant in Ottawa,
Canada. Using solvent extraction analysis with a detection
sensitivity of 50 ng/litre (or the XAD-2 resin extraction
method with a detection sensitivity of approximately 0.5
ng/litre), the authors did not detect any HEX in the
untreated water, but reported levels ranging from 57-110
ng/litre in the finished drinking-water. These results
suggest that HEX was introduced into the drinking-water
during the treatment process. However, the researchers did
not find the source of the HEX. Meier et al. (1985) found
that HEX can be produced through the chlorination of humic
acid.
Limited monitoring data from production sites revealed
that HEX was present in a spot sample at a level of 18
mg/litre (February 1977) and a range of 0.156-8.24 mg per
litre (over the month of January 1977) in the aqueous dis-
charge from the Memphis pesticide plant (US EPA, 1980c).
The calculated concentration of HEX in the Mississippi
River was 6 µg/litre (Carter, 1977). In the summer of
1977, shortly after these readings, a new waste-water
treatment plant began operation (Table 5). Prior to con-
struction of the plant, waste water flowed directly into
the Mississippi River or through one of its tributaries
(Elia et al., 1983). Voluntary improvements in controlling
the discharge from the Memphis plant resulted in reported
levels of 0.07 µg HEX/litre in the Mississippi River,
near the mouth of Wolf Creek (Velsicol Chemical Corpor-
ation, 1978). HEX has also been identified in the soil and
river sediments downstream from a USA manufacturing plant,
even after pesticide production was discontinued (US EPA,
1980c).
5.1.3. Soil
Ambient monitoring data for the terrestrial environ-
ment are not available, but it seems that these concen-
trations should be much lower than those in the aquatic
environment. Deposition of HEX from the atmospheric (and
aquatic) compartments into the terrestrial environment is
expected to be minimal. Similarly, direct release of HEX
into the terrestrial environment (i.e. as an impurity in
chlorinated pesticides) should be decreasing because of
regulatory controls on some products, with the possible
exceptions of disposal at waste sites, accidental spills,
and other illegal disposal methods.
Table 5. Concentrations of selected organic compounds in
influent waste water at the Memphis North treatment plant, 1978a
---------------------------------------------------------------
Concentration (µg/litre)b
Date No. of HEX HEX-BCHc HCBCHd Chlordane
samples
---------------------------------------------------------------
June 1 3 334 57 87
August 5 0.8 329 115 216
September 2 4 292 668 58
October-November 2 0.8 11 17 32
---------------------------------------------------------------
a From: Elia et al. (1983).
b Mean values for the number of samples indicated.
c Hexachlorobicycloheptadiene.
d Heptachlorobicycloheptene.
5.1.4. Food
HEX was qualitatively detected in fish samples taken
from water near a pesticide manufacturing plant in
Michigan (Spehar et al., 1977), but none was detected in
fish samples taken from the waters near the pesticide
manufacturing plant in Memphis (Velsicol Chemical Corpor-
ation, 1978; Bennett, 1982). No information regarding HEX
contamination of other foods is available.
5.2. General population exposure
There are insufficient data to determine the relative
contributions of the various sources of HEX to the
environment. There will be exposure to HEX present in
some commonly used pesticides, and possibly some flame
retardants, where HEX is a contaminant. The US EPA has
reported that exposure of humans to HEX from the air or
water should be extremely low, except in the case of
workers and residents near manufacturing, shipping, and
waste sites, and concluded that general population ex-
posure was not considered to be significant or substantial
(US EPA, 1982).
The only other estimates of relative source contri-
butions are from reports completed for the US EPA (Hunt &
Brooks, 1984) and the BUA (1988). The air releases from
manufacturing processes can come from the vents on reac-
tors, process and storage tanks, and as fugitive
emissions. Hunt & Brooks (1984) estimated that the total
quantity of HEX released from these sources was 8 tonnes
per year. In the Federal Republic of Germany and the
Netherlands, HEX emissions were estimated to be 400-500
kg/year. HEX can also be emitted into the air from the
incineration and landfilling of HEX-containing waste, the
most accurate estimate being 1 tonne per year. The total
annual estimated release of HEX to the environment in the
USA is 11.9 tonnes. These figures are only estimates
because of the limited available data. They are given
simply to indicate the relative magnitude of HEX emissions
into the environment.
Exposure limit values for various countries are given
in Appendix 1.
5.3. Occupational exposure
Occupational exposure can occur both at HEX production
and processing facilities and at other locations where
HEX-containing waste is present. For example, the highest
reported workplace air concentrations of HEX were measured
at the Louisville, Kentucky, USA, waste-water treatment
plant, which received a slug of HEX discharged by a waste
hauler. Four days after the plant was closed, air concen-
trations in the primary treatment area ranged from 3.05
to 11.0 mg/m3 (270 to 970 ppb) (Morse et al., 1979).
During the clean-up operations air concentrations as high
as 133 mg/m3 (11 800 ppb) were reported (Kominsky &
Wisseman, 1978).
In 1982, the Velsicol Chemical Corporation used the
Southern Research Institute (SRI) sampling method at the
Memphis (Tennessee) and Marshall (Illinois) facilities to
evaluate possible exposure of workers to HEX vapour and
the effectiveness of engineering controls. Tables 6 and 7
show the HEX concentrations measured at various points.
At the Memphis facility almost one-half of the worker
8-h time-weighted average (TWA) air HEX concentrations
were at or above the USA Threshold Limit Value of 0.11
mg/m3 (0.01 ppm) (OSHA, 1989). At the Marshall plant all
six TWA values were above 0.11 mg/m3. It should be noted
that in Tables 6 and 7 the results of employee monitoring
are reported without regard to respirator use. Respirators
are required to be worn in operations in these plants
where HEX exposure is possible.
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Appendix 1
(Table 21).
Table 6. Summary of hexachlorocyclopentadiene monitoring, Memphis, Tennessee, USAa
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
HEX Process operator 2 445 0.009-0.011 0.009
HEX No. 1 operator 5 432 0.006-0.033 0.015
HEX No. 2 process operator 5 418 0.006-0.029 0.014
HEX No. 2 cyclo operator 5 417 0.001-0.048 0.017
HEX No. 2 chlorine operator 6 415 0.004-0.0161 0.035
a) HEX Bottoms drumming 1 50 0.016
HEX Area sample control room 12 476 0.002-0.018 0.009
HEX Brinks filter cleaning 2 387 0.004-0.006 0.005
Formulations HEX drummers 4 407 0.002-2.0337 0.010
Material HEX railroad tank car 1 279 0.013 0.008
handling unloading
Endrin R2 filter operator 1 281 0.003
Endrin R1 operator 1 334 0.002
Chlorendic No. 1 operator 2 437 0.0077-0.0102 0.008
anhydride
Chlorendic No. 2 operator D34 2 440 0.0107-0.0198 0.014
anhydride
Chlorendic No. 2 operator R6 2 437 0.0065-0.0169 0.011
anhydride
Chlorendic Packaging operator 1 396 0.035 0.031
anhydride
----------------------------------------------------------------------------------------
Table 6 (contd.)
----------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrationsb TWAb
(min) (ppm) (ppm)
----------------------------------------------------------------------------------------
Chlorendic Area sample - control 3 475 0.0003-0.0014 0.001
anhydride room
Heptachlor No. 1 operator 2 407 0.007-0.009 0.007
Heptachlor No. 2 operator 2 415 0.006-0.009 0.007
Heptachlor 237 operator 2 392 0.006-0.019 0.011
Heptachlor Utility operator 1 363 0.006 0.005
Heptachlor Cleaning sparkler filter 3 44 0.002-0.005 0.0003
a) ceiling sample 1 15 0.006
----------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming
that the only chemical exposure occurred during the sampling period.
Table 7. Summary of hexachlorocyclopentadiene monitoring, Marshall, Illinois, USAa
--------------------------------------------------------------------------------------
Unit Description No. of Average Range of sample Average
samples duration concentrations TWAb
(min) (ppm) (ppm)
--------------------------------------------------------------------------------------
Chlordane No. 1 operator 8 451 0.0091-0.0316 0.017
Chlordane No. 2 operator 8 455 0.008 -0.0195 0.013
Chlordane No. 3 operator 8 451 0.0002-0.0325 0.014
Chlordane Area sample - 13 433 0.0002-0.0254 0.016
North control room
Chlordane Area sample - 10 435 0.001-0.0276 0.015
South control room
Chlordane HEX filter changing 1 15 0.1322
Chlordane Waste handling HEX 6 307 0.0006-0.0606 0.020
a) HEX mud drumming - 2 15 0.0005-0.0061
ceiling sample
b) Loading HEX waste 2 15 0.1199-0.2325
truck - ceiling sample
c) Sump pit dumping - 2 15 0.0333-0.1129
ceiling sample
--------------------------------------------------------------------------------------
a From: Levin (1982a).
b ppm = parts of HEX per million parts of air by volume.
TWA = 8-h time-weighted average. The TWA calculation was made assuming that the
only chemical exposure was during the sampling period.
6. KINETICS AND METABOLISM
6.1. Absorption, retention, distribution, metabolism,
elimination, and excretion
6.1.1. Oral
In a study by Mehendale (1977), male Sprague-Dawley
rats (225-250 g body weight) were administered 5 µmol
of 14C-HEX (approximately 5.5 mg/kg) by oral intubation
as 0.2 ml of a solution in corn oil. The total 14C ac-
tivity contained in the dose was approximately 1 µCi. The
animals were maintained in metabolism cages and the urine
and faeces were collected. About 35% of the administered
dose was collected in the urine and only 10% was collected
in the faeces. More than 87% of the 14C activity in the
urine and more than 60% of the activity in the faeces
appeared during the first day. Only a small amount
(approximately 0.5%) of the original dose was recovered
in the kidneys and liver. The author speculated that, in
view of the low total recovery of the administered dose, a
major part of the dose (> 50%) had been excreted through
the lung. This speculation was later proven to be unwar-
ranted because subsequent studies (Dorough, 1979), in
which exhaled air and lung and tracheal tissues were ana-
lysed, showed that this was not the case. There is strong
evidence to suggest that, after oral dosing with HEX, at
least part of the faecal contents contained a volatile
constituent that could be readily lost if the samples were
dried and powdered, as they were in this case. An extrac-
tion procedure, using the major tissues and excreta, fol-
lowed by thin-layer chromatography, showed that at least
four water-soluble (polar) metabolites were produced, but
not identified, after oral dosing.
In a study designed to re-examine some of the findings
and observations of Mehendale (1977), Dorough (1979)
investigated the accumulation, distribution, and excretion
of 14C-HEX after its administration to rats and mice
either as a single oral dose or as a component of their
diet. The principal results of this study were reported by
Dorough & Ranieri (1984). The animals used were male and
female Sprague-Dawley rats, weighing between 200 and 250 g
body weight, and male and female Sprague-Dawley albino
mice, weighing between 25 and 30 g. Two female rats were
dosed, by gavage, with HEX (20 mg/kg) in 0.9 ml of corn
oil and were immediately placed in separate metabolism
cages through which air was drawn at 600 ml/min. The
evacuated air was passed through two high efficiency
traps. Since less than 1% of the administered dose was
recovered from the traps, it was considered to be conclus-
ive evidence that the pulmonary route is not of major
importance in the excretion of HEX (Dorough, 1979).
Dorough (1979) conducted single dose studies by admin-
istering, with a dosing needle, either 2.5 or 25 mg 14C-
HEX/kg body weight (dissolved in 0.9 ml of corn oil for
rats and in 0.2-0.3 ml for mice). The animals were killed
at 1, 3, or 7 days after dosing, and samples of muscle,
brain, liver, kidneys, fat, and either ovaries or testes
were removed and analysed for 14C activity. Urine and
faeces were also collected during the period between
dosing and tissue sample collection. No appreciable dif-
ferences due to sex or species were found in the excretion
patterns. The liver, kidneys, and fat were the most
important deposition sites for 14C residues in both rats
and mice, the levels in the kidneys of rats and in the
liver of mice being the highest.
In the same study (Dorough, 1979), rats and mice were
also placed on diets containing 1, 5, or 25 mg 14C-HEX
per kg. Assuming a daily food intake of 15 g for rats and
5 g for mice, this would give daily dose rates of 0.066,
0.330, and 1.666 mg/kg for rats and 0.182, 0.910, and 4.55
mg/kg for mice. Feed was replaced in the feeders every
12 h to minimize the loss of 14C-HEX (from volatiliz-
ation), and the feeding study was carried out for 30 days.
During this period, rats and mice were killed at 1, 3, 7,
12, 15, or 30 days. The surviving animals were then
returned to a normal diet for up to 30 days and, during
this post-treatment period, animals were killed at 1, 3,
7, 15, or 30 days after the last exposure. The total
excretion (urine and faeces) of the radiolabel ranged from
63-79% of the consumed 14C-HEX, which was significantly
lower than that found in the single-dose study (73-96%).
In all cases, the liver, kidneys, and fat contained the
highest amounts of 14C, and it appeared that a steady
state for these levels was reached after 15 days of the
feeding phase. A good correlation was observed between
the level of HEX in the diet and the 14C-levels found in
all the examined tissues. In a separate experiment with
male rats, in which the bile duct was cannulated and a
single dose of 14C-HEX (25 mg/kg) was administered
orally, only 16% of the dose was excreted in the bile. The
extraction characteristics of the radiocarbon compounds in
the excreta showed that they were primarily polar metab-
olites, some of which were capable of being converted to
organic-soluble compounds after acid-catalysed hydrolysis.
In a comparative study of the pharmacokinetics
of 14C-HEX after intravenous and oral dosing, Yu &
Atallah (1981) dosed Sprague-Dawley rats (240-350 g body
weight) with either 3 or 6 mg 14C-HEX (specific activity:
0.267 mCi/mmol). The doses ranged from 8.5 to 25.6 mg/kg.
Shortly after oral dosing, 14C activity appeared in the
blood and reached a maximum after approximately 4 h.
The 14C activity appeared in most of the tissues
analysed at 8, 24, 48, 72, 96, and 120 h after dosing.
Following oral dosing, there were higher residue levels in
the kidneys and liver than in any other tissue, although
these levels were generally much lower than those observed
after intravenous dosing. For example, at 24 h after
dosing, the kidneys and liver were found to contain only
0.96 and 0.75%, respectively, of the administered oral
dose, while these organs retained 2.92 and 4.68%, respect-
ively, of the administered intravenous dose. A higher
proportion (15.07%) of the 14C activity was found in the
digestive system (duodenum and large and small intestines)
after oral dosing. Coupled with the increased rate and
extent of faecal excretion after oral administration
(approximately 72%), compared to that after intravenous
dosing (approximately 20%), this would suggest that only a
fraction of the orally administered dose was absorbed.
About 17% of the oral dose was excreted in the urine.
Both urinary and faecal metabolites were again
characterized as polar because of their insolubility in
organic solvents. Unchanged HEX was not detected in any
of the samples examined. Only 11% of the 14C content was
soluble in organic solvents and a further 32% was con-
verhydrolysis. This indicated, perhaps, the formation of
metabolic ester conjugates.
Lawrence & Dorough (1982) made a comparative study of
the uptake, disposition, and elimination of HEX after
administering radiolabelled 14C-HEX by the intravenous
(10 µg/kg), inhalation (24 µg/kg), and oral routes (6 mg
per kg) to Sprague-Dawley rats weighing between 175 and
250 g, respectively. They noted that while doses in the
microgram range were useful for monitoring the urinary and
faecal excretion of HEX, much higher doses (about 6 mg/kg
in 0.5 ml of corn oil, and with a 4-fold increase in
radiocarbon activity) were necessary to obtain levels in
the principal organs that could be measured with any pre-
cision. Indeed, the doses administered orally were some
250 and 600 times the inhaled and intravenous doses,
respectively. In agreement with other researchers, these
authors attributed the lack of measurable levels in the
organs, following the administration of low doses, to the
poor bioavailability of HEX when given by the oral route.
The total radiolabel recovery immediately after the admin-
istration of the dose was 98.0 ± 5.3% (mean ± S.D.). Rats
dosed orally eliminated 2-3 times more of the dose in the
faeces than those dosed by the intravenous or inhalation
route. A maximum blood level was reached at approximately
2 h after dosing. The peak was broad with similar blood
concentrations between 2 and 5 h, perhaps indicating that
absorption occurred along the gastrointestinal tract over
this period in a quasi-steady state with elimination.
Biliary excretion was again confirmed as being greater
after oral dosing than after intravenous or inhalation
dosing, but it still only accounted for 18% of the admin-
istered dose. This observation agreed with previous
studies and, more importantly, with the report of Yu &
Atallah (1981), who administered comparable dose levels by
the oral route. Lawrence & Dorough (1982) also reported
that the faecal material contained predominantly polar or
unextractable material, as did the bile. These authors
considered that this was a clear indication that 14C-HEX
was extensively metabolized to polar products by the gut
contents, since only approximately 50% of 14C-HEX was
recovered when it was added to rat stomach contents that
were then immediately extracted with hexane.
A more recent comparative study (El Dareer et al.,
1983) essentially confirmed the findings of Yu & Atallah
(1981) and Lawrence & Dorough (1982). Male Fischer-344
rats with an average body weight of 169 g were dosed at
a level of 4.1 and 61 mg/kg with approximately 1 ml of a
solution of 14C-HEX dissolved in a 1:1:4 mixture of
Emulphor EL620, ethanol, and water. Little radioactivity
appeared as exhaled 14CO2.
6.1.2. Inhalation
In studies by Dorough (1980) and Lawrence & Dorough
(1981, 1982), rats were exposed to 14C-HEX vapour in a
specially designed, single animal inhalation exposure sys-
tem. Each animal was exposed to the vapours in a rodent
respirator, with the exhaust vapours from the system pass-
ing through a filter pad made from expanded polyurethane
foam. The flow rate and concentration of HEX was measured
prior to and after passing through the respirator contain-
ing the exposed animal. The difference between the amounts
of HEX in the input and output was assumed to be equi-
valent to the retained dose. Rats were exposed for a
period of 1 h and received doses in air which ranged from
1.4 to 37.4 mg/kg body weight (Lawrence & Dorough, 1981).
Immediately after the 1-h exposure, the recovery of the
dose retained by the animal was 91.8 ± 8.5% (mean ± S.D.).
Exposed animals were immediately placed in metabolism
cages for 72 h, during which time faeces, urine, and
expired air were collected. The animals were then killed
and certain of their tissues analysed for 14C activity.
Less than 1% of the retained radiocarbon was expired
during the 24-h period immediately following exposure, and
no radiocarbon was detected as 14CO2. Only about 69%
of the inhaled dose was recovered, which was much lower
than that recovered after intravenous (85%) or oral dosing
(82%). Since recovery of the dose immediately after the
administration of the inhalation dose was approximately
92%, the reduced recovery during the 72-h post-dosing
period led to the speculation that a volatile metabolite
was formed during this period, but attempts to collect and
identify this metabolite were not successful.
No kinetic parameters were reported in either of the
publications by Lawrence & Dorough (1981, 1982), although
blood concentration-time data during the 1-h exposure and
the following 6 h were presented. Elimination during the
subsequent 6 h appeared to relate to a complex pharmaco-
kinetic model with a terminal rate comparable to that
reported for the intravenous route, the half-life being
approximately 30 h.
The elimination via the bile was relatively low (8%)
after inhalation exposure, compared with 13 and 18% after
intravenous or oral administration of the same dose
(5 µg/kg) (Lawrence & Dorough, 1982). The fraction of
the dose recovered in the faeces and urine (23 and 33%,
respectively) was about the same as that recovered after
the intravenous dose, except that more was recovered in
the urine than in the faeces after the inhalation
exposure, while the reverse was observed after the intra-
venous dose.
A comparative study of the uptake, distribution, and
elimination of 14C-HEX (El Dareer et al., 1983) confirmed
and extended the conclusions reached by Lawrence & Dorough
(1981, 1982) concerning pulmonary exposure. Individual
Fischer-344 rats weighing between 125 and 190 g (with an
average weight of 169 g) were placed in metabolism cages
and exposed by inhalation. The dose received by each rat
over a 2-h exposure period was calculated from the total
amount of radioactivity recovered from the tissues,
faeces, urine, and exhaled air. The animal fur was not
included. The dose received by the exposed animals was
between 1.3 and 1.8 mg/kg body weight. The animals were
killed at either 6 or 24 h after they were removed from
the inhalation exposure. Whole blood, plasma, liver,
kidneys, voluntary muscle (gastrocnemius), subcutaneous
fat, brain, skin (ears), and the residual carcass (except
for the skin and fur which were discarded) were analysed
for 14C activity, as were the urine, faeces, and exhaled
air. The principal sites of deposition were the lungs,
kidneys, and liver. Only approximately 1% of the radio-
label was identified as 14CO2. No intact HEX was found
in any of the tissues; the majority of the radiolabel
extracted was polar (water soluble). These findings were
similar to those of Lawrence & Dorough (1981, 1982).
6.1.3. Dermal
No studies on the pharmacokinetics or distribution of
dermally applied HEX were found in a survey of the pub-
lished literature. Although no qualitative studies or
estimates of the uptake of HEX through skin were found,
studies have been reported in which discoloration of the
skin was observed after the dermal application of HEX
(Treon at al., 1955; IRDC, 1972). In these reports, toxic
response, leading to death, was observed in several
instances, which would suggest that HEX was absorbed
transdermally into the systemic circulation.
6.1.4. Comparative studies
Each of the four major studies (Yu & Atallah, 1981;
Lawrence & Dorough, 1981, 1982; El Dareer et al., 1983) of
the uptake and distribution of HEX involved more than one
route of uptake. One objective of each of these studies
was to compare the exposure routes. The observations made
were as follows:
* The principal routes of excretion were via the urine
and faeces. Considerably more of the administered dose
was excreted in the faeces after oral administration
than after dosing by the intravenous or inhalation
route, probably as a consequence of the increased
biliary excretion after oral dosing and the interac-
tion or metabolism of the dose by gut and faecal con-
tents. More of the administered dose was excreted in
the urine than in the faeces after inhalation
exposure, while the reverse was the case after intra-
venous administration.
* Biliary excretion occurred after administration by
each of the three routes. For similar doses, the frac-
tion of the dose eliminated by this route was in the
order oral > intravenous > inhalation.
* Comparative distribution to the major organs and tis-
sues is presented in Tables 8, 9, and 10. The princi-
pal organs to which HEX was distributed by the sys-
temic circulation were the kidneys and liver. The
lungs and trachea contained the highest concentrations
of HEX after inhalation exposure.
* There was a significantly higher retention of 14C in
the carcass, at 72 h post-dosing, after dosing by the
inhalation and intravenous routes than after oral
dosing (Table 10).
6.1.5. In vitro studies
Yu & Atallah (1981) examined the ability of liver,
faecal, and gut homogenates to metabolize HEX in vitro.
In an apparent first-order kinetic process, HEX was
metabolized by gut content, faecal, and liver homogenates
with half-lives of 10.6, 1.6, and 14.2 h, respectively.
When mercuric chloride (HgCl2) was added to the gut and
faecal homogenates as a bacteriocide, the half-lives were
increased to 17.2 and 6.2 h, respectively, indicating that
the gut and faecal flora contributed significantly to the
metabolism of HEX. Denaturation of the liver homogenate
had virtually no effect on the in vitro metabolic rate
indicating, perhaps, that there was only limited involve-
ment of liver microsomes or other enzyme-dependent process.
Table 8. Distribution of radioactivity (expressed as percentage of administered
dose) from 14C-HEX in rats dosed by various routesa
---------------------------------------------------------------------------------
Oral dose Intravenous Inhalation dose
Low doseb High doseb doseb Group Ac Group Bb
(4.1 mg/kg) (61 mg/kg) 0.59 mg/kg (1.0 mg/kg) (1.4 mg/kg)
---------------------------------------------------------------------------------
Faeces 74.5 ± 2.8 65.3 ± 6.9 34.0 ± 1.0d 28.7 ± 4.3 47.5 ± 6.4
Urine 35.5 ± 2.5 28.7 ± 4.2 15.8 ± 1.4 41.0 ± 4.8 40.0 ± 6.6
Tissues 2.4 ± 0.6 2.4 ± 0.1 39.0 ± 1.0 28.9 ± 1.6 11.5 ± 0.8
CO2 0.8 ± 0.0 0.6 ± 0.0 0.1 ± 0.0 1.4 ± 0.3 1.0 ± 0.5
Other volatile 0.2 ± 0.0 0.3 ± 0.0 0.1 ± 0.0
compounds
Total recovery 118 ± 3.0e 97 ± 7.0 89 ± 2.0 100 100
---------------------------------------------------------------------------------
a Adapted from: El Dareer et al. (1983). Values represent the mean percentage
of dose ± S.D. for three rats.
b At 72 h after dosing or exposure.
c At 6 h after exposure.
d Plus intestinal contents.
e For an unexplained reason, the total recovery for this dose was higher than
theoretical. If the percent recoveries for this dose are "normalized" to
100%, differences in distribution for the two doses are minimal, indicating
that no saturable process is operative in this dose range.
Table 9. Fate of radiocarbon (expressed as percentage of
administered dose) after oral, inhalation, and intravenous
exposure of rats to 14C-HEXa
--------------------------------------------------------------
Cumulative percent of dose
Oralb Intravenousc Inhalationd
--------------------------------------------------------------
24-h
Urine 22.2 ± 1.8 18.3 ± 5.2 29.7 ± 4.5
Faeces 62.2 ± 8.0 21.1 ± 7.1 17.0 ± 7.5
48-h
Urine 24.0 ± 1.9 20.7 ± 5.6 32.5 ± 5.1
Faeces 67.7 ± 5.1 30.4 ± 1.7 21.0 ± 7.5
72-h
Urine 24.4 ± 1.9 22.1 ± 5.7 33.1 ± 4.5
Faeces 68.2 ± 5.1 47.4 ± 1.9 23.1 ± 5.7
Body 0.2 ± 0.2 15.7 ± 7.8 12.9 ± 4.7
Total Recovery 92.8 ± 4.7 85.2 ± 4.8 69.1 ± 9.6
--------------------------------------------------------------
a Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
b Dose (7 µg/kg body weight) administered in 0.5 ml corn oil.
c Dose (5 µg/kg body weight) administered in 0.2 ml
saline:propylene glycol:ethanol (10:4:1) by injection into
the femoral vein.
d Doses administered as vapours over a 1-h exposure period
to achieve doses of about 24 µg/kg body weight.
El Dareer et al. (1983) incubated 14C-HEX with homo-
genates of liver, faeces, and intestinal (large and small)
contents, as well as with whole blood and plasma. Samples
were taken at 0, 5, and 60 min. The results, presented in
Table 11, clearly demonstrated the chemical reactivity of
HEX and its ability to bind components of biological
material.
Table 10. Distribution of HEX equivalentsa in tissues and
excreta of rats 72 h after oral, inhalation, and intravenous
exposure to 14C-HEXb,c
-----------------------------------------------------------------
Sample Oral dose Inhaled dose Intravenous dose
(6 mg/kg)d (24 µg/kg) (10 µg/kg)
-----------------------------------------------------------------
ng/g of tissue
Trachea 292 ± 170 107.0 ± 65.0 3.3 ± 1.7
Lungs 420 ± 250 71.5 ± 55.2 14.9 ± 1.1
Liver 539 ± 72 3.6 ± 1.9 9.6 ± 1.1
Kidneys 3272 ± 84 29.5 ± 20.2 22.3 ± 0.6
Fat 311 ± 12 2.8 ± 0.4 2.3 ± 0.2
Remaining carcass 63 ± 40 1.3 ± 0.6 0.5 ± 0.1
percentage of dose
Whole body 2.8 ± 1.1 12.9 ± 4.7 31.0 ± 7.8
Urine 15.3 ± 3.3 33.1 ± 4.5 22.1 ± 5.7
Faeces 63.6 ± 8.5 23.1 ± 5.7 31.4 ± 1.9
Total recovery 81.7 ± 6.7 69.1 ± 9.6 84.6 ± 4.6
-----------------------------------------------------------------
a One HEX equivalent is defined as the amount of radiolabel
equivalent to 1 ng of HEX, based on the specific activity of
the dosing solution.
b Adapted from: Dorough (1980) and Lawrence & Dorough (1982).
c All values are the mean ± S.D. of three replicates.
d It should be noted that the oral dose was 250 and 600 times
that of the inhaled and intravenous doses, respectively.
This was necessary because residues were not detected in
individual tissues of animals treated orally at doses of
5-25 µg/kg.
6.2. Metabolic transformation
No primary metabolites or conjugates of HEX have been
identified. The data available on the pharmacokinetics of
HEX after dosing by the oral, inhalation, and dermal
routes are presented in sections 6.1.1, 6.1.2, and 6.1.3.
In studies by Dorough (1980) and Lawrence & Dorough
(1982), the principal routes of excretion were shown to be
via the urine and faeces. No unchanged HEX was found in
either, indicating that HEX was involved in extensive
metabolism.
In studies with rats and mice fed a diet containing 1,
5, or 25 mg 14C-HEX/kg, 63-79% of the consumed HEX was
recovered in the urine and faeces (Dorough, 1979; Dorough
& Ranieri, 1984). The extraction characteristics of the
radiocarbon compounds in the excreta showed that they were
primarily polar metabolites, some of which were trans-
formed to organic-soluble compounds after acid-catalysed
hydrolysis.
Table 11. Extractability of 14C-HEX and radioactivity derived from
saline and various biological preparationsa
----------------------------------------------------------------------
Preparation Time First Extraction Second Extraction Pellet
(min) Organicb Aqueous Organic Aqueous
----------------------------------------------------------------------
Saline 0 99.6 (92.4) 0.4
5 99.1 (92.8) 0.9
60 98.8 (94.6) 1.2
Liver 0 55.0 (74.4) 8.0 24.5 1.0 11.6
5 42.8 (49.7) 15.2 15.0 4.7 22.2
60 11.1 18.8 5.9 2.4 51.8
Plasma 0 22.2 (61.7) 7.2 50.2 0.8 19.6
5 19.7 (66.3) 25.0 33.6 2.0 19.6
60 1.4 43.4 21. 3.9 30.2
Whole blood 0 16.2 (60.4) 3.8 27.9 1.2 50.8
5 2.8 21.6 13.4 1.6 60.6
60 0.6 27.4 12.0 1.4 58.6
Faeces 0 90.0 (93.7) 0.6 8.0 0.2 1.2
5 83.4 (87.8) 0.8 9.0 0.6 6.2
60 40.5 (61.0) 2.8 31.3 3.0 22.4
Intestinal 0 93.7 (94.7) 0.6 4.6 0.2 1.0
contents 5 82.8 (89.5) 1.6 8.6 1.0 5.9
60 66.3 (87.0) 4.6 15.4 2.4 11.3
----------------------------------------------------------------------
a From: El Dareer et al. (1983). Values represent the percentage of
the total radioactivity in the respective fraction.
b Values in parentheses represent the percentage of the
radioactivity in the fraction as HEX.
In a comparative study of the pharmacokinetics of HEX
after intravenous or oral dosing at 8.5-25.6 mg/kg (Yu &
Atallah, 1981), urinary and faecal metabolites were again
characterized as polar because of their poor solubility in
organic solvents. No unchanged HEX was found. Only 11%
of the 14C content of the excreta was soluble in organic
solvents, and a further 32% of the extract was converted
to organic-soluble compounds after acid-catalysed hydro-
lysis. This indicated, perhaps, that metabolite ester
conjugates had been formed.
Yu & Atallah (1981) also performed in vitro metabolic
studies in which they incubated HEX with liver, faecal,
and gut-content homogenates (see section 6.1.5).
After Lawrence & Dorough (1982) had dosed rats by the
oral, inhalation, and intravenous routes with 14C-
labelled HEX at 6 mg/kg, 24 µg/kg and 10 µg/kg, respect-
ively, they found that the faecal and bile contents con-
tained mostly polar metabolites. These authors suggested
that HEX was rapidly metabolized to polar products, since
only about 50% of the HEX was recovered when it was added
to rat gut contents and immediately extracted with n-hex-
ane. In addition, the authors noted that approximately
15.8 ± 4% of the radiolabel that appeared in the faeces
within 24 h after dosing was volatile, indicating that a
catabolite was probably produced.
El Dareer et al. (1983) dosed rats by the inhalation
route so that individual animals received between 1.3 and
1.8 mg/kg body weight over a 2-h exposure period. They
were killed 6 or 24 h after removal from exposure. No in-
tact HEX was found in any of the tissues, and the majority
of the extractable material was polar (water soluble), in
accordance with the findings of Lawrence & Dorough (1981,
1982). As a part of this same study, El Dareer et al.
(1983) incubated 14C-HEX with homogenates of liver,
faeces, and intestinal (large and small) contents, as well
as with whole blood and plasma. These in vitro studies
were designed to assess the reactivity and binding charac-
teristics of HEX. The results, presented in Table 8,
clearly show chemical lability of HEX and its ability to
bind the components of biological material (see section
6.1.2).
Despite these efforts to characterize HEX metabolism,
no metabolites were identified. This observation suggests
that an attempt to rectify this deficiency would be a high
priority.
6.3. Reaction with body components
The toxic mechanisms of hexachlorocyclopentadiene are
not well understood. HEX has been shown to react with
olefins and other organic molecules such as aromatic com-
pounds. Using the available data, especially those from
studies comparing the various routes of exposure, a very
general and hypothetical rationale can be developed to
suggest possible reactions with body tissue.
The reactivity of HEX shows a high potential for
transformation and reaction with other chemicals. Absorp-
tion from the gastrointestinal contents is relatively
inefficient, probably due to the interaction of HEX with
the gastrointestinal contents and metabolism by intestinal
flora. The fact that HEX did not appear to be interactive
with the gastrointestinal epithelia in the kinetic studies
discussed in this chapter was probably due to its dilution
in the carrier vehicle, as well as its interaction with,
and metabolism by, the gut contents. However, in short-
term repeated oral dosing studies (SRI, 1981a,b), at doses
of 19 mg/kg or more, inflammation and hyperplasia was
noted in the forestomach (see Table 16). In addition, the
interaction of HEX after dermal contact was marked by a
distinct discoloration of the skin. This suggests a "site
of uptake" interaction, probably similar to that observed
in the lungs after pulmonary uptake.
During inhalation and the passage of HEX through the
lung tissue to reach the systemic circulation, metabolism
to water-soluble compounds probably occurs and HEX is
eliminated through the kidneys. However, an intravenous
dose may be bound unchanged to blood components (e.g.,
haemoglobin) and remain attached until reaching the liver.
The relatively slow elimination of the radiolabel from the
systemic circulation after intravenous dosing with 14C-
HEX (approximate terminal half-life of 30 h) suggests a
bioaccumulation potential, at least for some of the
metabolites, since little HEX appears to remain in the
tissues.
Rand et al. (1982b) showed that the cellular level in
lung tissue underwent significant changes after HEX inha-
lation. HEX vapour, administered by the inhalation route,
in addition to binding to epithelial lung tissue, was
found to bind to the extracellular lining in the lung.
Binding to bronchiolar Clara cells, which contribute
important materials to the extracellular lining of the
peripheral airways, was observed after inhalation exposure
in rats and monkeys (Rand et al., 1982a). HEX was also
found, in in vitro studies, to bind to the components of
whole blood, plasma, liver, and faecal homogenates and to
gastrointestinal contents (El Dareer et al., 1983). Thus,
irrespective of the route of administration, the principal
sites of toxic action seem to be the lungs, liver, and
kidneys. This observation is supported by the results from
the toxicity testing reported in chapter 8.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
The effects of HEX on microorganisms have been
studied in aqueous and soil systems. Many of the aqueous
concentrations used in these experiments exceeded the
upper limit of aqueous solubility (0.8-2.1 mg/litre).
These concentrations were usually obtained by using an
organic solvent vehicle to disperse the chemical in aque-
ous media. The environmental significance of the results
should be interpreted with this aspect in mind.
Cole (1953) inoculated 10 strains of common human and
animal pathogens into growth media that contained various
concentrations of HEX. The inhibiting concentration, or
lowest concentration in which no growth was observed after
96 h of contact, ranged from 1-10 mg HEX/litre. The
Addition of 5 or 10 mg HEX/litre to sewage effluent inocu-
lated with Salmonella typhosa was found to be more effec-
tive than similar concentrations of chlorine in reducing
counts of total bacteria, coliforms, and S. typhosa (Cole,
1954). Yowell (1951) also reported, in a patent appli-
cation, that HEX has antibacterial properties; standard
phenol coefficients for Ebertnella typhi (Salmonella
typhi) and Staphylococcus aureus were 25 and 33, at 21 and
23 mg HEX/litre, respectively. These findings indicate
that concentrations of HEX at or slightly above its aque-
ous solubility limit are toxic to several types of
pathogens.
In contrast, tests with other microorganisms have
shown some ability to withstand HEX exposure. Twenty-three
strains of organisms (type unspecified), when added to
aqueous media containing HEX at 1000 mg/litre, were able
to metabolize the compound to a varying degree. Analysis
of the medium after 14 days indicated a HEX removal of
2-76%, depending on the organism used (Thuma et al.,
1978).
Rieck (1977a) found no effects on natural populations
of bacteria, actinomycetes, or fungi after a 24-day incu-
bation of a sandy loam soil treated with 1 or 10 mg HEX/kg
dry weight. It was concluded that no significant detrimen-
tal effects on microbial populations would result from
contamination of soils with these levels of HEX.
The effects of HEX on three ecologically important
microbial processes have been reported (Butz & Atallah,
1980). Results on cellulose degradation by the fungus
Trichoderma longibrachiatum indicated that a suspension
of HEX inhibited cellulose degradation at a concentration
of 1 mg/litre or more in a liquid medium. The calculated
7-day EC50 was 1.1 mg/litre. Extrapolations for 1- and
3-day EC50 values were both reported to be 0.2 mg/litre.
The decrease in toxicity over the 7-day period was
attributed to adaptation by T. longibrachiatum.
HEX inhibited anaerobic sulfate reduction by the bac-
terium Desulfovibrio desulfuricans when HEX was present in
suspension in a liquid medium. After a 3-h contact period,
growth inhibition was observed at HEX concentrations of
10-100 mg/litre, and there was no growth at 500 or 1000
mg/litre. Similarly, growth inhibition was observed at 1
and 10 mg/litre after a 24-h contact period, and there was
no growth at 50-1000 mg/litre. HEX was considered to be
slightly toxic to D. desulfuricans (Butz & Atallah,
1980).
The third part of the study by these investigators
(Butz & Atallah, 1980) focused on the effects of HEX on
urea ammonification by a mixed microbial culture in moist
soil. The results indicated that HEX concentrations of
1-100 mg/kg (dry weight) were not toxic to soil organisms
responsible for urea ammonification. The EC50 increased
from 104 mg/kg at day 1 to 1374 mg/kg at day 14. The
authors suggested that the low toxicity and its decrease
over time in this experiment may have been due to adsorp-
tion of the toxicant onto soil particles, as well as to
potential adaptation by the organism. Adsorption onto soil
particles may also account for the lack of toxicity in the
study of Rieck (1977a).
Walsh (1981) reported unpublished data on the effects
of HEX on four species of marine algae, obtained according
to the method described by Walsh & Alexander (1980). The
7-day EC50 was calculated as the concentration that
caused a 50% decrease in growth compared with the control,
as estimated by absorbance at 525 nm. The 7-day EC50
values reported indicated a wide range of susceptibility
among the species tested. Isochrysis galbana and
Skeletonema costatum were the most susceptible species,
the average 7-day EC50 values being about 3.5 and 6.6 µg per
litre, respectively. The average value for Porphyridium
cruentum was 30 µg/litre, while that for Dunaliella
tertiolecta was 100 µg/litre. Other tests with S.
costatum indicated that the direct algicidal effect of HEX
was less pronounced than its effect on growth. After 48 h
of exposure to HEX at 25 µg/litre, mortality, as indi-
cated by staining and cell enumeration, was only 4%
(Walsh, 1983).
7.2. Aquatic organisms
7.2.1. Freshwater aquatic life
Several studies are available on the effects resulting
from exposure of freshwater aquatic life to various con-
centrations of HEX.
Results from acute toxicity tests with HEX have been
reported for a number of freshwater fish species (Table
12). The 96-h LC50 value for fathead minnow larvae in a
flow-through test with measured toxicant concentration was
7 µg/litre (Spehar et al., 1977, 1979). Values obtained
for adult fathead minnows in static tests with unmeasured
toxicant concentrations ranged from 59 to 180 µg/litre
(Henderson, 1956; Buccafusco & LeBlanc, 1977). Reported
96-h values for goldfish, channel catfish, and bluegills
were also within this range (Buccafusco & LeBlanc, 1977;
Podowski & Khan, 1979; Khan et al., 1981).
Sinhaseni et al. (1982) reported the biological
effects of HEX on rainbow trout (Salmo gairdneri) exposed
to 130 µg HEX/litre in a non-recirculating flow-through
chamber. Oxygen consumption, measured polarographically,
increased by 193% within 80 min and then gradually
decreased until the fish died (after approximately 5 h).
Vehicle controls showed no effects after 76 h of exposure.
When added to normal trout mitochondria, HEX increased
basal oxygen consumption. The authors concluded that HEX
uncoupled oxidative phosphorylation.
Sinhaseni et al. (1983) continued their research on
the respiratory effects of HEX on intact rainbow trout.
Acclimated rainbow trout were exposed to 130 µg HEX/litre
in a flow-through well-water circuit, which was designed
to allow measurements of oxygen consumption in fish.
Again, HEX increased oxygen consumption rates (186 ± 24%),
the maximum rates being nearly the same as in the previous
experiment (approximately 84 min). The oxygen consumption
decreased until death (after approximately 6.5 h). Control
trout, exposed to the same concentration of the vehicle
(acetone) used to disperse HEX, showed no changes. The
authors reported profound respiratory stimulation, and HEX
appeared to uncouple oxidative phosphorylation. Sinhaseni
et al. (1983) postulated that HEX intoxication in the
intact animal may be due to increased oxygen consumption
and impaired oxidative ATP synthesis resulting from the
mitochondrial uncoupling action of HEX.
Table 12. Acute toxicity data for freshwater species exposed to HEX
---------------------------------------------------------------------------------------------------------------------------
Acute
no-effect
Species Methoda LC50 (µg/litre)b concentration Comments Reference
24-h 48-h 96-h (µg/litre)
---------------------------------------------------------------------------------------------------------------------------
Cladoceran S,U 93.0 52.2 ND 32 17 °C, soft water Vilkas (1977)
Daphnia magna (78.9-109.6) (44.8-60.9)
Cladoceran S,U 130 39 ND 18 22 °C, soft water Buccafusco &
Daphnia magna (68-260) (30-52) Leblanc (1977)
Fathead minnow FT,M NR NR 7.0 3.7 25 °C, soft water Spehar et al.
(larvae, < 0.1 g) (1977, 1979)
Pimephales promelas
Fathead minnow (1-1.5 g) S,U 115 110 104 NR Hard water, Henderson
Pimephales promelas acetone soln. (1956)
93 78 78 NR Soft water,
acetone soln.
75 59 59 NR Hard water,
emulsion
(no acetone)
Fathead minnow (0.72 g) S,U 240 210 180 87 22 °C, soft water Buccafusco &
Pimephales promelas (170-320) (180-250) (160-220) Leblanc (1977)
Goldfish NR NR NR 78 NR No details given Podowski & Khan
Carassius auratus (1979)
Channel catfish (2.1 g) S,U 190 150 97 56 22 °C, soft water Buccafusco &
Ictalurus punctatus (140-250) (130-180) (81-120) Leblanc (1977)
Bluegill (0.45 g) S,U 170 150 130 65 22 °C, soft water Buccafusco &
Lepomis macrochirus (140-210) (120-180) (110-170) Leblanc (1977)
---------------------------------------------------------------------------------------------------------------------------
a S = static, FT = flow-through, U = unmeasured concentrations, M = measured concentrations.
b Numbers in parentheses show 95% confidence interval. ND = Not determined. NR = Not reported.
Spehar et al. (1977, 1979) conducted 30-day early-life
stage flow-through toxicity tests with fathead minnows
(Pimephales promelas) using measured concentrations and
1-day-old larvae. The 96-h LC50 value was 7 µg/litre.
The 96-h mortality data indicated a sharp toxicity
threshold, such that 94% survival was observed at 3.7 µg
per litre, 70% at 7.3 µg/litre, and 2% at 9.1 µg/litre.
At the end of the 30-day exposure period, mortality was
only slightly higher, with 90% survival at 3.7 µg/litre,
66% at 7.3 µg/litre, and 0% at 9.1 µg/litre. These
results indicated that the median lethal threshold, the
lowest concentration causing 50% mortality, was reached
within 4 days. In addition, the HEX residues found in
fathead minnows at the end of the 30-day tests were low
(< 0.1 µg/g), and a BCF value of < 11 was reported
(Spehar et al., 1979). The authors concluded that the
toxicity data and the BCF values indicated that HEX was
non-cumulative in fish, i.e. it did not bioconcentrate in
fish as a result of continuous low-level exposure to HEX.
The growth rate of surviving larvae, measured in terms of
both body length and weight, did not decrease signifi-
cantly at any of the concentrations tested, compared with
the controls. This was true even at 7.3 µg/litre, a level
greater than the calculated LC50 value. Based on these
toxicity and growth data, Spehar et al. (1977, 1979) con-
cluded that 3.7 µg/litre is the highest concentration of
HEX that produces no adverse effects on fathead minnow
larvae. No other chronic toxicity data for any freshwater
species are available.
7.2.2. Marine and estuarine aquatic life
Among marine invertebrates, the 96-h LC50 values for
HEX range from 7 to 371 µg/litre (Table 13) (US EPA,
1980a). Except where indicated, these results were
obtained from static tests with nominal concentrations of
HEX. The highest LC50 by far was for the polychaete
Neanthes arenaceodentata, which is an infaunal organism
living in the sediment. The two shrimp species tested
were more sensitive to HEX by a factor of 10 or more.
The static LC50 value reported by US EPA (1980a) for
the grass shrimp, Palaemonetes pugio, was slightly higher
than that for the mysid shrimp, Mysidopsis bahia (Table
13). However, the LC50 for the mysid shrimp was consider-
ably lower in a flow-through test than in the static test.
Similarly, the LC50 value was lower when calculated
from actual measurements of HEX concentrations in the test
solutions (measured concentration) than when calculated
according to the concentrations based on amounts orig-
inally added to test solutions (nominal concentrations).
Table 13. Acute toxicity data on estuarine marine
organisms exposed to HEXa
----------------------------------------------------------
Species Methodb Salinity 96-h LC50c
(o/oo) (µg/litre)
----------------------------------------------------------
Polychaete S,U 28 371
Neanthes arenaceodentata (297-484)
Grass shrimp S,U 22 42
Palaemonetes pugio (36-50)
Mysid shrimp S,U 24 32
Mysidopis bahia (27-37)
Mysid shrimp FT,U 24 12
Mysidopsis bahia (10-13)
Mysid shrimp FT,M 24 7
Mysidopsis bahia (6-8)
Pinfish S,U 22 48
Lagodon rhomboides (41-58)
Spot S,U 22 37
Leiostomus xanthurus (30-42)
Sheepshead minnow S,U 24 45
Cyprinodon variegatus (34-61)
----------------------------------------------------------
a Adapted from: US EPA (1980a) and Mayer (1987).
b S = static; FT = flow-through; U = unmeasured
concentrations; M = measured concentrations.
c Numbers in parentheses show 95% confidence interval.
The acute toxicity values for HEX were similar for
each of three estuarine and marine fish species tested (US
EPA, 1980a). The static 96-h LC50 values based on
unmeasured concentrations for spot, sheepshead minnow, and
pinfish varied only between 37 and 48 µg/litre (Table 13).
7.3. Terrestrial organisms and wildlife
In a USA patent application, HEX was reported to be
nontoxic to plants in concentrations at which it was an
effective fungicide (Yowell, 1951). Test solutions were
prepared by adding HEX at various proportions to attaclay
and a wetting agent, and they were then mixed with water.
The concentrations of HEX applied to plants as an aqueous
spray were 0.1, 0.2, 0.5, and 1.0%. Slight injury
(unspecified) to Coleus blumei was reported at 1.0% HEX,
but lower concentrations were not harmful. Similarly, HEX
was added to horticultural spray oil and an emulsifier at
various proportions and then mixed with water. The concen-
tations of HEX in the prepared spray were 0.25 and 0.5%.
No injury to C. blumei was observed at these concen-
trations. No data are available concerning the effects of
HEX on amphibians, reptiles, birds, or mammals other than
those routinely used in laboratory testing.
7.4. Population and ecosystem effects
The ecological effects of HEX have not been studied at
the ecosystem, population, or community levels.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Acute toxicity studies
8.1.1. Acute oral, inhalation, and dermal toxicity
The data from acute toxicity studies using HEX are
summarized in Table 14. Caution should be exercised in
comparing the various studies. The acute toxicity (LD50
and LC50) may be affected not only by the species and
age of the animals used in the experimental tests, but
also by the strain. In addition, the purity of the com-
pound and the nature of the contaminants may affect the
toxicity, as can the experimental method and the vehicle
used. These details (when specified by the researchers)
have been summarized in the table.
For the rat, oral LD50 values ranged from 425 to 926
mg/kg for males, and from 315 to 926 mg/kg for females.
Values for mice and rabbits were within the same range.
Acute inhalation experiments involved exposure
durations from 3.5 to 4 h, and LC50 values for male rats
ranged from 18.1 to 35.0 mg/m3 (1.6 to 3.1 ppm) and for
female rats from 35.0 to 40.0 mg/m3 (3.1 to 3.5 ppm).
The mouse, rabbit, and guinea-pig values ranged from 23.7
to 80.2 mg/m3 (2.1 to 7.1 ppm).
There are few data available on dermal toxicity.
LD50 values of < 200 mg/kg in male rabbits and 340-780
mg/kg in females have been reported.
In spite of variations in LD50 values in the differ-
ent studies, these data suggest that HEX is moderately
toxic when administered orally. The acute toxicity of HEX
by the dermal route is quite similar to its acute oral
toxicity. HEX is much more toxic by the inhalation route
of exposure than by the dermal or oral routes.
8.1.2. Eye and skin irritation
IRDC (1972) tested HEX for eye irritation by instil-
ling 0.1 ml HEX into the eyes of New Zealand white rabbits
for 5 min or 24 h before washing. All the rabbits died on
or before the ninth day of the observation period. Treon
et al. (1955) reported HEX to be a primary skin irritant
in rabbits (strain unspecified) at a dose level of 250
mg/kg, and IRDC (1972) reported HEX to be a dermal irri-
tant because of the oedema that appeared after application
of 0.5 ml HEX. In this study, intense discoloration of
the skin was noted. In the study of Treon et al. (1955),
monkeys (strain unspecified) were also tested, and dis-
coloration of the skin was noted even at low doses (0.05
ml of 10% HEX) (Table 15).
Table 14. Acute toxicity studies of HEX
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Oral LD50
Rat (unspecified) oral gavage M: 510 mg/kg Treon et al.
young adult (5% solution F: 690 mg/kg (1955)
of peanut oil)
Rat (Charles River CD) oral gavage M + F: 926 IDRC (1968)
young adult (corn oil) mg/kg
Rat (Sprague-Dawley) M + F: 651 Dorough (1979)
young adult mg/kg
Rat (Fischer-344) oral gavage M: 425 mg/kg SRI (1980a)
weanling (corn oil) F: 315 mg/kg
Mouse (unspecified) M + F: 600 Dorough (1979)
mg/kg
Mouse (B6C3F1) oral gavage M + F: 680 SRI (1980a)
weanling (corn oil) mg/kg
Rabbit (Albino, strain oral gavage F: 640 mg/kg Treon et al.
unspecified) adult (5% peanut oil) (1955)
Dermal LD50
Rabbit (unspecified) skin painted F: 780 mg/kg Treon et al.
adult (1955)
Rabbit (unspecified) skin painted M: < 200 mg/kg IDRC (1972)
adult F: 340 mg/kg
Inhalation LC50
Rat (Carworth) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 35.0 (1955)
mg/m3 (3.1 ppm)
Rat (Sprague-Dawley) inhalation 4.0-h LC50 Rand et al.
young adult M: 18.1 mg/m3 (1982a)
(1.6 ppm)
F: 39.6 mg/m3
(3.5 ppm)
Mouse (unspecified) inhalation 3.5-h LC50 Treon et al.
young adult M + F: 23.7 (1955)
mg/m3 (2.1 ppm)
----------------------------------------------------------------------
Table 14 (contd.)
----------------------------------------------------------------------
Species (strain) age Route (vehicle) Resultsa Reference
----------------------------------------------------------------------
Rabbit (unspecified) inhalation 3.5-h LC50 Treon et al.
adult F: 58.8 mg/m3 (1955)
(5.2 ppm)
Guinea-pig inhalation 3.5-h LC50 Treon et al.
(unspecified) adult M + F: 80.2 (1955)
mg/m3 (7.1 ppm)
----------------------------------------------------------------------
a M = male; F = female.
Table 15. Primary eye and dermal irritation
--------------------------------------------------------------------------------
Study Species (strain) Results Reference
age
--------------------------------------------------------------------------------
Primary eye Rabbit (New Zealand Severe eye irritant (0.1 IRDC (1972)
irritation white) adult ml for 5 min or 24 h); all
died by day 9 of study
Primary dermal Rabbit (unspecified) Moderate skin irritant Treon et al.
irritation adult (250 mg/kg); one (1955)
application
Primary dermal Rabbit (New Zealand Severe skin irritant IRDC (1972)
irritation white) adult (200 mg/kg); all males died
Primary dermal Monkey (unspecified) Mild skin discoloration Treon et al.
irritation adult (0.05 ml of 10% HEX solution) (1955)
--------------------------------------------------------------------------------
Shell Research Limited conducted a study of the skin-
sensitizing potential of 98.8% pure HEX (Shell Research
Limited, 1982). Guinea-pigs (310-370g) were placed at
random into single sex groups of 10 animals and housed in
groups of five animals. Range-finding tests were conducted
to determine the concentrations of test material to be
used for intradermal induction, topical induction, and
topical challenge. Two male and two female guinea-pigs
were injected intradermally on each side of the midline
with 0.1 ml of several dilutions (0.5, 1, 5, and 10 mg HEX
per ml HEX corn oil). Filter paper patches with 0.3 ml of
a 1% or 2% dilution of test material in corn oil were
applied to two other groups. On the basis of the results
of the range-finding studies, the following tests and HEX
concentrations were selected for use: intradermal induc-
tion, 0.5 mg/litre; topical induction, 20.5 mg/litre; and
topical challenge, 10 mg/ml (all in corn oil). In the
sensitizing test, all four animals given an intradermal
injection of 0.5 mg HEX/ml suffered necrosis, while top-
ical applications at 10 or 20.5 mg/litre produced slight
redness or no difference from surrounding skin. However,
all 20 test animals showed positive responses 24 and 48 h
after removal of the challenge patches. The researchers
concluded that HEX is a skin sensitizer.
8.2. Short-term exposure
8.2.1. Oral
In a range-finding study using groups of five male and
five female Fischer-344 rats, there were no deaths when 12
doses of 25, 50, or 100 mg/kg were given in 16 days (SRI,
1980b). With the same dosing schedule, one out of five
males and four out of five females died when the dose was
200 mg/kg, and five out of five males and four out of five
females died when the dose was 400 mg/kg. In the same
study, B6C3F1 mice died when given doses of 400 or 800
mg/kg, but not when given doses of 50, 100, or 200 mg/kg.
Both rats and mice exhibited pathological changes of the
stomach wall at all but the lowest dose level.
The short-term toxicity of HEX is summarized in Table
16. These short-term toxicity studies on B6C3F1 mice and
Fischer-344 rats were conducted by SRI (1981a,b) under
contract with the National Toxicology Program (NTP), and
the results were reported by Abdo et al. (1984). In the
mouse study (SRI, 1981a), HEX (94.3-97.4% pure) in corn
oil was administered by gavage at dose levels of 19, 38,
75, 150, and 300 mg/kg to 10 mice of each sex, 5 days/week
for 13 weeks (91 days). At the highest dose level (300
mg/kg), all 10 male mice died by day 8 and three females
died by day 14. In female mice, the liver was enlarged.
Toxic nephrosis in females at doses of 75 mg/kg or more
was characterized by distensions in the proximal convol-
uted tubules, with basophilia in the inner cortical zone
and cytoplasmic vacuolization. However, male mice admin-
istered 75 mg/kg or more did not show these effects. Dose
levels of 38 mg/kg or more caused lesions in the fore-
stomach, including ulceration in both males and females,
as well as increased kidney and liver weights. The no-
observed-adverse-effect level (NOAEL) in mice was 19 mg/kg
and the lowest-observed-adverse-effect level (LOAEL) was
38 mg/kg.
In the rat study (SRI, 1981b), HEX in corn oil was
administered by gavage at dose levels of 10, 19, 38, 75,
and 150 mg/kg to groups of 10 male and female F-344 rats.
At 38 mg/kg or more, mortality and toxic nephrosis were
observed in both males and females. The male rats treated
with 19 mg/kg showed no significant effects, but female
rats had lesions in the forestomach. Similar lesions were
observed in males given 38 mg/kg or more. There was a
dose-related depression of body weight gain (relative to
the controls) and female rats had increased kidney and
liver weights. The NOAEL in rats was 10 mg/kg, and the
lowest-observed-effect level (LOEL) was 19 mg/kg.
A summary of the results of these two experiments
appears in Table 17.
Table 16. Short-term toxicity of HEX
------------------------------------------------------------------------------------------------------
Study Species Dose Results Effects at LOEL Reference
or lowest dose
------------------------------------------------------------------------------------------------------
90-day rat 10, 19, 38, 75, and NOAEL: 10 mg/kg lesions of forestomach SRI (1981b)
feeding 150 mg/kg (by gavage) LOEL: 19 mg/kg in female rats at
study 19 mg/kg
14-week rat 0.11, 0.56, and 0.226 NOEL: 0.226 mg/m3 no statistically Rand et al.
inhalation mg/m3 (5 days/week) LOEL: NE significant effects (1982a)
study
14-week monkey 0.11, 0.56, and 0.226 NOEL: 0.2 ppm no effects noted Alexander
inhalation mg/m3 (5 days/week) LOEL: NE et al.
study (1980)
------------------------------------------------------------------------------------------------------
NE = not established.
NOAEL = no-observed-adverse-effect level.
NOEL = no-observed-effect level.
LOEL = lowest-observed-effect level.
Table 17. Toxicological parameters for mice and rats administered technical grade HEX
in corn oil by gavage for 91 daysa
---------------------------------------------------------------------------------------
Species Sex Dose Mortality Relative Forestomach Forestomach Toxic
(strain) (mg/kg) weight inflammation hyperplasia nephrosis
gainb
---------------------------------------------------------------------------------------
Mice male 0 1/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 36% 0/10 0/10 0/10
38 0/10 + 9% 2/10 2/10 0/10
75 0/10 - 9% 7/10 8/10 0/10
150 0/10 - 45% 7/10 9/10 0/10
300 10/10 7/10 8/10 0/10
Mice female 0 0/10 0/10 0/10 0/10
(B6C3F1) 19 0/10 + 13% 0/10 0/10 0/10
38 0/10 - 13% 2/9 2/9 0/9
75 0/10 - 13% 6/10 9/10 10/10
150 0/10 - 25% 10/10 10/10 10/10
300 3/10 - 38% 7/9 9/9 7/10
Rats male 0 3/10 0/10 0/10 0/10
(F-344) 10 1/10 - 4% 0/10 0/10 0/10
19 1/10 - 8% 0/10 0/10 0/10
38 1/10 - 20% 4/10 5/10 10/10
75 3/10 - 49% 9/10 9/10 9/10
150 7/10 - 57% 8/9 8/9 8/10
Rats female 0 1/10 0% 0/10 0/10 0/10
(F-344) 10 2/10 + 4% 0/10 0/10 0/10
19 1/10 - 5% 2/10 2/10 0/10
38 1/10 - 2% 2/10 5/10 10/10
75 3/10 - 30% 9/10 9/10 10/10
150 5/10 - 33% 9/10 9/10 10/10
---------------------------------------------------------------------------------------
a From: SRI (1981a,b).
b Relative weight gain was calculated as follows:
Dose group value - control group value
-------------------------------------- x 100
Control group value
8.2.2. Short-term inhalation toxicity
Rand et al. (1982a) conducted a range-finding study in
which groups of 10 male and 10 female Sprague-Dawley rats
were exposed to atmospheres of 0.25, 1.24, or 5.65 mg
HEX/m3 (0.022, 0.11, or 0.5 ppm), 6 h/day, 5 days/week
for a total of 10 exposures. Nine male rats and one female
rat exposed to 5.65 mg/m3 were moribund after five to
seven exposures. These rats had dark red eyes, laboured
breathing, and pale extremities. There were no mortalities
in the other exposure groups. However, the males in the
medium- and high-dose groups lost weight during the study
and reduced mean liver weights and pathology were noted.
The NOAEL for HEX exposure in this study was 0.25 mg/m3
and the LOEL was 1.24 mg/m3.
Fourteen-week inhalation studies have been carried out
on rats and monkeys (Alexander et al., 1980; Rand et al.,
1982a,b) and the results are summarized in Table 16.
Groups of 40 male and 40 female Sprague-Dawley rats
weighing 160-224 g and groups of 12 Cynomolgus monkeys
weighing 1.5-2.5 kg were exposed to HEX, 6 h/day, 5 days
per week, for 14 weeks. Levels of exposure were 0, 0.11,
0.56, and 2.26 mg/m3 (0, 0.01, 0.05, and 0.20 ppm). In
monkeys, there were no mortalities, adverse clinical
signs, weight gain changes, pulmonary function changes,
eye lesions, haematological changes, clinical chemistry
abnormalities, or histopathological abnormalities at any
dose level tested. Thus, the NOEL for monkeys was at least
2.26 mg/m3 for this exposure period, but the LOEL was
not established. Male rats had a transient appearance of
dark-red eyes at 0.56 and 2.26 mg/m3. At 12 weeks, there
were marginal but not statistically significant increases
in haemoglobin concentration and erythrocyte count in
males exposed to 0.11 mg/m3, females exposed to 0.56
mg/m3, and males and females exposed to 2.26 mg/m3.
There were small but not statistically significant changes
in mean liver weight of all groups of treated rats and
similar changes in the kidneys of all treated males. No
treatment-related abnormalities in gross pathology or
histopathology were observed. On this basis, the NOEL in
rats was 2.26 mg/m3, but the LOEL was not established.
In a further study by Rand et al. (1982b), no ultra-
structural changes were observed in monkeys that could be
attributed to the inhalation of HEX vapour. Exposure was
identical to that of the previous study (Rand et al.,
1982a). There was a statistically significant (P < 0.01)
increase in the mean number of electronlucent inclusions
in the apex and base of the Clara cells in exposed ani-
mals, as compared with the controls. According to some
researchers (Evans et al., 1978), Clara cells respond to
injury by regression to a more primitive cell type. Rand
et al. (1982b) noted that some of the ultrastructural
changes in the exposed animals resembled those of the
Evans study. It is not known what effect these changes
might cause. The Clara cell contributes important
materials to the extracellular lining of the peripheral
airways, and if this effect of HEX vapour causes a change
in the content of the contributed material, then the
extracellular lining may be altered and breathing may be
subsequently impaired (Rand et al., 1982b). This obser-
vation of lung effects coincides with those of other
researchers (Dorough, 1979, 1980; Lawrence & Dorough,
1981, 1982). Furthermore, in inhalation studies with HEX
occasional statistically significant increases in the
haemoglobin level and red blood cell counts of rats have
been noted, which may be manifestations of the impairment
of respiratory functions.
In 1984, the US National Toxicology Program (NTP)
completed a short-term, 90-day HEX inhalation study on
B6C3F1 mice and F-344 rats (NTP, 1984a,b). In the basic
study, ten rats and ten mice of each sex were placed at
random into five exposure and control groups. The rats and
mice were exposed to nominal concentrations of 0.45, 1.70,
4.52, 11.3, or 22.6 mg/m3 (0.04, 0.15, 0.4, 1.0, or 2.0
ppm) for 6 h/day, 5 days/week for 13 weeks. In the female
mouse study, 6 out of 10 of the control animals died,
while none of the animals in the male control population
died. The six animal deaths in week 7 were attributed to
a defective feeder insert. Mortality in both the rats and
mice was high in the two highest-dose groups; all rats and
mice in these groups died in the first five weeks of the
study. Posterior paresis and listlessness were observed
in mice at 4.52 and 11.3 mg/m3. Compound-related histo-
pathological alterations were observed in the respiratory
tracts of male and female mice exposed to 1.7 mg/m3 or
more. These changes included: necrosis, acute and
chronic inflammation, hyperplasia or squamous metaplasia
of the nasal, laryngeal, tracheal, bronchial, and bronchi-
olar epithelia of the affected animals. No compound-
related effects were observed in mice at the lowest dose,
and so this level could be considered the NOEL (NTP,
1984a).
Clinical signs resulting from HEX exposure included
posterior paresis in all rats exposed to 1.7 mg/m3 or
more, listlessness in all rats exposed to 4.52 mg/m3 or
more, and eye irritation and respiratory distress in all
rats exposed to 11.3 mg/m3 or more. As in the case of
mice, HEX caused significant histological alterations in
the respiratory tracts of rats at the two highest doses.
Changes of a less-marked degree were observed in the
respiratory tracts of rats receiving 4.52 mg/m3. No
compound-related changes were seen in any organ of male
and female rats exposed to the lowest dose, and so this
level could be considered the NOEL. In addition, compound-
related effects of a secondary stress-related nature were
seen in a number of other organs of rats of both sexes at
the two highest doses. These includes lymphoid depletion
of the spleen and thymus, degeneration of the seminiferous
tubules and decreased lytoplasmic vacuolization of the
adrenal cortex.
Some basic clinical pathology and histopathology
examinations were undertaken simultaneously in both
studies (NTP, 1984a,b). All effects were similar to those
noted in the previous toxicity studies. In the rat study
(NTP, 1984b), HEX nephrotoxicity was examined in more
depth. HEX was not found to be nephrotoxic at these ex-
posure levels, nor did it appear that it was myelotoxic.
8.2.3. Short-term dermal toxicity
No short-term dermal toxicity studies are available.
8.3. Long-term exposure
8.3.1. Long-term oral toxicity
No long-term oral toxicity studies have been reported.
8.3.2. Long-term inhalation toxicity
In view of the absence of long-term studies on the
inhalation of HEX, the following studies were examined.
Treon et al. (1955) exposed guinea-pigs, rabbits, rats,
and mice to a HEX (89.5% pure) concentration of 3.73
mg/m3 (0.33 ppm), 7 h/day, 5 days/week, for 25-30
exposure days. The guinea-pigs survived 30 exposures,
whereas rats and mice did not survive five exposures, and
four out of six rabbits did not survive 25 exposures.
Using a lower concentration of 1.70 mg/m3 (0.15 ppm),
guinea-pigs, rabbits, and rats survived 150 7-h exposures
over a 7-month period. This level was too high to conduct
a long-term study in mice since four out of five animals
did not survive. The rats, guinea-pigs, and rabbits toler-
ated 1.7 mg/m3 and did not exhibit any treatment-related
effects. Thus, the NOEL for rats, guinea-pigs, and rabbits
was 1.7 mg/m3 over the 7-month period. Due to the high
mortality, a NOEL for mice could not be established.
A long-term (30 weeks) inhalation study in rats with
technical grade HEX (96% pure with hexachlorobuta-1,3-
diene and octachlorocyclopentene as impurities) was con-
ducted by the Shell Toxicology Laboratory (D.G. Clark et
al., 1982). Four groups of eight male and eight female
Wistar albino rats were exposed to HEX at nominal concen-
trations of 0, 0.56, 1.13, and 5.65 mg/m3 (0, 0.05, 0.1,
and 0.5 ppm), 6 h/day, 5 days/week, for 30 weeks and were
observed for a 14-week recovery period without HEX
exposure. At the highest dose level, four males and two
females died. In males, there was depressed body weight
gain at the highest dose, relative to controls, beginning
at 7 weeks of exposure and persisting throughout the
remainder of the study. Females in the two highest-dose
groups had lower body weights at the end of the recovery
period than did the controls. At the highest dose, there
were pulmonary degenerative changes, ranging from epi-
thelial hyperplasia and oedema to epithelial ulceration
and necrosis, in both sexes, the males being affected more
severely. There were also mild degenerative changes in
the liver (bile duct hyperplasia and inflammatory cell
infiltration) and kidneys (protein casts in tubules and
pigmented cortical tubules) in a few rats, and kidney
weights were significantly increased in the females at 30
weeks. After 30 weeks of study, no biologically signifi-
cant toxicity was noted in animals that were exposed to
0.56 or 1.13 mg/m3. Thus, the NOEL in rats exposed to
HEX vapour in this study (6 h/day, 5 days/week, for 30
weeks) was 0.56 mg/m3, and the LOEL was 1.13 mg/m3.
A long-term inhalation study by the National Toxi-
cology Program started in January 1986. The animal
exposure has been completed and results will be published
as soon as the pathology review is completed.
8.3.3. Long-term dermal toxicity
No long-term dermal toxicity studies have been
reported.
8.3.4. Principal effects and target organs
Repeated exposure of several animal species to levels
of HEX vapour in the range of 1.13 to 2.26 mg/m3 (0.1-0.2
ppm) has been found to cause pulmonary degenerative
changes (Treon et al., 1955; D.G. Clark et al., 1982; Rand
et al., 1982a,b). In addition, Treon et al. (1955)
reported diffuse degeneration of the brain, heart, and
adrenal glands and necrosis of the liver and kidney
tubules, together with severe pulmonary hyperaemia and
oedema. In many instances, acute bronchitis and intersti-
tial pneumonitis also occurred. Necrosis of the epithelium
of the primary, secondary, and tertiary bronchi was
observed. At later stages, reacting inflammatory cells
migrated into the wall and the mucosa of the bronchi and
alveoli. In rabbits, the walls of the alveoli were covered
by a hyaline or fibrinoid membrane. Possibly, some of the
changes found by Treon et al. (1955) were caused by
impurities in the HEX preparation. Acute exposure by the
oral and dermal routes also affects the respiratory system
(Kommineni, 1978; SRI, 1980a). Death from acute exposure
by any tested route seemed to be associated with respir-
atory failure (Lawrence & Dorough, 1982).
There are insufficient data to identify the site that
is the most sensitive to prolonged, repeated exposure to
HEX. In comparing routes of administration, researchers
found that damage to the lungs occurred regardless of
which route was used (Lawrence & Dorough, 1982). When HEX
is administered orally to animals, the kidneys may be the
most sensitive site, since short-term dosing of rats and
mice was found to cause nephrosis, especially in females
(SRI, 1981a,b). Although the oral route may not be a
significant route of exposure for human beings, the fact
that the kidneys are a possible target organ in short-term
exposure indicates that low-level, prolonged systemic
exposure from any ambient route may affect the kidneys.
The liver has also been shown, in several of the labora-
tory studies, to be affected by HEX.
The available literature does not cite any single
mechanism to explain HEX toxicity. HEX vapour irritates
the respiratory tract, leading to death by respiratory
failure after bronchopneumonia (D.G. Clark et al., 1982).
The degenerative changes that have been observed in the
liver and kidneys are mild and unlikely to contribute to
the chemical's lethality (NAS, 1978; SRI, 1980a,b).
The difficulty in studying HEX because of its high
reactivity and volatility has also created problems in
identifying its metabolites. Several questions remain as
to whether the same metabolites are formed after various
routes of exposure and whether it is the administered HEX
or its metabolites that cause the lung injuries seen with
various dosing regimens. Furthermore, the strong ability
of HEX to interact with other compounds, especially
organic molecules, can lead to many other effects, such as
haemoglobin binding. Little is known about the interac-
tions of HEX with other chemicals in animal or human
tissue.
8.4. Developmental and reproductive toxicity
The teratogenic potential of HEX has been evaluated in
pregnant Charles River CD-1 rats that were administered
HEX (98.25%) in corn oil, by gastric intubation, at dose
levels of 3, 10, and 30 mg/kg per day from days 6 to 15 of
gestation. A control group received the vehicle (corn oil)
at a dose volume of 10 ml/kg per day. All the rats sur-
vived, and there was no difference in mean maternal body
weight gain between the dosed groups and controls. There
were no differences in the mean number of implantations,
corpora lutea, live fetuses, mean fetal body weights, or
male/female sex ratios among any of the groups, and there
were no statistical differences in malformation or devel-
opmental variations, compared with the controls, when
external, soft tissue, and skeletal examinations were
performed (IRDC, 1978).
Murray et al. (1980) evaluated the teratogenic poten-
tial of HEX (98%) in CF-1 mice and New Zealand white
rabbits. Mice were dosed at 0, 5, 25, or 75 mg HEX/kg per
day by gavage during days 6-15 of gestation, while rabbits
received the same dose during days 6-18 of gestation. The
fertility of the treated mice and rabbits was not signifi-
cantly different from that of the control groups. In the
mice, there was no evidence of maternal toxicity, embryo-
toxicity, or teratogenic effects. A total of 249-374
fetuses (22-33 litters) was examined in each dose group.
In rabbits, maternal toxicity was noted at a dose level of
75 mg/kg (diarrhoea, weight loss, and mortality), but
there was no evidence of maternal toxicity at the lower
levels. There were no embryotoxic effects at any dose
level. Although there was a two-fold increase over con-
trols in the proportion of fetuses with 13 ribs at 75
mg/kg, this was considered to be a minor skeletal vari-
ation. The authors concluded that HEX was not teratogenic
at the levels tested (Murray et al., 1980).
Chernoff & Kavlock (1982) tested 28 compounds, includ-
ing HEX, by an in vivo screening procedure. According to
the researchers, the underlying hypothesis was that most
prenatal insults would manifest themselves postnatally as
reduced viability and/or impaired growth. Twenty-five
Oravid CD-1 mice were administered HEX orally at or near
the maternal minimal tolerated dose (MTD). The MTD was
considered to be that dose resulting in either significant
weight reduction during the treatment period, mortality,
or other signs of toxicity. There were no differences in
maternal weight gain, number of live offspring or average
weight between the HEX-treated animals and controls when
HEX was administered orally for 8-12 days at 45 mg/kg.
Gray & Kavlock (1984) extended the observation period
proposed by Chernoff & Kavlock (1982, 1983) to 250 days to
determine whether neonatal weight reductions persisted
throughout life and whether other serious abnormalities or
mortality resulted from exposure to HEX. CD-1 pregnant
mice were orally exposed to HEX (45 mg/kg) on days 8 to 12
of gestation, which is within the period of major embryo-
nic organogenesis. Females were weighed throughout dosing
and on day 19 of gestation. They were allowed to deliver
and the litters were counted and weighed at 1 and 3 days
of age. The animals were observed at approximately 250
days of age. During the postmortem examination of males,
body weight and the weights of the liver, testes, seminal
vesicles, and right kidney were recorded. HEX did not
produce any statistically significant developmental
effects in this study.
Studies on the teratogenic potential of inhaled HEX
were not found in the review of the scientific literature.
8.5. Mutagenicity
Goggelman et al. (1978) found that HEX was not muta-
genic, either before or after liver microsomal activation,
at 2.7 mmol/litre in an Escherichia coli K12 back-
mutation system. In this test there was a 70% survival of
bacteria at 72 h. HEX was not tested at higher concen-
trations because it was cytotoxic to E. coli. A previous
report from the same laboratory (Greim et al., 1977) indi-
cated that HEX was also non-mutagenic in Salmonella
typhimurium strains TA1535 (base-pair mutant) and TA1538
(frame-shift mutant) after liver microsomal activation.
However, no details of the concentrations tested were
given. Although tetrachlorocyclopentadiene is mutagenic
in these systems, probably through metabolic conversion to
the dienone, it appears that the chlorine atoms at the C-1
position of HEX hinder metabolic oxidation to the corre-
sponding acylating dienone (Greim et al., 1977).
A study conducted by the Industrial Bio-Test Labora-
tories (IBT, 1977) also suggested that HEX is not muta-
genic in S. typhimurium. Both liquid HEX and its vapour
were tested with and without metabolic activation. The
vapour test was carried out in desiccators with the TA100
strain of S. typhimurium only. It is not clear from
vapour test data that sufficient amounts of HEX or
adequate exposure times (30, 60, and 120 min) were used.
Longer exposures in the presence of HEX vapour may be
necessary for a potential mutagenic effect to be seen.
At concentrations of up to 1.25 mg/litre in the
presence of an S-9 liver activating system, HEX was not
mutagenic in the mouse lymphoma mutation assay. Mutageni-
city could not be evaluated at higher concentrations
because of the cytotoxicity of HEX (Litton Bionetics,
Inc., 1978a). This assay uses L5178Y cells that are het-
erozygous for thymidine kinase (TK+/-) and are sensitive.
The mutation is scored by cloning with bromodeoxyuridine
in the absence of thymidine. HEX is highly toxic to these
cells, particularly in the absence of an activating system
(at 0.04 ml/litre), and the positive control, dimethyl-
nitrosamine, was mutagenic at 0.5 ml/litre.
Williams (1978) found that HEX (10-6 mol/litre) was
inactive in the liver epithelial culture (hypoxanthine-
guanine-phosphoribosyl transferase locus) mutation assay.
At 10-5 mol/litre it also failed to stimulate DNA repair
in hepatocyte primary cultures. Negative results were also
obtained in an additional unscheduled DNA synthesis assay
(Brat, 1983).
One study provided by the US National Toxicology
Program (NTP) (Haworth et al., 1983) demonstrated a lack
of mutagenicity of HEX (98% pure). In S. typhimurium
strains TA98, TA100, TA1535, and TA1537, levels of up to
3.3 µg/plate were not mutagenic without activation, and
levels of up to 100 µg/plate were not mutagenic after
microsomal activation. Higher levels could not be tested
because of excessive cell dealth. Zimmering et al. (1985)
tested Drosophila by the sex-linked recessive lethal test
(SLRL), either by feeding doses of 40 ppm for 3 days or by
giving a single injection of 2000 ppm or 3000 ppm (volume
not specified). The vehicle used was 10% ethyl alcohol,
which did not totally dissolve the HEX. HEX (98%) was
first assayed in the SLRL test in adult feeding exper-
iments. When negative results were obtained, the chemical
was retested by injecting < 1 day-old Canton-S wild-type
Drosphila males. The results of the injection test were
reported to be inconclusive.
HEX has also been assayed in the mouse dominant lethal
test (Litton Bionetics, Inc., 1978b). In this assay, 0.1,
0.3, or 1.0 mg HEX/kg was administered by gavage to 10
male CD-1 mice for 5 days and the mice were then mated
throughout spermatogenesis (7 weeks). This test determines
whether the compound induces lethal genetic damage to the
germ cells of males. There was no evidence of dominant
lethal activity, at any dose level, based on any parameter
(e.g., fertility index, implantations per pregnancy,
average resorptions per pregnancy).
8.6. Cell transformation
The ability of HEX to induce morphological transfor-
mation of BALB/3T3 cells in vitro has been studied by
Litton Bionetics, Inc. (1977).
The selection of test doses was based on previous
cytotoxicity tests using a wide range of HEX concen-
trations at 0.0, 0.01, 0.02, 0.039, 0.078, and 0.156 mg
per litre. The cultures were exposed for 48 h, which was
followed by an incubation period of 3-4 weeks. The
cultures were observed daily. The doses selected allowed a
cell survival of 80-100% compared with controls (solvent
only). This high survival rate permitted an evaluation of
in vitro malignant transformation in cultures treated
with HEX as compared with the solvent controls. 3-Methyl-
cholanthrene at a dose level of 3 mg/litre was used as a
positive control. Results indicated that HEX was not
responsible for cell transformation.
8.7. Carcinogenicity
Bioassays of HEX for possible carcinogenicity have not
been conducted. As noted previously (section 8.3.2), the
NTP has completed a study on HEX for carcinogenicity by
the inhalation route in rats and mice, but the results
were not available when this monograph was being prepared.
9. EFFECTS ON HUMANS
9.1. General population exposure
There is very little detailed information available on
the human health effects of HEX exposure. Acute human
exposure has been reported in homes near waste sites where
disposal of HEX has occurred (C.S. Clark et al., 1982;
Elia et al., 1983). The odour threshold has been stated
to be 1.92 µg/m3 (0.17 ppb), but there appears to be
great individual variation. According to a study completed
by the A.D. Little Co. for the Occidental Chemical
Corporation, the 100% panel recognition concentration was
1.92 µg/m3 (0.17 ppb v/v)a, but the study design and
methodology were not reported. The US EPA (1982) estimated
that exposure of the general population to HEX in air
and/or water would be extremely low.
Treon et al. (1955) reported that members of a
research group conducting toxicity tests developed head-
aches when they were accidentally exposed to unknown
concentrations of HEX. The HEX escaped into the room when
an aerated exposure chamber was opened.
In a 48-block area surrounding a contaminated sewer
line in Kentucky, USA, questionnaires were sent to a
selected sample of residents. A total of 212 occupants
were surveyed. Only 3.8% of the residents reported an
unusual odour. The most common symptoms were stomach aches
(5.2%), burning or watery eyes (4.7%), and headaches
(4.7%). There was no association between the frequency of
symptoms and the distance of houses from the contaminated
sewer line. No significant ambient air concentrations of
HEX were found in these areas (Kominsky et al., 1978).
9.2. Occupational exposure
The US National Institute for Occupational Safety and
Health (NIOSH, 1980) stated that 1427 workers were occu-
pationally exposed to HEX. Officials from the Velsicol
Chemical Corporation estimated that 157 employees had been
potentially exposed to HEX in their production and pro-
cessing facilities.
A well-documented incident of acute human exposure to
HEX occurred in March 1977 at the Morris Forman Wastewater
Treatment Plant in Louisville, Kentucky, USA (Wilson et
al., 1978; Morse et al., 1979; Kominsky et al., 1980). The
details of the incident are included in the original NIOSH
Hazard Evaluation and Technical Assistance Report Number
TA-77-39 (Kominsky et al., 1978), which is available from
the US National Technical Information Service (NTIS). This
--------------------------------------------------------
a Memorandum from G. Leonardos to P. Levins on Hooker
special priority samples (odour properties).
treatment facility was contaminated with approximately 6
tonnes of HEX and octachlorocyclopentene, a waste product
of HEX manufacture (Morse et al., 1979). The contamination
was traced to an illegal dumping in one large sewer line
that passed through several populated areas. Concen-
trations of HEX detected in the sewage at the plant were
as high as 1000 mg/litre, and levels in the sewer line
were up to 100 mg/litre. Air samples taken from the sewer
line showed HEX concentrations to be as high as 4.5
mg/m3 (400 ppb). Although the airborne concentrations of
HEX at the time of the exposure in the treatment facility
were not known, airborne concentrations in the primary
treatment areas (screen and grit chambers) ranged between
3.05 and 10.96 mg/m3 (270 and 970 ppb) 4 days after the
plant had closed. It should be noted that the ACGIH
8 h-TWA for HEX was 0.1 mg/m3 (10 ppb) in 1977. Workers
tried to remove an odoriferous and sticky substance from
the bar screens and grit collection system by using steam
during clean-up of the contamination. This procedure pro-
duced a blue haze which permeated the primary treatment
area. The airborne HEX concentration of the blue haze was
reported to be 217 µg/m3 (19.2 ppm) (Kominsky et al., 1980).
The US Centers for Disease Control (CDC) and NIOSH
sent representatives to the plant with questionnaires
about the type and duration of symptoms (Morse et al.,
1979; Kominsky et al., 1980). In all, 193 employees were
identified as having been potentially exposed for 2 or
more days during the 2 weeks before the plant was closed
(Morse et al., 1979). The questionnaire was sent to each
of these 193 workers and 145 (75%) responded. Workers with
complaints of mucous membrane irritation were given a
physical examination, and blood and urine samples were
collected for clinical screening by an independent labora-
tory. Data were also collected on the exposure levels and
symptoms experienced by several people who had been
acutely exposed to the chemical vapours.
The results of the CDC and NIOSH questionnaires showed
that the odour of HEX had been detected by 94% of the
workers before the onset of symptoms. The most common
symptoms reported were eye irritation (59%), headaches
(45%), and throat irritation (27%) (Table 18). Of the 41
plant workers examined, six had physical signs of eye
irritation (i.e. lacrimation or redness) and five had
signs of skin irritation. Laboratory analyses of blood
and urine specimens from these workers showed marginal
increases in lactic dehydrogenase activity in 27% of cases
and proteinuria in 15%. Three weeks later, no abnormali-
ties were detected in the blood and urine tests. After six
weeks, some of the clinical symptoms persisted in 25-45%
of the employees (Morse et al., 1978).
Table 18. Symptoms of 145 waste-water treatment
plant employees exposed to HEX (Louisville,
Kentucky, USA, March 1977)a
----------------------------------------------------
Symptom No. of Percent of
employees employees
with symptoms with symptoms
----------------------------------------------------
Eye irritation 86 59
Headache 65 45
Throat irritation 39 27
Nausea 31 21
Skin irritation 29 20
Cough 28 19
Chest pain 28 19
Difficult breathing 23 16
Nervousness 21 14
Abdominal cramps 17 12
Decreased appetite 13 9
Decreased memory 6 4
Increased saliva 6 4
----------------------------------------------------
a From: Morse et al. (1978).
Although there was difficulty in measuring the level
of exposure received by the plant workers, more than 50%
of the clean-up crew were monitored. Laboratory tests
showed no significant abnormalities in renal function
tests, complete blood counts, or urinalysis, but several
minimal or mild abnormalities were found in liver function
tests (Kominsky et al., 1980). The abnormalities in 18 out
of 97 clean-up workers are listed in Table 19. These
people also had physical signs of mucous membrane irri-
tation. A more detailed correlation between acute exposure
level data and symptomatology was reported for nine adults
(Kominsky et al., 1980). The data appear in Table 20. The
exposure levels could not be estimated accurately because
of prior exposure or because the worker had used protec-
tive equipment.
Table 19. Abnormalities detected in clean-up
workers at the Morris Forman treatment plant,
Louisville, Kentucky, USAa
-------------------------------------------------
Laboratory test Normal range Abnormal results
Range No.b
-------------------------------------------------
Serum glutamate
oxaloacetate 7-40 mU/ml 40-49 5
transaminase 50-59 1
60-69 4
70-79 0
80-89 1
90-99 1
Serum alkaline 30-100 mU/ml 100-109 3
phosphatase 110-119 1
120-129 1
Serum total 0.15-10 mg/dl 1.0-1.9 1c
bilirubin
Serum lactate 100-225 mU/ml 230-239 1
dehydrogenase
-------------------------------------------------
a From: Kominsky et al. (1980).
b For individuals with more than one serial
blood test, only the most abnormal result is
tabulated.
c Associated with a serum glutamate oxaloacetate
transaminase activity of 66 mU/ml.
Table 20. Individual exposure symptomatology correlations at the Morris Forman treatment plant (Louisville, Kentucky, USA)a
-------------------------------------------------------------------------------------------------------------------------------------
No. of Estimated airborne Immediate symptoms Persistence of symptoms Laboratory
workers exposureb results
-------------------------------------------------------------------------------------------------------------------------------------
1 19.2 ppm HEX and 650 ppb lacrimation; skin irritation on face 1.5 h post-exposure: fatigue; normal results
OCCP for several seconds and neck; dyspnoea and chest erythema of exposed skin; eye 4 days
(no protective equipment) discomfort;nausea (several min later) irritation subsided in 1 day; post-exposurec
chest discomfort persisted
several days
3 7083 ppb HEX and 446 ppb lacrimation; irritation of exposed asymptomatic at 2 h, except normal results
OCCP for several seconds skin for soreness around eyes 7 days post-
(half-face respirator) exposure in
one workerc
2 40-52 ppb HEX and slight eye irritation no symptoms after cessation of normal results
9-21 ppb OCCP exposure 7 days post-
(half-face respirator) exposure in one
workerc
2 exact exposure unknown slight skin irritation faces felt "puffy" and "windburned" none available
(half-face respirator) for 1-2 days after exposure; this
was noted also by friends and
family; no residual skin lesions.
1 980 ppb HEX for 15 min; irritated eyes; nasal irritation eyes felt "dry and irritated" for none available
OCCP not measured and sinus congestion after 2 weeks 2-3 days after exposure; nasal
(no protective equipment) of intermittent exposures irritation ceased within 1-2 days
of cessation of exposure.
-------------------------------------------------------------------------------------------------------------------------------------
a From: Kominsky et al. (1980).
b OCCP = Octachlorocyclopentene.
c Laboratory work was same as carried out on clean-up crew.
Hazards to workers in treatment plants can result from
chemical compounds contained in the industrial waste
treated in municipal waste-water plants. The HEX-contain-
ing wastes from a pesticide manufacturer were treated in a
municipal waste-water treatment plant at Memphis,
Tennessee, USA. In 1978, the workers at this plant
reported symptoms similar to those reported by workers in
the Louisville plant referred to above. The air and waste-
water were monitored, and analyses of urine and blood,
liver function tests, and illness symptom questionnaires
were completed. Workers from another waste treatment
plant, where no pesticide wastes had been received, were
used as a control group. No statistical differences in
urine HEX concentrations or liver function tests between
the exposed and control groups were found, although
differences in levels of HEX-related compounds (co-
contaminants) were detected (Elia et al., 1983).
9.3. Epidemiological studies
Mortality studies have been carried out on workers
involved in the production of HEX or formulation of HEX
products. Shindell et al. (1980) studied a cohort of 783
current and former workers who had been employed at the
Velsicol Chemical Corporation plant at Marshall, Illinois,
between 1946 and 1979. The purpose of the study was to
evaluate the overall health status of all employees who
had been present during the manufacture of chlordane for 3
months or more. There were no significant differences in
mortality rates between these employees and the overall
USA population. The observed value for deaths from all
causes, including heart disease and cancer, was less than
the expected value in the overall USA population.
Shindell et al. (1981) completed another epidemiologi-
cal study for the Velsicol Chemical Corporation at its
Memphis, Tennessee, plant covering the period 1952-1979.
This coincided with the manufacture of heptachlor, a
pesticide made from HEX. The reseachers studied 1115
current and former employees who had worked for 3 months
or more. Again, there was no significant difference in
mortality between the control and exposure groups.
Concomitant with the study performed by Shindell et
al. (1980), Wang & MacMahon (1979) conducted a retrospec-
tive mortality study of workers employed at the Marshall
and Memphis plants, where chlordane and heptachlor were
manufactured between 1946 and 1976. They studied 1403
males who had worked at the plants for more than 3 months.
There were 113 observed deaths compared with 157 expected
deaths, giving a standardized mortality ratio (SMR) of 72.
Among the various causes of death, the two highest SMRs
were 134 for lung cancer and 183 for cerebrovascular
disease, but only the latter figure was statistically sig-
nificant (P < 0.05). The excess mortality due to cerebro-
vascular disease was not related to the duration of
exposure or to the latency period, and occurred only after
termination of employment. Shindell & Ulrich (1986)
updated their 1980 data set with additional worker data.
There was no information specific to HEX exposure.
Buncher et al. (1980) studied the mortality of workers
at a chemical plant that produced HEX. They examined 341
workers (287 male and 54 female), together with their
health records, who had worked at the plant for at least
90 days between 1 October 1953 and 31 December 1974. Their
vital status was determined through 1978. The expected
numbers of deaths, based on the USA population and
specific for sex, age, and calendar year, were calculated.
The SMR for all causes of death was 69. Deaths caused by
specific cancers, all cancers, and diseases of the circu-
latory and digestive systems were also fewer than
expected. The authors noted that the time that had elapsed
since the initial exposure (25 years at most) reduced the
power of the study to detect cancers that may have a
10-40 year latent period.
Similar studies have been performed on a cohort of
workers in the Shell International Petroleum plants in the
Netherlands. These reports have been reviewed extensively
in Environmental Health Criteria 91: Aldrin and Dieldrin
(WHO, 1989).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
The general population is not at risk from exposure to
HEX. However, people living near HEX processing and pro-
duction facilities, as well as handlers of the chemical
and its waste, are at risk. Exposure to HEX can occur
through several different routes. However, HEX is much
more toxic when inhaled than when ingested or following
dermal contact. Skin exposure studies have shown that HEX
can cause irritation, together with visceral changes that
are similar to those that result from oral administration.
Inhalation studies in animals have shown that HEX vapour
is very irritating, repeated exposure to 1.13-2.26 mg/m3
(0.1-0.2 ppm) causing pulmonary pathological changes.
Acute toxic symptoms, including headaches, nausea, dizzi-
ness, and respiratory distress, have been reported. Based
on a 90-day inhalation study in mice and rats, a NOEL of
0.45 mg/m3 (0.04 ppm) has been estimated. No information
is available on the long-term effects of a single exposure
or of continuous exposure to HEX.
A carcinogenic study of HEX has been completed (but
not evaluated) by the US National Toxicology Program. In
vitro mutagenicity and cell transformation tests yielded
negative results, as did an in vivo mouse dominant lethal
assay at the levels tested. There was no evidence of
teratogenicity in oral exposure studies in which three
species were examined.
The toxic effects of HEX exposure on the human respir-
atory system are of major concern. Although the long-term
toxicity data are limited, systemic toxic effects of HEX
inhalation have been observed after short-term exposure,
suggesting that long-term inhalation exposure to low con-
centrations of HEX could cause adverse health effects.
Limited epidemiological studies of workers exposed to HEX
at levels above those known to induce adverse health
effects have been conducted, although concomitant exposure
to other chemicals was known to have occurred. These
workers complained of headaches, eye and skin irritation,
nausea, dizziness, and respiratory distress, as did
individuals living near areas where there were HEX
releases.
10.2. Evaluation of effects on the environment
Release of HEX into the environment can result from
the production, processing and use of HEX, disposal of
waste containing HEX or from products contaminated with
HEX. Only a small proportion of the total amount of HEX
released into the environment from production, processing,
and use can be expected to persist beyond a few days.
However, waste disposal has resulted in HEX persisting in
soil, sediment, and ground water. There is little monitor-
ing information on the levels of HEX in air, water, and
sediment. The exposure of organisms in the aquatic
environment to HEX is therefore difficult to quantify.
HEX may undergo photolysis, hydrolysis, and biodegra-
dation. In water, photolysis is the dominant process in
direct sunlight, hydrolysis being the next most important
degradation route. Volatilization to the atmosphere occurs
from both water and soil. Biodegradation occurs in soil
under both aerobic and anaerobic conditions and in sewage
sludge. In water and aquatic sediment, biodegradation is
initially limited. Information on the adaptation of micro-
organisms to degrade HEX is limited. HEX has a calculated
tropospheric residence time of approximately 5 h.
Laboratory studies suggest that HEX is relatively
immobile in soil, particularly in soil with a high organic
content. However, leaching and movement in ground water
has been reported in field studies.
HEX has been shown to be toxic to aquatic life at a
level of 1-100 µg/litre. However, little information has
been obtained under field conditions (long-term exposure
at low concentration in the presence of sediment). The
actual hazard to aquatic life is, therefore, difficult to
assess. HEX is toxic to aquatic microorganisms but less
toxic to soil microorganisms. Exposure of terrestrial
organisms, except at or near disposal sites, would be
expected to be low.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
* The general population is not at risk from exposure
to HEX, except in the case of people residing near
contaminated areas.
* The long-term human health effects of continuous low-
level exposure are not known. Handlers of the product
and its waste, as well as sewage workers, are at
risk.
* Results of laboratory studies indicate that HEX
should be degraded rapidly in the environment by
photolysis, hydrolysis, or biodegradation. The rela-
tive importance of these processes varies with the
medium. HEX is not a widespread environmental con-
taminant and the available data suggest that it is
only found associated with production, processing and
disposal sites. HEX does not bioaccumulate.
* Acute laboratory tests show that HEX is highly toxic
to aquatic microorganisms, invertebrates, and fish,
but less toxic to soil microorganisms. However,
information obtained under environmentally realistic
conditions is limited. The potential hazard to the
general environment is expected to be low.
11.2. Recommendations for protection of human health and
the environment
* Occupational exposure to HEX should be minimized by
the use of closed systems. Guidelines for the dis-
posal of HEX and HEX wastes should be followed.
* Environmental monitoring is needed to examine the
persistence and fate of HEX in all media near pro-
duction, processing and disposal sites, and also
hazardous waste incinerators. Monitoring data are
required for HEX in drinking-water, and in surface,
shower, and ground water.
12. FURTHER RESEARCH
* Biomarker technology should be developed to indicate
the possibility of past or current actions of HEX.
Such biomarkers could be stable metabolites derived
from HEX and its impurities that are present in the
original preparation.
* Research is needed on the metabolic, degradative, and
reactive products to understand the fate of HEX in
human beings and the environment.
* Further study of the apparent disparity between degra-
dation under laboratory conditions and that observed
in the environment is needed.
* The efficacy and safety of current disposal methods
should be evaluated and their present and future
health impacts assessed.
* Developmental and reproductive studies of HEX need to
be conducted, with emphasis on the inhalation route of
exposure.
* Methods for the early warning of the presence of low
levels of HEX should be developed.
REFERENCES
ABDO, K.M., MONTGOMERY, C.A., KLUWE, W.M., FARNELL, D.R., & PREJEAN, J.D.
(1984) Toxicity of hexachlorocyclopentadiene: subchronic (13-week)
administration by gavage to F344 rats and B6C3F1 mice. J. appl. Toxicol.,
4(2): 75-81.
ALEXANDER, D.J., CLARK, G.C., JACKSON, G.C., HARDY, C.J., STREET, A.E.,
HEYWOOD, R.H., BUIST, D., PRENTICE, D.E., & ISAACS, K.R. (1980)
Subchronic inhalation toxicity of hexachlorocyclopentadiene in monkeys
and rats, Huntingdon, Huntingdon Research Centre, 373 pp (Report
VCL14M/791081) (Prepared for Velsicol Chemical Corporation, Chicago).
ATALLAH, Y.H., WHITACRE, D.M., & BUTZ, R.G. (1980) Fate of
hexachlorocyclopentadiene in the environment. Paper presented at the 2nd
Chemical Congress of the North American Continent, Las Vegas, Nevada, 29
August, 1980, Chicago, Velsicol Chemical Corporation.
BELL, M.A., EWING, R.A., & LUTZ, G.A. (1978) Reviews of the environmental
effects of pollutants. XII. Hexachlorocyclopentadiene, Washington, DC, US
Environmental Protection Agency (Report EPA-600/1-78-047) (NTIS PB 80-
122963).
BENNETT, B. (1982) Hexachlorocyclopentadiene analyses of Mississippi
river fish, Athens, Georgia, US Environmental Protection Agency, Region
IV (Unpublished report).
BENOIT, F.M. & WILLIAMS, D.T. (1981) Determination of
hexachlorocyclopentadiene at the nanogram per liter level in drinking
water. Bull. environ. Contam. Toxicol., 27: 303-308.
BOYD, K.W., EMORY, M.B., & DILLON, H.K. (1981) Development of personal
sampling and analytical methods for organochlorine compounds. In:
Chemical hazards in the workplace, Washington, DC, American Chemical
Society, pp. 49-64 (ACS Symposium Series No. 149).
BRAT, S.V. (1983) The hepatocyte primary culture/DNA repair assay on
compound hexachlorocyclopentadiene using rat hepatocytes in culture,
Valhalla, New York, American Health Foundation, Naylor Dana Institute for
Disease Prevention.
BRIGGS, G.G. (1973) A simple relationship between soil adsorption of
organic chemicals and their octanol/water partition coefficients. In:
Proceedings of the 7th British Insecticide and Fungicide Conference,
Brighton, England, 19-22 November, 1973, Croydon, British Crop Protection
Council, Vol. 1, pp. 83-86 (Research Report).
BUA (BERATERGREMIUM FUR UMWELTRELEVANTE ALTSTOFFE) (1988)
[Hexachlorocyclopentadiene] Weinheim, Germany, VCH Verlagsgesellschaft
(BUA Report No. 25) (in German).
BUCCAFUSCO, R.J. & LEBLANC, G.A. (1977) Acute toxicity of
hexachlorocyclopentadiene to bluegill (Lepomis macrochirus), channel
catfish (Ictalurus punctatus), fathead minnow (Pimephales promelas), and
the water flea (Daphnia magna) (Unpublished report prepared for Velsicol
Chemical Corporation, Chicago).
BUNCHER, C.R., MOOMAW, C., & SIRKOSKI, E. (1980) Mortality study of
Montague Plant-Hooker Chemical, Cincinnati, Ohio, University of
Cincinnati Medical Center, Division of Epidemiology and Biostatistics
(Unpublished report prepared for Hooker Chemical Corporation).
BUTZ, R.G. & ATALLAH, Y.H. (1980) Effects of hexachlorocyclopentadiene on
three microbial functions (VCC Project No. 482428, Report No. 8)
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
BUTZ, R.G., YU, C.C., & ATALLAH, Y.H. (1982) Photolysis of
hexachlorocyclopentadiene in water. Ecotoxicol. environ. Saf., 6: 347-
357.
CALLAHAN, M.A., SLIMAK, M.W., GABEL, N.W., MAY, I.P., FOWLER, C.F.,
FREED, J.R., JENNINGS, P., DURFEE, R.L., WHITMORE, F.C., MAESTRI, B.,
MABEY, W.R., HOLT, B.R., & GOULD, C. (1979) Water-related environmental
fate of 129 priority pollutants: II. Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic
aromatic hydrocarbons, nitrosamines, miscellaneous compounds, Washington,
DC, US Environmental Protection Agency, Monitoring and Data Supervision
Division, Office of Water Planning and Standards (Report EPA-440/4-79-
029b).
CARTER, M.R. (1977) Legal affidavit field in the State of Georgia, Fulton
County, dated 14 June, 1977. Testimony concerning estimates of total
daily discharge of HEX from Velsicol Chemical Corporation, Atlanta,
Athens, Georgia, US Environmental Protection Agency.
CHERNOFF, N. & KAVLOCK, R.J. (1982) An in vivo teratology screen
utilizing pregnant mice. J. Toxicol. environ. Health, 10: 541-550.
CHERNOFF N. & KAVLOCK, R.J. (1983) A teratology test system which
utilizes postnatal growth and viability in the mouse. In: Waters, M.,
Sandhu, S., Lewtas, J., Claxton, L., Chernoff, N., & Nesnow, S., ed.
Short-term bioassays in the analysis of complex mixtures III, New York,
London, Plenum Publishing Corporation, pp. 417-427.
CHOPRA, N.M., CAMPBELL, B.S., & HURLEY, J.C. (1978) Systematic studies on
the breakdown of endosulfan in tobacco smokes: Isolation and
identification of the degradation products from the pyrolysis of
endosulfan I in a nitrogen atmosphere. J. agric. food Chem., 26: 255-258.
CHOU, S.F.J. & GRIFFIN, R.A. (1983) Soil, clay, and caustic soda effects
on solubility, sorption and mobility of hexachlorocyclopentadiene,
Springfield, Virginia, National Technical Information Service, 54 pp (PB
84-116060) (Environmental Geology Notes No. 104).
CHOU, S.F.J., GRIFFIN, R.A., CHOU, M.M, & LARSON, R.A. (1987) Products of
hexachlorocyclopentadiene (C-56) in aqueous solution. Environ. Toxicol.
Chem., 6: 371-376.
CLARK, C.S., MEYER, C.R., GARTSIDE, P.S., MAJETI, V.A., & SPECKER, B.
(1982) An environmental health survey of drinking water contamination by
leachate from a pesticide waste dump in Hardeman County, TN. Arch.
environ. Health, 37(1): 9-18.
CLARK, D.G., BLAIR, D., MARTIN, J., HENDY, R., PILCHER, A., & WIGGINS, D.
(1982) Thirty-week chronic inhalation study of hexachlorocyclopentadiene
(HEX) in rats, Tunstall, United Kingdom, Shell Toxicology Laboratory
(Experiment No. 1760, Report No. SBGR.81) (Permission for use granted by
M.J. Sloan, Shell Oil Co., Washington).
COLE, E.J. (1953) Chemotherapeutic and pharmacologic aspects of
hexachlorocyclopentadiene, Laramie, University of Wyoming, Department of
Veterinary Sciences and Bacteriology (Master's Thesis).
COLE, E.J. (1954) Treatment of sewage with hexachlorocyclopentadiene.
Appl. Microbiol., 2: 198-199.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in the air
environment, Research Triangle Park, North Carolina, US Environmental
Protection Agency (Report EPA-600/3-80-084).
DAL MONTE, R.P. & YU, C.C. (1977) Water solubility of MC-984 and hex
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DELEON, I.R., MABERRY, M.A., OVERTON, E.B., RASCHKE, P.C., REMELE, P.C.,
STEELE, C.F., WARREN, V.L., & LASETER, J.L. (1980a) Rapid gas
chromatographic method for the determination of volatile and semivolatile
organochlorine compounds in soil and chemical waste disposal site
samples. J. chromatogr. Sci., 18: 85-88.
DELEON, I.R., BROWN, N.J., COCCHIARA, J.P., MILES, S.K., & LASETER, J.L.
(1980b) Determination of trace levels of hexachlorocyclopentadiene and
octachlorocyclopentene in body fluids. J. anal. Toxicol., 4: 314-317.
DILLON, H.K. (1980) Development of air sampling and analytical methods
for toxic chlorinated organic compounds, Birmingham, Alabama, Southern
Research Institute, 76 pp (NTIS PB 80-193279).
DOROUGH, H.W. (1979) The accumulation, distribution and dissipation of
hexachlorocyclopentadiene (C56) in tissues of rats and mice, 27 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. (1980) Disposition of 14C-hexachlorocyclopentadiene (C56)
in rats following inhalation exposure, 53 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
DOROUGH, H.W. & RANIERI, T.A. (1984) Distribution and elimination of
hexachlorocyclopentadiene in rats and mice. Drug chem. Toxicol., 7(1):
73-89.
EICHLER, D.L. (1978) Quantitative analyses of mixtures containing trace
amounts of pesticides. Internal memorandum to M.R. Zavon, Hooker
Chemicals and Plastics Corporation, Niagara Falls, New York, 3 pp.
EL DAREER, S.M., NOKER, P.E., TILLERY, K.F., & HILL, D.L. (1983)
Investigations on the basis for the differential toxicity of
hexachlorocyclopentadiene administered to rats by various routes. J.
Toxicol. environ. Health, 12: 203-211.
ELIA, V.J., CLARK, C.S., MAJETI, V.A., GARTSIDE, P.S., MACDONALD, T.,
RICHDALE, N., MEYER, C.R., VAN MEER, G.L., & HUNNINEN, K. (1983) Chemical
exposure at a municipal wastewater treatment plant. Environ. Res., 32:
360-371.
EVANS, M.J., CABRAL-ANDERSON, L.J., & FREEMAN, G. (1978) Role of the
Clara cell in renewal of the bronchiolar epithelium. Lab. Invest., 38:
648-655.
GOGGELMAN, W., BONSE, G., HENSCHLER, D., & CREIM, H. (1978) Mutagenicity
of chlorinated cyclopentadiene due to metabolic activation. Biochem.
Pharmacol., 27: 2927-2929.
GRAY, L.E., Jr & KAVLOCK, R.J. (1984) An extended evaluation of an in
vivo teratology screen utilizing postnatal growth and viability in the
mouse. Teratog. Carcinog. Mutagen., 4: 403-426.
GREIM, J., BIMBOES, D., GOGGELMANN, W., & KRAMER, M. (1977) Mutagenicity
and chromosomal aberrations as an analytical tool for in vitro detection
of mammalian enzyme-mediated formation reactive metabolites. Arch.
Toxicol., 39: 159-169.
HAWLEY, G.G., ed. (1977) Condensed chemical dictionary, 9th ed., New
York, Van Nostrand Reinhold Co.
HAWORTH, S., LOWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E. (1983)
Salmonella mutagenicity test results for 250 chemicals. Environ.
Mutagen., 5(Suppl. 1): 3-142.
HENDERSON, C. (1956) Bio-assay investigations for International Joint
Commission, Niagara Falls, New York, Hooker Electrochemical Company, 17
pp (Unpublished report).
HUNT, G.E. & BROOKS, G.W. (1984) Source assessment for
hexachlorocyclopentadiene, Research Triangle Park, North Carolina, Radian
Corporation (Unpublished report prepared for the US Environmental
Protection Agency).
IBT (1977) Mutagenicity of PCL-HEX incorporated in the test medium tested
against five strains of S. typhimurium and as a volatilate against tester
strain TA-100, Northbrood, Illinois, Industrial Bio-Test Laboratories.
IRDC (1968) Hexachlorocyclopentadiene and octachlorocyclopentene: Acute
oral toxicity LD50 in male albino rats, Mattawan, Michigan, International
Research and Development Corporation, 4 pp (Unpublished report prepared
for Velsicol Chemical Corporation, Chicago).
IRDC (1972) Acute toxicity studies in rats and rabbits, Mattawan,
Michigan, International Research and Development Corporation, 21 pp
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
IRDC (1978) Hexachlorocyclopentadiene: Teratology study in rats,
Mattawan, Michigan, International Research and Development Corporation,
17 pp (Unpublished report prepared for Velsicol Chemical Corporation,
Chicago).
IRISH, D.D. (1963) Halogenated hydrocarbons: II. Cyclic.
Hexachlorocyclopentadiene. In: Patty, F.A., ed. Industrial hygiene and
toxicology, 2nd revised ed., New York, Chichester, Brisbane, Toronto,
John Wiley & Sons, pp. 1333-1363.
IRPTC (1989) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
KENAGA, E.E. (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol. environ.
Safety, 4: 26-38.
KENAGA, E.E. & GORING, C.A.I. (1980) Relationship between water
solubility, soil sorption, octanol/water partitioning, and
bioconcentration of chemicals in biota. In: Eaton, J.C., Parrish, P.R., &
Hendricks, A.C., ed. Aquatic toxicology, Philadelphia, American Society
of Testing and Materials, pp. 78-115 (ASTM STP 707).
KHAN, M.A.Q., SUDERSHAN, P., FEROZ, M., & PODOWSKI, A.A. (1981)
Biotransformations of cyclodienes and their photoisomers and
hexachlorocyclopentadiene in mammals and fish. In: Khan, M.A.Q. &
Stanton, R.H., ed. Toxicology of halogenated hydrocarbons: Health and
ecological effects, Oxford, New York, Pergamon Press, pp. 271-288.
KILZER, L., SCHEUNERT, I., GEYER, H., KLEIN, W., & KORTE, F. (1979)
Laboratory screening of the volatilization rates of organic chemicals
from water and soil. Chemosphere, 8: 751-761.
KLOSKOWSKI, R., SCHEUNERT, I., KLEIN, W., & KORTE, F. (1981) Laboratory
screening of distribution, conversion and mineralization of chemicals in
the soil-plant-system and comparison to outdoor experimental data.
Chemosphere, 10: 1089-1100.
KOMINSKY, J.R. & WISSEMAN, C.L. (1978) Morris Forman wastewater treatment
plant, Metropolitan Sewer District, Louisville, KY, Cincinnati, Ohio,
National Institute of Occupational Safety and Health (NIOSH Hazard
Evaluation and Technical Assistance Report No. TA 77-39) (NTIS PB 82-
178088).
KOMINSKY, J.R., WISSEMAN, C.L., & MORSE, D.L. (1980)
Hexachlorocyclopentadiene contamination of a municipal wastewater
treatment plant. Am. Ind. Hyg. Assoc. J., 41: 52.
KOMMINENI, C. (1978) Pathology reports. Animal portion of the Louisville
sewage study, Cincinnati, Ohio, National Institute of Occupational Safety
and Health (NIOSH Report No. TA 77-39).
LAWLESS, E.W., VON RUMKER, R., & FERGUSON, T.L. (1972) The pollution
potential in pesticide manufacturing, Springfield, Virginia, US
Department of Commerce, National Technical Information Service (NTIS PB
213782/3) (Prepared for the US Environmental Protection Agency by Midwest
Research Institute).
LAWRENCE, L.J. & DOROUGH, H.W. (1981) Retention and fate of inhaled
hexachlorocyclopentadiene in the rat. Bull. environ. Contam. Toxicol.,
26: 663-668.
LAWRENCE, L.J. & DOROUGH, H.W. (1982) Fate of inhaled
hexachlorocyclopentadiene in albino rats and comparison to the oral and
iv routes of administration. Fundam. appl. Toxicol., 2: 235-240.
LEVIN, A.A. (1982a) Hexachlorocyclopentadiene: Response to issues raised
at US EPA Test Rules Meeting of March 17, 1982, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Docket No.
40-8249078).
LEVIN, A.A. (1982b) Hexachloropentadiene: Follow-up and additional
information to the 17 March, 1982 Meeting, Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances.
LICHTENBERG, J.J., LONGBOTTON, J.E., & BELLAR, T.A. (1987) Analytical
methods for the determination of volatile non-polar organic chemicals in
water and water-related environments. In: Suffet, I.H. & Malaiyandi, M.,
ed. Organic pollutants in water: Sampling, analysis and toxicity testing.
A Symposium of the 188th Meeting of the American Chemical Society,
Philadelphia, 29-31 August, 1984, Washington, DC, American Chemical
Society, pp. 63-81.
LITTON BIONETICS, INC. (1977) Evaluation of hexachlorocyclopentadiene; in
vitro malignant transformation in BALB/3T3 cells, Kensington, Maryland,
Litton Bionetics, Inc., 7 pp (LBI Project No. 29840) (Prepared for
Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978a) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse lymphoma forward mutation assay,
Kensington, Maryland, Litton Bionetics, inc., 10 pp (LBI Project No.
20839) (Prepared for Velsicol Chemical Corporation, Chicago).
LITTON BIONETICS, INC. (1978b) Mutagenicity evaluation of
hexachlorocyclopentadiene in the mouse dominant lethal assay, Kensington,
Maryland, Litton Bionetics, Inc., 13 pp (LBI Project No. 20862).
LOOK, M. (1974) Hexachlorocyclopentadiene adducts of aromatic compounds
and their reaction products. Aldrichem. Acta, 7(2): 1974.
LU, P.Y., METCALF, R.L., HIRWE, A.S., & WILLIAMS, J.W. (1975) Evaluation
of environmental distribution and fate of hexachlorocyclopentadiene,
chlordane, heptachlor, and heptachlor epoxide in a laboratory model
ecosystem. J. agric. food Chem., 23: 967-973.
MACEK, R.J., PETROCELLI, S.R., & SLEIGHT, B.H., III (1979) Considerations
in assessing the potential for, and significance of, biomagnification of
chemical residues in aquatic food chains. In: Marking, L. & Kimerle,
R.A., ed. Aquatic toxicology, Philadelphia, American Society for Testing
and Materials, pp. 251-268.
MAYER, F.L. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida, US Environmental Protection Agency
(Report EPA-600/8-017).
MEHENDALE, H.M. (1977) Chemical reactivity-absorption, retention,
metabolism and elimination of hexachlorocyclopentadiene. Environ. Health
Perspect., 21: 275-278.
MEIER, J.R., RINGHAND, H.P., COLEMAN, W.E., MUNCH, J.W., STREICHER, R.P.,
KAYLOR, W.H., & SCHENCK, K.M. (1985) Identification of mutagenic
compounds formed during chlorination of humic acid. Mutat. Res., 157:
111-122.
MOLOTSKY, H.M. & BALLWEBER, E.G. (1957) Hexachlorocyclopentenones: US
Patent No. 2,795,608, June 11, 1957, Chicago, Velsicol Chemical
Corporation.
MORSE, D.L., LANDRIGAN, P.J., & FLYNT, J.W. (1978) Internal CDC report
concerning hexachlorocyclopentadiene contamination of a municipal sewage
treatment plant, Louisville, Kentucky, Atlanta, Georgia, Centers for
Disease Control.
MORSE, D.L., KOMINSKY, J.R., & WISSEMAN, C.L., III (1979) Occupational
exposure to hexachlorocyclopentadiene (How safe is sewage?). J. Am. Med.
Soc., 241: 2177-2179.
MURRAY, F.J., SCHWETZ, B.A., BALMER, M.F., & STAPLES, R.E. (1980)
Teratogenic potential of hexachlorocyclopentadiene in mice and rabbits.
Toxicol. appl. Pharmacol., 53: 497-500.
NAS (1978) Kepone/mirex/hexachlorocyclopentadiene: An environmental
assessment, Washington, DC, National Academy of Sciences (NTIS PB 280-
289).
NEUMEISTER, C. & KURIMO, R. (1978) Determination of
hexachlorocyclopentadiene and octachlorocyclopentene in air. Presented at
the ACGIH Conference, Los Angeles, May 1978, Cincinnati, Ohio, American
Conference of Governmental Industrial Hygienists.
NIOSH (1979) Manual of analytical methods, 2nd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1-5 (DHEW
(NIOSH) Pub. No. 77-157-A).
NIOSH (1980) Quarterly hazard summary report: Hexachlorocyclopentadiene,
Cincinnati, Ohio, National Institute for Occupational Safety and Health.
NTP (1984a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 128 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
NTP (1984b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 196 pp (Unpublished report prepared for the National
Toxicology Program, National Institutes of Health, Birmingham, Alabama).
OSHA (US DEPARTMENT OF LABOR, OCCUPATIONAL SAFETY AND HEALTH
ADMINISTRATION) (1989) Air contaminants: Final rule -
Hexachlorocyclopentadiene. Fed. Reg., 54(12): 2464.
PETERS, J.A., TACKETT, K.M., & EIMUTIS, E.C. (1981) Measurement of
fugitive hydrocarbon emissions from a chemical waste disposal site.
Presented at the 74th Annual Meeting of the Air Pollution Control
Association, Philadelphia, 21-26 June, 1981, Dayton, Ohio, Montesanto
Research Corporation.
PODOWSKI, A.A. & KHAN, M.A.Q. (1979) Fate of hexachlorocyclopentadiene in
goldfish (Carassius auratus). Paper presented at the American Chemical
Society Meeting, Honolulu, April 1979, Washington, DC, American Chemical
Society.
PODOWSKI, A.A. & KHAN, M.A.Q. (1984) Fate of hexachlorocyclopentadiene in
water and goldfish. Arch. environ. Contam. Toxicol., 13: 471-481.
RAND, G.M., NEES, P.O., CALO, C.J., ALEXANDER, D.J., & CLARK, G.C.
(1982a) Effects of inhalation exposure to hexachlorocyclopentadiene on
rats and monkeys. J. Toxicol. environ. Health, 9: 743-760.
RAND, G.M., NEES, P.O., CALO, C.J., CLARKE, G.C., & EDMONDSON, N.A.
(1982b) The Clara cell: An electron microscopy examination of the
terminal bronchioles of rats and monkeys following inhalation of
hexachlorocyclopentadiene. J. Toxicol. environ. Health, 10: 59-72.
RIECK, C.E. (1977a) Effect of hexachlorocyclopentadiene on soil microbe
populations, Lexington, Kentucky, University of Kentucky, Agronomy
Department (Unpublished report prepared for Velsicol Chemical
Corporation, Chicago).
RIECK, C.E. (1977b) Soil metabolism of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
RIECK, C.E. (1977c) Volatile products of 14C-hexachlorocyclopentadiene,
Lexington, Kentucky, University of Kentucky, Agronomy Department
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
ROBERTS, C.W. (1958) Chemistry of hexachlorocyclopentadiene. Chem. Ind.,
1 February: 110.
SHELL RESEARCH LIMITED (1982) Toxicology of insecticide intermediates:
The skin sensitizing potential of hexachlorocyclopentadiene, Tunstall,
United Kingdom, Shell Research Ltd., Sittingbourne Research Centre
(Report No. SBGR 82.225).
SHINDEL, S. & ULRICH, S. (1986) Mortality of workers employed in the
manufacture of chlordane. An update. J. occup. Med., 28(7): 497-501.
SHINDELL & ASSOCIATES (1980) Report of epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Marshall, Illinois,
January 1946-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SHINDELL & ASSOCIATES (1981) Report of the epidemiologic study of the
employees of Velsicol Chemical Corporation plant, Memphis, Tennessee,
January 1952-December 1979, Milwaukee, Wisconsin, Shindell and Associates
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1982)
Hexachlorocyclopentadiene increases oxygen consumption by intact rainbow
trout and isolated heart mitochondria. Sixty-sixth Annual Meeting of the
Federation of American Societies for Experimental Biology, New Orleans,
15-23 April, 1982. Fed. Proc., 41(5): 1580 (abstract).
SINHASENI, P., D'ALECY, L.G., HARTUNG, R., & SHLATER, M. (1983)
Respiratory effects of hexachlorocyclopentadiene on intact rainbow trout
(Salmo gairdneri) and on oxidative phosphorylation of isolated trout
heart mitochondria. Toxicol. appl. Pharmacol., 67: 215-223.
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1977) A rapid
assessment of the toxicity of three chlorinated cyclodiene insecticide
intermediates to fathead minnows, Duluth, Minnesota, US Environmental
Protection Agency, Environmental Research Laboratory (Report EPA-600/3-
77-099).
SPEHAR, R.L., VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.A. (1979) Toxicity
and bioaccumulation of hexachlorocyclopentadiene, hexachloronorbornadiene
and heptachloronorbonene in larval and early juvenile fathead minnows,
Pimephales promelas. Bull. environ. Contam. Toxicol., 21: 576-583.
SPRINKLE, C.L. (1978) Leachate migration from a pesticide. Waste disposal
site in Hardeman County, Tennessee, Washington, DC, US Department of
Interior, US Geological Survey (Waste Resources Investigations 78-128).
SRI (1980a) Acute toxicity report on hexachlorocyclopentadiene (C53607)
in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama, Southern
Research Institute, 44 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1980b) Repeated-dose toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats and B6C3F1 mice, Birmingham, Alabama,
Southern Research Institute, 33 pp (Unpublished report prepared for the
National Toxicology Program).
SRI (1981a) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in B6C3F1 mice, Birmingham, Alabama, Southern Research
Institute, 137 pp (Unpublished report prepared for the National
Toxicology Program).
SRI (1981b) Subchronic toxicity report on hexachlorocyclopentadiene
(C53607) in Fischer-344 rats, Birmingham, Alabama, Southern Research
Institute, 144 pp (Unpublished report prepared for the National
Toxicology Program).
STEVENS, J.E. (1979) Chlorinated derivatives of cyclopentadiene. In:
Kirk-Othmer encyclopedia of chemical technology, 3rd ed., New York,
Chichester, Brisbane, Toronto, John Wiley & Sons, Vol. 5, pp. 791-797.
THIELEN, D.R., OLSEN, G., DAVIS, A., BAJOR, E., STEFANOVSKI, J., &
CHODKOWSKI, J. (1987) An evaluation of microextraction/capillary column
gas chromatography for monitoring industrial outfalls. J. chromatogr.
Sci., 25(1): 12-16.
THUMA, N.K., O'NEILL, P.E., BROWNLEE, S.G., & VALENTINE, R.S. (1978)
Biodegradation of spilled hazardous materials. In: Control of hazardous
materials spills, Rockville, Maryland, Information Transfer, Inc., pp.
217-220.
TREON, J.F., CLEVELAND, F.P., & CAPPEL, J. (1955) The toxicity of
hexachlorocyclopentadiene. Arch. ind. Health, 11: 459-472.
UNGNADE, H.E. & MCBEE, E.T. (1958) The chemistry of
perchlorocyclopentenes and cyclopentadienes. Chem. Rev., 58: 249-254.
US EPA (1977) Chemical Hazard Information Profile:
Hexachlorocyclopentadiene (CHIP), Washington, DC, US Environmental
Protection Agency, TSCA Interagency Testing Committee.
US EPA (1980a) Summary of UWF Co-op Data on hexachlorocyclopentadiene and
hexachlorobutadiene, Gulf Breeze, Florida, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished laboratory data).
US EPA (1980b) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards (Report EPA-440/5-80-055)
(NTIS PB 292-436).
US EPA (1980c) Ambient water quality criteria for
hexachlorocyclopentadiene, Washington, DC, US Environmental Protection
Agency, office of Water Regulations and Standards (Report EPA-440/5-80-
055) (Unpublished data).
US EPA (1982) Hexachlorocyclopentadiene: Response to the Interagency
Testing Committee. Fed. Reg., 47(250): 58023-58025.
US EPA (1989) Toxic chemical release inventory: Online printout of April
1989, Washington, DC, US Environmental Protection Agency (Available from
the US National Library of Medicine).
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish.
Res. Board Can., 36: 1040-1048.
VELSICOL CHEMICAL CORPORATION (1978) TSCA Sec. 8(E): Submission 8EHQ-
06780208. Chlorinates in Mississippi River catfish and carp, 1978
(Unpublished report prepared for Velsicol Chemical Corporation, Chicago)
(Submitted to the US Environmental Protection Agency, Office of Toxic
Substances, Washington).
VELSICOL CHEMICAL CORPORATION (1979) Confirmation of HEX and HEX-BCH
residues in human urine. Analytical method No. 0682, Chicago, Velsicol
Chemical Corporation.
VELSICOL CHEMICAL CORPORATION (1984) Comments on draft health effects
document for hexachlorocyclopentadiene, Chicago, Velsicol Chemical
Corporation.
VELSICOL CHEMICAL CORPORATION (1986) Air sampling and monitoring at
Velsicol production facilities, Chicago, Velsicol Chemical Corporation.
VERSCHUEREN, K. (1977) Handbook of environmental data on organic
chemicals, New York, Van Nostrand Reinhold Co.
VILKAS, A.G. (1977) The acute toxicity of hexachlorocyclopentadiene to
the water flea, Daphnia magna straus, Tarrytown, New York, Union Carbide
Environmental Services (UCES Project No. 11506-03-05) (Prepared for
Velsicol Chemical Corporation, Chicago).
WALSH, G.E. (1981) Effects of chlordane, heptachlor and
hexachlorocyclopentadiene on growth of marine unicellular algae, Gulf
Breeze, Florida, US Environmental Protection Agency, Environmental
Research Laboratory (Unpublished report).
WALSH, G.E. (1983) Cell death and inhibition of population growth of
marine unicellular algae by pesticides. Aquat. Toxicol., 3: 209-214.
WALSH, G.E. & ALEXANDER, S.V. (1980) A marine algal bioassay method:
Results with pesticides and industrial wastes. Water Air Soil Pollut.,
13: 45-55.
WANG, H.H. & MACMAHON, B. (1979) Mortality of workers employed in the
manufacture of chlordane and heptachlor. J. occup. Med., 21: 745-748.
WEAST, R.C. & ASTLE, M.J. (1980) CRC handbook of chemistry and physics,
60th ed., Boca Raton, Florida, CRC Press, Inc.
WEBER, J.B. (1979) Adsorption of HEX by Cape Fear loam soil, Research
Triangle Park, North Carolina State University (Unpublished report
prepared for Velsicol Chemical Corporation, Chicago).
WHITMORE, F.C., DURFEE, R.L., & KHATTAK, M.N. (1977) Evaluation of a
technique for sampling low concentrations of organic vapors in ambient
air, Atlanta, Georgia, US Environmental Protection Agency (NTIS PB 279-
672).
WHO (1989) Environmental Health Criteria 91: Aldrin and Dieldrin, Geneva,
World Health Organization, 335 pp.
WILLIAMS, C.M. (1978) Liver cell culture systems for the study of
hepatocarcinogens. Proceedings of the 12th International Cancer Congress,
XII. International Cancer Congress Symposium No. 2: Chemical Oncogenesis
and Mutagenesis, October 1978, New York, London, Plenum Publishing
Corporation.
WILSON, J.A., BALDWIN, C.P., & MCBRIDE, T.J. (1978) Case history:
Contamination of Louisville, Kentucky Morris Foreman treatment plant.
Hexachlorocyclopentadiene. In: Control of hazardous material spills,
Miami, Florida, Hazardous Material Control Research Institute, pp. 170-
177.
WOLFE, N.L., ZEPP, R.G., SCHLOTZHAVER, P., & SINK, M. (1982)
Transformation pathways of hexachlorocyclopentadiene in the aquatic
environment. Chemosphere, 11(2): 91-101.
YOWELL, H.L. (1951) Fungicidal compositions containing
hexachlorocyclopentadiene: US Patent 2538509, Washington, DC, US Patent
Office.
YU, C.C. & ATALLAH, Y.H. (1977a) HEX hydrolysis at various pHs and
temperatures, Chicago, Velsicol Chemical Corporation (Project No. 482428,
Report No. 8) (Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1977b) Photolysis of hexachlorocyclopentadiene,
Chicago, Velsicol Chemical Corporation (Project No. 482428, Report No. 4)
(Laboratory report).
YU, C.C. & ATALLAH, Y.H. (1981) Pharmacokinetics and metabolism of
hexachlorocyclopentadiene in rats, Chicago, Velsicol Chemical Corporation
(Project No. 482428, Report No. 10).
ZEPP, R.G., BAUGHMAN, G.L., & SCHLOTZHAUER, P.F. (1979) Dynamics of
processes influencing the behavior of hexachlorocyclopentadiene in the
aquatic environment. Paper presented at the 178th National Meeting of the
American Chemical Society, Washington, 9-14 September, 1979, Washington,
DC, American Chemical Society, Division of Environmental Chemistry.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R.C. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20 coded
compounds tested for the National Toxicology Program. Environ. Mutagen.,
7: 87-100.
APPENDIX 1
Information on guidelines, recommendations, and stan-
dards used in various countries is given in Table 21.
Table 21. Guidelines, recommendations and standards used in various countries/areasa
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
Australia recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1983
weighted average
short-term exposure limit 0.3 (0.03)
Belgium recommendation air/occupational threshold limit value/time- 0.1 (0.01) 1988
weighted average
Canada regulation air/occupational threshold limit value/time- 0.1 (0.01) 1980
weighted average
Canada regulation transport specific transportation 1987
regulations
Finland recommendation air/occupational time-weighted average 1.0 (0.1) 1989
skin short-term exposure limit 3 (0.3) 1989
Netherlands recommendation air/occupational time-weighted average/ 0.11 (0.01) 1986
occupational
Federal regulation waste "toxic waste" subject to 1981
Republic specific handling,
of Germany transport, treatment,
storage, and disposal
regulation/permits
Switzerland regulation air/occupational time-weighted average 0.1 (0.01) 1987
USA regulation water ambient 1 µg/litre 1980
water quality criteria
(organoleptic)
----------------------------------------------------------------------------------------------------
Table 21 (contd.)
----------------------------------------------------------------------------------------------------
Country Type Medium/situation Exposure limit description Valueb Date
or remark
----------------------------------------------------------------------------------------------------
USA (ACGIH) recommendation air/occupational time-weighted average 0.1 (0.01) 1980
USA regulation air/occupational time-weighted average 0.1 (0.01) 1989
USA regulation water/land notification of spill 1983
of 0.45 kg (1 lb)
in 24-h period
USA regulation waste transport "toxic waste" subject to 1980
specific handling,
transport, treatment,
storage and disposal
regulation/permits
USA draft drinking-water lifetime 7 µg/kg 1990
recommendation per day
USSR regulation air/occupational threshold limit value 0.01 (0.001) 1989
USSR regulation water maximum allowable 1 mg/litre 1985
concentration
USSR regulation air/ambient short-term exposure limit 0.001 1987
Yugoslavia regulation air/occupational time-weighted average 0.1 1985
----------------------------------------------------------------------------------------------------
a From: IRPTC (1989)
b Unless stated otherwise, units are mg/m3. The value in parts per million is given in parentheses.
RESUME
L'hexachlorocyclopentadiène (HEX) est un liquide dense
et ininflammable, de couleur jaune pâle à jaune verdâtre,
et qui possède une odeur piquante caractéristique. Le HEX
est très réactif; il donne lieu à des réactions d'addition
et de substitution et à des réactions de Diels-Alder.
Aux Etats-Unis d'Amérique, la Velsicol Chemical Cor-
poration est actuellement le seul producteur de HEX. En
Europe, il est produit aux Pays-Bas par la Société Shell.
Les chiffres de production sont confidentiels mais on
estime que 3600 à 6800 tonnes de HEX sont produites
actuellement aux Etats-Unis. En 1988, la production
mondiale était d'environ 15 000 tonnes (BUA, 1988). Le
HEX est utilisé comme intermédiaire dans la production de
nombreux pesticides, mais quelques pays en ont limité
l'emploi à la fabrication de certains pesticides organo-
chlorés. Il est également utilisé pour la fabrication de
retardateurs de flamme, de résines et de colorants.
Au cours de la production et de la transformation du
HEX, de petites quantités sont libérées dans l'environne-
ment. Ce peut être également le cas lorsqu'il constitue
une impureté de certains des produits pour lesquels il
sert d'intermédiaire. La libération de HEX peut interve-
nir pendant ou après le rejet. On ne dispose que de
données limitées sur la surveillance des concentrations de
cette substance dans l'environnement. D'après ces données,
il semble que le HEX soit présent essentiellement dans le
compartiment aquatique et y soit associé aux sédiments et
aux matières organiques, sauf là où il y a eu rejet ou
libération du produit. D'après les études en laboratoire,
il y a sorption du HEX par la plupart des particules du
sol. Toutefois, on a fait état d'un lessivage et d'un
mouvement dans les eaux souterraines.
Aux Etats-Unis d'Amérique, on estime que 5,9 tonnes de
HEX sont libérées annuellement dans le milieu (US EPA,
1989). En République fédérale d'Allemagne et aux Pays-
Bas, environ 400 à 500 kg de HEX ont été libérés dans
l'atmosphère en 1987 (BUA, 1988). En raison des propriétés
physiques et chimiques du HEX, il ne devrait subsister
qu'une faible fraction de ces émissions.
En s'appuyant sur les données de laboratoire disponi-
bles, on a modélisé la destinée et le transport du HEX
dans l'atmosphère et calculé que son temps de séjour dans
la troposphère était d'environ 5 h. On a fait état d'un
transport atmosphérique de HEX à partir d'une zone de
stockage de déchets et de puits au cours du traitement de
rejets industriels.
Dans l'eau, le HEX peut subir une photolyse, une
hydrolyse et une biodégradation. Dans les eaux peu
profondes son temps de demi-photolyse est inférieur à une
heure. Dans les eaux plus profondes où la photolyse est
exclue, le temps de demi-hydrolyse varie de plusieurs
jours à environ trois mois et la biodégradation est encore
beaucoup plus lente. Le HEX se volatilise à la surface de
l'eau à une vitesse qui dépend de la turbulence et du
degré de sorption par les sédiments.
En raison de sa faible solubilité dans l'eau, le HEX
devrait être relativement immobile dans le sol. Toutefois
on en a trouvé dans des eaux souterraines. La volatili-
sation de cette substance qui se produit très vraisembla-
blement à la surface du sol est d'autant plus importante
que la teneur du sol en matières organiques est plus
faible. Les résultats d'études en laboratoire indiquent
que l'hydrolyse chimique et la métabolisation microbienne,
qu'elles soient aérobie ou anaérobie, devraient réduire la
teneur des sols en HEX.
En principe, le HEX devrait avoir un pouvoir de bio-
amplification notable en raison de sa forte lipophilicité
(log du coefficient de partage octanol/eau). Toutefois
les données expérimentales ne corroborent pas cette hypo-
thèse. Des études sur animaux d'expérience ont montré que
le 14C-HEX est à la fois métabolisé et excrété dans les
quelques heures qui suivent l'administration par voie
orale, la rétention dans l'organisme étant très faible.
Le facteur de bioconcentration à l'état stationnaire est
inférieur à 30 chez les poissons. Les facteurs de bio-
accumulation calculés à partir de modèles d'écosystèmes à
court terme indiquent que le potentiel d'accumulation est
modéré. Il semblerait donc que le HEX et ses métabolites
ne persistent pas et ne s'accumulent pas en quantités
importantes dans les systèmes biologiques.
On a montré qu'à faibles concentrations, le HEX était
toxique pour la faune aquatique. Des cas de mortalité par
intoxication aiguë (exposition de 48 à 96 h) ont été
observés chez des crustacés et des poissons dulçaquicoles
et marins à des concentrations nominales de 32 à
180 µg/litre, dans des systèmes statiques dont l'eau
n'était pas renouvelée au cours de l'épreuve. Etant donné
que le temps de demi-photolyse est inférieur à une heure,
la concentration en HEX devrait avoir diminué de manière
importante au cours de la période d'exposition utilisée
dans ces études. Les seules études au cours desquelles on
a mesuré les concentrations de HEX dans de l'eau courante
ont donné une valeur de la CL50 à 96 h de 7 µg/litre
pour le vairon américain et une crevette de mer. Les
épreuves effectuées sur ces deux espèces ont donné,
respectivement pour la CL10 et la CL40, des valeurs de
3,7 et de 0,7 µg/litre.
Des épreuves statiques de sept jours effectuées sur
des algues marines à des concentrations nominales allant
de 3,5 à 100 µg/litre ont fait ressortir une réduction
moyenne de la croissance (CE50), qui était fonction de
l'espèce. En milieu aqueux, le HEX est toxique pour de
nombreux microorganismes à des concentrations nominales de
0,2 à 10 mg/litre, c'est-à-dire à des valeurs sensiblement
plus importantes que celles qui sont nécessaires pour tuer
la plupart des animaux et des plantes aquatiques. Le HEX
semble être moins toxique pour les microorganismes terri-
coles que pour les microorganismes aquatiques, probable-
ment en raison de l'adsorption du HEX sur la matrice
constituée par le sol.
On pense que l'exposition devrait être faible mais les
données actuellement disponibles sont insuffisantes pour
permettre de déterminer les effets de l'exposition au HEX
sur la flore et la faune terrestres.
La résorption du HEX non modifié est minimale du fait
de sa réactivité vis-à-vis des membranes et des tissus de
l'organisme et plus particulièrement, du contenu des voies
digestives. Après administration par voie orale, percuta-
née ou par inhalation, la majeure partie du 14C-HEX
radiomarqué est retenue au niveau des reins, du foie, de
la trachée et des poumons des animaux d'expérience. Une
fois résorbé, le HEX est métabolisé et rapidement excrété,
principalement dans les urines, un peu moins dans les
matières fécales et à raison de moins de 1%, dans l'air
expiré. La durée nécessaire pour l'élimination complète
est d'environ 30 h quelle que soit la voie d'adminis-
tration. Après inhalation ou administration par voie
intraveineuse, le composé parent ne se retrouve plus dans
les excréta; on a isolé des métabolites fécaux et uri-
naires mais ils n'ont pas été identifiés. C'est d'ailleurs
la raison pour laquelle il est très difficile de se faire
une idée de la pharmacocinétique du HEX et d'élucider son
mode d'action.
La CL50 par inhalation (sur une période d'environ
4 h) est de 17,9 mg/m3 chez le rat mâle et de 39,1 mg
par m3 chez la ratte. Bien qu'il existe quelques
différences d'une espèce à l'autre, notamment entre les
cobayes, les rats, les lapins et les souris, les vapeurs
de HEX sont extrêmement toxiques pour toutes les espèces
étudiées. C'est par inhalation qu'il se révèle le plus
toxique, par comparaison avec l'administration orale ou
percutanée, et il se montre également extrêmement irri-
tant. Quelle que soit la voie d'administration, toute
exposition aiguë entraîne des effets généraux pathologi-
ques au niveau des poumons, du foie, des reins et des
surrénales.
L'administration de HEX par voie orale à des rats
(30 mg/kg et par jour) et à des souris (75 mg/kg et par
jour) pendant 91 jours a déterminé une néphrose ainsi
qu'une inflammation et une hyperplasie de l'estomac
antérieur. On n'a pas relevé de signes manifestes après
exposition de souris ou de rats à qui l'on avait fait
inhaler du HEX à raison de 2,26 mg/m3 (0,2 ppm), six
heures par jour, cinq jours par semaine pendant 14
semaines. A la concentration de 1,69 mg/m3 (0,15 ppm),
on a observé seulement une petite irritation. Des rats
exposés de la même manière à 5,65 mg/m3 (0,5 ppm) de
HEX pendant 30 semaines ont présenté des modifications
histopathologiques au niveau du foie, des voies respi-
ratoires et des reins. Une autre étude du même genre
portant sur des rats et des souris et qui s'est prolongée
pendant 90 jours a révélé des effets respiratoires à
partir de 4,52 mg/m3 (0,4 ppm). Le HEX ne s'est pas
révélé mutagène lors d'épreuves in vitro, qu'il y ait ou
non activation métabolique. Il s'est également révélé
inactif dans les épreuves de dominance létale chez la
souris. Administré par voie orale à des rats et à des
souris, il ne s'est pas révélé tératogène, mais on ne
dispose d'aucune donnée relative à sa tératogénicité
éventuelle après exposition par voie respiratoire. On ne
dispose que de données limitées sur l'exposition humaine
au HEX et sur ses effets. On a observé des incidents
isolés au cours desquels il y a eu forte irritation des
yeux, du nez, de la gorge et des poumons. Cette irritation
était généralement brève, les victimes commençant à
récupérer dès cessation de l'exposition. Après une expo-
sition de courte durée on n'a noté aucune différence
statistiquement significative au niveau de certaines
enzymes hépatiques entre les groupes exposés et les
groupes témoins. On ignore quels peuvent être les effets
à longue échéance sur la santé humaine d'une exposition
continue à de faibles concentrations de HEX ou d'une
exposition intermittente à des fortes concentrations. Les
personnes qui sont amenées à manipuler cette substance ou
des déchets qui en contiennent, ainsi que les égoutiers,
travailleurs des usines de traitement d'eaux usées et
personnes qui résident à proximité de décharges, pour-
raient être exposés au risque du fait des possibilités de
contact avec cette substance ou avec les déchets qui
résultent de sa fabrication.
La base de données dont on dispose n'est pas suffi-
sante pour qu'on puisse évaluer la cancérogénicité du HEX.
Le Programme toxicologique national des Etats-Unis (NTP) a
procédé à des études d'inhalation sur des rats et des
souris pendant toute la durée de leur vie. Une fois qu'un
rapport sur la pathologie observée aura été publié, on
aura une meilleure idée des effets à long terme éventuels
de l'exposition au HEX. En ce qui concerne la cancéro-
génicité de cette substance, il faudra différer les tra-
vaux dans l'attente des résultats des épreuves effectuées
par le NTP. Le Centre international de recherche sur le
cancer a examiné les données existantes sur le HEX et l'a
classé dans le Groupe 3 (ce qui indique qu'en raison
d'insuffisances quantitatives ou qualitatives importantes,
il n'est pas possible de déterminer, au vu des résultats
disponibles, s'il y a présence ou absence d'effets
cancérogènes). Un certain nombre d'études épidémiologiques
sont citées dans la littérature; on n'a pas fait état
d'une incidence accrue de cancers, de localisation
quelconque, qui puisse être attribuée au HEX ou à ses
métabolites.
RESUMEN
El hexaclorociclopentadieno (HEX) es un líquido denso
de color amarillo pálido o verdoso, no inflamable, con un
olor acre característico. Su masa molecular relativa es de
272,77 y es poco soluble en agua. El HEX es muy reactivo
y experimenta reacciones de adición, sustitución y de
Diels-Alder.
En los EE.UU., la Velsicol Chemical Corporation es la
única empresa que actualmente produce HEX. En Europa lo
fabrica la Shell Chemical Corporation en los Países Bajos.
Los datos de producción son propiedad de las empresas,
pero se calcula que al año se producen en los EE.UU. entre
3600 y 6800 toneladas de HEX. En 1988, la producción
mundial fue de aproximadamente 15 000 toneladas (BUA,
1988). Aunque el HEX se utiliza como intermedio en la
producción de numerosos plaguicidas, algunos países han
restringido su empleo en la fabricación de ciertos plagui-
cidas organoclorados. También se utiliza en la producción
de pirorretardantes, resinas y tintes.
Durante la fabricación y elaboración del HEX, se
liberan pequeñas cantidades de la sustancia al medio
ambiente. También puede liberarse cuando aparece en forma
de impureza en algunos de los productos para los que sirve
de intermedio. El HEX puede liberarse tanto durante como
después de su evacuación. Sólo se dispone de datos limi-
tados de vigilancia de los niveles ambientales de HEX.
Esos datos indican que aparece principalmente en el
compartimento acuático y que se asocia a los sedimentos y
la materia orgánica del fondo salvo en los lugares en los
que se ha producido evacuación o liberación. En el labora-
torio, el HEX se adsorbe sin dificultad a la mayoría de
los tipos de partículas del suelo. Sin embargo, se han
comunicado casos de lixiviación y de movimiento en aguas
subterráneas.
En los EE.UU., se calcula que la liberación total de
HEX al medio ambiente en un año es de 5,9 toneladas (US
EPA, 1989). En la República Federal de Alemania y los
Países Bajos, se emitieron en 1987 alrededor de 400-500 kg
(BUA, 1988). Dadas las características físicas y químicas
del HEX, es de esperar que solo persista una pequeña
fracción de esas emisiones.
Basandose en los datos de laboratorio disponibles, se
ha formulado un modelo sobre el destino y el transporte
del HEX en la atmósfera y se ha calculado que tiene un
tiempo de residencia de aproximadamente 5 horas en la
troposfera. Se ha comunicado la existencia de transporte
atmosférico de HEX desde una zona en la que se almacenan
desechos y a partir de las cisternas durante el trata-
miento de desechos industriales.
En el agua, el HEX puede experimentar fotolisis,
hidrólisis y biodegradación. En aguas poco profundas,
tiene una semivida fotolítica de < 1 h. En aguas más
profundas, donde la fotolisis se ve impedida, se ha
observado que la semivida hidrolítica puede variar entre
varios días y aproximadamente tres meses, mientras que es
de prever que la biodegradación se produzca con más
lentitud. Se sabe que el HEX se volatiliza en las aguas
superficiales, y que la tasa de volatilización varia con
la turbulencia y con la adsorción a los sedimentos.
Debido a su baja solubilidad en el agua, el HEX debe
ser relativamente inmóvil en el suelo. Sin embargo, se ha
detectado la sustancia en aguas subterráneas. La volatili-
zación, que tiene más probabilidades de producirse en la
superficie del suelo, guarda relación inversa con los
niveles de materia orgánica en éste. Los resultados de
estudios en laboratorio indican que la hidrólisis química
y el metabolismo microbiano, tanto aeróbico como anaeró-
bico, deben reducir los niveles de HEX en los suelos.
En teoría, el potencial de biomagnificación del HEX
debe ser importante debido a su elevada lipofilia (log
coeficiente de partición octanol/agua). Este extremo, sin
embargo, no ha sido demostrado en pruebas experimentales.
Los estudios en animales de laboratorio han demostrado que
el 14C-HEX es metabolizado y excretado durante de las
primeras horas que siguen la administración de una dosis
por vía oral, y que la proporción que queda retenida en el
organismo es pequeña. En los peces, los factores de
bioconcentración en estado estable son < 30. Los factores
de bioacumulación derivados de modelos de ecosistemas a
corto plazo indican un moderado potencial de acumulación.
Así pues, parece que el HEX y sus metabolitos no persisten
ni se acumulan en gran medida en los sistemas biológicos.
Se ha demostrado que el HEX en bajas concentraciones
es tóxico para los organismos acuáticos. Se ha observado
letalidad en exposiciones agudas (48 a 96 h) en crustáceos
y peces de agua dulce y salada, en concentraciones nomi-
nales de 32-180 µg/litro en sistemas de exposición
estática en los que el agua no se renovó durante la
prueba. Puesto que la semivida fotolítica es < 1 h, la
concentración de HEX habría disminuido sustancialmente
durante el periodo de exposición utilizado en esos
estudios. En los únicos estudios en los que se usó agua
corriente y se midieron las concentraciones de HEX, se
obtuvieron valores de la CL50 en 96 horas de 7 µg por
litro en Pimephales promelas y un camarón de mar. Los
ensayos realizados con esas dos especies dieron un valor
de CL10 de 3,7 y un valor de CL40 de 0,7 µg por litro,
respectivamente.
En ensayos estáticos de siete días con algas marinas
se observó una reducción mediana del crecimiento (CE50)
a concentraciones nominales que variaron entre 3,5 y
100 µg/litro, según la especie.
En medios acuosos, el HEX resulta tóxico para
numerosos microorganismos en concentraciones nominales de
0,2-10 mg/litro, es decir, niveles sensiblemente mayores
que los necesarios para destruir a la mayoría de los
animales o vegetales acuáticos. El HEX parece menos tóxico
para los microorganismos en el suelo que en el medio
acuático, probablemente a causa de la adsorción del HEX a
la matriz del suelo.
Aunque cabe esperar que la exposición sea reducida,
actualmente no se dispone de bastante información para
determinar los efectos de la exposición al HEX en la
vegetación o la fauna terrestres.
La absorción de HEX sin modificar es mínima debido a
su reactividad con las membranas y los tejidos del organ-
ismo y especialmente con el contenido del tracto gastro-
intestinal. La mayor parte del 14C-HEX radiomarcado
queda retenido en el riñón, el hígado, la tráquea y los
pulmones de los animales tras la administración por vía
oral, cutánea o respiratoria. El HEX absorbido es metab-
olizado y excretado rápidamente, sobre todo en la orina,
menos en las heces y < 1% en el aire expirado. El tiempo
de eliminación terminal es de unas 30 horas, con inde-
pendencia de la vía de administración. Tras la inhalación
o la administración intravenosa, no se excreta HEX sin
modificar; los metabolitos fecales y urinarios se han
aislado pero no se han identificado. La falta de identifi-
cación de los metabolitos representa uno de los prin-
cipales obstaculos para evaluar la farmacocinética y los
mecanismos potenciales de acción del HEX.
En la rata, la CL50 aguda por inhalación (durante un
periodo de aproximadamente 4 h) es de 17,9 mg/m3 en el
macho y 39,1 mg/m3 en la hembra. Aunque hay algunas
diferencias interespecíficas entre cobayos, conejos, ratas
y ratones, los vapores de HEX son sumamente tóxicos para
todas las especies ensayadas. Su toxicidad parece máxima
cuando se administra por inhalación, en comparación con la
administración oral y cutánea, y es un irritante primario
fuerte. Los efectos sistémicos de la exposición aguda,
con independencia de la vía de administración, comprenden
cambios patológicos en el pulmón, el hígado, el riñón y
las glándulas suprarrenales.
La administración oral a corto plazo a ratas (38 mg/kg
al día) y ratones (75 mg/kg al día) durante 91 días
produjo nefrosis e inflamación e hiperplasia de la región
anterior del estómago. No se observaron signos manifiestos
cuando se expuso a ratas o ratones por inhalación a 2,26
mg/m3 (0,2 ppm), 6 h/día, 5 días/semana, durante 14
semanas. Con 1,69 mg/m3 (0,15 ppm) sólo se observó una
ligera irritación. La exposición de ratas a la inhalación
de 5,65 mg/m3 (0,5 ppm) durante 30 semanas provocó
cambios histopatológicos en el hígado, el tracto respira-
torio y el riñón. En un estudio de inhalación a corto
plazo de HEX en ratones y ratas durante 90 días se
observaron efectos en el sistema respiratorio a 4,52
mg/m3 (0,4 ppm) o más. No se ha demostrado que el HEX
sea mutagénico en ensayos in vitro , con o sin activación
metabólica. También resultó inactivo en ensayos de letali-
dad dominante en el ratón. Tampoco se ha demostrado que
sea teratogénico en ratas y ratones por exposición oral;
no se dispone de datos sobre la teratogenicidad del HEX
tras la exposición por inhalación.
Sólo se dispone de datos limitados sobre los efectos
de la exposición al HEX en la salud humana. Se han
producido incidentes aislados en los que el HEX provocó
fuerte irritación de los ojos, la nariz, la garganta y los
pulmones. Por lo general esa irritación fue breve, y la
recuperación se inició en cuanto cesó la exposición. No
se observaron diferencias estadísticamente significativas
en ciertas enzimas hepáticas entre grupos expuestos y
grupos testigo tras la exposición a corto plazo. Se
desconocen los efectos a largo plazo en la salud humana de
la exposición continua a bajos niveles y/o la exposición
aguda intermitente. Se ha demostrado que los manipuladores
del producto y de sus desechos, así como las personas que
trabajan en la depuración de aguas residuales o que viven
en las proximidades de los lugares de evacuación corren
riesgo debido al potencial de exposición a la sustancia
química o a los residuos de su fabricación.
La base de datos no es lo bastante amplia ni adecuada
para evaluar la carcinogenicidad del HEX. El Programa
Nacional de Toxicología de los EE. UU. ha llevado a cabo
un bioensayo de inhalación durante toda la vida en ratas y
ratones. Cuando se publique el informe patológico, se
comprenderá mejor los efectos a largo plazo de la exposi-
ción al HEX. La evaluación de la carcinogenicidad deberá
demorarse hasta que estén disponibles los resultados del
bioensayo del Programa. El Centro Internacional de Inves-
tigaciones sobre el Cáncer evaluó los datos existentes
para el HEX y clasificó la sustancia en el Grupo 3 (lo que
indica que, debido a limitaciones importantes de orden
cualitativo o cuantitativo, no puede interpretarse que los
estudios demuestren ni la existencia ni la ausencia de
efecto carcinogénico). En la bibliografía se citaron
varios estudios epidemiológicos; no se notificaron aumen-
tos de la incidencia de neoplasmas en ninguna localización
que pudieran atribuirse al HEX o sus metabolitos.
